Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/2004/29. The contractual start date was in May 2013. The final report began editorial review in December 2015 and was accepted for publication in July 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Standing et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Overview of the research
This research study used qualitative methods to investigate patients’, family members’ and professionals’ views about, and experiences of, implantable cardioverter defibrillator (ICD) implantation and deactivation. This chapter presents a synopsis of the study context, rationale, aims and objectives, including an overview of the project team, patient and public involvement (PPI), ethics approval and the subsequent structure of the report.
Context and rationale for the research
In the UK there are 100,000 sudden cardiac deaths per year,1 80% of which are attributable to ventricular tachyarrhythmia. 2 ICDs are recommended for patients at high risk of sudden cardiac death [primary prevention (PP)] and for survivors of cardiac arrest [secondary prevention (SP)]. 2,3 All ICDs combine both a shock function (to treat fast heart rhythms) with a pacing function (to treat slow heart rhythms). In some cases, the pacing function may be very sophisticated and can provide so-called cardiac resynchronisation therapy (CRT) for the treatment of heart failure. CRT itself may be provided by a pacemaker [cardiac resynchronisation therapy with pacemaker (CRT-P)] or in conjunction with an ICD [cardiac resynchronisation therapy with defibrillator (CRT-D)]. The great majority of ICDs are provided for PP for patients with chronic heart failure. They increase life expectancy but may be associated with adverse effects, including unnecessary or inappropriate shocks, device complications, increased hospitalisation and anxiety and depression. 4 Consequently, decision-making about an ICD for individuals should consider the benefit (averting sudden death) against possible future harms, including adverse effects and the potential need for deactivation towards the end of life.
Implantable cardioverter defibrillator implantation rates in the UK vary geographically and are increasing, but the extent to which patients are engaged in decision-making, and what their information and support needs are, is unclear. 5,6 Shared decision-making (SDM), whereby doctors and patients make joint decisions informed by best evidence and aligned with patient values and preferences,7 is associated with improvements in patient involvement, knowledge and accurate risk perception, leading to informed values-based choices and increased treatment adherence. 8,9 There is a dearth of information relating to the role that potential ICD patients can and want to have in the decision-making process and what potential recipients need to know before they decide to have ICD therapy. 5 It is unclear how possible benefits and harms are communicated to patients during the clinical encounter and how patients make sense of this information. A 2014 review10 identified that patients faced with ICD-related decisions often misunderstood the functionality of ICDs, or overestimated their benefit, and the authors recommended a SDM approach to achieve improved patient outcomes.
At the end of life, shocks from ICDs may cause unnecessary pain and suffering to the patient and distress for carers and family members. 11,12 In these circumstances, deactivation may be the most appropriate management. However, there is a paucity of evidence to reflect if, how and when deactivation conversations take place in the UK.
There are clear gaps in the existing body of research with regard to the information needs and preferences of potential ICD recipients with respect to both implantation and deactivation.
The outputs of this study help to address these gaps by providing data on current decision-making while also establishing the information needs, values and preferences of ICD recipients, carers/family members and clinicians, so that decision-making involving patients can be effectively supported. Most patients are not aware of the possibility of ICD deactivation when they have the device implanted and, for those who are, it is unclear when, how and from whom [e.g. general practitioner (GP), cardiologist, heart failure nurse, palliative care clinician] they receive this information. 6,13,14 Little is known about how patients make sense of information about ICDs and what patients’ information needs and preferences are with respect to implantation and deactivation. 5,6,15,16 By documenting current decision-making throughout the care pathway, and by exploring patients’, family members’ and clinicians’ views of decision-making, we have addressed these knowledge gaps to help determine how to better support SDM about ICD implantation and deactivation.
Aims and objectives
Aim
To critically explore lay and professional views on, and experiences of, ICD implantation and deactivation (towards the end of life) and to examine how this information can be used to support SDM.
Objectives
-
To explore patients’, family members’ and clinicians’ views and experiences of decision-making about ICD implantation and deactivation (towards the end of life).
-
To establish how and when ICD risks, benefits and consequences are communicated to patients (including deactivation).
-
To determine patients’, family members’ and clinicians’ information and decision-support needs in the context of SDM.
-
To identify the individual and organisational facilitators and barriers to discussions about implantation and timely decision-making about deactivation.
-
To inform (1) early-stage development of decision support for ICD implantation and (2) improvements in advance care planning for ICD recipients.
Project team
The project was led by Professor Richard Thomson, expert in SDM, and Professor Catherine Exley, medical sociologist and qualitative researcher, in close co-ordination with Dr Kerry Joyce, Senior Research Associate, who was a co-investigator and responsible for the day-to-day project management until September 2014. Dr Joyce’s role was taken over by Holly Standing, Research Assistant, from August 2014, with additional support from Dr Darren Flynn, Senior Research Associate and Practitioner Health Psychologist, from September 2015. The core project team met, on average, once a month to discuss the day-to-day practicalities of running the project, recruitment and data analysis.
The Project Advisory Group consisted of members of the project team and Professor Julian Hughes (Honorary Professor of Philosophy of Ageing and Consultant in Old Age Psychiatry), Dr Stephen Lord and Dr Janet McComb (consultant cardiologists), Dr Paul Paes (Consultant in Palliative Medicine), Mr Tom Bryden, Mr Tom Twedell, Mr Paul Cuskin and Mr Steve Whitely (patient representatives), Mrs Trudie Lobban, Member of the Most Excellent Order of the British Empire (MBE) (founder and trustee of the Arrhythmia Alliance: The Heart Rhythm Charity) and Dr Daniel Matlock (Consultant in Palliative Medicine). The Project Advisory Group provided feedback and advice, and reviewed progress on all aspects of the study.
Patient and public involvement
Active, sustained and non-tokenistic engagement with patients is a crucial component of our research and echoes our commitment to conducting research that supports patients in adopting a more proactive role in decision-making. Public and patient engagement was initiated prior to the development of the outline application and was led by one of the co-investigators, Dr Kerry Joyce. Mrs Trudie Lobban MBE, Founder and Trustee of Arrhythmia Alliance, was contacted at the pre-application stage. Trudie enhanced the focus of the project on patients’ needs by encouraging us to concentrate on both implantation and deactivation decisions, explaining that deactivation is often a key issue that patients and relatives would like to discuss in advance. In this way, deactivation would be addressed in advance rather than leaving it until close to the end of life, when it is often the family/carers who are faced with making the decision rather than the patient themselves, causing additional distress at an extremely emotional time. This benefit of early PPI was used as an example of good practice by INVOLVE, a national advisory group ‘to support active public involvement in NHS, public health and social care research’. 17 Trudie subsequently joined the research team as both a co-applicant and a member of our study advisory group. Trudie provided service user input into all stages, including the development of patient/carer information resources, data analysis, report writing, interpretation and dissemination of study findings. Two patient/carer representatives (with an interest in end-of-life issues) joined the initial project advisory group. The Arrhythmia Alliance and the North of England Cardiovascular Network also reviewed the research proposal and their comments were incorporated, specifically with respect to strengthening the focus on decision-making across the care pathway, including more than one secondary care centre and emphasising patient benefit.
Ethics approval
Ethics approval for this study was obtained on 26 April 2013 from the National Research Ethics Service Committee North East-Sunderland (reference: 13/NE/0105). Two ethics amendments were subsequently given a favourable opinion, on 14 November 2013 (to amend slightly the strategy for recruiting patients to the study and to be able to include patients eligible for a defibrillator in combination with CRT) and 12 May 2014 (a change to the method of approaching bereaved relatives and widening of the eligibility criteria to 12–18 months post bereavement). All potential participants were provided with information about the study and there was an opportunity to address questions to the researchers prior to participation. Individuals were informed that their participation was entirely voluntary and that they were free to withdraw at any time without reason. All personal identifying information was removed to protect confidentiality.
Structure of the report
Chapter 2 presents the background literature on the implantation and deactivation of ICDs, the rationale for the current study and the study aim and objectives. Chapter 3 details the methods used to address the study objectives, which consisted of observation of consultations, in-depth interviews and interactive workshops. An overview of the context and the participants recruited within each of the three phases is presented in Chapter 4. The findings of the interviews and workshops with reference to implantation issues are presented in Chapter 5. Chapter 6 discusses the findings of interviews and workshops with reference to deactivation issues. Chapter 7 presents a summary of the study findings, along with a discussion in relation to the previous literature, the implications of findings for supporting better SDM about ICDs, the strengths and limitations of the current study and future directions for research.
Chapter 2 Background
In the UK there are 100,000 sudden cardiac deaths per year,1 80% of which are attributable to ventricular tachyarrhythmia. 2 ICDs are recommended for patients at high risk of sudden cardiac death [primary prevention (PP)] and for survivors of cardiac arrest [secondary prevention (SP)]. 2,3 All ICDs combine a shock function (to treat fast heart rhythms) with a pacing function (to treat slow heart rhythms). In some cases, the pacing function may be very sophisticated and can provide so-called CRT for the treatment of heart failure. CRT itself may be provided by a pacemaker (CRT-P) or in conjunction with an ICD (CRT-D). The majority of ICDs are used for PP in patients with chronic heart failure. ICDs increase life expectancy but may be associated with adverse effects, including unnecessary or inappropriate shocks, device complications, increased hospitalisation and anxiety and depression. 4 The reported incidence of adverse effects varies greatly from 20% to 60%. 4 Shocks may be painful and distressing and have been compared to being kicked in the chest by a horse or being struck by lightning. 18 Thus, decision-making about an ICD for the individual should include the weighing up of benefits (i.e. averting sudden death) against possible future harms, including adverse effects and the potential need for deactivation towards the end of life.
Implantable cardioverter defibrillator implantation rates in the UK vary geographically and are increasing,19,20 but the extent to which patients are engaged in decision-making and what their information and support needs are, is unclear. 5,6 SDM, whereby doctors and patients make joint decisions informed by best evidence and aligned with patient values and preferences,7 is associated with improvements in patient involvement, knowledge and accurate risk perception, which leads to informed values-based choices and increased treatment adherence. 8,9 There is a dearth of information relating to the role that potential ICD patients can and want to have in the decision-making process, and what potential recipients need to be informed about before they decide to have ICD therapy. 5 It is unclear how possible benefits and harms are communicated to patients during the clinical encounter and how patients make sense of this information. Indeed, data on patients’ expectations of ICDs suggest that survival benefits might be substantially overestimated,21 and improvements to how information on the clinical rationale for ICDs (including the fact that simple ICDs do not confer any symptom or quality-of-life benefits) is communicated to patients and their relatives may be warranted. It has been shown that patients eligible for different therapies might not be fully aware of the benefits and harms of each option,15,16 and the psychosocial impacts of available treatment or management options rarely feature in risk communication. 22 We also do not know how decisions to implant an ICD device align with what is important to the individual patient. However, recent work locally suggests that patients who are more involved in decisions about ICD implantations may be less inclined to have a device fitted. 23
Near the end of life, shocks from ICDs may cause unnecessary pain and suffering for the patient and distress for carers and family members. 11,12 Currently, research suggests that one in four patients experiences shocks during the last month of life. 13 The end-of-life trajectory is difficult to predict for patients with end-stage heart failure, although shocks do act as a marker of deterioration for some and may actually be a useful trigger for the palliative process; in these circumstances, deactivation can be the most appropriate management. Research from the USA suggests that clinicians discuss deactivation of ICDs with only a small subset of patients and, in the majority of cases, these discussions take place only a few hours or days before death. 13 There is a paucity of evidence to reflect if, how and when deactivation conversations take place in the UK. A focus group study with US ICD outpatients (approximately half of whom had experienced at least one shock) found that none recalled discussions about deactivation or knew that deactivation was a possibility. 14 From patients’ perspectives, barriers to deactivation conversations included a reluctance to begin advance care planning discussions and a lack of knowledge about ICD function. 11,14 Reasons for clinicians failing to initiate deactivation conversations were: a lack of knowledge about ICD function; poor quality doctor–patient relationships; the assumption that responsibility for discussing deactivation lies elsewhere; erroneous beliefs that patients are already aware that the device can be deactivated; and the belief that clinicians can accurately predict which patients will experience shock (and distress) towards the end of life. 11,24 This research suggests a level of unease around initiating deactivation discussions. The consequence of this lack of discussion is unnecessary or unwanted shocks towards the end of life. Indeed, one survey demonstrated that nearly half of all hospices in the USA had experience of a patient getting shocked by their ICD because no one thought to have a discussion about turning the ICD off. 25 The National Institute for Health and Care Excellence (NICE) guidelines2 on ICDs published in 2014 present, very briefly, details about the possible need for ICD deactivation, and a regional policy for the deactivation/reactivation of ICDs26 tends to be technical in style and the patient perspective is largely absent. Furthermore, a critical review of six clinical practice guidelines27 for ICD therapy reported that they tended to focus on evidence of device effectiveness, with minimal consideration of impacts on patient quality of life and psychosocial well-being, including the involvement of patients/relatives in decisions.
The implantation and deactivation of ICDs present interesting ethical challenges. The ethical issues around decision-making in connection with ICD implantation are, at first sight, no different from any other treatment decisions. The patient must be able to consent to the treatment, he/she must have the capacity to make the decision – by understanding, retaining, weighing up and communicating their decision – and must make it freely without coercion. 28 Furthermore, the decision can readily be discussed in terms of the four principles of medical ethics. 29 Thus, the person’s autonomy must be respected: they must be able to make the decision themselves having been fully informed about the consequences of having or not having the treatment. Implantation is undertaken in order to do the patient some good (beneficence). Unnecessary shocks (which are painful and distressing), however, would be one way in which the person might be harmed and would go against the principle that the doctor should do no harm (non-maleficence). Finally, the principle of justice also seems pertinent because there are issues around resource allocation.
Another layer of ethical complexity is added to these more ‘mundane’ ethical considerations once deactivation is considered. From a procedural perspective, within the framework of the Mental Capacity Act 2005,30 if the patient has capacity, he or she should make the decision; if the person lacks capacity, a decision must be made in his or her best interests. The purely ethical considerations around deactivation, however, are clearly broader than the more straightforward application of the four principles. For instance, the doctrine of ordinary and extraordinary means is clearly relevant to deactivation because, at the end of life, shocks could be considered both ineffective (defined as no longer leading to the goal that was originally intended) and, thus, futile as well as burdensome. This could, therefore, be considered an extraordinary treatment and, as such, there would be no moral obligation to provide it. 31 The temporality of deactivation discussions is important here. Some of the ethical issues are underpinned by deeper philosophical concerns, for example about the shape of our lives and about the role of health care within our conceptions of life’s purposes. The importance we attach to how our lives end is relevant to a narrative view of ethics. 31 In addition, it may be that deactivation needs to be considered in the context of the relationships of care and trust that exist between a patient and his or her health-care professionals. Finally, the sensitive nature and difficulty of the discussions that might have to be had at implantation about deactivation should alert us to the importance of the inner dispositions, which constitute the requisite professional virtues of fidelity, compassion, prudence, fortitude and integrity. 32 In other words, one of the ethical requirements is that those involved in the sensitive conversations about implantation and deactivation should approach patients in the right way, that is, they should be disposed correctly, which in turn means that they should be on the patient’s side but should act in a manner that is true to all the complexities that have to be weighed up in the course of the SDM conversation with the patient.
Despite these obvious complexities, there is a paucity of research on attitudes to, and practices around, ICD deactivation6 and there have been few systematic attempts to develop decision support for either patients or clinicians to facilitate conversations about ICD implantation and deactivation during end-of-life care. Published research (mostly from the USA) is limited in two key ways. First, it tends to consider decision-making in tertiary care (specialist centres), ignoring what happens earlier in the pathway. Second, the research has generally not sought to change practice; rather, the description of patient experience is viewed as an end in itself. The current study has increasing resonance with the prominence of SDM in NHS reforms33 and the increasing importance attached to advance care planning. 34 By documenting current decision-making throughout the care pathway, and by exploring patients’, family members’ and clinicians’ views of decision-making, we aimed to address these knowledge gaps and to better understand the support needed to enhance SDM about ICD implantation and deactivation.
Rationale
Health need
Implantable cardioverter defibrillator implantation rates are increasing in the UK, with a 10-year mean growth rate of 14.9% for ICDs from 2000 to 2010,35 with more recent figures showing a rate of 72 per 1 million in 2013/14 compared with 66 per 1 million in 2012,36 but with marked geographic variation. 19,20 We do not know if, when or how the risks (including inappropriate shocks and psychopathology), benefits (increases in survival) and consequences (including deactivation) of ICDs are discussed with patients, nor do we know the extent to which patients wish to be engaged in decision-making about ICDs. 5 In terms of implantation decisions, we aimed to provide guidance on how to improve existing decision support and to provide better support for SDM to ensure that implantation decisions are appropriate and fit with the values and preferences of patients. With respect to deactivation decision-making, we sought to better understand how discussions currently take place, and the information needs and preferences of patients at different points in the patient journey, in order to feed into appropriate advance care planning. Together with the ethical imperatives to conduct this piece of research, there are likely to be additional benefits in terms of improving the quality of life for patients with ICDs throughout the pathway and especially when facing end-of-life decisions. In addition, this research should help to reduce any anxiety in clinical staff and carers/family members, given the absence of clear national guidelines/protocols, and to reduce complicated grief among the bereaved associated with poor quality of death. 6
Expressed need
Increasing importance is attached to SDM33 and advance care planning34 in current NHS strategy. Decision-making about ICD implantation is a preference-sensitive decision for which the SDM model is fitting. For example, potential risks (e.g. inappropriate shocks, device complications, psychopathology) and possible consequences of an ICD (e.g. deactivation during end-of-life care) are likely to hold varying levels of importance for different people when they are deliberating between having an ICD fitted or not. Indeed, a recent study found that informed patients who are more involved in SDM may make decisions that contrast to those driven by the strict application of evidence-based guidelines in a more traditional and paternalistic approach to decision-making. 37 This accords with the finding that decision aids used in contexts in which both an invasive and a conservative treatment option are available result in a 25% reduction in patients choosing the invasive option. 8 Thus, there is a cogent, evidence-based argument for the adoption of the SDM model in the context of decisions about ICDs, which is reflected in the American College of Cardiology’s call for implementation of (and research into) models of SDM in cardiology. 38 Furthermore, the existing evidence base shows that the topic of ICD deactivation is seldom discussed in advance. 13 The study sought to examine ways in which to address the timeliness of deactivation conversations while exploring how these might best be supported.
Sustained interest and intent
Rates of ICD implantations are increasing and, with our ageing population, demand is likely to continue to escalate in the future. From both an ethical and practical perspective (in terms of current resource constraints), it is important that patients are supported to make decisions in line with their individual values and preferences, thus ensuring that decisions are appropriate for each individual patient, and that unnecessary or inappropriate implantations are avoided. In line with the increased importance of advance care planning within the NHS, decision-making about future ICD deactivation is an important issue to address, particularly in light of the absence of guidance on deactivation in current NICE guidelines. 2 At a regional level, the North of England Cardiovascular Network guidance26 on deactivation and reactivation of ICDs goes some way to addressing this deficit, but the document is largely technical in nature, with neither the patient perspective nor the importance of SDM featuring strongly. There is therefore potential for these findings to inform both regional and national guidelines about ICDs.
Capacity to generate new knowledge
There are clear gaps in the existing body of research with regard to the information needs and preferences of potential ICD recipients with respect to both implantation and deactivation. This study aimed to address these gaps by providing data on current decision-making while also establishing the information needs, values and preferences of ICD recipients, carers/family members and clinicians, so that decision-making involving patients can be effectively supported. Most patients are not aware of the possibility of ICD deactivation when they have the device implanted and, for those who are, it is unclear when, how and by whom (e.g. GP, cardiologist, heart failure nurse, palliative care clinician) they receive this information. Little is known about how patients make sense of information about ICDs and what patients’ information needs and preferences are with respect to implantation and deactivation. Existing research in this area has been conducted primarily in the USA and is limited to mainly descriptive accounts, which do not attempt to change practice and which focus on decision-making in tertiary care to the detriment of understanding and supporting decision-making earlier in the pathway.
Generalisable findings and prospects for change
By incorporating what is important to the individual patient in decision-making about ICDs, resources can be better targeted to ensure that those people who, after discussion of the benefits and risks of device implantation, make the decision to have an ICD are better equipped to deal with the consequences of an ICD on quality of life and the possible need for deactivation towards the end of life. There is also likely to be transferable learning about how to broach discussions about withdrawal of analogous assistive devices during end-of-life care. 39 This work is directly relevant to those working in clinical practice on individual patient preferences for information giving, levels of involvement in decision-making and how best to support patients and their families with regard to discussions about implantation and deactivation of ICDs. The dual focus on both ICD implantation and deactivation decisions reflects the government’s stated commitment to SDM,33 as well as the importance attached to advance care planning in the NHS. 34 Against this backdrop, our study is timely, particularly with the observation that there is a need for better understanding of how to implement SDM within existing services. 7
Building on existing work
Members of our research team have led and contributed to several studies on variation in the use of ICDs,19 patient involvement in decision-making about cardiac devices,23 patient and clinician perceptions of ICD decision-making,40 and the development of decision support. 41,42 To date, much of the existing (mostly North American) evidence is concerned with what happens in specialist centres, with little consideration of earlier stages of decision-making, particularly in secondary care for initial referral decisions. Equally, there is little available evidence about how decisions on deactivation are made, but a strong suggestion that these are generally made late, and close to death, without planning in advance. 13 This study builds on existing knowledge to (1) support the process of SDM with ICD patients and relatives and (2) disseminate learning to improve advance care planning for ICD patients.
Aim and objectives
Aim
To critically explore lay and professional views about, and experiences of, ICD implantation and deactivation (towards the end of life) and to examine how this information can be used to support SDM.
Objectives
-
To explore patients’, family members’ and clinicians’ views and experiences of decision-making about ICD implantation and deactivation (towards the end of life).
-
To establish how and when ICD risks, benefits and consequences are communicated to patients (including deactivation).
-
To determine patients’, family members’ and clinicians’ information and decision-support needs in the context of SDM.
-
To identify the individual and organisational facilitators and barriers to discussions about implantation and timely decision-making about deactivation.
-
To inform (1) the early-stage development of decision support for ICD implantation and (2) improvements in advance care planning for ICD recipients.
During the inception of the research, a US option grid, booklet and video was published to support SDM about ICD therapy. 43 Consequently, the final objective was amended to focus on the provision of guidance to improve existing decision support and on supporting the process of SDM with ICD patients and relatives.
