Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/2000/13. The contractual start date was in October 2012. The final report began editorial review in October 2014 and was accepted for publication in December 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Michael Bowen reports financial support from the College of Optometrists, London, during the conduct of the study and outside the submitted work as full-time employee of the College of Optometrists. Dave Edgar reports financial support from City University London during the conduct of the study and outside the submitted work and other support from the College of Optometrists during the conduct of the study and outside the submitted work. Beverley Hancock reports financial support from the College of Optometrists outside the submitted work. Sayeed Haque reports support from the College of Optometrists during the conduct of the study and outside the submitted work, and support from the University of Birmingham during the conduct of the study and outside the submitted work. Sayeed Haque provides statistical advice to the College of Optometrists Small Grants holders under a paid consultancy agreement. Rakhee Shah reports support from The Outside Clinic during the conduct of the study and outside the submitted work. Sarah Buchanan reports support from the Thomas Pocklington Trust during the conduct of the study and outside the submitted work. Steve Iliffe reports grants from the European Commission during the conduct of the study and grants from the Thomas Pocklington Trust outside the submitted work, and other support from University College London, during the conduct of the study and outside the submitted work. James Pickett reports other support from the Alzheimer’s Society outside the submitted work and during the conduct of the study. John-Paul Taylor reports support from the Institute for Neuroscience, Newcastle University, outside the submitted work and during the conduct of the study; and support from the Northumberland, Tyne and Wear NHS Trust, Newcastle upon Tyne, outside the submitted work and during the conduct of the study. Neil O’Leary reports support from Trinity College Dublin outside the submitted work and during the conduct of the study and grant funding from The Irish Longitudinal Study on Ageing (TILDA) during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Bowen et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Dementia and sight loss
Recent estimates of the number of people in the UK with some form of dementia range from 670,0001 to 835,000. 2 Dementia prevalence increases with age, rising dramatically from 1.7% of people aged between 65 and 69 years to affect > 40% of people over the age of 95 years. 2 It is estimated that by 2021 over 1 million people in the UK will have dementia, a figure expected to rise to over 2 million by 2051,2 reflecting increased longevity. The 2011 Census report Population and Household Estimates for England and Wales3 indicated that the percentage of the population aged ≥ 65 years was 16.4%, the highest recorded in any census. There has been a notable increase in the frequency of the oldest-old, with 430,000 residents aged ≥ 90 years in 2011, compared with 340,000 in 2001 and 13,000 in 1911. 3 The costs of dementia care in the UK were estimated to be approximately £23B per year in 2010,4 and in 2014 alone dementia cost the UK economy £26B. 2
The Royal National Institute of Blind People (RNIB) estimates that almost 2 million people in the UK live with sight loss that has a significant impact on their daily lives, and predicts that this figure will rise to almost 4 million by 2050. 5 Over 50% of sight loss is avoidable; this figure includes people with sight loss which, at least in part, is caused by wearing spectacles that are not of the optimum strength. One in four people aged ≥ 75 years is living with sight loss, and in the population aged > 85 years this rises to one in three. 6 Both dementia and sight loss are increasingly prevalent with age, and the UK’s ageing demographic will result in many more people living with both dementia and sight loss.
Two-thirds of people with dementia live in private households and one-third live in some form of institutional care setting. 1 Many older people with sight loss live in care homes and there is evidence that they are subject to a disproportionally high burden of visual loss, with an estimated 30% of those who are visually impaired living in care homes. 7 The proportion of the total UK population living in care homes was estimated to be 0.55% in 2011 (approximately 350,000 people) and this proportion is likely to rise to 0.85% (600,000 people) by 2031. 7
There is increasing evidence of significant disturbances to visual function in Alzheimer’s disease and other types of dementia, which may affect different aspects of visual performance including contrast sensitivity, colour vision, spatial awareness and depth perception,8 and hallucinations. 9,10 Studies investigating sight loss and dementia have revealed shared changes in nervous system physiology and suggest that the prevalence of sight loss in people with dementia is higher than that in the general population of older people without dementia. 10 People with dementia not only suffer the general visual problems associated with ageing but also experience deficits of higher level visual processing including reading, object recognition and spatial localisation as a result of the damage to, or degeneration of, the brain,11 which can make the differential diagnosis of ‘eye problems’ from functional vision loss caused by dementia or, for example, stroke more difficult. The effects of having both serious sight loss and dementia concurrently are much more severe than those resulting from either dementia or sight loss alone. 10 Dementia alone often has a significant impact on quality of life; however, the ability of a person with dementia to cope with visual impairment (VI) is reduced, which can impact significantly on his or her activities of daily living and cognitive performance. 8
Visual impairment
A variety of definitions of VI have been used in prevalence studies. All of these definitions use criteria for VI based on levels of visual acuity (VA). VA is determined from the size of the smallest line of letters or symbols on a chart which a person can read. The definitions all express VA in terms of Snellen’s fraction (e.g. 6/12, 6/36 or 20/40), where the numerator of the fraction is the standard testing distance (6 metres in most of the world) and the denominator is a measure of the size of the letters on the line of the smallest letters that can be read. There is no standardisation regarding the VA level at which an individual becomes classified as visually impaired. 11 Definitions of the degrees of VI and blindness vary from country to country, with those set by the World Health Organization and those used in the USA and UK influencing the cut-off points for VA adopted in VI prevalence studies. 11
The World Health Organization criterion for VI is poor vision resulting from any cause including uncorrected refractive error. They differentiate between visual impairment and blindness, and their VA cut-off points for VI are:
-
visual impairment – VA < 6/18 but ≥ 3/60 binocularly with presenting correction [International Classification of Diseases, Tenth Edition (ICD-10),12 categories 1 and 2]
-
blindness – VA of < 3/60 in the better eye with presenting correction (ICD-10,12 categories 3–5) or visual field of no greater than 10 degrees in radius around central fixation.
In the USA, VI is defined as the best corrected VA < 6/12 (equivalent to < 20/40) in the better-seeing eye. 13
Since 2003, people with VI in the UK can be classified as either ‘sight impaired’ or ‘severely sight impaired’. Prior to 2003, sight-impaired and severely sight-impaired people were referred to as ‘partially sighted’ and ‘blind’, respectively. There is no legal definition of sight impaired but it is defined in common use as being ‘substantially and permanently handicapped by defective vision caused by congenital defect, illness or injury’. 14 Guidance is given as to defects of binocular VA and visual field that could lead to registration as sight impaired:15
-
3/60 to 6/60 with full field
-
up to 6/24 with a moderate restriction of the field, opacities or aphakia
-
6/18 or better with a gross field defect.
The definition of blindness (severely sight impaired) in the UK is: ‘So blind as to be unable to perform any work for which eyesight is essential’. 14 Guidance is given as to defects of binocular VA and visual field which could lead to registration as severely sight impaired:
-
acuity < 3/60
-
acuity > 3/60 but < 6/60 with significantly contracted field
-
acuity > 6/60 but with substantially contracted fields, especially inferiorly.
It should be noted that neither definition nor the associated guidance makes any reference to near vision, to an individual’s occupation or to any other disabilities they may have.
Common causes of sight loss and visual impairment
An estimated 1.87 million people live with sight loss in the UK. Approximately 360,000 are registered as sight impaired or severely sight impaired. 5,6 Age-related macular degeneration (AMD) is by far the main cause of registrable VI (sight impaired or severely sight impaired) in the adult UK population. 16 Other major causes are diabetic eye disease and glaucoma. However, 1.64 million of the people with sight loss have mild or moderate loss, much of which is correctable. 6 The primary causes of correctable visual loss are cataract and uncorrected refractive error. 7,11
Age-related macular degeneration
Age-related macular degeneration can be defined as ageing changes in the central area of the retina (the macula) occurring in people aged ≥ 55 years in the absence of any other obvious cause. 17 AMD is the leading cause of irreversible severe visual loss in high-income countries in individuals aged > 60 years. 18 It is the most common cause of adult blind registration in many high-income countries, including the UK. 16 However, those registered are an underestimate of the true prevalence of the condition because many with early AMD do not qualify for registration and some elect not to opt for registration.
There are many classifications of AMD; one classification is late AMD, geographic atrophy and neovascular AMD, which have prevalences of 4.8%, 2.6% and 2.5%, respectively, in the UK population aged ≥ 65 years. 19 An alternative classification is into two types: dry (non-exudative) or wet (exudative). Dry AMD is a slow progressive disease which accounts for 90% of cases. The wet type is much less common but can be more devastating. All useful central vision can be lost within days of wet AMD developing. As a result of recent developments in wet AMD treatment, some resultant VI can now be successfully treated. 7 Approximately 1.5 million people are living with the early stage of AMD. 20
Cataract
Cataract is loss of transparency of the crystalline lens. Age-related cataract is the most common form. 21 A classic symptom is a slow, gradual, painless progressive reduction in the quality of vision. 22 Not all cataract types are equally deleterious to vision; for example, posterior subcapsular opacities develop near the posterior pole of the lens and can have a dramatic effect on vision owing to their location. 23 Age-related cataract is the most common cause of reversible blindness worldwide. The prevalence of cataract increases with age, with an increased number of advanced cataracts in the older population. 24,25 Acquired cataracts and dementia are both common age-related problems and it is, therefore, likely that they will coexist. 26
Cataract prevalence estimates depend on the definition of when the normal ageing and opacification of the crystalline lens reaches the point at which it becomes sufficient to bring a diagnosis of cataract. Often this point is based on when the cataract causes a significant effect on some aspect(s) of visual performance, normally standard VA measurements. In a study in North London in 1998,27 significant cataract was defined as VA < 6/12, attributable to cataract, in one or both eyes. In this sample of 1547 people aged > 65 years, prevalence of cataract for the whole sample was 30%. Cataract prevalence (including aphakia or pseudophakia) increased with age from 16% (65–69 years) to 24% (70–74 years), 42% (75–79 years) and 59% (80–84 years), to 71% in people aged ≥ 85 years.
Visual impairment resulting from cataract is potentially remediable via surgical removal of the existing lens, replacing it with an intraocular implant. Cataract surgery is now the most commonly performed surgical intervention carried out on the NHS in England,28 with an increase of almost 100,000 cases per annum compared with a decade ago. In England in 2011/12, a total of 337,000 cataract operations were performed as day cases, with the mean age of those undergoing the cataract surgery being 74.4 years. 29 Referral for cataract surgery can be initiated by either the optometrist or the general practitioner (GP). Action on Cataracts30 suggested direct optometrist referral according to locally agreed protocols and there are now many such projects with audited outcomes and high conversion rates from referral to surgery.
Diabetic retinopathy
Diabetic retinopathy, a complication of diabetes, is a chronic, progressive, potentially sight-threatening disease of the retinal microvasculature. 31 Retinopathy which affects the macular region of the eye, responsible for providing optimum VA, is often separately referred to as diabetic maculopathy. There are a number of different classifications of diabetic retinopathy, often with overlap between the different classifications. All refer to the two basic mechanisms which can lead to visual loss: that is, the risk of new vessel growth in retinopathy and the risk of damage to the central part of the macula, known as the fovea. 31 If abnormal new vessels are present, the retinopathy is described as proliferative retinopathy, while in the absence of new vessels it is known as non-proliferative or background retinopathy.
A population screening study in Liverpool reported the prevalence of diabetic retinopathy to be 25.3% in type 1 diabetes and 45.7% in type 2 diabetes. 32 In 2010, an estimated 748,000 people were living with background diabetic retinopathy and 85,000 would be classified as falling into non-proliferative and proliferative diabetic retinopathy combined (the more advanced stages of background diabetic retinopathy). 20 Diabetic maculopathy, which can lead to sight loss more rapidly, was expected to be present in 188,000 people in 2010. 20 When studies are stratified for duration of eye disease and age, there is an increase in diabetic retinopathy in older people and in those with long-standing disease. 33
There is clear evidence that sight-threatening diabetic retinopathy has a recognisable latent or early symptomatic stage. 32 Regular monitoring and vigilant treatment can help to prevent the disease progression which can lead to blindness. All those with diabetes, either type 1 or type 2, are offered an annual appointment for screening for diabetic retinopathy as part of the NHS diabetic eye screening programme. The screening can be carried out in hospital, GP or optometric practice locations and involves taking a fundus photograph which is subsequently graded. 33
Glaucoma
Glaucoma, or, more correctly, the glaucomas, is a group of eye diseases that have in common progressive structural damage to the optic nerve head, resulting in a characteristic glaucomatous optic neuropathy, with functional loss of the corresponding visual field and which can lead to blindness if left untreated. Glaucoma is second to AMD as the most common cause of severe sight impairment (blindness) in adults in the UK and Ireland. 15,16,34,35 Primary open-angle glaucoma (POAG) is a chronic, generally bilateral, although asymmetrical, disease, characterised by progressive damage of the optic nerve shown by glaucomatous changes affecting the optic disc, the retinal nerve fibre layer and/or the visual field. 36 POAG accounts for 75–95% of glaucoma among people from white ethnic groups37 and is the most common form of glaucoma in the UK. The prevalence of POAG in the UK population aged > 40 years is estimated to be 2.0%. Prevalence rises steeply with age, from 0.3% at 40 years of age to 3.2% at 70 years. 38 Based on a Bayesian meta-analysis, the pooled prevalence of open-angle glaucoma was estimated to be 6% in white populations, 16% in black populations and 3% in Asian populations. 39
In 2010, there were estimated to be 266,000 people living with detected glaucoma20 and an additional 191,000 people with undetected glaucoma in the UK. 29 A pooled prevalence analysis estimated the number with undetected disease to be as high as 380,000. 40 A further 513,000 people were estimated to have ocular hypertension (OHT), which is elevated intraocular pressure (IOP) in the eye without any other signs of glaucoma but which increases the risk of subsequently developing glaucoma. 20 The prevalence of OHT in those aged > 50 years has been estimated to be between 3.7% and 7.6%. 41
UK community optometrists provide the majority of primary eye care and are responsible for approximately 95% of referrals for suspected glaucoma to secondary care. 42 Late presentation with advanced disease is a risk factor for blindness from glaucoma. 43 Late detection may result from patients not engaging with community eye care, from a failure of health professionals to identify the disease at an early stage or from unusually rapid disease progression. The VI caused by glaucoma is irreversible but the disease is treatable, although successful management is more difficult with late-stage presentation. 36
Uncorrected or undercorrected refractive error
The term refractive error refers to errors in the optical performance of the eye resulting in a distorted or defocused image on the retina. Refractive error is usually not constant throughout life. One reason for recommending that people have regular optometric sight tests is to identify and correct those changes in refractive error that occur over time. People may not be wearing the required lens powers in their spectacles to fully correct their current refractive error. This can occur for various reasons, including people leaving too long an interval between sight tests, people declining to have the recommended prescription for their refractive error made up into spectacles or failing to wear the correct pair of spectacles. People who are not wearing the appropriate strength of lenses to correct their refractive error are referred to as having undercorrected or uncorrected refractive errors. These can be resolved using appropriate spectacles following an eye examination by an eye health professional. The prevalence of undercorrected or uncorrected refractive errors in the UK older population has been reported to be between 9%27 and 40%44 for VI defined as VA < 6/12. However, this 40% figure was obtained from a small, enriched sample of patients admitted to a department of geriatric medicine with acute illness and was unrepresentative of older people in general. The most recent of these studies was published in 1998 and there is a need for updated estimates of undercorrected or uncorrected refractive error to reflect changes in clinical practice, in health and social care policy, and in the life-course of individuals in the population.
Summary of common causes
A review of studies into correctable VI in older people estimated that 20–50% of older people have undetected reduced vision. 11 This wide range of estimates reflects, in part, the different criteria and cut-off points used in studies to define VI. 45 The majority of people with undetected reduced vision in these studies had reduced vision resulting from undercorrected (or uncorrected) refractive error and cataract, and hence their visual loss is potentially correctable following an optometric sight test by the provision and use of a pair of spectacles or contact lenses of the appropriate prescription, or cataract extraction, respectively. 11
Dementia and causes of sight loss develop independently. As people age, they are at an increased risk of developing dementia and having serious sight loss, and hence there will inevitably be people with both conditions. 10
The Prevalence of Visual Impairment in Dementia (PrOVIDe) study limited its scope to the five conditions listed above, as these are the most common eye-related conditions associated with sight loss. Many other eye conditions and non-ocular conditions lead to significant sight loss, notably stroke, head injuries and other neurological conditions. These conditions, particularly those more common in older age, can also make significant contributions to visual dysfunction. However, these conditions fall outside the remit of the current study.
Provision of eye-care services in the UK
The vast majority of UK optometrists work in primary care community optical practices, hospitals or domiciliary settings. Most optometrists work in community optical practices, from where they are the major providers of primary eye-care services. Optometrists are trained to perform sight tests, which include refraction and detection of signs of injury, disease or abnormality in the eye.
Secondary eye care is delivered by the Hospital Eye Service (HES). Secondary eye care is usually provided by a team of eye-care professionals including ophthalmologists, optometrists and orthoptists. There is increasing integration between primary and secondary eye care, with primary care optometrists involved in enhanced schemes to provide a range of services within a community-based setting, often in conjunction with GPs and ophthalmologists, in order to case-find and monitor eye conditions. 46–49
Provision of General Ophthalmic Services and NHS eye examinations in the UK
The vast majority of primary care optometrists have a contract with the NHS via local Clinical Commissioning Groups to provide sight tests to eligible persons. The provision of General Ophthalmic Services (GOS) was largely uniform across the UK until the introduction of devolved powers to Wales and Scotland. This, together with NHS restructuring, created a more diverse provision, with the emergence of a less rigid approach to the provision of primary eye care in some parts of the UK. Examples that illustrate these changes are the Welsh Eye Care Initiative, introduced in 2003, which has evolved into the Eye Health Examination Wales, and the new GOS contract in Scotland, which commenced in 2006. The PrOVIDe study is limited to England, where change to the GOS provision has been more limited.
The GOS regulations require practitioners to be satisfied that a NHS sight test is clinically necessary. 47 In general, people aged between 16 and 70 years at the time of the sight test will not normally have their sight tested under the GOS more frequently than every 2 years. Similarly, people aged ≥ 70 years will not normally have a sight test more frequently than every 12 months. However, under certain circumstances the practitioner can carry out a sight test at a shorter interval than stated above ‘either at the practitioner’s initiative for a clinical reason, or because the patient presents him/herself to the practitioner with symptoms or concerns which might be related to an eye condition’. 50 These recommended minimum sight test intervals are reduced for certain patients; for example, for a diabetic patient of any age the minimum interval between sight tests is reduced to 1 year. 50
In 2012/13, there were 12.3 million NHS-funded sight tests in England,51 with 5.5 million (44.4%) of these carried out for patients aged ≥ 60 years. 51 Private sight tests are an option and an estimated 5.6 million of these were carried out in 2011/12. 52 A section of a survey by the RNIB of people aged > 60 years asked how regularly they had sight tests; 53% reported having annual sight tests, 35% reported having a sight test every 2 years and 11% reported having sight tests less frequently. 53 It has been suggested that the uptake of eye examinations among people with dementia is considerably lower than that in the population without dementia of a similar age. 8,54 A telephone survey by Shah et al. 55 found that 93% of optometrists stated willingness to examine people with dementia, but evidence of the uptake and quality of vision care in this group of the population is lacking.
Provision of domiciliary sight tests
The majority of NHS sight tests are conducted on ophthalmic practitioners’ premises. In 2012/13, 3.2% of NHS tests were domiciliary examinations, conducted in the individual’s place of residence or at a day centre. 51 Anyone eligible for a NHS sight test qualifies for a domiciliary sight test if they are unable to attend a high-street practice unaccompanied because of physical or mental illness or disability. 50 The number of domiciliary sight tests has risen steadily since 2002/3, and the 2012/13 total of 407,000 was an increase of almost 60% compared with a decade earlier. This increase could be attributed to the ageing UK population, with more older people seeking eye examinations in their own home, although there is no evidence to directly support this. 51
Prevalence of visual impairment in people with dementia
Literature searches for the prevalence of VI and eye disease in the elderly, and for the prevalence of VI in people with dementia, revealed a lack of good-quality prevalence data on the topics.
Search strategy
A PubMed search for prevalence of VI and eye disease in the elderly was conducted using the following search strategy: (elderly[tiab] OR “aged”[MeSH Terms] OR geriatric[tiab] OR older) AND (“eye diseases” OR “eye disease” OR “eye diseases” OR “visual acuity” OR “vision disorder” OR “vision disorders” OR “visual impairment” OR “refractive error” OR “refractive errors” OR glaucoma OR presbyopia OR myopia OR astigmatic OR astigmatism OR cataract OR cataracts OR “retinal diseases”[MeSH Terms] OR “retinal diseases”[All Fields] OR retinopathy OR retinopathies OR “diabetic eye disease” OR “macular degeneration” OR “low vision”) AND ((“epidemiology”[Subheading] OR “prevalence”[All Fields] OR “prevalence”[MeSH Terms]) OR epidemiology[tiab]).
The search was initially carried out in 2011, prior to submission of a formal proposal to the National Institute for Health Research (NIHR), and identified 9035 papers. It has been updated regularly, most recently in April 2014 when 11,104 papers were identified. These studies vary as to whether the study deals with a single eye disease, or several; whether it only addresses eye disease prevalence or includes it in a study of several pathologies. Only a small number of papers found via this search, and from reference list searches of review articles and reports, specifically address the prevalence of VI in a large sample of elderly people in the UK, although a wider body of international prevalence data exists.
A search of PubMed was conducted for papers dealing with the prevalence of eye disease and/or VI in people with dementia using the following terms: (dementia OR alzheimer’s[tiab]) AND (“eye diseases” OR “eye disease” OR “eye diseases”[MeSH Terms] OR “visual acuity” OR “vision disorder” OR “vision disorders” OR “visual impairment” OR “refractive error” OR “refractive errors” OR glaucoma OR presbyopia OR myopia OR astigmatic OR astigmatism OR cataract OR cataracts OR retinopathy OR “retinal disease” OR “diabetic eye disease” OR “macular degeneration” OR “low vision”) AND (“epidemiology”[Subheading] OR epidemiology[tiab] OR prevalence[tiab] OR prevalence[MeSH Terms]). The search retrieved 295 papers in April 2014. Approximately 10 were prevalence studies and none of these were UK papers.
Review of population-based UK studies into prevalence of visual impairment in older people
Table 1 summarises the key features of the most relevant studies. Although the studies are few in number, comparisons between them are made difficult by the variations in population and methods used. The cut-off point in terms of VA employed to define VI was ‘worse than 6/12’ in some studies and ‘worse than 6/18’ in others, with prevalences for both criteria reported in some studies. There were also variations in the methods used for recording VA; presenting VA was always measured but sometimes binocularly only, sometimes monocularly (with best monocular VA recorded as presenting acuity), and sometimes both monocularly and binocularly. Nor was it always used in the prevalence estimates. 59 Older studies used Snellen VA charts,44,58 while more recent studies have used logarithm of minimum angle of resolution (logMAR) charts of various types. Testing distances varied between the standard 6-m distance and 3 m. Settings varied from broadly national56,57 to local. 27,44,58 Sample sizes ranged from > 14,00056 to around 200. 44,58 The sample ages were generally ≥ 65 years, although the Medical Research Council (MRC) study investigated a sample aged ≥ 75 years. 56
| Survey (year), reference | Location (sample size); age range | Method of recording VA | Sample size for VA testing | VA cut-off point defining VI | Prevalence (%) aged ≥ 65 years (95% CI) | Prevalence (%) aged ≥ 75 years (95% CI) |
|---|---|---|---|---|---|---|
| Study 1: MRC trial (1995–8), Evans et al., 200256 | Great Britain (n = 15,126); aged ≥ 75 years | Presenting binocular VA | 14,600 | < 6/12 | N/A | 19.9 (17.8 to 22.0) |
| < 6/18 | 12.4 (10.8 to 13.9) | |||||
| Pinhole corrected | < 6/18 | 10.2a | ||||
| Study 2: Jack et al., 199544 | Liverpool, UK (n = 200); aged ≥ 65 years | Presenting binocular VA (Snellen) | 200 | < 6/12 | 50.5a | N/A |
| Study 3: North London Study (1995–6), Reidy et al., 199827 | North London, UK (n = 1547); aged ≥ 65 years | Presenting monocular VA. Pinhole-corrected VA (logMAR) | 1547 | < 6/12 | 30.2 (24.8 to 35.5) | N/A |
| Study 4: NDNS (1994–5), van der Pols et al., 200057 | Mainland, UK (n = 2060); aged ≥ 65 years | Best monocular VA, with or without a pinhole | 1362 (not cognitively impaired) | < 6/12 | 28.3a | 39.3a |
| 1362 (not cognitively impaired) | < 6/18 | 14.3a | 21.0a | |||
| 125 (cognitively impaired) | < 6/18 | 64.8a | N/A | |||
| Study 5: Wormald et al., 199258 | London, socially deprived area, UK (n = 207); aged ≥ 65 years | Presenting binocular VA (Snellen) and monocular VA | 207 | < 6/12 | 14.5 (10.3 to 20.0) | 26.4 (18.9 to 35.6) |
| 207 | < 6/18 | 7.7 (4.5 to 12.2) | 14.2 (7.5 to 20.8) | |||
| Study 6: Melton Mowbray, Lavery et al., 198859 | Melton Mowbray, UK (n = 677); aged > 75 years | Best monocular VA (Snellen) | 529 Post-refraction data |
< 6/12 | N/A | 26.2a |
Some people with dementia will have been included in the sample for most studies, as cognitive impairment was not an exclusion criterion. However, only two studies assessed cognitive impairment: Jack et al. 44 specifically excluded those with severe cognitive impairment, while the National Diet and Nutrition Survey (NDNS)’s study analysed those with cognitive impairment separately. 57 Only two studies separately analysed data from a subgroup of participants who lived in care homes. 44,57
Study 1
The MRC study was a large cluster randomised trial taking place in 106 general practices with participants aged ≥ 75 years. VA data were obtained from 14,403 participants and VA was measured using the logMAR Glasgow acuity cards at 3 m. 56 Presenting VA was measured first binocularly and then monocularly. Where presenting VA was worse than 0.5 (equivalent to < 6/18) in either eye, VA was remeasured with a pinhole disc. The pinhole disc is an opaque disc into which a small hole (the pinhole) has been drilled. The effect of the pinhole is to reduce the effective pupil size of the participant. If the participant’s loss of vision is the result of an out-of-focus image on the retina (which should be correctable with the appropriate prescription in the participant’s spectacles), the pinhole can improve the participant’s vision by reducing the size of the blur circles on the retina. All participants with pinhole VA of worse than 0.5 were referred to an ophthalmologist. VA of the better eye was used if binocular VA was not available.
-
Using the criterion of binocular presenting VA < 6/18, 12.4% [95% confidence interval (CI) 10.8% to 13.9%] of the sample had VI.
-
With a pinhole, the prevalence of VI in the better eye was 10.2% using the ‘worse than 6/18’ criterion. The pinhole could only be used successfully on 62% of those with presenting VA < 6/18 in either eye.
-
Using the criterion of binocular presenting VA < 6/12, 19.9% (95% CI 17.8% to 22.0%) of the sample had VI.
-
The prevalence of VI was higher in women and rose steeply with age.
-
The main causes of VI were extracted from GP notes or hospital records. 45
-
When refractive error is excluded from the list, the main causes become AMD (52.9%), cataract (35.9%), glaucoma (11.6%), diabetic eye disease (3.4%) and myopic degeneration (4.2%).
Comment
-
This study had a large sample size covering many areas of Britain and was representative of the UK in terms of mortality and deprivation.
-
No refraction was carried out.
-
Visual acuity was recorded by trained nurses and may not be as accurately recorded as would be the case with optometrists, ophthalmic nurses or ophthalmologists.
Study 2
Jack et al. 44 conducted a prospective study of 200 consecutive mentally competent patients aged ≥ 65 years (mean age 80 years) admitted to hospital with an acute illness. Binocular VA was measured with a Snellen chart at 6 m. Patients with VA of ≤ 6/18 had a full ophthalmic assessment from an ophthalmologist, and a decision was made as to the main cause of VI if more than one potential cause was found.
-
Using the criterion of binocular presenting VA < 6/12, 50.5% of the sample had VI.
-
Of those with VI, 40% were caused by refractive errors and 37% by cataract; a total of 79% were considered as being due to reversible causes.
-
A total of 76% of those admitted to the hospital following a fall had VI.
Comment
-
The last line read correctly on the Snellen chart was taken as the participant’s VA. This was common practice in those studies reported here which used Snellen charts and is a weakness of such studies. A participant able to read most of the letters on a line will not have those successes counted towards their overall VA, which will be determined by a line of larger letters which was read correctly in its entirety. With modern logMAR charts, not available for these early studies, every letter read correctly by the participant counts towards the VA recorded, even if these letters are from a line on the chart that was not read correctly in its entirety. Choosing the last line read correctly on a Snellen chart will tend to underestimate VA and overestimate VI.
-
Patients with severe cognitive impairment were excluded from the study. The sample was an enriched one comprising a selective population of patients admitted to a department of geriatric medicine with an acute illness.
-
The eye examination by the ophthalmologist was carried out only on those with VI. A full refraction was performed on a proportion of those with VI but the number is not stated.
-
An unstated but small number of participants lived in care homes. No effect of location was found when comparing prevalence of VI in participants living in care homes and participants living in their own homes.
Study 3
Reidy et al. 27 conducted a cross-sectional survey using two-stage cluster random sampling in north London. Participants were recruited from general practices and 1547 were examined. Monocular VA was recorded with a logMAR chart at 6 m and was repeated with a pinhole. An autorefractor was used to determine refractive error. VA, autorefraction and visual fields were assessed by trained ophthalmic nurses and a comprehensive eye examination was performed by ophthalmologists.
-
With a criterion of bilateral presenting VA of < 6/12, 30.2% (95% CI 24.8% to 35.5%) of the sample had VI.
-
A total of 72% of those with VI as defined above were classed as potentially remediable.
-
The population prevalence of refractive error causing VI in one or both eyes was 9% (95% CI 7.0% to 11.4%).
-
The age-standardised prevalence of poor vision was significantly higher in residents of the most underprivileged areas.
-
The prevalence of cataract causing VI in one or both eyes was 30% (95% CI 25.1% to 35.3%).
-
The prevalence of open-angle glaucoma and suspected glaucoma was 3% (95% CI 2.3% to 3.6%) and 7% (95% CI 5.4% to 8.4%), respectively.
-
Reasons given for the high level of undetected and untreated morbidity in the population included low levels of attendance at the primary care optometrists or failure to purchase corrective spectacles; suboptimal integration of vision checks into the general primary care of older people; and people’s perceptions of the extent to which their vision has gradually diminished and the point at which help should be sought.
Comment
-
The examination of each participant by an ophthalmologist is a strength of this study.
-
Unusually, the proportion of VI reported was bilateral VI, rather than the more common binocular VI or VI based on the acuity of the better eye.
-
Results on socioeconomic background of the sample were described as ‘tentative’, but the association between the degree of underprivilege and cataract and uncorrected/undercorrected refractive error is informative.
-
A potential strength of the study is the use of an autorefractor to determine refractive error. However, it is unclear how the autorefractor results were used. There is no indication that the current/habitual spectacle correction was measured using a focimeter, so the autorefractor findings could not be used to estimate the change in refraction from the current spectacles. Nor does the VA appear to have been recorded with the participant wearing the autorefractor result. Instead, it seems likely that the pinhole VA recorded monocularly was used to identify VI resulting from uncorrected/undercorrected refractive error.
Study 4
A randomised cross-sectional survey of people living in their own homes or in care homes was carried out as part of the NDNS. 57 Participants were recruited from 80 randomly selected postcode areas of mainland Britain. Acuity measurements were made by a trained nurse in the participant’s place of residence. VA was measured in 1487 participants (1362 not cognitively impaired and 125 cognitively impaired) who received a nurse visit. LogMAR monocular distance VA was measured using the Glasgow acuity card test at 3 m, without any spectacle correction, and repeated with a pinhole. Monocular VAs were then recorded again, both with and without a pinhole, but with the participant now wearing any distance spectacles.
-
With a criterion of best monocular VA of < 6/12, 28.3% of the sample had VI.
-
With a criterion of best monocular VA of < 6/18, 14.3% of the sample had VI.
-
Visual impairment showed a strong positive linear trend with age.
-
Visual impairment was more common in participants living in care homes, with an age-adjusted odds ratio (OR) of 2.59 (95% CI 2.23 to 2.96).
-
Visual impairment was more common in women, with an age-adjusted OR of 1.55 (95% CI 1.21 to 1.89).
-
Of the cognitively impaired participants, 64.8% had VI when the ‘VA worse than 6/18 in the better eye’ criterion was applied.
-
Visual acuity improved by 0.2 logMAR units with a pinhole in 21.2% of participants in the non-cognitively impaired group.
Comment
-
A short memory questionnaire was used to identify participants with cognitive impairment to identify those potential participants for whom proxy consent was needed. There is no indication that any subjects were excluded on the basis of the assessment.
-
This was a national survey which is unusual in including both participants living in their own homes and those living in care homes.
-
Visual acuity measurements were recorded by trained nurses in participants’ homes rather than by optometrists, ophthalmic nurses or ophthalmologists. Standardisation of lighting conditions was not possible and variations would affect the accuracy of results.
-
No refraction was carried out and no causes of VI, apart from those where uncorrected/undercorrected refractive error was suspected, were identified.
-
This is the only study reviewed to have included an analysis of people with cognitive impairment as a subpopulation of the sample.
-
The paper states that ‘The highest Glasgow Acuity Card score from any of the measurements in the better eye is defined here as the best visual acuity’. 57 These measurements include the pinhole VA.
Study 5
A 1992 cross-sectional random sample survey by Wormald et al. 58 recruited 207 participants aged ≥ 65 years from an inner-London general practice. Binocular VA was recorded using a Snellen chart at 6 m, and monocular VA was recorded using a Sonksen Silver chart at 3 m, with a pinhole if 6/9 was not achieved. An improvement of greater than one line in VA monocularly or binocularly was taken as evidence of potential benefit from refraction. The cause of VI was identified by a consultant ophthalmologist, and where participants had more than one possible cause for their visual loss, a clinical decision was made as to the main cause.
-
Using a criterion of presenting VA of < 6/18 in either eye, 7.7% (95% CI 4.5% to 12.2%) of the sample had low vision.
-
A total of 27% of subjects would have benefited from refraction, based on the pinhole results.
-
Cataract was responsible for 63% of VI based on better eye VA of < 6/12 and excluding uncorrected/undercorrected refractive error.
-
A small number (n = 17) of examinations were conducted in participants’ own homes, and 41% of these participants were found to be visually impaired.
-
The prevalence of clinically obvious predisposing changes at the macula associated with visual loss in at least one eye was 25%.
Comment
-
The last line read correctly on the Snellen chart was taken as the VA. No refraction was carried out, with best corrected VA estimated with a pinhole.
-
With a criterion of best presenting acuity of < 6/12, 14.5% (95% CI 10.3% to 20.0%) were calculated to have VI.
-
One strength of the study is the assessment of each participant by an ophthalmologist and the identification of the cause of VI.
-
The high prevalence of cataract will reflect the much lower numbers of cataract surgery procedures carried out in the early 1990s than today.
-
This study is one of the few to record near VA; failure to achieve N6 in a subject with good distance vision served as an indication of the need for a new prescription for reading spectacles.
Study 6
This early 1980s study of residents of Melton Mowbray59 aged > 75 years comprised 529 participants. Monocular VA was recorded with existing spectacles (if any) and unaided vision in each eye was recorded for all subjects using a Snellen chart at 6 m. Best monocular VA was recorded following a full refraction by an optometrist. Subjects also received an ophthalmological examination.
-
Using the criterion of post-refraction best monocular acuity of < 6/12, 26.2% had VI.
-
Of the sample, 11.2% were unable to read N8 post refraction.
-
Of the sample, 10.4% were examined at home or in hospital.
Comment
-
The last line read correctly on the Snellen chart was taken as the VA.
-
A notable strength of the study is that all of the VA measurements and the determination of refractive error were undertaken by an optometrist.
-
Although both the VA with any current spectacles and the unaided vision were recorded on presentation, only unaided vision data are stated in the paper. Neither the proportion of participants presenting with VI nor the undercorrected element of VI were stated.
-
Another strength of the study is the recording of near VA (NVA) with what was presumably the post-refraction correction.
The studies reviewed above are 15–40 years old and reveal inconclusive data on prevalence, largely owing to methodological differences and definitions of VI. However, it is clear that many cases of VI in older people can be either prevented or successfully treated. Evans and Rowlands11 concluded that there is overwhelming evidence that a very large proportion of older people do not receive appropriate eye care and many of these people could be helped by cataract surgery or appropriate refractive correction (spectacles). Improvement in VA is not the only benefit from refractive correction, as behavioural and psychological problems in people with dementia are reduced when VI is prevented or corrected, with a corresponding positive impact on quality of life. 60 Data from older people with dementia have rarely been analysed separately in population studies of VI. The exception is the NDNS, in which 64.8% of the cognitively impaired participants had VI using the ‘VA worse than 6/18 in the better eye’ criterion. 57
A meta-analysis of the prevalence and causes of blindness and low vision in the UK highlighted the urgent need for improved UK epidemiological data, particularly for visual loss among the non-community-dwelling older population. 7 Care home residents were excluded from one VI population study;56 in others, they were possibly included but not separately analysed,27,58,59 or made up a very small proportion of an already small sample. 44 Only the NDNS study included a large care home residency subsample (23.8%), and these residents were more likely to have VI, with an OR of 2.59 when compared with people living in the community. 57
Review of population-based international studies into prevalence of visual impairment in older people
Methodological and sample differences between UK and international population studies, plus health-care variations between countries, make comparisons between studies difficult. A methodological strength of a number of international population studies is the measurement of best corrected VA, obtained after a subjective refraction. 61–64 For presenting VI using the criterion of best VA of < 6/12, prevalences vary from 6.9% in the SEE study of those aged 65–84 years (binocular VA),63 to 7.3%, calculated from the Melbourne study for those aged ≥ 60 years (best monocular VA),61 and to 11.7% in the Baltimore Eye Survey of those aged ≥ 40 years (best monocular VA). 65 For VI defined by best monocular presenting VA of < 6/18, the prevalence calculated from the Melbourne study data was 2.7%. 61 All of these prevalences are lower than those from UK studies, with the younger age of the international cohorts likely to be a major contributory factor.
For best-corrected VI for VA of < 6/12, the prevalences calculated for those aged ≥ 60 years from the two Australian studies (both best monocular VA) were 2.7% for Melbourne61 and 6.5% for Blue Mountains,62 with 5.8% reported in the Rotterdam study of those aged ≥ 55 years. 64 The only UK study using comparable methodology was Melton Mowbray, in which a prevalence of 26.2% was obtained from their sample aged > 75 years. 59 For VA of < 6/18, the best corrected VI prevalences were 1.9% for Melbourne61 and 2.5% for Rotterdam. 64
International studies of people living in care homes have reported high prevalences of VI. In Alabama, USA, the presenting VI based on binocular VA of < 6/12 was 57% in a sample in which those with Mini-Mental State Examination (MMSE) scores of < 13 were excluded. 66 In another US study which, like PrOVIDe, included people with all levels of cognitive impairment, presenting VI (best monocular VA of < 6/12) was found in 38% of participants, with best corrected VI of 29%. The sample included 40% who had severe cognitive impairment (MMSE score of 0–9) and 70% who had MMSE score of ≤ 18. The dearth of UK VI data from care homes has been highlighted. 7
Quality of life and visual impairment
Visual impairment, especially in older people, can lead to functional impairment which may adversely affect quality of life. 67 In a ranking of common chronic conditions, which can affect the ability of older people to perform essential tasks,68 VI was ranked third, behind arthritis and heart disease. A number of instruments have been used to measure quality of life. Some assess visual function status (e.g. Visual Function Index and National Eye Institute Visual Function Questionnaire), some are vision-specific quality-of-life questionnaires (e.g. Low Vision Quality of Life Questionnaire) and some use generic health-related quality-of-life instruments (e.g. EuroQol Questionnaire). 69 Measurements of VA and other measures of visual performance, such as contrast sensitivity, are highly correlated with activities of daily living. 9
Performance on various tasks of daily living was assessed in a study of > 2500 participants aged between 65 and 84 years, which excluded those with Standardised Mini-Mental State Examination (sMMSE) scores of < 18 (i.e. those with severe or moderate cognitive impairment). 70 For mobility tasks, at least 50% of participants were disabled [using a cut-off point for disability of one standard deviation (SD) below the population mean performance] if their VA was worse than logMAR 1.0 (6/60). However, for the more demanding visual task of face recognition, disability occurred when VA was < 0.3 (6/12). 70 Interestingly, > 90% of those with a VA of < 0.3 (6/12) were disabled for the visually demanding task of reading at 90 words per minute.
A nested trial within the national MRC Elderly Trial collected data using the 25-item National Eye Institute Visual Function Questionnaire instrument from 1745 participants aged > 75 years. For the analysis, VI was defined in two ways: either presenting VA in the better eye of < 6/12 or VA in the better eye of < 6/18. There was a strong association between VI, using either definition, and reported difficulties with general vision, near activities and social functioning. 45 Although there was a strong association between VA and questionnaire scores, VA accounted for only 20% of a combined score derived from each of the subscales of vision function. The authors note that psychological factors, such as having an optimistic or pessimistic outlook on life or adopting coping strategies to help counteract the impact of VI, may impact on the way people with equivalent levels of sight loss report their general level of visual function. Evidence supporting this view came from the 21% of those with VA of < 6/18 who reported having no problems with their general vision. This percentage was lower for specific activities such as near activities, for which only 11% of those with VA of < 6/18 reported having no problems.
A 2012 RNIB-funded UK study conducted by NatCen Social Research (McManus and Lord71) analysed many aspects of the circumstances of adults with sight loss, using data from two Britain-wide surveys: the Life Opportunities Survey and the Understanding Society Survey. Comparisons were made between participants with self-reported sight loss and the remainder of the population after controlling for differences in age and sex. Notably, 31% of participants with sight loss reported being dissatisfied with life overall, compared with 10% of those without sight loss. A greater proportion of participants with sight loss (94%) experienced some kind of restriction to their ability to take part in society than those without sight loss (83%). Respondents with sight loss were more likely to have experienced difficulty accessing health services (33% of the subsample) than those without sight loss (18%).
Mental well-being
Sight loss is frequently associated with negative feelings including frustration, anger and feeling low. These feelings are part of the normal grieving process, with grief in this context being for loss of sight. Studies in the USA and Canada estimate the prevalence of depression in people attending low-vision clinics with AMD, the leading cause of registrable VI in the older UK population, to be approximately 30%. 60,72,73 A typical study examined 151 adults aged > 60 years (mean age 80 years) with VI living in the community. The definition used for VI was presenting better eye VA of ≤ 6/18, together with VA of ≤ 6/30 in the other eye. The proportion of participants with depression, as determined by standard criteria using a validated instrument for diagnosis of depression (Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition74 Disorders), was 32.5%, which is approximately twice the prevalence of depression in the general population using similar diagnostic methods. 73 The authors noted that the prevalence of depression in their sample of people with VI was similar to prevalences of depression found in patients with life-threatening diseases, such as cancer.
In the UK, the MRC trial of the assessment and management of older people in the community collected data on depression and anxiety, in addition to measuring VA in order to identify participants with VI. 56 The instruments used to assess depression and anxiety were the Geriatric Depression Scale and the anxiety subscale of the General Health Questionnaire, respectively. VI was defined as presenting binocular VA of < 6/18 and depression by a GDS score of ≥ 6. Participants with VI had slightly raised levels of anxiety compared with participants who were not visually impaired when only age and sex were controlled. Control of other confounders removed any association between anxiety and VI. The prevalence of depression in the VI subgroup was 13.5% and was statistically significantly greater than in those without VI (4.6%; p < 0.001). 75 This 13.5% prevalence of depression was lower than that found in other studies, but the MRC trial had a sample drawn from the general population rather than from low-vision and outpatient clinics. The OR, following adjustment for age and sex, for depression with VI was 2.69 (95% CI 2.03 to 3.56), which reduced to 1.26 (95% CI 0.94 to 1.70) when other confounders, notably activities of daily living, were controlled. Although VI is one factor contributing to depression in people with sight loss, depression can be attributed to other factors, notably impairment to functional activities of daily living. It was suggested that VI leads to difficulties with functional activities, which can then result in depression. 75
Another UK study investigated the visual and psychosocial factors, including depression and adjustment to sight loss, which influenced participants with VI who had self-reported difficulties with a range of visual activities. 76 The instruments used to assess the participants’ depression and the level of adjustment to their sight loss were the Geriatric Depression Scale and the 19-item Acceptance and Self-Worth Adjustment Scale. The 100 participants with VI had an average age of 81 years. Visual parameters, including distance VA, NVA and contrast sensitivity, accounted for 28–50% of the variance in self-reported limitations in visual activities. However, depression and levels of adjustment were also statistically significant contributors to limitations in activities, with depression notably accounting for 17% of the variance in self-reported mobility function.
There is evidence that interventions to enhance visual performance can contribute to a reduction in depression symptoms. A sample of 95 adults with a mean age of 77 years (range 65–89 years) with VI were assessed before and after a rehabilitation package, which included provision of low-vision services and optical aids. After controlling for other factors, the rehabilitation package accounted for 10% of the variance in depression, with both the low-vision clinical service and the optical aids remaining statistically significant contributors60 to the reduction in depression symptoms over time.
The 2012 RNIB NatCen study compared many aspects of well-being in those with and those without sight loss, after controlling for age and sex differences. 71 Participants with sight loss (14%) were more likely than those without sight loss (2%) to have been feeling unhappy or depressed a lot more than usual. A loss of self-confidence, that occurred a lot more often than usual, was more prevalent in those with sight loss (11%) than in those without (1%). There was a fourfold difference in the proportion of participants reporting dissatisfaction in their health between those with sight loss (57%) and those without sight loss (14%). Participants with sight loss were approximately twice as likely to have fewer than three people to whom they felt close (15% compared with 8%). An overall well-being index was calculated using the Short Warwick–Edinburgh Mental Well-being Scale, which gave a score of between 7 and 35 for each individual, where the higher the score the better the individual’s well-being. Participants with sight loss had a lower mean score (22.70) than those without sight loss (25.86).
The National Institute for Health and Care Excellence (NICE) guideline on depression in adults with a chronic physical health problem recommends that primary care practitioners, who presumably include optometrists, should screen high-risk groups for depression and initiate a referral to the GP for those who screen positive for depression. 77 The association between VI and depression is complex, with the VI contributing to the depression and the depression contributing to the functional disability associated with the VI. 72 There is widespread agreement that a holistic approach involving collaborative care should be adopted for patients with moderate to severe depression who also have a chronic physical health problem such as VI. 56,72,77
Falls
The National Institute for Health and Care Excellence reports that 30% of people aged > 65 years and 50% of people aged > 80 years fall at least once per year. Falls are estimated to cost the NHS more than £2.3B per year. 78 Older people living in care homes or in sheltered housing are more likely to fall than those living in their own homes. 79,80 This increased risk results in part from care home residents often being frailer and more likely to have more of the other fall-related risk factors than those living in their own homes. 81 In a study of > 9000 participants, those with a cognitive impairment had an OR for falls of 2.3 when compared with those with no cognitive impairment. 82
There is considerable evidence that VI, measured in various ways and defined using acuity cut-off points for VI of < 6/12 or < 6/18, is a significant and independent risk factor for falls, with an OR of approximately 2.5. 44,81,83,84 This reflects the significant input provided by vision, both central and peripheral, to balance control. 85,86 Balance when standing was significantly impaired in AMD compared with controls when a mental arithmetic secondary task was introduced. 86 The visual system also provides vital information regarding the location and size of hazards that may lie in the path of people as they ambulate. 80 In a UK cross-sectional study of patients aged ≥ 65 years who had undergone hip fracture surgery, those with presenting VI (binocular VA of < 6/18) were compared with the non-VI group. Significantly more of the participants with VI had fallen in the previous 5 years. Participants with VI were also less likely to have had an optometric eye examination in the 3 years prior to their fall and less likely to have been wearing their glasses at the time of the fall. 87
Although NICE guideline 161 states that vision assessment and referral has been a component of successful multifactorial falls prevention programmes, NICE found no evidence that referral for correction of vision as a single intervention for older people living in the community is effective in reducing the number of people falling. 78 Elliott81 notes that this conclusion was based largely on the results of two randomised controlled trials, and identifies several possible sources of bias, including non-representative participants and potential bias introduced by the trials not being double-blind, both of which could have affected the results of these and other clinical trials.
There are many possible causes of falls, some specific to the person who falls, suggesting that programmes designed to prevent falls should be tailored to the individual. 88 Most falls result from a combination of contributory factors, one of which can be VI. Specialised falls services are provided nationally in the UK, offering advice, providing rehabilitation services and aiming to prevent further falls in those with a history of falling. As part of their assessment, the majority of falls services carry out a vision check, but these vary in terms of frequency and methods used. Reciprocal referral between optometrists and local falls services is recommended to improve continuity of care. 85 It is important to consider the role played in falls by elements of visual performance other than VA, including factors such as reduced contrast sensitivity, visual fields and binocular vision. 88
Reporting standards
Strengthening the Reporting of Observational Studies in Epidemiology statement
The study endeavoured to comply wherever possible with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement, which contains recommendations to improve the quality of reporting of observational studies. 89 The STROBE checklist, with annotations giving details of PrOVIDe’s compliance with each item on the checklist, is available in Appendix 1.
Standards for reporting and measurement of visual impairment
A report, The Prevalence of Visual Impairment in the UK: A Review of the Literature,45 commissioned by the RNIB, concluded with a section on the standards for reporting and measurement in studies investigating the prevalence of VI. The following recommendations were made and have been followed insofar as they were feasible in PrOVIDe:
The Working Group recommended that vision assessment in population-based studies should include a measurement of visual acuity using logMAR charts at distance and near under standardised conditions.
Information collected should record: (i) monocular and binocular distance presenting visual acuity, whether a method of vision correction is used (e.g. spectacles) and, if so, the type and power of vision correction device; (ii) monocular and binocular near presenting visual acuity at 40 cm, whether a method of vision correction is used (e.g. spectacles) and, if so, the type and power of vision correction device; (iii) monocular and binocular best-corrected visual acuity at distance and near, following refraction using an age-appropriate addition for near acuity.
We recommend the use of validated questionnaires or scales for measuring self reported vision problems or vision related quality of life. We emphasize the need to thoroughly test all questions before use in surveys.
Reproduced with permission from RNIB45
Aims and objectives
Previous research suggests that VI, often preventable, is not uncommon in the UK older population. The risks of VI and dementia both increase with age, suggesting that a proportion of people with dementia will also have undiagnosed VI, but evidence to support this is lacking owing to a dearth of research. This, together with evidence of the effects of VI on general well-being, prompted identification of the main research questions and associated objectives.
The main research questions are:
What is the prevalence of a range of vision problems in people with dementia aged 60–89 years and to what extent are these conditions undetected or inappropriately managed?
The four primary objectives of the study were:
-
to measure the prevalence of a range of vision problems in people with dementia
-
to compare the prevalences found in objective 1 with the published data on the general population in a comparable age range
-
to identify and describe reasons for any underdetection or inappropriate management of VI in people with dementia
-
to recommend interventions to improve eye care for people with dementia and further research in this area.
The secondary objectives of the study were:
-
to identify any differences in the level of undetected or inappropriately managed VI between those living in their own homes and those living in care homes
-
to determine estimates for the percentages of those with dementia likely to be able to perform successfully elements of the eye examination
-
to relate vision problems in people with dementia to data from functional and behavioural assessments.
Chapter 2 Methods
Study design
The study had two stages. Stage 1 was a cross-sectional study to establish the prevalence of a range of vision problems among people with dementia. Stage 2 was a qualitative study that used focus groups and interviews to explore and describe issues around detection and management of vision problems among people with dementia from the perspectives of affected individuals, family carers, professional care workers and optometrists.
Stage 1 was an observational cross-sectional study. PrOVIDe endeavoured to follow the STROBE statement for reporting of observational studies wherever possible. The STROBE recommendations have been developed to improve the quality of reporting of observational studies. 89 An annotated version of the STROBE checklist, giving details of PrOVIDe’s compliance with each item, can be found in Appendix 1.
Throughout the report, the term ‘potential participant’ is used to describe individuals who were approached by a member of the research team for involvement in the study. The term ‘participant’ is used to describe individuals who were formally recruited into the study. The term ‘family carer’ is used to describe a family member or friend who cares, unpaid, for their relative or friend. The term ‘care worker’ is used to describe someone who is employed to support individuals with everyday tasks, in their own homes or in residential care settings. The term ‘formal consultee’ has been used to describe a personal consultee who was required to give approval on behalf of individuals who lacked mental capacity to provide informed consent to participate. The term ‘informal consultee’ refers to a family member or personal carer whose opinion was sought regarding an individual’s participation in the study; this was applicable for individuals who had the capacity to provide informed consent to participate. An informal consultee role was sought for all potential participants and participants to provide additional assurance that participation in the study was supported by an independent source concerned with the interests of the participant.
Setting
Participants were recruited from 20 NHS sites in six regions of England. Sites were selected to ensure the recruitment of participants living in rural, urban and city locations and to encourage the participation of people from black and minority ethnic groups. It was originally envisaged that sites in four regions would be included: East Anglia, the North East, North Thames and Thames Valley. Two more regions, Yorkshire and the Humber and the North West, were added later when recruitment rates were lower than predicted in the original regions and concerns were raised that the recruitment target might not be achieved within the time scale. The regions referred to were those defined by the NIHR Dementias and Neurodegenerative Diseases Research Network (DeNDRoN), which subdivided England into nine regions.
Regions and sites were selected in consultation with DeNDRoN. Local network co-ordinators identified local principal investigators willing to support the study and then advised the research team on the numbers of participants that they predicted could be recruited in the time frame, and site-specific recruitment targets were agreed.
Approvals
The study received NHS ethics approval in September 2012 in advance of the scheduled start date. NHS research and development approval was sought from the NHS sites including primary care trusts where participants would be recruited from care homes. The first sites granted approval in November 2012, with the remaining sites gradually joining the study over the next 3 months. Recruitment began in November 2012.
The Association of Directors of Adult Social Services (ADASS) was consulted on whether or not social care research ethics approval was also required for recruitment through care homes. ADASS concluded that NHS ethics approval was sufficient but advised the study team to write to the directors of adult social care in all (n = 47) participating local authorities to inform them of the study and offer additional information if required. One authority declined to support the study.
Sampling
Stage 1
The study sample was people with dementia (any type) aged 60–89 years. To ensure that the spectrum of care was represented, the sample included people living in their own homes and people living in residential care (such as care homes and hospital inpatient wards). An additional inclusion criterion was that, in the case of individuals lacking mental capacity to provide informed consent to participate, a formal consultee was required who could give approval on the individual’s behalf. The opinion of a family member or professional carer in the role of informal consultee was sought in respect of all participants as to whether or not participation would be appropriate. The exclusion criteria were as follows:
-
Individuals who had been in hospital in the preceding 2 weeks following an acute illness, or who had major delirium or a major infection.
-
Individuals unable to understand English, as consent procedures and the eye examination were conducted in English.
-
Individuals unable to comply with the requirements of the eye examination; although it was expected that some participants would not be able to comply with all elements of the examination, individuals were excluded if they were unable to co-operate with the simplest procedures.
-
Individuals participating in a clinical drugs trial, because the eye examination involved the administration of tropicamide eye drops (Mydriacyl, Alcon Laboratories Ltd, Surrey, UK) and all of the potential drug interactions could not be determined. This exclusion criterion was added on completion of the pilot study.
Sample size
As described in Chapter 1, the prevalence of conditions causing VI has been estimated to be as high as 50%. 44 For the stage 1 cross-sectional study, a sample size of 385 was required to allow detection of an estimated prevalence of 50% with 5% precision and 95% confidence. If the prevalence of a condition was greater than or less than 50%, the study would have required a sample size smaller than 385. It was anticipated that some participants would be unable to perform all of the tests undertaken in a standard optometric examination, but there were no published data on the percentages of those with dementia who would be unable to perform individual elements of the examination. In the absence of published data, a decision was made to assume a worst-case scenario that up to 50% of the subjects might be unable to complete a particular test. This increased the maximum sample for stage 1 to 770 (385 × 2).
