Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/2000/05. The contractual start date was in October 2012. The final report began editorial review in October 2015 and was accepted for publication in March 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Claire Hulme is a member of the National Institute for Health Research Health Technology Assessment Commissioning Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Closs et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Dementia and pain are common and poorly managed
It has been estimated that 44.4 million people worldwide have dementia,1 including 670,000 people in England, and that one in three people aged > 65 years will develop the condition. 2 Dementias are chronic neurodegenerative syndromes that are most common in older people. They include Alzheimer’s disease, vascular dementia and frontotemporal dementia,3 and are associated with multiple changes in the brain, causing deterioration in cognitive performance as well as changes in behaviour, personality and communicative functioning. 4,5 Pain is also common among older people, with 45% of people aged > 65 years experiencing chronic pain. 6 A national study in the USA suggested that > 10% of people in the community aged > 65 years have dementia and that, of these, over 60% experience bothersome pain and more than 40% have pain that limits their activities. 7 In the UK the Care Quality Commission (2014)8 investigated people’s experiences of dementia care as they moved between care homes and hospitals, and found that the inability to communicate about pain was one of the most important experiences reported by people and their families. They found that assessments to identify and manage pain were variable, putting people with dementia at risk unnecessarily. 8 The identification and treatment of pain has frequently been poor among people with dementia in both acute9,10 and long-term settings. 11–13
The policy context
The policy imperative for this work is considerable. In England, the 5-year National Dementia Strategy14 (implemented in 2009) prioritised the need to improve the quality of care for people with dementia in general hospitals (objective 8). Then, in 2015, the National Institute for Health and Care Excellence (NICE) published a dementia pathway that emphasised the importance of recognising and treating pain. 15 In addition, two sets of national guidance have been published, one for assessing16 and another for managing17 the pain of older people, each of which considers issues related to cognitive impairment in this group. Although these are useful additions to the information available for clinicians, we do not yet know how to assess and manage the pain of people with cognitive impairment effectively, particularly in acute settings.
The impact of dementia on the experience and expression of pain
Patients with advancing dementia sustain progressive impairments in their cognitive, linguistic and social skills. Nevertheless, they are susceptible to the same potentially painful conditions as those without dementia, and there is little evidence to suggest that they experience less pain than their counterparts without cognitive impairment. 18 While they may experience pain, they may be unable either to understand what they are feeling or to verbalise that they are (or were) in pain. This makes it impossible for health-care professionals (HCPs) to rely on the clinical ‘gold standard’ of self-report for assessing pain in those who are severely cognitively impaired. For those who still have the ability to communicate, their inability to remember, interpret and respond to recent pain may limit their reports to ‘here and now’ experiences. 19
As 88–95% of people with dementia have difficulties with verbal communication,20,21 the recognition and assessment (and therefore management) of pain in this group is a significant challenge for those caring for them. The lack of appropriate pain management may then lead to functional decline, slow rehabilitation, disturbances in sleep routine, depression, agitation, poor appetite, impaired movement, increased risk of falling and a poorer quality of life. 22–24
Pain in hospital patients with dementia
It has been estimated that the cost of health care for people with dementia is around £1.2B, of which hospital inpatient stays account for 44%. 25 Dementia increases the length of hospital admission by an average of 4 days to > 23 days,26,27 resulting in an increase in complications28 and the risk of iatrogenic harm through polypharmacy. 29
An acute hospital ward may be a disorienting and distressing environment for a person with dementia due to heightened/unescapable noise, bright lighting and unfamiliar staff and surroundings. A study undertaken in one UK hospital showed that 95% of patients with advanced dementia were in pain as assessed using three observational pain tools [Abbey Pain Scale, Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC) and the Doloplus] as part of a randomised controlled trial in palliative care. 30 Research suggests that hospital patients with dementia are less likely to receive pain control than those without. 31
Poor pain control in the context of the acute environment is associated with neuropsychiatric symptoms, particularly aggression and anxiety,32 as well as behavioural responses such as agitation, vocalisations and withdrawal. 33 Neuropsychiatric symptoms affect over 75% of people with dementia admitted to acute hospitals and can increase the risk of mortality and cognitive decline. 34 Neuropsychiatric symptoms are particularly challenging for clinical staff to manage, and are often associated with suboptimal care or inappropriate prescriptions of antipsychotic medications. 35 Consequently, people with dementia are at higher risk of adverse events during their hospital stay36 and are more likely than their counterparts without cognitive impairment to spend an extended time in hospital. 37–39
There are no behaviours which are exclusively associated with pain, increasing the difficulty of identifying its presence. In people with dementia, many behaviours generally considered to indicate pain may also indicate boredom, hunger, depression, disorientation or other problems. 40 These behaviours lack specificity, some observational pain assessment tools may detect distress as well as pain, and there may be overlap between the two.
As it has been estimated that in general hospitals the prevalence of dementia on acute wards is around 40%,41,42 and more than 45% of those aged > 65 years have chronic pain,6 it is inevitable that hospital staff will regularly provide care for the substantial number of patients who suffer from both of these invisible but highly debilitating conditions. For clinical staff the challenge of interpreting the behaviour of the many patients who have both pain and dementia is considerable, militating against a simple approach to the assessment of pain.
The assessment of pain for people with dementia
In general, because of the subjectivity of pain, self-report is considered to be the gold standard for pain assessment. Although people with mild to moderate dementia are often able to report their pain verbally or use simple visual or numerical pain intensity assessment tools, these options are not feasible for use with people with later-stage dementia in whom communication ability is severely impaired. 43,44 As a result, previous work has shown that pain is frequently underdetected and poorly managed in people with dementia, in both long-term and acute care. 31,45–47
In the absence of accurate self-report it has been necessary to develop observational tools to be used in both research and practice based on the interpretation of behavioural cues as a proxy for the presence of pain. This approach has resulted in a proliferation in the number of pain assessment instruments developed to identify behavioural indicators of pain in people with dementia and other cognitive impairment. The most structured of these are predominantly based on guidance published by the American Geriatrics Society,48 which presents six domains for pain assessment in older adults. These include facial expression, negative vocalisation, body language, changes in activity patterns, changes in interpersonal interactions and mental status changes. However, the interpretation of many of these behaviours is complex when applied to dementia because of the considerable overlap with other common behavioural symptoms or cognitive deficits which may confound an assessment, manifesting from boredom, hunger, anxiety, depression or disorientation. 40 This increases the complexity of identifying the presence of pain accurately in patients with dementia and raises questions about the validity of existing instruments. The psychometric properties, discriminative properties and clinical utility of currently available instruments are as yet unclear. As a result, there is no clear guidance for clinicians and care staff on the effective assessment of pain, or how this should inform treatment and care decision-making. A large number of systematic reviews have been published which analyse the relative value and strength of evidence of existing pain tools. There is a need for guidance on the best evidence available and for an overall comprehensive synthesis.
Most pain tools for use with people with dementia have been developed within long-term care, and more work is required to establish whether or not the use of pain tools is feasible in the acute hospital and if these tools are reliable in detecting pain. There have been no studies in the UK exploring how pain is detected and managed in people with dementia on acute hospital wards. Recognising pain in people with dementia has been described as a ‘guessing game’ by some HCPs,49 and the Counting the Cost: Caring for People with Dementia on Hospital Wards report37 identified that 51% of carers and nurses were dissatisfied with their ability to detect pain and 71% of hospital staff wanted more training in recognising pain.
Dementia, pain and decision-making
The detection and management of pain are cognitive activities associated with decision-making. Pain detection involves identifying information cues (e.g. from a formal assessment tool, patient self-report, observation of behaviour) that would indicate a patient is experiencing pain. Clinicians then evaluate those cues to reach a judgement regarding the nature of a patient’s pain, before making a decision regarding what to do to manage that pain (the decision process). If individuals fail to assess a patient’s pain effectively, or detect that they have pain but then decide not to do anything to manage it, pain can be poorly controlled. The use of decision support tools can aid clinicians in the decision-making process, improve both the processes and outcomes of care50 and subsequently lead to an improvement in the quality of care for patients. In this study we aimed to develop decision support tools to assist clinicians with both the process of judgement (identification of pain) and decision-making (what actions to take on the basis of the judgement made). Figure 1 provides an overview of the theoretical framework that guided this study. 51,52
FIGURE 1.
Theoretical framework for decision-making.
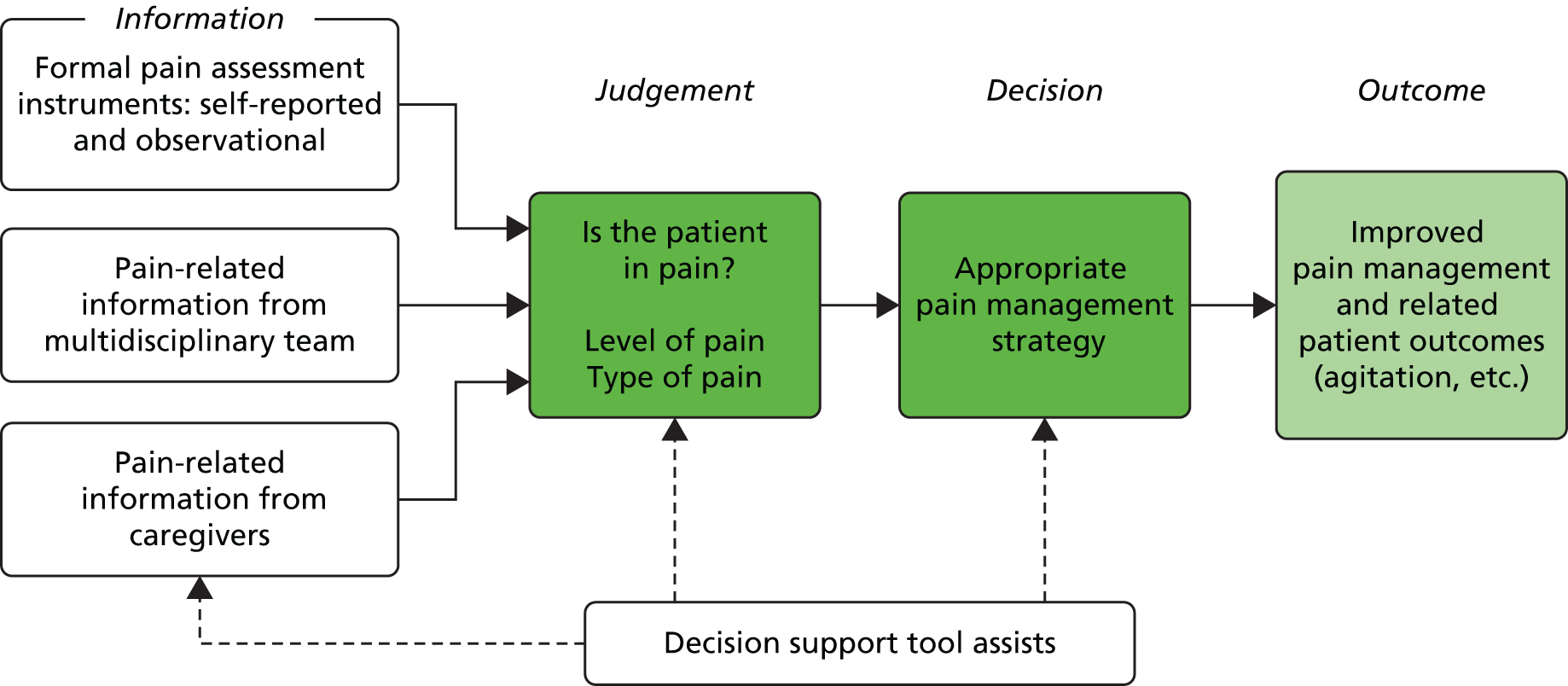
The framework shows the three main sources of information likely to be used by clinical staff as a basis for making judgements about patients’ pain. This judgement then leads to a decision about how to manage the pain in order to produce the outcome of reduced pain and related outcomes for the patient. This theoretical framework suggests that a decision support tool could assist in the acquisition of appropriate information; the accuracy of judgements; and the making of optimal decisions. Taken together, these should improve outcomes for patients.
There is a paucity of research in this area, with little having been reported in the UK. One recent study undertaken in Australia, which examined the complexities of pain assessment and management for hospitalised older people, identified four key aspects of care: communication among nurses and between older patients and nurses; strategies for pain management; environmental and organisational aspects of care; and complexities in the nature of pain. 53 The intricacies of meeting the analgesic needs of older patients were emphasised, especially for those with communication deficits. There has been no similar research undertaken in the NHS in the UK.
Aims and objectives
The aim of the work reported here, therefore, was to inform the development of a decision support tool to be used by staff in hospital settings, to aid in the recognition, assessment and management of pain among people with dementia. In order to achieve this, two studies were undertaken (Figure 2).
FIGURE 2.
Overview of project and preliminary development of PADDS.

The first study was a systematic review of systematic reviews of observational pain assessment instruments, referred to as the meta-review, which had the following three objectives:
-
to identify all tools which are available to assess pain in adults with dementia
-
to identify the settings and patient populations with which they had been used
-
to assess their reliability, validity and clinical utility.
The second was a multisite observational study of current pain assessment and management practices in a range of wards within four hospital sites across the UK, which had the following four objectives:
-
to identify what information is currently elicited and used by clinicians when detecting and managing pain in patients with dementia in acute hospital settings
-
to explore the existing process of decision-making for detecting and managing pain in patients with dementia in acute hospital settings
-
to identify the role (actual and potential) of carers in this process
-
to explore the organisational context in which HCPs operate with regard to this decision-making process.
Development of intervention and follow-on study: health economics and technical specifications
Data from the observational study were analysed from a health economics perspective, with the aim of developing data collection instruments for the feasibility evaluation of the intervention. The focus of the health economics analysis was twofold: first, to identify cost categories and resource use associated with the intervention developed in this project; and second, design of the instruments, which involved exploration of the use of outcome measures to assess proxy issues and generate hypotheses about the domain of impact. A set of health economic data collection forms was developed as the result of this work.
Finally, on the basis of the evidence gathered within this research project, we compiled a technical requirements specification document for the design of a Pain And Dementia Decision Support (PADDS) system.
Study Steering Committee project oversight
A Study Steering Committee (SSC) was established in accordance with Health Service and Delivery Research (HSDR) guidance. The SSC consisted of four experts in the areas of dementia and pain research, together with a representative for carers of patients of dementia.
The SSC and project investigators met three times during the project (June 2014, January 2015 and June 2015) with a final teleconference at the end of the project (1 October 2015). Regular updates on the project were circulated to the chairperson and the SSC by e-mail. Documents shared with the SSC included internal reports, manuscripts for publication, protocol amendments, National Institute for Health Research (NIHR) reports and any significant correspondence with NIHR or sponsor.
Summary
This study was funded by the NIHR HSDR programme (reference number 11/2000/05). The project aimed to generate a theory-based intervention to be used as the basis for providing better-quality care in the assessment and management of pain in people with dementia in hospital settings. The objectives were to identify the best observational pain assessment tool(s) currently available; to understand and support the decision-making processes of HCPs in hospital wards; and to provide insights into how carers’ expertise can be incorporated into the decision-making process. In order to achieve this we undertook two studies, a systematic review of systematic reviews (meta-review) and an observational study, and developed the foundations for the development and implementation of a decision support intervention (see Figure 2).
Structure of the report
The structure and content of each section of this report are outlined below.
Patient and public involvement
The involvement of people with dementia and their carers was an important part of the research process. The composition and participation of our lay advisory group (LAG) are described in Chapter 2, finishing with some reflections on the processes and timing of its involvement.
Meta-review
A systematic review of systematic reviews of observational methods of assessing pain in people with cognitive impairments was undertaken (see Chapters 3 and 4). Given the plethora of instruments that have been developed over the past decade, and the large number of systematic reviews that have produced inconclusive findings, we undertook a systematic review of systematic reviews (meta-review) in order to establish the psychometric properties and clinical utility of existing tools. This review is available as an open-access publication. 54
Observational study
An observational study of current pain assessment and management practices in a range of wards in four hospital sites across the UK was undertaken (see Chapters 5 and 6). This study provided valuable insights into the complexities related to pain communications, documentation and interventions, as well as the influence of the organisation and the ward environment faced by clinical staff when assessing and managing pain in patients with dementia.
Health economics
A health economics analysis was designed to develop methods for assessing costs of implementing a new decision support intervention (see Chapter 8). This analysis was linked to the observational study. It aimed to identify resource use associated with the intervention developed in this project, to develop health economic data collection forms and to explore use of the outcome measures to assess proxy issues and generate hypotheses about the domain of impact.
Discussion and proposal for a new decision support tool
The findings from these two studies are integrated and used to design a new decision support tool (the PADDS system), which requires testing for acceptability and feasibility in acute care settings (see Chapter 8). At the outset of this project it was anticipated that a discrete tool, such as an algorithm, would be developed and subjected to preliminary feasibility testing. However, our findings presented a far more complex picture than anticipated, requiring the development of a broader and more complex support intervention. This involved a conceptual shift, leading to a substantially different approach to decision support. We have discussed the reconceptualisation of pain assessment and management as a distributed, as opposed to a linear, process in more detail elsewhere. 55
As with many projects focusing on sensitive or hard-to-reach populations, ethics, research and development approvals and the recruitment of participants took longer than anticipated for the observational study. Following the development of the preliminary support tool, it became evident that a different approach to testing its feasibility from that originally envisaged was required, namely a realist feasibility evaluation. As the tool and the evaluation required would be substantially different from those in the original protocol, we required further ethics, research and development approvals in order to proceed with the evaluation. Unfortunately, there was insufficient time at the end of the project to complete these processes. We intend to submit a full proposal to the NIHR in order to continue the work by undertaking a realist evaluation of the PADDS’ usability, clinical utility and cost-effectiveness. Such a realist feasibility evaluation is a prerequisite for the refinement of the PADDS system, which may then be rigorously evaluated in a randomised controlled trial.
Chapter 2 Patient and public involvement
Introduction
Patient and public involvement was an important part of the research process. We involved people with dementia and their family members (‘carers’). Patient and public involvement informed the analysis of the findings from our exploratory study on how pain is currently detected, assessed and managed in patients with dementia in hospital settings, and the conclusions we drew from our study. The Alzheimer’s Society (AS) Research Network was instrumental in establishing our connection with the carers of persons with dementia via its research engagement manager.
The lay advisory group
A LAG was established at the beginning of the project. The group was invited to have an oversight and advisory role, informing the running of the project and providing advice on specific aspects of the research if necessary. It was made up of members of the AS Research Network, which is composed of carers and people with dementia who have received training in research methodology.
The LAG was made up of 16 members, one of whom was also a member of our SSC. It was agreed that the group would meet once a year and that they would receive project updates by e-mail, supplemented by further e-mail or telephone contact as and when needed.
More specifically, the involvement of the LAG members took place at three crucial moments in the life of the project:
-
at the beginning
-
on analysis of findings of the meta-review and start of the exploratory study
-
on completion of the exploratory study and initial work towards the development of a decision support intervention.
Each of these three moments of involvement is further detailed below, followed by reflections on how the process informed the development of the project.
The lay advisory group intervention at crucial times of project activities
Beginning of the project
The AS volunteers were consulted about the overall topic and approach of the study at a ‘speed dating’ event held after the themed call was announced by NIHR. Through this, the lay applicants came to be involved in the study and were recruited based on people’s interest after that event. They all provided useful insight and thoughts on the design of the project and where challenges might lie. This consultation resulted in changes and additions to the protocol and funding application. The group helped to review the information sheets and consent forms to make them more readable and user-friendly.
Mid-way through the meta-review and start of the exploratory study
A meeting with the LAG took place in February 2014 at the point when we were in the process of analysing the results of the systematic search for systematic reviews of pain assessment tools, and we had begun data collection for the exploratory study. The meeting was attended by eight lay members and three project team members. Two lay members who had difficulties travelling to the meeting were consulted by e-mail, and their feedback was included in the notes of the meeting which were circulated to all members of the group.
Part of the meeting was dedicated to discussing the findings of the meta-review. Patients’ and carers’ voices seem to be absent in studies of patient assessment scales for persons with dementia. At this meeting we reflected and shared ideas on pain assessment tools we had identified through the review, and we discussed the implications of the design of an alternative decision support tool to assist in pain assessment for patients with dementia.
In the second half of the meeting, the discussion was focused on those carer-facing tools intended to communicate a patient’s preferences and needs to hospital staff, including the patient’s experience of pain. These tools are paper forms known as, for example, ‘patient/carer’s passport’, ‘10 things about me’ or ‘know who I am’,and they are being introduced in hospitals across the NHS, including the sites we studied, as part of an effort to increase awareness and understanding of dementia. Usually carers and relatives are asked to fill in such forms at the time of the patient’s admission to hospital. The group was invited to comment on these kinds of forms and share any experiences they may have had with them.
Mid-way through the analysis of main study findings and start of the development of the intervention
A second meeting with the LAG took place in March 2015, to discuss the initial findings from the exploratory study with the group as we began work on the development of the decision support intervention. The meeting was attended by six lay members and two project team members, with follow-on activities taking place by e-mail that also included those who were unable to attend.
The discussion that took place during this meeting extended beyond pain assessment, touching on several points of interest raised by the participants, including the problem of hospital wards understaffing, the importance of a relationship of trust between patients and clinicians, specifically, the issue of carers experiencing distrust from clinicians (‘I felt a degree of suspicion’), and the importance of considering the patient as an ‘integrated whole’, as opposed to focusing only on the most acute medical condition.
As a follow-on from the meeting, the group was consulted by e-mail about the design of research instruments for the health economics evaluation of the intervention. In preparation for this phase of the research, a questionnaire was developed to collect baseline data from patients, carers and members of staff. The group was invited to comment on this new instrument, still in draft form, specifically, on whether they thought that participants would be able to answer the questions about hospital stays/contact with health services themselves, or if this should be completed by a research nurse, and if they could identify anything missing from the questionnaire (e.g. ‘Who would the patient typically see in hospital in respect of their dementia?’).
Finally, the present report was circulated by e-mail to the group at the end of the study (September 2015) to gather any further comments and feedback, and subsequent amendments were made.
How the lay advisory group intervention influenced the research process
Guidance on pain assessment and management for patients with dementia recommends that carers are be involved in the process, essentially to provide information that may help staff recognise patient-specific pain cues. The use of dementia awareness forms, such as those mentioned above, currently in use in the NHS, are part of this need to gather and share information about the patient as a person.
The contribution of the members of the LAG to our research was of great value for the interpretation of those findings from our exploratory study regarding the sharing of information in a hospital context particularly in relation to the pain recognition, assessment and management processes. Through the discussion with the group it became apparent that this is more complex than it may at first appear for a variety of reasons. For example, two main aspects came to the fore. First, there may be disagreement between family members of a person with dementia over what constitutes ‘the usual’ for the person and their likes or dislikes, and there is no clear answer to how to deal with this disagreement in the hospital context. Second, there is a real concern among the carers about the ‘labelling’ of their loved one associated with the use of the dementia awareness forms. This insight was taken into account during the analysis of the findings and included in the discussion in one of our manuscripts submitted for publication (Lichtner et al. 100).
The group also contributed advice on the design of research instruments. Its input on the design of the health economics questionnaire was particularly valuable.
Examples of the contributions from the group are provided in Table 1, illustrated with quotations from notes of meetings and e-mails from group members.
| Involvement | Contribution to the study |
|---|---|
| Advice on research instruments/documentation for use by patient and public involvement (i.e. information sheets, consent forms and health economics questionnaires) | Gathering lay views on research instruments was especially important to make them more readable and manageable. In particular, in relation to the health economics questionnaire to be filled in by patients with dementia and/or carers, there were concerns over:
|
| Carers’ and patients’ perspectives on the pain assessment and management process with the new tool in development | Members of the group stressed important points confirmed by the exploratory study, such as:
|
| Carers’ and patients’ perspectives on the use of dementia awareness forms currently in use in NHS hospitals and information-sharing with staff | The group agreed that the carers should be asked about the person with dementia’s needs and usual behaviours. Some also recounted how in their experience this had not happened (‘he was restless and nobody spoke to me at all’) |
| The group agreed that the lack of power of attorney is an issue and commented that this is a vital point; that family members can hold up the care of the patient because, for example, nobody has power of attorney; how ‘we should all have it’ (before dementia onset); that it is ‘complicated because of the financial implications’; and how it is difficult for those who do not have relatives and do not know who to ask | |
| Discussion took place over the trade-off between facilitating person identification and protecting confidentiality. This was a major point of discussion – the practice of ‘labelling people’ (e.g. with a butterfly or a flower). Some members of the group had issues with any labelling practice, such as ‘putting stickers at the end of beds’. Patients risk losing their identity (‘you are not Mrs Blog, but a patient with dementia’) and risk not been treated as a whole person (‘I know my mother was not seen holistically because she had dementia’). So, for example, they may not have been given physiotherapy (‘this happened with my mother, they would not give me any equipment after her hip replacement’) |
Evaluation of lay advisory group process
Our project aimed to deliver a decision support intervention to assist HCPs in the care of patients with dementia. A core aspect of this intervention was a decision support tool aimed at staff working in hospital clinical areas. There is a challenge in having meaningful patient and public involvement activities through a research process aimed at developing a working tool for staff – a tool that patients and the public may never use or get access to. They are key stakeholders in the overall process (and the outcomes of patient care delivered with the support of the tool), but not direct end-users of the new tool. Good practices of user-centred design in tool development include involving a varied range of stakeholders, but greater attention is paid often to the direct end-users. We believe that we were successful in overcoming this challenge, and that we were able to involve carers in a meaningful and lucrative way throughout the research.
The members of the LAG provided a complementary perspective over the findings of our study, and important points came out of the discussion that we then took into account at the time of tool development. The timing of the consultation was also important; meeting at ‘transition points’ in the research process enabled feedback from the group to be incorporated into the analysis of findings both for the systematic review and exploratory study, and also into the conclusions of the study, as disseminated in manuscripts for publications.
Face-to-face communication with members of the group happened through yearly meetings. The LAG meetings lasted 3 hours, over lunch, to give enough time for travelling, and they were held at King’s College London, the site of one of the investigators (AC), and a central location. The meetings did not have a formal agenda; rather, they were open and relaxed discussions and provided an opportunity to exchange ideas and inform the group of the progress of the project. Overall, this seemed a good format, allowing space for project presentations to update the group about the progress of the project, as well as substantial time for discussion.
We were aware that family members of persons with dementia are often elderly, possibly with health conditions that make travelling more difficult for them. However, all of the group had access to computers, and we investigated the possibility of using teleconferencing or videoconferencing facilities [such as Skype™ (Microsoft Corporation, Redmond, WA, USA)], as an alternative to meeting in person. We consulted on this with the group, and preference was expressed for meeting face to face. It was therefore agreed that those who could not attend would be consulted by e-mail and their feedback included in the notes of the meeting to be circulated to all members. One of the members who could not attend in person recommended our project to the AS as an exemplar in lay members’ participation, including those who were unable to attend in person. An e-mail was sent to the AS network stating that ‘this project would make an ideal example to take to Society regional meetings to try to explain research projects to the general membership’. Through the process it became apparent that difficulties in attending events in person is a major barrier for patient and public involvement, especially for persons with dementia, and that this is something that needs to be taken into account when planning patient and public involvement activities.
Finally, we never asked the group whether they were happy to be referred to as ‘carers’. Indeed ‘carer’ is not an uncontested label56 and, in retrospect, we should have consulted the group on this. Towards the end of the project one of the group members pointed out that instead of carer she would prefer ‘family supporter (I prefer to call myself that rather than carer)’ and, although this was not raised as an issue, her message was a reminder for future patient and public involvement activities with family members of patients with dementia to consult on this at the start of the research process.
The Alzheimer’s Society Conference
As the research project was coming to its end, in June 2015, we attended the AS research conference. We presented findings from the study to a varied audience including researchers (n = 75), some of whom were clinicians or health professionals, AS staff and trustees (n = 45) and, most importantly for the purpose of patient and public involvement, 72 volunteers, of whom 66 had a personal experience of dementia, including six people with dementia.
Conclusion
Patient and public involvement was very important for the conduct of the research and the conclusions of the study. Through their insight and experiences the members of the LAG helped us to problematise the findings and draw more nuanced conclusions. This chapter, along with the rest of the report, was circulated to the advisory group members, who commented favourably.
Chapter 3 Meta-review: methods
Introduction
This meta-review aimed to identify existing assessment tools with validation data concerning people with dementia. It presents a thorough synthesis of current systematic review literature concerning the psychometric properties and clinical utility of pain assessment tools for the assessment of pain in adults with dementia, and provides a detailed picture of the state of the field in the complex task of assessing pain. 54
For ease of reference, in this report we refer to our systematic review of systematic reviews as a ‘meta-review’. We call the systematic reviews considered for inclusion in the meta-review ‘reviews’, and refer to publications included in the reviews as ‘studies’. We use the term ‘records’ to refer to the bibliographic data of publications of reviews (for the most part retrieved through online database searches). The terms ‘scales’, ‘tools’ and ‘instruments’ (pain assessment) are used interchangeably. The process of the meta-review followed guidance from the Cochrane Collaboration57 and the Joanna Briggs Institute (JBI). 58 In undertaking this meta-review, our aims were as follows:
-
to identify all tools available to assess pain in adults with dementia
-
to identify the settings and patient populations with which they had been used
-
to assess their reliability, validity and clinical utility.
Criteria for considering reviews for inclusion
Definitions of criteria for inclusion of reviews in the meta-review followed an adapted setting, population, intervention, comparison, method of evaluation (SPICE) structure. 58 We included systematic reviews of pain assessment tools involving adults with dementia or with cognitive impairment. Dementia and cognitive impairment were defined according to the US National Library of Medicine’s medical subject heading (MeSH) vocabulary. Dementia was defined as ‘an acquired organic mental disorder with loss of intellectual abilities of sufficient severity to interfere with social or occupational functioning’. 59 The dementia MeSH term covered more specific subheadings such as Alzheimer disease or vascular dementia. Cognition disorder was defined as ‘disturbances in the mental process related to thinking, reasoning, and judgment’60 (distinct from, not including, delirium). We did not include learning disorders, defined as ‘Conditions characterized by a significant discrepancy between an individual’s perceived level of intellect and their ability to acquire new language and other cognitive skills’. 61 Examples of learning disorders of this type are dyslexia, dyscalculia and dysgraphia.
We included reviews regardless of setting (e.g. acute or nursing/care homes), type, location or intensity of pain (e.g. acute pain, persistent), and outcomes of the pain assessment (e.g. patients being in pain or not). Reviews were included if they provided psychometric data for the pain assessment tools and were available in English. We excluded publications, such as narrative reviews or case reports, which did not provide psychometric data or were not categorised as systematic reviews62 (Table 2 shows our systematic review definition).
| SPICE category | Criteria | Definitions |
|---|---|---|
| Setting | Reviews pertaining to any setting | Settings are, for example, acute hospitals, nursing homes, community settings |
| Patient population | Reviews of studies limited to adults with dementia or cognitive impairment | Dementia defined as:[. . .] an acquired organic mental disorder with loss of intellectual abilities of sufficient severity to interfere with social or occupational functioning. The dysfunction is multifaceted [. . .]National Library of Medicine59 |
| All stages of dementia in adults were considered (e.g. mild, severe) | Cognitive impairment defined as cognition disorder:Disturbances in the mental process related to thinking, reasoning, and judgmentNational Library of Medicine60Does not include learning disorders. (Source: MeSH vocabulary – www.nlm.nih.gov/mesh/) | |
| Intervention | Reviews of studies of the assessment of pain and of pain assessment tools. Reviews that included management of pain considered if they also covered assessment of pain | Pain assessment as defined by the International Association for the Study of Pain:[. . .] entails a comprehensive evaluation of the patient’s pain, symptoms, functional status, and clinical history [. . .] |
| All forms of pain were considered (e.g. acute pain, persistent), without distinction by location of pain (e.g. abdominal pain) | [. . .] The assessment process is essentially a dialogue between the patient and the health care provider that addresses the nature, location and extent of the pain, and looks at the patient’s daily life, and concludes with the pharmaceutical and nonpharmaceutical treatment options available to manage itPowell et al., pp. 67–7863Evaluation tools may be used in this process:To varying degrees, these tools attempt to locate and quantify the severity and duration of the patient’s subjective pain experience [. . .]Powell et al., pp. 67–7863 | |
| Reviews of studies of pain assessment were included, irrespective of the outcomes of the assessment (e.g. patients being in pain or not) | ||
| Evaluation (method of) | Systematic reviews only were included | Definition of systematic review:
|
| Additional criteria | Reviews were included only if with data and/or assessment of reliability and/or validity and/or clinical utility | Reliability:[. . .] the degree to which the measurement is free from measurement error [. . .]Mokkink et al.65 |
| Validity:[. . .] the degree to which the [instrument] measures the constructs(s) it purports to measure [. . .]Mokkink et al.65 | ||
| Inclusion limited to English language | Clinical utility: ‘the usefulness of the measure for decision-making’ (i.e. to inform further action, such as the administration of analgesics)66 |
Search methods for identification of reviews
The following databases were searched: MEDLINE, All Evidence Based Medicine Reviews [including Cochrane Database of Systematic Reviews, American College of Physicians (ACP) Journal Club, Database of Abstracts of Reviews of Effects, Cochrane controlled trials reports, Cochrane Methodology Register, Health Technology Assessment, and NHS Economic Evaluation Database], EMBASE, PsycINFO, and the Cumulative Index to Nursing and Allied Health Literature; the searches were all carried out on the same date (12 March 2013). Additional searches included The JBI Library (The JBI Database of Systematic Reviews and Implementation Reports) and the Centre for Reviews and Dissemination database. Further data were retrieved through reference chaining. No grey literature was sought.
