Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/1023/01. The contractual start date was in May 2012. The final report began editorial review in July 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Daniel Prieto-Alhambra has received unrestricted research and educational grants from Amgen and Bioibérica S.A. Nigel Arden reports personal fees from Merck Sharp & Dohme (MSD), grants and personal fees from Roche, personal fees from Smith and Nephew, personal fees from Q-Med, personal fees from Nicox, personal fees from Flexion, personal fees from Bioibérica and personal fees from Servier. Cyrus Cooper has received consultancy fees, lecture fees and honoraria from Amgen, GlaxoSmithKline, Alliance for Better Bone Health, Eli Lilly, Pfizer, Novartis, MSD, Servier, Medtronic and Roche. M Kassim Javaid has in the last 5 years received honoraria for travel expenses, speaker fees and/or advisory committees from Lilly UK, Amgen, Servier, MSD, Medtronic, Internis, Consilient Health and Jarrow Formulas. He also serves on the Scientific Committee of the National Osteoporosis Society and International Osteoporosis Foundation. Andrew Judge has received consultancy fees, lecture fees and honoraria from Servier, UK Renal Registry, Oxford Craniofacial Unit, IDIAP Jordi Gol and Freshfields Bruckhaus Deringer, is a member of the Data Safety and Monitoring Board (which involved receipt of fees) from Anthera Pharmaceuticals, Inc., and received consortium research grants from Roche.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Judge et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
There is a marked disparity in the delivery of secondary fracture prevention for hip fracture across England, despite national guidelines, which is of concern to clinicians, patients and commissioners. Given the substantial societal burden of hip fractures and the subsequent increased risk of fracture, understanding the causes and consequences of this disparity is a matter of urgent priority within the NHS.
Osteoporosis is a common bone disease affecting 3 million patients in the UK. The clinical and public health implications are substantial owing to the mortality, morbidity and cost of medical care associated with osteoporotic fractures. 1 Of all of the types of osteoporotic fracture, hip fractures are the most costly and are a major public health problem because of an ageing population. Hip fractures usually occur as result of a low-impact falls in individuals with underlying bone fragility due to osteoporosis. 2,3 Approximately 87,000 hip fractures occur annually in the UK, with a cost (including medical and social care) amounting to about £2.3B per year. 1,3 Length of stay accounts for the majority of overall hospital costs, and has been estimated to be between £5600 and £12,000 per case. 1 After discharge from hospital, the cost of complex home and institutional care for people who make a poor recovery is very high, with average additional costs for health and social aftercare previously estimated to be £25,000 in the first 2 years. 1
Importantly, patients experiencing hip fracture after low-impact trauma are at considerable risk of subsequent falls, osteoporotic fractures and premature death. 4–6 The risk of second hip fracture ranges from 2.3% to 10.6%, in which the majority of second hip fractures occurred within a few years of the first hip fracture. 7,8 It has been estimated that 55.6% of hip fracture patients had at least one fall within 12 months, 11.8% sustained a fall-related fracture and 5% sustained a fall-related hip fracture. 9 Mortality during the first year after fracture ranges from 8.4% to 36%. 4
Current guidelines in fracture prevention
The onset of osteoporosis is asymptomatic and it is often recognised only after an older person falls and sustains a fracture. There have been widespread calls to improve the identification and treatment of hip fracture patients to reduce the risk of further falls, fractures and mortality. 1,4,10 The risk of further fracture can be reduced by up to half with bone protection therapy. 1,11–13 As most fractures result from a fall, interventions to reduce the risk of falls may be effective in preventing further such events; however, direct evidence is lacking. Over the past decade, guidance from a number of professional bodies has been published for the management of hip fracture patients [British Orthopaedic Association (BOA) Blue Book,1 Scottish Intercollegiate Guidelines Network (SIGN),14 National Institute for Health and Care Excellence (NICE)13,15]. NICE technology appraisal (TA) guidelines TA 160/16113 are related to the effectiveness of bone protection therapy and clinical guideline (CG) 21 relates to falls prevention. 15 In the UK, secondary prevention of fracture is underutilised and widely neglected. 1 As a consequence, compliance with NICE publications TA 16113 and CG 2115 is low. Audits by the National Hip Fracture Database (NHFD)16,17 and the Royal College of Physicians18 suggest that the situation is improving but still inadequate, such that prior to discharge only 66% of hip fracture patients were on bone protection medication and 81% received a falls assessment. 17
As almost half of all hip fracture patients have had a prior fracture,4 responding to the first fracture provides a golden opportunity to prevent the second. The BOA Blue Book1 provided guidance on secondary prevention of fragility fractures. A comprehensive service should consist of osteoporosis assessment, including a dual-energy X-ray absorptiometry (DXA) scan to measure bone density, if appropriate, treatment with bone protection therapy in osteoporosis patients, falls risk assessment and systems to improve adherence and persistence with therapy. Organising such services is challenging owing to the multidisciplinary care that patients require. 3 The 2011 NICE hip fracture CGs make specific recommendations regarding the treatment and multidisciplinary management of patients including liaison and integration of services. A fracture liaison service (FLS) is the recommended model proposed by the Department of Health to organise secondary fracture prevention services1 in a ‘one-stop shop’ setting delivered by a nurse specialist supported by a lead clinician (‘champion’) in osteoporosis. 19 However, currently only 30% of hospitals in England have established a FLS. 17 A single model incorporating all components of secondary fracture prevention has not been mandated. Current practice is for various combinations of these components to be used within a hospital (and in some cases no components are used).
Current knowledge
The clinical effectiveness of co-ordinator-based models of care has been demonstrated, in terms of improving the uptake of appropriate osteoporosis management such as measuring bone density and the use of antiresorptive drug therapy. 4,10 There is growing evidence of these models’ cost-effectiveness20,21 and that they can provide cost savings to the NHS. 4,10,22,23 Evidence is emerging on the ability of co-ordinator-based systems to reduce the incidence of hip fractures. A review of the Glasgow Osteoporosis and Falls Strategy reported that hip fracture rates in the city had reduced by 7.3% over the decade, compared with a 17% increase in fracture rates for the entire population of England over the same period. 19,24 These findings are consistent with observational data from the USA by Dell, who reported a 37.2% reduction in hip fracture rates. 25 However, the strongest evidence on effectiveness has recently been provided by an Australian study that was designed as a prospective observational trial with a concurrent control group in which, compared with standard care, targeted identification and management significantly reduced the risk of refracture by > 80%. 26
Across the UK there is variation in the care pathway of the treatment and management of hip fracture patients and in the way secondary fracture prevention services are structured and organised. Even with a co-ordinator-based system in place, the structure of services can vary between hospitals. For example, hospitals use different models of orthogeriatric care, in which some hospitals now have specialised orthogeriatric wards and in others patients are seen on the trauma ward. Some hospitals may co-ordinate the care of hip fracture patients only while such patients are admitted as inpatients, whereas others have ensured that their osteoporosis service is integrated across primary care to monitor patients’ adherence to bone protection therapy.
Aims
The aim of this study was to characterise the delivery of secondary fracture prevention services over the past decade across hospitals in the region. Using qualitative research methods we have identified the reasons why hospitals chose their specific model of service delivery and assessed barriers to change. Using a natural experimental design27 we have established the cost-effectiveness of different models of care and the impact that changes to the delivery of care have had on altering trends in refracture rates, NHS costs and life expectancy.
Objectives
Objective 1: characterise secondary prevention of hip fracture across hospitals in a region of England (work stream 1)
The first phase of this project was to comprehensively describe and explore the variation and disparity in secondary fracture prevention services offered to hip fracture patients across hospitals in a region of England, and to identify the dates of key changes made to service delivery over the past decade.
Objective 2: identify the reasons why hospitals chose their specific model of service delivery and assess barriers to change (work stream 2)
The aims of the qualitative study were to (1) ascertain the reasons why each hospital has adopted their current and most recent models of care; (2) establish factors that facilitate or act as barriers to changes in service delivery; and (3) identify the elements of care of hip fracture patients that health professionals think are most effective.
Objective 3: evaluate the impact that changes to the delivery of secondary fracture prevention has had on health outcomes by altering trends in hip refracture rates, NHS costs and life expectancy (work stream 4)
A natural experimental study design27 evaluates the impact that changes hospitals in the region have made to the way in which they deliver secondary fracture prevention services for hip fracture patients has had on a range of health outcomes (mortality, second hip fracture and other fragility fractures).
Objective 4: establish the cost-effectiveness of different hospital models for delivery of secondary fracture prevention (work stream 3)
The health economics work calculates the hospital and non-hospital costs associated with hip fracture in the year of fracture and subsequent years and evaluate the costs (quality-adjusted) life expectancy and cost-effectiveness of the different hospital models of care.
Design and methodology
Characterise secondary prevention of hip fracture across hospitals in a region of England (work stream 1)
In order to collect information on models of care for secondary fracture prevention at each hospital, we conducted a service evaluation which comprised:
-
developing a questionnaire to collect information on staffing levels and new appointments over the past decade, procedures for case finding, osteoporosis assessment (including location of DXA scanner), falls assessments, treatment initiation and follow-up, as well as integration across primary, secondary and community care
-
identifying health professionals at each hospital to complete the questionnaire through a regional network of clinicians working in osteoporosis management.
Identify the reasons why hospitals chose their specific model of service delivery and assess barriers to change (work stream 2)
This qualitative research component involved the following stages:
-
identifying a local collaborator at each hospital through a regional network of clinicians and, with their input, identifying other health-care professionals and service mangers working at the hospital in secondary fracture prevention
-
conducting qualitative interviews with 3–5 health-care professionals or service managers at each hospital
-
thematic analysis conducted using codes to identify themes and subthemes.
Evaluate the impact that changes to the delivery of secondary fracture prevention has had on health outcomes by altering trends in hip refracture rates, NHS costs and life expectancy (work stream 3)
A natural experiment study design was used to assess the clinical effectiveness of changes in service delivery in terms of reducing mortality and secondary fracture rates using the following procedure:
-
trends in rates of 30-day mortality, 1-year mortality and secondary hip fracture established from Hospital Episodes Statistics (HES) data for hospitals in the region
-
interventions, or changes in service delivery, and their corresponding dates were identified from the service evaluation conducted in work stream 1
-
interrupted time series analysis and Cox proportional hazards regression modelling used to analyse impact of each intervention on outcomes of interest.
Establish the cost-effectiveness of different hospital models for delivery of secondary fracture prevention (work stream 4)
An economic analysis of the costs and cost-effectiveness of different services will be undertaken as described below.
-
Data from HES and the Clinical Practice Research Datalink (CPRD) will be used to identify the hospital and non-hospital costs of a hip fracture.
-
A Markov model will be developed to evaluate costs and cost-effectiveness of each model of care (using a measure of clinical effectiveness from work stream 3).
-
Utility scores (from literature search) will be used to provide the weights required to calculate the quality-adjusted life-years (QALYs) of the different models of care under evaluation. 28
Chapter conclusion
This chapter has provided the relevant background information to introduce the health-care need for this area of research. Each of the four work streams are outlined, along with the research methodology involved. These four work streams are described in detail in Chapters 2–8.
Chapter 2 Characterisation of secondary fracture prevention services at hospitals across a region of England, and identification of key changes in service delivery over the past 10 years
Introduction
In this chapter, we present findings of a service evaluation to look at the organisation of orthogeriatric and FLS available to patients being treated for a hip fracture. This was carried out at 11 acute hospitals in one region of England. We also identify the dates of key changes to service delivery, such as the appointment of fracture liaison nurses and orthogeriatricians. This work package was designed to inform the natural experiment study design described in Chapter 5, in which we look at the clinical effectiveness and cost-effectiveness of the interventions identified here.
We will initially begin by describing the evident variations in the structure of orthogeriatric and FLS across the different hospitals. Exploring the similarities and differences between these services in more detail will help clinicians and commissioners in England to identify gaps in care and provide them with information about which services to develop if necessary, which may help to reduce unwarranted variation.
We will then present the key time points of interest we have identified when interventions occurred, and justify our reasons for these selections.
Current knowledge of variations in delivery of fracture prevention services
Studies have shown a major variation in how hospitals organise their services for the treatment of hip fracture patients and management of secondary fracture prevention. The NHFD is a national audit from the Royal College of Physicians that includes information on the number of orthogeriatric sessions and specialist nurses per centre to deliver secondary fracture prevention. 17 Although the number of orthogeriatric hours per week is modestly related to reported hip fracture numbers, there was a wide variation in FLS service delivery. In 2013, 62% of hospitals reported that they had no fracture liaison nurse, 27% reported up to one whole-time equivalent (WTE) post and 11% reported more than one WTE. Furthermore, in those centres with a specialist nurse, there was no relationship between volume of fragility fractures and number of staff (Figure 1).
FIGURE 1.
The relationship between reported number of WTE specialist nurses for secondary fracture prevention and estimated number of fragility fracture patients seen in that hospital per year. Each data point represents a hospital that returned > 0 WTE of specialist nurse in the NHFD 2014 report. The number of fragility fractures per hospital was estimated using five times the number of proximal femoral fractures. The line shows a lowess plot with a bandwidth of 0.9.

These variations in services using even crude metrics such as staff resource are too great to be explained by local case mix and volume of hip fracture patients across hospitals. Establishing the differences between service models and levels of care in more details will allow clinicians and commissioners to identify existing gaps in care, and could help to reduce unwarranted variation across hospitals.
Current guidelines
A number of professional bodies have provided national guidance for the care of hip fracture patients reflecting two key aspects. The first type of guidance relates to optimising initial recovery after hip fracture and focuses around the provision of patient-centred care29 within three broad categories:
-
optimising surgical procedure – including early timing of surgery, preoperative appropriate correction of comorbidities and type of implant
-
early mobilisation
-
multidisciplinary management from admission to discharge.
The second type of guideline relates to secondary fracture prevention and there are many more guidelines available, including guidance from Canada22,30,31 the USA32 and the UK. 1,14,15,33 The ‘Capture the Fracture’ initiative from the International Osteoporosis Foundation (IOF) provides additional international guidance. 34 Guidelines describe a comprehensive fracture prevention service consisting of four main components:
-
case finding those at risk of further fractures
-
undertaking an evidence-based osteoporosis assessment, including a DXA scan to measure bone density, if appropriate
-
treatment initiation in accordance with guidelines for both bone health and falls risk reduction
-
monitoring and improving adherence to recommended therapies.
Such services require multidisciplinary care from orthogeriatrics, rheumatology, falls services and primary care. 3 Running such a service can be challenging and, as a result, such services often require a dedicated co-ordinator to provide a link between the different multidisciplinary teams involved in the care pathway. This is known as a co-ordinator-based system of care. 10 This co-ordinated, multidisciplinary approach to patient care is known as a FLS. 19 FLSs have now been introduced internationally. 35,36 In the UK, the Department of Health has proposed a model of best practice which is delivered by a nurse specialist supported by a lead clinician (‘champion’) in osteoporosis. 1 In addition, The Care of Patients with Fragility Fractures, published by the BOA, recommends fully integrating orthogeriatricians into the way the fracture service works in order to best meet the complex needs of hip fracture patients. 1
However, despite the guidance that is in place, there is no overall agreement on the best way to organise these services, and a single model comprising all elements of a FLS has not been mandated.
Changes in hip fracture care over the past decade
Guidance from a number of professional bodies for the management of hip fracture patients (BOA Blue Book,1 SIGN14 and NICE29,33) has led to key changes in hip fracture care across the UK. The NHFD collects data on staffing levels as well as on many other aspects of care, and has shown a significant increase in the number of consultant grade orthogeriatric hours per week since reporting began in 2009, and a particularly sharp upwards trend in the number of fracture liaison nurse hours per week since 2012. 16,17,37,38
Aim
The aim of this work package was to comprehensively describe the models of care for secondary fracture prevention for hip fracture patients across hospitals in one region of England, and describe the similarities and differences that exist across them.
In addition, this work package will identify key changes in these services that have occurred at each hospital over the past 10 years. This will form the basis of the ‘natural experiment’ described in Chapter 5, where the clinical effectiveness of changes to the care pathway established here will be evaluated.
Methods
The service evaluation was conducted in one regional area of England. There are 11 hospitals in this region which receive patients with acute hip fracture. Detail on the variation in size of these hospitals, in terms of catchment population and number of hip fracture patients seen each year, was taken from the NHFD 2013 Report. 17
Data for the service evaluation were collected using a questionnaire designed to identify key changes to service delivery over the past 10 years and characterise the level of service, closely based on the IOF Capture the Fracture Best Practice Framework,34 which defines 13 standards for an effective fracture liaison nurse. A copy of the questionnaire is included in Appendix 1, but, briefly, data were collected on the following aspects of care:
-
dates of employment of orthogeriatricians, fracture liaison nurses, falls nurses and clinical osteoporosis ‘champions’, their role in co-ordinating care and clinical contact, and changes they introduced when appointed
-
type of wards (trauma, geriatric orthopaedic rehabilitation unit, other rehabilitation ward) and date opened
-
presence of any service-level agreement for delivery of secondary fracture prevention
-
co-ordination of multidisciplinary care and across inpatients, outpatients and primary care
-
staff responsible for case finding
-
DXA scanner location and referrals
-
monitoring
-
falls assessments and other assessments.
A regional network of clinicians who work in osteoporosis services helped to identify health-care professionals at each hospital who would be best placed to complete the questionnaire, including fracture liaison nurses, orthogeriatricians, geriatricians, rheumatologists, general practitioners (GPs) with a special interest in osteoporosis, trauma surgeons, anaesthetists, endocrinologists and trauma nurses. Initially, one person at each hospital was approached to complete the questionnaire, and further health-care professionals were identified if additional information was needed. To allow for clarification, the health-care professional completed the questionnaire in the presence of one of the research team. This enabled participants to elaborate on any of the more complex details of each component of care.
Comparison of services across hospitals
To allow direct comparison of service levels between hospitals, staffing levels were calculated as WTEs and as ratios in terms of the number of WTEs per 1000 hip fracture patients, using data on the number of hip fractures reported in the 2013 NHFD report. 17 For simplicity, we grouped all nurses working in fracture prevention (e.g. trauma nurse, osteoporosis nurse specialist) together under the title ‘fracture liaison nurse’. In addition, we identified and described variations in how the four main elements of a fracture prevention service (identification, investigation, initiation and monitoring) are co-ordinated and conducted.
Identification of key changes in service delivery
Information on changes in service delivery, such as the appointment of a new member of staff, the opening of a new ward or changes in procedures for referring patients for DXA scans, was assembled and presented to the study team, which included a number of clinicians. Together, the team identified the dates of the key changes to feed into the analysis in work stream 3. Once a list of changes was assembled for each hospital, the list was sent to a health-care professional at that hospital to ensure that they agreed with the changes that had been identified.
Results
Current disparity in fracture prevention across hospitals
Staffing levels across hospitals in 2013
Table 1 provides the WTEs and proportion of specialist staff per 1000 hip fracture patients at each hospital, including orthogeriatricians, fracture liaison nurses, falls nurses, lead clinicians and ‘osteoporosis champions’. This highlights the differences in staffing levels across hospitals. For example, two hospitals had no consultant-level orthogeriatric support, while one hospital had a full-time consultant orthogeriatrician in place supported by five WTE lower-grade orthogeriatric staff.
| Hospital | Specialist staff (levels expressed as WTE) | ||||
|---|---|---|---|---|---|
| Consultant OG | Other orthogeriatric support | FLN | Orthopaedic/specialist nurse | Lead clinician | |
| 1 | 0 | 0.5 WTE 2.3 : 1000 patients |
1 WTE 4.5 : 1000 patients |
0 | 1 (rheumatologist) |
| 2 | 1.0 WTE 2 : 1000 patients |
1 WTE 2 : 1000 patients |
0 | 0 | 1 (OG) |
| 3 | 0.1 WTE 0.3 : 1000 patients |
1 WTE 3 : 1000 patients |
0 | 0 | 0 |
| 4 | 0.5 WTE 1.3 : 1000 patient |
0.2 WTE 0.5 : 1000 patients |
0.5 WTE 1.3 : 1000 patients |
0 | 1 (rheumatologist) |
| 5 | 0.33 WTE 1.5 : 1000 patients |
0.6 WTE (trust-grade OG) 3.7 : 1000 patients |
0 | 0 | 0 |
| 6 | 0.5 WTE 2.1 : 1000 people |
0 | 0 | 1 WTE 4 : 1000 patients |
1 (rheumatologist) |
| 7 | 0.7 WTE 1 : 1000 patients |
0.75 WTE 1 : 1000 patients |
0 | 1.8 WTE 2.6 : 1000 patients |
1 (rheumatologist) |
| 8 | 1 WTE (1.6 : 1000 patients) |
5 WTE 8 : 1000 patients |
0 | 0 | 1 (OG) |
| 9 | 0 | 0.25 WTE 1 : 1000 patents |
0 | 2 WTE 8 : 1000 patients |
2 (specialist nurse-outpatient, orthopaedic surgeon-inpatient) |
| 10 | 0.9 WTE 2 : 1000 patients |
1 WTE 2 : 1000 patients |
1.6 WTE 3 : 1000 patients |
2.2 WTE 4.6 : 1000 patients |
1 (rheumatologist) |
| 11 | 0.4 WTE 2.3 : 1000 patients |
1 WTE 5.7 : 1000 patients |
0.2 WTE 1 : 1000 patients |
0 | 1 (OG) |
Several hospitals still had no fracture liaison or specialist nurses in post as of April 2013, while one hospital had 3.8 WTE of specialist nurses. When considering the number of hip fracture patients seen at each hospital, the variation in staffing becomes even more pronounced, with overall orthogeriatric staff ranging from 1 WTE per 1000 patients to 9.6 WTE per 1000 patients, and overall nursing staff ranging from zero input to 7.6 WTE per 1000 patients.
Co-ordinator-based models of care
Information was gathered on the role of orthogeriatricians and fracture liaison nurses in the fracture prevention service. It is clear that some hospitals operated a nurse-led model, in which nurses were responsible for case finding, osteoporosis assessment and making treatment recommendations. In other hospitals, such roles were performed by consultant orthogeriatricians. At some hospitals, specialist nurses such as orthopaedic nurses were essentially performing the role of a fracture liaison nurse despite no one officially being employed in that role. The majority of services were inpatient led only, with only a few services being integrated with outpatient care and, in some cases, also community care. One hospital operated a service which was mainly outpatient led.
The majority of hospitals followed NICE implementation guidelines, holding multidisciplinary team meetings on a regular basis to co-ordinate care between orthopaedics, rheumatology and any other departments involved in care. Some hospitals also used multidisciplinary paperwork, but only two hospitals reported conducting multidisciplinary ward rounds. Only one of the hospitals in the study demonstrated little co-ordination of care and a lack of agreed protocols and meetings with staff from other departments.
Case finding
Fracture liaison nurses or orthogeriatricians were generally responsible for undertaking case finding at most hospitals in an inpatient setting. When questioned about methods for case finding, most hospitals reported ward rounds and multidisciplinary team meetings as the most widely used methods. This interaction was often done informally by liaising with trauma and orthopaedic surgeons and other staff. At one hospital, health-care professionals attended joint trauma meetings which were followed by a joint trauma round that included an orthogeriatrician, an orthopaedic surgeon and a registrar 5 days per week. Computer systems were also important for logging trauma referrals and admissions, allowing staff to search the database for hip fracture patients. Nurses could then look at patient notes to distinguish low-impact, osteoporotic fractures from those caused by high-impact trauma using locally agreed criteria. Table 2 outlines some of the case-finding procedures used at the hospitals with varying methods.
| Hospital | Who? | How? | Patient groups |
|---|---|---|---|
| 8 | OG, orthopaedic surgeons, registrar | Preoperative joint trauma round, joint trauma meeting | All aged > 60 years with a fragility fracture |
| 9 | Osteoporosis nurse specialist | Referred for outpatient appointment | All inpatient hip fractures |
| 10 | FLN in liaison with OG | Computer system logs admissions and referrals | All aged > 50 years with a fragility fracture |
| 11 | FLN in liaison with OG | Postoperative ward rounds | All aged > 50 years with a fragility fracture |
Osteoporosis assessment
Most hospitals performed an initial inpatient osteoporosis assessment approximately 2 days postoperatively. In several hospitals this assessment was performed preoperatively and in some hospitals the timing of this could vary depending on the needs and condition of the patient, although other hospitals were less flexible. The assessments were performed by a fracture liaison nurse or by one of the orthogeriatric team. Protocols were in place at most hospitals for assessing the patient’s risk of further fractures and identifying other comorbidities which may influence treatment choice. When nurses conducted the assessment, they were often supported and advised by orthogeriatricians and rheumatologists in more complex cases. As shown in Table 3, two hospitals differed by undertaking the osteoporosis assessment in an outpatient setting, whereas the majority of other hospitals conducted theirs on the ward 2 days after surgery.
| Hospital | Who? | Where? | When? | Assessment |
|---|---|---|---|---|
| 1 | Rheumatologist | Outpatient appointment | Postoperatively | DXA report reviewed before deciding whether or not patient needs an assessment |
| 2 | OG | On ward and often in a second outpatient appointment | Preoperatively/early postoperatively | Pro forma for integrated care, FRAX® used in patients < 75 years Certain patients seen again by surgeons in outpatients |
| 8 | OG | On ward | 2–3 days postoperatively | Protocol in place, bloods often done in A&E |
| 9 | Osteoporosis nurse specialist | Outpatient appointment | Postoperatively | 15-minute appointment using FRAX® tool |
These outpatient assessments also varied in length and content; in one case a rheumatologist gave all patients a 30-minute assessment, in the second hospital the assessment was performed by an osteoporosis nurse specialist and lasted 15 minutes except in more complex cases when longer appointments could be given.
All hospitals reviewed in this study referred patients < 75 years of age for a DXA scan and initiated treatment without a scan in those > 75 years, in compliance with NICE guidelines. 13 Patients were often referred for a scan by the same clinician who undertook the osteoporosis assessment, although in two cases these referrals had to be approved separately by the rheumatology department. In another case, a clinician made a recommendation for a DXA scan on a patient’s notes and it was left to junior members of the team to follow up on this. At one hospital, a pro forma letter was sent to the patient’s GP, which had to be signed by the GP for DXA referral.
At most hospitals, DXA scanners were located off site, either at a different hospital or at a community hospital, while in several cases DXA scanners were provided by private companies. Scans were performed at varying times post discharge, ranging from 2–3 weeks to 8–12 weeks for an appointment. Only one hospital had a DXA scanner on site and was able to scan patients as inpatients providing that they were well enough.
The DXA reports were usually sent to the clinician responsible for making treatment recommendations, including orthogeriatricians, rheumatologists, fracture liaison nurses and GPs. In most cases, results were communicated to GPs as well as hospital staff.
Falls assessments and prevention
All hospitals provided a falls risk assessment alongside the initial osteoporosis assessment. Hospitals also had various other services available for more comprehensive multifactorial risk assessments looking at cognitive, physical and environmental risk factors such as balance and gait and hazards around the home. If necessary, patients were referred to other specialty clinics if they suspected there was an underlying medical condition putting them at risk of falling. Some hospitals ran a falls clinic in their outpatient department, while one hospital undertook a full assessment of patients while they were inpatients, which began on admission and was conducted by a multidisciplinary falls team. This hospital offered a very comprehensive service, with a specialist falls nurse and a falls champion on each ward. Table 4 shows how the nature, timing and location of the assessment varied across the hospitals studied.
| Hospital | Inpatient assessment | Further assessments |
|---|---|---|
| 1 | Community falls prevention service in place but it is unclear if hip fracture patients are accessing this service | |
| 2 | Assessed by OG, occupational therapist and physiotherapist | Patients may be referred for outpatients appointment for physiotherapy or to community occupational therapists |
| 6 | Assessed by senior nurses, physiotherapists and occupational therapists | Geriatricians have a falls clinic in outpatient department for more formal assessment, which is rarely needed as the ward assessment is so comprehensive |
| 10 | 20-minute ward assessment by FLN | If necessary, patients are referred to one of several community hospitals around region for 1.5-hour assessment by a physiotherapist. Patients may even be seen in their own home |
Treatment initiation
At 8 out of the 11 hospitals, osteoporosis treatment was prescribed within inpatients for those aged > 75 years in line with NICE guidelines. 13 Orthogeriatricians were generally able to make treatment recommendations and prescriptions. Fracture liaison nurses were also able to make treatment recommendations in some cases, and these were written up in patient notes and later prescribed by doctors. At one hospital in which the fracture prevention service was mainly run in an outpatient setting, an osteoporosis nurse specialist made treatment recommendations and sent these to the patient’s GP, who was left to initiate treatment.
For those aged < 75 years who had received a DXA scan, treatment was initiated in outpatient clinics or delegated to primary care pending the results of the DXA scan. This also applied to patients who were unable to commence treatment as inpatients for other reasons. Treatment recommendations were usually communicated to GPs via a discharge summary.
Monitoring and treatment adherence
Seven of the 11 hospitals reported undertaking monitoring of patients after discharge in a secondary care setting. Methods for monitoring included telephone calls and questionnaires to check how patients were getting on with their medication. One hospital offered a follow-up appointment 6 weeks post discharge at a fracture clinic run by an orthogeriatrician to those patients who were discharged to their own home. However, most hospitals referred patients to an outpatient clinic only if there were more serious complications, such as fractures while on treatment, or if the patient was severely osteoporotic. At the remaining hospitals, all monitoring was delegated to primary care.
Changes in service delivery at hospitals in the past decade
The changes in service delivery at each hospital are summarised in Table 5. These were identified through the service evaluation questionnaire, with additional information supplemented from the qualitative work. Members of the project working group agreed on the key changes that had occurred based on the data collection, with significant input from clinicians around what factored as a key change.
| Hospital | Date | Change |
|---|---|---|
| 1 | November 2012 | FLN appointed |
| 2 | May 2005 August 2007 |
OG appointed Second OG appointed. Metabolic bone clinic started conducting falls assessments |
| 3 | June 2011 | OG and matron for hip fracture unit appointed |
| 4 | 2006 May 2009 2011 |
OG appointed FLN appointed (began doing falls assessment in June) Community rehabilitation introduced |
| 5 | September 2009 | Trust-grade OG appointed |
| 6 | November 2005 2011 |
OG appointed Trauma specialty nurse appointed |
| 7 | July 2004 June 2007 October 2007 |
Geriatrician appointed, hip fracture ward opened A second trauma specialty nurse appointed Specialty doctor in geriatrics appointed |
| 8 | March 2009 | Clinical lead OG appointed |
| 9 | November 2003 April 2005 2009 |
Orthopaedic nurse specialist appointed Osteoporosis nurse specialist appointed Staff-grade OG |
| 10 | December 2004 May 2006 May 2008 November 2009 November 2011 |
FLN appointed FLNs and consultant lead appointed, comprehensive trauma FLNs appointed Consultant OG appointed New monitoring pathway and two geriatricians appointed |
| 11 | February 2011 November 2011 |
Consultant geriatrician appointed FLN appointed |
Looking specifically at how WTE of specialist staff changed between 2003 and 2013 (Table 6), we see a general increase in the number of consultant orthogeriatricians and specialist nurses across most hospitals in the region, reflecting new guidelines for both optimal recovery after hip fracture and secondary fracture prevention introduced during this period.
| Hospital | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||||
| OG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FLN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.5 |
| 2 | ||||||||||
| OG | 0 | 0 | 0.6 | 0.6 | 0.6 | 2 | 2 | 2 | 2 | 2 |
| FLN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | ||||||||||
| OG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0.3 |
| FLN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | ||||||||||
| OG | 0 | 0 | 0 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| FLN | 0 | 0 | 0 | 0 | 0 | 0 | 1.3 | 1.3 | 1.3 | 1.3 |
| 5 | ||||||||||
| OG | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 4.5a | 4.5 | 4.5 |
| FLN | 8.8 | 8.8 | 8.8 | 8.8 | 8.8 | 0 | 0 | 0 | 0 | 0 |
| 6 | ||||||||||
| OG | 0 | 0 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 |
| FLN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 |
| 7 | ||||||||||
| OG | 0 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| FLN | 1.3 | 1.3 | 1.3 | 1.3 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 |
| 8 | ||||||||||
| OG | 1 | 1 | 1 | 1 | 1 | 1 | 1.6 | 1.6 | 1.6 | 1.6 |
| FLN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | ||||||||||
| OG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FLN | 0 | 4 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| 10 | ||||||||||
| OG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 |
| FLN | 3 | 3 | 1.9 | 3 | 3 | 3b | 3 | 3 | 3 | 7.6 |
| 11 | ||||||||||
| OG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.3 |
| FLN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Discussion
Description of current services
In conducting a service evaluation designed to look at changes in service delivery at a set of hospitals over the past 10 years, a number of observations were noted. Although there has been an overall increase in service provision in terms of both orthogeriatric and fracture liaison nurse appointments, a considerable variation remains in how fracture prevention services at 11 NHS hospitals in one region of England are organised. The largest disparities were in how the service was run (an inpatient service vs. an outpatient service) and in the levels of staffing provided for the FLS service. Some hospitals had only a consultant-led service in order to improve perioperative care of fracture patients, whereas other hospitals had a nurse-led service to tackle fracture prevention. Few hospitals have both a fracture liaison and orthogeriatric service, as is recommended by CGs. 1,13,15,29
Details on staffing levels across hospitals were already provided by the NHFD; however, this evaluation showed that the role of certain staff members such as orthogeriatricians also varied between hospitals in terms of their role in case finding, osteoporosis assessment, treatment initiation and monitoring. In some cases, these roles were actually performed by more junior members of staff. Although some hospitals reported having an orthogeriatrician in post, their role in fracture prevention may be extremely limited.
Changes in service models
The previous decade has demonstrated marked increases in the provision of both orthogeriatric and/or FLS nurse staff in each of the hospitals. Only in one hospital was a service discontinued. However, the increase in service provision is variable, with little relationship to the number of hip fracture patients. By the end of the period of observation, the number of orthogeriatric staff per 1000 hip fracture patients varied from 0 to 4.3. The equivalent number of FLS nurses to numbers of hip fractures varied from 0 to 9.3. This variability is greater than one would expect for local differences in NHS structure or patient case mix and again demonstrates the need to relate service investment with clinical effectiveness in order to demonstrate the relative value of such services.
Strengths and limitations
A major strength of this evaluation is the heterogeneity of NHS hospitals examined, from smaller district general hospitals to large tertiary major trauma centres, adding to the generalisability of the report’s findings. A limitation is the reliance on clinicians’ recall and understandings of events over the last decade. Although human resources departments can confirm the appointment of new staff, changes may often come about by alterations in the role and responsibilities of existing staff members.
Summary
Although this work stream initially set out to look at changes in fracture prevention services at hospitals in the past 10 years, an important outcome of this work was the detailed description of where similarities and differences in care pathways between different hospitals lay. In addition, this work highlights where gaps have existed in the care provided at one hospital, compared with others in the region.
The work so far provided an overview of the variations in models of care provided by hospitals in one region of England. There is more variability in service provision than can be accounted for by local variations, highlighting the need to link investment in these specialist posts with patient outcomes and effectiveness.
Chapter 3 Identifying the reasons why hospitals chose their specific model of service delivery and assessing barriers to change
Introduction
In this chapter, we present findings from the qualitative component of the study. This had three initial aims: (1) to find out how and why hospitals adopted their models of care; (2) to identify how secondary fracture prevention services can be successfully implemented, with a focus on barriers and enablers to change; and (3) to identify the elements of care that health-care professionals think are most effective. These three aims were addressed using concepts from extended Normalisation Process Theory (NPT) to provide a theoretical basis. In addition, because the process of business case development was a key issue in fracture service planning and delivery, we aimed to describe the experiences of clinicians and service managers of making business cases for FLSs.
This chapter provides a brief background to the qualitative study, describes the methods used and presents our findings. Our findings and discussion will be presented in two parts. Part 1 presents findings relating to the first three aims about how and why secondary fracture prevention services can be successfully implemented in secondary care, and we discuss the place of these findings in relation to current knowledge. Part 2 then describes findings relating to the fourth aim, focusing more specifically on the experiences of clinicians and service managers of making business cases for a FLS, and discusses these in relation to the current literature. After the two findings and discussion sections, we present the study’s strengths and limitations and a concluding section.
Background
Chapter 1 demonstrated that there is considerable variation in how fracture prevention services are organised and delivered in the region. 39 Despite findings from a number of national and international studies that demonstrate the efficacy of FLS,36 only 40% of hospitals in the UK deliver this service. 40 To date, no study has explored how best to implement these services or make effective business cases to obtain funding.
Implementation of complex interventions
The implementation of complex interventions is increasingly being studied using implementation theory. 41 Implementation research comprises a number of approaches and theoretical stances in order to help understand something of the complexity of change within health services. One such theory, extended NPT42 – which builds on two previous theoretical models, the Normalisation Process Model43 and NPT44 – describes how the collective actions of agents drives the implementation of new services. This provides a counterpoint to network perspectives whereby the implementation of innovations is viewed as a process of transmission and stabilisation through networks,45,46 and psychological perspectives that prioritise the role of individuals in instigating change. 47,48 The theory builds on previous iterations of the theory by combining the notion of implementation as a social process with psychological and network approaches to increase our understanding of the phenomena.
Extended Normalisation Process Theory
According to the theory, the successful implementation of an intervention is based on the ability of agents to fulfil four criteria, described using constructs. 42 These are outlined in Table 7.
| Construct | Description |
|---|---|
| ‘Capacity’ | Implementing an intervention depends on participants’ capacity to co-operate and co-ordinate their actions |
| ‘Potential’ | Translating capacity into action depends on participants’ commitment to operationalise the intervention |
| ‘Capability’ | The capability of participants to enact the intervention depends on its workability and integration into everyday practice |
| ‘Contribution’ | The implementation of an intervention over time depends on participants’ contributions to enacting it by investing in meaning, commitment, effort and appraisal |
Making business cases for a fracture liaison service
A potential barrier to the implementation of FLSs is the challenge of making effective cases to obtain funding. 32 Funding for the commissioning of new services is obtained by writing business cases and presenting them to managerial bodies in the trust. These cases may be developed by a range of professionals. Central to this process are the clinicians and service managers working within the department. Finance managers are also involved in costing developments and calculating their potential income generation. Clinicians and service managers from other departments may also have a role, along with patient representatives. If supported, these may be referred to local Clinical Commissioning Groups (CCGs) for final funding approval, especially in the case of larger-scale service developments.
Commissioning (purchasing) processes within the NHS are complex. In April 2013, responsibility for commissioning services changed from the Department of Health and local primary care trusts (PCTs) led by managers, to NHS England, a new organisation overseeing 211 GP-led local CCGs. These currently control 65% of the NHS budget and operate within defined geographical regions. The rationale of this restructure is that GPs are best placed to understand the clinical needs of the local population and are therefore able to utilise resources most effectively. 49 The introduction of CCGs has coincided with a rise in the number of foundation trusts in England. Foundation trusts are independent organisations responsible for providing over half of NHS hospital, mental health and ambulance services operating in local regions. They are contracted to deliver services that have been commissioned by local CCGs and NHS England and are regulated by an independent body called Monitor. A board of directors is responsible for setting budgets for the financial year and establishing targets and health-care priorities. 50
Commissioning has been targeted by the Department of Health as a means of raising standards in the NHS and ensuring that resources are evenly distributed. 1 The NHS Commissioning Board states that funding decisions should be systematic and transparent and that considerations should include cost-effectiveness, improvements in care quality and the strategic plans of NHS trusts. 51 Core public health priorities, prioritising the distribution of resources in key disease areas, have also been identified. 52
There is some support available for clinicians and service managers on how to develop business cases for a FLS. This includes written guidance,32,53 training courses54 and business case templates. 55 According to this support, business cases should describe the health profile of the population, including the numbers of fractures, outline service design with reference to established fracture prevention guidelines, outline costs, particularly how the service will generate savings,55 and utilise a number of different stakeholder groups. 32 More general guidance on the processes of designing services and making business cases also exists and some CCGs have developed business case templates to assist in this process. 56
Several studies have explored the experiences of commissioners of making purchasing decisions in the UK, including those operating under the now abolished PCTs57,58 and the CCGs. 59,60 These have examined relational aspects of commissioning57,61,62 and identified factors that impact on decision-making. 58,63 Recent research has also explored the new roles of GPs in the commissioning process. 64,65 However, only one study has explored the experiences of providers in acute care of making business cases. 66
Aims
This study enabled us to understand how secondary fracture prevention services can be effectively organised and successfully implemented in secondary care to help to inform the implementation and integration of these services into practice. It also provided information about experiences of business case development for FLSs and suggestions for effective strategies for health-care professionals and service managers about how best to develop business cases for FLSs in the future.
Methods
Sample
The sample comprised 43 professionals from all 11 hospitals in one region in England who were involved in the delivery or organisation of secondary fracture prevention after hip fracture. These included orthogeriatricians, fracture prevention nurses, trauma nurses, hospital practitioners in osteoporosis, surgeons and service managers. Participants were purposively sampled to include a range of characteristics such as profession and years in role and to ensure that they were adequately drawn from each of the 11 hospitals. 67
Potential participants were identified by the clinical lead/champion in osteoporosis and an operational service manager in trauma. In three waves of recruitment, the study team approached potential participants by e-mail to provide them with a brief overview of the study, including a participant information booklet for further information. If no response was received within 2 weeks, the e-mail was followed up with a telephone call. Snowball sampling68 was used such that participants recommended other professionals involved in fracture prevention. In total, 82 health-care professionals were contacted to take part in the study, of whom 43 agreed to take part. The remainder either declined or were unavailable. Rather than us aiming to achieve saturation,69 criterion sampling was applied to ensure an adequate range of professionals to enable us to address the issues under study. 70
Ethics approval
Ethics approval was provided by the University of Oxford’s Central University Research Ethics Committee in 2012, reference number MSD-IDREC-C1-2012-147. Each NHS trust involved provided research and development approval. Participants all provided their written consent prior to interview. This consent process emphasised the aims of the research and that participation was voluntary. Participants were also asked to provide their consent to audio-recording and to the inclusion of anonymous quotations in study publications.
Interview procedure
A qualitative researcher (Sarah Drew) undertook interviews in 2013. All interviews except one were conducted face to face at the participants’ workplaces; one interview took place by telephone. Interviews were between 30 and 50 minutes long. A topic guide (see Appendix 2) was used to help to ensure that similar questions were asked of all participants. The topic guide was structured into three areas. The first topic area was based on the four core elements of a fracture prevention service outlined by the IOF as part of the Capture the Fracture initiative. 34 This helped the researcher to explore participants’ views on the best models of care for the secondary fracture across the four main components of a fracture prevention service and co-ordination of care. The second area covered in interviews was structured around the four constructs of extended NPT to enable exploration of participants’ experiences of implementing fracture prevention services. The third area explored in interview was participants’ experiences of making business cases for a FLS, factors that they felt informed the decision of commissioners to approve the new service and what they considered to be the best strategies for making them. The interviewer used standard qualitative interview methods such as ‘probing’ to help achieve depth in the interviews. 71 After the first four interviews we revisited the topic guide and made amendments to it to ensure that it enabled us to successfully explore the issues under study. The topic guide shown in this report is the final topic guide used in the study. All interviews, including the first four pilot interviews, are included in the final data set.
Data analysis
Interviews were audio-recorded, transcribed, anonymised and imported into the qualitative data analysis software NVivo (QSR International, Warrington, UK). Analysis took place in two parts. Part 1 used coding and an abductive approach to address how and why secondary fracture prevention services can be successfully implemented in secondary care using extended NPT to provide theoretical basis to the analysis. Part 2 was an inductive thematic analysis, albeit that it was focused on exploring participants’ experiences of making business cases for a FLS, factors that they felt informed the decision of commissioners to approve the new service and what they considered to be the best strategies for making them.
Part 1: how and why secondary fracture prevention services can be successfully implemented in secondary care using extended Normalisation Process Theory
Analysis was undertaken using an abductive approach. 72 This involved initial thematic analysis, which comprised inductive coding of data to identify themes and subthemes in the responses. 73 Coded data were then transposed onto the four constructs of extended NPT. In order to compare and contrast participants’ responses, data were then displayed on charts using the framework approach to data organisation. 74
Part 2: exploring participants’ experiences of making business cases for a fracture liaison service
Data were analysed using a thematic approach to identify themes and subthemes in the responses. 73 A framework approach to data organisation was also applied here to facilitate cross-comparison of responses. 74
In both parts of the analysis, 20% of the interview transcripts were independently coded by another member of the team (RG-H) and codes were compared and discussed to arrive at a single code list which was refined as the analysis progressed. 75 Descriptive accounts of the data were then generated and discussed.
Results
The sample comprised five service managers and 38 health professionals: eight fracture prevention nurses, four orthogeriatricians, four geriatricians, two GP osteoporosis specialists, five consultant trauma orthopaedic surgeons, eight rheumatology consultants, two orthopaedic nurses, one trauma matron, one matron for the hip fracture unit, one falls co-ordinator, one falls nurse, one bone densitometry specialist. Between three and seven participants from each hospital took part in the study. The range of time spent in their current roles ranged from < 1 year to 32 years. Time spent working at the hospital ranged from < 1 year to 27 years. Of the 43 participants, 33 had experience of making business cases for a FLS and only these participants have been included in the second part of the analysis. Participants’ key characteristics are displayed in Table 8, but we present summarised information only in order to avoid the potential for identification of participants.
| Professional group | Number of participants | Years spent working in current roles (range) | Years spent working at the hospital (range) | Number of these participants who had experience of making business cases for a FLS |
|---|---|---|---|---|
| Fracture prevention nurses | 8 | 1–15 | 1–27 | 5 |
| OGs | 4 | 4–13 | 4–16 | 4 |
| Geriatricians | 4 | 3–21 | 2–6 | 4 |
| GP osteoporosis specialists | 2 | 10–43 | 10–20 | 2 |
| Consultant trauma orthopaedic surgeons | 5 | < 1–32 | 2–19 | 3 |
| Rheumatology consultants | 8 | 5–25 | 5–25 | 6 |
| Additional nursing staff (orthopaedic nurses, trauma matrons, matrons for hip fracture units) | 4 | 3–14 | 3–25 | 3 |
| Falls co-ordinators | 1 | 1 | 20 | 0 |
| Falls nurses | 1 | 23 | 23 | 0 |
| Bone densitometry specialists | 1 | 11 | 12 | 1 |
| Service managers | 5 | 2–6 | < 1–5 | 5 |
Findings and subsequent discussions from the two parts of the study are presented in the following sections.
Part 1: using extended Normalisation Process Theory to understand how and why secondary fracture prevention services can be successfully implemented, barriers and enablers to change and elements of care seen as most effective
Findings
A summary of the themes identified and their relation to the four main constructs of extended NPT are outlined in Table 9.
| ‘Capacity’ | ‘Potential’ | ‘Capability’ | ‘Contribution’ |
|---|---|---|---|
| Role of dedicated fracture prevention co-ordinator | High levels of support for introducing service | Fracture prevention co-ordinators ‘freeing up’ professionals previously engaged in care | Multidisciplinary team meetings |
| Multidisciplinary paperwork: protocols and pro forma records | Lack of support for introducing service from some professionals | Lack of time to deliver intervention | Clinical databases |
| Multidisciplinary teamwork: multidisciplinary team meetings, joint ward rounds | Relationships between different professional groups | Lack of capacity to administer DXA scans | Internal monitoring systems |
| Positive working relationships | Multidisciplinary team working | Challenges faced by service users in accessing services | External monitoring systems linked to funding |
| Location of professionals close to the service and each other | Role of fracture prevention co-ordinator | ||
| Challenge of securing co-operation and communication with GPs | Varying commitment from practitioners in primary care | ||
| High workload in primary care impacting on time spent implementing intervention | |||
| Written communication with GPs, especially discharge summaries and DXA reports | |||
| Potential role of fracture prevention co-ordinators in primary care |
Health-care professionals’ views about issues that affect the implementation of services using the four constructs of extended NPT are presented in more detail below.
Capacity
Implementing the service depends on participants’ capacity to co-operate and co-ordinate their actions.
Participants’ capacity to co-operate and co-ordinate their actions was achieved by using dedicated fracture prevention co-ordinators who organised important processes of care and oversaw the delivery of the service. The presence of co-ordinators was seen as a key element in effective interventions because they managed the relationships between different professional groups to ensure that they were aligned with the aims and objectives of the service. Co-ordination was achieved using ‘multidisciplinary paperwork’, such as protocols and pro formas, that meant information was accessible and shared between participants. Multidisciplinary working such as multidisciplinary team meetings also facilitated communication, enabling the team to discuss patients and jointly develop policy. As a result of these factors, co-operation between participants in secondary care was high.
The physical location of professionals in relation to the centre of the service influenced the levels of communication and enthusiasm for the intervention. Participants’ capacity to co-operate and co-ordinate with GPs was, therefore, viewed as challenging. Additional factors that were seen to impact on GPs’ investment in the intervention included the lack of reimbursement for delivering fracture prevention services in relation to other conditions, and high workloads. To facilitate communication, participants suggested improving written communications such as discharge summaries and DXA reports. However, participants also recognised the limitations of these strategies. Introducing fracture liaison nurses into the community was suggested as a means of providing advice and guidance to GPs and overseeing the implementation of services in primary care. Quotations from participants supporting these findings are included below.
[As the fracture prevention co-ordinator] I’m the key link in it all to be honest; it’s very much me who kind of sits in the middle really. I will link out to anybody any service that the patient needs.
Participant identification (ID): 002
[The fracture prevention team] have a separate Monday morning meeting as well to discuss every patient individually. So I think because of that there is a lot of communication both verbally and written as well, so I don’t think we have any issues there at all.
Participant ID: 003
The meetings that we attend [are useful as] you kind of gain a mutual professional respect.
Participant ID: 004
There’s not been a huge amount of engagement with primary care . . . they seem to be very – two separate camps: there’s what actually happens in trauma and then there’s what happens in primary care, and the communication is difficult.
Participant ID: 009
GPs get probably 400 or 500 letters a day, do they read everything? Hopefully they do.
Participant ID: 035
GPs are fantastic, but how can they be experts and know everything . . . and that’s why I think we have a duty to them and to our patients to inform appropriately.
Participant ID: 042
Potential
Translating capacity into action depends on participants’ commitment to operationalise the fracture prevention service.
Professionals were enthusiastic about the introduction of services, and such enthusiasm enabled services to be started and to continue to deliver effective care. Participants described having considered services to be necessary before they were implemented and identified the importance of a ‘strong lead’ in the introduction of a new service. Counter to this, they often characterised less supportive professionals as ‘difficult’ and ‘obstructive’ potential barriers to implementation. It was clear that, in some cases, opposition of colleagues to the introduction of a new service was founded on the complex, hierarchical relationships between different professional groups. However, as these individuals were a minority, their presence was not seen as presenting an insuperable barrier to implementation.
In addition, continued commitment of the multidisciplinary team to enacting the intervention was enabled and facilitated by the fracture prevention co-ordinators who managed different professional cultures within the multidisciplinary team, and ensured that their aims and objectives were aligned. One of the main challenges encountered was the sense that GPs were not as committed to the enactment of the intervention. Quotations from participants supporting these findings are included below [quotations reproduced with permission from Drew S, Judge A, Cooper C, Javaid MK, Farmer A, Gooberman-Hill R. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteopors Int 2016;27:1719–27, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode)].
I mean we were so desperate for it that we bent over backwards to make it work. I mean it was never a problem in the sense that if it’s a service you’ve been asking for a very long time, if someone finally provides it you’re going to make sure it works and give that individual as much support as they need to come into the system.
Participant ID: 001
So there’s some people who historically are the ones that are resistant to change are just dissatisfied, disgruntled, have lost enthusiasm, just mistrust, have become jaded with the system . . . Sometimes it can be very obstructive.
Participant ID: 006
I think those particular individuals probably looked down on geriatricians and so they didn’t like it that a geriatrician was coming and saying . . . this is when you operate and they didn’t like being told what to do.
Participant ID: 005
It’s just about encouraging people to know that they all have a role, we all have a responsibility to deliver the quality care and all of us are important in making that so. You know each of you can’t do it without the other and it’s actually about ownership and responsibility.
Participant ID: 026
It needs a strong lead, it needs a me or equivalent of me really . . . In terms of the orthopaedic team, you know you’re working with orthopaedic nurses so it’s a different culture set . . . So you need to sort of pull in the ethos.
Participant ID: 026
I think the amount of interest from general practice is variable and I think the variability to be honest on the whole is average if you’re lucky. And I think there isn’t an engagement I don’t feel, in the majority of primary care, and ownership of secondary prevention fracture.
Participant ID: 027
Capability
The capability of users to enact the components of a fracture prevention service depends on those components’ potential for workability and integration into everyday practice.
Participants saw the workability and integration of services into everyday practice as high when FLSs provided a new layer of service provision. Their presence freed up the capacity of other professionals previously responsible for undertaking this role and otherwise did not change the content of their work. However, there were also considerable barriers to implementation. Participants viewed some services as under-resourced and understaffed, which limited the time they were able to dedicate to effective patient care. Administrative and communications work was described as particularly time-consuming. Participants also felt that clinicians lacked equipment to deliver certain aspects of patient care, and this sometimes included poor access to DXA scanners. Finally, the organisation and delivery of these services presented patient barriers to access. For instance, participants felt that some patients struggled to reach services because of difficulties with public transport; this was particularly problematic for patients living long distances away. Transport costs were also seen as a barrier for patients with limited financial resources. Quotations from participants supporting these findings are included below [quotations reproduced with permission from Drew S, Judge A, Cooper C, Javaid MK, Farmer A, Gooberman-Hill R. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteopors Int 2016;27:1719–27, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode)].
[The introduction of the service was not that difficult because] the model is that [the fracture prevention co-ordinators] just come in and do everything. So we’re not asking other people to do much else for hip fracture . . . We’re not even asking the trauma team to identify the hip fractures because the fracture liaison service is basically doing everything.
Participant ID: 007
I think there are obviously different demands on people’s time which sometimes means care is delayed.
Participant ID: 018
There is one orthogeriatrician who does all that work but of course she can’t be here 24 hours a day, 7 days a week so there have been some patients who will occasionally come through and slip through the net.
Participant ID: 001
We do at least 3500 scans a year with one scanner and two people . . . We have to meet the government targets, the 6-week diagnostic targets and we are just about making those targets with difficulty.
Participant ID: 025
Our biggest barrier is obviously the fact that we have to drag our patients from [another town] down to [the city] . . . So I guess it’s a demographics and the location in relation of distance travelled, that means sometimes people are reluctant [to attend].
Participant ID: 004
Contribution
Participants’ contributions to enacting a fracture prevention service depend on them investing in meaning, commitment, effort and appraisal.
Fracture prevention co-ordinators did not change the clinical work that was undertaken. Rather, their introduction changed the way the work was organised and delivered. Multidisciplinary meetings were used to sustain the potential and capacity of professionals involved in service delivery.
Clinical databases also enabled providers to deliver services over time as databases helped to define individual roles and ensured that work was not duplicated. Poor data quality presented difficulties, making it challenging for providers to consistently identify patients with hip fracture and to deliver clinical practice. Such data also presented obstacles to monitoring outcomes and this was seen as playing a vital role in ensuring that work was carried out to high standards over time. Monitoring operated in two ways. First, it enabled clinicians within the service to assess levels of adherence to bone protection therapies and evaluate service delivery, allowing them to reconfigure services if necessary. Second, it was a means of linking activity to funding mechanisms such as the Best Practice Tariff76 and Quality and Outcomes Framework77 in primary care, which potentially provided an important impetus for sustaining and developing high-quality services. The respective influence of these funding mechanisms was described as variable. Although participants saw the Best Practice Tariff as useful to provide financial benefit, they saw the Quality and Outcomes Framework as less valuable because it did not. Quotations from participants supporting these findings are included below [quotations reproduced with permission from Drew S, Judge A, Cooper C, Javaid MK, Farmer A, Gooberman-Hill R. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteopors Int 2016;27:1719–27, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode)].
I can modify [the guidelines for assessment] how I want to. You know because it doesn’t work perfectly at the beginning and things change and you know the hospital and the government introduce new requirements so you have to modify your form.
Participant ID: 017
It depends which doctors have been on the night before as to how much has been put onto [the computer system] . . . some of it turns out to be rubbish.
Participant ID: 010
[Auditing] it would be helpful because if you know what your compliance rate is you know whether or not you’re having an impact. So if you’ve got a service whereby you’re suggesting that they have a treatment and then it turns out that no one actually takes these, well then why are you wasting your resources on trying to you know.
Participant ID: 024
We have a score card so we can get all the compartments of the Best Practice Tariff . . . so we keep a track of what things are, which things are going well, which things we are not performing very well and obviously look for the reasons for any shortcomings.
Participant ID: 012
Discussion
The study used extended NPT42 to understand how and why hospitals adopted their models of care for the prevention of secondary fractures after hip fracture and identify how secondary fracture prevention services can be successfully implemented, with a focus on barriers and enablers to change and the elements of care that health-care professionals think are most effective. With regard to successful implementation, professionals’ capacity or levels of communication and co-operation were influenced by their distance in relation to the centre of the service. As a result, communication with GPs was viewed as challenging. Potential or enthusiasm for enacting the intervention was generally high, although participants identified exceptions. Shared commitments were facilitated by multidisciplinary team working. However, GPs were seen to be less committed to delivering fracture prevention.
Introduction of fracture prevention co-ordinators was advocated because their inclusion enhanced professionals’ capability to deliver the service, ‘freeing up’ other members of the team who had been previously responsible for this role. As a result the service could be easily integrated with existing services and the involvement of co-ordinators was seen as central to effective services. In addition, enthusiasm and leadership of professionals enabled services to be implemented and was a key reason for the introduction of new services. However, a lack of time and equipment and the challenges of accessing the services for some patients hindered implementation and had the potential to reduce the effectiveness of services for patients. Participants identified strategies to facilitate the delivery of the service over time, and they saw that good delivery was seen across the key elements of effective care. Strategies included multidisciplinary team meetings, clinical databases recording aspects of patient care, and monitoring to enable professionals to adapt and change the service when necessary.