This led to a decision to divide the sample into two groups: group 1 was people living in their own homes and group 2 was people living in care. Although the group sizes were not defined to specifically support ‘between-group’ comparisons, the use of equal numbers in the two groups would provide scope for some comparative analysis between groups 1 and 2. With the determined sample size, there would be at least 80% power to detect a difference between proportions of 50% and 42% (or less) in two equal-sized groups. The initial sampling strategy for both groups was to stratify by age and sex to match as closely as possible the best estimates available at the time for the general dementia population. In both group 1 and group 2 the PrOVIDe target sample was one-third male and two-thirds female; 30% of males were aged between 60 and 74 years and 70% of males were aged between 75 and 89 years; and 15% of females were aged between 60 and 74 years and 85% of females were aged between 75 and 89 years.
Stage 2
The population under study in stage 2 was extended beyond people with dementia to include family carers, care workers working in care settings and optometrists. This process of triangulation – posing similar questions to different sources – was employed to increase the validity of findings. Stage 1 participants who had mental capacity to consent themselves into the study and who were able to participate in an interview were considered for inclusion in stage 2. Purposive sampling was applied to identify participants across the age range, of both sexes, and to include individuals who might be particularly informative because of a history of eye disease.
Family carers were also identified through their involvement in stage 1 in that they were the named next of kin or the informal consultee of stage 1 participants. The method of data collection with family carers was focus groups, and the named carers of stage 1 participants living in an area where focus groups were planned were invited to participate. Similarly, the care workers approached for stage 2 worked in care homes that had assisted with recruitment of stage 1 participants.
Optometrists were sampled using the membership of the College of Optometrists (CoO). The College had 10,086 members representing in excess of 70% of the members of the profession registered to practise. The method of data collection used for optometrists was focus groups. All College members living or working in an area where focus groups were planned were invited to participate.
Recruitment of participants
In stage 1, the initial approach to potential participants was made by a member of the direct care team, a member of DeNDRoN, local co-ordinating research staff or a member of the CoO research team. The aim of this initial contact was to inform the potential participant and carers of the study and its remit. This was achieved by direct face-to-face contact (e.g. in a clinic), by telephone or by letter. Potential participants and carers were provided with written information on the study’s purpose, methods and risks, and what was required of participants.
Subsequently, potential participants and personal and professional carers were contacted by either a DeNDRoN staff member or a member of the research team to determine if the potential participant and family member/carer were willing to be involved in the study. They were also given the opportunity to ask any questions or raise any concerns they might have had at that stage. Potential participants were given a minimum of 1 week to consider their possible participation in the study. If the potential participant and carer felt confident and comfortable to participate in the study, consent forms were completed.
Ethically, and for validity of the findings, it was important and appropriate to include participants who lacked mental capacity to consent within the tenets of the Mental Capacity Act (2005). 90 In recognition of the complexities surrounding informed consent and capacity in people with dementia, all research workers involved in the study received formal NIHR Clinical Research Network training in good clinical practice, informed consent, the ethics of consent and the Mental Capacity Act. 90 In the case of individuals unable to consent for themselves, a formal consultee (family member/dependant/friend) was asked for their opinion. The formal consultee was asked to take into account any advance decisions or previously expressed wishes and feelings, and consider the potential participant’s best interests. In group 1, 67.9% of participants were able to consent themselves into the study and 32.1% required a consultee. In group 2, 27.6% were able to consent themselves into the study and 72.4% required a consultee. The consultee was provided with an information sheet, containing the same information as that for a participant able to consent for themselves, and was asked to sign a consultee declaration form. As an additional measure to safeguard participants’ interests, for participants with the capacity to consent an informal consultee was asked to give their opinion on the potential participant’s suitability to take part in the study.
In stage 2, potential participants were contacted by a member of the research team. During stage 1, the examining optometrist assessed participants for their ability to cope with an interview, and those who were considered to be able to do so were asked if they were willing to be contacted about stage 2. Individuals who were subsequently selected through purposive sampling were initially telephoned by a member of the research team to check their willingness to consider participation in stage 2. This was followed by a letter of invitation and further written information about what the interview would entail. A second telephone call was made at least 1 week later to answer any questions and, if the individual agreed, to set a date and time for the interview to take place. The appointment was confirmed with a follow-up letter. Formal consent was obtained on the day of the interview prior to commencing data collection.
Potential focus group participants (family carers and optometrists) were sent a letter of invitation and an information sheet about the focus groups. The sheet included details of how to contact the research team with any queries. Those wishing to attend the focus groups indicated this by returning a contact form (by fax, e-mail or prepaid reply envelope). On receipt of this form, an acknowledgement was sent to the participant, together with further details about the focus group venue. Non-responders were not contacted again.
Early pilot work suggested probable difficulties in recruiting care home care workers to attend focus groups. Only one focus group was arranged, with the assistance of a care home group manager. Additional data from care workers were collected through interviews. With assistance from stage 1 recruiters, purposive sampling was used to identify and target care homes that might be willing to take part. The initial invitation to participate in stage 2 was made by a letter accompanied by a participant information sheet. Formal consent to take part in focus groups or interviews was taken on the day of the data collection procedure, prior to any data collection taking place.
Data collection
Stage 1
Stage 1 comprised three elements:
-
a sMMSE
-
functional and behavioural assessment questionnaires completed by a carer
-
an eye examination, that is, a full optometric examination in line with requirements of the GOS sight test and the CoO guidance document C4. 91
Standardised Mini-Mental State Examination
The MMSE is a tool used by clinicians to help them diagnose and assess dementia. It was originally developed by Marshall Folstein92 in 1975. The MMSE is available in several versions. A standardised version (sMMSE) of the test was used for the PrOVIDe study. The sMMSE consists of a series of 12 questions or tests designed to assess orientation, memory, attention and calculation, recall and language. Each element is scored and a maximum score of 30 is possible. The study recruiter carried out sMMSE on the day that the participant was consented into the study, once consent had been obtained.
Functional and behavioural assessment questionnaires
Two questionnaires were used to provide background information on the participant’s functional and behavioural state. This allowed comparison between cases with and without VI, as well as providing background information on individuals for whom it was not possible to perform certain elements of the eye examination.
The two questionnaires employed were established tools: the Bristol Activities of Daily Living Scale (BADLS)93 and the Cambridge Behavioural Inventory – Revised (CBI-R). 94 The BADLS was selected as it is a well-established, validated, carer-rated instrument. It was selected as a means of gathering additional information about the extent to which participants’ dementia impacted their day-to-day life. It was considered to offer scope for some evaluation of the interactions between dementia, vision and daily living activities. The BADLS assesses the performance of 20 activities of living, using four statements describing different levels of performance and a fifth ‘not applicable’ option. The respondent is asked to select the statement that most closely describes the individual’s performance over the preceding 2 weeks.
The CBI-R was selected to provide scope to conduct analyses to explore the possible relationships between vision, vision problems and the levels of cognitive impairment encountered within the sample population. The CBI-R describes behavioural changes using 45 items arranged into 10 sections. It is designed to be completed by a carer who states the frequency of a behaviour using a five-point scale ranging from ‘never’ to ‘constantly’. If a question does not apply, a ‘not applicable’ response should be used.
The eye examinations
Each optometrist aimed to perform a full optometric examination in line with the requirements of the GOS sight test and professional guidance. 91 Participants were offered a choice between having their eye examination in an optometric practice (normal community setting) and having it in their own home (domiciliary examination). A number of community optometrists volunteered to assist with the study by performing practice-based eye examinations. However, the number was small for the wide geographical spread of the recruitment area, which meant that most study participants would have been required to travel some distance to their appointment if they wished to have an examination in an optometric practice. All study participants chose to have a domiciliary eye examination.
Examinations were carried out by an optometrist from The Outside Clinic (TOC), a company specialising in providing domiciliary eye care nationwide. During recruitment of optometrists, a number of TOC optometrists expressed interest. TOC was willing to support its optometrists interested in working on PrOVIDe; it extended this support to allow the study to use TOC software to gather participants’ data from the eye examinations and its experienced logistics team to co-ordinate the arrangement of appointments.
Further factors that contributed to the research team’s decision to involve TOC included the rigorous training its staff undergo, the clearly evidenced and rigorous audit and clinical governance mechanisms used by the company, its bespoke tablet-based electronic patient data-management system, and its familiarity with working in Clinical Commissioning Groups across England. The strong clinical governance framework in place at TOC provided assurance that the research data collected were reliable, valid, comprehensive and consistent. The record-keeping system for clinical records was electronic, facilitating data extraction and analysis for the study.
The Outside Clinic’s optometrists are trained and experienced in dealing with people with illness or disability, including people with dementia, and all had the necessary and specialised equipment for conducting eye examinations in the home. Following several meetings and a review of TOC’s processes, systems and clinical governance framework, it was determined that working with TOC and its optometrists would offer significant advantages to PrOVIDe.
Fourteen optometrists were recruited to perform the eye examinations and data collection for the study. Three optometrists were required in each of the initial four regions to cover the wide geographical areas in which participants lived. Two more were recruited later to cover the recruitment of participants from the two additional regions. The optometrists received additional training from the PrOVIDe project manager on study-specific requirements. Additional tests that the optometrists were required to perform on study participants included using the logMAR chart for recording vision and VA and the use of a specific cataract grading scale when grading cataracts.
Participants and family members/care workers received advance notice of the optometrist’s visit. They were contacted 3–5 days before the appointment to confirm the suitability of the appointment date. If the date was not suitable, TOC sent another appointment date by post. On the day of the examination, the examining optometrist telephoned the named point of contact approximately 1 hour before the appointment to give an accurate time of arrival.
On arrival at the participant’s place of residence, the optometrist introduced him- or herself to the participant and family member or carer, explaining who they were and the reason for their visit. The optometrist set up his or her equipment in the room allocated for the eye examination (in care homes) or in the most appropriate available room (in the participant’s own home). Where there was a choice of rooms, the ability to achieve dim illumination was a major factor in the final selection.
Prior to the eye examination commencing, participants were asked if there were any tasks (vision related) that they found particularly difficult. Where a participant was unable to respond appropriately, advice was taken from carers and/or care home staff as to the cognitive and other abilities of the patient. The tests constituting the eye examination were normally conducted in the order described in Figure 1, but the over-riding principle was that the optometrist should adopt a flexible approach throughout, and the order of tests was adapted as required to suit the individual patient. The examinations were performed according to the cognitive and physical abilities of the participant, with the emphasis on objective assessments and omitting tests (both objective and subjective) as required by the needs of individual participants. This approach was consistent with the current CoO professional guidance on examining patients with dementia,91 although not all examination data were required for the PrOVIDe study.
FIGURE 1.
Elements of the eye examination. a, The ocular health examination and pupil dilatation were able to be carried out at any of the points indicated by the dashed arrows: their place in the order of examination elements was not rigidly fixed.

Tests carried out in a typical PrOVIDe eye examination
History and symptoms
These were obtained from the participant and/or the carer. For care home residents, the optometrist requested access to the participant’s care record or asked a member of staff to provide the relevant information from the record. Where necessary, input was sought from the carer or care home staff regarding the participant’s history and symptoms. Information was gathered regarding the participant’s current visual status and spectacle wear, their general health, previous ocular history and family general health.
Vision and visual acuity
Distance vision and VA were recorded using either the Thomson Test Xpert 3Di Test chart (Thomson Software Solutions, Welham Green, UK) or TOC’s own computerised test chart. Both test charts allow the presentation of letters using either logMAR or Snellen progression of letter sizes. The optometrist would normally use a 3-m testing distance, and if shorter testing distances were required both test charts would adjust the letter size to compensate for the distance at which the chart was used. No mirror was used during VA testing in this study. LogMAR VA was assessed using letters from the Roman alphabet, with five letters on each line of letters presented. However, the optometrist would attempt to obtain vision and VA measurements using symbols if standard letters did not prove successful. Vision and VA were measured on a letter-by-letter basis.
The procedure followed by the research optometrists when deciding when to terminate the acuity measurement is described in the following example. The participant in this illustrative example correctly identified three of the five letters on the smallest line of letters that they could read (i.e. all of the letters on the next smallest line of letters were read incorrectly or could not be read at all). The optometrist would then present a different line of five letters of the same size and check how many letters the participant could read on this second line. If this number was again three letters out of five, the acuity measurement would terminate at that point for that eye. However, if the participant read a different number of letters on this second line of letters of the same size, for example four letters, the optometrist would present a third line of five letters of the same size. If three letters were read on this third line, the acuity measurement would be terminated, and three letters on this line would contribute to this participant’s VA. If four letters were read on this third line, the acuity measurement would be terminated and four letters on this line would contribute to this participant’s VA. In summary, the optometrist would always check the ability of the participant to correctly identify letters on the lowest line of letters that they could read by presenting a second line of letters of the same size, and would present a third line (or more) of letters of that size if required and if co-operation allowed. The aim of this approach was to seek confirmation of the number of letters the participant correctly identified on the lowest line of letters they could read. However, with the PrOVIDe participant base it was unlikely that these methods would be successful with every participant. Co-operation levels would vary between participants, and could vary in a single participant during the eye examination. Therefore, the optometrist was instructed to take a pragmatic approach to the recording of vision and VA, using their professional judgement to decide when a test should be terminated if co-operation was no longer adequate to justify further assessment.
Whenever participant co-operation permitted, the optometrist measured the distance vision (if no previous spectacles were available) or distance VA with the participants’ existing spectacles and with the prescription determined during the PrOVIDe examination. The optometrists recorded vision and VA for the right and left eyes separately. This allowed the prevalence of VI to be calculated based on best monocular VA.
NVA was measured by means of a conventional eye examination approach using a standard Faculty of Ophthalmologists near-vision chart. The near-point scale used in these charts does not have a consistent progression between sizes of print, and in this respect it is similar to Snellen charts used for distance VA measurement. For example, the difference in size between N6 print and N5 print on the near vision chart is 1.2×, while the difference between N24 and N18 is 1.33×.
Near visual acuity was recorded as the smallest print size a participant could read at their preferred reading distance. Angular near vision reading acuity, which would require measurement of the smallest print size read at a fixed working distance with a fixed addition, was not recorded in this study. Rather than specify a particular number of words or lines of print that had to be read in order to give credit for that print size, the optometrists regarded the participant as being able to read a line of print if that line of print could be read fluently. All participants were encouraged to read the smallest size of print possible. The optometrist always aimed to provide optimum NVA at the preferred near working distance. Letters were available on the back of the near vision chart for those participants unable to read words.
Whenever participant co-operation permitted, the optometrist also measured NVA with the participants’ existing spectacles for near vision. The optometrists recorded NVA for the right and left eyes separately. This allowed the prevalence of presenting near-vision loss to be calculated based on best monocular VA.
Binocular function
This was assessed using the cover test and by testing ocular motility. The cover test is a dissociation test in which each eye is covered in turn while the patient fixates on a specified target at a given distance. The practitioner observes the eye movements and diagnoses the anomaly, if any. The ocular motility test is used to assess the extraocular muscles and their associated neural pathways. These tests were included in the eye examination but were not part of the PrOVIDe study.
Pupil reactions
The integrity of the pupillary pathways is assessed by shining a torch into each of the participant’s eyes in turn. Testing pupil reactions was included in the eye examination but was not part of the PrOVIDe study.
Tonometry
This is performed to measure the IOPs in the eyes. Tonometry is an objective measurement during which the only requirement from the participant is to keep their eyes open and maintain reasonably steady fixation. This test is used as part of a battery of tests (the others being visual field examination and optic nerve head examination) to detect glaucoma. The Icare tonometer was used (Icare Finland Oy, Vantaa, Finland). The Icare is a contact technique (i.e. involves contact between the tonometer probe and the cornea) that can be performed without the use of a topical anaesthetic. A comparison between the Icare tonometer and the Goldmann applanation tonometer (Haag Streit AG, Bern, Switzerland), the current reference standard method of measuring IOP, concluded that there is a good agreement between the two methods of IOP measurement. In addition, the Icare tonometer is easy to use and records consistent readings rapidly, with minimal training required. 95 The TOC optometrists working on the study were all familiar with the Icare and used this device routinely prior to their participation in the project.
The objective and subjective determination of refractive error
This allows the strength of the spectacle prescription to be determined. Retinoscopy is an objective technique used by optometrists to determine the refractive error of the eye by observing the movement of light reflected from the fundus. It was attempted in all the study examinations to determine the prescription objectively. Wherever possible, the retinoscopy result was modified subjectively using a conventional monocular subjective refraction with the patient reading letters or symbols presented on the logMAR test chart.
On completion of the determination of each participant’s refractive error (their spectacle prescription), the distance VAs for the right and left eyes were recorded (whenever participant co-operation permitted) and, in most cases, a binocular VA (VA with both eyes together) was also recorded. The participant’s working distance for doing near tasks was recorded and informed the determination of the near addition, which is the extra element of power required by older people to provide comfortable, clear vision for near visual tasks. This initial estimate of near addition was refined, where possible, and NVAs were recorded.
Ocular health examination
At this stage in the test the participant’s pupils were dilated using tropicamide eye drops to facilitate the examination of the eye’s structures behind the iris, notably the lens, the retina and other back-of-the-eye structures. Both 0.5% and 1% Tropicamide Minims® (Bausch + Lomb, Surrey, UK) (single-dose containers) were available to the optometrist, who chose the concentration based on their clinical assessment of the participant. An examination of the anterior eye (eyelids, cornea, conjunctiva and iris) would take place prior to examination of the posterior surfaces of the eye. Prior to pupillary dilatation, the likelihood of the pupil dilatation provoking an acute angle-closure glaucoma (ACG) attack (see Glossary) was assessed using the pen light test for anterior chamber depth estimation. 96 The risk of provoking an acute ACG attack with tropicamide is very low, with zero cases of ACG reported in almost 4000 dilatations. 97 However, participants judged at risk of an ACG attack were not dilated and were examined through undilated pupils.
Following the instillation of tropicamide eye drops, the participant’s pupils were normally sufficiently dilated after approximately 20 minutes. Tropicamide is a drug that can reduce accommodation (the ability to alter the focus of the eyes) but PrOVIDe participants were aged > 60 years and would therefore have no accommodation. Once the pupils were fully dilated, the examination of the fundus followed, using a direct hand-held ophthalmoscope. As a further check on the possibility of a dilatation-induced acute ACG attack, post-dilatation IOPs were measured using the Icare tonometer. A significant increase in IOP could be an indicator of a pending ACG attack, and if this was suspected the optometrist remained and monitored the participant for a further hour.
Optometrists use a variety of methods to grade the severity of AMD, diabetic retinopathy and cataract. For this study, AMD was classified into dry and wet (neovascular) AMD and then graded as mild, moderate or severe. Diabetic retinopathy was graded as background diabetic retinopathy; mild, moderate or severe non-proliferative retinopathy; proliferative retinopathy; and diabetic maculopathy. When calculating the prevalence of AMD, diabetes and glaucoma in this study, a participant was considered as being positive for the condition irrespective of the severity recorded by the optometrist.
Optometrists regularly detect and monitor patients with different types and grades of cataract. The three most common types of age-related cataract (nuclear, posterior subcapsular or cupuliform, and cortical) are often graded by community optometrists as mild, moderate or severe. In addition to this grading, a sketch of the lens changes observed may form part of the clinical record. Some community optometrists use more sophisticated slit-lamp based grading systems for cataract, such as The Lens Opacities Classification System (LOCS) III. 98 Systems such as LOCS III, also often used in research studies, require the use of a slit-lamp biomicroscope. The slit lamp normally used in community optometric practices is a bulky piece of equipment, unsuitable for domiciliary use, and was not available for PrOVIDe.
PrOVIDe optometrists used the cataract grading section of the Optometric Grading Scale (Figure 2) as the main method for grading cataract for PrOVIDe participants. The research team contacted the designer, Mr RM Pearson, for approval to use the Optometric Grading Scale99 when grading cataracts in study participants. Free-text descriptions and diagrams were also used if necessary.
FIGURE 2.
Optometric Grading Scale for grading age-related cataract. 99 © Richard M Pearson. Reproduced with permission.
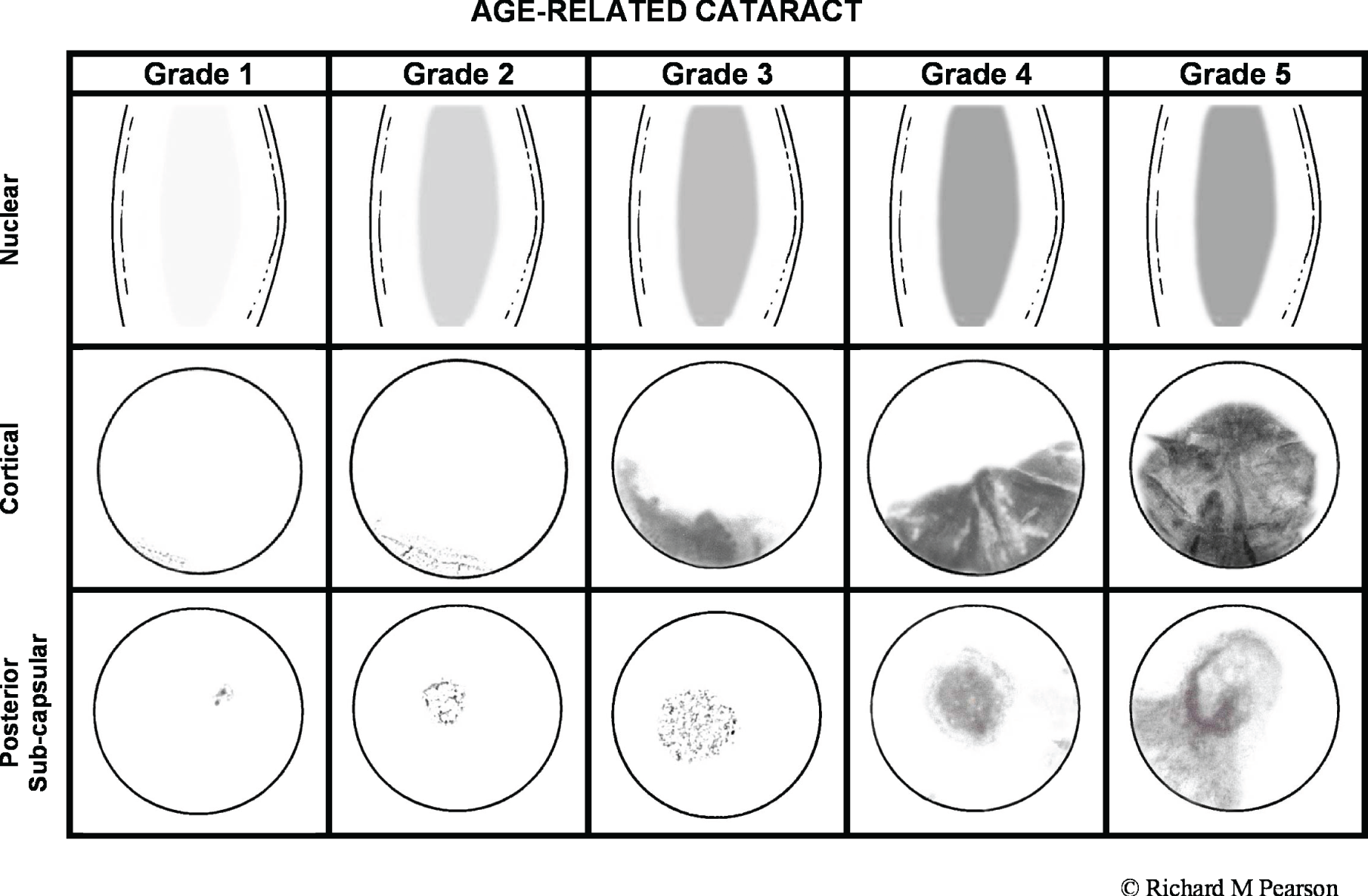
Visual fields
This was by far the most demanding of the tests attempted in the study eye examination. In the first half of the recruitment and data collection period, optometrists were advised to request this test for all participants for whom the test was clinically necessary or if the optometrist felt that the participant would be able to understand and complete the test. This requested test was carried out by a member of TOC’s field staff team on a different day from the actual eye examination. The visual field tests were conducted using either the Humphrey® Frequency Doubling Technology perimeter (Carl Zeiss Meditec AG, Jena, Germany) or the OCULUS Easyfield® perimeter (OCULUS Optikgeräte GmbH, Wetzlar, Germany). In the second half of the data collection period, optometrists were advised to return to their normal practice of requesting the test only when it was clinically necessary, owing to practical and logistical difficulties encountered when arranging the fields appointments and the additional burden on participants of having a second visit.
Conclusion
The examination concluded with verbal and written advice to the participant and/or carer based on the optometrist’s findings. This included advice on any referral required, on the use of the participant’s existing or any new spectacles or other treatments advised, and on the recommended date of the next eye examination. Information explaining the effects of the tropicamide drops, including the symptoms and signs of an ACG attack and advice on the action to take in the unlikely event of this occurring, were printed on the form containing the spectacle prescription.
Where participant co-operation was poor, the optometrist moved the ocular health examination forwards in the eye examination. This allowed the optometrist to focus on ophthalmoscopy through dilated pupils whenever possible, which is the key test for detection of eye disease and does not require high levels of participant co-operation.
Optometrists asked carers for their views on whether or not the extent to which a participant was able to co-operate with the examination reflected his or her usual state. These views were noted on the record card.
Participants, their relatives and carers were informed in writing of how to raise any concerns they might have regarding the study or any aspect of the eye examination. Concerns were reported initially to the PrOVIDe research team, who liaised with TOC and recruiters as required. All concerns or complaints were documented and addressed.
Pilot study
Recruitment of participants for the pilot study commenced at the end of November 2012 and the main study began on 15 February 2013. The pilot largely involved group 1 participants, as there were some outstanding approvals relating to group 2 participants. Data from the pilot are not presented separately in this report. Feedback from recruiters during the pilot study led to minor changes being made to procedures relating to arranging and scheduling eye examinations, but no changes were made to data collection.
A recruiter query arose during the pilot study about whether or not to include subjects who were already taking part in other studies involving clinical drug trials of pharmaceutical products. This was discussed at the Steering Group meeting held at the end of the pilot study; the group agreed a modification to the exclusion criteria to exclude those participating in clinical trials, because the possible interactions between trial drugs, particularly if a new product, and the tropicamide eye drops were unknown.
Main study
As there were no changes to data collection after the pilot study, the eye examination data collected from the 21 participants who took part in the pilot were used in the main study; therefore, the eye examination data collection period began in December 2012 and finished in April 2014.
Stage 2
Qualitative data collection techniques were employed in stage 2 through a combination of focus groups and interviews. Focus groups are particularly useful when the aim of data collection is to bring together individuals with a shared knowledge to explore common or contrasting opinions and experiences. 100 For this reason, focus groups were employed with two populations: family carers of people with dementia, and optometrists. With any population a minimum of three focus groups is recommended to reduce selection bias,101 and in this study there was a total of 10 focus groups from these two populations: five with family carers and five with optometrists.
Each focus group lasted for between 1 and 1.5 hours. The key question areas were the frequency and relevance of sight tests for people with dementia, reported experiences of eye care and barriers to eye care. The focus group question schedules are in Appendix 2.
Focus groups were also planned with professional carers. The recommended number of participants is a minimum of four and a maximum of 12, but pilot work with care homes indicated that there would be difficulties in recruiting a sufficient number of participants. Indeed, it was possible to arrange only one focus group, and so interviews were used to elicit the views of additional care home representatives. Each interview was conducted face to face by the same interviewer using a semistructured interview schedule, which can be found in Appendix 3.
Interviews were also used to collect the views of people with dementia. Data saturation can usually be achieved with around 30 interviews. 102 In this study, 36 interviews were conducted with an equal number of male and female participants, covering the full sample age spectrum of 60–89 years. Interviews took place in participants’ homes and participants were invited to have a carer present if they wished. Nearly all interviewees (n = 30) exercised this option, which proved to be helpful, as carers were able to answer recall questions which participants found difficult. Carers often also offered their own contributions to the interview discussion, thereby providing additional data. The length of interviews varied but usually ranged between 15 and 30 minutes; the variation was largely dependent on the individual’s ability to respond as a result of their cognitive state and the extent of the carer’s contribution to the discussion. Focus groups and interviews were audio-recorded and then transcribed in preparation for data analysis.
Interviews with people with dementia were conducted over a 12-month period between May 2013 and April 2014. Two focus groups were arranged in November 2013 to facilitate the piloting of the sampling and recruitment procedures and the focus group schedule. The remaining eight focus groups with family carers and optometrists took place between February and May 2014. Data collection with care workers was completed in June 2014.
Stage 1 data analysis plan
Study outcomes
Distance vision
The calculation and analysis of the prevalence of the different types of VI in our sample addressed several of the PrOVIDe study objectives, namely primary objectives 1 and 2, and secondary objectives 1, 2 and 3 (see Chapter 1, Aims and objectives). In PrOVIDe, the three types of VI investigated were as follows:
-
Presenting VI, based on best monocular VA recorded with the participant wearing their current distance spectacles or unaided if no spectacles were worn for distance vision.
-
Post-refraction (or best corrected) VI, based on best VA recorded with the participant wearing the prescription determined by the PrOVIDe optometrist.
-
Uncorrected or undercorrected VI, which includes those participants who had presenting VI but who were not visually impaired wearing the prescription determined by the PrOVIDe optometrist.
As discussed in Chapter 1 (see Visual impairment), a variety of definitions of VI have been used in prevalence studies. All of these definitions use criteria for VI based on levels of VA as recorded on Snellen progression VA charts. The two most commonly used cut-off points used to define VI in terms of VA are (1) VA < 6/12 Snellen and (2) VA < 6/18 Snellen. The logMAR equivalent of 6/12 Snellen is 0.30, so a VA of < 6/12 is equivalent to 0.32 or worse logMAR acuity, and this was one of the cut-off points taken in the PrOVIDe study to define VI. There is no full logMAR line equivalent to 6/18 Snellen; reading all of the letters of a 0.5 logMAR line is equivalent to 6/19 Snellen, and reading all the letters on the 0.4 logMAR line is equivalent to 6/15 Snellen. The logMAR equivalent of 6/18 Snellen is 0.4771, so in mathematical terms a logMAR acuity of 0.48 is worse than 6/18 Snellen. Therefore, 0.48 or worse logMAR acuity was the second cut-off point used in the PrOVIDe study to define VI.
For distance vision, our data analyses included descriptive statistics of VI prevalence in the whole sample and for groups 1 and 2 separately, with tests for differences in proportions between groups. Multivariable ORs were calculated for each type of VI by age, sex and group, and for uncorrected/undercorrected VI by sMMSE score. Prevalence data were presented using descriptive statistics for our four target eye conditions (AMD, cataract, diabetic retinopathy and glaucoma) for the whole sample and for groups 1 and 2 separately, with tests for differences in proportions between groups. Multivariable ORs were calculated for each eye condition by age, sex and group. The clinically determined causes of post-refraction VI were presented using descriptive statistics. The proportion of carers providing support to the participant with dementia during the eye examination was tabulated for the whole sample and for groups 1 and 2 separately, with tests for differences in proportions between groups. Improvements in VA between pre and post refraction were presented for the sample as a whole and for groups 1 and 2 separately, with tests for the statistical significance of any improvements between groups.
Near vision
The two cut-off points in terms of NVA used to define impaired near vision were (1) best monocular NVA of worse than N8 and (2) best monocular NVA of worse than N10. Our analyses included descriptive statistics of the prevalence of near vision loss in the whole sample and for groups 1 and 2 separately, with tests for differences in proportions. Multivariable ORs were calculated for each type of near vision loss by age, sex and group, and for uncorrected/undercorrected VI by sMMSE score. Improvements in NVA between pre and post refraction were presented for the sample as a whole and for groups 1 and 2 separately, with tests for the statistical significance of any improvements between groups.
Ability of participants to complete individual elements of the eye examination
This was secondary objective 2 of the study (see Chapter 1, Aims and objectives). Our data analyses included descriptive statistics of the proportions of the whole sample able to complete each key test in the eye examination, and proportions for groups 1 and 2 separately, with tests for differences in proportions between groups. Multivariable ORs were calculated for each key test with age, sex and group as covariates to assess their independent associations with the completion of each examination component. Any association between participants’ level of cognition, as assessed by their sMMSE score, and their ability to complete key elements of the eye examination was summarised using descriptive statistics and multivariable ORs calculated for each test, with age, sex and group as covariates.
Patient management on completion of the eye examination
Descriptive statistics were presented for the proportions of the sample referred to participants’ GP or to the HES.
Analysis of the effects of visual impairment on function (Bristol Activities of Daily Living Scale) and behaviour (Cambridge Behavioural Inventory – Revised)
The results from the visually impaired and non-visually impaired participants were compared for both instruments and ORs were derived, with age, sex and group as covariates. This was secondary objective 3 of the study (see Chapter 1, Aims and objectives).
Statistical methods
In stage 1, for descriptive statistics, the mean and SD were used to describe data that were close to normally distributed, and the median and interquartile range (IQR) were used to describe data that were strongly non-normally distributed. All relevant data reported in the text of Chapter 3 are in the following style ‘count/percentage (95% CI)’ and will appear as ‘N/N, PP% (PP to PP)’. When data are stated as proportions in tables, the 95% CI is given. Pearson chi-squared tests were performed to test the difference between group proportions, using Yates’ continuity correction when appropriate (two-group comparisons), and through Monte Carlo simulation (empirical distribution) when the number of cases in a given group was low. When the results of chi-squared tests are reported, the degrees of freedom have been recorded where appropriate. Logistic regression was used to calculate adjusted ORs when attempting to control for covariates and when investigating independent effects on binary outcomes. For our exploratory analyses of VI and behavioural and functional ability, ordinal logistic regression was used in some of the item responses to the BADLS and CBI-R instruments. We have used ‘binary logistic regression’ and ‘logistic regression’ synonymously, as is conventional, with an extra qualification for ordinal logistic regression for the analyses indicated. Initial analyses as described above were performed on complete cases: that is, only on participants in whom all relevant data were present for a particular tabulation, estimate, model or test.
For all tests, p < 0.05 was considered significant, with the Bonferroni correction applied when required. All data were analysed using SPSS version 21 (IBM Corporation, Armonk, NY, USA) and R (an open-source programming package from the R Foundation for Statistical Computing, Vienna, Austria).
Missing data plan
For all proportion estimates, and for all regression analyses, examining the independent associations of age, sex, sMMSE scores (where appropriate) and residential status (living in residential care or own home) with study outcomes (i.e. VI status, near vision loss, presence of eye conditions and the effects of VI on behaviour and function), the primary analysis was a complete-case analysis (i.e. it included only participants in each analysis in whom the relevant variables were fully observed).
Given that different study outcomes would be missing in different participants and for varying reasons (e.g. some participants might not have been able to complete the recording of presenting or post-refraction VA at distance and/or near vision, while for others dilatation of the pupil might not have been possible), this may lead to some inconsistency in our separate analysis of each study outcome. Complete-case analysis also leads to less efficient estimates, because it does not use the partial information available on participants. Perhaps most crucially, it may lead to bias in unadjusted estimates if the data are not missing completely at random. For example, our VI rate estimates from complete cases would be unbiased only if those participants in whom we did/could not measure VA were expected to have the same rate of VI as those with observed VA. That assumption seems implausible. Missing data can even lead to bias in regression modelling on complete cases if the causes of missing data are not fully explained by the covariates in each model (missing at random assumption). 103 This assumption is unlikely to be realistic given that our regression models adjust only for age, sex, sMMSE (where appropriate) and residential status.
To improve the plausibility of our prevalence estimates and our regression analysis assumptions, a multiple imputation procedure employing chained equations104,105 was performed as a sensitivity analysis. Simply stated, under multiple imputation, observed values of variables are used to predict missing values in other variables. For our data, we used age, sex, location (living in residential care or own home), region, site and examining optometrist as predictor variables, all of which were fully observed in the sample. Other variables with some missing data included in the imputation model were time since last eye examination; presenting and post-refraction distance and NVA; sMMSE score; presenting and post-examination AMD; glaucoma and diabetes; post-examination diabetic retinopathy; presenting cataract and post-examination cataract severity (left and right eye); BADLS total score; and CBI-R total score. Thus, for each variable with missing data, the observed values of all other variables in this set would be used to predict the missing values. A total of 30 multiply imputed data sets were generated and the estimates in each imputed data set were pooled to obtain a result, the standard error of which accounted for the random variation across data sets owing to the uncertainty from missing data. All proportion estimates and their standard errors were calculated on the logistic scale within each imputation and combined similarly.
Additionally, for cognitive performance, we operated under the assumption that missing sMMSE scores were indicative of lower cognitive performance and were informatively missing. 103 Thus, even if two participants had similar characteristics but one had missing and the other had non-missing sMMSE scores, we would expect there to be a difference in their cognitive performance. To address this, we performed a pattern mixture model with a conditional expectation difference of 10 units between observed and missing sMMSE scores.
Extrapolating prevalence to the UK dementia population
Prevalence estimates for VI and its causes in the wider UK population of those aged 60–89 years with dementia were calculated by comparing the estimated distributions of characteristics in this target population with the PrOVIDe sample characteristics and calibrating estimates of rates of VI and of ocular conditions (AMD, cataract, diabetic retinopathy and glaucoma) accordingly. The two-stage clustered sampling of six regions and then 20 sites used in PrOVIDe was accounted for in this prevalence estimation, as was the stratified sampling by age (60–74 years, 75–89 years), sex and location in each site (see Chapter 3, Demographics and characteristics of the sample, Recruitment). The use of clustered sampling would result in larger standard errors and wider CIs of estimates from the so-called design effect, whereas stratification acts to reduce sampling variability and, in effect, reduce the standard error and the width of CIs. 106
Post-stratification population calibration weights were derived using the estimate counts of the population aged between 60 and 89 years with dementia in various strata. Two reliable joint distributions (cross-tabulations) of the population were obtained2 and used for calibration: (1) age group and sex; and (2) age group and residency (residential care or own home). Age groups were in 5-year bands – 60–64 years, 65–69 years, 70–74 years, 75–79 years, 80–84 years and 85–89 years – and residency was dichotomised as ‘living in the community’ (own home) or ‘living in care’ (residential care). A raking procedure107 was performed to derive weights to calibrate on these two population distributions (age–sex and age–residency). Multiply imputed data were used (see Missing data plan) to account for non-completion of components of the eye examination or other sources of missing data in outcomes within the sample. Thus, a single weight factor could be calculated for all outcomes, with the imputation addressing representativeness in the sample. All proportions and their standard errors were calculated on the logistic scale within each imputation108 and combined using Rubin’s rules. 109
All analyses were performed using the survey package in R. 110
Comparison of prevalence with other UK studies of visual impairment in older people
Using a similar approach to the prevalence estimation for the wider UK dementia population, estimated rates of VI in PrOVIDe were also reweighted for greater comparability with two nationally representative studies of VI in the elderly. 56,57 This was achieved by applying post-stratification weights to the PrOVIDe data. Rather than being derived using estimated distributions of characteristics from the UK population aged 60–89 years with dementia, these weights were instead derived from the distributions of participant characteristics in the NDNS and MRC studies.
The NDNS reported the cross-tabulations of age–sex and age–residency, where age groups were in bands of 65–74 years, 75–84 years and ≥ 85 years, and residency was ‘community’ or ‘institution’ (the latter has been interpreted as equivalent to living in residential care). The MRC study reported cross-tabulations of age–sex, where age groups were in bands of 75–79 years, 80–84 years, 85–89 years and ≥ 90 years, and participants lived in the community only. NDNS and MRC participants in age categories that fell outside the age range of PrOVIDe were excluded for these comparative analyses (≥ 85 years for NDNS and ≥ 90 for MRC), as were participants living in residential care for the MRC comparison. In turn, participants not in the age ranges of NDNS or MRC were excluded in each respective comparison. Thus, the age range used when comparing NDNS and PrOVIDe was 65–84 years and the age range used when comparing MRC and PrOVIDe was 75–89 years.
An alternative approach would have been to simply examine the rate of VI in each age–sex stratum available; however, these strata become increasingly small, and estimates within them become increasingly imprecise, with additional issues of multiple testing. Both of these issues would limit the ability to conclude whether or not any differences were credible. The rationale, therefore, was to compare as large an overlapping sample as possible for each of these two comparator studies in order to maximise the power to detect any meaningful differences, while using weighting in the PrOVIDe estimates to control for differences in sampling proportions of age and sex (and residency for NDNS).
From a statistical perspective, both comparator studies had differences in estimating and reporting VI prevalences. The MRC reported that non-response was higher in females than in males, and in older age groups than in younger age groups, but did not adjust for this in estimates. The NDNS used a weighting factor to adjust and report overall prevalence to reflect the UK population, but this was not accompanied by a CI to assess its precision. The NDNS did not report any CIs for any of their estimated prevalences, whereas the MRC reported CIs with an adjustment for the clustered sampling of the study. For the comparison of PrOVIDe estimates with the NDNS in the comparable/overlapping age group of 65–84 years, it was only possible to use VI rates and CIs calculated crudely from reported counts in age strata and there was no information on the weights in the subset of age strata. From reported MRC estimates the mean VI rate in those aged 75–89 years was calculated and combined with the cluster-adjusted standard errors across the three relevant age strata to obtain approximate cluster-adjusted CIs on the rate of presenting VI for distance vision.
Stage 2 data processing and qualitative analysis
In stage 2, audio recordings of the interviews and focus group discussions were transcribed and then analysed using framework analysis. 111 The process consists of five stages:
-
Familiarisation with the data – key ideas and recurrent themes are listed.
-
Identifying a thematic framework – data are sifted and sorted into key issues, concepts and themes.
-
Indexing – systematic application of the framework to the data while judging the significance and meaning of the data.
-
Charting – lifting the data from the original source into charts.
-
Mapping and interpretation of the chart contents – perceptions, accounts and experiences are compared and contrasted, patterns and connections are sought and explanations for differences are considered.
Reliability and validity were achieved through independent analysis of the data by several members of the research team. There was public and participant involvement (PPI) in data analysis: transcripts, data interpretation and reporting were reviewed by the PPI member of the Project Steering Group.
Chapter 3 Stage 1 results
Demographics and characteristics of the sample
Recruitment
Study recruitment began in November 2012 and was closed in February 2014, by which time 808 participants had been recruited (Table 2). Recruitment targets were stratified by age and sex to reflect the age/sex distribution of the UK dementia population at that time. The median age of the whole sample was 81 years (IQR 76–85 years); the median ages for participants living in their own homes (group 1) and in care homes (group 2) were 80 years (IQR 74–84 years), and 83 years (IQR 79–86 years), respectively.
| Demographics | Group 1 | Group 2 | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | ||||||
| Age (years) | 60–74 | 75–89 | 60–74 | 75–89 | 60–74 | 75–89 | 60–74 | 75–89 | |||
| Recruited | 66 | 126 | 53 | 198 | 443 | 25 | 95 | 26 | 219 | 365 | 808 |
| Withdrawn | 11 | 13 | 2 | 28 | 54 | 5 | 15 | 1 | 25 | 46 | 100 |
| Study participants | 55 | 113 | 51 | 170 | 389 | 20 | 80 | 25 | 194 | 319 | 708 |
The peak period of recruitment of group 1 participants occurred between February and July 2013, while for group 2, care home participants, the peak period of recruitment was between September 2013 and January 2014. The study profile is illustrated in Figure 3.
FIGURE 3.
Study profile: stage 1.
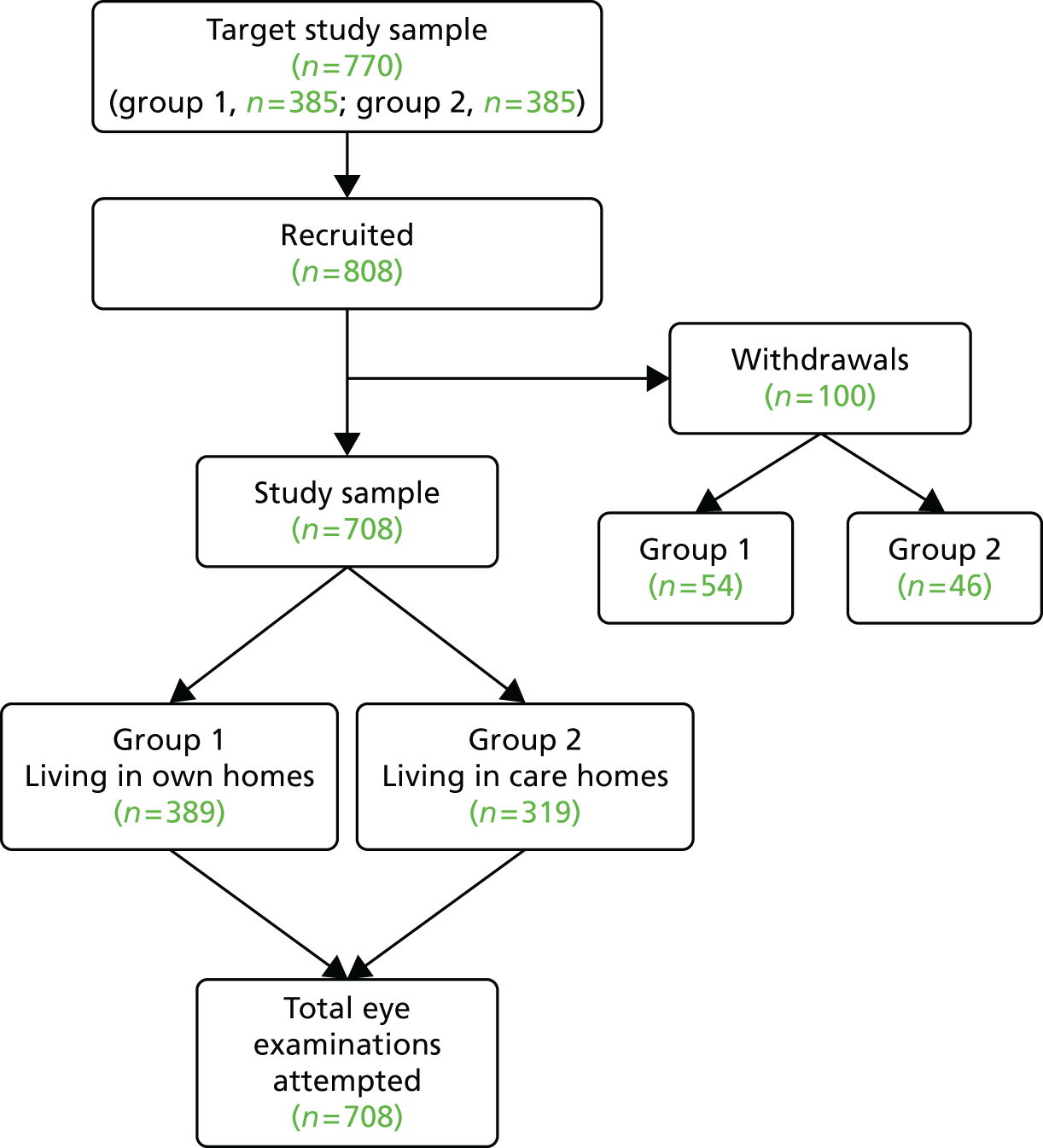
Table 3 shows a comparison between the age, sex and group profile of the final PrOVIDe sample and the 2014 UK population data from the Alzheimer’s Society. 2 Compared with these UK population data, the PrOVIDe sample has a lower percentage of males and females in the 60–64 years, 65–69 years, 70–74 years and 75–79 years ranges, a higher percentage of males and females in the 75–79 years category and a higher percentage of females, but a lower percentage of males, in the 85–89 years category. For place of residence, those participants living in their own homes were under-represented in the PrOVIDe sample compared with the Alzheimer’s Society data for all age ranges apart from the 85–89 years range, in whom the PrOVIDe sample was oversampled by 2.7%. There was better sampling agreement for those living in care homes, apart from the 80–84 years range, who were oversampled in PrOVIDe by 7.7%.
| Age group (years) | Population (frequency) | Population (%) | PrOVIDe sample (frequency) | PrOVIDe sample (%) | Under-/oversampling (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| 60–64 | 16,256 | 15,611 | 2.6 | 2.5 | 9 | 13 | 1.3 | 1.8 | –1.3 | –0.7 |
| 65–69 | 32,286 | 25,467 | 5.1 | 4 | 24 | 26 | 3.4 | 3.7 | –1.7 | –0.3 |
| 70–74 | 40,126 | 37,250 | 6.3 | 5.9 | 44 | 35 | 6.2 | 4.9 | –0.1 | –1.0 |
| 75–79 | 75,093 | 50,580 | 11.8 | 8 | 74 | 55 | 10.5 | 7.8 | –1.3 | –0.2 |
| 80–84 | 105,187 | 67,040 | 16.5 | 10.5 | 137 | 91 | 19.4 | 12.9 | +2.9 | +2.4 |
| 85–89 | 118,932 | 51,818 | 18.7 | 8.2 | 151 | 49 | 21.3 | 6.9 | +2.6 | –1.3 |
| Community | Care | Community | Care | Community | Care | Community | Care | Community | Care | |
| 60–64 | 23,177 | 8690 | 3.6 | 1.4 | 18 | 4 | 2.5 | 0.6 | –1.1 | –0.8 |
| 65–69 | 47,743 | 10,010 | 7.5 | 1.6 | 31 | 19 | 4.4 | 2.7 | –3.1 | +1.1 |
| 70–74 | 57,326 | 20,050 | 9 | 3.2 | 57 | 22 | 8.1 | 3.1 | –0.9 | –0.1 |
| 75–79 | 86,194 | 39,480 | 13.6 | 6.2 | 86 | 43 | 12.1 | 6.1 | –1.5 | –0.1 |
| 80–84 | 114,086 | 58,140 | 17.9 | 9.1 | 109 | 119 | 15.4 | 16.8 | –2.5 | +7.7 |
| 85–89 | 61,810 | 108,940 | 9.7 | 17.1 | 88 | 112 | 12.4 | 15.8 | +2.7 | –1.3 |
Geographical distribution of participants
Six regions, with multiple sites in each region (a total of 20 sites), were involved in the recruitment of potential participants. Figure 4 shows the locations of participating sites.
FIGURE 4.
Locations of participating recruitment sites.
Each site proposed its own recruitment targets for group 1 and group 2 participants based on various factors, including the size of the trust, geographical location, and local support for ENRICH (Enabling Research in Care Homes) and access to care homes.
Recruitment was co-ordinated by the central DeNDRoN team. Table 4 presents the number of participants recruited, the number of participants who withdrew and the number of participants who completed the eye examination for each of the six regions.
| Region | Group 1 | Group 2 | ||||
|---|---|---|---|---|---|---|
| Recruited | Withdrawn | Participants | Recruited | Withdrawn | Participants | |
| East Anglia | 73 | 8 | 65 | 45 | 4 | 41 |
| North East | 118 | 18 | 100 | 117 | 15 | 102 |
| North Thames | 147 | 11 | 136 | 88 | 13 | 75 |
| Thames Valley | 100 | 15 | 85 | 65 | 6 | 59 |
| North West | 5 | 2 | 3 | 8 | 0 | 8 |
| Yorkshire | 0 | 0 | 0 | 42 | 8 | 34 |
| Study total | 443 | 54 | 389 | 365 | 46 | 319 |
Withdrawals from the study
The 100 out of 808 potential recruits who withdrew from the study before the eye examination represent 12.4% of recruits. One-third of withdrawals were attributable to death (16%) or to deterioration in the health of the participant or carer (17%). One-quarter of withdrawals (25%) were attributable to the participant and/or carer changing their mind after consenting. One-fifth of withdrawals (20%) were attributed to difficulties in appointment scheduling. Four individuals were withdrawn from the study because they were found to be ineligible. The remaining 18 individuals who withdrew from the study did not report a specific reason.
Levels of cognitive impairment in the sample
Participants’ level of cognition was assessed using the sMMSE instrument. It was not possible to complete the sMMSE instrument for 54 out of 708 participants, 52 of whom lived in care homes. These participants mainly comprised those for whom no coherent responses were obtained when attempting the test, and so could not be assessed using the sMMSE, and a small number who were unavailable, asleep or unco-operative on the day of recruitment, and so the test was not carried out. From logistic regression, after adjusting for age and sex, there is a statistically significant group effect on the probability of being unable to obtain a sMMSE score, with the OR for those in care homes of 42.7 (95% CI 12.9 to 264.3; p < 0.001). As a result of the small number of participants in group 1 in whom it was not possible to obtain a sMMSE score (only two participants with missing sMMSE scores), the precision of the OR is low, hence the wide CI. There was a statistically significant age (per year) effect [OR 0.95 (95% CI 0.91 to 1.00); p = 0.04] but no evidence for an independent sex effect [OR 0.63 (95% CI 0.31 to 1.21); p = 0.18].
The mean total sMMSE score, out of a maximum of 30, for the sample as a whole was 16.7 (95% CI 16.1 to 17.3). The mean score for group 1 was 19.8 (95% CI 19.2 to 20.5) and for group 2 was 12.1 (11.2 to 13.1), with a statistically significant group effect from linear regression when adjusted for age and sex: –7.6 (95% CI –8.8 to –6.5; p < 0.001). There was no evidence for independent age or sex effects, with the estimated differences (95% CI) per year of 0.03 (–0.05 to 0.11; p = 0.49) and between males and females of 1.09 (–0.05 to 2.24; p = 0.06).
For the analysis of the range of sMMSE scores in our sample (Tables 5 and 6), each participant was allocated to one of five levels of cognitive impairment based on their total sMMSE score: severe cognitive impairment (sMMSE score of 0–9), moderate cognitive impairment (10–20), mild cognitive impairment (21–24), very mild cognitive impairment (25–27) and no cognitive impairment (28–30). In the context of the PrOVIDe study, the term mild cognitive impairment is being taken to include people in the sMMSE range of 21–24, although mild cognitive impairment has a different meaning in the general literature, where it often refers to people without dementia but who have a performance of 1.5–2 SDs lower on cognitive (usually memory) testing than age-matched norms. People in this group typically have subtle cognitive and functional impairment and are an ‘at risk’ group for future development of dementia.
| sMMSE score (degree of cognitive impairment) | Full sample (N = 654)a | Group 1 (N = 387) | Group 2 (N = 267) | Difference in proportions between groups 1 and 2, %b (95% CI); p-value | |||
|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | ||
| 0–9 (severe cognitive impairment) | 21.1 (138) | 18.1 to 24.5 | 9.3 (36) | 6.7 to 12.8 | 38.2 (102) | 32.4 to 44.3 | –28.9 (–35.7 to –22.1); < 0.01 |
| 10–20 (moderate cognitive impairment) | 39.8 (260) | 36.0 to 43.6 | 34.9 (135) | 30.2 to 39.9 | 46.8 (125) | 40.7 to 53.0 | –11.9 (–19.9 to –4.0); < 0.01 |
| 21–24 (mild cognitive impairment) | 22.2 (145) | 19.1 to 25.6 | 30.5 (118) | 26.0 to 35.4 | 10.1 (27) | 6.9 to 14.5 | 20.4 (14.2 to 26.5); < 0.01 |
| 25–27 (very mild cognitive impairment) | 12.7 (83) | 10.3 to 15.5 | 18.1 (70) | 14.5 to 22.4 | 4.9 (13) | 2.7 to 8.4 | 13.2 (8.3 to 18.2); < 0.01 |
| 28–30 (no cognitive impairment) | 4.3 (28) | 2.9 to 6.2 | 7.2 (28) | 4.9 to 10.4 | 0 (0) | 0 to 1.8 | 7.2 (4.3 to 10.1); < 0.01 |
| sMMSE score (degree of cognitive impairment) | 60–64 years (N = 22) | 65–69 years (N = 50) | 70–74 years (N = 79) | 75–79 years (N = 129) | 80–84 years (N = 228) | 85–89 years (N = 200) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | |
| 0–9 (severe cognitive impairment) | 14.3 (3) | 3.6 to 36.0 | 20.5 (9) | 9.0 to 31.9 | 20.5 (15) | 11.4 to 29.7 | 23.1 (28) | 15.1 to 30.0 | 23.9 (51) | 17.2 to 28.4 | 17.6 (32) | 11.4 to 22.0 |
| 10–20 (moderate cognitive impairment) | 47.6 (10) | 25.1 to 67.3 | 15.9 (7) | 6.3 to 27.4 | 31.5 (23) | 19.7 to 40.6 | 33.1 (40) | 23.3 to 39.8 | 42.3 (90) | 33.1 to 46.2 | 49.5 (90) | 38.0 to 52.2 |
| 21–24 (mild cognitive impairment) | 19.0 (4) | 6.0 to 41.0 | 31.8 (14) | 16.7 to 42.7 | 19.2 (14) | 10.4 to 28.3 | 25.6 (31) | 17.1 to 32.5 | 23.0 (49) | 16.5 to 27.5 | 18.1 (33) | 11.8 to 22.5 |
| 25–27 (very mild cognitive impairment) | 4.8 (1) | 0.2 to 24.9 | 20.5 (9) | 9.0 to 31.9 | 17.8 (13) | 9.4 to 26.9 | 13.2 (16) | 7.5 to 19.6 | 9.9 (21) | 5.9 to 13.9 | 12.6 (23) | 7.6 to 16.9 |
| 28–30 (no cognitive impairment) | 14.3 (3) | 3.6 to 36.0 | 11.4 (5) | 3.7 to 22.6 | 11.0 (8) | 4.8 to 19.5 | 5.0 (6) | 1.9 to 10.3 | 0.9 (2) | 0.2 to 3.5 | 2.2 (4) | 0.6 to 5.4 |
| Missing data,a % (n) | 4.5 (1) | 12.0 (6) | 7.6 (6) | 6.2 (8) | 6.6 (15) | 9.0 (18) | ||||||
It should be noted that sMMSE scores do not equate to dementia severity. They only give a measure of cognitive function, which may be influenced by many factors, including education, motor ability and vision. 112 Similarly, treatments for dementia, notably cholinesterase inhibitors, may have improved sMMSE scores in some of our participants to levels within the more normal range. Other participants may have, at the time of their assessment, achieved a score on the sMMSE in the relatively normal range but will still have significant functional impairment as a consequence of their cognitive impairment. As a result of these functional impairments, not detectable using the relatively insensitive sMMSE, these participants still have clinically defined dementia. Nor does sMMSE pick up on executive dysfunction particularly well, so relatively normal scores may be found in people with frontal variants of dementia.