The search strategy used a combination of text words and established indexing terms such as MeSH (see Appendix 1). The search was structured by the relevant SPICE concepts. Search terms were identified by comparing published search strategies adopted by reviews in similar areas,64,67 or on the subject of pain or pain management tools, not specifically for the same patient population,68 using the search strategy for retrieving reviews outlined by Montori et al. 69 Detailed search strategies were optimised for each electronic database searched.
Selection of reviews
Four reviewers (DD, MB, VL and PE) screened all search results, initially on the basis of title and abstract and then the full text of potentially eligible papers (see Figure 3). The results of the search were divided into two sets among the reviewers, so that each review was first screened by two of the reviewers independently, then assessed, again independently, by the other two reviewers. When consensus could not be reached, the reviews were referred to a third party (SJC).
Assessment of methodological quality of included reviews
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance on systematic reviews encourages reviewers ‘to think ahead carefully about what risks of bias (methodological and clinical) may have a bearing on the results of their systematic reviews’. 62 In our meta-review, the risk of bias may reside in each review considered for inclusion, as well as in the original studies that make up that review. We did not access the original studies to be able to accurately judge their quality or risk of bias. For each review, we assessed risk of bias in terms of how the review was conducted and the criteria applied for inclusion/exclusion. Critical appraisal was carried out by two independent reviewers, using the A MeaSurement Tool to Assess systematic Reviews (AMSTAR) systematic review critical appraisal tool. 70 Critical appraisal and evaluation of potential bias were carried out at the time of data extraction, after screening was completed on the basis of the inclusion criteria.
Data extraction and management
Data were extracted by two reviewers independently using a set of data extraction forms which were developed for the meta-review: (1) the AMSTAR checklist;70 (2) two forms for data about the reviews; and (3) one form for data about the tools. The third included a field for data extraction on the user-centredness of the tools, informed by Long and Dixon’s71 work on the development of health status instruments. 71 The data extraction forms were both paper based and built into a Microsoft Access® database (Microsoft Corporation, Redmond, WA, USA). At the time of data extraction, the reviews eligible for inclusion were screened further on the basis of availability of psychometric data of tools. At this point, we found that some of the reviews initially identified as being eligible for inclusion in the meta-review did not provide psychometric data of tools and were subsequently excluded (this is discussed in detail in Chapter 4). Data about the characteristics of the tool (e.g. tool design and instructions for use) were extracted from the reviews; we did not search for, or retrieve, the original tools.
The search retrieved 441 potentially eligible unique records. After screening titles and abstracts, and removing duplicates, we obtained the full text of 183 records and assessed these for eligibility. We identified 23 reviews as being potentially eligible for inclusion, of which 13 were excluded as they did not provide data on the psychometric properties of the tools. The remaining set included 10 records reporting data from eight reviews (the Schofield et al. 200572 review was reported in three separate studies;72–74 we have combined the results of this) (Figure 3). The tables of included and excluded reviews are listed in Appendix 2. The reviews were synthesised using a narrative synthesis approach.
FIGURE 3.
Flow chart of retrieved sources and screening process. Reproduced from Lichtner et al. 54 © 2014 Lichtner et al. ; licensee BioMed Central. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
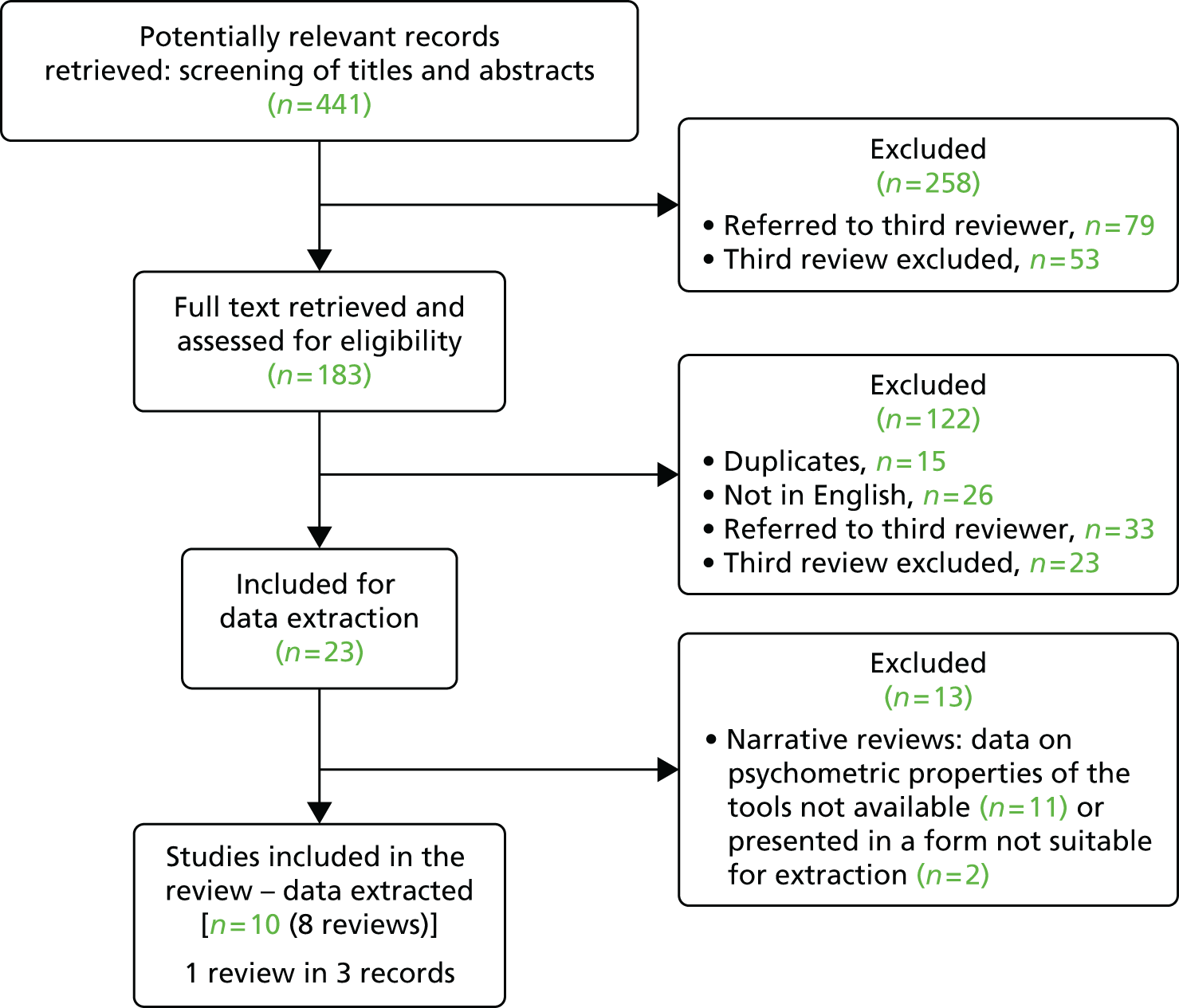
Further details about the methods are provided in additional files (see Appendix 3).
Chapter 4 Meta-review: results
Introduction
The findings are structured as follows. First, we briefly summarise the reviews considered at the time of data extraction, but excluded owing to lack of data on psychometric properties of the tools. Second, we describe the reviews included in our analysis, specifically their methods and our quality assessment of them. Third, we describe the findings of these included reviews regarding the characteristics, psychometric properties, feasibility of use and clinical utility of pain assessment tools. We conclude with the reviews’ overall assessment of these tools.
Description of excluded reviews
Thirteen of the 23 reviews were excluded because they did not provide suitable data for extraction (these data were absent or not reported in a format suitable for extraction). Several of these were narrative reviews. They varied in length and details of reporting. Tools were analysed in the broader context of, for example, pain physiology, pain prevalence, and patients’ attitudes and beliefs about pain. For some, the areas of focus were the processes of pain assessment and/or pain management/interventions in the elderly population, including (but not always limited to) persons with dementia (e.g. in Rutledge et al. ,75 Rutledge and Donaldson,76 Andrade et al. 77 and Miller and Talerico78) and/or in specific types of pain (e.g. orofacial pain, Lobbezoo et al. 79). One review80 focused on the barriers to successful pain assessment, identifying non-use of assessment tools as one such barrier.
Two reviews were updated by, or related to, a later review by the same authors (Rutledge et al. 75 is an update of Rutledge and Donaldson76 – both excluded; Herr et al. 81 is related to Herr et al. 82 – the last included in our study – and aimed at reviewing the methods of the previous study).
Two reviews83,84 were reviews of reviews, and they were very specific in their aims, namely to identify pain assessment tools for adults with cognitive impairment recommended for use by paramedics83 and district nurses. 84 They identified two and four reviews, respectively, all of which were among those that we retrieved. Thus, the reasons for exclusion in their case were their nature as reviews of reviews (overviews, as opposed to systematic reviews), that they provided no data for extraction and on the grounds of repetition.
Two other reviews81,85 did report and discuss psychometric data, but not in a form suitable for this meta-review. Their choice of reporting may suggest authors’ methodological concerns for the comparability and presentation of the data in quantitative form, given the heterogeneity of the studies and their methods.
Description of included reviews
Each review included in this meta-review analysed between 8 and 13 tools (Table 3). The most frequently reviewed tools included the Abbey Pain Scale, NOn-communicative Patient’s Pain Assessment Instrument (NOPPAIN), PACSLAC, Pain Assessment for the Dementing Elderly (PADE), Checklist of Nonverbal Pain Indicators (CNPI) and Pain Assessment IN Advanced Dementia (PAINAD). Reviews had searched the literature across a variety of date ranges, from 1980 to 2010. The number of individual studies included in each review varied from 972 to 29,64 although the number of included studies in some reviews was ambiguous. The reasons for this ambiguity were twofold. First, the number of studies included in each review was different for each tool, thus making it difficult to aggregate in one number (‘number of included studies’). Second, the studies included in each review were each found to have reported one or more studies aimed at evaluating a tool. Thus, a number of included studies of ‘1’ may actually have referred to a larger number of studies conducted.
| Tool | Systematic review, authors and year of publication | Number of reviews per tool | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Corbett et al., 201267 | Herr et al., 200682 | Park et al., 201087 | Qi et al., 201286 | Schofield et al., 200572 | Smith, 200588 | van Herk et al., 200766 | Zwakhalen et al., 200664 | ||
| Abbey Pain Scale | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |
| ADD protocol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| Behavior Checklist | ✓ | ✓ | 2 | ||||||
| CNPI | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| Comfort Checklist | ✓ | 1 | |||||||
| CPAT | ✓ | 1 | |||||||
| Doloplus-2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| DS-DAT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| ECPA | ✓ | 1 | |||||||
| ECS | ✓ | 1 | |||||||
| EPCA-2 | ✓ | 1 | |||||||
| FACS | ✓ | 1 | |||||||
| FLACC | ✓ | 1 | |||||||
| MPS | ✓ | 1 | |||||||
| MOBID/MOBID-2 | ✓ | ✓ | ✓ | 3 | |||||
| NOPPAIN | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |
| OPBT | ✓ | ✓ | 2 | ||||||
| PACSLAC | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |
| PADE | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| PAINAD | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| PAINE | ✓ | 1 | |||||||
| PATCOA | ✓ | ✓ | ✓ | 3 | |||||
| PATCIA | ✓ | 1 | |||||||
| PBM | ✓ | 1 | |||||||
| PPI | ✓ | 1 | |||||||
| PPQ | ✓ | 1 | |||||||
| RaPID | ✓ | 1 | |||||||
| REPOS | ✓ | 1 | |||||||
The reviews aimed to summarise the available evidence by means of a comprehensive overview. Three reviews82,86,87 also explicitly aimed at an evaluation of the evidence, that is to critically evaluate the existing tools, or to identify key components and analyse the reported psychometric properties of tools. Two reviews64,82 reported a systematic method for evaluation of the tools.
Not all reviews made explicit their assessment of the quality of the studies or risk of bias, or assessment of the scales considered. When this was done, the reviews highlighted the methodological limitations of both studies and scales. For example, in one review64 the overall assessment was ‘generally moderate’, with 11 points being the highest score out of the 20-point evaluation scale applied, and only four of the 12 tools examined reaching this score [these were Doloplus-2, L’Échelle Comportementale pour Personnes Âgées (ECPA), PACSLAC and PAINAD]. The heterogeneity of study designs and/or inconsistencies made aggregation of findings in the reviews difficult and/or methodologically inappropriate. For example, Zwakhalen et al. 64 stressed the ‘Considerable heterogeneity in terms of design (retrospective vs. prospective), method (pain in vivo vs. observational methods), research population (different types of dementia, different levels of impairment, different settings) and conceptualisation of pain’, making their results hard to compare.
We assessed the quality of the systematic reviews through the use of the AMSTAR questionnaire (original questionnaire adapted to a binary scoring: 1 if item is present, 0 if unclear, absent or not applicable) (Tables 4 and 5). The mean score was about 4.9, with a range of 188 to 1086 out of a maximum score of 11.
| Systematic review, authors and year of publication | AMSTAR question | |||||
|---|---|---|---|---|---|---|
| Q1: was an ‘a priori’ design provided? | Q2: was there duplicate study selection and data extraction? | Q3: was a comprehensive literature search performed? | Q4: was the status of publication (i.e. grey literature) used as an inclusion criterion? | Q5: was a list of studies included and excluded provided? | Q6: were the characteristics of the included studies provided? | |
| Corbett et al., 201267 | 1 | 0 | 1 | 0 | 1 | 1 |
| Herr et al., 200682 | 1 | 0 | 1 | 1 | 0 | 1 |
| Park et al., 201087 | 1 | 0 | 1 | 0 | 1 | 1 |
| Qi et al., 201286 | 1 | 1 | 1 | 0 | 1 | 1 |
| Schofield et al., 200572 | 1 | 0 | 1 | 0 | 0 | 0 |
| Smith, 200588 | 0 | 0 | 0 | 0 | 0 | 1 |
| van Herk et al., 200766 | 1 | 0 | 1 | 0 | 0 | 0 |
| Zwakhalen et al., 200664 | 1 | 0 | 0 | 1 | 0 | 0 |
| Systematic review, authors and year of publication | AMSTAR question | Total score | ||||
|---|---|---|---|---|---|---|
| Q7: Was the scientific quality of the included studies assessed and documented? | Q8: Was the scientific quality of the included studies used appropriately in formulating conclusions? | Q9: Were the methods used to combine the findings of studies appropriate? | Q10: Was the likelihood of publication bias assessed? | Q11: Was the conflict of interest stated? | ||
| Corbett et al., 201267 | 0 | 1 | 1 | 0 | 1 | 7 |
| Herr et al., 200682 | 1 | 0 | 1 | 0 | 0 | 6 |
| Park et al., 201087 | 1 | 0 | 0 | 1 | 0 | 5 |
| Qi et al., 201286 | 1 | 1 | 1 | 1 | 1 | 10 |
| Schofield et al., 200572 | 0 | 1 | 0 | 0 | 0 | 3 |
| Smith, 200588 | 0 | 0 | 0 | 0 | 0 | 1 |
| van Herk et al., 200766 | 0 | 0 | 0 | 0 | 0 | 2 |
| Zwakhalen et al., 200664 | 0 | 1 | 0 | 0 | 1 | 4 |
| Mean score | 4.875 | |||||
Most (six out of eight) reviews presented an a priori design and a comprehensive literature search (question 1; question 3). However, in general, the reporting lacked detail. For example, as shown in Tables 4 and 5, the list of included/excluded studies was provided in only three reviews,67,86,87 the explicit involvement of two or more independent reviewers (question 2) was reported in only one review,86 only three reviews67,82,86 explained the methods used to combine findings (question 9) and only one review86 seemed to have assessed the likelihood of publication bias. This lack of detail in reporting may be because of restrictions on word limits in publications. We did not contact the authors to obtain missing data.
Reviews’ findings: the pain assessment tools
In total, 28 pain assessment tools were assessed in the eight reviews. Nine tools [Abbey Pain Scale, Assessment of Discomfort in Dementia (ADD) protocol, CNPI, Discomfort Scale – Dementia of the Alzheimer’s Type (DS-DAT), Doloplus-2, NOPPAIN, PACSLAC, PADE and PAINAD] were assessed in five or more reviews. One tool [Mobilization-Observation-Behavior-Intensity-Dementia (MOBID) pain scale] was assessed in three reviews. Three tools [Behavior Checklist, Observational Pain Behaviour Tool (OPBT) and Pain Assessment Tool in Confused Older Adults (PATCOA)] were assessed in two reviews. The remaining 15 tools were each assessed in one review.
It should be noted that there seem to be different versions of PACSLAC, with a preliminary tool comprising 60 items, then a later version modified to 36 items. A Dutch version, PACSLAC-D, was also mentioned. There seems to be ambiguity about which version of the tool the data were reported about. 67 Similar ambiguity was found in relation to the PADE questionnaire; it was unclear which version – or which of its subscales – was studied for psychometric properties. Equally, the MOBID scale has been studied in two different versions. It is also unclear how much the Abbey Pain Scale had been refined across the studies carried out to evaluate it (including a Japanese version).
Description of the tools
The reporting of the tools’ content and intended use was undertaken differently by different reviews, making it difficult to provide a comprehensive comparative descriptive summary of all 28 tools. Six of the eight reviews64,66,67,72,87,88 provided summary tables giving an overview descriptions of the tools’ designs, but these summaries focus on a varied range of aspects, including target population,66 number of items,64,66,67 type of behaviours identified,72,82,88 number of dimensions/behaviours,64 presence of the American Geriatric Society (AGS) categories66,82,87 and scoring range. 64,66
From the eight reviews, it appeared that most tools (24 out of 28) were observational, requiring observations undertaken by HCPs. However, reviewers’ classification of these observational tools varied: the Face, Legs, Activity, Cry, Consolability scale (FLACC) was described as a ‘behavioural scale’ as opposed to observational;82 the Abbey Pain Scale, PADE and Pain Assessment In Noncommunicative Elderly persons (PAINE) were classified by one review67 as ‘caregiver or informant rating scales’; the same review67 classified the ADD tool as ‘interactive’ – that is an ‘interactive method’ including ‘a physical and affective needs assessment, a review of the patient’s history, and the administration of analgesic medication’. 67 In a number of reviews no specific classification is made. Among the remaining four tools of the 28, one, Present Pain Intensity (PPI), relied on patient self-reporting, while another, Proxy Pain Questionnaire (PPQ), was described as relying on caregivers reporting, and compared pain experienced currently with pain experienced the previous week.
Twenty-five of the 28 tools appeared to include an assessment of pain intensity. Three tools (ADD protocol, Behavior Checklist and OPBT) aimed at determining simply the presence or absence of pain, with no scoring or rating of pain intensity. In the case of two tools [Doloplus-2 and Rotterdam Elderly Pain Observation Scale (REPOS)] binary scores were summed up and the total score interpreted as presence/absence of pain.
Methods of scoring and rating of pain varied, from scores made of counting checkmarks – that is yes/no binary responses (item present or absent), to a variety of rating systems; total scores ranges varied from 0–6 to 0–60 {i.e. 0–6 [CNPI], 0–9 [PATCOA], 0–10 [FLACC, MOBID, PAINAD], 0–14 [Edmonton Classification System (ECS)], 0–25 [OPBT], 0–27 [DS-DAT], 0–30 [Doloplus], 0–44 [ECPA], 0–54 [Rating Pain In Dementia (RaPID)], 0–60 [PACSLAC]}. Likert scales, binary scores, multiple choice and visual analogue scale (VAS) systems were mentioned in the reviews; in three cases [PADE, Pain Assessment Tool for use with Cognitively Impaired Adults (PATCIA), PPQ] different rating systems are used in the same tool (Likert scales/VASs; Likert scales/binary scores).
Only 2 out of 28 tools (CNPI and NOPPAIN) appeared to be designed explicitly for pain assessment both at rest and during movement, although data about this aspect of the tool’s design may have been missing for the other tools. For one tool (Doloplus-2) the score was reported to reflect the progression of the pain experienced, rather than the patient’s pain experienced at a specific moment in time.
Two tools (REPOS and the ADD protocol) combined assessment with guidelines for intervention (this is discussed further in Settings in which the tools were studied).
Three reviews66,82,87 explicitly analysed the tools in terms of whether or not their design applied the AGS48 guidelines and categories of potential pain indicators in older persons, namely facial expressions, verbalisations/vocalisations, body movements, changes in interpersonal interactions, changes in activity patterns or routines, and mental status. These reviews covered 15 tools [Abbey Pain Scale, ADD protocol, Behavior Checklist, CNPI, DS-DAT, Doloplus-2, Facial Action Coding System (FACS), FLACC, MOBID, NOPPAIN, PACSLAC, PADE, PAINAD, PATCOA and the Pain Behavior Method (PBM)].
Settings in which the tools were studied
The tools were studied in a variety of settings and with varied patient populations. The terminology used to describe settings varied, and those which appeared to be in non-acute settings included long-term care, nursing homes, dementia care units, psychogeriatric units, rehabilitation facilities, aged care facilities, residential care facilities, long-term care facilities and palliative care, but also included geriatric clinics, care homes, residential and skilled care facilities, long-term-care dementia special care units and a residential dementia care ward.
The terminology used to refer to hospital settings also varied, with reference either to patients and/or type of services. For example, terms used included hospital patients in a long-term stay department, psychiatric hospital setting, hospital medical care unit, dementia special care units in hospital, hospital patients and older hospital patients.
Tools’ psychometric data
Reliability
The reliability of pain assessment scores was measured using inter-rater reliability (agreement between raters), test–retest (extent to which a tool achieved the same result on two or more occasions when the condition was stable) or intrarater reliability (agreement of scores from the same rater at different time points) and internal consistency. There were no reliability data available for four of the tools (ECS, PATCIA, OPBT and Behaviour Checklist). Overall, reliability measures were carried out on small samples of patients and raters, so data for all of the tools were limited.
Inter-rater reliability
This was calculated in different ways for each of the tools. Methods included percentage agreement, kappa coefficients, correlation coefficients, and intraclass correlation coefficients. The variation in calculation of reliability of the different tools made drawing direct comparisons difficult. Percentage agreement is the least robust measurement of reliability, and was used to calculate agreement for the FACS (43–93%), CNPI (93%), DS-DAT (84–94%), PACSLAC (94%), PATCOA (56.5–100%), NOPPAIN (82–100%) and ADD protocol (86–100%). The kappa coefficient measures agreement between two observers and takes into account the agreement expected by chance. It is therefore a more robust measure than percentage agreement. For kappa coefficients, a value of 0.6 or above indicates moderate agreement. Kappa coefficients were provided for the FLACC (0.404), Mahoney Pain Scale (MPS) (0.55–0.77), CNPI (0.625–0.819), MOBID (0.05–0.90), MOBID-2 (0.44–0.90) and NOPPAIN (0.70–0.87). Correlation coefficients were used to assess agreement for the following tools: FACS (0.82–0.92), PAINE (0.711–0.999), RaPID (0.97), DS-DAT (0.61–0.98) and PAINAD (0.72–0.97). Measures of inter-rater reliability using intraclass correlations were as follows: Certified nursing assistant Pain Assessment Tool (CPAT; 0.71), PBM (range 0.10–0.87), DS-DAT (0.74), Doloplus-2 (range 0.77–0.90 total scale and 0.60–0.96 subscales), PACSLAC (range 0.77–0.96), PADE (range 0.54–0.96), ECPA (0.80), Elderly Pain Caring Assessment 2 (EPCA-2; range 0.852–0.897), MOBID (range 0.70–0.96) and Abbey Pain Scale (range 0.44–0.845). There were no inter-rater reliability data provided for the PPQ.
Overall, the majority of the tools assessed had moderate to good inter-rater reliability. However, there were limitations in terms of the sample sizes used to evaluate their reliability.
Test–retest and intrarater reliability
Intrarater reliability was not assessed for the FLACC, MPS, PBM, PPI, PAINAD, PATCOA, ECPA, EPCA-2 and the ADD protocol. Evaluations of intrarater reliability included percentage agreement, correlation, kappa, Nygård test–retest and intraclass correlations. In terms of intrarater reliability, the variation in calculations made direct comparison across the tools difficult and the use of small sample sizes indicated that all of the results should be treated with caution. Percentage agreement for intrarater reliability was provided for the FACS (79–93%); correlations for the FACS (0.88–0.97), PAINE (0.711–0.999) and RaPID (> 0.75), DS-DAT (0.6); kappa coefficients for MOBID-2 [0.41–0.83 (pain behaviour], 0.48–0.93 (visual pain recordings); Nygård test–retest for the CNPI (0.23–0.66); and intraclass correlations for the CPAT (0.67), REPOS (0.90–0.96), PACSLAC (0.72–0.96), PADE (0.70–0.98), MOBID (0.60–0.94) and Abbey Pain Scale (0.657). As with inter-rater reliability, the values indicate moderate to good temporal stability.
Internal consistency
Internal consistency data were available for the MPS (total scale = 0.76; subscales range 0.68–0.75), PAINE (0.75–0.78), RaPID (0.79), REPOS (0.49), CNPI (0.54–0.64), Doloplus-2 (0.668–0.82), PACSLAC (0.74–0.92), PADE (0.24–0.88), PAINAD (0.5–0.74), PATCOA (0.44), ECPA (0.70), EPCA-2 (0.73–0.79), MOBID (0.82–0.91), MOBID-2 (0.82–0.84) and Abbey Pain Scale (0.645–0.81). There was considerable variation in the internal consistency of scales, with the MOBID and MOBID-2 indicating the highest internal consistency and the PADE, PATCOA and PAINAD having some of the lowest ratings.
Validity
The validity of the pain tools was primarily explored using concurrent and/or criterion validity (correlation of the pain scale with other pain scores or a benchmark criterion) and/or discriminant and/or predictive validity (e.g. ability to discriminate, or predict between pain on movement and at rest). Some reviews, for example those by Zwakhalen et al. 64 and Herr et al. ,82 also provided brief insight into the conceptual foundation of the measures and ways content validity was explored. As with measures of reliability, there was considerable variation in how the validity of tools was assessed. Three tools had no validity assessment (the Comfort Checklist, the PATCIA and the OPBT). The NOPPAIN instrument also had little overall formal validity assessment.
Content validity
In general, only limited insight was provided into the conceptual foundation of the tools (as opposed to the tool’s purpose). For the vast majority of tools, their derivation, and thus the implied conceptual basis, lay in literature reviews and/or clinical and/or research experts in pain and older patients with dementia. In the case of other tools, for example the Abbey Pain Scale, the basis was unclear or, as with the Behavior Checklist, no information was provided. Two of the measures, Doloplus-2 and ECPA, were adapted from measures originally developed for a different patient group, namely young children. In contrast, the purpose of all the measures was commonly outlined. It is notable that some were developed for particular users (CPAT, for certified nursing assistant care providers; NOPPAIN, for nursing assistants), another for research purposes (DS-DAT) and two as decision support tools (the ADD protocol and the REPOS).
Concurrent and criterion validity
Concurrent and criterion validity were measured by comparing the scores of one tool to another, by comparing one tool’s scores with nurse/doctor ratings of pain or through comparison with self-report (using VASs). The following is a summary of the comparisons (ranges refer to correlation coefficients):
-
CPAT (r = 0.22; p = 0.076) was compared with DS-DAT (r = 0.25; p = 0.048)
-
PAINAD compared with the DS-DAT (range 0.56–0.76)
-
DS-DAT compared with the Pittsburgh Agitation Scale (r = 0.51) and the Cohen-Mansfield Agitation Inventory (r = 0.25)
-
Doloplus-2 compared with the PAINAD (r = 0.34) and PACSLAC (range 0.29–0.38)
-
REPOS compared with PAINAD (range 0.61–0.75)
-
FACS was compared with PBM (range 0.02–0.41)
-
PAINE compared with PADE (r = 0.65)
-
PADE compared with Cohen-Mansfield Agitation Inventory (range 0.30–0.42)
-
PPI compared with the Memorial Pain Assessment subscale (r = 0.67), Verbal Scale (r = 0.54), RAND® Health Survey (RAND Corporation, Santa Monica, CA, USA) and Dartmouth Primary Care Cooperative Information Project chart (r = 0.72)
-
RaPID compared with McGill Pain Scale (range 0.8–0.86).
Comparisons with proxy pain reports (doctor or nurse) were as follows: MPS (r = 0.86), PAINAD (r = 0.84), the PBM (range 0.62–0.73), MOBID (range 0.41–0.64), Abbey Pain Scale (r = 0.586), PACSLAC (range 0.35–0.54) and REPOS (range –0.12 to 0.39).
Comparison with self-report (using a VAS) comprised RaPID (range 0.8–0.86), EPCA-2 (r = 0.846), DS-DAT (range 0.56–0.81), PAINAD (r = 0.75 pain VAS and r = 0.76 discomfort VAS), ECPA (r = 0.67), Doloplus-2 (range 0.31–0.65), PPI (r = 0.55), CNPI (range 0.30–0.50), PATCOA (r = 0.41) and PBM (range 0.11–0.30).
Overall, the tools that had the highest correlations with each other were the RaPID compared with the McGill Pain Scale, the REPOS compared with PAINAD and the PPI compared with the Memorial Pain Assessment subscale. The MPS and the PAINAD had the highest correlation with nurse/doctor ratings of pain, and the RaPID with self-reports of pain/discomfort. There was no one scale that appeared to be superior to the others (or applicable as a gold standard), and no consistency in comparisons across the scales.
Discriminant validity
Discriminant validity was measured by comparing scores before or after a painful event. Several of the reviews reported that tools had discriminant or predictive validity without providing data; this included the reviews of the FACS and PBM. Other scales with a significant difference in scores pre and post interventions/events included the CPAT, CNPI, DS-DAT, PACSLAC, MOBID, Abbey Pain Scale, ADD protocol and the Behavior Checklist.
Construct validity
Construct validity was measured by comparing scores to medication use or prescription of medications. The PPQ scores were correlated to pain medication use (correlation coefficient range 0.37–0.55), and patients assessed with the PADE on psychoactive medications had significantly higher scores on the physical and verbal agitation subscales. With the PAINAD there was a significant fall in score after the administration of pain medication, and the EPCA-2 was correlated with the prescription of opioids (r = 0.782) and non-opioids (r = 0.730).
Feasibility and clinical utility
The feasibility of a tool is ‘its applicability in daily practice’, including aspects such as ease of use and time required to administer it, whereas clinical utility is ‘the usefulness of the measure for decision-making’, that is, to inform further action, such as the administration of analgesics. 66 Data on feasibility and clinical utility of tools were very limited. Often data were not available in the reviews or, when data were available, limitation often pertained to a lack of data in the original studies (e.g. reviewers stating the item was not reported and could therefore not be assessed). More specifically, feasibility data were completely absent for six tools (Comfort Checklist, FLACC, PAINE, PATCOA, PPI and PPQ); clinical utility data were completely or substantially absent for seven tools (ECS, FACS, MPS, PAINE, PBM, PPQ and RaPID). For four tools reviewers explicitly noted that claims of feasibility (e.g. time required to administer the tool) were made from the authors of the study without supporting evidence (Abbey Pain Scale, Doloplus-2, PACSLAC and PADE). There were also two instances (PACSLAC and MOBID) of conflicting data on clinical utility and feasibility from the different reviews, possibly because of an ambiguous reference to different versions of the same tool.
Specific evaluation for feasibility appears to have been carried out only for three tools (CPAT, MPS and PATCIA). In the first two of these cases the evaluation was done by use of questionnaires. It also appeared that users of the Abbey Pain Scale were asked for feedback in the context of the psychometric testing of the tool. In was unclear whether or not the ADD protocol was also assessed for feasibility.
