Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/1016/04. The contractual start date was in July 2012. The final report began editorial review in October 2015 and was accepted for publication in March 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Llewellyn et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Costing at the patient level in health care makes sense. NHS clinicians treat patients, so they should readily connect with individual patient costs. More broadly, understanding cost drivers at the patient level should enable better resource allocation not only along care pathways within hospitals, but also across the whole NHS economy. Patient-level information and costing systems (PLICSs) have the potential to produce a granular analysis of the cost of individual pathways both within trusts [in this report the term ‘trusts’ refers to both foundation trusts (FTs) and NHS trusts, unless otherwise specified], from referral to discharge, and across 1 ‘year of care’ (for chronic conditions that cross organisational boundaries).
In 2009, when PLICSs were first introduced, the Department of Health (DH) gave the following definition:
[PLICS] relates to the primary functions of health providers, which are to diagnose and treat patients . . . [it] is derived from tracing resources used by an individual patient in diagnosis and treatment and calculating the expenditure of those resources using the actual costs incurred by the organisation.
DH1
The use of the term ‘actual costs’ in the above definition heralded a shift in hospital costing methodology from a mainly ‘top-down’ mode of cost allocation to a more direct approach, based on the principles of activity-based costing,2 whereby every effort is made to cost all the interventions and events that could be associated with individual patients. 1 In the NHS, it has been known for some time that a minority of patients drive costs, but PLICS data give specificity to this knowledge; Blunt and Bardsley3 found that 3% of patients account for 45% of costs. PLICSs give the opportunity to discern where, on the care pathway, these costs are incurred.
At the time when the government implemented PLICSs as a new costing methodology, the benefits were expected to be (1) an enhanced ability to understand financial drivers enabling cost benchmarking at patient, specialty and hospital level; (2) ‘dramatically improved clinical ownership’ of costs that would enable a comparison of the cost profiles of different clinicians for similar patients; (3) a detailed knowledge of individual patient costs to inform patient classification, rather than reliance on the average cost; (4) to progress payment by results (PbR) through calculating ‘a long-term sustainable price to an efficient provider’; and (5) informed dialogue between providers and commissioners. 1
In 2012, the Nuffield Trust report supported the use of PLICSs to generate cost savings, but commented that such savings will be achieved only if clinicians actually use PLICSs to investigate how their decisions impact on costs and warned that trusts could use PLICSs to identify unprofitable service areas and, subsequently, pull out of providing them. 3
How trusts can use patient-level information and costing systems
Research indicates the potential for the use of PLICSs in hospitals. The reliability of reference costs (the only widely available hospital cost data before PLICSs were introduced) for benchmarking has been found to be questionable. 4 If trusts are willing to share their PLICS data externally for benchmarking, performances can be enhanced only if there is an internal evaluation of the processes that led to the externally shared results. 5 PLICSs can provide the data for this internal examination of processes. In many clinical areas, especially complex care in which expert judgement is exercised, there is acknowledged variability in resource use between different clinicians (or teams of clinicians). 6 The NHS Institute reported that £3B could be saved if every NHS organisation performed as well as the upper quartile in key areas [e.g. reducing length of stay (LoS), reducing pre-operation bed-days and managing variation in outpatient appointments and emergency admissions]. 7 Current costing practice under Health Resource Groups (HRGs) masks such variability at the consultant level. 8
Care integration along pathways within trusts should enhance the quality of patient care while also making cost savings. 9,10 PLICSs illuminate the care pathway within trusts from admission to discharge, so cost savings associated with care integration along the pathway can be identified.
How patient-level information and costing systems can be used across organisational boundaries
For chronic conditions, such as cancer, heart disease, diabetes mellitus and asthma, tracking patient pathways has been advocated for decades but such pathways have never fully been established. 11 The year-of-care model for long-term conditions that cross organisational boundaries emphasises costed care pathways and anticipates year-of-care prices. 12 PLICSs can potentially cost 1 year of care across organisational boundaries by identifying the direct and indirect costs associated with all the events, procedures and clinical interactions for an individual patient trajectory. If commissioners have access to PLICS data, they can use it to develop tariff currencies to enable commissioning on the basis of care pathways and year of care.
Research aims
Against this background, we aim to analyse the potential of PLICSs in four areas:
-
cost improvement through enhanced technical efficiency
-
better allocative efficiency of resources and congruence with patient preferences within health-care economies (first, within and between trusts; second, between primary and secondary/tertiary care; and, third, along care pathways and year of care)
-
understanding clinical variation in resource use and the relationships between cost and quality
-
greater clinical engagement through more clinical ownership of costs and information systems.
These research aims make reference to both technical and allocative efficiency. Technical efficiency implies the use of resource inputs (e.g. labour, capital or technology) to maximum advantage in terms of either intermediate outputs (e.g. numbers of patients treated) or outcomes (e.g. numbers of patients benefiting from treatment), whereas allocative efficiency implies allocating resources in such a way as to maximise health-care outcomes. 13 In terms of available comparative statistics on outcomes, hospitals use short-term survival rates, health recovery rates after treatment in hospital and changes in waiting times. 14
Technical efficiency implies two criteria: (1) producing as much as possible with a given set of inputs and (2) producing at minimum cost. 15 Therefore, technical efficiency has both transformative (or productive technological) and cost-minimisation elements. Technical efficiency can also be observed under two conditions: (1) an output-transformative approach, which maximises outputs while keeping inputs fixed; and (2) an input approach, which minimises inputs while keeping outputs fixed. 16 In health care, technical efficiency is a necessary, but not sufficient, condition for allocative efficiency because decisions on where to best allocate resources (e.g. acute or community care) are made prior to the achievement of technical efficiency. By contrast, allocative efficiency implies technical efficiency because resources are put to best use only under conditions of technical efficiency. Both technical and allocative efficiency lower hospital costs. 17
Concerns have been raised that technical cost efficiency can be pursued to the detriment of quality of care; however, limited evidence is available and what evidence there is generally employs data envelopment analysis. With this technique, Nayar and Ozcan18 found that improving technical efficiency did not compromise quality of care, Clement et al. 19 reported that lower technical efficiency was linked to worse risk-adjusted quality outcomes and Mark et al. 20 identified patient safety as a mechanism to improve technical efficiency. However, a cross-country data envelopment analysis revealed that enhanced technical efficiency is apparent in some countries with good care outcomes, but also in those with more modest achievements. 21
Another issue regarding technical efficiency is to question the circumstances under which it is achieved. Leibenstein22 argued that technical or ‘X-efficiency’ should not be assumed, as a benign economic climate, especially when allied to a lack of competitive pressures, as it will lead individuals to work less hard to the extent that outputs are not maximised for a certain level of inputs. Although ‘efficiency’ has often been seen as a matter of dry calculation rather than an ethical issue, whenever the resources dedicated to health care result in less health benefit than would have been possible, there are moral implications. 23,24
Research questions
Our research questions are:
-
How are NHS trusts and commissioners using PLICSs?
-
How can PLICSs be used to benefit the total health economy (focusing on cost improvement, resource allocation across services and settings, linking costs with quality and clinical engagement)?
In the first research question, ‘NHS trusts’ refers to FTs and non-FTs. We surveyed all NHS trusts – both FTs and non-FTs. However, all four of our case studies were FTs, so in the case study chapters the ‘trust’ is a FT (see Chapter 3 for more details).
Contribution and significance of the research
Since 2009, the use of PLICSs in the trusts has not been mandatory; however, Monitor (the economic regulator for health) is now planning to use PLICSs to set prices for funding [see Chapter 2 for details on Monitor’s merger with the Trust Development Authority (TDA) in 2015, which occurred during the writing of this report]. Despite the significance of PLICSs, in terms of both policy and impact on NHS service delivery, it is understudied academically. At the time of writing, to our knowledge, there are only two relevant academic reports: one (Chapman and Kern)2 mentions PLICSs only in the context of activity-based costing, and the other (Blunt and Bardsley)3 investigated PLICSs at only one hospital.
In the past, health commentators have pointed out that policy strands frequently cut across each other; indeed, there is often transparent inconsistency between health policy objectives. 25 The NHS is currently characterised by pressures for both competition and collaboration. Although the Health and Social Care Act 201226 requires competition and collaboration, the two are often at variance; when they conflict, some commentators argue that competition will dominate. 27,28 Eighty-two of the 282 clauses in the bill are about competition, so it appears that enabling competition is the governmental intent. 28 In terms of greater competition, however, the professionally dominated NHS has resisted change in the past and may do so again. 29 In 2011, the British Medical Association polled its members on the health and social care bill reforms; > 80% said that they were mostly or very unwelcome and > 50% said that the role of Monitor to promote competition was the most damaging of the reforms. 30
In broad policy terms, PLICSs could be mobilised to support either competition or collaboration. Competition can enable basic cost awareness. As mentioned above, PLICSs are sophisticated costing tools that can support technical efficiency and hence cost improvement in the trusts. A multiplicity of individually competing sellers (providers) and buyers (commissioners) is the essence of market competition and may improve technical or X-efficiency. However, such a situation leads to fragmentation. In a huge and very complex multiagency organisation like the NHS, which is facing ever-increasing demand, collaboration is required to avoid duplication and enable effective resource allocation. As discussed above, PLICSs have the potential to be used in collaborative endeavours to integrate care across organisational boundaries. An important aspect of this research is to track the use of PLICSs against the background of both competition and collaboration in the UK health-care economy.
The structure of this report
We next turn to the policy background for PLICSs (see Chapter 2), before detailing our research methodology (see Chapter 3). The empirical chapters then commence with an analysis of survey findings (see Chapter 4). The four case studies are presented next. First, we discuss ‘Gertrude’ (see Chapter 5) and then ‘Sybil’ (see Chapter 6); both are generalist FTs and early implementers of PLICSs. The third case study chapter discusses ‘Chelsea’ (see Chapter 7), a specialist FT which also has considerable expertise in PLICSs. The last empirical chapter focuses on ‘St Winifred’s’ (see Chapter 8), a FT which implemented a PLICS during our research. Finally, we garner our arguments and evidence to (1) address our research questions and objectives; and (2) discuss the wider implications of our findings. The discussion chapter (see Chapter 9) ends with concluding comments and suggestions for future research.
Chapter 2 Policy background
In this chapter, we set out the policy context for our investigation of current practice and future potential for PLICSs. In Chapter 1, we discussed the policy pressures for both competition and collaboration in the NHS. After outlining our literature search strategy, in the first part of this chapter we explore other aspects of the broad policy background (see The broad policy background) and in the second part we look more closely at the specific policy context for our empirical findings on PLICSs in the four case studies (see The specific policy context for the use of patient-level information and costing systems). Although this distinction is somewhat artificial, we think that there are broad and specific dimensions to the impact of policy on the use of PLICSs.
Academic literature search strategy
The academic literature search strategy used the University of Manchester online library, Manchester e-scholar (University of Manchester, Manchester, UK) and Mendeley databases, version 1.16 (Mendeley, London, UK), through carefully considered keyword combinations. However, at the time of writing, there are few academic articles on PLICSs. To interrogate the policy background for PLICSs, we accessed relevant grey literature and blogs through organisation websites (e.g. the DH, Monitor, NHS Choices, NHS England, The King’s Fund and the Health Foundation). We also created and used a reference database of articles from Healthcare Finance and the Health Service Journal as leading, influential practitioner-oriented publications that have discussed PLICSs. Our time frame for the search was 2000 onwards, because PLICSs are a relatively recent development (from approximately 2006 onwards).
We searched, reviewed and synthesised literature throughout all stages of our research to keep up to date with both new publications, particularly on patient-level costing, and relevant policy developments in the area, for example mandatory policy documents released by Monitor and NHS England. Our approach was narrative in style, being non-linear and iterative, in accordance with the recommendations of Cronin et al. 31 for social science research.
Part 1: the broad policy background
In the past, Monitor and the DH differed somewhat in their approach to PLICSs. Monitor concentrated more on service line reporting (SLR) (see Service line reporting and patient-level information and costing systems), seeing the implementation of PLICSs as a natural progression from SLR once this is in place, whereas the DH promoted costs at the patient level, recognising the value attached to the added granularity of PLICSs. 32 Now, PLICSs are a type of costing system that, under Monitor, look set to become a pricing model. The Healthcare Financial Management Association (HFMA)33 reported that: ‘Monitor regards accurate and comparable patient level cost data as fundamentally important in supporting the development of pricing mechanisms’. Monitor proposes that the first year of mandatory costing based on PLICSs will be 2018–19 for acute trusts. 34
Recent developments in NHS costing/pricing and patient-level information and costing systems
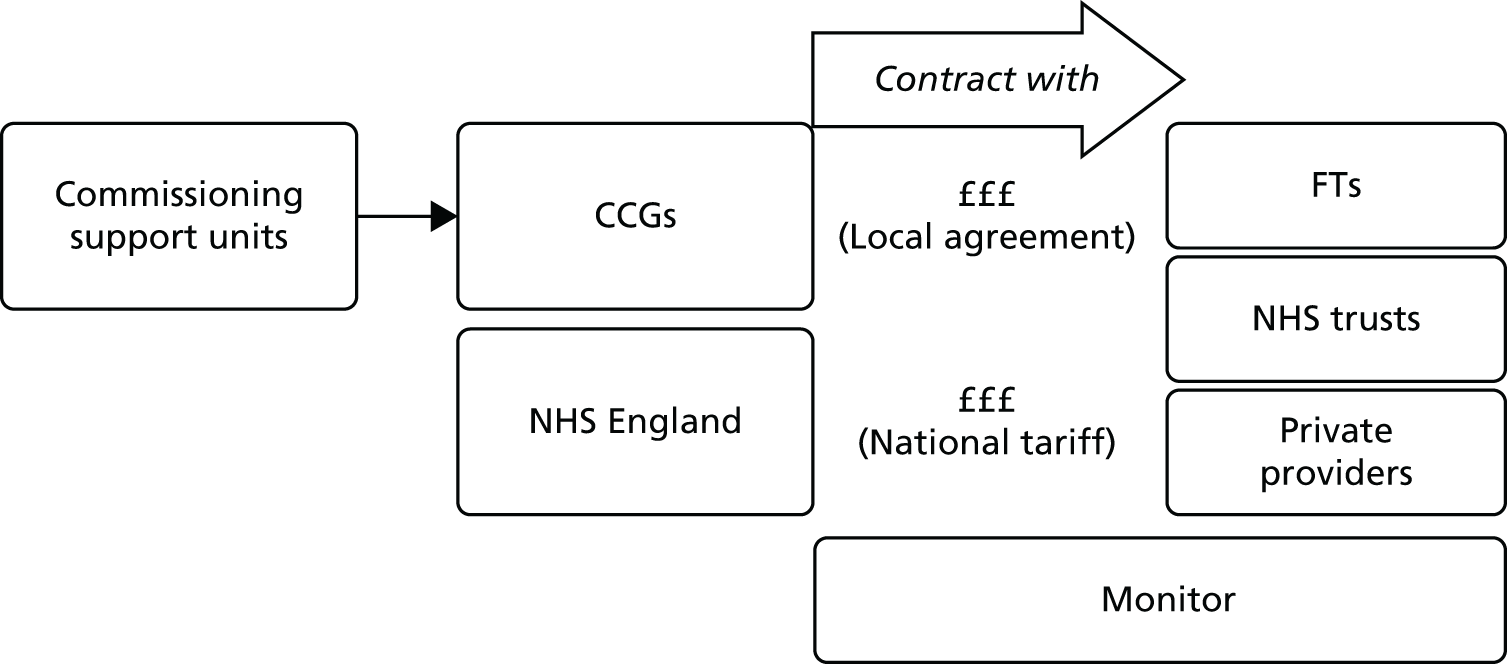
Following the Health and Social Care Act 2012,26 Monitor and NHS England took responsibility for the NHS payment system, a responsibility that previously lay with the DH. The 2012 Act prescribes that NHS England should specify currencies (i.e. units of health care for which there can be a national price), whereas Monitor’s duty is to set those prices. 35 Clinical commissioning groups (CCGs) and health and well-being boards constitute a partnership at the next level. Figure 1 portrays current accountabilities, partnerships and monetary flows in NHS England.
FIGURE 1.
Accountabilities and partnerships in the NHS in England. GP, general practitioner. a, Monitor has now merged with the TDA to become NHS Improvement.
A particularly controversial aspect of the Health and Social Care Act 2012 was to make the standard tariff a maximum, thus allowing providers to compete on price (see Chapter 1, Contribution and significance of the research). There appears to be potential for price competition between trusts; Blunt and Bardsley3 reported that in the majority of cases in which the trust’s tariff funding was under PbR (see Payment by results: the national tariff), they incurred costs which were > 10% higher or lower than the tariff and only 17% of cases were within 10% of the tariff. Another aspect of competition is the enhanced role for the private sector, under the political remit of ‘any qualified provider’. With Monitor as the economic regulator, this may signal a more market-based approach associated with price competition,36 for which PLICSs could supply the most sophisticated data. However, Simon Stevens, the Chief Executive Officer (CEO) of NHS England in 2014, remarked recently that ‘It’s unlikely the NHS will ever fully return to Lansley [the former Health Minister]’s price setting system.’37 As yet, it is uncertain what developments there will be on price competition within the national tariff.
There have been tariff consultations. In 2013, Monitor and NHS England published a discussion paper on reforms to the tariff, inviting comment. 38 Following the consultation, Monitor stated: ‘we consider on balance that a rollover [continuing] approach for national prices is the most appropriate approach for the 2014/15 National Tariff Payment System. We propose to apply this approach to determining prices for 2014/15 only; for 2015/16, we are likely to propose a different method for setting national prices, based on updated cost data (which is likely to include reference costs data as well as potentially PLICS data).’39
Another consultation document was issued for the proposed 2015–16 tariff. 40 Tariff proposals for 2015–16 included a 3.8% efficiency savings requirement and marginal rates of 50% for emergency admissions and specialised services. (Trusts have had to find 4% savings annually between 2010 and 2015.) These mandated efficiency targets are referred to as Cost Improvement Programmes (CIPs) at the trusts, including our case study sites. Issues around efficiency savings and marginal tariffs for both emergency admissions and specialised services were raised repeatedly in our interviews at these case study sites. The marginal rate rule restricts tariff payments to only a percentage of the full national average cost when activity (number of patients treated) exceeds a baseline value (commonly the activity level of the previous year).
In any event, providers ‘resoundingly rejected’ the proposed new tariff for 2015–16. Seventy-five per cent of providers (by share of supply) made formal objections; this was well over the 51% threshold for objections that triggers either a referral to the Competition and Markets Authority or another consultation with providers on the basis of revised prices which take account of the formal objections. 41 After providers rejected the new tariff proposals for 2015–16, Dowler42 reported that Monitor and NHS England made a £500M bid to providers to try to agree prices before the start of 2015–16; this bid included a reduced efficiency savings target of 3.5% and uplifts on the cap on payments for specialised services and emergency admissions to 70% (see Exceptions to payment on the national average tarriff). This bid is known as the Enhanced Tariff Option (ETO). The NHS Confederation reported in April 2015 that the majority of NHS trusts and FTs have signed up for the ETO, with the remainder continuing on 2014–15 tariffs – the default tariff rollover. 43 However, it is worth noting that 30 of the 241 trusts that rejected the ETO included all 10 members of the Shelford Group, which comprises major teaching and specialist trusts, such as Great Ormond Street Hospital for Children and Moorfields Eye Hospital. 44 The influential Shelford Group is now expected to engage in further negotiations, both locally and nationally. 45 In addition, those who rejected the ETO are not eligible for Commissioning for Quality and Innovation (CQUIN) payments, worth up to 2.5% of income. Dowler45 reports that providers that accepted the ETO felt ‘blackmailed’ by the threat of losing CQUIN monies (as recommended by Darzi,46 CQUIN money has been top-sliced and paid since 2009).
Providers that signed up to the new ETO will be required to share cost data (including PLICSs) in a new ‘efficiency collaborative’ established by Lord Carter (in 2014, Lord Carter was appointed to be Chair of the NHS Procurement and Efficiency Board). 47 In relation to the Carter efficiency collaborative, Simon Stevens remarked: ‘At the moment 22 trusts are signed up to sharing data on their [PLICSs] costs so we get a granular look at where the efficiency opportunities are sitting . . . I would like to see the vast majority of trusts participating in that.’ In an interim report, the Lord Carter review48 commented: ‘By 2019–20, the review believes £5bn a year could be saved across staffing, medicines, everyday items and estates.’
However, some commentators viewed the clear rejection of the originally proposed 2015–16 tariff as indicating a new era in NHS cost improvement. At The King’s Fund, Murray49 remarked: ‘It signals that the policy of implementing year-on-year reductions in the prices paid to hospitals for their services has reached the end of the line . . . the two main ways used to reduce NHS costs over the last few years – limiting staff salary increases and reducing payments to hospitals – have now been largely exhausted.’
Such views heighten the relevance of care integration across organisational boundaries to both achieve cost savings and improve the care delivered to patients. Allegedly, as a result of the ‘listening exercise’ which followed controversy over the bill,50 the NHS Health and Social Care Act 2012 places responsibilities on NHS England, CCGs and the health and well-being boards (see Figure 1) to better co-ordinate care. 51 Evaluation of the 16 integrated care pilots set up in 2009 demonstrated lower than expected outpatient and elective care but, perhaps surprisingly, no change on emergency admissions. 52 However, clear evidence to substantiate both improved patient satisfaction with integrated care and cost savings is often still lacking. Perhaps unavoidably, proxies for cost savings are often used, such as the avoidance of unnecessary hospital admissions. 53 Clearly, deriving cost savings from counterfactuals is a subjective exercise.
In 2015, there were arguments that integrated care should also be seen in terms of its potential for improved population health. 50 However, such a suggestion does not solve the complexities of clearly demonstrating the benefits of integrated care. Nevertheless, many commentators prescribe integrated care, including a redistribution of resources across health economies to transfer care (when safe to do so) from secondary providers to primary and community services. 9,10,54 We argue that PLICSs could be important tools for promoting care integration – including a transfer of resources from secondary to primary care – but our evidence casts considerable doubt over whether or not this will occur (see The potential for patient-level information and costing systems to cost cross-organisational care pathways).
In summary, costing and pricing models are, potentially, significant drivers for more effective resource distribution across health economies55–57 but, as will be argued in this research report, the specificity of the costing (or pricing) model and its mode of implementation are fundamental to success. Consequently, from an historical perspective, we briefly review costing and pricing models next, before discussing the national tariff and the role of Monitor.
History: the development of costing and pricing models in the NHS
Since the 1970s, NHS financial management policy has been inclined to oscillate between cost benchmarking and market pricing. This oscillation has, at least in part, been driven by the choice of cost object, to which there are three broad alternatives: department or specialty; intervention or diagnosis; or the patient. These alternatives are not, of course, necessarily mutually exclusive. In the 1970s, work was initiated on specialty costing (e.g. Magee et al. 58 and Pugh59). This provided grounds for exploratory work on patient costing. 60 Körner61 recommended the general adoption of specialty costing and associated patient costing through analysing patient groups. Taylor62 confirmed that such patient costing was feasible. This early work clearly formed the basis of a future agenda for SLR and PLICSs (see Service line reporting and patient-level information and costing systems).
Meanwhile, the Griffiths Inquiry63 heralded the introduction of market principles into the NHS. Subsequent to the Griffiths Inquiry, in 1983, four management budgeting pilot sites were initiated;61 these largely proved to be unsuccessful in using costs at a patient level but, in the 1986 Resource Management Initiative,64 they spearheaded improved patient information. Enthoven’s65 notions on the use of competitive markets in health care to enhance cost efficiency proved highly influential on the (then) UK Prime Minister, Margaret Thatcher. In 1991, she introduced the NHS and Community Care Act 1990;66 this brought about a NHS internal market to enable competition, which was assumed to drive greater cost-effectiveness and better resource allocation.
The expectation at the time was that contracting between purchasers [health authorities and general practitioner (GP) fund holders] and providers would become more sophisticated, developing from block grants to (1) cost and volume specifications; and (2) cost-per-patient case negotiation. 67 These patient costs were an early forerunner of PLICSs. In the internal market, hospitals had to set prices; in theory, these prices were cost based as, under the internal market, hospitals were not intended to make a profit. 64,68,69 However, these cost-based prices were not robust for three reasons: (1) hospitals’ cost information was inadequate for price-setting;70,71 (2) price differences did not convey information because currencies (the procedures of which were being costed) were not consistently defined; and (3) prices did not adequately reflect provider efficiency because lower or higher prices did not always signal lower or higher cost. 64,68 Moreover, if the present mix of services and their mode of delivery is not optimal, cost-based pricing does not incentivise providers to engage in service reconfiguration. 72
In 1997, the New Labour government announced a policy shift away from the internal market instituted by the Conservatives, back to a cost benchmarking regime. However, this shift was, at least in part, rhetorical because the cost benchmarking was mandatory and would herald funding on the basis of national average costs (rather than providers’ actual costs), although, initially, providers were not cognisant of the intended new funding initiative. 73
Payment by results: the national tariff
There is, currently, a tariff based on national average costs for health-care interventions. This national tariff is derived from reference costs aggregated to HRGs (trusts are mandated to provide reference costs for their services). Since 2003, the funding scheme PbR, based on national tariffs, has been rolled out to fund health-care activity. 74 Examples of the 2014–15 national tariff are £8652 for a major hip operation for trauma with major complications and £339 for a prostate or bladder neck minor endoscopic procedure, done as an outpatient appointment. 75
Under PbR there are strong monetary incentives for increased activity (i.e. to treat more patients). Trusts have incentives to reduce costs and shorten LoS to boost productivity. 76 If income under the tariff exceeds costs, hospitals make a surplus which can be retained. 77 Conversely, if income under the tariff is less than the cost to the provider, the trust will lose money on the activity concerned. Under PbR, revenue to the trusts is usually the full average cost.
Developments on the national tariff
Payments under the national tariff, set under PbR, constitute about one-third of all NHS expenditure. 78 Although it was intended that all hospital services be funded through the national tariff, many are still on block contracts or locally agreed prices.
The scope of the tariff
Calkin79 reported that in the acute sector 66.6% of services were under PbR, with 33.4% being on block contracts or locally agreed prices; for mental health 66% were on block contracts, with 33.4% locally agreed; and for community services, 90% were on block contracts with 10% locally agreed. But even in the acute sector, many trusts were well below the 66.6% average. At one of our case study sites, only 19.6% of services were under PbR, but the vast majority were locally agreed. The trusts commented on the generally advantageous prices secured through local agreement rather than national tariffs. Dowler80 reported that PLICSs may have given trusts an ‘unfair advantage’ in tariff negotiations at both a local and national level, not least because of the current information asymmetry (in the trusts’ favour) of PLICS data.
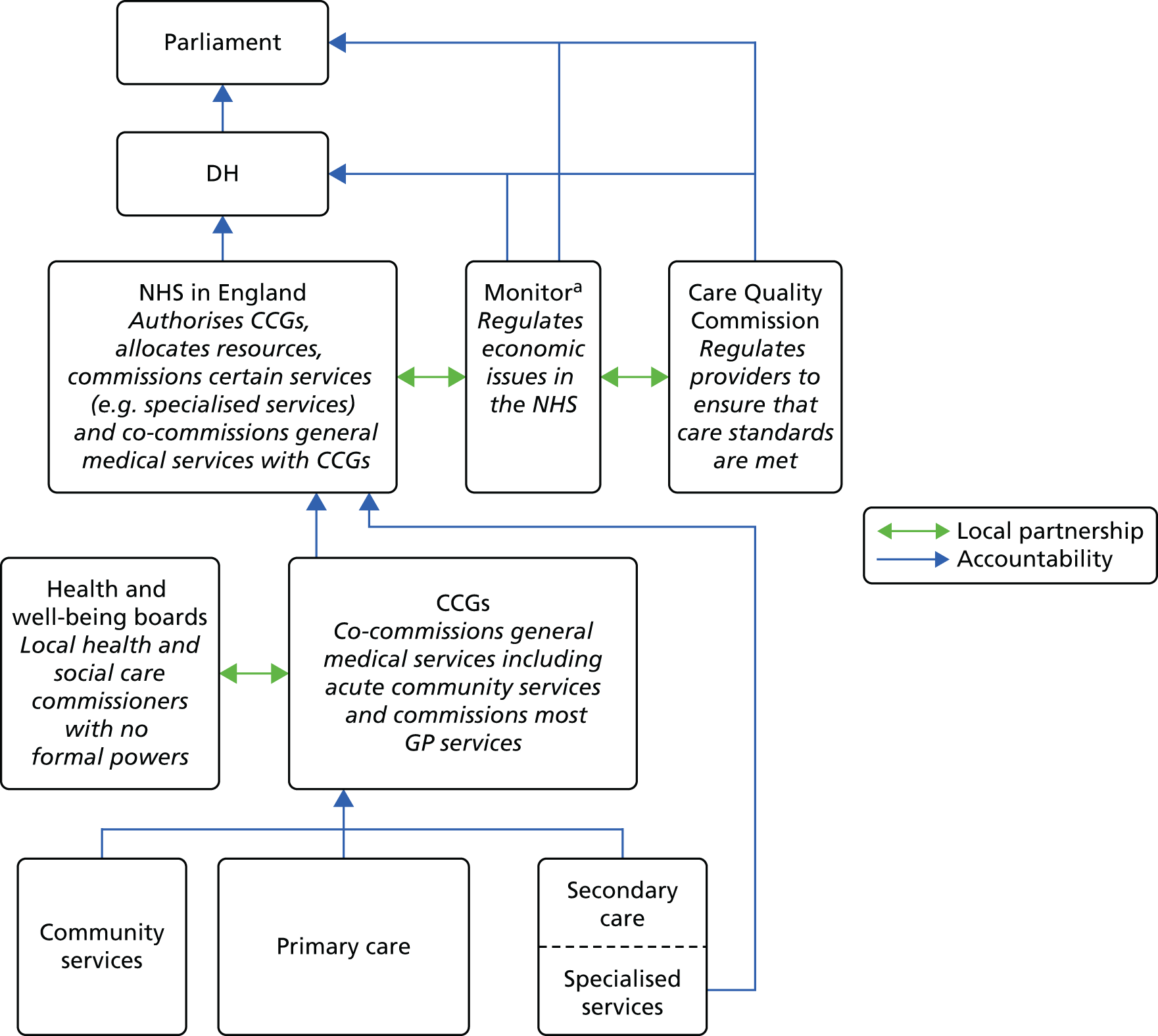
Figure 2 illustrates the contracting regime for secondary-care providers.
Commissioning support units (CSUs) were established in 2012 to support CCGs (see Figure 2). The role of CSUs is described as follows: ‘CCGs are likely to need support in carrying out: transformational commissioning functions, such as service redesign and transactional commissioning functions, such as market management, healthcare procurement, contract negotiation and monitoring, information analysis, and risk stratification.’82 As explained in Chapter 4, our commissioning survey was sent out to CSUs rather than CCGs because they were thought to be more able to give an overview about the actual and potential use of PLICS data for the commissioning function.
Exceptions to payment on the national average tariff
One exception to payment for activity on the basis of the full average cost is for admissions from accident and emergency (A&E) departments. A marginal rate for emergency admissions was introduced in 2009–10 and took full effect in 2010–11. Under this policy, if the value of a trust’s emergency admissions exceeds the previous year’s activity, then the trust receives only 30% of the income for the additional activity. This marginal payment was much commented upon at our case study sites, where there was disquiet about its impact. The marginal rate rule was introduced by the DH ‘in response to concerns about growth in the volume of patients being admitted to hospital as emergencies.’40 This is evidenced by, for example, a calculated 11.8% increase in emergency admissions from 2004–5 to 2008–9, suggesting that the thresholds for admission to hospital have reduced. 83 However, a submission from the Foundation Trust Network84 found there to be only a 6.6% rise in the average number of admissions from 2008–9 to 2012–13 and, on this basis, called for the policy on marginal payments to be abandoned. Despite this submission, Monitor and NHS England decided to retain a marginal rate rule for 2014–15. 40
From the outset of PbR it was recognised that specialised services are frequently more expensive than non-specialised care, so a ‘top-up’ was applied. For example, in 2012–13 there was a 50% top-up for specialised children’s services. 85 NHS England now directly commissions specialised services, which account for around 14% of the total NHS budget. 86 The commissioning budget for non-specialised services is devolved from NHS England to the CCGs. Figure 3 depicts these responsibilities and their associated, vertical monetary flows.
FIGURE 3.
NHS commissioning responsibilities and monetary flows.

One of our case study sites was a specialist centre for cancer so these arrangements were highly relevant to the staff members. Indeed, the introduction of a PLICS at this site was, in part, driven by the need to understand the financial consequences of some of their specialised services moving on to the national tariff; they anticipated that this development would have a negative impact on income for the trust.
In their consultation document for the proposed 2015–16 tariff, Monitor and NHS England87 note the ‘relatively rapid rate of activity and cost growth in acute services without national prices, particularly specialised services.’ NHS England35 described the cost growth in specialised services as ‘unaffordable’. In response to this cost growth in specialised services in the acute sector, it was proposed that only 50% of prices be paid over a prescribed baseline. 43 However, as outlined above, Monitor and NHS England offered what were effectively financial inducements to providers to accept its proposed 2015–16 tariff, including an increase to 70% of the marginal rate for specialised services.
Best practice tariffs (BPTs) are defined as follows: ‘A best practice tariff is a national tariff that has been structured and priced to incentivise and adequately reimburse care that is high quality and cost-effective.’88 These were introduced in 2010, in the first instance covering four procedures with the intent to incentivise day-case surgery for cholecystectomy, pay for best practice for stroke and hip fracture and streamline the care pathway for cataract surgery. For example, there was a 24% increase in price for cholecystectomies undertaken as day cases, which led to a 7% increase in patients treated as day-cases. 89 By 2013–14 there were 18 BPTs. 79 For 2015–16, Monitor and NHS England proposed a new heart failure BPT and some adjustments to existing BPTs.
Advantages and disadvantages of the tariff and payment by results
Under PbR, payments for activity have had advantages, including reduced waiting times and improved access to planned care. 90 An evaluation of PbR undertaken in 2009, using Scotland as a comparator, concluded that PbR led to LoS falling more rapidly and the proportion of day cases increasing more quickly. 91 Appleby et al. 92 stated that PbR is most suited for elective care and is less appropriate when less rather than more activity is required.
The Nuffield Trust reasoned that the cost efficiency of the whole NHS economy will improve only when payment systems cover whole care pathways and create incentives to deliver the ‘right care in the right setting’,93 thus improving allocative efficiency. Currently, payments under PbR are made to geographically separate organisational units; in consequence, although ‘unbundling’ the tariff is possible, the present funding regime does not encourage care integration across services and settings.
Moreover, PbR can have a negative impact on innovation because new technologies change the patient pathway and require staff training. Therefore, although innovation can improve productivity, at least in the short term, innovation can reduce patient throughput and, therefore, income under the tariff. 94
In sum, the adverse effects of PbR are:
-
increased hospital admissions
-
unco-ordinated care across settings
-
undertreatment
-
cost shifting to other budgets
-
cherry-picking lower-risk cases
-
up-coding or misreporting. 79
We found examples of all of these disadvantages in our case studies. Before looking more closely at various aspects of the context for issues raised in the case studies, we review the key role of Monitor in determining NHS payment systems.
The role of Monitor
Monitor is the regulator for health in England. It ensures the maintenance of essential health services in the event of a provider being in serious difficulties. Monitor is responsible for the NHS payment system, which must enable quality and efficiency. More widely, Monitor also ensures patient choice, guards against poor purchasing on behalf of patients and makes judgements on any anticompetitive behaviour by providers. 95,96
It should be noted that, in June 2015, during the writing of this report, Monitor and the TDA were merged97 to form NHS Improvement. In some circles, Monitor was seen as the government’s ‘stick’ with the TDA as the NHS ‘carrot’ because it worked with trusts to achieve foundation status. 98 The performance of the TDA has been less than impressive: set up in 2012 with a goal of making all NHS trusts FTs by 2014, this target was revised in 2014 to at least 2018, and trusts’ deficits may reach £2.1B in 2015. 99 Dixon100 reports that both Monitor and the TDA had run ‘compliance’ regimes, arguing that, given the challenges ahead, a more supportive ‘commitment’ approach may enable more ‘NHS improvement’.
Monitor was dubbed ‘Ofhealth’;101 regulating the financial performance of NHS providers since 2004, its role has been described as ‘totemic . . . a highly charged symbol of the political debate’. 102 In April 2013, Monitor’s remit was extended to all providers of health and adult social services. Debate has focused largely on Monitor’s powers over both competition and collaboration, along with scepticism, in some quarters, over ‘Ofhealth’s’ independence.
The reforms proposed by the coalition government (from 2010 to 2015), as set out by Andrew Lansley, the Secretary of State for Health at the time, in the proposed health and social care bill, referred to Monitor’s duty to promote competition in health care, a role that was vigorously opposed by Nick Clegg, leader of the Liberal Democrat Party, a coalition partner at the time. 103 A focus on promoting competition was controversial in itself, but particularly so in the light of McKinsey (the international management consultancy)’s involvement in drawing up the bill. McKinsey’s clients have included 15 of the 22 largest health-care and pharmaceutical companies. 104 Monitor’s promotion of competition may facilitate its access to the lucrative UK health-care market. At the time of writing, the head of Monitor is a former McKinsey employee, as is its Director of Strategy. During intense lobbying over the bill, the Chair of the Royal College of General Practitioners referred to concerns over ‘conflicts of interest’, commenting that ‘private companies that have advised the Government on how to dismantle the old system stand to derive commercial benefits from the new one’. 105 The government proved unable to quell all the disquiet. In April 2011 there was a ‘pause’ for the ‘listening exercise’ over their health reforms.
At the time of writing, in terms of their background, very few (just 21) of Monitor’s 337 staff had experience of running or being employed in a hospital and only seven had a clinical background. The Chair of the Public Accounts Committee cautioned that this ‘damages Monitor’s credibility and ability to diagnose problems and develop solutions.’106
After amendments, the Health and Social Care Bill was passed in 2012. Subsequently, Monitor’s role remains one of ensuring ‘that choice and competition operate in the best interests of patients [and preventing] . . . anti-competitive behaviour by commissioners or providers where it is against patients’ interests’. 107 However, CCGs will now not have to put all services out to tender and the private sector will be unable to ‘cherry-pick’ profitable, less complex procedures, although it remains unclear how Monitor will guard against the latter. 108 It should also be noted that the number of private patients treated in NHS hospitals has gone up by 58% since 2010. 109 NHS FTs may now take up to 50% of their income from private patients. 108 In 2013, Monitor published a review aimed at securing fairer competition between the NHS trusts and private and voluntary sector providers. 36 NHS England responded by stating, inter alia, that some of Monitor’s proposals would ‘increase tariff variability . . . and potentially put the continuation of certain services at risk’. 110
Monitor is now tasked to ‘enable better integration of care so services are less fragmented and easier to access’. 107 However, integration and collaboration between services, including moving some services out of secondary care, may result in trusts losing income, and Monitor’s focus on the bottom-line results for FTs may, therefore, impede collaboration. 111 Moreover, enabling service integration and ensuring both choice and competition through preventing anticompetitive behaviour will be a difficult balance to strike. For example, a proposed merger between Norfolk and Suffolk Mental Health Trusts should reduce duplication, but was seen as a move that would adversely affect patient choice and competition. 102 Commenting on the likely discrepancy between enabling competition and achieving care integration, Hudson27 stated that the ‘most likely outcome is that as providers proliferate and competitive tendering becomes the norm, integration will become more difficult.’
Evidence on this shows that, between April and December 2013, of 57 contracts (valued at £510M) awarded, 39 (70%) went to private firms, at a total value of £450M. In a different study, a Freedom of Information request to CCGs demonstrated that the private sector won 40% of contracts put out to tender, whereas NHS providers were awarded 41%. 112 An investigation carried out for the British Medical Journal reported that ‘private sector providers have secured a third of the contracts to provide NHS clinical services . . . in England since the Health and Social Care Act came into force in April 2013’;113 however, as the authors point out, it is hard to assess the degree of penetration of the NHS by private firms because many high-value awards to the NHS are for acute care which the private sector is not equipped to provide.
Historically, there has often been transparent discrepancy between health policy objectives. 25 The inconsistency between promoting competition and enabling care integration is a key issue for Monitor’s regulatory role.
Part 2: the specific policy context for the use of patient-level information and costing systems
Here, we discuss three policy initiatives of, arguably, a more local nature, which impact on how PLICSs are being used at our case study sites: SLR; costing care pathways across organisational boundaries; and clinical engagement with costs.
As mentioned earlier, PLICSs have developed within the context of SLR and service line management (SLM). At our case study sites, SLR/SLM tended to be the operational mode for financial management, whereas PLICSs were more of a strategic tool.
Service line reporting and patient-level information and costing systems
Hospital organisation has always been ‘federal’ in nature,114 whereby the clinical specialty (or ‘service line’) is a natural organisational grouping. SLR and SLM form the context within which PLICSs have emerged in the trusts. Service lines are, basically, specialties that SLM casts into profit as well as cost centres. Specialties have had cost reporting responsibilities since ‘clinical directorates’ were introduced as an aspect of the internal market reforms of the early 1990s (see History: the development of costing and pricing models in the NHS). Clinical directors (usually senior clinicians) manage specialties as ‘directorates’ (semi-autonomous, self-managed units); this autonomy is coupled with financial responsibility for the directorate budget. 57,115 So, the organisational building blocks were already in place for SLR/SLM to calculate profit (or loss) for a service line (there can be 10–20 service lines depending on the size of the trust). 6 Monitor developed the concept of SLR/SLM with McKinsey, the management consultancy;116 it identifies SLR as ‘measuring a Trust’s profitability by each of its service lines, rather than just at an aggregated level’. 117 The expectation is that SLR/SLM will have relevance, for both financial and operational aspects of trust management, through driving organisational structure, strategy, performance management and information management. 118
As discussed in detail in Chapters 5–8, PLICSs are more of a strategic tool at the trusts. For example, PLICs are used in business cases to evaluate the financial impact of investment in a particular specialty. Operational financial management occurs through SLR/SLM. Clinicians are naturally aligned to their specialty, which forms a focus for their interests and allegiances. 119 Monitor anticipates that SLM and PLICSs will enable more clinical engagement in resource management: ‘Through SLM, clinicians can play a far more influential role, driving performance and making better use of resources to improve quality and patient care’. 117 Unsurprisingly, the main benefits of SLR – for both clinicians and managers – are increased autonomy and access to any surplus/profit accruing to the service line. 116
However, taking the whole health economy perspective, a disadvantage of SLR/SLM is likely to be that their success rests on maximising income and minimising costs for a service line, neglecting what this implies for other service lines in a trust and for primary and community services. Service complexity increases cost. 120–122 Not all trusts can disinvest complex services, for example blood and marrow transplantation, to focus investment on less complex and, therefore, potentially more profitable areas. In our case studies we found that trusts dealt with this by cross-subsidising loss-making specialties through ‘contributions’ from surplus-generating specialties. There was, however, some frustration in the profitable specialties insofar as they were frequently not able to retain ‘their own income’ under the tariff.
Furthermore, again from a whole health economy perspective, a service line may be profitable, at least in part, because some primary-care referrals are inappropriate (i.e. ‘false positives’ who are discharged after first appointment). This ‘false-positives’ problem may well be exacerbated by SLM because service lines can retain their own profits, indicating that, alongside SLM, there should be costing that crosses organisational boundaries. However, our evidence shows that, although there is awareness of the potential for PLICSs to cost care pathways, such costing is not currently available outside the trusts. Within the trusts there is work that uses PLICSs to cost internal care pathways (see Chapters 5–8).
The potential for patient-level information and costing systems to cost cross-organisational care pathways
Costing care pathways that cross organisational boundaries necessitates robust systems for identifying and then costing community services. In terms of identifying activities in the community, there are no currencies, no definitions and no adequate data collection systems [Claire Yarwood, NHS England (Greater Manchester) 18 June 2015, personal communication]. Evidently, therefore, PLICSs are not being used to cost community services. In turn, the potential for PLICSs to inform care integration is severely curtailed.
Community services comprise 10% of the NHS budget. 123 The HFMA33 produced a discussion paper on community services, in which it noted that PLICSs are an appropriate methodology for community services and summarised the issues that impede their costing: the lack of a tariff; the absence of detailed descriptions of services; limited use of electronic data systems; issues with data quality and data capture; commissioners giving lower priority to community services; and the wave of mergers and acquisitions in community services, which have distracted from costing as a priority.