The principal reason for analysing numbers/percentages of participants in different categories of dementia was to establish that the PrOVIDe sample included an adequate distribution of severity of cognitive impairment across the sMMSE range, and the data presented in Table 5 demonstrate that this aim was achieved. Almost 40% of the sample (260/654) had moderate cognitive impairment (sMMSE score of 10–20), with approximately 20% in each of the mild and severe groups (145/654 and 138/654, respectively). At the time of assessment, 4.3% of the sample (28/654) revealed no cognitive impairment, scoring 28, 29 or 30 on the sMMSE instrument. The difference between distributions of non-missing sMMSE categories in groups 1 and 2 was statistically significant (χ12 p < 0.01).
The variations in the proportions of our sample with different categories of dementia with age are presented in Table 6. There is a difference in the distribution of non-missing cognitive impairment categories with age (overall chi-squared test, using Monte Carlo adjustment for small cell counts, p < 0.001). This trend is also evident in the scatterplot of sMMSE score versus age in Figure 5. The curved, dashed line in the scatterplot is a ‘Friedman super-smoother line’, a non-parametric regression estimator based on local linear regression with adaptive bandwidths. It is essentially a fitted line following the mean of y at a given x, which is required to be smooth but allowed to curve upwards and downwards. 113
FIGURE 5.
Scatterplot of sMMSE score vs. age.
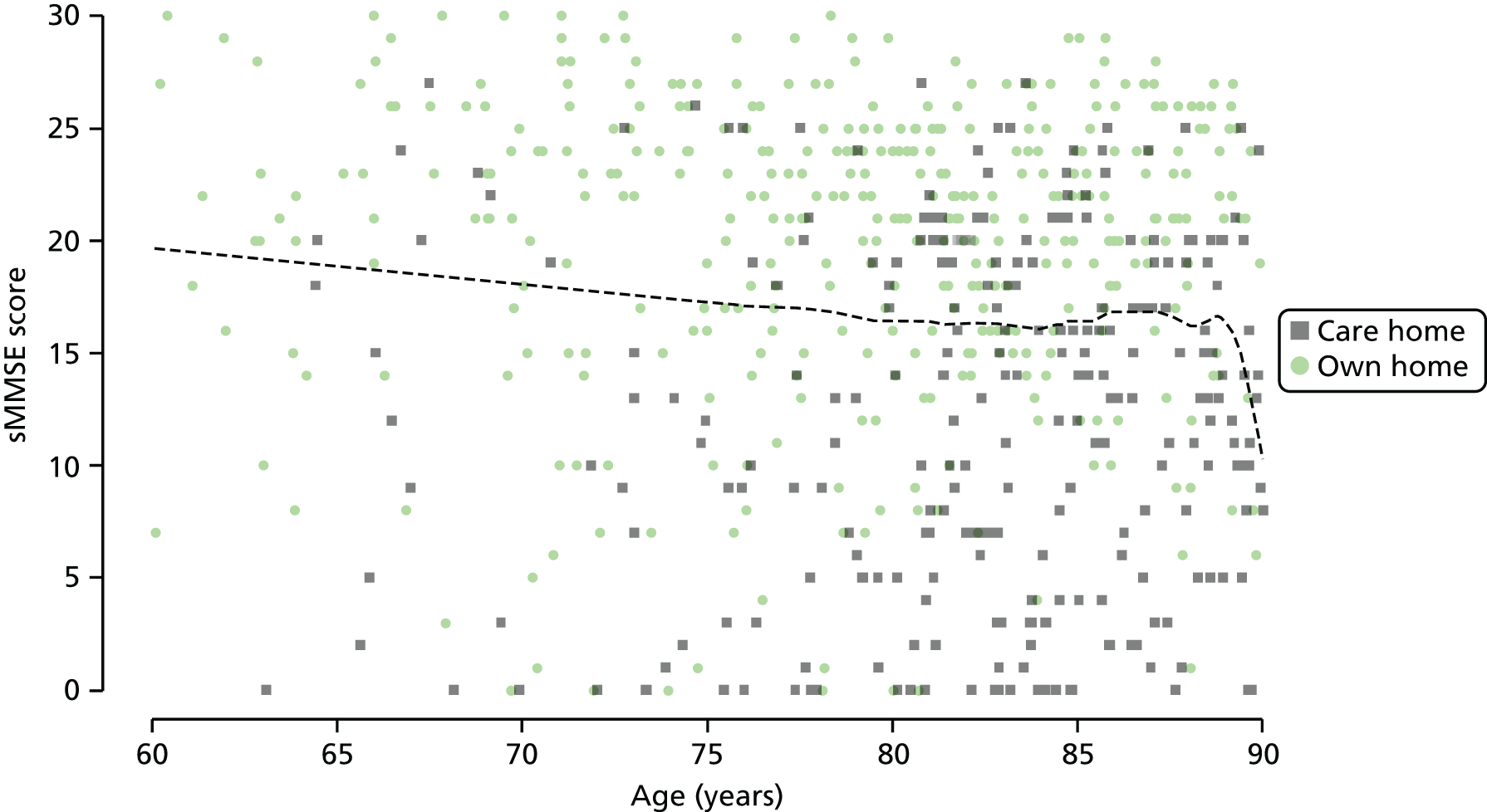
Eye examination data
Eye examination data were collected by 14 TOC optometrists. The average number of eye examinations performed by each optometrist was 51 (SD 35, range 11–131). Location was a major factor affecting the number of eye examinations by each optometrist, as each worked in a specific region.
Optometrists were able to perform an eye examination, although not necessarily a full eye examination, on all participants living in their own homes (group 1; n = 389). Optometrists were unable to perform any part of the eye examination on eight participants living in care homes (group 2; n = 319). The ages and sMMSE scores for these eight participants were: 85 years, sMMSE = 13; 63 years, sMMSE = 0; 82 years, sMMSE = 7; 85 years, sMMSE = 16; 87 years, sMMSE = 19; 80 years, sMMSE = 5; 85 years, sMMSE not possible; and 73 years, sMMSE not possible. Hence an eye examination, albeit often not a full eye examination, was performed on the remaining 311 participants in group 2. A full eye examination was sometimes not possible because optometrists were unable to perform one or more elements of the eye examination on the participant (see Ability of participants to complete individual elements of the eye examination). Eleven participants were examined twice by TOC optometrists, in line with TOC policy for situations in which the first examination failed to yield adequate results, or if the patient or carer requested a second attempt, or in instances where a recheck examination was indicated (e.g. if a participant failed to adapt well to new spectacles). It is unlikely that a second examination would have been attempted in most of these cases had the participant been examined as part of normal optometric domiciliary care, and therefore only data from the participant’s first eye examination have been included in the analysis.
Prevalence of visual impairment
Prevalence of VI (Table 7) is described in terms of:
-
presenting VI, based on best monocular VA recorded with the participant wearing their current distance spectacles or unaided if no spectacles were worn for distance vision
-
post-refraction (or best corrected) VI, based on best VA recorded with the participant wearing the prescription determined by the PrOVIDe optometrist
-
uncorrected or undercorrected VI, which includes those participants who have presenting VI but who were not visually impaired wearing the prescription determined by the PrOVIDe optometrist.
| Type of VI | Full sample (N = 708) | Group 1 (N = 389) | Group 2 (N = 319) | Difference in proportions between groups 1 and 2%a (95% CI); p-value | |||
|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | ||
| VA < 6/12 | |||||||
| Presenting | 32.5 (191) | 28.7 to 36.5 | 21.8 (82) | 17.8 to 26.4 | 51.4 (109) | 44.5 to 58.3 | –29.6 (–37.9 to –21.3); < 0.001 |
| Missing data | 16.9 (120) | 3.3 (13) | 33.5 (107) | ||||
| Uncorrected or undercorrected | 14.3 (84) | 11.7 to 17.5 | 10.6 (40) | 7.8 to 14.3 | 21.0 (44) | 15.8 to 27.2 | –10.4 (–17.0 to –3.6); < 0.01 |
| Missing data | 17.2 (122) | 3.3 (13) | 34.2 (109) | ||||
| Post refraction | 18.1 (107) | 15.2 to 21.5 | 11.4 (43) | 8.4 to 15.1 | 30.2 (64) | 24.2 to 36.9 | –18.8 (–26.1 to –11.5); < 0.001 |
| Missing data | 16.7 (118) | 2.8 (11) | 33.5 (107) | ||||
| VA < 6/18 | |||||||
| Presenting | 16.3 (96) | 13.5 to 19.6 | 10.6 (40) | 7.8 to 14.3 | 26.4 (56) | 20.7 to 33.0 | –15.8 (–22.8 to –8.7); < 0.001 |
| Missing data | 16.9 (120) | 3.3 (13) | 33.5 (107) | ||||
| Uncorrected or undercorrected | 7.7 (45) | 5.7 to 10.2 | 5.1 (19) | 3.2 to 7.9 | 12.4 (26) | 8.4 to 17.8 | –7.3 (–12.7 to –2.0); < 0.01 |
| Missing data | 17.2 (122) | 3.3 (13) | 34.2 (109) | ||||
| Post refraction | 8.6 (51) | 6.6 to 11.3 | 5.6 (21) | 3.6 to 8.5 | 14.2 (30) | 9.9 to 19.7 | –8.6 (–14.2 to –3.0); < 0.001 |
| Missing data | 16.7 (118) | 2.8 (11) | 33.5 (107) | ||||
Flow charts that illustrate the stages involved in identifying each of the three types of VI, and which present the numbers of visually impaired for each type of VI, are given in Figure 6 for the VI criterion of VA < 6/12, and in Figure 7 for VA < 6/18.
FIGURE 6.
Identification of presenting VI, post-refraction VI and uncorrected/undercorrected VI for best monocular VA < 6/12 criterion. a, Includes one participant for whom VA could not be measured post refraction but presenting VA could be measured.
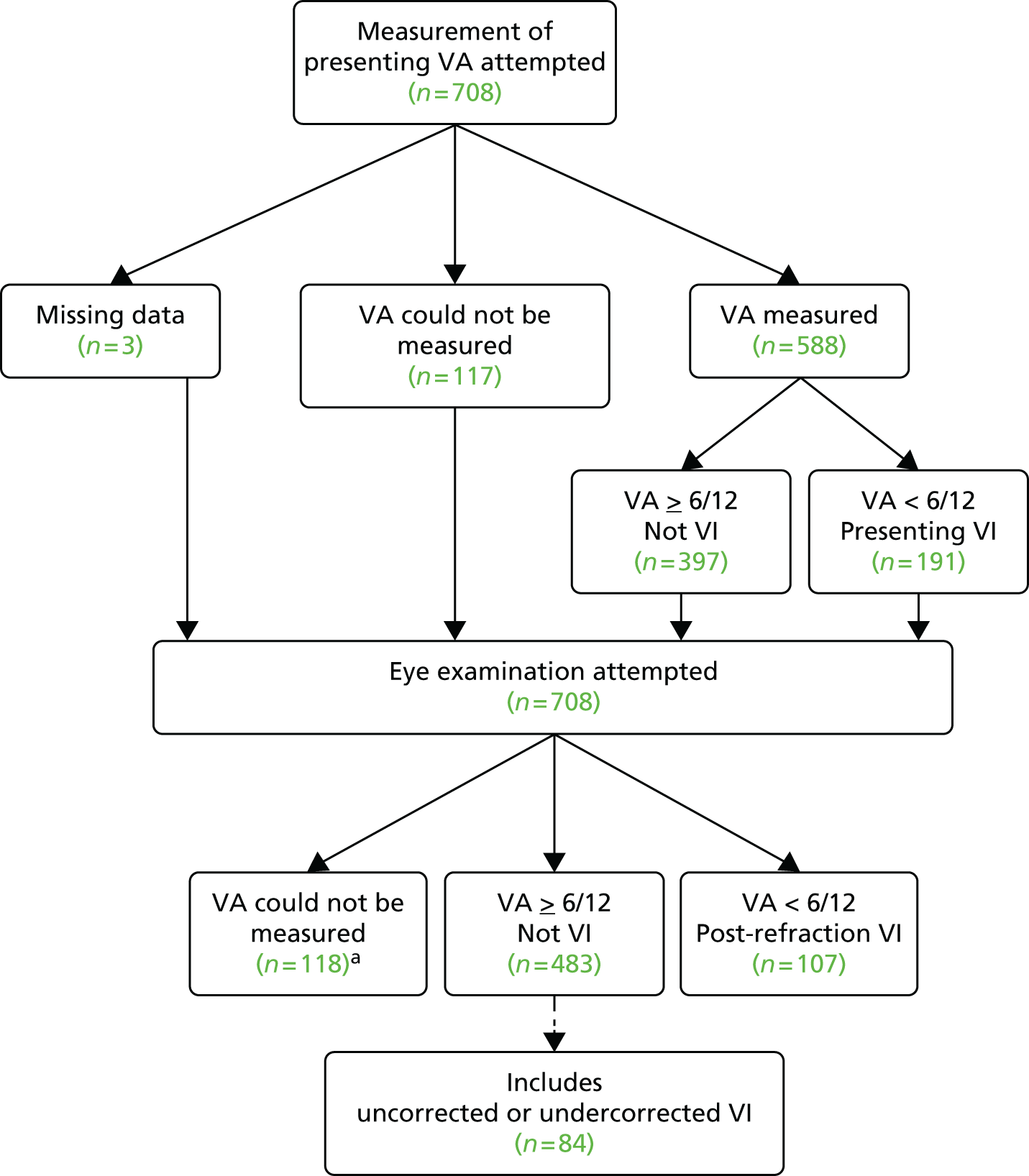
FIGURE 7.
Identification of presenting VI, post-refraction VI and uncorrected/undercorrected VI for best monocular VA < 6/18 criterion. a, Includes one participant in whom VA could not be measured post refraction but presenting VA could be measured.

-
Presenting VI It was not possible to measure the presenting best monocular VA in 117 out of 708 participants (16.5%, 95% CI 13.9% to 19.5%), with 11 out of 389 (2.8%, 95% CI 1.5% to 5.2%) in group 1, and 106 out of 319 (33.2%, 95% CI 28.1% to 38.7%) in group 2. In 0.4% (3/708) of participants, the presenting best monocular VA data were missing. The overall prevalence of VI defined by the criterion of VA < 6/12 was 191 out of 588 (32.5%, 95% CI 28.7% to 36.5%) and for VA < 6/18 was 96 out of 588 (16.3%, 95% CI 13.5% to 19.6%) (see Table 7).
-
Post-refraction VI Post-refraction VAs could not be recorded in 118 out of 708 participants (16.7%, 95% CI 14.2% to 19.7%). The overall prevalence of VI for VA < 6/12 was 107 out of 590 (18.1%, 95% CI 15.2% to 21.5%) and for VA < 6/18 was 51 out of 590 (8.6%, 95% CI 6.6% to 11.3%).
-
Uncorrected or undercorrected VI The prevalence of uncorrected or undercorrected VI was 84 out of 586 (14.3%, 95% CI 11.7% to 17.5%) for VA < 6/12 and 45/586 (7.7%, 95% CI 5.7% to 10.2%) for VA < 6/18.
-
Group 1 versus group 2 Differences in prevalence between groups 1 and 2 were statistically significant for both the 6/12 and 6/18 criteria for presenting, uncorrected or undercorrected and post-refraction VI (see Table 7).
Factors associated with visual impairment
The variation in prevalence with age for each type of VI (VA < 6/12 and VA < 6/18) is shown in Table 8. The highest prevalences for presenting and post-refraction VI were in the 80–84 years and 85–89 years age groups, with a more even distribution of prevalences across the age bands for uncorrected or undercorrected VI. The variation in prevalence with category of severity of cognitive impairment for VA < 6/12 is shown in Table 9 and for VA < 6/18 is shown in Table 10. Prevalences for presenting and post-refraction VI for both cut-off points were highest in the severe category for cognitive impairment, with prevalences tending to reduce with lesser degrees of cognitive impairment. There was a similar trend for uncorrected/undercorrected VI for VA < 6/18. However, for uncorrected/undercorrected VI for VA < 6/12, there was a more even distribution of prevalences across the categories of cognitive impairment. The data in Tables 8–10 were analysed by logistic regression analysis, summarised in Table 11.
| Type of VI | 60–64 years (N = 22) | 65–69 years (N = 50) | 70–74 years (N = 79) | 75–79 years (N = 129) | 80–84 years (N = 228) | 85–89 years (N = 200) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | |
| VA < 6/12 | ||||||||||||
| Presenting | 5.0 (1) | 0.3 to 26.9 | 21.6 (8) | 10.4 to 38.7 | 24.2 (16) | 14.9 to 36.6 | 24.1 (26) | 16.6 to 33.4 | 36.9 (69) | 30.1 to 44.3 | 41.8 (71) | 34.3 to 49.6 |
| Missing data | 9.1 (2) | 26.0 (13) | 16.5 (13) | 16.3 (21) | 18.0 (41) | 15.0 (30) | ||||||
| Uncorrected or undercorrected | 0.0 (0) | 0.0 to 20.0 | 16.2 (6) | 6.8 to 32.7 | 10.6 (7) | 4.7 to 21.2 | 13.9 (15) | 8.2 to 22.2 | 16.6 (31) | 11.7 to 22.9 | 14.9 (25) | 10.0 to 21.4 |
| Missing data | 9.1 (2) | 26.0 (13) | 16.5 (13) | 16.3 (21) | 18.0 (41) | 16.0 (32) | ||||||
| Post refraction | 5.0 (1) | 0.3 to 26.9 | 5.4 (2) | 0.9 to 19.5 | 13.4 (9) | 6.7 to 24.5 | 11.0 (12) | 6.1 to 18.8 | 20.2 (38) | 14.9 to 26.8 | 26.6 (45) | 20.3 to 34.1 |
| Missing data | 9.1 (2) | 26.0 (13) | 15.2 (12) | 15.5 (20) | 17.5 (40) | 15.5 (31) | ||||||
| VA < 6/18 | ||||||||||||
| Presenting | 5.0 (1) | 0.3 to 26.9 | 13.5 (5) | 5.1 to 29.6 | 10.6 (7) | 4.7 to 21.2 | 10.2 (11) | 5.4 to 17.9 | 15.0 (28) | 10.3 to 21.1 | 25.9 (44) | 19.6 to 33.3 |
| Missing data | 9.1 (2) | 26.0 (13) | 16.5 (13) | 16.3 (21) | 18.0 (41) | 15.0 (30) | ||||||
| Uncorrected or undercorrected | 5.0 (1) | 0.3 to 26.9 | 13.5 (5) | 5.1 to 29.6 | 4.5 (3) | 1.2 to 13.6 | 6.5 (7) | 2.9 to 13.4 | 5.3 (10) | 2.7 to 9.9 | 11.3 (19) | 7.1 to 17.3 |
| Missing data | 9.1 (2) | 26.0 (13) | 16.5 (13) | 16.3 (21) | 18.0 (41) | 16.0 (32) | ||||||
| Post refraction | 0.0 (0) | 0.0 to 20.0 | 2.7 (1) | 0.1 to 15.8 | 6.0 (4) | 1.9 to 15.3 | 3.7 (4) | 1.2 to 9.7 | 9.6 (18) | 5.9 to 14.9 | 14.2 (24) | 9.5 to 20.6 |
| Missing data | 9.1 (2) | 26.0 (13) | 15.2 (12) | 15.5 (20) | 17.5 (40) | 15.5 (31) | ||||||
| Type of VI | Severe (0–9) (N = 138) | Moderate (10–20) (N = 260) | Mild (21–24) (N = 145) | Very mild (25–27) (N = 83) | None (28–30) (N = 28) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | |
| VA < 6/12 | ||||||||||
| Presenting VI | 48.1 (37) | 36.6 to 59.7 | 36.2 (89) | 30.2 to 42.6 | 25.7 (37) | 18.9 to 33.8 | 28.0 (23) | 19.0 to 39.2 | 3.6 (1) | 0.2 to 20.2 |
| Missing data | 44.2 (61) | 5.4 (14) | 0.7 (1) | 1.2 (1) | 0.0 (0) | |||||
| Uncorrected or undercorrected VI | 13.2 (10) | 6.8 to 23.3 | 17.1 (42) | 12.8 to 22.6 | 11.8 (17) | 7.2 to 18.5 | 15.9 (13) | 9.0 to 26.0 | 3.6 (1) | 0.2 to 20.2 |
| Missing data | 44.9 (62) | 5.8 (15) | 0.7 (1) | 1.2 (1) | 0.0 (0) | |||||
| Post-refraction VI | 33.8 (26) | 23.6 to 45.5 | 19.4 (48) | 14.7 to 24.9 | 13.9 (20) | 8.9 to 20.9 | 12.2 (10) | 6.3 to 21.7 | 0 (0) | 0 to 15.0 |
| Missing data | 44.2 (61) | 4.6 (12) | 0.7 (1) | 1.2 (1) | 0 (0) | |||||
| Type of VI | Severe (0–9) (N = 138) | Moderate (10–20) (N = 260) | Mild (21–24) (N = 145) | Very mild (25–27) (N = 83) | None (28–30) (N = 28) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | |
| VA < 6/18 | ||||||||||
| Presenting | 29.9 (23) | 20.2 to 41.5 | 19.5 (48) | 14.9 to 25.1 | 9.7 (14) | 5.6 to 16.1 | 11.0 (9) | 5.5 to 20.3 | 0.0 (0) | 0 to 15.0 |
| Missing data | 44.2 (61) | 5.4 (14) | 0.7 (1) | 1.2 (1) | 0.0 (0) | |||||
| Uncorrected or undercorrected | 11.8 (9) | 5.9 to 21.8 | 10.6 (26) | 7.2 to 15.3 | 3.5 (5) | 1.3 to 8.3 | 4.9 (4) | 1.6 to 12.7 | 0.0 (0) | 0 to 15.0 |
| Missing data | 44.9 (62) | 5.8 (15) | 0.7 (1) | 1.2 (1) | 0.0 (0) | |||||
| Post refraction | 16.9 (13) | 9.6 to 27.5 | 9.3 (23) | 6.1 to 13.8 | 6.2 (9) | 3.1 to 11.9 | 6.1 (5) | 2.3 to 14.3 | 0.0 (0) | 0 to 15.0 |
| Missing data | 44.2 (61) | 4.6 (12) | 0.7 (1) | 1.2 (1) | 0.0 (0) | |||||
| Type of VI | Variable | OR | p-value | 95% CI |
|---|---|---|---|---|
| VA < 6/12 | ||||
| Presenting VI | Age (per year) | 1.05 | < 0.01 | 1.02 to 1.08 |
| Sex (male) | 0.71 | 0.08 | 0.48 to 1.04 | |
| Group 2 | 3.19 | < 0.001 | 2.19 to 4.66 | |
| Post-refraction VI | Age (per year) | 1.07 | < 0.001 | 1.03 to 1.12 |
| Sex (male) | 0.80 | 0.35 | 0.50 to 1.27 | |
| Group 2 | 2.71 | < 0.001 | 1.74 to 4.26 | |
| Uncorrected/undercorrected VI | Age (per year) | 1.00 | 0.83 | 0.97 to 1.04 |
| Sex (male) | 0.69 | 0.16 | 0.41 to 1.14 | |
| Group 2 | 2.19 | < 0.01 | 1.30 to 3.73 | |
| sMMSE (per unit score) | 1.00 | 0.87 | 0.97 to 1.04 | |
| VA < 6/18 | ||||
| Presenting VI | Age (per year) | 1.05 | 0.01 | 1.01 to 1.10 |
| Sex (male) | 0.93 | 0.78 | 0.58 to 1.50 | |
| Group 2 | 2.52 | < 0.001 | 1.59 to 4.03 | |
| Post-refraction VI | Age (per year) | 1.10 | < 0.01 | 1.04 to 1.17 |
| Sex (male) | 1.84 | 0.05 | 1.00 to 3.37 | |
| Group 2 | 2.26 | 0.01 | 1.23 to 4.20 | |
| Uncorrected/undercorrected VI | Age (per year) | 0.99 | 0.56 | 0.94 to 1.04 |
| Sex (male) | 0.45 | 0.04 | 0.21 to 0.92 | |
| Group 2 | 1.99 | 0.05 | 0.99 to 4.02 | |
| sMMSE (per unit score) | 0.95 | 0.03 | 0.91 to 1.00 | |
Relationship between Standardised Mini-Mental State Examination score and visual acuity
An alternative method of presenting the relationship between participants’ level of cognitive impairment, as measured by sMMSE score, and their visual performance as defined by their level of VA, is shown in the box and whisker plots in Figure 8. Participants with best monocular VA of > 6/12 have been classified as ‘level 0’, participants with VA between 6/12 and 6/18 as ‘level 1’, and those with VA worse than 6/18 as ‘level 2’. There is a trend for presenting VA to decline with decreasing sMMSE score. For post-refraction VA, sMMSE scores are higher in level 0 participants than in those in either level 1 or level 2. Figure 8c illustrates the change in VA level plotted against sMMSE score; for example, a participant who improved from < 6/18 (level 2) to > 6/12 (level 0) would be classified as –2 in terms of change in VA level. There is a trend for those participants with the greatest changes in level (improvement in VA) to have lower sMMSE scores.
FIGURE 8.
Box and whisker plots of sMMSE score for VA defined by VA > 6/12 (level 0), for VA between 6/12 and 6/18 (level 1), and for VA < 6/18 (level 2), for (a) presenting VA; (b) post-refraction VA; and (c) box and whisker plot of sMMSE score for change in VA level between presenting and post-refraction measurement.

Logistic regression analysis
For both presenting and post-refraction VI there were statistically significant independent age and group differences for both the VA < 6/12 and VA < 6/18 criteria (see Table 11), with adjusted OR estimates (per year) of between 1.05 and 1.10 (p ≤ 0.01). There was little evidence for consistent independent sex differences on VI.
For uncorrected/undercorrected VI defined by VA < 6/12, there was no evidence for any independent age or sex effects (see Table 11). After adjusting for age, sex and group there was no evidence for any independent effect of sMMSE score, OR 1.00 (95% CI 0.97 to 1.04; p = 0.87). After adjusting for age, sex and sMMSE score there was a statistically significant group difference, with the OR for those participants living in care homes compared with those living at home being 2.19 (95% CI 1.30 to 3.73; p < 0.01).
For uncorrected/undercorrected VI defined by VA < 6/18, there was no evidence of any independent change with age (p = 0.56). Sex, sMMSE score and group all exhibited statistically significant independent differences on the prevalence of VI with OR estimates of 0.45 (male vs. female, p = 0.04), 0.95 (sMMSE, per unit score, p = 0.03) and group 1.99 (care homes vs. own homes, p = 0.05) (see Table 11).
Time elapsed since last eye examination
Data were gathered on the time that had elapsed since each participant’s last eye examination. Although some participants were able to give an exact date (usually taken from the form on which their last spectacle prescription was written), for the majority of participants this was an approximate date. Figure 9 shows these data for groups 1 and 2 separately, presented as a bar chart. No data on the time since last eye examination could be obtained from 7 out of 708 participants, all of whom lived in care homes.
FIGURE 9.
Time elapsed since participants’ last eye examination for groups 1 and 2.
These data are presented as a histogram in Figure 10. The research optometrists recorded the time since last eye examination using the following non-linear scale: 0–1 month, 1–2 months, 2–3 months, 3–4 months, 4–5 months, 5–6 months, 6–12 months, 12–24 months, 24–36 months, 36–48 months, 48–60 months, 60–72 months, 72–84 months, 84–96 months, 96–108 months and 108–120 months.
FIGURE 10.
Histogram of time elapsed since participants’ last eye examination for group 1 (participants living in their own homes) and group 2 (participants living in care homes).
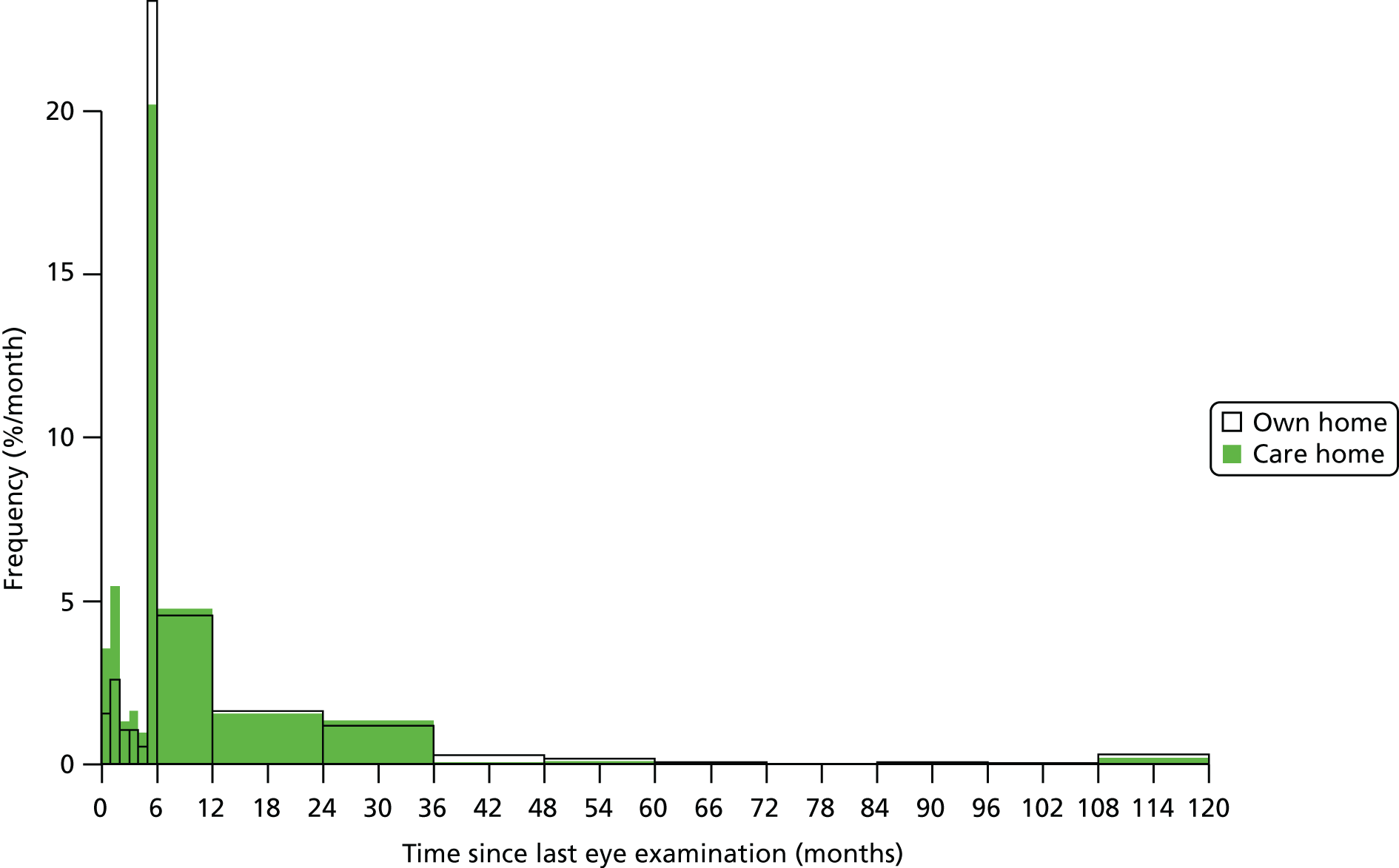
Predictors of time since last eye examination
The time since last eye examination was recorded in intervals that varied from 0–1 month and 1–2 months, up to 108–120 months, with the time intervals becoming increasingly coarse as time increased. The independent (of age, sex and sMMSE score) associations of the time interval since the last eye examination were examined using two different regression analyses that included age, sex, sMMSE score and group (participants living in care homes or living in their own homes) as covariates. These were (1) a continuous ‘coarse’ regression with a log-link function, modelling times within intervals using a latent model and giving rate ratios114 and (2) an ordinal logistic regression model giving ORs for a change from one time interval to a later one. Analysis 1 estimated a rate ratio of 0.78 (95% CI 0.65 to 0.93) for group, implying a reduction of 22% in the time since the last eye examination in care home residents, compared with those living in their own homes. Analysis 2 estimated an OR of 0.70 (95% CI 0.51 to 0.97) for group, implying an increased probability for a shorter time interval between eye examinations for care home residents than for those living in their own homes. Neither analysis provided evidence for an association of time since last eye examination with age, sex or sMMSE score. In summary, living in a care home is independently (of age, sex and sMMSE score) associated with a shorter interval since last eye examination than living at home.
Independent association between time since last examination and uncorrected/undercorrected visual impairment
The time interval since last eye examination was examined as a predictor for uncorrected/undercorrected VI. The mid-points of all time intervals were calculated and time since last examination was then added as a continuous covariate to the primary model for uncorrected/undercorrected VI, including age, sex, group and sMMSE scores. For VI defined as VA of < 6/12, there appeared to be no association (independent of age, sex, sMMSE score and group) between the time since last eye examination and the probability of having uncorrected/undercorrected VI, with an OR of 1.04 (95% CI 0.89 to 1.19). However, for VI defined as VA of < 6/18, there was evidence of an association (independent of age, sex and group) between the time since last eye examination and the probability of having uncorrected/undercorrected VI, with an OR of 1.23 (95% CI 1.04 to 1.42). Here, ORs were expressed as the increase in odds for VI for every year increase in the time since last eye examination.
The independent association of group (participants living in care homes or their own homes) with VI in this analysis had adjusted ORs of VI of 2.24 (95% CI 1.32 to 3.82) for VA of < 6/12 and 2.34 (95% CI 1.14 to 4.86) for VA of < 6/18 in those living in care homes versus those living in their own homes. Thus, an individual living in a care home was more likely to have uncorrected/undercorrected VI than an individual living in their own home, even if both had the same time interval since their last eye examination. These ORs were slightly increased compared with the model when it does not include the time since last eye examination: 2.19 (95% CI 1.30 to 3.73) for VA of < 6/12 and 1.99 (95% CI 0.99 to 4.02) for VA of < 6/18. This finding reflects the shorter time since the last eye examination in those living in care homes versus those living in their own homes.
Prevalence of specified eye conditions
Before the reporting of the prevalence of eye conditions, it should be pointed out that optometrists use a variety of methods to grade the severity of AMD, diabetic retinopathy and cataract. For this study, AMD was classified into dry and wet (neovascular) AMD and graded as mild, moderate or severe. Diabetic retinopathy was graded as background diabetic retinopathy; mild, moderate or severe non-proliferative retinopathy; proliferative retinopathy; and diabetic maculopathy. When calculating the prevalence of AMD, diabetic retinopathy and glaucoma in this study, a participant was considered as being positive for the condition irrespective of the severity recorded by the optometrist.
The prevalences of the four specified conditions (AMD, cataract, diabetic retinopathy and glaucoma) are presented in Table 12. Some participants had more than one of these conditions. A total of 185 out of 695 participants (26.6%) (108/388, 27.8%, of group 1 participants, and 77/307, 25.1%, of group 2 participants) already had an intraocular lens (IOL) in at least one eye following cataract surgery. Currently these participants have been classified in this table as not having cataract, even if there was cataract in one eye. The rationale for this decision was that for these participants cataract was not impairing their vision under binocular conditions. For a more detailed analysis of participants with IOLs see Factors associated with the PrOVIDe specified conditions.
| Condition | Full sample (N = 708) | Group 1 (N = 389) | Group 2 (N = 319) | Difference in proportions between groups 1 and 2, %a (95% CI); p-value | |||
|---|---|---|---|---|---|---|---|
| % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | ||
| AMD | 17.7 (123) | 15.0 to 20.8 (2.0) | 18.3 (71) | 14.7 to 22.6 (0.3) | 17.0 (52) | 13.1 to 21.8 (4.1) | 1.3 (–4.7 to 7.3); 0.73 |
| Cataractb | 59.0 (410) | 55.2 to 62.7 (1.8) | 55.9 (217) | 50.8 to 60.9 (0.3) | 62.9 (193) | 57.2 to 68.2 (3.8) | –7.0 (–14.6 to 0.7); 0.08 |
| Diabetic retinopathy | 2.0 (14/699) | 1.1 to 3.4 (N/A) | 2.0 (7/389) | 0.8 to 3.8 (N/A) | 2.3 (7/310) | 1.0 to 4.8 (N/A) | –0.3 (–2.9 to 1.9); 0.79 |
| Glaucomac | 7.1 (49/694) | 5.3 to 9.3 (2.0) | 8.0 (31/388) | 5.6 to 11.3 (0.3) | 5.7 (18/306) | 3.6 to 9.3 (4.1) | 2.3 (–2.0 to 6.2); 0.35 |
Factors associated with the PrOVIDe specified conditions
Cataract, glaucoma and AMD all become more prevalent with increasing age in the general UK population. Table 13 presents prevalence data for each condition, with our age range (60–89 years) subdivided into six equal 5-year ranges. This subdivision also facilitates comparison with published studies (see Discussion), which include participants in a variety of age groups. The cataract grading scale (see Chapter 2, Data collection, Stage 1) grades cataract from 0 to 5, according to the density of the opacification, where grade 0 is no cataract visible to the PrOVIDe optometrist on ophthalmoscopy. Grades 1–5 are visible to the optometrist and the prevalence of these cataracts is presented in Table 13. Grade 1 cataract is unlikely to have a significant effect on the participants’ VA, unlike grades 2–5. Therefore, the prevalences of cataracts of grades 2–5 are also given in Table 13.
| Condition | 60–64 years (N = 22) | 65–69 years (N = 50) | 70–74 years (N = 79) | 75–79 years (N = 129) | 80–84 years (N = 228) | 85–89 years (N = 200) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | |
| AMD | 0 (0) | 0.0 to 19.2 (4.5) | 2.0 (1) | 0.1 to 12.0 (0) | 5.2 (4) | 1.7 to 13.5 (2.5) | 10.2 (13) | 5.7 to 17.1 (0.8) | 14.7 (33) | 10.4 to 20.1 (1.3) | 37.3 (72) | 30.5 to 44.6 (3.5) |
| Cataract (grades 1–5) | 38.1 (8) | 19.0 to 61.3 (4.5) | 46.0 (23) | 32.1 to 60.5 (0) | 59.7 (46) | 47.9 to 70.6 (2.5) | 62.0 (80) | 53.0 to 70.3 (0) | 63.8 (143) | 57.1 to 70.1 (1.8) | 56.1 (110) | 48.9 to 63.1 (2.0) |
| Cataract (grades 2–5) | 14.3 (3) | 3.8 to 37.4 (4.5) | 24.0 (12) | 13.5 to 38.5 (0) | 28.6 (22) | 19.1 to 40.2 (2.5) | 37.2 (48) | 29.0 to 46.2 (0) | 42.9 (96) | 36.3 to 49.6 (1.8) | 42.8 (83) | 35.4 to 49.6 (2.0) |
| Diabetic retinopathy | 9.5 (2) | 1.7 to 31.8 (4.5) | 2.0 (1) | 0.1 to 12.0 (0) | 1.3 (1) | 0.1 to 8.0 (2.5) | 3.9 (5) | 1.4 to 9.3 (0.8) | 2.2 (5) | 0.8 to 5.4 (1.3) | 0 | 0.0 to 2.4 (3.5) |
| Glaucoma | 14.3 (3) | 3.8 to 37.4 (4.5) | 6.0 (3) | 1.6 to 17.5 (0) | 7.1 (6) | 3.2 to 16.8 (2.5) | 5.5 (7) | 2.4 to 11.4 (0.8) | 7.1 (16) | 4.3 to 11.5 (1.3) | 8.3 (16) | 5.0 to 13.3 (3.5) |
Cataract prevalence increases initially with age but then reaches a plateau, which reflects the increasing proportion of participants who have undergone cataract surgery and had an IOL implant in one or both eyes with age. For ease of interpretation of the data we have until now regarded participants who have an IOL in one eye as not having cataract on presentation. A more detailed breakdown of the crystalline lens status of the sample is given in Table 14.
| Cataract/IOL status | 60–64 years (N = 22) | 65–69 years (N = 50) | 70–74 years (N = 79) | 75–79 years (N = 129) | 80–84 years (N = 228) | 85–89 years (N = 200) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | |
| No cataract (grade 0) | 52.4 (11) | 30.3 to 73.6 (4.5) | 46.0 (23) | 32.1 to 60.5 (0) | 20.8 (16) | 12.7 to 31.8 (2.5) | 17.1 (22) | 11.2 to 24.9 (0) | 8.0 (18) | 5.0 to 12.6 (1.8) | 5.2 (10) | 2.6 to 9.5 (3.0) |
| Cataract (grades 1–5) | 38.1 (8) | 19.0 to 61.3 (4.5) | 46.0 (23) | 32.1 to 60.5 (0) | 59.7 (46) | 47.9 to 70.6 (2.5) | 62.0 (80) | 53.0 to 70.3 (0) | 63.8 (143) | 57.1 to 70.1 (1.8) | 56.1 (110) | 49.4 to 63.7 (3.0) |
| IOL in one eye and no cataract in the other eye | 0 | 0.0 to 19.2 (4.5) | 0 | 0.0 to 8.9 (0) | 0 | 0.0 to 5.9 (2.5) | 0.8 (1) | 0.0 to 4.9 (0) | 0.9 (2) | 0.2 to 3.5 (1.8) | 1.0 (2) | 0.2 to 4.1 (3.0) |
| IOL in one eye and grades 1–5 cataract in the other eye | 0 | 0.0 to 19.2 (4.5) | 4.0 (2) | 0.7 to 14.9 (0) | 6.5 (5) | 2.4 to 15.2 (2.5) | 6.2 (7) | 2.4 to 11.3 (0) | 6.7 (13) | 3.3 to 9.9 (1.8) | 8.8 (15) | 4.5 to 12.7 (3.0) |
| IOL in both eyes | 9.5 (2) | 1.7 to 31.8 (4.5) | 4.0 (2) | 0.7 to 14.9 (0) | 13.0 (10) | 6.7 to 23.0 (2.5) | 14.7 (19) | 9.3 to 22.3 (0) | 21.4 (48) | 16.4 to 27.5 (1.8) | 29.4 (57) | 23.2 to 36.4 (3.0) |
The influence of IOLs on the prevalence of cataract with age is illustrated in Figure 11, with the proportion of participants with IOLs almost doubling between 75–79 and 85–89 years, while the proportion of participants with cataract actually falls in the oldest age group.
FIGURE 11.
Prevalence of cataract and IOLs stratified into six age categories.
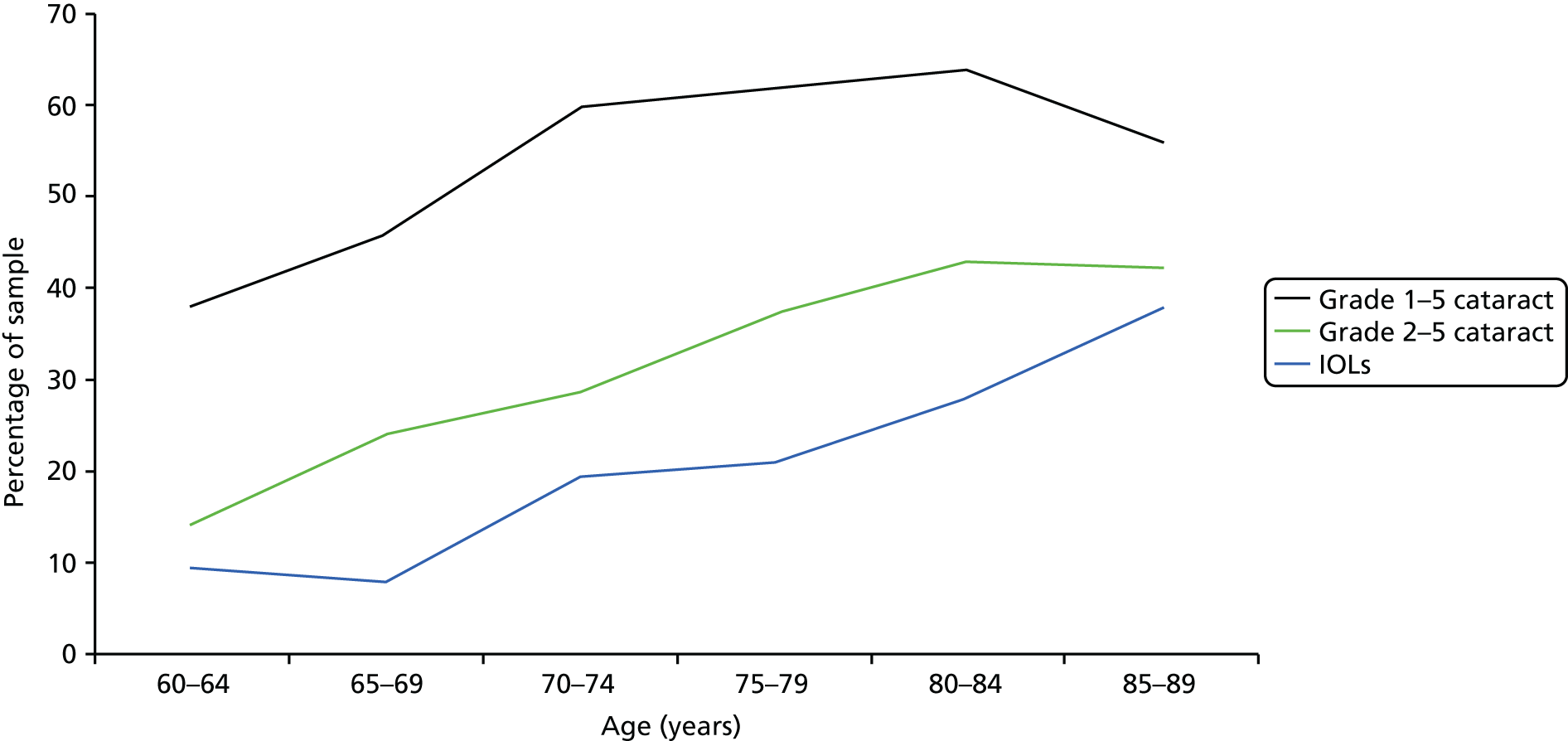
The results of the multivariable logistic regression analysis on the presence of each condition are presented in Table 15. After adjusting for sex and group there was a statistically significant age effect for AMD, cataract grades 1–5 and cataract grades 2–5 (p < 0.001). There is weak evidence for an independent age effect on diabetic retinopathy and no evidence for one on glaucoma/OHT, with OR estimates of 0.93 (per year, p = 0.06) and 1.01 (per year, p = 0.51), respectively.
| Condition | Variable | OR | p-value | 95% CI |
|---|---|---|---|---|
| AMD | Age (per year) | 1.18 | < 0.001 | 1.13 to 1.24 |
| Sex (M) | 0.79 | 0.31 | 0.50 to 1.24 | |
| Group 2 | 0.59 | 0.01 | 0.38 to 0.90 | |
| Cataracta (grades 1–5) | Age (per year) | 1.14 | < 0.001 | 1.10 to 1.18 |
| Sex (M) | 0.80 | 0.35 | 0.51 to 1.28 | |
| Group 2 | 0.90 | 0.66 | 0.56 to 1.45 | |
| Cataracta (grades 2–5) | Age (per year) | 1.11 | < 0.001 | 1.08 to 1.14 |
| Sex (M) | 0.81 | 0.22 | 0.57 to 1.14 | |
| Group 2 | 0.96 | 0.83 | 0.68 to 1.36 | |
| Diabetic retinopathy | Age (per year) | 0.93 | 0.06 | 0.87 to 1.00 |
| Sex (M) | 3.69 | 0.03 | 1.19 to 13.80 | |
| Group 2 | 1.90 | 0.26 | 0.62 to 5.89 | |
| Glaucoma or OHT | Age (per year) | 1.01 | 0.51 | 0.98 to 1.06 |
| Sex (M) | 1.43 | 0.18 | 0.85 to 2.39 | |
| Group 2 | 0.68 | 0.17 | 0.39 to 1.16 |
There was a statistically significant sex effect for diabetic retinopathy (male vs. female OR 3.69) when adjusted for age and group (p = 0.03). However, there were only 14 participants in the sample with diabetic retinopathy, so all the OR estimates for this condition should be interpreted with caution. There is little evidence of an independent sex effect for AMD (p = 0.31), cataract grades 1–5 (p = 0.35), cataract grades 2–5 (p = 0.22) or glaucoma/OHT (p = 0.18).
After adjusting for age and sex, there was a statistically significant group effect for AMD (group 2 vs. group 1, OR 0.59; p = 0.01). There was no evidence for an independent group effect for cataract grades 1–5 (p = 0.66) and cataract grades 2–5 (p = 0.83), and weak evidence for a group effect on diabetic retinopathy and glaucoma/OHT with OR estimates (group 1 vs. group 2) of 1.90 and 0.68, respectively (p = 0.26 and p = 0.17).
Causes of visual impairment
A total of 102 out of 708 participants were classified post refraction as visually impaired based on their VA being < 6/12; 47 out of 708 participants were classified post refraction as visually impaired based on their VA being < 6/18. Binocular VA was used, with best monocular substituted when binocular VA was not available, to facilitate comparison with other UK studies. 44,56 Any VI resulting from uncorrected or undercorrected refractive error has now been corrected, so refractive error has been eliminated as a possible cause. The eye condition(s) identified by the examining optometrist as being the cause(s) of their VI are given in Table 16. For some participants two possible causes were recorded, and in these cases both causes have been included in Table 16.
| Condition(s) | VA < 6/12 (N = 102) | VA < 6/18 (N = 47) | ||
|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | |
| AMD alone | 16.7 (17) | 10.3 to 25.6 | 19.1(9) | 9.6 to 33.7 |
| Cataract alone | 44.1 (45) | 34.4 to 54.3 | 31.9 (15) | 19.5 to 47.3 |
| Diabetic retinopathy alone | 0 | 0.0 to 4.5 | 0 | 0.0 to 9.4 |
| Glaucoma alone | 2.0 (2) | 0.3 to 7.6 | 4.3 (2) | 0.7 to 15.7 |
| Cataract and AMD | 20.6 (21) | 13.5 to 30.0 | 29.8 (14) | 17.8 to 45.1 |
| Cataract and diabetic retinopathy | 1.0 (1) | 0.1 to 6.1 | 0 | 0.0 to 9.4 |
| Cataract and glaucoma | 4.9 (5) | 1.8 to 11.6 | 0 | 0.0 to 9.4 |
| Glaucoma and AMD | 2.9 (3) | 0.8 to 9.0 | 4.3 (2) | 0.7 to 15.7 |
| Other | 7.8 (8) | 3.7 to 15.3 | 10.6 (5) | 4.0 to 23.9 |
To facilitate comparison with other studies, the data in Table 16 have been re-presented in Table 17 but with the totals for each of the four conditions presented alone. The total is more than 100%, as approximately 30% of participants had more than one potential cause for their loss of vision.
| Condition | VA < 6/12 (N = 102) | VA < 6/18 (N = 47) | ||
|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | |
| AMD | 40.2 (41) | 30.8 to 50.4 | 53.2 (25) | 38.2 to 67.6 |
| Cataract | 70.6 (72) | 60.6 to 79.0 | 61.7 (29) | 46.4 to 75.1 |
| Diabetic retinopathy | 1.0 (1) | 0.1 to 6.1 | 0 | 0.0 to 9.4 |
| Glaucoma | 9.8 (10) | 5.1 to 17.7 | 8.5 (4) | 2.8 to 21.3 |
| Other | 7.8 (8) | 3.7 to 15.3 | 10.6 (5) | 4.0 to 23.9 |
An alternative approach to the analysis of causes of VI is for a clinician to make a forced choice of the single condition most likely to be the cause of the visual loss is shown in Table 18. The process adopted to identify a single cause was for two researchers (RS and DE) to review each visually impaired participant’s eye examination record and independently identify a single causative condition for each participant. Any differences in opinion were resolved by discussion. Two cases required discussion for clarification, following which RS and DE agreed on the single causative condition for each case.
| Condition | VA < 6/12 (N = 102) | VA < 6/18 (N = 47) | ||
|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | |
| AMD alone | 36.3 (37) | 27.2 to 46.4 | 48.9 (23) | 34.3 to 63.7 |
| Cataract alone | 48.0 (49) | 38.1 to 58.1 | 36.1 (17) | 23.1 to 51.5 |
| Diabetic retinopathy alone | 1.0 (1) | 0.1 to 6.1 | 0 (0) | 0.0 to 9.4 |
| Glaucoma alone | 6.9 (7) | 3.0 to 14.1 | 4.3 (2) | 0.7 to 15.7 |
| Other | 7.8 (8) | 3.7 to 15.3 | 10.6 (5) | 4.0 to 23.9 |
Cataract was the primary cause in 49 out of 102 (48.0%, 95% CI 38.1% to 58.1%) of cases of post-refraction VI (for VA of < 6/12 criterion). This VI is potentially remediable. AMD was the primary cause in 37 out of 102 (36.3%, 95% CI 27.2% to 46.4%) cases. For the VA of < 6/18 criterion, AMD was the cause in 23 out of 47 (48.9%, 95% CI 34.3% to 63.7%) cases and cataract in 17 out of 47 (36.1%, 95% CI 23.1% to 51.5%) cases.
Fundus examination and dilatation
Optometrists were requested to attempt a dilated fundus examination on all suitable participants. However, participants were not dilated unless full informed consent was given by the participant or personal or professional carer. Sixty-seven per cent (476/708) of participants had dilated fundus examinations and 28.2% (200/708) of participants were not dilated. The reasons for not dilating are given in Table 19. Pupillary dilatation was not possible in a further 4.5% (32/708) of participants because the optometrist was unable to measure the IOP before dilatation, a requirement of TOC examination protocol.
| Reasons for not dilating | % of participants (n) (N = 200) |
|---|---|
| Raised IOP or a large IOP difference between the two eyes | 4.5 (9) |
| Pre-existing glaucoma | 8.5 (17) |
| Narrow anterior chamber angles | 4.5 (9) |
| Participant declined dilatation | 16 (32) |
| Lack of informed consent to dilate | 14 (28) |
| Poor co-operation | 14 (28) |
| Reasons for not dilating not explained or unclear | 27 (54) |
| Other (e.g. adequate fundus view obtained without dilatation) | 12 (24) |
Registration as sight impaired and severely sight impaired
Nine out of 687 participants (1.3%) were registered severely sight impaired (blind) or sight impaired (partially sighted). Eight were registered severely sight impaired (four in each group) and one group 2 participant was registered sight impaired. Optometrists were unable to obtain information on registration from 21 participants.