Relationship to current literature
This study contributes to existing work on the delivery of fracture prevention by describing aspects of successful implementation, including barriers and enablers. Implementation of good services was seen as key to delivery of effective care for patients. Findings reflect those from previous studies that have advocated the use of fracture prevention co-ordinators in organising care. 10,32,34–36 The BOA also recommends enhancing co-ordination between primary and secondary care by developing comprehensive discharge summaries and introducing fracture prevention co-ordinators into the community. 1 Undertaking service audits and introducing cost-saving incentives have also been recognised as a means of ensuring that the service develops over time. 32 An additional finding from this study is the role of potential or actual commitments of professionals to enact the intervention and the role of fracture prevention co-ordinators in mobilising collective commitments within the multidisciplinary team.
Findings on the challenges of implementing these services can be placed in the context of recent work conducted on service delivery for other conditions. A lack of co-operation and co-ordination within secondary care and between primary and secondary care has been recognised across the NHS. 78–80 Furthermore, a lack of time to deliver care, lack of access to equipment and the inability of some sociodemographic groups to access services have also been identified. 81
Further research
It was clear in our qualitative work that communication with primary care was sometimes challenging, and research could further explore reasons for this in order to inform the design of possible improvements. Primary care clinicians have key roles in initiation of medication and ongoing monitoring of patients and further research could complement the findings presented here. Future research should also consider patients’ experiences to ensure that future service developments fully meet their needs and preferences. 82 Falls prevention is also critical in the prevention of further fractures1,82 and a future study exploring the experiences of delivering these services would contribute to a ‘system-wide’ understanding of the challenges of undertaking fracture prevention.
Part 2: exploring the experiences of clinicians and service managers of developing and making business cases for a fracture liaison service
In the second part of the qualitative research we explored the experience of business case development for a FLS. This was addressed through the latter part of the interviews, as described in Methods. Of the 43 participants in the study as a whole, 33 had experience of making cases (Table 10), and we analysed the material from these interviews in relation to this topic. In the findings presented here, we describe health-care professionals’ views and experiences of making business cases for a FLS; identify factors they think impact on the decision of managerial bodies to approve the service; and explore what they feel are the most successful strategies for making an effective case.
| Professional group | Number of participants | Years spent working in current role (range) | Years spent working at the hospital (range) |
|---|---|---|---|
| Fracture prevention nurses | 5 | 2–11 | 2–11 |
| OGs | 4 | 4–13 | 4–16 |
| Geriatricians | 4 | 3–21 | 2–6 |
| GP osteoporosis specialists | 2 | 10–43 | 10–20 |
| Consultant trauma orthopaedic surgeons | 3 | < 1–32 | 4–19 |
| Rheumatology consultants | 6 | 5–25 | 5–25 |
| Additional nursing staff (orthopaedic nurses, trauma matrons, matrons for hip fracture units) | 3 | 3–14 | 3–14 |
| Bone densitometry specialists | 1 | 11 | 12 |
| Service managers | 5 | 2–6 | < 1–5 |
Experiences of making business cases for a fracture liaison service
Participants felt that constructing business cases was challenging and at times frustrating. It was seen to be time-consuming, a problem made more difficult by their conflicting clinical commitments. Processes of approving the cases were viewed as ‘tedious’ and ‘incredibly cumbersome’. Participants felt that the propensity of operational and senior managers to remain in their posts for short periods of time exacerbated this problem, as it meant that any progress for developing the case was lost when they left. Having multiple tiers of management to approve the service was also viewed as a hindrance.
Clinicians had varying levels of confidence in their ability to make effective business cases and in their understanding of the approval processes. Some felt that it was unsuited to their skill set, while others felt that they lacked sufficient training. Collection of relevant data was viewed as a challenge, as the quality of data available was seen to be limited. Others were unsure how to demonstrate improvements in care quality.
Funding structures within the hospital were also viewed as an impediment to service development. The compartmentalisation of budgets meant that there were disagreements about which departments should be responsible for funding the service. These challenges were seen to arise from the necessary interdisciplinary working required to deliver the service, both within secondary care and between primary and secondary care. 1
The importance of effective communication and co-operation between stakeholders was emphasised. However, experiences of this were variable. Although some identified examples of successful co-working, others felt that some of their colleagues were more ‘resistant to change’. Participants also thought that relationships between clinicians and operational service managers were varied. Although some provided examples of effective working relationships, some service managers felt that they needed more support from clinicians in developing business cases and this view was reciprocated by a number of participating clinicians. In general, participants thought that increasing the level of co-operation between commissioners and providers (including trust managers) would be beneficial. Quotations from participants supporting these findings are included below.
[Making business cases is a] total and utter brick wall . . . and after a while you do lose the will to live.
Participant ID: 029
I will never be able to write a good business case, because I can’t. I don’t like doing them, I get bored, it’s not where my skills are so don’t ask me to do it.
Participant ID: 009
You constantly find you’ve got to go digging around for statistics. You’re trying to find numbers from data that isn’t collected . . . [it’s] all done on percentages and sort of guesswork.
Participant ID: 008
If you start getting into areas of complexity, so frailty complexity, where you get different services crossing: whose budget is it? It’s harder to cost them . . . just things like this don’t fit into categories.
Participant ID: 007
[The service manager’s] priorities are dependent on their interests.
Participant ID: 030
We felt that we were setting up and driving towards an improved Hip Fracture Service . . . but we were doing it without the support of our management.
Participant ID: 038
Views about fracture liaison service approval
Participants identified factors they thought impacted on the decision of managerial bodies to approve the service. Financial considerations were viewed as the most important factor and participants felt under pressure to demonstrate the potential for short-term cost savings. This was seen to be difficult when making business cases for FLSs, as fracture prevention tends to generate savings in the longer term.
The majority of clinicians thought that quality of care was given a low priority by funders, a view that was not shared by service managers. Political priorities such as dementia, as well as national guidelines, were seen as factors. This presented a further problem, as participants felt that osteoporosis was viewed as a low national priority and that, as a result, commissioners were less likely to approve the service. In addition, they thought that approval was hindered by a perception that osteoporosis should be managed in primary care and linked this to the increasing concentration of funds in the community. 83
Clinicians thought that services were more likely to receive funding if their audit results and clinical outcomes data suggested that they were ‘failing’. This was a source of frustration for participants at one hospital, who felt that they had had to wait for their service to ‘deteriorate’ before their business case was approved.
Participants thought that the personality and persistence of the ‘local champions’, the clinicians leading the case,32 influenced funding decisions along with the clinical interests of GP commissioners. Both of these factors suggest that participants felt commissioners did not always make funding decisions based on the principles of evidence-based health care. Quotes supporting factors seen to determine whether or not a FLS is approved are shown below [quotations reproduced with permission from Drew S, Gooberman-Hill R, Farmer A, Graham L, Javaid MK, Cooper C, et al. Making the case for a fracture liaison service: a qualitative study of the experiences of clinicians and service managers. BMC Musculoskelet Disord 2015;16:274, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode)].
The powers that be don’t want to put the money into it because you’re not going to see an instant result.
Participant ID: 015
It has kind of lost the quality of care a little bit . . . quality doesn’t pay the bills unfortunately.
Participant ID: 023
What tends to happen in health care is that you get certain things that become a certain flavour of the month. So lets look at this, this and this disease. Right what are we going to do in hospitals, whose doing that, right we think this is a priority would you like to do something in this area.
Participant ID: 026
I think it often comes down to the individual people who are doing it . . . you’ve got [a local champion in another hospital] sort of waving his little flag and everybody listens. Well if you hadn’t have had a him you may not have had that service.
Participant ID: 023
He who shouts loudest gets most.
Participant ID: 001
We have this problem with commissioning the local general practitioners, if you have a clinical lead who has a strong interest in dementia services then there will be lots of money put into dementia services.
Participant ID: 001
If the chairman of a CCG had had a mother with a hip fracture [laughs] that CCG, I can tell you, someone would push it through.
Participant ID: 008
Best ways of making a business case for a fracture liaison service
Participants identified a number of strategies for developing effective business cases. To help develop the skills of clinicians in making business cases, participants thought it was important that training courses, templates and toolkits were made more widely available. They also felt that capitalising on the skills and knowledge of clinicians and service managers in other hospitals would be useful, and advocated the introduction of mentorship schemes or support networks.
The importance of using a range of different types of evidence was highlighted. Empirical sources of evidence included CGs and academic research. A number of participants referred here to research conducted on the Glasgow FLS that showed the service contributed to a significant reduction in hip fractures within the city, compared with substantial rise across England. 19 To address the perception of osteoporosis as a low national priority, two clinicians thought that it would be useful to try to align the aims of the service with national priorities such as dementia. 52 Quotes on the best ways of making a business case for a FLS are given below [quotations reproduced with permission from Drew S, Gooberman-Hill R, Farmer A, Graham L, Javaid MK, Cooper C, et al. Making the case for a fracture liaison service: a qualitative study of the experiences of clinicians and service managers. BMC Musculoskelet Disord 2015;16:274, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode)].
You can use other [services as] comparators and the USA seems to be quite a good thing to beat doctors with at the moment so if you look at outcome in the best centres compared to the average centres, which is quite a good . . . we do this but [another trust] do this, what’s the difference? Well they’ve got 15 fracture liaison nurses and everyone doing this . . . [the trust] tend to benchmark things across local groups but if you’ve got national comparators those are quite good.
Participant ID: 021
I think patients’ voice can have more power, especially if we are looking at situations where we feel that patients may be at risk and I think that obviously helps to drive the changes as well.
Participant ID: 012
You also need to get allies on side who will help you and support you . . . people who are key, for example, [a GP], she wasn’t involved in commissioning or anything but she is very well respected in her field.
Participant ID: 004
I think the managers are too separate and it should be more embedded and integrated so that we work together and we come up with clinical designs and models, they tell us we’re stupid and spending too much money, and then we work it out and come up with a compromise for it.
Participant ID: 009
It’s through no fault of anyone’s but these are human organisations and you’ve got to engage with these things on a human level . . . It’s much easier to say no to someone who you’ve been saying no repeatedly to for the last five years and where you’re not regularly seeing them.
Participant ID: 024
Other, more practical, sources of data included the use of outcomes data, such as HES,84 to make cost-effectiveness calculations. Using outcomes data to benchmark the service against local and national comparators was seen as a means of demonstrating the need for service improvement. Involving patient representatives or using feedback questionnaires was viewed as a way of responding to the increasing need to involve patients in service design. 85
Promoting effective communication and co-operation between stakeholders was perceived to be a priority. Within the hospital, participants thought it was important to canvass the support of service providers early on in the process as a way of galvanising support, anticipating and mitigating objections and ensuring that input was received from a range of stakeholders. Service managers thought that the ‘local champions’ leading the case53 had an important role in securing the support of their colleagues. Obtaining support from ‘prominent clinicians’ was seen as a way of gaining the ‘respect’ of commissioners.
Participants felt that working more closely with purchasers would facilitate the process of making business cases. Strategies to achieve this were approaching commissioners early on in the process so they could provide feedback and liaising with them informally.
Discussion
This component of the study has explored the experiences of clinicians of making a business case for a FLS, identified factors that are seen to inform the decisions of managerial bodies to approve the service and identified what they consider to be the best ways of making a business case for a FLS. Participants felt that constructing business cases was challenging. Frustrations included the longevity of the process, a perceived lack of time and skills among clinicians for making the cases and a lack of available evidence for demonstrating potential improvements in quality of care. In addition, the compartmentalisation of hospital budgets meant that it was sometimes unclear which department was responsible for funding the service. The relationship between stakeholders involved in making the cases was seen to be variable and the levels of co-operation between purchasers and providers were generally low.
Participants identified a number of factors that they felt impacted on the decisions of managers to approve the service. Financial considerations were perceived to be the most important factor and clinicians felt that quality of care was afforded a low priority, a view not shared by service managers. Approval was seen to be hindered by the perception of osteoporosis as a low national priority. Participants thought that the personality and persistence of the clinical champion leading the case was important, along with the clinical interests of commissioners. Both of these factors suggest that participants did not think that commissioners consistently adhered to the principle of evidence-based health care. Strategies for making effective cases included the provision of training courses, templates, toolkits and mentorship schemes for stakeholders. Using different types of evidence to demonstrate the potential for care improvements was advocated and included national guidelines, academic research, outcomes data to demonstrate the cost-effectiveness of the proposed service and audit data. Strategies were suggested for developing effective working relationships between stakeholders when making business cases and improving the relationship between commissioners and providers.
Findings in this study support those from previous work that have explored providers’ experiences of commissioning. These studies showed that clinicians found the processes to be time-consuming66 and the collection of data challenging on account of the often low quality of data available. 66,86 Challenges of managing the relational aspects of commissioning have also been highlighted. 61,62,66 Research has found varying levels of communication and co-operation between purchasers and providers. Although one study found that the roles of purchasers and providers were well integrated,66 others, including ours, suggest that this is not the case. 61,62 Previous work exploring the processes of commissioning has been largely focused on the experiences of providers working under the old PCTs. Ours succeeds in demonstrating that these challenges remain under the new commissioning bodies. It also highlights additional problems such as the lack of confidence clinicians feel in developing business cases and the difficulty of navigating the funding structures in the NHS.
A number of the factors participants felt impacted on funding decisions were in accordance with guidelines issues by the NHS Commissioning Board. 52 These included quality of care, cost-effectiveness and the use of national guidelines. However, clinicians felt commissioners placed a greater emphasis on financial considerations than on these other factors. A belief that the clinical interests of GP commissioners impacted on decision-making reflects findings of previous studies. 86,87 Although the objective of introducing the CCGs was to ensure that the views of clinicians were represented in commissioning decisions,51 this suggests that the views of commissioners and providers may not always be in accord.
This study identified strategies to develop effective business cases that support existing recommendations. Guidance recommends using multiple sources of evidence to construct cases, including outcomes data to demonstrate potential cost savings, academic research and national guidelines. 53,66,88 Existing recommendations have also highlighted the importance of developing effective co-operation and communication between the stakeholders involved. Developing a multidisciplinary working group led by a clinical lead has been advocated as a means of achieving this. 53,54
Findings echo previous research that has suggested clinicians support more integrated working between health-care purchasers and providers. 66 As with this research, existing guidance suggests contacting commissioners early in the process to establish a funding remit for the service. 54 Another suggested strategy has been the introduction of commissioners into the multidisciplinary working group tasked with developing the case. 53 To enable the clinical lead to work effectively, guidance also recommends introducing protected time for the clinical lead and operational service manager to work on the case. 53 Doing so would address the lack of time participants identified as a barrier to developing the service.
Strength and limitations for the complete qualitative study
Qualitative research methods were an appropriate means of exploring the views of health-care professionals and managers on implementing fracture prevention service, identifying effective models of care and making business cases for FLSs. Using robust strategies to analyse the data, such as independent double-coding by two researchers, provides confidence that the analysis presented here reflects these views. As stated, the study did not aim to achieve data saturation but used criterion sampling70 to explore a diverse range of views, and this was successfully achieved. However, the time constraints of service managers meant that we were able to recruit only five participants from this group. This lack of representation made it difficult to fully examine how service managers’ opinions on making business cases differed from those of other participants. In exploring the implementation of fracture prevention services and experiences of making business cases, participants were asked to recall their experiences, which may have resulted in bias. In addition, the study was limited to one region in England and this could limit the transferability of findings to other areas. 89 However, the variation in service delivery in the region and the homogeneity of views suggest that it is unlikely that this presents a limitation.
Undertaking an abductive analysis72 to enable us to use extended NPT42 is potentially challenging, as data must be coded into constructs while it is ensured that they are not ‘forced’ into pre-defined categories. We avoided this problem by performing an initial inductive analysis73 to identify factors that may impact on the implementation of services and then transposing them onto the theory. Doing so meant that any factors that did not ‘fit’ within the theory would have been identified. However, we found that the theory was able to account for all of the issues relating to service implementation.
Conclusions
The study has successfully used extended NPT42 to understand implementation of services for secondary fractures after hip fracture, with a focus on barriers and enablers. Clinicians and service managers described these in some detail, and saw good provision as central to effective care. Drawing on their own experiences, participants identified enthusiasm, leadership, communication and the presence of co-ordinators as key to the introduction and delivery of effective services. Furthermore, given the importance of business case construction for provision of services, we also explored and have described views and experiences of making business cases for FLSs. We have highlighted the challenges of business case development and some ways in which these can be addressed. It is hoped that these findings may be used by clinicians and service managers to develop effective fracture prevention services in the future and to make successful business cases for funding of services.
Chapter 4 Data sources
Introduction: the use of routinely collected data for research
Routinely collected health data are widely used by regulators (e.g. Medicines and Healthcare products Regulatory Agency, European Medicines Agency) and policy-makers (such as NICE) to inform disease epidemiology/burden, health-care resource use, drug or device safety and real-world use of existing technologies in actual NHS settings.
There is a breadth of computerised health records data available for research, both in the UK and Europe-wide. Potential sources of such information include drug utilisation databases, primary care records, secondary care outpatient data, hospital admission(s) episodes, drug registries (e.g. the British Society for Rheumatology Biologics Register registry of antirheumatic biologic therapies), device registries (e.g. the National Joint Registry), and even patient-centred outcomes as collected during routine clinical practice (the Patient-Reported Outcomes Database) (Figure 2).
FIGURE 2.
Examples of existing sources of routinely collected data in the UK and Europe-wide. BSRBR, British Society for Rheumatology Biologics Register; CMBD, Conjunto Mínimo Baásico de Datos (National Information System for Hospital Data); DANBIO, The Danish Registry for Biologic Therapies in Rheumatology; EU, European Union; NJR, National Joint Registry; PROM, patient-reported outcome measure.
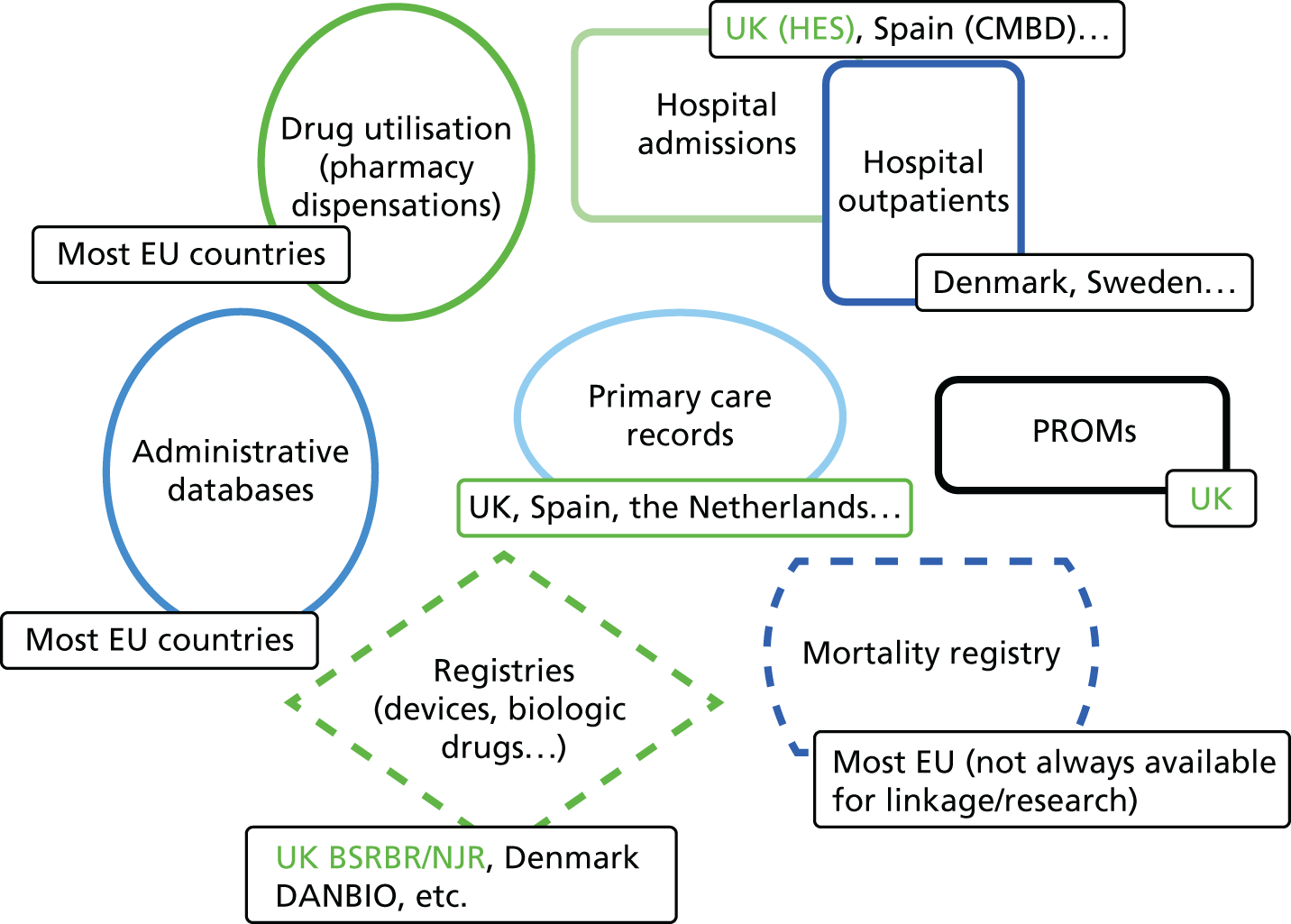
The Clinical Practice Research Datalink
General practitioners and the NHS: why is the Clinical Practice Research Datalink a valuable source of data?
Primary care health services play an essential role in the UK NHS. GPs working in NHS primary care play the role of gatekeepers to the system: access to secondary care is possible only after referral from a GP practice, with the exception of urgent attention in hospital emergency departments.
In addition, GPs are a key part of NHS health-care continuity: they receive clinical letters from secondary care (consultants and other health professionals) to inform or suggest shared therapeutic plans, and they are responsible for most repeat prescriptions for long-term conditions. As part of this, they also receive hospital discharge letters regarding all patients registered with them; the information contained, including inpatient treatments and diagnoses, among others, is then used by GPs to co-ordinate outpatient care (Figure 3). All of this adds value to the information gathered in primary care medical records.
FIGURE 3.
The role of GPs in the NHS, and the flow of information into primary care GP records. A&E accident and emergency.
The CPRD gathers information from computerised primary care records, which can be enriched with other digital linked data from a number of data sets, providing a unique source of information for epidemiological research.
The Clinical Practice Research Datalink
Jointly funded by the National Institute for Health Research (NIHR) and the Medicines and Healthcare products Regulatory Agency, the CPRD (formerly General Practice Research Datalink) is a unique data source, used extensively by researchers worldwide for observational and interventional research. Such studies have resulted in > 900 published articles and a huge number of conference presentations.
The core CPRD Gp OnLine Data (GOLD) version of the database was used as the source from which patient-level bespoke data sets were extracted. It comprises anonymised clinical records for a representative > 10 million people living in the UK. For this sample, the data set contains information on any diagnoses (primary and secondary care based) recorded by GPs in primary care records using Read/OXMIS codes, clinical measurements taken in primary care (including body mass index, smoking, alcohol drinking and others), routine laboratory results, referrals, GP prescriptions [identified using British National Formulary (BNF) codes90] and administrative information (date of registration in the primary care practice, date of death, etc.). The encoding of patient identifiers ensures anonymisation of the data. A full list of medical codes used to identify hip fractures can be found in Appendix 3.
This variety of data was provided in a number of text files, which have been checked, imported and linked in a database management system (MySQL, Oracle Corporation, Cupertino, CA, USA) using programming (Python; www.python.org). The required variables have then been extracted from the database in the right format to be analysed using conventional statistical packages.
Linked hospital and mortality data
About 60% of the primary care practices contributing to CPRD have agreed to linkage to the HES and Office for National Statistics (ONS) data. 84,91 These data sources provide information on hospital admission episodes in NHS hospitals and mortality (date and cause of death), respectively.
Hospital Episode Statistics and ONS information was also combined with the core CPRD GOLD data set to improve the completeness and accuracy (of diagnoses, inpatient procedures and mortality) of the data analysed.
Hospital Episode Statistics
Introduction
The HES database gathers clinical information on the records for hospital admission episodes in England, including both those in NHS hospitals and those in the independent sector that are commissioned by the NHS. Data on such episodes have been recorded from 1987, and only a sample (around 10%) of the episodes were included before that time.
Inpatient data are available from 1989–90 onwards; these were initially delivered every financial year but more recently have been delivered on a monthly basis. Since 2006, data collection and preparation have been undertaken by the Secondary Uses Service, which is part of the Health and Social Care Information Centre.
The data collected in HES are submitted by participating hospitals in all NHS trusts in England for reimbursement purposes. Although it is primarily an administrative database, HES is designed to allow also for secondary use for research.
Data available in Hospital Episode Statistics
For all NHS hospital admission episodes, outpatient and accident and emergency (A&E) appointments in England, each HES record contains the following information:
-
hospital diagnoses and procedures
-
administrative data – date of admission/discharge, etc.
Hospital inpatient data are available from 1989 onwards, outpatient appointments data are available from 2003 and A&E information has been recorded since 2007.
A bespoke anonymous patient ID (called gen HESid) is created for each individual, and used to track patients through the data set while preserving confidentiality. The patid (allocated to each patient from his or her practice) is used to enable linkage of HES data to other relevant data sets, such as ONS mortality and CPRD data.
Hospital admissions in Hospital Episode Statistics
Of the different types of information available within the HES database, this study used a data set including hospital admission episodes. Such records contain information on a primary hospital diagnosis (reason for admission), a number of secondary/concomitant diagnoses (comorbidity) and a number of in-hospital procedures.
In addition, information on patient complexity used for reimbursement is also available in the form of Healthcare Resource Group (HRGs), which can be used to calculate the cost to the NHS of each hospital admission episode.
Chapter 5 Clinical effectiveness of service models of care following hip fracture: natural experimental study
Introduction
The clinical effectiveness of orthogeriatric and FLS has been previously demonstrated with varying study designs. Two trials from Scotland randomised patients admitted with a hip fracture to either stay on the orthopaedic ward or be transferred to a rehabilitation ward. The rehabilitation ward was characterised by a weekly case conference and ward rounds by both geriatric and orthopaedic staff; this was the orthogeriatric model. 92,93 One trial demonstrated a significant reduction in discharge to institutional care and length of stay,92 while the other demonstrated an increased number of comorbidities diagnosed and treated. 93 A retrospective obesrvational study comparing 30-day mortality between 37 Australian centres with and without any type of orthogeriatric service demonstrated that 30-day mortality was lower in those sites with an orthogeriatric service. 94 Although Charlson comorbidities were recorded, a number of key potential confounders such as pre-fracture health status and deprivation were not recorded; in addition, the presence/absence of an orthogeriatric service did not affect mortality in major trauma centres.
In terms of secondary fracture prevention, the current literature has confirmed that well-meaning patient and/or physician education strategies are ineffective. 95 The current consensus supports more intensive service models of specialist nurse-led services:10,32
-
Observed differences in hip fracture rates before and after introduction of a fracture prevention service: South California S Permanente, CA, USA, has demonstrated a 37% reduction in hip fracture rates with an associated 2–6 times increase in DXA rates and prescribing rates. 96 However, there was no contemporary control arm and only three pre-intervention data points to accurately model an exponential fracture rate trajectory. Importantly, the Kaiser secondary fracture prevention model requires an integrated information system linking hospital admissions, primary care physician visits, bone density scanning and pharmacy dispensing to case find, assess, initiate and detect discontinuation, which, while an aspiration, is not available within the NHS at present.
-
Using osteoporosis drug therapy rates:
-
The Glasgow FLS, UK, used 8-year audit data to inform a Markov model of secondary fracture prevention. 20 Using this model they were able to demonstrate only a 7% reduction in fractures at 5 years. However, the prescribing rate in the control group was estimated from national audit data; all patients were assumed to remain on treatment for 5 years despite no active monitoring programme; the effect of treatment on fracture rate reductions was estimated using published trials; the benefit for 5 more years as an off-treatment effect was assumed but remains unproven in clinical studies. In addition, those unable to take oral bisphosphonates were not switched to injectable therapies that are now available but kept on oral calcium and vitamin D supplements; this resulted in a disappointing 54% initiation rate.
-
A study in Alberta, Canada, alternated the presence of a fracture liaison nurse between two emergency centres every month for patients presenting with a wrist fracture. They demonstrated significant improvements in prescribing of bone agents of 34/55 (62%) compared with 8/47 (17%). However, the trial was stopped prematurely because of the clear benefit in treatment initiation before effects on bone density or fracture rates could be assessed. 97 Patients with cognitive impairment or patients admitted to hospital were excluded and no health economic data were collected.
-
A study in Toronto has published cost-effectiveness results on treatment initiation and 1-year adherence rates in co-ordinator versus non-co-ordinator settings and demonstrated significant cost savings. 98 Again, this study did not directly measure refracture rates, estimated treatment initiation and adherence in the non-co-ordinator setting and used fracture reduction rates based on clinical trials.
-
-
Using observed fracture rates from patients who did or did not attend a specialist service: the Concord study, Australia, demonstrated an 80% reduction in fractures over 5 years. 26 However, using patients who did not attend the specialist clinic as the ‘comparator’ group resulted in significant immortal time bias and selection bias in those who attended were more likely to be healthier and have fewer comorbidities. Finally, only 20% of all fragility fracture patients attend the specialist service, as those with cognitive impairment and other serious comorbidities were excluded; this is an important limit to generalisability of the service in terms of extension to all fragility fracture patients.
The wide variation in effect size from 7% to 80% from the published literature illustrates the magnitude of potential biases but also the lack of consistency between service models. No study has used robust outcomes such as refracture rates as highlighted by recent systematic reviews. 95,99 A recent meta-analysis demonstrated a trend towards improved bone mineral density testing rates and treatment initiation. 10,36
However, data on key clinical outcomes are scarce,100 especially in the context of actual practice conditions in a NHS setting. It has been demonstrated elsewhere that lower mortality is associated with both an orthogeriatric service94 and the introduction of a dedicated fracture nurse. 101 Recent studies have also reported that co-ordinator-based programmes can significantly reduce the rate of total hip fracture admissions96 and refracture rate after a non-vertebral fracture. 26,101 Despite these data, a recent audit in the UK found that only 37% of local health services provide any kind of FLS. 40 Importantly for both orthogeriatric and FLS services, significant variation exists in how such services are structured. 3,39
Two recent systematic reviews have confirmed that patient/physician education strategies are ineffective. 26,36,95 They identified major deficiencies in current literature, which included (1) using treatment initiation as the primary outcome97 – this is a poor surrogate given the low adherence to therapy; (2) non-contemporary or estimated control data;20,25 (3) effect sizes based on randomised controlled trials (RCTs) for the drug;20,98 and (4) attendee versus non-attendee designs. These are subject to significant selection bias and left censoring. 26,102 The reported range of fracture reduction from a FLS from 7% to 80% evidences the magnitude of effect of these biases. This is unhelpful when using the potential benefits to guide prioritisation for commissioners and policy-makers. In this chapter we will summarise the key findings from using the variability of timing of orthogeriatric and FLS services to infer estimates of effectiveness.
Aims
To evaluate the impact that changes to the delivery of secondary fracture prevention have had on health outcomes by altering trends in:
-
hip refracture rates
-
life expectancy – 30 days and 1 year.
Methods
Data sources
A cohort of hip fracture patients treated at hospitals in the South Central region using English national HES data was linked to the national mortality data from 1999 to 2011. The data came from a linked data set of English national HES and data from death certification built by the team that developed and manages the Oxford record linkage study. The HES data set includes records of all inpatient episodes undertaken in NHS trusts in England, including acute hospitals. Information about deaths comes from death certificates held by the ONS, which includes information on the date of death. A cohort of hip fracture patients (4260 patients per year in South Central) was identified through OPCS Classification of Interventions and Procedures version 4 (OPCS-4) operation codes103 and International Classification of Diseases, Tenth Edition (ICD-10) diagnostic codes. 104 The hospital provider code allowed identification of the 11 hospitals in the region.
Information on hip fracture hospital admissions was obtained from the HES database. Each record relates to a ‘finished consultant episode’: the period of time an individual spends under the care of one NHS consultant. Private procedures are excluded from HES, as there is no requirement for private hospitals to provide routine data. Mortality data from the ONS were linked and extracted using an encrypted patient identifier.
Patients
All patient episodes containing an ICD-10 code indicating hip fracture (S72.0, S72.1, S72.2 or S72.9) as the primary diagnosis and with a start date occurring within the study period (1 April 2003–31 March 2013) were identified (Figure 4). Hip fracture episodes were excluded if they indicated the patient was < 60 years of age, admitted as a day-case, admitted as a non-emergency or admitted as a result of trauma such as a transport accident (external cause ICD-10 codes: V01–V99). Hip fracture episodes were excluded if it was indicated that the patient had been admitted for any previous hip fracture (regardless of eligibility criteria) any time between 1 April 1999 and 31 March 2013. Provider codes and treatment site codes were used to identify episodes occurring within the 11 hospitals in the region of interest.
FIGURE 4.
Population flow diagram.
It is important to establish the laterality of the second versus index hip fracture to avoid double counting the index fracture. Given that the ICD-10 codes do not indicate laterality, we used the OPCS codes from NICE CG124 2011:29 W191, W241, Z762 + W192, Z762 + W193, Z762 + W242, Z763 + W192, Z763 + W193, Z763 + W242, W371, W381, W391, W461, W462, W482, W471, W472, W481, W931, W941 and W951. Eighty-six per cent of primary fractures and 79% of second hip fractures had relevant procedure codes.
Outcomes
The primary outcome of interest was time to second hip fracture within 2 years of a primary hip fracture. To ensure that this was a separate fracture and not the first fracture recoded, we counted second hip fractures only if admitted in a separate ‘continuous inpatient spell’ (CIPS) and at least 30 days after admission for the primary fracture. Secondary outcomes of interest were (1) time to death within 30 days; (2) time to death within 1 year following a primary hip fracture admission; and (3) non-hip fractures.
Interventions
The primary exposure (‘intervention’) was the implementation within individual hospitals of specific change to the model of post-hip fracture care. Information on the nature and timing of such changes had been obtained through a detailed evaluation of hip fracture services within the 11 hospitals of interest over the last decade, and was carried out prior to data being obtained. 39 Dates for the introduction or expansion of either an orthogeriatric or a FLS model of post-hip fracture care occurring throughout the study period were identified a priori (Table 11 and see Appendix 4).
| Hospital | Primary hip fractures, n | Age (years), mean (SD) | Sex (% female) | 2-year secondary hip fractures | 2-year major non-hip fractures | 30-day mortality | 1-year mortality | Time points of change to post-hip fracture model of care | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Proportion (%)a | n | Proportion (%)a | n | Proportion (%)a | n | Proportion (%)a | Nurse-led FLS | OG | ||||
| 1 | 3115 | 82.9 (8.3) | 74.8 | 146 | 5.0 | 79 | 2.5 | 309 | 10.6 | 949 | 31.3 | – | – |
| 2 | 3943 | 82.9 (8.2) | 74.2 | 161 | 4.4 | 59 | 1.5 | 378 | 9.9 | 1176 | 30.3 | – | May 2005, August 2007 |
| 3 | 1858 | 82.6 (8.1) | 73.6 | 80 | 4.2 | 50 | 2.7 | 178 | 9.7 | 586 | 31.2 | – | – |
| 4 | 2819 | 82.9 (8.3) | 75.8 | 99 | 4.1 | 57 | 2.0 | 242 | 9.3 | 753 | 28.5 | May 2009 | October 2006 |
| 5 | 1837 | 82.8 (8.1) | 73.9 | 56 | 3.4 | 41 | 2.2 | 154 | 8.7 | 528 | 29.7 | – | September 2009b,c |
| 6 | 1030 | 82.7 (8.1) | 73.8 | 41 | 4.1 | 28 | 2.7 | 60 | 5.5 | 238 | 22.6 | – | November 2005d |
| 7 | 5895 | 82.8 (8.2) | 76.6 | 206 | 3.7 | 173 | 2.9 | 489 | 8.8 | 1687 | 29.2 | June 2007c | July 2004 |
| 8 | 4937 | 83.0 (8.1) | 74.3 | 191 | 4.0 | 150 | 3.0 | 481 | 9.7 | 1549 | 31.7 | -– | March 2009 |
| 9 | 1994 | 83.1 (8.1) | 75.0 | 76 | 4.2 | 46 | 2.3 | 194 | 9.7 | 562 | 28.5 | April 2005 | – |
| 10 | 4218 | 82.9 (8.3) | 74.1 | 154 | 4.1 | 118 | 2.8 | 417 | 9.9 | 1213 | 28.9 | May 2006, May 2008 | November 2009b,c |
| 11 | 1506 | 82.7 (8.2) | 73.9 | 78 | 5.2 | 44 | 2.9 | 131 | 9 | 421 | 29.1 | – | – |
| Whole region | 33,152 | 82.9 (8.2) | 74.8 | 1288 | 4.2 | 845 | 2.6 | 3033 | 9.5 | 9662 | 29.8 | ||
Confounders
Confounding factors controlled for were age, sex, Index of Multiple Deprivation (IMD) score and Charlson Comorbidity Index (none, mild, moderate and severe).
Sample size calculation
1. Interrupted time-series analysis
The required sample size is dependent on (1) the number of data points available for analysis and (2) the number of observations within each data point. There is no gold standard, but it is generally agreed that the more data points and observations available the better. 105 A general recommendation is for at least 10 pre- and post-intervention data points,106 but there is a lack of consensus within the literature. The Cochrane Effective Practice and Organisation of Care Review Group (EPOC) review suggest three or more data points within each section of the time series, but this would be dependent on the number of observations available. 107 A minimum of 100 observations at each point of the time series is considered desirable, to achieve an acceptable level of variability of the estimate at each time point. 108 However, this will also depend on the prevalence of outcome being estimated. Hence for rare outcomes such as second hip fracture, numbers required at each time point will be higher than for the more common mortality end points.
2. Survival regression models
We used a two-sided log-rank test for equality of survival curves, with 80% power at a 5% level of significance (alpha). In the pre-intervention (control group) we assumed a 1-year mortality rate of 30%, 30-day mortality of 10% and a 2-year second hip fracture rate of 6%. For the mortality outcomes there is no loss to follow-up as information on date of death is obtained through linked ONS mortality data, while for second hip fracture we allow for 30% loss to follow-up owing to mortality. The required sample sizes for each outcome were as follows:
-
To detect a 5% absolute difference in 1-year mortality (30% pre intervention vs. 25% post intervention), equivalent to a hazard ratio (HR) of 0.81, the total sample size required is 1214 patients in each group (2428 in total with 683 expected events) assuming equal-sized groups where the intervention is in the middle of the time series. For an intervention occurring towards the end of the time series, allowing for unequal-sized groups in a ratio of 4 : 1, we require 3068 patients in the time period before the intervention and 1023 in the post-intervention period, with 1186 expected events.
-
To detect a 3% absolute difference in 30-day mortality (10% vs. 7%), a HR of 0.69, assuming equal-sized groups, requires 1356 in each group (a total of 2712 patients and 231 events). With unequal-sized groups in the ratio of 4 : 1, this is 3514 pre intervention and 1171 post intervention (4685 total with 441 events).
-
To detect a 3% absolute difference in second hip fracture (6% vs. 3%), a HR of 0.49 requires 890 in each for equal-sized groups (1780 total with 68 events). For a 4 : 1 ratio, 2504 pre intervention and 835 post intervention are required (3338 total with 152 events).
Statistical analysis
1. Interrupted time-series
Data were aggregated in the form of age- and sex-standardised quarterly proportions of each outcome of interest. A segmented linear regression model was specified for each outcome, which divides the time series of biannual proportions into pre- and post-intervention segments while controlling for baseline trend:
Here, Yt is the proportion of outcome within time point (i.e. 3-monthly period) t. β0 estimates the baseline level of the outcome at the beginning of the time series. β1 estimates the pre-intervention trend, β2 estimates the change in level immediately following the intervention and β3 estimates the change in post-intervention trend. A full model was specified which included regression terms for all interventions to be analysed, and a final parsimonious model was derived by way of removing non-significant regression terms (p ≥ 0.1) in a process of backward elimination. The presence of autocorrelation was tested using the Durbin–Watson test.
As described in the sample size calculation, for the interrupted time series models, there is a balance to be had between the number of pre- and post-intervention data points, and the number of observations available at each time point. On initial explorative analyses it was apparent that their statistical power would be limited to conduct segmented linear regression using aggregated quarterly proportions at a hospital level. This was because of the number of observations available at each time point, and being able to estimate this accurately, particularly for rare outcomes such as second hip fracture. In addition, given that the pre-analysis service evaluation identified multiple interventions for several hospitals, with most occurring towards the end of the time series, this limited the number of data points available pre and post intervention. It was decided a before-and-after impact analysis using time to event data would be more suitable for the primary analysis, as used elsewhere to evaluate the implementation of osteoporosis guidelines. 101 Furthermore, this allowed adjustment to be made for confounding factors such as sex, age and comorbidities. The results of the time-series analyses are therefore presented as a secondary analysis.
2. Survival regression models
Time to second hip fracture was estimated for the time period after each intervention relative to the time period before. Each hospital was analysed separately. Patients were censored on date of the outcome of interest, date of death, date of loss to follow-up or end of study period. Given that a high mortality rate could significantly overestimate the incidence and effect sizes,109 the competing risk of death was accounted for using Fine and Gray regression modelling. 110 This specifies a model for the hazard of the subdistribution, which generates failure events while keeping patients who experience a competing event at risk so that they can be included as not having a chance of failing. In the presence of competing risk, we focus on the cumulative incidence of an event occurring, and this can be estimated by modelling the subhazard distribution. The regression model produces subhazard ratios (SHRs), in which a SHR of 1 implies no association, a SHR of > 1 implies an increased cumulative incidence of outcome and a SHR of < 1 implies decreased cumulative incidence. Hence the interpretation is similar to that of the more familiar Cox regression model. The index date for these models was the end date for the primary hip fracture CIPS or 30 days after primary hip fracture admission if the CIPS finished before this time, as defined above. Confounding factors relating to case mix were adjusted for. It was decided a priori to exclude primary hip fracture episodes admitted 12 months after an intervention in order to account for any lag in the effect of an orthogeriatrician or FLS appointment on secondary fracture prevention and the fact that bone therapy takes at least 6 months to influence fracture rates. Primary hip fracture episodes starting after 31 March 2011 were not included owing to insufficient (i.e. < 2 years) follow-up before the end of the study period. In addition, assessment of linear trend over time was carried out using a piecewise Cox proportional hazards model in which linear splines were fitted to quarterly time points in separate sections of the time series corresponding to before and after intervention dates. The proportional hazards assumption was checked using Schoenfeld residuals.
Fine and Gray survival models were likewise used to evaluate intervention impact on major non-hip fracture (proximal humerus, rib, pelvis, forearm/wrist and spine) within the 2 years following primary hip fracture. Major non-hip fractures were included any time after the date of primary hip fracture.
Evaluation of impact on post-fracture mortality was carried out using a similar approach to above with the use of Cox proportional hazards regression modelling. A lag period of 3 months after each intervention date was used, during which primary hip fracture admissions were excluded from analyses, as it was expected that interventions may require less time to impact mortality rates than required for establishing better methods for secondary fracture prevention. Primary hip fracture episodes were removed from analyses of 30-day and 1-year mortality if admitted after 31 December 2012 or 31 March 2012, respectively, so as to allow for sufficient follow-up before the end of the study period.
A fixed-effects meta-analysis was used to pool estimates of impact on each health outcome under study for orthogeriatric and FLS interventions across hospitals of interest in the region. Estimated impact of interventions with a pre-existing linear trend (p < 0.05) for any of the health outcomes under study were not included in the corresponding meta-analysis of that health outcome in order to address the potential bias of secular trend.
Results
Survival analysis
A total of 33,152 hospital episodes were identified as pertaining to a primary hip fracture. The total number over the study period per hospital of interest ranged from 1030 to 5895. The proportion of female admissions significantly changed over time, decreasing from 78.2% (2003–4) to 72.0% (2012–13). Mean age increased slightly over the study period from 82.7 years (2003–4) to 83.1 years (2012–13).
Two of the 13 interventions could not be analysed in relation to time to second hip fracture owing to insufficient pre- or post-intervention follow-up time once a 12-month lag period was introduced. Interventions that were preceded by a significant pre-intervention trend in health outcome were not evaluated in relation to that particular health outcome owing to the bias of a pre-existing secular trend likely to be incorporated in such estimates of intervention impact. For this reason two interventions were not evaluated in relation to 1-year post-fracture mortality.
Second hip fracture
There were 1288 patients identified as sustaining a second hip fracture, at an average annual directly age- and sex-standardised proportion of 4.2%. This proportion remained stable throughout the study period (p-trend = 0.11), with no significant change in annual rate within any year of follow-up. Of the patients identified as having sustained a second hip fracture, 883 (69%) had both procedure and laterality codes for both index and second fracture. For 96% of this subset of patients with known procedure and laterality, the second fracture occurred at the contralateral side to the index fracture; that is only 4.4% of these patients sustained a second hip fracture on the same side as the index fracture.
The SHRs from the survival models showed no evidence for an impact on time to second hip fracture (Figure 5) following any of the interventions when analysed separately or when pooled by type of intervention: orthogeriatrician [SHR 0.95, 95% confidence interval (CI) 0.79 to 1.15] or FLS (SHR 1.03, 95% CI 0.85 to 1.26). Analyses of intervention impact on time to second hip fracture remained unchanged when stratified by sex, age (< 75 years or ≥ 75 years) or Charlson Comorbidity Index score (0 or ≥ 1).
FIGURE 5.
Forest plot of SHRs for 2-year second hip fracture, by type of change in service delivery. a, Prior orthogeriatrician; b, prior FLS; and c, prior FLS and orthogeriatrician.
Thirty-day and 1-year mortality
The average annualised age- and sex-standardised proportions for 30-day and 1-year mortality were 9.5% (n = 3033) and 29.8% (n = 9663), respectively. Overall, age- and sex-standardised 30-day mortality declined from 11.8% in 2003–4 to 7.1% in 2011–12 (p-trend < 0.001) (Figure 6). When compared with the year 2003, 30-day mortality remained stable until 2005–6 but significantly decreased thereafter. Similarly, the age- and sex-standardised 1-year mortality of 33.1% in 2003–4 remained stable until 2006–7 and thereafter decreased markedly to 26.0% in 2011–12 (overall p-trend < 0.001).
FIGURE 6.
Annual and quarterly regional trends in mortality (30 days and 1 year) and second hip fracture (2 years) after primary hip fracture during the study period. Reproduced with permission from Hawley SJ, Javaid MK, Prieto-Alhambra D, Arden N, Lippett J, Sheard S, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing 2016;45:236–42, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode).
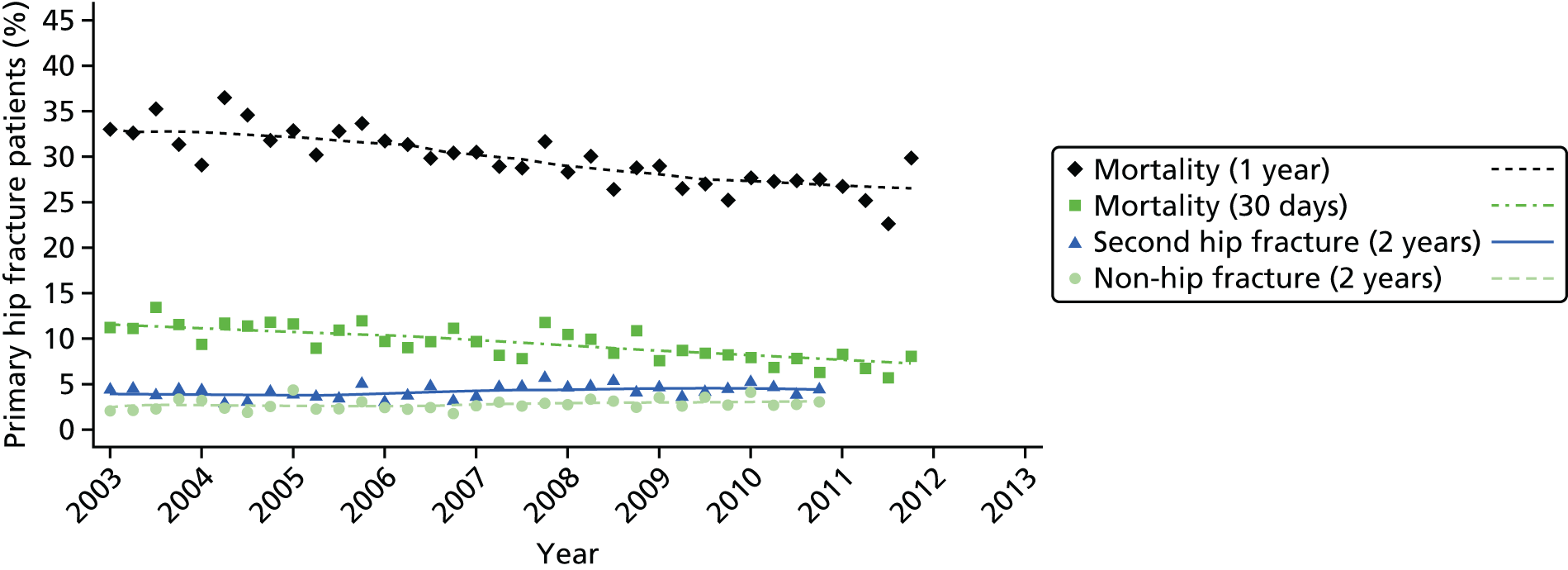
The pooled estimated impact of introducing an orthogeriatrician on 30-day and 1-year mortality was HR 0.73 (95% CI 0.65 to 0.82) and HR 0.81 (95% CI 0.75 to 0.87), respectively (Figures 7 and 8). Thirty-day and 1-year mortality were likewise reduced following the introduction of a FLS: HR 0.80 (95% CI 0.71 to 0.91) and HR 0.84 (95% CI 0.77 to 0.93), respectively.
FIGURE 7.
Forest plot of HRs for 30-day mortality, by type of change in service delivery. a, Prior orthogeriatrician; b, prior FLS; and c, prior FLS and orthogeriatrician. Reproduced with permission from Hawley SJ, Javaid MK, Prieto-Alhambra D, Arden N, Lippett J, Sheard S, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing 2016;45:236–42, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode).
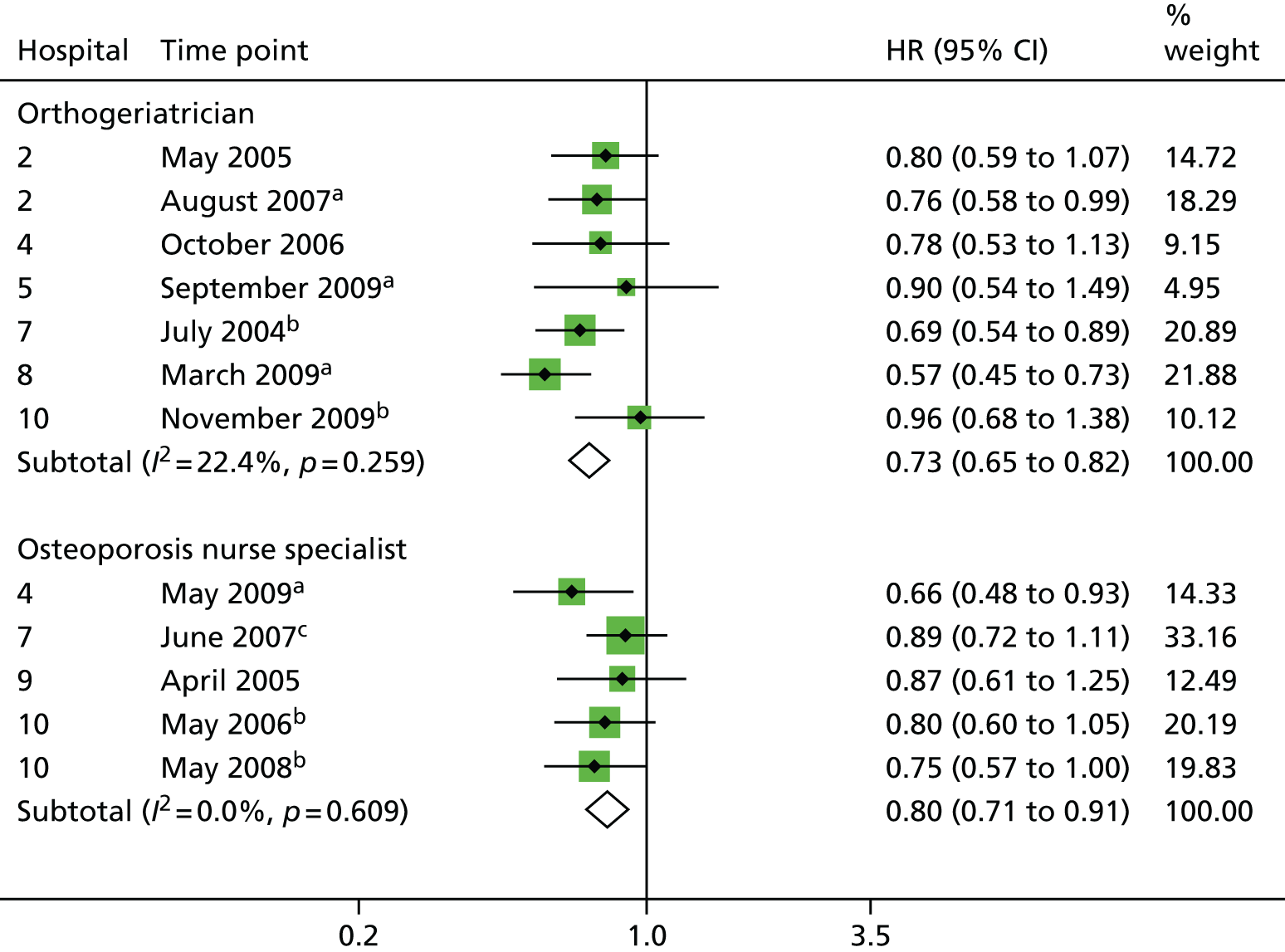
FIGURE 8.
Forest plot of HRs for 1-year mortality, by type of change in service delivery. a, Prior orthogeriatrician; b, prior FLS. Reproduced with permission from Hawley SJ, Javaid MK, Prieto-Alhambra D, Arden N, Lippett J, Sheard S, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing 2016;45:236–42, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode).

The reductions in mortality were seen whether introducing an orthogeriatric or FLS model of care for the first time or as part of an expansion of an existing service. For example, 1-year mortality was reduced following the appointment of a second orthogeriatrician within Hospital 2 in August 2007, compared with service delivery during the time with one orthogeriatrician already in post (HR 0.78, 95% CI 0.67 to 0.91). Likewise, adding two extra nurses and a consultant ‘champion’ for osteoporosis to the FLS model of care in Hospital 10 in May 2008 was associated with a further reduction in 1-year mortality (HR 0.76, 95% CI 0.63 to 0.92), compared with the relatively less intensive FLS in place before this intervention (see Figure 7).
Non-hip fractures
Overall, 2.8% of index hip fracture patients went on within 2 years to have a major non-hip fragility fracture requiring hospital admission. At a regional level, the rate of subsequent major non-hip fracture after primary hip fracture increased from 1.9% in 2003 to 2.5% in 2009 (p-trend = 0.03) (see Figure 6). Pooled SHRs indicated no significant impact of either orthogeriatrician or FLS (Figure 9).
FIGURE 9.
Forest plot of SHRs for 2-year major non-hip fracture, by type of change in service delivery interrupted time-series analysis. a, Prior orthogeriatrician; b, prior FLS. Reproduced with permission from Hawley SJ, Javaid MK, Prieto-Alhambra D, Arden N, Lippett J, Sheard S, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing 2016;45:236–42, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode).
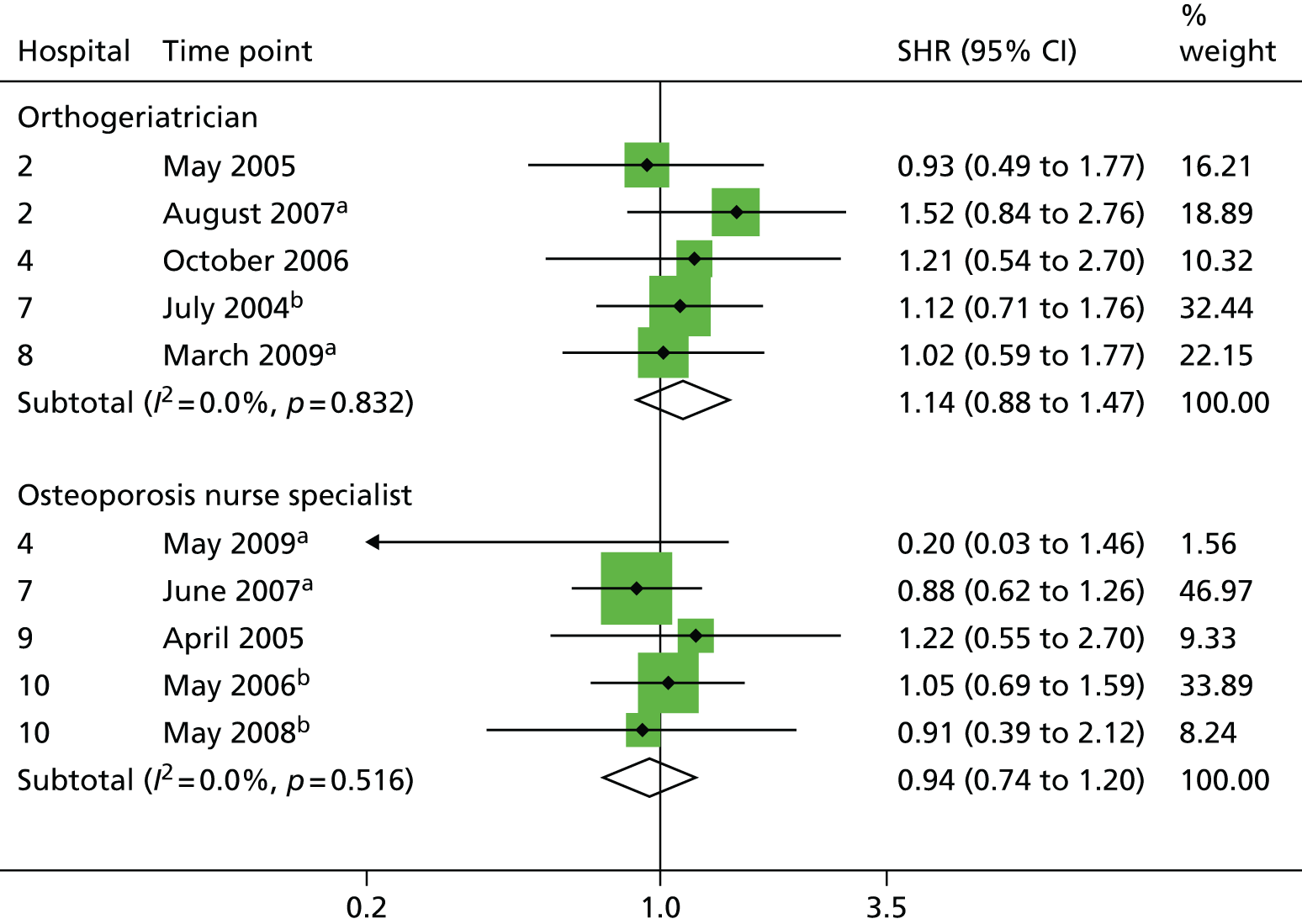
The results of the interrupted time series analysis are presented for the five biggest hospitals in the region only because of the limited number of observations available at each time point. Figures 10 and 11 present a visual representation of the quarterly trends in rates of mortality and second hip fracture at each of these individual hospitals. Using a segmented linear regression approach, no interventions were associated with either a step or a slope change in 2-year secondary hip fracture (Table 12). For 30-day and 1-year mortality, large effect sizes were observed for the estimated step changes (Tables 13 and 14), but none reached conventional levels of statistical significance owing to limited statistical power.
