Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 13/05/12. The contractual start date was in November 2014. The final report began editorial review in September 2015 and was accepted for publication in January 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Chambers et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
This rapid review was undertaken to address a topic suggested by Professor Erika Denton, National Clinical Director for Diagnostics at NHS England, and identified as a priority by the Department of Health Research and Development Committee. The purpose of the evidence synthesis is to assess the evidence base for diagnostic services provided outside hospital settings, for example in the community or in general practice. The findings will be expected to provide actionable messages for the NHS and/or identify priorities for further research.
Diagnostic tests and their results are fundamental to clinical decision-making. In the UK NHS, general practitioners (GPs) and other primary care professionals have traditionally had a limited ability to access such tests directly. Instead, a common model is for GPs to refer patients for blood, tissue or imaging tests at the local hospital, sometimes resulting in waits for appointments and availability of test results. Offering more diagnostic tests in the community could enable faster and earlier diagnosis of common conditions and avoid unnecessary referrals. Other potential benefits include greater convenience and lower costs for patients and possibly a reduction in numbers of missed appointments. The Health and Social Care Information Centre has reported that in the year to March 2014, over 4.3 million patients accounted for 6.8 million missed outpatient appointments, at an estimated cost of £108 per appointment. 1
Diagnostic tests cover the whole range of clinical conditions, and this review, although not aiming at exhaustive coverage, will include those tests and conditions most relevant to primary care and community settings. A potentially important distinction is between tests (e.g. most types of imaging) that require specialist equipment and/or staff to administer and those that can be administered by any health professional or the patient him- or herself in any setting or at home. Following a preliminary mapping of the literature and further discussion with stakeholders, it was decided to focus primarily on the first group of tests in view of the broader implications for the NHS of any changes to the way these services are delivered, for example in relation to staff training, workforce composition and requirements for equipment and suitable premises in which to perform the tests.
Greater availability of diagnostic testing in primary care is supported by both policy and technological drivers. Current NHS policy supports initiatives aimed at early diagnosis of long-term conditions with an emphasis on management in the community as far as possible. The remainder of this introduction briefly discusses the technological, economic and social factors relevant to the provision of diagnostic services in primary care and community settings.
Technology
Technological drivers are based on a range of advances whose effect is to increase the speed, size and range of devices suitable for primary care that can provide accurate measurements of a wide range of biochemical, microbiological and haematological parameters. 2 Glucometers have transformed the speed and ease of obtaining accurate blood glucose levels, and urine dipsticks have removed the need for microscopy in many patients. 2 Electronics increase the portability and ease of use, and reduce the cost, of electrocardiograms (ECGs), ultrasound, spirometry and pulse oximetry. Mobile magnetic resonance imaging (MRI) is more easily accommodated on GP premises. Partially stimulated by direct-to-consumer demand, an array of point-of-care (POC) tests, either in the marketplace or in the developmental pipeline, offers the potential to transform cardiac services, diabetic services and genetic testing. Devices such as endoscopes are easier and safer to use.
Diagnostic services that could, in future, be delivered closer to home include blood tests, audiology, plain film X-rays, ultrasound, 70% of pathology (non-slide-based, non-specialist work), echocardiology, endoscopy, colposcopy, international normalised ratio (INR) testing, 24-hour ECG monitoring, exercise ECG testing, ambulatory blood pressure (BP) monitoring, nerve conduction studies, Helicobacter pylori tests and lung function tests. 3 A polyclinic-type diagnostic service in Whitstable already offers digital X-ray, ultrasound, echocardiography, upper endoscopy and mobile MRI. 4
Equipment requirements for general practice are not generally well documented. Our desk-based research identified a chapter on Equipment and Premises in General Practice in the Oxford Textbook of Primary Medical Care. 5 The Royal Australian College of General Practice maintains accreditation standards around practice equipment in the Royal Australian College of General Practice Standards for General Practice (4th Edition). 6 Otherwise, requirements for equipment are defined by professional associations with little recognition of which should be housed within primary care. Surveys of equipment in general practice are fairly uncommon. A 2010 survey of equipment among GPs in Ireland found that 83% of practices had an ECG machine, 80% had a 24-hour BP monitor and 64% had a spirometer. 7 It is unclear how such figures might map to a UK context, given that prevalence may relate to both investment and rurality. However, a survey in a UK cardiac network found that 85% had 12-lead ECG machines, close to the corresponding figure in the Irish study, and 91% used these on a weekly or more frequent basis. GP and practice characteristics are influential, while ‘learning-by-using’ also affects the adoption of medical equipment in a general practice setting. 8
Access to imaging and other tests remains relatively restricted. To ensure smoother patient pathways and speedier diagnoses, GPs need increased access to imaging, equivalent to that available to hospital doctors, on the basis of the clinical needs of patients. With improved access to imaging, the Chief Scientific Officer promoted an initiative to improve access to so-called ‘physiological measurements’. 3 General practitioners with a special interest (GPwSIs) can perform diagnostic procedures, including endoscopy, in primary care settings. However, an expanded role may compromise the essential roles and function of general practice and has implications for service capacity, facilities and trained staff. Although championing a drive to improve services and diagnostics in the community, those working in primary care are keen that it is not seen merely as a ‘conduit’ to deliver secondary care-type services – the extra services must be integrated in a model that enshrines the values, philosophy and strategic function of general practice. 3
Economics
Increases in test utilisation are driven by guidelines to screen more patients and monitor them more frequently. 9 Diagnostic services, traditionally provided and housed in acute hospitals, have struggled to cope with demand. 10 Increased demand often leads to increased delays and longer waiting lists. Diagnostic departments have sought to redesign their services with a market focus. However, they find it difficult to match the pace and scale of demand.
Efforts have been made to identify those diagnostic tests that offer the best clinical and economic value for primary care. A ‘Horizon Scanning’ approach, funded by the National Institute for Health Research (NIHR), has produced a series of structured assessment reports to examine the research evidence, including technical accuracy, clinical utility and cost-effectiveness. 11 Pressures are increasing to perform more tests, more rapidly, reducing referrals, keeping patients informed and reducing the risk of diagnostic errors. Reductions in health service funding demand that improved performance is balanced with improved cost-effectiveness. 2 Such an approach, although technically invaluable, stops short of concerns around service redesign. Material concerns (including the test platform, equipment, reagents and supplies) must extend to cover health professionals, their roles, their relations and the sociocultural context in which testing occurs. More operational research is required into health system requirements and the impact of technologies on diagnostic accuracy, retesting and diagnostic delays. 12 Factors associated with reconfiguration in the health system relate to skills, training, cost, equipment, premises, and referral linkages between primary and secondary care.
Social factors
Primary care physicians need to be empowered to redesign diagnostic services. For them to do this requires that perceived barriers and facilitators within current diagnostic services be identified and then overcome. A 2013 review of qualitative studies revealed that primary care clinicians believed that POC testing improved diagnostic certainty, targeting of treatment, self-management of chronic conditions and clinician–patient communication and relationships. 13 At the same time, clinicians expressed concerns about test accuracy, over-reliance on tests, undermining of clinical skills, cost and limited usefulness. Additional factors include diagnostic uncertainty, patient anxiety and litigation, among others. 2
Tests that are developed do not necessarily mirror what GPs and other practice staff (e.g. practice nurses, district nurses and midwives) actually want. 2 The diagnostic test industry does not always consider which tests GPs would use most often, and which tests to prioritise for research and development. 2
The primary focus of this review is on services rather than individual tests. For example, one model of service is a community diagnostic centre offering a range of tests such as radiography, ultrasound, spirometry and electrocardiography, as described by Hollins et al. 14 Other models include mobile services providing a specific imaging technology such as MRI, and condition-specific services for conditions such as suspected respiratory disease. In assessing the evidence, it is important to take account of the specific needs of tests to be administered in primary care/community settings (both clinical criteria and practical requirements such as premises, equipment and workforce training).
Chapter 2 Review methods
The review aimed to address the following questions:
-
What models of community diagnostic services currently exist (in the UK and internationally)?
-
What is the evidence for quality, safety and clinical effectiveness of different models of diagnostic service provision outside hospital settings?
-
Which tests are most commonly provided and is there any evidence for an effect on outcomes?
-
Is there any evidence to support a broader range of diagnostic tests being provided in the community? This question was interpreted to refer to:
-
tests that are not currently offered in community settings but which could be appropriate for such use
-
organisational models, such as larger diagnostic centres, compared with single-service models.
-
Protocols were developed to guide the overall methods and conduct of the review and subsequently for each of the focused reviews reported in Chapters 4–6. Copies of these documents are available on the project website. 15
We performed the review in two stages. We carried out an initial mapping exercise to assess the quantity and nature of the available research evidence. Full details of the mapping exercise are presented in Chapter 3. The results of this exercise, together with discussions involving the review team, our funders and clinical experts, were then used to identify areas to focus on in more detail, as follows.
-
Logistics of diagnostic modalities in primary care (see Chapter 4): this review reflects the finding from the mapping exercise of a variety of different diagnostic technologies with different implications arising from being located in primary care. It was agreed that it would be helpful to characterise key technologies (e.g. radiology, audiology, POC testing) against a common set of logistic and service delivery considerations. This review was undertaken as a framework map and synthesis.
-
Diagnostic ultrasound services (see Chapter 5): a more detailed focused review of ultrasound services was also performed. The mapping exercise revealed some evidence on provision of these services and ultrasound is used in diagnosing a wide range of conditions. In addition to seeking comparative evidence around different service models, this review focused on issues around equipment (small portable scanners, including handheld devices, are available) and training.
-
Primary care-led diagnostic pathway (see Chapter 6): this review covered the differential diagnosis of breathlessness and examined issues related to decision-making and the availability of multiple tests in primary care/community settings.
Protocols were developed for each of these focused reviews before further searching was started. Full details of review methods are reported in the relevant chapters. The findings are reported separately, but overall implications for service delivery and organisation are considered in the overall discussion section (see Chapter 7).
Chapter 3 Literature mapping exercise
Introduction
The objective of this initial phase of the review was to map and broadly describe the published literature on diagnostic testing services in community and primary care settings, particularly that relevant to the UK NHS and similar health-care systems. We intended to use the results of this mapping exercise to guide decisions about further focused review work. As a mapping review seeks to characterise a large body of literature by quantity and study characteristics, we did not perform formal data extraction or quality assessment. As the aim was to acquire a broadly representative, not exhaustive, sample, we did not perform any grey literature or citation searches at this stage and restricted the search to one bibliographic database.
For the purposes of this mapping exercise, we applied broad inclusion criteria and in particular did not seek to exclude studies of tests and programmes that were described as ‘screening’ rather than ‘diagnostic’. The terminology related to diagnostic and screening tests is not always clear or consistently applied in the literature and we therefore adopted an inclusive approach to scanning and coding the references identified by the literature search.
Methods
A single database [Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R), 1946 to present] was searched for the mapping exercise. The search strategy combined terms around ‘primary care’ and ‘diagnostics’, and included both medical subject heading (MeSH) and free-text searches. In addition, methodological search filters were applied to retrieve two different study types:
-
reviews (including non-systematic reviews)
-
comparative studies.
Results were further limited by date to 2000 to current (December 2014) to enable us to map a reasonable range of literature without including older studies of limited relevance to current practice. The full search strategies and further details of the search filters are provided in Appendix 1.
Search results were stored in a reference management database (EndNote X7.5, Thomson Reuters, CA, USA) and exported to Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) for coding purposes. Records that appeared potentially relevant were coded as far as possible for condition/population studied, intervention/technology, country, setting, type of study (e.g. primary research, systematic review or narrative review) and focus of study (service delivery, test performance or both). Studies were selected for coding that mentioned any kind of test (diagnostic or screening, including questionnaires or similar) when it appeared that a substantive component of the testing, diagnosis, analysis and interpretation took place in a primary care or similar setting. We tried to exclude studies from settings in which the findings would clearly not be relevant to service delivery in the UK NHS (e.g. tropical diseases in low-income countries). Coding was based on title and abstract (where available) only. A coding scheme covering the major conditions and technologies expected to be found in the literature was developed by one of the authors (DC). Studies dealing with multiple conditions and/or technologies were coded as such so that each study had only one condition and one technology code. A code of ‘unclear’ was also available for any studies in which the specific condition or technology could not be identified from the available information.
The findings were synthesised narratively by condition and technology. For studies judged to be most relevant to service delivery issues, key details were extracted and tabulated.
Results
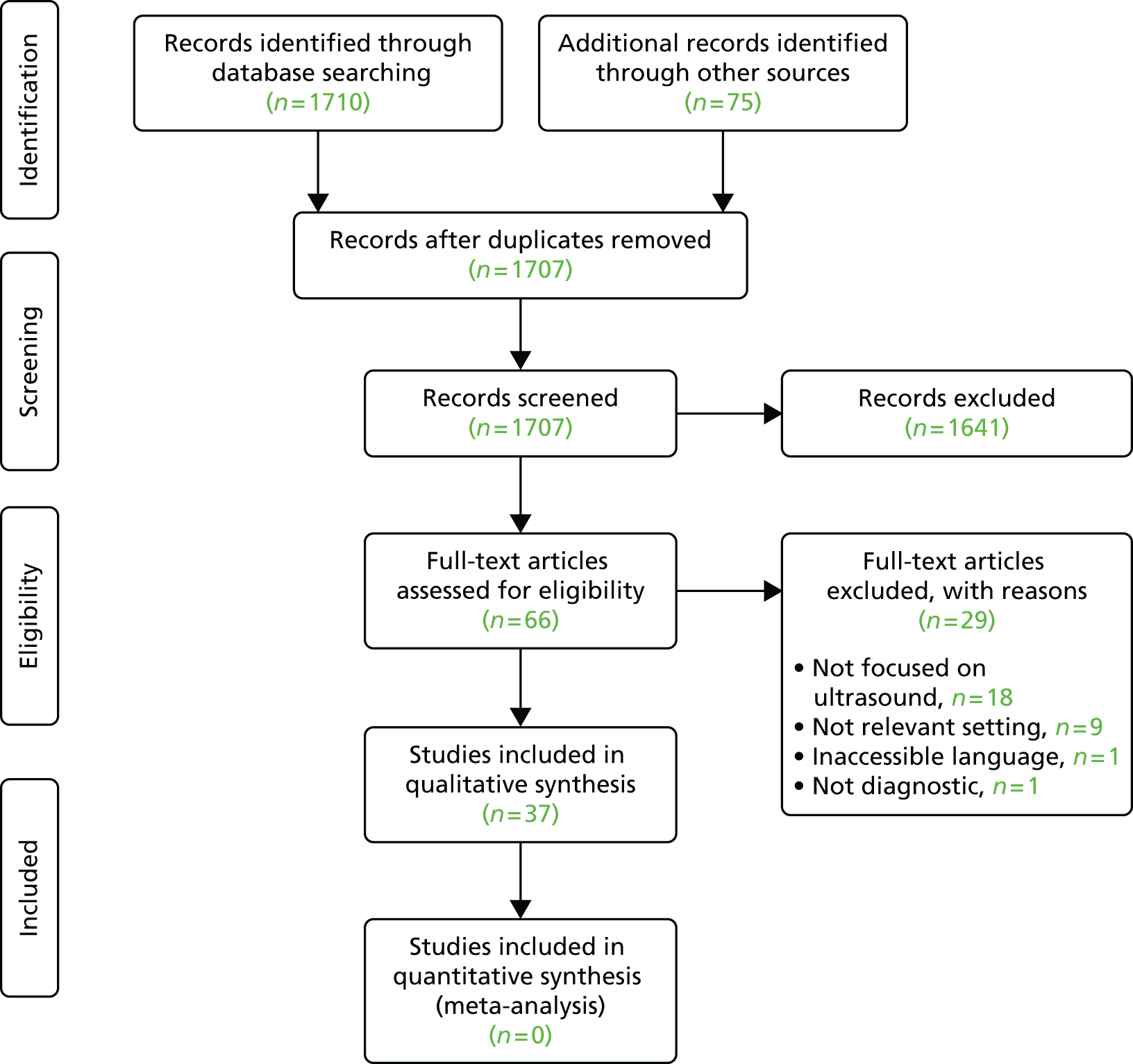
The searches identified 2644 records, of which 309 were identified by the review filter. Overall, we coded 302 records (11.4%) as meeting the inclusion criteria for the mapping exercise. This total includes 30 records included after discussion of 46 queries on inclusion between the authors involved (AB and DC).
Summary of findings
Comparatively few studies (eight reviews and 20 comparative studies) focused on different models of providing diagnostic services. Two of the most relevant studies were those by Pallan et al. 16 and Voutilainen and Kunnamo. 17 We are aware that other studies of this type, not included in the search results, would be picked up by a search of additional data sources (including grey literature). Most studies focused on test performance (e.g. diagnostic accuracy in a primary care population/setting). More details and examples of these types of service-focused studies are listed below.
The largest number of studies (10 reviews and 23 comparative studies) looked at diagnosis of mental health conditions, followed by cancer, cardiac disease, acute infections, dementia and chronic obstructive pulmonary disease (COPD)/respiratory disease (Table 1). Other conditions formed a large miscellaneous group but included studies of primary care diagnosis of Helicobacter pylori infection, other gastrointestinal (GI) conditions and low-back pain. Many studies examined multiple technologies, questionnaires/scales or POC tests (Table 2). A sizeable group of studies (26 comparative studies) examined test behaviour in primary care, that is, health professional and patient attitudes and behaviours towards diagnostic testing and interventions aimed at influencing these. Studies of COPD mainly evaluated use of spirometry in primary care settings and addressed some issues relevant to service delivery.
| Population/condition | Review filter | Comparative study filter | Total |
|---|---|---|---|
| Mental health | 10 | 23 | 33 |
| Cancer | 9 | 22 | 31 |
| Heart disease | 2 | 26 | 28 |
| Acute infection | 4 | 20 | 24 |
| Dementia | 6 | 10 | 16 |
| Multiple conditions (using the same diagnostic technology or technologies) | 3 | 12 | 15 |
| Diabetes | 14 | 14 | |
| COPD | 1 | 12 | 13 |
| Neurological conditions | 2 | 6 | 8 |
| HIV | 5 | 5 | |
| Other STI (syphilis 1; unclear 4) | 5 | 5 | |
| Chlamydia | 2 | 1 | 3 |
| Asthma/allergy | 3 | 3 | |
| Liver disease | 2 | 2 | |
| IBS/IBD | 2 | 2 | |
| Renal disease | 1 | 1 | |
| Other | 12 | 87 | 99 |
| Total | 55 | 247 | 302 |
| Technology | Review filter | Comparative study filter | Total |
|---|---|---|---|
| Questionnaires/scales | 22 | 49 | 71 |
| Multiple | 19 | 36 | 55 |
| Other | 3 | 53 | 56 |
| POC testing | 8 | 34 | 42 |
| Test behaviour | 26 | 26 | |
| Unclear | 1 | 8 | 9 |
| Ultrasounda | 6 | 6 | |
| Imaging | 6 | 6 | |
| Endoscopy | 6 | 6 | |
| Spirometry | 6 | 6 | |
| Natriuretic peptides | 2 | 4 | 6 |
| ECG | 5 | 5 | |
| X-ray | 4 | 4 | |
| MRI | 2 | 2 | |
| Skin prick | 2 | 2 | |
| Total | 55 | 247 | 302 |
Reviews
The search using the review filter identified 309 records, of which 55 (18%) were coded as potentially relevant; of these, 37 appeared to be systematic or non-systematic reviews and the remainder were other types of study retrieved because of the sensitivity of the filter.
Of all coded reviews, eight were classified as focusing mainly on service (service delivery, organisation, staffing, patient experience/outcomes, etc.);18–25 16 were classified as focusing mainly on both service and test performance; and the remaining 31 were mainly concerned with test performance (diagnostic accuracy, etc.). The largest groups of reviews dealt with mental health (10 papers), cancer (nine papers) and dementia (six papers). Twelve reviews covered other conditions including obesity and alcohol abuse. The main types of diagnostic test reported by these reviews were questionnaires and scales (22 reviews) and POC testing (eight reviews), while 19 reviews were coded as covering multiple diagnostic technologies.
Among the reviews focusing on service delivery-related topics (Table 3), the largest group was related to cancer diagnosis and screening. 21,23,24 Others dealt with various conditions including chronic fatigue syndrome,19 depression,20 respiratory disease22 and arthritis. 25 Five reviews appeared to consider diagnostic tests and technologies in the context of broader patient management pathways. 19–22,25
| Reference | Condition | Technology | Service topic |
|---|---|---|---|
| Baricchi et al. 201218 | General | Pathology tests | Test ordering |
| Bayliss et al. 201419 | Chronic fatigue syndrome | General | Physician behaviour |
| Bijl et al. 200420 | Depression | General | Clinical effectiveness |
| Mitchell et al. 200821 | Cancer | General | Diagnostic delay |
| O’Byrne et al. 201022 | Respiratory disease | General | Referral |
| O’Malley et al. 200223 | Cancer | Mobile clinics | Population screening |
| Smith et al. 201424 | Cancer | General | Workforce |
| Villeneuve et al. 201325 | Arthritis | General | Referral |
Comparative studies
A total of 247 records, identified by the search terms combined with a comparative studies filter, were coded as potentially relevant for at least one type of diagnostic test or technology. It was difficult to assess how many of these were genuine comparative studies based on the limited information available from the title and abstract.
In terms of conditions (see Table 1), the largest numbers of comparative studies dealt with mental health conditions (n = 23), heart disease (n = 26) and cancer (n = 22). However, 87 studies related to conditions not covered by our pre-specified list of common diseases. The most commonly studied single technologies (see Table 2) were questionnaires/scales (n = 49 studies) and POC tests (n = 34). A substantial number of studies (n = 26) dealt with test behaviour (primarily physicians’ attitudes and behaviour around ordering or performing diagnostic tests).
Twenty16,17,26–43 of the coded comparative studies were considered to relate to aspects of service delivery and organisation (Table 4) and 30 dealt with both service delivery and test performance/accuracy. The remaining coded comparative studies were mainly relevant to aspects of test accuracy in primary care or community settings.
| Reference | Condition | Technology | Service topic |
|---|---|---|---|
| Arber et al. 200626 | Heart disease | General | Physician behaviour |
| Ayoub et al. 200927 | Osteoporosis | General | Service models |
| Chan et al. 200528 | Attention deficit hyperactivity disorder | General | Physician behaviour |
| Chey et al. 200529 | Gastro-oesophageal reflux disorder | General | Physician behaviour |
| Hay et al. 200930 | Peripheral vascular disease | General imaging | Costs |
| Madurell et al. 201031 | Acute infection | Rapid antigen detection | Decision-making |
| Maserejian et al. 201432 | Musculoskeletal pain | General imaging | Physician behaviour |
| Murchie et al. 201233 | Cancer | General | Diagnostic delay |
| Nucci et al. 200434 | Diabetes | General | Population screening |
| Oliveria et al. 200235 | Cancer | General | Workforce |
| Pallan et al. 200516 | General | Ultrasound | Service models |
| Parkins and Edgar 201136 | Glaucoma | General | Referral |
| Pearson et al. 200637 | Respiratory disease | General | General |
| Pérez-Martínez and Puente-Muñoz 200638 | Neurology | General | Referral |
| Peterson and Peterson 200439 | Heart disease | Cardiac catheterisation | Safety |
| Poon et al. 200440 | General | General | Test result management |
| Smeeth et al. 200341 | Visual impairment | General | Clinical effectiveness |
| Tsianakas et al. 201042 | Sickle cell/thalassaemia | Genetic testing | Physician attitudes |
| Urban et al. 201243 | General | General | Workforce |
| Voutilainen and Kunnamo 200517 | Cancer | Endoscopy | Service models |
As far as could be judged from the title and abstract, the majority of the comparative studies were focused on specific conditions such as cancer or heart disease and included a range of diagnostic technologies (noted as ‘general’ in Table 4). A few studies covered particular diagnostic tests or technologies, for example genetic testing,42 ultrasound16 and endoscopy. 17 The studies were conducted in a range of different health-care systems, with only three performed in the UK. 16,36,37
Only three of the coded studies primarily looked at service models for the delivery of diagnostic tests in primary care or community settings. 16,17,27 Other studies examined related topics such as clinicians’ behaviour and attitudes,28,29,32 workforce issues (e.g. whether doctors or other health-care personnel administer the tests),35,43 resource use and costs,30 and impact of community diagnostics on decision-making about patient management (e.g. prescribing and referrals to other health professionals). 31,36,38 One study compared cancer diagnostic delays across different health systems with a key role for primary care. 33
Discussion
Main findings and implications
The search results indicated a substantial body of research on diagnostic testing in primary care and community settings. However, only three primary studies that reviewed or compared different models of service were found. 16,17,27 and there were no reviews in this category. Among either reviews (including all studies identified using the review filter) or primary studies, most papers that were coded as potentially relevant to the topic focused on aspects of test accuracy and performance in primary care/community settings and populations.
The reviews that were more directly relevant to service delivery dealt with a range of conditions and topics (see Table 3). A major theme of these papers was to examine diagnostic tests in the context of patient management pathways and decision-making processes rather than in isolation. This finding helped to support the subsequent decision of the review team to propose to examine the evidence base around a selected primary care diagnostic pathway (symptom-based pathways for patients presenting with breathlessness) and its individual components in more depth (see Chapter 6).
Studies identified with the help of the comparative studies filter covered a similar range of technologies and conditions to those documented by the reviews. As with the reviews, the focus was mainly on test accuracy rather than on service delivery, although several service-focused papers were identified (see Table 4). Many papers dealt with multiple diagnostic technologies for a particular condition or group of conditions.
The findings of the mapping exercise suggested that it would be appropriate to examine a particular diagnostic technology in some depth, as this had not been done by any of the reviews that we identified. Ultrasound was chosen because it is a key diagnostic technology for a wide range of clinical conditions; provision of diagnostic ultrasound in the community has been possible since the 1990s44 and recent developments in equipment could potentially change the balance between different models of service;45 and improving access to ultrasound to support early diagnosis of cancer (particularly ovarian cancer) is a priority for the NHS.
Another finding from the mapping exercise that seemed to require further analysis was the wide range of diagnostic technologies included and the different implications for providing them in community settings. The requirements for equipment, staff (including training) and premises, and associated costs, differ widely between, for example, in vitro POC tests and MRI. In view of this, we proposed to carry out a further piece of work to identify key logistic and service delivery considerations associated with the introduction and ongoing provision of diagnostic services in community or primary care settings (see Chapter 4).
One of the largest groups of studies looked at questionnaires and scales for diagnosis of mental health or neurological conditions. Although important, the delivery of these tests is not influenced by the same range of considerations that affects diagnostic technologies such as endoscopy or spirometry, as there is no requirement for special equipment or premises. Papers on questionnaires and scales were, therefore, excluded from further consideration in the review.
An important negative finding from this mapping exercise is that the published evidence does not include an adequate description or evaluation of the wide range of service models currently being commissioned (or that might feasibly be commissioned) for diagnostic services in the NHS. In particular, services (e.g. mobile laboratories, diagnostic co-operatives and open-access diagnostic services) may be designed to impact on primary care without necessarily being located in a primary care or community setting. This limits our ability to assess the relevance of the evidence found to future commissioning decisions. Even a fully comprehensive mapping review will be of little practical use to decision-makers if the published evidence does not reflect the range of services being commissioned in practice. We proposed to explore this issue further by mapping the range of current NHS diagnostic ultrasound services as part of the focused review of that technology (see Chapter 5).
Limitations
This mapping exercise was designed to provide a preliminary scoping assessment of the extent and nature of the research evidence relevant to community diagnostic services. Insights provided by examining and coding the search results are intended to assist the review team in deciding where to concentrate our efforts for the remainder of the project. As such, we did not expect the search to be in any way comprehensive: only one bibliographic database (MEDLINE) was searched and we did not examine other potential sources of references such as grey literature and internet searching. Filters for reviews and comparative studies were used in the searches in an attempt to identify the best available evidence and to optimise use of the time and resources available. However, these filters trade off sensitivity and specificity, and some of the studies retrieved by the review search were not identifiable as systematic or narrative literature reviews.
Papers were coded as potentially relevant if they referred to diagnostic or similar tests performed in primary care or community settings. Coding sought to obtain a broad picture of the literature but meant that many studies coded were peripheral to the main objectives of the project. In particular, we coded numerous studies that evaluated diagnostic scales and questionnaires; subsequent discussion indicated that findings of such studies would not be generalisable to diagnostic technologies with greater resource requirements for, for example, equipment.
The coding of records for inclusion was shared between two researchers, and uncertainties were resolved by discussion with a small sample of records being checked by a second reviewer. Use of simple classification system for health conditions sped up the coding process but meant that a higher than optimum proportion of records was classified under the heading of ‘other conditions’. All coding decisions were based on information available in the record title and abstract, if available, as it was not felt to be justified to obtain full-text papers for a preliminary literature mapping exercise.
Conclusions
The results of the mapping exercise suggest that there is a relatively large body of research on diagnostic testing in primary care/community settings, with 302 papers (11.4% of those identified by the search strategy) being coded as potentially relevant. However, only a few studies evaluated or described models of service, with most focusing on test accuracy in primary care populations. The search used broad search terms and covered only one database, so it was designed to be representative, not comprehensive. The review team has no reason to believe that these results are not broadly representative of the overall body of evidence.
Examination of the identified papers supported the need for further focused reviews to synthesise the research evidence in more depth and identify any implications for practice and research. The review team proposed that they might focus on:
-
ultrasound, as a key diagnostic technology affected by recent developments in technology (see Chapter 5)
-
the widely differing logistic and service delivery implications of introducing different diagnostic technologies in primary care/community settings (see Chapter 4)
-
diagnostic pathways delivered in primary care, using differential diagnosis of breathlessness as an exemplar of a symptom-driven pathway (see Chapter 6).
Based on the results of the mapping exercise, it appeared that at least some evidence would be available to inform each of these areas. Given the diverse nature of these three focused reviews, separate protocols and search strategies were developed for each.
Chapter 4 Logistics of diagnostic modalities in primary care: a framework map and synthesis
Executive summary
Introduction
Recent years have witnessed rapid and significant advances in diagnostic technologies and their supporting technological and communications infrastructures. Numbers of tests performed continue to increase as patients, carers and clinicians seek more accurate and rapid diagnoses and treatment selection. 2 With such demand comes an imperative to locate testing procedures as close as possible to the point of first presentation by the patient, typically requiring service reconfiguration from acute hospitals to primary care. The reconfiguration of diagnostic services involves multifactorial considerations relating to the workforce, equipment and communication among stakeholders. The synthesis team has devised a framework entitled STEP-UP (Skills, Training, Equipment, Premises, User Perspectives and Primary–secondary interface) as a lens through which to examine 13 primary care diagnostic topics [audiology, cardiac services (including specifically ECG and echocardiography), diabetic services, endoscopy, genetic testing, MRI, POC testing, radiology/X-ray, respiratory tests (including specifically spirometry) and ultrasound]. Topic selection sought to examine a wide variety of logistic and implementation considerations and to model disease-specific, system specific and technology-specific topics. These 13 topics were not intended to be either exhaustive or mutually exclusive.
Methods
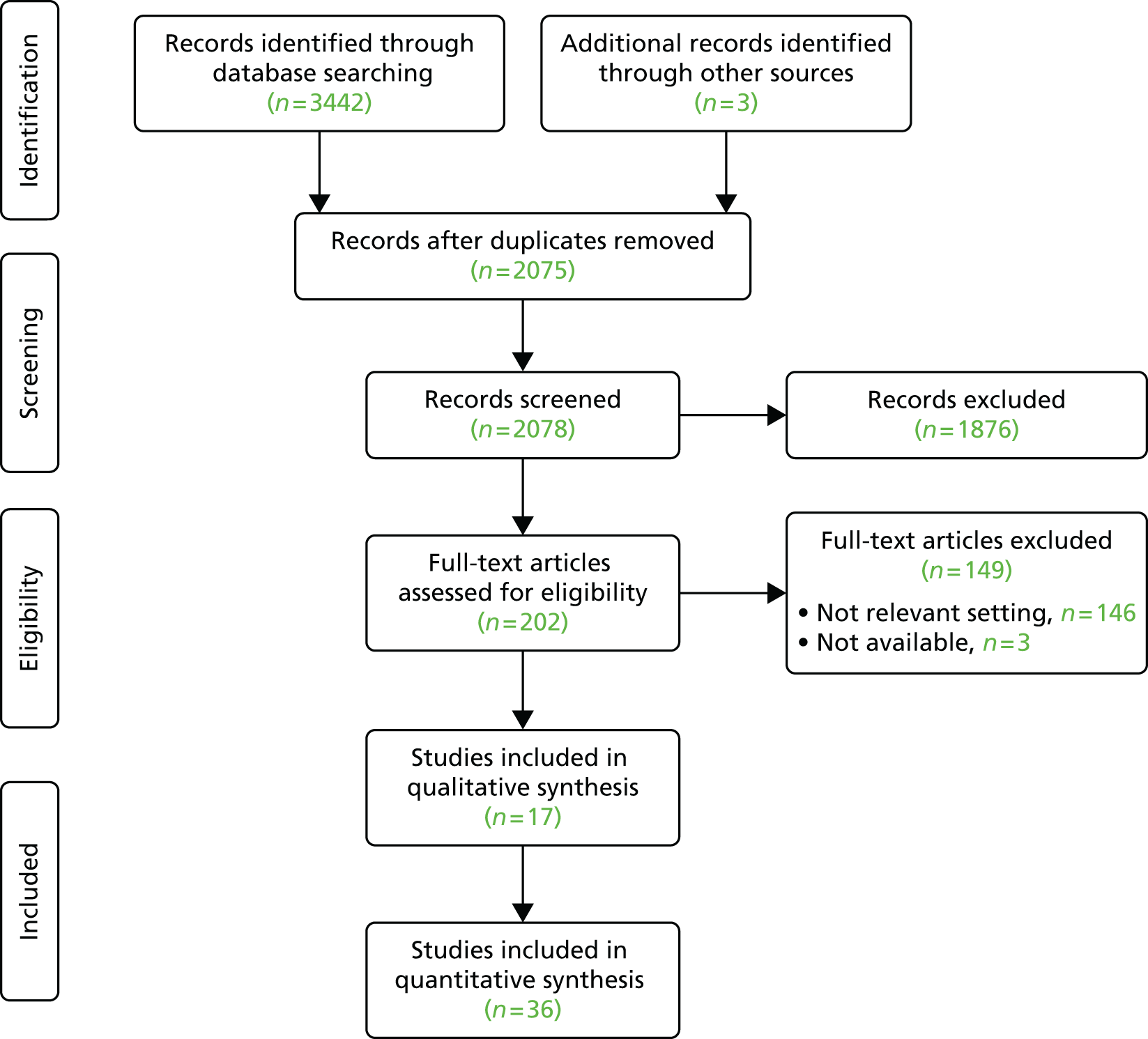
A systematic mapping approach, standardised across 13 topics, was used to identify, map and present findings from key items from the literature. As the focus of the mapping was descriptive and interpretive, not analytical, the methodology prioritised identification of systematic reviews, randomised controlled trials (RCTs) and qualitative studies, together with UK-based evidence sources. The objective was to identify factors and considerations determining the uptake and utilisation of diagnostic modalities in a primary and community care setting. Sensitive searches were conducted using PubMed Clinical Queries and PubMed Special Queries and The Cochrane Library (including the Database of Abstracts of Reviews of Effects). Modalities were also searched on PubMed in conjunction with the MeSH term ‘Great-Britain’, and The King’s Fund Centre Library database was searched by modality. The time period covered was from 2000 to September 2015. Coverage of logistic issues such as equipment, skills and training in the titles and abstracts of journal articles was minimal. Therefore, an extensive process of examination of full text and follow-up of references in context was employed to populate the STEP-UP framework for each topic. UK sources such as the NHS Horizon Scanning Centre and the NIHR Diagnostic Evidence Co-operative (DEC), Oxford, were scrutinised. Each topic summary includes an overview map, an examination of the type and nature of the evidence and a brief summary under each of the main headings of the STEP-UP framework. In recognition of the mapping function of the synthesis and its interpretative nature, no attempt was made to assess included items for quality.
Results
A total of 673 items for inclusion was identified across the 13 topics. Information on logistic considerations was diffuse, uneven and incomplete. Coverage of new technologies was relatively strong and, when specialist primary care professional associations exist (e.g. endoscopy), training and skills requirements were well specified. However, little direct evidence exists for equipment requirements, implementation issues and the impact on the primary–secondary care interface. A methodological challenge relates to the intensive search and find process required to identify relevant information submerged within lengthy full-text articles and position statements. The diverse range of considerations identified across the 13 topics illustrates the importance of a multifactorial decision-making process.
Skills
Many modalities require a wide range of contextual and/or technical skills: in administering the test, in interpreting the results and in managing the consequences. Genetic tests, although comparatively easy to administer, make considerable demands on specialist knowledge in interpretation and skills in genetic counselling. Radiology/X-ray and other types of imaging (MRI and ultrasound) require interpretation, possibly necessitating follow-up and expert advice from specialists in secondary care. Such a need may translate into a hybrid model utilising telemedicine technologies. Inadequate administration of tests may require repeat testing, either in the primary care setting or following referral to secondary care. It may also impair effective use of telediagnosis. Generally, three main routes have been identified for addressing the potential skills deficit. For some roles the GPwSI is seen as a route by which to fortify diagnostic expertise within primary care (e.g. endoscopy and genetics). For other scenarios, the extended role of the advanced nurse practitioner, either with a specific diagnostic function (e.g. ultrasound or spirometry) or within a management pathway (e.g. respiratory or diabetic specialist nurses), is emphasised. A third route, although not fully exploited, is to bring in a specialist professional, either on a sessional basis or as an employee of the primary care organisation (e.g. radiology or genetic counselling). Alternative models, not covered by this report, include the use of shared diagnostic services within a primary care consortium, mobile testing services and the use of commercial providers.
Training
Training may be delivered through specialist courses, attachments to acute specialist departments or manufacturers of diagnostic equipment. A particular concern relates to whether or not the number of cases to be seen in a practice justifies a significant investment of time and resources in training (e.g. endoscopy). The opportunity costs for the consultation and other aspects of primary care required by pursuit of specialisation are highlighted in the related GPwSI literature. A tension is identified between the professional interests of associations charged with assuring both the quality of procedures and the existence of their professional group and the need to engage with a wider primary care workforce. For example, data on the safety of endoscopy in acute hospitals were initially viewed as prohibitive to wider primary care involvement. In reality, triaging the complexity of particular cases and specific populations (e.g. children, pregnant women and the elderly), particularly for invasive modalities, may increase the accessibility of primary care diagnostics while preserving specialisation in secondary care.
Equipment
The cost and manageability of technologies is extremely variable across topic areas. Some technologies have benefited from moves towards miniaturisation (e.g. ultrasound) or to more end-user friendly versions of a technology (e.g. flexible sigmoidoscopy). Others have seen a trend towards popularisation, either in the health-care professions generally (e.g. POC testing) or through direct-to-consumer marketing (e.g. genetic tests), from which primary care might potentially benefit. Concerns about safety persist, either from the diagnostic equipment itself (e.g. radiation from radiology/X-ray) or from consumables (e.g. glutaraldehyde) or from ancillary equipment to support administration or analysis of the test results (e.g. electrical equipment). Ancillary equipment requirements are not widely documented (e.g. continuous pulse oximetry is recommended when intravenous sedation is required for endoscopy), while requirements for garments, gloves, goggles and glasses should not be overlooked. For newer technologies, the outputs of the NIHR DEC, Oxford, are useful. However, these outputs focus on narrower health technology assessment (HTA) perspectives of the technology, with correspondingly less attention to health services delivery and organisational issues.
Premises
Diagnostic technologies place particular requirements on the physical location in which a test is administered. In some cases the focus is on the housing and safe administration of the equipment (e.g. endoscopy or radiology). Specialist premises, that is, rooms dedicated to a particular diagnostic modality, are particularly prohibitive in legacy primary care premises. Even when premises are purpose-built, changes in technologies and their associated requirements, and a lack of specifications for an integrated multipurpose approach across technologies, make accommodation problematic. Patient flows (e.g. additional seating and queuing) need examination at a specific practice level. Requirements are not necessarily technology-driven. For example, audiological requirements for a quiet environment, even for simple hearing tests, may be difficult to accommodate and may particularly be compromised in a multipurpose environment. The requirements of accommodation are sparsely populated in Health Building Guidance, although the document Health Building Note 11-01, Facilities for Primary and Community Care Services,46 is a very helpful starting point. The number of potential cases seen versus the particular spatial requirements of the technology and its administration becomes particularly important when contemplating specialised, dedicated accommodation.
User perspectives
Generally, across primary care, the delivery of diagnostic modalities locally meets increasing demands for improved access and, by implication, enhanced equity. Patients prefer services that may be reached easily and that will not necessitate extensive time away from their day-to-day responsibilities. Prompt test results and the joining up of diagnosis and treatment are important considerations for patient and primary care provider alike. On the other hand, impaired access may function as a disincentive to inappropriate utilisation and, particularly, overtesting. There is little evidence to inform whether or not improved general practice access to testing increases uptake of tests or whether or not a greater awareness of resource use deters general practice staff from initiating testing. Patients presenting with medically unexplained symptoms may be assuaged with offers of more, and more immediately available, tests. More critically, the appropriateness of testing, rather than utilisation rates per se, becomes a key issue. Finally, given that diagnostic services function as a gateway to subsequent health service use, improved access to diagnosis might potentially defer current bottlenecks to other points in the patient care pathway.
Primary–secondary interface
Although the STEP-UP framework encourages a holistic view of primary care considerations, diagnostic services function in a whole-system health system environment. Ramifications of system change at the primary care first point of call are not fully explored in the evaluation literature. Earlier detection may result in earlier and more effective treatment and, thus, result in overall cost savings. Unintended consequences for patient and provider behaviours require careful examination, especially when resulting in increased utilisation or needless duplication. The impact on uptake of direct-to-consumer products and on commercial diagnostic services or private health care is equally important in a whole-system perspective.
Discussion
The multifactorial nature of decisions on diagnostic services, and indeed on general practice-level change more generally, is emphasised by the STEP-UP framework. Observations are discussed under the modalities themselves and the future research and synthesis agenda.
Candidate modalities
The Synthesis team members were constrained by limited data on current UK practice and uptake of diagnostic services. For long-established technologies (e.g. endoscopy), it is unclear whether the extent of spread has been determined by demand or by logistic considerations such as the limited availability of premises or the costs of equipment. In particular, the implications of a concerted attempt to improve diagnostic provision, involving the introduction of diverse modalities within a relatively intensive period, are unclear. Mechanisms for prioritisation, strategically and in an individual practice, are also complex and unclear. Several commentators observe on the importance of identifying particular barriers and constraints at a micro level, as well as acknowledging the role of guidance from the National Institute for Health and Care Excellence (NICE) or professional associations in driving forward initiatives. The variability of context makes it difficult to translate this mapping review directly into actionable recommendations for primary care. At the same time, the STEP-UP lens offers the possibility of a more holistic and consistent approach to evaluation. Economic evaluation, with its whole-systems perspective, its approach to itemisation of particular components and its functionality for handling multiattribute decisions, appears to afford an opportunity to implement STEP-UP considerations in a more technical, consistent and decision-specific manner.
Methodological considerations
As a conceptual framework, STEP-UP is vulnerable to a mismatch between the logistic information required to complete the map and a research agenda focused on innovations and/or on the evaluation of effectiveness. Position statements and professional standards are typically underpinned by implicit and non-articulated assumptions about the stage of diffusion at which a particular technology finds itself. So, for example, early documents state requirements for setting up an endoscopy unit but the costings are now outdated and the specification is time-bound. Extensive use of the STEP-UP framework across a wide variety of topics reveals its general utility for a consistent approach that highlights similarities and contrasts across very different technologies. As such, STEP-UP offers a viable framework for extending evaluation beyond the current narrow interpretation of HTA towards important considerations of service delivery and organisation. Considered reflection leads us to suggest the possible extension of the STEP-UP framework to include three additional components: Public perspectives, Economics and Drivers (STEPPED-UP). Although the inclusion of economics in the extended framework is unsurprising, it should be noted that the STEPPED-UP framework introduces this in the context of service delivery and not in the more common, limited application of individual diagnostic technologies.
Conclusion
The evidence map and synthesis (Table 5) provides a rapid synoptic view of leading areas of development for primary and community care diagnostics and a potential mechanism for identifying and specifying future areas for development (for synthesis, primary research and policy). In particular, the need for whole-system evaluations, economic evaluations and a mechanism for better organising and presenting information on aspects relating to service delivery and organisation, possibly analogous to the NIHR DECs, has been highlighted. Further data on current levels of diagnostic provision in primary care and future priorities are required. Finally, the focus of this synthesis on staff and equipment located in primary care has necessarily constrained the scope of the review and subsequent recommendations. Alternative models, such as consortium approaches, direct access, mobile testing services, outreach initiatives and use of commercial laboratories, require a similarly systematic examination.
| Diagnostic technology | Human resources | Logistics | Communications and relationships | ||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | User perspective | Primary–secondary interface | ||
| Clinician | Patient | ||||||
| Audiology | ○ | ○ | ○ | ○ | ⨁ | ⨁ | ○ |
| Pneumatic otoscopy | ○ | ⨁ | ⨁ | Ø | ○ | Ø | Ø |
| Tympanometry | ⨁ | ⨁ | ⨁ | Ø | ○ | ⨁ | ○ |
| Cardiac services | ○ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ⨁ |
| BNP | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ○ |
| ECG | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ⨁ |
| Echocardiography | ○ | ○ | ⨁ | ⨁ | ⨁ | ⨁ | ○ |
| Diabetic services | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ⨁ |
| Endoscopy | ⨂ | ○ | ⨂ | ⨂ | Ø | ⨂ | ○ |
| Genetic testing | ⨂ | ○ | ⨁ | Ø | ○ | ⨁ | ○ |
| MRI | ○ | ○ | ⨂ | ⨂ | Ø | Ø | ○ |
| POC testing | ○ | ○ | ⨁ | Ø | ○ | ⨁ | ○ |
| CRP | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ○ |
| Radiology/X-ray | ○ | ⨁ | ○ | ○ | ⨁ | ⨁ | ○ |
| Respiratory tests | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ○ |
| Pulse oximetry | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ○ |
| Spirometry | ○ | ⨁ | ⨁ | ⨁ | ⨁ | ○ | ⨁ |
| Ultrasound | ○ | ⨁ | ⨁ | ⨁ | ⨁ | ⨁ | ⨁ |
Background
Recent years have witnessed increasing momentum towards improved access to diagnostic services for GPs, allied health professionals and other primary and community care staff. 47,48 Department of Health documents such as Care Closer to Home49 and Our NHS Our Future: NHS Next Stage Review – Leading Local Change50 outline a need to achieve change through ‘disruptive innovation’, that is, change involving radical service redesign and an emphasis on devolving key aspects of care pathways from secondary to primary care. 51 Other drivers for increased use of diagnostic services in primary care include rapid and significant advances in test technologies (Technology); increases in numbers of tests performed (Economics) and pressure from patients and carers for more accurate and rapid diagnoses (Social Drivers). 2 For further discussion of these issues, see Chapter 1.
This framework map and synthesis was undertaken within a larger review project looking at provision of diagnostic services in community settings. Initial literature mapping revealed a variety of diagnostic modalities with different implications for being located in primary care (see Chapter 3). In addition to examining one modality, diagnostic ultrasound (see Chapter 5), in detail, the review team believed that it would be helpful to characterise modalities against a common set of logistic and service delivery considerations. They could then populate these considerations with data relating to existing modalities and the framework could become a template for evidence gathering for potential and future technologies.
Hypotheses tested in the review (review questions)
Review question
What are the logistic and service delivery considerations associated with the introduction and ongoing provision of diagnostic services in community or primary care settings? These should include implications for NHS organisations (e.g. related to provision of staff, premises, training and equipment, costs and cost-effectiveness) and patients (e.g. related to changes in management/pathways, acceptability to patients, accuracy of diagnosis and longer-term clinical outcomes).
Objectives
The objectives of this mapping review are:
-
to develop a series of maps of logistic factors (STEP-UP) relating to a pre-agreed list of diagnostic modalities of potential use in primary care
-
to populate those maps with evidence from rigorous or relevant studies
-
to use the maps to produce an analysis of logistic considerations
-
to identify needs for further research and development to inform future research priorities.
Scope
This review covers logistic and service delivery considerations associated with the introduction and ongoing provision of diagnostic services in community or primary care settings. It is not possible to anticipate technologies that are currently being developed and tested in acute hospital settings but that have not been used in primary care. For inclusion, technologies should offer some evidence of trialled and or uptake in a primary care or community care setting. Evidence for effectiveness and cost effectiveness is not the focus of the review, serving only as a backdrop to considerations about logistic and service delivery factors.
The emphasis of this mapping review is in identifying, summarising and digesting existing data sources, not in performing original analyses. The deliverables, therefore, relate to the STEP-UP maps, not to detailed assessments of each individual technology.
Appendix 2 sets out the innovative STEP-UP framework used to guide the review process. This framework allows us to:
-
define the scope of the search strategy
-
define inclusion and exclusion criteria to specify the types of studies to be included in the final report
-
construct summary tables of all included studies to present key information and findings
-
synthesise the evidence from the included studies.
Review methods
The framework map and synthesis did not attempt to identify all relevant evidence or to search exhaustively for all evidence that meets the inclusion criteria; instead, the search approach sought to identify the key evidence of most relevance to the review question. Evidence identification privileged systematic reviews, quantitative studies (including RCTs), qualitative studies and UK studies. In addition, substantive items such as position statements, standards and guidance and editorials were included. Although the last are understandably viewed with caution in the context of an effectiveness review, they were considered appropriate for this logistic ‘map’. The emphasis was on identifying factors considered important, not to authoritatively determine their contribution to test development and implementation.
Protocol development
The protocol for the review was developed iteratively between the School of Health and Related Research, University of Sheffield, and NIHR Health Services and Delivery Research. A copy of the study protocol is available on the study website. 15
Literature searching
An efficient search strategy was devised, in response to an initial scoping search which revealed that information on logistic issues was rarely identifiable in the title and abstracts of journal articles and usually could be retrieved only from full text.
Identification of key systematic reviews
Searches were conducted for systematic reviews on each diagnostic technology on the Centre for Reviews and Dissemination databases, the PubMed Clinical Queries search engine using the Broad (Sensitivity) filter for Diagnosis, and searches of The Cochrane Library and the Turning Research Into Practice database.
Identification of key quantitative studies
Searches were conducted for key clinical studies on each diagnostic technology on the PubMed Clinical Queries search engine using the Broad (Sensitivity) filter for Diagnosis. Searches were also conducted on the Cochrane Trials Register.
Identification of key qualitative studies
Searches were conducted for key qualitative studies on each diagnostic technology on the PubMed Special Queries – Health Services Research search engine using the Broad (Sensitivity) filter for Qualitative Research.
Identification of other substantive evidence
Searches were conducted for other substantive studies on each diagnostic technology on the PubMed Special Queries – Health Services Research search engine using the Broad (Sensitivity) filters for Appropriateness, Costs and Economics. The King’s Fund Library was also searched for substantive items.
Identification of UK studies
Studies conducted in the UK were identified in three ways:
-
Geographical terms for ‘united kingdom’, ‘uk’, ‘britain’, ‘England’, ‘Scotland’, ‘Wales’, ‘Ireland’ were used to retrieve records from the PubMed database.
-
Searches by technology, with no geographical limitations given its UK focus, were conducted on The King’s Fund database.
-
Similarly, geographical terms for ‘united kingdom’, ‘uk’, ‘britain’, ‘England’, ‘Scotland’, Wales, Ireland, combined with the technology and words such as ‘equipment, logistics, premises, facilities’ were used to retrieve items from Google Scholar (Google Inc., Mountain View, CA, USA). By harnessing full-text retrieval, this search added value over the title-and-abstract-based approach listed above.
Retrieved items were sifted against the inclusion/exclusion criteria listed in the following section. A bibliography of key items was compiled for each technology. This bibliography was used to retrieve full texts when available and to perform citation searches. As additional relevant items were retrieved, further citation searches were performed for these in turn until saturation of relevant items was achieved.
Inclusion/exclusion criteria
Population
People requiring diagnostic services for any medical condition (excluded: universal screening and monitoring, including pregnancy). Studies that described screening for selective populations (e.g. by age, gender, ethnic group) or for individuals indicated to be at risk were included provided that the identified factors were either common or concentrated in the UK population. Excluded were psychological and psychiatric conditions (e.g. depression) and neurological conditions (e.g. dementia).
Intervention
Diagnostic services where a complete diagnostic (but not necessarily treatment) pathway was provided in a primary care or community setting by primary care/community staff using any type of equipment. Open-access services provided to GPs by a hospital using its premises, equipment and staff were treated as a comparator intervention. (Studies examining telemedicine that links primary with secondary care were excluded from this review because of extent of dependency on secondary care support. However, such initiatives should be factored into any decisions.)
Comparator
Hospital-based diagnostic services (open access or traditional). ‘Outreach’ services using hospital-based staff to deliver services in community settings were also relevant comparators.
Outcomes and study designs
The main focus was research studies conducted in any developed world setting that evaluated community diagnostic services against a comparator. Audits, service evaluations, descriptive studies, economic evaluations and qualitative research studies were included if they had been conducted in a UK setting. Systematic reviews with no geographic limits or where geographic limits include UK settings were also eligible for inclusion. In addition, relevant expert opinion or reports from professional bodies identifying practical issues related to the provision of community diagnostic services were included. Articles focusing on test performance with no examination of subsequent health services outcomes were excluded.
Other limits
Given that this was a mapping review, articles without an abstract were excluded. Studies were to be written in English and published from 2000 onwards.
Studies were included in the framework map and synthesis in accordance with Table 6.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Adults and/or children presenting in primary care without a previous definitive diagnosis | Community diagnostics for healthy patient groups (i.e. those without an indication related to a chronic health condition). This exclusion covers:
|
| Intervention | Delivery of one or more services to a small group of patients (typically 8–10 patients) simultaneously. Only studies including the delivery of the intervention by one or more specialist HCPs met the inclusion criteria of the review | Tests carried out as part of national screening programmes do not count as a diagnostic test/procedure for the purposes of this review |
| Comparator | Other methods of organisation of treatment (with the exception of qualitative research and surveys, only studies with a comparator group are included) | |
| Outcome | Patient outcomes; health services outcomes; patient and carer satisfaction; resource use |
Given the focus of the review on models of service, studies that report only on the diagnostic accuracy of modalities have not been included.
Data extraction including development of the data extraction tool
A standardised data extraction form was designed to specify and capture relevant information from the studies on a broad range of factors related to community diagnostics (see Appendix 2). A narrative commentary was undertaken to discuss identified aspects of each test technology.
Quality assessment
This mapping review sought to identify logistic factors or themes. Given the interpretive nature of this work it was not considered necessary to undertake formal quality assessment. However, high-quality evidence sources were interrogated in seeking the best possible study designs.
Synthesis
Data were extracted and tabulated. This tabulation was used to inform the narrative synthesis in Results of the framework map and synthesis. The innovative STEP-UP framework is used for comparison and analysis (see Appendix 2).
Studies included in the review
Table 7 summarises the results of the review by type of test and study type.
| Diagnostic technology | Systematic reviews | Quantitative | Qualitative | UK | Other | Totala |
|---|---|---|---|---|---|---|
| Audiology | 6 | 27 | 9 | 14 | 40 | 83 |
| Cardiac services | 6 | 14 | 5 | 19 | 18 | 54 |
| ECG | 8 | 9 | 3 | 6 | 9 | 29 |
| Echocardiography | 4 | 16 | 5 | 5 | 25 | 49 |
| Diabetic services | 5 | 10 | 6 | 4 | 13 | 34 |
| Endoscopy | 6 | 16 | 5 | 14 | 15 | 43 |
| Genetic testing | 2 | 25 | 24 | 16 | 34 | 79 |
| MRI | 1 | 11 | 0 | 5 | 11 | 24 |
| Point of care testing | 11 | 31 | 5 | 3 | 24 | 60 |
| Radiology/X-ray | 0 | 7 | 1 | 8 | 23 | 32 |
| Respiratory tests | 3 | 6 | 2 | 12 | 17 | 39 |
| Spirometry | 8 | 39 | 5 | 9 | 46 | 111 |
| Ultrasound | 2 | 12 | 0 | 12 | 22 | 36 |
| Total | 62 | 223 | 70 | 127 | 297 | 673 |
Results of the framework map and synthesis
Audiology
Definition: overall service delivery framework within which audiometry (hearing tests) are used to assess ability to hear different sounds and to determine if there are any problems (adapted from NHS Choices).
This map includes otoacoustic emissions; pneumatic otoscopy; pure tone audiometry; spectral gradient acoustic reflectometry; tympanometry.
This map excludes universal newborn hearing screening.
Contains public sector information licensed under the Open Government Licence v3.0. 52
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
The complexity of audiological investigation requires that GPs work with a wide range of staff. Some services for adults are now provided in a primary care setting by GPwSIs in otology. Some staff encounter initial difficulties when using the tympanometer or the pneumatic otoscope. However, a wide range of audiological investigations can be performed by community staff not specialising in audiology. In the UK, good practice guidance and standards and quality audit tools have been developed for audiology services.
STEP-UP summary statement With regard to SKILLS, audiology is attributed a MODERATE degree of implementation difficulty owing to initial difficulties in using the tympanometer or the pneumatic otoscope and ongoing concerns about the appropriateness of diagnosis and its implications for secondary care.
Training
Specialist GPs may be trained in the initial diagnosis and management of hearing and balance disorders, particularly in adults. Practice nurses can support delivery of procedures requiring basic skills such as basic hearing aid trouble-shooting, relaxation training for those with tinnitus and vertigo, and in gentle mobility training for those with vertigo. Skills in tympanometry and pneumatic otoscopy can be acquired through short courses.
STEP-UP summary statement With regard to TRAINING, audiology is attributed a MODERATE degree of implementation difficulty owing to the facility of short courses to increase confidence in use of equipment.
Equipment
Equipment most typically used by a practice would include a tympanometer (Table 8) and a pneumatic otoscope (Table 9). Audiology requires a wide range of equipment. Requirements may include flexible fibre-optic nasendoscopy, Hopkins rod endoscopy, a soundproof facility, audiometry, tympanometry and microscopy. Over 75% of all patients will require an additional investigation or procedure. With the introduction of portable and handheld equipment, tympanometry has become more feasible for a primary care setting. Future-proofing of audiology equipment requires provision for otoacoustic emissions technology, initially used for newborn hearing screening but with potential in small children and the aged.
| Description of technology | ||||||
|---|---|---|---|---|---|---|
| Tympanometer: equipment for examining the condition of the middle ear and mobility of the eardrum (tympanic membrane) and the conduction bones by creating variations of air pressure in the ear canal. Tympanometry is an objective test of middle-ear function | ||||||
| Brief summary of effectiveness/cost-effectiveness in primary care | ||||||
| Tympanometry has a high sensitivity and specificity in diagnosing middle ear effusion in young children.53 Use of a tympanogram, over visual inspection of photographs, improved agreement between clinicians and with expert observers.54 In children with AOM, pneumatic otoscopy is the preferred diagnostic method with tympanometry used to confirm the diagnosis and document the duration of effusion55 | ||||||
| Skills | ||||||
| Tympanograms are ‘Type A’ (normal), ‘Type B’ (pathology of the middle ear) and ‘Type C’ (indicating poor eustachian tube function). Good agreement with experts.56 Many GPs unclear on the significance of negative pressure (Type C).57 GPs expressed preference over pneumatic otoscopy based on ease of use and interpretation.57 Machine self-explantory and easy to use57 | ||||||
| Training | ||||||
| Three-hour workshop (Australia) improved GP confidence in tympanometry and pneumatic otoscopy.57,58 Supported by online resource (ePROM).59 GPs required further training. Six-hour tympanometry and otitis media course (Denmark)53 Diagnosis changed in 26.4% after tympanometry plus GP training (Denmark)60 Nurses trained in 2-hour course on tympanometry for AOM. Observed reliable test results in excluding middle ear effusions after AOM61 |
||||||
| Equipment | ||||||
| Need for hygiene. Tympanometer and probe tip should be clean (i.e. free from dust and dirt and compliant with local infection control standards).62 Tympanometers shall meet the performance and calibration requirements of BS EN 60645–5.62 Safe equipment [< 5 years old, regularly maintained and calibrated (checked daily) and appropriate to clinical needs63]. Some tympanometers are difficult to handle61,64 | ||||||
| Premises | ||||||
| Health Building Note 11-01, Facilities for Primary and Community Care Services,46 includes provision for an adult hearing test room and a paediatric hearing test room | ||||||
| Clinician | ||||||
| Problems reported by primary health-care staff in understanding the meaning of the displayed figures and using them to quality assure the measurement, deriving a reliable curve, obtaining an airtight sealing and understanding what the curves means for the clinical decision. Other problems include handling the tympanometer and getting children to co-operate.53,61 Results obtained at fewer than half of asymptomatic visits owing to young, unco-operative children, inexperienced nurses, and relative rarity of exclusive test results.61 Slow diffusion as technology is difficult to handle and understand.53 Useful in communicating diagnosis to carers57 | ||||||
| Patient | ||||||
| No details given, except in unco-operative children61 | ||||||
| Primary–secondary interface | ||||||
| No details given | ||||||
| Skills | Training | Equipment | Premises | Clinician | Patient | Primary–secondary interface |
| ⨁ | ⨁ | ⨁ | ○ | ○ | ⨁ | ○ |
| Description of technology | ||||||
|---|---|---|---|---|---|---|
| Pneumatic otoscopy is an examination that allows determination of the mobility of a patient’s tympanic membrane in response to pressure changes. The normal tympanic membrane moves in response to pressure. Immobility may be due to fluid in the middle ear, a perforation or tympanosclerosis, among other reasons | ||||||
| Brief summary of effectiveness/cost-effectiveness in primary care | ||||||
| Pneumatic otoscopy improves diagnosis by between 15% and 26%, compared with usual otoscopy.65 Taking into account direct and indirect costs, pneumatic otoscopy is cheaper than tympanometry or acoustic reflectometry.66 Pneumatic otoscopy can be as effective as or better than tympanometry and acoustic reflectometry66 | ||||||
| Skills | ||||||
| Pneumatic otoscopy was seen as a more difficult technical skill than tympanometry57 | ||||||
| Training | ||||||
| Skills can be acquired through short courses | ||||||
| Equipment | ||||||
| Requires otoscope with pneumatic bulb and speculum tips with rubber rings to create proper seal67 | ||||||
| Premises | ||||||
| Health Building Note 11-01, Facilities for Primary and Community Care Services,46 includes provision for an adult hearing test room and a paediatric hearing test room | ||||||
| Clinician | ||||||
| Most important barrier was uncertainty on whether there was true drum immobility or whether their technique was simply inadequate.57 Some GPs ‘gave up’ on pneumatic otoscopy.57 Teachers identified improved diagnostic accuracy as main facilitator for use and for teaching.68 All physicians reported lack of availability of equipment as main barrier to use. Main barrier to teaching reported by teachers was that they did not use it themselves. Uptake of pneumatic otoscopy in UK general practice is not known68 Evidence Assessment66 concludes that, for typical clinician, pneumatic otoscopy should be easier than other diagnostic methods, not borne out by qualitative study57 |
||||||
| Patient | ||||||
| No information available | ||||||
| Primary–secondary interface | ||||||
| No information available | ||||||
| Skills | Training | Equipment | Premises | Clinician | Patient | Primary–secondary interface |
| ○ | ⨁ | ⨁ | ○ | ○ | Ø | Ø |
STEP-UP summary statement With regard to EQUIPMENT, audiology is attributed a MODERATE degree of implementation difficulty because, beyond basic equipment, many needs for additional equipment will require follow-up in secondary care.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 includes provision for an adult hearing test room and a paediatric hearing test room. Audiological testing must be performed in soundproofed accommodation built to International Standards Organisation (ISO) 8253-1 and ISO 8253-2 standards for acoustic test methods and suitable for children. Despite the growth of purpose-built clinics and the increasing miniaturisation of audiology equipment, the inability of premises to accommodate treatment rooms for audiology services has been a reported obstacle to the relocation of hospital-based provision.
STEP-UP summary statement With regard to PREMISES, audiology is attributed a MODERATE degree of implementation difficulty owing to the specific requirement for a soundproofed room.
User perspective
Generally, GPs found tympanometry and pneumatic otoscopy to be acceptable to carers and children, although some GPs stated that they preferred not to use pneumatic otoscopy as children sometimes found it uncomfortable. 57 Some GPs thought tympanometry was particularly useful for communicating with carers about ear disease, offering tangible proof to parents of the GP’s diagnosis and support of the management plan. 57 Many GPs were unclear about the significance of negative pressure tympanometry57 readings in general practice, which could potentially generate unnecessary GP follow-ups.
General practitioners believed that more training and experience was needed to become confident with pneumatic otoscopy than was needed for tympanometry. 57 GPs could teach themselves how to perform tympanometry after the relatively brief introduction to the equipment, with the main challenge being interpretation of the tympanograms, which required them to refer back to written information they were given during training. Most GPs said that they would choose to continue to use tympanometry.
STEP-UP summary statement With regard to USER PERSPECTIVES, audiology is attributed a LOW degree of implementation difficulty in the light of only minor GP concerns with equipment and the referral pathway and difficulties with investigations involving small children.
Primary–secondary interface
The Good Practice Guide, specifically for adults with tinnitus,69 recommends strategies for tinnitus assessment, management and referral at four different levels of the service: primary care, local community-based tinnitus services, specialist hospital-based centres and supra-specialist assessment centres. Patient routes through the system were to be determined by clinical assessment and specific referral criteria, designed with service efficiency and equity of patient care in mind. A systematic review of outreach services in primary care found a direct counterpoint between the needs of the patient and the needs of the service. 70 Reported disadvantages concerned administrative costs, accommodation costs and inefficient use of specialists’ time. Comparative studies showed that more patients expressed a preference for outreach clinics than for hospital-based clinics, and measures of patient satisfaction and convenience generally were higher for outreach clinics. A self-contained satellite facility within a GP practice sought to enable 5000–7000 people to receive secondary care hearing services in a primary care setting. The project explored the benefits to patients and to the health economy of shifting care closer to home. 71
Traditionally, patients needing a hearing aid would be referred by their GP to the audiology service, with one appointment for assessment and one for fitting. Advances in technology72 mean that some patients can be assessed and fitted at the same appointment.
STEP-UP summary statement With regard to the PRIMARY–SECONDARY INTERFACE, audiology is attributed a MODERATE degree of implementation difficulty owing to dependence on secondary services for further investigation and yet the possibility of streamlined hearing aid pathways.
Conclusion
Provision of audiology services in primary care is complex, in that it not only requires a wide variety of equipment and, optimally, an audiology-friendly environment, but also may necessitate access to other diagnostic services, most notably endoscopy or radiology. Table 10 summarises the overall findings. There is little evidence available to examine the impact of acquisition of audiology-related skills by primary care staff. Studies on provision of specialist outreach clinics (i.e. a hospital specialist operating in a primary care environment) offer supplementary data on equipment utilisation and premises. Current models being favoured in primary care audiology involve direct-access referral clinics or provision of services under contract by commercial (or, alternatively, independent) providers.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| Audiology | ○ | ○ | ○ | ○ | ⨁ | ⨁ | ○ |
| Systematic reviews | A81 | A42, A73 | A32, A37, A65 | ||||
| Quantitative studies | A4, A5, A6, A11, A31, A46, A50, A63, A78 | A46, A50, A60, A72 | A12, A13, A35, A62, A71, A75 | A54 | A2, A8, A22, A23, A38, A39, A45 | ||
| Qualitative studies | A68 | A1 | A1, A15, A29, A80 | A80 | A15, A29 | ||
| UK studies | A27, A33 | A20, A27 | A16, A34 | A24 | A69 | A23, A27, A38, A40, A48, A83 | |
| Other significant evidence | A3, A7, A17, A21, A25, A26, A31, A36, A52, A55, A67, A76 | A30, A41, A66 | A9, A10, A14, A18, A19, A28, A43, A44, A47, A49, A51, A59, A61, A64, A70, A74, A77, A79 | A25, A26, A53 | A56, A57, A58, A82 | ||
| Pneumatic otoscopy | ○ | ○ | ○ | ○ | ○ | ○ | Ø |
| Tympanometry | ○ | ⨁ | ○ | ○ | ○ | ○ | Ø |
Cardiac services
Definition: cardiac tests are used to diagnose and treat heart disease, such as heart failure and AF. Some tests are non-invasive; others are more invasive but potentially are more useful for diagnosis of heart disease.
This map includes BNP, blood tests (specifically in the context of heart disease) (see also Point-of-care testing), 24-hour ambulatory BP monitoring.
This map excludes chest X-ray (see Radiology/X-ray), electrocardiography (see Electrocardiography), echocardiography (see Echocardiography), telecardiology.
BNP, B-type natriuretic peptide.
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3. The findings are summarised in Table 11.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| Cardiac services | ○ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ○ |
| Systematic reviews | C6, C29, C30, C33, C34 | C38 | |||||
| Quantitative studies | C3, C7, C26, C50 | C12, C17, C19, C36, C42, C47, C49, C51, C54 | C35 | ||||
| Qualitative studies | C26 | C4, C21, C32, C37 | |||||
| UK studies | C7, C26, C39, C44, C45, C46 | C2, C5, C8, C9, C12, C25, C41, C48, C54 | C32, C35, C37 | C20 | |||
| Other significant evidence | C15, C40 | C18 | C1, C10, C11, C13, C14, C16, C22, C23, C24, C27, C43, C49, C53 | C31, C52 | |||
Skills
Based on clinical scenarios, GP experts have concluded that natriuretic peptides testing should be the routine test for suspected heart failure where referral for diagnostic testing is considered appropriate. Abnormal natriuretic peptides testing should be followed up with referral for echocardiography. 73 In a survey on barriers to effective management of heart failure, the majority of respondents who diagnosed left ventricular systolic dysfunction (LVSD) used ECGs, chest radiography and clinical assessment, the exception being nurses. Around one-quarter of the nurses and half of the GPs were confident about interpreting the results of an ECG, whereas most cardiologists and general physicians were confident. 74
STEP-UP summary statement With regard to SKILLS, cardiac services is attributed a MODERATE degree of implementation difficulty owing to the range of diagnostic scenarios and investigations.
Training
Education and training in cardiac imaging should meet the present requirements specified by the General Medical Council (GMC) in curricula in cardiology and cardiac radiology, the future demands of revalidation and the requirements of allied professional groups. 75 The British Society of Echocardiography (BSE) (www.bsecho.org), the British Society for Cardiovascular Magnetic Resonance (BSCMR) (www.bscmr.org) and the British Society for Cardiac Imaging (www.bsci.org.uk) have endorsed voluntary accreditation procedures for their members.
A survey of 170 general practices in one cardiac network suggests that training of staff in use of cardiac equipment is variable. 76 Training updates were not provided for 12-lead ECG in 20 practices, static BP in 26 practices, ambulatory BP in 21 practices and Holter monitors in 10 practices. Responding practices indicated that staff require additional training in 12-lead ECG (n = 17), static BP (n = 3), ambulatory BP (n = 11) and Holter monitors (n = 8).
A new extended GP role in cardiology was developed and piloted to enable GPs to diagnose and manage patients with mild to moderate heart failure or atrial fibrillation (AF) and to use diagnostics effectively in primary care. 77 Training entailed GPs participating in a four-session short course with ongoing clinical supervision. A mixed-methods evaluation found that participating GPs perceived the extended GP role as a professional development opportunity with the potential to reduce health-care utilisation and costs, through a reduction in referrals, while meeting the patient’s wishes for the provision of care closer to home. Patient experience of the new GP service was positive.
STEP-UP summary statement With regard to TRAINING, cardiac services is attributed a LOW degree of implementation difficulty owing to the widespread availability of appropriate training and accreditation, although underutilisation of training in basic cardiac equipment remains a concern.
Equipment
A survey of 170 general practices in one cardiac network suggests that the provision of cardiac equipment, and the training of staff in its use, is variable. 76 Service contract providers included local hospitals and equipment suppliers, while practice nurses are the group of staff most likely to be responsible for the equipment on a day-to-day basis. Following the purchase and installation of equipment, there is a need for appropriate maintenance and training to ensure optimal use and patient safety. Guidance and standards regarding equipment maintenance and training within primary care premises is difficult to identify,76 typically being scattered across a range of stakeholder professional association.
With regard to emerging technologies, Table 12 offers a specific framework assessment for B-type natriuretic peptide (BNP). Implementation of BNP will incur initial expenses including the purchase or hire of the analyser. Quality control issues relate to equipment and the purchase of limited shelf-life reagent strips. The cost of each BNP test is likely to be higher when relatively small numbers of tests are required, and will reduce with volume. 80 A key source for an evaluation of the BNP POC testing kit is a 2011 Horizon Scanning report. 81
| Description of technology | ||||||
|---|---|---|---|---|---|---|
| BNP involves taking blood from a patient and testing it, often in a GP surgery Brief summary of effectiveness/cost-effectiveness in primary care. BNP is produced from heart muscle cells as a pro-hormone (proBNP) and released into the cardiovascular system in response to ventricular dilatation and pressure overload. The pro-hormone is secreted as BNP and NT-proBNP. POC BNP testing devices measure either BNP or NT-proBNP (the latter has a longer half-life). Different models have been identified and evaluated by the Horizon Scanning report A systematic review,78 including 12 studies from primary care, found that BNP and NT-proBNP are useful diagnostic tools to identify patients with heart failure in primary care. Results agree with a 2009 HTA report, using individual patient data meta-analysis, when both BNP and NT-proBNP had high sensitivities for diagnosis of heart failure.79 NICE guidelines on the diagnosis of heart failure recommend using BNPs in place of ECG |
||||||
| Skills | ||||||
| Not specified | ||||||
| Training | ||||||
| Not specified | ||||||
| Equipment | ||||||
| Ten studies used TRIAGE BNP test, one study used the ADVIA-Centaur® BNP Assay and one study used the Abbott AxSYM® BNP Microparticle Enzyme Immunoassay78 | ||||||
| Premises | ||||||
| No details given | ||||||
| Clinician | ||||||
| No details given | ||||||
| Patient | ||||||
| No details given | ||||||
| Primary–secondary interface | ||||||
| Abnormal natriuretic peptides testing should be followed up with referral for echocardiography | ||||||
| Skills | Training | Equipment | Premise | Clinician | Patient | Primary–secondary interface |
| ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ○ |
STEP-UP summary statement With regard to EQUIPMENT, cardiac services is attributed a LOW degree of implementation difficulty owing to developments in POC testing and the increased portability of equipment.
Premises
Most aspects of cardiac services have been accommodated in primary care for many years, typically specified for the ‘near patient testing room’. 46 The implications of equipment purchases such as ECG or echocardiography are reviewed elsewhere (see Electrocardiography and Echocardiography). This review was not able to identify information on the implications of providing an increased range of cardiac testing facilities in primary care (i.e. BNP), specifically in relation to implications for the premises.
STEP-UP summary statement With regard to PREMISES, cardiac services is attributed an UNCERTAIN degree of implementation difficulty owing to the lack of information on the facilities required.
User perspective
A study in UK general practice examined management of suspected heart failure. 82 Some GPs requested a full blood count and urea and electrolytes on all of their patients with suspected heart failure. Very few GPs reported sending a patient for echocardiography to confirm a diagnosis of heart failure. Most GPs mentioned the adequacy of facilities as an obstacle to diagnosing and managing patients with heart failure, specifically lack of access to an echocardiogram. Many GPs mentioned poor access to echocardiography as a barrier to diagnosing patients with suspected heart failure. Those few GPs and practices who admitted to routinely arranging echocardiograms for patients suspected of having heart failure were also more likely to report treating patients with angiotensin-converting enzyme inhibitors.
STEP-UP summary statement With regard to the USER PERSPECTIVE, cardiac services is attributed a LOW degree of implementation difficulty owing to the non-invasive nature of most cardiac tests. However, the non-availability of echocardiology services remains a significant barrier.
Primary–secondary interface
Open-access services (echocardiography, stress testing, Holter monitoring) may simply provide an investigation or add value by providing interpretation and advice. 83 Open-access services may be provided either on hospital premises or in partnership with primary care in a community setting and should be quality assured with appropriate governance arrangements and regular systematic audit. An alternative to a primary care-based approach involves using a local NHS trust laboratory. A NHS Improvement report84 concludes that this is often a more cost-effective option, citing a likely correlation between the number of tests and the cost per test. Points to consider are the length of time for the results to be returned, the courier service required to transport the samples to the provider, transport difficulties for patients and the importance of a robust service-level agreement.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, cardiac services is attributed a LOW degree of implementation difficulty owing to existing pathways for the management of heart disease and heart failure.
Conclusion
Benefits of a BNP service include rapid results, convenience for patients, early diagnosis and commencement of treatment. Centralising in one agreed location allows one GP practice to serve several surgeries, allowing results to be available within a few days or even on the same day. Benefits include that it is convenient for the patient, it is cheaper than doing the test in individual surgeries, and results may still be available within an acceptable time span (depending on the agreement). This type of service may involve the transportation of samples from or to the testing centre, and this must be factored into planning.
In some parts of the UK, the introduction of BNP has led to increased numbers of echo tests being performed without an increase in true positives. This means that if the test is used indiscriminately, false-positive results may outnumber true positives. A local agreement may help to decide who should order the test or when access to ordering a BNP test is appropriate. A further consideration when calculating costs is that a percentage of those with positive BNP results will require an echocardiogram. The cost of the BNP test will need to be added to the cost of an echocardiogram. Many studies have identified savings with an increase in the number of appropriate referrals once those testing negative had been set on alternative pathways of care. N-terminal pro B-type natriuretic peptide (NT-proBNP) concentrations increase with age in the normal population. Some areas, through collaboration and agreement have introduced age-related ‘cut-off’ values when deciding which patients to refer for further investigations.
The NHS Improvement Heart Improvement Network has identified examples in the UK where BNP testing has been used as a means of ‘rule-out’ for echocardiography. 80 Some documents include economic information and cost analysis for the projects. NICE guidelines on the initial diagnosis of chronic heart failure and referral for echocardiography recommend use of BNP in combination with clinical assessment. 85 NICE guidelines recommend measurement of serum natriuretic peptides in patients with suspected heart failure without previous myocardial infarction, and those with previous myocardial infarction should be referred for an urgent echocardiogram. 85 Although several hospital laboratories carry out BNP testing, few return results within a day. POC BNP testing can considerably reduce turnaround time and could lead to earlier initial treatment, more timely referral and less uncertainty for patients. Using POC BNP levels to quickly rule out heart failure could allow more rapid initiation of investigation of other causes of dyspnoea.
A UK HTA carried out a comprehensive systematic review and meta-analysis of all studies comparing the diagnostic accuracy of BNP testing with clinical examination by cardiologists in heart failure in all settings until 2006. 79 The review included 20 studies on the accuracy of BNP for the diagnosis of clinically defined heart failure. Four studies took place in primary care, one used the POC test and three used laboratory-based tests. These demonstrated a slightly lower sensitivity (84%) but similar specificity (73%). Eight of 16 studies reporting data on the accuracy of NT-proBNP for the diagnosis of clinically defined heart failure were conducted on samples from patients presenting in primary care (pooled sensitivity of 90%, a specificity of 60%). The HTA report also highlighted that the utility of BNP testing will depend on the pre-test probability of chronic heart failure in the patient. 79 BNP testing would contribute important diagnostic information, as a negative test would reduce the post-test probability. A US technology assessment gave similar results. 86
A systematic review and meta-analysis of diagnostic accuracy of BNP testing for heart failure in the emergency department, in which 9 of the 11 studies used the Triage system, showed that the ≤ 100 pg/ml cut-off value used in most studies has a sensitivity of 0.93 and a specificity of 0.66. 87 Two other systematic reviews that included the same studies achieved similar results. 88,89 However, most studies on the Triage BNP system were performed in a US emergency setting, so cut-off values for primary care may be different.
Although a study investigating the impact of POC NT-proBNP testing with the Cobas h 232 (Roche Diagnostics Limited, Rotkreuz, Switzerland) has yet to be carried out, feedback on its ease of use has been documented. 90 In this study, the nurses on duty in the coronary care unit, who operated the device, found it simple to learn and handle.
The Horizon Scanning report observes that most published studies have investigated the use of BNP in an emergency department or hospital setting. 81 It concludes that more studies are required on the diagnostic accuracy of POC BNP tests in primary care.
Electrocardiography
Definition: an ECG is a simple test used to check the heart’s rhythm and electrical activity (NHS Choices).
This map includes portable ECGs.
This map excludes N/A.
N/A, not applicable.
Contains public sector information licensed under the Open Government Licence v3.0. 91
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
Wireless ECG equipment is easy to use. The challenge with ECG devices of any type lies in interpretation. A systematic review of ECG interpretation accuracy studies found that both physicians and computer software frequently made errors, compared with expert electrocardiographers; however, there was also frequent disagreement in interpretation between experts. 92 When interpreting ECG studies, it is important to distinguish the effect of using a simpler ECG from the effect of using a non-expert to interpret the trace. Thus, relatively poor results of single-lead ECGs interpreted by GPs were similar to results obtained for 12-lead ECGs when read by GPs. 93 In contrast, two studies found high sensitivity and high specificity when a bipolar thumb ECG was read by a cardiologist and a bipolar ECG was read by an experienced GP, respectively.
STEP-UP summary statement With regard to SKILLS, ECG is attributed a LOW degree of implementation difficulty owing to the fact that a large majority of practices have the equipment, although interpretation is variable.
Training
The interpretation of readings from the ECG is included in standard medical and other health-care professional (HCP) training. NICE guidance94 notes that skill can be lost in general practice if it is not used frequently, and commissioners should ensure that training on interpretation is offered, linked to local need (the national training cost is quoted to be £105 per person). Some practices may feel that they are too small to develop expertise in ECG interpretation, which may present opportunities for local practices to work together rather than refer to secondary care. In a survey of cardiac equipment use in a cardiac network, a higher proportion of registered nurses than GPs had received formal training in the use of ECG machines, although the interpretation of ECG recordings is predominantly undertaken by GPs rather than by registered nurses. 76 Most ECG machines have interpretative software, but combining interpretative software with GP interpretation does not improve the sensitivity of diagnosis significantly. The evidence suggests that GPs can detect AF on ECGs accurately, given the appropriate training. 95
STEP-UP summary statement With regard to TRAINING, ECG is attributed a LOW degree of implementation difficulty owing to the availability of training provision, although many training needs are not fulfilled.
Equipment
A new ECG machine with installation and initial training costs approximately £2000, including value-added tax (VAT). 94 Increasingly portable ECG machines have become an attractive option. Handheld ECG devices allow ECG readings to be stored on a portable device for review at a later time. Unlike 12-lead ECGs, which require electrodes to be placed on the patient and connected to the ECG, handheld devices have integral electrodes. The Horizon Scanning report96 documents devices that can be used very quickly in primary care for screening or in acutely unwell patients, as well as a monitoring device over a period of time. Two devices could be used for screening for AF in the general population. 97 One is a finger probe similar to that used in general practice for pulse oximetry; the other is a modified BP monitor as used by patients to monitor their BP at home. 98–100 The latter could be used either by people monitoring their own BP to self-screen for AF or by primary care professionals to opportunistically screen patients. These devices are able to adjust their sensitivity to optimise their value as screening devices.
The AliveCor Heart Monitor and AliveECG apps (Alive Technologies, Ashmore, QLD, Australia) are, respectively, a pocket-sized ECG recorder and a mobile device application for the analysis and communication of the results. 101
STEP-UP summary statement With regard to EQUIPMENT, ECG is attributed a LOW degree of implementation difficulty owing to the availability of a wide range of relatively cheap and acceptably accurate equipment that can be matched to clinical need.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates ECG in the ‘examination/therapy room’ (contains public sector information licensed under the Open Government Licence v3.0). In the remaining literature, little detail is available of the premises in which ECGs, portable or not, are to be stored, cleaned or maintained. This corresponds with the findings from the survey of equipment from a UK cardiac network. 76
STEP-UP summary statement With regard to PREMISES, ECG is attributed an UNCERTAIN degree of implementation difficulty owing to insufficient detail of storage requirements.
User perspective
The ease of use, convenience and portable nature of ECG devices means that their implementation could see a better rate of patient compliance than that for a Holter monitor in patients who use the device for home recordings. 102
STEP-UP summary statement With regard to USER PERSPECTIVE, ECG is attributed a LOW degree of implementation difficulty owing to ease of use, particularly of wireless devices.
Primary–secondary interface
Excessive delays for cardiac patients waiting for a hospital appointment led Harrow Primary Care Trust (PCT) to establish a mobile cardiac service delivered in community-based settings: in GP surgeries, in community health-care centres and in patients’ homes. 103 The service used a portable ECG system to automatically store and analyse recorded ECG data using existing computer hardware. The service has reduced waiting times and improved patient care.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, ECG is attributed a LOW degree of implementation difficulty owing to the already-integrated nature of the diagnostic and management pathway.
Conclusion
The overall STEP-UP summary for ECG is presented in Table 13. The increasing portability, ease of use and low prices of ECG equipment has led to almost universal diffusion in NHS primary care; therefore, issues relate to choice of the most appropriate equipment for practice needs, including an optimal mix of static and portable equipment. The reduction in the number of leads in ECG systems makes them easier to use and more acceptable to both patient and clinician. Of particular value is the potential for small ECG devices to replace the 24-hour Holter monitor tests. Organisational issues concerning loans of, and maintenance for, equipment to patients may need to be addressed. Similar concerns will relate to ambulatory BP monitoring.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| ECG | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ⨁ |
| Systematic reviews | EC20, EC22, EC24, EC26 | EC11, EC12, EC20, EC28 | |||||
| Quantitative studies | EC18 | EC3, EC10, EC17, EC19, EC21, EC27 | EC15 | EC22 | |||
| Qualitative studies | EC7, EC9 | ||||||
| UK studies | EC16 | EC4, EC8, EC10, EC16 | EC14 | ||||
| Other significant evidence | EC6 | EC16 | EC1, EC3, EC5, EC16, EC24, EC29 | EC2 | |||
Echocardiography
Definition: an echocardiogram is a test that uses sound waves to create pictures of the heart. The picture is more detailed than a standard X-ray image. An echocardiogram does not expose the body to radiation.
This map includes portable and fixed echocardiography.
This map excludes other forms of ultrasound (see Ultrasound).
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
Echocardiography is pivotal in the management of heart failure as it can make the diagnosis, determine the aetiology and help plan treatment. 104 Community echocardiography is also indicated for heart murmurs, AF and hypertension. 105 An echocardiography service must be integral to a general local plan for heart failure to avoid isolated open-access echocardiography. A UK study comparing community echocardiography with hospital echocardiography found that community echocardiography gave comparable results with traditional hospital echocardiography for LVSD detection and for significant valvular disease detection. 106
STEP-UP summary statement With regard to SKILLS, echocardiography is attributed a MODERATE degree of implementation difficulty owing to difficulties in diagnosis.
Training
Echocardiography should be performed and reported by operators trained to the standards set by the of BSE, that is, they should hold Accreditation in Adult Transthoracic Echocardiography or Adult Community Echocardiography. The BSE has endorsed voluntary accreditation procedures for their members. BSE accreditation in community echocardiography qualifies a GPwSI in standard echocardiography, being equivalent to the BSE adult accreditation process. The provision of echocardiography services, regardless of their setting, must link with staff training and continuing professional development (CPD), which in turn informs workforce planning. In the UK study comparing community echocardiography with community echocardiography, the community echocardiogaphy was performed by a cardiology trained research fellow. 106 The authors identified a need to assess whether or not other HCPs (e.g. nurses) could be trained to successfully to provide similar accuracy with community echocardiography.
STEP-UP summary statement With regard to TRAINING, echocardiography is attributed a MODERATE degree of implementation difficulty owing to the need for an extended period of certificated training.
Equipment
A standard echocardiogram is required for all patients with suspected heart failure and is the required quality standard for Community Echocardiography. 104 The type of study is mainly determined by the clinical question, but also depends on the level of experience of the operator and the type of machine. Standard machines are usually required for standard and advanced studies. Advances in ultrasound technology have led to the development of smaller echocardiography machines that may be transported more easily to different sites in the community. However, studies have not looked at the performance of these smaller echocardiogram machines in community settings where conditions may be different, for example in terms of suitable couches, lighting and lack of an immediate second opinion.
STEP-UP summary statement With regard to EQUIPMENT, echocardiography is attributed a LOW degree of implementation difficulty owing to the ease of use and portability of acceptable devices.
Premises
Community service settings for echocardiography may include mobile units, community hospitals/diagnostic centres, primary care polyclinics/primary care centres/super surgeries, GP practice surgeries and other appropriate locations such as walk-in facilities or high-street settings. Although the performance of smaller echocardiogram machines in community settings has been found to be comparable with that of larger machines in an acute setting, the conditions may be different, for example in terms of the non-availability of suitable couches and the presence of suboptimal lighting. 106
STEP-UP summary statement With regard to PREMISES, echocardiography is attributed a LOW degree of implementation difficulty owing to the flexibility of locales and settings in which the technology may be administered.
User perspective
A UK qualitative study reports numerous barriers to use of echocardiography in primary care. These include lack of availability of electrocardiography, chest radiography and echocardiography, and lack of confidence in interpreting results. GPs are keen to have access to echocardiography, and, where access is provided, they use it appropriately. 82 In another qualitative UK study, cardiologists and nurses were more confident in using and interpreting echocardiography reports than GPs107 but expressed frustration that patients were not routinely referred for echocardiography testing and about the poor quality of referral information.
STEP-UP summary statement With regard to USER PERSPECTIVE, echocardiography is attributed a LOW degree of implementation difficulty owing to the ease of use and lack of an invasive procedure.
Primary–secondary interface
The organisation of services should be based around a BSE-accredited department but organised as a network, avoiding barriers between community and hospitals. Services should extend between hospital and community bases as part of a continuum of care, irrespective of organisational barriers. The service should complement existing hospital-based provision and be integral to the delivery of heart failure services in a health community. The service should be linked to the hospital-based service for second opinions and clinical back-up and to ensure quality assurance.
The favoured model of care is probably the one-stop heart failure clinic, offering diagnosis and initial treatment while liaising with GPs and nurses. 108 A community echocardiography service must always be considered in the context of the whole patient pathway for those with suspected heart failure. The Rapid Access Heart Failure Clinic locates echocardiography within a heart failure one-stop shop linking secondary care-based staff with primary care staff for ongoing care and tertiary staff for specific specialist advice.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, echocardiography is attributed a MODERATE degree of implementation difficulty owing to the need for secondary care support in training, support and referral.
Conclusion
Echocardiography remains a skill that continues to challenge significant numbers of primary care staff, including GPs. Although the administration of the technology is comparatively easy, the challenge lies in the interpretation of the readings. Some GPs may welcome the opportunity to pass on the responsibility for definitive diagnosis and resultant communication to secondary care. Substantive personal and organisational barriers remain in integrating the use of echocardiography into an integrated pathway that spans both primary and secondary care. The STEP-UP summary for echocardiography is presented in Table 14.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| Echocardiography | ○ | ○ | ⨁ | ⨁ | ⨁ | ⨁ | ○ |
| Systematic reviews | EH31, EH34 | EH24, EH32 | |||||
| Quantitative studies | EH5, EH21, EH22, EH23, EH33 | EH8, EH26, EH28, EH29, EH39, EH45 | EH13 | EH37, EH41, EH43, EH48 | |||
| Qualitative studies | EH12, EH16, EH20, EH25, EH37, EH47 | ||||||
| UK studies | EH7, EH35 | EH37, EH40, EH41 | |||||
| Other significant evidence | EH3, EH17, EH42, EH44 | EH3, EH4 | EH6, EH7, EH9, EH10, EH11, EH14, EH18, EH26, EH27, EH30, EH35, EH36, EH38, EH46, EH49 | EH1, EH2, EH7, EH15, EH19 | |||
Diabetic services
Definition: tests to establish the presence of diabetes or to monitor the progress of the condition, especially its sequelae relating to eye and foot consequences.
This map includes HbA1c, diabetic retinopathy tests, diabetic neuropathy tests.
This map excludes POC tests of more general use (see Point-of-care testing).
HbA1c, glycated haemoglobin.
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
Diabetes is a complicated, multisystem condition. It typically proves a complex condition to integrate into diagnostic testing. However, general practice, in the UK and elsewhere, has extensive experience in managing the condition in a community setting. It is not a considerable extension to include diabetes testing in a primary care-orientated pathway of care. Dipstick tests for microalbuminuria are convenient, but their accuracy is uncertain. An Oxfordshire study compared results of single dipstick tests and sequences of dipstick and laboratory tests with a clinical testing strategy based on current guidelines to assess the accuracy and estimate costs of testing. 109 Testing strategies involving dipstick and laboratory measurements or dipstick tests had similar accuracy. The costs of using dipstick tests were, overall, lower than laboratory-based testing. The authors concluded that dipstick testing in this study did not reliably identify diabetes patients with microalbuminuria. Although dipstick testing would decrease testing costs, it could either fail to diagnose most patients with microalbuminuria or increase the numbers of patients retested, depending on the dipstick used.
STEP-UP summary statement With regard to SKILLS, diabetic services are attributed a LOW degree of implementation difficulty owing to the existence of recognised pathways and guidance.
Training
Biochemical tests can be diagnostic and often necessary for monitoring metabolic and endocrine diseases, so it is important for GPs to know which tests are useful in a primary care setting and how to interpret these tests and understand their limitations. A GMC core competency is to understand the use and main limitations of tests commonly used in primary care to investigate and monitor metabolic or endocrine disease, for example fasting blood glucose, glycated haemoglobin (HbA1c), urinalysis for glucose and protein, urine albumin-to-creatinine ratio, near-patient testing for capillary glucose, lipid profile and thyroid function tests, and uric acid tests. 110 Blood glucose meters are a good example of POC testing, and central to result quality is high-quality training, robust internal quality control, external quality assurance schemes and effective process management. 111
STEP-UP summary statement With regard to TRAINING, diabetic services are attributed a LOW degree of implementation difficulty owing to the wide availability of training provision.
Equipment
In patients with existing diabetes, HbA1c monitoring is usually performed every 3–6 months. It typically involves a nurse visit or phlebotomist for venepuncture, with follow-up 1–2 weeks later to discuss results. POC testing could provide more immediate therapeutic decisions and result in fewer patient visits.
Typically, the POC HbA1c device uses a finger-stick drop of blood applied to a reagent cartridge, which is then inserted in a desktop analyser, where the analysis is performed, and HbA1c is reported. A prospective controlled trial comparing POC testing and standard laboratory testing in an urban primary care clinic showed that POC testing availability resulted in more frequent intensification of therapy when baseline HbA1c was ≥ 7.0%. HbA1c fell significantly in the POC testing group but not in the standard care group. 112
STEP-UP summary statement With regard to EQUIPMENT, diabetes services are attributed a LOW degree of implementation difficulty owing to the increasing availability of POC tests and other portable equipment.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services, locates near-patient testing services in the near-patient testing room. 46 No specific detail was identified relating to the implications of storage and maintenance of increasing numbers of POC tests.
STEP-UP summary statement With regard to PREMISES, diabetic services is attributed an UNCERTAIN degree of implementation difficulty owing to insufficient detail on storage and stock maintenance requirements.
User perspective
Diagnostics for diabetes can include POC testing, screening for diabetic retinopathy and a variety of tests for specific diabetes-associated disorders. A survey by the MaDOx (Monitoring and Diagnosis in Oxford) team found that the majority of UK clinicians would like to use POC devices for HbA1c testing,113 but the reasons for their low uptake are unclear. In England, routine diabetes care and diabetic retinopathy screening (DRS) are principally managed in primary care, whereas treatment for retinopathy takes place in secondary care. Patients appear to confuse routine retinal photography at optometry practices during eye examinations with DRS. 114 The same study observed differences between patients screened at GP and those screened at optometrist practices, identifying that ease of making an appointment, including its time and navigating home after the mydriasis drops, etc., appeared less problematic at GP practices.
STEP-UP summary statement With regard to USER PERSPECTIVE, diabetic services are attributed a LOW degree of implementation difficulty owing to accepted pathways and standards of care.
Primary–secondary interface
Initiatives to break the reported primary–secondary divide in diabetes care include a West Sussex initiative in which services have been redesigned so that the primary care diabetes specialist nurses are employed by the PCT and work with nurses and GPs in general practice, but are based at the secondary care diabetes centre. 115 This model of care has enabled the primary care diabetes specialist nurses to function as part of the wider diabetes team, which encompasses all primary and secondary care clinicians, while not being isolated from their secondary care colleagues.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, diabetic services are attributed a LOW degree of implementation difficulty owing to the existence of integrated pathways across primary–secondary care, notwithstanding the continuing ‘divide’ in some circumstances.
Conclusion
Diabetic services has a long tradition of being delivered as a two-tier service across the primary–secondary interface. The distinction is becoming increasingly blurred, as is the case for cardiac services (as described earlier). In examining these technologies it is most important to first define the optimal management pathway and then decide where to locate the various diagnostic interventions. A key consideration, therefore, is the existence of guidelines that make the basis for intervention, and for specific roles, manifestly clear for both patients and clinicians. Table 15 presents the STEP-UP summary map for diabetic services.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| Diabetic services | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ⨁ |
| Systematic reviews | D1, D8, D22, D26, D30 | ||||||
| Quantitative studies | D24 | D3, D7, D14, D16, D17, D18, D25 | D21 | D10 | |||
| Qualitative studies | D2, D7 | D9 | D6, D9, D29 | ||||
| UK studies | D28 | D11 | D29 | D11 | |||
| Other significant evidence | D12, D19, D23, D32 | D27, D31 | D13, D15, D20, D33, D34 | D4, D5 | |||
Endoscopy
Definition: an endoscope is a long, thin, flexible tube that has a light source and a video camera at one end. Images of the inside of the body are relayed to a television screen. Endoscopes can be inserted into the body through a natural opening, such as the mouth and down the throat, or through the anus (NHS Choices).
This map includes flexible sigmoidoscopy.
This map excludes colonoscopy.
Contains public sector information licensed under the Open Government Licence v3.0. 116
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
Many GPwSIs in gastroenterology will probably have developed skills or expertise in endoscopy during their hospital training. Competent GPs should be able to demonstrate that they have sufficient skills to ensure the safe and effective practice of adult endoscopy. Endoscopy procedures in primary care are typically performed on a selected population of otherwise fit patients, making comparison with secondary care unhelpful and irrelevant. A 2015 systematic review of the safety, competency and cost-effectiveness of nursing staff providing GI endoscopy services, although not examining a primary care context, reported the collective experiences of nurses working in metropolitan areas under the strict supervision and guidance of a specialist gastroenterologist. 117 Nurse endoscopists were less cost-effective per procedure at year 1 than services provided by physicians, due largely to the increased need for subsequent endoscopies, specialist follow-up and primary care consultations. However, the studies clustered between 1999 and 2001, making applicability problematic.
STEP-UP summary statement With regard to SKILLS, endoscopy in primary care is attributed a HIGH degree of implementation difficulty owing to the range of skills and competencies required, the risk of serious consequences and the time taken to acquire requisite competencies.
Training
Training for endoscopy is regulated by the Joint Advisory Group on Gastroenterology (JAG). All training must take place at JAG-approved units. Initially, all practitioners have to attend a formal training course (a JAG-compliant course) in that particular procedure. In addition, the GP should have knowledge of the 15 endoscopy workforce competencies that pertain to a primary care setting. In 2002, 96% of GPs performing endoscopy had undergone training in, and continued to work in, a consultant-led endoscopy unit. 118 The same survey concluded that simple diagnostic endoscopies could be performed safely in the primary care setting, leaving secondary care units to concentrate on patients requiring sedation, those who are acutely ill and those who require therapeutic procedures. 118 Primary Care Society for Gastroenterology guidelines suggest that no unit should offer fewer than 200 cases of any flexible endoscopy per year and the endoscopist should have continuing association with a secondary care unit for a wider exposure to case mix. Although the accreditation of a practitioner as fit to provide endoscopy services is outlined by JAG, providing endoscopy services goes beyond this and requires fulfilment of the formal GPwSI accreditation process. Support for endoscopy in primary care may be achieved through active membership of a professional society, such as the Primary Care Society for Gastroenterology or the British Society of Gastroenterology.
STEP-UP summary statement With regard to TRAINING, endoscopy in primary care is attributed a MODERATE degree of implementation difficulty owing to the availability of courses, the clear definition of a curriculum, the existence of specialist groups and the variety of training methods available that mitigate, to some degree, the wide range of skills and competencies required.
Equipment
Endoscopy ‘incurs significant cost through its high usage rate of consumables coupled with significant capital costs (eg, endoscope systems)’. 119 When endoscopy is performed in a setting outside a hospital unit, such as a cottage hospital, diagnostic and treatment centre or GP surgery, the facilities, staffing and equipment should be of the same quality. In 1994, a specification was produced for an endoscopy unit. 120 This included upper GI endoscopes and a fibre sigmoidoscope, a light source, a procedure trolley, an endoscopy trolley, three suction machines, an automated washing machine and three pulse oximeters. Anaesthetic and resuscitation facilities must be provided on site for the management of sedated patients. Adequate equipment must be available, together with safe facilities for its cleaning and maintenance. Cleaning and decontamination of endoscopes should comply with British Society for Gastroenterology (BSG) and Control of Substances Hazardous to Health (COSHH) guidelines. 121 The BSG has published recommendations in a report on aldehyde disinfection and health in endoscopy units. 122 Glutaraldehyde is most commonly used for disinfection of flexible GI endoscopes, and it is toxic, an irritant and allergenic.
STEP-UP summary statement With regard to EQUIPMENT, endoscopy in primary care is attributed a HIGH degree of implementation difficulty owing to the capital costs of purchase, the expense of consumables, the precautions required for cleaning and maintenance and the risks associated with glutaraldehyde.
Premises
Requirements for an endoscopy unit, typically based in an acute hospital, are outlined in Health Building Note 52123 and in the Users Guide to Achieving a JAG Compliant Endoscopy Environment. 124 Similarly, guidelines from the World Endoscopy Organization specify design principles for a digestive disease endoscopy unit. Health Building Note 11-01, Facilities for Primary and Community Care Services, suggests that ‘most invasive procedures and certain procedures using rigid endoscopes can take place in a generic treatment room’ (contains public sector information licensed under the Open Government Licence v3.0). 46 Endoscopy needs to be practised in a purpose-designed area with adequate space and easy accessibility. Instruments, their accessories, monitoring equipment and all the necessary drugs need to be readily to hand. The standards of care in purpose-built, well-equipped primary care premises need not fall noticeably short of those in a general hospital, particularly when staff are well trained and experienced in endoscopy. Endoscopy should be performed in a unit that complies with the JAG guidelines for safe endoscopy. In general practice any endoscopy facility is likely to be used for multiple purposes, but due consideration must be given to the care, storage and security of endoscopy equipment.
STEP-UP summary statement With regard to PREMISES, endoscopy in primary care is attributed a HIGH degree of implementation difficulty owing to the high capital costs of specific accommodation for equipment and the requirements to provide a safe environment for operation of the equipment and for cleaning.
User perspective
Consumer preference is frequently cited as a major driver for a move towards primary care endoscopy services. However, the public is relatively poorly informed of the risks, precautions and the importance of staff training and experience as they contribute to safe endoscopy. Endoscopy procedures should be performed to recognised levels of safety and accuracy, ensuring patient comfort and satisfaction. The lack of severe symptoms, fear of pain, concerns of sedation, comorbidity and competing life demands have been reported by patients as barriers to performing an endoscopic investigation. 125
In a qualitative study of UK ethnic groups, focus groups were used to explore barriers to the uptake of flexible sigmoidoscopy screening among UK ethnic minority populations. Common barriers across all ethnic groups were anxiety regarding the invasiveness of the test, the bowel preparation and fear of a cancer diagnosis. 126
STEP-UP summary statement With regard to the USER PERSPECTIVE, endoscopy in primary care is attributed an assessment of UNCERTAIN, given that attitudes of patients and staff to endoscopy in primary care are not well explored.
Primary–secondary interface
Hospital-based endoscopy depends largely on highly trained specialists. These specialists have acquired a wider clinical knowledge of gastroenterology over and above simple endoscopic interpretation. Conflicts of access and perceived vested interest are best resolved by improving communication between GPs, specialists, patient groups, purchasers and provider units. Alternatives to a GP-based community service include greater open access to hospital facilities by GPs and outreach clinics performed by hospital-based consultants in community hospitals or individual/group practices. 127
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, endoscopy in primary care is attributed a MODERATE degree of implementation difficulty given existing working relationships with secondary care but the need to refer more serious or complex cases to an acute hospital setting.
Conclusion
The potential to offer endoscopy in primary care has been long recognised and it may make an important potential contribution to the disease management pathway. It no longer holds the high level of risk that it was once perceived to have. Developments in more flexible diagnostic tools have helped to make it easier to conduct. It continues to occasion fear among patients, particularly those who are older and female. The physical requirements for the premises, for example for disinfection and storage of the equipment, may be prohibitive in an ordinary-sized general practice. However, these requirements may more easily be accommodated in purpose-built clinics or community hospitals. Table 16 presents the overall STEP-UP summary map.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| Endoscopy | ⨂ | ○ | ⨂ | ⨂ | Ø | ⨂ | ○ |
| Systematic reviews | En33, En40 | En7, En21, En41 | En41 | ||||
| Quantitative studies | En4, En12, En31 | En35, En39 | En6, En8, En9, En10, En13, En20, En23, En30 | En37, En38, En42 | |||
| Qualitative studies | En22, En34 | En3, En28, En34 | |||||
| UK studies | En4, En16 | En15 | En8, En9, En10, En13, En14, En20, En23, En30, En32 | En27, En32 | |||
| Other significant evidence | En1 | En2, En11, En15, En18 | En14, En19, En29, En43 | En17, En24, En26 | En5, En25, En36 | ||
Genetic testing
Definition: genetic testing is a type of medical test that identifies changes in chromosomes, genes or proteins. The results of a genetic test can confirm or rule out a suspected genetic condition or help determine a person’s chance of developing or passing on a genetic disorder.
This map includes testing for inherited genetic conditions and for genetic risk factors indicating a predisposition to particular conditions, for example cardiac conditions.
This map excludes newborn heel screening.
Definition is public domain information reproduced from US National Library of Medicine: Genetics Home Reference. 128
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
Skills for genetic testing relate to understanding which patients to test, what tests to order and how to interpret the results of these genetic tests. Importantly, they also require being able to handle the counselling and communication issues associated with testing and the test result. 129 A Dutch survey130 of knowledge of genetics and genetic tests revealed general levels to be poor, with GP knowledge being poorer than that of paediatricians and gynaecologists.
STEP-UP summary statement With regard to SKILLS, genetic testing is attributed a HIGH degree of implementation difficulty owing to inadequate knowledge of genetics, challenges in interpretation and the need for skills in communicating and handling the outcome of the test.
Training
Generally, primary care practitioners receive minimal training in clinical genetics. Various methods are required to support practitioners to develop these genetic skills and knowledge, including changes to the undergraduate curriculum. The empirical evidence of the learning needs of practitioners in relation to genetic testing reveals widespread deficits in knowledge and skills, and low confidence levels. Skills-based training, clinical scenarios and assessment have all been identified as factors that can promote learning.
Primary care providers are interested in learning more about who should receive genetic testing and what tests are available. Educational efforts should build on primary care providers’ prior knowledge base, highlight the clinical relevance of genetic medicine to primary care practice and emphasise red flags: cues to alert primary care providers to a potential genetic contribution. 131 Nurses are on the front line and are expected to recognise patterns of disease that may indicate a possible genetic link, educate the family about the implications of a potential genetic susceptibility and refer the family for counselling. Many studies of nurses’ knowledge of genetics reveal gaps in professional competence and/or education. 132 The authors suggested that each nurse should, thus, acquire a minimum basic knowledge of genetics. 133
STEP-UP summary statement With regard to TRAINING, genetic testing is attributed a MODERATE degree of implementation difficulty owing to the need to receive significant training and ongoing support in offering genetic services.
Equipment
Genetic testing is expensive and time-consuming, given the sheer scale of the genes that need to be examined. However, the development of direct-to-consumer testing is likely to reduce this financial burden. A systematic review of genetic tests in primary care was published in 1999. 134 This mapping review has been unable to find a more recently published review. Specific searching for such a systematic review is recommended before considering whether or not to commission further work.
STEP-UP summary statement With regard to EQUIPMENT, genetic testing is attributed a LOW degree of implementation difficulty owing to increasing numbers of direct-to-consumer POC tests.
Premises
The mapping review was unable to identify any items that specifically described requirements to house genetic testing facilities in general practice or community premises. Health Building Note 11-01, Facilities for Primary and Community Care Services, locates near-patient testing services (such as blood and gas) in the ‘near-patient testing room’ (contains public sector information licensed under the Open Government Licence v3.0). 46 This Health Building Note does not provide detailed design guidance on specific rooms and spaces. It is unclear what tests should be provided and what the implications are with regard to the management of stock.
STEP-UP summary statement With regard to PREMISES, genetic testing is attributed an UNCERTAIN rating for implementation difficulty owing to the shortage of studies describing how tests might be accommodated. Co-ordinated approaches with regard to storage may relate to POC tests.
User perspective
Concerns are expressed about potential harm from the inappropriate use of genetic testing. It is believed that primary care practitioners are reluctant to adopt responsibility for genetic testing. In a survey of family physicians, respondents felt that genetic tests would be more useful for breast cancer and haemochromatosis than for Alzheimer’s disease, heart disease or diabetes. Individuals who believed themselves more familiar with genetic tests were more likely to anticipate that genetic testing would impact significantly on their future practice. 135
The findings from qualitative studies of patient viewpoints report misunderstandings concerning genetic tests, for example that genetic tests are more predictive than they actually are, and that they are predictive of behaviours for which no markers have in fact yet been discovered. 136 A qualitative study of genetic testing for risk of coronary heart disease found that the test was acceptable. 137 The findings from qualitative studies of provider viewpoints report that cost of testing consistently appears as the most frequently cited barrier to genetic testing. 138–140
STEP-UP summary statement With regard to USER PERSPECTIVES, genetic testing has LOW implementation difficulty for patients and MODERATE difficulty for clinicians, because patients already have access to direct-to-consumer genetic tests but GPs are cautious about the ramifications of interpretation.
Primary–secondary interface
Studies in the UK have evaluated community genetic counsellors acting as outreach workers134 from the genetics clinic to liaise with local general practices. Such practitioners are either genetic nurses141 or genetic counsellors, and they can fulfil a dual role of filtering referrals to the geneticist and providing basic genetic information. 142
Genetic nurse counsellors, specifically in a cancer genetics context, were found to perform not significantly differently from conventional cancer genetic services in two RCTs in Scotland and Wales. 143 The development of primary care specialists, possibly working in conjunction with community genetic counsellors, has been suggested to offer an intermediate point of referral between general practice and specialist genetic clinics. 142
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, genetic testing is attributed a MODERATE degree of implementation difficulty owing to a high dependence on secondary care expertise, support and follow-up provision.