Chapter 3 Research methodology
Overview of study design
To understand fully the views and experiences of those involved in decision-making about ICD implantation and deactivation, and how such discussions are enacted in practice, requires the use of qualitative methods. In this study, we used a combination of observations of consultations, in-depth individual interviews and interactive group workshops. The study was not longitudinal in nature, that is, we did not follow a particular cohort of patients throughout the care pathway. To reflect the diversity and range of patients’ experiences, we recruited people before and after ICD implantation, as well as people who declined ICD, people considering prospective deactivation and bereaved relatives. We also adhered to the consolidated criteria for reporting qualitative research (COREQ) guidelines. 44
Patients can be referred for an ICD for either PP of sudden cardiac death or as SP after they have experienced a cardiac arrest. PP patients are likely to have more time to discuss and reflect on their decisions about an ICD than those patients who are referred for SP. With this in mind, and because of the different nature of the decision-making process (after a cardiac event), we focused pre-implantation interviews on patients referred for PP. However, to achieve sufficient numbers and to understand fully the nature of decision-making for the two different groups, we did not exclude patients with SP ICDs. We also included patients eligible for, or living with, CRT: patients eligible for a CRT device can also choose to have CRT alone or CRT-D. The decision is presented to patients as the option of having something to improve symptoms or the option of having something to improve symptoms as well as something to increase the chances of living longer. It is important, therefore, that we captured this group of patients in our sample to understand the full range of options available and the nuances of the decision-making process. Moreover, CRT-D is a common option for ICD patients, and excluding this group of patients would limit how representative the study sample was in terms of the wider population of defibrillator patients.
Data collection and analysis for this study followed the principles of the constant comparative method. 45 This is an iterative process whereby data collection and analysis occurred concurrently, with earlier interviews informing subsequent ones and continuing until no new information emerged. The initial phases used observations and interviews to explore lay and professional views and experiences of decision-making about ICD and CRT-D implantation and deactivation. Observations were conducted at one specialist centre, where ICDs are implanted, and at two district general hospitals (DGHs), with ICDs implanted at one of these. We selected these study sites because they provide different pathways to ICD implantation and, therefore, may provide different insights into decision-making between implanting and non-implanting clinicians, and the importance of different organisational approaches, such as the role of the specialist nurse. Our rationale was to understand patient/clinician interactions, information sharing and decision-making in implanting centres and to explore what happens earlier in the pathway by including two DGHs offering contrasting approaches to patient referral, one of which was cardiologist led and the other of which was nurse led. In-depth interviews were conducted with patients, family members and clinicians at the above sites. We also recruited from two additional sites (one in tertiary care and the other in secondary care) to conduct clinician interviews. For the deactivation conversations, patients and family members were recruited through cardiac physiologists, cardiologists or palliative care physicians directly involved in the patients’ care.
In the final stage of the study, these data were used in the interactive group workshops with patients/family members and clinicians to validate the findings of the initial phases and to provide a vehicle to explore their ideas and views about how the findings could be used to support better SDM about ICD implantation and deactivation, as well as to inform advance care planning for ICD recipients.
Phase 1: observations
In order to understand decision-making across the care pathway, non-participant observation46 was conducted by two researchers (KJ and HS) to familiarise themselves with the clinical environment and patient pathways. Specifically, we were interested in learning more about:
-
the nature of ICD consultations (how ICDs are portrayed within the clinical encounter, the focus and duration of interactions, who interacts with whom, who initiates/ends interactions, how the physical environment might constrain/promote the delivery of good-quality decision-making)
-
the nature of decision-making interactions (how information is shared and by whom, how risk communication is enacted and by whom, how the topic of possible future deactivation is approached and by whom)
-
the patient’s journey through the care pathway (what happens and when, key decision points regarding ICDs and how are these negotiated, the stage of the pathway at which risks, benefits and consequences – e.g. possible future deactivation – are communicated to patients).
Patients received a letter about the study with their outpatient appointment letter explaining that a researcher might be present at his/her clinic appointment to observe the consultation. This letter included clear details of how to opt out of the study (see Appendix 1). The letter of invitation included an opt-out reply slip which patients could return to the receptionist/member of clinical team on arrival to enable the opportunity to opt out without having to approach the issue face to face. The consultant cardiologist further relayed the information at the beginning of the consultation and offered a final chance to opt out. Patients had the opportunity to reflect on their decision and discuss their participation in the study with family members/significant others before deciding whether or not to participate (there was usually a period of several weeks between appointment letter and actual appointment). We chose an opt-out process of consent for the observations for three reasons: (1) we wished to avoid overburdening patients at a time when they are already receiving large volumes of information about ICDs; (2) clinicians would be seeking written consent for device implantation so asking for written consent to observe the consultation may result in confusion over what the patient is actually consenting to; and (3) obtaining written consent would lengthen the consultation and place additional demands on the consultant cardiologist.
Experienced qualitative researchers (KJ and HS) observed consultations with patients being considered for ICD. Initially, it was hoped that we would also be able to observe consultations that focused on deactivation, but this was not possible because of the nature and timing of these discussions. It is unknown how many deactivation conversations took place during the study period. We were made aware of very few deactivation conversations, and in some cases the researchers were not notified in time to permit attendance and observation of these consultations.
Our observations helped to understand how conversations about ICD implantation and subsequent deactivation are enacted. Our approach avoided imposing structure on the observations and brief field notes were written up immediately after the observation period. 47 Prior to beginning data collection, a pilot observation was conducted opportunistically, after which the researcher discussed the consultation with the consultant cardiologist in order to shape an observation grid and coding frame as a guide to their note-taking around the key components of a typical ICD consultation (see Appendix 2). This included, but was not limited to, discussions of diagnosis and checking understanding; communication of the risk of sudden cardiac death; presentation of the ICD, with risks and benefits information; and the impact of the ICD on quality of life and, in some cases, the capacity of the ICD to change the mode of death. It should be noted that this is not a deep ethnographic study and the purpose of the observations was to provide context for subsequent interviews. Consequently, the observations enabled us to understand how decision-making about ICD implantation and deactivation was enacted in different settings and the nature of ICD consultations and decision-making interactions (what is said and what remains unsaid), including the patient’s journey through the care pathway. Notes were taken by hand either during or immediately after the consultation in order to minimise the presence of a researcher within the clinic environment. Observations at each site took place prior to beginning recruitment for phase 2, thus providing local context and helping to inform the purposive sampling strategy and interview guides for in-depth interviews with patients, relatives and clinicians.
Phase 2: in-depth interviews
Interviews were conducted by KJ and HS with patients (aged > 18 years), partners/relatives (aged > 18 years) and clinicians to explore understandings and experiences of, and the factors that influence, decisions about (1) ICD implantation, and (2) deactivation towards the end of life. We excluded patients and family members who lacked capacity to participate in the study and patients and family members who were unable to speak English. Interviews were conducted at a time and place convenient to the interviewee. The majority of interviews were conducted face to face, but a small group of people preferred to be interviewed by telephone. We ensured that patient and family member interviews were conducted during times when support from the clinical team was available in the event that any of the interviewees experienced undue distress. We also made participants aware of sources of support and further information where necessary, including Arrhythmia Alliance, Patient Advice and Liaison Service, and Cruse bereavement support (appropriate information was included in the participant information sheet and was reiterated at the beginning of the interviews).
One-to-one, in-depth interviews are the method of choice where the issue under investigation requires detailed exploration of sensitive issues. In-depth interviews are particularly instructive where the current evidence base is limited,47 as they enable space and flexibility for the interviewee to shape the issues discussed, rather than the direction being predetermined by the researchers’ interests or hypotheses. The interviews investigated how decision-making about ICDs is currently undertaken and if, and at what stage, device deactivation is addressed; patients’ and clinicians’ information and support needs regarding ICDs in general and regarding deactivation in particular; patients’, carers’ and clinicians’ views about how best to support decision-making about the implantation and deactivation of ICDs; and individual and organisational facilitators and barriers to discussions about implantation and timely decision-making about deactivation.
Purposive sampling was used to capture a broad range of views and experiences and, as data collection progressed, we deliberately sought out individuals who had different views or experiences to challenge our analysis. During recruitment we sought to interview people about both implantation and deactivation decisions and purposively sampled so that the views and experiences of the following groups of patients and/or family members were included.
With regard to implantation decisions:
-
before implantation – patients referred for an ICD from secondary care
-
before implantation – patients recruited from implanting centres who elected to have a device implanted
-
after implantation – ICD patients who had a device implanted (we purposively sampled to capture a range of experiences from those recently implanted to others who have lived with the device for up to 12 months)
-
declined ICD – patients who were referred for an ICD but who declined [at either secondary (declined referral) or tertiary (declined device) stages of the pathway].
With regard to deactivation decisions:
-
patients who were prospectively considering device deactivation
-
bereaved family members (4–18 months post bereavement).
Where possible, we sought to interview prospectively patients who were considering deactivation via tertiary care (e.g. advanced cancer patients). However, discussions about deactivation often take place only a few days (or even hours) before death,13 which presented practical and ethical issues with regard to interviewing patients and relatives. Where deactivation was close to the end of life, and we were unable to speak to patients, we sought to interview family members of patients 4–18 months post bereavement.
Our aim was to continue with interviews until no new information emerged (theoretical saturation). The interview schedule focused on understandings of and feelings about ICDs; experiences of decision-making about implantation; if, where, when and how ICD deactivation was discussed; availability and appropriateness of information; preferences for information and decision support; and exploration of patient pathways. In addition, we included some specific prompts to tap into the values and ethical viewpoints that underpinned peoples’ decisions. Interviews were audio-recorded with written consent, transcribed verbatim and anonymised. Permission was sought from participants to retain contact details for the purposes of inviting them to participate in workshops in phase 3.
Clinician interviews
As already noted, clinicians were recruited from the five sites (two tertiary care centres and three DGHs) in total. By including two additional sites, we were able to purposively sample a range of views and experiences that took account of geographic variation in the referral pathway. Increasing the potential sampling population also served to protect the anonymity of clinicians who participated in the interviews. Clinician interviews considered both implantation and deactivation decisions and involved purposively sampling a range of clinical specialties including secondary and tertiary care cardiologists, heart failure nurse specialists, cardiac physiologists and palliative care physicians. Copies of the letter of invitation for participation in an interview, participant information sheet, consent form and interview schedules can be found in Appendix 3.
Recruitment strategy for interviews
A summary of the recruitment process is shown in Figure 1.
FIGURE 1.
Recruitment process for patient interviews.

We used a slightly different approach to recruit bereaved relatives: a letter of invitation, together with an information sheet, was sent by post to bereaved relatives identified by the physiologists at the tertiary centre, which holds a register of all those people who died with an active ICD in situ and all those people who had their device deactivated before death. We included both groups of patients in our approach (i.e. those who had their device deactivated before death and those whose device was deactivated afterwards). If interested in participating, the individual was asked to return the accompanying consent to contact form. In the first instance, we contacted bereaved relatives 4–6 months post bereavement; however, we were faced with low levels of participation so the eligibility criterion was widened to 12–18 months post bereavement. It was also made clear in the invitation that relatives could choose to be interviewed with a friend or family member if preferred. Equally, if those people contacted did not feel that they were the most appropriate person to complete the interview, they were invited to pass the information on to whoever was most closely involved in the care of their loved one. The letter about the research was accompanied by a cover letter from a cardiologist member of the study team (see Appendix 3). The purpose of the covering letter was to reassure potential participants that the researchers had no access to the clinical data of their loved one and to emphasise that there was no obligation to take part in the research. The consultant’s contact details were also included in the letter to provide a point of contact in the instance that someone encountered distress from our recruitment approach.
Data analysis: interviews
Data collection and analysis was an iterative process. 45 NVivo version 7 (QSR International, Warrington, UK) was used to facilitate data management and retrieval. The research team (KJ, HS, CE initially, then HS and CE) met regularly to discuss emergent themes and to resolve any discrepancies and determine theoretical saturation. Independent coding and cross-checking by members of the research team (KJ, HS, CE) helped to ensure validity of interpretation. A common coding frame was used across all data sets to allow for comparison between the different data sources. Data were examined for deviant cases. 47 The emergent findings were discussed on a regular basis with other members of the active research team as well as with the wider study advisory group.
Phase 3: interactive group workshops with clinicians and patients/relatives
Two interactive group workshops (2 hours in duration), involving Microsoft PowerPoint (Microsoft Corporation, Redmond, WA, USA) presentations, small group work and plenary discussions, were conducted with (1) clinicians and (2) patients/relatives. These were aimed at validating the findings of the initial phases and as a vehicle to explore their ideas and views about how the findings could be used to support better SDM about ICD implantation and deactivation, as well as informing advance care planning for ICD recipients.
Workshops were audio-recorded and were facilitated by two researchers (DF and HS). Field notes were taken during workshops, which were audio-recorded.
Recruitment of workshop participants
Patients/relatives and clinicians who had provided written consent to be contacted about later stages of the study were invited to take part in the workshops. The Northern England Cardiovascular Disease Strategic Clinical Network lead also e-mailed a request for participation in an attempt to recruit additional clinicians who had not participated in the earlier phases of the research. Patient representatives of Cardiomyopathy UK (www.cardiomyopathy.org/), Pumping Marvellous (http://pumpingmarvellous.org/) and the British Heart Foundation (www.bhf.org.uk/) were also invited to attend the patient/relative workshop, which took place in a community setting.
Data collection and analysis strategy for workshops
The clinician workshop took place within a tertiary care centre. Following an overview of the aims, objectives and methods, including a brief overview of SDM, attendees were presented with a summary of the key findings from phases 1 and 2 and were invited to comment on whether or not they resonated with their views and experiences in a group plenary. This was followed by group work, in which attendees were allocated to one of two small groups to consider the following issues, with reference to the generic question ‘How can patients and their relatives be better supported to make informed values-based decisions about ICD implantation/deactivation in partnership with clinicians?’:
-
Who should discuss the pros and cons of ICDs with patients/relatives?
-
When should a discussion about the pros and cons of ICDs with patients/relatives take place?
-
What information should be provided to patients/relatives?
-
How could specific barriers to SDM be overcome?
The workshop concluded with key issues identified from each small group being discussed in a plenary session. A copy of the clinician workshop slides can be found in Appendix 4.
The patient/relative workshop was convened in a community setting. The structure was similar to the clinician workshop, commencing with an overview of the study aims, objectives and methods, including a brief overview of SDM, followed by a summary of the key findings from phases 1 and 2 and a subsequent opportunity to comment on/discuss them within a group plenary session. This was followed by small group work that also focused on eliciting their ideas on how the findings could be used to support better SDM about ICD implantation and deactivation. A structured small group exercise with reference to a summary of preference-sensitive decision points and opportunities for discussions with patients/relatives across the generic heart failure pathway (Figure 2) was used to elicit their ideas on the following questions:
-
What information about options (ICD, CRT-D or doing nothing/active monitoring) should be provided to patients/relatives?
-
Who should discuss the pros and cons of the options (ICD, CRT-D or doing nothing/active monitoring) with patients and their relatives?
-
When should a discussion with patients and their relatives about deactivation of ICDs/CRT-Ds take place?
FIGURE 2.
Decision points and opportunities for discussions with patients/relatives about ICDs.
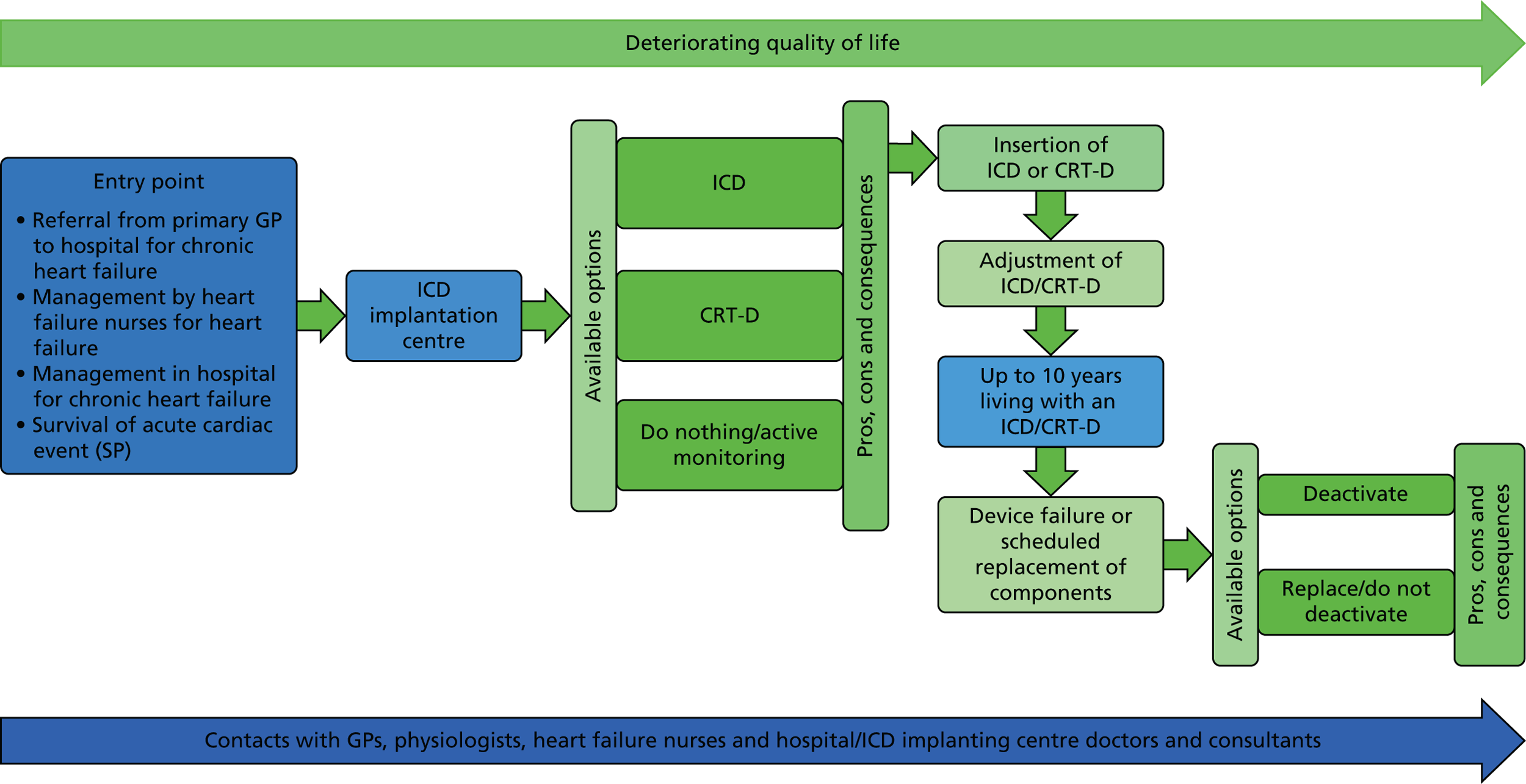
A copy of the patient/relative workshop slides and structured exercise can be found in Appendix 4.
Field notes and transcriptions of audio-recordings from the workshops were subjected to functional content analysis (use of a priori and emergency coding with the specific function of identifying generic categories of information) for discussion with the wider research team to establish whether or not the descriptions of our findings (presented in Chapters 5 and 6) accorded with the understandings of the participants, and to inform recommendations on how to support better SDM about ICDs.
Chapter 4 Profile of participants in each phase of the study
Phase 1: observations
A total of 38 consultations involving clinicians and patients being considered for both ICD implantation and deactivation were observed across three sites (July 2013 to January 2015). Consultations ranged in length from around 10 to 30 minutes.
-
Site 1: a tertiary care (implanting) centre – 29 observations of two different cardiologists consulting with their patients.
-
Site 2: DGH, nurse-led clinics – three observations of two heart failure nurses consulting with patients.
-
Site 3: DGH – six observations of one cardiologist consulting with patients.
Owing to a lesser frequency of potential ICD candidates seen in secondary care clinics, and the difficulties in managing to observe a consultation (owing to some patients showing improvement in the repeat echo and discussion of the possibility of an ICD or CRT-D no longer being appropriate), lower numbers of observations were conducted in secondary care than initially anticipated.
Phase 2: in-depth interviews
A total of 80 interviews were undertaken with patients/relatives and clinicians (Table 1).
| Patient and family members (and context) | n |
|---|---|
| Patient interviews | |
| Pre implantation (secondary care) | 4 |
| Pre implantation (tertiary care) | 9 |
| Decliners (secondary care) | 5 |
| Decliners (tertiary care) | 3 |
| Post implantation | 18 |
| Post implantation (experience of psychological sequelae) | 3 |
| Prospective deactivation | 2 |
| Total patient interviews | 44 |
| Bereaved relatives interviews | |
| Bereaved spouse | 4 |
| Bereaved spouse and daughter (dyad) | 2 |
| Bereaved son and daughter-in-law (dyad) | 1 |
| Total bereaved relatives interviews | 7 |
| Total patient and family member interviews | 51 |
| Clinician group (and context) | |
| Implanting cardiologists (tertiary care) | 5 |
| Cardiologists (secondary care) | 5 |
| Arrhythmia nurses (tertiary care) | 1 |
| Secondary care and community heart failure nurses | 6 |
| Cardiac physiologists | 4 |
| Health psychologists | 2 |
| Palliative care clinicians | 6 |
| Total clinician interviews | 29 |
| Overall total | 80 |
Fifty-one interviews were conducted with patients and/or relatives in total; of these, seven interviews were conducted with bereaved relatives. Forty-four interviews were undertaken with patients: 33 men and 11 women, aged between 47 and 85 years [mean 68.4 years, standard deviation 10.3 years]. The majority of patients (n = 34) had been offered an ICD for PP and 10 people had received one for SP. Eleven patients had a diagnosis of cardiomyopathy and the remaining patients (n = 33) were diagnosed with ischaemic heart disease. Twenty-seven interviews were conducted with patients alone and 17 were conducted with patients along with a family member (usually a spouse) present. The largest proportion of patients was recruited via tertiary care following implantation of an ICD, or they were recruited in tertiary and secondary care contexts while considering ICD therapy. We were able to interview eight patients who declined an ICD (one of whom accepted CRT but not the ICD), but only two people who had decided in advance to have their ICDs deactivated were interviewed. Of the seven bereaved relative interviews, four were with spouses, in each case the widow of the deceased. A further three group interviews were conducted. These included two interviews with the spouse and daughter of the deceased and one interview with the son and daughter-in-law of the deceased. Our sampling strategy was successful in recruiting the range of medical/clinical specialties involved in the treatment and care of ICD patients across the pathway.
Phase 3: interactive group workshops
A total of 11 clinicians participated in the group workshop from the following specialties: two physiologists; two implanting cardiologists; two consultant cardiologists; one palliative care consultant; one clinical psychologist; two heart failure nurses; and a public health registrar working with the Cardiovascular Clinical Research Network. The patient/relative workshop was attended by 12 participants: five patients, six relatives and a patient representative from a charitable organisation.