For decades, patient pathways for long-term conditions such as cancer, heart disease, renal disease, diabetes mellitus and asthma have been advocated but have never fully emerged. 11 We note the potential for PLICSs to produce ‘year-of-care’ estimates to assist in the management of long-term conditions. We anticipated that all four of our case study sites would use PLICSs to cost care pathways. However, in the event, although three used PLICSs to cost internal care pathways, only one of our four sites had a clear focus on costing care pathways across organisational boundaries and estimating year-of-care costs when these were being developed for frailty. However, it is recognised that new care models, including (1) integrated acute and primary care initiatives and (2) acute care collaboratives, are essential to effective care delivery; NHS England has chosen 50 ‘vanguard’ sites for such models. 124 PLICSs would be appropriate to build a cost-evidence base for these vanguards.
Patient-level information and costing systems and clinical engagement
It has long been apparent that decisions made by senior doctors largely determine hospital costs. 125,126 Specifically, Hillman et al. 127 estimated that doctors’ decision-making accounted for up to 70% of hospital expenditure. In the 1980s, Griffiths63 recommended that in order to ‘involve the clinicians more closely in the management process . . . Clinicians must participate fully in decisions about priorities in the use of resources’. However, research carried out at the time showed clearly that disseminating cost data to clinicians resulted in little change in work patterns or expenditures. 128
However, as mentioned earlier, during the 1990s, the new role of clinical director of a specialty (or support service) was introduced. 57,129 This medical manager model was successful in imbuing key senior clinicians with some cost awareness. 57,130,131 As discussed earlier, SLR/SLM builds on the natural allegiances of clinicians to their specialties. But, if specialties become fully fledged ‘business units’, with an outlook focused on cost improvement and the ‘bottom line’, this could impede other key health policy objectives, such as integrated services and more care in the community.
In terms of cost improvement, PLICSs are a form of ‘bottom-up’ costing, which captures the lowest possible level of detail to cost all events (e.g. consultations with clinicians and rehabilitation) and clinical interventions (treatments, theatre time, diagnostic tests, physiotherapy) along a patient pathway. These close links that PLICSs create between costs and patient care foster better clinician engagement than was found with previous costing initiatives and should result in more financially informed clinicians. 7 Through PLICSs, variation in costs per patient is clearly evident. Equally, PLICS costs can be aggregated to investigate variation between consultants, specialties, HRGs and trusts. Illuminating cost variation between consultants proved to be a powerful, if somewhat contentious, practice at the case study sites.
Overall, PLICSs can be an entry point for clinicians into costing discourses. Unlike other costing initiatives, PLICSs take the patient as the cost object (i.e. all costs are related to patients, rather than to diagnoses or medical interventions). These patient-level costs can engender clinical engagement, but may also encounter resistance when mobilised to change clinical practices.
Summary
Exploring the policy background to PLICSs reveals complexity, even conflict, in terms of the policy drivers for PLICS use. On the one hand, financial stringency and a competitive environment incentivise providers to use PLICSs for cost improvement and tariff negotiations, while militating against sharing PLICS data with commissioning bodies. At the specialty level, the use of SLR/SLM incentivises both clinicians and managers to argue for more autonomy and any surplus/profit accruing to service line to be retained within the specialty. Therefore, at both levels, there are tendencies towards fragmentation, with PLICS data being used to serve the financial interests of the trusts, at the provider level, and those of the specialty, at the level below. The increasing presence of the private sector in UK health care can only intensify these competitive pressures.
On the other hand, taking a whole NHS health economy perspective, key health policy goals are integrated services with more primary and community care. In this context, PLICSs have the potential to ensure better allocative efficiency for resources between primary and secondary (or tertiary) care. PLICSs also illuminate the patient pathway and have the potential to enable sophisticated analyses of individual pathways both within trusts and across 1 year of care, for chronic conditions for which the patient pathway crosses organisational boundaries. Such potential will be fulfilled only if there is collaboration between providers and commissioners, with the sharing of PLICS data.
In the chapters that follow Chapter 3, we provide empirical evidence, from a national survey and four case studies, on how these different policy drivers are playing out as we investigate PLICS use.
Chapter 3 Methodology
Introduction
The study was undertaken between July 2012 and October 2015, comprising a large-scale survey of the whole population of English NHS trusts (both FT and non-FT) and four case studies of FTs at different geographical locations in England.
Practitioner team member involvement
A notable aspect of the study was the involvement of practitioner team members: Dr Mahmood Adil (Senior Clinician), Mr Tony Whitfield (Provider Director of Finance) and Mrs Claire Yarwood (Commissioner Director of Finance). As President of the HFMA, Mr Whitfield was instrumental in setting up access to the organisation’s network of finance professionals. Dr Adil brought expertise from previous work on clinical engagement with costing. Mrs Yarwood provided expertise in commissioners’ use of costing data, drawing on experience as the Finance Director (FD) at NHS England (Greater Manchester). All three practitioners were active as core team members and helped to develop the research design in response to the changing NHS financial management landscape during the study period.
Research design
Health care is a complex, open system. Complex systems are defined as those ‘that incorporate a dynamic, emergent, creative, and intuitive view of the world’. 132 When researching complex, open systems it is clear that the experimental approach is not appropriate. 133 However, ‘natural’ experiments do arise in open systems: ‘natural experiments are common in the organisation and management of health care . . . [and] . . . can indicate the scale and nature of [policy] impacts’. 134 As identified in Policy background, policy initiatives have driven the development of PLICSs. The Chartered Institute of Management Accountants32 identifies three natural experiments in the differential approaches to PLICS use: (1) for cost improvement in the context of financial challenge; (2) to underpin a case for investment or reconfiguration of services; or (3) as a better management tool.
These natural experiments in differential PLICS use constitute general themes which could be explored through either quantitative or qualitative methodologies; we chose to examine them through mixed-methods research. 135–137 This approach involves using both quantitative and qualitative methods in tandem to enhance the overall strength of the study. 138 Specifically, we employed ‘sequential exploratory design’ (Figure 4) to conduct quantitative data collection and analysis before qualitative data collection. 136 A sequential exploratory strategy uses the initial quantitative results to inform secondary qualitative data collection, with the two forms of data remaining separate but connected. 139 This approach can be particularly useful when unexpected results arise from the quantitative stage. 140
FIGURE 4.
Methodology: sequential exploratory design.

Quantitative data collection, undertaken through a survey, was the first stage of the research. Using a survey provides a numeric or quantitative description of attitudes, opinions or trends of a population, usually by studying a sample of that population. 141 Informed by analyses of these survey data, the second stage was conducted at four case study sites. Case study research is well developed in the study of organisations (e.g. Yin142). Llewellyn143 argues that case studies seek to develop a holistic understanding of specific phenomena. As a ‘strategy of enquiry’, case studies enable an in-depth exploration of a process, activity or event. 144 Case studies also enable a strong emphasis on context and are recognised as particularly important in evaluating the impact of policy initiatives. 145
Research methods
Research at the case study sites was conducted through a variety of methods, including semistructured interviews, documentary analysis and some limited observational research. Observational research focused on the practice of actors and was ‘essentially exploratory’. 146
The primary data collection method was semistructured interviews. The open design of semistructured interviews can be contrasted with the use of a standardised questionnaire, because an open approach to interview questions is less likely to constrain the variety of possible responses. 147
We wanted to link the data from individual interviews with the emergent PLICSs strategy at the organisational level. To accomplish this, we triangulated the interview data with documentary analyses of the following: samples of PLICS data; business cases for investment; service line reports; presentations on PLICSs; and strategy documents used in discussions at board level.
Patient-level information and costing systems implementation and use are understudied. In the absence of existing studies, documentary analysis provides material for researchers in the form of, for example, written plans. 148 Table 1 shows our documentary sources.
| Number | Document | About | Date | Trust |
|---|---|---|---|---|
| 1 | Financial report | Surgery | December 2013 | Case study 1 |
| 2 | Business case template | Staffing | April 2014 | Case study 1 |
| 3 | SLR | Women’s services | April 2014 | Case study 1 |
| 4 | PLICSs comparison | Pain injection centre | May 2012 | Case study 2 |
| 5 | PLICSs comparison | Stroke patients | June–December 2012 | Case study 2 |
| 6 | Financial review | Haematology unit | May 2012 | Case study 3 |
| 7 | PbR analysis | Complex activity | November 2012 | Case study 3 |
| 8 | Presentation | Benchmarking | April 2012 | Case study 3 |
| 9 | Presentation | PLICSs introduction and implementation | Undated | Case study 3 |
| 10 | Presentation | Understanding the tariff | February 2012 | Case study 3 |
| 11 | Financial review | Haematology | Undated | Case study 3 |
| 12 | Report on HRG calculations | Haematology | April 2010–March 2011 | Case study 3 |
| 13 | Board meeting papers | SLR-PLC implementation | March–October 2014 | Case study 4 |
| 14 | Presentations | Shortlisted PLICS suppliers | August 2014 | Case study 4 |
| 15 | Briefing paper | The case for PLICSs | June 2013 | Case study 4 |
| 16 | Report | Transformation programme closure | April 2014 | Case study 4 |
| 17 | Financial review | SLR 2013–14 | December 2014 | Case study 4 |
| 18 | Presentations | Annual conference NBG | April 2014 | NBGa |
| 19 | PLICSs comparison | Consultants | Undated | NBG |
| 20 | Presentation | SLR paediatrics | December 2012 | NBG |
| 21 | PLICSs comparison | Urology | July 2013 | NBG |
| 22 | SLR report Microsoft PowerPoint® presentationb | Whole trust | March 2012 | NBG |
| 23 | PLICSs report PowerPoint presentation | Whole trust | 2011–12 | NBG |
| 24 | Business unit review PowerPoint presentation | Surgery | December 2011 | NBG |
| 25 | Guidance document | SLR principles | 2011–12 | NBG |
Methodology and ontology
In the case studies and semistructured interviews we followed a ‘critical realist’ approach149,150 that delineates the extent to which individuals and groups can be studied in the same way as natural systems and through which it is accepted that the nature of the object determines the form of its science. 150 Working from participants’ views, we paid attention to ‘what works’ with PLICSs, specifically in which contexts and with which mechanisms, thus conforming to critical realist research guidelines for social science research. 151–153
Critical realism stems from the work of Roy Bhaskar150,154,155 and has been explicated by others. 149,153,156–158 Critical realists hold that a real world exists. For the natural world, they see a significant distinction between the nature of reality and people’s knowledge of this reality. By contrast, in social science, the research ‘object’ is primarily the meanings that people attribute to their social world; therefore, a clear distinction between the social world and people’s knowledge of it is not possible. 150 Archer149 (pp. 154–90) includes a third reality – the practical. In addition to the natural and the social, the practical is the domain of non-linguistic human practice. Accounting falls into this world of practice but, naturally, people also talk about accounting in the social world and, in doing so, attribute meaning to it. In consequence, this research is informed by critical realism in acknowledging ‘differentiated realities’;159 specifically we locate accounting in both the social and practical domains. Our mixed-methods design (see Work packages) reflects this in that we conducted semistructured interviews to identify meanings and gathered multiple accounting documents which included accounting calculations resulting from practical activity.
Work packages
Work package 1: the survey
Work package 1 has three stages: a preliminary online pilot survey, a main online survey and a follow-up analysis of survey results. Sue and Ritter160 recommend (1) using online surveys for professional groups, (2) piloting test information with intended professionals in the field and (3) pre-notifying respondents of the survey to increase the response rate (see also Ritter and Sue161). We followed these recommendations by:
-
piloting the survey online with finance professionals at one case study site
-
giving our potential respondents advance notice of the main survey (specifically, in the month before distribution we placed a short description of its content and purpose in the HFMA’s professional journal, Healthcare Finance, which is widely read by the health-care finance community in England)
-
selecting the two final case study sites through a third stage follow-up analysis (two case study sites were pre-selected and under way as the pilot survey was being conducted).
Stage 1: pilot study
For PLICS use in the acute sector we identified 11 hypotheses (listed below), drawing from the initial literature review undertaken for the proposal. We piloted our survey to 35 NHS provider trusts in England, with organisations targeted from each of the 10 (former) strategic health authority (SHA) geographical regions. The questionnaire was distributed online by e-mail to either Directors of Finance personally, or to their offices via administrative contacts. The questions in the pilot survey were based on our ‘hypotheses about PLICS use’:
In relation to research question 1 (How are NHS trusts and commissioners using PLICSs?), the pilot survey to providers included 11 hypothesis-based questions on the possible uses for PLICSs:
-
to identify how much a particular patient costs using direct and attributed costs
-
to ascertain whether that cost was more or less than income received under the tariff
-
to engage clinicians with costing issues
-
to identify resource variation and, hence, cost between consultants
-
to inform consultants as to how their decisions impact on cost
-
to reduce LoS when this impacts upon cost
-
to understand the relationship between cost and quality
-
to understand the benefit achieved through BPT
-
to lobby for exemption from the tariff (or flexibilities under the tariff)
-
to prepare a business case for investment in the specialty
-
prepare for the newer environment encompassing ‘any qualified provider’.
From a NHS economy perspective, PLICSs should inform clinician, management or commissioner decisions on service redesign and referral management. This was the reasoning behind our second research question, ‘How can PLICSs be used to benefit the total health economy (focusing on cost improvement, resource allocation across services and settings, linking cost with quality, and clinical engagement)?’. For the pilot study, we defined a local health economy group as one ‘that includes commissioner and provider representatives and/or multiple other partner relationships, within a specific locality, either as part of a one-off project or longer term initiative’.
We included five hypothesis-based questions about the possible uses of PLICSs in local health economies with a further four hypothesis-based questions about the use of PLICSs in dialogue between acute trusts and commissioners (again our hypotheses were derived from existing literature):
-
Has service redesign for integration, including moving services to primary care when cost-effective and in line with patient preferences, been undertaken?
-
Are services between acute trusts shared to create centres of excellence?
-
Can unnecessary admissions and referrals (false positives) to acute care be reduced?
-
Can unnecessary diagnostics, interventions and treatments be reduced?
-
Can LoS be reduced, when appropriate, while maintaining high-quality care?
We also asked about the extent to which PLICS data are used in dialogue between acute trusts and commissioners:
-
Do you belong to a local health economy group?
-
If yes, do you discuss and analyse PLICS data in this group?
-
-
Have you redesigned services and estimated/calculated savings on the basis of PLICS data?
-
Has service redesign incorporated patient preferences or been prompted by patient preferences?
However, the pilot survey results and initial interviews at the case study sites showed that the extent to which PLICS data are shared across different providers, especially between providers and commissioners, is very limited. Specifically, as reported in Pilot survey results, none of the pilot survey respondents answered the nine questions about the use of PLICSs between providers, or between providers and commissioners – indicating that these questions did not apply to them or did not make sense in the context.
Pilot survey results
There were a total of 11 respondents to the pilot survey, which equates to a response rate of 22.9%. This response rate is close to the average of 24.8% for most online surveys. 162 Most respondents took between 2 and 3 minutes to answer 20 questions in total. We performed analyses of the pilot results and triangulated with data from the initial interviews at the pre-selected case study sites. With regard to the first 11 questions, pilot results chimed with actors’ accounts and provided evidence to develop the main survey prior to full distribution. For example, in line with interview accounts from finance professionals in the pre-selected case studies, almost all of the trusts replying to the pilot survey were using PLICSs. Also, despite more than half of trusts confirming that they were considering using PLICSs to understand the relationship between cost and quality, none was actively doing so.
The trusts also reported limited activity in terms of membership of a local health economy group. We asked in the pilot survey if trusts belonged to a local health economy group, if PLICS data were discussed and analysed as part of this group and if savings had been calculated or services redesigned on the basis of PLICS data. Around half of the pilot survey respondents confirmed membership of a local health economy group; however, none of the respondents replied positively in terms of discussing PLICS data as part of this group. There was, consequently, no evidence in the pilot survey responses that PLICS data were being discussed or used in any context across local health economies.
Stage 2: main study
The 11 provider survey questions asked in the pilot survey had proved robust; therefore, we included them in the main survey.
Changes to the main survey on the use of patient-level information and costing systems in acute trusts, consequent on the pilot and team discussion
In preparation for the main survey we sense-checked the pilot survey design and analysis with practitioner team members and also with finance professionals at one of the pre-selected case study sites. We were advised that (1) respondents would be willing to undertake a survey taking 10 minutes (the pilot had taken only 2–3 minutes); (2) respondents may not understand the wording of some of the questions; and (3) further questions should be included. Accordingly, first, we adapted the wording of some of our initial pilot survey questions to accord with current health finance usage. Specifically, we adapted the wording of six questions from the pilot survey (the wording of five stayed the same). Second, we increased the number of questions by adding a further 16, including, for example, questions about how PLICS information is shared and used, both internally and externally; whether or not provider trusts regarded PLICS data as commercially sensitive; and, on a scale of 1–4, trusts’ level of engagement with clinicians in costing, from limited (e.g. board level only: level 1) to advanced (e.g. routine joined-up clinical/finance working across all specialties: level 4) (see Appendix 2 for full main survey questionnaire).
In response to advice from Claire Yarwood, the commissioner team member, the main survey questions on local health economy group activity were redesigned into a four-part matrix. Respondents were asked (1) if they belonged to a group (that discussed finance and costing); (2) if this group was within their own trust; (3) if this group was part of a local health economy of providers and commissioners; and (4) if this group covered a larger geographical footprint [e.g. a National Benchmarking Group (NBG) – see Exploratory observational work]. This reframing of questions relating to local health economy group membership (to include group meetings that were internal to trusts and those that were external) sought to address the disappointing response to local health economy-based questions in the previous pilot survey. Finally, in the main survey, we asked if PLICS data had been used or influenced decision-making in any of the groups selected, with options to indicate the appropriate group.
The full main survey analysis is discussed in Chapter 4.
Stage 3: further case study selection and initial analysis
In our third stage of work package 1, the main survey was analysed, additional interviews were conducted and findings were validated to inform the next phase of the research.
Further case study selection and follow-up interviews
After analysing the results of the main survey we selected the two further case studies on the basis of leading-edge practice with PLICSs, particularly in relation to our four aims, and our four tracer groups (stroke; mastectomy; blood-related cancers; fractured neck of femur – see Table 2). We drew on the expertise of our practitioner team members to guide and confirm our choices of case study selection. To confirm (or question) that our selection was appropriate, we conducted additional interviews with the FD or cost accountant at the two provisionally selected trusts. These interviews confirmed that our choices were appropriate.
Broad survey findings
The main survey data analysis (see Chapter 4) further supported the finding that PLICS data were not being freely used between different providers or between providers and commissioners. However, there were instances of data sharing between networks of providers as part of a NBG (see Exploratory observational work).
Risk assessment and mitigation
As part of the project management strategy, all project tasks, milestones and related activity were tracked using a green–amber–red document. The team used this tool to identify and prioritise tasks according to their status as ‘completed’ (green), ‘incomplete but achievable through current design’ (amber) and ‘incomplete with significant challenges identified’ (red). The document was employed during project meetings and updated iteratively.
Risk in relation to work package 1
The original research proposal risk assessment in relation to work package 1 was as reported below.
Our risk assessment is based on three strategies to reduce a potential poor response rate:
First, Dr Mahmood Adil (a research team member) is currently working at the Department of Health. He contributed to this year’s (2011) ‘DH Reference Cost Survey’, which includes questions on PLICS. This will facilitate access to the e-mail list of the trusts who responded positively to this (and the similar 2010 DH survey) enabling us to use a targeted approach. It is evident from these two surveys that the response rate has increased (from 80% to 85%) among over 400 NHS organisations indicating a high level of interest in PLICS. Hence, we hope to achieve a comparable response level. We are seeking more sophisticated data than was gathered by the DH but we will use the same list to achieve high response as well as depth of information from the relevant individuals/organisations. In line with the affiliations of our practitioner team members, we will seek approval from the Healthcare Financial Management Association to badge our survey with their logo along with those of the Universities of Manchester and Bristol. We anticipate that this badging will increase interest and, hence, response.
Second, we have already selected two of the case study sites (as described below and in the original proposal) so we are not wholly reliant on the survey for case site selection. We will commence work on these two sites at the same time as we undertake the survey, thus employing an element of parallel working in our fundamentally sequential (quantitative followed by qualitative) research design. This will be enabled by the project manager working with the PI on the logistics of the survey whilst the research fellow and other team members work on the already selected case study sites.
Third, we will be guided by the pilot in terms of the impact of the number of questions (i.e. we will reduce the number of questions if this proves to be an impediment). We will also follow up rapidly on any non-responding Trusts, using if necessary the trust finance and accounting contacts of Dr Adil and Mr Whitfield (another team member, FD at Salford and leading PLICS practitioner).
Actual risk contingencies in relation to work package 1
First, we surveyed the whole population of NHS provider trusts rather than targeting only the respondents to the previous 2011 DH reference cost survey. The pilot results indicated that the majority of acute trusts were using PLICSs and, therefore, a whole-population survey was more appropriate.
Second, the decision to badge and distribute the main survey through contacts made with the HFMA was revised after the first wave of distribution. Our main contact at HFMA, Steve Brown, agreed to publish our notice to HFMA members about the survey and implemented the first distribution of the survey to this group. Unfortunately, the response rate was disappointing, at < 10%. This was possibly attributable to the high volume of HFMA e-mails to members on a range of different health-care costing issues. Therefore, we subsequently embarked upon our own full-scale distribution of the main survey directly from the University of Manchester to include all provider trusts in England. We conducted three additional follow-up e-mails to all recipients; the final main survey response rate percentage was 45.8%.
As indicated above, the parallel working on the main survey and pre-selected case study sites was successful and supported our completion of project milestones in line with the original proposed timeline. Furthermore, analysing the pilot survey results in parallel with interviews at the first two case study sites informed the decision on the main survey length.
Our commissioner team member (Claire Yarwood) and our external Project Advisory Group (see Appendix 1) indicated that, to their knowledge, currently commissioners did not use PLICS data. To further investigate this issue, we also interviewed six commissioners in particular roles (e.g. a commissioner programme director, a commissioner director of quality and provider management, a commissioner strategic health analyst and a commissioner acting director of finance). These interviews confirmed that PLICS data were not being used. However, our sources thought PLICS data to be potentially very relevant for redesigning care and better integration across the care pathway.
The above commissioners also reflected on the impact of the Health and Social Care Act 2012 and subsequent NHS reorganisation. These commissioner accounts highlighted that all commissioning organisations in England were restructuring, with significant changes in personnel and accountability. Several commissioners confirmed that newly formed CSUs were providing business intelligence services to CCGs and would be more likely than commissioners to be aware of the potential for PLICSs in commissioning decisions. Therefore, to find out about the potential of PLICS data for commissioners, managing directors of CSUs were targeted as survey respondents. The results from this CSU survey confirmed that PLICS data were not being shared between providers and commissioners, but that commissioners recognised the future potential value of access to PLICS data (see Chapter 4 and Appendix 3 for the CSU survey questions).
Work package 2: case studies
In the second phase of the project, we undertook four case studies at different geographical regions in England. Two of the case studies were pre-selected. The first pre-chosen site, case study 2, was well advanced in its use of PLICSs and was therefore selected because of its leading-edge implementation. The second pre-selected site, case study 4, was in the planning stages of implementing PLICSs and developing expertise in PLICS use. For research question 2 on the use of PLICSs for the whole health economy, we were interested in a particular pathway at case study 4, which crossed organisational boundaries between the acute trust and community services.
The remaining two case study sites were chosen through survey data analysis. They both reported level 4 clinical and financial collaboration, which meant that they self-identified as having ongoing collaborative engagement between finance and clinical colleagues. In addition, case study 1 was chosen for its reported involvement in clinical costing projects and because the trust is a long-term user of PLICSs. A key respondent at case study 1 conveyed to us how different PLICS use is between a large acute provider (similar to his organisation) and a more specialist trust (such as case study 3). We therefore pursued this line of enquiry, selecting case study 3 (from the possible level 4 engagement sites) because this site was a specialist trust with well-developed PLICSs. In addition, both case study 1 and case study 3 reported membership of a NBG in their survey response.
Case study site settings and tracer conditions
The development of PLICSs was locally specific across all four case study sites. Within each setting, the awareness and use of PLICS data were different; there was also differential interest across specialties. To capture the different contexts of use for PLICS data between specialties, the team identified a number of potential tracer conditions in the original proposal. These ‘tracers’ were revisited and selection was confirmed following the initial interview process. For the pre-selected sites, we continued to collect data on the ‘stroke’ (case study 2) and ‘frail elderly: fractured neck of femur’ (case study 4) pathways, focusing on specific diagnostic codes for each condition. The selection of the tracer ‘mastectomy’ at case study 1 followed initial interviewing and documentary analysis that demonstrated the use of PLICS data for development of this treatment pathway. Similarly, for case study 3 the tracer condition ‘blood-related cancers’ was chosen after documents were collated, identifying a role for PLICS data in decisions about service redesign in this area. For further information about documentary evidence gathered at each site see Table 1.
Data collection
For each of the four case study sites, a senior team member took the lead role for data collection, with support from either the research fellow or research project manager (see Table 2). We identified a minimum of 10 interviewees at each site. After follow-ups and supplementary work, the team conducted 45 interviews over the four case studies. Additionally, as indicated previously, several commissioners (n = 6) were interviewed, as well as three provider finance managers, who discussed their work with a NBG, and one clinician with a strong interest in PLICSs. The total number of interviewees for the study was 54 (see Chapters 5–8 for case study interviews and Appendix 4 for all additional interviewees). All interviews were audio-recorded and transcribed as Microsoft Word® 2010 (Microsoft Corporation, Redmond, WA, USA) documents, except in one instance in which consent to audio-record was withheld and hand-written notes taken instead.
The first point of contact at each site was usually the director of finance and his or her office. In parallel to interviewing FDs, cost accountants, and clinical costing and information technology specialists, the medical director (MD) at each site was invited for interview. If appropriate, the CEO was also interviewed. The case study lead at each site decided on certain specialties for further investigation, drawing on initial interview data analysis and evidence gathered in preparation for the study (e.g. PLICSs-related articles published in professional health finance journals and magazines). At all four case study sites, clinical directors with decision-making responsibilities were approached for interview. For a number of clinical directorates in each trust, we also interviewed business or general managers. The team also targeted various specific job roles within each case study that were relevant for PLICSs implementation, including, for example, the quality improvement lead, service improvement manager and PLICSs/SLR project manager (Table 2 shows case study details; see Chapters 5–8 for case study interview schedules).
| Case study (pseudonym) | Lead | Support | Tracer | Number of interviews |
|---|---|---|---|---|
| Gertrude | Professor Naomi Chambers | Chris Wood (Research Project Manager) | Mastectomy | 12 |
| Sybil | Professor Sue Llewellyn | Chris Wood | Stroke | 11 |
| Chelsea | Professor Sue Llewellyn | Christos Begkos (Research Fellow) | Blood-related cancers | 11 |
| St Winifred’s | Professor Sheila Ellwood | Christos Begkos | Frail elderly: fractured neck of femur | 11 |
In reporting our case study findings, the chapter ordering is as follows: we have presented Gertrude first, followed by Sybil, because both trusts are large acute providers with integrated community services, and both have significant research and teaching portfolios. Chelsea (see Chapter 7), as indicated, is a specialist provider with substantial funding for research and development in specific treatment areas. All three of these trusts have well-established PLICSs reporting structures and use PLICSs strategically to manage their organisations. The organisation that is the subject of the final case study presented, St Winifred’s, is a new implementer of PLICSs and has undertaken several procurement and clinical engagement projects, in relation to adopting PLICSs, during the course of the study. St Winifred’s is also involved in pertinent (to our research questions) cross-organisational work between the acute trust and local community services, with the potential for PLICS data to underpin the development of that interorganisational pathway (see Chapter 8).
Exploratory observational work
The team undertook exploratory observational work in two areas: the PLICSs implementation process at St Winifred’s and at a NBG.
St Winifred’s
The case study lead at St Winifred’s employed exploratory observational work to track the PLICS implementation process. As an invited member of the project board for PLICSs implementation, Professor Sheila Ellwood observed project meetings and analysed the papers and reports under discussion (see Table 1 for documentation gathered). Utilising this level of access within the trust, the project gathered data on, for example, how processes were designed to select appropriate PLICSs, and the methods devised for engaging clinicians in this process.
National Benchmarking Group
Around one-third of NHS trusts in England belong to a NBG in which PLICS data are shared. A benchmarking group was named in several responses to the main survey. We were introduced to the group at the Gertrude site and benchmarking was a theme at case study interviews. We decided to conduct exploratory observational work with this benchmarking group. Two team members attended the group’s annual meeting of around 70 provider organisations. The hosting software organisation requested consent from all benchmarking group members for our team to be present during their annual meeting. Notes were taken at this meeting. The main topic for group members was issues about data comparability to ensure accurate benchmarking. Three further follow-up meetings were undertaken with individual trust members of the benchmarking group. The notes taken during these meetings concerned local and one-off projects through which financial information was being used in the trusts to develop or improve service line performance, for example the improvement of clinical coding and reference cost returns. This initial exploratory work informed our approach to the questions on benchmarking when conducting interviews at Gertrude and Chelsea.
Data analysis
Work package 1
The data analysis for the survey focused on the use, and potential use, of PLICSs to further inform the interview questions over and above our hypotheses from the literature. The online survey software Qualtrics (Qualtrics Insight Platform, Provo, UT, USA) was used for the design and distribution of the survey, to monitor and follow up responses, and to perform initial analyses on the data set. The automated response rate calculator and descriptive statistical tools in Qualtrics enabled the team to undertake analyses while the survey remained open for new responses, enabling the case study leads to draw on the survey findings to develop interview questions during the second work package data collection phase.
To conduct the analysis of the complete survey data set all raw data were extracted from Qualtrics into Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) as comma-separated value (CSV) files. The research fellow first exported these files into the SPSS software package, version 22 (IBM SPSS Statistics, New York, NY, USA). Using this package, the team conducted non-response bias analysis136 by comparing respondents with non-respondents using a number of known characteristics for the population of trusts (see Chapter 4). We decided to use SPSS instead of Qualtrics for the final analysis of the survey data because the process of exporting the raw data into Microsoft Excel CSV files allowed the research team to manually check the automated descriptive statistics produced in Qualtrics. The comprehensive numerical databases created and verified manually in Microsoft Excel were later used to produce all graphs and tables presented in the survey chapter.
Work package 2
The interview transcripts for all four case study sites were collated and reviewed by each case study lead. A sample of six transcripts was analysed through an initial hand-coding exercise, in which content was organised into themes. Using this method, the team generated a draft theme code template (see Appendix 5). This template was an iterative document, allowing the team flexibility to develop the coding exercise as each new transcript was analysed. This collaborative approach to analysis proved successful and the team proceeded to hand code all case study transcripts to complete a first pass of the interview data set. This preparatory work on theme coding enabled two researchers to review each transcript; specifically, the case study lead at each site distributed their transcript analysis to another lead (who was not present at the interview) for comment. Once all hand-coding was completed, the research fellow imported this data set into the qualitative data analysis software package NVivo, version 10 (QSR International, Warrington, UK). All documents, secondary data and interview transcripts were imported to the software and were categorised according to case study site. NVivo was used to generate codes, segment text and filter the data, and to describe, label and group together different themes. By using qualitative software, the core team enhanced our ability to ‘sort, sift, search and think through the identifiable patterns as well as idiosyncrasies [in our data set]’. 163
The reliability of coding methods was strengthened by employing recommendations from Gibbs;164 the team routinely cross-checked for coding drift and used independently derived results to confirm coding accuracy (i.e. team members shared their analyses as a group to verify coding criteria and amend the code theme template to ensure consistency between researchers).
Patient and public involvement
In the initial months of the project, the North West Patient and Public Involvement (PPI) forum assisted us in advertising for potential PPI representatives for the project. We received one response to the PPI advert but unfortunately, because of ill health, this person was unable to attend the first Project Advisory Group meeting. Fortunately, a colleague (Bill Burgoine) of the person with ill health was available and was invited to attend instead. He attended each of the Project Advisory Group meetings, and provided feedback and comments at several stages of the research design and report write-up. With a background in information technology system development, Bill Burgoine quickly understood the concept of PLICSs and the potential value to the health service. As an active member of his local community (e.g. he sits on the local Healthwatch committee as a patient representative), Bill Burgoine contributed significantly during Project Advisory Group meetings. The team has taken guidance from him in terms of writing with a broad, non-accounting audience in mind, especially when our chapter content was dealing with more technical aspects of accounting literature.
Research site ethical approvals
The study did not require registration with the NHS National Research Ethics Service (NRES) according to section 2.3.2 of the NRES research governance guidance amendment. The guidance states that, as of 1 September 2011, all studies focusing on management, including NHS staff, are exempt from the full NRES application process. However, we sought and secured local research and development office approval for site access at each of the four case study sites.
Table 3 summarises our dissemination activity to date in accordance with that planned in our original proposal.
| Output type | Date | Title | Organisation/venue | Planned/completed |
|---|---|---|---|---|
| Press release | September 2012 | Study to examine PLICSs potential | HFMA | Completed |
| Press release | May 2013 | Feature news item in Healthcare Finance | HFMA | Completed |
| Conference abstract | June 2013 | Patient-level costing and information systems (PLICS): does PLICS enable cost savings, service integration and better understanding of cost drivers in healthcare systems? | European Health Management Association | Completed |
| Presentation | January 2014 | Patient-level costing and information systems (PLICS): current use and future potential | HFMA Annual Conference (learning laboratory) | Completed |
| Social media | May 2014 | Open up on costs to improve NHS care | Policy@Manchester | Completed |
| Feature article | May 2014 | Open book accounting can lead to a more honest NHS | Health Service Journal | Completed |
| Feature article | January 2015 | Achieving the benefits of patient-level costing – open book or can’t look? | Public Money and Management | Completed |
| Academic/practitioner workshop | December 2015 | TBC | Project team final academic/practitioner workshop | Planned: invitations sent out |
| Conference presentation | April 2016 | TBC | Institute for Health Improvement (Gothenburg, Sweden) | Planned: abstract submitted |
Chapter 4 Patient-level information and costing systems national survey
Introduction
This chapter presents the empirical findings from the study’s main survey to all provider organisations. Although the DH has recently conducted surveys assessing the extent of PLICSs implementation among providers (in 2012 and 2013),165 this survey explores how PLICSs are being used by finance staff and clinicians rather than focusing only on whether or not a PLICS is implemented. Therefore, the survey’s findings relate to the study’s first research question, which explores how NHS trusts and commissioners are using PLICSs. As explained in Chapter 3, we found through several sources that commissioners do not have access to PLICS data. To explore the potential for PLICSs in the context of commissioning, we were advised to survey CSUs rather than commissioners (see Chapter 3 for an explanation). The results of the CSUs survey are analysed in Commissioning support units’ use of patient-level information and costing systems.
The survey’s questions were informed by the study’s research objectives. The team’s three practitioner members advised on the survey’s language to ensure that it was understandable to practitioners. A pilot was initially distributed to 35 provider organisations, and the final survey was also tested with two finance professionals at the Sybil case study site. The main distribution followed Sue and Ritter’s160 recommendations (see Chapter 3).
The online survey was distributed to the FDs of 242 NHS providers via e-mail. The HFMA provided the original mailing list, which was extended by team members to cover the whole population of trusts. The survey ran for 3 months and was closed in February 2013, after three follow-up e-mails to all recipients. The survey had 111 total responses and a response rate of 45.8%. Responses were reviewed individually, and both an Excel and a SPSS database were created. The response data were cleaned and 12 responses were excluded due to partial or duplicate answers, leading to a final response rate of 40.9%.
Non-response bias was tested, using Pearson’s chi-squared goodness-of-fit measure. Respondents were compared with non-respondents using a number of known characteristics for the population of trusts. Therefore, the analysis focused on the type of trust (acute, mental health or community), on the former SHA geographical location of the trust and on FT status. No significant differences were identified between respondents and non-respondents for the type of trust criterion. No bias was found in 9 out of 10 SHA geographical locations, except for the north-west SHA variable. Further analysis compared PLICSs implementation only among north-west trusts, finding no significant differences between respondents and non-respondents. This finding suggests that north-west trusts had a higher uptake in PLICSs and were more likely to respond to the survey. Finally, the very high response rate from FTs caused bias in the FT status variable. However, Monitor strongly encourages FTs to implement patient-level costing systems; therefore, FTs were more likely to answer the PLICSs survey. Hence, the non-response bias analysis indicates an overall good fit.
The following section describes the results of the PLICS national survey to provider organisations, focusing on the use of PLICSs and its future potential. It explores providers’ ownership of PLICSs, how providers use PLICSs (such as connecting cost with quality and for year-of-care costing purposes) and how they share and discuss PLICS data. The chapter continues by exploring CSUs’ awareness and use of PLICSs. The chapter then concludes with a brief summary of the survey findings.
Patient-level information and costing systems ownership in provider organisations
The survey first explored whether or not trusts were implementing PLICSs and producing service line reports. Approximately 64% of the respondents indicated that, as of 2013, their organisations were currently using a PLICS. Evidence also suggested that PLICS implementation is gaining momentum, with 45% of the respondents not using a PLICS indicating that it was likely or very likely that a PLICS would be implemented in the 12 months following the date of the survey response (February 2013) (Figure 5).
FIGURE 5.
Main survey: PLICSs implementation.
Moreover, as illustrated in Figure 6, 88% of respondents indicated that their trusts already produced service line reports, which measure the profitability of a trust’s service lines. An additional 11% indicated that they were considering producing SLR information within the next 12 months. Therefore, 99% of trusts stated that they were already producing, or were considering producing, SLR information highlighting that SLR systems are the norm among provider organisations. Such a high ownership rate is not surprising, because Monitor strongly encourages FTs to adopt the SLM approach and produce information under the SLR format. Furthermore, 7% of organisations that had PLICSs in place stated that they had used it for over 24 months, while only 27% of them had been using it for < 1 year.
FIGURE 6.
Main survey: producing SLR information.
Most of the organisations using PLICSs stated moderate implementation (Figure 7). Specifically, approximately 56% of respondents indicated moderate implementation, 23% of respondents indicated that PLICSs were fully implemented in clinical costing and reporting schedules at their trusts, while only 19% of respondents suggested that PLICS implementation was still in the early stages (e.g. pilot reports).
FIGURE 7.
Main survey: stages of PLICS implementation.
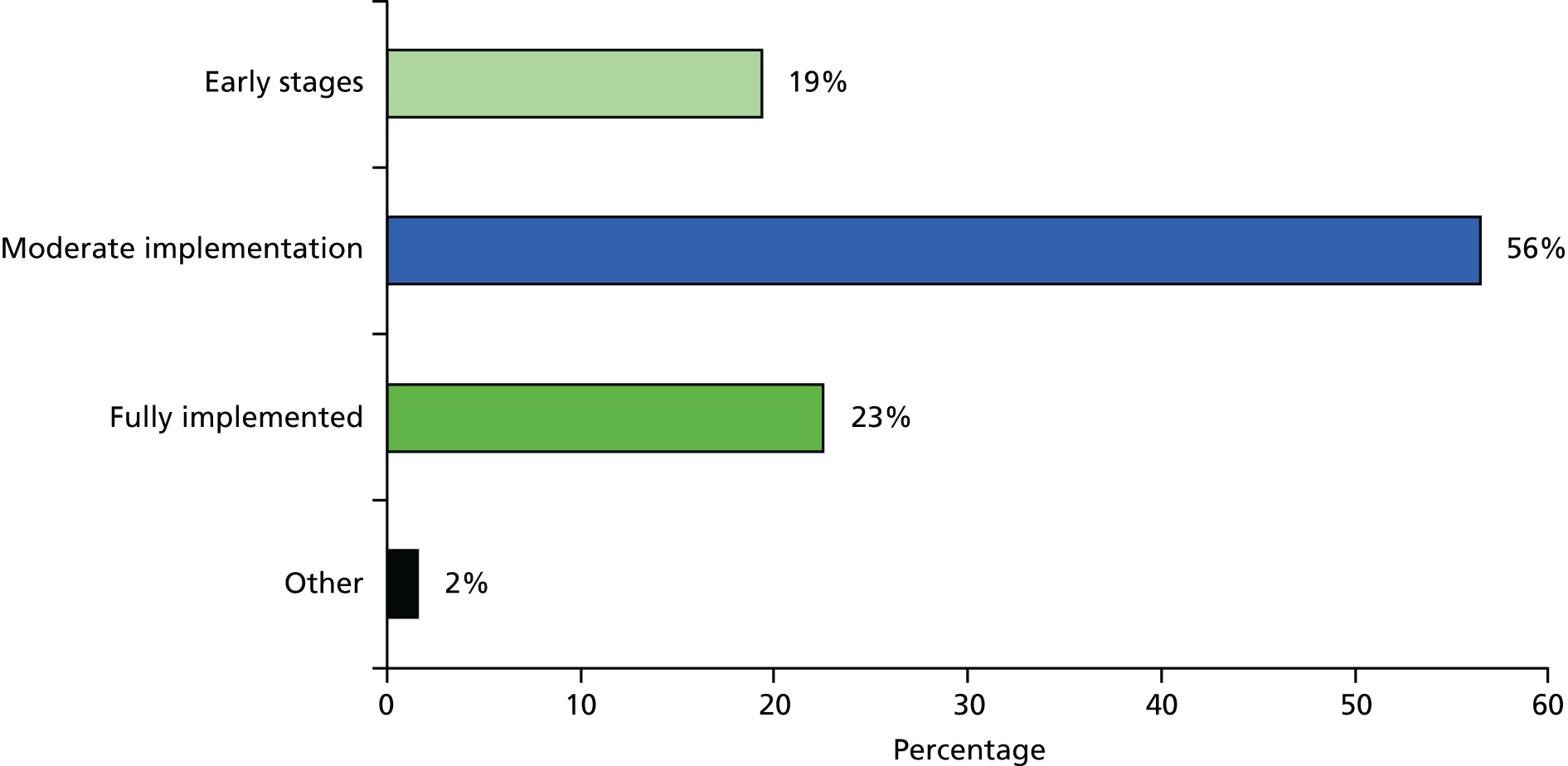
In addition, as indicated in Figure 8, most organisations (58%) using PLICSs stated that they produced PLICS information on a quarterly basis, and 23% reported a monthly frequency. Only a small proportion of respondents indicated producing PLICS information on a biannual or annual basis.
FIGURE 8.
Main survey: frequency of PLICSs reporting.
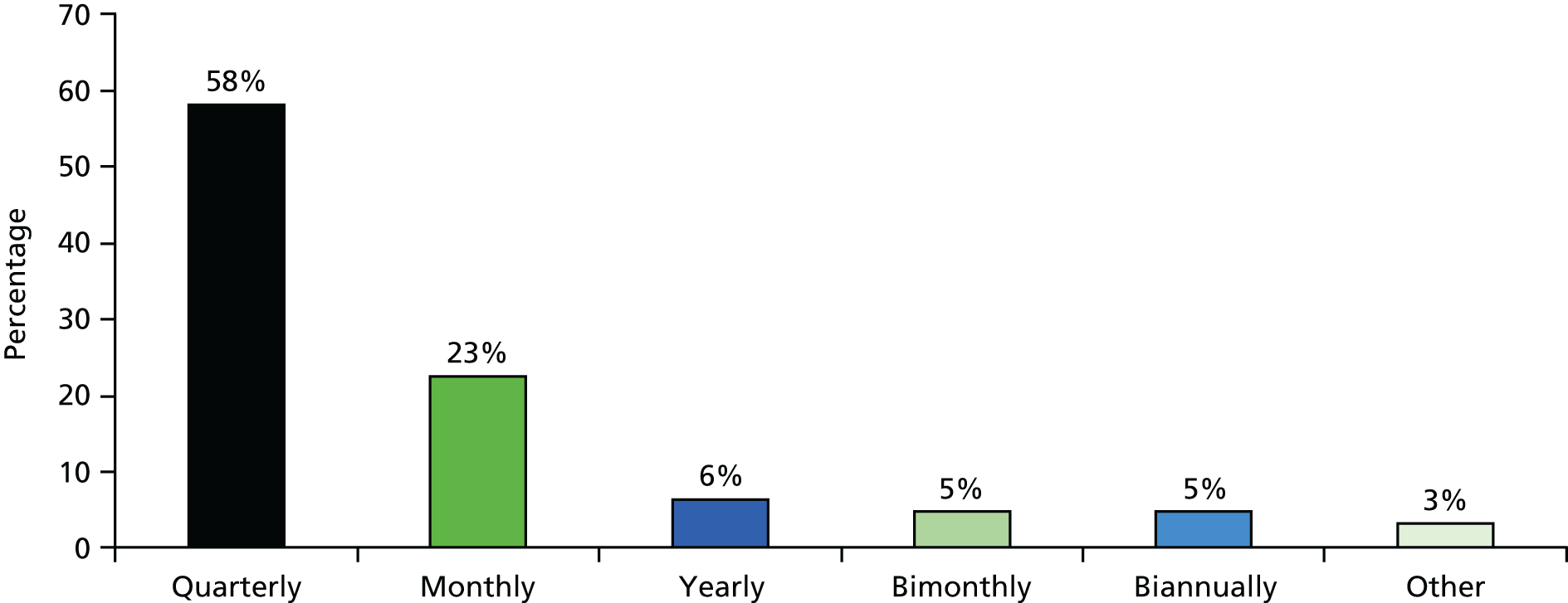
Providers’ use of patient-level information and costing systems in broad medical and support areas
The survey also produced findings on the broad areas in which PLICSs are used. In Figure 9, the results suggest that PLICSs are extensively used to identify costs in a wide range of activities, from wards, operating theatres and medical staff, to radiology, pathology and drugs.