Conclusion
Because of the uncertainties around the benefits and harm from genetic testing, the clinical implications of a specific genetic test will require careful evaluation, including information about cost-effectiveness, before widespread adoption is recommended. 142 With thousands of tests being developed it is important to ensure that these are matched to meaningful intervention. Rather than focusing on the organisation and service delivery of specific individual tests, the priority is to organise services that can adapt to emerging technologies, their interpretation and their implications for patient support and counselling. Table 17 presents the STEP-UP summary map for genetic testing.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| Genetic testing | ⨂ | ○ | ⨁ | Ø | ○ | ⨁ | ○ |
| Systematic reviews | G15 | G3 | |||||
| Quantitative studies | G1, G2, G7, G25, G28, G33, G58, G63, G71 | G16, G25, G51, G52, G53 | G21 | G6, G23, G31, G58, G71 | G40, G78 | G1, G4, G63 | |
| Qualitative studies | G55, G70 | G6, G13, G27, G46, G43, G47, G48, G49, G56, G57, G62, G65, G69, G74, G76, G79 | G5, G11, G64, G69 | G13, G69 | |||
| UK studies | G30, G67 | G22 | G60, G73 | G23, G47, G48, G54, G59, G66, G75 | G71, G75, G78 | G71 | |
| Other substantive evidence | G7, G8, G9, G14, G35, G67 | G12, G17, G18, G19, G26, G37, G38, G45, G61, G72 | G4, G24, G29, G32, G34, G39, G42, G44, G50, G77 | G34, G35, G36, G41 | G10, G20, G34, G68 | ||
Magnetic resonance imaging
Definition: MRI is a type of scan that uses strong magnetic fields and radio waves to produce detailed images of the inside of the body (NHS Choices).
This map includes portable MRI and (exceptionally for this review) commercial service provision.
Contains public sector information licensed under the Open Government Licence v3.0. 144
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
To provide a MRI service requires that staff undertake sufficient current MRI practice and a sufficient number of examinations to maintain competency in every area of MRI. Staff interpreting the images and providing a clinical report should fulfil the following requirements:145
-
Be a UK-registered radiologist on the GMC Specialist Register undertaking sufficient current clinical practice in that modality. A consultant radiologist must have undertaken planned regular clinical MRI sessions in their current job plan.
-
Be a radiographer currently registered with the Health and Care Professions Council and have performed regular sessions of MRI examinations within the last 12 months.
-
Have a minimum of 1 year’s experience.
-
Maintain their CPD in accordance with their professional body guidelines.
-
Meet the specification set out in the National Occupational Standards for Imaging (RD5: produce MRI images for diagnostic purposes). 145
STEP-UP summary statement With regard to SKILLS, MRI is attributed a MODERATE degree of implementation difficulty owing to its requirements in terms of experience and training.
Training
The BSCMR has endorsed voluntary accreditation procedures for their members. These have no statutory role but are provided to allow both medical and non-medical practitioners to demonstrate a specified level of experience in an appropriate educational environment. In the case of MRI, the BSCMR and the British Society for Cardiac Imaging support the criteria developed by the US-based Society for Cardiovascular Magnetic Resonance and do not separately accredit individuals.
STEP-UP summary statement With regard to TRAINING, MRI is attributed a MODERATE degree of implementation difficulty owing to the ongoing need for professional development and updating.
Equipment
Magnetic resonance imaging has entered common use as part of the standard equipment portfolio over the last 10–15 years. The following are commissioned requirements for MRI equipment that should be met or exceeded: fixed or mobile units shall contain one full-body MRI scanner with a magnetic strength of at least 1.5 Tesla; complies with the Safety Guidelines for Magnetic Resonance Equipment in Clinical Use146 as updated; and is superseded and replaced from time to time.
STEP-UP summary statement With regard to EQUIPMENT, MRI is attributed a HIGH degree of implementation difficulty owing to the prohibitive cost of an installation.
Premises
There is no specific provision for MRI in Health Building Note 11-01, Facilities for Primary and Community Care Services. 46 Commissioners will need to consider mobile or static sites. All facilities, including mobile units, are required to have a minimum of a patient reception and waiting area either on the unit or nearby, access to a toilet and access to appropriate levels of security. We found no specific guidance on housing MRI units in primary care.
STEP-UP summary statement With regard to PREMISES, MRI is attributed a HIGH degree of implementation difficulty owing to the exacting requirements for facilities and supplies.
User perspective
Because MRI equipment is typically housed in secondary care, we were unable to find qualitative research relating to attitudes to MRI specifically in a community setting. Items on perspectives of MRI provided by acute hospitals from GPs and their referred patients would indirectly inform this issue. However, these will require a supplementary search strategy.
STEP-UP summary statement With regard to USER PERSPECTIVE, MRI is attributed an UNCERTAIN degree of implementation difficulty owing to the shortage of published experience on MRI in a primary care context.
Primary–secondary interface
A MRI service needs to work with other providers to offer an integrated service. Key to the successful integration of MRI services is consideration of the management pathway, irrespective of which facilities are located in the primary or secondary sectors of local health service provision. The London NHS Diagnostic Service was established by the InHealth Group in 2007 following competitive tendering. 51 Local PCTs and federated general practice groups utilise the service, which charges for imaging services including ultrasound, plain film and MRI at standard tariff costs. This popular model of service delivery avoids the heavy capital expenditure required for purchasing the MRI equipment.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, MRI is attributed a MODERATE degree of implementation difficulty owing to the dependence on secondary care support facilities and/or commercial providers.
Conclusion
Ultimately, MRI provision did not meet the criteria for this review in terms of services and staff managed in a primary care setting. The most common model was of service provision under contract by an external provider. This sidesteps many of the considerations from the STEP-UP framework, for example training and equipment, except from a commissioner and quality assurance customer perspective (Table 18). The implications of offering MRI services have not been explored and represent a potential line of future exploration.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| MRI | ○ | ○ | ⨂ | ⨂ | Ø | Ø | ○ |
| Systematic reviews | M17 | ||||||
| Quantitative studies | M2 | M5, M6, M8, M9, M12, M18, M20, M22, M23, M24 | |||||
| Qualitative studies | |||||||
| UK studies | M2 | M12, M18, M21, M22 | |||||
| Other significant evidence | M4 | M1, M3, M7, M14 | M16 | M10, M11, M13, M15, M19 | |||
Point-of-care testing
Definition: POC testing is defined as any analytical test performed for an individual by a HCP outside the conventional laboratory setting. 147
This map includes CRP, D-dimer, faecal occult blood, haemoglobin, HbA1c, Infectious disease testing, INR, lipid profiles, nose/throat swab for influenza, platelet count, procalcitonin biomarkers, quantitative β-human chorionic gonadotropin, throat swab for group A streptococci, urine albumin–creatinine ratio, urine leucocytes or nitrite, urine pregnancy test, urine strips and whole-blood lactate.
This map excludes BNP (see Cardiac services), blood glucose, HbA1c (see Diabetic services), cholesterol screening, pregnancy tests and respiratory POC tests (see Respiratory tests).
CRP, C-reactive protein.
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
To practise competently, users of POC testing equipment must have the knowledge and skills for safe and effective practice when working without direct supervision; they must recognise and work within the limits of competence, keeping knowledge and skills up to date, and take part in appropriate learning and practice activities that maintain and develop their competence and performance. 148
Standard operating procedures would include clinical governance, risk management and also implementation of risk reduction strategies. This would be achieved by careful patient selection, internal and external quality control procedures, a regular audit process, and ensuring relevant, complete and accurate documentation of the clinic process.
The internal quality control supplied by the manufacturer should be performed at the start of each clinic, and external quality assurance, provided either by collaboration with a hospital laboratory or through a quality assurance scheme, such as the National External Quality Assessment Service, should be performed regularly.
STEP-UP summary statement With regard to SKILLS, POC tests are attributed a MODERATE degree of implementation difficulty owing to the exacting requirements for quality assurance.
Training
Training of health professionals to manage POC testing should include an understanding of the specific POC device used, setting up and using the device, recording of results and quality assurance materials, health and safety, disposal of sharps, and the COSHH regulations. 121
STEP-UP summary statement With regard to TRAINING, POC tests are attributed a MODERATE degree of implementation difficulty owing to the need to identify which to use, what they mean and how to integrate them into existing clinical practice.
Equipment
As with all in vitro diagnostic equipment, new POC equipment should be thoroughly evaluated prior to clinical implementation to ensure that reliable results are consistently obtained. 149 In the UK, this is performed on a voluntary basis at the manufacturers’ discretion by the Medicines and Healthcare products Regulatory Agency (MHRA) of the Department of Health. Calibration should be performed by the manufacturers, using the same procedure as conventional laboratory systems. Calibration as performed by the manufacturers typically cannot be altered by the operator. New POC coagulometers are currently under evaluation with the MHRA and will have further field evaluations in the primary care setting. 150 A Cochrane review concluded that a POC biomarker to guide antibiotic treatment of acute respiratory infections in primary care can significantly reduce antibiotic use. 151
An illustrative STEP-UP framework assessment, for C-reactive protein (CRP), is given in Table 19.
| Description of technology | ||||||
|---|---|---|---|---|---|---|
| CRP is a protein in the blood that can quickly be measured from a finger-prick blood sample using a POC test. When CRP is low in acute exacerbations of COPD, patients are unlikely to benefit from antibiotics | ||||||
| Brief summary of effectiveness/cost-effectiveness in primary care | ||||||
| Cochrane review concluded that CRP, to guide antibiotic treatment of acute respiratory infections in primary care, can significantly reduce antibiotic use.151 A major HTA trial (the PACE study) is currently under way and due to report in June 2018152 | ||||||
| Skills | ||||||
| No details available – unlikely to be complex – requirement for quality control | ||||||
| Training | ||||||
| No details available – unlikely to be complex – requirement for knowledge on interpretation and practical application | ||||||
| Equipment | ||||||
| Simple POC test requiring only a finger-prick sample. Also analysis equipment | ||||||
| Premises | ||||||
| No evidence for impact on premises | ||||||
| Clinician | ||||||
| Interviews with 20 clinicians to gather in-depth feedback on use of the test, will help plan general uptake should the CRP POC test prove worthwhile (PACE study protocol)152 | ||||||
| Patient | ||||||
| Interviews with 20 patients to gather in-depth feedback on use of the test, which will help plan general uptake should the CRP POC test prove worthwhile (PACE study protocol)152 | ||||||
| Primary–secondary interface | ||||||
| Implications unclear at present; potential to reduce antibiotic administration | ||||||
| Skills | Training | Equipment | Premise | Clinician | Patient | Primary–secondary interface |
| ○ | ○ | ⨁ | Ø | ⨁ | ⨁ | Ø |
STEP-UP summary statement With regard to EQUIPMENT, POC testing is attributed a LOW degree of implementation difficulty owing to the ease of testing and ready availability of equipment and supplies.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates near-patient testing services (such as blood and gas) in the ‘near-patient testing room’ (contains public sector information licensed under the Open Government Licence v3.0). This document does not provide detailed design guidance on specific rooms and spaces and refers to the following for guidance on generic rooms and spaces: Health Building Note 00-03, Clinical and Clinical Support Spaces. 153 It is unclear what tests should be provided and what the implications are with regard to the management of stock.
STEP-UP summary statement With regard to PREMISES, POC testing is attributed an UNCERTAIN rating for implementation difficulty owing to the shortage of studies describing how tests might be accommodated. Co-ordinating approaches with regard to storage may relate to genetic tests.
User perspective
Paradoxically, the greater the ease with which tests might be severed from existing secondary care domination of supply by primary care services, the correspondingly greater the likelihood of these tests bypassing primary care altogether, by going direct to consumer. GPs with different levels of experience of using diagnostic POC tests had similar perceptions that they would help to reassure patients and lead to more effective targeted treatment without alienating or upsetting patients. 13 Overall, primary care clinicians believed that POC tests increase diagnostic certainty, help target treatment, educate and empower patients, and improved the relationship between clinicians and patients by enhancing communication and shared decision-making. 13 Clinicians were also concerned about cost, over-reliance (in that POC tests could undermine clinical skills) and limited usefulness. Some issues are not unique to POC tests, but also apply to laboratory testing in general. In addition to the barriers to POC test use identified here, other reasons for lack of widespread use may include lack of needs assessments of primary health-care clinicians, resulting in discordance between the tests that they want/would use frequently, and those that are produced. A published review13 focused on blood POC tests in primary care, so further research is needed to confirm whether or not the issues raised here apply to other types of POC tests.
STEP-UP summary statement With regard to USER PERSPECTIVES, attitudes to POC testing between patients and clinicians differed, resulting in a MODERATE rating for implementation difficulty for clinicians and a LOW rating for implementation difficulty for patients.
Primary–secondary interface
In seeking to reduce variation, some PCTs are commissioning laboratories to provide a satellite service from the central laboratory to primary care sites. Testing in primary care reduces the dependency of primary care clinicians on hospital-based services, many of which are outside their own control.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, POC testing is attributed a MODERATE degree of implementation difficulty, as it may facilitate earlier diagnosis but has a potentially unpredictable effect on demand for secondary care services.
Conclusion
Evidence so far indicates that POC testing systems have great potential for primary care with clear quality-of-life benefits for patients. Table 20 presents the overall STEP-UP summary map. The implementation of POC testing technologies requires collaboration between manufacturers, pathology laboratories and general practice, as well as adherence to a recognised external quality assurance scheme. Diagnostic technologies need to improve both accuracy and precision.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| POC testing | ○ | ○ | ⨁ | Ø | ○ | ⨁ | ○ |
| Systematic reviews | P23 | P2, P10, P17, P22, P37, P38, P39, P44, P52 | P26 | ||||
| Quantitative studies | P7, P11, P18, P31, P32, P40, P45, P60 | P48 | P3, P5, P15, P19, P24, P27, P30, P31, P33, P40, P41, P45, P51, P58 | P12 | P9, P29, P45, P53 | P18, P47, P60 | |
| Qualitative studies | P6, P8, P59 | P57, P59 | |||||
| UK studies | P11 | P41 | P29 | ||||
| Other substantive evidence | P20, P56 | P4, P28, P36, P55 | P1, P4, P14, P16, P21, P25, P30, P34, P35, P42, P43, P46, P49, P50, P52, P54, P56 | P13 | |||
The increased availability of a test is likely to increase the usage of that test. Increased use may indicate previously unmet need, constitute an inappropriate response or simply reflect a growth in demand. In practices where desktop analysers are introduced, the rate of testing increases, but these extra tests do not inform changes in diagnosis or management. 154 However, such studies often fail to acknowledge the effect of tests in reducing the uncertainty experienced by both doctor and patient.
From the perspective of GPs, likely benefits of introducing POC tests include increased diagnostic certainty, more efficient care and fewer (re)consultations. 13 Barriers to the implementation of POC tests must be addressed, some by primary care and others elsewhere. The accuracy of POC tests in primary care populations must be addressed by manufacturers. Policy-makers and clinicians should carefully consider the role and impact of POC tests in primary care in relation to GP roles. Against a backdrop of reductions in health service funding, attention must be paid to how POC tests are to be funded. 13
Radiology/X-ray
Definition: an X-ray is a quick and painless procedure commonly used to produce images of the inside of the body (NHS Choices).
This map includes chest X-ray and partial CT scanning.
This map excludes echocardiography and MRI.
CT, computed tomography. Contains public sector information licensed under the Open Government Licence v3.0. 155
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
Radiology is a well-documented profession with well-developed education, training and curricula. For example, the Royal College of Radiologists (RCR) produces a Specialty Training Curriculum for Clinical Radiology. 156 Training must include the equipment and its intended application. It will also include related topics such as health and safety, radiation protection and control of infection. 157 RCR guidance for clinical radiographers emphasises quality assurance issues. It would be challenging for a small radiological unit based in primary care to achieve the adherence to standards and quality control required by a fully-established radiological department.
STEP-UP summary statement With regard to SKILLS, radiology/X-ray is attributed a MODERATE degree of implementation difficulty due to the need to satisfy the expectations created by the curriculum and quality assurance procedures.
Training
Patients should have timely access to appropriate imaging provided by a service with proven clinical governance structures in place, by appropriately trained staff using appropriately maintained equipment, with underlying compliant ‘24/7’ information technology (IT) support. Comprehensive clinical imaging services in the NHS provide patients, GPs and other referrers and commissioners with direct access to essential core services. The quality of any clinical service can be judged by patient outcomes, patient safety, patient and user experience and efficiency. The RCR and the Society and College of Radiographers (SCoR) have developed the Imaging Services Accreditation Standard (ISAS) and the associated accreditation scheme. Patients, GPs, referrers and commissioners can be assured that ISAS-accredited services are delivering the highest quality of services.
STEP-UP summary statement With regard to TRAINING, radiology/X-ray is attributed a HIGH degree of implementation difficulty owing to extensive and specialist training requirements.
Equipment
One study in primary care reports that patient management by the GP changed in 60% of patients following chest X-ray. Chest X-ray substantially reduced the number of referrals and initiation or change in therapy, and more patients were reassured by their GP. This confirms that chest X-ray is an important diagnostic tool for GPs. 158
STEP-UP summary statement With regard to EQUIPMENT, radiology/X-ray is attributed a LOW degree of implementation difficulty owing to established practices and procedures for using X-ray equipment.
Premises
An imaging cluster will require such facilities as X-ray rooms, image control/reporting rooms and changing rooms. These facilities should be clustered together, alongside other dedicated imaging rooms, where provided. 159 Rooms have to meet the requirements of the Ionising Radiations Regulations 1999,160 which have much stricter limits than earlier regulations on radiation exposure to the public from man-made sources of radiation.
STEP-UP summary statement With regard to PREMISES, radiology/X-ray is attributed a MODERATE degree of implementation difficulty owing to the need for accommodation with radiation protection and shielding.
User perspective
When ordering radiological tests, clinicians in primary care consider such factors as the potential impact on the clinical outcome for the patient, the probability of significant findings based on the clinical picture and the sensitivity/specificity of the test. Urgent cases in which the report has an impact on the immediate management of the patient need to be reported at the time of examination and to be made available to the referrer. All reports should be communicated electronically via a reporting system that can push both key images and the report directly into the patient management system. The timeliness of test, and then the result, is thus a key question for patients when they are referred for an imaging test.
STEP-UP summary statement With regard to USER PERSPECTIVES, radiology/X-ray is attributed a LOW degree of implementation difficulty owing to the familiarity of X-ray arrangements.
Primary–secondary interface
The Department of Health has been seeking to facilitate imaging tailored to the needs of primary care, and thus combat a perceived secondary care imaging agenda. When GPs have access to diagnostic testing from primary care and clear referral guidelines, they are likely to use imaging resources as efficiently as hospital doctors. If implemented correctly, improving imaging access should shorten the patient pathway, facilitate better patient care and produce savings across the health-care economy. Traditional providers of NHS clinical imaging based in secondary care hospitals must seek to balance demands from both secondary and primary care for their services. A London-based group explored the effect on patient management of direct access to diagnostic imaging. 161 Three core components supported appropriate GP referral guidelines, structured referral forms and clinical triage with telephone feedback to GPs suggesting alternative tests or contraindications to testing. Thirty-two per cent of patients referred for echocardiography were found to have an abnormal report but only 29% of these were referred to secondary care, as the majority were managed in primary care.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, radiology/X-ray is attributed a MODERATE degree of implementation difficulty owing to uncertainties about the new provider arrangements.
Conclusion
Imaging services are facing what has been described as a paradigm shift. Although the increase in competitiveness and a market orientation ultimately holds the prospect of higher standards and expectations, this is being realised on only a small local scale at present. There is considerable uncertainty in this particular area of diagnostic provision (Table 21 gives a STEP-UP summary), making it very difficult to predict what will happen in the short term.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| Radiology/X-ray | ○ | ⨁ | ○ | ○ | ⨁ | ⨁ | ○ |
| Systematic reviews | |||||||
| Quantitative studies | R11, R26, R27 | R7, R16, R19, R20 | |||||
| Qualitative studies | R3 | ||||||
| UK studies | R4, R21 | R2, R12 | R9 | R1, R22, R31 | |||
| Other substantive evidence | R15, R28, R29 | R6, R13, R14, R21, R23 | R2, R5, R10, R15, R17, R18, R29, R32 | R2 | R8, R17, R18, R24, R25, R30 | ||
Respiratory tests
Definition: respiratory tests measure how much air is moved in and out of the lungs, how successful the lungs are at getting oxygen into the blood stream and if there are problems in the lungs that can be seen in images of the lungs.
This map includes lung function tests.
This map excludes spirometry (see Spirometry) and CRP (see Point-of-care testing).
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
A systematic review of 30 primary care studies from across the world162 evaluated the diagnostic ability of GPs in relation to respiratory diseases, such as acute respiratory infections, tuberculosis, asthma and COPD. In relation to asthma and COPD, studies show either overdiagnosis or underdiagnosis. With increasing affordability and recognition of its clinical applications, there is an increasing interest of the role of the oximeter in primary care. Data concerning the influence of pulse oximetry on patient management and on the extent of oximetry use in the general practice setting are scarce. Several studies identify the role and potential of the oximeter as a screening tool in assessing hypoxia in primary care. 163–166
STEP-UP summary statement With regard to SKILLS, respiratory tests are attributed a LOW degree of implementation difficulty owing to the existence of well-established testing technologies.
Training
A draft working document outlines possible roles for a GPwSI, using respiratory medicine as a model. It envisages the role of a GPwSI in respiratory medicine as primarily one of leadership and service development. A modified draft document proposes a hybrid framework that combines a generic Royal College of General Practitioners (RCGP) framework with a respiratory disease-specific framework. 167
STEP-UP summary statement With regard to TRAINING, respiratory tests are attributed a LOW degree of implementation difficulty owing to the ease of use of most equipment.
Equipment
Table 22 offers an illustrative framework assessment for pulse oximeters. Pulse oximeters are increasingly used during endoscopy and other diagnostic procedures, and as part of pulmonary function testing. Data on the role of pulse oximeters in detecting hypoxia in general practice are limited. A minority of GPs reported that they used a pulse oximeter to measure pulse rate or to assess respiratory status. 2,177
| Description of technology | ||||||
|---|---|---|---|---|---|---|
| Pulse oximeters give non-invasive estimation of arterial haemoglobin oxygen saturation and are routinely used in primary care,168 useful in initial assessment, ongoing monitoring and in both acute and chronic clinical situations. NICE guidelines on the management of COPD169 advocate pulse oximetry as screening tool in identifying patients eligible for long-term oxygen therapy | ||||||
| Brief summary of effectiveness/cost-effectiveness in primary care | ||||||
| Oximetry offers objective measure of respiratory compromise. It helps in diagnosis and management of several common respiratory diseases in primary care. There is little evidence of its clinical utility in primary care2 | ||||||
| Skills | ||||||
| Routine use of pulse oximetry in patients suspected of having community-acquired pneumonia would detect clinically unrecognised hypoxaemia, thereby identifying patients requiring hospitalisation.170,171 British Thoracic Society guideline update (2004) for management of community-acquired pneumonia in adults recommended that pulse oximetry, with appropriate training, should become increasingly available to GPs and others responsible for out-of-hours assessment of patients, for assessment of severity and oxygen requirement for patients with community-acquired pneumonia and other acute respiratory illnesses172 | ||||||
| Training | ||||||
| Appropriate training is required. No professional guidance is available | ||||||
| Equipment | ||||||
| Pulse oximetry available in 3.9% of practices and 37.5% of out-of-hours services (2006).173 Pulse oximeter inaccurate in anaemic patients. Cold or poorly perfused peripheries give false readings, as do poorly positioned or dirty probes. Normal SpO2 can occur in patients with abnormal blood pH or CO2 levels; arterial or capillary blood gas sampling and analysis are needed. Owing to their small size, user-friendliness and affordable prices, handheld pulse oximeters are available in primary care.174 Prices vary from £50 to £300. Sizes vary from small pocket-sized models to bench-top displays | ||||||
| Premises | ||||||
| No details given | ||||||
| Clinician | ||||||
| Pulse oximetry is underused (only 1.8% of primary care referrals to accident and emergency with acute exacerbation of asthma had documented pulse oximetry)173 | ||||||
| Patient | ||||||
| No details | ||||||
| Primary–secondary interface | ||||||
| The British Thoracic Society guidelines for emergency oxygen use in adult patients state that, in emergency situations, pulse oximetry should be checked in all breathless175,176 and acutely ill patients, and should be regarded as ‘the fifth vital sign’172 | ||||||
| Skills | Training | Equipment | Premise | Clinician | Patient | Primary–secondary interface |
| ⨁ | ⨁ | ⨁ | ⨁ | ⨁ | ⨁ | ○ |
Pulse oximeters are available, highly portable and increasingly becoming less costly to purchase. 178 If pulse oximeters are being considered for a practice/community setting, it may be worth discussing this with colleagues who use pulse oximeters regularly. The NICE Centre for Evidence Based Purchasing has published an evidence review, market review and buyers’ guide for pulse oximeters, providing a list of pulse oximeters with evidence of their accuracy and performance. 179
STEP-UP summary statement With regard to EQUIPMENT, respiratory tests are attributed a LOW degree of implementation difficulty owing to portability, increasingly cheap equipment and ease of use.
Premises
Little detail is available on the implications of respiratory tests for primary care premises. If patients are being assessed in an area with a high level of artificial light (e.g. operating theatre fluorescent lighting), this can falsely reduce the readings. 180,181
STEP-UP summary statement With regard to PREMISES, respiratory tests is attributed an UNCERTAIN degree of implementation difficulty owing to lack of detail on storage and stock space requirements.
User perspective
Interviews with family practitioners174 found that they considered pulse oximetry especially valuable when they were on an out-of-office-hours shift, as a supportive tool to decide whether to send a patient to an emergency department or to refer to a medical specialist. They considered pulse oximetry an adjunct in diagnostic assessment of patients, not a full diagnostic tool by itself.
To reduce the likelihood of inaccurate readings in patients undergoing pulse oximetry, HCPs should always ensure that a patient’s nail varnish is removed, if present, that a patient’s hand is warmed, if it is cold on presentation, and that the probe is correctly positioned and clean.
STEP-UP summary statement With regard to USER PERSPECTIVES, respiratory tests are attributed a LOW degree of implementation difficulty, as they tend to be well understood and well tolerated.
Primary–secondary interface
Several countries have developed respiratory assessment units to improve the diagnosis of respiratory diseases, such as asthma and COPD, and to overcome problems with misdiagnosis. A community respiratory assessment unit was established in London to optimise diagnosis of respiratory disease by providing focused history-taking, quality-assured spirometry and evidence-based guideline-derived management advice. 182 Based in a secondary care hospital, this was a nurse-led facility, staffed by two specialist respiratory nurses with extensive experience of caring for respiratory diseases in both hospital and the community. A 4-year review found that one-third of suggested diagnoses of COPD by the GP were incorrect, resulting in the inappropriate prescribing of inhaled therapies. The authors identified significant financial, ethical and safety implications and highlighted a need for either diagnostic centres (community respiratory assessment unit) or alternative peripatetic practice-based services operating to quality-controlled standards.
STEP-UP summary statement With regard to the PRIMARY–SECONDARY INTERFACE, respiratory tests are attributed a MODERATE degree of implementation difficulty owing to dependencies on follow-up secondary care diagnosis and treatment.
Conclusion
The overall STEP-UP summary map for respiratory tests is presented in Table 23. The evidence base to support pulse oximetry is limited, but there are several areas in which further work may demonstrate benefits from the application of this technology in primary care. 183 Quantitative studies involving the rate of admission to hospital of acute respiratory illness might evaluate this device further in acute illness scenarios. Further qualitative studies could examine GPs’ experiences of the use of portable oximeters. It is conceivable that the oximeter could be used in health promotion, for example in encouraging patients to give up smoking. Although no clinician would base treatment solely on the oximeter’s readings, there is some evidence for the usefulness of pulse oximetry in general practice. It is not yet clear whether or not its use has any effect on diagnosis or patient-defined outcomes.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | Clinician perspective | Patient perspective | Primary–secondary interface | |
| Respiratory tests | ⨁ | ⨁ | ⨁ | Ø | ⨁ | ⨁ | ○ |
| Systematic reviews | Re22 | Re20, Re31 | |||||
| Quantitative studies | Re37 | Re6, Re14, Re25, Re26, Re36 | Re3 | ||||
| Qualitative studies | Re33 | Re13 | |||||
| UK studies | Re15, Re32, Re28, Re39 | Re4, Re5, Re10, Re11 | Re12, Re27, Re29 | Re21 | |||
| Other substantive evidence | Re7, Re8, Re16, Re17, Re18, Re30, Re39 | Re10 | Re1, Re3, Re9, Re19, Re23, Re24, Re34, Re35, Re38 | ||||
Spirometry
Definition: spirometry is a simple test used to help diagnose and monitor certain lung conditions by measuring how much air someone can breathe out in one forced breath (NHS Choices).
This map includes portable spirometry.
This map excludes other respiratory tests (see Respiratory tests).
Contains public sector information licensed under the Open Government Licence v3.0. 184
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
A study of spirometry carried out by GPs in primary care found that, in 95% of cases, fewer than five trials were required to achieve the highest-quality grade, concluding that spirometries undertaken in general practice are of acceptable quality and reproducible in only 60% of measurements. 185 Although the study involved only small numbers of diagnoses, it was concluded that the practice nurses required more training than they were originally given, again highlighting the importance of high-quality training if spirometry is to be used successfully in primary care. A study conducted with community pharmacists found that 73% of spirometries carried out by pharmacists were of acceptable quality, as judged by lung function experts in an acute setting. 186 Ongoing challenges related to the selection of a suitable spirometric screening test and to maintaining the quality of spirometric tests in primary care. A further study investigating the quality of spirometry in primary care found that > 15% of the tests being sent from primary care to specialists to analyse lacked complete data. 187 The results showed unacceptable quality in the provision of spirometry in primary care for patients with COPD, suggesting that adequate training must be given if spirometry is to be performed appropriately in primary care.
STEP-UP summary statement With regard to SKILLS, spirometry is attributed a MODERATE degree of implementation difficulty owing to the skills required to obtain a valid reading.
Training
Spirometry should be performed only by people who have been trained and assessed to Association for Respiratory Technology & Physiology, or equivalent, standards by recognised training bodies in the performance and interpretation of spirometry. Most COPD training courses include training in spirometry. Spirometry can be performed by any health-care worker who has undergone the appropriate training and who keeps his or her skills up to date. 188 However, a RCT in Australia, in which 26 intervention practices received comprehensive spirometry training and 14 control practices provided usual care, concluded that training in spirometry did not result in any measurable improvement in the use of spirometry, quality of management of asthma or patient outcomes in primary care. 189
A longitudinal study in Denmark demonstrated that improved education of staff enhanced the use of spirometry in hospital outpatients with COPD, indicating the importance of staff training. 190 Further education has also been shown to increase the use of spirometry by GPs191 and also improve their capacity to diagnose clear-cut pathologies.
STEP-UP summary statement With regard to TRAINING, spirometry is attributed a LOW degree of implementation difficulty owing to the success of short training courses for skills acquisition.
Equipment
In its Spirometer Users and Buyers Guide, the National Asthma Council Australia provides a useful list of factors to consider when purchasing a spirometer. 192 Several devices are available on the market, and one study reviewed the technical properties of 10 different spirometers designed for use in general practice. 193 Spirometers should be regularly cleaned and sterilised, as they may become reservoirs of micro-organisms. In a small study of 16 spirometers in South Australia, microbiological contamination was present in three. 194 The frequency of spirometer disinfection did not match the manufacturers’ recommendations, highlighting a need for stricter hygiene measures for spirometer maintenance in general practices. A Dutch study examined 50 desktop spirometers in general practices and found that, on average, they slightly overestimated forced expiratory volume (FEV) and forced vital capacity (FVC) values, with some devices showing substantial deviations. 195 Spirometers, therefore, need to be calibrated yearly and verified before each session.
STEP-UP summary statement With regard to EQUIPMENT, spirometry is attributed a LOW degree of implementation difficulty owing to improvements in ease of use, cost and portability.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates spirometry testing services in an ‘examination/therapy room’ (contains public sector information licensed under the Open Government Licence v3.0). Initial testing could occur in community locations or surgery waiting rooms, but diagnosis should be confirmed in line with recommendations from NICE and the British Thoracic Society.
STEP-UP summary statement With regard to PREMISES, spirometry is attributed a LOW degree of implementation difficulty owing to the facility to apply portable spirometry in a variety of primary care settings.
User perspective
Evidence suggests that spirometry is underutilised and that guidelines that recommend spirometry to confirm airflow obstruction among patients with suspected COPD are not routinely followed. 196 Reported barriers to the use of spirometry include poorly designed and unduly complex spirometers with too many confusing parameters of limited value, lack of availability of spirometers, poor or no teaching in medical schools and the perceived lack of an evidence base demonstrating the value and cost-effectiveness of spirometry. In a UK context only specialist registrars and GPwSIs in undergraduate education spontaneously cited spirometry as a diagnostic tool. 197 GPs stated that they now felt that they had more access to spirometry than in the past and they were highly aware of the value of spirometry, perhaps reflecting its inclusion as a quality marker in the NHS GPs’ contract. GPs in the study commented on the need for retraining, confirmed by a further study which showed that only 33% of GPs were confident in interpreting spirometry and 58% were confident in using spirometers. 198 Health-system barriers specific to spirometry use were not identified, suggesting that the availability of spirometry was not a perceived barrier.
STEP-UP summary statement With regard to USER PERSPECTIVES, spirometry is attributed a LOW implementation difficulty for clinicians and a MODERATE difficulty for patients. The equipment is becoming easier to use but may require multiple attempts for a patient to make their technique acceptable.
Primary–secondary interface
The increased availability of spirometers in primary care offers the potential for wider use. Newer spirometers are user-friendly and have the capacity for self-monitoring. With the advent of telemedicine and internet transmission of data, many more patients could have access to a diagnostic screening and/or monitoring.
Konstantikaki et al. 199 compared an open spirometry programme with a case-finding programme providing spirometry to high-risk subjects selected by primary care physicians. The proportion of newly diagnosed COPD was 27.9% in the case-finding programme, compared with 8.4% in the open spirometry programme. The average cost for a new diagnosis of COPD was €173 in the open spirometry programme and €102 in the case-finding programme. Thus, a case-finding programme involving primary care physicians was more cost-effective than an open spirometry programme for the identification of new cases of COPD.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, spirometry is attributed a LOW degree of implementation difficulty owing to existing referral pathways for asthma, COPD, etc.
Conclusion
Spirometry equipment has frequently figured in GP practices, alongside other requisite equipment such as ECGs. It is similarly benefiting from the move to miniaturisation, as well as from demand for end-user friendly devices. However, the availability of the technology should not disguise the fact that interpreting spirometry readings, and indeed deciding to use the equipment in the first place, when readily available, remains a significant barrier to the effective utilisation of the technology. The STEP-UP summary for spirometry is presented in Table 24.
| HUMAN RESOURCES | LOGISTICS | COMMUNICATIONS AND RELATIONSHIPS | ||||
|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | User perspective | Primary–secondary interface | |
| Spirometry | ○ | ⨁ | ⨁ | ⨁ | ⨁ | ○ |
| Systematic reviews | Sp38, Sp65, Sp79, Sp94, Sp103, Sp106, Sp107 | Sp21 | ||||
| Quantitative studies | Sp1, Sp2, Sp16, Sp19, Sp52, Sp54, Sp55, Sp62, Sp63, Sp87 | Sp8, Sp9, Sp15, Sp43, Sp75, Sp87, Sp89, Sp90, Sp92, Sp97 | Sp6, Sp14, Sp16, Sp18, Sp27, Sp36, Sp47, Sp49, Sp55, Sp60, Sp61, Sp64, Sp88, Sp100 | Sp2, Sp57, Sp59, Sp68, Sp108 | ||
| Qualitative studies | Sp5, Sp39, Sp50, Sp85 | |||||
| UK studies | Sp22, Sp31, Sp33, Sp35, Sp54, Sp61, Sp84, Sp110, Sp111 | Sp34, Sp109 | Sp42, Sp45, Sp46, Sp48, Sp71 | Sp58, Sp95, Sp101 | ||
| Other substantive evidence | Sp3, Sp7, Sp11, Sp20, Sp23, Sp24, Sp25, Sp28, Sp41, Sp67, Sp69, Sp70, Sp74, Sp80, Sp81, Sp90, Sp91, Sp99, Sp102, Sp105 | Sp4, Sp12, Sp26, Sp30, Sp43, Sp44, Sp53, Sp76, Sp77, Sp96 | Sp10, Sp17, Sp20, Sp29, Sp32, Sp51, Sp72, Sp73, Sp78, Sp82, Sp86, Sp93, Sp98 | Sp83, Sp104 | Sp40 | |
Ultrasound
Definition: an ultrasound scan, sometimes called a sonogram, is a procedure that uses high-frequency sound waves to create an image of part of the inside of the body (NHS Choices).
This map includes portable and fixed ultrasound.
This map excludes echocardiography (see Echocardiography) and neonatal screening for dysplasia of the hip.
Contains public sector information licensed under the Open Government Licence v3.0. 200
STEP-UP summary
NB: a full account of the evidence used in compiling this STEP-UP map can be found in Appendix 3.
Skills
Imaging must be undertaken by trained and experienced practitioners, and, even then, perfect images may not be obtained in every patient. 201 Ultrasound images can be saved or printed for reference, but, unlike MRI scans and computed tomograms, performance and interpretation are much more dependent on the skill and experience of the operator. This limits ultrasound’s use for the skull, chest and abdomen. Ultrasound has no known long-term side effects, but it has a more limited scope than other imaging methods.
STEP-UP summary statement With regard to SKILLS, ultrasound is attributed a MODERATE degree of implementation difficulty owing to its being very operator dependent.
Training
The fact that it is safe to carry out, is relatively inexpensive and can be provided in most clinical facilities makes ultrasound one of the most commonly requested examinations in the field of diagnostic imaging. Ultrasound examinations are undertaken by practitioners from a wide range of professional backgrounds and in many different clinical settings. The National Ultrasound Steering Group of the British Medical Ultrasound Society recommended in 2008 that local clinical governance boards be established to oversee the training, supervision and audit of all providers of ultrasound imaging services. 202 Many factors affect the quality of ultrasound examinations, including appropriate training, experience, the equipment itself, clinical leadership, audit, general support and having sufficient time to undertake the examination and compile a clinically relevant report. Clear, effective clinical leadership is also essential if the ultrasound service provider is to achieve timely, accurate, clinically relevant reports. Ultrasound practitioners for whom statutory registration is not possible can apply for voluntary registration with the Public Voluntary Register of Sonographers, which is administered by the College of Radiographers.
STEP-UP summary statement With regard to TRAINING, ultrasound is attributed a LOW degree of implementation difficulty owing to the widespread availability of training courses and opportunities.
Equipment
The latest portable machines produce images that are almost the same quality as that of the larger machines; they are easy to use, durable and cost as little as £5000. 201 Images obtained with a portable ultrasound machine are usually not stored, and a decision on how to act on the scan is made at the time by the clinician doing the scan. The role of the operator is critical, and matching the operator knowledge and competence level to the equipment features is essential, to include the key machine characteristics, how these characteristics match to clinical need, features to be considered when purchasing a machine, and the associated environmental and organisational systems needed to support the efficient use of the machine. Physicians in many medical specialties are thought to be using POC scanning, but the scale on which this is happening is not yet clear; these scans are not systematically recorded in the same way as those performed by imaging experts. In 2002 a cost analysis and an assessment of quality of GP scans, based on a clinical audit and a postal survey of patients’ preferences in the Grampian region of Scotland, reported that the unit cost of a scan was higher in the practice than at the hospital. 203 However, when all of the costs of a scanning episode were considered, the total and average costs were lower in the practice because of the avoidance of hospital visits.
STEP-UP summary statement With regard to EQUIPMENT, ultrasound is attributed a LOW degree of implementation difficulty owing to its portability, its ease of use and the absence of radiation risk.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates ultrasound services in a generic treatment room. A key consideration is the size of the machine relative to the size of the room in which it is to be used. Other issues to be considered include the scanning couch and operator seating, the display monitor, the room heating and lighting, hygiene, infection and cleanliness, and electrical and IT provision. 204
STEP-UP summary statement With regard to PREMISES, ultrasound is attributed a LOW degree of implementation difficulty owing to increasing developments in miniaturisation and portability.
User perspective
A patient preference study for 500 patients from a GP list and 250 consecutive patients scanned at a Grampian general practice found that patients preferred to be scanned at the practice. They were prepared to wait up to an extra 5 days to enact their preference. 203 Patients were prepared to accept a reduction in the accuracy of scanning of up to 3.5% in being able to realise their choice. Patients were not concerned about which member of staff actually carried out the ultrasound scan.
Skills and willingness were not sufficient factors in themselves to prompt rural family practitioners to utilise ultrasound. 205 Economic considerations (i.e. equipment cost and remuneration) were seen as more important. 205
STEP-UP summary statement With regard to USER PERSPECTIVES, ultrasound is attributed a LOW degree of implementation difficulty owing to it being well accepted among clinicians and well tolerated among patients.
Primary–secondary interface
The number of ultrasound examinations performed by imaging experts has increased on average by 5.2% every year for the past 10 years, according to Department of Health data, and imaging experts are struggling to keep up with the growing demand. With increasing pressure on ultrasound services owing to the number of requests, changing patterns of service delivery and a shortfall in the qualified workforce, there have been concerns that the quality of some ultrasound examinations has been affected. 201 A 2002 audit of a small series of patients demonstrated that the use of an ultrasound scanner at a Grampian general practice reduced the number of hospital scans, outpatient and inpatient visits, and emergency admissions. 203 Blackpool, Fylde and Wyre Hospitals Foundation Trust extended primary care ultrasound as a NHS improvement initiative. 206 Long waiting times for routine ultrasound examination, accompanied by patients arriving late for appointments due to parking difficulty, and a limited choice for patients in terms of time and location of appointment, required an innovative service response. The trust negotiated the use of a PCT facility, off the main district general hospital site, and acquired a portable ultrasound machine to provide community-based ultrasound. Improvements included 30–40% of routine outpatient work being performed off the main site (more local scanning with better parking), improved patient satisfaction and increased patient choice of scanning venue.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, ultrasound is attributed a LOW degree of implementation difficulty owing to its being comparatively easily implemented in primary care and the likely shifting demand from secondary care.
Conclusion
Ultrasound is one of the fastest growing areas of diagnostic technology application, excluding POC testing. There is substantive concern that this might not be translating into clinical benefit. Ultrasound, notwithstanding its portability and ease of use, carries requirements for quality assurance. These must be monitored and met if the technology is to achieve its potential clinical benefit. Furthermore, there is a need to examine the impact of ultrasound scans on the referral and secondary care pathways. The STEP-UP summary map for ultrasound is presented in Table 25.
| Human resources | Logistics | Communications and relationships | |||||
|---|---|---|---|---|---|---|---|
| Skills | Training | Equipment | Premises | User perspective | Primary–secondary interface | ||
| Ultrasound | ○ | ○ | ⨁ | ⨁ | ⨁ | ⨁ | ○ |
| Systematic reviews | U18, U19 | ||||||
| Quantitative studies | U5, U13, U28 | U1, U7, U31 | U28, U30 | U4, U5, U17, U23 | |||
| Qualitative studies | |||||||
| UK studies | U36 | U22 | U4, U32 | U36 | U36 | U2, U8, U10, U11, U12, U14 | |
| Other substantive evidence | U1 | U22, U25, U26, U27, U34 | U3, U6, U9, U12, U15, U16, U20, U21, U33, U34, U35 | U9 | U8, U16, U24, U29 | ||
Discussion
Summary of evidence
A total of 673 items for inclusion was identified across the 13 topics. Information on logistic considerations was diffuse, uneven and incomplete. Coverage of new technologies was relatively strong and, where specialist primary care professional associations exist (e.g. endoscopy), training and skills requirements were well specified. However, little direct evidence exists for equipment requirements, implementation issues and the impact on the primary–secondary care interface. A methodological challenge relates to the intensive search and find process required to identify relevant information submerged within lengthy full-text articles and position statements. The diverse range of considerations identified across the 13 topics illustrates the importance of a multifactorial decision-making process.
The multifactorial nature of decisions on diagnostic services, and, indeed, on general practice-level change more generally, has been emphasised by the use of the STEP-UP framework. Observations are discussed under the modalities themselves and the future research and synthesis agenda.
Skills
Many modalities require a wide range of contextual, interpretative and/or technical skills: in administering the test, in interpreting the results and in managing the consequences. Genetic tests, although comparatively easy to administer, make considerable demands on specialist knowledge in interpretation and skills in genetic counselling. Radiology/X-ray and other types of imaging (MRI and ultrasound) require interpretation, possibly necessitating follow-up and expert advice from specialists in secondary care. Such needs may translate into a hybrid model utilising telemedicine technologies. The inadequate administration of tests may require repeat testing, either in the primary care setting or following referral to secondary care. It may also impair the effective use of telediagnosis. Three main routes were identified for addressing the potential skills deficit. For some roles, the GPwSI is the route by which to fortify diagnostic expertise within primary care (e.g. endoscopy and genetics). For other scenarios the extended role of the advanced nurse practitioner, either with a specific diagnostic function (e.g. ultrasound or spirometry) or in a management pathway (e.g. respiratory or diabetic specialist nurses), is emphasised. A third route, although not fully exploited, is to bring in a specialist professional, either on a sessional basis or as an employee of the primary care organisation (e.g. radiology or genetic counselling). Alternative models, not covered by this report, include the use of shared diagnostic services within a primary care consortium, mobile testing services and the use of commercial providers.
A survey of equipment in a cardiac network revealed another important role in the practice team, that of the person responsible for the equipment. 76 Most typically, this is a practice nurse, and so not necessarily the person using the equipment, thus requiring that the equipment is in good working order, remains safe and is supported by the availability of supplies of consumables. The supply of diagnostic equipment and consumables is not well covered in the research literature. In particular, there is a need to accompany the purchase of equipment with maintenance schedules and equipment replacement policies to ensure the effective continued use of equipment, where needed.
Training
Training may be delivered through specialist courses, attachments to acute specialist departments or manufacturers of diagnostic equipment. A particular concern relates to whether or not the number of cases to be seen in a practice justifies a significant investment of time and resources in training (e.g. endoscopy). The opportunity costs for the consultation and other aspects of primary care required by pursuit of specialisation are highlighted in the related GPwSI literature.
A palpable tension exists between the professional interests of associations charged with assuring both the quality of procedures and the existence of their professional group and the need to engage with a wider primary care workforce. For example, historically, data on the safety of endoscopy in acute hospitals were initially viewed as prohibitive to wider primary care involvement. In reality, triaging the complexity of particular cases and specific populations (e.g. children, pregnant women and the elderly), particularly for invasive modalities, may increase the accessibility of primary care diagnostics while preserving specialisation in secondary care.
For modalities such as echocardiography and ultrasound, stepped approaches have been developed whereby the type of equipment and reliability of the test result are commensurate with the criticality of the clinical pathway. However, the need for accreditation can vary across modalities, often being associated not so much with risk as with how mobilised and active the relevant professional associations are.
The survey by Day et al. 76 revealed that little attention is being given to requirements for training, especially in relatively low-cost items of equipment. There is a need to accompany the purchase of equipment with schedules for training so that they can be used to best effect.
Equipment
The cost and manageability of technologies is extremely variable across topic areas. Some technologies have benefited from moves towards miniaturisation (e.g. ultrasound) or to more end-user friendly versions of a technology (e.g. flexible sigmoidoscopy). Others have seen a trend towards popularisation, either in the health-care professions generally (e.g. POC testing) or through direct-to-consumer marketing (e.g. genetic tests), from which primary care might potentially benefit. Concerns about safety persist, either from the diagnostic equipment itself (e.g. radiation from radiology/X-ray) or from consumables (e.g. glutaraldehyde) or from ancillary equipment to support administration or analysis of the test results (e.g. electrical equipment). Ancillary equipment requirements are not widely documented (e.g. continuous pulse oximetry is recommended when intravenous sedation is required for endoscopy), whereas requirements for garments, gloves, goggles and glasses should not be overlooked. For newer technologies, the outputs of the NIHR DEC, Oxford, are useful. However, these focus on narrower HTA perspectives of the technology, with correspondingly less attention given to health services delivery and organisational issues.
Premises
Diagnostic technologies place particular requirements on the physical location in which a test is administered. In some cases the focus is on the facilities and safe administration of the equipment (e.g. endoscopy or radiology). Specialist premises, that is, rooms dedicated to a particular diagnostic modality, are particularly prohibitive on legacy primary care premises. Even where premises are purpose-built, changes in technologies and their associated requirements, and a lack of specifications for an integrated multipurpose approach across technologies, make accommodation problematic. Patient flows (e.g. additional seating and queuing) need examination at a specific practice level. Requirements are not necessarily technology-driven. For example, audiological requirements for a quiet environment, even for simple hearing tests, may be difficult to accommodate and may particularly be compromised in a multipurpose environment. A particular deficiency relates to the facilities to store equipment and consumables. Although this most obviously relates to sizeable items of equipment, it must not be overlooked for the storage of portable devices or in large volumes of consumables, for example POC or genetic test kits.
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 recognises that ‘as technology improves, more direct diagnosis will be undertaken in primary and community care settings’ (contains public sector information licensed under the Open Government Licence v3.0). The building note acknowledges that near-patient testing will require bench-top equipment, whereas digital diagnosis will comprise ultrasound scans, resting ECGs and radiography. It suggests that some of these activities may be ‘delivered on a timetabled basis’ from generic rooms, such as the consulting/examination room equipped to carry out resting ECGs and the treatment room equipped to carry out ultrasound scans and/or resting ECGs. In addition, the near-patient testing room would be used for blood-gas analysis. It suggests that a ‘docking station may also be required to accommodate mobile vehicles carrying diagnostic equipment such as MRI, CT [computed tomography] and mammography scanners’. It specifies that the internal route to the docking station should be well planned, passing the main reception desk and waiting area.
Of particular importance is the degree to which developments in the adoption of one technology may facilitate accommodating a similar technology across diagnostic groups, for example ECGs and spirometers, echocardiographs and ultrasound or X-ray and MRI. Developments in POC testing will, thus, probably impact on cardiac and diabetic diagnostic services. Equally unexplored are antagonisms between potential diagnostic technologies in terms of the space they consume or the type of activity that they necessitate. For example, providing for the safe cleaning of endoscopes may impact on the non-availability of the required space for patient examination or care.
User perspectives
Generally, across primary care, the delivery of diagnostic modalities locally meets increasing demands for improved access and, by implication, enhanced equity. Patients prefer services that may be reached easily and that will not necessitate extensive time away from their day-to-day responsibilities. Several qualitative studies refer to the patient experience of difficulties in parking when attending secondary care for tests. Prompt test results and the joining up of diagnosis and treatment are important considerations for patient and primary care provider alike. On the other hand, impaired access may function as a disincentive to inappropriate utilisation and may control overtesting. There is little evidence to inform whether improved general practice access to testing increases uptake of tests or whether a greater awareness of resource use deters general practice staff from initiating testing. Patients presenting with medically unexplained symptoms may be assuaged with offers of more, and more immediately available, tests. More critically, the appropriateness of testing, rather than utilisation rates per se, becomes a key issue. Finally, given that diagnostic services function as a gateway to subsequent health service use, improved access to diagnosis might potentially defer current bottlenecks to other points in the patient care pathway.
Primary–secondary interface
Whereas the STEP-UP framework encourages a holistic view of primary care considerations, diagnostic services function in a whole-system health system environment. The ramifications of system change at the primary care first point of call are not fully explored in the evaluation literature. Earlier detection may result in earlier and more effective treatment and, thus, in overall cost savings. Unintended consequences for patient and provider behaviours require careful examination, especially when resulting in increased utilisation or needless duplication. The impact on uptake of direct-to-consumer products and on commercial diagnostic services or private health care is equally important in a whole-system perspective.
Candidate modalities
The synthesis team members were constrained by limited data on current UK practice and uptake of diagnostic services. For long-established technologies (e.g. endoscopy) it is unclear whether the extent of spread has been determined by demand or by logistic considerations such as the limited availability of premises or the costs of equipment. In particular, the implications of a concerted attempt to improve diagnostic provision, involving the introduction of diverse modalities within a relatively intensive period, are unclear.
Mechanisms for adoption and prioritisation, strategically and in an individual practice, are also complex and unclear. Several commentators observe on the importance of identifying particular barriers and constraints at a micro level, as well as acknowledging the role of guidance from NICE or professional associations in driving forward initiatives. The variability of context makes it difficult to translate this mapping review directly into actionable recommendations for primary care. At the same time, the STEP-UP lens offers the possibility of a more holistic and consistent approach to evaluation. Economic evaluation, with its whole-systems perspective, its approach to itemisation of particular components and its functionality for handling multiattribute decisions, appears to afford an opportunity to implement STEP-UP considerations in a more technical, consistent and decision-specific manner.
Strengths and limitations of this review
The conduct of this mapping review was challenging. Information to populate the STEP-UP framework was diffuse and required full-text examination of numerous sources. In particular, information on the spatial implications of the technology for the primary care premises was lacking. The principal exception to this was endoscopy, for which a health building note exists.
When examining a technology, it proved problematic to identify what the current uptake of a technology was likely to be. Where prevalence was considered high, for example for ECGs or spirometry, the emphasis of the evidence was on migration to more portable, handheld technologies. Expensive equipment, such as MRI (and, by implication, CT scanning), was becoming more compact and, consequently, less expensive. Consumer demands shaped a corresponding reaction in primary care with regard to POC and genetic testing. A further consequence was to reduce any likely demand to house laboratory facilities in a primary care setting. Other contexts, such as cardiac services and diabetic services, were affected to a partial degree by developments in POC testing. All of these variables made comparability across technologies problematic, particularly in terms of stage of innovation and extent of available information.
A further complication relates to a dilemma concerning technology or service. STEP-UP reports could examine a service as a whole or a specific technology. In fact, some of the services examined in this report, such as cardiac services and audiology services, incorporate specific technology summaries as well as trying to summarise at a service level. This mixed approach proved problematic. It is preferable to either single out a particular technology – to conduct a more extended and holistic technology assessment – but with corresponding difficulty in identifying implications for more system-based considerations such as premises or the primary secondary interface; or conduct a service-based assessment which privileges whole-system considerations but perhaps lacks granularity for skills, training and equipment considerations.
Several workforce planning documents were identified from The King’s Fund Library catalogue. These reports, from PricewaterhouseCoopers,207,208 may help to inform the workforce requirements (as itemised under the skills and training headings for all modalities), but they are not available online. Time constraints meant that it was not possible to consult these in their physical location.
Methodological limitations of the included studies
As a conceptual framework STEP-UP is vulnerable to a mismatch between the logistic information required to complete the map and a research agenda focused on innovations and/or on the evaluation of effectiveness. Position statements and professional standards are typically underpinned by implicit and non-articulated assumptions about the stage of diffusion at which a particular technology finds itself. So, for example, early documents state requirements for setting up an endoscopy unit but the costings are now outdated and the specification is time-bound. Extensive use of the STEP-UP framework across a wide variety of topics reveals its general utility for a consistent approach that highlights similarities and contrasts across very different technologies.
As such, STEP-UP offers a viable framework for extending evaluation beyond the current narrow interpretation of HTA towards important considerations of service delivery and organisation. Considered reflection leads us to suggest the possible extension of the STEP-UP framework to include three additional components: Public perspectives, Economics and Drivers (STEPPED-UP). Although the inclusion of Economics in the extended framework is unsurprising, the STEPPED-UP framework introduces this in the more relevant context of service delivery and not in the more common, limited application of individual diagnostic technologies. In our experience we also found it useful to separate providers and patients under the User perspective heading, in recognition that some tests may be viewed as attractive by patients but might occasion concern among service providers, or vice versa. A further modification, although not implemented in this report, is to distinguish between skills and training for administration and skills and training for interpretation. Furthermore, communication skills for handling the implications of a positive test may be particularly important for some types of otherwise easily administered testing, most notably genetic tests.
Research implications
Many studies of diagnostic modalities focus almost exclusively on technical accuracy. The MaDOx initiative sought to extend this to include reliability, diagnostic accuracy, impact on outcomes and cost-effectiveness. 209 This mapping review has revealed that an even wider breadth of scope is required when seeking to implement these technologies in a primary care environment. Additional considerations relate to the skills and training required by the staff, the physical properties of the equipment and the facilities required for its use and storage of equipment and consumables. Furthermore, there is the impact of the use of the equipment, and any consequential changes in procedures, on those delivering and those receiving the service. Finally, examination of the impact on the primary–secondary interface, in the context of a pathway of care, is considered essential if the diagnostic technologies are to be integrated into overall patient management.
Horizon Scanning reports from the Oxford Horizon Scanning Programme (http://madox.org/) exist for many of the diagnostic technologies under review. These offer a useful structure on which to hang this logistics review and they particularly address the domain of Equipment in the STEP-UP model. Horizon Scanning reports sometimes afford passing mention to implementation issues such as quality assurance and training, but this occurs unevenly and incompletely. The coverage of these useful reports might be enhanced by either (1) extending their structure to include the dimensions addressed by the STEP-UP framework or (2) commissioning a parallel set of research products specifically addressing service delivery and organisation issues, either from the same agency or from a comparable agency with specific expertise in health services management and implementation issues. However, it is to be noted that the last report was dated 2013.
Other diagnostics to explore
It was not possible, for logistic reasons, to perform a STEP-UP map for every candidate technology. Primary exclusions include CT scanning and colonoscopy. In terms of the specific detail, these may be populated from similar sources to the exemplars in this report. In the absence of a detailed assessment, considerations may be informed by the assessments for radiology and MRI (for CT scans) and for endoscopy (for colonoscopy). However, specific technical details and qualitative studies on the patient and clinician views and attitudes towards the technology are required to supplement the overall maps.
Alternative methods of delivery
The specific remit of this mapping review, that is, to look at implications for staff and equipment located in primary or community care, has necessarily excluded some innovative approaches that transect the primary and secondary care divides. Although some of these issues and examples have been included opportunistically in the STEP-UP sections entitled ‘primary care interface’, we have identified a need to systematically examine models, from the literature or good practice, that include some of the following:
-
commercial providers
-
diagnosis and treatment centres
-
direct access
-
mobile laboratories
-
outreach clinics
-
rapid-access clinics
-
telemedicine.
Several of the above options, including direct access, rapid access clinics and telemedicine, are briefly summarised in a report by the Health Services Management Centre. 210 However, almost a decade has elapsed since this rapid review was conducted. The companion review of NHS experience in the same topic area reported that diagnostics was the least well-populated direction for innovation. 211 This suggests a fruitful area for future examination and review.
Conclusions
Delays or deficiencies in diagnostic services often prove a bottleneck for the patient pathway through treatment. Often the quickest access to tests is achieved by admitting the patient, often unnecessarily, to hospital. Three proposed routes by which to alleviate this bottleneck are (1) to move diagnostic services out of hospital and locate them in primary care, (2) to use independent sector services to increase capacity and (3) to develop POC testing in hospitals. The first of these is the sole emphasis of this report. It may therefore be useful to examine the other options with equal attention, perhaps using the revised version of the STEP-UP framework (see Discussion) to facilitate comparability. 212 Implementation strategies for new diagnostic tests require a structured business plan, including performance management following introduction. 213 This approach should seek to involve commissioners, specialists and GPs.
The evidence map and synthesis provides a rapid synoptic view of leading areas of development for primary and community care diagnostics and a potential mechanism for identifying and specifying future areas for development (for synthesis, primary research and policy). In particular, the need for whole-system evaluations, economic evaluations and an improved system for organising and presenting information on aspects relating to service delivery and organisation, possibly analogous to the NIHR DEC, has been highlighted. Further data on current levels of diagnostic provision in primary care and future priorities are required. Finally, the focus of this synthesis on staff and equipment located in primary care has necessarily constrained the scope of the review and subsequent recommendations. Alternative models, such as consortium approaches, direct access, mobile testing services, outreach initiatives and the use of commercial laboratories, require a similarly systematic examination.
Chapter 5 Focused review: community diagnostic ultrasound services
Introduction
The literature mapping exercise reported in Chapter 3 revealed a lack of published systematic reviews looking at community provision of specific diagnostic technologies. Diagnostic ultrasound was chosen as a topic for further review based on both the published evidence identified by the mapping exercise, which suggested that there were some relevant published studies,16,203 and advice from clinical experts about the need for evidence to guide policy and practice. Following implementation of the Health and Social Care Act 2012,214 local Clinical Commissioning Groups (CCGs) have had increased flexibility to commission new models of community services to replace or supplement hospital-based services. The Act also places a duty on CCGs to take account of evidence from research in their decision-making.
Ultrasound appears to be a suitable diagnostic technology for use in primary care and community settings, being relatively inexpensive, safe and non-invasive. 215 In addition to potential benefits for patients and the health-care system, the main issues that need to be considered in evaluating provision of diagnostic ultrasound in community or primary care settings are equipment, staffing and workload, and training. Training may be required for health professionals newly required to perform ultrasound scans and for GPs to enable them to make optimum use of new service models.
The traditional model of service for non-emergency ultrasound scanning in the UK NHS involved secondary care radiology departments performing the examination following an outpatient appointment for patients referred by GPs. Since the 1990s, many areas have developed direct-access or open-access services, whereby patients referred by GPs are scanned using hospital facilities without being seen by a secondary care specialist. The location of services outside hospital settings has also been possible for many years. This model of service is driven by increasing demand, the relatively low cost of the equipment and improvements including improved imaging quality and the availability of smaller, more portable scanners (including handheld devices).
However, wider access to ultrasound scans may increase the risk of the technology being used inappropriately. There is some evidence that rates of inappropriate requests are high for ultrasound scanning, compared with other types of imaging. 216,217 In addition to the increased likelihood of excessive imaging and its associated costs, direct access to ultrasound services may lead to additional diagnostic tests and unnecessary treatment. 158 The potential effect on patient anxiety of being referred for a scan is also a factor to consider, although this should be balanced against the reassurance provided by a negative scan result.
In 1990, the RCR released guidelines for radiologic referral218 and several subsequent studies showed that the application of these guidelines led to a considerable decrease in inappropriate radiological requests, such as for X-rays. 219 However, such guidelines require GPs and other primary care staff to be trained appropriately in use of the equipment. This would also be the case for other types of imaging such as ultrasound. Community diagnostic ultrasound services therefore embody a complex decision problem with the potential to impact on the activities of the primary care clinic and, more widely, on referral pathways into secondary care.
Methods
The review aimed to address the following question: what is known about the implications of different ways of providing diagnostic ultrasound services in community or primary care settings? This was defined to include implications for both NHS organisations (e.g. related to provision of staff, premises, training and equipment, costs and cost-effectiveness) and patients (e.g. related to changes in management/pathways, acceptability to patients, accuracy of diagnosis and longer-term clinical outcomes).
Inclusion/exclusion criteria
Inclusion and exclusion criteria for the review were as follows.
Population
People requiring diagnostic ultrasound for any condition (excluded: population screening and monitoring, including pregnancy). Studies described as screening could be included if the people being screened were identified by having a specific risk factor for a medical condition (rather than through a screening programme offered to all individuals on the basis of age and/or gender) and the identified factors were either common or concentrated (e.g. common in a particular minority ethnic group) in the UK population.
Intervention
Ultrasound provided in a primary care or community setting by primary care/community staff using any type of equipment (including portable ultrasound devices). Open-access services provided to GPs by a hospital using its premises, equipment and staff were treated as a comparator intervention.
Comparator
Hospital-based diagnostic ultrasound services (open access or traditional). ‘Outreach’ services using hospital-based staff to deliver services in community settings were also considered to be relevant comparators.
Outcomes and study designs
The main focus was research studies in developed countries that evaluate community diagnostic ultrasound services and have a comparator; given the importance of context, we also included evaluative studies without a comparator group [e.g. audits and service evaluations (UK only)] and descriptive studies providing usable information about service delivery in UK settings. Systematic reviews, UK-relevant economic evaluations/cost studies and qualitative research studies were also eligible for inclusion.
In addition, we included relevant expert opinion pieces or reports from professional bodies that identify and/or discuss practical issues related to the provision of community diagnostic ultrasound services (e.g. staffing, training, equipment and premises) in UK settings. 220
Outcomes of interest included patient outcomes (e.g. waiting times, acceptability, changes to diagnosis or management, and any clinical outcomes) and service/process outcomes (e.g. costs/resource use, cost-effectiveness, needs for training, premises and equipment). Resource-use outcomes include any implications for test ordering by GPs. 221
Given that the focus of the review was on models of service, studies reporting on the diagnostic accuracy of ultrasound in community settings for particular conditions were not included unless they were considered relevant to the primary–secondary care interface, as defined in the review protocol (e.g. they contained information on referrals or changes to diagnosis or management pathways).
Searching
The following bibliographic databases were searched from 1995 to February 2015:
-
MEDLINE via Ovid SP
-
EMBASE via Ovid SP
-
The Cochrane Library
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
-
Web of Science.
The start date of 1995 was based on evidence from the initial mapping exercise (see Chapter 3) that ultrasound began to appear in UK primary care settings from the mid-1990s. 215 Search strategies are presented in Appendix 4.
Citation searches were performed in Google Scholar for papers citing key studies included in the review: first authors Wordsworth,203 Everett,222 van Gurp,223 Robinson,215 Scholten-Peeters224 and Pallan. 16
Internet and grey literature
The objective of this part of the review was to identify providers and models of service for diagnostic ultrasound in the UK NHS (primarily England).
The following internet and grey literature searches were performed:
-
Internet searches using the Google (Google Inc., Mountain View, CA, USA) search engine (see Appendix 4 for details)
-
OpenGrey (European grey literature database): www.opengrey.eu/
-
NIHR DEC, Oxford: www.oxford.dec.nihr.ac.uk/.
The first 100 results from Google searches were examined. Websites of companies providing diagnostic ultrasound services in NHS community settings and of ‘NHS community diagnostic centres’ (providing diagnostic ultrasound alongside other tests) were searched in more detail to identify any evaluations or fuller descriptions of the services and any information on governance, accreditation and similar issues. These services appeared to meet the criteria for comparator interventions in the protocol and were poorly covered by the published literature.
Study selection
Search results were stored in a reference management database (EndNote X7), where decisions on inclusion/exclusion were recorded. The selection of studies for inclusion (scanning of titles/abstracts and full-text publications) was initially carried out by one reviewer. Uncertainties were resolved by discussion and consensus among the review team.
Included studies were classified on the basis of quality and relevance as level 1, level 2 or level 3, as follows:
-
Level 1: comparative studies of community diagnostic ultrasound services from developed countries. This category also includes full publications of non-comparative and descriptive studies from UK settings, UK-relevant economic evaluations/cost studies and qualitative research.
-
Level 2: non-comparative empirical studies from non-UK developed country settings that include relevant information about community diagnostic ultrasound services. This category also includes conference abstracts providing limited information on UK services.
-
Level 3: non-empirical studies, for example conceptual papers and expert opinion, that include relevant information about community diagnostic ultrasound services.
Data extraction and quality assessment
Included experimental or observational studies (level 1 or 2) were assessed for quality using the following tools:
-
case series: Canadian quality appraisal tool for case series225
-
cohort and other non-randomised comparative studies: Downs and Black checklist226
-
cost studies: checklist from Drummond et al. 227
-
diagnostic studies: QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool as adapted by the Cochrane Collaboration. 228
Data were extracted from level 1 and 2 studies using forms/tables set up in advance and piloted on a small number of studies. Data extraction and quality assessment were checked by a second reviewer. Any discrepancies were resolved by discussion and consensus, with reference to a third reviewer if necessary. Basic details were tabulated for included level 3 studies and these were not formally assessed for quality.
Synthesis of evidence
Patient-related and service-related issues (as defined above) were used as a framework for a narrative synthesis. Evidence was grouped by type of service model and where appropriate by indications/patient groups covered. The synthesis aimed to provide an analysis of the quality of evidence and the strength of conclusions which can be drawn from current studies. We also aimed to identify evidence gaps to inform future research.
Results
Systematic review of published literature
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the systematic review is presented in Figure 1.
Included studies and characteristics
Three papers met the inclusion criteria for level 1 studies. 203,222,229 Ten English-language papers,16,223,230–237 four papers published in other languages238–241 (including one thesis) and three conference abstracts242–244 were classified as level 2 studies. Seventeen level 3 studies were also included. 44,45,205,215,220,224,245–255
Full details of the included level 1 and 2 studies can be found in the data extraction tables (see Appendix 5) and below. Appendix 6 summarises the main characteristics and findings of the included level 3 studies.
Quality assessment results
Because of the varied designs of the included studies, a number of different quality assessment tools were used to assess the risk of bias of individual studies. Level 3 studies were not formally assessed for quality. Overall, the quality of the included studies was low. There were no randomised trials included and many studies had no control group. The findings of the quality assessment are discussed in more detail below.
One level 1 study229 and two level 2 studies with control groups16,223 were assessed using the Downs and Black checklist226 (see Appendix 7). The study by Salihefendic et al. 229 was of low quality, with many important features missing or not clearly reported. The fact that English was not the first language of the lead author made the study often difficult to understand and interpret. The two level 2 studies clearly described their participants and interventions, and used appropriate methods of analysis, but there was no indication that possible confounding factors were taken into account. The study by van Gurp et al. 223 had no control group; instead, it compared outcomes following referral to a community echocardiography service with what physicians said they would have done in the absence of the service.
Studies classed as case series, including two level 1 studies,203,222 were assessed using a checklist developed by the Institute of Health Economics in Alberta, Canada. 225 This tool has considerable similarities with the Downs and Black checklist226 but was specifically developed to assess studies without a control group. The results (see Appendix 7) indicated that the studies had several limitations in addition to those associated with a case series design. For example, both level 1 studies were conducted at one centre and both appeared to have a retrospective design, which increases an already fairly high risk of bias. Other questions were difficult to assess because of reporting limitations.
Two studies16,203 that were also among the few to compare different models of service reported elements of costs or cost-effectiveness. In view of the importance of this outcome, the quality of the economic component was assessed using the checklist of Drummond et al. 227 A critical abstract from the NHS Economic Evaluation Database (NHS EED) was available for the study by Pallan et al. 16 Both studies appeared to adopt a NHS perspective (i.e. wider societal costs and benefits were not included), but this was not stated explicitly. Limitations of the study by Wordsworth and Scott203 were noted above, particularly the lack of real data for the comparison group, and these should be taken into account when interpreting the economic aspect of the study. The authors reported carrying out a sensitivity analysis to examine the effects of varying the assumptions made, but no details of this were reported. Overall, the findings of this study provide only limited evidence to support the cost-effectiveness of ultrasound scanning by GPs in the surgery.
The main limitations of the Pallan et al. 16 study, as noted by the NHS EED commentary, were its retrospective design, its poor reporting of costs and the absence of any sensitivity analysis to explore uncertainty in the findings. These limitations make it difficult to assess the reliability of the authors’ conclusions, as discussed further below.
Finally, three diagnostic accuracy studies were assessed using the version of the QUADAS tool adopted by the Cochrane Collaboration (see Appendix 7). 228 This tool was chosen because of the ease of application and a level of detail sufficient for the needs of this project, which is not a review of diagnostic accuracy. Although the reporting was not always clear, the quality of the three studies appeared generally moderate to good. The major exception was the study by Grubel,231 in which only a proportion of the patients received a reference standard test, leading to a risk of partial verification bias. The study by Thoomes-de Graaf et al. 236 aimed to assess agreement between physiotherapists and radiologists, with the radiologist’s diagnosis being treated in effect as a reference standard. There were no major quality issues with this study, although, as with other diagnostic studies in the review, it was not explicitly reported whether or not any unclear or uninterpretable results were recorded.
Summary of included study characteristics
The included studies focused on ultrasound scans for a variety of sites/conditions, cardiac (echocardiography), GI and musculoskeletal being the most common (Table 26). Several studies examined general ultrasound services offering scans for a range of conditions, while a group of studies from Finland examined the use of ultrasound for the diagnosis of sinusitis in the primary care setting.
| Study (authors and year) | Site | |||||
|---|---|---|---|---|---|---|
| Heart | GI | Sinus | MSK | Other | General | |
| Level 1 | ||||||
| Everett and Preece 1996222 | ✓ | |||||
| Wordsworth and Scott 2002203 | ✓ | |||||
| Salihefendic et al. 2009229 | ✓ | |||||
| Level 2 | ||||||
| Goldberg et al. 2003230 | ✓ | |||||
| Grubel 2011231 | ✓ | |||||
| Heikkinen et al. 2005232 | ✓ | |||||
| Laine et al. 1998233 | ✓ | |||||
| Landers and Ryan 2014234 | ✓ | |||||
| Mäkelä and Leinonen 1996235 | ✓ | |||||
| Pallan et al. 200516 | ✓ | |||||
| Thoomes-de Graaf 2014236 | ✓ | |||||
| van Gurp et al. 2013223 and van den Brink 2013253 | ✓ | |||||
| Varonen et al. 2003237 | ✓ | |||||
| Abstracts/non-English publications | ||||||
| Blanchet and Thierry 2015238 | ✓ | |||||
| de la Figuera et al. 2012239 | ✓ | |||||
| Esquerra et al. 2012240 | ✓ | |||||
| Evangelista et al. 2013241 | ✓ | |||||
| Gallagher et al. 2012242 | ✓ | |||||
| Hoyer et al. 2007243 | ✓ | |||||
| Singh et al. 2009244 | ✓ | |||||
| Level 3 | ||||||
| Aitken 199944 | ✓ | |||||
| Bono and Campanini 2007245 | ✓ | |||||
| Cardiac Networks Co-ordinating Group 2006220 | ✓ | |||||
| Colquhoun et al. 1995246 | ✓ | |||||
| Doddy 2009247 | ✓ | |||||
| Hölscher et al. 2013248 | ✓ | |||||
| Hussain et al. 2004249 | ✓ | |||||
| Mjølstad et al. 2012250 | ✓ | |||||
| NIHR DEC Oxford 201445 | ✓ | |||||
| Ottenheijm et al. 2011251 | ✓ | |||||
| Robinson et al. 1997215 | ✓ | |||||
| Scholten-Peeters et al. 2014224 | ✓ | |||||
| Senior et al. 2003252 | ✓ | |||||
| Siu et al. 2013205 | ✓ | |||||
| Vicente-Molinero et al. 2009254 | ✓ | |||||
| Xiao 2003255 | ✓ | |||||
Topics relevant to the review question covered by the included studies are summarised in Table 27. The included level 1 and 2 studies were mainly concerned with comparing or describing service models and with workforce and training issues. Level 3 papers also covered these topics but this group also included studies looking at equipment (primarily the role of handheld devices) and attitudes towards community diagnostic ultrasound service, particularly health professionals’ attitudes as a barrier (or facilitator) to setting up such services.
| Study (authors and year) | Service models | Equipment | Workforce/training | Barriers/attitudes |
|---|---|---|---|---|
| Level 1 | ||||
| Everett and Preece 1996222 | ✓ | |||
| Wordsworth and Scott 2002203 | ✓ | |||
| Salihefendic et al. 2009229 | ✓ | |||
| Level 2 | ||||
| Goldberg et al. 2003230 | ✓ | ✓ | ||
| Grubel 2011231 | ✓ | ✓ | ||
| Heikkinen et al. 2005232 | ✓ | |||
| Laine et al. 1998233 | ✓ | |||
| Landers and Ryan 2014234 | ✓ | ✓ | ||
| Mäkelä and Leinonen 1996235 | ✓ | |||
| Pallan et al. 200516 | ✓ | |||
| Thoomes-de Graaf et al. 2014236 | ✓ | |||
| van Gurp et al. 2013223 and van den Brink 2013253 | ✓ | |||
| Varonen et al. 2003237 | ✓ | |||
| Abstracts/non-English publications | ||||
| Blanchet and Thierry 2015238 | ✓ | |||
| de la Figuera et al. 2012239 | ✓ | |||
| Esquerra et al. 2012240 | ✓ | |||
| Evangelista et al. 2013241 | ✓ | |||
| Gallagher et al. 2012242 | ✓ | |||
| Hoyer et al. 2007243 | ✓ | ✓ | ||
| Singh et al. 2009244 | ✓ | |||
| Level 3 | ||||
| Aitken 199944 | ✓ | ✓ | ||
| Bono and Campanini 2007245 | ✓ | |||
| Cardiac Networks Co-ordinating Group 2006220 | ✓ | ✓ | ✓ | |
| Colquhoun et al. 1995246 | ✓ | ✓ | ||
| Doddy 2009247 | ✓ | ✓ | ||
| Hölscher et al. 2013248 | ✓ | |||
| Hussain et al. 2004249 | ✓ | |||
| Mjølstad et al. 2012250 | ✓ | ✓ | ||
| NIHR DEC Oxford 201445 | ✓ | |||
| Ottenheijm et al. 2011251 | ||||
| Robinson et al. 1997215 | ✓ | ✓ | ||
| Scholten-Peeters et al. 2014224 | ✓ | |||
| Senior et al. 2003252 | ✓ | |||
| Siu et al. 2013205 | ✓ | ✓ | ✓ | |
| Vicente-Molinero et al. 2009254 | ✓ | |||
| Xiao 2003255 | ✓ | |||
Outcomes examined in the included studies were categorised as clinical effectiveness (including diagnostic accuracy and yield), process/system, patient acceptability and costs/resource use (Table 28). Most level 1 and 2 studies reported clinical and/or process outcomes, with only two16,203 reporting on patient acceptability and costs. Two level 3 studies44,247 examined patient acceptability. Some level 3 papers were relatively discursive or conceptual and did not report outcomes per se but are included in the table for ease of comparison.
| Study (authors and year) | Clinical effectiveness | Process/system | Patient acceptability | Costs/resource use |
|---|---|---|---|---|
| Level 1 | ||||
| Everett and Preece 1996222 | ✓ | |||
| Wordsworth and Scott 2002203 | ✓ | |||
| Salihefendic et al. 2009229 | ✓ | ✓ | ✓ | ✓ |
| Level 2 | ||||
| Goldberg et al. 2003230 | ✓ | |||
| Grubel 2011231 | ✓ | |||
| Heikkinen et al. 2005232 | ✓ | |||
| Laine et al. 1998233 | ✓ | |||
| Landers and Ryan 2014234 | ✓ | |||
| Mäkelä and Leinonen 1996235 | ||||
| Pallan et al. 200516 | ✓ | ✓ | ✓ | |
| Thoomes-de Graaf et al. 2014236 | ✓ | |||
| van Gurp et al. 2013223 and van den Brink 2013253 | ✓ | ✓ | ||
| Varonen et al. 2003237 | ✓ | |||
| Abstracts/non-English publications | ||||
| Blanchet et al. 2015238 | ✓ | |||
| de la Figuera et al. 2012239 | ✓ | ✓ | ||
| Esquerra et al. 2012240 | ✓ | |||
| Evangelista et al. 2013241 | ✓ | |||
| Gallagher et al. 2012242 | ✓ | |||
| Hoyer et al. 2007243 | ✓ | |||
| Singh et al. 2009244 | ✓ | |||
| Level 3 | ||||
| Aitken 199944 | ✓ | |||
| Bono and Campanini 2007245 | ||||
| Cardiac Networks Co-ordinating Group 2006220 | ||||
| Colquhoun et al. 1995246 | ||||
| Doddy 2009247 | ✓ | |||
| Hölscher et al. 2013248 | ✓ | |||
| Hussain et al. 2004249 | ||||
| Mjølstad et al. 2012250 | ||||
| NIHR DEC Oxford 201445 | ||||
| Ottenheijm et al. 2011251 | ✓ | |||
| Robinson et al. 1997215 | ||||
| Scholten-Peeters et al. 2014224 | ✓ | |||
| Senior et al. 2003252 | ✓ | |||
| Siu et al. 2013205 | ✓ | |||
| Vicente-Molinero et al. 2009254 | ✓ | |||
| Xiao 2003255 | ||||
The following sections examine the included studies in more detail using the topics and outcomes in Tables 27 and 28 as a framework.
Studies comparing/describing service models
The principal service models for diagnostic ultrasound are listed in Table 29. Rapid-access clinics may be hospital based or community based and are relevant only to certain indications, for example suspected heart failure. The other models mentioned could be applied to general diagnostic ultrasound services or services targeted at specific conditions. ‘Traditional’ access to ultrasound, through referral by a hospital clinician, is not considered in this report, which deals only with community ultrasound.
| Secondary care setting | Community/primary care setting |
|---|---|
| Secondary care ‘traditional’ (via consultant referral) | Community diagnostic centre (offering multiple diagnostic services) |
| Secondary care ‘open access’ | Community outreach from secondary care |
| Rapid-access clinic (condition specific) | GP scanning by GP in surgery |
| Scanning by radiographer in GP surgery (employed by GP?) | |
| Mobile service delivered at GP surgery |
Studies describing/evaluating service models are those by Everett and Preece,222 Wordsworth and Scott,203 Salihefendic et al. ,229 Pallan et al. 16 (mobile), van Gurp et al. 223 (community open access) and Landers and Ryan234 (community palliative care) and conference abstracts by Gallagher et al. 242 and Singh et al. 244 (echocardiography clinics). Of these, only Salihefendic et al. 229 compared primary care services with and without direct ultrasound access. Unfortunately, poor reporting of the methods and results made this study difficult to interpret and greatly limited its usefulness to the review.
The other two included level 1 studies were non-comparative evaluations of services provided in the UK (England222 and Scotland203). In both studies, ultrasound scanning was carried out at a GP practice/health centre and primarily by GPs. These studies were performed in the late 1990s to early 2000s, so may not reflect current practice. As discussed below (see Table 33), the results of both studies were generally favourable for the community diagnostic ultrasound service model but, given their reported limitations, they must be considered as relatively weak evidence. However, we have not been able to find any more recent and/or rigorous research.
Pallan et al. 16 evaluated a community-based mobile service compared with a hospital-based open-access service. The community service was described as an ‘independent, radiographer-led service’. It appears likely that this service was delivered by a private sector provider rather than a NHS community organisation; this limits its relevance to the review question as originally defined as the study evaluated two ‘comparator’ interventions. Given that the grey literature search located numerous examples of services commissioned from private sector providers, Pallan et al. ’s16 study clearly addressed an intervention that remains relevant and a comparison for which further research is needed.
The conference abstracts by Gallagher et al. 242 and Singh et al. 244 both dealt with the role of echocardiography for heart failure diagnosis in the community. Both abstracts reported limited details of the models of service under study. Singh et al. 244 may be evaluating a hospital-based service and Gallagher et al. 242 may be evaluating community outreach, both of which meet the criteria for comparator interventions rather than the main focus of the review. We have not been able to locate full publications for these studies, limiting the conclusions that can be drawn from them.
Finally, Landers and Ryan234 reported an evaluation of ultrasound in the management of ascites in a community palliative care service. The authors reported that bedside ultrasound allowed scanning and subsequent treatment to be undertaken at home in many cases, which was convenient for the patients and potentially cost saving for the health service. There was an implicit comparison with a model of care based on hospital admission for investigation and treatment, but no comparative data were reported.
Other studies offering insight on service models
Level 3 papers by Robinson et al. 215 and Colquhoun et al. 246 suggest that the hospital open-access model was widely favoured in the UK in the late 1990s. If there was a general acceptance of this model, this may explain the relative lack of research on alternatives. Interestingly, the 1999 thesis by Aitken44 evaluating patient and health professional opinions on community ultrasound services pays little attention to open access as an alternative model of service and concentrates on the implications of transferring a ‘conventional’ hospital-based service to a community setting.
The results of the internet and grey literature search reported below demonstrate the existence of NHS community diagnostic/primary care centres offering ultrasound and other tests, but we have found no evaluations of these in the published literature.
Equipment
Provision of ultrasound scanning in community settings has been favoured by the development of portable devices, culminating in the availability of handheld or ‘pocket’ ultrasound. We located two reviews of handheld devices for echocardiography. 252,255 A third, more up-to-date review examines portable ultrasound devices in other indications, with echocardiography being due for consideration in a separate publication. 45 Although the use of handheld devices appears well established, this review identifies limitations of the evidence base for outcomes other than diagnostic accuracy and identifies an extensive list of research gaps that we have drawn on in our own research recommendations. The Welsh Cardiac Networks Co-ordinating Group also identified areas of uncertainty around the use of handheld scanners for echocardiography. 220
Three primary studies provided additional evidence on the use of handheld devices in community settings (Table 30). 205,247,250 The papers by Doddy247 and Mjølstad et al. 250 provide limited evidence for the effectiveness of handheld scanners in view of their small sample size, lack of a control group247 and reliance on a surrogate outcome measure. 250 There is some overlap with the literature on workforce and training as discussed in the following section. The review also included a conceptual paper on equipment for pre-hospital ultrasound in stroke. 248 The report by the Welsh Cardiac Networks Co-ordinating Group suggests equipment requirements for echocardiography services. 220
| Study | Indication | Setting | Staff | Details of equipment | Findings relevant to equipment |
|---|---|---|---|---|---|
| Doddy 2009247 | General | Primary care (England) | GPwSI in ultrasound | Handheld systems [SonoSite instruments (FUJIFILM SonoSite Inc.) mentioned, no further details] | Considers handheld systems ideal for use in GP surgery. Mentions initial outlay of £18,000–25,000. Reference to funding of machine/service now out of date |
| Mjølstad et al. 2012250 | Echocardiography | Primary care (Norway) | Seven GPs | Portable scanner capable of B-mode and colour flow imaging | Compares handheld with standard echocardiography; findings for septal mitral annular excursion did not differ significantly |
| Siu et al. 2013205 | General | Primary care (Canada) | Family physicians | Not applicable | Survey reveals GPs ready to use handheld ultrasound but faced with barriers |
Workforce and training issues
Numerous studies examine training for GPs and others to perform ultrasound in the community (see Table 27). Further details are provided in Table 31 for level 2 studies and Table 32 for level 3 studies. The amount and level of training varies widely across studies, from short courses designed for use of ultrasound in specific indications243 to comprehensive training programmes designed for clinicians wanting to offer a wide range of ultrasound services. 245 The available research looks at training of predominantly GPs to use ultrasound technology (a study on physiotherapists236 was an exception). No data were available on training or employment of radiographers in community or primary care settings. A general limitation of many of the included studies is that they look only at one or a small number of people being trained.
| Study (authors and year) | Indication | Setting | Staff being trained | Type and amount of training | Findings relevant to training |
|---|---|---|---|---|---|
| Grubel 2011231 | Abdominal | Community practice | Gastroenterologist | Daily supervised ultrasound over 6 months; > 1000 scans in total | Specific standards of training required to demonstrate competence in ultrasound have not yet been defined |
| Hoyer et al. 2007243 | Echocardiography | Primary care (training delivered at cardiology centres) | 48 GPs | Structured training programme of about 4 hours in use of handheld echocardiography for heart failure diagnosis | After first training session, concordance between GPs and cardiologists for presence of heart failure was 80% at centre 1 and 78% at centre 2. Authors concluded that training allowed previously inexperienced GPs to apply handheld echocardiography for heart failure diagnosis |
| Laine et al. 1998233 | Sinus | Primary care centres | 12 GPs | GPs at one centre received standard information on the use of the ultrasound machine from the manufacturer; the other centre had used this machine for 6 years | GPs produced a high number of false-positive diagnoses. More education and practice could improve accuracy of diagnosis |
| Thoomes-de Graaf et al. 2014236 | MSK | Primary care | Physiotherapists | Physiotherapists were required to have at least 1 year of experience in diagnostic ultrasound and to have performed at least 100 shoulder ultrasounds after graduating | Physiotherapists with more experience (> 200 ultrasounds) and more training (advanced course) showed greater agreement with radiologists |
| Varonen et al. 2003237 | Sinus | Primary care | 35 GPs from nine health centres | GPs received a 1.5-hour small-group tutorial on ultrasound from an experienced specialist. GPs at one health centre had used sinus ultrasound for several years (sensitivity and specificity were calculated on results from this centre) | Authors stated that a short tutorial can improve GPs’ accuracy in sinus ultrasound examination |
| Non-English language | |||||
| Esquerra et al. 2012240 | Abdominal | Hospital radiology department and primary care centre | 2 GPs | 100 hours of teaching followed by 112 hours based in the hospital radiology department | For the primary diagnosis, GP scans had a sensitivity of 95.5% (95% CI 91.8% to 99.2%) and specificity of 94.3% (95% CI 90.2% to 98.5%). Authors concluded that it is feasible for trained GPs to perform low complexity diagnostic abdominal ultrasound |
| Evangelista et al. 2013241 | Echocardiography | Primary care centre | 1 GP | Basic training recommended for non-cardiologists (no further details reported) | Agreement between GP and cardiologist was good or very good. Authors concluded that pocket echocardiography performed by a GP as an extension of clinical assessment provides an early diagnosis of significant cardiac lesions that may improve therapeutic management of people with hypertension |
| Study (authors and year) | Indication | Setting | Staff being trained | Type and amount of training | Findings relevant to training |
|---|---|---|---|---|---|
| Bono and Campanini 2007245 | General | Italian primary care | GPs | Requirements for certification as GP ultrasonographer:
|
Programme has been designed to ensure quality of diagnostic ultrasound by GPs |
| Cardiac Networks Co-ordinating Group 2006220 | Echocardiography | NHS in Wales | Cardiac physiologists; echocardiographers; GPs; specialist nurses | 4 years for cardiac physiologists, 2 years part-time for others. Training for existing GPs/nurses needs locum cover (GP) or fitting in with other duties (nurse) | A minimum of 2 years is required to train a practitioner to BSE standards |
| Doddy 2009247 | General | NHS primary care in England | GP | Over 100 hours of Royal College of Radiology training, including a course on musculoskeletal ultrasound at postgraduate certificate level 1 | The choice of training courses for GPs is growing and ranges from a basic general course to specialist applications relevant to primary care |
| Hussain et al. 2004249 | Urinary tract symptoms | NHS primary care in England | GP | 15 telemedicine sessions in which images from GP scans were transmitted to a university expert | Results show feasibility of teleultrasound in primary care. GP benefited from regular training and supervision and achieved satisfactory competence in scanning prostate, bladder and kidneys |
| Mjølstad et al. 2012250 | Echocardiography | Primary care in Norway | 7 GPs | 8 hours of training from a cardiologist certified in echocardiography | After limited training GPs were able to assess a marker of left ventricular function in 87% of patients using pocket ultrasound. There was no significant difference between GPs’ and cardiologists’ findings |
| Siu et al. 2013205 | General | Primary care | Family physicians | 14 out of 18 respondents to a survey had previous training in ultrasonography. Most had received training through a continuing education course | Training issues (confidence, reliability and skill maintenance) were a barrier to physicians using ultrasound in community settings |
Full professional training requires at least 2 years of study220 and developing existing staff has implications for resource use in terms of providing cover/scheduling work.
Two studies243,250 looked at short training programmes for GPs to use handheld equipment for echocardiography. In these studies total training time was approximately 4 hours and 8 hours, respectively. In both studies, GPs achieved good levels of accuracy relative to the study’s reference standard. However, study authors noted that, given the limitations of current equipment, handheld scanner results cannot be regarded as definitive, so GP scan is not a substitute for full echocardiography by a cardiologist. Further research is needed to assess the role of handheld ultrasound to support decision-making for patients with possible LVSD in primary care. 250
One study described a full training programme for GPs to operate as ultrasonographers. 245 A limitation of this study was that at the time of writing it appeared that no courses had actually taken place, so the study describes a consensus view of a desirable training programme for GPs. [In this respect compare the RCR document Ultrasound Training Recommendations for Medical and Surgical Specialties256 (not included in the review because it is not primarily directed at community settings), which recognises three levels of expertise and provides detailed training recommendations for each level by indication.]
Other studies describe individual training experiences of varying intensity. 231,240,247 In the UK, Doddy247 reported having completed over 100 hours of training, comparable with the amount of teaching in the programme described by Esquerra et al. 240 Grubel’s231 training for GI ultrasound included performing supervised ultrasound daily for 6 months. This training was acquired while working in Germany and the author noted that in the USA it is unusual for GI ultrasound to be performed in community settings.
Two studies in Finland evaluated the use of ultrasound for diagnosis of sinusitis by GPs. 233,237 In one study, GPs who had received only basic information from the ultrasound machine manufacturer produced a high number of false-positive diagnoses and the authors suggested that more attention to education and training could improve their performance. 233 In a later study, GPs received 1.5 hours of small group training and high rates of accuracy were received. The authors commented that even a short period of training can improve accuracy. 237 However, it should be noted that the diagnostic accuracy results appear to have come from a centre where the GPs had substantial experience of diagnostic ultrasound, and the findings may not be applicable to less experienced GPs.
The study by Siu et al. 205 examined attitudes to providing ultrasound in community settings among family physicians in Yukon, Canada. Although training was not the main topic of this study, training-related issues (e.g. confidence and maintenance of skills) were identified as key barriers to doctors offering this type of service.
Attitudes/barriers to community provision
A small number of papers, all classified as level 3 studies, addressed attitudes or barriers to provision as their main focus. 44,205,215,224,238,246 Studies from the UK/England suggest that most health professionals preferred open access to hospital services rather than facilities based in general practice. 215,246 This seems to be a key finding, although the most relevant study215 (the other source being expert opinion) dates from 1997 and is not high quality, so the findings may not be applicable to current practice. Also in the late 1990s, Aitken’s44 PhD thesis investigated attitudes to transferring ultrasound services (including monitoring of pregnancy as well as diagnostic ultrasound) to community settings. The research involved focus groups and a questionnaire study with relatively large samples of health professionals (n = 353) and patients (n = 495). Overall, health professionals regarded ultrasound as a suitable service to be provided in the community and patients would be happy to use such a service. However, given the broad scope of ultrasound services covered by the thesis, the relevance of the findings to specifically diagnostic ultrasound is uncertain. Several potential problems were identified, particularly by hospital-based clinicians. These included issues related to communication, quality control and training. Radiographers were concerned about possible isolation when working in the community.
A more recent paper by a GPwSI in ultrasound also shows some awareness of barriers to ultrasound by GPs. 247 Barriers mentioned include access to training and funding for providing the service, although the author believes that these can be overcome. Doddy argues that the perceived benefits to patients are such that all general practices should have access to diagnostic ultrasound for appropriate indications.
Another potential barrier to community ultrasound would arise if secondary care professionals displayed a negative attitude towards this type of service. In the Netherlands, Scholten-Peeters et al. 224 found that hospital doctors (radiologists and orthopaedic surgeons) had low confidence in diagnostic musculoskeletal ultrasound performed by physiotherapists or GPs. Scans performed in primary care were often repeated in secondary care, although this was not quantified. It is interesting that the findings of this recent study echo those of Robinson et al. 215 from 1997.
In a non-UK context, respondents to the survey of Siu et al. 205 identified equipment costs and remuneration as the major barriers to ultrasound in the community, followed by issues related to training. These barriers were evidently considerable, as none of the respondents was currently providing a service in this setting, although most recognised the advantages, had relevant training and were keen to do so.
Siu et al. ’s205 findings are broadly in line with the results of a qualitative study (involving interviews and focus groups) of French GPs. 238 Again, none of the eight participants was currently providing an ultrasound service in the community; seven were interested in doing so and five had received training. However, the participants identified numerous barriers: the authors summarised the main themes as lack of experience with ultrasound in general practice; mastering ultrasound scanning techniques; uncertain relevance of ultrasound scanning in general practice; the GPs’ own reluctance to use the technique; and possible legal issues.
Clinical effectiveness (includes diagnostic accuracy/yield)
All three level 1 studies reported outcomes related to clinical effectiveness of primary care ultrasound (Table 33). 203,222,229 Everett and Preece reported that GP ultrasound had a sensitivity of 97% and specificity of 98% for establishing fetal viability in women with bleeding in early pregnancy. The main clinical benefit was that women with a viable fetus could be given a strong reassurance that they were not at high risk of miscarriage. This enabled referral to hospital to be avoided, saving a journey for the patient and the cost of a hospital ultrasound scan for the health service. However, this was not a controlled study and the authors recognised the need for further research to compare different ways of delivering diagnostic ultrasound for women with bleeding in early pregnancy.
| Study (authors and year) | Application of ultrasound | Main clinical findings |
|---|---|---|
| Everett and Preece 1996222 | Obstetric | 240 women with bleeding in early pregnancy were scanned. Community ultrasound scanning had a sensitivity of 97% and specificity of 98% for predicting fetal survival to the 20th week of pregnancy. No fetal heart movement was detected in 117 women and all subsequently miscarried |
| Wordsworth and Scott 2002203 | General | 131 patients were scanned over 6 months. GP scanning reduced the number of referrals (14.8% vs. 29.9%), emergency hospital admissions (7.8% vs. 13.4%) and hospital scans (0.8% vs. 23.6%) |
| Salihefendic et al. 2009229 | Abdominal | 383/1539 patients in the experimental group and 175/1878 in the control group were scanned. Abdominal ultrasound resulted in a change in anticipated patient management in 54% of patients, including a reduction in anticipated referrals to medical specialists |
The other level 1 UK study reported that having ultrasound scanning available in general practice for abdominal, gynaecological and obstetric problems reduced outpatient referrals, hospital scans and emergency admissions. 203 This study is also limited by the lack of a control group. The results for patients using the GP ultrasound service were compared with a consensus view of how that patient would have been managed had the service not been available.
The only study with a control group that reported any outcomes related to clinical effectiveness of a community ultrasound service was that of Salihefendic et al. 229 These authors compared two primary care clinics in Bosnia-Herzegovina, with and without direct access to abdominal diagnostic ultrasound. The usefulness of this study is limited by unclear reporting but the authors stated that abdominal ultrasound was valuable in guiding patient management. It was unclear how the impact on patient management differed between the experimental and control groups.
Eight studies classified as level 2 assessed the diagnostic accuracy of primary care/community ultrasound but in most cases with some attention to wider clinical implications, making it appropriate to discuss them in this section (Table 34). 230–233,236,237,239 There were contrasting findings across this group of studies, partly explained by differences between different indications. In two studies of musculoskeletal ultrasound, accuracy was found to be poor in community practice in Australia230 and levels of agreement between community physiotherapists and radiologists were slight to moderate for most shoulder pain diagnoses. 236 These findings cast doubt on the value of community ultrasound for these conditions/settings. A further two studies examined abdominal ultrasound. An investigation of scans performed by a gastroenterologist in a US community setting indicated that the results were useful for guiding clinical management in two-thirds of cases and findings were generally supported by subsequent reference tests. 231 However, Heikkinen et al. ,232 in a long-term study of patients with functional dyspepsia, reported that repeat ultrasound rarely changes the diagnosis and hence it was not recommended.
| Study (authors and year) | Application of ultrasound | Main clinical findings |
|---|---|---|
| Goldberg et al. 2003230 | Musculoskeletal | The accuracy of diagnostic ultrasound was 0.38, sensitivity was 0.24 and specificity was 0.61. There were 155 false negatives and 51 false positives based on the diagnostic ultrasound reports (out of 336 patients) |
| Grubel 2011231 | Abdominal | 310 patients underwent ultrasound. Ultrasound findings guided clinical management in two-thirds of patients (exact numbers not reported). A normal ultrasound result was subsequently confirmed in 35 out of 40 patients (88%). Abnormal findings were confirmed in 41 out of 44 patients (93%). Ultrasound missed three (4%) significant clinical lesions but no malignancy was overlooked |
| Heikkinen et al. 2005232 | Abdominal | Repeated ultrasound examination in people with functional dyspepsia is not recommended and rarely changes the diagnosis |
| Laine et al. 1998233 | Sinus | Sensitivity of GP ultrasound was 61% and specificity was 53%. For ultrasound combined with clinical examination, sensitivity was 70% and specificity was 37%. Poor accuracy of ultrasound was mainly due to a high number of false-positive diagnoses |
| Thoomes-de Graaf et al. 2014236 | Musculoskeletal | Agreement between physiotherapists and radiologists is borderline substantial for full-thickness tears and slight to moderate for other diagnoses |
| van Gurp et al. 2013223 | Echocardiography | GPs using a community open-access service reported that they referred fewer patients to a cardiologist than they would have done without the service and managed more patients themselves. In 127 cases (82%), the GP thought that the echocardiogram was beneficial to decision-making |
| Varonen et al. 2003237 | Sinus | With practice and training, primary care physicians can perform sinus ultrasound as accurately as specialists, potentially reducing unnecessary use of antibiotics for patients with acute maxillary sinusitis |
| de la Figuera et al. 2012239 | Echocardiography | Data on 684 patients who underwent echocardiography at four health centres in 2006–7 were analysed. 84% of requests were appropriate and 79.7% showed the presence of disease. Echocardiography results influenced decision making in > 35% of cases: 17.1% were referred to a cardiologist, 10.5% had their treatment changed and 9.6% were referred for additional tests |
Two studies of ultrasound for diagnosis of sinusitis in Finnish primary care identified problems caused by a high rate of false-positive diagnoses233 but, given appropriate training, GPs could diagnose as accurately as specialists,237 with the potential to reduce unnecessary antibiotic use. Two studies of echocardiography were identified. A study of a community-based open-access service in the Netherlands223 was accompanied by a critical commentary. 253 The study authors concluded that the open-access service reduced referrals and allowed more patients to be managed in primary care, although again there was no control group and comparison was against what the primary care physician reported they would have done in the absence of the service. The author of the commentary criticised the lack of connection between the open-access service and local hospitals. The commentary also stated that the ultrasound technicians might have had insufficient experience and the cardiologists involved in the study were provided with insufficient information. In conclusion, the commentary author argued that open-access echocardiography may lead to a ‘low-budget pseudo-consult[ation] of the cardiologist, whilst the patient thinks he is getting a state-of-the-art treatment’. 253
Overall, these studies indicate that community ultrasound can guide patient management and potentially reduce referrals, at least in some indications/settings. However, the contradictory results and generally poor quality of the studies, particularly the absence of true control groups, make it difficult to draw any firm conclusions.
Conference abstracts by Gallagher et al. 242 and Singh et al. 244 are discussed separately, as they deal with different service models for echocardiography (Table 35). Gallagher et al. 242 studied a community outreach clinic giving GPs access to natriuretic peptide testing followed by echocardiography in the community if required. The main outcome measure in this non-comparative retrospective study was diagnostic yield (see Table 35). The authors concluded that specialist review in the community offers the opportunity for older patients to access specialist care in a timely manner. 242 Singh et al. 244 studied a one-stop diagnostic clinic for heart failure. The setting for this clinic was probably secondary care, although it was run by a primary care physician specialist. As with Gallagher et al. ,242 the main outcome measure for the study was the diagnostic yield and there was no comparison group. The ability to provide alternative diagnoses for most patients without LVSD was seen as an advantage of the one-stop clinic over open-access echocardiography. Unfortunately, neither study appears to have been published in full, which makes it difficult to evaluate them or place them in the context of the review question.
| Study (authors and year) | Application of ultrasound | Main clinical findings |
|---|---|---|
| Gallagher et al. 2012242 | Echocardiography | GPs were given direct access to natriuretic peptide testing followed by facilitated access to echocardiography in the community. Of 66 patients who completed assessment over 6 months, 37 had echocardiography; 23 were abnormal and 17 were diagnosed with heart failure |
| Singh et al. 2009244 | Echocardiography | Of 1008 patients referred to a one-stop diagnostic clinic, 292 (29%) had confirmed LVSD on echocardiography. For the 716 without LVSD, an alternative cause for symptoms was identified in 578 (81%) |
Four studies classed as level 3 evidence reported issues related to clinical effectiveness, although these were limited. Hölscher’s review article assesses the potential use of diagnostic ultrasound in the pre-hospital treatment of people with stroke, but it is described as a concept paper and does not report any hard data. 248 Ottenheijm et al. 251 published the protocol for a randomised trial of diagnostic ultrasound-guided therapy in patients with shoulder pain. The primary outcome is patient-perceived recovery at 52 weeks. Full details of the trial had not been published in time for inclusion in the report.
Finally, two review articles should be mentioned for the sake of completeness. Senior et al. ,252 focusing mainly on accuracy and technical outcomes, compared handheld echocardiography with traditional echocardiography and BNP testing for diagnosis of BNP in the community. A review article in Spanish by Vicente-Molinero et al. 254 searched a range of sources including INAHTA (International Network of Agencies for Health Technology Assessment), PubMed and websites of scientific societies for the years 1996–2006. This broad-ranging review presented few details of the included studies and was used primarily as a means by which to identify references for possible inclusion in our review.
Overall, the evidence base for clinical effectiveness of community ultrasound is very limited and, in particular, there is a lack of controlled studies comparing different ways of delivering ultrasound services.
Evidence on process/system outcomes (e.g. waiting times)
Only one level 1 study provided evidence on process or health system-related outcomes. 203 The study by Wordsworth and Scott203 included an evaluation of the quality of GP ultrasound scans by a consultant radiologist. Fifty of 131 scans could not be evaluated, but overall the quality of scans was sufficient to allow scanning to continue at the practice, subject to the further training of GPs in specific areas. It was also recommended that one GP, rather than two, should perform ultrasound scans to achieve a sufficient volume of scanning. 203 The authors also noted that 6 of the 131 patients scanned received earlier access to treatment as a result of being scanned at the practice.
Five of the level 2 and 3 studies published in English provided evidence on process/system outcomes. Makela et al. 235 reported that, when available, ultrasound was used to diagnose maxillary sinusitis in Finnish primary care in 82–92% of cases. The paper by Pallan et al. 16 compared a radiographer-led community diagnostic ultrasound service with a hospital open-access service and found that waiting times for appointments were shorter for the community service (mean 17.44 vs. 44.53 days). Eighteen out of 23 GPs who used both services stated that waiting times influenced their decision about where to refer a patient. Pallan et al. 16 also followed the pathways of three pairs of patients referred to secondary care following an abnormal scan. The time interval between initial ultrasound investigation and referral ranged from 1 to 168 days, although the reasons for this were not reported. The authors reported that, overall, there were no systematic differences between the two services.
An important finding from the study by Scholten-Peeters et al. 224 was that, according to Dutch orthopaedic surgeons and radiologists, the diagnostic process for people with musculoskeletal problems was rarely focused on the referral diagnosis from primary care. Only 47 (22.1%) respondents stated that it was, compared with 133 (62.4%) answering negatively and 23 (10.8%) not applicable. Combined with the negative opinions of respondents about diagnostic ultrasound performed in primary care, this would suggest that the process of diagnosis may be suboptimal, although it is unclear if the results are likely to be generalisable to the UK setting.
In the Canadian health system, Siu et al. 205 reported, based on a survey with a 44% response rate, that family physicians were not currently using ultrasound in community office settings. This was attributed in part to the availability of ultrasound technicians locally to perform scans following referral. The authors noted that bedside ultrasonography performed by a physician would most often be focused on a particular clinical question and could be used to inform a decision about referral for further investigation (including ultrasonography).
Process and/or system outcomes reported in van Gurp et al. ’s223 study of community echocardiography in the Netherlands included waiting times and GP adherence to the cardiologist’s advice and to Dutch clinical guidelines for heart failure. Waiting time for echocardiography via referral was estimated at 5 weeks, compared with 6 days via the community open-access service. GPs followed specific advice from the cardiologist evaluating the scan in 25 out of 31 cases (81%). In one case the advice was not followed and in five cases the outcome was not known. Adherence to clinical guidelines was considered suboptimal, in that 13 out of 55 patients with suspected heart failure were referred to echocardiography without evidence of an abnormal ECG or BNP result.
A study published as a conference abstract243 and two studies published in Spanish240,241 focused on training GPs for handheld echocardiography241,243 or abdominal ultrasound. 240 The studies are discussed above, but in terms of health system outcomes, they indicate that training programmes can be delivered successfully at different centres and levels of intensity. By contrast, the barriers to GP ultrasound in the French health system identified by Blanchet and Thierry238 included uncertainty about various process- or system-related factors, for example impact on length of consultations and relationships with secondary care specialists.
Patient acceptability
The four studies that reported on patient views of ultrasound in the community all reported positive findings. 16,44,203,247 The studies used different methods to assess patients’ views. Wordsworth and Scott carried out a discrete choice experiment which showed that patients strongly preferred scanning at the GP surgery to the hospital alternative. This study was performed in a rural area of Scotland and the hospital was 30 miles from the surgery, so the results may not be generalisable to more urban areas. Patients showed no strong preferences as to who performed the ultrasound scan (e.g. the GP or the radiographer). 203 In the study by Pallan et al. ,16 patients (and referring GPs) were asked to rate their satisfaction with a community ultrasound service and a hospital open-access service on a 1–5 scale. Eighty-six per cent of patients rated the community service at 4 or 5 (the highest levels of satisfaction) compared with 76% for the hospital service. GPs were markedly more satisfied with the community service than with the hospital service. This was the only study to compare the views of users of different types of service.
Turning to level 3 evidence, the majority of patients who took part in Aitken’s focus groups and surveys indicated that, hypothetically, they were happy to use a community ultrasound service. 44 Percentages of patients responding positively in the surveys were 76% in a pilot survey and 72% in the main survey. Focus groups identified convenience and improved access as key reasons for preferring community ultrasound. Finally, Doddy states in his opinion article that his patients are ‘delighted to be scanned at the surgery’, although no evidence is presented to support this. 247
Costs/resource use
Two included studies reported on costs or resource use. 16,203 Wordsworth and Scott performed a cost analysis of GP versus hospital ultrasound scanning. The main finding was that cost per scan was higher for GP scanning (£36.37 vs. £20.32) but the average cost per episode was lower (£148 vs. £183). Pallan et al. 16 calculated the cost per abnormality detected for community and hospital ultrasound services and found that the cost was higher for the community service [£107.69, 95% confidence interval (CI) £90.61 to £132.71 vs. £77.35, 95% CI £63.76 to £98.30]. The authors argued that reduced waiting times and high patient and GP satisfaction could justify the higher costs for the community service.
Mapping of current practice (internet search)
Included items and characteristics
Published articles
Two published articles not identified by the database searches234,247 were picked up by the internet search and included in the synthesis below. An ongoing systematic review of ultrasound for musculoskeletal disorders in primary care was identified from the PROSPERO systematic reviews register. 257 Other online articles were retained as useful background papers. The remaining articles were judged not relevant to the review or added little to the evidence available from the peer-reviewed literature.
Companies providing diagnostic ultrasound services
The searches revealed details of services provided by 22 companies, most of which provided a range of diagnostic (and in some cases clinical) services in addition to ultrasound (see Appendix 8). However, one of these appeared to be not mainly focused on ultrasound (InHealth), one was mainly serving hospitals rather than community settings (Independent Vascular Services), and one appeared to be an exclusively private service (The Ultrasound Centre). Details provided by the company websites were highly variable. Commonly reported features include time standards for appointments and report delivery and patient satisfaction ratings. Links to the NHS, including experience of the radiographers and others providing the service and roles of NHS consultants in audit and governance, were emphasised by most of the companies. It appeared that many staff worked part-time for the companies and the remainder of the time for NHS organisations (primarily hospital trusts).
Services were described as being generally commissioned through the ‘any qualified provider’ system. All services are regulated by the Care Quality Commission (our search found one Care Quality Commission inspection report). All of the services appeared to follow basically the same model of care, namely a mobile service operating through GP surgeries and other community sites and staffed by radiographers (and health-care assistants), with access to consultant radiologists as required.