Chapter 5 Findings: implantation issues
In this chapter, we present our analysis related to implantation, where we have drawn on and combined data from all three phases of the study in order to critically examine the different perspectives and experiences of those involved in decision-making about ICDs. To protect anonymity, all participants have been given pseudonyms; when presenting patient quotations, to give further context, alongside names we provide information about age and if the ICD had been given for PP or SP.
This chapter begins with a description of themes on how ICDs are presented to potential recipients, outlining the key information that should be given to facilitate SDM as demonstrated using data from interviews and workshops with patients, family members and clinicians. This is followed by a discussion of some of the key factors involved in decision-making. Finally, the potential impact on the outcome of the key actors involved in the decision-making process is discussed.
Information provision to patients/relatives about the rationale for implantable cardioverter defibrillator therapy
As discussed in Chapter 1, the pathway to receiving an ICD is complex as there are a number of potential routes by which patients may come to be offered a device. The patient’s pathway is dependent upon several factors, including if the device is being offered for PP or SP. Furthermore, the pathway for PP patients may also vary depending on if the patient’s heart failure is being managed in the community or in a secondary care setting. Here, we outline some of the key information provided to patients when deciding whether or not to have an ICD implanted.
The purpose of the pre-implantation consultations was to establish the patient’s need for an ICD. The interviews and workshops indicated that there was variability in the timing of the initial ICD discussion. In particular, patients who were being managed for heart failure in a secondary care setting sometimes had an initial discussion about the device before accepting a referral to an implanting centre (and some patients declined the device at this point). However, it was evident from the observations, interviews and workshops that the majority of patients had limited knowledge about the device at the time of the implanting centre consultation. This may be due to one or more possibilities, most probably a combination of patients being unable to recall fully or to understand information provided on ICDs prior to referral to the implanting centre or limited discussion about ICDs before referral.
Conveying information on risk of sudden death and the implantable cardioverter defibrillator role in modifying this
Patients’ levels of awareness regarding their risk of sudden cardiac death appear to be dependent on whether the device is offered for PP or SP. For the majority of patients, including those sampled in this study, the device is more commonly offered for PP purposes. As such, these individuals are less likely to have any prior awareness of their risk of dangerous arrhythmias and sudden cardiac death. This information was often met with shock and disbelief by patients and their families; interviewees described difficulties processing the offer of the device:
She [wife] went through a period of time saying ‘you’re 48 years old there’s nothing been wrong with you, you haven’t fainted, you haven’t been sick, you’re not off work’, you know,’ you’ve never been off work’, blah de blah de blah . . . ‘But why are they fitting anything?’
Dan, 48 years old, PP, post implant, ICD
Patients’ responses indicated that they often had difficulty marrying the idea that they needed an ICD with their own views of their health status. Their experiences and perception of themselves did not fit with their preconceived categories of people who may need life-saving medical intervention.
Furthermore, it was acknowledged by clinicians that the role of ICDs as a protective medical intervention may make it harder for some patients to process:
I wouldn’t say there are common misunderstandings. I think the bit that people have difficulty with is about understanding risk. And so the idea is that they’re a risk modifier. It doesn’t remove risk . . . and we’re not talking about an absolute ‘oh you’re going to die without having this’. It’s all about modifying risk and I think that’s a very difficult concept for many people.
Dr Rosemary, implanting cardiologist
The quotation above indicates that there are potentially two complex issues that need to be conveyed to patients in terms of risk. There is the patient’s risk of experiencing a dangerous arrhythmia and there is also the ICD’s role in managing this risk. Understanding the role of the ICD as a risk modifier is discussed in greater detail in Function of the device and Fostering patients’ and their relatives’ expectations about living with the device.
Data from the observations and clinician interviews show that communicating risk information repeatedly emerged as a challenging aspect of the information-giving process. Clinicians used different ways of presenting such information to patients. Particular reference was given to numerical risk information and the extent to which this was a useful tool in consultations. However, although it was acknowledged that numerical risk data could be useful, clinicians had concerns about the extent to which patients are able to make sense of such information. For example, Dr Oak described how he used elastic language in the first instance to convey risk information to patients, followed by numerical information if considered ‘helpful’:
No, no, no I don’t use numbers. I, I do later on, when I come to the end, but I start off not by using numbers. I use words like, ‘Very unlikely, but, but slightly more likely than somebody to whom one could compare.’ Whether that be me, if they look about the same age, or slightly older than me, or there’s no one else, or if there’s somebody with them like a partner or I might say, ‘Your friend who isn’t known to have heart disease’. But I then say, ‘It could still happen to me or it could still happen to them because we don’t know what my arteries look like.’ Or something like, I might say something like that to emphasise the fact that it’s not, I, I want to try and get a few things over, firstly that it’s not a very, very high risk, because always they go away thinking they’re going to die in the next 10 seconds or the next week, and they may have to wait quite a while for this thing to be put in. And secondly because in absolute terms, actually the risk isn’t that high on a day-to-day basis. And, and so I try, I try and get that over and I try and say, ‘The risk is not very high but it could happen, and it’s something that could happen over years rather than weeks or months, even though, of course, it’s a day-to-day risk.’ . . . But then I do, later on, say that, ‘The, the absolute risk is of the order of 1%–5% depending on where they fit in the . . .’. Or sometimes I might say, if I don’t, you know, if the conversation’s gone in a way which I think might, numbers might not be helpful, I, I say, ‘Very low risk, moderate risk or, or at the higher end of the risk.’
Dr Oak, implanting cardiologist
During observations, clinicians, including Dr Oak, largely gave patients verbal comparisons rather than numbers to position the patient in terms of their risk of sudden cardiac death. Many clinicians indicated that they tried to tailor the manner in which they presented information in response to cues from the patient. On the importance of tailoring information, see Tailored information below.
Function of the device
In addition to conveying risk information, establishing the need for the device involves explaining the functioning by which the device manages a patient’s risk of sudden cardiac death. Through the observations of clinic appointments, interviews and workshops, it was evident that there was no standard way in which clinicians delivered this information; rather, clinicians developed their own methods. One approach in particular that stood out as being particularly useful for patients in understanding the function of the device was personification or anthropomorphism.
Defibrillators are mechanical devices, electrical devices that in and of themselves will not make you feel any different, and, but the device that . . . we would use for you would not make you feel any better, would not make you feel any different, its job essentially is to help you live as long as you can by taking away the risk of an arrhythmic or a heart rhythm abnormality, not that it does away with the heart rhythm abnormality, but it allows it to be treated in a way that it wouldn’t be treated if you didn’t have a paramedic in your chest if you like.
Dr Birch, implanting cardiologist explaining to a patient
Personifications, for example describing the ICD as a paramedic or a sentry, appeared to be a useful means of explaining the role of the ICD, as this enabled the device to be presented in a way that was both easy to understand and easily memorable. The success of such techniques was evidenced by the ease with which many patients were able to effortlessly recall these during the interviews when asked what they remembered and understood about the function of the device:
It’s like a sentry; it’s on guard, waiting.
Iris, 76 years old, PP, post implant, CRT-D
Furthermore, it is possible that such techniques will also have a role in fostering positive views about the device, humanising this piece of machinery that is being fitted to the body.
As noted earlier in Dr Rosemary’s quotation, the ICD is a risk modifier rather than a risk eliminator; as such, it is essential that patients understand that, even with the device in place, it is still possible for them to experience an arrhythmia that could kill them. It was evident in the patient and relative interviews that many did not appreciate or fully understand this distinction:
The only thing that puzzles me now is why it never shocked . . . why didn’t the ICD shock him to put it right? . . . so when he did die 2 days afterwards, I rang up to see if I could keep the appointment just to question, would that ICD have shocked him while he was in there, when he had collapsed? You know.
Mary, bereaved relative of ICD patient
Several of the bereaved relatives expressed misunderstandings regarding the role of the ICD in managing their deceased relatives’ condition and were confused as to why the device had failed to activate and save the patient’s life. This appeared to be particularly acute in cases in which the device had never given a defibrillation and the patient had died.
Implantation procedure
The data from this study demonstrate variation in the extent to which patients felt informed about the implantation procedure itself, suggesting the need for attention to be given to discussing the practicalities of the procedure during pre-implantation consultations. Some patients reported that they were ill prepared for the experience. For example, one patient recounted her shock at the number of clinicians involved in the procedure:
I can’t remember the amount of people [in the operating theatre], I counted at least 10 . . . That was fright, that was, actually that was quite intimidating because you’ve got all these people, again you’re lying flat in a bed, strapped down going, ‘Oh . . .’, ‘I’m just here to . . .’, ‘Oh that’s lovely’.
Molly, 60 years old, PP, post implant, CRT-D
Being unprepared about the implantation procedure had the potential to be distressing for patients, which could leave them feeling vulnerable and exposed. The possibility of discomfort and distress during implantation is enhanced by the use of local anaesthetic as the patient is conscious and cognisant throughout the procedure.
Tailored information
Both patients and clinicians recognised the importance of adequately informing patients about the ICD in order to allow them to make an informed decision. Clinicians must endeavour to strike a balance between giving patients as much information as they need without overloading them with information.
It will range from people who will just say, ‘Well, if you think it’s in my best interest, I’ll happily go along with it’, versus the others who want to know the chapter and verse.
Dr Thyme, non-implanting cardiologist
Yes. I’m, I’m an intelligent man. I have always wanted doctors to tell me. Dr Ash, the cardiologist in [DGH S04], some people find very difficult because one of the nurses described it to me is that he thinks out loud and you get all the information that’s going through his brain. Now I want that, I can handle that. I don’t want it neatly packaged into something that, talking down to the patient, they think I’ll be able to handle . . . I like that, I like to be told what it is they want to do, why, and what it’s going to be like.
Bob, 67 years old, PP, pre implant
Dr Ash was aware of the challenge of communicating information about ICDs and of the difficulty of not being able to provide patients with a definitive answer regarding the ‘best’ option for treatment/management/prevention of sudden cardiac death:
I suspect a lot of patients leave the room maybe more bewildered than when they came I because I’m not able to, sort of, say, ‘look, this is what you should do.’ Some patients say, ‘What should I do?’ and it’s very hard to advise them, but again, if they say them I just try and tell them what I’d want my dad to do, if they were my dad or something like that.
Dr Ash, secondary care cardiologist
The above quotation reflects an issue of recognising the value of expert advice but also that the patient’s values and preferences may differ from the clinician’s. The patient was asking for advice ‘What would you do?’. As a result the clinician can be drawn into saying what their values and preferences would be if he/she was making a decision for themselves, as opposed to using reflective listening to explore and elicit a patient’s preferences and values.
Both clinicians and patients recognised the variation between individuals about the level of information they wanted. Some people wanted very detailed information and others preferred to be told the key points. One suggestion to improve the process of providing information was that a menu of options could be provided to patients allowing them to choose the level of information they desired:
I know there’ll come a point where you [clinicians] don’t want to overload people with information and then it’ll be up to people, somehow, to decide, ‘I don’t want lots of information, give me, I don’t know, the bronze information, not the silver or the gold.’ But I’m a gold one, I want it all ’cause it’s going to be important for me. ’Cause I’ll only be thinking about it anyway. So if you can think of it and you think you should tell your patient, tell your patient. I think . . . there will be a way to say it that is the right way for everybody. And I know not everybody wants all the information. But I need it from the medical people.
Gwen, 53 years old, PP, post implant
However, some patients expressed frustration in eliciting the level of information they desired from the clinicians that they encountered. Clinicians suggested that they sometimes based their assumptions on what patients wanted to know and their desire for involvement in the decision-making process, based on their clinical judgement (as opposed to explicitly using strategies to elicit patients’ information needs and desire to engage in decision-making). Furthermore, patients such as Ted (56 years old, PP, post implant, ICD) also expressed a desire for information to be tailored to issues of key importance to their lives:
‘Well that’s an issue I don’t want to touch on’ that kind of thing, ‘Well actually it’s an issue that I want to talk about’, you know, it might, ‘It’s affecting my life.’ So there needs to be more emphasis on that, the support element.
So listening and empathy?
Listening and empathy, but it’s because what affects one person doesn’t affect somebody else.
Tailoring information to the specific issues of importance to individual patients was a challenge for implanting cardiologists, who had often never met the patient prior to the consultation about implantation. Consequently, owing to the lack of therapeutic alliance, the preferences and values of patients were unknown to clinicians and, if not skilfully elicited, hindered tailoring of information to enable patients to make an informed implantation decision.
Written information
In addition to information that can be given verbally, ICD information booklets are also available; information booklets referenced by participants in this study included those developed by the British Heart Foundation and Arrhythmia Alliance. Our study indicated that these could potentially be a useful tool for patients when deciding whether or not to have an ICD. One of the key benefits of this written information was that it is a resource that patients can refer back to following their consultation. It was suggested that it may be useful for these information booklets to be supplied to patients who may be eligible for an ICD in advance of their implanting centre consultation, which would afford patients the opportunity to formulate potential questions that they may have about the device.
It might be sort of, ‘In the event that you have to have, at some later date – take this.’ Because – and that was the first time – I sat down and read it, or lay down and read it, and you know, I come to understand it all.
Patrick, 78 years old, SP, post implant, ICD
However, it was evident in both the clinician interviews and the clinician workshop that there were concerns that providing patients with information about ICDs prior to their attendance at the implanting centre may raise expectations of receiving a device among individuals who may later be found to be ineligible by the implanting clinicians.
No I would want to make sure they were eligible before I went into any information about how it is and . . . some people have actually said that it’s been mentioned in the past, I might give, I tend to give the British Heart Foundation ICD leaflet. I’ve posted that to a lady this week who’s seen a cardiologist and, in fact, it was the patient’s daughter who asked me, was asking me questions, so I felt as though she was leading round to something, so, I was a little non-committal and she said, she’s been told she might need a special pacemaker.
Nurse Mint, heart failure nurse
Furthermore, some practical issues were also identified regarding written information. The first relates to the availability of the information. Disparities were evident in the extent to which the information booklets were available across the different NHS trusts included in the study. Indeed, clinicians reported experiencing issues maintaining an adequate supply of the booklets, particularly those that needed to be paid for, such as those from the Arrhythmia Alliance.
Second, although patients, and their families, expressed a preference for information that comes from trusted sources such as well-known charities, some clinicians expressed some reservations about the information contained within these. As a result, some clinicians preferred to develop their own information, as this afforded them the opportunity to alter and update the contents as they thought necessary.
Some of the national information is wrong, you know? People writing things, and you look at it, and you go, ‘That’s the wrong thing to say’, you can’t correct it . . . In the BHF [British Heart Foundation] book, it tells defibrillator patients they get two kinds of shocks. Well an electric shock is an electric shock. I wouldn’t describe it as two kind of shocks. So patients say ‘is that the big one or the little one?’ and you go . . . it’s hard to say ‘well actually that nationally revered, you know, venerated organisation, is not well written’, so each department tends to develop their own, rather than going for a generic thing . . . If somebody misinterprets something, I can rewrite it much easier.
Ms Forsythia, physiologist
However, this preference for having local information at different NHS trusts, each of which develops its own resources, further compounds the issue of providing standardised and reliable information to all patients.
Patients also indicated that GP letters could be a potentially useful source of information. However, this was the case only if the letters were written in non-technical and jargon-free language (plain English). Patients expressed frustration upon receiving letters that they were unable to understand:
You see I don’t understand what I can, what these anterior segments is, I haven’t a clue. That’s what the echocardiogram showed. An ejection fraction of, you know. If I had a criticism I would say, ‘please use layman’s language’.
Emily, 84 years old, PP, pre implant
Presenting implantable cardioverter defibrillator therapy as a choice
As with the great majority of medical interventions, having an ICD implanted is a choice; patients always have the option of not having the ICD. However, there was clearly variation in the extent to which patients, and their families, perceived that they had a choice about the ICD, with many viewing the device as a necessity:
I don’t think we ever thought that [there was a choice] because when it was presented to him about the ICD it was a case of, ‘If you don’t have this done–’, well, in fact that was the words of one of the consultants. I wasn’t there at the time, but Alan told me that she had said to him, ‘If you don’t have this in you could die’.
Cathy, bereaved relative, SP, deactivated post mortem
This suggested that the option of not having an ICD is not always presented as a valid choice to patients facing implantation.
Importance of time and checking on patient understanding
In both individual interviews and the workshop, clinicians expressed concerns about patients’ abilities to retain the information given to them. Although they said that they endeavoured to provide the patient with as much information as they needed, clinicians often believed that much of this was forgotten soon after the consultation. However, having time to reflect was an important factor in patients’ decision-making processes:
It took us 2 or 3 month you [know], ’cause I didn’t even read the leaflet. I just tossed it to one side when I first got it . . . [partner’s name] read it first and she says, ‘You want to read this.’ ‘Aye, I’ll read it tomorrow, aye, I’ll read it tomorrow.’ And I eventually did read it, you [know]. But I thought to myself, ‘I’ll read it in my time.’ You [know]?
Alex, 53 years old, PP, pre implant
For patients being offered an ICD for PP, being afforded time to consider the information, discuss with appropriate others and return to clinicians with any additional questions was perceived as extremely beneficial.
Dr Elm had prepped me, and I’d been able to talk to [name of GP] about it in advance, I didn’t, incidentally, get on the web . . . So, I mean, I think where I am placed, and particularly having cardiologist [secondary care] and the rehab nurses, and . . . I’ve talked a little bit about it to [first name], the GP. I think I’ve had lots of chances to talk about it.
Bob, 67 years old, PP, post implant, ICD
However, SP patients, for clinical reasons (i.e. to minimise the risk of an early recurrent episode) were often given little time to consider their decision. Nonetheless, as SP patients have also experienced the trauma of a recent cardiac event, this may further limit their ability to process information given to them about the ICD.
Perhaps there needs to be a checking of understanding. Perhaps that’s necessary. Because I’m sure I probably seemed awake and alert and I very quickly realised that, you know, I, I was in hospital – You know, I woke up and thought, you know, ‘I’m in hospital’ . . . But obviously what I now realise was traumatic for my body obviously did have these effects that lasted longer than possibly it was obvious that it had done.
Isobel, 67 years old, SP, post implant
These data suggest the need for checks to ensure that patients have retained the necessary information about the device. Such checks of understanding would be useful throughout the pathway between the initial implanting consultation and the decision, as well as beyond any implantation.
Fostering patients’ and their relatives’ expectations about living with the device
There was variation in the extent to which patients were aware of the therapeutic purpose of the ICD or the negative impacts of living with an ICD. One important aspect of this was the understanding that the ICD is a risk modifier that offers potential survival benefits; however, a simple ICD does not confer any symptom improvement to the recipient (some devices such as a CRT-D, a specialist form of pacemaker, can offer symptom improvement). Erroneous expectations of symptom improvements and quality-of-life benefits are likely to influence patients’ decision-making.
I try to make a very clear distinction between feeling better and living longer. And I try to make it quite clear, if it’s a straight ICD, that this is not about feeling better, this is about prolonging life expectancy, so that their anticipation is not that they will feel better as a result of that process.
Dr Thyme, non-implanting cardiologist
Such confusion about the likelihood of symptom improvement was often related to a conflation of ICDs with other devices with which the patient was more familiar, such as pacemakers. As such, a major aspect of the information giving process is to distinguish between these devices.
I don’t think they realise what that box is actually going to do for them. I think a lot of people think, ‘I’m getting a pacemaker’. That’s what they, because their friends have got one and sometimes I say, ‘But yours is a very special device, yours has got other properties’, and they’ll say, ‘oh have I got the sort of, the deluxe model’.
Nurse Mint, heart failure nurse
As pacemakers are more widely utilised in the treatment of heart failure and have been around for several decades, many patients being offered an ICD possess some prior knowledge of these devices. Many patients have heard of pacemakers and often know someone, such as a friend or family member, who has received this type of device, whereas relatively few patients have prior knowledge of an ICD. Clinicians have to ensure that patients understand the differences between these devices and that they are aware of which device they are being offered.
We batter that [calling the ICD a pacemaker] out of them. Because there is a strong disincentive to call it a pacemaker, because it causes so much confusion. So any time a defibrillator patient says they’ve got a pacemaker in we say, you know ‘it’s like a pacemaker, but don’t use that word because it causes confusion with everybody’.
Ms Forsythia, physiologist
In addition to the potential for unrealistic expectations about improvements to symptoms, if patients erroneously believe that they are receiving a pacemaker, this may leave them potentially unprepared for the defibrillating function of the device. Despite clinicians’ reported attempts to try and prevent patients from confusing ICDs with pacemakers, it was evident that there were still some patients who possessed a degree of confusion, several of whom referred to the ICD as a pacemaker throughout their interview:
She [cardiologist] said she had sent a letter to [implanting centre S01] for putting my name forward for a pacemaker.
Jim, 80 years old, PP, pre implant
Furthermore, one bereaved relative expressed no knowledge of the fact that her husband had a defibrillator, believing that he had only a pacemaker.
The key difference between ICDs and pacemakers is the ICD’s capacity to deliver a defibrillation. Defibrillation is the main function by which the device mediates the patient’s risk of sudden cardiac death. In addition to the inability of a simple ICD to offer symptom benefit, patients should be made aware prior to implantation that the device has the potential for adverse impacts on quality of life. The experience of defibrillation itself has the potential to be distressing to patients.
Your ICD will not improve your quality of life and we know that in some patients it makes their quality of life worse, particularly those who are unhappy with inappropriate shocks or those who get very obsessed with their heart function and their device.
Dr Rosemary, implanting cardiologist
Although the defibrillation, or shocking function, is the key mechanism by which the device may extend a patient’s life, it is also this function that has the potential to be psychologically distressing. Indeed, several patients were interviewed who had experienced psychological sequelae following the experience of shocks.
Variations were apparent in the manner in which clinicians discussed the defibrillations; many employed humorous comparisons:
Well, a lot, a lot of people tend to say it’s like a, being kicked in the chest by a camel, but, or, I mean I don’t know how they know that’s what it feels like. But it’s, it’s, it’s a bit of a kinda, I think it’s a bit of a lighter way to tell them, so that they know it’s going to cause a bit of discomfort, but kinda not, not quite making light of it.
Mr Broom, physiologist
As with anthropomorphising the device, comparing the shocking function of the device to being kicked in the chest by a horse or camel may be useful, as this is an image that might be easily recalled by some patients. Another common method employed by clinicians to illustrate the shocking function of the device was to hit the desk very hard and suddenly. These methods appeared to be successful in instilling in patients the appropriate expectations of the defibrillation. Discussion of the shocking function and how this may be experienced appear to be an essential element of the information given to patients pre implantation.
Impact of the implantable cardioverter defibrillator on physical appearance
Another important consideration in decision-making is the potential impact of the ICD on the appearance of a patient’s body.
A patient I’ve already met but, mentioned but who is considering having an ICD fitted and she is very body conscious. She’s a young woman and she did talk about, ‘well what will it look like? Well, what’s that going to be like, having this thing that’s visible?’ . . . So that was certainly a concern for her.