Specific evaluation of clinical utility appeared to have been undertaken for the PATCIA tool, and possibly for the ADD protocol and PAINAD.
It must be stressed that when reviews assessed or mentioned the feasibility and/or clinical utility of the tools, the two aspects were often confounded (reviewers, authors or users typically drawing conclusions from ease of use or brevity of a scale to its usefulness).
Specific dimensions of feasibility assessed were time to complete the assessment (e.g. to complete a checklist), availability of instructions on how to use the tool and/or availability of guidelines on how to score pain, and training needs.
Six tools were reported to be ‘easy to use’ (Abbey Pain Scale, Behavior Checklist, CNPI, CPAT, MPS and NOPPAIN), two were considered manageable/acceptable (ECPA and RaPID) and four were judged to be complex (ADD protocol, DS-DAT, PATCIA and PADE). Conflicting views on the ease of use or complexity of the tools were apparent for five tools (Doloplus-2, MOBID, PACSLAC, PAINAD and PBM).
Instructions for use and/or guidelines on scoring were reported to be available for 13 tools (Abbey Pain Scale, CNPI, CPAT, Doloplus-2, DS-DAT, ECS, FACS, MPS, MOBID, NOPPAIN, PACSLAC, PAINAD and REPOS), with varied assessments in terms of clarity or complexity of the instructions.
Training in the use of the tool was judged as necessary for 10 tools (EPCA-2, MPS, NOPPAIN, PADE, PACSLAC, PAINAD, ADD protocol, CPAT, DS-DAT and MOBID), four of which seemed to require significant training (ADD protocol, CPAT, DS-DAT and MOBID). For six tools it was stated that the authors of studies creators of tools did not report the level and length of training required (Abbey Pain Scale, CNPI, Doloplus-2, PAINAD, PACSLAC and REPOS). For the majority of the tools, however, data about training were not available (Behavior Checklist, Comfort Checklist, ECPA, ECS, FACS, FLACC, OPBT, PATCIA, PAINE, PATCOA, PBM, PPI, PPQ and RaPID).
Specific dimensions of clinical utility were less straightforward. The availability of cut-off scores and interpretation of scores for decision-making appeared to be the two dimensions supporting evidence of clinical utility. The presence of cut-off scores contributes to achieving clinical utility, for example to help discriminate between presence and absence of pain, or to couple the scale with a treatment algorithm. Otherwise, general statements were available related to evidence of use in clinical settings and evidence of clinical utility, the latter being dependent on the former.
Cut-off scores appeared to be available only for REPOS and Doloplus-2, although in the latter case the scores still need to be validated. The availability of guidance on how to interpret the scores and for further action following assessment was variably reported. Data on this were missing for 18 tools (ADD protocol, Behavior Checklist, Comfort Checklist, DS-DAT, ECS, EPCA-2, FACS, FLACC, MPS, MOBID, PATCIA, PAINE, PATCOA, PBM, PPI, PPQ, RaPID and REPOS). For four tools it appeared that interpretations of the scores were available (Abbey Pain Scale, Doloplus-2, CNPI and CPAT), although in two of these they were deemed unclear (CNPI and CPAT). For five tools, it appeared interpretations of the scores were not available (ECPA, NOPPAIN, OPBT, PACSLAC and PADE) and a further four were reported as lacking guidance for further action following assessment (CNPI, CPAT, Doloplus-2 and NOPPAIN). It was unclear whether or not PAINAD does provide interpretation of scores.
Suggestive evidence of use in clinical settings was reported for two tools; the Abbey Pain Scale has been incorporated into the Australian pain guidelines, while the ADD protocol was introduced in 57 long-term care facilities together with an education strategy for 12 months, and the evaluation was done in a study with 32 nurses in 25 facilities. It is otherwise unclear whether any of the other tools were actually used in practice beyond a period of research or testing of the tool. In one example (NOPPAIN), the testing of the tool was done by use of video-recording of an actress portraying a bed-bound patient, and it remains unclear whether further testing was done in clinical setting or with patients at bedside.
With the exception of the ADD protocol and the REPOS, there was no mention of how the tools would inform intervention (e.g. choice of treatment). We found an overall lack of clarity in the descriptions of the ADD protocol, but it seems that one of its strengths is that it links observation of behaviour with interventions. Similarly, one review suggested that the clinical utility of REPOS potentially resides in its combination with a decision tree to assist in determining interventions after pain assessment. 86
Summary of findings
There was a consensus among the reviews that the current evidence on validation and clinical utility of the tools is insufficient. The overall conclusion was that there is a need for further psychometric testing of each tool. Two reviews recommended that the focus should be on studying existing scales rather than creating new ones,64,72 although one review also suggested that there may be a need to revisit the tools’ conceptual foundations. 82
Recommendations for further research and testing of the tools included the involvement of culturally diverse populations64,82,87 and the provision of scoring methods and guidelines for interpretation in the evaluation of the scale. 87 Finally, a need for research emerged to link assessment with treatment algorithms. 66
Some of the reviews also concluded with recommendations for practice; for example, the use of at least two different pain assessment approaches at the same time in clinical practice and two different tools in research;67 the importance of a comprehensive approach to pain assessment beyond the use of tools;82 and the need to involve social workers in regular holistic multidisciplinary pain assessment (in nursing homes), with training in the use of the scales. 87
Among the tools selected by the reviews as possible best candidates, albeit on limited evidence, were the DS-DAT, Doloplus-2, MPS, PACSLAC, PAINAD, Abbey Pain Scale, and ECPA. However, there was no clear consensus on the single best tool. A summary of the conclusions and recommendations from each review is given in Table 6.
| Systematic review, authors and year of publication | Tools included in the review | Conclusions and recommendations |
|---|---|---|
| Corbett et al., 201267 | 12 tools: Abbey Pain Scale; ADD protocol; CNPI; Doloplus-2; DS-DAT; EPCA-2; NOPPAIN; PACSLAC-D; PADE; PAINAD; PAINE; and PPI | The majority of tools available require validation in people with dementia. Instruments appear sensitive to changes in pain intensity during treatment studies. Agreement between tools is limited. Some not validated in non-English-speaking populations. Use of at least two different assessment approaches recommended |
| Herr et al., 200682 | 10 tools: Abbey Pain Scale; ADD protocol; CNPI; DS-DAT; Doloplus-2; FLACC; NOPPAIN; PACSLAC; PADE; and PAINAD | A number of tools demonstrate potential, but are in early stages of development and testing. With the exception of the DS-DAT and Doloplus-2, tools have limited testing beyond the initial study setting and sample. No testing done for use with ethnic minority older adults with dementia. Strong evidence of reliability for only one tool, the DS-DAT, and none of the tools has demonstrated strong support for validity. This may reflect a need to revisit the tools’ conceptual foundation |
| There is no standardised tool based on non-verbal behavioural pain indicators in English that may be recommended for broad adoption in clinical practice | ||
| Authors emphasise that identification of pain indicators using a standardised tool is only one step in a complex diagnostic process. Use of a tool to identify pain behaviours should be integrated within a comprehensive approach to pain assessment in this population. One tool included in this review, the ADD protocol, is an example of such an approach | ||
| Smith, 200588 | Eight tools: ADD protocol; CNPI; DS-DAT; Comfort Checklist; Observed Pain Behavior Scale; PADE; PAINAD; and PPQ | Each tool has merit and limitations. Need to further test tools reviewed |
| Qi et al., 201286 | 10 tools: Abbey Pain Scale, CNPI; CPAT; Doloplus-2; MPS; MOBID; NOPPAIN; PACSLAC; PAINAD; and REPOS | No single tool identified to be recommended with confidence for use across both acute and long-term care settings. Limitations include absence of cut-off scores indicating pain. Three of the tools, the MPS, the PACSLAC and the PAINAD, show most promising outcomes and potential for use in acute and long-term care settings |
| Park et al., 201087 | 11 tools: Abbey Pain Scale; ADD protocol; Behavior Checklist; CNPI; DS-DAT; MOBID; NOPPAIN; PACSLAC; PADE; PAINAD; and PATCOA | Of the 11 scales reviewed, the PACSLAC appears to be the best pain assessment scale for non-verbal older adults with cognitive impairments or dementia. However, more psychometric testing is recommended. Future research on pain scales for this population should identify which scales are reliable and valid at various levels of cognitive impairment |
| Some of the reviewed scales do not provide either scoring methods or interpretations of scores. Therefore, it is recommended that scoring methods and their accurate interpretation be included in the assessment of these scales. In addition to behavioural and emotional pain indicators, future studies should focus on accurate pain assessment in culturally diverse older adults | ||
| Social workers should be involved in a regular holistic multidisciplinary pain assessment (in nursing homes) and be trained in the use of the scales | ||
| Schofield et al., 200572 | Nine tools: Abbey Pain Scale; ADD; CNPI; Doloplus-2; DS-DAT; NOPPAIN; PADE; PAINAD; and PACSLAC | Assessment tools such as a verbal rating scale are the preferred method for assessing pain, and behavioural scales should be used only when severe cognitive impairment is present |
| Collectively, the tools reviewed corroborate the use of common behavioural indicators of pain, but do not demonstrate sufficient evidence for the use of a particular scale | ||
| Need to focus on scales that already exist and spend more time validating them. Most promising scales for practice and research: the PACSLAC, Abbey Pain Scale and Doloplus-2 | ||
| van Herk et al. 200766 | 13 tools: Abbey Pain Scale; ADD; Behavior Checklist; CNPI; Doloplus-2; DS-DAT; FACS; NOPPAIN; PACSLAC; PADE; PAINAD; PATCOA; and PBM | PAINAD seems the best feasible scale for practice. Suggests need to develop cut-off scores for scales to indicate whether or not to provide interventions for pain – need to link assessment with treatment algorithms |
| Zwakhalen et al., 200664 | 12 tools: Abbey Pain Scale; CNPI; Doloplus-2; ECPA; ECS; NOPPAIN; Observational Pain Behavior Tool; PACSLAC; PADE; PATCIA; PAINAD; RaPID | None of these assessment scales was convincingly the most appropriate and, therefore, a preferable scale for assessing pain in elderly people with dementia. PAINAD, PACSLAC, Doloplus-2 and ECPA show the best (but moderate) psychometric qualities (none scored more than 12 points out of 20) among those reviewed. For implementation of one of these tools in clinical practice, two further criteria were added: scale items specifically geared towards elderly persons with dementia and most comprehensively tested in clinical settings. After adding these criteria to the psychometric properties, PACSLAC and Doloplus-2 appear the most appropriate scales among those currently available Recommendations for further research:
|
The ADD protocol was mentioned as an example of a more comprehensive approach for the identification of pain, beyond the use of an assessment tool as ‘a standardised tool is only one step in a complex diagnostic process’. 82 There was also agreement on recommending that patients with dementia can often reliably verbalise their pain, suggesting therefore that the use of observational scales should be limited to patients who demonstrably cannot reliably verbalise their pain.
This meta-review did not cover a new tool recently developed in France by the Doloplus Collective team – Algoplus. 89 To our knowledge, this tool had not yet been included in a systematic review.
Conclusions
The assessment of pain in patients with dementia is challenging for clinicians, because of some patients’ inability to verbalise the nature of their pain, and clinicians’ inability to understand other pain-related cues. This review highlighted the state of the evidence base in relation to pain assessment tools, and provided insights into current gaps in our understanding. We identified a total of 28 tools that could possibly be used in clinical practice to help with this process. However, we cannot at present recommend any particular tool for use in any clinical setting because of the lack of comprehensive evidence on the reliability, validity, feasibility or clinical utility of any one particular tool. These findings are discussed in Chapter 9.
Chapter 5 Observational study: methods
Introduction
This project hypothesised that a rigorously developed decision support tool could help clinicians, carers and people with dementia by improving pain assessment and management in acute hospital settings. To inform the design of such a tool, an exploratory study was conducted to understand how pain is currently recognised, assessed and managed among patients with dementia in representative acute settings in the UK, through the lens of decision-making theory.
Aims and objectives
The overall aim of this exploratory study was to investigate how HCPs and others recognised, assessed and managed pain in patients with dementia in a range of acute settings. This was to provide the basis for the development of a decision support tool to improve the management of pain for this population.
The study addressed the following objectives:
-
to identify what information is currently elicited and used by clinicians when detecting and managing pain in patients with dementia in acute hospital settings
-
to explore the existing process of decision-making for detecting and managing pain in patients with dementia in acute hospital settings
-
to identify the role (actual and potential) of carers in this process
-
to explore the organisational context in which HCPs operate with regard to this decision-making process.
Theoretical framework
Pain is multidimensional, consisting of sensory, cognitive, affective and social components. The focus of this study was physical pain, although we acknowledged that pain and emotional distress are closely linked, as distress may exacerbate pain symptoms and vice versa. Pain experiences are associated with a multiplicity of factors that are unique to each individual and, even in the absence of cognitive impairment, they are very difficult to communicate meaningfully to other people. 90,91
We conceptualised pain assessment and management as involving decision-making processes, such as the accurate interpretation of the patient’s pain experience (an assessment or judgement), and taking appropriate actions to ameliorate the pain (making treatment decisions). This is explained in more detail in Chapter 1 (see Figure 1). There are a variety of theories of judgement and decision-making. 52 A common central element is the existing information that supports judgements and decisions. This includes the type of information, how it is gathered and where it is found. For example, in the hypothetico-deductive reasoning model of decision-making,51,92 individuals process information to make a judgement, defined as ‘an assessment between alternatives’. 93 Information is gathered through ‘cue acquisition’, for example, clinical information about a patient such as the patient’s verbal reports of pain or observation of their behaviour. Hypotheses are then generated to explain and interpret the information, and more information is gathered if needed, until a hypothesis is chosen that is supported by the majority of the evidence or information.
In investigating how HCPs and others recognise, assess and manage pain in patients with dementia, the research design and research instruments for this study were focused on information (types and availability of information sources, clinicians’ information needs and methods of recording information), individuals’ processes of perception, judgement and decision-making, as well as any tools used in the process and documentation of patients’ pain. The model of judgement and decision-making focused on linear processes as shown in Chapter 1 (see Figure 1) was used to guide data collection.
Design
This study was a multiple case site study, with embedded units of analysis (individuals, wards and organisations), approached with ethnographic methods. Case studies involve an empirical design that focuses on describing phenomena within their real-life context94 and appropriate to exploratory objectives.
Setting
Four case sites (NHS hospital trusts, each with one or more hospitals) were sampled to provide varying settings for acute care: one in the south of England, two in the north of England and one in Scotland. One of the four organisations used electronic patient record systems, whereas the others used paper for medical and nursing notes. Criteria for sampling included the type of hospital (tertiary referral centre/secondary care) and the type of service provision available to HCPs in the hospital (e.g. a specialist pain management team, dementia outreach team). In each site two wards were initially selected for data collection, with additional wards approached where access to participants was found particularly challenging. The selection was theoretically driven to ensure that there was representation from a variety of clinical settings in acute care where patients with dementia may be cared for (e.g. orthopaedic, acute medicine, care of the elderly) across the sample, as shown in Table 7. This approach was used to ensure a detailed, comparative overview of how pain is currently detected and managed in patients with dementia in a wide range of acute care settings would be derived.
| Case site | Types of ward/medical specialty |
|---|---|
| H1 | Vascular surgery; care of the elderly |
| H2 | Medicine for the elderly; continuing care |
| H3 | Stroke rehabilitation; elderly medicine (three wards); surgery |
| H4 | Surgical/orthopaedic; acute medical admissions |
Data collection
Data collection was undertaken by research fellows at each of the four sites. Data were collected at three of the sites (case sites H1, H2 and H4) by one researcher each (VL, NG and KJ), and by two researchers, in turn, at case site H3 (SC and CS). In each case site, a variety of data collection methods were used to provide multiple sources of evidence for addressing the research questions. Non-participant observation of HCPs and health-care assistants (HCAs) interacting with patients who have dementia was used to identify how information appears to be identified and elicited in order to detect and manage pain, and the care processes that currently take place. This included observing patients at bedside; a focus on how and where pain was discussed and documented; interactions between HCPs/HCAs, patients and carers; interactions between members of the multidisciplinary team (MDT); and availability of resources such as pain specialist services. An observation protocol derived from the theoretical framework was used to guide data collection.
Semistructured interviews lasting approximately 15–60 minutes were carried out with staff (HCAs, nurses, doctors, other members of the MDT) and patients’ family members (‘carers’). Interview guides were used flexibly (see Appendix 4), with the freedom to explore any other relevant issues specific to the site. For the most part these focused on exploring people’s perceptions of how pain was detected and managed in each of the wards, how carers were involved in the process, how the process may be improved and what an effective decision support tool would look like (e.g. format, content and resources). Interviews were recorded and transcribed verbatim, with the exception of those conducted in case site H3 which were recorded using hand-written notes. In site H3 recruitment was particularly difficult, even with the help of a local research (dementias and neurodegeneration network) nurse. Staff were protective of patients and appeared to feel that tape-recording of interviews was unacceptable (even though we had ethical approval). Access to patients had to be negotiated particularly carefully in this site because of the gatekeeping role of ward nursing staff. Note-taking ensured that we did capture relevant data, in a slightly different form.
We looked for any existing policies and procedures in place in the unit and/or organisation that were specifically focused on the detection and management of pain. We also audited patients’ medical and nursing notes for documentation of pain assessment, action taken, pain reassessment and records of prescribed analgesics.
Initial coding of the data was undertaken, collaboratively, with research staff from each site to agree themes and coding categories, and then brought to the whole research team at regular meetings. Analysis was carried out in parallel to data collection and when no new themes emerged from any of the sites, it was considered that saturation had been reached. Data were collected between May 2013 and July 2014.
Participants
Eligible participants were aged > 65 years with a recorded diagnosis of dementia. The degree and type of dementia, and presence of pain, were not recruitment criteria, as we were interested in whether and how potential, as well as actual, presence of pain was addressed by staff in the wards. However, patients in the wards we sampled were likely to have undergone medical procedures, or to be recovering from falls, for example, and it would be highly likely that pain was being experienced.
The sampling of staff and carers for interviews included all members of staff caring for patients in the wards included in the study, in addition to managers and specialists of relevant hospital services. Carer interviews were limited to the family of the patients participating in our study.
The number and length of observation or interviews required to provide an adequate overview of ward-based activities was governed by a notional guide; reaching a specified target (number of participants) was not our concern. Our research was informed by the principles of theoretical sampling and theoretical saturation,95 rather than those usually found in quantitative sampling. Initially, our sampling was done purposefully, on the basis of the aims of the research and the availability of cases (patients who consented). As data collection progressed, we began the analysis and this informed our further data collection activities.
Ethics, consent and permissions
Ethical approval was obtained both in England (National Research Ethics Committee Yorkshire and The Humber – Leeds West – Research Ethics Committee reference number 12/YH/0363) and Scotland (Scotland A Research Ethics Committee, Edinburgh – Research Ethics Committee reference number 13/SS/0006). The process to recruit patients was informed by the Mental Capacity Act 200596 and the Mental Health (Care and Treatment) (Scotland) Act 2003. 97 This recruitment process included written consent by patients or agreement from a carer consultee, patients’ capacity assessment to consent, consultation with staff and assent of carers. 98 Interviewees gave their written consent and were informed of the audio-recording. The NHS trusts participating in this study granted access to the researchers, who complied with local requirements for data collection. Data were anonymised at the time of data collection.
Data analysis
Data for analysis from the observational study consisted of verbatim transcripts of observation sessions, field notes of medical and nursing records, notes and transcripts of interviews. Data were indexed using NVivo (version 10, QSR International, Warrington, UK) qualitative analysis software and subjected to thematic analysis. Both inductive and deductive approaches were applied, with dimensions of decision-making (information/pain cues and documentation; judgement/pain assessment; decision/pain management) providing initial categories for indexing data, but with a variety of other themes (e.g. about the context of care) emerging from the data.
Data from observations and audits of patients’ records were coded separately from interview transcripts. The themes generated from the interview data tended to be related to decision-making processes. These included types of information used, judgments and rationales for decisions. Participants explained their thought processes and actions, and field notes from observations illustrated the context of care and what ‘actually happened’ in terms of patient care and patient–staff interaction. The audit of patients’ nursing and medical notes provided evidence of how and when documentation of pain assessment and management was undertaken. Data from the three sources were compared and contrasted, and then integrated. This provided a more nuanced understanding of the processes and events concerned with the recognition, assessment and management of pain. For example, activities planned for set times actually took place at different times (or not at all), and the documentation of assessment was often too brief to convey the richness of the different aspects of actual pain-related activities and interactions between staff and patients.
The strategy for the multisite qualitative data analysis emerged through a process of team meetings, sharing of documents and reflections among the interdisciplinary team of researchers and investigators. It became apparent that a large number and varied range of themes were emerging from the analysis, but it was agreed that a focus on dimensions of decision-making was necessary to answer the research questions. The senior research fellow in the team (VL) led the multisite analysis, which was then discussed with the research team and verified by other researchers (CS, LR and KJ). Transcripts were scrutinised to identify themes or categories, which were then used to code the data. Subsets of the data set were coded by three of the researchers (VL, KJ and RL). The senior research fellow checked a sample of each subset to verify consistency in the analysis. Data in each theme were examined for negative cases or contradictory findings. 99 To increase transparency in the analytic process, team meetings of the researchers from each of the sites convened with the project analysis group (including also SJC, NA, JK and MB), on a regular basis during, and after, the data collection period. This ensured consistency between the four sites. Emerging themes were compared, contrasted and discussed within the group and with the wider project team until a consensus was reached.
At the end of the study a two-page information sheet summarising the project was sent to participants (see Appendix 5).
Chapter 6 Observational study: findings
The findings from the ward observations, interviews and audit are presented in Chapter 6. Parts of this chapter are reproduced from Lichtner V, Dowding D, Allcock N, Keady J, Sampson EL, Briggs B, et al. The assessment and management of pain in patients with dementia in hospital settings: a multi-case exploratory study from a decision making perspective. BMC Health Serv Res 2016;16:427. 100 © 2016 The Author(s). This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Participants’ characteristics
Thirty-one patients with dementia participated in the study and were observed for a total of 170 hours at bedside (Tables 8 and 9). A total of 52 health-care staff and four carers were interviewed (Table 10). Interviews were supplemented with informal conversations recorded in field notes with staff and carers during observations. Among the health-care staff interviewed across the four case sites were seven HCAs, 31 nurses (staff nurses, sisters and specialists), three doctors in training, five medical consultants, a pharmacist, a physiotherapist and four clinical educators. Ward management was informed that all members of staff were invited to take part in interviews, but there were challenges in finding time when they would be available and researchers tried to minimise disruption to normal work activities.
| Data | Case study site | Total | |||
|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | ||
| Patients observed (number) | 8 | 7 | 9 | 7 | 31 |
| Mean patient age, years (range) | 83 (77–87) | 84 (75–93) | 88 (79–99) | 85 (75–94) | 88 (75–99) |
| Patient sex | Male, n = 1; female, n = 7 | Male, n = 2; female, n = 5 | Male, n = 4; female, n = 5 | Male, n = 4; female, n = 3 | Male, n = 11; female, n = 20 |
| Observation time (approximate number of hours) | 71 | 45 | 22 | 32 | 170 |
| Time in the field (approximate number of hours) | 161 | 167 | 73 | 85 | 486 |
| Site | Periods per site | Minimum duration of a period | Maximum duration of a period | Number of days | Number of hours (approximate) |
|---|---|---|---|---|---|
| H1 | 29 | 30 minutes | 6 hours and 20 minutes | 22 | 71 |
| H2 | 18 | 1 hour and 15 minutes | 4 hours | 14 | 45 |
| H3 | 15 | 30 minutes | 3 hours | 8 | 22 |
| H4 | 15 | 50 minutes | 3 hours and 40 minutes | 10 | 32 |
| Data collection | Case study site | Total | |||
|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | ||
| Interviews with staff | 24 | 13 | 7 | 8 | 52 |
| Interviews with carers | 1 | 3 | 0 | 0 | 4 |
Patients included in the study had a mean age of 88 years (range 75–99 years). A number of challenges were encountered in recruiting patients for all case sites. Among them was the lack of a documented diagnosis of dementia in ward notes despite strong indications that the condition was present, and many patients had no available carer.
Observations took place at the bedside, and included any HCP interacting with the patient. It did not follow specific members of staff. As a consequence, we do not have data about the ‘current shift’ from the HCP perspective, so data collection activities could not be recorded in terms of shifts.
Our initial research protocol planned for ‘40 shifts per case sites’ (i.e. 20 shifts per hospital ward), but this had to be interpreted as ‘periods of observations’. For the purpose of reporting data collection activities, a period of observation was defined as a period of continuous observation at a patient’s bedside in any 1 day. One or more periods per patient could be done per day of data collection.
Our periods of observations ranged from 30 minutes to more than 6 hours (see Table 9).
Themes emerging from qualitative analysis
The analysis identified four overarching themes which are discussed below: communicating pain with dementia; carer–clinician communication; use of analgesics; and putting a picture together.
Communicating pain with dementia
In assessing and managing a patient’s pain, the guiding principle used by staff was to rely on self-report (the patient vocalising their pain), with the patient being the main information source.
However, for many of the patients observed in the study, communication barriers of various sorts hindered a patient’s ability to provide staff with information on their pain experience. These included issues related to language and cognitive impairment, the impact of patterns of work on a patient’s ability to communicate, and issues of trust and familiarity. We describe and discuss each of these aspects (see Language and cognitive impairment, Patterns of work, time, location and division of labour and Trust and familiarity).
Language and cognitive impairment
Patients with severe dementia showed significant communication difficulties. Interviewees explained that questions about pain should be rephrased to account for this. For example, when questions were asked for the purpose of gathering and recording a pain score in a form structured with the three options ‘mild, moderate and severe’, these were to be translated into words patients could understand, taking into account the ability of each individual:
Somebody might have no concept of what moderate means, for example.
Nurse specialist, H1
Patients appeared to be using various gestures, postures, bodily movements, behavioural prompts, metaphorical expressions and a combination of these in what was interpreted as an expression and communication of pain. Data from interviews suggested that nurses and clinicians also looked at physical and behavioural signs to understand patients’ pain. One interviewee commented that the identification of these non-verbal communication cues depended largely on staff skills, experience, knowledge and perceptions and added that ‘we need to get staff to think differently’ (nurse, H3).
Some patients made use of metaphorical expressions to communicate their experience of pain. For example, a patient explained the pain in her knee as ‘It’s murder, it’s awful. For quite a while it’s alright and then suddenly it’s [sic] murder’ (field notes, H1) and another patient expressed headache as ‘red hot’ (field notes, H3). An interviewee suggested that ‘it is not always what is said, but how it is said’ (nurse, H3) that gives a cue of a patient’s pain.
Patterns of work, time, location and division of labour
The challenges raised by cognitive impairment in patients were compounded by the organisational context and time frames of staff interactions with patients. Organisational routines and staffing numbers meant that the majority of patients’ encounters with staff during their hospital stay were brief, sometimes extremely brief, and encounters with senior staff members were less frequent. One patient said, ‘they are always dashing’ (field notes, H1), or in the words of a carer, ‘it’s just a fleeting glance, they talk to them and off they go’ (carer, H2):
As an FY1 [foundation year 1 doctor], if I’m only seeing the patients for a few minutes at a time then the rest of the time looking at their investigations or trying to organise things, I don’t usually, I wouldn’t say that I actually get to know the patient as a person [. . .]
Foundation doctor, H2
Furthermore, given the organisation of staff work over rotas and shifts, a patient’s communication about their pain was not always with the same member of staff but may have involved several different HCAs, nurses and doctors. There was awareness among staff that patients with dementia would need more time than usual to communicate pain. For example, a local pain dementia care plan guidance (H1) did include instructions for nurses and HCAs to give enough time to the patient to communicate, but in practice this was a matter of minutes. Indeed, in interviews the lack of time was often voiced by the nurses as a concern:
There’s not enough of us, and we just haven’t that time.
Staff nurse, H1
Patients shouldn’t go a long period of time without their pain being reassessed [. . .] certainly you’ve got to go back, it does recommend half an hour, but as I said, it’s quite difficult to get back within that period of time.
Senior charge nurse, H2
These brief encounters required the patient to be ready to answer questions and to recall their pain experience with little or no forewarning. Moreover, these opportunities at times occurred while patients were otherwise engaged in eating or sleeping, or when they were not prepared to discuss pain.
Patients were directed to use a call button at bedside (a ‘buzzer’) to request assistance. Patients with more severe dementia appeared not to recognise the purpose of the buzzer or forgot it was there, thus their ability to communicate pain was severely limited. In addition, calls for help, including those using a buzzer, could not always be answered immediately, leading to distress and confusion for the patients concerned. Some patients seemed to have no memory of having used the buzzer when a staff member arrived. In other cases, patients expressed a disinclination to use the buzzer and disturb busy staff, or they did not know what the buzzer was ‘for’, thus rendering it unhelpful to the person with dementia. Cases were also recorded where patients verbally reported pain, but at a time when there were no staff members present. During bedside observations it became apparent that it was necessary to be in close proximity to the patient in order to communicate, and in the same bay or at bedside, as patients rarely left their bed or chair. At times, patients’ needs surfaced out of background noise, or while the staff were in the room with other patients, and patients were able to attract their attention through verbal communication or behaviour. However, this was not always the case, and some patients were alone and without interaction for relatively long periods. Many of the ward routines, such as note-keeping and handovers, took place away from the bedside, thus minimising a clinician’s opportunity to communicate with the patient during periods of alertness, or to detect subtle changes in expression and engagement.
Trust and familiarity
Clinicians were aware that relationships of trust and familiarity were important for a patient to communicate their pain. One Elderly Medicine Consultant explained: ‘if you feel that somebody cares about you then I’m sure it makes it easier to express it if you’ve got pain’ (medical consultant, H1), and a student nurse with a HCA background stated, ‘if you are connected with your patient, if you, they know you and they trust you, if you build that rapport, that itself will allow you better access to how their pain is’ (student nurse, H4). However, it seemed to the researchers that establishing these relationships was not facilitated by the brief time frames available for communication and patterns of interaction.
Carer–clinician communication
Relatives, visitors and carers represented an important information source in the recognition, assessment and management of pain. One ward sister explained the reasons for involving carers in the process of pain recognition, assessment and management in terms of ‘how well’ the staff know a patient compared with carers, carers’ ability to communicate on behalf of the patient, and how HCPs would be guided by carers in the management of their loved one’s pain.
[carers] would know the patient much better than we would and they would be able to assess whether [the patients with dementia] they’re in pain or how they’re feeling, and communicate with us. So yes we often ask for input from the carers or the family members to guide us in how we’re managing the patients.
Deputy sister, H4
Carers were observed as acting as messengers on behalf of the patient, and helped recognise and interpret pain cues. An example of this process is described in a quote from a carer interview:
My mum has a terrible habit, even though we know as a family, when she needs to go to the bathroom she starts shaking her leg and she was doing that in hospital and she was getting in a panic, she actually was crying, she went in with a really bad urine infection which was causing a lot of pain at the time [. . .]
Carer, H2
However, the observations conducted in this study showed that the majority of staff communications with relatives were concerning medicolegal reasons of consent or about discharge arrangements, and less about ‘the needs of how [patients] are and how we can help them here’ (staff nurse, H1). This observation is strengthened by the lack of documented communication with carers found in patient records. In part, this was also because of the unavailability of family members in a number of cases (something also supported by findings of an internal audit in case site H3 reported by an interviewee, where more than half of respondents said they did not ask a relative ‘because the family were not there’). Several carers perceived staff to be occupied and were therefore reluctant to initiate a conversation, whereas others expressed that they did not perceive themselves as experts in the knowledge of the person they care about. Some were elderly and had dementia themselves. Family conflicts, domestic violence, poverty and deprivation also seemed to be complicating factors in some cases.
Staff expressed the belief that there is a need for clinicians to improve communication skills with carers. They explained this as having the ability to elicit the right information from the right relative/carer, and to assess the trustworthiness of these information sources. One nurse described ‘difficult conversations’ (H1), which required assertiveness and a degree of certainty to be acting on behalf of the patient:
I hear a conversation at the nurses’ station between nurses about another patient. A nurse spoke with the niece, says the patient is OK at home. The other nurse says the niece may not know, may only see her once a week, to talk with (social worker? assistance?), that they may have a completely different picture.
Field notes, H1
The skill that the juniors need to have is in digging out, ferreting out the information that is relevant to a person [. . .] in order for us to make an informed decision [. . .] it’s not just, it’s not just asking the question, ‘How was your relative before they came into hospital?’ It’s really understanding the nitty-gritty of the details, [. . .] of course I don’t have the time, unfortunately, to do all of that for every patient, so [. . .]