NHS service specification
The internet search also found a standard NHS specification for non-obstetric ultrasound,258 covering the following indications:
-
general abdominal: includes assessment of the aorta, biliary tract, gallbladder, inferior vena cava, kidneys, liver, pancreas, retroperitoneum and spleen
-
gynaecological: including transabdominal and transvaginal
-
renal/bladder/prostate
-
scrotal/testicular
-
musculoskeletal
-
vascular.
There are various exclusions from the service specification, including scans for suspected cancer, ultrasound-guided procedures and obstetric care, as well as parts of the body not listed above. The NHS service specification also provides for scans to be undertaken in secondary care where this is an integral part of a clear clinical pathway. 258
NHS diagnostic centres (or similar names)
Websites were identified for ‘diagnostic centres’ run by private sector companies in Rotherham (Care UK), Havant (Care UK), Buckinghamshire (Care UK) and Woking (Virgin Care). These centres provide a range of diagnostic tests, including ultrasound. The websites provided little information that could be helpful for evaluation of the service.
Primary care centres
Websites were also identified for ‘primary care centres’: Bingfield (InHealth), Keyworth (Diagnostic Healthcare), George Street (Diagnostic Healthcare), Duncan Street (Diagnostic Healthcare) and Lordship Lane (InHealth). These differed from the ‘diagnostic centres’ by being integrated with GP surgeries rather than specialising in diagnostic services, but resembled them in offering ultrasound alongside other diagnostic services. Again, the websites provided limited information.
Service description/specification
Published service specifications for non-obstetric ultrasound from Nottingham City CCG, Milton Keynes CCG, Manchester PCT and Ipswich CCG were identified. All were closely based on the standard NHS service specification described above. 258
Summary of findings
Evidence from the internet search results suggests that community-based services run by non-NHS providers with links to the NHS for governance and quality control are feasible and, in some cases, well established. Where reported, levels of patient satisfaction are high. It is possible that the characteristics of patients referred to these services are different from those of patients referred to secondary care services (including ‘open access’ services); this makes it difficult to compare the two types of service. The limited evidence also suggests that these services support the stated aims of providing patients with services close to home, avoiding unnecessary referrals and allowing more patients to be managed in primary care.
For practical reasons, it was possible to examine only a small proportion of the search results. The results examined were ranked highly for relevance by the search engine but the findings should not be regarded as a comprehensive list of current services. Furthermore, the possibility cannot be ruled out that some of the webpages found relate to services that are no longer operating.
Discussion
Main findings
Service models
To the best of our knowledge this is the first systematic review to examine community and primary care ultrasound from the perspective of service delivery and organisation. We found very little evidence to guide decision-making about models of service for diagnostic ultrasound services. The best evidence from the UK is over 10 years old, with no rigorously designed experimental studies of different service models. Very few studies compare community and secondary care services16,203 or even describe UK community-based services. 222 We have found no recent studies from the UK.
Open access for GPs to ultrasound facilities based in secondary care became widely available during the 1990s. Research published in 1997 suggested that this model of care was favoured by GPs and secondary care clinicians. 215 There was little interest in ultrasound services based in GP surgeries or in training GPs to perform ultrasound. The limited number of studies located meant that we were unable to form a clear picture of the current situation, although we identified one article by an enthusiastic GP who had undertaken training and was offering diagnostic ultrasound services which were popular with his patients. 247 Funding structures and training opportunities at the time this article was written did not appear to encourage GPs to take this route and it appears likely that the numbers offering ultrasound in average-size practices would be small.
Lack of research may reflect general satisfaction with the prevalent ‘open-access’ model. However, increasing pressure to provide services in the community combined with ability to commission services from ‘any qualified provider’ has favoured the emergence of other models (primarily mobile services operating at community sites and delivered by private providers who may share staff with NHS organisations). We found that such services are widespread in the English NHS, although the extent of this diffusion is difficult to quantify. The only study in our review that evaluated a mobile service found that, compared with a hospital open-access service, waiting times were shorter but costs per abnormality detected were marginally higher. 16 CCGs have also begun commissioning other types of service such as ‘community diagnostic centres’, primarily from private sector providers. Such centres would offer ultrasound scanning alongside other diagnostic/imaging tests.
Services tend to offer scanning for a standard range of indications for ultrasound as defined by the NHS non-obstetric service specification. Limited evidence suggests that alternative/innovative uses may be possible, for example in community palliative care. 234
Clinical effectiveness
The few studies that reported clinical effectiveness outcomes were mostly of poor methodological quality. Two studies indicated that community ultrasound can guide patient management and potentially reduce unnecessary referrals, at least for some indications/settings. 203,223 A further study highlighted problems with poor reliability and quality of scans performed in community settings. 230 The contradictory results and generally poor quality of the studies, particularly the absence of true control groups, make it difficult to draw any firm conclusions.
One of the main potential benefits of community diagnostic services is the potential for patients to obtain quicker access to diagnosis, and, if necessary, treatment, than through referral to secondary care. There was limited evidence related to this in studies included in the review. Wordsworth and Scott reported that 6 out of 131 patients in their study ‘received earlier access to [surgical] treatment, because their diagnoses had been made earlier as a result of having a scan at the practice’. 203 In the absence of a control group, it was unclear what would have happened to these patients had scanning not been available (e.g. outpatient referral or referral for direct access scan) and so the benefit of GP ultrasound scanning was difficult to quantify. Pallan et al. 16 found that waiting times for an appointment were substantially shorter for a community-based mobile service than for a hospital ‘open access’ service. However, it was unclear if this translated into improved access to treatment. The authors mapped the pathways of three pairs of patients referred to secondary care after an abnormal scan and reported no systematic differences between the two services.
Cost-effectiveness
Two studies in the review reported on costs of different models of ultrasound services. Wordsworth and Scott reported that cost per scan was higher for GP than hospital scanning but the cost per episode was lower. 203 Pallan et al. 16 found that the cost per abnormality detected was higher for a radiographer-led community service than a hospital open-access service. The authors argued that reduced waiting times and high patient and GP satisfaction could justify the higher costs for the community service. The limited quantity and quality of evidence around the cost-effectiveness of community ultrasound services in UK settings is particularly important in view of the recent emergence of new models of service, such as privately operated mobile services and diagnostic centres offering multiple testing services at a single site.
Attitudes
A recent survey of radiologists and orthopaedic surgeons in the Netherlands indicated that they have a low opinion of the quality and appropriateness of ultrasound scanning in primary care, and scans performed in primary care are often repeated after referral to hospital. 224 Many clinicians in this study did not focus on the referral diagnosis from primary care but in effect restarted the diagnostic process from scratch. Lack of confidence by secondary care clinicians in scans performed by their primary care and community colleagues would have considerable implications for resource use and would be a major barrier to the successful implementation of community-based services. We found no evidence on the attitudes of secondary care specialists in the UK. However, the research conducted by Robinson et al. 215 in the 1990s suggested that the delivery of ultrasound was seen as a role for secondary care, despite the then-policy of encouraging primary care and community services. 44
Training/workforce
The studies included in the review deal with training of GPs and other doctors. Studies are split between those evaluating long periods of intensive training220,231,245 and those arguing that short training programmes (hours to days) equip doctors adequately to perform, for example, screening echocardiography using a handheld scanner. 243,250 The evidence thus suggests that training needs will vary depending on the role of diagnostic ultrasound scanning in the practice of the individual or the service that employs them.
None of the evidence we examined looked at training for health professionals other than doctors, despite the fact that a high proportion of ultrasound scans are delivered by radiographers and/or ultrasonographers. In particular, there was no consideration of the training needs of ultrasonographers working in community settings, how these needs might differ from those of hospital-based staff and how best to deliver training to those working outside hospital structures. Links to secondary care may be important for governance and quality control of a community-based service, as highlighted in the critique of a community echocardiography service in the Netherlands. 223,253
Equipment
A major development in the field of ultrasound has been the availability of handheld or ‘pocket’ devices. These are cheaper and more easily transportable than traditional scanners and as such have facilitated the development of services outside hospital settings. However, a recent report identified many uncertainties around training needs, impact on clinical decision-making and cost-effectiveness. 45 One study included in the review highlighted that scans performed with handheld devices at the bedside may not be definitive but would have a role in guiding decisions on further investigations, including additional ultrasound scans. 205
Strengths and limitations
We aimed to be inclusive in terms of study designs and sought information from a range of other sources in addition to bibliographic databases. The internet search enabled us to identify some useful items of grey literature and articles not indexed by the databases (e.g. articles from non-academic professional periodicals such as the Health Service Journal). We were also able to identify a selection of current NHS services and companies offering services to the NHS, and extract relevant data from their websites.
While including evidence from a wide range of study designs, we attempted to assess the quality (risk of bias) of included publications when possible. This involved the use of several different tools and checklists in an attempt to provide the reader with the most appropriate quality assessment for each type of included study. We have also included some reports that we were unable to quality assess, for example conference abstracts, when these provided useful information about models of service. In addition to quality assessment, we categorised evidence into levels based on study design, setting and relevance to the review question. This approach has been used in other systematic reviews259 and is useful when a small subset of core studies is supplemented by studies that meet inclusion criteria but are less useful in addressing the review question (e.g. lower external validity) or address only limited aspects of the question.
The limitations of the evidence base have been discussed above. Possible limitations of the review process include the possibility of omitting relevant studies published before our cut-off date of 1995. We found relevant evidence dating from 1997,215 so this possibility cannot be ruled out. However, developments in equipment as well as changes in the organisation of the NHS mean that older literature is decreasingly relevant to the scope of the review, justifying the decision to stop at 1995.
A further possible limitation of the review concerns the selective internet searching. Although the results made a valuable contribution to the review, the websites and pages included represent a snapshot of current services rather than a comprehensive list. Information about the services currently being commissioned in the NHS is contained in many and disparate sources and is constantly changing. The results appear fairly representative but a full picture of the range of services currently available would require a survey of commissioning organisations with intensive follow-up to obtain a reasonable response rate. Such a survey was outside the scope of this project.
Relationship to other research
We are not aware of any other systematic reviews addressing the specific topic of ultrasound services in primary care/community settings, although a protocol for a review of ultrasound imaging for musculoskeletal disorders in primary care was located. 257 Based on the details provided, this review appeared to have more of a descriptive/qualitative focus and, as such, its findings will complement our review with its emphasis on issues affecting service delivery. Although not strictly systematic reviews, the Horizon Scanning reports published by the NIHR DEC in Oxford also represent relevant synthesised evidence, and some of the recommendations of the DEC report on portable ultrasound devices45 are drawn on below.
Conclusions
The limitations of the evidence base identified by this focused rapid review suggest that there are urgent needs for further data collection and research to guide decision-making in relation to diagnostic ultrasound services.
Implications for health care
We have identified the following implications for health care:
-
The evidence suggests uncertainty about the training needs of different staff groups, for example GPs, nurses and radiographers, particularly any specific needs for community-based services. This also applies to needs for the teaching/training of students and GP trainees. 45
-
Further uncertainty surrounds the implications of different models of service for quality control and governance. Relevant standards have been published by the RCR and Society and College of Radiographers. 204
-
The evidence we were able to locate suggests that the monitoring and evaluation of changes to services at the local level through appropriate audit and data collection is currently limited.
Implications for research
We have identified the following priority suggestions for research.
-
Studies to compare different models of ultrasound service, particularly to compare GP-based and hospital-based services with (nurse-led?) ‘diagnostic centres’. This could include general diagnostic ultrasound services and/or specific indications, for example echocardiography. Outcomes could include waiting times, access to treatment if required and patient satisfaction. Although randomised studies using a cluster design may be feasible in principle, in practice such research would probably involve an observational study design, for example controlled before-and-after or interrupted time series. The Nuffield Trust has recently published a guide to evaluating new service initiatives using retrospective matched control methods. 260 Given access to suitable data, these methods could be valuable for comparing different service models for ultrasound and other diagnostic interventions.
-
Research to understand the workforce implications of different models of care as above, including workforce needs, staff training and development, and job satisfaction. This overlaps with the first implication for health care above, but involves research to inform longer-term planning as distinct from short-term service development.
-
It would also be valuable to compare patterns of demand for ultrasound scanning, both across areas with different types of service provision and over time within an area. This would be helpful in understanding the extent to which increases in availability of services may create demand that would not otherwise exist, and the implications of this for patient flow within the health system.
-
A better understanding is needed of the barriers to and facilitators of the process of developing ultrasound services in primary care/community settings, including both the process of transferring services from secondary care to primary care settings and supporting the growth and development of new service models.
-
Rigorous economic evaluation(s) of different models of service using modelling or data collected from clinical studies should be performed to complement the studies mentioned above.
-
Integration of information from diagnostic ultrasound with results of other imaging tests and how this feeds into patient management pathways. This could include investigation of the usefulness of POC ultrasound performed by GPs and other trained health professionals. 45
Chapter 6 Primary care/community-led diagnostic pathways for the assessment of breathlessness
Introduction
Breathlessness (shortness of breath or dyspnoea) is a common symptom associated with a range of acute and chronic conditions, including COPD, asthma, lung cancer, heart failure, AF and obesity. Outcomes for these conditions can be improved with an early diagnosis. In the NHS, diagnostics have traditionally been offered in hospital settings. However, rising demand year on year is contributing to overloading in the secondary care system that impacts on patient pathways, despite relatively recent recommendations to increase the hours of access. The provision of diagnostics in the community or in primary care has the potential to ease flow through the health system as well as increase access to an early diagnosis. However, obtaining a differential diagnosis for a condition such as COPD requires training in the delivery of investigations and increased utilisation of resources and equipment. Therefore, a primary care-led diagnostic service would need to account for implementation costs and benefits accordingly.
International guidelines exist for primary care diagnosis of lung conditions; however, not all breathlessness is associated with a lung condition and not all lung conditions initially present with breathlessness. A number of national and international models of care and pathways currently exist for primary care and/or community diagnosis for symptoms of breathlessness, including rapid-access breathlessness clinics and diagnostic pathways. For example, one aim of providing a rapid-access clinic is to allow multimorbidities to be accounted for in a setting dedicated to breathlessness diagnostics. Another approach is the federated model, which links a network of GP practices to form a collaborative effort in providing diagnostic and follow-up services. However, there is no published consensus about the clinical effectiveness or cost-effectiveness or the acceptability for service users of any particular service model.
The aim of this systematic subreview, within the overall package of work examining services in primary care, was agreed in consultation with NIHR. The purpose was to assess the evidence base for diagnostic services provided outside hospital settings (such as community or general practice) for undiagnosed individuals presenting with symptoms of breathlessness.
Methods
Research questions
The study aimed to address the following research questions:
-
What service models and pathways for breathlessness diagnostics delivered in primary care or community settings currently exist in the UK and internationally?
-
What evidence is there for the quality, safety, feasibility, acceptability, clinical effectiveness and cost-effectiveness of such models and pathways?
-
What are the barriers to, and facilitators of, developing and implementing such service models and pathways?
Inclusion/exclusion criteria
Participants
People with symptoms of breathlessness who have a suspected condition such as COPD or heart failure (not people who have a diagnosis and are being monitored/managed in a primary care setting; not screening an ‘at risk’ population for a particular condition).
Intervention
Service models and pathways that are designed to be initiated in primary care or the community in order to make a differential diagnosis from a presenting symptom of breathlessness. This could include specific diagnostic technologies such as spirometry, although the effectiveness of such technologies will not be assessed.
Comparator
Service delivery models or pathways developed to assist diagnosis from breathlessness symptoms and delivered in secondary care settings. This is included comparison of the clinical effectiveness, cost-effectiveness, quality, safety, feasibility and acceptability of the intervention between settings.
Outcomes
Any measure of effect on patient management, outcomes, patient experiences or costs/resource use including training.
Setting
Diagnostics delivered in a primary care or community setting.
Study design
Systematic literature reviews and primary studies of any design that describe or evaluate a pathway of care for breathlessness. These include retrospective/prospective studies and audits as well as experimental, observational and economic evaluations. Qualitative studies that reported service user or staff views about the barriers to, and facilitators of, service development and use of the pathways (received care or decision-making) were eligible for inclusion.
Identification of studies
A comprehensive search of key bibliographic databases was performed in July 2015. The MEDLINE strategy combined terms around ‘primary care’, ‘dyspnoea’, ‘spirometry’ and ‘diagnostics’, and included both MeSH and free-text searches. Terms were combined using Boolean operators and database-specific syntax. The MEDLINE strategy was subsequently adapted in accordance with the other databases searched as part of the review. Results were limited by date to 2000 to current. The search strategies are provided in Appendix 9.
The databases searched were:
-
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R), 1946–present
-
EMBASE via Ovid, 1974–present
-
Cochrane Database of Systematic Reviews via The Cochrane Library, 1996–present
-
Cochrane Central Register of Controlled Trials (CENTRAL) via The Cochrane Library, 1898–present
-
Database of Abstracts of Reviews of Effects (DARE) via The Cochrane Library, 1946–present
-
HTA database via The Cochrane Library, 1989–present
-
NHS Economic Evaluation Database (NHS EED) via The Cochrane Library, 1968–present
-
CINAHL, 1960–present
-
Science Citation Index Expanded (SCI-EXPANDED) via Web of Science, 1900–present
-
Conference Proceedings Citation Index- Science (CPCI-S) via Web of Science, 1990–present.
Selection of studies
Citations retrieved via the searching process were uploaded to an EndNote database. This database of study titles and abstracts was independently screened by two reviewers and any queries regarding inclusion were resolved by consulting other team members. Full-text copies of all citations coded as potentially relevant were then retrieved for systematic screening.
Studies that evaluated the effectiveness of new services or changes to the referral pathway were given the coding ‘intervention studies’. Papers that reported the views or perceptions of staff or patients were coded as ‘views studies’.
Extraction of data and synthesis methods
Studies that met the inclusion criteria following the selection process above were read in detail and data were extracted. Three members of the research team carried out the data extraction. The data extraction and synthesis was carried out separately for the subgroups of intervention and views papers. Following examination of these different forms of evidence, we integrated the findings via use of a conceptual model.
Intervention studies data analysis
The heterogeneity and limited study design of the included work precluded summarising the effectiveness of the interventions via meta-analysis. The effectiveness review findings are therefore reported using narrative synthesis methods, including tabulation of characteristics of the included studies, and examination of outcomes by characteristics such as intervention content and study design. The relationships between studies and outcomes within these typologies are scrutinised.
Views studies
The qualitative and survey data were synthesised using thematic synthesis methods to develop an overview of recurring perceptions of potential obstacles to successful outcomes in the data. This method comprises familiarisation with each paper and coding of the finding sections (which constitute the ‘data’ for the synthesis) according to key concepts within the findings. Although some data may directly address the research question, sometimes information, such as barriers to and facilitators of implementation, has to be inferred from the findings, as the original study might not have been designed to have the same focus as that of the review question.
Integrating the data
We used a conceptual modelling method to integrate findings from examination of the intervention and views literature. This was intended to provide an evidence-based overview of elements of the referral pathway that were reported in the identified literature.
Quality appraisal strategy
Quality assessment of the higher quality intervention studies was based on the Cochrane criteria for judging risk of bias (The Cochrane Collaboration). This evaluation method classifies studies in terms of sources of potential bias within studies: selection bias, performance bias, attrition bias, detection bias and reporting bias (see Appendix 10). The conference abstract papers were not quality assessed, as they provided only limited data; these papers are highlighted in the synthesis. Tools used for the assessment of quality are provided in Appendix 10.
The assessment of quality for the qualitative papers was carried out using an eight-item tool adapted from the Critical Appraisal Skills Programme tool for qualitative studies (see Appendix 10). The quality scoring for each study is presented in tabular form across each of the eight items.
Results of review of intervention studies
We identified 36 studies that were relevant to our examination of models and pathways for patients presenting with breathlessness in the community. 105,182,187,195,261–292 These papers evaluated the effectiveness of additions to diagnostic services or technology available to GPs that might be utilised for a patient presenting with breathlessness. Four pairs of papers were linked,105,182,195,270,274,278 with the first of each pair reporting earlier findings and the second updating with a more recent set of data. Therefore, there were 28 unique studies across the group of included papers. Figure 2 provides an overview of the process of inclusion/exclusion, which resulted in the inclusion of 32 intervention studies.
Type of evidence available
Study design
Table 36 summarises the range of study designs in the included set of papers. As can be seen, we identified six RCTs and one further paper reporting a non-randomised comparator study. We found a sizeable literature retrospectively examining service data or patient data. This was commonly reported via conference presentation abstracts rather than published peer-reviewed journal papers. Although the data contained in these conference publications tended to be limited, we made the decision to include conference abstracts in the review, to provide a more complete indication of where work in this area was being carried out.
| Study design | Study (authors and year) |
|---|---|
| RCT | Burgos 2011264 |
| Burri et al. 2012265 | |
| Lusardi et al. 2006279 | |
| Masa et al. 2011280 | |
| Poels et al. 2008284 | |
| Tomonaga et al. 2011288 | |
| Non-RCT | Akhtar and Wilson 2005261 |
| Cohort | Van der Mark et al. 2014289 |
| Before and after | Carr et al. 2011267 |
| aCallister et al. 2011266 | |
| aSchermer et al. 2013195 | |
| Retrospective case note/service data analysisa | Cawley et al. 2011268 |
| Lodewijks-van der Bolt et al. 2007278 | |
| Starren et al. 2012182 | |
| Walker et al. 2006290 | |
| van Heur et al. 2010105 | |
| Wolfenden et al. 2006292 | |
| aBackler et al. 2011262 | |
| aDiar Bakerly and Roberts 2009271 | |
| aHarris et al. 2012273 | |
| aLau et al. 2014277 | |
| aMcNeil et al. 2012281 | |
| aMetting et al. 2013282 | |
| aO’Herlihy et al. 2013283 | |
| aPunwani et al. 2014285 | |
| aSallaway et al. 2011286 | |
| aWilley et al. 2013291 | |
| Cross-sectional | Chavannes et al. 2004269 |
| Hassett et al. 2006274 | |
| Jones et al. 2005275 | |
| Thijssing et al. 2014287 | |
| White et al. 2007187 | |
| aDenis et al. 2013270 | |
| aFois et al. 2014272 | |
| aKaplan and Lerner 2013276 | |
| Borg et al. 2010263 |
Country of origin
Fourteen of the papers originated from the UK; six of these were conference abstracts (Table 37). None of the RCTs originated in the UK, although the non-randomised controlled study261 was carried out in the UK (Leicester).
| Country | Study (authors and year) |
|---|---|
| UK | Akhtar and Wilson 2005261 |
| Carr et al. 2011267 | |
| Hassett et al. 2006274 | |
| Jones et al. 2005275 | |
| Starren et al. 2012182 | |
| Walker et al. 2006290 | |
| White et al. 2007187 | |
| Wolfenden et al. 2006292 | |
| aBackler et al. 2011262 | |
| aCallister et al. 2011266 | |
| aDiar Bakerly and Roberts 2009271 | |
| aHarris et al. 2012273 | |
| aLau et al. 2014277 | |
| aPunwani et al. 2014285 | |
| The Netherlands | Chavannes et al. 2004269 |
| Lodewijks-van der Bolt et al. 2007278 | |
| Poels et al. 2008284 | |
| Thijssing et al. 2014287 | |
| van Heur et al. 2010105 | |
| van der Mark et al. 2014289 | |
| aDenis et al. 2013270 | |
| aMetting et al. 2013282 | |
| aSchermer et al. 2013195 | |
| USA | Cawley et al. 2011268 |
| aWilley et al. 2013291 | |
| Canada | Sallaway et al. 2011286 |
| aKaplan and Lerner 2013276 | |
| Spain | Burgos 2011264 |
| Masa et al. 2011280 | |
| Switzerland | Burri et al. 2012265 |
| Tomonaga et al. 2011288 | |
| Italy | Lusardi et al. 2006279 |
| aFois et al. 2014272 | |
| The Republic of Ireland | aMcNeill et al. 2012281 |
| aO’Herlihy et al. 2013283 | |
| Australia | Borg et al. 2010263 |
Type of intervention
We developed a typology to categorise interventions described in the literature. We used the term ‘interventions’ to include both experimental and observational studies when there is an evaluation of change, or potential change, to the pathway for patients with breathlessness in the community. The three main categories of interventions we identified were additional services, additional technology and additional access (Table 38).
| Intervention | Study (authors and year) |
|---|---|
| Additional diagnostic services in the community | |
| Mobile spirometry unit | Jones et al. 2005275 |
| Community respiratory assessment unit | Hassett et al. 2006274 |
| Starren et al. 2012182 | |
| Community breathlessness clinic | aLau et al. 2014277 |
| Community COPD clinic | aDiar Bakerly and Roberts 2009271 |
| aO’Herlihy et al. 2013283 | |
| Community spirometry clinic | aMcNeill et al. 2012281 |
| Additional diagnostic technology in the GP surgery | |
| Trained GPs using spirometry | Borg et al. 2010263 |
| Carr et al. 2011267 | |
| Chavannes et al. 2004269 | |
| Lusardi et al. 2006279 | |
| aHarris et al. 2012273 | |
| aDenis et al. 2013270 | |
| aKaplan and Lerner 2013276 | |
| aSchermer et al. 2013195 | |
| Trained practice nurses using spirometry | Akhtar and Wilson, 2005261 |
| Burgos 2011264 (access to a web application) | |
| Additional information accompanying spirometry results | Poels et al. 2006284 |
| BNP testing in primary care | Burri et al. 2012265 |
| POC testing analyser | Tomonaga et al. 2011288 |
| Diagnostic algorithm | van der Mark et al. 2014289 |
| Additional access to diagnostic services in secondary care | |
| Open-access echocardiography service | Lodewijks-van der Bolt et al. 2007278 |
| van Heur et al. 2010105 | |
| Open-access spirometry clinic | Walker et al. 2006290 |
| Wolfenden et al. 2006292 | |
| aSallaway et al. 2011286 | |
| Open-access pulmonary function clinic | aBackler et al. 2011262 |
| Self-referral chest radiography clinic | aCallister et al. 2011266 |
Outcomes measured
In the analysis of this set of literature, we focused on outcomes relating to patient pathways and models of service delivery, rather than on clinical outcomes. However, we recognise that there is inevitably some blurring of this distinction as, for example, clinical presentation will impact on the referral pathway. Table 39 summarises the main range of outcomes that were measured and reported in the identified studies. Included in these categories are specific measures relating to individual tests (such as FVC for spirometry, D-dimer or BNP levels), as will be described in the narrative presentation of the studies. It can be seen from Table 39 that there was considerable heterogeneity in the outcomes which were considered to represent ‘success’ when evaluating these initiatives.
| Outcome | Study (authors and year) |
|---|---|
| Accuracy/quality of spirometry testing | Akhtar and Wilson 2005261 |
| Borg et al. 2010263 | |
| Burgos 2011264 | |
| Carr et al. 2011267 | |
| Lusardi et al. 2006 279 | |
| White et al. 2007187 | |
| Fois et al. 2014272 | |
| Schermer et al. 2013195 | |
| Willey et al. 2013291 | |
| Diagnostic accuracy | Masa et al. 2011280 |
| Poels et al. 2006284 | |
| Tomonaga et al. 2011288 | |
| van der Mark et al. 2014289 | |
| Walker et al. 2006290 | |
| van Heur et al. 2010105 | |
| White et al. 2007187 | |
| Backler et al. 2011262 | |
| Metting et al. 2013282 | |
| Number of further tests/diagnostic certainty | Burri et al. 2012265 |
| Cawley et al. 2011268 | |
| Chavannes et al. 2004269 | |
| Number with abnormality/no abnormality detected | Hassett et al. 2006274 |
| Lodewijks-van der Bolt et al. 2007278 | |
| Starren et al. 2012182 | |
| Wolfenden et al. 2006292 | |
| Backler et al. 2011262 | |
| Callister et al. 2011266 | |
| Diar Bakerly and Roberts 2009271 | |
| aLau et al. 2014277 | |
| McNeill et al. 2012281 | |
| O’Herlihy et al. 2013283 | |
| Punwani et al. 2014285 | |
| Sallaway et al. 2011286 | |
| Time to treatment | Burri et al. 2012265 |
| Number of referrals made to specialist service | Carr et al. 2011267 |
| Chavannes et al. 2004269 | |
| Hassett et al. 2006274 | |
| Starren et al. 2012182 | |
| Thijssing et al. 2014287 | |
| Backler et al. 2011262 | |
| Callister et al. 2011266 | |
| Pattern/type of referrals | Hassett et al. 2006274 |
| Lodewijks-van der Bolt et al. 2007278 | |
| Van Heur et al. 2010105 | |
| Wolfenden et al. 2009292 | |
| Diar Bakerly and Roberts 2009271 | |
| Harris et al. 2012273 | |
| Cost of service/test | Cawley et al. 2011268 |
| Jones et al. 2005275 | |
| Patient satisfaction | Hassett et al. 2006274 |
| GP-related outcomes (satisfaction/perceived usefulness/feasibility/confidence) | Thijssing et al. 2014287 |
| White et al. 2007187 | |
| Backler et al. 2011262 | |
| Kaplan and Lerner 2013276 | |
| GP usage | Hassett et al. 2006274 |
| Jones et al. 2005275 | |
| Starren et al. 2012182 | |
| Thijssing et al. 2014287 | |
| Van Heur et al. 2010105 | |
| Diar Bakerly and Roberts 2009271 | |
| McNeill et al. 2012281 | |
| Metting et al. 2013282 | |
| Punwani et al. 2014285 (appropriateness of usage) | |
| Number of GPs trained | Denis et al. 2013270 |
Study quality
We assessed the quality of the journal papers to establish those with stronger study designs and less potential for bias. As the included papers included a range of designs, we used appropriate tools for intervention studies, cohort studies and cross-sectional studies (see Appendix 10). The quality of the literature was limited by a lack of studies using comparator designs. Of the eight intervention studies, four had issues with potential selection bias and two had issues with potential attrition bias. Many of the cross-sectional studies also had potential issues with selection of the sample.
Analysis of interventions by typology
Additional diagnostic services in the community
Seven papers182,271,274,275,277,281,283 examined new services in the community that could be relevant to patients presenting with breathlessness. These services encompassed a mobile spirometry unit,275 a community respiratory assessment unit,182,274 a community breathlessness clinic,277 a community COPD clinic271,283 and a community spirometry clinic. 281 The majority of these studies (n = 5) reviewed service data, with none using more rigorous designs, and four were in the form of conference abstracts. The quality of this set of papers was, therefore, generally low. Five of the studies were carried out in the UK182,271,274,275,277 and two were carried out in Ireland. 281,283 The three full papers will be outlined first.
The Jones et al. study,275 in Plymouth, provided in-service training for staff, and also a mobile spirometry clinic to GPs who were known to own a spirometer. The take-up of staff education was low, with half of the 14 practices that were offered the training accepting. The spirometry results were interpreted by a trained respiratory nurse leading the service and a GP with a respiratory specialist interest. There was a high rate of diagnosis of COPD among the 98 patients who were assessed over a 3-month period, with only six found to have normal lung function. The authors report that the cost of the study was £107 per new patient diagnosed with COPD and that the GPs were satisfied with the service (there are no data regarding this). The paper provides limited evidence regarding pathways or models, as it provides no detail regarding any criteria for patient referral (such as presenting symptoms). It is assumed, rather than documented, that patients might have presented with breathlessness. It describes the service as ‘a structured COPD diagnostic and management service’, and reports data for diagnosis of COPD only. It is also presumed that the patients diagnosed were seen by the mobile clinic, rather than by the GPs who had received training, as this is not documented.
The Hassett et al. 274 paper and the Starren et al. 182 paper appear to be evaluating the same community respiratory assessment unit in London (although all but one of the authors are different, and this connection is not made in the later paper). The unit was based in a hospital, although it was described as a ‘community service’, and provided outreach services to practices further from the base. It was staffed by a specialist respiratory nurse for the first 2 years, and was subsequently run by a community respiratory nursing team. The earlier paper focuses on referral rates, reporting that 364 patients were referred in first 12 months of the service. Sixteen GP practices were initially included, with 17 added 6 months later. Analysis of the referral outcomes highlighted that suspected or established diagnoses were often not confirmed. One-quarter of patients referred for suspected or confirmed COPD had no abnormalities detected on assessment, and 34% of those referred for suspected or confirmed asthma had no detectable airway narrowing. The authors report a high take-up of the service among GPs (all but two had used it), and high satisfaction rates among GPs and patients (51% of GPs rated themselves as satisfied and 46% rated themselves as very satisfied with the service; 88% agreed that patients had benefited from the service; and 99% of patients rated their experience as good).
Four years of data from (presumably) the same unit are examined in the Starren et al. 182 paper. This article specifically reports that unexplained breathlessness was one of five common reasons for referral to the service (79/1156 referrals). Forty-one of these patients were diagnosed as having a non-respiratory cause of breathlessness, 14 had a restrictive defect, six were diagnosed with COPD, asthma could not be excluded in 17 and for one no explanation for symptoms could be found. The authors echo the finding of the earlier paper in concluding that the unit reduced diagnostic inaccuracy, and suggested that it prevented referrals that might otherwise have been made to specialists. Approximately one-third of COPD diagnoses made in the community were incorrect, with potentially high levels of inappropriate prescribing. However, the study was unable to draw meaningful conclusions regarding asthma referrals.
A further UK study277 assessed the potential clinical utility of a community breathlessness clinic for detecting heart failure and other cardiorespiratory disorders. The clinic offered a ‘one-stop testing’ service by a tertiary heart failure team. Breathlessness was the presenting symptom in 82% of the 191 patients assessed over 2 years. A new cardiac diagnosis was made in 17% of patients and a new respiratory diagnosis was made in 10% of patients. The authors highlighted that high levels (38%) of patients were found to have no cardiac or respiratory disease on assessment by the clinic, and thus the service prevented the need for secondary level investigations or review.
Two conference abstracts (one reporting a study in the UK and one reporting a study in Ireland) evaluated community COPD clinics. The first of these271 is of limited value to this review, as it reports data from both new patients to the clinic and those transferred from secondary care, and also includes patients with an existing diagnosis of COPD. The authors concluded that community COPD clinics provide easy access to specialist advice. They also found that inappropriate referrals to these clinics did not appear to be a problem (although this may be related to the referral sources and type of patients included). The second abstract (from a team in Ireland)283 provides limited data, beyond reporting that 50% of patients seen by a COPD satellite clinic were diagnosed with COPD, 25% were diagnosed with asthma and 25% had no significant respiratory problem. The authors concluded that community clinics could provide a time-saving and cost-saving alternative to the traditional hospital-based outpatient clinic model.
The final study in this group (also from the Republic of Ireland281) evaluated a community spirometry clinic. Of the 104 patients seen, 82 were tested and 35 were found to have normal spirometry results. The success of the service was reportedly compromised by the underusage of available appointments.
Additional diagnostic technology/information in the general practitioner surgery
The largest subgroup of studies we identified (14 papers) evaluated the provision of technology in GP surgeries (see Table 38). All but three265,284,288 of these examined the use of spirometry by primary care staff. The papers considered the potential value of spirometry during a consultation, the accuracy of spirometry carried out in primary care, and whether or not training courses could enhance the quality of spirometry. Five of the studies261,264,265,279,284 used controlled designs.
An evaluation of the value of spirometry during GP consultations in Italy279 found that there was no significant difference in levels of diagnostic accuracy between GPs who had access to spirometry and those who did not. The study examined GP diagnosis of patients who presented with cough (the majority of patients), dyspnoea, wheezing or chest tightness. There was agreement on diagnosis between GPs and specialists in 78.6% of cases in a group who had been randomised to receive 8 hours of training and had access to spirometry. This compared with 69.2% in the group who had received no training and carried out consultations without spirometry (p = 0.35).
Harris et al. 273 evaluated the validity of spirometric testing performed in the community, by retrospectively examining records of patients who had been found to have abnormal screening. Although the data are very limited and mainly relate to patients with established COPD diagnosis, it indicates that the majority of patients referred following community testing using spirometry were found to have COPD. The paper therefore provides contrary findings to other studies that indicate poor accuracy of community diagnosis.
The potential value of spirometry for aiding GP decision-making was examined in a study from the Netherlands. 269 GPwSIs in spirometry were given 6 hours of training. Case scenarios were used to assess GP decision-making at intervals over the 12-month period following training. The number of diagnoses considered by the GPs reduced over the follow-up period, which the authors described as being due to improved diagnostic skills and less uncertainty. However, the study found that, based on responses to case scenarios, there might be an increase in additional testing and also in referral rates to specialists.
A randomised controlled study examined whether or not additional information accompanying spirometry results was of benefit to GP decision-making. 284 The study found that additional information provided by a computerised system had no detectable benefit on the accuracy of diagnosis by GPs. There was no difference in accuracy of diagnosis between the groups who had received additional textual notes regarding the test results, with those who had received only the results (COPD: odds ratio 1.08, 95% CI 0.7 to 1.66; asthma: odds ratio 1.13, 95% CI 0.7 to 1.8; absence of respiratory disease: odds ratio 1.32, 95% CI 0.61 to 2.86).
The only study from the UK261 which used a controlled design (non-randomised) evaluated the accuracy of spirometry carried out by practice nurses in Leicester. The work compared the accuracy of practice nurse spirometry with spirometry in a centralised laboratory. The study found that the absolute values of FEV, FVC, etc., were lower and hence the COPD would be classified as more severe if measured by nurses than if measured in a specialised laboratory (significant difference in measurement of FEV and FVC; p < 0.05), with the potential for overdiagnosis of COPD severity (and, therefore, the potential for over-referral).
A UK-based before-and-after study267 aimed to assess the impact of an educational intervention on the quality of spirometry. The study found a positive impact on assessment quality, and a potential reduction in referrals to specialist services following GP training. Before the intervention, 38% of post-bronchodilator spirometry tests were technically flawed. After the intervention, 2% were flawed. Over the study time period, the proportion of chest clinic referrals following assessment fell from 62% of patients to 32% of patients.
An interactive five-module spirometry training course for GPs and other clinic staff in the Netherlands is described in conference abstracts by Denis et al. 270 and Schermer et al. 195 In the first abstract, the authors recommend that formal training is required for spirometry to be used effectively in primary care by GPs and practice nurses, and that refresher courses are important to maintain skills. The second abstract describes programmes for practice nurses and practice nurses and assistants. The study seems to indicate that structured spirometry training has a limited effect on test quality (for one course, 39.1% of tests were adequate before training and 51.0% were adequate after training: odds ratio 1.60, 95% CI 1.12 to 2.30; for the other course, 45.3% of tests were adequate before training and 44.1% were adequate after training: odds ratio 0.93, 95% CI 0.65 to 1.33).
A paper which supports the limited effect of training on the quality of spirometry comes from Australia. 263 Nurses and physiotherapists in rural health facilities received 14 hours of training. Retrospective review of test quality found that only 37%, 60% and 58% of tests at 5, 7 and 9 months, respectively, had met established criteria. The authors concluded that the course did not provide sufficient skill to perform spirometry to the required quality.
Kaplan and Lerner276 recommend a training programme and tool for interpreting spirometry as increasing GP intention to use spirometry and ability to interpret results. However, there are no data in this abstract to support this recommendation.
A paper from a team from Spain264 indicated the need for not only training but ongoing support in interpretation. This study evaluated the use of spirometry by trained practice nurses who were randomised to have access or no access to a web application. Over a 12-month period, the intervention group presented 71.5% high-quality spirometries and the control group presented 59.5% high-quality tests (p < 0.001). The additional web-based support increased the proportion of high-quality spirometry tests carried out (by around 20%), and decreased the percentage of very low-quality spirometries with maintenance of quality over a 1-year period. The application was rated as being acceptable by GPs and useful by nurses.
Three papers evaluated other technologies that may be available in primary care. 265,288,289 One study evaluated the provision of BNP testing in primary care265 and one examined results following the use of a POC testing analyser in GP practices. 288 The final study in this group reported the development and testing of an algorithm which GPs could use to support a diagnosis of asthma. 289
Burri et al. 265 carried out a RCT in Switzerland to evaluate whether or not adding BNP testing in primary care would improve the evaluation and diagnosis of patients with breathlessness. The study found that use of BNP in primary care improved diagnostic certainty (further diagnostic testing 33% of patients in the BNP group vs. 45% of patients in the control group; p = 0.02), and time to initiation of treatment (66% of patients began appropriate treatment on the day of presentation in the intervention group vs. 53% in the control group; p = 0.02). There appeared to be little impact on service delivery outcomes with the number of hospitalisations for dyspnoea, number of days in hospital, and number of outpatient visits not significantly different between the groups at 3 or 12 months.
Another Swiss study288 assessed the value of having a three-in-one POC testing analyser (cardiac troponin T, N-terminal pro-brain natriuretic peptide, D-dimer) in primary care. Patients presenting with chest pain or symptoms of dyspnoea were assessed by GPs using conventional diagnosis methods or GPs with the addition of the analyser. In the intervention group the working diagnosis made at first visit was confirmed at second visit in 76% of cases. For the control group the diagnosis was confirmed in 60% of cases (p = 0.002). Acute coronary syndromes were correctly ruled out in 92% of patients in the intervention group versus 78% in the control group. The authors concluded that use of the tests improved GP diagnostic accuracy.
An algorithm that could assess the likelihood of developing asthma was described in a cohort study from the Netherlands. 289 The Clinical Asthma Prediction Score is calculated on the basis of age, family history, reported symptoms, environmental factors and allergies. The evaluation of the tool found that a score of < 3 had a negative predictive value for asthma of 78.4%. A score of > 7 had a positive predictive value of 74.3%. The authors concluded that the scoring system for pre-school children with symptoms could be a useful decision aid in asthma diagnosis.
Additional access to diagnostic services in secondary care
This group of seven studies can be considered to be on the borderline of meeting our inclusion criteria, as all but one evaluate diagnostic services which were provided in secondary care. However, we have included them in the review as they describe a change to a typical pathway, with GPs referring patients directly for diagnostic testing rather than referring to a specialist. One study in particular reported a significant change to traditional models, by exploring the introduction of patient self-referral for chest radiography. 266
The introduction of an open-access echocardiography service in the Netherlands was evaluated in linked papers by Lodewijks-van der Bolt et al. 278 and Van Heur et al. 105 Patients in the study were referred to the open-access service by GPs following examination, and the echocardiogram was carried out without referral to a cardiologist. The study reports that 107 out of 471 patients were referred for dyspnoea. The authors concluded that the service identified around one-quarter of patients with no cardiac abnormality who might otherwise have been referred to a cardiologist (and, therefore, the service prevented referrals). However, there are no data in the paper making this comparison. The second paper adds more recent data and compares the first 250 patients with the last. The study found that fewer referrals for dyspnoea were made in the more recent year (2007) than in 2002. This article reports that the proportion of patients with no cardiac abnormality found on testing increased over the lifetime of the study (32.8% in 2007 vs. 22% in 2002). It is unclear how these data link to referral patterns, as this could indicate a greater prevention of referrals to secondary care, as more patients with no abnormality were seen in the clinic. However, it also could indicate that GPs were referring more patients with less severe symptoms to the service.
Three papers investigated outcomes following introduction of an open-access spirometry clinic. Walker et al. 290 evaluated a spirometry clinic in London in a ‘health suite in the local community’. A respiratory technical officer performed spirometry on the patients (who were smokers or ex-smokers over 40 years with respiratory symptoms) and a consultant respiratory physician reviewed the data to produce a report that was sent back to the referring GP. Of those referred, 39% had no existing diagnosis, 30% had a diagnosis of asthma and 29% had a diagnosis of COPD. Following testing, 128 patients were newly diagnosed with asthma or COPD and 46 patients had their diagnosis changed. The authors concluded that the spirometry clinic increased rates/accuracy of diagnosis and improved treatment.
Another paper reporting a study carried out in London292 evaluates a different service from the one in the Walker et al. 290 study. This open-access spirometry clinic operated one morning each week in a hospital. GPs could refer with a suspected or actual diagnosis and spirometry was carried out by a consultant respiratory physician. The study found that just over half of referrals with suspected or actual COPD were found to have airway obstruction. Airway obstruction was rarely observed in patients referred for stated or suspected asthma. The authors stated that spirometry is needed to establish correct diagnosis of COPD, and so referrals for suspected or stated COPD were appropriate. However, home peak flow monitoring rather than referral may be more helpful for those with suspected asthma.
A conference abstract reporting an open-access spirometry clinic in Canada286 found, in contrast to the above study, that a significant proportion of referrals to the service yielded abnormal results. There are limited data regarding the setting or operation of the service.
An open-access pulmonary function clinic in the UK262 was reported to have resulted in 72% of patients not being referred to an outpatient chest clinic, with an estimated saving of £1800 to the health service. The sample that this is based on is small (18 referrals) and, as the document is a conference abstract, there are limited data.
Callister et al. 266 evaluated a UK self-referral radiography clinic, which was provided as part of an awareness campaign to improve the early detection and diagnosis of lung cancer. During the first 7 weeks, 95 patients presented for self-referral chest radiography, of whom three had confirmed lung cancer. There was a 30% increase in the number of GP-ordered chest radiographs, compared with the same period in 2010 (mean 557 (SD 65) per week vs. mean 428 (SD 55), respectively; p < 0.001), and a 70% increase in fast-track lung cancer clinic referrals, compared with 2010 as a whole [mean 16.0 (SD 6.0) per week vs. mean 9.4 (SD 3.1), respectively; p < 0.05].
Summary of evidence from intervention studies
Seven papers provide evidence that additional services in the community, including mobile clinics and community clinics, can be a useful means of differentiating between patients who have no significant respiratory problems or cardiac disease and may be managed in community services, and those who may need referral to a specialist (see Table 38). Around one-third to one-quarter of patients may have no abnormalities detected on assessment. It should be noted that this evidence comes from studies that have less rigorous study designs. However, the findings have high applicability, as they were carried out in either the UK or Ireland.
Fourteen papers provide evidence regarding the use of additional technology in community settings (see Table 38). The papers exploring the use of spirometry, by either GPs or other practice staff, provide evidence of limited impact on diagnostic decision-making, and conflicting evidence of impact on referrals. One study suggested a reduction in referrals to a chest clinic and another suggested the potential for increased referrals to specialists. A number of studies raised concerns regarding the quality and accuracy of spirometry testing in the community, with variable outcomes following targeted training. One paper indicated that training, together with web-based support, may improve the quality of spirometry. The quality of evidence in this group of studies is more robust, with five studies using controlled designs (four RCTs).
The three papers265,288,289 examining other (non-spirometry) technologies suggested that POC testing can have a positive impact on GP differential diagnosis, diagnostic certainty and time to treatment, although the impact on specialist referral is less clear. A simple algorithm for asthma also may be a useful decision-aid.
Only three261,267,273 of the papers in this group examining the use of technologies in primary care were from the UK, although there appear to be no features of the studies, which would suggest that they would not be applicable to the UK health-care context.
Seven studies provide evidence regarding the provision of open-access diagnostic services in secondary care (see Table 38). As with the provision of additional services in the community group of papers, there are data supporting a beneficial impact in terms of a reduction in referrals to specialist services. This is associated with high numbers of patients found to have no abnormality in these clinics. There is also an indication in this set of papers that these services could reduce misdiagnosis.
The papers as a group report an uncertain effect on referrals (sometimes rates increased and sometimes they reduced). Referral rates as a measure of intervention effectiveness can be perceived as a crude measure. Patients who would not have been referred may be now appropriately referred as a positive outcome, although, equally, referral may be seen as a negative outcome, as patients might have been referred who would have been better managed in the community.
Results of views review
From the database searches (see Figure 2), a total of nine qualitative papers were identified that met the criteria for inclusion. Through scrutiny of the reference lists related to these papers, a further four papers were identified, giving a total of 13 included qualitative papers. However, one of these papers could not be obtained, giving 12 papers reporting 10 studies (see Table 40). Six papers relevant to the review question were identified that reported views of staff or patients and used cross-sectional survey methods to collect data (level 3). One of these papers could not be obtained, giving five papers reporting five studies (see Table 41). Seventeen papers reporting 15 studies were therefore included in the review of views and perceptions of staff or patients.
Characteristics of included studies
We developed a three-level grading system for the included papers based on relevance to the research question and richness of data. Thus, level 1 papers were those that explored views and experiences, using interview, focus group or observation methods, of diagnosis pathways in general practice where breathlessness was undifferentiated. Level 2 papers use these same qualitative methods, with a focus on a particular condition where breathlessness presents as a typical symptom, for example COPD, asthma, lung cancer or heart failure. Level 3 papers utilise cross-sectional methods to identify aspects of diagnosing breathlessness-related conditions, such as the degree of spirometry use in a population, or the perceived extent of confidence that staff have in using a spirometer or interpreting results.
Consistent with other subsections of this review study, level 1 and level 2 papers have been subjected to quality assessment using a tool aligned with the study design. Level 3 papers have not been formally assessed for quality but are critiqued, where applicable, in the synthesis of findings.
Qualitative studies
Of the 12 qualitative papers, one was considered a level 1 paper, having a focus on undifferentiated dyspnoea. The remaining 11 papers (level 2) focused on a range of conditions for which dyspnoea was the main symptom (e.g. COPD and asthma) or one of a number of potential symptoms (such as lung cancer, heart failure and pulmonary arterial hypertension). Table 40 provides a summary of the included qualitative papers.
| Study (authors and year) | Country | Population and sample size | Condition | Focus of paper | Data collection methods | Data analysis methods |
|---|---|---|---|---|---|---|
| Level 1 papers | ||||||
| Roberts et al. 2011197 | UK | HCPs, medical undergraduates, junior doctors, GPs, specialist registrars | Dyspnoea | Reasons for the underuse of spirometry in diagnosis of breathlessness | Focus groups | Thematic coding |
| Level 2 papers | ||||||
| Armstrong et al. 2012293 | UK | Patients, 30 | Pulmonary arterial hypertension | Patients’ experience of the trajectory to receiving diagnosis | Interviews | Thematic analysis |
| Birt et al. 2014294 | UK | Patients, 35 | Lung cancer (and other conditions) | Factors impacting on pathways prior to referral | Interviews | Framework analysis |
| Corner et al. 2005295 | UK | Patients, 22 | Lung cancer | Delayed diagnosis | Interviews | Thematic analysis |
| Corner et al. 2006296 | UK | Patients, 22 | Lung cancer | Reasons for delay in seeking care | Interviews | Constant comparison |
| Dennis et al. 2010297 | Australia | HCPs and GPs, 18 | Asthma | Difficulties faced by GPs when trying to make a diagnosis | Focus groups | Thematic coding |
| Goeman et al. 2005298 | Australia | HCPs and GPs, 49 | Asthma | GPs’ priorities for achieving optimal outcomes; barriers | Nominal group technique | Thematic coding |
| Joo et al. 2013198 | USA | HCPs and PCPs, 12 | COPD | Attitudes and barriers to performing spirometry | Focus groups | Thematic coding |
| Khunti et al. 200282 | UK | HCPs, unsure: 18 practices | Heart failure | Perceived obstacles to diagnosis and management | Interviews | Open coding; constant comparison |
| Tod et al. 2008299 | UK | Patients, 20 | Lung cancer | Factors in delayed diagnosis | Interviews | Framework analysis |
| Walters et al. 2005300 | Australia | Patients, 38; HCPs and GPs, 16 | COPD | Barriers to use of spirometry | Interviews and focus groups | Thematic coding |
| Walters et al. 2008301 (same study as above) | Australia | Patients, 14; HCPs and GPs, 16 | COPD | Attitudes influencing diagnosis | Interviews and focus groups | Thematic coding |
One level 1 study was published in the UK197 and sampled HCPs, although it was not focused specifically on primary care. Nine level 2 studies reported in 11 papers were published in the UK (n = 682,293–296,299), Australia (n = 4297,300–302) and the USA (n = 1198). Five papers sampled patient-only populations, four sampled HCPs only and one study reported in two papers sampled a mixed population of HCPs and patients. COPD was the condition of interest in two studies reported in three papers,198,300,301 lung cancer in three studies reported in four papers,295,296,299 asthma in two studies,297,302 heart failure in one study82 and pulmonary arterial hypertension in one study293 (see Table 42).
Three included studies used focus group methods,197,198,297 six used interviews82,293–296,299 and two combined these two methods300,301 to collect data. A further study302 utilised the nominal group technique to obtain GPs’ views about optimising patient outcomes. The analysis of qualitative data was mainly thematic, with two studies using constant comparison methods and two using the framework method. In general, studies that sampled patients were concerned with the illness trajectory, including diagnosis, particularly delay of diagnosis. Those sampling HCPs were interested in barriers to making a diagnosis, including attitudes towards the use of spirometry.
Cross-sectional studies
In five level 3 studies,191,303–306 telephone or mailed questionnaires were utilised to explore diagnosis of COPD/asthma and/or the use of spirometry in primary care. Two of these papers304,306 had higher applicability, being published in the UK. The remaining three papers were published in Belgium,303 Italy305 and the USA. 191 All were condition specific, focusing on COPD or a combination of COPD and asthma (Table 41).
| Study (authors and year) | Country | Population and sample size | Condition | Focus of paper | Data collection methods | Response rate |
|---|---|---|---|---|---|---|
| Level 3 papers | ||||||
| Boffin et al. 2006303 | Belgium | GPs, n = 197 | COPD/asthma | Use of spirometers by GPs | Telephone questionnaire | 81% |
| Bolton et al. 2005304 | UK | Practitioners at 227 primary care practices Patients, n = 125 |
COPD | Spirometry in primary care (availability, training, interpretation of results) | Mailed questionnaire | 61.6% |
| Caramori et al. 2005305 | Italy | All general practices | COPD | Extent of spirometry use in primary care | Mailed questionnaire | Almost 100% (2474/2475) |
| Halpin et al. 2007306 | UK | Total from 2001 and 2005: GPs, n = 85; practice nurses, n = 121 | COPD | Confidence of practitioners in diagnosing COPD | Telephone questionnaire | Not reported |
| Kaminsky et al. 2005191 | USA | General practices, n = 29 | COPD | Use of spirometers to diagnose COPD | Mailed questionnaire | 51% |
Quality assessment
Included level 1 and level 2 studies were assessed for quality using an adapted version of the CASP qualitative research checklist, as outlined in the methods section. Ten studies met most of the assessment criteria to a greater or lesser extent (see Appendix 10). The area that was least reported across all studies was the relationship between researchers and participants. Reporting of ethical considerations was mixed, with half of the papers not discussing consent or confidentiality issues. One study was reported in two papers,300,301 the latter being a brief summary of the former and presenting less detail generally about the study. Most of the papers presented findings that included rich data, for example quotations, to verify the discussed themes.
However, our research question focused on diagnosis of undifferentiated dyspnoea in primary care rather than condition-specific diagnosis and management. All but one of the papers197 focused on condition-specific dyspnoea, and one paper sampled hospital staff as well as GPs. 197 This meant that we could not extract all of the data from each paper; rather, we extracted only relevant sections of text. This has resulted in a data set that varies in quantity and relevance.
Results of the review
Theoretical framework for presenting the results: model of pathways to treatment
The Andersen Model of Total Patient Delay is a five-stage model describing decisional processes that might lead to delays in eventual treatment. 307 Critiques of this model, not least owing to the potentially value laden aspect of ‘delay’ and the inability of the model to convey the complexity of the diagnostic pathway, have led to the development of a revised framework, the Model of Pathways to Treatment. 308 This model does not specify a particular sequence of events in the lead-up to diagnosis and treatment; rather, it allows for a range of ways of entering and timings along the pathway, as well as capturing dynamic movements between stages. 309
The initial stages of the Model of Pathways to Treatment are concerned mainly, but not always, with the patient, and include ‘detection of bodily changes’, which, for the purposes of this review, would include some degree of breathlessness. A patient may decide, for whatever reason, not to consult a HCP at this stage. The model then moves to a position where the patient perceives there is a reason to seek attention from a HCP; in our review this would be in the community, although in reality a patient may well have sought advice elsewhere first.
The first consultation with a HCP is where the bodily change is discussed and advice is sought. At this stage the views and experiences of patients and HCP views are both relevant and there is interaction between the two population groups. Typically, investigations are carried out or requested to rule in or out particular conditions based on HCP knowledge and skills. In our review, we have obtained evidence from studies that explore the diagnostic pathway experiences as well as attitudes to diagnostic procedures in both patients and HCPs in primary care. In particular, we are interested in diagnostic procedures that are new to primary care or the community that would historically have required a referral to a hospital. Spirometry is a procedure that can be carried out in primary care without referral, and some of the included papers address the extent to which this is occurring and barriers to its use.
Differential diagnosis is important so that management can be tailored to specific conditions such as COPD, asthma, lung cancer, heart failure and pulmonary arterial hypertension. We have utilised the Model of Pathway to Treatment308 to organise the data extracted from included views studies, as it allows the diagnostic pathway to be analysed at various stages and from the viewpoint of patients and HCPs. It also affords us an opportunity to critically assess the model and its usefulness in developing pathways of care for a particular presentation.
Views/perceptions of community diagnostic pathways for breathlessness
In this section we present a synthesis of the findings from included qualitative and cross-sectional studies focusing on the views of patients and HCPs about the diagnostic pathway in regard to breathlessness (dyspnoea). As described above, we use the Model of Pathway to Treatment308 as a framework from which to analyse the data and draw out factors that influence the diagnostic process at each stage. As already discussed, only one paper197 fulfilled the research question in terms of its focus on undifferentiated dyspnoea; therefore, the remaining data will be addressed in the context of different conditions.
Patient appraisal and self-management
Four qualitative studies reported in five included papers293–296,299 explored the views and experiences of patients with symptoms of breathlessness alone or with other symptoms. The first stage of the Model of Pathway to Treatment, that is, patient appraisal and self-management, was addressed in these studies and described in a similar way. Typically, the studies report that, far from seeking medical help at the first onset of breathlessness, the symptom was ‘contained’ and self-managed for a time, partly owing to the insidious nature of dyspnoea which makes it difficult for patients to discern as a change in health status. 294–296 For those with comorbidities it could be difficult to identify changes in health deemed important enough to trigger help-seeking. 294 Symptoms were played down or ‘normalised’ or attributed to something other than illness, such as smoking behaviour or ageing. 294 Patients also reportedly used strategies that delayed help-seeking, such as avoidance, perseverance or ‘covering up’ the symptom. Such strategies provided a degree of protection for the patient and family members. 293 Some patients remained stoic or did not want to bother HCPs, remembering a time when seeking medical advice was costly. Anxiety regarding a feared diagnosis was another factor in delayed help-seeking. 299 Self-management of symptoms accompanied by the strategies described above were generally the initial coping behaviours and this stage could last for weeks or several months.
Decision to consult health-care professionals and arrange appointments
According to findings from three included qualitative studies with service user participants,293,294,299 patients experiencing symptoms of breathlessness eventually made a decision to seek medical advice from their GP according to ‘triggers’. Triggers to help-seeking included the symptom becoming severe and/or a decline in the patient’s health status. 293,294 Family and friends were often instrumental in persuading patients to seek help and would even make appointments on their behalf. 293,294,299 For some patients there was recognition that the symptom was not related to existing conditions or that the symptom was not responding as expected. A few participants were prompted to seek advice following a media campaign, from which they recognised the symptoms as potentially linked to lung cancer. 294 A further study that sought the views of GPs identified the need for patient education at this stage to raise awareness of the need to seek help. 298
First consultation with health-care professionals
Views of both patients and HCPs were represented at this stage of the model, as it describes interactions and consultations between the patient and the GP about this particular symptom. However, the initial consultation did not necessarily lead to a diagnosis, with patients reporting iterations of ineffective treatments resulting in another period of appraisal and self-management before reconsulting the GP. 294 For some patients, a number of doctors were consulted before an accurate diagnosis was offered. Diagnostic uncertainty was more likely to occur in primary care where that actual diagnosis was relatively rare, such as for pulmonary hypertension. 293
It was also reported that some patients had attended their GP practice while experiencing symptoms but had not mentioned them to the GP, or the patient had mentioned them but perceived that the GP had been dismissive. 294 For others, a consultation triggered by a different condition led to the identification of a respiratory problem. 294 Tod et al. 299 highlighted the role of families as advocates for patients when they perceived that symptoms were not being investigated.
Diagnosis
Owing to the increased relevance to the review question, this section includes a larger, although still limited, body of evidence from eight qualitative82,197,198,293,295–298,300,301 and five cross-sectional191,303–306 studies.
The patient pathway from a reported symptom of breathlessness at this point was contingent on the condition being ruled in or out by the GP and on primary care resources, access to diagnostic equipment, medical knowledge and skill in performing and interpreting diagnostic information. Therefore, diagnostic activity is reported to take place variously in the GP practice and as a shared activity between primary and secondary care settings, with referrals to specialists a common feature of the pathway. One study noted that conditions could be labelled differently over time; for example, an initial diagnosis of asthma could change to one of emphysema. HCPs also reported that medical language has altered over time, with ‘COPD’ being a relatively recent diagnosis. 300,301
Referral to a specialist
Referrals to specialists by GPs depended on the medical condition and the investigations required for a diagnostic work-up. For example, echocardiography was reported to assist in identifying cases of heart failure. However, Khunti et al. 82 reported a lack of systematic diagnostic work-up among GPs for this condition, perhaps due to a lack of awareness regarding the usefulness of echocardiogram. Many GPs preferred ECG and referral for an X-ray because it was ‘easier to organise’ and ‘less expensive’ than accessing echocardiography, particularly for non-fundholding practices. There was also a reported lack of trust at the interface between primary and secondary care. Some single-handed GPs referred their patients to a rapid-access cardiology unit at the hospital, whereas others requested access through cardiologists. Lack of time to assess patients for heart failure was cited as another barrier in primary care diagnostics, particularly as the population with suspected heart failure are elderly and can require more time to undress for physical examinations.
For patients with breathlessness and accompanying symptoms suggestive of lung cancer, the process of specialist referral was rapid once the symptom had been recognised by the GP, possibly because of greater awareness of recent UK guidance for cancer symptoms. 295,296
Office spirometry
Spirometry was one investigation that could be conducted in the GP office and was particularly associated with the differentiation between a diagnosis of COPD and one of asthma. A number of included studies explored the perceived usefulness of office spirometry from the point of view of the clinicians involved. No included study was identified that looked at patient views and experiences of spirometry in this setting.
In the four qualitative studies exploring GP views about performing spirometry, there were contradictory findings. Joo et al. 198 reported that GPs felt confident in diagnosing COPD without the use of spirometry, particularly if risk factors such as smoking were apparent and trialled treatments for COPD appeared to be working. The results from referred spirometry were reported as confusing for GPs, furthering their confidence in using non-spirometric diagnostic strategies. Practical considerations were also reported, such as lack of time to perform spirometry297 and the patient’s unwillingness to return for follow-up investigations. Goeman et al. 298 found that Australian GPs felt uncomfortable about their ability to use spirometers properly and that few practices had access to a spirometer. These findings highlighted a gap between national guidelines and current practice. However, Roberts et al. 197 found that GPs regarded spirometry as an essential diagnostic tool, albeit with acknowledgement of the need for training and retraining. This study sampled GPs in the UK who may be incentivised by criteria in the new GMC contract. They also had a special interest in medical education, and, therefore, their perceptions of spirometry use might differ from those of the broader UK GP population.
Five included cross-sectional studies assessed the use and perceived usefulness of spirometers in the diagnosis of COPD and/or asthma. Boffin et al. 303 found that although 38% of Flemish GPs had access to a spirometer, 66% had never or almost never used one. One in five GPs was using a spirometer at the time of the study and a similar number had used spirometers but stopped doing so. Seventy per cent were using a peak flow meter, suggesting that GPs recognised the importance of assessing lung function. Reasons for not using spirometers or stopping spirometer use included the time it takes during the consultation, and a lack of knowledge and skill. Eighty-six per cent of GPs responded that training in spirometry ought to be provided. Of users, 71% had attended educational sessions, of which 64% were organised by vendors of spirometers. A study carried out in Italy305 concluded that GPs probably only diagnose the most severe cases of COPD and that 70% of these will have used spirometry. They suggest that this could be improved by offering better educational programmes. Following a workshop that included a presentation and hands-on experience of how to use a spirometer in a US-based study,191 the main reasons given for not carrying out spirometry were reimbursement, time, lack of familiarity with testing and uncertainty with interpretation of results. The authors point towards a lack of awareness or acceptance of the value of lung function testing among primary care practitioners.
By contrast, two studies304,306 found that access to spirometers and perceived confidence in diagnosis using a spirometer was higher in the UK. Over 80% of practices in one study304 reported possessing a spirometer and 160 out of 187 of these used it in every case of suspected COPD. However, 44% rarely used a spirometer. Over half of those using spirometry were confident in its use, and these practices used spirometry more than those who reported less confidence. However, only one-third of using practices were confident about interpreting the results. Most spirometry was carried out by a nurse, with only one-third carried out by a GP. Halpin et al. 306 reported that practice nurses were less confident (55%) in diagnosing COPD than GPs (80%), although GP confidence had increased from 2001 to 2005, whereas that of practice nurses remained the same at the end of this period. Bolton et al. 304 concluded that reported misdiagnosis of COPD could benefit from more widespread use of spirometry. More GPs than practice nurses reported feeling confident in differentiating COPD from asthma.
For patients, diagnosis was received with mixed emotions, and the way that the diagnosis was conveyed by HCPs was important; for example, there were reports of a lack of empathy. GPs might have come across some conditions, such as pulmonary hypertension, only rarely, and this might also be the first time that a patient had heard of the condition. Patients often felt ill-informed, returning home to look for information on the internet, with upsetting consequences. 293 Similarly, Khunti et al. 82 found differing views between GPs within practices about diagnosing heart failure, as well as diagnostic confusion about whether a condition was respiratory or cardiac related. In another study, continuing education for GPs to promote better care was a priority for Australian GPs. 298
Summary of the views review findings
Differing perspectives, different conditions
This limited body of views and perspectives from a combination of level 1, level 2 and level 3 evidence provides some insight into the way in which patients and HCPs enter and interact in a theoretical pathway from the onset of breathlessness symptoms to diagnosis. Each enters the pathway at a different stage and, rather than a linear progression, there are often iterations of consultation leading to diagnosis. For patients, the psychological barriers to disclosing a symptom such as dyspnoea can lead to delay in diagnosis, as GPs may not have the full patient history at their disposal. As patient history-taking is an initial indication on the care pathway, GPs need to be aware of the potential sensitivities that patients may have regarding disclosure.
Findings from included studies highlight the barriers to developing a single pathway for diagnosis of breathlessness (dyspnoea) symptoms in primary care. The evidence base lacks strength in terms of how practitioners might move forward in this endeavour. It also shows the heterogeneity of conditions that could be diagnosed from a symptom of breathlessness, of both respiratory and cardiac origin, some of which are not covered here owing to a lack of identified relevant publications. Such conditions are also associated with other symptoms, for example cough in lung cancer and wheezing in asthma as well as a range of symptoms and signs, such as oedema, related to heart failure.
Barriers to breathlessness diagnostic pathway in primary care
The evidence synthesis indicates a number of barriers to diagnostics for breathlessness in primary care. The first consideration is that patients might not want to reveal their breathlessness symptoms owing to a perceived lack of importance attributed to the symptoms, fear of receiving a challenging diagnosis or a wish not to be a burden on family, friends or the health service. Although diagnostic pathways are reliant to some extent on patient history, the wishes of patients are of great importance in diagnostic decision-making. Second, patients may not feel satisfied with the consultation and, as a result, may not return for follow-up sessions at the practice. Third, GPs may not identify the symptom as indicative of a relatively rare condition, resulting in delayed or misguided referrals for investigations.
Evidence for attitudes towards diagnostics in primary care suggested that ease of access to certain facilities, such as echocardiography, would increase referrals for patients with suspected heart failure. 82 Evidence for views about and the extent of use of spirometry in primary care was mixed, with some GPs preferring to prescribe treatments and see how they impacted on the symptom rather than measure lung function. 198,297 Lack of time during the consultation, lack of access to spirometers and lack of confidence in using spirometers or interpreting the results were reported barriers to spirometry use in primary care.
Integrating the intervention and views data
Figure 3 provides a conceptual model summarising elements of the pathway for patients with breathlessness that are described in the literature. We developed this model from analysis of the interventions and outcomes described in the intervention literature, combined with analysis of the themes emerging from the views studies.
FIGURE 3.
Conceptual pathway model.
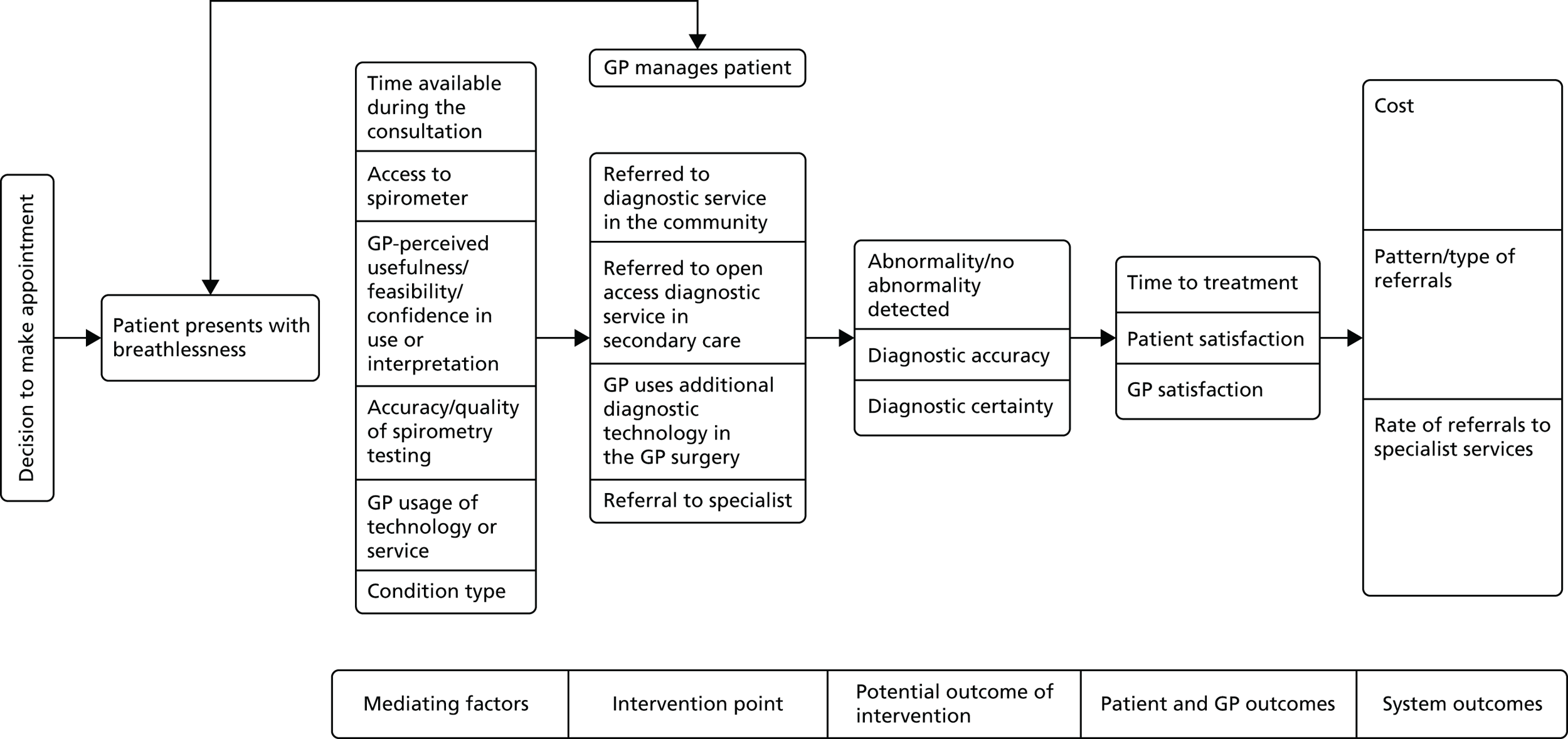
The initial element of the model recognises the influence of patient decision-making in regard to whether or not to seek a consultation with a GP. The views literature highlighted a lack of importance attributed to the symptoms, fear of receiving a challenging diagnosis or a wish not to be a burden on family, friends or the health service, all of which could delay presentation. Following patient presentation, the next phase of the model outlines elements described in the literature that may act as mediating factors (influences) on the selection of various patient management options. These include factors reported in the views literature (such as time available during the consultation and condition type) and elements reported in the intervention studies (such as accuracy of spirometry). The following section of the model details potential GP management options, including direct referral to a specialist service, and the initiatives outlined in the intervention papers. These are potential points for interventions with GPs to be put in place. We have indicated the repeat cycle of patient presentation and GP management described in the qualitative studies by a two-way arrow. The final sections of the model detail the potential outcomes at different levels that were described in the included studies: immediately following an intervention, for patients and GPs, and impacts on the wider system/services.
Discussion
Thirty-six studies were included in the review of interventions in the pathway for patients presenting with breathlessness. Our search of the literature found few studies using higher-quality comparator designs, although we identified evidence relating to a range of different interventions, and a body of work that was carried out in the UK.
Although the limited robustness of the evidence should be fully recognised, the included research suggests that additional services in the community (such as mobile or community clinics) can be a useful means of differentiating between patients who may be managed in community services and those who need referral to a specialist. Similarly, the provision of open-access diagnostic services in secondary care may reduce the referral of patients with no abnormality to specialists and/or may reduce the numbers of patients misdiagnosed. The findings regarding spirometry use in GP surgeries suggest a limited impact on diagnostic decision-making, and conflicting evidence regarding the impact on referrals. This may be linked to the reported limited quality and accuracy of much spirometry carried out in the community. A small number of studies evaluating other technologies, such as POC testing or algorithms, suggest that these may impact on GP differential diagnosis, diagnostic certainty and time to treatment. However, the impact of these technologies on referral rates to specialists is unclear.
The studies as a group provide limited consistency in evidence, and also highlight the challenge of measuring effectiveness. Although a reduction in specialist referrals for some conditions that can be managed in primary care may be advantageous, a reduction in early referrals to specialists for other conditions may have a negative impact on optimal and cost-effective management and outcomes.
Seventeen papers were included in the review of views and perceptions of staff or patients. Findings from these papers were structured within the Models of Pathway to Treatment framework. 307–309 Eight included views studies were based in the UK, increasing the applicability of findings in these studies to NHS settings. For example, one study that explored diagnosis of heart disease found that there was a perceived lack of access to echocardiography in primary care which influenced the GP decision-making process. Patients were referred for chest radiography instead, as it was easier and less costly.
In this literature, there was acknowledgement of the benefits of spirometry among a group of UK-based GPwSIs in education. Cross-sectional studies also revealed higher levels of access to spirometers and greater confidence in their use in the UK, suggesting differences across international health-care settings. Authors suggested that incentives through quality framework measures as well as regular training might act as motivators to spirometry use. However, spirometry use remains low in the UK, and, although the evidence suggests that UK-based practice nurses are more likely than GPs to perform spirometry, they are less confident in doing so.
Comparison of the findings from the intervention and views studies provides insights into the evidence relating to spirometry in primary care. The intervention studies highlighted the limited impact and accuracy of much spirometry, with the views papers providing potential explanations for this by reporting that some GPs prefer to prescribe treatments rather than test lung function, and limited confidence in testing or interpreting results. Although the views review suggested that training might increase the usage of spirometry in the UK, the intervention studies provide a cautionary note to this, with evidence that training may not increase the quality of testing.
The views data also may provide some insight into the unclear effect on referrals reported in much of the intervention literature. The association made by many of the intervention studies was that high numbers of patients with no abnormality seen in the new services were evidence of a reduction in specialist referrals. An alternative explanation is that GPs use these additional services for patients who might have more borderline/less severe symptoms, and who would not have been referred for specialist assessment. In support of this possibility, views studies reporting attitudes towards diagnostics in primary care suggested that ease of access to certain facilities, such as echocardiogram, would increase referrals for patients with suspected heart failure.
Limitations
Although the review included a reasonable volume of work, the limited quality of the included intervention literature requires the study findings to be treated with caution. The views review found little literature which related to patients with undiagnosed breathlessness, and highlighted the heterogeneity in conditions that could be associated with breathlessness.
Structuring qualitative findings in the Models of Pathway to Treatment framework307–309 also highlighted the extended pathway that includes patient decision-making when breathlessness symptoms occur. Delays in presentation, along with practice-related factors, can lead to delayed diagnosis.
Implications for health services
-
There is limited evidence derived from a range of study designs that community clinics, including mobile services, might be useful in providing a way of distinguishing between patients whose respiratory problems might be managed in the community and those who require referral to a specialist.
-
More robust evidence, along with qualitative and cross-sectional views data, suggests that the use of technology such as spirometry for diagnosing breathlessness in primary care is mixed in terms of access to technology, impact on diagnostic decision-making and impact on referrals. There is conflicting evidence regarding whether referrals may increase or decrease as a result of spirometry use in primary care.
-
Quantitative and qualitative evidence suggests that confidence and accuracy in using equipment and interpreting results varies in primary care. A small body of work associates increased confidence in using the technology with having received training, although the evidence is mixed regarding training having a substantial impact on the accuracy of testing.
-
There is a small body of evidence to suggest that POC testing can positively impact on differential diagnosis, diagnostic certainty and time to treatment in primary care.
-
A larger body of quantitative evidence suggests that open access to secondary care diagnostic services can reduce referrals to specialists, as well as reducing the risk of misdiagnosis.
-
Qualitative evidence highlights barriers to carrying out diagnostic procedures in primary care, such as the extended time required during consultations, and also the beliefs and attitudes of providers towards specific diagnostic procedures.
-
The literature highlights the range of conditions to which dyspnoea can be attributed, all of which are characterised by other signs and symptoms as well as psychological and social impact on the patient. This diversity is a barrier to developing a single pathway for breathlessness.
Implications for research
-
The review of available literature highlights a lack of robust evidence in the area of service models for differential diagnosis of breathlessness in primary care, with much of the literature, particularly the qualitative data, focusing on particular conditions rather than patient pathways.
-
The evidence indicates that some interventions may be more worthwhile than others, providing a rationale for further evaluation of these technologies or practices. Examples include mobile and community clinics and open-access clinics in secondary care. As noted for diagnostic ultrasound services in Chapter 5, when randomised trials are not feasible, research and evaluation may use a variety of observational study designs making use of routinely available data. 260
-
The evidence base was underdeveloped, with a need for more and higher-quality studies in the area. A key area of uncertainty relates to the value of spirometry testing in GPs’ surgeries and similar settings. There is a need for research both to clarify the effects of testing on subsequent patient management and to evaluate methods of providing training and support to improve the quality of spirometry in primary care.
Conclusions
Limited evidence from intervention studies indicates that the provision of services in the community, POC testing and open-access testing may have a positive impact on the diagnostic pathway for breathlessness, in terms of appropriate referral to specialists and a reduction in misdiagnosis. Qualitative and cross-sectional studies highlight a complex interplay of patient, practitioner and organisational factors influencing the diagnostic pathway for breathlessness. Patient data highlight an iterative process from symptom onset to diagnosis that may involve a number of consultations. Practitioners in primary care vary in their attitude towards the use of diagnostic technology based on accessibility, motivation, confidence, skills and knowledge. Although there is the suggestion that there should be improved access to diagnostic tools with regular training and financial incentives, the literature emphasises that the use of technologies such as spirometry must be carried out to high standards, and that training may not lead to these standards being achieved.
Chapter 7 Discussion and conclusions
Main findings
Following an initial broad literature mapping exercise, we conducted three separate focused reviews examining the evidence base around community diagnostic services from three different angles: the diverse logistic and practical implications of implementing a wide range of diagnostic technologies; a focus on a specific widely used diagnostic technology (ultrasound); and diagnostic pathways for patients presenting with breathlessness, a symptom associated with a range of possible underlying causes. We have identified at least seven main models of service that are delivered in primary care/community settings and, in most cases, with the possible involvement of community/primary care staff (Table 42). Not all of these models are relevant to all types of diagnostic test. The current organisation of the UK NHS means that some types of service can potentially be commissioned from private sector providers, which makes it difficult to generalise about who is involved in providing the service.
| Community/primary care setting | Secondary care setting (comparators) |
|---|---|
| Community diagnostic centre (offering multiple diagnostic services or specialising in a single test); possibly non-NHS providera | Secondary care ‘traditional’ (via consultant referral) |
| Community outreach from secondary carea | Secondary care ‘open access’ |
| GPwSI (offering test in addition to normal GP services) | Rapid-access clinic (condition-specific) |
| Specialist nurse or advanced nurse practitioner [dedicated to test (e.g. spirometry) or condition (e.g. diabetes)] | |
| Mobile service delivered at GP surgery or other community setting (possibly by non-NHS provider)a | |
| Shared services within a primary care consortium (e.g. GP federation) | |
| Telediagnosis (interpretation/advice from secondary care) |
In general, there is a lack of evidence comparing different service models directly. The focused review of ultrasound services (see Chapter 5) found only two such studies,16,229 one of which was difficult to interpret due to language difficulties. Other studies used weaker designs, for example comparing outcomes of screening in the community with hypothetical outcomes in the absence of such a service. 203,223 The review of breathlessness pathways (see Chapter 6) included a broader range of studies, including a number of UK studies, but most studies that evaluated new services were uncontrolled and many were presented at conferences rather than being published in peer-reviewed journals, limiting the data available.
Most of the research evidence included in this report evaluates some aspect of the quality, safety and/or clinical effectiveness of diagnostic testing in community or primary care settings. In the review of ultrasound scanning (see Chapter 5), a wide variety of applications of the technology was examined, including cardiac, GI, musculoskeletal and palliative care. There was some evidence that community services can guide decision-making, reduce referrals to secondary care and provide patients with quicker access to scanning and possibly subsequent treatment, if required. 16,203 The lack of true control groups in most of the included studies makes it difficult to assess how a community-based service, for example with GPs or nurses performing scans, compares with the alternatives (particularly the widely favoured model of GP open access to secondary care ultrasound services). The best evidence included in this review did not raise any major concerns about the quality or safety of ultrasound scanning in the community, given the availability of appropriate training and audit. 203 However, one study from Australia found a poor rate of accuracy of diagnoses based on musculoskeletal community ultrasound scans,230 suggesting that there may be variation across indications and/or settings.
Compared with the ultrasound review, the review of diagnostic pathways for patients with breathlessness (see Chapter 6) found a larger body of evidence (although still of limited quality) and more studies from UK settings. The findings suggest that community-based services can be useful in identifying patients who can be managed in primary care and avoiding unnecessary referrals to secondary care. However, a similar finding was reported for open-access services in secondary care and there were no studies that compared these different service models. The option for GPs to manage a patient with breathlessness by a pragmatic trial of medication rather than referral for further diagnostic tests was not considered in the included studies.
Although this review included all types of diagnostic test used in the assessment of breathlessness, the majority of studies that assessed a specific test focused on spirometry. This is an important test, particularly in the diagnosis of COPD, but we found limited evidence of an impact of spirometry performed in GP surgeries on diagnostic decision-making and contradictory evidence of an impact on referrals. There is some evidence from the UK indicating that the accuracy of spirometry in the community is often suboptimal and there is also uncertainty over whether or not accuracy can be improved by training or educational interventions. An important output of this part of the project is the conceptual model (see Figure 3) which integrates evidence from intervention studies and qualitative research to summarise the wide range of factors that may affect longer-term outcomes of different service models available to GPs in diagnosing and managing patients with breathlessness.
A major limitation of the evidence in both the ultrasound and breathlessness pathway reviews was a lack of good-quality evidence on the cost-effectiveness of different models of different service provision. For example, only two studies included in each review reported on service costs. Decision-making around provision of diagnostic tests in community and primary care settings would ideally be supported by evidence not only on the costs and benefits of different service models (including costs associated with reconfiguring services) but also on associated practical and logistic issues. The other main section of this report (see Chapter 4) addresses this need. Our framework map and synthesis cover a broad range of diagnostic technologies and use an innovative framework, termed STEP-UP, to map the literature around logistic factors and considerations affecting their uptake and use. The STEP-UP framework covers skills, training, equipment, premises, user perspectives and the primary–secondary interface. The diverse range of tests and factors covered emphasises the multifactorial nature of decisions around diagnostic service provision.
A key finding of this part of the project (in line with the focused reviews in Chapters 5 and 6) is the limited availability of data and evidence on current UK practice and uptake of diagnostic services. In particular, the implications of attempting to improve diagnostic test provision by introducing several new services within a short time are unclear. The context in which new services are introduced and evaluated will differ between, for example, different conditions or pathways and different types of setting (e.g. city vs. rural). This variation makes it difficult to provide specific actionable messages from the mapping review. However, the STEP-UP framework offers the possibility of developing a rigorous approach to the evaluation and implementation of proposals for new community diagnostic services. The combination of the STEP-UP framework with an economic evaluation from a whole-system perspective appears particularly worthy of consideration.
Our experience suggests that there may be a need for additional evidence and data resources for decision-makers on diagnostic service delivery and organisation, possibly organised along the lines of the NIHR DECs. There appears to be a particular need to improve access to data on current levels and models of diagnostic provision in primary care and to project forward to address future priorities.
Strengths and limitations
This project was conducted by a team of investigators with diverse backgrounds and expertise, and followed protocols developed in advance (topics and protocols for Chapters 4–6 were guided by the results of the initial mapping exercise). Particular strengths of the report include the initial mapping phase to gauge the quantity and quality of the evidence base and to guide selection of topics for detailed investigation; the use of systematic and documented search strategies, including extensive grey literature and internet searching; and risk-of-bias assessment supplemented by classifying evidence according to overall quality and relevance, enabling us to concentrate on synthesis of the most useful evidence. We thought that it was important to adopt an inclusive approach to reviewing this topic, including all types of study and both qualitative and quantitative evidence. This complicated some aspects of the review process, for example risk-of-bias assessment, but had the advantage of facilitating a report that reflects the complex range of factors that may influence decision-making regarding the location of diagnostic testing services closer to patients in primary care and community settings. Overall, the report documents the strengths and extensive limitations of the currently available evidence and provides suggestions for a clear research agenda underpinned by a practical methodological framework (STEP-UP) that can be used to support the development of future studies and evaluations at different levels of the health-care system.
We have not tried to cover all types of diagnostic technology in equal depth, choosing instead to provide focused reviews on logistics (see Chapter 4), on a specific widely used diagnostic technology (see Chapter 5) and on the components of pathways for diagnosing the underlying cause of breathlessness (see Chapter 6). Although this decision was informed by our initial mapping exercise, it inevitably means that some diagnostic tests have received relatively little attention. In particular, in vitro/POC diagnostic tests (covered only in Chapter 4) are the focus of a good deal of interest and may be suitable for both administration and interpretation by community/primary care settings. However, we believe that our decision to concentrate on those technologies with the greatest implications for possible changes to models of service delivery is justified based on the specific objectives of this study. The NIHR DECs have been set up to research the clinical effectiveness and cost-effectiveness of in vitro diagnostics, with the centre in Oxford having a particular interest in tests suitable for primary care. 11
The three main strands of the project (logistics map and framework synthesis, ultrasound review and breathlessness pathway review) were specified in the overall protocol and conducted largely in parallel. The different approaches of the framework synthesis and the two focused reviews complemented one another and we intend to integrate them fully in further outputs from this project. However, there was inevitably some overlap between the logistics framework synthesis and the other two reviews in relation to ultrasound and respiratory tests. If permitted by the project timetable and resources, an alternative approach would have been to develop and validate the framework as the first stage of the process. This might have enabled us to bring together evidence around effectiveness and practical requirements for service delivery in a more seamless fashion. Such an approach could be considered for future evidence synthesis projects that seek to evaluate service delivery across a broad range of technologies and indications.
The project has inevitably been constrained by the time and staff resources available and this has limited our ability to carry out all review processes in duplicate. We do not believe that this is likely to have had a major impact on, for example, decisions on inclusion of studies, but the possibility of some effect at the margins cannot be ruled out.
An important limitation on the use of systematic review methods to obtain and synthesise evidence about service delivery and organisation in the UK NHS is the difficulty of obtaining, from publicly available sources, up-to-date information about what models of service are commissioned, where and from which providers. We identified many websites and pages describing relevant services but a comprehensive listing would have required extensive contacts with (> 200) CCGs and other NHS organisations, which was not possible with the methods and resources available to this project.
Implications for practice
-
Current policy in the NHS in the UK (specifically in England) favours the development of diagnostic services in community and primary care settings. 310 This is part of a general trend towards developing new models of care centred on primary care and allowing patients to be seen and treated close to home rather than in hospital. Across the broad range of diagnostic technologies considered (in differing degrees of detail) in this report, the two main areas with implications for practice appear to be (1) training, workforce and governance issues and (2) views and attitudes at the primary–secondary care interface.
-
The reviews reported in Chapters 4–6 all consider aspects of training for health professionals delivering diagnostic services in the community. The findings suggest a need to both improve training and adapt it to the needs of health professionals working in community settings. The limited evidence included in the ultrasound review suggested that training needs will vary depending on the role of diagnostic ultrasound scanning in the practice of the individual or the service that employs them. None of the evidence looked at training for health professionals other than doctors. In particular, the training needs of ultrasonographers working in community settings, how these needs might differ from those of hospital-based staff and how best to deliver training to those working outside hospital structures have not been considered.
-
A link to secondary care may be important for governance and quality control of a community-based service, as highlighted in the critique of a community echocardiography service in the Netherlands. 223,253 However, the review of logistic issues (see Chapter 4) noted that problematic issues may arise around the role of professional bodies whose function is both to oversee quality and safety and to represent the interests of particular professional groups.
-
We found little evidence related to the primary–secondary care interface in the UK NHS. The ultrasound review included a UK study from 1997215 indicating little interest in community-based services from either GPs or hospital radiology departments (in terms of providing support and training). Open access to secondary care facilities was widely favoured. This is understandable, as this model allows hospitals to maintain their services and equipment while GPs can see their patients without having to worry about the potential workload and training implications of providing a service in the community or their own surgeries. However, open-access services are always at risk of additional costs if GPs order unnecessary tests. More recently, a study in the Netherlands224 found that orthopaedic surgeons largely distrusted ultrasound scans performed by physiotherapists in primary care. The diagnostic process was frequently restarted after referral rather than using the information supplied by the referring physiotherapist.
-
In the review of differential diagnosis of breathlessness, one study that explored diagnosis of heart disease found that there was a perceived lack of access to echocardiography in primary care which influenced the GP decision making process. 82 Patients were referred for chest X-ray instead as it was easier and less costly. These studies suggest that barriers arising at the primary–secondary care interface can hinder the effective operation of community diagnostic services.
Implications for research
Specific research implications arising from individual focused reviews are presented in the relevant chapters. Overall research themes for community diagnostic services in general are summarised here. These reflect the main findings and implications for practice presented in Implications for practice.
-
There is a need for studies to compare the outcomes of different service models using robust study designs. Comparisons of ‘true’ community-based services (using community staff for test administration, interpretation and decisions about treatment/referral/further testing) with secondary care-based open-access services and rapid-access clinics would be particularly valuable. There are specific needs for economic evaluations and for studies that incorporate the effects of diagnostic decision-making in the community on the wider health system. For example, does the provision of services in the community stimulate demand for testing that would not otherwise exist (supply-induced demand), potentially leading to overtesting and unnecessary investigations and appointments further along the patient pathway? Research on wider system impacts could investigate, for example, whether diagnostic testing in the community genuinely improves patient access or merely shifts demand from one part of the health system to another, with no overall benefit to the patient. Economic evaluations designed to support changes in services should take into account the costs of reconfiguring services, additional training, etc., as well as the costs and benefits of different service models.
-
Research into logistic and practical factors that can affect decision-making around diagnostic service provision could be based around a specific technology or focus on the needs of a particular condition or management pathway. For example, specific recommendations on research around training have recently been provided by the NIHR Oxford DEC. 45 The STEP-UP framework presented here, or its extended version (STEPPED-UP, also incorporating Public perspectives, Economics and Drivers), could be used as a framework for planning programmes of research and evaluation that reflect the complex range of factors that may influence decision-making in this area.
-
The above-noted difficulty in identifying what services are being commissioned and keeping up with local evaluations suggests that there may be a need to improve the availability of information in this area to decision-makers, researchers and the public. Preliminary research could be undertaken to establish if this is a true gap in the information resources available and if NHS decision-makers would find such information helpful. Given that some studies included in the reviews in this report were presented at conferences but not published, it would seem sensible to examine the barriers to full publication of NHS service evaluations and interventions that could potentially overcome them.
Conclusions
Overall, the evidence base for community- and primary care-based diagnostic services is limited, with very few controlled studies comparing different models of service. There is evidence from different settings that community-based services can reduce referrals to secondary care and allow more patients to be managed in primary care. Evidence on quality (including diagnostic accuracy and appropriateness of test ordering) and safety of such services is mixed.
In the absence of clear evidence of superior clinical effectiveness and cost-effectiveness, the expansion of community-based services has been driven by other factors. These include government policies to encourage moving services out of hospitals; the promise of reduced waiting times for diagnosis and potentially treatment; the availability of a wider range of suitable tests and/or cheaper, more user-friendly equipment (e.g. handheld ultrasound scanners); and the ability of commercial providers to bid for NHS contracts, potentially offering new and more flexible models of service. However, service development also faces a number of barriers, including issues related to staffing, training, governance and quality control. Perceptions and attitudes of health professionals and patients are particularly influential in the absence of clear evidence-based conclusions. Drivers and barriers vary according to the diagnostic technology involved and other contextual factors which can be addressed using the STEP-UP framework developed in this report.
The limited quantity and quality of the available research evidence means that there are many potential areas in which further research would be beneficial. In addition to assessing the clinical effectiveness and cost-effectiveness of different service models using controlled study designs, research should take into account the wider costs and benefits of reorganising services (e.g. additional training or new premises). It will also be important to ensure that new services are genuinely effective for patients and do not merely redistribute pressures from one part of the patient pathway to another.
Acknowledgements
We thank Dr Lindsay Blank for her help with quality assessment for the review of breathlessness pathways reported in Chapter 6. We are grateful to Dr Michelle Horspool and Dr Mike Tomson for facilitating feedback from clinicians and to all those who provided feedback on sections of the draft report.
Contributions of authors
Duncan Chambers (Research Fellow) contributed to the systematic review methodology, study selection, data extraction, quality assessment, report writing and liaison with clinical experts. He also contributed to the preliminary literature mapping exercise and led the review reported in Chapter 5.
Andrew Booth (Reader in Evidence Based Information Practice) contributed to the systematic review methodology – conception of review, review methodology, study selection, data extraction, quality assessment and report writing. He also contributed to the preliminary literature mapping exercise and led the review reported in Chapter 4, including conception and development of the STEP-UP framework and associated methodology.
Susan K Baxter (Research Fellow) contributed to the systematic review methodology, study selection, data extraction, quality assessment and report writing. She also led the quantitative review reported in Chapter 6.
Maxine Johnson (Research Fellow) contributed to the systematic review methodology, study selection, data extraction, quality assessment and report writing. She also led the qualitative review reported in Chapter 6.
Katherine C Dickinson (Information Specialist) contributed to the literature searching and report writing. She also performed searches for the preliminary literature mapping exercise and the reviews reported in Chapters 5 and 6.
Elizabeth C Goyder (Professor of Public Health) contibuted to the systematic review methodology, clinical input and advice, and liaison with clinical experts.
Data sharing statement
No new data have been created by this research.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Health and Social Care Information Centre . One in 50 Outpatients Who Miss an Appointment Fail to Attend Three or More Further Appointments Within Three Months 2014. www.hscic.gov.uk/article/4801/One-in-50-outpatients-who-miss-an-appointment-fail-to-attend-three-or-more-further-appointments-within-three-months (accessed 19 October 2016).
- Thompson M, Plüddemann A, Price CP, Heneghan C. Emerging diagnostic technologies in primary care: what’s on the horizon?. Br J Gen Pract 2013;63:177-8. http://dx.doi.org/10.3399/bjgp13X665107.
- The Future Direction of General Practice: A Roadmap. London: RGCP; 2007.
- Rosen R, Parker H. New Models of Primary Care: Practical Lessons from Early Implementers. London: Nuffield Trust; 2013.
- van den Hombergh P, Schers HJ, Dijkers FW, Jones R, Jones R, Britten N, et al. Oxford Textbook of Primary Medical Care. Oxford: Oxford University Press; 2004.
- Standards for General Practices (4th Edition). Melbourne, VIC: Royal Australian College of General Practitioners; 2007.
- Bourke J, Bradley CP. Factors associated with staffing provision and medical equipment acquisition in Irish general practice. Ir Med J 2012;105:338-40.
- Bourke J, Roper S. The influence of experiential learning on medical equipment adoption in general practices. Health Policy 2014;118:37-4. http://dx.doi.org/10.1016/j.healthpol.2014.05.004.
- Doll H, Shine B, Kay J, James T, Glasziou P. The rise of cholesterol testing: how much is unnecessary. Br J Gen Pract 2011;61:e81-8. http://dx.doi.org/10.3399/bjgp11X556245.
- Johnson P. Acute care. Testing times for diagnostics. Health Serv J 2013;123:22-3.
- NIHR . Diagnostic Evidence Co-Operatives (DECs) 2015. www.nihr.ac.uk/about/diagnostic-evidence-co-operatives.htm (accessed 27 August 2015).
- Engel N, Ganesh G, Patil M, Yellappa V, Pant Pai N, Vadnais C, et al. Barriers to point-of-care testing in India: results from qualitative research across different settings, users and major diseases. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0135112.
- Jones CH, Howick J, Roberts NW, Price CP, Heneghan C, Plüddemann A, et al. Primary care clinicians’ attitudes towards point-of-care blood testing: a systematic review of qualitative studies. BMC Fam Pract 2013;14. http://dx.doi.org/10.1186/1471-2296-14-117.
- Hollins L, Elliott-Rotgans D, Eaton M. A viable plan for virtual reporting. Health Serv J 2011;121:22-4.
- National Institute for Health Research . HS&Amp;DR Evidence Synthesis Centre n.d. www.nets.nihr.ac.uk/projects/hsdr/130512 (accessed 6 December 2016).
- Pallan M, Linnane J, Ramaiah S. Evaluation of an independent, radiographer-led community diagnostic ultrasound service provided to general practitioners. J Public Health 2005;27:176-81. http://dx.doi.org/10.1093/pubmed/fdi006.
- Voutilainen M, Kunnamo I. A survey of open-access endoscopy in primary health care centres: outcome of gastric carcinoma patients diagnosed by general practitioners compared with hospital-referred endoscopy. Dig Liver Dis 2005;37:119-23. http://dx.doi.org/10.1016/j.dld.2004.09.020.
- Baricchi R, Zini M, Nibali MG, Vezzosi W, Insegnante V, Manfuso C, et al. Using pathology-specific laboratory profiles in clinical pathology to reduce inappropriate test requesting: two completed audit cycles. BMC Health Serv Res 2012;12. http://dx.doi.org/10.1186/1472-6963-12-187.
- Bayliss K, Goodall M, Chisholm A, Fordham B, Chew-Graham C, Riste L, et al. Overcoming the barriers to the diagnosis and management of chronic fatigue syndrome/ME in primary care: a meta synthesis of qualitative studies. BMC Fam Pract 2014;15. http://dx.doi.org/10.1186/1471-2296-15-44.
- Bijl D, van Marwijk HW, de Haan M, van Tilburg W, Beekman AJ. Effectiveness of disease management programmes for recognition, diagnosis and treatment of depression in primary care. Eur J Gen Pract 2004;10:6-12. http://dx.doi.org/10.3109/13814780409094220.
- Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer 2008;98:60-7. http://dx.doi.org/10.1038/sj.bjc.6604096.
- O’Byrne L, Darlow C, Roberts N, Wilson G, Partridge MR. Smoothing the passage of patients from primary care to specialist respiratory opinion. Prim Care Respir J 2010;19:248-53. http://dx.doi.org/10.4104/pcrj.2010.00028.
- O’Malley AS, Lawrence W, Liang W, Yabroff R, Lynn J, Kerner J, et al. Feasibility of mobile cancer screening and prevention. J Health Care Poor Underserved 2002;13:298-319. http://dx.doi.org/10.1353/hpu.2010.0711.
- Smith AA, Kepka D, Yabroff KR. Advanced practice registered nurses, physician assistants and cancer prevention and screening: a systematic review. BMC Health Serv Res 2014;14. http://dx.doi.org/10.1186/1472-6963-14-68.
- Villeneuve E, Nam JL, Bell MJ, Deighton CM, Felson DT, Hazes JM, et al. A systematic literature review of strategies promoting early referral and reducing delays in the diagnosis and management of inflammatory arthritis. Postgrad Med J 2013;89:231-40. http://dx.doi.org/10.1136/postgradmedj-2011-201063rep.
- Arber S, McKinlay J, Adams A, Marceau L, Link C, O’Donnell A. Patient characteristics and inequalities in doctors’ diagnostic and management strategies relating to CHD: a video-simulation experiment. Soc Sci Med 2006;62:103-15. http://dx.doi.org/10.1016/j.socscimed.2005.05.028.
- Ayoub WT, Newman ED, Blosky MA, Stewart WF, Wood GC. Improving detection and treatment of osteoporosis: redesigning care using the electronic medical record and shared medical appointments. Osteoporos Int 2009;20:37-42. http://dx.doi.org/10.1007/s00198-008-0635-x.
- Chan E, Hopkins MR, Perrin JM, Herrerias C, Homer CJ. Diagnostic practices for attention deficit hyperactivity disorder: a national survey of primary care physicians. Ambul Pediatr 2005;5:201-8. http://dx.doi.org/10.1367/A04-054R1.1.
- Chey WD, Inadomi JM, Booher AM, Sharma VK, Fendrick AM, Howden CW. Primary-care physicians’ perceptions and practices on the management of GERD: results of a national survey. Am J Gastroenterol 2005;100:1237-42. http://dx.doi.org/10.1111/j.1572-0241.2005.41364.x.
- Hay JW, Lawler E, Yucel K, Guo A, Balzer T, Gaziano JM, et al. Cost impact of diagnostic imaging for lower extremity peripheral vascular occlusive disease. Value Health 2009;12:262-6. http://dx.doi.org/10.1111/j.1524-4733.2008.00438.x.
- Madurell J, Balague M, Gomez M, Cots JM, Llor C. Impact of rapid antigen detection testing on antibiotic prescription in acute pharyngitis in adults. FARINGOCAT STUDY: a multicentric randomized controlled trial. BMC Family Pract 2010;11. http://dx.doi.org/10.1186/1471-2296-11-25.
- Maserejian NN, Fischer MA, Trachtenberg FL, Yu J, Marceau LD, McKinlay JB, et al. Variations among primary care physicians in exercise advice, imaging, and analgesics for musculoskeletal pain: results from a factorial experiment. Arthritis Care Res 2014;66:147-56. http://dx.doi.org/10.1002/acr.22143.
- Murchie P, Campbell NC, Delaney EK, Dinant GJ, Hannaford PC, Johansson L, et al. Comparing diagnostic delay in cancer: a cross-sectional study in three European countries with primary care-led health care systems. Fam Pract 2012;29:69-78. http://dx.doi.org/10.1093/fampra/cmr044.
- Nucci LB, Toscano CM, Maia AL, Fonseca CD, Britto MM, Duncan BB, et al. A nationwide population screening program for diabetes in Brazil. Rev Panam Salud Publica 2004;16:320-7. http://dx.doi.org/10.1590/S1020-49892004001100005.
- Oliveria SA, Altman JF, Christos PJ, Halpern AC. Use of nonphysician health care providers for skin cancer screening in the primary care setting. Prev Med 2002;34:374-9. http://dx.doi.org/10.1006/pmed.2001.0995.
- Parkins DJ, Edgar DF. Comparison of the effectiveness of two enhanced glaucoma referral schemes. Ophthalmic Physiol Opt 2011;31:343-52. http://dx.doi.org/10.1111/j.1475-1313.2011.00853.x.
- Pearson M, Ayres JG, Sarno M, Massey D, Price D. Diagnosis of airway obstruction in primary care in the UK: the CADRE (COPD and Asthma Diagnostic/management REassessment) programme 1997-2001. Int J Chron Obstruct Pulmon Dis 2006;1:435-43. http://dx.doi.org/10.2147/copd.2006.1.4.435.
- Pérez-Martínez DA, Puente-Muñoz AI. Prior authorisation to visit the neurologist from primary care may not be necessary: the findings of a prospective, controlled study. Rev Neurol 2006;43:388-92.
- Peterson LF, Peterson LR. The safety of performing diagnostic cardiac catheterizations in a mobile catheterization laboratory at primary care hospitals. Angiology 2004;55:499-506. http://dx.doi.org/10.1177/000331970405500505.
- Poon EG, Gandhi TK, Sequist TD, Murff HJ, Karson AS, Bates DW. ‘I wish I had seen this test result earlier!’: dissatisfaction with test result management systems in primary care. Arch Intern Med 2004;164:2223-8. http://dx.doi.org/10.1001/archinte.164.20.2223.
- Smeeth L, Fletcher AE, Hanciles S, Evans J, Wormald R. Screening older people for impaired vision in primary care: cluster randomised trial. BMJ 2003;327. http://dx.doi.org/10.1136/bmj.327.7422.1027.
- Tsianakas V, Calnan M, Atkin K, Dormandy E, Marteau TM. Offering antenatal sickle cell and thalassaemia screening to pregnant women in primary care: a qualitative study of GPs’ experiences. Br J General Pract 2010;60:822-8. http://dx.doi.org/10.3399/bjgp10X532602.
- Urban E, Ose D, Joos S, Szecsenyi J, Miksch A. Technical support and delegation to practice staff – status quo and (possible) future perspectives for primary health care in Germany. BMC Med Inform Decis Mak 2012;12. http://dx.doi.org/10.1186/1472-6947-12-81.
- Aitken V. Community Ultrasound: A Study of the Factors Influencing Transfer into Primary Care in the Context of a Shift in Service Provision. London: King’s College, University of London; 1999.
- Portable Ultrasound Devices no. Horizon Scan Report 0036. Oxford: NIHR DEC Oxford; 2014.
- Health Building Note 11-01: Facilities for Primary and Community Care Services. London: Department of Health; 2013.
- Wallace P, Bowling A, Roberts JA. Better services and more choice in the NHS. BMJ 2006;333:56-7. http://dx.doi.org/10.1136/bmj.333.7558.56.
- Imison C, Naylor C, Maybin J. Under One Roof: Will Polyclinics Deliver Integrated Care?. London: The King’s Fund; 2008.
- Care Closer to Home. London: DH; 2008.
- Our NHS Our Future: NHS Next Stage Review – Leading Local Change. London: DH; 2008.
- Birchall D. Primary care access to diagnostics: a paradigm shift. Br J Radiol 2010;83:101-3. http://dx.doi.org/10.1259/bjr/88648707.
- NHS Choices . Hearing Tests n.d. www.nhs.uk/Conditions/Hearing-tests/Pages/Introduction.aspx (accessed December 2016).
- Lous J, Ryborg CT, Damsgaard JJ, Munck AP. Tympanometry in general practice: use, problems and solutions. Fam Pract 2012;29:726-32. http://dx.doi.org/10.1093/fampra/cms045.
- Blomgren K, Pitkäranta A. Is it possible to diagnose acute otitis media accurately in primary health care?. Fam Pract 2003;20:524-7. http://dx.doi.org/10.1093/fampra/cmg505.
- American Academy of Pediatrics Subcommittee on Otitis media With Effusion . Otitis media with effusion. Pediatrics 2004;113:1412-29. http://dx.doi.org/10.1542/peds.113.5.1412.
- Green LA, Culpepper L, de Melker RA, Froom J, van Balen F, Grob P, et al. Tympanometry interpretation by primary care physicians. A report from the International Primary Care Network (IPCN) and the Ambulatory Sentinel Practice Network (ASPN). J Fam Pract 2000;49:932-6.
- Abbott P, Rosenkranz S, Hu W, Gunasekera H, Reath J. The effect and acceptability of tympanometry and pneumatic otoscopy in general practitioner diagnosis and management of childhood ear disease. BMC Fam Pract 2014;15. http://dx.doi.org/10.1186/s12875-014-0181-x.
- Rosenkranz S, Abbott P, Reath J, Gunasekera H, Hu W. Promoting diagnostic accuracy in general practitioner management of otitis media in children: findings from a multimodal, interactive workshop on tympanometry and pneumatic otoscopy. Qual Prim Care 2012;20:275-85.
- Kaleida PH, Ploof DL, Kurs-Lasky M, Shaikh N, Colborn DK, Haralam MA, et al. Mastering diagnostic skills: Enhancing Proficiency in Otitis Media, a model for diagnostic skills training. Pediatrics 2009;124:e714-20. http://dx.doi.org/10.1542/peds.2008-2838.
- Johansen EC, Lildholdt T, Damsbo N, Eriksen EW. Tympanometry for diagnosis and treatment of otitis media in general practice. Fam Pract 2000;17:317-22. http://dx.doi.org/10.1093/fampra/17.4.317.
- Laine MK, Tähtinen PA, Ruuskanen O, Löyttyniemi E, Ruohola A. Can nurses exclude middle-ear effusion without otoscopy in young asymptomatic children in primary care?. Scand J Prim Health Care 2015;33:115-20. http://dx.doi.org/10.3109/02813432.2015.1030174.
- Recommended Procedure for Tympanometry. Bathgate: British Society of Audiology; 2014.
- Policy Document. London: British Association of Audiological Physicians; 2002.
- Patricoski C, Ferguson S. Which tympanometer is optimal for an outpatient primary care setting?. J Fam Pract 2006;55:946-52.
- Clarke LR, Wiederhold ML, Gates GA. Quantitation of pneumatic otoscopy. Otolaryngol Head Neck Surg 1987;96:119-24.
- Takata GS, Chan LS, Morphew T, Mangione-Smith R, Morton SC, Shekelle P. Evidence assessment of the accuracy of methods of diagnosing middle ear effusion in children with otitis media with effusion. Pediatrics 2003;1122:1379-87. http://dx.doi.org/10.1542/peds.112.6.1379.
- Ponka D, Baddar F. Pneumatic otoscopy. Can Fam Physician 2013;59.
- Ouedraogo E, Labrecque M, Côté L, Charbonneau K, Légaré F. Use and teaching of pneumatic otoscopy in a family medicine residency program. Can Fam Physician 2013;59:972-9.
- Provision of Services for Adults with Tinnitus. A Good Practice Guide. London: Central Office of Information; 2009.
- Powell J. Systematic review of outreach clinics in primary care in the UK. J Health Serv Res Policy 2002;7:177-83. http://dx.doi.org/10.1258/135581902760082490.
- Pushing the Boundaries: Evidence to Support the Delivery of Good Practice in Audiology. London: NHS Improvement; 2010.
- Smith P, Mack A, Davis A. A multicenter trial of an assess-and-fit hearing aid service using open canal fittings and comply ear tips. Trends Amplif 2008;12:121-36. http://dx.doi.org/10.1177/1084713808316976.
- Campbell SM, Fuat A, Summerton N, Lancaster N, Hobbs F. Diagnostic triage and the role of natriuretic peptide testing and echocardiography for suspected heart failure: an appropriateness ratings evaluation by UK GPs. Br J Gen Pract 2011;61. http://dx.doi.org/10.3399/bjgp11x583218.