FIGURE 9.
Main survey: activities and cost classification.
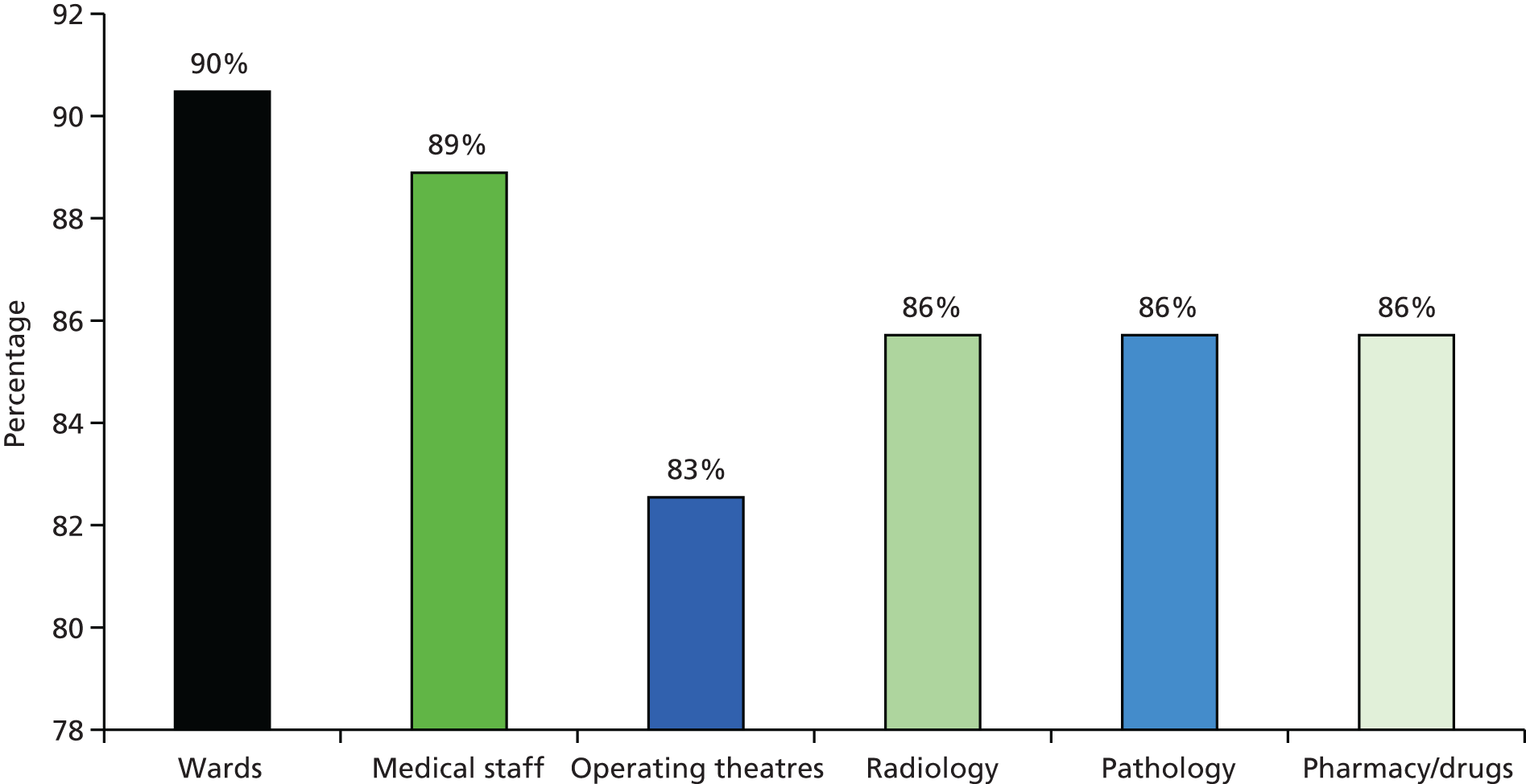
Organisations utilise PLICSs in reference cost exercises. Since the financial year of 1997–8, it has been mandatory for all NHS providers to report their reference costs across designated areas to the DH; by contrast, PLICSs are strongly recommended for FTs, but their status remains optional at the time of writing. Reference costs refer to the average unit costs of providing NHS-funded services to NHS patients in England. 166 As shown in Figure 10, approximately 74% of trusts reported employing their PLICSs for their reference cost returns, with an additional 16% indicating that they were considering such actions in the 12 months following the date of the survey response (February 2013). Only 10% of trusts that have PLICSs reported not using them for reference cost returns. This finding highlights the organisations’ confidence in the granularity of PLICSs information. Such confidence might prove a precursor to how the tariff will be set in the future. Such findings also agree with Monitor’s report on the 2013–14 patient-level cost collection, which states that approximately 70% of trusts that have implemented PLICSs submitted patient-level costs to Monitor. 34
FIGURE 10.
Main survey: reference cost returns.
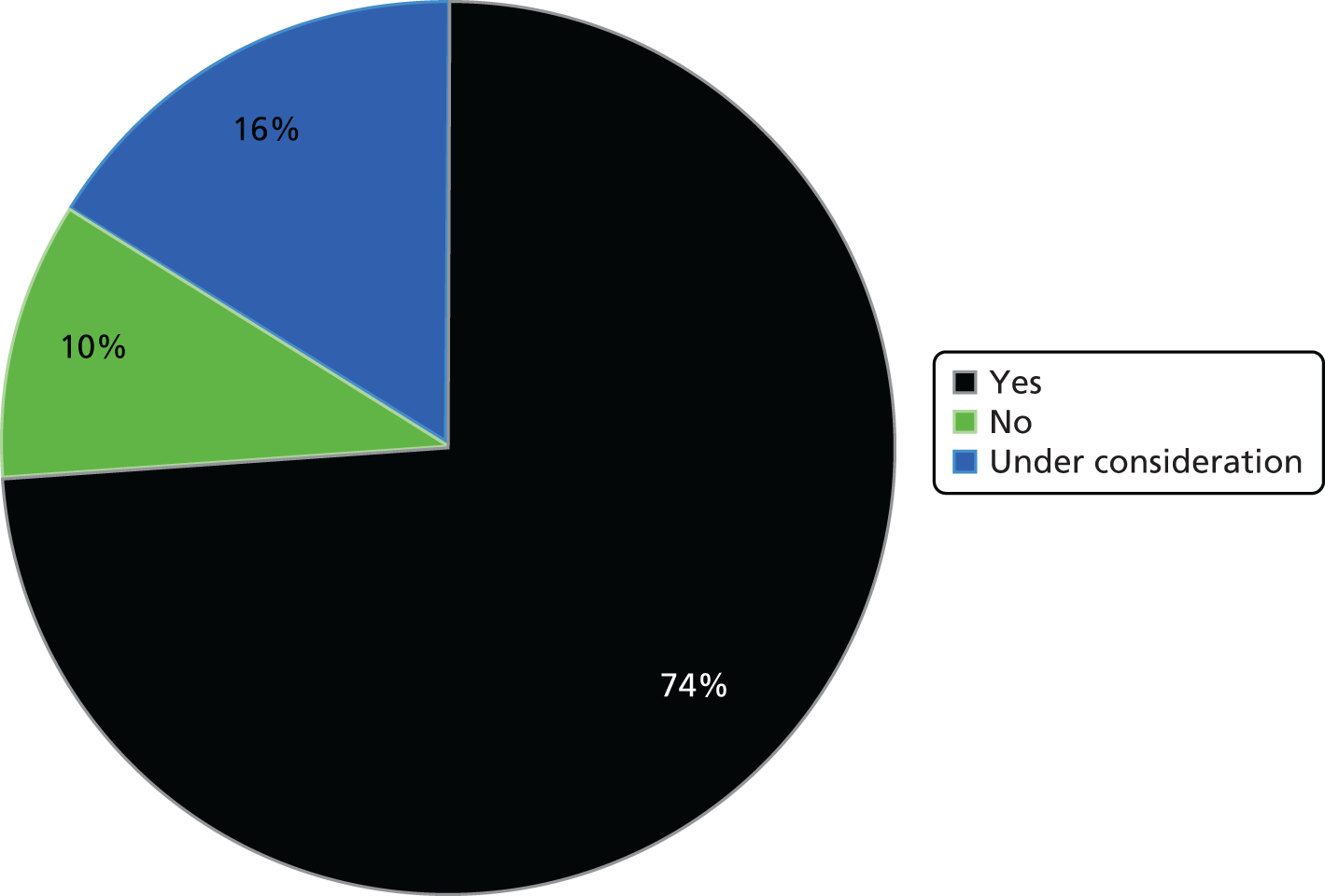
Patient-level information and costing systems use for linking cost with quality
Approximately 80% of trusts reported that their senior management or their board of directors do not use PLICS data to look at the relationship between cost and quality (Figure 11). Respondents indicated that the assessment of quality is difficult to measure, which is directly in line with findings from all four case study sites. However, some respondents in open-ended responses stated that their trusts are moving towards linking cost with quality, as evidenced in the following open-ended responses:
The assessment of quality is a difficult thing to measure, the trust captures a range of quality indicators alongside PLICS costs, and presents them as a balanced scorecard.
Deputy FD of acute trust
We are looking to set up a forum for clinicians and senior managers to look at cost and quality information.
FD of acute trust
We are just beginning to use the data [PLICSs] in projects which are assessing quality.
Head of costing, performance and commissioning of acute trust
A clinical outcome project is currently being developed by the Trust.
Senior finance manager of specialist trust
We are moving towards this [linking PLICSs and quality data].
Senior cost accountant of acute trust
Just finishing implementation so expect to use [PLICSs] with clinical KPIs [Key Performance Indicators] when complete.
Chief finance officer of integrated trust
FIGURE 11.
Main survey: cost and quality.
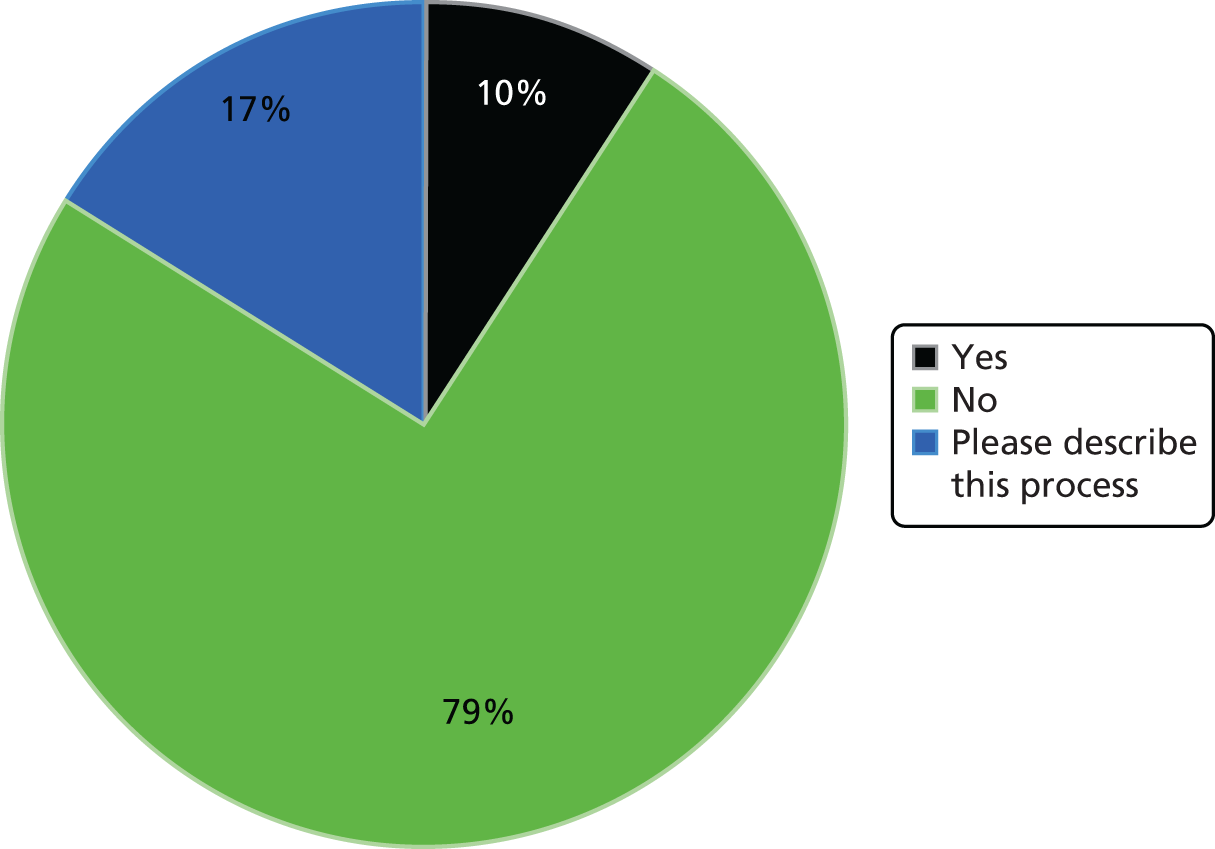
Patient-level information and costing systems use for year-of-care costing
Furthermore, respondents indicated that they do not use PLICSs for year-of care costing. Approximately 60% of respondents stated that their trusts do not collect data or produce reports in a way that would allow costing a patient on the basis of 1 year of care, mostly because of data quality issues and underdeveloped and disparate costing systems (Figure 12). The FD of a community trust remarked in an open-ended response how costing across services and settings is still very underdeveloped.
Costing is still at a relatively embryonic stage. Formally there is no activity and costing link to hospital admissions. NHS culture and structure designed around acute hospital system e.g. incentivising hospitals to detect dementia, when vast majority is in community/ primary care!
FD of community trust
FIGURE 12.
Main survey: year of care.
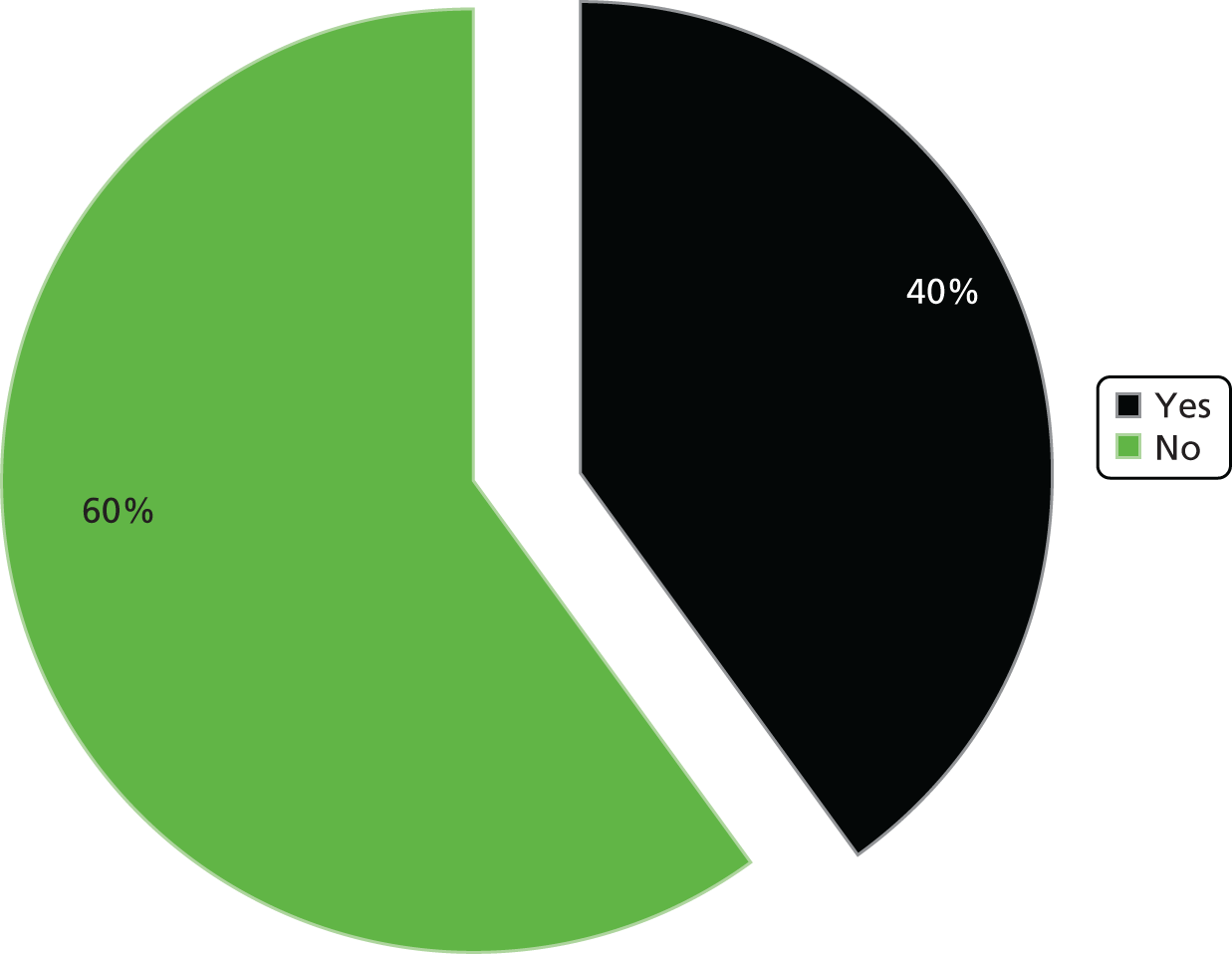
Types of patient-level information and costing systems use
Survey results also suggested that PLICSs are broadly used for clinical engagement and cost-improvement purposes, as well as for comparing within and between trusts. Results indicated that 88% of respondents use PLICSs to engage clinicians with costing issues, while 86% use PLICSs to compare specialties under SLR and to ascertain whether costs are more or less than the income received under the tariff. These are described in Figure 13 as ‘engage clinicians’, ‘compare specialties’ and ‘compare against tariff’. Furthermore, 83% of respondents indicated using PLICSs to identify how much a particular patient costs using direct and attributed costs. Other significant uses of PLICSs, indicated by most of the survey’s respondents, include using PLICSs to identify how LoS impacts upon cost (described as ‘identify LoS’), to prepare business cases (BCs) for investments in a specialty (described as ‘Prepare BCs’), to inform consultants of how their decisions impact on cost (described as ‘inform consultants’) and to benchmark services against other providers (described as ‘benchmark’).
FIGURE 13.
Main survey: types of PLICS use.
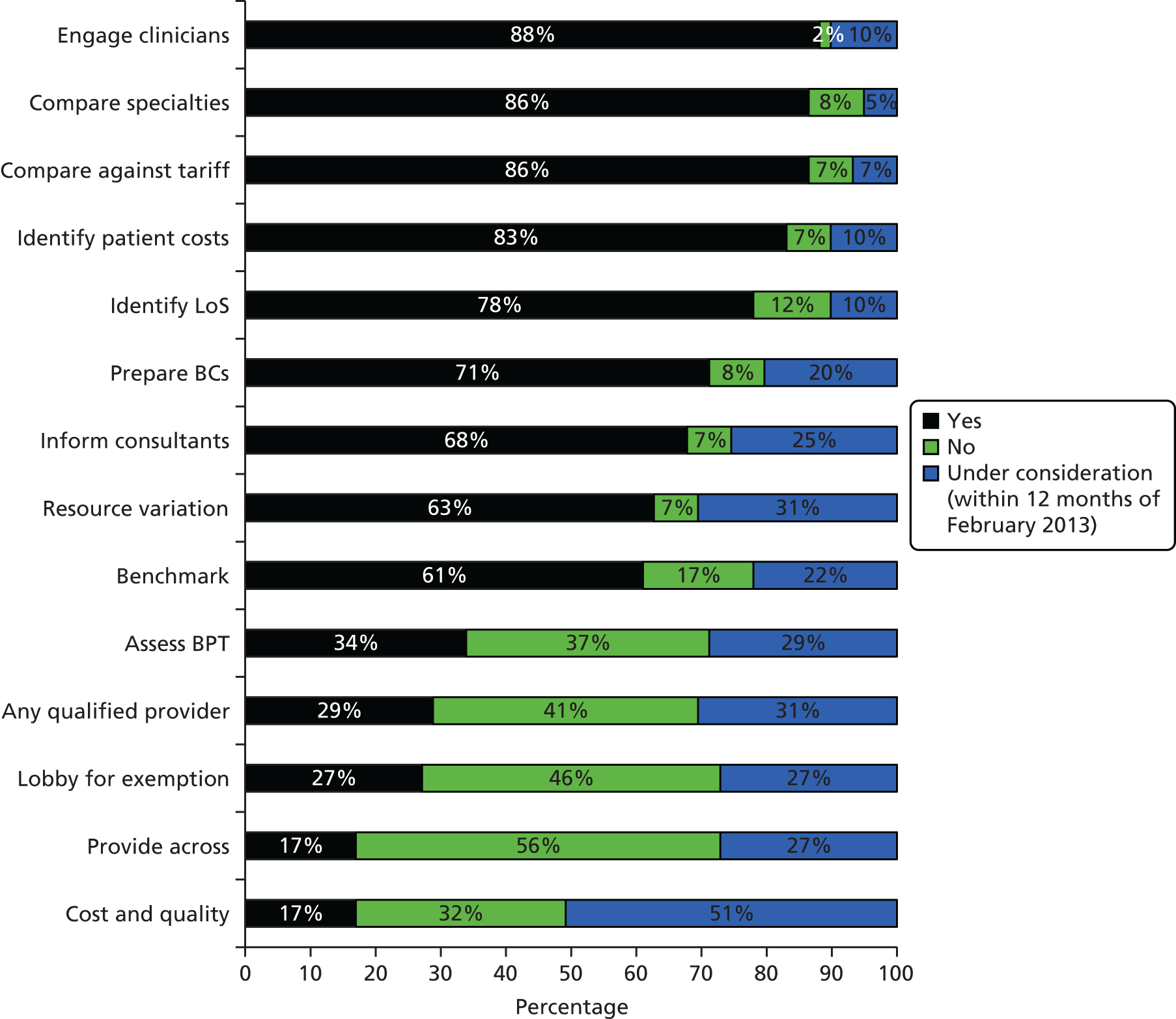
However, results suggested that PLICSs are much less used (see Figure 13) for any qualified provider purposes and for better allocative efficiency of resources across a network of provider organisations. Specifically, only 34% of respondents stated that they used PLICSs to understand the benefit achieved through a BPT, only 29% used PLICSs to prepare for the newer environment encompassing ‘any qualified provider’, only 27% used PLICSs to lobby for exemption from the tariff and 17% used PLICSs to provide services across more than one trust, for example a network of providers or a joint venture (described as ‘provide across’). Furthermore, only 17% of respondents indicated using PLICSs to understand the relationship between cost and quality; however, 51% of respondents were considering such use within the next 12 months.
Sharing and discussing patient-level information and costing systems
The survey also explored the extent to which PLICS data are shared and discussed within and across organisations (Figure 14).
FIGURE 14.
Main survey: sharing of PLICS data.
The results suggest that patient-level information is extensively shared with senior management and clinicians, to some extent with Monitor and the DH, and hardly ever with commissioning and governing bodies. Specifically, 98% and 88% of respondents indicated that their trusts shared PLICS information directly with senior management and clinicians, respectively. However, only 13% of trusts shared PLICS data with the DH and Monitor, and just 5% shared these with commissioners and governors. Although many trusts use PLICSs for their reference cost returns, few of them share PLICS data for other reasons. This aligns with our interview data, which suggest that providers share information only on a ‘need-to-know’ basis and are reluctant to share information on profitable areas with commissioning bodies out of fear of reduced funding.
Furthermore, many respondents indicated that their trusts are members of patient-level costing benchmarking clubs or groups. Such findings highlight that providers are hesitant to share PLICS data outside their organisational borders, unless they belong to a benchmarking group in which confidentiality to members is ensured.
Providers’ hesitancy in sharing patient-level information is also evident in how trusts consider PLICS data to be commercially sensitive. As illustrated in Figure 15, approximately three-quarters of respondents indicated that their trusts regard their PLICS data as commercially sensitive, highlighting that the possibility that using PLICSs for better resource allocation across the health economy is much reduced.
FIGURE 15.
Main survey: commercial sensitivity of PLICS data.
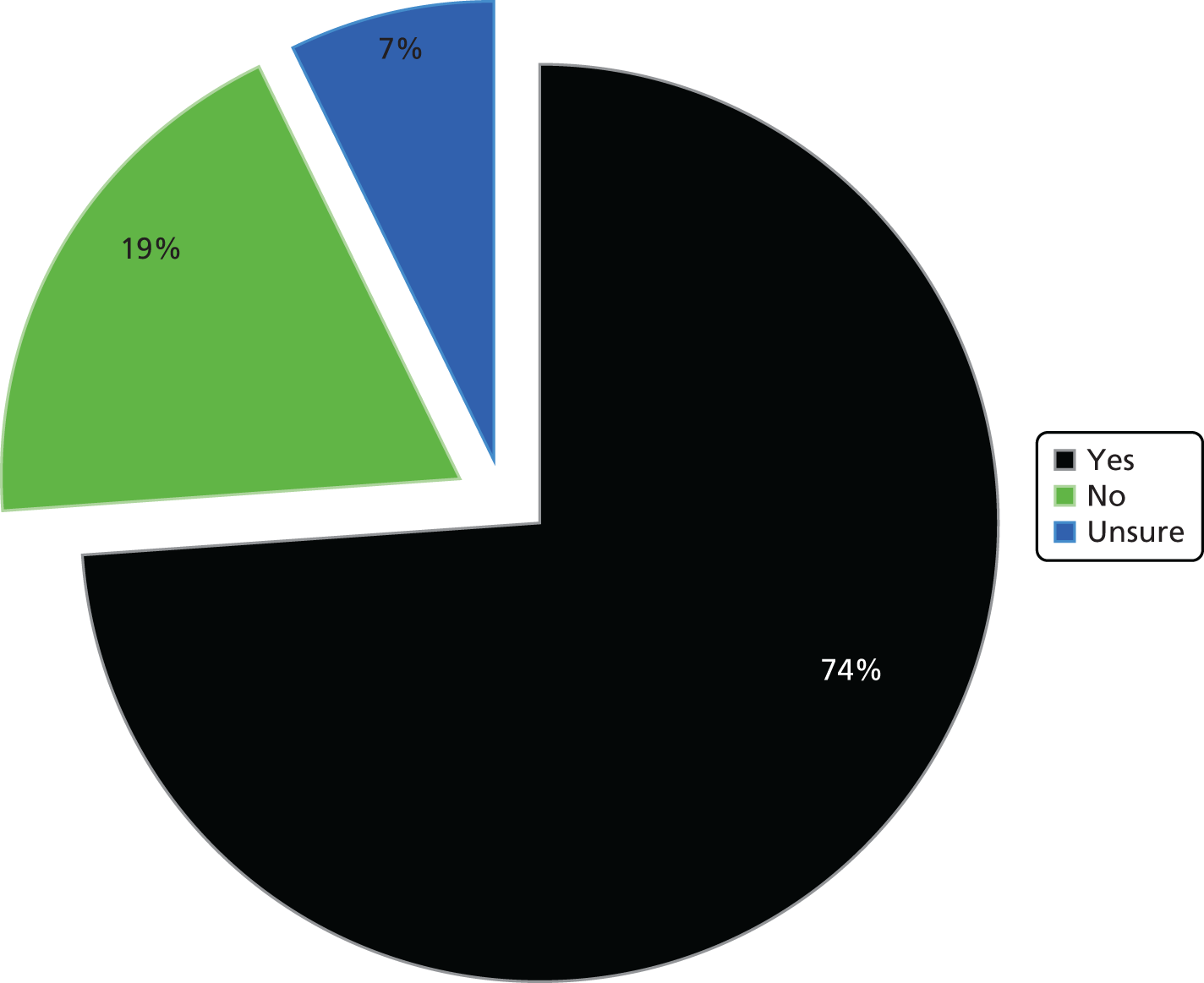
Survey results also showed that PLICS data are moderately discussed within organisations (Figure 16). Specifically, 50% of respondents indicated being a member of a group within their trust that discussed PLICS data, such as a SLR group, PbR/reference cost group or CIP group. Furthermore, 23% of respondents stated that such membership is under consideration within the next 12 months, and only 23% are not members of any group that discusses PLICS data.
FIGURE 16.
Main survey: membership in discussion groups within and across organisations.
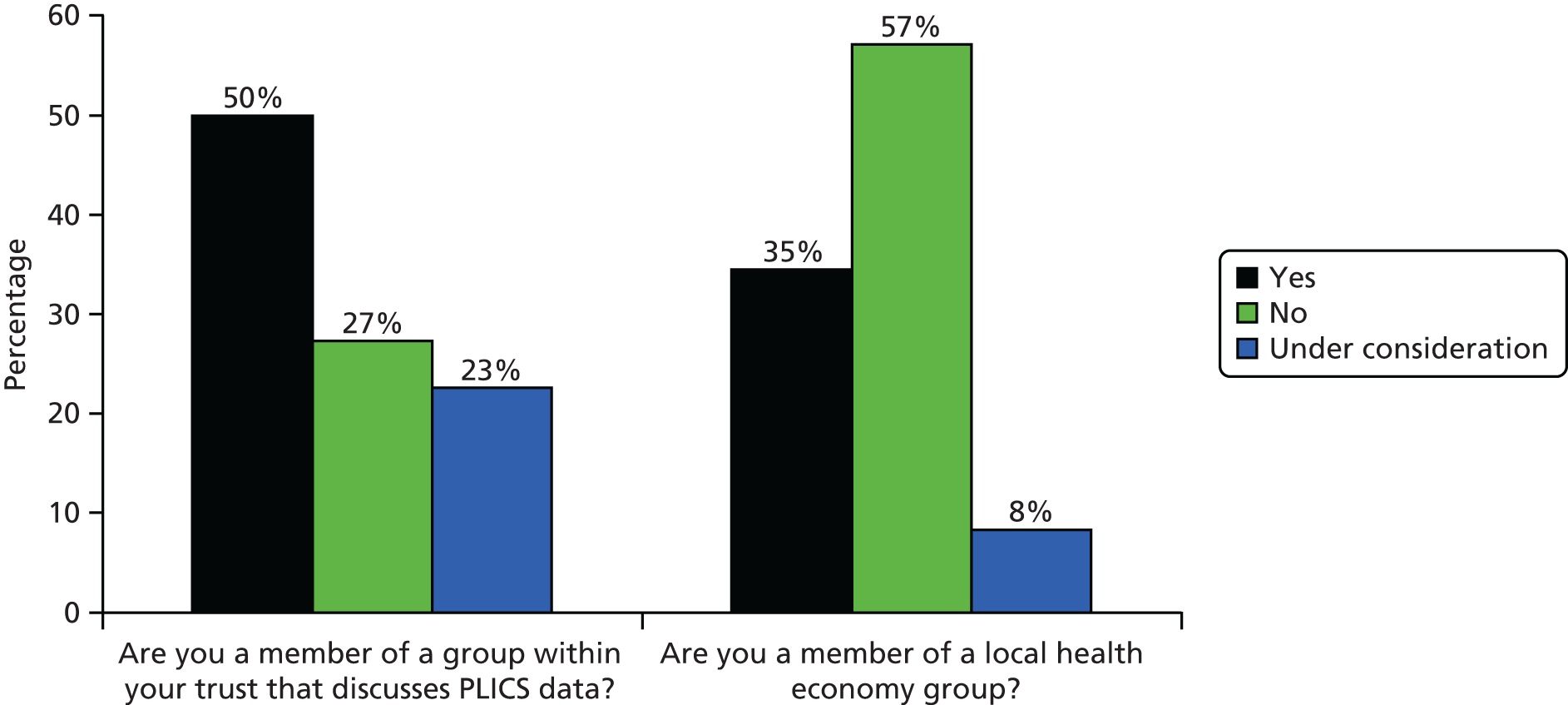
However, such discussions are less evident across organisational borders. Only 35% of respondents indicated being a member of a local health economy group (see Chapter 3 for an explanation of ‘local economy group’), as part of either a one-off project or a longer-term initiative, with only an additional 8% of respondents considering such membership for 12 months after February 2013.
Commissioning support units’ use of patient-level information and costing systems
Originally, we intended to investigate PLICS use through a single survey that would be distributed to both providers and commissioners. However, the commissioning landscape was changed considerably because of the Health and Social Care Act 2012,26 with CCGs assuming formal status on April 2013. Therefore, PLICS use was initially explored through a provider survey and then further explored through a second survey directed to CSUs, following the advice of practitioners and interviews with people in commissioning roles (see Chapter 3 for details).
A CSU survey was designed and a mailing list of all 18 CSU managing directors was created. The survey was dispatched to them and followed up at regular intervals. A total of eight responses were received, three of which were duplicate (answered by the same CSU more than once) and one response was blank. Thus, after cleaning the data, four valid responses remained with a response rate of 22.2%. This was clearly disappointing but could, at least in part, indicate that CSUs had little knowledge or understanding of PLICSs. The survey questionnaire can be found in Appendix 3.
Survey results indicated that 75% of CSU respondents were aware of patient-level costs, 67% of whom were also aware of PLICSs. All of the respondents indicated that ‘they would advise their CCG clients to obtain patient-level costing data from the trusts’. Furthermore, all of the respondents also indicated that ‘they would work with their CCG clients to create a community database of patient-level costing information (e.g. for patients with long-term conditions treated outside of the trusts)’.
The survey also produced findings on how useful patient-level costs would be for respondents’ CCG clients (Figure 17). All of the respondents indicated that if they had access to patient-level costs, then this would prove useful or very useful for comparing costs against the tariff, negotiating the tariff with providers, linking costs with patient outcomes, linking costs with quality, redesigning services and meeting CIP targets.
FIGURE 17.
Commissioning support unit survey: usefulness of patient-level costs for CCG clients.
The low response rate from CSUs makes it difficult to assess PLICSs’ current use for commissioning purposes. However, the results suggest that there is certainly interest and future potential in PLICSs. The low response rate also indicated that a CCG survey would most probably have been unproductive.
Summary
The survey explored how providers use, share and discuss PLICS data. Survey results indicated that SLR systems are more extensively implemented than PLICSs among providers. Such a finding is in line with evidence from the study’s case study sites, which were also more focused on SLR. However, survey findings suggest that PLICSs are widely used across a range of activities.
Similar to case study results, respondents indicated that PLICSs are extensively used for clinical engagement and cost-improvement purposes, such as focusing on LoS as a cost driver and informing consultants of their patient-level costs. However, PLICS data are not yet extensively used to link cost and quality; the open-ended comments indicated that this is mostly because of coding and accuracy issues.
The survey also highlighted that PLICSs are largely not discussed or shared with other providers, commissioners or governing bodies. Providers treat PLICS data as commercially sensitive in nature. This militates against using PLICSs to underpin opportunities for integrated care across services and settings.
Furthermore, the survey results informed the subsequent qualitative stage, in accordance with the study’s sequential explanatory research design (see Chapter 3). Specifically, an analysis of the individual responses enabled the research team to identify two of the study’s case sites, based on their reported high level of engagement. The survey findings on the commercial sensitivity of PLICSs led the research team to investigate how two of the case study sites share data through a NBG. Survey results on the use of PLICSs showed trusts’ high use of PLICSs to engage clinicians and low use of PLICSs to connect cost and quality. Moreover, results suggested that trusts widely use PLICSs to prepare business cases for investment, which led the research team to collect business cases and financial reviews from the study sites.
Chapter 5 The Gertrude case study
Introduction
This organisation is anonymised under the name Gertrude; it is a very large full-service district general acute hospital trust. It also provides community health care and additionally offers significant tertiary services. It is close to other hospitals of similarly high reputations. It enjoys long-standing FT status. The trust reported level 4 engagement with PLICS data in our survey, which implies that collaborative working between clinical and finance teams is the norm across all specialties and both professional groups share finance and quality data to improve outcomes.
Brief background to the case
Gertrude Hospitals Trust provides services across two sites, employs > 13,000 staff, has > 2 million patient contacts per year, delivers nearly 7000 babies and has an annual budget of > £1B. It has a commercial directorate that drives strategic business planning, several successful joint ventures with private-sector partners, thriving charitable funds and a private health-care offering for an extensive number of specialties.
The trust was one of the first implementers of PLICSs. The philosophy of patient costing has been around for a long time. The organisation is characterised by stability in the financial management function with a low turnover of very senior staff in recent years. The broad consensus from the interviews was that the finance function is seen as one that provides information and support rather than exerting pressure to achieve financial balance. That pressure comes from the clinical and operational leaders.
Guide to interviewees
At Gertrude we interviewed two senior financial managers, an operations manager and eight clinicians with organisational roles, including the MD. At four of the seven interviews with clinical directors, the business manager was also present. In addition, we interviewed the managing director of the NBG of which Gertrude was a member (Table 4).
| Interviewee number | Role/job title | Date | Code |
|---|---|---|---|
| 1 | Associate Director of Finance | 10 May 2013 and 10 March 2014 | F2-B |
| 2 | Director of Finance | 10 May 2013 | F1-B |
| 3 | MD | 14 May 2013 | MD-B |
| 4 | Deputy Director of Operations | 14 June 2013 | OD-B |
| 5 | Clinical Director | 22 August 2013 | CD2-B |
| 6 | Director of Orthopaedics & Plastics, with acting Business Manager | 22 August 2013 | M1-B and M2-B |
| 7 | Clinical Director, with Business manager | 6 December 2013 | CD3-B and M3-B |
| 8 | Clinical Director, with Business Manager | 6 December 2013 | CD4-B and M4-B |
| 9 | Clinical Director | 9 December 2013 | CD5-B |
| 10 | Clinical Director | 10 January 2014 | CD6-B |
| 11 | Clinical Director, with Business Manager | 30 January 2014 | CD7-B and M5-B |
| 12 | Managing Director, NBG | 10 March 2014 (with F2-B) | IT1-B |
Empirical findings
General
The respondents were clear about the rationale for PLICSs. There was an overwhelming consensus from those interviewed that the costs of treating patients were not always, but should be, known to the organisation. The interviews revealed that PLICSs appeared to be well embedded in the organisation. They were the cornerstone of business cases for investment in clinical services. It was noted, for example, that:
[PLICS] is getting better in terms of presentation . . . when I first started, one of the main issues was you’d get different answers from the same question, whether you spoke to ops, whether you spoke to finance or contract monitoring, because we’re all talking a different language – to the answer you wanted [laughs]. And I think now it’s a little bit better; it’s more aligned, so . . . we’re usually being performance managed on the same piece of data . . .
CD7-B
There were, however, contradictory statements from others who did not know why there were two systems, PLICSs and integrated SLR, running side by side; they felt that PLICSs were ‘clunky’ and resource intensive, and that alternatives were preferable.
What you do with PLICS is, you work out every month how much each individual patient’s care has cost. With ISLR [integrated SLR] we use the same data, but in about 4 hours we can process an entire month’s data . . . We don’t line it up, we don’t spend hours trying to line it up at patient level.
F2-B
Will I use PLICS [to support the redesign of a clinical pathway]? Probably not. I will – I think it unlikely that I will use PLICS. I probably will use – I will use the service line reporting stuff because I think that’s – because service line reporting here, the stuff that [the Associate Director of Finance]’s leading on, we’re finally beginning to get under the trading accounts, the internal trading accounts. That’s becoming valuable.
M1-B
One respondent talked about the development of an in-house patient education and research costing system in order to cost all of the training and research as well as the patient care activities, which are significant for Gertrude.
In addition, there was frustration from some interviewees, in contrast to statements about patient-level costing having been in existence for a while, that despite PLICSs, there was some artificiality about some aspects of the costs and they did not have the financial information required.
We’re not really set up to do it. So I mean we can’t immediately say yes, OK, transplant, we know how much that costs. We’ve worked it all out. We should be able to do it. It would be much better if we did.
CD4-B
In summary, therefore, there was some variation in the confidence that respondents had in how PLICSs are to be constructed and how they are used in practice.
Cost improvement
The examples given in the interviews of how PLICSs were used to identify and reduce costs included length of the hospital inpatient stay, theatre utilisation (including early starts and over-runs), therapist salary costs and medical devices. The argument was advanced that a focus on direct costings on which clinicians had an influence, rather than overhead costs, was likely to be more productive. An example here was when the orthopaedic clinicians, who used external PLICSs benchmarking as evidence to build their case, challenged the current model of care provided by the therapists, because they judged their recharge cost to be too high.
They’ve [the orthopaedic surgeons] become hugely engaged in wanting to challenge the working model in therapies . . . They’re thinking the cost is too high and they’re wanting to negotiate about changes in skill mix, changes in the way that they run the clinical model to reduce the cost ultimately.
OD-B
At Gertrude, the PLICS helps to identify the costs of clinical practice variation. The ways that PLICSs are used to improve clinical quality, as well as to reduce costs, is dealt with in more detail in Using patient-level costing and information systems to relate cost to quality. The financial aspects of clinical practice variation are depicted in cost curve graphs and examples were given of their use in breast surgery and in dermatology (Figures 18–21 provide examples in relation to breast surgery). The histograms show relative efficiency by consultant and Tables 5 and 6 show profitability (variance is the difference between income and costs). A definition of the purpose of a cost curve was given as follows:
. . . a cost curve, which will show a small number of patients costing a small amount, a small number costing a large amount and then a big chunk in the middle which is the rump of the activity you do for a given HRG and actually, by understanding what drives the cost on that rump and saying, ‘How can we modify the care pathway for that rump of patients?’ we can drag that cost curve over to the left-hand side and generate savings whilst, at the same time, improving care for the patient.
F2-B
FIGURE 18.
Gertrude: example of cost curve for unilateral major breast procedures without complications. FCE, finished consultant episode.
FIGURE 19.
Gertrude: example of cost analysis, finished consultant episodes (£2.5K–5K). CNST, Clinical Negligence Scheme for Trusts.

FIGURE 20.
Gertrude: example 2 of consultant-cost comparisons for unilateral major breast procedures without complications.