Medical consultant, H1
Use of analgesics
The most common pain treatment used in the study sites was analgesic medication. Indeed pain management and pharmacological management of pain often appeared to be one and the same. A limited number of non-pharmacological pain management strategies were used, such as patient repositioning, but these were not frequently observed. Clinicians considered potential side effects of medications, including confusion, when making treatment decisions.
In certain wards, depending on the ‘type of patients’, pain medications were prescribed routinely to all patients. For example, in a ward in case site H4, an orthopaedic surgery ward, patients received ‘pain relief already regularly on their charts for their surgical procedures’ and this prescription was administered ‘every 6 hours’ even if patients did not report pain (staff nurses, H4). This standard acute pain medication prescription followed an established protocol. The prescription was provided by the anaesthetists at the time of surgery. More complex cases, or when pain was clearly not under control, required escalating out of the routine prescription through contact with a specialist pain team and trialled combinations of different analgesic drugs. In other sites, pain medication was administered on an ad hoc basis depending on the patient’s medical condition. In these cases, pain management was commonly approached in a trial-and-error mode, titrating the dose gradually and assessing the patient’s response.
Importantly, the process of (re)assessment for the purpose of establishing the most appropriate medication encountered similar communication challenges as described above, as not all patients were effectively communicating changes in their pain. Judging the level of titration, or the appropriate step in the analgesic ladder, relied on clinicians’ knowledge or ‘sense’ of what the expected pain medication for a given medical condition would be:
Obviously a knowledge of the reason why they’re in hospital and if they’d had a particular surgery, of knowledge of what is happening within the body. [. . .] And how much pain relief somebody would need for that.
Deputy sister, H4
Pain relief medication prescribed to be administered as and when needed [pro re nata (PRN)] was usually considered part of this process of titration, but could not be used effectively with patients with dementia who would not request additional pain relief.
‘Putting a picture together’
Overall, understanding a person’s pain in these acute hospital wards involved investigative work and ‘putting a picture together’ of an individual’s pain (‘we’re trying to build a picture’, staff nurse, H1). This process required time and availability of information from various sources, including carers, the MDT assessment, administration of medication and the patient’s response.
The observation of the context of care and document analysis revealed that patient information was shared through face-to-face encounters and written documents such as patient records, medical and nursing notes, transfer reports, checklists, care plans and drug charts. The drug chart was frequently referred to by the majority of team members, and a number of staff respondents in the study stated that they used information available on the drug chart to assess, reassess and review both medication and care plans.
Importantly, however, paper-based documentation was fragmented, not easily accessible or poorly organised. The various documents retained and used by different HCPs were kept ‘in silos’:
So quite a lot of nursing work has to come down in silos so we have nutrition, tissue viability, falls, dementia, and it doesn’t necessarily speak to each other on paper, which I think we’ve quite siloed risk assessments that it’s then difficult to put together holistically.
Nurse manager, H2
In one ward, the intentional rounding forms (for routine comfort checks) were filled in every 2 hours, in what appeared to have become an administrative, rather than investigative, exercise. Staff respondents in the study raised concerns over the large amount of paperwork, some of which they considered redundant:
When we fill in care plans, we’ve got the specialist assessment [forms] and they say the same things as your care plans.
Sister, H1
We also identified ambiguity in documenting the absence of pain. The interviewees reported the tendency to assume that the patient is not in pain if patients’ pain is not recorded in the documents:
If there’s nothing written, I suppose I would assume the question hasn’t. . . has the question been asked? I don’t know, I probably from a personal point of view [. . .] I would have asked it but probably not documented that there was no pain. If I don’t see anything written I would assume that the patient hasn’t complained of pain but I suppose what I can’t say is that they’ve been asked if they’ve got any pain.
Doctor, H1
No use of decision support tools was observed in any of the settings studied for pain assessment or management. In one site (H1), the Abbey Pain Scale101 was recommended in the local set of documentation, but was not available, or appeared not to be known, to the staff. Instead the site used a pain care plan, which was written anew for each patient in a loosely structured form, with narrative entries at assessment. This was used with all patients, with or without dementia, and with bare information recorded regarding patient experience of pain and/or what intervention was used.
One site utilised electronic documentation and at the time of the study it was in the early stages of implementing the Abbey Pain Scale in electronic form. However, no data could be collected regarding this and no staff were observed using it. A manager reported how the tool had been trialled, but ‘not well used on the ward’, that the criteria for using it were unclear and that it ended up being used as a ‘tick-box exercise’ (ward manager, H3). Wards in case site H2 had recently implemented a generic pain assessment (GPA) form, developed locally, to be used alongside the PACSLAC tool. 102 In the period of our study, for the patients we observed in this site, the GPA was often with a patients’ drug chart, but left blank or showing only initial entries without follow-up:
Nurses have so many assessments now to do that it’s, they’ve kind of lost their credibility a bit, [the GPA form] it’s just seen as a form and a tick-box exercise [. . .] it’s another thing to do and yet they have a hugely frantic day [. . .]
Clinical educator, H2
Conclusion
Pain assessment and management are complex activities that embrace physiological, emotional, cognitive and social dimensions. Pain is often described as a private experience, but in reality it regularly requires public expression in order to obtain relief. When caring for people with dementia, the complexity of communication is exacerbated by the challenges that the progressive cognitive and functional decline present. These complex social processes occur within hospital contexts and routines organised mainly around the needs of the organisation rather than those of patients. This complexity may, at least in part, explain why simple tools available to assess pain in patients with dementia are not well used in practice. Future decision support interventions need to take this complexity into account during their development, reorganising frames and quality of time for communication with patients, making a more varied range of pain management interventions routine, and devising tools that bring together information to provide a ‘picture of a patient’s pain’ accessible to all involved, within an overall framework of person-centred care.
Chapter 7 Foundations for the development of the decision support tool
The overall aim of this research was to develop a decision support tool to assist hospital staff with the assessment and management of pain in patients with dementia. Having first gathered evidence on existing observational pain assessment tools through the systematic review, and having evaluated ways in which pain is currently assessed and managed in hospital settings, analytic work was then undertaken to produce an initial design for the decision support intervention. This chapter describes the methodology applied for this purpose and the associated research activities. It is organised into two sections, the first describing the overall methodology and the different possible options that were considered and prioritised, and the second focusing on the design of the new tool and briefly explaining the methods and rationale specifically for the design of this tool.
Methodology for the design of a new intervention
In designing our intervention we have followed the guidance for theory-based intervention design,103–106 and have taken inspiration from user-centred design approaches. 107,108
Our aim was to achieve an improvement intervention based on both evidence and theory. 109 The data gathered through the observational study were our main evidence, supplemented with the literature. The main theoretical frame of reference was the decision-making theory51,52 underlying traditional pain assessment models, although other theories were also explored in connection with the range of different themes emerging from the analysis of the data.
The work towards the identification of the intervention was undertaken in parallel with the analysis of the data from the observation study (as described in the Chapter 5). Although the initial aim of the research was the development of a decision support tool, we did not exclude the possibility that other forms of interventions would be as (or more) appropriate. The team was open to the exploration of all possible options, and to then select and prioritise those more strongly supported by the evidence and that had the potential to address the issues with pain recognition, assessment and management identified by our study. Once generated, one (or more) interventions feasible for implementation in NHS hospital settings would then be selected for further development (feasibility being an important aspect of any successful improvement strategy,109 in light of current improvement and policy initiatives in the areas of dementia, pain, and organisation of hospital services (e.g. Department of Health14,110).
The method was iterative and collaborative, involving the whole team of researchers and investigators. Their individual contributions reflected their multidisciplinary areas of expertise which included pain management, mental health of elderly people, occupational therapy, improvement initiatives in acute care settings, decision-making and decision support design, user-centred design and health economics.
In practice, the process unfolded with the following iterative and collaborative activities:
-
Identifying in the data areas for improvement, specifically, identifying the data issues, gaps, and problems in current hospital practice.
-
Periodically writing-up and circulating analysis documents among the team to support discussion.
-
Brainstorming ideas for improvement strategies during analysis meetings.
-
Discussion with the SSC (January 2015 meeting). The SSC reminded the team that in designing any intervention, metrics and methods to assess its feasibility and effectiveness would also have to be included.
-
Collating all ideas into one whole final table, containing proposals and their rationales (hypothesis/theory). More than 30 intervention components were collated, of various expected impact and level of feasibility (a summary in Table 11). They ranged from systemic health services reorganisation (e.g. involving staff level and skill mix, empowering of HCPs, integration of mental health services into hospital care), changing local practices (e.g. making pain teams routine in all wards, keeping the patient in HCPs’ ‘horizon of observation’), and improving documentation and communication among the MDT.
-
A final analysis workshop, with Skype teleconference (with Dawn Dowding participating from Columbia University, New York, NY, USA) was held to prioritise and select the intervention.
Following guidance about the development of evidence/theory-based interventions,103 the workshop was structured around seven key conceptual stages that summarise the improvement intervention proposals:
-
how is pain understood and known
-
the problem(s) identified in pain assessment and management
-
components of the proposed intervention
-
mechanisms that would make the proposed intervention work
-
expected outcomes
-
metrics to evaluate effectiveness
-
conditions (contextual factors) necessary for the intervention to work as intended.
-
-
Large flipchart sheets of paper – one for each stage – were laid out on a long table and potential interventions and related elements were mapped onto them with sticky notes (Figure 4). This facilitated making assumptions explicit (e.g. about mechanisms and contextual factors), and prioritising interventions that had the potential to be more feasible in the NHS context. The team agreed that this would be a complex intervention around documentation that would facilitate HCPs in ‘making sense’ of a patient’s pain and support judgements and decisions (later we called this PADDS; we describe this in Chapter 8).
-
Finalising a design rationale document, explaining rationales, hypotheses and assumptions for the chosen intervention (see Methods for the design and evaluation of the decision support tool). This was shared via e-mail with the members of the SSC.
| Proposed improvement strategy | Rationale |
|---|---|
| ‘Could it be pain?’ Make this question ‘routine’ | The routine should facilitate noticing a patient’s pain cues |
| All hospital wards to extend the range of pain management strategies available for inpatients and extend clinician’s ‘repertoire for action’. These may include dedicated time for comforting actions; comfort measures; physiotherapy services and involvement of pain specialists and acute pain services | Staff should be aware that ‘something can be done about pain’, even in hospital settings, and for both chronic and acute pain |
| Having a richer ‘repertoire of action’ should empower the HCPs; empowerment should counter the phenomenon of ‘if I can’t do anything about it, why ask?’ | |
| Resource to allow pain specialist teams to cover rehabilitation and medical wards. Ensure that pain specialist teams are regularly present on site. More generally, expand pain teams and pain services to elderly care acute environments to cater for both acute and chronic pain | More patients will be referred to pain specialists, albeit ‘informally’, and receive a specialist consultation |
| Will contribute to a (stronger, more positive) discourse about the importance of attention to pain and chronic pain management in this setting, not exclusively in community care | |
| Create opportunities for patients’ communication. Know each patient as best as you can and develop a relationship of trust and familiarity. This can be facilitated by not dismissing patients’ incoherent stories, spending time in the patient’s bay (be visible, keeping the patient in your ‘horizon of observation’) and performing documentation tasks in the patient’s room instead of in corridors or offices | By knowing the patient, the HCP/HCA will be able to recognise and interpret signs of pain/discomfort; by knowing the HCP/HCA, the patient will feel confident he/she can trust him/her and confidently communicate the pain experience |
| Knowing the patient will support HCP/HCA communication with the patient’s carers | |
| Create opportunities for carers to act as advocates and interpreters of patients’ pain-related behaviours. Ensure flexibility, so that carers who may have difficulty in visiting at prescribed times can discuss the patient’s individual pain cues with staff, in person or by telephone, and ensure that this information is recorded and accessible | Improved identification and understanding of individuals’ unique pain cues will enhance HCPs ability to provide appropriate interventions at appropriates times to improve management of pain |
| Organise the ward so as to support the elderly patient in functioning independently. Involve occupational therapists in facilitating the development of an occupationally rich environment. This is especially relevant for long periods of hospitalisation | Observing the patient in action will provide informative cues for pain recognition (i.e. the right information for the right decision) |
| It will give patients opportunities for meaningful engagement and, therefore, assist in the affective component of pain and/or contribute to prevent pain from occurring | |
| Facilitate HCP/HCA ‘making a picture’ of a patient’s pain. Improve data quality. Improve documentation of pain. Avoid silos and fragmentation. Avoid the expression no complaint of pain (‘no c/o pain’) in the nursing or medical notes; use a more precise notation in documentation. A more precise notation could maintain the same level of conciseness, but with less ambiguity; for example, with the expressions ‘no pain noticed’ or ‘appears not to be in pain’. Adopt electronic prescribing and computer-supported administration of medication (e.g. barcode scanning of medicines to match with patients at the time of administration) | Improve judgements and decision by assisting sense-making |
| Improve HCP/HCAs communication about a patient’s pain | |
| Electronic administration of medication will improve accuracy of recording the time of administration |
FIGURE 4.
Design team analysis workshop: working out components, rationales, expected outcomes and assumptions (the ‘programme theory’). Potential interventions and related elements were mapped with (a) sticky notes and organised into key components; (b) how pain is understood; (c) components; (d) mechanisms; (e) outcomes; and (f) conditions.





In summary, our analysis and exploration of potential interventions led us to a range of intervention components, targeted at different levels of the organisation, from the individual member of staff to ward-level or hospital-wide initiatives. As we acknowledge the potential for such a wide-scope multifaceted complex intervention, the focus of our research was on those elements that aim to support decision-making at the bedside. These include improving the recording of pain assessment/management and creating a more complete pain picture. Therefore, we proceeded with the design of an intervention of this kind.
Methods for the design and evaluation of the decision support tool
As mentioned in the introduction (see Methodology for the design of a new intervention), we approached the design of the intervention on the basis of the principles of implementation science and user-centred design. The former suggests the need for a ‘programme theory’ as the basis of implementation and evaluation; the latter suggests starting with high-level (conceptual) design before moving on to low-level design (the actual working of the system and the ‘look and feel’ of the system). The three phases inform each other as propositions/ideas are discussed, and mock-ups are tested and evaluated (Figure 5).
FIGURE 5.
Three phases for the design of a theory-based intervention.
We completed the first steps of the first two phases by developing the foundations for a programme theory (Table 12, further developed in the proposal for the PADDS system) and the requirements specifications document (version 1; see Appendix 6). These were based on the discussions and analytic work done by the team, written up by the senior research fellow (VL), with documents circulated for comments among the team. The requirement specification document was written with reference to the requirements specifications for electronic prescribing systems published by NHS Connecting for Health. 111
| Conceptual elements of the programme theory | Findings, propositions, hypotheses |
|---|---|
| The problem we aim to address | The decision-making problem we identified is multidimensional and it involves:
|
| The components of our intervention intended to mitigate or solve the problems | Our intervention entails a new documentation process and chart or electronic space that enables connecting all available information about the presence or absence of pain to make a patient-specific pain picture that can provide an overview to inform collective decision-making. The intervention will include these components:
|
| This will need to be digital. We are aware of the inherent risks associated with a pain medication chart that is separate from the drug chart: a new documentation process and chart dedicated to a patient’s pain that also includes pain medication data cannot be physically separated from the documentation of the patient’s other medical prescriptions. For reasons of patient safety, and given the design constraints of paper, the new documentation process and information space we propose will augment existing electronic patient record/electronic prescribing systems | |
| The intervention’s expected outcomes | The intervention is expected to have direct and indirect, and immediate and medium-term outcomes |
Direct and immediate outcomes:
|
|
Indirect, medium-term outcomes:
|
|
| The metrics and methods for assessing these outcomes | Assessment clearly linked to prescription |
| Administration clearly linked to assessment | |
| Number of days patients appear content and are able to meaningfully engage | |
| Shorter stays in hospital | |
| Less recourse to specialist care in the community | |
| Carers’ satisfaction | |
| Involvement and shared decision-making documented in patient records | |
| Decreased use of neuroleptics | |
| Greater variety and frequency of use of appropriate non-pharmacological pain management interventions | |
| The mechanisms: from our input to expected outcomes | The chart is expected to change individual cognitive work and decision-making tasks from ‘mental computation’ to ‘pattern recognition’, through:
|
| This reorganisation of available information will facilitate the building of mental models (‘patterns of pain’ or ‘pain pictures’) both in general and for individual patients. It will therefore enable more effective triggering of the identification of pain and more effective use of pain management strategies | |
| Pattern recognition tasks are less error prone than mental computational tasks and therefore a change from computation to pattern recognition is expected to lead to improved quality and safety | |
| The conditions necessary for the intervention to work and outcomes to be achieved (context and implementation strategy) | There are prerequisites for the effectiveness of our intervention. We identified the following:
|
The third stage, the low-level design, including codesign sessions with users and evaluation, required more time and different expertise than those available within the research team and was therefore left to further research. This third stage will incorporate a health economic evaluation, using the outcome measures developed in this study. However, given that the proposed intervention is more complex than anticipated, further development of this part of the evaluation will be essential.
At this point the high-level design envisages a ward-based electronic health record module designed to ‘slot into’ existing systems, augmenting rather than replacing existing electronic or paper forms or records where a patient’s pain is documented. The actual design of this module will be strongly influenced by potential users during the codesign process.
Chapter 8 Health economics
Background
There has been little research about the cost or cost-effectiveness of interventions to identify or manage pain for individuals with dementia. For example, a recent review112 identified only five randomised controlled studies published since 2003 targeting behavioural disturbances and behavioural interventions targeting pain; none of these considered the cost or cost-effectiveness of the interventions. Similarly, a systematic review, of the same year, considered the effectiveness of interventions targeting pain or behaviour in dementia. The study included 16 papers, but again none assessed cost or cost-effectiveness. 113 However, evidence suggests that dementia is costly. The onset of behavioural disturbances such as agitation are often linked with pain114 and result in more frequent medical examinations and hospitalisation, thus higher costs. 115
Aim and objectives
The aim of the economic substudy was to develop health economic data collection forms that may be used in a future economic evaluation of a decision tool for pain management in people with dementia.
The data collection forms would comprise health-care and personal social care resources used by this population over the course of their hospital stay and subsequently, post discharge, in the community, and would be an outcome measure congruent for use in an economic evaluation in line with NICE guidelines. 116 In addition, recommendations for how these forms might be administered was deemed important.
With respect to resource use, while in the UK there is increasing use of NHS administrative records for collection of these data for use in economic evaluation, for personal social care there is considerable variation in the configuration of services and recording systems, so use of this type of tool for collecting detailed information of resource use is likely to continue to be necessary. 117 With respect to the outcome measure, cognitive challenges suggest that in some contexts a proxy measure, completed by a supporter or HCP, might be appropriate.
A first draft of the data collection forms focusing on resource use would be developed drawing on existing literature in the field. These data collection forms would be designed to capture health- and social-care resource use associated with the intervention and its implementation, and any subsequent health and social care used in the community post discharge. The forms would be designed to allow tick-box completion wherever possible. Feeding into the semistructured interviews with clinical staff, patients’ and carers’ opinion would be sought in respect of:
-
health- and social-care resource use that should be included
-
acceptability/feasibility of draft questionnaires and their content
-
the optimal method and timing of data collection.
The data collections forms would be adapted in line with the results from the interviews. The semistructured interviews would also provide a forum in which to assess the feasibility of patient self-completion of outcome measures suitable for use in an economic evaluation. This would include the European Quality of Life-5 Dimensions (EQ-5D), the EQ-5D proxy version (www.euroqol.org/home.html), Dementia Quality of Life Measure (DEMQOL) and the DEMQOL proxy (developed for completion by carers of people with dementia). 118
Within the original protocol a feasibility study was to be undertaken. The decision tool, developed in the overarching study, was to be assessed for feasibility and acceptability in acute hospital care settings. As part of this planned feasibility study it was intended that we would also test the acceptability and feasibility of the economic data collection forms (outcome measures and resource use). The forms could be revised in light of the results from the feasibility study and used in any subsequent randomised controlled study.
However, over the course of the overarching study it became clear that the feasibility study would not be undertaken, and no decision tool was developed. As such, the aim of health economic component of the study became more modest, with focus lying on the development of the health economic data collection forms. The feasibility and acceptability may be tested in any subsequent feasibility study (outside of the study reported here).
Methods
The data collection forms for use in economic evaluation were developed based on a review of the literature and on the ward observations made by the research team (described in Chapter 6). In the case of the latter, a workshop was convened of field researchers and wider research team members including methodologists, clinicians and experts in dementia. A semistructured interview schedule was used that addressed the following areas:
-
resource use associated with the patient group while they stay in an acute hospital setting
-
the professionals involved in patient care and the tasks these professionals undertake
-
the types of assessments or therapy patients have had while in hospital
-
the medication types and frequency, specifically with regard to pain
-
the reasons for admission, that is, comorbidities.
Notes were taken during the workshop, and content analysis was used to identify elements to include in the design of research instruments.
The focus of the literature review, undertaken in 2013/14, initially lay on pain identification and/or management in individuals with dementia, and cost-effectiveness. Although this was not a systematic review, we used key search terms ‘pain’, ‘dementia’ and ‘cost-effectiveness’. We were unable to locate any previous cost-effectiveness studies, and this was borne out by systematic reviews of the effectiveness of interventions in this area which, it was anticipated, would identify studies in which the cost-effectiveness would be undertaken alongside the clinical effectiveness. As highlighted earlier, Achterberg et al. 112 in their review of interventions targeting behavioural disturbances and behavioural interventions targeting pain, and Pieper et al. 113 in their review of interventions targeting pain or behaviour in dementia, did not report on any studies that included cost-effectiveness. As such, we spread our net over a wider area to identify randomised controlled studies that included cost-effectiveness analyses of dementia treatments.
Based on the findings from the literature review, the results from the workshops and previous data collection forms produced within the Academic Unit of Health Economics (University of Leeds), draft resource-use data collection forms were then designed. The forms also included the two potential quality of life measures that might be used within a subsequent randomised controlled trial: the EQ-5D, three levels (EQ-5D-3L), a measure of health-related quality of life used in the estimation of quality-adjusted life-years, and the DEMQOL,118 which is designed to work across dementia subtypes and care arrangements, and can be used at all stages of dementia.
Once completed, the draft data collection forms were then distributed among the researchers and LAGs for feedback, and comments was received on their acceptability.
Results
The review of the literature revealed that adaptations of the Client Service Receipt Inventory (CSRI) were typically used in economic evaluations alongside clinical trials for this population. 119–121 The CSRI collects details of resources use across health and social care, as well as information on areas such as housing. It has been successfully used in the collection of information on costs, service utilisation, income and related matters. 122 The CSRI in its original form collects data across a wide range of variables including socioeconomic data, education, employment and income. Clearly some of these variables were likely to be surplus to the requirements of the resource use required within this study. We also drew on previous resource-use data collection forms, developed in house for use with similar populations. For example, data collections used within the Pilot Trial of STOP delirium! (PiTSTOP) study; a study of a delirium prevention programme. 123 Finally, the workshop informed the content of the draft resource-use form.
The team analysis workshop was conducted on 7 April 2014 with five project researchers/investigators from across a range of disciplines, and opinion was sought with respect to identifying resource use, professionals’ time, assessment, therapy and medication, as well as reasons for admission. As a result, the following cost categories were identified:
-
Resource use, including staff involved in direct patient care-related tasks, such as feeding, clothing, toilet care, supported walking and patient comforting. The supportive family members in some of the tasks challenged this cost category ‘family members are not considered part of the care team’. Resource use included time spent with the patient. For example, it was noted that during the end-of-life stage, the palliative care teams may become involved and staff may find it very useful if a family member is there to observe changes in the patient. If no family members can be present at night, it may be possible for a nurse to stay with the patient if time and workload allow.
-
The professionals involved in patient care and the tasks these professionals undertake. Included in this cost category are staff members from a range of specialisms (beyond only doctors and nurses) for example chaplains, HCAs, physiotherapists, social workers and all others covered by overhead figures.
-
The types of assessments or therapy patients have had while in hospital. This included general tests such as blood tests and X-rays or specialised assessments such as the Waterloo score to assess risk of pressure ulcers. Therapy may include physiotherapists’ interventions to encourage the patient to move, as some pain is caused by not moving about.
-
The medication types and frequency, specifically with regard to pain, including times of drug rounds.
-
The reasons for admission, that is, comorbidities. For example, trauma is a common reason for older patients to be admitted to hospital.
Data collection forms
The data collection forms were designed to capture health- and social-care resource use within the acute sector, and also included community-sector resource use. As highlighted, they were based on results from the analysis of data from the observational study by way of the researchers’ analysis workshops and previous data collection forms, including the CSRI and those produced within house for similar populations. Based on the results, including the importance of input from both formal and informal carers in recording resource use, and acting as a proxy for the person with dementia for completion of the health-related quality-of-life measures, the research instruments were designed to allow tick-box completion wherever possible. Three types of data collection form were developed:
-
a patient questionnaire
-
an informal carer ‘family or friends’ questionnaire
-
a formal carer ‘staff’ questionnaire.
The patient questionnaire was designed to be interview administered. Questions regarding resource use while in hospital are asked, including department, type of assessments and/or operations. The patient is also asked about the health-care services they have used in the last month while not in hospital, and previous hospital attendance prior to their current stay. As previously highlighted (see Aims and objectives) we included DEMQOL and EQ-5D prior to eliciting feedback from the researchers and LAG. The patient is asked to complete a 0–10 pain VAS, the EQ-5D-3L and the DEMQOL. The purpose of the supporter and formal carer questionnaires was to collect and validate information about the health of the person they provide support or care to. The EQ-5D-3L proxy and DEMQOL proxy, a 31-item interviewer-administered questionnaire is included.
Feedback from researchers and lay advisory group
The questionnaires were distributed among the researchers and the LAG. As there was no intervention developed, they could only comment on the acceptability in relation to the patient group.
One member felt that all questionnaires were quite lengthy and questioned whether or not anyone would take the time to complete them. They felt that a person with dementia would have great difficulty answering all the questions, and that completion of more than 10 questions would be unrealistic. Another member felt that background questions for the supporter about education and questions about employment and living situation were intrusive. Another suggested the questions about how many hours worked/sick days were intrusive.
Conclusion
Questionnaires for use in economic evaluations of a cohort of individuals with dementia were developed with input from researchers, HCPs and laypeople (see Appendix 7). Further testing is needed to assess whether or not the data collection forms need to be reduced, as per the concerns voiced in the feedback from researchers and LAG. There is typically a tension in collecting data between participant burden and having a core data set that allows the study question to be answered in a robust manner. It is unfortunate that, within the confines of this substudy, we were not able to undertake further assessment within a feasibility study, as further research is required to test the acceptability and suitability of the questionnaires and the method of data collection (e.g. interview administered or carer/self-completion). The initial feedback suggests that piloting of these research instruments is needed prior to use, and that adjustments may need to be made to make the forms easier to use and more acceptable to users.
The workshop also revealed the intensive staff input to direct patient care-related tasks, such as feeding, clothing, toilet care, supported walking and patient comforting, and the potential, or not, of input of informal carers within the acute sector. This suggests that a micro-costing study to assess this input would be a valuable part of any future feasibility study for this population.
Chapter 9 Discussion, conclusions and a way forward
Here we discuss the findings of the meta-review, the observational study, the strengths and limitations of the two studies, and then the implications of both for practice, policy and research. The chapter finishes with a proposal for a new decision support tool designed to improve the recognition, assessment and management of pain for patients with dementia in hospital wards. Parts of this chapter are reproduced from Lichtner et al. 100 © 2016 The Author(s). This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Discussion of meta-review findings
The objective of our meta-review was to identify which tools are available to assess pain in adults with dementia, in which settings and patient population they have been used, and evaluate their reliability, validity, feasibility and clinical utility. We found a relatively large number of reviews on this topic and a considerable number of pain assessment tools available for the population with cognitive impairment. Of the reviews we evaluated, only one86 met all of the quality criteria outlined in the AMSTAR checklist, and would be considered a high-quality review. The review by Herr et al. 82 had an associated website in which 17 tools (specifically for use in nursing homes) are listed and commented on in detail. 124
The systematic reviews commonly situated their choice of interventions to review in the context of the challenges associated with assessing pain in patients who were cognitively impaired. Although there was recognition that the gold standard for pain assessment was the patient’s own assessment, this was unlikely to be possible with this patient population if the person was severely cognitively impaired, thus leading to the use of observational scales. The tools included in these reviews were therefore for the greater part observational, but showed a broad variation of measures and methods of assessing pain in adults with dementia. The foundation and focus of an observational scale is how the pain is manifested or made known, for example, on the basis on the AGS’s pain indicators. 48 An additional conceptual foundation resided on differentiation between aspects of pain, such as ‘the sensory-discriminative and motivational-affective aspects’. 125 Very few tools appeared to have any strong theoretical underpinning to the development.
Overall, there was no single tool that appeared to be more reliable and valid than the others. There was considerable variation in how reliability and validity of the tools were assessed. The majority of reliability and validity assessments were carried out on small samples in one or two different studies, so the applicability of tools across settings has yet to be evaluated. Similar conclusions can be drawn in relation to the feasibility and clinical utility of the tools. These findings have implications for research, which we briefly discuss over four points.
First, given the large number of existing tools identified by our meta-review, it seems inappropriate to develop further tools on the basis of the same conceptual foundations. Instead, researchers need to either envisage new assessment instruments on the basis of different conceptual foundations, or concentrate on extending the psychometric evidence base for the existing tools. There is a need to evaluate the tools on a wider scale, across a variety of patients and clinical settings, using rigorous methods and larger sample sizes. The studies need to ensure that the definitions of cognitive impairment and type of pain that is being assessed are clearly defined, to enable comparisons across populations. Although rigorous research is needed, it must be noted that the accuracy of tools is difficult to assess when an objective biological marker or other gold standard criterion is lacking – such as is the case for pain intensity. 85,86,126
Second, research should be conducted in clinical practice to assess the feasibility and clinical utility of the tools, and thus their potential for use in everyday clinical practice. Tools may be more useful in detecting relative changes in individual patients than differences between patients. 76 Tools that showed high reliability in research may not demonstrate high reliability in routine clinical practice if they are not administered as intended. 127 Pain assessment tools designed for research purposes, in order to aggregate and compare data across patients, do not necessarily transfer easily and effectively to clinical settings for everyday use. In general, it has been suggested that measurement tools developed for the purpose of evaluation of policy-making128,129 or routine general screening127 might have ‘no meaning’ at the frontline of care.
Third, research on the clinical utility of the tools should include evaluation of their impact in terms of choice of treatment and patient outcomes. This question was considered in one of the excluded reviews:126 the reviewers found no evaluations of the effect of protocols such as algorithms or pathways for assessing pain in inpatients (including those with dementia), and they reported ‘a study in cancer patients [that] found opioid-related over sedation and other adverse effects increased substantially after implementation of pain assessment on a numerical scale routinely with other vital signs’ (Helfand and Freeman,126 citing Hurley et al. 130). Herr et al. 82 reported that ‘the use of the ADD protocol was associated with a significant increase in the use of pharmacologic [. . .] and non-pharmacologic comfort interventions’. Alternatively, the use of the wrong pain assessment tool might reduce the likelihood that pain treatment will be initiated or contribute to acute exacerbations of pain (as could be inferred by data from Smith88) or may be found to have no effect on the quality of pain management. 131
Finally, an instrument must be relevant to ‘the condition, setting and participants in the health interaction, in particular the patient and clinical users’. 132 This includes the notion of user-centredness, defined as ‘the extent to which an instrument faithfully captures both the content of the health care user’s views and the ways in which their views are expressed’. 133 As a number of the systematic reviews and other literature makes clear, pain is a subjective experience, thus, the associated measurement gold standard is the patient’s own assessment. 66 However, where the patient is severely cognitively impaired, alternative ways to assess the patient’s level of pain must be found. Resort is made to behavioural indicators and groups of cues. 134 Ideally, these aspects would be used to assess expressed pain when the patient is at rest and when moving. Against this context, user-centredness, in terms of the actual patient’s self-assessment of pain, is unachievable. The next best option, from a user-centred perspective, becomes the assessment of a person who is most familiar with the patient in their everyday life in a hospital or other care setting – what Herk et al. 66 called a ‘silver standard’. It is critical, however, that the assessor has a high familiarity with the patient. It can plausibly be argued that the more this is achieved, the greater the likelihood of a closer fit of the proxy’s view with the patient’s own experience. At the same time, overfamiliarity may lead to an attenuation of the observer’s focus on potential pain clues. However, as Smith88 commented, one is rarely in ‘an ideal situation’ where the direct care providers know, and are thus highly familiar, with the care recipient’s personal habits and history. Thus, rather than seeking to review the degree of the user-centredness of tools in this area, it is important to evaluate the guidance on a tool’s use (that is who should administer the intervention and in what range of situations) and then explore whether or not it is actually used in the indicated way in everyday clinical practice. Exploration and findings on clinical utility and feasibility of use provide suggestive evidence of potential for use in everyday practice. Such evidence is a necessary but not sufficient condition for a tool’s actual use. It is notable that little or no insight is provided into the guidance over who should administer the tools in any of the systematic reviews. To gain insight into this aspect of use and possible additional evidence on a tool’s actual use in practice, one would need to examine the original instrument designers’ papers and validation studies, rather than rely on a systematic reviewer’s observations or summaries. Notwithstanding, it can be recommended that these two aspects of tool use (guidance and actual use) are incorporated into tools reviews and, given this patient group, development of evidence on the actual use of the tools in clinical practice.