Dr Aspen, psychologist
However, our data suggest that this was not an isolated incident. For example, Dan felt self-conscious about the physical appearance of the ICD:
I think one of the big subconscious things for me, in, for a personal point of view, is how protruding it is, you know, when I, when I take me [sic] shirt off, you know, it’s quite evident that it’s there. I thought in my mind’s eye at the time, that, where you know, that it would be a lot more you know, less sightly to see. You know, it, they are a fairly big thing, if you like. I am, I am a fairly slight person anyways and I think, for me, consciously, if I was away on holiday and was having to take me top off anywhere, subconsciously that’d be on my mind.
Dan, 48 years old, PP, post implant, ICD
It was evident that not all participants in this study felt fully informed about the size of the device prior to implantation. Some, such as Phil (68 years old, SP, post implant, ICD), discovered, following implantation, that the device was much larger than they had expected:
They said it was about the size of a box of matches . . . They should of [sic] said, what was it, Swan Vestas [Republic Technologies (UK) Ltd, High Wycombe, UK]!
A big box of matches? So it was bigger than you expected it to be?
And a damn site more painful.
Implantation scars, and the way in which the device can protrude, can leave some people feeling self-conscious about their changed bodies, and others reported experiencing ongoing pain or discomfort:
Yeah, it hurts when I lie on me [sic] left side and I find that very strange. It’s as though, where’s it [going] to [fall] out. And actually sometimes when you, you go over the scar it hurts, it’s very strange . . . But when I lie on my left side and I’m like in a really comfortable position, oops, no it wakes me up. It’s as though it’s squashing together. And it’s, you can see it. Now you know where they go in . . . It’s a neat scar, but it’s got a lump . . . Well I was told, because, I’m not exactly Twiggy, you wouldn’t see a thing which just shows that’s wrong.
Molly, 60 years old, PP, post implant, CRT-D
Those patients, and their families, who were able to see the ICD before the procedure reported that this was useful. Furthermore, this was something that was reinforced at the ICD support group.
And she, she did show the size and they did explain how they put them in. She did, she explained everything . . . they even passed one around. ’Cause they passed the one, they passed a pacemaker around as well to show you the difference. But do you know, they’re getting smaller all the time ’cause his is quite round and large, and it’s evident, you can see it. But I’ve seen some on the telly and they’re not, they’re not very big.
Wife of Burt, 74 years old, SP, post implant, ICD
Being able to see and touch the device appeared to facilitate patients’ development of realistic expectations regarding the impact of the device on their bodies.
Many people are involved in implantation decisions
At its core, SDM involves more than two actors. The findings of this study indicated that decision-making around ICD implantation, consistent with other preference-sensitive decisions,45 often involves multiple actors, including the patient and implanting clinician dyad but also family members, significant others and other clinicians.
Clinicians
It was evident from the study data that clinicians have a key role in implantation decisions. Depending on the patient pathway, there are various clinicians who may be involved, including heart failure nurses and both non-implanting and implanting cardiologists. Implanting cardiologists have a key role in the process of decision-making about ICDs. Implanting cardiologists were often viewed as the experts to whom the decision should be deferred by many of the patients interviewed. This is perhaps not surprising when patients are referred to specialist tertiary care services, and this is likely to imbue these ‘specialist’ clinicians with more ‘status’ than others. Many patients felt that if the implanting cardiologist believed that they needed the device, then they would accept and follow this advice:
I mean, if he says I’ve got to have one I will, I will have one. I mean I’m, wouldn’t say, ‘Oh no, no, I’m not having one’ you know. If he thinks I should have it I’ll have it . . .
Doris, 73 years old, PP, pre implant, CRT-D
Although there were exceptions, the majority of patients expressed how they trusted and valued the clinicians’ expert opinions, but this was balanced against what they themselves regarded to be important:
There comes a case where you’re dealing with professionals, it’s like any business if you’re dealing with a professional, you’ve got to look at his advice, weigh it up, and in the main, you should go with that professional’s advice. After you’ve done your various . . . your own checks and balances. Well you must, otherwise you’re wasting your time. Why go to a financial adviser if you’re going to listen to him and then ignore what he tells you?
Adrian, 76 years, PP, post implant
In addition to the implanting cardiologist, interviewees often suggested a role for other health-care professionals, in particular those with whom they already had a relationship. Many patients were already under the care of a heart failure nurse with whom they had a continuing relationship, in contrast to their relationship with an implanting cardiologist.
Dr Oak had a big file there, but that’s all he knew of me. Where, I mean [heart function nurse]’s seen me since I first came out of coronary care, and she knows the bits that frighten me and that sort of thing.
Bob, 67 years old, PP, pre implant, ICD
Patients valued the opinion of those clinicians who they felt knew them personally and welcomed the opportunity to discuss their decision with these trusted others.
Data from both clinicians and patients indicated a potential role for psychologists in the decision-making process. Under current practice, psychologists are not routinely involved in the pre-implantation assessment of patients. Indeed, several clinicians (non-psychologists) indicated that they used their clinical judgement to assess the risk of a psychological impact on patients of ICD implantation:
If I see someone where there is that potential, where I get a gut feeling that it’s a potential issue. Somebody who has had a history of mental health issues in the past or someone who comes across as being quite anxious and introverted, then I would often talk about . . . that I’ve had some patients where this has had a real negative impact upon how they view life and they have become very obsessed with their heart or with their device and it’s had a really negative impact in that way. I might briefly mention something like that but I wouldn’t routinely talk about it.
Dr Rosemary, implanting cardiologist
Participants in the patient workshops indicated that they would have appreciated some specialist psychological support at the time of implantation and stated that the psychological impact had been greater than expected. This appeared to be particularly acute among SP patients who had experienced a cardiac event prior to the implantation of the device:
A lot of patients, especially the secondary prevention are, you can see them, they are like rabbits in headlights. It’s such a shock, they’ve had a cardiac arrest recently, they, all of a sudden they’ve got this big lump of metal in their chest to keep them alive and it’s, it’s probably pretty daunting.
Mr Jasmine, physiologist
As such, it was suggested that early contact with psychologists may be beneficial during the decision-making process and this could facilitate the development of a relationship that stretches beyond implantation.
I mean there are, there are quite a few that have psychological issues of this device that’s going to give them a shock, to a point when, I think we had one patient in particular, who has had psychological issues since, more or less, day one. It’s got worse and worse, to a point where it is now turned off because he said if he didn’t get it turned off he’d pull it out his chest. So that’s how they can affect people.
Mr Jasmine, physiologist
It was evident from the interviews with those who had experienced shocks that this had the potential to leave a lasting psychological impact:
I thought me whole chest had exploded. I didn’t even think what it was when it happened, you know, you just . . . I know why they don’t tell you, you know, they hadn’t prepared me for it but I don’t think they should . . . No, because . . . I was on diazepam for ages after it ’cause you think it, if it happens again it happens again. I didn’t know whether I was supposed to stand up or what I was supposed to do ’cause they didn’t tell you, tell me what to do if I was shocked mind. That’s something I think, that would be a good idea, ‘If in the event you do get a shock this is what you do.’
Florence, 67 years old, SP, post implant, ICD
The experience of a defibrillation was extremely unpleasant for the majority of patients and had the potential to have a lasting psychological impact. Access to psychological support throughout the ICD pathway may be helpful to reduce this impact.
Family
Beyond clinicians, other important actors involved in the decision-making process included a patient’s significant other and family members. Although variation was evident in the extent to which patients chose to engage family members and significant others in the decision-making process, the majority of patients chose to include at least their partners in the decision. Clinicians appeared to recognise the importance of partners in the decision-making process and recognised that they would have their own concerns about the device relating to what to do if the device discharged and their own safety, for example. Some of the clinicians referred to the work they undertook to engage the partner as well as the patient:
And again it often seems to be the partner that takes the bulk of the stress with them, the patient is often come across anyway quite philosophical of what has done is what is designed to do, ‘I’m still here and just get on with it’. It’s usually the partner looking completely frazzled by the whole thing and terrified and really stressed about it all and you often spend a lot more time talking to the partner than the actual patient.
Dr Sage, implanting cardiologist
Furthermore, it was also suggested that engaging partners and significant others in the implantation discussions had an added benefit in that there would be an additional person to absorb and retain information.
Patients
A potential role was also identified for other ICD patients. Speaking to someone who had been through the same experience was clearly of value but, importantly, was seemingly different from speaking to a clinician.
It all gets a bit abstract. It’s very difficult to put my finger on it. But it was, it was definitely there, a certain, just a certain quality. I don’t know. And it’s not empathy, he [other patient] was empathic, ’cause we didn’t dwell on emotional, emotionally on things. It was him imparting his knowledge to me actually. He was sharing it with me and it was a real feeling of sharing. If I had to bring it into a couple of words, it was sharing and it was on a, it was meeting as equals. That, that really was what was happening.
Gwen, 53 years old, PP, post implant, CRT-D
Several patients indicated that they felt it might be beneficial to speak to someone who was already living with the device:
Anybody who’s living with one, even if you can just phone them up or, oh no, that would be difficult ’cause you can’t give out numbers. But there must be a way you can get to talk to somebody, or even talk to people before you have it done saying, you know, ‘He hasn’t told us much what they do . . .’ Of course that might put people off so it’s maybe not, that’s not a good thing.
Molly, 60 years old, PP, post implant, CRT-D
Patients also recognised that talking to other patients would be valuable to support decision-making about whether or not to accept an ICD, as it would present opportunities to gain a realistic view of life with the device. Furthermore, there was a suggestion that contact with other patients and/or patient groups could continue to have value throughout the patient pathway.
Many patients appreciated interactions with clinicians who elicited and took into account their own values and beliefs. Patients’ own values were an essential component in the decision-making process about ICD implantation. The decision-making process involves a weighing of the benefits of having the device against the potential risks/adverse effects, the relative importance of which will depend on an individual patient’s values. For example, some patients indicated that they initially thought that the costs of the device in terms of hospital visits outweighed the potential benefit of the device as an insurance against sudden cardiac death:
I didn’t like the idea of the faff of repeatedly . . . ’cause he’d sort of mentioned that I had to come back to, for battery recharges, for this and for, and for all the . . . And I felt like I was kind of OK really. So as an insurance policy it didn’t really sell it to me, because it seemed like an awful lot of effort for a chance thing.
Mark, 47 years old, PP, post implant, ICD
In the case of Mark, it was not until he perceived an increase in his risk of sudden cardiac death coupled with a change in his personal circumstances following the birth of his first child that he was willing to reconsider device implantation.
Patients living with heart failure are likely to be faced with increasing levels of disability affecting their everyday lives; as such, the possibility of the device facilitating the patients’ involvement in these activities could be an important decision-making factor. This was particularly evident among patients offered a CRT-D, as these devices could potentially alleviate their symptoms:
Keeping up with my family who are young, walking along with my children who are in the late teens and twenties, my peers, socialising, lots of things that, like, you know, preparing a meal. So I talked about those mundane things with him and what they were like. And he, you know, he said, ‘Even given all the risk that are there’, ’cause the risks are substantial, I suppose. That I really wanted an end to these problems if that was possible. So it was how much I wanted it. It was almost as if, we know that there, there are potential problems but it’s, kind of, is it worth it for what you might get out of that? Even though there are no promises, because all the way he said, ‘There are no promises that it’ll make any difference’.
Gwen, 53 years old, PP, post implant, CRT-D
In addition to the mundane activities of daily life, patients also expressed certain valued activities that they were not prepared to give up. As such, decision-making about device implantation was balanced against their ability to keep doing these activities.
I mean as long as I can keep playing bowls and what have you I’m quite happy, you know, so . . .
Doris, 73 years old, PP, pre implant, CRT-D
Some patients indicated that maintaining a high level of quality of life was more important to them than the potential survival benefit offered by the ICD:
Because I know if I get started on some other procedures it’s going to affect my life. Now I don’t know how much life I have got left in me. Let’s live it what I’ve got. And I’m OK. So if all of a sudden you say I have to go into hospital and I’m gonna be, I can’t do this for 6 month and I can’t do this, I am going to be sitting around a loppy dog that will drive me around the bend and that is wasting my life. By the time I am fit enough to do the things, I might not be fit enough to do the things if you follow my lead.
Ernie, 83 years old, ICD decliner
A specific factor that influenced patient decision-making was the driving restrictions imposed by the presence of the ICD. According to guidance from the Driver and Vehicle Licensing Agency, in all cases of ICD for sustained ventricular arrhythmia associated with incapacity, driving must stop for 6 months from the date of implantation (and resumption of driving requires fulfilment of specific criteria), while ICD implantation is a permanent bar to group 2 licensing (bus and lorry drivers); as such, it has a strong influence on patient decision-making. 48 For some patients, the prospect of not being able to drive outweighed the potential benefits conferred by having the device. The impact of driving implications was particularly acute among those who lived in rural areas who were heavily dependent on their cars for their independence. Clinicians recognised the importance of driving restrictions on decision-making. As such, clinicians tried to manage the impact of this information by carefully timing where it was placed in the consultation. There was the suggestion that discussing this restriction too early could have the potential to cause patients to dismiss the idea of the device:
I’ve tried to wait to tell them about driving at the very end of any discussion. That’s where I’ll say ‘No, driving’, not in the initial discussion . . . ’Cause if you tell them they can’t drive for another year, 6 months, day 1, first thing, that’s it, they don’t listen to anything else.
Mr Jasmine, physiologist
For many patients, the ability to drive is a quality-of-life issue. Retaining quality of life and the ability to engage in these valued activities may outweigh the potential benefits that the ICD confers in terms of survival.
A final consideration in decision-making was patients’ perceived ability to live with the shocking function of the device. Clinicians suggested that a perceived inability to live with the uncertainty of when the device might fire was one of the most common reasons why patients chose not to have the device implanted:
It’s about whether or not they would want to live with something that could give them a shock, perhaps it’s the way I explain it I guess. But it, there are a few people of an anxious disposition who go away and who chose not to have it.
Dr Oak, implanting cardiologist
Information provision about defibrillations at the pre-implantation stage may facilitate the development of strategies to cope more effectively with anxiety issues that may arise once the device has been implanted.
It is also important to note that the defibrillating function of the ICDs also has the potential to alter the patient’s mode of death. While the ICD is activated, patients are unlikely to experience a quick and unexpected death. Some patients may have preferences for how they wish to die, which may influence their decision-making about ICD implantation:
Well one that springs to mind is the guy who wants to drop down dead out walking with his mates and that . . . it was very clear . . . it wasn’t so much him declining it was him telling me what he wanted and me saying what he wants is not compatible with this device, therefore, don’t have it.
Dr Rosemary, implanting cardiologist
The shocking function of the ICD and its potential to alter the mode of death may actually be incompatible if a patient has specific preferences regarding death. However, a patient’s values about his or her mode of death, or about the perceived benefit of the ICD, may not be stable over time. As the patient ages, the survival benefit offered by the ICD may be viewed less positively, and the potential for a quick and relatively painless death may be viewed more positively. As such, the impact of the device on a patient’s mode of death is something that should be considered and returned to regularly while the patient is living with the device; as the patient’s condition changes, so too may their opinion of the device.
Chapter 6 Findings: deactivation issues
In this chapter we outline some of the key features concerning decision-making about ICD deactivation, including how and when conversations about deactivation occur, as well as their content and the barriers and facilitators to these types of conversations taking place. This chapter begins by outlining some of the issues concerning the deactivation discussions; in particular, this focuses on the timing of these discussions and who is, and who should be, involved. This includes an outline of what clinicians, patients and their relatives said about current practice regarding these discussions. We examine participants’ suggestions for how discussions about deactivation could be improved. It is important to note that we were able to interview only two people (Fred and Nancy) who had chosen to have their ICD deactivated. Both people were in receipt of palliative care, and Nancy had been experiencing unpleasant shocks prior to deactivation. We also interviewed seven bereaved relatives about their experiences, but some of these people had not been aware that their relatives had had an ICD (see Fostering expectations about living with the device in patients and their relatives). Nonetheless, the wider group of patients, their families and clinicians had important views on this issue.
Implantable cardioverter defibrillators are devices intended to prevent the occurrence of sudden cardiac death as the result of an arrhythmia. Once the patient has been fitted with the device the battery lasts, on average, 5 years before it requires replacing. Over the time of living with the device, it is possible that the patient’s condition may change; they may experience deterioration in their heart failure or they may develop another life-limiting condition, such as cancer. As a result, it may no longer be appropriate for the device to remain activated. If the device remains activated during the last months, weeks or days before death, the patient is at risk of experiencing unpleasant and potentially distressing shocks, and the device could be considered to be potentially prolonging the suffering of these individuals, taking away the possibility of a relatively quick and painless death.
Timing of deactivation discussions
Our data provide evidence of different views of clinicians and patients/family members about whether or not patients should be informed about the deactivation of ICDs prior to implantation. There appear to be several potential opportunities across the patient pathway when these discussions could be held and these are discussed in relation to their respective benefits.
Both patients and clinicians were asked about their opinions of when was the best, or an appropriate, time to discuss deactivation. In particular, we elicited views about the appropriateness of advance discussions where the issue of deactivation is introduced pre implantation. It was apparent from our data that there was a lack of consensus between patients and clinicians, and among clinicians, regarding the appropriateness of advance deactivation conversations. One argument for the inclusion of deactivation in pre-implantation discussions was the suggestion that this information could influence how the patient feels about the device and may therefore be an important factor to inform their decision-making about implantation.
It’s a tricky one. But I think that would be the ideal place [for deactivation discussions], would be before [implantation]. ‘Cause once you’ve got it, if you then decide that, ‘Well I don’t really want that because of X, Y and Z’ . . . then it seems to have ‘Had I known . . .’ decision. I think if it’s going to be talked about it should be talked about beforehand, definitely.
Mr Jasmine, physiologist
Indeed, patient interviews suggested that some patients, such as Fred (70 years old, SP, deactivated ICD patient), regretted the decision to have the device implanted when they were faced with deactivation:
I think if there was more discussion about them and about the whys and the wherefores.
So back at the time, before you have it implanted, kind of, more look to the future and what might happen?
Definitely . . . I think at the time when I had the heart attack, and before the ICD, if I’d have known what was coming, I wouldn’t have had it . . . I definitely wouldn’t have had it, no.
And is that because of the personal impact on you or is that, for you, or are you back thinking about the costs again there?
I think it would be both. I think I am practical – if nothing’s going to help, nothing’s going to help, and that’s what it is about. And what’s the point in clutching at straws?
Fred’s misgivings about the device took into account both personal and broader health service (economic) factors. Overall, he felt that the decision to have an ICD was a mistake. Furthermore, Fred’s account indicates that his view is not specific to the ICD, but rather reflects his feelings about medical interventions more broadly; he had also decided to discontinue enzyme replacement therapy, as he no longer perceived any benefit from it. Fred’s account indicates that he might have benefited from greater discussion of the future and the potential outcomes of the ICD at the time of implantation, as these factors were important in his decision-making.
A number of clinicians, in particular palliative care physicians, drew comparisons between heart failure and oncology services, and the manner in which medical interventions are presented:
It’s not exactly a parallel situation, but it’s not dissimilar to chemotherapy. I don’t give chemotherapy, but obviously I work closely with oncologists who do and many of those I’ve seen having the discussions and many of the discussions I’ve heard about subsequently have explained that, ‘This treatment may have beneficial effects but we’d need to review the effectiveness with repeat scans after a certain interval, and there may be a time when it’s no longer appropriate.’ I think anything that you do to somebody needs an exit strategy as well as an entry strategy.
Dr Basil, palliative care clinician
Dr Basil highlights how chemotherapy is not presented as something that will be used indefinitely for the remainder of the patient’s life, but rather as something that will be routinely reviewed for its appropriateness and clinical effectiveness. He indicates that such an approach would be appropriate for ICD patients, as it would highlight that there may come a point when the device is no longer appropriate, enabling patients to develop realistic expectations regarding the long-term outcomes of the device.
Furthermore, advance discussions about deactivation can provide patients, and their families, with an awareness that deactivation is a possibility in the future.
And that sounds like, you know, not putting the patient first, but if you can have an easier conversation [about deactivation] with the patient then the patient will feel more comfortable having that conversation. And so it’s not just to make it easier to have the conversation for us, it’s to make it easier for the patient to have the conversation as well, and all the family. And it’s just a simple sentence, ‘Your device will be switched off at some point, you can’t live forever’, you know.
Nurse Cypress, heart failure nurse specialist
Several clinicians spoke of advance discussions in terms of ‘sowing a seed’ by introducing the potential need for deactivation early so that it can be built upon in subsequent consultations. As Nurse Cypress suggests, the advance discussions do not need to be in-depth, unless specifically desired by the patient, but rather should present an opportunity to start to set expectations and understandings about the future. As such, when the time comes that deactivation is a consideration, this will be a concept with which the patient, and their families, are familiar.
Indeed, some clinicians recounted experiences where advance discussions had successfully facilitated the end-of-life pathway:
I think the family certainly were aware that the ICD needed to be deactivated at some point. So, I think the cardiologist had had a conversation previously, not to say, ‘We need to deactivate it now’, but to say ‘there will be some point that we need to deactivate this’ because the gentleman has been becoming more frail over the preceding months. So it wasn’t a complete shock for the family and they recognised that that was what was happening, that the patient was dying and they wanted their dad to be as comfortable as possible.
Dr Buckthorn, palliative care consultant
The findings suggest that early advance discussions about deactivation can be useful and may facilitate a realisation in patients and family members that a patient is approaching the end of his or her life. Advance conversations about deactivation and other end-of-life issues allow for planning, whereby the patient may be moved away from an active treatment plan to a more palliative approach. Clinicians indicated that, in their experiences, difficulties in the deactivation process occurred when there had been a failure to engage with the patient about future possibilities and appropriate planning had not taken place. Failure to plan for the end of life often resulted in deactivation decisions occurring in the last days or hours of the patient’s life, or not at all, and deactivation decisions being made quickly.
[If] they’ve got some other problem like cancer and are reaching the end of their life, and we have to make decisions quite quickly, unless it’s been thought through already, about their ICD.
Dr Mulberry, palliative care consultant
It seems that there is often a failure to recognise when a patient is approaching the end of life. In these instances, where deactivation often occurs out of necessity because the patient is close to death, the patient often cannot be engaged in the decision, and there were even some instances recounted of occasions on which deactivation did not take place until post mortem.
In spite of participants acknowledging the benefits of advance discussions about deactivation, our data suggest a lack of agreement regarding the timing of these conversations. For example, there appeared to be variation in the extent to which clinicians chose to engage in these discussions at the time of implantation; some gave only cursory attention to these issues:
Well again, not in any great detail perhaps sadly. But so it’s often a throwaway line along the lines of, ‘If it’s, if it’s shocking you when it shouldn’t or you’re dying of something else then the, there is the option to switch it off.’ And I’ll be honest, not everyone has that discussion.