- Hancock HC, Close H, Fuat A, Murphy JJ, Hungin AP, Mason JM. Barriers to accurate diagnosis and effective management of heart failure have not changed in the past 10 years: a qualitative study and national survey. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-003866.
- Accreditation in Cardiac Imaging: A Statement from the BCS Imaging Council. London: British Cardiovascular Society; n.d.
- Day A, Oldroyd C, Godfrey S, Quinn T. Availability of cardiac equipment in general practice premises in a cardiac network: a survey. Br J Cardiol 2008;15:141-4.
- Pollard L, Rogers S, Shribman J, Sprigings D, Sinfield P. A study of role expansion: a new GP role in cardiology care. BMC Health Serv Res 2014;14. http://dx.doi.org/10.1186/1472-6963-14-205.
- Balion C, Don-Wauchope A, Hill S, Santaguida PL, Booth R, Brown JA, et al. Use of Natriuretic Peptide Measurement in the Management of Heart Failure. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- Mant J, Doust J, Roalfe A, Barton P, Cowie M, Glasziou P, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13320.
- NHS Improvement . Heart Improvement Programme Brain-Type Natriuretic Peptide (BNP) – An Information Resource for Cardiac Networks 2007.
- MaDOx . Point-Of-Care-B-Type-Natriuretic-Peptide-Testing 2011.
- Khunti K, Hearnshaw H, Baker R, Grimshaw G. Heart failure in primary care: qualitative study of current management and perceived obstacles to evidence-based diagnosis and management by general practitioners. Eur J Heart Fail 2002;4:771-7. http://dx.doi.org/10.1016/S1388-9842(02)00119-8.
- Ray S. Commissioning of Cardiac Services – A Resource Pack from the British Cardiovascular Society. London: British Cardiovascular Society; 2011.
- NHS Improvement . Heart Failure: Use of BNP NTproBNP Testing in Primary Care to Facilitate Early Diagnosis n.d. www.evidence.nhs.uk/QIPP (accessed 4 January 2016).
- Guideline 108: Chronic Heart Failure National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: NICE; 2010.
- Balion C, Santaguida PL, Hill S, Worster A, McQueen M, Oremus M, et al. Testing for BNP and NT-proBNP in the diagnosis and prognosis of heart failure. Evid Rep Technol Assess 2006;142:1-147.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure?. JAMA 2005;294:1944-56. http://dx.doi.org/10.1001/jama.294.15.1944.
- Latour-Pérez J, Coves-Orts FJ, Abad-Terrado C, Abraira V, Zamora J. Accuracy of B-type natriuretic peptide levels in the diagnosis of left ventricular dysfunction and heart failure: a systematic review. Eur J Heart Fail 2006;8:390-9. http://dx.doi.org/10.1016/j.ejheart.2005.10.004.
- Korenstein D, Wisnivesky JP, Wyer P, Adler R, Ponieman D, McGinn T. The utility of B-type natriuretic peptide in the diagnosis of heart failure in the emergency department: a systematic review. BMC Emerg Med 2007;7. http://dx.doi.org/10.1186/1471-227X-7-6.
- Alehagen U, Janzon M. A clinician’s experience of using the Cardiac Reader NT-proBNP point-of-care assay in a clinical setting. Eur J Heart Fail 2008;10:260-6. http://dx.doi.org/10.1016/j.ejheart.2008.01.005.
- NHS Choices . Electrocardiogram n.d. www.nhs.uk/conditions/electrocardiogram/pages/introduction.aspx (accessed December 2016).
- Salerno SM, Alguire PC, Waxman HS. Competency in interpretation of 12-lead electrocardiograms: a summary and appraisal of published evidence. Ann Intern Med 2003;138:751-60. http://dx.doi.org/10.7326/0003-4819-138-9-200305060-00013.
- Mant J, Fitzmaurice DA, Hobbs FD, Jowett S, Murray ET, Holder R, et al. Accuracy of diagnosing atrial fibrillation on electrocardiogram by primary care practitioners and interpretative diagnostic software: analysis of data from screening for atrial fibrillation in the elderly (SAFE) trial. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39227.551713.AE.
- ECG Capability Within Primary Care. Dorset: NHS Dorset CCG; 2015.
- Harris K, Edwards D, Mant J. How can we best detect atrial fibrillation?. J R Coll Physicians Edinb 2012;42:5-22.
- Diagnostic Technology: Hand-held ECG Monitors for the Detection of of Atrial Fibrillation (AF) in Primary Care. Oxford: MaDOx; 2013.
- Stott DJ, Dewar RI, Garratt CJ, Griffith KE, Harding NJ, James MA, et al. RCPE UK Consensus Conference on ‘approaching the comprehensive management of atrial fibrillation: evolution or revolution?’. J R Coll Physicians Edinb 2012;42:34-5. http://dx.doi.org/10.4997/JRCPE.2012.S01.
- Wiesel J, Wiesel D, Suri R, Messineo FC. The use of a modified sphygmomanometer to detect atrial fibrillation in outpatients. Pacing Clin Electrophysiol 2004;27:639-43. http://dx.doi.org/10.1111/j.1540-8159.2004.00499.x.
- Wiesel J, Fitzig L, Herschman Y, Messineo FC. Detection of atrial fibrillation using a modified microlife blood pressure monitor. Am J Hypertens 2009;22:848-52. http://dx.doi.org/10.1038/ajh.2009.98.
- Stergiou GS, Karpettas N, Protogerou A, Nasothimiou EG, Kyriakidis M. Diagnostic accuracy of a home blood pressure monitor to detect atrial fibrillation. J Hum Hypertens 2009;23:654-8. http://dx.doi.org/10.1038/jhh.2009.5.
- AliveCor Heart Monitor and AliveECG App for Detecting Atrial Fibrillation (Medtech Innovation Briefing). London: NICE; 2015.
- Oresko JJ, Duschl H, Cheng AC. A wearable smartphone-based platform for real-time cardiovascular disease detection via electrocardiogram processing. IEEE Trans Inf Technol Biomed 2010;14:734-40. http://dx.doi.org/10.1109/TITB.2010.2047865.
- Dee H, Pope J. Harrow Primary Care Trust takes cardiac care into the community. Br J Healthcare Comput Inf Manag 2009.
- Chambers J, Fuat A, Liddiard S, McDonagh T, Monaghan M, Nihoyannopoulus P. Community echocardiography for heart failure: a consensus statement from representatives of the British Society of Echocardiography, the British Heart Failure Society, the Coronary Heart Disease Collaborative and the Primary Care Cardiovascular Society. Br J Cardiol 2004;11:399-402.
- van Heur LMSG, Baur LHB, Tent M, Lodewijks-van der Bolt CLB, Streppel M, Winkens RAG, et al. Evaluation of an open access echocardiography service in the Netherlands: a mixed methods study of indications, outcomes, patient management and trends. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-37.
- Jeyaseelan S, Goudie BM, Pringle SD, Donnan PT, Sullivan FM, Struthers AD. Agreement between community echocardiography and hospital echocardiography in patients suspected of having left ventricular systolic dysfunction. Postgrad Med J 2005;81:777-9. http://dx.doi.org/10.1136/pgmj.2005.033605.
- Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7382.196.
- Sparrow N, Adlam D, Cowley A, Hampton JR. The diagnosis of heart failure in general practice: implications for the UK National Service Framework. Eur J Heart Fail 2003;5:349-54. http://dx.doi.org/10.1016/S1388-9842(03)00046-1.
- Nagrebetsky A, Jin J, Stevens R, James T, Adler A, Park P, et al. Diagnostic accuracy of urine dipstick testing in screening for microalbuminuria in type 2 diabetes: a cohort study in primary care. Fam Pract 2013;30:142-52. http://dx.doi.org/10.1093/fampra/cms057.
- The RCGP Curriculum: Clinical Modules Version Approved 18 May 2015 for Implementation from 5 August 2015. 3.17 Care of People with Metabolic Problems. London: RCGP; 2015.
- Portogallo C, Barlow I. Implementing a quality assurance system for point-of-care blood glucose monitoring. J Diabet Nurs 2010;14:63-7.
- Miller CD, Barnes CS, Phillips LS, Ziemer DC, Gallina DL, Cook CB, et al. Rapid A1c availability improves clinical decision-making in an urban primary care clinic. Diabetes Care 2003;26:1158-63. http://dx.doi.org/10.2337/diacare.26.4.1158.
- Howick J, Cals JW, Jones C, Price CP, Plüddemann A, Heneghan C, et al. Current and future use of point-of-care tests in primary care: an international survey in Australia, Belgium, The Netherlands, the UK and the USA. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005611.
- Hipwell AE, Sturt J, Lindenmeyer A, Stratton I, Gadsby R, O’Hare P, et al. Attitudes, access and anguish: a qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005498.
- Burgess S, McHoy A. Breaking the primary-secondary care divide in diabetes. J Diabet Nurs 2007;11.
- NHS Choices . Endoscopy n.d. www.nhs.uk/conditions/endoscopy/Pages/Introduction.aspx (accessed December 2016).
- Stephens M, Hourigan LF, Appleyard M, Ostapowicz G, Schoeman M, Desmond PV, et al. Non-physician endoscopists: a systematic review. World J Gastroenterol 2015;21:5056-71. http://dx.doi.org/10.3748/wjg.v21.i16.5056.
- Galloway JM, Gibson J, Dalrymple J. Endoscopy in primary care – a survey of current practice. Br J Gen Pract 2002;52:536-8.
- Lee SS, Enns R. The hidden realities of endoscopy unit budgeting. Can J Gastroenterol Hepatol 2014;28:619-20. http://dx.doi.org/10.1155/2014/208532.
- [No authors listed] . Gastrointestinal endoscopy in general practice. Gut 1994;35. http://dx.doi.org/10.1136/gut.35.10.1342.
- Health and Safety Executive . Control of Substances Hazardous to Health (COSHH) 2002.
- Cowan RE, Manning AP, Ayliffe GA, Axon AT, Causton JS, Cripps NF, et al. Aldehyde disinfectants and health in endoscopy units. British Society of Gastroenterology Endoscopy Committee. Gut 1993;34:1641-5. http://dx.doi.org/10.1136/gut.34.11.1641.
- Health Building Notes 52 Vol 2 Accommodation for Day Care Endoscopy Units. London: HMSO; 1994.
- Follows R, Johnston D. User’s Guide to Achieving a JAG Compliant Endoscopy Environment. London: Royal College of Physicians; 2011.
- Oikonomidou E, Anastasiou F, Pilpilidis I, Kouroumalis E, Lionis C. Upper gastrointestinal endoscopy for dyspepsia: exploratory study of factors influencing patient compliance in Greece. BMC Gastroenterol 2011;11. http://dx.doi.org/10.1186/1471-230X-11-11.
- Austin KL, Power E, Solarin I, Atkin WS, Wardle J, Robb KA. Perceived barriers to flexible sigmoidoscopy screening for colorectal cancer among UK ethnic minority groups: a qualitative study. J Med Screen 2009;16:174-9. http://dx.doi.org/10.1258/jms.2009.009080.
- Voss M, Forward LM, Smits CA, Duvenage R. Endoscopy outreach: how worthwhile is it?. S Afr Med J 2010;100. http://dx.doi.org/10.7196/SAMJ.3708.
- US National Library of Medicine . Genetics Home Reference n.d. https://ghr.nlm.nih.gov (accessed December 2016).
- Brennan KS. Clinical implications of pharmacogenetic and microarray testing for advanced practice nurses. J Am Assoc Nurse Pract 2015;27:246-55. http://dx.doi.org/10.1002/2327-6924.12237.
- Baars MJ, Henneman L, Ten Kate LP. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: a global problem. Genet Med 2005;7:605-10. http://dx.doi.org/10.1097/01.gim.0000182895.28432.c7.
- Trinidad SB, Fryer-Edwards K, Crest A, Kyler P, Lloyd-Puryear MA, Burke W. Educational needs in genetic medicine: primary care perspectives. Community Genet 2008;11:160-5. http://dx.doi.org/10.1159/000113878.
- Burke S, Kirk M. Genetics education in the nursing profession: literature review. J Adv Nurs 2006;54:228-37. http://dx.doi.org/10.1111/j.1365-2648.2006.03805.x.
- Chapman DD. Cancer genetics. Semin Oncol Nurs 2007;23:2-9. http://dx.doi.org/10.1016/j.soncn.2006.11.002.
- Emery J, Watson E, Rose P, Andermann A. A systematic review of the literature exploring the role of primary care in genetic services. Fam Pract 1999;16:426-45. http://dx.doi.org/10.1093/fampra/16.4.426.
- Mainous AG, Johnson SP, Chirina S, Baker R. Academic family physicians’ perception of genetic testing and integration into practice: a CERA study. Fam Med 2013;45:257-62.
- Klitzman RL. Misunderstandings concerning genetics among patients confronting genetic disease. J Genet Couns 2010;19:430-46. http://dx.doi.org/10.1007/s10897-010-9307-z.
- Middlemass JB, Yazdani MF, Kai J, Standen PJ, Qureshi N. Introducing genetic testing for cardiovascular disease in primary care: a qualitative study. Br J Gen Pract 2014;64:e282-9. http://dx.doi.org/10.3399/bjgp14X679714.
- Bernhardt BA, Zayac C, Pyeritz RE. Why is genetic screening for autosomal dominant disorders underused in families? The case of hereditary hemorrhagic telangiectasia. Genet Med 2011;13:812-20. http://dx.doi.org/10.1097/GIM.0b013e31821d2e6d.
- Friedman L, Cooper HP, Webb JA, Weinberg AD, Plon SE. Primary care physicians’ attitudes and practices regarding cancer genetics: a comparison of 2001 with 1996 survey results. J Cancer Educ 2003;18:91-4. http://dx.doi.org/10.1207/S15430154JCE1802_11.
- Anderson B, McLosky J, Wasilevich E, Lyon-Callo S, Duquette D, Copeland G. Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol 2012;2012. http://dx.doi.org/10.1155/2012/298745.
- Shickle D, Hapgood R, Qureshi N. The genetics liaison nurse role as a means of educating and supporting primary care professionals. Fam Pract 2002;19:193-6. http://dx.doi.org/10.1093/fampra/19.2.193.
- Emery J, Hayflick S. The challenge of integrating genetic medicine into primary care. BMJ 2001;322:1027-30. http://dx.doi.org/10.1136/bmj.322.7293.1027.
- Torrance N, Mollison J, Wordsworth S, Gray J, Miedzybrodzka Z, Haites N, et al. Genetic nurse counsellors can be an acceptable and cost-effective alternative to clinical geneticists for breast cancer risk genetic counselling. Evidence from two parallel randomised controlled equivalence trials. Br J Cancer 2006;95:435-44. http://dx.doi.org/10.1038/sj.bjc.6603248.
- NHS Choices . MRI Scan n.d. www.nhs.uk/conditions/MRI-scan/Pages/Introduction.aspx (accessed December 2016).
- Diagnostic Tests Direct Access Magnetic Resonance Imaging Service. London: Department of Health; 2013.
- Device Bulletin: Safety Guidelines for Magnetic Resonance Imaging Equipment in Clinical Use. London: Medical Devices Agency; 2007.
- Kost GJ, Kost GJ. Principles and Practices of Point-of-Care Testing. Philadelphia, PA: Lippincott Williams & Wilkins; 2002.
- The Code: Standards of Conduct, Performance and Ethics for Nurses and Midwives. London: Nursing and Midwifery Council; 2008.
- Gardiner C, Machin S, Fitzmaurice DA, Murray ET. The Primary Care Perspective, Chapter 3. Newmarket: Hayward Medical Press; 2002.
- van Vugt SF, Broekhuizen BDL, Lammens C, Zuithoff NPA, de Jong PA, Coenen S, et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: diagnostic study. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2450.
- Aabenhus R, Jensen JU, Jorgensen KJ, Hrobjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst Rev 2014;11. http://dx.doi.org/10.1002/14651858.cd010130.pub2.
- National Institute for Health Research . Primary Care Use of a C-Reactive Protein (CRP) Point of Care Test (POCT) to Help Target Antibiotic Prescribing to Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD) Who Are Most Likely to Benefit (The PACE Study) n.d. http://www.nets.nihr.ac.uk/projects/hta/123312 (accessed 6 December 2016).
- Health Building Note 00-03: Clinical and Clinical Support Spaces. London: Department of Health; 2000.
- Murray ET, Fitzmaurice DA, McCahon D. Point of care testing for INR monitoring: where are we now?. Br J Haematol 2004;127:373-8. http://dx.doi.org/10.1111/j.1365-2141.2004.05154.x.
- NHS Choices . X-ray n.d. http://www.nhs.uk/conditions/X-ray/Pages/Introduction.aspx (accessed December 2016).
- RCR . Specialty Training Curriculum for Clinical Radiology 2014. www.rcr.ac.uk/sites/default/files/docs/radiology/pdf/Curriculum_CR_28_October_2014_FINAL.pdf (accessed 19 October 2016).
- College of Radiographers and Royal College of Radiologists . The Imaging Services Accreditation Scheme Standard: Statements, Rationales and Criteria 2013. www.sor.org/system/files/article/201502/isas_standard_v2.1_jan_2013.pdf (accessed 19 October 2016).
- Speets AM, Hoes AW, van der Graaf Y, Kalmijn S, de Wit NJ, van Swijndregt AD, et al. Upper abdominal ultrasound in general practice: indications, diagnostic yield and consequences for patient management. Fam Pract 2006;23:507-11. http://dx.doi.org/10.1093/fampra/cml027.
- Health Building Note Number 6. London: The Stationery Office; 2001.
- Ionising Radations Regulations 1999. London: The Stationery Office; 1999.
- Wilson S, Fromont C, Langley R, Chescoe S, Grootoonk S. A Study of the Effect on Patient Management of GP Direct Access to Diagnostic Imaging Tests. The London Outcomes Audit: Prepared by InHealth; 2010.
- José BP, Camargos PA, Cruz Filho ÁA, Corrêa Rde A. Diagnostic accuracy of respiratory diseases in primary health units. Rev Assoc Med Bras 2014;60:599-612. http://dx.doi.org/10.1590/1806-9282.60.06.021.
- Potter VA. Pulse oximetry in general practice: how would a pulse oximeter influence patient management?. Eur J Gen Pract 2007;13:216-20. http://dx.doi.org/10.1080/13814780701574762.
- Bewick T, Greenwood S, Lim WS. What is the role of pulse oximetry in the assessment of patients with community-acquired pneumonia in primary care?. Prim Care Respir J 2010;19:378-82. http://dx.doi.org/10.4104/pcrj.2010.00049.
- King T. Pulse oximetry from past to present. J Clin Engineer 2011;36:129-31. http://dx.doi.org/10.1097/JCE.0b013e318223cbad.
- Gunstone C. Pulse oximetry in primary care. Br J Gen Pract 2011;61. http://dx.doi.org/10.3399/bjgp11X588394.
- Pinnock H, Netuveli G, Price D, Sheikh A. General practitioners with a special interest in respiratory medicine: national survey of UK primary care organisations. BMC Health Serv Res 2005;5. http://dx.doi.org/10.1186/1472-6963-5-40.
- Kelly C. The use of pulse oximetry in primary care. Br J Prim Care Nurs 2008;2.
- Chronic Obstructive Pulmonary Disease (COPD) Quality Standard (QS10). NICE: London; 2004.
- Levin KP, Hanusa BH, Rotondi A, Singer DE, Coley CM, Marrie TJ, et al. Arterial blood gas and pulse oximetry in initial management of patients with community-acquired pneumonia. J Gen Intern Med 2001;16:590-8. http://dx.doi.org/10.1046/j.1525-1497.2001.016009590.x.
- Krueger P, Loeb M, Kelly C, Edward HG. Assessing, treating and preventing community acquired pneumonia in older adults: findings from a community-wide survey of emergency room and family physicians. BMC Fam Pract 2005;6. http://dx.doi.org/10.1186/1471-2296-6-32.
- Guidelines for the Management of Community Acquired Pneumonia in Adults 2004 Update. London: British Thoracic Society; 2004.
- Cunningham S, McMurray A. The availability and use of oxygen saturation monitoring in primary care in order to assess asthma severity. Prim Care Respir J 2006;15:98-101. http://dx.doi.org/10.1016/j.pcrj.2006.01.005.
- Schermer T, Leenders J, van den Bosch W, Wissink A, Smeele I, Chavannes N. Pulse oximetry in family practice: indications and clinical observations in patients with COPD. Fam Pract 2009;26:524-31. http://dx.doi.org/10.1093/fampra/cmp063.
- British Thoracic Society Standards of Care Committee . BTS guidelines for the management of community acquired pneumonia in adults. Thorax 2001;56:IV1-64. http://dx.doi.org/10.1136/thx.56.suppl_4.iv1.
- Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64:iii1-55. http://dx.doi.org/10.1136/thx.2009.121434.
- Thompson M, Mayon-White R, Harnden A, Perera R, McLeod D, Mant D. Using vital signs to assess children with acute infections: a survey of current practice. Br J Gen Pract 2008;58:236-41. http://dx.doi.org/10.3399/bjgp08X279689.
- Holmes S, Peffers SJ. PCRS-UK Opinion Sheet No. 28: Pulse Oximetry in Primary Care. Primary Care Respiratory Society; 2009.
- Project Initiation Document: Pulse Oximeters. National Health Service Center for Evidence-based Purchasing; 2009.
- Amar D, Neidzwski J, Wald A, Finck AD. Fluorescent light interferes with pulse oximetry. J Clin Monit 1989;5:135-6. http://dx.doi.org/10.1007/BF01617888.
- Primary Care Opinion Sheet: Pulse Oximeters. Primary Care Respiratory Society; 2015.
- Starren ES, Roberts NJ, Tahir M, O’Byrne L, Haffenden R, Patel IS, et al. A centralised respiratory diagnostic service for primary care: a 4-year audit. Prim Care Respir J 2012;21:180-6. http://dx.doi.org/10.4104/pcrj.2012.00013.
- Ingram G, Munro N. The use (or otherwise) of pulse oximetry in general practice. Br J Gen Pract 2005;55:501-2.
- NHS Choices . Spirometry n.d. www.nhs.uk/Conditions/spirometry/Pages/Introduction.aspx (accessed December 2016).
- Leuppi JD, Miedinger D, Chhajed PN, Buess C, Schafroth S, Bucher HC, et al. Quality of spirometry in primary care for case finding of airway obstruction in smokers. Respiration 2010;79:469-74. http://dx.doi.org/10.1159/000243162.
- Castillo D, Guayta R, Giner J, Burgos F, Capdevila C, Soriano JB, et al. COPD case finding by spirometry in high-risk customers of urban community pharmacies: a pilot study. Respir Med 2009;103:839-45. http://dx.doi.org/10.1016/j.rmed.2008.12.022.
- White P, Wong W, Fleming T, Gray B. Primary care spirometry: test quality and the feasibility and usefulness of specialist reporting. Br J Gen Pract 2007;57:701-5.
- Walters JA, Hansen EC, Johns DP, Blizzard EL, Walters EH, Wood-Baker R. A mixed methods study to compare models of spirometry delivery in primary care for patients at risk of COPD. Thorax 2008;63:408-14. http://dx.doi.org/10.1136/thx.2007.082859.
- Holton C, Crockett A, Nelson M, Ryan P, Wood-Baker R, Stocks N, et al. Does spirometry training in general practice improve quality and outcomes of asthma care?. Int J Qual Health Care 2011;23:545-53. http://dx.doi.org/10.1093/intqhc/mzr039.
- Lange P, Andersen KK, Munch E, Sørensen TB, Dollerup J, Kassø K, et al. Quality of COPD care in hospital outpatient clinics in Denmark: the KOLIBRI study. Respir Med 2009;103:1657-62. http://dx.doi.org/10.1016/j.rmed.2009.05.010.
- Kaminsky DA, Marcy TW, Bachand M, Irvin CG. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care 2005;50:1639-48.
- Burton D, Johns DP, Swanney MP. Spirometer Users’ and Buyers’ Guide. Melbourne, VIC: National Asthma Council Australia; 2011.
- Liistro G, Vanwelde C, Vincken W, Vandevoorde J, Verleden G, Buffels J. Technical and functional assessment of 10 office spirometers: a multicenter comparative study. Chest 2006;130:657-65. http://dx.doi.org/10.1378/chest.130.3.657.
- Hancock KL, Schermer TR, Holton C, Crockett AJ. Microbiological contamination of spirometers: an exploratory study in general practice. Aust Fam Physician 2012;41.
- Schermer T, Grootens-Stekelenburg J, Rauws J, Denis J, Heijdra Y, de Jongh F, et al. Impact of two spirometry training programs on test quality in Dutch general practices. Eur Respir J 2013;42.
- Barr RG, Celli BR, Martinez FJ, Ries AL, Rennard SI, Reilly JJ, et al. Physician and patient perceptions in COPD: the COPD resource network needs assessment survey. Am J Med 2005;118:1415-e9.
- Roberts NJ, Smith SF, Partridge MR. Why is spirometry underused in the diagnosis of the breathless patient: a qualitative study. BMC Pulm Med 2011;11. http://dx.doi.org/10.1186/1471-2466-11-37.
- Joo MJ, Sharp LK, Au DH, Lee TA, Fitzgibbon ML. Use of spirometry in the diagnosis of COPD: a qualitative study in primary care. COPD 2013;10:444-9. http://dx.doi.org/10.3109/15412555.2013.766683.
- Konstantikaki V, Kostikas K, Minas M, Batavanis G, Daniil Z, Gourgoulianis KI, et al. Comparison of a network of primary care physicians and an open spirometry programme for COPD diagnosis. Respir Med 2011;105:274-81. http://dx.doi.org/10.1016/j.rmed.2010.06.020.
- NHS Choices . Ultrasound Scan n.d. www.nhs.uk/Conditions/Ultrasound-scan/Pages/Introduction.aspx (accessed December 2016).
- Wittenberg M. Will ultrasound scanners replace the stethoscope?. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g3463.
- National Ultrasound Steering Group . Ultrasound Clinical Governance 2008. www.bmus.org/policies-guides/ClinicalGovernanceInUltrasound-061108.pdf (accessed 28 August 2015).
- Wordsworth S, Scott A. Ultrasound scanning by general practitioners: is it worthwhile?. J Public Health Med 2002;24:88-94. http://dx.doi.org/10.1093/pubmed/24.2.88.
- Standards for the Provision of an Ultrasound Service. London: RCR; 2014.
- Siu T, Chau H, Myhre D. Bedside ultrasonography performed by family physicians in outpatient medical offices in Whitehorse, Yukon. Can J Rural Med 2013;18:43-6.
- NHS Improvement – Diagnostics . Radiology Case Studies 2013 2013. http://webarchive.nationalarchives.gov.uk/20130221101407/http:/www.improvement.nhs.uk/diagnostics/RadiologyCaseStudies.aspx#Ult (accessed 1 August 2016).
- Baseline Report on Diagnostics Activity and Workforce Issues. London: NHS London; 2008.
- An Analytical Based Workforce Review of Community Focused Care and Diagnostics. London: NHS London; 2008.
- Malone B. AACC Clinical Laboratory News. Spotlight on Point-of-Care Testing; 2012.
- Singh D. Making the Shift: Key Success Factors. Birmingham: Health Services Management Centre and NHS Institute for Innovation and Improvement, University of Birmingham; 2006.
- Parker H. Making the Shift: A Review of NHS Experience. Birmingham: University of Birmingham; 2006.
- Carlisle D. Brain teaser: how to find a cure for diagnostics’ ills. Health Serv J 2004;114:14-5.
- Thompson M, Price C, Van den Bruel A. Innovation in diagnostics and healthcare: improving bench to bedside processes for testing. Oxford: Department of Primary Care Health Sciences, University of Oxford; 2011.
- Health and Social Care Act 2012. London: The Stationery Office; 2012.
- Robinson L, Potterton J, Owen P. Diagnostic ultrasound: a primary care-led service?. Br J Gen Pract 1997;47:293-6.
- Lackner K, Krug B, Stützer H, Sechtem U, Heindel W. A prospective study to assess the need for requested radiological studies. Rofo 1996;165:4-9. http://dx.doi.org/10.1055/s-2007-1015706.
- Van Breuseghem I, Geusens E. Assessment of the appropriateness of requested radiological examinations for outpatients and the potential financial consequences of guideline application. JBR-BTR 2006;89:8-11.
- Making Best Use of a Department of Clinical Radiology. Guidelines for Doctors. London: RCR; 1990.
- Oakeshott P, Kerry SM, Williams JE. Randomized controlled trial of the effect of the Royal College of Radiologists’ guidelines on general practitioners’ referrals for radiographic examination. Br J Gen Pract 1994;44:197-200.
- Recommendations on the Delivery of Community Echocardiography in Wales. Cardiff: Cardiac Networks Co-ordinating Group; 2006.
- Landry BA, Barnes D, Keough V, Watson A, Rowe J, Mallory A, et al. Do family physicians request ultrasound scans appropriately?. Can Fam Physician 2011;57:e299-304.
- Everett CB, Preece E. Women with bleeding in the first 20 weeks of pregnancy: value of general practice ultrasound in detecting fetal heart movement. Br J Gen Pract 1996;46:7-9.
- van Gurp N, Boonman-De Winter LJ, Meijer Timmerman Thijssen DW, Stoffers HE. Benefits of an open access echocardiography service: a Dutch prospective cohort study. Neth Heart J 2013;21:399-405. http://dx.doi.org/10.1007/s12471-013-0416-9.
- Scholten-Peeters GG, Franken N, Beumer A, Verhagen AP. The opinion and experiences of Dutch orthopedic surgeons and radiologists about diagnostic musculoskeletal ultrasound imaging in primary care: a survey. Man Ther 2014;19:109-13. http://dx.doi.org/10.1016/j.math.2013.08.003.
- Mogam C, Guo B, Schopflocher D, Harstall C. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. Edmonton, AB: Institute of Health Economics; 2012.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. http://dx.doi.org/10.1136/jech.52.6.377.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, JJ D, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 100. Oxford: The Cochrane Collaboration; 2009.
- Salihefendic N, Spahovic H, Cabric E, Hrgovic Z. Social and medical yield and consequences of ultrasonography in primary health care. Acta Informatica 2009;17:32-5.
- Goldberg JA, Bruce WJ, Walsh W, Sonnabend DH. Role of community diagnostic ultrasound examination in the diagnosis of full-thickness rotator cuff tears. ANZ J Surg 2003;73:797-9. http://dx.doi.org/10.1046/j.1445-2197.2003.02790.x.
- Grubel P. Evaluation of abdominal ultrasound performed by the gastroenterologist in the office. J Clin Gastroenterol 2011;45:405-9. http://dx.doi.org/10.1097/MCG.0b013e3181eeb472.
- Heikkinen M, Räsänen H, Färkkilä M. Clinical value of ultrasound in the evaluation of dyspepsia in primary health care. Scand J Gastroenterol 2005;40:980-4. http://dx.doi.org/10.1080/00365520510015845.
- Laine K, Määttä T, Varonen H, Mäkelä M. Diagnosing acute maxillary sinusitis in primary care: a comparison of ultrasound, clinical examination and radiography. Rhinology 1998;36:2-6.
- Landers A, Ryan B. The use of bedside ultrasound and community-based paracentesis in a palliative care service. J Prim Health Care 2014;6:148-51.
- Mäkelä M, Leinonen K. Diagnosis of maxillary sinusitis in Finnish primary care. Use of imaging techniques. Scand J Prim Health Care 1996;14:29-35. http://dx.doi.org/10.3109/02813439608997065.
- Thoomes-de Graaf M, Scholten-Peeters G, Duijn E, Karel Y, van den Borne MPJ, Beumer A, et al. Inter-professional agreement of ultrasound-based diagnoses in patients with shoulder pain between physical therapists and radiologists in the Netherlands. Man Ther 2014;19:478-83. http://dx.doi.org/10.1016/j.math.2014.04.018.
- Varonen H, Savolainen S, Kunnamo I, Heikkinen R, Revonta M. Acute rhinosinusitis in primary care: a comparison of symptoms, signs, ultrasound, and radiography. Rhinology 2003;41:37-43.
- Blanchet T, Thierry R. Obstacles a La Pratique De l’echographie Par Le Medecin Generaliste Au Cabinet: Etude Qualitative 2015.
- de la Figuera M, Fernández J, Fernández MI, Castelló M, Canadell J. Suitability and performance of echocardiogram in primary care. Aten Primaria 2012;44:190-8. http://dx.doi.org/10.1016/j.aprim.2011.03.007.
- Esquerra M, Roura Poch P, Masat Tico T, Canal V, Maideu Mir J, Cruxent R. Abdominal ultrasound: a diagnostic tool within the reach of general practitioners. Aten Primaria 2012;44:576-83. http://dx.doi.org/10.1016/j.aprim.2011.07.016.
- Evangelista L, Juncadella E, Copetti S, Pareja A, Torrabadella J, Evangelista A. Diagnostic usefulness of pocket echography performed in hypertensive patients by a general practitioner. Med Clin 2013;141:1-7. http://dx.doi.org/10.1016/j.medcli.2012.10.029.
- Gallagher J, Travers B, Ledwidge M, Weakliam D, Buckley A, White B, et al. Diagnosing heart failure: the role of a community outreach clinic. Eur J Heart Fail 2012;11.
- Hoyer C, Guenther S, Rost C, Stoerk S, Buck T, Erbel R, et al. Supra-regional success of a structured training program for general practitioners enabling performance of handheld echocardiography and point-of-care BNP testing and use for heart failure diagnosis. Eur J Heart Fail 2007;6. http://dx.doi.org/10.1016/s1567-4215(07)60061-7.
- Singh R, Fuat A, Brennen G, Murphy JJ. Providing added value to diagnosis and treatment of heart failure: six years on. The role of a hospital primary care physician specialist led heart failure clinic. Eur J Heart Fail 2009;8.
- Bono F, Campanini A. The METIS project for generalist ultrasonography. J Ultrasound 2007;10:168-74. http://dx.doi.org/10.1016/j.jus.2007.09.003.
- Colquhoun MC, Waine C, Monaghan MJ, Struthers AD, Mills PG. Investigation in general practice of patients with suspected heart failure: how should the essential echocardiographic service be delivered?. Br J Gen Pract 1995;45:517-19. http://dx.doi.org/10.1136/hrt.74.4.335.
- Doddy J. Using ultrasound in primary care. GP Online 2009. www.gponline.com/using-ultrasound-primary-care/article/876835 (accessed 30 April 2015).
- Hölscher T, Dunford JV, Schlachetzki F, Boy S, Hemmen T, Meyer BC, et al. Prehospital stroke diagnosis and treatment in ambulances and helicopters – a concept paper. Am J Emerg Med 2013;31:743-7. http://dx.doi.org/10.1016/j.ajem.2012.12.030.
- Hussain P, Deshpande A, Shridhar P, Saini G, Kay D. The feasibility of telemedicine for the training and supervision of general practitioners performing ultrasound examinations of patients with urinary tract symptoms. J Telemed Telecare 2004;10:180-2. http://dx.doi.org/10.1258/135763304323070850.
- Mjølstad OC, Snare SR, Folkvord L, Helland F, Grimsmo A, Torp H, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract 2012;29:534-40. http://dx.doi.org/10.1093/fampra/cms009.
- Ottenheijm RP, Joore MA, Walenkamp GHI, Weijers RE, Winkens B, Cals JW, et al. The Maastricht Ultrasound Shoulder pain trial (MUST): Ultrasound imaging as a diagnostic triage tool to improve management of patients with non-chronic shoulder pain in primary care. BMC Musculoskelet Disord 2011;12. http://dx.doi.org/10.1186/1471-2474-12-154.
- Senior R, Galasko G, McMurray JV, Mayet J. Screening for left ventricular dysfunction in the community: role of hand held echocardiography and brain natriuretic peptides. Heart 2003;89:24-8. http://dx.doi.org/10.1136/heart.89.suppl_3.iii24.
- van den Brink RB. Open access echocardiography: from high budget to low budget referral, but what about quality of patient care and delayed referral to the cardiologist?. Neth Heart J 2013;21:396-8. http://dx.doi.org/10.1007/s12471-013-0454-3.
- Vicente-Molinero Á, Aznar-Cantín S, Yáñez-Rodríguez F. Ecografía en Atención Primaria: estado de la cuestión. Semergen – Medicina De Familia 2009;35:58-61. http://dx.doi.org/10.1016/S1138-3593(09)70422-8.
- Xiao HB. Hand-held echocardiography for primary care. Br J Cardiol 2003;10:235-40.
- Ultrasound Training Recommendations for Medical and Surgical Specialties, Second Edition. London: RCR; 2012.
- Karaczun M, Smith T, Fleetcroft R, Stockl A. What Is the Application of Ultrasound Imaging in Primary Care for Musculoskeletal Disorders? n.d. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015015905 (accessed 27 August 2015).
- Any Qualified Provider Diagnostics Non-Obstetric Ultrasound (NOUS) Implementation Packs. London: Department of Health; 2013.
- Hewitt C, Gilbody S, Brealey S, Paulden M, Palmer S, Mann R, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13360.
- Davies A, Ariti C, Georghiou T, Bardsley M. Evaluation of Complex Health and Care Interventions using Retrospective Matched Control Methods. London: Nuffield Trust; 2015.
- Akhtar R, Wilson A. A comparison of spirometry in general practice and a pulmonary function laboratory. Prim Care Respir J 2005;14:215-20. http://dx.doi.org/10.1016/j.pcrj.2004.12.005.
- Backler KE, Leech L, Purdon M, Roberts D, Lord Z, Buttery RC. Direct access pulmonary function testing for primary care. Thorax 2011;66:A109-A10. http://dx.doi.org/10.1136/thoraxjnl-2011-201054c.105.
- Borg BM, Hartley MF, Fisher MT, Thompson BR. Spirometry training does not guarantee valid results. Respir Care 2010;55:689-94.
- Burgos F. Quality of forced spirometry in primary care, impact on the COPD treatment. Arch Bronconeumol 2011;47:224-5. http://dx.doi.org/10.1016/j.arbres.2011.03.001.
- Burri E, Hochholzer K, Arenja N, Martin-Braschler H, Kaestner L, Gekeler H, et al. B-type natriuretic peptide in the evaluation and management of dyspnoea in primary care. J Internal Med 2012;272:504-13. http://dx.doi.org/10.1111/j.1365-2796.2012.02552.x.
- Callister MEJ, Milton R, Darby M, Robertson RJ, Plant PK, Malhotra P, et al. Patient self-referral for chest X-ray to increase early detection of lung cancer in Leeds, United Kingdom. J Thorac Oncol 2011;6:S1153-S4.
- Carr R, Telford V, Waters G. Impact of an educational intervention on the quality of spirometry performance in a general practice: an audit. Prim Care Respir J 2011;20:210-3. http://dx.doi.org/10.4104/pcrj.2011.00006.
- Cawley MJ, Pacitti R, Warning W. Assessment of a pharmacist-driven point-of-care spirometry clinic within a primary care physicians office. Pharm Pract 2011;9:221-7. http://dx.doi.org/10.4321/S1886-36552011000400007.
- Chavannes N, Schermer T, Akkermans R, Jacobs JE, van de Graaf G, Bollen R, et al. Impact of spirometry on GPs’ diagnostic differentiation and decision-making. Respir Med 2004;98:1124-30. http://dx.doi.org/10.1016/j.rmed.2004.04.004.
- Denis J, Rauws J, Verschuur Veltman M, Smeele I. CASPIR course. Practical spirometry for primary care, a nation wide programme. Eur Respir J 2013;42.
- Diar Bakerly N, Roberts JA. Consultant-led specialist community COPD clinic: does it work?. Thorax 2009;64.
- Fois AG, Manca S, Ostera S, Zeminian D, Glisenti F, Meloni GM, et al. Telespirometry performed by general practitioners for early diagnosis of obstructive airway disease: our experience. Eur Respir J 2014;44.
- Harris M, Church S, Agarwal S. Validity of spirometry performed in the primary care setting. Eur Respir J 2012;40.
- Hassett R, Meade K, Partridge MR. Enhancing the accuracy of respiratory diagnoses in primary care: a report on the establishment of a community respiratory assessment unit. Prim Care Respir J 2006;15:354-61. http://dx.doi.org/10.1016/j.pcrj.2006.10.003.
- Jones R, Whittaker M, Hanney K, Shackell B. A pilot study of a mobile spirometry service in primary care. Prim Care Respir J 2005;14:169-71. http://dx.doi.org/10.1016/j.pcrj.2004.12.003.
- Kaplan A, Lerner J. Spirometry interpretation for primary care: a validation study. Eur Respir J 2013;42.
- Lau C, Barron A, McDonagh S, Screeche-Powell C, Francis DP, Mayet J, et al. A heart-failure led one-stop diagnostic service for breathlessness: Initial experiences and diagnostic yield. Eur Heart J 2014;35.
- Lodewijks-van der Bolt CL, Baur LH, Lenderink T, Guldemond F, Nijhof J, Winkens R, et al. The Dutch experience of open access echocardiography. Neth Heart J 2007;15:342-7. http://dx.doi.org/10.1007/BF03086012.
- Lusuardi M, De Benedetto F, Paggiaro P, Sanguinetti CM, Brazzola G, Ferri P, et al. A randomized controlled trial on office spirometry in asthma and COPD in standard general practice: data from spirometry in asthma and COPD: a comparative evaluation Italian study. Chest 2006;129:844-52. http://dx.doi.org/10.1378/chest.129.4.844.
- Masa JF, González MT, Pereira R, Mota M, Riesco JA, Corral J, et al. Validity of spirometry performed online. Eur Respir J 2011;37:911-18. http://dx.doi.org/10.1183/09031936.00011510.
- McNeill M, Sheehy M, Mc Donnell T. Spirometry outreach clinics for the midlands: to determine the feasibility of spirometry testing being provided in primary care centres by respiratory scientists. Ir J Med Sci 2012;181.
- Metting E, Riemersma R, Robbert S, Van Der Molen T, Janwillem K. Description of a Dutch well-established asthma/COPD service for primary care. Eur Respir J 2013;42.
- O’Herlihy J, O’Toole P, Gallagher J, McDonnell TJ. COPD satellite clinic. Ir J Med Sci 2013;182.
- Poels PJ, Schermer TR, Schellekens DP, Akkermans RP, de Vries Robbé PF, Kaplan A, et al. Impact of a spirometry expert system on general practitioners’ decision making. Eur Respir J 2008;31:84-92. http://dx.doi.org/10.1183/09031936.00012007.
- Punwani R, Draper A, Loke T, Keddie Z, O’Brien M. Community Pharmacy Referrals Project (CoPhaR): increasing awareness and early diagnosis of respiratory disease via a direct pathway to secondary care. Lung Cancer 2014;83:S31-S2. http://dx.doi.org/10.1016/S0169-5002(14)70084-4.
- Sallaway K, Rae R, Road JD, FitzGerald JM. The utility of a walk-in spirometry laboratory in the diagnosis of asthma and COPD. Am J Respir Crit Care Med 2011;183. http://dx.doi.org/10.1164/ajrccm-conference.2011.183.1_meetingabstracts.a1512.
- Thijssing L, van der Heijden JP, Chavannes NH, Melissant CF, Jaspers MWM, Witkamp L. Telepulmonology: effect on quality and efficiency of care. Respir Med 2014;108:314-18. http://dx.doi.org/10.1016/j.rmed.2013.10.017.
- Tomonaga Y, Gutzwiller F, Luscher TF, Riesen WF, Hug M, Diemand A, et al. Diagnostic accuracy of point-of-care testing for acute coronary syndromes, heart failure and thromboembolic events in primary care: a cluster-randomised controlled trial. BMC Fam Pract 2011;12. http://dx.doi.org/10.1186/1471-2296-12-12.
- van der Mark LB, van Wonderen KE, Mohrs J, van Aalderen WM, ter Riet G, Bindels PJ. Predicting asthma in preschool children at high risk presenting in primary care: development of a clinical asthma prediction score. Prim Care Respir J 2014;23:52-9. http://dx.doi.org/10.4104/pcrj.2014.00003.
- Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J 2006;28:945-52. http://dx.doi.org/10.1183/09031936.06.00019306.
- Willey V, Simon S, Akkineni S, Reinhold J, Kelly B, Kim E, et al. Preliminary evaluation of comprehensive pharmacist-led services to patients with respiratory diseases within a community-based medical home. J Am Pharm Assoc 2013;53.
- Wolfenden H, Bailey L, Murphy K, Partridge MR. Use of an open access spirometry service by general practitioners. Prim Care Respir J 2006;15:252-5. http://dx.doi.org/10.1016/j.pcrj.2006.05.007.
- Armstrong I, Rochnia N, Harries C, Bundock S, Yorke J. The trajectory to diagnosis with pulmonary arterial hypertension: a qualitative study. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2011-000806.
- Birt L, Hall N, Emery J, Banks J, Mills K, Johnson M, et al. Responding to symptoms suggestive of lung cancer: a qualitative interview study. BMJ Open Respir Res 2014;1. http://dx.doi.org/10.1136/bmjresp-2014-000067.
- Corner J, Hopkinson J, Fitzsimmons D, Barclay S, Muers M. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax 2005;60:314-19. http://dx.doi.org/10.1136/thx.2004.029264.
- Corner J, Hopkinson J, Roffe L. Experience of health changes and reasons for delay in seeking care: a UK study of the months prior to the diagnosis of lung cancer. Soc Sci Med 2006;62:1381-91. http://dx.doi.org/10.1016/j.socscimed.2005.08.012.
- Dennis SM, Zwar NA, Marks GB. Diagnosing asthma in adults in primary care: a qualitative study of Australian GPs’ experiences. Prim Care Respir J 2010;19:52-6. http://dx.doi.org/10.4104/pcrj.2009.00046.
- Goeman DP, Hogan CD, Aroni RA, Abramson MJ, Sawyer SM, Stewart K, et al. Barriers to delivering asthma care: a qualitative study of general practitioners. Med J Aust 2005;183:457-60.
- Tod AM, Craven J, Allmark P. Diagnostic delay in lung cancer: a qualitative study. J Adv Nurs 2008;61:336-43. http://dx.doi.org/10.1111/j.1365-2648.2007.04542.x.
- Walters JA, Hansen E, Mudge P, Johns DP, Walters EH, Wood-Baker R. Barriers to the use of spirometry in general practice. Aust Fam Physician 2005;34:1-3.
- Walters JA, Hansen EC, Walters EH, Wood-Baker R. Under-diagnosis of chronic obstructive pulmonary disease: a qualitative study in primary care. Respir Med 2008;102:738-43. http://dx.doi.org/10.1016/j.rmed.2007.12.008.
- Goeman D, Beanland C, George J, Douglass J. What role can the community nurse play in regard to asthma, COPD and dementia?. Respirology 2014;19.
- Boffin N, Van der Stighelen V, Paulus D, Van Royen P. Use of office spirometers in Flemish general practice: results of a telephone survey. Monaldi Arch Chest Dis 2006;65:128-32.
- Bolton CE, Ionescu AA, Edwards PH, Faulkner TA, Edwards SM, Shale DJ. Attaining a correct diagnosis of COPD in general practice. Respir Med 2005;99:493-500. http://dx.doi.org/10.1016/j.rmed.2004.09.015.
- Caramori G, Bettoncelli G, Tosatto R, Arpinelli F, Visonà G, Invernizzi G, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis 2005;63:6-12. http://dx.doi.org/10.4081/monaldi.2005.651.
- Halpin DM, O’Reilly JF, Connellan S, Rudolf M. Confidence and understanding among general practitioners and practice nurses in the UK about diagnosis and management of COPD. Respir Med 2007;101:2378-85. http://dx.doi.org/10.1016/j.rmed.2007.06.010.
- Andersen BL, Cacioppo JT. Delay in seeking a cancer diagnosis: delay stages and psychophysiological comparison processes. Br J Soc Psychol 1995;34:33-52. http://dx.doi.org/10.1111/j.2044-8309.1995.tb01047.x.
- Walter F, Webster A, Scott S, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy 2012;17:110-8. http://dx.doi.org/10.1258/jhsrp.2011.010113.
- Scott SE, Walter FM, Webster A, Sutton S, Emery J. The model of pathways to treatment: conceptualization and integration with existing theory. Br J Health Psychol 2013;18:45-6. http://dx.doi.org/10.1111/j.2044-8287.2012.02077.x.
- Five Year Forward View. Leeds: NHS England; 2014.
- Brown L, Carne A, Bywood P, McIntyre E, Damarell R, Lawrence M, et al. Facilitating access to evidence: Primary Health Care Search Filter. Health Information Libraries J 2014;31:293-302. http://dx.doi.org/10.1111/hir.12087.
- Davis A, Smith P. Adult hearing screening: health policy issues – what happens next?. Am J Audiol 2013;22:167-70. http://dx.doi.org/10.1044/1059-0889(2013/12-0062).
- Pronk M, Kramer SE, Davis AC, Stephens D, Smith PA, Thodi C, et al. Interventions following hearing screening in adults: a systematic descriptive review. Int J Audiol 2011;50:594-609. http://dx.doi.org/10.3109/14992027.2011.582165.
- Davis A, Smith PA, Booth M, Martin M. Diagnosing patients with age-related hearing loss and tinnitus: supporting gp clinical engagement through innovation and pathway redesign in audiology services. Int J Otolaryngol 2012;2012. http://dx.doi.org/10.1155/2012/290291.
- Krishnamurti S. Audiology service models in a family physician practice setting. Perspect Audiol 2010;6:24-32. http://dx.doi.org/10.1044/poa6.1.24.
- Whiteford CL, White S, Stephenson M. Effectiveness of nurse-led clinics on service delivery and clinical outcomes in adults with chronic ear, nose and throat complaints: a systematic review protocol. JBI Database System Rev Implement Rep 2013;11:23-37. http://dx.doi.org/10.11124/jbisrir-2013-1091.
- Gruen R, Weeramanthri T, Knight S, Bailie R. Specialist outreach clinics in primary care and rural hospital settings (Cochrane Review). Commun Eye Health 2006;19.
- Bowling A, Bond M. A national evaluation of specialists’ clinics in primary care settings. Br J Gen Pract 2001;51:264-9.
- Rose E. Audiology. Aust Fam Physician 2011;40:290-2.
- Pichichero ME, Poole MD. Comparison of performance by otolaryngologists, pediatricians, and general practioners on an otoendoscopic diagnostic video examination. Int J Pediatr Otorhinolaryngol 2005;69:361-6. http://dx.doi.org/10.1016/j.ijporl.2004.10.013.
- Royal College of Physicians . Specialty Spotlight – Audiovestibular Medicine n.d. www.rcplondon.ac.uk/education-practice/advice/specialty-spotlight-audiovestibular-medicine (accessed 1 August 2016).
- El-Shunnar SK, Hoare DJ, Smith S, Gander PE, Kang S, Fackrell K, et al. Primary care for tinnitus: practice and opinion among GPs in England. J Eval Clin Pract 2011;17:684-92. http://dx.doi.org/10.1111/j.1365-2753.2011.01696.x.
- Harkin H. A nurse-led ear care clinic: sharing knowledge and improving patient care. Br J Nurs 2005;14:250-4. http://dx.doi.org/10.12968/bjon.2005.14.5.17658.
- Gander PE, Hoare DJ, Collins L, Smith S, Hall DA. Tinnitus referral pathways within the National Health Service in England: a survey of their perceived effectiveness among audiology staff. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-162.
- Bentur N, Valinsky L, Lemberger J, Ben Moshe Y, Heymann AD. Primary care intervention programme to improve early detection of hearing loss in the elderly. J Laryngol Otol 2012;126:574-9. http://dx.doi.org/10.1017/S0022215112000072.
- Steinbach WJ, Sectish TC. Pediatric resident training in the diagnosis and treatment of acute otitis media. Pediatrics 2002;109:404-8. http://dx.doi.org/10.1542/peds.109.3.404.
- MacClements JE, Parchman M, Passmore C. Otitis media in children: use of diagnostic tools by family practice residents. Fam Med 2002;34:598-603.
- Kinshuck AD, Derbyshire SG, Wickham M, Lesser THJ. Current Demand for ENT Outpatient Services in the UK 2010. www.priory.com/family_medicine/ENT_services.htm (accessed 1 August 2016).
- Ayshford CA, Johnson AP, Chitnis JG. What is the value of ENT specialist outreach clinics?. J Laryngol Otol 2001;115:441-3. http://dx.doi.org/10.1258/0022215011907965.
- Foust T, Eiserman W, Shisler L, Geroso A. Using otoacoustic emissions to screen young children for hearing loss in primary care settings. Pediatrics 2013;132:118-23. http://dx.doi.org/10.1542/peds.2012-3868.
- Muderris T, Yazıcı A, Bercin S, Yalçıner G, Sevil E, Kırıs M. Consumer acoustic reflectometry: accuracy in diagnosis of otitis media with effusion in children. Int J Pediatr Otorhinolaryngol 2013;77:1771-4. http://dx.doi.org/10.1016/j.ijporl.2013.08.019.
- Anon . Wyg Supports £124 M Lift Project 2006. www.Hdmagazine.Co.Uk/Story.Asp?Storycode=2035925.
- Earis H, Reynolds S. Deaf and Hard-of-Hearing People’s Access to Primary Health Care Services in North East Essex. NHS North East Essex; 2009.
- Henry JA, Zaugg TL, Myers PJ, Kendall CJ, Michaelides EM. A triage guide for tinnitus. J Fam Pract 2010;59:389-93.
- Hofstetter PJ, Kokesh J, Ferguson AS, Hood LJ. The impact of telehealth on wait time for ENT specialty care. Telemed J E Health 2010;16:551-6. http://dx.doi.org/10.1089/tmj.2009.0142.
- Operational Research Modelling: Transferring ENT/Audiology Services into a Community Setting (Shine 2012 Final Report). The Health Foundation; 2014.
- Improving Access to Audiology Services in England. London: Department of Health; 2007.
- Audiology Pilot Sites. London: NHS Improvement; 1900.
- NHS Improvement . Shaping the Future: Strengthening the Evidence to Transform Audiology Services 2011.
- Siemens Hearing Aids . HearCheck Screener n.d. www.bestsound-technology.co.uk/nhs/equipment/hear-check/ (accessed 2 August 2016).
- Chime Social Enterprise . Welcome to Chime Social Enterprise – Audiology Services n.d. www.chimehealth.co.uk (accessed 2 August 2016).
- Campbell C. Now hear this: the case for community based hearing care. Health Service Journal n.d. www.hsj.co.uk/sectors/commissioning/audiology-today/now-hear-this-the-case-for-community-based-hearing-care/5066966.article (accessed 14 January 2014).
- McKnight T. Why patients prefer community based services. Health Service Journal n.d. www.hsj.co.uk/sectors/commissioning/audiology-today/why-patients-prefer-community-based-services/5070182.article (accessed 28 April 2014).
- National Service Framework for Coronary Heart Disease. London: Department of Health; 2000.
- National Service Framework for Coronary Heart Disease. London: Department of Health; 2005.
- Fahey T, Schroeder K. Cardiology. Br J Gen Pract 2004;54:695-702.
- Petersen S, Rayner M, Wolstenholme J. Coronary Heart Disease Statistics: Heart Failure Supplement; 2002 Edition; British Heart Foundation Statistics Database 2002. London: British Heart Foundation; 2010.
- Scarborough P, Bhatnagar P, Wickramasinghe K, Smolina K, Mitchell C, Rayner M. Coronary Heart Disease Statistics 2010 Edition. London: British Heart Foundation; 2010.
- Murphy JJ, Chakraborty RR, Fuat A, Davies MK, Cleland JG. Diagnosis and management of patients with heart failure in England. Clin Med 2008;8:264-6. http://dx.doi.org/10.7861/clinmedicine.8-3-264.
- Hill SA, Balion CM, Santaguida P, McQueen MJ, Ismaila AS, Reichert SM, et al. Evidence for the use of B-type natriuretic peptides for screening asymptomatic populations and for diagnosis in primary care. Clin Biochem 2008;41:240-9. http://dx.doi.org/10.1016/j.clinbiochem.2007.08.016.
- Scott MA, Price CP, Cowie MR, Buxton MJ, Scott MA. Cost-consequences analysis of natriuretic peptide assays to refute symptomatic heart failure in primary care. Br J Cardiol 2008;15:199-206.
- Gabhainn SN, Murphy AW, Kelleher C. A national general practice census: characteristics of rural general practices. Fam Pract 2001;18:622-6. http://dx.doi.org/10.1093/fampra/18.6.622.
- Doliwa PS, Frykman V, Rosenqvist M. Short-term ECG for out of hospital detection of silent atrial fibrillation episodes. Scand Cardiovasc J 2009;43:163-8. http://dx.doi.org/10.1080/14017430802593435.
- Somerville S, Somerville J, Croft P, Lewis M. Atrial fibrillation: a comparison of methods to identify cases in general practice. Br J Gen Pract 2000;50:727-9.
- Kearley K, Selwood M, Van den Bruel A, Thompson M, Mant D, Hobbs FR, et al. Triage tests for identifying atrial fibrillation in primary care: a diagnostic accuracy study comparing single-lead ECG and modified BP monitors. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004565.
- Diagnostic Technology: Handheld HeartScan ECG Monitor for Detecting Atrial Fibrillation in Primary Care. Oxford: MaDOx; 2009.
- Chronic Heart Failure. National Clinical Guidelines for Diagnosis and Management in Primary and Secondary Care. London: NICE; 2003.
- Hackett D. Cardiac Workforce Requirements in the UK. London: British Cardiac Society; 2005.
- Galasko GI, Lahiri A, Senior R. Portable echocardiography: an innovative tool in screening for cardiac abnormalities in the community. Eur J Echocardiogr 2003;4:119-27. http://dx.doi.org/10.1053/euje.4.2.119.
- Kanavos P, van den Aardweg S, Schurer W. Diabetes Expenditure, Burden of Disease and Management in 5 EU Countries. London: London School of Economics Health and Social Care; 2012.
- World Health Organization . Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation 2011.
- Plüddemann A, Price CP, Thompson M, Wolstenholme J, Heneghan C. Primary care diagnostic technology update: point-of-care testing for glycosylated haemoglobin. Br J Gen Pract 2011;61:139-40. http://dx.doi.org/10.3399/bjgp11X556290.
- Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The Diabetes Care From Diagnosis Research Team. BMJ 1998;317:1202-8. http://dx.doi.org/10.1136/bmj.317.7167.1202.
- St John A, Davis TM, Goodall I, Townsend MA, Price CP. Nurse-based evaluation of point-of-care assays for glycated haemoglobin. Clin Chim Acta 2006;365:257-63. http://dx.doi.org/10.1016/j.cca.2005.09.003.
- Howse JH, Jones S, Hungin AP. Screening and identifying diabetes in optometric practice: a prospective study. Br J Gen Pract 2011;61:436-42. http://dx.doi.org/10.3399/bjgp11X583227.
- Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem 2010;56:44-52. http://dx.doi.org/10.1373/clinchem.2009.130641.
- Kennedy L, Herman WH, Strange P, Harris A. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabet Care 2006;29:1-8. http://dx.doi.org/10.2337/diacare.29.01.06.dc05-1058.
- Khunti K, Stone MA, Burden AC, Turner D, Raymond NT, Burden M, et al. Randomised controlled trial of near-patient testing for glycated haemoglobin in people with type 2 diabetes mellitus. Br J Gen Pract 2006;56:511-17.
- Bubner TK, Laurence CO, Gialamas A, Yelland LN, Ryan P, Willson KJ, et al. Effectiveness of point-of-care testing for therapeutic control of chronic conditions: results from the PoCT in General Practice Trial. Med J Aust 2009;190:624-6.
- National Service Framework for Diabetes: Standards. London: Department of Health; 2001.
- Weqas . Wales External Quality Assessment Scheme 2016. www.weqas.com (accessed 2 August 2016).
- Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11170.
- Brown JB, Harris SB, Webster-Bogaert S, Porter S. Point-of-care testing in diabetes management: what role does it play?. Diabetes Spectr 2004;17:244-8. http://dx.doi.org/10.2337/diaspect.17.4.244.
- Levetan CS, Dawn KR, Robbins DC, Ratner RE. Impact of computer-generated personalized goals on HbA(1c). Diabetes Care 2002;25:2-8. http://dx.doi.org/10.2337/diacare.25.1.2.
- Laurence C, Gialamas A, Yelland L, Bubner T, Ryan P, Willson K, et al. A pragmatic cluster randomised controlled trial to evaluate the safety, clinical effectiveness, cost effectiveness and satisfaction with point of care testing in a general practice setting – rationale, design and baseline characteristics. Trials 2008;9. http://dx.doi.org/10.1186/1745-6215-9-50.
- Eborall H, Davies R, Kinmonth AL, Griffin S, Lawton J. Patients’ experiences of screening for type 2 diabetes: prospective qualitative study embedded in the ADDITION (Cambridge) randomised controlled trial. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39308.392176.BE.
- Guidance and Competences for The Provision Of Services Using Practitioners With Special Interests (PwSIs): Adult Endoscopy (Upper & Lower GI). London: RCGP, Department of Health; n.d.
- Acute Upper Gastrointestinal Bleeding: Management. London: Royal College of Physicians; 2012.
- Williams JG, Roberts SE, Ali MF, Cheung WY, Cohen DR, Demery G, et al. Gastroenterology services in the UK. The burden of disease, and the organisation and delivery of services for gastrointestinal and liver disorders: a review of the evidence. Gut 2007;56:1-113. http://dx.doi.org/10.1136/gut.2006.117598.
- EGD, Training and Credentialing of Family Physicians in (Position Paper). American Academy of Family Physicians; 2015.
- Mulder CJ, Jacobs MA, Leicester RJ, Nageshwar RD, Shepherd LE, Axon AT, et al. Guidelines for designing a digestive disease endoscopy unit: report of the World Endoscopy Organization. Dig Endosc 2013;25:365-75. http://dx.doi.org/10.1111/den.12126.
- O’Dowd A. GPs should be freed up to make more endoscopy referrals, cancer charity says. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g3602.
- O’Riordan M, Doran G, Collins C. Access to diagnostics in primary care and the impact on a primary care led health service. Ir Med J 2015;108:53-5.
- Wolf CR, Smith G, Smith RL. Science, medicine, and the future: pharmacogenetics. BMJ 2000;320. http://dx.doi.org/10.1136/bmj.320.7240.987.
- Rafi I, Qureshi N, Lucassen A, Modell M, Elmslie F, Kai J, et al. ‘Over-the-counter’ genetic testing: what does it really mean for primary care?. Br J Gen Pract 2009;59:283-7. http://dx.doi.org/10.3399/bjgp09X395021.
- Royal College of General Practitioners . RCGP Curriculum: 3.02 The Clinical Example On Genetics in Primary Care 2014.
- Andermann A, Blancquaert I. Genetic screening: a primer for primary care. Can Fam Physician 2010;56:333-9.
- Jackson L, Goldsmith L, Skirton H. Guidance for patients considering direct-to-consumer genetic testing and health professionals involved in their care: development of a practical decision tool. Fam Pract 2014;31:341-8. http://dx.doi.org/10.1093/fampra/cmt087.
- Saukko PM, Ellard S, Richards SH, Shepherd MH, Campbell JL. Patients’ understanding of genetic susceptibility testing in mainstream medicine: qualitative study on thrombophilia. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-82.
- Murray E, Pollack L, White M, Lo B. Clinical decision-making: physicians’ preferences and experiences. BMC Fam Pract 2007;8. http://dx.doi.org/10.1186/1471-2296-8-10.
- Myers MF, Chang MH, Jorgensen C, Whitworth W, Kassim S, Litch JA, et al. Genetic testing for susceptibility to breast and ovarian cancer: evaluating the impact of a direct-to-consumer marketing campaign on physicians’ knowledge and practices. Genet Med 2006;8:361-70. http://dx.doi.org/10.1097/01.gim.0000223544.68475.6c.
- Emery J, Walton R, Murphy M, Austoker J, Yudkin P, Chapman C, et al. Computer support for interpreting family histories of breast and ovarian cancer in primary care: comparative study with simulated cases. BMJ 2000;321:28-32. http://dx.doi.org/10.1136/bmj.321.7252.28.
- McKelvey KD, Evans JP. Cancer genetics in primary care. J Nutr 2003;133:3767S-72S.
- Holtzman NA, Marteau TM. Will genetics revolutionize medicine?. New Engl J Med 2000;343. http://dx.doi.org/10.1056/NEJM200007133430213.
- American College of Preventive Medicine . Genetic Testing Time Tool: A Resource from the American College of Preventive Medicine 2010. www.acpm.org/?GeneticTestTTClinic (accessed 6 December 2016).
- Carroll JC, Cappelli M, Miller F, Wilson BJ, Grunfeld E, Peeters C, et al. Genetic services for hereditary breast/ovarian and colorectal cancers – physicians’ awareness, use and satisfaction. Community Genet 2008;11:43-51. http://dx.doi.org/10.1159/000111639.
- Javitt GH. Policy implications of genetic testing: not just for geneticists anymore. Adv Chronic Kidney Dis 2006;13:178-82. http://dx.doi.org/10.1053/j.ackd.2006.01.003.
- Brandt R, Ali Z, Sabel A, McHugh T, Gilman P. Cancer genetics evaluation: barriers to and improvements for referral. Genet Test 2008;12:9-12. http://dx.doi.org/10.1089/gte.2007.0036.
- Our Health, Our Care, Our Say: A New Direction for Community Services. London: The Stationery Office; 2006.
- Making the Best Use of a Department of Radiology. London: RCR; 2007.
- Standards for the Communication of Critical, Urgent and Unexpected Significant Radiological Findings. London: RCR; 2008.
- Safety Guidelines for Magnetic Resonance Imaging Equipment in Clinical Use – MHRA Device Bulletin 2007. London: MHRA; 2007.
- Department of Health . Health Building Note 11-01: Facilities for Primary and Community Care Services 2013.
- Brealey SD. Influence of magnetic resonance of the knee on GPs’ decisions: a randomised trial. Br J Gen Pract 2007;57:622-9.
- Global Information . The World Market for Point of Care (POC) Diagnostics 2015 2015. www.giiresearch.com/report/kl253350-world-market-point-care-diagnostics.html (accessed 21 December 2015).
- Deo S, Sohoni MG. Optimal decentralization of early infant diagnosis of HIV in resource-limited settings. MASOM 2015;17:191-207. http://dx.doi.org/10.1287/msom.2014.0512.
- Fitzmaurice DA, Hobbs FDR, Murray ET, Holder RL, Allan TF, Rose PE. Randomised controlled trial of oral anticoagulation using computerised decision support (CDSS) and near patient testing (NPT). Arch Intern Med 2000;160:2343-8. http://dx.doi.org/10.1001/archinte.160.15.2343.
- Aabenhus R, Jensen JU. Procalcitonin-guided antibiotic treatment of respiratory tract infections in a primary care setting: are we there yet?. Prim Care Respir J 2011;20:360-7. http://dx.doi.org/10.4104/pcrj.2011.00064.
- Schuetz P, Muller B, Christ-Crain M, Stolz D, Tamm M, Bouadma L, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;9. http://dx.doi.org/10.1002/14651858.cd007498.pub2.
- Gialamas A, St John A, Laurence CO, Bubner TK. Point-of-care testing for patients with diabetes, hyperlipidaemia or coagulation disorders in the general practice setting: a systematic review. Fam Pract 2010;27:17-24. http://dx.doi.org/10.1093/fampra/cmp084.
- Poller L, Keown M, Chauhan N, van den Besselaar AMHP, Tripodi A, Shiach C, et al. Reliability of international normalised ratios from two point of care test systems: comparison with conventional methods. BMJ 2003;327. http://dx.doi.org/10.1136/bmj.327.7405.30.
- Shiach CR, Campbell B, Poller L, Keown M, Chauhan N. Reliability of point of care prothrombin time testing in a community clinic: a randomized crossover comparison with hospital laboratory testing. Br J Haematol 2002;119:370-5. http://dx.doi.org/10.1046/j.1365-2141.2002.03888.x.
- Plüddemann A, Thompson M, Price CP, Wolstenholme J, Heneghan C. The D-dimer test in combination with a decision rule for ruling out deep vein thrombosis in primary care: diagnostic technology update. Br J Gen Pract 2012;62:e393-5. http://dx.doi.org/10.3399/bjgp12X641645.
- Wood F, Brookes-Howell L, Hood K, Cooper L, Verheij T, Goossens H, et al. A multi-country qualitative study of clinicians’ and patients’ views on point of care tests for lower respiratory tract infection. Fam Pract 2011;28:661-9. http://dx.doi.org/10.1093/fampra/cmr031.
- Cooke J, Butler C, Hopstaken R, Dryden MS, McNulty C, Hurding S, et al. Narrative review of primary care point-of-care testing (POCT) and antibacterial use in respiratory tract infection (RTI). BMJ Open Respir Res 2015;2. http://dx.doi.org/10.1136/bmjresp-2015-000086.
- Stone MA, Burden AC, Burden M, Baker R, Khunti K. Near patient testing for glycated haemoglobin in people with type 2 diabetes mellitus managed in primary care: acceptability and satisfaction. Diabet Med 2007;24:792-5. http://dx.doi.org/10.1111/j.1464-5491.2007.02175.x.
- Shephard MD. Cultural and clinical effectiveness of the ‘QAAMS’ point-of-care testing model for diabetes management in Australian Aboriginal medical services. Clin Biochem Rev 2006;27:161-70.
- Chaudhry R, Scheitel SM, Stroebel RJ, Santrach PJ, Dupras DM, Tangalos EG. Patient satisfaction with point-of-care international normalized ratio testing and counseling in a community internal medicine practice. Manag Care Interface 2004;17:44-6.
- Cherryman G. Imaging in primary care. Br J Gen Pract 2006;56:563-4.
- Health and Social Care Act 2012: Chapter 7, Explanatory Notes. London: The Stationery Office; 2012.
- Ionising Radiation (Medical Exposure) Regulations 2000 (Statutory Instrument 2000 No. 1059). London: The Stationery Office; 2000.
- Yelland M. Diagnostic imaging for back pain. Aust Fam Physician 2004;33:415-9.
- White PM, Halliday-Pegg JC, Collie DA. Open access neuroimaging for general practitioners – diagnostic yield and influence on patient management. Br J Gen Pract 2002;52:33-5.
- Speets AM, van der Graaf Y, Hoes AW, Kalmijn S, Sachs AP, Rutten MJ, et al. Chest radiography in general practice: indications, diagnostic yield and consequences for patient management. Br J Gen Pract 2006;56:574-8.
- Royal College of General Practitioners . Quality Imaging Services for Primary Care: A Good Practice Guide 2013. www.rcgp.org.uk/news/∼/media/Files/CfC/RCGP-Quality-imaging-services-for-Primary-Care.ashx (accessed 4 January 2016).
- O’Riordan M, Collins C, Doran G. Access to Diagnostics: A Key Enabler for a Primary Care Led Health Service 2013. www.lenus.ie/hse/bitstream/10147/292726/1/Access%20to%20diagnostics%202013.pdf (accessed 19 October 2016).
- Sibbald B. Direct access to diagnostic services. Br J Gen Pract 2009;59:e144-e5. http://dx.doi.org/10.3399/bjgp09X420563.
- Roland M, McDonald R, Sibbald B, Boyd A, Fotaki M, Gravelle H, et al. Outpatient Services and Primary Care. A Scoping Review of Research into Strategies for Improving Outpatient Effectiveness and Efficiency. Manchester: National Primary Care Research and Development Centre; 2006.
- Simpson GC, Forbes K, Teasdale E, Tyagi A, Santosh C. Impact of GP direct-access computerised tomography for the investigation of chronic daily headache. Br J Gen Pract 2010;60:897-901. http://dx.doi.org/10.3399/bjgp10X544069.
- DAMASK (Direct Access to Magnetic Resonance Imaging: Assessment for Suspect Knees) Trial Team . Effectiveness of GP access to magnetic resonance imaging of the knee: a randomised trial. Br J Gen Pract 2008;58:e1-8. http://dx.doi.org/10.3399/bjgp08X342651.
- An Outcomes Strategy for People with Asthma and COPD. London: Department of Health; 2011.
- An Outcomes Strategy for COPD and Asthma: NHS Companion Document. London: Department of Health; 2012.
- Healthcare Commission . Clearing the Air: National Study of Chronic Obstructive Pulmonary Disease 2006.
- Harrison B, Stephenson P, Mohan G, Nasser S. An ongoing Confidential Enquiry into asthma deaths in the Eastern Region of the UK, 2001-2003. Prim Care Respir J 2005;14:303-13. http://dx.doi.org/10.1016/j.pcrj.2005.08.004.
- Feiner JR, Severinghaus JW, Bickler PE. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: the effects of oximeter probe type and gender. Anesth Analg 2007;105:18-23. http://dx.doi.org/10.1213/01.ane.0000285988.35174.d9.
- Gruffydd-Jones K, Small I, Fletcher M, Bryant T. The Primary Care Respiratory Society-UK Quality Award: development and piloting of quality standards for primary care respiratory medicine. Prim Care Respir J 2013;22:353-9. http://dx.doi.org/10.4104/pcrj.2013.00065.
- Plüddemann A, Thompson M, Heneghan C, Price C. Pulse oximetry in primary care: primary care diagnostic technology update. Br J Gen Pract 2011;61:358-9. http://dx.doi.org/10.3399/bjgp11X572553.
- Marks GB. Evaluation of patients with symptoms of chronic lung disease in primary care. Prim Care Respir J 2013;22:145-7. http://dx.doi.org/10.4104/pcrj.2013.00054.
- Harris SKA. The Experiences and Monitoring of People Living With Chronic Obstructive Pulmonary Disease and Utilising Long-Term Oxygen Therapy 2012.
- Vaughn R, Carter R, Duncan M. An outreach spirometry service for Greater Glasgow health board: does it help in the diagnosis. Eur Respir J 2004;24.
- Enright P. Does screening for COPD by primary care physicians have the potential to cause more harm than good?. Chest 2006;129:833-5. http://dx.doi.org/10.1378/chest.129.4.833.
- Strong M, Green A, Goyder E, Miles G, Lee AC, Basran G, et al. Accuracy of diagnosis and classification of COPD in primary and specialist nurse-led respiratory care in Rotherham, UK: a cross-sectional study. Prim Care Respir J 2014;23:67-73. http://dx.doi.org/10.4104/pcrj.2014.00005.
- Parkes G, Plüddemann A, Heneghan C, Price CP, Wolstenholme J, Thompson M. Spirometry in primary care for case finding and management of chronic obstructive pulmonary disease primary care diagnostic technology update. Br J Gen Pract 2011;61:698-9. http://dx.doi.org/10.3399/bjgp11X606799.
- Wilt TJ, Niewoehner D, Kim C, Kane RL, Linabery A, Tacklind J, et al. Use of Spirometry for Case Finding, Diagnosis, and Management of Chronic Obstructive Pulmonary Disease (COPD). Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- Lin K, Watkins B, Johnson T, Rodriguez JA, Barton MB. Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2008;148:535-43. http://dx.doi.org/10.7326/0003-4819-148-7-200804010-00213.
- Haroon SM, Adab PA, Jordan RE. Case finding for COPD in primary care: a systematic review. Prim Care Respir J 2012;21:354-7. http://dx.doi.org/10.4104/pcrj.2012.00060.
- Bunker J, Hermiz O, Zwar N, Dennis SM, Vagholkar S, Crockett A, et al. Feasibility and efficacy of COPD case finding by practice nurses. Aust Fam Physician 2009;38:826-30.
- Association for Respiratory Technology and Physiology . A Guide To Performing Quality Assured Diagnostic Spirometry 2013. www.artp.org.uk/en/professional/artp-standards/index.cfm/QADS%20Apr%202013 (accessed 19 October 2016).
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. http://dx.doi.org/10.1183/09031936.05.00034805.
- National Institute for Health and Care Excellence . Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management n.d. www.nice.org.uk/guidance/cg101/chapter/1-Guidance (accessed 19 October 2016).
- NHS Information Centre . Indicators for Quality Improvement: Full Indicator List 2012.
- Cartright S. An Evaluation of Screening for COPD Against the National Screening Committee Criteria 2012.
- Smith SF, Roberts NJ, Partridge MR. Comparison of a web-based package with tutor-based methods of teaching respiratory medicine: subjective and objective evaluations. BMC Med Educ 2007;7. http://dx.doi.org/10.1186/1472-6920-7-41.
- Levy ML, Quanjer PH, Booker R, Cooper BG, Holmes S, Small I. General Practice Airways Group. Diagnostic spirometry in primary care: proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations-a General Practice Airways Group (GPIAG) document, in association with the Association for Respiratory Technology & Physiology (ARTP) and Education for Health. Prim Care Respir J 2009;18:130-47. http://dx.doi.org/10.4104/pcrj.2009.00054.
- Barr RG, Stemple KJ, Mesia-Vela S, Basner RC, Derk SJ, Henneberger PK, et al. Reproducibility and validity of a handheld spirometer. Respir Care 2008;53:433-41.
- Gibbons D. A comprehensive guide to buying a spirometer. Prof Nurse 2005;20:45-8.
- Spirometry in Primary Care for COPD Case Finding, Self-Monitoring (Horizon Scan Report 0015) and Remote Technology. Oxford: MaDOx; 2011.
- Schermer TR, Verweij EH, Cretier R, Pellegrino A, Crockett AJ, Poels PJ. Accuracy and precision of desktop spirometers in general practices. Respiration 2012;83:344-52. http://dx.doi.org/10.1159/000334320.
- International Primary Care Respiratory Group . Opinion No 1 GPIAG Opinion No 7 (v2) Spirometry 2004.
- Salas T, Rubies C, Gallego C, Muñoz P, Burgos F, Escarrabill J. Technical requirements of spirometers in the strategy for guaranteeing the access to quality spirometry. Arch Bronconeumol 2011;47:466-9. http://dx.doi.org/10.1016/j.arbres.2011.06.005.
- Petty TL, Doherty DE. The National Lung Health Education Program: roots, mission, future directions. Respir Care 2004;49:678-83.
- Buffels J, Degryse J, Liistro G. Diagnostic certainty, co-morbidity and medication in a primary care population with presumed airway obstruction: the DIDASCO2 study. Prim Care Respir J 2009;18:34-40. http://dx.doi.org/10.3132/pcrj.2008.00047.
- Cranston JM, Crockett AJ, Moss JR, Pegram RW, Stocks NP. Models of chronic disease management in primary care for patients with mild-to-moderate asthma or COPD: a narrative review. Med J Aust 2008;188.
- Petty TL. Benefits of and barriers to the widespread use of spirometry. Curr Opin Pulm Med 2005;11:115-20. http://dx.doi.org/10.1097/01.mcp.0000150948.13034.78.
- Moore PL. Practice management and chronic obstructive pulmonary disease in primary care. Am J Med 2007;120:S23-S7. http://dx.doi.org/10.1016/j.amjmed.2007.04.009.
- Barton C, Proudfoot J, Amoroso C, Ramsay E, Holton C, Bubner T, et al. Management of asthma in Australian general practice: care is still not in line with clinical practice guidelines. Prim Care Respir J 2009;18:100-5. http://dx.doi.org/10.3132/pcrj.2008.00059.
- Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009;374:721-32. http://dx.doi.org/10.1016/S0140-6736(09)61290-3.
- Wilt TJ, Niewoehner D, Kane RL, MacDonald R, Joseph AM. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine Tob Res 2007;9:21-32. http://dx.doi.org/10.1080/14622200601078509.
- Marina N, Bayón JC, López de Santa María E, Gutiérrez A, Inchausti M, Bustamante V, et al. Economic assessment and budgetary impact of a telemedicine procedure and spirometry quality control in the primary care setting. Arch Bronconeumol 2016;52:24-8. http://dx.doi.org/10.1016/j.arbres.2015.02.012.
- Anderson S. Office spirometry: don’t just blow it off. Med Econ 2004;81:63-5.
- Laerum F, Eik-Nes S, Fonnebo V, Heilo A, Johnsen R, Stray-Pedersen B, et al. Use of Ultrasonography in the Primary Health Care Setting. Oslo: Norwegian Knowledge Centre for the Health Services (NOKC); 2001.
- Wise J. Everyone’s a radiologist now. BMJ 2008;336:1041-3. http://dx.doi.org/10.1136/bmj.39560.444468.AD.
- Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician 2012;58:e172-8.
- Kane D, Grassi W, Sturrock R, Balint PV. Musculoskeletal ultrasound – a state of the art review in rheumatology. Part 2: Clinical indications for musculoskeletal ultrasound in rheumatology. Rheumatology 2004;43:829-38. http://dx.doi.org/10.1093/rheumatology/keh215.
- Anon . ‘Ultrasound on demand’ – how will it work in the UK?. Br J Cardiol 2015;22:89-90.
- RCR . Standards for the Provision of an Ultrasound Service 2014.
- Grant L, Appleby J, Griffin N, Adam A, Gishen P. Facing the future: the effects of the impending financial drought on NHS finances and how UK radiology services can contribute to expected efficiency savings. Br J Radiol 2014.
- Nicholson BD, Thompson M, Price CP, Heneghan C, Plüddemann A. Home-use faecal immunochemical testing: primary care diagnostic technology update. Br J Gen Pract 2015;65:156-8. http://dx.doi.org/10.3399/bjgp15X684229.
- Wang K, Gill P, Wolstenholme J, Price CP, Heneghan C, Thompson M, et al. Non-contact infrared thermometers for measuring temperature in children: primary care diagnostic technology update. Br J Gen Pract 2014;64:e681-3. http://dx.doi.org/10.3399/bjgp14X682045.
- Goel RM, Patel KV, Borrow D, Anderson S. Video capsule endoscopy for the investigation of the small bowel: primary care diagnostic technology update. Br J Gen Pract 2014;64:154-6. http://dx.doi.org/10.3399/bjgp14X677680.
- Mahtani KR, Miller A, Rivero-Arias O, Heneghan C, Price CP, Thompson M, et al. Autoimmune markers for the diagnosis of rheumatoid arthritis in primary care: primary care diagnostic technology update. Br J Gen Pract 2013;63:553-4. http://dx.doi.org/10.3399/bjgp13X673919.
- Khangura J, Van den Bruel A, Perera R, Heneghan C, Price CP, Wolstenholme J, et al. Point-of-care testing for coeliac disease: primary care diagnostic technology update. Br J Gen Pract 2013;63:e426-8. http://dx.doi.org/10.3399/bjgp13X668401.
- Plüddemann A, Thompson M, Wolstenholme J, Price CP, Heneghan C. Point-of-care INR coagulometers for self-management of oral anticoagulation: primary care diagnostic technology update. Br J Gen Pract 2012;62:e798-800. http://dx.doi.org/10.3399/bjgp12X658476.
- Plüddemann A, Thompson M, Price CP, Wolstenholme J, Heneghan C. Point-of-care testing for the analysis of lipid panels: primary care diagnostic technology update. Br J Gen Pract 2012;62:e224-6. http://dx.doi.org/10.3399/bjgp12X630241.
- Plüddemann A, Heneghan C, Price CP, Wolstenholme J, Thompson M. Point-of-care blood test for ketones in patients with diabetes: primary care diagnostic technology update. Br J Gen Pract 2011;61:530-1. http://dx.doi.org/10.3399/bjgp11X588600.
- Plüddemann A, Heneghan C, Thompson M, Wolstenholme J, Price CP. Dermoscopy for the diagnosis of melanoma: primary care diagnostic technology update. Br J Gen Pract 2011;61:416-17. http://dx.doi.org/10.3399/bjgp11X578142.
Appendix 1 Search strategies for literature mapping exercise
Search strategies/filters
Reviews strategy
-
primary care.tw.
-
general practi$.tw.
-
primary health care.tw.
-
Community Mental Health Services/
-
Family Practice/
-
Home Care Services/
-
Physicians, Family/
-
Community Health Services/
-
Community Health Nursing/
-
Community Pharmacy Services/
-
Community Health Workers/
-
Preventive Health Services/
-
or/1-12 Primary Care filter
-
*Diagnostic Services/
-
*Clinical Laboratory Services/
-
*Genetic Testing/
-
*Mobile Health Units/
-
diagnostic service$.ti,ab.
-
clinical laboratory service$.ti,ab.
-
genetic test$.ti,ab.
-
mobile health$ unit$.ti,ab.
-
mobile health$ clinic$.ti,ab.
-
(point of care testing or point-of-care testing or POCT or near patient testing or near-patient testing).ti,ab.
-
diagnos$.ti,ab.
-
test$.ti,ab.
-
24 or 25
-
26 and primary care.tw.
-
or/14-23,27 Diagnostics terms
-
13 and 28
-
Meta-Analysis as Topic/
-
meta analy$.tw.
-
metaanaly$.tw.
-
Meta-Analysis/
-
(systematic adj (review$1 or overview$1)).tw.
-
exp Review Literature as Topic/
-
(review adj3 literature).ti,ab.
-
or/30-36
-
cochrane.ab.
-
embase.ab.
-
(psychlit or psyclit).ab.
-
(psychinfo or psycinfo).ab.
-
(cinahl or cinhal).ab.
-
science citation index.ab.
-
bids.ab.
-
cancerlit.ab.
-
or/38-45
-
reference list$.ab.
-
bibliograph$.ab.
-
hand-search$.ab.
-
relevant journals.ab.
-
manual search$.ab.
-
or/47-51
-
Review/
-
Comment/
-
Letter/
-
Editorial/
-
animal/
-
human/
-
57 not (57 and 58)
-
or/53-56,59
-
37 or 46 or 52 or 53
-
61 not 60 Reviews filter
-
29 and 62
-
limit 63 to yr=“2000 –Current” Date limit
Comparative studies strategy
-
primary care.tw.
-
general practi$.tw.
-
primary health care.tw.
-
Community Mental Health Services/
-
Family Practice/
-
Home Care Services/
-
Physicians, Family/
-
Community Health Services/
-
Community Health Nursing/
-
Community Pharmacy Services/
-
Community Health Workers/
-
Preventive Health Services/
-
or/1-12 Primary Care filter
-
*Diagnostic Services/
-
*Clinical Laboratory Services/
-
*Genetic Testing/
-
*Mobile Health Units/
-
diagnostic service$.ti,ab.
-
clinical laboratory service$.ti,ab.
-
genetic test$.ti,ab.
-
mobile health$ unit$.ti,ab.
-
mobile health$ clinic$.ti,ab.
-
(point of care testing or point-of-care testing or POCT or near patient testing or near-patient testing).ti,ab.
-
diagnos$.ti,ab.
-
test$.ti,ab.
-
24 or 25
-
26 and primary care.tw.
-
or/14-23,27 Diagnostics terms
-
13 and 28
-
clinical trial.pt.
-
comparative study.pt.
-
30 or 31 Comparative studies filter
-
29 and 32
-
limit 33 to yr=“2000 –Current” Date limit
Search filters
Primary care search filter taken from Brown et al. 311
Reviews filter adapted from the SIGN search filter resource: www.sign.ac.uk/methodology/filters.html#systematic.
Appendix 2 STEP-UP framework
| Human resources | |
|---|---|
| SKILLS Skill mix Extended roles Inappropriate test ordering Accuracy Errors Delay in diagnosis Quality assurance |
TRAINING Training needs Training in using equipment Training in interpretation Training costs Duration |
| Logistics | |
| EQUIPMENT Equipment for modality Equipment for analysis Consumable costs Maintenance |
PREMISES Cost of premises Space for equipment Space for consumables Space for staff Space for patients/waiting areas, etc. Health and safety Risk assessment |
| Communications and relationships | |
| USER PERSPECTIVE Waiting times Acceptability Repeat procedures |
PRIMARY–SECONDARY INTERFACE Referrals Changes to diagnosis pathways Changes to management pathways Health service utilisation Relationships between staff Specialist support Attitudes of secondary providers General management |
Appendix 3 Evidence and study identifiers for STEP-UP maps
Audiology
Definition: overall service delivery framework within which audiometry (hearing tests) are used to assess ability to hear different sounds and to determine if there are any problems (adapted from NHS Choices).
This map includes otoacoustic emissions; pneumatic otoscopy; pure tone audiometry; spectral gradient acoustic reflectometry; tympanometry.
This map excludes universal newborn hearing screening.
Contains public sector information licensed under the Open Government Licence v3.0. 52
Background
Ten per cent of adults in England experience hearing loss and would benefit from hearing aids or other forms of hearing management. However, 76% of these adults do not have hearing aids or other management. The impact of this unmet need is substantial and has been linked to depression, social isolation, employment problems, loss of independence and dementia. 312 Hearing problems are widely reported as underdiagnosed and undertreated. 313
NHS Audiology services are complex health systems in complex environments. They provide ‘end-to-end’ care with newborn screening, diagnostic assessment of patients, dispensing of hearing aids and appropriate follow-up to ensure that good outcomes are obtained. 314 Historically, audiology services were commissioned from the acute sector and have had a low priority. First presentation in the UK is usually to the GP.
Audiology clinics were among the first diagnostic services to be relocated in primary care under the provisions of GP fundholding. As of 2014, 44% of CCGs were commissioning community-based hearing services to move care towards a patient-centred service. Neonatal hearing screening is excluded from the remit of this evidence map. Diagnostic interventions are required for developmental issues for children, occurrence of otitis media and the onset of age-related hearing degeneration in older patients. In addition, some GPs will investigate occupation-related hearing problems. In the UK, the vast majority of ear, nose and throat (ENT) problems are treated by the GP without reference to ENT.
Placing diagnostic audiology services in a community setting may improve access and availability of services, potentially leading to earlier presentation and intervention. The location of audiology services in a locality may improve equity of service provision in overcoming barriers related to transport services or finding extended time to attend an appointment. Hearing/balance problems often accompany systemic disorders (e.g. cardiovascular disease, diabetes or cancer) in which GPs already intervene. 315 Detecting hearing problems at an earlier stage may lead to the enhanced use of audiological services by geriatric and paediatric populations.
We did not identify any specific systematic reviews relevant to the locating of audiology diagnostic services in a primary care setting. A review by the Joanna Briggs Institute is examining the effectiveness of nurse-led ENT clinics on service delivery and clinical outcomes. 316 However, the protocol for the Joanna Briggs Institute review does not distinguish between locations of the clinics and, therefore, has limited applicability. This proposed review does not have a specific focus on diagnostic interventions. Systematic reviews on the provision of specialist outreach clinics in primary care may be of relevance when looking at issues beyond those related to skills, roles and personnel. 70,317 In addition, several UK-based national evaluations of specialist outreach clinics may inform the relative advantages of services delivered at a primary care location. 318
STEP-UP summary
Skills
An audiogram is a hearing test conducted under ideal listening conditions in a soundproof booth. The test includes different pitches and intensities and the results are conveyed in graphical form. When hearing loss is present, an audiogram helps to distinguish between conductive loss (outer/middle ear) and sensorineural loss (cochlea/cochlear nerve). 319 Hearing tests for children vary according to their age and level of understanding. For babies this may simply involve checking their neuronal response to sound or using behavioural observation audiometry. For children aged between 8 months and 3 years, visual reinforcement audiology is used rewarding a child for turning to a sound by seeing a toy or puppet. For children older than 3 years, play audiometry can be used, with very similar results to an adult audiogram. Each ear can be tested separately. 319
Audiological investigation includes diagnosis of permanent childhood hearing loss, speech disorders and balance problems in children. Staff with whom GPs need to work include audiologists; vestibular rehabilitationists; tinnitus therapists; clinical, educational and family psychologists; otologists; speech and language therapists; educational audiologists; specialist social workers; and teachers. It may be helpful to distinguish between ‘the small, specifically medical requirement, and the large, specifically audiological testing requirement’, with the latter more completely reflecting the scale of audiology required to be done by community staff who do not specialise in audiology.
Few studies have compared the performance of specialist otolaryngologists with that of GPs in aspects of audiological practice. One such study compared the performance of US, South African and Greek otolaryngologists, paediatricians and GPs in recognising acute otitis media (AOM) and otitis media with effusion (OME) as presented in an otoendoscopic video evaluation test. 320 Overall, the average correct diagnosis on the otoscopic video exam by otolaryngologists was significantly superior that by to GPs in all three countries.
There are no structured audiovestibular services for adults in primary care in the UK, although some services for adults are now provided in a primary care setting by GPwSIs in otology. Some providers have developed community-based services for common problems and hub-and-spoke services would support the provision of more rapid effective care for this group of patients. A significant proportion of paediatric audiological services, including hearing surveillance programmes, are provided by second-tier community services. In the UK, good practice guidance and standards and quality audit tools have been developed for audiology services. The Royal College of Physicians’ document on audiovestibular medicine321 specifies requirements.
STEP-UP summary statement With regard to SKILLS, audiology is attributed a MODERATE degree of implementation difficulty owing to initial difficulties in using the tympanometer or the pneumatic otoscope and ongoing concerns about the appropriateness of diagnosis and its implications for secondary care.
Training
A survey of UK GP management of tinnitus322 revealed that almost all responders (99%) routinely performed otoscopy, but only 31% routinely performed a tuning fork test. When asked directly if tinnitus sufficiently impacted on their practice to warrant dedicated training, 28% of responders indicated that it did. Some indicated that they would approve of succinct (≈1 hour) training, either online or as part of a broader ENT workshop. When GPs were invited to comment on how they felt tinnitus management in primary care could be improved, the dominant theme was a desire for concise accessible training on tinnitus management.
The British Association of Audiological Physicians (BAAP) acknowledges the role of training for specialist GPs and practice nurses. 63
Several papers describe training for tympanometry for GPs (Australia)57,58 and for GPs and practice nurses (Denmark). 53 A 3-hour workshop to improve skills in use of tympanometry and pneumatic otoscopy provided GPs with a portable tympanometer. 57 GPs used their own otoscopes with an appropriate insufflator bulb supplied to enable pneumatic otoscopy. Content and outcomes for the course are described in an earlier report. 58 The workshop included pre-reading and access to an online training resource [Enhancing Proficiency in Otitis Media (ePROM)],59 a didactic presentation on otitis media, expert presentations and demonstrations of use of tympanometry and pneumatic otoscopy, hands-on experience in pneumatic otoscopy and tympanometry and guided video examples. These brief training workshops were shown to significantly improve GP confidence for use of the techniques and for diagnosing OME.
The Danish course offered an update on middle ear disease, theoretical and practical information on tympanometry, technical presentation of two different tympanometers and practice performance of tympanometry. 53 Six weeks after the course, participants were asked about their experience with tympanometry, problems before and after the course, their experience with tympanometry in the last 2 weeks and how they evaluated the different elements of the course. The 1-day course improved the knowledge and practical skills of the participating GPs and nurses.
The effect of introducing tympanometry in combination with training to GPs was investigated in a prospective study with 40 GPs in Denmark. 60 Before the study, tympanometry instruction sessions were organised, during which otolaryngologists discussed their experience of middle ear diagnostics with the GPs. The diagnosis was changed in 26.4% after information from the tympanometry.
The BAAP recognises the importance of maintaining a safe environment through the training of staff. This relates to countering the threat of potential clinical risk through continuing education, working with peers and not single-handed, Deaf awareness training, child protection awareness, and training in infection control and protocols for sedation and general anaesthesia. 63
Numerous studies highlight the lack of otolaryngology (ENT) teaching in GP training and suggest both the degradation and the inadequacy of knowledge that GPs have acquired as medical students, particularly when managing complex ENT issues. 323 Nurses with specialised ENT training can facilitate training of GPs and community nurses by running study sessions and providing ongoing support.
Considerable scope exists for the greater involvement of primary care in the management of patients with tinnitus. However, most GPs and their colleagues in primary care may need a specific programme of updating, education and training. 69 A well-trained primary care team could provide initial advice, exclude the existence of wax or external ear infections or other conditions which may contribute to patient’s perceptions of tinnitus and give the necessary treatment. 324 Professional audiology associations suggest that a specifically trained primary HCP with an appropriate documented level of competency in audiology (e.g. GPwSI in audiology/ENT) will be able to manage these patients and will triage the others according to agreed criteria. The majority of patients with non-troublesome tinnitus and those with additional hearing difficulty will be managed by the first level of audiology services. Others will be referred directly to specialist and supraspecialist audiology centres (the second and third levels of care). An educational intervention targeting GPs in Israel achieved an increase of numbers undertaking a hearing test from 19% to 49%, with little change in the control groups over a 2-year period. 325
Several authors note that a diagnosis of AOM is problematic, pointing out the importance of formularized training which is typically expected to be, but is not universally delivered as, part of paediatric resident training. 326 In Texas, only 66% of family practice residents used a pneumatic otoscope, and half of the residents had insufficient criteria in diagnosing AOM. Education in diagnostic criteria and equipment increased the use of the pneumatic otoscope. 327
Three nurses were trained to perform tympanometry and spectral gradient acoustic reflectometry in checking for AOM. 61 In one training session, lasting approximately 2 hours, the team taught the nurses the principles of tympanometry and spectral gradient acoustic reflectometry and how to perform examinations with these devices. During the study visits, one of the three nurses performed examinations. However, the clinical usefulness was reduced by the fact that these test results were obtained only at fewer than half of the asymptomatic visits owing to young unco-operative children, inexperienced nurses and the relative rarity of exclusive test results. The nurses were inexperienced when starting to perform examinations, and they gradually became more experienced during the study. Thus, in clinical practice, nurses would perform better if they were already experienced with the devices when starting to perform routine ear controls. The tympanometer (MicroTymp2, Welch Allyn, Skaneateles Falls, NY, USA) used in the study has been found slightly difficult to handle, and better success rates could be obtained with a more easily handled tympanometer. 64
STEP-UP summary statement With regard to TRAINING, audiology is attributed a MODERATE degree of implementation difficulty owing to the facility of short courses to increase confidence in the use of equipment.
Equipment
Equipment most typically used by a practice would include tympanometer (see Table 8) and a pneumatic otoscope (see Table 9). Interventions following hearing screening generally involve referral to a hearing specialist or hearing aid rehabilitation. 313 The complexity of audiology equipment requirements in primary care can be extrapolated from examining ENT outpatient clinics. Seventy per cent of patients seen in one outpatient ENT clinic had some sort of diagnostic or treatment procedure, including 36 (31%) endoscopic examinations: both laryngoscopy and rigid nasoendoscopy. 328 Twenty-five patients (22%) needed a pure tone audiogram, 11 (10%) needed aural microscopy and two needed skin testing. Eighteen patients (16%) required complex imaging, 12 had CT scans, two had barium swallows, two had MRIs, one had ultrasound and one had a sialogram. 328 In 2001, a prospective study of 1155 consecutive patients seen by one consultant in two different GP surgeries in Birmingham required such specialist equipment as audiometric assessment, the use of the flexible fibre-optic nasendoscope or rigid Hopkins rod rhinoscope, plain radiographs, examination under the operating microscope and skin testing for allergy. 329 This equipment was available in the base hospital ENT outpatient department but may not be available in a primary care setting. Seventy-six per cent of all patients required an additional investigation or procedure (audiometric assessment 49%; flexible nasendoscope 14%; rigid nasendoscope 10%; radiology 4%; examination under microscope 4%; skin testing 5%; minor procedures 2%). There was very little difference between the proportions of new and follow-up patients needing these procedures. The usual consultation time was 12 minutes.
Ear, nose and throat outpatients are required to have the following range of equipment if they are to be considered by ENT UK (https://entuk.org/) as an adequate outpatient service: flexible fibre-optic nasendoscopy, Hopkins rod endoscopy, a soundproof facility, audiometry, tympanometry and microscopy. Most would support the availability of skin tests and plain radiography, although they are used in varying degrees. To avoid an extra visit to a hospital, these facilities would need to be made available in the GP surgery. However, the cost of purchasing and maintaining the equipment would deter most GPs from such investment.
Indicative equipment requirements are included in a BAAP policy document. 63 In addition to purchase cost, the cleaning and maintenance of the instruments need to be considered. Audiometers require regular calibration. Endoscopes require disinfection with glutaraldehyde between patients and adequate facilities that adhere to health and safety legislation are needed. Unless equipment is regularly used, the expense of purchasing and maintaining it and of training practice staff to care for it may not be justified. Expenditure on equipping and supporting frequently used ENT hospital outpatient departments and improving patient access to them might potentially represent better value for money.
With the introduction of portable and handheld equipment, tympanometry has become more feasible for a primary care setting. Future proofing of audiology equipment requires provision for otoacoustic emissions technology, initially used for newborn hearing screening but shown to have potential in small children330 and the aged. Acoustic reflectometry is a technique based on a sonar that enables the diagnosis of middle ear effusion. It has been suggested as offering a potential application for both patients and GPs. 331
In an evidence assessment, Takata et al. 66 conclude that, taking into account direct and indirect costs, pneumatic otoscopy is cheaper than tympanometry or acoustic reflectometry. Pneumatic otoscopy could be as effective as or better than tympanometry and acoustic reflectometry. However, the authors conclude that, for the typical clinician, pneumatic otoscopy should be easier to use than other diagnostic methods,66 an observation not borne out in a qualitative investigation. 57 For this evidence assessment, the critical issue relates to the degree of training needed for the clinician to be as effective with pneumatic otoscopy as examiners in the studies reviewed in the report.
The BAAP recognises that clinical risk should be minimised by providing safe equipment (< 5 years old, regularly maintained and calibrated and appropriate to the clinical needs of the service, etc.). 63 Similarly, infection control, in relation to both equipment and consumables, is an important consideration. Good hygiene and infection control procedures are essential,62 especially with regard to tympanometry and the pneumatic otoscope. Single-use disposable tips should be used, if available. 62
STEP-UP summary statement With regard to EQUIPMENT, audiology is attributed a MODERATE degree of implementation difficulty owing to the fact that, beyond basic equipment, many needs for additional equipment will require follow-up in secondary care.
Premises
Space requirements for audiology equipment figure prominently in logistic considerations around the siting of audiology clinics. Health Building Note 11-01, Facilities for Primary and Community Care Services,46 includes provision for an adult hearing test room and a paediatric hearing test room.
Audiological services typically require a consulting room and a supporting nurse’s room. 63 Despite the growth of purpose-built clinics and the increasing miniaturisation of audiology equipment, the inability of premises to accommodate treatment rooms for audiology services has been a reported obstacle to the relocation of hospital-based provision. Audiology places a particular requirement not only on the size of premises but also to take into account ‘the acoustic features for those [with] audiology departments’. 332 Acoustic protection for hearing tests require rooms with special environmental characteristics. 46 There is limited evidence on health and safety aspects of audiology equipment.
The BAAP recognises the importance of maintaining a safe environment in countering potential clinical risk. 63 Relevant issues relate to safe equipment and minimisation of the risk of cross-infection.
STEP-UP summary statement With regard to PREMISES, audiology is attributed a MODERATE degree of implementation difficulty owing to the specific requirement for a soundproofed room.
User perspective
A Danish study reports slow dissemination of tympanometry despite the fact that reimbursement from the Danish National Health Service means that the equipment can be paid within 2 years in an average clinic with 85 tympanometries per year. The authors explain the slow diffusion, stating that the technology is difficult to understand and difficult to handle for the average GP. 53
An Australian study interviewed GPs after a brief 3-hour course on tympanometry and otoscopy. 57 Most GPs found that both tympanometry and pneumatic otoscopy were acceptable to carers and children, although some GPs stated that they preferred not to use pneumatic otoscopy as children sometimes found it uncomfortable. Some GPs thought tympanometry was particularly useful for communicating with carers about ear disease, offering tangible ‘proof’ to parents of the GP diagnosis and support of the management plan.
Most GPs stated that both tympanometry and pneumatic otoscopy could assist in the diagnosis of otitis media without creating an undue time burden during the consultation. 57 However, this did not translate into a strong intention to use the techniques in the future. Whereas some GPs believed the techniques had improved the accuracy of their diagnoses of otitis media, for others uncertainty was created when the findings did not agree with their clinical judgement. The techniques were seen to have most value in the diagnosis and monitoring of OME. There was less agreement that the techniques were useful in diagnosis of AOM. Several GPs stated that neither tympanometry nor pneumatic otoscopy was needed for the diagnosis of AOM, as they were guided by other symptoms and signs.
Some GPs were concerned that by detecting effusions which were not clinically important there might be unnecessary health costs to patients and the community through increased GP follow-up and referral to audiologists and otolaryngologists. 57 Many GPs were unclear on the significance of negative pressure (type C) tympanometry57 readings in general practice (see Table 8), which could potentially generate unnecessary GP follow-ups.
Tympanometry versus otoscopy
General practitioners expressed a preference for tympanometry based on ease of use and interpretation. 57 GPs believed that more training and experience was needed to become confident with pneumatic otoscopy than tympanometry.
Tympanometry
Three-hour training was reported by most GPs as adequate to allow them to incorporate tympanometry in their practice, the machine being largely self-explanatory and easy to use. 57 GPs could teach themselves how to perform tympanometry after the relatively brief introduction to the equipment, with the main challenge being interpretation of the tympanograms, which required them to refer back to written information they were given during training. Most GPs (9 out of 13) said that they would choose to continue to use tympanometry. However, GPs perceived the cost of the tympanometer to be prohibitive, given the low reimbursement for this service in the Australian universal health-care system. One participant expressed a strong intention to use tympanometry in the future and another to use both tympanometry and pneumatic otoscopy.
Pneumatic otoscopy
Pneumatic otoscopy was seen as the more difficult technical skill. 57 The most important barrier to using pneumatic otoscopy was GP uncertainty over whether there was true drum immobility or their technique was simply inadequate. Some GPs ‘gave up’ on pneumatic otoscopy.
Although by no means all those potentially presenting to audiological services in a primary context may be experiencing functional deafness, an important additional consideration relates to the patient experience of primary care venues. GP practices or primary care clinics may be less likely to have communication aids, such as text telephones, and their waiting room procedures (e.g. use of tannoy systems) may be considered inappropriate. 333
There are no data on repeat procedures per se. However, evidence examining the utilisation of audiological equipment found that patients attending ENT outreach clinics held in GP surgeries with basic equipment often required an extra visit to hospital for further investigations. For such clinics to be cost-effective requires that they be well equipped and regularly used. In rural areas, community hospitals could provide this. However, in large cities where a hospital is usually only a short distance away, a user-friendly outpatient clinic would appear to offer relative advantage.
STEP-UP summary statement With regard to USER PERSPECTIVES, audiology is attributed a LOW degree of implementation difficulty in the light of only minor GP concerns with equipment and the referral pathway and difficulties with investigations involving small children.
Primary–secondary interface
General practitioners are required to identify the most common conditions and refer to the appropriate management pathway. Effective management of adult tinnitus patients requires quick triage and referral to an appropriate professional, given the potential for serious health concerns, and the often high level of distress experienced by tinnitus patients. 334 Excessive waiting times have been addressed by a National Audiology Programme and variable care by a Good Practice Guide. The Good Practice Guide,69 specifically for adults with tinnitus, informed commissioners and service managers about how to improve the service while still meeting the 18-week target. The Good Practice Guide69 recommends strategies for tinnitus assessment, management, and referral at four different levels of the service: primary care (GPs), local community-based tinnitus services (audiologists and hearing therapists), specialist hospital-based centres (multidisciplinary teams that include audiologists, hearing therapists, ENT specialists, audiovestibular physicians and clinical psychologists) and supra-specialist assessment centres (multidisciplinary teams that can offer more complex audiological assessments, neurosurgical interventions and radiotherapy). Patient routes through the system were to be determined by clinical assessment and specific referral criteria, designed with service efficiency and equity of patient care in mind.
Alternatives to community provision of audiological services include outreach provision. A systematic review of outreach services in primary care found a direct counterpoint between the needs of the patient and the needs of the service. 70 Reported disadvantages concerned administrative costs, accommodation costs and inefficient use of specialists’ time. Comparative studies showed that more patients expressed a preference for outreach clinics than for hospital-based clinics, and measures of patients’ satisfaction and convenience generally were higher for outreach clinics. Studies did not show any consistent difference in health outcome between outreach and hospital-based clinics. Outreach clinics had higher direct costs to the health system than hospital-based clinics. Audiology clinics have been proposed as a prime candidate for the use of telehealth services. 335
In one experience, 49% of cases required an audiological assessment, whereas 41% of patients required only an audiogram or a tympanogram. Consultant travelling time to outreach clinics is a further consideration. One study suggests that an estimated 492 additional patients (43%) could have been seen in the travelling time. Travelling to a distant site may also require two sessions of fixed NHS commitment. The evidence on patient satisfaction with outreach clinics is mixed, with patients being more concerned with the content rather than the location of their consultation. Benefits in terms of improved communication between GPs and hospital specialists are not always realised given a large workload.
A 2012 project,336 led by the Cardiff and Vale University Health Board in partnership with Cardiff University’s School of Mathematics, focused on ENT and audiology outpatient services and aimed to improve to ENT and audiology services by shifting care closer to home. A self-contained satellite facility within a GP practice sought to enable 5000–7000 people to receive secondary care hearing services in a primary care setting. The project explored the benefits to patients and the health economy of shifting care closer to home. The team also developed a toolkit to assist in decision-making and calculation of time and related costs. The model was developed with input from ENT and audiology staff, but can be applied to any specialty. As a consequence, ‘do not attend’ rates reduced from 13% to under 1%. There was a 62% reduction in patient travel time, 67% reduction in patient travel distance and 60% reduction in patient travel costs. This constituted a saving of £4.80 per appointment. With regard to patient satisfaction, patients gave the move an average score of 9 out of 10, with 70% giving a 10 out of 10 rating.
The Department of Health report Improving Access to Audiology Services in England337 included a specific commitment to apply service improvement tools. Eighteen audiology improvement pilot sites338 were set up to support and develop innovative ways to demonstrate measurable benefits of whole pathway redesign. 314,338 One project was Triage in Primary Care at University Hospitals of Leicester NHS Trust. 71,339 Traditionally, patients needing a hearing aid would be referred by their GP to the audiology service: one appointment for assessment and one for fitting. Advances in technology (‘open fit’ hearing aids)72 mean that some patients can be assessed and fitted at the same appointment. If suitable patients can be identified before they arrive at audiology (i.e. if triaged in primary care), then the process becomes efficient for patients and service providers. The HearCheck Screener340 can identify age-related hearing problems and predict people who could benefit from intervention. It is a simple, low-cost, handheld device that produces a fixed series of six pure tones. The screening devices, which cost approximately £100 each, were loaned to the GPs in the pilot. Calibration is needed every 3 years and the devices are expected to last around 5 years. GPs were better able to brief patients on their condition, telling them that they may get a hearing aid at one appointment if suitable. At the end of the project, the audiology team was working with 11 practices. Two of the four original practices used health-care assistants to carry out the triage on their behalf.
Sherwood Forest Hospitals NHS Foundation Trust sought to overcome service bottlenecks by piloting direct access from primary care for patients with tinnitus. All local GPs were able to refer into the service. Clear referral criteria were drawn up for GPs to ensure that appropriate patients would be referred. The clinic was set up on the Choose and Book system to allow referrals to be accepted electronically. Data on all waiting times were recorded and a detailed patient satisfaction questionnaire was completed by all directly referred tinnitus patients. The numbers of patients requiring or requesting subsequent ENT consultations were recorded to ensure that all aspects of an efficient service were considered.
One hybrid model involves specialist outreach clinics administering hearing tests in the context of a hospital-based service and then to deliver the follow-up via a specialist outreach clinic sited within primary care. This obviates the need for specialist equipment to be housed in the primary care premises. However, if further tests are subsequently required, the need to be referred back to the hospital for testing could prove frustrating for clinician and patient alike.