Discussion of observational study findings
The overall aim of the observational study was to investigate how HCPs and others recognised, assessed and managed pain in patients with dementia in a range of acute settings.
The main information source was claimed to be the patient, as the sole individual with insight into their pain. However, cognitive impairment, communication difficulties, the organisation and context of the ward, all of which hindered access to this source of knowledge. As shown in other contexts of care,135 the communication difficulties experienced by patients with dementia were interactional in the sense that they ‘arise, in part, from their cognitive deficits’, but were also ‘occasioned by, or contingent on, the other’s contributions in interaction’. The hospital routines and environments generated a modality of interaction that challenged the communication abilities patients with dementia may have had. In fact, a similar finding was discussed about hospital inpatients, in general,136 that ‘the ward social system may also have an effect on patients’ communications [. . .] [in the sense that] the very structure of the work situation discourages patients from communicating clearly’. 136
The social context of the ward environment also critically shaped health professionals’ ability to recognise and respond appropriately to a patient’s communication of pain. The time frames for patient interaction, the number of patients on each ward and the expectations set up by the ward routines all influenced HCPs’ and HCAs’ abilities to perceive, recognise and manage a patient’s pain. Observational studies have revealed the complexity of pain management in acute settings and the barriers to optimal pain relief, such as staff’s attentiveness to pain cues, interruptions when assessing pain and reconciling varying interpretations of pain from multiple sources. 137,138 The typology of patients present in a ward – whether the ward was an admission unit, surgery ward or care of the elderly ward – affected the assumptions and expectations regarding pain and how it should be addressed. This finding echoes that of a study based in a surgical ward unit in the USA where ‘[p]ain assessment was rooted in a reference typology of clients based on surgical procedure’138 and nurses ‘expected’ a ‘certain kind of pain’. The type of patients in each ward influenced staff expectations about their pain and how it should be routinely treated. In turn, this routine also affected what could be expected about a patient’s pain and whether or not pain may be detected and recognised as pain (Figure 6).
FIGURE 6.
Systemic links between HCAs’/HCPs’ (individuals) perceptions and (organisational) routines. Reproduced from Lichtner et al. 100 © 2016 The Author(s). This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

Hypothetico-deductive reasoning92 posits that clinicians would initially act on cues given by patients that might suggest pain was present. Being alert and receptive to a pain cue would be the first step in detecting and then managing the patients’ pain. However, the findings from this study showed that cues that were potentially indicative of the presence of pain could be missed or go unrecognised. Clisset et al. 139 defined ‘missed opportunities’ in dementia care as occasions when ‘opportunities presented themselves for HCPs to make some connection with the person with dementia but they seemed unable or unwilling to do so, often by choosing to end the interaction as quickly as possible’. In this study, missed opportunities manifested in several forms. For example, opportunities to detect, and manage, pain were missed when the patient was expressing pain but a clinician was not present (colocated) at that moment, when a pain cue was atypical of that which a clinician might usually interpret as pain, or when the cue did not seem to merit further investigation as it appeared to fall within acceptable limits.
Analysis of data also revealed organisational routines with an almost exclusive reliance on medication for managing pain. This type of approach is reliant on patients being able to communicate the presence of pain and changes in their pain after administration of medication. However, the communication difficulties caused by dementia drastically alter the effectiveness of this process. Other pain management interventions (such as repositioning, physiotherapy or physical activity, or patient engagement in meaningful activities) may offer the patients more opportunities to express and communicate their experience of pain and are included in best practice guidelines. 48 Thus it can be argued that reliance on pharmaceutical interventions – and the absence of any other forms of interventions – not only reduces the opportunities for patients with dementia to have their pain more effectively managed, but greatly limits the opportunities for HCPs to assess pain.
The central finding is that the recognition, assessment and management of pain in patients with dementia in hospital wards is a complex, dynamic, sense-making process, involving a number of information sources and individuals at different times and in different places (Figure 7). Pain cues generated by patients are interpreted by ward staff in light of their own knowledge, experiences and expectations, and are influenced by ward environments, which have their own routines and operate within a wider organisational culture.
FIGURE 7.
Complex, non-linear factors that influence pain recognition, assessment and management.
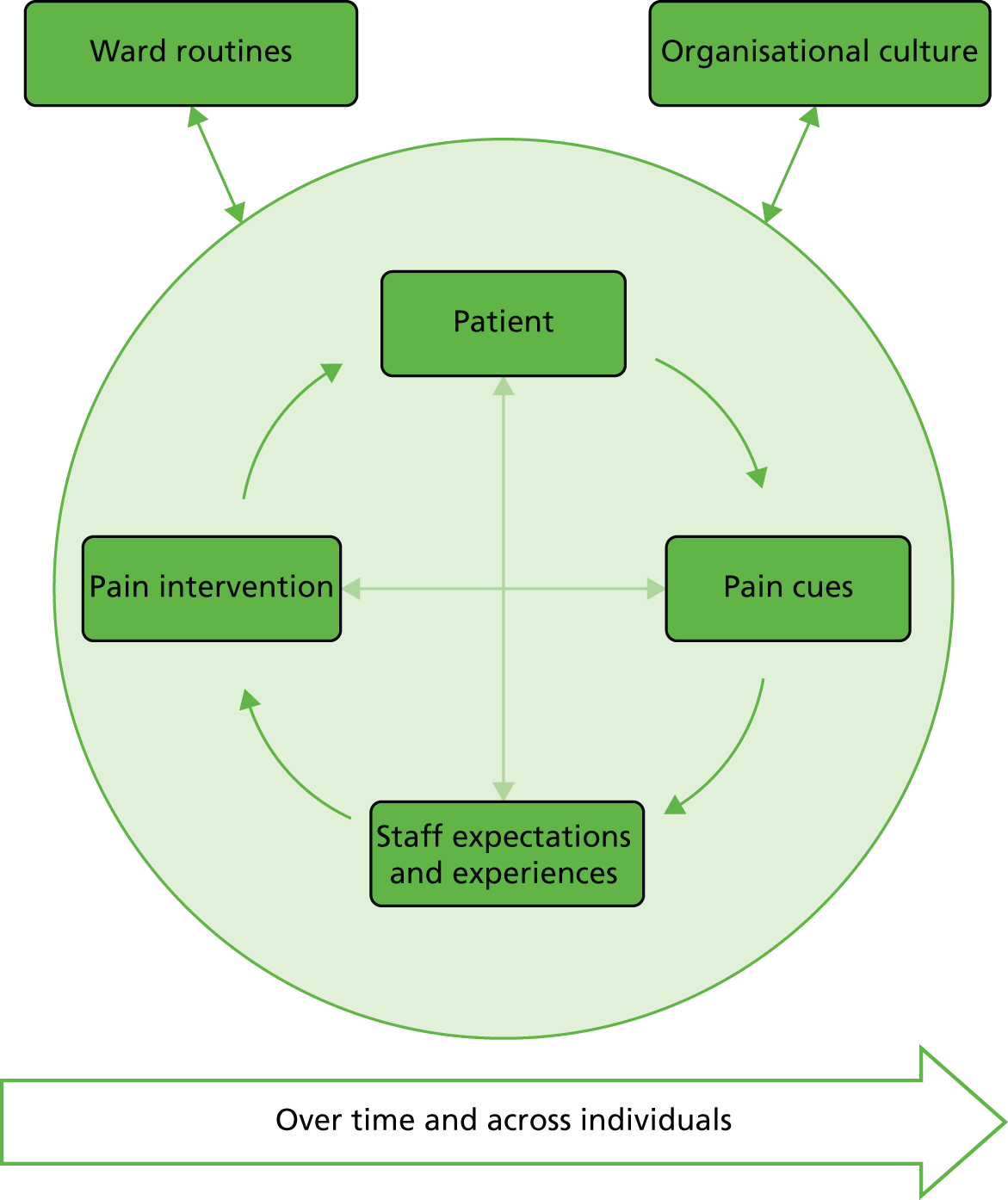
At present the timing, personnel and recording are disparate and it may be that a system which integrates all of these factors could significantly improve the structures, processes and outcomes of pain management for patients.
In terms of the health economics study, questionnaires for use in economic evaluations of a cohort of patients with dementia were developed with input from researchers, HCPs and laypeople. Further research is required to test the acceptability and suitability of the questionnaires. Depending on the structure of the intervention, any economic evaluation would be required to take account of the time and staff resources involved in pain assessment and delivery of pain relief.
Strengths and limitations
Meta-review
We analysed 23 reviews for data extraction and included data from eight of these. Although we could have been stricter in our interpretation of the criteria for a systematic review and excluded a greater number of potentially eligible records before reaching the stage of data extraction, this would not necessarily have restricted the analysis to reviews with higher AMSTAR scores. It would have given us a smaller number of records for data extraction and, although this would have saved us time in the data extraction process, we do not believe that it would have changed the final outcome and the findings. In addition, it would have reduced the number and range of tools assessed.
We analysed 28 tools included in eight reviews. However, other tools are also available and were included in some of the reviews we excluded because they did not provide psychometric data for extraction. The review by Stolee et al. ,85 for example, covers 30 tools, including the PPQ, the PBM, the 21-Box Scale and the Pain Thermometer, among others.
It is possible that more systematic reviews would have been identified if grey literature had been searched and non-English-language papers had been included. However, we would expect the English translation of the titles of these to be indexed in the databases we searched; we did not limit our searches by language, yet we did not retrieve any non-English-language systematic reviews.
As with all reviews, there was a risk of publication bias. We checked further available data, when readily available, such as in supplementary tables online67 but we did not contact the authors to obtain missing data. This is a limitation of the review, but our unit of analysis was reviews rather than original studies. As part of the scoping work it was decided not to contact the authors either of the reviews or of the original studies.
In our assessment of the reliability and validity of the tools, we did not attempt a meta-analysis; we are aware that studies had been counted in various reviews and their results could have been counted more than once. However, we do not believe this to be an issue for our narrative synthesis.
Our search did not specifically target reviews reporting on the feasibility or clinical utility of the tools, thus our evidence may be more limited on these aspects than it might have been otherwise.
We attempted to minimise bias in the review process by involving a review team with diverse expertise, specifically by having each review assessed independently by at least two members of the team, by having the whole team of reviewers involved at every stage of the process, and by not excluding reviews on the basis of our assessment of their quality.
Importantly, this meta-review does not cover a new tool developed in France by the Doloplus Collective team – Algoplus. 89 To our knowledge, this tool has not yet been included in a systematic review.
The original protocol proposed undertaking two systematic reviews. One was intended to provide an overview of existing tools to map out potential available assessment tools and the settings where they have been evaluated. The findings of this overview were then to be used as a focus for the second review, to explore the psychometric properties and clinical utility of tools to assess pain in older people with any degree of cognitive impairment in acute settings.
However, the pain experts on the team were aware of numerous systematic reviews which had already been undertaken into the identification of best-available observational pain tools. Rather than replicate work, it was agreed that a systematic review of all published systematic reviews would cover the aims of both reviews more efficiently. Hence, the reported meta-review which identified 28 tools, their reliability and validity well as the settings in which they had been tested. On completion of this meta-review it was agreed that there was little to be gained from an additional review.
Even if a systematic review of feasibility and clinical utility had been undertaken, our meta-review had already indicated that virtually all available tools at that time had been devised for long-term, rather than acute settings – the focus of our work. It was therefore likely that little of direct relevance would be found. Having commenced the observational study, preliminary findings suggested that the emphasis on tool use and validation may be a secondary consideration, thus comprising only a small part of the complex processes and communications involved in pain recognition, assessment and management we were observing.
The large amount of work done to date on developing tools for the assessment of pain in people who have dementia has had a narrow focus on the tools themselves, largely ignoring the influence of the complex systems within which they may be used. Our findings from the first year of this study suggested that tools (though available) were rarely used and more would be gained from shifting the emphasis away from tool selection and towards contextual issues related to personnel, contexts and communications related to the recognition, assessment and management of pain.
Observational study
There are few in-depth qualitative studies about pain assessment and management in patients with dementia in hospital settings (e.g. Manias53 and Bolster and Manias140) and, to our knowledge, none have been conducted through the lens of decision-making theory. This perspective has informed data collection and analysis, which remained focused mainly on dimensions of information and communication.
Core aspects of the study design were observations at the bedside, providing an almost unique opportunity to experience hospital patient care from the patient’s vantage point. It is possible that there may have been some observer (Hawthorne) effect, but observations were designed as ‘non-participant’ in the sense that field researchers did not take part in patient care activities. As it is often the case in ethnographic work, the researchers attempted to ‘become part of the furniture’, by spending time in the settings they studied so that staff became familiar with them and carried on with their activities as if researchers were not there observing. 141 The researchers attempted to minimise any impact their presence at bedside or in the ward may have on staff activities, or their use of tools, or staff interaction with the patient. On the other hand, having the researcher at the bedside did inevitably affect the patients’ interaction with the environment and patients’ experience of their hospital stay, for example from being alone for long hours to being with some company (we reflected on this in a paper142 written at the time of data collection).
The opportunity cost of this approach involved the loss of observation of concurrent activities outside the patient view, several of which could have involved decisions about a patient’s pain. However, this was compensated for by additional observations in the ward, for example, at nurses’ stations, or doctors’ offices. Challenges in recruitment of both patients and interviewees limited the number of participants in the study, but meant that a large number of hours were spent in the ward, for example while waiting for family to visit the patients identified as potential participants.
Details of limitations in patient data, carer involvement and the intended final feasibility study are given below.
Patient data
Identifying patients with a diagnosis of dementia for inclusion in the study was compromised by the fact that such diagnoses were not systematically recorded in the medical notes. Ideally, all elderly patients should benefit from assessment by a geriatrician, including a clear indication of whether or not a patient is suffering from dementia and/or pain. Ideally, this information should then be effectively shared with the rest of the MDT taking care of the patient (including HCAs). However, in practice this was not necessarily the case. In the centres where this work was done, older patients with dementia were not all admitted to ‘geriatric’ wards, they were in almost all areas of the hospital, and not all were assessed by a geriatrician. This means that many could not benefit from a thorough comprehensive geriatric assessment and the information necessary for recruitment was not readily available to researchers.
In one of the four sites data collection had additional challenges, mainly because of gatekeeping and protectiveness of staff towards patients with dementia. The difficulties encountered in collecting data in H3, and especially the lack of audio-recording, meant that H3 data could not be treated in the same way as the other data points. We were more cautious in taking the field notes of the interviews as exactly what the participant said during the interview and in the knowledge we had gained of the practices in each of the wards visited. Fewer hours of observation recorded gave us less material for data analysis.
It is part of ethnographic research methods that the researcher and the context where the research takes place has a strong impact on the data. The researcher is the lens through which observations are done and field notes recorded. Observations were guided by an observation protocol, but were inevitably affected by the researchers’ own background and research experience. This resulted in different levels of detail, depth and scope across the data for all the four sites, not only H3. We found value in the difference as it offered opportunities for challenging assumptions and exploring complexities.
Carer involvement
A very small number of carers took part in recorded interviews, but conversations took place with all carers who were informed of the study at the time of recruitment and the field notes of these brief conversations were included in the analysis. The fact that so few carers could be recruited is an important finding. Many of the dementia patients who were considered potential participants for the study either had no carer or had an elderly spouse who was too unwell to visit or take part. Some had family in other parts of the country with whom they were no longer in touch. Of those we met and who agreed for their elderly relative to participate in the study, only a few agreed to take part in recorded interviews. It cannot be assumed that this group of patients is typical, but it is possible that many dementia patients have no carer who can act as their interpreter or advocate.
Researchers had opportunistic informal conversations with other carers visiting elderly patients who were not part of the study but appeared to have cognitive impairments. As we did not have ethical permission to interview this group of carers, we could not use their comments as research data. However, the content of these conversations added depth to our understanding of the ‘formal’ data.
Despite data for this group being limited, there is considerable scope for more effective future involvement of carers in the assessment and management of pain in ward settings, albeit ‘carers’ can include not only family members, but also anyone outside of the hospital context who knows the patient well in their everyday life (friends, neighbours, staff of other NHS/social care services). In lieu of the hoped-for sample of carers, members of the LAG for the project made an extremely valuable contribution to our understanding of the potential for carers to be more involved in the pain management of dementia patients in hospital settings.
Finally, our findings are mainly based on wards that relied on paper-based information systems (medical, nursing notes and drug charts); one case site had an electronic system in use, but we were unable to collect data on how this was used in practice to communicate information about patients. A consultant from a ward in another site that was planning to implement a hospital-wide electronic patient record system expressed the belief that this would facilitate aggregating and sharing information in one place accessible to all, and would reduce fragmentation and dispersion of information. The literature, however, suggests that electronic records may introduce fragmentation when each piece of information is recorded in a separate screen,143 but we leave this as a question for further research.
Feasibility study
We had hoped to develop a decision support tool and to carry out a feasibility testing of this as a third and final part of this study. However, this development and evaluation was left outside of the scope of this project for two main reasons: complexity of the outcome and time constraints.
-
Complexity of the outcome: at the outset of the project, it was assumed that a decision-making support tool could be devised based on the evidence generated, and that this might take the form of an algorithm or some other structured tool alongside a validated pain assessment tool. However, the completed systematic review and four-site observational study showed that a more complex decision support system, within the context of a complex intervention, would be needed than was initially envisaged. This required a new research approach (realist evaluation) requiring research skills and resources not held by the existing research team. Setting this up would have required more resources than were available.
-
Time constraints: as with most research, recruitment for this project was difficult. Access to wards was challenging and research governance processes time-consuming. The sensitive nature of recruitment for patients with dementia and the lack of routine documentation of the diagnosis of dementia (in order to identify potential recruits) meant that obtaining sufficient data took longer than anticipated. Although we had been optimistic that we would be able to undertake a pilot feasibility study within the remaining time on the project, once we had completed the analysis of the observational study, it became clear that this would not be possible. The development and implementation of the proposed complex decision support system and its evaluation would take considerably longer and require more and different resources than originally planned for a simpler intervention.
Specifically, additional time would be needed to:
-
identify and negotiate access to new sites which have established ward-based electronic documentation systems across all health-care disciplines
-
develop a computerised pain assessment and management module, which can be tailored to the requirements of, and be interoperable with, different systems
-
develop an implementation package to provide education and support in the sites involved.
We attempted to run focus groups to develop and test our initial ideas for the PADDS system intervention, but were unable to complete ethical approval processes in time. Therefore, the final section of the study focused on developing a preliminary specification for the PADDS system tool and an outline bid to undertake a realist evaluation.
It is in the nature of qualitative exploratory research that unexpected findings emerge and flexibility in methods is required. Therefore, it does not come as a surprise that our exploratory observational study did not develop exactly as originally planned. However, there are strengths and value in flexible exploratory qualitative methods, and these are found in the complexities and contextual factors they can reveal and the new research questions they can propose. We believe this research offers an example of how to deliver valuable results, albeit not exactly as originally planned in study protocols.
Health economics evaluation analysis
The work undertaken was important, generating preliminary data collection forms. The forms need piloting and refinement prior to use, but they constitute a strong base from which to further develop the economic evaluation of the proposed PADDS system intervention.
Implications of the studies
For practice
Although our findings corroborate existing recommendations for the assessment of pain among people who have cognitive impairments in long-term settings144,145 and management of acute and chronic pain,146–148 we found that wider organisational issues require consideration in order to improve practice in acute settings. In particular, this includes the diverse communications involved in pain recognition and assessment, the need for rapid access to all relevant information in some kind of central documentation system, an expansion of methods of managing pain by using non-pharmacological approaches to complement analgesics and the potential for greater carer involvement.
The recognition and assessment of pain
The most significant part of the information gathering and sense-making process is in self-report, capturing the pain experiences from the patients themselves. People with dementia who are able to communicate verbally may use a range of metaphors and analogies drawn from their life experience to communicate their pain. The ability to self-report pain has been shown to be possible for the majority of patients with mild to moderate cognitive impairment. 47 Patients need to be given sufficient time to express their pain; questions should be patiently repeated or reworded when not understood47 and patients should not be interrupted during their responses. 149 To do this empathically requires the development of a relationship with patients who have dementia.
Where these vulnerable patients are unable to self-report through verbal communication, effort is required to understand cues unique to each person that may indicate the presence of pain. These cues may form part of more formal observations of changes in behaviour (possibly using an observational pain tool) alongside the input of carers (friends and family) who are familiar with that person’s behaviour, allowing a more accurate understanding of the meaning of their specific behavioural pain cues.
When overall ‘pain pictures’ are informed by the person with dementia, the documentation of the patient’s pain (both assessment and management) may allow their pain to be understood and documented from a person-centred perspective. To achieve this, it is important to build a relationship with the person with dementia, their carer and other support networks as necessary, in order to elicit self-reports and/or maximise understanding of individuals’ idiosyncratic methods of communication, including what is normal for them, and what drugs or other interventions are known to work or not work for them. This process is in line with the gold standard of person-centred care, which has been established as a critical element in dementia care. 150,151 Such relationships should be forged at the hospital admission point and continue until discharge. Ideally, the HCP–patient relationship should be stable and continuous throughout this time in order to minimise, first, the potential for increased environmental disorientation caused by the hospital surroundings and, second, the missed opportunities that may occur in everyday care.
However, there is a perennial difficulty in interpreting the meaning of behaviours which indicate some kind of distress. Information cues (such as patients verbalising their pain, patients’ behaviours, bodily postures, or facial expressions) are often not recognised by staff as indicating the presence or absence of pain or are misinterpreted as ‘behavioural problems’. Distress may be due to pain, but it may also have other causes that the patient is unable to communicate. We cannot at present recommend any single observational pain assessment tool from those studied for use in an acute clinical setting, due to the lack of comprehensive evidence on the reliability, validity, feasibility or clinical utility of any of the tools. Nevertheless, it is worth bearing in mind that all are based on similar indicators of the presence of pain, as identified by the AGS. 48 These include facial expressions, verbalisations and vocalisations, body movements, changes in interpersonal interactions, changes in activity patterns or routines, and mental status changes. These are all aspects of behaviour which, when observed and understood, may indicate the presence of pain or other kinds of distress. The use of these cues in some form is an important part of the pain recognition and assessment process. Familiarity with the patient, supported by carers’ input, should enable ward staff to untangle possible causes of distressed behaviours, and to attempt analgesic trials where pain is a likely cause. 152
Documentation of pain-related information
Related to the issue of disparate and incomplete communication of pain by the patient is the need for easy access to comprehensive information about all aspects of pain for an individual patient. We observed that different professional groups tended to seek and record different types of information in different types of documentation. Thus diagnoses and causes of pain, medications prescribed and administered, pain scores and carers’ narratives, were spread out within the clinical setting. It is desirable that clinicians should be able to access all this quickly and easily in one location, accelerating the creation of an ‘overall picture’ of pain. This would most effectively be achieved through the use of electronic documentation, designed to streamline and integrate existing systems rather than creating additional forms to complete.
It is worth adding that inconsistency in the recording of the diagnosis of dementia may also be improved through such a centralised system. It may be possible to link dementia diagnoses to pain information as part of the overall picture created by electronic documentation.
Pain management
The provision of medication was the main response to pain in the wards studied, with other interventions rarely observed. There are numerous evidence-based policy documents which advocate the use of non-pharmacological interventions for both acute and chronic pain, including information provision, relaxation, distraction, trans-cutaneous electrical nerve stimulation, manual and massage therapies, and warming and cooling interventions and exercise. 48,146–148 Increasing knowledge and skills in how, when and with whom to use such therapies, along with necessary resources, would be a useful adjunct to the use of analgesic drugs.
The role of carers
Finally, the involvement of carers can help clinicians and patients to overcome communication barriers. Family members or friends who are familiar with patients may be able to decipher idiosyncratic behaviours that they know indicate the presence of pain for particular individuals. At present, information provided by carers, when available, is not well integrated into the information system in use, or is not documented. Increasingly in the UK paper forms are being introduced in hospitals where family members are asked to describe the person’s usual behaviour, likes and dislikes (these are variably known as ‘patient passports’, ‘10 things about me’, or ‘know who I am’, and they are part of a drive to increase awareness of dementia in hospital. Unfortunately these do not seem to be integrated with a patient medical record. It should be acknowledged that challenges are often encountered in clinician–carer relationships, and staff’s perceived need ‘to manage the family’153 (e.g. when carers insist that nurses ‘provide additional or further analgesics when not clinically indicated’153). Carers’ and patients’ concerns over ‘labelling’ should also be taken into account when using such forms. However, a relationship-centred care approach would reframe the carer as part of the identity of the patient, and this would shift the balance from trying to seek out information from carers, to them being actively involved in its creation.
In summary, self-report and observations of behaviour and information from carers form the core of pain assessment, and these need to be undertaken, recorded and shared systematically by the MDT. The range of pain management methods should be widened, and expanding the role of carers has considerable potential to improve the recognition, assessment and management of pain in hospital settings.
For organisations and systems
The challenges associated with pain assessment and management in patients with dementia have underlying contributing factors linked to wider cultural and organisational arrangements at a hospital level, organisation of the ward and ward routines, the information systems in use, skill mix and individual clinicians’ beliefs. Although this project has focused on some of these factors, such as the information systems in use, addressing the root causes of suboptimal pain care in patients with dementia is likely to require complex interventions in staff education, improvements in resources and organisational infrastructures, and change in culture and routine practice. There are valid and powerful arguments to be made regarding the importance of improved infrastructure and education around pain management, particularly within the cost-effectiveness profile of dementia care (with estimated costs in the UK of £20 billion each year). 154 Behavioural symptoms and institutionalisation are key factors in dementia’s large costs to the economy, both of which are closely linked to pain management. 155
For research
The vast majority of the research concerned with managing pain in people with dementia has taken place in care homes, with most being undertaken in the USA, Europe and Australia. Very little research has considered issues encountered in busy acute hospital settings. Furthermore, most research in the field has focused on the development and validation of observational pain instruments. Less effort has been dedicated to the contextual factors influencing their use. Not only should suitable assessment tools be used, but also the relative contributions of factors such as improved use of time, improved multidisciplinary communication and a more varied range of resources for managing pain need to be understood.
There is a need for a valid and reliable observational pain tool with good feasibility and clinical utility to be available for use with people who have dementia. Many have already been developed, but rather than creating yet another, further research should be conducted on existing observational pain assessment tools to establish their psychometric properties, clinical utility and feasibility in acute settings and, provide much needed guidance on their appropriate use in clinical practice.
Realist studies of clinical practice where decision support tools have been implemented could explore the relevance of contextual contributory factors. This should be followed by clinical trials of the effectiveness of the interventions likely to have the most positive impact.
There is also a need for research to evaluate integrated approaches to pain management, considering behavioural symptoms, prescription patterns and institutionalisation, in order to embed pain management within the overall treatment and care a person receives throughout their journey with the condition. In particular, the role of carers in the interpretation of pain cues has considerable potential, and evidence on how this potential may best be developed in acute settings would be of value. The strong reliance on analgesic medications to manage pain is unsurprising in acute settings, but greater use of evidence-based complementary approaches may be effective in improving pain and quality of life. An evaluation of methods of implementation of such approaches, including education about, and structured use of, such interventions with patients who have dementia would be a helpful addition to knowledge in this field.
The key findings of the observational study were the three prerequisites of time, interdisciplinary communication/documentation and the availability of a range of pain management resources (Figure 8). Together, improvements in these areas should facilitate the creation of an ‘overall picture of pain’ to support clinical decision-making for optimum pain management. Recognising and assessing pain involves a degree of guesswork, underpinned by medical knowledge and experience (‘what is usual for this medical case?’), the most common ‘typologies’ of patients in each ward, and the type of ward (e.g. surgery or elderly care).
FIGURE 8.
The key elements to obtain a dynamic, patient-specific, overall picture of pain. Reproduced with permission from SAGE from Closs SJ, Lichtner V. Management of pain in peple with dementia: time for a change of approach. Pain News 2016;14:76–8, box 2. 156 © Authors.
When pain is recognised, it is understood through a dynamic sense-making process shared across individuals in the MDT in what staff, in this study, referred to as ‘a picture’ of the patient. This key finding has implications for the development of decision support interventions, in that they would need to ensure that they assist with staff identification of pain cues, in part through allowing sufficient time and adequate location to do so. Notably, individuals may have their own unique time-related patterns of pain, related to circadian rhythms, which are known to be disrupted in dementia, and the timing of the assessment would need to consider this.
Further research is needed to ascertain whether or not staff education to improve the understanding of the importance of giving sufficient/quality time to patients and patient-centred communication could improve pain recognition (and therefore management).
The creation and development of an overall picture of pain, specific for the patient, is both a prerequisite for planning and undertaking a trial of therapy and a result of this therapeutic trial. In the sites we studied there was a lack of connection between documentation of the information about decisions to act (decision to prescribe, decision to administer), the feedback from the action (e.g. outcome of administration of analgesics) and the reassessment that informs the ensuing decisions and/or actions. This lack of connection represented a considerable challenge to the understanding of the patient pain and effectiveness of therapy. On the part of staff, it involved the cognitive process of reassembling the pieces, requiring memory, ‘mental computation’ and time. Research is needed to ascertain whether or not centralising how all the relevant pain information is documented and displayed can save time and energy in reaching an overall picture of each patient’s pain.
Given a ward’s division of labour and organisation of work over shifts, effective documentation is essential for composing this ‘overall picture’ over time, each information item acquiring value when aggregated with and compared with others. Decision tools would need to assist in the integration of the distributed pain information, allowing a central point where the picture of a patient’s pain is created, over time and across individuals. Furthermore, it would have to assist with action and decision taking in a dynamic fashion that supports the trial and error nature of pain management and options for intervention.
The paper-based information systems currently in use for the distributed work processes involved in pain assessment and management did not facilitate the perception of such a holistic ‘picture of pain’. Single pain assessments at one point in time, even if they were done with the aid of one of the many pain assessment tools available, did not provide sufficient information about a patient’s pain; even when repeated they risked remaining as isolated information points because they were not connected in a common, accumulated picture of a patient’s pain and the effectiveness of any pain mitigating care given. Much of the literature concerned with the assessment and management of pain for people with cognitive impairment has focused on the development and validation of observational instruments designed for the purpose of assessment. 54 A number of studies have shown that these tools are not often used in clinical practice, a finding that was supported in our data. This may be because the tools do not facilitate the rapid creation of the ‘overall picture of pain’ and, therefore, do not assist clinicians with their decision-making about pain in this vulnerable group of patients.
Conclusions
The main conclusions derived from the project reported here which could guide future research studies were the following:
-
There are no existing observational pain assessment tools for patients with dementia that have yet been demonstrated to have good validity, reliability, feasibility and clinical utility. No single tool can be recommended in preference to any other for general use in hospital settings.
-
Future assessment tools should:
-
have a strong theoretical underpinning
-
elicit self-reports and identify cues from the patient first (at rest and on movement), then from those who are familiar with them.
-
-
Clear opportunities for interactions between patients, carers and staff are needed, allowing time for the identification and understanding of pain.
-
The influence of the social context of wards should be recognised, incorporating assumptions about pain according to patient type, etc.
-
The present reliance on medication provision (using trials) to alleviate pain should be supplemented with other non-pharmacological interventions.
-
Clear and effective communication between all the individuals involved in the care of the patient is needed.
-
Centralised records of all pain assessment and intervention information are needed.
-
Guidance on the use of assessment and/or decision support tools should be available.
Each of these conclusions needs to be evaluated in future research. It would be helpful to know how these suggestions for change may influence the recognition, assessment and management of pain for patients who have dementia. They are interlinked and while each may have its own impact and place within patient care, the interactions between them may also be significant.