Dr Oak, implanting cardiologist
Others indicate that the issue of deactivation was not part of their routine implantation discussion at all:
Generally I don’t because of the . . . at that point in time they’re not really . . . it doesn’t seem quite the right time to have that conversation. I mean, it’s certainly something I would think about. But I can’t think that I’ve ever had a ‘we’re going to put this in and think about deactivating’ conversation. I mean it might be something that perhaps we should think about. Certainly, I don’t as a matter of routine and I can’t think . . . I’ve ever had a conversation, ‘well, we’re going to put this in but we could turn it off at some point if you want’ conversation. . . . it’s slightly counterintuitive to think about end-of-life care at the time you are implanting the device.
Dr Rosemary, implanting cardiologist
Furthermore, it was apparent that some clinicians were opposed to the idea of including deactivation in the pre-implantation discussions, believing that too much focus on this idea at the time of implantation could be potentially harmful:
Yeah. The biggest decision is, is death. That’s the biggest decision, and we [physiologists] have to disagree with everybody else, that death cannot be mentioned when you’re putting a defibrillator in too strongly. How could you? How could you say to a patient, ‘We’re going to give you this device that’s going to save your life, but when you’re going to die, think about this conversation, now.’ It just doesn’t work. I think you’d be, you’d be giving mixed messages. So we have in the waiting area, our sole means of communication is, there is an Arrhythmia Alliance leaflet up that says, ‘Dying’. Hopefully, that, that is enough for a family member, sitting waiting for dad or mum to come out. They’ll look and go, ‘Oh I hadn’t thought of that.’
Ms Forsythia, physiologist
Ms Forsythia suggests that raising the issue of deactivation at the time of implantation has the potential to cause confusion and distress, giving patients mixed messages. Rather than engage in potentially uncomfortable conversations about death and the end of life, it seems that it is hoped that the literature available in the waiting room might be read and help patients and/or their families come to the realisation themselves that it is something that they may need to consider.
In addition to concerns about the appropriateness of including a discussion of deactivation at the time of implantation, clinicians also expressed concerns about the ability of patients to retain this information if it was given earlier in the pathway:
It’s no different to other aspects of medicine where if you’re able to offer somebody a cure to a problem, then that is the thing that they most need and most want, and whether they’re in that position to be able to take on board any of those things and really embrace it, I’m not so sure about . . . I think it would be a mistake to try and move everything upstream and say that when we put the ICDs in we need to be having much more upfront discussions about end of life ’cos I just don’t think it’s realistic for patients.
Dr Mulberry, palliative care consultant
However, an alternative approach was suggested, whereby deactivation forms part of an ongoing conversation that is introduced early on, potentially at implantation, and is then followed up throughout the patient pathway:
. . . [deactivation] should be acknowledged at the time of insertion, so that when they come for checks that they’re not just coming for checks of how frequently it’s fired and how they’re doing. Discuss the relative burdens and risks of having it and how they feel about continuing to have it.
Dr Buckthorn, palliative care consultant
Trigger points for discussions about deactivation
In addition to implantation, it was suggested that the issue of deactivation should be returned to throughout the patient pathway, to ensure that patients are informed and involved in these decisions. One suggested means of insuring that deactivation conversations take place in a timely manner was to identify trigger points for the discussions and to build these into the care of ICD patients.
It would be good to make sure that there was a trigger there to just be saying ‘Has this patient changed significantly? Is the ICD still appropriate? Should we be initiating a discussion about it?’ would be useful.
Dr Mulberry, palliative care clinician
I suppose it can be kind of key points in someone’s illness as well and whether things like hospital admissions for whatever reason, something that’s a necessity to a change and care environment, means that something has potentially changed, erm, and almost a kind of near-miss.
Dr Echinacea, palliative care clinician
These trigger points could occur at different stages throughout the patient pathway to ensure that these conversations were conducted with all relevant individuals. These may include the development of another life-limiting condition, a deterioration in the patient’s heart failure, recurrent hospital admissions or listing on a palliative care register. The production of do not attempt resuscitation (DNAR) forms or advanced directives were suggested to be the final trigger point by which to indicate that deactivation conversations should have occurred. It seemed from the interviews that this was not current practice and a recognition that the ICD needed to be deactivated often did not occur until very late in the end-of-life pathway, if at all:
And unfortunately what does happen is that it takes the patient to be really on their death bed and then they’ve got no, they’re not cognitive, well they’re not aware, they haven’t got the cognition that things are going to be switched off and but that their, it’s the families that suffer the trauma as well, you know.
Nurse Cypress, heart failure nurse
Failure to engage in deactivation conversations can cause distress for both the patient and their family if the device needs to be deactivated when the patient is in the last moments of life.
Finally, a need for deactivation to be an ongoing consideration in the care of ICD patients was identified:
[It] isn’t a one-off it’s an ongoing dialogue and an ongoing process; and not something that you can – I would imagine, it’s not something that you can do in a ten minute outpatient appointment.
Dr Echinacea, palliative care consultant
It was suggested that, rather than there being one time point for deactivation discussions, the decision-making process should take place over time:
I think that the discussion is not a one off, it’s going to be part of a series of consultations, which perhaps take place with their, their usual specialist at a more frequent interval than they would have been used to having a review in the past.
Dr Basil, palliative care consultant
As Dr Basil indicates, the decision-making process may be an escalation of the reviews that the patient is used to having. The ideal pathway would include an introduction to the idea of deactivation at the time of implantation, which is revisited across the pathway, as and when necessary, and conversations may become more frequent as the patient’s condition deteriorates.
Actors involved in deactivation decisions
A key theme that emerged from the data was the lack of clarity regarding whose responsibility it is to instigate deactivation conversations and engage patients in the decision-making process. There are several key groups of clinicians who may be appropriate to discuss the deactivation decision. These include cardiologists, palliative care clinicians, physiologists, heart failure nurses and primary care clinicians. The following sections explore participants’ accounts of each of these.
Potential increased role for cardiologists in deactivation decisions?
Clinicians from other specialisms suggested that cardiologists may be best placed to make deactivation decisions, but stated that deactivation created a conflict between withholding or withdrawing treatment in the context of end of life:
The decision about switching off the ICD had come from the cardiologist – whereas, I think it would be very difficult as a non-cardiologist to make the call; whereas when it was a cancer diagnosis then it was – I suppose it was that decision about: Well, we wouldn’t be offering resuscitation to this person but . . . it – again, I suppose it’s that withdrawing or withholding treatment while they’re deemed the same; and I think particularly for ICDs it’s a very physical act that you have to do . . . than just – it feels to be a slight difference . . .
Dr Echinacea, palliative care
Although implanting cardiologists are arguably the most knowledgeable about ICDs, they have limited involvement in the care of patients following the ICD implantation; they are unlikely to have regular contact with the patient, and, thus, they may not be best placed to take the lead on deactivation discussions.
I’m not sure how many of these patients are, or the patients that it’s relevant to switch the ICDs off – I’m genuinely not sure how many of them are seeing cardiologists, a lot of the patients I end up seeing are housebound really, so they’re not having regular contact with the [cardiologist], and that causes a problem.
Dr Mulberry, palliative care consultant
As the above quotation suggests, this problem may be particularly difficult for patients who are being managed in the community, who are unlikely to come into contact with cardiologists, particularly those at the tertiary centre. As such, it is problematic to assume that these clinicians will take the lead on deactivation discussions. This indicates a need for other clinicians to be involved in and to raise these discussions, such as GPs and heart failure nurses.
Furthermore, some patients recounted difficulties expressing their preferences about deactivation to cardiologists who were focused on active treatment:
My experience just with Dr Acacia at DGH N08, and what he actually said, ‘Well, you’ll basically drop down dead as soon as I switch it off.’ It was so unkind. I wouldn’t dare say that to anyone . . . Like I say, it was just my wife saying, ‘Oh, I’m not ready for that. I couldn’t watch that, Fred.’ So we left it. And I think that’s what Dr Acacia was up to – ‘At least go home and talk about it.’ You know, I wasn’t going to change my mind; I just didn’t want a heart transplant.
Fred, 70 years old, SP, deactivated ICD patient
Fred described how, having found out that his heart function had deteriorated significantly and that he was nearing the end of life, he had decided that he wished to withdraw from medical interventions. However, upon requesting this from the cardiologist at his local hospital, he reported being told that he would die immediately. Although Fred was aware that this was untrue, it was extremely distressing to hear and had a big impact on his wife. Fred indicated that he believed this to have been a manipulation on the part of the clinician to try and force him to reconsider the possibility of having a heart transplant.
Heart failure nurses’ and physiologists’ roles in deactivation decisions
During the clinician interviews and workshops, significant emphasis was given to the role of heart failure nurses and physiologists in deactivation conversations and decision-making, as they had regular contact with the patients. In their interviews, some of the heart failure nurses demonstrated how they had opted to take on responsibility themselves for engaging in these discussions, and which they worked into their routine visits.
And maybe not on that visit [first] but maybe a subsequent visit, I would draw the conversation round to the fact that, you know, as everybody’s life draws to a close, this ICD device will try and activate, and I have actually seen it activating on a dying patient before and it wasn’t pleasant, that one day the decision will have to be made that this box will have to be deactivated, you know, in the most sensitive way that I can, because when the heart naturally wants to stop and end of life is drawing nigh that this box would try to deliver a shock, it would be unpleasant, unkind. Two words I also use for the more mature patient is that, when we all get to that stage of life where it’s the dying stage of life, I use the words: dignity; respect – older people really, really want, that is one of the main requirements of when life’s limited and that somebody, either the spouse or the children, will have to make the decision about switching the box off. I’ve found most people are quite accepting of that fact and that there’s been no huge adverse, oh shock, and they think, ‘well, oh yes, if I’m going to die’, and, as I said before, some people think, ‘well if I’ve got cancer then I don’t want my heart to keep going on’, so they accept that it will have to be turned off at some stage and I always like to involve the patient in this conversation and the decision-making, rather than leaving it to, potentially, an elderly wife to make that decision. In my experience, as I say, most people accept this as part of the whole package.
Nurse Mint, heart failure nurse
These data suggest that early engagement in deactivation discussions may facilitate the involvement of the patient in the decision-making process, and that patients can respond well to this conversation about the end of life, as they understand that this is a natural part of life.
As well as heart failure nurses, it is arguably physiologists who have the most regular contact with ICD patients following implantation. Patients attend physiologist clinics yearly, if not 6-monthly, to check the functioning of the device (the timing depends on whether or not the patient has a remote monitoring system; those with remote monitoring are required to attend yearly check-ups, whereas those without remote monitoring are required to attend 6-monthly check-ups). As such, these appointments were suggested as potential opportunities to engage patients in discussions about deactivation:
I suppose as physiologists we see the patients on a regular basis to follow the device up. So I think realistically we could do it. But we’re a bit kinda focused, because we do the follow up clinics, but then we don’t know, we don’t tend to learn much more about the patients from a medical point of view. So like we don’t see other concerns like where a GP would see more, more of the general kinda medical concerns and that. We just tend to see the patients and check the device and kind of say ‘It’s fine.’ So although I think we are in a good position some ways, I think maybe there are other professionals out there who are in, in a position to do it as well.
Mr Broom, physiologist
This quotation alludes to some of the potential challenges to physiologists taking on this responsibility. First, physiologists are primarily trained to deal with the technical side of the ICD, such as checking if the device has fired and tweaking programming; they may have received limited training in communication skills. In particular, they are unlikely to have received training in how to manage difficult and potentially distressing conversations such as deactivation. A second issue is that of time. Like all clinicians, physiologists are confined by time constraints, and it would be potentially problematic and unrealistic to expect them to engage with deactivation decision-making in the timeframe currently available to them for consultations.
Palliative care role in deactivation decisions
The findings of this study also indicate the need for a greater involvement from palliative care services for cardiac patients. Palliative care services were recognised as having an important role in patient care as patients approach the end of life. However, it was also noted that significant work would be required in order to build a relationship between cardiology and palliative care services, where a link does not traditionally exist:
I think that’s been proven in different studies or different area where there are good heart failure and palliative care, where there’s joint working there’s been huge investment of time. Clinicians are keen to invest that time. So I can’t say directly but I think it’s to do with that historical link and actually we don’t see a huge number of people here in the hospice here referred with end-stage heart failure. That may change and I hope it does, but I think it will take some investment of time in the hospice.
Dr Buckthorn, palliative care consultant
Dr Buckthorn indicates that one of the potential barriers to a relationship between palliative care and heart failure services is a historical link with oncology services. Indeed, it became evident through the interviews and workshops that there were differences in the provision between the different sites involved in the study. Whereas some NHS trusts had invested time and effort in establishing a relationship between palliative care and heart failure services, others had not; as such, the level of involvement patients had from palliative care was dependent upon their location.
Where palliative care services were involved in the care of ICD patients only at a late stage, the impact they could have was limited. Some bereaved relatives, such as Shirley and Lynn (the wife and daughter of an ICD patient deactivated pre mortem), indicated that they received little support from palliative care services, as there was a failure to recognise that the patient was nearing the end of life:
Ours was a bad experience.
And I think it’s because – in my view it’s because he wasn’t cast, as he left the hospital, as palliative, but I don’t know how.
Lynn and Shirley’s account of their relative’s end-of-life experience indicated that it had been rushed and stressful, for both the patient and his family. There had been limited involvement of palliative care and little planning in advance; the patient’s ICD was deactivated eventually but only hours before his death. They indicated that earlier involvement from palliative care services would have been beneficial; however, they were not sure at what point in the pathway this would be.
Palliative care clinicians themselves expressed frustration at cardiologists who they felt often left conversations and decision-making about deactivation to them rather than taking responsibility for leading these discussions themselves:
Subsequently I spoke to the, I couldn’t get hold of the consultant himself, but to the registrar who’d also seen the patient in clinic and I said, ‘What was the plan regarding the defibrillator?’, ‘Oh, Doctor so and so’s going to discuss that with him the next time he comes to clinic.’ And that struck me, it struck me then that it was quite a reticence to discuss deactivation of the devices, even when the specialist who’d arranged for it to go in had referred to another specialist for the patient to discuss end-of-life care . . . I thought that was a bit of surrendering of responsibilities to be honest.
Dr Basil, palliative care consultant
Dr Basil clearly viewed cardiologists as capable of engaging patients in deactivation discussion; however, this was something that they were reticent to do, preferring instead to pass these responsibilities on to other specialties.
Rather than being the responsibility of a specific group of clinicians, some participants suggested that it was more important that discussions and decision-making took place with someone with whom the patient was familiar and comfortable. Who is involved in the decision-making process is likely to be patient dependent and to vary according to the patient’s condition and where they are in the pathway. For instance, for patients who are being managed in the community, the responsibility would probably fall to heart failure nurses and GPs. However, cardiologists may be more actively involved in patients being managed in secondary and tertiary care.
It’s hard to say ’cos I think that for any given patient somebody’s going to be taking the lead and it’s not always clear-cut by role who that should be, so it may either be the GP, it’s conceivably a heart failure nurse, it’s conceivably a MacMillan nurse or our palliative care service, and I think that’s probably right but there will be some situations where cardiologists are leading that, although as people deteriorate and they become housebound, that becomes a bit greyer, but I think what all of those other people need is to be able to access the cardiologists who’ve discussed if they’re thinking of switching it off.
Dr Mulberry, palliative care clinician
The patient and the importance of their family in deactivation decisions
Clinicians recognised the importance of engaging patients in discussions about deactivation and establishing the patient’s wishes in terms of continued therapy:
Patients who do have terminal illnesses, we talk them through the consequences of turning it off too early, versus turning it off too late . . . So, a patient who has cancer, I suppose, is the biggest one. You say, ‘Well, what’s your quality of life, now? Do you still want to be rescued?’ ‘Yes, I do definitely. I’ve still got things to do.’ ‘When do you think that things are going to change?’ ‘Well, I don’t know.’ ‘So, you let us know when you think things are changing.’ We don’t want shocks delivered to patients in their last 24/48 hours of their life. I think we’re all in agreement with that.
Ms Forsythia, psychologist
It is important that patients, and families, are engaged in discussions and decision-making about deactivation. However, several of the interviews with bereaved relatives suggested that it had fallen to them to raise the issue of the ICD when their loved one was at the end of life:
So, we were given the opportunity of what to do, but, whatever we did he wouldn’t recover. So, we chose to withdraw the breathing machine and the tubes and everything and just keep him comfortable really. The consultant was walking out and I said, ‘What happens with the’ because we said not to resuscitate him, a DNR [do not resuscitate], and they wrote that on the notes. I said, ‘What happens to this implant that he has, the device? Oh, they didn’t know, did they?’.
Ivy, bereaved relative, ICD patient deactivated pre mortem
These individuals expressed concerns that this responsibility should not fall to patients, as they may lack the capacity to raise the issue of the ICD in these situations:
I’m thinking about my father-in-law who’s got early onset Alzheimer’s. He probably would forget that he had a device, so it wouldn’t cross his mind to mention it. Neighbours of ours both have dementia. If it happened to one of them . . . you’re reliant upon medical staff who are completing the operations to read the notes and understand. If the patient doesn’t understand or if the patient forgets.
Katy, bereaved relative of ICD patient deactivated pre mortem
In Katy’s experience of her father’s death and ICD deactivation, clinicians had not recognised that he had an ICD that needed to be deactivated. Katy’s father had what was referred to as an unrecoverable stroke, and the decision to turn off life-support had been made by the family. However, the implications of this for the ICD had not been identified by the clinicians, and it had been the family who raised the issue of what should happen to the device. This had caused them some concerns about what would occur in the case of other patients who may not have the capacity to raise the issue of the ICD and who may not have close family or friends to do this on their behalf. Katy suggested that important information about a patient, such as the presence of an ICD, could be recorded in such a way that it can be accessed by health-care professionals in an urgent situation. This could also potentially be used to draw attention to the ICD, and the fact it needs to be deactivated, towards the end of life.
You would think, in this day and age, with all the communication facilities that we have, that you could just provide a QR [quick response] code that somebody could scan to find out what medication you’re on, what devices you have, what illnesses you have. Things that you need to know before operations.
Katy, bereaved relative, ICD patient deactivated pre mortem
Other relatives interviewed, such as Shirley and Lynn (the bereaved wife and daughter of ICD patient deactivated pre mortem), felt that their decision to have the device deactivated was over-ridden by clinicians:
He wasn’t really progressing and it was obvious that he was really end of life. So we decided all we wanted to do was to bring him home. And then Nurse Cypress who’s the heart failure nurse at the DGH S02 and came out to see him and we talked – he was compos mentis, we talked about – he knew he was dying.
And he wasn’t frightened to die.
He wasn’t frightened and he knew he was dying and he planned everything, and we talked about deactivating the device. Nurse Cypress phoned up the consultant at the Implanting Centre 1 and she said his heart looked alright and she didn’t feel it was time to deactivate it.
Shirley and Lynn recounted how after coming to a decision regarding deactivation with their relative’s heart failure nurse, with whom they had an established relationship, this decision was over-ridden by someone else, which made the end-of-life experience more distressing as the patient had to be deactivated in the last hours of life.
Benefits of advance deactivation discussions for patients and their families
During the interviews with patients, it was evident that advance discussions had the potential to position the idea of deactivation as something positive:
Well there are the end-of-life considerations. I’ve read somewhere something . . . And I thought, ‘Oh yes . . . well I’m aware that, that this could happen. I think, yes, if, if I was seriously ill and near the end of life I wouldn’t want the thing shocking me.
Isobel, 67 years old, SP, post implant
For Isobel, being informed about deactivation, and that this may at some point be an issue that she needs to consider, was not a frightening or distressing concept; rather, she recognised that the shocking function of the device would be inappropriate as she approached end of life and it was not something that she wished to experience. As such, the potential to deactivate the device in advance of this was viewed positively.
Furthermore, awareness of deactivation and having advance conversations about deactivation afforded some patients a sense of control over their situation.
I went and I looked and found out. It gave me a bit of feeling of, sort of, control. You haven’t really got great control or any autonomy I suppose. But it gave me a sense of that, really. And it was as if I’d left no stone unturned and my eyes are wide open about what I was going through.
Gwen, 53 years old, PP, post implant, CRT-D
Throughout her interview it was obvious that Gwen desired to be fully informed about the CRT-D and the potential outcomes of life with the device. At times she described frustration in her encounters with clinicians, where she faced difficulties eliciting the level of information she desired. Gwen expressed how having information about the CRT-D was important as she wished to process the potential outcomes in advance. This could then be retained and returned to at such point in her future at which it needs to be dealt with.
Furthermore, it was evident among some of the bereaved relatives whose partner had been deactivated pre mortem that deactivation of the ICD was viewed positively and was seen as a natural part of the end-of-life process:
You can’t fire, start a car when there’s no petrol in it, yeah? . . . Yeah, I’ve made this up . . . It’s no good firing your starter motor, starting your car, when there’s no petrol in it. So it seems that this thing [ICD] was superfluous.
Janet, bereaved relative of ICD patient, deactivated pre mortem
Janet’s husband had reached a level of deterioration at which she could recognise that he was coming towards the end of life, or, to follow her analogy, ‘out of petrol’, and, as such, she no longer saw the ICD as having a purpose. The device firing in the event of a dangerous heart rhythm would do little to change his overall condition; at this point, the purpose of the device as a protection against sudden cardiac death was futile.
Barriers to timely deactivation decision-making
Themes identified in the context of ICD deactivation included several salient barriers to timely discussions with patients/relatives: reticence of secondary/tertiary care clinicians to discuss end-of-life care, and mechanisms for appropriate identification of patients who would benefit from a discussion about deactivation, as well as ethical issues.
Reticence of clinicians and patients to engage in end-of-life conversations
Reticence among clinicians to engage in conversations about end-of-life issues appeared to act as a barrier to timely decision-making about deactivation. Indeed, palliative care clinicians suggested that the culture of cardiology, which often focused on achieving a fix for problems, may be a barrier; discussing deactivation may be seen as akin to admitting failure, that there is nothing more you can do for this patient:
With a 10% ejection fraction [a measurement for the amount of blood the left ventricle pumps out with each contraction which is used in diagnosing and tracking heart failure] you just think, really? What are we going to achieve with this? So in many ways it doesn’t surprise me that there’s a reluctance to talk about that, as with a lot of medicine often that’s kind of seen as a failure of what we’ve tried to achieve, whereas in palliative medicine we try to embrace it as a natural part of the life death cycle. But I don’t know that’s the same view that all clinicians would hold.
Dr Echinacea, palliative care consultant
It was suggested in both interviews and the clinician workshop that training in delivering bad news and engaging in difficult conversations would be extremely beneficial to many clinicians. This may be likely to instil greater confidence in clinicians undertaking these conversations.