Support for participant during the eye examination
Presence of a carer during the eye examination
Although the presence of a carer (personal or professional) was desirable during the eye examination, in some cases no one was present to support the participant (Table 20). Optometrists were unable to perform any part of the eye examination on eight participants living in care homes and hence this information was not recorded in these eight cases. There was a statistically significant difference between the proportions in group 1 and group 2 for the presence of a relative, for the presence of a professional carer and for nobody being present to provide support during the eye examination (χ12 p < 0.001). Notably, no carer was present in one-quarter of eye examinations, with 8.5% of examinations in group 1 conducted without a carer and 43.4% in group 2 conducted without a carer.
| Support present during eye examination | Sample for which eye exam attempted (N = 700) | Group 1 (N = 389) | Group 2 (N = 311) | Difference in proportions between groups 1 and 2, %a (95% CI) | |||
|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | ||
| Relative (personal carer) | 64.0 (448) | 60.3 to 67.5 | 89.5 (348) | 85.9 to 92.2 | 32.2 (100) | 27.1 to 37.7 | 57.3 (51.0 to 63.6) |
| Staff (professional carer) | 12.0 (84) | 9.7 to 14.7 | 2.1 (8) | 1.0 to 4.2 | 24.4 (76) | 19.8 to 29.7 | –22.3 (–27.6 to –17.1) |
| Nobody present | 24.0 (168) | 20.9 to 27.4 | 8.5 (33) | 6.0 to 11.8 | 43.4 (135) | 37.9 to 49.1 | –34.9 (–41.4 to –28.5) |
Contribution of carer to successful completion of key elements of the eye examination
The presence or absence of a carer was analysed for successful and unsuccessful completion of five key elements of the eye examination. These were: (1) objective assessment of refractive error; (2) subjective assessment of refractive error; (3) VA measurement; (4) fundus examination by ophthalmoscopy; and (5) tonometry. For each of these elements, the unadjusted rate ratios (95% CI) were as follows:
-
objective refraction: OR 1.3 (0.7 to 2.5)
-
subjective refraction: OR 1.5 (0.6 to 4.0)
-
VA measurement: OR 1.9 (1.2 to 3.0)
-
ophthalmoscopy: OR 1.6 (0.9 to 2.9)
-
tonometry: OR 1.7 (0.8 to 3.8).
From this analysis, the presence of a carer was associated with a statistically significant positive impact on the ability of the participant to successfully complete VA measurement. For the other four tests, although failing to reach statistical significance, the point estimates are all positive, and for tonometry and ophthalmoscopy are close in magnitude to that for VA measurement.
Change in distance visual acuity from pre to post refraction
Mean improvement in distance visual acuity
The mean improvement in VA (using best monocular VA before and after refraction) for the sample as a whole was 0.09 logMAR units (SD 0.15 logMAR units, range –0.10 to 1.40 logMAR units). The mean improvement in VA for group 1 participants was also 0.09 logMAR units (SD 0.15 logMAR units, range –0.10 to 1.40 logMAR units), with an improvement of 0.10 logMAR units (SD 0.17 logMAR units, range –0.02 to 1.20 logMAR units) for group 2. This improvement is equivalent to reading a further five letters, or one line extra, on the logMAR chart. The improvements in VA were statistically significant (Wilcoxon) for the sample as a whole (p < 0.01), for group 1 (p < 0.01) and for group 2 (p < 0.01) participants.
The mean VA and mean improvement in VA from presentation to post refraction for the four main conditions are presented in Table 21.
| Condition (n) | Mean VA (SD) | Mean improvement in VA (SD) | Wilcoxon test for VA improvement | Measurement of VA not possible | Others |
|---|---|---|---|---|---|
| AMD (101) | 0.27 (0.22) | 0.08 (0.10) | p < 0.01 | 8 | CF-3 HM-6 NPL-1 |
| Diabetic retinopathy (12) | 0.21 (0.12) | 0.10 (0.10) | p = 0.02 | 2 | – |
| Glaucoma (49) | 0.15 (0.17) | 0.13 (0.18) | p < 0.01 | 7 | – |
| Cataract (grades 1–5) (317) | 0.19 (0.15) | 0.10 (0.17) | p < 0.01 | 62 | CF-3 HM-3 LP-1 NPL-1 |
| Cataract (grades 2–5) (209) | 0.22 (0.15) | 0.11 (0.18) | p < 0.01 | 36 | CF-1 LP-1 NPL-1 |
Improvement in visual acuity post refraction based on lines of improvement on distance test charts
As an alternative to mean improvements in VA, the change in VA following refraction can be described by the percentage of the sample for which acuity improved by one or more lines on standard VA charts. These data are shown in Table 22 for improvements in VA of greater than one line, or greater than or equal to two or three lines on a logMAR chart (> 0.10, ≥ 0.20 and ≥ 0.30 logMAR units, respectively).
| Improvement in VA | Full sample (N = 584) | Group 1 (N = 376) | Group 2 (N = 208) | Difference in proportions between groups 1 and 2, %a (95% CI); p-value | |||
|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | ||
| > 0.10 logMAR units | 24.8 (145) | 21.4 to 28.6 | 23.1 (87) | 19.0 to 27.8 | 27.9 (58) | 22.0 to 34.6 | –4.8 (–12.6 to 3.1); 0.24 |
| ≥ 0.16 logMAR units | 20.4 (119) | 17.2 to 23.9 | 19.1 (72) | 15.4 to 23.6 | 22.6 (47) | 17.2 to 29.0 | –3.5 (–10.8 to 3.9); 0.38 |
| ≥ 0.20 logMAR units | 17.8 (104) | 14.8 to 21.2 | 16.5 (62) | 13.0 to 20.7 | 20.2 (42) | 15.1 to 26.4 | –3.7 (–10.7 to 3.3); 0.26 |
| ≥ 0.30 logMAR units | 6.8 (40) | 5.0 to 9.3 | 6.1 (23) | 4.0 to 9.2 | 8.2 (17) | 5.0 to 13.0 | –2.1 (–6.9 to 2.8); 0.35 |
Also included is the percentage of the sample who improved by ≥ 0.16 logMAR units. This criterion was chosen as it represents an improvement greater than the average change in acuity between two lines on a Snellen chart, which is 0.154 log units. 57 Although all the distance VA measurements in PrOVIDe were made using charts with logMAR progression of letter sizes, previous studies that have investigated VI in older people have often used charts with Snellen progression. 44,58,59 Snellen charts have several disadvantages compared with logMAR charts, notably the unequal progression in letter sizes between lines on the chart. However, to permit comparisons between PrOVIDe results and those obtained in previous studies that used Snellen charts, the ‘equivalent’ improvement in terms of lines of Snellen acuity has also been calculated.
Data are presented for groups 1 and 2 separately and there were no statistically significant differences between the proportions in each group who improved in VA post refraction for each of the chosen acuity levels. It is notable that in 17.8% of the sample (16.5% in group 1 and 20.2% in group 2) VA improved by at least two lines on the logMAR chart following refraction.
Loss of near visual acuity
The NVA levels on the standard near vision charts used during the eye examination were N4, N5, N6, N8, N10, N12, N14, N18, N24 and N48. The median NVA for the sample as a whole pre refraction was N6 (IQR N5–N8), recorded with the participant wearing their current spectacles, if any, for near vision. Median NVA post refraction was N5 (IQR N5–N6), recorded with the participant wearing the prescription for near vision found in the PrOVIDe examination. All near acuities recorded were based on best monocular NVA. The median NVA for group 1 pre refraction was N6 (IQR N5–N8) and N5 (IQR N5–N6) post refraction. The median NVA for group 2 pre refraction was N8 (IQR N6–N8) and N6 (IQR N5–N8) post refraction. Figure 12 illustrates the distribution of NVA pre and post refraction for the sample as a whole.
FIGURE 12.
Near visual acuity pre and post refraction.

Prevalence of near vision loss
Best monocular pre- and post-refraction NVAs were available for 475 participants. It was not possible to record NVA in 157 (22.2%) participants. There were missing data pre refraction for 76 (10.7%) participants. The two cut-off points in terms of NVA used to define impaired near vision were best monocular NVA < N8 and best monocular NVA < N10. Prevalences of presenting impaired near vision, uncorrected or undercorrected impaired near vision, and impaired near vision post refraction are presented in Table 23.
| Type of near vision loss | Full sample (N = 475) | Group 1 (N = 336) | Group 2 (N = 139) | Difference in proportions between groups 1 and 2, %a (95% CI); p-value | |||
|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | ||
| Presenting near vision loss | |||||||
| NVA < N8 | 16.2 (77) | 13.1 to 19.9 | 13.4 (45) | 10.0 to 17.6 | 23.0 (32) | 16.5 to 31.1 | –9.6 (–18.0 to –1.2); 0.01 |
| NVA < N10 | 8.4 (40) | 6.2 to 11.4 | 6.3 (21) | 4.0 to 9.5 | 13.7 (19) | 8.6 to 20.8 | –7.4 (–14.2 to –0.60); 0.01 |
| Uncorrected or undercorrected near vision loss | |||||||
| NVA < N8 | 10.3 (49) | 7.8 to 13.5 | 9.8 (33) | 7.0 to 13.6 | 11.5 (16) | 6.9 to 18.3 | –1.7 (–8.4 to 5.0); 0.70 |
| NVA < N10 | 6.9 (33) | 4.9 to 9.7 | 5.4 (18) | 3.3 to 8.5 | 10.8 (15) | 6.4 to 17.5 | –5.4 ( –11.6 to 0.80); 0.06 |
| Post-refraction near vision loss | |||||||
| NVA < N8 | 5.9 (28) | 4.0 to 8.5 | 3.6 (12) | 1.9 to 6.3 | 11.5 (16) | 6.9 to 18.3 | –7.9 (–14.1 to –1.8); < 0.01 |
| NVA < N10 | 1.5 (7) | 0.6 to 3.1 | 0.9 (3) | 0.2 to 2.8 | 2.9 (4) | 0.9 to 7.7 | –2.0 (–5.4 to 1.50); 0.20 |
-
Presenting near vision loss For the NVA < N8 cut-off point the prevalence of presenting near vision loss was 16.2%, reducing to 8.4% for NVA < N10. For both criteria the differences in prevalence between groups 1 and 2 were statistically significant (χ12 p = 0.01).
-
Post-refraction near vision loss Following refraction the prevalence of near vision loss had fallen to 5.9% for NVA < N8 and to 1.5% for NVA < N10. There remained a statistically significant difference between the proportions of those with near vision loss post refraction between groups 1 and 2 for NVA < N8 but not for NVA < N10 (χ12 p < 0.01 and p = 0.20, respectively). Of the 93 participants who had a valid post-refraction NVA but no valid presenting NVA, 26 and 15 participants had NVA < N8 and < N10, respectively, giving VI rates of 28.0% and 16.1% compared with the rates in the ‘overlapping’ sample, as per Table 23, of 5.9% and 1.5%.
-
Uncorrected or undercorrected near vision loss For the NVA < N8 cut-off point the overall prevalence of uncorrected or undercorrected near vision loss was 10.3%, with no statistically significant difference between groups 1 and 2 (χ12 p = 0.70). Similarly, there is no statistically significant difference between groups 1 (5.4%) and 2 (10.8%) for the < N10 criterion (χ12 p = 0.06).
Factors associated with near vision loss
For presenting near vision loss, using logistic regression models, statistically significant independent group differences were found for both the NVA < N8 and NVA < N10 criteria, with adjusted OR estimates of 2.08 and 2.56 (care homes vs. own homes); p < 0.01 (Table 24). There was no evidence for independent age and sex effects on near vision loss.
| Type of near vision loss | Variable | OR | p-value | 95% CI |
|---|---|---|---|---|
| NVA < N8 | ||||
| Presenting near vision loss | Age (per year) | 1.00 | 0.98 | 0.96 to 1.04 |
| Sex (M) | 1.00 | 1.00 | 0.59 to 1.66 | |
| Group 2 | 2.08 | < 0.01 | 1.23 to 3.51 | |
| Post-refraction near vision loss | Age (per year) | 1.06 | 0.04 | 1.01 to 1.12 |
| Sex (M) | 0.79 | 0.45 | 0.42 to 1.44 | |
| Group 2 | 2.71 | < 0.01 | 1.51 to 4.94 | |
| Uncorrected/undercorrected near vision loss | Age (per year) | 0.98 | 0.50 | 0.94 to 1.03 |
| Sex (M) | 1.04 | 0.89 | 0.56 to 1.91 | |
| Group 2 | 1.48 | 0.26 | 0.74 to 2.88 | |
| sMMSE (per unit score) | 1.01 | 0.76 | 0.96 to 1.06 | |
| NVA < N10 | ||||
| Presenting near vision loss | Age (per year) | 1.01 | 0.73 | 0.96 to 1.07 |
| Sex (M) | 1.01 | 0.97 | 0.50 to 1.98 | |
| Group 2 | 2.56 | < 0.01 | 1.30 to 5.05 | |
| Post-refraction near vision loss | Age (per year) | 1.20 | < 0.01 | 1.08 to 1.37 |
| Sex (M) | 0.63 | 0.38 | 0.20 to 1.67 | |
| Group 2 | 2.32 | 0.07 | 0.95 to 6.02 | |
| Uncorrected/undercorrected near vision loss | Age (per year) | 0.98 | 0.54 | 0.93 to 1.04 |
| Sex (M) | 1.09 | 0.82 | 0.51 to 2.27 | |
| Group 2 | 2.51 | 0.02 | 1.13 to 5.53 | |
| sMMSE (per unit score) | 1.01 | 0.78 | 0.95 to 1.07 | |
For uncorrected/undercorrected near vision loss defined by either criterion, there was no evidence for any independent age, sex or sMMSE differences. There was a statistically significant group effect for the NVA < N10 criterion, with estimated adjusted OR of 2.51 (95% CI 1.13 to 5.53; p = 0.02). There was no evidence for a group effect for the NVA < N8 criterion (p = 0.26).
For post-refraction near vision loss for both the NVA < N8 and NVA < N10 criteria, there was a statistically significant independent age effect, with OR estimates of 1.06 (per year, p = 0.04) and 1.20 (per year, p < 0.01), respectively. There was a statistically significant independent group effect for NVA < N8, with the estimated OR for those participants living in care homes compared with those living at home being 2.71 (95% CI 1.51 to 4.94; p < 0.01), and some evidence for a similar independent group effect for NVA < N10, with OR 2.32 (95% CI 0.95 to 6.02; p = 0.07). There was no evidence of an independent sex effect.
There were 233 out of 708 (32.9%) participants with missing NVA data. There were statistically significant independent group and sMMSE differences in the occurrence of missing data with estimated ORs for unobserved NVA of 0.87 (95% CI 0.84 to 0.89) (per unit score) for sMMSE (p < 0.001) and 3.21 (95% CI 2.06 to 5.04) for care homes versus own homes (p < 0.001). There was no evidence of an independent change with age and little evidence of a sex difference (p = 0.07).
Improvement in near visual acuity post refraction based on the number of lines of improvement on near test charts
The change in NVA following refraction can be described by the percentage of the sample for whom NVA remained unchanged and the proportion for whom NVA improved by one or more lines on the NVA charts (Table 25). There was no statistically significant difference between groups for any of the improvements in NVA (Wilcoxon rank-sum test, p = 0.26).
| Improvement in NVA | Full sample (n = 475) | Group 1 (n = 336) | Group 2 (n = 139) | Difference in proportions between groups 1 and 2%,a (95% CI) | |||
|---|---|---|---|---|---|---|---|
| % (n) | 95% CI | % (n) | 95% CI | % (n) | 95% CI | ||
| No improvement | 55.6 (264) | 51.0 to 60.1 | 53.3 (179) | 47.8 to 58.7 | 61.2 (85) | 52.5 to 69.2 | –7.9 (–18.1 to 2.3) |
| One line | 28.6 (136) | 24.7 to 33.0 | 31.0 (104) | 26.1 to 36.2 | 23.0 (32) | 16.5 to 31.1 | 8.0 (–1.1 to 17.0) |
| Two lines | 9.3 (44) | 6.9 to 12.3 | 10.1 (34) | 7.2 to 14.0 | 7.2 (10) | 3.7 to 13.2 | 2.9 (–3.0 to 8.8) |
| Three lines | 3.4 (16) | 2.0 to 5.5 | 4.2 (14) | 2.4 to 7.1 | 1.4 (2) | 0.2 to 5.6 | 2.8 (–0.7 to 6.1) |
| More than three lines | 3.2 (15) | 1.8 to 5.3 | 1.5 (5) | 0.5 to 3.6 | 7.2 (10) | 3.7 to 13.2 | –5.7 (–10.7 to –0.7) |
Ability of participants to complete individual elements of the eye examination
The proportions of the sample who were able to complete key elements of the eye examination (objective and subjective determination of refractive error, measurement of VA, tonometry, pupil dilatation, visual fields and the fundus examination) are given in Table 26. The difference in proportions between those in groups 1 and 2 who were able to complete each of these elements was statistically significant (χ12 p< 0.01).
| Eye examination element | Full sample (N = 708a) | Group 1 (N = 389b) | Group 2 (N = 319c) | Difference in proportions between groups 1 and 2,% (95% CI) | Adjusted OR (95% CI) group 2 vs. group 1 | Adjusted OR p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | % (n) | 95% CI (missing %) | ||||
| Objective assessment | 88.1 (624) | 85.5 to 90.4 (11.9) | 92.0 (358) | 88.8 to 94.4 (8.0) | 83.4 (266) | 78.7 to 87.2 (16.6) | 8.6 (3.5 to 13.8) | 0.64 (0.32 to 1.27) | 0.20 |
| Subjective assessment | 80.9 (573) | 77.8 to 83.7 (19.1) | 95.1 (370) | 92.3 to 97.0 (4.9) | 63.6 (203) | 58.1 to 68.9 (36.4) | 31.5 (25.5 to 37.5) | 0.26 (0.14 to 0.49) | < 0.01 |
| VA measurement | 83.2 (589) | 80.2 to 85.8 (16.8) | 97.4 (379) | 95.2 to 98.7 (2.6) | 65.8 (210) | 60.3 to 71.0 (34.2) | 31.6 (25.9 to 37.3) | 0.22 (0.08 to 0.51) | < 0.01 |
| Tonometry | 94.8 (671) | 92.8 to 96.2 (5.2) | 99.2 (386) | 97.6 to 99.8 (0.8) | 89.3 (285) | 85.3 to 92.4 (10.7) | 9.9 (6.1 to 13.7) | 0.23 (0.05 to 0.80) | 0.03 |
| Dilatation | 67.2 (476) | 63.6 to 70.7 (32.8) | 81.0 (315) | 76.6 to 84.7 (19.0) | 50.5 (161) | 44.9 to 56.1 (49.5) | 30.5 (23.5 to 37.5) | 0.27 (0.09 to 0.68) | 0.01 |
| Visual fields | 30.0 (110) | 25.4 to 35.0 (70) | 52.5 (104) | 45.3 to 59.6 (47.5) | 3.6 (6) | 1.5 to 7.9 (94) | 48.9 (40.9 to 57.0) | Not included | N/A |
| Fundus examination | 91.2 (646) | 88.9 to 93.2 (8.8) | 97.4 (379) | 95.2 to 98.7 (2.6) | 83.7 (267) | 79.1 to 87.5 (16.3) | 13.7 (9.1 to 18.4) | 0.46 (0.19 to 1.02) | 0.06 |
The visual fields data are from those 116 participants in whom a fields test was attempted, of whom 110 could complete the test. These data should be interpreted with caution as, in addition to these 116 participants, there were 251 participants for whom the optometrists decided that the visual fields test, although clinically desirable, would not be possible because of the participant’s level of co-operation. However, some of these 251 participants might have been able to complete the fields test had it been carried out. Nevertheless, it is probable that the visual fields test would not have been possible in the majority of these 251 participants. Assuming that none of these 251 participants could have completed the visual fields test, an estimated 30% of participants (110/367) were able to complete the visual fields test.
Logistic regression models were also used, with age, sex and group (care home or own home) as covariates to assess their independent associations with the completion of each examination component. When adjusted for age and sex there was a statistically significant independent group difference for subjective examination, VA measurement, tonometry and dilatation, with the estimated ORs for those participants living in care homes compared with those living in their own homes ranging from 0.22 to 0.27 (p ≤ 0.03), that is to say living in a care home was likely to be associated with lower completion rates of these elements of the eye examination. There was some evidence of a group difference in the ability to complete the fundus examination, with an estimated OR of 0.46 (95% CI 0.19 to 1.02; p = 0.06). There was little evidence for a group difference in the ability of the participant to complete the objective determination of refractive error, with an estimated OR for group 2 versus group 1 of 0.64 (95% CI 0.32 to 1.27; p = 0.20).
Patient management on completion of the eye examination
As a result of the eye examination, 47 participants (6.7%) were referred to their GP for management or for onward referral to the HES. The reasons for these referrals are given in Table 27. Three of the four participants referred to the diabetic medical retina clinic were referred to arrange annual diabetic screening because the participants were not currently participating in the NHS Diabetic Eye Screening programme. Three of the five referrals for external eye conditions were to the participants’ GP for their management, rather than for onward referral to the HES. Almost 60% of referrals were for either possible cataract extraction/capsulotomy or suspected glaucoma/OHT.
| Reason for referral (referral clinic) | % (95% CI) of participants with the suspected condition (n) (N = 47) |
|---|---|
| Cataract | 19.1 (9.6 to 33.7) (9) |
| Diabetic medical retina | 8.5 (2.8 to 21.3) (4) |
| External eye | 10.6 (4.0 to 23.9) (5) |
| Pre-existing glaucoma | 8.5 (2.8 to 21.3) (4) |
| Suspect glaucoma | 31.9 (19.5 to 47.3) (15) |
| OHT | 4.3 (0.7 to 15.7) (2) |
| Laser YAG capsulotomy | 4.3 (0.7 to 15.7) (2) |
| Orthoptic anomaly | 4.3 (0.7 to 15.7) (2) |
| Other medical retina (AMD) | 4.3 (0.7 to 15.7) (2) |
| Not specified | 4.3 (0.7 to 15.7) (2) |
On completion of the eye examination, optometrists advised 700 participants (98.9%) of the recommended date of their next eye examination. The remaining eight participants who could not be advised of a recommended date were also those on whom optometrists were unable to perform any part of the eye examination. The recommended date of next eye examination was 1 year in 576 out of 700 (82.3%) participants and 2 years for the remaining 124 out of 700 (17.7%) participants. A total of 444 out of 700 (63.4%) participants were advised that a new or changed spectacle prescription would be beneficial, based on improvement in VA and/or subjective improvement in performance with the new/changed prescription, with 244 out of 700 (34.8%) advised that no change in spectacle prescription was necessary. A total of 26 out of 700 (3.7%) participants were advised that their current spectacles were unserviceable owing to fair wear and tear, and hence new spectacles would be beneficial. These percentages total more than 100% because some participants were advised both that a new or changed prescription would be beneficial and that their current spectacles were unserviceable, and others were advised that no change in prescription was necessary but that their current spectacles were unserviceable.
Some participants were asked, before any new spectacles were prescribed, to compare subjectively the performance of their current spectacles with the performance of the prescription found by the research optometrist. Although this was not an obligatory procedure for optometrists from TOC, it was preferred practice where patient co-operation permitted. However, even when the optometrist decided that co-operation was sufficient to attempt this procedure, it was not possible for many PrOVIDe participants to make this comparison because the challenge of remembering the appearance of a letter chart and comparing that appearance subjectively with a previous appearance of the chart was too demanding, particularly because this comparison would have been attempted towards the end of the subjective examination when participant fatigue often impaired performance. Nevertheless, 27.7% of participants who were advised of a new or changed spectacle prescription voluntarily reported a noticeable subjective improvement in VA with the new prescription when compared with the old prescription, while 3.2% found no subjective improvement in VA with the new prescription. Of those participants who were advised of a new or changed spectacle prescription by the optometrist, 50.5% were dispensed new spectacles at the time of the eye examination and 39.6% declined new spectacles.
Level of cognition and outcomes of the eye examination
Association between the ability of participants to perform elements of the eye examination and their level of cognition
An analysis of participants’ ability to complete various elements of the eye examination and their overall level of cognition as measured by the total sMMSE score is summarised in Table 28. For the objective determination of refractive error by retinoscopy, there was no statistically significant difference between the sMMSE scores (Wilcoxon rank-sum test, p = 0.13) for those participants on whom optometrists could complete retinoscopy and those participants in whom retinoscopy was not possible. However, for the other elements of the eye examination tested (visual fields was not tested) there was a statistically significant difference (p < 0.01 for each of the other tests) between the sMMSE scores for participants for whom the test was possible and those participants for whom the test was not possible. Notably, the mean sMMSE score was 18.4 in those participants for whom subjective refraction was possible, compared with 6.8 in those for whom subjective refraction was not possible. Similarly, the mean sMMSE score was 18.2 in those participants for whom VA measurement was possible, compared with 4.9 in those for whom VA measurements were not possible. The visual fields data are from those 116 participants in whom a fields test was attempted.
| Test | n | Mean sMMSE score (SD) | Adjusted ORa (95% CI) | OR p-value |
|---|---|---|---|---|
| Objective assessment | ||||
| Possible | 607 | 14.8 (8.8) | 1.25 (0.84 to 1.87) | 0.27 |
| Not possible | 47 | 16.8 (8.0) | ||
| Subjective assessment | ||||
| Possible | 557 | 18.4 (6.9) | 4.62 (3.25 to 6.72) | < 0.01 |
| Not possible | 96 | 6.8 (6.9) | ||
| VA measurement | ||||
| Possible | 578 | 18.2 (6.9) | 18.90 (10.33 to 38.1) | < 0.01 |
| Not possible | 76 | 4.9 (6.3) | ||
| Tonometry | ||||
| Possible | 634 | 17.0 (7.9) | 2.67 (1.44 to 5.27) | < 0.01 |
| Not possible | 20 | 8.0 (7.3) | ||
| Dilatation | ||||
| Possible | 613 | 17.4 (7.7) | 4.21 (2.57 to 7.24) | < 0.01 |
| Not possible | 41 | 6.5 (6.9) | ||
| Visual fields | ||||
| Possible | 110 | 21.9 (4.9) | Not attempted | N/A |
| Not possible | 6 | 16.3 (6.3) | ||
| Fundus examination | ||||
| Possible | 613 | 17.2 (7.7) | 2.75 (1.76 to 4.41) | < 0.01 |
| Not possible | 41 | 9.0 (9.4) | ||
Using logistic regression models, after adjustment for age, sex and group (care home or own home) there was a statistically significant independent association between sMMSE and participants’ ability to complete the subjective examination, VA measurement, tonometry, dilatation and fundus examination, with the estimated ORs for those with lower sMMSE scores ranging from 2.7 to 18.9 per 10-unit score increase of sMMSE from 0 (p < 0.01 for each) (see Table 28). There was no evidence of an independent sMMSE association with the ability of the participant to undergo an objective refraction, with an estimated OR of 1.25 (95% CI 0.84 to 1.87; p = 0.27). Logistic regression analysis was not carried out for the visual fields data.
Potential bias in Standardised Mini-Mental State Examination scoring in participants with visual impairment
The sMMSE instrument can be modified for participants with a sensory loss that could affect their performance. In particular, for participants with loss of vision, their performance on the three sMMSE items which involve participants reading the words ‘close your eyes’, writing a complete sentence and copying a design could be adversely affected. An alternative version of the instrument, MMSE blind, is often used with visually impaired participants. With MMSE blind, the three items requiring significant visual input are omitted and the test is scored out of a maximum of 27 rather than the usual 30. The results obtained out of a maximum of 27 for MMSE blind are then rescaled to give a score out of 30.
In PrOVIDe, the sMMSE instrument was used on all participants, both visually impaired and non-visually impaired. The sMMSE test was delivered at recruitment, at which point the recruiter would, in general, be unaware if the participant was visually impaired or not. It is possible that there was overestimation of cognitive impairment through the use of sMMSE in visually impaired participants, who might have been unable to complete correctly these three items requiring visual input as a result of their VI rather than because of any cognitive impairment. To investigate the possible bias (underestimation) on the PrOVIDe sMMSE scores in patients who were visually impaired, a linear regression model was fitted to the sMMSE scores, adjusting for age, sex, residential status and presenting VI ordered in terms of severity: ‘better than 6/12 (none)’, ‘6/12 to 6/18’ and ‘worse than 6/18’. The coefficient point estimates and statistical significances were then compared with those of a model of MMSE blind scores (Table 29).
| Coefficient | Estimate | 95% CI | p-value |
|---|---|---|---|
| sMMSE score | |||
| Intercept | 22.62 | 16.12 to 29.13 | < 0.001 |
| Age (per year) | –0.04 | –0.12 to 0.04 | 0.303 |
| Sex: male | 0.68 | –0.40 to 1.76 | 0.216 |
| Care home | –4.93 | –6.10 to –3.76 | < 0.001 |
| VA 6/12 to 6/18 | –1.70 | –2.75 to –0.65 | 0.002 |
| VA < 6/18 | –0.68 | –1.90 to 0.53 | 0.268 |
| MMSE blind score | |||
| Intercept | 23.34 | 16.80 to 29.87 | < 0.001 |
| Age | –0.05 | –0.13 to 0.03 | 0.210 |
| Sex: male | 0.81 | –0.27 to 1.88 | 0.143 |
| Care home | –4.96 | –6.12 to –3.80 | < 0.001 |
| VA 6/12 to 6/18 | –1.52 | –2.58 to –0.47 | 0.005 |
| VA < 6/18 | –0.73 | –1.95 to 0.48 | 0.237 |
No substantive differences were observed in the direction and statistical significance of predictors for both measures of cognitive performance, although there is an indication of higher MMSE blind scores in both non-visually impaired participants and moderately visually impaired participants. Of the 654 participants with measured sMMSE scores, 149 had identical MMSE blind scores and 493 were within a single score difference. The 95% limits of agreement between sMMSE and MMSE blind scores were 1.43 and –1.33, with a mean difference score of –0.07 (MMSE blind – sMMSE). The correlation between sMMSE scores and MMSE blind scores was 0.994. A Deming regression (allowing for measurement error in both dependent and independent variables) was fitted to blind MMSE score with sMMSE score as the only predictor: this yielded a regression slope estimate with a CI between 0.989 and 1.001, where a slope of 1 with no variance would indicate perfect agreement between the scores. All of this illustrates that the use of the sMMSE and not of MMSE blind, even in participants with VI, is not likely to lead to different conclusions on a population level.
Analysis of effects of visual impairment on function (Bristol Activities of Daily Living Scale) and behaviour (Cambridge Behavioural Inventory – Revised)
Analysis of Bristol Activities of Daily Living Scale data
The results from the visually impaired and non-visually impaired participants were compared for all 20 activities of the BADLS for both VA < 6/12 (Table 30) and VA < 6/18 (Table 31). ORs were derived from ordinal logistic regression models, with age, sex and group as covariates, and are estimates of the independent effect of VI on these function activities. Presenting VA has been used for the analysis, as it best represents the participants’ visual status when the BADLS data were collected. Correction for multiple comparisons across the related items (and, thus, related hypotheses) in the BADLS has been applied using the Bonferroni correction. This modifies the statistical significance cut-off point from 0.05 to 0.05/20 or 0.0025. For VI defined by VA < 6/12, all the BADLS items have estimated ORs > 1.0 (range from 1.11 to 2.19) for participants with VI, compared with participants without VI, indicating an adverse association of VI with each of these activities. Applying the conservative significance level of 0.0025, only ‘toilet/commode’, with an estimated OR of 2.19 (95% CI 1.36 to 3.53), and ‘telephone’, with an OR of 1.89 (95% CI 1.26 to 2.85), were statistically significant (p = 0.001 and p = 0.002, respectively). There is some evidence of an independent VI association with ‘drink’, with an estimated OR of 1.93 (95% CI 1.22 to 3.06), and ‘teeth’, with an OR of 1.86 (95% CI 1.22 to 3.06) (p = 0.005 and 0.004, respectively).
| BADLS function activity | Number analysed | OR VI vs. non-VI (95% CI) | p-value |
|---|---|---|---|
| Food | 350 | 1.50 (0.94 to 2.42) | 0.092 |
| Eating | 485 | 1.75 (1.06 to 2.9) | 0.029 |
| Drink | 396 | 1.93 (1.22 to 3.06) | 0.005 |
| Drinking | 487 | 1.83 (0.84 to 4.04) | 0.129 |
| Dressing | 480 | 1.66 (1.11 to 2.49) | 0.014 |
| Hygiene | 476 | 1.7 (1.13 to 2.54) | 0.011 |
| Teeth | 470 | 1.86 (1.22 to 2.84) | 0.004 |
| Bath/shower | 466 | 1.36 (0.91 to 2.02) | 0.13 |
| Toilet/commode | 474 | 2.19 (1.36 to 3.53) | 0.001 |
| Transfers | 479 | 1.12 (0.68 to 1.84) | 0.644 |
| Mobility | 477 | 1.26 (0.83 to 1.89) | 0.271 |
| Orientation: time | 469 | 1.11 (0.76 to 1.63) | 0.581 |
| Orientation: space | 466 | 1.45 (0.97 to 2.19) | 0.071 |
| Communication | 478 | 1.15 (0.75 to 1.77) | 0.515 |
| Telephone | 416 | 1.89 (1.26 to 2.85) | 0.002 |
| Housework/gardening | 352 | 1.15 (0.73 to 1.81) | 0.543 |
| Shopping | 364 | 1.63 (1.03 to 2.61) | 0.039 |
| Finances | 350 | 1.7 (1.09 to 2.66) | 0.019 |
| Games/hobbies | 427 | 1.17 (0.79 to 1.74) | 0.427 |
| Transport | 347 | 1.91 (1.14 to 3.24) | 0.014 |
| BADLS function activity | Number analysed | OR VI vs. non-VI (95% CI) | p-value |
|---|---|---|---|
| Food | 350 | 1.96 (1.03 to 3.82) | 0.044 |
| Eating | 485 | 1.36 (0.71 to 2.49) | 0.333 |
| Drink | 396 | 1.87 (1.04 to 3.35) | 0.035 |
| Drinking | 487 | 1.21 (0.47 to 2.83) | 0.673 |
| Dressing | 480 | 1.2 (0.71 to 2.00) | 0.494 |
| Hygiene | 476 | 1.46 (0.86 to 2.45) | 0.158 |
| Teeth | 470 | 1.70 (0.99 to 2.9) | 0.052 |
| Bath/shower | 466 | 1.46 (0.87 to 2.45) | 0.151 |
| Toilet/commode | 474 | 1.28 (0.71 to 2.28) | 0.4 |
| Transfers | 479 | 0.66 (0.33 to 1.24) | 0.213 |
| Mobility | 477 | 1.24 (0.74 to 2.06) | 0.418 |
| Orientation: time | 469 | 1.14 (0.70 to 1.85) | 0.605 |
| Orientation: space | 466 | 1.42 (0.85 to 2.39) | 0.18 |
| Communication | 478 | 1.24 (0.72 to 2.08) | 0.429 |
| Telephone | 416 | 2.08 (1.22 to 3.56) | 0.007 |
| Housework/gardening | 352 | 1.62 (0.89 to 2.98) | 0.114 |
| Shopping | 364 | 1.94 (1.07 to 3.54) | 0.03 |
| Finances | 350 | 1.87 (1.06 to 3.30) | 0.03 |
| Games/hobbies | 427 | 1.12 (0.68 to 1.83) | 0.662 |
| Transport | 347 | 2.55 (1.29 to 5.08) | 0.007 |
For VI defined by VA < 6/18, all items had estimated ORs > 1.0 (range from 1.12 to 2.55), apart from ‘transfers’, which had an estimated OR of 0.66 (95% CI 0.33 to 1.24) (p = 0.21). There is some evidence of an independent VI association with ‘telephone’, with an estimated OR of 2.08 (95% CI 1.22 to 3.56), and ‘transport’, with an OR of 2.55 (95% CI 1.29 to 5.08) (p = 0.007 for both).
Analysis of Cambridge Behavioural Inventory – Revised data
Results from the visually impaired and non-visually impaired participants were compared for all 45 items of the CBI-R for both VA < 6/12 (Table 32) and VA < 6/18 (Table 33). ORs were derived from ordinal logistic regression models, with adjustments made for age, sex and residential status, and are estimates of the independent association of VI with these behavioural activities. Presenting VA has been used for the analysis, as it best represents the participants’ visual status when the CBI-R data were collected. Correction for multiple comparisons has been applied using the Bonferroni correction. This modifies the statistical significance cut-off point from 0.05 to 0.05/45 or 0.0011.
| CBI-R | Number analysed | OR VI vs. non-VI (95% CI) | p-value |
|---|---|---|---|
| Memory and orientation | |||
| CMO 1: has poor day-to-day memory (e.g. about conversations, trips, etc.) | 482 | 0.92 (0.63 to 1.36) | 0.681 |
| CMO 2: asks the same questions over and over again | 488 | 0.89 (0.61 to 1.28) | 0.526 |
| CMO 3: loses or misplaces things | 468 | 1.18 (0.8 to 1.73) | 0.401 |
| CMO 4: forgets the names of familiar people | 490 | 1.25 (0.87 to 1.81) | 0.227 |
| CMO 5: forgets the names of objects and things | 478 | 1.34 (0.92 to 1.94) | 0.124 |
| CMO 6: shows poor concentration when reading or watching television | 483 | 1.39 (0.95 to 2.03) | 0.087 |
| CMO 7: forgets what day it is | 484 | 1.24 (0.86 to 1.81) | 0.256 |
| CMO 8: becomes confused or muddled in unusual surroundings | 483 | 1.31 (0.9 to 1.89) | 0.160 |
| Everyday skills | |||
| CES 1: has difficulties using electrical appliances (e.g. television, radio, cooker, washing machine) | 442 | 1.55 (1.04 to 2.31) | 0.03 |
| CES 2: has difficulties writing (letters, Christmas cards, lists, etc.) | 448 | 2.27 (1.5 to 3.45) | 0 |
| CES 3: has difficulties using the telephone | 441 | 1.89 (1.27 to 2.82) | 0.002 |
| CES 4: has difficulties making a hot drink (e.g. tea/coffee) | 429 | 1.67 (1.08 to 2.57) | 0.02 |
| CES 5: has problems handling money or paying bills | 398 | 1.89 (1.2 to 3.02) | 0.006 |
| Self-care | |||
| CSC 1: has difficulties grooming self (e.g. shaving or putting on make-up) | 479 | 1.81 (1.24 to 2.66) | 0.002 |
| CSC 2: has difficulties dressing self | 484 | 1.66 (1.13 to 2.42) | 0.009 |
| CSC 3: has problems feeding self without assistance | 477 | 1.76 (1.1 to 2.8) | 0.018 |
| CSC 4: has problems bathing or showering self | 473 | 1.55 (1.05 to 2.28) | 0.026 |
| Abnormal behaviour | |||
| CAB 1: finds humour or laughs at things others do not find funny | 476 | 1.38 (0.91 to 2.09) | 0.132 |
| CAB 2: has temper outbursts | 490 | 1.12 (0.76 to 1.65) | 0.549 |
| CAB 3: is unco-operative when asked to do something | 481 | 1.04 (0.71 to 1.54) | 0.828 |
| CAB 4: shows socially embarrassing behaviour | 485 | 1.2 (0.79 to 1.82) | 0.397 |
| CAB 5: makes tactless or suggestive remarks | 477 | 1.48 (0.97 to 2.25) | 0.067 |
| CAB 6: acts impulsively without thinking | 485 | 1.14 (0.77 to 1.69) | 0.51 |
| Mood | |||
| CMOOD 1: cries | 460 | 1.04 (0.69 to 1.56) | 0.855 |
| CMOOD 2: appears sad or depressed | 466 | 1.01 (0.69 to 1.48) | 0.944 |
| CMOOD 3: is very restless or agitated | 470 | 1.31 (0.9 to 1.9) | 0.152 |
| CMOOD 4: is very irritable | 470 | 1.03 (0.7 to 1.5) | 0.883 |
| Beliefs | |||
| CBELIEFS 1: sees things that are not really there (visual hallucinations) | 473 | 1.47 (0.95 to 2.25) | 0.08 |
| CBELIEFS 2: hears voices that are not really there (auditory hallucinations) | 472 | 1.4 (0.84 to 2.3) | 0.188 |
| CBELIEFS 3: has odd or bizarre ideas that cannot be true | 464 | 1.28 (0.85 to 1.94) | 0.237 |
| Eating habits | |||
| CEH 1: prefers sweet foods more than before | 462 | 1.16 (0.78 to 1.71) | 0.472 |
| CEH 2: wants to eat the same foods repeatedly | 464 | 0.95 (0.61 to 1.46) | 0.806 |
| CEH 3: her/his appetite is greater, s/he eats more than before | 472 | 1.6 (1.04 to 2.46) | 0.031 |
| CEH 4: table manners are declining, e.g. stuffing food into mouth | 469 | 1.44 (0.9 to 2.3) | 0.128 |
| Sleep | |||
| CSLEEP 1: sleep is disturbed at night | 469 | 1.43 (0.99 to 2.09) | 0.06 |
| CSLEEP 2: sleeps more by day than before (cat naps, etc.) | 470 | 1.45 (1 to 2.11) | 0.051 |
| Stereotypic and motor behaviours | |||
| CSMB 1: is rigid and fixed in her/his ideas and opinions | 461 | 0.81 (0.54 to 1.2) | 0.297 |
| CSMB 2: develops routines from which she or he cannot easily be discouraged, e.g. wanting to eat or go for walks at fixed times | 467 | 1.03 (0.68 to 1.56) | 0.894 |
| CSMB 3: clock watches or appears pre-occupied with time | 466 | 0.92 (0.59 to 1.41) | 0.696 |
| CSMB 4: repeatedly uses the same expression or catchphrase | 474 | 0.97 (0.66 to 1.43) | 0.891 |
| Motivation | |||
| CMOTIVATION 1: shows less enthusiasm for his or her usual interests | 470 | 1.00 (0.68 to 1.45) | 0.988 |
| CMOTIVATION 2: shows little interest in doing new things | 474 | 0.88 (0.6 to 1.28) | 0.504 |
| CMOTIVATION 3: fails to maintain motivation to keep in contact with friends or family | 462 | 1.00 (0.67 to 1.48) | 0.997 |
| CMOTIVATION 4: appears indifferent to the worries and concerns of family members | 473 | 1.21 (0.82 to 1.78) | 0.328 |
| CMOTIVATION 5: shows reduced affection | 472 | 0.98 (0.64 to 1.51) | 0.944 |
| CBI-R | Number analysed | OR VI vs. non-VI (95% CI) | p-value |
|---|---|---|---|
| Memory and orientation | |||
| CMO 1: has poor day-to-day memory (e.g. about conversations, trips, etc.) | 482 | 0.72 (0.45 to 1.18) | 0.193 |
| CMO 2: asks the same questions over and over again | 488 | 0.81 (0.51 to 1.31) | 0.397 |
| CMO 3: loses or misplaces things | 468 | 1.12 (0.69 to 1.82) | 0.657 |
| CMO 4: forgets the names of familiar people | 490 | 1.09 (0.69 to 1.74) | 0.709 |
| CMO 5: forgets the names of objects and things | 478 | 1.07 (0.66 to 1.73) | 0.79 |
| CMO 6: shows poor concentration when reading or watching television | 483 | 0.88 (0.54 to 1.43) | 0.606 |
| CMO 7: forgets what day it is | 484 | 1.17 (0.73 to 1.88) | 0.516 |
| CMO 8: becomes confused or muddled in unusual surroundings | 483 | 1.18 (0.74 to 1.87) | 0.486 |
| Everyday skills | |||
| CES 1: has difficulties using electrical appliances (e.g. television, radio, cooker, washing machine) | 442 | 1.72 (1.03 to 2.91) | 0.038 |
| CES 2: has difficulties writing (letters, Christmas cards, lists, etc.) | 448 | 2.17 (1.24 to 3.89) | 0.008 |
| CES 3: has difficulties using the telephone | 441 | 1.61 (0.97 to 2.68) | 0.067 |
| CES 4: has difficulties making a hot drink (e.g. tea/coffee) | 429 | 1.34 (0.77 to 2.33) | 0.292 |
| CES 5: has problems handling money or paying bills | 398 | 1.77 (0.98 to 3.31) | 0.064 |
| Self-care | |||
| CSC 1: has difficulties grooming self (e.g. shaving or putting on make-up) | 479 | 1.55 (0.96 to 2.51) | 0.072 |
| CSC 2: has difficulties dressing self | 484 | 1.69 (1.05 to 2.72) | 0.03 |
| CSC 3: has problems feeding self without assistance | 477 | 1.49 (0.84 to 2.58) | 0.166 |
| CSC 4: has problems bathing or showering self | 473 | 1.61 (0.99 to 2.62) | 0.057 |
| Abnormal behaviour | |||
| CAB 1: finds humour or laughs at things others do not find funny | 476 | 1.4 (0.84 to 2.29) | 0.189 |
| CAB 2: has temper outbursts | 490 | 1.5 (0.93 to 2.42) | 0.098 |
| CAB 3: is unco-operative when asked to do something | 481 | 1.6 (0.99 to 2.57) | 0.055 |
| CAB 4: shows socially embarrassing behaviour | 485 | 1.07 (0.63 to 1.79) | 0.798 |
| CAB 5: makes tactless or suggestive remarks | 477 | 2.14 (1.29 to 3.5) | 0.003 |
| CAB 6: acts impulsively without thinking | 485 | 1.35 (0.83 to 2.17) | 0.226 |
| Mood | |||
| CMOOD 1: cries | 460 | 1.2 (0.70 to 2.04) | 0.492 |
| CMOOD 2: appears sad or depressed | 466 | 0.95 (0.58 to 1.57) | 0.854 |
| CMOOD 3: is very restless or agitated | 470 | 1.68 (1.04 to 2.70) | 0.033 |
| CMOOD 4: is very irritable | 470 | 1.21 (0.75 to 1.94) | 0.437 |
| Beliefs | |||
| CBELIEFS 1: sees things that are not really there (visual hallucinations) | 473 | 1.52 (0.90 to 2.54) | 0.109 |
| CBELIEFS 2: hears voices that are not really there (auditory hallucinations) | 472 | 1.42 (0.75 to 2.58) | 0.266 |
| CBELIEFS 3: has odd or bizarre ideas that cannot be true | 464 | 1.46 (0.88 to 2.39) | 0.14 |
| Eating habits | |||
| CEH 1: prefers sweet foods more than before | 462 | 1.16 (0.71 to 1.88) | 0.557 |
| CEH 2: wants to eat the same foods repeatedly | 464 | 1.05 (0.60 to 1.81) | 0.862 |
| CEH 3: her/his appetite is greater, she or he eats more than before | 472 | 1.91 (1.14 to 3.18) | 0.013 |
| CEH 4: table manners are declining, e.g. stuffing food into mouth | 469 | 1.22 (0.66 to 2.18) | 0.511 |
| Sleep | |||
| CSLEEP 1: sleep is disturbed at night | 469 | 1.45 (0.92 to 2.31) | 0.112 |
| CSLEEP 2: sleeps more by day than before (cat naps etc.) | 470 | 1.4 (0.88 to 2.24) | 0.155 |
| Stereotypic and motor behaviours | |||
| CSMB 1: is rigid and fixed in her/his ideas and opinions | 461 | 0.89 (0.53 to 1.47) | 0.644 |
| CSMB 2: develops routines from which s/he can not easily be discouraged e.g. wanting to eat or go for walks at fixed times | 467 | 1.39 (0.82 to 2.33) | 0.21 |
| CSMB 3: clock watches or appears pre-occupied with time | 466 | 0.93 (0.52 to 1.61) | 0.787 |
| CSMB 4: repeatedly uses the same expression or catch phrase | 474 | 1.17 (0.73 to 1.88) | 0.506 |
| Motivation | |||
| CMOTIVATION 1: shows less enthusiasm for his or her usual interests | 470 | 0.92 (0.57 to 1.48) | 0.73 |
| CMOTIVATION 2: shows little interest in doing new things | 474 | 1 (0.62 to 1.61) | 0.994 |
| CMOTIVATION 3: fails to maintain motivation to keep in contact with friends or family | 462 | 1.26 (0.76 to 2.08) | 0.366 |
| CMOTIVATION 4: appears indifferent to the worries and concerns of family members | 473 | 1.15 (0.70 to 1.88) | 0.572 |
| CMOTIVATION 5: shows reduced affection | 472 | 0.88 (0.49 to 1.54) | 0.663 |
For VI defined by VA < 6/12, 75% of behaviours (36/45) have ORs that are > 1.0 (range from 1.01 to 2.27) for participants with VI, compared with participants without VI, indicating an adverse relationship of VI with each of these activities. Seven behaviours have ORs < 1.0 (range 0.81–0.98), five of which are from the ‘motivation’ and ‘stereotypic and motor behaviours’ domains; none of these ORs are statistically significant (p-values range from 0.30 to 0.94). Applying the conservative 0.0011 cut-off point for statistical significance, only ‘has difficulties writing (letters, Christmas cards, lists, etc.)’ has an OR that is statistically significant, with an estimated OR of 2.27 (95% CI 1.50 to 3.45; p< 0.001). There is some evidence of an independent VI association with ‘has difficulties using the telephone’, with an estimated OR of 1.89 (95% CI 1.27 to 2.82; p = 0.002), ‘has problems handling money or paying bills’, with an OR of 1.89 (95% CI 1.20 to 3.02; p = 0.006), and ‘has difficulties grooming self (e.g. shaving or putting on make-up)’, with an OR of 1.81 (95% CI 1.24 to 2.66; p = 0.002).
For VI defined by VA < 6/18, 75% of behaviours (36/45) have ORs that are > 1.0 (range from 1.05 to 2.17) for participants with VI compared with participants without VI, indicating an adverse relationship of VI with each of these activities. Eight behaviours had ORs < 1.0 (range 0.72–0.95), of which two are in the ‘motivation’ domain, two are in the ‘stereotypic and motor behaviours’ domain and three are in the ‘memory and orientation’ domain; none of these eight ORs is statistically significant (p-values range from 0.19 to 0.85). Applying the conservative 0.0011 cut-off point for statistical significance, no OR is statistically significant. There is evidence of an independent VI association with ‘has difficulties writing (letters, Christmas cards, lists, etc.)’, with an estimated OR of 2.17 (95% CI 1.24 to 3.89; p = 0.008), and ‘makes tactless or suggestive remarks’, with an OR of 2.14 (95% CI 1.29 to 3.5; p = 0.003).
Missing data sensitivity results
The primary variables, VA and sMMSE score, exhibited considerable rates of missingness owing to participants being unable to complete either or both components. Presenting distance VA was unmeasured (missing) in 120 participants (17%), and for presenting NVA this figure was 232 (33%) participants. The sMMSE score was unmeasurable (missing) in 54 (7%) participants. In 43 participants (6%), all three of these variables were unmeasured. There was a strong association between missingness on distance VA and missingness on sMMSE scores, missingness on NVA and missingness on sMMSE scores, and missingness on distance VA and missingness on NVA (all p < 0.001). Table 34 shows the combinations of missingness on these three variables.
| Presenting distance VA | Presenting NVA | sMMSE | |
|---|---|---|---|
| Not missing | Missing | ||
| Not missing | Not missing | 463 (65.4%) | 8 (1.1%) |
| Not missing | Missing | 114 (16.1%) | 3 (0.4%) |
| Missing | Not missing | 5 (0.7%) | 0 (0.0%) |
| Missing | Missing | 72 (10.2%) | 43 (6.1%) |
For the primary results relating to distance VI, ocular conditions and near vision loss, which have been presented for complete cases in Tables 7, 12 and 23, the overall sample estimates of prevalence (accounting for missing data using multiple imputation) are given in Table 35. It is apparent that the prevalence of distance VI and near vision loss are greater than estimated from the complete-case analysis, reflecting that the prevalence of VI is higher by location, and by age, and that these factors are also associated with difficulty obtaining VA measurements; therefore, participants with missing data are more likely to have poorer VA. Prevalences of ocular conditions are similar to those reported in the complete-case analysis in Table 12, reflecting that these assessments were mostly without missing data compared with the measurements of distance and NVA.
| Acuity status/ocular condition | Imputed prevalence (%) | |
|---|---|---|
| Estimate | 95% CI | |
| Distance VI | ||
| Presenting (VA < 6/12) | 37.7 | 33.3 to 42.3 |
| Presenting (VA < 6/18) | 21.9 | 18.4 to 25.8 |
| Post refraction (VA < 6/12) | 24.8 | 19.2 to 31.4 |
| Post refraction (VA < 6/18) | 13.4 | 10.1 to 17.7 |
| Under-/uncorrected (VA < 6/12) | 14.5 | 11.3 to 18.4 |
| Under-/uncorrected (VA < 6/18) | 8.7 | 6.1 to 12.2 |
| Ocular conditions | ||
| AMD | 18.4 | 15.7 to 21.5 |
| Cataract (1–5, no IOL) | 58.6 | 54.9 to 62.2 |
| Cataract (2–5, no IOL) | 37.7 | 34.2 to 41.4 |
| Diabetic retinopathy | 2.4 | 1.4 to 4.0 |
| Glaucoma | 8.8 | 6.8 to 11.2 |
| Near vision loss | ||
| Presenting (NVA < N8) | 27.9 | 24.2 to 31.9 |
| Presenting (NVA < N10) | 19.9 | 15.4 to 25.4 |
| Post refraction (NVA < N8) | 19.6 | 16.1 to 23.7 |
| Post refraction (NVA < N10) | 9.2 | 6.5 to 12.9 |
| Under-/uncorrected (NVA < N8) | 11.4 | 9.0 to 14.3 |
| Under-/uncorrected (NVA < N10) | 13.1 | 9.6 to 17.8 |
For the regression results relating to VI presented in Table 11, there was no change in the substantive conclusions under the imputation model. Compared with the complete-case analysis, the independent associations of age, sex, sMMSE score (where investigated) and location with VI (presenting VI, post-refraction VI or uncorrected/undercorrected VI) were all unchanged in direction and statistical significance. Point estimates tended to be slightly increased for the OR of presenting and post-examination VI in participants in residential care versus community resident participants. These ORs for uncorrected/undercorrected VI in participants in residential care versus participants resident in their own homes were slightly attenuated in the multiple imputation analysis compared with the complete-case analysis, but the differences were small: approximately a 10% reduction in the ORs.
Similarly for results relating to Table 15 for the four target eye conditions (AMD, cataract, diabetic retinopathy and glaucoma) there were no changes in the substantive conclusions under the imputation model compared with our initial findings. The independent associations of age, sex and location with each ocular condition were unchanged in direction and statistical significance compared with the complete-case analyses. In addition, for our findings relating to NVA presented in Table 24, there were no substantive changes in conclusions under the multiple imputation model compared with the complete-case analysis.
Tables reporting the ORs and inferences relevant to Tables 11, 15 and 24, but under the multiple imputation model, can be found in Appendix 4 for further detailed comparisons.
Extrapolating prevalence to the UK dementia population
The methods used to calculate the weights assigned to each stratum were described in Chapter 2 (see Stage 1: data analysis plan, Extrapolating prevalence to the UK dementia population). These weights are given in Table 36; higher weights indicate calibration addressing undersampling relative to the target population, and lower weights indicate oversampling. The weights, normalised such that they sum to the PrOVIDe sample size, range from 0.44 to 2.86 (a ratio of 6.50) exhibiting large differences in sampling probabilities across these strata.
| Age group (years) | Living in own home | Living in care home | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| 60–64 | 1.77 | 1.22 | 2.86 | 1.98 |
| 65–69 | 2.18 | 1.38 | 0.69 | 0.44 |
| 70–74 | 1.04 | 1.22 | 0.94 | 1.1 |
| 75–79 | 1.18 | 1.05 | 1.06 | 0.95 |
| 80–84 | 1.24 | 1.08 | 0.57 | 0.49 |
| 85–89 | 0.72 | 0.97 | 1.00 | 1.35 |
The extrapolated prevalence estimates for each outcome for the UK population of those aged 60–89 years with dementia are given in Table 37.
| Acuity status/ocular condition | Extrapolated population prevalence (%) | |
|---|---|---|
| Estimate | 95% CI | |
| Distance VI | ||
| Presenting (VA < 6/12) | 34.6 | 29.3 to 40.3 |
| Presenting (VA < 6/18) | 20.3 | 16.7 to 24.6 |
| Post refraction (VA < 6/12) | 22.4 | 16.4 to 29.9 |
| Post refraction (VA < 6/18) | 12.2 | 8.8 to 16.6 |
| Under-/uncorrected (VA < 6/12) | 13.6 | 10.5 to 17.4 |
| Under-/uncorrected (VA < 6/18) | 8.3 | 5.9 to 11.6 |
| Ocular conditions | ||
| AMD | 17.7 | 14.7 to 21.0 |
| Cataract (1–5, no IOL) | 57.7 | 52.4 to 62.9 |
| Cataract (2–5, no IOL) | 36.7 | 32.5 to 41.1 |
| Diabetic retinopathy | 2.3 | 1.2 to 4.4 |
| Glaucoma | 8.4 | 6.5 to 10.7 |
| Near vision loss | ||
| Presenting (NVA < N8) | 25.9 | 22.1 to 30.1 |
| Presenting (NVA < N10) | 17.8 | 13.2 to 23.6 |
| Post refraction (NVA < N8) | 17.5 | 13.1 to 23.0 |
| Post refraction (NVA < N10) | 8.0 | 5.2 to 12.0 |
| Under-/uncorrected (NVA < N8) | 11.1 | 8.7 to 14.1 |
| Under-/uncorrected (NVA < N10) | 11.9 | 8.3 to 16.7 |
Further prevalence estimates of distance VI for the populations with dementia aged 60–74 years, 65–89 years and 75–89 years are provided in the additional tables in Appendix 5.