The way forward
Our findings suggest that a reconceptualisation of how we approach the assessment and management of pain for patients with dementia in hospital is needed. Rather than relying on the traditional linear concept of assessing pain, providing an intervention and reassessing, a broader, more systemic approach is needed. We hypothesise that systemic thinking needs to replace the current focus on improving individual tasks (e.g. history-taking, pain intensity assessment, analgesic prescribing). This requires thinking in a different way about care, where, for example, a ward may be understood as a complex system containing many components (staff, patients, equipment, visitors, etc.) and there are linkages and interactions between the components that constitute the entirety of that defined system. Systems thinking of this kind has been defined in many ways,157 but should be goal-orientated (e.g. to ensure good pain management).
The patient’s self-report should still be used as the gold standard wherever possible, including careful conversations with patients about their pain and the judicious use of self-report pain scales if they are appropriate, usually for acute pain. This requires staff to ensure that they spend sufficient time with patients, and where feasible their carers, to identify the presence of different types and locations of pain; and that staff learn to elicit and understand patient self-reports of pain and to recognise individuals’ unique pain cues.
The pain-related information elicited by different staff and informal carers is not currently effectively communicated between all those involved, so strategies are needed to ensure that everyone has easy access to all the necessary information. Records of such information are currently kept in different forms in different locations and, consequently, individual records tend to be incomplete. These need to be centralised in some way so that comprehensive accounts of a patient’s pain are rapidly accessible by all staff.
The almost exclusive use of medication to alleviate pain should be documented and linked with pain reports over time. Analgesics could be supplemented with other non-pharmacological approaches, some of which may be benign and soothing (e.g. gentle massage) and others which may have a positive effect on rehabilitation, such as exercise therapy.
The use of observational pain assessment tools needs to be pragmatic, using the best (in the top seven of those reviewed), preferably locally available tools, which staff may be familiar with, increasing the likelihood of their use. Over time, one of the many tools available may be demonstrated to be superior to the others, at which point its adoption into practice should be considered.
The disparate activities described above need to be integrated into the complex, dynamic and multidisciplinary sense-making activity of hospital care. This led us (following analysis and debate reported in Chapter 7) to a proposal for a new kind of pain decision support intervention, using a more systemic approach, as outlined in the next section.
Proposal for a new decision support intervention
Given that structured, observational pain assessment tools do not appear, on their own, to provide the information needed for clinicians to understand and manage the pain of people with dementia, we are suggesting a broader approach.
Three key practical problems related to effective pain management for people with dementia, which are amenable to change, have been identified. These include patients’ pain not being noticed and understood as pain; a lack of integration of relevant information required for collective sense-making of the patient’s pain; and poor feedback mechanisms to inform the empirical (trial) approach to pain management.
It would be ambitious to attempt to change an entire ‘ward system’, so we are proposing an intervention which focuses on these three problem areas and makes links and interactions between key elements of the processes become visible, and rapidly accessible. This is the PADDS system tool.
The PADDS system tool would be a module designed to ‘slot into’ existing centralised electronic health record system systems, which would be strongly influenced by potential users during the codesign process. It would be accessible to all ward staff involved in the care of patients with dementia (and others, especially those with communication difficulties). It would be used to record different types of pain-related information elicited by different staff at different times. It would integrate all this information and produce detailed graphical displays of chronological events including assessments and interventions, alerts and reminders for assessments and interventions, options for interventions other than medications and narrative information from staff, family and friends. It is intended to be flexible enough to be implemented in a range of different contexts and organisational cultures, but requires a pre-existing electronic platform into which it may be integrated. A preliminary specification for the essential elements of the PADDS system is given in Appendix 6.
At this stage the focus is on the PADDS tool, which comprises an electronic information module to integrate and display all the relevant pain-related information. This is one part of the PADDS intervention, which is intended to include not only the PADDS tool but also an educational package to support staff, patients and carers in understanding (each to an appropriate extent) the rationale for and functions of the PADDS tool: the importance of knowing the patient; strategies for effective pain communication; the use of non-pharmacological approaches to pain management to augment the use of analgesics; and an implementation plan for incorporating the PADDS tool into routine practice.
Before wide implementation of the PADDS system in NHS hospitals can take place, its feasibility and impact should be evaluated, through initial pilot and then wider clinical trial. We propose that the PADDS system pilot and evaluation would comprise part of a complex intervention, being codesigned with ward staff in order to accommodate contextual issues, then introduced into the existing electronic ward health record alongside an education and implementation package. This would require a realist evaluation which would need to ascertain first, whether the PADDS system is associated with improved pain recognition, assessment and management for patients with dementia, and, second, whether or not staff find that the PADDS system is a useable and useful augmentation of existing electronic documentation systems. Within such a study the objectives would be to:
-
codesign the PADDS electronic pain module with NHS clinical staff and carers
-
codesign an implementation strategy for PADDS with users, with representation of each professional group and managers in the selected wards/units
-
develop an educational package to support the implementation of the PADDS tool by staff, patients and carers, along with skills in the use of non-pharmacological pain interventions
-
introduce the PADDS using the implementation strategy [designed in (b) above]
-
assess the feasibility of outcome measures of the PADDS, for use in a future trial of the intervention’s effectiveness
-
identify potential mechanisms through which the PADDS leads to these outcomes
-
understand potential contextual factors of PADDS use that are necessary for the mechanisms to be triggered
-
develop a programme theory to support a trial of a refined version of the PADDS, including the identification of contextual factors that need to be in place, and the contexts in which the PADDS can provide most benefit, to inform wider implementation and evaluation.
The meta-review and observational study have provided the evidence for a theoretical basis for the PADDS system which can then be developed and tested following Medical Research Council guidance for evaluation of complex interventions. 109 The implementation of the PADDS system has the potential to move practice towards widespread improvements in how the pain of people with dementia (and others) have their pain managed in hospital.
Acknowledgements
Lay advisory group
We are particularly grateful to our LAG, who were contacted via the AS. They were a participative group, ready to speak out, whose commitment was evident, and whose input was crucial in improving various aspects of the project as we went along. We would like to thank (alphabetically) Mrs Rosemary Copsey, Sandra Duggan, Andra Houchen, Mr U Hla Htay, Matt Murray, Andrew Pepper, Mrs Lynne Ramsay, Sylvia Wallach Squire, Sue Tucker and Barbara Di Vita. Three other members did not give permission to be named here.
Contributors
Our thanks to Sara Campbell and Nita Gorasia for contributing to data collection for the exploratory study and Lesley Jones (Advanced Nurse Practitioner), Greater Manchester West Mental Health NHS Foundation Trust, for providing expert advice and input on pain and dementia.
Study Steering Committee
We would like to thank the chairperson (Peter Crome) and members (Sandra Duggan, Roger Knaggs, Bridget Penhale and Pat Schofield) of the SSC for their wise counsel and support throughout the project.
Contributions of authors
S José Closs (Professor, Nursing Research, Pain Assessment) was the chief investigator for the project, contributed to securing the funding, the overall conduct of the study and led the writing of the final report for publication.
Dawn Dowding (Professor, Nursing, Clinicial Decision Making, Nursing Informatics) conceived the study, secured the funding, contributed to the systematic review, the analysis of the data from the observational study and the design of the decision support intervention.
Nick Allcock (Professor, Nursing and Pain Research) was the principal investigator leading research in one of the four sites where the exploratory study took place, contributed to the analysis of the data of the exploratory study and to the overall conduct of the project.
Claire Hulme (Professor, Health Economics) led the health economics aspects of the project.
John Keady (Professor, Older People’s Mental Health Nursing) was the principal investigator leading research in one of the four sites where the exploratory study took place, contributed to the analysis of the data of the exploratory study and to the overall conduct of the project.
Elizabeth L Sampson (Reader, Old Age Psychiatry and Dementia) was the principal investigator leading research in one of the four sites where the exploratory study took place, contributed to the analysis of the data of the exploratory study and to the overall conduct of the project.
Michelle Briggs (Professor, Nursing and Pain Research) led the systematic review, contributed to the analysis of the data of the exploratory study and to the overall conduct of the project.
Anne Corbett (Senior Lecturer, Dementia Research) led the user involvement, contributed to the writing of previous related publications and to the overall conduct of the project.
Philip Esterhuizen (Lecturer, Nursing Ethics) contributed to the systematic review and the exploratory study, with data collection and analysis.
John Holmes (Senior Lecturer, Liaison Psychiatry of Old Age) contributed to analysis of the data of the exploratory study.
Kirstin James (Occupational Therapist and Researcher, Acute Care Medicine; Older Person’s Well-Being) contributed to the exploratory study, to data collection and analysis, to the writing of previous related publications and the findings report, and to the overall conduct of the project.
Reena Lasrado (Research Associate, Mental Health Social Work) contributed to data analysis in the exploratory study, to the writing of previous related publications and to the overall conduct of the project.
Andrew Long (Professor, Health Systems Research) contributed to the systematic review, to the analysis of the data of the exploratory study and to the overall conduct of the project.
Elizabeth McGinnis (Nurse Consultant, Tissue Viability) contributed to the exploratory study by facilitating access to setting and recruitment.
John O’Dwyer (Research Officer, Health Economics/Economic Evaluation) contributed to the health economics aspects of the project and designed the health economics questionnaires.
Caroline Swarbrick (Research Fellow, Dementia and Ageing Research) participated in the design of the study, contributed to the exploratory study with data collection and analysis, to the writing of previous related publications and to the overall conduct of the project.
Valentina Lichtner (Senior Research Fellow, Health Informatics and Decision Making) led the project management of the four research centres and co-ordinated all project activities, contributed to the systematic review, the exploratory study, the data collection and analysis, and led the design of the decision support intervention.
Publications
The research described in this report has been presented in a range of publications and at a variety of conferences.
Journal papers
Closs SJ, Lichtner V. Management of pain in people with dementia in hospital: time for a change of approach. Pain News 2016;14:76–8.
Lichtner V, Dowding D, Esterhuizen P, Corbett A, Closs SJ, Long A, Briggs M. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatrics 2014;14:138. URL www.biomedcentral.com/1471-2318/14/138/abstract (accessed 29 January 2016).
Dowding D, Lichtner V, Allcock N, Briggs M, James K, Keady J, et al. Using sense-making theories of decision making to aid understanding of the recognition, assessment and management of pain in patients with dementia in acute hospital settings: a UK multisite study. Int J Nurs Stud 2016;53:152–62. URL: www.sciencedirect.com/science/article/pii/S002074891500259X (accessed 29 January 2016).
Lichtner V, Dowding D, Closs SJ. The relative meaning of absolute numbers: the case of pain intensity scores as decision support systems for pain management of patients with dementia. BMC Med Inform Decis Mak 2015;15:1–11. http://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-015-0233-8 (accessed 29 January 2016).
Lichtner V, Dowding D, Allcock N, Keady J, Sampson EL, Briggs M, et al. The assessment and management of pain in patients with dementia in hospital settings: a multi-case exploratory study from a decision making perspective. BMC Health Services Research 2016;16:427.
Conference presentations
Lichtner V. The everyday ethics of field work research with vulnerable patients. Stud Health Technol Inform 2014;205:813–17. URL: www.ncbi.nlm.nih.gov/pubmed/25160300 (accessed 29 January 2016).
Dowding D on behalf of the team. Assessing and Managing Pain in Patients with Dementia in Acute Care Settings: Developing a Decision Support Tool. Proceedings of the 12th International Congress on Nursing Informatics, Taipei, Chinese Taipei, 21–25 June 2014.
Allcock N, James K on behalf of the team. The Detection and Management of Pain in Patients with Dementia in Acute-Care Settings: Development of a Decision Tool [poster]. Scottish Pain Research Community (SPaRC) Fourth Annual Scientific Meeting, Dundee, 27 March 2014.
Allcock N, James K. Emerging Findings from the Research Study: The Detection and Management of Pain in Patients with Dementia in Acute-Care Settings [poster]. Scottish Pain Research Community (SPaRC) Fifth Annual Scientific Meeting, Dundee, 27 March 2015.
Closs SJ, Lichtner V on behalf of the pain and dementia team. Recognition, Assessment and Management of the Pain of Hospital Patients with Dementia: Uncovering the Complexities [poster]. British Pain Society Annual Scientific Meeting, Glasgow, 21–23 April 2015.
Briggs M, Esterhuizen P, Closs SJ, Long A, Corbett A, Dowding D, Lichtner V. Pain Assessment for People with Dementia: a Review of Systematic Reviews of Pain Assessment Tools [poster]. British Pain Society Annual Scientific Meeting, Glasgow, 21–23 April 2015.
James K, Allcock N. Occupational Enrichment: A Role for Occupation in Pain Recognition, Assessment and Management for People with Dementia in Hospital Settings [poster]. British Pain Society Annual Scientific Meeting, Glasgow, 21–23 April 2015.
James K, Allcock N. The Role of Occupation in Pain Assessment and Management for People for Dementia in Hospital Settings: A Finding from the Pain and Dementia Study [oral presentation]. International Association of Geriatricians and Gerontologists Conference, Dublin, Ireland, 23–26 April 2015.
Dowding D, Lichtner V, Esterhuizen P, Corbett A , Closs S, Long A, Briggs M. Pain Assessment Tools for Individuals with Cognitive Impairment: A Meta-Review [poster]. Eastern Nursing Research Society Annual Scientific Sessions, Washington DC, WA, 15–17 April 2015.
Lasrado R, Swarbrick C, Keady J on behalf of the team. Generating Pain Stories To Inform Clinical Decision-Making In Acute Hospital Care For People With Dementia: Process And Positioning [poster]. Alzheimer’s Society Annual Research Conference, Manchester, UK, 29–30 June 2015.
Esterhuizen P, Lichtner V. The Reflective Researcher: Yours, Mine and Ours. Sigma Theta Tau International Phi Mu Chapter, 2nd Biennial Nursing Scholarship Conference, Leeds, UK, 19 June 2015.
Briggs M, Lichtner V, Esterhuizen P, Dowding D, Closs SJ on behalf of the team. The Detection and Management of Pain in Patients with Dementia in Hospital: Exploring the Impact of Social Factors. British Sociological Association Medical Sociology Group Annual Conference, York, UK, 9–11 September 2015.
Dowding D, Closs SJ, Lichtner V on behalf of the team. Recognition, Assessment And Management Of The Pain Of Hospital Patients with Dementia: Uncovering the Complexities. Advancing Science, Improving Lives: National Institute of Nursing Research 30th Anniversary Symposium and Poster Session, Washington, DC, WA, 13 October 2015.
Data sharing statement
Data may be accessed via the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Alzheimer’s Disease International . Dementia Statistics 2014. www.alz.co.uk/research/statistics (accessed 14 July 2014).
- Prime Minister’s Challenge on Dementia. London: Department of Health; 2012.
- Dementia UK. London: Alzheimer’s Society; 2007.
- McKeith I, Cummings J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol 2005;4:735-42. http://dx.doi.org/10.1016/S1474-4422(05)70219-2.
- Alzheimer’s Disease Facts and Figures. New York, NY: Alzheimer’s Association Public Policy Office; 2015.
- Bridges S. Health Survey for England – 2011: Chapter 9, Chronic Pain. Leeds: The Health and Social Care Information Centre; 2012.
- Hunt LJ, Covinsky KE, Yaffe K, Stephens CE, Miao Y, Boscardin WJ, et al. Pain in community-dwelling older adults with dementia: results from the National Health and Aging Trends Study. J Am Geriatr Soc 2015;63:1503-11. http://dx.doi.org/10.1111/jgs.13536.
- Cracks in the Pathway. People’s Experiences of Dementia Care as They Move between Care Homes and Hospitals. Newcastle upon Tyne: Care Quality Commission; 2014.
- DeWaters T, Faut-Callahan M, McCann JJ, Paice JA, Fogg L, Hollinger-Smith L, et al. Comparison of self-reported pain and the PAINAD scale in hospitalized cognitively impaired and intact older adults after hip fracture surgery. Orthop Nurs 2008;27:21-8. http://dx.doi.org/10.1097/01.NOR.0000310607.62624.74.
- de Souto Barreto P, Lapeyre-Mestre M, Vellas B, Rolland Y. Potential underuse of analgesics for recognized pain in nursing home residents with dementia: a cross-sectional study. Pain 2013;154:2427-31. http://dx.doi.org/10.1016/j.pain.2013.07.017.
- Horgas AL, Tsai PF. Analgesic drug prescription and use in cognitively impaired nursing home residents. Nurs Res 1998;47:235-42. http://dx.doi.org/10.1097/00006199-199807000-00009.
- Horgas AL, Dunn K. Pain in nursing home residents. Comparison of residents’ self-report and nursing assistants’ perceptions. Incongruencies exist in resident and caregiver reports of pain; therefore, pain management education is needed to prevent suffering. J Gerontol Nurs 2001;27:44-53. http://dx.doi.org/10.3928/0098-9134-20010301-08.
- Maxwell CJ, Dalby DM, Slater M, Patten SB, Hogan DB, Eliasziw M, et al. The prevalence and management of current daily pain among older home care clients. Pain 2008;138:208-16. http://dx.doi.org/10.1016/j.pain.2008.04.007.
- Living Well with Dementia: A National Dementia Strategy. London: Department of Health; 2009.
- Dementia Pathway. Manchester: NICE; 2015.
- The Assessment of Pain in Older People: National Guidelines. Concise Guidance to Good Practice Series, No 8. London: Royal College of Physicians; 2007.
- Abdulla A, Bone M, Adams N, Elliott AM, Jones D, Knaggs R, et al. Evidence-based clinical practice guidelines on management of pain in older people. Age Ageing 2013;42:151-3. http://dx.doi.org/10.1093/ageing/afs199.
- Stokes G. Challenging Behaviour in Dementia: A Person-Centred Approach. Oxford: Speechmark Publishing Ltd; 2000.
- Leong IY, Nuo TH. Prevalence of pain in nursing home residents with different cognitive and communicative abilities. Clin J Pain 2007;23:119-27. http://dx.doi.org/10.1097/01.ajp.0000210951.01503.3b.
- Kunz M, Scharmann S, Hemmeter U, Schepelmann K, Lautenbacher S. The facial expression of pain in patients with dementia. Pain 2007;133:221-8. http://dx.doi.org/10.1016/j.pain.2007.09.007.
- Husebo BS, Strand LI, Moe-Nilssen R, Borgehusebo S, Aarsland D, Ljunggren AE. Who suffers most? Dementia and pain in nursing home patients: a cross-sectional study. J Am Med Dir Assoc 2008;9:427-33. http://dx.doi.org/10.1016/j.jamda.2008.03.001.
- Buffum MD, Sands L, Miaskowski C, Brod M, Washburn A. A clinical trial of the effectiveness of regularly scheduled versus as-needed administration of acetaminophen in the management of discomfort in older adults with dementia. J Am Geriatr Soc 2004;52:1093-7. http://dx.doi.org/10.1111/j.1532-5415.2004.52305.x.
- Jacobs JM, Hammerman-Rozenberg R, Cohen A, Stessman J. Chronic back pain among the elderly: prevalence, associations, and predictors. Spine 2006;31:E203-7. http://dx.doi.org/10.1097/01.brs.0000206367.57918.3c.
- Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain 2009;145:276-8. http://dx.doi.org/10.1016/j.pain.2009.04.007.
- Luengo-Fernandez R, Leal J, Gray A. Dementia 2010. The Economic Burden of Dementia and Associated Research Funding in the United Kingdom. Cambridge: Alzheimer’s Research Trust; 2010.
- Fulop G, Strain JJ, Fahs MC, Schmeidler J, Snyder S. A prospective study of the impact of psychiatric comorbidity on length of hospital stays of elderly medical-surgical inpatients. Psychosomatics 1998;39:273-80. http://dx.doi.org/10.1016/S0033-3182(98)71344-1.
- Lyketsos CG, Steele C, Galik E, Rosenblatt A, Steinberg M, Warren A, et al. Physical aggression in dementia patients and its relationship to depression. Am J Psychiatry 1999;156:66-71. http://dx.doi.org/10.1176/ajp.156.1.66.
- Fields SD, MacKenzie CR, Charlson ME, Sax FL. Cognitive impairment. Can it predict the course of hospitalized patients?. J Am Geriatr Soc 1986;34:579-85. http://dx.doi.org/10.1111/j.1532-5415.1986.tb05763.x.
- Zekry D, Herrmann FR, Grandjean R, Meynet MP, Michel JP, Gold G, et al. Demented versus non-demented very old inpatients: the same comorbidities but poorer functional and nutritional status. Age Ageing 2008;37:83-9. http://dx.doi.org/10.1093/ageing/afm132.
- Sampson EL, Jones L, Thuné-Boyle IC, Kukkastenvehmas R, King M, Leurent B, et al. Palliative assessment and advance care planning in severe dementia: an exploratory randomized controlled trial of a complex intervention. Palliat Med 2011;25:197-209. http://dx.doi.org/10.1177/0269216310391691.
- Sampson EL, Gould V, Lee D, Blanchard MR. Differences in care received by patients with and without dementia who died during acute hospital admission: a retrospective case note study. Age Ageing 2006;35:187-9. http://dx.doi.org/10.1093/ageing/afj025.
- Sampson EL, White N, Lord K, Leurent B, Vickerstaff V, Scott S, et al. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: a longitudinal cohort study. Pain 2015;156:675-83. http://dx.doi.org/10.1097/j.pain.0000000000000095.
- Ryden MB, Feldt KS, Oh HL, Brand K, Warne M, Weber E, et al. Relationships between aggressive behavior in cognitively impaired nursing home residents and use of restraints, psychoactive drugs, and secured units. Arch Psychiatr Nurs 1999;13:170-8. http://dx.doi.org/10.1016/S0883-9417(99)80003-X.
- Spencer K, Foster PE, Whittamore KH, Goldberg SE, Harwood RH. Staff confidence, morale and attitudes in a specialist unit for general hospital patients with dementia and delirium – a qualitative study. Int J Geriatr Psychiatry 2014;29:1315-17. http://dx.doi.org/10.1002/gps.4178.
- Corbett A, Husebo BS, Achterberg WP, Aarsland D, Erdal A, Flo E. The importance of pain management in older people with dementia. Br Med Bull 2014;111:139-48. http://dx.doi.org/10.1093/bmb/ldu023.
- Watkin L, Blanchard MR, Tookman A, Sampson EL. Prospective cohort study of adverse events in older people admitted to the acute general hospital: risk factors and the impact of dementia. Int J Geriatr Psychiatry 2012;27:76-82. http://dx.doi.org/10.1002/gps.2693.
- Counting the Cost: Caring for People with Dementia on Hospital Wards. London: Alzheimer’s Society; 2009.
- Young J, Hood C, Woolley R, Gandesha A, Souza R. Report of the National Audit of Dementia Care in General Hospitals 2011. London: Healthcare Quality Improvement Partnership, Royal College of Psychiatrists; 2011.
- Mukadam N, Sampson EL. A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr 2011;23:344-55. http://dx.doi.org/10.1017/S1041610210001717.
- Jordan AI, Regnard C, Hughes JC. Hidden pain or hidden evidence?. J Pain Symptom Manage 2007;33:658-60. http://dx.doi.org/10.1016/j.jpainsymman.2007.02.026.
- Laurila JV, Pitkala KH, Strandberg TE, Tilvis RS. Detection and documentation of dementia and delirium in acute geriatric wards. Gen Hosp Psychiatry 2004;26:31-5. http://dx.doi.org/10.1016/j.genhosppsych.2003.08.003.
- Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009;195:61-6. http://dx.doi.org/10.1192/bjp.bp.108.055335.
- Closs SJ, Barr B, Briggs M. Cognitive status and analgesic provision in nursing home residents. Br J Gen Pract 2004;54:919-21.
- Maclean W. Identifying and managing pain in people with dementia. Nurs Res Care 2003;5:428-30. http://dx.doi.org/10.12968/nrec.2003.5.9.11668.
- Cook AK, Niven CA, Downs MG. Assessing the pain of people with cognitive impairment. Int J Geriatr Psychiatry 1999;14:421-5. http://dx.doi.org/10.1002/(SICI)1099-1166(199906)14:6<421::AID-GPS932>3.0.CO;2-Y.
- Archibald C. People with Dementia in Acute Hospital Settings. Stirling: University of Stirling, Dementia Services Development Centre; 2002.
- Closs SJ, Barr B, Briggs M, Cash K, Seers K. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J Pain Symptom Manage 2004;27:196-205. http://dx.doi.org/10.1016/j.jpainsymman.2003.12.010.
- American Geriatrics Society Panel on Persistent Pain in Older persons . The management of persistent pain in older persons. J Am Geriatr Soc 2002;50:205-24. http://dx.doi.org/10.1046/j.1532-5415.50.6s.1.x.
- Kovach CR, Griffie J, Muchka S, Noonan PE, Weissman DE. Nurses’ perceptions of pain assessment and treatment in the cognitively impaired elderly. It’s not a guessing game. Clin Nurse Spec 2000;14:215-20. http://dx.doi.org/10.1097/00002800-200009000-00011.
- Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Bevene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223-38. http://dx.doi.org/10.1001/jama.293.10.1223.
- Dowding D, Thompson C. Using judgement to improve accuracy in decision-making. Nurs Times 2004;100:42-4.
- Thompson C, Dowding D. Essential Decision Making and Clinical Judgement for Nurses. Edinburgh: Churchill Livingstone; 2009.
- Manias E. Complexities of pain assessment and management in hospitalised older people: a qualitative observation and interview study. Int J Nurs Stud 2012;49:1243-54. http://dx.doi.org/10.1016/j.ijnurstu.2012.05.002.
- Lichtner V, Dowding D, Esterhuizen P, Closs SJ, Long AF, Corbett A, et al. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr 2014;14. http://dx.doi.org/10.1186/1471-2318-14-138.
- Dowding D, Lichtner V, Allcock N, Briggs M, James K, Keady J, et al. Using sense-making theory to aid understanding of the recognition, assessment and management of pain in patients with dementia in acute hospital settings. Int J Nurs Stud 2016;53:152-62. http://dx.doi.org/10.1016/j.ijnurstu.2015.08.009.
- Hughes N, Locock L, Ziebland S. Personal identity and the role of ‘carer’ among relatives and friends of people with multiple sclerosis. Soc Sci Med 2013;96:78-85. http://dx.doi.org/10.1016/j.socscimed.2013.07.023.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.
- Joanna Briggs Institute Reviewers’ Manual 2008 Edition. University of Adelaide, SA: Joanna Briggs Institute; 2008.
- National Library of Medicine . Medical Subject Headings, 2014 MeSH, MeSH Descriptor Data – Dementia – Tree Number C10.228.140.380 and F03.087.400 – Unique ID D003704 2014. www.nlm.nih.gov/cgi/mesh/2014/MB_cgi?mode=&term=Dementia&field=entry (accessed 16 June 2014).
- National Library of Medicine . Medical Subject Headings, 2014 MeSH, MeSH Descriptor Data – Cognition Disorders – Tree Number F03.087.250 – Unique ID D003072 2014. www.nlm.nih.gov/cgi/mesh/2014/MB_cgi?mode=&term=Cognition+Disorders&field=entry (accessed 16 June 2014).
- National Library of Medicine . Medical Subject Headings, 2014 MeSH, MeSH Descriptor Data – Learning Disorders – Tree Number C10.597.606.150.550, C23.888.592.604.150.550, F03.550.350.500 and F03.550.450 – Unique ID D007859 2014. www.nlm.nih.gov/cgi/mesh/2014/MB_cgi?mode=&term=Learning+Disorders&field=entry (accessed 16 June 2014).
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000100.
- Powell RA, Downing J, Ddungu H, Mwangi-Powell F, Kopf A, Patel NB. Guide to Pain Management in Low-Resource Settings. Seattle, WA: IASP; 2010.
- Zwakhalen SM, Hamers JP, Abu-Saad HH, Berger MP. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatr 2006;6. http://dx.doi.org/10.1186/1471-2318-6-3.
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010;63:737-45. http://dx.doi.org/10.1016/j.jclinepi.2010.02.006.
- van Herk R, van Dijk M, Baar FP, Tibboel D, de Wit R. Observation scales for pain assessment in older adults with cognitive impairments or communication difficulties. Nurs Res 2007;56:34-43. http://dx.doi.org/10.1097/00006199-200701000-00005.
- Corbett A, Husebo B, Malcangio M, Staniland A, Cohen-Mansfield J, Aarsland D, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol 2012;8:264-74. http://dx.doi.org/10.1038/nrneurol.2012.53.
- Liu JL, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al. Systematic reviews of clinical decision tools for acute abdominal pain. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10470.
- Montori VM, Wilczynski NL, Morgan D, Haynes RB. Optimal search strategies for retrieving systematic reviews from MEDLINE: analytical survey. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38336.804167.47.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-10.
- Long AF, Dixon P. Monitoring outcomes in routine practice: defining appropriate measurement criteria. J Eval Clin Pract 1996;2:71-8. http://dx.doi.org/10.1111/j.1365-2753.1996.tb00029.x.
- Schofield P, Clarke A, Faulkner M, Ryan T, Dunham M, Howarth A. Assessment of pain in adults with cognitive impairment: a review of the tools. Int J Disabil Hum Dev 2005;4:59-66. http://dx.doi.org/10.1515/IJDHD.2005.4.2.59.
- Schofield P. Assessment and management of pain in older adults with dementia: a review of current practice and future directions. Curr Opin Support Palliat Care 2008;2:128-32. http://dx.doi.org/10.1097/SPC.0b013e3282ffb406.
- Schofield P, Clarke A, Faulkner M, Ryan T, Dunham M, Howarth A. Assessment of pain in older people. Where are we now and what needs to be done?. Int J Disabil Hum Dev 2006;5:3-8. http://dx.doi.org/10.1515/IJDHD.2006.5.1.3.
- Rutledge DN, Donaldson NE, Pravikoff DS. Update. Pain assessment and documentation. Special populations of adults. OJCI 2002;5:1-49.
- Rutledge DR, Donaldson NE. Pain assessment and documentation. Part II – special populations of adults. OJCI 1998;1:1-29.
- Andrade DC, Faria JW, Caramelli P, Alvarenga L, Galhardoni R, Siqueira SR, et al. The assessment and management of pain in the demented and non-demented elderly patient. Arq Neuropsiquiatr 2011;69:387-94. http://dx.doi.org/10.1590/S0004-282X2011000300023.
- Miller LL, Talerico KA. Pain in older adults. Annu Rev Nurs Res 2002;20:63-88.
- Lobbezoo F, Weijenberg RA, Scherder EJ. Topical review: orofacial pain in dementia patients. A diagnostic challenge. J Orofac Pain 2011;25:6-14.
- McAuliffe L, Nay R, O’Donnell M, Fetherstonhaugh D. Pain assessment in older people with dementia: literature review. J Adv Nurs 2009;65:2-10. http://dx.doi.org/10.1111/j.1365-2648.2008.04861.x.
- Herr K, Bursch H, Ersek M, Miller LL, Swafford K. Use of pain-behavioral assessment tools in the nursing home: expert consensus recommendations for practice. J Gerontol Nurs 2010;36:18-29. http://dx.doi.org/10.3928/00989134-20100108-04.
- Herr K, Bjoro K, Decker S. Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review. J Pain Symptom Manage 2006;31:170-92. http://dx.doi.org/10.1016/j.jpainsymman.2005.07.001.
- Lord B. Paramedic assessment of pain in the cognitively impaired adult patient. BMC Emerg Med 2009;9. http://dx.doi.org/10.1186/1471-227X-9-20.
- While C, Jocelyn A. Observational pain assessment scales for people with dementia: a review. Br J Community Nurs 2009;14:438-42. http://dx.doi.org/10.12968/bjcn.2009.14.10.44496.
- Stolee P, Hillier LM, Esbaugh J, Bol N, McKellar L, Gauthier N. Instruments for the assessment of pain in older persons with cognitive impairment. J Am Geriatr Soc 2005;53:319-26. http://dx.doi.org/10.1111/j.1532-5415.2005.53121.x.
- Qi NS, Brammer JD, Creedy DK. The psychometric properties, feasibility and utility of behavioural-observation methods in pain assessment of cognitively impaired elderly people in acute and long-term care: a systematic review. JBI Libr Syst Rev 2012;10:977-1085.
- Park J, Castellanos-Brown K, Belcher J. A review of observational pain scales in nonverbal elderly with cognitive impairments. Res Soc Work Pract 2010;20:651-64. http://dx.doi.org/10.1177/1049731508329394.
- Smith M. Pain assessment in nonverbal older adults with advanced dementia. Perspect Psychiatr Care 2005;41:99-113. http://dx.doi.org/10.1111/j.1744-6163.2005.00021.x.