FIGURE 10.
Quarterly regional trends in second hip fracture (2 years) after primary hip fracture during the study period, by hospital. The grey bars represent the date of the interventions in each hospital. The dotted line represents a specialist nurse intervention at the hospital that did not treat hip fracture. (a) Hospital 2; (b) Hospital 4; (c) Hospital 7; (d) Hospital 8; and (e) Hospital 10.

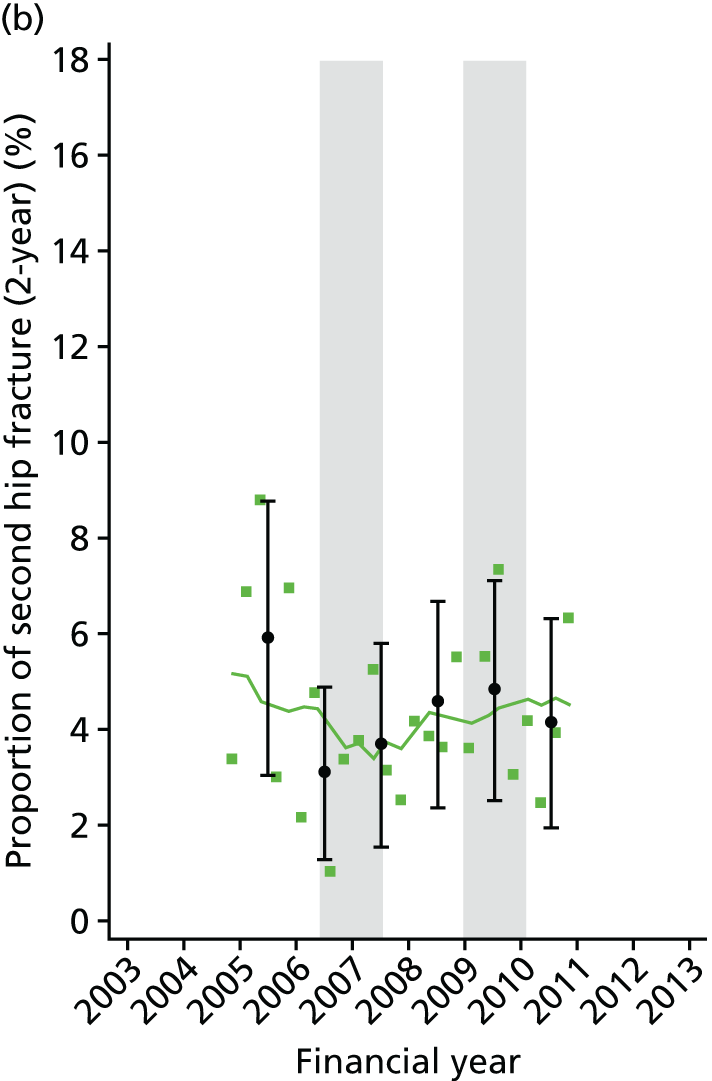
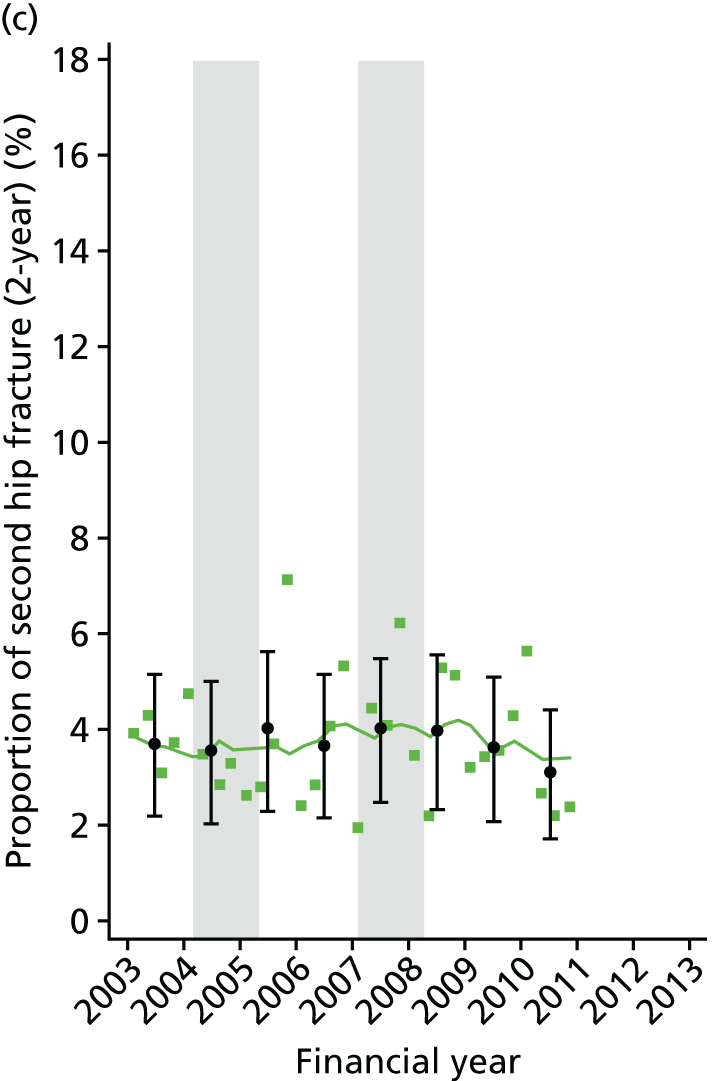
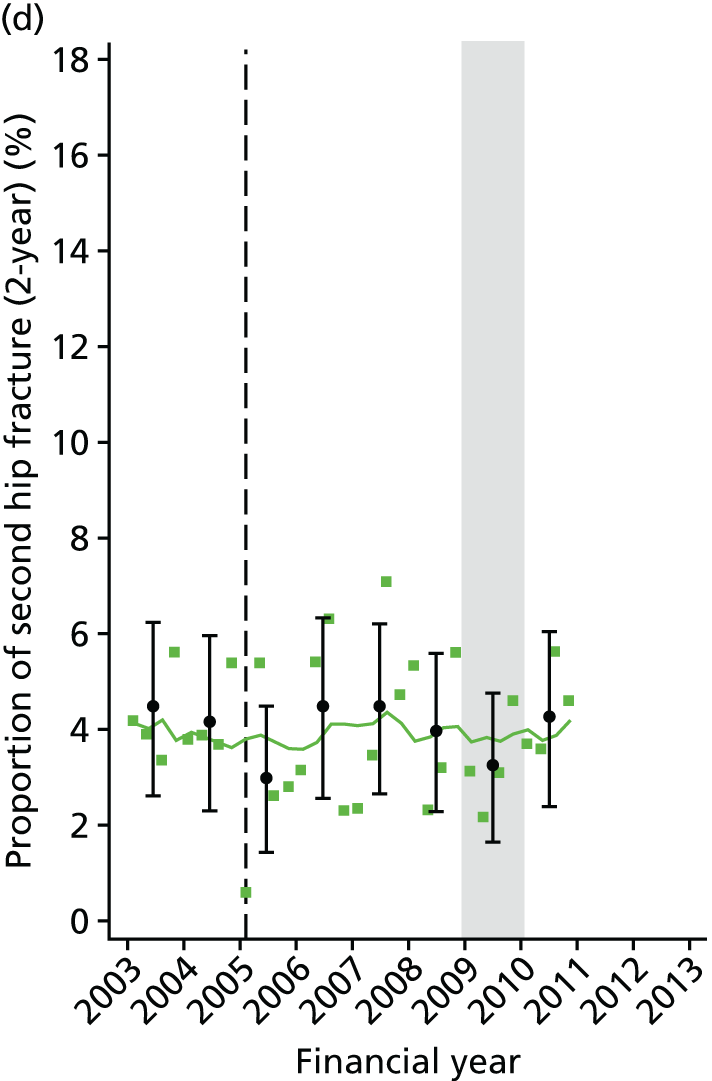
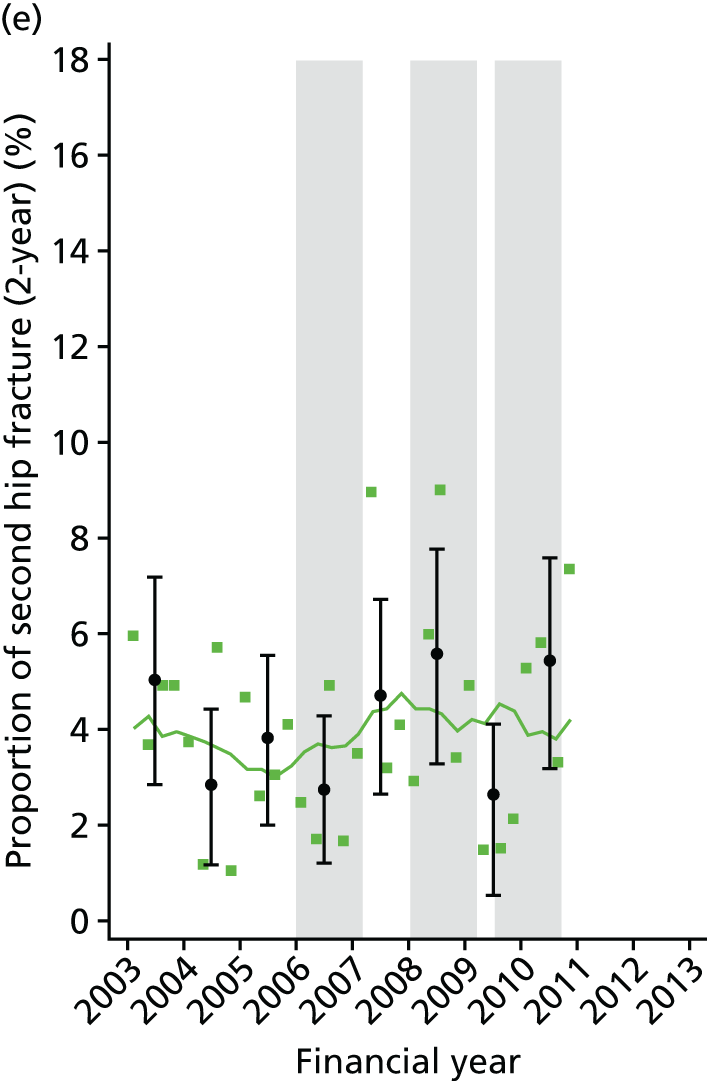
FIGURE 11.
Quarterly regional trends in mortality (30 days and 1 year) after primary hip fracture during the study period, by hospital. The grey bars represent the date of the interventions in each hospital. The dotted line represents a specialist nurse intervention at the hospital that did not treat hip fracture. (a) Hospital 2; (b) Hospital 4; (c) Hospital 7; (d) Hospital 8; (e) Hospital 10.
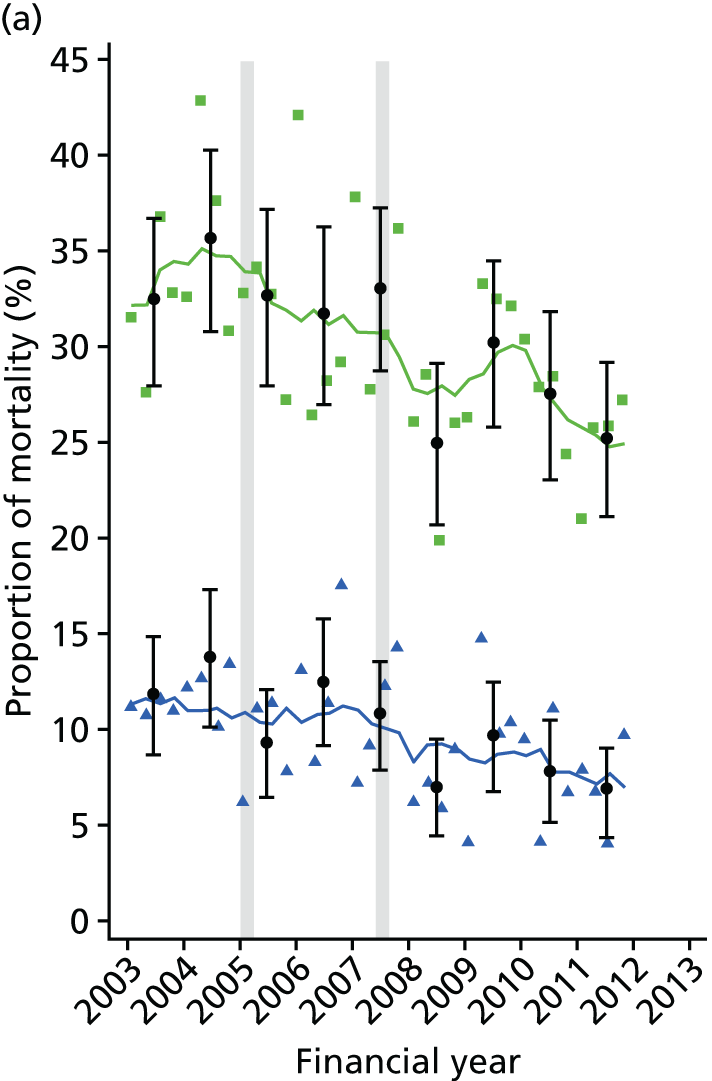

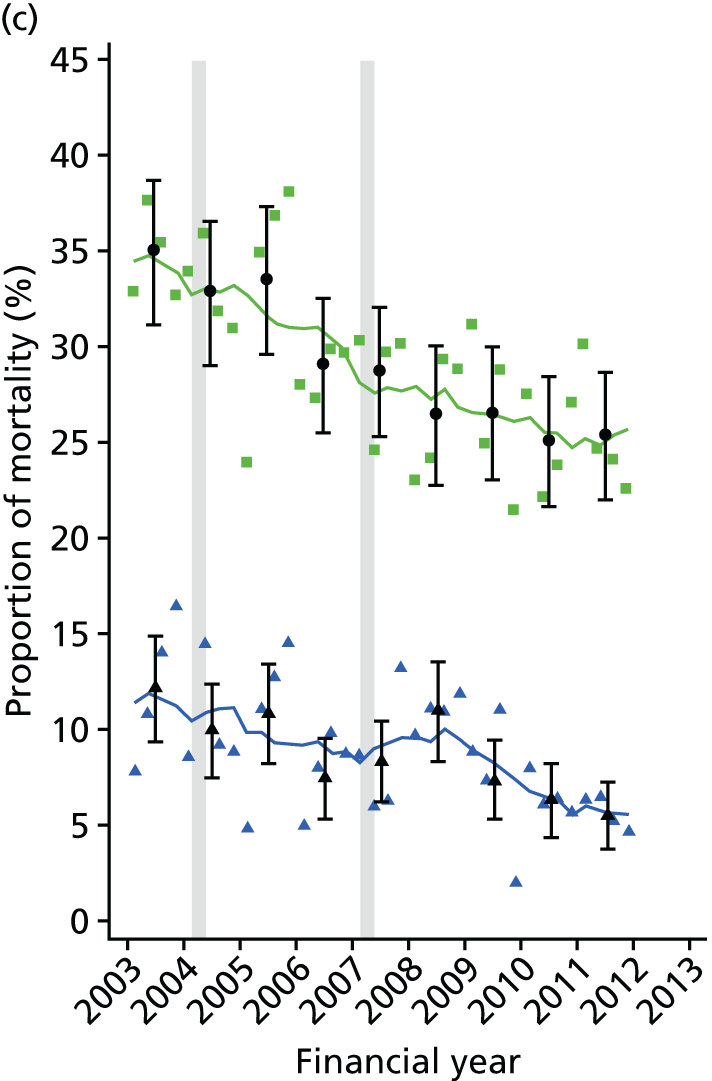
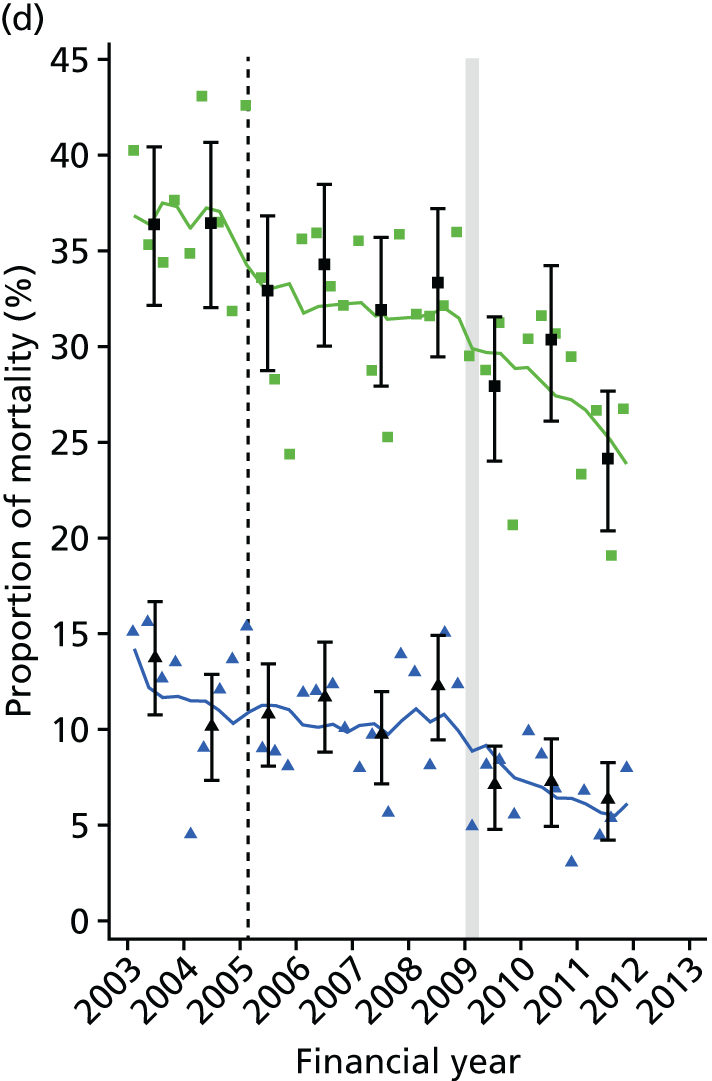
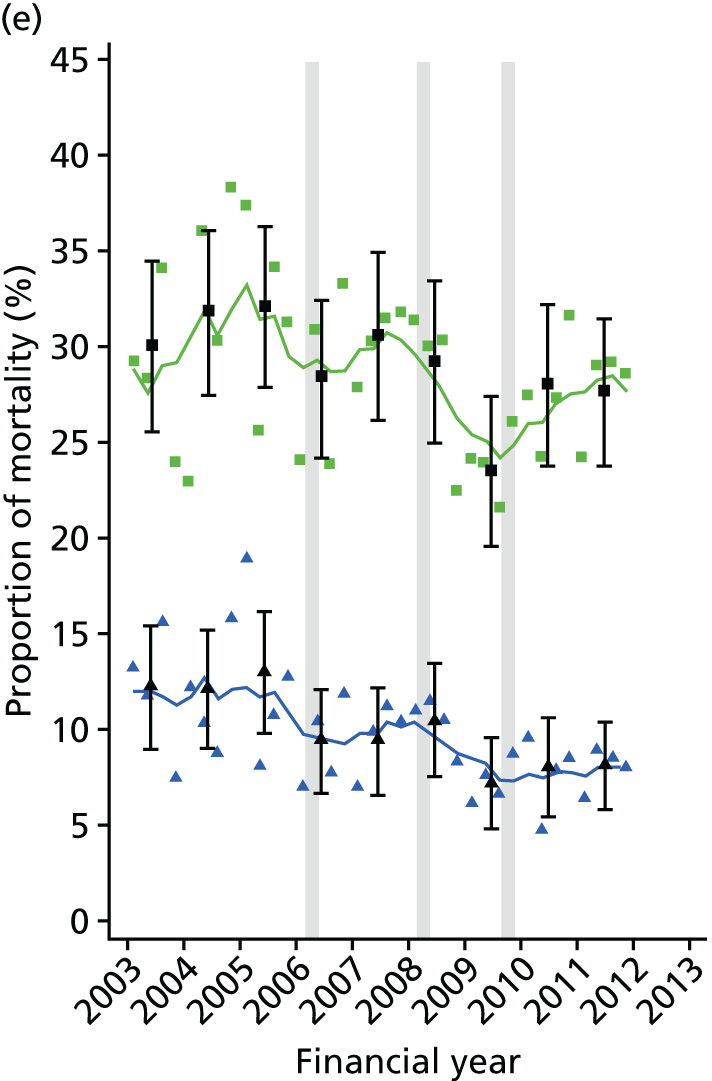
| Hospital | Baseline trend | Step change | Slope change | Step change | Slope change | Step change | Slope change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β1 | p-value | β2 | p-value | β3 | p-value | β4 | p-value | β5 | p-value | β6 | p-value | β7 | p-value | |
| Hospital 10 | ||||||||||||||
| Full model | –0.23 | 0.25 | 2.12 | 0.430 | 2.89 | 0.590 | –2.61 | 0.470 | 0.35 | 0.530 | ||||
| Parsimonious model | < 0.01 | 0.96 | ||||||||||||
| Hospital 8 | ||||||||||||||
| Full model | 0.04 | 0.67 | –0.53 | 0.81 | 0.16 | 0.78 | ||||||||
| Final model | 0.04 | 0.27 | ||||||||||||
| Hospital 2 | ||||||||||||||
| Full model | –0.71 | 0.81 | 2.99 | 0.39 | –0.45 | 0.55 | 2.08 | 0.63 | 0.5 | 0.47 | ||||
| Parsimonious model | –0.02 | 0.69 | ||||||||||||
| Hospital 7 | ||||||||||||||
| Full model | 0.11 | 0.8 | 0.20 | 0.95 | –0.37 | 0.47 | 1.77 | 0.39 | 0.24 | 0.38 | ||||
| Parsimonious model | < 0.01 | 0.89 | ||||||||||||
| Hospital 4 | ||||||||||||||
| Full model | –0.33 | 0.51 | –2.56 | 0.57 | 0.44 | 0.49 | –2.53 | 0.51 | 0.68 | 0.5 | ||||
| Parsimonious model | –0.02 | 0.75 | ||||||||||||
| Hospital | Baseline | Intervention 1 | Intervention 2 | Intervention 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline trend | Step change | Slope change | Step change | Slope change | Step change | Slope change | ||||||||
| β1 | p-value | β2 | p-value | β3 | p-value | β4 | p-value | β5 | p-value | β6 | p-value | β7 | p-value | |
| Hospital 10 | ||||||||||||||
| Full model | 0.00 | 1.00 | –3.93 | 0.23 | 0.42 | 0.55 | –0.67 | 0.89 | –1.52 | 0.28 | 3.11 | 0.54 | 1.21 | 0.36 |
| Parsimonious model | –0.14 | < 0.001 | ||||||||||||
| Hospital 8 | ||||||||||||||
| Full model | < 0.01 | 0.98 | –3.75 | 0.088 | < 0.01 | 0.99 | ||||||||
| Parsimonious model | < 0.01 | 0.96 | –3.75 | 0.073 | ||||||||||
| Hospital 2 | ||||||||||||||
| Full model | 0.40 | 0.42 | –4.84 | 0.24 | –0.09 | 0.91 | –2.79 | 0.45 | –0.49 | 0.44 | ||||
| Parsimonious model | –0.16 | 0.001 | ||||||||||||
| Hospital 7 | ||||||||||||||
| Full model | 0.7 | 0.45 | –4.1 | 0.28 | –0.73 | 0.45 | 2.28 | 0.35 | –0.33 | 0.34 | ||||
| Parsimonious model | –0.18 | < 0.001 | ||||||||||||
| Hospital 4 | ||||||||||||||
| Full model | –0.49 | 0.56 | –1.57 | 0.74 | 0.73 | 0.45 | –4.51 | 0.25 | –0.11 | 0.86 | ||||
| Parsimonious model | –0.19 | 0.011 | ||||||||||||
| Hospital | Baseline | Intervention 1 | Intervention 2 | Intervention 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline trend | Step change | Slope change | Step change | Slope change | Step change | Slope change | ||||||||
| β1 | p-value | β2 | p-value | β3 | p-value | β4 | p-value | β5 | p-value | β6 | p-value | β7 | p-value | |
| Hospital 10 | ||||||||||||||
| Full model | 0.43 | 0.22 | –8.07 | 0.10 | 0.61 | 0.56 | –4.89 | 0.48 | –2.76 | 0.19 | 6.89 | 0.36 | 2.08 | 0.29 |
| Parsimonious model | –0.13 | 0.063 | ||||||||||||
| Hospital 8 | ||||||||||||||
| Full model | –0.08 | 0.72 | –1.64 | 0.66 | –0.44 | 0.36 | ||||||||
| Parsimonious model | –0.31 | 0.003 | ||||||||||||
| Hospital 2 | ||||||||||||||
| Full model | 0.74 | 0.33 | –6.99 | 0.26 | –0.55 | 0.65 | –3.05 | 0.58 | –0.43 | 0.65 | ||||
| Parsimonious model | –0.26 | 0.001 | ||||||||||||
| Hospital 7 | ||||||||||||||
| Full model | –0.29 | 0.8 | –1.51 | 0.75 | 0.12 | 0.92 | –1.51 | 0.62 | –0.06 | 0.89 | ||||
| Parsimonious model | –0.32 | < 0.001 | ||||||||||||
| Hospital 4 | ||||||||||||||
| Full model | –0.36 | 0.79 | –3.02 | 0.7 | 0.22 | 0.89 | 2.01 | 0.74 | –0.25 | 0.79 | ||||
| Parsimonious model | –0.3 | 0.01 | ||||||||||||
Discussion
Main findings
In this study we observed an overall decline in mortality after primary hip fracture across the region as a whole from financial year 2003 to financial year 2011. Despite this increased survivorship following primary hip fracture, rates of second hip fracture remained relatively stable across the region. It was demonstrated from pooling estimates by intervention type that the introduction and/or expansion of orthogeriatric and FLS models of care was significantly associated with reduced post-hip fracture mortality. An alternative way of expressing this beneficial impact is using the number needed to treat,111 which is the number of hip fracture patients needing to be treated following an intervention in order to prevent one excess death, compared with service delivery as it was before the intervention. Assuming a pre-intervention survival of 90% at 30 days and using the pooled estimates of intervention impact, the numbers of patients needed to treat to avoid one excess death at 30 days after a service model intervention are 12 and 17 for orthogeriatric and FLS type interventions, respectively. However, neither orthogeriatric nor FLS interventions evaluated here, either individually or pooled by type, had any significant impact on time to second hip fracture.
Mortality
We found the average annual proportion of primary hip fracture patients dying within 30 days and 1 year to be 9.5% and 29.8%, respectively, which is consistent with previous reports in a similar population. 112,113 The overall downwards trend in mortality after hip fracture identified here between 2003 and 2011 is also consistent with previous findings. 114
The beneficial impact on mortality following the introduction and/or expansion of an orthogeriatric model of care, as found here, is a plausible finding given our knowledge of specific details of changes to models of care identified from the pre-analysis service evaluation. For example, it was reported in Hospital 8 that the appointment of an orthogeriatric clinical lead for hip fracture care meant that 90% of patients were seen preoperatively for optimisation for surgery. Likewise, the service evaluation identified that the appointment of a second orthogeriatrician in Hospital 2 was reported to have led to hip fracture patients being taken to theatre quicker and in better condition. These are likely to be important factors, given previous evidence that trauma-related complications play a key role in post-hip fracture mortality,115 and that earlier surgery is associated with lower risk of death. 116 A recent study similarly reported that hospitals with orthogeriatric services were found to have significantly lower mortality after hip fracture than hospitals without such services,94 and findings from RCTs also demonstrate the benefits of geriatric hip fracture care on delirium117 and mortality. 118 It should also be noted that later on in Chapter 6 of this report, using national-level data from the CPRD database, there was a reduction in 30-day mortality (but not in 1-year mortality) between October 2007 and September 2008 (the publication of the BOA Blue Book and the NICE TA 161). There are hospitals (4, 5, 8 and 10) in which interventions occur at around the time of, or after publication of, these guidelines. It is possible that, in these hospitals, some of the changes made to the service models may be a reflection of the guidance and adherence to them. Our findings are in keeping with the UK NICE CGs, which recommend orthogeriatric assessment and continued review of patients admitted with hip fracture,3 and the BOA’s publication The Care of Patients with Fragility Fractures, which states that ‘senior medical input – from a consultant orthogeriatrician . . . is now essential in the good care of fragility fracture patients’. 1
The reasons for a significant decrease in mortality following the introduction and/or expansion of a FLS model of care are not as clear as for the orthogeriatric model. An environment of better co-ordination of multidisciplinary care with better communication between staff following the introduction and/or expansion of a FLS is likely to play a role. In addition, the comprehensive assessment and inspection of routine bloods and medical history that an osteoporosis nurse specialist carries out may contribute to the identification of secondary diseases and comorbidities. Furthermore, patients receiving zoledronic acid have previously been shown to be at a reduced risk of death,119 with a much greater reduction than could be attributed solely to lower fracture incidence on treatment. 120 The mechanisms that mediate the remainder of the drugs effect on mortality are not known. 120 Given that treatment recommendation is within the remit of the FLS, it may be that this is a contributory factor in the process in reduced long-term mortality. It is also likely that introducing or expanding a FLS entails wider underlying changes to service delivery that could also contribute to the reduction in morality following the implementation of change. Similar to our findings here, implementation of osteoporosis guidelines by a fracture nurse has previously been shown to be associated with a 33%101,121 reduction in post-fracture mortality following any index fragility fracture, thereby prompting the conclusion that measures to prevent fractures also reduce mortality. 101
Second hip fracture
We report here an average annual direct age- and sex-standardised proportion of 4.2% for second hip fracture within 2 years of a primary hip fracture. This is in accord with previous studies,122–124 although direct comparisons are difficult owing to different lengths of follow-up time used. We found that the regional incidence of refracture remained stable during the study period (see Figure 6), consistent with a recent analysis of trend in second hip fracture in Olmsted County, MN, USA. 8
In this context, the BOA’s publication The Care of Patients with Fragility Fractures states that the most effective health-care solution for fracture patients is the FLS, routinely delivered by a nurse specialist supported by a lead clinician in osteoporosis. 1 In a review of co-ordinator-based systems for secondary fracture prevention, Marsh et al. 10 reported that such systems appeared to be able to overcome barriers to osteoporosis intervention and treatment in fragility fracture patients. It is surprising, therefore, to find a null effect on hip refracture risk following the introduction or expansion of an orthogeriatric or a FLS model of care. One reason may be because of patients’ inadequate adherence to prescribed therapies. During the period of the study, the FLSs that were analysed focused on identification, investigation and initiation of bone therapy, leaving monitoring to primary care services. However, the adherence of patients within NHS primary care services is poor, at < 40% by 12 months. 125 This highlights the importance of incorporating monitoring within the FLS scope to reliably close the secondary prevention care gap. Compounding this issue is that the earliest demonstrated time to antiosteoporosis treatment efficacy in terms of hip fracture risk reduction is 6–36 months. 126 Specifically, zoledronic acid has been shown to reduce clinical fracture risk after hip fracture only after 12 months. 119 In the present analysis, 35% and 63% of second hip fractures within 2 years occurred within the first 6 and 12 months, respectively. This is consistent with the clinical audit of the FLS programme at Glasgow,127 and an extraordinarily high risk of early second hip fracture in a large Danish study. 128 The high rate of early refracture combined with the delayed onset of fracture risk reduction associated with therapies recommended or dispensed within the orthogeriatric and FLS models of care studied here may have contributed to our finding of no impact on refracture risk. In order to detect whether or not the interventions had an impact on longer-term refracture risk, we carried out a post-hoc analysis of second hip fracture risk, in which extended follow-up (when possible) and a time-varying hazards model were used to focus only on risk in the second and third years after a primary hip fracture. However, no significant changes in pooled estimates were found. Data on treatment initiation and adherence were not available in the HES data set and, therefore, this issue could not be further investigated here.
It has been noted that the risk of subsequent fracture after hip fracture can be offset by increased post-fracture mortality,129 which has been used elsewhere to explain increasing refracture trends during 2000–10. 130 It is possible that this dependency of fracture risk on mortality may be a contributory factor to the lack of beneficial impact on second fracture rate here observed. Given the overall ensuing improvement in survival after the introduction and/or expansion of orthogeriatric and FLS models of care, refracture risk would be expected to increase over the period of time from which excess mortality was reduced.
To our knowledge, this is the first study to evaluate orthogeriatric or FLS models of care in terms of impact on hip refracture rate after hip fracture. Our finding of no impact may seem surprising given the positive results from the Kaiser Southern California Healthy Bones Program (Kaiser SCAL) study. 96 Kaiser SCAL reported an overall 37% reduction in hip fracture rate associated with the introduction of intensive champion-led multidisciplinary osteoporosis disease management programmes across 11 medical centres. Key differences in study design between the Kaiser SCAL study and our own may account for the dissimilar findings, notably their consideration of all hip fractures rather than only refracture and that the authors estimated the exponential rise in hip fractures for 2006 from observed rates in 1997–9. Furthermore, the Kaiser SCAL programme took place in a highly integrated setting in which members received > 95% of all medical care at the centres involved in the programme.
Implementation of osteoporosis and fall prevention guidelines has also been reported to be associated with a significant reduction in subsequent fracture risk after a non-vertebral index fragility fracture. 101 Similarly, fracture patients receiving care within the intervention programme at Concord Repatriation General Hospital (Sydney, NSW, Australia) were found to be at 80% reduced risk of subsequent fracture, compared with patients electing standard care. 26 It is likely, however, that a proportion of the difference reported reflects selection bias with worse comorbidities in those who did not attend and better health-seeking behaviour in those who did (95% remained on initial treatment throughout the study period). It is also important to note that these previous analyses considered refracture after any index fragility non-vertebral fracture as opposed to our focus on hip fracture.
Strengths and limitations
A major strength of the present analysis is the use of a natural experimental design for which each hospital acted as its own control in a before-and-after impact analysis. The comprehensive nature of the HES database allowed for case-mix adjustment to estimates of intervention impact, thereby aiding transparency of such comparisons over the time course through the controlling for age, sex and Charlson Comorbidity Index. Clinical audit findings provided detailed descriptions of all major changes to service delivery of post-hip fracture care and secondary fracture prevention during the study period in each relevant hospital, enabling the timing and nature of each intervention to be described a priori. Our definition of primary hip fracture was robust and addressed the possibility of erroneously identifying readmissions as second hip fractures by only counting second hip fractures sustained in a separate CIPS and after 30 days after the primary admission.
A main limitation of our analysis is that confounding events coinciding with the interventions of interest here evaluated cannot be ruled out. We consider this is unlikely given that the estimated impact on each health outcome was consistent across hospitals and for interventions that occurred at different time points over the study period. Secular trend may also have introduced bias into impact estimates; however, this issue was addressed by excluding from analyses those estimates of impact for interventions that were preceded by a significant trend in the respective health outcome (two estimates of impact on 1-year mortality were hence excluded). Although we have pooled service model interventions according to a broad definition of either orthogeriatric or FLS models of care, further aspects of these interventions were subject to variation and were not able to be included, such as processes used to case find, DXA scanning and the undertaking of falls prevention. Although such details were not controlled for in analyses, details of each intervention are provided (see Appendices 5–8). We were also unable to compare, at the patient level, rates of diagnostic testing and medication use. It also needs to be noted that given our concentration on health outcomes after hip fracture in this evaluation, our findings may not reflect the effectiveness of models of care among patients having sustained a non-hip fragility fracture. A limitation is that routine hospital admissions data are collected for administrative rather than research purposes, and concerns have been raised over the completeness and accuracy of such data. Last, HES data underestimate the total number of admissions by excluding a minority of privately funded procedures, but this unlikely to bias the observed results.
Conclusions
In conclusion, this study provides evidence that the introduction and/or expansion of orthogeriatric and FLS models of care are associated with a large beneficial effect on subsequent mortality after hip fracture. There was no evidence for a reduction in second hip fracture rate, but the effect on non-hip fracture remains unanswered, as these outcomes cannot be ascertained within the secondary care setting of this study.
Chapter 6 Effect of national guidelines on rates of hip fracture, non-hip fracture and life expectancy using national data sets
Introduction
For patients admitted with a fragility fracture of the hip, there remains wide variation in clinical outcomes including mortality between sites even after case-mix adjustment. 17 The purpose of CGs is to inform decision-making to deliver high value health care across the health-care system and minimise deleterious variations. Guidelines are based on evidence and then take into account feasibility, equity and politics to develop policy and national guidance. 131 In the UK, secondary prevention of fracture is underutilised and widely neglected. 1 One of the metrics for the success of a guideline is its effect on important clinical outcomes.
Over the past decade guidance from a number of professional bodies has been published for the management of hip fracture patients (BOA Blue Book,1 SIGN,14 NICE13,15). NICE TAs 160/16113 are related to the effectiveness of bone protection therapy and CG 21 is related to falls prevention. 15 Audits by the NHFD38 and the Royal College of Physicians Audit18 suggest that the situation is improving, but still inadequate, such that prior to discharge only 66% of hip fracture patients were on bone protection medication and 81% received a falls assessment. 38
Aim
Using an interrupted time series approach, we will examine the effect national guidelines have had on altering trends in refracture rates, life expectancy (30 days and 1 year) and proportion of patients taking bone-strengthening drugs within 1 year after fracture.
Methods
Data sources
The data set used for this analysis was extracted from the CPRD, which comprises computerised medical records for > 8% of the UK population chosen to be representative of the wider UK population. We used linked ONS data to obtain information on mortality.
Patients
From the CPRD we identified index hip fracture cases occurring between 1 April 1999 and 31 March 2013, defined as patients with no previous hip fracture in the preceding 3 years. Patients with < 3 years from registration into a participating GP practice were, therefore, not included. A list of the Read codes used is included in Appendix 3. Patients < 50 years of age were excluded, as were patients registered in a GP practice outside England or Wales, as we had ONS data for these countries only and the NICE guidelines here evaluated were not implemented in Scotland or Northern Ireland.
Selection of control patients
Each hip fracture case was matched to two control patients without a Read code for a hip fracture on age (within 1 year), sex and GP practice and who were registered at the practice at the index date of the matched patient with hip fracture.
Interventions
The interventions here analysed were key national guidelines published within the study period and were identified prior to statistical analysis. Five specific guidelines were evaluated: NICE CG 21 (November 2004),15 NICE TA 87 (January 2005),33 BOA Blue Book (September 2007),1 NICE TA 161 (October 2008)13 and Best Practice Tariff for inpatient hip fracture care (April 2010). 76 The time points of these interventions are displayed in Figure 12.
FIGURE 12.
Time points of interest over study period.

The interventions can be divided into two broad groups: those with a sole pharmacological content and those with service delivery/falls interventions.
National Institute for Health and Care Excellence CG 2115 focused on assessment and prevention of falls in older people including after fragility fractures of the hip. A number of interventions were recommended, including individualised multifactorial interventions including strength and balance training, home hazard assessment and intervention, vision assessment and referral, and medication review with modifications/withdrawal, in addition to specific interventions, when appropriate, such as cardiac pacing, education and information giving. NICE TA 8733 focused on the clinical thresholds for the use of different pharmacological agents in postmenopausal women who have sustained a clinically apparent osteoporotic fracture. The BOA Blue Book in 20071 outlined the key standards for the care of hip fracture patients in the acute setting as well as secondary fracture prevention from both the bone health and the falls perspectives. NICE TA 16113 updated the guidance for TA 87 and included strontium ranelate. The Best Practice Tariff76 formalised the standards from the Blue Book care during the acute phase and then linked them to changes in reimbursement for trusts at the patient level.
Outcomes
Refracture
The primary outcome of interest was the proportion of index hip fracture patients sustaining a second hip fracture within 2 years. To reduce the likelihood of identifying a recoding of the primary fracture event, only second hip fracture events occurring after a washout period of 6 months were considered using the same method as in Chapter 5. A secondary outcome measures was the proportion of index hip fracture patients sustaining a subsequent non-hip major fracture (including fractures of the pelvis, proximal humerus, rib, spine and wrist/forearm) within 2 years.
Mortality
Evaluation was also made on the impact of guidelines on the proportion of index hip fracture patients dying within (1) 30 days and (2) 1 year. The mortality data were linked to the English national HES data using the national mortality data from 1999 to 2011. Mortality was also modelled as the difference in mortality between index hip fracture patients and matched control patients.
Bone-strengthening drugs
The proportion of index hip fracture patients initiating bone-strengthening drugs within 1 year was modelled among treatment-naive patients (defined as no bone-strengthening prescription within the preceding 6 months from index hip fracture date), as was the proportion initiating the same medications within 4 months post index hip fracture. Bone-strengthening drugs included were bisphosphonates, strontium ranelate, teraparatide, denosumab and selective oestrogen receptor modulators. Incident antiosteoporosis medication use within 1 year was also analysed separately for males and females given the paucity of guidelines for men, reflecting the differences in evidence base. As a sensitivity analysis we considered separately the proportion of bisphosphonate-naive index hip fracture patients who received one or more bisphosphonate prescriptions within the time period of (1) 2–6 months and (2) 10–14 months post index hip fracture date.
Statistical analysis
Data were aggregated, separately for cases and controls, in the form of age- and sex-standardised biannual proportions of each outcome of interest. The national guidelines were evaluated using an interrupted time series analysis. 108 A segmented linear regression model was specified for each outcome. A full model was specified which included regression terms for all interventions to be analysed and a final parsimonious model was derived by way of removing non-significant regression terms (p ≥ 0.1) in a process of backward elimination. The presence of autocorrelation was tested using the Durbin–Watson test. All Durbin–Watson statistics were close to the value of two and above the lower bound; therefore, we accepted the null hypothesis of no autocorrelation.
Owing to insufficient data points between NICE CG 21 (November 2004)15 and NICE TA 87 (January 2005),33 these two interventions were evaluated as one, as were the BOA Blue Book (September 2007)1 and NICE TA 161 (October 2008)13 (see Figure 12). Aggregated data points between interventions to be evaluated as one were, therefore, excluded from analyses. In order to minimise the number of data points removed from the time series, interventions occurring within the first or last month of a 6-month period were considered to have occurred at either the beginning or the end, respectively. Aggregated data points were not included in analyses of a particular outcome if follow-up time before the end of the study period was insufficient.
Results
Within the participating GP practices contributing data to the CPRD in England and Wales, we identified 11,243 eligible patients as having sustained an index hip fracture within the study period, and 21,606 control patients. Baseline characteristics for these patient groups are reported in Appendices 5 and 6. Over the 13 years, for both cases and controls, there was an increase in the proportion of men with hip fracture and, in addition, patients became older, with more severe comorbidities and greater prior bisphosphonate use both ever and in the last 6 months. With the matching, there was little difference in gender and age stratification between cases and controls. However, there was a slightly higher rate of severe Charlson comorbidities and greater prior bisphosphonates use in the cases than in the controls.
Table 15 reports the different outcomes during the study period by calendar year. Among case patients between April 1999 and September 2011, 238 (2.3%) suffered a subsequent hip fracture between 6 months and 2 years post index hip fracture. Similarly, 231 (2.3%) of index hip fracture patients sustained a major non-hip fracture within the first full 2 years after the primary event. The averaged proportion of index hip fracture patients initiating bone-strengthening therapy within 1 year was 25.7%, with post-index hip fracture mortality 6.3% at 30 days and 22.6% at 1 year.
| Yeara | Index hip fracture | Second hip fracture (2 years), n (%) | Major non-hip fracture (2 years), n (%) | Mortality (30 days), n (%) | Mortality (1 year), n (%) | Incident any antiOP medication use (1 year), n (%) | Incident non-BP antiOP medication use (1 year), n (%) | Incident BP use (1 year), n (%) | BP prevalenceb (10–14 months), n (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1999 | 861 | 19 (2.2) | 18 (2.1) | 69 (8) | 218 (25.3) | 47 (5.7) | 1 (0.1) | 46 (5.5) | 30 (3.6) |
| 2000 | 859 | 24 (2.8) | 22 (2.6) | 58 (6.8) | 203 (23.6) | 42 (5.2) | 0 (0) | 42 (5.2) | 30 (3.7) |
| 2001 | 834 | 20 (2.4) | 16 (1.9) | 51 (6.1) | 170 (20.4) | 65 (8.3) | 1 (0.1) | 64 (8.2) | 50 (6.4) |
| 2002 | 862 | 23 (2.7) | 23 (2.7) | 70 (8.1) | 222 (25.8) | 84 (10.5) | 1 (0.1) | 83 (10.4) | 78 (9.8) |
| 2003 | 882 | 24 (2.7) | 18 (2) | 64 (7.3) | 206 (23.4) | 112 (14) | 2 (0.3) | 112 (13.9) | 89 (11.1) |
| 2004 | 818 | 27 (3.3) | 24 (2.9) | 56 (6.9) | 182 (22.3) | 126 (17.1) | 3 (0.4) | 126 (17.1) | 94 (12.7) |
| 2005 | 847 | 18 (2.1) | 17 (2) | 69 (8.2) | 203 (24) | 225 (29.7) | 13 (1.7) | 213 (28.1) | 168 (22.1) |
| 2006 | 829 | 20 (2.4) | 18 (2.2) | 54 (6.5) | 194 (23.4) | 267 (36.9) | 19 (2.6) | 253 (34.8) | 187 (25.7) |
| 2007 | 820 | 18 (2.2) | 15 (1.8) | 51 (6.2) | 197 (24) | 256 (36.4) | 17 (2.4) | 243 (34.2) | 164 (23.1) |
| 2008 | 803 | 18 (2.2) | 20 (2.5) | 42 (5.2) | 172 (21.4) | 290 (42.2) | 44 (6.4) | 252 (36.4) | 177 (25.6) |
| 2009 | 758 | 9 (1.2) | 16 (2.1) | 40 (5.3) | 180 (23.8) | 278 (43.3) | 28 (4.4) | 254 (39.5) | 168 (26.1) |
| 2010 | 725 | 14 (1.9) | 16 (2.2) | 26 (3.6) | 132 (18.2) | 283 (45.9) | 25 (4.1) | 262 (41.9) | 174 (27.8) |
| 2011 | 669 | 4 (1.2)c | 8 (2.5)c | 27 (4) | 122 (18.2) | 292 (50.7) | 21 (3.6) | 275 (47.3) | 190 (32.7) |
| 2012 | 676 | 36 (5.3) | 68 (19.8)c | 145 (49.2)c | 16 (5.4)c | 134 (44.8)c | |||
| Overall | 11,243 | 238 (2.3) | 231 (2.3) | 713 (6.3) | 2469 (22.6) | 2513 (25.7) | 191 (2.0) | 2359 (24.1) | 1682 (17.2) |
The number of index hip fractures recorded per annum fell from 861 in 1999 to 676 in 2012 (see Table 15). When the years 1999–2000 were compared with the years 2010–11, the percentage of post-index hip fracture patients who sustained a second hip fracture declined from 2.5% to 1.7% (p = 0.17). For the same study years, the proportion of patients sustaining a major non-hip fracture remained the same at 2.3% (p = 0.95).
Post-index hip fracture mortality declined over the course of the study period (1999–2000 compared with 2011–12), both at 30 days and at 1 year, from 7.4% to 4.7% (p = 0.002) and from 24.5% to 18.8% (p = 0.001), respectively.
As with the trends in prior antiosteoporosis medication use, among treatment-naive index hip fracture patients at baseline the initiation of antiosteoporosis medication within 12 months increased markedly during the study period (1999–2000 compared with 2011–12) from 8.1% to 53.9% (p < 0.001). It is worth noting the gap between men and women in initiating antiosteoporosis treatment, with relatively fewer men than women starting any antiosteoporosis medication by 12 months, and that this gap increased over time (Figure 13). Although the overall trend was for an increase in incident use of antiosteoporosis medication post hip fracture, when analysed in age bands those < 75 years had the highest proportion at the beginning of the period of observation but by 2012 had the lowest proportion, with those aged 75–84 years having the highest proportion (Figure 14). The overall increase was mainly driven by bisphosphonate prescriptions (Figure 15); an average of 24.1% of hip fracture cases initiated a bisphosphonate prescription within 12 months of an index hip fracture as opposed to 2.0% initiating a non-bisphosphonate bone-strengthening drug. Indeed, the only other increase in medication use apart from bisphosphonates was seen in strontium (see Figure 15). This increase in bisphosphonate use was itself mainly driven by use of alendronate (Figure 16). Of interest is that not only did bisphosphonate initiation increase markedly, but among treatment-naive hip fracture cases at baseline the prevalence of bisphosphonate use at 10–14 months post index hip fracture also increased over the study period from < 5% to > 30% (Figure 17).
FIGURE 13.
Incident antiosteoporosis medication use stratified by sex. Reproduced with permission from Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, et al. Anti-osteoporosis medication prescriptions and incidence of secondary fracture among primary hip fracture patients in England and Wales: an interrupted time-series and economic analysis. J Bone Miner Res 2016; in press.
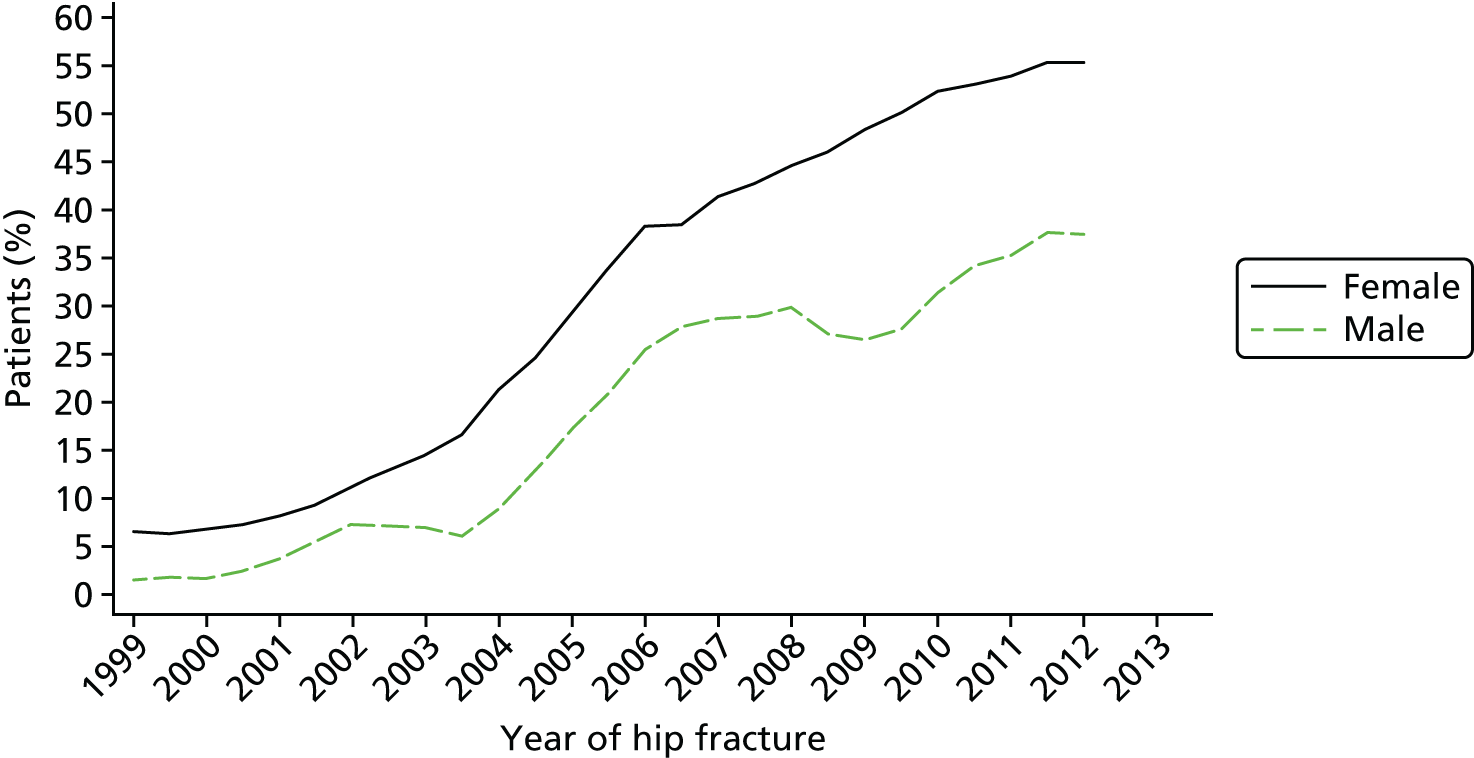
FIGURE 14.
Incident antiosteoporosis medication use stratified by age group. Reproduced with permission from Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, et al. Anti-osteoporosis medication prescriptions and incidence of secondary fracture among primary hip fracture patients in England and Wales: an interrupted time-series and economic analysis. J Bone Miner Res 2016; in press.
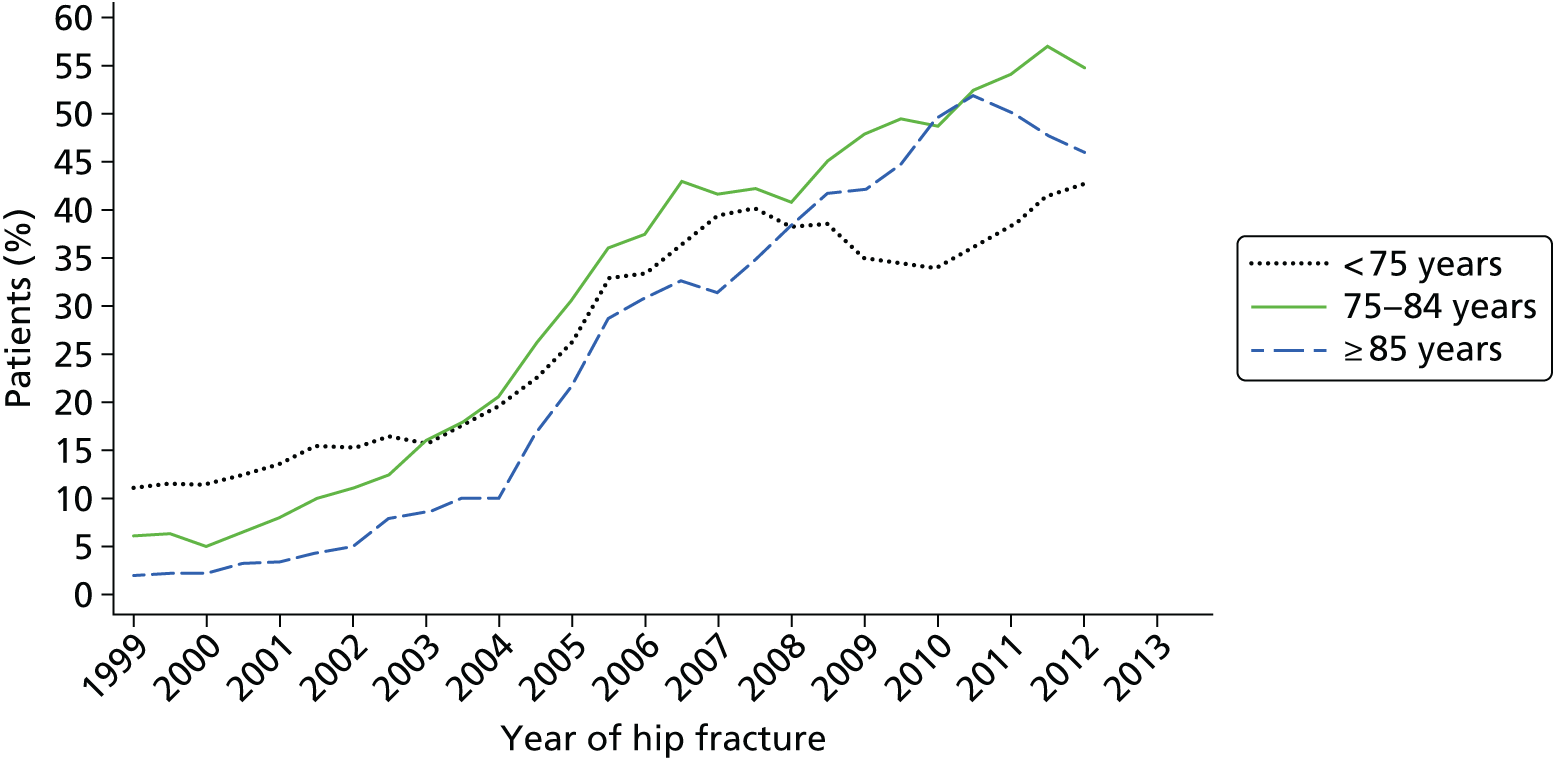
FIGURE 15.