. . . there’s a great need still to help people with their general communication skills, and specifically their communication skills around end-of-life planning discussions. Better skilled clinicians would have more confidence to embark on these discussions and, you know, more, more readily accessible set of tools to have the discussion in the first place, that’s a large part of it.
Dr Basil, palliative care consultant
In addition, there was a sense from some of the clinicians, particularly cardiologists, that death and end-of-life issues were not something that the majority of patients were comfortable discussing:
There’s a time when people want, want to talk about death and stuff like that. Most patients don’t, they come to see you ’cause they want to get better. They don’t want to be told, ‘Well, you’re going to die.’ So they don’t want to talk about it, so it’s hard to bring it up if they don’t want to talk about it. And so there is an appropriate time to bring these things up, but not, you know, these are patients who, you know, you only put defibrillators in if you think their prognosis is reasonable, isn’t that right? So I think, at that stage, I don’t think it’s, they don’t want to hear that.
Dr Elm, non-implanting cardiologist
Dr Elm indicated that he perceives a conflict in discussing issues pertaining to death when patients are seeing him in order to be better. However, the extent to which this opinion was based on direct interaction with patients who had expressed their desire not to engage in these conversations or was just an assumption that he held about the type of information patients wished to receive in a clinical interaction was not clear.
Indeed, our data suggest that patients and their families preferred to be informed about end-of-life issues in advance. Furthermore, some interviewees actually recounted how they had tried to initiate discussions about the end of life with health-care professionals but that these had been dismissed. In Gwen’s account below, she indicates that her attempt to elicit information about the impact of the ICD on end-of-life issues was dismissed by the health-care professionals. She suggests that this was because they did not view her as being in a category of patients who require a conversation about the end of life because this was an event that was still some way in the future for her:
I asked a nurse about end-of-life matters and she was quite ready to dismiss it and not talk about it with me. So she didn’t, so I never had a chat with anybody about it. I’ve just read about it. But it was important to me, so I went and looked it up myself . . . I thought, ‘This is what it must be like for the nurses . . .’ This is somebody coming to get one implanted, and she’s 50, OK. She’s not in that box [near end of life]. She’s not one of those. So it’s not time. But actually it’s very important for me to think about that, right at the end, because I think about things in detail and what that might mean. And, for me, it’s important to visit it emotionally, think about it, and come to some sort of terms with it, and then I can leave it. And when it arises later on I will refresh, ‘Yeah OK, I know about this and this is something to consider.’ I have to, sort of, pick it all apart and look at that detail. I know not everybody, I think most people mustn’t want to do that, because if most people wanted to do that, by now, it would be, the nurse would’ve said, ‘Oh yeah, that’s what happens. And at the end of your life we like to turn into part of your, if you’re a DNR [do not resuscitate] then we do this and so on and we’ll need to tell your family and it’s part of the process.’ So I think, ‘oh well, I must be out of, a bit out of the ordinary, either ’cause I’m younger’, although I wouldn’t have thought I’m the only young person who’s got a device, by any means. I’m not even as young as some. But it must be something that they, that certain nurses who are dealing with, maybe the palliative care or something like that, might be the ones who talk about it more. But it was just left out.
Gwen, 53 years old, PP post implant, CRT-D
Gwen’s desire to engage in these conversations indicates that there is not a ‘one-size fits all’ approach to discussing and dealing with end-of-life issues. Although a minority of patients may wish to delay considering these issues until they are directly faced with them, most patients, like Gwen, wished to gather information in advance that they can return to when the time comes, preferring to have an awareness of what the future is likely to hold.
Identification of patients who would benefit from a discussion about deactivation
Another potential barrier to timely deactivation discussions was the difficulty in identifying patients who needed to engage in these decisions. The reasons for this were two-fold. First, heart failure is relatively unpredictable in its trajectory, meaning that many patients experience cycles of deterioration and improvement, which makes it difficult to judge when the patient is approaching the end of life.
We have to recognise, of course, some people won’t want their devices to be turned off and, and there is a big difference between people who are reaching the end of their lives from cancer, which is more or less fairly predictable trajectory, not entirely, but rather more than less. Compared with something like heart failure where you can suddenly become very unwell, but suddenly get quite a lot better again, even though you may only be expected to live for another small number of months.
Dr Basil, palliative care
A second issue is the relatively limited knowledge about ICDs that exists outside the cardiology specialty, so that identification of devices suitable for deactivation would not necessarily be a priority for many clinicians. Indeed, some of the bereaved relatives’ accounts discussed earlier, such as those of Shirley and Lynn and Ivy, indicate that the presence of the ICD was not identified by clinicians. There was a suggestion that there may be the need to raise the profile of ICDs:
‘Cos I think, in everything that . . . people have got to remember . . . so I’m thinking about even people who have come in to A&E [accident and emergency] who will have ICDs, there’s so much that has to go on in terms of assessment and management, but that will probably be on the back burner but it’s about just raising the profile of ICDs.
Dr Mulberry, palliative care
It was argued that raising the profile of ICDs would facilitate the earlier identification of patients for whom deactivation may be a possibility. In particular in the acute and community settings this could facilitate the identification of patients earlier in the end-of-life pathway. The workshop with clinicians indicated that there would be particular difficulties in identifying historical ICD patients who may have been fitted with a device for several years and were now possibly approaching the stage at which deactivation would be appropriate. If these individuals were not being routinely followed up by cardiologists or heart failure nurses, it would be unlikely that they would be engaged in much discussion about these issues. Clinician participants indicated that in some hospitals measures were already in place to counter some of the issues discussed above. Specifically, there were measures to try to educate non-specialist clinicians about ICDs. However, barriers were also described in attempts to engage clinicians outside the specialty of cardiology in education sessions about the devices:
I think the problem is sometimes with these sessions, like if you offer them out, people think that ’cause they’re cardiology related, it’s only for cardiology staff, kind of thing. But they don’t realise it’s for anyone. But I think them kind of sessions work, so if you, and they have like a training manual in the hospital, if you offered like a course, just like an hour’s course or something, where you are explaining, just to talk, talk through with them, and if you had like a range of staff I think that would really help.
Mr Broom, physiologist
Clinicians spoke of their frustration at having engaged a patient in the deactivation decision-making process only for this not to be followed up by other clinicians:
I hate it when you have an initial discussion with a patient, but then nobody tells you until the last minute, and you think, ‘Oh, it’s not what I wanted for this man. I wanted it to be better organised.’ Sometimes, we get it better than others, and that’s where we have lots of teams of people involved. The Macmillan nurses, and, well, everybody who was involved in that team process, goes, ‘I have had a patient with a defibrillator in, I know who to ring’, and that’s how it works.
Ms Forsythia, physiologist
This failure to continue the decision-making process through the patient pathway meant that deactivation was often rushed when it became apparent that the patient was in the last days or hours of life.
Ethical concerns: deactivation as analogous to assisted suicide
Another barrier to timely deactivation was concern about the ethical connotations of withdrawing treatment. There was some suggestion that deactivation could be, and was, viewed by some of the patients as akin to suicide:
[A] patient who’s a Roman Catholic priest actually still refuses to have it switched off even though he should have ’cause he considers that similar to suicide basically . . . I’ve told him very clearly I think it’s inappropriate, he’s not going to have it switched off, that’s fine. I don’t think we can switch it off against his will. I don’t think? So we just have to leave it there and if it keeps on defibrillating so be it . . . the worry about him is he’ll just be kept unnecessarily alive ’cause the poor guy is just at the end of his life now.
Dr Ash, secondary care cardiologist
It was evident that some patients held erroneous beliefs that the ICD was keeping them alive and, as such, were uncomfortable with the idea of deactivation, assuming that this would lead to immediate death.
I don’t want to be euthanised . . . Natural is all. And I think my life is sort of natural, if I might put it, I don’t want to, euthanasia doesn’t come into my . . . if I die I die and I suppose there are some people say ‘Well if it’s a painful death just turn it off’. No, not me.
Richard, 76 years old, PP, post implant
An interesting conflict is apparent in Richard’s account. He appears to believe that dying with the device in place but activated would be a natural death and that deactivating the device would result in an unnatural death. However, there is no acknowledgement of the unnaturalness of having a device such as an ICD fitted to the body.
It was suggested that such concerns about deactivation may be related to a lack of understanding about the limits of the device:
I think recognising that in that context they would die with an ICD whether or not there was a rhythm which could be shocked.
Dr Buckthorn, palliative care clinician
Dr Buckthorn highlights the importance of understanding the limits of the effect of the ICD, the purpose of which is to protect against dangerous heart rhythms; however, it is not infallible and cannot protect against the development of other life-limiting conditions.
Furthermore, it was suggested that the view of deactivation as akin to suicide could be inadvertently reinforced by the type of language used to refer to it:
Like I say the switching off I, is, it just sounds so final and, I don’t know, like you’ve got, like switching off life-support, I suppose that’s the analogy that springs to mind.
Dr Echinacea, palliative care clinician
Indeed, clinicians indicated throughout the interviews that they modified the language they used in order to increase the acceptability of deactivation conversations.
I always use examples with my patient, say, ‘If you were dying of cancer would you want something to be firing in your chest when you’re breathing your last with your family around you?’ And that sort of makes ’em think, ‘Yeah, well if that’s what it’s going to do’ that might make them more accepting of why and, and when it’s to be switched off, you know.
Nurse Cypress, heart failure nurse specialist
A number of clinicians reported using a similar technique to create a hypothetical situation in which the patient may have developed another life-limiting condition. The purpose of this was to establish in a patient’s mind the potential of a possible turn of events whereby the functioning of the ICD may no longer be appropriate. This would facilitate in the patient a positive view of deactivation as something that would alleviate potential pain and suffering in the last days of life, rather than a negative thing.
Chapter 7 Summary and conclusions
The combined analysis of our data provides detailed insights into clinicians’, patients’ and relatives’ views and experiences of decision-making about ICD implantation and deactivation (objective 1), including how and when ICD risks, benefits and consequences are communicated to patients (objective 2). This enabled the identification of patients’, family members’ and clinicians’ information and decision-support needs in the context of SDM (objective 3), including a range of facilitators and barriers to patient engagement in preference and values-based discussions about implantation, and timely decision-making about implantation and deactivation (objective 4). During the inception of the research, an option grid and video was published in the USA to support SDM about ICD therapy. 43 Consequently, the final objective was amended to focus on the provision of guidance to improve existing decision support and on supporting the process of SDM with ICD patients and relatives.
Summary of implantation issues
During the pre-implantation period, patients reported variable levels of knowledge about the severity and/or gravity of their condition and the associated risk of sudden cardiac death. In particular, patients being considered for ICD therapy as a PP strategy were frequently unaware of the clinical rationale for ICD therapy until their consultation with a tertiary (implanting centre) care specialist. This contributed to some patients, and their relatives, experiencing negative reactions such as a sense of shock and disbelief. This appeared to confound their capability to process adequately the information being conveyed to them about the rationale for the ICD and to differentiate ICDs from CRT-Ds and pacemakers (the latter of which was a core issue in relation to patient confusion and unrealistic expectations about the benefits of ICD therapy). Interviews with clinicians affirmed these findings. They acknowledged that communicating the rationale for ICD therapy, and the fact that the simple ICD does not confer any symptom or quality-of-life benefit, was a challenging task. Patients often reported being unaware of the availability of choice, including no active therapy (no ICD) as an option available to them.
Clinicians were observed to use, and reported the use of, metaphors to describe the function of an ICD (e.g. anthropomorphising the device by describing it as a sentry on guard duty against cardiac events) alongside the use of verbal descriptors and numerical risk information during consultations with patients. The use of verbal descriptors (using elastic language such as low, medium and high risk) was influenced by clinicians’ perceptions of their patients’ capability to comprehend probabilistic information.
Issues related to the need to understand the rationale for ICD therapy (i.e. as a risk modifier, as opposed to a risk eliminator, for sudden cardiac death and not as a treatment to improve their symptoms) was also evident from interviews with bereaved relatives of ICD patients.
The primary function of an ICD is to deliver a shock (defibrillation) that has the ‘benefit’ of preventing sudden cardiac death. However, appropriate and, in particular, inappropriate defibrillations (shocks) can be extremely distressing for patients and are likely to be a key contributing factor to the development of mental health problems such as depression and anxiety. Clinicians used a range of metaphors to describe the experience of defibrillation from an ICD, ranging from likening a shock to being ‘kicked in the chest by a camel’ to using physical actions such as ‘hitting a desk with their fists’ to evoke a vivid image in patients’ minds.
Variability in the way in which clinicians related information about the outcomes of implantation on appearance (owing to the protrusion of the implanted device in patients’ chests and post-surgical scars) was also evident. Interviews with patients revealed that they were often not fully informed about these important implications, including potential negative consequences in terms of perceived body image. There was also evidence from patient interviews that information about the surgical procedure to implant an ICD in terms of the complexity and the use of local (as opposed to general) anaesthesia was not always conveyed by clinicians prior to implantation, which was a source of considerable distress to some patients.
Patients, relatives and clinicians acknowledged the importance of providing ‘balanced’ information on the risks and consequences of ICD therapy, including the provision of information on the surgical procedure used to implant the device. However, concerns were raised about the detrimental impact of information overload, and there was a need to identify and fulfil the information needs of individual patients/relatives, as well as to gauge their desire for involvement in decision-making, which patients often felt was suboptimal. Many patients reported experiencing frustration at clinicians’ failures to recognise/address their personal information needs, which would help them to form personal preferences and values in relation to the options available. In many cases, this went beyond the prevention of sudden cardiac death to considerations about their values towards mode of death and the impact of ICDs on their capability to retain a driving licence, which was key to quality of life for many. This was compounded by a lack of time and opportunities for the development of a rapport with patients in consultations; indeed, on many occasions, the implanting cardiologist had not previously had any contact with the patient prior to the implantation consultation.
Clinicians expressed variable access to, use of and perceived value of patient educational materials produced by charities such as the British Heart Foundation and the Arrhythmia Alliance, which could further compound the issues of patient understanding about the rationale for ICD therapy, including an awareness of the associated benefits, risks and consequences of ICD implantation before, during and after any implantation procedure.
This was further complicated by clinicians often reporting that patient retention of information was suboptimal; a common remedial strategy suggested by clinicians was ‘information checks’ during subsequent clinical contacts across the pathway.
Adequate time for patients and their relatives to reflect on information provided by clinicians and the options available was considered an important prerequisite for engagement in effective SDM. Consistent with other contexts, multiple actors were important influences on decision-making about ICD therapy, including expert and trusted clinicians (in particular heart failure nurses with whom many patients in the current study had developed a close and continuing relationship) and close family members.
Summary of deactivation issues
We identified evidence of differing views of clinicians and patients/family members about whether or not patients should be informed about the deactivation of ICDs prior to implantation. However, our data suggest that patients, and their families, preferred to be informed about end-of-life issues in advance. Such discussions could be hypothetical in nature, offering an introduction to the idea that a patient’s condition may deteriorate or that they might be diagnosed with an additional life-limiting condition, at which time the shocking function of the device may no longer be considered appropriate and may diminish the quality of life remaining.
These views were shared by some clinicians who described an initial discussion to ‘plant the seed’ regarding deactivation and to address unrealistic expectations about ICD therapy, but other clinicians intimated that deactivation was not a routine component of the pre-implantation discussions; this was driven by a clinical belief that discussing end-of-life issues alongside a device to prevent cardiac death (which appeared to be an illogical juxtaposition of information on implantation and deactivation) may lead to harm to patients, in particular for patients offered ICD therapy as a SP strategy following a recent cardiac event.
Indeed, there was a lack of consensus among clinicians about when in the pathway a conversation about deactivation should occur. Introducing the idea of deactivation prior to implantation appeared to be a particularly contentious issue, with some clinicians expressing concerns about the distal nature of this ‘advance decision’ that may or may not have to be implemented; however, others were concerned about the potential for information and/or discussions about deactivation carrying a risk of communicating mixed messages to patients.
However, we identified evidence of decisional regret associated with a lack of patient awareness about the long-term consequences of ICD therapy related to deactivation; for example, one patient reported that if he was aware of the issues around deactivation, then he would not have chosen to have an ICD. Clinicians also described patients/relatives experiencing difficulties in the deactivation process owing to a failure to engage with them about future possibilities that inhibited them from developing appropriate planning for end-of-life care in the context of ICD deactivation.
Potential trigger points for deactivation discussions embedded within a formalised protocol were suggested by clinicians to ensure that opportunities for appropriate and timely discussions about deactivation could occur with patients and families, with an emphasis placed on the need for regular monitoring and review over time in terms of the clinical effectiveness and appropriateness of continued ICD therapy, a process described by one palliative care clinician as ‘an entry and exit strategy’ when discussing the option of ICD therapy. Specific trigger points that could result in a deactivation discussion included diagnosis of an additional life-limiting condition, deterioration in heart failure, frequent hospitalisations and placement on a palliative care register, with the production of a DNAR order being a final trigger point. However, clinicians suggested that there might be difficulties with, and challenges to, introducing a formalised protocol with regard to identifying trigger points for historical cases in which patients had been living with an ICD for a number of years and are unaware of deactivation issues. Nevertheless, opportunities to identify historical cases could be facilitated via ‘trigger points’ such as appointments for battery replacement, or, where patients are being cared for in the community, appointments with their GPs (emphasising a potential role for primary care clinicians in deactivation discussions).
A lack of consensus on who is best placed to initiate discussions about deactivations at these trigger points was apparent from interviews with a range of clinical specialists involved in the ICD patient pathway. Assertions that cardiologists are best placed were based on their expertise with the device itself, but were countered by arguments that other clinicians, such as primary care clinicians and heart failure nurses, are more closely involved in an individual patient’s continuing care (and thus are more familiar with their preferences and values). There were also calls for greater involvement from palliative care clinicians, but a key barrier was a lack of perceived interprofessional collaboration in the care of cardiac patients in some of the research sites. Palliative care clinicians themselves expressed frustration at cardiologists who they felt often surrendered conversations about deactivation to them.
Consistent with implantation issues, patients and relatives valued having an expert and trusted clinician, with whom they had developed a positive therapeutic alliance, to initiate a discussion about deactivation. A role for heart failure nurses or physiologists in initiating deactivation conversations was highlighted by patients, as they had the most established relationships and continued contact with them. However, nurses are clinically trained, whereas physiologist training is focused on the technical elements of ICD function; therefore, currently nurses may be better placed and more likely to have had appropriate training for engaging patients/relatives in deactivation discussions than physiologists. For physiologists to take responsibility for initiating discussions about deactivation, this would involve taking on additional duties that, at present, are not recognised as part of the physiologist role.
The crucial role of patients’ preferences and values in making decisions about deactivation was acknowledged, including the benefits of decisions about deactivation being made in advance, which would provide patients with a sense of control over their situations. Interviews also emphasised that decision-making does not occur outside consultations in a social vacuum; rather, the patient’s family is often closely involved in deactivation discussions and decisions, frequently acting as a patient’s proxy towards the end of life if no advance discussion had taken place.
Summary of the barriers and facilitators to shared decision-making in the context of implantable cardioverter defibrillator therapy
Arguably the most salient barriers to SDM were discordant clinician and patient/relative preferences and views on information needs with regard to ICD therapy. These were strong contributing factors to: (1) patients expressing variable levels of knowledge about the severity/gravity of their condition and associated risk of sudden cardiac death, including details about the surgical implantation procedure for ICDs, and, importantly, the clinical rationale for ICD therapy (i.e. as a risk modifier as opposed to a risk eliminator for sudden cardiac death and not as a treatment to improve their symptoms); (2) clinicians’ variable disclosure and methods of conveying information about the benefits, risks and consequences of ICD therapy; (3) patients lacking awareness of choice, in particular, the reasonable ‘preference-sensitive’ option of no active therapy (no ICD); (4) clinicians avoiding discussions about end-of-life issues, which, to some extent, was driven by beliefs about the ‘illogical juxtaposition’ of presenting information about implantation alongside deactivation (beliefs that patients would be harmed by discussing end-of-life issues alongside discussing a device to prevent cardiac death); and (5) a lack of consensus among clinicians about when in the pathway a conversation about deactivation should occur (and who should do this) with perceived suboptimal interprofessional collaboration in the care of cardiac patients in some of the research sites.
Facilitators to SDM in the context of ICD therapy were close involvement of a patient’s relatives in the decision-making process (in the context of deactivation, this facilitated acting as a patient’s proxy towards the end of life); the existence of positive therapeutic alliances with expert and trusted clinicians (and the role of heart failure nurses, GPs and, possibly, physiologists in the deactivation discussions); the provision of sufficient time for patients and their relatives to reflect on information provided to them; clinicians using strategies such as information checks across multiple consultations; raising deactivation as a possibility (which also served to address unrealistic expectations about ICD therapy); and potential trigger points for deactivation discussions being embedded within a formalised protocol for appropriate and timely discussions about deactivation.
Interpretation of findings in relation to the literature on shared decision-making
Several themes identified in the current study resonate with findings of previous research on SDM about ICD therapy and the wider theoretical research on SDM.
The involvement of multiple actors in the decision-making process in the context of ICD therapy is consistent with the concept of ‘distributed decision-making’, which describes decision-making as a continuing event taking place over multiple encounters with a range of different people. 49 This was driven to a large extent by the complexity of the ICD pathway, the multiple clinicians involved, the close involvement of relatives and the widening of this complexity when end-of-life issues emerge. As seen in other preference-sensitive decision-making contexts, such as decision-making about thrombolysis in acute stroke care,42 patients valued the views and support of expert and trusted clinicians (in this context, heart failure nurses with whom many patients in the current study had developed a close and continuing relationship) and close family members.
Sepucha and colleagues50–52 describe the concept of decision quality, whereby preference/value-concordant decisions are made by patients who are knowledgeable about their condition, the options available to them and their personal preferences and values associated with the options. Our findings suggest that information exchange in SDM encounters should go beyond the presentation of options, with information on the clinical rationale for ICD therapy, including the patient’s diagnosis, also playing an integral part in engaging patients in SDM to enable them to understand their condition and probable prognosis. This is particularly important given that simple ICD therapy does not have symptom or quality-of-life benefit and the appropriateness of presenting a ‘do nothing’ option (i.e. no ICD therapy) as a valid choice.
Our findings concur with previous research that reported that patients/relatives want to be actively involved in decision-making about implantation and deactivation, and the majority want to be informed about deactivation at the pre-implantation stage,53 which can have important benefits such as an improved sense of control for patients, while preparing both patients and families for later end-of-life discussions. Patients and relatives want to be offered a choice and sought balanced information about the available options [pros and cons, in particular, the potential impact on psychological well-being and quality of life in the short and long term (deactivation)], and involvement in decision-making. Patients/relatives who participated in the group workshop unanimously agreed that patients would benefit from a clear overview of the pathway and associated decision points (see Figure 2) to facilitate the process of SDM.