- Rat P, Jouve E, Pickering G, Donnarel L, Nguyen L, Michel M, et al. Validation of an acute pain-behavior scale for older persons with inability to communicate verbally: Algoplus®. Eur J Pain 2011;15:198.e1-10.
- Melzack R. From the gate to the neuromatrix. Pain 1999:121-6. http://dx.doi.org/10.1016/S0304-3959(99)00145-1.
- Schiavenato M, Craig KD. Pain assessment as a social transaction: beyond the “gold standard”. Clin J Pain 2010;26:667-76. http://dx.doi.org/10.1097/ajp.0b013e3181e72507.
- Elstein AS. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, MA: Harvard University Press; 1978.
- Dowie J, Llewelyn H, Hopkins A. Analysing How We Reach Clinical Decisions. London: Royal College of Physicians; 1993.
- Yin RK. Case Study Research: Design and Methods. Thousand Oaks, CA: Sage Publications, Inc.; 2003.
- Coyne IT. Sampling in qualitative research. Purposeful and theoretical sampling; merging or clear boundaries?. J Adv Nurs 1997;26:623-30. http://dx.doi.org/10.1046/j.1365-2648.1997.t01-25-00999.x.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Mental Health (Care and Treatment) (Scotland) Act 2003. London: The Stationery Office; 2003.
- Monroe TB, Herr KA, Mion LC, Cowan RL. Ethical and legal issues in pain research in cognitively impaired older adults. Int J Nurs Stud 2013;50:1283-7. http://dx.doi.org/10.1016/j.ijnurstu.2012.11.023.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. http://dx.doi.org/10.1136/bmj.320.7226.50.
- Lichtner V, Dowding D, Allcock N, Keady J, Sampson EL, Briggs B, et al. The assessment and management of pain in patients with dementia in hospital settings: a multi-case exploratory study from a decision making perspective. BMC Health Serv Res 2016;16. http://dx.doi.org/10.1186/s12913-016-1690-1.
- Abbey J, Piller N, De Bellis A, Esterman A, Parker D, Giles L, et al. The Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs 2004;10:6-13. http://dx.doi.org/10.12968/ijpn.2004.10.1.12013.
- Fuchs-Lacelle S, Hadjistavropoulos T. Development and preliminary validation of the pain assessment checklist for seniors with limited ability to communicate (PACSLAC). Pain Manag Nurs 2004;5:37-49. http://dx.doi.org/10.1016/j.pmn.2003.10.001.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Qual Saf 2015;24:228-38. http://dx.doi.org/10.1136/bmjqs-2014-003627.
- Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol 2010;29:1-8. http://dx.doi.org/10.1037/a0016939.
- Walshe K. Understanding what works – and why – in quality improvement: the need for theory-driven evaluation. Int J Qual Health Care 2007;19:57-9. http://dx.doi.org/10.1093/intqhc/mzm004.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Hevner A, Chatterjee S, Hevner A, Chatterjee S. Design Research in Information Systems: Theory and Practice. New York, NY: Springer-Verlag; 2010.
- Hevner AR, March ST, Park J, Ram S. Design science in information systems research. MIS Q 2004;28:75-105.
- Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Department of Health, The Rt Hon Norman Lamb . Policy Paper. 2010 to 2015 Government Policy: Dementia 2015. www.gov.uk/government/publications/2010-to-2015-government-policy-dementia (accessed 3 February 2016).
- Connecting for Health . EPrescribing Functional Specification for NHS Trusts Baselined v1.0 – 25th Jan 2007 2007. www.connectingforhealth.nhs.uk/systemsandservices/eprescribing/baselinefunctspec.pdf (accessed 21 August 2015).
- Achterberg WP, Pieper MJ, van Dalen-Kok AH, de Waal MW, Husebo BS, Lautenbacher S, et al. Pain management in patients with dementia. Clin Interv Aging 2013;8:1471-82. http://dx.doi.org/10.2147/CIA.S36739.
- Pieper MJ, van Dalen-Kok AH, Francke AL, van der Steen JT, Scherder EJ, Husebø BS, et al. Interventions targeting pain or behaviour in dementia: a systematic review. Ageing Res Rev 2013;12:1042-55. http://dx.doi.org/10.1016/j.arr.2013.05.002.
- Husebo BS, Ballard C, Cohen-Mansfield J, Seifert R, Aarsland D. The response of agitated behavior to pain management in persons with dementia. Am J Geriatr Psychiatry 2014;22:708-17. http://dx.doi.org/10.1016/j.jagp.2012.12.006.
- Colucci L, Bosco M, Fasanaro AM, Gaeta GL, Ricci G, Amenta F. Alzheimer’s disease costs: what we know and what we should take into account. J Alzheimers Dis 2014;42:1311-24.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Martin A, Jones A, Mugford M, Shemilt I, Hancock R, Wittenberg R. Methods used to identify and measure resource use in economic evaluations: a systematic review of questionnaires for older people. Health Econ 2012;21:1017-22. http://dx.doi.org/10.1002/hec.1766.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9100.
- Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease (review of Technology Appraisal No. 111): a systematic review and economic model. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16210.
- Woods RT, Bruce E, Edwards RT, Elvish R, Hoare Z, Hounsome B, et al. REMCARE: reminiscence groups for people with dementia and their family caregivers – effectiveness and cost-effectiveness pragmatic multicentre randomised trial. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16480.
- Banerjee S, Hellier J, Romeo R, Dewey M, Knapp M, Ballard C, et al. Study of the use of antidepressants for depression in dementia: the HTA-SADD trial – a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17070.
- Beecham JK, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. Canterbury: PSSRU, University of Kent; 1999.
- Heaven A, Cheater F, Clegg A, Collinson M, Farrin A, Forster A, et al. Pilot trial of Stop Delirium! (PiTStop) – a complex intervention to prevent delirium in care homes for older people: study protocol for a cluster randomised controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-47.
- Hughes JC. Thinking Through Dementia. Oxford: Oxford University Press; 2011.
- Scherder E, Oosterman J, Swaab D, Herr K, Ooms M, Ribbe M, et al. Recent developments in pain in dementia. BMJ 2005;330:461-4. http://dx.doi.org/10.1136/bmj.330.7489.461.
- Helfand M, Freeman M. Assessment and management of acute pain in adult medical inpatients: a systematic review. Pain Med 2009;10:1183-99. http://dx.doi.org/10.1111/j.1526-4637.2009.00718.x.
- Lorenz KA, Sherbourne CD, Shugarman LR, Rubenstein LV, Wen L, Cohen A, et al. How reliable is pain as the fifth vital sign?. J Am Board Fam Med 2009;22:291-8. http://dx.doi.org/10.3122/jabfm.2009.03.080162.
- Collins A. Measuring What Matters to Patients at the Frontline [Video Webinar] 2014. www.health.org.uk/multimedia/video/measuring-what-matters-to-patients-at-the-frontline/ (accessed 10 February 2016).
- Roland M, Campbell S. Successes and failures of pay for performance in the United Kingdom. N Engl J Med 2014;370:1944-9. http://dx.doi.org/10.1056/NEJMhpr1316051.
- Hurley AC, Volicer BJ, Hanrahan PA, Houde S, Volicer L. Assessment of discomfort in advanced Alzheimer patients. Res Nurs Health 1992;15:369-77. http://dx.doi.org/10.1002/nur.4770150506.
- Mularski RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med 2006;21:607-12. http://dx.doi.org/10.1111/j.1525-1497.2006.00415.x.
- Dixon P, Long A. Broadening the criteria for selecting and developing health status instruments. Outcomes Briefing 1995;6:4-14.
- Greenhalgh J, Long AF, Brettle AJ, Grant MJ. Reviewing and selecting outcome measures for use in routine practice. J Eval Clin Pract 1998;4:339-50. http://dx.doi.org/10.1111/j.1365-2753.1998.tb00097.x.
- Closs SJ, Cash K, Barr B, Briggs M. Cues for the identification of pain in nursing home residents. Int J Nurs Stud 2005;42:3-12. http://dx.doi.org/10.1016/j.ijnurstu.2004.05.012.
- Jones D. A family living with Alzheimer’s disease: the communicative challenges. Dementia 2015;14:555-73. http://dx.doi.org/10.1177/1471301213502213.
- Elder RG, Skipper JK, Leonard RC. Social Interaction and Patient Care. Philadelphia, PA: Lippincott; 1965.
- Manias E, Botti M, Bucknall T. Observation of pain assessment and management – the complexities of clinical practice. J Clin Nurs 2002;11:724-33. http://dx.doi.org/10.1046/j.1365-2702.2002.00691.x.
- Lauzon Clabo LM. An ethnography of pain assessment and the role of social context on two postoperative units. J Adv Nurs 2008;61:531-9. http://dx.doi.org/10.1111/j.1365-2648.2007.04550.x.
- Clissett P, Porock D, Harwood RH, Gladman JR. The challenges of achieving person-centred care in acute hospitals: a qualitative study of people with dementia and their families. Int J Nurs Stud 2013;50:1495-503. http://dx.doi.org/10.1016/j.ijnurstu.2013.03.001.
- Bolster D, Manias E. Person-centred interactions between nurses and patients during medication activities in an acute hospital setting: qualitative observation and interview study. Int J Nurs Stud 2010;47:154-65. http://dx.doi.org/10.1016/j.ijnurstu.2009.05.021.
- Draper J. Ethnography: principles, practice and potential. Nurs Stand 2015;29:36-41. http://dx.doi.org/10.7748/ns.29.36.36.e8937.
- Lichtner V. The everyday ethics of field work research with vulnerable patients. Stud Health Technol Inform 2014;205:813-17.
- Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: the nature of patient care information system-related errors. J Am Med Inform Assoc 2004;11:104-12. http://dx.doi.org/10.1197/jamia.M1471.
- Hadjistavropoulos T, Herr K, Turk DC, Fine PG, Dworkin RH, Helme R, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain 2007;23:1-43. http://dx.doi.org/10.1097/AJP.0b013e31802be869.
- Hadjistavropoulos T, Herr K, Prkachin KM, Craig KD, Gibson SJ, Lukas A, et al. Pain assessment in elderly adults with dementia. Lancet Neurol 2014;13:1216-27. http://dx.doi.org/10.1016/S1474-4422(14)70103-6.
- Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al. Guidance on the management of pain in older people. Age Ageing 2013;42:i1-57. http://dx.doi.org/10.1093/ageing/afs199.
- RCN Pain Knowledge and Skills Framework for the Nursing Team. London: Royal College of Nursing; 2015.
- Schug SA, Palmer GM, Scott DA, Halliwell R, Trinca J. Acute Pain Management: Scientific Evidence. Melbourne, VIC: Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine; 2015.
- McDonald DD, Fedo J. Older adults’ pain communication: the effect of interruption. Pain Manag Nurs 2009;10:149-53. http://dx.doi.org/10.1016/j.pmn.2009.03.003.
- Fossey J, Masson S, Stafford J, Lawrence V, Corbett A, Ballard C. The disconnect between evidence and practice: a systematic review of person-centred interventions and training manuals for care home staff working with people with dementia. Int J Geriatr Psychiatry 2014;29:797-80. http://dx.doi.org/10.1002/gps.4072.
- Dewar B, Nolan M. Caring about caring: developing a model to implement compassionate relationship centred care in an older people care setting. Int J Nurs Stud 2013;50:1247-58. http://dx.doi.org/10.1016/j.ijnurstu.2013.01.008.
- van der Steen JT, Regnard C, Volicer L, Van den Noortgate NJA, Sampson EL. Detecting pain or distress in people with dementia: an appraisal of two strategies. EJPC 2015;22:110-13.
- Fry M, Chenoweth L, MacGregor C, Arendts G. Emergency nurses perceptions of the role of family/carers in caring for cognitively impaired older persons in pain: a descriptive qualitative study. Int J Nurs Stud 2015;52:1323-31. http://dx.doi.org/10.1016/j.ijnurstu.2015.04.013.
- The 20 Billion Question: An Inquiry into Improving Lives Through Cost Effective Dementia Services. London: House of Commons, All Party Parliamentary Group on Dementia; 2011.
- Ballard C, Smith J, Husebo B, Aarsland D, Corbett A. The role of pain treatment in managing the behavioural and psychological symptoms of dementia (BPSD). Int J Palliat Nurs 2011;17:420-4. http://dx.doi.org/10.12968/ijpn.2011.17.9.420.
- Closs SJ, Lichtner V. Management of pain in peple with dementia: time for a change of approach. Pain News 2016;14:76-8.
- Arnold RD, Wade JP. A definition of systems thinking: a systems approach. Procedia Comput Sci 2015;44:669-78. http://dx.doi.org/10.1016/j.procs.2015.03.050.
- Lichtner V, Dowding D, Closs SJ. The relative meaning of absolute numbers: the case of pain intensity scores as decision support systems for pain management of patients with dementia. BMC Med Inform Decis Mak 2015;15. http://dx.doi.org/10.1186/s12911-015-0233-8.
- Wyatt J, Spiegelhalter D. Field trials of medical decision-aids: potential problems and solutions. Proc Annu Symp Comput Appl Med Care n.d.:3-7.
- Richardson JE, Ash JS, Sittig DF, Bunce A, Carpenter J, Dykstra RH, et al. Multiple perspectives on the meaning of clinical decision support. AMIA Annu Symp Proc 2010;2010:1427-31.
- Royal College of Physicians . National Early Warning Score (NEWS): Standardising the Assessment of Acute-Illness Severity in the NHS 2012. www.rcplondon.ac.uk/resources/national-early-warning-score-news (accessed August 2015).
- Hilligoss B, Moffatt-Bruce SD. The limits of checklists: handoff and narrative thinking. BMJ Qual Saf 2014;23:528-33. http://dx.doi.org/10.1136/bmjqs-2013-002705.
- Agency for Healthcare Research and Quality . Patient Safety Primer: Alert Fatigue 2015. psnet.ahrq.gov/primer.aspx?primerID=28 (accessed 4 February 2016).
- AGS Panel on Persistent Pain in Older Persons . The management of persistent pain in older persons. J Am Geriatr Soc 2002;50:205-24. http://dx.doi.org/10.1046/j.1532-5415.50.6s.1.x.
- National Patient Safety Agency . Design for Patient Safety: Guidelines for the Safe On-Screen Display of Medication Information – v.1 – Ref. No 1023 2010. www.nrls.npsa.nhs.uk/resources/collections/design-for-patient-safety/?entryid45=66713 (accessed 4 February 2016).
- The Academy of Medical Royal Colleges . Standards for the Design of Hospital In-Patient Prescription Charts 2011. www.aomrc.org.uk/doc_view/9416-standards-for-the-design-of-hospital-in-patient-prescription-charts (accessed 4 February 2016).
- Smith HS. Opioid metabolism. Mayo Clin Proc 2009;84:613-24. http://dx.doi.org/10.1016/S0025-6196(11)60750-7.
- Kelly J, Wright D. Medicine administration errors and their severity in secondary care older persons’ ward: a multi-centre observational study. J Clin Nurs 2012;21:1806-15. http://dx.doi.org/10.1111/j.1365-2702.2011.03760.x.
- Belden JL, Grayson R, Barnes J. Defining and Testing EMR Usability: Principles and Proposed Methods of EMR Usability Evaluation and Rating 2009. http://s3.amazonaws.com/rdcms-himss/files/production/public/FileDownloads/HIMSS_DefiningandTestingEMRUsability.pdf (accessed 4 February 2016).
- Brezis M. Warning: false sense of certainty from the illusion of accuracy and precision in measurements. Accredit Qual Assur 2011;16:599-602. http://dx.doi.org/10.1007/s00769-011-0821-y.
- Tufte E. Envisioning Information. Cheshire, CT: Graphics Press; 1990.
- Tufte E. The Visual Display of Quantitative Information. 2nd edn. Cheshire, CT: Graphics Press; 2001.
- Sittig DF, Murphy DR, Smith MW, Russo E, Wright A, Singh H. Graphical display of diagnostic test results in electronic health records: a comparison of 8 systems. J Am Med Inform Assoc 2015;22:900-4. http://dx.doi.org/10.1093/jamia/ocv013.
- Brighton and Sussex Medical School . DEMQOL: Dementia Quality of Life Measure n.d. www.bsms.ac.uk/research/cds/research/demqol.aspx (accessed October 2016).
Appendix 1 Literature search strategy
| SPICE categories | Search terms |
|---|---|
| Patient population: adults with dementia or cognitive impairment | 1. Dementia.mp. 2. Alzheimer.mp 3. exp Dementia/ 4. exp Alzheimer Disease/ 5. 1 or 2 or 3 or 4 6. exp Cognition Disorders/ 7. Cognitive impairment.mp. 8. Cognitive function*.mp. 9. exp mental retardation/ 10. 6 or 7 or 8 or 9 11. 5 or 10 |
| Intervention: pain assessment | 12. (Assess$ adj5 pain).mp. 13. (Measur$ adj5 pain).mp. 14. (Scale$ adj5 pain).mp. 15. (Rating adj5 pain).mp. 16. exp Pain Measurement/ 17. exp Pain/di 18. *Pain Measurement/mt 19. *Pain Measurement/ 20. (Pain adj3 tool$).mp. 21. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 |
| 22. 11 and 21 | |
| Limited to study design: reviews | 23. meta-analysis.mp. 24. meta-analysis.pt. 25. review.pt. 26. search:.tw. 27. 23 or 24 or 25 or 26 |
| 28. 22 and 27 |
Appendix 2 Included and excluded reviews
List of included reviews
| Review |
|---|
| Corbett A, Husebo B, Malcangio M, Staniland A, Cohen-Mansfield J, Aarsland D, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol 2012;8:264–74 |
| Herr K, Bjoro K, Decker S. Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review. J Pain Symptom Manage 2006;31:170–92 |
| Smith M. Pain assessment in nonverbal older adults with advanced dementia. Perspect Psychiatr Care 2005;41:99–113 |
| Qi NS, Brammer JD, Creedy DK. The psychometric properties, feasibility and utility of behavioural-observation methods in pain assessment of cognitively impaired elderly people in acute and long-term care: a systematic review. JBI Lib Syst Rev 2012;10:977–1085 |
| Park J, Castellanos-Brown K, Belcher J. A review of observational pain scales in nonverbal elderly with cognitive impairments. Res Soc Work Pract 2010;20:651–64 |
| Schofield P, Clarke A, Faulkner M, Ryan T, Dunham M, Howarth A. Assessment of pain in adults with cognitive impairment: a review of the tools. Int J Disabil Hum Dev 2005;4:59–66 |
| van Herk R, van Dijk M, Baar FP, Tibboel D, de Wit R. Observation scales for pain assessment in older adults with cognitive impairments or communication difficulties. Nurs Res 2007;56:34–43 |
| Zwakhalen SM, Hamers JP, Abu-Saad HH, Berger MP. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatr 2006;6:3 |
List of excluded reviews and reason for inclusion
| Review | Reason for exclusion |
|---|---|
| Ni Thuathail A, Welford C. Pain assessment tools for older people with cognitive impairment. Nurs Stand 2011;26:39–46 | Narrative review. The review covers several tools, with very little information of each. Comparison is difficult, as no table is provided |
| Lord B. Paramedic assessment of pain in the cognitively impaired adult patient. BMC Emerg Med 2009;9:20 | Narrative review. No data on psychometric properties |
| While C, Jocelyn A. Observational pain assessment scales for people with dementia: a review. Br J Community Nurs 2009;14:438–42 | A review of reviews. No data on psychometric properties of the tools |
| Rutledge DN, Donaldson NE, Pravikoff DS. Update. Pain assessment and documentation. Special populations of adults. OJCI 2002;5:1–49 | Broad review of pain in a variety of different patient groups – of which adults with cognitive impairment a subset. No data on psychometric properties of the tools to extract |
| Rutledge DR, Donaldson NE. Pain assessment and documentation. Part II – special populations of adults. OJCI 1998;1:1–29 | No data on psychometric properties of the tools. Review updated in Sampson et al.30 |
| Andrade DC, Faria JW, Caramelli P, Alvarenga L, Galhardoni R, Siqueira SR, et al. The assessment and management of pain in the demented and non-demented elderly patient. Arq Neuropsiquiatr 2011;69:387–94 | Narrative review, including physiology, assessment and management of pain. No data on psychometric properties of the tools |
| Scherder E, Oosterman J, Swaab D, Herr K, Ooms M, Ribbe M, et al. Recent developments in pain in dementia. BMJ 2005;330:461–4 | Narrative review. No data on psychometric properties of tools |
| Lobbezoo F, Weijenberg RAF, Scherder EJA. Topical review: orofacial pain in dementia patients. A diagnostic challenge. J Orofac Pain 2011;25:6–14 | Narrative review. No data on psychometric properties of the tools. Focus: orofacial pain |
| Herr K, Bursch H, Ersek M, Miller LL, Swafford K. Use of pain-behavioral assessment tools in the nursing home: expert consensus recommendations for practice. J Gerontol Nurs 2010;36:18–29; quiz 30–1 | Expert reviewers were asked to rate each tool and provide a score; no data on psychometric properties of tools. The way in which the data are presented makes it unusable for our purposes |
| McAuliffe L, Nay R, O’Donnell M, Fetherstonhaugh D. Pain assessment in older people with dementia: literature review. J Adv Nurs 2009;65:2–10 | A narrative review with no data on tools. Focus: barriers to successful pain assessment |
| Miller LL, Talerico KA. Pain in older adults. Annu Rev Nurs Res 2002;20:63–88 | Narrative review. No data on psychometric properties of the tools |
| Helfand M, Freeman M. Assessment and management of acute pain in adult medical inpatients: a systematic review. Pain Med 2009;10:1183–99 | Narrative review. No data on psychometric properties of the tools |
| Stolee P, Hillier LM, Esbaugh J, Bol N, McKellar L, Gauthier N. Instruments for the assessment of pain in older persons with cognitive impairment. J Am Geriatr Soc 2005;53:319–26 | Thirty tools reviewed comparatively in overview tables. Reliability and validity data reported but in a form unusable for our purposes |
Appendix 3 Systematic review methods: additional files
The following files are available on BMC Geriatrics, at the web address given below, providing further details on the methods applied in conducting the systematic review.
Additional file 1: literature search
Details of the retrieval process, including databases searched, adjustments to the search strategy first optimised for the MEDLINE (via Ovid) database, for use in other databases, and detailed search strategies for each database.
Additional file 2: notes on screening titles abstracts
Details of the screening process on the basis of title and abstract of the records retrieved, how it was shared among the reviewers so that each record was screened by two independent reviewers, and the number of records referred for a third reviewer.
Additional file 3: notes on screening full text
Details of the screening process on the basis of the full text of the records retrieved, how it was shared among the reviewers so that each record was screened by two independent reviewers and the number of records referred for a third reviewer.
Additional file 4: data extraction forms
The data extraction forms used to build a data extraction database and extract data for the meta-review.
Additional file 5: summary of reviews
Overview of the included reviews, with a description of the aims and the methods used for including and analysing the studies, their quality assessment of the studies, and the tools examined.
Additional file 6: sample of studies’ methodological weaknesses identified by the reviews
A sample of studies’ methodological challenges and weaknesses identified in/by the reviews, organised by type of issue (e.g. sampling, tool design).
Additional file 7: summary of the tools – characteristics
Summary of the data extracted from the reviews providing a classification and description of the tools.
Additional file 8: summary of the tools – settings of use
Summary of the data extracted from the reviews pertaining to the setting, where the tools had been studied, tested or used for clinical practice.
Additional file 9: summary of the tools – reliability and validity
Summary of the data extracted from the reviews regarding the reliability and validity of each tool.
Additional file 10: summary of tools – content-validity-user centredness
Analysis of the data extracted from the reviews regarding conceptual foundation of each tool and ways content validity was explored. These aspects also pertain to the user-centredness of the tools.
Additional file 11: tools concurrent and criterion validity comparison table
Summary of the data on the concurrent and criterion validity of the tools, extracted from the reviews.
Additional file 12: summary of tools – feasibility
Summary of the data on the feasibility of each tool, extracted from the reviews, analysed in terms of ‘dimensions’ of feasibility: ease of use, time to complete, availability of instruction and guidelines, training required.
Additional file 13: summary of tools – clinical utility
A summary of the data on the clinical utility of each tool, extracted from the reviews, analysed in terms of ‘dimensions’ of clinical utility (availability of cut-off scores and interpretation of scores for decision-making) and overall evidence.
Appendix 4 Observational study data collection/research instruments
Appendix 5 Feedback to participants
Appendix 6 The Pain And Dementia Decision Support system: functional requirements specifications
Version 1.2, October 2015.
| Date | Version | Authors | Description |
|---|---|---|---|
| 5 August 2015 | 0.0 | Valentina Lichtner | First draft, for discussion |
| 21 August 2015 | 0.1 | José Closs | Review and comments |
| 27 August 2015 | 1.0 | Valentina Lichtner | Edits in response to review. Added section 2.4. Completed first draft of Appendix |
| 14 September 2015 | 1.0 | Dawn Dowding | Comments to requirements FR.02, FR.05, FR.09, FR.p-a.01 |
| 21 September 2015 | 1.1 | Valentina Lichtner | Edits in response to review |
| 22 September 2015 | 1.1 | SSC | Comments to requirements Figure 9, FR.09, FR.p-a.03 |
| 5 October 2015 | 1.2 | Valentina Lichtner | Edits in response to SSC comments |
| Anticipated change | When |
|---|---|
| The requirements specification for the PADDS system are expected to change as a result of the feasibility evaluation of the system in use | Feasibility evaluation planned for 2016, to be confirmed |
Documents generated by this study providing additional information.
| References |
|---|
| Dowding D, Lichtner V, Allcock N, Briggs M, James K, Keady J, et al. Using sense-making theories of decision making to aid understanding of the recognition, assessment and management of pain in patients with dementia in acute hospital settings: a UK multisite study. Int J Nurs Stud 2015;53:152–6255 http://www.sciencedirect.com/science/article/pii/S002074891500259X (accessed 29 January 2016) |
| Lichtner V, Dowding D, Allcock N, Keady J, Sampson EL, Briggs B, et al. The assessment and management of pain in patients with dementia in hospital settings: a multi-case exploratory study from a decision making perspective. BMC Health Serv Res 2016;16:427100 |
| Lichtner V, Dowding D, and Closs SJ. The Relative Meaning of Absolute Numbers: The Case of Pain Intensity Scores as Decision Support Systems for Pain Management of Patients with Dementia. BMC Med Inform Decis Making 2015;15:1–11158 http://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911–015–0233–8 (accessed 29 January 2016) |
| CFH, ePrescribing Functional Specification for NHS Trusts Baselined v1.0 – 25 January 2007 Available: http://www.connectingforhealth.nhs.uk/systemsandservices/eprescribing/baselinefunctspec.pdf (accessed 21 August 2015)111 |
| Term | Acronym | Definition |
|---|---|---|
| Computerised Decision Support System | CDSS | Also known as Clinical Decision Support System |
| CDSS are ‘active knowledge systems which use two or more items of patient data to generate case-specific advice’159 | ||
| CDSS refer specifically to electronic tools in support of decision making. For a more general definition of decision support tools, not limited to computerised ones, see Decision Support System (DSS) | ||
| Decisions Support System | DSS or DST | Also known as decision tool |
| A tool that helps clinicians to make the right decision for the right patient at the right time. Mechanisms for decision support may include: templates to guide data acquisition, algorithms, provision of guidance, default options, or the display of ‘a snapshot of a patient’s disease state’.160 Such tools may, or may not, be computerised | ||
| For a definition of computerised DSS, see CDSS | ||
| Early Warning Score | EWS | A process and chart to record observations of a patient’s physiological parameters and score allocated to their measurement. It often includes a section related to pain – and a pain score – as a ‘fifth vital sign’. A national version is available161 (the NEWS) to standardise these systems across the NHS acute settings; however, modified versions (also known as MEWS) are also in use |
| See also EWS and MEWS | ||
| Electronic Patient Record | EPR | Patients’ clinical notes recorded on a digital system. The record includes medical notes; it may include nursing notes; it may also include (or link to systems for) orders and/or results for lab tests, digital pictures of scans, medical prescriptions |
| Electronic prescribing | ePrescribing | Electronic systems to support both the prescribing of medicines and ‘facilitate and enhance the communication of a prescription or medicine order, aiding the choice, administration and supply of a medicine through knowledge and decision support and providing a robust audit trail for the entire medicines use process’111 |
| Electronic Prescribing and Medicine Administration system | EPMA | Electronic systems to support both the prescribing of medicines and their administration to patients in acute settings. Medicine administration may include the use of barcodes on both patients’ wristbands and on medicines, and barcode scanners to match patients to their medicines |
| Health-care assistant | HCA | Assistant practitioners, usually to nurses, with tasks of direct patient care involving activities of daily living (e.g. assisting patients with meals or bathing) in acute settings. They may also be responsible for periodic observations of patients’ vital signs and recording EWS |
| Health-care professional | HCP | A nurse, doctor, pharmacist, therapist or other member of the clinical team caring for patients in hospital |
| Modified Early Warning Score | MEWS | See EWS |
| Multidisciplinary team | MDT | The group of HCPs and HCAs who care for a patient at any given time |
| National Early Warning Score | NEWS | See EWS |
| Patient Administration System | PAS | Information system for the administration of a hospital’s clinical activities, such as bookings of appointments or recording of hospital admissions; usually the main source of patients’ demographic information used within other clinical systems |
| Patient’s Passport | A form to be used by patient’s family members to describe the person’s likes and dislikes and usual behaviour. These forms are usually paper based and variably known as, for example, ‘Patient passports’, ‘10 things about me’, ‘Know who I am’ |
Contents
| Summary |
|
Summary
This document contains the specifications for a set of functional requirements for an electronic decision support system to assist with the recognition, assessment and management of pain, specifically for patients with dementia. We call this a Pain And Dementia Decision Support (PADDS) system. The system as outlined in these requirements is intended to be used primarily in hospital settings.
The requirements outlined in this document emerged out of an in-depth exploratory study conducted in three acute NHS trusts in England and one acute NHS board in Scotland, through research funded by the NIHR HSDR programme (HSDR – 11/2000/05). These specifications are intended as a first set of requirements to guide software development for the PADDS.
It is expected that in the first instance the PADDS system will be developed within a single hospital organisation which has ward-based EHRs. The PADDS system will then be tested for usability and feasibility.
These requirements specifications are to be revised, amended and completed once the system has been put in place and its use, usability and clinical utility evaluated.
1. About this document
1.1 Purpose
This document describes the functional requirements of an electronic Pain And Dementia Decision Support (PADDS) system.
1.2 Definition
A PADDS system is an electronic system aimed at aiding the recognition, assessment and management of pain in patients with dementia. It may be implemented in acute wards within a complex improvement intervention, including changes to the organisation of care and documentation practices (also known as a PADDS intervention).
1.3 Audience
This document is aimed at software developers and is intended to guide the design and building of the PADDS system.
It may be of interest to managers, healthcare professionals and other stakeholders involved in the implementation of PADDS interventions to gain a better understating of the role of the PADDS system within the complex intervention.
1.4 Expected benefits
The PADDS system is expected to facilitate distributed sense-making and decision making of the MDT with regard to the recognition, assessment and management of pain in a patient with dementia. This includes:
-
Assisting with individuals’ initial ‘noticing and bracketing’ of pain cues (i.e. identification that there may be pain) – including creating opportunities for noticing, and for patients communication of the patient’s pain, with carers’ contribution whenever possible.
-
Assisting in the making sense of the information to identify that it may be pain, the type of pain and how it manifests, within the ‘building of a picture’ of a patient’s pain – sense-making is both an individual and group activity and it happens over time.
-
Assisting with decision taking and actions (pain management), in a dynamic fashion that supports the empirical (trial) nature of pain management, over time, distributed across individuals.
Direct and immediate beneficial outcomes from the use of the PADDS systems are expected to be:
-
Improved documentation of pain assessment. This includes, for example, documentation of absence of pain as well as presence, and whether the assessment is based on clinician’s inference from a patient behaviour or from patients’ verbal reports.
-
Improved documentation of the trial of therapy. This includes, information on effectiveness of the trial (e.g. patient is feeling better); performance of the trial (e.g. drug was administered at a given time); outcome of trial (e.g. strategy abandoned, change in prescription); personalisation of the pain management (i.e. what helps the individual with their pain). The trial can include other forms of intervention than medicines.
-
Improved shared understanding of the patient pain and the best ways to manage it.
Overall, these outcomes are believed to contribute to better, patient-centred, care and improvement in the management of the patient’s pain.