In 2011, the audiology department from Devon Primary Care NHS Trust transferred to a new organisation: CHIME Social Enterprise Community Interest Company, an independent, not-for-profit company (www.chimehealth.co.uk/about-chime). At the time of writing (2014), CHIME was the only audiology department in the UK that had moved to this independent status, with other services expressing a wish that they too could make such a move. 341 By 2014, Specsavers had contracts for adult hearing services with 95 CCGs. 342,343
STEP-UP summary statement With regard to the PRIMARY–SECONDARY INTERFACE, audiology is attributed a MODERATE degree of implementation difficulty owing to dependence on secondary services for further investigation and yet the possibility of streamlined hearing aid pathways.
Conclusion
The provision of audiology services in primary care is complex in that it not only requires a wide variety of equipment and, optimally, an audiology-friendly environment, but also may necessitate access to other diagnostic services, most notably endoscopy or radiology. There is little evidence available to examine the impact of acquisition of audiology-related skills by primary care staff. Studies on provision of specialist outreach clinics (i.e. a hospital specialist operating in a primary care environment) offer supplementary data on equipment utilisation and premises. Current models being favoured within primary care audiology involve direct-access referral clinics or the provision of services under contract by commercial (or, alternatively, independent) providers.
Audiology (A1–83)
A1. Abbott P, Rosenkranz S, Hu W, Gunasekera H, Reath J. The effect and acceptability of tympanometry and pneumatic otoscopy in general practitioner diagnosis and management of childhood ear disease. BMC Fam Pract 2014;15:181.
A2. Ayshford CA, Johnson AP, Chitnis JG. What is the value of ENT specialist outreach clinics? J Laryngol Otol 2001;115:441–3.
A3. Benge S, Williamson I. Hearing impairments part 1: an overview of glue ear. Br J School Nurs 2011;6:69–72.
A4. Bentur N, Valinsky L, Lemberger J, Ben Moshe Y, Heymann AD. Primary care intervention programme to improve early detection of hearing loss in the elderly. J Laryngol Otol 2012;126:574–9.
A5. Bhatia P, Mintz S, Hecht BF, Deavenport A, Kuo AA. Early identification of young children with hearing loss in federally qualified health centers. J Dev Behav Pediatr 2013;34:15–21.
A6. Blomgren K, Pitkäranta A. Is it possible to diagnose acute otitis media accurately in primary health care? Fam Pract 2003;20:524–7.
A7. Blomgren K, Haapkylä J, Pitkäranta A. Tympanometry by nurses – can allocation of tasks be optimised? Int J Pediatr Otorhinolaryngol 2007;71:7–10.
A8. Bowling A, Bond M. A national evaluation of specialists’ clinics in primary care settings. Br J Gen Pract 2001;51:264–9.
A9. BAAP. Policy Document. London: BAAP; 2002.
A10. British Society of Audiology. Recommended Procedure Tympanometry. Bathgate: British Society of Audiology; 2014.
A11. Buchanan CM, Pothier DD. Recognition of paediatric otopathology by general practitioners. Int J Pediatr Otorhinolaryngol 2008;72:669–73.
A12. Chianese J, Hoberman A, Paradise JL, Colborn DK, Kearney D, Rockette HE, et al. Spectral gradient acoustic reflectometry compared with tympanometry in diagnosing middle ear effusion in children aged 6 to 24 months. Arch Pediatr Adolesc Med 2007;161:884–8.
A13. Clarke LR, Wiederhold ML, Gates GA. Quantitation of pneumatic otoscopy. Otolaryngol Head Neck Surg 1987;96:119–24.
A14. Davis A, Smith P. Adult hearing screening: health policy issues – what happens next? Am J Audiol 2013;22:167–70.
A15. Davis A, Smith PA, Booth M, Martin M. Diagnosing patients with age-related hearing loss and tinnitus: supporting GP clinical engagement through innovation and pathway redesign in audiology services. Int J Otolaryngol 2012;2012:290291.
A16. Department of Health. Building Note 12, Supplement 3–ENT and Audiology Clinics/Hearing Aid Centres. NHS Estates; 1994.
A17. Department of Health. Transforming Adult Hearing Services for Patients with Hearing Difficulty: A Good Practice Guide. London: Department of Health; 2007. p. 40.
A18. Department of Health. Improving Access to Audiology Services. 2007. URL: www.dh.gov.uk/prod consum dh/groups/dh digitalassets/@dh/@en/documents/digitalasset/dh 101860.pdf (accessed 28 August 2015).
A19. Department of Health. Provision of Services for Adults with Tinnitus. A Good Practice Guide. London: Central Office of Information; 2009.
A20. Earis H, Reynolds S. Deaf and Hard-of-Hearing People’s Access to Primary Health Care Services in North East Essex. NHS North East Essex.
A21. Smith PA, Evans PI. Hearing assessment in general practice, schools and health clinics: guidelines for professionals who are not qualified audiologists. Education Committee of the British Society of Audiology. Br J Audiol 2000;34:57–61.
A22. Eikelboom RH, Mbao MN, Coates HL, Atlas MD, Gallop MA. Validation of tele-otology to diagnose ear disease in children. Int J Pediatr Otorhinolaryngol 2005;69:739–44.
A23. Eley KA, Fitzgerald JE. Quality improvement in action. Direct general practitioner referrals to audiology for the provision of hearing aids: a single centre review. Qual Prim Care 2010;18:201–6.
A24. El-Shunnar SK, Hoare DJ, Smith S, Gander PE, Kang S, Fackrell K, et al. Primary care for tinnitus: practice and opinion among GPs in England. J Eval Clin Pract 2011;17:684–92.
A25. Erkkola-Anttinen N, Laine MK, Tähtinen PA, Ruohola A. Parental role in the diagnostics of otitis media: can layman parents use spectral gradient acoustic reflectometry reliably? Int J Pediatr Otorhinolaryngol 2015;79:1516–21.
A26. Erkkola-Anttinen N, Tähtinen PA, Laine MK, Ruohola A. Parental role in the diagnostics of otitis media: can parents be taught to use tympanometry reliably? Int J Pediatr Otorhinolaryngol 2014;78:1036–9.
A27. Fonseca S, Forsyth H, Neary W. School hearing screening programme in the UK: practice and performance. Arch Dis Child 2005;90:154–6.
A28. Foust T, Eiserman W, Shisler L, Geroso A. Using otoacoustic emissions to screen young children for hearing loss in primary care settings. Pediatrics 2013;132:118–23.
A29. Gander PE, Hoare DJ, Collins L, Smith S, Hall DA. Tinnitus referral pathways within the National Health Service in England: a survey of their perceived effectiveness among audiology staff. BMC Health Serv Res 2011;11:162.
A30. Department of Health. Getting the Right Start: National Service Framework for Children. Standard for Hospital Services. London: Department of Health; 2003.
A31. Green LA, Culpepper L, de Melker RA, Froom J, van Balen F, Grob P, Heeren T. Tympanometry interpretation by primary care physicians. A report from the International Primary Care Network (IPCN) and the Ambulatory Sentinel Practice Network (ASPN). J Fam Pract 2000;49:932–6.
A32. Gruen R, Weeramanthri TS, Knight S, Bailie R. Specialist outreach clinics in primary care and rural hospital settings (Cochrane Review). Commun Eye Health 2006;19:31.
A33. Harkin H. A nurse-led ear care clinic: sharing knowledge and improving patient care. Br J Nurs 2005;14:250–4.
A34. Health Building Note 11-01. Facilities For Primary And Community Care Services. URL: www.gov.uk/government/uploads/system/uploads/attachment_data/file/148509/HBN_11-01_Final.pdf (accessed 19 October 2016).
A35. Helenius KK, Laine MK, Tähtinen PA, Lahti E, Ruohola A. Tympanometry in discrimination of otoscopic diagnoses in young ambulatory children. Pediatr Infect Dis J 2012;31:1003–6.
A36. Henry JA, Zaugg TL, Myers PJ, Kendall CJ, Michaelides EM. A triage guide for tinnitus. J Fam Pract 2010;59:389–93.
A37. Hind SE, Haines-Bazrafshan R, Benton CL, Brassington W, Towle B, Moore DR. Prevalence of clinical referrals having hearing thresholds within normal limits. Int J Audiol 2011;50:708–16.
A38. Hoare DJ, Gander PE, Collins L, Smith S, Hall DA. Management of tinnitus in English NHS audiology departments: an evaluation of current practice. J Eval Clin Pract 2012;18:326–34.
A39. Hofstetter PJ, Kokesh J, Ferguson AS, Hood LJ. The impact of telehealth on wait time for ENT specialty care. Telemed J E Health 2010;16:551–6.
A40. Hospital Development. Wyg Supports £124m Lift Project. 2006. URL: www.Hdmagazine.Co.Uk/Story.Asp?Storycode=2035925 (accessed 28 August 2015).
A41. Royal College of Physicians. Audiovestibular Medicine. URL: www.rcplondon.ac.uk/sites/default/files/audiovestibular_medicine.pdf (accessed on 4 January 2016).
A42. Pronk M, Kramer SE, Davis AC, Stephens D, Smith PA, Thodi C, et al. Interventions following hearing screening in adults: a systematic descriptive review. Int J Audiol 2011;50:594–609.
A43. British Standards. ISO 8253-1 and BS EN ISO 8253-2 Standards for Acoustic Audiometric Test Methods. London: British Standards Institution; 1998.
A44. Johansen EC, Lildholdt T, Damsbo N, Eriksen EW. Tympanometry for diagnosis and treatment of otitis media in general practice. Fam Pract 2000;17:317–22.
A45. Johnson CE, Danhauer JL, Koch LL, Celani KE, Lopez IP, Williams VA. Hearing and balance screening and referrals for Medicare patients: a national survey of primary care physicians. J Am Acad Audiol 2008;19:171–90.
A46. Kaleida PH, Ploof DL, Kurs-Lasky M, Shaikh N, Colborn DK, Haralam MA, et al. Mastering diagnostic skills: Enhancing Proficiency in Otitis Media, a model for diagnostic skills training. Pediatrics 2009;124:e714–20.
A47. Khoza-Shangase K, Kassner L. Automated screening audiometry in the digital age: exploring uhear™ and its use in a resource-stricken developing country. Int J Technol Assess Health Care 2013;29:42–7.
A48. Kinshuck AJ, Derbyshire SG, Wickham M, Lesser THJ. Current Demand for ENT Outpatient Services in the UK. Family Medicine Online; 2010. URL: www.priory.com/family_medicine/ENT_services.htm.
A49. Krishnamurti S. Audiology service models in a family physician practice setting. Perspectiv Audiol 2010;6:24–32.
A50. Laine MK, Tähtinen PA, Ruuskanen O, Löyttyniemi E, Ruohola A. Can nurses exclude middle-ear effusion without otoscopy in young asymptomatic children in primary care? Scand J Prim Health Care 2015;33:115–20.
A51. Lous J, Ryborg CT, Damsgaard JJ, Munck AP. Tympanometry in general practice: use, problems and solutions. Fam Pract 2012;29:726–32.
A52. MacClements JE, Parchman M, Passmore C. Otitis media in children: use of diagnostic tools by family practice residents. Fam Med 2002;34:598–603.
A53. McKnight T. Why patients prefer community based services. Health Serv J 2014.
A54. Muderris T, Yazıcı A, Bercin S, Yalçıner G, Sevil E, Kırıs M. Consumer acoustic reflectometry: accuracy in diagnosis of otitis media with effusion in children. Int J Pediatr Otorhinolaryngol 2013;77:1771–4.
A55. Newman CW, Sandridge SA. Hearing loss is often undiscovered, but screening is easy. Cleve Clin J Med 2004;71:225–32.
A56. NHS Improvement. Pushing the Boundaries: Evidence to Support the Delivery of Good Practice in Audiology. London: NHS Improvement; 2010. URL: www.improvement.nhs.uk/LinkClick.aspx?fileticket=zRsxjLXTeCw%3d&tabid=56 (accessed 28 August 2015).
A57. NHS Improvement. Shaping the Future: Strengthening the Evidence to Transform Audiology Services. London: NHS Improvement; 2011. URL: www.improvement.nhs.uk/audiology/documents/Shaping the Future.pdf (accessed 28 August 2015).
A58. NHS Improvement. Audiology Pilot Sites. NHS Improvement. URL: www.improvement.nhs.uk/audiology/pilotsites.html (accessed 28 August 2015).
A59. Campbell C. Now Hear This: The Case for Community Based Hearing Care. Health Service Journal. 2014. URL: www.hsj.co.uk/sectors/commissioning/audiology-today/now-hear-this-the-case-for-community-based-hearing-care/5066966.article (accessed 19 October 2016).
A60. Ouedraogo E, Labrecque M, Côté L, Charbonneau K, Légaré F. Use and teaching of pneumatic otoscopy in a family medicine residency program. Can Fam Physician 2013;59:972–9.
A61. Patricoski C, Ferguson S. Which tympanometer is optimal for an outpatient primary care setting? J Fam Pract 2006;55:946–52.
A62. Patterson BM, Renaud M. Routine hearing screening in primary care for adult populations using distortion product Otoacoustic Emissions testing. J Am Acad Nurse Pract 2012;24:400–4.
A63. Pichichero ME, Poole MD. Comparison of performance by otolaryngologists, pediatricians, and general practitioners on an otoendoscopic diagnostic video examination. Int J Pediatr Otorhinolaryngol 2005;69:361–6.
A64. Ponka D, Baddar F. Pneumatic otoscopy. Can Fam Physician 2013;59:962.
A65. Powell J. Systematic review of outreach clinics in primary care in the UK. J Health Serv Res Policy 2002;7:177–83.
A66. National Deaf Children’s Society. Quality Standards in Paediatric Audiology – Guidelines for the Early Identification and Audiological Management of Children with Hearing Loss. National Deaf Children’s Society; 2000.
A67. Rose E. Audiology. Aust Fam Physician 2011;40:290–2.
A68. Rosenkranz S, Abbott P, Reath J, Gunasekera H, Hu W. Promoting diagnostic accuracy in general practitioner management of otitis media in children: findings from a multimodal, interactive workshop on tympanometry and pneumatic otoscopy. Qual Prim Care 2012;20:275–85.
A69. Cardiff and Vale University Health Board, School of Mathematics CU. Operational Research Modelling: Transferring ENT/Audiology Services into a Community Setting (Shine 2012 Final Report). The Health Foundation; 2014.
A70. Siemens HearCheck. URL: http://hearing.siemens.com/uk/01-professional/03-partner-solutions/01-hear-check/hear-check.jsp (accessed 28 August 2015).
A71. Smith P, Mack A, Davis A. A multicenter trial of an assess-and-fit hearing aid service using open canal fittings and comply ear tips. Trends Amplif 2008;12:121–36.
A72. Steinbach WJ, Sectish TC. Pediatric resident training in the diagnosis and treatment of acute otitis media. Pediatrics 2002;109:404–8.
A73. Takata GS, Chan LS, Morphew T, Mangione-Smith R, Morton SC, Shekelle P. Evidence assessment of the accuracy of methods of diagnosing middle ear effusion in children with otitis media with effusion. Pediatrics 2003;1123:1379–87.
A74. Teppo H, Revonta M, Lindén H, Palmu A. Detection of middle-ear fluid in children with spectral gradient acoustic reflectometry: a screening tool for nurses? Scand J Prim Health Care 2006;24:88–92.
A75. Teppo H, Revonta M. Comparison of old, professional and consumer model acoustic reflectometers in the detection of middle-ear fluid in children with recurrent acute otitis media or glue ear. Int J Pediatr Otorhinolaryngol 2007;71:1865–72.
A76. The American Academy of Family Physicians; American Academy of otolaryngology-Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis media With Effusion. Otitis media with effusion. Pediatrics 2004;113:1412–29.
A77. Department of Health. Provision of Services for Adults with Tinnitus: A Good Practice Guide. London: Department of Health; 2009.
A78. Wall TC, Senicz E, Evans HH, Woolley A, Hardin JM. Hearing screening practices among a national sample of primary care pediatricians. Clin Pediatr 2006;45:559–66.
A79. Weinstein BE. Tool kit for screening otologic function of older adults1. Am J Audiol 2013;22:179–82.
A80. Wensing M, van de Lisdonk E, van Weel C, van den Hoogen F, Schattenberg G, Grol R. Hearing disability in older adults: patient and doctor delay in primary medical care. J Am Geriatr Soc 2001;49:1398–9.
A81. Whiteford C, White S, Stephenson M. Effectiveness of nurse-led clinics on service delivery and clinical outcomes in adults with chronic ear, nose and throat complaints: a systematic review protocol. JBI Database Syst Rev Implemen Reports 2013;11:23–37.
A82. Zapala DA, Stamper GC, Shelfer JS, Walker DA, Karatayli-Ozgursoy S, Ozgursoy OB, et al. Safety of audiology direct access for medicare patients complaining of impaired hearing. J Am Acad Audiol 2010;21:365–79.
A83. CHIME Health. Recruitment. URL: http://chimehealth.co.uk/web/data/chime-2014-recruitment-1.pdf (accessed 28 August 2015).
Cardiac services
Definition: cardiac tests are used to diagnose and treat heart disease, such as heart failure and AF. Some tests are non-invasive; others are more invasive but potentially more useful for diagnosis of heart disease.
This map includes BNP, blood tests (specifically in the context of heart disease) (see also Point-of-care testing) and 24-hour ambulatory BP monitoring.
This map excludes chest X-ray (see Radiology/X-ray), electrocardiography (see Electrocardiography), echocardiography (see Echocardiography) and telecardiology.
Background
Cardiovascular diseases are the most common causes of premature death. The National Service Framework for Coronary Heart Disease344 sets out national standards for the diagnosis of coronary heart disease with explicit recognition of the role of primary care teams. A further National Service Framework chapter, Arrhythmias and sudden cardiac death,345 was published in 2005, which emphasised that patients with long-term conditions may be managed in primary care. It also highlighted better access to effective management of arrhythmias in all areas, including primary care. Developments in cardiac diagnostic services include 24-ambulatory BP monitoring. 346
Around 900,000 people in the UK currently have heart failure. 347 The ageing population and improved survival of individuals with ischaemic heart disease are likely to lead to a continuing rise in the prevalence of heart failure. The incidence of heart failure in the UK is less clear but the crude rate has been estimated to be 1.3 cases per 1000 population per year for those aged ≥ 25 years. The British Heart Foundation reported the incidence of heart failure in the UK in 2009 as 39.1 per 100,000 person-years. 348 Overall, a GP with a patient population of 2000 will care for approximately 40–50 patients with heart failure and see two or three new cases each year.
Cardiac imaging equipment is therefore required in the primary care setting. The principal modalities of cardiac imaging are echocardiography (see Echocardiography), invasive contrast angiography, nuclear imaging, cardiac MRI (see Magnetic resonance imaging) and cardiac CT. Cardiac positron emission imaging is available in a few specialised centres, but not in primary care. Significant investment in cardiac services since the introduction of the National Service Framework for Coronary Heart Disease has enabled primary care teams to receive funding for equipment. However, it is difficult to track expenditure on equipment, making it problematic to know which cardiac diagnostic equipment is currently available in primary care. 76
STEP-UP summary
Skills
A UK study used 540 clinical scenarios, involving permutations of presenting symptoms (cough, bilateral ankle swelling, dyspnoea, fatigue) and levels of risk of cardiovascular disease, together with cardiovascular/chest examination and ECG result, rated by GP experts. The experts concluded that natriuretic peptide testing should be the routine test for suspected heart failure when referral for diagnostic testing is considered appropriate. 73 Abnormal natriuretic peptides testing should be followed up with referral for echocardiography (see Echocardiography). A postal survey sought to identify services accessed by PCTs for patients with chronic heart failure. 349 Natriuretic peptides were used by 61 (26%) PCTs and direct access to echocardiography was available to 163 (72%). The survey highlighted the variation of service models with different implications for the cost-effectiveness of service models for diagnosing heart failure.
In a survey on barriers to effective management of heart failure the majority of survey respondents who diagnosed LVSD used ECGs, chest radiography and clinical assessment, the exception being nurses. Around one-quarter of the nurses (23%) and half of the GPs (49%) were confident in interpreting the results of an ECG, whereas most cardiologists and general physicians (both 87%) were confident. One-third of nurses (33%) and two-thirds of GPs (65%) were confident in interpreting the results of an echocardiogram, whereas the majority of cardiologists and general physicians (98% and 85%, respectively) were confident. Cardiologists were more likely to use ECGs and chest X-rays (64% and 63%, respectively) than other groups; clinical assessment was almost never used by any group. This was reflected in the confidence levels in interpreting these results. 74
STEP-UP summary statement With regard to SKILLS, cardiac services is attributed a MODERATE degree of implementation difficulty owing to the range of diagnostic scenarios and investigations.
Training
Performance and interpretation of cardiac imaging requires a high level of skill, appropriate equipment and systems of governance and quality control. Cardiac imaging should be performed and interpreted only by suitably qualified and experienced individuals working in departments with appropriate facilities. Education and training in cardiac imaging should meet the present requirements specified by the GMC in curricula in cardiology and cardiac radiology, the future demands of revalidation and the requirements of allied professional groups. Improvement in the quality of cardiac imaging in the UK is a common aim of all members of the Imaging Council. 75
Staff training can have a significant impact on quality control. A survey of 170 general practices in one cardiac network suggests that the training of staff in the use of cardiac equipment is variable. 76 Training updates were not provided for 12-lead ECG in 20 practices (31%), static BP in 26 practices (39%), ambulatory BP in 21 practices (62%) and Holter monitors in 10 practices (71%). Responding practices indicated that staff require additional training in 12-lead ECG in 17 practices (27%), static BP in three practices (5%), ambulatory BP in 11 practices (32%), and Holter monitor in eight practices (57%).
A new extended GP role in cardiology was developed and piloted to enable GPs to diagnose and manage patients with mild to moderate heart failure or AF and to use diagnostics effectively in primary care. 77 Training entailed GPs participating in a four-session short course with ongoing clinical supervision. A mixed-methods evaluation found that participating GPs perceived the extended GP role as a professional development opportunity with the potential to reduce health-care utilisation and costs, through a reduction in referrals, while meeting the patient’s wishes for the provision of care closer to home. Patient experience of the new GP service was positive. The standard of clinical practice was judged acceptable. Referrals fell during the study period. This 2014 report recommended further development and continuing evaluation of the model.
The BSE, the BSCMR (www.bscmr.org) and the British Society for Cardiac Imaging have endorsed voluntary accreditation procedures for their members. Accreditation has no statutory role but is provided to allow medical and non-medical practitioners to demonstrate that they have achieved a specified level of experience in an appropriate educational environment. Departmental accreditation procedures are primarily a quality improvement scheme designed to facilitate the introduction of quality control and to demonstrate that staff, equipment and processes reach specified standards.
STEP-UP summary statement With regard to TRAINING, cardiac services is attributed a LOW degree of implementation difficulty owing to the widespread availability of appropriate training and accreditation, although the underutilisation of training in basic cardiac equipment remains a concern.
Equipment
A survey of 170 general practices in one cardiac network suggests that provision of cardiac equipment, and training of staff in its use, is variable. 76 Few peer-reviewed or authoritative recommendations were found for provision of cardiac equipment such as 12-lead ECG machines, Holter monitors or BP monitors (static or ambulatory) in primary care. Practices were asked whether or not they had a service contract, a maintenance contract and an identified responsible individual for the specified equipment. Service contract providers included local hospitals and equipment suppliers, while practice nurses are the group of staff most likely to be responsible for the equipment on a day-to-day basis.
Following the purchase and installation of equipment, there is a need for appropriate maintenance and training to ensure optimal use and patient safety. 76
Guidance and standards regarding equipment maintenance and training on primary care premises is difficult to identify,76 typically being scattered across a range of stakeholder professional association.
The implementation of BNP will incur initial expenses including the purchase or hire of the analyser. Quality control issues relate to equipment and the purchase of limited shelf-life reagent strips. The cost of each BNP test is likely to be higher when relatively small numbers of tests are required and will reduce with volume. 80 A key source for an evaluation of the BNP POC testing kit is a 2011 Horizon Scanning report. 81 A systematic review of RCTs concluded that for diagnosis in primary care, low BNP values may be used to rule out heart failure but, owing to poor specificity, high values cannot be used to rule in the condition. 350
STEP-UP summary statement With regard to EQUIPMENT, cardiac services is attributed a LOW degree of implementation difficulty owing to developments in POC testing and the increased portability of equipment.
Premises
Most aspects of cardiac services have been accommodated in primary care for many years. Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates near-patient testing services (such as blood and gas) in the ‘near-patient testing room’ (contains public sector information licensed under the Open Government Licence v3.0).
Implications of equipment purchases such as ECG or echocardiography are reviewed elsewhere (see Electrocardiography and Echocardiography). The review was not able to identify information on the implications of providing an increased range of cardiac testing facilities in primary care, specifically in relation to implications for the premises. In particular, there was no information on the implications of stock maintenance and storage of POC tests such as BNP.
STEP-UP summary statement With regard to PREMISES, cardiac services is attributed an UNCERTAIN degree of implementation difficulty owing to a lack of information on the facilities required.
User perspective
A study in UK general practice examined the management of suspected heart failure. 82 Some GPs requested a full blood count and urea and electrolytes on all their patients with suspected heart failure. Many GPs mentioned that they would arrange for chest radiography and some would arrange a 12-lead ECG. Some GPs excluded a diagnosis of heart failure on the basis of clinical examination and occasionally with the addition of a normal chest radiography. Very few GPs reported sending a patient for echocardiography to confirm a diagnosis of heart failure. A few GPs, who were mainly single-handed practitioners, reported that they would send all their patients to the rapid assessment cardiology clinic at the local hospital. 82
Most GPs mentioned the adequacy of facilities as an obstacle to diagnosing and managing patients with heart failure, specifically a lack of access to an echocardiogram. Another obstacle was not having enough time to deal with patients suspected of having heart failure. Even when practitioners were aware of the evidence about the validity of investigations or signs, the principal obstacle was the high demand by elderly patients, including the time these patients take getting dressed and undressed. 82
Some obstacles were directly related to the organisational aspects of services. Many GPs mentioned poor access to echocardiography as a barrier to diagnosing patients with suspected heart failure. Doctors also perceived that they were more likely to refer younger patients to the hospital. Not being fundholders was also viewed as a major obstacle, as these practices did not have open access to echocardiography. The GPs also reported interface problems with providers, who they perceived did not trust them to use the services appropriately.
Those few GPs and practices that admitted to routinely arranging echocardiograms for patients suspected of having heart failure were also more likely to report treating patients with angiotensin-converting enzyme inhibitors. These GPs were also more likely to be aware of the impact of heart failure on patients’ morbidity and mortality.
Heart failure is perceived to be a difficult diagnosis to make in general practice because of problems with subtlety of clinical symptoms and signs; difficulty in differential diagnosis, especially in elderly patients with comorbidity; time constraints and generally increasing clinical and administrative workload for GPs, and lack of availability of diagnostic tests, including electrocardiography, chest radiography and echocardiography. 107 Other factors include a lack of confidence in interpreting the results of these, inertia or fear of initiating action because of anxieties about committing to an intensive course of action, including investigations, initiation, titration and monitoring of treatment, and patients’ choices, including reluctance to be investigated or treated further.
STEP-UP summary statement With regard to the USER PERSPECTIVE, cardiac services is attributed a LOW degree of implementation difficulty owing to the non-invasive nature of most cardiac tests. However, the non-availability of echocardiology services remains a significant barrier.
Primary–secondary interface
Open-access services (echocardiography, stress testing, Holter monitoring) provided by secondary care have an important role to play. The service may simply provide an investigation or add value by providing interpretation and advice. 83 Open-access services may be provided either on hospital premises or in partnership with primary care in a community setting and should be quality assured with appropriate governance arrangements and regular systematic audit. A potential advantage is the inbuilt access to specialist cardiology opinion in the event of abnormal investigations.
A HTA systematic review and economic model of different diagnostic strategies for heart failure in primary care79 analysed the impact of varying the sensitivity/specificity for BNP on the cost-effectiveness of BNP versus echocardiography. Heart failure referrals costs the NHS more than £5M per annum, use of BNP could result in a 30–40% reduction in referral to cardiology outpatient departments, resulting in a 25–40% cost saving. 80 NICE estimated a whole-pathway saving of almost £4M (2010) following the introduction of the test. 85
Only one economic evaluation focusing on POC BNP in a primary care setting is believed to exist. 351 This concluded that the adoption of BNP in primary care is likely to lead to fewer delayed diagnoses for symptomatic heart failure patients at a very small increased cost relative to referring all patients for an ECG. Results were subject to significant uncertainty relating to such parameters as the relative unit cost of the BNP test and ECG, and the accuracy and sensitivity of the assays. 81
An alternative to a primary-care based approach involves using a local NHS trust laboratory. The NHS improvement report84 concludes that this is often a more cost-effective option, citing a likely correlation between the number of tests and the cost per test. The more tests being done, the greater the likely cost–benefit. Quality control, equipment updates and staff training issues are routinely addressed within the remit of the local service provider. Points to consider are the length of time for the results to be returned (from < 24 hours up to 1 week), the courier service required to transport the samples to the provider, transport difficulties for patients and the importance of a robust service-level agreement.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, cardiac services is attributed a LOW degree of implementation difficulty owing to existing pathways for the management of heart disease and heart failure.
Conclusion
Benefits of a BNP service include rapid results, convenience for patients, early diagnosis and commencement of treatment. Centralising in one agreed location allows one GP practice to serve several surgeries: allowing results to be available within a few days, or even on the same day. Benefits include convenience for the patient, it is cheaper than doing the test in individual surgeries, and results may still be available within an acceptable time span (depending on the agreement). This type of service may involve the transportation of samples from or to the testing centre and this must be factored into planning.
In some parts of the UK, the introduction of BNP has led to increased numbers of echo tests being performed without an increase in true positives. This means that if the test is used indiscriminately, false-positive results may outnumber true positives. A local agreement may help to decide who should order the test or when access to ordering a BNP test is appropriate. A further consideration when calculating costs is that a percentage of positive BNP results will require an echocardiogram is also indicated. The cost of the BNP test will need to be added to the cost of an echocardiogram. Many studies have identified savings with an increase in the number of appropriate referrals once those testing negative had been set on alternative pathways of care. NT-proBNP concentrations increase with age in the normal population. Some areas, through collaboration and agreement, have introduced age-related ‘cut-off’ values when deciding which patients to refer for further investigations.
The NHS Improvement Heart Improvement Network has identified examples in the UK when BNP testing has been used as a means of ‘rule-out’ for echocardiography. 80 Some documents include economic information and cost analysis for the projects. NICE guidelines on the initial diagnosis of chronic heart failure and referral for echocardiography recommend use of BNP in combination with clinical assessment. 85 NICE guidelines recommend the measurement of serum natriuretic peptides in patients with suspected heart failure without previous myocardial infarction, and those with previous MI should be referred for an urgent echocardiogram. 85 Although several hospital laboratories carry out BNP testing, few return results within a day. POC BNP testing can considerably reduce turnaround time and could lead to earlier initial treatment, more timely referral and less uncertainty for patients. Using POC BNP levels to quickly rule out heart failure, could allow more rapid initiation of investigation of other causes of dyspnoea.
A UK HTA carried out a comprehensive systematic review and meta-analysis of all studies comparing the diagnostic accuracy of BNP testing to clinical examination by cardiologists in heart failure in all settings until 2006. 79 The review included 20 studies on the accuracy of BNP for the diagnosis of clinically defined heart failure. Four studies took place in primary care, one used the POC test and three used laboratory-based tests. These demonstrated a slightly lower sensitivity (84%) but similar specificity (73%). Eight of 16 studies reporting data on the accuracy of NT-proBNP for the diagnosis of clinically defined heart failure were conducted on samples from patients presenting in primary care (pooled sensitivity of 90%, a specificity of 60%), The HTA report also highlighted that the utility of BNP testing will depend on the pre-test probability of chronic heart failure in the patient. 79 BNP testing would contribute important diagnostic information as a negative test would reduce the post-test probability. A US technology assessment gave similar results. 86
A systematic review and meta-analysis of diagnostic accuracy of BNP testing for heart failure in the emergency department, in which 9 of the 11 studies used the triage system, showed that the ≤ 100 pg/ml cut-off value used in most studies has a sensitivity of 0.93 and specificity of 0.66. 87 Two other systematic reviews that included the same studies achieved similar results. 88,89 However, most studies on the triage BNP system were performed in a US emergency setting and so cut-off values for primary care may be different.
Although a study investigating the impact of POC NT-proBNP testing with the Cobas h232 has yet to be carried out, feedback on its ease of use has been documented. 90 In this study the nurses on duty in the coronary care unit, who operated the device, found it simple to learn and handle. The Horizon Scanning report observes that most published studies have investigated the use of BNP in an emergency department or hospital setting. 81 It concludes that more studies are required on the diagnostic accuracy of POC BNP tests in primary care.
Cardiology (C1–54)
C1. British Cardiovascular Society. Accreditation in Cardiac Imaging. URL: www.bcs.com/documents/Accreditation_in_Cardiac_Imaging.pdf (accessed 28 August 2015).
C2. A’Court C, Stevens R, Sanders S, Ward A, McManus R, Heneghan C. Type and accuracy of sphygmomanometers in primary care: a cross-sectional observational study. Br J Gen Pract 2011;61:e598–603.
C3. Adlam D, Silcocks P, Sparrow N. Using BNP to develop a risk score for heart failure in primary care. Eur Heart J 2005;26:1086–93.
C4. Alehagen U, Janzon M. A clinician’s experience of using the Cardiac Reader NT-proBNP point-of-care assay in a clinical setting. Eur J Heart Fail 2008;10:260–6.
C5. Ashworth M, Gordon K, Baker G, Deshmukh A. Sphygmomanometer calibration: a survey of one inner-city primary care group. J Hum Hypertens 2001;15:259–62.
C6. Balion C, Don-Wauchope A, Hill S, Santaguida PL, Booth R, Brown JA, et al. Use of Natriuretic Peptide Measurement in the Management of Heart Failure. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. URL: www.ncbi.nlm.nih.gov/books/NBK179184/ (accessed 28 August 2015).
C7. Campbell SM, Fuat A, Summerton N, Lancaster N, Hobbs FR. Diagnostic triage and the role of natriuretic peptide testing and echocardiography for suspected heart failure: an appropriateness ratings evaluation by UK GPs. Br J Gen Pract 2011;61:e427–35.
C8. Chapman AR, Leslie SJ, Walker SW, Bickler C, Denvir MA. Potential costs of B-type natriuretic peptide for the identification of people with heart failure in primary care in Scotland – a pilot study. J R Coll Physicians Edinb 2015;45:27–32.
C9. Collinson PO. The cost effectiveness of B-Type natriuretic peptide measurement in the primary care setting-a UK perspective. Congest Heart Fail 2006;12:103–7.
C10. British Cardiovascular Society. Commissioning of Cardiac Services – A Resource Pack from the British Cardiovascular Society. 2011. URL: www.bcs.com/documents/Commissioning_of_Cardiac_Services_finalv21.pdf (accessed 28 August 2015).
C11. Cowie MR. B type natriuretic peptide testing: where are we now? Heart 2004;90:725–6.
C12. Day A, Oldroyd C, Godfrey S, Quinn T. Availability of cardiac equipment in general practice premises in a cardiac network: a survey. Br J Cardiol 2008;15:141–4.
C13. Department of Health. National Service Framework for Coronary Heart Disease. London: Department of Health; 2000.
C14. Department of Health. National Service Framework for Coronary Heart Disease. Chapter 8: Arrhythmias And Sudden Cardiac Death. London: Department of Health; 2005.
C15. Doll H, Shine B, Kay J, James T, Glasziou P. The rise of cholesterol testing: how much is unnecessary. Br J Gen Pract 2011;61:e81–8.
C16. Fahey T, Schroeder K. Cardiology. Br J Gen Pract 2004;54:695–702.
C17. Fuat A, Murphy JJ, Brennan G, Mehrzad AA, Johnston JI, Smellie WSA. Suspected heart failure in primary care – the utility of N-terminal proB-Type natriuretic peptide (NTproBNP) as a pre-screening test for secondary care referral: a real life study. Heart 2005;91(Suppl. I):A22; and Eur Heart Fail J 2005;4(Suppl. 1):128.
C18. Fuat A, Murphy JJ, Brennan G, Mehrzad AA, Johnston JI, Smellie WSA. Screening for suspected heart failure with N-terminal proB-Type natriuretic peptide (NTproBNP) in primary care: Money Well Spent? Heart 2005;91(Suppl. I):A56; and Eur Heart Fail J 2005;4(Suppl. 1):128.
C19. Fuat A, Murphy JJ, Hungin AP, Curry J, Mehrzad AA, Hetherington A, et al. The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 2006;56:327–33.
C20. Fuat A. Improving diagnosis and management of heart failure across the primary-secondary care interface – a review of the evidence base and the Darlington service delivery model. Clin Focus Prim Care 2005;1:43–8.
C21. Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ 2003;326:196.
C22. Gabhainn SN, Murphy AW, Kelleher C. A national general practice census: characteristics of rural general practices. Fam Pract 2001;18:622–6.
C23. Galasko GI, Lahiri A, Senior R. Portable echocardiography: an innovative tool in screening for cardiac abnormalities in the community. Eur J Echocardiogr 2003;4:119–27.
C24. Galasko GI, Barnes SC, Collinson P, Lahiri A, Senior R. What is the most cost-effective strategy to screen for left ventricular systolic dysfunction: natriuretic peptides, the electrocardiogram, hand-held echocardiography, traditional echocardiography, or their combination? Eur Heart J 2006;27:193–200.
C25. Hamilton W, Round A, Goodchild R, Baker C. Do community based self-reading sphygmomanometers improve detection of hypertension? A feasibility study. J Public Health Med 2003;25:125–30.
C26. Hancock HC, Close H, Fuat A, Murphy JJ, Hungin AP, Mason JM. Barriers to accurate diagnosis and effective management of heart failure have not changed in the past 10 years: a qualitative study and national survey. BMJ Open 2014;4:e003866.
C27. Heart Improvement Programme Brain-type Natriuretic Peptide (BNP). An Information Resource for Cardiac Networks. URL: www.gmccsn.nhs.uk/files/3113/5246/5920/NHS_Improvement_-_Brain-type_Natriuretic_Peptide_BNP_2007.pdf (accessed 28 August 2015).
C28. Hill SA, Balion CM, Santaguida P, McQueen MJ, Ismaila AS, Reichert SM, et al. Evidence for the use of B-type natriuretic peptides for screening asymptomatic populations and for diagnosis in primary care. Clin Biochem 2008;41:240–9.
C29. Horizon Scanning Report 0019 28 September 2011. Point-of-Care B-type Natriuretic Peptide Testing. URL: www.oxford.dec.nihr.ac.uk/reports-and-resources/horizon-scanning-reports/horizon-scan-reports-non-dec-funded (accessed 7 September 2016).
C30. Kelder JC, Cowie MR, McDonagh TA, Hardman SM, Grobbee DE, Cost B, et al. Quantifying the added value of BNP in suspected heart failure in general practice: an individual patient data meta-analysis. Heart 2011;97:959–63.
C31. Kelshiker A, Hannah, D. Telecardiology Speeds Referrals. Independent Nurse; 2008.
C32. Khunti K, Hearnshaw H, Baker R, Grimshaw G. Heart failure in primary care: qualitative study of current management and perceived obstacles to evidence-based diagnosis and management by general practitioners. Eur J Heart Fail 2002;4:771–7.
C33. Korenstein D, Wisnivesky JP, Wyer P, Adler R, Ponieman D, McGinn T. The utility of B-type natriuretic peptide in the diagnosis of heart failure in the emergency department: a systematic review. BMC Emerg Med 2007;7:6.
C34. Latour-Pérez J, Coves-Orts FJ, Abad-Terrado C, Abraira V, Zamora J. Accuracy of B-type natriuretic peptide levels in the diagnosis of left ventricular dysfunction and heart failure: a systematic review. Eur J Heart Fail 2006;8:390–9.
C35. Leslie SJ, McKee SP, Imray EA, Denvir MA. Management of chronic heart failure: perceived needs of general practitioners in light of the new general medical services contract. Postgrad Med J 2005;81:321–6.
C36. Lim TK, Dwivedi G, Hayat S, Collinson PO, Senior R. Cost effectiveness of the B type natriuretic peptide, electrocardiography, and portable echocardiography for the assessment of patients from the community with suspected heart failure. Echocardiography 2007;24:228–36.
C37. MacKenzie E, Smith A, Angus N, Menzies S, Brulisauer F, Leslie SJ. Mixed-method exploratory study of general practitioner and nurse perceptions of a new community based nurse-led heart failure service. Rural Remote Health 2010;10:1510.
C38. Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess 2009;13(32).
C39. Murphy JJ, Chakraborty RR, Fuat A, Davies MK, Cleland JG. Diagnosis and management of patients with heart failure in England. Clin Med 2008;8:264–6.
C40. NICE. Chronic Heart Failure. National Clinical Guidelines for Diagnosis and Management in Primary and Secondary Care. London: NICE; 2003.
C41. NHS Improvement. Heart Failure: Use of BNP/NTproBNP testing in primary care to facilitate early diagnosis. URL: www.evidence.nhs.uk/QIPP (accessed 4 January 2016).
C42. Nielsen OW, Cowburn PJ, Sajadieh A, Morton JJ, Dargie H, McDonagh T. Value of BNP to estimate cardiac risk in patients on cardioactive treatment in primary care. Eur J Heart Fail 2007;9:1178–85.
C43. Penney MD. Natriuretic peptides and the heart: current and future implications for clinical biochemistry. Ann Clin Biochem 2005;4212:432–40.
C44. Petersen S, Rayner M, Wolstenholme J. Coronary Heart Disease Statistics: Heart Failure Supplement 2002 edition. British Heart Foundation; 2002.
C45. Pollard L, Rogers S, Shribman J, Sprigings D, Sinfield P. A study of role expansion: a new GP role in cardiology care. BMC Health Serv Res 2014;14:205.
C46. Scarborough P, Bhatnagar P, Wickramasinghe K, Smolina K, Mitchell C, Rayner M. Coronary Heart Disease Statistics 2010 Edition. British Heart Statistics Database. URL: www.heartstats.org (accessed 28 August 2015).
C47. Schaufelberger M, Bergh CH, Caidahl K, Eggertsen R, Furenäs E, Lindstedt G, et al. Can brain natriuretic peptide (BNP) be used as a screening tool in general practice? Scand J Prim Health Care 2004;22:187–90.
C48. Scott MA, Price CP, Cowie MR, Buxton MJ. Cost-consequences analysis of natriuretic peptide assays to refute symptomatic heart failure in primary care. Br J Cardiol 2008;15:199–206.
C49. Senior R, Galasko G, McMurray JV, Mayet J. Screening for left ventricular dysfunction in the community: role of hand held echocardiography and brain natriuretic peptides. Heart 2003;89(Suppl. 3):iii24–8.
C50. Smellie WSA, Duffy V, Cawley P. The diagnostic accuracy and utility of natriuretic peptides in a community population of patients with suspected heart failure, using near patient and laboratory assay methods. Br J Gen Pract 2006;56:327–33.
C51. Vaes B, Stalpaert S, Tavernier K, Thaels B, Lapeire D, Mullens W, et al. The diagnostic accuracy of the MyDiagnostick to detect atrial fibrillation in primary care. BMC Fam Pract 2014;15:113.
C52. Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005;294:1944–56.
C53. Williams D. Setting up a cardiovascular clinic in primary care. Nurs Stand 2006;20:45–50.
C54. Zaphiriou A, Robb S, Murray-Thomas T, Mendez G, Fox K, McDonagh T, et al. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail 2005;7:537–41.
Electrocardiography
Definition: an ECG is a simple test used to check the heart’s rhythm and electrical activity (NHS Choices).
This map includes portable ECGs.
This map excludes N/A.
Contains public sector information licensed under the Open Government Licence v3.0. 91
Electrocardiograms are seen as standard provision for many types of investigation. A survey of Irish GP rural practices in 2001 found that practices were most likely to include equipment such as ECGs and oxygen. 352 Developments in cardiac diagnostic services include 24-hour ambulatory BP monitoring and ambulatory electrocardiography for the diagnosis of arrhythmias in primary care. 346 Given that the availability of ECG equipment is already likely to be highest among the equipment examined in this report, this section will selectively take a brief look at handheld ECG devices.
The wireless, electrode-free and portable nature of devices means fewer lifestyle restrictions on the patient. Patients can bring the device directly to their GP, who can ‘review’ the recorded ECGs and analyse them directly. ECGs can be done on the spot by GPs, during a patient’s medical review, to assess both rate and rhythm control, rather than organising a separate ECG appointment.
Current NICE guidelines for management of AF recommend patient-activated event recording to detect AF, if the AF is symptomatic and episodes last longer than 24 hours. Existing guidelines do not contain any recommendations about the use of electrode-free handheld patient-activated ECG devices.
STEP-UP summary
Skills
Wireless ECG equipment is easy to use. The challenge with ECG devices of any type lies in interpretation. A systematic review of ECG interpretation accuracy studies found that both physicians and computer software frequently made errors, compared with expert electrocardiographers; however, there was also frequent disagreement in interpretation between experts. 92 The largest study investigated the ability of 42 GPs and 41 practice nurses to detect AF on ECGs generated during the SAFE (Screening for Atrial Fibrillation in the Elderly) study. 93 Overall, primary care practitioners could not detect AF on an ECG with sufficient accuracy to guide therapy (GP sensitivity 80%, specificity 92%; practice nurse sensitivity 77%, specificity 85%).
When interpreting ECG studies, it is important to distinguish the effect of using a simpler ECG from the effect of using a non-expert to interpret the trace. 95 Who reads the ECG appears to be a much more important factor than how the reading is obtained. Thus, relatively poor results of single-lead ECGs when interpreted by GPs were similar to the results obtained for 12-lead ECGs when read by GPs. 93 In contrast, two studies found high sensitivity (92% and 96%) and high specificity (96% and 98%) when a bipolar ‘thumb’ ECG was read by a cardiologist and a bipolar ECG was read by an experienced GP, respectively. 353,354
Interpretative software depends on the diagnostic algorithm that it uses. Three studies that evaluated the accuracy of computer software for detecting AF found similar results even though different software was employed. 95 This suggests that there may be some consistency between the algorithms used. Improvements in diagnostic algorithms in the future may make interpretative software good enough to become a diagnostic gold standard.
Quality control of the interpretation of ECGs is an important aspect of diagnosis of AF in primary care. 95 Potential strategies to address this are to provide training to HCPs who regularly read ECGs or to have ECGs centrally read. However, in connection with the latter, the performance of cardiologists when they had no patient contact was significantly poorer than when they were the ordering physician (72 vs. 94%).
STEP-UP summary statement With regard to SKILLS, ECG is attributed a LOW degree of implementation difficulty owing to the fact that a large majority of practices have the equipment, although interpretation is variable.
Training
The NICE guidance in relation to management of transient loss of consciousness advises that all service providers should have capacity to undertake 12-lead ECGs and that NHS England area teams should ensure that GP surgeries are able to undertake initial assessments with these patients and interpret the results competently. The guidance notes that skill can be lost in general practice if it is not used frequently and commissioners should ensure that training on interpretation is offered linked to local need (the national training cost is quoted to be £105 per person). 94 No additional training is required. 355 The interpretation of readings from the ECG is included in standard medical and other HCP training. Some practices may feel that they are too small to develop expertise in ECG interpretation and it may present opportunities for local practices to work together rather than refer to secondary care.
In a survey of cardiac equipment use in a cardiac network, 64 responding practices (85%) had 12-lead ECG machines and 58 (91%) used these on a weekly or more frequent basis. 76 Eighty per cent of these machines had a service contract and an identified responsible individual in the practice to oversee this role. A higher proportion of registered nurses (n = 64, 97%) had received formal training in the use of ECG machines than GPs (n = 47, 73%), although the interpretation of ECG recordings is predominantly undertaken by GPs (n = 60, 94%) rather than registered nurses (n = 8, 13%). Substantive evidence on the training required to obtain and sustain competence in ECG interpretation is not available. Twenty-seven per cent of respondents reported that their practice members require additional training in 12-lead ECG use.
Most ECG machines have interpretative software, but combining interpretative software with GP interpretation does not improve the sensitivity of diagnosis significantly. The evidence suggests that GPs can detect AF on ECGs accurately, given appropriate training. 95
STEP-UP summary statement With regard to TRAINING, ECG is attributed a LOW degree of implementation difficulty owing to the availability of training provision, although many training needs are not fulfilled.
Equipment
A new ECG machine with installation and initial training costs about £2000 including VAT. 94 The reference standard test in diagnosing AF, a 12-lead ECG, is readily available, non-invasive and relatively inexpensive. Its main drawbacks are that it is time-consuming to use and requires some degree of privacy to perform. A single-lead ECG avoids the need for the patient to remove clothing and is quicker to perform than a 12-lead ECG. However, inevitably some information is lost, which may lead to a reduced ability to detect AF.
Increasingly, portable ECG machines have become an attractive option. Handheld ECG devices allow ECG readings to be stored on a portable device, for review at a later time, by transferring the data to a personal computer, ‘playing back’ the ECG on the device screen or transmitting the reading via ‘telemedicine’. Unlike 12-lead ECGs, which require electrodes to be placed on the patient and connected to the ECG, handheld devices have integral electrodes. They require a patient’s thumbs, fingers or palms to be placed on the device, and, in some cases, the holding of the device against the chest. The devices are battery powered and can store multiple ECG tracings. Some devices include an analysis function, whereby they instantly alert the user if a trace indicates an arrthymia.
The Horizon Scanning report documents devices that can be used very quickly in primary care for screening or in acutely unwell patients, as well as a monitoring device over a period of time. 96,356 The Merlin wristwatch (www.medgadget.com) is the most compact and portable device and may be ideal to use as an event recorder by patients at home. Memory capacity is important for devices used as event recorders at home. Some devices have capacity for storing approximately 100 readings of 30 seconds each. Other devices are independent of a telemedicine system, so they can be purchased and will operate on a standalone basis. Devices which have an analysis function to identify when a patient is in AF would be valuable for nurse-led or GP-led opportunistic screening for AF. A new device, the ‘Cardiobip’™ (NewCardio), is yet to be available commercially (www.newcardio.com/products-cardio-bip.php). It uses telemedicine and is operated by holding the device against the chest and touching two integral finger electrodes.
Two devices could be used for screening for AF in the general population. 97 One is a finger probe similar to that used in general practice for pulse oximetry; the other is a modified BP monitor as used by patients to monitor their BP at home. 98–100 The latter could be used either by people monitoring their own BP to self-screen for AF or by primary care professionals to opportunistically screen patients. These devices are able to adjust their sensitivity to optimise their value as screening devices. Studies evaluating newer technologies (e.g. finger probes and modified BP readings) suggest that a sensitivity of > 90% could be achieved while maintaining reasonable specificity. 95
A UK study355 performed in six general practices assessed the performance of a modified BP monitor and two single-lead ECG devices, as diagnostic triage tests for the detection of AF. One thousand ambulatory patients aged ≥ 75 years were diagnosed using a modified BP monitor and single-lead ECG devices, compared with reference standard of 12-lead ECG, independently interpreted by cardiologists. All three devices had a high sensitivity and are useful for ruling out AF. WatchBP is a better triage test than Omron autoanalysis because it is more specific. The study concluded that WatchBP performs better as a triage test for identifying AF in primary care than the single-lead ECG monitors. It does not require expertise for interpretation and its diagnostic performance is comparable with single-lead ECG analysis by cardiologists. It could be used opportunistically to screen elderly patients for undiagnosed AF at regular intervals and/or during BP measurement.
The AliveCor Heart Monitor and AliveECG apps are, respectively, a pocket-sized ECG recorder and a mobile device application for analysis and communication of the results. 101 Two fingers from each hand are placed on the AliveCor Heart Monitor to record an ECG, which is transmitted wirelessly to the AliveECG app. Two clinical studies reported that the AliveCor Heart Monitor and the AliveECG app have sensitivity above 85% and specificity above 90% in identifying AF. An AliveCor Heart Monitor unit costs £62.49, excluding VAT; the AliveECG app is free of charge. If proven to be accurate and cost-effective in the primary care setting, these devices could improve the detection and management of AF, thus reducing the well-known risks of stroke, transient ischaemic attacks or heart failure in these patients.
STEP-UP summary statement With regard to EQUIPMENT, ECG is attributed a LOW degree of implementation difficulty owing to the availability of a wide range of relatively cheap and acceptably accurate equipment that can be matched to clinical need.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates ECG in the ‘Examination/Therapy room’ (contains public sector information licensed under the Open Government Licence v3.0). In the remaining literature, little detail is available of the premises in which ECGs, portable or not, are to be stored, cleaned or maintained. This corresponds with the findings from the survey of equipment from a UK cardiac network. 76
STEP-UP summary statement With regard to PREMISES, ECG is attributed an UNCERTAIN degree of implementation difficulty owing to insufficient detail about storage requirements.
User perspective
Portable devices are designed to be easy to use; it has been reported that 4-year-old children were able to use such a device. The ease-of-use, convenience and portable nature of these devices means implementation could see a better rate of patient compliance than to a Holter monitor in patients who use the device for home recordings. Future developments may see this technology being integrated into smartphones for wider applicability in clinical and home settings. 102
STEP-UP summary statement With regard to the USER PERSPECTIVE, ECG is attributed a LOW degree of implementation difficulty owing to the ease of use, particularly of wireless devices.
Primary–secondary interface
Excessive delays for cardiac patients waiting for a hospital appointment led Harrow PCT to establish a mobile cardiac task force based in the community, involving GPs, specialist nurses and health-care assistants supported by hospital-based heart disease specialists. 103 The service was delivered in community-based settings: in GP surgeries, community health-care centres and in patients’ homes. The service used a portable ECG system to automatically store and analyse recorded ECG data using existing computer hardware. Every general practice in the area is networked to a central server, allowing patients’ cardiac data to be requested by individual GPs and the results returned to them electronically. The service has reduced waiting times and improved patient care.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, ECG is attributed a LOW degree of implementation difficulty owing to the already integrated nature of the diagnostic and management pathway.
Conclusion
The increasing portability, ease of use and low prices of ECG equipment has led to an almost universal diffusion in NHS primary care. Issues therefore relate to choice of the most appropriate equipment for practice needs, including an optimal mix of static and portable equipment. The reduction in the number of leads in ECG systems makes them easier to use and more acceptable to both patient and clinician. Of particular value is the potential for small ECG devices to replace the 24-hour Holter monitor tests. Organisational issues concerning loans of, and maintenance for, equipment to patients may need to be addressed. Similar concerns will relate to ambulatory BP monitoring.
Electrocardiography (EC1–29)
EC1. NICE. Alive Cor Heart Monitor and Alive ECG App for Detecting Atrial Fibrillation. 2015. URL: www.nice.org.uk/guidance/mib35 (accessed 28 August 2015).
EC2. Backman W, Bendel D, Rakhit R. The telecardiology revolution: improving the management of cardiac disease in primary care. J R Soc Med 2010;103:442–6.
EC3. Barclay JL, Macfarlane P, Potts S, Leslie SJ. Evaluation of a new device for the transmission of electrocardiograms by email. J Telemed Telecare 2008;14:219–20.
EC4. Caldwell JC, Borbas Z, Donald A, Clifford A, Bolger L, Black A, et al. Simplified electrocardiogram sampling maintains high diagnostic capability for atrial fibrillation: implications for opportunistic atrial fibrillation screening in primary care. Europace 2012;14:191–6.
EC5. Day A, Oldroyd C, Godfrey S, Quinn T. Availability of cardiac equipment in general practice premises in a cardiac network: a survey. Br J Cardiol 2008;15:141–4.
EC6. Doliwa PS, Frykman V, Rosenqvist M. Short-term ECG for out of hospital detection of silent atrial fibrillation episodes. Scand Cardiovasc J 2009;43:163–8.
EC7. Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ 2003;326:196.
EC8. Gabhainn SN, Murphy AW, Kelleher C. A national general practice census: characteristics of rural general practices. Fam Pract 2001;18:622–6.
EC9. Gnani Shamini. Influences on general practitioners’ use of pre-hospital thrombolysis: a qualitative study. J Public Health 2004.
EC10. Goudie BM, Jarvis RI, Donnan PT, Sullivan FM, Pringle SD, Jeyaseelan S, et al. Screening for left ventricular systolic dysfunction using GP-reported ECGs. Br J Gen Pract 2007;57:191–5.
EC11. Monitoring and Diagnosis in Oxford. Diagnostic Technology: Hand-Held ECG Monitors for the Detection of Atrial Fibrillation (Af) in Primary Care. Oxford: Monitoring and Diagnosis in Oxford; 2013.
EC12. Monitoring and Diagnosis in Oxford. Diagnostic Technology: Handheld HeartScan ECG Monitor for Detecting Atrial Fibrillation in Primary Care. Oxford: Monitoring and Diagnosis in Oxford; 2009.
EC13. Harris K, Edwards D, Mant J. How can we best detect atrial fibrillation? J R Coll Physicians Edinb 2012;42(Suppl. 18):5–22.
EC14. Hannah D, Pope J. Harrow Primary Care Trust takes cardiac care into the community. British Journal of Healthcare Computing and Information Management 2009.
EC15. Hobbs FD, Jones MI, Allan TF, Wilson S, Tobias R. European survey of primary care physician perceptions on heart failure diagnosis and management (Euro-HF). Eur Heart J 2000;21:1877–87.
EC16. NHS Dorset CCG Joint Primary Care Commissioning Committee. ECG Capability Within Primary Care. Dorset: NHS Dorset CCG; 2015. URL: www.dorsetccg.nhs.uk/Downloads/aboutus/Primary%20Care%20Commissioning%20Committee/5%20August%202015/10.4%20ECG%20within%20Primary%20Care%20report%20050815.pdf (accessed 19 October 2016).
EC17. Kearley K, Selwood M, Van den Bruel A, Thompson M, Mant D, Hobbs FR, et al. Triage tests for identifying atrial fibrillation in primary care: a diagnostic accuracy study comparing single-lead ECG and modified BP monitors. BMJ Open 2014;4:e004565.
EC18. Kelder JC, Cramer MJ, van Wijngaarden J, van Tooren R, Mosterd A, Moons KG, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011;124:2865–73.
EC19. Lim TK, Collinson PO, Celik E, Gaze D, Senior R. Value of primary care electrocardiography for the prediction of left ventricular systolic dysfunction in patients with suspected heart failure. Int J Cardiol 2007;115:73–4.
EC20. Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008;9:56.
EC21. Mant J, Fitzmaurice DA, Hobbs FD, Jowett S, Murray ET, Holder R, et al. Accuracy of diagnosing atrial fibrillation on electrocardiogram by primary care practitioners and interpretative diagnostic software: analysis of data from screening for atrial fibrillation in the elderly (SAFE) trial. BMJ 2007;335:380.
EC21. Mant J, McManus RJ, Oakes RA, Delaney BC, Barton PM, Deeks JJ, et al. Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care. Health Technol Assess 2004;8(2).
EC22. Mooney H. ECG and arrhythmic services. The heart of diagnostics. Health Serv J 2012;122:S10–13.
EC23. Oresko JJ, Duschl H, Cheng AC. A wearable smartphone-based platform for real-time cardiovascular disease detection via electrocardiogram processing. IEEE Trans Inf Technol Biomed 2010;14:734–40.
EC24. Salerno SM, Alguire PC, Waxman HS. Competency in interpretation of 12-lead electrocardiograms: a summary and appraisal of published evidence. Ann Intern Med 2003;138:751–60.
EC25. Somerville S, Somerville J, Croft P, Lewis M. Atrial fibrillation: a comparison of methods to identify cases in general practice. Br J Gen Pract 2000;50:727–9.
EC26. Stergiou GS, Karpettas N, Protogerou A, Nasothimiou EG, Kyriakidis M. Diagnostic accuracy of a home blood pressure monitor to detect atrial fibrillation. J Hum Hypertens 2009;23:654–8.
EC27. Stott DJ, Dewar RI, Garratt CJ, Griffith KE, Harding NJ, James MA, et al. RCPE UK Consensus Conference on ‘approaching the comprehensive management of atrial fibrillation: evolution or revolution?’. J R Coll Phys Edinb 2012;42:3. URL: www.rcpe.ac.uk/sites/default/files/files/supplement-18.pdf (accessed 28 August 2015).
EC28. Wiesel J, Fitzig L, Herschman Y, Messineo FC. Detection of atrial fibrillation using a modified microlife blood pressure monitor. Am J Hypertens 2009;22:848–52.
EC29. Williams P, Bond C, Hannaford P, Ritchie L. Influences on general practitioners’ use of pre-hospital thrombolysis: a qualitative study. J Public Health 2004;26:38–41.
Echocardiography
Definition: an echocardiogram is a test that uses sound waves to create pictures of the heart. The picture is more detailed than a standard X-ray image. An echocardiogram does not expose the body to radiation.
This map includes portable and fixed echocardiography.
This map excludes other forms of ultrasound (see Ultrasound).
Patients with breathlessness and ankle oedema presenting to their GP may have heart failure. Echocardiography is the key investigation for assessing suspected heart failure patients. In 2003, the availability of echocardiography in the UK was rated as suboptimal. 82,357
STEP-UP summary
Skills
Echocardiography is pivotal in the management of heart failure as it can make the diagnosis, determine the aetiology and aid the planning of treatment. 104 Community echocardiography is also indicated for heart murmurs, AF and hypertension. In a study from the Netherlands, GPs used the open-access echocardiography service efficiently: only 24% of referrals did not yield relevant disease. 105 An echocardiography service must be integral to a general local plan for heart failure to avoid isolated open-access echocardiography. Community and hospital-based medical care should be located on a continuum.
Echocardiography in heart failure has a place alongside BNP and 12-lead ECG. If these investigations are normal, further investigation by electrocardiography is not indicated. Diagnosis of diastolic heart failure remains difficult and must be made from the clinical context and a complex echocardiogram. NICE guidelines state that Doppler two-dimensional (2D) echocardiographic examination should be performed to exclude important valve disease, assess the systolic (and diastolic) function of the (left) ventricle and detect intracardiac shunts. 85 NICE guidelines state that echocardiography should be performed on high-resolution equipment by experienced operators trained to the relevant professional standards. 85 Need and demands should not compromise quality. The reporting of echocardiography should be performed by those experienced in doing so.
A UK study106 comparing community echocardiography with hospital echocardiography in 136 suspected heart failure patients found that community echocardiography gave results comparable with traditional hospital echocardiography for LVSD detection and for significant valvular disease detection.
STEP-UP summary statement With regard to SKILLS, echocardiography is attributed a MODERATE degree of implementation difficulty owing to difficulties in diagnosis.
Training
Echocardiography services must include provision for training, quality assurance, CPD, referral for a second opinion for difficult studies and clinical back-up if problems are identified by the echocardiography procedure. The BSE has endorsed voluntary accreditation procedures for their members. The accreditation has no statutory role but aims to allow medical and non medical practitioners to demonstrate that they have achieved a specified level of experience in an appropriate educational environment. Some GPwSIs or practitioners with a special interest are already expert and experienced echocardiographers, with increasing numbers likely to be trained in general cardiology at diploma courses (e.g. South Middlesex and Bradford). Courses discuss the indications for and interpretation of echoardiography reports and also offer practical exposure to echocardiography. BSE accreditation in community echocardiography qualifies a GPwSI or practitioner with a special interest in standard echocardiography, being equivalent to the BSE adult accreditation process. It differs in reflecting a specific community focus, rather than hospital-based case mix. The accreditation involves a written examination and a log book of 200 cases. Accreditation is gained with experience and training in a BSE-accredited department or under supervision using a portable machine in the community, with regular sessions at a hospital department.
There is no definitive source of information on workforce levels needed to provide community echocardiography services. However, the British Cardiac Society report of June 2005358 suggests that each acute hospital should have a consultant cardiologist with a special interest in heart failure who spends two programmed activities per week (one programmed activity for a medical consultant = 4 hours) directly leading to the management of patients with heart failure including diagnosis and monitoring. In addition, the report suggest that 2.1 whole-time equivalent non consultant specialists per million population (nurse, physiologist, GPwSI, non-consultant medical) are needed to provide diagnostic services for patients with heart failure and 14.6 whole-time equivalent per million population are needed to provide monitoring services.
The provision of echocardiography services, regardless of their setting, must link with staff training and CPD, which in turn informs workforce planning. The recommendations in the Welsh Guidelines on Community Echocardiography endorse the BSE accreditation and reaccreditation process. 220 A Royal College of Physicians’ report highlighted the difficulty in recruiting suitable technicians for echocardiography. It cites the Whittington hospital, which established its own graduate training programme, but emphasises that solutions should preferably be national not local.
A minimum of 2 years is required to train a practitioner to BSE-accredited standards, whether they are a cardiology registrar, a cardiac physiologist, a GPwSI, a new entrant echocardiographer, a heart failure specialist nurse or other practitioner with a special interest. BSE accreditation in community echocardiography began in October 2004 and will qualify a GPwSI or a practitioner with a special interest in standard echocardiography. The process involves a written examination and a log book of 200 cases.
Echocardiography should be performed and reported by operators trained to the standards set by the BSE (i.e. they should hold accreditation in adult transthoracic echocardiography or adult community echocardiography). Echocardiographic images and data should be recorded and archived; ideally, this should be a digital system that enables telemedicine links. Clinical interpretation of the technical echocardiogram report should be provided by an appropriate clinical specialist (i.e. cardiologist or GPwSI in cardiology). Community echocardiogarphy services should be linked to a local hospital-based echocardiography department. A local quality assurance system should be developed.
Notwithstanding acute shortages of staff who are trained and competent in performing and interpreting echocardiograms, the need and demand for echocardiography should not compromise quality. Quality assurance is of paramount importance in the delivery of all echocardiography services, including those in which patients are directly referred from primary care. BSE accreditation is mandatory for those performing community echocardiography.
As of 2010, Bradford City Teaching PCT offered a postgraduate diploma in cardiology for primary care practitioners with a special interest in conjunction with the University of Bradford. It was not possible to determine if this is still running.
In the UK study106 comparing community echocardiography with community echocardiography, the community echocardiography was performed by a cardiology trained research fellow. The authors identified a need to assess whether or not other HCPs (e.g. nurses) could be trained to successfully to provide similar accuracy with community echocardiography.
STEP-UP summary statement With regard to TRAINING, echocardiography is attributed a MODERATE degree of implementation difficulty owing to the need for an extended period of certificated training.
Equipment
A ‘standard echocardiogram’ is required for all patients with suspected heart failure and is the required quality standard for community echocardiography. Echocardiograms should be performed on high-quality equipment, which includes high-resolution 2D imaging, tissue harmonic imaging, full-spectral Doppler and colour flow mapping (and, ideally, tissue Doppler capability). The recommended lifespan of echocardiography scanners is 5–7 years. 220
Echocardiography includes at least five separate types of study. 104 The type of study is mainly determined by the clinical question, but also depends on the level of experience of the operator and the type of machine. Standard machines are usually required for standard and advanced studies. Portable systems are capable of ultrasonic stethoscopy and screening alone. 104 However, these divisions are not stable, as portable systems vary in their capability and simpler versions of advanced systems are being introduced for basic functions and without the ability to perform stress or contrast studies.
Advances in ultrasound technology have led to the development of smaller echocardiography machines that may be transported more easily to different sites in the community. Studies have found that smaller echocardiography machines, when used in hospitals by experts, are accurate for detecting LVSD and valvular disease. 252,359 However, studies have not looked at the performance of these smaller echocardiogram machines in community settings where conditions may be different, such as suitable couches, lighting and a lack of immediate second opinion.
The standard (‘standard transthoracic study, ‘traditional’) echocardiogram takes between 30 and 40 minutes to perform. 75 It is regarded as a formal cardiological investigation and should be documented and archived. The limited (‘focused’ or ‘point of care’) echocardiogram is directed at answering specific clinical questions (e.g. changes since a baseline echocardiogram). The procedure takes 5–10 minutes and should also lead to archived material and a formal report. 104,220
STEP-UP summary statement With regard to EQUIPMENT, echocardiography is attributed a LOW degree of implementation difficulty owing to the ease of use and portability of acceptable devices.
Premises
Community service settings for echocardiography may include mobile (including self-contained) units, community hospitals/diagnostic centres, primary care polyclinics/primary care centres/super surgeries, GP practice surgeries and other appropriate location (e.g. walk-in facilities, high street settings). Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates echocardiography in the ‘Treatment room’ (contains public sector information licensed under the Open Government Licence v3.0). This document does not provide detailed design guidance on specific rooms and spaces and refers to the following for guidance on generic rooms and spaces: Health Building Note 00-03, Clinical and Clinical Support Spaces. 153
Open-access community-based services can be located in a community hospital, clinic or GP surgery. The service should complement existing hospital-based provision and be integral to the delivery of heart failure services in a health community. There is no place for isolated stand-alone open-access echocardiography. The service should be delivered by an operator who is BSE accredited. The operator could be a GPwSI or a practitioner with a special interest.
Mobile echocardiography is typically provided by a third party (other than existing primary or secondary care providers), usually a private company. The mobility of the staff and equipment is able to service demand across a large geographical area. Portable systems can equally be provided in a community hospital, clinic or GP surgery but must match the type of echocardiograms performed. Standard echocardiograms require high-quality imaging, including second harmonics together with colour mapping and both pulsed and continuous wave spectral Doppler. Archiving is essential. The service must be integral to delivery of heart failure services and should be delivered by an operator who is BSE accredited. The service should be linked to the hospital-based service (preferably electronic) for second opinions and clinical back-up and to ensure quality assurance. Links to the hospital-based service will also facilitate training and CPD for staff, services and destabilisation of local provision.
Although the performance of smaller echocardiogram machines in community settings has been found to be comparable with that of larger machines in an acute setting, conditions may be different, such as the non-availability of suitable couches and the presence of suboptimal lighting. 106
STEP-UP summary statement With regard to PREMISES, echocardiography is attributed a LOW degree of implementation difficulty owing to the flexibility of locales and settings in which the technology may be administered.
User perspective
A UK qualitative study reports numerous barriers to the use of echocardiography in primary care, such as the lack of availability of diagnostic tests, including electrocardiography, chest radiography and echocardiography, and the lack of confidence in interpreting the results of these. The lack of access to echocardiography is a major obstacle. 82 GPs are keen to have access to echocardiography and, where provided, use it appropriately. 82
A qualitative study of heart failure found that echocardiographic findings were used by 97% of cardiologists, 91% of general physicians, 52% of salaried GPs, 35% of GP partners and 31% of nurses. Cardiologists and nurses were more confident than GPs in using and interpreting echocardiography reports but expressed frustration about patients not routinely referred for echocardiography testing and the poor quality of referral information. Most participants felt that it was important to educate patients about their illness but some expressed concerns about informing patients of their diagnosis as this might lead to anxiety. 107
Perceived handicaps included the variability of open-access echocardiography in the same locality; several open-access services had been funded through pharmaceutical sponsorship but disappeared as ‘monies dried up’. A further perceived problem was variability in echocardiography reporting, some by technicians and some by clinicians, and a lack of guidance for using the procedure or for standardising request forms. The reasons given included uncertainty about the importance of results and interpretation of technical reports, not being able to cope with echocardiography, many preferred to refer the patient to a consultant, and distance to nearest echocardiography clinic may inconvenience patients.
STEP-UP summary statement With regard to USER PERSPECTIVE, echocardiography is attributed a LOW degree of implementation difficulty owing to the ease of use and lack of invasive procedure.
Primary–secondary interface
The demand for echocardiography already exceeds the capacity of current services. GPwSI and practitioners with a special interest are expected to collaborate with hospital-based departments to accommodate increases in demand. The organisation of services should be based around a BSE-accredited department but organised as a network, avoiding barriers between community and hospitals. PCTs and hospital trusts must recognise that the needs of echocardiography extend beyond service delivery to include quality assurance, training and continuing education. Support will usually be provided by a hospital-based BSE-accredited department. The BSE-accredited department and community echocardiographer should ideally have an electronic link for second-opinions, quality assurance and clinical back-up.
The favoured model of care is probably the one-stop heart failure clinic, offering diagnosis and initial treatment while liaising with GPs and nurses for maintenance and palliative services. A community echocardiography service must always be considered in the context of the whole patient pathway for those with suspected heart failure. Services should extend between hospital and community bases as part of a continuum of care irrespective of organisational barriers. Alternatives to community echocardiography include open-access provision and standard provision from a hospital department. Structured, protocol-based referral and reporting processes must be built on effective communications between primary and secondary care. Provision should be subject to regular audit and/or peer review. A study of prescribed diuretics for indications of heart failure suggested that GPs should have a low threshold for referring patients for echocardiography. 108
The rapid-access heart failure clinic locates echocardiography within a heart failure ‘one-stop shop’ linking secondary care-based staff with primary care staff for ongoing care and tertiary staff for specific specialist advice. A patient with suspected heart failure will receive a clinical assessment, echocardiogram, ECG, BNP and chest radiography. The echocardiogram may have been performed as part of (1) an open-access hospital-based service, (2) a community-based service or (3) a mobile echocardiography service provided it has been conducted under BSE-accredited conditions. Each member of staff performing echocardiography should normally carry out at least 100 tests per year.
General practitioners are less likely to use open-access echocardiography when reports were technical and lacked a clinical opinion than when a clinician guided report was available. Local organisational factors around the provision of diagnostic services, such as open-access echocardiography, resources, lack of cardiologists and professional interactions between primary and secondary care shaped practice and decision-making processes among GPs. 107
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, echocardiography is attributed a MODERATE degree of implementation difficulty owing to the need for secondary care support in training, support and referral.
Conclusion
Echocardiography remains a skill with which continues to challenge significant numbers of primary care staff, including GPs. While administration of the technology is comparatively easy (cp. Ultrasonography), the challenge lies in interpretation of the readings. Some GPs may welcome the opportunity to pass on the responsibility for definitive diagnosis and resultant communication to secondary care. Substantive personal and organisational barriers remain in integrating the use of echocardiography into an integrated pathway that spans both primary and secondary care.
Echocardiography (EH1–49)
EH1. Backman W, Bendel D, Rakhit R. The telecardiology revolution: improving the management of cardiac disease in primary care. J R Soc Med 2010;103:442–6.
EH2. Barclay JL, Macfarlane P, Potts S, Leslie SJ. Evaluation of a new device for the transmission of electrocardiograms by email. J Telemed Telecare 2008;14:219–20.
EH3. British Cardiac Society. Cardiac Workforce Requirements in the UK. London: British Cardiac Society; 2005.
EH4. British Society of Echocardiography, British Heart Failure Society, CHD Collaborative and Primary Care Cardiovascular Society. A Consensus Statement from Representatives of the British Society of Echocardiography, British Heart Failure Society, CHD Collaborative and Primary Care Cardiovascular Society. URL: www.bsecho.org/media/13235/community_echocardiography_for_heart_failure.pdf (accessed 28 August 2015).
EH5. Caldwell JC, Borbas Z, Donald A, Clifford A, Bolger L, Black A, et al. Simplified electrocardiogram sampling maintains high diagnostic capability for atrial fibrillation: implications for opportunistic atrial fibrillation screening in primary care. Europace 2012;14:191–6.
EH6. British Society of Cardiovascular Imaging, British Society of Cardiac Computed Tomography. Cardiac Imaging A Report from the National Imaging Board March 2010. URL: www.bsci.org.uk/downloads/category/1-guidelines?download=2:cardiac-imaging-report (accessed 28 August 2015).
EH7. Community Echocardiography Recommendations. Recommendations on the Delivery of Community Echocardiography in Wales. URL: www.wales.nhs.uk/sites3/documents/338/Community%20Echocardiography%20Recommendations.pdf (accessed 28 August 2015).
EH8. Culp BC, Mock JD, Chiles CD, Culp WC. The pocket echocardiograph: validation and feasibility. Echocardiography 2010;27:759–64.
EH9. Dalen H, Haugen BO, Graven T. Feasibility and clinical implementation of hand-held echocardiography. Expert Rev Cardiovasc Ther 2013;11:49–54.
EH10. Day A, Oldroyd C, Godfrey S, Quinn T. Availability of cardiac equipment in general practice premises in a cardiac network: a survey. Br J Cardiol 2008;15:141–4.
EH11. Duvall WL, Croft LB, Goldman ME. Can hand-carried ultrasound devices be extended for use by the noncardiology medical community? Echocardiography 2003;20:471–6.
EH12. Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ 2003;326:196.
EH13. Gabhainn SN, Murphy AW, Kelleher C. A national general practice census: characteristics of rural general practices. Fam Pract 2001;18:622–6.
EH14. Galasko GI, Lahiri A, Senior R. Portable echocardiography: an innovative tool in screening for cardiac abnormalities in the community. Eur J Echocardiogr 2003;4:119–27.
EH15. Galasko GI, Henein M. Direct access echocardiography. Int J Cardiovasc Imaging 2006;22:29–31.
EH16. Gnani S. Influences on general practitioners’ use of pre-hospital thrombolysis: a qualitative study. J Public Health (Oxf) 2004;26:38–41.
EH17. Harris K, Edwards D, Mant J. How can we best detect atrial fibrillation? J R Coll Physicians Edinb 2012;42(Suppl. 18):5–22.
EH18. Hannah D, Pope J. Harrow Primary Care Trust takes cardiac care into the community. Br J Healthcare Comput Inf Manag 2009.
EH19. Hickling JA, Nazareth I, Rogers S. The barriers to effective management of heart failure in general practice. Br J Gen Pract 2001;51:615–18.
EH20. Hobbs FD, Jones MI, Allan TF, Wilson S, Tobias R. European survey of primary care physician perceptions on heart failure diagnosis and management (Euro-HF). Eur Heart J 2000;21:1877–87.
EH21. Jeyaseelan S, Goudie BM, Pringle SD, Donnan PT, Sullivan FM, Struthers AD. A critical re-appraisal of different ways of selecting ambulatory patients with suspected heart failure for echocardiography. Eur J Heart Fail 2007;9:55–61.
EH22. Jeyaseelan S, Goudie BM, Pringle SD, Donnan PT, Sullivan FM, Struthers AD. Agreement between community echocardiography and hospital echocardiography in patients suspected of having left ventricular systolic dysfunction. Postgrad Med J 2005;81:777–9.
EH23. Kelder JC, Cramer MJ, van Wijngaarden J, van Tooren R, Mosterd A, Moons KG, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011;124:2865–73.
EH24. Khunti K. Systematic review of open access echocardiography for primary care. Eur J Heart Fail 2004;6:79–83.
EH25. Khunti K, Hearnshaw H, Baker R, Grimshaw G. Heart failure in primary care: qualitative study of current management and perceived obstacles to evidence-based diagnosis and management by general practitioners. Eur J Heart Fail 2002;4:771–7.
EH26. Kimura BJ, DeMaria AN. Technology insight: hand-carried ultrasound cardiac assessment – evolution, not revolution. Nature Clin Prac Cardiovasc Med 2005;2:217–23.
EH27. Kimura BJ, Shaw DJ, Agan DL, Amundson SA, Ping AC, DeMaria AN. Value of a cardiovascular limited ultrasound examination using a hand-carried ultrasound device on clinical management in an outpatient medical clinic. Am J Cardiol 2007;100:321–5.
EH28. Lafitte S, Alimazighi N, Reant P, Dijos M, Zaroui A, Mignot A, et al. Validation of the smallest pocket echoscopic device’s diagnostic capabilities in heart investigation. Ultrasound Med Biol 2011;37:798–804.
EH29. Liebo MJ, Israel RL, Lillie EO, Smith MR, Rubenson DS, Topol EJ. Is pocket mobile echocardiography the next-generation stethoscope? A cross-sectional comparison of rapidly acquired images with standard transthoracic echocardiography. Ann Intern Med 2011;155:33–8.
EH30. Lim TK, Collinson PO, Celik E, Gaze D, Senior R. Value of primary care electrocardiography for the prediction of left ventricular systolic dysfunction in patients with suspected heart failure. Int J Cardiol 2007;115:73–4.
EH31. Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008;9:56.
EH32. Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess 2009;13(32).
EH33. Mant J, Fitzmaurice DA, Hobbs FR, Jowett S, Murray ET, Holder R, et al. Accuracy of diagnosing atrial fibrillation on electrocardiogram by primary care practitioners and interpretative diagnostic software: analysis of data from screening for atrial fibrillation in the elderly (SAFE) trial. BMJ 2007;335:380.
EH34. Mant J, McManus RJ, Oakes RA, Delaney BC, Barton PM, Deeks JJ, et al. Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care. Health Technol Assess 2004;8(2).
EH35. Mooney H. ECG and arrhythmic services. The heart of diagnostics. Health Serv J 2012;122:S10–3.
EH36. NICE. Chronic Heart Failure. National Clinical Guidelines for Diagnosis and Management in Primary and Secondary Care. London: NICE; 2003.
EH37. Sandler DA, Steeds RP, Hadfield JW. Opening access to echocardiography for general practitioners in North Derbyshire. Br J Cardiol 2000;7:94–100.
EH38. Senior R, Galasko G. Cost-effective strategies to screen for left ventricular systolic dysfunction in the community – a concept. Congest Heart Fail 2005;11:194–8, 211.
EH39. Senior R, Galasko G, McMurray JV, Mayet J. Screening for left ventricular dysfunction in the community: role of hand held echocardiography and brain natriuretic peptides. Heart 2003;89(Suppl. 3):24–8.
EH40. Shah S, Davies MK, Cartwright D, Nightingale P. Management of chronic heart failure in the community: role of a hospital based open access heart failure service. Heart 2004;90:755–9.
EH41. Sparrow N, Adlam D, Cowley A, Hampton JR. The diagnosis of heart failure in general practice: implications for the UK National Service Framework. Eur J Heart Fail 2003;5:349–54.
EH42. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2013;26:567–81.
EH43. van Heur LM, Baur LH, Tent M, Lodewijks-van der Bolt CL, Streppel M, Winkens RA, et al. Evaluation of an open access echocardiography service in the Netherlands: a mixed methods study of indications, outcomes, patient management and trends. BMC Health Serv Res 2010;10:37.
EH44. Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr 2014;27:683–e1.
EH45. Weiner RB, Wang F, Hutter AM, Wood MJ, Berkstresser B, McClanahan C, et al. The feasibility, diagnostic yield, and learning curve of portable echocardiography for out-of-hospital cardiovascular disease screening. J Am Soc Echocardiogr 2012;25:568–75.
EH46. Whalley GA, Wasywich CA, Walsh H, Doughty RN. Role of echocardiography in the contemporary management of chronic heart failure. Expert Rev Cardiovasc Ther 2005;3:51–70.
EH47. Williams P, Bond C, Hannaford P, Ritchie L. Influences on general practitioners’ use of pre-hospital thrombolysis: a qualitative study. J Public Health 2004;26:38–41.
EH48. Williams SG, Currie P, Silas JH. Open access echocardiography: a prospective audit of referral patterns from primary care. Int J Clin Pract 2003;57:136–9.
EH49. Zamorano JL, Moreno R, Alburquerque C. Echocardiography performed by physicians outside of echo-labs – is it possible? Eur Heart J 2002;23:908–9.
Diabetic services
Definition: tests to establish the presence of diabetes or to monitor the progress of the condition, especially its sequelae relating to eye and foot consequences.
This map includes HbA1c, diabetic retinopathy tests, diabetic neuropathy tests.
This map excludes POC tests of more general use (see Point-of-care testing).
Diabetes and its complications pose a major financial burden. 360 In the UK NHS treatment costs for diabetes amount to £13.8B annually. 360 Around 80% of health-care costs in the UK arise from inpatient care and medication costs for treating diabetes-related complications. Maintaining good glycaemic control through regular monitoring and treatment with glucose-lowering medications reduces the risk of developing future complications and is relatively cheap, accounting for approximately 17% of total NHS costs. 360 Glycaemic control is monitored in people with diabetes by measuring HbA1c. HbA1c testing may be used either to diagnose diabetes361 or for long-term monitoring of glycaemic control in people with a diagnosis of diabetes. Test results from HbA1c monitoring are used to make treatment decisions, and treatment thresholds will differ depending on the country, the patient’s previous HbA1c test result and individual clinician preferences. The monitoring of HbA1c usually takes place in primary care settings where the patient needs to attend to have a venous blood sample taken. The sample is then sent for analysis using high-performance liquid chromatography, which is typically performed in a central laboratory. The test result is reported back to the clinician within a few days and the patient is then often required to make a second visit to the health centre or clinic to discuss the result with the physician or a specialist nurse.
New technologies have emerged facilitating the measurement of HbA1c levels in blood, and thus contributing to a more streamlined and thereby improved patient–clinician encounter. 362 POC bench-top devices use a finger-stick capillary blood sample to provide a rapid test result. This means that the patient receives the result of their test typically within a few minutes.
STEP-UP summary
Skills
Diabetes is a complicated multisystem condition. As a consequence, it typically proves a complex condition to integrate into diagnostic testing, with many modalities being required to handle the condition and its eventual sequelae. However, general practice, in the UK and elsewhere, has extensive experience in managing the condition in a community setting. It is not a considerable extension to include diabetes testing in a primary care-orientated pathway of care. Universal screening for diabetes in general practice has been found to be low yield. 363 More selective testing procedures are, therefore, required at a practice level.
Various devices are now available to measure HbA1c outside the laboratory. One study sought to assess the performance of these POC instruments in the hands of nursing staff. Only one of four POC devices for HbA1c compared favourably with a gold-standard chromatography method in a central laboratory. On the basis of results obtained by nursing staff, only the Bayer DCA, of the four devices tested, could be recommended for measurement of HbA1c outside the laboratory. 364
Similarly, dipstick tests for microalbuminuria are convenient, but their accuracy is uncertain. An Oxfordshire study aimed to assess the utility of urine dipstick testing for microalbuminuria in type 2 diabetes. 109 Results of single dipstick tests and sequences of dipstick and laboratory tests were compared with a clinical testing strategy based on current guidelines to assess the accuracy and estimate costs of testing. Testing strategies involving dipstick and laboratory measurements or dipstick tests had similar accuracy. The costs of using dipstick tests were overall lower than those of laboratory-based testing. The authors concluded that dipstick testing in this study did not reliably identify diabetes patients with microalbuminuria. Although dipstick testing would decrease testing costs, it could either fail to diagnose most patients with microalbuminuria or increase the numbers of patients retested depending on the dipstick used.
It has been suggested that finger-prick tests might be administered by optometrists in the high street as a route to detecting undiagnosed diabetes. A prospective study in the north of England found that screening in optometric practices provides an efficient opportunity to screen at-risk individuals who do not present to conventional medical services, and is acceptable and appropriate. 365 Optometrists represent a skilled resource that could provide a screening service. Refinement of the list of risk factors used to make them more sensitive was suggested by the study. However, some have expressed concern that finger-prick tests do not represent a sufficient work-up for undiagnosed people with diabetes.
STEP-UP summary statement With regard to SKILLS, diabetic services are attributed a LOW degree of implementation difficulty owing to the existence of recognised pathways and guidance.
Training
Biochemical tests can be diagnostic and often necessary for monitoring metabolic and endocrine diseases, so it is important for GPs to know which tests are useful in a primary care setting and how to interpret these tests and understand their limitations. A GP does not only need to be able to undertake the relevant diabetes tests; they must also be able to interpret the results, assess the likely consequences in terms of the impact of the disease (e.g. neuropathy and retinopathy) and know how to manage the patient and the patient management pathway. Primary care professionals have built up considerable expertise in managing patients with diabetes and the tests are largely well established. A new domain of knowledge is POC testing for diabetes. A GMC Core Competency is to ‘Understand the use and main limitations of tests commonly used in primary care to investigate and monitor metabolic or endocrine disease, e.g. fasting blood glucose, HbA1c, urinalysis for glucose and protein, urine albumin: creatinine ratio, “near patient testing” (POC testing) for capillary glucose, lipid profile and thyroid function tests, and uric acid tests’. 110
Blood glucose meters are a good example of POC tests, and central to result quality is high-quality training, robust internal quality control, external quality assurance schemes and effective process management. 111 In the past there have been a significant number of critical clinical incidents that occurred as a consequence of inpatients being treated on the basis of erroneous blood glucose meter results. HCPs were, therefore, encouraged to improve blood glucose meter result quality and reduce risks to patients.
STEP-UP summary statement With regard to TRAINING, diabetic services are attributed a LOW degree of implementation difficulty owing to the wide availability of training provision.
Equipment
In patients with existing diabetes, HbA1c monitoring is usually performed every 3–6 months. It typically involves a nurse visit or phlebotomist for venepuncture, with follow-up 1–2 weeks later to discuss results. POC testing could provide more immediate therapeutic decisions and lead to fewer patient visits. This might result in improved diabetic control and practice efficiency. POC tests for HbA1c demonstrated improved outcomes when used in a rural primary care clinic. 112 Greater intensification of therapy occurred when the HbA1c was available at the time of the consultation (51% vs. 32%) and HbA1c fell in the intervention group (8.4% to 8.1%; p = 0.04). Clearly, use of the POC testing or, at a minimum, availability of the result, should be co-ordinated with the consultation process.
Typically, the POC HbA1c device uses a finger-stick drop of blood applied to a reagent cartridge, which is then inserted in a desktop analyser, where the analysis is performed, and HbA1c is reported (as percentage and mmol/mol). The time to result is between 5 and 10 minutes. Some systems allow measurement of the urine albumin–creatinine ratio using a different reagent cassette. A wide range of literature reports on the accuracy of different POC HbA1c tests. Several commentators question the suitability of many POC instruments for the accurate measurement of HbA1c. 366 A study comparing eight HbA1c measurement devices to investigate imprecision, accuracy and bias reported that only three fell within the clinically relevant range. 366
A prospective controlled trial comparing POC testing and standard laboratory testing in an urban primary care clinic showed that POC testing availability resulted in more frequent intensification of therapy when baseline HbA1c was ≥ 7.0% (51% vs. 32% of patients; p = 0.01). HbA1c fell significantly in the POC test group (from 8.4 to 8.1%; p = 0.04) but not in the standard care group (from 8.1 to 8.0%; p = 0.31). 112 A primary care study among patients receiving active insulin titration showed that POC testing resulted in a greater proportion achieving HbA1c< 7.0% than those with laboratory measurement. 367 In contrast, a second RCT conducted in general practice in Leicestershire, UK, showed no significant change in the proportion of patients with HbA1c < 7.0% when using POC testing at 12-months’ follow-up. 368 However, the investigators noted that it was ‘difficult to organise their management of patients in such a way as to maximise the benefit from rapid testing for intervention group patients’, implying that the results were not discussed with the patient at the time of the clinic visit. The study also indicated that POC testing was highly acceptable to patients and staff and confirmed there may be benefits such as time saving, reduced anxiety and impact on patient management and job satisfaction. 368 However, the study also identified high pre-existing levels of satisfaction with diabetes care and the survey failed to attribute increased patient satisfaction to rapid testing.
A large RCT undertaken in Australia found that POC testing was non-inferior to pathology laboratory testing in relation to the proportion of patients showing an improvement in their test results from baseline. 369 A pragmatic UK RCT reported that near-patient testing for HbA1c alone does not lead to outcome or cost benefits in managing people with type 2 diabetes in primary care, finding a non-statistical total cost difference of diabetes related care (£390 in the control group and £370 in the POC test group). 368 However, this study had not managed to change how patients were managed and so presumably had not managed to influence the number of clinic visits. Further research is required into the use of rapid testing within an optimised patient management model. 368
The UK RCT used the Bayer DCA 2000 which requires a finger-prick sample rather than formal venesection, with the result being ready in about 6 minutes. To observe National Service Framework for diabetes,370 requirements for quality control and both internal and external quality assurance procedures were used. 371
A UK HTA report of a study in diabetes clinics indicated providing that near-patient testing of HbA1c results seemed to improve the process of care and aspects of patient satisfaction. The report recommended a prospective RCT of near-patient testing in diabetes clinics. 372
STEP-UP summary statement With regard to EQUIPMENT, diabetes services are attributed a LOW degree of implementation difficulty owing to the increasing availability of POC tests and other portable equipment.
Premises
The review was unable to identify specific material relating to the implications of primary care diagnostic services in diabetes for premises. Front-line diabetic services have a long tradition of being delivered in primary care. Health Building Note 11-01, Facilities for Primary and Community Care Services, locates near-patient testing services (such as blood and gas) in the ‘near-patient testing room’ (contains public sector information licensed under the Open Government Licence v3.0). 46 This document does not provide detailed design guidance on specific rooms and spaces and refers to the following for guidance on generic rooms and spaces: Health Building Note 00-03, Clinical and clinical support spaces. 153 No specific detail was identified relating to the implications of storage and maintenance of increasing numbers of POC tests.
STEP-UP summary statement With regard to PREMISES, diabetic services is attributed an UNCERTAIN degree of implementation difficulty owing to insufficient detail on storage and stock maintenance requirements.
User perspective
Diagnostics for diabetes can include POC testing, screening for diabetic retinopathy and a variety of tests for specific diabetes-associated disorders. POC tests enable a clinician to perform the test, discuss of the test result and change medication, when necessary, during a single consultation. POC devices have been evaluated in some health-care settings. 367 Few studies have been carried out in the UK, where widespread adoption of these technologies has been slower. A survey by the MaDOx team found that the majority of UK clinicians would like to use POC devices for HbA1c testing113 but the reasons for low uptake are unclear. A study in the USA has shown that the main reasons for not adopting these new technologies are concerns about cost and instrument accuracy. 373 Many patients prefer POC tests for HbA1c testing because they get the result quickly and have the opportunity to discuss the result during a single consultation. 373–375
Approximately 20% of people invited for DRS do not attend, with minority ethnic populations and people living in deprived areas less likely to attend and more likely to have worse retinopathy. 114 Deprivation alone does not explain all of the uptake variability between GP practices and regions. Major barriers to attending can include misunderstandings about the importance of diabetes and personal risk factors, and patients’ lack of awareness, psychological factors or practical obstacles. 114,376
In England, routine diabetes care and DRS are principally managed in primary care, whereas treatment for retinopathy takes place in secondary care. Issues surrounding diabetic retinopathy, therefore, have practice implications for medical and health professionals working in both settings. 114 Patients appear to confuse routine retinal photography at optometry practices during eye examinations with DRS. 114 Optometry photography may, therefore, impair more comprehensive coverage. The team also observed differences between patients screened at GP and those screened at optometrist practices, identifying that ease of making an appointment, including its time, and navigating home after the mydriasis drops, etc., appeared less problematic at GP practices. Furthermore, making patients responsible for arranging appointments in some regions, combined with encountering delays, could undermine the perceived importance of DRS. They also identified patients’ misperceptions about their attendance regularity. The successful implementation of a new care pathway should address these factors and improve DRS attendance.
STEP-UP summary statement With regard to the USER PERSPECTIVE, diabetic services are attributed a LOW degree of implementation difficulty owing to accepted pathways and standards of care.
Primary–secondary interface
Initiatives to break the reported primary secondary divide in diabetes care include a West Sussex initiative in which services have been redesigned so that that the primary care diabetes specialist nurses are employed by the PCT and work with nurses and GPs in general practice, but are themselves based at the secondary care diabetes centre. This model of care has enabled the primary care diabetes specialist nurses to function as part of the wider diabetes team, which encompasses all primary and secondary care clinicians, while not being isolated from their secondary care colleagues. Communication was set to improve once primary and secondary care computer systems were linked. Logistic barriers include the various computer systems used in the different practices, as the primary care diabetes specialist nurses have not been trained in either their use or their integration. In addition, practices do not have access to all of the results of blood tests carried out at the hospital. Therefore, further blood samples may be taken or decisions may be made based on results from the general practice system with no knowledge that the hospital may have more recent data. Communication was set to improve once primary and secondary care computer systems were linked.
The success of the model is attributed to a team approach towards managing diabetes services and patient care, aiming to prevent omission, fragmentation and duplication of services, while improving communication between primary and secondary care diabetes services. 115
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, diabetic services are attributed a LOW degree of implementation difficulty owing to the existence of integrated pathways across primary–secondary care, notwithstanding the continuing ‘divide’ in some circumstances.
Conclusion
Diabetic services have a long tradition of being delivered as a two-tier service across the primary and secondary interface. Increasingly, the distinction is becoming blurred, as is the case for cardiac services (described in the previous section). In examining these technologies, it is most important to first define the optimal management pathway and then decide where to locate the various diagnostic interventions. A key consideration, therefore, is the existence of guidelines that make the basis for intervention, and for specific roles, manifestly clear for both patients and clinicians.
Diabetes services (D1–34)
D1. Al-Ansary L, Farmer A, Hirst J, Roberts N, Glasziou P, Perera R, et al. Point-of-care testing for Hb A1c in the management of diabetes: a systematic review and metaanalysis. Clin Chem 2011;57:568–76.
D2. Brown JB, Harris SB, Webster-Bogaert S, Porter S. Point-of-care testing in diabetes management: what role does it play? Diabet Spectrum 2004;17:244–48.
D3. Bubner TK, Laurence CO, Gialamas A, Yelland LN, Ryan P, Willson KJ, et al. Effectiveness of point-of-care testing for therapeutic control of chronic conditions: results from the PoCT in General Practice Trial. Med J Aust 2009;190:624–6.
D4. Burgess S, McHoy A. Breaking the primary-secondary care divide in diabetes. J Diabet Nurs 2007;11:99.
D5. Department of Health. National Service Framework for Diabetes: Standards. 2001/026. London: Department of Health; 2001.
D6. Eborall H, Davies R, Kinmonth A-L, Griffin S, Lawton J. Patients’ experiences of screening for type 2 diabetes: prospective qualitative study embedded in the ADDITION (Cambridge) randomised controlled trial. BMJ 2007;335:490.
D7. Galasko G, Lahiri A, Barnes SC, Collinson P, Senior R. Screening for left ventricular systolic dysfunction in the community by use of hand-held echocardiography, N terminal pro-brain natriuretic peptide or both: a cost effectiveness analysis. Circulation 2003;108:IV–777.
D8. Gialamas A, St John A, Laurence CO, Bubner TK. Point-of-care testing for patients with diabetes, hyperlipidaemia or coagulation disorders in the general practice setting: a systematic review. Fam Pract 2010;27:17–24.
D9. Hipwell AE, Sturt J, Lindenmeyer A, Stratton I, Gadsby R, O’Hare P, et al. Attitudes, access and anguish: a qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open 2014;4:e005498.
D10. Howick J, Jones C, Price C, Plüddemann A, Heneghan C, M T. Point of care tests for monitoring, diagnosis and reducing referrals: a quantitative survey. In SWSAPC Annual Meeting Abstracts. South West Society for Academic Primary Care (SWSAPC). 2013.
D11. Howse JH, Jones S, Hungin AP. Screening and identifying diabetes in optometric practice: a prospective study. Br J Gen Pract 2011;61:e436–42.
D12. Royal College of General Practitioners. Care of People with Metabolic Problems. URL: www.rcgp.org.uk/∼/media/Files/GP-training-and-exams/Curriculum-2012/RCGP-Curriculum-3-17-Metabolic-Problems.ashx (accessed 28 August 2015).
D13. Kanavos P, van den Aardweg S, Schurer W. Diabetes Expenditure, Burden of Disease and Management in 5 EU Countries. London: London School of Economics; 2012.
D14. Kennedy L, Herman WH, Strange P, Harris A; GOAL AIC Team. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabetes Care 2006;29:1–8.
D15. Kennedy L, Herman WH, GOAL A1C Study Team. Glycated hemoglobin assessment in clinical practice: comparison of the A1cNow point-of-care device with central laboratory testing (GOAL A1C Study). Diabetes Technol Ther 2005;7:907–12.
D16. Khunti K, Stone MA, Burden AC, Turner D, Raymond NT, Burden M, et al. Randomised controlled trial of near-patient testing for glycated haemoglobin in people with type 2 diabetes mellitus. Br J Gen Pract 2006;56:511–17.
D17. Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The Diabetes Care From Diagnosis Research Team. BMJ 1998;317:1202–8.
D18. Laurence C, Gialamas A, Yelland L, Bubner T, Ryan P, Willson K, et al. A pragmatic cluster randomised controlled trial to evaluate the safety, clinical effectiveness, cost effectiveness and satisfaction with point of care testing in a general practice setting – rationale, design and baseline characteristics. Trials 2008;9:50.
D19. Leca V, Ibrahim Z, Lombard-Pontou E, Maraninchi M, Guieu R, Portugal H, et al. Point-of-care measurements of HbA(1c): simplicity does not mean laxity with controls. Diabetes Care 2012;35:e85.
D20. Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem 2010;56:44–52.
D21. Levetan CS, Dawn KR, Robbins DC, Ratner RE. Impact of computer-generated personalized goals on HbA(1c). Diabetes Care 2002;25:2–8.
D22. Matteucci E, Giampietro O. Point-of-care testing in diabetes care. Mini Rev Med Chem 2011;11:178–84.
D23. Miller CD, Barnes CS, Phillips LS, Ziemer DC, Gallina DL, Cook CB, et al. Rapid A1c availability improves clinical decision-making in an urban primary care clinic. Diabetes Care 2003;26:1158–63.
D24. Nagrebetsky A, Jin J, Stevens R, James T, Adler A, Park P, et al. Diagnostic accuracy of urine dipstick testing in screening for microalbuminuria in type 2 diabetes: a cohort study in primary care. Fam Pract 2013;30:142–52.
D25. Petersen JR, Finley JB, Okorodudu AO, Mohammad AA, Grady JJ, Bajaj M. Effect of point-of-care on maintenance of glycemic control as measured by A1C. Diabetes Care 2007;30:713–15.
D26. Plüddemann A, Price CP, Thompson M, Wolstenholme J, Heneghan C. Primary care diagnostic technology update: point-of-care testing for glycosylated haemoglobin. Br J Gen Pract 2011;61:139–40.
D27. Portogallo C, Barlow I. Implementing a quality assurance system for point-of-care blood glucose monitoring. J Diabetes Nurs 2010;14:63–7.
D28. St John A, Davis TM, Goodall I, Townsend MA, Price CP. Nurse-based evaluation of point-of-care assays for glycated haemoglobin. Clin Chim Acta 2006;365:257–63.
D29. Stone MA, Burden AC, Burden M, Baker R, Khunti K. Near patient testing for glycated haemoglobin in people with Type 2 diabetes mellitus managed in primary care: acceptability and satisfaction. Diabet Med 2007;24:792–5.
D30. Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11(17).
D31. WEQAS. Wales External Quality Assessment Scheme. URL: www.weqas.co.uk/dsp_frame.cfm?link=schemes (accessed 28 August 2015).
D32. Westgard JO, Darcy T. The truth about quality: medical usefulness and analytical reliability of laboratory tests. Clin Chim Acta 2004;346:3–11.
D33. World Health Organization (WHO). Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated Report of a WHO Consultation. Geneva: WHO; 2011. URL: www.who.int/diabetes/publications/diagnosis_diabetes2011/en/ (accessed 7 September 2016).
D34. Wood JR, Kaminski BM, Kollman C, Beck RW, Hall CA, Yun JP, et al. Accuracy and precision of the Axis-Shield Afinion hemoglobin A1c measurement device. J Diabetes Sci Technol 2012;6:380–6.
Endoscopy
Definition: an endoscope is a long, thin, flexible tube that has a light source and a video camera at one end. Images of the inside of the body are relayed to a television screen. Endoscopes can be inserted into the body through a natural opening, such as the mouth and down the throat, or through the anus (NHS Choices).
This map includes flexible sigmoidoscopy.
This map excludes colonoscopy.
Contains public sector information licensed under the Open Government Licence v3.0. 116
Endoscopy is the most common day-case procedure offered by the NHS and is frequently cited as a service that could be performed in primary care. 118 Concerns have previously been expressed over safety, supervision, patient acceptability and cost-effectiveness. Initially, experiences from secondary care were extrapolated as a basis for British Society of Gastroenterology recommendations, with reservations expressed regarding whether or not endoscopy in primary care could be cost-effective. 120 A 2002 survey demonstrated that endoscopy services could be performed successfully and safely in primary care. 118 In the face of long waiting lists in acute hospitals and resources released by fundholding, several endoscopy units were set up in primary care.
Endoscopy in primary care includes the use of gastroscopy, flexible sigmoidoscopy and colonoscopy. Services are typically delivered by GPs or nurses, with GPs being most likely to deliver the endoscopy procedure. Issues of training, qualifications, experience and premises are of prime concern because they are the essential to safe practice in endoscopy. Existing knowledge and practice is contained in guidance from the British Society of Gastroenterology and the Primary Care Endoscopy Group and in building guidance aimed at small acute hospitals.
STEP-UP summary
Skills
High standards of patient care, the avoidance of complications, accuracy in diagnosis, appropriate therapeutic manoeuvres and the cost-effective use of equipment depend on an endoscopy service being provided by skilled professionals. Many GPwSIs in gastroenterology will probably have developed skills or expertise in endoscopy during hospital training. Competent GPs should be able to demonstrate that they have sufficient skills to ensure the safe and effective practice of adult endoscopy. A GPwSI in endoscopy will necessarily be able to demonstrate their competence in the use and interpretation of endoscopy. Commissioners subsequently need to ensure that the GP has the specific competences to meet the requirements of their service specification. 377
Evidence suggests that more straightforward cases are handled in primary care, with more complex cases being directed straight to an acute hospital. For example, almost all patients who develop acute GI bleeding are managed in hospital; there is no published literature relating to primary care and, therefore, the Royal College of Physicians/NICE guideline focuses on hospital care. 378 Because of the risks, therapeutic gastroscopy, colonoscopy and endoscopy in children should be confined to hospitals, where support for the management of complications is present. Endoscopy procedures in primary care are typically ‘performed on a selected population of otherwise fit patients’, making comparison with secondary care unhelpful and irrelevant. 118
A 2015 systematic review of the safety, competency and cost-effectiveness of nursing staff providing GI endoscopy services, although not examining a primary care context, reported the collective experiences of nurses working in metropolitan areas under the strict supervision and guidance of a specialist gastroenterologist. 117 Nurse endoscopists were less cost-effective per procedure at year 1 than services provided by physicians, owing largely to the increased need for subsequent endoscopies, specialist follow-up and primary care consultations. However, the studies clustered between 1999 and 2001, making applicability problematic. 117
More diagnostic endoscopies could be undertaken by trained nurses. 379 However, a systematic review,117 based on studies from metropolitan units in which nurse endoscopists performed under the close supervision of specialists, found that the nurses were less cost-effective once readmissions and other consequences after 1 year had been taken into account. It is recommended that two people should assist with every endoscopy: a nurse to care for the patient and an assistant to help with the use of instruments and accessories.
STEP-UP summary statement With regard to SKILLS, endoscopy in primary care is attributed a HIGH degree of implementation difficulty owing to the range of skills and competencies required, the risk of serious consequences and the time taken to acquire the requisite competencies.
Training
Training for endoscopy is regulated by the JAG. All training must take place at JAG-approved units. Initially, all practitioners have to attend a formal training course (a JAG-compliant course) in that particular procedure. These courses include the principles and practice of safe endoscopy as well as the indications and contraindications for each type of procedure. GPwSIs should demonstrate that they have pursued training in line with JAG recommendations or have their competency assessed by a local accredited JAG trainer. In addition, the GP should have knowledge of the 15 endoscopy workforce competencies that pertain to a primary care setting. In 2002, 96% of GPs performing endoscopy had undergone training in, and continued to work in, a consultant-led endoscopy unit. About half of the same sample had undergone training courses in endoscopy. 118
The RCGP considers that the training of GPwSIs in endoscopy must be considered ongoing and involve support from specialists. The GP must maintain a sufficient workload377 and continue to access support from other endoscopists and specialists. In 2002, the numbers of endoscopies performed in each primary care unit suggested an inefficient use of resources, but this was attributed to the fact that most units were considered to be still in their infancy. The Primary Care Society for Gastroenterology guidelines suggest that no unit should offer fewer than 200 cases of any flexible endoscopy per year and the endoscopist should have continuing association with a secondary care unit for a wider exposure to case mix. 377 Clearly, if more investigative work is performed in primary care then those GPs involved will be less available for core general practice.
A 2002 survey concluded that simple diagnostic endoscopies could be performed safely in the primary care setting, leaving secondary care units to concentrate on patients requiring sedation, those who are acutely ill and those who require therapeutic procedures. 118 The survey left unanswered questions about community-based endoscopy. These include the effectiveness of referral guidelines, reporting systems, numbers of failed or inadequate examinations requiring referral to specialist units, and the effects on workload in primary care where doctors in a partnership are involved in intermediate care provision. The economics of service provision was not investigated in the survey and neither was morbidity. 118
Requirements from the RCGP are outlined for induction, mentoring, accreditation, reaccreditation and CPD. The GP will require appropriate professional insurance cover (e.g. medical defence organisation or NHS Litigation Authority). Professional documents emphasise the need for flexible and varied approaches to training and assessment, tailored towards the service that the GP will deliver. 377 Assessment may include any combination of the following:
-
observed practice
-
demonstration of skills under direct observation by a specialist clinician
-
reflective practice
-
log book/portfolio of achievement
-
observed communication skills, attitudes and professional conduct
-
evidence of gained knowledge via attendance at accredited courses or conferences.
Although the accreditation of a practitioner as fit to provide endoscopy services is outlined by JAG, providing endoscopy services goes beyond this and requires fulfilment of the formal GPwSI accreditation process. 377
The literature illustrates innovative approaches to endoscopy training. Endoscopy training is a particular candidate for simulation-based approaches. To develop a safe and effective endoscopy service, competent GPs are expected to demonstrate knowledge of the curriculum areas listed in the RCGP guidance. 377
The development of GPwSIs in gastroenterology is supported in primary care. However, their clinical effectiveness and cost-effectiveness need to be researched. A comparatively strong body of evidence relates to their participation in diagnostic services, and the consequent need ‘to develop and implement appropriate training and stringent assessment to ensure patient safety’. 379
Training in instrument handling and disinfection forms an integral part of the training of nurses and other endoscopy assistants and it is essential that all staff involved in endoscopy are kept up to date with an ever-changing technology. These considerations are applicable wherever endoscopy is practised. GPs and their funding bodies must recognise them when considering establishing endoscopy in the community.
Support for endoscopy in primary care may be achieved through active membership of a professional society such as the Primary Care Society for Gastroenterology (www.pcsg.org.uk) or the British Society of Gastroenterology. These associations also offer a mechanism for clinical governance. The British Society of Gastroenterology also hosts a national nurse endoscopist group.
The education and training of staff in health and safety matters is essential and should include the use of personal protective equipment and handling of spillage procedures. Compliance with legislation and regulations places a considerable demand on training and facilities for primary care providers, making the extent to which the use of these facilities justifies such levels of provision critical to a commissioning decision.
Guidance documents emphasise the extended length of time required to train a GPwSI in endoscopy. As a consequence, they suggest that extended contracts of longer than 3 years’ duration should be considered. 377 The same guidance suggests that all appropriate training should be completed within a 5-year framework.