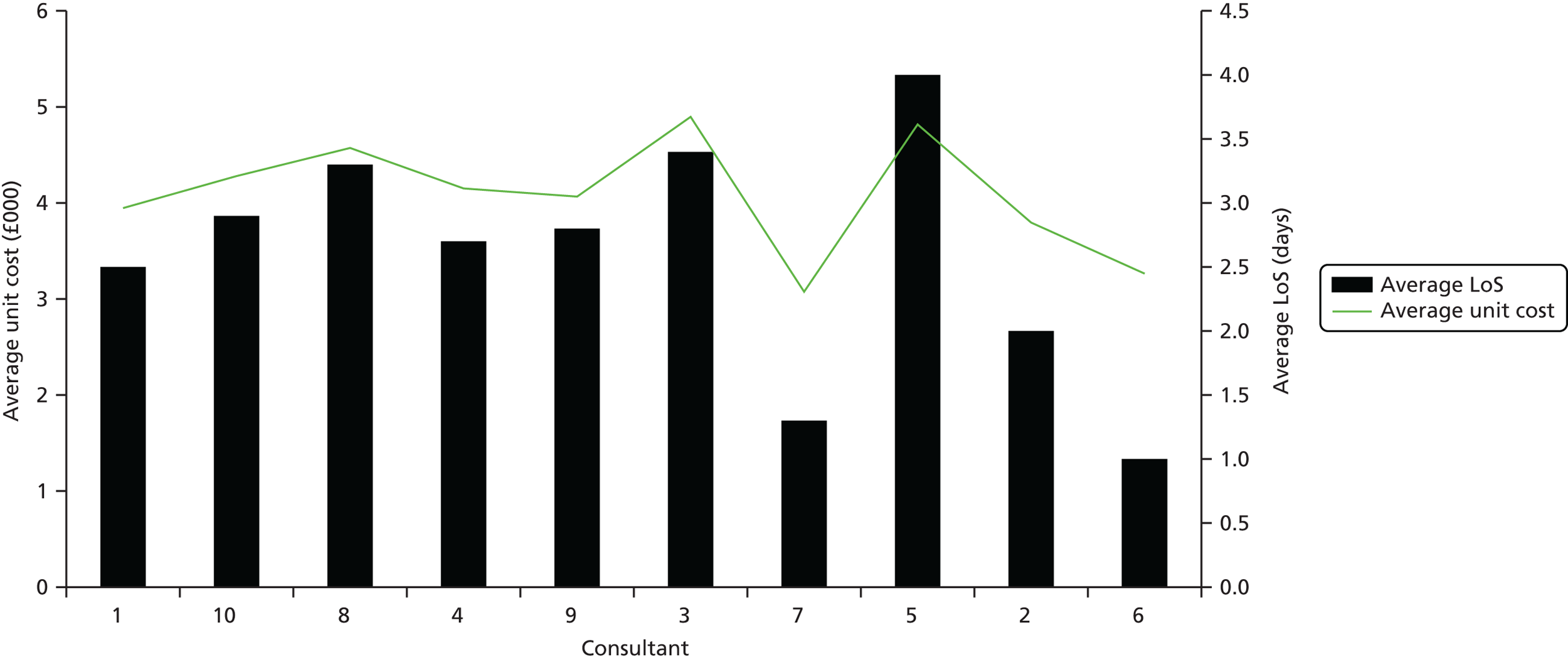
FIGURE 21.
Gertrude: example 4 of consultant cost comparisons for unilateral major breast procedures without complications.
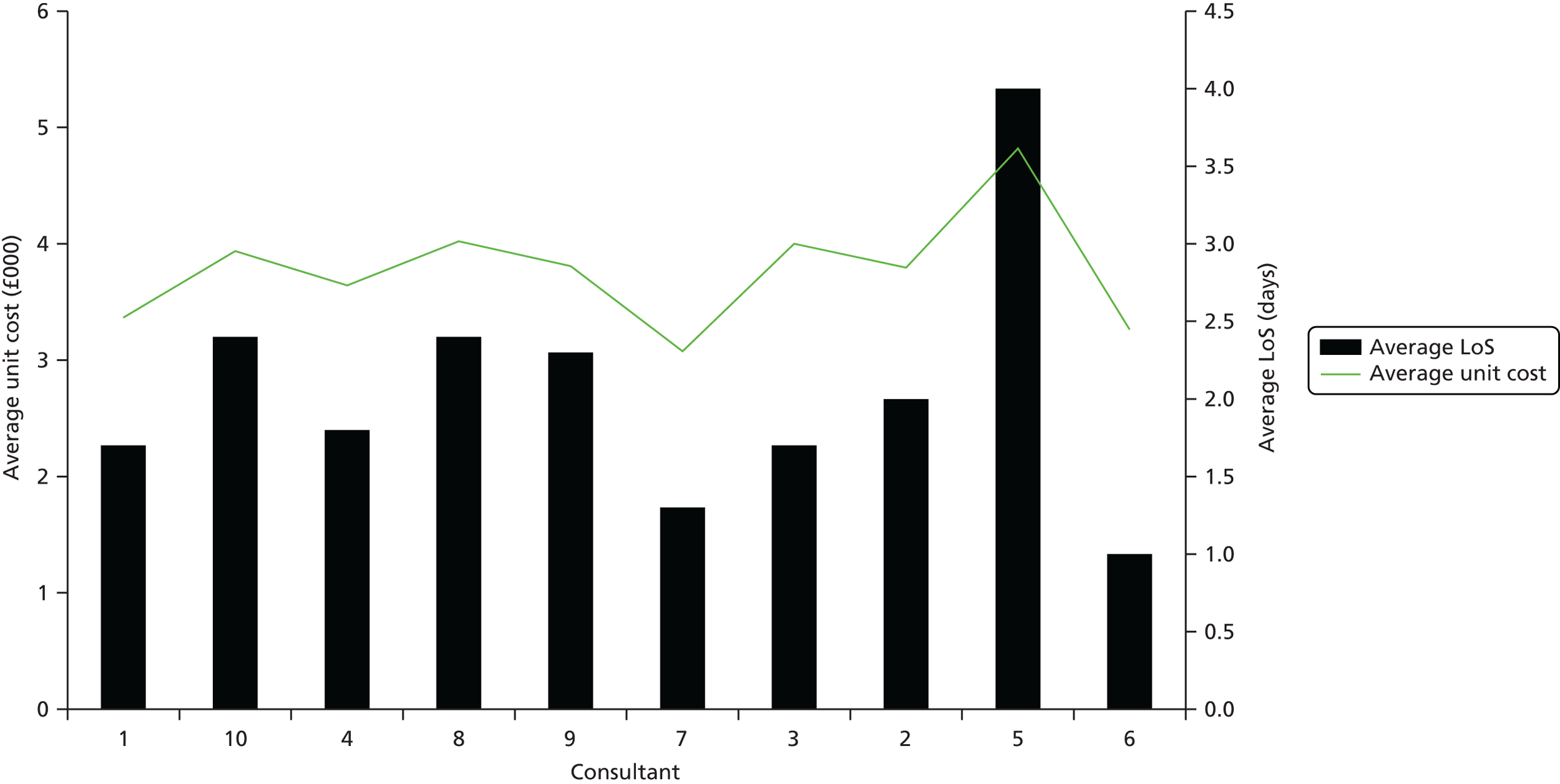
| Consultant | FCEs | Total cost (£) | Income (£) | Variance (£) | Average unit cost (£) | Average LoS (days) |
|---|---|---|---|---|---|---|
| Consultant 1 | 24 | 94,744 | 62,687 | –32,057 | 3948 | 2.5 |
| Consultant 10 | 21 | 89,859 | 61,874 | –27,985 | 4279 | 2.9 |
| Consultant 8 | 18 | 82,320 | 47,355 | –34,965 | 4573 | 3.3 |
| Consultant 4 | 18 | 74,700 | 46,046 | –28,654 | 4150 | 2.7 |
| Consultant 9 | 16 | 65,069 | 40,674 | –24,395 | 4067 | 2.8 |
| Consultant 3 | 5 | 24,489 | 15,221 | –9268 | 4898 | 3.4 |
| Consultant 7 | 4 | 12,308 | 10,276 | –2032 | 3077 | 1.3 |
| Consultant 5 | 1 | 4819 | 2569 | –2250 | 4819 | 4 |
| Consultant 2 | 1 | 3795 | 2569 | –1226 | 3795 | 2 |
| Consultant 6 | 1 | 3264 | 2569 | –695 | 3264 | 1 |
| Grand total | 109 | 455,367 | 291,840 | –163,527 |
| Consultant | FCEs | Total cost (£) | Income (£) | Variance (£) | Average unit cost (£) | Average LoS (days) |
|---|---|---|---|---|---|---|
| Consultant 1 | 16 | 53,853 | 40,396 | –13,457 | 3366 | 1.7 |
| Consultant 10 | 16 | 63,001 | 41,104 | –21,897 | 3938 | 2.4 |
| Consultant 4 | 13 | 47,354 | 32,689 | –14,665 | 3643 | 1.8 |
| Consultant 8 | 13 | 52,288 | 33,397 | –18,891 | 4022 | 2.4 |
| Consultant 9 | 12 | 45,710 | 30,120 | –15,590 | 3809 | 2.3 |
| Consultant 7 | 4 | 12,308 | 10,276 | –2032 | 3077 | 1.3 |
| Consultant 3 | 3 | 11,999 | 7707 | –4292 | 4000 | 1.7 |
| Consultant 2 | 1 | 3795 | 2569 | –1226 | 3795 | 2 |
| Consultant 5 | 1 | 4819 | 2569 | –2250 | 4819 | 4 |
| Consultant 6 | 1 | 3264 | 2569 | –695 | 3264 | 1 |
| Grand total | 80 | 298,391 | 203,396 | –94,995 |
Gertrude is a member of a national patient costing benchmarking group (hereafter NBG) which, at the time of the interviews during which this was discussed (March 2014), had 71 members drawn from hospital trusts across England. Each organisation freely shared its patient-level costs, but not its income. Benchmarking was then used to identify services in individual trusts that may or may not be profitable and may or may not be more expensive to run than similar ones in other trusts, and to understand the cost drivers. This was explained thus:
So there’s two things you can do with PLICS. The first is to say there’s a service that’s unprofitable and we can . . . say, ‘Well, which of the HRGs in that service . . . are unprofitable?’ Using this benchmarking data, what we can now do is to say, ‘Are we for those particular HRGs, not only losing money against the tariff, but are we also expensive compared with our peer group?’ Well actually we’re the same as our peer group. Now [second] if we’re the same as our peer group, that would indicate to us that there’s an issue with the tariff, but if we’re loss-making and we’re more expensive than our peer group, there should in theory be an opportunity to do something about that.
F2-B
Gertrude has used this facility to identify cardiology, thoracic surgery and orthopaedics as potentially high-cost services. The manager of the benchmarking group, one of the interviewees, remarked that, although comparisons were made between trusts, in some organisations there may be missed opportunities for internal benchmarking.
[Internal benchmarking is] . . . not really [used], because principally, clinical practice is the most difficult thing to change. So I discover that this consultant does something better and this consultant does something worse, what’s the probability that you will convince consultants to do something different?
IT1-B
Although PLICSs are revealing the costs of treating patients and thus providing data to indicate potential efficiencies, one of the interviews described counterpressures, particularly ‘protocolised working’, which were driving up costs.
So if you look at A&E for example. So the 4-hour target comes into A&E. What does that mean? That means that the nurse who’s seeing the patient at the front for triage is ticking every single box for every single test. So there’s no chance that the patient will breach because you forgot to do a test, which he needed. And because the nurses aren’t senior enough to think about, you know . . . that patient needs . . . they just tick every box.
CD7-B
Because we’ve moved into this kind of protocolised way of working, people are just not thinking. And that’s the biggest thing. So on our ward, you know, when you request a blood test, the junior doctors because they’re busy will request all the blood tests for the whole week because there’s one box that says the next 5 days’ requests. You just tick it. All the tests for the next 5 days are requested automatically. So of course if you’re a busy junior doctor you don’t want to be doing it every day. You just do it on a Monday morning; you know it’s done for the rest of the week.
CD7-B
Clinical engagement
Gertrude hospitals have been using PLICSs for a while to engage clinicians to solve financial problems.
We took out, at that time [2006/7], patient-level cost information, and profitability information to all of the directorates . . . we then got the Clinical Directors, talking to their departments about, with support from us and information people, the profitability of their service and the financial issues we’re facing. I reckon at that time we spoke to about 4000 of our staff about it and we turned the corner that year, I think that was the year we came in with the £50M surplus . . .
F2-B
The use of PLICSs (and other financial management tools such as SLR) has provided clinicians (by which the respondents mainly meant doctors) at Gertrude with an alternative lens and language through which to view and talk about the services they provide. Not all clinicians are interested in the financial aspects and, in addition to those who are and those who are not engaged, there is a third group who can be helped to engage.
There’s a group of clinicians who are interested and there’s a group who, frankly, have got other things to do with their lives . . . my next tack now is to send in this hit squad to help provide them with the information and to draw the data out of their manual bits of data that they’ve got so that they’ve got confidence about the data and then they can decide to do something about the care pathway.
F2-B
The core business of Gertrude is delivering excellent health care rather than providing a financial return for investors, so it was not surprising that the former is the first priority of the clinicians.
. . . people use the word world class a lot . . . and we’re driven by that. And so we’re . . . just constantly innovating our services from a clinical perspective. And it may be the fact that the financial elements of it is done in shadow form and slightly behind the curve, is a good thing because it means the clinicians in the team are focused on what clinical excellence is. And by the way, if it’s financially really good, then that’s the Holy Grail, isn’t it?
M1-B
In addition, clinicians reported being happiest when doing the work for which they were professionally trained, while using what they perceived as the most effective tactics for getting more investment into their own service.
I love medicine. I love this non-business business that we run. We’ve made it into a business, and that’s what the government are trying to do, and of course it isn’t a business. And there is no common sense to how the money is allocated. The people who shout loudest, get the most. Call the NHS. The louder you shout, the more money you get. And to shout loud, you need plenty of time, so you’ve not got to be too busy. I love it. I love medicine.
CD5-B
However, challenging clinicians on efficiency also harnesses their competitive instinct:
Most of them [clinicians] are very proud people and don’t want to do something that is making a loss. The turnaround in Orthopaedics has been remarkable; they’ve saved millions of pounds because there is an enthusiasm there to deliver (a) a good quality of care to their patients and (b) to do it efficiently and effectively. They’ve attacked almost every aspect so they negotiated better prices with suppliers, they’ve honed down the variety of consumables they use. They’ve put effort into establishing how they can reduce their length of stay, they’re getting more efficient usage out of their theatres.
F2-B
The respondents highlighted the value of professional managers and clinicians working jointly on scrutinising the financial data to come up with innovative solutions, and a belief that both groups want most of all the best possible clinical outcomes. The importance of translating and interpreting the language of finance to make it accessible to clinicians was underlined. One respondent dwelt at some length on what the process of effective engagement of clinicians looked like:
If you can present the sort of data we’re talking about, patient-level or physician-level type data, covering real costs, I think most clinicians engage with that very readily. You know, as long as it’s analysed and presented in a way that allows them to see the key issues, given their levels of experience, they then will engage because it’s meaningful. You know, it makes sense to them and it allows them to start thinking laterally, thinking creatively, innovate, with the right support around them to look at different solutions, different ways of doing it. The trick, with all of this, is to marry it to the clinical outcome developments that are starting. They’re well developed in some areas and if you get the clinical outcome measures really, really well defined, that enables you to focus strategically on what you want to do operationally, and then you – and then of course you’re looking at the costs of doing that and you’re starting to improve health care with efficient use of costs . . .
CD2-B
The other enablers of clinical engagement included the development of a ‘high-trust’ and flexible organisation culture.
What we’ve really found is that people want freedom to . . . make decisions independently of the bureaucracy of the organisation . . . if you’re living within your budgets you should be able to make a decision to recruit someone without having to go to some kind of decision-making panel . . . that’s the kind of thing that we’ve been playing around with in terms of the organisational culture, how might you best incentivise staff . . . There are two directorates where we’ve allowed them to spend surplus on redeveloping services within their directorate as a reward for the fact that they made a surplus.
OD-B
The attainment of some financial literacy has also led the clinicians to grasp some of the pricing anomalies (see the section below on the impact of using PLICSs to uncover anomalies in tariffs and block contracts). The illogicality of some of the tariff setting may inadvertently drive frustration and, ultimately, disengagement because of a perception that it can block best quality of care.
. . . the tariff that we get paid for a consultation that’s not face to face, is £23–24 plus market forces factor. So, it’s about 30-odd quid. The tariff for a new consultant appointment is about £170 plus market forces. So it’s over £200. The time that that senior nurse . . . will spend with the patient on the phone, might be 30, 40 minutes. And they will get a proper consultation from someone who really knows their stuff. They could come to a clinic and they could see . . . a junior doctor for 10 minutes, and the junior doctor will refer them for tests that they don’t necessarily need. And they’ll come back, and they’ll bounce around in outpatients for a while. It costs the health economy more, worse for the patient, takes longer to get care. Everything about it is not a great way to deliver the care. But £23–30 doesn’t really cover the costs of everything that’s been invested into that nursing member of staff. The work that they go away and do afterwards . . .
M4-B
A second barrier to engagement is the plausibility of the data:
The success we’ve had in really rolling that out in a way that gives it some meaning is limited by the fact that people just don’t believe the data in the first instance . . .
OD-B
There’s nothing like getting clinicians in the room and the data being obviously wrong. You disengage people.
CD7-B
A third barrier to engagement was a lack of buy-in for the management accounting approach used:
We are very, very old fashioned here, and I personally hate variances. I think we should look at actuals. I think we should look at what we earn, versus what we spend. And we should be able to share profitability information with clinicians and with people who deliver services.
M4-B
The other barrier was that the PLICS did not focus as much on the cultural side of the organisation, as it was seen principally as a technical managerial tool.
I think we’ve got a very technical and slightly commercial edge around the set-up . . . There’s a question then about . . . what we have the capability to do in that system, [does it] have the traction it could and should have within our own organisation in terms of how we use it . . . how we use it in . . . decision-making and that’s the bit where, perhaps the service line management bit of the organisation, the cultural bit of how we use that information is perhaps not quite as advanced as the technical reporting system bit . . . we are relatively traditional and hierarchical as a structure and so there’s a strong sense of wanting to retain central control on certain things . . .
OD-B
Using patient-level information and costing systems to relate cost to quality
Respondents explained that Gertrude had a goal around relating costs to clinical quality and patient experience, but that currently the measures on costs, waits and outcomes were all reported separately.
We’ve got a formal programme board that’s chaired by the chief executive and its complete ambition is to combine the work that we need to do around cost reduction and productivity improvement with what’s coming out of the Francis recommendations around quality and experience improvement. I suppose the phase that we’re in right now is how best we can define the indicators that will measure our success or otherwise in doing that. . . . we haven’t landed on what that looks like yet or the how, so we’re very much in the . . . development stage of defining what those indicators should be and how best we can make those links together . . .
OD-B
The main operational way in which our informants related PLICSs to quality initiatives was with regard to the use of PLICSs to support the redesign of the patient care pathway. An example of some years ago was given about the negotiation of a new local tariff for simultaneous mastectomy and breast reconstruction surgery, which resulted in a new service with a better clinical outcome and experience for patients. One informant implicitly relates cost with quality in his plea for more focus on lean methodologies:
The biggest influence for me is lean methodologies and actually applying them in health because I think we talk lean really well in the NHS but I don’t know that we necessarily do it . . . taking that principle of understanding bottle necks and flow . . . and then asking the clinicians if we do it this way, can you tell me is this a clinically good thing or a clinically not such a good thing because we’re not going to do anything that’s not clinically good . . .
M1-B
It was reported that PLICSs were used for an in-depth analysis process called ‘deep dives’ (see the description in Brown167).
There was a request from our medical specialties directorate, to be in the next deep dive, which is interesting . . . diabetes and ophthalmology want to be in the next deep dive . . . I explained to him . . . that it was my view that if you go to any one of our 80-odd specialties in the hospital, you’ll find each of them do six or seven things for 80% of the time that they’re there . . . if you want to make a material difference to your cost base, you can do that either by doing some functional analysis, which says, how much do I spend on nursing? How much do I spend on doctors? What do pacemakers cost me? Or you can look at those six or eight things and say, how can I redesign the patient care pathway around those six or eight things, to maintain or improve quality, and to reduce cost at the same time? So they were asking, in their business planning review, to be the next one, because they say they want to streamline the way they do their cataract surgery. They want to streamline the things that they do. But again, it’s too early for me to sit here and say, we’ve saved this much money by redesigning this patient care pathway.
F2-B
Other reported initiatives, which were in the early stages, included the use of Hospital Episode Statistics data compiled by Birmingham University and, in particular, nursing resource-use data to understand the impact of different patient acuity on wards, and, in time, associations between staffing levels and mortality, bed sores and so on. The results thus far have been unexpected at the individual patient level, thereby suggesting that resource-use differences between elective and non-elective cases were not as straightforward as had been thought.
. . . [Nursing acuity data showed] the variability and the cost of patients [was different] from what I was expecting it to be, to what it actually is . . . There are clearly groups of patients that are more dependent, and others that are less dependent. Not just the elective and non-elective mix . . . We’ve all been getting our costs wrong.
F2-B
A couple of examples were given in which higher costs were related to higher quality and which present an organisation like Gertrude with a conundrum: human immunodeficiency virus services that focus on ‘lost to follow-up’ cases, which is really important for preventing individual health deterioration and spread of infection in the community, but is not financially rewarding; and joint replacement revisions, which can be very costly, but which a centre of excellence such as Gertrude should be doing. One respondent commented that costs were always related to current interventions and not to the wider impact on the patient’s quality of life, for example, the economic benefits to patients of being able to return to work, enjoy family life and so on.
The other side of the coin is the interventions . . . you know, an intervention A costs a lot of money, but it’s effective in terms of that long-term health economic benefit for the patient . . . But the data is looking – most of the commissioning or the approach to approval and licensing, et cetera, is only focusing on the cost of the intervention period. But that isn’t the cost. The cost is the patient out of work, the patient, you know . . .
CD2-B
One respondent remarked on the human costs of relentlessly driving high quality with efficiency gains:
What you lose if you’re not careful, is the compassion in the care, you’re just processing people and you forget they’re people. And the more you push people, the worse their support gets, the closer they get to that point where they change over from looking after people, to getting a job done . . .
CD5-B
The same respondent also outlined the tension between the values of a clinician, who aims to provide the best possible care for their individual patient, and those of a corporate manager aiming to deliver the most efficient and financially viable service.
You know, I was looking after a man with COPD [chronic obstructive pulmonary disease] yesterday on our unit. And he came and he’s had 30 admissions in the last 3 years, on average 7 days per admission. The first thing I go and look in is, how many times has he been in, what’s his average length of stay? He’s a lovely man. He’s desperately ill. He needs really first-class care. But you’ve got this dichotomy. When people know this is . . . loss making . . . there is a worry about not delivering the best kind of care. You know, I spent yesterday saying, ‘Does he really need to come to HDU [high dependency unit] at £2500 a day?’ I’m getting £1000 for this, if I’m lucky. You know, does he really need – ? Yes, he does need to come to HDU.
CD5-B
Resource allocation across services and settings
Respondents described lots of scenarios in which the costs, and the pricing and funding systems, inhibited seamless working and the provision of care in the right place for patients. Care provided in the community service arm costs the trust more than in the hospital arm because the latter is rewarded by tariff rather than by the block contract.
We’re very borough focused and we work very closely with our Local Authority colleagues. So there’s lots of tensions about [the community service] being part of [Gertrude] again, not least in that they are paid a tariff for what they do and we have a block contract for what we do. So in a sense in terms of the service redesign it’s kind of difficult to shift things, because there’s a perverse incentive isn’t there to kind of send things up to the hospital which makes them money. There’s not much incentive for me to do more work because I’m not going to get any more money for it. I can’t employ more – you know I could say, ‘Right, we’ll see all these children,’ but we wouldn’t get the money to either employ doctors or nurses to see them.
CD6-B
The tariff was considered particularly inappropriate for funding the care for people with chronic disease.
The tariff-based system for long-term conditions and probably for acute care in long-term conditions is crazy. It’s inappropriate, it has a number of misaligned incentives, it breaks across collaborative working and it’s not in the best interests of patients. It’s not aligned to pathway approaches and it’s organisationally focused and not patient focused. So if you could do anything to bring in year-of-life tariffs or something much more intelligent a way about how we deal with long-term conditions, that would be a major change in the way we deal with health care.
MD-B
Respondents did not generally share PLICS data outside the organisation, except with the NBG and locally with other neighbouring trusts in a very limited way to look at cost comparisons (e.g. within the Academic Health Science Centre).
In addition, there was significant nervousness about sharing PLICS data with commissioners for fear of the consequences.
If we get to the level where we’re forced to . . . share with our commissioners, the detail of what it’s costing, then they will cherry-pick. They will say, ‘Well, I’m paying you this much money; it’s more than it’s costing you so I’m going to pay you less,’ but they’re not going to pay us more for the things that we’re losing money on. We’ve got real examples of that going on at the moment, this specialist commissioning where they want to force you down to the average tariff on things where you’re being paid more, but they won’t bring up the tariff where you’re being paid less and it’s all very one-sided at the moment, it’s hugely difficult. So I would not expect to share patient-level cost data on this . . . if we were forced to do that [share PLICS data] I think we might question about whether we actually did it any more.
F2-B
The exception to this was when the trust was aiming to provide a new service (the example given was simultaneous mastectomy and breast reconstruction) and had shared costs with local commissioners with whom they had a good relationship. The outcome was a price that related to patient costs and a better clinical outcome because it was for one operation rather than two separate ones.
Using patient-level information and costing systems to identify anomalies in tariffs and block contracts
One of the consequences of PLICSs in this case study is that greater transparency about patient costs has uncovered the extent of the anomalies in the relative payments to hospitals for elective and for non-elective activity.
I, as the clinical director for the emergency medical services, have to provide at 4 o’clock in the morning a service to – for example, let’s say your hand gets squashed, for whatever reason. You come out of the club, you fall over and you land on your hand. I will have to triage that through the Emergency Department. That involves a nurse. It will then need a radiologist to come and . . . do some radiology. It will then need a doctor to interpret the X-ray, and then it will require a plasterer or a bandager to strap up the hand. And I will get paid £58 for that at 4 o’clock in the morning.
CD5-B
Within non-elective activity, there exists a perverse incentive to admit patients rather than treat and discharge within 1 day because of the payment triggered by admission, amounting to financial punishment for sending patients home quickly.
We’ve become so efficient, we get people home quickly. We have 23,000 admissions a year and 14,000 of those go home within 1 day, so they get a non-tariff. So if I send my COPD [chronic obstructive pulmonary disease] patient home in less than 1 day, I get a maximum of £247. I send 14,000 of my patients home in 1 day; 8500 [patients] and . . . about 300 elderly care patients stay longer than 1 day. If you went to Queen’s [not real name] and took the same data, they have 21,000 admissions, 6000 of theirs stay for less than 1 day and the rest stay for greater than 1 day, so they get a tariff on about 17,000. If you look at St Christopher’s [not real name] who have 57,000 admissions, because there’s about four hospitals, 6000 are under 1 day, and 50,000 are over 1 day. The proportion is completely skewed.
CD5-B
This quotation is illuminated in the numbers given in Table 7.
| Hospital | Approximate total number of emergency admissions/year | LoS, n | |
|---|---|---|---|
| < 1 day (n) | > 1 day (n) | ||
| Gertrude | 23,000 | 14,000 | 9000 |
| Queen’s | 21,000 | 6000 | 15,000 |
| St Christopher’s | 57,000 | 6000 | 51,000 |
This kind of anomaly is also in danger of leading to clinician disengagement:
. . . [my brother] just laughs at the way the NHS works. I described it to him, and he said, ‘Well, there’s no point in calling this a business, it’s a joke’. This is nothing like a business. A business aims to be efficient and make money. This is not aimed at being efficient and making money, you’re being efficient and losing money . . . the tariff system is just a joke. We are losing money furiously. We’re looking at a £5M overspend this year in my directorate. And I get beaten to death about this.
CD5-B
The same respondent indicated that emergency care was a particular area of clinician disengagement:
I think the NHS is two bodies. There’s the bit that likes to do the nice bit, which is easy, 9 till 5, Monday to Friday. And then there’s the other bit that nobody wants to talk about, called emergency care. And our medical director said, 3 years ago now when things were really bad across the whole country, when Mid Staffordshire was occurring, ‘Emergency care is the river that runs through the hospital.’ My colleagues very quickly changed that to, ‘It’s the sewer that runs through the hospital that everybody runs away from’. Nobody wants to get involved with it. For me to keep my physicians involved in medical care is a nightmare. They all want to go and be specialists, ‘I want to be an endocrinologist, but I don’t want that lot’. They know that’s a nightmare.
CD5-B
Morever the tariff anomalies can drive inappropriate behaviour.
So, COPD [chronic obstructive pulmonary disease] is a good example, again. If a patient comes in with COPD, the tariff is £990. By coding better, I can improve my tariff. Now, this may be a simple COPD, but I will start looking for the ways I can make it into a non-simple COPD. If at any point, without being dishonest or cheating or not playing by the Monopoly [Hasbro, Pawtucket RI, USA] rules that they’ve put in place, if for example the CO2 goes above six or the PO2 goes below eight, I can increase the tariff to about £2500. Now I know most of the ambulance men will stick lots of oxygen on my COPD patients, that will drive their CO2 up. It’s inappropriate management by the Metropolitan Ambulance Service, but it will guarantee the CO2 is seven when they arrive. The first thing my junior doctors do, a blood gas. So it’s seven. So I just made that £2500. If this patient needs non-invasive ventilation at any point, an additional £1000. If I then put in some complex coding, you know, they live alone, they’re a complex elderly patient, I can get probably another £1600.
CD5-B
But it won’t work in A&E. The problem is, this does not work – there are only 11 codes in A&E, and the highest one is £247. And that’s of course a cardiopulmonary resuscitation, which for us is nothing. The cardiopulmonary resuscitation is easy, but that’s our highest code. So if a patient comes in and we jump up and down on their chest for a few minutes, we get the best code we can.
CD5-B
One of the respondents more positively felt that PLICSs could ultimately be used to charge commissioners a fair price for services using best practice clinical pathways. Another example was the way in which PLICS data from different hospitals were used to develop a national tariff for bone marrow transplants:
I did a piece of work with the commissioners – it must be about 5 years ago now – so historically, the bone marrow transplant tariff across the city was very, very different. So for the same procedure, one hospital was getting paid sixty grand; another hospital would be getting paid a hundred and fifty grand. This has just grown up historically with locally agreed tariffs and things . . . And then because the commissioners wanted to get underneath this and try and work – try and harmonise the tariff across the city and then ultimately, nationally. And so what I did, was we went through 30 or 40 bone marrow transplant patients that we’d done here at [Gertrude] and counted every single item, every blood test, every X-ray, every scan, and worked it all up, and came up with an average price for different types of transplants. And then we tested that at [a different hospital], because they were one of the ones who were charging a lot of money. And when they also did their . . . itemised costing, it actually was very close to what we came out with. And that then became adopted as initially the city tariff; it’s now the national tariff.
CD7-B
We have seen that PLICS data are rarely shared outside the organisation, but have been used on occasion to negotiate local and determine national tariffs. One manager argued for the importance of relational contracting to drive realistic prices:
The ability to negotiate on a local basis . . . your relationships with your commissioners, your history with them . . . Have you done what you said you would do? Is your performance good?
M4-B
Summary
There are four main themes arising from this case study with regard to the use of PLICSs.
Patient-level information and costing systems are well embedded in the organisation, particularly for cost improvement, benchmarking and business planning purposes and there is good evidence of collaborative working between clinical and financial teams, corroborating the report of level 4 engagement in the survey. There remains a broader question about traction in the organisation for two reasons. First, clinicians say that their ‘first love’ is their clinical work and their desire to develop clinical innovations, so finances will always be a secondary consideration. Second, there may be a missed opportunity thus far to use PLICSs as an organisation cultural tool for transformational change, rather than as a technical tool.
There remains a challenge in the usability of PLICSs; for some, the allocation of costs appear somewhat artificial, and the system somewhat ‘clunky’, and for others there is an ongoing need to get better at translation and interpretation of the data for easier use by clinicians. There is a danger in thinking that PLICS data are ‘true’ costs. They are indicative and can point out areas of financial concern, but there is always a trade-off between usefulness and cost of precision.
The anomalies in NHS funding of services may drive clinical disengagement. There were many examples of what appeared to the respondents to be nonsensical prices for treatments and services.
There is a low level of trust, although not from all respondents, between the trust and its commissioners and, therefore, reluctance to share cost information. This means that commissioners are rather in the dark. Given the context of tariff anomalies, there is an argument for foregrounding the development of close and strong relationships between commissioners and providers to overcome some of the paradoxes in the system, to consider the potential of relational contracting and, as part of that, to share an aspiration to pay for and receive a fairer price from local and national commissioners for the cost of activities.
Chapter 6 The Sybil case study
Introduction
This hospital is anonymised under the name Sybil; it is a large, multispecialist trust offering integrated hospital and community care. Sybil was selected to be a case study before the research began (see Chapter 3). The trust was one of the first in the country to implement a PLICS (in 2007). It reported level 4 engagement with the PLICS data, so the trust had in-depth experience of its use. Access had been arranged between the research team and the trust at this early stage to enable aspects of the research to start promptly. The trust is a lead trauma centre, forms the base for a local research network into stroke and is a regional referral centre for neurosciences.
Brief background to the case
Sybil is a large teaching trust with 850 beds and 19 theatres, providing elective and emergency inpatient services, day-case services, outpatient services, diagnostic and therapeutic services, and adult and children’s community services. It serves a local population of 220,000 but also provides regional services (as described above and below). The trust employs 6700 staff and has a turnover of £474M. It is one of the highest performing hospitals in the NHS on patient safety, standardised hospital mortality and staff engagement. A Quality Improvement Strategy to reduce harm to patients is at the centre of its mission.
The trust has a structure of four broad divisions:
-
Division of Healthcare, primarily for the local population
-
Division of Surgery, including specialist cancer services and surgery, providing services across the region
-
Division of Neurosciences and Renal Medicine, including the major trauma centre, providing services across the region
-
Division of Clinical Support Services and Tertiary Medicine, including critical care, providing services across the region.
The patient-level information and costing system at Sybil
A PLICS was introduced within the context of SLR. A sense of the detail in the PLICS is given in Table 8 for 10 patients treated for transient ischaemic attack (defined as a ‘mini-stroke’, i.e. a brief period of neurological dysfunction, caused by an interruption of the blood supply to the brain or eye, which can be a precursor to a stroke). Stroke is one of the tracer conditions in the research.
| Patient | Care pathway cost (£) | Total cost (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A&E | AHPs and other support | Corporate and overhead | LoS | Other | Pathology | Pharmacy | Radiology | ||
| Patient 1 | 129 | 1 | 143 | 264 | 77 | 14 | 58 | 128 | 814 |
| Patient 2 | 130 | 2 | 203 | 577 | 65 | 17 | 42 | 170 | 1206 |
| Patient 3 | 102 | 9 | 393 | 1304 | 147 | 12 | 46 | 2013 | |
| Patient 4 | 107 | 1 | 199 | 921 | 78 | 10 | 41 | 1357 | |
| Patient 5 | 108 | 2 | 351 | 106 | 125 | 21 | 97 | 810 | |
| Patient 6 | 127 | 1 | 165 | 442 | 66 | 3 | 54 | 858 | |
| Patient 7 | 107 | 1 | 199 | 921 | 78 | 10 | 41 | 1357 | |
| Patient 8 | 130 | 2 | 203 | 223 | 65 | 23 | 42 | 119 | 807 |
| Patient 9 | 95 | 1 | 278 | 467 | 87 | 27 | 71 | 162 | 1188 |
| Patient 10 | 95 | 1 | 299 | 586 | 87 | 28 | 71 | 206 | 1373 |
| Highest | 130 | 9 | 393 | 1304 | 147 | 28 | 97 | 206 | 2013 |
| Average | 113 | 2 | 243 | 581 | 88 | 17 | 56 | 157 | 1178 |
| Lowest | 95 | 1 | 143 | 106 | 65 | 3 | 41 | 119 | 807 |
Guide to interviewees
A list of the 14 interviewees, in the order in which they were interviewed, is given in Table 9. They include the three finance staff most closely associated with the operation of the PLICS and the two directors of finance who were in post during the research. The other nine interviewees were clinical or managerial staff who, we were advised, had involvement or interest in the PLICS.
| Role/job title | Interview date | Code |
|---|---|---|
| Corporate Accountant 1 | 9 October 2012 | F1-S |
| Deputy Director of Finance | 17 October 2012 | F2-S |
| Corporate Accountant 2 | 6 February 2013 | F3-S |
| Consultant Stroke Physician and Senior Lecturer in Stroke | 26 February 2013 | C1-S |
| Anaesthetist | 21 March 2013 | C2-S |
| Director of Finance until 1 May 2014 | 28 March 2013 | FD1-S |
| Quality Improvement Lead | 17 April 2013 | M1-S |
| Head of Information, Management and Technology along with two clinical coders | 29 April 2013 | M2-S CC1-S CC2-S |
| Cardiovascular Programme Lead | 28 August 2013 | M3-S |
| MD | 28 October 2013 | MD-S |
| Clinical Director for Theatres | 28 January 2014 | CD1-S |
| Director of Finance from 1 May 2014 | 23 July 2014 | FD2-S |
Empirical findings
As in the previous chapter, this section is organised around our PLICS-related research objectives and questions, encompassing the following: cost improvement, clinical engagement, linking costs with quality and resource allocation across services and settings. We have also included a section on tariff because, as with the other cases, this was an issue of prime importance for the Sybil trust.
We begin with a comment from the MD who emphasised the key specialties for the Sybil trust:
If an organisation doesn’t know where it’s going, you’re in big trouble. So this place – trauma, the brain, the gut, dermatology, research . . . That doesn’t mean other colleagues are not important; but they’re the big things that really set us apart.
MD-S
Cost improvement
Trusts can reduce costs by doing things more efficiently; staff at Sybil discussed theatre utilisation and LoS in this context, but it was reported as being easier to meet CIP targets if the hospital can generate more income. In this regard, clinicians at Sybil discussed working at capacity and accurate coding.
Meeting Cost Improvement Programme targets
The Deputy FD commented that PbR has been advantageous for the Sybil trust because the staff had the capacity to undertake additional activity and some of the income generated could be used to meet CIP targets.
Payment by results has been brilliant for [Sybil] because we’ve been great at doing more activity within relatively the same amount of capacity. So we’ve earned money. And that’s been a vehicle through which we’ve been able to tag some of that as being cost saving.
F2-S
One of the corporate accountants commented that the clinicians could see the link between working at capacity and avoiding making a loss.
Once you start using data the clinical director can say, ‘Well actually, yeah, I can see why we’re making a loss. And it’s because we’re getting income for seeing this many patients. But actually, we’re running a service that has the capacity to see 20% more patients. So we’re paying for more in capacity than what we really need to run on’.
F1-S
However, the MD commented that he was finding it increasingly difficult to ensure that cost saving was not detrimental to patient care.
The bit about costing is important. When I first came into post, obviously we had to sign off CIPs every year, Monitor charged the medical director to give an assurance that those CIPs will not have an effect on patient quality and, even more importantly, on patient safety. In 2010, it was ‘sign it off, not a problem.’ 2011, bit of thought, signed it off. 2012, bit different . . . Could that [CIP] have an effect on patient care? And guarding about something which had an unintended consequence and nobody had realised.
MD-S
The MD also remarked that the CIP target was a powerful driver to make changes to service delivery.
In this organisation one needs to find £15 million every year [to meet the CIP]. That’s been for the past 3 years, and probably for the next 5 years or even longer. So, knowledge that we will not be able to provide services as we do now . . . So that’s a real powerful driver for change which led into thinking, ‘How can we deliver care differently?’
MD-S
As pointed out above, Sybil has 19 theatres, so one key area for care delivery was ensuring that theatre utilisation was efficient.
Using theatres efficiently and avoiding expensive theatre equipment
To investigate theatre utilisation, the organisation needed a costing system that captured actual knife-to-skin time.
Let’s take theatres for example. [Now] we actually capture theatre usage based on the amount of cutting time there is.
F2-S
This ‘cutting time’ variable reflected the issue that staff should limit avoidable downtime.
It’s the flow into theatre that needs to be corrected, and it’s the utilisation. That’s the only way you can make money. If you’re not operating, you’re not making money. So I’m sounding a mercenary, but that is the reality of it. You know, it’s £12 a minute; there are 10 theatres down there, it’s a huge amount of money.
CD1-S
Sometimes avoiding downtime was as simple as having this stark cost of ‘£12 a minute’ to put to a surgeon who was frequently late into theatre.
We identified that cases were starting late and a common factor was that one surgeon was always late. A bit traditional, I suppose, in their attitude; but it was pointing out to them that, OK; you’re 20 minutes late, but actually eight people have been waiting for you and the infrastructure has been £12 a minute while you’re taking your kids to school. So what can we do to solve it? And he changed his practice.
CD1-S
One clinical director pointed out that the private sector would not tolerate slow surgeons, so presumably they would not put up with late ones!
It’s [the private sector that’s] much better at it [PLICSs]. They cost in the staffing and they don’t carry slow surgeons. Whereas we will carry slow surgeons because we’ve appointed them we’ll keep them, whereas they just slide them out.
CD1-S
The MD summarised the patient flow issues for theatres.
Within orthopaedics, mapping the flow of patients, the times which patients spend in each aspect of their care, for example, the pre-op assessments. Then their surgery and utilising information on the theatre, theatre/man system [captures] when patients arrive in the theatre, the time the operation starts, how long the operation takes, the efficient utilisation of those [theatre] lists, the patient’s recovery, use of enhanced recovery to facilitate patients being discharged quicker. So, using all of that to streamline the process and to ensure that more cases are being done.
MD-S
Another issue was to move work between theatres so that they were all working at capacity.
Theatre – are we using that as efficiently as possible. So, do all of the non-elective cases have to be on the non-elective emergency list or can some of them be moved into theatres where there’s some space on those lists?
MD-S
Equally, some simple procedures were transferred from theatres with a highly expensive infrastructure to a more appropriate setting.
Hand surgery [should be] profitable, we knew the patient-level costing for hand surgery. Simple surgery I think the tariff’s about £800, a bit of soft tissue moulding but we were still making a loss, we realised we were operating on them in a laminar-flow [a system which circulates filtered air to reduce infection] theatre which is hugely expensive infrastructure. And actually, by transferring them to a minor operating suite, you reverse the financial losses. It becomes profitable again and you can carry on delivering the service.
CD1-S
The clinical director for theatres commented on how the cost system had highlighted the expense of many of the kits and, consequently, the need to restrict their use.
A lot of kits are brought in on loan equipment for surgeons to use, they have a huge cost. We identified they were using probably about a quarter of a million pounds a year of loan equipment, we have tried to cut that down, it’s quite hard to, just use the analogy of boys and toys.
CD1-S
However, sometimes, the costing system was just not sophisticated enough to provide the right information to decide whether an expensive piece of equipment was being used only for the patients who really required it because the cost was being apportioned over all patients.
One of the things I have looked at is, in certain procedures in the neurosurgical bit, there are some very expensive screws. I don’t know, £2000 a go or something like that. Actually, if you were wanting to understand the cost of neurosurgical patients, you’d want to know which patients had had these screws and how many. But the system doesn’t do that. Those screws get apportioned over the whole.
FD2-S
However, for some larger pieces of equipment it was possible to persuade surgeons to target the patients who really required the expensive option.
I mean one of the hip surgeons was using implants that you just couldn’t make a profit on. Once you pointed out to them that I appreciate you need to use these implants for some people, but you’re using them all the time, they take it on board. Nobody wants to see their hospital struggle.
CD1-S
Another issue for hospitals in cost improvement is to ensure that cases are coded correctly, so that income flows are sufficient to enable the organisation to reduce costs in some areas; this is particularly important for any high-cost operations.
Getting the coding right
One technical issue is that patients are coded to the right category to ensure that the Sybil trust is fairly reimbursed through the tariff. One anaesthetist had worked in a hospital that had a coder present in theatre.
In [Hospital Y] they actually had a person employed from the coding department in theatre so they could, in real time, code and actually have the clinician there so if there were questions they needed to ask, they could actually ask them just to get the correct code.
C2-S
One coder commented that clinicians had been uninterested in coding but were now engaged because accurate coding can ensure that patients are coded to the HRG, which maximises income for the trust and specialty.
There’s a genuine thirst of interest [from clinicians] about coding ‘cos now – you know when we did it like 6 years ago before payment by results it was just for information. They weren’t that keen on it but now it’s at the forefront of everything: it’s real life to them.
CC1-S
Another coder commented that sometimes accurate coding only entailed incorporating the patient’s condition.
Just making people [clinicians] aware of the importance of correct coding, because the difference in tariffs between a fit person and an unwell person for the same procedure is quite substantial.
C2-S
Unwell patients tend to stay longer in hospital; trying to reduce LoS while still ensuring safe care is fundamental to cost improvement.
Length of stay as a cost driver
The MD commented on the importance of best practice on LoS.