Incident antiosteoporosis medication use stratified by medication type. OP, osteoporosis; SERM, selective oestrogen receptor modulator. Reproduced with permission from Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, et al. Anti-osteoporosis medication prescriptions and incidence of secondary fracture among primary hip fracture patients in England and Wales: an interrupted time-series and economic analysis. J Bone Miner Res 2016; in press.
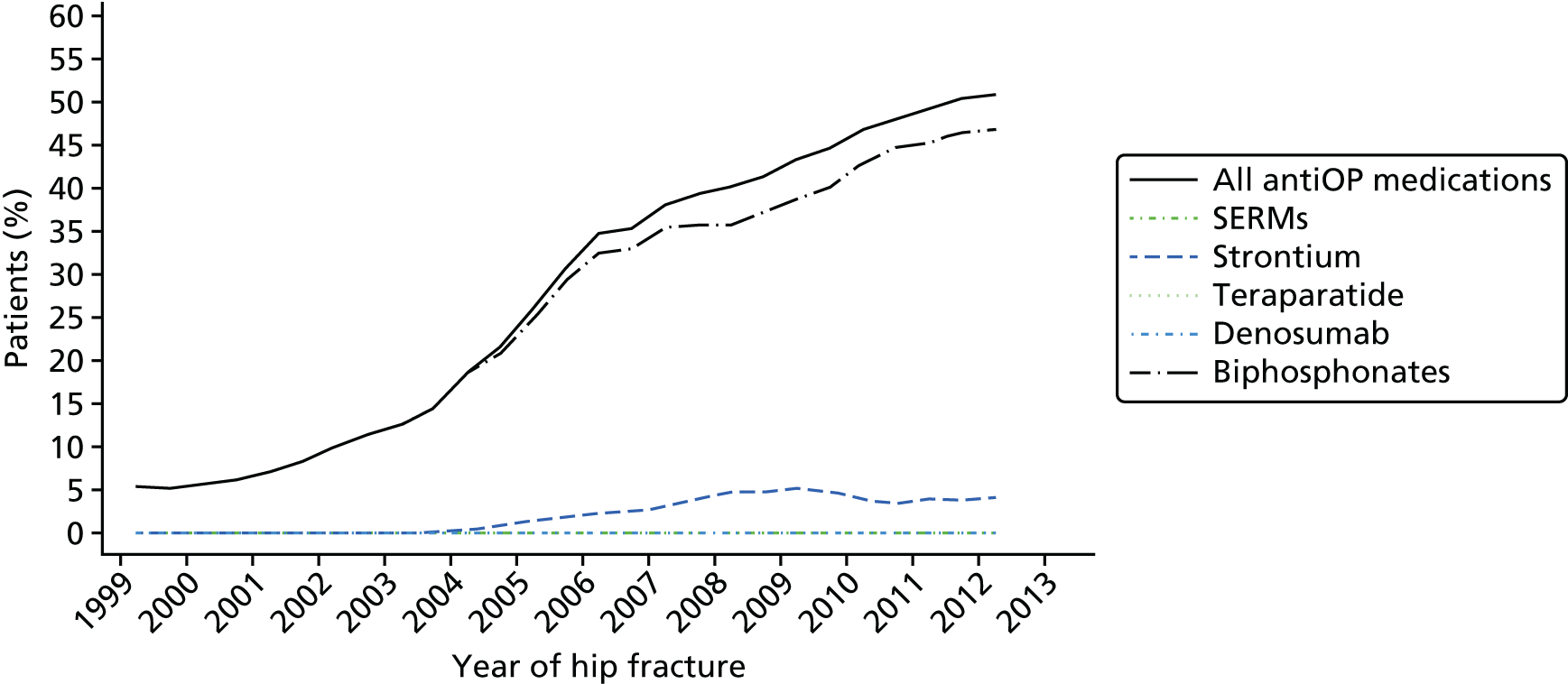
FIGURE 16.
Bisphosphonate use among treatment-naive hip fracture cases at baseline within 12 months, stratified by bisphosphonate type. Reproduced with permission from Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, et al. Anti-osteoporosis medication prescriptions and incidence of secondary fracture among primary hip fracture patients in England and Wales: an interrupted time-series and economic analysis. J Bone Miner Res 2016; in press.
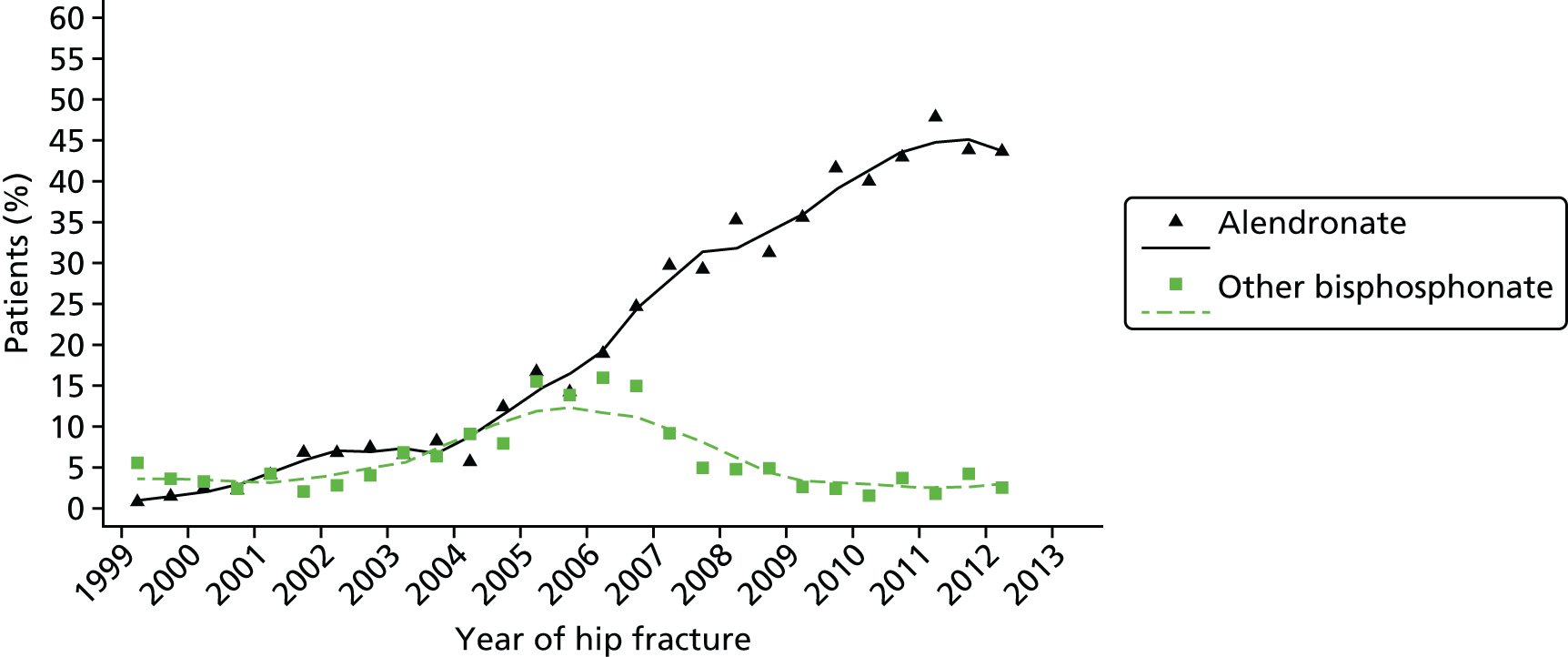
FIGURE 17.
Bisphosphonate use among treatment-naive hip fracture cases at baseline, period prevalence between 2–6 and 10–14 months.
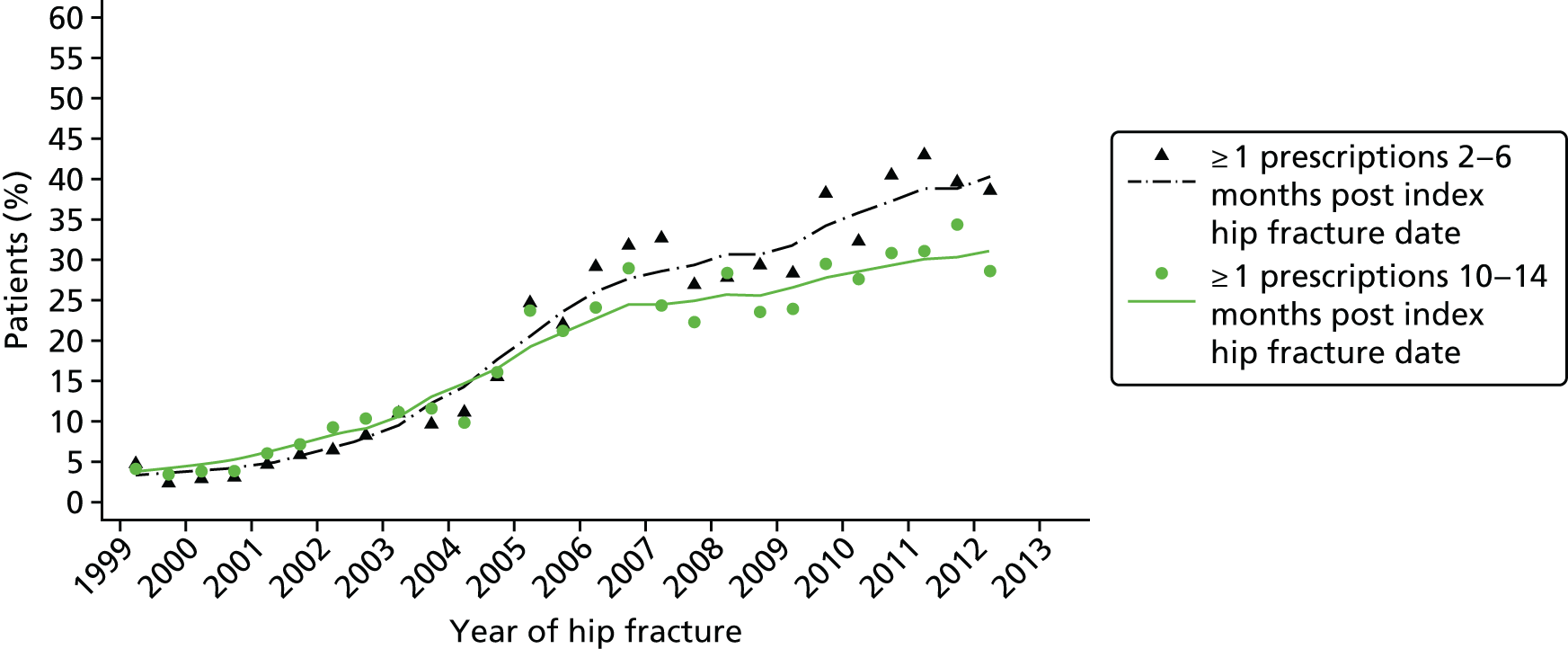
Segmented linear regression results
For simplicity, the figures referred to below are from the final parsimonious segmented linear regression analyses, with the coefficients reported in Appendix 7. Results are expressed for each intervention in terms of immediate ‘step’ and/or ‘trend’ change in each aggregated outcome measure under study. Estimates from full models with forced inclusion of each intervention are also reported in Appendix 8.
Index hip fracture and refracture
The number of primary hip fractures occurring per 6 months between 1999 and 2013 are shown in Figure 18a. When the time series was compared with publication of the guidelines under evaluation, there was a significant trend-change in the number of index hip fractures occurring following the October 2004–March 2005 period wherein the NICE CG 21 and NICE TA 87 were published. Prior to this period the biannual proportions were stable, whereas a post-intervention trend of –7.17 (95% CI –8.75 to –5.6; p < 0.001) index hip fractures per 6 months was detected (see Figure 18a). This association was unchanged for aggregated proportions of overall hip fractures (see Figure 18b).
FIGURE 18.
(a) Number (1999/2000–2012/13) of primary hip fractures; and (b) overall number of primary and secondary hip fractures.

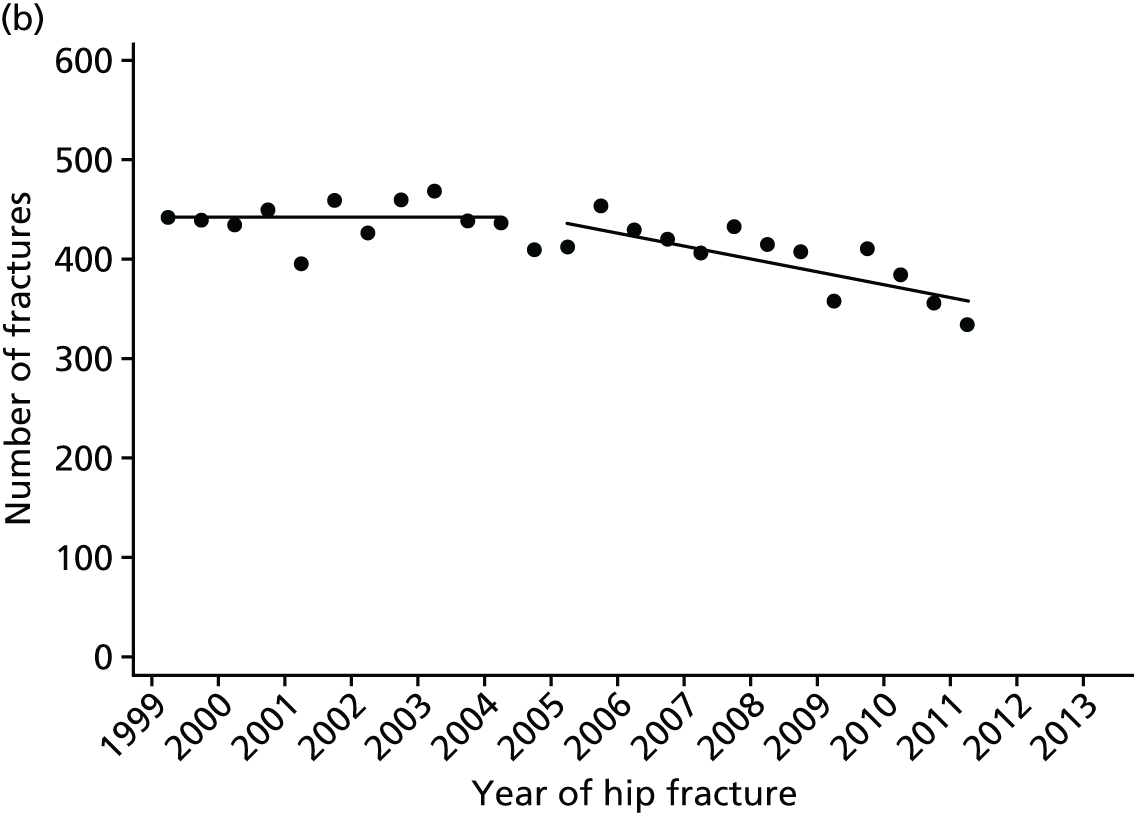
Considering refracture, an initial stable rate of 2.49% (95% CI 2.12% to 2.87%) was estimated for subsequent hip fracture, although a step-change reduction of –0.95% (95% CI –1.67 to –0.23; p = 0.012) between October 2007 and September 2008 (publication of the BOA Blue Book1 and NICE TA 16113) was found (Figure 19a). This reduction in refracture at the hip was not observed for subsequent major non-hip fracture (see Figure 19b). There was no evidence associated with any of the other guidelines evaluated for a change in level or trend in biannual proportions (see Figure 19).
FIGURE 19.
(a) Post-index date second hip fracture: cases (black circle); and (b) post-index date major non-hip fracture: cases (black circle) and controls (green triangle).
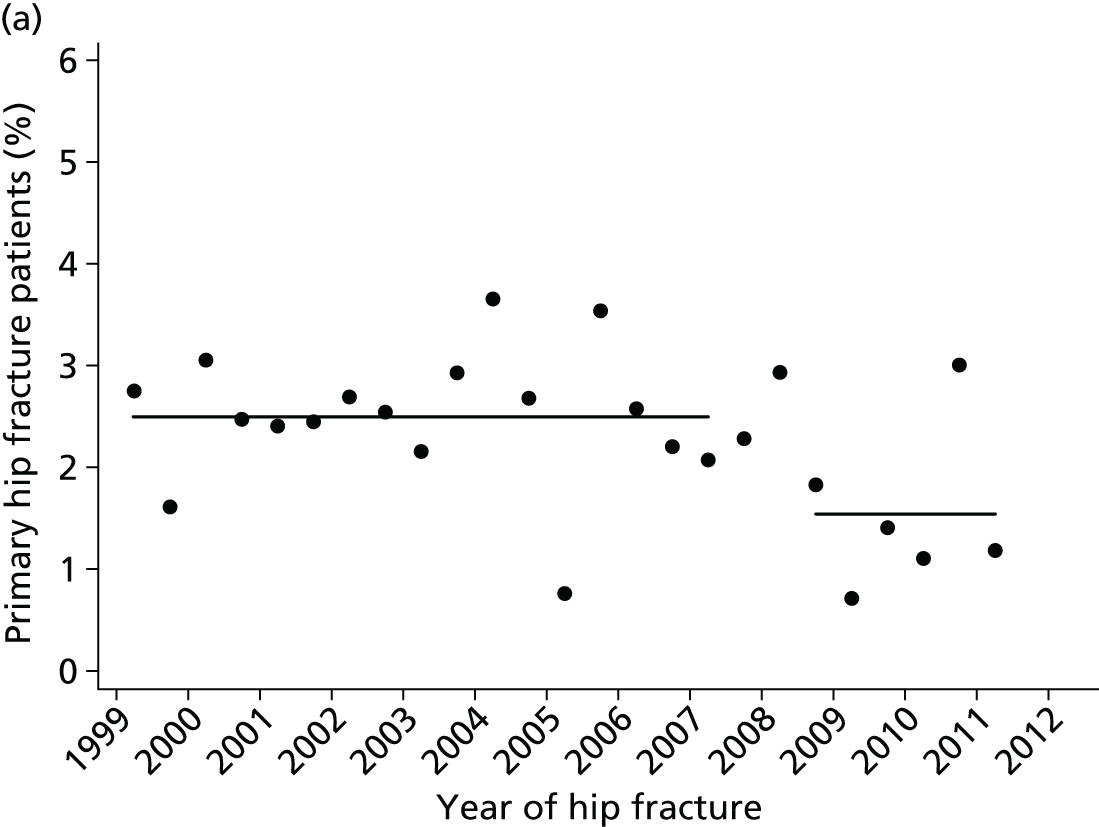

Mortality
In relation to the guidelines evaluated here, a significant step-change reduction in 30-day mortality occurred of –2.81% (95% CI –3.73 to –1.85; p ≤ 0.001) between October 2007 and September 2008 (publication of the BOA Blue Book1 and NICE TA 16113) (Figure 20a). Although this step change was not reflected in 1-year mortality following the same publications, a significant reduction in 1-year mortality of –5.56% (95% CI –7.59 to –3.52; p < 0.001) was seen immediately following the introduction of the Best Practice Tariff in April 201076 (Figure 21a). No other step change or trends were found in 30-day or 1-year mortality. When a ‘difference in differences’ analysis was carried out for mortality between cases and controls, the significant reduction in 30-day mortality remained (see Figure 20b), as it did for 1-year mortality (see Figure 21b); however, for the difference in 1-year mortality between cases and controls, there was a trend increase following the October 2007 to September 2008 period (the publication of the BOA Blue Book1 and the NICE TA 16113) of 0.98% (95% CI 0.16 to 1.8; p = 0.022) in biannual proportions that persisted throughout the rest of the study period (see Figure 21b).
FIGURE 20.
(a) Post-index date mortality within 30 days among cases (black circle) and controls (green triangle); and (b) post-index date 30-day mortality: difference in differences (black circle) between cases (blue diamond) and controls (green triangle).
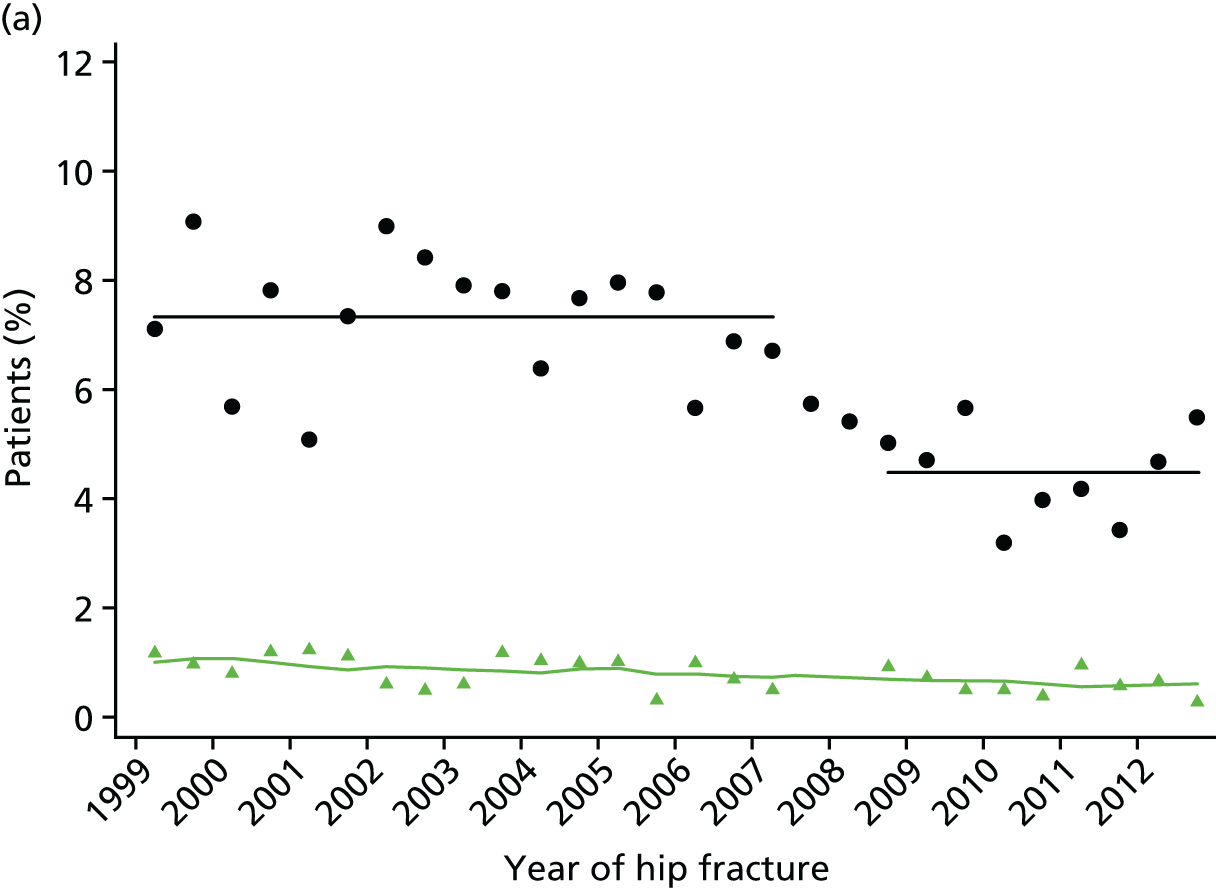

FIGURE 21.
(a) Post-index date mortality within 1 year among cases (black circle) and controls (green triangle); and (b) post-index date 1-year mortality: difference in differences (black circle) between cases (blue diamond) and controls (green triangle).
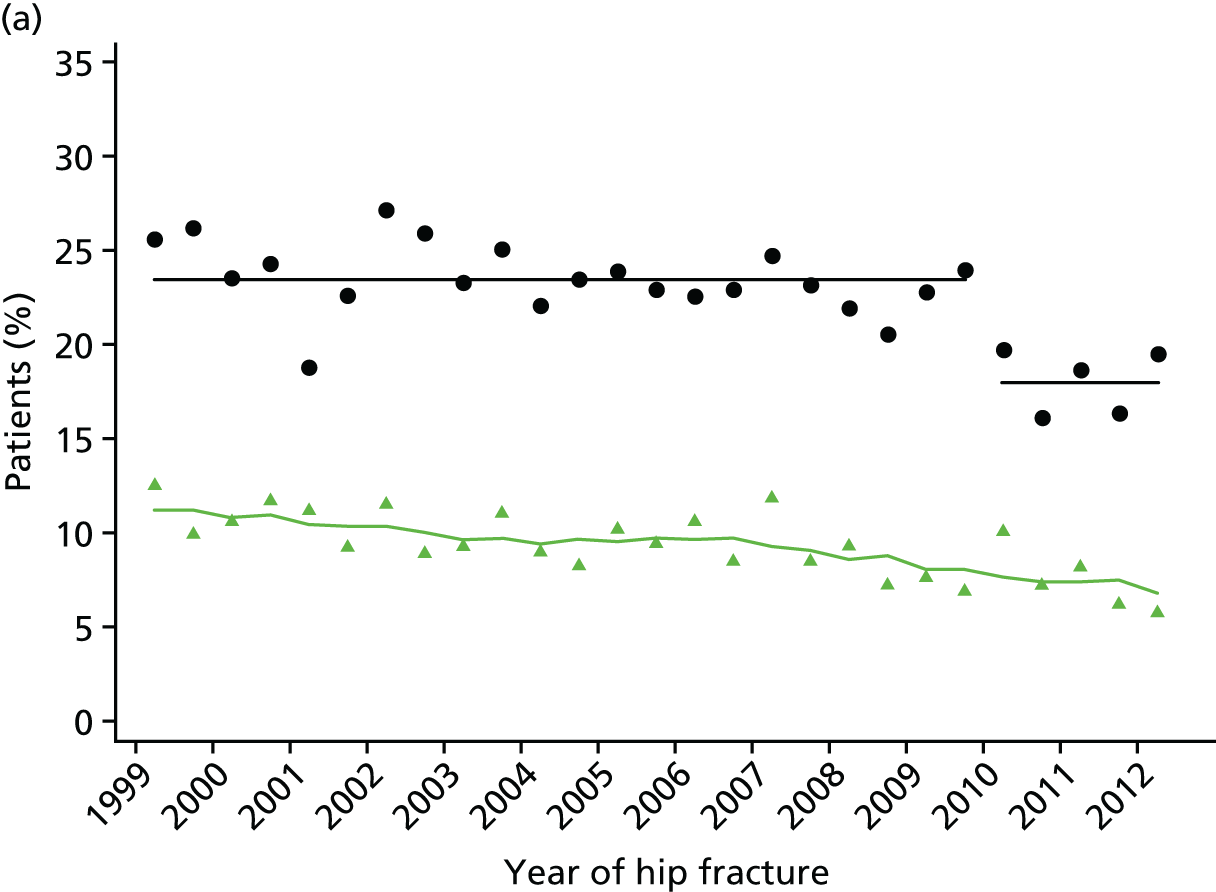

Bone-strengthening drugs
The time series of biannual proportions of treatment-naive index hip fracture patients receiving an incident prescription for a bone-strengthening drug in the first year following hip fracture is presented in Figure 20b. This shows an initial upwards trend in the proportion receiving such a prescription of 1.1% per 6 months, with a marked step change of 14.5% (95% CI 11.1% to 17.8%; p ≤ 0.001) taking place between pre-publication of NICE CG 21 (November 2004)15 and post-publication of the NICE TA 87 (January 2005). 33 These publications were also associated with a small increase (0.49%, 95% CI –0.05% to 1.03%; p = 0.073) in the prior upwards trend (see Figure 20b). Similar estimates in both a trend and a step change following these publications were also found in the time series of antiosteoporosis medication initiation in the first 4 months after index hip fracture date (Figure 22a).
FIGURE 22.
Any antiosteoporosis medication post index date among cases (black circle) and controls (green triangle) within (a) 4 months; and (b) 12 months.
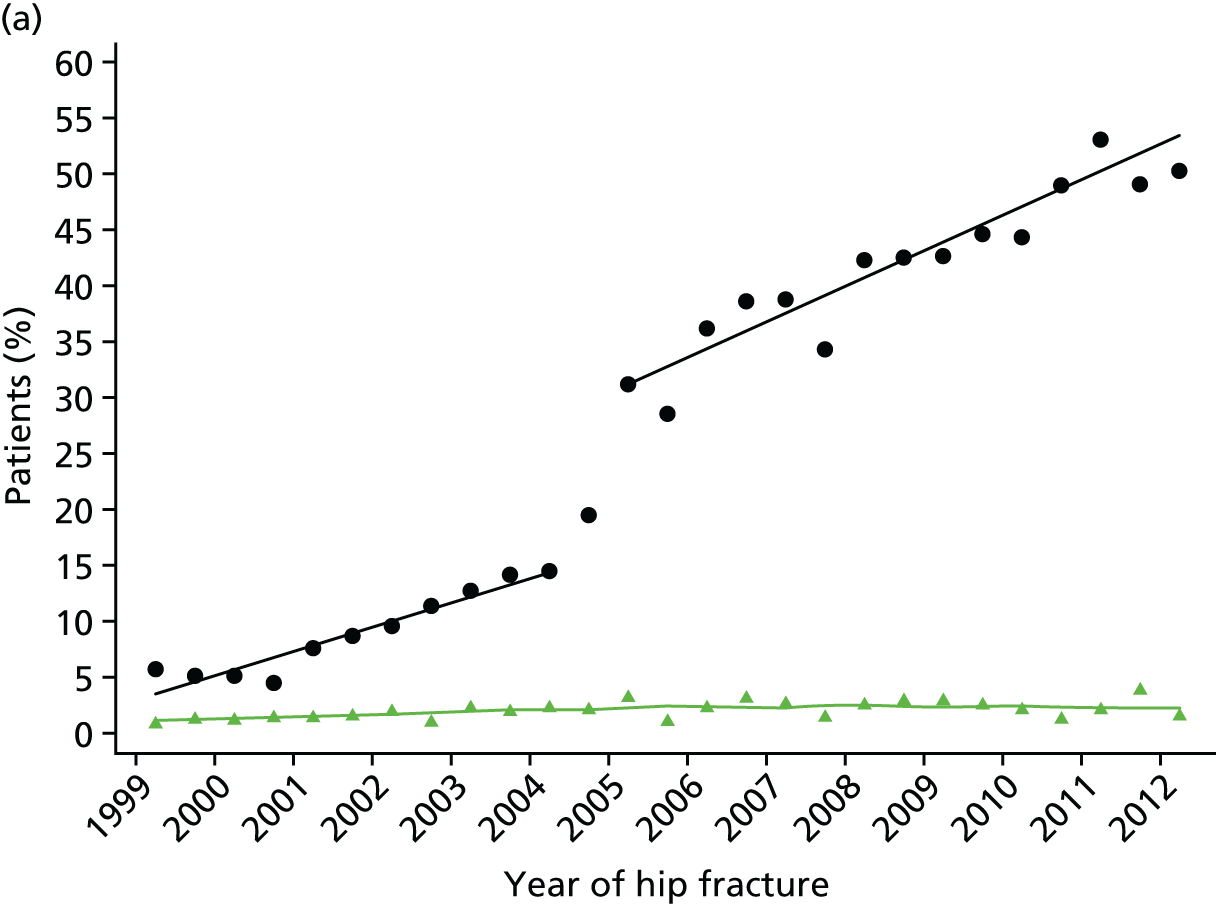
In analyses of the biannual proportions of patients with at least one bisphosphonate prescription at 10–14 months following index hip fracture, an overall trend increase of 0.96% (95% CI 0.62% to 1.27%; p < 0.001) per 6 months was detected. In addition, a step-change increase of 8.71% (95% CI 5.04% to 12.4%; p < 0.001) was observed between the pre-publication of NICE CG 21 (November 2004)15 and post-publication of the NICE TA 87 (January 2005)33 (Figure 23). However, a modest step-change decrease of –3.79% (95% CI –7.4% to –0.17%; p = 0.041) was found to occur between October 2007 and September 2007 (publication of the BOA Blue Book1 and NICE TA 16113) (see Figure 23).
FIGURE 23.
Bisphosphonate prescription 10–14 months post index date among treatment-naive individuals at baseline among cases (black circle) and controls (green triangle).
When analyses of any antiosteoporosis medication within 1 year were stratified by sex, the step-change increase associated with the time period October 2004–March 2005 was seen among both men (9.07%, 95% CI 3.68% to 14.5%) and women (15.2%, 95% CI 11.1% to 19.3%) (see Appendix 9). Among men, however, this time period was also associated with a trend increase of 1.74% (95% CI 0.94% to 2.53%; p < 0.001) per 6 months that was then followed by a step decrease of –7.52% (95% CI –14.1% to –0.95%; p = 0.027) following the October 2007–September 2008 time period (see Appendix 9). Coefficients from full models stratified by sex are also reported in Appendix 10.
A summary of the associations between the guidelines and changes in outcomes from the interrupted time series models is shown in Table 16.
Discussion
In this analysis, we have been able to demonstrate significant temporal associations with a number of national guidelines, suggesting that these guidelines have positively impacted on clinical decision-making and then patient outcomes. Of the health outcomes studied, only non-hip refractures were not associated with the introduction of guidelines. The guidelines focused on medication choices, standards of inpatient care and falls assessment.
It was of interest that each outcome was affected by only one guideline. It is unclear why the BOA Blue Book1 affected only 30-day mortality while the Best Practice Tariff76 affected only the 1-year mortality.
The strengths of this analysis include the use of national data representative of England and Wales, and the use of an interrupted time series approach that controls for baseline level and trend in estimating the intervention impact on each outcome of interest. 108
One limitation of the analysis was the fewer than eight data points before or after some interventions, as eight has been suggested elsewhere as a reasonable number for an interrupted time series analysis. 132 We did, however, have three or more data points within each section of the time series, as is stated as the minimum required for inclusion of an interrupted time series analysis in a Cochrane EPOC Review Group review. 107 A major limitation was the need to link a number of interventions that were introduced within a short interval, for example NICE CG 2115 and TA 87. 33 However, given that the focus of NICE CG 2115 is on falls, it is more likely that the association with prescribing was through NICE TA 87. 33 In addition, during the time of TA 87, the first-line antiosteoporosis medication alendronate became available as a generic medicine and is likely to have contributed significantly to the observed associations with prescribing (see Figure 16). Although we were able to measure the association of guidelines on the average changes in outcomes, it would be of interest to examine if the timing of the guidelines was also associated with a reduction in variability of outcomes between sites. The use of routinely collected data with no individual validation of fracture events recorded is another limitation of the analysis; however, validation of hip and vertebral fracture coding has been carried out previously and been shown to be accurate. 133
Chapter 7 Primary care and hospital care costs for hip fracture patients
Introduction
In this chapter, we report the costs associated with the use of primary and hospital care resources resulting from a hip fracture. The current evidence on the economic burden of hip fracture on the UK health services is limited and outdated. However, it is important to have robust and up-to-date evidence of the economic impact of hip fracture and its main drivers. Such data are essential to inform decisions about changes in health service delivery aimed at achieving greater efficiency and better patient care. Furthermore, such information is key to investment and disinvestment decisions regarding new osteoporosis and hip fracture prevention interventions as these are driven by cost-effectiveness analysis,29 in which a key input is the long-term cost of hip fracture. Hence our aim is to use large primary and secondary care administrative data sets to determine primary care and hospital care costs in the year of the hip fracture and the following year.
Aims
Hip fractures are a major public health problem in terms of patient morbidity, mortality and costs to health and social care services. The incidence of hip fracture increases steeply with age owing to higher rates of osteoporosis and falls in the ageing population. Hip fractures account for the majority of osteoporotic fragility fractures and for > 40% of the estimated burden of osteoporosis worldwide. 134 In 2010, there were an estimated 600,000 incident hip fractures in the European Union, costing an estimated €20B and accounting for 54% of the total costs of osteoporosis. 135 In the UK, the annual number of hip fractures is expected to increase from 79,000 to 104,000 by 2025. 135 Existing estimates of the health and social care costs of hip fractures in the UK range from £2B to £3B,135,136 but UK estimates on hip fracture costs are limited and outdated. Hence the primary aim of this chapter is to estimate the primary care and hospital care costs of hip fracture up to 2 years post event for both index fracture and subsequent fracture, using large patient-level data sets representative of the UK hip fracture population. Second, we compare costs before and after the event to explore the impact of significant comorbidities in individuals with hip fracture. Finally, we report the main predictors of long-term costs following hip fracture.
Existing research
We undertook a literature review to identify UK-specific costing studies of hip fracture patients published from 1990 to 1 December 2013. The following databases were searched: EMBASE, MEDLINE, Global Health, CAB Abstracts, American Economic Association’s electronic bibliography (EconLit), NHS Economics Evaluation Database (NHS EED) and Health Technology Assessment (HTA) and Web of Science. Search terms related to hip fracture and costs were used to identify papers of interest in the databases and are available on request from the authors. We subsequently performed a search of the database using terms relating to the UK (UK, United Kingdom, Britain, England, Wales, Scotland, Ireland, NHS and National Health Service). A total of 65 papers were identified, of which 13 presented costs based on patient-level data and 52 presented costs citing results from other studies or from national databases of costs (Figure 24). We focused on the studies reporting costs based on patient-level data. Data were extracted from each study using a predefined pro forma.
FIGURE 24.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram for literature search of patient-level UK costing studies.
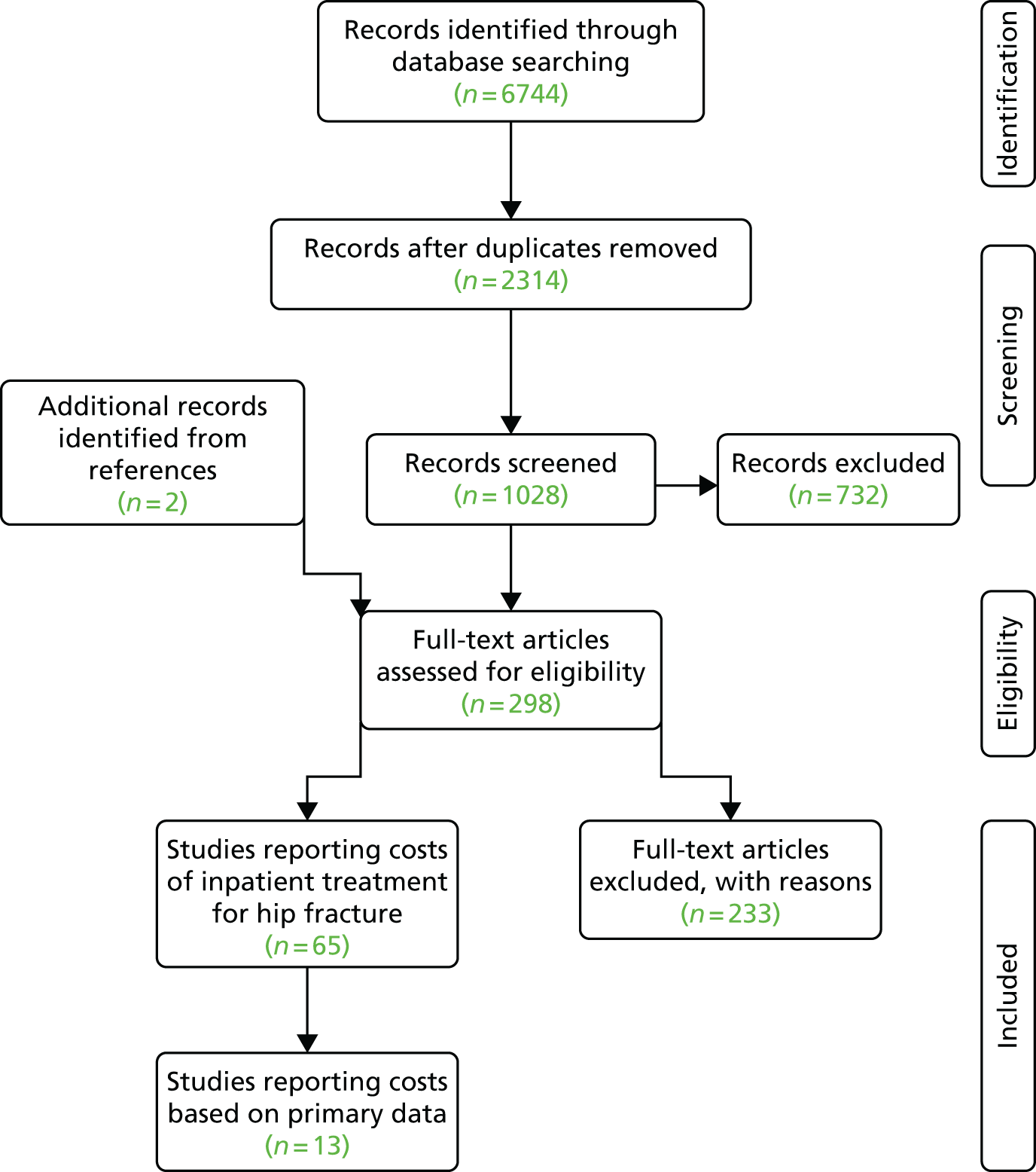
We identified four studies published between 2010 and 2013, seven studies published between 2000 2009 and two studies published before 2000. We also found considerable heterogeneity across the studies in terms of study populations (e.g. all hip fractures, hip fractures > 50/60/65/70 years, women only or admitted from care homes), setting (e.g. single hospital or administrative data sets for a region), sample sizes (10 to 2427 patients), types of resource use included (e.g. inpatient care, outpatient care, primary care, community hospital or care home), time horizon (period over which costs were included) and methods to estimate the costs (e.g. micro costing or valuing HRGs). These help to explain the significant variability in the reported costs; for example, the reported costs for acute inpatient admission with hip fracture varied from £4202 to £16,452 (2012–13 prices).
Focusing on the four more recent studies, Gutiérrez et al. 137 reported costs based on 2427 women aged > 50 years identified in a primary care research database. The time horizon of the analysis was 12 months post hip fracture. The authors reported the costs to be £6176 in the first year after hip fracture and £5083 during the acute admission (2012–13 prices). Sahota et al. 138 estimated costs based on a sample of 100 hip fracture patients admitted from nursing or residential home. The time horizon of analysis was from hospital admission to discharge. Using HRGs to inform the unit costs, these authors reported the acute inpatient stay to cost £7468 (2012–13 prices). Thakar et al. 139 reported a prospective study that included all hip fractures admitted into a single hospital over a 5-year period. These authors then compared 144 cases with complications requiring surgery against 288 control patients. The time horizon of the costs was from acute admission to hospital discharge including rehabilitation and patients with complications were reported to cost £12,137, compared with £4202 for patients without complications (2012/13 prices). Finally, Kazi and Acharya140 estimated the costs of acute inpatient stay post hip fracture to be £8654 based on 11 hip fracture admissions to a single hospital (2012–13 prices).
Existent UK data on primary care and hospital care costs associated with hip fractures have several limitations. Hence there is significant scope to improve the evidence base by using large primary care and secondary care administrative data sets to estimate short- and long-term costs and resource use using samples that are representative of the hip fracture population and that are large enough to explore in detail potential drivers of costs.
Methods
Setting and data sources
We used two sets of data sources to estimate the costs associated with a hip fracture. For hospital costs, we used the HES database and for primary costs we used CPRD GOLD (see Chapter 4 for description of data sources). We adopted the same incidence-based approach to identify hip fracture patients in both sets of data and estimate the costs of hip fracture.
Hospital Episode Statistics data set
Data were obtained from the HES database for a representative region of the UK covering a population of around 4 million people and with 11 NHS hospitals treating fragility fractures. This database captures all hospital NHS patient care, as well as private patients treated in NHS hospitals and care delivered by treatment centres (including private providers) funded by the NHS. It contains anonymised patient administrative information (such as date of admission and discharge, admission method, age, sex and length of stay), diagnosis (ICD-10) and procedures codes (OPCS-4). We extracted inpatient care data from April 1999 to March 2013, hospital outpatient activity from April 2003 and A&E attendances from April 2007. Deaths were obtained from the linked HES and ONS mortality database, which captures deaths occurring in and out of hospital.
Clinical Practice Research Datalink data set
The CPRD GOLD database contains data on patient consultations entered by the GP, medical history, referrals data, tests and all pharmaceutical prescriptions on the GP system. The data extracted consisted of patients with a first ever clinical or referral record of hip fracture occurring from 1 January 1999 onwards and with at least 3 years of GP registration prior to the index date. Hip fracture was identified using pre-defined Read codes (see Appendix 3). The CPRD GOLD data set was linked to HES and ONS (mortality) records. About 60% of the primary care practices contributing to CPRD have agreed to linkage to the HES and ONS data. When HES data could be linked to CPRD, records consisted of inpatient care data from April 1999 to March 2012.
Study participants
To be consistent, we used the same approach to identify patients with a hip fracture in the HES and CPRD data sets. We searched the hospital finished consultant episodes for patients > 60 years of age who had had an emergency hospital admission with a primary ICD-10 diagnosis code for hip fracture (S72.0–S72.2, S72.9) between April 2003 and March 2013 (HES data set) and April 2003 and March 2012 (CPRD data set). We extracted all primary (CPRD) and hospital (HES) records before and after that admission. A number of exclusion criteria were applied to minimise misclassification: (1) day cases were excluded by imposing a condition that patients had to stay at least one night in hospital, unless death occurred in the first 24 hours of admission; (2) individuals who had had a previous hip fracture between April 1999 and March 2003 were excluded to reduce duplicate coding of hip fractures that occurred before the period of analysis but led to repeat hospital admissions due to complications or unresolved sequelae; and (3) patients were also excluded if they had had a hip fracture due to trauma, such as transport accidents, identified using ICD-10 codes (V01–V99).
When estimating hospital costs in the year before and after fracture we included only patients with an index admission after 1 April 2008, to ensure that outpatient and emergency attendances costs would be included. We refer to this set of results as ‘total hospital’ costs and contacts. Conversely, we used the whole HES data set (April 2003–April 2013) to report costs due to hospitalisation, critical care and day cases, and benefit from the increased statistical power. We refer to these results as ‘hospitalisation’ costs. When estimating primary care costs, we included only patients who were registered with a GP at the time of index hip fracture admission.
Second hip fractures were identified using the same approach as for the index fracture. To ensure that they were separate fractures and not hospital readmissions resulting from adverse effects of the index fracture, we counted second hip fractures only if admitted in a separate CIPS from index admission and at least 30 days after admission for the primary fracture. A CIPS is made up by one or more hospital spells (i.e. time patient stays in one hospital) and is defined as a continuous period of care within the NHS, regardless of any transfers to another hospital. A hospital spell starts with the index admission, involves treatment by one or more consultants (i.e. finished consultant episodes) and ends when the patient dies or is discharged from hospital.
Primary care costs
Primary care contacts included GP consultations in clinic/surgery, telephone contacts and out-of-office visits. It also included nurse face-to-face and non-face-to-face contacts and contacts with other community health-care professionals (e.g. health visitor or physiotherapist). GP and nurse consultations excluded repeat prescriptions for which the patient was not seen, notes and reports and laboratory/radiology requests and results. Following previous research,141 GPs were identified using the following codes: senior partner; partner; associate; non-commercial local rota of less than 10 GPs; commercial deputising service; GP registrar; sole practitioner; and GP retainer. Nurses were identified using practice nurse; community-based nurse; hospital nurse; school nurse; and other nursing and midwifery. CPRD records listing administrative staff, such as secretaries, IT staff, practice and fund managers and receptionists, were not counted as a clinical direct contact with the patient and were, therefore, excluded from the costs. Following a previous study,142 we only counted one consultation per day if more than one was recorded per patient. Primary care contacts and tests were costed using unit costs from national cost databases (Table 17). 143 We excluded from our costs tests that are routinely performed as part of a primary care consultation, such as blood pressure measurement, to avoid double counting. Pharmaceuticals were costed by matching each prescribed medication to a BNF code, moving from the most detailed level (subparagraph) to the top level (chapter) until a match was found. 90 The number of medications per patient stratified by BNF code were then multiplied by the respective unit costs. The unit costs for each BNF code concerned the net ingredient cost per item prescribed reported in the Health and Social Care Information Centre Prescription Cost Analysis. 145 These were estimated using the average for each BNF level (from subparagraph to chapter) using the number of items prescribed as weights. Primary care costs were computed by multiplying the number of contacts/test/prescribed items by their unit costs. Costs per patient were then summed across these different resource categories and aggregated into monthly and annual amounts for the purposes of the analysis.
| Categories | Unit cost (£) | Source |
|---|---|---|
| Consultations/contacts | ||
| GP consultation at clinic | 60 | PSSRU 2013143 |
| GP consultation in surgery | 41 | PSSRU 2013143 |
| GP consultation by telephone | 25 | PSSRU 2013143 |
| GP consultation out of office | 104 | PSSRU 2013143 |
| Nurse face-to-face consultation | 38 | Reference Costs 2012–13144 |
| Nurse non-face-to-face consultation | 23 | Reference Costs 2012–13144 |
| Health visitor/social worker | 50 | Reference Costs 2012–13144 |
| Chiropodist/chiropractor/osteopath | 42 | Reference Costs 2012–13144 |
| Physiotherapist | 50 | Reference Costs 2012–13144 |
| Dentist | 115 | Reference Costs 2012–13144 |
| Dietitian | 71 | Reference Costs 2012–13144 |
| Speech therapist | 89 | Reference Costs 2012–13144 |
| Other | 34 | Reference Costs 2012–13144 |
| Testsa | ||
| Haematology | 3 | Reference Costs 2012–13144 |
| Clinical biochemistry | 1 | Reference Costs 2012–13144 |
| Microbiology | 7 | Reference Costs 2012–13144 |
| Cytology | 17 | Reference Costs 2012–13144 |
| Immunology | 5 | Reference Costs 2012–13144 |
Hospital costs
Each finished consultant episode in a hospital spell was assigned into a 2012–13 HRG using standard software. HRGs consist of standard groups of clinically similar treatments that consume a common set of health-care resources. All resource use was valued using 2012–13 prices that were obtained from the schedule of reference costs for NHS trusts. 144 Total costs per patient were aggregated into monthly and annual amounts for the purposes of the analysis.
Statistical analysis
We determined the marginal costs attributable to a hip fracture by comparing costs in the year before and after the index hip fracture. The national total annual primary and hospital costs of hip fracture were determined by multiplying the incidence of hip fracture in the UK (79,243 in 2010) by the estimated costs per hip fracture. 135
The HES database was censored in 31 March 2013, and complete follow-up was not available for all cases. Hence we report total hospital costs for those patients with complete follow-up data at years 1 and 2 following hip fracture and for the whole sample after adjusting for censoring using the methodology developed by Lin. 146 Costs are reported as means together with their 95% CIs, obtained from 1000 bootstrap estimates.
Predictors of primary care and hospitalisation costs of hip fracture were estimated using a generalised linear model (GLM). After reviewing the literature, we examined the following predictors of costs in the year of the hip fracture: age at fracture; type of fracture (head and neck S72.0; pertrochanteric S72.1; subtrochanteric S72.2; unspecified S72.9); sex; Charlson Comorbidity Index score (complications at fracture and occurring up to 3 years before fracture);147 place of residence pre and post fracture (own home or care home: residential or nursing home); occurrence of second hip fracture; history of other osteoporatic major fragility fractures requiring hospitalisation pre and post hip fracture (spine, wrist, pelvis, rib, humerus and other identified with ICD-10104 diagnosis codes S22, S32, S42, S52.0–S52.3, S22.5 and S22.6); primary hip replacement and revision;148 complications of internal orthopaedic devices (ICD-10 code T84) and infection or haemorrhage following procedure (T81.0 and T81.4); dislocation (M24.3 and M24.4); malunion and non-union of fracture (M84.0–M84.2); periprosthetic fracture (M96.6); other/unspecified postprocedural musculoskeletal disorders (M96.8, M96.9); sequelae of fractures of the femur (T93.1); hip luxations (S73.0); ethnicity (white and non-white); year of hip fracture; and income deprivation measured by the IMD. We assumed that once a patient moved to a care home they would remain there for the rest of their life. We included only covariates that had a frequency of at least 100 patients in the samples available. Selection of covariates for the final model included the type of hip fracture and other covariates from the list specified that were found to be significant and met the inclusion criteria. For hospitalisation costs, a variable was deemed to be statistically significant if p < 0.01 to account for the large sample size and 95% CIs were reported for ease of comparison with other studies. For primary care costs, a variable was deemed to be statistically significant if p < 0.05. The choice of the GLM model family and link functions was informed by the modified Park test and the Box–Cox test, respectively. Model fit was assessed using Pregibon link test and different family and link functions were compared using Akaike’s information criterion. Univariate analyses in continuous variables were performed using Student’s t-tests. All analyses were performed using Stata version 12 (StataCorp, College Station, TX, USA).
Results
Patient sample
Hospital care data set
Between 1 April 2003, and 31 March 2013, 33,152 patients were identified as having had a hip fracture. The mean age of the sample was 83 years (SD 8.2 years) and 75% were female. The majority of the population was of white ethnicity. Baseline characteristics of the study participants are shown in Table 18. Women were older than men (+2.0 years) and less likely to have a history of complications (Charlson Comorbidity Index score of 1.1 vs. 1.7 in men) at index hip fracture.
| Characteristics | n (%) |
|---|---|
| Age (years), mean (SD) | 82.7 (8.2) |
| Type of hip fracture | |
| Fracture of head and neck of femur | 25,335 (76.4) |
| Pertrochanteric fracture | 6590 (19.9) |
| Subtrochanteric fracture | 913 (2.8) |
| Unspecified fracture of femur | 315 (1.0) |
| Males | 8355 (25.2) |
| White ethnicity | 31,287 (98.9) |
| CCI, mean (SD) | 1.26 (1.57) |
| History of comorbidities recorded in previous hospitalisations | |
| Dementia | 6101 (18.4) |
| Pulmonary disease | 4594 (13.9) |
| Diabetes | 3841 (11.6) |
| Source of admission at index fracture | |
| Own home | 27,985 (84.4) |
| Care home or temporary accommodation | 3681 (11.1) |
| Another hospital | 1415 (4.4) |
| Unknown | 35 (0.1) |
Primary care data set
Between 1 April 2003, and 31 March 2012, 4433 patients were identified as having had a hip fracture in England and in a primary care practice with linkage to the HES and ONS data. These represent 62% of all hip fracture patients identified using the Read codes (see Chapter 6) within the participating GP practices contributing data to the CPRD in England and Wales (n = 7155), between April 2003 and March 2012. The majority of the population was of white ethnicity, 82% of the sample were female and the average age was 82.7 years (SD 8.0 years). Baseline characteristics of the study participants are shown in Table 19. Compared with the patient sample in the hospital care data set, the proportion of patients with dementia was smaller in the primary care data set and a higher proportion of patients were admitted from their own home at index fracture.
| Characteristics | n (%) |
|---|---|
| Age (years), mean (SD) | 82.7 (8.0) |
| Type of hip fracture | |
| Fracture of head and neck of femur | 3197 (72.1) |
| Pertrochanteric fracture | 1081 (24.4) |
| Subtrochanteric fracture | 123 (2.8) |
| Unspecified fracture of femur | 32 (0.7) |
| Males | 988 (22.3) |
| White ethnicity | 3888 (87.7) |
| Charlson Comorbidity Index, mean (SD) | 1.20 (1.51) |
| History of comorbidities recorded in previous hospitalisations | |
| Dementia | 608 (13.7) |
| Pulmonary disease | 649 (14.6) |
| Diabetes | 497 (11.2) |
| Source of admission at index fracture | |
| Own home | 3895 (87.9) |
| Care home or temporary accommodation | 421 (9.5) |
| Another hospital | 114 (2.8) |
| Unknown | 3 (0.1) |
Patient outcomes and hospitalisation costs (Hospital Episode Statistics data set)
The average follow-up of the cohort was 2.6 years (median 1.8 years; SD 2.5 years) from index hip fracture, during which time 6.6% of patients suffered a second hip fracture (Table 20). Mortality at 30 days and 1 year was estimated to be 9.4% and 31.2%, respectively. After index fracture, the majority of patients were recorded as being discharged to their own home (49%), or transferred to another hospital (23%) or to a care home (18%). Ten per cent of patients died in hospital during the index admission. The number of patients in a care home increased to 24% after 1 year of complete follow-up. In contrast, after a second hip fracture, 32% of patients were discharged to a care home after hospital discharge, which increased to 40% among patients with at least 1 year of follow-up. Hospital readmissions for any reason with inpatient stay in the year following the index hip fracture totalled 0.9 [median 0, SD 1.4, interquartile range (IQR) 0–19] per patient or 1.8 (median 1, SD 1.6, IQR 1–19) per patient readmitted. Of these readmissions, 57% were emergency admissions and accounted for 18.1 (median 0, SD 35.9, IQR 0–22) additional days in hospital per patient or 37.2 (median 23, SD 43.9, IQR 8–50) additional days per patient readmitted. About 50% of diagnosis codes in non-emergency inpatient admissions following index admission were hip fracture related.
| Patient outcomes | n (%) |
|---|---|
| Follow-up time in years, mean (SD) | 2.6 (2.5) |
| Second hip fracture | 2206 (6.6) |
| Time to second hip fracture in years, mean (SD) | 2.2 (2.0) |
| Surgery or implant-related complicationsa | |
| Within index fracture hospital | 1015 (3.1) |
| Within 1 year of fractureb | 1942 (6.4) |
| Hip replacement surgeryc | |
| Within index fracture hospitalisation | 1522 (5.0) |
| Within 1 year of fractureb | 1781 (5.9) |
| Hip revision surgeryc | |
| Within index fracture admission | 247 (0.7) |
| Within 1 year of fractureb | 463 (1.5) |
| Mortality | |
| Within 30 daysd | 3101 (9.4) |
| Within 1 yearb | 9492 (31.2) |
| Discharge destination following index fracture admission | |
| Own home | 16,126 (48.6) |
| Care home or temporary accommodation | 5957 (18.0) |
| NHS hospital | 7453 (22.5) |
| Unknown | 371 (1.1) |
| Dead | 3245 (9.8) |
| Care home within 1 year of index fractureb | 7409 (24.4) |
| Total length of hospital stay within 1 year of fracture,b mean (SD) | |
| Initial hospitalisation | 20.5 (20.0) |
| Emergency hospitalisations after discharge | 6.9 (19.0) |
| Non-emergency hospitalisations after discharge | 11.3 (28.6) |
| Total | 38.6 (41.2) |
| Hospital inpatient readmissions within 1 year of fractured | |
| Emergency, mean (SD) | 0.5 (0.9) |
| Non-emergency, mean (SD) | 0.4 (0.9) |
| Total, mean (SD) | 0.9 (1.4) |
| Initial hospitalisation costs (index admission to discharge) (£) | |
| Primary hip fracture, mean (SD) | 8663 (4605) |
| Second hip fracture, mean (SD) | 8544 (4178) |
| Hospitalisation costs within 1 year of fractureb (£) | |
| Emergency-related costs, mean (SD) | 10,854 (7268) |
| Non-emergency-related costs, mean (SD) | 2972 (7896) |
| Total, mean (SD) | 13,826 (11,016) |
| Hip fracture related hospitalisation costs within 1 year of admissionb (£), mean (SD) | 10,375 (6962) |
The hospitalisation costs associated with index admission for primary hip fracture were £8663 (median £8049, SD £4605) compared with £8544 (median £8049, SD £4112) for the second hip fracture. Length of stays in the index admission were 20.5 (median 14, SD 20.0, IQR 9–307) and 20.8 (median 15, SD 18.8, IQR 9–139) days for primary and second hip fracture, respectively. For patients suffering a subsequent hip fracture (6.6%), the hospital admission following the second hip fracture resulted in significantly higher length of stay (2.0 days; p < 0.001; 18.8 vs. 20.8 days) and costs (£406; p < 0.001; £8138 vs. £8544) relative to the index fracture. Within the first year following primary hip fracture, the total hospitalisation costs were estimated to be £13,826 (median £10,425, SD £11,016), of which 75% were because of hip fracture-related admissions (£10,375, median £8050). The distribution of hospitalisation costs was skewed (Figure 25) with a small proportion of cases staying in hospital for a whole year resulting in very high costs (36 patients had hospitalisation costs above £100,000). Hospitalisation costs and length of stay were highly correlated (Spearman’s correlation coefficient: 0.82; p < 0.001).