The prevalences for distance VI and near vision loss are generally higher in these population estimates, and CIs are wider, than in the PrOVIDe sample rates, reflecting the design effect and the large variation in calibration weights.
Comparison of prevalence with other UK studies of visual impairment in older people
The methods used to reweight the estimated rates of VI in PrOVIDe to allow greater comparability with two nationally representative studies of VI in the elderly were described in Chapter 2 (see Stage 1: data analysis plan, Comparison of prevalence with other UK studies of visual impairment in older people). In the NDNS,57 the crude (unweighted, unclustered) rates of VI defined by VA < 6/12 and VA < 6/18 in those aged 65–84 years were calculated as 17.9% and 7.4%, respectively. In the MRC study,56 the crude rates of VI defined by VA < 6/12 and VA < 6/18 in those aged 75–89 years were calculated as 18% and 11.0%, respectively. Reweighted PrOVIDe estimates are given alongside these comparative estimates in Table 38.
| Distance VI | Comparator study | Age range overlap (years) | n PrOVIDe | n comparator | PrOVIDe reweighted VI rate (%) | Comparator VI rate (%)a | ||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |||||
| Presenting (VA < 6/12) | NDNS | 65–85 | 486 | 1027 | 23.7 | 20.0 to 27.7 | 17.9 | 15.6 to 20.4 |
| Presenting (VA < 6/18) | NDNS | 65–85 | 486 | 1027 | 11.9 | 8.5 to 16.3 | 7.4 | 5.9 to 9.2 |
| Presenting (VA < 6/12) | MRC | 75–89 | 283 | 13,819 | 28.7 | 21.3 to 37.4 | 18.0 | 14.5 to 21.5 |
| Presenting (VA < 6/18) | MRC | 75–89 | 283 | 13,819 | 10.4 | 7.1 to 15.1 | 11.0 | 9.3 to 12.7 |
Point estimates of presenting VI rates are higher for PrOVIDe than for the NDNS subsample for both definitions of VI, suggesting a meaningful difference between these populations, although there is only weak evidence to rule out no difference between prevalence rates. For presenting distance VI defined by VA < 6/12, the point estimates of prevalence are meaningfully lower in the MRC subsample than in the age-similar participants of PrOVIDe who are living in their own homes. Although this difference of 10.7% is imprecisely estimated, there is some evidence of a difference between true prevalence rates. For presenting VI defined by VA < 6/18, estimates are slightly higher in the MRC subsample than in the age-similar participants of PrOVIDe who are living in their own homes, but these differences are neither statistically significant nor very large in magnitude.
It should be noted that even though age, sex and residency differences in the samples have been largely accounted for, there may of course be systematic differences between the samples even after reweighting. In particular, how participants were recruited and how representative they are of certain regions cannot be addressed. It may be that participants of the same age, sex and residency relative to their own populations (dementia for PrOVIDe, non-dementia for the main NDNS sample and mixed for MRC) were generally healthier or less healthy in MRC or NDNS than those in PrOVIDe, or that their health-care access was better. NDNS participants were sampled by postcodes directly through residential addresses, whereas PrOVIDe and the MRC study participants were sampled by regional clusters through public health services, and this may have led to differences in those recruited.
Key findings from stage 1
-
Optometrists usually recommend that people have annual sight tests from the age of 70 years and every 2 years before that unless there are clinical reasons for more frequent testing. In the PrOVIDe study, 22% reported not having had a test in the past 2 years: this included 19 participants who had not been tested in the past 10 years.
-
The prevalence of presenting VI was 32.5% (95% CI 28.7% to 36.5%) and 16.3% (95% CI 13.5% to 19.6%) for VA < 6/12 and 6/18 respectively, in people aged 60–89 years, generally higher than in comparable data from prevalence studies on the general population after adjustment for age and sex. Notably, 51.4% (95% CI 44.5% to 58.3%) and 26.4% (95% CI 20.7% to 33.0%) of participants living in care homes were visually impaired using the VA < 6/12 and VA < 6/18 cut-off points, respectively.
-
Visual impairment was correctable with an up-to-date spectacle prescription (uncorrected/undercorrected VI) for 14.3% (95% CI 11.7% to 17.5%) of participants for VA < 6/12 and 7.7% (95% CI 5.7% to 10.2%) for VA < 6/18.
-
Even with the best spectacle correction, VI remained for 18.1% (95% CI 15.2% to 21.5%) and 8.6% (95% CI 6.6% to 11.3%) of participants for VA < 6/12 and < 6/18, respectively.
-
Cataract was the primary clinically determined cause in 48.0% of cases of post-refraction VI (for VA < 6/12 criterion). This VI is potentially remediable. AMD was the primary cause in 36.3% of cases. For VA < 6/18, AMD was the cause in 48.9% of cases and cataract was the cause in 36.1%.
-
Distance VA improved by two or more lines on a logMAR chart post refraction in 17.8% of participants.
-
16.2% of participants could not read standard newspaper-size print with their current spectacles; however, almost two-thirds of these participants could read this print with up-to-date spectacles.
-
While research studies rarely include substantial numbers of people with dementia living in care homes, the PrOVIDe study had 319 care home residents (44%). The unadjusted rates of all types of VI were between 2 and 2.5 times greater for care home residents than for participants living in their own homes. After age and sex adjustments, the higher rates of VI persisted in those living in care homes.
-
After adjustment for age, sex and group, cognitive impairment assessed by sMMSE score had a significant independent association with uncorrected/undercorrected VI (VA < 6/18) (p = 0.03) but there was no evidence for an independent association of sMMSE with VI defined as VA < 6/12.
-
Exploratory analysis found evidence for deficits in some vision-related aspects of function and behaviour in visually impaired participants compared with non-visually impaired participants.
-
There was no evidence that management of VI in people with dementia differed from that in the general population of older people. The percentage of participants advised of a change in spectacle prescription post refraction was consistent with the national figure. The PrOVIDe referral rate was 6.7%, higher than the national figure of 5% for the population as whole, but this could be due to the older age-profile of PrOVIDe participants.
-
When extrapolated to the UK wider population with dementia, following post-stratification calibration and imputation, prevalences of VI are higher, with wider CIs, than the PrOVIDe sample rates.
-
For VA < 6/12, the extrapolated prevalences for presenting, post-refraction and uncorrected/undercorrected VI were 34.6% (95% CI 29.3% to 40.3%), 22.4% (95% CI 16.4% to 29.9%), and 13.6% (95% CI 10.5% to 17.4%), respectively.
-
For VA < 6/18, the extrapolated prevalences for presenting, post-refraction and uncorrected/undercorrected VI were 20.3% (95% CI 16.7% to 24.6%), 12.2% (95% CI 8.8% to 16.6%) and 8.3% (95% CI 5.9% to 11.6%).
-
-
For the primary results relating to distance VI and near vision loss, when accounting for missing data using multiple imputation, the prevalences are greater than estimated from the complete-case analysis. Prevalences of ocular conditions are similar to those reported in the complete-case analysis.
-
For the regression results relating to distance VI, near vision loss and the four eye conditions, there was no change in the substantive conclusions under the imputation model compared with the complete-case analysis.
-
Point estimates of presenting VI rates are higher for PrOVIDe than for the NDNS subsample for both definitions of VI, suggesting a meaningful difference between these populations, although there is only weak evidence to rule out no difference between prevalence rates. For presenting distance VI defined by VA < 6/12, the point estimates of prevalence are meaningfully lower in the MRC subsample than in the age-similar participants of PrOVIDe who are living in their own homes. It should be noted that there may be systematic differences between the samples even after reweighting.
Chapter 4 Stage 2 results
Introduction
Stage 2 involved the collection of qualitative data from four participant sets:
-
people with dementia
-
family carers of people with dementia
-
professional care workers working in care homes
-
optometrists.
The basic topic guide used with all four participant sets covered the same key areas: experiences of eye examinations, attitudes towards eye care, and opinions on if or how eye care for people with dementia could be improved. This approach, using triangulation of participants to explore similar areas from different perspectives, resulted in the identification of overlapping themes when framework analysis was applied to the data. The data generated a total of 111 categories across the four sets of participants, although this number includes duplications; for example, cataract was a data category in every set of participants. These categories were synthesised into six common themes:
-
eye examinations
-
domiciliary eye care
-
spectacles
-
cataract
-
improving the eye care of people with dementia
-
quality of life.
The structure of this chapter is a description of the characteristics of study participants followed by an exposition of the six themes.
Characteristics of the sample
Interviews with people with dementia
Interviews were conducted with 36 people with dementia, all of whom had participated in stage 1. Their key characteristics are listed in Table 39.
| Interview | Region | Group | Sex | Age (years) | Carer present | sMMSE score |
|---|---|---|---|---|---|---|
| 1 | North Thames | 1 | Female | 88 | Son | 23 |
| 2 | North East | 1 | Female | 79 | Daughter | 24 |
| 3 | North East | 1 | Female | 79 | Husband | 23 |
| 4 | North East | 1 | Female | 76 | None | 22 |
| 5 | North East | 1 | Male | 75 | Wife | 27 |
| 6 | Thames Valley | 1 | Male | 68 | Wife | 27 |
| 7 | Thames Valley | 1 | Female | 76 | Daughter | 24 |
| 8 | Thames Valley | 1 | Male | 80 | Wife | 24 |
| 9 | Thames Valley | 1 | Male | 60 | Wife | 30 |
| 10 | North Thames | 1 | Female | 86 | Husband | 20 |
| 11 | Thames Valley | 1 | Male | 63 | Wife | 20 |
| 12 | Thames Valley | 1 | Male | 72 | Wife | 25 |
| 13 | Thames Valley | 1 | Male | 82 | Wife | 22 |
| 14 | North East | 1 | Male | 81 | Wife | 24 |
| 15 | North East | 1 | Male | 69 | Wife | 30 |
| 16 | North East | 1 | Female | 81 | None | 21 |
| 17 | North East | 1 | Female | 62 | None | 16 |
| 18 | North East | 1 | Male | 69 | Wife | 24 |
| 19 | North Thames | 1 | Female | 79 | Partner | 12 |
| 20 | North Thames | 1 | Female | 67 | Husband | 23 |
| 21 | Thames Valley | 2 | Female | 74 | None | 26 |
| 22 | North Thames | 2 | Male | 88 | Son | 11 |
| 23 | Thames Valley | 2 | Female | 89 | Daughter | 24 |
| 24 | North East | 1 | Male | 84 | Wife | 22 |
| 25 | North East | 1 | Male | 78 | Wife | 22 |
| 26 | North East | 1 | Male | 67 | Wife | 26 |
| 27 | North East | 1 | Male | 74 | Wife | 26 |
| 28 | North Thames | 2 | Female | 80 | None | 20 |
| 29 | North Thames | 2 | Female | 79 | None | 19 |
| 30 | Thames Valley | 1 | Female | 80 | Friend | 8 |
| 31 | Thames Valley | 1 | Female | 67 | Husband | 30 |
| 32 | East Anglia | 1 | Female | 81 | Husband | 22 |
| 33 | East Anglia | 1 | Male | 83 | Wife | 24 |
| 34 | East Anglia | 1 | Male | 87 | Wife | 26 |
| 35 | East Anglia | 1 | Male | 82 | Wife | 27 |
| 36 | East Anglia | 1 | Female | 73 | Husband | 15 |
The majority of participants (n = 31) lived in their own homes. Participants were informed that they were welcome to have someone present at the interview and all but two of those living at home and two living in a care home had a relative or friend in attendance.
Stage 2 interviews were initiated in May 2013, within 6 months of the commencement of stage 1, and all of the interviewees were recruited from the four regions originally involved in the study. Purposive sampling was employed to ensure that the sample comprised participants living in all types of location: city, urban and rural.
The sample was evenly divided between males and females and encompassed the full age range for study participants, 60–89 years, with a mean age of 77.5 years for women and 75.6 years for men.
Family carer focus groups
A total of 33 family carers attended across the five focus groups. The demographics of the sample are listed in Table 40. As the table shows, 20 of these focus group participants were caring for a relative at home and 13 had relatives in care homes, so the focus groups provided insights into experiences in both care settings.
| Region | ID | Carer for | Relationship | Resides at |
|---|---|---|---|---|
| North East focus group | CFG 1.1 | Wife | Husband | Home |
| CFG 1.2 | Mother | Daughter | Home | |
| CFG 1.3 | Mother | Son | Care home | |
| CFG 1.4 | Mother-in-law | Daughter-in-law | Care home | |
| CFG 1.5 | Husband | Wife | Home | |
| North Thames focus group | CFG 2.1 | Wife | Husband | Home |
| CFG 2.2 | Mother-in-law | Daughter-in-law | Home | |
| CFG 2.3 | Brother | Brother | Home | |
| CFG 2.4 | Sister | Sister | Home | |
| CFG 2.5 | Mother and father | Daughter | Home | |
| CFG 2.6 | Father | Daughter | Home | |
| CFG 2.7 | Mother | Son | Home | |
| North Thames focus group | CFG 3.1 | Father | Daughter | Care home |
| CFG 3.2 | Husband | Wife | Home | |
| CFG 3.3 | Mother | Daughter | Home | |
| CFG 3.4 | Wife | Husband | Home | |
| CFG 3.5 | Wife | Husband | Home | |
| CFG 3.6 | Mother | Daughter | Care home | |
| CFG 3.7 | Mother | Daughter | Care home | |
| CFG 3.8 | Sister | Sister | Care home | |
| CFG 3.9 | Mother-in-law | Daughter-in-law | Care home | |
| Thames Valley focus group | CFG 4.1 | Husband | Wife | Home |
| CFG 4.2 | Husband | Wife | Care home | |
| CFG 4.3 | Grandmother | Granddaughter | Home | |
| CFG 4.4 | Wife | Husband | Care home | |
| CFG 4.5 | Mother | Daughter | Care home | |
| CFG 4.6 | Wife | Husband | Home | |
| CFG 4.7 | Wife | Husband | Home | |
| North East focus group | CFG 5.1 | Mother | Daughter | Care home |
| CFG 5.2 | Mother | Daughter | Care home | |
| CFG 5.3 | Mother | Daughter | Care home | |
| CFG 5.4 | Husband | Wife | Home | |
| CFG 5.5 | Mother | Son | Home |
Optometrist focus groups
A total of 34 optometrists participated across five focus groups (Table 41). Four regionally organised groups each had five or six participants working in a range of optometric settings. A fifth focus group (focus group 2) was arranged with the optometrists who examined patients for the PrOVIDe study to capitalise on their experiences of working in domiciliary care.
| Focus group | ID | Primary work setting |
|---|---|---|
| 1 | OFG 1.1 | Low vision/locum |
| OFG 1.2 | Independent practice/diabetic retinopathy | |
| OFG 1.3 | Independent practice | |
| OFG 1.4 | Independent practice | |
| OFG 1.5 | Independent practice | |
| OFG 1.6 | Independent practice | |
| 2 | OFG 2.1 | TOC domiciliary |
| OFG 2.2 | TOC domiciliary | |
| OFG 2.3 | TOC domiciliary | |
| OFG 2.4 | TOC domiciliary | |
| OFG 2.5 | TOC domiciliary | |
| OFG 2.6 | TOC domiciliary | |
| OFG 2.7 | TOC domiciliary | |
| OFG 2.8 | TOC domiciliary | |
| OFG 2.9 | TOC domiciliary | |
| OFG 2.10 | TOC domiciliary | |
| OFG 2.11 | TOC domiciliary | |
| 3 | OFG 3.1 | Independent practice |
| OFG 3.2 | Hospital | |
| OFG 3.3 | Hospital | |
| OFG 3.4 | Community practice/hospital practice | |
| OFG 3.5 | Part-time | |
| OFG 3.6 | Independent practice/locum work | |
| 4 | OFG 4.1 | Glaucoma clinic/independent practice/domiciliary work |
| OFG 4.2 | Community optometrist | |
| OFG 4.3 | Independent practice | |
| OFG 4.4 | Independent practice/hospital | |
| OFG 4.5 | Independent practice | |
| OFG 4.6 | Hospital/independent practice | |
| 5 | OFG 5.1 | Hospital |
| OFG 5.2 | Hospital/private practice | |
| OFG 5.3 | Community practice/hospital/domiciliary | |
| OFG 5.4 | Hospital | |
| OFG 5.5 | Community practice/domiciliary |
Professional care workers
Professional care worker perspectives were explored through one focus group comprising representatives from a care home company in North Thames, and individual interviews with care workers and managers employed by a range of companies (Table 42).
| ID | Role |
|---|---|
| CHFG 1 | Company dementia manager |
| CHFG 2 | Reception and administrator |
| CHFG 3 | Care home manager |
| CHFG 4 | Care team manager |
| CHFG 5 | Relief care team manager |
| CH interviewee 1 | Deputy care home manager |
| CH interviewee 2 | Senior carer |
| CH interviewee 3 | Carer |
| CH interviewee 4 | Dementia unit care manager |
| CH interviewee 5 | Dementia care unit manager |
| CH interviewee 6 | Care assistant |
| CH interviewee 7 | Care assistant |
| CH interviewee 8 | Carer |
| CH interviewee 9 | Nurse |
| CH interviewee 10 | Nurse |
| CH interviewee 11 | Head of care |
Theme 1: eye examinations
This theme describes how eye examinations were arranged, the frequency of examinations and experiences from patient, carer and professional perspectives. People with dementia were asked about their experiences of eye examinations, how often they had an examination, where they went and what their experiences had been.
Provision of the eye examination
In terms of who performed the eye examination, there were variations in responses among interviewees; some had tended to see the same optometrist or at least had visited the same practice over the years, while others moved around. None of the 31 interviewees living in their own homes had experienced a domiciliary eye examination prior to the study.
Conversely, in care homes, most eye examinations were conducted by a company specialising in the provision of domiciliary services. Care workers described how an optometrist from the company usually made several visits to the care home during the course of a year and carried out several examinations. The number of examinations conducted during one visit could be as many as 20 in 1 day, although it was unclear if these were all conducted by one optometrist. The company often sent two people but, from the care workers’ accounts, one of these appears to have been either a clinical assistant or a dispensing optician. Care workers reported that it was rare for a resident to continue to be seen by their previous optometrist following admission to the care home; two thought that ‘private’ optometrists were reluctant to carry out a domiciliary visit.
There was a view, expressed mainly by optometrists but also by some participants in all other categories, that it was desirable to see the same optometrist in order to provide continuity of care. For example:
It’s better and easier to go the same place because they know him, he knows them and that makes it easier.
Wife of interviewee 9
In my opinion it would be like going to a doctor’s, you’d like to see the same one, because he knows when he makes his notes, what he’s put down, and then you get to know that person. The continuity is better, and you feel better. But then even if you go to the opticians, you’ll see different people at different times, it’s not quite the same.
CFG 2.2
If you go to Mr X in Chiswick High Road, he’s been there a long time, and you go back again, he’s got your file on you and everything else. But you go to [name of optometrist chain], the young girl’s gone back to Poland, the other chap’s gone back to wherever and it’s all different again, at the end of the day.
CFG 2.3
I think something that’s quite important, there isn’t continuity in care these days, so many people dot around which practice they’ll go to. And as people get older, if they can be encouraged, that continuity of care I think is so important. And I don’t know perhaps how we could put that over to try and encourage that.
OFG 3.3
I think it’s kind of nicer for patients to be able to go back to where they’ve gone; you’ve got the history and the stuff that makes your life a lot easier, and makes the decision-making more straightforward.
OFG 4.1
Once they’ve found an optometrist they are happy with, stick with them, don’t be tempted by the latest special offer when they go shopping because continuity really helps everybody, patient and practitioner alike.
OFG 4.2
Frequency of eye examinations
Most interviewees stated that they had an eye examination annually or approximately every 2 years. The main reason stated by those who did not do this was that they thought that there was nothing wrong with their eyes or that they could see through their current spectacles and did not need to see an optometrist.
Reports from family carers were consistent with this; most of them said that their relative had regular (annual or 2-yearly) eye examinations both before and after the onset of dementia. Again, exceptions to this were linked with a perceived lack of need.
Nan had cataracts removed about 12 years ago and after that her eyesight improved dramatically, as you would expect. And so since then she hasn’t felt she needed to have her eyes tested again, because she was so happy with them.
CFG 4.3
There was just one case of someone not having an eye examination following difficulties with a previous examination:
Some of you have already said something about problems coping with certain aspects of the eye exam, is that right?
Yes, that’s right. That was a number of years ago, and that was the reason, she hadn’t had an eye test for 4 or 5 years, I suppose.
When participants were asked for a view on how often people with dementia should have an examination, the most commonly expressed view was that annual examinations were necessary. Generally, family carers and optometrists thought that an annual eye exam was desirable and that 2 years between examinations would be too long for most people.
Specifically with dementia, it would be a great source of confusion annoyance, irritation, puzzlement to my wife if she were having problems with her eyes and she didn’t have them checked annually; to go 2 years would really be just too far in terms of the time involved.
CFG 4.6
However, some participants – particularly some care workers – thought that there was a need for more frequent testing, as often as 6-monthly, in the belief that as a person’s dementia progressed there was a need to check if their sight had also deteriorated. For example:
I would say for some of them, yearly, but if their dementia’s starting to go down that little bit, or they’re not finding their way around it could be their eyes, you know, so I would say that, I would say a little bit more often.
CH interviewee 2
Experiences of eye examinations
Participants were asked to share their experiences of eye examinations conducted on people with dementia. Interviewees generally were satisfied with their eye examinations and did not perceive any problems, but some family carers and care workers expressed doubts about how optometrists could conduct a full examination because of the cognitive impairment associated with dementia, particularly those elements of the examination which required a judgement or some type of recall response from the person being examined.
I have real concerns about what he can see and how you assess what he can see. With hallucinations, with lack of concentration, lack of ability to focus, all the things that go along with the dementia. When he was first tested he read the eye chart as a book, trying to make words out of the letters, but at least that showed that he could see the letters and the last time, which was a year later, he couldn’t identify the letters. And I honestly don’t know whether it’s vision, or whether there’s a fault between the eye and the brain.
CFG 3.2
I have doubts when they put things and say ‘is this better and is that better?’ because you don’t remember from one to the next. So I’m not confident that she gives the right response, and therefore she can’t get a proper diagnosis and a proper prescription.
CFG 3.3
I was identifying with what other people were saying, you know, how difficult it is to know to what extent they’re giving the right answers. She just doesn’t see things a lot of the time and there’ve been so many occasions where I’ve tried to point something out and she just will not see it. And if she can’t see it, I’m doubtful about the value of the eye test. There is the other side to it, the health part, which is obviously, as you were saying, objective, and worthwhile.
CFG 3.4
I think some parts of the eye examination though, are quite threatening for someone who doesn’t understand.
CFG 4.3
Well just from my own personal experience of having my eyes tested, they ask lots of questions ‘is this clearer with this lens or that lens’ and it’s quite a lengthy process when you’re having your eyes checked and to get the right spectacles for you. And if they can’t answer, those questions, I don’t understand sometimes how they’re getting the right glasses for them.
CH interviewee 10
Problems with the eye examination
Not all family carers had observed a relative having an eye examination. Very few reported going into the examination room in a practice. Carers of stage 1 participants living in their own homes had mostly been at home when the PrOVIDe examination was conducted, but few had been present if their relative had been examined in a care home. Of those who had been present at an examination in a practice (not a PrOVIDe examination), a small number identified problems they had encountered. The problems included difficulties with communication and understanding test instructions. Carers explained the effect of dementia on a person’s ability to understand and respond. For example:
You know the one where you have to count the dots or whatever, they move around, and he couldn’t, it was inconclusive. They wanted to do it again. And they weren’t really explaining why, he had to do it again, and he was getting a bit frustrated with that.
CFG 2.6
There were definitely a few instructions that my mum struggled to understand. There was one machine and she really struggled to sit in the seat and get the right placing for. My sister said there was something they said would take 10 minutes and took about 30 or something, so that seemed to be a bit of lack of confidence, a bit of not understanding the instructions, but as far as we know they muddled through.
CFG 1.2
Our first language is Aramaic, then obviously we came here, she worked on her English. Now, she’s forgotten all that, gone back to the Aramaic. So when we go, this is our problem, the letters, this is the thing. So obviously something to do with dementia, rather than the eyesight.
CFG 2.4
Care home staff also described examinations in which significant cognitive impairment led to communication problems and lack of co-operation.
The pressures test or ‘can you see out of this eye, can you see best out of this, can you read the numbers well’. If you’ve got the majority, say 20 residents, and 18 of those residents aren’t able to verbally communicate, or understand what you’re even asking them, how are you possibly going to conduct a reasonable eye test on that person?
CH interviewee 3
We did have one [person] that would not get her eyes tested whatsoever. She wouldn’t have nothing on her face, it used to distress her too much, so we just couldn’t do it.
CH interviewee 4
I had a lady, looked after her a long time, and she wouldn’t let anybody look at her eyes. I mean she didn’t like anyone very close up at anything, although we did try and try and try . . . so they could never get her eyes tested to see how bad she had got.
CH interviewee 2
Although family carers gave relatively few examples of difficulties with the ‘mechanics’ of the eye examination, some expressed concern about the way eye-care professionals – optometrists and hospital staff – interacted and their understanding of the impact of dementia. The first in the next group of quotations is from an optometrist who talked about taking her grandmother for an eye examination.
My nana had dementia. I accompanied her for an eye examination. The consulting room had all the state of art equipment but the optometrist had no experience in examining a patient with dementia. It was visible through the course of the eye examination.
OFG 2.6
It would be really good if opticians had dementia training. We got some bifocals for my husband, he’d never worn bifocals before, and I thought ‘well it’ll be easier for him than switching’. It was a complete disaster, he never wore them. And I feel that the optician ought to have warned me that that was a bad choice.
CFG 3.2
The optician just couldn’t grasp the concept she had dementia. When she wouldn’t do the peripheral test, he just couldn’t take that in and he got cross with her and then she got upset.
CFG 4.5
One of the worst ones, was the hospital department, and the girl there – I won’t even go into it ‘cause I could’ve hit her – when she did put something in wrong, she said ‘what did you do!’ and I said ‘she can’t remember’; ‘well how can I deal with her if she doesn’t know!’, I said ‘she doesn’t know what she did’, ‘well look, tell me, just tell me what you did’, I said ‘she doesn’t know’. I could’ve murdered her, this woman had no concept of dementia whatsoever and she was really aggressive this . . . optometrist, or whatever you want to call her. And another time they had to go in a little room, on their own to be tested, and I said ‘I’ll come in with her’, ‘no you can’t’, I said ‘she needs me with her’, ‘no, you stay out here’, I thought ‘you haven’t got a clue’ and that angered me.
CFG 5.3
There were several pleas from family carers for optometrists to understand the need to involve carers in the consultation. The main explanation for this was that a carer knew that communicating with the individual in certain ways would be more likely to elicit an appropriate response or to reduce the individual’s anxiety. For example:
You mention the word ‘dementia’ but the person I dealt with, did not seem to have the slightest clue of the implications that this would mean for mobility, for understanding, for me having to be there. Medically they might not want it but I said ‘you must accept this, I’m afraid, because you just won’t get anything to happen if I’m not there’.
CFG 4.6
Another thing, if the relative is there, they are going to be more at ease than if it’s just a stranger doing something to them. I think it will make it more successful.
CFG 5.1
For some family carers, the main area of concern was not the actual eye examination but the issue of accessibility to the practice (or to a hospital appointment); they explained the difficulties associated with getting ready for an appointment, travel, parking at the venue, etc.
I think it is a massive logistical exercise, taking somebody to get their eyes examined. It’s not straightforward to do.
CFG 3.1
What I have had a problem with, is mobility, because of her eyesight. Because I’ve got to take her in, that’s really where I’ve had the problems, because everyone’s supposed to be disablement friendly, but not everywhere is.
CFG 2.5
I had difficulties simply because I got to the point where to get my wife anywhere was such a problem, to get her into the car, that was a major achievement, and she might get in and then get out again, and that was the end of it.
CFG 4.6
Optometrists talked about the problems of the eye examination from their perspective. As the next set of quotations demonstrates, the problems included the range of tests, the patient’s reactions, and the patient’s emotional state and memory problems.
The other thing that I find is that quite a few of the dementia patients don’t seem to be able to cope with the mirror, so you’ve got to do a direct measurement of vision. So Kay’s pictures would solve that problem. I mean I often use a logMAR chart, if I’m just not getting any sensible visions.
OFG 1.3
I think the biggest problem for a young optometrist who is coming in to see a patient with dementia is they probably think ‘I should try and do everything’ and frankly, you just can’t. I mean, if you’ve got somebody on a slit lamp and you’re trying to see the peripheral fundus and they’re just always looking to one way, you’ve got to come to a point where that person, the young practitioner has to realise ‘I can do this, this and this’ and that’s the hard bit, working out what you can do and what you can’t, and what would be reasonable not to do.
OFG 1.2
It doesn’t matter what equipment you have, it comes back to the fear value. You start shining a light in somebody’s eye, whether it’s a direct or indirect ophthalmoscope; a direct ophthalmoscope I think is worse ‘cause you’re right in their face; indirect, you’re shining a light in their eyes, and they’re not happy, they don’t know why you’re doing it. So it’s not always what kit we’ve got, it’s what kit they will accept, and sometimes the simpler the better, but it can be so difficult.
OFG 3.3
The patients’ difficulty in doing certain tests, the fields test is probably the most obvious, maybe tonometry as well, where you can’t get all the pieces of the diagnostic jigsaw you need to enable you to make as robust a management decision as you might like to do. Which might have several consequences, it might lead to over-referrals, if for instance you can’t get an adequate view of the disc and you don’t have normal fields and pressures, you might feel your only option is to refer that patient. But it also might mean, conversely, that you might not refer that patient, when you should refer that patient.
OFG 4.2
Well they vary don’t they? And sometimes you can’t even get near them, and they’re aggressive, there are times when it’s exceedingly difficult and there’s sometimes you wouldn’t think there’s anything wrong. They co-operate, they answer the questions, may not be sensible answers, not the right answers, but they come up with answers, and you can often in the early stages get good acuities and you can do well. But then there comes a point when you can’t.
OFG 5.5
In the early stages of dementia, I manage to get to a position where they go through the whole routine, and they then make what appears quite a logical decision to make a purchase, and then once you’ve made the spectacles up, and I’ve had this before, ‘what glasses? I didn’t order any glasses’. And I think that there are barriers or challenges all the way through that we need to address.
OFG 1.1
However, as far as optometrists were concerned, two of the biggest problems when examining someone with dementia were related to communication. The first problem was not knowing that someone had dementia. This excerpt from one focus group demonstrates the consistency of experience.
Right. The biggest problem, I don’t know if you would class this as a barrier, the biggest problem is not knowing that the patient has dementia.
My biggest problem is the family saying nothing and I’ve had three patients in the last month where the family say nothing. And I’ve taken the patient in the room and you think there’s something funny going on here, you’re asking their medical history and they’re very vague, they insist that they’ve never been to an eye hospital and you can see the implant glinting in the light, and then eventually you work it out. But I think the biggest problem is not being told that the patient has dementia, and that’s three people in the last month, different practices.
I would agree with that, yes.
I would strongly agree with that as well.
There’s also the patients that haven’t been diagnosed as well.
Where you’re kind of talking to them and you’re thinking . . .
Well some of the members of the family just say they’re a bit eccentric.
But I mean that’s slightly harder because we’re not necessarily the person to diagnose that, but, definitely not being told about it makes it more difficult.
Optometrists from the second focus group, all of whom worked for TOC, had more experience of examining people with dementia and were able to describe being alert to ‘clues’ that might suggest that someone had dementia. For example, it was a clue ‘if the patients repeat things they have already told me earlier in the examination’ (OFG2.4). However, another explained that they could go through the full test but have no clue that the patient had been given a diagnosis (OFG2.2).
Informing the optometrist was something that was discussed in the interviews with people with dementia and their carers. About half of the interviewees had not told the optometrist but in many instances this was because the dementia had been diagnosed recently, since the person’s last eye examination. It was evident from responses that many of these interviewees had not considered telling their optometrist, but some thought that it was something they would discuss next time, as this sample conversation illustrates:
Do they know [at the optician’s] that you have problems with your memory?
Probably not because I haven’t been to them for a long time. It was your team that last sorted my glasses out.
I think the last time you saw them you were probably just waiting for diagnosis so they probably don’t. But I’m not 100% sure if we didn’t mention it. But we will next time because then they will know to be patient.
The second communication difficulty reported by optometrists occurred when they saw a patient accompanied by a carer or care worker who had insufficient knowledge about the patient or their eye-care history. They gave examples in which their impression was that the accompanying care worker was the newest or least experienced member of staff with no knowledge of the patient. The first of the following two quotations explains the problem, while the second is from an optometrist who explained the difference it can make when the accompanying care worker is someone who knows the resident.
Often, from care homes, there’s quite a lot of carers, a lot of patients, and some carers have no idea, they don’t have the information on the patient, so they don’t know if the patient is struggling with their distance vision or near vision, and then the patient doesn’t know, so often you have no history whatsoever to go from.
OFG 3.2
I think for me, one of the barriers, again particularly in secondary care, is how well you can liaise with other people around the person as well. So if you’ve got a care assistant who, who doesn’t know anything about the person and who’s just come with them in the taxi that day, then that makes life difficult whereas if you’ve got somebody who’s a key worker and can understand the person’s needs, and can perhaps give you some insight as to what the function is, so that you’re trying to gauge, like their coping for cataract and so on. I think that makes a big difference.
OFG 5.1
It was interesting to note that family carers had said that they wanted optometrists to take more notice of them and that, in their focus groups, the optometrists also stated how useful it was to have the involvement of the families. This quotation succinctly described the scenario.
Communication with carers is vital. It makes such a difference to have the communication two ways between all of them.
OFG 3.1
Some optometrists also talked about multidisciplinary communication, which they thought was inadequate in many cases. A very small number of optometrists gave examples of contacting an individual’s GP or other health-care professional to acquire more information or insight, but several other optometrists described how they would be unable to do the same because of time constraints related to the allotted time for an appointment.
Theme 2: domiciliary eye care
The discussions that informed theme 1 were mostly related to experiences in community practice; the exceptions were when participants were referring to experiences in care homes, where most residents had a domiciliary eye examination conducted. Theme 2 is concerned specifically with the provision of domiciliary eye care. It covers patient and professional experiences of domiciliary eye care and then moves on to describe issues that were raised about its provision.
The experience of domiciliary eye care
In stage 1 of the study all participants had a domiciliary eye examination. It was interesting to note during the stage 2 interviews that, before participating in the study, nearly all of the interviewees and family carers had been unaware that domiciliary eye care was available. Those who had known of domiciliary eye care did not realise that people with dementia would be eligible; they thought that it was for people who were ‘immobile’ or ‘very disabled’.
Some people with dementia and carers expressed surprise at how extensive the examination was and the amount of equipment used. Now that they knew about it, they thought it useful to know for future reference, and some said that they would like to have future examinations at home.
Patients and next of kin are surprised about the quality of eye examination given by the optometrists in a domiciliary setting. It could be because TOC optometrists have more time and this allows them to perform a thorough eye examination not a Cowboy eye test.
OFG 2.10
Did you know it’s possible to have a sight test done at home?
No, beforehand, I don’t think we did.
No.
Well I thought maybe, obviously if people are housebound and can’t get out there must be some provision for them to have it but I thought maybe your own optician would be able to come out although I didn’t know to what extent they’d be able to do it because you’ve got to carry the equipment and stuff around.
Did you know it was possible to have your eyes tested at home before we arranged it this time?
We thought you had to be really disabled. My mother died when she was 97 and it wasn’t until she was 96 that she had her eyes done at home. We thought you had to be really old and disabled you know.
I think when you see all the complicated equipment at the opticians you don’t think that you can replicate that at home.
CFG 1.3
Although researchers did not expressly ask for views on the eye examination performed for the study, many of the stage 1 participants and their carers volunteered complimentary comments on the experience. When considered collectively, the following string of comments describes what can be perceived as the benefits and positive aspects of a domiciliary eye examination:
I thought he was great. A nice person. Very tranquil. Just great.
Interviewee 19
I can only compare with what I have at [my practice]. Personally I would say that the one he had with the people here was more thorough than what I have.
Wife of interviewee 34
She really liked someone coming to the house, there’s nothing like being treated a bit special. We like the fact that he came here and he took a bit more time, I suppose you’re not being rushed through. He was here like an hour and it wouldn’t have been like that at the opticians it would have been 10, maybe 15 minutes. I think that was the difference.
Daughter of interviewee 2
The biggest difference was, I think, dignity, if that’s the right word. He was fantastic, he just treated her not like a VIP [very important person] but, you know, he was just great, if there was anything that she didn’t understand, he almost didn’t let her know she didn’t understand ‘cause he explained it, he wasn’t talking to me he was talking to her.
CFG 1.2
I have to say, when Mum had her eyes done by whoever . . . you sent, no problem at all. He was charming, you know, he was . . . he obviously understood who he was dealing with and . . . was appropriate.
CFG 4.5
The care from the home visit was much better than the local one in the high street. Because they understand, well that’s how it seemed to me, the person who came was excellent, and cared about mum’s eyesight and things like that.
CFG 2.7
It was superb. Very very thorough. They were thorough, polite, they were kind, patient in their attitude. Excellent.
Were you aware of any difference between having your eye test at [local branch of optometrist chain] and the eye test this time?
This one was far more thorough.
I don’t tend to go in the booth with you, I take you to the optician but I don’t go in the booth with you. It probably was better. They were particularly caring and understood.
And the extent of the equipment they had for making their diagnosis.
Did the eye examination take longer, having it done at home?
Well I think the extent to which my eyes were tested was far greater.
Interviewee 12 and wife
Oh [the domiciliary eye examination] was a lot more relaxed. We have to get all ready, get on the bus then you’ve got all the trouble of waiting for the buses to come home.
This is so much nicer.
That’s why we kept putting it off really. That’s why we missed one year and put it off to a second year because every time we went to go, one of us wasn’t well. When there’s two of you there’s always one of you got something. You think ‘I can’t do it this week I’ll make an appointment next week’ and then something else crops up. And it goes on and on. But when you’re having it done at home, if you’re not feeling quite up to it you can still have it done.
Interviewee 10 and husband
The optometrists from the four regionally based focus groups varied in the amount of experience each one had of conducting domiciliary visits. Those with little experience were sceptical about how much could be done, or whether or not an examination carried out at home was as good as those conducted in a practice. However, those who provided domiciliary eye examinations as part of their role extolled the virtues of testing at home, explaining how the patient was often more relaxed in their own environment and how the optometrist could also assess the impact of the environment on the patient’s sight. This exchange is an example of the comparative experiences:
I used to do home visits. And I wouldn’t consider doing fields actually, in a home visit really, I’m not having a go at you, believe me. I just wouldn’t.
I often find it sometimes easier to do a home visit on a dementia patient, I find that I get more useful results. I find stuff like ophthalmoscopy easier to do, because they’re in their home environment; stuff like pressures is always easier when they’re in their home environment as well. They’re more relaxed, their reading is better, they have access to things that they normally read, so you can always try to test out things in their home environment. So it’s often more of a useful, it’s a more functional eye test.
It’s interesting you say you find ophthalmoscopy, I haven’t done domiciliary for years, but I’m surprised you say that ophthalmoscopy is easier. I would have thought quite the reverse, unless you’re dilating them, ‘cause you can’t make the room as dark.
But they’re less distressed, so they’ll keep still.
As Table 41 showed, some of the optometrists had roles involving domiciliary care but many worked in community or hospital settings only. The availability of equipment was explored as a factor in community optometrists’ provision of domiciliary eye care and most of those who did not currently do domiciliary work thought that they did not have the right equipment to do so. This contrasted with TOC optometrists, all of whom thought that they were well equipped, as exemplified by this quotation from their focus group.
I feel very set up for a domiciliary eye examination with TOC. I am able to give a good test to provide the best possible eye care for a patient with dementia.
OFG 2.10
Provision of domiciliary care services
Not all optometrists are obliged to provide domiciliary care but under GOS regulations they should be able to advise a patient on how to access domiciliary care. The community optometrists who did not provide a domiciliary service were asked if they could identify a domiciliary services provider for a patient if required. Two optometrists said that they knew of individual practitioners; otherwise, responses indicated that their only knowledge related to specialist domiciliary care companies, and even then most had only a theoretical knowledge of their existence and limited experience of how they operated.
This area of questioning gave rise to discussions about the whole area of how domiciliary care is provided and funded. Although the comments about the domiciliary eye examinations conducted for the PrOVIDe study were complimentary, the comments from focus group participants and care home interviewees suggested some dissatisfaction with the system of domiciliary care provision.
Care workers described eye-care arrangements in the care home. Usually the care home had an arrangement with a domiciliary services provider who would send an optometrist to the care home several times a year. The optometrist usually had a list of who was scheduled for an examination, comprising new admissions and those for whom the annual examination was due. The optometrist was not usually the same person on each visit, suggesting consequent impact on continuity of care:
It was OK, but I noticed it’s somebody different that comes every time, so you know, I would think, you know, the continuity’s not there that, you would get probably possibly yourself if you were going to the optician.
CH interviewee 5
Not all care workers had first-hand experience of the eye examination. Of those who had observed or assisted a resident during an examination, some described perceived limitations in the thoroughness of the examination, albeit with the caveat that this was only their impression. Care workers explained that the optometrist would see several people in one session; in one home it was reported that as many as 20 people might be seen in one day. There were concerns that examinations sometimes felt ‘rushed’.
They whizz through.
They whizz through? OK, tell me about that . . .
Make of that what you will!
CH interviewee 5
I thought they did really well in the morning but when they came back in the afternoon, I did feel that they just had had enough and they were, they were a bit rushed and they just wanted to get finished and go because they’d got other people to see. I did feel that, I definitely noticed the difference in the afternoon.
CH interviewee 8
As much as possible we’re trying to encourage the optometrists, to come later in the afternoon when the residents are more settled and relaxed because sometimes they come in the morning or too late. We’ll tell them ‘come on this day then, because it’s less hectic’ because if everything is rush rush rush the residents feel that as well and that’s the time that they don’t co-operate.
CH interviewee 11
As described in Theme 1: eye examinations, care workers queried how much of the examination could be accomplished with someone with dementia, particularly with regard to people with advanced or end-stage dementia, but some suggested that limitations to the examination were at least in part linked to the optometrist. This care worker described this in some detail.
Some [optometrists] have a better understanding. And as I said before, some will say, ‘don’t worry, there’s other things I can check’ and have the patience, the understanding, the knowledge to work alongside a resident with those conditions at that stage. And I’ve also witnessed opticians who are like ‘well if they can’t do this, there’s no point, I can’t do it’ kind of thing. Which I understand because if that person doesn’t have an understanding of the dementia and the effect that dementia can have on that resident they’re thinking ‘well they’re not responding, I’m getting no response and there’s only so much I can do’ so, you know, I’ve witnessed both them things.
I don’t want to put words in your mouth, I just want to check, make sure what you’re saying. Do you ever think that they give up too quickly or . . .?
Yeah, like I said, I have worked where I’ve seen very patient people that have come to test these residents’ eyes, who sympathise, empathise and have the time and patience, and maybe it is have knowledge and understanding, I don’t know, but I’ve also witnessed where it’s just a case of, you know, ‘there’s no response and I’m getting nothing so can I have the next patient’ kinda thing, and that doesn’t help that resident who can’t speak or show any facial expression to respond to what you’re asking, but might have really bad eyes and might need them glasses, who would be able to wear them ‘cause wouldn’t take them off or whatever, but for them reasons don’t get that proper eye test.
CH interviewee 3
The potential for domiciliary examinations to be rushed or incomplete was also a concern for optometrists, as evidenced by comments about the purpose of the eye examination, limitations of the fee structure and the potential for exploitation of the system. There was no question that optometrists believed that people with advanced dementia should have some sort of eye examination but, cognisant of the perceived limitations already expressed, some thought that a comprehensive eye examination was not always possible and, if this was the case, wondered if a full examination fee should be paid. A ‘health check’ to identify conditions which it was in the interest of the patient to treat – for example, finding glaucoma that needed eye drops to prevent pain and/or further VI – should not incur a full fee. It was noted that an optometrist might not know in advance that an examination would be limited or even not possible, and if the optometrist was an independent practitioner attending a single patient, there would be an issue regarding how to compensate him or her for the time booked out of practice. However, focus group participants were wary of the ethical and financial considerations of domiciliary service provision in care homes when multiple examinations might be conducted and the majority of residents might not benefit from a full examination. These dilemmas are clear in the following extract from one focus group comprising mainly independent practitioners.
It’s a difficult one. On the very advanced dementia patients, I don’t like examining them because I don’t like claiming a fee. What are you going to do, 5 minutes and then claim a full fee, especially a domiciliary fee.
I don’t think the amount of time you spend, or what you can get done, should dictate how you claim something, it’s a matter of what is the good you can do for that patient surely.
You get the odd one where you think it’s really worthwhile of course, people who’ve been ignored or had a stroke and maybe got early dementia, but there’s so many people with advanced dementia and you think really this is a money exercise.
There was some overlap between this theme and theme 5 – improving services for people with dementia – so further detail is provided in Theme 5: improving the eye care of people with dementia. Similarly, the debate around the dilemma of providing eye examinations for people with advanced dementia is compounded by the additional factor of prescribing and dispensing new spectacles. There were suspicions that some dispensing is unnecessary as many people with dementia do not wear their spectacles. This is the focus of theme 3.
Theme 3: spectacles
Spectacles as a theme describes problems related to the failure to wear spectacles as prescribed and to optometrist dispensing decisions.
Not wearing spectacles
A phenomenon that first emerged during the interviews was of people with dementia not wearing their spectacles. It was also reported by some family carers during the focus groups but was particularly reinforced during the interviews with care workers.
Three interviewees, all with prescriptions for reading and distance lenses, stated that they did not wear spectacles because they felt that they did not need them; one person did not know where her spectacles were. The interviewer asked the other two people to put on their spectacles and then asked about changes in vision; both people reported improved vision with the spectacles on. One of them repeatedly stated that she did not wear spectacles because she ‘didn’t need them’ but she kept a magnifying glass to hand and stated that she used it ‘if I want to read something, you know. That’s when I use it, to look up something for the television’ (interviewee 1).
All three interviewees had two pairs of spectacles but did not appear to understand this or why they had two pairs. For example:
Right. Do you have two pairs of glasses?
Yes.
So, what are they for?
I don’t know. I’ve never used them. Those are the ones they left here with me. And then there are these. I didn’t want to throw them away, they are the ones that your optician gave me. [takes them out of a case] There they are. But I haven’t used them yet.
A fourth interviewee stated that he only wore his glasses ‘occasionally’ but, when making the appointment for the interview, his wife stated that she thought the money spent recently on new spectacles was ‘a waste of money because he refuses to wear them’ (interviewee 11).
Several family carers said that their relative would not wear spectacles and that they did not understand why, especially when the individual had worn spectacles for many years before the onset of dementia.
Mam’s thing is ‘I can see quite well without my glasses, I don’t need them, I can see what I need to see without me glasses’.
CFG 1.4
As far as I can remember from being a child, she’s always had glasses, always, and this last couple of years, she seems to get angry with them on her face, and she’ll take them off, and she’ll look at them as if ‘what am I doing with these’, and she’ll fling them on the floor.
CFG 5.2
She doesn’t wear them, I’ve never seen her wear them, she’s been prescribed glasses for reading, for watching television and she doesn’t wear them.
CFG 5.5
Those whose relatives lived in care homes often attributed the absence of spectacles to the failures of staff: staff not knowing the resident, high staff turnover, pressure of work and staff saw it as a low priority to ensure that spectacles were worn. However, care workers also reported residents’ refusal to wear spectacles as a major issue. The suggestion that failure to wear spectacles was attributable to staff turnover or to staff being unfamiliar with residents was refuted by care workers’ descriptions of how spectacle wearing was well documented in care plans. They were aware that relatives often blamed them, even though the care workers had tried, unsuccessfully, to persuade the resident to wear their spectacles. These care workers explained the problems they faced.
It does sound like I’m making an excuse of why they haven’t got their glasses, but that’s not the case. I can only speak for the residents that are in my care and the shifts that I’ve worked on, and I can honestly say we’ve got more than a good handful of residents in this home, who I know should wear glasses, have been given glasses, but will not wear them. Or we’ll put them on and not 2 minutes later they’ll take them off and you’ll put them on, then that resident will become agitated because you’re trying to put glasses on and they clearly don’t want them glasses on or they don’t understand that they need them glasses, and in which case you’re going to cause an incident or upset somebody.
CH interviewee 3
We’ve got a gentleman at our place and he’s mobile, you give him his glasses first thing in the morning, first thing he does is take them off, he doesn’t want to know, doesn’t, but, he can’t see enough to even feed himself, so now we have to assist him with feeding because he doesn’t want to wear his glasses. There’s nothing you can do to get him to want to wear his glasses, you try throughout the day and he just takes them off, throws them away, doesn’t want to know.
CHFG 2
The reasons why people refused to wear their spectacles were something that some carers speculated on but none really knew. One suggestion was that the persistence of long-term memory and loss of short-term memory meant that people with dementia remembered the time when they did not wear glasses and did not remember that they needed them now. Another hypothesis was that a dementia sufferer’s world ‘shrank’ and an adaptive response (unconsciously) was to go without anything that was no longer perceived to be necessary. A third theory concerning people who had different spectacles for distance and near vision was that confusion and fear of wearing the wrong ones resulted in neither pair being worn. But, in essence, the reasons why some people with dementia refuse to wear their spectacles were not answered by this study.
Missing and broken spectacles
A second problem was that of ‘missing spectacles’. Focus group participants caring for relatives at home reported incidences of spectacles going missing, but this was more commonly associated with people living in care homes.
My mother hides her glasses, hides everything. So that’s the problem with actually getting her to wear her glasses. She’ll put them in socks, anything, in drawer. One pair we don’t even know where in the world it might be, we’ve looked everywhere.
CFG 2.7
I think it’s about 2008 when she started having dementia. We actually noticed it because she wasn’t eating. Also she’d seem to lose her glasses very quickly. And the ones which she’s had most recently, they’ve gone, they’ve disappeared as well, even with her name on. And none of the staff, in the home she’s in now, have got a clue where they are.
CFG 3.8
Care workers identified missing spectacles as a major problem and again they felt that they were often unfairly blamed. Just as CFG2.7 explained how her mother hid her spectacles, care workers described how residents would hide spectacles or leave them lying around, or how a resident would pick up another resident’s spectacles (one care worker reported finding 30 pairs in one resident’s room). Several care workers explained that the company dispensing the spectacles marked them with the resident’s name and sometimes whether they were for distance or near vision; still, they went missing.
Another problem, particularly in care homes, was the frequency with which spectacles were broken. These two care workers summed up the difficulties they faced:
Oh, bane of our lives. If I could make a way for glasses not to go missing I’d be a rich person. It’s either they get broken because they have them in their hands, sitting on them, they’ve put them somewhere, or [they are removed by other residents]. You know they’ve got their names on and everything but they just go, it’s just like they vanish for good forever and it’s like ‘but you only had them on last night’. We’re not bad at it, but some glasses do go missing, I agree, and that’ll be in every home, any home that says that they don’t, they’re liars.
CH interviewee 2
You see that lady? You’d open her bag and she’d have maybe five six, seven pairs of glasses, and then you have other family members saying ‘where’s my mum’s glasses, she hasn’t had them on’ but you can’t get in, it’s an intrusion to open that lady’s bag, you’ve got to do it discreetly, which can cause a lot problems but yeah, it is a problem.
CH interviewee 10
Despite the difficulties of individuals refusing to wear spectacles and missing or broken spectacles, there was general agreement that it was important for people to wear their spectacles, if they could be persuaded to do so without causing distress.
The cost of spectacles
A considerable number of participants introduced the issue of the cost of spectacles. Although most of these avoided express statements that spectacles were too expensive or overpriced, they said things such as ‘the frames were £150 but you want nice frames’ (interviewee 2); ‘so by now, I’ve spent twelve hundred pounds on glasses in 15 months’ (CFG 4.2); ‘I wouldn’t go to [optician’s name] because I ended up with a pair of glasses which cost me £400 because they are experts at putting prices on’ (CFG 2.1); ‘to keep spending out money for glasses that are never gonna come out the bag’ (CFG 3.6). A small number of people overtly remarked on the deterrent effect of cost:
Can I ask, why did you leave it for five years before having your eyes tested?
I never had the money. Fluctuating income.
Does the cost of spectacles make you reluctant to have your eyes tested, in case you need new spectacles?
Depends on your pocket. If your pocket is a bit low it makes you reluctant to go and get your eyes tested.
Interviewee 22
It’s a big outlay, you know, if they’re paying three or four hundred pounds for a pair of glasses, it can actually put you off going.
CFG 5.4
Overall, the comments suggest that cost was not an explicit barrier to acquiring spectacles for most participants, but the references to cost raise a question about the extent to which the cost of spectacles is a concern for some people.
Spectacle dispensing
Two issues emerged in relation to spectacle dispensing: the factors that should be taken into consideration when prescribing or dispensing, and a concern about unnecessary dispensing, particularly in care homes.
Several optometrists made the point that, to avoid confusion, it was better to keep things as they were as far as possible and avoid unnecessary changes if new spectacles were required after an eye examination. This meant not changing the type of lenses, for example moving from single vision lenses to varifocals, or the style of frame. This view was echoed by family carers who illustrated the point with examples of unsuccessful changes:
My wife, a couple of years ago she went, and they gave the prescription for her glasses, she normally wears varifocals, and they gave her some reading glasses, they thought that would be better than trying to use the reading part of the lens. She’s never used them, never used them at all.
CFG 3.4
We got some bifocals for my husband, he’d never worn bifocals before, and I thought well it’ll be easier for him than switching. It was a complete disaster, he never wore them. And I feel that the optician ought to have warned me that that was a bad choice.
CFG 3.2
Beforehand, my mum did wear glasses for short sight, for close work, not all the time, occasionally when she really needed them, and then as she aged she obviously needed them more, and they got to a point where she needed two pairs of glasses. At this point she was a few years into the dementia, and that was hopeless, it was much worse than having one pair of glasses, because she could not cope with the ‘which glasses’, she just couldn’t cope with the concept of two pairs of glasses. I think it was because throughout her life it was ‘my glasses’, not ‘which glasses’.
CFG 5.1
My wife needs both reading and distance glasses and they said would you like to try these varifocal things and we paid over £300 for a pair of those and you only used them for about a week and you couldn’t get used to them at all.
Because the eyes aren’t the same and I couldn’t you know. It didn’t work, I couldn’t.
So we had to go back and get separate glasses for distance and reading which cost again.
Returning to the subject of whether or not all new dispensing is necessary, and the association with theme 2 (domiciliary eye care), care workers were asked about the procedure for ordering spectacles for care home residents. If residents had capacity to make decisions and control their financial affairs, the decision was theirs. If they lacked capacity, the need for new spectacles was referred to the family. Care workers did say that there were instances, although rare, when families refused to make the purchase on the basis that the spectacles were a waste of money as they would not be worn, would go missing or did not improve the individual’s quality of life.
Despite this defined procedure for ordering spectacles, there were reports from some family carers of spectacles ‘appearing’ even though the carer had not been consulted. It would appear that this could happen only if the care home resident was eligible for free spectacles as this would remove the need for a relative to agree but, as the next quotation demonstrates, this was not always the case.