In terms of using costing information, for instance, how could things change if every place adhered to better practice and adhered to the top 25% in terms of length of stay? For instance, how many beds could be taken out if care was more aligned in terms of being joined up with the community, then that money could be reinvested.
MD-S
He also remarked on the significance of ‘joined-up’ thinking with community provision to facilitate discharge. The new FD at Sybil pointed out the advantage that accrued to Sybil in being an integrated provider.
There are some things we can do . . . that that we wouldn’t have done when that community service was being delivered by a PCT [primary care trust], if we discharge a patient from our ward to our community service, if we can do it cheaper than we can on the ward, we’ll do it.
FD2-S
However, LoS does not drive all costs; the new FD commented that sometimes nursing acuity reflected the need to observe vulnerable patients to avoid falls.
You’ve still got ward-based costs apportioned to individual patients based on their length of stay. So how much is nursing as a percentage of the total cost base? And yet it’s done on average length of stay. Well, length of stay isn’t a determinant, necessarily, of the level of intensity of nursing. Is acuity the right model? Some would say it is. [But] where a particular patient has a particular need, it might not be acuity driven; it might be just risk of falls, but they need observation.
FD2-S
The former FD also points out that long LoS is inevitable for some atypical patients; a better way to release resources is to see where care can be delivered differently for the typical patients.
[The] PLICS [identifies] the patient that costs you an absolute fortune ‘cos they were here forever and [we] did lots of things to them. They’re pretty atypical and probably when you understand those cases, there wasn’t an awful lot that you could have done differently because of the complex medical and social environments in which that patient lives. What’s much more interesting is being able to work across areas where there are volumes; where small differences in that care pathway, small differences – but repeated lots and lots of times, give you the bigger opportunity to liberate some [resources].
FD1-S
Clinical engagement
The former FD and the Deputy FD acknowledged that the finance function and its reports used to be remote from the rest of the organisation.
The message that X [the FD] and I got from the Chief Executive was the finance was distant from the rest of the organisation. They always just reported things and they never really engaged in any real way with the wider organisation.
F2-S
The great thing about producing financial information that nobody looks at is that you convince yourself you’re doing a brilliant job.
FD1-S
The finance team emphasised that patient-level data were required for clinical engagement, not least because the clinicians have signed those data off, implying clinical ownership through coding or other forms of engagement.
We’ve now got, not top-down data that you [the clinicians] don’t trust in terms of contribution, but bottom-up patient-level data that you’ve input into, that you’ve actually signed off at all of these stages. Therefore the contribution kind of analysis that comes out of that, you’ve accepted as being true because it’s met your quality standards.
FD2-S
One of the team also pointed out that ‘headline’ figures were available for each service line.
Our patient-level costing information feeds automatically into our service line reporting position. So that gives us the profitability of each of the service lines. So that’s what most of the clinicians see as a headline figure every single month and that’s what they engage with.
F1-S
In addition, services lines could be compared on ‘bubble charts’, which visually portrayed both the size and the profitability (or otherwise) of the service line.
So a bubble chart, each circle represents a specialty and you want to be up here. And your position and your colour represents your improvement or failure. So the size of the bubble is your business and the position above the line is profitability or failure. You’re small and unprofitable, but you do want to be up here, it’s about growing your bubble and moving it up the chart.
CD1-S
The former FD remarked on the size of the divisions in financial terms, emphasising the importance of cost information for decision-making.
So in this organisation [there’s] £400M [turnover and] four divisions, so some consultants are leading divisions with a turnover of £150M. And when those people started to engage and we were telling them the financial constraints their appetite to want information [grew] . . . This is all about wanting to deliver high-quality care but [being] realistic that money was always going to be a constraint; they needed to get information from us, from finance, that was going to properly allow them to make some judgement calls. And so we redoubled our efforts; that was a kind of renaissance point for us – with service line management [and] with PLICS.
FD1-S
However, the MD mentioned that PLICS data were a resource that was available to him rather than information that he consulted routinely. (The managing director of each division would be more likely to see PLICS data.)
I wouldn’t see it [PLICS data] necessarily, unless I asked for it, but if I and the Finance Director, felt uncomfortable or wanted to know the details, obviously that detail is there and can be accessed.
MD-S
Clinical variation and scorecards
Questioning clinical resource use has been mentioned in the context of expensive equipment in theatre; a related issue is highlighting variation between consultants, which can have both clinical and financial implications.
We’ve got standards of care, a consultant may decide they’re going to operate in a different way and ignore that [standard] . . . So that variation between practices, yeah, we would look at that. I think it’s important for transparency that everybody knows what that is. I’d expect colleagues to be shining the light on variation in practice because that has got clinical consequences as well as financial consequences.
MD-S
However, there were differing views on the introduction of scorecards or placemats (guides showing position against national benchmarks) for consultants. One clinical director thought that they were ignored, but the new FD was of the view that they were the latest clinical engagement technique.
We introduced score cards for the consultants which I think other places are taking on now, which told them their profitability, their infection, their utilisation, their start times. And actually, interestingly, they didn’t log onto them. They didn’t utilise that at all. You just kept it for them.
CD1-S
So all of its energy is here, now trying to link up that clinical record and the information to be able to play back to clinicians on individual scorecards – scorecard is the wrong word but, oh, like placemats or something – that would say this was your practice for all the different types of your procedure, how you compare to some national benchmarks, etc.
FD2-S
One clinical director made the broad point that, for some consultants, their engagement with private practice may deflect attention away from their NHS responsibilities.
You can’t incentivise us with money because we’re reasonably well paid anyway. The ones that have – like orthopaedic surgeons with half a million pound private practices, it’s very hard to be engaged with your £60,000 a year practice because half a million pounds is hard to earn.
CD1-S
Indeed, the NHS can become the place of ‘last resort’ for complex and hence costly work.
One of our clinical directors pointed out to us . . . that most straightforward orthopaedic work is now in the private sector . . . which is across the road from this hospital. And of course it’s our same clinicians . . . [doing this straightforward work] with the certain knowledge that if it is complicated he can refer the patient back to himself in his other place in the NHS.
FD1-S
The next section discusses the issue of using PLICSs to relate cost to quality of care, an issue which should engender clinical engagement.
Using patient-level information and costing systems to relate cost to quality
As mentioned above, a quality improvement strategy is central to the mission at Sybil. However, there was little evidence of PLICSs being used to relate cost to quality at the patient level.
The first point raised by the former FD was that health-care professionals seem to find it hard to measure quality so that it can be related to cost.
[In] health services, we all talk about quality but we find it hard to describe it in a way a manufacturer would and come up with a measured specification.
FD1-S
However, he went on to say that the stroke service was able to come up with a service specification which could be costed.
What was good about . . . the stroke service was [they said] ‘this is what we want to see happen to patients’. And therefore we had to kind of rebuild the cost model from this specification [and] attach the cost to it, and then do a model that [was] clinically deliverable as well as financially affordable.
FD1-S
He also mentioned that if the money available to the organisation was insufficient to provide a quality service, then the consequence was that the service had to be subsidised from elsewhere.
If . . . we’re not able to deliver high-quality care for the amount of money our commissioners give us and we’re not in debate with them that they should pay us more, then we have to agree with another part of the clinical [services] . . . within the organisation that they’re happy to subsidise [this activity].
FD1-S
The new FD described an example in which spending more on quality initially (e.g. the patient being longer in theatre) could be justified by later savings on LoS and, more importantly, avoiding harm.
It would be really interesting for us to be able to understand and demonstrate [for example] that longer in theatre is both clinically safer and cheaper as a consequence. Hell, that would be a brilliant place to be. And by the way, the things that you’re saving are the cost of a surgical site infection, or longer length of stay because you’ve not got the mobility right, or whatever it is.
FD2-S
He went on to reflect that BPTs should reflect this kind of scenario. He also pointed out that if Sybil could demonstrate the costs of best practice, they avoided the risk of tariff underfunding.
I would hope that at some point this idea of moving to best practice tariffs becomes a kind of reality. Well, the best way for you to not experience financial risk is for your best practice tariff to be set at your cost base. Therefore, you’ve got no risk because yours are seen to be the benchmark costs.
FD2-S
He also pointed out that insurers for private patients will make additional payments, for example for nursing acuity (or intensity).
I have first-hand experience, having sat as a director of the joint venture company at the [X hospital], that in the insurance market, it is possible to be paid for a higher level of nursing against certain criteria. So that already exists, and the regulator must be looking across the sector and looking at the multiple players to how their tariffs work. All we need to do is to be able to influence it. You know, if you’re going to influence tariffs, or people setting tariffs, you actually have to have the data to back it up.
FD2-S
In the same vein as BPTs, the next section explores the issues around a particular project to avoid harm to patients, which also saved on resources.
The project to link the avoidance of pressure sores with cost savings
The quality improvement lead mentioned that the CEO had wanted to relate cost with quality, starting out with the cost savings that would accrue to the prevention of pressure sores, but he felt that the inability to control for other types of harm impeded this project.
The CEO started to think about how we demonstrate a link between improving quality outcomes and cost; and we discussed, saying well if we work in the elderly care ward and we eliminate every pressure ulcer but in terms of realising the cost of that if you’ve still got problems with discharge and you’ve still problems with other types of harm happening, so infections, patients falling, then you can’t realise the cost of reducing pressure ulcers.
M1-S
However, he did acknowledge that there was literature that linked pressure sores to increased LoS.
If the literature says that patients who get pressure ulcers stay an extra 2 days, for example, and we reduce pressure ulcers on this ward by 50%, what does that mean in terms of cost for those patients? It was really, really crude.
M1-S
On this basis, the quality lead got in touch with the finance team, but the project seems to flounder because each patient identifier had to be entered separately.
We approached the finance team and said if we get a list of patient numbers, the patient identifiers, can we then pull out the patient-level cost data? And they said yes, you can but it’s manual . . . The idea was we [the Quality Improvement Team] would do that in the short term and in the meantime we would be working with the [finance] team to try and work out a way of automating it. So we give them a list of numbers and then they give the data out, but they couldn’t do it in the end . . . [and] we couldn’t do it because it took far too long for each patient.
M1-S
The corporate accountant remembered this meeting. She seemed to think that the project may have progressed.
About 18 months ago I did spend some time with them [the Quality Improvement team] to show them the patient-level costing information because they were thinking, well, what we’d like to do is to be able to show that, you know, harm is reducing, length of stay is decreasing, but the readmission rate isn’t coming up at the same time. They wanted to show areas of patient care, but actually attach some financial figures to it. They were really interested in the patient level-costing tool and I think they did start using some of that information.
F1-S
In summary, despite the focus on quality at Sybil, the availability of a sophisticated PLICS and the willingness of the finance team to work with the quality team, according to the quality lead, work on relating quality to costs had stalled over fairly simple technical issues (i.e. it took too long to extract the patient-level data and the process could not be automated).
Resource allocation across services and settings
The MD at Sybil was involved with a regional reconfiguration to avoid clinical variation and duplication. He spoke of the tension this posed in terms of Monitor’s anticompetitive regulations.
Currently money is wasted because of variation and duplication. So tonight there will be 10 hospitals across this conurbation trying to service a rota for acute surgery. Ten sets of juniors, 10 sets of registrars, 10 sets of consultants, 10 sets of anaesthetists, 10 sets of theatres, and all of the stuff that goes with it . . . That’s not using public money wisely, is it? . . . The variations come from the need for duplication because everywhere has got to have something . . . the financial sovereignty of organisations could be maintained to prevent Monitor breathing down my neck, I think, for me, the difficulty is not describing it; it’s trying to satisfy regulatory bodies that this is not anticompetitive and it’s not detrimental to the financial standing and sovereignty of organisations, which often, in this conurbation, are FTs.
MD-S
But, with regard to moving activity out of Sybil into the community, unsurprisingly, there was scepticism.
Well, I was involved in the closure of a large [mental health] hospital – it costs precisely twice as much to look after a patient in the community as it did in the institution. So what I think PLICS helps us to do is to [understand this better] . . . there’s often statements made as if community is good for patients and good financially. I don’t know if there’s enough evidence around to say whether we really understand what the true cost of community care is. I’m not sure we even understand what we mean by community care!
FD1-S
The point was also made that if activity is transferred to the community, there is less income available to offset fixed costs.
Some people say it must be cheaper to put things out into the community. What they forget is that as soon as they move some of the cheap and cheerful things out of the hospital, the unit cost of what’s left goes up. Because actually the fixed cost is still the fixed cost. I can’t slice an end off the building and say, we’ll knock that bit down and paper it up again each time a service moves out.
F2-S
As mentioned earlier, Sybil is an integrated provider with access to its own community provision, but even then there were sometimes issues in transferring patients over because of the differing funding mechanisms.
We’ve started using patient-level information to understand what the financial position is of the community strand of anticoagulation [treatment to prevent blood clotting] and the acute strand of anticoagulation. We’re finding that the acute side is funded on a cost per activity – a price per activity basis. So we get paid [by the commissioners] for every patient that we treat, but the community side is under a block contract, well, if we put more into the community, if it’s under a block, we won’t get paid any extra for doing it. So the financial implications of doing that don’t support what we want to do, which is best for the patient.
F1-S
However, when Sybil created its community provision, Monitor’s regime stated that this had to generate a surplus.
When community services were outside the hospital the need to make a surplus wasn’t there. They [community services] had a breakeven duty. Monitor’s regime says you have to make a surplus. So a GP sat there said to me, well, I can’t get my head round this, let’s make it simple, a service that used to cost £100 when we provided it, you want £105 because you’ve got to make a surplus.
F2-S
One clinician reflected on the popular and hence political dimension of hospital closures to concentrate resources in fewer centres.
We have still got too many hospitals per capita. And so you need to shut the hospitals down. But then this is where the motivation between politics and local support for their A&E comes in against the actual strategic health need for a population.
C2-S
Finally, this chapter turns to the related issues of tariffs, the different profitability profiles of specialties and cross-subsidisation.
Tariffs, profitability and cross-subsidisation
The Deputy Director of Finance commented on the proportion of work that was under the national tariff and the inherent risk attached to locally negotiated tariffs which were, generally, more favourable but insecure over the longer term, as the political push intensified for more work to come under the national tariff. This situation created risk for the trusts because their locally agreed income was not assured over time.
About 65% of activity is [reimbursed through the national tariff]. Maybe 70%. But there’s still a huge chunk that’s at local tariff. And that’s a risk for us.
F2-S
The issue of more favourable local tariffs, along with the – sometimes – advantageous national tariffs, resulted in staff at the Sybil trust being cautious about sharing their PLICS data with commissioners.
We are still careful about what we share with commissioners, so we will only – it’s more on a bit of a need-to-know kind of basis.
F1-S
Of the specialties that were under a national tariff, some enjoyed favourable tariffs, whereas others did not.
Neuroscience is a big-time specialty and pretty advantageous tariffs, and there may be other specialties that aren’t. Now, although neuroscience generates significant money for the organisation, they can’t be seen to get special privileges because one of the reasons that they’re generating a lot of money for the organisation is because of advantageous tariffs.
MD-S
The MD commented above that neuroscience cannot be seen to be treated favourably, but subsequently he acknowledged that profitable specialties will be considered for expansion, if this fits with corporate strategy.
If you’re chair of the division that incorporates neurosciences, you can say very legitimately, ‘Well, we’re generating all this income and we need this infrastructure to support it and we need this number of consultants and we need to expand’, so whether it [neuroscience expansion] fits the strategic direction of the organisation is very important.
MD-S
The current strategy at Sybil was to effectively cross-subsidise loss-making services by making the profitable ones contribute more to the cost of overheads.
There are some services that are never going to make money. That doesn’t mean that that money should be spent wilfully; what it does mean is to provide a full portfolio of services that commissioners feel is necessary to service local need with some that are profitable and some that aren’t. So, the overall bit is important. That’s the bit over contribution to trust overheads. It’s a shared, collective thing, if everybody is aware of that, then that’s going to be the ethos of the organisation.
MD-S
The new FD felt that, for the Sybil trust, refining the SLR on a contribution basis was more important than in-depth patient costing.
I think what [Sybil] would do is to pursue – instead of the patient level and going to the bottom, is to pursue the service line reporting contribution and understanding it, and to try to iterate that more frequently. You could move the way in which you actually manage financially from a traditional I&E [income and expenditure] you know, to a kind of contribution service line reporting approach.
FD2-S
The MD commented that differentiation of service lines was necessary to identify whether a speciality was profitable or loss-making.
Cardiology was very much under spotlight. How could cardiology be losing money? The issue was that it was getting wrapped up in general medicine. We hadn’t got our service line costing forensic enough, so it was difficult to be really clear where the issue was in terms of the financial difficulties. Those service lines weren’t as crisp as they are now. Once you’ve got your service line crisp, then you can look at every aspect of the patient care.
MD-S
Unsurprisingly, the clinicians in profitable services were acutely aware that their profit could be negated by their higher contribution, even though the ethos of the organisation was, supposedly, around shared collective endeavour.
The trust always point out your profit, loss and your contribution. So it’s quite interesting in that you can make a profit and you have your costs, but your contribution to the organisation may wipe out your profit.
CD1-S
Additionally, it was becoming clear that, at the executive level, there was no longer an absolute commitment to maintaining a comprehensive portfolio of services.
At the strategy advisory group, the Chief Executive is saying he isn’t precious about which services we provide anymore. Bloody hell, that’s comes out of the Chief Executive’s mouth! Actually the services we want to provide should be the services that are in tune with the core delivery of [the Sybil]. I suspect there’ll be a bit of bargaining that’ll be done at a political level across the city, you know, you want to do neurology, well I want to do cancer. OK, because to be honest, everybody can’t do everything.
F2-S
Indeed, the MD remarked that disinvestments were being discussed, in the context of financial position.
That [disinvestment in services] might be because of reorganisation of services or it might be because it isn’t the way that we would wish to go. So I wouldn’t want to say what the services are because some of them we’re looking at currently, it’s sensitive stuff. But yes, we are looking at services that may be delivered differently, and with this organisation, part of that will be financial consideration.
MD-S
The next section provides a brief summary of the issues at the Sybil trust.
Summary
Compared with the Chelsea trust, and in line with the Gertrude trust, the Sybil trust was more concerned with SLR and less focused on the accuracy of the PLICS. This, of course, reflected the nature of the Sybil and the Gertrude trusts as large, complex, multispecialty teaching hospitals that use cross-subsidisation, setting a higher level of contribution to overheads for profitable specialties, to maintain a full portfolio of services. However, at the Sybil trust, there were indications that this commitment to a comprehensive set of services was under review, not least for financial reasons. This review was understandable in the context of the regional initiative to rationalise services and create further specialisation in dedicated centres to avoid duplication and variation in terms of the quality of care delivered to patients.
Given the continued focus on the quality improvement strategy and the longevity of the PLICS, more progress on demonstrating the cost of patient harms might have been expected, particularly as the CEO had shown a personal interest in this project. Another area that seems not to have realised its full potential is the integration between the acute and community arms of the Sybil trust. However, as one of the UK’s best-performing hospitals, the Sybil trust did appear to be exploiting its PLICS well to understand cost drivers, with a particular focus on the efficient utilisation of its 19 theatres.
Chapter 7 The Chelsea case study
Introduction
This hospital is anonymised under the name Chelsea. It was selected from our survey as a case study site. In addition, at the Gertrude site, it was mentioned as having a ‘leading edge’ in its use of PLICSs; it reported level 4 engagement with the PLICS data and, as a specialist site, its inclusion offered an interesting contrast to our large multispecialist trust case studies.
Brief background to the case
Chelsea is one of Europe’s leading centres for the treatment of cancer. It serves a local population of 3.5 million and treats 40,000 patients per year. As a specialist centre, 26% of patients come from outside the local area. The hospital has an annual budget of £190M and employs 2500 staff. A PLICS was introduced in 2008 within the context of SLR. The hospital participates in a national patient cost benchmarking group.
Chelsea’s portfolio comprises:
-
NHS services for radiotherapy, chemotherapy, specialist surgery for rare and complex cancer, and support and diagnostics.
-
Research and education through the Chelsea School of Oncology. The Chelsea is also the largest early-phase trial unit in the world.
-
Joint venture with an international private provider.
-
Chelsea charity, which is the second largest hospital charity in the country.
A sense of the scope and level of detail in the hospital’s PLICS is given by the following patient pathway (source: An Introduction to Patient Level Information & Costing Systems, Trust internal document):
-
Patient X attends for a review with a consultant (£280) and positron emission tomography–computed tomography is undertaken to assist with diagnosis (£700).
-
The patient is admitted for robotic surgery for prostate cancer (total cost £13,654). This total cost consists of theatre costs (consumables £3162 + nursing and anaesthetics £1585 + other £2101); a ward stay of 3 days (£630); medical costs (both theatre and ward, £1105); pathology costs (£1001); blood costs (£510); and specialist nursing (£125). For example, theatre consumables for this patient included items such as 10 swabs (£4–50); one two-way catheter (£8.55); a urometer (£5.93); and a sterile hypodermic needle (£0.02).
-
Later the patient attends for a follow-up in clinic (£175).
Financial management strategy
The finance team highlighted the following five features of their financial management strategy:
-
Divisional performance reviews take place monthly; they are used, inter alia, to interrogate divisions on any of their HRGs that have been identified as high cost.
-
Income is distributed to divisions using cost allocation from PLICSs. This incentivises divisions to validate their methodologies.
-
Service line position is used to set some CIP targets. The rationale of this is that those with an adverse position should be more able to make savings.
-
There is a trust-wide Directory of Care. This covers all patient pathways to allow patient-level activity to be benchmarked against national or locally agreed protocols for treatment. Variations against these protocols can, therefore, be confronted and discussed at the level of the clinical team.
-
All business cases for investment must use PLICSs to analyse the service line position. If the service line is loss-making, the trust will not invest without a complete understanding of the drivers behind the financial position. 168
Financial position
Over and above the use of the PLICS for cost improvement, clinical engagement, comparing cost with quality and clinical protocols to link across services, the specialist nature of Chelsea drove one of its key diagnostic uses of the PLICS – to ascertain the extent to which the complexity of the treatments and care delivered was compensated via the tariff and lobby for change if the tariff was thought to be inadequate (see Tariffs and lobbying for change).
Tariff issues were significant because, although the trust was profitable overall, it was making a loss on admitted PbR activity. Financial viability was maintained through the private patient joint venture, the use of charitable monies for financing new developments and negotiating local prices with commissioners for activities not historically included in PbR, such as chemotherapy and radiotherapy. However, PbR was extended to chemotherapy and radiotherapy in the 2013–14 guidance, albeit in a phased manner in recognition of the impact on specialist trusts of a move from locally agreed to national prices. 166 At the time of the research (2013–14), it was not entirely clear how the inclusion of chemotherapy and radiotherapy in PbR would play out financially for Chelsea but there had been, for some time, knowledge that having most of the activity at locally agreed rates was a strategic risk. At the trust, there was considerable concern about this issue both within the finance team and more widely in the clinical community.
Guide to interviewees
As mentioned in Chapter 3, in terms of the clinicians, we generally sought to interview clinical directors and the MD. These designations were not used at Chelsea, but the clinicians interviewed had the status and responsibilities of clinical directors. In some instances we approached the clinicians directly; in others we were guided by the finance team, which advised us that the individual concerned had a special interest in PLICSs. During the time of the research the Director of Finance was on long-term sick leave, hence we interviewed the deputy. Table 10 gives details of the 12 interviewees at Chelsea.
| Role/job title | Date | Code |
|---|---|---|
| Deputy Director of Finance | 10 December 2013 | F1-C |
| Finance Manager Income and Costing | 14 August 2013 | F2-C |
| Interim Chief Executive | 1 April 2014 | CE-C |
| Quality Manager Haematology | 28 May 2014 | M1-C |
| Consultant Clinical Oncologist | 16 July 2014 | CD1-C |
| Consultant Haematologist and Head of the Stem Cell Transplant Programme | 30 September 2013 | CD2-C |
| Consultant in Anaesthesia and Critical Care, Director of Acute and Critical Care | 9 May 2014 | CD3-C |
| Consultant Haematologist, Director of the Haematology and Transplant Unit, with Business Manager | 30 May 2014 | CD4-C and M2-C |
| Consultant Plastic Surgeon | 19 May 2014 | C1-C |
| Consultant Surgeon Urology | 3 February 2014 | CD5-C |
| Consultant Clinical Oncologist | 19 August 2014 | CD6-C |
Empirical findings
Chelsea is a relatively small, specialist hospital that has a degree of independence from the major multispecialist trusts nearby. One respondent compared Chelsea to a fast-moving pirate gunboat surrounded by large tankers.
The thing about [Chelsea] is it’s a small organisation and it’s got a very close relationship. That doesn’t mean to say we’re all happy-clappy bunnies, but we know each other very well . . . it’s a bit like a Somali gunboat in the middle of tankers, it has a great deal of ability to change direction, be very agile on its feet and it has to be, because if it stays still, one of these great big supertankers . . . would just drive straight over us.
CD3-C
As in previous chapters, this section is organised around our PLICS-related research objectives and questions, encompassing cost improvement, clinical engagement, linking costs with quality and resource allocation across services and settings. For Chelsea we also include two other important PLICS-related issues: Tariffs and lobbying for change and Any qualified providers, joint ventures and private patients.
As discussed in Chapter 2, as a specialist trust undertaking complex cancer cases, Chelsea had particular problems with PbR tariffs; it used the PLICS as a diagnostic tool to analyse tariff shortfalls and to try to influence tariff setting. Chelsea had also undertaken a joint venture with an American private health-care provider. The PLICS data were the basis of this partnership.
Before presenting the evidence on the specific uses, one point to emphasise is that the PLICS is mostly a strategic tool at the Chelsea site. Operational financial management occurs through monthly budgets and SLR.
The patient-level information and costing system is a strategic tool at the Chelsea site
The finance team identified these strategic uses.
[The] PLICS is more a strategic tool, we only run ours quarterly, it’s going to inform business cases, help us do evaluations of services, inform our pricing, it’s going to help work we’re doing around clinical outcomes and how we can start bringing those things together.
F1-C
Another pertinent issue, especially regarding clinical engagement with PLICSs, is that the clinical specialties are managed on budgets that do not directly reflect the income they generate.
Service line reporting comes out of PLICS. But the issue with trying to have a conversation with the divisions is they’ve got a very different view of the world because they’ve got budgets, that’s their monthly stuff and that’s how they’re actually being managed.
F2-C
Another related aspect of the strategic use of PLICSs is that its messages could adversely impact on patient care.
[Y]ou could use PLICS, if somebody was really hard core . . . detrimentally [and] affect patient care . . . for example, if you coldly used the business model . . . that comes out of PLICS, you would be . . . ‘[This] is completely losing us loads of money. Let’s not invest in that, let’s get rid of it.’ So you would be taking away that service . . .
M2-C
With this context in place, we first discuss the use of PLICSs in cost improvement, which, as discussed in Chapter 2, the government has organised as CIPs.
Cost improvement
The first point made by one of the clinicians is that, in the past, units could meet their CIP by generating more income rather than by reducing costs.
The problem is you can’t use service expansion as a quasi, you can’t say, ‘To keep our CIP target by having the same costs, I’m doing 4% more’. Last year that wasn’t allowed.
CD3-C
With this restriction in place, cost improvement focused on increasing day cases, reducing LoS and identifying areas in which tariffs were not being claimed.
Day cases, length of stay and unfunded work
The use of PLICSs has given more rigour to traditional modes of cost improvement, such as increasing the number of day cases and reducing LoS.
We’ve been working very hard in the last 5 or 6 years to try and move services from an inpatient to a day-treatment basis. We had a lot of success with that . . . The inpatient bed provision here has reduced by nearly a third in the last 5 years.
CE-C
An example of this was sentinel lymph node treatment:
One effective thing which we did was the – from a 2-day stay of a sentinel lymph node to a day care procedure – now we do about 170, 180 patients of these cases in a year and all of them, 99% are day cases.
C1-C
There was also a rather entrepreneurial clinician who looked to accommodate the first nights of his inpatients elsewhere! It should be recognised, however, that this is a ‘saving’ only if the bed is being used by another patient, because the fixed costs are still incurred if the bed is empty. So, although ‘putting them up at the Premier Inn™’ [Whitbread Group PLC, Dunstable, UK] may feature as a saving on a specialty budget, it is not necessarily a saving to the trust.
Some of our patients travel from quite far, we’ll bring them in the night before, so it costs us another £400. So why don’t we just go to Premier Inn across the road and say, ‘Well, can you put our patients up for £29?’ and we’ll save more than £350 straightaway. There’s little things like that – and it gets quite exciting, ‘I like it, we’re actually making a difference here’.
CD5-C
Clinicians recognised that the impact of LoS was non-linear.
The difference between profit and loss is all about length of stay. If the length of stay is long, the hospital is in crisis. If it’s short, it’s in profit. And the difference is a non-linear relationship. It’s not a linear one . . . All you need is a few long-stay patients to completely throw your average.
CD3-C
Managers used PLICSs to pinpoint work that was not being funded.
[O]ur endocrinology department is quite a small department. The clinicians are really enthusiastic . . . We wanted to do a complete service review . . . I started looking at some activity and I was . . . where’s the income, there’s no income attached to that, and then what transpired was we hadn’t gone to commissioners historically and asked for any money. And basically I went to the commissioners, did a proposal and they agreed it. So that’s an extra hundred thousand pounds a year into the service that we found through going through PLICS[s].
M1-C
Even where work attracted a tariff, some areas at the Chelsea site were always loss-making.
Loss-making health resource groups
Chelsea undertook work to identify the biggest loss-making HRGs:
Any inpatient stay you lose money, thousands and thousands of pounds. So we started looking at the high, the most expensive HRGs in the trust and the ones that had the biggest margin of deficit, so [for us] it was . . . basically the haematology [patients].
M1-C
Furthermore, costs were compared within the NBG.
So she [the Deputy Director of Finance] has done some work on clinical haematology where she’s grouped patients based on cost . . . So she could say, ‘As the cost goes like that, is there any correlation between that and comorbidities?’. If you benchmark that against the rest of the world – which is what we did through the [benchmarking] group – you’ll see that correlation. So if the cost is going like that with the complexity but the income is flat lining then, we sit up.
F1-C
Figure 22 quantifies income and cost, demonstrating that it is most problematic for emergency inpatients.
FIGURE 22.
Chelsea: clinical haematology service line position.

The financial position of the haematology department exemplifies the strategic risks as more of the Chelsea’s activity moves onto PbR. The Chief Executive identified this as a driver for initiating a PLICS.
Having so much of our activity outside of the national tariff was a big strategic risk . . . it’s certainly identified as one of the reasons why we drove so hard to get what I think is quite a sophisticated patient-level information costing system in place.
CE-C
We next present evidence on the in-depth work being done at the Chelsea site on understanding the impact of the tariff.
Tariffs and lobbying for change
The first point made by the finance team on tariffs is that clinicians should not use arguments about inadequate tariffs as a way of avoiding efficiency savings.
There’s winners and losers on the tariff, aren’t there? The difficulty is, when I talk to clinicians it’s very easy for them to say the tariff isn’t right . . . There are tariff elements, but there’s also efficiency savings that you can make and it’s trying not to detract from the second.
F2-C
Staff at Chelsea recognised the strategic risk of having favourable local prices, given that national tariffs for chemotherapy and radiotherapy were being introduced.
Up until April this year we had local prices for chemotherapy and radiotherapy. These only became a national tariff on the 1st of April. So we went from an organisation that was 70% funded through local arrangements last year, to one that’s 70% funded through PbR . . . luckily we’ve been able to manage this year.
F2-C
This situation raised the issue of whether or not this specialist trust which had had a PLICS for 6 years could seek to influence the process of tariff setting.
We are very, very privileged to have – one of our professors is the chair of the clinical reference group [CRG] for X service . . . that particular CRG was one of the first to be formed. So [we were well positioned] in terms of price setting and tariffs and things like that to influence that work . . . Even though there’s still things that he [the professor/chair] would argue are not just right, it’s only with the PLICS data to support that we are in the position we are for X service.
F1-C
One clinician felt that Chelsea should be a pioneer in setting a tariff that was advantageous for it.
[We are] looking at PLICS as a potential tool to try to address both our local issues and to try and iron out how that was going to impact nationally. We can be part of somebody else’s costing model or we can pioneer and take it forward and actually set the tariff. It might be somewhat self-serving, but ultimately, if we get it right for us, then who cares about how it works for everybody else?
CD2-C
Even though there had been anxiety that national tariffs for chemotherapy and radiotherapy would disadvantage Chelsea, that was not always the case.
So, radiology is now more secure because, with the [national] tariff, radiology now actually attracts money. And, speaking to my radiology colleagues, they actually feel they do pretty well out of it. They get a lot of inward investment and they seem to be making money.
CD2-C
The Chelsea has two satellite services; one was judged to be particularly profitable as regards their chemotherapy service.
We had a chemotherapy service at X satellite. They were earning a fortune from the delivery of the chemotherapy under our governance. So we said to them, look . . . it should be an open book thing. We don’t want you to lose money. We don’t want you to make money. What we would like [is] for the patients to have access to local chemotherapy of a high standard.
CD1-C
For inpatient work at the Chelsea, although transplants were profitable, most procedures made a loss.
The way that the tariff has generally been set for the transplant-related work, it generates a significant surplus. For the non-transplant in-patient work where it’s complex and very time consuming and often with very expensive drugs, it always generates a loss. And as a general rule, I think there’s a sort of an acceptance . . . that anything that requires a patient to be in hospital because of the staffing and infrastructure requirement, almost invariably generates a significant loss for the organisation.
CD4-C
One of the business managers cited a specific inpatient loss-making example:
For example, an induction chemotherapy patient normally stays in for about a month, the tariff that we get is about £3000 . . . whereas our cost of the ward is about £700 a night and each patient that comes through costs about 20 grand.
M1-C
Patient-level information and costing systems also informed calculations on the PbR underfunding of complex care. Along with input from the NBG, work at the Chelsea site identified four hypotheses on the drivers of this underfunding:
-
Reference cost calculations allocate cost on the basis of LoS rather than intensity of care, resulting in the undercosting of complex care.
-
Reference costs are collected on a finished-consultant-episode (FCE) basis and then converted to spells of care to construct tariffs. This results in distortions because organisations define FCEs differently.
-
Patients with cancer are expensive to treat. HRGs are procedure driven, and tariffs will average the cost of treating patients with cancer and those without. Therefore, specialist trusts for cancer patients will be underfunded.
-
Trusts with a more complex case mix will treat a lower proportion as day cases. Day cases and elective inpatients attract the same payment but incur different levels of cost; thus, trusts with a complex case mix are underfunded.
The findings on the above hypotheses were:
-
This hypothesis was upheld: underfunding was calculated to be £3.7M.
-
This aspect of tariff construction was found to benefit Chelsea by £1.1M.
-
Chelsea has a high proportion of patients with cancer: 86% compared with a national average of 19% for the same mix of HRGs. The financial impact of this was a loss of £1.8M, which is strong evidence for the introduction of separate HRGs for patients with and without cancer.
-
The day case rate at Chelsea was 43% lower than the national average for its case mix. The financial impact of this was £3.0M.
(Source: Analysis of the PbR Tariff and Underfunding of Complex Activity. Trust internal document.)
However, one clinician thought that some tariffs had become too generous:
People look at tariffs on a regular basis, someone looked [at] them for penile implant surgery, they used to be around £3500 but they [implants] vary in price from around £1200 to £10,000, depending on which model. So if you’re putting in let’s say a £7000 prosthesis, you’re not going to hit the target. So someone changed the tariff to £7500. I think it’s wrong. The more expensive ones are made by a different company and give you more girth. You know, most of these men are really satisfied they can just get an erection . . . that’s a good example of the way the tariff has been bastardised, in my view.
CD5-C
Although managers and clinicians did not, generally, question PbR and tariffs, some were worried about the potential impact of identifying profitable and unprofitable services.
So my concern is there will come a point where they’ll [Chelsea senior management] go, ‘Why should we be doing this, when the hospital down the road, is doing all the stuff that’s making the money and referring all the stuff that’s really complex and costly to us?’. But at some point someone’s got to do those operations.
CD5-C
The chief executive commented that the board had raised the issue of disinvesting in unprofitable areas and felt that Monitor was behind this suggestion.
The real question is, ‘What is a comprehensive cancer centre’? Why would we divest ourselves of something that’s not as profitable; just because we understand the profitability question . . . But I think, when we were first a foundation trust, the board were pushing at that question; mainly I think because the regulator and others said you should be divesting yourself of things that are not profitable.
CE-C
As also occurs at the other case study sites, the current position at Chelsea equates to cross-subsidisation.
There are some service lines that will make money and some that will make less money and ultimately it all feeds the bottom line. Ultimately, within that, there will be informal cross-subsidy in that the better performing services will be subsidising the not so well performing services.
CD2-C
The Deputy Director of Finance made the point that Monitor was currently exploring alternatives to PbR.
I think Monitor will probably question PbR. They may even price services rather than activity because there is always a perverse incentive with PbR . . . It pays you for what you do, so it does incentivise you to do more activity. So I think they will look at alternatives. On the flip side of that, if activity drops then you could be destabilising services.
F1-C
A PLICS was introduced in 2008 at Chelsea. The finance team had had considerable time to involve clinicians with the PLICS. They had concluded that mandatory PLICS use in business cases for expansion offered the best leverage.
Clinical engagement
The first point made by the finance team was to pinpoint the big cultural shift in the clinical community.
There’s a very big change in the NHS, clinicians are actually starting to understand the business.
F2-C
Business cases
The finance team at Chelsea made a strategic decision to engage clinicians with the PLICS through making PLICS data a requirement for all business cases involving investment. The Deputy Director of Finance comments:
We also have made it [PLICS] a requirement of businesses cases . . . So for any big business case, we’d expect to see a 15% improvement in the service line position as a result of the proposals.
F1-C
This focus on the use of business cases as leverage to engage clinicians with finance was echoed by the cost accountant:
In all honesty, the way that it’s [clinical engagement] worked best for us has been through business cases. You need clinicians’ buy in, and part of that buy in is where they can genuinely see that something better is going to happen for their patients.
F2-C
The service being considered for investment could be loss-making but, nevertheless, a full analysis of the financial position (present and future) using PLICSs may secure resources. 168 This was the situation regarding the business case for a proposed merger between two units, the haematology transplant unit (HTU) and the young oncology unit (YOU).
The past loss-making service line positions for the HTU and the YOU were analysed using the PLICS data and are presented in Table 11.
| Contribution breakdown (£) | Time period for unit | |||
|---|---|---|---|---|
| 2010–11 | 2011–12 (baseline) | |||
| YOU | HTU | YOU | HTU | |
| Tariff income | 2,064,063 | 10,129,514 | 2,603,369 | 11,483,313 |
| Total cost | 4,177,971 | 11,024,269 | 4,287,128 | 12,636,595 |
| Contribution | –2,113,909 | –894,755 | –1,683,759 | –1,168,282 |
| Total contribution | –3,008,664 | –2,852,041 | ||
The cost accountant remarks on their proposed merger:
In clinical haematology, there is a ward [the HTU] in desperate need of updating, it was very old, very tired, and there’s a capacity issue as well. When they [clinicians] said we want a new build, it was right, OK, let’s have a look. We’re [the trust] losing quite a lot of money [in your service line] . . . Through that business case, bringing the young oncology unit and clinical haematology into one area, you’ve got staffing efficiencies . . . And we could also look if we’re bringing people in for chemotherapy, do we want them in as day cases? . . . that business case was approved by the board on the understanding – they weren’t expecting a profit situation, but they wanted to see a better contribution, going forward, to overheads.
F2-C
The Chelsea board of directors agreed in 2012 that any loss-making services that required capital investment would have to show at least a 15% improvement, the business case for the merger estimated income growth of 30.5%, on the basis of staffing efficiencies and release of rent, when set against an allowance for mixed-sex accommodation in the new unit [Business Case (Calculations): Integrated YOU and HTU, 2012. Internal trust document].
One difficulty in achieving the promised income growth was income instability when tariffs changed.
Part of the criticism from Monitor is that, and it’s a criticism from ourselves as well, is that tariffs change year on year. So a business case in 1 year might be effective and then all of a sudden the next year, oh crikey, the tariff’s dropped so that’s very difficult to plan in a system where your income shifts are so great year on year.
F2-C
Despite these problems for financial planning, one of the consultants in clinical haematology was satisfied that his personal commitment to understanding and using the PLICS data in the business case had delivered dividends in terms of the capital spend he had secured for his unit.
I wouldn’t say just because of this [understanding PLICS] we’ve managed to secure a £15 million capital investment, but it has helped. I think the confidence that the trust, the organisation, has in our service . . . They believe the activity plan, they believe that that increase in growth will generate income, and it gets supported with capital spend . . . now we’ve got a new ward and we’re also in advanced stages that we’re going to get a new outpatient facility . . . I suppose there is a self-serving part to this, but I think you definitely can see the advantages of doing that [incorporating costing in the business case], because I knew it would work.
CD4-C
The above consultant was one of the clinical champions for the use of the PLICS data at Chelsea; he copresented both internally and externally with the Deputy Director of Finance on the topic of ‘Efficiency or anomaly: Using PLICSs/SLR to understand national tariff’.
Clinical champions
The PLICS was introduced by the finance team in 2008, but they soon recognised the need for clinical champions, along with support at board level.
You need your board-level buy-in, you need your clinical champions, it needs to be something that’s embedded throughout the organisation. And when we originally had PLICS brought in – it started as a finance tool within the costing team – but it needs to have that gravitas, I think, to really start to make some differences.
F1-C
The clinical champion from haematology, along with the Deputy Director of Finance, identified the following reasons why senior clinicians and the finance team need to work together:
-
to ensure that coding is accurate and to select the optimal HRG classification
-
to assist in developing reference costs that are meaningful
-
to ensure the necessary clinical ownership for the acceptance and meaningful use of PLICS data
-
finance personnel require clinical expertise to help them understand patient cost variations.
(Source: Efficiency or Anomaly: Using PLICS/SLR to Understand National Tariff. Trust internal document.)
The Deputy Director of Finance’s strategy was to identify clinical champions in loss-making services and work with them to interrogate the cost and income data.
We’re empowering them [clinicians] to take ownership of it, this is not a finance tool. And I would want them to interrogate the information themselves, raise questions with us, rather than us going out to do that individually . . . we do have clinical champions against every speciality . . . at the moment we’re focusing on surgery. Surgery is where we have the most loss making services . . . We haven’t gone big bang, we haven’t got the engagement that we want across all areas to the same extent.
F1-C
Clinical variation on resource use
Another key issue for clinical engagement is to pinpoint the areas in which there is cost variability between clinicians for the same procedure.
What you’re looking for when you’re looking at [patient cost] variability is some consultant who is doing some different practice to somebody else. And then you can challenge that variability.
F1-C
An answer to idiosyncratic practice is to ensure that clinicians are working to protocols, an added benefit for patients.
Has this cohort of patients received two, three, four times more radiology tests than you would have expected on that patient pathway? Is there a common factor in the type of patient or is it a more junior consultant who is just a bit unsure? Is there a training issue? There are other benefits as well, the protocol can go to the patients in a leaflet . . . this is what’s going to happen to you [the patient] so they know exactly.
F2-C
However, access to bottom-up PLICS data also informs clinicians on costs.
Clinicians’ challenge on the basis of cost data
Having costing expertise can also enable clinicians to challenge where they think internal changing is unfair; the following quotation relates to resentment over theatre costs.
I’m one of those nuisance doctors, all right? I am one of the few that can actually read a spreadsheet . . . Theatre costs, for example . . . So, what’s the theatre time we have and, OK, per minute it’s this much, and the costing on PLICS is, about twice what it [actually] costs. I know what their staffing costs are . . . because I’ve seen the budget line so I know exactly how much the total salary – the total budget is. I know how many theatre hours she’s providing for that. It’s a back of the cigarette packet calculation. The PLICS costs are double that.
CD3-C
In a similar way, clinicians are astounded by the way overheads work.
For example, I see a patient in outpatients, I would just assume there was just my cost as I’m the only person involved. I didn’t realise that, actually, from that £107 that we’re getting [from the tariff] they [finance] will take a little bit for the cleaner, they’ll take little bit for estates, they’ll take a little bit for reception, they’ll take a little bit for the consultant, a little bit for the nurse, a little bit for the lighting . . . I mean I was just gobsmacked by it.
CD5-C
Using patient-level information and costing systems to relate cost to quality
One clinician pointed out that the new regulatory regime had, perhaps inevitably, driven an emphasis on cost rather than quality.