FIGURE 25.
Distribution of hospitalisation costs in the year after primary hip fracture.

Figure 26 reports the hospitalisation costs in the months before and after primary hip fracture. The annual cost in the year of the fracture was estimated to be £10,860 (95% CI £10,710 to £11,011) higher than the previous year. The highest costs occur in the first 6 months post hip fracture dropping sharply afterwards to pre-fracture levels of expenditure and remaining fairly constant throughout the second year post fracture. The 2-year survivors show a similar pattern of costs relative to all patients. However, while the costs in the second year after hip fracture remain numerically higher than in the year pre-fracture (£112, 95% CI –£29 to £274) this was not statistically significant.
FIGURE 26.
Hospitalisation costs in the months before and after primary hip fracture. a, Complete cases, including those who died in that year.Reproduced with permission from Leal J, Gray AM, Prieto-Alhambra D, Arden NK, Cooper C, Javaid MK, et al. Impact of hip fracture on hospital care costs: a population based study. Osteoporos Int 2016;27:549–58, under a Creative Commons licence (https://creativecommons.org/licenses/by-nc/4.0/legalcode).
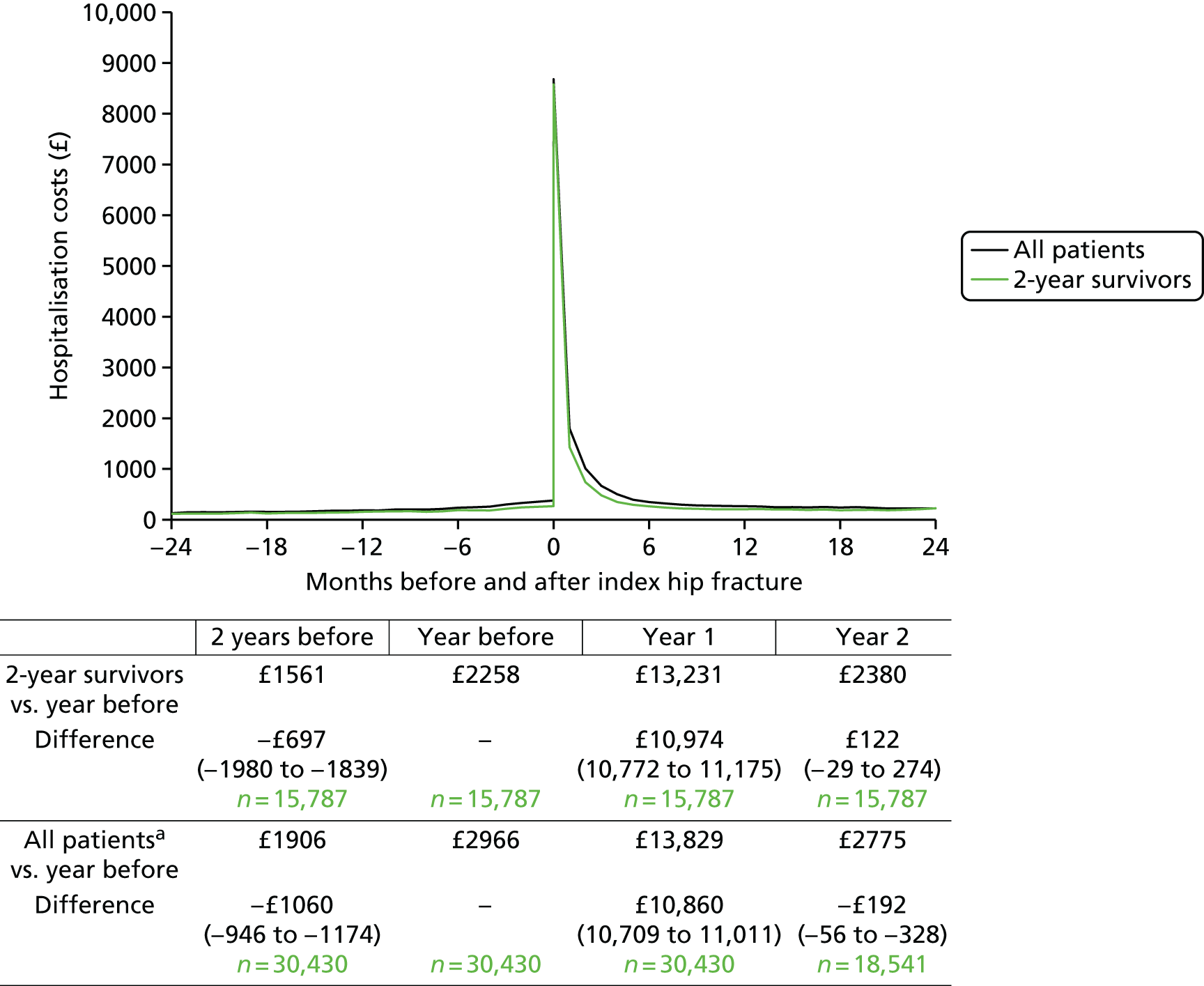
Patient outcomes and primary care costs (Clinical Practice Research Datalink data set)
The average follow up of the cohort was 2.5 (median 1.9, SD 2.3) years from index hip fracture, during which time 7.2% of patients suffered a second hip fracture (Table 21). Mortality at 30 days and 1 year was estimated to be 5.7% and 26.3%, respectively; these rates were lower than the mortality rates found in the hospital care data set. After index fracture, the majority of patients were recorded as being discharged to their own home (55%), or transferred to another hospital (19%) or to a care home (19%). The number of patients in a care home increased to 23% after 1 year of complete follow-up. GP contacts in the year of the hip fracture amounted to 8.3 (median 6, SD 9.3) per person, of which 77% were clinic or surgery appointments and the rest were telephone contacts and out-of-office visits. In the same year, 67 (median 43, SD 86.6) medications were prescribed per patient including non-medication items (e.g. wound management bandages). Excluding non-pharmaceutical items, the most common type of medications were for the cardiovascular system (BNF chapter 2), accounting for 33% of all medications, and the central nervous system (BNF chapter 4), accounting for 19%, followed by gastrointestinal (BNF chapter 1) and nutrition and blood systems (BNF chapter 9) accounting for 12% each, and endocrine system (BNF chapter 6) accounting for 10%. 90
| Patient outcomes | n (%) |
|---|---|
| Follow-up time in years, mean (SD) | 2.5 (2.3) |
| Second hip fracture | 320 (7.2) |
| Time to second hip fracture in years, mean (SD) | 2.0 (1.9) |
| Mortality | |
| Within 30 days | 252 (5.7) |
| Within 1 year | 1028 (26.3) |
| Discharge destination following index fracture admission | |
| Own home | 2436 (55.0) |
| Care home or temporary accommodation | 855 (19.3) |
| NHS hospital | 835 (18.8) |
| Unknown | 41 (0.9) |
| Dead | 266 (6.0) |
| Care home within 1 year of index fracture | 706 (22.5) |
| Primary care contacts within 1 year of fracture, mean (SD) | |
| GP consultation at clinic/surgery | 6.5 (7.2) |
| GP consultation out of office | 0.8 (2.0) |
| GP telephone contact | 1.1 (2.5) |
| Nurse contacts (surgery/out of office/telephone) | 0.2 (0.8) |
| Other health-care professionals contacts (surgery/out of office/telephone) | 0.6 (2.1) |
| Medications and non-pharmaceuticals prescribed | 66.8 (86.6) |
| Primary costs within 1 year of fracturea (£), mean (SD) | |
| GP contacts | 358 (409) |
| Contacts with nurse and other health-care professionals | 76 (365) |
| Medications and non-pharmaceuticals | 614 (1586) |
| Tests taken | 111 (144) |
| Total costs | 1065 (1798) |
The primary care costs associated with primary hip fracture were £1065 (median £660, SD £1798), of which medications and non-pharmaceuticals accounted for £614 (median £248, SD £1586) of the costs and GP contacts accounted for £358 (median £246, SD £409).
Figure 27 reports the primary care costs in the months before and after primary hip fracture. Similar to hospital care, there is an increase in costs before hip fracture, with the highest costs occurring in the first 6 months post hip fracture. Nonetheless, although the costs for all patients in the first year after hip fracture were numerically lower than the year pre fracture (£26, 95% CI –£102 to £50), this was not statistically significant. However, when we considered only the 2-year survivors, we found the costs in the first and second year post hip fracture to be significantly higher than the costs in the year prior to hip fracture. Compared with the year prior to the hip fracture, primary care costs, in patients who were alive 2 years post hip fracture, were £256 (95% CI £160 to £352) and £273 (95% CI £167 to £380) higher in the first and second year following hip fracture, respectively. This was mostly led by a significant increase in GP contacts and in the costs of medications and non-pharmaceuticals.
FIGURE 27.
Primary care costs in the months before and after primary hip fracture. Two-year survivors concerns patients who did not die within 2 years post hip fracture and follow-up data were available for this period. a, Complete cases, including those who died in that year.
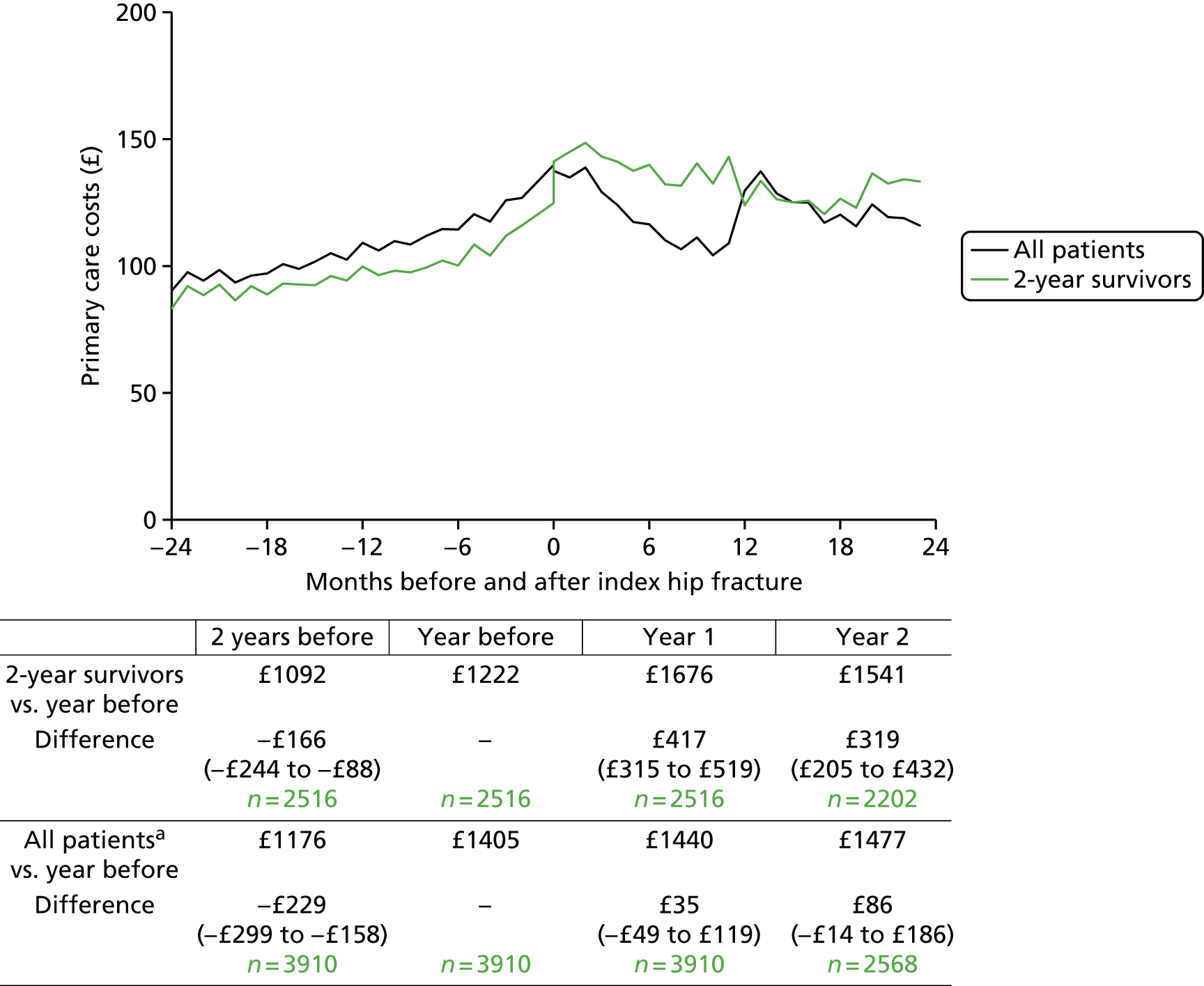
Total hospital costs before and after index fracture
Patients who had a primary hip fracture after 1 April 2008 and complete 1-year follow-up (44%, n = 14,552) were used to compare hospital resource use and total costs in the years before and after the fracture (Table 22). In the year of the hip fracture, patients had 1.03 additional hospital admissions (i.e. inpatient stay, day cases and regular day/night attenders) (p < 0.001), 27.9 additional hospital inpatient days (p < 0.001), 0.54 additional A&E contacts (p < 0.001) and 0.01 additional outpatient visits (p < 0.001), compared with the previous year.
| Resource use, annual | Sample | Total | A&E | Outpatient care | Inpatient care | Length of stay |
|---|---|---|---|---|---|---|
| Resource use | ||||||
| Before index fracture | 14,552 | – | 0.71 | 3.10 | 1.20 | 8.29 |
| After, complete casesa | 14,552 | – | 1.26 | 3.13 | 2.23 | 36.14 |
| Difference | – | 0.54** | 0.03** | 1.03** | 27.85** | |
| Costs | ||||||
| Before index fracture | 14,552 | £3299 | £105 | £295 | £2899 | – |
| After, complete casesa | 14,552 | £14,264 | £202 | £297 | £13,765 | – |
| Difference (95% CI) | £10,964** (£10,767 to £11,161) | £96** (£93 to £101) | £2 (–£9 to £12) | £10,865** (£10,671 to £11,060) | – | |
| After, censor-adjusted (95% CI) | 17,274 | £14,163 (£14,008 to £14,317) | £207 (£204 to £210) | £303 (£293 to £313) | £13,653 (£13,501 to £13,812) | – |
Including both outpatient and emergency contacts, the total costs were estimated at £14,264 (95% CI £14,092 to £14,436) in the year of the fracture, of which 96% (£13,635) was because of inpatient stay and critical care. Unadjusted for other covariates, men had significantly higher total hospital costs than women (£1188; p < 0.001). Having a hip fracture resulted in additional costs of £10,964 (95% CI £10,767 to £11,161), compared with the year prior to hip fracture. Adjusting for censoring, the 1-year costs were similar to the complete-case analysis at £14,163 (95% CI £14,008 to £14,317). The costs in the first 2 years following hip fracture (2 years) adjusted for censoring were £16,302 (95% CI £16,097 to £16,515), compared with £16,270 using only the complete cases (n = 12,155).
Primary care and hospital care costs 1 year and 2 years post index fracture
The primary care and hospital care costs in the first year and second year (conditional on surviving the first year) post hip fracture per sex and age group are reported in Table 23. The total primary care and hospital care costs in the year of the hip fracture were £15,329, of which £14,264 was a result of hospital care and £1065 was a result of primary care. Conditional on surviving the first year, the total primary care and hospital care costs in the second year after the hip fracture were £4242, of which £3072 was a result of hospital care and £1170 was a result of primary care.
| Resource use, annual | Primary care costs in year 1 (SD) | Hospital costs in year 1 (SD) | Primary care costs in year 2 (SD) | Hospital costs in year 2 (SD) |
|---|---|---|---|---|
| Men | ||||
| All ages | £1051 (£1708) | £15,131** (£11,349) | £1252 (£1879) | £3614** (£7302) |
| Age 60–69 years | £882* (£954) | £14,659** (£13,623) | £821 (£1322) | £3042 (£7022) |
| Age 70–79 years | £1161 (£2085) | £15,278** (£12,628) | £1279 (£1913) | £3471 (£7248) |
| Age 80–89 years | £1085 (£1765) | £15,485** (£10,637) | £1420* (£2196) | £4058 (£7738) |
| Age ≥ 90 years | £874 (£1087) | £14,210 (£9590) | £1037 (£1089) | £2848 (£5575) |
| Women | ||||
| All ages | £1069 (£1823) | £13,943 (£10,311) | £1149 (£2111) | £2908 (£6905) |
| Age 60–69 years | £1338 (£1730) | £12,212 (£11,118) | £1322 (£2062) | £2906 (£8135) |
| Age 70–79 years | £1278 (£2197) | £13,830 (£10,981) | £1353 (£2679) | £3032 (£6488) |
| Age 80–89 years | £1065 (£1777) | £14,430 (£10,294) | £1101 (£2008) | £2993 (£7325) |
| Age ≥ 90 years | £830 (£1518) | £13,538 (£9389) | £950 (£1405) | £2519 (£5478) |
Annual costs of hip fractures in the UK
The total annual costs associated with all incident hip fractures (in the year of hip fracture) in the UK among those aged ≥ 50 years (n = 79,243) were estimated at £1215M (Table 24).
| Primary and hospital care costs | Total |
|---|---|
| Annual incident hip fractures in the UK | 79,243 |
| Annual primary care costs, absolute | £1065 |
| Annual inpatient care costs, absolute | £13,765 |
| Annual A&E costs, absolute | £202 |
| Annual outpatient care costs, absolute | £297 |
| Annual cost per incident hip fracture, absolute | £15,329 |
| Total cost, absolute (£M) | £1215 |
Predictors of primary care costs in first year following hip fracture
The predictors of hospitalisation costs are shown in Table 25. A GLM with gamma family and identify link function had the best fit. Adjusting for all covariates, there were no statistically significant differences in primary care costs between the types of hip fractures. Costs were inversely associated with age (–£15 per additional year) and positively associated with multiple deprivation, with costs decreasing with more deprived patients. A higher Charlson Comorbidity Index score at index hip fracture increased primary costs by approximately £131 per additional unit of the Charlson Comorbidity Index score.
| Variables | Mean | 95% CI | p-value |
|---|---|---|---|
| Type of hip fracture | |||
| Head and neck | Reference | ||
| Pertrochanteric | –£53 | –£155 to £49 | 0.308 |
| Subtrochanteric | £329 | –£111 to £769 | 0.142 |
| Unspecified | –£143 | –£551 to £266 | 0.493 |
| Age at hip fracture (centred on 82 years) | –£15 | –£21 to –£8 | < 0.001 |
| Year at hip fracture (centred on year 2008) | £54 | £33 to £74 | < 0.001 |
| Indices of multiple deprivation | |||
| Least deprived (1) | Reference | ||
| 2 | –£81 | –£221 to £59 | 0.260 |
| 3 | –£121 | –£263 to –£19 | 0.091 |
| 4 | £6 | –£148 to £161 | 0.937 |
| Most deprived (5) | –£165 | –£316 to –£14 | 0.032 |
| CCI score | £131 | £86 to £175 | < 0.001 |
| Constant | £1065 | £941 to £1188 | < 0.001 |
| Number of observations | 3899 | ||
| Residual degrees of freedom | 3888 | ||
| Link test (p-value for y-hat square) | 0.215 | ||
Predictors of hospitalisation costs in first year following hip fracture
The predictors of hospitalisation costs are shown in Table 26. A GLM with gamma family and identify link function had the best fit. Adjusting for all covariates, men had higher hospitalisation costs than women (£910, 95% CI £679 to £1141) and higher length of stay (4.5 days). Furthermore, costs were positively associated with age (£45 per additional year) and inversely associated with income, with costs rising with income deprivation. A higher Charlson Comorbidity Index score at index hip fracture increased hospitalisation costs by approximately £694 per additional unit of the Charlson score. Transiting to a care home for the first time after index fracture was associated with higher hospital costs (£5583, 95% CI £5197 to £5970) and a longer length of stay (22 days) relative to patients who went back to their previous accommodation, possibly indicating poorer health.
| Mean | 95% CI | p-value | |
|---|---|---|---|
| Type of hip fracture | |||
| Head and neck | Reference | ||
| Pertrochanteric | –£266 | –£505 to –£28 | 0.029 |
| Subtrochanteric | £491 | –£137 to £1119 | 0.125 |
| Unspecified | £610 | –£432 to £1653 | 0.251 |
| Gender | |||
| Female | Reference | ||
| Male | £910 | £679 to £1141 | < 0.001 |
| Age at hip fracture (centred on 82 years) | £47 | £35 to £60 | < 0.001 |
| Year at hip fracture (centred on year 2008) | –£99 | –£135 to –£63 | < 0.001 |
| Indices of income deprivation (× 100) | £31 | £18 to £45 | < 0.001 |
| CCI score (up to 3 years prior to fracture) | £695 | £622 to £768 | < 0.001 |
| Death within 30 days of hip fracture | –£4672 | –£4906 to –£4437 | < 0.001 |
| Death between 31 days and 1 year | £2549 | £2246 to £2853 | < 0.001 |
| Living in care home before hip fracture | –£595 | –£896 to –£294 | < 0.001 |
| Moving to care home after hip fracture (new) | £5583 | £5197 to £5970 | < 0.001 |
| Second hip fracture | £9198 | £8059 to £10,337 | < 0.001 |
| Major fragility fracture requiring hospitalisation post hip fracture | £5705 | £4434 to £6975 | < 0.001 |
| Surgical complications within index admissiona | £5694 | £4849 to £6538 | < 0.001 |
| Surgical complications after discharge from index admissiona | £10,552 | £10,352 to £10,753 | < 0.001 |
| Malunion and non-union of fractureb | £4613 | £2396 to £6830 | < 0.001 |
| Periprosthetic fracturec | £9569 | £6302 to £12,835 | < 0.001 |
| Hip luxationsd | £14,266 | £7630 to £20,902 | < 0.001 |
| Sequelae of fractures of the femure | £8463 | £3545 to £13,381 | < 0.001 |
| Constant | £10,547 | £10,346 to £10,748 | < 0.001 |
| Number of observations | 30,282 | ||
| Residual degrees of freedom | 30,261 | ||
| Link test (p-value for y-hat square) | 0.41 | ||
| AIC | 20.98 | ||
Holding all else constant, having a second hip fracture within the same year as the primary one was associated with an additional £9198 (95% CI £8059 to £10,337) of expenditure in hospitalisation costs. Major (non-hip) fragility fractures requiring hospitalisation post hip fracture were also found to be significantly associated with higher hospitalisation costs (£5705, 95% CI £4434 to £6975). Among hip fracture-related complications, surgical complications within and after index admission were the most frequently reported and were associated with higher costs and length of stay (22 and 25 days, respectively) relative to no complications. Periprosthetic fracture was also associated with significantly higher hospitalisation costs relative to patients without this, at £9569 (95% CI £6302 to £12,835). All of these cost differences remained significant after adjusting for length of stay.
Finally, patients who died within 30 days were associated with lower hospitalisation costs (–£4672, 95% CI –£4906 to –£4437), mostly as a result of lower length of stay, than those who survived the first 30 days (mean of 11.3 days vs. 41.9 days). Patients who died after 30 days had higher costs (£2549, 95% CI £2246 to £2853) than the survivors, again mostly because of a longer length of stay (mean of 53.2 days vs. 38.3 days).
Conclusion
This chapter presents the immediate and medium-term (up to 2 years) primary care and hospital care costs of hip fracture in a large representative sample of patients in the UK. We also adjusted the costs for health-care resource use prior to the index fracture, explored the main variables influencing the costs and estimated the costs of all incident hip fractures in the UK. Furthermore, we found that hip fracture survivors are associated with higher primary care costs than in the years before hip fracture and that second hip fracture within the first year of index fracture is a major driver of additional hospitalisation costs.
Although previous studies have reported costs of hip fracture in the UK, few were based on patient-level data or on recent populations. Moreover, previous cost estimates have been mostly informed by studies using small sample sizes (between 10149 and 2427 patients137), and a variety of time horizons from time to initial discharge following acute admission138–140,150 up to 12 months post admission. 137,149 When the results of these previous studies were inflated to 2012–13 prices, we found considerable variability in the reported 1-year costs after hip fracture, with estimates between £6176137 and £20,470. 137,149 However, these studies focused solely on women with hip fractures. Hence our study contributes significantly to the evidence base regarding hip fractures by identifying and following large populations of hip fracture patients in primary and hospital care up to 2 years before and after the index event.
The major component of costs in the first year following hip fracture was hospital resource use with primary costs accounting for only 7% (£1065) of the total costs (£15,329). However, in the second year post hip fracture, the proportion of total costs (£4242) resulting from primary care use increased to 28% (£1170) among those who survived the first year. Furthermore, we found primary care costs to increase significantly after hip fracture among 2-year survivors, making these an important component of hip fracture costs.
In contrast, we found hospital costs to be high in the first 6 months after hip fracture, falling thereafter to levels of expenditure similar to the year before fracture. The same cost profile was observed in patients with a second hip fracture; however, initial admission costs were higher in the second fracture than in the first. Acute hospitalisation costs due to index fracture accounted for 61% (£8663) of total 1-year costs and these costs were similar between primary and secondary hip fractures, representing about 20 days of inpatient stay. Hospital costs in the year following hip fracture were estimated to be £14,264, representing 36 days of inpatient stay, with the majority of costs being associated with hip fracture-related hospitalisations (75%). Furthermore, the 2-year costs at £16,289 show that the majority of costs (88%, £14,269) occur in the year after the index hip fracture.
Comparing primary and hospital care costs before and after hip fracture showed these to gradually increase in the last 6 months prior to fracture, suggesting a worsening in health that may be associated with the risk of fracture. Such a pattern in costs is consistent with that which has been reported in diseases such as stroke. 151 In addition, men experienced higher hospital costs following a hip fracture than women, even after adjusting for several covariates. This is of concern, as men are more likely than women to be underdiagnosed and have lower treatment uptake rates for osteoporosis before and after a fragility fracture. 36 There was no significant difference in primary care costs in the year of the hip fracture between men and women.
Using the annual number of incident hip fracture cases, we were able to extrapolate our findings to the UK as a whole and estimate the annual impact of all incident hip fractures on primary care and hospital services in the year of the event. This was estimated to be £1215M per year and, if incidence is to rise by 32% in 2025,135 the costs will increase to £1604M per year. Furthermore, given the high marginal annual costs of hip fracture per patient (£10,964), there is a considerable economic incentive to fund research aimed at identifying cost-effective ways of improving the uptake of osteoporosis therapies and the implementation of embedded care pathways across healthcare services to effectively reduce avoidable fractures. Such data would aid decision-makers to implement policy decisions at a local and a national level. Another major consequence of fragility fractures are the consequent increases in non-health-care costs, resulting from the use of social care services, admission to care homes and provision of unpaid care by friends and relatives. 152 Further research is needed to determine these costs for both hip and non-hip fragility fractures.
The main drivers of first-year hospital costs were found to be mostly events related with hip fracture. Having a second hip fracture in the same year as the index fracture was associated with higher costs (£9198). However, hip fracture-related complications or subsequent fractures were not found to be significantly associated with primary care costs. Another interesting finding with implications for economic evaluations concerning hip fracture management concerns the costs associated with mortality following hip fracture. Interventions that can improve survival may result in cost savings or reduce future consumption of hospital resources.
In addition to the above, we recognise the following limitations. The hospital data were confined to a single region in the UK. However, this region has a representative rate of hip fracture cases with similar sex and age distribution to that of the rest of the UK. 17 Furthermore, the length of stay of index admission of a hip fracture is similar to that reported across the UK (19 vs. 20 days in 2012)17 and by capturing actual NHS activity our results are strongly generalisable. Although data sets such as HES capture key variables influencing costs of hospital stay such as diagnosis and procedures during episodes of hospitalisation, they are not comprehensive in recording other morbidity and severity measures. Nonetheless, the validity of the HES data set in identifying hip fracture cases has been shown to be very high. 17 Furthermore, the ascertainment of diagnosis and comorbidities occurs mostly in patients with admitted patient care (inpatient stay or day cases), and a better understanding is required of the reasons for outpatient and emergency contacts. In addition, it would have been useful to have linked hospital data with social care records and estimate the costs beyond the health-care setting. This is, however, very limited with the current administrative data sets in the UK. Hence, despite these limitations, the quality of the primary and hospital care data sets and the large sample sizes allowed us to make robust estimates of the health-care costs of hip fracture and the impact of patient characteristics such as age, sex and deprivation and hip fracture-related complications.
In conclusion, we report the impact of hip fracture on health-care costs and its predictors in the UK. Our findings highlight the impact of hip fracture and the importance of preventing hip refractures.
Chapter 8 Cost-effectiveness analysis of models of care for secondary prevention of hip fracture
Introduction
In this chapter, we report the cost-effectiveness analysis of the delivery of secondary fracture prevention following index hip fracture in NHS hospitals. No firm evidence or evidence-based consensus exist as to which type of secondary fracture prevention services for hip fracture would be optimal. Hence the aim is to determine whether or not introducing an orthogeriatrician or a fracture liaison nurse, as part of the delivery of secondary fracture prevention services, is cost-effective in the English NHS, compared with standard care. The availability of large primary and secondary care administrative data sets allows robust estimation of the impact of the different models of care in terms of morbidity, survival, costs and cost-effectiveness.
Aims
Hip fractures are a major public health problem in terms of morbidity, mortality and health-care and social care costs. Hip fractures account for the majority of osteoporotic fragility fractures and for > 40% of the estimated burden of osteoporosis worldwide. 134 However, half of all hip fracture patients have had a prior fracture and responding to the first fracture is essential to prevent the second. In 2007, guidance on secondary prevention of fragility fractures recommended a comprehensive service consisting of osteoporosis assessment including a DXA scan to measure bone density, if appropriate, treatment with bone protection therapy in osteoporosis patients, falls risk assessment and systems to improve adherence and persistence with therapy. 1 The 2011 NICE guideline recommended a formal multidisciplinary management of the patients with hip fracture including liaison or integration with services such as primary care, bone health, falls prevention and social services. 29 The Department of Health recommends a FLS to organise the secondary fracture prevention services to be delivered by a nurse specialist supported by a lead clinician in osteoporosis. However, despite these recommendations, no firm evidence or evidence-based consensus exist as to which model of secondary prevention care should be mandated across the NHS. As a result, current practice reflects a significant variation across NHS hospital providers in terms of the adoption of FLS and their structure. Therefore, the aim of this work is to determine whether or not introducing an orthogeriatrician or a fracture liaison nurse for post-hip fracture care in hospital is cost-effective when compared with usual care in the English NHS.
Existing research
Two recently published systematic review assessed the economic evidence concerning the prevention of osteoporotic fractures. 36 These reviews identified three studies assessing the cost-effectiveness of models of care for the prevention of fractures: one in the UK,20 one in Canada97 and one in Australia. 21 The three cost-effectiveness studies used decision models (Markov models) to examine the impact of secondary fracture prevention interventions such as the presence of a hospital osteoporosis case manager and FLSs. The main outcome measure used across the three studies was QALYs and the time horizon of the analysis varied from 10 years to lifetime.
The Canadian study by Majumdar et al. 97 focused specifically on a hip fracture population and examined the impact of a hospital-based osteoporosis case manager in improving the rates of osteoporosis treatment, compared with usual care. The role of the case manager was to educate patients, arrange bone mineral density tests, provide prescriptions and communicate with primary care doctors. The time horizon of the analysis was lifetime and the authors report the intervention to be both cost saving and more effective than usual care. However, the model was based on published literature and a small clinical trial of 220 patients followed up for 1 year. Furthermore, the clinical trial did not evaluate the impact of the intervention in terms of refracture rates, quality of life or life expectancy. Rather, the trial evaluated, as main outcomes, the receipt of bisphosphonate therapy, bone mineral density testing and appropriate care. Hence assumptions and published literature were required to link the trial outcomes with the main outcomes of the cost-effectiveness analysis, namely QALYs. Furthermore, the clinical trial excluded patients with hip fracture admitted from a care home, who are likely to represent a significant proportion of the hip fracture population.
The Australian study by Cooper et al. 21 compared an outpatient-based FLS for patients presenting with a fragility fracture with patients treated in primary care (standard care). The FLS involved additional input from a specialist clinic and resulted in increased usage of bone scans, laboratory investigations and medications compared with primary care. The time horizon of the analysis was 10 years. The authors reported the FLS to be highly cost-effective with an incremental cost-effectiveness ratio (ICER) below AU$20,000 per QALY gained compared with standard care (primary care). The model was based on a 4-year prospective observational study of patients with fragility fracture which included a concurrent control group treated in primary care. The observational study informed the clinical effectiveness and the natural history inputs of the decision model. However, the authors were not able to identify the components of the FLS that accounted for its clinical effectiveness. Furthermore, no results were provided for the subgroup of hip fracture patients and the authors did not extrapolate the findings to lifetime. Another limitation concerns the cost component of the analysis which was informed by a variety of sources such as a burden of illness study, assumptions and the prospective observational study. In addition, health-care costs used in the model included only the treatment of fractures, and excluded other health-care usage resulting from having a fracture.
The UK study by McLellan et al. 20 compared a FLS for the prevention of further fractures with care in the absence of FLS. The FLS consisted of having an osteoporosis nurse identifying low-trauma fracture patients in hospital, educating and inviting them to the fracture risk-assessment clinic where a treatment recommendation was made, if appropriate, on the basis of assessment of future potential fracture risk. The treatment recommendation was endorsed by the lead consultant and sent to the patient’s GP for the initiation of treatment in primary care. The time horizon of the analysis was lifetime. The authors reported the FLS to be more effective and less costly than no FLS. However, there are important limitations to this study. As in the Australian study, no results were provided for a population solely composed by hip fracture patients. Furthermore, although the study was informed by 8 years of audit data collected by the West Glasgow FLS, there was no comparable control group. Hence the authors had to rely on published literature and assumptions to model the impact of FLS on fractures relative to its absence. The remaining model inputs were also derived from a range of sources and fracture populations, and various assumptions were required to synthesise the data.
Existent cost-effectiveness evidence is limited but seems to suggest that secondary prevention services for fractures can be cost-effective and even cost saving. Nonetheless, the most cost-effective approach to provide secondary prevention service following hip fractures remains unclear. There is, therefore, significant scope to bridge this gap by using large primary and secondary care administrative data sets supported by a systematic and detailed characterisation of the services provided across NHS providers. Such a framework should allow the impact of the different components of a FLS to be compared in a robust and precise manner.
Methods
Interventions under study
We estimated the cost-effectiveness of three models of secondary fracture prevention for all patients with a hip fracture admitted to a NHS hospital:
-
introduction of an orthogeriatrician model of post-hip fracture care
-
introduction of a fracture liaison nurse model of post-hip fracture care
-
usual post-hip fracture care (no orthogeriatrician or fracture liaison nurse).
In addition, the cost-effectiveness of these models of care was assessed in patient subgroups in terms of age at hip fracture (60, 70, 80 and 90 years), sex (male or female) and Charlson Comorbidity Index before hip fracture.
Model structure
A decision-analytic model was developed to evaluate the costs, (quality-adjusted) life expectancy and cost-effectiveness of the different models of secondary care hip fracture prevention under evaluation. Given the natural history of hip fracture progression with recursive events, the most appropriate type of model was judged to be a Markov model which was developed in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). The model was used to simulate the natural history of the hip fracture population across health states representing the history of hip fracture and major non-hip fractures and hospital discharge to the patient’s own home or to a care home. Hence the following health states were defined (Figures 28 and 29):
-
death within 30 days following first or second hip fracture admission
-
second hip fracture
-
other major fragility non-hip fractures requiring hospitalisation
-
history of primary hip fracture
-
history of major fragility non-hip fractures requiring hospitalisation
-
history of second hip fracture
-
history of second hip fracture and major fragility non-hip fractures
-
death within year.
FIGURE 28.
Model structure and health states in the first year of simulation. (a) Discharge to care home; and (b) discharge to own home. Reproduced with permission from Leal J, Gray AM, Hawley S, Prieto-Alhambra D, Delmestri A, Arden NK, et al. Cost-effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: a population based study. J Bone Miner Res 2016; in press.
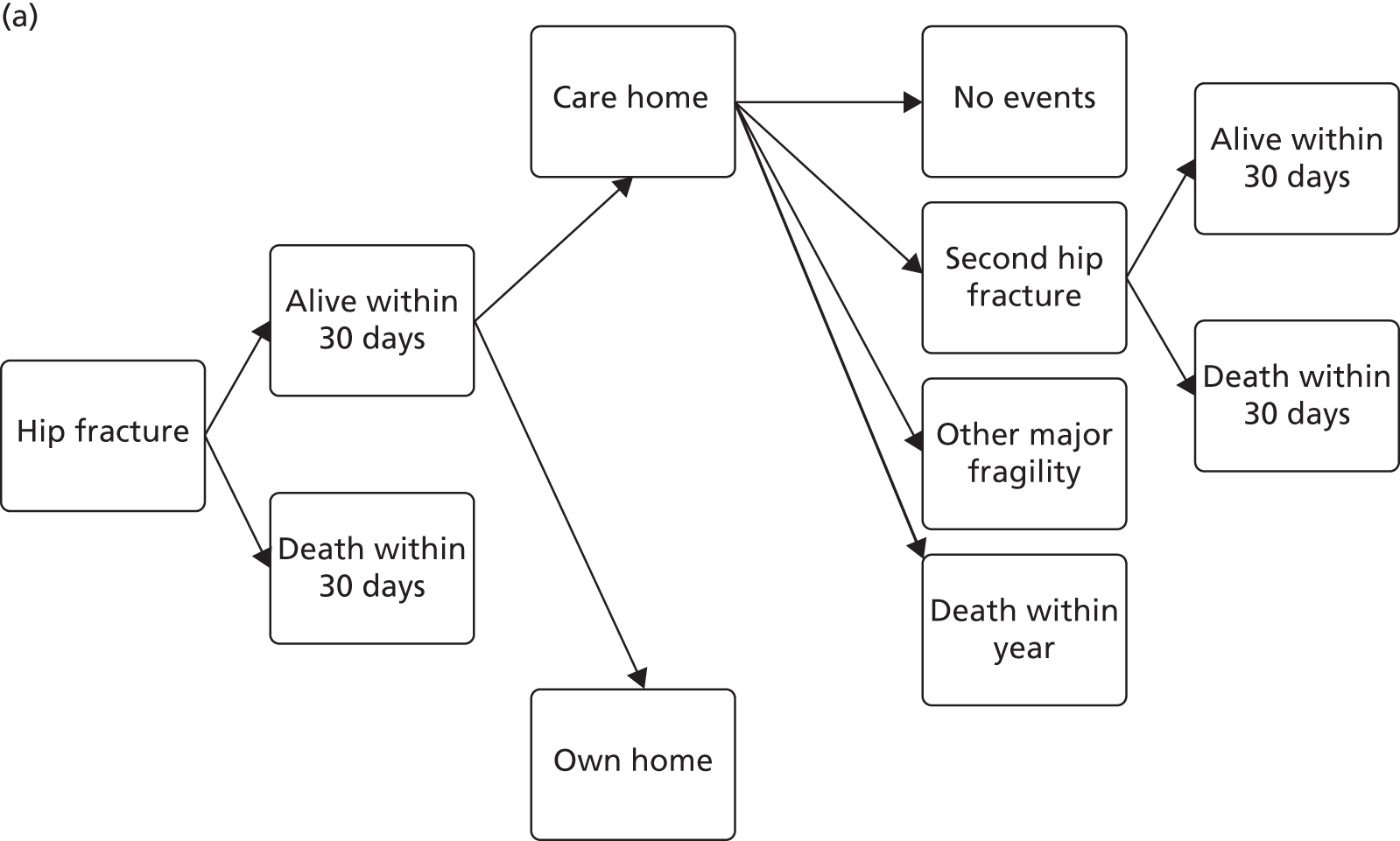
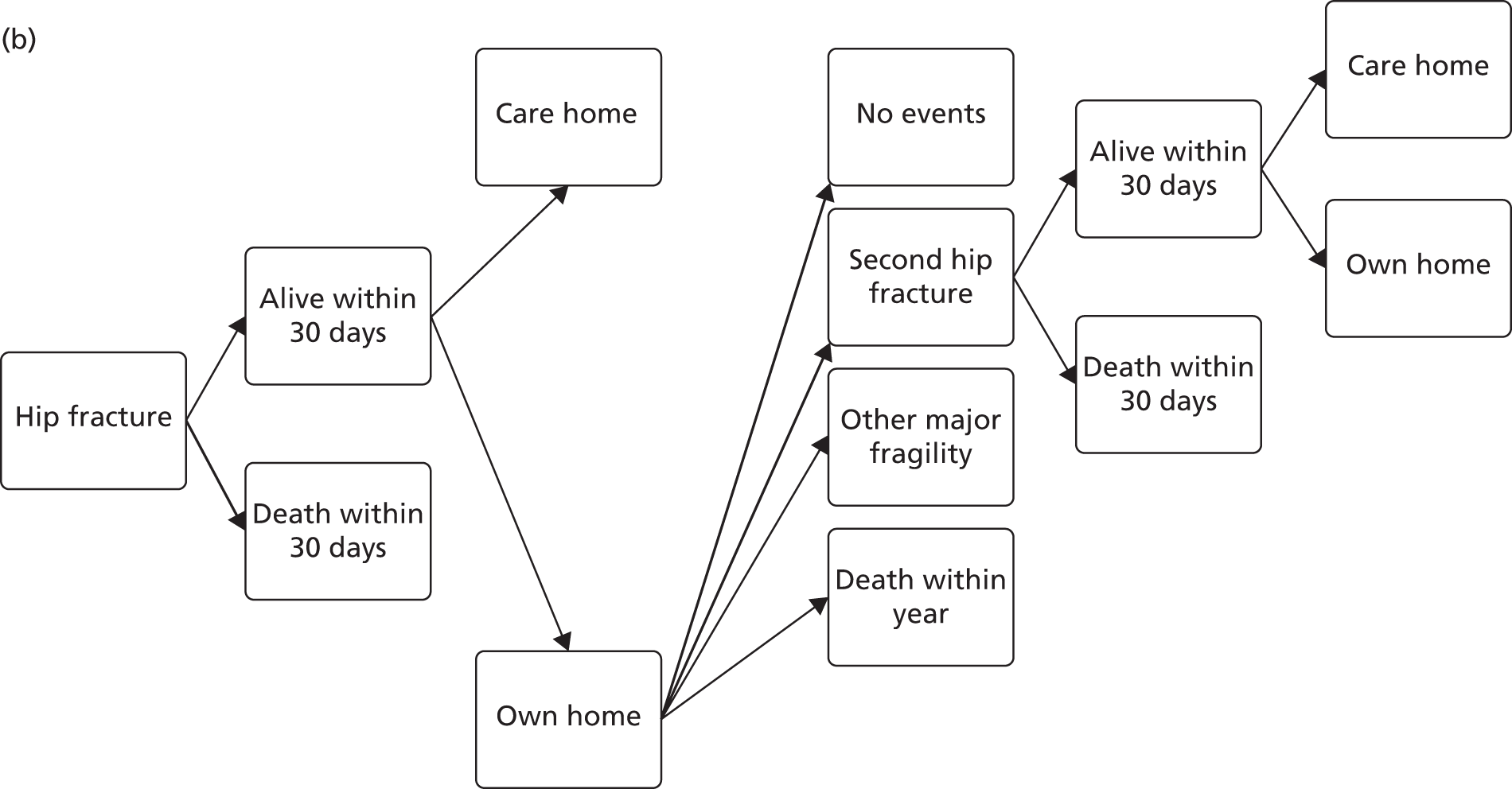
FIGURE 29.
Model structure and health states in the years following the first hip fracture. (a) Discharge to care home; and (b) discharge to own home.
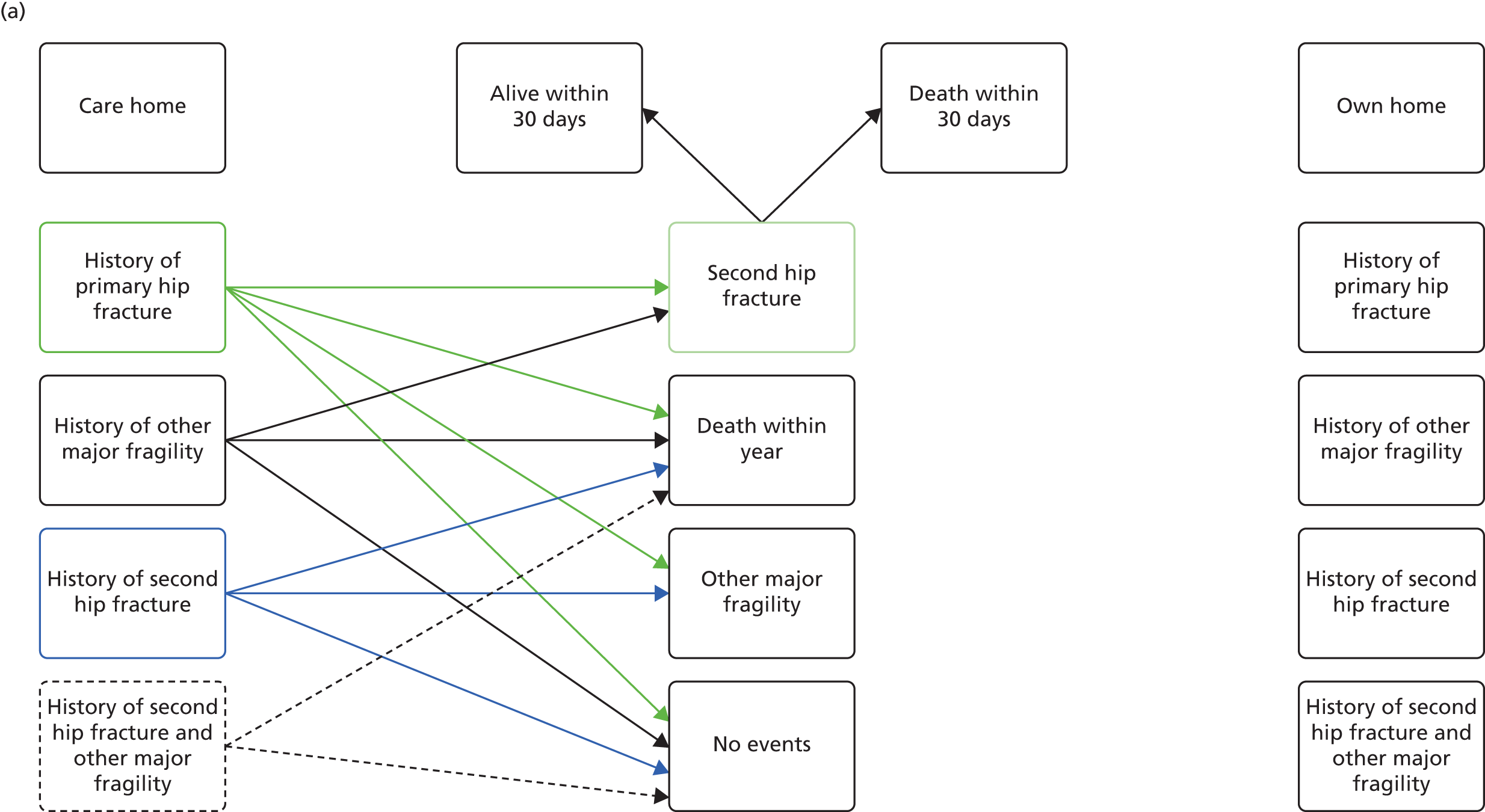
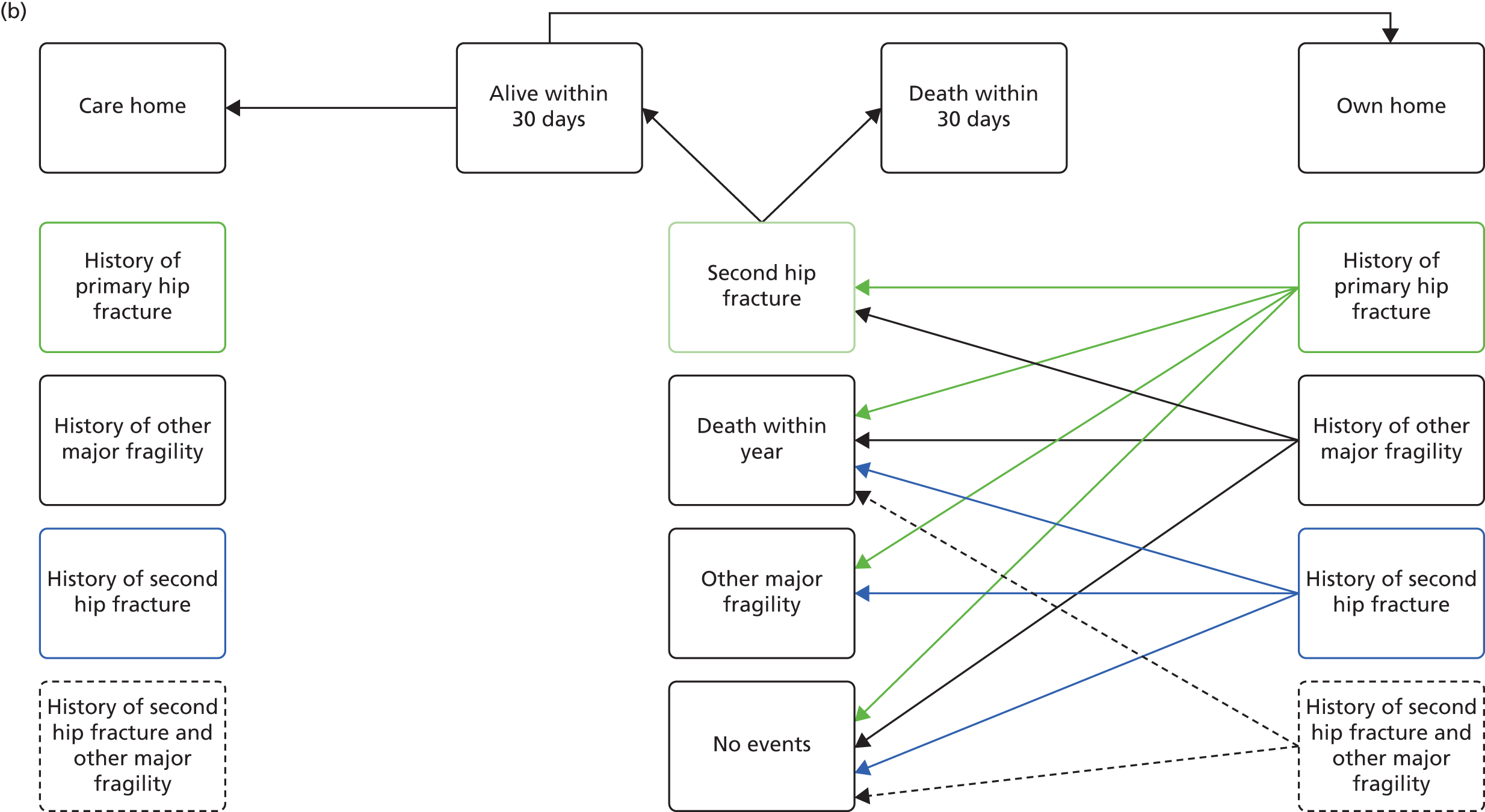
The model had three absorbing states: history of second hip fracture and major fragility non-hip fractures (progressing only to death after reaching this state), death within 30 days following hip fracture and death within year. We assumed that if a patient transited to a care home they would remain there for the rest of their lifetime.
The model structure and assumptions were informed by the hip fracture, the needs of the decision problem and discussions with clinical experts, health economists, statisticians and epidemiologists involved in the project. This meant that an iterative process was used to define the model structure when the agreed conceptual framework was revisited given the results of the data analysis and new findings in the published literature.
The time horizon of the analysis was lifetime and the population moved between health states according to defined transition probabilities. A cycle length of 1 year was considered appropriate given the natural history of hip fracture patients. Half-cycle correction was performed using the lifetable approach. 153
The model simulated the transition of a cohort of patients with an index hip fracture through the health states over time, to estimate expected costs and outcomes (see Figures 28 and 29). For example, at the start of the simulation, patients with a hip fracture could die within 30 days or be discharged home or to a care home (nursing or residential care home). In the same cycle, patients could then develop a second hip fracture, other major fragility fracture requiring hospitalisation (non-hip such as pelvic, spine, wrist, humerus and rib), have no further events or die (see Figure 28). If patients experienced a second hip fracture, they could die within 30 days or, if alive, be discharged to a care home or their own home. In the next cycles, patients were allowed to continue progressing if they had not yet reached an absorbing state (see Figure 29).
Finally, the model adopted the perspective of the NHS and Personal Social Services. All costs and effects were discounted beyond the first year of simulation using an annual discount rate of 3.5%, based on current UK government recommendations. The price year was 2012–13 and, when necessary, costs were adjusted to that year using the UK health sector pay and prices inflation factor.
Data sources
The data sources used to inform the model inputs consisted of HES database, CPRD GOLD and published literature.
Hospital Episode Statistics data set
The HES database contained all hospital NHS patient care, in the form of ‘finished consultant episodes’, and linked mortality data from the ONS on 33,152 patients with hip fracture who attended one of the 11 hospitals in a region of England between 1 April 2003 and 31 March 2013. Chapters 5 and 7 describe the methodology underlying the construction of the HES data set. Briefly, we identified HES records for all patients > 60 years of age who had had an emergency hospital admission with a primary ICD-10 diagnosis code for hip fracture (S72.0–S72.2, S72.9) between April 2003 and March 2013. Patients were excluded if they were day cases, had a previous history of hip fracture or had the hip fracture as a result of a trauma accident. The resulting HES data set was used to estimate the following model inputs:
-
time to second hip fracture
-
time to major non-hip fragility fracture
-
probability of death within 30 days of admission due to hip fracture
-
probability of being discharged to a care home after hip fracture
-
time to death
-
effectiveness of orthogeriatrician and fracture liaison nurse relative to usual care
-
hospital costs in the year of hip fracture and subsequent years.
Clinical Practice Research Datalink data set
The CPRD GOLD database covered primary care data from 4433 patients with hip fracture, between 1 April 2003 and 31 March 2012, registered with CPRD GOLD practices and linked with HES and ONS data sets. These patients represent 62% of all hip fracture patients identified using the Read codes (see Chapter 6) within the participating GP practices contributing data to the CPRD within England and Wales (n = 7155), between April 2003 and March 2012. Chapter 7 describes the methodology in detail. Briefly, we identified hip fracture patients in the CPRD GOLD data set using the same criteria as for the HES data set. About 60% of the primary care practices contributing to CPRD have agreed to linkage to the HES and ONS data. The HES records for which linking to CPRD was possible consisted to admitted patient care data from April 1999 to March 2012. The resulting CPRD GOLD data set was used to estimate the primary care costs in the year of hip fracture and subsequent years.
Model inputs
Risk equations for second hip fracture, non-hip fragility fracture, all-cause mortality and admission to care home
The HES database was used to develop the risk equations for the following events: second hip fracture, major non-hip fragility fracture, admission to care home (nursing or residential) following hip fracture and death. Major non-hip fracture requiring hospitalisation was defined as spine, wrist, pelvis, rib, humerus or other fragility fractures and identified with ICD-10 diagnosis codes: S22, S32, S42, S52.0–S52.3, S22.5 and S22.6. 104 The criteria followed for identifying hip fractures were also applied to major non-hip fractures, that is, the relevant ICD-10 codes had to be primary diagnosis, > 60 years of age at fracture, emergency admission and no trauma accident.
The risk equations for second hip fracture and major non-hip fracture consisted of multivariate semiparametric proportional hazards survival models derived with time to event determined in continuous time from the onset of first hip fracture, using the censor date of death or the date of administrative censoring (31 March 2013).
The risk equations for all-cause mortality included two logistic models to capture the high mortality in the first 30 days after first and second hip fracture, and a Gompertz proportional hazards survival model for the subsequent years. Time to death was modelled in continuous time, using patient’s current age as time at risk to better extrapolate beyond the observed follow-up period. 154
The risk equations to predict the probability of a care home admission (nursing or residential) following hip fracture (first and second) consisted of two logistic models. Admissions and discharges to a care home were identified in HES using the variables ‘admission source’ and ‘discharge destination’ and the codes for temporary accommodation and nursing/residential home. We assumed that if a patient had been admitted or discharged to a nursing/residential home at any point they would remain in a care home for the rest of their lifetime. This was to reduce the potential risk of misclassification regarding the admission source. Hence the estimated equations focused solely on patients who were not in a care home before the hip fracture event and the dependent variable was new admission to a care home.