He went into a care home and his glasses disappeared for 5 weeks and that was because the optician had been and taken away everyone’s glasses in order to get them new ones. Goodness knows who consented to that exam. Then we took part in the study and he had his eyes tested. And then he moved to another home where – surprise, surprise – I walked in one day and there was an optician testing everyone’s eyes and getting people to sign the consent form for the exam. So I think some interesting things go on. Ah, this is a money spinner! They tested his eyes, when he wasn’t due a test, and provided two pairs of NHS glasses, reading and distance. And he wasn’t entitled to NHS glasses.
CFG 4.2
This was an isolated case but may be the sort of incident that a couple of optometrists had in mind when they expressed fears that dispensing in care homes was open to exploitation.
The thing is, something’s gone wrong when the actual prescribing rate is higher than the national average. And something has also gone wrong, because, if anything, I actually prescribe less. When people just go in and they change the glasses time after time after time, I am suspicious that there is something.
OFG 5.3
The companies I’m talking about, give glasses every year because they [can’t see the old ones] so they find something or they use the last time records, and they give new glasses, and they’re all sat in a pot in the office, because they’re not worn.
OFG 5.5
Theme 4: cataracts
Although cataract is only one of the conditions that can cause VI, it is extremely common . As the results of stage 1 suggest, a large percentage of people with dementia will develop a cataract. Therefore, the effect and treatment of cataracts was discussed with every participant set.
The topic of cataract was discussed during the interviews with people with dementia. They were asked if they would agree to surgery if they developed a cataract that progressed to the stage of causing VI. Nearly all interviewees said that they would. Several of the interviewees had already had cataract surgery and reported successful outcomes.
Family carers were, similarly, asked for their views on their relative having cataract surgery if this was advised. In principle, carers supported surgery if they thought that it would have a positive effect on their relative’s life. However, they expressed concerns about how their relative would react, physically and emotionally.
I think we would probably say ‘yes’ but I think we would ask the question ‘is it worth the upheaval’ because we know it would be quite an upheaval and distressing for her when any big event is coming, whether it’s good or bad, she focuses on it a lot.
CFG 1.2
But would they understand what was happening? That’d be my worry.
CFG 2.7
I’ve only got reservations because of her health. Her physical health.
CFG 3.9
Is it worth doing it? You’d have it done if you felt it was worth the risks and her going through it, otherwise I don’t think that I would put her through it. And she’s 85 and has other medical issues going on anyway. Doesn’t become top of the list, then.
CFG 2.2
Family carers also queried whether or not there would be any complications arising from surgery and their relative’s ability to cope with pain, eye drops and dressings. The comparative drawbacks of local and general anaesthetic were also raised, with concerns expressed about both. The following exchange between two carers illustrates how they weighed the benefits against the risks as applied to the individual.
My dilemma is, I don’t think Mum is distressed about it. When she went for the eye test, the guy was quite amazed by what she could read with the cataracts that she did have, but reading is just, it’s just nothing to her so I don’t think she’s distressed by them so is it worth the distress of going to the hospital having the bandages and all that trauma?
In a slightly alternative interpretation of that, it would be the distress [my wife] would experience if she couldn’t do it, rather that the distress she’s currently experiencing, because if she’s constantly questioning me ‘why can’t I see out of this eye, why can I see better here and not here, why can’t I, I can’t see that’ you know?
Care workers were more hesitant about supporting cataract surgery, reflecting the fact that most of the residents they cared for had reached an advanced stage of dementia. They identified the same potential barriers but thought that the ability to cope would be compromised by the levels of cognitive impairment. They described the challenging behaviours displayed by some residents and how they thought this would impact on the ability of those residents to tolerate surgery.
If they’ve advanced dementia, to send them out to the hospital to have an operation would be too traumatising for that person; they’ll be totally traumatised and it’ll last for days and it’s not worth it sometimes, it’s too traumatic for them.
CH interviewee 4
There’s no cure for dementia, it’s just going to get progressively worse and so you would think that if there was some way that you could improve their life, not through the dementia but like improving their eyesight, you would think well, you know, you’d want to sort of go for it. But that person would be under a lot of stress, depending on what their understanding of what was going on. Going into hospital they’d be under a lot of stress, it would be a lot of pressure for the family as well, and then afterwards there’s the recovery, and everything so it’s a very difficult decision to make, and I really don’t know. Your automatic thing is ‘if it improves them have the operation’ but it’s not as black and white as that. When they get to a certain stage of the dementia, they’d lose the understanding of what you’re saying to them and then they’re suddenly taken away and in this hospital, they would be so frightened upset, it would just be awful.
CH interviewee 8
Prior to the start of the study, the research team piloted the focus group questions with carers at a dementia conference. They raised an interesting question with regard to performing cataract surgery at an earlier stage for people with dementia. That is to say: if a person has a cataract that has not progressed to the stage of needing surgery, and then that person is diagnosed with dementia, should they be offered surgery before the cognitive impairment progresses to the state at which capacity to consent is lost or the ability to cope with the demands of surgery is compromised? When this was raised with carers and care workers, they agreed that early intervention would be a better option than having to address capacity issues that may arise from deferring the surgery to a later point in time.
I think it’d probably be better to do it early on, if possible.
CFG 1.1
I think if someone is naturally anxious, I think it would be much better for them to have something done at an earlier stage.
CFG 1.5
At the moment, that’s where we are, the cataracts aren’t ripe and my dad can’t read, and he loves reading, so we’re kind of a bit stuck until they’re ready to be taken out. It’s really crucial to try and get resolved while he is still quite able to do things.
CFG 2.6
Take it off there and then. It’s not rocket science!
CH Interviewee 2
Yeah, definitely. It’s the better of the two options isn’t it.
CH Interviewee 8
When the same question was put to optometrists, their reaction was mostly one of surprise, suggesting that this was not something they had previously thought of. But as they talked through the advantages and disadvantages of early intervention, the majority came down in favour of early intervention. Discussing scenarios in which people developed a cataract at a later stage of dementia, optometrists debated the benefits and risks of surgery, citing the same factors as carers and care workers.
The impact of cataracts versus surgery on quality of life was another topic of discussion, and this is addressed in Theme 6: quality of life.
Theme 5: improving the eye care of people with dementia
This theme emerged from discussions about the following:
-
optometrists’ skills and training needs
-
the development of the specialist practitioner
-
revision of the examination fees structure.
Optometrists’ skills and training needs
Theme 1 included problems associated with conducting an eye examination. This gave rise to discussions about the extent to which members of the optometry profession were confident and competent to provide eye care for people with dementia and how they could be helped to improve their skills and knowledge. Asked about how they felt about their personal clinical skills, optometrists said that they needed more information about dementia to improve their understanding of the patient’s experience and how to adapt their procedures, and provide them with insight into the ethical-legal aspects of consent and capacity, as the following examples demonstrate.
It’s not just the testing, I think maybe we do need to know more about how dementia affects patients so it’s not specifically to do with their vision. I was reading last night about peripheral awareness and to do with visual hallucinations similar to Charles Bonnet Syndrome but connected to the dementia. Now, to be honest, that’s not something I really would ever have considered until I was reading it last night.
OFG 1.1
I think a lot more information about dementia and what patients with dementia are going through would be useful. A way of adapting your routine, or ways of thinking, processes involved, would be very useful. It would not only open the optometrist’s mind to what the patient is experiencing, but also give us avenues to experience to empathise better with the patient. I think that’s very important.
OFG 3.1
I think the issue of consent is a big thing really, understanding. I think from a training point of view from optometrists we don’t really have training on what consent is, and valid consent, and informed consent and power of attorney.
OFG1.4
They discussed the different ways in which training could be provided, and their preferences were for interactive methods such as peer review, workshops and talks from people involved in dementia care rather than distance learning materials. However, the general feeling was that skills and confidence were best developed through experience: exposure to people with dementia but with access to peers and mentors who could guide and advise.
Maybe that’s where our peer review training comes into it, dementia cases as part of the case scenarios. And maybe for young practitioners, encouraged to ask more experienced practitioners ‘I had this really difficult patient the other day, what do I do when they won’t press the button on the visual field machine’, feeling the courage to say, to admit, that ‘we didn’t know what to do in that circumstance, what does everyone else do?’. And I think that’s where the peer review, or maybe mentoring, over and above the pre-registration year, would be really useful, to have someone pick up the phone and say ‘oh gosh, what did I do’. And our young practitioners don’t have that. They don’t have anyone to go to.
OFG 4.5
It all comes down to exposure to these patients and experience. And, the more you do, you know, the more you learn. So you can’t, you can’t get away from the fact that you need to constantly be honing your skills, with this group of patients, if you want to get better.
OFG 3.4
The Outside Clinic’s optometrists explained how their confidence and competence quickly built up through experience, but they had more exposure to people with dementia than did the average high-street optometrist. The clinical governance procedures and supervision for new optometrists operated by the company also meant that, when they first started work, they had opportunities to ‘shadow’ more experienced optometrists. The importance of such opportunities was discussed by optometrists in other groups who talked about the need for supervision and some form of mentorship.
Development of the specialist practitioner
A second suggestion to improve services was to explore the potential for developing a specialist practitioner role, someone not necessarily limited to working with people with dementia but who would specialise in older people. This could accommodate eye care for many people with dementia because the prevalence of both dementia and the major eye conditions causing VI increases with age. They acknowledged that work was needed to explore the feasibility of this role, including assessing how many practitioners would be needed, what training would be required and if sufficient professionals would be interested in this type of role.
An alternative suggestion was to develop a community eye health service in the NHS, rather like community dental services, with salaried optometrists. This linked into the next area that optometrists thought needed attention: the service structure around examination fees.
Revision of the fees structure
The need to allow more time when examining people with dementia was identified by all participant groups. In the interviews with people with dementia, perceptions about what constituted good practice were often associated with the optometrist taking time. Family carers raised the need for the optometrist to allow time to involve them and care workers commented on the number of appointments that were completed in a session, and wondered if sufficient time was allowed for each examination:
You feel as if, it’s rushed because there’s so many, or even when there’s that few, if you know what I mean, they probably don’t get the time that’s needed, in all fairness.
CH interviewee 3
So they get through 20 [residents in a session]?
Yes, they whizz through, yeah.
They whizz through? OK, tell me about that . . .
Make of that what you will.
CH interviewee 5
There was broad agreement from optometrists that examining a patient with dementia can take more time than examining most other patients, and that appointment scheduling, particularly in large or busy practices or multiples, does not allow for this.
I think they have special needs. And quite often, they need more time than any other patients that we see. And somehow with the present system of one size fits all, it doesn’t really work . . . there’s more to think about before we have a final solution there but it’s quite obvious that to give the proper level of care, we need to give them the proper level of our time.
OFG 3.4
Despite the time constraints of ‘the system’, optometrists discussed ways to overcome the lack of time. ‘Over-running’ – taking longer than the scheduled time – can have a knock-on effect for the rest of the schedule, affecting patients and staff, and the only alternative is to prepare for this. One suggested approach was to book a longer or double appointment. Some optometrists said that they did this, but others identified two difficulties. The first was anticipating the need if the patient was not previously known to the practice as someone with dementia. The second difficulty concerned funding. Although some optometrists said that they or their practices were prepared to absorb the cost of a longer appointment, this was not the case everywhere, hence the necessity to review the fees structure to meet patient needs:
Something needs to be done about that at a higher level, at an NHS level, where we can get proper remuneration for looking after these people properly, because it’s completely unfair on the patient that they have to be whacked through when they’re going, the majority of them will probably be going, to the big opticians because the majority of patients are being seen by them and 20 minutes, if they’re lucky 30 minutes, that’s no way enough. No way.
OFG 4.3
Another suggestion was that, on some occasions, it may be desirable to bring the patient back for a second appointment to complete the examination and prescribing. The next quotation summarised this well:
Taking your time with [the examination] is a double-edged sword, because sometimes if you take too long, you lose their concentration. There is a delicate balance of making the patient comfortable, but also getting through what you need to do. I’ve often found that sometimes it’s easier to do it in chunks and get them to come back, for little visits, rather than doing it all in one sitting. Having a couple of visits, tends to make them a little bit more familiar with the environment that you’re in, and more approachable. So the fear factor tends to reduce, the second or third visit, and then you find you can do more, with them, at that point.
OFG 3.1
Again, the issue of funding was raised as a consideration when scheduling multiple appointments, but optometrists described this as something that should be overcome rather than remaining an insurmountable barrier.
There must be somewhere in the system, that allows, in special cases, to get those patients back again if you haven’t got the right . . . level of information to make a decision.
OFG 3.4
Theme 6: quality of life
The inter-relationship of vision/VI and dementia and the impact on quality of life was a thread that ran through all themes but emerged as being of such significance that it merits separate explanation.
As this chapter has shown, dementia can impact many aspects of eye care: the ability to complete an eye examination, wearing spectacles and decisions about cataract surgery. Asked about the importance of regular eye examinations, all parties agreed that it was desirable to complete at least some form of health check and ideally a complete eye examination with the aim of identifying and correcting any loss of vision. As one optometrist succinctly said, ‘confusion and visual impairment, is a much worse combination than visual impairment on its own’ (OFG 4.5). A second optometrist pointed out that ‘the combination of visual impairment and dementia is a difficult thing and leads to further deterioration in dementia’ (OFG 5.2).
Similarly, there was agreement that people should be provided with spectacles of the correct prescription, fit for purpose, and encouraged to wear them wherever possible, but this was said against a backdrop of an acknowledgement that ensuring that someone wore their spectacles was not always achievable.
Carers eloquently described the importance of vision for relatives with dementia:
Eyesight is so precious and if somebody’s life is being impacted upon so dramatically with Alzheimer’s or dementia, I think to have the additional thing of going blind would be awful. For my mind, I think I would want them to have as much done as possible to keep them as healthy as possible so they can enjoy doing what they can enjoy.
CFG 1.5
My wife’s been an avid reader all her life and given that she has no short-term memory, this is really part of some of the remnants of who she really is and reading is instinctive with her. I explained before, how painful it would be to her not to be able to see. It doesn’t matter to me that she’s still reading the book that she’s been on for the last 18 months, and she can be anywhere in it on any day, but the fact is, that satisfies her, that pleases her, that fulfils something in her, to be able to do that.
CFG 4.6
Many of the optometrists in the study recognised the importance of carer perspectives on what was best for the individual. For example:
And I think that’s really interesting for optometrists to hear the carers’ perspective, as well, because, again it’s about that hearing the patient’s life isn’t it? It’s easy for me to say that because I’ve got a good understanding of what’s impinging on his world. But I do think you’ve got to listen to the carers and see what is impinging on the world, what really will make life better for them, for the person that they’re living with.
OFG 5.1
The collective comments of focus group participants described how, as the level of cognitive impairment increases, care becomes more complex. Carers, care workers and optometrists all talked about the pros and cons, the risks and benefits, the advantages and disadvantages of intervention in the context of the underlying question: ‘what would be best for the individual?’. The answer was not as simple as saying that there is a certain level of cognitive impairment at which stage there would be no point in performing an eye examination or providing spectacles.
Quality of life was also a major factor in respect of decisions about cataract surgery. As previously described in theme 4, family carers thought that surgery would be worthwhile if it had a positive effect on their relative’s life, but they had concerns about how their relative would react, both physically and emotionally. The quotation below is lengthy but provides a case study of what happened with one lady who was found to have cataracts, described by her daughter, CFG5.1:
There was no suggestion of doing anything about it when I had previously spoken, about a year, 18 months beforehand to the optician. He said ‘well, it’s not, she might not be able to lie still enough to have the operation’, he was sort of saying it’s not a good idea to have this dealt with and at that point it wasn’t so bad. Up to the present date, she’s virtually blind, her glasses make no difference whatsoever, she’s not particularly interested in the television. She has a notepad and it’s a bit like her comfort blanket to be honest, her notepad and her pen, and she does a little word puzzle, very simple, but this is what she does, and everybody knows that mum goes around with her notepad and she asks for it all the time, and now she can’t see to write, at all. And it’s just making life so terrible, it’s completely changed everything. She’s anxious because she can’t see and because she hasn’t got the comfort of this little activity that gave her some purpose, she’s upset, you know . . . Well, up to date, things have now moved on. Because at the memory clinic test, after that I spoke to the nurse who came and told her about mum’s eye problems, she then got in touch with me shortly after that and said ‘would you like to be part of the study’ and I said ‘well if it’ll help mum in any way, yes’. So now down the line, we’ve had The Outside Clinic come and test her eyes again and through that she’s been referred to the hospital, we’ve seen the consultant ophthalmologist, who was absolutely brilliant, you know, exceeded my expectations, and mum’s on a waiting list now to have the cataracts removed. She’s going to have them removed under general anaesthetic, for two reasons, because she has a head tremor and also she’s got very bad osteoporosis so she’s got a big curvature of the spine, and so the consultant suggested that it would be better to do them both in one go under general anaesthetic, and I couldn’t have asked for anything, a better solution than that. It hasn’t happened yet, so I can’t tell you what the final outcome is, and the waiting time’s been longer than I thought it would be, but I’m hoping in a couple months, another couple of months which will be about 6 months after her original consultation, I’m hoping it’ll be done. Which will be the best outcome we could have really. And I’m hoping that it’ll restore her sight for her to be able to do what she wants to do. The other thing which the consultant suggested, which is brilliant and shows some thought about mum’s situation, is that she suggested putting in the new lenses so that she can see to read without glasses. What she will need is one pair of glasses for distance, which is fine, that’ll mean you know when she just goes out with me in the car, we take her anywhere we can take the glasses and she’ll hopefully be able to see her surroundings. But to actually have eyes that work, to do what she needs to do most of the time, it’ll be fantastic, and it will make a huge difference to her.
CFG 5.1
The daughter telephoned the CoO a month later; because she had talked about the events leading to her mother’s planned surgery in the focus group, she wanted to provide an update. Her mother had undergone the surgery 2 days earlier. The daughter had visited her mother, who was thrilled with the result because she could see again. The daughter stated that her mother appeared to have improved cognitively and emotionally and felt that the decision to proceed with surgery, despite fears, had been completely justified by the outcome.
Summary
Using the qualitative methods of interviews and focus groups, stage 2 explored issues relating to eye care for people with dementia from the perspectives of people with dementia, carers and optometrists.
People with dementia and their carers (familial and professional) were aware of the importance of eye examinations and the benefits of correcting VI, where possible, with spectacles or cataract extraction. The data suggest that most people with dementia had regular eye examinations, but significant cognitive impairment as the dementia progresses can present challenges. All parties described situations that could have been improved by optometrists having greater awareness and understanding of dementia or by better communication between optometrists and the person with dementia and their carers.
Domiciliary eye care provided by optometrists with experience of caring for people with dementia was well received and appreciated by study participants and is one option for overcoming some of the difficulties described by some carers. Yet most people with dementia living in their own homes (and their family carers) were unaware that they were eligible for this type of eye examination. Conversely, in care homes, domiciliary eye examinations are the predominant form of eye-care provision, but this elicited some concerns about uniform application of procedures and the potential for the system to become underefficient.
The wearing of prescribed spectacles and intervention for cataracts are the two most common forms of correction for VI and their value is equally important for people with dementia as for the general population. However, significant cognitive impairment can lead to individuals becoming reluctant to wear their spectacles and ethical issues arise with regard to capacity to consent to treatment. There were understandable and well-argued concerns put forward regarding the pros and cons and the benefits and drawbacks of intervention and balancing these with achieving optimum quality of life.
Suggestions for improving eye-care provision for people with dementia included more training and awareness raising of optometrists, reviewing the current fees structure which can militate against optometrists providing eye examinations in the best way for individuals, and improving communication between optometrists and carers.
Appreciating the value of multiple perspectives is a key feature of this stage of the study, and the difficulties, problems or limitations perceived by one set of participants were often shared by the other sets of participants. However, each participant approached the topic through their personal situation: as someone in receipt of eye care (people with dementia), someone supporting the eye care of individuals (carers and care workers) or someone providing eye care (optometrists). Theme 6 demonstrates the importance of sharing and understanding these various perspectives to improve the quality of life for people with dementia.
Chapter 5 Discussion
This chapter discusses key findings from the combined results of stages 1 and 2 in relation to the study objectives and existing related research. The mixed-methods approach to data collection – prevalence data from eye examinations and qualitative research from focus groups and interviews – allowed the research team to investigate a comprehensive set of objectives as set out below.
Objectives
The four primary objectives of the study were:
-
to measure the prevalence of a range of vision problems in people with dementia
-
to compare the prevalences found in objective 1 with the published data on the general population in a comparable age range
-
to identify and describe reasons for any underdetection or inappropriate management of VI in people with dementia
-
to recommend interventions to improve eye care for people with dementia and further research in this area.
The secondary objectives of the study were:
-
to identify any differences in the level of undetected or inappropriately managed VI between those living in their own homes and those living in care homes
-
to determine estimates for the percentages of those with dementia likely to be able to perform successfully elements of the eye examination
-
to relate vision problems in people with dementia with data from functional and behavioural assessments.
Before discussing the study findings, it is appropriate to highlight the strengths and limitations of the study.
Strengths of the study
The sample is broadly representative of the overall dementia population aged ≥ 60 years in England, in that it encompasses a wide age range, both sexes, people living at home and in care homes, and participants who were recruited from several regions. The study is notable in that VI in people who have cognitive impairment has rarely been investigated in the UK, an exception being the NDNS study57 in which just 8.4% of the sample had cognitive impairment. The high proportion of care home residents in PrOVIDe (44%) permitted comparison for many parameters between those living in their own homes and those living in care homes. There are very limited modern data on VI in care home residents. Residents of care homes were included in the NDNS study, constituting 23.8% of the sample. 57 Another UK study included a ‘small’ proportion of care home residents. 44 Otherwise, they have been either excluded56 or not identified. 27,58,59
Data were collected by skilled optometrists with particular expertise and experience in domiciliary eye care and in working with people with disability. This contrasts with other major VI studies in which data were collected by non-eye-care professionals. 56,57 The quality of data was also enhanced by the optometrists’ use of equipment appropriate to domiciliary settings. This equipment is available to all UK optometrists but evidence from focus groups suggested that not all optometrists use this standard of equipment in a domiciliary setting.
Unusually for UK population studies of VI, a full optometric eye examination, including the determination of refractive error, was carried out in PrOVIDe. This made possible the measurement of VA post refraction with participants wearing their up-to-date spectacle prescription, which allowed the assessment of the prevalence of presenting VI, post-refraction VI, and uncorrected or undercorrected VI.
PrOVIDe also measured NVA and distance VA, whereas most UK studies of VI have been limited to the measurement of distance VA. 27,44,56,57 Near vision is an important aspect for many older people, and in PrOVIDe NVA was measured with the participant wearing their current near spectacles (if any) and measured again post refraction with the participant wearing their optimum near vision prescription determined by the optometrist. Recording near VAs is a recommendation in Standards for Reporting and Measurement in studies of VI prevalence. 45 The only previous study reporting NVAs dates from the 1980s. 59
Optometric data were supported wherever possible by data on the degree of cognitive impairment, using sMMSE, and data on participants’ functional and behavioural state using the BADLS and CBI-R. This allowed the study to consider the person with dementia as a whole.
The qualitative stage of the study involved people with dementia, their family carers, professional care workers working in care homes, and optometrists; this generated insights from different perspectives on a range of aspects of eye care. The overall number of stage 2 participants was 119, a considerable sample size for a qualitative study.
Limitations of the study
Sampling bias is possible owing to quota-sampling and response bias. In group 1 particularly, it is likely that some volunteers and/or their carers were more health-orientated than the general population. In addition, the PrOVIDe regional sample may not be fully representative of the general UK population.
It is a strength that the stage 1 sample of over 700 participants represented a wide spectrum of people with dementia in terms of age, cognitive impairment and setting, and the sample size was sufficiently powered to meet the primary objectives. However, there was no specific intention to sufficiently power the sample sizes of groups 1 and 2 to enable detection of differences between groups, nor of associations of VI with age, sex or cognitive impairment. It was also acknowledged at the design stage that the sample size would not allow for comparisons to be made on the basis of ethnicity. However, participants were recruited from a range of demographic areas to encourage a heterogeneous population.
A considerable proportion of VA assessments (≈17%) and sMMSE assessments were not available owing to a range of factors, notably poor participant co-operation, and this proportion varied across age (≈9% to 26%) and between groups 1 (≈3%) and 2 (≈33%). Unavailable VA and sMMSE assessments are likely to come from patients with lower VA and greater cognitive impairment. Findings from ‘complete-case’ adjusted analyses will not suffer from any bias if sufficient covariates are included and missing data are ‘missing at random’, whereas those from unadjusted analyses will be somewhat biased in this likely case. However, given that the proportion of missing data is higher in group 2 and increases with age, this is likely only to underestimate the negative association of these factors on VI, that is, leading to conservative findings. On the other hand, as discussed in Chapter 2 (see Stage 1 Data analysis plan, Missing data plan), different study outcomes would be missing in different participants and for varying reasons, which may lead to some inconsistency in our separate analysis of each study outcome. Complete-case analysis also leads to less efficient estimates, because it does not use the partial information available on participants. Notably, it may lead to bias if the causes of missing data are not fully explained by the covariates in each model, which is likely as our regression models only adjusted for age, sex, sMMSE (where appropriate) and location.
To improve the plausibility of our regression analysis assumptions, a multiple imputation procedure was carried out, with the results discussed in Chapter 3 (see Missing data sensitivity results). There were no changes in the substantive conclusions relating to our key tables, Tables 11, 15 and 24, under the imputation model compared with the complete-case analysis.
There is a small risk that participants with VI were systematically underscored in the sMMSE because of their inability to complete 10% of the items that require visual input, thereby receiving a lower score. A rudimentary sensitivity analysis was performed modelling both sMMSE scores and MMSE blind scores, and this showed no change in the conclusions regarding the relationship between either sMMSE or MMSE blind and age, sex, VI and group. Further work to calibrate the modified MMSE blind scoring system with that of the sMMSE could more thoroughly address this issue.
Monocular VAs were recorded at both distance and near, both before and after the refraction. Binocular VA was recorded post refraction for distance vision for the majority of (62%), but not for all, participants. There is wide variation in the methods used to record VA in previous studies (e.g. best monocular VA, binocular VA, best monocular pinhole VA, binocular VA when available and best monocular when not, etc.). For comparison of VI prevalence with published studies, best monocular VAs have been used, as these were recorded both before and after refraction for virtually all PrOVIDe participants in whom VA could be measured. When PrOVIDe prevalence data are compared with studies that measured binocular VA, this makes comparisons less robust.
The Outside Clinic has robust quality control mechanisms regarding record keeping, etc. (see Chapter 2, Data collection, Stage 1). Every record card was checked by the project manager and any concerns regarding the data collection were raised with the relevant optometrist. However, there were no additional internal quality controls regarding the results of the eye examination. No internal checks have been reported in other UK population studies investigating VI apart from the MRC trial,56 in which the trained nurses recording VA received regular quality control visits.
The slit-lamp biomicroscope is normally a bulky table-mounted piece of equipment unsuitable for domiciliary use. A number of cataract grading scales are used in research studies;98 however, these require the use of a slit-lamp. Optometrists carrying out a domiciliary eye examination normally detect and grade cataract using a hand-held direct ophthalmoscope. Hand-held ophthalmoscopes were used in PrOVIDe, and cataract was graded using the published but unvalidated cataract section of the Optometric Grading Scale. 99
The causes of VI in the PrOVIDe sample have mostly been identified by the examining optometrists, and not definitively made, or confirmed, by an ophthalmologist. This limitation was inherent to the study design. The accuracy of optometrists’ diagnoses of eye conditions has been investigated; cataract, alone or with other conditions, was correctly diagnosed in almost 95% of cases. 115 For retinal disease, which includes AMD and diabetic retinopathy, the conditions were correctly diagnosed in > 70% of cases. 49,115 Accuracy of glaucoma diagnosis by optometrists is lower, at around 30% of cases. 116 However, glaucoma had already been diagnosed in almost 85% of cases in PrOVIDe and was confirmed by the participants’ glaucoma medication or evidence of glaucoma surgery. The remaining cases were based on the assumption that 50% of those referred as suspects would have the disease. A detection rate of 50% was chosen rather than 30%, as the prevalence of glaucoma increases with age, from 2% of those aged > 40 years to almost 10% in those > 75 years old. 117
Prevalence of visual impairment
Methods of recording VA in previous studies vary between Snellen and logMAR; research settings vary with some VA measurements taken in participants’ own homes and some in dedicated clinics; some studies use best monocular VA to define VI while others use binocular VA; age profiles of the samples vary; some studies exclude those living in care homes, and so on. These factors must be borne in mind when comparing PrOVIDe prevalence rates for VI with other studies. Two studies, the NDNS and MRC,56,57 provided sufficient details of participants’ age, sex and, in the case of the NDNS, location in either their own home or residential care, to facilitate reweighting of the estimated rates of VI in PrOVIDe to allow statistical comparison between rates of presenting VI for distance vision.
Presenting visual impairment
Using the VI prevalence criterion of best monocular VA < 6/12, the overall prevalence in PrOVIDe was 32.5% (95% CI 28.7% to 36.5%) for VA < 6/12. The lower age limit for comparable UK studies is usually 65 years and, when adjusted to exclude participants aged 60–64 years (see Table 8), the VI prevalence increases to 33.5% (95% CI 29.6% to 37.5%).
A prevalence of 28.3% is quoted in the best comparator data from the NDNS study,57 a national study which also used best monocular VA data, with a sample aged ≥ 65 years. The reweighted PrOVIDe prevalence estimate was 23.7% (95% CI 20.0% to 27.7%) compared with our estimate of 17.9% (95% CI 15.6% to 20.4%) for NDNS for participants in each study aged between 65 and 85 years, suggesting a meaningful difference between these populations, although there is only weak evidence to discount no difference between prevalence rates. There are several possible explanations for this difference in prevalence rates between PrOVIDe and NDNS. VA measurements in NDNS were recorded by trained nurses rather than by optometrists, ophthalmic nurses or ophthalmologists and some VA measurements were obtained using a pinhole. VA was recorded in the participants’ place of residence, and standardisation of lighting conditions was not possible. Although the fieldwork during which VA was assessed was conducted in 1994/5, so approximately 20 years before PrOVIDe, the NDNS has the strength of being a truly nationally representative sample from a random sampling frame of over 20,000 addresses from 799 postcode sectors across the UK. Cognitively impaired participants were excluded from this NDNS estimate of VI prevalence, although their data were presented separately for the VA < 6/18 cut-off point and are discussed below. Therefore, this comparison between prevalence rates in PrOVIDe and NDNS is between a sample with dementia and a sample in which subjects ‘classified as mentally impaired by a memory test’ were excluded.
Other UK studies in this age group found prevalences of 14.5% (95% CI 10.3% to 20.0%),58 30.2% (95% CI 24.8% to 35.5%)27 and 50.5%. 44 All three studies were local in their setting and insufficient data were provided to allow reweighting of PrOVIDe prevalence rates to permit direct comparison between their estimates and those of PrOVIDe. In addition, these studies range from 17 to 33 years old. The prevalence figure of 50.5% from Jack et al. 44 is often quoted and was based on a sample with a similar age range to that of PrOVIDe (≥ 65 years) and which contained a small but unstated number of participants living in care homes. Their study sample was small (n = 200) and comprised a selective population of patients admitted to a department of geriatric medicine with acute illness, and was unrepresentative of older people in general. Snellen VA was recorded, with the last line read correctly taken as the participant’s VA. These factors, notably selection bias, seem likely to have contributed to the high prevalence of VI reported in this study.
The large-scale MRC national trial in the 75–89 years age group reported a lower prevalence of 19.9% (95% CI 17.8% to 22.0%),56 but the study excluded those living in care homes. The reweighted PrOVIDe prevalence estimate was 28.7% (95% CI 21.3% to 37.4%) compared with 18.0% (95% CI 14.5% to 21.5%) for the MRC study for participants in each study aged between 75 and 89 years who were living in their own homes. There is some evidence of a difference between prevalence rates, suggesting a meaningful difference between these populations. Again, in the MRC study VA was recorded by trained nurses who may not be as reliable as optometrists in their measurements. Most of the MRC fieldwork (99.7%) was conducted between 1995 and 1998, at least 16 years before the typical date of PrOVIDe assessments.
Using the prevalence criterion for VI of the best monocular VA being < 6/18, the overall prevalence of presenting VI in the PrOVIDe sample was 16.3% (95% CI 13.5% to 19.6%). For those aged 65–89 years the prevalence of presenting VI was 16.7% (95% CI 13.8% to 20.1%). A prevalence of 14.3% is quoted in the NDNS. 57 The reweighted PrOVIDe prevalence estimate was 11.9% (95% CI 8.5% to 16.3%), compared with our estimate of 7.4% (95% CI 5.9% to 9.2%) for NDNS for participants in each study aged between 65 and 85 years, again suggesting a meaningful difference between these populations, although there is only weak evidence to discount no difference between prevalence rates.
Wormald et al. 58 reported a prevalence of 7.7% (95% CI 4.5% to 12.2%). However, although the paper notes that participants were a random sample of those aged ≥ 65 years from a large GP practice register, the age and sex breakdown of the sample or of the register is not presented. In addition, only 8.2% of Wormald et al. ’s sample was examined in their own homes, and the prevalence of VI among this subset was 41%. As a result of these factors, no substantive comparison with PrOVIDe can be made.
In the small subset of the NDNS sample (n = 125) who had cognitive impairment, 64.8% had VI. 57 The authors noted that it is unclear to what extent cognitive impairment affected the VA measurements, which were recorded by nurses.
When comparing group 1 with group 2, using the 6/12 criterion the unadjusted rate ratio of presenting VI among those in care homes was almost two-and-a-half times greater than for those living in their own homes (51.4%/21.8%). For the 6/18 criterion, the unadjusted rate ratio of presenting VI for group 2 versus group 1 was similar (26.4%/10.6%). There was a statistically significant group difference for both criteria after adjusting for age and sex (p < 0.001). One possible explanation is that those living in their own homes have more regular eye examinations; however, those living in residential care had a 22% reduction in the time since their last eye examination compared with those living in their own homes and there was an increased probability for a shorter interval between eye examinations for those in residential care compared with those living in their own homes (see Chapter 3, Eye examination data, Time elapsed since last eye examination). People living in care homes often have multiple comorbidities, which could increase their risk of developing eye conditions. Similar unadjusted rate ratios of presenting VI in care home residents and those living in their own homes were found in the NDNS for the 6/12 criterion (56.8%/22.4%), with an even greater ratio for the 6/18 criterion (35.6%/9.9%). 57
Previous studies of care home residents have reported high prevalence rates of vision problems both in the UK118 and in Australia. 119 It has been suggested that VI may be a contributory factor to older people being placed in care homes. 57
In summary, approximately one in three of the PrOVIDe sample had presenting VA < 6/12 and one in six had presenting VA < 6/18. Prevalences of VI are generally higher in the PrOVIDe dementia population than in comparable studies of older people that exclude or have lower proportions of participants with dementia. Based on unadjusted rate ratios, presenting VI was approximately two-and-a-half times more common in those living in care homes, and a clear, higher rate of VI existed for those living in care homes, even after controlling for age and sex differences.
Uncorrected or undercorrected visual impairment
For some PrOVIDe participants with presenting VI, VA improved sufficiently with the spectacle prescription found during the eye examination that they were no longer classed as visually impaired. These participants had uncorrected or undercorrected VI, for which there are few robust UK prevalence data and none in older people with cognitive impairment. One UK study included the determination of refractive error by an optometrist,59 but the prevalence of uncorrected/undercorrected VI was not reported. Another obtained autorefractor measurements on participants27 but VA does not appear to have been recorded with the participant wearing the spectacle prescription determined by the autorefractor. In a third study,44 a full refraction was performed on a proportion of those with VI, but the number is not stated.
In studies where the improvement in VA that can be produced by an up-to-date spectacle correction has been investigated, the post-refraction VA has usually been estimated from the best pinhole VA recorded monocularly. 27,56–58 Based on this estimate of post-refraction VA, and knowing the presenting VA for each participant, the prevalence of uncorrected or undercorrected refractive error can be estimated. The pinhole disc has been used in most of the comparator studies on VI in older people. The theory supporting its use is that if the participant’s loss of vision is the result of an out-of-focus image on the retina (which should be correctable with the appropriate prescription in the participant’s spectacles), then the pinhole can improve the participant’s vision by reducing the size of the blur circles on the retina. If the loss of vision is purely due to some pathological cause, such as AMD, then the pinhole should not improve vision. However, the pinhole has several limitations. It reduces the illumination of the image on the retina, which can impair vision. Furthermore, in a patient with cataract or other ocular media anomalies, the pinhole can be aligned with a ‘tunnel’ of clearer vision in a lens with cataract, which leads to an improvement in vision through the pinhole. However, when the pinhole is removed any attempt by the optometrist to achieve the same level of vision with the normal pupil size may fail owing to scattering of light through the more opaque portions of the lens. 11 Older patients in general, and particularly those with cognitive impairment, may have problems with use of the pinhole. This is evidenced by the statement in the paper reporting the MRC study56 that ‘use of the pinhole was not straightforward in this elderly population and only 62% of people with visual acuity less than 6/18 in either eye completed a pinhole test satisfactorily’.
Using the VI prevalence criterion of best monocular VA < 6/12, the overall prevalence of uncorrected or undercorrected VI in the PrOVIDe sample was 14.3% (95% CI 11.7% to 17.5%) for VA < 6/12. In the PrOVIDe sample aged 65–89 years the prevalence of uncorrected or undercorrected VI was 14.8% (95% CI 12.0% to 18.0%). The only comparator study found a prevalence of 9% (95% CI 7.0% to 11.4%) based on ‘refractive error causing VI [one or both eyes]’. 27 Methodological information on how this figure was obtained was not fully reported but, as the pinhole was used in this study, it is likely that improvement with a pinhole from VA of < 6/12 to VA of ≥ 6/12 was the basis on which this prevalence figure was calculated.
Using the VI prevalence criterion of best monocular VA < 6/18, the overall prevalence of uncorrected or undercorrected VI in PrOVIDe was 7.7% (95% CI 5.7% to 10.2%). In the PrOVIDe sample aged 65–89 years the VI prevalence was 7.8% (95% CI 5.7% to 10.3%). The only comparator study found uncorrected or undercorrected refractive error to be the main cause of VI in 40% of their sample. 44 Methodological weaknesses suggest that these data are likely to be unreliable for comparison purposes.
For the subgroup of the PrOVIDe sample aged 75–89 years, uncorrected or undercorrected VI was 7.8% (95% CI 5.6% to 10.7%). The MRC trial45 estimated the prevalence of uncorrected or undercorrected VI to be 3.2% of their population aged ≥ 75 years. However, this figure of 3.2% is suspect because of the deficiencies of the pinhole test. The MRC study reports comments on the difficulties of conducting the pinhole test in older people, which resulted in 38% of missing data. In addition, in the MRC trial, VA recording was carried out by trained nurses, rather than by optometrists who may be more reliable in their VA measurements. Any attempt at a comparison with the PrOVIDe post-refraction prevalence estimate is further hampered by a non-comparable age–sex distribution.
Many of the studies quoted above do not have age–sex breakdowns from which to assess these distributions,27,44 and thus it is not possible to make any meaningful comparisons with PrOVIDe prevalence estimates. Another salient point when attempting to compare PrOVIDe prevalence estimates with previous studies is the currency of the published data: the Melton Mowbray study59 dates from 1987, Wormald et al. 58 dates from 1992, Jack et al. 44 dates from 1995, and the North London study dates from 1998. 27 The current ageing population, combined with a changing health services and health-care policy landscape in the last 20–30 years, is a confounding factor for any attempted comparisons between a 2014 study and these studies from the twentieth century.
When comparing group 1 with group 2 using the 6/12 criterion, the unadjusted rate of uncorrected or undercorrected VI among people living in care homes was almost twice that of those living in their own homes (21.0%/10.6%). For the 6/18 criterion, the unadjusted rate ratio of uncorrected or undercorrected VI was even greater (12.4%/5.1%). There was a statistically significant independent group difference for both criteria after adjusting for age, sex and sMMSE scores (p < 0.01 for 6/12 and p = 0.05 for 6/18). However, as reported in Chapter 4, there are difficulties in ensuring that people living in care homes wear their spectacles. Prescribing new spectacles with the up-to-date prescription will not guarantee that these will be worn by all those who would benefit from them.
These prevalence findings for uncorrected or undercorrected VI have possible consequences for increasing the likelihood of falls. Risk factors for falls include age,78 living in care homes,57,80 cognitive impairment82 and VI. 44,83,84,120 Falls are estimated to cost the NHS more than £2.3B per year, and 50% of people aged > 80 years fall at least once per year. 78 If the figure of approximately 14% of participants having correctable VI (using the VA < 6/12 criterion) is generalisable to the whole population with dementia, and if regular spectacle wearing could be promoted, this could reduce the number of falls, particularly in care homes, and even a modest cut in falls rate should produce significant NHS cost savings.
In summary, 14.3% of the PrOVIDe sample had VI (VA < 6/12) that was correctable with their up-to-date spectacle prescription. For VA < 6/18 the equivalent figure was 7.7%. There is a particular issue in care homes, where 21.0% of participants were unnecessarily visually impaired using the 6/12 criterion. Correcting this element of VI could impact positively on NHS costs, quality of life and reduced admission to care homes.
Post-refraction visual impairment
Following the determination of refractive error, the measurement of VA allows identification of those who, despite wearing their up-to-date spectacle prescription, remain visually impaired. The prevalence of post-refraction VI in the PrOVIDe sample was 18.1% (95% CI 15.2% to 21.5%) for best monocular VA < 6/12, reducing to 8.6% (95% CI 6.6% to 11.3%) for VA < 6/18.
For the subgroup aged 75–89 years, post-refraction VI for VA < 6/12 was 20.4% (95% CI 16.8% to 24.1%), lower than the prevalence of 26.2% (95% CI 22.4% to 30.3%) calculated from data from the best comparator study in the early 1980s. 59 Factors contributing to the higher prevalence in the comparator study include the recruitment of participants aged ≥ 90 years (although they were only approximately 5% of the sample of 529), the use of a Snellen chart requiring every letter of the 6/18 line to be read in order for the participant to be classified as having achieved that level of acuity, and the recruitment of participants from one market town in England. 59 Using the pinhole method for obtaining monocular VAs which are then used to estimate post-refraction VI, the MRC trial derived a prevalence of VI of 10.2%,56 for a sample of participants aged ≥ 75 years for VA < 6/18, similar to the prevalence in PrOVIDe of 9.9% (95% CI 7.4% to 13.1%) for the subgroup aged 75–89 years.
In a comparison of PrOVIDe groups 1 and 2, the unadjusted rate of post-refraction VI was approximately two-and a-half-times greater among those living in care homes using both criteria: 30.2% versus 11.4% for < 6/12 and 14.2% versus 5.6% for < 6/18, respectively. There was a statistically significant group difference for both criteria after adjusting for age and sex (p ≤ 0.01).
In summary, with best possible spectacle correction VA < 6/12 is present in 18.1% of this sample of people with dementia. For VA < 6/18 the equivalent figure is 8.6%. Based on unadjusted rate ratios, post-refraction VI is approximately two-and-a-half times more likely among those living in care homes.
Causes of visual impairment, excluding refractive error
Excluding VI from uncorrected or undercorrected refractive error, 102 participants were classified post refraction as being visually impaired based on binocular VA (or best monocular VA if binocular VA not available) being < 6/12, and 47 participants post refraction were visually impaired based on VA being < 6/18. Comparisons with other studies are difficult, as a variety of definitions were used for the conditions causing VI. Most studies27,44,58,59 determined clinical status following an examination by an ophthalmologist, while in the MRC study45 cause was established from GP notes with supporting evidence for some participants from a survey of the ophthalmologists who last examined each participant.
Criterion for visual impairment: visual acuity < 6/12
As previously shown in Table 17, where more than one possible cause of VI is included for each participant, cataract (70.6%, 95% CI 60.6% to 79.0%) and AMD (40.2%, 95% CI 30.8% to 50.4%) predominate, followed by glaucoma (9.8%, 95% CI 5.1% to 17.7%) and diabetic retinopathy (1.0%, 95% CI 0.1% to 6.1%). There is no equivalent comparator with these data. Reidy et al. 27 used the 6/12 cut-off point and included the possibility of more than one cause, but their data for each condition include participants who have VA < 6/12 in one or both eyes. Therefore, for example, a participant with AMD reducing right-eye acuity to 6/18 but with left-eye acuity of 6/6 was classified in the Reidy et al. 27 study as having VI caused by AMD. In PrOVIDe and other studies, it is the participants’ binocular VA or best monocular VA that is used when classifying participants as VI. Therefore, for the example given above, either binocular VA or best monocular VA would be at least 6/6 for this participant who would not be classified as VI.
Where a single cause of VI was identified in PrOVIDe, cataract was responsible for almost half of the cases of VI (48.0%, 95% CI 38.1% to 58.1%) and all of these cases were potentially remediable with cataract surgery. AMD accounted for over one-third of cases (36.3%, 95% CI 27.2% to 46.4%). Glaucoma accounted for 6.9% (95% CI 3.0% to 14.1%) and the figure for diabetic retinopathy was 1.0% (95% CI 0.1% to 6.1%).
In a small sample of 30 participants aged ≥ 65 years, Wormald et al. 58 used the same criteria as PrOVIDe. Cataract was the principal cause of VI in 63% of participants and AMD was the principal cause in 20% of participants. However, this study was published in 1992 when fewer cataract surgeries were performed; this is reflected in the 26.6% of PrOVIDe participants who had an intraocular implant in one or both eyes, compared with 5.8% of Wormald et al. ’s sample who were aphakic.
Criterion for visual impairment: visual acuity < 6/18
Where more than one cause for VI was included (see Table 17), AMD (53.2%, 95% CI 38.2% to 67.6%) and cataract (61.7%, 95% CI 46.4% to 75.1%) are again the most frequent causes, but AMD is more common and cataract less so than with the 6/12 cut-off point because AMD is more likely to be the cause of VI as participants’ VAs worsen. Glaucoma (8.5%, 95% CI 2.8% to 21.3%) is a minor contributor. In the only comparator, the MRC study,45 AMD caused a similar proportion of VI (52.9%, 95% CI 49.2% to 56.5%). However, cataract was identified as a cause in 35.9% (95% CI 31.7% to 40.1%) of MRC participants. One explanation could be the procedure in PrOVIDe for optometrists and the research team to record two potential causes, which occurred in 34% of cases, compared with only 16% in the MRC trial.
Where a single cause was identified, AMD (48.9%, 95% CI 34.3% to 63.7%) overtook cataract (36.1%, 95% CI 23.1% to 51.5%) as the leading cause of VI, with glaucoma making a small contribution (4.3%, 95% CI 0.7% to 15.7%). Comparator studies attribute lower contributions from AMD to VI (14%,58 23%44) but these studies date from the early 1990s when cataract surgery was less frequently performed.
In summary, the PrOVIDe data should be interpreted with caution as they rely on causes reported by optometrists. However, for the VA < 6/12 criterion, almost half of VI was caused by cataract, which is potentially remediable, and approximately one-third was caused by AMD.
Prevalence of specified eye conditions
Comparisons between PrOVIDe estimates of prevalence for the eye conditions and estimates from population studies are limited by differences between studies in their definitions of conditions and variations in sample demographics. 121 Furthermore, the PrOVIDe estimates are based on identification of the condition by an optometrist rather than by an ophthalmologist (see Chapter 5, Limitations of the study). The domiciliary eye examinations carried out in PrOVIDe precluded the use of the bulky slit-lamp biomicroscope. This limited the optometrist to direct hand-held ophthalmoscopy for assessment of the ocular media and fundus, rather than using the clinically superior examination techniques of a slit-lamp assessment of the ocular media and binocular indirect ophthalmoscopy to examine the fundus.
The overall prevalence of AMD in PrOVIDe was 17.7% (95% CI 15.0% to 20.8%), rising to 18.3% (95% CI 15.5% to 21.4%) when adjusted to include only those aged between 65 and 89 years (see Table 12). A Bayesian meta-analysis estimate of UK prevalence for late stage AMD for those aged > 65 years is approximately 9.9%,19 but the PrOVIDe estimate will include those with less severe AMD (see Chapter 2, Data collection, Stage 1, The eye examinations). A European study of people aged ≥ 65 years, which included the UK among its seven centres, graded fundus photographs according to the Rotterdam staging system, in which Grade 0 represented no AMD and Grade 4 was late-stage neovascular AMD. The prevalence of AMD Grades 1–4 was 52.4%, reducing to 15.9%, similar to the PrOVIDe prevalence, when those with Grade 1 (mildest visible signs of AMD) were excluded. 122
Prevalence of cataract in PrOVIDe was 59.0% (95% CI 55.2% to 62.7%) for grades 1–5, reducing to 38.0% (95% CI 34.5% to 41.7%) when grade 1 (minimal visible cataract) was excluded. The best comparator data come from the Eye Diseases Prevalence Research Group (EDPRG),123 which included data from major population studies in the USA, Europe and Australia. 121 Definitions of significant cataract varied between studies contributing to these estimates but all of these definitions are likely to exclude early cataract (grade 1 in PrOVIDe). The prevalence of cataract in those aged > 60 years was calculated for this report from published EDPRG data to be 37.8%,123 similar to the grades 2–5 estimate from PrOVIDe.
Glaucoma prevalence in PrOVIDe was estimated to be 7.1% (95% CI 5.3% to 9.3%), increasing to 7.2% (95% CI 5.4% to 9.5%) when adjusted to include only those aged between 70 and 89 years. A Bayesian meta-analysis estimated a pooled prevalence of open-angle glaucoma in white populations aged > 70 years to be approximately 6%. 39 This figure is similar to that from PrOVIDe, although the meta-analysis had no upper age limit and the PrOVIDe figure was not limited to open-angle glaucoma nor to white populations.
The prevalence of diabetic retinopathy in PrOVIDe was 2.0% (95% CI 1.1% to 3.4%) of the overall sample, and the prevalence of diabetes was 12.0% (95% CI 9.8% to 14.7%). The prevalence of diabetes in the adult population in England in 2013 was estimated to be 6.0%,124 with the higher prevalence in PrOVIDe reflecting the older population in this study. In a UK diabetic population screening study, 25.3% of type 2 diabetics had some form of diabetic retinopathy. 32 In a US non-Hispanic white diabetic population aged > 65 years the estimated prevalence of diabetic retinopathy was 26.4% (95% CI 21.4% to 32.2%). 121 The PrOVIDe sample was predominantly white and the diabetics were predominantly type 2; therefore, applying this approximate figure of one-quarter of diabetics having diabetic retinopathy32,121 to the 12% of diabetics in PrOVIDe gives an estimate of 3% of the total sample having diabetic retinopathy, a figure which lies within the 95% CI for diabetic retinopathy in the PrOVIDe sample.
In summary, comparisons with published population studies should be interpreted with caution. However, when assumptions are made in an effort to compare like-with-like, the prevalences of the four conditions in PrOVIDe are similar to those in the best comparator studies.
Factors affecting visual impairment prevalence data
The prevalences of presenting and post-refraction VI increased significantly with age after adjusting for sex and group for both VI criteria (p ≤ 0.01), confirming previous UK studies. 44,56,57 However, after adjusting for sex, group and sMMSE score, there was no evidence for an independent age effect on uncorrected/undercorrected VI. This suggests that when post-refraction VI (frequently the result of age-related eye conditions) is excluded, then uncorrected/undercorrected VI (the result of wearing an incorrect spectacle prescription) is not dependent on the age of the participant. In addition, for VI defined as VA < 6/18 there was evidence of an association (independent of age, sex, sMMSE score and group) between the time since last eye examination and the probability of having uncorrected/undercorrected VI, with an OR of 1.23 (95% CI 1.04 to 1.42). Here, ORs are expressed as the increase in odds of VI for every year increase in the time since last eye examination, at a fixed age, sex, group and sMMSE score. There was no evidence of a similar association for VI defined as VA < 6/12.
There was no evidence of any independent sex difference for presenting VI, unlike most other UK studies,44,56,57 nor for post-refraction VI or for uncorrected/undercorrected VI for VA < 6/12. There was a statistically significant independent sex effect for uncorrected/undercorrected VI for VA < 6/18, with an adjusted OR for males versus females of 0.45.
The prevalence of AMD, when adjusted for sex and group, increased significantly with age, with an OR of 1.18 (95% CI 1.13 to 1.24), as found in other studies. 121 Similarly, there was an independent age effect on the prevalence of the combination of cataract (grades 1–5 or grades 2–5) and an IOL in one or both eyes, again consistent with other studies. 123 As the population of the UK rapidly ages,3 age-related eye disease and VI will become greater public health issues. There was no evidence for an independent age effect for glaucoma, which was found in other studies;39,121 however, the definitive studies are meta-analyses or used data pooled from a number of population studies. No evidence of independent sex differences was found for any conditions apart from diabetic retinopathy (adjusted OR for males vs. females 3.69, 95% CI 1.19 to 13.80). This result should be interpreted with caution, as only 14 participants had diabetic retinopathy.
Association between Standardised Mini-Mental State Examination and Mini-Mental State Examination blind scores
The sMMSE instrument can be modified for participants with loss of vision, to compensate for their performance on the three sMMSE items which require significant visual input. 125 MMSE blind is often used with visually impaired participants, and the three items requiring significant visual input are omitted and the test score out of 27 is rescaled to give a score out of the usual maximum of 30. In PrOVIDe, the sMMSE instrument was used at recruitment on all participants, at a stage when the recruiter would not normally be aware if the participant was visually impaired or not. This could lead to an overestimation of cognitive impairment through the use of sMMSE in visually impaired participants.
To test this possible effect, a linear regression model was fitted to the sMMSE scores, adjusting for age, sex, residential status and presenting VI [ordered in terms of severity ‘better than 6/12 (none)’, ‘6/12–6/18’, ‘worse than 6/18’]. The coefficient point estimates and statistical significances were then qualitatively compared with those of a model of sMMSE blind scores (see Table 29). A rudimentary sensitivity analysis was performed, modelling both sMMSE scores and MMSE blind scores, and this showed no change in the conclusions regarding the relationship between either sMMSE or MMSE blind and age, sex, VI and residential status. The difference in the mean sMMSE and MMSE blind scores according to these models was approximately 0.75, with a higher mean score for MMSE blind. This difference is much lower than the maximum possible difference of 3. There is an indication of higher MMSE blind scores in both non-visually impaired participants and in moderately visually impaired participants.
In summary, the prevalences of presenting and post-refraction VI increase significantly with age. There is no independent age effect for uncorrected/undercorrected VI. The prevalences of both AMD and cataract increase significantly with increasing age after adjustment for sex and group. No independent sex effect was found for AMD, cataract or glaucoma.
Improvement in visual acuity post refraction
Distance acuity
The mean improvement in VA following refraction was 0.09 logMAR units, equivalent to almost one line improvement on the logMAR chart, with no significant differences between groups 1 and 2. Similar improvements were measured for AMD, cataract, glaucoma and diabetic retinopathy. All mean improvements were statistically significant. Clinical significance is more relevant and best described by the percentages of participants who achieved improvements in acuity greater than set criteria (see Table 22).
As noted in Chapter 3 (see Change in distance visual acuity from pre to post refraction, Improvement in visual acuity post refraction based on lines of improvement on distance test charts), the percentage of the sample who improved by ≥ 0.16 logMAR units has also been recorded. This criterion was chosen as it represents an improvement greater than the average change in acuity between two lines on a Snellen chart, which is 0.154 log units. 57 Although all of the distance VA measurements in the PrOVIDe study were made using charts with logMAR progression of letter sizes, previous studies that have investigated VI in older people have often used charts with Snellen progression. 44,58,59 Snellen charts have several disadvantages compared with logMAR charts, notably the unequal progression in letter sizes between lines on the chart. However, to permit comparisons between PrOVIDe results and those obtained in previous studies that used Snellen charts, the ‘equivalent’ improvement in terms of lines of Snellen acuity has also been calculated.
There was an improvement equivalent to more than one line on a Snellen chart in 20.4% of participants, an improvement that clinicians often regard as clinically significant. When improvement in distance VA was assessed using a pinhole in a sample of comparable age (n = 207), it was estimated that 27% would have benefited from a refraction by virtue of an increase of VA of greater than one Snellen line. 58
In PrOVIDe, 17.8% of participants improved by two or more lines on the logMAR chart (≥ 0.20), a figure similar to NDNS (21.2%), again using a pinhole to estimate post-refraction VA. 57 The test–retest reliability of logMAR charts similar to those used in the PrOVIDe study, namely Bailey–Lovie and Early Treatment Diabetic Retinopathy Study (ETDRS) charts, have 95% confidence limits for change of ± 0.12 logMAR units for Bailey–Lovie and ± 0.14 logMAR units for ETDRS charts;126 therefore, changes greater than this are unlikely to occur by chance. Similar methods to the PrOVIDe study were used by Lavery et al. ,59 in which optometrists carried out a full refraction and measured the improvement in VA based on best monocular VA. Using Snellen acuity, 6.8% of the sample who had spectacles improved their VA by at least two lines. This is equivalent to an improvement of approximately three lines or more on a logMAR chart, and 6.8% of the PrOVIDe sample improved their VA by three logMAR lines or more.