If you’re not financially viable then Monitor will come to call and the institution gets shut down. Quality has perhaps come second to that.
CD2-C
Another clinician expressed the view that the Chelsea site had started working on linking cost and quality (in the sense of clinical outcomes), even in the absence of a national framework.
Obviously in time, it would be nice if we could actually relate it [cost] to improved outcomes, but there’s nothing nationally to relate cost either to quality or to outcomes. We’ve actually taken a lead in terms of outcomes in the Chelsea, and I think we’re not too far away from being able to link the two.
CD1-C
A manager also pointed to the absence of any national quality framework.
I think it’s quite difficult to measure outcome apart from patient deaths and relating that to treatment . . . there isn’t an established national data set that’s mandated for every patient diagnosed with cancer . . . it’s the sort of thing that you could bring in.
M2-C
Another clinician’s expectation was that higher quality and lower cost were correlated.
Just chase the quality and forget about the cost attached to it. Or, obviously, keep an eye on it. Make sure it’s not putting the costs up, but by and large, most quality measures tend to be in alignment with costs savings. But, if you say, ‘We’re doing this to save money’; you tend to immediately result in a lesser [clinical] engagement.
CD3-C
The finance team were hopeful about linking the PLICS data with clinical outcomes.
The clinical outcomes team are looking at cancer outcomes and I’m hoping that linking in with them will be a way of me being able to identify [the patients] within PLICS.
F2-C
This expectation was also present in the clinical community.
It’s not just about money you are [also] looking at improving the quality . . . We’ve got big CQUIN targets in the Trust. The financial penalties for not achieving quality indicators are now sufficiently large that the two [cost and quality] run together. So, certainly for investment through the CQUIN targets, we’ve been able to use some of the patient-level costing to demonstrate financial sustainability of the service . . . the next part of is then [measuring] quality. So . . . we can then feed back to square the circle.
CD2-C
However, the clinical lead for the Clinical Outcomes Group pointed out a problem in relating costs to clinical outcomes – the finance office only costed procedures they could charge for.
An example is cervical cancer, the curative treatment here is chemoradiotherapy. They [finance] said to us that something like 70% of the patients were treated with chemotherapy and 10% of the patients were treated with radiotherapy . . . That’s not true, that’s against the NICE guidelines . . . The patients got chemoradiotherapy but they [finance] said they [the patients] only got chemotherapy because that’s the bit they charge for.
CD6-C
Indeed, one manager remarked that the drive for income generation could actually distort resource allocation in a publicly funded NHS.
In the States, some procedures are offered because of the financial benefit to the hospital . . . protons [proton beam therapy] is quite widely used for prostate cancers [but data are] showing that the outcomes from having proton treatment is no different than having standard radiotherapy . . . when we’ve been developing our proton process, we’ve not looked at [the impact for] prostate patients but from an income generation perspective, it could be quite lucrative.
M2-C
One clinician pointed out that outcomes should not be related to treatments without taking account of relevant patient and tumour differences.
You’ve got to be very careful with outcomes, not to directly link it to treatments whilst ignoring patient and tumour-related factors. So whatever outcome data you have, those are the three components. People, all the time, ignore patient and tumour related differences.
CD1-C
He went on to explain these patient differences.
There’s a global score [for patients], which is performance status. So, looking at you, you look like performance status zero. If you had chronic bronchitis and one leg and you were diabetic and so on, you might be performance status 2. You might spend a lot of your time sitting in a chair at home. So those are sort of international standards. Quite crude . . . That’s how fit is the patient, because the patient’s fitness is, obviously, a huge determining factor for outcomes.
CD1-C
In terms of tumour-related differences, there is a staging assessment.
I treat cervical cancer . . . The survival from stage 1 disease is 95% because, you know what, you’d be hard pushed not to be cured, my patients have got much bigger tumours, they’ve been found much later, some of them are still called stage 1 but usually are 2 or 3 or whatever. But my chance of curing them is completely different because they’ve got a really big tumour now . . . in the grand scheme of the UK we’d [at the Chelsea] look rubbish . . . [so we] document our patients really tightly, so we can go, yes, but they all had 4-cm tumours.
CD6-C
There is, however, a moral hazard element to this increased emphasis on outcomes.
What they’ve done in surgery now, is they’re focusing on individual surgical outcomes. So the surgeons now are very aware of their outcomes. A potential danger is that they would look at the list of patients and think, hmm, they’re riskier, I’m just not going to go there.
CD1-C
Another aspect of quality services and improved outcomes is the concentration of expertise in specialist centres, which should reduce costs and enhance outcomes.
Centres of expertise as quality assurance
This next clinician has been proactive in promoting change to create a smaller number of centres of expertise.
I submitted a paper to NHS England to say that radiotherapy is provided by 50 providers at the moment, that’s just grown up in an ad hoc way . . . there’s a great deceit going on because you might go to your local oncologist, a very nice person, and get your treatment locally and you’re very happy. What you don’t know is actually there’s a difference in quality. Small centres, they specialise in four or five different cancers; they can’t really keep abreast with that number of subspecialisations, but their patients never know that. So the proposal is to actually have partnerships and go from 50 down to 20, for instance. And then you’ve got much bigger teams and critical mass, much greater interest in clinical trials, cross-cover and all the rest of it. So that’s been proposed and NHS England seem to like it.
CD1-C
The same clinician identified patient throughput as an enabler of expertise.
I think there’s an acceptance that throughput is promoting expertise, and should provide better outcomes. We haven’t really got granular evidence to support that in radiotherapy, but people accept the principle. So things like brachytherapy, we do 150 radioactive iodines a year; some centres are doing 10. That doesn’t make any clinical sense. [And] doesn’t make any financial sense. An awful lot in the NHS is ad hoc, provider-led, and totally irrational.
CD1-C
Another issue, in terms of public awareness, is the lack of transparency on the performance of small centres.
At the moment, poor service, poor quality, is under the radar. We publish all our results here, but the small centres don’t publish anything. So how do you know they’re bad? With difficulty. So I think, yeah, it’s a real problem within the NHS.
CD1-C
The creation of centres of excellence is an aspect of our research focus on better allocative efficiency of resources within health-care economies. Next, we explore work at Chelsea on care pathways. As a specialist centre doing tertiary work, they did not work with GPs on care pathways but they did look to improve pathways within the trust. This work was organised under the label of ‘Directory of Care’.
Care pathways and the Directory of Care
Managers at the Chelsea were aware that using PLICSs along a care pathway could identify the potential for moving services to more cost-effective settings, but one manager concluded that there had been little progress on this.
[W]hen we set out the potential [pathway] uses of PLICS [it] was that it would benefit the whole health economy so that individual organisations would move services away from them to maybe a community if that was more cost effective. And they might lose income but it would be better for the patient and it would be better for the economy and the area . . . it’s quite – it’s a nice idea. I mean we haven’t found much of that yet.
M1-C
However, work on internal pathways within the trust was ongoing. At Chelsea, the objectives of the Directory of Care were to:
-
create a directory at disease-group level
-
standardise pathways across the trust: include standard care plans and written protocols that are accessible to GPs and patients
-
improve service quality and patient experience
-
reduce clinical variation
-
support auditable reporting.
As a result of implementing the Directory of Care, it was anticipated that it should be possible to:
-
release savings through increases in efficiency
-
enhance benchmarking through information transfer between centres.
(Source: An Introduction to Patient Level Information & Costing Systems. Trust internal document.)
The finance team were looking to cost care pathways but had been tasked with identifying the areas in which protocols were not being adhered to.
Another area . . . is a Directory of Care. So all the clinical teams are pulling together very standardised protocols for patients. So if a patient presents with X, we would expect them to come in and within that first appointment, we would expect them to have a CT scan on their abdomen, a profile pathology test and three lots of chemotherapy. Pathology costs, drug costs, costs associated with the consultant, nurse . . . So you can build up a profile of these patients and what you expect the cost to be. What I have been tasked with is looking at what’s actually happening to these patients compared to that standardised pathway.
F2-C
An example is taking blood before operations in theatre.
Looking at the patient pathway, are there any potential savings? A very simple example . . . when you go through it with the clinician, that’s four bloods being ordered going down to theatre, generally we only use two. Let’s go for two – that’s 400 quid saving, straight up.
F2-C
Having standardised, clinically effective pathways can be used as a mechanism for arguing for BPTs but, as a specialist trust, Chelsea found that the DH was not receptive.
I’ve worked with ‘A’ [senior clinician] on developing best practice tariffs . . . we put a proposal to the Department of Health, which unfortunately they didn’t take forward because they were looking for schemes that could run across the whole country rather than quite a specialist area . . . And we tried our best to develop that pathway and put a price around what that should cost. But unless you get that buy in nationally there’s only so far you can go with things like that.
F1-C
The clinicians felt that best practice pathways should attract BPTs.
The head and neck cancer patients should be having certain scans, a named specialist nurse, speech therapy input, a dental assessment before radiotherapy. I mean, we know that sometimes they do and sometimes they don’t. There might be a way of saying, well actually, if you do it properly, you get paid more.
CD1-C
Any qualified providers, joint ventures and private patients
As mentioned earlier, the income from private patients is one part of the Chelsea portfolio that ensures its financial viability. Income from private patients is about £10M per year, representing about 6% of total income. However, prior to the PLICS implementation, although the finance team knew the income, they did not know the associated costs.
Previously our private patient costs – 5, 6 years ago, we couldn’t tell you what our trading account was for private patients. They were just another set of patients. So we knew what the income was, we had 2–3 million, but we didn’t have a PLICs system, so we didn’t know what costs were associated.
F2-C
Now private patients are part of a joint venture with [X] – a private provider. This £14M joint venture was set up in 2013 to create a new cancer clinic for private patients.
We developed the trading account [for private patients] with the PLICS system but now we’re in joint partnership with X, an American company, so they’re private patients with the joint venture. They still trade with us, we will still do tests for them, et cetera, and we charge the joint venture, the joint venture’s owned by the Chelsea and X. And there’s a profit share agreement and all the rest of it.
F2-C
The following clinician argued that much of the income from patients under the joint venture was invested in NHS services.
What the [Chelsea] has seen working in partnership with X is that, rather than a private provider generating huge surplus, it’s a partnership and a substantial proportion of that money is then used to reinvest into NHS services, so impacts upon what our cost improvement plan would be, what business cases might be approved, what capital developments might go ahead, all as a consequence of working in a partnership with a private company . . . turning around what was a very small element of private practice, making it substantially larger.
CD4-C
The same clinician stated that the financial growth in income from private patients was not so much the greater numbers, but the ability of the partner in the joint venture to negotiate with the insurance companies.
In fact, there’s been a modest increase in numbers but the actual financial growth has been quite significant because, you know, negotiated tariffs. If you’ve got a – you could describe it as being a relatively ruthless or more sophisticated international company negotiating what the tariff is, you’re going to get preferential terms as opposed to an NHS organisation negotiating with BUPA [British United Provident Association Ltd, London, UK], that’s made a huge difference I think.
CD4-C
He ended by reflecting on the different ethos the private-sector partner had brought to the negotiating table.
You don’t like to think of yourself as a bit part-time and a bit rubbish at doing things but looking back at it, the [Chelsea] had private activity but it kind of just bumbled along. Whereas if you bring in people with a completely different background who don’t come from an NHS perspective and are ruthless about saying, we’re in this to make money, then, not surprisingly, it generates a greater dividend.
CD4-C
Another perspective on joint ventures was that by working with a private-sector provider you avoid losing activity to them.
Pathology, we’re in the process of a joint venture tender with a third party provider . . . Bulk is everything for the pathology service and you can drive down the costs by doing that . . . it’s about removing the risk and retaining activity by pushing into the private sector, but retaining some of that income, then we don’t lose it somewhere else. And by working with a qualified provider then you don’t put yourself in competition with them.
CD2-C
One of the finance team reflected on whether or not qualified providers could move in on the Chelsea site’s core activity in radiotherapy and chemotherapy.
I think it would be difficult for people [any qualified providers] to come in on radiotherapy because it’s a million pound for a LINAC [linear accelerator] and then the bill for a bunker. Not to say it couldn’t happen, but chemotherapy’s a much easier one. . . . what will stop Boots [Boots UK Ltd, Nottingham, UK] from coming in and starting to do the very, very simple chemotherapy, where it’s just oral, pick up a tablet and walk away with it, that could potentially be a big issue for us.
F2-C
As mentioned earlier, Chelsea has made a successful business case to be a national site for proton beam therapy, although, based on the US experience, there are suggestions that it may not be more successful than conventional radiotherapy. 169 One manager put this in the context of private practice.
[The Proton Beam Therapy Centre] is a Department of Health-driven facility, as far as I’m aware, there’s not been any discussion about private practice within that. But I’m sure you know in a few years . . . they’ll be proton units all around the country that are private practice driven and then that’s down to patient choice . . . [But] if a prostate patient was saying I want protons and I’ll pay out of my own pocket, I think the clinician has got a responsibility to explain why that treatment is just as good as this treatment and educate them more about the risks of one against the other and having that evidence-based practice as well.
M2-C
This last comment reflects the view that patient choice to pay for treatment should be made in light of the evidence on efficacy and that clinicians in private practice have a responsibility to provide this.
Summary
Our research at the Chelsea site was conducted at a crucial time for the trust. The majority of its work had been funded by commissioners at locally agreed prices. This was recognised as a strategic risk. In 2013–14 the tariff was extended to radiotherapy and chemotherapy, effectively reversing the situation at Chelsea from being 70% funded under local arrangements to 70% funded under PbR (as estimated by the finance team). A major reason for the introduction of the PLICS was to quantify this strategic risk.
Overall, we found considerable evidence on the use of PLICSs for cost improvement and clinical engagement. In contrast, although many saw the potential for PLICSs to relate cost to clinical outcomes and, although this work was being taken forward, definitive results were not yet available. The PLICS was used to cost care pathways in the trust under the Directory of Care remit, but there was no evidence of any initiatives to extend this work outside the trust boundaries. Finally, the PLICS was now employed to construct a trading account for private patients and had informed negotiations on tariffs with the private health-care insurance companies.
Chapter 8 The St Winifred’s case study
Introduction
St Winifred’s is a FT providing acute care from four hospital sites and community care across the shire county of Winifredshire, which has a population of 550,000. St Winifred’s has an annual budget of £230M and employs 3700 staff (St Winifred’s Annual report 2013/14. Trust internal document). The FT provides a wide range of services organised into four directorates: elective care, emergency care, integrated care and support services. It has a large service transformation programme and it is at the vanguard of developing integrated services, in particular, integrating care for the frail elderly. In 2013, following the appointment of a new FD in 2012, St Winifred’s decided to introduce a PLICS. The PLICS would support the trust’s strategic aims of providing high-quality services and service integration. The procurement process started in late 2013; subsequently, implementation of the PLICS commenced in early 2014.
Research approach and guide to interviews
The research team examined information used in the transformation programme and followed the implementation of a PLICS. A member of the research team had observer status at meetings relating to procurement of the PLICS and development. As in the other case study sites, we undertook interviews with senior personnel (Table 12).
| Role | Date | Code |
|---|---|---|
| MD to mid-2013 | April 2013 | MD1-W |
| Chief Executive | April 2013 | CE-W |
| FD from late 2012 | April 2013 | F1-W |
| Contracts Manager | April 2013 | F2-W |
| Associate Director Operations | May 2014 | AD-W |
| Consultant Orthogeriatrician | May 2014 | C1-W |
| Service Improvement Manager | May 2014 | IM-W |
| Associate MD Emergency/Gynaecology | January 2015 | C2-W |
| PLICS Project/Costing Manager | January 2015 | F3-W |
| MD from late 2013 | January 2015 | MD2-W |
| Associate MD Elective Services | April 2015 | C3-W |
Interviews were held with senior personnel at St Winifred’s over a 2-year period. The design of the PLICS was followed as it was implemented and specific developments, in particular care of the frail elderly, were investigated in relation to the PLICS design and potential use.
Background
The transformation programme and the frail elderly project
Following the transfer of Winifredshire Community Services to St Winifred’s FT in 2011, the FT embarked on a transformation journey to reduce any overlaps between hospital and community services and strengthen partnerships with local GPs and other agencies. Ten areas fell under the umbrella of the transformation programme, including care of the frail elderly.
In early 2013, the MD at St Winifred’s explained the frailty project and his concerns for financial information.
Well the frailty project that I’ve been involved in and leading over the last 3 years since I came to [Winifredshire] has been a call to cutting the costs of frailty. And that meant it was to do with cutting both the human and the financial costs of a system that was failing frail older people. And the intention of the project is to deliver the right care, in the right place, at the right time, for frail older people from the point of a frailty crisis until their recovery to whatever long-term state they go back to.
MD1-W
The approach is to redesign pathways and pool resources across acute care, community, primary care and social services.
So there is a pathway following a frailty crisis, and that means we have to define which is the patient group, what is the current pathway, what should the new pathway look like . . . successive attempts to do it have floundered on the basis of people arguing over who’s got the money and what is it for. So cutting the cost of frailty, the frailty project is looking across these boundaries, having an agreed set of redesigned principles, and then trying to align the incentives in the leadership to deliver the changes so that we deliver the right care, right place, right time, every time.
MD1-W
The frailty project comprises four major redesign principles: choose to admit, acute old age specialist care, discharge to assess and maximising rehabilitation/re-enablement.
Choose to admit, which means that if you’ve got a frailty presentation, that’s falls, off legs [cannot walk], confused, or other presentations in an older person where there is no clear reason to go into hospital for say a stroke or myocardial infarction or broken hip, the default should not be to an emergency hospital bed, the default should be to an Emergency Community Response . . . got a 2-hour Emergency Community Response in [Winifredshire] we can respond to frailty crises in the person’s own home, rather than admitting to hospital.
MD1-W
The second principle requires that frail patients admitted to hospital are under the care of an old age specialist team within the first 24 hours and are looked after by that team until discharged and ‘that’s required investment of a 50% increase in Old Age Specialists in this hospital’. The third principle is ‘discharge to assess’:
Discharge to assess . . . the most radical principle because currently our discharge from acute hospitals is based on assessment about discharge potential and need. And that’s where it all gets to go down ‘cos you can have 40 people saying no to their discharge, whereas it’s in the absolute primary interest of the individual to get out of the acute bed as soon as possible when their acute needs have been dealt with. So discharge to assess is you go into a community setting following your acute medical episode and you get further assessed there for your ongoing needs, rather than doing any of that further assessment in hospital. And then the fourth principle is maximising your assessment rehabilitation reablement within post-acute care, so nobody is prematurely placed into long-term care.
MD1-W
The pathway, as explained, requires significant investment in community services and disinvestment (or redeployment) of hospital beds. The MD saw this as the financial problem.
We’re in disagreement on the costings with the Council and the Clinical Commissioning Group, regarding the financial model. Because the concern is if they support this to max they might end up with getting people coming out of hospital very fast, and who’s going to look after the patients, and what’s going to happen to them. But the big financial gain is you maximise the independence of older people at the end of the pathway and you reduce long-term care needs by about a third, based on evidence. So your continuing health-care costs go down by a third, your long-term social care support services go down by a third, and that pays for that investment.
MD1-W
All this work is concerned with looking across boundaries and considering what ‘is right’ for the patient, but the financial flows do not enable cost savings to be evident and may disincentivise efficient and effective working. The trust had used a ‘Big Four’ accounting firm170 to model the financial savings indicated above.
Well we got [accounting firm] to run the model on it, they said, ‘You maximise the model within 5 years, you save the economy of [Winfredshire] £23M a year’. That’s the overall saving to the formal care system. Most of the cost saving is in reduced long-term care costs.
MD1-W
In order to try and access this gain, St Winifred’s had been working with the accounting firm on a financial risk share in which funding is pooled on a capitation basis.
Easiest way to do it is not take a subgroup but take 75+ population, institute these four redesigned principles, look at the total costs of care for that group, the long-term social care, long-term NHS care, acute care, rehabilitation, that financial bundle . . . use that financial bundle to restructure our services, work in partnership, then we agree a risk share [through pooling our resources] and a financial gain from getting the model right across the system. So it’s that accountable care partnership approach . . . in a fully modelled and costed system.
MD1-W
The MD felt that this arrangement could be best achieved through a pooled budget based on capitation. Indeed, he felt that using a capitation basis enabled a fifth principle of including prevention work as well by maximising early intervention primary care – ’identify needs at an early stage, sort them out’. The MD was keen to emphasise that this collaborative approach is fundamental to redesigning services for older people. He was sceptical that a PLICS could help to improve decision-making because PLICSs tend to look at the costs by organisation. But, what was paramount was a whole-system approach:
We need to have good financial information . . . I want that information across our whole system, or as much of the whole system as possible, because essentially the redesign work that I’m trying to bring about is about whole-system redesign, right care, right place.
MD1-W
Therefore, the MD was looking for partnership working, pooling budgets and looking at the whole patient. PLICSs are organisation based; they unbundle the patient by costing each episode in each organisation, but the MD wanted to look at the patient as a whole:
I think unbundling’s dangerous, I think it just introduces perverse incentives, and then we spend our life fighting over who’s doing what bit of the bundle.
MD1-W
He preferred a capitation basis or year-of-care approach for each patient both for funding and costing the patient and as a risk arrangement:
If you pool the health and social care budget for the elderly then you have a tariff system based on that. And then you’d have to have a partnership or an accountable lead provider who then competes for that business to run integrated care services.
MD1-W
The risk arrangement would be through a formal partnership arrangement with all relevant parties.
But you need a provider partnership as well, you need a pooled budget for health and social care ideally, and then you need a partnership arrangement for the people that deliver that care. And that could include Age UK providing some of the service, it could include elements of the private-sector providing elements. You can define the boundaries of that, but you do need the provider partnership as well as the pooled budget. Now I’m not saying that’s for all services, but I think for integrated services for frail elderly.
MD1-W
A risk-sharing arrangement with pooled budgets based on 1 year of care
In the past, a general risk-sharing arrangement had operated between St Winifred’s and its deficit-bearing host primary care trust (PCT). The MD for elective services claimed that St Winifred’s was ‘paid about 85% typically’ of the tariff. The contracts manager explained the risk share as a system whereby the PCT received a discount and made a fixed payment, while penalties would not be applied to the FT; thus, the risks shared between FT and PCT were as follows:
Well the risk of penalties for us and the risk around the fact the PCT didn’t have enough money for them so they couldn’t afford the level of activity.
F2-W
In early 2013, a more formal risk-sharing arrangement was considered.
A Big Four accounting firm was engaged to provide an options analysis for a risk-sharing arrangement for integrated care for the Winifredshire health economy (St Winifred’s FT, the CCG and the local authority). Key components of the analysis were the definition of the components for inclusion, the services in scope, the payment mechanism and the contractual structure.
It was concluded that a demographically bounded population would be most beneficial and ‘older people – those who are over 75 [years]’ would be a good fit for the focus. The scope of services would be assessment and care planning, non-elective, elective, mental health services, domiciliary care, residential care and reablement. Although it was recognised that the inclusion of primary care, ambulance services and the third sector would also be beneficial, the added complexity before the commissioning landscape matured was considered too problematic. A bespoke payment mechanism that included elements of a capitated budget alongside outcome-based performance payments was recommended. The existence of a contractual structure, that is, the mechanism by which the providers are wrapped up into the risk-sharing agreement, was considered necessary for both the commissioning process and service delivery. There would be a joint commissioning body, the Commissioner for Older People’s Services funded by health and social care. At the provider level, three potential options were considered: joint venture, prime contractor and alliance-style contracting. A provider joint venture was recommended to be the integrator of the system and procure the services, make investment decisions and hold or distribute surpluses.
The risks would be shared as shown in Table 13.
| Risk holder | Type of risk |
|---|---|
| Commissioners | Demographic change |
| Shared between providers of health and social care | Total system delivery cost |
| Demand and capacity for specific service | |
| Delivering service/user outcomes (including quality) | |
| Individual providers | Unit delivery cost of intervention |
The accounting firm reported in 2013, but the scheme was not implemented in the study period. The MD left St Winifred’s in summer 2013, but the frailty project remained part of St Winifred’s transformation programme.
Why a decision to procure a patient-level information and costing system?
The provision of cost information
St Winifred’s’ chief executive trained as an accountant in the NHS and held senior finance posts before moving across to management. He had a clear vision of how to develop services and costing, but in early 2013 he, like the MD, was unconvinced of the need for a PLICS. He explained the current way of looking at cost information at board level.
So we’re in transition, so budget . . . the standard Board report is very much focused on the expenditure side of the business and we’re moving now towards a more service line approach. And the way that we’ve started to move forward is we use contribution statements now. . . . we’re trying to get into contribution statements by speciality and what we also use are bubble charts. So every quarter we see bubble charts at management board level and we periodically share them with the full board. My view is that they help to present a picture and they’re quite good when we’re making strategic investment decisions. So we’ve been doing some work recently looking at the Shakeford Hospital [one of St Winifred’s’ four hospital sites] and whether we should expand certain specialties, so we’ve been looking at the bubbles for ophthalmology for example.
CE-W
The bubble diagrams as presented to the St Winifred’s Board in 2012 are shown in Figure 23.
FIGURE 23.
St Winifred’s: bubble chart presentation of service line profitability to the board.
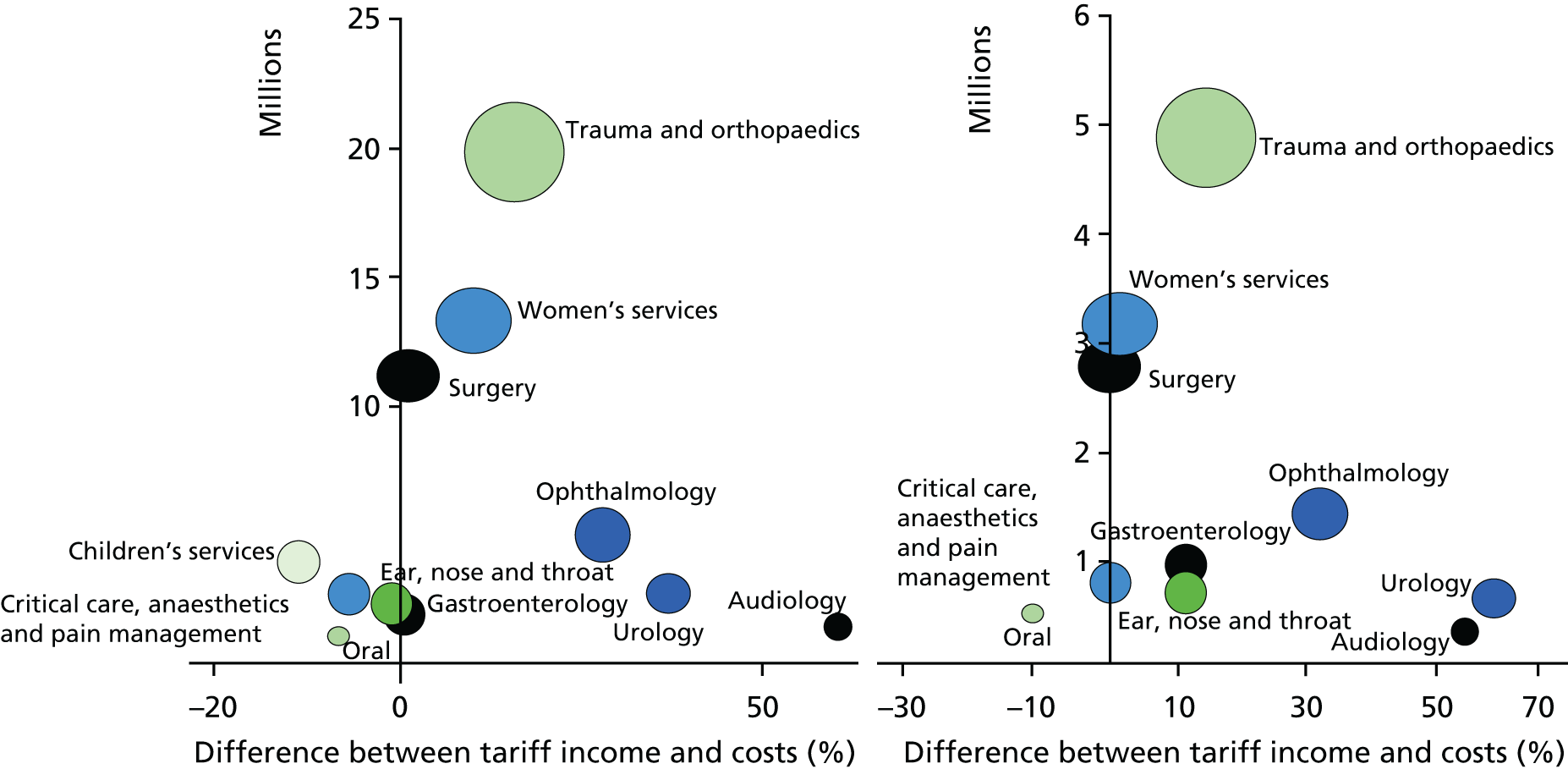
The chief executive explained how they are interpreted.
What the bubble tries to do is to capture firstly the size of the enterprise by size of bubble, but then it also demonstrates its relative level of turnover, so compared to similar specialties in similar size organisations so you get a view as to whether we’re under or over trading for that particular speciality, but then also we’re looking at its profitability. So to the right of the diagram, the further right the more the profit, the higher up the chart the more there appears to be more activity than your average market . . . So they’re a kind of a strategic marketing tool because, you know, if you’ve got a high margin speciality that appears to be low in terms of market share there’s a real opportunity to grow that and that’s where we use it.
CE-W
The chief executive saw the trust moving to more sophisticated costing as a development of the reference cost exercise.
We’ve been doing reference cost for years and you tend to do that in a reasonably logical way, but we tend to do it in fits and starts so maybe quarterly or annually we’re allocating costs for support services for example. I’d like us to pick up particularly some of the bigger cost areas on a regular basis.
CE-W
He admitted that he was ‘unfamiliar with today’s PLICS solution’, but thought previously that the trust would have had ‘a transfer pricing [a system of charging between different departments of the hospital for units of activity] approach to it’ and that ‘would need to be standard cost [a predetermined estimate of what it should cost for each unit of activity (not actual costs)] . . .’. He discussed the transformation agenda at St Winifred’s and the concentration on pathway redesign and team structure. He felt that there was ‘very little evidence’ to ensure that change was cost-effective. However, he felt that it was not cost that was driving change:
It’s what patients want, it’s what our GP commissioners want. So even if the economics didn’t stack up, if you’ve got patients and commissioners wanting it you’re going to end up moving in that direction.
CE-W
Nevertheless, there was an impetus to have better cost information. The non-executive directors exerted some pressure for better information on investments.
For example, Community Emergency Response Team which is a 2-hour response, you may have had this described to you for patients who either present in A&E or could be discharged from the hospital. We’ve put a resource into that and we’ve pulled a figure out of the air in terms of we expect them to discharge 60 patients a week, . . . we need to be more sophisticated . . . when I talk to the non-execs [non-executive directors] at the board . . . they want to know what today’s productivity unit cost looks like for those patients and whether it’s a good business decision today, but then if it isn’t they’ll want us to demonstrate how much more productive our community staff need to be to make it less expensive than a hospital admission.
CE-W
For St Winifred’s, the chief executive saw the payment mechanism for community services changing, perhaps using a year-of-care approach and the costing necessarily having to follow a patient-year-of-care approach (rather than a patient episode).
There’s no way we’re going to stick to a block tariff arrangement for Community Services that’s just untenable in the long run, so we could move to year of care tariff and, you know, it’s been talked about for a few years, it would seem to make sense for long-term conditions, for me it would make sense for care of the elderly, the frail elderly. If you’ve got a year of care tariff in effect you’re transferring a lot of that risk to the provider, so I think what we would then need is a better means of quantifying the use of services by those individual patients that are covered by that tariff. So you start to look at whether patients are outside the norm.
CE-W
Ideally, costing would have a wider scope than is generally the case for PLICS and include treatment in a community setting and even primary care costs as well.
You’d bundle the whole thing up. You’d want all of those individual transactions to be costed against that income for the year of care tariff . . . and to know which patients to target [for cost improvement].
CE-W
He went on to explain how separating out expensive patients and working where necessary on patient education, GP interventions or measures such as equipment installation could alleviate the situation. Having all the costs identified at the patient level could be illuminating.
Analysis in the past has all shown us . . . small number of patients, big amount of resource. You want to know who those small number of patients are, and if you’ve got all these contacts going on with different teams left, right and centre there’s a danger you miss that. Pull out one patient and see what their resource use is in its entirety, and that might provide the solution . . . It is sophisticated. The patient becomes the cost bucket, in effect. But that’s my vision for how you’d really start to get to change the dynamics of this.
CE-W
At the time of interview the chief executive did not know whether or not St Winifred’s would implement a PLICS in the near future, but a new FD had recently joined St Winifred’s. She had experience of a PLICS at her previous trust and the chief executive commented that ‘what she seemed to be particularly good at was challenging clinical practice and clinical variation’.
The new FD was very keen to change the current costing system at St Winifred’s starting with SLR. She had strong views on how costing information should be constructed and distributed:
I’m used to service line reporting down to a contribution [contribution is defined at St Winifred’s as revenue income minus direct costs. It excludes overhead costs] level, so you only look at influenceable costs. The beast we have at the moment, . . . has your traditional bubble charts and . . . it’s very, very, very crude and rude and I wouldn’t want decisions made on the back of it because it isn’t done at contribution level. It’s an attempt to smear all the trust overheads across every specialty and like any absorption [absorption costing absorbs all costs into the cost object (patient). It determines full costs (the total cost of the patient)] method, it’s so open to well if you did it this way, it would give us a result, or that way it would give the other result.
F1-W
An advocate of responsibility costing, she was keen not to discredit cost information by the inclusion of remote costs in cost information distributed within the trust:
I’m going to be putting in a service line reporting system during this year, which . . . sets a contribution target level for each specialty and each division. So it’ll have direct costs . . . number of bed-days, your time in theatre, your pathology, your pharmacy, your imaging, the direct costs you’ve incurred as a result of having that thing done to you . . . [The current approach] is broadly spreading all the costs in the ledger across all the activity we do, but you know, it’s attributing the cost of the board, it’s attributing the cost of everything into all of the activity and it’s just too blunt at the moment.
F1-W
The FD envisaged bringing in a new version of SLR and a PLICS; she wanted to move SLR from ‘a top-down attempt at SLR at totally absorbed cost’ as shown in the previous bubble charts (see Figure 23) to ‘top-down SLR at contribution level and PLICS’. She developed a case for a proposed PLICS.
The case for a patient-level information and costing system
The case for a PLICS was put forward in a briefing paper to the St Winifred’s Trust board in summer 2013. The paper stated that SLR and a PLICS were a strategic fit with the St Winifred’s 2013/14 objectives, namely to:
-
Provide high-level care – PLICS supports understanding variations in care.
-
Develop our services – PLICS highlights which services contribute most to the trust.
-
Integrate our services – pathway views of cohorts of patients and variation assists understanding of resources for deployment in new models of care.
-
Develop our people – PLICS, user-friendly, drill-down information.
-
Provide a sustainable future – granular insight into results and variation in impact of HRGs/specialties/consultants to improve patient experience and quality/efficiency of service delivery.
Preliminary discussions and product demonstrations were held with several suppliers with an established track record of delivery to NHS organisations. This enabled an estimation to be made of the optimal solution as well as an indication of the likely capital and revenue requirement and the impact on internal staff resource required to support deployment.
The approximate costs were:
-
capital outlay (hardware and software): £90,000
-
non-recurrent revenue expenditure (implementation and training): £90,000
-
recurrent revenue expenditure: £15,000.
The management board agreed the business case in January 2014. The system would be chosen based on weighted selection criteria (Table 14).
| Criteria | Weighting |
|---|---|
| Cost | 35 |
| Functionality | |
| Ease of use for clinicians | 15 |
| Flexibility of system to tailor/extend ourselves | 15 |
| Functionality to produce statutory information | 5 |
| Driver for data quality improvement | 10 |
| Ease of in-house maintenance of system | 5 |
| Supplier competence/resilience | 15 |
| Total functionality weighting | 65 |
Cost was a significant factor, but functionality was much more important. In terms of functionality, the ease of use by clinicians, the flexibility to tailor the system to the needs of the trust and supplier competence and resilience were seen as equally important.
Four PLICS suppliers were invited to submit a bid and show their systems to clinicians and senior personnel at the trust. All four are established firms for the supply of PLICSs to NHS provider organisations, serving from 4% to 34% of all NHS providers with a PLICS. 165
The chosen system was inevitably a compromise.
There were two frontrunners, one of which had got a very sophisticated costing machine and a pretty good dashboard. The one we went for, the costing machine itself wasn’t that sophisticated but it does the job. . . . It [still] feels pretty sophisticated and certainly quite challenging . . . But their dashboards were very good and very well presented to the group, which I have to admit surprised me and surprised our director of finance. But the bottom line actually came down to the cost. I think the one we went with was far cheaper and although I think that both the FD and I would have preferred the more expensive supplier, we couldn’t justify it on the grounds of cost.
F3-W
Although the PLICS project manager liked the flexibility of the more expensive systems, ‘these are tough times in the NHS and we can’t have everything we want’. Cost had outweighed the functionality of costing in the PLICS.
The patient-level information and costing system implementation
A project board was established and a member of the research team had observer status on the board. The project board was established to oversee the SLR PLICS implementation activity delivered primarily via the Project Planning Group.
The approach to implementation was set out as a series of stages in the project board papers:
-
identify and set up activity data sources
-
costing assumptions
-
set up costing system and reconcile
-
income assumptions
-
set up Qlikview, version 1.0 (QlikTech International AB, Lund, Sweden) dashboards
-
develop dashboards in conjunction with clinicians and operational managers.
Impact of clinicians on the patient-level information and costing system design
The FD was keen for the PLICS to be driven by clinicians and sought various ways to engage them. Clinicians were invited to the presentations where the PLICSs from the four preferred suppliers were showcased and their views were sought. The FD also held various workshops to promote PLICS use. A new MD joined St Winifred’s in late 2013. He became the lead for PLICS implementation and promoted clinical engagement.
The MD saw the project as a clinical project:
Ultimately, if PLICS is going to work, and does more than monitoring and makes strategic and financial decisions. If it’s going to change practice, which ultimately is where the biggest benefit is, then you need a clinician role in it.
MD2-W
He was keen to lead and promote the FD’s desire for the system to be driven by clinicians:
I think the first thing to do is to get the clinical engagement and make sure the whole PLICS project actually produces things that people believe in and are reliable. And in the, sort of the PLICS’ journey, we’ve had clinical engagement from the Associate Medical Directors and a degree of, you know, co-design, particularly actually discussing the refinements about, and how the system would work and the demarcation [boundaries] between clinical areas and service lines, which is a very important thing to start with.
MD2-W
The clinicians influenced the design of the system. In any absorption costing system there are different ways in which costs can be attributed. At St Winifred’s, the PLICS aimed to reflect how the clinicians worked; costs were not attributed to individual consultants if that did not reflect current practice:
We’ve got much more teamworking and ‘physician of the week’. So we’re trying to make some sense of that by . . . aggregating costs to groups of clinicians, or functional units within the trust and that will need refinement.
MD2-W
The MD felt that there needed to be clinical input into issues relating to ‘case mix and cross-subsidy’ and, like the FD, felt that it was important to provide information for clinicians that did not include overheads.
Overheads are something that clinicians find incredibly difficult. So, it’s absolutely right to leave them out, because they’re a confusing issue at the moment, but they will have to be played in, because they’re a big factor, they will remove the contribution in a lot of cases. But at the moment, I think they are a distraction because people, instead of thinking about their services, they’re thinking about why the overheads are so big.
MD2-W
The MD stressed that PLICS development is iterative: ‘we will probably change things, and we’ll change how we present things and indeed what the definitions are underneath it’. He saw it as a ‘start of a journey’. The implementation of PLICS had been discussed at the Medical Staff Committee and various engagement meetings, but there had been modest success in clinical engagement:
But, you know, it is difficult. You invite people to come along, you only tend to get the enthusiasts until you have some practical examples of how it affects them.
MD2-W
Although the FD was enthusiastic about the PLICS being driven by clinicians and the MD chaired the PLICS implementation board, there were other drivers. The PLICS project manager noted at the start of the process that she could ‘see clearly that there was an interest, particularly from general managers’. The non-executive directors were also particularly supportive of the introduction of a PLICS (see Board, directorate and deep dive). Nevertheless the project manager felt that the clinicians had significantly influenced the design of the system:
Clinicians influenced the way in which we attributed income or didn’t attribute income to individual patient episodes, because there was some debate about how we attributed non-PBR income – or the contractual adjustment and things like marginal rate.
F3-W
Therefore, in order for clinicians to have confidence in the system, the PLICS showed consistent tariff income against the patient record (it did not change if a threshold had been reached and marginal tariff paid). Similarly, cost items were recorded on the basis of whether or not the clinicians could influence them – at specialty level or trust-wide level. Likewise, the clinicians influenced how incentives and penalties from contracting were treated in the PLICS:
Emergency readmissions, we initially had planned to actually charge a patient with the re-admission deduction – or at least [attribute it] at a specialty level, but we were overruled on that and were told that . . . had to be held at a trust-wide level. They wanted the benefit of a best practice tariff [which provides a higher tariff recorded at patient level] but they didn’t want the penalties.
F3-W
However, to the extent that this was pragmatic, the project board wanted to start with a workable assumption but, as the MD indicated above, changes could be made later if necessary. The project manager held individual meetings with senior clinicians on the design of the dashboard and this often led to refinements in the costing methodology. The clinicians had input in the choice of system and the stages of implementation – how activity is measured and costing and income assumptions.
Patient-level information and costing systems and other financial information systems
The PLICS was introduced alongside improved SLR. SLR operates at directorate and division level. PLICS provides more granularity, but quality information is not included within the PLICS. The PLICS holds activity at patient level that has costs attached and, through a contract management system, income attributed. Although quality data are not held in the PLICS, quality information may be looked at alongside the PLICS.
. . . the two would coexist in the same forum and that’s probably more important than actually trying to have a technical solution.
MD2-W
There is also no pathway identifier currently within the PLICS, that is, a separate code is not included on the patient record that denotes a pathway. Development of non-financial information and pathway indicators are future developments.
[The FD] is very keen to get non-financial data connected to it. I would really like to go down the care pathway route, but I think it’s not an easy or quick win because we don’t generate a care pathway unless it’s an elective admission.
F3-W
The PLICS is aligned to SLR, but there are difficulties in aligning the PLICS with the budgeting system; budget responsibility does not follow service lines or the PLICS. This is most apparent when looking at service departments. The dashboards are set out by division, then down to directorate, and then down to service lines. However, whereas management and budget responsibility sits within one directorate or division, the PLICS attributes cost to patients across directorates; this is the case for outpatients, various service departments and some wards.
So you wouldn’t expect the manager of outpatients to be able to use it [the PLICS] easily. And the same with things like diagnostics, we have struggled with finding appropriate views for them to use.
F3-W
Similarly, the PLICS does not map across to reference costs easily. There are prescribed rules for compiling reference costs that do not reflect how St Winifred’s operates:
For reference costs we have to report in certain HRGs but actually, for PLICS we’ve organised it around how we actually work. For community services within reference costs you report on first or follow-up visit . . . But actually, the way we managerially run ourselves is geographically. So we have integrated teams in the districts . . . And that’s how we manage our business. So for PLICS we’ve set it up so ‘the HRGs’ [the cost objects] are staff groups.
F3-W
Therefore, there has to be adjustments in the ‘HRG costs’ held in the PLICS for community services for reference cost purposes. There are other differences from reference costs, such as the inclusion of excess bed-days in the PLICS that are excluded in reference costs. At St Winifred’s, the PLICS is not primarily designed for pricing purposes but for removing waste and variation. However, the necessary adjustments are identified in the PLICS to enable the production of reference costs.
Patient-level information and costing systems reporting and potential uses
By December 2014, the PLICS was operational for financial year 2013/14 data and work commenced on the current year’s data and refining the reporting.
Board, directorate and deep dive
In December 2014, the FD presented the summary information on SLR and PLICSs to the board. The PLICS introduced at St Winifred’s provides service line reports at contribution level, that is, without overheads. These are presented in bubble charts by division and specialty (Figures 24 and 25, respectively). Board members were enthusiastic about the improved performance reporting and its potential use. The non-executives were particularly supportive:
I am led to believe that the governors have been – and actually I know that it’s true because when I’ve been to present to board, I’ve heard the non-execs [sic] saying that they have been desperate for this information for a long time. So I feel that there’s been quite a lot of pressure from the non-execs to see this information.