Notwithstanding the above focus on GPs, commissioners may also develop specialist endoscopy services using other practitioners (e.g. nurses and other HCPs). Competences for NHS-employed staff providing specialist care in community settings are typically assessed through the knowledge and skills framework. 377
STEP-UP summary statement With regard to TRAINING, endoscopy in primary care is attributed a MODERATE degree of implementation difficulty owing to the availability of courses, the clear definition of a curriculum, the existence of specialist groups and the variety of training methods available that mitigate, to some degree, the wide range of skills and competencies required.
Equipment
Endoscopy ‘incurs significant cost through its high usage rate of consumables coupled with significant capital costs (e.g. endoscope systems)’. 119 When endoscopy is performed in a setting outside a hospital unit, such as in a cottage hospital, diagnostic and treatment centre or GP surgery, facilities, staffing and equipment should be of the same quality. 2 GPs and their funding bodies must consider whether or not the associated high capital outlay is justified and whether or not it is cost-effective to provide a service to their own and their colleagues’ patients. The availability of primary care endoscopy services may help to challenge any monopolies among NHS or commercial providers.
Anaesthetic and resuscitation facilities must be provided on site for the management of sedated patients. Adequate equipment must be available, together with safe facilities for its cleaning and maintenance. Endoscopes and their accessories are expensive and relatively delicate, requiring careful handling and correct maintenance. The BSG has recommended that the care, maintenance and use of endoscopes and the numerous accessories involved requires a high level of technical competence among medical and nursing staff, comparable with that in the operating theatre.
In a US context the American Academy of Family Physicians reports that economics favour the hospital laboratory over provision in family practice. 380 Equipment, including video and photography equipment, intravenous medications and supplies, and nursing staff are provided as part of an overall service. However, provision in a hospital environment, at a set fee, is consistently higher than when the procedure is performed in the family physician’s office. Although the health economics and funding systems are markedly different, it is likely similar considerations apply in the UK.
Rigid sigmoidoscopy, a safe, simple, inexpensive procedure, is being displaced increasingly by fibre sigmoidoscopy. The technique is reportedly easy to learn. The demands for colonoscopy and fibre sigmoidoscopy far exceed availability in the UK. These services will inevitably expand rapidly in the next few years.
In 1994 a specification was produced for an endoscopy unit. 120 This included upper GI endoscopes and a fibre sigmoidoscope, a light source, a procedure trolley, an endoscopy trolley, three suction machines, an automated washing machine and three pulse oximeters. In 2002, 75% of the endoscopes used were made by Olympus and the remaining were predominantly Pentax endoscopes. 118 The examination needs to be performed on a ‘tipping’ trolley so that the patient may be tilted head or feet down. The life expectancy of the equipment will require replacement over a 5- to 10-year period. Constant improvements to technology are taking place and equipment may become obsolete before the end of its natural life. Underutilisation of equipment will invariably lead to waste. Correspondence from Canada reports that supplies and consumables accounted for approximately half of all expenditure in an endoscopy unit. 119
Cleaning and decontamination of endoscopes should comply with BSG and COSHH guidelines. 121 In a 2002 survey, units using flexible endoscopes all used automatic washing machines to sterilise the instruments and the most commonly used sterilising fluids were glutaraldehyde (66%) and peracetic acid (33%). 118 The BSG has published recommendations in a report on aldehyde disinfectants and health in endoscopy units. 122 Glutaraldehyde is most commonly used for the disinfection of flexible GI endoscopes and it is toxic, irritant and allergenic. The disinfection of flexible endoscopes should be performed in the automated washer/disinfector following the thorough brushing of the channels. The disinfection of equipment should take place away from clinical areas and where suitable extraction and ventilation equipment can be utilised. 120
The cost of endoscopy reflects such factors as the cost of capital investment, staffing, disposables, accessories and the number of procedures undertaken. Capital expenditure required to establish an endoscopy unit requires a considerable investment even when only relatively simple diagnostic procedures are planned.
STEP-UP summary statement With regard to EQUIPMENT, endoscopy in primary care is attributed a HIGH degree of implementation difficulty owing to the capital costs of purchase, the expense of consumables, the precautions required for cleaning and maintenance and the risks associated with glutaraldehyde.
Premises
Requirements for an endoscopy unit, typically based in an acute hospital, are outlined in Health Building Note No. 52123 and in the User’s Guide to Achieving a JAG Compliant Endoscopy Environment. 124 These documents have some, albeit limited, applicability for general practice. Similarly, guidelines from the World Endoscopy Organization specify design principles for a digestive disease endoscopy unit. 381 Health Building Note 11-01, Facilities for Primary and Community Care Services, suggests that ‘most invasive procedures and certain procedures using rigid endoscopes can take place in a generic treatment room’. 46
Building costs may involve erecting new accommodation or adapting existing buildings. The costs of installing fume extraction equipment may also be a factor. Standards of care in purpose-built, well-equipped primary care premises need not fall noticeably short of those in a general hospital, particularly when staff are well-trained and experienced at endoscopy. 118 Endoscopy should be performed in a unit that complies with JAG guidelines for safe endoscopy. 124 When conscious sedation is used, facilities for resuscitation and recovery should be available to the same standard as in an acute hospital. Endoscopy needs to be practised in a purpose-designed area with adequate space and easy accessibility. Instruments, their accessories, monitoring equipment and all the necessary drugs need to be readily to hand. Staff must be able to move freely, and at times quickly, around a patient. Patients may need room for manoeuvre onto trolleys before, during and after endoscopy. Staff need room in which to perform resuscitation manoeuvres. Space is required for appropriate equipment. Suction needs to be available to clear the mouth and pharynx of saliva and any regurgitation of gastric contents. Oxygen must be available. In general practice, any endoscopy facility is likely to be used for multiple purposes but due consideration must be given to the care, storage and security of endoscopy equipment. A separate, properly equipped cleaning and disinfection area will be required to fulfil the COSHH requirements. 121
A separate, and perhaps shared, appropriately staffed patient preparation and recovery area will be required and depending on the workload and patient turnover, space will be needed for one or more trolleys. Monitoring facilities and supplemental oxygen will also need to be available in this area. From time to time patients require overnight admission for observation during unexpectedly prolonged recovery.
STEP-UP summary statement With regard to PREMISES, endoscopy in primary care is attributed a HIGH degree of implementation difficulty owing to the high capital costs of specific accommodation for equipment and the requirements to provide a safe environment for operation of the equipment and for cleaning.
User perspective
Consumer preference is frequently cited as a major driver for a move towards primary care endoscopy services. However, the public is relatively poorly informed of the risks, the precautions and the importance of staff training and experience as they contribute to safe endoscopy. Endoscopy procedures should be performed to recognised levels of safety and accuracy, ensuring patient comfort and satisfaction. The lack of severe symptoms, fear of pain, concerns of sedation, comorbidity and competing life demands have been reported by patients as barriers to performing an endoscopic investigation. 125 A Greek qualitative study, recruiting from primary care patients, found that those refusing upper endoscopy were predominantly female and over the age of 50 years. 125
In a qualitative study of UK ethnic groups, focus groups were used to explore barriers to the uptake of flexible sigmoidoscopy screening among UK ethnic minority populations. Anxiety regarding the invasiveness of the test, the bowel preparation and fear of a cancer diagnosis were common barriers across all ethnic groups. Language difficulties, failure to meet religious sensitivities and the expression of culturally influenced health beliefs were all discussed as specific barriers to uptake. Ethnically tailored health promotion and GP involvement were recommended as ways of overcoming such barriers. 126
STEP-UP summary statement With regard to the USER PERSPECTIVE, endoscopy in primary care is attributed an assessment of UNCERTAIN given that attitudes of patients and staff to endoscopy in primary care are not well explored.
Primary–secondary interface
Hospital-based endoscopy depends largely on highly trained specialists. These specialists have acquired a wider clinical knowledge of gastroenterology over and above simple endoscopic interpretation. Conflicts of access and perceived vested interest are best resolved by improving communication between GPs, specialists, patient groups, purchasers and provider units. Current difficulties may occur when patients encounter difficulty in qualifying for an urgent referral, have to wait too long or experience varying quality in treatment. 382
Alternatives to a GP-based community service include greater ‘open’ access to hospital facilities by GPs and ‘outreach’ clinics performed by hospital-based consultants in community hospitals or individual/group practices. It has also been suggested that mobile units could provide a peripatetic service comparable with radiography or breast screening. Reports have suggested that open access may result in more appropriate selection of patients for whom the investigation is appropriate. 127
A survey of Irish GPs383 examined the availability of radiologic and endoscopic services. In all services, access to diagnostics for public patients is unacceptably long when compared with that for private patients. As a consequence, GPs may refer patients inappropriately to overcrowded emergency departments in order to access diagnostic tests. This can be an unnecessarily traumatic experience, particularly for elderly patients, and places an extra costly burden on hospital services. The vast majority of respondents indicated that increased access to diagnostics would facilitate them to reduce the number of referrals to both emergency and outpatient departments, reduce unnecessary admissions and improve the quality of referrals overall.
STEP-UP summary statement With regard to the PRIMARY–SECONDARY INTERFACE, endoscopy in primary care is attributed a MODERATE degree of implementation difficulty given existing working relationships with secondary care but the need to refer more serious or complex cases to an acute hospital setting.
Conclusion
The potential to offer endoscopy in primary care has been long recognised and it may make an important potential contribution to the disease management pathway. It no longer holds the high level of risk that it was once perceived to have. Developments in more flexible diagnostic tools have helped to make it easier to conduct. It continues to occasion fear among patients, particularly those who are older and female. Physical requirements for the premises, for example for disinfection and storage of the equipment, may be prohibitive in an ordinary-sized general practice. However, they may more easily be accommodated in purpose-built clinics or community hospitals.
Endoscopy (En1–43)
En1. Primary Care Society for Gastroenterology (See Appendix A of the Primary Care Society for Gastroenterology (PCSG) document, Guidelines for the appointment of General Practitioners with Special Interest in undertaking procedures in Gastrointestinal Endoscopy. URL: http://pcsg.org.uk/about (accessed 7 September 2016).
En2. Armstrong D, Enns R, Ponich T, Romagnuolo J, Springer J, Barkun AN. Canadian credentialing guidelines for endoscopic privileges: an overview. Can J Gastroenterol 2007;21:797–801.
En3. Austin KL, Power E, Solarin I, Atkin WS, Wardle J, Robb KA. Perceived barriers to flexible sigmoidoscopy screening for colorectal cancer among UK ethnic minority groups: a qualitative study. J Med Screen 2009;16:174–9.
En4. Bodger K, Eastwood PG, Manning SI, Daly MJ, Heatley RV. Dyspepsia workload in urban general practice and implications of the British Society of Gastroenterology Dyspepsia guidelines (1996). Aliment Pharmacol Ther 2000;14:413–20.
En5. Cardin F, Zorzi M, Furlanetto A, Guerra C, Bandini F, Polito D, et al. Are dyspepsia management guidelines coherent with primary care practice? Scand J Gastroenterol 2002;37:1269–75.
En6. Curvers WL, van Vilsteren FG, Baak LC, Böhmer C, Mallant-Hent RC, Naber AH, et al. Endoscopic trimodal imaging versus standard video endoscopy for detection of early Barrett’s neoplasia: a multicenter, randomized, crossover study in general practice. Gastrointest Endosc 2011;73:195–203.
En7. Delaney BC, Moayyedi P, Forman D. Initial management strategies for dyspepsia. Cochrane Database Syst Rev 2003;2:CD001961. Update in Cochrane Database Syst Rev 2005;4:CD001961.
En8. Delaney BC, Wilson S, Roalfe A, Roberts L, Redman V, Wearn A, et al. Cost effectiveness of initial endoscopy for dyspepsia in patients over age 50 years: a randomised controlled trial in primary care. Lancet 2000;356:1965–9.
En9. Delaney BC, Wilson S, Roalfe A, Roberts L, Redman V, Wearn A, et al. Randomised controlled trial of Helicobacter pylori testing and endoscopy for dyspepsia in primary care. BMJ 2001;322:898–901.
En10. Dent J, Jones R, Kahrilas P, Talley NJ. Management of gastro-oesophageal reflux disease in general practice. BMJ 2001;322:344–7.
En11. American Academy of Family Physicians. EGD, Training and Credentialing of Family Physicians in (position paper). Leawood, KS: American Academy of Family Physicians, 2015.
En12. Eisendrath P, Tack J, Devière J. Diagnosis of gastroesophageal reflux disease in general practice: a Belgian national survey. Endoscopy 2002;34:998–1003.
En13. Galloway JM, Gibson J, Dalrymple J. Endoscopy in primary care–a survey of current practice. Br J Gen Pract 2002;52:536–8.
En14. Anon. Gastrointestinal endoscopy in general practice. Gut 1994;35:1342.
En15. Royal College of General Practitioners. Guidance and Competences for the Provision of Services Using Practitioners with Special Interests (PWSIs) Adult Endoscopy (Upper & Lower GI). URL: www.rcgp.org.uk/clinical-and-research/clinical-resources/∼/media/62F4C7F149D04156BB33A4F21A35B7CA.ashx (accessed 28 August 2016).
En16. Department of Health/RCGP. Implementing a Scheme for GeneralPractitioners with Special Interests. London: Department of Health/RCGP; 2002. URL: www.gencat.cat/ics/professionals/recull/bibliografic/2007_3/Implementing.pdf (accessed 7 September 2016).
En17. NHS Estates. Health Building Note 52 Volume 2: Accommodation for Day Care Endoscopy Units. London: The Stationery Office; 1994.
En18. Primary Care Society for Gastroenterology. URL: www.pcsg.org.uk/about/ (accessed 6 December 2016).
En19. Lee SS, Enns R. The hidden realities of endoscopy unit budgeting. Can J Gastroenterol Hepatol 2014;28:619–20.
En20. Lochhead P, Phull P. Initial experience of direct-to-test endoscopic ultrasonography for suspected choledocholithiasis. Scott Med J 2015;60:85–9.
En21. Mason J, Hungin AP. Review article: gastro-oesophageal reflux disease–the health economic implications. Aliment Pharmacol Ther 2005;22(Suppl. 1):20–31.
En22. McNulty CA, Freeman E, Bowen J, Delaney BC. Variation in the use of H. pylori tests in UK general practice–a qualitative study. Aliment Pharmacol Ther 2005;21:1425–33.
En23. Meineche-Schmidt V, Jørgensen T. Investigation and therapy in patients with different types of dyspepsia: a 3 year follow-up study from general practice. Fam Pract 2000;17:514–21.
En24. Mulder CJ, Jacobs MA, Leicester RJ, Nageshwar Reddy D, Shepherd LE, Axon AT, et al. Guidelines for designing a digestive disease endoscopy unit: report of the World Endoscopy Organization. Dig Endosc 2013;25:365–75.
En25. National Clinical Guideline Centre (UK). Acute Upper Gastrointestinal Bleeding: Management. London: Royal College of Physicians; 2012.
En26. NHS Estates. Accommodation for Day Care: Endoscopy Unit. London: The Stationery Office; 1994.
En27. O’Dowd A. GPs should be freed up to make more endoscopy referrals, cancer charity says. BMJ 2014;348:g3602.
En28. Oikonomidou E, Anastasiou F, Pilpilidis I, Kouroumalis E, Lionis C; Greek General Practice Dyspepsia Group. Upper gastrointestinal endoscopy for dyspepsia: exploratory study of factors influencing patient compliance in Greece. BMC Gastroenterol 2011;11:11.
En29. O’Riordan M, Doran G, Collins C. Access to diagnostics in primary care and the impact on a primary care led health service. Ir Med J 2015;108:53–5.
En30. Quartero AO, Numans ME, de Melker RA, de Wit NJ. In-practice evaluation of whole-blood Helicobacterpylori test: its usefulness in detecting peptic ulcer disease. Br J Gen Pract 2000;50:13–6.
En31. Salo M, Collin P, Kyrönpalo S, Rasmussen M, Huhtala H, Kaukinen K. Age, symptoms and upper gastrointestinal malignancy in primary care endoscopy. Scand J Gastroenterol 2008;43:122–7.
En32. Schurer W, Kanavos P. Colorectal cancer management in the United Kingdom: current practice and challenges. Eur J Health Econ 2010;10(Suppl. 1):85–90.
En33. Stephens M, Hourigan LF, Appleyard M, Ostapowicz G, Schoeman M, Desmond PV, et al. Non-physician endoscopists: a systematic review. World J Gastroenterol 2015;21:5056–71.
En34. Stermer T, Hodgson S, Kavalier F, Watts S, Jones R. Patients’ and professionals’ opinions of services for people at an increased risk of colorectal cancer: an exploratory qualitative study. Fam Cancer 2004;3:49–53.
En35. ClinicalTrials.gov. The Alberta Primary Care Endoscopy (APC-Endo) Study. URL: https://clinicaltrials.gov/ct2/show/NCT01320826
En36. Voss M, Forward LM, Smits CA, Duvenage R. Endoscopy outreach: how worthwhile is it? S Afr Med J 2010;100:158.
En37. Voutilainen M, Kunnamo I. Open-access gastroscopy in primary health-care offices to prevent peptic ulcer-related hospitalization and mortality. Scand J Gastroenterol 2004;39:1289–92.
En38. Voutilainen M, Kunnamo I. A survey of open-access endoscopy in primary health care centres: outcome of gastric carcinoma patients diagnosed by general practitioners compared with hospital-referred endoscopy. Dig Liver Dis 2005;37:119–23.
En39. Walker T, Deutchman M, Ingram B, Walker E, Westfall JM. Endoscopy training in primary care: innovative training program to increase access to endoscopy in primary care. Fam Med 2012;44:171–7.
En40. Wilkins T, LeClair B, Smolkin M, Davies K, Thomas A, Taylor ML, et al. Screening colonoscopies by primary care physicians: a meta-analysis. Ann Fam Med 2009;7:56–62.
En41. Williams JG, Roberts SE, Ali MF, Cheung WY, Cohen DR, Demery G, et al. Gastroenterology services in the UK. The burden of disease, and the organisation and delivery of services for gastrointestinal and liver disorders: a review of the evidence. Gut 2007;56(Suppl. 1):1–113.
En42. Wong BC, Chan CK, Wong KW, Wong WM, Yuen MF, Lai KC, et al. Evaluation of a new referral system for the management of dyspepsia in Hong Kong: role of open-access upper endoscopy. J Gastroenterol Hepatol 2000;15:1251–6.
En43. Cowan RE, Manning AP, Ayliffe GA, Axon AT, Causton JS, Cripps NF, et al. Aldehyde disinfectants and health in endoscopy units. British Society of Gastroenterology Endoscopy Committee. Gut 1993;34:1641–5.
Genetic testing
Definition: genetic testing is a type of medical test that identifies changes in chromosomes, genes, or proteins. The results of a genetic test can confirm or rule out a suspected genetic condition or help determine a person’s chance of developing or passing on a genetic disorder.
This map includes testing for inherited genetic conditions and for genetic risk factors indicating a predisposition to particular conditions, e.g. cardiac conditions.
This map excludes newborn heel screening.
Definition is public domain information reproduced from US National Library of Medicine: Genetics Home Reference. 128
Background
Over-the-counter genetic tests are becoming increasingly plentiful. 384 Primary care health professionals will increasingly be exposed to commercial testing both when patients are contemplating genetic testing and when they return after being given the results of such tests. 385 Health professionals increasingly need to become ‘genetically literate’. 142 It has been estimated that at least 1 in 10 of the patients seen in primary care has a disorder with a genetic component. Three main themes of genetics in primary care relate to identifying patients with, or at risk of, a genetic condition, the clinical management of genetic conditions and communicating genetic information (including genetic counselling). 386
Genetic screening tests can involve molecular, biochemical and other types of analyses, or even the use of family history questionnaires, to predict which individuals are at risk of developing or transmitting (or both) a genetic condition. 387 Some tests are strong predictors of disease occurrence, but many are subject to a high degree of uncertainty. It can be difficult for those who have positive screening results to decide how best to proceed, as the proposed interventions vary greatly depending on the disease in question, and they are not always highly effective and might also involve certain risks. 387
Predictive genetic tests differ from traditional medical tests in part because they directly affect other individuals who may not have wanted such information. Depending on family size, the number of possibly affected family members can be quite large and the risk is never trivial. Another way in which predictive genetic tests differ from traditional medical tests is the time frame they span. Traditional medical tests typically ascertain an individual’s condition at that moment, whereas genetic tests purport to reveal something about a possible future state or condition. Strictly speaking, predictive tests are not new in medicine. Cholesterol levels have been used for years to predict cardiac disease, and nuclear imaging routinely guides the need for further intervention in patients with chest pain. However, genetic testing presents a new set of conditions and new levels of complexity and uncertainty not found in traditional predictive tests.
STEP-UP summary
Skills
Skills for genetic testing relate to understanding which patients to test, what tests to order and how to interpret the results of these tests. Importantly, they also require being able to handle the counselling and communication issues associated with testing and the test result. 129 A Dutch survey of knowledge of genetics and genetic tests revealed general levels to be poor, with GP knowledge being poorer than that of paediatricians and gynaecologists. 130 Knowledge of DNA (deoxyribonucleic acid) testing was particularly deficient. The factors associated with higher knowledge scores included more recent graduation, having taken an elective course in genetics and providing genetic counselling in their own practice. This suggests that GPs will demonstrate an improved knowledge of genetics with the changing workforce dynamics and changes in emphasis in medical education and other HCPs. At least for the moment, the supply of genetics professionals is inadequate to meet increasing demand and the expansion of genetic knowledge will cause the primary care physician to be increasingly called on to provide genetic services.
STEP-UP summary statement With regard to SKILLS, genetic testing is attributed a HIGH degree of implementation difficulty owing to inadequate knowledge of genetics, challenges in interpretation and the need for skills in communicating and handling the outcome of the test.
Training
Generally, primary care practitioners have received minimal training in clinical genetics. Various methods are required to support practitioners to develop these genetic skills and knowledge, including changes to the undergraduate curriculum. Postgraduate training and assessment in primary care should include approaches to developing and examining the outlined genetic skills. However, these will require clinical teachers themselves to acquire good genetic knowledge and skills.
As with GPs, empirical evidence of the learning needs of practitioners in relation to genetic testing reveals widespread deficits in knowledge and skills, and low confidence levels. Provision of nursing education in genetics is uneven. Significant progress has been made in the identification of learning outcomes for nurses. Research on the delivery of genetics education is limited. Skills-based training, clinical scenarios and assessment have all been identified as factors that can promote learning. Many studies of nurses’ knowledge of genetics reveal gaps in professional competence and/or education. 132 New initiatives are under way to support genetics education and its integration into professional practice. Further research is needed on the most effective forms of educational delivery. E-learning extends the possibility of this type of training across a wide number of staff scattered across general practices.
The role of computer decision support and clinical decision aids is mentioned by some authors. 388 The additional cost of software, and the time spent to learn and use such tools, needs to be factored in alongside equipment costs.
Clinicians need guidance to help them introduce genetic tests, communicate their results and explain their implications. 389 Primary care providers are interested in learning more about who should receive genetic testing and what tests are available. Training in counselling and risk communication is desired, as are ‘just-in-time’ resources to guide clinical decisions. 131 Primary care providers are eager to learn about genetic medicine. Educational efforts should build on primary care providers’ prior knowledge base, highlight the clinical relevance of genetic medicine to primary care practice, and emphasise ‘red flags’: cues to alert primary care providers to a potential genetic contribution. Shared decision-making has been advocated, and 75% preferred this approach with their patients. Physicians who preferred their patients to play an active role in decision-making were more likely to report encouraging patients to look for information, and to report having enough time to discuss decisions in visits. 390
The science of genetics will impact every aspect of health care, from primary care to specialised care. 133 Nurses are on the front line and are expected to recognise patterns of disease that may indicate a possible genetic link, educate the family about the implications of a potential genetic susceptibility and refer the family for counselling. Each nurse should, thus, acquire a minimum basic knowledge of genetics. Those who educate and counsel should attend formal education provision.
Given the complexity and limitations of genetic testing, there is a need to develop and disseminate clinical guidelines and to educate physicians. 391 Clinicians need guidance to help them introduce genetic tests, communicate their results and explain their implications. 389 Additional, more innovative methods are required, including management guidelines and computerised pedigree drawing and decision support. 392 Online resources will become increasingly useful for both health professionals and their patients.
STEP-UP summary statement With regard to TRAINING, genetic testing is attributed a MODERATE degree of implementation difficulty owing to the need to receive significant training and ongoing support in offering genetic services.
Equipment
Genetic testing is expensive and time-consuming given the sheer scale of the genes that need to be examined. Although robotics and high-throughput sequencing greatly speed sequencing, the process remains expensive and laborious. Commercial sequencing may take 3 or 4 weeks for results. 393 Existing screening methods possess reduced sensitivity and specificity in comparison with this current gold standard of sequence analysis. The necessary pre-test and post-test counselling that must accompany such testing is best measured in hours, not minutes. A systematic review of genetic tests in primary care was published in 1999. 134 This mapping review has been unable to find a more recently published review. Specific searching for such a systematic review is recommended before considering whether or not to commission further work.
STEP-UP summary statement With regard to EQUIPMENT, genetic testing is attributed a LOW degree of implementation difficulty owing to increasing numbers of direct to consumer POC tests.
Premises
The mapping review was unable to identify any items that specifically described requirements to house genetic testing facilities in general practice or community premises. Health Building Note 11-01, Facilities for Primary and Community Care Services, locates near-patient testing services (such as blood and gas) in the ‘near-patient testing room’ (contains public sector information licensed under the Open Government Licence v3.0). 46 This document does not provide detailed design guidance on specific rooms and spaces, and refers to the following for guidance on generic rooms and spaces: Health Building Note 00-03, Clinical and Clinical Support Spaces. 153 It is unclear what tests should be provided and what the implications are with regard to the management of stock.
STEP-UP summary statement With regard to PREMISES, genetic testing is attributed an UNCERTAIN rating for implementation difficulty owing to the shortage of studies describing how tests might be accommodated. Co-ordinated approaches with regard to storage may relate to POC tests.
User perspective
Commentators question how quickly genetics will deliver clinically useful tests and knowledge to primary care and if it will initially raise more questions than answers. 394 Concerns are expressed about the potential harm from the inappropriate use of genetic testing. It is believed that primary care practitioners are reluctant to adopt responsibility for genetic testing. In a survey of family physicians,135 respondents felt that genetic tests would be more useful for breast cancer and hemochromatosis than for Alzheimer’s disease, heart disease or diabetes. Individuals who believed themselves more familiar with genetic tests were more likely to anticipate that genetic testing would impact signifianctly on their future practice (23.1% vs. 13.4%). Respondents had little exposure to direct-to-consumer genetic tests, but most felt that they were more likely to cause harm than benefit. 135
Qualitative studies on attitudes to genetic tests in primary care, from both practitioner and patient perspectives, are plentiful. This mapping study has focused on studies of genetic tests in general or studies that include multiple conditions, in the interests of transferability. However, numerous qualitative studies were found for individual genetic conditions. This mapping review did not identify any qualitative systematic reviews of attitudes to genetic tests specifically in a primary care setting, although such reviews in general health contexts do exist. Given the plentiful nature of qualitative evidence, a systematic review, specifically of genetic tests in primary care, may be warranted. A more comprehensive search should be undertaken before considering whether or not to commission further work.
Unlike cholesterol level or a pap smear, a genetic test directly examines one’s unique genetic code and thereby addresses individuality on a deeper level. Fears of genetic discrimination are widespread and, partly for these reasons, genetic information typically occupies a privileged position with respect to privacy and informed consent. Findings from qualitative studies of patient viewpoints report misunderstandings concerning genetic tests, for example that genetic tests are more predictive than they actually are, and that they are predictive of behaviours for which no markers have in fact yet been discovered. 136 A qualitative study of genetic testing for risk of coronary heart disease found that the test was acceptable. However, patients were unclear how to interpret the meaning of the test and rarely used it to initiate appropriate action. 137
Findings from qualitative studies of provider viewpoints report that cost of testing consistently appears as the most frequently cited barrier to genetic testing. 138–140 GPs also rate their baseline knowledge of genetics as uniformly poor. 395 GPs are most confident when eliciting family history and providing psychosocial support and least confident when discussing risks/benefits of genetic testing and in counselling. GPs were more likely to refer to genetics counselling services when they were confident in their knowledge of referral criteria and core competencies in genetics, and when they were aware of the programme and where to refer. 396
STEP-UP summary statement With regard to USER PERSPECTIVES, genetic testing has LOW implementation difficulty for patients and MODERATE difficulty for clinicians because patients already have access to direct to consumer genetic tests but GPs are cautious about the ramifications of interpretation.
Primary–secondary interface
Studies in the UK have evaluated community genetic counsellors acting as outreach workers134 from the genetics clinic to liaise with local general practices. Such practitioners are either genetic nurses141 or genetic counsellors, and they can fulfil a dual role of filtering referrals to the geneticist and providing basic genetic information. 142 Genetic nurse counsellors, specifically in a cancer genetics context, were found to perform not significantly differently from conventional cancer genetic services in two RCTs in Scotland and Wales. 143 Although the nurses were located in regional genetics services the possibility of locating such nurses in primary care or, indeed, of offering liaison nurse roles located in general practice should be investigated. Such roles hold significant workforce requirements. Development of primary care specialists, possibly working in conjunction with community genetic counsellors, has been suggested to offer an intermediate point of referral between general practice and specialist genetic clinics. A third allied model is to train a specific practitioner from each practice in genetic skills to act as an in-house expert, supported by electronic resources. 142
A study from the USA found that communication between primary care physicians and genetics specialists is suboptimal. Improvement is needed in identifying and referring adult patients to genetic services. 397 Primary care physicians are less comfortable than specialists with identifying patients for referral and with discussing genetics. 398 The largest barriers to referral were lack of programme awareness and limited knowledge regarding patient eligibility, improved insurance coverage and antidiscrimination legislation.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, genetic testing is attributed a MODERATE degree of implementation difficulty owing to a high dependence on secondary care expertise, support and follow-up provision.
Conclusion
Because of the uncertainties around the benefits and harm from genetic testing, the clinical implications of a specific genetic test will require careful evaluation, including information about cost-effectiveness, before widespread adoption is recommended. 142 With thousands of tests being developed, it is important to ensure that these are matched to meaningful intervention. Rather than focusing on the organisation and service delivery of specific individual tests, the priority is to organise services that can adapt to emerging technologies, their interpretation and their implications for patient support and counselling.
Genetics (G1–79)
G1. Aalfs CM, Smets EM, de Haes HC, Leschot NJ. Referral for genetic counselling during pregnancy: limited alertness and awareness about genetic risk factors among GPs. Fam Pract 2003;20:135–41.
G2. Acheson LS, Stange KC, Zyzanski S. Clinical genetics issues encountered by family physicians. Genet Med 2005;7:501–8.
G3. Andermann A, Blancquaert I. Genetic screening: a primer for primary care. Can Fam Physician 2010;56:333–9.
G4. Andermann AA, Watson EK, Lucassen AM, Austoker J. The opinions, expectations and experiences of women with a family history of breast cancer who consult their GP and are referred to secondary care. Community Genet 2001;4:239–43.
G5. Anderson B, Anderson J, McLosky E, Wasilevich S, Lyon-Callo S, Duquette D, et al. Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol 2012;2012:298745.
G6. Baars MJ, de Smit DJ, Langendam MW, Adèr HJ, ten Kate LP. Comparison of activities and attitudes of general practitioners concerning genetic counseling over a 10-year time-span. Patient Educ Couns 2003;50:145–9.
G7. Baars MJ, Henneman L, ten Kate LP. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: a global problem. Genet Med 2005;7:605–10.
G8. Bankhead C, Emery J, Qureshi N, Campbell H, Austoker J, Watson E. New developments in genetics-knowledge, attitudes and information needs of practice nurses. Fam Pract 2001;18:475–86.
G9. Barr OG, McConkey R. Supporting parents who have a child referred for genetic investigation: the contribution of health visitors. J Adv Nurs 2006;54:141–50.
G10. Battista RN, Blancquaert I, Laberge AM, van Schendel N, Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genomics 2012;15:34–45.
G11. Bernhardt BA, Zayac C, Pyeritz RE. Why is genetic screening for autosomal dominant disorders underused in families? The case of hereditary hemorrhagic telangiectasia. Genet Med 2011;13:812–20.
G12. Blaine SM, Carroll JC, Rideout AL, Glendon G, Meschino W, Shuman C, et al. Interactive genetic counseling role-play: a novel educational strategy for family physicians. J Genet Counsel 2008;17:189–95.
G13. Brandt R, Ali Z, Sabel A, McHugh T, Gilman P. Cancer genetics evaluation: barriers to and improvements for referral. Genet Test 2008;12:9–12.
G14. Brennan KS. Clinical implications of pharmacogenetic and microarray testing for advanced practice nurses. J Am Assoc Nurse Pract 2015;27:246–55.
G15. Burke S, Kirk M. Genetics education in the nursing profession: literature review. J Adv Nurs 2006;54:228–37.
G16. Burke S, Stone A, Bedward J, Thomas H, Farndon P. A ‘neglected part of the curriculum’ or ‘of limited use’? Views on genetics training by nongenetics medical trainees and implications for delivery. Genet Med 2006;8:109–15.
G17. Burke W, Emery J. Genetics education for primary-care providers. Nat Rev Genet 2002;3:561–6.
G18. Burton H. Education in Genetics for Health Professionals. Report to the Wellcome Trust. Cambridge: Public Health Genetics Unit; 2002.
G19. Carroll JC, Rideout AL, Wilson BJ, Allanson JM, Blaine SM, Esplen MJ, et al. Genetic education for primary care providers: improving attitudes, knowledge, and confidence. Can Fam Physician 2009;55:e92–9.
G20. Carroll JC, Cappelli M, Miller F, Wilson BJ, Grunfeld E, Peeters C, et al. Genetic services for hereditary breast/ovarian and colorectal cancers – physicians’ awareness, use and satisfaction. Community Genet 2008;11:43–51.
G21. Carroll JC, Wilson BJ, Allanson J, Grimshaw J, Blaine SM, Meschino WS, et al. GenetiKit: a randomized controlled trial to enhance delivery of genetics services by family physicians. Fam Pract 2011;28:615–23.
G22. Challen K, Harris H, Benjamin CM, Harris R. Genetics teaching for non-geneticist health care professionals in the UK. Community Genet 2006;9:251–9.
G23. Challen K, Harris H, Kristoffersson U, Nippert I, Schmidtke J, Ten Kate LP, et al. General practitioner management of genetic aspects of a cardiac disease: a scenario-based study to anticipate providers’ practices. J Commun Genet 2010;1:83–90.
G24. Chapman DD. Cancer genetics. Semin Oncol Nurs 2007;23:2–9.
G25. Clyman JC, Nazir F, Tarolli S, Black E, Lombardi RQ, Higgins JJ. The impact of a genetics education program on physicians’ knowledge and genetic counseling referral patterns. Med Teacher 2007;29:e143–50.
G26. Dhar SU, Alford RL, Nelson EA, Potocki L. Enhancing exposure to genetics and genomics through an innovative medical school curriculum. Genet Med 2012;14:163–7.
G27. Elwyn G, Iredale R, Gray J. Reactions of GPs to a triage-controlled referral system for cancer genetics. Fam Pract 2002;19:65–71.
G28. Emery J, Walton R, Murphy M, Austoker J, Yudkin P, Chapman C, et al. Computer support for interpreting family histories of breast and ovarian cancer in primary care: comparative study with simulated cases. BMJ 2000;321:28–32.
G29. Emery J, Watson E, Rose P, Andermann A. A systematic review of the literature exploring the role of primary care in genetic services. Fam Pract 1999;16:426–45.
G30. Emery J, Hayflick S. The challenge of integrating genetic medicine into primary care. BMJ 2001;322:1027–30.
G31. Friedman L, Cooper HP, Webb JA, Weinberg AD, Plon SE. Primary care physicians’ attitudes and practices regarding cancer genetics: a comparison of 2001 with 1996 survey results. J Cancer Educ 2003;18:91–4.
G32. American College of Preventive Medicine. Genetic Testing Time Tool A Resource from the American College of Preventive Medicine Clinical Reference. URL: www.acpm.org/?GeneticTestgClinRef (accessed 4 January 2016).
G33. Glasspool DW, Fox J, Coulson AS, Emery J. Risk assessment in genetics: a semi-quantitative approach. Stud Health Technol Inform 2001;842:459–63.
G34. Godard B, Kääriäinen H, Kristoffersson U, Tranebjaerg L, Coviello D, Aymé S. Provision of genetic services in Europe: current practices and issues. Eur J Hum Genet 2003;11(Suppl. 2):13–48.
G35. Greendale K, Pyeritz RE. Empowering primary care health professionals in medical genetics: how soon? How fast? How far? Am J Med Genet 2001;106:223–32.
G36. Harvey A. From lab to lifestyle: translating genomics into healthcare practices. New Genet Soc 2011;30:309–27.
G37. Hayflick SJ, Eiff MP. Will the learners be learned? Genet Med 2002;4:43–4.
G38. Hetteberg C, Prows CA. A checklist to assist in the integration of genetics into nursing curricula. Nurs Outlook 2004;52:85–8.
G39. Holtzman NA, Marteau TM. Will genetics revolutionize medicine? N Engl J Med 2000;343:141–4.
G40. Hyland F, Kinmonth AL, Marteau TM, Griffin S, Murrell P, Spiegelhalter D, et al. Raising concerns about family history of breast cancer in primary care consultations: prospective, population based study. Women’s Concerns Study Group. BMJ 2001;322:27–8.
G41. Jackson L, Goldsmith L, Skirton H. Guidance for patients considering direct-to-consumer genetic testing and health professionals involved in their care: development of a practical decision tool. Fam Pract 2014;31:341–8.
G42. Javitt GH. Policy implications of genetic testing: not just for geneticists anymore. Adv Chronic Kidney Dis 2006;13:178–82.
G43. Klitzman RL. Misunderstandings concerning genetics among patients confronting genetic disease. J Genet Couns 2010;19:430–46.
G44. Knottnerus JA. Community genetics and community medicine. Fam Pract 2003;20:601–6.
G45. Laberge AM, Fryer-Edwards K, Kyler P, Lloyd-Puryear MA, Burke W. Long-term outcomes of the ‘Genetics in Primary Care’ faculty development initiative. Fam Med 2009;41:266–70.
G46. Mainous AG, Johnson SP, Chirina S, Baker R. Academic family physicians’ perception of genetic testing and integration into practice: a CERA study. Fam Med 2013;45:257–62.
G47. Mathers J, Greenfield S, Metcalfe A, Cole T, Flanagan S, Wilson S. Family history in primary care: understanding GPs' resistance to clinical genetics – qualitative study. Br J Gen Pract 2010;60:e221–30.
G48. McCahon D, Holder R, Metcalfe A, Clifford S, Gill P, Cole T, et al. General practitioners’ attitudes to assessment of genetic risk of common disorders in routine primary care. Clin Genet 2009;76:544–51.
G49. McCann S, MacAuley D, Barnett Y. Genetic consultations in primary care: GPs’ responses to three scenarios. Scand J Prim Health Care 2005;23:109–14.
G50. McKelvey KD, Evans JP. Cancer genetics in primary care. J Nutr 2003;133(Suppl. 11):3767–72.
G51. Metcalf MP, Tanner TB, Buchanan A. Effectiveness of an online curriculum for medical students on genetics, genetic testing and counseling. Med Educ Online 2010;15.
G52. Metcalfe S, Hurworth R, Newstead J, Robins R. Needs assessment study of genetics education for general practitioners in Australia. Genet Med 2002;4:71–7.
G53. Metcalfe S, Seipolt M, Aitken M, Flouris A. Educating general practitioners about prenatal testing: approaches and challenges. Prenat Diagn 2005;25:592–601.
G54. Middlemass JB, Yazdani MF, Kai J, Standen PJ, Qureshi N. Introducing genetic testing for cardiovascular disease in primary care: a qualitative study. Br J Gen Pract 2014;64:e282–9.
G55. Morgan S, McLeod D, Kidd A, Langford B. Genetic testing in New Zealand: the role of the general practitioner. N Z Med J 2004;117:U1178.
G56. Mountcastle-Shah E, Holtzman NA. Primary care physicians’ perceptions of barriers to genetic testing and their willingness to participate in research. Am J Med Genet 2000;94:409–16.
G57. Murray E, Pollack L, White M, Lo B. Clinical decision-making: physicians' preferences and experiences. BMC Fam Pract 2007;8:10.
G58. Myers MF, Chang MH, Jorgensen C, Whitworth W, Kassim S, Litch JA, et al. Genetic testing for susceptibility to breast and ovarian cancer: evaluating the impact of a direct-to-consumer marketing campaign on physicians’ knowledge and practices. Genet Med 2006;8:361–70.
G59. Phelps C, Platt K, France L, Gray J, Iredale R. Delivering information about cancer genetics via letter to patients at low and moderate risk of familial cancer: a pilot study in Wales. Fam Cancer 2004;3:55–9.
G60. Rafi I, Qureshi N, Lucassen A, Modell M, Elmslie F, Kai J, et al. ‘Over-the-counter’ genetic testing: what does it really mean for primary care? Br J Gen Pract 2009;59:283–7.
G61. Royal College of General Practitioners. RCGP Curriculum: Genetics in Primary Care. URL: www.gmc-uk.org/3_02_Genetics_in_Primary_Care_May_2014.pdf_56885088.pdf (accessed 28 August 2015).
G62. Robins R, Metcalfe S. Integrating genetics as practices of primary care. Soc Sci Med 2004;59:223–33.
G63. Rose PW, Watson E, Yudkin P, Emery J, Murphy M, Fuller A, et al. Referral of patients with a family history of breast/ovarian cancer – GPs’ knowledge and expectations. Fam Pract 2001;18:487–90.
G64. Saukko PM, Ellard S, Richards SH, Shepherd MH, Campbell JL. Patients’ understanding of genetic susceptibility testing in mainstream medicine: qualitative study on thrombophilia. BMC Health Serv Res 2007;7:82.
G65. Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians' preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomarkers 2013;17:219–25.
G66. Shakespeare T. Genetics, Big Brother and the GP. Br J Gen Pract 2006;56:167–8.
G67. Shickle D, Hapgood R, Qureshi N. The genetics liaison nurse role as a means of educating and supporting primary care professionals. Fam Pract 2002;19:193–6.
G68. Starfield B, Holtzman NA, Roland MO, Sibbald B, Harris R, Harris H. Primary care and genetic services. Health care in evolution. Eur J Public Health 2002;12:51–6.
G69. Stermer T, Hodgson S, Kavalier F, Watts S, Jones R. Patients’ and professionals’ opinions of services for people at an increased risk of colorectal cancer: an exploratory qualitative study. Fam Cancer 2004;3:49–53.
G70. Telner DE, Carroll JC, Talbot Y. Genetics education in medical school: a qualitative study exploring educational experiences and needs. Med Teach 2008;30:192–8.
G71. Torrance N, Mollison J, Wordsworth S, Gray J, Miedzybrodzka Z, Haites N, et al. Genetic nurse counsellors can be an acceptable and cost-effective alternative to clinical geneticists for breast cancer risk genetic counselling. Evidence from two parallel randomised controlled equivalence trials. Br J Cancer 2006;95:435–44.
G72. Trinidad SB, Fryer-Edwards K, Crest A, Kyler P, Lloyd-Puryear MA, Burke W. Educational needs in genetic medicine: primary care perspectives. Community Genet 2008;11:160–5.
G73. Walter FM, Emery JD. Genetic advances in medicine: has the promise been fulfilled in general practice? Br J Gen Pract 2012;62:120–1.
G74. Welkenhuysen M, Evers-Kiebooms G. General practitioners and predictive genetic testing for late-onset diseases in Flanders: what are their opinions and do they want to be involved? Community Genet 2002;5:128–37.
G75. Westwood G, Pickering RM, Latter S, Lucassen A, Little P, Karen Temple I. Feasibility and acceptability of providing nurse counsellor genetics clinics in primary care. J Adv Nurs 2006;53:591–604.
G76. Williams JL, Collingridge DS, Williams MS. Primary care physicians’ experience with family history: an exploratory qualitative study. Genet Med 2011;13:21–5.
G77. Wolf CR, Smith G, Smith RL. Science, medicine, and the future: Pharmacogenetics. BMJ 2000;320:987–90.
G78. Hyland F, Kinmonth AL, Marteau TM, Griffin S, Murrell P, Spiegelhalter D, et al. Raising concerns about family history of breast cancer in primary care consultations: prospective, population based study. Women’s Concerns Study Group. BMJ 2001;322:27–8.
G79. Wood ME, Stockdale A, Flynn BS. Interviews with primary care physicians regarding taking and interpreting the cancer family history. Fam Pract 2008;25:334–40.
Magnetic resonance imaging
Definition: MRI is a type of scan that uses strong magnetic fields and radio waves to produce detailed images of the inside of the body (NHS Choices).
This map includes portable MRI and (exceptionally for this review) commercial service provision.
This map excludes N/A.
N/A, not applicable.
Contains public sector information licensed under the Open Government Licence v3.0. 144
Background
The drive to improve primary care access to imaging services is encapsulated in the Department of Health’s 2008 documents Care Closer to Home49 and NHS Next Stage Review: Leading Local Change,50 chaired by Lord Darzi. These documents champion the introduction of change through ‘disruptive innovation’, that is, change involving radical service redesign, centred on improving the quality of care that patients receive and with an emphasis on devolving key aspects of care pathways from secondary to primary care ‘to empower the frontline’. 399
As part of the reshaping of imaging services in primary care, independent-sector providers – possibly the most high-profile of which is the London NHS Diagnostic Service – have been closely involved in the planning, delivery and auditing of direct-access imaging in primary care. 51 The response from primary care in London has been positive. For example, the Croydon Federation of General Practices audited its interaction with the London NHS Diagnostic Service, concluding that the service provides a high-quality service and rapid access to imaging in a way that provides ‘value-for-money’, improves clinical management, and enhances the patient experience. The perception in primary care is that improved access redefines standards of service delivery. Patient-centricity is promoted through attempts to optimise choice and convenience by providing imaging not only closer to home but also at an appropriate time. The timeliness of the service has improved, with a maximum waiting time for diagnostics of 2 weeks (compared with standard 6-week waiting times at many local secondary care institutions). The nature of the interaction between primary care and the independent provider is also reported to be ‘mould-breaking’ in terms of the ‘can-do’, proactive, facilitative and communicative attitudes of the provider, held up in contrast to relationships with local secondary-care providers. The Croydon Federation has concluded that there is an overwhelming ‘buy in’ from local general practices and their patients. Clinical management in primary care has been facilitated and onward referral to secondary care reduced by one-third. The beneficial effect of direct-access imaging on the effective functioning of the Department of Health 18-week referral-to-treatment targets in secondary care centres is also cited by all parties as a real and objective outcome of the service. 51 A range of independent-sector providers is similarly supplying direct-access imaging to primary care elsewhere in the country by using static and mobile MRI scanners within PCTs and GP practices, and through mobile ultrasound services. The London NHS Diagnostic Service differs in being centrally procured and by effectively acting as a formal pilot scheme for primary care direct access. 51
STEP-UP summary
Skills
To provide a MRI service requires that staff undertake sufficient current MRI practice, and a sufficient number of examinations to maintain competency in every area of MRI. Staff interpreting the images and providing a clinical report should fulfil the following requirements:145
-
UK Registered Radiologists on the GMC Specialist Register undertaking sufficient current clinical practice within that modality. A consultant radiologist must have undertaken planned regular clinical MRI sessions in their current job plan.
-
Radiographers currently registered with the Health and Care Professions Council who have performed regular sessions of MRI examinations within the last 12 months.
-
A minimum of 1 year’s experience.
-
All staff maintain their CPD in accordance with their professional body guidelines.
-
Meet the specification set out in the ‘National Occupational Standards for Imaging’ (RD5: produce MRI images for diagnostic purposes).
STEP-UP summary statement With regard to SKILLS, MRI is attributed a MODERATE degree of implementation difficulty owing to the requirements for experience and training.
Training
The BSCMR (www.bscmr.org) has endorsed voluntary accreditation procedures for their members. These have no statutory role but are provided to allow both medical and non-medical practitioners to demonstrate a specified level of experience in an appropriate educational environment. In the case of MRI, the BSCMR and British Society for Cardiac Imaging support the criteria developed by the US-based Society for Cardiovascular Magnetic Resonance and do not separately accredit individuals.
In this rapidly changing field, the following standards are currently applicable:
-
Making the Best Use of a Department of Radiology, 6th edition – RCR 2007400
-
Standards for the Communication of Critical, Urgent and Unexpected Significant Radiological Findings – RCR 2008401
-
Safety Guidelines for Magnetic Resonance Imaging Equipment in Clinical Use – MHRA Device Bulletin 2007. 402
STEP-UP summary statement With regard to TRAINING, MRI is attributed a MODERATE degree of implementation difficulty owing to the ongoing need for professional development and updating.
Equipment
Magnetic resonance imaging has entered common use as part of the standard equipment portfolio over the last 10 to 15 years. 159 Three distinct classes of MRI scanners are marketed and sold by manufacturers and suppliers:
-
specialised MRI scanners, which have been designed for niche applications, such as orthopaedics
-
whole-body imaging systems, where the technology has improved to allow for higher-field strength magnets at lower cost
-
specialised cryogenic and non-cryogenic electromagnets, designed for interventional and imaging work.
The following are commissioned requirements for MRI equipment, which should meet or exceed the following:
-
Fixed or mobile units shall contain one full-body MRI scanner with a magnetic strength of at least 1.5 Tesla.
-
Equipment should comply with the Guidelines for Magnetic Resonance Equipment in clinical use, MHRA (2007) as updated, superseded and replaced from time to time. 146
-
Equipment should be a maximum of 7 years old.
-
Electrical safety testing is required annually with regular maintenance and quality assurance testing.
-
Details of maintenance contracts to include regular and emergency service cover must be provided.
-
Replacement schedule must be available with the maximum age of equipment of 7 years.
Most MRI scanners are currently only utilised on a ‘9 to 5’ basis, and therefore these high-cost assets are not delivering value for a significant proportion of any given day. The extension of working hours would utilise the equipment more efficiently and might also offer appointments at times that are more convenient for the patient. Routine maintenance could be performed out of hours, with quality assurance procedures performed prior to a clinical session.
STEP-UP summary statement With regard to EQUIPMENT, MRI is attributed a HIGH degree of implementation difficulty owing to the prohibitive cost of an installation.
Premises
There is no specific provision for MRI in Health Building Note 11-01, Facilities for Primary and Community Care Services. 403 Commissioners will need to consider mobile or static sites. All facilities, including mobile units, are required to have a minimum of a patient reception and waiting area, either on the unit or nearby, access to a toilet and access to appropriate levels of security. We found no specific guidance on housing MRI units in primary care. However, elements of the government document Facilities for Diagnostic Imaging and Interventional Radiology (Health Building Note 6) will apply to primary care premises. 159 This document claims to have recognised trends in primary care imaging but does not acknowledge a primary care context for MRI (chapter 13 on magnetic resonance imaging, pp. 118–32). 159 The requirements for space for conventional MRI as specified by this document would be prohibitive to a general practice setting. More realistic is to consider the place of mobile MRI units and their requirements (chapter 14 on Mobile vehicle scanning units to include CT/MRI and positron emission tomography). 159 A mobile unit will require space for a control area, an examination room and, if needed, a technical room. There will be no room for patient support areas, which will, therefore, have to be provided within the remaining primary care facilities.
STEP-UP summary statement With regard to PREMISES, MRI is attributed a HIGH degree of implementation difficulty owing to exacting requirements for the facilities and supplies.
User perspective
Access to MRI is seen as a way to reduce diagnostic uncertainty. Early access to MRI compared with referral to an orthopaedic specialist did not alter GPs’ diagnosis or treatment plans but significantly increased their therapeutic confidence. 404 Because MRI equipment is typically housed in secondary care we were unable to find qualitative research relating to attitudes to MRI specifically in a community setting. Items on perspectives of MRI provided by acute hospitals from GPs and their referred patients would indirectly inform this issue. However, these will require a supplementary search strategy.
STEP-UP summary statement With regard to USER PERSPECTIVE, MRI is attributed an UNCERTAIN degree of implementation difficulty owing to the shortage of published experience on MRI in a primary care context.
Primary–secondary interface
A MRI service needs to work with other providers to offer an integrated service. This includes third-sector organisations providing help and support for patients. The role of local clinical networks is particularly important. It is equally important to achieve good engagement with all stakeholders. Key to the successful integration of MRI services is consideration of the management pathway, irrespective of which facilities are located in the primary or secondary sectors of local health service provision.
An alternative to primary care services housing their own staff and equipment in their premises is offered by the London NHS Diagnostic Service. The London NHS Diagnostic Service was established by the InHealth Group in 2007 following competitive tendering. Local PCTs and federated general practice groups utilise the service, which charges for imaging services including ultrasound, plain film and MRI at standard tariff costs. MRI reporting is provided by groups of NHS-based consultant radiologists operating within an independent organisation affiliated with the private provider. The aim is to offer access to diagnostic imaging services comparable with those provided in secondary care. 51 This popular model of service delivery avoids the heavy capital expenditure required for purchasing the MRI equipment.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, MRI is attributed a MODERATE degree of implementation difficulty owing to dependence on secondary care support facilities and/or commercial providers.
Conclusion
Ultimately, MRI provision did not meet the criteria for this review in terms of services and staff managed in a primary care setting. The most common model was of service provision under contract by an external provider. This sidesteps many of the considerations from the STEP-UP framework, for example training and equipment, except from a commissioner and quality assurance customer perspective. The implications of offering MRI services have not been explored and represent a potential line of future exploration.
Magnetic resonance imaging (M1–24)
M1. Royal College of Radiologists. Framework for Primary Care Access to Imaging ‘Right Test, Right Time, Right Place’. London: Royal College of Radiologists, RCGP; 2006.
M2. Barter S, Drinkwater K, Remedios D. National audit of provision of MRI services 2006/07. Clin Radiol 2009;64:284–90.
M3. Birchall D. Primary care access to diagnostics: a paradigm shift. Br J Radiol 2010;83:101–3.
M4. Bjerager M, Palshof T, Dahl R, Vedsted P, Olesen F. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract 2006;56:863–8.
M5. Brealey SD. Influence of magnetic resonance of the knee on GPs’ decisions: a randomised trial. Br J Gen Pract 2007;57:622–9.
M6. Brealey SD, Atwell C, Bryan S, Coulton S, Cox H, Cross B, et al. The DAMASK trial protocol: a pragmatic randomised trial to evaluate whether GPs should have direct access to MRI for patients with suspected internal derangement of the knee. BMC Health Serv Res 2006;6:133.
M7. Cherryman G. Imaging in primary care. Br J Gen Pract 2006;56:563–4.
M8. DAMASK (Direct Access to Magnetic Resonance Imaging: Assessment for Suspect Knees) Trial Team. Cost-effectiveness of magnetic resonance imaging of the knee for patients presenting in primary care. Br J Gen Pract 2008;58:e10–16.
M9. Brealey SD. Influence of magnetic resonance of the knee on GPs’ decisions: a randomised trial. Br J Gen Pract 2007;57:622–9.
M10. Department of Health. Our health, Our Care, Our Say: A New Direction for Community Services. London: Department of Health; 2006.
M11. Dhillon V, Creiger J, Hannan J, Hurst N, Nuki G. The effect of DXA scanning on clinical decision making by general practitioners: a randomised, prospective trial of direct access versus referral to a hospital consultant. Osteoporos Int 2003;14:326–33.
M12. Gough-Palmer AL, Burnett C, Gedroyc WM. Open access to MRI for general practitioners: 12 years’ experience at one institution – a retrospective analysis. Br J Radiol 2009;82:687–90.
M13. Greaves C, Gilmore J, Bernhardt L, Ross L. Reducing imaging waiting times: enhanced roles and service-redesign. Int J Health Care Qual Assur 2013;26:195–202.
M14. National Audit Office. Managing High Value Equipment in the NHS in England National Audit Office. 2011. URL: www.improvement.nhs.uk/diagnostics/RadiologyKeyResources/DHpublications.aspx (accessed 28 August 2015).
M15. NICE. Referral Guidelines for Suspected Cancer. London: NICE; 2005.
M16. NHS Estates. Facilities for Diagnostic Imaging and Interventional Radiology. London: The Stationery Office; 2001. URL: www.gov.uk/government/uploads/system/uploads/attachment_data/file/149183/HBN_6_V1_DSSA.pdf (accessed 28 August 2015).
M17. Olisemeke B, Chen YF, Hemming K, Girling A. The effectiveness of service delivery initiatives at improving patients’ waiting times in clinical radiology departments: a systematic review. J Digital Imag 2014;27:751–78.
M18. Robling MR, Houston HL, Kinnersley P, Hourihan MD, Cohen DR, Hale J, et al. General practitioners’ use of magnetic resonance imaging: an open randomized trial comparing telephone and written requests and an open randomized controlled trial of different methods of local guideline dissemination. Clinical Radiol 2002;57:402–7.
M19. RCR. Making the Best Use of a Department of Clinical Radiology. Guidelines for Doctors. London: RCR; 2003.
M20. Simpson GC, Forbes K, Teasdale E, Tyagi A, Santosh C. Impact of GP direct-access computerised tomography for the investigation of chronic daily headache. Br J Gen Pract 2010;60:897–901.
M21. Szczepura A, Clark M. Creating a strategic management plan for magnetic resonance imaging (MRI) provision. Health Policy 2000;53:91–104.
M22. Thomas R, Cook A, Main G, Taylor T, Galizia Caruana E, Swingler R. Primary care access to computed tomography for chronic headache. Br J Gen Pract 2010;60:426–30.
M23. Thomas RE, Grimshaw JM, Mollison J, McClinton S, McIntosh E, Deans H, et al. Cluster randomized trial of a guideline-based open access urological investigation service. Fam Pract 2003;20:646–54.
M24. White PM, Halliday-Pegg JC, Collie DA. Open access neuroimaging for general practitioners – diagnostic yield and influence on patient management. Br J Gen Pract 2002;52:33–5.
Point-of-care testing
Definition: POC testing is defined as any analytical test performed for an individual by a HCP outside the conventional laboratory setting. 147
This map includes CRP, D-dimer, faecal occult blood, haemoglobin, HbA1c, infectious disease testing, INR, lipid profiles, nose/throat swab for influenza, platelet count, procalcitonin biomarkers, quantitative β-human chorionic gonadotropin, throat swab for group A streptococci, urine albumin–creatinine ratio, urine leucocytes or nitrite, urine pregnancy test, urine strips and whole-blood lactate.
This map excludes BNP (see Cardiac services), blood glucose, HbA1c (see Diabetes services), cholesterol screening, pregnancy tests and respiratory POC tests (see Respiratory tests).
Background
Point-of-care tests are a significant growth area in the worldwide market of diagnostics. As the NHS moves its focus closer to the patient primary care, doctors and commissioners need to take stock of which new POC tests should be used, and why. 2 POC tests have been developed for many common blood tests, such as lipid profiles, HbA1c, CRP, INR and urine albumin-to-creatinine ratio. The traditional set of POC tests includes blood glucose testing, blood gas and electrolytes analysis, rapid coagulation testing, rapid cardiac markers diagnostics, drugs of abuse screening, urine strips testing, pregnancy testing, faecal occult blood analysis, haemoglobin diagnostics, infectious disease testing and cholesterol screening. In the past 5–10 years the following tests were added: HbA1c, BNP, whole-blood lactate, D-dimer and CRP. 405
Point-of-care testing holds potential benefits for primary care in trading off accuracy against availability. 406 POC testing assumes that an immediate result actually makes a difference to the patient’s management and outcome. The technical complexity and specialist nature of many diagnostic technologies sees them being placed in centralised laboratories, typically processing samples from multiple general practice facilities. Centralisation of diagnostic provision in this way may lead to long delays between the collection of samples and the receipt of results at the general practices. The receipt of results has, to a certain extent, been speeded up with the advent of communication technologies including, first, fax and second, e-mails and text messages. However, there is no immediate way of reducing the time taken to collect and process samples, other than locating the processing facilities nearer to the POC, either by delivering results at the practice itself or through using mobile facilities. Delays in testing and, for some tests, the need to travel to a secondary care facility may result in fewer patients undergoing tests and to delays in initiating treatment, where necessary.
An international survey in 2014 attempted to identify demand for POC tests in five different countries. 113 As well as the UK and the USA, it included two countries with leading reputations for POC testing. The survey also identified those conditions for which GPs would most like to have availability of/access to tests. In both cases selective data of UK results are presented below (Tables 44 and 45) against cumulative international supply or demand to minimise the effect of national practice.
| Condition | Per cent (n) |
|---|---|
| UTI | 47 (521) |
| PE/DVT | 43 (478) |
| Diabetes | 35 (385) |
| Acute cardiac disease | 25 (282) |
| INR/anticoagulation | 18 (199) |
| Pregnancy | 16 (178) |
| Anaemia | 15 (162) |
| Heart failure | 11 (124) |
| COPD/asthma | 10 (116) |
| Chest infection/cough/LRTI | 9 (102) |
| UK (N = 1109), % (n) | Total (N = 2770), % (n) | |
|---|---|---|
| Urine pregnancy test | 80 (887) | 81 (2236) |
| Urine leucocytes or nitrite | 90 (993) | 81 (2234) |
| Blood glucose | 69 (760) | 80 (2209) |
| INR | 43 (476) | 31 (852) |
| Haemoglobin | 16 (174) | 28 (784) |
| Faecal occult blood | 13 (143) | 20 (567) |
| Throat swab for group A streptococci | 15 (164) | 20 (547) |
| C-reactive protein | 15 (163) | 19 (528) |
| Quantitative β-human chorionic gonadotropin | 17 (193) | 19 (520) |
| HbA1c | 17 (183) | 15 (406) |
| Nose/throat swab for influenza | 6 (61) | 12 (328) |
| Platelet count | 15 (163) | 10 (290) |
Certain conditions were not afforded prominence in the UK but appeared in the lists of other countries. These are included below in the context of a possible Horizon Scan of emerging priorities.
-
Australia: chronic and acute renal conditions (excluding urinary tract infection).
-
Belgium and the Netherlands: infections and sexually transmitted diseases.
-
Belgium: acute and chronic renal impairment.
-
Netherlands: appendicitis.
-
USA: strep throat, influenza, infectious mono and sexually transmitted diseases.
STEP-UP summary
Skills
To practise competently, the user of POC testing equipment must have the knowledge and skills for safe and effective practice when working without direct supervision; they must recognise and work within the limits of competence, keeping knowledge and skills up to date and take part in appropriate learning and practice activities that maintain and develop their competence and performance. 148
A primary concern relating to POC is quality assurance. In the mid-1980s and 1990s, several groups of pathologists, particularly clinical chemists, produced guidelines for decentralised laboratory work. Collaboration between pathology laboratories and primary care is essential if POC testing is to be safely and effectively utilised. Primary care practitioners cannot ignore the issues of both internal and external quality control steps in the validation of test results. To produce standardised and clinically effective anticoagulation management, correct procedures need to be followed in terms of internal and external quality control procedures, liaison with the local laboratory for assessing precision and accuracy of the system and standard operating procedures to ensure optimum care.
Standard operating procedures would include clinical governance, risk management and also implementation of risk reduction strategies. This would be achieved by careful patient selection, internal and external quality control procedures, a regular audit process and ensuring relevant, complete and accurate documentation of the clinic process.
The internal quality control supplied by the manufacturer should be performed at the start of each clinic, and external quality assurance provided either by collaboration with a hospital laboratory or through a quality assurance scheme, such as National External Quality Assessment Service, should be performed regularly.
Liaison with a local laboratory is essential for support with staff training and the external quality assurance scheme and to refer patients difficult to control to designated specialist care, or if there are any concerns with regard to POC device performance or results.
STEP-UP summary statement With regard to SKILLS, POC tests is attributed a MODERATE degree of implementation difficulty owing to exacting requirements for quality assurance.
Training
The training of health professionals to manage POC should include an understanding of the specific POC device used, setting up and using the device, recording of results and quality assurance materials, health and safety, disposal of sharps and the COSHH regulations. 121
A Department of Health evaluation compared Thrombotrak with a manual system using the same thromboplastin. The authors of the report concluded that, ‘The system is extremely simple to use and requires no previous knowledge of coagulation instrumentation]. The two hour training given on-site was completely adequate and, backed up by the instruction manual, ensured trouble free user operation’. 407
STEP-UP summary statement With regard to TRAINING, POC tests is attributed a MODERATE degree of implementation difficulty owing to the need to identify which to use, what they mean and how to integrate them within existing clinical practice.
Equipment
As with all in vitro diagnostic equipment, new POC equipment should be thoroughly evaluated prior to clinical implementation to ensure that reliable results are consistently obtained. 149 In the UK, this is performed on a voluntary basis at the manufacturers’ discretion by the MHRA of the Department of Health. Although no specific guidelines for the INR calibration of POC devices are currently available, it is accepted that calibration should be performed by the manufacturers using the same procedure as conventional laboratory systems. Calibration as performed by the manufacturers typically cannot be altered by the operator. New POC coagulometers are currently under evaluation with the MHRA and will have further field evaluations in the primary care setting. The MHRA investigates, first, the electrical, mechanical and microbiological safety of the device and, second, the performance in terms of ease of use, reliability, imprecision, comparability, calibration, sensitivity and interfering factors.
Studies comparing CRP with procalcitonin found that procalcitonin has greater sensitivity and specificity for distinguishing bacterial and viral infection in hospital settings. However, studies show that procalcitonin does not yet add sufficient value for decision-making in primary care,150,408,409 whereas CRP POC testing appears better in correctly predicting the absence of radiographic pneumonia. 150 A Cochrane review concluded that a POC biomarker (e.g. CRP) to guide antibiotic treatment of acute respiratory infections in primary care can significantly reduce antibiotic use. 151 Studies included in the final analysis included randomised and cluster RCTs from England, Wales and six other countries to demonstrate that using CRP POC tests in primary care can significantly reduce the initial prescribing rate of antibiotics.
Until fairly recently, high-quality evidence in the form of RCTs to support POC testing has, however, been lacking. A systematic review limited to lipids, HbA1c, INR and urinary albumin-to-creatinine ratio determinations identified RCTs, but none was of sufficiently similar design to allow a meta-analysis. 410 Measurements of HbA1c, albumin-to-creatinine ratio and lipids are of structured care. A RCT conducted in UK general practice randomised patients in practices to have their results by POC test or from the central laboratory. 368 The POC test results were available at the time of the consultation with the GP and the outcome measure was the proportion of patients achieving good metabolic control (i.e. HbA1c < 7.0%). On completion of the trial after 12 months, there was no significant difference in the proportion of patients within the therapeutic range (37% vs. 38%). However, the management of patients in the POC test group was not different from that in the group who had normal care. The difference between the results of the primary and secondary care trials may be due to the presence of better-controlled patients in the UK study compared with the Australian study, all of whom were on insulin.
For a Department of Health evaluation, Thrombotrak was compared with a manual system using the same thromboplastin. Differences in the measured INR were ‘small enough to be deemed acceptable’. 154 The trial demonstrated that nurse-led primary care anticoagulation clinics using POC testing and computerised decision support software had comparable outcomes in terms of INR control with routine hospital outpatient management. 407 The nurses involved were trained by laboratory personnel before managing anticoagulation clinics. Training involved theoretical aspects of anticoagulation management, including INR monitoring and measures of control, as well as practical training in using the POC device and computerised decision support software, and quality control procedures.
A study exploring discrepancies of approximately 20% in INR between two POC systems concluded that systems require improved International Sensitivity Index calibration by manufacturers along with better methods of quality control. 411 However, even in so-called expert laboratories there can be significant differences in the INR obtained. 154 An editorial discusses quality issues in relation to providing a safe INR service outside the laboratory. 154 One randomised crossover trial comparing the use of a coagulometer within a community clinic and a hospital laboratory showed that results generally compared well. 412
Although studies have demonstrated that the use of POC tests for the measurement of INR is feasible and practical, concerns are expressed about discrepancies between results obtained on near-patient testing devices and those obtained in hospital laboratories. However, small differences will naturally occur because of differences in the thromboplastin reagent used to derive the result. 154 More significantly, none of the abovementioned reports indicate significant clinical management differences associated with the use of POC tests.
For other tests that might anticipate strong demand in primary care (e.g. POC-tests for lipids and HbA1c, and non-invasive bilirubin meters), there is often little evidence of clinical utility in primary care. 2 The Horizon Scanning series has examined several POC technologies. The topics are itemised in Appendix 4. POC testing for D-dimer in conjunction with the Wells criteria can rule out lower-leg deep-vein thrombosis in about half of patients presenting with suspected deep-vein thrombosis in primary care. 2,413
STEP-UP summary statement With regard to EQUIPMENT, POC testing is attributed a LOW degree of implementation difficulty owing to ease of testing and ready availability of equipment and supplies.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates near-patient testing services (such as blood and gas) in the ‘near-patient testing room’ (contains public sector information licensed under the Open Government Licence v3.0). This document does not provide detailed design guidance on specific rooms and spaces and refers to the following for guidance on generic rooms and spaces: Health Building Note 00-03, Clinical and Clinical Support Spaces. 153 It is unclear what tests should be provided and what the implications are with regard to the management of stock.
STEP-UP summary statement With regard to PREMISES, POC testing is attributed an UNCERTAIN rating for implementation difficulty owing to the shortage of studies describing how tests might be accommodated. Co-ordinating approaches with regard to storage may relate to genetic tests.
User perspective
Point-of-care tests do not simply offer improved accessibility to GPs and other practice staff. By far the largest potential market for POC is believed to be the patient self-management market. For example, the monitoring of anticoagulation through INR testing technologies is offered by increasingly accessible and affordable technologies. Paradoxically, the greater the ease with which tests might be severed from existing secondary care domination of supply by primary care services, the correspondingly greater the likelihood of these tests bypassing primary care altogether, by going direct to consumer. Clearly, the expansion of POC testing facilities in primary care should factor in the supply and demand of self-management technologies. 154
A qualitative systematic review of seven studies has summarised the perceived benefits of POC tests. 13 GPs welcomed POC tests because they offered the prospect of enhanced immediate diagnosis and treatment. Diagnostic POC tests reduced diagnostic certainty and increased confidence in clinical decisions. POC tests were particularly welcomed as a way of ruling out serious infections. This was found for both GPs who had used and those who had not used diagnostic POC tests.
Although POC tests were perceived, on the whole, to enhance patient care (if the tests were accurate), exceptions were noted. Diagnostic POC tests would not be helpful when serious complications arise from viral illnesses. It was believed that patients would be convinced, reassured and more satisfied in their GP’s decisions if POC tests had been used, compared with if they had received no test. In particular, a test result confirming a GP’s decision not to prescribe antibiotics would help them to ‘sell’ this decision to patients and manage patient expectations for antibiotics, leading to shared decisions with patients. GPs with different levels of experience of using diagnostic POC tests had similar perceptions that they would help to reassure patients and lead to more effective targeted treatment without alienating or upsetting patients.
A general assumption is that patients would like to have POC tests available. However, the qualitative review found that several participants were concerned that patients may not like testing. 13,414 This may be a particular concern for procedures involving children. 13 Not all GPs feel comfortable interpreting and explaining diagnostic test results, particularly intermediate results which could lead to increased uncertainty in patients. They expressed a need for training. 13 Overall, primary care clinicians believe that POC tests increase diagnostic certainty, help target treatment, educate and empower patients, and improve the relationship between clinicians and patients by enhancing communication and shared decision-making. Clinicians were also concerned about cost, over-reliance – in that POC tests could undermine clinical skills – and limited usefulness. 13
Some issues are not unique to POC testing, but also apply to laboratory testing in general. In addition to the barriers to POC test use identified here, other reasons for the lack of widespread use may include a lack of needs assessments of primary health-care clinicians, resulting in discordance between the tests that they want/would use frequently and those that are produced.
The findings of the qualitative systematic review emphasise, for POC tests specifically but also for community diagnostics more generally, that the issues are not simply technical and organisational. The attitudes of primary care clinicians are integral to understanding if and how POC tests are to be implemented more widely. Further qualitative systematic reviews associated with community diagnostic technologies will help in understanding and anticipating the user (in the broad sense of clinician and patient). In particular, communicating the benefits of individual POC tests more widely and more precisely may help in stimulating more widespread adoption of POC testing in primary care. The review focused on blood POC tests in primary care; further research is needed to confirm whether or not the issues raised here apply to other types of POC tests (e.g. urine tests, respiratory samples).
Both GPs and patients appear to find using CRP acceptable, with one Dutch study showing CRP POC testing to have little effect on GP workload in 50% of practices. 415 Patients were satisfied to be provided with the results of a reassuringly low CRP POC test rather than receiving an antibiotic prescription. CRP testing has a role as an adjunct in effective communication with patients; clinicians and patients all recommend seeing the CRP in conjunction with the overall assessment and caution against over-reliance on CRP results in isolation of clinical assessment.
Moreover, procalcitonin has not yet been proven to be suitable for deployment as a POC test in general practice, with a turnaround time (depending on the system) of 18–30 minutes; CRP POC tests, by contrast, have a turnaround time of < 5 minutes, thereby giving a result within the ambit of a standard NHS GP consultation.
Primary care staff may derive increased satisfaction from being able to resolve patient problems without being dependent on referral for tests or on a further delayed encounter when results are available. However, referral for confirmatory tests and for treatment may still be required, so this advantage does not apply in all circumstances.
Studies of patient satisfaction consistently reveal that most patients like POC INR testing as it is typically more convenient for them, contributing to patient-centred care. A UK GP trial found no significant differences in the satisfaction of UK patients receiving POC testing and that of patients who had their HbA1c levels from the central laboratory. 416 In contrast, an Australian trial found that satisfaction generally increased for patients, POC test device operators and GPs over the course of the trial. 364 An observational study also found a significant increase in patient satisfaction after POC testing, with patients reporting that POC testing was convenient and motivated them to manage their condition better. 417 Quantitatively significantly more patients preferred a POC INR testing service when compared with usual care. 418 Patients demonstrated improved capacity to make appointments, spent less time at the appointment, experienced less pain and received improved communication about their medication dosage. 418
STEP-UP summary statement With regard to USER PERSPECTIVES, attitudes to POC testing between patients and clinicians differ, resulting in a MODERATE rating for implementation difficulty from clinicians and a LOW rating for implementation difficulty for patients.
Primary–secondary interface
Provision of primary care POC test diagnostic services is variable and may be dependent on the corresponding provision of local hospital facilities. In seeking to reduce variation, some PCTs are commissioning laboratories to provide a satellite service from the central laboratory to primary care sites. Such laboratories employ a hub-and-spoke method of delivery but have not been evaluated for clinical effectiveness.
The General Medical Services Contract offers remuneration for a ‘national enhanced service’ for anticoagulation services, which includes the development and maintenance of a register, a call and recall system, appropriate training and audit procedures. Funding for the service is given for four different levels of service, with level 4 accruing the highest funding.
Testing in primary care reduces dependency of primary care clinicians on hospital-based services, many of which are outside their own control. Increasingly, primary care stakeholders are being involved in the specification and development of hospital-based diagnostic services operating, in fact, as the informed customer. Other dependencies, that will necessarily continue for most modalities, lie in access to training, advice and specialist referral.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, POC testing is attributed a MODERATE degree of implementation difficulty as it may facilitate earlier diagnosis but has a potentially unpredictable effect on demand for secondary care services.
Conclusion
Evidence so far indicates that POC testing systems have great potential for primary care with clear quality-of-life benefits for patients. The implementation of POC testing technologies requires collaboration between manufacturers, pathology laboratories and general practice as well as adherence to a recognised external quality assurance scheme. Diagnostic technologies need to improve both accuracy and precision.
The increased availability of a test is likely to increase the usage of that test. Increased use may indicate previously unmet need, may constitute an inappropriate response or may simply reflect a growth in demand. In practices where desktop analysers are introduced, the rate of testing increases, but these extra tests do not inform changes in diagnosis or management. 154 However, such studies often fail to acknowledge the effect of tests in reducing the uncertainty experienced by both doctor and patient.
From the perspective of GPs, the likely benefits of introducing POC tests include increased diagnostic certainty, more efficient care, and fewer (re)consultations. 13 Barriers to the implementation of POC tests must be addressed, some by primary care and others elsewhere. The accuracy of POC tests in primary care populations must be addressed by manufacturers. Policy-makers and clinicians should carefully consider the role and impact of POC tests in primary care in relation to GP roles. Against a backdrop of reductions in health service funding, attention must be paid to how POC tests are to be funded. 13
Point of care (P1–60)
P1. Aabenhus R, Jensen JU. Procalcitonin-guided antibiotic treatment of respiratory tract infections in a primary care setting: are we there yet? Prim Care Respir J 2011;20:360–7.
P2. Aabenhus R, Jensen JUS, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst Rev 2014;11.
P3. Aspevall O, Kjerstadius T, Lindberg L, Hallander H. Performance of Uricult Trio assessed by a comparison method and external control panels in primary healthcare. Scand J Clin Lab Invest 2000;60:381–6.
P4. Briggs C, Carter J, Lee SH, Sandhaus L, Simon-Lopez R, Vives Corrons JL. ICSH Guideline for worldwide point-of-care testing in haematology with special reference to the complete blood count. Int J Lab Hematol 2008;30:105–16.
P5. Bubner TK, Laurence CO, Gialamas A, Yelland LN, Ryan P, Willson KJ, et al. Effectiveness of point-of-care testing for therapeutic control of chronic conditions: results from the PoCT in General Practice Trial. Med J Aust 2009;190:624–6.
P6. Butler CC, Simpson S, Wood F. General practitioners' perceptions of introducing near-patient testing for common infections into routine primary care: a qualitative study. Scand J Prim Health Care 2008;26:17–21.
P7. Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant GJ. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ 2009;338:b1374.
P8. Cals JW, Chappin FH, Hopstaken RM, van Leeuwen ME, Hood K, Butler CC, et al. C-reactive protein point-of-care testing for lower respiratory tract infections: a qualitative evaluation of experiences by GPs. Fam Pract 2010;27:212–8.
P9. Chaudhry R, Scheitel SM, Stroebel RJ, Santrach PJ, Dupras DM, Tangalos EG. Patient satisfaction with point-of-care international normalized ratio testing and counseling in a community internal medicine practice. Manag Care Interface 2004;17:44–6.
P10. Cooke J, Butler C, Hopstaken R, Dryden MS, McNulty C, Hurding S, et al. Narrative review of primary care point-of-care testing (POCT) and antibacterial use in respiratory tract infection (RTI). BMJ Open Respir Res 2015;2:e000086.
P11. de Lusignan S, Nitsch D, Belsey J, Kumarapeli P, Vamos EP, Majeed A, et al. Disparities in testing for renal function in UK primary care: cross-sectional study. Fam Pract 2011;28:638–46.
P12. de Vries C, Doggen C, Hilbers E, Verheij R, IJzerman M, Geertsma R, et al. Results of a survey among GP practices on how they manage patient safety aspects related to point-of-care testing in every day practice. BMC Fam Pract 2015;16:9.
P13. Deo S, Sohoni M. Optimal decentralization of early infant diagnosis of HIV in resource-limited settings. M&SOM 2015;17:191–207.
P14. Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 2014;14:239–49.
P15. Fitzmaurice DA, Hobbs FDR, Murray ET, Holder RL, Allan TF, Rose PE. Randomised controlled trial of oral anticoagulation using computerised decision support (CDSS) and near patient testing (NPT). Arch Intern Med 2000;160:2343–8.
P16. Gardiner C, Machin S. Anticoagulant Near Patient Testing (NPT), Oral Anticoagulation Management and Stroke Prevention. In Fitzmaurice DA, Murray ET, editors. The Primary Care Perspective, Chapter 3. Newmarket: Hayward Medical Press; 2002.
P17. Gialamas A, St John A, Laurence CO, Bubner TK. Point-of-care testing for patients with diabetes, hyperlipidaemia or coagulation disorders in the general practice setting: a systematic review. Fam Pract 2010;27:17–24.
P18. Gialamas A, Laurence CO, Yelland LN, Tideman P, Worley P, Shephard MD, et al. Assessing agreement between point of care and laboratory results for lipid testing from a clinical perspective. Clin Biochem 2010;43:515–18.
P19. Gialamas A, Yelland LN, Ryan P, Willson K, Laurence CO, Bubner TK, et al. Does point-of-care testing lead to the same or better adherence to medication? A randomised controlled trial: the PoCT in General Practice Trial. Med J Aust 2009;191:487–91.
P20. Gill JP, Shephard MD. The conduct of quality control and quality assurance testing for PoCT outside the laboratory. Clin Biochem Rev 2010;31:85–8.
P21. Glover S, Bajorek BV. Exploring point-of-care testing of capillary blood in warfarin management. J Pharm Pract Res 2008;38:300.
P22. Health Quality Ontario. Point-of-care international normalized ratio (INR) monitoring devices for patients on long-term oral anticoagulation therapy: an evidence-based analysis. Ontario Health Technol Assess Series 2009;9:1.
P23. Hirst JA, Price CP, Stevens RJ, Farmer AJ. Protocol for a systematic review of the accuracy of point-of-care HbA1c tests.
P24. Howick J, Cals JW, Jones C, Price CP, Plüddemann A, Heneghan C, et al. Current and future use of point-of-care tests in primary care: an international survey in Australia, Belgium, The Netherlands, the UK and the USA. BMJ Open 2014;4:e005611.
P25. Huckle D. Point-of-care diagnostics: an advancing sector with nontechnical issues. Expert Rev Mol Diagn 2008;8:679–88.
P26. Jones CH, Howick J, Roberts NW, Price CP, Heneghan C, Plüddemann A, et al. Primary care clinicians’ attitudes towards point-of-care blood testing: a systematic review of qualitative studies. BMC Fam Pract 2013;14:117.
P27. Khunti K, Stone MA, Burden AC, Turner D, Raymond NT, Burden M, et al. Randomised controlled trial of near-patient testing for glycated haemoglobin in people with type 2 diabetes mellitus. Br J Gen Pract 2006;56:511–17.
P28. Kost GJ. Goals, Guidelines and Principles for Point of-Care Testing. In Kost GJ, editor. Principles & Practice of Point-of-Care Testing. Hagerstwon, MD: Lippincott Williams & Wilkins; 2002.
P29. Laurence CO, Gialamas A, Bubner T, Yelland L, Willson K, Ryan P, et al. Patient satisfaction with point-of-care testing in general practice. Br J Gen Pract 2010;60:e98–104.
P30. Laurence CO, Moss JR, Briggs NE, Beilby JJ. The cost-effectiveness of point of care testing in a general practice setting: results from a randomised controlled trial. BMC Health Serv Res 2010;10:165.
P31. Laurence C, Gialamas A, Yelland L, Bubner T, Ryan P, Willson K, et al. A pragmatic cluster randomised controlled trial to evaluate the safety, clinical effectiveness, cost effectiveness and satisfaction with point of care testing in a general practice setting - rationale, design and baseline characteristics. Trials 2008;9:50.
P32. Laurence C, Gialamas A, Yelland L, Bubner T, Ryan P, Willson K, et al. A pragmatic cluster randomised controlled trial to evaluate the safety, clinical effectiveness, cost effectiveness and satisfaction with point of care testing in a general practice setting–rationale, design and baseline characteristics. Trials 2008;9:50.
P33. Little P, Turner S, Rumsby K, Jones R, Warner G, Moore M, et al. Validating the prediction of lower urinary tract infection in primary care: sensitivity and specificity of urinary dipsticks and clinical scores in women. Br J Gen Pract 2010;60:495–500.
P34. Global Information. The World Market for Point of Care (POC) Diagnostics. Kalorama Information; 2015. URL: www.giiresearch.com/report/kl253350-world-market-point-care-diagnostics.html (accessed 28 August 2015).
P35. Murray ET, Fitzmaurice DA, McCahon D. Point of care testing for INR monitoring: where are we now? Br J Haematol 2004;127:373–8.
P36. Nursing and Midwifery Council. The Code: Standards of Conduct, Performance and Ethics for Nurses and Midwives. London: Nursing and Midwifery Council; 2008.
P37. Plüddemann A, Heneghan C, Price CP, Wolstenholme J, Thompson M. Point-of-care blood test for ketones in patients with diabetes: primary care diagnostic technology update. Br J Gen Pract 2011;61:530–1.
P38. Plüddemann A, Thompson M, Price CP, Wolstenholme J, Heneghan C. The D-dimer test in combination with a decision rule for ruling out deep vein thrombosis in primary care: diagnostic technology update. Br J Gen Pract 2012;62:e393–5.
P39. Plüddemann A, Thompson M, Price CP, Wolstenholme J, Heneghan C. Point-of-care testing for the analysis of lipid panels: primary care diagnostic technology update. Br J Gen Pract 2012;62:e224–6.
P40. Australian Government Department of Health. Point of Care Testing in General Practice Trial Report – Executive Summary. URL: www.health.gov.au/internet/main/publishing.nsf/Content/poctgenpract-reportexecsum (accessed 1 April 2010).
P41. Kitchen DP, Kitchen S, Jennings I, Woods TA, Fitzmaurice DA, Murray ET, et al. Point of care INR testing devices: performance of the Roche CoaguChek XS and XS Plus in the UK NEQAS BC external quality assessment programme for healthcare professionals: four years’ experience. J Clin Pathol 2012;65:1119–23.
P42. Poller L, Keown M, Chauhan N, den Besselaar AM, Tripodi A, Shiach C et al. Reliability of international normalised ratios from two point of care test systems: comparison with conventional methods. BMJ 2003;327:5–6.
P43. National Institute for Health Research. Primary Care Use of a C-Reactive Protein (CRP) Point of Care Test (POCT) to Help Target Antibiotic Prescribing to Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD) who are Most Likely to Benefit (The PACE Study). URL: www.nets.nihr.ac.uk/__data/assets/pdf_file/0020/130664/PRO-12-33-12.pdf (accessed 28 August 2015).
P44. Schuetz P, Müller B, Christ-Crain M, Stolz D, Tamm M, Bouadma L, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;9:CD007498.
P45. Shephard MD. Cultural and clinical effectiveness of the ‘QAAMS’ point-of-care testing model for diabetes management in Australian aboriginal medical services. Clin Biochem Rev 2006;27:161–70.
P46. Shephard M. Point-of-Care Testing in Australia: the status, practical advantages, and benefits of community resiliency. Point Care 2013;12:41–5.
P47. Shephard MD. Influence of geography on the performance of quality control testing in the Australian Government’s point of care testing in general practice trial. Clin Biochem 2009;42:1325–7.
P48. Shephard MD, Mazzachi BC, Watkinson L, Shephard AK, Laurence C, Gialamas A, et al. Evaluation of a training program for device operators in the Australian Government’s Point of Care Testing in General Practice Trial: issues and implications for rural and remote practices. Rural Remote Health 2009;9:1189.
P49. Shephard MD, Spaeth B, Mazzachi BC, Auld M, Schatz S, Loudon J, et al. Design, implementation and initial assessment of the Northern Territory Point-of-Care Testing Program. Aust J Rural Health 2012;20:16–21.
P50. Shephard M, Shephard A, Watkinson L, Mazzachi B, Worley P. Design, implementation and results of the quality control program for the Australian government's point of care testing in general practice trial. Ann Clin Biochem 2009;46:413–19.
P51. Shiach CR, Campbell B, Poller L, Keown M, Chauhan N. Reliability of point-of-care prothrombin time testing in a community clinic: a randomized crossover comparison with hospital laboratory testing. Br J Haematol 2002;119:370–5.
P52. St John A. The evidence to support point-of-care testing. Clin Biochem Rev 2010;31:111–19.
P53. Stone MA, Burden AC, Burden M, Baker R, Khunti K. Near patient testing for glycated haemoglobin in people with type 2 diabetes mellitus managed in primary care: acceptability and satisfaction. Diabet Med 2007;24:792–5.
P54. Thompson M, Plüddemann A, Price CP, Heneghan C. Emerging diagnostic technologies in primary care: what’s on the horizon? Br J Gen Pract 2013;63:177–8.
P55. Tirimacco R, Glastonbury B, Laurence CO, Bubner TK, Shephard MD, Beilby JJ. Development of an accreditation program for point of care testing (PoCT) in general practice. Aust Health Rev 2011;35:230–4.
P56. Tirimacco R. Evolution of point-of-care testing in Australia. Clin Biochem Rev 2010;31:75–80.
P57. van Bokhoven MA, Pleunis-van Empel MC, Koch H, Grol RP, Dinant GJ, van der Weijden T. Why do patients want to have their blood tested? A qualitative study of patient expectations in general practice. BMC Fam Pract 2006;7:75.
P58. van Vugt SF, Broekhuizen BD, Lammens C, et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: diagnostic study. BMJ 2013;346:f2450.
P59. Wood F, Brookes-Howell L, Hood K, Cooper L, Verheij T, Goossens H, et al. A multi-country qualitative study of clinicians’ and patients’ views on point of care tests for lower respiratory tract infection. Fam Prac 2011;28:661–9.
P60. Yelland LN, Gialamas A, Laurence CO, Willson KJ, Ryan P, Beilby JJ. Assessing agreement between point of care and pathology laboratory results for INR: experiences from the Point of Care Testing in General Practice Trial. Pathology 2010;42:155–9.
Radiology/X-ray
Definition: an X-ray is a quick and painless procedure commonly used to produce images of the inside of the body (NHS Choices).
This map includes chest X-ray and partial CT scanning.
This map excludes echocardiography and MRI.
Contains public sector information licensed under the Open Government Licence v3.0. 155
Background
The Department of Health’s document Our Health, Our Care, Our Say spelled out a commitment to providing more diagnostic testing in primary care. 399 This is seen as a route to reducing the total number of secondary care referrals and speeding up the patient journey when referral is appropriate. For this to occur requires that GPs are comfortable with a greater range of diagnostic tests and confident that better direct access to testing will not lead to a reduction in patient care and outcome.
Radiology involves three components: acquiring a digital image, using a computer to analyse the computer-based image and information management (including picture archiving and storage). Increasingly it involves the transmission of images through communication technologies so that images and reports can be viewed anywhere in the NHS. GPs are beginning to take an interest in seeing their patients’ medical images, especially with the development of special interests and primary-to-primary referrals. Concern has been expressed that ‘as the availability and power of imaging grows, skills in history taking and physical examination decline’. 419
Consultation for a road map for the RCGP in 2007 found general support for the concept of community hospitals, or a modern variant of a diagnostic centre, assuming that they were properly resourced. The report articulated that ‘The time has come for diagnostic radiology to sever its secondary care cord and move into primary care’. Facilities based in community hospitals would house MRI, CT and ultrasound, with GPs and radiologists working together to get the right diagnosis. 3
There is a drive to improve support for patients at home and in the community by enhancing primary care services and thus avoiding unnecessary referrals to secondary care. For this to be successful, there needs to be appropriate support for clinical colleagues in primary care.
It is well established that early diagnosis is key to the management of such patients. Not only does such early diagnosis lead to better outcomes but a negative investigation can exclude disease, reassuring the patient and doctor and avoiding further unnecessary anxiety.
It is, therefore, timely to consider what constitutes best practice in clinical imaging services focusing on primary care. In doing so this guide will support local NHS clinical imaging departments in remaining the preferred provider of imaging services to their local CCGs.
There is increasing momentum in improving access to diagnostic imaging for primary care physicians and allied health professionals. 419 Although direct access arrangements have existed for several years in many centres in the UK, the shift of diagnostic resource from secondary to primary care creates a fundamental change regarding how service provision may operate.
Many hospital trusts have developed direct access services for plain radiography provision to primary care over the past few years, with a smaller proportion providing access to ultrasound and MRI. A high proportion of independent hospitals also offer direct access to imaging to GPs, usually through local engagement with PCTs. Hence the RCR continues to promote its Making the Best Use of a Department of Radiology document. 218 However, primary care physicians in many locations feel that there are significant limitations in their freedom to access imaging, particularly CT and MRI, at secondary care locations. Furthermore, deficiencies persist in relation to the timeliness and convenience of access to imaging and the communication of imaging results provided by hospital trusts. In short, there are issues relating to the level of ‘customer service’ provided by secondary care to primary care physicians. 51
Clinical Commissioning Groups have strong incentives to promote direct access to diagnostics. Many CCGs are seeking to improve the efficiency of the service supplied by the imaging providers with whom they choose to contract. There is an impetus to reduce waiting times for diagnostics to ≤ 2 weeks; to improve patient choice and convenience in terms of the location and time of scanning; and to refocus diagnostic provision onto primary care. Many PCTs are embracing the independent sector market to achieve timeliness of diagnostics provision and to devolve access to diagnostics far more widely and ‘closer to home’. 51 This ‘power shift’ from secondary care to primary care and from NHS-led provision to the independent sector has major implications for UK radiologists and radiology services. 51
STEP-UP summary
Skills
Radiology is a well-documented profession with well-developed education, training and curricula. For example, the RCR produces a Specialty Training Curriculum for Clinical Radiology. 156 Training must include the equipment and its intended application. It will also include related topics such as health and safety, radiation protection and control of infection. 157 RCR guidance for clinical radiographers emphasises quality assurance issues. It would be challenging for a small radiological unit based in primary care to achieve the adherence to standards and quality control required by a fully-established radiological department.
STEP-UP summary statement With regard to SKILLS, radiology/X-ray is attributed a MODERATE degree of implementation difficulty owing to the need to satisfy the expectations created by the curriculum and quality assurance procedures.
Training
General practitioners and GP educators must ensure that primary care is armed with the appropriate knowledge and tools to take advantage of the shift in the imaging paradigm. 419 The Health and Social Care Act 2012420 directed a specific focus on the commissioning of clinical imaging services, under the ‘any qualified provider’ agenda. Commissioners must ensure that clinical and quality standards are met. Imaging must be undertaken in line with accepted, agreed evidence-based guidelines, with full knowledge and application of statutory obligations, in particular the Ionising Radiation (Medical Exposure) Regulations 2000 (Statutory instrument 2000 No. 1059)421 and subsequent amendments.
Patients should have timely access to appropriate imaging provided by a service with proven clinical governance structures in place, by appropriately trained staff using appropriately maintained equipment, with underlying compliant 24/7 IT support. Imaging services should have knowledge of, and access to, necessary onward referral pathways.
Comprehensive clinical imaging services in the NHS provide patients, GPs and other referrers and commissioners with direct access to essential core services. The quality of any clinical service can be judged by patient outcomes, patient safety, patient and user experience and efficiency. Five components need specific attention:
-
patient access
-
patient information
-
GP/referrer access
-
clinically appropriate imaging
-
integration into pathways of care.
The RCR and the SCoR have developed the ISAS and the associated accreditation scheme. Patients, GPs, referrers and commissioners can be assured that ISAS-accredited services are delivering the highest quality of services.
Potential sites for primary care clinical services include larger health-care centres, stand-alone diagnostic centres and, in some situations, mobile options. Commissioners need to balance improved access against the potential increased cost and reduced efficiency of service/staff utilisation.
An important issue when agreeing such standards within an imaging service for primary care is that the patients’ care and well-being is not compromised either by unnecessary delays in the result of an imaging study being available or by an incomplete or substandard report being produced because not all of the required information or expertise was available within a deadline.
Patient choice requires that patients have access to relevant information about the quality of any imaging service that is available to them. Such information should include details of the qualifications and experience of those HCPs providing the service and details of how such a service is integrated into the care pathway. Referring clinicians should inform the patient of the purpose of the investigation and how it may affect their management. Although they may seek to minimise anxiety that serious life-threatening conditions might be uncovered, such conversations should occur before the referral, with both referrer and patient being aware of the limitations of imaging and the possibility of no definitive result or outcome.
STEP-UP summary statement With regard to TRAINING, radiology/X-ray is attributed a HIGH degree of implementation difficulty owing to extensive and specialist training requirements.
Equipment
One study in primary care reports that patient management by the GP changed in 60% of patients following chest radiography. Chest radiography substantially reduced the number of referrals and initiation or change in therapy, and more patients were reassured by their GP. This confirms that chest radiography is an important diagnostic tool for GPs. 158
STEP-UP summary statement With regard to EQUIPMENT, radiology/X-ray is attributed a LOW degree of implementation difficulty owing to established practices and procedures for using X-ray equipment.
Premises
Radiation protection is a major consideration when using X-ray equipment. Health Building Note 11-01, Facilities for Primary and Community Care Services, locates X-ray services in a purpose-built ‘X-ray room’ with lead shielding (contains public sector information licensed under the Open Government Licence v3.0). 46 It suggests the idea of an ‘imaging cluster’ that will require such facilities as X-ray rooms, image control/reporting room and changing rooms, suggesting that these facilities should be clustered together alongside other dedicated imaging rooms, where provided. Health Building Note 11-01 also provides an illustration of the imaging cluster concept. 46 Examination rooms housing X-ray equipment should incorporate radiation shielding to contain the controlled radiation area. Rooms have to meet the requirements of the Ionising Radiations Regulations 1999,160 which have much stricter limits than earlier regulations on radiation exposure to the public from man-made sources of radiation. Chapter 4 of Health Building Note 6, entitled ‘General X-ray imaging or radiography’,159 includes specifications for space and for basic equipment. It does not specifically outline requirements for a primary care setting.
STEP-UP summary statement With regard to PREMISES, radiology/X-ray is attributed a MODERATE degree of implementation difficulty owing to the need for accommodation with radiation protection and shielding.
User perspective
When ordering radiological tests, clinicians in primary care consider such factors as the potential impact on the clinical outcome for the patient, the probability of significant findings based on the clinical picture and the sensitivity/specificity of the test. 422 Secondary considerations include patient reassurance, medico-legal and compensation issues and the costs and risks of the investigation. It is not always possible to realise benefits from a normal examination into patient reassurance. 158 In one primary care study, only 50% of patients felt reassured by having had a chest radiograph and 25% recorded that the radiograph was of little value to them because no referral or treatment followed the investigation. Decision-making in primary care, especially in patients with chronic conditions, is less often influenced by the radiological report.
Once patients have had an imaging investigation, they expect the results to be available to their GP without delay. It is desirable to report the majority of cases within 1 working day. Urgent cases in which the report has an impact on the immediate management of the patient need to be reported at the time of examination and to be made available to the referrer. All reports should be communicated electronically via a reporting system that can push both key images and the report directly into the patient management system. This needs to incorporate a clear audit trail with regard to receipt/action. An agreed system should be in place for the handling of urgent or unexpected results in line with the RCR guidance.
Timeliness of test, and then the result, is thus a key question for patients when referred for an imaging test. Convenience of time and place is a third important consideration. A prompt and appropriate response helps in meeting patients’ psychological and social needs.
STEP-UP summary statement With regard to USER PERSPECTIVES, radiology/X-ray is attributed a LOW degree of implementation difficulty owing to the familiarity of X-ray arrangements.
Primary–secondary interface
The Department of Health has been seeking to facilitate imaging tailored to the needs of primary care, and thus combat a perceived ‘secondary care imaging agenda’. The independent sector is involved in this process, particularly because the provision of diagnostics by the independent sector has created new expectations for imaging services, especially in relation to waiting times and quality of service. The involvement of the independent sector in shifting imaging from secondary to primary care is perceived as being ‘disruptively innovative’. It is viewed as important in the continued drive to achieve ‘zero waits’ for the diagnostic aspect of clinical pathways. In support of this, tariffs for primary care direct access have been unbundled from overall clinical pathway tariffs. 51
When GPs have access to diagnostic testing from primary care and clear referral guidelines they are likely to use imaging resources as efficiently as hospital doctors. 423 However, local variation in direct access to imaging tests remains, especially for the more complex investigations. Imaging must be undertaken primarily to benefit patients. If implemented correctly, improving imaging access should shorten the patient pathway, facilitate better patient care and produce savings across the health-care economy. One study reported a change in proposed management for 60% of patients referred for chest radiography from primary care. 424 Benefits to the referring practice included streamlined referrals to secondary care (first-time referral to the appropriate specialty), an overall reduction in secondary care referrals and improved prescribing.
The aspirational goal for clinical imaging services is to have a no-wait culture through appropriate and timely ‘choose and book’ arrangements. Almost all imaging should be performed as soon as possible from receipt of referral; this should normally occur within 2 weeks. This does, however, have clear implications for resources but would be expected to deliver tangible benefits, not only in shortening patient pathways but also in reducing secondary care referrals and hospital admissions.
A secondary care provider seeking to compete in the ‘direct-access imaging market’ will probably need to increase the local equipment and staffing resource. 51
Radiology services, and radiologists themselves, will face significant pressure either to demonstrate optimised efficiency or to adapt to new working arrangements tailored to increase throughput and work intensity. The radiology profession may experience pressure to undergo benchmarking of its performance. Radiology departments (including radiologists) may need to become more ‘customer-friendly’, both to primary care professionals and to their patients. The way in which reports are structured and communicated by radiologists will also need to be adapted to for primary care. 51
Those commissioning imaging services should seek to offer an integrated pathway across primary and secondary care. They should seek to avoid destabilising other necessary imaging services to the population for which they are responsible, for example 24/7 emergency access, which requires an increased workforce and resource overheads. The use of alternative providers may result in a reduction in staff and resources that compromises other emergency and other urgent imaging services within the locality. A further concern is education and training issues. If a significant volume of imaging is delivered outside the traditional NHS providers, this will potentially reduce trainees’ access to appropriate learning opportunities and material, as already experienced with, for example, the outsourcing of ultrasound and musculoskeletal MRI. 425 Commissioning groups should not allow patient care to be disadvantaged by inappropriate financial drivers.
When primary care commissioning is supported with auditable standards for waiting times and turnaround times, there is the prospect that follow-up examinations could be booked at the time of the first consultation. The radiological report can, therefore, be available in a timely fashion, reducing GP frustration and patient delay. Traditional providers of NHS clinical imaging, based in secondary care hospitals, must seek to balance demands from both secondary and primary care for their services. Decisions on providing such services must be patient-centred and seek to secure the best outcome for the patients.
Despite pessimistic predictions, direct access to diagnostics in general practice appears to increase demand for testing but does not reduce appropriateness of testing or diagnostic yield. 383,423,426 Direct access to CT scanning and MRI led to a good diagnostic yield in a study from Edinburgh. When guidelines for chronic daily headache were provided to GPs facilitating appropriate access to CT of the brain, the referral rate was only 1.2%. A London-based group explored the effect on patient management of direct access to diagnostic imaging. Three core components supported appropriate GP referrals – (1) referral guidelines, (2) structured referral forms and (3) clinical triage with telephone feedback to GPs – suggesting alternative tests or contraindications to testing. 383,423,426 Thirty-two per cent of patients referred for echocardiography were found to have an abnormal report but only 29% of these were referred to secondary care as the majority were managed in primary care. Overall, 71% of patients referred for diagnostic imaging were managed in the primary care setting. 161
Alternatively, when direct-access imaging is supplied locally by the independent sector, secondary care providers may have to downsize, impacting on radiologist recruitment and existing work plans and patterns. Radiologists might adopt a mixed portfolio, working for both their NHS employer independent-sector providers. Increasingly, secondary care radiologists will be required to provide ‘second opinions’ on imaging and reports sourced externally. Such demands will likely have to be factored into future job planning. 51
Logistical difficulties relating to independent provision of direct-access diagnostics may occur when suboptimal clinical referral information or lack of access to previous imaging in the primary care setting increase the need for unnecessary subsequent investigation or referral. Nevertheless, the shift to direct access for imaging seems so strong that such considerations are unlikely to prove ‘show stoppers’. 51 This paradigm shift in imaging provision is likely to have wide-reaching effects on the way in which radiologists practise, and requires proactive involvement by UK radiology bodies. 51 The limited uptake of direct access by GPs highlights a need to engage GPs in the planning and implementation of new services. 427
Direct access may result in reduced waiting times from presentation to testing and treatment. 427 A review found that direct access to diagnostic tests allows GPs to manage a substantial number of patients who would otherwise have been referred to the hospital outpatient department. 428 Limited information is available on costing associated with increased access to diagnostics for GPs versus hospital-based access only. An appropriate costing model would factor in the cost of an initial outpatient department appointment, the average number of patients referred for CT from the OPD and the average number given a further follow-up appointment minus the cost of the direct access to CT and reduction in referral to outpatients departments. 429 The DAMASK (Direct Access to Magnetic Resonance Imaging: Assessment for Suspect Knees) RCT for direct access to MRI for knee problems in the UK resulted in increased costs, offset by a statistically significant improvement in health-related quality of life. 430
In a survey of Irish GPs, O’Riordan et al. 383,426 examined the availability of radiologic and endoscopic services. In all services, access to diagnostics for public patients is unacceptably long when compared with that for private patients. As a consequence, GPs refer patients inappropriately to overcrowded emergency departments in order to access diagnostic tests. This can be unnecessarily traumatic for elderly patients, and places an extra costly burden on hospital services. The vast majority of GPs indicated that increased access to diagnostics would help them to reduce the number of referrals to emergency and outpatient departments, reduce unnecessary admissions and improve the overall quality of referrals.
STEP-UP summary statement With regard to the PRIMARY–SECONDARY INTERFACE, radiology/X-ray is attributed a MODERATE degree of implementation difficulty owing to uncertainties about the new provider arrangements.
Conclusion
Imaging services are facing what has been described as a paradigm shift. Although the increase in competitiveness and a market orientation ultimately holds the prospect of higher standards and expectations, this is being realised on only a small local scale at present. There is considerable uncertainty in this particular area of diagnostic provision, making it very difficult to predict what will happen in the short term.
Radiology (R1–32)
R1. Anon. Making a call on the mobile. Health Serv J 2014;124:30–31.
R2. Birchall D. Primary care access to diagnostics: a paradigm shift. Br J Radiol 2010;83:101–3.
R3. Carlin LE, Smith HE, Henwood F. To see or not to see: a qualitative interview study of patients’ views on their own diagnostic images. BMJ Open 2014;4:e004999.
R4. Centre for Workforce Intelligence. Securing the Future Workforce Supply. Clinical Radiology Stocktake. 2012. URL: www.cfwi.org.uk/publications/securing-the-future-workforce-supply-clinical-radiology-stocktake (accessed 28 August 2015).
R5. Cherryman G. Imaging in primary care. Br J Gen Pract 2006;56:563–4.
R6. College of Radiographers. A Strategy for the Education and Professional Development of Therapeutic Radiographers. London: College of Radiographers; 2000.
R7. DAMASK (Direct Access to Magnetic Resonance Imaging: Assessment for Suspect Knees) Trial Team. Effectiveness of GP access to magnetic resonance imaging of the knee: a randomised trial. Br J Gen Pract 2008;5:e1–8.
R8. Department of Health. Our health, Our Care, Our Say: A New Direction for Community Services. London: Department of Health; 2006.
R9. NHS Estates. Facilities for Diagnostic Imaging and Interventional Radiology. Health Building Note Number 6. London: The Stationery Office; 2001.
R10. Holm T. Consumer Guide for the Purchase of X-ray Equipment. Geneva: World Health Organization, Collaborating Centre for General and Continuing Radiological Education; 2000.
R11. Honkanen PO, Rautakorpi UM, Huovinen P, Klaukka T, Palva E, Roine R, et al. Diagnostic tools in respiratory tract infections: use and comparison with Finnish guidelines. Scand J Infect Dis 2002;34:827–30.
R12. Royal College of General Practitioners. Quality Imaging Services for Primary Care: A Good Practice Guide. 2013. URL: www.rcgp.org.uk/news/∼/media/Files/CfC/RCGP-Quality-imaging-services-for-Primary-Care.ashx (accessed 4 January 2016).
R13. The Imaging Services Accreditation Scheme Standard. Statements, Rationales and Criteria January 2013. 2013. URL: www.sor.org/system/files/article/201502/isas_standard_v2.1_jan_2013.pdf (accessed 28 August 2015).
R14. Royal College of Radiologists. Specialty Training Curriculum for Clinical Radiology. URL: www.rcr.ac.uk/sites/default/files/docs/radiology/pdf/Curriculum_CR_28_October_2014_FINAL.pdf (accessed 28 August 2015).
R15. Khan I, Alamri AF, Baig MIA, Iftikhar R. Radiographic examination of patients with musculoskeletal problems in general practice. Life Sci J 2013;10.
R16. Miller P, Kendrick D, Bentley E, Fielding K. Cost-effectiveness of lumbar spine radiography in primary care patients with low back pain. Spine 2002;27:2291–7.
R17. Moore A. What’s the diagnosis? Health Serv 2005;115(Suppl.):2–4.
R18. Nolan A. Technology. Positive image. Health Serv J 2005;115(Suppl. 5).
R19. O’Riordan M, Collins C, Doran G. Access to Diagnostics: A Key Enabler for a Primary Care Led Health Service. Irish College of General Practitioners; 2013. URL: www.lenus.ie/hse/bitstream/10147/292726/1/Access%20to%20diagnostics%202013.pdf (accessed 28 August 2015).
R20. O’Riordan M, Doran G, Collins C. Access to diagnostics in primary care and the impact on a primary care led health service. Ir Med J 2015;108:53–5.
R21. Quality Assurance Agency for Higher Education. Radiography. Benchmark Statement: Health Care Programmes Phase 1. London: Quality Assurance Agency for Higher Education; 2001. pp. 28.
R22. Roland MP. Outpatient Services And Primary Care: A Scoping Review of Research into Strategies for Improving Outpatient Effectiveness and Efficiency. 2006. URL: www.medicine.manchester.ac.uk/primarycare/npcrdc-archive/Publications/Studyinghealthcare_Roland_finalreport.pdf (accessed 28 August 2015).
R23. Royal College of Radiologists. Investing in the Clinical Radiology Workforce – the Quality and Efficiency Case. RCR; 2012. URL: www.rcr.ac.uk/docs/radiology/pdf/RCR_CRWorkforce_June2012.pdf (accessed 28 August 2015).
R24. Sibbald B. Direct access to diagnostic services. Br J Gen Pract 2009;59:e144–5.
R25. Simpson GC, Forbes K, Teasdale E, Tyagi A, Santosh C. Impact of GP direct-access computerised tomography for the investigation of chronic daily headache. Br J Gen Pract 2010;60:897–901.
R26. Speets AM, van der Graaf Y, Hoes AW, Kalmijn S, Sachs AP, Rutten MJ, et al. Chest radiography in general practice: indications, diagnostic yield and consequences for patient management. Br J Gen Pract 2006;56:574–8.
R27. Stapley S, Sharp D, Hamilton W. Negative chest X-rays in primary care patients with lung cancer. Br J Gen Pract 2006;56:570–3.
R28. RCGP. The Future Direction of General Practice: A Roadmap. RCGP; 2007.
R29. NHS Improvement. Diagnostics: Towards Best Practice in Interventional Radiology. Leicester: NHS Improvement; 2012.
R30. White PM, Halliday-Pegg JC, Collie DA. Open access neuroimaging for general practitioners – diagnostic yield and influence on patient management. Br J Gen Pract 2002;52:33–5.
R31. Wilson S, Fromont C, Langley R, Chescoe S, Grootoonk S. A Study of the Effect on Patient Management of GP Direct Access to Diagnostic Imaging Tests – The London Outcomes Audit. In Health; 2010.
R32. Yelland M. Diagnostic imaging for back pain. Aust Fam Physician 2004;33:415–19.
Respiratory tests
Definition: respiratory tests measure how much air is moved in and out of the lungs, how successful the lungs are at getting oxygen into the blood stream and if there are problems in the lungs that can be seen in images of the lungs.