2. Introduction
There has long been recognition that the assessment of pain in patients with dementia in hospital is challenging and difficult to manage effectively. Challenges are linked to the subjective nature of pain as an experience, patients’ difficulties in recall and interpretation of pain, behavioural signs of pain being altered in unexpected ways in patients with dementia, and barriers in communication. The context of hospital wards contributes to further complexity, as patients may not be well known to staff, and both the illness and the environment may create distress for the patient that adds to and confounds signs of pain. There are significant consequences related to the inadequate management of pain in hospital settings, including slower functional rehabilitation, longer hospital stays and lower quality of life, making improvements in this area of care urgent and important.
There is currently no single reliable mechanism or method that could be recommended for staff to use in hospitals for identifying and assessing pain in patients with dementia. It should be noted that recognising absence of pain is of equal importance in assessment, in order to avoid the unnecessary use of pain medications with associated side effects.
Decisions support systems (DSS) have been shown to improve care processes and patient outcomes in other clinical areas and are one of the ways in which pain assessment and management could be better supported in hospital settings. Traditionally DSS provide guidance on the basis of data about the patient and pre-set rules or algorithms, such as ‘cut-off’ points for generating alerts. In pain assessment and management of patients who are able to verbalise their pain, the patient pain intensity scores may be used, for example, to trigger an increased or reduced use of analgesics.
In the case of the assessment and management of pain in patients with dementia in hospital settings, given the inherent uncertainties about their pain and the limited staff knowledge of the patient, decision support systems cannot be based on standardised pre-set automated algorithms on the basis of inferred data points. Instead, the system needs to support HCPs judgements (recognition/assessments of pain) and decisions (management strategies) through:
-
The progressive, cumulative building of a ‘picture of pain’ that is specific for that patient that staff can use to ‘make sense’ of the patient’s signs as signs of pain (or not pain). This involves the meaningful display of an aggregated summary of all pain-related information gathered at different points in time by the different HCPs and HCAs involved in the care of the patient, through graphical displays that support individuals’ cognitive processes of pattern recognition.
-
Providing a reminder of the options for pain management that are inclusive of non-pharmacological interventions.
-
Providing a set of alerts on the basis of trends and unusual signs rather than activated by single individual data points.
This is what we call the PADDS system. In the following sections we first describe brief use cases for this system, to identify users, other systems, and activities involved with the PADDS; we then present what is required in the design of the system, for the system to work as intended (functional requirements specifications). Evidence and rationales in support of each of the requirements listed is provided in the appendix. Further information about the underlying rationale for PADDS is available in the documents listed at the front of this document (see Table 15).
PADDS is recommended for use with patients with dementia, but may be used with all patients, especially those with communication difficulties.
PADDS is recommended for use with patients with or without identified pain or established presence of pain, as a reminder at any point in time that patient’s signs ‘could be pain’.
3. Use and interconnections with other systems
The PADDS system will be used in two modalities:
-
PADDS will be used for the care of individual patients, to support recognition, assessment and management of their pain. This includes the identification of patient specific pain cues, the establishment of pain intensity scores for EWS systems, the assessment of the presence, causes, quality and intensity of pain, the decisions about analgesics or other pharmacological or non-pharmacological pain management strategies, the assessment of the effectiveness of these strategies.
-
PADDS will be used for the management of activities pertaining to all patients present in the ward at a given time for which PADDS is used; PADDS will work as a system HCPs can use to monitor the quality of care for these patients. In prioritising the implementation of systems requirements, those pertaining to this second modality can be considered optional.
PADDS is an electronic system embedded and interconnected with existing hospital EPR and ePrescribing/EPMA systems. It is envisaged that most of the data displayed in PADDS will be entered through other systems in use for the care of the patient. These include: ePrescribing/EPMA systems for prescription and administration of pharmaceutical pain management strategies; EWS systems for periodic observations of a patient’s pain; clinical notes for observation and assessment of patients’ that may involve signs of pain such as specialist nurse assessments, physiotherapists’ notes, medical rounds. Should any of these systems be paper-based at the time of implementation of PADDS in the hospital wards, there should be temporary arrangements for data to be copied from these paper charts to PADDS in near real-time. However, it is an essential requirement that data entry in PADDS is not proposed to HCPs as a documenting task in addition to existing practices of documentation to be continued in parallel; rather PADDS should replace or augment existing documentation tasks.
PADDS can be used together with ePrescribing systems by displaying the interconnection between the prescription (by doctors), the administration of the medicine (by nurses) and the patient’s response to it (as assessed by HCPs or HCAs). Developers of ePrescribing systems may want to consider the possibility of alerts generated through data available in PADDS appearing in the ePrescribing system at the time analgesics are prescribed. As this pertains to the design of the ePrescribing systems, further details on this are outside the scope of this document.
It is assumed that access to PADDS will be controlled in the same way that access to other hospital systems is controlled (e.g. with NHS Smartcards, username and password combinations). Access to PADDS should be granted through these information governance/access systems to all HCPs and HCAs who care for the patient. It is also assumed that actions performed within the system will be auditable.
Relevant information about the patient’s pain provided by family members/informal carers (‘carers’) MUST be included in PADDS. However, it is not envisaged at this stage that carers, nor patients, will have direct access to PADDS. The possibility of patient/carer access to PADDS SHOULD be explored as a further development of the PADDS system at a later stage, as a new release or system update.
It is assumed that the PADDS screen will display all the necessary demographic information about the patient as per all other hospital systems and that this information will be obtained via interfacing with other clinical or PAS systems. The specification for these remains outside the scope of this document.
Figures 9 and 10 illustrate the expected direct users of the system together with their goals in using this system, and the interconnections with other systems.
FIGURE 9.
The PADDS technical specifications: use cases (modality 1). IT, information technology.

FIGURE 10.
The PADDS technical specifications: use cases (modality 2). IT, information technology.
Note: These diagrams present a simplified generic range of users of PADDS and its connected systems identified through generic professional roles (e.g. nurses, doctors). A sample of possible interconnected systems are shown in boxes. Text displayed in ovals presents the main purposes for use of these systems. All these are not intended to be comprehensive; they need to be interpreted in the light of specifics staff roles and systems in use in each hospital context. Uses and use as represented in the diagram are not sequential (as in a workflow) but can happen in any order at any time of the patient stay in hospital. The dotted line for carers’ input to the system indicates carers’ access to the system should be granted – though this may be done on a subsequent version of the system.
4. Requirements
Each of the requirements listed below has a priority level assigned to it. Throughout this document, the following apply:
-
Requirements containing the word MUST are mandatory; the PADDS system cannot be expected to work as intended if they are not implemented. Delivery priority: 1
-
Requirements containing the word SHOULD are recommended; the PADDS system can begin to be put into use without them, while possibly implemented at a later stage. Delivery priority: 2
-
Requirements containing the word MAY are intended as optional. Delivery priority: 3
4.1 Usability and safety
Detailed usability and safety requirements are left outside of the scope of this document. However, it is assumed that they will be part of system design and implementation. The following are a reminder that these aspects of system design and implementation are a priority. It is assumed that the system MUST be:
-
safe
-
secure
-
accessible – both in terms of location and access to hardware
-
flexible
-
intuitive
-
fast
Assessment for usability and safety MUST be carried out prior to system roll-out.
4.2 Functional requirements
The following requirements pertain to the recording and display within PADDS of information regarding single patient records for the current admission (as per use case/modality 1 described in section 3).
| Ref. | Description | Priority |
|---|---|---|
| FR.01 | The system MUST be available, used and continue to be updated from the time of a patient’s admission, in admission units, to other clinical areas when/where the patient is transferred, until discharged from the hospital | 1 |
| FR.02 | The PADDS system MUST capture and display in a meaningful way any pain-related information about the patient from any other system in use in the organisation (e.g. EWS, EPR, EPMA) if available. Examples of this type of information include: clinical assessments, therapeutic interventions, patient’s preferences, patient specific cues of pain, individual coping mechanisms | 1 |
| This information MUST be accessible and visible in real time in PADDS, as soon as data are entered in the other system(s) | ||
| FR.03 | If and when the user needs to add information to PADDS, the user MUST do this through updating the appropriate other relevant IT system feeding data to PADDS (e.g. EWS, pain management plans). Links to the appropriate systems MUST be available in PADDS and access across systems MUST be seamless | 1 |
| Should local hospital policies opt for the use of PADDS instead of alternative systems, THEN facilities for data entry MUST be available in PADDS for the user to enter the missing information directly in the system | ||
| This is a general requirement applying across all others as appropriate | ||
| FR.04 | Details of the person who entered any of the information displayed in PADDS MUST be available. This MAY be done at user’s further action – e.g. mouse over, right click or pop-up box | 1 |
| FR.05 | HCPs MUST be able to add to the PADDS system a narrative (free text) summary description of the patient’s pain (or absence of it) as currently understood by the MDT. This may include, for example, presence of both chronic and acute pain, patient specific cues of pain, individual coping mechanisms. The PADDS system may present prompts to the users on the kind of information that may be recorded in this section | 1 |
| Alternatively, IF this information is otherwise available in other systems in use in the organisation (e.g. EPR), THEN the system MUST capture this automatically | ||
| The PADDS system MUST clearly display this information | ||
| FR.06 | The patient hospital length of stay MUST be clearly visible, as well as the time in the current clinical area | 1 |
| FR.07 | HCPs MUST be able to add to the PADDS system information about the pain care plan or pain management strategy agreed for the patent with the MDT, if pain is deemed to be present | 1 |
| Alternatively, IF this information is otherwise available in other systems in use in the organisation (e.g. EPR), THEN the system MUST capture this automatically | ||
| FR.08 | It MUST be possible to limit access to the generation and maintenance of pain management plans, to individuals, specialties, sites or user types or a combination of the above | 1 |
| FR.09 | HCPs MUST be able to add to the PADDS system narrative (free text) information about the patent’s pain provided by patient’s relatives or carers from outside the hospital. The PADDS system SHOULD present prompts to the users on the kind of information that may be recorded in this section | 1 |
| Alternatively, IF this information is otherwise available in other systems in use in the organisation (e.g. ‘Patients’ passports’), THEN the system SHOULD capture this automatically | ||
| The PADDS system MUST clearly display this information and its provenance | ||
| FR.10 | As and when information available in PADDS is updated in time and accumulates, then older information MUST be made available | 1 |
| This MAY be done by displaying the information upon users’ further action (e.g. as pop-up box, or separate screen) | ||
| FR.11 | The system SHOULD remind the HCPs of alternative pain management strategies – such as repositioning, referral to therapists, etc. | 2,3 |
| This MAY be done through alerts generated by the PADDS system in conjunction with specific patterns or trends in pain intensity scores. Examples of these may be recurrent pain with movement that may benefit by physiotherapy, or pain associated with distress that may benefit by mental health support | ||
| FR.12 | The PADDS system MAY be able to generate alerts on the basis of trends of the patient’s signs of pain (e.g. steadily increasing pain intensity) or unusual signs (e.g. sudden increase in pain intensity) | 3 |
| FR.13 | Within PADDS, it SHOULD be possible to set automatic reminders customised for each patient. These may be for example reminders about tasks to complete or time ‘alarms’ when activities are due (e.g. times for reassessment) | 2 |
| FR.14 | Information from PADDS MAY be made available for the patient discharge, e.g. giving HCPs the option to add a PADDS report to the patient discharge summary | 3 |
| FR.15 | All actions performed within the system MUST be date-, time- and user-stamped and be auditable | 1 |
| FR.16 | Users MAY be able to access the system from remote places as agreed locally and according to security requirements | 3 |
| FR.m.01 | The PADDS system MUST capture and display the current pain medicines list, all dose changes, medicines stopped and started with dates, the reasons why and the prescriber details | 1 |
| Information displayed MUST be derived from the current complete medicine(s) list of the patient | ||
| This list MUST be limited to medications prescribed for the management or treatment of pain. A link to the prescribing system with the complete list of medications MUST be provided | ||
| FR.m.02 | HCPs MUST be able to add to the PADDS system a narrative (free text) explanation of the rationale for the prescription of the pain medication as currently understood by the prescriber and MDT. This may include, for example, the explanation of a therapeutic trial that include the prescription of PRN medication and the review of its use | 1 |
| Alternatively, IF this information is otherwise available in other systems in use in the organisation (e.g. EPR), THEN the system MUST capture this automatically | ||
| FR.m.03 | Pain medication(s) that a patient is being prescribed prior to admission SHOULD be visible within the system | 2,3 |
| This MAY be minimal information e.g. drug name alone. Details on form, strength, dose, administration instructions, MAY be available upon user further action, for example as mouse-over or pop-up screen | ||
| FR.m.04 | It MUST NOT be possible for medicines to be added, changed, or deleted from within PADDS. This is a safety requirement and MUST NOT be possible to override this. | 1 |
| FR.m.05 | In the future it MAY be possible to capture and display information about a patient’s phenotype/genome relevant to pain medication, for example for CYP3A4 and CYP2D6 enzymes. | 3 |
| FR.m-a.01 | The PADDS system MUST display the actual times the drugs were administered for each day | 1 |
| The PADDS system MUST NOT utilise the time of data entry as a proxy for time of administration, unless this is within a specified reasonably short, time range (e.g. 5 minutes) | ||
| FR.m-a.02 | The PADDS system MUST capture and display the doses of pain medications that were scheduled but not given | 1 |
| IF information relevant to the missed dose is available on the system for prescribing/administration of medicines (e.g. reasons for doses not given coded in EPMA), THEN this MUST be available in PADDS | ||
| FR.m-a.03 | It SHOULD be possible to visualise the times of planned administration of prescribed pain medication | 2 |
| This SHOULD be in a manner that makes clear that these are planned but not yet administered (e.g. displayed in lighter colour or symbols) | ||
| FR.p-a.01 | The PADDS system MUST capture and display information about the assessment of the patient’s pain. This MUST be done also in case of when pain is found to be absent | 1 |
This information MAY derive from:
|
||
| This information may include numerical pain scores, but it MUST also include textual descriptions. These SHOULD be structured; the PADDS system should present the user with possible options to choose from; as well as an option to add new entries if those available to not fit the patient’s case | ||
| FR.p-a.02 | For the purpose of visualising pain assessment information in an overview, this information may be coded. The PADDS system MUST then ask the owner of the information to assign the appropriate code. Should the owner of the information not be available, it MUST be possible for others to do this | 1 |
| The codes used for this purpose MUST be consistent with any pain intensity rating system used locally. The 0–3 pain intensity range and associated traffic light colour system MAY be used for this purpose | ||
| Descriptive (narrative) details on the original assessment that is associated with each code SHOULD be available upon user further action, for example as mouse-over or pop-up screen | ||
| FR.p-a.03 | For any pain intensity score displayed in PADDS for the patient, it MUST be made clear whether this was communicated verbally by the patient or inferred by the HCP/HCA or member of family/carer | 1 |
| This MAY be done, for example, by use of different colours or symbols in case of scores verbalised by patients from those resulting from HCP/HCA’s own judgement | ||
| FR.t.01 | The PADDS system MUST display information about any non-pharmacological pain management therapy given to the patient. This may include, for example, sessions of physiotherapy | 1 |
Similarly to the prescription and administration of medicines, this SHOULD include both planned therapy and actual administration of the therapy:
|
||
| As for the administration of medicines, the PADDS system MUST NOT utilise the time of data entry as a proxy for time of administration, unless this is within a specified reasonably short, time range (e.g. 5 minutes) | ||
| FR.v.01 | The PADDS system MUST generate and display clearly trends and patterns over a period of time, of HCPs and HCAs actions and/or patient responses. It is expected that this will be done by use of graphical visualisations of data over time | 1 |
| As information will build up over time, visualisations MUST adjust accordingly | ||
| FR.v.02 | Graphical visualisations of data over time MUST be done in chronological order (i.e. it MUST NOT be done in inverse chronological order) | 1 |
| FR.v.03 | The connection between treatment and patient response over time MUST be made clear to the user | 1 |
| This MUST be done by means of effective visualisation of data about administration of medicines and other pain therapies, and data about pain assessment | ||
| This MAY be done, for example, by use of graphical display of these data over time, with the possibility of filtering different layers of data – such as with radio buttons or menu options to display data about: medicines administration, missed doses, administration of PRN, therapy sessions, and coded pain assessments done with use of pain intensity scales/EWS | ||
| FR.v.04 | The trend and pattern of the patient’s pain experience over time MUST be made clear to the user | 1 |
| This MUST be done by means of effective visualisation of data about pain assessment | ||
| This MAY be done, for example, by use of colour-coded symbols for presence/absence of pain and different levels of pain intensity |
The following requirements pertain to the recording and display of information regarding all (or a subset of) patient records available within the PADDS system at any one time in a given clinical area (as per use case/modality 2 described in section 3).
| Ref. | Description | Priority |
|---|---|---|
| FR.w.01 | The user MAY be able to access a summary overview of all patients for which PADDS is active | 3 |
| This MAY be done by filtering for specific clinical areas (e.g. specific hospital wards) | ||
| FR.w.02 | The overview MAY display a row of summary data pertaining to PADDS for each of the patients | 3 |
| This MUST include patient identifiers (e.g. name/bed number/NHS number) | ||
| Information MAY include: An assessment generated by the system of how much information is available in each patient record available on PADDS Whether important information is missing in PADDS Whether the patient’s pain intensity scores suggest an increasing trend Whether PADDS contain alerts or reminders that need to be addressed The date/time of the last pain assessment Some of this information may be conveyed, for example, by the presence of different alerting icons |
||
| FR.w.03 | It MAY be possible for the user to generate customised alerts pertaining to the entire set of patients, or subsets | 3 |
| FR.w.04 | PADDS may transmit some of this summary information to hospital whiteboards, as and when required | 3 |
5. Hypotheses and rationales for system design
Table 19 provides a summary of the supporting evidence and rationales associated with each of the requirements listed in this document. These rationales may serve as hypotheses to be verified with the evaluation of the PADDS system.
| Requirement [Ref] | Evidence, rationales and/or hypotheses |
|---|---|
| Usability and safety | The six points identified in Section 4.1 are those also identified as ‘Key Features’ of ePrescribing systems in NHS CFH specifications.111 They are keystones for successful adoption |
| FR.01 | The PADDS system works by accumulating and synthesising information about the patient pain as gained by the MDT over time. The process of gathering information and making sense of the patient’s pain starts at admission and continues throughout hospitalisation, until the patient is discharged |
| The PADDS system is intended for hospital use only and does not include, in this version, inter-organisational use after discharge | |
| FR.02 | Opportunities to gather information about a patient’s pain occur during a variety of clinical and daily activities, such as, for example, general assessment at admission, pre-operative assessments, or physiotherapists’ interaction with the patient. Any of the clinical notes about the patient may contain information pertaining to the patient’s pain. When these notes are kept on paper, information remains fragmented and may not be accessible to all. It is this information that the system should capture and display so that it is made available to all members of the MDT in aggregated form |
| The graphical display of this information must be organised so as to convey meaning (i.e. for staff to be able to make sense of the patient’s pain). This may be done through highlights of connections of different data points, source of data, contextual relevance, etc. | |
| FR.03 | It is essential that the PADDS system does not add to staff burden of data entry and documentation tasks; it is also essential that information/data for PADDS does not duplicate, or take away from, documentation in other clinical systems (e.g. medical or nursing notes) |
| The best way to achieve this twofold requirement, is for users to complete data entry/documentation tasks as they would if the PADDS system were not implemented – in their usual, respective clinical notes – and for the PADDS system then to capture these automatically and display as appropriate | |
| FR.04 | When details of the person who entered any of the information displayed in PADDS are available, other members of the MDT may be able to discuss any issues further in person |
| FR.05 | While coded or numerical data, such as pain intensity scores and equivalent labels (such as ‘moderate’ or ‘severe’ pain), may be useful for purposes of tracking progress of therapy over time, they are not sufficient to make sense of the patient specific pain experience and how best to manage it. A narrative text is essential for this purpose |
| This is supported by theories of cognition which distinguish between paradigmatic mode and narrative modes of thinking, each appropriate to different purposes: ‘The narrative mode [. . .] organises context-sensitive knowledge into temporal plots that emphasise part–to-whole relations in order to develop meaningful, holistic understandings of particular events or identities’162 | |
| However, the use of this free text should be carefully assessed during pilot implementation and feasibility study, with the aim of exploring how to code key elements of this narrative text using descriptive labels. It may then be possible to provide structure to this information, and this will then make possible to customise the system to provide decision support (e.g. system triggers for individual symptoms) | |
| FR.06 | The time spent in the clinical area is a clue to how well the patient may be known by the HCPs/HCA. If this information is clearly available, the user may be able to better assess the volume and quality of the information recorded in PADDS |
| FR.07 | Once the presence of pain has been established, there will be a pain management strategy in place and a pain care plan of how to implement it. Both are likely to be ‘tentative’– (i.e. to be revised, adjusted and fine-tuned depending on the patient response to treatment). It is important that both strategy and care plan are explained in order for other members of the MDT to be able to assess whether and how they have been implemented, and/or revised, and patient’s response |
| In traditional paper-based documentation systems, pain management is often documented only as recorded prescription in the drug chart, but the rationale for the prescription and its role in the therapeutic trial is not explained (e.g. whether it needs to be revised after x number of days). Alternatively, structured care plans may be used, more or less standardised across all patients, not providing information specific enough for each patient | |
| See also Req. FR.m.02 for explicit reference to pain care plan/management strategy specifically by use of medicines | |
| FR.08 | While read/write access to PADDS must be granted to all members of the MDT – including HCAs – pain management plans is expected to be a prerogative of clinical roles in charge for this task |
| FR.09 | The involvement of family and carers in pain assessment is recommended by national guidelines: ‘Pain behaviours differ between individuals, so assessment should include insights from familiar carers and family members to interpret the meaning of their behaviours’16 |
| Although not all patients with dementia have relatives/informal carers able to provide information about the patient pain (usual cues, coping strategies), when available, this information helps shorten the time of staff’ sense-making process about the patient’s and the time of the therapeutic trial. Whenever possible, this information should be gathered and added to the PADDS system as part of the patient-specific pain picture | |
| Involvement of relatives/informal carers in shared decision making about the patient’s pain may remain outside of hospital routine activities; to counter this risk, a reminder may be placed within PADDS to prompt staff. However the use of this needs to be carefully assessed, so as not to add to alert fatigue. ‘The term alert fatigue describes how busy workers (in the case of health care, clinicians) become desensitized to safety alerts, and as a result ignore or fail to respond appropriately to such warnings’163 | |
| FR.10 | A period of hospitalisation may last for more than a week; during this time a large volume of data may be generated about the patient’s pain. There is a need to prioritise the most recent information while not losing the possibility of comparing current data to older data |
| As usual practice in other clinical systems, older data may be placed ‘off screen’ and made accessible ‘upon request’ | |
| FR.11 | Pain management strategies in hospital often rely almost completely on medication, despite guidance recommending a range of possible alternative strategies should also be considered164 |
| Other pain management interventions include, for example: repositioning, physiotherapy, or patient engagement in meaningful activities. These would offer patients more opportunities to communicate their experience of pain | |
| As part of the decision support role of the PADDS system, reminders of these alternative options should be provided as prompts for the MDT | |
| FR.12 | The inherent uncertainty about the pain of a patient with dementia undermines the possibility of alerting algorithm based on unique data points, such as, for example, when pain intensity = 3, then increase administration of pain medication. Instead, the PADDS system can generate alerts on the basis of a range of data points, such as trends or unusual signs |
| FR.13 | Requirement FR.12 intends to give users the ability to set-up their own prompts and reminders to share with the other members of the MDT. These may be patient-specific and/or context-specific reminders, and to be set flexibly by members of the MDT |
| Decision support tools often act through prompts and alerts – and these are also included in the PADDS system | |
| FR.14 | In order to facilitate continuity of care in a patient’s pain management after discharge from hospital, information about the patient’s pain (what sense has been made of it inside the hospital, and what has been done about it) may be transmitted to clinicians in the community/primary care. This may be done by adding data from PADDS to discharge summaries |
| FR.15 | This is a keystone security requirement. The text has been borrowed from Connecting for Health specifications from ePrescribing systems (Ref GEN.OS.003)111 |
| FR.16 | Requirement of remote access matches equivalent ones for ePrescribing systems and may be implemented for those PADDS systems interlinked with ePrescribing systems that are accessible remotely. (See Connecting for Health specifications from ePrescribing systems -Ref GEN.OS.004)111 |
| FR.m.01 | Pain medicines are one of the most used and most important methods for pain management, and this is why if/when they have been prescribed, this information must be included in the PADDS system |
| For reasons of safety, information about medicines must always be accurate, up-to-date and derived from the complete drug chart (or the electronic equivalent) | |
| The display of medicines information should follow NPSA165 and Academy of Medical Royal Colleges guidance166 | |
| FR.m.02 | This is a more specific instance (pertaining to pain management by the use of medication) of the more general requirement FR.07 – that the pain care plan and pain management strategy should be clearly and explicitly documented |
| See FR.07 for further details | |
| FR.m.03 | Medication prescribed and used for/by the patient prior to admission to hospital may give cues on the presence of pain and may provide information about strategies that have been tried in the past |
| This is therefore useful information to contribute to ‘building the picture’ about a patient’s pain | |
| This requirement is not for purposes of medicine reconciliation – this is left outside of the scope of this document, and should be part of prescribing systems and processes | |
| FR.m.04 | Information about medicine use must be displayed in PADDS as read-only data. If it were possible to amend pain medication through the PADDS system, the prescriber would lose potential safety-critical data otherwise available in the drug chart (or electronic equivalent), such as drug–drug interactions or allergies, with serious risks for the patient |
| FR.m.05 | Genomic information is expected to emerge in the future that may explain different metabolic responses to medicines. For example, the CYP3A4 and CYP2D6 enzymes are known to influence a patient response to pain medication167 |
| The availability of this information in PADDS may aid HCPs in interpreting patient’s response to therapy | |
| A similar requirement is included in functional specifications of ePrescribing systems [Requirement GEN.OS.019]111 | |
| FR.m-a.01 | Regular medicines are prescribed for administration at specified intervals, often corresponding to pre-established times (e.g. times of drug rounds). However, for a variety of reasons, the actual time the administration of the medicine takes place may differ from the pre-established time slots. In order for information about a patent’s pain derived from pain assessments to be understood in light of the therapeutic trial (and vice-versa), it is essential that the timing of administration of therapy and timing of assessment are accurate. This is also supported by a study published by the Journal of Clinical Nursing in 2012,168 discussing how ‘the fact that the medicine has been signed as being given, when in some cases it is not, makes it difficult for the prescribers to evaluate the effectiveness of the treatment and if carried out repeatedly could lead to loss of control of the patient’s disease’ |
| In order to assess patient’s response to therapy, pain assessment should follow administration of therapy, with enough time for the therapy to have the desired effect | |
| FR.m-a.02 | Information about missed doses of regular medicines may provide cues on how and why the patient’s pain appear to be like. For example, a patient refusing pain medication may be a sign that the pain is under control, or a missed dose for other reasons (e.g. patient absent from the ward at time of ward round) may explain why the patient appear to be in greater pain than expected |
| FR.m-a.03 | Information about planned doses of regular medicines may help plan and organise the scheduling of assessments by other members of the MDT |
| FR.p-a.01 | As explained in Req. FR.02, opportunities to gather information about a patient’s pain occur during a variety of clinical activities. Information about a patient’s pain may be gathered for the specific purpose of pain assessments but also during other assessments of the patient (e.g. general assessments at admission). Any and all of these assessments should add to the ‘overall picture’ of the patient’s pain offered through PADDS |
| See also Req. FR.02 | |
| For the need for narrative, see also Req. FR.05 | |
| FR.p-a.02 | In order to track trends over time, such as progress in pain control, the complex information about the patient’s pain experience may be condensed to the uni-dimension of pain intensity. Pain intensity is a continuous phenomenon that can be translated to an ordinal scale, such as numerical ranked data from zero (no pain) to x (the maximum possible pain), or to ranked adjectives such as mild, moderate, severe (pain) |
| The national EWS system uses the 0–3 range, where 0 = no pain, 1 = mild, 2 = moderate, 3 = severe. Other systems may be used, provided a degree of consistency is maintained across the hospital context | |
| Consistency across systems is a key principle for usability and safety requirement169 | |
| FR.p-a.03 | Patients with dementia may have difficulties verbalising their pain experience. Clinicians may ‘guess’ the presence and intensity of the patient’s pain by observing the patient’s behaviour, vocalisation or facial expressions (‘pain cues’). When such informed ‘guesses’ are documented in narrative form, they may be qualified, for example, through the expression ‘patient appears to be in pain’. The inherent degree of uncertainty in such assessments should be maintained even when pain assessment is documented through coded means (e.g. with numerical scores). The loss of this information may lead to a false sense of certainty,170 overconfidence, and HCP/HCAs paying less attention to further signs/pain cues that may otherwise disconfirm the assessment |
| FR.t.01 | Similarly to the display of data about administration of pain medication and of subsequent pain assessment (needed to assess the effectiveness of the medication), there is a need to document and display the administration of any other non-pharmacological pain relief and any subsequent pain assessment |
| The absence of data about administration of non-pharmacological pain relief should be a warning to the MDT of possible over reliance on medication at the detriment of other possible therapeutic interventions the patient may benefit from | |
| FR.v.01 | The display of trends over time should aid pattern recognition which in turn should aid HCPs/HCAs making sense of the patient’s pain |
| For guidance on how best to display information over time, see the work by Edward Tufte171,172 | |
| Sittig et al.173 provide criteria for the evaluation of the display of trends of clinical data over time | |
| FR.v.02 | There is evidence that electronic clinical systems have been built displaying data over time in inverse chronological order.173 This was found to be contributing to HCPs’ errors and should be avoided |
| FR.v.03 | The juxtaposition of different data types can aid users in drawing connections between objects or events – in this case the connection between therapeutic interventions and assessments of the patient’s pain to confirm whether the intervention had (or not) the expected effect. These different data correspond to activities (administration, assessment) carried out by different individuals at different times; an effective display of these data in the PADDS system can aid the MDT share information and support their judgement of the progress of the therapeutic trial, and control of the patient pain |
| FR.v.04 | This requirement is linked to requirements above about displaying data about activities of administration of therapy and assessment |
| This requirement focuses more specifically on the overall effect that must be produced through PADDS of conveying a meaningful picture of the patient’s pain experience (or absence of pain). This ‘picture’ will be built over time through the accumulation of data in the PADDS system |
Appendix 7 Health economics data collection forms
List of abbreviations
- ADD
- Assessment of Discomfort in Dementia
- AGS
- American Geriatric Society
- AMSTAR
- A MeaSurement Tool to Assess systematic Reviews
- AS
- Alzheimer’s Society
- CNPI
- Checklist of Nonverbal Pain Indicators
- CPAT
- Certified nursing assistant Pain Assessment Tool
- CSRI
- Client Service Receipt Inventory
- DEMQOL
- Dementia Quality of Life Measure
- DS-DAT
- Discomfort Scale – Dementia of the Alzheimer’s Type
- ECPA
- L’Échelle Comportementale pour Personnes Âgées
- ECS
- Edmonton Classification System
- EPCA-2
- Elderly Pain Caring Assessment 2
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions, three levels
- FACS
- Facial Action Coding System
- FLACC
- Face, Legs, Activity, Cry, Consolability scale
- GPA
- generic pain assessment
- HCA
- health-care assistant
- HCP
- health-care professional
- HSDR
- Health Service and Delivery Research
- JBI
- Joanna Briggs Institute
- LAG
- lay advisory group
- MDT
- multidisciplinary team
- MeSH
- medical subject heading
- MOBID
- Mobilization-Observation-Behavior-Intensity-Dementia pain scale
- MOBID-2
- Mobilization-Observation-Behavior-Intensity-Dementia-2 pain scale
- MPS
- Mahoney Pain Scale
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NOPPAIN
- NOn-communicative Patient’s Pain Assessment Instrument
- OPBT
- Observational Pain Behaviour Tool
- PACSLAC
- Pain Assessment Checklist for Seniors with Limited Ability to Communicate
- PADDS
- Pain And Dementia Decision Support
- PADE
- Pain Assessment for the Dementing Elderly
- PAINAD
- Pain Assessment IN Advanced Dementia
- PAINE
- Pain Assessment In Noncommunicative Elderly persons
- PATCIA
- Pain Assessment Tool for use with Cognitively Impaired Adults
- PATCOA
- Pain Assessment Tool in Confused Older Adults
- PBM
- Pain Behavior Method
- PPI
- Present Pain Intensity
- PPQ
- Proxy Pain Questionnaire
- RaPID
- Rating Pain In Dementia
- REPOS
- Rotterdam Elderly Pain Observation Scale
- SPICE
- setting, population, intervention, comparison, method of evaluation
- SSC
- Study Steering Committee
- VAS
- visual analogue scale