F3-W
FIGURE 24.
St Winifred’s: contribution margin by directorate.
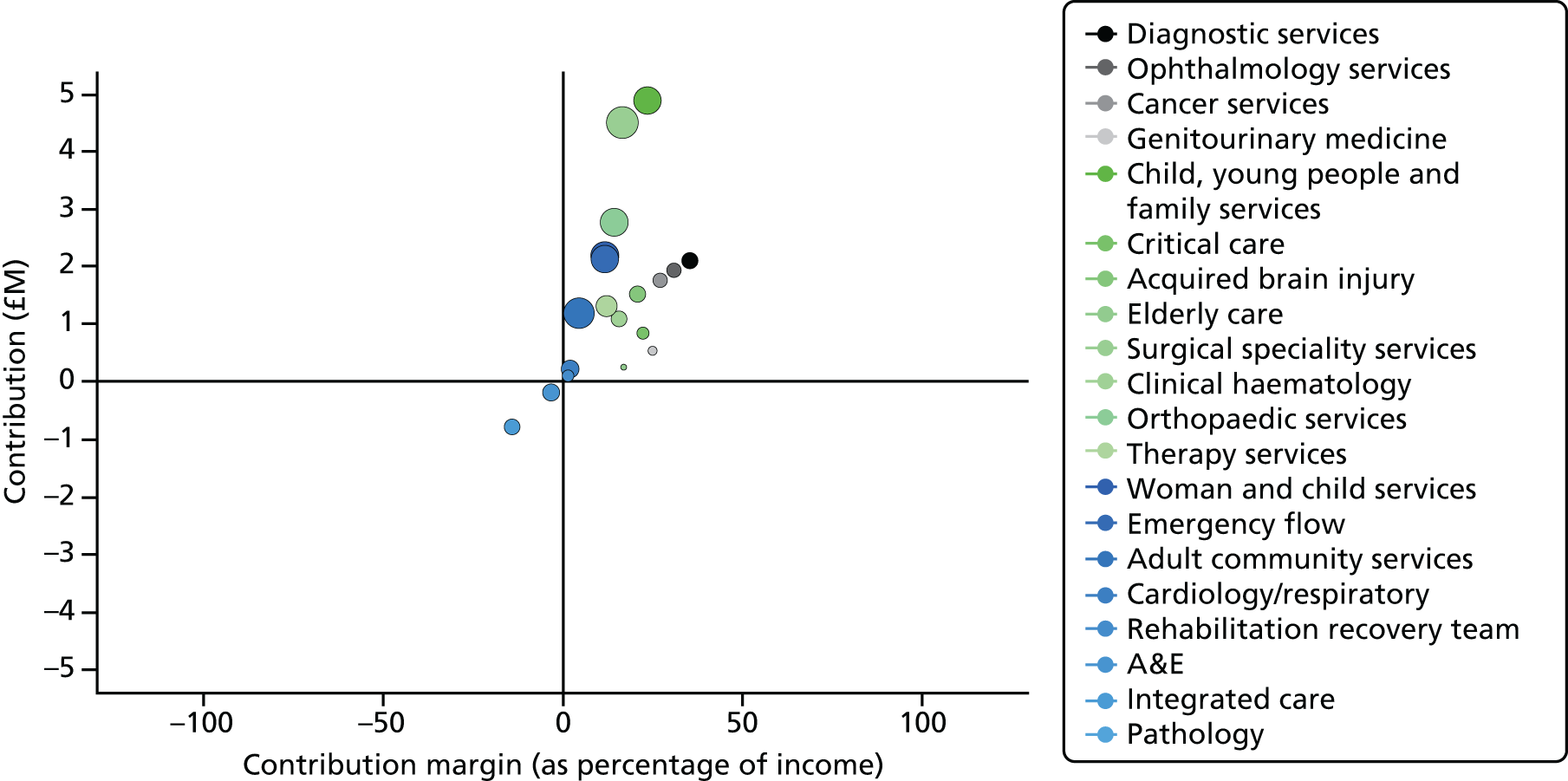
FIGURE 25.
St Winfred’s: contribution margin by specialty. CDC, Child Development Centre; CHC, continuing health care; CMDT, Community Multidisciplinary Team; ENT, ear nose and throat; T&O, trauma and orthopaedics.
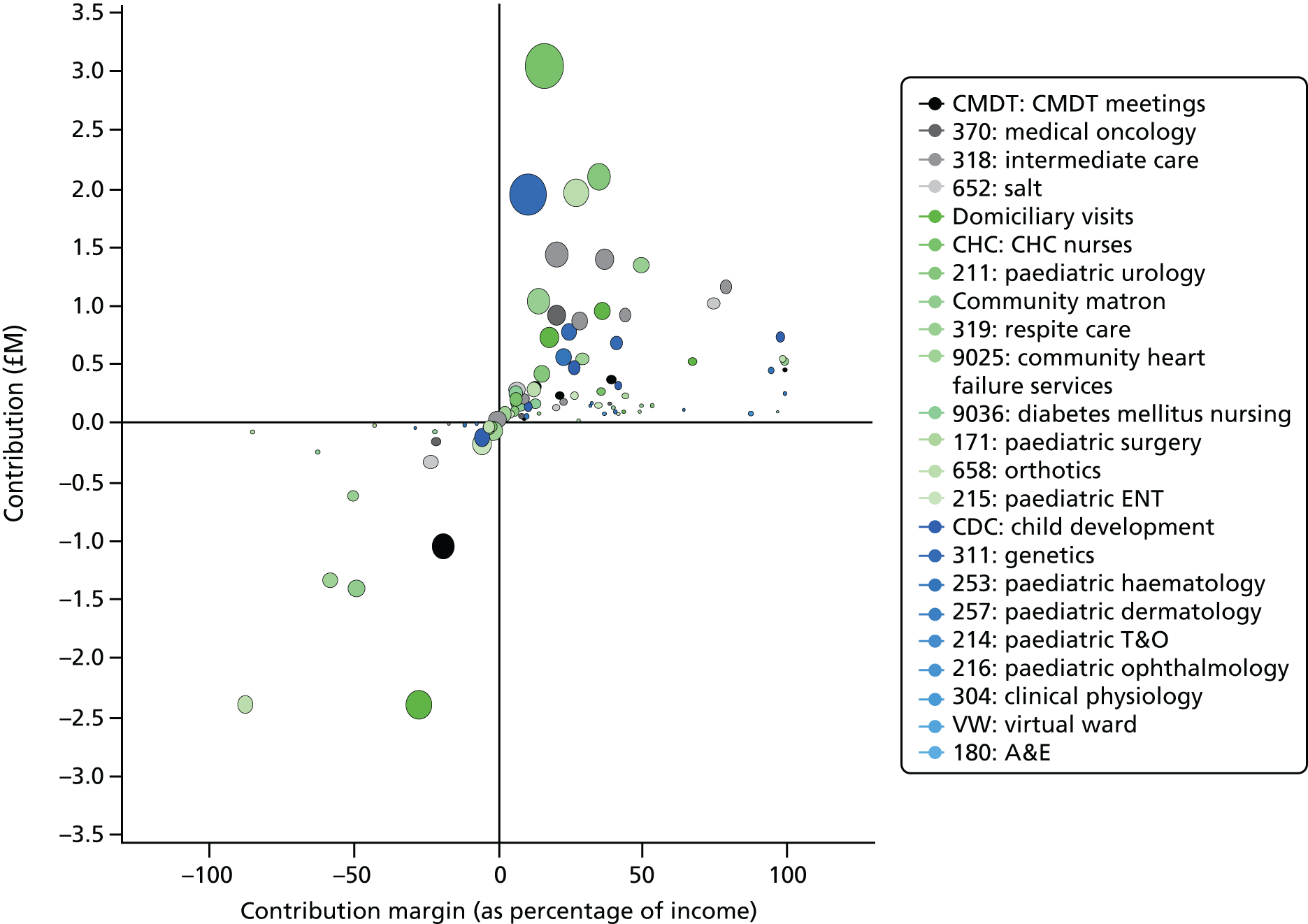
At directorate level, more detailed information was reported, although this was at an early stage:
I can see myself in my obs [sic] and gynae [sic] departmental meeting which we have every month, to have this data projected and say, ‘Look; this is what we have spent as a division.’ And within that division, by consultant, this is what they’ve done. And by consultant, for these procedures, this is the breakdown. I’ve been looking at that now and it’s really, really interesting.
C2-W
However, some board members were sceptical of this approach:
I railed at service line reporting. You don’t wait for something bad to happen [and be reported through service lines] and then look at the cost to patients for it. [You should identify] what are the steps that need to be there? Put those in and then review. But I’m not going to wait for something bad to happen and then report on the financial consequences of it. So there’s a difference in my philosophy.
C3-W
Nevertheless, the associate MD found the system useful when undertaking job planning and discussing performance with clinicians:
I’ve used it for job planning and because this is elective care division I’m primarily working with surgeons . . . type A personalities that they are, they like to feel that they are the best in the world or in the country or in the region at doing whatever they’re doing. And what service line reporting for me has been able to show with some of them that actually, sorry, chum, but you did this, this is the final short consequence and actually you’re losing more money than you’re bringing in.
C3-W
At board and directorate level, standard outlier reports are prepared: at board level – the five highest and five lowest contributing specialties; at directorate level – the top ten most expensive patients and the most variable HRGs. However, it is envisaged that the PLICS can be used to respond to specific queries immediately. The dashboards available to view within the Qlikview SLR document are supported by 48 million rows of data.
Trust activity, cost and income can be viewed in virtually any combination and at any level:
-
divisional
-
directorate
-
specialty
-
consultant (or professional carer)
-
HRG
-
diagnosis
-
primary procedure
-
patient
-
patient event
-
cost type
-
resources (e.g. pathology tests).
The nature and complexity of the dashboards mean that they will be continually developed to improve the quality of the underlying data, the quality of the costing and mapping processes and the presentation of the charts and tables in the dashboards. However, as the project manager pointed out:
It will never be real time. It will always be quarterly in arrears and we think it will take about 6 weeks after the quarter end to get it done, because we have to wait for the ledger to close and for activity information.
F3-W
At St Winifred’s there will be targeted ‘deep-dive’ reviews; these will be investigations exploring the detailed information held within the PLICS. The system includes the following data sets at patient level: admitted patient care episodes; A&E attendances; outpatient attendances; community contacts; ward stay; radiology; pathology; drugs (high-cost drugs and some named patient drugs); blood products; theatres; intensive care unit; and special care baby unit. The dermatology specialty volunteered for a pilot deep-dive review.
The PLICS is used to support business cases and wider strategy reviews (e.g. to support a business case for an additional consultant dermatologist).
Frail elderly: fractured neck of femur
The service development unit at St Winifred’s was looking to use the PLICS to support measuring the impact of pathway changes for fractured neck of femur.
We have mapped out the patient’s project, the whole pathway right from fracture right through to the end point.
AD-W
The PLICS enables the cost of the care pathway to be identified, but not directly; patients are recorded on the PLICS according to their primary diagnoses, not a pathway. Figure 26 shows the (simplified) pathway. The pathway focuses on transforming care for the frail elderly including discharging to assess and achieving best practice aspects, such as the involvement of an orthogeriatrician. The pathway starts before the operation and finishes 3 months after the patient leaves the hospital. This current service is regarded as the gold standard; for example, the pre-operation assessment was undertaken by a consultant orthogeriatrician. The consultant orthogeriatrician stated that he personally sees:
. . . these patients 3 months after their hip fracture to do the ‘falls assessment’ in a clinic.
C1-W
FIGURE 26.
St Winifred’s: fractured neck of femur pathway (frail elderly). CERT, Community Emergency Response Team; D2A, discharge to assess; EDD, expected date of discharge; MDT, multidisciplinary team; OT, occupational therapy; PT, physiotherapy.
Table 15 shows the summarised costs held in the PLICS for 10 (randomly selected) patients with a main diagnosis of fractured neck of femur and aged > 60 years (HRG code S720). A more detailed presentation of the PLICS costs held for these 10 patients is shown in Appendices 6 and 7.
| Care spell ID | Costs (£) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medical staff | Theatres | Prostheses | Diagnostic | Therapies | Drugs | Other support | Non-pay costs | Ward | Overhead | Total inpatient | A&E | Community | Total related cost | |
| 1 | 728 | 412 | 1356 | 63 | 191 | 20 | 487 | 418 | 1064 | 427 | 5166 | 226 | 9881 | 15,273 |
| 2 | 683 | 604 | 308 | 34 | 91 | 81 | 209 | 469 | 541 | 183 | 3203 | 268 | 3289 | 6760 |
| 3 | 2386 | 810 | 41 | 349 | 473 | 54 | 1126 | 594 | 2844 | 705 | 9382 | 109 | 0 | 9491 |
| 4 | 1255 | 987 | 241 | 135 | 273 | 92 | 696 | 597 | 1524 | 611 | 6411 | 278 | 2128 | 8817 |
| 5 | 2514 | 710 | 1116 | 114 | 197 | 57 | 559 | 418 | 1209 | 427 | 7417 | 96 | 0 | 7418 |
| 6 | 882 | 732 | 1356 | 82 | 151 | 84 | 348 | 299 | 1123 | 305 | 5362 | 159 | 2509 | 8030 |
| 7 | 1065 | 462 | 1356 | 139 | 336 | 52 | 835 | 716 | 2201 | 733 | 7895 | 213 | 6767 | 14,875 |
| 8 | 2136 | 0 | 0 | 99 | 506 | 31 | 1273 | 6 | 4242 | 655 | 8947 | 184 | 0 | 9131 |
| 9 | 2735 | 803 | 1205 | 29 | 169 | 48 | 418 | 358 | 967 | 366 | 7098 | 69 | 688 | 7855 |
| 10 | 967 | 0 | 0 | 141 | 214 | 69 | 530 | 291 | 1819 | 272 | 4303 | 247 | 0 | 4550 |
| Grand total | 15,351 | 5520 | 6979 | 1185 | 2601 | 588 | 6481 | 4166 | 17,534 | 4684 | 65,089 | 1849 | 25,262 | 92,200 |
| Average | 1535 | 552 | 698 | 119 | 260 | 59 | 648 | 417 | 1753 | 468 | 6509 | 185 | 2526 | 9220 |
St Winifred’s PLICS includes the hospital and community activities shown on the pathway map (see Figure 26). However, the pathway includes activities not held in the PLICS, such as undertaking therapy assessments. Conversely, the PLICS identifies costs not focused on in the transformation pathway (see Figure 26), for example the cost of prostheses. In Table 15, patient costs are shown across A&E, inpatient spell and community care. This integrated costing is rare within PLICSs, which have traditionally focused on hospital services and have not included pathway codes (pathways are more commonly used for elective care). However, the PLICS at St Winfred’s will not encompass all the costs; the whole-patient view as originally put forward by the MD leading the frailty project (and envisaged in the sophisticated risk-sharing arrangement) is not shown; only the costs on the pathway that fall within the integrated trust are shown.
St Winifred’s is one of the few PLICS users that have included community services on the system (see Table 15). The community costs are attributed via integrated health teams and specialist services, such as podiatry and specialist nursing for leg ulcers. This reflects how St Winifred’s community services are structured and how they are managed, but does not reflect the requirements for reference costs. It is evident from looking at these sample patients that patients coded to S720 (fractured neck of femur) do not align clearly with the fractured neck of femur pathway and that patients frequently have comorbidities, which require deviations from the pathway. In addition, outcome data are missing – some patients have no prosthesis cost or follow-up community visits, which raises the question of whether or not they were treated. The most basic qualitative data (e.g. on mortality) are not in the PLICS. There is marked variation between patients at St Winifred’s; there will also be considerable variation between trusts because of how costs are accumulated on a patient-by-patient basis. For example, the clinical negligence costs on a patient-by-patient basis at St Winifred’s are quite different despite all the patients being elderly with a primary diagnosis of fractured neck of femur; this reflects the costing approach. The clinical negligence cost is allocated to specialties based on the Clinical Negligence Scheme for Trusts bill, which shows the number of clinicians in the post; the specialty cost is then attributed to patients on an event basis for community and A&E services and per bed-day for inpatient spells. Although the PLICS has the potential to assist in transformational change through pathway redesign, aligning the PLICS data and pathways is complex and at an early stage of development. The fractured neck of femur pathway for the frail elderly includes some transformational change, for example the discharge-to-assess principle. Including the community costs alongside hospital costs is very important; from the sample, it is clear that community costs can be as significant as those of the inpatient spell. However, aligning patients in the PLICS with pathways or treatment protocols is much more straightforward for elective surgical care in which treatment could be considered routine. For the frail elderly with many comorbidities and needs that span acute, community, primary and social care, the pathways for individual treatments and the tendency to cross pathways lead to very difficult problems of alignment. Appendices 6 and 7 show the more complex and non-uniform cost structure of frail elderly patients with fractured neck of femur as the primary diagnosis.
Commercial sensitivity
The PLICS is clearly envisaged to be used in internal benchmarking through examining the comparative performances of individual clinicians and clinical teams. However, the sharing of information outside the FT, particularly with commissioners, is regarded as harmful to St Winifred’s. The FT had previously been keen to disseminate its progress in the transformation programme (e.g. St Winifred’s held events open to delegates from all over the NHS) and it had had a risk-sharing scheme with the former PCT and its successor CCGs, but the nature of the environment had changed.
The MD in early 2013 explained the dangers he saw in sharing performance information:
Any comparisons with other organisations are seen as a threat, really, because of just the politics of the different organisations and different views of people in the system as to what should happen. I mean the overarching thing [here in the City of Wintry and in Winfredshire] is that [the nearby university teaching hospital] wants to swallow up [St Winifred’s]. And some people want that to happen as a systems solution, so sometimes data that you use to compare outcomes has been used maliciously to try and undermine the viability of services here.
MD1-W
He felt that there were:
. . . so many threats to the small organisation viability that sharing of information is very dangerous, in the way that it can be used and manipulated to undermine our organisation’s integrity.
MD1-W
He continues:
[I thought it was] . . . absolutely right, it goes to CQC [Care Quality Commission] and Monitor and the CCG and all the bodies that regulate and oversee our work.
MD1-W
However, the MD had deep concerns about the data being used by governing bodies to look system-wide. However, by 2015, the sharing of information, particularly to commissioners, had changed dramatically. The MD (at the time of writing) saw problems in sharing the PLICS information because of the present contracting environment and the nature of the local CCG:
We have quite a transactional CCG . . . it’s all about procurement . . . And, you know, it’s different in other CCGs where there’s a kind of a, how do we run health care, you know, for the benefit of the system and NHS PLC [patient-level costing] together and then you would use it, because it would be a tool to see where your inefficiencies are, where your problems are. Then you might, you know, you might change either the contracting basis or the pathways across primary and secondary care, etc. We’re not there really . . . our CCG sees themselves very much as a slave to procurement regulations [the requirement to tender] and they would see that kind of collaboration as being anticompetitive. I don’t agree with them, but that’s the way they see the world.
MD2-W
These views were echoed by the PLICS project manager and the associate MDs:
I think that giving out cost breakdowns would put us at a commercial disadvantage, particularly if we’re negotiating prices with commissioners.
F3-W
I think you would be hard-pushed to share that data because of the nature of the contracting arrangements. I don’t think it’s helpful. I don’t think the contracting arrangements are helpful.
C3-W
St Winifred’s had changed from being a FT with no PLICS that was keen to show off its transformation work in improving outcomes and efficiency and that was seeking a formal shared-risk approach in the local economy; it now had a sophisticated cost system, but in a new environment was guarded about releasing information relating to its costs to others, particularly local commissioners.
Summary
At the start of the research study in 2012, St Winifred’s did not have a PLICS, but was a FT at the leading edge of transforming care, in particular, integrating care for the frail elderly. During the study period, the FT introduced a PLICS that included both acute and community care and had the potential to look at costs along a care pathway. However, aligning costs held on the PLICS with care pathways is complex; patients cross multiple pathways and the PLICS data lack quality or outcome information. When making cost comparisons, considerable variation can arise from the costing approach. The PLICS was purchased from one of the four most popular suppliers, but implementation did not necessarily adhere to a standardised norm. The system was designed to meet the needs of the FT and to engage the clinicians. The new FD and the new MD were strong advocates of the PLICS, as were the non-executive directors. Emphasis was placed on user requirements and costs are largely reported at contribution level. The system is designed for use in St Winifred’s across all services (including community services). At St Winifred’s the aim was to ‘reduce waste, waiting and variation’. The PLICS is used alongside SLR to improve performance reporting at board and directorate level, support business cases, aid pathway redesign and improve efficiency. At the start of the project, St Winifred’s was very open in showcasing its transformation successes and its partnership working. It had considered a sophisticated, formal arrangement for risk sharing between all stakeholders for the care of the frail elderly (aged > 75 years). However, it became concerned about the commercial environment, particularly after the move from PCTs to CCGs, and regards cost data as commercially sensitive. Although the PLICS shows good potential for improving efficiency and resource allocation and, to some extent, assisting in moving care into different settings, the system fits most easily with elective routine procedures. It can be difficult to align PLICSs with care pathways when patients have comorbidities and use services within a number of settings. Furthermore, the full potential of PLICSs is hampered by an environment of commercial confidentiality.
Chapter 9 Discussion and concluding comments
How patient-level information and costing systems are used in the English NHS
In this chapter, we garner our arguments and evidence to, first, address our research questions and objectives and, second, discuss the wider implications of our findings.
Our research questions are:
-
How are NHS trusts and commissioners using PLICSs?
-
How can PLICSs be used to benefit the total health economy (focusing on cost improvement, resource allocation across services and settings, linking costs with quality and clinical engagement)?
The use of patient-level information and costing systems in the trusts
Our evidence shows that the primary use of PLICSs is for cost improvement in the trusts. Overall, we found a modest use of PLICSs to direct resource allocation across services and settings – although there was a major service transformation programme at St Winifred’s – and scant use of PLICSs to link costs with quality. Raman et al. 171 suggest that PLICSs can facilitate greater engagement because PLICS costs are at the patient activity level, such as diagnostic tests, care procedures and LoS, and such costs make sense to clinicians. We found that the use of PLICSs had engendered clinical engagement, although this was often limited to specific individuals rather than being widespread across the clinical community, making it difficult to conclude that there had been the ‘dramatically improved clinical ownership’ anticipated by the DH when it introduced PLICSs.
In terms of cost improvement, respondents indicated, primarily, that their use of PLICSs was driven by the requirement to meet government-imposed CIP targets. In our main survey (see Chapter 4), 50% of respondents stated that they were members of a CIP or similar initiative. Often, cost improvements in the trusts were achieved through cost savings (e.g. using less expensive equipment) rather than being driven by greater technical or allocative efficiency, although there was evidence of enhanced technical efficiency when better theatre utilisation resulted in higher numbers of patients treated per session. We found little evidence of PLICSs promoting allocative efficiency. Changing resource allocation in the trusts was being driven by the need to cross-subsidise specialties that were loss-making, using income from areas that were profitable under current tariffs. As mentioned earlier, technical efficiency means using resources to maximum advantage for either outputs (e.g. numbers of patients treated) or outcomes (e.g. numbers of patients benefiting from treatment), whereas allocative efficiency means allocating resources in such a way as to maximise health-care outcomes. 13
Given that, until comparatively recently, what the NHS delivered was driven mainly by the individual decisions of clinicians,172 enhanced clinical engagement seems appropriate. Owens et al. 173 argue that effective health care in the future must take into account both costs and the value of the care delivered (defined as ‘medical benefits that are commensurate with their costs’), pointing out that, currently, some care interventions may harm patients, whereas for other interventions, the benefits do not outweigh the costs, indicating low value. Another parameter of low value is when patients suffer adverse events. Vincent et al. 174 undertook a retrospective analysis in acute British hospitals, and found that around 10% of patients had an adverse event and around half of these were preventable. They estimated that ‘preventable adverse events could cost the NHS around £1bn a year in terms of additional bed-days’. Clinicians need to be involved in assessments of value, in working on how to release additional resources through eliminating low value and in decisions on how to reuse released resources. 175 In consequence, even more clinical engagement with costing information seems warranted in the future.
The wider policy agenda appeared to impact on how the trusts were using their PLICSs. As pointed out earlier, after the ‘listening exercise’, although the Health and Social Care Act 201226 requires both competition and collaboration, the two are often in conflict; when this occurs, some policy commentators argue that competitive forces will predominate27,28 or that competition will impede care integration. 176 This appeared to be the case in our study; our survey results show that 74% of respondents at the trusts regarded the PLICS data as commercially sensitive, only 5% shared PLICS data with commissioners (the same percentage shared with governors) and only 13% shared data with Monitor (the same percentage shared with the DH). As discussed in Better allocative efficiency of resources, the requirement to demonstrate to Monitor that collaborative initiatives were not anticompetitive was a theme reiterated in the interviews. Even St Winifred’s, at which staff had been eager to share progress on its service transformation programme and where there was a risk-sharing agreement with the local CCGs, did not share the PLICS information outside the trust when the system was implemented. The MD judged that the policy environment had changed and that commissioners now adopt a transactional view of commissioning (e.g. the separate contracting of inpatient, outpatient and community services rather than a holistic approach); thus, a pathway approach across acute, community and primary care may be seen as anticompetitive.
The use of patient-level information and costing systems by commissioners
To our knowledge, sophisticated PLICS data are held and used only by trusts. Our survey reveals that only 5% of the trusts using PLICS data shared these with commissioners. Another pertinent issue is that, for commissioners, the most immediate relevant ‘costs’ are the costs to their budgets of purchasing from providers rather than reimbursing the costs to providers of delivering services. In consequence, in this sense, commissioners have limited incentives to request PLICS data from the trusts.
As mentioned in Chapter 3, we were advised by our commissioner team member and our external Project Advisory Group that, to their knowledge at the time, commissioners did not use PLICS data. We also interviewed four commissioners who confirmed this and pointed us in the direction of CSUs as a good source of further intelligence on the potential for PLICSs in commissioning. The results of the CSU survey showed that PLICS data were not being shared between providers and commissioners, but commissioners recognised the future potential value of access to PLICS data. Provider interviews indicated that personnel at the trusts thought that sharing PLICS data with commissioners may result in the latter reducing monies under the tariff to clinical areas that were profitable, while not increasing monies to areas that were loss-making.
Although it is clear that, at the time of this study, the sharing of PLICS data on trust activities was severely curtailed, PLICS data could be developed for community services. However, as discussed in Chapter 2, there are no standard currencies (units of health care for which payment can be made, e.g. a ‘HRG’, a ‘year of care’ or a ‘mental health cluster’), no standard service definitions and no adequate data collection systems. It seems clear that, in the current environment, even if PLICS data did start to emerge in the community, using such data to cost care pathways between primary and secondary care or as evidence to relocate services from secondary care to community care would be likely to be challenged by secondary care providers on the grounds of data inadequacy or inaccuracy.
As mentioned earlier in the report (see Chapters 5 and 8), patient pathways for long-term conditions such as cancer, heart disease, renal disease, diabetes mellitus and asthma have been advocated for decades but have never been fully developed. 11 If PLICS data revealed the potential for considerable cost savings, this would have given impetus to cross-organisational care pathways, but our study has only modest early evidence of PLICS data emerging in the community. Moreover, we have very limited evidence of commissioners using PLICS data, pressing for its wider dissemination or advocating its development for community services. In light of the views that integration between primary, community and acute care along care pathways has the greatest potential for improving the patient experience and reducing costs,9,10,50,54 the lack of PLICSs for community services seems to represent a lost opportunity. Although the current government has looked to the market imperatives of competition and patient choice to drive health care, Jeremy Hunt, the Secretary of State for Health at the time of writing, has remarked that ‘market forces would not create good integrated community care’ and, in this respect, he identified that ‘ensuring commissioners had patient level cost data’ was a priority, but added that ‘at the moment CCGs don’t collect that data’. 177
The use of patient-level information and costing systems to benefit the total health economy
As outlined above, our evidence indicates that the use of PLICSs to benefit the total health economy, compared with individual trusts, is severely curtailed by:
-
trusts adopting the view that their PLICS data are commercially sensitive
-
trusts being unwilling to share their PLICS data externally, particularly with commissioners.
We recently argued that there should be an open-book approach to NHS finances, but this is unlikely to occur within an environment dominated by a business orthodoxy178 that is regulated, by Monitor, to a competitive ethos.
At the time of this study, to our knowledge, the only source of sophisticated PLICS data is trusts (we note, however, that the HFMA33 reports a case study on a community health services NHS trust that uses time and clinician-based costings to produce data on patient cost per minute). Jeremy Hunt has advocated that commissioners have access to PLICS data, but according to our survey only 5% of trusts share their PLICS data with commissioners. In addition, to make decisions on allocative efficiency between acute and community levels for the delivery of patient care, patient-level cost data comparisons between the two levels are required. As discussed above, not only are PLICS data lacking in the community, but the infrastructure (currencies, definitions and adequate data collection systems) to build a PLICS is also absent. As a result, we found little evidence of PLICSs being used to allocate resources across services and settings, other than in the trusts.
However, NHS England’s Five Year Forward View179 argues that community services must be central to new care models. To support moves towards more community care, the HFMA has recently established a Community Services Costing Practitioner Group; noting that Monitor ‘wants to see improvements in community services costing as part of its proposals to move the whole service to a patient-level costing model over the next seven years’, they propose to mandate the implementation of PLICSs across the whole NHS, including community services, although the HFMA remarks that ‘timescales remain ambitious for community providers’. 33 Patient-level costing was developed in the trusts and, as our study shows, there is considerable expertise in hospital settings. However, in the NHS, power and influence have traditionally resided in acute rather than community care, impeding knowledge sharing across their boundaries. 69,180,181 The current competitive ethos in the NHS increases pressures on trusts to act in their own interests rather than those of the total health economy. Competition may improve technical or X-efficiency22 (see Chapter 1, Contribution and significance of the research) by individual health-care providers, but a transactional approach to commissioning inhibits allocative efficiency.
Gertrude, Sybil and St Winifred’s were successful in acquiring the local community services from 2011/12 onwards when it was decided that PCTs (the forerunners to the CCGs) could not be providers as well as commissioners. At the time, these arrangements were termed ‘vertical integration’. As a result, all three of our generalist case study sites (Gertrude, Sybil and St Winifred’s) already offer integrated community services. In such instances any moves to shift care ‘out’ into the community may mean that care remains within the scope of trust activities. Therefore, perverse incentives can arise which impede the efficient reallocation of services. Our evidence indicates that the trusts were sometimes unconvinced about using their community services when the consequence would be that the care moved from being funded through the tariff to being covered under a block contract, in which funds would not increase to cover additional patients.
In the following sections we analyse and discuss our findings relative to our four research objectives:
-
cost improvement through enhanced technical efficiency
-
better allocative efficiency of resources and congruence with patient preferences in health-care economies (first, in and between trusts; second, between primary and secondary/tertiary care; and, third, along care pathways and year of care)
-
understanding clinical variation in resource use and the relationships between cost and quality
-
greater clinical engagement through more clinical ownership of costs and information systems.
Cost improvement through enhanced technical efficiency
In the survey, 83% of respondents stated that they use PLICSs to identify how much a particular patient costs using direct and attributed costs. Perhaps unexpectedly, given that patient-level costs generate costings at the individual patient level, in terms of cost improvement, at interview, we did not find much interest in outliers (i.e. either very high or very low patient costs for a particular intervention). Attention focused instead on ‘shifting the cost curve graph for the majority of patients’ (i.e. thinking of how to reduce costs for the majority of patients within the normal cost range). This focus was apparent at both Gertrude and Sybil, where clinicians remarked that, generally, small numbers of patients were very expensive because their care was, unavoidably, exceptionally complex, and that to make substantial savings small changes had to be made to the pathway for the greater volume of patients.
One part of the pathway for surgical patients, at both Gertrude and Sybil, where opportunities for greater technical efficiency were apparent, was theatre utilisation. Roberts et al. 182 point out that relatively simple changes, for example, theatre start times and over-runs, could be improve theatre utilisation. They point out that, for example, the intended start time may be 9 a.m., but the first case frequently does not begin until 10.30 a.m., resulting in over-runs; they advocate pre-selecting an investigated, prepared ‘golden’ patient the night before and fixing this patient at the beginning of the list. At Sybil, there are 19 theatres. The PLICS data indicated the costs of underutilisation. Throughput and hence technical efficiency improved by moving patients between theatre lists when spare capacity became apparent during the day, by ensuring that very expensive theatres were not used for non-complex cases and simply by highlighting the cost of theatre infrastructure lying idle – through delayed start times – to one surgeon who routinely arrived late because he took his children to school. Notably, for technical efficiency, one respondent commented that the NHS carried ‘slow surgeons’, whereas the private sector just ‘slid them out’.
At the specialist provider, Chelsea, cost improvement through technical efficiency was achieved, when possible, through switching patients who would have been inpatients to day cases (one respondent mentioned that, consequently, inpatient beds had reduced by one-third over the past 5 years) and reducing LoS and, therefore, raising throughput. The survey found that 78% of respondents use PLICSs to identify how LoS impacts on cost.
Most controversially, respondents at Chelsea, Sybil and Gertrude commented that cost improvement for the trust as a whole could be achieved through simply disinvesting in high-cost areas of clinical work. As mentioned earlier, Blunt and Bardsley3 warned of this. One respondent remarked that this was the upshot if the PLICS data were ‘coldly applied’, another that the board was pushing for disinvestments because it perceived this to be the message from Monitor. Although, at interview, this was clearly a sensitive area, the indications were that at both Sybil and Gertrude disinvestment was an active issue. In our survey, 55% of respondents stated that services had been terminated or moved to a different provider as the result of a trust initiative, whereas 14% said that the PLICS had informed this decision.
Better allocative efficiency of resources
Our main finding on using PLICSs for resource allocation in trusts was to support loss-making areas from service lines that made a surplus under the tariff, although, as indicated above, the possibility of disinvestment in some loss-making specialties was under active consideration. Before any cross-subsidisation occurred, PLICSs were used to ascertain which HRGs were loss-making within any loss-making specialty. Both Gertrude and Chelsea were part of a NBG and both trusts used PLICSs benchmarking data to determine whether or not the extent of the loss on a particular HRG was comparable within the benchmarking peer group. If the loss was comparable, that was taken to indicate a tariff issue, but if the loss was less for the peer group (or there was no loss) then the HRG was closely scrutinised for cost inefficiencies before any cross-subsidisation occurred. In the survey, 61% of respondents stated that they use PLICSs to benchmark services against other providers.
In terms of patient preferences, National Voices183 stated that the biggest frustration for patients, service users and carers was the lack of joined-up care; they want ‘continuity of care, smooth transitions between care settings, and services that are responsive to all their needs together’. In the survey, only 2% of trusts reported sharing PLICS data with patient groups; however, 86% of respondents stated that patient preferences had influenced service redesign as part of a trust initiative, with 5% saying that PLICS data had informed this decision. At interview, we found few indications that patient preferences were actively sought in decisions on resource allocation across services and settings or in relation to integrated care. Respondents sometimes spoke for patients; for example, at St Winifred’s it was thought that community services were more of an ‘ideology’ for patients and commissioners even if the cost evidence was not robust. At Chelsea, local access to high-standard chemotherapy was discussed and facilitated but it was also pointed out that, sometimes, patients preferred local access, not realising that this was sometimes not of the standard that could be obtained in a specialist centre. Respondents identified the tariff-based system as a major disincentive to ‘joined-up’ care because payments were made to organisations rather than for pathways, and it was thought that ‘unbundling’ the tariff only resulted in organisations ‘fighting’ over who got how much money for which part of the pathway.
With respect to resource allocation between trusts, at Sybil, the MD, who was leading a regional reconfiguration of services to reduce care variation and duplication between sites, spoke of difficulties in satisfying Monitor that the proposal was not anticompetitive and did not jeopardise the financial standing or sovereignty of the trust. This echoes the proposed merger between two mental health trusts (mentioned earlier), which was seen as detrimental to patient choice and competition102 and the blocking of the merger between Poole Hospital NHS Foundation Trust and Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust on the grounds of ‘substantial lessening of competition’. 184 It is also congruent with warnings to trusts that sharing information that could be seen as commercially sensitive is anticompetitive. 185
In terms of PLICS use in decisions on resource allocation between primary and secondary/tertiary care, in the survey, 52% of respondents said services had been moved to a different care setting as part of a trust initiative and 13% said the PLICS informed this decision. The main interview evidence on resource allocation between primary and acute care comes from St. Winifred’s; its transformation programme sought to reduce duplication between the trust and community services and also to strengthen partnerships with local GPs. To this end, St Winifred’s set out to pool resources and redesign pathways between acute, community and primary care and social services. During our research, the trust introduced a sophisticated PLICS that could have been used to track costs along a pathway that included both acute and community services. However, the trust became concerned about commercial sensitivity and, at the time of writing, it looked unlikely that the PLICS data would be shared with commissioners. At Sybil, one respondent commented that, although their community services were on a block contract, Monitor required it to generate a surplus on both the acute and community arms of the trust. This implied that community services at the trusts would be more expensive than in the community, because in the community, the requirement was only to break even.
In terms of resource allocation along care pathways in the trusts, we discussed our findings on the use of PLICSs to improve theatre utilisation in Chapter 6. Aside from theatre utilisation, respondents at interview did not often refer to PLICSs as tools for pathway redesign and those that did thought it too early to comment on its potential in this respect. However, in the survey, of the 88% of respondents who stated that their organisation had been involved in service redesign, 28% said that PLICSs had informed the decision.
The year-of-care funding model is broad in scope. For example, The King’s Fund presentation included the following elements: primary care; ambulance support; community-based care; acute care; transitory social care funding; and wider social care. With respect to year-of-care PLICS costing, in the survey, in answer to the question, ‘does the organisation collect data or produce reports in a way that would allow costing a patient on the basis of “year of care”?’, 39% of respondents answered ‘yes’. However, this reported figure should be treated with caution because, for the reasons outlined above, we assume that respondents were aware only of the acute part of the year-of-care costs. St Winifred’s were working towards a year-of-care model but, as discussed in Chapter 8, they had distinct reservations about sharing the data from their recently implemented PLICS. At interview, respondents at St Winifred’s indicated that they thought a block contract for community services was untenable and that a year-of-care approach was the future funding mode but it required adequate assessment because it transferred all the risk to the provider.
Understanding clinical variation in resource use and the relationships between cost and quality
Variability in resource use at the consultant level is masked under HRG costing,8 so PLICSs have considerable potential in this regard. However, PLICS data on clinical variation in resource use should be used in conjunction with data on clinical outcomes because, clearly, in the longer term, it is sometimes cost-effective to pay for higher quality. As Owens et al. 173 argue, both current and downstream costs (which result from low quality) should be taken into account in any value-based assessment of care.
In the survey, 63% of respondents stated that they use PLICSs to identify resource variation and, hence, cost between consultants, but only 17% use PLICSs to understand the relationship between cost and quality (defined in the survey as ‘clinical outcomes’). This situation begs the question of whether or not any resource variation between clinicians is justified by better clinical outcomes. At interview, respondents commented favourably on the use of PLICSs to identify clinical variation. At St Winifred’s, reducing variation was one of the primary reasons for introducing a PLICS, but respondents were divided on the question of whether or not clinicians were open to changing their practice when shown the PLICS costs. Other clinicians did refer to the imperative to include downstream costs (e.g. longer time in theatre may improve clinical outcomes) but they recognised that making such links was not currently possible.
One issue is the existence of discrete, disconnected reporting lines for cost and quality (in terms of clinical outcomes). At Gertrude, at the time of our study, measures on costs, waits and outcomes were all reported separately. Respondents reported that the trust had a goal of linking costs with quality and the patient experience, but work was still in its early stages. At Sybil, the CEO had an interest in linking cost and quality, particularly in relation to the cost of harms to patients. The quality improvement lead investigated the possibility of matching PLICS data to their patient data on pressure sores, but concluded that making the link was not feasible because high numbers of patients were involved and the data download could not be automated.
At Chelsea, respondents thought that they had taken a lead in collating clinical outcomes for cancer and that they ‘weren’t too far away’ from linking outcomes with costs. They pointed out that there is no national mandated cancer data set for clinical outcomes. The clinical lead for clinical outcomes identified another problem in relating cost to quality – the costing office costed only what they could charge for and some of the best curative procedures undertaken at Chelsea did not have a tariff.
Finally, on linking costs with quality, BPTs are intended to promote and adequately reimburse high-quality and cost-effective care. 88 In the survey, 34% of respondents reported using PLICSs to understand the benefit achieved through BPT. Respondents at Chelsea and Sybil did not feel that best practice pathways were rewarded with BPTs; the argument at Chelsea was that the DH were interested in BPTs only in very high-volume areas.
Greater clinical engagement through more clinical ownership of costs and information systems
When PLICSs were introduced, the DH1 anticipated ‘dramatically improved clinical ownership’ of costs. In the survey, 88% of trusts reported sharing PLICS data with clinicians, but our findings indicate that clinical ownership does not occur without the finance function strategising over how to engage clinicians.
The finance staff at the four case studies had different strategies to engage clinicians with PLICS data. At Gertrude, there had been a substantial effort to communicate the advantages of PLICSs to clinicians. The Assistant Director of Finance spoke of meeting with 4000 staff over 1 year. He subsequently formed a ‘hit squad’ to go out to assist with the use of PLICSs in relation to specific issues encountered in the directorate. The natural focus for the interest, loyalty and commitment of clinicians is their clinical area. 119 The prime benefits of devolved authority to directorate level, from the point of view of both clinicians and directorate managers, are increased autonomy and access to the surplus (or profit) of the service line. 116 The Assistant Director of Finance spoke of two directorates that had made a surplus and were rewarded by being allowed to use these surpluses to redevelop their services. However, at Gertrude, operational decisions were made through SLR rather than PLICSs; this was the same at Sybil.
In the survey, 58% of respondents reported receiving PLICS reports quarterly, with only 23% having a monthly PLICS report. At Sybil, respondents pointed out that the number that clinicians engaged with was the headline profitability figure in the service line report, which they received monthly. The finance department used bubble charts as ‘strategising artefacts’186 to facilitate consensus and collaboration between the finance department and the clinicians; the latter were encouraged to move their bubble up the chart (to increase their profitability) and, if they were profitable, to grow their bubble (to increase their activity).
At Chelsea, finance managers identified a big change in the NHS, namely that clinicians were actually starting to understand ‘the business’. Their main strategy to promote this engagement was making PLICSs a requirement for business cases. They pointed out that PLICS reports were done only quarterly and PLICSs were actually strategic tools to understand, for example, the financial consequences of business cases for clinical investment. This strategy of engaging the clinicians through business cases fits naturally with the creation of ‘PLICS clinical champions’ (another strategy at Chelsea), because any clinician who has submitted a business case relying on PLICS data is, potentially, a future clinical champion for the costing methodology.
At St Winifred’s, the PLICS was implemented during our study. A newly appointed FD, who was a PLICS enthusiast, sought buy-in from clinicians over the choice of PLICS supplier and the way that the PLICS worked. Specifically, it was agreed that clinicians should be involved in decisions about continued cross-subsidisation and should have access to the PLICS reports without overheads. However, despite this strategy to involve clinicians in contentious areas, overall, the finance team described their success in engaging clinicians as ‘modest’.
It should be noted that, although a few clinicians do complete an MBA (Master of Business Administration) or PhD (Doctor of Philosophy) on their own initiative,187 generally, clinicians do not have formal financial training. 126,188,189 Indeed, they may lack the time to engage in training or management activities. 190 Therefore, their decisions on resource allocation and strategies to reduce costs may not always be fully informed. For example, the clinician who was enthusiastic about patients staying at the Premier Inn rather than in hospital on the night before surgery neglected the hospital’s high fixed costs and did not consider whether or not the trust was able to fill the bed with another patient; the imputed cost saving may have been made against the service line budget but it did not necessarily represent a saving to the trust as a whole.