This map includes lung function tests.
This map excludes spirometry (see Spirometry) and CRP (see Point-of-care testing).
The Department of Health outcomes strategy for people with COPD and asthma431 and its companion document432 outline the aspirations for high-quality care in COPD and asthma. There is, however, evidence of suboptimal care, including substantial variation in standards in COPD care across England433 and deficiencies in the assessment of the acute asthma attack. 434
STEP-UP summary
Skills
A systematic review of 30 primary care studies from around the world162 evaluated the diagnostic ability of GPs in relation to respiratory diseases, such as acute respiratory infections, tuberculosis, asthma and COPD. In relation to asthma and COPD, studies show either overdiagnosis or underdiagnosis. Variation for asthma varied from 54% underdiagnosis to 34% overdiagnosis, whereas for COPD this ranged from 81% for underdiagnosis to 86.1% for overdiagnosis. For acute respiratory infections, inclusion of a complementary test for diagnosis led to an improvement in diagnostic accuracy. GPs showed a low level of knowledge about tuberculosis. The review highlighted a significant need to improve the skills and knowledge of GPs.
With increasing affordability and recognition of its clinical applications, there is an increasing interest of the role of the oximeter in primary care. Data concerning the influence of pulse oximetry on patient management and on the extent of oximetry use in the general practice setting are scarce. Several studies identify the role and potential of the oximeter as a screening tool in assessing hypoxia in primary care. 163–166 Pulse oximetry technology has relevance to COPD, asthma, community-acquired pneumonia and paediatric assessment, as well as for preparations for a possible influenza pandemic, trends in primary care emergency-care provision, demographic changes and alterations in the case mix of patients encountered in primary care. Other obvious trends include the increasing prevalence of adults with congenital heart disease encountered by GPs. Increased use of pulse oximetry (supported by training and quality assurance) must be appropriate. Clinically appropriate testing should ensure that the expected health benefits exceed the expected negative consequences by a sufficiently wide margin that the test is worth doing.
Factors affecting displayed readings and causing errors relate mainly to light transmission, perfusion or pulse detection. Pulse oximetry can be inaccurate in patients with poor peripheral circulation, excessive sweating, presence of carbon monoxide (e.g. in smokers), nail varnish/synthetic nails, light interference, as a motion artefact, abnormal haemoglobin and dark or jaundiced skin. 435 Documentation of the pulse oximetry test, as with all clinical procedures, is essential. This should detail the actual reading, any activity (at rest or walking), whether the patient was breathing room air or oxygen (if so, what flow rate and percentage) and any other factors that might have influenced the reading (e.g. tremor or cold hands).
STEP-UP summary statement With regard to SKILLS, respiratory tests are attributed a LOW degree of implementation difficulty owing to the existence of well-established testing technologies.
Training
A draft working document outlines possible roles for a GPwSI, using respiratory medicine as a model. It envisages the role of a GPwSI in respiratory medicine as primarily one of leadership and service development (i.e. as a GP lead in respiratory medicine in a primary care organisation). This contrasts with specialties, such as ENT or dermatology, when the GPwSI has a predominantly clinical role. In addition, the draft document envisaged that the ‘gold standard‘ qualification for a GPwSI would be a diploma in respiratory medicine. A modified draft document proposes a hybrid framework that combines a generic RCGP framework with a respiratory disease-specific framework. Improving access to spirometry was agreed as a top priority by GPwSIs in respiratory care. 167 The Primary Care Respiratory Society UK has developed quality standards which can act as the basis for a training programme. 436 GPwSIs in respiratory care are envisaged to support a nurse-led spirometry service. 167
The guideline recommends that pulse oximetry should be available in all locations where emergency oxygen is used, including primary care. Pulse oximetry should be used if considering oxygen administration in acute stroke and myocardial infarction, as high flow oxygen has been shown to produce vasospasm and potentially worsen outcomes.
STEP-UP summary statement With regard to TRAINING, respiratory tests are attributed a LOW degree of implementation difficulty owing to the ease of use of most equipment.
Equipment
Pulse oximeters give a non-invasive estimation of the arterial haemoglobin oxygen saturation. 437 The gold standard for measurement of oxygen saturation remains arterial blood gas analysis. Arterial blood gas analysis is invasive, painful, time-consuming and costly, provides only intermittent information on patient status, and there is a delay between sampling and results. The measurement of pulse oximetry is a suggested disease management strategy to identify patients with chronic lung disease who would benefit from long-term oxygen therapy. 438 Performing spirometry for the detection of airflow obstruction in symptomatic patients will probably identify those who will benefit from bronchodilator therapy. However, as yet there are no feasible tests to assist in targeting β2-agonist and antimuscarinic inhaled therapy specifically, nor are there tests to identify who will benefit from other therapeutic options. Pulse oximeters are increasingly used during endoscopy and other diagnostic procedures, and as part of pulmonary function testing. Data on the role of pulse oximeters in detecting hypoxia in general practice are limited. A minority of GPs reported that they used a pulse oximeter to measure pulse rate (9%) or to assess respiratory status (20%). 177
It is not possible, within the constraints of this mapping review, to cover all types of respiratory equipment, so pulse oximetry has been used as an example. Spirometry is covered separately (see Spirometry) and also features in the accompanying review on breathlessness symptoms.
Pulse oximeters are available, highly portable and increasingly less costly to purchase. 181 Many models are available for purchase from numerous manufacturers. 437 Prices can vary from around £50 to £300. Sizes vary from small pocket-sized models to bench-top displays. Smaller versions are more easily ‘mislaid’. Considerations when choosing the instrument include size, battery capability and robustness. 179 In primary care, especially when carried in diagnostic bags, the equipment needs to be reliable, reproducible, safe, accurate, robust, portable, cost-effective and simple to use. 178 If buying pulse oximeters for a practice/community setting is being considered, it may be worth discussing with colleagues who use pulse oximeters regularly (especially if they use pulse oximeters in a community setting, e.g. respiratory nurse specialists). The NICE Centre for Evidence Based Purchasing has published an evidence review, market review and buyers’ guide for pulse oximeters, providing a list of pulse oximeters with evidence of accuracy and performance. 179 Pulse oximetry is as useful in the management of children as in adults, and the same ranges are applicable. Many ‘adult’ pulse oximeters can be used in children over the age of 2 years. Below the age of 2 years more specialised oximeters are generally preferred.
Most available pulse oximeters are accurate between oxygen saturations of 70% and 100%, with a range of ± 2%. The oximeters are calibrated during manufacture and most have an internal check system to ensure that calibration remains valid. Those using the machine are responsible for ensuring that it is in good working order, stored correctly (according to manufacturers’ recommendations) and regularly serviced. Probes should be appropriate for the site of monitoring (e.g. finger or ear sensor), cleaned regularly and checked for obvious faults such as loose connections or wires.
The use of pulse oximetry in primary care is still in its infancy. 183 Its advantages are that it is quick, non-invasive, reproducible and accurate in most circumstances, and minimal training is necessary. Its disadvantages are that it needs maintenance, has accuracy limitations in some patients and may not provide a clinical benefit to patient care if used indiscriminately. Practices planning to use oximetry in daily practice need to consider how they will handle an isolated low reading in an otherwise well patient, and under what circumstances readings should be taken. The implications for an individual general practice depend on the population demographic and the resources or time used by having an oximeter in the surgery. A portable pulse oximeter costs £300–400 and needs recalibration every 3 years. If a practice considers it appropriate to buy spirometry equipment and ECG machines, purchase of an oximeter is of a similar degree.
Probes are an infection control risk. Cleaning procedures, according to manufacturers’ recommendations, must be performed between patients. 181 Dirty probes can also reduce the accuracy of results because the light will not be effectively transmitted. Risks associated with pulse oximetry include burns and pressure ulcers but this is due to prolonged use at a single site, which would rarely be an issue in primary care.
STEP-UP summary statement With regard to EQUIPMENT, respiratory tests are attributed a LOW degree of implementation difficulty owing to portability, increasingly cheap equipment and ease of use.
Premises
Little detail is available on the implications of respiratory tests for primary care premises. If patients are being assessed in an area with a high level of artificial light (e.g. operating theatre fluorescent lighting), this can falsely reduce the readings. 180,181
STEP-UP summary statement With regard to PREMISES, respiratory tests are attributed an UNCERTAIN degree of implementation difficulty owing to the lack of detail on storage and stock space requirements.
User perspective
Interviews with family practitioners174 found that they considered pulse oximetry especially of value when on an out-of-office-hours shift, as a supportive tool to decide whether or not to send a patient to an emergency department or to refer them to a medical specialist. The family practitioners also considered pulse oximetry of additional value to direct their decisions on which medication to prescribe in case of an exacerbation of COPD. All interviewed family practitioners stated that a pulse oximeter should be included in the standard equipment package of a practitioner. They considered pulse oximetry as an adjunct in diagnostic assessment of patients, not a full diagnostic tool by itself.
Pulse oximetry, as an alternative to arterial blood gas monitoring, avoids the pain associated with that invasive procedure. A qualitative study of patients’ experience of spirometry found that whereas patients were generally ambivalent about spirometry, they unanimously expressed abhorrence and dread associated with arterial blood gas. 439 Patients also expressed variation in a clinician’s proficiency in undertaking the procedure accompanied by relief when they were able to perform this speedily, successfully and with minimal discomfort. Local anaesthesia mitigates the painfulness of the arterial blood gas procedures but this is not universally offered.
To reduce the likelihood of inaccurate readings in patients undergoing pulse oximetry, HCPs should always ensure that a patient’s nail varnish is removed, if present, a patient’s hand is warmed if cold on presentation and that the probe is correctly positioned and clean. Two particular challenges arise when measuring oxygen saturation in young children. First, they may be difficult to examine and not want to stay still while the oximeter reading is taken. Second, small digits (fingers) are more likely to have poor perfusion and a reading may not be obtainable.
STEP-UP summary statement With regard to USER PERSPECTIVES, respiratory tests are attributed a LOW degree of implementation difficulty as they tend to be well understood and well tolerated.
Primary–secondary interface
Several countries have developed respiratory assessment units to improve the diagnosis of respiratory diseases such as asthma and COPD and to overcome problems with misdiagnosis. These services are heterogeneous and may include spirometry,187 review of medical history440 or radiography and oxygen saturation assessments. 274 The review may consist of paper-based information or may involve a face-to-face review or consultation292 with components of the diagnostic services mentioned above. 274 The services are also delivered by a diverse range of HCPs such as respiratory nurses,274 GPs275 or respiratory specialists. 290 Centralisation of spirometry via a dedicated service has previously been discussed as a solution but has not been trialled in the UK. 441 To date, UK models have included within-practice services, peripatetic services and centralised services, usually in a local hospital.
A community respiratory assessment unit was established to optimise diagnosis of respiratory disease by providing focused history-taking, quality-assured spirometry and evidence-based guideline-derived management advice. 182 Based in a London secondary care hospital, this was a nurse-led facility, staffed by two specialist respiratory nurses with extensive experience of caring for respiratory diseases in both hospital and the community. Access to a respiratory specialist for advice was always available. All local GPs had access to the service. GPs were informed about the unit by means of a letter from the executive director of the PCT, as well as a personal visit from the community respiratory assessment unit nurses to each primary care practice. A 4-year review found that one-third of suggested diagnoses of COPD by the GP were incorrect, resulting in the inappropriate prescribing of inhaled therapies. The authors identified significant financial, ethical and safety implications and highlighted a need for either diagnostic centres (community respiratory assessment unit) or alternative peripatetic practice-based services operating to quality-controlled standards. Similar findings have been reported in a UK cross-sectional study. 442
STEP-UP summary statement With regard to the PRIMARY-SECONDARY INTERFACE, respiratory tests is attributed a MODERATE degree of implementation difficulty owing to dependencies on follow-up secondary care diagnosis and treatment.
Conclusion
The evidence base to support pulse oximetry is limited, but there are several areas in which further work may demonstrate benefits from the application of this technology in primary care. 183 Quantitative studies involving the rate of admission to hospital of acute respiratory illness might evaluate this device further in acute illness scenarios. Further qualitative studies could examine GPs’ experiences of the use of portable oximeters. It is conceivable that the oximeter could be used in health promotion, for example in encouraging patients to give up smoking. Although no clinician would base treatment solely on its readings, there is some evidence for the usefulness of pulse oximetry in general practice. It is not yet clear whether or not its use has any effect on diagnosis or patient-defined outcomes.
Respiratory (Re1–39)
Re1. Amar D, Neidzwski J, Wald A, Finck AD. Fluorescent light interferes with pulse oximetry. J Clin Monit 1989;5:135–6.
Re2. Bausewein C, Jolley C, Reilly C, Lobo P, Kelly J, Bellas H, et al. Development, effectiveness and cost-effectiveness of a new out-patient Breathlessness Support Service: study protocol of a phase III fast-track randomised controlled trial. BMC Pulm Med 2012;12:58.
Re3. Bewick T, Greenwood S, Lim WS. What is the role of pulse oximetry in the assessment of patients with community-acquired pneumonia in primary care? Prim Care Respir J 2010;19:378–82.
Re4. British Thoracic Society. BTS Guidelines for the Management of Community Acquired Pneumonia in Adults. Thorax 2001;56(Suppl. 4):IV1–64.
Re5. O’Driscoll BR, Howard LS, Davison AG on behalf of the British Thoracic Society. BTS guideline for emergency oxygen use in adult patients. Thorax 2008;63(Suppl. VI):i1–68.
Re6. Cunningham S, McMurray A. The availability and use of oxygen saturation monitoring in primary care in order to assess asthma severity. Prim Care Respir J 2006;15:98–101.
Re7. Department of Health. An Outcomes Strategy for COPD and Asthma: NHS Companion Document 2012. URL: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_134000 (accessed 28 August 2015).
Re8. Department of Health. An Outcomes Strategy for People with Asthma and COPD. 2011. URL: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_127974 (accessed 28 August 2015).
Re9. Feiner JR, Severinghaus JW, Bickler PE. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: the effects of oximeter probe type and gender. Anesth Analg 2007;105(Suppl. 6):18–23.
Re10. Gruffydd-Jones K, Small I, Fletcher M, Bryant T. The Primary Care Respiratory Society UK Quality Award: development and piloting of quality standards for primary care respiratory medicine. Prim Care Respir J 2013;22:353–9.
Re11. Guidelines for the Management of Community Acquired Pneumonia in Adults 2004 Update. URL: www.britthoracic.org.uk/ClinicalInformation/Pneumonia/PneumoniaGuidelines/tabid/136/Default.aspx (accessed 28 August 2015).
Re12. Gunstone C. Pulse oximetry in primary care. Br J Gen Pract 2011;61:497.
Re13. Harris SKA. The Experiences and Monitoring of People Living with Chronic Obstructive Pulmonary Disease and Utilising Long-Term Oxygen Therapy. Doctoral dissertation. University of Otago; 2012.
Re14. Harrison B, Stephenson P, Mohan G, Nasser S. An ongoing Confidential Enquiry into asthma deaths in the Eastern Region of the UK, 2001-2003. Prim Care Respir J 2005;14:303–13.
Re15. Healthcare Commission. Clearing the Air: National Study of Chronic Obstructive Pulmonary Disease. 2006. URL: http://archive.cqc.org.uk/_db/_documents/COPD_report1_200607272728.pdf (accessed 28 August 2015).
Re16. Healthcare Improvement Scotland. Asthma Services for Children and Young People Clinical Standards. 2007. URL: www.healthcareimprovementscotland.org/previous_resources/standards/asthma_services_for_children_a.aspx (accessed 28 August 2015).
Re17. Healthcare Improvement Scotland. British Guideline on the Management of Asthma. May 2009 (revised 2012). URL: www.brit-thoracic.org.uk/Portals/0/Guidelines/AsthmaGuidelines/sign101%20Jan%202012.pdf (accessed 28 August 2015).
Re18. Healthcare Improvement Scotland. Chronic Obstructive Pulmonary Disease (COPD) Services – Clinical Standards and Evaluation. URl: www.healthcareimprovementscotland.org/default.aspx?page=11931 (accessed 28 August 2015).
Re19. Holmes S, SJ Peffers. PCRS-UK Opinion Sheet No. 28: Pulse Oximetry in Primary Care. 2009. URL: www.pcrs-uk.org (accessed 28 August 2015).
Re20. Horizon Scanning Report 0004 2 April 2009. Diagnostic Technology: Pulse Oximetry in Primary Care. 2009.
Re21. Ingram G, Munro N. The use (or otherwise) of pulse in general practice. Br J Gen Pract 2005;55:501–2.
Re22. José BP, Camargos PA, Cruz Filho ÁA, Corrêa Rde A. Diagnostic accuracy of respiratory diseases in primary health units. Rev Assoc Med Bras 2014;60:599–612.
Re23. Kelly C. The use of pulse oximetry in primary care. Br J Prim Care Nurs 2008;2.
Re24. King T. Pulse oximetry from past to present. J Clin Engineer 2011;36:129–31.
Re25. Krueger P, Loeb M, Kelly C, Edward HG. Assessing, treating and preventing community acquired pneumonia in older adults: findings from a community-wide survey of emergency room and family physicians. BMC Fam Pract 2005;6:32.
Re26. Levin KP, Hanusa BH, Rotondi A, Singer DE, Coley CM, Marrie TJ, et al. Arterial blood gas and pulse oximetry in initial management of patients with community-acquired pneumonia. J Gen Intern Med 2001;16:590–8.
Re27. Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64(Suppl. 3):iii1–55.
Re28. Marks GB. Evaluation of patients with symptoms of chronic lung disease in primary care. Prim Care Respir J 2013;22:145–7.
Re29. NHS Centre for Evidence-based Purchasing. Project Initiation Document: Pulse Oximeters. NHS Centre for Evidence-based Purchasing; 2009.
Re30. National Institute for Health and Care Excellence. National Guidelines for the management of chronic obstructive pulmonary disease in adults in primary and secondary care, 2004. Thorax 2004;59(Suppl. 1):S1–S232.
Re31. NIHR DEC. Horizon Scan Reports. 2009. URL: www.oxford.dec.nihr.ac.uk/reports-and-resources/horizon-scanning-reports/horizon-scan-reports-non-dec-funded (accessed 28 August 2015).
Re32. Pinnock H, Netuveli G, Price D, Sheikh A. General practitioners with a special interest in respiratory medicine: national survey of UK primary care organisations. BMC Health Serv Res 2005;5:40.
Re33. Potter VA. Pulse oximetry in general practice: how would a pulse oximeter influence patient management? Eur J Gen Pract 2007;13:216–20.
Re34. Primary Care Respiratory Society UK. Opinion No.28 Opinion Pulse Oximetry in Primary Care. Primary Care Respiratory Society UK.
Re35. Plüddemann A, Thompson M, Heneghan C, Price C. Pulse oximetry in primary care: primary care diagnostic technology update. Br J Gen Pract 2011;61:358–9.
Re36. Schermer T, Leenders J, in’t Veen H, van den Bosch W, Wissink A, Smeele I, et al. Pulse oximetry in family practice: indications and clinical observations in patients with COPD. Fam Pract 2009;26:524–31.
Re37. Thompson M, Mayon-White R, Harnden A, Perera R, McLeod D, Mant D. Using vital signs to assess children with acute infections: a survey of current practice. Br J Gen Pract 2008;58:236–41.
Re38. Thompson M, Plüddemann A, Price CP, Heneghan C. Emerging diagnostic technologies in primary care: what's on the horizon? Br J Gen Pract 2013;63:177–8.
Re39. Williams S, Ryan D, Price D, Langley C, Fletcher M, Everden P. General practitioners with a special clinical interest: a model for improving respiratory disease management. Br J Gen Pract 2002;52:838–43.
Spirometry
Definition: spirometry is a simple test used to help diagnose and monitor certain lung conditions by measuring how much air someone can breathe out in one forced breath (NHS Choices).
This map includes portable spirometry.
This map excludes other respiratory tests (see Respiratory tests).
Contains public sector information licensed under the Open Government Licence v3.0. 184
Spirometry measures air flow and volume. Spirometry identifies the presence of airflow obstruction; this may be reversible as in asthma or fixed as in COPD. Spirometry is the gold standard for the diagnosis, assessment and monitoring of COPD, and may assist the diagnosis of asthma. It can also contribute to the diagnosis of other causes of dyspnoea. There is no single diagnostic test for COPD. Spirometry is used to confirm or refute COPD after comprehensive clinical history has identified risk factors and symptoms. There is, however, evidence of suboptimal care, including variation in the quality of primary care spirometry.
Background information on the clinical effectiveness and cost-effectiveness of spirometry is largely derived from the primary care diagnostic technology update entitled Spirometry in primary care for case finding and management of chronic obstructive pulmonary disease. 443 A 2005 technology assessment from the US Agency for Healthcare Research and Quality is also useful. 444
STEP-UP summary
Skills
No systematic reviews specifically examine the effects of migrating spirometry testing from a hospital context to primary care. A diagnostic technology update has summarised much of the evidence, with an emphasis on effectiveness. 443 Two systematic reviews444,445 conducted for the US Preventative Service Task Force have looked at the use of spirometry for population screening for COPD. A relevant review protocol exploring case finding for COPD in primary care using a wide range of methods is available, but the systematic review itself does not yet seem to have been published. 446
A study of spirometry carried out by GPs in primary care found that in 95% of cases fewer than five trials were required to achieve the highest quality grade, concluding that spirometries undertaken in general practice are of acceptable quality and reproducible in only 60% of measurements. 185 The results of this study suggest a need for high-quality initial training and refresher training for spirometry.
Another study investigated the feasibility of practice nurses undertaking case-finding spirometry in general practice. 447 Although the study involved only small numbers of diagnoses, it was concluded that the practice nurses required more training than they were originally given, again highlighting the importance of high-quality training if spirometry were to be used successfully in primary care. In a small study of 45 patients in Leicester, Akhtar and Wilson261 compared the results of spirometry testing in primary care with those obtained at a pulmonary function laboratory. They also explored whether differences were due to technique or equipment. They concluded that spirometry results obtained by practice nurses were lower than those obtained in a laboratory, leading to practice nurses overestimating severity.
A study conducted with community pharmacists found that 73% of spirometries carried out by pharmacists were of acceptable quality, as judged by lung function experts in an acute setting. 186 Ongoing challenges related to selection of a suitable spirometric screening test and to maintaining the quality of spirometric tests in primary care.
A further study investigating the quality of spirometry in primary care found > 15% of the tests being sent from primary care to specialists to analyse lacked complete data. 187 Almost 40% of those that were complete were reported by specialists to be unacceptable. The results showed unacceptable quality in the provision of spirometry in primary care for patients with COPD, suggesting that adequate training must be given if spirometry is to be performed appropriately in primary care.
Poorly performed spirometry can lead to incorrect diagnosis and treatment. All HCPs undertaking diagnostic spirometry should meet accredited quality assured standards. Standards for diagnostic spirometry have been published, including a suggested process for working through a spirometry trace. 448 Primary care practitioners should be encouraged to perform spirometry wherever the quality has been demonstrated to be good (against American Thoracic Society/European Respiratory Society quality goals). 449
STEP-UP summary statement With regard to SKILLS, spirometry is attributed a MODERATE degree of implementation difficulty owing to the skills required to obtain a valid reading.
Training
According to the document A Guide to Performing Quality Assured Diagnostic Spirometry, spirometry should be performed only by people with appropriate training (training standards defined in the document). 448 Training should cover both performance of the test and interpretation of the findings. Poorly performed spirometry produces misleading results. Training for operators, with regular updates and quality audits, is fundamental. Spirometry manufacturers can provide training in the use of their equipment, and some run spirometry courses. Most COPD training courses include training in spirometry.
The National Institute for Health and Care Excellence recommends that health professionals involved in caring for COPD patients should have access to spirometry and be able to interpret the results. 450
Spirometry can be performed by any health-care worker who has undergone appropriate training and who keeps his or her skills up to date. The GP Quality and Outcomes Framework451 requires diagnosis in primary care to be confirmed post bronchodilator. The 2010 NICE guidance similarly recommends spirometry be conducted post bronchodilator. 452
The evidence base in general encourages use of training programmes. 81 However, a RCT in Australia, where 26 intervention practices received comprehensive spirometry training and 14 control practices provided usual care, concluded that training in spirometry did not result in any measurable improvement in the use of spirometry, quality of management of asthma or patient outcomes in primary care. 189
A longitudinal study in Denmark demonstrated that the improved education of staff enhanced the use of spirometry in hospital outpatients with COPD, indicating the importance of staff training. 190 Further education has also been shown to increase GPs’ use of spirometers191 and also improve their capacity to diagnose clear-cut pathologies. 269 New methods for teaching spirometry should be evaluated. E-learning holds several advantages in this context and is especially beneficial in helping trainees with data interpretation. 453
Advances in technology and widespread internet and mobile phone use open up the possibility of automated data transfer from community- or home-based spirometry to primary care teams (or respiratory support team). 443 The use of remote and home monitoring is currently under investigation. The need for accreditation is under review.
STEP-UP summary statement With regard to TRAINING, spirometry is attributed a LOW degree of implementation difficulty owing to the success of short training courses for skills acquisition.
Equipment
Spirometry measures air flow and volume using varying types of equipment, all of which should conform to international standards. The American Thoracic Society and European Respiratory Society have jointly published comprehensive guidelines on the minimum performance specifications for spirometers. 454 These standards have been interpreted in a UK context by the General Practice Airways Group. 454 Many handheld or desktop devices have evidence for good accuracy compared with respiratory laboratory standards. 193,455 However, it is considered essential to follow up reduced handheld/screening spirometry with full quality assured diagnostic spirometry to confirm or refute the findings.
The document A Guide to Performing Quality Assured Diagnostic Spirometry448 specifies the following equipment requirements and standards:
-
a spirometer which meets the ISO standard 267823
-
one-way mouthpieces and nose clips
-
bacterial and viral filters (as indicated in selected patients)
-
height measure and weighing scales – calibrated according to manufacturer’s instructions
-
nebuliser or single-patient-use volumatics (for post-bronchodilator spirometry and reversibility testing)
-
single-patient use mask/mouthpiece for nebuliser
-
short-acting bronchodilators as per guidelines.
The document also specifies requirements for quality assurance. A user-friendly guide to buying a spirometer has been produced. 456 The National Asthma Council Australia provides a useful list of factors to consider when purchasing a spirometer in its Spirometer Users and Buyers Guide. 192
Handheld and desktop spirometers detect flow volume and rates using a variety of flow sensors. Several devices are available on the market, and one study reviewed the technical properties of 10 different spirometers designed for use in general practice. 193 The devices were tested in laboratory and primary care settings, and user-friendliness was assessed. For an overview of devices on the market and their reported accuracy, see the detailed spirometry report at the Department of Primary Health Care MaDOx. 457
Some devices can transfer data by telephone (landline or mobile phone). Many have storage capacity and allow for use in the field (for surveys or patient use), so data can be downloaded at a later date for analysis.
Disposables include mouthpieces and other consumables. Spirometers should be regularly cleaned and sterilised, as they may become reservoirs of micro-organisms. In a small study of 16 spirometers in South Australia, microbiological contamination was present in three. 194 The three practices concerned all reported having a written spirometer-cleaning protocol in place. The frequency of spirometer disinfection did not match the manufacturers’ recommendations, highlighting a need for stricter hygiene measures for spirometer maintenance in general practices.
A Dutch study examined 50 desktop spirometers in general practices and found that, on average, they slightly overestimated FEV and FVC values, with some devices showing substantial deviations. 458 Spirometers, therefore, need to be calibrated yearly and verified before each session. A preventative spirometer maintenance and quality assurance regimen will include:
-
regular cleaning
-
calibration checks
-
equipment maintenance to ensure that the spirometer is operating correctly
-
regular review to ensure ongoing test quality.
Regular validation of the calibration is ideally performed with an accurate syringe before each testing session. The overall performance of the spirometer can be checked by regularly testing a healthy subject. Records should be kept of each calibration and the test results from healthy subjects. Ideally, a spirometer should have a graphical display to allow technical errors to be detected. It should be able to produce a hard copy. Regular calibration is essential. Some spirometers need to be calibrated before each session using a calibration syringe; others hold their calibration between annual services.
Three types of spirometer are commonly used in primary care:
-
Small, handheld meters which provide digital readings are the cheapest option and small enough to fit into a medical bag. The lack of graphs can make it difficult to judge when the procedure is complete. Predicted charts and a calculator are needed to interpret the results.
-
Portable meters with integral printers are more expensive but they undertake all of the calculations, including reversibility. Small displays of the volume–time graph help to monitor the blow and the print-out includes a flow–volume loop.
-
Systems that work with a computer to display a graph and provide a print-out. Integral memories allow data to be recorded outside the practice and uploaded when convenient. 459
The Spanish agency CatSalut has produced very helpful technical requirements for spirometers and calibration syringes. 460
STEP-UP summary statement With regard to EQUIPMENT, spirometry is attributed a LOW degree of implementation difficulty owing to improvements in ease of use, cost and portability.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates spirometry testing services in an ‘examination/therapy room’ (contains public sector information licensed under the Open Government Licence v3.0). In an economic analysis on the economics of initiating office spirometry in a six-man internal medicine group in Kirkville, MO,461 the spirometry laboratory occupied < 147 square feet. Initial testing could occur in community locations or surgery waiting rooms, but diagnosis should be confirmed in line with recommendations from the NICE and the British Thoracic Society. 443
STEP-UP summary statement With regard to PREMISES, spirometry is attributed a LOW degree of implementation difficulty owing to the facility to apply portable spirometry in a variety of primary care settings.
User perspective
Two qualitative studies have looked at attitudes of doctors to the use of spirometry. Accessibility of equipment does not figure prominently as an identified barrier. This corroborates quantitative studies that show that, even when equipment is available, as few as one-third of patients receive a spirometry investigation. 462
Evidence suggests that spirometry is underutilised and that guidelines that recommend spirometry to confirm airflow obstruction among patients with suspected COPD are not routinely followed. Not all potential users accept the value of spirometry as a tool that will impact on practice or patient welfare. 463 Some doctors fail to use spirometry as they believe that little or nothing can be done to help patients who continue to smoke. 196 Reported barriers to the use of spirometry include poorly designed and unduly complex spirometers with too many confusing parameters of limited value, lack of availability of spirometers, poor or no teaching in medical schools and the perceived lack of an evidence base demonstrating the value and cost-effectiveness of spirometry. 464
In a UK context only, specialist registrars and GPwSIs in undergraduate education spontaneously cited spirometry as a diagnostic tool. 197 When asked explicitly about spirometry, other grades cited unfamiliarity and inability to interpret the results as key factors inhibiting their use of spirometry. Junior doctors (Foundation Year 2) specifically noted lack of encouragement, reinforcement or even basic information about obtaining spirometric equipment from senior colleagues, whereas respiratory specialist registrars and GPs viewed spirometry as essential. 197
General practitioners stated that they now felt that they had more access to spirometry than in the past and they were highly aware of the value of spirometry, perhaps reflecting its inclusion as a quality marker in the NHS General Practitioners’ contract. GPs in the study commented on the need for retraining, confirmed by a further study which showed that only 33% of general practices were confident at interpreting spirometry and 58% were confident at using spirometers. 304
The UK study may be limited by including only GPs who were uniformly enthusiastic about spirometry, given their special interest in teaching undergraduates and the fact that they had been recruited via an educational update session. 197 A focus group study in Chicago, IL, found that, in general, primary care physicians believed that spirometry was not necessary to confirm the diagnosis of COPD. 198 Health system barriers specific to spirometry use were not identified, suggesting that the availability of spirometry was not a perceived barrier. The findings suggest that the use of spirometry among primary care physicians may first require the establishment of their belief that spirometry is actually useful in diagnosing COPD. A study of 25 GP practices in the USA found that 75% failed to use spirometry in their diagnosis of COPD. 465 The reasons cited for non-use included lack of time and staffing. 465
The availability of equipment seems to be a less important factor, with spirometers often available but not used. Almost 75% of Australian GPs reported having a spirometer in their practice, but only 12% had used it to review patients with asthma within the year prior to the study. 466 Similar underuse has been observed in primary care in Sweden and in Spain. A 2008 study from the USA produced more optimistic conclusion, with the authors reporting that 74% of primary care physicians responding to a questionnaire said that they used spirometry in the diagnosis of COPD, although the actual frequency of use was not measured.
Spirometry is a reliable, simple, non-invasive, safe and non-expensive procedure. 467 However, there are some challenges with its acceptability. A false positive could lead to unnecessary diagnostic testing and stress. A false negative can lead to a false sense of being healthy; a smoker may not give up smoking, for example, leading to further problems later in life. Spirometry has been suggested as a motivational tool to help smokers to quit smoking. However, the evidence does not offer a clear conclusion on whether or not spirometry increases motivation and success in quitting smoking. 468 The impact of providing a smoker with a ‘healthy’ reading is not clear.
STEP-UP summary statement With regard to USER PERSPECTIVES, spirometry is attributed a LOW implementation difficulty for clinicians and a MODERATE difficulty for patients. The equipment is becoming easier to use but may require multiple attempts for a patient to make their technique acceptable.
Primary–secondary interface
The increased availability of spirometers in primary care offers the potential for wider use. Newer spirometers are user-friendly and have the capacity for self-monitoring. With the advent of telemedicine and internet transmission of data, many more patients could have access to a diagnostic screening and/or monitoring.
Konstantikaki et al. 199 compared an open spirometry programme with a case-finding programme providing spirometry to high-risk subjects selected by primary care physicians. A network of primary care physicians was created after invitation and all participants received training on COPD and spirometry. The study team visited 12 primary care settings over a 1-year period. Spirometry was performed in all eligible participants. The proportion of newly diagnosed COPD was 27.9% in the case-finding programme, compared with 8.4% in the open spirometry programme (p < 0.0001). The average cost for a new diagnosis of COPD was €173 in the open spirometry programme and €102 in the case-finding programme. Thus, a case-finding programme involving primary care physicians was more cost-effective for identification of new cases of COPD than an open spirometry programme.
A Spanish study has examined the economic impact of telespirometry, compared with standard spirometry. 469 Although telespirometry costs more per unit than standard spirometry (€47.80 vs. €39.70) (2013), the quality of the telespirometry procedure is superior (84% good quality vs. 61%). The cost-effectiveness analysis concludes that telespirometry is 23% more expensive and 46% more effective. Health-care costs consequently fall as the number of lung function tests performed by telespirometry rises.
A US office spirometry study conducted 1179 spirometry tests and clinic revenue showed a $40,000 surplus over operating costs over 1 year. 470 The service often resulted in the new diagnosis of obstructive lung disease that had not been previously made. This became the foundation for initiating care for these patients. Having on-site service in this small community saved patients the time and cost of travelling 100 miles to have spirometry carried out in a specialist’s office. 470
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, spirometry is attributed a LOW degree of implementation difficulty owing to the existing referral pathways for asthma and COPD, etc.
Conclusion
Spirometry equipment has frequently figured in GP practices, alongside other requisite equipment such as ECGs. It is similarly benefiting from the move to miniaturisation as well as from demand for end-user friendly devices. However, the availability of the technology should not disguise the fact that interpreting spirometry readings, and indeed deciding to use the equipment in the first place where it is readily available, remains a significant barrier to effective utilisation of the technology.
Spirometry (Sp 1–111)
Sp1. Abramson MJ, Schattner RL, Sulaiman ND, Del Colle EA, Aroni R, Thien F. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J 2012;21:167–73.
Sp2. Akhtar R, Wilson A. A comparison of spirometry in general practice and a pulmonary function laboratory. Prim Care Respir J 2005;14:215–20.
Sp3. Anderson S. Office spirometry: don't just blow it off. Med Econ 2004;81:63–5.
Sp4. ARTP. A Guide To Performing Quality Assured Diagnostic Spirometry 23 April 2013. URL: www.artp.org.uk/en/professional/artp-standards/index.cfm/QADS%20Apr%202013 (accessed 28 August 2015).
Sp5. Barr RG, Celli BR, Martinez FJ, Ries AL, Rennard SI, Reilly JJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med 2005;118:1415.
Sp6. Barr RG, Stemple KJ, Mesia-Vela S, Basner RC, Derk SJ, Henneberger PK, et al. Reproducibility and validity of a handheld spirometer. Respir Care 2008;53:433–41.
Sp7. Barton C, Proudfoot J, Amoroso C, Ramsay E, Holton C, Bubner T, et al. Management of asthma in Australian general practice: care is still not in line with clinical practice guidelines. Prim Care Respir J 2009;18:100–5.
Sp8. Blair KA, Evelo AJ. COPD: overview and survey of NP knowledge. Nurse Pract 2013;38:18-26.
Sp9. Bolton CE, Ionescu AA, Edwards PH, Faulkner TA, Edwards SM, Shale DJ. Attaining a correct diagnosis of COPD in general practice. Respir Med 2005;99:493–500.
Sp10. Booker R. Spirometry in primary care: in need of development. Prof Nurse 2004;19:6–7.
Sp11. Booker R. Best practice in the use of spirometry. Nurs Stand 2005;19:49–54.
Sp12. Borg BM, Hartley MF, Fisher MT, Thompson BR. Spirometry training does not guarantee valid results. Respir Care 2010;55:689–94.
Sp13. Buffels J, Degryse J, Liistro G. Diagnostic certainty, co-morbidity and medication in a primary care population with presumed airway obstruction: the DIDASCO2 study. Prim Care Respir J 2009;18:34–40.
Sp14. Buffels J, Degryse J, Heyrman J, Decramer M. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO Study. Chest 2004;125:1394–9.
Sp15. Bunker J, Hermiz O, Zwar N, Dennis SM, Vagholkar S, Crockett A, et al. Feasibility and efficacy of COPD case finding by practice nurses. Aust Fam Physician 2009;38:826–30.
Sp16. Lei Burton D, LeMay KS, Saini B, Smith L, Bosnic-Anticevich S, Southwell P, et al. The reliability and utility of spirometry performed on people with asthma in community pharmacies. J Asthma 2015;52:913–9.
Sp17. Cartwright S. An Evaluation of Screening for COPD against the National Screening Committee Criteria, April 2012. URL: http://legacy.screening.nhs.uk/policydb_download.php?doc=360 (accessed 28 August 2015).
Sp18. Castillo D, Guayta R, Giner J, Burgos F, Capdevila C, Soriano JB, et al. COPD case finding by spirometry in high-risk customers of urban community pharmacies: a pilot study. Respir Med 2009;103:839–45.
Sp19. Chavannes N, Schermer T, Akkermans R, Jacobs JE, van de Graaf G, Bollen R, et al. Impact of spirometry on GPs’ diagnostic differentiation and decision-making. Respir Med 2004;98:1124–30.
Sp20. Coates AL, Graham BL, McFadden RG, McParland C, Moosa D, Provencher S, et al. Spirometry in primary care. Can Respir J 2013;20(1):13–21. [Erratum published in Can Respir J 2013;20:122.]
Sp21. Cranston JM, Crockett AJ, Moss JR, Pegram RW, Stocks NP. Models of chronic disease management in primary care for patients with mild-to-moderate asthma or COPD: a narrative review. Med J Aust 2008;188(Suppl. 8):50–2.
Sp22. Department of Health and RCGP. Guidelines for the Appointment of GPSI With the Role of Service Development. London: Department of Health and RCGP; 2002.
Sp23. Department of Health. An Outcomes Strategy for People with Asthma and COPD. 2011. URL: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_127974 (accessed 28 August 2015).
Sp24. Derom E, van Weel C, Liistro G, Buffels J, Schermer T, Lammers E, et al. Primary care spirometry. Eur Respir J 2008;31:197–203.
Sp25. Primary Care Respiratory Society UK. Diagnosis and Management of COPD in Primary Care: A Guide for those Working in Primary Care. Curdworth: Primary Care Respiratory Society UK; 2007.
Sp26. Swanney MP, Eckert B, Johns DP, Burton D, Crockett AJ, Guy P, et al. Spirometry Training Courses: A Position Paper of the Australian and New Zealand Society of Respiratory Science and the Thoracic Society of Australia and New Zealand. URL: https://assets.nationalasthma.org.au/resources/205-213-spirotrainingposition.pdf (accessed 28 August 2015).
Sp27. Enright P, Quanjer P. Spirometry for COPD is both underutilized and overutilized. Chest 2007;132:368–70.
Sp28. Enright P. Does screening for COPD by primary care physicians have the potential to cause more harm than good? Chest 2006;129:833–5.
Sp29. Enright P. The use and abuse of office spirometry. Prim Care Respir J 2008;17:238–42.
Sp30. Fernández Villar A, Represas Represas C. Training of primary care health professionals and quality in spirometry. Arch Bronconeumol 2014;50:259.
Sp31. Pinnock H, Netuveli G, Price D, Sheikh A. General practitioners with a special interest in respiratory medicine: national survey of UK primary care organisations. BMC Health Serv Res 2005;5:40.
Sp32. Gibbons D. A comprehensive guide to buying a spirometer. Prof Nurse 2005;20:45–8.
Sp33. Gruffydd-Jones K, Pinnock H, Loveridge C, Wolfe S, McArthur R, Bellamy D. Conflicting standards for diagnostic spirometry within-session repeatability are confusing. Prim Care Respir J 2012;21:136.
Sp34. Gruffydd-Jones K, Small I, Fletcher M, Bryant T. The Primary Care Respiratory Society UK Quality Award: development and piloting of quality standards for primary care respiratory medicine. Prim Care Respir J 2013;22:353–9.
Sp35. Gruffydd-Jones K. The framework for general practitioners with a special interest in respiratory medicine. Primary Care Resp J 2003;12:35:939.
Sp36. Gunstone C. Pulse oximetry in primary care. Br J Gen Pract 2011;61:497.
Sp37. Hancock KL, Schermer TR, Holton C, Crockett AJ. Microbiological contamination of spirometers – an exploratory study in general practice. Aust Fam Physician 2012;41:63–4.
Sp38. Haroon SM, Adab PA, Jordan RE. Case finding for COPD in primary care: a systematic review. Prim Care Respir J 2012;21:354–7.
Sp39. Harris SKA. The Experiences and Monitoring of People Living with Chronic Obstructive Pulmonary Disease and Utilising Long-Term Oxygen Therapy. PhD Thesis. Dunedin: University of Otago; 2012.
Sp40. Hassett R, Meade K, Partridge MR. Enhancing the accuracy of respiratory diagnoses in primary care: a report on the establishment of a Community Respiratory Assessment Unit. Prim Care Respir J 2006;15:354–61.
Sp41. Hill K, Goldstein RS, Guyatt GH, Blouin M, Tan WC, Davis LL, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ 2010;182:673–8.
Sp42. Holt K. Improving early detection of COPD: the role of spirometry screening assessment. Prof Nurse 2004;20:31–3.
Sp43. Holton C, Crockett A, Nelson M, Ryan P, Wood-Baker R, Stocks N, et al. Does spirometry training in general practice improve quality and outcomes of asthma care? Int J Qual Health Care 2011;23:545–53.
Sp44. Burton D, Johns DP, Swanney MP. Spirometer Users’ and Buyers’ Guide. Melbourne, VIC: National Asthma Council Australia; 2011. URL: www.artp.org.uk/download.cfm/docid/69500FB9-3FA1-4D9D-B8C77C902C7CE082 (accessed 28 August 2015).
Sp45. Ingram G, Munro N. The use (or otherwise) of pulse in general practice. Br J Gen Pract 2005;55:501–2.
Sp46. Jenkins CR. Spirometry performance in primary care: the problem, and possible solutions. Prim Care Respir J 2009;18:128–9.
Sp47. Johns DP, Burton D, Walters JA, Wood-Baker R. National survey of spirometer ownership and usage in general practice in Australia. Respirology 2006;11:292–8.
Sp48. Jones R, Whittaker M, Hanney K, Shackell B. A pilot study of a mobile spirometry service in primary care. Prim Care Respir J 2005;14:169–71.
Sp49. Jones RC, Price D, Ryan D, Sims EJ, von Ziegenweidt J, Mascarenhas L, et al. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med 2014;2:267–76.
Sp50. Joo MJ, Sharp LK, Au DH, Lee TA, Fitzgibbon ML. Use of spirometry in the diagnosis of COPD: a qualitative study in primary care. COPD 2013;10:444–9.
Sp51. José BP, Camargos PA, Cruz Filho ÁA, Corrêa Rde A. Diagnostic accuracy of respiratory diseases in primary health units. Rev Assoc Med Bras 2014;60:599–612.
Sp52. Kaminsky DA, Marcy TW, Bachand M, Irvin CG. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care 2005;50:1639–48.
Sp53. Kelly S. A partnership approach to raising community spirometry standards. Primary Health Care 2011;21.
Sp54. Kernick DP. The economic perspective of respiratory general practitioners with a special interest (GPwSIs): proceed with caution. Primary Care Resp J 2003;12:108–9.
Sp55. Koefoed MM. Spirometry Utilization among Danish Adults Initiating Medication Targeting Obstructive Lung Disease. Doctoral dissertation. Syddansk Universitet; 2013.
Sp56. Koefoed MM, Søndergaard J, Christensen RP, Jarbøl DE. General practice variation in spirometry testing among patients receiving first-time prescriptions for medication targeting obstructive lung disease in Denmark: a population-based observational study. BMC Fam Pract 2013;14:113.
Sp57. Konstantikaki V, Kostikas K, Minas M, Batavanis G, Daniil Z, Gourgoulianis KI, et al. Comparison of a network of primary care physicians and an open spirometry programme for COPD diagnosis. Respir Med 2011;105:274–81.
Sp58. Primary care at the heart of respiratory medicine in the UK. Lancet Respir Med 2014;2:83.
Sp59. Lange P, Andersen KK, Munch E, Sørensen TB, Dollerup J, Kassø K, et al. Quality of COPD care in hospital outpatient clinics in Denmark: the KOLIBRI study. Respir Med 2009;103:1657–62.
Sp60. Leuppi JD, Miedinger D, Chhajed PN, Buess C, Schafroth S, Bucher HC, et al. Quality of spirometry in primary care for case finding of airway obstruction in smokers. Respiration 2010;79:469–74.
Sp61. Levy ML, Quanjer PH, Booker R, Holmes S, Small I. Standards for diagnostic spirometry within-session repeatability in primary care. Prim Care Respir J 2012;21:252–3.
Sp62. Levy ML, Quanjer PH, Booker R, Cooper BG, Holmes S, Small I. General Practice Airways Group. Diagnostic spirometry in primary care: proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations-a General Practice Airways Group (GPIAG) document, in association with the Association for Respiratory Technology & Physiology (ARTP) and Education for Health. Prim Care Respir J 2009;18:130–47.
Sp63. Licskai CJ, Sands TW, Paolatto L, Nicoletti I, Ferrone M. Spirometry in primary care: an analysis of spirometery test quality in a regional primary care asthma program. Can Respir J 2012;19:249–54.
Sp64. Liistro G, Vanwelde C, Vincken W, Vandevoorde J, Verleden G, Buffels J. Technical and functional assessment of 10 office spirometers: a multicenter comparative study. Chest 2006;130:657–65.
Sp65. Lin K, Watkins B, Johnson T, Rodriguez JA, Barton MB. Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2008;148:535–43.
Sp66. Lusuardi M, De Benedetto F, Paggiaro P, Sanguinetti CM, Brazzola G, Ferri P, et al. A randomized controlled trial on office spirometry in asthma and COPD in standard general practice: data from spirometry in asthma and COPD: a comparative evaluation Italian study. Chest 2006;129:844–52.
Sp67. MacIntyre NR. The American Association for Respiratory Care and the National Lung Health Education Program: assuring quality in spirometry. Respir Care 2004;49:587–8.
Sp68. Marina N, Bayón JC, López de Santa María E, Gutiérrez A, Inchausti M, Bustamante V, et al. Economic assessment and budgetary impact of a telemedicine procedure and spirometry quality control in the primary care setting. Arch Bronconeumol 2016;52:24–8.
Sp69. Masa JF, González MT, Pereira R, Mota M, Riesco JA, Corral J, et al. Validity of spirometry performed online. Eur Respir J 2011;37:911–18.
Sp70. Michael Roberts C, Abedi MK, Barry JS, Williams E, Quantrill SJ. Predictive value of primary care made clinical diagnosis of chronic obstructive pulmonary disease (COPD) with secondary care specialist diagnosis based on spirometry performed in a lung function laboratory. Primary Health Care Res Dev 2009;10:49-53.
Sp71. Miller MR. Spirometry in primary care. Prim Care Respir J 2009;18:239–40.
Sp72. Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J 2005;26:153–61.
Sp73. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38.
Sp74. Moore PL. Practice management and chronic obstructive pulmonary disease in primary care. Am J Med 2007;120(Suppl. 8):23–7.
Sp75. Murray S, Cytryn KN, Barrett TJ, Meinzer RL. Outcomes evaluation of a skill-based workshop targeting the use of spirometry in chronic obstructive pulmonary disease. CE Measure 2010;4:50–7.
Sp76. National Clinical Guideline Centre. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. URL: www.nice.org.uk/nicemedia/live/13029/49425/49425.pdf (accessed 28 August 2015).
Sp77. National Institute for Health and Care Excellence. Chronic Obstructive Pulmonary Disease (COPD) Quality Standard (QS10). URL: www.nice.org.uk/guidance/qualitystandards/chronicobstructivepulmonarydisease/copdqualitystandard.jsp (accessed 28 August 2015).
Sp78. International Primary Care Respiratory Group, General Practice Airways Group. Spirometry. URL: www.canahome.org/files/spirometry.pdf (accessed 28 August 2015).
Sp79. Parkes G, Plüddemann A, Heneghan C, Price CP, Wolstenholme J, Thompson M. Spirometry in primary care for case finding and management of chronic obstructive pulmonary disease primary care diagnostic technology update. Br J Gen Pract 2011;61:698–9.
Sp80. Partridge MR. Enhancing the diagnosis and management of COPD in primary care. Multidiscip Respir Med 2014;9:62.
Sp81. Pérez-Padilla R, Vázquez-García JC, Márquez MN, Menezes AMB. Spirometry quality-control strategies in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care 2008;53:1019–26.
Sp82. Petty TL, Doherty DE. The National Lung Health Education Program: roots, mission, future directions. Respir Care 2004;49:678–83.
Sp83. Petty TL. Benefits of and barriers to the widespread use of spirometry. Curr Opin Pulm Med 2005;11:115–20.
Sp84. Richardson C. Benefit or burden? The rise of GPs with a special interest. Primary Care Report 2002;4:38–9.
Sp85. Roberts NJ, Smith SF, Partridge MR. Why is spirometry underused in the diagnosis of the breathless patient: a qualitative study. BMC Pulm Med 2011;11:37.
Sp86. Salas T, Rubies C, Gallego C, Muñoz P, Burgos F, Escarrabill J. Technical requirements of spirometers in the strategy for guaranteeing the access to quality spirometry. Arch Bronconeumol 2011;47:466–9.
Sp87. Schermer TR, Crockett AJ, Poels PJ, van Dijke JJ, Akkermans RP, Vlek HF, et al. Quality of routine spirometry tests in Dutch general practices. Br J Gen Pract 2009;59:e376–82.
Sp88. Schermer TR, Verweij EH, Cretier R, Pellegrino A, Crockett AJ, Poels PJ. Accuracy and precision of desktop spirometers in general practices. Respiration 2012;83:344–52.
Sp89. Schermer TR, Akkermans RP, Crockett AJ, van Montfort M, Grootens-Stekelenburg J, Stout JW, et al. Effect of e-learning and repeated performance feedback on spirometry test quality in family practice: a cluster trial. Ann Fam Med 2011;9:330–6.
Sp90. Schermer TR, Jacobs JE, Chavannes NH, Hartman J, Folgering HT, Bottema BJ, et al. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease (COPD). Thorax 2003;58:861–6.
Sp91. Schermer T, Eaton T, Pauwels R, van Weel C. Spirometry in primary care: is it good enough to face demands like World COPD Day? Eur Respir J 2003;22:725–7.
Sp92. Smith SF, Roberts NJ, Partridge MR. Comparison of a web-based package with tutor-based methods of teaching respiratory medicine: subjective and objective evaluations. BMC Med Educ 2007;7:41.
Sp93. Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009;374:721–32.
Sp94. Parkes G, Plüddemann A, Heneghan C, Price CP, Wolstenholme J, Thompson M. Spirometry in primary care for case finding and management of chronic obstructive pulmonary disease primary care diagnostic technology update. Br J Gen Pract 2011;61:698–99.
Sp95. Starren ES, Roberts NJ, Tahir M, O'Byrne L, Haffenden R, Patel IS, et al. A centralised respiratory diagnostic service for primary care: a 4-year audit. Prim Care Respir J 2012;21:180–6.
Sp96. Steenbruggen I, Mitchell S, Severin T, Palange P, Cooper BG. Harmonising spirometry education with HERMES: training a new generation of qualified spirometry practitioners across Europe. Eur Respir J 2011;37:479–81.
Sp97. Strong M, Green A, Goyder E, Miles G, Lee AC, Basran G, et al. Accuracy of diagnosis and classification of COPD in primary and specialist nurse-led respiratory care in Rotherham, UK: a cross-sectional study. Prim Care Respir J 2014;23:67–73.
Sp98. The NHS Information Centre. Indicators for Quality Improvement: Full Indicator List. The NHS Information Centre; 2012.
Sp99. Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma 2006;43:75–80.
Sp100. Van Schayck C, Loozen JMC, Wagena E, Akkermans RP, Wesseling GJ. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ 2002;324:1370.
Sp101. Vaughan RCR, MacIntyre D. An outreach spirometry service for Greater Glasgow Health Board: does it help in diagnosis? Eur Respir J 2006;28:945–52.
Sp102. Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J 2006;28:945–52.
Sp103. Wallace LD, Troy KE. Office-based spirometry for early detection of obstructive lung disease. J Am Acad Nurse Pract 2006;18:414–21.
Sp104. Walters JA, Hansen EC, Johns DP, Blizzard EL, Walters EH, Wood-Baker R. A mixed methods study to compare models of spirometry delivery in primary care for patients at risk of COPD. Thorax 2008;63:408–14.
Sp105. White P, Wong W, Fleming T, Gray B. Primary care spirometry: test quality and the feasibility and usefulness of specialist reporting. Br J Gen Pract 2007;57:701–5.
Sp106. Wilt TJ, Niewoehner D, Kane RL, MacDonald R, Joseph AM. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine Tob Res 2007;9:21–32.
Sp107. Wilt TJ, Niewoehner D, Kim C-B, Kane RL, Linabery A, Tacklind J, et al. Use of Spirometry for Case Finding, Diagnosis, and Management of Chronic Obstructive Pulmonary Disease (COPD). Evidence Report/Technology Assessment No. 121 (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-02-0009.) AHRQ Publication No. 05-E017-2. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
Sp108. Wolfenden H, Bailey L, Murphy K, Partridge MR. Use of an open access spirometry service by general practitioners. Prim Care Respir J 2006;15:252–5.
Sp109. Yawn BP, Yawn RA. Spirometry testing education in medical schools: a missed opportunity? Prim Care Respir J 2005;14:21–4.
Sp110. Strong M, South G, Carlisle R. The UK Quality and Outcomes Framework pay-for-performance scheme and spirometry: rewarding quality or just quantity? A cross-sectional study in Rotherham, UK. BMC Health Serv Res 2009;9:108.
Sp111. Strong M, Green A, Goyder E, Miles G, Lee AC, Basran G, et al. Accuracy of diagnosis and classification of COPD in primary and specialist nurse-led respiratory care in Rotherham, UK: a cross-sectional study. Prim Care Respir J 2014;23:67–73.
Ultrasound
Definition: an ultrasound scan, sometimes called a sonogram, is a procedure that uses high-frequency sound waves to create an image of part of the inside of the body (NHS Choices).
This map includes portable and fixed ultrasound.
This map excludes echocardiography (see Echocardiography) and neonatal screening for dysplasia of the hip.
Contains public sector information licensed under the Open Government Licence v3.0. 200
Ultrasound provides instant images and can be used at the bedside or in an office for rapid diagnosis, whereas other imaging methods take time to process. Ultrasound images can be saved or printed for reference, but, unlike MRI scans and CT scans, performance and interpretation are much more dependent on the skill and experience of the operator. Ultrasound examinations are undertaken by practitioners from a wide range of professional backgrounds and in many different clinical settings.
General practitioner ultrasound scanning appears to offer an alternative to traditional hospital-based services. Over recent years the technology has become more portable and less prohibitive in cost. As early as 2002, the question ‘Ultrasound scanning by general practitioners: is it worthwhile?’203 was being asked. At approximately the same time the Norwegian Knowledge Centre for the Health Services471 assessed the available evidence on the use of ultrasonography in the primary health care setting. They concluded that, notwithstanding the increasing availability of high-quality, cheap and compact equipment, there was very limited evidence for the diagnostic accuracy and clinical benefit of using diagnostic ultrasound in primary care. This contrasts with an abundance of literature regarding its use in acute hospitals. This imbalance makes the ‘transfer value’ for ultrasound technology uncertain, particularly given that the primary care population is one of low disease prevalence. Knock-on effects for secondary care were also incapable of being anticipated at this time. Training and education for ultrasound has implications for the continuum of medical education: basic medical education – further education in general medical practice – certification procedures (accreditation) and documented CPD (recertification). Furthermore, the cost implications for primary health-care services were unknown. The review concluded that there is a need for clinical studies based on general practice to address the diagnostic value and clinical effect (including the cost-effectiveness) of ultrasonography use. Fundamentally, against a backdrop of decreasing costs, greater portability and more common usage, this situation remains unchanged.
STEP-UP summary
Skills
Ultrasound is a highly operator-dependent imaging modality and requires skills that take time to acquire. Imaging must be undertaken by trained and experienced practitioners and, even then, perfect images may not be obtained in every patient. Regardless of who undertakes them or where they are undertaken, ultrasound examinations must be of a high quality as they have a direct effect on patient management. Skill mix in ultrasound in primary care has grown organically, with boundaries often ill-defined.
Faster diagnosis has to be offset against ‘risks to the patient from false positive diagnoses that generate additional procedures or tests, and false negatives that miss potentially serious pathology’. 201 Medical ultrasonography is ‘fraught with scope for diagnostic error’. 201 Ultrasound images can be saved or printed for reference, but, unlike MRI scans and CT scans, performance and interpretation are much more dependent on the skill and experience of the operator. Ultrasound has a reputation as a simple, easy test. It is easy to perform but interpreting the results is not so easy and features can be overlooked. 472 Ultrasound takes clear images of muscle, soft tissue, fluid and bone surfaces, but it is not good at penetrating bone or air. This limits its use in the skull, chest and abdomen. Ultrasound has no known long-term side effects, but it has a more limited scope than other imaging methods. There are limits to the depth of penetration that can be achieved, so images of structures deep inside the body can be difficult to capture, particularly in obese patients.
STEP-UP summary statement With regard to SKILLS, ultrasound is attributed a MODERATE degree of implementation difficulty owing to its being very operator dependent.
Training
Ultrasound, if carried out correctly in the appropriate clinical situation, is one of the most effective diagnostic tools in health care. As ultrasound machines become cheaper, more reliable and highly portable, GPs are increasingly using them to make on-the-spot diagnoses of many conditions without having to consult an imaging expert. 201 The fact that it is safe to carry out, relatively inexpensive and can be provided in most clinical facilities makes ultrasound one of the most commonly requested examinations in the field of diagnostic imaging. Ultrasound examinations are undertaken by practitioners from a wide range of professional backgrounds and in many different clinical settings. Guidance emphasises that whoever does the training must be trained, as ultrasound require both experience and expertise. There is no physical risk to the patient but there is a risk of false negatives or false positives. 472
The National Ultrasound Steering Group of the British Medical Ultrasound Society recommended in 2008 that local clinical governance boards be established to oversee the training, supervision and audit of all providers of ultrasound imaging services. 202 The RCR recommends that ultrasound training for medical non-radiologists should be to the same standard as for radiologists, albeit restricted to the relevant area of their clinical expertise. Providers should ensure that ultrasound users are adequately trained and that this is maintained through adequate audit and quality assurance systems.
Many factors affect the quality of ultrasound examinations, including appropriate training, experience, the equipment itself, clinical leadership, audit, general support and having sufficient time to undertake the examination and compile a clinically relevant report. Those undertaking ultrasound examinations, regardless of their professional background, must meet the standards of best clinical practice, substantiated by appropriate audit and good governance processes. Clear, effective clinical leadership is also essential if the ultrasound service provider is to achieve timely, accurate, clinically relevant reports. Documents relating to ultrasound service provision are available from organisations such as the RCR, the SCoR, the British Medical Ultrasound Society and the former United Kingdom Association of Sonographers (the United Kingdom Association of Sonographers merged with the SCoR on 1 January 2009). Ultrasound providers are encouraged to seek accreditation from ISAS. In England it is a legal requirement that providers of diagnostic and screening ultrasound services are registered with the Care Quality Commission.
Machines should not be used away from specified clinical areas and operators must be clearly instructed not to go beyond defined and agreed protocols. Focused ultrasound training standards have been published by the RCR. 256 Support from the manufacturer is an important consideration when purchasing a machine. Such support should include the availability of specialists for particular clinical applications, the availability of appropriate training courses and resources for repair and maintenance. Manufacturers should specify the minimum number of years over which they will supply spare parts and the necessary resources for repairs.
Rapid changes in technology make it important to undertake a formal review and replacement policy. High-specification ultrasound scanners will often have a longer useful life than basic or middle-range equipment. Depending on the outcomes of a review, a decision can then be made whether to continue to use the equipment or to obtain a replacement machine. By far the most important reason for replacement is a clinically significant change in performance as revealed by regular quality assurance or unfavourable image quality when compared with newer ultrasound machines, leading to an increase in inconclusive reports.
An employer or manager should record the statutory or voluntary registration status of all ultrasound practitioners. Ultrasound practitioners should be registered with the relevant statutory regulatory body, where appropriate, or with the relevant voluntary registration body. Ultrasound practitioners must hold recognised qualifications, including qualifications approved by the Consortium for the Accreditation of Sonographic Education, or equivalent, either from overseas or in the UK. Consortium for the Accreditation of Sonographic Education-accredited universities and professional bodies such as the RCR and Royal College of Obstetricians and Gynaecologists offer a range of different training methods.
Employment and registration
Ultrasound practitioners come from a wide range of professional backgrounds, including radiologists, radiographers, nurses, midwives, physicists, physiotherapists, obstetricians and clinical scientists. Ultrasound practitioners who are medically qualified will be registered with the GMC as a doctor with a licence to practise. Ultrasound practitioners not registered with the GMC will often be registered with a statutory regulatory body such as the Health and Care Professions Council or the Nursing and Midwifery Council. Examples include radiographers, physiotherapists and some clinical scientists. Ultrasound practitioners for whom statutory registration is not possible can apply for voluntary registration with the Public Voluntary Register of Sonographers which is administered by the College of Radiographers. Statutory registration for sonographers was recommended by the Health and Care Professions Council to the Secretary of State for Health in 2009 but new groups are not receiving statutory registration except where evidence demonstrates a level of risk to the public that warrants the costs of regulation.
Different bodies have different interpretations of what experienced means in practice. One study found that doctors need to be involved in more than 200 cases during training to develop an acceptable level of competence. The RCR’s guidelines on ultrasound training for non-radiologists give varying minimum number of examinations depending on the specialty. 256 Available courses are modelled on training for radiographers and the time commitment is impractical, such as block release courses, for full-time clinicians.
STEP-UP summary statement With regard to TRAINING, ultrasound is attributed a LOW degree of implementation difficulty owing to the widespread availability of training courses and opportunities.
Equipment
The absence of ionising radiation, the ability to deliver in the community closer to the patient’s home and the comparatively low cost of the equipment make ultrasound the first-choice examination for many clinical conditions. Ultrasound machines, once the size of washing machines and used solely by radiologists and sonographers in radiology departments, have become cheaper, smaller and more portable. Miniaturisation enables the latest models to be pocket sized. Ultrasound machines use variation in the way that high-pitched sound waves penetrate different tissues to generate images. Patients are not exposed to the risks of ionising radiation associated with CT or radiography. It is also now cheap, costing £56 for a scan of more than 20 minutes compared with £217 for a MRI scan (2013 NHS tariffs). The latest portable machines produce images that are almost the same quality as that of the larger machines; and they are easy to use, durable and cost as little as £5000. 201 Images obtained with a portable ultrasound machine are usually not stored, and a decision on this is made at the time by the clinician doing the scan.
Ultrasound machines are increasingly used by non-radiologists as part of the clinical examination or to assist in practical procedures such as the insertion of a central line. The number of GPs buying their own ultrasound machines has also gradually increased. The price of an ultrasound machine has reduced significantly to £5000–10,000 (2010), resulting in more widespread use. 472
Ultrasound scanners can be physically moved with ease, presenting a risk that machines may be inappropriately used for clinical tasks for which they were never intended and to which they may be ill suited. The role of the operator is critical and matching the operator knowledge and competence level to the equipment features is essential, to include the key machine characteristics, how these characteristics match to clinical need, features to be considered when purchasing a machine, and the associated environmental and organisational systems needed to support efficient use of the machine. In addition, service providers will need to ensure that the equipment continues to perform at the required level and have strategies for withdrawing it from service when this is no longer the case.
Ideally, ultrasound scanners should provide excellent images of diagnostic quality at all times. However, this is not implementable as an objective description of machine performance. However good the machine might be technically, some patients will present insurmountable challenges. The user must be clear about which applications are paramount for each scanner, devise metrics of quality and take and archive representative images encapsulating desired features. This enables the machine performance to be mapped over time. The user should specify, as precisely as possible, the investigation(s) for which each machine is optimised. Representative images indicating the performance of each machine should be archived on an annual basis and these should be monitored as part of the audit system in place in the department, and also with any bench-top testing that takes place. 204
The choice of scanner should be matched to the type and nature of the workload. However, occasionally functionality in primary care may be sacrificed with reduced size and weight. For the purposes of review and audit, all images obtained should be recorded, stored on a picture archiving and communication system and linked to the report. However, this may not always be part of the functionality of a portable device. Physicians in many medical specialties are thought to be using POC scanning, but the scale on which this is happening is not yet clear: these scans are not systematically recorded in the same way as scans performed by imaging experts. Essential and desirable functions are covered in the document Standards for the Provision of an Ultrasound Service. 204
In 2002 a cost analysis and an assessment of quality of GP scans, based on a clinical audit and a postal survey of patients’ preferences in the Grampian region of Scotland, reported that the unit cost of a scan was higher in the practice than at the hospital. 203 However, when all of the costs for a scanning episode were considered, the total and average costs were lower in the practice because of the avoidance of hospital visits. The results showed that the quality of GP scanning, subject to further training, was considered to be sufficient to continue scanning at the practice. Although the study provides some evidence to support GP scanning, further research on diagnostic accuracy and alternative models of care is still required.
The potential uses of ultrasonography in primary care include screening for abdominal aortic aneurysm,473 musculoskeletal diagnosis474 and echocardiography (already covered in Echocardiography). Further research is required to examine specific issues relating to risk and safety.
In August 2015, the British Journal of Cardiology reported the launch of an ‘ultrasound on demand’ scheme. 475 The monthly fee-based scheme offers a basic service with a further range of high-specification ultrasound packages on a pay-as-you-go basis. This allows ultrasound services to be tailored to both current and future needs as demand and patient workload change. ‘Ultrasound on demand’, launched by Philips, is a flexible scheme with 50 options and access to advanced facilities, such as three-dimensional transoesophageal echocardiography, which previously may have been limited by the restrictions of upfront investment costs. Staff can explore new imaging options and upgrade and alter systems without locking in capital expenditure budgets. Additional functionality is only charged when used. Coupled with this is the benefit of advanced data analytics, which provide in-depth information on utilisation of services. These data are often difficult to obtain from traditional IT systems and should facilitate strategic planning and rationalisation of service delivery. It remains to be seen how this scheme compares with traditional leasing systems.
STEP-UP summary statement With regard to EQUIPMENT, ultrasound is attributed a LOW degree of implementation difficulty owing to its portability, ease of use and the absence of radiation risk.
Premises
Health Building Note 11-01, Facilities for Primary and Community Care Services,46 locates ultrasound services in a generic treatment room. The environment in which a scanner is housed has a demonstrable effect on its performance. The size of the machine relative to the size of the room in which it is to be used is a key consideration. Other issues to be considered include the scanning couch and operator seating, the display monitor, the room heating and lighting, hygiene, infection and cleanliness and electrical and IT provision. 476 The couch, the seating, the transducer and the display should be ergonomically compatible. The size of the monitor will probably be a compromise between being able to accommodate it and not occasioning operator fatigue through an overly small screen.
Room lighting should be subdued but not render movement hazardous. Excess room temperatures must be avoided. The electrical supply should support the demands of the scanner, the couch and any accessories. IT links to picture archiving and communication systems are important and trailing leads are to be avoided. 476 A key consideration in harnessing the diagnostic technology effectively is provision of ‘safe, comfortable areas for “stacking” the patients so that the expensive equipment and staff can work to maximum efficiency’. 477
STEP-UP summary statement With regard to PREMISES, ultrasound is attributed a LOW degree of implementation difficulty owing to increasing developments in miniaturisation and portability.
User perspective
A patient preference study for 500 patients from a GPs’ list and 250 consecutive patients scanned at a Grampian general practice found that patients preferred to be scanned at the practice. They were prepared to wait up to an extra 5 days to enact their preference. 203 Patients were prepared to accept a reduction in the accuracy of scanning of up to 3.5% in being able to realise their choice. Patients were not concerned about which member of staff actually carried out the ultrasound scan.
Skills and willingness were not sufficient factors in themselves to prompt rural family practitioners to utilise ultrasound. 205 Economic considerations (i.e. equipment cost and remuneration) were seen as being more important. 205 Significantly, 78% had undergone training and used ultrasound in another setting; 94% reported that they would consider using ultrasonography in their medical practice office.
STEP-UP summary statement With regard to USER PERSPECTIVES, ultrasound is attributed a LOW degree of implementation difficulty owing to its being well accepted among clinicians and well tolerated among patients.
Primary–secondary interface
The number of ultrasound examinations performed by imaging experts has increased on average by 5.2% every year for the past 10 years, according to Department of Health data, and imaging experts (radiologists, radiographers and sonographers) are struggling to keep up with the growing demand. Their numbers are increasing, but not quickly enough, and some health-care providers are choosing not to invest in their expertise. The Centre for Workforce Intelligence projects a 45% increase in demand for ultrasonography by 2025 as a result of population ageing and the growing popularity of this kind of scanning. 201
With increasing pressure on ultrasound services as a result of the number of requests, changing patterns of service delivery and a shortfall in the qualified workforce, there have been concerns that the quality of some ultrasound examinations has been affected. Sometimes large groups of patients have had to be recalled for repeat ultrasound examinations. Reports and images of examinations performed by one provider are also not always available to others, with individual scans being repeated before treatment in secondary care is initiated. A 2002 audit of a small series of patients demonstrated that use of an ultrasound scanner at a Grampian general practice reduced the numbers of hospital scans, outpatient and inpatient visits, and emergency admissions. 203
The RCR is concerned about a national shortage of trained sonographers to deliver training. To train others, radiology departments will need additional staff, space and equipment. It is not sufficient to rely on the goodwill of the local radiologists. Ultimately, ultrasonography should be taught to everyone in medical school. However, there will be a skills gap in ultrasonography for some time to come.
Blackpool Fylde and Wyre Hospitals Foundation Trust extended primary care ultrasound as a NHS Improvement initiative. 206 Long waiting times for routine ultrasound examination, accompanied by patients arriving late for appointments owing to parking difficulty, and a limited choice for patients in terms of time and location of appointment required an innovative service response. The trust negotiated the use of a PCT facility, off the main district general hospital site, and acquired a portable ultrasound machine to provide community-based ultrasound. They also changed sonographer rotas to ensure that as much scanning time as possible was undertaken off the main site. Some sonographers required initial mentoring in total independent practice but there was no resistance to this change of working practice. Booking systems were centralised, offering all patients a choice of scanner site and more choice regarding the time of the scan. Improvements included 30–40% of routine outpatient work being performed off the main site (more local scanning with better parking), improved patient satisfaction and increased patient choice of scanning venue.
STEP-UP summary statement With regard to PRIMARY–SECONDARY INTERFACE, ultrasound is attributed a LOW degree of implementation difficulty owing to its being comparatively easily implemented in primary care and likely shifting demand from secondary care.
Conclusion
Ultrasound is one of the fastest growing areas of diagnostic technology application, excluding POC testing. There is substantive concern that this might not be translating into clinical benefit. Ultrasound, notwithstanding its portability and ease of use, carries requirements for quality assurance. These must be monitored and met if the technology is to achieve its potential clinical benefit. Furthermore, there is a need to examine the impact of ultrasound scans on the referral and secondary care pathways.
Ultrasound (U1–36)
U1. Alamri AF, Khan I, Baig MI, Iftikhar R. Trends in ultrasound examination in family practice. J Family Community Med 2014;21:107–11.
U2. Anon. ‘Ultrasound on demand’ − how will it work in the UK. Br J Cardiol 2015;22:89–90.
U3. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician 2012;58:e172–8.
U4. Brasted C. Ultrasound Waiting Lists: Rational Queue or Extended Capacity? Health Care Management Science Publisher; 2008.
U5. Caplan RH, Wester SM, Lambert PJ, Rooney BL. Efficient evaluation of thyroid nodules by primary care providers and thyroid specialists. Am J Manag Care 2000;6:1134–40.
U6. Carlisle D. Brain teaser: how to find a cure for diagnostics' ills. Health Serv J 2004;114:14–5.
U7. Day A, Oldroyd C, Godfrey S, Quinn T. Availability of cardiac equipment in general practice premises in a cardiac network: a survey. Br J Cardiol 2008;15:141–4.
U8. Department of Health. Direct Access to Diagnostic Tests for Cancer: Best Practice Referral Pathways for General Practitioners. London: Department of Health; 2012.
U9. Edwards H. Let’s all jump on the ultrasound bandwagon: further debate on the use of ultrasound. Ultrasound 2010;18:4–7.
U10. Grant L, Appleby J, Griffin N, Adam A, Gishen P. Facing the future: the effects of the impending financial drought on NHS finances and how UK radiology services can contribute to expected efficiency savings. Br J Radiol 2012;85:784–91.
U11. Hawtin KE, Hameed S, Ramachandran R, Harvey CJ, Lim AK, Gishen P, et al. Provision of a ‘same-day’ ultrasound service in an inner-city NHS trust: report on experience and lessons learned after the first 2 years. Clin Radiol 2010;65:40–6.
U12. Healthcare Commission. An Improving Picture?: Imaging Services in Acute and Specialist Trusts. London: Healthcare Commission; 2007.
U13. Heikkinen M, Räsänen H, Färkkilä M. Clinical value of ultrasound in the evaluation of dyspepsia in primary health care. Scand J Gastroenterol 2005;40:980–4.
U14. Radiology Case Studies. URL: http://webarchive.nationalarchives.gov.uk/20130221101407/http:/www.improvement.nhs.uk/diagnostics/RadiologyCaseStudies.aspx#Ult (accessed 28 August 2015).
U15. Royal College of Radiologists. Focused Ultrasound Training Standards. London: RCR; 2012. URL: www.rcr.ac.uk/focused-ultrasound-training-standards (accessed 28 August 2015).
U16. Imison C, Williams S, Smith J. Toolkit to Support the Development of Primary Care Federations. London: RCGP; 2010.
U17. Ingeman ML, Ormstrup TE, Vedsted P. Direct-access to abdominal ultrasonic investigation from general practice – the role in earlier cancer diagnosis. Fam Pract 2015;32:205–10.
U18. Kane D, Balint PV, Sturrock R, et al. Musculoskeletal ultrasound – a state of the art review in rheumatology. Rheumatology 2004;43:823–8.
U19. Laerum F, Eik-Nes S, Fonnebo V, Heilo A, Johnsen R, Stray-Pedersen B, et al. Use of Ultrasonography in the Primary Health Care Setting. Oslo: The Norwegian Knowledge Centre for the Health Services; 2001.
U20. Malone B. Spotlight on Point-of-Care Testing. AACC Clinical Laboratory News; 2012.
U21. Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med 2011;364:749–57.
U22. National Ultrasound Steering Group. Ultrasound Clinical Governance. British Medical Ultrasound Society; 2008. URL: www.bmus.org/policies-guides/ClinicalGovernanceInUltrasound-061108.pdf (accessed 28 August 2015).
U23. Pallan M, Linnane J, Ramaiah S. Evaluation of an independent, radiographer-led community diagnostic ultrasound service provided to general practitioners. J Public Health 2005;27:176–81.
U24. Parker H. Making the Shift: A Review of NHS Experience. Birmingham: University of Birmingham; 2006.
U25. PricewaterhouseCoopers. Baseline Report on Diagnostics Activity and Workforce Issues. London: NHS London; 2008.
U26. PricewaterhouseCoopers. An Analytical Based Workforce Review of Community Focused Care and Diagnostics. London: NHS London; 2008.
U27. RCR. Ultrasound Training Recommendations for Medical and Surgical Specialties. 2nd edn. RCR; 2010.
U28. Salihefendic N, Spahovic H, Cabric E, Hrgovic Z. Social and medical yield and consequences of ultrasonography in primary health care. Acta Inform Med 2009;17:32–35.
U29. Singh D. Making the Shift: Key Success Factors. Birmingham: Health Services Management Centre and NHS Institute for Innovation and Improvement, University of Birmingham; 2006.
U30. Siu T, Chau H, Myhre D. Bedside ultrasonography performed by family physicians in outpatient medical offices in Whitehorse, Yukon. Can J Rural Med 2013;18:43–6.
U31. Speets AM, Hoes AW, van der Graff Y, Kalmijn S, De Wit NJ, van Swijndregt AD, et al. Upper abdominal ultrasound in general practice: indications, diagnostic yield and consequences for patient management. Fam Pract 2006;23:507–11.
U32. RCR, Society and College of Radiographers. Standards for the Provision of an Ultrasound Service. London: RCR; 2014.
U33. Thompson M, Price CPP, Van den Bruel A, et al. Innovation in Diagnostics and Healthcare: Improving Bench to Bedside Processes for Testing. Oxford: Department of Primary Care Health Sciences, University of Oxford; 2011.
U34. Wise J. Everyone’s a radiologist now. BMJ 2008;336:1041–3.
U35. Wittenberg M. Will ultrasound scanners replace the stethoscope? BMJ 2014;348:g3463.
U36. Wordsworth S, Scott A. Ultrasound scanning by general practitioners: is it worthwhile? J Public Health Med 2002;24:88–94.
Appendix 4 Search strategies and related information for Chapter 5
Bibliographic database searches
Overview/methods
-
MEDLINE via Ovid SP.
-
EMBASE via Ovid SP.
-
The Cochrane Library [Cochrane Database of Systematic Reviews (CDSR) CENTRAL, DARE, NHS EED, HTA].
-
CINAHL.
-
Web of Science.
-
Citation searches of key titles.
Grey literature searches.
Along with some general searching via a search engine such as Google, the following websites will be searched:
-
Oxford DEC
websites of service providers.
The grey literature element of the searches is likely to be informed by our contact with clinical experts, who may be able to suggest particular resources/websites of interest.
Table of results
Please note: the dates on which the searches were performed and any keywords applied to the results are included in the table below.
| Results | Keywords | |
|---|---|---|
| MEDLINE | 399 | $$MEDLINE |
| EMBASE | 786 | $$Embase |
| Cochrane CDSR | 6 | $$Cochrane; $$CDSR |
| Cochrane CENTRAL | 69 | $$Cochrane; $$CENTRAL |
| Cochrane DARE | 0 | $$Cochrane; $$DARE |
| Cochrane NHSEED | 1 | $$Cochrane; $$NHSEED |
| Cochrane HTA | 0 | $$Cochrane; $$HTA |
| CINAHL | 88 | $$CINAHL |
| Web of Science | 1012 | $$WoS |
| Total | 2361 | |
| Total after duplicates have been removed |
Search strategies
MEDLINE
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R).
Date range searched: 1946 to present.
-
primary care.tw. (73,475)
-
general practi$.tw. (63,013)
-
primary health care.tw. (15,134)
-
Community Mental Health Services/ (16,715)
-
Family Practice/ (59,984)
-
Home Care Services/ (27,904)
-
Physicians, Family/ (14,697)
-
Community Health Services/ (27,031)
-
Community Health Nursing/ (18,468)
-
Community Pharmacy Services/ (2926)
-
Community Health Workers/ (3300)
-
Preventive Health Services/ (10,983)
-
or/1-12 (266,862)
-
*Diagnostic Services/ (1050)
-
*Clinical Laboratory Services/ (94)
-
*Genetic Testing/ (12,574)
-
*Mobile Health Units/ (1937)
-
diagnostic service$.ti,ab. (1039)
-
clinical laboratory service$.ti,ab. (121)
-
genetic test$.ti,ab. (12,848)
-
mobile health$ unit$.ti,ab. (52)
-
mobile health$ clinic$.ti,ab. (21)
-
(point of care testing or point-of-care testing or POCT or near patient testing or near-patient testing).ti,ab. (1722)
-
diagnos$.ti,ab. (1,713,087)
-
test$.ti,ab. (2,238,771)
-
24 or 25 (3,649,705)
-
26 and primary care.tw. (24,271)
-
or/14-23,27 (51,331)
-
13 and 28 (25,005)
-
Ultrasonography/ (63,040)
-
ultrasonic diagnos$.ti,ab. (1831)
-
(ultrasound adj3 imaging$).ti,ab. (10,337)
-
(ultrasonic adj3 imaging$).ti,ab. (1257)
-
sonograph$.ti,ab. (44,065)
-
ultrasound scan$.ti,ab. (7157)
-
Echocardiography/ (67,752)
-
(echocardiography or echocardiogram).ti,ab. (85,365)
-
(echo adj2 cardi$).ti,ab. (555)
-
us.fs. (203014) – extra line added in after protocol agreed? .fs = floating subheading for ultrasound
-
or/30-39 (350,310)
-
29 and 40 (429)
-
limit 41 to yr=“1995 –Current” (399)
EMBASE
Database: EMBASE.
Date range searched: 1974 to 9 February 2015.
-
primary care.tw. (94,182)
-
general practi$.tw. (80,437)
-
primary health care.tw. (17,154)
-
general practice/ (70,635)
-
home care/ (48,838)
-
general practitioner/ (66,185)
-
community care/ (48,583)
-
community health nursing/ (25,539)
-
pharmacy/ (56,513)
-
health auxiliary/ (3304)
-
preventive health service/ (21,752)
-
or/1-11 (416,412)
-
*preventive health service/ (10,515)
-
*laboratory diagnosis/ (7391)
-
*genetic screening/ (10,737)
-
diagnostic service$.ti,ab. (1325)
-
clinical laboratory service$.ti,ab. (154)
-
genetic test$.ti,ab. (18,829)
-
mobile health$ unit$.ti,ab. (48)
-
mobile health$ clinic$.ti,ab. (23)
-
(point of care testing or point-of-care testing or POCT or near patient testing or near-patient testing).ti,ab. (2587)
-
diagnos$.ti,ab. (2,318,322)
-
test$.ti,ab. (2,853,121)
-
22 or 23 (4,722,933)
-
24 and primary care.tw. (33,613)
-
or/13-21,25 (80,950)
-
12 and 26 (44,826)
-
echography/ (232,573)
-
ultrasonic diagnos$.ti,ab. (2176)
-
(ultrasound adj3 imaging$).ti,ab. (13,738)
-
(ultrasonic adj3 imaging$).ti,ab. (1435)
-
sonograph$.ti,ab. (57,024)
-
ultrasound scan$.ti,ab. (9892)
-
echocardiography/ (135,270)
-
(echocardiography or echocardiogram).ti,ab. (129,663)
-
(echo adj2 cardi$).ti,ab. (1157)
-
or/28-36 (452,549)
-
27 and 37 (824)
-
limit 38 to yr=“1995 –Current” (786)
The Cochrane Library
#1 (primary care):ti,ab,kw
#2 (general practi*):ti,ab,kw
#3 (primary health care):ti,ab,kw
#4 MeSH descriptor: [Community Mental Health Services] this term only
#5 MeSH descriptor: [Family Practice] this term only
#6 MeSH descriptor: [Home Care Services] this term only
#7 MeSH descriptor: [Physicians, Family] this term only
#8 MeSH descriptor: [Community Health Services] this term only
#9 MeSH descriptor: [Community Pharmacy Services] this term only
#10 MeSH descriptor: [Community Health Workers] this term only
#11 MeSH descriptor: [Preventive Health Services] this term only
#12 (or #1–#11)
#13 MeSH descriptor: [Diagnostic Services] this term only
#14 MeSH descriptor: [Clinical Laboratory Services] this term only
#15 MeSH descriptor: [Genetic Testing] this term only
#16 MeSH descriptor: [Mobile Health Units] this term only
#17 (diagnostic service*):ti,ab
#18 (clinical laboratory service*):ti,ab
#19 (genetic test*):ti,ab
#20 (mobile health* unit*):ti,ab
#21 (mobile health* clinic*):ti,ab
#22 (point of care testing or point-of-care testing or POCT or “near patient testing” or “near-patient testing”):ti,ab
#23 diagnos*:ti,ab
#24 test*:ti,ab
#25 #23 or #24
#26 (primary care):ti,ab,kw
#27 #25 and #26
#28 (or #13–#22, #27)
#29 #12 and #28
#30 MeSH descriptor: [Ultrasonography] this term only
#31 (ultrasonic diagnos*):ti,ab
#32 (ultrasound near/3 imaging*):ti,ab
#33 (ultrasonic near/3 imaging*):ti,ab
#34 sonograph*:ti,ab
#35 (ultrasound scan*):ti,ab
#36 MeSH descriptor: [Ultrasonography] this term only
#37 (echocardiography or echocardiogram):ti,ab
#38 (echo near/2 cardi*):ti,ab
#39 (or #30–#38)
#40 #29 and #39 Publication Year from 1995 to 2015
Cumulative Index to Nursing and Allied Health Literature
| Search ID# | Search terms | |
|---|---|---|
| □ | S39 | S28 AND S38 |
| □ | S38 | S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 |
| □ | S37 | TI echo N2 cardi* OR AB echo N2 cardi* |
| □ | S36 | TI ( echocardiography or echocardiogram ) OR AB ( echocardiography or echocardiogram ) |
| □ | S35 | (MH “Echocardiography”) |
| □ | S34 | TI ultrasound scan* OR AB ultrasound scan* |
| □ | S33 | TI sonograph* OR AB sonograph* |
| □ | S32 | TI ultrasonic N3 imaging* OR AB ultrasonic N3 imaging* |
| □ | S31 | TI ultrasound N3 imaging* OR AB ultrasound N3 imaging* |
| □ | S30 | TI ultrasonic diagnos* OR AB ultrasonic diagnos* |
| □ | S29 | (MH “Ultrasonography”) |
| □ | S28 | S11 AND S27 |
| □ | S27 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S26 |
| □ | S26 | S24 AND S25 |
| □ | S25 | TI primary care OR AB primary care |
| □ | S24 | S22 OR S23 |
| □ | S23 | TI test* OR AB test* |
| □ | S22 | TI diagnos* OR AB diagnos* |
| □ | S21 | TI ( (point of care testing or point-of-care testing or POCT or near patient testing or near-patient testing) ) OR AB ( (point of care testing or point-of-care testing or POCT or near patient testing or near-patient testing) ) |
| □ | S20 | TI mobile health* clinic* OR AB mobile health* clinic* |
| □ | S19 | TI mobile health* unit* OR AB mobile health* unit* |
| □ | S18 | TI genetic test* OR AB genetic test* |
| □ | S17 | TI clinical laboratory service* OR AB clinical laboratory service* |
| □ | S16 | TI diagnostic service* OR AB diagnostic service* |
| □ | S15 | (MM “Mobile Health Units”) |
| □ | S14 | (MM “Genetic Screening”) |
| □ | S13 | (MM “Clinical Laboratories”) |
| □ | S12 | (MM “Diagnostic Services”) |
| □ | S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 |
| □ | S10 | (MH “Community Health Workers”) |
| □ | S9 | (MH “Community Health Nursing”) |
| □ | S8 | (MH “Community Health Services”) |
| □ | S7 | (MH “Physicians, Family”) |
| □ | S6 | (MH “Home Health Care”) |
| □ | S5 | (MH “Family Practice”) |
| □ | S4 | (MH “Community Mental Health Services”) |
| □ | S3 | TI primary health care OR AB primary health care |
| □ | S2 | TI general practi* OR AB general practi* |
| □ | S1 | TI primary care OR AB primary care |
Web of Science
| # 43 | 1012 | #42 AND #33 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 42 | 214,889 | #41 OR #40 OR #39 OR #38 OR #37 OR #36 OR #35 OR #34 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 41 | 875 | TS=(echo near/2 cardi*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 40 | 80,759 | TS=(echocardiography or echocardiogram) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 39 | 22,367 | TS=(ultrasound scan*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 38 | 41,540 | TS=(sonograph*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 37 | 4012 | TS=(ultrasonic near/3 imag*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 36 | 21,771 | TS=(ultrasound near/3 imag*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 35 | 4086 | TS=(ultrasonic diagnos*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 34 | 74,311 | TS=Ultrasonograph* Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 33 | 50,783 | #32 AND #13 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 32 | 199,981 | #31 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 31 | 45,861 | #30 AND #29 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 30 | 134,510 | TS=(primary care) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 29 | 4,124,771 | #28 OR #27 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 28 | 3,063,325 | TS=(test*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 27 | 1,330,265 | TS=(diagnos*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 26 | 207 | TS=(“near-patient testing”) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 25 | 207 | TS=(“near patient testing”) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 24 | 650 | TS=(POCT) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 23 | 4726 | TS=(point-of-care testing) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 22 | 11,169 | TS=(point of care testing) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 21 | 1868 | TS=(mobile health* clinic*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 20 | 1046 | TS=(mobile health* unit*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 19 | 2693 | TS=(clinical laboratory service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 18 | 13,056 | TS=(diagnostic service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 17 | 917 | TS=(Mobile Health Unit*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 16 | 130,577 | TS=(Genetic Test*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 15 | 2693 | TS=(Clinical Laboratory Service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 14 | 13,056 | TS=(Diagnostic Service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 13 | 327,081 | #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 12 | 7060 | TS=(Preventive Health Service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 11 | 7844 | TS=(Community Health Worker*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 10 | 1604 | TS=(Community Pharmacy Service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 9 | 9727 | TS=(Community Health Nursing) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 8 | 33,166 | TS=(Community Health Service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 7 | 21,656 | TS=(family physician*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 6 | 14,835 | TS=(Home Care Service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 5 | 42,922 | TS=(Family Practice) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 4 | 10,099 | TS=(Community Mental Health Service*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 3 | 68,124 | TS=(primary health care) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 2 | 124,796 | TS=(general practi*) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
| # 1 | 134,510 | TS=(primary care) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=1995-2015 |
Internet and grey literature searches
Google searches
((“primary care”) AND diagnostic* AND (ultrasonography OR ultrasound OR ultrasonic OR sonography OR sonograph OR echocardiography OR echocardiogram))
Results: http://tinyurl.com/l3usvtx
(((“primary care”) AND diagnostic* AND (ultrasonography OR ultrasound OR ultrasonic OR sonography OR sonograph OR echocardiography OR echocardiogram))) site:.ac.uk
(((“primary care”) AND diagnostic* AND (ultrasonography OR ultrasound OR ultrasonic OR sonography OR sonograph OR echocardiography OR echocardiogram))) site:.nhs.uk
((“primary health care”) AND diagnostic* AND (ultrasonography OR ultrasound OR ultrasonic OR sonography OR sonograph OR echocardiography OR echocardiogram))
Results: http://tinyurl.com/mhcv8um
http://tinyurl.com/k2ebqy4%20site:.ac.uk
http://tinyurl.com/ls9mvxv%20site:.nhs.uk
((“family practice”) AND diagnostic* AND (ultrasonography OR ultrasound OR ultrasonic OR sonography OR sonograph OR echocardiography OR echocardiogram))
Results: http://tinyurl.com/k4hzk7o
http://tinyurl.com/mjovk6k%20site:.ac.uk
http://tinyurl.com/mrm7p52%20site:.nhs.uk
((“community health services”) AND diagnostic* AND (ultrasonography OR ultrasound OR ultrasonic OR sonography OR sonograph OR echocardiography OR echocardiogram))
Results: http://tinyurl.com/lc9gbho
http://tinyurl.com/ky85re3%20site:.ac.uk
http://tinyurl.com/m9eupph%20site:.nhs.uk
((“point of care testing” OR “point-of-care testing” OR POCT OR “near patient testing” OR “near-patient testing”) AND diagnostic* AND (ultrasonography OR ultrasound OR ultrasonic OR sonography OR sonograph OR echocardiography OR echocardiogram))
Results: http://tinyurl.com/mtsken6
Searches of specified websites
Companies providing services
InHealth
Ultrasound AND diagnosis = 101 results: www.inhealthgroup.com/search/gss/ultrasound%20AND%20diagnosis
Results include word variations.
Sonograph AND diagnosis = 3 results:
www.inhealthgroup.com/search/gss/sonograph%20AND%20diagnosis
Results include word variations.
Echocardiograph AND diagnosis = 94 results: www.inhealthgroup.com/search/gss/echocardiograph%20AND%20diagnosis
Results include word variations.
CareUK
Ultrasound AND diagnosis: www.careuk.com/search/careuk/Ultrasound%20diagnosis
Ultrasound AND diagnostics: www.careuk.com/search/careuk/ultrasound%20diagnostics
Sonograph AND diagnosis = 0 results
Echocardiograph AND diagnosis = 0 results
Fountain Medical Diagnostic Services
No search feature.
Diagnostic Healthcare
Search feature does not produce relevant results.
Ultrasound is available as an option from the list of services on the home page.
GP Care
Ultrasound + diagnosis = 63 results: www.gpcare.org.uk/site/search/?q=Ultrasound%2Bdiagnosis+&srchBtn=&m=
Ultrasound + diagnostics = 57 results: www.gpcare.org.uk/site/search/?q=Ultrasound%2Bdiagnostics+&srchBtn=&m=
Sonograph + diagnosis = 17 results: www.gpcare.org.uk/site/search/?q=Sonograph%2Bdiagnosis+&srchBtn=&m=
Sonograph + diagnostics = 5 results: www.gpcare.org.uk/site/search/?q=Sonograph%2Bdiagnostics+&srchBtn=&m=
Echocardiograph + diagnosis = 0 results
Echocardiograph + diagnostics = same 5 results as sonograph+diagnostics
Services for specific conditions
Arrhythmia (South Gloucestershire)
Hosted on GP Care website that was searched as part of item 1 (see previous page).
Community cardiology service (Imperial College Healthcare NHS Trust)
Searches for medical technologies do not provide any results.
There are a handful of results for ‘diagnostics’, although this is obviously searching across the whole Imperial College Health care website and results may therefore not be relevant to the community cardiology service.
Dedicated diagnostic centres
Harvant NHS Diagnostic Centre
No search feature.
Mid & South Buckinghamshire NHS Diagnostic Centre
No search feature.
Rotherham NHS Diagnostic Centre (located at Community Health Centre)
No search feature.
Additional searches
Open Grey
A search for “Primary care” AND ultrasonography AND daignos* found the following thesis: Community ultrasound A study of the factors influencing transfer into primary care in the context of a shift in service provision (http://hdl.handle.net/10068/509919).