The set of candidate covariates for each equation included time-invariant factors (i.e. sex, age and Charlson Comorbidity Index at first hip fracture) and time-variant factors such as: occurrence or history of second hip fracture, occurrence or history of major non-hip fracture, and admission from care home or own home.
The following process was used to estimate the risk equations. First, binary covariates (e.g. sex, history of fracture, admission source) were included only if they occurred at least 100 times, given the large sample size of the data set. Second, a multivariate model was fitted and all covariates with p > 0.3 were discarded. The final model was selected in a backwards stepwise regression at p < 0.05. The robustness of the final model specification was tested by performing forwards stepwise regression and varying the p-value in the first step (e.g. p > 0.2, p > 0.1). The parametric form of the underlying hazard was examined graphically and models were chosen according to the Akaike’s information criterion for exponential, Weibull and Gompertz parametric forms. The proportional hazards assumption was tested using the Schoenfeld residuals155 in Cox models with the same covariates as the parametric ones and the specification of the independent variable was tested using the Pregibon link test. 156 Internal validation of the risk equations was performed by comparing the predictions, as cumulative failure, against the observed incidence of events and death, Kaplan–Meier cumulative failure, over the follow-up period. Statistical analysis was carried out using Stata version 12.
The mean follow-up time of patients was 2.6 years (SD 2.5 years) and 84,717 patient-years of data were available to estimate the risk equations. Table 27 reports the number of events available to estimate the risk equations. Tables 28 and 29 report the fully specified risk equations.
| Event | Total | Annual event ratea |
|---|---|---|
| Death | 19,084 | 0.225 |
| Second hip fracture | 2206 | 0.026 |
| Major non-hip fracture | 1464 | 0.017 |
| Event | Care home after first hip fracture | Care home after second hip fracture |
|---|---|---|
| Patient-years | 24,879 | 1599 |
| Patients | 24,879 | 1599 |
| Number of events | 4869 | 278 |
| Functional form | Logistic | Logistic |
| Parameters, mean (SE) | ||
| Constant | –10.490 (1.398) | –4.120 (0.777) |
| Female | 0.152 (0.040) | |
| Age at first hip fracture | 0.173 (0.034) | |
| (Age at first hip fracture)2 | –0.0008 (0.0002) | |
| Age at second hip fracture | 0.030 (0.009) | |
| CCI score | 0.042 (0.011) | |
| p > χ2 | < 0.001 | < 0.001 |
| Event | Second hip fracture | Non-hip fracture | 30-day all-cause mortality after first hip fracture | 30-day all-cause mortality after second hip fracture | All-cause mortality post 30 days |
|---|---|---|---|---|---|
| Patient-years | 59,740 | 60,557 | 32,989 | 2197 | 62,907 |
| Patients | 29,888 | 29,888 | 32,989 | 2197 | 29,888 |
| Number of events | 2206 | 1464 | 3101 | 173 | 13,008 |
| Functional form | Weibull | Weibull | Logistic | Logistic | Gompertz |
| Parameters, mean (SE) | |||||
| Constant | –6.951 (0.244) | –6.867 (0.298) | –8.705 (0.242) | –6.264 (1.014) | –7.471 (0.143) |
| ρ | 1.099 (0.018) | 1.259 (0.024) | |||
| γ | 0.012 (0.005) | ||||
| Female | 0.117 (0.055) | 0.481 (0.077) | –0.505 (0.043) | –0.623 (0.186) | –0.436 (0.020) |
| Age at first hip fracture | 0.042 (0.003) | 0.028 (0.004) | 0.075 (0.003) | 0.072 (0.002) | |
| Age at second hip fracture | 0.048 (0.012) | ||||
| Care home | 0.451 (0.055) | 0.236 (0.057) | 0.360 (0.170) | 2.092 (0.219) | |
| CCI score | 0.269 (0.010) | 0.655 (0.062) | |||
| Non-hip fracture | 0.371 (0.117) | ||||
| Second hip fracture | 0.377 (0.121) | ||||
| History of non-hip fracture | 0.152 (0.053) | ||||
| History of second hip fracture | 0.286 (0.113) | 0.246 (0.044) | |||
| Age × care home | –0.020 (0.003) | ||||
| Age × CCI | –0.006 (0.001) | ||||
| p > χ2 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Residential and nursing home
The NHFD reported the discharge destination from all NHS Trust hospitals to be 10.8% to residential care and 10.1% to nursing care in 2013. 17 We multiplied these proportions by the number of hip fracture admissions recorded in that year (n = 61,508) and estimated the proportion of patients discharged to a nursing home out of all patients discharged to a care home (48%). We assumed this proportion to apply to all hip fracture patients simulated in the model discharged to a care home.
Effectiveness of models of care
Chapter 5 describes the statistical analysis to estimate the effectiveness of introducing an orthogeriatrician and a fracture liaison nurse relative to usual care in terms of:
-
30-day mortality following primary hip fracture
-
1-year mortality following primary hip fracture
-
2-year risk of developing second hip fracture following primary hip fracture.
In the base case, we assumed that the effect of the new models of care on mortality relative to usual care would not be felt beyond the first year following the primary hip fracture. We also assumed that there would be no difference between the three models of care regarding mortality after the second hip fracture. However, we assumed that the relative effect on the development of a second hip fracture would be valid only for the first 2 years post primary hip fracture. These assumptions were explored in sensitivity analysis. The impact of an orthogeriatrician and a fracture liaison nurse was modelled by converting the transition probabilities into rates (assuming exponential distribution), multiplying the rates by the respective HRs and then converting the resulting rates back into probabilities. Table 30 reports the relative effectiveness estimates used to inform the model. Two sets of effectiveness values were explored. Base-case analysis consisted of using the pooled effectiveness estimates associated with introducing an orthogeriatrician or a fracture liaison nurse relative to the usual care that was being provided before (e.g. nothing, fracture liaison nurse or orthogeriatrician). This fits with the remit of the effectiveness work presented in Chapter 5, makes use of all data available and minimises the impact of hospital outliers. In sensitivity analysis, we used effectiveness estimates concerning the comparison of orthogeriatrician or fracture liaison nurse relative to hospitals that had no previous orthogeriatrician or fracture liaison nurse.
| OG | 95% CI | FLN | 95% CI | |
|---|---|---|---|---|
| Pooled estimates (base case) | ||||
| 30-day mortality | 0.73 | 0.65 to 0.82 | 0.80 | 0.71 to 0.91 |
| 1-year mortality | 0.81 | 0.75 to 0.87 | 0.84 | 0.77 to 0.93 |
| 2-year second hip | 0.95 | 0.79 to 1.15 | 1.03 | 0.85 to 1.26 |
| Relative to no previous OG or FLN | ||||
| 30-day mortality | 0.79 | 0.63 to 0.99 | 0.87 | 0.61 to 1.25 |
| 1-year mortality | 0.79 | 0.70 to 0.90 | 0.74 | 0.60 to 0.91 |
| 2-year second hip | 1.00 | 0.73 to 1.37 | 1.40 | 0.77 to 2.56 |
Intervention costs
The costs of introducing an orthogeriatrician or a fracture liaison nurse were estimated using 2012–13 prices. Following clinical advice, a fracture liaison nurse was assumed to work with hip fracture patients at 1 WTE, whereas an orthogeriatrician was assumed to work at 0.75 WTE capacity (this is also similar to the average WTE reported across hospitals with an orthogeriatrician; see Chapter 2). The annual costs of an orthogeriatrician and a fracture liaison nurse per hip fracture patient were estimated by multiplying the respective WTE by the total annual costs (salary, salary on-costs, qualification costs, overheads and capital overheads) and dividing these by 450 hip fracture patients (average patients seen per year across the 11 hospitals in the HES data set with an orthogeriatrician and/or fracture liaison nurse)17 (Table 31). The intervention of an orthogeriatrician and a fracture liaison nurse was deemed to occur only in the first year post primary hip fracture and, therefore, was not costed in the remaining years of simulation. We assumed that there would be no significant change in resources at hospital level within the primary hip fracture hospital admission because of an orthogeriatrician or a fracture liaison nurse beyond what was already captured in the HRGs. Hence any difference in costs between the models of care were assumed to be because of the intervention costs, any additional hip fractures occurring and changes to patient’s longevity. We evaluated the impact of these assumptions in sensitivity analysis.
| Predictors | Annual costs (£)a | WTE | Annual costs (£) | Per hip fracture patient (£) (450 patients) | Source |
|---|---|---|---|---|---|
| FLN (grade 7) | 90,078 | 1 | 90,078 | 200 | PSSRU 2013143 |
| OG (consultant) | 252,003 | 0.75 | 189,002 | 420 | PSSRU 2013143 |
Primary care costs
Information on primary care costs was obtained from the 4433 patients with hip fracture identified in the CPRD database (Table 32). The methodology used to derive the primary cost data set is described in Chapter 7. Briefly, we estimated the following cost items:
-
GP consultations in clinic, GP surgery, telephone contacts, out-of-office visits
-
nurse face-to-face and non-face-to-face contacts
-
other community health-care professionals contacts (e.g. health visitor, physiotherapist)
-
pharmaceuticals and tests.
| Cost equation | Year of first hip fracture | Subsequent years |
|---|---|---|
| Patient-years | 3910 | 7373 |
| Number of patients | 3910 | 2568 |
| Distributional form | Gamma | Gamma |
| Link function | Identity | Identity |
| Parameters, mean (SE) | ||
| Constant | 1251 (39) | 1161 (40) |
| Death within 30 days | –1197 (52) | |
| Death within year | –689 (36) | –437 (52) |
| Living in care home | 126 (39) | |
| Major non-hip fracture | 502 (264) | |
Each primary care contact and test was costed using unit costs from national cost databases (see Table 17). 143 Following a previous study,142 we counted only one consultation per day. Pharmaceuticals were costed using the number of prescriptions stratified by BNF code90 per patient. The unit costs for each BNF code concerned the net ingredient cost per item prescribed reported in the Health and Social Care Information Centre Prescription Cost Analysis. 145
Hospital costs
Information on hospital costs was obtained from 33,152 patients with hip fracture identified in the HES data set. HES provided details of all admissions and contacts in English hospitals funded by the NHS. We used the same methodology in Chapter 7 to estimate the annual hospitalisation costs for each health state of the model. Briefly, each hospital contact was valued using the 2012/13 HRG English tariff and the annual costs were estimated using a GLM with a gamma distribution, for the relationship between the variance and conditional mean, and a link function. The choice of the GLM family and link functions was informed by the modified Park test and the Box–Cox test, respectively. Model fit was assessed using Pregibon’s link test and different family and link functions were compared using Akaike’s information criterion. To assess the annual hospital care costs by health state, we included the following predictors: sex; current age; age at hip fracture (first and second); living in a care home (nursing or residential); 30-day mortality following hip fracture; 1-year mortality following hip fracture; second hip fracture; major non-hip fracture requiring hospitalisation; history of second hip fracture; and history of major non-hip fracture. Tables 33 and 34 report the cost equations.
| Predictors | Hospitalisation costs in year of first hip fracture | Probability of hospitalisation in the years post first hip fracture | Hospitalisation costs in subsequent years (conditional on hospitalisation) | Hospitalisation costs in year of second hip fracture (subsequent years to first hip fracture) |
|---|---|---|---|---|
| Patient-years | 30,430 | 29,133 | 10,243 | 1166 |
| Number of patients | 30,430 | 18,213 | 8604 | 1166 |
| Distributional form | Gamma | Logistic | Gamma | Gamma |
| Link function | Identity | Identity | Identity | |
| Parameters, mean (SE) | ||||
| Constant | 11,462 (559) | –0.449 (0.029) | 10,795 (1133) | 23,206 (3166) |
| Death within 30 days of hip fracture | –5110 (129) | –3560 (589) | ||
| Death within year of hip fracture | 2979 (169) | 5391 (1091) | ||
| Living in care home | 3168 (149) | 0.273 (0.030) | 2676 (259) | 1053 (520) |
| Age at hip fracture | 24 (7) | –108 (36) | ||
| Current age | –55 (13) | |||
| Female | –1265 (129) | –0.328 (0.032) | –1039 (268) | –1908 (762) |
| Major non-hip fracture | 5964 (707) | |||
| Second hip fracture | 10,017 (635) | |||
| History of major non-hip fracture | 0.364 (0.087) | |||
| History of second hip fracture | 0.383 (0.069) | 993 (472) | ||
| Predictors | Hospitalisation costs if major fracture occurs (subsequent years to first hip fracture) | Probability of hospitalisation given death | Hospitalisation costs if death occurs (conditional on hospitalisation) |
|---|---|---|---|
| Patient-years | 968 | 9282 | 5404 |
| Number of patients | 899 | 9282 | 5404 |
| Distributional form | Gamma | Logistic | Gamma |
| Link function | Identity | Identity | |
| Parameters, mean (SE) | |||
| Constant | 11,582 (1057) | 3.970 (0.269) | 19,401 (1489) |
| Living in care home | 2001 (606) | –0.209 (0.043) | 896 (235) |
| Current age | –0.038 (0.003) | –144 (17) | |
| Female | –2953 (1096) | –0.251 (0.053) | |
Care home costs
The annual costs of institutionalisation in a nursing and residential home were obtained from data published by the Personal Social Services Research Unit,143 and estimated at £39,000 per year (£750 per week × 52 weeks) and £27,664 per year (£532 per week × 52 weeks), respectively.
Quality of life in patients with hip fracture
A literature search was conducted to identify studies reporting preference-based quality of life for patients with hip fracture [e.g. European Quality of Life-5 Dimensions (EQ-5D), EQ Visual Analogue Scale, Health Utilities Index 2, Health Utilities Index 3, Short Form questionnaire-6 Dimensions, standard gamble, time trade-off, etc.]. The following databases were searched for studies published between 1 January 1990 and 1 April 2014: EconLit, EMBASE, Global Health, MEDLINE, NHS EED and HTA and Web of Science. Literature reviews and reference lists of the papers found in the literature review were also searched for additional studies. Data were extracted from each study using a pro forma.
We identified 64 studies reporting preference-based quality of life data in populations with hip fracture (Figure 30). We synthesised data from studies reporting absolute utility values at different follow-up times using a metaregression approach. These corresponded to 32 populations of hip fracture patients, 187 observations and 21,085 hip fracture patients. Each observation was weighted by the respective sample size divided by the variance of the mean utility value and synthesised using a linear mixed-effects model. The following predictors were examined: follow-up time (months), follow-up time to the power of two, and the use of the EQ-5D instrument to elicit the utilities. The resulting model was used to predict the EQ-5D utility values of hip fracture patients at the following time points: onset of the fracture (0.44 at 0 months), 6 months (0.52) and 18 months (0.65) (Table 35). We assumed changes in the mean utility values between onset of hip fracture and 1 year and between 1 year and 2 years post hip fracture to be straight line transitions and hence used the predict utility values at the mid-point time periods. We assumed the utility value for the hip fracture population to remain constant after the first year post hip fracture (i.e. 0.65). Finally, we assumed second hip fracture and major non-hip fractures requiring hospitalisation to be associated with the same utility values in the year of the event as those at the onset of hip fracture (0.44). The most common non-hip fractures requiring hospitalisation were pelvic fractures.
FIGURE 30.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) diagram for literature review of preference-based quality of life studies in hip fracture populations.
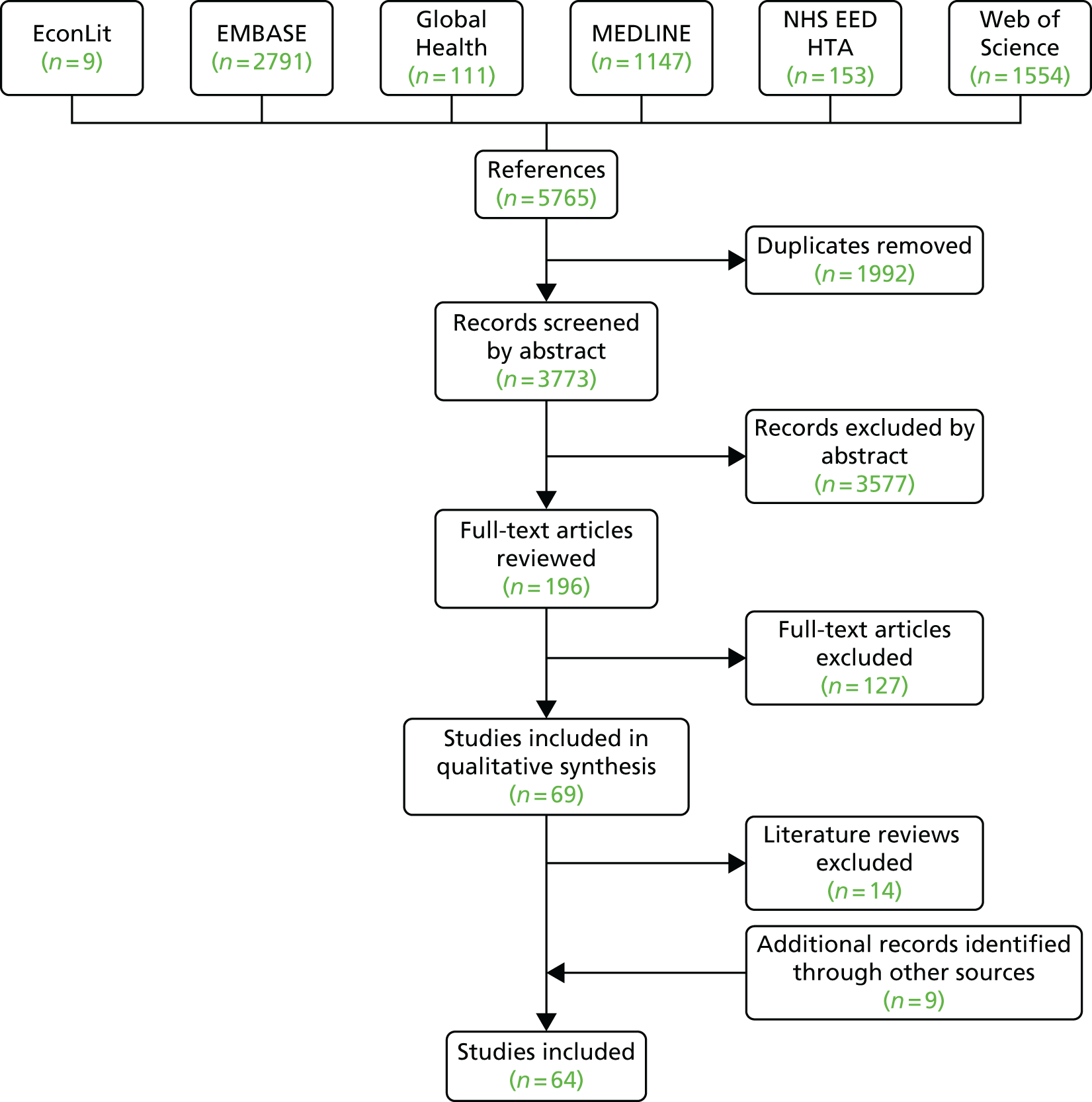
| Linear mixed-effects model | ||
|---|---|---|
| Number of observations | 187 | |
| Number of groups | 32 | |
| Parameters | Mean | SE |
| Follow-up time (months) | 0.0180 | 0.0021 |
| Follow-up time (months2) | –0.0003 | 0.00006 |
| EQ-5D | –0.1912 | 0.0881 |
| Constant | 0.6187 | 0.0776 |
| Random effects (SD) | 0.0000 | |
| Probability > χ2 | 0.0000 | |
| Utility predictions | Mean | 95% CI |
| Utility at onset of hip fracture | 0.44 | 0.22 to 0.66 |
| Utility at 6 months | 0.52 | 0.30 to 0.74 |
| Utility at 18 months | 0.65 | 0.40 to 0.89 |
Analysis
A model of care was deemed to be cost-effective if the ICER was below £30,000 per QALY gained. The ICER was estimated by dividing the difference in mean costs (ΔC) by the difference in mean effects (ΔE) (life-years and QALYs) for a given model of care compared with its next best alternative. As more than two models of care were compared, to facilitate the identification of the most cost-effective option we used the net benefit framework. 157 This consists of estimating the net monetary benefit (NMB) of each model of care:
where E and C represent the mean effects (QALYs and life-years) and costs, respectively, for a given model of care and λ represents the maximum willingness to pay per unit of effect (i.e. £30,000 per QALY gained). The most cost-effective model of care is identified as being the one with the highest NMB. Finally, the internal validity of the model was checked using sensitivity analysis (extreme values) and by comparing the model outputs with the data used to build the model.
Sensitivity and uncertainty analysis
Model parameters and structural assumptions were evaluated in one-way and probabilistic sensitivity analysis. The key uncertainties in the model structure were identified during the discussions for the conceptual framework. The distributions for the regression coefficients informing the several models described were obtained by bootstrapping the sample and re-estimating the regression models. This ensured the correlation between coefficients to be fully captured. The choice of distributions used for the remaining parameters was made according to recommended practice. 158 Relative effectiveness measures (i.e. HRs) were modelled using a log-normal distribution. Parameters concerning proportions/probabilities were modelled using beta distributions.
A cost-effectiveness acceptability curve was constructed159 using the NMB results and analysis of covariance methods were used to determine the proportion of variance in the incremental costs and QALYs saved explained by parameter uncertainty. 158 Finally, the overall contribution of the model inputs to the decision uncertainty was explored using the expected value of perfect information (EVPI). The EVPI per patient was estimated non-parametrically. 158
The EVPI for the total population who stand to benefit from reducing the decision uncertainty was also estimated. This required information on the period over which information about the decision will be useful, T (5-, 10- and 15-year scenarios), and the number of hip fractures in England, Pt,
where Pt was 70,000 in England and the discount rate used, r, was 3.5%.
Results
Representative patient
Two cohorts of 1000 identical men and women were used to simulate a representative patient aged 83 years at hip fracture, with an average pre-admission Charlson Comorbidity Index score of 1.2 and living in their own home before the fracture.
For our male cohort, the introduction of an orthogeriatrician and a fracture liaison nurse would result in a reduction of 26 (95% CI 17 to 34) and 19 (95% CI 9 to 28) deaths within 30 days of primary hip fracture, respectively, compared with usual care (Table 36). Within 1 year of primary hip fracture, the reduction in deaths by introducing an orthogeriatrician and a fracture liaison nurse, compared with usual care, was 58 (95% CI 42 to 71) and 46 (95% CI 27 to 63), respectively. Over the lifetime of the cohort, when compared with usual care, there would be an increase of 0.18 (95% CI 0.14 to 0.22) and 0.14 (95% CI 0.09 to 0.19) life-years (undiscounted) spent in their own home if an orthogeriatrician or a fracture liaison nurse were to be introduced, respectively.
| Patient outcomes | Usual care | FLN | OG |
|---|---|---|---|
| Deaths within 30 days of primary hip fracture | 115 | 96 | 89 |
| Deaths within 1 year of primary hip fracture | 322 | 275 | 264 |
| Second hip fracture | 107 | 115 | 113 |
| Major non-hip fractures requiring hospitalisation | 55 | 58 | 59 |
| LYs in a care homea | 0.40 | 0.43 | 0.43 |
| LYs in own homea | 2.51 | 2.65 | 2.69 |
For our female cohort, the introduction of an orthogeriatrician and a fracture liaison nurse would result in a reduction of 16 (95% CI 11 to 22) and 12 (95% CI 5 to 18) deaths within 30 days of primary hip fracture, respectively, compared with usual care (Table 37). Within 1 year of primary hip fracture, the reduction in deaths by introducing an orthogeriatrician and a fracture liaison nurse, compared with usual care, was 42 (95% CI 31 to 52) and 33 (95% CI 20 to 46), respectively. Over the lifetime of the cohort, when compared with usual care, there would be an increase of 0.17 (95% CI 0.13 to 0.22) and 0.13 (95% CI 0.08 to 0.19) life-years (undiscounted) spent in their own home if an orthogeriatrician or a fracture liaison nurse were to be introduced, respectively.
| Patient outcomes | Usual care | FLN | OG |
|---|---|---|---|
| Deaths within 30 days of primary hip fracture | 77 | 64 | 60 |
| Deaths within 1 year of primary hip fracture | 221 | 187 | 179 |
| Second hip fracture | 171 | 179 | 177 |
| Major non-hip fractures requiring hospitalisation | 132 | 137 | 138 |
| LYs in a care homea | 0.72 | 0.76 | 0.76 |
| LYs in own homea | 3.67 | 3.81 | 3.85 |
Owing to better survival, the numbers of second hip fractures and major non-hip fractures were higher with the introduction of an orthogeriatrician or a fracture liaison nurse, compared with usual care, in both the female and the male cohorts (see Tables 36 and 37).
The average discounted hospital cost per male patient was £23,825 with an orthogeriatrician, compared with £23,690 and £23,037 with a fracture liaison nurse and usual care. Mean primary care costs associated with an orthogeriatrician were also higher, £3523, than a fracture liaison nurse and usual care (£3469 and £3275, respectively). Mean care home costs were higher if an orthogeriatrician was introduced (£13,946) compared with having a fracture liaison nurse (£13,710) or usual care (£12,789). Combining all health and social care costs included in the model, mean discounted costs were £41,714 when an orthogeriatrician was introduced, £41,068 when a fracture liaison nurse was introduced and £39,101 for standard care (Table 38). The discounted average QALYs gained by male patients were 1.74 with an orthogeriatrician, 1.72 with a fracture liaison nurse and 1.62 with usual care.
| Usual care | FLN | OG | |
|---|---|---|---|
| Male cohort | |||
| Total costs (95% CI) | £39,101 (£37,798 to £40,514) | £41,068 (£39,530 to £42,623) | £41,714 (£40,265 to £43,262) |
| Intervention | 0 | £200 | £420 |
| Hospital care | £23,037 | £23,690 | £23,825 |
| Primary care | £3275 | £3469 | £3523 |
| Care home | £12,789 | £13,710 | £13,946 |
| Total LYs (95% CI) | 2.68 (2.56 to 2.79) | 2.83 (2.70 to 2.96) | 2.88 (2.75 to 3.00) |
| Total QALYs (95% CI) | 1.62 (1.54 to 1.70) | 1.72 (1.64 to 1.80) | 1.74 (1.66 to 1.83) |
| Female cohort | |||
| Total costs (95% CI) | £50,573 (£49,226 to £52,276) | £52,472 (£50,935 to £54,340) | £53,104 (£51,559 to £54,974) |
| Intervention | 0 | £200 | £420 |
| Hospital care | £23,881 | £24,372 | £24,463 |
| Primary care | £4716 | £4895 | £4947 |
| Care home | £21,976 | £23,006 | £23,274 |
| Total LYs (95% CI) | 3.89 (3.77 to 4.03) | 4.04 (3.91 to 4.19) | 4.08 (3.95 to 4.24) |
| Total QALYs (95% CI) | 2.38 (2.29 to 2.48) | 2.48 (2.38 to 2.58) | 2.50 (2.40 to 2.61) |
For our female cohort, the average discounted hospital cost per patient was £24,463 with an orthogeriatrician, compared with £24,372 and £23,881 with a fracture liaison nurse and usual care. As with male patients, the average primary care costs associated with an orthogeriatrician were higher, £4947, than those associated with a fracture liaison nurse and usual care (£4895 and £4716, respectively). Mean care home costs were considerably higher than for male patients and introducing an orthogeriatrician was associated with highest costs (£23,274) compared with a fracture liaison nurse (£23,006) or usual care (£21,976). Combining all health and social care costs included in the model, mean discounted costs were £53,104 when an orthogeriatrician is introduced, £52,472 when a fracture liaison nurse is introduced and £50,573 if usual care is provided (see Table 38). The discounted average QALYs gained by female patients were 2.50 with an orthogeriatrician, 2.48 with a fracture liaison nurse and 2.38 with usual care.
After combining costs and outcomes in an incremental cost-effectiveness analysis, and at a £30,000 per QALY threshold, the most cost-effective model of care was introducing an orthogeriatrician (Table 39). The probability of adding an orthogeriatrician being the most cost-effective option at £30,000/QALY was estimated to about 70% across both sexes.
| Difference in costs, £ (95% CI) | Difference in LYs (95% CI) | Difference in QALYs (95% CI) | ICER (£/LY) | ICER (£/QALY) | Probability that is the most cost-effective at £30,000/QALY | |
|---|---|---|---|---|---|---|
| Male cohort | ||||||
| Usual care | – | – | – | – | – | 0% |
| FLN vs. usual care | 1967 (1266 to 2592) | 0.157 (0.096 to 0.215) | 0.097 (0.057 to 0.133) | £12,492 | £20,302 | 30% |
| OG vs. FLN | 646 (–132 to 1503) | 0.044 (–0.026 to 0.125) | 0.028 (–0.016 to 0.078) | £14,640 | £23,271 | 70% |
| Female cohort | ||||||
| Usual care | – | – | – | – | – | 0% |
| FLN vs. usual care | 1899 (1272 to 2515) | 0.148 (0.092 to 0.204) | 0.091 (0.056 to 0.127) | £12,871 | £20,794 | 30% |
| OG vs. FLN | 632 (–125 to 1443) | 0.043 (–0.026 to 0.119) | 0.027 (–0.017 to 0.075) | £14,683 | £23,189 | 70% |
Figure 31 reports the cost-effectiveness acceptability curve and EVPI per female patient associated with the different models of care. The population EVPI over 5 years was estimated to be £23M at the £30,000 per QALY gained threshold. This suggests that undertaking additional major commissioned research work to further reduce decision uncertainty is likely to be of significant benefit.
FIGURE 31.
Cost-effectiveness acceptability curve and EVPI per female patient. The curves provide the probability of the models of care being the most cost-effective option at any willingness-to-pay value for an additional QALY gained. Please note that the curves of the models of care add to one at any given point. The EVPI curve provides the value of EVPI at any willingness-to-pay value for an additional QALY gained. OG, orthogeriatrician.
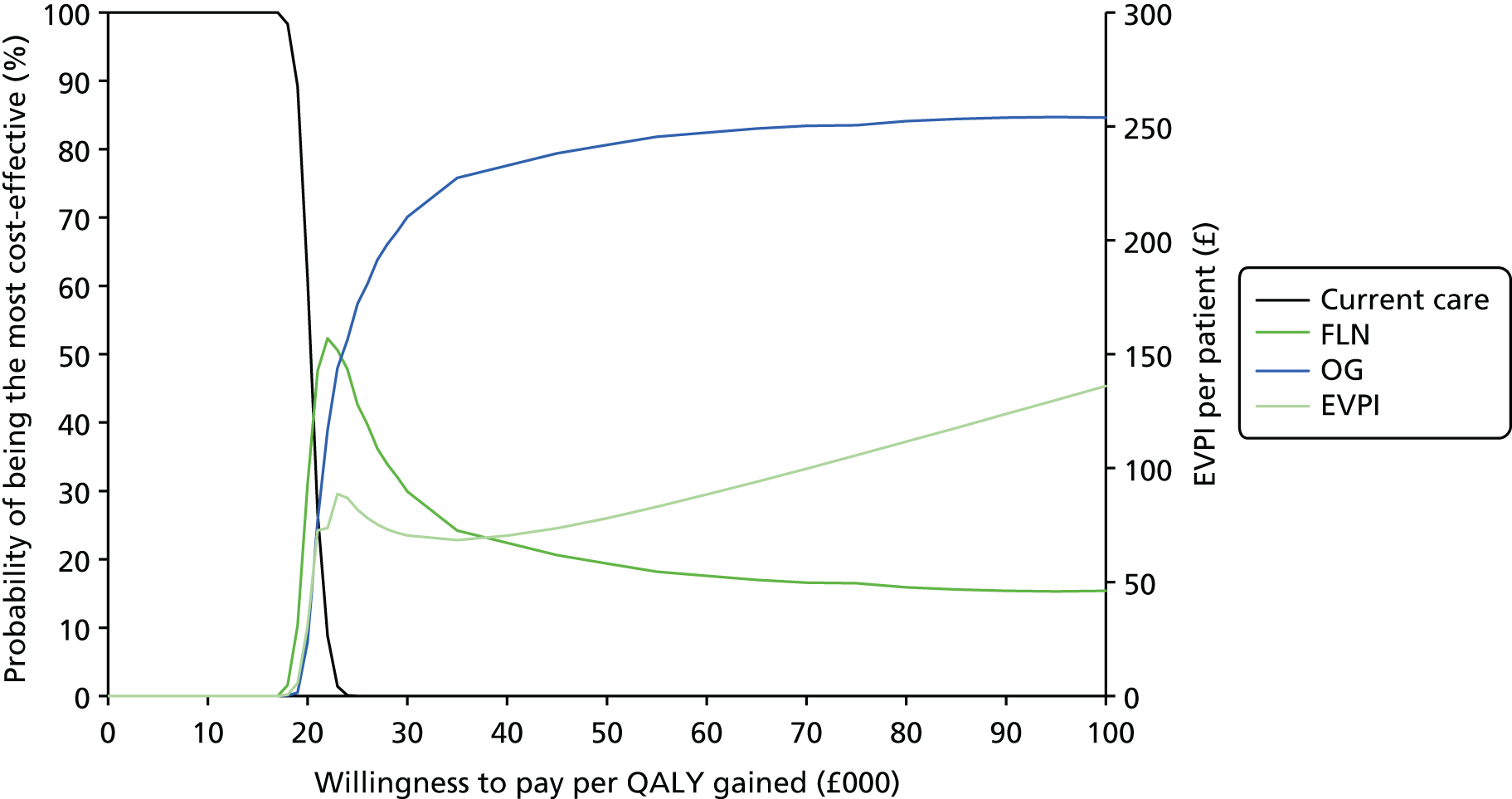
Figure 32 reports the parameters according to their impact on the variance of incremental costs and QALYs from the comparison of introducing an orthogeriatrician with introducing a fracture liaison nurse. Variance in the estimated incremental costs was dominated by the variation in the following variables: relative effectiveness inputs, regression models predicting hospital costs, natural history inputs (e.g. death within 30 days, time to second hip fracture), and the likelihood of being discharge to a care home following hip fracture. Variance in the incremental QALYs was dominated by the variance in effectiveness inputs and natural history inputs.
FIGURE 32.
Orthogeriatrician vs. fracture liaison nurse: analysis of covariance analysis of proportion of sum of squares for incremental QALYs saved and incremental costs explained by the uncertainty in the model. The horizontal axis represents the variation in incremental costs and QALYs that is associated with the uncertainty in the model inputs. Reproduced with permission from Leal J, Gray AM, Hawley S, Prieto-Alhambra D, Delmestri A, Arden NK, et al. Cost-effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: a population based study. J Bone Miner Res 2016; in press.
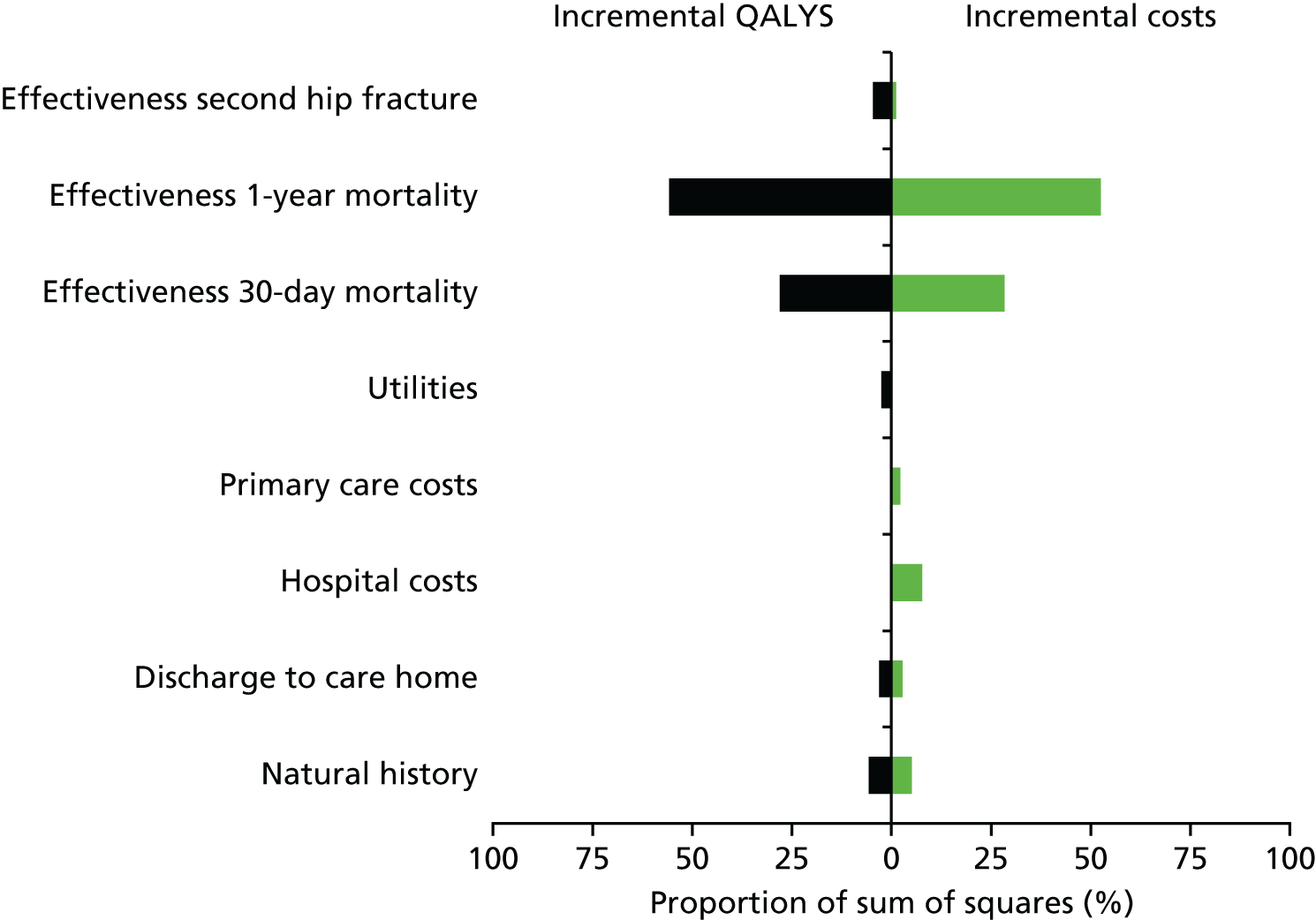
Sensitivity analysis
Table 40 reports the results of a range of other sensitivity analyses using the female cohort. Overall, the results were robust to changes in the majority of assumptions. Excluding social care costs from the analysis resulted in the model of care with an orthogeriatrician becoming cost-effective below the £20,000 per QALY threshold. The results were also robust to using relative effectiveness measures concerning the comparison of orthogeriatrician or fracture liaison nurse relative to hospitals that had no previous orthogeriatrician or fracture liaison nurse rather than the pooled estimates. Finally, the assumptions regarding the costs of the models of care had an impact in terms of the results. Using the number of hip fractures reported in the smallest hospital in the HES data set (220 per year) to estimate the costs per patient of the models of care resulted in introducing a fracture liaison nurse becoming the most cost-effective option.
| Female cohort | FLN vs. usual care | OG vs. FLN |
|---|---|---|
| Base case | £20,794 | £23,189 |
| Primary and hospital care costs only (excluding care home costs) | £9046 | £13,039 |
| Relative effectiveness estimates (where comparator is no OG or FLN rather than pooled estimate) | Dominated | £21,798a |
| Relative effectiveness for 30-day mortality also applying to second hip fracture | £20,754 | £23,140 |
| Effectiveness over mortality beyond first year post hip fracture into lifetime | £16,944 | £18,052 |
| Effectiveness over second hip fracture extending into lifetime | Extendedly dominated | £20,036a |
| Effectiveness over major non-hip fractures requiring hospitalisation (OG: HR 1.14; FLN: HR 0.94, see Chapter 5) | £20,439 | £26,559 |
| OG and FLN costs without qualification costs (£300 and £177 per patient, respectively) | Extendedly dominated | £20,230a |
| OG at 1 WTE (£560 per patient) | £20,794 | £28,418 |
| FLN at 0.25 WTE (£50 per patient) | £19,066 | £28,798 |
| FLN at 0.60 WTE (£120 per patient) | £19,817 | £26,172 |
| 220 hip fracture patients per year seen by OG or FLN (intervention costs at £859 and £409 per patient, respectively) | £22,922 | £31,784 |
| 690 hip fracture patients per year seen by OG or FLN (intervention costs at £274 and £131 per patient, respectively) | £19,929 | £20,307 |
| OG and FLN result in savings of £1000 per patient in management and test costs during first hip fracture admission | £9947 | £23,189 |
| OG and FLN result in savings of £500 per patient in management and test costs during first hip fracture admission | £15,312 | £23,189 |
| OG and FLN result in savings of £100 per patient in management and test costs during first hip fracture admission | £19,603 | £23,189 |
| OG and FLN result in additional £100 per patient in management and test costs during first hip fracture admission | £21,749 | £23,189 |
| OG and FLN result in additional £500 per patient in management and test costs during first hip fracture admission | Extendedly dominated | £25,401a |
| OG and FLN result in additional £1000 per patient in management and test costs during first hip fracture admission | Extendedly dominated | £29,573a |
| Utility value for major non-hip fracture (0.52 and not 0.44) | £20,597 | £23,094 |
| Utility value after first year post hip fracture (0.70 and not 0.65) | £19,628 | £21,955 |
Subgroup analysis
We further assessed the cost-effectiveness of the models of care in several subgroups of hip fracture patients defined according to their age, sex and Charlson Comorbidity Index score at index hip fracture (Table 41). For patients up to the age of 80 years, the model of care with an orthogeriatrician was the most cost-effective option. For patients aged 90 years, introducing a fracture liaison nurse became the most cost-effective option if the Charlson Comorbidity Index score at index hip fracture was 5.
| Patient subgroup | FLN vs. usual care | OG vs. FLN |
|---|---|---|
| Female cohort | ||
| Age 60 years and CCI score of 0 | £14,431 | £20,735a |
| Age 70 years and CCI score of 0 | £16,221 | £19,988a |
| Age 80 years and CCI score of 0 | £19,377 | £21,808a |
| Age 90 years and CCI score of 0 | £23,318 | £25,408a |
| Age 60 years and CCI score of 1 | £13,718 | £19,651a |
| Age 70 years and CCI score of 1 | £15,919 | £19,631a |
| Age 80 years and CCI score of 1 | £19,410 | £22,027a |
| Age 90 years and CCI score of 1 | £23,728 | £26,048a |
| Age 60 years and CCI score of 3 | £12,934 | £18,583a |
| Age 70 years and CCI score of 3 | £15,732 | £19,685a |
| Age 80 years and CCI score of 3 | £19,811 | £22,854a |
| Age 90 years and CCI score of 3 | £24,913 | £27,710a |
| Age 60 years and CCI score of 5 | £12,878 | £18,665a |
| Age 70 years and CCI score of 5 | £16,102 | £20,490a |
| Age 80 years and CCI score of 5 | £20,746 | £24,330a |
| Age 90 years and CCI score of 5 | £26,712a | £30,049 |
| Male cohort | ||
| Age 60 years and CCI score of 0 | £13,807 | £19,425a |
| Age 70 years and CCI score of 0 | £15,623 | £19,474a |
| Age 80 years and CCI score of 0 | £18,692 | £21,844a |
| Age 90 years and CCI score of 0 | £22,683 | £25,965a |
| Age 60 years and CCI score of 1 | £13,426 | £18,917a |
| Age 70 years and CCI score of 1 | £15,544 | £19,525a |
| Age 80 years and CCI score of 1 | £18,904 | £22,278a |
| Age 90 years and CCI score of 1 | £23,315 | £26,848a |
| Age 60 years and CCI score of 3 | £13,191 | £18,719a |
| Age 70 years and CCI score of 3 | £15,780 | £20,144a |
| Age 80 years and CCI score of 3 | £19,714 | £23,613a |
| Age 90 years and CCI score of 3 | £25,070 | £29,162a |
| Age 60 years and CCI score of 5 | £13,634 | £19,554a |
| Age 70 years and CCI score of 5 | £16,611 | £21,593a |
| Age 80 years and CCI score of 5 | £21,198 | £25,811a |
| Age 90 years and CCI score of 5 | £27,680a | £32,450 |
Conclusion
Existent cost-effectiveness evidence suggests that secondary prevention services for fractures can be cost-effective and even cost saving. Nonetheless, there is considerable uncertainty regarding the most cost-effective approach to provide secondary prevention services following hip fractures.
At current NICE thresholds of £20,000 to £30,000 per QALY gained, our cost-effectiveness analysis suggests that it is cost-effective to introduce a hospital-based orthogeriatrician or a fracture liaison nurse, as part of the delivery of secondary hip fracture prevention services, compared with usual care. Furthermore, our results show that, for this group of patients, the most cost-effective option would be introducing an orthogeriatrician. These results are consistent with those reported in previous cost-effectiveness analysis of models of care for the secondary prevention of osteoporotic fractures, despite the difference in the options of care being evaluated. 20,21,97 However, in contrast with previous work, our study benefits from the availability of large primary and secondary care administrative data sets that enabled the robust estimation of the impact of the models of care in terms of survival, prevention of second hip fracture, primary care and hospital care costs and cost-effectiveness. Furthermore, our results did not show orthogeriatrician or a fracture liaison nurse to be cost saving compared with standard care as was reported in the cost-effectiveness analyses by McLellan et al. 20 and Majumdar et al. 97 However, this is the first cost-effectiveness analysis in this area being informed by large primary and secondary care data records that allowed the robust estimation of the short- and long-term costs experienced by patients with a hip fracture.
Irrespective of how patients were stratified in terms of their age, sex and Charlson Comorbidity Index score at index hip fracture, our results suggest that it is cost-effective to introduce an orthogeriatrician or a fracture nurse compared with standard care. This is largely because of the impact that these models of care have on patient survival rather than on the prevention of further hip fractures or other major non-hip fractures. Hence the orthogeriatrician or fracture liaison nurse options appear to be more cost-effective when the outcome measure used is life-years (lower ICERs) rather than QALYs (e.g. an orthogeriatrician compared with a fracture liaison nurse was £14,683 per life-year saved compared with £23,189 per QALY). This is because quality of life is a function of events such as second hip fracture and major non-hip fractures for which there is no significant effect from introducing an orthogeriatrician or a fracture liaison nurse compared with usual care. Furthermore, longer survival may also have higher health-care and social care costs relative to usual care which may not be outweighed by the gains in quality of life.
There is considerable uncertainty in the evidence informing the model, particularly the relative effectiveness of an orthogeriatrician compared with a fracture liaison nurse on survival and prevention of second hip fracture and the natural history of hip fracture. This is reflected in the very large EVPI values of £23M to £73M, conditional on the expected lifetime of the models of care. The EVPI gives the maximum amount of funding required for further research to reduce the decision uncertainty about which of the models of care is the most cost-effective option. The large incidence of hip fractures every year that will be affected by the decision together with the considerable uncertainty about whether or not an orthogeriatrician is more effective than a fracture nurse warrant caution about the model results. Hence our work suggests undertaking additional major commissioned research work to further reduce the decision uncertainty, with particular emphasis on clinical trials comparing the different models of secondary care prevention services.
There are several limitations to consider. First, the effectiveness data were not informed by a clinical trial, as such data are not available. In addition, it was not possible to robustly evaluate more options of models of secondary prevention care, such as introducing an orthogeriatrician together with a fracture nurse compared with usual care, as this was limited by the range and number of services currently provided in the region studied. Furthermore, it was not possible to directly compare the introduction of a fracture nurse relative with an orthogeriatrician but rather this was compared indirectly in terms of their effectiveness relative to the same reference group (i.e. usual care).
Second, the models of care did not significantly affect the prevention of second hip fractures as had been expected at the outset of the study. However, the apparent lack of effect on second hip fractures may be a result of the relatively short time horizon used to evaluate it (2 years) and the relative small number of patients with a second hip fracture (2206 patients overall) available to evaluate its impact (see Chapter 6 for further discussion). Nonetheless, despite these limitations, the effectiveness data used to inform the cost-effectiveness analysis were based on a very large administrative data set supported by a careful survey of the services provided in each. This allowed us to robustly identify when and what type of changes occurred in the delivery of services and, thus, inform the statistical analysis.
Third, we did not separate the different types of fragility-related fractures that the patients could suffer post discharge. We focused on major non-hip fractures requiring hospitalisation given their relative large impact in terms of health-care costs and the feasibility of tracking these in the administrative health-care records. Furthermore, we did not separate non-hip fractures by type so that we could benefit from having a larger sample to estimate the costs. Other fragility fractures not resulting in hospitalisation were still included in the model as part of the primary and hospital care cost equations (captured in the constant term).
Fourth, although a great proportion of the data used was derived from health-care records of patients with hip fracture, we had to obtain health state utility values from a review of the published literature. These data were then synthesised using a metaregression approach to inform on the changes of utility conditional on time since hip fracture and the use of the EQ-5D instrument. However, it was not possible to reliably estimate utility values for non-hip fractures or the additional impact these may have on the quality of life of individuals with a history of hip fracture. Hence assumptions were needed that were fully explored in sensitivity analysis. Furthermore, owing to the cohort nature of the Markov model and its lack of memory, it was not possible to model the trajectory of utility values that took into account both time since primary hip fracture and history of events such as non-hip fractures and second hip fracture. An alternative would have been to analyse the Markov model as an individual-based model (i.e. microsimulation) so that we could keep track of each individual’s history and adjust the utility values accordingly. 160 This would have allowed for capturing better the utility trajectory of the hip fracture patients conditional on the history of events.
Finally, we used the perspective of the NHS and social care services to inform our analysis. Hence other important cost categories not relevant to the perspective adopted were not included in the analysis. For example, we excluded some important economic considerations for people with hip fracture and their families, such as unpaid care provided by friends and family and home adaptation costs as a consequence of the fracture.
Hence to address several of the limitations above we performed extensive internal validity checks complemented by the exploration of different parameter and structural scenarios in the sensitivity analysis.
In conclusion, our work suggests that it may be cost-effective to introduce an orthogeriatrician or a fracture nurse as part of secondary care prevention fracture services for patients with a hip fracture. Further research is needed to make more informed decisions with a focus on estimating the effectiveness of these models of care informed by clinical trials.
Chapter 9 Dissemination to clinicians, NHS managers and patients
Throughout the project we presented our findings to a local group of health-care professionals working in fracture prevention services in the South Central region [the ‘FRISCy (Fracture Reduction in South Central PolicY) network’]. Towards the end of the project, we also arranged to present findings to the Bath and Bristol Fracture Prevention Network, another regional network of clinicians with less familiarity with the background to the study. Finally, we arranged a workshop with patient members of the National Osteoporosis Society. The objectives of these routes of dissemination were to:
-
inform key stakeholders (clinicians, service managers, commissioners) as to the variations in care pathways and the cost-effectiveness of fracture liaison and orthogeriatric services, thereby driving best practice and investment in these services
-
ensure that our interpretation of findings fit with clinicians’ own experiences and were agreed with key stakeholders
-
ensure that patients agreed with our interpretation of findings and get their perspective on particular points to highlight in dissemination.
Health-care professional workshops: participants and methods
The FRISCy workshops were attended by 20 health-care professionals, including consultant rheumatologists, osteoporosis champions, fracture liaison nurses, osteoporosis nurses, orthogeriatricians, service mangers and commissioners. Each of these worked in fracture prevention services in the South Central region. The final workshop also included a group of 10 patient representatives. The Bristol and Bath Bone Society comprised a similar number of health-care professionals working in fracture prevention services in the Bath and Bristol areas. These regional meetings were well attended, with a contribution from each hospital in the region. Using existing networks of osteoporosis specialists proved a more effective and efficient way of communicating findings to stakeholders than holding a national conference, which would be more poorly attended owing to the busy schedules of such individuals and the need to travel.
Over four different meetings with the FRISCy network, results of the following work streams were presented:
-
variations in fracture prevention services across a region of England
-
implementation of FLSs
-
clinicians’ experiences of making business cases
-
costs of hip fracture to the NHS
-
clinical effectiveness of different models of care.
An overview of all findings was presented to the Bath and Bristol Bone Society.
Conclusions following health-care professional workshops
The stakeholders were very interested in the results of this study. They felt that it was a very worthwhile project, particularly given that many of them were trying, or had previously tried, to get funding towards a FLS at their respective hospitals. The findings fitted with their own experiences, and they agreed that medication adherence was a real problem and likely to explain the lack of effect of services on refracture rates.
Patient perspectives of findings
Patients have been engaged with the interpretation and dissemination of findings throughout the course of the study. Two patient representatives were recruited to sit on the project advisory board, one with a previous hip fracture and one who cared for his wife who had osteoporosis and dementia. They were sent documentation providing summaries of findings and results throughout the course of the study, and asked to provide their own insight and interpretation. Our patient and public representatives commented that this was a valuable study to inform commissioners of the most effective service and the associated costs of this service, and distil out the best practice from each hospital.
Furthermore, at the end of the study we met with a group of six patients with osteoporosis, five of whom had experienced a prior fragility fracture. The patients had been on various osteoporosis medications for varying lengths of time. At this event we discussed differences in the models of care identified as part of this study and patients’ experiences and preferences surrounding each. Specific points of discussion, highlighted by both the patient group and our patient representatives, are explained below in the following sections: Osteroporosis assessment, Treatment initiation, Adherence and monitoring and Multidiscplinary care.
The final results of the clinical effectiveness analysis and the costs of hip fracture were presented to a regional group of patient members of the National Osteoporosis Society. They were very enthusiastic about the findings and fully agreed with the interpretation provided, as laid out in this report.
Patients felt that the burden of osteoporosis is often underappreciated, particularly that surrounding the associated loss of independence. Many were aware of existing variations and gaps in care at certain hospitals. Overall, patients welcomed research being done into care around osteoporosis diagnosis and treatment and agreed with the interpretation of the findings as presented here.
Osteoporosis assessment
Only one of the hospitals studied had a DXA scanner available on site so they could perform the DXA scan while in an inpatient setting. At every other hospital patients had to travel to a different location for their DXA scan at a later date. In addition, some hospitals conducted the osteoporosis assessment at an outpatient appointment, too, so this could require patients to attend two separate outpatient appointments. There were mixed feelings about the impact of patients needing to attend extra outpatient appointments, with some feeling that this was not an excuse for missing appointments and that patients need to take more responsibility for their own care. They did agree that this would be more problematic for older and frailer people relying on public transport, and that DXA scans and consultant appointments should be offered together. They felt that mobile DXA scans were an excellent idea if the scanner was not available at the local hospital.
Treatment initiation
As discussed in Chapter 2, many hospitals with FLSs now initiate osteoporosis treatment for patients within the hospital setting, while several hospitals still send treatment recommendations to patients’ GPs. All patients within the group were initially prescribed treatment by their GP. One patient was experiencing problems with this, having been waiting 6 weeks at that point in time for his or her GP to take action following a treatment recommendation made by a hospital consultant. There was a sense that GPs often lacked knowledge and interest in osteoporosis, compared with other diseases such as cancer and heart disease. There is a lack of information available in GP surgeries about the risks of osteoporosis, and patients wished that they had had more information before they fractured and were diagnosed. Some patients have previously researched bone-strengthening treatments themselves when they found that their GP lacked knowledge about the options available, and had made their own choices about which treatment they wanted. Others felt that GPs also lacked training in diet and nutrition, with one person having taken calcium supplements for years with their GP never mentioning that it would be helpful to take vitamin D supplements alongside. The patients also felt that GPs were too busy to read all of the letters they received from hospital staff, and they sympathised with this and understood that GPs could not be experts in everything. Although hospitals with established FLSs and orthogeriatric services are taking over more of the responsibility for treatment initiation and monitoring, patients felt that it was still more convenient to visit GPs rather than attend any additional hospital appointments. Several patients were members of their local Patient Participation Group, which they thought was an excellent way of expressing their concerns to GPs.
Adherence and monitoring
All of the patients involved had been monitored after discharge by the FLS at their local hospital. They were initially sent a letter, and if they did not respond then they were contacted by telephone, and referred for DXA scans every 2–5 years post fracture (depending on the severity of their osteoporosis and age). The group were very surprised by the low rates of adherence, but could understand why many people failed to take their treatment. The asymptomatic nature of osteoporosis means that you cannot tell if the treatment is working, making people less likely to stay on it. They felt that their follow-up DXA scans helped them to monitor how their treatment was working for them. Patients agreed that this was a potential reason why changes to service delivery had no effect on rates of second fracture, and felt that more monitoring after discharge was important. Many patients are not aware that there are alternative medications available if they have side effects from their first treatment and this should be more clearly explained.
Multidisciplinary care
One particular point our patients agreed with was the importance of multidisciplinary care for hip fracture patients, as was also highlighted by many of the clinicians interviewed. Patients were aware of how other medical conditions can greatly hinder recovery following a major fragility fracture, and that multidisciplinary care, in their own experience, can help to pick up on pre-existing medical conditions.
Impact of research findings
Findings have also been presented to the National Osteoporosis Society, Public Health England, and at various other meetings and events. Specific impacts of the findings are described below.
NHS England/Public Health England
This study will be useful to analysts estimating the burden of hip fracture and osteoporosis and the long-term cost-effectiveness of interventions for the prevention and management of these conditions. Previous estimates of the health and social care costs of hip fractures in the UK range from £2B to £3B per year, but UK cost data on hip fracture were limited. The results of our study show that hip fractures are an enormous cost to the health-care system. Furthermore, the findings highlight the impact of complications following initial hospital discharge as a main driver of costs and the importance of preventing hip refractures.
This 1-year hospital cost of hip fracture was used by the multistakeholder FLS implementation group economic model which includes the National Osteoporosis Society (third sector), the Royal College of Physicians Clinical Effectiveness Evaluation Unit, Fragility Fracture Audit Programme FLS-Database Workstream, NHS England CCG gateway and Public Health England as a robust up-to-date estimate of the economic impact of hip fracture and potential benefits of a FLS at the local CCG level as well as the national level. These data will be used by NHS England to inform decisions about health service changes aimed at achieving greater efficiency and better patient care within the NHS. These findings also received significant press coverage in several daily newspapers as well as ITV news following presentation at the 2015 IOF-ESCEO (European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis) conference, where they will have been seen by the general public as well as clinicians, NHS commissioners and policy-makers. The articles emphasised the increasing number of hip fractures and the financial implications, and made the case for policy-makers to prioritise bone health through the universal provision of FLSs.