Near acuity
Near acuity has rarely been recorded in previous studies, although its measurement under standardised conditions is recommended in population studies of VI. 45 Standard newspaper print is approximately N8 size,127 and 16.2% (95% CI 13.1% to 19.9%) (n = 77) of the 475 PrOVIDe participants for whom NVA was recorded both pre and post refraction were unable to read N8 print with their current near vision spectacles (see Table 23). Almost two-thirds of this visual loss (n = 49) was correctable with the optimum spectacle prescription for near vision. However, this proportion of participants achieving N8 acuity with their up-to-date spectacle prescription when this was not achievable with their current spectacles should be interpreted with caution, as other important aspects of near visual function were not evaluated in PrOVIDe. Although the research optometrists recorded the VA as the smallest print that could be read fluently, the ability to carry out sustained reading of this size of print comfortably (critical print size) was not assessed, nor was reading speed. The ability of a participant to read N8 print over a short period of time does not necessarily mean that he or she will be capable of reading newsprint in comfort for a reasonable period of time. It is estimated that in order to read print comfortably an acuity ‘reserve’ is required;128 for example, for someone to read N8 print comfortably for a reasonable period of time, they would usually need have a NVA of N4.
In the Melton Mowbray study, the only other UK population study to record NVA following an optometrist’s refraction,59 11.2% of the sample were unable to read N8 after refraction, almost twice the 5.9% in PrOVIDe. The reason for this difference is unknown.
There was no evidence for independent sex effects on near vision loss. There was a statistically significant independent group effect for presenting near vision loss for both near vision loss criteria, for post-refraction near vision loss for the NVA < N8 criterion, and for uncorrected/undercorrected near vision loss for the NVA < N10 criterion. This group effect is similar to that found for distance VI. There were also some similarities with distance VI with regard to the effects of age; the prevalences of post-refraction near vision loss increased significantly with age after adjusting for sex and group for both near vision loss criteria, consistent with the increased incidence of age-related eye conditions. No other independent age effects were found for presenting or uncorrected/undercorrected near vision loss.
In terms of the number of extra lines on the near vision chart that could be read following refraction, 28.6% read one extra line, 9.3% read two extra lines and 6.6% read three or more lines (see Table 25). Improvements of more than three lines occurred in 7.2% of those in care homes, and improvements in NVA of this magnitude have the potential to turn reading books and newspapers from being a chore into being a pleasure; for example, an improvement of four lines from N10 to N4 should allow the participant, previously unable to read standard newspaper print of N8 size, to have sufficient acuity reserve to be able to read N8 comfortably.
In summary, mean improvement in VA post refraction was approximately one line of logMAR acuity. Distance acuity improved by two or more lines post refraction in 17.8% of participants. Of the participants, 16.2% could not read standard newspaper-size print with their current spectacles, and for almost two-thirds of these participants this was correctable.
Clinical limitations of the eye examination
Patient dependent
Visual acuity and determination of refractive error
Cognitive impairment and the patient’s level of co-operation can limit an optometrist’s ability to perform a complete eye examination. The PrOVIDe study is unique in quantifying these limitations for key tests and in relation to the participant’s level of cognitive impairment.
In just eight cases (1.1%) the optometrist was unable to carry out any part of the eye examination. The determination of refractive error by objective methods using a retinoscope was possible in both eyes of almost 90% of participants (see Chapter 3, Ability of participants to complete individual elements of the eye examination). Focus group and anecdotal evidence from community optometrists suggests that optometrists have concerns that elements of the eye examination, notably VA and subjective refraction, are unlikely to be possible in people who have dementia. However, both tests were possible in > 80% of participants. The pinhole test was also possible in > 80% of those in whom it was attempted. However, the optometrists decided, based on their assessment of the participant’s co-operation and cognition, not to attempt the pinhole test in > 70% of the sample. The MRC trial, in their national sample of older people, noted that the pinhole test was not straightforward, proving unsuccessful in 38% of those in whom the test was attempted. 56
Optometrists in focus groups reported other difficulties faced during eye examinations of people with dementia, including the inability of some people to cope with VA charts presented at 6 m by means of a mirror. Charts viewed directly (e.g. Kay pictures) may be preferred. The CoO Guidance document C5 The domiciliary eye examination130 states the equipment used in domiciliary examinations should include a ‘portable test chart (preferably illuminated)’, and although Kay pictures are available only in booklet form, similar charts can be selected in portable computerised systems.
Evaluating the health of the eyes
Visual fields examination is a challenge in the domiciliary setting. 129 This test is requested by TOC optometrists when clinically necessary and if, in the professional judgement of the optometrist, the individual could cope with the demands of the test. Although visual fields tests were requested for only 21% of participants, the test was possible in > 90% of those in whom it was attempted.
Tonometry, using the Icare tonometer, was possible in almost 95% of all participants and in > 99% of those in group 1 (see Table 26). The importance of tonometry in older people suspected of having dementia is reinforced by the increasing prevalence of glaucoma with age,40 and elevated IOP is a major risk factor for POAG.
Examination of the retina and remainder of the fundus is facilitated by pupil dilatation. Ophthalmoscopy becomes more difficult in patients with cataract or if the patient’s pupil diameter reduces, and both factors are exacerbated by increasing age. Pupil dilatation with tropicamide eye drops was attempted on all participants, and was possible in two-thirds. Reasons for not dilating included the absence of informed consent and the participant declining dilatation (see Table 19); poor participant co-operation accounted for only 14% of non-dilatations.
Ophthalmoscopic examination of the fundus was possible in > 90% of participants and almost 98% in group 1. This examination is crucial in the detection of AMD, glaucoma and other rarer conditions that can cause significant loss of vision or blindness. In this test the optometrist places the ophthalmoscope around 1–2 cm from the patient’s eye and directs bright illumination into the eye. The optometrist is also in the patient’s personal space. All these factors could cause distress, which makes the level of co-operation achieved impressive.
For all but one of these tests, the sMMSE scores for those in whom the test was possible were statistically significantly higher than for those in whom the test was not possible (see Table 28). The exception was the objective assessment of refractive error using a retinoscope. These differences were confirmed from logistic regression, after adjustment for age, sex and group. It should be noted that sMMSE scores were missing for 54 participants, who are more likely to be older and more likely to live in care homes than participants for whom sMMSE scores are available.
Group 1 participants were significantly more likely than group 2 participants to be able to undertake each key test (apart from objective assessment of refractive error by retinoscopy) (see Table 26). The differences between groups were greatest for subjective assessment (95.1% vs. 63.6%), the measurement of VA (97.4% vs. 65.8%), dilatation (81% vs. 50.5%) and visual fields (52.5% vs. 3.6%). When adjusted for age and sex, this statistically significant independent group effect persisted for the subjective examination, VA measurement, tonometry and dilatation (p ≤ 0.03). There was some evidence of a group effect for ability to complete the fundus examination (p = 0.06), but little evidence for a group effect for the ability of the participant to complete the objective determination of refractive error (p = 0.20).
In summary, most key elements of the routine optometric eye examination could be successfully completed on > 80% of participants. Objective tests were more likely to be successfully completed than subjective tests, notably retinoscopy and fundus examination. Visual fields and the pinhole test were possible in a minority of participants. For all key tests, with the exception of retinoscopy, sMMSE scores were significantly lower in those unable to complete the tests. Participants living in care homes were significantly less likely to be able to undertake most key tests.
Setting dependent
The CoO guideline C5 The Domiciliary Eye Examination130 notes that people able ‘to attend a practice for examination should be encouraged to do so, since it is recognised that a dedicated consulting room is the optimum environment in which to conduct their eye examination . . .’. It follows on: ‘. . . for the patient who is unable to access a community practice the optimum environment will be their place of residence’. It is desirable for the examination to take place in the environment best suited to the individual, and taking vulnerable people or those with challenging behaviour to an optometrist’s practice may increase stress levels and make them feel intimidated. 129 Optometrists in focus groups who had experience of domiciliary examinations reported some advantages to examining people in their own homes, including that people are more relaxed at home than in a practice environment. In addition, there is access to the reading material used by the person, and their habitual visual environment, notably lighting, can be assessed. From the public perspective, very few people with dementia who were interviewed in stage 2 or carers in focus groups had been aware of the availability of domiciliary services prior to participating in PrOVIDe. Despite the rapid increase in numbers of domiciliary sight tests over the past decade,51 there remains a need for greater publicity of the service.
A theme from optometrist focus groups was the importance of the presence of a carer with good knowledge of the patient throughout the examination. This view is supported by the CoO guideline C5: ‘Wherever possible, the domiciliary visit should take place when a relative or carer is present’. 130 Not all optometrists appreciate the potential benefits of having a carer present as, in the family carers’ focus groups, pleas were made for optometrists to understand the need to involve carers. For PrOVIDe participants living at home, a carer was present in almost 90% of examinations. However, for participants living in care homes, > 40% of examinations were conducted with no carer present. Some optometrists in focus groups reported that the presence of a carer or care worker was no guarantee that the carer knew the patient well, but someone with good knowledge of the person being examined may be able to provide some or all of the history and symptoms91,129 and may help the individual feel more at ease.
The presence of a carer during the eye examinations conducted during stage 1 of PrOVIDe had a positive impact on the successful completion of all five key elements of the eye examination that were evaluated. These five were: (1) objective assessment of refractive error; (2) subjective assessment of refractive error; (3) VA measurement; (4) fundus examination by ophthalmoscopy; and (5) tonometry, and this positive impact was statistically significant for VA measurement with an unadjusted rate ratio of 1.9 (95% CI 1.2 to 3.0).
In summary, a domiciliary setting may offer some advantages, allowing the visual environment to be assessed. There remains a need to publicise domiciliary services. Having a carer present with good knowledge of the person being examined is beneficial and may have a positive impact on the successful completion of key elements of the eye examination.
Practitioner dependent
The need for optometrists examining people with dementia to have adequate training and experience was a message that emerged from focus groups. Family carers had experiences of optometrists (outside PrOVIDe) who lacked understanding of the impact of cognitive impairment on the examination and who failed to communicate adequately. Non-TOC optometrist focus group members thought that they would benefit from further knowledge of dementia in general and training in the management in optometric practice of people with dementia. Specific topics included how best to adapt usual eye examination routines to accommodate the cognitively impaired and the need for greater information on issues of consent and capacity. Preferences were for interactive rather than didactic approaches to training, and the limitations of the didactic approach to optometric training in glaucoma have been published. 131
Continuing Education and Training and Continuing Professional Development training is readily available to optometrists in a variety of forms. However, where there is a desire for greater experience in examining people with dementia, the opportunities to gain this by observing practitioners with expertise in this area, and/or having peers/mentors who could advise their less-experienced colleagues, are not currently available.
The development of optometrists who are specialist practitioners was suggested. There are long-standing UK precedents for the development of specialist optometrists in contact lenses and orthoptics and, more recently, in therapeutics132 and glaucoma. 133,134 However, the process of establishing a specialist qualification for optometrists is lengthy, requiring the drafting of a syllabus, core competencies, training programmes and assessment procedures. 135 The viability of a specialist qualification should be a topic for further research. Through the CoO Higher Qualifications framework, optometrists can already obtain Professional Certificates in low vision, medical retina and glaucoma, and Professional Higher Certificates in low vision, glaucoma and contact lenses. 136
Excluding the TOC focus group, most optometrists who did not currently undertake domiciliary work felt that they did not have adequate equipment to carry out a satisfactory examination in a home environment.
In summary, optometrists identified the need for further, preferably interactive, training in dementia and its management in practice. A specialist higher optometric qualification in the care of older people could improve eye care. The viability of this specialist qualification should be a topic for further research.
Quality of optometric practice
Degree of satisfaction with eye examination
In general, stage 2 interviewees were satisfied with their eye examinations prior to the PrOVIDe examination, although some carers expressed doubts about the ability of optometrists to carry out the subjective elements of the eye examination. The CoO guideline C491 stresses the need for the optometrist to be flexible during the examination, adapting techniques and using alternative appropriate methods. The PrOVIDe optometrists examined according to these guidelines and were able to conduct subjective examinations on > 80% of participants, although subjective refraction was less likely to be possible as the degree of cognitive impairment progressed. There was frequent praise from participants and carers for the PrOVIDe optometrists, particularly for their understanding of how to relate to and communicate with the participants. The thoroughness of the examination and the length of time allocated also received favourable comment.
Communication between carers and health-care professionals
Focus group optometrists commented that family carers did not always inform them that their relative had dementia, and that this information frequently emerged during the examination. Knowing this from the outset would make the subsequent examination more efficient and a better experience for all parties.
Optometrists expressed the desirability of continuity of optometric care for people with dementia. Continuity depends, at the practice level, on patients returning to the same practice and, at the individual level, on requesting the same optometrist. Some interviewees reported continuity in their choice of practice and optometrist, while others moved around. CoO guideline C5130 states that domiciliary eye examinations should not be seen as a ‘one-off’ service but as part of the provision of continuing care to the patient. Continuity of care may be even less likely for people living in care homes, where most eye examinations are carried out by optometrists working for companies that supply domiciliary services.
Time allocated for the eye examination and the NHS fee structure
The need to allow sufficient time to conduct the eye examination in people with cognitive impairment was a recurrent theme from participants, carers and optometrists. The CoO guideline C4 notes that optometrists ‘should be prepared to take longer to complete the eye examination’91 if the patient’s responses are slow. There is considerable variation in the duration of optometric eye examinations even in people without cognitive impairment. 55 Optometrists in Scotland can carry out a maximum of 20 sight tests per day but no maximum applies in England. 137 As a result, optometrists in England are often scheduled to carry out sight tests every 20 minutes (or less) throughout the working day. Individuals who require a longer examination than the norm can play havoc with appointment scheduling, affecting other patients and other practice staff. Booking longer appointment slots for people with cognitive impairment attracts no extra NHS funding, nor does the alternative of the patient returning for a second appointment. Family carers expressed concerns about the logistical challenges of bringing their relative to the optometrist’s practice for an eye examination, so returning for a second appointment may not be a realistic option for some people with dementia. In the UK, apart from Scotland, the current NHS sight test fee is £21.10,138 which is reported to be less than half of the actual cost of providing a sight test. 52 This fee leads to a situation in which NHS sight tests outside Scotland are financially viable to a practice only when subsidised by the purchase of spectacles. 52,139 Overall expenditure on GOS has fallen in real terms since the 1950s, a situation not common to any other service provided across the NHS. 139
Although focus group comments regarding the PrOVIDe domiciliary eye examinations were complimentary, some concerns were expressed regarding eye-care provision in care homes. There was potential for examinations to be rushed, against the ethos of the CoO guideline C4. When examining people with advanced dementia, it is not always possible to carry out a full sight test. All agreed that an eye health check in people with dementia was valuable, but optometrists queried if a partial sight test should attract the full NHS sight test fee in instances when a full test is not possible. The current NHS fee structure does not compensate the optometrist who may have taken time out of practice to carry out a domiciliary sight test but who is unable to claim the fee because the patient was not sufficiently co-operative to complete most of, or any of, the sight test. This could be a disincentive for independent practitioners to provide domiciliary services.
Spectacle dispensing
When dispensing new spectacles to people with dementia, optometrists reported that it was good practice to minimise change from the type of lenses (e.g. separate pairs for distance and near vision, bifocal, progressive power lenses) and frames (e.g. style and colour) used in previous spectacles, as changes may be confusing and contribute to a refusal to wear the new spectacles.
In summary, stage 2 interviewees were generally satisfied with the quality of eye examinations. It is desirable for the practice to be informed, when an appointment is made, that the person to be examined has dementia, and for the optometrist to be informed of this prior to beginning the examination. Continuity of care is desirable but not always achieved, especially in care homes. Allowing more time for the eye examination is desirable but not always possible owing to practice timetabling difficulties. The NHS sight test fee structure does not take into account the particular issues arising when people with dementia have sight tests (e.g. extra time required, only partial sight tests possible, no test possible). When dispensing spectacles, unnecessary changes should be kept to a minimum.
Inappropriate management of visual impairment in people with dementia
Inappropriate management of spectacle prescriptions
For spectacle prescriptions, inappropriate management could be revealed if large changes in spectacle prescription occurred over a short period of time. Determination of refractive error is subject to variability. If the same person is examined by two different optometrists under identical conditions, the variability, or reproducibility, of the two measurements of refractive error can be calculated. 140 The reproducibility of refractive error in older people with cognitive impairment is unknown. However, the reproducibility of subjective refractive findings in people aged < 60 years without cognitive impairment is approximately ± 0.75 dioptres (D). 140,141 Furthermore, the multiple eye examinations from which this ± 0.75 D figure was calculated were completed over a relatively short period of time, during which refractive errors of younger people would be unlikely to alter. However, refractive errors can change more rapidly in older people with cataract, especially nuclear sclerosis cataract, and in the weeks following cataract surgery. For these reasons, the 95% reproducibility limits are likely to be wider, possibly considerably wider, than ± 0.75 D in people with cognitive impairment. Furthermore, people with dementia may not be wearing their current spectacles or, as noted in focus groups, their own spectacles, so any change in prescription noted by the optometrist may be based on an erroneous baseline. All of these factors make the identification of inappropriate management difficult. Nevertheless, in PrOVIDe the optometrists had access for some participants to the prescription statement issued at the previous sight test, on which the date of that sight test was recorded. When this previous sight test took place a short time before the PrOVIDe examination, it was possible to compare the PrOVIDe spectacle refraction with the prescription issued at the previous eye examination. Of the 6.2% (n = 44) of PrOVIDe examinations that took place within 2 months of the previous examination, there was evidence of potential inappropriate refractive management in only one case.
If there was large-scale inappropriate management of spectacle prescriptions at the eye examination previous to the PrOVIDe examination, this could result in a higher than usual proportion of participants being advised that a change in spectacle prescription would be beneficial. The proportion advised of a change following their eye examination was 63.4%. This figure is almost identical to the percentage (63.5%) of ‘changed or new’ spectacle prescriptions for people of all ages in the UK52 and, although not age-matched, supports the view that inappropriate management is unlikely to be occurring on a large scale in the PrOVIDe sample.
Inappropriate management of eye conditions and dispensing of spectacles
If inappropriate management of eye conditions had been detected, this could have resulted in a higher than expected referral rate following the PrOVIDe eye examination. The PrOVIDe referral rate was 6.7% (n = 47), higher than the national figure for people of all ages of 5% reported in 2011/12. 52 However, a higher percentage of referrals could be expected in older people as a result of the greater prevalence of eye conditions in this population.
Only nine PrOVIDe referrals were for potential cataract surgery out of a total of 209 participants with grades 2–5 cataract. Referral for possible cataract surgery in PrOVIDe was made according to the Action on Cataracts guidelines, which require three criteria to be satisfied before referral: VA is reduced as a result of the cataract; visual symptoms, as a result of the cataract, are impacting on the individual’s lifestyle; and the affected individual is willing to undergo surgery to remove the cataract. 30 Although VA was often reduced as the result of cataract (see Table 21), in the majority of cases quality of life may not have been affected sufficiently to warrant referral and/or the participant/carer was unwilling to undergo/agree to surgery. Cataract surgery was often discussed during the eye examination with the participant and/or carer, allowing the participant’s family to make an informed decision based on quality-of-life issues. The low referral rate for cataract surgery, together with the high proportion of participants with an intraocular implant in at least one eye (26.6%), suggests that there is no evidence of inappropriate management of cataract in the PrOVIDe sample. However, although cataract surgery had been carried out in over one-quarter of the sample, an unknown proportion of these participants had surgery before they developed dementia.
In summary, there is no evidence of a high proportion of inappropriate management of either spectacle prescriptions or eye conditions in the PrOVIDe sample. This conclusion must be interpreted with caution, as diagnosis and appropriate management of eye conditions were not confirmed by an ophthalmologist. One case was identified in which possible mismanagement of the spectacle prescription had occurred.
Intervals between eye examinations and spectacle dispensing
The consensus among optometrists and carers was that annual eye examinations were appropriate for most people with cognitive impairment, although some care workers thought that more frequent testing was required. In the ‘Older people and eye tests’ survey of those aged > 60 years, 53% thought that people should have annual sight tests and 32% thought that the interval should be every 2 years. 53 Annual GOS eye examinations for people aged ≥ 70 years are possible under the Department of Health’s Memorandum of Understanding50 and this age group will include the majority of those with dementia. 2 Those aged < 70 years may be entitled to an eye examination annually, or more frequently, at the practitioner’s initiative for a clinical reason. If annual eye examinations are to become the norm for all with cognitive impairment, an alteration to the Memorandum of Understanding will be required to permit this for those < 70 years.
It is estimated that 77% of PrOVIDe participants had their previous eye examination within the previous 2 years and 59% within the previous year (see Figures 9 and 10). These results are broadly similar to those from the Older People and Eye Tests survey53 (88% and 53%, respectively). In the NDNS57 a lower proportion (44.8%) of respondents reported having had an eye test in the previous 12 months. In surveys in Australia, Canada and the USA, a similar proportion (60–70%) of older adults had visited an eye-care provider, although not necessarily an optometrist, in the previous year. 142 Data from the Optical Confederation survey for 2011/12 give an average interval between eye examinations of 27 months overall and 30 months for those of working age,52 which is broadly consistent with findings in PrOVIDe (see Figures 9 and 10).
The results of two regression analyses revealed that living in residential care is independently (of age, sex, group and sMMSE score) associated with a shorter interval since the last eye examination than living at home. In addition, when the time interval since the last eye examination was added as a continuous covariate to the primary model for uncorrected/undercorrected VI (see Table 11), then for VA < 6/18 it emerged that a person living in a care home was more likely to have uncorrected/undercorrected VI than a person living in their own home, even if both had the same time interval since their last eye examination. Taken together, these two findings raise the possibility that eye examinations may be more difficult to conduct in care homes than in people’s own homes, given the current level of optometric training. However, this suggestion should be treated with caution and as a signpost to possible further research. Any future research in this area should incorporate a larger set of measured potential confounders in the time since last examination and VI model. These could include factors involved in a person’s admission to a care home, socioeconomic factors (e.g. education) and general health factors, to account for the fact that these may influence frequency of eye examinations and also influence VI. Access to records of the previous eye examination would be crucial to assessing the factors involved in the non-correction/undercorrection of VI.
A total of 154 (22.0%) PrOVIDe participants had not had an eye examination for more than 2 years. The main reasons offered in stage 2 for not attending for optometric examination were the belief that nothing was wrong with the eyes and having no problems with current spectacles, reasons that have previously been reported. 11,53 Other reasons for older people not attending for regular eye examinations included difficulties in travelling to the practitioner’s practice,53 the cost of glasses53 and the assumption that a reduction in vision is a normal process as people age. 11 The longer the intervals between examinations, the greater the changes in prescription that are likely to be found.
Concerns were expressed during interviews regarding the possibility of the unnecessary dispensing of new spectacles, particularly in care homes. In PrOVIDe, almost 40% of participants were dispensed new glasses, with approximately 10% of the remaining participants taking their prescription for spectacles which may have been made up into spectacles elsewhere. The costs of new spectacles can deter older people from accessing eye care. 53,143 There was some evidence from stage 2 that the cost of spectacles could be an issue. People with limited financial resources can get financial assistance in the form of a ‘voucher’ that can be used towards the cost of spectacles,144 although not everyone may be aware of their entitlement. Additionally, despite relatively low incomes, some older people would still not qualify for assistance.
In summary, although one-fifth of participants had not had an eye examination for > 2 years, nearly 80% of PrOVIDe participants were having regular eye examinations. However, the possibility of the sample and/or their carers being more health conscious than the norm suggests this conclusion should be treated with caution. Some concerns were expressed regarding the potential for unnecessary dispensing of spectacles, particularly in care homes.
Issues connected with wearing spectacles
Carers, particularly in care homes, noticed some people with dementia being reluctant to wear their spectacles. The reason given by interviewees was that the glasses were not needed, even for those interviewees who had successfully worn glasses before the onset of dementia. This may be an accurate assessment of the situation, as some people will have clearer vision without their spectacles, especially if their refractive error has changed or if they are wearing someone else’s glasses, and so on, but in some cases a participant’s decision that glasses are not needed is unlikely to have been made on the basis of better VA without the spectacles. There is no obvious explanation for this phenomenon, although carers suggested that short-term memory loss reinforced long-term memories of not requiring spectacles for distance, or that glasses were being shed along with other items considered unnecessary in the ‘shrinking world’ of the participant.
Missing and broken spectacles were another issue and reported to be common in care homes. Labelling spectacles with the wearer’s name, date of supply and purpose (e.g. for distance or near tasks) facilitates the return of spectacles to the rightful owner. 91 Carers can also benefit from the optometrist providing a brief report that includes the person’s visual problems, the purpose of the spectacles and a recommended date for re-examination. 129 Labelling current spectacles with names and functions would also assist optometrists when they carry out an eye examination.
Stage 2 data revealed that some people with dementia refused to wear their glasses, although the exact reasons for this were unknown. In the PrOVIDe study it could be argued that some participants classified as having uncorrected/undercorrected VI are not correctable in practical terms because they will not wear any glasses prescribed. To investigate this possibility, those cases in which the research optometrists recorded that spectacles were ‘lost’ or were ‘damaged or in poor condition’ were investigated. Broken glasses would have been recorded as ‘damaged or in poor condition’, but so too would damage that fell short of the spectacles being broken, for example very scratched lenses. The proportion of participants with damaged/poor-condition glasses in the uncorrected/undercorrected VI category was compared with the proportion in the remainder of the sample, to test if the proportion of damaged/poor-condition glasses was significantly different between the two groups.
For VI defined by the VA < 6/12 cut-off point, and for uncorrected/undercorrected VI, 6 out of 84 (7.1%, 95% CI 3.3% to 14.7%) participants had lost or damaged spectacles. Of the six participants with lost or damaged spectacles, three had lost their spectacles and the remaining three participants had damaged spectacles. For the remainder of the sample, 41 out of 624 (6.6%, 95% CI 4.9% to 8.8%) had lost or damaged spectacles.
For VI defined by the VA < 6/18 cut-off point, and for uncorrected/undercorrected VI, 4 out of 45 (8.9%, 95% CI 3.5% to 20.7%) participants had lost or damaged spectacles. Of the four participants with lost or damaged spectacles, two had lost their spectacles and the remaining two had damaged spectacles. For the remainder of the sample, 43 out of 663 (6.5%, 95% CI 4.9% to 8.6%) had lost or damaged spectacles.
There is no evidence from this analysis to suggest that the proportion of participants with lost or damaged spectacles is greater in the uncorrected/undercorrected VI group than in the remainder of the sample. However, the numbers with lost or damaged glasses are low in the uncorrected/undercorrected VI group for both the VA < 6/12 and VA < 6/18 cut-off points, so this result should be interpreted with caution.
In summary, there is the potential for people with dementia to be wearing inappropriate spectacles owing to loss, breakage or cost. Labelling spectacles with the wearer’s name and the purpose for which they should be worn can be helpful.
Expedited cataract surgery
In stage 1 of PrOVIDe, cataract of all grades, clinically significant cataract (grades 2 and above) and IOLs in one or both eyes were all common occurrences, found in 59%, 38% and 27%, respectively, of the sample. Cataract was identified in stage 1 as a major cause of VI at both the VA < 6/12 and VA < 6/18 cut-off points. As a result, the issue of when to intervene and initiate cataract surgery was a topic discussed in interviews and focus groups in stage 2, which led to consideration of the problem of capacity as cognitive impairment progresses. Participants interviewed were generally positive about cataract surgery and those who had undergone surgery reported positive outcomes. Family carers appreciated the possible benefits but had concerns regarding the physical and emotional demands on their relatives. Care workers expressed concerns about how those with more severe cognitive impairment might cope with surgery. Each focus group was asked if it would be beneficial to offer expedited cataract surgery to those with both cataract and dementia before the person’s cognitive impairment was sufficiently advanced to compromise surgery or before they lacked capacity to consent. Carers and care workers all agreed that early intervention would be beneficial; the idea was also supported by optometrists, although not unanimously.
The benefits of cataract surgery in care home residents were demonstrated in a small US study. 145 One inclusion criterion was a sMMSE score of ≥ 13 that excluded those with more severe cognitive impairment. The mean and SDs of the sMMSE scores for the intervention (21.1, SD 5.3) and control groups (19.7, SD 5.3) suggest that many of the subjects had mild to moderate cognitive impairment. Cataract surgery significantly improved short-term vision-targeted quality-of-life measures, in addition to improvements in VA. In a small-scale Japanese study of 20 subjects and 20 controls, the grade of cognitive impairment after cataract surgery improved in 12 patients (60%), was unchanged in seven patients (35%) and was worse in one patient (5%). Mean scores on the Revised Hasegawa Dementia Scale in the cataract surgery group improved from 12.5 (SD 5.3) points preoperatively to 16.6 (SD 6.2) points postoperatively; the improvement was significant (t = −5.02; p < 0.0001). 146 VI as a result of cataract may cause stress to attentional mechanisms, and cognitive performance may improve following cataract surgery in some patients with early cognitive impairment. 26 In patients with more advanced cognitive impairment the benefits of cataract extraction are less clear.
The benefits of expedited cataract extraction, as measured by the effect on falls, are more equivocal. In one UK study limited to female subjects, expedited first eye cataract extraction significantly reduced the risk of recurrent falls, the overall rate of falls and the fracture risk, although there was no significant difference in the rate of first falls. No subjects had sMMSE scores of < 15, and the distribution of sMMSE scores (median 27 in both the expedited group and controls) and range of scores (15–30 and 18–30, respectively) suggests a sample containing a minority of cognitively impaired subjects. However, the cataract extractions in the intervention group were expedited by only 11 months on average, and surgery expedited by several years could bring greater benefit. 79 A similar study investigated the effects of second eye surgery on falls, finding no significant difference for any of the measures of falls between the groups with expedited second eye cataract surgery and those remaining on the waiting list for second eye surgery. 147 Based on these studies, a Cochrane review included in its implications for practice that ‘Expedited first eye cataract surgery for people on a waiting list significantly reduces rate of falls compared with waiting list controls’. 148 Recommendations for clinical practice for the management of those at moderate or high risk of falling, which includes people with dementia, included having regular eye examinations and early referral for first eye cataract extraction as appropriate. 81
Another small-scale study investigated the effects of expediting binocular cataract extraction by 6 months in the cognitively impaired. 149 Preliminary results suggested that expedited cataract surgery in people with dementia has the potential to improve behavioural measures while decreasing neuropsychiatric symptoms and carer distress. The intervention also reduced the decline of cognitive function as measured by sMMSE scores. In summary, further research is needed into expedited first eye cataract surgery in those with cognitive impairment.
Visual impairment and behavioural and functional ability
This exploratory analysis, using ordinal logistic regression, compared data from the BADLS and CBI-R instruments for the visually impaired and non-visually impaired subsamples for both VI cut-off criteria based on presenting VA. Adjustments were made for age, sex and residential status, and the resulting ORs are estimates of the independent effect of VI on these function activities and behaviours. To compensate for the number of multiple comparisons the conservative Bonferroni correction was used, altering the cut-off p-values to 0.0025 for BADLS and to 0.0011 for the CBI-R instrument. All 20 BADLS items had ORs of > 1.0, which indicates an adverse effect of VI on each of these activities, for the VA < 6/12 criterion, and 19 out of 20 were > 1.0 for the VA < 6/18 criterion. For the VA < 6/12 criterion, ORs were significant for the ‘toilet/commode’ and ‘telephone’ activities, both of which are dependent on visual input for successful completion. For the VA < 6/18 criterion, there were no statistically significant estimated ORs for any activity.
For the CBI-R, for both the VA < 6/12 and the VA < 6/18 criteria, 75% of the 45 behaviours have ORs of > 1.0, indicating an adverse affect of VI. The only behaviour with a statistically significant OR for VI defined by VA < 6/12 was ‘has difficulties writing (letters, Christmas cards, lists, etc.)’, for which good visual performance is crucial, but there was also some evidence for a VI effect for other behaviours requiring efficient processing of visual input, namely ‘has difficulties using the telephone’, ‘has problems handling money or paying bills’ and ‘has difficulties grooming self (e.g. shaving or putting on make-up)’. No behaviours had significant ORs for the VA < 6/18 criterion, although there was some evidence for an independent VI effect on ‘has difficulties writing (letters, Christmas cards, lists, etc.)’ and for a behaviour with less obvious links to vision, ‘makes tactless or suggestive remarks’.
Visual impairment is frequently associated with an increased risk of visual hallucinations, and the prevalence of visual hallucinations in dementia is common, particularly, for example, in the Lewy body dementias. 150,151 In this context, the co-occurrence of VI and cognitive impairment/dementia is likely to be additive. An interesting future question arising out of our findings is whether or not correcting VI can lead to a clinical reduction in visual hallucinations. There is weak evidence for an adverse effect of VI on visual hallucinations from PrOVIDe data, with estimated ORs of 1.47 (95% CI 0.95 to 2.25; p = 0.08) and 1.52 (95% CI 0.90 to 2.54; p = 0.11) for the VA < 6/12 and VA < 6/18 criteria, respectively.
This preliminary investigation suggests that VI in the cognitively impaired causes deficits in some activities of daily living and behaviours that are vision-related. The association between VI and various aspects of daily living, both social and physical, is well documented. 11,45,69–71,76,145 However, people with moderate to severe cognitive impairment have often been excluded from these studies. 56,70,145 PrOVIDe uses only VA to define VI, and other aspects of visual function are known to influence visual performance, notably contrast sensitivity70,76 and visual fields. 76 Psychosocial factors, such as coping strategies and having a pessimistic attitude to life, may also affect how people with VI report on their visual function. 45 Nevertheless, the measurement of VA used in PrOVIDe has been found to be the best predictor of self-reported ‘vision-related activity limitation’. 76 Cataract surgery, which can, at a stroke, eliminate VI for some people, significantly improved vision-related quality of life in elderly patients, and cognitive impairment and depressive mental status also improved in parallel with improvement in vision-related quality of life. 152
The importance of considering each individual and their needs in terms of quality of life was commonly raised in focus groups. Although the benefits of improving VA are undeniable, there was an expressed need to weigh this against the potential risks of distress caused by an eye examination, trying to force people to wear spectacles if they do not want to, or having cataract surgery. Minimising stress during the eye examination to allow a thorough eye health assessment is important. Optometrists should take into account the carer’s perspective on what is best for the person with dementia.
In summary, an exploratory analysis found some significant deficits in vision-related aspects of function and behaviour in the visually impaired participants that can impact on quality of life. Optometrists and carers should work together to maximise quality of life for each individual.
Generalisability
The possibility of sampling bias limits, to a degree, the generalisability of the PrOVIDe findings. In group 1 particularly, the likelihood of some participants and/or their carers being more health-orientated than the general population is suggested by the high proportion of participants who had undergone a recent eye examination (see Figure 9). While it might be expected that group 2 participants would have regular eye examinations organised by their care home, it was more surprising to find this in group 1, because participants and/or carers would have to make a specific effort to organise an eye examination.
Participants in PrOVIDe were aged between 60 and 89 years at recruitment, and our results may not be generalisable to those with dementia outside this age range. However, a strength of PrOVIDe was the inclusion of the full range of cognitive impairment, including 27% of participants who either were unable to be assessed using the sMMSE or scored ≤ 9 on the sMMSE.
Although PrOVIDe participants were recruited from a range of demographic areas to encourage a heterogeneous population comprising a range of ethnicities, data on ethnicity were not collected, as the sample size would not have allowed for analysis of variations between ethnic groups.
Recruitment targets were stratified by two wide age groups and sex to reflect the age–sex distribution of the UK dementia population at the time. To achieve the overall sample recruitment target in the duration of the project, there was some over- and under-recruitment to several strata. This, and the non-ignorable proportion of unobserved distance VA and NVA within the sample (missing data), limits the generalisability of extrapolating our unadjusted prevalence estimates to the wider UK population with dementia. To obtain more plausible prevalence estimates within the sample, multiple imputation (not just for complete cases) was used. To then calibrate our prevalence estimates to the latest-available wider UK data from the Alzheimer’s Society,2 within the sample and post-stratification population calibration weights were derived and extrapolated population prevalences were calculated. We also accounted for the clustering effect of our sampling by regions and by sites within regions. Given all of this, extrapolated prevalences for distance VI and near vision loss (1) are higher in these population estimates than the PrOVIDe sample rates, and (2) have wider CIs. Feature 1 reflects the undersampling and increased difficulty in obtaining VA measures from people more likely to have worse VA. Feature 2 reflects the design effect and the large variation in calibration weights.
Chapter 6 Conclusions
Summary
This is the first large-scale investigation of VI in the dementia population in England. The overall prevalences of presenting VI were 32.5% and 16.3% for VA < 6/12 and VA < 6/18, respectively, in people with dementia aged 60–89 years. Prevalence estimates of presenting VI in those with dementia from PrOVIDe are generally higher, after adjusting for age and sex differences, than estimates from previous population studies of older people that used comparable methods and which either excluded or had low proportions of participants with dementia. There is a lack of robust comparative prevalence data for uncorrected/undercorrected VI; prevalences in PrOVIDe were 14.3% for VA < 6/12 and 7.7% for VA < 6/18. Distance acuity improved by two or more lines post refraction in 17.8% of participants.
For post-refraction VI, the element of VI that remains even with best spectacle correction, prevalences were 18.1% and 8.6% for VA < 6/12 and VA < 6/18, respectively. For VA < 6/12, almost half of this VI was the result of cataract, and is potentially remediable: approximately one-third was caused by AMD.
When extrapolated to the wider UK population with dementia, following post-stratification calibration and imputation, prevalences of VI are generally higher, with wider CIs, than the PrOVIDe sample rates. For VA < 6/12, the extrapolated prevalences for presenting, post-refraction and uncorrected/undercorrected VI were 34.6% (95% CI 29.3% to 40.3%), 22.4% (95% CI 16.4% to 29.9%) and 13.6% (95% CI 10.5% to 17.4%), respectively. For VA < 6/18, the extrapolated prevalences for presenting, post-refraction and uncorrected/undercorrected VI were 20.3% (95% CI 16.7% to 24.6%), 12.2% (95% CI 8.8% to 16.6%) and 8.3% (95% CI 5.9% to 11.6%), respectively.
While research studies rarely include substantial numbers of people with dementia living in care homes, the PrOVIDe study included 319 care home residents (group 2). The unadjusted rate ratios of presenting, uncorrected/undercorrected and post-refraction VI were all two to two-and-a-half times greater in those living in care homes, and the differences in proportions between groups 1 and 2 were statistically significant. After adjusting for age and sex (and, for uncorrected/undercorrected VI only, for sMMSE), the group effect remained statistically significant. Presenting VI prevalence in participants living in care homes was 51.4% for VA < 6/12 and 26.4% for VA < 6/18, and, for both VI criteria, > 40% of this presenting VI was correctable with spectacles.
The prevalences of presenting and post-refraction VI increased significantly with age after adjusting for sex and group for both VI criteria. However, after adjusting for sex, group and sMMSE score, there was no evidence of an independent age effect on uncorrected/undercorrected VI. There was no consistent evidence for any independent sex effect. For uncorrected/undercorrected VI for VA < 6/18, women were more likely to be in this category.
Near vision is rarely investigated in VI studies; 16.2% of PrOVIDe participants could not read standard newspaper-size print with their current spectacles; however, almost two-thirds of these participants could read standard newspaper-size print with up-to-date spectacles. After adjusting for age and sex (and, for uncorrected/undercorrected VI only, for sMMSE), there was a significant independent group effect for presenting near vision loss for both the < N8 and the < N10 criteria, for post-refraction loss for the < N8 criterion and for uncorrected/undercorrected loss for the < N10 criterion.
There was no evidence that the management of VI in people with dementia differed from that in the general population of older people. The percentage of participants advised of a change in their spectacle prescription post refraction was consistent with the national figure. In terms of the management of eye disease, if the study had identified considerable inappropriate management it could have resulted in a higher than expected referral rate following the PrOVIDe eye examination. Although the PrOVIDe referral rate was 6.7%, higher than the national figure of 5%,52 the higher percentage of referrals could be expected owing to the age range of the sample.
An exploratory ordinal logistic regression analysis found significant deficits in some vision-related aspects of function and behaviour in the visually impaired participants, compared with the non-visually impaired participants.
Key messages
The high prevalence of participants with uncorrected/undercorrected VI, the disproportionately high prevalence of VI in care home residents and the high proportion of those with VI due to potentially remediable cataract suggest that eye care for people with dementia could be enhanced by attention to the following:
-
more information about eye care for people with dementia and carers
-
better communication between optometrists and carers and between optometrists and other health-care professionals
-
tailoring the eye examination, spectacle dispensing and treatment of eye problems to meet the needs of the individual
-
providing professional development, training and guidance for optometrists.
More information about eye care for people with dementia and carers
The results have demonstrated that it is possible for optometrists to conduct most of the key components of the eye examination on > 80% of people with dementia in this study, with visual fields being the exception. The important health checks of tonometry and ophthalmoscopy were possible in > 90% of participants. Those participants unable to carry out some elements of the examination were usually people classified, using the sMMSE, as having severe cognitive impairment. However, the qualitative data suggest that some carers and care workers were unsure if people with dementia could have a full eye examination if they had difficulty answering questions. This indicates a need to increase their awareness about the purpose, scope and limitations of eye examinations in order to encourage uptake of eye examinations in line with health-care recommendations.
Better communication between optometrists and carers and between optometrists and other health-care professionals
The qualitative data revealed that communication between optometrists and those responsible for caring for people with dementia could be improved. Ensuring that optometrists know when they are dealing with someone with cognitive impairment would enable them to tailor the examination to meet the individual’s needs. This would include involving a family member whenever possible, something that family carers identified as being highly relevant. Where individuals having an eye examination are accompanied by a professional care worker, it is important that the care worker knows the individual and has the relevant information to hand; optometrists should ensure that they make time to contact the care home for further information if necessary.
Tailoring the eye examination, spectacle dispensing and treatment of eye problems to meet the needs of the individual
Improving VA, identifying possible causes of VI and referring the patient for medical intervention when necessary are the main responsibilities of the optometrist when examining an older person with cognitive impairment. However, the needs of the individual and quality-of-life issues should be taken into consideration by the attending optometrist and discussed with carers. This may impact on decisions regarding the desirability of subjecting an individual to a full eye examination, if this is likely to cause substantial distress, minimising unnecessary changes when prescribing and dispensing spectacles and possible referral for cataract surgery or other interventions.
Providing professional development, training and guidance for optometrists
The PrOVIDe study was led by the CoO, the professional, scientific and examining body for optometry in the UK, working for the public benefit. More than 70% of UK optometrists are members, which places the College in an excellent position to increase professional awareness of eye care for people with dementia by providing information and guidance and opportunities for professional development, and exploring the possibility of a mentorship scheme.
Key implications for practice
Table 43 provides a list of the key implications for practice, together with the audiences for which these implications are relevant.
| Key implication | Relevant audiences |
|---|---|
| More information about eye care for people with dementia and carers |
|
| Better communication between optometrists and carers and between optometrists and other health-care professionals |
|
| Tailoring the eye examination, spectacle dispensing and treatment of eye problems to meet the needs of the individual |
|
| Providing professional development, training and guidance for optometrists |
|
Limitations of the study (see Chapter 5 for full details)
-
Sampling bias is possible owing to quota-sampling and response bias. Some participants and their carers might have been more health-orientated than the general population. In addition, the PrOVIDe regional sample may not be fully representative of the general UK population.
-
A considerable proportion of VA assessments (≈17%) and sMMSE assessments were not available owing to a range of factors, notably poor participant co-operation, and this proportion varied across age (≈9% to 26%) and between group 1 (≈3%) and group 2 (≈33%). To improve the plausibility of our regression analysis assumptions, a multiple imputation procedure was carried out, with the results discussed in Chapter 3 (see Missing data sensitivity results). There were no changes in the substantive conclusions relating to our key tables, Tables 11, 15 and 24, under the imputation model, compared with the complete-case analysis.
-
Monocular VAs were recorded at both distance and near, both before and after the refraction. When PrOVIDe prevalence data are compared with those from studies that measured binocular VAs, this makes comparisons less robust.
-
The causes of VI in the PrOVIDe sample have mostly been identified by the examining optometrists, and not definitively made, or confirmed, by an ophthalmologist. This limitation was inherent to the study design.
Recommendations for research
Further improvements to eye care for people with dementia may be recommended depending on the results of further research. Four areas have emerged from this study with recommendations for further research:
-
development of an eye-care pathway for people with dementia
-
early intervention for cataract
-
the role of the specialist practitioner
-
eye care for other vulnerable groups.
Development of an eye-care pathway
The first recommendation is for research into the development of an eye-care pathway for people with dementia, which could be supported by local commissioners, that considers what should happen in terms of eye care when an individual is diagnosed with dementia. This could include the following questions:
-
What information do individuals and carers need to promote uptake of eye examinations?
-
How regularly should people with dementia have an eye examination? Is there a need for more frequent eye examinations than is currently advised for the general population of similar age?
-
In acknowledgement of the problems regarding spectacles for people with dementia (increased incidence of spectacles being broken or lost) should there be additional financial support for spectacle provision? For example, should there be financial subsidies to provide spectacles made from materials less likely to break?
-
What are the barriers to and facilitators of providing continuity of eye care for individuals?
-
What modifications are required to the current structure for GOS funding of sight tests in both community practice and domiciliary practice? This could include establishing minimum requirements for a health check when there are difficulties in completing a full eye examination, and providing adequate remuneration for the extra time often required for the examination of people with dementia.
-
Should the threshold for considering cataract surgery be lower for people with dementia than for the general population?
The last research question is related to the second area of research recommendations.
Early intervention for cataract
People with dementia who were interviewed for PrOVIDe said that they would have cataract surgery if needed. Carers described balancing the risks, burdens and benefits of cataract surgery against the impact on quality of life. The potential for different outcomes in decision-making depending on who is responsible suggests that it would be preferable for the decision to be made while an individual had mental capacity to decide. This gave rise to the second recommendation for research, that there should be research into the effects of early cataract intervention for people in the early stages of cognitive impairment. Should this research demonstrate benefits from early intervention, these could lead to local commissioning of expedited cataract surgery when clinically necessary.
The role of the specialist optometric practitioner
The third recommendation for research is to explore the potential of developing the role of a specialist optometric practitioner for people with dementia. This would include establishing the competencies for the role, training requirements and feasibility. Initial research should consider the possibility of insufficient interest from the optometric profession and consider if this could be accommodated within the alternative of a specialty for working with older people. Research should also explore how the role would be positioned in the current mixed economy of health-care provision; that is, would specialists be independent practitioners or employed by the NHS in hospital, or in community/domiciliary settings? If this research suggested that there is potential for the specialist optometric practitioner, this role could be developed by the CoO, who already offer higher qualifications in other specialisms (e.g. contact lenses, low vision).
Eye care for other vulnerable groups
The findings of the PrOVIDe study suggest that optometric care for people with dementia could be improved. It follows that research should also be conducted into the prevalence of undetected or uncorrected VI and the provision of optometric care for other vulnerable groups. Based on the study findings regarding the lack of awareness of domiciliary eye care, one such target group would be older people with chronic illness and disability who have difficulty accessing community-based optometric practice. Depending on the outcome of this research, local commissioners and optometric professional organisations could become involved in improving eye care for these groups.
Acknowledgements
Contributions of authors
Michael Bowen (Director of Research) was the chief investigator, led the design and development of the study, oversaw all aspects of the study, and contributed to the stage 2 focus groups, to data analysis, and to the writing and editing of the final report.
David F Edgar (Professor, Clinical Optometry) was the clinical advisor, contributed to the development and design of the study, acted as the lead for stage 1, supported stage 2 focus groups, contributed to analysis of the eye examination data (stage 1) and co-ordinated the writing of the report (stage 1).
Beverley Hancock (Researcher, Qualitative Methods) contributed to the design and development of the study, supported recruitment to both stages of the study, acted as the lead researcher for stage 2 for data collection and analysis, and was central to the writing and editing of the final report.
Sayeed Haque (Senior Lecturer, Medical Statistics) contributed to the design and development of the project, acted as a member of the Steering Group and contributed to the statistical analysis of the eye examination data.
Rakhee Shah (Optometrist) was the project manager and co-ordinated stage 1 data collection, supervised study optometrists and collected data for stage 1, conducted analysis of the eye examination data, supported stage 2 focus groups and contributed to the drafting of the final report.
Sarah Buchanan (Research Director, Sight Loss, Older People) contributed to the design and development of the project from initiation, and advised on sight loss in older people. She was a member of the Steering Group and contributed to the review of the study findings and to the final report.
Steve Iliffe (Professor, Primary Care for Older People) was a member of the Steering Group and advised on collaborations with DeNDRoN and ENRICH and research on older people. He contributed to the development and design of the study, and contributed to the review of the study findings and to the final report.
Susan Maskell (PPI representative) was a member of the Steering Group, and contributed to the design and development of the study, and the participant recruitment materials. She reviewed the study findings, contributed to the final report and provided PPI representation throughout the study.
James Pickett (Head of Research, Alzheimer’s Society) contributed to the development and design of the study, advised on Alzheimer’s disease research and data, was a member of the Steering Group, and contributed to the review of the study findings and the editing of the final report.
John-Paul Taylor (Senior Clinical Lecturer and Honorary Consultant, Old Age Psychiatry) contributed to the development and design of the study, provided clinical and research expertise, was a member of the Steering Group and contributed to the review of the study findings and to the final report.
Neil O’Leary (Statistician and Research Fellow) contributed to the statistical analysis of the eye examination data and provided additional statistical advice and support.
The study team is particularly grateful to Neil O’Leary and Sayeed Haque for their extensive additional work to support the team in responding to peer-review comments and with the additional analyses conducted to address feedback at all stages of the reporting process.
Additional acknowledgements
Michael Clarke (Consultant Ophthalmologist and Honorary Lecturer, Ophthalmology, Newcastle Eye Centre, Royal Victoria Infirmary, Newcastle upon Tyne, UK) was a member of the Steering Group and provided ophthalmological expertise.
Dr Lesley Hall (National Portfolio Manager for Non-Commercial Studies, DeNDRoN) provided advice and support on study set-up and recruitment to stage 1.
We are most grateful to Mr Damian Kenning, Managing Director of TOC, and his dedicated staff (optometrists and head office team) for their generous provision of services throughout stage 1 data collection.
Professor David Thomson (Thomson Software Solutions, Welham Green, UK) kindly loaned four versions of Test Chart 2000 for use in the early stages of the study.
Mr RM Pearson kindly gave permission for his Optometric Grading Scales to be used in the study for the grading of cataract.
The Support Team at the CoO, Rosa Pepe (Project Co-ordinator), Martin Cordiner (Head of Research), Martha Dankwa (Research Administrator) and Marilyn Spidell (Research Administrator), provided invaluable support with project co-ordination and the administrative and managerial processes that are vital in delivering a study from start to end.
Finally, a special thanks to everyone who participated in the study.
Publication
Bowen M, Edgar DF, Hancock B, Haque S, Shah R, Buchanan S, et al. A proposal for a UK Dementia Eye Care Pathway. Optom Pract 2015;16:71–6.
Data sharing statement
The PrOVIDe study has generated a rich data set. The PrOVIDe team is keen to support the principles of good practice with regard to managing and sharing these data for the benefit of the scientific community and in accordance with NIHR recommendations.
For research involving samples or information from human participants, data must be managed and shared in a way that safeguards the confidentiality and anonymity of participants and is consistent with the terms of consent signed by participants. Within these constraints the PrOVIDe team would welcome applications from researchers to access PrOVIDe data. As no suitable national or local repository exists, the data are lodged with the CoO, and are held and managed in accordance with the terms set out in the original protocol and the College’s own data protection policies.
Information about accessing PrOVIDe data and about the way the data are held and managed is available by contacting the corresponding author, Michael Bowen, at the CoO: michael.bowen@college-optometists.org or researchteam@college-optometrists.org.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405-12. http://dx.doi.org/10.1016/S0140-6736(13)61570-6.
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK: Update. London: Alzheimer’s Society; 2014.
- 2011 Census – Population and Household Estimates for England and Wales, March 2011. London: Office for National Statistics; 2012.
- Luengo-Fernandez R, Leal J, Gray A. Dementia 2010. Oxford: Alzheimer’s Research Trust, University of Oxford; 2010.
- Future Sight Loss UK (1): The Economic Impact of Partial Sight and Blindness in the UK Population. London: RNIB; 2009.
- Royal National Institute of Blind People . Sight Loss UK 2013: The Last Evidence 2013. www.rnib.org.uk/sites/default/files/Sight_loss_UK_2013.pdf (accessed 4 October 2015).
- Evans J, Wormald R. Prevalence and Causes of Blindness and Low Vision in the UK from 2010 to 2020: Modelling the Impact of Incorporating Estimates of Vision Loss in Non-Community Dwelling Adults. London: Vision 2020 UK; 2011.
- McKeefry D, Bartlett R. Improving Vision and Eye Health Care to People with Dementia. Thomas Pocklington Trust; 2010.
- Chapman FM, Dickinson J, McKeith I, Ballard C. Association among visual hallucinations, visual acuity, and specific eye pathologies in Alzheimer’s disease: treatment implications. Am J Psychiatry 1999;156:1983-5.
- Jones RW, Trigg R. Dementia and Serious Sight Loss. Chiswick: Thomas Pocklington Trust; 2007.
- Evans BJ, Rowlands G. Correctable visual impairment in older people: a major unmet need. Ophthalmic Physiol Opt 2004;24:161-80. http://dx.doi.org/10.1111/j.1475-1313.2004.00197.x.
- International Classification of Diseases. Geneva: WHO; 1990.
- All Vision Impairment. Bethesda, MD: National Eye Institute; n.d.
- Department of Health . Certificate of Vision Impairment: Explanatory Notes for Consultant Ophthalmologists and Hospital Eye Clinic Staff n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/213286/CVI-Explanatory-notes-in-DH-template.pdf (accessed 4 October 2015).
- Evans J. Causes of Blindness and Partial Sight in England and Wales 1990–1. London: Office of Population Census and Surveys; 1995.
- Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England & Wales. BMC Public Health 2006;6. http://dx.doi.org/10.1186/1471-2458-6-58.
- Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844-51. http://dx.doi.org/10.1016/j.ophtha.2012.10.036.
- Owen CG, Fletcher AE, Donoghue M, Rudnicka AR. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom?. Br J Ophthalmol 2003;87:312-17. http://dx.doi.org/10.1136/bjo.87.3.312.
- Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 2012;96:752-6. http://dx.doi.org/10.1136/bjophthalmol-2011-301109.
- Minassian D, Reidy A. Future Sight Loss in the Decade 2010 to 2020: An Epidemiological and Economic Model. Buckinghamshire: Epivision and RNIB; 2009.
- The Royal College of Ophthalmologists . Cataract Surgery Guidelines 2010. www.rcophth.ac.uk/wp-content/uploads/2014/12/2010-SCI-069-Cataract-Surgery-Guidelines-2010-SEPTEMBER-2010.pdf (accessed 4 October 2015).
- Pesudovs K, Elliott DB. Refractive error changes in cortical, nuclear, and posterior subcapsular cataracts. Br J Ophthalmol 2003;87:964-7. http://dx.doi.org/10.1136/bjo.87.8.964.
- Rabbetts RB. Cataract and the nodal point fallacy. Optometry Pract 2009;10:167-70.
- Tan AG, Wang JJ, Rochtchina E, Mitchell P. Comparison of age-specific cataract prevalence in two population-based surveys 6 years apart. BMC Ophthalmol 2006;6. http://dx.doi.org/10.1186/1471-2415-6-17.
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, et al. The Framingham eye study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–5. Surv Ophthalmol 1980;24:335-610.
- Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol 2011;95:17-23. http://dx.doi.org/10.1136/bjo.2009.165902.
- Reidy A, Minassian DC, Vafidis G, Joseph J, Farrow S, Wu J, et al. Prevalence of serious eye disease and visual impairment in a north London population: population based, cross sectional study. BMJ 1998;316:1643-6. http://dx.doi.org/10.1136/bmj.316.7145.1643.