Implications for practice
There is considerable potential for PLICSs to inform allocative efficiency in the sense of ensuring that resources are located where they can be best utilised to achieve technical efficiency, but this potential is not being realised because of the lack of PLICSs in the community and, relatedly, its non-availability to commissioners. Although this is, at least in part, a policy issue, practitioners, particularly commissioners, could be instrumental in developing PLICSs in the community and in negotiating with providers over access to their PLICS data. However, the most relevant costs to commissioners are the charges against their budgets for purchasing from providers. They do not reimburse the costs to providers of delivering services, so it could be said that commissioners have a limited incentive to negotiate with the trusts to obtain their PLICS data.
Three of our case studies were integrated trusts; in these hospitals, knowledge of the PLICS costs (when these were below the tariff) strengthened a perverse incentive to retain patients in acute care (when they were being funded through the tariff) rather than to move them into their community setting, which was covered by a block contract, as in the latter case funds did not increase to cover additional patients.
The separate reporting lines on costs and clinical outcomes in the trusts severely impede the consideration of costs in relation to outcomes, making it impossible to make assessments of value and, in particular, to judge if higher costs are justified by better outcomes either in aggregate or at the level of the individual clinician. Merging reporting lines on costs and clinical outcomes appears to be a clear avenue for making progress with PLICSs. There has been some success in involving senior clinicians with PLICSs but closer relationships with the finance team are warranted to ensure that the cost-based decisions made by clinicians benefit both the reported financial position of the specialty and the trust as a whole.
Concluding comments
Overall, we found competitive forces rather than collaborative cross-organisational initiatives to be driving the use of PLICSs and, in turn, technical efficiency to be more of a concern than allocative efficiency. This seems likely to continue as Monitor develops PLICSs into a rule-based pricing mechanism. 34 However, many policy commentators advocate collaborative, integrated care, including transferring care and resources from acute providers to primary and community services, as both better for patients and more cost-effective. 9,10,54 However, it is still unclear if equivalent health-care interventions are cheaper outside acute care. 191,192 If PLICSs are developed to include community services they have a significant role as evidence in this debate but, at the time of writing, for the reasons outlined above, their potential is not being realised.
There were important uses of PLICSs that we did not anticipate when we set our research questions and objectives. For example, at Chelsea – as a specialist trust – one of the prime uses of PLICSs was demonstrating that HRG costing underfunded complex, specialist care. The principles underpinning PLICSs as a bottom-up system are similar to those driving activity-based costing. 2 One of the strengths of activity-based costing is revealing the way in which traditional top-down costing methods (like HRG costing) overcost routine products or services, but undercost complex products or services. 193 Thus, at Chelsea, much of the work with the PLICS was to identify the funding shortfall under the HRG-based tariff for their complex care and to press for boosting the tariff.
Another development was the use of PLICSs to cost, and thus identify, the profitability of private patients and potentially, drive initiatives, for example proton beam therapy, which were financially rewarding to the trusts. Proton beam therapy may be increasingly offered in private practice within and outside trusts but respondents indicated that the evidence to date did not show benefit to patients over and above standard treatment. Owens et al. 173 prescribe the identification of low-value treatments, but low clinical value may not equate to low financial value. In a health-care environment becoming progressively more business oriented, respondents felt that there was a role for clinicians in educating patients (who may be desperately anxious to obtain the latest, technologically advanced, treatments) about new interventions that are financially lucrative to hospitals but may be of little or no additional clinical value compared with standard procedures.
Study limitations and suggestions for future research
We undertook a survey, followed by four case studies to investigate the use of PLICSs in the English NHS. In the main, our survey findings were reflected and could be further interrogated and expanded at interview. However, as described in Better allocative efficiency of resources, in the cases of service redesign and moving services to different settings, the findings diverged somewhat, with more indications that this was happening in the survey evidence as opposed to in the interview findings, although there was a major service transformation programme at St Winifred’s and survey respondents may have included, for example, improving theatre utilisation as service redesign. To further investigate this, we reviewed all the open-ended survey comments on service redesign and moving care to a different setting but, although examples were given, unfortunately the comments did not help us to understand the discrepancy in findings in the two areas.
As was expected, our four case studies demonstrated both commonalities and differences in their use of PLICSs. In particular, Chelsea, a specialist trust caring for patients with complex needs, had a distinct focus. However, we cannot claim to have covered all the current and emergent uses for PLICSs. We were unable to identify any significant PLICS use in the community. Moreover, although there is considerable potential for PLICSs in the commissioning function, within the scope and time frame for our research, we were unable to pinpoint any commissioner utilisation of PLICSs. There is also the matter of patients whose care frequently crosses organisational and funding boundaries, for example mental health patients. We were unable to comment on PLICSs in relation to these patients, first, because there were no robust PLICS data in the community and, second, because we did not raise mental health as a specific issue either in our survey or at interview in the case studies.
In terms of generalisation from our four case studies we would anticipate, but are unable to confirm, that our findings would be broadly indicative of PLICS practice in the NBG to which two of our case study sites belonged. Our survey revealed that about one-third of all trusts participate in this group. We found that trusts view their fellow members as a peer group for cost issues. For example, as discussed in Chapter 7, trusts used peer group comparisons in the NBG to determine whether losses on HRGs were indicative of a tariff issue or resulted from cost inefficiencies. This degree of confidence in cost comparability between members indicates that, in the main, our findings will be robust across this group.
We purposefully chose three of our case study sites as ones that were well advanced in their use of PLICSs (i.e. they may have had the leading edge in their practice). Clearly, in such circumstances, one would not anticipate that our findings would be applicable to all trusts or indeed to all clinicians and finance staff in our chosen trusts. Llewellyn and Northcott194 point out that some views are superior to others in terms of discerning ‘what’s going on’ in organisations; this pre-eminence can flow from more knowledge, better judgements or simply holding organisational roles that enable more insight into ‘what’s going on’ in a particular area of organisational practice.
For future research we make three suggestions. First, we would surely advocate tracking and evaluating any emerging uses of PLICSs in the community and by commissioners, particularly for cross-organisational collaborative initiatives, for example the 50 vanguard sites for new care models chosen by NHS England. 124 Second, current plans to deliver the ‘Five Year Forward View’ emphasise care redesign within the context of local health systems rather than individual trusts. 195 Among the key challenges for vanguards and initiatives to deliver the ‘Five Year Forward View’ will be measuring their impact on costs but also determining if they can improve value through linking costs with clinical outcomes. Researching the extent to which PLICSs feature in these cost calculations and value estimates will be important. Third, currently, we found that the trusts designed and used their PLICSs to meet their management needs and to incentivise appropriate actions. The prospect of a mandatory, rule-based and centralised policy agenda for PLICSs begs the question of whether or not the divergent PLICS uses we observed can survive and flourish; this question too is worthy of future attention and investigation.
Acknowledgements
We gratefully acknowledge the HSDR funding which enabled us to undertake this research. We thank our practitioner team members (Mahmood Adil, Tony Whitfield and Claire Yarwood) for their advice and support throughout the project. We also acknowledge the very valuable comments and guidance of our Project Advisory Group (Jane Broadbent, Bill Burgoine, Christopher Haley, Stephen Kennedy, Richard Laughlin, David Peat, Pam Stapleton, Philip Thomas and Emidia Vagnoni). Finally, we thank the staff who gave willingly of their time to participate in the study, as our research relied on their goodwill.
Contributions of authors
This report was co-authored by five project team members. Each chapter had one lead author and all chapters were reviewed in draft form by all authors.
The Principal Investigator, Sue Llewellyn, was the lead author for Chapters 1, 2, 6, 7 and 9.
Naomi Chambers was the lead author for Chapter 5.
Sheila Ellwood was the lead author for Chapter 8.
Christos Begkos was the lead author for Chapter 4.
Chris Wood was the lead author for Chapter 3 and undertook copy-editing tasks for the final published report.
Sections of the report were also reviewed in draft form by members of the Project Advisory Group (see Appendix 1).
Publication
Ellwood S, Chambers N, Llewellyn S, Begkos C, Wood C. Debate: achieving the benefits of patient-level costing – open book or can’t look? Public Money Manag 2015;35:69–70.
Data sharing statement
All study data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Definition: Patient Level Information and Costing Systems. London: DH; 2009.
- Chapman C, Kern A. Costing in the National Health Service: From Reporting to Managing. London: Chartered Institute of Management Accountants; 2010.
- Blunt I, Bardsley M. Use of Patient-Level Costing To Increase Efficiency In NHS Trusts. London: Nuffield Trust; 2012.
- Northcott D, Llewellyn S. The ‘ladder of success’ in healthcare: the UK national reference costing index. Manag Account Res 2003;14:51-66. http://dx.doi.org/10.1016/S1044-5005(02)00032-X.
- Holloway J, Hinton M, Francis G, Mayle D. Identifying Best Practice in Benchmarking. Wokingham: Chartered Institute of Management Accountants Publishing; 1999.
- Doors Open on Patient Level. HFMA; 2009.
- Adil M. Meeting of Minds. Westchester, IL: Healthcare Financial Management Association; 2010.
- Moreea A, Ronald P. PLICS – the curse of averages. Health Serv Rev 2011:5-7.
- Curry N, Ham C. Clinical and Service Integration: The Route to Improve Outcomes. London: The King’s Fund; 2010.
- Ward S. New incentives must support integration. Healthc Finance 2011.
- Maynard A. The Darzi reforms: just words?. Br J Healthc Manag 2008;14. http://dx.doi.org/10.12968/bjhc.2008.14.6.29567.
- The LTC Year of Care Funding Model. London: The King’s Fund; 2013.
- Palmer S, Torgerson DJ. Economic notes: definitions of efficiency. BMJ 1999;318. http://dx.doi.org/10.1136/bmj.318.7191.1136.
- Bridge M. Public Service Productivity Estimates: Healthcare 2013. London: Office for National Statistics; 2015.
- Greene WH, Pesaran MH, Schmidt P. Handbook Of Applied Econometrics: Microeconometrics. London: Blackwell Publishers Ltd; 1997.
- Porcelli F. Measurement of Technical Efficiency. A Brief Survey on Parametric and Non-Parametric Techniques 2009. www2.warwick.ac.uk/fac/soc/economics/staff/fporcelli/porcelli_dea_sfm.pdf (accessed 21 January 2016).
- Byrnes P, Valdmanis V, Charnes A, Cooper WW, Lewin AY, Seiford LM. Data Envelopment Analysis: Theory, Methodology, and Applications. New York, NY: Springer; 1994.
- Nayar P, Ozcan YA. Data envelopment analysis comparison of hospital efficiency and quality. J Med Syst 2008;32:193-9. http://dx.doi.org/10.1007/s10916-007-9122-8.
- Clement JP, Valdmanis VG, Bazzoli GJ, Zhao M, Chukmaitov A. Is more better? An analysis of hospital outcomes and efficiency with a DEA model of output congestion. Health Care Manag Sci 2008;11:67-7. http://dx.doi.org/10.1007/s10729-007-9025-8.
- Mark BA, Jones CB, Lindley L, Ozcan YA. An examination of technical efficiency, quality, and patient safety in acute care nursing units. Policy Polit Nurs Pract 2009;10:180-6. http://dx.doi.org/10.1177/1527154409346322.
- Retzlaff-Roberts D, Chang CF, Rubin RM. Technical efficiency in the use of health care resources: a comparison of OECD countries. Health Policy 2004;69:55-72. http://dx.doi.org/10.1016/j.healthpol.2003.12.002.
- Leibenstein H. Allocative efficiency vs.’ X-efficiency’. Am Econ Rev 1966;56:392-415.
- Culyer AJ. The morality of efficiency in health care – some uncomfortable implications. Health Econ 1992;1:7-18. http://dx.doi.org/10.1002/hec.4730010105.
- Culyer AJ. The bogus conflict between efficiency and vertical equity. Health Econ 2006;15:1155-8. http://dx.doi.org/10.1002/hec.1158.
- Sassi F, Le Grand J, Archard L. Equity versus efficiency: a dilemma for the NHS. If the NHS is serious about equity it must offer guidance when principles conflict. BMJ 2001;323:762-3. http://dx.doi.org/10.1136/bmj.323.7316.762.
- Health and Social Care Act 2012. London: The Stationery Office; 2012.
- Hudson B. Competition and Collaboration in the ‘New NHS’. London: Centre for Health and the Public Interest; 2015.
- Sell S. Forcing Monitor to Promote Integration Won’t Change ‘Heart’ of Health Bill 2011. www.gponline.com/forcing-monitor-promote-integration-wont-change-heart-health-bill/article/1070703 (accessed 31 July 2015).
- Walshe K, Ham C. Can the government’s proposals for NHS reform be made to work?. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d2038.
- Johnstone R. BMA Calls for Withdrawal of ‘Risky’ Health Plans. Public Finance, Chartered Institute of Public Finance and Accountancy; 2011.
- Cronin P, Ryan F, Coughlan M. Undertaking a literature review: a step-by-step approach. Br J Nurs 2008;17:38-43. http://dx.doi.org/10.12968/bjon.2008.17.1.28059.
- A Picture of Health: Service Cost Data in Performance Management in the NHS. London: Chartered Institute of Management Accountants; 2009.
- Healthcare Finance Management Association . Patient level costing for community services. Healthc Finance 2015.
- 2013/14 Patient Level Cost Collection: Review and Lessons for the Future. London: Monitor; 2015.
- NHS Payment System. London: NHS England; 2015.
- Peedell C. For ‘Liberating the NHS’ Read ‘Dismantling the NHS’. Banbury: NHSCA Publications; 2011.
- McLellan A. Stevens is Dismantling the Recent Past to Make Way for the NHS’s Future. Health Services Journal; 2015.
- A Fair Playing Field for the Benefit of NHS Patients: Monitor’s Independent Review for the Secretary of State for Health. London: Monitor; 2013.
- National Tariff 2014/15: A Consultation Notice. London: Monitor; 2013.
- Monitor and NHS England’s Review of the Marginal Cost Rate Rule. London: Monitor; 2013.
- Broad M. Objections Throw Pricing Plans into Disarray 2015. www.hospitaldr.co.uk/blogs/tag/tariffs (accessed 9 June 2015).
- Dowler C. Exclusive: Providers Win £500m in New Tariff Deal. Health Services Journal; 2015.
- The 2015/16 National Tariff. NHS Confederation; 2015.
- Health Investor ‘Voluntary’ Tariff Accepted by Majority of NHS Providers . HealthInvestor 2015. www.healthinvestor.co.uk/ShowArticle.aspx?ID=3940 (accessed 22 June 2015).
- Dowler C. There are no Winners to be Found in the Battle over NHS Prices. Health Services Journal; 2015.
- Darzi L. High Quality Care For All. London: The Stationery Office; 2008.
- NHS Procurement and Efficiency. London: DH; 2014.
- Review of Operational Productivity in NHS Providers: Interim Report June 2015. London: Crown Copyright; 2015.
- ‘Disarray’ as Providers Resoundingly Reject 2015/16 Tariff Proposals. National Health Executive; 2015.
- Alderwick H, Ham C, Buck D. Population Health Systems: Going Beyond Integrated Care. London: The King’s Fund; 2015.
- 2010 to 2015 Government Policy: Health and Social Care Integration. London: DH; 2015.
- National Evaluation of Integrated Care Pilots. London: Nuffield Trust; 2015.
- Thiel V. Where is the Evidence for Promoting Integrated Care?. Health Services Journal; 2014.
- Shaw S, Rosen R, Rumbold B. What is Integrated Care?. London: Nuffield Trust; 2011.
- Ellwood S. The public manager in 2010: the NHS financial manager in 2010. Public Money Manag 2000;20:23-30. http://dx.doi.org/10.1111/1467-9302.00198.
- Jones MJ, Mellett HJ. Determinants of changes in accounting practices: accounting and the UK Health Service. Crit Perspect Account 2007;18:91-121. http://dx.doi.org/10.1016/j.cpa.2005.05.003.
- Llewellyn S. ‘Two-way windows’: clinicians as medical managers. Organ Stud 2001;22:593-62. http://dx.doi.org/10.1177/0170840601224003.
- Magee CC, Osmolski RJ. Specialty costs: an aid to planning and control. Hosp Health Serv Rev 1980;76:203-6.
- Pugh RG. Specialty costing: a new perspective. N Z Med J 1985;98:1040-3.
- Jones T, Prowle M. Health Service Finance. London: CAET; 1982.
- Körner E. A Report on the Collection and Use of Financial Information in the NHS. London: Department of Health and Social Security; 1984.
- Taylor DJ. Patient-Based Costing in the Hospital. Bristol: West Midlands Regional Health Authority; 1984.
- Griffiths R. NHS Management Enquiry. London: Department of Health and Social Security; 1983.
- Ellwood S. Full-cost pricing rules within the National Health Service internal market-accounting choices and the achievement of productive efficiency. Manag Account Res 1996;7:25-51. http://dx.doi.org/10.1006/mare.1996.0002.
- Enthoven AC. Reflections on the Management of the National Health Service: An American Looks at Incentives to Efficiency in Health Services Management in the UK. London: Nuffield Provincial Hospitals Trust; 1985.
- NHS and Community Care Act 1990. London: The Stationery Office; 1990.
- Ellwood S. Cost Methods for NHS Healthcare Contracts. Savona: CIMA Research Foundation; 1992.
- Ellwood S, Bourne M, Sutcliffe C. Management Accounting in Healthcare. London: Chartered Institute of Management Accountants; 1996.
- Llewellyn S. Purchasing power and polarized professionalism in British medicine. Account Audit Account J 1997;10:31-59. http://dx.doi.org/10.1108/09513579710158702.
- Bates K, Brignall TJ. Rationality, politics and healthcare costing. Financ Account Manag 1993;9:27-44. http://dx.doi.org/10.1111/j.1468-0408.1993.tb00143.x.
- Jones CS. Developing financial accountability in British acute hospitals. Financ Account Manag 1999;15:1-20. http://dx.doi.org/10.1111/1468-0408.00071.
- Street A, Maynard A. Payment by results: qualified ambition?. Health Econ Policy Law 2007;2325:445-8. http://dx.doi.org/10.1017/s1744133107004306.
- Llewellyn S, Northcott D. The average hospital. Account Organ Soc 2005;30:555-83. http://dx.doi.org/10.1016/j.aos.2004.05.005.
- Code of Conduct for Payment by Results. London: DH; 2005.
- 2014/15 National Tariff Payment System. London: Monitor; 2013.
- Mannion R, Marini G, Street A. Implementing payment by results in the English NHS: changing incentives and the role of information. J Health Organ Manag 2008;22:79-88. http://dx.doi.org/10.1108/14777260810862425.
- Street A, Maynard A. Activity based financing in England: the need for continual refinement of payment by results. Health Econ Policy Law 2007;21:419-27. http://dx.doi.org/10.1017/s174413310700429x.
- Taylor L. NHS England and Monitor consult on PbR reform. PharmaTimes 2013. www.pharmatimes.com/Article/13–05–16/NHS_England_and_Monitor_consult_on_PbR_reform.aspx (accessed 29 October 2013).
- Calkin C. Knowing the Business (Webinar). Bristol: HFMA; 2013.
- Dowler C. Patient data may give NHS an unfair edge. Health Serv J 2012;122:10-1.
- Imison C. How is the New NHS structured?. London: The King’s Fund; 2015.
- Commissioning Support: Key Facts. London: NHS Commissioning Board; 2012.
- Blunt I, Bardsley M, Dixon J. Trends in Emergency Admissions in England 2004–9: Is Greater Efficiency Breeding Inefficiency?. London: Nuffield Trust; 2010.
- Emergency Admissions Marginal Rate Review: Call for Evidence. Foundation Trust Network; 2013.
- A Simple Guide to Payment by Results. London: DH; 2012.
- NHS Commissioning. NHS England; 2015.
- National Tariff Payment System: A Consultation Notice. London: Monitor; 2014.
- Best Practice Tariff: Questions and Answers. London: British Society for Rheumatology; 2015.
- McDonald R, Zaidi S, Todd S, Konteh F, Hussain K, Roe J, et al. A Qualitative and Quantitative Evaluation of the Introduction of Best Practice Tariffs. Manchester: Institute of Population Health, University of Manchester, University of Nottingham; 2012.
- Is the Treatment Working?. London: Audit Commission; 2008.
- Farrar S, Yi D, Sutton M, Chalkley M, Sussex J, Scott A. Has payment by results affected the way that English hospitals provide care? Difference-in-differences analysis. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3047.
- Appleby J, Harrison T, Hawkins L, Dixon A. Payment by Results: How Can Payment Systems Help to Deliver Better Care?. London: The King’s Fund; 2012.
- Charlesworth A, Hawkins L, Marshall L. NHS Payment Reform: Lessons from the Past and Directions for the Future. London: Nuffield Trust; 2015.
- Llewellyn S, Procter R, Harvey G, Maniatopoulos G, Boyd A. Facilitating technology adoption in the NHS: negotiating the organisational and policy context – a qualitative study. Health Serv Deliv Res 2014;2.
- Patient Level Cost Collection: Review and Lessons for the Future. London: Monitor; 2015.
- Monitor . NHS Providers 2015. www.nhsproviders.org/influencing-and-policy/regulation/monitor/ (accessed 24 September 2015).
- Government Announces Monitor and Trust Development Authority Move to Single Leadership to Deliver Increased Support to Hospitals. London: DH; 2015.
- Welcoming NHS Improvement. medConfidential; 2015.
- Blagg H. ‘Outrageous’ Payout. NHS Chief’s Golden Handshake Is ‘Sickening Reward for Failure’ 2015. www.unitelive.org/outrageous-payout/ (accessed 5 October 2015).
- Dixon J. NHS Improvement: Can Monitor/TDA Adapt to the Future?. London: The Health Foundation; 2015.
- Stirling A. Monitor Explained in 500 Words. Pulse; 2013.
- Seddon N. NHS reforms: patient choice has gone out with the bathwater. The Telegraph 2011. www.telegraph.co.uk/news/health/8575791/NHS-reforms-Patient-choice-has-gone-out-with-the-bathwater.html (accessed 29 October 2013).
- Mulholland H, Stratton A. Nick Clegg threatens to veto health reforms over role of NHS regulator. The Guardian 2011. www.theguardian.com/politics/2011/may/18/nick-clegg-veto-health-reforms-role-nhs-regulator (accessed 24 September 2015).
- Byrne JA. Inside McKinsey. Bloomberg Business; 2002.
- Rose D. The firm that hijacked the NHS. Mail on Sunday 2012. www.dailymail.co.uk/news/article-2099940/NHS-health-reforms-Extent-McKinsey--Companys-role-Andrew-Lansleys-proposals.html (accessed 1 November 2013).
- Monitor: Regulating NHS Foundation Trusts. London: House of Commons; 2014.
- About Monitor. London: Monitor; 2013.
- Craig E. ‘The End of the NHS As We Know It’? A Guide to the Health and Social Care Act. Full Fact; 2013.
- Campbell D. Far more NHS contracts going to private firms than ministers admit, figures show. The Guardian 2015. www.theguardian.com/society/2015/apr/25/far-more-nhs-contracts-going-to-private-firms-than-ministers-admit-privatisation (accessed 22 June 2015).
- NHS England’s Initial Response to Monitor’s Fair Playing Field Review Recommendations. London: NHS England; 2013.
- Ham C, Smith J. Removing the Policy Barriers to Integrated Care in England. London: Nuffield Trust; 2010.
- Hughes D. Private firms ‘win 70% of NHS contracts’. BBC News 2014. www.bbc.co.uk/news/health-25660555 (accessed 22 June 2015).
- Anon . A third of NHS contracts awarded since health act have gone to private sector, BMJ investigation shows. BMJ 2014;349. http://dx.doi.org/10.1136/bmj.g7606.
- Bourn M, Ezzamel M. Costing and budgeting in the national health service. Financ Account Manag 1986;2:53-71. http://dx.doi.org/10.1111/j.1468-0408.1986.tb00027.x.
- Ezzamel M, Willmott H. Corporate governance and financial accountability: recent reforms in the UK public sector. Account Audit Account J 1993. http://dx.doi.org/10.1108/eum0000000001937.
- Bury E, Carter K, Feigelman M, Grant J. How service line management can improve hospital performance. Health Int 2007;7:54-65.
- How Service Line Reporting Can Improve the Productivity and Performance of NHS Foundation Trusts. London: Monitor; 2008.
- Service Line Management – An Overview. London: Monitor; 2009.
- Richman J. Medicine and Health. London: Longman Publishing Group; 1987.
- Jones R. A case of the emperor’s new clothes?. Br J Healthc Manag 2008;14:460-1. http://dx.doi.org/10.12968/bjhc.2008.14.10.31300.
- Jones R. Limitations of the HRG tariff: efficiency comparison. Br J Healthc Manag 2009;15:40-3. http://dx.doi.org/10.12968/bjhc.2009.15.1.37899.
- Jones R. A maximum price tariff. Br J Healthc Manag 2010;16:146-7. http://dx.doi.org/10.12968/bjhc.2010.16.3.46824.
- Annual Report and Accounts 2012–13. London: The Stationery Office; 2013.
- New Care Models – Vanguard Sites. London: NHS England; 2015.
- Hunter DJ. Doctors as managers: poachers turned gamekeepers?. Soc Sci Med 1992;35:557-66. http://dx.doi.org/10.1016/0277-9536(92)90349-U.
- Jones CS, Dewing IP. The attitudes of NHS clinicians and medical managers towards changes in accounting controls. Financ Account Manag 1997;13:261-80. http://dx.doi.org/10.1111/1468-0408.00037.
- Hillman AL, Nash DB, Kissick WL, Martin SP. Managing the medical-industrial complex. N Engl J Med 1986;315:511-13. http://dx.doi.org/10.1056/NEJM198608213150810.
- Wickings I, Coles JM, Flux R, Howard L. Review of clinical budgeting and costing experiments. Br Med J 1983;286:575-8. http://dx.doi.org/10.1136/bmj.286.6364.575.
- Kitchener M. The ‘bureaucratization’ of professional roles: the case of clinical directors in UK hospitals. Organization 2000;7:129-54. http://dx.doi.org/10.1177/135050840071007.
- Braithwaite J, Westbrook MT. A survey of staff attitudes and comparative managerial and non-managerial views in a clinical directorate. Health Serv Manage Res 2004;17:141-66. http://dx.doi.org/10.1258/0951484041485629.
- Magnezi R, Elzam L, Kliker Y, Kedem R, Fire G, Wilf-Miron R. Cost awareness when prescribing treatment. Br J Healthc Manag 2010;16. http://dx.doi.org/10.12968/bjhc.2010.16.2.46416.
- Plsek PE, Greenhalgh T. Complexity science: the challenge of complexity in health care. BMJ 2001;323:625-8. http://dx.doi.org/10.1136/bmj.323.7313.625.
- Barnes M, Matka E, Sullivan H. Evidence, understanding and complexity: evaluation in non-linear systems. Evaluation 2003;9:265-84. http://dx.doi.org/10.1177/13563890030093003.
- Petticrew M, Cummins S, Ferrell C, Findlay A, Higgins C, Hoy C, et al. Natural experiments: an underused tool for public health?. Public Health 2005;119:751-7. http://dx.doi.org/10.1016/j.puhe.2004.11.008.
- Bryman A. Mixed Methods: A Four-Volume Set. London: Sage; 2006.
- Cresswell JW. Research Design: Qualitative, Quantitative and Mixed Methods Approaches. London: Sage; 2003.
- Tashakkori A, Teddlie C. Handbook of Mixed Methods in Social & Behavioral Research. Thousand Oaks, CA: Sage; 2003.
- Plano Clark VL, Creswell JW. Designing and Conducting Mixed Methods Research. Thousand Oaks, CA: Sage; 2007.
- Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. London: Sage; 2009.
- Morse JM. Approaches to qualitative-quantitative methodological triangulation. Nurs Res 1991;40:120-3. http://dx.doi.org/10.1097/00006199-199103000-00014.
- Babbie E. Survey Research Methods. Belmont, CA: Wadsworth; 1990.
- Yin RK. Case Study Research. London: Sage; 1991.
- Llewellyn S. The role of case study methods in management accounting research: a comment. Br Account Rev 1992;24:17-31. http://dx.doi.org/10.1016/S0890-8389(05)80063-0.
- Stake RE. The Art of Case Study Research. Thousand Oaks, CA: Sage; 1995.
- Bryman A. Research Methods and Organization Studies. London: Unwin Hyman; 1989.
- Silverman D. Interpreting Qualitative Data: Methods for Analyzing Talk, Text and Interaction. London: Sage; 2006.
- Flick U. An Introduction to Qualitative Research. London: Sage; 2009.
- Shaw S, Elston J, Abbott S. Comparative analysis of health policy implementation: the use of documentary analysis. Policy Stud 2004;25:259-66. http://dx.doi.org/10.1080/0144287042000288451.
- Archer MS. Being Human: The Problem of Agency. Cambridge: Cambridge University Press; 2000.
- Bhaskar R. The Possibility of Naturalism. London: Routledge; 1979.
- Danermark B, Ekstrom M, Jakobsen L, Karlsson JC. Explaining Society: Critical Realism in the Social Sciences. Oxford: Routledge; 2002.
- Pawson R, Tilley N. Realist Evaluation. London: Sage; 2004.
- Sayer A. Realism and Social Science. London: Sage; 2000.
- Bhaskar R. A Realist Theory of Science. Leeds: Leeds Books; 1975.
- Bhaskar R, Archer MS, Bhaskar R, Collier A, Lawson T, Norrie A. Critical Realism: Essential Readings. London: Routledge; 1998.
- Archer MS. Realist Social Theory: The Morphogenetic Approach. Cambridge: Cambridge University Press; 1995.
- Fairclough N. Peripheral vision discourse analysis in organization studies: the case for critical realism. Organ Stud 2005;26:915-39. http://dx.doi.org/10.1177/0170840605054610.
- Lawson T, Backhouse R. New Directions in Economic Methodology. London: Routledge; 1994.
- Llewellyn S. Case studies and differentiated realities. Qual Res Account Manag 2007;4:53-68. http://dx.doi.org/10.1108/11766090710732505.
- Sue VM, Ritter LA. Conducting On-Line Surveys. Thousand Oaks, CA: Sage; 2007.
- Ritter LA, Sue VM. Introduction to using online surveys. New Direct Eval 2007;2007:5-14. http://dx.doi.org/10.1002/ev.230.
- Penwarden R. Response Rate Statistics for Online Surveys – What Numbers Should You Be Aiming For? 2014. www.fluidsurveys.com/university/response-rate-statistics-online-surveys-aiming/ (accessed 12 May 2015).
- Lu C-J, Shulman SW. Rigor and flexibility in computer-based qualitative research: introducing the Coding Analysis Toolkit. Int J Mult Res Appr 2008;2:105-17. http://dx.doi.org/10.5172/mra.455.2.1.105.
- Gibbs G. Analyzing Qualitative Data. London: Sage; 2007.
- Reference Costs 2012–2013. London: DH Publications; 2013.
- Reference Costs 2013–2014. London: DH Publications; 2014.
- Brown S. The Right Level. Healthcare Finance; 2008.
- Robinson S. Making a Mark. Healthcare Finance; 2012.
- Emanuel EJ, Pearson SD. It costs more but is it worth more?. New York Times 2012. www.opinionator.blogs.nytimes.com/2012/01/02/it-costs-more-but-is-it-worth-more/?_r=1 (accessed 22 July 2016).
- Anonymous . Big 4 Accounting Firms n.d. www.accountingverse.com/articles/big-4-accounting-firms.html (accessed 11 September 2016).
- Raman G, Robinson S, Barham L. Engaging Clinicians in Costing. Healthcare Finance; 2012.
- Stevens S. Reform strategies for the English NHS. Health Aff 2004;23:37-44. http://dx.doi.org/10.1377/hlthaff.23.3.37.
- Owens DK, Qaseem A, Chou R, Shekelle P. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med 2011;154:174-80. http://dx.doi.org/10.7326/0003-4819-154-3-201102010-00007.
- Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. BMJ 2001;322:517-19. http://dx.doi.org/10.1136/bmj.322.7285.517.
- Crump B, Adil M. Can quality and productivity improve in a financially poorer NHS?. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4638.
- Davenport A. We need clarity around how competition law will affect new care models. Health Serv J 2015. www.hsj.co.uk/comment/we-need-clarity-around-how-competition-law-will-affect-new-care-models/5089426.fullarticle (accessed 11 September 2016).
- West D. Exclusive: patient choice is not key to improving performance, says Hunt. Health Serv J 2014. www.hsj.co.uk/sectors/acute-care/exclusive-patient-choice-is-not-key-to-improving-performance-says-hunt/5077051.fullarticle (accessed 20 September 2015).
- Chambers N, Llewellyn S, Adil M, Wood C, Begkos C, Ellwood S. Open book accounting can lead to a more honest NHS. Health Serv J 2014. www.hsj.co.uk/home/innovation-and-efficiency/open-book-accounting-can-lead-to-a-more-honest-nhs/5071602.article (accessed 11 September 2016).
- Reforming the Payment System for NHS Services: Supporting the Five Year Forward View. London: Monitor; 2015.
- Coulter A. Shifting the balance from secondary to primary care. BMJ 1995;311:1447-8. http://dx.doi.org/10.1136/bmj.311.7018.1447.
- Currie G, Suhomlinova O. The impact of institutional forces upon knowledge sharing in the UK NHS: the triumph of professional power and the inconsistency of policy. Public Admin 2006;84:1-30. http://dx.doi.org/10.1111/j.0033-3298.2006.00491.x.
- Roberts S, Saithna A, Bethune R. Improving theatre efficiency and utilisation through early identification of trauma patients and enhanced communication between teams. BMJ Qual Improv Reports 2015;4.
- Principles for Integrated Care. London: National Voices; 2011.
- Spencelayh E, Dixon J. Mergers in the NHS 2014. www.health.org.uk/publication/mergers-nhs (accessed 21 September 2015).
- Kilpatrick B. Are competition warnings to NHS trusts a sign of things to come?. The Guardian 2012. www.theguardian.com/healthcare-network/2012/aug/23/competition-authority-warning-nhs-trusts (accessed 21 September 2015).
- Jarzabkowski P, Spee AP, Smets M. Material artifacts: practices for doing strategy with ‘stuff’. Eur Manag J 2013;31:41-54. http://dx.doi.org/10.1016/j.emj.2012.09.001.
- Cork N, Devine CA. Educating clinician leaders: can the NHS benefit from the US MD/MBA experience?. Clin Med 2015;15:404-6.
- Kurunmäki L. A hybrid profession – the acquisition of management accounting expertise by medical professionals. Account Organ Soc 2004;29:327-47. http://dx.doi.org/10.1016/S0361-3682(02)00069-7.
- Preston AM, Cooper DJ, Coombs RW. Fabricating budgets: a study of the production of management budgeting in the National Health Service. Account Organ Soc 1992;17:561-93. http://dx.doi.org/10.1016/0361-3682(92)90014-J.
- O’Riordan C, McDermott A. Clinical managers in the primary care sector: do the benefits stack up?. J Health Organ Manag 2012;26:621-40. http://dx.doi.org/10.1108/14777261211256945.
- Shepperd S, Doll H, Broad J, Gladman J, Iliffe S, Langhorne P, et al. Early discharge hospital at home. Cochrane Database Syst Rev 2009;1. http://dx.doi.org/10.1002/14651858.cd000356.pub3.
- Steventon A, Bardsley M, Billings J, Georghiou T, Lewis G. An Evaluation of the Impact of Community-Based Interventions on Hospital Use. London: Nuffield Trust; 2011.
- Kaplan R, Bruns W. Accounting and Management: A Field Study Perspective. New York, NY: Harvard Business School Press; 1987.
- Llewellyn S, Northcott D. The ‘singular view’ in management case studies. Qual Res Organ Manag 2007;2:194-207. http://dx.doi.org/10.1108/17465640710835355.
- Delivering the Forward View: NHS Planning Guidance 2016/17–2020/21. London: NHS England; 2015.
Appendix 1 Project Advisory Group members
The Project Advisory Group members were:
-
Jane Broadbent (Professor), Royal Holloway, University of London.
-
Bill Burgoine, patient/public advisor.
-
Christopher Haley (Clinician), Stockport NHS.
-
Stephen Kennedy (Deputy Director of Finance, Salford Royal NHS Foundation Trust).
-
Richard Laughlin (Professor), King’s College London.
-
David Peat (Commissioning Development Director, retired), public advisor.
-
Pam Stapleton (Professor), University of Manchester.
-
Philip Thomas (Divisional Clinical Director), Brighton & Sussex University NHS Trust.
-
Emidia Vagnoni (Professor), University of Ferrara.
Appendix 2 Questionnaire to provider organisations
Appendix 3 Questionnaire to commissioning support units
Appendix 4 List of additional interviewees
Additional interviews conducted outside the four main case study sites are detailed in Table 16.
| Role/job title | Organisation type | Interview date |
|---|---|---|
| Programme Director | Commissioner | 24 April 2013 |
| Divisional Clinical Director | Acute Provider | 13 May 2013 |
| Programme Manager, Stroke Association | Commissioner | 28 August 2013 |
| Director of Quality and Provider Management | Commissioner | 5 September 2013 |
| Acting Director of Finance | Commissioner | 9 September 2013 |
| Strategic Health Analyst For Stroke Pathway Redesign | Commissioner | 19 September 2013 |
| Director of Primary Care | Commissioner | 28 March 2014 |
| Finance Manager | NBG member | 8 May 2014 |
| Finance Manager | NBG member | 9 May 2014 |
| Finance Manager | NBG member | 9 May 2014 |
Appendix 5 Theme code template for transcript analysis
Template produced for thematic analysis of interview transcripts
Final version
-
Cost improvement
-
Tariff
-
Cross-subsidisation between specialties consequent on tariff inequities
-
Sharing for benchmarking
-
Performance review
-
Service redesign
-
Business development/joint ventures
-
Any qualified provider/threats and opportunities
-
Patient cost variability
-
Research and teaching costs
-
SLM/SLR
-
– Transfer pricing
-
-
-
Clinical engagement
-
Productivity
-
Adherence to protocols
-
Sharing for benchmarking
-
Investment/disinvestment/business cases
-
-
Cost and quality
-
Paying for quality
-
– Best practice tariffs
-
– Nursing acuity
-
-
-
Services and settings
-
Tariff
-
Year-of-care costing
-
Shared care protocols
-
Patient pathway
-
-
Commissioning
-
National versus local tariffs
-
Needs assessment
-
-
Other
-
Trust status: FTs
-
Accuracy of information system
-
NHS reforms
-
PLICS critique
-
Coding for a result
-
Patient preferences
-
Monitor
-
Private patients
-
Appendix 6 St Winifred’s: sample of 10 patients > 60 years old – fractured neck of femur (patient-level information and costing system data)
| Patient-level information | Patient ID | Total | Average | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| Age range (years) | 90–100 | 60–70 | 90–100 | 70–80 | 60–70 | 90–100 | 80–90 | 80–90 | 70–80 | 100–110 | ||
| Spell LoS (days) | 35 | 20 | 55 | 10 | 7 | 5 | 12 | 35 | 6 | 20 | 205 | 21 |
| Costing system data (£) | ||||||||||||
| Blood | 290 | 290 | 287 | 867 | 87 | |||||||
| Clinical negligence | 207 | 89 | 195 | 295 | 207 | 148 | 354 | 76 | 177 | 32 | 1780 | 178 |
| Consultants | 241 | 292 | 918 | 515 | 2001 | 369 | 315 | 853 | 2185 | 366 | 8055 | 806 |
| Drugs | 11 | 78 | 47 | 79 | 48 | 78 | 37 | 29 | 41 | 68 | 516 | 52 |
| Emergency department | 72 | 72 | 7 | |||||||||
| General staffing | 11 | 5 | 12 | 15 | 11 | 8 | 18 | 7 | 9 | 3 | 99 | 10 |
| High-cost drugs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Imaging | 8 | 6 | 137 | 84 | 19 | 18 | 25 | 11 | 10 | 28 | 346 | 35 |
| Junior medics | 453 | 377 | 1443 | 693 | 480 | 489 | 692 | 1283 | 521 | 601 | 7032 | 703 |
| Medical staffing | 33 | 14 | 24 | 48 | 33 | 24 | 57 | 1 | 29 | 0 | 264 | 26 |
| Non-pay – other | –27 | –12 | –17 | –39 | –27 | –20 | –47 | 4 | –23 | 2 | –207 | –21 |
| Non-patient care activities | –12 | –5 | –54 | –18 | –12 | –9 | –21 | –75 | –11 | –31 | –248 | –25 |
| Operating theatres | 412 | 604 | 810 | 987 | 710 | 732 | 462 | 803 | 5520 | 552 | ||
| Other clinical supplies/services | 251 | 107 | 180 | 358 | 251 | 179 | 430 | 1 | 215 | 0 | 1972 | 197 |
| Other diagnostics | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 12 | 1 |
| Other specialist nursing | 141 | 60 | 214 | 202 | 141 | 101 | 242 | 186 | 121 | 77 | 1485 | 149 |
| Other support | 336 | 144 | 900 | 479 | 336 | 240 | 575 | 1081 | 288 | 450 | 4829 | 483 |
| Overhead | 427 | 183 | 705 | 611 | 427 | 305 | 733 | 655 | 366 | 272 | 4684 | 468 |
| Pathology | 54 | 27 | 210 | 50 | 95 | 63 | 113 | 87 | 18 | 113 | 830 | 83 |
| Pharmacy services | 9 | 4 | 7 | 13 | 9 | 6 | 15 | 2 | 8 | 1 | 74 | 7 |
| Prostheses/implants/devices | 1356 | 308 | 41 | 241 | 1116 | 1356 | 1356 | 1205 | 6979 | 698 | ||
| Therapies | 191 | 91 | 473 | 273 | 197 | 151 | 336 | 506 | 169 | 214 | 2601 | 260 |
| Ward and other settings | 1064 | 541 | 2844 | 1524 | 1209 | 1123 | 2201 | 4242 | 967 | 1819 | 17,534 | 1753 |
| Total inpatient | 5166 | 3203 | 9382 | 6411 | 7322 | 5362 | 7895 | 8947 | 7098 | 4303 | 65,089 | 6509 |
| A&E | 226 | 268 | 109 | 278 | 96 | 159 | 213 | 184 | 69 | 247 | 1849 | 185 |
| Community contacts | 9881 | 3289 | 0 | 2128 | 0 | 2509 | 6767 | 0 | 688 | 0 | 25,262 | 2526 |
| Total related cost | 15,273 | 6760 | 9491 | 8817 | 7418 | 8030 | 14,875 | 9131 | 7855 | 4550 | 92,200 | 9220 |
Appendix 7 St Winifred’s: sample of 10 patients > 60 years old – fractured neck of femur (patient-level information and costing system data) community analysis
| Patient-level information | Patient ID | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Number of contacts | 32 | 17 | 0 | 10 | 0 | 21 | 32 | 0 | 4 | 0 |
| Costing system data (£) | ||||||||||
| IHT A | 7629 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IHT B | 110 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IHT C | 317 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IHT D | 1824 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IHT E | 0 | 1740 | 0 | 0 | 0 | 0 | 5778 | 0 | 0 | 0 |
| IHT F | 0 | 1486 | 0 | 0 | 0 | 0 | 777 | 0 | 0 | 0 |
| Podiatry/diabetes | 0 | 63 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IHT G | 0 | 0 | 0 | 2128 | 0 | 2509 | 0 | 0 | 688 | 0 |
| IHT H | 0 | 0 | 0 | 0 | 0 | 0 | 122 | 0 | 0 | 0 |
| SPN leg ulcer | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 0 | 0 |
List of abbreviations
- A&E
- accident and emergency
- BC
- business case
- BPT
- best practice tariff
- CCG
- Clinical Commissioning Group
- CEO
- chief executive officer
- CIP
- Cost Improvement Programme
- CQUIN
- commissioning for quality and innovation
- CSU
- commissioning support unit
- CSV
- comma-separated value
- DH
- Department of Health
- ETO
- Enhanced Tariff Option
- FCE
- finished consultant episode
- FD
- finance director
- FT
- foundation trust
- GP
- general practitioner
- HFMA
- Healthcare Financial Management Association
- HRG
- health resource group
- HTU
- haematology transplant unit
- LoS
- length of stay
- MD
- medical director
- NBG
- national benchmarking group
- NRES
- National Research Ethics Service
- PbR
- payment by results
- PCT
- primary care trust
- PLICS
- patient-level information and costing system
- PPI
- patient and public involvement
- SHA
- Strategic Health Authority
- SLM
- service line management
- SLR
- service line reporting
- SPSS
- Statistical Package for the Social Sciences
- TDA
- Trust Development Authority
- YOU
- young oncology unit