Fracture liaison service workshop
Although national guidelines recommend service models targeting secondary fracture prevention at patients following a fragility fracture, data on the clinical effectiveness of these services are rare. The increasing burden of hip fractures is also a concern in other countries across Europe, North America and Australasia. Data from this study showed that both the introduction and the expansion of an orthogeriatric service or a FLS were effective in reducing 30-day and 1-year mortality following a hip fracture. This information was presented at an international workshop held in Milan that was targeted at health-care professionals who were either in the process of setting up a FLS or considering it. The findings of this study support the effectiveness of fracture prevention services in reducing mortality and may persuade more hospitals worldwide to adopt this model of care and set up a FLS.
Clinical Practice Research Datalink codes for fragility fracture
As part of this study we created a robust list of CPRD Read codes for fragility fractures, osteoporosis-related medications and comorbid conditions. A rigorous procedure was used to generate the lists, with input from two clinicians who each generated two separate lists and then discussed and agreed on any discrepancies. Generating such a thorough and extensive list is a time-consuming process and organisations often do not share lists of codes with each other once these are generated, so the procedure is often repeated. We now have a thorough and extensive list of Read codes which can be used by other researchers studying osteoporosis or fragility fractures. We have shared this list with researchers from the Falls and Fragility Fracture Audit Programme being undertaken by the Royal College of Physicians. This is a national clinical audit designed to audit the care that patients with fragility fractures and inpatient falls receive in hospital and to facilitate quality improvement initiatives.
Establishing levels of fracture prevention services in hospitals in Spain
Despite national and international guidance from various professional organisations such as the BOA, NICE and the IOF, there is major variation in the care pathway for the treatment and management of hip fracture patients and in the way secondary fracture prevention services are organised across hospitals in the UK. This study began by establishing whether or not a FLS or other type of fracture prevention service (such as an orthogeriatric-led service) was in place at 11 different hospitals in one region of England. A questionnaire for clinicians was developed to gather this information and showed large variations in services across hospitals in one region of England. This work was presented to a group of clinicians in Spain. Following this presentation, the group that we met with intend to use this questionnaire to gather information about FLSs across hospitals in Spain. This will enable them to identify hospitals with the most need for improvement to target for a programme to develop FLSs.
Chapter 10 Final conclusions
Guidance
National guidance from a number of professional bodies both within and outside the UK has been published for the management of hip fracture patients,1,14,15,22,32 in addition to international guidance such as the ‘Capture the Fracture’ initiative from the IOF. 34 According to this guidance, a comprehensive secondary fracture prevention service should consist of four main components – case finding, osteoporosis assessment, treatment initiation, and treatment adherence and monitoring1 – in addition to falls risk assessment and management. Organising such services is challenging owing to the multidisciplinary care patients require. 3 Expert consensus recommends that the optimal service model for effective delivery of secondary fracture prevention services in hospitals requires a co-ordinator-based system of care to provide a link between all of the multidisciplinary teams involved in fracture prevention;10 this is known as a FLS. The model proposed by the Department of Health in the UK is that delivered by a nurse specialist supported by a lead clinician (‘champion’) in osteoporosis. 1
Evidence of clinical effectiveness and cost-effectiveness
The evidence regarding the clinical effectiveness and cost-effectiveness of FLS models that have been used to inform this guidance is limited and has many important weaknesses. The review of the Glasgow Osteoporosis and Falls Strategy reported that hip fracture rates in the city had reduced by 7.3% over the decade 1998–2008, compared with a 17% increase in fracture rates for the entire population of England. 24,35 However, the prescribing rate in the control group was estimated from national audit data; all patients were assumed to remain on treatment for 5 years despite no active monitoring programme from the FLS; and the effect of treatment on fracture rate reductions was estimated using published trials. Data from the Kaiser SCAL study reported a 37.2% reduction in hip fracture rates. 25 However, this was, in essence, a screening programme and there was no contemporary control arm, and the reduction in hip fractures may have been largely driven by a reduction in primary rather than secondary hip fractures. In the Concord study of non-vertebral fracture,21,26 patients electing to receive care within the intervention programme were found to be at an 80% reduced risk of subsequent fracture, compared with patients remaining within standard care. However, using patients who did not attend the specialist clinic as the ‘comparator’ group meant that those who attended were more likely to be healthier and have fewer comorbidities, and so have a lower risk of refracture and higher adherence to therapy. A subsequent trial assessing the effect of the monitoring service on adherence was unable to show a significant difference. 161 The non-randomisation of allocation to co-ordinated versus standard care may have influenced persistence to therapy in those on the intervention programme (95% remaining on initial treatment). Finally, only 20% of all fragility fracture patients attend the specialist service, as those with cognitive impairment and other serious comorbidities were excluded, limiting the generalisability of the study findings.
There are three studies assessing the cost-effectiveness of models of care for the prevention of fractures. The Canadian study by Majumdar et al. 97 reported the intervention to be both cost saving and more effective than usual care. However, the model was based on published literature and a small clinical trial of 220 patients followed up for 1 year. The clinical trial did not evaluate the impact of the intervention in terms of refracture rates, quality of life or life expectancy, and excluded patients with hip fracture who were admitted from a care home. The Australian study by Cooper et al. 21 compared an outpatient-based FLS with patients treated in primary care, and reported the FLS to be highly cost-effective. No results were provided for the subgroup of hip fracture patients, and the authors did not extrapolate the findings to lifetime. The UK study by McLellan et al. 20 compared a FLS for the prevention of further fractures with the absence of FLS using audit data from the Glasgow FLS. The authors reported the FLS to be more effective and less costly than no FLS. However, no results were provided for a population solely composed of hip fracture patients and there was no comparable control group. Hence, the authors had to rely on published literature and assumptions to model the impact of FLS on fractures relative to its absence. The remaining model inputs were also derived from a range of sources and fracture populations resulting in several assumptions as to how best to synthesise the available data.
This highlights the need for robust data on key clinical outcomes and cost-effectiveness of FLS models in order to prioritise, guide and inform commissioning decisions.
Main findings
The service evaluation provided evidence of significant variation in the way 11 hospitals in a region of England organise and structure the delivery of secondary fracture prevention for hip fracture. This included variation in current levels of staffing of professionals involved in providing FLS: across hospitals in the region, overall orthogeriatric staff ranged from 1 WTE per 1000 patients to 9.6 WTE per 1000 patients, and overall fracture liaison or specialist nursing staff ranged from zero input to 7.6 WTE per 1000 patients. There were different types of co-ordinator-based models of care which could be either consultant-led or nurse-led services. There was also variation in the processes used by each hospital to case find, assess for osteoporosis and risk of future falls, initiate bone protection treatment and undertake falls prevention and monitor patients. By characterising the changes hospitals made to service delivery over the past decade, this demonstrated marked increases in the provision of orthogeriatric and/or FLS nurse staff in each of the hospitals, although there was little relationship of staffing levels to the size of hospitals’ hip fracture catchment population. The scale of variation in the way hospitals in the region organise their services highlights the need to link investment in these specialist posts with impact on clinical outcomes and cost-effectiveness.
Quality of evidence: although within a regional area of England, a strength of this evaluation is the heterogeneity of NHS hospitals examined from smaller district general hospitals to large tertiary major trauma centres, adding to generalisability. The service evaluation enabled us to characterise the dates and timing of key changes to service delivery together with underlying detail of processes used to case find, assess for osteoporosis, initiate treatment and monitor. This formed detailed data on the interventions of interest used in subsequent analyses. A limitation is the reliance on clinicians’ recall and understandings of events over the past decade.
A qualitative research study was undertaken to understand how and why secondary fracture prevention services could be successfully implemented. A total of 43 semistructured interviews were conducted with health-care professionals and managers involved in delivering secondary fracture prevention within 11 hospitals in the region; these included orthogeriatricians, fracture prevention nurses, hospital practitioners in osteoporosis and service managers. The capacity of health-care professionals to co-operate and co-ordinate their actions was achieved by using dedicated fracture prevention co-ordinators to organise important processes of care. However, participants described securing communication and co-operation with GPs as challenging. Individual potential and commitment to operationalise services was generally high. Shared commitments were promoted through multidisciplinary team working, facilitated by fracture liaison co-ordinators. Health-care professionals had the capacity to deliver multiple components of services when co-ordinators ‘freed up’ time. Aside from the difficulty of co-ordination with primary care, FLSs were seen as highly workable and easily integrated into practice. Nevertheless, successful implementation was threatened by understaffed and under-resourced services, a lack of capacity to administer DXA scans and difficulties that patients encountered in accessing services. To ensure ongoing service delivery, the contributions of health-care professionals were shaped by planning in multidisciplinary team meetings, the use of clinical databases to identify patients and define the composition of clinical work, and monitoring to improve clinical practice. Identifying issues that impact on the implementation of facture prevention services after hip fracture provides information to health-care professionals and service managers on how best to implement services for patients in the future.
The qualitative study also explored the experiences of clinicians and service managers of developing and making business cases for a FLS. Challenges in the development of business cases included collecting all of the relevant data and negotiating compartmentalised budgets that impeded service development. Participants described communication and co-operation between providers and commissioners as variable. They felt that financial considerations were the most important factor in funding decisions, while improved quality of care was less influential. Other factors included national guidelines and political priorities. The personalities of clinicians who were championing services, and the clinical interests of commissioners, were seen to influence the decision-making process. Participants identified a number of strategies that they thought were particularly helpful in the successful development of business cases. These included support, enhancements to co-operation between stakeholders, and the demonstration of potential cost-effectiveness and improved quality of care. Participants felt that the work of commissioners and providers should be better integrated and suggested strategies for doing this. The study provides information to health-care professionals and service managers about how best to develop business cases for a FLS in the future.
Quality of evidence: the study did not aim to achieve data saturation but used criterion sampling to explore a diverse range of views, and this was successfully achieved. However, only five service managers were recruited and their lack of representation made it difficult to fully examine how their opinions on making business cases differed from those of other participants. FLS are complex interventions, and a strength of the study is the use of NPT as a theoretical framework in order to help to understand something of the complexity of change within health services. Robust strategies were used to analyse the data, such as independent double-coding by two researchers, providing confidence that the analysis presented reflects views of participants. Participants were asked to recall their experiences and this may have resulted in bias. The study is on the perspectives of professionals working in secondary care and hence does not reflect the views of those in primary care settings.
A natural experimental study design was used to evaluate the clinical effectiveness of orthogeriatric and nurse-led FLS models of post-hip fracture care in terms of impact on mortality and rates of second hip fracture. The interventions were broadly defined as the introduction or expansion of either an orthogeriatric or a FLS model of post-hip fracture care, with information on the nature and timing of such changes obtained through the service evaluation. HES data linked to ONS mortality records were obtained on 33,152 hip fracture patients across the 11 hospitals in the region between 2003 and 2013. Of these patients, 1288 (4.2%) sustained a secondary hip fracture within 2 years, while for 30-day and 1-year mortality this was 9.5% (n = 3033) and 29.8% (n = 9663), respectively. Overall, age- and sex-standardised 1-year mortality declined from 33.1% to 26.0% from 2003/4 to 2011/12. In contrast, the proportion of second hip fractures remained stable throughout the study period. The pooled estimated impact of introducing an orthogeriatrician on 30-day and 1-year mortality was HR 0.73 (95% CI 0.65 to 0.82) and HR 0.81 (95% CI 0.75 to 0.87), respectively. Thirty-day and 1-year mortality were likewise reduced following the introduction or expansion of a FLS: HR 0.80 (95% CI 0.71 to 0.91) and HR 0.84 (95% CI 0.77 to 0.93), respectively. There was no significant impact on time to secondary hip fracture following any of the interventions when analysed separately or when pooled by type of intervention: orthogeriatrician (SHR 0.95, 95% CI 0.79 to 1.15) or FLS (SHR 1.03, 95% CI 0.82 to 1.31). The study provides evidence that the introduction and/or expansion of such services was associated with a large beneficial effect on subsequent mortality. Reassuringly, the effect was consistent across hospitals and for interventions that occurred at different time points over the study period. There was no evidence for a reduction of second hip fracture.
Quality of evidence: our preferred approach would have been to use an interrupted time series design, as this would have given the highest level of evidence of effect. However, there was insufficient statistical power to do so, owing to either (a) not enough pre- and post-intervention time points, either as interventions occurred at the beginning or end of the time series, or having multiple interventions; or (b) insufficient numbers of observations at each time point for rare outcomes such as second hip fracture. In terms of levels of evidence, we instead used the next best observation study design, using a before-and-after impact survival analysis in which each hospital acted as its own control. As this method can introduce bias by not accounting for pre-existing secular trends, we excluded from analyses interventions that were preceded by a significant trend in the respective health outcome. Strengths of the survival model are that it allows for greater adjustment of confounding factors, and the ability to account for competing risk of death with second hip fracture outcome. A strength of the study is that interventions were identified a priori through the audit before data were obtained, and the statistician analysing the data was blinded to what the intervention was and to the audit findings (knowing only the date the intervention occurred). A main limitation is that other events coinciding with the interventions of interest here evaluated cannot be ruled out, such as the publication of national guidelines. This is unlikely given that the estimated impact on each health outcome was consistent across hospitals and for interventions that occurred at different time points over the study period. Further limitations are that we could not assess outcomes seen outside a secondary care setting, as these were not captured in the routine data, and that we could not assess osteoporosis medication use and adherence to treatment.
A natural experimental study design was used to assess the effect national guidelines have had on altering trends in refracture rates, life expectancy (30 days and 1 year) and the proportion of patients taking bone-strengthening drugs within 1 year after fracture. Five specific guidelines were evaluated: NICE CG 21 (November 2004),15 NICE TA 87 (January 2005),33 BOA Blue Book (September 2007),1 NICE TA 161 (October 2008)13 and Best Practice Tariff for inpatient hip fracture care (April 2010). 76 Data from the CPRD linked to ONS mortality records were obtained on 11,243 primary hip fracture cases aged > 50 years occurring between 1 April 1999 and 31 March 2013. Initiation of antiosteoporosis medication within 12 months of primary hip fracture increased markedly during the study period (1999–2000 compared with 2011–12) from 8.1% to 53.9%. However, sex differences were observed, with fewer men initiating treatment than women, and this gap increased over time. Importantly, among treatment-naive hip fracture patients the prevalence of bisphosphonate use at 10–14 months post index hip fracture increased over the study period from < 5% to > 30%, suggesting that more patients were adhering to treatment. A step-change reduction in subsequent hip fracture of –0.95% (95% CI –1.67% to –0.23%) between October 2007 and September 2008 (publication of the BOA Blue Book1 and NICE TA 16113) was found. However, this was not observed for subsequent major non-hip fracture. A significant step-change reduction in 30-day mortality occurred of –2.81% (95% CI –3.73% to –1.85%) between October 2007 and September 2008 (publication of the BOA Blue Book1 and NICE TA 16113), with no effect on 1-year mortality. However, a significant reduction in 1-year mortality of –5.56% (95% CI –7.59% to –3.52%) was seen immediately following the introduction of the Best Practice Tariff in April 2010. There was a marked step change in the proportion of primary hip fracture patients receiving an incident prescription for a bone-strengthening drug of 14.5% (95% CI 11.1% to 17.8%) taking place between pre-publication of NICE CG 21 (November 2004)15 and post-publication of the NICE TA 87 (January 2005). 33 The proportion of patients prescribed at least one bisphosphonate at 10–14 months following index hip fracture showed a step-change increase of 8.71% (95% CI 5.04% to 12.4%) between pre-publication of NICE CG 21 (November 2004)15 and post-publication of the NICE TA 87 (January 2005)33 followed by a modest step-change decrease of –3.79 (95% CI –7.4 to –0.17) between October 2007 and September 2007 (publication of the BOA Blue Book1 and NICE TA 16113). The study provides evidence of significant temporal associations with a number of national guidelines suggesting that these guidelines have positively impacted on clinical decision-making and then on patient outcomes.
Quality of evidence: strengths include the large number of primary hip fractures and the generalisability of the CPRD cohort to the general UK population. We used an interrupted time series analysis that allows for baseline level and pre-intervention trend and is self-controlled by design. Hence the results are valid in the presence of a pre-existing downwards secular trend. We were able to look at trends in both hip-fracture and non-hip fracture outcomes, and treatment initiation and adherence to osteoporosis medication. The main limitation is the possibility that changes in outcomes here associated with national guidelines were confounded by other events occurring during the same time period. The use of routinely collected data with no individual validation of fracture events recorded is another limitation of the analysis; however, validation of hip and vertebral fracture coding has been carried out previously and been shown to be accurate.
A health economics study was conducted to estimate the primary care and hospital costs of hip fracture up to 2 years post fracture and compare costs before and after the index fracture. For hospital costs, we used the HES database of 33,152 hip fracture patients and for primary costs we used CPRD GOLD with 4433 hip fracture patients, over the years 2003 to 2013. Within the first year following primary hip fracture, the total hospitalisation costs were estimated to be £13,826 (median £10,425, SD £11,016), of which 75% were a result of hip fracture-related admissions (£10,375, median £8050). The total hospital cost (including outpatient and emergency care) in the year of the fracture was estimated to be £14,264 (95% CI £14,092 to £14,436), which was £10,964 (95% CI £10,767 to £11,161) higher than the previous year. The primary care costs associated with index admission for primary hip fracture were £1065 (median £660, SD £1798), of which medications and non-pharmaceuticals accounted for £614 (median £248, SD £1586) of the costs and GP contacts accounted for £358 (median £246, SD £409). When we considered only the 2-year survivors, compared with the year prior to the hip fracture, primary care costs were £256 (95% CI £160 to £273) and £273 (95% CI £167 to £380) higher in the first and second year following hip fracture, respectively. This was mostly led by a considerable increase in GP contacts and in the costs of prescribed items. Hence the total primary care and hospital care cost in the year of the hip fracture was estimated to be £15,329, which, when extrapolated to all incident hip fractures in the UK among those aged ≥ 50 years (n = 79,243), resulted in a cost of £1215M in the year of the fracture. There is a strong economic incentive to prioritise research funds towards identifying the best approaches to prevent index and subsequent hip fractures. Furthermore, the total primary care and hospital care cost in the second year after hip fracture, conditional on surviving the first year, was estimated to be £4242, of which £3072 was a result of hospital care and £1170 was a result of primary care.
A cost-effectiveness analysis was undertaken to determine whether or not introducing an orthogeriatric or a nurse-led FLS model for post-hip fracture care in hospital is cost-effective when compared with usual care in the English NHS. A decision analytic (Markov) model was developed to evaluate the costs, (quality-adjusted) life expectancy and cost-effectiveness of the different models of secondary care hip fracture prevention under evaluation. The data sources used to inform the model inputs consisted of HES, CPRD GOLD and published literature. Data on clinical effectiveness of orthogeriatric and FLS models were obtained through the natural experimental study. For male patients, combining all health and social care costs included in the model, mean discounted costs were £41,714 when an orthogeriatrician is introduced, £41,068 when a fracture liaison nurse is introduced and £39,101 for usual care. The discounted average QALYs gained by male patients were 1.74 with an orthogeriatrician, 1.72 with a fracture liaison nurse and 1.62 with standard care. For female patients, mean discounted costs were £53,104 when an orthogeriatrician is introduced, £52,472 when a fracture liaison nurse is introduced and £50,573 if usual care is provided. The discounted average QALYs gained by female patients were 2.50 with an orthogeriatrician, 2.48 with a fracture liaison nurse and 2.38 with standard care. After combining costs and outcomes in an incremental cost-effectiveness analysis, and at a £30,000 per QALY threshold, the most cost-effective model of care was introducing an orthogeriatrician. The probability of adding an orthogeriatrician being the most cost-effective option at £30,000/QALY was estimated to about 70% across both sexes. The population EVPI over 5 years was estimated to be between £23M and £73M at the £30,000 per QALY gained threshold. This suggests that undertaking additional major research work to further reduce decision uncertainty is likely to be of significant benefit.
Quality of evidence: a strength is that the Markov model structure and assumptions were informed by the hip fracture, the needs of the decision problem and discussions with clinical experts, health economists, statisticians and epidemiologists involved in the project. This meant that an iterative process was used to define the model structure. The work benefits from the availability of large primary and secondary care administrative data sets that enabled the robust estimation of the impact of the models of care in terms of survival, prevention of second hip fracture, primary care and hospital care costs and cost-effectiveness. Limitations were that we had to obtain health state utility values from a review of the published literature. Furthermore, it was not possible to reliably estimate utility values for non-hip fractures or the additional impact these may have on the quality of life of individuals with a history of hip fracture. Hence, assumptions were needed but these were fully explored in sensitivity analysis. The work does not allow for non-health-care costs, resulting from the use of social care services, admission to care homes and provision of unpaid care by friends and relatives.
Research in context
The finding that orthogeriatric and FLS models of care are associated with lower mortality is consistent with other findings in the literature. 94,101,121 It is very plausible that the role of the orthogeriatrician has implications for the mortality risk for hip fracture patients. The mechanism is highlighted through characterisation in the service evaluation and understanding of the changes made to service delivery by orthogeriatricians appointed at hospitals in the region to lead care for hip fracture patients, with examples given as follows.
Hospital 2
The involvement of an orthogeriatrician in the care of hip fracture patients has ensured that the majority of patients admitted with a hip fracture are now seen preoperatively and the orthogeriatrician attends the daily trauma meetings and does a daily ward round (assisted by an elderly care specialist registrar). This allows patients to reach theatre quicker and with less physical deterioration by optimising any preoperative condition, such as pre-existing medical comorbidity and acute conditions, ensuring that those taking warfarin are identified and assessing fitness for anaesthesia. Involving the orthogeriatrician in postoperative care ensures early identification and treatment of complications such as chest infections and myocardial infarction. Rehabilitation goals are set with the orthogeriatrician leading multidisciplinary team meetings.
The impact of introducing an orthogeriatrician at this hospital on 30-day and 1-year mortality was HR 0.76 (95% CI 0.58 to 0.99) and HR 0.78 (95% CI 0.67 to 0.91), respectively. In the year pre intervention the number of deaths at 30 days and 1 year was 45 (12.0%) and 121 (32.2%), respectively and, after intervention, this has now fallen to 29 (6.9%) and 108 (25.6%) deaths, respectively.
Hospital 7
The consultant geriatrician started to focus on hip fracture in July 2004. The geriatrician’s sessions have increased over time to 30.5 hours per week, and an extra session was added in January 2012. There is also a specialty doctor in geriatrics. The service has changed from providing reactive care to becoming a service that sees all patients preoperatively and postoperatively, assesses and manages falls and bone health, and provides discharge planning. Patients are now seen on a dedicated hip fracture ward. The hip fracture unit works in a collaborative fashion, and uses a care pathway and multidisciplinary paperwork. In the unit, two fast-track beds are available, and the unit operates within 36 hours to meet the Best Practice Tariff. The hip fracture unit has been crucial in building expertise and experience.
The impact of introducing an orthogeriatrician at this hospital on 30-day and 1-year mortality was HR 0.69 (95% CI 0.54 to 0.89) and HR 0.85 (95% CI 0.73 to 0.99), respectively. In the year pre intervention the number of deaths at 30 days and 1 year was 50 (8.5%) and 172 (29.4%), respectively, and, after intervention, this has now fallen to 38 (6.1%) and 162 (26.1%) deaths, respectively.
Hospital 8
On starting in post, the clinical lead orthogeriatrician reviewed the existing service. Based on this review, the orthogeriatrician changed the pathway after 6 to 8 months in post, moving the service towards an acute model of care for fragility fracture patients. There is now a joint trauma round that includes weekends run by the clinical lead orthogeriatrician and the trauma lead who started at the same time (March 2009) (this is now run jointly by the clinical lead and a consultant orthogeriatric surgeon who started in July 2012). There are six consultant ward rounds per week, resulting in a total of 7.5 Direct Clinical Care. There is a trauma meeting with orthopaedic surgeons at which they ensure that all patients are seen. All patients with fragility fractures who are admitted at the hospital are placed under the care of the orthogeriatric team. They see patients of all ages with any fragility fracture. Younger patients are identified and seen for further follow-up. Older fragility fracture patients (and all hip fracture patients whether aged over or under 70 years) are seen by day 2, and by day 3 on the ward. On the weekends there is another geriatrician who provides cover. Around 90% of patients are seen preoperatively to optimise them for surgery. This all matches the criteria given in the Best Practice Tariff. 76
The impact of introducing an orthogeriatrician at this hospital on 30-day and 1-year mortality was HR 0.57 (95% CI 0.45 to 0.73) and HR 0.81 (95% CI 0.70 to 0.95), respectively. In the year pre intervention the number of deaths at 30 days and 1 year was 68 (12.8%) and 176 (33.2%), respectively, and, after intervention, this has now fallen to 33 (6.5%) and 124 (24.3%) deaths, respectively.
The reasons for a significant decrease in mortality following the introduction and/or expansion of a FLS model of care are not as clear as for the orthogeriatric model. Implementation of osteoporosis guidelines by a fracture nurse has previously been shown to be associated with a 33% reduction in post-fracture mortality following any index fragility fracture, prompting the conclusion that measures to prevent fractures also reduce mortality. 101,121 While FLSs conceivably contribute to an environment of better co-ordination of care with better communication between staff, it may be that the appointment of such nurse specialists reflects wider underlying changes to a hospital’s service delivery that led up to the successful implementation and change in hospital care model. In addition, a RCT has indicated that patients receiving zoledronic acid were at 28% reduced risk of death, compared with those receiving placebo,119 yet only 8% of such a reduction can be attributed to lower fracture incidence on treatment. 120 The mechanisms that mediate the remainder of the drugs effect are not known, although an effect on cardiovascular events and pneumonia may play a role.
To our knowledge this is the first study to evaluate the FLS model in terms of impact on hip refracture rate after primary hip fracture. We found no evidence that FLS models of care reduce the risk of second hip fractures. The average annual age- and sex-standardised proportion of second hip fractures was just 4.2% for hospitals in this region of England, and remained unchanged and stable through the study period from 2003 to 2013. Although this might seem surprising, given the positive results reported by the Glasgow,24 Concord21 and Kaiser SCAL studies,25 there are many important key limitations associated with these studies, as described earlier, that may explain this discrepancy.
To try to understand the reason for the lack of association, it is necessary to tease apart the key elements of a FLS: case finding; osteoporosis assessment including a DXA scan to measure bone density if appropriate; treatment initiation with bone protection therapy in osteoporosis patients; and systems to improve adherence and persistence with therapy. As part of the qualitative study, we identified the elements of care of hip fracture patients that health professionals think are most effective in preventing secondary fractures after hip fracture. This included the processes for undertaking the four main components of a fracture prevention service and co-ordination of care.
Case finding
Participants felt that such patients were relatively easy to identify as they were invariably admitted and remained in hospital for a period of time and, therefore, presented a ‘captive audience’. Attendance of fracture prevention co-ordinators or other health-care professionals at daily preoperative trauma meetings was seen as effective at identifying patients at risk of further fractures. Perioperative or postoperative ward rounds were successfully used to identify patients, although participants were concerned that patients seen perioperatively could be missed if they were particularly ill or when staff were absent, as it meant that there was no one to identify cases. To mitigate this, participants emphasised the importance of a back-up such as computerised databases to identify patients retrospectively. The processes underlying case finding are done well for this element of a FLS.
Osteoporosis assessment
This was best done in an inpatient setting, when possible, to enable clinicians to initiate bone protection therapies more quickly. A risk of ‘losing’ patients after discharge was identified, as at that time they may not receive appointment letters, may be too frail or may forget to attend appointments. Participants were of the opinion that services should adhere to NICE guidelines by providing DXA scans to those aged < 75 years and treating those aged ≥ 75 without the absolute need for DXA. The location of the DXA scan, whether in an inpatient setting, an outpatient setting or a community hospital, was seen to impact on whether or not patients received it. Conducting a scan in the post-discharge outpatient setting gave patients time to recover from their operation, but this was tempered by concern about the failure of patients to attend appointments. This was seen as being particularly problematic when patients lived a long way from the scanner. The process of referral to scans was important; while some orthogeriatricians and fracture prevention nurses described how they were able to refer patients directly, others had to request an appointment via primary care. Interviewees felt that the latter approach was problematic, as it meant that patients had to wait longer, delayed the start of treatment and made it more likely that patients would ‘get lost’ in the system. In the < 75 years age group there is greater potential for patients to miss receiving osteoporosis assessment and not receive bone protection therapy if needed, although only a small minority of hip fractures patients are aged < 75 years.
Treatment initiation
Patients who were aged ≥ 75 years and who did not need a DXA scan should have their treatment initiated in an inpatient setting when possible. This meant that they could begin treatments more quickly and enabled clinicians to assess whether or not they could tolerate it. However, some clinicians were concerned that patients were sometimes too ‘shocked’ postoperatively to understand how and why they were taking the therapies, which could impact on adherence. Some participants were worried that patients were not being prescribed treatments while they were in an inpatient setting. One clinician had found it useful to put ‘checks’ in place so that his or her colleagues were able to spot if they had not been prescribed. Furthermore, treatments were not always included on discharge summaries. For those aged < 75 years who received a DXA scan or who needed treatments that could not be initiated immediately, therapies were initiated either in primary care or in an outpatient setting. However, there was a concern that this was not being done consistently in primary care and that GPs lacked ‘alertness’ about the importance of the therapies.
Further information is provided by correlating this with statistical analysis of national data from CPRD in Chapter 6. Figures 13–15 show that there has been a substantial increase in the prescribing of antiosteoporosis medication within 12 months of hip fracture, particularly since 2004. However, differences are observed stratified by age. In those aged > 85 years, prescribing rates have increased from 10% to 50%, and in those aged 75–84 years they have increased from 20% to 55%, but in those aged < 75 years the increase has been smaller, from around 20% to 40%. This would fit with qualitative data that younger patients may be missed or that in earlier years there was differential undertreatment of the older patient. An aspect not addressed in the qualitative study is a sex effect (see Figure 13), whereby the national increase in antiosteoporosis medication is much higher in women than in men. There is potential for the < 75 years age group, and for male patients, to be less likely to initiate osteoporosis therapy.
Monitoring
Participants were most concerned about the low levels of adherence to bone protection therapies and felt that monitoring had the potential to improve adherence to oral therapies. A number of participants thought that zolendronic acid could help improve adherence, as it is given at a clinic rather than at home and given once per year rather than being taken regularly. However, they also described how the provision of this therapy was often constrained by local guidelines as well as by the requirement to have good kidney function, an issue with many patients. Patients prescribed oral bisphosphonates had their monitoring delegated to primary care. However, there was a worry that this management was often ‘suboptimal’ and some participants were concerned that GPs did not always have enough knowledge to monitor patients effectively. On account of these problems, participants thought that more monitoring could be conducted by secondary care. However, there was no consensus on how this could be systematically achieved. Participants discussed the relative advantages and disadvantages of using questionnaires, telephone calls and outpatient appointments to perform monitoring.
Within this study, the service evaluation highlights how monitoring was undertaken by secondary care at seven sites and the remainder was delegated to GPs. Monitoring by secondary care included telephone calls and questionnaires. Only one hospital had a FLS that included a monitoring pathway, but, as this happened at the end of 2011, it was not possible to examine the impact of this on outcomes, as the HES data for our analysis were collected up to the end of 2013. The IOF adherence gap report162 highlights that up to 60% of patients who take a once-weekly bisphosphonate and nearly 80% who take a once-daily bisphosphonate discontinue treatment within 1 year.
Monitoring and adherence to therapy is the weakest element of FLS for hospitals within the region, and was highlighted as a clear cause for concern by health professionals in the qualitative study. This is a key element that could explain the lack of effectiveness of the FLS intervention.
The FLS model of secondary fracture prevention is centred on the efficacy of antiresorptive drugs. It is widely known that the risk of further fracture can be reduced by up to half with bone protection therapy. 1,11–13 The clinical effectiveness of these drugs is reviewed in NICE TA 161 guidance, in which it is noted that for non-vertebral fracture types, individual data on hip, leg, pelvis, wrist, hand, foot, rib and humerus fractures were sometimes provided, whereas some studies presented only data for all non-vertebral fractures grouped together. In their consideration of the evidence it is interesting that they note that ‘all these drugs have proven efficacy in reducing the incidence of vertebral fragility fractures in women with osteoporosis, but that there were differences between the drugs as to the degree of certainty that treatment results in a reduction in hip fracture’. They considered evidence for effectiveness to be robust for alendronate and risedronate, but not for etidronate, strontium and raloxifene, and the effect of teriparitide required more research. Data from 14 RCTs indicated that between 81% and 100% of patients persisted with bisphosphonates in the first year of treatment. This contrasts starkly with adherence and persistence outside the clinical trial setting, which is substantially lower.
Statistical analysis of our UK data from CPRD shows that the increase in bisphosphonate use is largely driven by the use of alendronate, with minimal use of other bisphosphonate therapies, for which the trend over the past decade has been largely flat. This is reassuring, given the evidence of effectiveness of alendronate from RCTs.
Although oral bisphosphonates have been demonstrated to be effective in a trial setting, there is a lack of generalisability in terms of real-world effectiveness, largely driven by issues in adherence and persistency in therapy. In particular, the oral bisphosphonates, although taken only weekly, have a very complicated method of administration that needs to be carefully followed to ensure reasonable bioavailability. Hence, the lack of association observed in our study may reflect the poor quality of the monitoring element of FLS in the hospitals in the region of study.
Problems relating to adherence and persistence with therapy can be overcome with intravenous (i.v.) bisphosphonates, which need administering only once per year. Data from RCTs have shown significant reductions in subsequent fracture risk among hip fracture patients on i.v. antiresorptive treatment in all patients, including those with cognitive impairment. 119 However, specifically in this trial, zoledronic acid has only been shown to significantly reduce the risk of second hip fracture after a primary hip fracture after 12 months, with the same observed for the composite outcome of all non-vertebral fractures. In addition, generalisability is primarily affected by the trial excluding patients with a life expectancy of < 6 months in the investigator’s judgement. We could not address this exclusion criterion within our study.
Our data from the natural experiment highlight that 35% and 63% of secondary hip fractures within 2 years occurred within the first 6 and 12 months, respectively. Further in our hip fracture population, the mortality rates were 9.5% and 29.8% at 30 days and 1 year, respectively, with an average life expectancy of just over 2 years.
Although i.v. medications such as zoledronic acid overcome problems with adherence to therapy monitoring, a move from oral to i.v. bisphosphonates may not be as clinically efficacious as perceived owing to (1) the high mortality within the first year of hip fracture; (2) the fact that the majority of second hip fractures occur within the first year; and (3) the effectiveness of zoledronic acid on reducing secondary fractures occurs after the first year. A further consideration is that many patients will not be eligible for treatment with zoledronic acid owing to their poor renal function.
Final conclusions
The finding in relation to the beneficial effects of orthogeriatrician and FLS models of care on reducing 30-day and 1-year mortality is a very positive one. The health economics analysis shows that these models of care are cost-effective.
We found that in hip fracture patients a FLS was not effective at reducing the risk of second hip fracture. Although this was initially a surprising finding, combining the data from both the qualitative and quantitative components of the study has helped us to understand the reasons behind the lack of a statistical association. There is a need for more monitoring of patients and work to enhance adherence to bisphosphonate therapy if FLSs are to provide effective care. This could be achieved by treating patients with i.v. zoledronic acid (a drug which has recently become generic), and through interventions intended to improve persistence with antiresorptive therapy. 163
For the project as a whole, a great strength is the use of a mixed-methods approach, using qualitative research models, together with statistical and health economic analysis that take advantage of large routinely collected data sets from primary and secondary care. The methodology used throughout was robust, with the use of extended NPT as appropriate for complex interventions, a natural experimental study design with before-and-after impact analysis, and Markov modelling. A strength of mixed-methods study is the ability to integrate findings from the different work streams. This was helpful in attempting to understand the reasons why no effect was observed for FLS models on second hip fracture outcome, when information was synthesised from qualitative and quantitative data in order to provide deeper understanding and learning. The observational nature of the study is a limitation, as the best evidence of effect would ideally be obtained through a RCT. To determine the clinical effectiveness and cost-effectiveness of FLS service models requires comparison of hospitals with and without a service, as well as between different service models. As a complex intervention there is also a need to fully understand the intervention, including which are the most effective key components. Given the complexity, projected sample size, ethical issues, cost and lag time in setting up such services, individualised or cluster randomised control designs are not logistically possible or feasible. Our mixed-methods approach using a natural experiment with large routinely available data sets is the most efficient approach to address the question. As this is not a trial, the limitations are mainly in relation to unobserved and unmeasured residual confounding. This is, however, minimised by using a quasi-experimental approach with a before-and-after time series design with each hospital acting as its own control, and provides the next best level of evidence as a study design. In this context the limitation would be through time-varying confounding such as the comorbidity profile of hip fracture patients changing over time. Characterisation of the intervention is essential, to understand mechanism of effect but also measurement error relating to timing of intervention. Although we conducted a service evaluation supplemented by qualitative interviews, this is still subject to recall bias. A strength of the study over a clinical trial is in respect of the generalisability of the study findings in a real-world setting, in which we are not restricted by inclusion criteria and capture patients with cognitive impairment.
This study is in hip fracture patients only. The effectiveness of a FLS for non-hip fracture patients remains unanswered. In this study we were able to look at only second hip refracture as an outcome, as other non-hip fractures are not captured by the routine data used in the study. So, the effectiveness of a FLS for hip fracture patients on non-hip fracture outcomes also remains unanswered.
To inform a decision on the value of undertaking further research in order to eliminate the uncertainty surrounding the decision of cost-effectiveness of FLS models of care, the EVPI over 5 years was estimated at £23M–73M at the £30,000 per QALY gained threshold. This suggests that undertaking additional major commissioned research work to further reduce decision uncertainty is likely to be of significant benefit.
Implications for practice
This study supports the hypothesis that improving clinical care after hip fracture can reduce mortality, suggesting that, in this frail elderly multimorbid population, opportunities exist to avoid preventable death in the short and long term. The estimated impact of introducing an orthogeriatrician on 30-day and 1-year mortality was HR 0.73 (95% CI 0.65 to 0.82) and HR 0.81 (95% CI 0.75 to 0.87), respectively. 30-day and 1-year mortality were likewise reduced following the introduction of a FLS: HR 0.80 (95% CI 0.71 to 0.91) and HR 0.84 (95% CI 0.77 to 0.93), respectively. Assuming a pre-intervention survival of 90% at 30 days, the number of patients needed to treat to avoid one excess death at 30 days is 12 and 17 for orthogeriatric and FLS-type interventions, respectively.
After combining costs and outcomes in an incremental cost-effectiveness analysis, and at a £30,000 per QALY threshold, the most cost-effective model of care was introducing an orthogeriatrician. The probability of adding an orthogeriatrician being the most cost-effective option at £30,000/QALY was estimated to about 70% across both sexes.
The mechanism by which an orthogeriatric model of care can reduce subsequent mortality is clearer than for the FLS model. It is a plausible finding that is consistent with existing literature and from our knowledge of how the service changed detailed in the service evaluation. Ensuring that patients are seen preoperatively for optimisation for surgery and that patients are being taken to theatre quicker and in better condition are likely to be important factors given previous evidence that trauma-related complications play a key role in post-hip fracture mortality,115 and that earlier surgery is associated with lower risk of death. 116 A recent study similarly reported that hospitals with orthogeriatric services were found to have significantly lower mortality after hip fracture than hospitals without such services,94 and findings from RCTs also demonstrate the benefits of geriatric hip fracture care on mortality. 118
The orthogeriatric model of care also reflects national guidance29 on optimising initial recovery after hip fracture through optimising surgical procedure, including early timing of surgery, preoperative appropriate correction of comorbidities and type of implant; early mobilisation; and multidisciplinary management from admission to discharge.
Services should be commissioned to improve the quality of clinical care in the preoperative period for patients admitted with a hip fracture. Orthogeriatric models of care are increasingly being implemented in hospitals across the NHS. The NHFD has shown a significant increase in the number of consultant grade orthogeriatric hours per week since reporting began in 2009. This study provides evidence of the clinical effectiveness and cost-effectiveness of this service model. Whether or not there is a threshold for maximising the reduction in mortality and whether or not there is a target-adjusted mortality rate remains to be tested.
In hip fracture patients a FLS was not effective at reducing the risk of second hip fracture. The lack of effect on second hip fracture highlights the complexity of secondary fracture prevention in its overlay across secondary and primary care, the four stages (identification, investigation, initiation and monitoring) and across bone health and falls prevention intervention. It is likely that not every FLS is automatically clinically effective. There is a need for more monitoring of patients and work to enhance adherence to bisphosphonate therapy if FLSs are to provide effective care. This underscores the need for commissioning of current FLS to include auditing of the FLS using standardised methods (e.g. IOF Capture the Fracture Best Practice Framework and Royal College of Physicians FLS Database national audit). It also highlights that light-touch FLSs are unlikely to be effective and the need for an experimental research study to formally test the clinical effectiveness and cost-effectiveness of more complex and expensive secondary fracture prevention services.
Scope for future work
-
Further research is urgently needed to assess the clinical effectiveness and cost-effectiveness of FLS models for non-hip fracture patients. This question cannot be answered using the natural experimental design of this study, as the routine data are not available. This question can be answered only through conducting a RCT.
-
For hip fracture patients, the clinical effectiveness and cost-effectiveness of a FLS on non-hip refracture outcomes remain unanswered.
-
For the cost-effectiveness analysis, although a great proportion of the data used was derived from health-care records of patients with hip fracture, we had to obtain health state utility values from a review of the published literature. It was not possible to reliably estimate utility values for non-hip fractures or the additional impact these may have on the quality of life of individuals with a history of hip fracture. To remove uncertainty in the decision model, high-quality data on utility values are required.
-
The qualitative study was focused solely on the perspectives of professionals working in secondary care. Further work could explore their experiences of engagement with fracture prevention services and service provision in primary care. This would offer a comprehensive, ‘system-wide’ perspective that would overarch the division between primary and secondary care.
-
Further qualitative research should explore the experiences of hip fracture patients and their significant others of accessing these services to add a ‘patient-centred’ context to the implementation of these services.
-
The study focused on fracture prevention rather than falls prevention services. We acknowledge that these are inter-related and this represents an area of further qualitative and quantitative study.
Acknowledgements
Contributions of authors
Andrew Judge contributed to the conception and design of the project, the acquisition or analysis of data and the interpretations of the findings of this work.
M Kassim Javaid contributed to the conception and design of the project and the interpretations of the findings of this work.
José Leal contributed to the conception and design of the project, the acquisition or analysis of data and the interpretations of the findings of this work.
Samuel Hawley contributed to the acquisition or analysis of data and the interpretations of the findings of this work.
Sarah Drew contributed to the acquisition or analysis of data and the interpretations of the findings of this work.
Sally Sheard contributed to the acquisition or analysis of data and the interpretations of the findings of this work.
Daniel Prieto-Alhambra contributed to the conception and design of the project and the interpretations of the findings of this work.
Rachael Gooberman-Hill contributed to the conception and design of the project, the acquisition or analysis of data and the interpretations of the findings of this work.
Janet Lippett contributed to the conception and design of the project and the interpretations of the findings of this work.
Andrew Farmer contributed to the conception and design of the project and the interpretations of the findings of this work.
Nigel Arden contributed to the conception and design of the project and the interpretations of the findings of this work.
Alastair Gray contributed to the conception and design of the project and the interpretations of the findings of this work.
Michael Goldacre contributed to the conception and design of the project and the interpretations of the findings of this work.
Antonella Delmestri contributed to the acquisition or analysis of data.
Cyrus Cooper contributed to the conception and design of the project and the interpretations of the findings of this work.
All authors contributed to drafting this report or revising it critically for important intellectual content and have approved the final version.
All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
In addition, the authors would like to acknowledge the contributions of Laura Graham (Royal Shrewsbury Hospitals NHS Trust) for her help in interpreting and writing up the findings of the business cases work and Professor Carl May (University of Southampton) for his help in analysing qualitative findings applying NPT to the data.
The authors would also like to acknowledge the support of the NIHR Musculoskeletal Biomedical Research Unit, University of Oxford.
Publications
Drew S, Sheard S, Chana J, Cooper C, Javaid MK, Judge A, et al. Describing variation in the delivery of secondary fracture prevention after hip fracture: an overview of 11 hospitals within one regional area in England. Osteopors Int 2014;25:2427–33.
Drew S, Gooberman-Hill R, Farmer A, Graham L, Javaid MK, Cooper C, et al. Making the case for a fracture liaison service: a qualitative study of the experiences of clinicians and service managers. BMC Musculoskelet Disord 2015;16:274.
Drew S, Judge A, May C, Farmer A, Cooper C, Javaid MK, Gooberman-Hill R, et al. Implementation of secondary fracture prevention services after hip fracture: a qualitative study using extended Normalisation Process Theory. Implement Sci 2015;10:57.
Drew S, Judge A, Cooper C, Javaid MK, Farmer A, Gooberman-Hill R. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteopors Int 2016;27:1719–27.
Hawley S, Javaid MK, Prieto-Alhambra D, Lippett J, Sheard S, Arden NK, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing 2016;45:236–42.
Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, et al. Anti-osteoporosis medication prescriptions and incidence of secondary fracture among primary hip fracture patients in England and Wales: an interrupted time series and economic analysis. J Bone Miner Res 2016; in press.
Leal J, Gray AM, Hawley S, Prieto-Alhambra D, Delmestri A, Arden NK, et al. Cost-effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: a population based study. J Bone Miner Res 2016; in press.
Leal J, Gray AM, Prieto-Alhambra D, Arden NK, Cooper C, Javaid MK, et al. Impact of hip fracture on hospital care costs: a population based study. Osteoporos Int 2016;27:549–58.
Data sharing statement
Anonymised data included in Chapter 2 are included within the report. We are unable to share data related to Chapter 3 owing to the nature of the data generated not being suitable for sharing. Data obtained for Chapters 5–8 were sought through the Health and Social Care Information Centre and CPRD. Please contact the lead author for further information.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- The Care of Patients with Fragility Fractures. London: BOA; 2007.
- Dennison E, Mohamed MA, Cooper C. Epidemiology of osteoporosis. Rheum Dis Clin North Am 2006;32:617-29. http://dx.doi.org/10.1016/j.rdc.2006.08.003.
- Chesser TJS, Handley R, Swift C. New NICE guideline to improve outcomes for hip fracture patients. Injury 2011;42:727-29. http://dx.doi.org/10.1016/j.injury.2011.06.002.
- Cooper C, Mitchell P, Kanis J. Breaking the fragility fracture cycle. Osteoporos Int 2011;22:2049-50. http://dx.doi.org/10.1007/s00198-011-1643-9.
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633-50. http://dx.doi.org/10.1007/s00198-009-0920-3.
- Johnell O, Kanis JA, Odén A, Sembo I, Redlund-Johnell I, Petterson C, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int 2004;15:175-79. http://dx.doi.org/10.1007/s00198-003-1514-0.
- George GHM, Patel S. Secondary prevention of hip fracture. Rheumatology 2000;39:346-49. http://dx.doi.org/10.1093/rheumatology/39.4.346.
- Melton LJ, Kearns AE, Atkinson EJ, Bolander ME, Achenbach SJ, Huddleston JM, et al. Secular trends in hip fracture incidence and recurrence. Osteoporos Int 2009;20:687-94. http://dx.doi.org/10.1007/s00198-008-0742-8.
- Lloyd BD, Williamson DA, Singh NA, Hansen RD, Diamond TH, Finnegan TP, et al. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the sarcopenia and hip fracture study. J Gerontology A Biol Sci Med Sci 2009;64A:599-60. http://dx.doi.org/10.1093/gerona/glp003.
- Marsh D, Åkesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int 2011;22:2051-65. http://dx.doi.org/10.1007/s00198-011-1642-x.
- Knopp J, Diner B, Blitz M, Lyritis GP, Rowe BH. Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials. Osteoporos Int 2005;16:1281-90. http://dx.doi.org/10.1007/s00198-004-1798-8.
- Black DM, Arden NK, Palermo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. J Bone Miner Res 1999;14:821-28. http://dx.doi.org/10.1359/jbmr.1999.14.5.821.
- Osteoporosis – Secondary Prevention Including Strontium Ranelate. London: NICE; 2008.
- Management of Hip Fracture in Older Patients: A National Clinical Guideline. Edinburgh: Scottish Intercollegiate Guidelines Network; 2009.
- Clinical Practice Guideline for the Assessment and Prevention of Falls in Older People. London: NICE; 2004.
- Boulton C, Burgon V, Cromwell D, Johansen A, Stanley R, Tsang C, et al. National Hip Fracture Database Report 2014. London: Royal College of Physicians; 2014.
- Johansen A, Wakeman R, Boulton C, Plant F, Roberts J, Williams A. National Hip Fracture Database Report 2013. London: Royal College of Physicians; 2013.
- National Audit of the Organisation of Services for Falls and Bone Health for Older People. London: Royal College of Physicians; 2009.
- Mitchell P. Fracture liaison services: the UK experience. Osteoporos Int 2011;22:487-94. http://dx.doi.org/10.1007/s00198-011-1702-2.
- McLellan A, Wolowacz S, Zimovetz E, Beard SM, Lock S, McCrink L, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int 2011;22:2083-98. http://dx.doi.org/10.1007/s00198-011-1534-0.
- Cooper MS, Palmer AJ, Seibel MJ. Cost-effectiveness of the concord minimal trauma fracture liaison service, a prospective, controlled fracture prevention study. Osteoporos Int 2012;23:97-107. http://dx.doi.org/10.1007/s00198-011-1802-z.
- Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010;182:1864-73. http://dx.doi.org/10.1503/cmaj.100771.
- Fracture Prevention Services: An Economic Evaluation. London: Department of Health; 2009.
- Skelton D, Neil F. NHS Greater Glasgow and Clyde Strategy for Osteoporosis and Falls Prevention 2006–2010: An Evaluation 2007–2009. Glasgow: HealthQWest and Glasgow Caledonian University; 2009.
- Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int 2011;22:457-60. http://dx.doi.org/10.1007/s00198-011-1712-0.
- Lih A, Nandapalan H, Kim M, Yap C, Lee P, Ganda K, et al. Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int 2011;22:849-58. http://dx.doi.org/10.1007/s00198-010-1477-x.
- Craig P, Cooper C, Gunnell D, Haw S, Lawson K, Macintyre S, et al. Using Natural Experiments to Evaluate Population Health Interventions: Guidance for Producers and Users of Evidence. London: Medical Research Council; 2011.
- Torrance GW. Measurement of health state utilities for economic appraisal: a review. J Health Econ 1986;5:1-30. http://dx.doi.org/10.1016/0167-6296(86)90020-2.
- The Management of Hip Fracture in Adults. London: NICE; 2011.
- Cheung AM, Feig DS, Kapral M, Diaz-Granados N, Dodin S. Canadian Task Force on Preventive Health Care . Prevention of osteoporosis and osteoporotic fractures in postmenopausal women: recommendation statement from the Canadian task force on preventive health care. CMAJ 2004;170:1665-7. http://dx.doi.org/10.1503/cmaj.1030757.
- Lentle B, Cheung AM, Hanley DA, Leslie WD, Lyons D, Papaioannou A, et al. Osteoporosis Canada 2010 guidelines for the assessment of fracture risk. Can Assoc Radiol J 2011;62:243-50. http://dx.doi.org/10.1016/j.carj.2011.05.001.
- Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE, McLellan A, et al. Supporting appendix A: making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 2012;27:2039-46. http://dx.doi.org/10.1002/jbmr.1698.
- Bisphosphonates (Alendronate, Etidonate or Risedronate), Selective Oestrogen Receptor Modulators (Raloxifene) and Parathyroid Hormone (Teriparatide) for the Secondary Prevention of Osteoporotic Fragility Fractures in Post Menopausal Women. London: NICE; 2005.
- Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, et al. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int 2013;24:2135-52. http://dx.doi.org/10.1007/s00198-013-2348-z.
- Mitchell PJ. Best practices in secondary fracture prevention: fracture liaison services. Curr Osteoporos Rep 2013;11:52-60. http://dx.doi.org/10.1007/s11914-012-0130-3.
- Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 2013;24:393-406. http://dx.doi.org/10.1007/s00198-012-2090-y.
- Currie CF, Fleming S, Partridge M, Plant F, Wakeman R, Williams A. The National Hip Fracture Database National Report 2010. London: Royal College of Physicians; 2010.
- Currie CP, Partridge M, Plant F, Roberts J, Wakeman R, Williams A. The National Hip Fracture Database National Report 2011. London: Royal College of Physicians; 2011.
- Drew S, Sheard S, Chana J, Cooper C, Javaid MK, Judge A. on behalf of REFReSH study group . Describing variation in the delivery of secondary fracture prevention after hip fracture: an overview of 11 hospitals within one regional area in England. Osteoporos Int 2014;25:2427-33. http://dx.doi.org/10.1007/s00198-014-2775-5.
- Treml J, Husk J, Lowe D, Vasilakis N. Falling Standards, Broken Promises. Report of the National Audit of Falls and Bone Health in Older People 2010. London: Royal College of Physicians; 2011.
- Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ 2013;347.
- May C. Towards a general theory of implementation. Implement Sci 2013;8. http://dx.doi.org/10.1186/1748-5908-8-18.
- May C. A rational model for assessing and evaluating complex interventions in health care. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-86.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. http://dx.doi.org/10.1177/0038038509103208.
- Rogers EM. The Diffusion of Innovation. New York, NY: Free Press; 1995.
- Rogers EM. A prospective and retrospective look at the diffusion model. J Health Commun 2004;9:13-9. http://dx.doi.org/10.1080/10810730490271449.
- Grol RP, Bosch MC, Hulscher ME, Eccles MP, Wensing M. Planning and studying improvement in patient care: the use of theoretical perspectives. Milbank Q 2007;85:93-138. http://dx.doi.org/10.1111/j.1468-0009.2007.00478.x.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. Psychological Theory Group. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. http://dx.doi.org/10.1136/qshc.2004.011155.
- Equity and Excellence: Liberating the NHS. London: The Stationery Office; 2010.
- A Short Guide to NHS Foundation Trusts. London: Department of Health; 2005.
- Commissioning Policy: Ethical Framework for Priority Setting and Resource Allocation. London: NHS Commissioning Board; 2013.
- Putting Patients First: The NHS England Business Plan for 2013/14–2015/16. London: NHS England; 2013.
- Mitchell P, Adekunle F. Fracture Liaison Services: Resource Pack. Surrey: Novartis; 2010.
- From Zero to FLS: Implementation and Beyond. Bath: National Osteoporosis Society; 2010.
- Mitchell P, Adekunle F, Mitchell P, Adekunle F. Fracture Liaison Services: Resource Pack. Surrey: Novartis; 2010.
- CDG Template for Approval in Principle for Service Change or New Service. London: NHS; 2012.
- Checkland K, Snow S, McDermott I, Harrison S, Coleman A. ‘Animateurs’ and animation: what makes a good commissioning manager?. J Health Serv Res Policy 2012;17:11-7. http://dx.doi.org/10.1258/jhsrp.2011.011010.
- Clarke A, Taylor-Phillips S, Swan J, Gkeredakis E, Mills P, Powell J, et al. Evidence-based commissioning in the English NHS: who uses which sources of evidence? A survey 2010/2011. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002714.
- Checkland K, Coleman A, McDermott I, Segar J, Miller R, Petsoulas C, et al. Primary care-led commissioning: applying lessons from the past to the early development of clinical commissioning groups in England. Br J Gen Pract 2013;63:e611-19. http://dx.doi.org/10.3399/bjgp13X671597.
- Petsoulas C, Allen P, Checkland K, Coleman A, Segar J, Peckham S, et al. Views of NHS commissioners on commissioning support provision. Evidence from a qualitative study examining the early development of clinical commissioning groups in England. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005970.
- Sampson F, O’Cathain A, Strong M, Pickin M, Esmonde L. Commissioning processes in primary care trusts: a repeated cross-sectional survey of health care commissioners in England. J Health Serv Res Policy 2012;17:31-9. http://dx.doi.org/10.1258/jhsrp.2011.010191.
- Zachariadis M, Oborn E, Barrett M, Zollinger-Read P. Leadership of healthcare commissioning networks in England: a mixed-methods study on clinical commissioning groups. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002112.
- Bravo Vergel Y, Ferguson B. Difficult commissioning choices: lessons from English primary care trusts. J Health Serv Res Policy 2006;11:150-4. http://dx.doi.org/10.1258/135581906777641749.
- Ashman I, Willcocks S. Engaging with clinical commissioning: the attitudes of general practitioners in East Lancashire. Qual Prim Care 2014;22:91-9.
- Sabey A, Hardy H. Prepared for commissioning? A qualitative study into the views of recently qualified GPs. Educ Prim Care 2013;24:314-20. http://dx.doi.org/10.1080/14739879.2013.11494195.
- Shaw SE, Smith JA, Porter A, Rosen R, Mays N. The work of commissioning: a multisite case study of healthcare commissioning in England’s NHS. BMJ Open 2013;3:e003341-e41. http://dx.doi.org/10.1136/bmjopen-2013-003341.
- Miles MB, Huberman AM. Qualitative Data analysis: A Sourcebook of New Methods. Thousand Oaks, CA: Sage; 1994.
- Atkinson R, Flint J. Sampling, Snowball: Accessing Hidden and Hard-to-Reach Populations. The A-Z of Social Research. London: Sage Publications; 2001.
- Saumure K, Given LM. The SAGE Encyclopedia of Qualitative Research Methods. Thousand Oaks, CA: Sage Publications; 2008.
- Baker SE, Edwards R. How Many Qualitative Interviews is Enough?. Southampton: National Centre for Research Methods; 2012.
- Ayres L, Given LM. The SAGE Encyclopedia of Qualitative Research Methods. Thousand Oaks, CA: Sage Publications; 2008.
- Shank G, Given LM. The SAGE Encyclopedia of Qualitative Research Methods. Thousand Oaks, CA: Sage Publications; 2008.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ 2000;320:114-16. http://dx.doi.org/10.1136/bmj.320.7227.114.
- Best Practice Tariffs. London: Department of Health; 2010.