- The NHS Atlas of Variation in Healthcare. London: Department of Health; 2011.
- Slade J. Eye Health Data Summary. London: UK Vision Strategy; 2014.
- Action on Cataracts – Good Practice Guidance. London: NHS; 2000.
- Diabetic Retinopathy Guidelines. London: Royal College of Ophthalmologists; 2012.
- Younis N, Broadbent DM, Harding SP, Vora JR. Prevalence of diabetic eye disease in patients entering a systematic primary care-based eye screening programme. Diabet Med 2002;19:1014-21. http://dx.doi.org/10.1046/j.1464-5491.2002.00854.x.
- Screening for Diabetic Retinopathy. London: NHS; 2014.
- Kelliher C, Kenny D, O’Brien C. Trends in blind registration in the adult population of the Republic of Ireland 1996–2003. Br J Ophthalmol 2006;90:367-71. http://dx.doi.org/10.1136/bjo.2005.075861.
- Bamashmus MA, Matlhaga B, Dutton GN. Causes of blindness and visual impairment in the West of Scotland. Eye 2004;18:257-61. http://dx.doi.org/10.1038/sj.eye.6700606.
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363:1711-20. http://dx.doi.org/10.1016/S0140-6736(04)16257-0.
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996;80:389-93. http://dx.doi.org/10.1136/bjo.80.5.389.
- Azuara-Blanco A, Burr J, Thomas R, Maclennan G, McPherson S. The accuracy of accredited glaucoma optometrists in the diagnosis and treatment recommendation for glaucoma. Br J Ophthalmol 2007;91:1639-43. http://dx.doi.org/10.1136/bjo.2007.119628.
- Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci 2006;47:4254-61. http://dx.doi.org/10.1167/iovs.06-0299.
- Burr JM, Mowatt G, Hernandez R, Siddiqui MA, Cook J, Lourenco T, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11410.
- Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 1996;103:1661-9. http://dx.doi.org/10.1016/S0161-6420(96)30449-1.
- Bowling B, Chen SD, Salmon JF. Outcomes of referrals by community optometrists to a hospital glaucoma service. Br J Ophthalmol 2005;89:1102-4. http://dx.doi.org/10.1136/bjo.2004.064378.
- Fraser S, Bunce C, Wormald R. Risk factors for late presentation in chronic glaucoma. Invest Ophthalmol Vis Sci 1999;40:2251-7.
- Jack CI, Smith T, Neoh C, Lye M, McGalliard JN. Prevalence of low vision in elderly patients admitted to an acute geriatric unit in Liverpool: elderly people who fall are more likely to have low vision. Gerontology 1995;41:280-5. http://dx.doi.org/10.1159/000213695.
- Tate R, Smeeth L, Evans J, Fletcher A. The Prevalence of Visual Impairment in the UK: A Review of Literature. London: RNIB; 2005.
- Park JC, Ross AH, Tole DM, Sparrow JM, Penny J, Mundasad MV. Evaluation of a new cataract surgery referral pathway. Eye 2009;23:309-13. http://dx.doi.org/10.1038/sj.eye.6703075.
- Parkins DJ, Edgar DF. Comparison of the effectiveness of two enhanced glaucoma referral schemes. Ophthalmic Physiol Opt 2011;31:343-52. http://dx.doi.org/10.1111/j.1475-1313.2011.00853.x.
- Ratnarajan G, Newsom W, Vernon SA, Fenerty C, Henson D, Spencer F, et al. The effectiveness of schemes that refine referrals between primary and secondary care – the UK experience with glaucoma referrals: the Health Innovation & Education Cluster (HIEC) Glaucoma Pathways Project. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002715.
- Sheen NJ, Fone D, Phillips CJ, Sparrow JM, Pointer JS, Wild JM. Novel optometrist-led all Wales primary eye-care services: evaluation of a prospective case series. Br J Ophthalmol 2009;93:435-8. http://dx.doi.org/10.1136/bjo.2008.144329.
- Federation of Ophthalmic & Dispensing Opticians . Memorandum of Understanding: Frequency of GOS Sight Tests 2002. www.aop.org.uk/uploads/uploaded_files/GOS/memorandum_on_frequencies.pdf (accessed 4 October 2015).
- Health and Social Care Information Centre . General Ophthalmic Services, Activity Statistics: England 2012 13 2013. www.hscic.gov.uk/catalogue/PUB11233/gene-opht-serv-acti-eng-12–13-repo.pdf (accessed 4 October 2015).
- Optics at a Glance 2012. London: Optical Confederation; 2013.
- Conway L, McLaughlan B. Older People and Eye Tests: Don’t Let Age Rob You of Your Sight. London: RNIB; 2007.
- Keller BK, Hejkal T, Potter JF. Barriers to vision care for nursing home residents. J Am Med Dir Assoc 2001;2:15-21. http://dx.doi.org/10.1016/S1525-8610(04)70148-6.
- Shah R, Evans BJ, Edgar D. A survey of the availability of state-funded primary eye care in the UK for the very young and very old. Ophthalmic Physiol Opt 2007;27:473-81. http://dx.doi.org/10.1111/j.1475-1313.2007.00506.x.
- Evans JR, Fletcher AE, Wormald RP, Ng ES, Stirling S, Smeeth L, et al. Prevalence of visual impairment in people aged 75 years and older in Britain: results from the MRC trial of assessment and management of older people in the community. Br J Ophthalmol 2002;86:795-800. http://dx.doi.org/10.1136/bjo.86.7.795.
- van der Pols JC, Bates CJ, McGraw PV, Thompson JR, Reacher M, Prentice A, et al. Visual acuity measurements in a national sample of British elderly people. Br J Ophthalmol 2000;84:165-70. http://dx.doi.org/10.1136/bjo.84.2.165.
- Wormald RP, Wright LA, Courtney P, Beaumont B, Haines AP. Visual problems in the elderly population and implications for services. BMJ 1992;304:1226-9. http://dx.doi.org/10.1136/bmj.304.6836.1226.
- Lavery JR, Gibson JM, Shaw DE, Rosenthal AR. Vision and visual acuity in an elderly population. Ophthalmic Physiol Opt 1988;8:390-3. http://dx.doi.org/10.1111/j.1475-1313.1988.tb01174.x.
- Horowitz A, Reinhardt JP, Boerner K. The effect of rehabilitation on depression among visually disabled older adults. Aging Ment Health 2005;9:563-70. http://dx.doi.org/10.1080/13607860500193500.
- Taylor HR, Livingston PM, Stanislavsky YL, McCarty CA. Visual impairment in Australia: distance visual acuity, near vision, and visual field findings of the Melbourne Visual Impairment Project. Am J Ophthalmol 1997;123:328-37. http://dx.doi.org/10.1016/S0002-9394(14)70128-X.
- Wang JJ, Foran S, Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains Eye Study. Clin Experiment Ophthalmol 2000;28:268-73. http://dx.doi.org/10.1046/j.1442-9071.2000.00315.x.
- Muñoz B, West SK, Rubin GS, Schein OD, Quigley HA, Bressler SB, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol 2000;118:819-25. http://dx.doi.org/10.1001/archopht.118.6.819.
- Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol 1998;116:653-8. http://dx.doi.org/10.1001/archopht.116.5.653.
- Tielsch JM, Sommer A, Witt K, Katz J, Royall RM. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol 1990;108:286-90. http://dx.doi.org/10.1001/archopht.1990.01070040138048.
- Owsley C, McGwin G, Scilley K, Meek GC, Dyer A, Seker D. The visual status of older persons residing in nursing homes. Arch Ophthalmol 2007;125:925-30. http://dx.doi.org/10.1001/archopht.125.7.925.
- Binns AM, Bunce C, Dickinson C, Harper R, Tudor-Edwards R, Woodhouse M, et al. How effective is low vision service provision? A systematic review. Surv Ophthalmol 2012;57:34-65. http://dx.doi.org/10.1016/j.survophthal.2011.06.006.
- La Plante MP, Carlson D. Disability in the United States: Prevalence and Causes, 1992. Washington, DC: Disability Statistics Rehabilitation Research and Training Centre, Institute of Age and Aging; 1996.
- Wang C-W, Chan CLW, Chi I. Overview of quality of life research in older people with visual impairment. Adv Aging Res 2014;3:79-94. http://dx.doi.org/10.4236/aar.2014.32014.
- West SK, Rubin GS, Broman AT, Muñoz B, Bandeen-Roche K, Turano K. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol 2002;120:774-80. http://dx.doi.org/10.1001/archopht.120.6.774.
- McManus S, Lord C. Circumstances of People with Sight Loss: Secondary Analysis of Understanding Society and Life Opportunities Survey. London: RNIB; 2012.
- Margrain TH, Nollett C, Smith DJ. Depression and vision loss in aging. Optometry Pract 2012;13:77-84.
- Brody BL, Gamst AC, Williams RA, Smith AR, Lau PW, Dolnak D, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology 2001;108:1893-900. http://dx.doi.org/10.1016/S0161-6420(01)00754-0.
- DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition-Text Revision. Washington D.C.: APA; 2000.
- Evans JR, Fletcher AE, Wormald RP. Depression and anxiety in visually impaired older people. Ophthalmology 2007;114:283-8. http://dx.doi.org/10.1016/j.ophtha.2006.10.006.
- Tabrett DR, Latham K. Factors influencing self-reported vision-related activity limitation in the visually impaired. Invest Ophthalmol Vis Sci 2011;52:5293-302. http://dx.doi.org/10.1167/iovs.10-7055.
- Depression in Adults with a Chronic Physical Health Problem: Treatment and Management. London: NICE; 2009.
- NICE Clinical Guideline 161 Falls: Assessment and Prevention of Falls in Older People. London: NICE; 2013.
- Harwood RH, Foss AJ, Osborn F, Gregson RM, Zaman A, Masud T. Falls and health status in elderly women following first eye cataract surgery: a randomised controlled trial. Br J Ophthalmol 2005;89:53-9. http://dx.doi.org/10.1136/bjo.2004.049478.
- Salonen L, Kivelä SL. Eye diseases and impaired vision as possible risk factors for recurrent falls in the aged: a systematic review. Curr Gerontol Geriatr Res 2012;2012. http://dx.doi.org/10.1155/2012/271481.
- Elliott DB. Falls and visual impairment: guidance for the optometrist. Optometry Pract 2012;13:65-76.
- Yamashita T, Noe DA, Bailer AJ. Risk factors of falls in community-dwelling older adults: logistic regression tree analysis. Gerontologist 2012;52:822-32. http://dx.doi.org/10.1093/geront/gns043.
- Close J, Ellis M, Hooper R, Glucksman E, Jackson S, Swift C. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet 1999;353:93-7. http://dx.doi.org/10.1016/S0140-6736(98)06119-4.
- National Service Framework for Older People. London: Department of Health; 2001.
- Focus on Falls. London: CoO; 2014.
- Kotecha A, Chopra R, Fahy RT, Rubin GS. Dual tasking and balance in those with central and peripheral vision loss. Invest Ophthalmol Vis Sci 2013;54:5408-15. http://dx.doi.org/10.1167/iovs.12-12026.
- Cox A, Blaikie A, Macewen CJ, Jones D, Thompson K, Holding D, et al. Optometric and ophthalmic contact in elderly hip fracture patients with visual impairment. Ophthalmic Physiol Opt 2005;25:357-62. http://dx.doi.org/10.1111/j.1475-1313.2005.00307.x.
- Morrison-Fokken A, Dunne M. Falls risk assessment in optometric practice: an introduction to non-visual and visual risk factors. Optometry Pract 2014;15:39-4.
- Vandenbroucke JP, von EE, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLOS Med 2007;4. http://dx.doi.org/10.1371/journal.pmed.0040297.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Examining the Patient with Dementia and Other Acquired Cognitive Impairment. London: CoO; 2012.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing 1996;25:113-20. http://dx.doi.org/10.1093/ageing/25.2.113.
- Wear HJ, Wedderburn CJ, Mioshi E, Williams-Gray CH, Mason SL, Barker RA, et al. The Cambridge Behavioural Inventory revised. Dement Neuropsychol 2002;2:102-7.
- Pakrou N, Gray T, Mills R, Landers J, Craig J. Clinical comparison of the Icare tonometer and Goldmann applanation tonometry. J Glaucoma 2008;17:43-7. http://dx.doi.org/10.1097/IJG.0b013e318133fb32.
- Elliott DB. Clinical Procedures in Primary Eye Care. London: Butterworth Heinemann Elsevier; 2007.
- Pandit RJ, Taylor R. Mydriasis and glaucoma: exploding the myth. A systematic review. Diabet Med 2000;17:693-9. http://dx.doi.org/10.1046/j.1464-5491.2000.00368.x.
- Chylack LT, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993;111:831-6. http://dx.doi.org/10.1001/archopht.1993.01090060119035.
- Pearson RM. Optometric grading scales: for use in everyday practice. Optometry Today 2003:39-42.
- Kitzinger J. Qualitative research. Introducing focus groups. BMJ 1995;311:299-302. http://dx.doi.org/10.1136/bmj.311.7000.299.
- Kruger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research. Thousand Oaks, CA: Sage Publications; 2000.
- Morse J. Determining sample size. Qual Health Res 2000;10:3-5. http://dx.doi.org/10.1177/104973200129118183.
- Rubin DB, Little RJA. Statistical Analysis with Missing Data. New York, NY: Wiley; 2002.
- Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Sim 2006;76:1049-64. http://dx.doi.org/10.1080/10629360600810434.
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681-94. http://dx.doi.org/10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R.
- Kish L. Survey Sampling. Oxford: Wiley; 1995.
- Deville J-C, Sarndal C-E, Sautory O. Generalized raking procedures in survey sampling. JASA 1993;88:1013-20. http://dx.doi.org/10.1080/01621459.1993.10476369.
- Korn EL, Graubard BI. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Survey Methodol 1998;23:193-201.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J Wiley & Sons; 2004.
- Lumley T. Analysis of complex survey samples. J Stat Softw 2004;9:1-19. http://dx.doi.org/10.18637/jss.v009.i08.
- Ritchie J, Spencer L, Brymann A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993;269:2386-91. http://dx.doi.org/10.1001/jama.1993.03500180078038.
- Friedman JH. A Variable Span Scatterplot Smoother. Stanford, CA: Stanford University Technical Laboratory for Computational Statistics; 1984.
- Heitjan D, Rubin D. Ignorability and coarse data. Ann Stat 1991;19:2244-53. http://dx.doi.org/10.1214/aos/1176348396.
- Pierscionek TJ, Moore JE, Pierscionek BK. Referrals to ophthalmology: optometric and general practice comparison. Ophthalmic Physiol Opt 2009;29:32-40. http://dx.doi.org/10.1111/j.1475-1313.2008.00614.x.
- Theodossiades J, Murdoch I. Positive predictive value of optometrist-initiated referrals for glaucoma. Ophthalmic Physiol Opt 1999;19:62-7. http://dx.doi.org/10.1046/j.1475-1313.1999.00410.x.
- Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension. London: NICE; 2009.
- Sturgess I, Rudd AG, Shilling J. Unrecognized visual problems amongst residents of Part III Homes. Age Ageing 1994;23:54-6. http://dx.doi.org/10.1093/ageing/23.1.54.
- Mitchell P, Hayes P, Wang JJ. Visual impairment in nursing home residents: the Blue Mountains Eye Study. Med J Aust 1997;166:73-6.
- Coleman AL, Stone K, Ewing SK, Nevitt M, Cummings S, Cauley JA, et al. Higher risk of multiple falls among elderly women who lose visual acuity. Ophthalmology 2004;111:857-62. http://dx.doi.org/10.1016/j.ophtha.2003.09.033.
- Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci 2013;54:ORSF5-13.
- Augood CA, Vingerling JR, de Jong PT, Chakravarthy U, Seland J, Soubrane G, et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE). Arch Ophthalmol 2006;124:529-35. http://dx.doi.org/10.1001/archopht.124.4.529.
- Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol 2004;122:487-94. http://dx.doi.org/10.1001/archopht.122.4.487.
- Diabetes UK . Diabetes: Facts and Stats Version 3 2014. www.diabetes.org.uk/Documents/About%20Us/Statistics/Diabetes-key-stats-guidelines-April2014.pdf (accessed 4 October 2015).
- Busse A, Sonntag A, Bischkopf J, Matschinger H, Angermeyer MC. Adaptation of dementia screening for vision-impaired older persons: administration of the Mini-Mental State Examination (MMSE). J Clin Epidemiol 2002;55:909-15. http://dx.doi.org/10.1016/S0895-4356(02)00449-3.
- Hazel CA, Elliott DB. The dependency of logMAR visual acuity measurements on chart design and scoring rule. Optom Vis Sci 2002;79:788-92. http://dx.doi.org/10.1097/00006324-200212000-00011.
- Wolffsohn JS, Cochrane AL. The practical near acuity chart (PNAC) and prediction of visual ability at near. Ophthalmic Physiol Opt 2000;20:90-7. http://dx.doi.org/10.1016/S0275-5408(99)00035-6.
- Lovie-Kitchin J. Reading with low vision: the impact of research on clinical management. Clin Exp Optom 2011;94:121-32. http://dx.doi.org/10.1111/j.1444-0938.2010.00565.x.
- Rashid K, Sheppard A. Domiciliary eye care-the practitioner’s perspective. Optometry Today 2013.
- Guidance for Professional Practice: The Domiciliary Eye examination. London: College of Optometrists; 2015.
- Myint J, Edgar DF, Murdoch IE, Lawrenson JG. The impact of postgraduate training on UK optometrists’ clinical decision-making in glaucoma. Ophthalmic Physiol Opt 2014;34:376-84. http://dx.doi.org/10.1111/opo.12126.
- Needle JJ, Petchey R, Lawrenson JG. A survey of the scope of therapeutic practice by UK optometrists and their attitudes to an extended prescribing role. Ophthalmic Physiol Opt 2008;28:193-20. http://dx.doi.org/10.1111/j.1475-1313.2008.00551.x.
- Marks JR, Harding AK, Harper RA, Williams E, Haque S, Spencer AF, et al. Agreement between specially trained and accredited optometrists and glaucoma specialist consultant ophthalmologists in their management of glaucoma patients. Eye (Lond) 2012;26:853-61. http://dx.doi.org/10.1038/eye.2012.58.
- Syam P, Rughani K, Vardy SJ, Rimmer T, Fitt A, Husain T, et al. The Peterborough scheme for community specialist optometrists in glaucoma: a feasibility study. Eye 2010;24:1156-64. http://dx.doi.org/10.1038/eye.2009.327.
- Myint J, Edgar DF, Kotecha A, Crabb DP, Lawrenson JG. Development of a competency framework for optometrists with a specialist interest in glaucoma. Eye 2010;24:1509-14. http://dx.doi.org/10.1038/eye.2010.62.
- College of Optometrists . Higher Qualifications Regulations (Legacy) 2014. www.college-optometrists.org/en/utilities/document-summary.cfm?docid=BA6614EB-AEF3–4216–82EABD8AF31A7969 (accessed 4 October 2015).
- The National Health Service (General Ophthalmic Services) (Scotland) Regulations 2006 with Amendments. London: The Stationery Office; 2012.
- Department of Health . The General Ophthalmic Services Contracts (Payments) Directions n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/327623/payment_directions_2014.pdf (accessed 4 October 2015).
- Bosanquet N. Liberating the NHS: Eye Care, Making a Reality of Equity and Excellence. London: Imperial College London; 2014.
- MacKenzie GE. Reproducibility of sphero-cylindrical prescriptions. Ophthalmic Physiol Opt 2008;28:143-50. http://dx.doi.org/10.1111/j.1475-1313.2008.00549.x.
- Shah R, Edgar DF, Rabbetts R, Harle DE, Evans BJ. Standardized patient methodology to assess refractive error reproducibility. Optom Vis Sci 2009;86:517-28. http://dx.doi.org/10.1097/OPX.0b013e31819fa590.
- Vela C, Samson E, Zunzunegui MV, Haddad S, Aubin MJ, Freeman EE. Eye care utilization by older adults in low, middle, and high income countries. BMC Ophthalmol 2012;12. http://dx.doi.org/10.1186/1471-2415-12-5.
- Smeeth L. Assessing the likely effectiveness of screening older people for impaired vision in primary care. Fam Pract 1998;15:24-9.
- Jessa Z, Evans BJ, Thomson DW, Rowlands G. Provision of NHS-funded spectacles in South London. Ophthalmic Physiol Opt 2009;29:641-7. http://dx.doi.org/10.1111/j.1475-1313.2009.00686.x.
- Owsley C, McGwin G, Scilley K, Meek GC, Seker D, Dyer A. Impact of cataract surgery on health-related quality of life in nursing home residents. Br J Ophthalmol 2007;91:1359-63. http://dx.doi.org/10.1136/bjo.2007.118547.
- Tamura H, Tsukamoto H, Mukai S, Kato T, Minamoto A, Ohno Y, et al. Improvement in cognitive impairment after cataract surgery in elderly patients. J Cataract Refract Surg 2004;30:598-602. http://dx.doi.org/10.1016/j.jcrs.2003.10.019.
- Foss AJ, Harwood RH, Osborn F, Gregson RM, Zaman A, Masud T. Falls and health status in elderly women following second eye cataract surgery: a randomised controlled trial. Age Ageing 2006;35:66-71. http://dx.doi.org/10.1093/ageing/afj005.
- Interventions for Preventing Falls in Older People Living in the Community (Review). London: John Wiley & Sons; 2013.
- Lerner AJ, Debanne SM, Belkin JK, Lass JH, Ogrocki PK, Riedel TM, et al. Visual and cognitive improvement following cataract surgery in subjects with dementia. Alzheimers Dement 2014;10:456-7. http://dx.doi.org/10.1016/j.jalz.2014.05.630.
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007;22:1689-707. http://dx.doi.org/10.1002/mds.21507.
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863-72. http://dx.doi.org/10.1212/01.wnl.0000187889.17253.b1.
- Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am J Ophthalmol 2008;146:404-9. http://dx.doi.org/10.1016/j.ajo.2008.05.014.
- About INVOLVE. 2015.
Appendix 1 Annotated Strengthening the Reporting of Observational Studies in Epidemiology checklist
The comments in the right-hand column of the checklist below give detail of compliance of the PrOVIDe study with the STROBE criteria.
| Item | Item number | Recommendation | PrOVIDe compliance |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | (a) Compliant: the cross-sectional design is included in both the title and the abstract |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | (b) Compliant | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | Compliant: pages xxiii, xxv and 1–16 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | Compliant: page 16, Aims and objectives |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | Compliant: page 17, Methods |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up and data collection | Compliant: pages 17–27 |
| Participants | 6 | Cross-sectional study: give the eligibility criteria, and the sources and methods of selection of participants | Compliant: pages 19–20, Recruitment of participants |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | Compliant, where applicable: pages 20–8 and, for additional diagnostc criteria, page 41 |
| Data sources/measurement | 8a | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | Compliant: pages 20–7, for Stage 1 (quantitative study) Data collection and pages 27–8 for Stage 2 (qualitative study) |
| Bias | 9 | Describe any efforts to address potential sources of bias | Compliant: page 17 (England-wide recruitment), pages 19–20 (inclusion of participants who lacked mental capacity to consent), pages 33–5 (matching sample profile with UK population with dementia), pages 29–30 (logistic regression was used to control for covariates), pages 30–1 (missing data plan), pages 68–70 (use of sMMSE blind) |
| Study size | 10 | Explain how the study size was arrived at | Compliant: pages 18–19 (Sample size) |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | Compliant: pages 20–7 (Stage 1 Data collection), pages 37–40 (Levels of cognitive impairment in the sample), page 41 (Prevalence of visual impairment) |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | (a) Compliant: pages 28–32 (Stage 1 Data analysis plan) |
| (b) Describe any methods used to examine subgroups and interactions | (b) None | ||
| (c) Explain how missing data were addressed | (c) Compliant: pages 30–1 (Missing data plan) | ||
| (d) Cross-sectional study: if applicable, describe analytical methods taking account of sampling strategy | (d) All prevalence estimates and their inferences were adjusted for the two-stage sample design and for the deviation of age, sex and residence distributions in the recruited sample from those of the target population. Page 31 (Extrapolating prevalence to the UK dementia population) | ||
| (e) describe any sensitivity analyses | (e) Compliant: pages 30–1 (Missing data plan) | ||
| Results | |||
| Participants | 13a | (a) Report numbers of individuals at each stage of study, e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up and analysed | (a) Compliant: pages 33–7 (Recruitment) (notably Figure 3) and pages 41–3 (notably Figures 6 and 7) |
| (b) Give reasons for non-participation at each stage | (b) Compliant: pages 33–7 (Recruitment), pages 41–3 (Prevalence of visual impairment) and pages 57–8 (Reasons for not dilating) | ||
| (c) Consider use of a flow diagram | (c) Compliant: see flow diagrams in Figures 1, 3, 6 and 7 | ||
| Descriptive data | 14a | (a) Give characteristics of study participants (e.g. demographic, clinical, social) and information on exposures and potential confounders | (a) Compliant: pages 33–40 (Demographics and characteristics of the sample). There is no exposure data in our data set. We had no access to social data |
| (b) Indicate number of participants with missing data for each variable of interest | (b) Compliant: Tables 5–10, pages 63–4 (Factors associated with near vision loss) | ||
| Outcome data | 15 | Cross-sectional study: report numbers of outcome events or summary measures | Compliant. The summary measures (counts) of all important outcome measures are given in Tables 7–10 (distance visual impairment), Table 12 (eye conditions) and Tables 21 and 22 (near vision loss) |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g. 95% CI). Make clear which confounders were adjusted for and why they were included | (a) Compliant. All estimates are available in the report in both unadjusted and adjusted (for age, sex and where appropriate sMMSE) ORs |
| (b) Report category boundaries when continuous variables were categorised | Compliant. For example, Table 5 (cognitive impairment categorised by sMMSE score), Table 8 (variations in VI prevalence with age), Tables 13 and 14 (prevalence of eye conditions with age), etc. | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | N/A | ||
| Other analyses | 17 | Report other analyses done, e.g. analyses of subgroups and interactions, and sensitivity analyses | Compliant: pages 30–31 (Missing data plan) for details of sensitivity analyses |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | Compliant: pages 137–9 (Summary) |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision Discuss both direction and magnitude of any potential bias |
Compliant: pages 114–115 (Limitations of the study) |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | Compliant: pages 116–134 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | Compliant: pages 134–135 (Generalisability) |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | Compliant: NIHR |
Appendix 2 Stage 2 focus group question schedules
Focus group for family carers
1. Introductions
Going around the group, can I ask you to introduce yourself, giving the name that you would like group members to use when addressing you?
2. Opening question
(‘Round Robin’ style question designed to be answered by all participants. The question should be something that everyone can answer.)
As you know, this research study is about visual impairment in people with dementia. But before we get onto that I’d like to start with a general question about eye examinations. I’ll go around the group again to ask:
-
How often do you think people should have their eyes tested?
(Reinforce that the question is to find out opinions, not to identify a right answer or to find out who is right and who is wrong.)
3. Follow-up
If the national recommendation was not identified by any group participant in response to question 2, explain what this is. Then ask the group for their views on this recommendation.
4–8. Key questions
Explain that I won’t go around the whole group in turn for the remaining questions that they can answer if and when they would like to.
4. I’d like to move on now to ask you some questions about eye care and the person with dementia that you care for:
-
Did they have regular eye tests before the dementia started?
[Follow up to find out if they still have eye tests, how regularly and when the last test was (before participation in stage 1 of this study.]
5. I’d like to hear about your experiences of your friend or relative’s eye tests:
-
Has anyone had any problems finding an optometrist to do the test?
-
Were you present at the test and if so do you remember/were you aware of any difficulties in conducting the eye test?
6. If the person you care for has an eye problem and you are willing to share this information with the group:
-
Did you know about the eye problem before they developed dementia?
-
As far as you are aware, has the dementia affected the management of the eye problem?
7. From my own experience I know that there are all sorts of things that are important when you are caring for people with dementia. Like making sure they have enough to eat and drink, continence, safety, and so on:
-
How important is it that people with dementia have their eyes tested?
-
If they need glasses, how important is it for them to wear them?
8. If your friend or relative needed hospital treatment for an eye problem, for example they had a cataract and removing it would help them to see better, how would you feel about them having that treatment?
9–10. Summary questions
9. I’ve covered the main things that I wanted to ask you about:
-
Is there anything that you would like to raise that hasn’t been covered?
10. Before we end, I have one final question. I’ll go around the group so that everyone has a chance to answer if you would like to:
-
If you could say one thing about eye care for people with dementia, that you would like health-care providers to hear, what would that be?
Focus group for care workers
1. Introductions
Going around the group, can I ask you to introduce yourself, giving the name that you would like group members to use when addressing you?
2. Opening question
(‘Round Robin’ style question designed to be answered by all participants. The question should be something that everyone can answer.)
As you know, this research study is about visual impairment in people with dementia. But before we get onto that I’d like to start with a general question about eye examinations. I’ll go around the group again to ask:
-
How often do you think people should have their eyes tested?
(Reinforce that the question is to find out options, not to identify a right answer or to find out who is right and who is wrong.)
3. Follow up
If the national recommendation was not identified by any group participant in response to question 2, explain what this is. Then ask the group for their views on this recommendation.
4–8. Key questions
Explain that I won’t go around the whole group in turn for the remaining questions, that they can answer if and when they would like to.
4. I’d like to move on now to ask you some questions about eye care and the people with dementia that you care for:
-
When people are first admitted to the care home, what do you do to find out about any history of eye problems?
5. What arrangements are made for people to have an eye test?
-
Has anyone had any problems finding an optometrist to do the test?
-
Have you been present at eye tests and if so do you remember/were you aware of any difficulties in conducting the eye test?
6. Are there any particular problems about providing eye care for people with dementia in care homes?
7. From my own experience I know that there are all sorts of things that are important when you are caring for people with dementia. Like making sure they have enough to eat and drink, continence, safety, and so on:
-
How important is it that people with dementia have their eyes tested?
-
If they need glasses, how important is it for them to wear them?
8. If someone with dementia needed hospital treatment for an eye problem, for example, they had a cataract and removing it would help them to see better, what is your view about them having that treatment?
9–10. Summary questions
9. I’ve covered the main things that I wanted to ask you about:
-
Is there anything that you would like to raise that hasn’t been covered?
10. Before we end, I have one final question. I’ll go around the group so that everyone has a chance to answer if you would like to:
-
If you could say one thing about eye care for people with dementia, that you would like health providers to hear, what would that be?
Focus group for optometrists
1. Introductions
Going around the group, can I ask you to introduce yourself, stating your profession, and giving the name that you would like group members to use when addressing you?
2. Opening question
(‘Round Robin’ style question designed to be answered by all participants. The question should be something that everyone can answer.)
As you know, this research study is about visual impairment in people with dementia. But before we get onto that I’d like to start with a general question about eye examinations. I’ll go around the group again to ask:
-
How often do you think people with dementia should have their eyes tested by an optometrist?
(Reinforce that the question is to find out opinions, not to identify a right answer or to find out who is right and who is wrong.)
3. Follow-up
If the national recommendation was not identified by any group participant in response to question 2, explain what this is. Then ask the group for their views on this recommendation.
4–7. Key questions
Explain that I won’t go around the whole group in turn for the remaining questions, that they can answer if and when they would like to.
4. I’d like to move on now to ask you some questions about eye care and the people with dementia that you examine:
-
What are the main barriers, if any, that you come across when examining someone with dementia?
-
How can these barriers be overcome?
5. Do you feel adequately prepared in terms of your professional training and experience for the examination of patients with dementia?
-
If not, what additional training do you think would be most useful?
-
What form of training would you prefer: hands-on training, via DVD, paper based, short courses, or some combination?
6. If you were asked to carry out a domiciliary examination on a patient with dementia, do you have access to the equipment required to perform an adequate domiciliary examination?
7. You are asked by a patient to carry out a domiciliary examination, but you are not able to provide this service yourself or through someone else in your practice:
-
Would you be able to recommend optometrists or dispensing opticians or companies who would be able to make a home visit to examine the patient?
8–9. Summary questions
8. I’ve covered the main things that I wanted to ask you about:
-
Is there anything that you would like to raise that hasn’t been covered?
9. Before we end, I have one final question. I’ll go around the group so that everyone has a chance to answer if you would like to:
-
If you could say one thing about eye care for people with dementia, that you would like health-care providers to hear, what would that be?
Appendix 3 Stage 2 interviews topic guide
IMPORTANT NOTE: the following question schedule is a guide and will be used flexibly and appropriately according to the abilities and responses of the interviewee.
1. I know that you had your eyes tested recently as part of this research study. When did you last have your eyes tested before that?
-
Ask about how often they normally have their eyes tested and whether this has changed in recent years.
2. Do you have spectacles?
-
Ascertain type of spectacles (reading, long distance, bifocals, progressive power lenses).
-
Ask about whether they wear their glasses as prescribed.
-
When did they last have new glasses or new lenses in their own frames?
3. Do you have a magnifying glass?
-
Did they get the magnifying glass from opticians or from a hospital?
-
Ask if they use their magnifying glass.
-
If used, establish what tasks magnifying glass is used for.
-
When did they last have their magnifying glass checked by the optician or at the hospital?
4. Does your eyesight cause you any problems?
5. Do you usually go the same optician to have your eyes tested?
-
Ask if they have any worries about seeing an optician (optometrist).
-
Ask if they find any problems coping with the eye test.
-
Ask about satisfaction with how the optician (optometrist) dealt with them.
-
Ask if the optician (optometrist) knows about their dementia.
6. Before you took part in the research study, did you know about the possibility of having an eye examination done at home?
7. Do you have/have you had any eye problems that meant you had to go to the hospital, for example, a cataract?
-
Ask about their experience, before or since they found out they had dementia.
-
If applicable, in their opinion, does/did having dementia make any difference to their treatment (look for positive as well as negative comments).
8. What is your opinion about eye care for people with dementia?
-
Anything that could be improved?
-
Any suggestions about how things could be improved?
9. Thank you. That’s all I wanted to ask you about. Is there anything that you would like to say that I haven’t asked you about? Anything you want to add?
Appendix 4 Odds ratios and inferences under the multiple imputation model
Imputed multivariable (adjusted) odds ratios for each type of visual impairment by age, sex, and group
| OR | 95% CI | p-value | |
|---|---|---|---|
| Presenting VI (VA < 6/12) (n observed = 588) | |||
| Age (per year) | 1.03 | 1.00 to 1.06 | 0.050 |
| Sex: male | 0.75 | 0.51 to 1.11 | 0.152 |
| Group: residential care | 3.66 | 2.40 to 5.57 | < 0.001 |
| Post-refraction VI (VA < 6/12) (n observed = 590) | |||
| Age (per year) | 1.03 | 0.99 to 1.07 | 0.121 |
| Sex: male | 0.82 | 0.51 to 1.32 | 0.417 |
| Group: residential care | 3.86 | 2.10 to 7.11 | < 0.001 |
| Under-/uncorrected VI (VA < 6/12) (n observed = 575) | |||
| Age (per year) | 1.01 | 0.97 to 1.04 | 0.793 |
| Sex: male | 0.75 | 0.45 to 1.23 | 0.247 |
| sMMSE (per unit score) | 1.00 | 0.97 to 1.04 | 0.903 |
| Group: residential care | 1.96 | 1.13 to 3.42 | 0.018 |
| Presenting VI (VA < 6/18) (n observed = 588) | |||
| Age (per year) | 1.02 | 0.98 to 1.05 | 0.326 |
| Sex: male | 0.95 | 0.57 to 1.58 | 0.843 |
| Group: residential care | 3.50 | 2.21 to 5.55 | < 0.001 |
| Post-refraction VI (VA < 6/18) (n observed = 590) | |||
| Age (per year) | 1.03 | 0.98 to 1.08 | 0.282 |
| Sex: male | 1.56 | 0.86 to 2.81 | 0.138 |
| Group: residential care | 3.98 | 2.12 to 7.50 | < 0.001 |
| Under-/uncorrected VI (VA < 6/18) (n observed = 575) | |||
| Age (per year) | 0.99 | 0.94 to 1.04 | 0.761 |
| Sex: male | 0.46 | 0.21 to 1.04 | 0.063 |
| sMMSE (per unit score) | 0.96 | 0.93 to 1.00 | 0.072 |
| Group: residential care | 1.87 | 0.92 to 3.81 | 0.085 |
Imputed multivariable (adjusted) odds ratios for age-related macular degeneration, cataract, diabetic retinopathy and glaucoma/ocular hypertension by age, sex and group
| OR | 95% CI | p-value | |
|---|---|---|---|
| Post-examination cataract (grades 2–5) (n observed = 695) | |||
| Age (per year) | 1.11 | 1.08 to 1.14 | < 0.001 |
| Sex: male | 0.80 | 0.57 to 1.13 | 0.208 |
| Group: residential care | 0.99 | 0.70 to 1.39 | 0.480 |
| Post-examination diabetic retinopathy (n observed = 694) | |||
| Age (per year) | 0.94 | 0.87 to 1.00 | 0.062 |
| Sex: male | 2.45 | 0.80 to 7.57 | 0.118 |
| Group: residential care | 2.23 | 0.75 to 6.62 | 0.147 |
| Post-examination glaucoma or OHT (n observed = 694) | |||
| Age (per year) | 1.01 | 0.97 to 1.05 | 0.528 |
| Sex: male | 1.38 | 0.82 to 2.31 | 0.227 |
| Group: residential care | 0.75 | 0.44 to 1.30 | 0.311 |
| Post-examination AMD (n observed = 694) | |||
| Age (per year) | 1.17 | 1.12 to 1.23 | < 0.001 |
| Sex: male | 0.79 | 0.50 to 1.23 | 0.292 |
| Group: residential care | 0.63 | 0.41 to 0.97 | 0.037 |
| Post-examination diabetes (n observed = 699) | |||
| Age (per year) | 0.98 | 0.95 to 1.02 | 0.318 |
| Sex: male | 1.14 | 0.71 to 1.83 | 0.582 |
| Group: residential care | 1.18 | 0.74 to 1.89 | 0.493 |
Imputed multivariable (adjusted) odds ratios for each type of near vision loss by age, sex, group, and (for undercorrected/uncorrected visual impairment) by Standardised Mini-Mental State Examination score
| OR | 95% CI | p-value | |
|---|---|---|---|
| Presenting NVA < N8 (n observed = 476) | |||
| Age (per year) | 0.98 | 0.95 to 1.01 | 0.250 |
| Sex: male | 1.06 | 0.71 to 1.58 | 0.772 |
| Group: residential care | 3.38 | 2.18 to 5.24 | < 0.001 |
| Post-refraction NVA < N8 (n observed = 568) | |||
| Age (per year) | 1.00 | 0.96 to 1.04 | 0.915 |
| Sex: male | 0.94 | 0.57 to 1.54 | 0.795 |
| Group: residential care | 5.52 | 2.92 to 10.45 | < 0.001 |
| Under-/uncorrected NVA < N8 (n observed = 468) | |||
| Age (per year) | 1.03 | 0.89 to 1.18 | 0.717 |
| Sex: male | 0.53 | 0.15 to 1.91 | 0.327 |
| sMMSE (per unit score) | 0.92 | 0.85 to 0.99 | 0.035 |
| Group: residential care | 3.70 | 0.69 to 19.92 | 0.127 |
| Presenting NVA < N10 (n observed = 476) | |||
| Age (per year) | 0.98 | 0.94 to 1.02 | 0.291 |
| Sex: male | 1.00 | 0.61 to 1.63 | 0.987 |
| Group: residential care | 4.59 | 2.27 to 9.28 | < 0.001 |
| Post-refraction NVA < N10 (n observed = 568) | |||
| Age (per year) | 1.03 | 0.95 to 1.11 | 0.521 |
| Sex: male | 0.70 | 0.33 to 1.47 | 0.343 |
| Group: residential care | 5.41 | 2.22 to 13.23 | < 0.001 |
| Under-/uncorrected NVA < N10 (n observed = 468) | |||
| Age (per year) | 1.02 | 0.86 to 1.21 | 0.811 |
| Sex: male | 0.59 | 0.14 to 2.47 | 0.466 |
| sMMSE (per unit score) | 0.93 | 0.87 to 1.01 | 0.075 |
| Group: residential care | 4.45 | 0.72 to 27.54 | 0.107 |
Appendix 5 Further prevalence estimates of distance visual impairment for the UK populations aged 60–74, 65–89 and 75–89 years with dementia
Projected prevalence (with 95% confidence intervals) of distance visual impairment in the population of people with dementia in the UK between 65 and 89 years old
| Population prevalence (%), estimate (95% CI) | |
|---|---|
| Distance VI | |
| Presenting (VA < 6/12) | 35.7 (30.4 to 41.3) |
| Presenting (VA < 6/18) | 20.7 (17.1 to 24.8) |
| Post refraction (VA < 6/12) | 22.9 (16.9 to 30.3) |
| Post examination (VA < 6/18) | 12.5 (9.1 to 17.0) |
| Under-/uncorrected (VA < 6/12) | 14.2 (11.1 to 18.0) |
| Under-/uncorrected (VA < 6/18) | 8.4 (6.1 to 11.4) |
Projected prevalence (with 95% confidence intervals) of distance visual impairment in the population of people with dementia in the UK between 75 and 89 years old
| Population prevalence (%), estimate (95% CI) | |
|---|---|
| Distance VI | |
| Presenting (VA < 6/12) | 38.2 (32.3 to 44.6) |
| Presenting (VA < 6/18) | 22.0 (18.2 to 26.3) |
| Post refraction (VA < 6/12) | 24.9 (18.2 to 33.1) |
| Post refraction (VA < 6/18) | 13.7 (9.9 to 18.5) |
| Under-/uncorrected (VA < 6/12) | 14.6 (11.3 to 18.7) |
| Under-/uncorrected (VA < 6/18) | 8.5 (6.3 to 11.3) |
Projected prevalence (with 95% confidence intervals) of distance visual impairment in the population of people with dementia in the UK between 65 and 74 years old
| Population prevalence (%), estimate (95% CI) | |
|---|---|
| Distance VI | |
| Presenting (VA < 6/12) | 26.6 (19.8 to 34.9) |
| Presenting (VA < 6/18) | 16.1 (9.6 to 25.8) |
| Post refraction (VA < 6/12) | 15.8 (9.0 to 26.5) |
| Post refraction (VA < 6/18) | 8.4 (3.9 to 17.3) |
| Under-/uncorrected (VA < 6/12) | 12.6 (7.1 to 21.2) |
| Under-/uncorrected (VA < 6/18) | 7.9 (3.3 to 18.0) |
Appendix 6 Public and participant involvement in the study
Public and participant involvement was a key element in the development and completion of the PrOVIDe study. We engaged the views of people with dementia and carers at the design stage to ensure that the perspectives of people living with dementia were at the centre of the project’s design and development. People with experience of caring for people with dementia were involved as coapplicants and in research roles. This ensured that the study was conceived, planned and executed from the carers’ perspective, always keeping in mind the best interests of people with dementia.
This appendix describes how PPI was integrated into all stages of the study.
Public and participant involvement in developing the research idea: step 1
George Hancock died at the age of 89 years after having dementia for approximately 10 years. For the first few years he lived with his wife, supported by his family. Five years before his death he and his wife went to live with his eldest daughter and her son. At first, family care centred on reassuring him about his failing memory and keeping him safe. As the dementia progressed, the main issues were diet, hydration, hygiene and continence. His wife and daughter were supported by the extended family and they felt that they were doing their best for him, right up to the time that he moved into a care home for the last few months of his life. But, looking back, they cannot remember him having an eye examination even though he wore glasses. Why? Was it because the family were more concerned with the more pressing physical and psychological needs? Or was eyesight overlooked as his cognitive state deteriorated and he no longer read or watched television or took interest in his surroundings? No one knows. But when the NIHR Dementia Themed Call for research proposals was announced in 2011, George’s experience struck a chord with his daughter-in-law (Beverley Hancock) who, by that time, was working as a research adviser to the CoO.
Public and participant involvement in developing the research idea: step 2
Michael Bowen at the CoO had already started discussions with Sarah Buchanan at the Thomas Pocklington Trust and the chief executive of the Alzheimer’s Society about conducting some pilot research into dementia and sight loss. This followed a multiagency conference, at which four people affected by dementia and sight loss contributed to conference presentations.
The CoO is a professional body for optometrists but it also has a role in promoting public interests, while the Thomas Pocklington Trust is a charitable organisation concerned with older people and sight loss. The Alzheimer’s Society is the UK’s leading research and service provision charity for Alzheimer’s and dementias. All three organisations have a tradition of PPI in developing their research programmes. With support from a leading academic in optometry (David Edgar), they drafted an outline research proposal and took it to a workshop organised by the Alzheimer’s Society in response to the NIHR Dementia Themed Call.
At the workshop researchers were invited to present their research idea to a series of small groups of Alzheimer’s Society Research Network volunteers and Alzheimer’s Society staff. After the workshop, Alzheimer’s Society informed the CoO team that the proposal was one of the most highly rated by participant feedback. The Alzheimer’s Society was willing to join the project as a coapplicant, and several Research Network volunteers (who were all currently or previously caring for people with dementia) had expressed interest in being part of a Steering Group. This was a great confidence booster as we saw it as confirming something we already thought, namely that the proposed research was addressing an important topic for people with dementia and their carers, and that people would be interested and want to participate.
Public and participant involvement in developing the research idea: step 3
The outline funding application was shortlisted and a Steering Group was formed. James Pickett, at that time Research Officer with the Alzheimer’s Society, and Susan Maskell, a Research Network volunteer who had cared for her mother when she had dementia, joined the Steering Group and contributed to the full application as coapplicants. Their experience of people with dementia, at both the organisational and the personal level, was invaluable in informing the application. The cost of PPI was factored into the funding application in accordance with INVOLVE guidelines. 153
Lay members of the respective Research Committees of the CoO and Thomas Pocklington Trust reviewed and contributed to the application prior to submission.
Public and participant involvement in managing the research study
The PPI representatives from the Thomas Pocklington Trust (Sarah Buchanan) and the Alzheimer’s Society (Susan Maskell and James Pickett) oversaw the management of the study in their role as members of the Steering Group, providing advice and information as required and asking the research team lots of questions.
Susan Maskell, in particular, was keen to assist as much as possible and was actively involved in several stages:
-
assisting with the design of participant information literature and letters of invitation to participate
-
supporting the development of the ethics submission to the Research Ethics Committee and helping to prepare the response to the Research Ethics Committee’s questions
-
offering to go into care homes to explain the study to people with dementia, their relatives and care workers
-
reading interview and focus group transcriptions and assisting with data analysis
-
adding her perspective to the presentation of research findings
-
reviewing the report to the NIHR.
Less easy to itemise are the numerous instances in which our PPI members made us review our practices and procedures by asking us why we were planning to do things in a certain way or by contributing an idea, opinion or the value of their experience.
Public and participant involvement in dissemination
The PPI representatives will be involved in disseminating the research findings. For example:
-
A range of journal submissions are planned and they will contribute as authors.
-
They will be actively involved in agreeing the content and design of leaflets and information on websites.
-
Susan Maskell contributed to a workshop on eye examinations for people with dementia at the CoO’s annual conference.
The PrOVIDe study team are grateful to the PPI members of the Steering Group for all the help and advice that they provided. Their continuing input will ensure that work will continue to make the study findings available as widely and as clearly as possible.
Impact of public and participant involvement
The Steering Group and Management Group were clear that there were several points in the development and delivery of the project where input from Susan Maskell as the PPI representative on the team was central to avoiding a design flaw or to identifying an effective solution to a problem. Overall, the consensus was that not only had the PPI involvement across the study enabled the project to be more relevant and engaging to potential participants, but it had also ensured that the study was technically more effective and efficient as a piece of research.
In addition to the direct benefits to the PrOVIDe project, the experience of PPI’s impact on developing and delivering good research led to the CoO and the Thomas Pocklington Trust further developing their internal PPI engagements to make more effective use of PPI in the design and delivery of future research.
Appendix 7 Revised protocol and explanatory note
Background
When PrOVIDe was initially approved for funding, the approach to recruitment set out in the final detailed proposal did not incorporate a recruitment approach that sought to stratify the final sample to reflect the best estimates of the demographics of the UK’s dementia population for characteristics of age and sex. The original approach proposed and approved by NIHR was to recruit to two groups: group 1 was to be people with dementia living in their own homes and group 2 was to be people with dementia living in residential care. As the detailed approach to recruitment developed, it was agreed by the steering group that stratified recruitment targets should be set based on the best available estimates for the age/sex profile for the UK dementia population as a whole.
Using the Alzheimer’s Society’s best estimates of these demographic characteristics for the UK dementia population, the study’s statistician developed a proposed approach with age/sex targets for recruiting, while maintaining the overarching sample targets. The intention of this stratification was to increase the likelihood that the final sample for the study would be representative of the wider UK’s dementia population, so far as age and sex were concerned. It is worth noting that the study was powered to achieve the primary objective of the study – to gather prevalence data on VI from all causes in the dementia population in sites across England – and the study was designed to allow the study to achieve the desired sample size (n = 385) even if there were high rates of missing data. We had no prior information regarding what the test completion rates would be, and therefore we allowed for up to a 50% missing data rate in this sample of people with dementia. This gave our overall sample size of 770 and we split this number into 385 participants living in care homes and 385 living in their own homes. This division was not based on a sample size calculation, and it should be noted that at this stage there was considerable uncertainty as to the size of any difference in prevalences of VI between these two groups of participants. However, this number of 385 participants in each group would allow us, provided the rates of missing data were not too high, to address the secondary objective of identifying any differences in the level of undetected or inappropriately managed VI between those living in their own homes and those living in care homes.
Rationale for changes
As noted, the initial changes to the recruitment strategy were to support the potential to achieve a final sample that was as representative as possible of the wider UK dementia population. The stratification of recruitment targets to reflect the wider population’s demographic characteristics was acknowledged to introduce additional challenges to the recruitment process, but it was felt to be worthwhile. This change was approved by NIHR prior to the commencement of recruitment to the study.
During the course of the study, it became clear that beyond the inherent challenges associated with recruiting people living with dementia to participate in research, there were additional barriers specific to recruiting people living with dementia who were living in residential care settings. As the project progressed, recruitment was very closely monitored, and towards the end of the planned recruitment phase it became clear that it would not be possible to achieve recruitment targets by the study completion date if the precise sample stratification was adhered to, for sex, age and, ultimately, for residential care settings. Following correspondence with NIHR regarding these issues, the research team carried out some preliminary analyses of the data collected up to that point to inform the decision-making process in relation to whether or not to seek to extend the duration of the recruitment phase (and, thus, the overall duration of the project), or to adjust the final sample size to reflect what was achievable. Two options were presented to NIHR: accept a slightly smaller final sample or extend the project. On 11 March 2014 NIHR approved the approach to accept a smaller sample size with less rigid adherence to sample stratification for age and sex.
Impact of changes
The preliminary analyses showed that the original sample size of 770 had been a conservative estimate. The project team had used such a cautious estimate because the best available prevalence data from previously published studies on VI in people living with dementia were so variable, and there were limitations associated with the best available data on the prevalence of key eye conditions/diseases for the general UK population as a whole. In addition, the proportion of missing data was unknown and likely to be high. The study was powered to take the ‘worst-case scenario’ for possible prevalence rates. Statistically, if the actual prevalence rates found in the study were > 50% or < 50%, then the sample required would be smaller than the planned 385. There was additional resilience built in through the inclusion of the second 385 participants in group 2. The preliminary analyses showed that the study would be adequately powered to address the primary research question with this adjusted approach.
Recruitment targets were stratified by two wide age groups and sex to reflect the age/sex distribution of the UK dementia population at the time. To achieve the overall sample recruitment target within the duration of the project there was some over- and under-recruitment to several strata. This and the non-ignorable proportion of unobserved distance VA and NVA in the sample (missing data) limits the generalisability of extrapolating our unadjusted prevalence estimates to the wider UK population with dementia. To obtain more plausible prevalence estimates within the sample, multiple imputation (not just for complete cases) was used. To then calibrate our prevalence estimates to the latest-available wider UK data from the Alzheimer’s Society,2 within-sample and post-stratification population calibration weights were derived and extrapolated population prevalences were calculated. We also accounted for the clustering effect of our sampling by regions and by sites within regions. Given all this, the impact overall of our changes was that the extrapolated prevalences for distance VI and near vision loss (1) are higher in these population estimates than the PrOVIDe sample rates, and (2) have wider CIs. Feature (1) reflects the undersampling and increased difficulty in obtaining VA measures from people more likely to have worse VA. Feature (2) reflects the design effect and the large variation in calibration weights.
The study team and NIHR considered these changes very carefully prior to making them and the final analysis of the data set shows that the decision to adjust the recruitment approach and the final sample size did not impact negatively on the overall value of the final data set. For the sake of transparency and clarity, it was agreed to include the final revised protocol and this explanatory note.
Glossary
- Acute angle-closure glaucoma
- A condition in which the pressure inside the eye increases rapidly, and which can be provoked by dilatation of the pupil in a predisposed eye.
- Aphakia
- An ocular condition in which the crystalline lens is absent. It is usually the result of surgical removal of cataract.
- Aphakic
- An eye without the crystalline lens.
- Binocular vision
- Vision that incorporates images from both eyes simultaneously. In normal binocular vision these images are fused into a single impression.
- Crystalline lens
- The lens inside the eye, situated behind the iris, which contributes to the formation of images on the retina.
- Domiciliary eye examination
- An eye examination undertaken at the place where the person being examined normally lives.
- Fundus
- The back portion of the interior of the eye visible with an ophthalmoscope.
- Glasgow acuity cards
- A visual acuity test which consists of a set of cards contained in a flip-card format.
- Intraocular lens implant
- A lens inserted into the eye to replace the crystalline lens after surgical removal of cataract.
- Logarithm of minimum angle of resolution chart
- A chart for testing visual acuity in which the size of the rows of letters varies in a logarithmic progression.
- Monocular
- Relating to one eye.
- Opacities
- Non-transparent structures (often cataract) in the eye.
- Ophthalmologist
- A medical specialist in the field of medical and surgical care of the eyes.
- Ophthalmoscope
- A piece of equipment for viewing the inside of the eye.
- Ophthalmoscopy
- Examination of the interior of the eye with an ophthalmoscope.
- Optometrist
- A person who practises the profession of optometry.
- Orthoptist
- A person who practises the profession of orthoptics, which is primarily the diagnosis and treatment of anomalies of binocular vision.
- Posterior subcapsular cataract
- A cataract, which is usually age-related, and which is situated towards the back of the crystalline lens.
- Refractive correction
- The prescription of spectacles (or contact lenses) to correct errors of focusing of the eye.
- Retinopathy
- A disease of the retina.
- Snellen charts
- A visual acuity test using a graduated series of Snellen letters.
- Tonometry
- The measurement of intraocular pressure with a tonometer.
- Visual acuity
- The ability to see distinctly the details of an object.
- Visual field
- The extent of the surrounding area which is visible to an eye looking steadily in a given position.
- Visual impairment
- Reduced visual performance, which can be defined in various ways.
List of abbreviations
- ACG
- angle-closure glaucoma
- AMD
- age-related macular degeneration
- BADLS
- Bristol Activities of Daily Living Scale
- CBI-R
- Cambridge Behavioural Inventory – Revised
- CI
- confidence interval
- CoO
- College of Optometrists
- D
- dioptre
- DeNDRoN
- Dementia and Neurodegenerative Diseases Research Network
- ENRICH
- Enabling Research in Care Homes
- GOS
- General Ophthalmic Services
- GP
- general practitioner
- HES
- Hospital Eye Service
- ICD-10
- International Classification of Diseases, Tenth Edition
- IOL
- intraocular lens
- IOP
- intraocular pressure
- IQR
- interquartile range
- logMAR
- logarithm of minimum angle of resolution
- MMSE
- Mini-Mental State Examination
- MRC
- Medical Research Council
- NDNS
- National Diet and Nutrition Survey
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NVA
- near visual acuity
- OHT
- ocular hypertension
- OR
- odds ratio
- POAG
- primary open-angle glaucoma
- PPI
- public and participant involvement
- PrOVIDe
- Prevalence of Visual Impairment in Dementia
- RNIB
- Royal National Institute of Blind People
- SD
- standard deviation
- sMMSE
- Standardised Mini-Mental State Examination
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
- TOC
- The Outside Clinic
- VA
- visual acuity
- VI
- visual impairment