However, we identified a range of issues that may have inhibited good SDM and prevented patients from making quality decisions about ICD therapy.
Consistent with previous research,15,16,24,54 several patients in our study held erroneous beliefs about the benefit of ICD therapy and seemed unaware of the adverse effects of ICDs and the potential threats to quality of life, such as the risk of psychological problems, driving restrictions and a negative impact on body image.
Clinicians’ values (beliefs/attitudes) and their patients’ preferences for information and involvement in decision-making were often discordant with each other, in particular with respect to the timing of deactivation discussions. We found evidence that clinicians relied on their clinical judgement to ‘diagnose’ their patients’ preferences for information about ICDs, including the method of conveying risk information (e.g. Dr Oak described how he used elastic language in the first instance, followed by numerical information if perceived to be ‘helpful’, to convey risk information to patients) and their desired level of involvement in decision-making. However, research has consistently shown that clinicians are typically very poor at accurately ‘diagnosing’ their patients’ preferences. 55,56 In the SDM literature, this has become known as the ‘silent misdiagnosis’, as clinicians are often unaware of their patients’ preferences or make incorrect assumptions and are unaware of the consequences that this might have for patients. 57
This silent misdiagnosis was evident in our findings and has important implications for SDM in the context of ICD implantation and deactivation. Misdiagnosing patient/family member preferences can:
-
Act as a salient barrier to engaging patients and relatives in SDM, which may result in patients receiving treatment and care that they do not want, or would not choose if they were fully informed.
-
Foster the development of unrealistic expectations of ICD therapy, in particular when risk information (outcome probabilities) is presented using different methods that can have a differential impact on perceptions of risk and decision-making in both clinicians and patients. 58
-
Lead to missed opportunities for optimising the costs of ICD treatment to the NHS by reducing any unwarranted variation in their use across the UK,59 as previous research has shown that patients often choose less conservative options when they are informed about the available options. In the context of our study, we found evidence that patients being considered for ICD implantation were often unaware of the option to ‘do nothing’.
We found evidence of another type of silent misdiagnosis in the context of ICD therapy, with regard to clinicians’ perceptions of a patient’s future disposition in terms of the impact an ICD may have on their psychological health and well-being. In the context of ICD therapy, this may provide an argument for the wider involvement of psychologists as further sources of ‘expertise’, which patients could use to more accurately identify their treatment preferences in order to inform their decision-making at the pre-implantation stage. In addition, for ICD patients who experience anxiety and depression following implantation, greater awareness and referral (self or clinician) to the NHS Improving Access to Psychological Therapies service may be a further preference-sensitive option that could be presented to patients.
We identified evidence of clinicians’ gatekeeping of information regarding ICD therapy. In addition to unmet information needs regarding implantation and deactivation, the option of not having an ICD was not always presented as a valid choice to patients. One possible reason for this is that clinicians may be less inclined to offer no active therapy (no ICD) as an option to patients who have experienced a cardiac event (SP). Furthermore, such patients themselves may be less inclined to consider having no intervention as their experiences of a cardiac event may instil in them a more acute perception of the potential benefits of ICDs. In PP, the balance of risks and benefits, the course of underlying disease and varying patient values may mean that not having an ICD is a more valid option.
A further key barrier to the provision of information on risks and benefits at the pre-implantation stage was the view held by some clinicians in our study that providing patients with information about ICDs prior to attendance at the implanting centre may raise patient expectations of the device, when later they may be deemed to be ineligible for an ICD by the implanting cardiologist.
Congruent with previous research,11,24 we identified a reticence among clinicians about raising the issue of deactivation with patients and relatives (especially pre implantation), including a lack of consensus on who should initiate discussions about deactivation and their timing across the pathway. In previous research,6,13,14,53 many patients and relatives reported not being informed about deactivation until a patient was nearing the end of life, when early discussion (pre implantation) would have been their preference. In contrast, several clinicians in our study held the view that discussing deactivation at the pre-implantation stage would cause unnecessary distress or send mixed messages about ICDs; however, this is also contrary to research that reported that the great majority of patients did not believe that this would lead to increased anxiety in patients or relatives. 53 Inattention to deactivation in NICE guidelines2 and a regional policy for the deactivation/reactivation of ICDs26 is likely to have been another factor influencing clinicians’ decisions to refrain from discussing deactivation of ICDs with patients and relatives.
Nevertheless, this withholding (intentionally or not) can have negative consequences, such as patients experiencing decisional regret and poor satisfaction with care received. We found evidence of decisional regret associated with a lack of patient awareness about the long-term consequences of ICD therapy related to deactivation. As well as a salient barrier to patient engagement in SDM, it is also felt by some authors that withholding information violates three principles that underpin international clinical research ethical guidelines, namely, respect for persons or autonomy, beneficence or a favourable balance of risks and potential benefits and justice or a fair distribution of the benefits and burdens of research. 60
How can the silent misdiagnosis and gatekeeping of information be addressed to overcome these key barriers to patient engagement in SDM? The literature can be dichotomised into ‘clinician pull’ and ‘patient push’ (or vice versa) interventions, including strategies targeting both sides of the consultation.
Patients’ preferences for involvement is influenced by demographics, experiences of illness and medical care, their beliefs on involvement and the interactions and relationships they experience with health-care professionals, which are likely to develop and change over time. 61,62 Good SDM requires two kinds of expertise, namely clinicians’ knowledge of efficacy and outcomes of treatment options and patients’ expertise on their values and preferences with regard to the available treatment options. 63 This assumes that patients possess both the willingness and capability to engage in SDM. Arguably, the latter is a prerequisite of the former, with active involvement in SDM requiring patients and clinicians to have acquired a complex range of knowledge, behaviours (skills) and confidence to enact those skills. This does not preclude the value of decision aids, but, as Flynn64 asks, ‘can a decision aid be a useful adjunct to the clinical process if patients lack awareness of the value of SDM; and perhaps crucially do not have the experience, knowledge, skills and/or confidence to engage meaningfully in discussions with clinicians about their healthcare?’.
Patient activation is a behavioural concept, which is important for active patient engagement and participation in SDM about their treatment and care, and is defined as ‘an individual’s knowledge, skill, and confidence for managing their health and health care’. 65 Low patient activation is an important barrier to SDM and there is evidence that this can be ameliorated with behavioural interventions,66 which appear to be more effective when they are tailored to support the individual’s level of activation (tailored coaching) and focus on skills (e.g. question formulation) and confidence building,67 which have been shown to be effective in increasing patient activation for up to 12 month post intervention. 68
Clinician push interventions can take many forms to increase the willingness and capability for SDM in order to actively support their patients to engage in SDM and, ideally, will be underpinned by an evidence-based implementation strategy to embed SDM in clinical systems and patients pathways. 69 A multicentre implementation project, MAking Good decisions In Collaboration (MAGIC) programme,70 underpinned by a theory-based model of SDM skills,69 was designed to explore how SDM can be embedded in both primary and secondary care settings
More than 270 clinical and non-clinical staff (including NHS management and general practice staff) participated in MAGIC. They were provided with the following active ingredients of the MAGIC programme:
-
skills development and engagement, for example introductory and advanced skills development workshops for participating clinicians
-
support with developing and implementing (or adapting existing) decision-support tools (brief patient decision aids)
-
facilitation and peer support, for example action learning forums that involved regular meetings with MAGIC staff and clinical teams to share experiences and learning
-
support to involve patients, which included activities such as convening patient forums and a patient activation intervention designed to increase awareness of SDM and as a prompt to initiate SDM discussions known as ‘Ask 3 Questions’ (What are my options? What are the possible benefits and risks? How can we make a decision together that is right for me?).
An independent evaluation of MAGIC71 found evidence that the programme successfully improved participants’ understanding and awareness of SDM and developed their skills and confidence to use SDM in practice; supported clinical teams to test and develop SDM tools and approaches; made changes to clinical practice; and provided opportunities to hear and understand the patient experience of SDM, which (via role-play exercises) facilitated connections between the theory of SDM and its use in routine practice. This challenged a key barrier to SDM: ‘we are doing this already’. The importance of training was heavily emphasised, with a key learning point being the aphorism ‘attitudes and skills trump tools’.
The lack of formal incentives for clinicians to engage in SDM has been discussed in the literature; for example, the existing incentive structure ‘Qualities and Outcomes Framework’72 can, in effect, disincentivise engagement in SDM. 71,73 However, there is a recent legal precedent from the UK Supreme Court in 2015, whereby clinicians are now required to take ‘reasonable care to ensure that the patient is aware of any material risks involved in any recommended treatment, and of any reasonable alternative or variant treatments’. 74 This precedent in UK law provides a strong incentive for clinicians to refrain from information gatekeeping and may lead to improved SDM by increasing their willingness (and wider infrastructure support) to engage in continuing professional development on SDM.
Implications for the development of decision-support tools
In addition to being an essential prerequisite for effective SDM, the provision of comprehensible and balanced information on the clinical rationale for ICD implantation (and the associated surgical procedure) and deactivation (and associated potential short- and long-term benefits and risks to daily quality of life) is critically important for patients’ adaptation to the device to reduce the risk of emotional distress. 75–79 Research has shown that 25% of ICD recipients experience depression or anxiety problems,80 with the majority receiving no formal treatment for psychological problems. 81 Only 3 out of 44 patients in our study disclosed psychological problems attributable to device implantation, which is lower than previous research. This may be due to different methods of data collection, which influence the disclosure of mental health problems (surveys vs. interviews), the timing of interviews and the different rates of comorbidities (chronic heart failure, chronic obstructive pulmonary disease, cerebrovascular disease and renal failure) which are reported to be important predictors of depression in ICD patients during the first 12 months post implantation. 82
Patients in our study demonstrated concerns about the impact of the ICD on the appearance of the body. Previous research has suggested that the impact of the device on body image may be particularly acute for women, owing to a cultural focus on their sexual attractiveness. 83 Indeed, concerns about the impact of the device on appearance have been suggested as the primary reason for women to opt for submammary device placement. 84 However, the findings of our study suggest that the impact of the ICD on body image is a concern for both male and female patients. A study of ICD patients from Sweden and Denmark found that approximately 60% of patients reported receiving information on potential detriments of the device to psychosocial/sexual functioning. 85 In our study, the impact of the device on psychosexual functioning was not raised as an issue, which may reflect cultural differences regarding the discussion of sexual topics between Sweden and Denmark and the UK. These findings suggest that it is important for information on psychosocial issues to be considered for inclusion in consultations and in updates to existing decision-support tools for use in the context of ICD therapy. Therefore, in addition to the moral imperative for SDM, informing patients about the risk of psychosocial problems could potentially reduce any negative impact on quality of life and reduce the risk of psychological morbidity; viewed this way, SDM is also a therapeutic intervention. 86
We identified a lack of standardised methods used to convey information about the nature of ICDs (and other options) and their pros and cons, including tools to support the acquisition of probabilistic information on the latter in both the short and long term. These issues are consistent with those reported by clinicians when conveying information to patients and relatives on risks and benefits of treating acute stroke with and without thrombolysis. 87
Owing to the lack of robust decision-support tools until recently, it is not surprising that clinicians reported variable techniques to communicate risk about ICD therapy, in particular the use of elastic language (low, medium and high) and percentages that can cause difficulties for patients’ understanding and interpretation. 88,89 Using numerical methods of communicating probabilities, and, in particular, natural frequencies with identical denominators and time horizons for outcome probabilities, can improve risk perceptions90 (e.g. out of 100 patients like you with heart failure who are implanted with an ICD, 25 will experience depression/anxiety to a level that warrants treatment over a 12-month period). Graphical methods, such as pictographs, to present natural frequencies (and other numerical outcome probabilities) also have a substantial evidence base (notwithstanding patients’ levels of health literacy) to demonstrate that they can improve risk perception. 91 Nonetheless, to account for individual patient preferences, multiple methods of presenting probabilistic information are recommended. 92–94
The impact of tools will also be enhanced when they are underpinned by an auditable structured development and testing process (ideally, co-produced and co-designed in partnership with patients/relatives and clinicians/researchers so that they reflect their perspectives, priorities and lived experiences) and utilise evidence-based methods to convey probabilistic information (using the methods described above) on outcome states deemed to be important by patients/relatives. 94
Strengths and limitations
The strengths of our research approach are numerous. We provide a clear audit trail of the methods and processes for generating the findings of our study (dependability). The first phase (observation) enabled us to familiarise ourselves with the clinical setting and provided us with important insights about the decision-making environment, the actors involved and the nature of ICD consultations, in order to optimise our sampling and data collection strategies (interview guides). Combining data collected from multiple perspectives – clinicians, patients and family members – alongside the use of peer debriefing (from members of the research team and steering group) and ‘sense checking’ of findings within interactive workshops served to maximise the likelihood that our findings are an accurate conceptual interpretation of participants’ views and experiences.
The findings of our study relating to how to broach discussions about the withdrawal of analogous assistive devices during end-of-life care are also likely to be transferable to how to broach discussions with patients about the withdrawal from, for example, renal dialysis. 39
A limitation that may be inferred from our sampling strategy is the small number of interviews undertaken with patients who were prospectively considering deactivation. However, from the outset of the project, the study team was aware that this would be a difficult group to sample. Consequently, the views and experiences of this group may be under-represented. The level of difficulty experienced with the recruitment of bereaved relatives was greater than unexpected. As a result of these difficulties, we expanded the inclusion criteria for this group from 4–6 months post bereavement to 4–18 months. Although this approach was successful in increasing the number of bereaved relatives recruited into the study, given the sometimes protracted period of time post bereavement, accurate recollection of the deactivation event and associated issues may have been compromised to some extent.
As the focus of our study was on decision-making about ICDs in secondary and tertiary settings, this study lacks data from the perspective of primary care clinicians such as GPs and practice or district nurses.
Suggested areas to consider in the future development of services
-
The clinical rationale for offering ICD therapy would benefit from being clearly communicated to patients and their relatives as early as possible in the patient pathway by skilled clinicians supported, where available, by codesigned information and tools, including live demonstrations and the manipulation of devices to support informed consent and SDM.
-
Patients desire more information on the surgical procedure to implant ICDs to support informed consent and SDM, which can facilitate the development of positive/protective preparatory coping strategies to minimise any impact on psychological well-being (decisional regret) and perceived body image.
-
Increased access to, and a greater role for, psychological support from appropriately qualified mental health professionals throughout the ICD pathway.
-
Signposting to support groups for patients with lived experience of heart failure/ICD therapy and their relatives, while acknowledging that these options are preference-sensitive and that patients would benefit from being provided with sufficient information to make an informed values-based decision.
-
There is a lack of consensus/ownership among clinicians about who should take responsibility for discussing deactivation. To address this, the issue of deactivation could be introduced early in the pathway and formalised within a standardised protocol with specific trigger points to enable more detailed and timely discussions with patients and relatives about deactivation.
Recommendations for future research
We identified a number of important areas that are prerequisites for good SDM and for maximising the likelihood of patients making ‘high-quality’ values-based decisions. The following would be fruitful avenues for further research:
-
Developing and evaluating the use of risk communication tools that convey balanced information on the benefits, risks/adverse effects and short- and long-term consequences of ICD implantation and deactivation (identified by our research as being important for patients), including training on SDM skills and risk communication techniques for clinicians involved in the treatment and care of ICD patients.
-
Developing and evaluating training for clinicians (SDM, risk communication and delivering bad news to patients/engaging in difficult conversations, which should be viewed as a key skill as part of the SDM process in this context).
-
Developing theory-based patient activation interventions for patients and their relatives early on in the pathway.
-
Developing ICD-specific decision quality measures (implantation and deactivation).
-
Developing appropriate interventions to prepare patients (in particular those in tertiary care) and family members for SDM with cardiologists throughout the ICD pathway provided by clinicians with frequent patient contact (e.g. heart failure nurses) who can act as centralised points of contact outside tertiary consultations.
-
Updating existing decision support for ICD implantation and deactivation decisions informed by our findings (available options and associated risks, benefits and consequences) as part of national decision-support material to reduce any unwarranted regional/national variation in ICD therapy and other health-care domains. Such tools would benefit from being codesigned in partnership with patients and clinicians in both primary and secondary care. A decision-support tool for ICD deactivation would potentially benefit from being embedded within advance care planning processes to ensure sharing of this information with all clinical staff across care settings (e.g. the Electronic Palliative Care Co-ordination systems that are being rolled out nationally).
-
Exploration of the role of primary care clinicians and information sharing between and within primary and secondary care clinicians involved in the care of ICD patients, including palliative care.
-
Developing a central role for heart failure nurses, physiologists and primary care clinicians in terms of providing ongoing care/initiating deactivation issues.
-
Fostering evidence-based strategies for interprofessional learning and collaboration between cardiology and palliative care teams in the context of ICD therapy.
-
Carrying out multicentre service evaluation studies of enhanced ICD pathway (training, tools and role extensions described above).
All the decisions around ICD therapy in the current study were taken in particular points in time and are, therefore, shaped by that context. Future research might consider the use of longitudinal interviews to explore the trajectory of experiences.
Strategy to translate findings into continuous professional development learning and training
Our approach to mobilisation of knowledge is multifaceted. First, a summary of the study findings will be produced for the Arrhythmia Alliance and Cardiomyopathy UK for distribution to patients/relatives. We shall also host a half-day dissemination event in 2016 in collaboration with the Arrhythmia Alliance and Cardiomyopathy UK, at which patients, family members and clinicians can discuss the study findings. Second, we shall present the findings at meetings of the Arrhythmia Alliance and other relevant patient organisations, as well as work with our advisory group and the Northern England Cardiovascular Network, to publicise the findings to clinicians and academics more broadly. Third, we shall present the findings to practitioners and academics in the field at national and international symposia. To engage with practitioners in the cardiology community, we shall present the findings at the Heart Rhythm Congress, and to connect with other academic audiences we plan to present findings at the Palliative Care Congress, the International Shared Decision Making Conference and the British Sociological Association Medical Sociology Conference. We shall also publish the study findings in a peer-reviewed, high-impact-factor, journal.
We shall work with the Northern England Cardiovascular Network and specialist palliative care teams to embed the patient voice into their operational policy for the deactivation of ICDs. We shall also cascade learning from this study to NICE to inform national guidelines on ICDs.
We shall share our interview findings with a team led by Dr Dawn Stacey at the University of Ottawa to inform the design and evaluation of a planned pilot RCT of an ICD decision-support tool.
Acknowledgements
First, we would like to thank all the patients, relatives and clinicians who allowed us to observe their consultations and who took part in interviews and workshops. We would like to express our thanks to Jan Fuller and Lavinia Miceli for their excellent administrative support. We are also grateful to the members of the project advisory group for their valuable advice throughout the study development process, in particular, to our patient representatives, Mr Tom Bryden, Mr Tom Twedell, Mr Paul Cuskin and Mr Steve Whitely, and to Mrs Jo Jerrome (at the time, Atrial Fibrillation Association Deputy Chief Executive Officer).
Contributions of authors
Holly Standing (Research Assistant) contributed to data collection (observations, interviews and workshops), data interpretation and the drafting of the report.
Catherine Exley (Professor of Qualitative Health Research) developed the study protocol and contributed to the study design, data interpretation and the drafting of the report.
Darren Flynn (Senior Research Associate and Practitioner Health Psychologist) contributed to design and delivery of interactive group workshops, data interpretation and drafting of the report.
Julian Hughes (Honorary Professor of Philosophy of Ageing and Consultant in Old Age Psychiatry) developed the study protocol and contributed to the study design, data interpretation and the drafting of the report.
Kerry Joyce (Senior Research Associate) led PPI, developed the study protocol and contributed to the study design and data collection (observations and interviews).
Trudie Lobban MBE (Founder and Trustee of Arrhythmia Alliance: The Heart Rhythm Charity) developed the study protocol and contributed to the study design, data interpretation and the drafting of the report.
Stephen Lord (Consultant Cardiologist) developed the study protocol and contributed to the study design, data interpretation and the drafting of the report.
Daniel Matlock (Consultant in Palliative Medicine) developed the study protocol and contributed to study design, data interpretation and the drafting of the report.
Janet M McComb (Consultant Cardiologist) developed the study protocol and contributed to the drafting of the report.
Paul Paes (Consultant in Palliative Medicine) contributed to the study design, data interpretation and the drafting of the report.
Richard G Thomson (Professor of Epidemiology and Public Health) developed the study protocol and contributed to study design, data interpretation and the drafting of the report.
Outputs and dissemination
Outputs delivered to date
Papers
Joyce K, Lord S, Matlock DD, McComb J, Thomson RG. Incorporating the patient perspective: a critical review of clinical practice guidelines for implantable cardioverter defibrillator therapy. J Interv Card Electrophysiol 2013;36:185–97.
Conference presentations
Standing H, Joyce K, Exley C, Hughes J, Lord S, Thomson RG. Information Needs in Decision-Making about Implantation and Deactivation of Cardioverter Defibrillators (ICDs), paper presented to ISDM/ISEHC 2015, Sydney Australia, 19–22 July 2015.
Clinical meetings/engagement events
Standing H, Thomson R, Exley C, Joyce KE, Lord S, Hughes J, et al. Decision-making about implantation of cardioverter defibrillators (ICDs) and deactivation during end of life care. Northern England CVD Network Event, Durham, 23 April 2015.
Scheduled outputs and outputs in preparation
Papers are in preparation for submission to Social Science and Medicine and Implementation Science.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- National Service Framework for Coronary Heart Disease. Modern Standards and Servie Models. London: DH; 2000.
- NICE . Implantable Cardioverter Defibrillators and Cardiac Resynchronisation Therapy for Arrhythmias and Heart Failure 2014. www.nice.org.uk/guidance/ta314 (accessed 8 October 2016).
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 Guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:e350-408. http://dx.doi.org/10.1161/CIRCUALTIONAHA.108.189742.
- Redhead AP, Turkington D, Rao S, Tynan MM, Bourke JP. Psychopathology in postinfarction patients implanted with cardioverter-defibrillators for secondary prevention. A cross-sectional, case-controlled study. J Psychosom Res 2010;69:555-63. http://dx.doi.org/10.1016/j.jpsychores.2010.06.002.
- Agård A, Löfmark R, Edvardsson N, Ekman I. Views of patients with heart failure about their role in the decision to start implantable cardioverter defibrillator treatment: prescription rather than participation. J Med Ethics 2007;33:514-8. http://dx.doi.org/10.1136/jme.2006.017723.
- Russo JE. Original research: deactivation of ICDs at the end of life: a systematic review of clinical practices and provider and patient attitudes. Am J Nurs 2011;111:26-35. http://dx.doi.org/10.1097/01.NAJ.0000406411.49438.91.