- Quality and Outcomes Framework. Leeds: Health and Social Care Information Centre; 2005.
- Mason B, Epiphaniou E, Nanton V, Donaldson A, Shipman C, Daveson BA, et al. Coordination of care for individuals with advanced progressive conditions: a multi-site ethnographic and serial interview study. Br J Gen Pract 2013;63:e580-8. http://dx.doi.org/10.3399/bjgp13X670714.
- Bevan G, Janus K. Why hasn’t integrated health care developed widely in the United States and not at all in England?. J Health Polit Policy Law 2011;36:141-64. http://dx.doi.org/10.1215/03616878-1191135.
- Stevens PE, O’Donoghue DJ. The UK model for system redesign and chronic kidney disease services. Semin Nephrol 2009;29:475-82. http://dx.doi.org/10.1016/j.semnephrol.2009.06.004.
- Limb M. NHS England slips further behind on its efficiency savings target. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f5297.
- Gooberman-Hill R. Qualitative approaches to understanding patient preferences. Patient 2012;5:215-23.
- Our NHS: Our Future. NHS Next Stage Review – Interim Report. London: Department of Health; 2007.
- Health and Social Care Information Centre . Hospital Episode Statistics. Secondary Hospital Episode Statistics n.d. www.hscic.gov.uk/hes (accessed 14 July 2015).
- NHS Patient Experience Framework. London: Department of Health; 2012.
- Sinclair E, Radford K, Grant M, Terry J. Developing stroke-specific vocational rehabilitation: a soft systems analysis of current service provision. Disabil Rehabil 2014;36:409-17. http://dx.doi.org/10.3109/09638288.2013.793410.
- Gridley K, Spiers G, Aspinal F, Bernard S, Atkin K, Parker G. Can general practitioner commissioning deliver equity and excellence? Evidence from two studies of service improvement in the English NHS. J Health Serv Res Policy 2012;17:87-93. http://dx.doi.org/10.1258/jhsrp.2011.010176.
- Taylor-Phillips S, Clarke A, Grove A, Swan J, Parsons H, Gkeredakis E, et al. Coproduction in commissioning decisions: is there an association with decision satisfaction for commissioners working in the NHS? A cross-sectional survey 2010/2011. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-004810.
- Given LM. The SAGE Encyclopedia of Qualitative Research Methods. Thousand Oaks, CA: Sage Publications; 2008.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- A Guide to Linked Mortality Data from Hospital Episode Statistics and the Office for National Statistics. Leeds: Health and Social Care Information Centre; 2015.
- Kennie DC, Reid J, Richardson IR, Kiamari AA, Kelt C. Effectiveness of geriatric rehabilitative care after fractures of the proximal femur in elderly women: a randomised clinical trial. BMJ 1988;297:1083-6. http://dx.doi.org/10.1136/bmj.297.6656.1083.
- Gilchrist WJ, Newman RJ, Hamblen DL, Williams BO. Prospective randomised study of an orthopaedic geriatric inpatient service. BMJ 1988;297:1116-18. http://dx.doi.org/10.1136/bmj.297.6656.1116.
- Zeltzer J, Mitchell RJ, Toson B, Harris IA, Ahmad L, Close J. Orthogeriatric services associated with lower 30-day mortality for older patients who undergo surgery for hip fracture. Med J Aust 2014;201:409-11. http://dx.doi.org/10.5694/mja14.00055.
- Sale JEM, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 2011;22:2067-82. http://dx.doi.org/10.1007/s00198-011-1544-y.
- Dell RG. S. R. Williams . K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 2008;90:188-94. http://dx.doi.org/10.2106/JBJS.H.00628.
- Majumdar SR, Lier DA, Beaupre LA, Hanley DA, Maksymowych WP, Juby AG, et al. Osteoporosis case manager for patients with hip fractures: results of a cost-effectiveness analysis conducted alongside a randomized trial. Arch Intern Med 2009;169:25-31. http://dx.doi.org/10.1001/archinte.169.1.25.
- Sander B, Elliot-Gibson V, Beaton DE, Bogoch ER, Maetzel A. A coordinator program in post-fracture osteoporosis management improves outcomes and saves costs. J Bone Joint Surg Am 2008;90:1197-205. http://dx.doi.org/10.2106/JBJS.G.00980.
- Sale JEM, Gignac MA, Hawker G, Frankel L, Beaton D, Bogoch E, et al. Decision to take osteoporosis medication in patients who have had a fracture and are ‘high’ risk for future fracture: a qualitative study. BMC Musculoskelet Disord 2011;12. http://dx.doi.org/10.1186/1471-2474-12-92.
- Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Key outcomes are usually not reported in published fracture secondary prevention programs: results of a systematic review. Arch Orthop Trauma Surg 2014;134:283-9. http://dx.doi.org/10.1007/s00402-011-1442-y.
- Huntjens KM, van Geel TC, Geusens PP, Winkens B, Willems P, van den Bergh J, et al. Impact of guideline implementation by a fracture nurse on subsequent fractures and mortality in patients presenting with non-vertebral fractures. Injury 2011;42:39-43. http://dx.doi.org/10.1016/S0020-1383(11)70011-0.
- Van der Kallen J, Giles M, Cooper K, Gill K, Parker V, Tembo A, et al. A fracture prevention service reduces further fractures two years after incident minimal trauma fracture. Int J Rheum Dis 2014;17:195-203. http://dx.doi.org/10.1111/1756-185X.12101.
- OPCS-4 Classification. Leeds: Health and Social Care Information Centre; n.d.
- International Classification of Diseases. Geneva: World Health Organization; n.d.
- Jandoc R, Burden AM, Mamdani M, Lévesque LE, Cadarette SM. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol 2015;68:950-6. http://dx.doi.org/10.1016/j.jclinepi.2014.12.018.
- Ramsey CR, Matowe L, Grill R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care 2003;19:613-23.
- Data Collection Checklist. Ottawa, ON: Institute of Population Health, University of Ottawa; 2002.
- Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharma Ther 2002;27:299-30. http://dx.doi.org/10.1046/j.1365-2710.2002.00430.x.
- Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc 2010;58:783-7. http://dx.doi.org/10.1111/j.1532-5415.2010.02767.x.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JASA 1999;94:496-509. http://dx.doi.org/10.1080/01621459.1999.10474144.
- Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492-5. http://dx.doi.org/10.1136/bmj.319.7223.1492.
- Roche JJW, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ 2005;331. http://dx.doi.org/10.1136/bmj.38643.663843.55.
- Roberts SE, Goldacre MJ. Time trends and demography of mortality after fractured neck of femur in an English population, 1968-98: database study. BMJ 2003;327:771-5. http://dx.doi.org/10.1136/bmj.327.7418.771.
- Wu TY, Jen MH, Bottle A, Liaw CK, Aylin P, Majeed A. Admission rates and in-hospital mortality for hip fractures in England 1998 to 2009: time trends study. J Public Health (Oxf) 2011;33:284-91. http://dx.doi.org/10.1093/pubmed/fdq074.
- Vestergaard P, Rejnmark L, Mosekilde L. Increased mortality in patients with a hip fracture-effect of pre-morbid conditions and post-fracture complications. Osteoporos Int 2007;18:1583-93. http://dx.doi.org/10.1007/s00198-007-0403-3.
- Simunovic N, Devereaux PJ, Sprague S, Guyatt GH, Schemitsch E, Debeer J, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ 2010;182:1609-16. http://dx.doi.org/10.1503/cmaj.092220.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001;49:516-22. http://dx.doi.org/10.1046/j.1532-5415.2001.49108.x.
- Vidan M, Serra JA, Moreno C, Riquelme G, Oritz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc 2005;53:1476-82. http://dx.doi.org/10.1111/j.1532-5415.2005.53466.x.
- Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. New Engl J Med 2007;357:1799-809. http://dx.doi.org/10.1056/NEJMoa074941.
- Colón-Emeric CS, Mesenbrink P, Lyles KW, Pieper CF, Boonen S, Delmas P, et al. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res 2010;25:91-7. http://dx.doi.org/10.1359/jbmr.090704.
- Huntjens KM, van Geel TA, van den Bergh JP, van Helden S, Willems P, Winkens B, et al. Fracture Liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Joint Surg Am 2015;96:1-8.
- Lawrence TM, Wenn R, Boulton CT, Moran CG. Age-specific incidence of first and second fractures of the hip. J Bone Joint Surg Br 2010;92:258-61. http://dx.doi.org/10.1302/0301-620X.92B2.23108.
- Chapurlat RD, Bauer DC, Nevitt M, Stone K, Cummings SR. Incidence and risk factors for a second hip fracture in elderly women. The study of osteoporotic fractures. Osteoporos Int 2003;14:130-6.
- Melton LJ, Ilstrup DM, Beckenbaugh RD, Riggs BL. Hip fracture recurrence. A population-based study. Clin Orthop Relat Res 1982;167:131-8.
- Li L, Roddam A, Gitlin M, Taylor A, Shepherd S, Shearer A, et al. Persistence with osteoporosis medications among postmenopausal women in the UK general practice research database. Menopause 2012;19:33-40. http://dx.doi.org/10.1097/gme.0b013e318221bacd.
- Inderjeeth CA, Chan K, Kwan K, Lai M. Time to onset of efficacy in fracture reduction with current anti-osteoporosis treatments. J Bone Miner Metab 2012;30:493-50. http://dx.doi.org/10.1007/s00774-012-0349-1.
- Langridge CR, McQuillian C, Watson WS, Walker B, Mitchell L, Gallacher SJ. Refracture following fracture liaison service assessment illustrates the requirement for integrated falls and fracture services. Calcif Tissue Int 2007;81:85-91. http://dx.doi.org/10.1007/s00223-007-9042-0.
- Nymark T, Lauritsen JM, Ovesen O, Röck ND, Jeune B. Short time-frame from first to second hip fracture in the Funen County hip fracture study. Osteoporos Int 2006;17:1353-7. http://dx.doi.org/10.1007/s00198-006-0125-y.
- Curtis JR, Arora T, Matthews RS, Taylor A, Becker DJ, Colón-Emeric C, et al. Is withholding osteoporosis medication after fracture sometimes rational? A comparison of the risk for second fracture versus death. J Am Med Dir Assoc 2010;11:584-91. http://dx.doi.org/10.1016/j.jamda.2009.12.004.
- Gibson-Smith D, Klop C, Elders PJ, Welsing PM, van Schoor N, Leufkens HG, et al. The risk of major and any (non-hip) fragility fracture after hip fracture in the United Kingdom: 2000–2010. Osteoporos Int 2014;25:2555-63. http://dx.doi.org/10.1007/s00198-014-2799-x.
- Whitworth JA. Best practices in use of research evidence to inform health decisions. Health Res Policy Syst 2006;4. http://dx.doi.org/10.1186/1478-4505-4-11.
- Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13:38-44. http://dx.doi.org/10.1016/j.acap.2013.08.002.
- Van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HG. The use of a large pharmacoepidemiological database to study exposure to oral corticosteroids and risk of fractures: validation of study population and results. Pharmacoepidemiol Drug Saf 2000;9:359-66. http://dx.doi.org/10.1002/1099-1557(200009/10)9:5<359::AID-PDS507>3.0.CO;2-E.
- Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726-33. http://dx.doi.org/10.1007/s00198-006-0172-4.
- Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013;8. http://dx.doi.org/10.1007/s11657-013-0136-1.
- Burge RT, Worley D, Johansen A, Bhattacharyya S, Bose U. The cost of osteoporotic fractures in the UK: projections for 2000–2020. J Med Econ 2001;4:51-62. http://dx.doi.org/10.3111/200104051062.
- Gutiérrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, et al. Study of the incremental cost and clinical burden of hip fractures in postmenopausal women in the United Kingdom. J Med Econ 2011;14:99-107. http://dx.doi.org/10.3111/13696998.2010.547967.
- Sahota O, Morgan N, Moran CG. The direct cost of acute hip fracture care in care home residents in the UK. Osteoporos Int 2012;23:917-20. http://dx.doi.org/10.1007/s00198-011-1651-9.
- Thakar C, Alsousou J, Hamilton TW, Willett K. The cost and consequences of proximal femoral fractures which require further surgery following initial fixation. J Bone Joint Surg Br 2010;92:1669-77. http://dx.doi.org/10.1302/0301-620X.92B12.25021.
- Kazi HA, Acharya A. Comparison of trauma and elective income in a district general hospital. Br J Med Pract 2011;4.
- Hippisley-Cox J, Vinogradova Y. Trends in Consultation Rates in General Practice 1995/1996 to 2008/2009: Analysis of the QResearch® Database. London: QResearch® and Health and Social Care Information Centre; 2009.
- Violato M, Gray A, Papanicolas I, Ouellet M. Resource use and costs associated with coeliac disease before and after diagnosis in 3,646 cases: results of a UK primary care database analysis. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0041308.
- Curtis LH. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- NHS Reference Costs 2012–2013. London: Department of Health; 2013.
- Prescription Cost Analysis, England – 2013. Leeds: Health and Social Care Information Centre; 2014.
- Lin DY. Linear regression analysis of censored medical costs. Biostatistics 2000;1:35-47. http://dx.doi.org/10.1093/biostatistics/1.1.35.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. http://dx.doi.org/10.1016/0021-9681(87)90171-8.
- OPCS Codes Relevant to Procedures Recorded in the NJR. Hemel Hempsted: National Joint Registry; 2013.
- Iglesias CP, Manca A, Torgerson DJ. The health-related quality of life and cost implications of falls in elderly women. Osteoporos Int 2009;20:869-78. http://dx.doi.org/10.1007/s00198-008-0753-5.
- Lawrence TM, White CT, Wenn R, Moran CG. The current hospital costs of treating hip fractures. Injury 2005;36:88-91. http://dx.doi.org/10.1016/j.injury.2004.06.015.
- Luengo-Fernandez R, Gray AM, Rothwell PM. A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke 2012;43:3343-51. http://dx.doi.org/10.1161/STROKEAHA.112.667204.
- Hansen L, Mathiesen AS, Vestergaard P, Ehlers LH, Petersen KD. A health economic analysis of osteoporotic fractures: who carries the burden?. Arch Osteoporos 2013;8. http://dx.doi.org/10.1007/s11657-013-0126-3.
- Barendregt JJ. The half-cycle correction: banish rather than explain it. Med Decis Making 2009;29:500-2. http://dx.doi.org/10.1177/0272989X09340585.
- Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia 2013;56:1925-33. http://dx.doi.org/10.1007/s00125-013-2940-y.
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239-41. http://dx.doi.org/10.1093/biomet/69.1.239.
- Pregibon D. Resistant fits for some commonly used logistic models with medical application. Biometrics 1982;38:485-98. http://dx.doi.org/10.2307/2530463.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. http://dx.doi.org/10.1002/(SICI)1099-1050(199611)5:6<513::AID-HEC237>3.0.CO;2-9.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force–3. Value Health 2012;15:812-20. http://dx.doi.org/10.1016/j.jval.2012.06.014.
- Ganda K, Schaffer A, Pearson S, Seibel MJ. Compliance and persistence to oral bisphosphonate therapy following initiation within a secondary fracture prevention program: a randomised controlled trial of specialist vs. non-specialist management. Osteoporos Int 2014;25:1345-55. http://dx.doi.org/10.1007/s00198-013-2610-4.
- The Adherence Gap: Why Osteoporosis Patients Don’t Continue with Treatment. A European Report Highlighting the Gap between the Beliefs of People with Osteoporosis and the Perceptions of their Physicians. IOF; 2005.
- White HJ, Bettiol SS, Perera R, Roberts NW, Javaid MK, Farmer AJ. A systematic review assessing the effectiveness of interventions to improve persistence with anti-resorptive therapy in women at high risk of clinical fracture. Fam Pract 2010;27:593-60. http://dx.doi.org/10.1093/fampra/cmq060.
Appendix 1 Evaluation questionnaire
Appendix 2 Interview guide for qualitative interviews
Theme 1
Current service provision – things that work well/not so well/improvements/best way of doing this:
-
case finding or identifying those at risk
-
assessing patients ‘at risk’
-
treating patients
-
monitoring patients.
Co-ordination of care – things that work well/not so well/improvements/best way of doing this:
-
within hospital services
-
with primary care services.
Theme 2
Change in services – most significant change in how services to prevent secondary fractures after hip fracture were delivered whilst at the hospital.
-
How easy/difficult process was.
Capability.
-
change in work done
-
access to resources
-
finances, staffing, technology.
Capacity.
-
How well everyone communicated with each other during the process.
-
How well everyone worked together to introduce the service.
Potential.
-
How useful YOU thought the service was that was being introduced/how useful you thought other colleagues felt about the service was that was being introduced.
Contribution.
-
Collection of outcomes data.
Appendix 3 Medical codes for identifying hip fractures in the Clinical Practice Research Datalink
| medcode | readcode | readterm |
|---|---|---|
| 5742 | 7K1D000 | Prmy open red+int fxn prox femoral #+screw/nail+plate device |
| 58817 | 7K1D011 | Prim open reduct # neck femur & op fix - Blount nail plate |
| 52395 | 7K1D012 | Prim op red # nck femur & op fix- Charnley compression screw |
| 97337 | 7K1D013 | Prim op red # nck femur & op fix - Deyerle multiple hip pin |
| 94714 | 7K1D014 | Prim open reduct # neck femur & op fix - Holt nail |
| 105352 | 7K1D015 | Prim open reduct # neck femur & op fix - Jewett nail plate |
| 56568 | 7K1D017 | Prim open red # neck femur & op fix - McLaughlin nail plate |
| 46258 | 7K1D018 | Prim open reduct # neck femur & op fix - Neufield nail plate |
| 65536 | 7K1D019 | Prim open reduct # neck femur & op fix - Pugh nail plate |
| 24493 | 7K1D01A | Prim open reduct # neck femur & op fix - Richards screw |
| 57884 | 7K1D01B | Prim open reduct # neck femur & op fix - Ross Brown nail |
| 57889 | 7K1D01D | Prim op red # nck femur & op fix- Zickel intramed nail plate |
| 9792 | 7K1D01E | DHS - Dynamic hip screw primary fixation of neck of femur |
| 12544 | 7K1D01F | Dynamic hip screw primary fixation of neck of femur |
| 33624 | 7K1D600 | Prmy open red+int fxn prox femoral #+screw/nail device alone |
| 34764 | 7K1D700 | Prmy open red+int fxn prox fem #+screw/nail+intramed device |
| 105803 | 7K1DE00 | Prim op red frac neck fem op fix us prox fem nail antirotatn |
| 41888 | 7K1G200 | Primary open reduction+external fixation of femoral fracture |
| 8719 | 7K1J000 | Cls red+int fxn proximal femoral #+screw/nail device alone |
| 53670 | 7K1J011 | Cl red intracaps frac neck femur fix-Garden cannulated screw |
| 40999 | 7K1J012 | Cl red intracaps fract neck femur fix - Smith-Petersen nail |
| 57514 | 7K1J013 | Cls red+int fxn prox femoral #+Richard’s cannulat hip screw |
| 35004 | 7K1J500 | Primary int fxn(no red) prox fem #+screw/nail device alone |
| 44594 | 7K1J600 | Primary int fxn(no red) prox fem #+scrw/nail+intramed device |
| 38856 | 7K1J700 | Primary int fxn(no red) prox fem #+screw/nail+plate device |
| 55386 | 7K1JB00 | Primary cls red+int fxn prox fem #+screw/nail device alone |
| 54819 | 7K1JC00 | Prim cls rd+int fxn prox fem #+screw/nail+intramdulry device |
| 46959 | 7K1JD00 | Primary cls red+int fxn prox fem #+screw/nail+plate device |
| 39322 | 7K1Jd00 | Closed reduction of intracapsular # NOF internal fixat DHS |
| 70018 | 7K1K300 | Primary external fixation(without reduction) prox femoral # |
| 102313 | 7K1K500 | Primary cls reduction+external fixation proximal femoral # |
| 6660 | 7K1L400 | Closed reduction of fracture of hip |
| 2225 | S30..00 | Fracture of neck of femur |
| 1994 | S30..11 | Hip fracture |
| 38489 | S300.00 | Closed fracture proximal femur, transcervical |
| 39984 | S300000 | Cls # prox femur, intracapsular section, unspecified |
| 69919 | S300100 | Closed fracture proximal femur, transepiphyseal |
| 65690 | S300200 | Closed fracture proximal femur, midcervical section |
| 52194 | S300300 | Closed fracture proximal femur, basicervical |
| 51861 | S300311 | Closed fracture, base of neck of femur |
| 17019 | S300500 | Cls # prox femur, subcapital, Garden grade unspec. |
| 34351 | S300600 | Closed fracture proximal femur, subcapital, Garden grade I |
| 33957 | S300700 | Closed fracture proximal femur, subcapital, Garden grade II |
| 36599 | S300800 | Closed fracture proximal femur, subcapital, Garden grade III |
| 34078 | S300900 | Closed fracture proximal femur, subcapital, Garden grade IV |
| 45779 | S300A00 | Closed fracture of femur, upper epiphysis |
| 49209 | S300y00 | Closed fracture proximal femur, other transcervical |
| 68229 | S300y11 | Closed fracture of femur, subcapital |
| 62966 | S300z00 | Closed fracture proximal femur, transcervical, NOS |
| 73981 | S301.00 | Open fracture proximal femur, transcervical |
| 50727 | S301000 | Opn # proximal femur, intracapsular section, unspecified |
| 72138 | S301100 | Open fracture proximal femur, transepiphyseal |
| 100771 | S301311 | Open fracture base of neck of femur |
| 38878 | S301500 | Open fracture proximal femur,subcapital, Garden grade unspec |
| 60885 | S301600 | Open fracture proximal femur,subcapital, Garden grade I |
| 67394 | S301700 | Open fracture proximal femur,subcapital, Garden grade II |
| 23803 | S301800 | Open fracture proximal femur,subcapital, Garden grade III |
| 51999 | S301900 | Open fracture proximal femur,subcapital, Garden grade IV |
| 96518 | S301A00 | Open fracture of femur, upper epiphysis |
| 68668 | S301y00 | Open fracture proximal femur, other transcervical |
| 73234 | S301y11 | Open fracture of femur, subcapital |
| 5301 | S302.00 | Closed fracture of proximal femur, pertrochanteric |
| 19117 | S302000 | Cls # proximal femur, trochanteric section, unspecified |
| 19387 | S302011 | Closed fracture of femur, greater trochanter |
| 48337 | S302012 | Closed fracture of femur, lesser trochanter |
| 45141 | S302100 | Closed fracture proximal femur, intertrochanteric, two part |
| 51216 | S302300 | Cls # proximal femur, intertrochanteric, comminuted |
| 8648 | S302400 | Closed fracture of femur, intertrochanteric |
| 44735 | S302z00 | Cls # of proximal femur, pertrochanteric section, NOS |
| 61733 | S303.00 | Open fracture of proximal femur, pertrochanteric |
| 67633 | S303000 | Open # of proximal femur, trochanteric section, unspecified |
| 101567 | S303100 | Open fracture proximal femur, intertrochanteric, two part |
| 97971 | S303300 | Open fracture proximal femur, intertrochanteric, comminuted |
| 39396 | S303400 | Open fracture of femur, intertrochanteric |
| 70479 | S303z00 | Open fracture of proximal femur, pertrochanteric, NOS |
| 28965 | S304.00 | Pertrochanteric fracture |
| 24276 | S30w.00 | Closed fracture of unspecified proximal femur |
| 58642 | S30x.00 | Open fracture of unspecified proximal femur |
| 18273 | S30y.00 | Closed fracture of neck of femur NOS |
| 10570 | S30y.11 | Hip fracture NOS |
| 38054 | S30z.00 | Open fracture of neck of femur NOS |
Appendix 4 Description of changes to orthogeriatric and fracture liaison service models of care as previously identified and described in Chapter 2
| Hospital | Date | Change | Description |
|---|---|---|---|
| 2 | May 2005 | Appointment of OG | Provided a liaison service. OG responsible for case finding, assessment and treatment initiation |
| August 2007 | Appointment of OG | Became clinical lead/osteoporosis ‘champion’. Allowed a full-time consultant to be present in orthogeriatrics throughout the year. Majority of patients seen preoperatively and in theatre quicker and in better condition. OG also involved in postoperative care, early identification and treatment of complications. Began doing falls assessments on ward | |
| 4 | October 2006 | Appointment of OG | Attends daily trauma meetings and conducts daily ward round |
| May 2009 | Appointment of osteoporosis nurse specialist | Assesses all inpatient hip fractures, assesses for falls risk (with referral to falls service if required), osteoporosis risk factors, and refers for DXA and blood tests. Makes treatment recommendations and a follow-up plan. Attends daily trauma meetings, screens inpatients. Responsible for case finding | |
| 6 | November 2005 | Appointment of OG | Carries out the initial assessment, preoperative and postoperative care and secondary fracture prevention assessment: attends trauma meetings, case finds, does falls and osteoporosis assessment, supports discharge planning, sets rehabilitation goals, reviews complex cases and does ward round |
| 5 | September 2009 | Appointment of OG | Brought extra support for OG already in post. Allowed weekend orthogeriatric cover and was responsible for case finding. Assessment of all hip fracture patients for osteoporosis risk factors, lifestyle factors, nutrition, treatment compliance and falls risk, and also initiate treatment. They co-ordinate multidisciplinary team meetings, attend trauma meetings, conduct pre- and post-operative assessments, and conduct a 4- to 6-week post-discharge follow-up if required |
| 7 | July 2004 | Appointment of geriatrician to hip fracture ward | Increased number of orthogeriatric sessions from 1 to 3 per week. Introduced service which sees all patients preoperatively and postoperatively, including falls and osteoporosis assessments, and discharge planning (increasing intensity over time). A dedicated hip fracture ward was opened at the same time |
| June 2007 | Appointment of trauma specialty nurse | Additional support for orthogeriatric team and management of bone health | |
| 8 | March 2009 | Appointment of OG | Became clinical lead. Introduced daily trauma rounds and six consultant ward rounds per week (four per week prior to this). Began assessing younger patients (prior to this those under 70 years would not be seen). 90% of patients seen preoperatively by consultant to optimise them for surgery. Standardised falls assessment and gait and balance assessments, with referral to community falls assessment if required |
| 9 | April 2005 | Appointment of osteoporosis nurse specialist | Runs the day-to-day osteoporosis clinic (outpatient). Conducts 15-minute appointment for osteoporosis assessment and conducts blood test and medication assessment at 6 months’ follow-up |
| 10 | May 2006 | Appointment of osteoporosis nurse specialist | Osteoporosis and fracture liaison nurse (0.5 WTE). Extra support for pre-existing FLS model in which patients received osteoporosis assessment with recommendation made and letter to GP. Not all hip fracture patients seen |
| May 2008 | Appointment of osteoporosis nurse specialist | Both an osteoporosis and fracture liaison clinical nurse specialist and a specialist practitioner – osteoporosis fracture liaison nurse specialist appointed. Co-ordination of inpatient care of all incident hip fracture patients. Consultant rheumatologist also appointed as osteoporosis ‘champion’. A new computer system put in place aided identification of hip fracture patients | |
| November 2009 | Appointment of OG | Rewrote admission paperwork and streamlined NOF pathway. Abnormal blood results were hence picked up by doctors rather than nurses |
Appendix 5 Baseline characteristics of cases (primary hip fracture patients)
| Yeara | Sex (% female) | Age (%) | Charlson comorbidities | Prior bisphosphonate use (ever) | Prior bisphosphonate useb | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 60–74 years | 75–84 years | ≥ 85 years | None | Mild | Moderate | Severe | |||||
| 1999 | 673 (78.2) | 149 (17.3) | 351 (40.8) | 361 (41.9) | 482 (56.0) | 286 (33.2) | 72 (8.4) | 21 (2.4) | 50 (5.8) | 30 (3.5) | 861 |
| 2000 | 680 (79.2) | 157 (18.3) | 337 (39.2) | 365 (42.5) | 475 (55.3) | 292 (34.0) | 63 (7.3) | 29 (3.4) | 94 (10.9) | 45 (5.2) | 859 |
| 2001 | 646 (77.5) | 152 (18.2) | 343 (41.1) | 339 (40.7) | 484 (58.0) | 250 (30.0) | 65 (7.8) | 35 (4.2) | 89 (10.7) | 55 (6.6) | 834 |
| 2002 | 689 (79.9) | 159 (18.5) | 364 (42.2) | 339 (39.3) | 501 (58.1) | 266 (30.9) | 68 (7.9) | 27 (3.1) | 117 (13.6) | 64 (7.4) | 862 |
| 2003 | 674 (76.4) | 155 (17.6) | 409 (46.4) | 318 (36.1) | 503 (57.0) | 279 (31.6) | 67 (7.6) | 33 (3.7) | 108 (12.2) | 78 (8.8) | 882 |
| 2004 | 630 (77.0) | 139 (17.0) | 336 (41.1) | 343 (41.9) | 470 (57.5) | 245 (30.0) | 78 (9.5) | 25 (3.1) | 129 (15.8) | 79 (9.7) | 818 |
| 2005 | 638 (75.3) | 135 (15.9) | 357 (42.2) | 355 (41.9) | 469 (55.4) | 268 (31.6) | 71 (8.4) | 39 (4.6) | 134 (15.8) | 88 (10.4) | 847 |
| 2006 | 618 (74.6) | 141 (17.0) | 329 (39.7) | 359 (43.3) | 410 (49.5) | 271 (32.7) | 85 (10.3) | 63 (7.6) | 152 (18.3) | 101 (12.2) | 829 |
| 2007 | 643 (78.4) | 130 (15.9) | 330 (40.2) | 360 (43.9) | 379 (46.2) | 265 (32.3) | 111 (13.5) | 65 (7.9) | 170 (20.7) | 109 (13.3) | 820 |
| 2008 | 587 (73.1) | 166 (20.7) | 288 (35.9) | 349 (43.5) | 391 (48.7) | 241 (30.0) | 106 (13.2) | 65 (8.1) | 177 (22.0) | 111 (13.8) | 803 |
| 2009 | 569 (75.1) | 148 (19.5) | 262 (34/6) | 348 (45.9) | 355 (46.8) | 236 (31.1) | 109 (14.4) | 58 (7.7) | 180 (23.8 | 115 (15.2) | 758 |
| 2010 | 534 (73.7) | 145 (20.0) | 251 (34.6) | 329 (45.4) | 312 (43.0) | 260 (35.9) | 102 (14.1) | 51 (7.0) | 162 (22.3) | 100 (13.8) | 725 |
| 2011 | 511 (76.4) | 110 (16.4) | 249 (37.2) | 310 (46.3) | 294 (44.0) | 224 (33.5) | 103 (15.4) | 48 (7.2) | 147 (22.0) | 87 (13.0) | 669 |
| 2012 | 502 (74.3) | 114 (16.9) | 242 (35.8) | 320 (47.3) | 328 (48.5) | 213 (31.5) | 90 (13.3) | 45 (6.7) | 150 (22.2) | 85 (12.6) | 676 |
| Overall | 8594 (76.4) | 2000 (17.8) | 4448 (39.6) | 4795 (42.7) | 5853 (52.1) | 3596 (32.0) | 1190 (10.6) | 604 (5.4) | 1859 (16.5) | 1147 (10.2) | 11,243 |
Appendix 6 Baseline characteristics of controls
| Yeara | Sex (% female) | Age (%) | Charlson comorbidities | Prior bisphosphonate use (ever) | Prior bisphosphonate useb | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 60–74 years | 75–84 years | ≥ 85 years | None | Mild | Moderate | Severe | |||||
| 1999 | 1292 (78.3) | 298 (18.1) | 701 (42.5) | 652 (39.5) | 1011 (61.2) | 495 (30.0) | 107 (6.5) | 38 (2.3) | 75 (4.5) | 44 (2.7) | 1651 |
| 2000 | 1314 (79.4) | 312 (18.9) | 673 (40.7) | 670 (40.5) | 1054 (63.7) | 458 (27.7) | 102 (6.2) | 41 (2.5) | 75 (4.5) | 43 (2.6) | 1655 |
| 2001 | 1245 (77.4) | 304 (18.9) | 680 (42.3) | 625 (38.8) | 1033 (64.2) | 466 (29.0) | 78 (4.9) | 32 (2.0) | 90 (5.6) | 55 (3.4) | 1609 |
| 2002 | 1318 (80.0) | 317 (19.3) | 722 (43.8) | 609 (37.0) | 1024 (62.1) | 457 (27.7) | 135 (8.2) | 32 (1.9) | 92 (5.6) | 62 (3.8) | 1648 |
| 2003 | 1305 (76.9) | 307 (18.1) | 811 (47.8) | 579 (34.1) | 1117 (65.8) | 427 (25.2) | 112 (6.6) | 41 (2.4) | 154 (9.1) | 102 (6.0) | 1697 |
| 2004 | 1213 (77.2) | 277 (17.6) | 665 (42.3) | 630 (40.1) | 1024 (65.1) | 403 (25.6) | 109 (6.9) | 36 (2.3) | 152 (9.7) | 95 (6.0) | 1572 |
| 2005 | 1226 (75.5) | 270 (16.6) | 704 (43.3) | 651 (40.1) | 1011 (62.2) | 424 (26.1) | 124 (7.6) | 66 (4.1) | 180 (11.1) | 115 (7.1) | 1625 |
| 2006 | 1189 (74.9) | 281 (17.7) | 651 (41.0) | 656 (41.3) | 923 (58.1) | 436 (27.5) | 157 (9.9) | 72 (4.5) | 205 (12.9) | 136 (8.6) | 1588 |
| 2007 | 1229 (78.4) | 258 (16.5) | 650 (41.5) | 659 (42.1) | 848 (54.1) | 429 (27.4) | 195 (12.4) | 95 (6.1) | 193 (12.3) | 122 (7.8) | 1567 |
| 2008 | 1139 (73.0) | 332 (21.3) | 572 (36.7) | 656 (42.1) | 827 (53.0) | 452 (29.0) | 181 (11.6) | 100 (6.4) | 217 (13.9) | 130 (8.3) | 1560 |
| 2009 | 1097 (75.5) | 296 (20.4) | 521 (35.8) | 637 (43.8) | 758 (52.1) | 428 (29.4) | 174 (12.0) | 94 (6.5) | 23 (16.0) | 154 (10.6) | 1454 |
| 2010 | 1039 (73.9) | 290 (20.6) | 502 (35.7) | 615 (43.7) | 733 (52.0) | 433 (30.8) | 160 (11.4) | 81 (5.8) | 220 (15.6) | 136 (9.7) | 1407 |
| 2011 | 976 (76.9) | 220 (17.3) | 497 (39.2) | 552 (43.5) | 647 (51.0) | 390 (30.7) | 153 (12.1) | 79 (6.2) | 216 (17.0) | 133 (10.5) | 1269 |
| 2012 | 965 (74.0) | 228 (17.5) | 484 (37.1) | 592 (45.4) | 747 (57.3) | 365 (28.0) | 127 (9.7) | 65 (5.0) | 229 (17.6) | 115 (8.8) | 1304 |
| Overall | 16,547 (76.6) | 3990 (18.5) | 8833 (40.9) | 8783 (40.7) | 12,757 (59.0) | 6063 (28.1) | 1914 (8.9) | 872 (4.0) | 2331 (10.8) | 1442 (6.7) | 21,606 |
Appendix 7 Estimated impact of interventions using segmented linear regression (parsimonious) models on all primary hip fracture patients
| Variables | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Primary hip fracture rate (i.e. number per half annum) | |||
| Intercept (β0) | 431 | 421 to 441 | < 0.001 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | |||
| Trend change after intervention 1 + 2 (β3) | –7.18 | –8.75 to –5.6 | < 0.001 |
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Overall hip fracture rate (i.e. number per half annum) | |||
| Intercept (β0) | 442.2 | 430.8 to 453.6 | < 0.001 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | |||
| Trend change after intervention 1 + 2 (β3) | –7.85 | –10.23 to –5.47 | < 0.001 |
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Second hip fracture (2 years) | |||
| Intercept (β0) | 2.49 | 2.12 to 2.87 | < 0.001 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | |||
| Trend change after intervention 1 + 2 (β3) | |||
| Level change after intervention 3 + 4 (β4) | –0.95 | –1.67 to –0.23 | 0.012 |
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Major non-hip fracture (2 years) | |||
| Intercept (β0) | 2.12 | 1.92 to 2.32 | < 0.001 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | |||
| Trend change after intervention 1 + 2 (β3) | |||
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Mortality (30 days) | |||
| Intercept (β0) | 7.30 | 6.74 to 7.85 | < 0.001 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | |||
| Trend change after intervention 1 + 2 (β3) | |||
| Level change after intervention 3 + 4 (β4) | –2.81 | –3.73 to –1.85 | < 0.001 |
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Mortality (30 days): difference between cases and controls | |||
| Intercept (β0) | 6.41 | 5.8 to 7.01 | < 0.001 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | |||
| Trend change after intervention 1 + 2 (β3) | |||
| Level change after intervention 3 + 4 (β4) | –2.546 | –3.55 to –1.54 | < 0.001 |
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Mortality (1 year) | |||
| Intercept (β0) | 23.6 | 22.7 to 24.6 | < 0.001 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | |||
| Trend change after intervention 1 + 2 (β3) | |||
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | –5.56 | –7.59 to –3.52 | < 0.001 |
| Trend change after intervention 5 (β7) | |||
| Mortality (1 year): difference between cases and controls | |||
| Intercept (β0) | 13.44 | 12.51 to 14.4 | < 0.001 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | |||
| Trend change after intervention 1 + 2 (β3) | |||
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | 0.98 | 0.16 to 1.8 | 0.022 |
| Level change after intervention 5 (β6) | –8.76 | –13.8 to –3.7 | 0.002 |
| Trend change after intervention 5 (β7) | |||
| Any incident antiOP medication use (4 months)a | |||
| Intercept (β0) | 0.57 | –2.82 to 3.96 | 0.73 |
| Baseline trend (β1) | 0.84 | 0.35 to 1.34 | 0.002 |
| Level change after intervention 1 + 2 (β2) | 13.3 | 8.7 to 17.8 | < 0.001 |
| Trend change after intervention 1 + 2 (β3) | 0.72 | –0.01 to 1.44 | 0.051 |
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Any incident antiOP medication use (1 year)a | |||
| Intercept (β0) | 2.52 | –0.01 to 5.05 | 0.05 |
| Baseline trend (β1) | 1.08 | 0.71 to 1.45 | < 0.001 |
| Level change after intervention 1 + 2 (β2) | 14.5 | 11.1 to 17.8 | < 0.001 |
| Trend change after intervention 1 + 2 (β3) | 0.49 | –0.05 to 1.03 | 0.073 |
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Bisphosphonate prevalence (2–6 months)a | |||
| Intercept (β0) | 0.84 | –2.55 to 4.22 | 0.61 |
| Baseline trend (β1) | 0.88 | 0.38 to 1.38 | 0.002 |
| Level change after intervention 1 + 2 (β2) | 7.57 | 1.54 to 13.6 | 0.017 |
| Trend change after intervention 1 + 2 (β3) | 2.1 | 0.55 to 3.66 | 0.011 |
| Level change after intervention 3 + 4 (β4) | –9.59 | –16.81 to –2.36) | 0.012 |
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | –1.74 | –3.78 to 0.31 | 0.091 |
| Bisphosphonate prevalence (10–14 months)a | |||
| Intercept (β0) | 1.31 | –1 to 3.61 | 0.25 |
| Baseline trend (β1) | 0.95 | 0.62 to 1.27 | < 0.001 |
| Level change after intervention 1 + 2 (β2) | 8.71 | 5.04 to 12.4 | < 0.001 |
| Trend change after intervention 1 + 2 (β3) | |||
| Level change after intervention 3 + 4 (β4) | –3.79 | –7.4 to –0.17 | 0.041 |
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
Appendix 8 Estimated impact of interventions using segmented linear regression (full) models on all primary hip fracture patients
| Variables | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Primary hip fracture rate (i.e. number per half annum) | |||
| Intercept (β0) | 424.4 | 396.6 to 452.1 | < 0.001 |
| Baseline trend (β1) | 0.75 | –3.34 to 4.84 | 0.7 |
| Level change after intervention 1 + 2 (β2) | –3.94 | –56.8 to 48.9 | 0.88 |
| Trend change after intervention 1 + 2 (β3) | –5.65 | –19.8 to 8.51 | 0.41 |
| Level change after intervention 3 + 4 (β4) | –24.5 | –98.4 to 49.4 | 0.49 |
| Trend change after intervention 3 + 4 (β5) | 6.9 | –26.3 to 40.1 | 0.67 |
| Level change after intervention 5 (β6) | –18 | –73.9 to 37.9 | 0.51 |
| Trend change after intervention 5 (β7) | –9.14 | –41.2 to 22.9 | 0.56 |
| Overall hip fracture rate (i.e. number per half annum) | |||
| Intercept (β0) | 433.6 | 403.4 to 463.8 | < 0.001 |
| Baseline trend (β1) | 1.13 | –3.33 to 5.58 | 0.596 |
| Level change after intervention 1 + 2 (β2) | –9.627 | –67.22 to 47.97 | 0.725 |
| Trend change after intervention 1 + 2 (β3) | –5.627 | –21.068 to 9.81 | 0.447 |
| Level change after intervention 3 + 4 (β4) | –28.9 | –109.5 to 51.6 | 0.45 |
| Trend change after intervention 3 + 4 (β5) | 6 | –30.2 to 42.2 | 0.73 |
| Level change after intervention 5 (β6) | 14.83 | –68.36 to 98 | 0.71 |
| Trend change after intervention 5 (β7) | –26.5 | –73.2 to 20.2 | 0.244 |
| Second hip fracture (2 years) | |||
| Intercept (β0) | 2.21 | 1.1 to 3.31 | 0.001 |
| Baseline trend (β1) | 0.068 | –0.0948 to 0.231 | 0.39 |
| Level change after intervention 1 + 2 (β2) | –1.17 | –3.28 to 0.931 | 0.25 |
| Trend change after intervention 1 + 2 (β3) | 0.06 | –0.504 to 0.625 | 0.82 |
| Level change after intervention 3 + 4 (β4) | –0.88 | –3.83 to 2.06 | 0.53 |
| Trend change after intervention 3 + 4 (β5) | –0.337 | –1.66 to 0.985 | 0.59 |
| Level change after intervention 5 (β6) | 0.583 | –2.46 to 3.62 | 0.69 |
| Trend change after intervention 5 (β7) | 0.25 | –1.46 to 1.95 | 0.76 |
| Major non-hip fracture (2 years) | |||
| Intercept (β0) | 2.09 | 1.41 to 2.77 | < 0.001 |
| Baseline trend (β1) | 0.02 | –0.08 to 0.12 | 0.72 |
| Level change after intervention 1 + 2 (β2) | 0.28 | –1.01 to 1.57 | 0.65 |
| Trend change after intervention 1 + 2 (β3) | –0.23 | –0.58 to 0.11 | 0.17 |
| Level change after intervention 3 + 4 (β4) | 0.78 | –1.03 to 2.59 | 0.37 |
| Trend change after intervention 3 + 4 (β5) | 0.08 | –0.74 to 0.89 | 0.85 |
| Level change after intervention 5 (β6) | 0.59 | –1.28 to 2.46 | 0.51 |
| Trend change after intervention 5 (β7) | 0.055 | –0.99 to 1.11 | 0.91 |
| Mortality (30 days) | |||
| Intercept (β0) | 7.26 | 5.78 to 8.74 | < 0.001 |
| Baseline trend (β1) | 0.03 | –0.19 to 0.25 | 0.8 |
| Level change after intervention 1 + 2 (β2) | 0.46 | –2.36 to 3.28 | 0.74 |
| Trend change after intervention 1 + 2 (β3) | –0.37 | –1.13 to 0.38 | 0.31 |
| Level change after intervention 3 + 4 (β4) | –1.89 | –5.86 to 2.05 | 0.33 |
| Trend change after intervention 3 + 4 (β5) | 0.67 | –1.1 to 2.44 | 0.44 |
| Level change after intervention 5 (β6) | –2.58 | –5.57 to 0.4 | 0.086 |
| Trend change after intervention 5 (β7) | 0.04 | –1.67 to 1.75 | 0.96 |
| Mortality (30 days): difference between cases and controls | |||
| Intercept (β0) | 6.17 | 4.49 to 7.85 | < 0.001 |
| Baseline trend (β1) | 0.05 | –0.2 to 0.29 | 0.69 |
| Level change after intervention 1 + 2 (β2) | 0.38 | –2.81 to 3.58 | 0.8 |
| Trend change after intervention 1 + 2 (β3) | –0.33 | –1.18 to 0.52 | 0.43 |
| Level change after intervention 3 + 4 (β4) | –2.51 | –6.97 to 1.96 | 0.25 |
| Trend change after intervention 3 + 4 (β5) | 0.82 | –1.19 to 2.83 | 0.4 |
| Level change after intervention 5 (β6) | –2.69 | –6.07 to 0.68 | 0.11 |
| Trend change after intervention 5 (β7) | –0.15 | –2.09 to 1.78 | 0.87 |
| Mortality (1 year) | |||
| Intercept (β0) | 24.7 | 21.8 to 27.5 | < 0.001 |
| Baseline trend (β1) | –0.1 | –0.53 to 0.32 | 0.61 |
| Level change after intervention 1 + 2 (β2) | –0.52 | –5.98 to 4.95 | 0.84 |
| Trend change after intervention 1 + 2 (β3) | 0.28 | –1.19 to 1.74 | 0.69 |
| Level change after intervention 3 + 4 (β4) | –4.6 | –12.2 to 3.04 | 0.22 |
| Trend change after intervention 3 + 4 (β5) | 1.56 | –1.87 to 5 | 0.35 |
| Level change after intervention 5 (β6) | –6.05 | –12.2 to 0.11 | 0.054 |
| Trend change after intervention 5 (β7) | –1.75 | –5.18 to 1.68 | 0.3 |
| Mortality (1 year): difference between cases and controls | |||
| Intercept (β0) | 13 | 10.1 to 15.8 | < 0.001 |
| Baseline trend (β1) | 0.1 | –0.32 to 0.52 | 0.62 |
| Level change after intervention 1 + 2 (β2) | –0.7 | –6.12 to 4.73 | 0.79 |
| Trend change after intervention 1 + 2 (β3) | –0.17 | –1.62 to 1.29 | 0.81 |
| Level change after intervention 3 + 4 (β4) | –2 | –9.59 to 5.6 | 0.59 |
| Trend change after intervention 3 + 4 (β5) | 1.97 | –1.45 to 5.38 | 0.24 |
| Level change after intervention 5 (β6) | –9.32 | –15.5 to –3.21 | 0.005 |
| Trend change after intervention 5 (β7) | –0.95 | –4.36 to 2.46 | 0.56 |
| Any incident antiOP medication use (4 months)a | |||
| Intercept (β0) | 0.47 | –2.92 to 3.87 | 0.77 |
| Baseline trend (β1) | 0.86 | 0.36 to 1.36 | 0.006 |
| Level change after intervention 1 + 2 (β2) | 9.54 | 3.08 to 16 | 0.006 |
| Trend change after intervention 1 + 2 (β3) | 1.97 | 0.24 to 3.7 | 0.028 |
| Level change after intervention 3 + 4 (β4) | –2.26 | –11.3 to 6.78 | 0.6 |
| Trend change after intervention 3 + 4 (β5) | –1.81 | –5.88 to 2.25 | 0.36 |
| Level change after intervention 5 (β6) | 4.8 | –2.5 to 12.1 | 0.18 |
| Trend change after intervention 5 (β7) | –0.61 | –4.67 to 3.45 | 0.75 |
| Any incident antiOP medication use (1 year)a | |||
| Intercept (β0) | 2.5 | –0.16 to 5.17 | 0.064 |
| Baseline trend (β1) | 1.09 | 0.69 to 1.48 | < 0.001 |
| Level change after intervention 1 + 2 (β2) | 11.6 | 6.55 to 16.7 | < 0.001 |
| Trend change after intervention 1 + 2 (β3) | 1.44 | 0.08 to 2.8 | 0.04 |
| Level change after intervention 3 + 4 (β4) | –0.57 | –7.68 to 6.53 | 0.87 |
| Trend change after intervention 3 + 4 (β5) | –1.52 | –4.71 to 1.67 | 0.33 |
| Level change after intervention 5 (β6) | 1.31 | –4.42 to 7.03 | 0.64 |
| Trend change after intervention 5 (β7) | 0.18 | –3 to 3.37 | 0.91 |
| Bisphosphonate prevalence (2–6 months)a | |||
| Intercept (β0) | 0.84 | –2.69 to 4.36 | 0.62 |
| Baseline trend (β1) | 0.88 | 0.36 to 1.4 | 0.003 |
| Level change after intervention 1 + 2 (β2) | 8.61 | 1.9 to 15.3 | 0.015 |
| Trend change after intervention 1 + 2 (β3) | 1.76 | –0.04 to 3.56 | 0.055 |
| Level change after intervention 3 + 4 (β4) | –12.1 | –21.5 to –2.75 | 0.015 |
| Trend change after intervention 3 + 4 (β5) | 1.91 | –2.31 to 6.13 | 0.35 |
| Level change after intervention 5 (β6) | –1.1 | –8.68 to 6.46 | 0.76 |
| Trend change after intervention 5 (β7) | –3.4 | –7.62 to 0.82 | 0.11 |
| Bisphosphonate prevalence (10–14 months)a | |||
| Intercept (β0) | 1.65 | –1.13 to 4.43 | 0.23 |
| Baseline trend (β1) | 0.89 | 0.48 to 1.3 | < 0.001 |
| Level change after intervention 1 + 2 (β2) | 9.28 | 3.98 to 14.6 | 0.002 |
| Trend change after intervention 1 + 2 (β3) | –0.02 | –1.44 to 1.4 | 0.98 |
| Level change after intervention 3 + 4 (β4) | –8.18 | –15.6 to –0.77 | 0.033 |
| Trend change after intervention 3 + 4 (β5) | 2.11 | –1.22 to 5.44 | 0.2 |
| Level change after intervention 5 (β6) | 0.19 | –5.79 to 6.17 | 0.95 |
| Trend change after intervention 5 (β7) | –2.46 | –5.79 to 0.87 | 0.14 |
Appendix 9 Estimated impact of interventions using segmented linear regression (parsimonious) models on all primary hip fracture patients, stratified by sex
| Variables | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Males | |||
| Any incident antiOP medication use (1 year)a | |||
| Intercept (β0) | 0.57 | –3.34 to 4.49 | 0.76 |
| Baseline trend (β1) | 0.67 | 0.09 to 1.24 | 0.026 |
| Level change after intervention 1 + 2 (β2) | 9.07 | 3.68 to 14.5 | 0.002 |
| Trend change after intervention 1 + 2 (β3) | 1.51 | 0.49 to 2.53 | 0.006 |
| Level change after intervention 3 + 4 (β4) | –7.52 | –14.1 to –0.95 | 0.027 |
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Bisphosphonate prevalence (10–14 months)a | |||
| Intercept (β0) | 2.83 | 1.1 to 4.56 | 0.003 |
| Baseline trend (β1) | |||
| Level change after intervention 1 + 2 (β2) | 7.98 | 4.06 to 11.9 | < 0.001 |
| Trend change after intervention 1 + 2 (β3) | 1.74 | 0.94 to 2.53 | < 0.001 |
| Level change after intervention 3 + 4 (β4) | –8.69 | –14.8 to –2.56 | 0.008 |
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Females | |||
| Any incident antiOP medication use (1 year)a | |||
| Intercept (β0) | 1.63 | –0.49 to 3.75 | 0.13 |
| Baseline trend (β1) | 1.48 | 1.23 to 1.73 | < 0.001 |
| Level change after intervention 1 + 2 (β2) | 15.2 | 11.1 to 19.3 | < 0.001 |
| Trend change after intervention 1 + 2 (β3) | |||
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
| Bisphosphonate prevalence (10–14 months)a | |||
| Intercept (β0) | 2.11 | –0.64 to 4.86 | 0.13 |
| Baseline trend (β1) | 1.04 | 0.65 to 1.42 | < 0.001 |
| Level change after intervention 1 + 2 (β2) | 9.59 | 5.22 to 14 | < 0.001 |
| Trend change after intervention 1 + 2 (β3) | –4.22 | –8.53 to 0.09 | 0.055 |
| Level change after intervention 3 + 4 (β4) | |||
| Trend change after intervention 3 + 4 (β5) | |||
| Level change after intervention 5 (β6) | |||
| Trend change after intervention 5 (β7) | |||
Appendix 10 Estimated impact of interventions using segmented linear regression (full) models on all primary hip fracture patients, stratified by sex
| Variables | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Males | |||
| Any incident antiOP medication use (1 year)a | |||
| Intercept (β0) | 0.57 | –3.46 to 4.6 | 0.76 |
| Baseline trend (β1) | 0.67 | 0.71 to 1.26 | 0.031 |
| Level change after intervention 1 + 2 (β2) | 6.6 | –1.08 to 14.3 | 0.087 |
| Trend change after intervention 1 + 2 (β3) | 2.33 | 0.27 to 4.39 | 0.029 |
| Level change after intervention 3 + 4 (β4) | –4.8 | –15.5 to 5.94 | 0.36 |
| Trend change after intervention 3 + 4 (β5) | –3.1 | –7.93 to 1.72 | 0.19 |
| Level change after intervention 5 (β6) | 4.42 | –4.24 to 13.1 | 0.3 |
| Trend change after intervention 5 (β7) | 1.67 | –3.15 to 6.5 | 0.47 |
| Bisphosphonate prevalence (10–14 months)a | |||
| Intercept (β0) | 0.65 | –3.33 to 4.62 | 0.74 |
| Baseline trend (β1) | 0.36 | –0.22 to 0.95 | 0.21 |
| Level change after intervention 1 + 2 (β2) | 5.56 | –2.02 to 13.1 | 0.14 |
| Trend change after intervention 1 + 2 (β3) | 1.45 | –0.58 to 3.48 | 0.15 |
| Level change after intervention 3 + 4 (β4) | –10.5 | –21.1 to 0.11 | 0.052 |
| Trend change after intervention 3 + 4 (β5) | 0.26 | –4.51 to 5.02 | 0.91 |
| Level change after intervention 5 (β6) | 1.39 | –7.16 to 9.95 | 0.73 |
| Trend change after intervention 5 (β7) | –0.78 | –5.54 to 4 | 0.73 |
| Females | |||
| Any incident antiOP medication use (1 year)a | |||
| Intercept (β0) | 3.14 | –0.2 to 6.49 | 0.064 |
| Baseline trend (β1) | 1.23 | 0.73 to 1.72 | < 0.001 |
| Level change after intervention 1 + 2 (β2) | 13.3 | 6.94 to 19.7 | < 0.001 |
| Trend change after intervention 1 + 2 (β3) | 1.14 | –0.57 to 2.85 | 0.18 |
| Level change after intervention 3 + 4 (β4) | 0.83 | –8.07 to 9.74 | 0.85 |
| Trend change after intervention 3 + 4 (β5) | –0.99 | –5 to 3.01 | 0.61 |
| Level change after intervention 5 (β6) | 0.27 | –6.92 to 7.45 | 0.94 |
| Trend change after intervention 5 (β7) | –0.31 | –4.32 to 3.69 | 0.87 |
| Bisphosphonate prevalence (10–14 months)a | |||
| Intercept (β0) | 1.77 | –1.59 to 5.14 | 0.28 |
| Baseline trend (β1) | 1.09 | 0.6 to 1.59 | < 0.001 |
| Level change after intervention 1 + 2 (β2) | 10.2 | 3.84 to 16.7 | 0.004 |
| Trend change after intervention 1 + 2 (β3) | –0.39 | –2.1 to 1.33 | 0.64 |
| Level change after intervention 3 + 4 (β4) | –7.94 | –16.9 to 1.03 | 0.079 |
| Trend change after intervention 3 + 4 (β5) | 2.52 | –1.51 to 6.55 | 0.2 |
| Level change after intervention 5 (β6) | –0.57 | –7.8 to 6.66 | 0.87 |
| Trend change after intervention 5 (β7) | –2.78 | –6.81 to 1.25 | 0.16 |
Glossary
- Charlson Comorbity Index
- An index of diseases which predicts 10-year mortality for a patient with comorbid conditions.
- Clinical Commissioning Group
- A NHS organisation which organises the delivery of NHS services in England.
- Continuous inpatient spell
- A continuous period of care in the NHS.
- Healthcare Resource Group
- A grouping of events or procedures performed in the NHS which use a similar level of resources.
- International Classification of Diseases, Tenth Edition
- A medical classification list produced by the World Health Organization and containing codes for, for example, diseases, injuries and symptoms.
- International Osteoporosis Foundation
- A global alliance of patient societies, research organisations, health-care professionals and international companies working to promote bone, muscle and joint health.
- National Osteoporosis Society
- A UK-based osteoporosis charity.
- Normalisation Process Theory
- A method to look at how the collective actions of agents drive the implementation of a new service.
- Office of Population Censuses and Surveys, Classification of Interventions and Procedures version 4
- A list of codes for operations, procedures and interventions performed.
- Periprosthetic fracture
- A fracture which occurs around the components of a total hip replacement.
List of abbreviations
- A&E
- accident and emergency
- BNF
- British National Formulary
- BOA
- British Orthopaedic Association
- CCG
- Clinical Commissioning Group
- CG
- clinical guideline
- CI
- confidence interval
- CIPS
- continuous inpatient spell
- CPRD
- Clinical Practice Research Datalink
- DXA
- dual-energy X-ray absorptiometry
- EQ-5D
- European Quality of Life-5 Dimensions
- EVPI
- expected value of perfect information
- FLS
- fracture liaison service
- GLM
- generalised linear model
- GOLD
- Gp OnLine Data
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICD-10
- International Statistical Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- ID
- identification
- IOF
- International Osteoporosis Foundation
- IQR
- interquartile range
- i.v.
- intravenous
- Kaiser SCAL
- Kaiser Southern California Healthy Bones Program
- NHFD
- National Hip Fracture Database
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- NPT
- Normalisation Process Theory
- ONS
- Office for National Statistics
- OPCS-4
- Office of Population Censuses and Surveys, Classification of Interventions and Procedures version 4
- PCT
- primary care trust
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SHR
- subhazard ratio
- TA
- technology appraisal
- WTE
- whole-time equivalent