- Elwyn G, Laitner S, Coulter A, Walker E, Watson P, Thomson R. Implementing shared decision making in the NHS. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c5146.
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2011;10. http://dx.doi.org/10.1002/14651858.cd001431.pub3.
- Joosten EAG, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom 2008;77:219-26. http://dx.doi.org/10.1159/000126073.
- Lewis KB, Stacey D, Matlock DD. Making decisions about implantable cardioverter-defibrillators from implantation to end of life: an integrative review of patients’ perspectives. Patient 2014;7:243-60. http://dx.doi.org/10.1007/s40271-014-0055-2.
- Goldstein N, Bradley E, Zeidman J, Mehta D, Morrison RS. Barriers to conversations about deactivation of implantable defibrillators in seriously ill patients: results of a nationwide survey comparing cardiology specialists to primary care physicians. J Am Coll Cardiol 2009;54:371-3. http://dx.doi.org/10.1016/j.jacc.2009.04.030.
- Kelley AS, Mehta SS, Reid MC. Management of patients with ICDs at the end of life (EOL): a qualitative study. Am J Hosp Palliat Care 2009;25:440-6. http://dx.doi.org/10.1177/1049909108320885.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004;141:835-8. http://dx.doi.org/10.7326/0003-4819-141-11-200412070-00006.
- Goldstein NE, Mehta D, Siddiqui S, Teitelbaum E, Zeidman J, Singson M, et al. ‘That’s like an act of suicide’: patients’ attitudes toward deactivation of implantable defibrillators. J Gen Intern Med 2008;23:7-12. http://dx.doi.org/10.1007/s11606-007-0239-8.
- Teno JM, Fisher ES, Hamel MB, Coppola K, Dawson NV. Medical care inconsistent with patients’ treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc 2002;50:496-500. http://dx.doi.org/10.1046/j.1532-5415.2002.50116.x.
- Woolf SH, Chan EC, Harris R, Sheridan SL, Braddock CH, Kaplan RM, et al. Promoting informed choice: transforming health care to dispense knowledge for decision making. Ann Intern Med 2005;143:293-300. http://dx.doi.org/10.7326/0003-4819-143-4-200508160-00010.
- INVOLVE . Examples of Public Involvement in Research Funding Applications: Decision Making About Implantation of Cardioverter Defibrillators (ICDs) and Deactivation During End of Life Care 2013. www.invo.org.uk/wp-content/uploads/2013/10/Example-4-publicinvolvement-in-funding-app2013.pdf (accessed 5 July 2016).
- Pycha C, Gulledge AD, Hutzler J, Kadri N, Maloney J. Psychological responses to the implantable defibrillator: preliminary observations. Psychosomatics 1986;27:841-5. http://dx.doi.org/10.1016/S0033-3182(86)72589-9.
- Cunningham AD, Plummer CJ, McComb JM, Lord SW, Cunningham MW, Toussaint JM, et al. The implantable cardioverter-defibrillator: postcode prescribing in the UK 1998–2002. Heart 2005;91:1280-3. http://dx.doi.org/10.1136/hrt.2004.048512.
- McComb JM, Plummer CJ, Cunningham MW, Cunningham D. Inequity of access to implantable cardioverter defibrillator therapy in England: possible causes of geographical variation in implantation rates. Europace 2009;11:1308-12. http://dx.doi.org/10.1093/europace/eup264.
- Stewart GC, Weintraub JR, Pratibhu PP, Semigran MJ, Camuso JM, Brooks K, et al. Patient expectations from implantable defibrillators to prevent death in heart failure. J Card Fail 2010;16:106-13. http://dx.doi.org/10.1016/j.cardfail.2009.09.003.
- Godolphin W. The role of risk communication in shared decision making. BMJ 2003;327:692-3. http://dx.doi.org/10.1136/bmj.327.7417.692.
- Langseth MS, Shepherd E, Thomson R, Lord S. Quality of decision making is related to decision outcome for patients with cardiac arrhythmia. Patient Educ Couns 2012;87:49-53. http://dx.doi.org/10.1016/j.pec.2011.07.028.
- Goldstein NE, Mehta D, Teitelbaum E, Bradley EH, Morrison RS. ‘It’s like crossing a bridge’: complexities preventing physicians from discussing deactivation of implantable defibrillators at the end of life. J Gen Intern Med 2008;23:2-6. http://dx.doi.org/10.1007/s11606-007-0237-x.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: Management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010;152:296-9. http://dx.doi.org/10.7326/0003-4819-152-5-201003020-00007.
- Operational Policy for the Deactivation/Reactivation of Implantable Cardioverter Defibrillator (ICD). Newcastle upon Tyne: NHS England Northern Clinical Networks and Senate; 2013.
- Joyce KE, Lord S, Matlock DD, McComb JM, Thomson R. Incorporating the patient perspective: a critical review of clinical practice guidelines for implantable cardioverter defibrillator therapy. J Interv Card Electrophysiol 2013;36:185-97. http://dx.doi.org/10.1007/s10840-012-9762-6.
- Consent: Patients and Doctors Making Decisions Together. London: GMC; 2008.
- Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Oxford and New York: Oxford University Press; 2008.
- Mental Capacity Act 2005. London: Her Majesty’s Stationery Office; 2005.
- Ashcroft RE, Dawson A, Draper H, McMillan J. Principles of Health Care Ethics. Chichester: John Wiley and Sons; 2007.
- Hursthouse R. On Virtue Ethics. Oxford: Oxford University Press; 1999.
- Equity and Excellence: Liberating the NHS. London: The Stationery Office; 2010.
- Capacity, Care Planning and Advance Care Planning in Life Limiting Illness. A Guide for Health and Social Care Staff. Leicester: Department of Health, National End of Life Care Programme; 2011.
- Cardiac Rhythm Management UK . National Clinical Audit Report 2010. www.ucl.ac.uk/nicor/audits/cardiacrhythm/documents/annual-reports/heart-rhythm10 (accessed 5 July 2016).
- National Institute for Cardiovascular Outcomes Research . National Audit of Cardiac Rhythm Management Devices 2013–14 n.d. www.ucl.ac.uk/nicor/audits/cardiacrhythm/documents/annual-reports/CRM_National_Annual_Report_2013-14 (accessed 5 July 2016).
- Ahmed N, Ellins J, Krelle H, Lawrie M. Person-Centred Care: From Ideas to Action 2014. www.health.org.uk/sites/health/files/PersonCentredCareFromIdeasToAction.pdf (accessed 5 July 2016).
- Brindis R, Spertus JA. President’s page: employing shared decision-making models to improve care and patient value: a cardiovascular professional initiative. J Am Coll Cardiol 2010;56:2046-8. http://dx.doi.org/10.1016/j.jacc.2010.11.004.
- Galla JH. Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians Association and the American Society of Nephrology. J Am Soc Nephrol 2000;11:1340-2.
- Matlock DD, Nowels CT, Masoudi FA, Sauer WH, Bekelman DB, Main DS, et al. Patient and cardiologist perceptions on decision making for implantable cardioverter-defibrillators: a qualitative study. Pacing Clin Electrophysiol 2011;34:1634-44. http://dx.doi.org/10.1111/j.1540-8159.2011.03237.x.
- Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, et al. A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Qual Saf Health Care 2007;16:216-23. http://dx.doi.org/10.1136/qshc.2006.018481.
- Flynn D, Nesbitt DJ, Ford GA, McMeekin P, Rodgers H, Price C, et al. Development of a computerised decision aid for thrombolysis in acute stroke care. BMC Med Inform Decis Mak 2015;15. http://dx.doi.org/10.1186/s12911-014-0127-1.
- University of Colorado . Patient Decision Aids – Implantable Cardioverter-Defibrillator (ICD) n.d. www.patientdecisionaid.org (accessed 5 July 2016).
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. http://dx.doi.org/10.1093/intqhc/mzm042.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. Thousand Oaks, CA: Sage Publications; 2006.
- Fetterman DM. Ethnography: Step-by-Step. London: Sage Publications; 2010.
- Silverman D. Doing Qualitative Research: A Practical Handbook. London: Sage Publications; 2005.
- GOV.UK . Guidance. Cardiovascular Disorders: Assessing Fitness to Drive 2016. www.gov.uk/guidance/cardiovascular-disorders-assessing-fitness-to-drive (accessed 12 October 2016).
- Rapley T. Distributed decision making: the anatomy of decisions-in-action. Sociol Health Illn 2008;30:429-44. http://dx.doi.org/10.1111/j.1467-9566.2007.01064.x.
- Sepucha K, Ozanne EM. How to define and measure concordance between patients’ preferences and medical treatments: a systematic review of approaches and recommendations for standardization. Patient Educ Couns 2010;78:12-23. http://dx.doi.org/10.1016/j.pec.2009.05.011.
- Sepucha KR, Levin CA, Uzogara EE, Barry MJ, O’Connor AM, Mulley AG. Developing instruments to measure the quality of decisions: early results for a set of symptom-driven decisions. Patient Educ Couns 2008;73:504-10. http://dx.doi.org/10.1016/j.pec.2008.07.009.
- Sepucha KR, Fowler FJ. Measuring Decision Quality: Where We Stand Today 2013. http://informedmedicaldecisions.org/wp-content/uploads/2013/03/Measuring_DQ_Sepucha_Fowler_Final.pdf (accessed 5 July 2016).
- Pedersen SS, Chaitsing R, Szili-Torok T, Jordaens L, Theuns DA. Patients’ perspective on deactivation of the implantable cardioverter-defibrillator near the end of life. Am J Cardiol 2013;111:1443-7. http://dx.doi.org/10.1016/j.amjcard.2013.01.296.
- Groarke J, Beirne A, Buckley U, O’Dwyer E, Sugrue D, Keelan T, et al. Deficiencies in patients’ comprehension of implantable cardioverter defibrillator therapy. Pacing Clin Electrophysiol 2012;35:1097-102. http://dx.doi.org/10.1111/j.1540-8159.2012.03448.x.
- Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making?. JAMA 1984;252:2990-4. http://dx.doi.org/10.1001/jama.1984.03350210038026.
- Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. BMJ 1999;319:766-71. http://dx.doi.org/10.1136/bmj.319.7212.766.
- Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6572.
- Hoffrage U, Lindsey S, Hertwig R, Gigerenzer G. Medicine. Communicating statistical information. Science 2000;290:2261-2. http://dx.doi.org/10.1126/science.290.5500.2261.
- Mulley AG. The need to confront variation in practice. BMJ 2009;339:1007-9.
- Sharkey K, Savulescu J, Aranda S, Schofield P. Clinician gate-keeping in clinical research is not ethically defensible: an analysis. J Med Ethics 2010;36:363-6. http://dx.doi.org/10.1136/jme.2009.031716.
- Deber RB, Kraetschmer N, Urowitz S, Sharpe N. Do people want to be autonomous patients? Preferred roles in treatment decision-making in several patient populations. Health Expect 2007;10:248-58. http://dx.doi.org/10.1111/j.1369-7625.2007.00441.x.
- Say R, Murtagh M, Thomson R. Patients’ preference for involvement in medical decision making: a narrative review. Patient Educ Couns 2006;60:102-14. http://dx.doi.org/10.1016/j.pec.2005.02.003.
- Coulter A. Implementing Shared Decision Making in the UK: A Report for the Health Foundation. London: Health Foundation; 2009.
- Flynn D, Forshaw M, Sheffield D. Health Psychology in Action. Chichester: Wiley-Blackwell; 2012.
- Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918-30. http://dx.doi.org/10.1111/j.1475-6773.2005.00438.x.
- Smith SG, Pandit A, Rush SR, Wolf MS, Simon CJ. The role of patient activation in preferences for shared decision making: results from a national survey of U.S. adults. J Health Commun 2016;21:67-75. http://dx.doi.org/10.1080/10810730.2015.1033115.
- Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff 2013;32:207-14. http://dx.doi.org/10.1377/hlthaff.2012.1061.
- Lorig K, Alvarez S. Re: Community-based diabetes education for Latinos. Diabetes Educ 2011;37. http://dx.doi.org/10.1177/0145721710393089.
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27:1361-7. http://dx.doi.org/10.1007/s11606-012-2077-6.
- King E, Taylor J, Williams R, Vanson T. The MAGIC Programme: Evaluation. An Independent Evaluation of the MAGIC (Making Good Decisions in Collaboration) Improvement Programme. London: Health Foundation; 2013.
- The Health Foundation . Implementing Shared Decision Making. Clinical Teams’ Experiences of Implementing Shared Decision Making As Part of the MAGIC Programme. Learning Report n.d. www.health.org.uk/sites/health/files/ImplementingSharedDecisionMaking.pdf (accessed 5 July 2016).
- Department of Health . Investing in General Practice – The New General Medical Services Contract 2003. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4071966 (accessed 8 October 2016).
- Coulter A, Collins A. Making Shared Decision-Making a Reality: No Decision About Me, Without Me. London: The King’s Fund; 2011.
- Montgomery V. Lanarkshire Health Board 2015. www.supremecourt.uk/decided-cases/docs/UKSC_2013_0136_Judgment.pdf (accessed 5 July 2016).
- Cinar FI, Tosun N, Kose S. Evaluation of an education and follow-up programme for implantable cardioverter defibrillator-implanted patients. J Clin Nurs 2013;22:2474-86. http://dx.doi.org/10.1111/jocn.12201.
- Vazquez LD, Conti JB, Sears SF. Female-specific education, management, and lifestyle enhancement for implantable cardioverter defibrillator patients: the FEMALE-ICD study. Pacing Clin Electrophysiol 2010;33:1131-40. http://dx.doi.org/10.1111/j.1540-8159.2010.02787.x.
- Dunbar SB, Dougherty CM, Sears SF, Carroll DL, Goldstein NE, Mark DB, et al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: a scientific statement from the American Heart Association. Circulation 2012;126:2146-72. http://dx.doi.org/10.1161/CIR.0b013e31825d59fd.
- Dunbar SB, Langberg JJ, Reilly CM, Viswanathan B, McCarty F, Culler SD, et al. Effect of a psychoeducational intervention on depression, anxiety, and health resource use in implantable cardioverter defibrillator patients. Pacing Clin Electrophysiol 2009;32:1259-71. http://dx.doi.org/10.1111/j.1540-8159.2009.02495.x.
- Eads AS, Sears SF, Sotile WM, Conti JB. Supportive communication with implantable cardioverter defibrillator patients: seven principles to facilitate psychosocial adjustment. J Cardiopulm Rehabil 2000;20:109-14. http://dx.doi.org/10.1097/00008483-200003000-00004.
- Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart 2002;87:488-93. http://dx.doi.org/10.1136/heart.87.5.488.
- Hoogwegt MT, Kupper N, Theuns DA, Zijlstra WP, Jordaens L, Pedersen SS. Undertreatment of anxiety and depression in patients with an implantable cardioverter-defibrillator: impact on health status. Health Psychol 2012;31:745-53. http://dx.doi.org/10.1037/a0028018.
- Hoogwegt MT, Kupper N, Jordaens L, Pedersen SS, Theuns DA. Comorbidity burden is associated with poor psychological well-being and physical health status in patients with an implantable cardioverter-defibrillator. Europace 2013;15:1468-74. http://dx.doi.org/10.1093/europace/eut072.
- Giudici MC, Carlson JI, Krupa RK, Meierbachtol CJ, Vanwhy KJ. Submammary pacemakers and ICDs in women: long-term follow-up and patient satisfaction. Pacing Clin Electrophysiol 2010;33:1373-5. http://dx.doi.org/10.1111/j.1540-8159.2010.02871.x.
- Walker RL, Campbell KA, Sears SF, Glenn BA, Sotile R, Curtis AB, et al. Women and the implantable cardioverter defibrillator: a lifespan perspective on key psychosocial issues. Clin Cardiol 2004;27:543-6. http://dx.doi.org/10.1002/clc.4960271019.
- Hoogwegt MT, Widdershoven JW, Theuns DA, Pedersen SS. Information provision, satisfaction and emotional distress in patients with an implantable cardioverter-defibrillator. Int J Cardiol 2014;177:586-8. http://dx.doi.org/10.1016/j.ijcard.2014.08.150.
- Flynn D, De Brôn A, Tomson D, Cameron A, Thomson RG. Barriers and Enabling Factors to Shared Decision Making in Primary Care for Mental Health Conditions n.d.
- Lie ML, Murtagh MJ, Watson DB, Jenkings KN, Mackintosh J, Ford GA, et al. Risk communication in the hyperacute setting of stroke thrombolysis: an interview study of clinicians. Emerg Med J 2015;32:357-63. http://dx.doi.org/10.1136/emermed-2014-203717.
- Thomson R, Edwards A, Grey J. Risk communication in the clinical consultation. Clin Med 2005;5:465-9. http://dx.doi.org/10.7861/clinmedicine.5-5-465.
- Gigerenzer G, Galesic M. Why do single event probabilities confuse patients?. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e245.
- Elwyn G, O’Connor AM, Bennett C, Newcombe RG, Politi M, Durand MA, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLOS ONE 2009;4. http://dx.doi.org/10.1371/journal.pone.0004705.
- Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns 2008;73:448-55. http://dx.doi.org/10.1016/j.pec.2008.07.023.
- Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38926.629329.AE.
- International Patient Decision Aid Standards (IPDAS) Collaboration . Update of the IPDAS Collaboration Background Document 2012. http://ipdas.ohri.ca/resources.html (accessed 5 July 2016).
- Flynn D, Ford GA, Stobbart L, Rodgers H, Murtagh MJ, Thomson RG. A review of decision support, risk communication and patient information tools for thrombolytic treatment in acute stroke: lessons for tool developers. BMC Health Serv Res 2013;13. http://dx.doi.org/10.1186/1472-6963-13-225.
Appendix 2 Observation materials
Observation grid
Date: Clinician:
| Indication (ICD only or CRT-D) | ||||||
|---|---|---|---|---|---|---|
| Information about condition and risk of sudden death | ||||||
| Information about device (what it looks like and how it works) | ||||||
| Device function (does not improve symptoms) | ||||||
| Philosophical/existential discussion around desired mode of death | ||||||
| Benefit of device | ||||||
| Risks of device (complications); risk communication (e.g. absolute/relative risk, natural frequencies, same denominator) | ||||||
| Risks of device (mental health) | ||||||
| Impact of device on quality of life (physical activity, sexual activity) | ||||||
| What might happen to the device in the future |
Observation coding frame
-
Implantation.
Understanding views and beliefs
-
Understanding of need for ICD/perception of illness.
-
Understanding of ICDs:
-
ICD as prevention not therapy
-
benefits
-
improvements.
-
-
Understanding procedure and risks.
-
Personalising/anthropomorphising.
-
Previous experiences.
-
ICD as reminder of heart problems.
-
Impact on identity:
-
disruption to ‘healthy person’ identity
-
becoming the ‘bionic man’.
-
-
Effects on significant others.
-
Reliance on hospitals.
-
Feelings about surgery.
-
Reconsideration of the device versus one off offer.
Information giving
-
Presentation of the device:
-
being tested for suitability
-
comparison to other treatments
-
risk
-
difficulties of communicating complex information.
-
-
Misunderstandings of the ICD:
-
denial of need.
-
-
Prior understandings/understanding at tertiary appointment.
-
Gaps in information provision – changes to mode of death.
-
Understanding of condition.
-
Information needs:
-
honest and open
-
tailored
-
ability to absorb information after heart event.
-
-
Hierarchies of knowledge.
-
Written information:
-
GP letters.
-
-
Support group/other patients.
-
Impact of negative information.
Decision-making
-
Driving
-
Living longer/survival.
-
Lack of choice.
-
Back-up for the future.
-
Improvements in daily life:
-
quality of life improvements
-
ability to do mundane daily activities
-
ability to do valued activities.
-
-
Age.
-
Experiential knowledge.
-
Time.
-
Views of others:
-
trusted others
-
other patients.
-
-
Clinician–patient relationship:
-
SDM
-
patient more than ‘file notes’
-
clinicians power to persuade
-
trust in clinicians
-
maintenance of relationship
-
hierarchies.
-
Living with the device
-
Expectations versus lived experience:
-
materiality
-
physical appearance
-
ability to forget the device
-
expectation of fix.
-
-
Support:
-
ongoing support from clinicians/hospital
-
social support.
-
-
Problems with the device.
-
Impacts of device:
-
impacts on daily life
-
disruption to other treatments
-
avoidance of activities
-
device as reassurance
-
impact on mind and body
-
impact on significant others
-
improvements to quality of life.
-
-
Future with ICD.
-
Shocks:
-
unaware of discharge
-
experience of others’ discharges
-
experience of own shocks
-
shocks = good thing
-
impact on significant others
-
expectation/anticipation of shocks.
-
Deactivation
-
Reversibility of decision.
-
Desire to keep it activated.
-
Patient decision versus clinical.
-
Clinical barriers:
-
triggers for discussion
-
identification of potential deactivation patients
-
difficulties ascertaining where patient is in discussions
-
negotiation between different clinician groups
-
clinician avoidance of end-of-life discussions
-
need to raise profile of ICDs
-
inappropriate terminology
-
need to routinise discussions.
-
-
Timing:
-
advanced discussions
-
‘right’ time for discussions.
-
-
Actors involved:
-
cardiologists
-
palliative care.
-
-
Ethical issues:
-
deactivation = suicide
-
active removal of treatment.
-
-
Understanding of deactivation:
-
confusion of process
-
patient misunderstandings
-
clinician misunderstandings
-
significance of the heart.
-
Appendix 3 Interview materials
Invitation letter
Participant information sheet
Consent form
Interview schedules
Interview topic guide (pre-implantation patients)
Interview topic guide (pre-implantation patients)
Interview topic guide (family)
Interview topic guide (decliners)
Interview topic guide (post-implantation patients)
Interview topic guide (post-implantation patients)
Interview topic guide (prospective deactivation)
Interview topic guide (cardiologists)
Interview topic guide (physiologists)
Interview topic guide (specialist nurse)
Interview topic guide (psychologists)
Interview topic guide (palliative care clinicians)
Clinician cover letter for bereaved relative interviews
Appendix 4 Workshop slides and materials
Health-care professional workshop slides
Patient/family member workshop slides and materials
List of abbreviations
- CRT
- cardiac resynchronisation therapy
- CRT-D
- cardiac resynchronisation therapy with defibrillator
- CRT-P
- cardiac resynchronisation therapy with pacemaker
- DGH
- district general hospital
- DNAR
- do not attempt resuscitation
- GP
- general practitioner
- ICD
- implantable cardioverter defibrillator
- MAGIC
- MAking Good decisions In Collaboration
- MBE
- Member of the Most Excellent Order of the British Empire
- NICE
- National Institute for Health and Care Excellence
- PP
- primary prevention
- PPI
- patient and public involvement
- SDM
- shared decision-making
- SP
- secondary prevention