Appendix 5 Data extraction tables for Chapter 5
Data extraction of level 1 studies
| Study (authors and year) | Everett and Preece 1996222 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | UK (England) |
| Setting | Community health centre |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Obstetric |
| Staff involved | Involved in doing the test and interpreting the results Senior midwife or GP (both had received ultrasound training at the local general hospital) |
| Research question/objectives | To compare the presence of foetal heart movement at the patient’s initial scan with subsequent fetal survival during the first 20 weeks of pregnancy |
| Research methods | The community ultrasound clinic was set up in 1986 and data were collected for the first 3 years. Data were recorded for the initial scan, the diagnoses of non-viable pregnancies and the outcome of viable pregnancies at 20 weeks (from the patient’s health centre records) |
| Results/data | 240 women with bleeding in early pregnancy were scanned. Community ultrasound scanning had a sensitivity of 97% and specificity of 98% for predicting fetal survival to the 20th week of pregnancy. Results of scanning were unclear in eight cases; six patients received a second scan and two were referred to hospital. No fetal heart movement was detected in 117 women and all subsequently miscarried. Authors stated that ‘many women were given the option of delaying their hospital admission to the following day, some of whom miscarried at home without being admitted to hospital’ |
| Main findings | Community ultrasound scanning made it possible to quickly identify if pregnancies were viable and make appropriate arrangements, avoiding unnecessary bed rest when miscarriage was inevitable and providing strong reassurance to women with viable pregnancies |
| Comments | This was not an evaluation of the general practice ultrasound clinic per se but it demonstrated potential benefits of the service in diagnosing patients more quickly and potentially avoiding hospital visits and/or admissions |
| Study (authors and year) | Wordsworth and Scott 2002203 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Cost analysis based on audit data; audit of scan quality; discrete choice experiment |
| Country | UK (Scotland) |
| Setting | Rural general practice |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | General |
| Staff involved | Involved in doing the test and interpreting the results Two GPs (source of training not reported) |
| Research question/objectives | To evaluate the impact of GP ultrasound scanning on the use of NHS resources and to elicit patient preferences around ultrasound scanning |
| Research methods | Cost analysis comparing costs of scanning at the health centre vs. scanning at the local teaching hospital; clinical audit of quality of GP scans; discrete choice experiment to elicit patient preferences for who performed the scan, where and acceptable levels of waiting time and accuracy. Effects on patient management compared actual patient management with consensus of what would have been done if GP scanning had not been available |
| Results/data | Analysis of costs and assessment of scan quality were based on 131 patients scanned over 6 months. Patient preference study used a random sample of 500 patients from practice list and 250 consecutive patients scanned at the practice |
| GP scanning reduced the number of referrals (14.8% vs. 29.9%), emergency hospital admissions (7.8% vs. 13.4%) and hospital scans (0.8% vs. 23.6%). Cost per scan was higher for GP scanning (£36.37 vs. £20.32) but the average cost per episode was lower [£148 vs. £183 (predicted)]. Assessment of scan quality revealed a need for further training and it was recommended that GPs should scan more patients per year | |
| Response rates for patient preference study were 41% for non-scanned patients and 37% for the scanned group. Patients preferred to be scanned at the practice and were prepared to wait an extra 5.5 days and accept a 3.5% reduction in scan accuracy for their choice. Who carried out the scan was not considered important | |
| Main findings | The results of the study provide some evidence to support GP scanning. Further research was recommended, specifically on relative accuracy of GP and hospital scans; effects of providing the service on GP workload and stress; and alternative models of care such as a radiographer scanning at the practice |
| Comments | A limitation of the study was its retrospective design and the fact that comparisons were based on hypothetical management rather than an actual control group. The study was performed in a rural setting and patient preferences could be different in an area with closer proximity to hospital services |
| Study (authors and year) | Salihefendic et al. 2009229 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Cohort |
| Country | Bosnia-Herzegovina |
| Setting | Primary care clinics |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Abdominal |
| Staff involved | Involved in doing the test and interpreting the results Two radiologists |
| Research question/objectives | To assess the influence of abdominal ultrasound on patient management in general practice |
| Research methods | Comparative study comparing a primary care clinic with direct access to ultrasound scanning (experimental) with a clinic accessing ultrasound by referral to secondary care (control). The reporting is very unclear but the study appears to be attempting to compare ultrasound performed by GPs in primary care clinics with ultrasound obtained by referral to secondary care. What the authors appear to have done is performed scans on patients referred from two primary care clinics, one with direct access to ultrasound and one without, that is, those who GPs say they would scan in each setting. Although not explicitly stated in the paper, they also appear to have examined the entire patient sample (via records or even by performing a scan on them as well) to identify patients with a theoretical indication for ultrasound. They must also have asked GPs about anticipated patient management before and after knowing the scan results but this is not reported in the methods |
| Results/data | 383 out of 1539 patients in the experimental group and 175 out of 1878 in the control group were scanned. Graph suggests that theoretical indications for ultrasound were present in 416 (experimental) and 336 (control) patients. In the control group, 46 patients (26%) had no abnormalities detected by ultrasound. Abdominal ultrasound resulted in a change in anticipated patient management in 54% of patients, including a reduction in anticipated referrals to medical specialists |
| Main findings | Authors stated that the use of ultrasound in primary care clinics reduces time to establish a final diagnosis, reduces the need for more expensive tests and can give an early diagnosis of benign and malignant tumours |
| Comments | The value of this study is limited by lack of clarity about the methods and findings |
Level 2 studies
English-language full papers
| Study (authors and year) | Goldberg et al. 2003230 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Retrospective diagnostic accuracy study |
| Country | Australia |
| Setting | Hospital assessment of tests performed in community settings |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Musculoskeletal |
| Staff involved | Involved in doing the test and interpreting the results Ultrasound scans were performed by 109 different radiologists on 336 patients. The majority of patients (number not reported) were referred by their doctor for consideration for surgical intervention for full-thickness rotator cuff tear |
| Research question/objectives | To assess the accuracy of diagnostic ultrasound examinations for diagnosis of full-thickness rotator cuff tears in general community practice |
| Research methods | Diagnostic ultrasound findings were compared with results of single-contrast arthrography (reference standard) and in 225 cases with findings at surgery. Arthrograms were interpreted by a consultant radiologist and one of the study authors (blinded to clinical and radiological findings). Time from diagnostic ultrasound to arthrography averaged 47 days (range 1–120 days). There were no reports that any patient suffered trauma between the two investigations |
| Results/data | The accuracy of diagnostic ultrasound was 0.38, sensitivity 0.24 and specificity 0.61. Positive and negative predictive values were 0.49 and 0.34, respectively. There were 155 false negatives and 51 false positives based on the diagnostic ultrasound reports. Findings from surgery and arthrography agreed in all cases, supporting use of the latter as reference standard |
| Main findings | Diagnostic ultrasound examination in general community settings is not a reliable tool for diagnosis of full-thickness rotator cuff tears |
| Comments | Authors noted that other studies reporting higher accuracy were relatively small and generally came from units with a special interest in ultrasound |
| Study (authors and year) | Grubel 2011231 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Retrospective diagnostic accuracy study |
| Country | USA |
| Setting | Community private practice |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Abdominal |
| Staff involved | Involved in doing the test and interpreting the results One gastroenterologist, who had undertaken formal ultrasound training as part of a German internal medicine programme |
| Research question/objectives | To assess the diagnostic accuracy and value for clinical management of ultrasound performed by a gastroenterologist in a community practice |
| Research methods | Records from March to December 2009 were retrospectively reviewed. Diagnostic accuracy of ultrasound was assessed for patients who subsequently underwent CT, MRI or endoscopic retrograde cholangiopancreatography. Influence of ultrasound findings on subsequent clinical management was also assessed |
| Results/data | 310 patients underwent ultrasound. Abdominal pain, nausea and vomiting, and abnormal liver function tests were the most common indications. Ultrasound findings guided clinical management in two out of three patients (exact numbers not reported). 84 patients subsequently had CT, MRI or endoscopic retrograde cholangiopancreatography within 1 month. A normal ultrasound result was confirmed in 35 out of 40 patients (88%). Abnormal findings were confirmed in 41 out of 44 patients (93%). Ultrasound missed three (4%) significant clinical lesions but no malignancy was overlooked |
| Main findings | Gastroenterologist-operated ultrasound provides fast and accurate information for the diagnosis and management of abdominal disorders |
| Comments |
| Study (authors and year) | Heikkinen et al. 2005232 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Cohort |
| Country | Finland |
| Setting | Primary care (patients with dyspepsia investigated by GPs in four health centres) |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Abdominal |
| Staff involved | Involved in doing the test and interpreting the results Ultrasound scanning was performed by an experienced radiologist blinded to the results of clinical, laboratory and endoscopic investigations |
| Research question/objectives | To assess the role of ultrasound in evaluating dyspepsia and to assess the long-term clinical relevance of minor findings found by ultrasound scanning in patients with functional dyspepsia |
| Research methods | 400 consecutive patients with dyspepsia were recruited. Following ultrasound and gastroscopy, patients were divided into two groups. Endoscopy-positive patients (140) had a final diagnosis identified by endoscopy, while endoscopy-negative patients (260) had no final diagnosis after endoscopy. After further diagnostic work-up, 55 of the endoscopy-negative patients were diagnosed with an organic disease; the remainder were invited to participate in a follow-up study. Follow-up patients received a repeat ultrasound scan 6–7 years after the initial scan and performed by the same radiologist |
| Results/data | In the endoscopy-negative group, gallstones were detected by ultrasound in 21 patients but were only considered to be the cause of symptoms in nine cases. No malignant lesions were detected. In the endoscopy-positive group a malignant tumour in the kidney was suspected in three patients but only one was confirmed. Several minor findings were revealed by ultrasound |
| Of 260 endoscopy-negative patients, 180 were eligible for follow-up ultrasound and 135 received it. Two significant findings were diagnosed: a small renal cancer and hydronephrosis | |
| Main findings | Wide untargeted use of abdominal ultrasound in evaluating patients with dyspepsia following a gastroscopy is not necessary. Repeated ultrasound examination in people with functional dyspepsia is not recommended and rarely changes the diagnosis |
| Comments | Relevant to diagnostic yield of ultrasound vs. no ultrasound in an unselected primary care population |
| Study (authors and year) | Laine et al. 1998233 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Prospective diagnostic accuracy study |
| Country | Finland |
| Setting | Primary care (two health centres covering populations of 30,000–37,000 people) |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Sinus |
| Staff involved | Involved in doing the test and interpreting the results 12 GPs performed ultrasound scans and interpreted the results. GPs at one centre received standard information on the use of the ultrasound machine from the manufacturer; the other centre had used this machine for 6 years. One of the study principal investigators also performed ultrasound scans but results were not reported |
| Research question/objectives | To assess the diagnostic accuracy of ultrasound, clinical examination and radiography for diagnosing acute maxillary sinusitis in primary care |
| Research methods | Ultrasound, clinical examination and radiography (interpreted by experienced radiologists) were performed on consecutive adult patients with suspected acute maxillary sinusitis. Sinus irrigation (reference standard) was performed as soon as possible after ultrasound and radiography |
| Results/data | Full data were available for 39 patients. Sensitivity of GP ultrasound was 61% and specificity 53%. For ultrasound combined with clinical examination, sensitivity was 70% and specificity 37%. Radiography was the most accurate test (sensitivity 61%; specificity 98%). Poor accuracy of ultrasound was mainly due to a large number of false-positive diagnoses |
| Main findings | Accuracy of ultrasound in primary care was lower than results reported from specialist practice. More attention should be paid to education and quality management in the use of ultrasound in primary care |
| Comments | Study limited by small sample size as noted by authors and attributed to unwillingness to undergo sinus puncture |
| Study (authors and year) | Landers and Ryan 2014234 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | New Zealand |
| Setting | Palliative care service |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Other (scans performed to assess need for drainage in patients with abdominal ascites and to place the drains safely |
| Staff involved | Involved in: doing the test; interpreting the results Specialist palliative care physician and palliative care registrar performed the scans. One of the operators undertook a 1-day course aimed at GPs and non-radiology specialists |
| Research question/objectives | To evaluate the use of portable ultrasound scanning in the management of abdominal ascites |
| Research methods | Patient data were prospectively entered into a database over a 12-month period. Charts of all patients were retrospectively reviewed to complete the database and check outcomes |
| Results/data | Forty-one scans were performed on 32 patients. All except three patients had cancer, with ovarian (n = 7) and pancreatic (n = 4) cancers being most common. Fluid was present in 19 cases and drains were placed in 17 of these. Twenty-five ultrasounds were completed at home and nine of these patients went on to have paracentesis (drainage). Clinical notes reported relief of symptoms in 8 out of 17 patients who received drainage. There were no major complications, although one procedure did not obtain any fluid and was abandoned |
| Main findings | Bedside ultrasound allowed some scans and subsequent procedures to be performed at home, increasing convenience for the patient and reducing time spent at the hospital |
| Comments | Identified limitations include small sample, retrospective data collection and poor quality of documentation, as well as being based on only one centre and two operators |
| Study (authors and year) | Mäkelaä and Leinonen 1996235 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | Finland |
| Setting | Primary care (14 health centres with varying facilities for imaging) |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Sinus |
| Staff involved | Involved in doing the test and interpreting the results 161 GPs agreed to participate; details of training were not reported |
| Research question/objectives | To assess the effect of imaging techniques on the diagnostic pattern of acute maxillary sinusitis in primary care |
| Research methods | GPs filled in a questionnaire for each consultation with a patient with suspected sinusitis covering symptoms and their duration; signs on physical examination; diagnostic methods used; diagnosis and how certain they were about it; treatment; control visits ordered; sick leaves; and referral to specialists. Diagnostic tests evaluated were ultrasound, radiography and clinical impression. Availability of imaging techniques was analysed as an independent variable but the behaviour of individual GPs was not evaluated |
| Results/data | GPs returned 502 questionnaires, of which 446 met inclusion criteria. Imaging was available in 337 cases (radiography 60, ultrasound 101 and both in 176); 109 cases could be judged only by clinical examination |
| When available, ultrasound was used in 82–92% of cases and radiography in 6–32%. Sinusitis was diagnosed in 84–88% of cases when only ultrasound or radiography could be used and 77% when both techniques were available. GPs’ confidence in their diagnosis was 39% for clinical examination only, 45% for radiography, 58% for ultrasound and 66% for both technologies. Authors suggested that the convenience of immediate availability of ultrasound may be important for its use. Sensitivity and specificity of ultrasound varied according to the cut-off point and classification used | |
| Main findings | When available, ultrasound is widely used for diagnosing sinusitis in Finnish primary care. Accuracy of diagnosis could be increased by improving the interpretation of ultrasound findings |
| Comments | All data based on GP reports/records |
| Study (authors and year) | Pallan et al. 200516 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Cohort |
| Country | UK (England) |
| Setting | Primary care |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | General |
| Staff involved | Involved in doing the test and interpreting the results Community service: independent radiographer Hospital service: NHS Trust staff |
| Research question/objectives | To assess the advantages and disadvantages of a radiographer-delivered, primary care-based diagnostic ultrasound service compared with a hospital open-access service |
| Research methods | Random samples of patients who had used the two services in 2001/2 were identified, as were all GP principals in the area. Waiting times, patient and GP satisfaction were assessed using postal questionnaires. Clinical quality was assessed by review of stored ultrasound images. Access to secondary care following an abnormal scan result was mapped using patient records. Unit cost data were collected for the cost study |
| Results/data | Response rates were 52.9% (100/189) for the community service patient survey, 44.6% (82/184) for the hospital patient survey and 80.6% (29/36) for the GP survey. Patient characteristics did not vary greatly between the groups. Mean wait for an appointment was 17.4 days (95% CI 15.8 to 19 days) for the community service and 44.5 days (95% CI 38.8 to 50.2 days) for the hospital service. Patients were highly satisfied with both services but GPs were markedly less satisfied with the hospital service. Access to secondary care was not systematically different between services. Quality of stored images and reports was comparable between services. Cost per abnormality detected was higher for the community service (£108 vs. £77) but the difference was not statistically significant |
| Main findings | The community service reduced waiting times and was of comparable quality with the hospital service. Authors considered that this benefit, together with high patient and GP satisfaction, may justify the possibly higher cost per abnormality detected |
| Comments | Retrospective study design |
| Study (authors and year) | Scholten-Peeters et al. 2014224 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Cross-sectional survey |
| Country | The Netherlands |
| Setting | Secondary care |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Musculoskeletal |
| Staff involved | Involved in doing the test and interpreting the results Not applicable |
| Research question/objectives | To evaluate the opinions and experiences of Dutch orthopaedic surgeons and radiologists about DMUS performed in primary care by physiotherapists or GPs |
| Research methods | Cross-sectional survey using a questionnaire developed by the authors with the aid of focus groups. Orthopaedic surgeons (n = 388) and radiologists (n = 450) in the South and Central regions of the Netherlands were invited to participate by e-mail |
| Results/data | Response rate was 213/838 (25.4%). Most respondents (93.4%) worked in a hospital. Over 90% of radiologists performed DMUS themselves, whereas most surgeons (93.5%) referred patients to a radiologist and only 1.9% performed DMUS themselves |
| Most respondents (86.3%) thought that radiologists were the most appropriate staff to perform DMUS, with only 2.9% choosing physiotherapists and 0.5% GPs. Among radiologists, 21.8% thought radiological technicians were most appropriate. Perceived advantages of DMUS in primary care included faster diagnosis and treatment (23.9%), avoiding referrals (21.1%), cost savings (21.1%) and better indication for referral (17.4%). 48% of respondents saw no advantages in primary care DMUS. Disadvantages mentioned were false-positive results (71.4%); lack of experience (70%); insufficient education (69.5%); not able to relate DMUS results to other forms of diagnostic imaging (65.7%); and false-negative results (65.3%). Only 5.6% believed that primary care DMUS had more advantages than disadvantages | |
| Most respondents trusted the DMUS knowledge of specific radiologists; smaller percentages trusted specific physiotherapists (13.3%) and GPs (3.4%). The majority of respondents repeated DMUS in secondary care for those who had a scan in primary care (no number/percentage reported) | |
| Main findings | Radiologists and orthopaedic surgeon in the Netherlands showed low trust in the DMS knowledge of physiotherapists and GPs. Results should be interpreted cautiously because of the low response rate and uncertain generalisability to other countries |
| Comments |
| Study (authors and year) | Thoomes-de Graaf et al. 2014236 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Cohort (diagnostic reliability) |
| Country | The Netherlands |
| Setting | Primary care physiotherapy |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Musculoskeletal |
| Staff involved | Involved in: doing the test; interpreting the results 13 physiotherapists with > 1 year of experience of diagnostic ultrasound, including > 100 diagnostic ultrasounds of the shoulder; nine experienced radiologists who specialised in musculoskeletal complaints and regularly performed diagnostic ultrasound of the shoulder |
| Research question/objectives | To evaluate the degree of agreement on the interpretation of diagnostic ultrasound in patients with shoulder pain between physiotherapists and radiologists |
| Research methods | Part of a larger cohort study. Patients who received diagnostic ultrasound from a primary care physiotherapist visited a radiologist within 1 week for a second scan. Patients and radiologists were blinded to the physiotherapist’s diagnosis. Four primary diagnostic categories were identified. Agreement was assessed using Cohen’s kappa. Kappa values of 0.81–1.00 were considered as almost perfect agreement; 0.61–0.80 substantial; 0.41–0.60 moderate; 0.21–0.40 fair; 0.01–0.20 slight; and < 0.01 poor. Subgroup analyses were performed based on experience and education of the physiotherapists |
| Results/data | 65 patients were enrolled and 13 physiotherapists and 9 radiologists performed diagnostic ultrasound. The overall kappa across all four diagnostic categories was 0.36 (95% CI 0.29 to 0.43), indicating fair agreement. There was good agreement on diagnosis of full-thickness tears (kappa 0.63, 95% CI 0.31 to 0.94). In subgroup analyses, kappa values were higher for more experienced physiotherapists [0.43 (95% CI 0.25 to 0.63) vs. 0.17 (95% CI –0.15 to 0.50)] and for those with advanced rather than basic training [0.43 (95% CI 0.27 to 0.60) vs. 0.09 (95% CI –0.30 to 0.48)] |
| Main findings | Agreement between physiotherapists and radiologists is borderline substantial for full-thickness tears and slight to moderate for other diagnoses. More experience and training of physiotherapists may increase reliability |
| Comments |
| Study (authors and year) | van Gurp et al. 2013223 and van den Brink 2013253 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Cohort |
| Country | The Netherlands |
| Setting | Primary care |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Echocardiography |
| Staff involved | Involved in doing the test and interpreting the results Test performed by ultrasound technicians Interpretation by cardiologists from Erasmus Medical Centre |
| Research question/objectives | To evaluate an open-access echocardiography service provided independently of the regional hospitals |
| Research methods | Patients referred for open access echocardiography between April 2011 and April 2012 were eligible for the study. Referring GPs were asked what they would have done with this patient if open-access echocardiography had not been available. After the GP had received the results and contacted the patient, they were asked by telephone what management had been initiated; whether or not they had followed the cardiologist’s advice; and if they thought the echocardiogram had been of benefit. GPs were also asked to estimate waiting time for echocardiography via referral to a cardiologist |
| Results/data | 155 patients were referred to the open-access service and full data were available for 105. GPs reported that they referred fewer patients to a cardiologist than they would have done without the service [36 (34%) vs. 97 (92%)] and managed more patients themselves [65 (62%) vs. 10 (10%)]. Cardiologist advice was reportedly followed in 25 out of 31 cases (81%). In 127 cases (82%), the GP thought the echocardiogram was beneficial for decision-making. Waiting time for echocardiography via referral was estimated at 5 weeks, compared with 6 days via the community open-access service Of 55 patients with suspected heart failure, the Dutch clinical guideline for heart failure was followed for 34 (62%); for 13 (24%) patients, neither ECG nor NT-proBNP was performed |
| Main findings | The open-access service reduced referrals to cardiologists, saved time and enabled GPs to manage more patients themselves. However, adherence to diagnostic guidance for heart failure was suboptimal |
| Comments | Study compares actual with hypothetical management rather than having a true control group Critique of this study in van den Brink 2013.253 Issues mentioned: open-access was independent of local hospitals; ultrasound technicians insufficiently trained at first and certification not mentioned; cardiologists had insufficient information; and uncertain legal consequences. Van den Brink stated that open-access echocardiography could mislead the patient into thinking they are receiving state-of-the-art treatment when this is not the case |
| Study (authors and year) | Varonen et al. 2003237 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Prospective diagnostic accuracy study |
| Country | Finland |
| Setting | Primary care (nine health centres) |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Sinus |
| Staff involved | Involved in doing the test and interpreting the results 35 GPs performed ultrasound scans and interpreted the results. GPs received a small group tutorial on the use of ultrasound from an experienced ENT specialist |
| Research question/objectives | To assess the accuracy of symptoms, signs and ultrasound findings for diagnosis in patients with clinically suspected AMS in the context of a randomised trial of AMS therapy, using radiography as a reference standard |
| Research methods | Patients with a clinical diagnosis of AMS were recruited. Data on symptoms and signs were collected for all patients. Ultrasound scans were classified as sinusitis or non-sinusitis for both maxillary sinuses. At one health centre, patients had sinus radiography between 15 and 60 minutes after the ultrasound examination, which acted as a reference standard. Ultrasound results were frozen and printed and later read by an ENT specialist blinded to the patient’s clinical status. Interpretations were compared with those of the GPs |
| Results/data | 150 patients were recruited, of whom 32 received radiography. Prevalence of AMS was 74 out of 148 (50%) in those with ultrasound results and 13 out of 32 (41%) in the subgroup with radiography results. Sensitivity of the best combination of signs/symptoms was 71% (95% CI 56% to 87%) and specificity 42% (95% CI 25% to 59%). Using radiography as reference standard, sensitivity of ultrasound was 92% (95% CI 83% to 100%) and specificity 95% (95% CI 87% to 100%) with the patient as unit of analysis. With sinuses as the unit of analysis, sensitivity of ultrasound was 71% (95% CI 59% to 82%) and specificity 91% (95% CI 85% to 98%). Agreement between GP and specialist interpretation of ultrasound was moderate (kappa 0.47). Authors noted that the use of ultrasound or radiography would reduce antibiotic prescriptions by a half in patients with clinically diagnosed AMS |
| Main findings | With practice and training, primary care physicians can perform sinus ultrasound as accurately as specialists, potentially reducing unnecessary use of antibiotics. Symptoms and clinical examination were not reliable in AMS diagnosis |
| Comments |
English-language conference abstracts
| Study (authors and year) | Gallagher et al. 2012242 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | Ireland? |
| Setting | Primary care |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Echocardiography |
| Staff involved | Involved in doing the test and interpreting the results Unclear who performed the test. Echocardiograms were reviewed by a consultant cardiologist |
| Research question/objectives | To evaluate outcomes from a community outreach clinic for diagnosis of heart failure |
| Research methods | GPs were given direct access to natriuretic peptide testing followed by facilitated access to echocardiography in the community. Echocardiograms were reviewed by a consultant cardiologist and patients with abnormal echocardiogram results were reviewed by a heart failure nurse specialist and a consultant cardiologist in a primary care centre. Referrals over the initial 6 months of the service were analysed |
| Results/data | Of 66 patients who completed assessment, 37 had echocardiography; 23 were abnormal and 17 patients diagnosed with heart failure |
| Main findings | Direct access to diagnostics and specialist review in the community offer the opportunity for elderly patients to access specialist care in a timely manner |
| Comments | Unclear if community service or hospital outreach |
| Study (authors and year) | Hoyer et al. 2007243 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | Germany |
| Setting | Not applicable |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Echocardiography |
| Staff involved | Involved in doing the test and interpreting the results Not applicable |
| Research question/objectives | To evaluate the supra-regional success of a structured training programme in handheld echocardiography and POC brain natriuretic peptide testing for GPs |
| Research methods | The training programme was delivered to 24 GPs at each of two cardiology centres. After each session, GPs were tested against experts in a multiple choice quiz |
| Results/data | In both centres, concordance of BNP quiz results was > 90% after the first session. Data for the echocardiography quiz are presented. Total training time for echocardiography was 225 ± 34 minutes at centre 1 and 236 ± 28 minutes at centre 2 |
| Main findings | GPs could determine presence or absence of heart failure using handheld echocardiography after < 4 hours of expert teaching |
| Comments | No full publication detected |
| Study (authors and year) | Singh et al. 2009244 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | UK (England) |
| Setting | One-stop diagnostic clinic |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Echocardiography |
| Staff involved | Involved in doing the test and interpreting the results |
| Unclear who performed and interpreted echocardiography. Clinic was run by a ‘primary care physician specialist’ | |
| Research question/objectives | To evaluate the role of a one-stop clinic in validating the diagnosis of heart failure in the community |
| Research methods | A retrospective study examined outcomes of patients referred to the clinic between January 2002 and December 2007. All patients received tests including trans-thoracic echocardiography |
| Results/data | In 6 years, 1008 patients were referred. Of these, 292 (29%) had confirmed LVSD on echocardiography. For the 716 without LVSD, an alternative cause for symptoms was identified in 578 (81%) |
| Main findings | Only 29% of patients with suspected heart failure in the community had proven LVSD. The ability to provide alternative diagnoses for most patients without LVSD was seen as an advantage of the one-stop clinic over open-access echocardiography |
| Comments | Unclear if community- or hospital-based service |
Foreign-language papers (limited data extraction)
| Study (authors and year) | Blanchet and Thierry 2015238 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Qualitative research (semi-structured interviews and focus group) |
| Country | France |
| Setting | Primary care (GPs from Savoie and Haut-Savoie) |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | General |
| Staff involved | Involved in doing the test and interpreting the results Not applicable |
| Research question/objectives | To identify barriers to use of ultrasound by GPs in the office setting |
| Research methods | An interview guide was developed and semistructured interviews were conducted with eight GPs. A focus group involved six GPs (two trainees were also present). Interviews were transcribed and data coded independently using the process of triangulation. Themes and subthemes were identified and agreed between the authors. NVivo software (QSR International, Warington, UK) was used to code and classify the data |
| Results/data | Data saturation was achieved by the individual interviews (no new themes emerged from the focus group). None of the GPs was currently using ultrasound; seven stated an interest in using it and five had undergone training. Fifteen principal themes were identified, which the authors summarised as lack of experience with ultrasound in general practice; mastering ultrasound scanning techniques; uncertain relevance of ultrasound scanning in general practice; the GPs’ own reluctance to use the technique; and possible legal issues |
| Main findings | In addition to identifying the barriers to use of ultrasound, the authors discussed how to overcome them, including teaching basic ultrasound to medical students and creating a degree in general practice ultrasound. They also suggested further research to better define the place of ultrasound in French general practice |
| Comments | Grey literature (thesis). In French with English abstract |
| Study (authors and year) | de la Figuera et al. 2012239 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative |
| Country | Spain |
| Setting | Primary care (four health centres) |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Echocardiography |
| Staff involved | Involved in doing the test and interpreting the results Not reported (assumed to be primary care staff but not explicitly stated) |
| Research question/objectives | To analyse the clinical adequacy of the application, performance and diagnostic decisions resulting from echocardiograms requested by GPs |
| Research methods | Retrospective review of medical records and echocardiography reports |
| Results/data | Data on 684 patients who underwent echocardiography in 2006–7 were analysed. 84% of requests were appropriate and 79.7% showed the presence of disease. Echocardiography results influenced decision-making in > 35% of cases: 17.1% were referred to a cardiologist, 10.5% had their treatment changed and 9.6% were referred for additional tests |
| Main findings | Use of echocardiography was highly appropriate and the results influenced clinical decision-making in a high percentage of cases. Authors concluded that echocardiography should be available to all GPs |
| Comments | Percentages used as reported, exact numbers not always clear |
| Study (authors and year) | Esquerra et al. 2012240 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Cohort (diagnostic reliability) |
| Country | Spain |
| Setting | Hospital radiology department and primary care centre |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | Abdominal |
| Staff involved | Involved in doing the test and interpreting the results GPs undertaking training in ultrasound; radiologists |
| Research question/objectives | To assess the effect of training GPs in low complexity abdominal ultrasound on their diagnostic competence |
| Research methods | Selected patients undergoing low complexity abdominal ultrasound were scanned independently by a GP and radiologist who were blinded to each other’s findings. The kappa score was calculated for the primary diagnosis (pathological vs. normal) and for the ultrasound for each abdominal organ. A kappa of 0.8 or more was considered to indicate a good level of training. An interim analysis was performed after 6 months of training and a final analysis at the end of the study (1 December 2006 to 31 March 2008) |
| Results/data | Two GPs underwent training involving a course of 100 hours of teaching followed by 112 hours based in the hospital radiology department. 120 patients needing low complexity abdominal ultrasound were selected from a total of 868 ultrasound examinations scheduled. In the interim analysis, kappa for the primary ultrasound diagnosis was 0.85. At the end of the study the overall kappa score was 0.89 (95% CI 0.82 to 0.98). Kappa values were high (> 70%) for individual organs except for the pancreas (0.38) and spleen (0.48). For the primary diagnosis, GP scans had a sensitivity of 95.5% (95% CI 91.8% to 99.2%) and specificity of 94.3% (95% CI 90.2% to 98.5%) |
| Main findings | It is feasible for trained GPs to perform low complexity diagnostic abdominal ultrasound |
| Comments | Small sample means results may not be widely generalisable |
| Study (authors and year) | Evangelista et al. 2013241 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Cohort (diagnostic reliability) |
| Country | Spain |
| Setting | Urban primary care centre |
| Application of ultrasound (e.g. echocardiography, GI, musculoskeletal, obstetric) | ‘Pocket’ echocardiography |
| Staff involved | Involved in doing the test and interpreting the results |
| GP trained in pocket echocardiography; expert cardiologist. GP had received recommended basic training for non-cardiologists | |
| Research question/objectives | To assess the diagnostic value of pocket echocardiography performed by a GP in patients with arterial hypertension |
| Research methods | Patients with arterial hypertension underwent examination by a GP using pocket echocardiography. Studies were assessed by an expert cardiologist, blinded to the GP’s findings, and analysed quantitatively by computer software |
| Results/data | Data for 393 consecutive patients seen between January 2011 and January 2012 were analysed. Pocket echocardiography took < 5 minutes and quality of images was good or acceptable in 98% of cases. Agreement between GP and cardiologist was rated very good (kappa > 0.83) for dimensions of the left ventricle, left atrium and ascending aorta, interventricular septum thickness, aortic regurgitation and aortic valve sclerosis. Agreement was rated good (kappa > 0.71) for mitral regurgitation and mitral valve calcification. Six out of 228 significant lesions diagnosed by the cardiologist were missed by the GP |
| Main findings | Pocket echocardiography performed by a GP as an extension of clinical assessment provides an early diagnosis of significant cardiac lesions which may improve therapeutic management |
| Comments | Appears to be based on a single GP, again limiting generalisability of findings |
Appendix 6 Characteristics of included level 3 studies for Chapter 5
| Study (authors and year) | Study design | Setting | Research question/methods | Main findings | Limitations |
|---|---|---|---|---|---|
| Aitken 199944 | Qualitative | English NHS | Qualitative study aiming to identify issues that are important when transfer of ultrasound services to a community setting is considered; understand the views of users and providers; and understand factors affecting the decision process. Used eight focus groups followed by a questionnaire study of 1167 service providers and users | Overall, 353 health professionals and 495 patients completed the questionnaire. In addition to obstetric monitoring, gynaecology and routine abdominal ultrasound were seen as services that could be community-based. Respondents suggested the service could be located at a health centre or GP surgery and staffed by radiographers. Key issues were communication (with secondary care), quality and training. Hospital-based clinicians were concerned about the quality of service possible in a community setting and the possibility that inappropriate funding could result in two inefficient services | Thesis, not peer-reviewed publication. Covers pregnancy monitoring as well as diagnostic ultrasound |
| Bono and Campanini 2007245 | Descriptive | Italian primary care | Describes training courses organised by METIS (scientific society of the Italian Federation of GPs) | Courses began in autumn 2006. Trained GP ultrasonographers expected to handle up to 40% of scan requests | No data on outcomes or updated reports located |
| Cardiac Networks Co-ordinating Group 2006220 | Expert group report | Welsh NHS | Expert group recommendations based on consensus statement from the BSE and other policy documents | Outlines possible models and workforce issues. Stresses the importance of quality assurance. Whatever model is adopted should link into a BSE-accredited hospital department | No updates located |
| Colquhoun et al. 1995246 | Editorial (expert opinion) | English NHS | Expert opinion as to the best way to provide access to echocardiography for GPs | Favours open access to hospital facilities as the best model of service | Expert opinion but represents views of BSE and British Cardiac Society |
| Doddy 2009247 | Descriptive | English NHS (general practice) | Describes author’s use of ultrasound as a GP, including training, costs, indications and perceived benefits | GP ultrasound has numerous benefits, including peace of mind and improved understanding for patients and avoidance of unnecessary referrals | Represents experience of one GPwSI |
| Hoelscher et al. 2013248 | Review | Emergency care | Literature review | Use of transcranial ultrasound for pre-hospital diagnosis of stroke has the potential to significantly improve management | Non-systematic review |
| Hussain et al. 2004249 | Feasibility study | English NHS (GP training) | Evaluation of real-time videoconferencing between a GP surgery and university department of radiology for training and supervision of GPs performing ultrasound of the bladder, prostate and kidneys. Fifteen patients were randomly selected for comparison of hard-copy and transmitted images | Of 105 transmitted images, 90% were classified as diagnostic and 10% were classified as non-diagnostic. Agreement of technical quality scores for transmitted and hard-copy images was poor (kappa = 0.04). Authors concluded that teleultrasound is feasible in primary care. The GP involved benefited from regular ultrasound training and supervision | Again, involved a single GPwSI |
| Mjølstad et al. 2012250 | Feasibility study | Norwegian primary care | Evaluation of GPs’ ability to evaluate left ventricular function using pocket ultrasound. Seven GPs participated and received 8 hours of supervised training. Their findings were compared with those of cardiologists | Among 92 patients, GPs were able to obtain a standard view and measure same (septal mitral annular excursion) in 87% of cases. Their findings did not differ from those of the cardiologists. Authors concluded that with targeted training GPs could use pocket ultrasound to assess left ventricular function | Still a relatively small sample |
| NIHR DEC Oxford 201445 | Review | Primary care | Horizon Scanning report (non-systematic literature review) on portable (POC) ultrasound | Several research gaps were identified covering identification of priority needs; evaluation of technical capabilities; and consensus over training, competency demonstration, medical legal issues and regulatory approval | Echocardiography excluded, no report found |
| Ottenheijm et al. 2011251 | RCT protocol | Dutch primary care | Protocol of RCT comparing therapy based on ultrasound findings vs. usual care for patients with shoulder pain | Trial to provide evidence on the cost-effectiveness of ultrasound for diagnostic triage in primary care | No published results identified: author contacted |
| Robinson et al. 1997215 | Survey | English NHS | Assessment of provision of ultrasound services to primary care in northern England and attitudes of GPs and radiologists towards training GPs to perform ultrasound. Based on postal surveys of GPs (67% response), practice managers (59%) and radiologists (68%) | Around half of GPs did not have direct access to ultrasound for obstetric referrals and 22% for non-obstetric referrals. 52% of GPs were moderately or very interested in ultrasound training but only 3/13 radiology departments were willing to provide training. 73% of GPs preferred an open-access service vs. 15% for a practice-based service | |
| Scholten-Peeters et al. 2014224 | Survey | Secondary care | To evaluate the opinions and experiences of Dutch orthopaedic surgeons and radiologists about DMUS performed in primary care by physiotherapists or GPs | Radiologists and orthopaedic surgeon showed low trust in the DMUS’s knowledge of physiotherapists and GPs | Low response rate (25%); results may not be generalisable |
| Senior et al. 2003252 | Review | Primary care | Review of pros and cons of handheld echocardiography and BNP for detecting LVSD | Further research is required but authors noted that plasma BNP estimation may rule out LVSD, leaving only those with high values requiring echocardiography | Non-systematic review |
| Siu et al. 2013205 | Survey | Primary care | Survey to determine current practice and opinions of family physicians in Yukon, Canada, regarding bedside ultrasonography performed in community settings | Respondents had substantial experience in bedside ultrasonography and the vast majority were ready to use the technology in community settings. However, the skills and willingness of the physicians have not translated into actual use. Respondents considered economics (equipment cost and remuneration) as the biggest barrier, followed by confidence, reliability and skill maintenance | Small sample; results may not be generalisable |
| Vicente-Molinero et al. 2009254 | Review | Primary care | Review of ultrasound in primary care based on searches on INAHTA, PubMed and scientific societies from 1996 to 2006 | A small number of relevant articles were found (no details or synthesis presented), contrasting with a high level of activity reported by the scientific societies in organising work groups, courses, etc. | Spanish language, used mainly as reference source |
| Xiao 2003255 | Review | Primary care | Review of early handheld echocardiography devices | Handheld echocardiography is expected to reduce referrals and waiting times for hospital echocardiography services. Its use is limited by inability to detect subtle changes and by the need for training | Non-systematic review |
Appendix 7 Quality assessment tables for Chapter 5
Quality assessments using the Downs and Black checklist226
| Checklist question | Salihefendic et al. 2009229 | Pallan et al. 200516 | van Gurp et al. 2013223 |
|---|---|---|---|
| 1. Aim clearly described | Yes | Yes | Yes |
| 2. Outcomes clearly described | No | Yes | Yes |
| 3. Patient characteristics clearly described | Yes? (See comments) | Yes? (See comments) | Yes |
| 4. Interventions clearly described | Yes | Yes | Yes |
| 5. Principal confounders clearly described | No | No | No |
| 6. Main findings clearly described | No | Yes | Yes |
| 7. Random variability for the main outcome provided | No | Yes | No |
| 8. Adverse events reported | No | No | No |
| 9. Loss to follow-up reported | No | Yes? (See comments) | Yes |
| 10. Actual p-value provided for main outcome | No | No | Yes |
| 11. Sample asked to participate representative of the population | Yes | Yes | Yes |
| 12. Sample agreed to participate representative of the population | Yes | Unable to determine | Yes |
| 13. Staff participating representative of the patient’s environment | Unable to determine | Yes | Yes |
| 14. Attempt to blind participants | No | No | No |
| 15. Attempt to blind assessors | No | No | No |
| 16. Data dredging results stated clearly | Unable to determine | Yes | Yes |
| 17. Analysis adjusted for length of follow-up | Yes? | Yes | Yes |
| 18. Appropriate statistics | Unable to determine | Yes | Yes |
| 19. Reliable compliance | Yes | Yes | Yes |
| 20. Accurate outcome measures | Unable to determine | Yes | Yes |
| 21. Same population | No | Yes? | Yes |
| 22. Participants recruited at the same time | Yes | Yes | Yes |
| 23. Randomised? | No | No | No |
| 24. Adequate allocation concealment? | No | No | No |
| 25. Adequate adjustment for confounders? | No | No | Unable to determine |
| 26. Loss to follow-up taken into account? | No | Unable to determine | No |
| 27. Power calculation | ? No sample size calculation, unclear what relevant sample size would be | ? Sample size calculation was reported | ? Sample size calculation was reported |
| Comments | 3. Mentions all patients plus exclusions; diagnosis only reported | 3. Inclusion criteria very limited but appropriate for this type of study; diagnosis only reported | No actual control group |
| 9. Percentage of patients for whom records were not available | 3. Brief inclusion criteria and table | ||
| 17. Similar follow-up for all | |||
| 25. Adjusted for ‘nesting’ |
Quality assessments using the Canadian Institute of Health Economics tool for case series
| Everett and Preece 1996222 | Wordsworth and Scott 2002203 | Heikkinen et al. 2005232 | Landers and Ryan 2014234 | Mäkelä and Leinonen 1996235 | |
|---|---|---|---|---|---|
| 1. Hypothesis/objective clearly stated? | Yes | Yes | Yes | Yes | Yes |
| 2. Participant characteristics described? | Partially reported | No | Yes | Yes | Yes |
| 3. More than one centre? | No | No | Yes | No | Yes |
| 4. Eligibility criteria stated? | Yes? | Yes | Partially reported | Yes? | Yes |
| 5. Participants recruited consecutively? | Yes? | Yes | Yes | Unclear | Unclear |
| 6. Participants at a similar point in the disease? | Yes? | Unclear | Unclear | Unclear | Yes? |
| 7. Intervention clearly described? | Yes | Yes | Yes | Yes | Partially reported |
| 8. Co-interventions reported? | No | No | Unclear | Yes | Yes |
| 9. Outcome measures established a priori? | Yes | Partially reported | Partially reported | Partially reported | Partially reported |
| 10. Outcomes measured with appropriate methods? | Yes | Yes | Yes | Unclear | Yes |
| 11. Outcomes measured before and after intervention? | No | No | Yes | Yes | No |
| 12. Were statistical tests appropriate? | Unclear? | Unclear | Yes | Unclear | Yes |
| 13. Length of follow-up reported? | Yes? (See comments) | Yes | Yes | No | Unclear |
| 14. Loss to follow-up reported? | Yes? | Yes (see comments) | Yes | Yes | Yes |
| 15. Estimates of random variability provided? | No | No | No | No | No |
| 16. Adverse events reported? | No? | Yes | No | Yes | No |
| 17. Conclusions supported by results? | Yes | Yes | Yes | Partially reported | Yes |
| 18. Competing interests and funding sources reported? | No | Partially reported | No | Partially reported | No |
| 19. Study conducted prospectively? | Unclear (see comments) | No | Yes | No | Yes |
| 20. Were outcome assessors blinded to intervention status? | No | No | No | No | No |
| Comments | 20-week assessment could be considered as follow-up? | Considers actual patient management (table 1) as case series | Case series because only one group in follow-up | No statistical tests or discussion but reason fairly clear | |
| Results reported for all included participants | Results reported for all patients (actual management) | Patients recruited consecutively to original study | 16. Adverse events of paracentesis, not ultrasound per se | ||
| Appears to be retrospective but not explicitly stated | Adverse events = issues indicating need for further training |
Quality assessment of economic studies
| Wordsworth and Scott 2002203 | Pallan et al. 200516 | |
|---|---|---|
| 1. Was a well-defined question posed in an answerable form? | Yes? | Yes? |
| 2. Was a comprehensive description of the competing alternatives given? | Yes | Yes |
| 3. Was the effectiveness of the programmes or services established? | Yes? | Can’t tell |
| 4. Were all the important relevant costs and consequences for each alternative identified? | Yes? | No? |
| 5. Were costs and consequences measured accurately in appropriate physical units? | Can’t tell | Can’t tell |
| 6. Were costs and consequences valued credibly? | Yes? | Can’t tell |
| 7. Were costs and consequences adjusted for differential timing? | No | No |
| 8. Was an incremental analysis of costs and consequences of alternatives performed? | Can’t tell | Yes |
| 9. Was allowance made for uncertainty in the estimates of costs and consequences? | Yes? | No |
| 10. Did the presentation and discussion of study results include all issues of concern to users? | Can’t tell | Can’t tell |
| Comments | 1. NHS viewpoint, although not stated explicitly | 1. NHS viewpoint, although not stated explicitly |
| 6. Source for costs cited | 6. Source of cost data not reported | |
| 7. But probably not relevant due to short time frame | 7. But probably not relevant due to short time frame |
Quality assessment using the QUADAS tool for diagnostic studies (Cochrane Collaboration version)
| Goldberg et al. 2003230 | Grubel 2011231 | Laine et al. 1998233 | Thoomes-de Graaf et al. 2014236 | Varonen et al. 2003237 | |
|---|---|---|---|---|---|
| 1. Was the spectrum of patients representative of the patients who will receive the test in practice? (Representative spectrum) | Yes | Yes | Unclear | Unclear | Unclear |
| 2. Is the reference standard likely to classify the target condition correctly? (Acceptable reference standard) | Yes | Yes | Yes | Yes | Yes |
| 3. Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? (Acceptable delay between tests) | Unclear | Yes | Yes | Yes | Yes |
| 4. Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? (Partial verification avoided) | Yes | No | Yes | Yes | No |
| 5. Did patients receive the same reference standard irrespective of the index test result? (Differential verification avoided) | Yes | Unclear | Yes | Yes | Yes |
| 6. Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? (Incorporation avoided) | Yes | Yes | Yes | Yes | Yes |
| 7. Were the reference standard results interpreted without knowledge of the results of the index test? (Index test results blinded) | Yes | Unclear | Unclear | Yes | Yes |
| 8. Were the index test results interpreted without knowledge of the results of the reference standard? (Reference standard results blinded) | Yes | Yes | Yes | Yes | Yes |
| 9. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? (Relevant clinical information) | Yes | Yes | Yes | Yes | Yes |
| 10. Were uninterpretable/intermediate test results reported? (Uninterpretable results reported) | Yes | Yes | Yes | Yes | Yes |
| 11. Were withdrawals from the study explained? (Withdrawals explained) | Yes | Yes | Yes | Yes | Yes |
| Comments | 10. There seem to be no uninterpretable results | 3. Reference test within 1 month | 1. Some potential patients did not consent | 1. Patients were trial participants | 1. Patients were trial participants |
| 11. All patients included in analysis | 4/5. Verification a major issue for this study, which is not a standard diagnostic study | 2. Radiologist finding treated as reference standard | |||
| 10. There seem to be no uninterpretable results or withdrawals | 10. No uninterpretable results? |
Appendix 8 Companies providing diagnostic ultrasound services
Appendix 9 Search strategies and related information for Chapter 6
Table of results
Searches performed: 3 July 2015.
| Results | |
|---|---|
| MEDLINE | 786 |
| EMBASE | 1624 |
| Cochrane CDSR | 27 |
| Cochrane CENTRAL | 198 |
| Cochrane DARE | 1 |
| Cochrane NHS EED | 2 |
| Cochrane HTA | 1 |
| CINAHL | 125 |
| Web of Science | 678 |
| Total | 3442 |
| Total after duplicates have been removed | 2075 |
Search strategies
MEDLINE
-
primary care.tw. (77,439)
-
general practi$.tw. (64,660)
-
primary health care.tw. (15,642)
-
Community Mental Health Services/ (16,875)
-
Family Practice/ (60,364)
-
Home Care Services/ (28,354)
-
Physicians, Family/ (14,826)
-
Community Health Services/ (27,489)
-
Community Health Nursing/ (18,588)
-
Community Pharmacy Services/ (3045)
-
Community Health Workers/ (3475)
-
Preventive Health Services/ (11,290)
-
or/1-12 (274,163)
-
dyspnea/ (16,034)
-
dyspn?ea.ti,ab. (33,559)
-
breathless$.ti,ab. (3655)
-
(short$ adj2 breath).ti,ab. (5496)
-
(breath$ adj2 difficult$).ti,ab. (1558)
-
orthopnea.ti,ab. (431)
-
spirometry/ (17,974)
-
spirometry.ti,ab. (11,698)
-
respiratory care.ti,ab. (1548)
-
respiratory service$.ti,ab. (73)
-
(community adj3 respiratory).ti,ab. (1421)
-
or/14-24 (75,124)
-
diagnostic services/ (1783)
-
nursing diagnosis/ (3904)
-
diagnos$.ti,ab. (1,781,010)
-
mobile health units/ (3052)
-
mobile health unit$.ti,ab. (53)
-
mobile health clinic$.ti,ab. (23)
-
rapid access clinic$.ti,ab. (38)
-
one stop shop$.ti,ab. (376)
-
(point of care testing or point-of-care testing or POCT or near patient testing or near-patient testing).ti,ab. (1867)
-
or/26-34 (1,788,486)
-
13 and 25 and 35 (914)
-
limit 36 to yr=“2000 –Current” (786)
EMBASE
-
primary care.tw. (99,530)
-
general practi$.tw. (83,237)
-
primary health care.tw. (17,910)
-
general practice/ (72,724)
-
home care/ (50,319)
-
general practitioner/ (69,001)
-
community care/ (49,886)
-
community health nursing/ (25,766)
-
pharmacy/ (58,551)
-
health auxiliary/ (3607)
-
preventive health service/ (22,758)
-
or/1-11 (431,957)
-
dyspnea/ (99,727)
-
dyspn?ea.ti,ab. (51,589)
-
breathless$.ti,ab. (5393)
-
(short$ adj2 breath).ti,ab. (10,087)
-
(breath$ adj2 difficult$).ti,ab. (2495)
-
orthopnea.ti,ab. (910)
-
spirometry/ (29,794)
-
spirometry.ti,ab. (17,853)
-
respiratory care.ti,ab. (2182)
-
respiratory service$.ti,ab. (130)
-
(community adj3 respiratory).ti,ab. (1819)
-
or/13-23 (154,821)
-
nursing diagnosis/ (3767)
-
diagnos$.ti,ab. (2,425,045)
-
mobile health unit$.ti,ab. (50)
-
mobile health clinic$.ti,ab. (24)
-
rapid access clinic$.ti,ab. (128)
-
one stop shop$.ti,ab. (533)
-
(point of care testing or point-of-care testing or POCT or near patient testing or near-patient testing).ti,ab. (2840)
-
or/25-31 (2,429,314)
-
12 and 24 and 32 (1770)
-
limit 33 to yr=“2000 –Current” (1624)
The Cochrane Library
#1 (primary care):ti,ab,kw
#2 (“general practi*”):ti,ab,kw
#3 (primary health care):ti,ab,kw
#4 MeSH descriptor: [Community Mental Health Services] this term only
#5 MeSH descriptor: [Family Practice] this term only
#6 MeSH descriptor: [Home Care Services] this term only
#7 MeSH descriptor: [Physicians, Family] this term only
#8 MeSH descriptor: [Community Health Services] this term only
#9 MeSH descriptor: [Community Health Nursing] this term only
#10 MeSH descriptor: [Community Pharmacy Services] this term only
#11 MeSH descriptor: [Community Health Workers] this term only
#12 MeSH descriptor: [Preventive Health Services] this term only
#13 (or #30–#38)
#14 MeSH descriptor: [Dyspnea] this term only
#15 dyspn?ea:ti,ab
#16 breathless*:ti,ab
#17 (short* near/2 breath):ti,ab
#18 (breath* near/2 difficult*):ti,ab
#19 orthopnea:ti,ab
#20 MeSH descriptor: [Spirometry] this term only
#21 spirometry:ti,ab
#22 (respiratory care):ti,ab
#23 (respiratory service*):ti,ab
#24 (community near/3 respiratory):ti,ab
#25 (or #14–#24)
#26 MeSH descriptor: [Diagnostic Services] this term only
#27 MeSH descriptor: [Nursing Diagnosis] this term only
#28 diagnos*:ti,ab
#29 MeSH descriptor: [Mobile Health Units] this term only
#30 (mobile health unit*):ti,ab
#31 (mobile health clinic*):ti,ab
#32 (rapid access clinic*):ti,ab
#33 (one stop shop*):ti,ab
#34 (point of care testing or point-of-care testing or POCT or “near patient testing” or “near-patient testing”):ti,ab
#35 {or #26-#34}
#36 #13 and #25 and #35 Publication Year from 2000 to 2015
Cumulative Index to Nursing and Allied Health Literature
| S35 | S12 AND S24 AND S34 |
| S34 | S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 |
| S33 | TI ( “point of care testing” or “point-of-care testing” or “POCT” or “near patient testing” or “near-patient testing” ) OR AB ( “point of care testing” or “point-of-care testing” or “POCT” or “near patient testing” or “near-patient testing” ) |
| S32 | TI “one stop shop*” OR AB “one stop shop*” |
| S31 | TI “rapid access clinic*” OR AB “rapid access clinic*” |
| S30 | TI “mobile health clinic*” OR AB “mobile health clinic*” |
| S29 | TI “mobile health unit*” OR AB “mobile health unit*” |
| S28 | (MH “Mobile Health Units”) |
| S27 | TI diagnos* OR AB diagnos* |
| S26 | (MH “Nursing Diagnosis”) |
| S25 | (MH “Diagnostic Services”) |
| S24 | S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 |
| S23 | TI community N3 respiratory OR AB community N3 respiratory |
| S22 | TI “respiratory service*” OR AB “respiratory service*” |
| S21 | TI “respiratory care” OR AB “respiratory care” |
| S20 | TI spirometry OR AB spirometry |
| S19 | (MH “Spirometry”) |
| S18 | TI orthopnea OR AB orthopnea |
| S17 | TI (breath* N2 difficult*) OR AB (breath* N2 difficult*) |
| S16 | TI short* N2 breath OR AB short* N2 breath |
| S15 | TI breathless* OR AB breathless* |
| S14 | TI dyspn?ea OR AB dyspn?ea |
| S13 | (MH “Dyspnea”) |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 |
| S11 | (MH “Community Health Workers”) |
| S10 | (MH “Pharmacy Service”) |
| S9 | (MH “Community Health Nursing”) |
| S8 | (MH “Community Health Services” |
| S7 | (MH “Physicians, Family”) |
| S6 | (MH “Home Health Care”) |
| S5 | (MH “Family Practice”) |
| S4 | (MH “Community Mental Health Services”) |
| S3 | TI “primary health care” OR AB “primary health care” |
| S2 | TI “general practi*” OR AB “general practi*” |
| S1 | TI “primary care” OR AB “primary care” |
Web of Science
| # 32 | 678 | #31 AND #23 AND #13 Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 31 | 1,181,490 | #30 OR #29 OR #28 OR #27 OR #26 OR #25 OR #24 Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 30 | 2033 | TS=(“point of care testing” or “point-of-care testing” or POCT or “near patient testing” or “near-patient testing”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 29 | 449 | TS=(“one stop shop*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 28 | 53 | TS=(“rapid access clinic*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 27 | 13 | TS=(“mobile health clinic*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 26 | 29 | TS=(“mobile health unit*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 25 | 29 | TS=(“mobile health unit*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 24 | 1,179,784 | TS=(diagnos*) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 23 | 19,976 | #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 22 | 1726 | TS=(community NEAR/3 respiratory) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 21 | 57 | TS=(“respiratory service*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 20 | 628 | TS=(“respiratory care”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 19 | 8267 | TS=(spirometry) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 18 | 286 | TS=(orthopnea) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 17 | 916 | TS=(breath* NEAR/2 difficult*) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 16 | 2952 | TS=(short* NEAR/2 breath) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 15 | 2324 | TS=(breathless*) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 14 | 3913 | TS=(dyspn?ea) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 13 | 113,080 | #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 12 | 541 | TS=(“Preventive Health Service*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 11 | 1585 | TS=(“Community Health Worker*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 10 | 169 | TS=(“Community Pharmacy Service*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 9 | 243 | TS=(“Community Health Nursing”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 8 | 561 | TS=(“Community Health Service*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 7 | 6628 | TS=(“family physician*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 6 | 874 | TS=(“Home Care Service*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 5 | 3730 | TS=(“Family Practice*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 4 | 567 | TS=(“Community Mental Health Service*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 3 | 10,717 | TS=(“primary health care”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 2 | 37,362 | TS=(“general practi*”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
| # 1 | 73,830 | TS=(“primary care”) Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan=2000-2015 |
Appendix 10 Quality assessment tables for Chapter 6
Tool for assessing the quality of intervention studies
| Potential risk of bias | Bias present? | Detail of concerns |
|---|---|---|
| 1. Selection bias: method used to generate the allocation sequence, method used to conceal the allocation sequence, characteristics of participant group(s) | Yes/no/unclear | |
| 2. Performance bias: measures used to blind participants and personnel and outcome assessors, presence of other potential threats to validity | Yes/no/unclear | |
| 3. Attrition bias: incomplete outcome data, high level of withdrawals from the study | Yes/no/unclear | |
| 4. Detection bias: accuracy of measurement of outcomes, length of follow-up | Yes/no/unclear | |
| 5. Reporting bias: selective reporting, accuracy of reporting | Yes/no/unclear |
Tool for assessing cohort studies
| 1. Did the study address a clearly focused question/issue? | ||
| 2. Is the research method (study design) appropriate for answering the research question? | ||
| 3. Were there enough subjects (employees, teams, divisions, organisations) in the study to establish that the findings did not occur by chance? | ||
| 4. Was the selection of the cohort/panel based on external, objective and validated criteria? | ||
| 5. Was the cohort/panel representative of a defined population? | ||
| 6. Was the follow up of cases/subjects long enough? | ||
| 7. Were objective and unbiased outcome criteria used? | ||
| 8. Are objective and validated measurement methods used to measure the outcome? | ||
| 9. Is the size effect practically relevant? | ||
| 10. How precise is the estimate of the effect? Were CIs given? | ||
| 11. Were reasonable attempts made to account for confounding factors? | ||
| 12. Can the results be applied to other settings? |
Tool for assessing the quality of survey studies
| Did the study address a clearly focused research question? | ||
| Is the study design appropriate for answering the research question? | ||
| Is the method of selection of the subjects clearly described? | ||
| Was the way the sample was obtained likely to be free of selection bias? | ||
| Was the sample of subjects representative with regard to the population to which the findings will be referred? | ||
| Was the sample size based on pre-study considerations of statistical power? | ||
| Was a satisfactory response rate achieved? | ||
| Are the measures (questionnaires) likely to be valid and reliable? | ||
| Was the statistical significance assessed? | ||
| Are CIs given for the main results? | ||
| Were reasonable attempts made to account for confounding factors? | ||
| Can the results be applied to other settings? |
Quality appraisal tool for qualitative studies
| 1. Was there a clear statement of the aim of the research? | Yes/no |
| 2. Is a qualitative methodology appropriate to address the aims of the research? | Yes/no |
| 3. Was the recruitment strategy appropriate to the aims of the research? | Yes/no/unclear |
| 4. Were the data collected in a way that addressed the research issue? | Yes/no/unclear |
| 5. Has the relationship between researcher and participant been adequately considered? | Yes/no |
| 6. Have ethical issues been taken into account? | Yes/no/unclear |
| 7. Was the data analysis sufficiently rigorous? | Yes/no |
| 8. Is there a clear statement of findings? | Yes/no |
Assessment of intervention studies (randomised and non-randomised controlled trials and before-and-after studies)
| Study and year | Selection bias | Performance bias | Attrition bias | Detection bias | Reporting bias | Other concerns |
|---|---|---|---|---|---|---|
| Akhtar and Wilson 2005261 | No | No Blinding not appropriate |
Yes | No | No | Non-randomised design Higher dropout from pulmonary function lab ‘first’ group |
| Burgos 2011264 | Yes | No Blinding not appropriate |
No | No | No | No information on method of allocation or concealment. Some baseline differences |
| Burri et al. 2012265 | Yes | No Blinding not appropriate |
No | No | No | Some baseline differences (p-values not given) |
| Carr et al. 2011267 | No | No Blinding not appropriate |
No | No | No | |
| Lusardi et al. 2006279 | Unclear | No Blinding not appropriate |
Yes | No | No | No reporting of groups at baseline 135/333 completed: no reporting of differences |
| Masa et al. 2011280 | Yes | No Blinding not appropriate |
No | No | No | No information on method of allocation or concealment Age difference between groups at baseline |
| Poels et al. 2006284 | No | No | No | No | No | |
| Tomonaga et al. 2011288 | Unclear | No Blinding not appropriate |
No | No | No | No information on method of allocation or concealment |
Assessment of cohort study
| Study and year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Comments/concerns |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Van der Mark et al. 2014289 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Assessment of cross-sectional studies (including service data analyses)
| Study and year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Comments/concerns |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borg et al. 2010263 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | No | Yes | Yes | |
| Cawley et al. 2011268 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | No | Yes | Yes | |
| Chavannes et al. 2004269 | Yes | Yes | Yes | No | Yes | NR | NR | Yes | Yes | Yes | Yes | Yes | Selection bias: GPwSIs in spirometry |
| Hassett et al. 2006274 | Yes | Yes | No | No | Yes | No | NA | Yes | Yes | No | Yes | Yes | 16 of 33 practices ‘selected’ (unclear) |
| Jones et al. 2005275 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | 7 of 14 practices declined |
| Lodewijks-van der Bolt et al. 2007278 | Yes | Yes | No | No | Yes | No | NA | Yes | Yes | No | Yes | Yes | Not clear how the sample was obtained |
| Starren et al. 2012182 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | No | Yes | Yes | |
| Thijssing et al. 2014287 | Yes | Yes | Yes | Yes | No | No | NA | Yes | No | No | Yes | Yes | All GPs had spirometer linked to computer |
| van Heur et al. 2010105 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | No | Yes | Yes | |
| Walker et al. 2006290 | Yes | Yes | No | Yes | Yes | No | NA | Yes | Yes | No | Yes | Yes | |
| White et al. 2007187 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | Yes | Yes | Yes | Convenience sample |
| Wolfenden et al. 2009292 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | No | No | Yes | Yes |
Quality assessment of level 1 and level 2 papers
| Study and year | Clear statement of the aims | Qualitative methodology appropriate | Recruitment strategy appropriate | Data collected to address research issue | Relationship researcher/participant | Ethical issues | Rigour of data analysis | Clear statement of findings | Details/comments |
|---|---|---|---|---|---|---|---|---|---|
| Level 1 | |||||||||
| Roberts et al. 2011197 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Low |
| Level 2 | |||||||||
| Armstrong et al. 2012293 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Low |
| Birt et al. 2014294 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Low |
| Corner et al. 2005295 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Low |
| Corner et al. 2006296 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Low |
| Dennis et al. 2010297 | Yes | Yes | Yes | Yes | No | Unclear | Yes | Yes | Low |
| Goeman et al. 2005298 | Yes | Yes | Yes | Yes | No | Yes | Unclear | Yes | Low |
| Joo et al. 2013198 | Yes | Yes | Yes | Yes | No | Unclear | Unclear | Yes | Some aspects are not reported in sufficient detail |
| Khunti et al. 200282 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Low |
| Tod et al. 2008299 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Low |
| Walters et al. 2005300 | Yes | Yes | Yes | Yes | No | No | Unclear | Yes | Brief summary paper |
| Walters et al. 2008301 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | This paper reports the same study as Walters et al. 300 |
Appendix 11 Search strategies for Chapter 4
Stage 1
| Setting | Primary Care or General Practice or Community Care |
| Intervention | Diagnostic Techniques and Procedures; Diagnostic Services; Diagnostic Tests or |
| One of the following modalities: | |
| Audiology; Point of Care Testing; Cardiac Services; ECG; Echocardiography; Diabetic Services; Endoscopy; Genetic Testing | |
| Laboratory Tests; Magnetic Resonance Imaging; Radiology/X-Ray | |
| Respiratory Tests; Ultrasound | |
| ‘direct access imaging’ ‘direct access mri’ ‘rapid access cardiology’ | |
| Factors/considerations | Barrier$or Facilitator$or Logistic$or Feasib$OR/organisation & administration |
Stage 2
| Domain | Concepts | Search terms |
|---|---|---|
| Setting | Primary Care or General Practice or Community Care | Family Practice |
| UK | Great Britain | |
| Modalities | Audiology | exp Diagnostic Techniques, Otological |
| Cardiac Services | exp Diagnostic Techniques, Cardiovascular | |
| ECG | ||
| Echocardiography | exp Echocardiography | |
| Diabetic Services | ||
| Endoscopy | exp Endoscopy | |
| Genetic Testing | exp Genetic Testing | |
| Laboratory Tests | ||
| Magnetic Resonance Imaging | exp Magnetic Resonance Imaging | |
| Point of Care Testing (haemoglobin A1c (HbA1c) and urine albumin: creatinine ratio (ACR) on patients with diabetes, total cholesterol, triglyceride and high density lipoprotein cholesterol on patients with hyperlipidaemia, and INR on patients on anticoagulant therapy) | Point-of-Care Systems | |
| Radiology/X-Ray | exp Radiography | |
| Respiratory Tests | exp Diagnostic Techniques, Respiratory System | |
| Ultrasound | exp Ultrasonography | |
| Factors/considerations | Skills & expertise: Skill mix; Extended roles; Inappropriate Test Ordering | Physician’s Practice Patterns |
| Training: Training Needs; Training Costs; Duration | ||
| Equipment: Equipment for modality and for analysis; consumable costs | Diagnostic Equipment Equipment Safety Equipment Design Equipment Failure Equipment Failure Analysis Maintenance |
|
| Premises: Cost of Premises; Health & Safety | ||
| User perspective: Waiting Times; Acceptability; Repeat Procedures | ||
| Primary–secondary interface: Referrals, Changes to Diagnosis or Management Pathways | /utilisation |
Appendix 12 Horizon Scanning reports
Horizon Scanning reports (http://madox.org/horizon-scanning-report) are compiled on priority technologies for specific clinical questions. They provide an overview of emerging technologies and related clinical advantages with specific reference to patient group and use, assessing importance and documenting previous research. The reports also identify the outstanding research questions as well as expected outcomes.
Each report is evaluated by a panel of experts and more detailed secondary reports are compiled on selected technologies. Reports are disseminated to the NIHR HTA programme and commissioners.
-
A portable handheld electronic nose in the diagnosis of cancer, asthma and infection.
-
Alternative sampling methods for collection of urine specimens in older adults.
-
Autoimmune markers for the diagnosis of rheumatoid arthritis in primary care.
-
Automated lung sound analysis for asthma.
-
Dermoscopy for the diagnosis of melanoma in primary care.
-
Diagnostic technology: genotyping polymorphisms affecting warfarin metabolism.
-
Diagnostic technology: handheld ECG monitors for the detection of AF in primary care.
-
Estimating central blood pressure and arterial stiffness in primary care using non-invasive automated pulse wave analysis.
-
Handheld HeartScan ECG monitor for detecting atrial fibrillation in primary care.
-
Handheld nerve conduction measurement devices for carpal tunnel syndrome.
-
iPhone, iPod and iPad (Apple Inc., Cupertino, CA, USA) add-on or plug-in medical devices.
-
Non-contact infrared thermometers.
-
POC blood test for ketones in diabetes patients.
-
POC B-type natriuretic peptide testing.
-
POC international normalised ratio coagulometers for self-management of oral anticoagulation.
-
POC test for CRP.
-
POC test for HbA1c.
-
POC test for cardiac troponin.
-
POC test for procalcitonin to improve the early diagnosis of serious bacterial infections in patients presenting in primary care.
-
POC test for the analysis of lipid panels.
-
POC test for total white blood cell count.
-
POC testing for coeliac disease.
-
POC testing for hepatitis C virus.
-
POC testing for thyroid-stimulating hormone.
-
POC tests for influenza in children.
-
POC urine albumin-to-creatinine ratio test for the early detection and management of renal disease and as a risk factor for cardiovascular disease.
-
Pulse oximetry in primary care.
-
Screening instruments for frailty in primary care.
-
Spirometry in primary care for COPD case finding, self-monitoring and remote technology.
-
Testing for Chlamydia trachomatis in primary care using self-taken swabs.
-
The D-dimer test for ruling out deep-vein thrombosis in primary care.
-
Transcutaneous bilirubin measurement.
Appendix 13 Data extraction tables for Chapter 6
Intervention papers
| Study (authors and year) | Akhtar and Wilson 2005261 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Comparative study. Non-randomised |
| Country | UK (Leicester) |
| Setting | Primary care |
| Training/equipment | |
| Staff involved | |
| Research question/objectives | To compare the accuracy of nurse use of spirometry vs. a centralised laboratory |
| Research methods | Forty-five patients were assessed at both their local practice by a nurse and a pulmonary function laboratory. The results obtained were compared |
| Results/data | Practice nurses underestimated FEV1 and FVC. The mean difference in FEV1 was 0.109 l (6.69%, 95% CI 2.88% to 9.51%) compared with a bellows spirometer, and 0.07 l (6.2%, 95% CI 0.89% to 8.25%) when the same type of spirometer was used. The mean difference in FVC was 0.413 l (15.0%, 95% CI 9.3% to 20.6%) when compared with bellows, and 0.267 l (10.2%, 95% CI 4.1% to 16.2%) when the same type of spirometer was used. All differences were statistically significant (p < 0.05). Agreement on categorisation of COPD was moderate (kappa 0.46) with practice nurses overestimating severity. Compared with Pulmonary Function Laboratory categorisation for the presence or absence of COPD using bellows spirometers, the sensitivity of practice nurse spirometry was 0.93 (95% CI 0.76 to 0.99) and specificity was 0.65 (95% CI 0.38 to 0.86) |
| Main findings | Spirometry results obtained by practice nurses were lower than those obtained in a specialised laboratory, with the potential for overdiagnosis of COPD severity |
| Comments |
| Study (authors and year) | Borg et al. 2010263 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | Australia |
| Setting | Rural health facilities |
| Training/equipment | A 14-hour spirometry course facilitated by respiratory scientists with at least 5 years’ experience |
| Staff involved | Nurses, physiotherapists |
| Research question/objectives | To determine whether or not a 14-hour course provides sufficient skill to obtain valid results and whether or not follow-up training improves validity |
| Research methods | Participants underwent follow-up assessments against ATS criteria 5, 7 and 9 months after the initial course. Further education was provided after the reviews at 5 and 7 months |
| Results/data | Fifteen participants from 10 sites completed the three reviews. Adherence to ATS criteria was 40% at 5 months, 67% at 7 months and 87% at 9 months. Retrospective review found that 37%, 60% and 58% of tests at 5, 7 and 9 months, respectively, met ATS criteria and had correctly selected the best test |
| Main findings | Authors concluded that a 14-hour spirometry course does not provide sufficient skill to perform spirometry to ATS criteria and short-term follow-up is important for improving test validity |
| Comments |
| Study (authors and year) | Burgos 2011264 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Comparative study 18 primary care centres: 12 randomised to intervention arm and 6 to control arm |
| Country | Spain |
| Setting | Primary care |
| Training/equipment | Nurses received 2 days’ training which included performing ‘several manoeuvres’ and discussion on standardisation. Nurses in intervention arm in addition had access to a website with educational content and via this able to ask questions of and receive feedback from a co-ordinator regarding the quality of spirometry Disposable and pre-calibrated pneumotachograph-based spirometer (Datospir 110) or an ultrasound transit time-based spirometer (EasyOne) used, connected to a computer. The computer program used was one module of an information and communication technology platform used to manage chronic patients |
| Staff involved | Thirty-four nurses, five co-ordinators and three telecommunication engineers |
| Research question/objectives | To evaluate a web-based application |
| Research methods | A total of 4581 patients with respiratory symptoms identified by 150 GPs and tested with spirometry by nurses who had access to web application or not. Acceptability of intervention reported by GPs and usability rated by nurses |
| Results/data | Over a 12-month period the intervention group presented 71.5% high-quality spirometries, the control group 59.5% high-quality tests (p < 0.001). No change over time for the intervention group whereas the quality in the control group reduced between months 1 and 12 (p = 0.011). The intervention nurses also carried out more spirometry tests over the study period. 97% of GPs rated the web functionality as acceptable, 26% reported problems of implementation. Nurse scores for usability over 50 for efficiency, effect, helpfulness and learning-ability |
| Main findings | The intervention increased the proportion of high-quality spirometry tests (by around 20%) and decreased the percentage of very low-quality spirometries. The application was perceived to be acceptable and useful |
| Comments |
| Study (authors and year) | Burri et al. 2012265 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Comparative study RCT |
| Country | Switzerland |
| Setting | Primary care |
| Training/equipment | BNP measured via collecting venous blood sample in a tube containing a rapid fluorescence immunoassay (from Biosite Diagnostics). All GPs were ‘repeatedly trained’ in the most appropriate use of BNP levels |
| Staff involved | No detail of who provided the training |
| Research question/objectives | To evaluate whether or not adding BNP testing in primary care would improve the evaluation and diagnosis of patients with breathlessness |
| Research methods | 323 patients seen by 29 primary care physicians: 160 in the control arm (conventional diagnosis) and 163 in the additional BNP intervention arm |
| Results/data | There was less further diagnostic testing in the BNP group (33% vs. 45% of patients p = 0.02). The time to initiation of appropriate treatment (associated with less further testing) reduced by 12 days in the BNP group (p = 0.01). In the BNP group 66% of patients began appropriate treatment on the day of presentation vs. 53% in the control group (p = 0,02). The number of hospitalisations for dyspnoea, the number of days in hospital or the number of outpatient visits were not significantly different between the groups at 3 or 12 months. There was no difference in severity of dyspnoea at 3 months; both groups had improved |
| Main findings | The use of BNP in primary care improved diagnostic certainty and time to initiation of treatment but appeared to have few benefits for service delivery costs at 3 or 12 months |
| Comments |
| Study (authors and year) | Carr et al. 2011267 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Before-and-after study |
| Country | UK (England) |
| Setting | Primary care (one general practice) |
| Training/equipment | Educational intervention involving a presentation by a GPwSI in respiratory medicine, a visit from a local secondary care consultant and a visit from a secondary care specialist nurse |
| Staff involved | GPs, nurses |
| Research question/objectives | To assess the impact of an educational intervention on the quality of spirometry |
| Research methods | Retrospective audit followed by re-audit following educational intervention |
| Results/data | Forty-five spirometry reports were assessed in each audit. Before the intervention, 17/45 (38%) post-bronchodilator spirometry tests were technically flawed. After the intervention, 1/44 (2%) was flawed. Chest clinic referrals fell from 28/45 (62%) to 14/44 (32%) |
| Main findings | The authors concluded that technical quality of spirometry can be audited and in-house education can significantly reduce spirometry errors with a possible follow-on effect on referrals |
| Comments |
| Study (authors and year) | Cawley et al. 2011268 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Retrospective cohort, patients who attended clinic 2008–10 |
| Country | USA |
| Setting | Spirometry clinic in a primary care physician office |
| Training/equipment | The spirometer Workstation software and flow transducer. Checked and calibrated at the beginning of each day |
| Staff involved | A pharmacist who was a registered pulmonary therapist and certified pulmonary function technologist |
| Research question/objectives | What was the added value of a pharmacist-led spirometry clinic? |
| Research methods | Patients > 8 years of age with cough, shortness of breath, pulmonary diagnosis or other pulmonary symptoms referred by physicians in the primary care clinic or other physicians outside the clinic for spirometry testing. Testing was carried out by a clinical pharmacist. The pharmacist also carried out patient interview, performed pulse oximetry, recommended pharmacological intervention, provided drug education and smoking cessation literature |
| Results/data | Estimated each patient would require a 1 hour 15 minutes appointment to carry out testing. Cost calculated as Medicare reimbursement fee = US$85.80 per patient. A total of 51 patients attended appointments offered (of 65 scheduled). Subsequent physician consultation or further testing was required for 27.4% of patients |
| Main findings | Authors report study demonstrates that pharmacists can accurately perform spirometry and optimise pharmaceutical care for patients, although only data relating to this are number requiring further services |
| Comments | Data mostly relate to characteristics of patients and describes the medications required, with few regarding accuracy or impact of clinic |
| Study (authors and year) | Chavannes et al. 2004269 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Cross-sectional |
| Country | Netherlands |
| Setting | Primary care |
| Training/equipment | 2 × 3-hour sessions of training with an interval of 1 month |
| Staff involved | 39 GPs with an interest in spirometry and involved in vocational training |
| Research question/objectives | To compare the achievements of trained GPs in spirometric diagnosis with an expert consensus panel (1) and to assess the influence of spirometry on the GPs’ decision-making (2) |
| Research methods | Standardised case descriptions (× 12) developed from actual cases, designed by a GP and pulmonologist. The cases depicted a range of respiratory conditions as well as normal respiration and their typical flow–volume curves. Participating GPs worked through two sets of six cases each that were assessed in random order by a research assistant within a period of 1 year. Assessment followed presentation of patient history and physical examination data including FEV, FEV1/FVC and flow–volume curves. GPs had to select a diagnosis from a list |
| Prior to the assessment the 12 cases had been judged by an independent panel including pulmonologists and the judgments of two researchers were in 100% concordance with these. These judgements formed the ‘gold standard’ | |
| Clinical outcomes were analysed, also the impact of spirometry on GP decision-making | |
| SPSS: univariate analyses and logistical regression | |
| Results/data | Three GPs dropped out, one retired and two lost interest A total of 444 cases were assessed. Concordance with the expert panel in terms of obstruction was 91.3% (95% CI 86.8% to 95.8%). Normal spirometry obtained 77.9% (95% CI 70.2% to 85.6%) correct answers, incorrect manoeuvres reached a score of 64.9% (95% CI 54.0% to 75.8%), and rare pathological curves were recognised in 41.3% (95% CI 32.1% to 50.5%) of cases. Positive predictive values (probability of righteously labelling disease) revealed a range of values between 0.87 (normal curves) and 0.49 (rare pathology) The number of diagnoses considered by the GPs reduced following the use of spirometry, creating less uncertainty (from a mean of 2 diagnoses per case, range 1–8 to a mean of 1.35, range 1–6). More than one diagnosis was considered in 59.6% (95% CI 55.1% to 64.1%) of cases before spirometry, whereas after spirometry more than one diagnosis was considered in 31.2% (95% CI 26.9% to 35.5%) of cases (odds ratio 0.266, 95% CI 0.200 to 0.353). The probability of referral changed from 6.0% (95% CI 3.8% to 8.2%) to 31.7% (95% 27.4% to 36.0%) as a result of spirometry (odds ratio 7.26, 95% CI 4.71 to 11.2) |
| Main findings | There was a reduction in the number of alternative diagnoses but an increase in referral rates and diagnostic prednisolone courses. The establishing of a diagnosis in patients with respiratory morbidity may have improved, but this probably leads to an increased use of additional diagnostic procedures or specialist care, at least initially. Trained GPs differentiated between normal and obstructive disease patterns, while indicators of rare and mixed pathology were often missed |
| Comments |
| Study (authors and year) | Hassett et al. 2006274 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Cross-sectional – analysis of referral patterns, questionnaire to GPs and patients |
| Country | UK (London) |
| Setting | A community respiratory assessment unit. The unit was based in a hospital adjacent to a respiratory nursing service by was described as a ‘community service’ and also provided peripatetic service to practices furthest away from base |
| Training/equipment | Nurses trained at Education for Health centre in use of spirometry. GPs sent educational materials on causes of breathlessness, asthma guidelines and aids to differential diagnosis. The nurses also visited each practice. Chairperson of PCT also wrote to GPs about the service |
| Staff involved | 2 × specialist nurses with experience in respiratory medicine, assistance provided by GP colleagues and a local respiratory consultant |
| Research question/objectives | To evaluate a community respiratory assessment unit service |
| Research methods | 16 GP practices initially included, with 17 added 6 months later. GPs referred patients for patients diagnosed or with suspected COPD or asthma or for unexplained cough or breathlessness. Analysis of referral data and satisfaction survey to GPs and patients |
| Results/data | 364 patients referred in first 12 months of service, age range 18–90 years, 36% smokers. Significant differences in referral rates between practices. Most frequent reason for referral was suspected or definite COPD (57% of referrals). One-quarter of these patients had no abnormalities detected. 28% of referrals were for definite or suspected asthma, with 34% of these having airway narrowing detected |
| There was a 48% response rate to questionnaire (n = 41), all but two GPs had used the service, 51% satisfied and 46% very satisfied with service, 88% felt patients had benefited from service. GPs reported that 58% of cases would have been referred to a specialist hospital respiratory outpatient clinic if the unit did not exist, 53% would have had a trial of therapy (answers not mutually exclusive). Patient satisfaction high (99% rated experience of unit as good, very good or excellent) | |
| Main findings | The reason for the referral (suspected COPD/asthma) was often not confirmed on assessment. Satisfaction rates for the unit were high |
| Comments | The paper presents data on average referral rates over the time frame of the unit’s existence; however, it provides no analysis |
| Study (authors and year) | Jones et al. 2005275 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Cross-sectional data |
| Country | UK (England: Plymouth) |
| Setting | GP practices |
| Training/equipment | Practice staff education (spirometry, data interpretation, COPD management). Spirometry clinics offered bronchodilator and steroid reversibility assessments |
| Staff involved | Trained respiratory nurse specialist experienced in practice nursing and a GP with a ‘respiratory special interest’ |
| Research question/objectives | To evaluate a mobile spirometry service |
| Research methods | Practices that were known to own a spirometer were offered practice staff education and/or spirometry clinics |
| Results/data | Fourteen practices randomly selected from those known to have a spirometer; seven accepted the service. Of those who declined, three reported already well organised, three failed to respond and one quoted time restraint issues. Total cost of the study was £6189 after deduction of set-up expenses of £107 per new patient diagnosed with COPD A total of 98 patients assessed during study; only six found to have normal lung function |
| Main findings | The service enabled the diagnosis and treatment of many new cases of COPD |
| Comments | Limited data (e.g. reported high satisfaction with service but no data) |
| Study (authors and year) | Lodewijks-van der Bolt et al. 2007278 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Analysis of referrals 2002–6 |
| Country | The Netherlands |
| Setting | Echocardiography service in a local hospital |
| Training/equipment | Hospital-based equipment |
| Staff involved | Staff in echocardiography unit |
| Research question/objectives | To evaluate an open-access echocardiography service |
| Research methods | Patients referred to the open access service by GPs following examination, 107 of 471 patients referred for dyspnoea. Echocardiography carried out without referral to a cardiologist |
| Results/data | No cardiac abnormality was found in 28% of patients |
| Main findings | The service identified around one-quarter of patients with no cardiac abnormality who may otherwise have been referred to a cardiologist (no data, however, making this comparison) |
| Comments | The paper predominantly contains clinical data regarding the characteristics of patients referred |
| Study (authors and year) | Lusuardi et al. 2006279 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Comparative study. RCT |
| Country | Italy |
| Setting | Primary care |
| Training/equipment | Two educational meetings of 4 hours each provided to GPs by ‘reference specialists’. Portable electronic spirometer (Spirobank Office) |
| Staff involved | GPs and pulmonary specialists |
| Research question/objectives | To assess the value of consultations including spirometry |
| Research methods | A total of 57 centres and 570 GPs were recruited to either the standard consultation arm of the study or consultation using spirometry intervention arm. Following the consultations referrals were sent to pulmonary specialists (with the GP diagnosis/intervention removed). The GP diagnosis and specialist diagnosis were then compared to calculate the rate of agreement for each group. The main symptoms at the moment of the consultation were cough in the majority of patients, followed by dyspnoea, wheezing and chest tightness |
| Results/data | Of the 224 patients with one or more respiratory symptoms assessed, there was agreement on diagnosis between GPs and specialists in 78.6% of cases in the intervention group compared with 69.2% in the standard evaluation group (p = 0.35). In the intention-to-treat analysis, the respective percentages of concordant diagnosis were 57.9 and 56.7 (p = 0.87). The average time for each visit was 14 ± 5.2 minutes; the mean time required to instruct patients for spirometry was 5.6 ± 3.1 minutes; the performance of spirometry took on average 6.4 ± 3.5 minutes. Spirometry findings in the normal range were obtained for 61.8% of patients. A diagnosis of asthma was made by GPs in 32.1% of patients and COPD in 29.1% |
| Main findings | There was no significant difference between diagnostic accuracy for GPs who had access to spirometry and those who did not. The authors associated this with the study being underpowered. The authors note that regular use of spirometry tended to decrease progressively within a few months if there are no reinforcing recalls or retraining, despite most GPs rating spirometry as useful |
| Comments | The study had issues with recruitment and randomisation |
| Study (authors and year) | Masa et al. 2011280 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Comparative study, randomised crossover |
| Country | Spain |
| Setting | Online community spirometry (patient in primary care has consultation with pulmonary specialist in hospital pulmonary function laboratory) |
| Training/equipment | Spirometer VMax 20, spirometry performed in line with recommended standards/guidelines. Software analysed if end-of-test criteria had been met. Calibration checks carried out. Technician remotely controlled the computer that had the spirometer software installed (VMax SensorMedic) using another computer with VNC Free Edition software. Two additional computers with webcam used for teleconference |
| Staff involved | Two technicians with 15 years each experience carried out the spirometry, consultations scheduled to take 20 minutes |
| Research question/objectives | To assess the value of online spirometry |
| Research methods | Patients aged 14–74 years, entered spirometer room alone, asked to sit in front of computer and initiate the spirometer under instruction via teleconference. One in five patients also had conventional spirometry performed in person by technician |
| Results/data | Data for 261 of 283 patients; only five patients were unable to complete the spirometry successfully. Most patients were smokers or ex-smokers with generally mild dyspnoea. Online vs. conventional difference intention-to-treat analysis: FVC, p = 0.492; FEV, p = 0.211; FEV/FVC%, p = 0.466 |
| Main findings | Online spirometry values were not significantly different from those obtained via conventional procedures |
| Comments |
| Study (authors and year) | Poels et al. 2008284 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Comparative study. Cluster RCT |
| Country | The Netherlands |
| Setting | Primary care |
| Training/equipment | Spirometry expert system (SpidaXpert). Provides levels of FEV1/FVC and FEV1 and also a textual interpretation which provides information on and suggestions for additional testing/treatment options |
| Staff involved | An expert panel |
| Research question/objectives | To investigate the effect of supplementing test results with additional information on GP decision-making |
| Research methods | A total of 78 GPs visited by a research assistant and given a presentation with a number of case studies to work through to discuss their diagnosis and management. First, only medical history and medications presented for GP to decide diagnosis/management; second, GPs received the spirometry test results and were asked to reconsider diagnosis and management in light of this information. Intervention group GPs received spirometry test results, flow–volume curve, graphical curve and textual notes. GPs in control group received test results, flow–volume curve and volume–time curve (sham/placebo information) only. An expert panel of two chest physicians, a GP with specific expertise in spirometry and a health scientist agreed diagnosis for the case studies used |
| Results/data | There was no difference between the groups in terms of agreement between GP diagnosis and expert panel diagnosis for COPD odds ratio 1.08 (95% CI 0.7 to 1.66), asthma odds ratio 1.13 (95% CI 0.7 to 1.8), absence of respiratory disease (odds ratio 1.32, 95% CI 0.61 to 2.86) |
| Main findings | Additional information (support) provided by a computerised system had no detectable benefit on GP accuracy of diagnosis |
| Comments |
| Study (authors and year) | Starren et al. 2012182 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Four years of data from operation of service |
| Country | UK (London) |
| Setting | Community respiratory assessment unit based in a hospital |
| Equipment/training | Spirometry carried out following national and international guidance. Calibration completed on a weekly basis and cleaning on a daily basis |
| Staff involved | Was staffed by a specialist respiratory nurse for first 2 years, subsequently run by a community respiratory nursing team. Team had access to a professor of respiratory medicine/consultant in integrated respiratory medicine |
| Research question/objectives | To audit a centralised community respiratory service |
| Research methods | All local GPs had access to the service |
| Results/data | A total of 1156 referrals were received over the 4 years (range 217–348 per year). 65% of patients referred smoked. 162 did not attend and 30 could not complete the spirometry assessment. GPs of 49% of newly referred patients stated they would have referred to a specialist outpatient clinic if the service had not been available. 28 of the 32 practices used the service. Half of those referred for suspected COPD were confirmed as having it (138 of 265). 79 were referred for unexplained breathlessness: 41 of these were diagnosed as having non respiratory cause, 14 had a restrictive defect, 6 were diagnosed with COPD, asthma could not be excluded in 17, and for one no explanation for symptoms could be found |
| Main findings | The community respiratory assessment unit reduced diagnostic inaccuracy. Approximately one-third of COPD diagnoses made in the community were incorrect with potentially high levels of inappropriate prescribing. The study was unable to draw meaningful conclusions regarding asthma referrals |
| Comments | The same service as the Hassett et al.274 study. However, although it references this study it does not say that it is linked and the authors are different |
| Study (authors and year) | Thijssing et al. 2014287 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Cross-sectional data |
| Country | The Netherlands |
| Setting | GP practices |
| Training/equipment | KSYOS TeleConsultation system, GPs received 1 hour of on-site training to use if were not already using. Dedicated help desk available |
| Staff involved | A total of 158 GPs and 32 local pulmonologists |
| Research question/objectives | To evaluate a web-based teleconsultation service |
| Research methods | GPs who were in possession of a spirometer that could be linked to a computer were recruited. GPs could use telepulmonology consultation for any patients they considered suitable. GP completed patient medical history and uploaded four PDF files of the patient’s spirometry results via the teleconsultation system and sent to the local pulmonologist. Pulmonologist required to respond within 2 working days: e-mail/telephone reminders sent if this exceeded. Pulmonologist provided description of findings, diagnostic considerations, advice and asked any additional questions. Answered two mandatory questions – diagnosis COPD? Yes/no/unsure; is a visit to a specialist required? Yes/no/inapplicable. GP could send one more round of questions |
| Results/data | A total of 1958 consultations were sent by 158 GPs between April 2009 and November 2012. COPD diagnosed in 23%, unsure 16%, no 61%. The authors report that 69% of teleconsultations were made for the purpose of receiving advice, and of these 18% led to referral. They calculated that 31% of referrals were intended to prevent a physical referral, and 68% of prevented a referral (402 patients). Overall percentage of prevented referrals was calculated to be 27% |
| 92% of GPs indicated that they had learned from the pulmonologist’s response. Response was received an average of 18 hours after request sent | |
| Main findings | Teleconsultation was perceived to be a useful service by GPs and reduced numbers who might otherwise be referred |
| Comments | Calculation of referrals prevented based on total number of patients referred, divided by total GP self-report of whether they would have referred |
| Study (authors and year) | Tomonaga et al. 2011288 |
|---|---|
| Type of study/document | Empirical level 1 ✓ |
| Empirical level 2 | |
| Level 3 | |
| Study design | Comparative study, RCT |
| Country | Switzerland |
| Setting | Primary care |
| Training/equipment | A 3-in-1 POC testing analyser: a bedside Cardiac Reader (Roche Diagnostics) that assesses cardiac troponin T, N-terminal pro-brain natriuretic peptide, D-dimer in heparinised venous blood within 8–12 minutes. GPs in the intervention group received advice on interpretation of the test results |
| Staff involved | GPs |
| Research question/objectives | To evaluate the addition of POC testing to GP diagnosis |
| Research methods | A total of 218 patients presenting with chest pain or symptoms of dyspnoea were assessed by GPs using conventional diagnosis methods or GPs with the addition of POC testing. Physicians could choose which biomarker tests were necessary. Patients returned 3 weeks later and the physicians reviewed the diagnosis |
| Results/data | Total of 79 clinicians from 68 practices. In the intervention group the working diagnosis made at first visit was confirmed at second visit in 76% of cases. For the control group the diagnosis was confirmed in 60% of cases (p = 0.002). The tests showed good sensitivity and specificity. Acute coronary syndromes were correctly ruled out in 92% of patients in the intervention group vs. 78% in the control group |
| Main findings | Use of the tests improved diagnostic accuracy for heart failure, thromboembolic events, acute coronary syndromes |
| Comments | The assessment of accuracy was in most cases GP diagnosis on the second visit. Some patients were referred on and diagnostic accuracy was assessed by specialist report for them (numbers of these not specified) |
| Study (authors and year) | Van der Mark et al. 2014289 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Cohort study |
| Country | The Netherlands |
| Setting | Primary care |
| Training/equipment | None |
| Staff involved | GPs |
| Research question/objectives | To evaluate the usefulness of a questionnaire assessing the likelihood of developing asthma |
| Research methods | A total of 771 children aged 1–5 years presenting at GP surgery with shortness of breath/coughing/wheezing were assessed. Presence of asthma versus no asthma later assessed at 6 years. These data were used to develop a prediction system (Clinical Asthma Prediction Score). This has a scale of 0–11 and is based on age, family history, reported symptoms, environmental factors and allergies |
| Results/data | A score of < 3 had a negative predictive value for asthma of 78.4%. A score of > 7 had a positive predictive value of 74.3% |
| Main findings | The scoring system for pre-school children with symptoms can be a useful decision aid in asthma diagnosis |
| Comments |
| Study (authors and year) | Walker et al. 2006290 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Clinical records reviewed |
| Country | UK (Liverpool, England) |
| Setting | Practices within one district |
| Training/equipment | Non-computerised wedge bellows spirometer (Vitalograph), volume and time calibration performed daily and comparison against a biological control carried out weekly. Bronchodilator reversibility testing also available |
| Staff involved | A total of 29 GPs were able to refer to service, where a respiratory technical officer with more than 25 years of experience performed spirometry. A consultant respiratory physician reviewed spirometry data to produce a report which was sent back to referees: obstructive, normal or restrictive, and likely diagnosis of asthma, COPD or other. Management left entirely to referring GP |
| Research question/objectives | To evaluate an open-access spirometry clinic |
| Research methods | GPs advised on appropriate patients to refer (smokers or ex-smokers > 40 years with respiratory symptoms). Referred using standard pro forma to a ‘health suite in the local community’ |
| Results/data | A total of 1508 referrals were made January 1999 to December 2003 (2.6% of the patient population in that area). Median referral rate was 1.8%; 28% of referrals did not meet age or smoker referral criteria. Of those referred, 39% had no existing diagnosis, 30% had asthma and 29% had COPD. Following testing, 128 patients were newly diagnosed with asthma or COPD and 46 patients had their diagnosis changed. In total, 22 of the 139 COPD patients (17%) were referred to secondary care within 6 months of testing. No onward referral data were provided for other patients |
| Main findings | The spirometry clinic increased rates/accuracy of diagnosis and improved treatment |
| Comments | Additional data available on pharmacological management |
| Study (authors and year) | van Heur et al. 2010105 This paper adds more recent data to the Lodewijks-van der Bolt et al. 2010278 paper |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Analysis of referrals December 2002–March 2007. In 2007 GPs were sent a questionnaire on management |
| Country | The Netherlands |
| Setting | Echocardiography service in local hospital |
| Training/equipment | Echocardiography in hospital, Philips SONOS 5500 system, ECONCERT digital storage and retrieval system |
| Staff involved | Staff in echocardiography unit: performed by one of four cardiologists with experience in cardiac imaging or a resident under supervision. Results were interpreted by the cardiologist |
| Research question/objectives | To evaluate an open-access echocardiography service |
| Research methods | Patients referred to the open-access service by GPs following examination. Echocardiography was carried out without referral to a cardiologist. Results of the examination were returned to the GP with a comment on how to manage the patient |
| Results/data | A total of 625 patients were referred over the time period of the study. The service had been used by 81% of GPs in the local area over this time (average one patient per GP per year). Of the referrals, 32% had been for dyspnoea; 24% of the echocardiograms showed no disease. Patients were recommended for referral to cardiology in 28.7% of cases, and the GP followed this advice and made the referral in 71% of these Comparing the first 250 patients with the last 250, fewer referrals for dyspnoea were made in 2007, more for cardiac murmur or other indications. The proportion of patients with no cardiac abnormality found increased (32.8% in 2007 vs. 22% in 2002). More advice was given to GPs in later patients |
| Main findings | The majority of patients referred had a cardiac abnormality, although the finding of an abnormality following referral decreased slightly over the years |
| Comments | Action taken by GP finding based on survey data |
| Study (authors and year) | White et al. 2007187 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Cross-sectional |
| Country | UK (London) |
| Setting | Primary care |
| Training/equipment | Practices provided with an electronic handheld spirometer (MicroLoop®, Micro Medical) with personal computer-based software. Staff received training provided at the lung function laboratory of the local respiratory medicine unit of one group 2-hour session followed by two 3-hour individual clinical tuition sessions. Practices received reimbursement of £10 for the cost of each spirometry test. The built-in electronic spirometry interpreter was switched off for the duration of the study |
| Staff involved | Tests were carried out by practice nurses in five of the practices and by a care assistant in one |
| Research question/objectives | To evaluate the usefulness of remote specialist reporting of spirometry |
| Research methods | Test results were e-mailed as attachments to primary care clinicians (GPs in five practices and a practice nurse in one) and to the local respiratory specialists for remote interpretation. The interpretation of the results was evaluated in terms of acceptability of the test, diagnosis, severity of impairment, management advice and confidence in the interpretation of the test. Analysis of agreement on acceptability, diagnosis and severity was evaluated by comparing interpretation by the primary care clinician with that by the specialist |
| Results/data | Six practices e-mailed 312 tests over 3 months. Clinically significant disagreements between GPs and specialists were identified in regard to diagnosis in 49/168 (29%) tests (κ = 0.39, 95% CI 0.25 to 0.55) and in regard to severity in 62/191 (32%) tests (κ = 0.53, 95% CI 0.43 to 0.63) |
| Main findings | Remote reporting of primary care spirometry results was feasible and provided useful additional information for GPs. However, there were high levels of unsatisfactory quality of community-performed spirometry |
| Comments |
| Study (authors and year) | Wolfenden et al. 2006292 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study. Review of referral patterns |
| Country | UK (London) |
| Setting | Open-access spirometry clinic in a hospital |
| Training/equipment | Not reported |
| Staff involved | Consultant respiratory physician |
| Research question/objectives | To examine the type of patients referred to an open-access spirometry clinic |
| Research methods | A total of 200 patient referrals were examined from 10 general practices. GPs could refer with a suspected or actual diagnosis, to a clinic one morning each week. Spirometry carried out by consultant respiratory physician |
| Results/data | A total of 51% of referrals were for diagnosed or suspected COPD. Just over half of these were found to have airway obstruction. Airway obstruction was rarely observed in those patients referred for stated or suspected asthma |
| Main findings | Spirometry needed in order to establish correct diagnosis of COPD so referrals for suspected or stated were appropriate. Home peak flow monitoring rather than referral may be more helpful for those with suspected asthma |
| Comments |
Conference abstracts
| Study (authors and year) | Backler et al. 2011262 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | UK (England) |
| Setting | Secondary care open-access pulmonary function testing service |
| Training/equipment | Not reported |
| Staff involved | Respiratory physiologists and consultants |
| Research question/objectives | Early evaluation of open-access service |
| Research methods | Referrals for pulmonary function testing were received directly from GPs. Quality assured spirometry, gas diffusion and static lung volume tests were performed. Feedback was obtained by GP and patient questionnaires |
| Results/data | Data for 18 referrals were analysed. Pre- and post-test diagnoses agreed in 11 (61%) cases. Four referred patients had normal test results, eight had COPD, three asthma and three ‘other diagnosis’. Thirteen (72%) were referred to this service instead of the outpatient chest clinic. All GPs were satisfied or very satisfied with the service |
| Main findings | Authors stated that the service has been successfully started, the main difficulty being low awareness in primary care. Three-quarters of patients who used the service have subsequently been managed in primary care, with an estimated saving of £1800 to the health system |
| Comments | Secondary care open access |
| Study (authors and year) | Callister et al. 2011266 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Before-and-after study |
| Country | UK (England) |
| Setting | Local NHS services, including self-referral chest X-ray service |
| Training/equipment | Not reported |
| Staff involved | GPs, community health educators, community matrons, respiratory nurses and pharmacists |
| Research question/objectives | To evaluate a self-referral chest X-ray service provided as part of an intervention to improve early detection and diagnosis of lung cancer |
| Research methods | Public awareness of lung cancer symptoms was assessed using the validated Cancer Awareness Measure. A social marketing approach was used to increase awareness and promote early referral for chest X-ray among health professionals and the public. A service was established where patients aged ≥ 50 years could self-present for chest X-ray after 3 weeks of cough or other chest symptom without medical referral. All community HCPs were encouraged to direct symptomatic patients to the self-referral service |
| Results/data | Pre-campaign market research involving 630 members of the public revealed poor knowledge of early lung cancer symptoms. The marketing communication campaign, HCP campaign and self-referral chest X-ray service all began in January 2011. During the first 7 weeks 95 patients presented for self-referral chest X-ray, of whom three had confirmed lung cancer. There was a 30% increase in the number of GP-ordered chest X-ray compared with the same period in 2010 (mean 557, SD 65 per week vs. mean 428, SD 55, respectively; p < 0.001), and a 70% increase in fast-track lung cancer clinic referrals compared with 2010 as a whole (mean 16.0, SD 6.0 per week vs. mean 9.4, SD 3.1, respectively; p < 0.05) |
| Main findings | Authors stated that further data on chest X-ray rates and number of lung cancer diagnoses, stage and treatment, and outcomes will be needed to evaluate this approach |
| Comments | Main focus is awareness campaign |
| Study (authors and year) | Denis et al. 2013270 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 | |
| Level 3 ✓ | |
| Study design | Non-comparative (descriptive study) |
| Country | The Netherlands |
| Setting | Primary care |
| Training/equipment | CASPIR is an interactive course for GPs and their staff. The course consists of five modules and a yearly refresher course. The course was developed in co-operation with GPwSIs, respiratory technicians and pulmonary physicians |
| Staff involved | GPs, nurses |
| Research question/objectives | Descriptive evaluation of CASPIR programme |
| Research methods | Descriptive summary of programme outcomes |
| Results/data | Since the programme began in 2008, 270 base courses have been given, 3136 GPs and 2146 nurses have participated and 150 refresher courses have followed. More than 90% of participants passed all modules and exams. An estimated 80% of the target population of health professionals have completed the training |
| Main findings | Authors stated that formal training is required for spirometry to be used effectively in primary care. Refresher courses are important to maintain skills |
| Comments |
| Study (authors and year) | Diar Bakerly and Roberts 2009271 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | UK (England) |
| Setting | Community COPD clinic |
| Training/equipment | Not reported |
| Staff involved | Consultant respiratory physician and respiratory nurse consultant |
| Research question/objectives | To evaluate a consultant-led specialist community COPD clinic intended to deal mainly with severe or difficult to diagnose COPD |
| Research methods | Referral criteria were developed before the service was introduced and included a chest radiograph and spirometry prior to referral. The clinic was widely advertised to clinicians in primary and secondary care |
| Results/data | A total of 203 patients were seen, of whom 117 (58%) were new patients and the remainder were follow-ups or transfers of care from secondary care clinics. Average waiting time from referral was 30.2 days. 65% of patients were referred from primary care, 18% from secondary care and 13% from the integrated COPD team. 97 (83%) patients had COPD as the primary diagnosis and only eight (6%) did not have COPD, with three referrals considered inappropriate. 26 (22.2%) new patients required extra tests including blood tests, full pulmonary function tests and CT scans; however, most blood tests were conducted in general practice |
| Main findings | Authors concluded that community COPD clinics provide easy access to specialist advice. Inappropriate referrals to these clinics did not appear to be a problem. These clinics may therefore have a role in the management of patients with COPD |
| Comments | Outreach |
| Study (authors and year) | Fois et al. 2014272 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 | |
| Level 3 ✓ | |
| Study design | Non-comparative study |
| Country | Italy |
| Setting | Primary care |
| Training/equipment | Short course on performance and interpretation of telespirometry |
| Staff involved | GPs, specialists |
| Research question/objectives | To evaluate whether or not telespirometry performed in GPs’ offices can improve early diagnosis of obstructive airway disease |
| Research methods | Ten GPs enrolled current or ex-smokers with or without respiratory symptoms and people with respiratory symptoms but no history of asthma or COPD. Telespirometry data were sent online for interpretation by a pulmonary specialist and the results returned to GPs in real time |
| Results/data | A total of 198 patients were enrolled and telespirometry quality was acceptable in 76% of cases |
| Main findings | Telespirometry, performed by GPs, may improve the early diagnosis of obstructive airway disease |
| Comments | Interpretation in secondary care |
| Study (authors and year) | Harris et al. 2012273 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | UK |
| Setting | Community (primary care) |
| Training/equipment | Not reported |
| Staff involved | Trained general practice staff |
| Research question/objectives | To evaluate the validity of spirometric testing performed in the community |
| Research methods | Retrospective study of 405 patients found to have abnormal screening spirometry performed in the community |
| Results/data | The majority (45%) of patients had moderate COPD. 32% and 11% were found to have severe and very severe COPD, respectively. The mean FEV1 in the community (1.52 l) was slightly higher than the pulmonary function laboratory (1.49 l) |
| Main findings | |
| Comments | Mainly relates to patients with already-established COPD diagnosis, so only limited relevance to review |
| Study (authors and year) | Kaplan and Lerner 2013276 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 | |
| Level 3 ✓ | |
| Study design | Survey |
| Country | Canada |
| Setting | Primary care |
| Training/equipment | Teaching programme and tool for interpretation of spirometry |
| Staff involved | Primary care physicians |
| Research question/objectives | To assess trainees’ attitudes to the training programme and tool |
| Research methods | Post-programme survey |
| Results/data | Response rate was 88% (121/138). Most participants (63%) were not currently performing spirometry. Almost all (98%) found the tool helpful for interpreting spirometry, with 93% intending to use it in practice in the future |
| Main findings | Authors concluded that the training programme and spirometry interpretation tool enable most participants to feel comfortable about interpreting spirometry results |
| Comments |
| Study (authors and year) | Lau et al. 2014277 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | UK (England) |
| Setting | Community breathlessness service |
| Training/equipment | Not reported |
| Staff involved | Members of a tertiary heart failure team |
| Research question/objectives | To assess the potential clinical utility of a breathlessness clinic for detecting heart failure and other cardiorespiratory disorders |
| Research methods | Primary care physicians were invited to refer patients for one-stop testing, including ECG, BNP, spirometry, echocardiography, cardiopulmonary exercise testing and clinical assessment |
| Results/data | Of 191 patients assessed over 2.6 years, breathlessness was the presenting symptom in 82%, oedema in 12% and cough in 4%. Sixty-four patients were known to have pre-existing cardiac conditions (including 13 with heart failure) and 56 had pre-existing respiratory conditions. A cause for the patient’s breathlessness was found in 80% with a new cardiac diagnosis made in 17% and new respiratory diagnosis in 10%; 38% of patients were reassured with no requirement for further secondary-level investigations or review |
| Main findings | Only a small proportion of subjects with unselected referral to a community breathlessness service had heart failure requiring further expert investigation and management. Authors concluded that this approach offers a streamlined assessment with the additional potential for fully reassuring patients of the absence of significant cardiac or respiratory disease |
| Comments | Outreach |
| Study (authors and year) | McNeill et al. 2012281 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 | |
| Level 3 ✓ | |
| Study design | Non-comparative study |
| Country | The Republic of Ireland |
| Setting | Primary care |
| Training/equipment | Not reported |
| Staff involved | Respiratory scientists, GPs |
| Research question/objectives | To assess the value of respiratory scientist-led spirometry clinics in primary care |
| Research methods | Spirometry clinics were held at four primary care centres. Clinics were intended for people who had not previously undergone spirometry. Patients were referred directly to the clinics and were selected according to GOLD guidelines |
| Results/data | Of 104 patients given appointments, 82 were tested, of whom 35 had normal spirometry, 41 had COPD, 2 had possible restrictive lung disease and 2 unreliable data. One centre used only 50% of the available slots |
| Main findings | Authors concluded that the success and efficiency of this type of service is dependent on GP support for the spirometry clinics |
| Comments | Outreach |
| Study (authors and year) | Metting et al. 2013282 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 | |
| Level 3 ✓ | |
| Study design | Non-comparative study |
| Country | The Netherlands |
| Setting | Primary care |
| Training/equipment | Not reported |
| Staff involved | GPs, technicians, specialists (pulmonologists) |
| Research question/objectives | To describe a service implemented in the Netherlands to help GPs in diagnosing and managing patients with asthma or COPD |
| Research methods | GPs can refer patients with evidence of respiratory problems to the service for assessment and advice. Patients complete a history questionnaire, the Clinical COPD Questionnaire and the Asthma Control Questionnaire, and spirometry is performed by a trained technician. The pulmonologist inspects the data online, without seeing the patient, and sends the GP the results along with a diagnosis and treatment advice |
| Results/data | The service has included ≈12,000 patients from 359 GPs and ≈2000 new patients are included yearly. In 78%of cases, the specialist was able to diagnose patients based on online information |
| Main findings | Authors concluded that this service has proven to be a feasible collaboration system between GPs and specialists |
| Comments | Interpretation in secondary care |
| Study (authors and year) | O’Herlihy et al. 2013283 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | The Republic of Ireland |
| Setting | Community COPD clinic |
| Training/equipment | Not reported |
| Staff involved | Members of COPD outreach team |
| Research question/objectives | To evaluate a COPD satellite clinic based in a primary care centre |
| Research methods | Review of patient records |
| Results/data | Of the patients reviewed (number not reported), 50% had COPD and 25% had asthma diagnoses confirmed; the remainder had no significant respiratory problem |
| Main findings | The authors concluded that satellite clinics can provide a time saving and cost-saving alternative to the traditional hospital based outpatient clinic model for those thought to have COPD |
| Comments | Outreach |
| Study (authors and year) | Punwani et al. 2014285 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | UK (England) |
| Setting | Community pharmacies in south-west London |
| Training/equipment | Pharmacists attended standardised training sessions to help them identify people at risk of COPD and/or lung cancer for referral to secondary care |
| Staff involved | Pharmacists from 43 pharmacies |
| Research question/objectives | To evaluate a community pharmacy pathway to increase awareness and early diagnosis of lung cancer |
| Research methods | A referral template was created based on the two week rule pathway for suspected lung cancer. Patients were referred to local secondary care clinics where clinical assessment, chest X-ray and spirometry were performed. Feedback was obtained via questionnaires |
| Results/data | Of 66 referrals over 6 months, 55 were considered appropriate. A new diagnosis of COPD was made in 30% of patients referred |
| Main findings | Community pharmacists are an acceptable and underused resource for identifying and referring patients with symptoms of previously undiagnosed COPD who may also be at risk of lung cancer |
| Comments | Appears to meet inclusion criteria assuming patients presented to pharmacist complaining of breathlessness |
| Study (authors and year) | Schermer et al. 2013195 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Comparative before-and-after study |
| Country | The Netherlands |
| Setting | Primary care |
| Training/equipment | CASPIR and Cohesie spirometry training programmes |
| Staff involved | GPs and practice nurses (CASPIR); practice nurses and assistants (Cohesie) |
| Research question/objectives | To evaluate the impact of the two training programmes on quality of spirometry tests |
| Research methods | Random samples from practices’ spirometry databases were reviewed before and after training. Primary outcome was the proportion of ‘adequate’ tests |
| Results/data | Twenty-nine practices (15 CASPIR and 14 Cohesie) took part and 1065 tests were reviewed. For CASPIR 39.1% of tests were adequate before training and 51.0% after training (odds ratio 1.60, 95% CI 1.12 to 2.30). Before CASPIR training two practices (13.3%) reached the desired performance level (60% adequate tests); after training seven (46.7%) did so. For the Cohesie programme, pre- and post-training rates were 45.3% vs. 44.1% (odds ratio 0.93, 95% CI 0.65 to 1.33) for the primary outcome. At pre-training four Cohesie practices (28.6%) reached the performance level, but after training only one practice (7.1%) did so |
| Main findings | The authors concluded that structured spirometry training seems to improve test quality but does not necessarily produce desired levels of quality in every practice |
| Comments |
| Study (authors and year) | Sallaway et al. 2011286 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 ✓ | |
| Level 3 | |
| Study design | Non-comparative study |
| Country | Canada |
| Setting | Open-access spirometry laboratory (no further details reported) |
| Training/equipment | Not reported |
| Staff involved | Not reported |
| Research question/objectives | To describe the pattern of referrals and characteristics of patients referred from primary care with suspected asthma or COPD |
| Research methods | Referral records from October 2008 to March 2010 were analysed |
| Results/data | Of 2140 patients who attended for spirometry, 1671 (78%) were investigated by post-bronchodilator spirometry; of these, 428 were classified as obstructive (according to American Thoracic Society guidelines) |
| Main findings | A significant proportion of referrals to the service yielded abnormal results. The authors stated that a continued effort to improve access to spirometry and promote these services is essential. As availability increases, emphasis should be placed on educating HCPs in the importance of spirometry for early detection and monitoring of pulmonary disease |
| Comments | The setting was unclear, may be secondary care open-access service |
| Study (authors and year) | Willey et al. 2013291 |
|---|---|
| Type of study/document | Empirical level 1 |
| Empirical level 2 | |
| Level 3 ✓ | |
| Study design | Non-comparative study |
| Country | USA |
| Setting | Community-based primary care practice |
| Training/equipment | Not reported |
| Staff involved | Pharmacist(s) |
| Research question/objectives | To describe the experience and preliminary outcomes of pharmacist-led care for patients with respiratory disorders |
| Research methods | Medical record review of patients referred to the pharmacist by physicians for investigation of respiratory symptoms between May 2011 and September 2012 |
| Results/data | Pharmacist interventions included detailed history taking and spirometry. Thirty-four patients were assessed by spirometry, which met American Thoracic Society quality standards in 82.5% of tests. The pharmacist made various recommendations that were implemented, including smoking cessation, medications and specialist referral |
| Main findings | The authors concluded that these preliminary results support pharmacists expanding their role by providing patient care services, including spirometry, to patients with respiratory disorders |
| Comments |
Qualitative data extractions
| Citation | Armstrong et al. 2012293 |
| Country | UK |
| Setting | Community |
| Dyspnoea/specific condition | Pulmonary hypertension |
| Application of model/pathway | Anderson’s model of total patient delay |
| Research question/objectives | To investigate patient experiences of the pathway to receiving a diagnosis of PAH to inform care provision |
| Research methods | Data collection: Face-to-face interviews, experience of living with PAH Mapping of ‘individual journey’ using pictorial representation |
| Analysis: Thematic analysis | |
| Population/sample | Patients: members of the Pulmonary Hypertension Association (n = 30) |
| Age: mean 56.3 years. Gender: 18 female; 12 male | |
| Recruitment | From the Pulmonary Hypertension Association UK database |
| Main findings relevant to the research question | General Road to diagnosis was emotional for both the patient and their family. Use of Anderson’s Model to structure findings |
| Specific issues Many delayed visit to a doctor for over 1 year A patient appraisal process was evident when symptoms, including breathlessness, had been present for extended periods, having gradually crept on Breathlessness had different meanings (e.g. getting unfit, getting old or a symptom of a virus) Smokers who had recently given up assumed smoking to cause the breathlessness Period of perseverance; tried to rectify reduced fitness level (younger participants continued regardless) For some, avoidance strategies were used and activities were adapted to the severity of breathlessness Covering up of symptoms Delayed decision to seek medical opinion until either patient or family member acknowledged the seriousness Diagnosis was not necessarily made at first visit to a doctor and for all participants there was a phase of attendance with no outcome Misdiagnosis was common; PAH reported as not being recognised or understood by clinicians Many tests carried out and repeated Multiple referrals to specialists Owing to not having a diagnosis, patients tried to carry on as normal even when symptoms were worsening Diagnosis provoked positive and negative emotions. For some, the way that diagnosis was disclosed was reported as inappropriate or lacking empathy. Positive experiences included acknowledgement from staff of the delay in reaching a diagnosis Some patients felt ill informed about PAH and for some it was the first they had heard of it Patients felt more reassured at specialist centres where they received more information |
|
| Potential limitations Range in time since diagnosis (< 1 year to > 10 years). The health service could have changed during this time. However, the authors point out that patients’ reflections were similar regardless of the time lapse |
| Citation | Birt et al. 2014294 |
| Country | UK |
| Setting | Primary care referrals to specialist respiratory clinics |
| Dyspnoea/specific condition | Lung cancer |
| Application of model/pathway | Model of pathways to treatment |
| Research question/objectives | To explore symptom appraisal and help-seeking decisions for patients referred to specialist respiratory services with lung cancer symptoms |
| Research methods | Data collection: face-to-face interviews. Open-ended questions to explore the participant’s appraisal of symptoms and help-seeking decisions |
| Analysis: framework analysis | |
| Population/sample | Patients (n = 35) |
| n = 17 diagnosed with lung cancer, n = 18 diagnosed with other conditions | |
| Age: mean 65 years. Gender: 15 female; 20 male | |
| Recruitment | When patients were referred to specialist respiratory clinics by GPs who reported symptoms of potential lung cancer |
| Main findings relevant to the research question | General Little evidence that patients received adequate advice from their GPs about symptom monitoring or reasons to return for review |
| Specific issues There were similarities in patient appraisal of lung cancer to that of other conditions When a symptom was difficult to recognise or was attributed to ageing or smoking, or could be self-managed, the appraisal phase was extended Difficult to recognise a change in respiratory function, particularly in the presence of comorbidities such as COPD Some participants alerted to the symptom during consultations for other conditions Alternative explanations for the cause, such as anticipated changes with age, or smoking Some tried to contain and self-manage symptoms; occasionally there was a reported dislike of attending the doctor’s surgery Decision to consult a HCP triggered by patient, disease, health-care factors. Symptoms recognised by family/friends as presented in the ‘Be Clear on Cancer Campaign’ No reports that smoking inhibited help-seeking Limited access to health care and competing responsibilities delayed help-seeking for a few Over half consulted GP within 30 days of noticing symptoms; usually interpreting it as a chest infection If symptom appraised as self-limiting or not requiring medical attention (e.g. allergy, muscle strain), help-seeking was delayed until symptoms did not resolve as expected For older participants, symptoms were often attributed to the ageing process General satisfaction with outcome of first consultation unless prescribed treatment not effective. In this case the process of reappraisal started. Not always easy decision to consult a doctor again A minority reported GP as dismissive of symptoms and concerns |
| Citation | Corner et al. 2005295 (from reference list); Corner et al. 2006296 (from reference list) |
| Country | UK |
| Setting | Primary/secondary care interface |
| Dyspnoea/specific condition | Lung cancer |
| Application of model/pathway | Similar to model of pathways to treatment (although the model is not cited) |
| Research question/objectives | To explore pathway to diagnosis among a group of patients recently diagnosed with lung cancer |
| Research methods | Data collection: interviews. To map pre-diagnosis symptom history and the events leading up to diagnosis |
| Analysis: event charts of the pathway to diagnosis; thematic analysis | |
| Population/sample | Patients with recently diagnosed lung cancer (n = 22) |
| Age: median = 68 years (range 42–82 years). Gender: 10 female, 12 male | |
| Recruitment | From outpatient clinics |
| Main findings relevant to the research question | General Event charts highlighted delays in diagnosis attributed to patients and GP. GP attendance led to speedy outcomes which may indicate response to recent guidelines |
| Specific issues Thirty symptoms recorded (breathlessness 5/22), all of which were reported as new leading up to diagnosis Patients did not always act on symptoms even when noticeable Symptoms were often self-managed until this became difficult Symptoms were often not interpreted as serious (unless accompanied by haemoptysis) or signifying lung cancer Time between first recalled changes in health and action by patient: median 7 months However, the time lapse between trigger to act and visiting the GP tended to be short Half of participants recalled symptoms lasting > 1 year before visiting the GP For most there was little or no contact with the GP during this time Little association with change of health and being ill and, even for smokers, the possibility of lung cancer was not considered (usually attributed to increasing age instead) Lack of evidence that smokers expected to be treated promptly, possibly due to campaigns that leave smoking patients feeling that they do not deserve the same care as others. This may also lead to delay in help-seeking |
|
| Potential limitations Not all patients had chest symptoms and other symptoms were experienced. Patient reports of their symptoms differ from those listed in guidance for lung cancer diagnosis |
| Citation | Dennis et al. 2010297 |
| Country | Australia |
| Setting | Primary care |
| Dyspnoea/specific condition | Asthma |
| Application of model/pathway | None reported |
| Research question/objectives | To explore difficulties faced by GPs when trying to diagnose asthma in adults, and whether or not patient characteristics influence the process |
| Research methods | Data collection: focus groups. Current evidence for making the diagnosis of asthma |
| Analysis: thematic analysis | |
| Population/sample | GPs (n = 18) |
| Gender: 5 female, 13 male | |
| 15 had > 10 years of experience as a GP | |
| Recruitment | Purposeful sampling of a database of research-interested GPs/identified through membership database of local Divisions of General Practice |
| Main findings relevant to the research question | General Reported GP confidence in theoretical stages of diagnosis process but more challenging in reality. Spirometry was a challenge, especially when few practice nurses were available to assist. Patient willingness to know about their condition was a factor in diagnosis; some patients may not disclose their symptoms |
| Specific issues GPs were familiar with Australian guidelines for asthma management GPs confident about symptoms and signs of asthma but less so about using spirometry to diagnose asthma Lack of confidence particularly apparent in diagnosing children or elderly but more in those with history of allergies such as hay fever, or young, fit adults GPs recognised the need to rule out conditions such as lung cancer and COPD The issue of time was raised in different contexts, such as consultation availability and time to discuss with patient and carry out spirometry and other tests, as well as continuity of care over time. It could take several visits to confirm a diagnosis Willingness of patient to become involved in decision-making was reported as having impact on accurate diagnosis. Lack of patient engagement may delay diagnosis for another time Doctor–patient relationship important Sometimes challenging to persuade patients to return for follow-up visits, resulting in symptom management (this was especially a problem for professionals who are busy at work) Patients with comorbidities tended to miss symptoms of asthma or attribute the symptoms to other conditions Some patients were already taking antiasthma medication by the time they first consulted their GP Patients who smoked or were overweight might not disclose their symptoms for fear of a lecture on healthy lifestyle |
|
| Potential limitations Some issues raised by GPs likely to be exacerbated by primary care organisation in Australia (less relevant to countries with different systems such as NHS) |
|
| Comments | Applicability In Australia, general practice operates as fee-for-service, funded by Medicare. Patients do not have to register with a particular GP or practice. This impacts continuity of care, as patients may visit different GPs or practices. GPs were not confident about using spirometry and few had a practice nurse. The role of the practice nurse in Australia is less developed than in UK; many have not taken post-registration training in respiratory care and therefore may be less confident in using spirometry |
| Citation | Goeman et al. 2005298 |
| Country | Australia |
| Setting | Primary care |
| Dyspnoea/specific condition | Asthma |
| Application of model/pathway | None reported |
| Research question/objectives | To ascertain GPs’ priorities for achieving optimal outcomes in people with asthma, and the barriers they face in delivering care |
| Research methods | Data collection: the nominal group technique (highly structured meeting to gain information from experts): ‘What do you think is needed to achieve best outcomes in people with asthma?’ |
| Population/sample | GPs (n = 49): urban (n = 34), rural (n = 15) |
| Recruitment | Invitation and advertisement through the Royal Australian College of General Practitioners and Divisions of General Practice |
| Main findings relevant to the research question | General Gap between guidelines and GP priorities. 3 + Visit Plan dealt with some issues |
| Specific issues Patient education ranked highest priority for GP groups Continuing medical education was a high priority, particularly in asthma diagnosis and severity (under-over medication) Detecting and diagnosing asthma early and correctly Use of and access to spirometry (expense and confidence in correct use) Lack of time and access limited opportunity for continued education |
|
| Potential limitations Not reported |
| Citation | Joo et al. 2013198 |
| Country | USA |
| Setting | Primary care |
| Dyspnoea/specific condition | COPD |
| Application of model/pathway | None reported |
| Research question/objectives | To identify attitudes and barriers of primary care physicians to performing spirometry for patients with possible COPD |
| Research methods | Data collection: focus groups |
| Population/sample | Primary care physicians (n = 12) |
| Age: mean 40 years. Gender: six female, six male | |
| Mean time in general practice: 11.9 years | |
| Recruitment | Invitations to all internal medicine primary care physicians from one urban academic medical centre with at least one outpatient clinic per week |
| Main findings relevant to the research question | General Primary care physicians were not reluctant to use the label patients as having COPD |
| Specific issues Perspective on spirometry use based on whether a pre-existing or new diagnosis Most COPD patients had a pre-existing diagnosis before attending Primary care physicians did not routinely confirm the diagnosis when spirometry had not been used in the initial diagnosis, especially where there were comorbidities If risk factors for COPD were present (age, smoking, etc.) the diagnosis would not be reinvestigated Most believed that spirometry was not needed to diagnose COPD as they felt confident in diagnosing from patient history and effects of inhalers. There was a perception that management would not change regardless of spirometry results. There was a lack of confidence/confusion regarding pulmonologist definition of airway disease. Patient-related factors and their lack of willingness to attend for follow-up tests owing to costs and lack of time; transportation and health service insurance issues were also factors |
|
| Potential limitations Primary care physicians from a single academic, urban institution. Therefore, findings may not be generalisable to all primary care physicians |
| Citation | Khunti et al. 200282 |
| Country | UK |
| Setting | Primary care |
| Dyspnoea/specific condition | Heart failure |
| Application of model/pathway | None reported |
| Research question/objectives | To explore GPs’ accounts of their management of patients with heart failure and identify the perceived obstacles to diagnosis and management |
| Research methods | Semistructured interviews (individual and group): how GPs diagnose and manage patients with suspected heart failure. Perceived obstacles to diagnosis and management Constant comparison analysis |
| Population/Sample | GPs (18 practices) |
| Recruitment | From practices randomised for a larger study |
| Main findings relevant to the research question | General GPs keen to have access to echocardiography |
| Specific issues Heart failure frequently suspected where patient presents with breathlessness or ankle oedema Reliance on a number of signs and symptoms for diagnosis No systematic method of heart failure diagnosis reported Differing views reported about diagnostic procedures, even within the same GP practice Chest X-ray preferred as easy to arrange and inexpensive. Some diagnosed by trial of diuretics Few reported referring for echocardiography to confirm; many not aware of its usefulness. Those who were aware reported long waiting lists as a barrier A few single-handed practices reported referring to rapid assessment cardiology clinic at the local hospital Other arrangements included requesting a consultant to arrange access Inadequacy of facilities was a reported barrier to diagnosis, especially echocardiogram, for which there was lack of access owing to not being fund-holders and lack of trust from providers that may perceive the service is not used appropriately Lack of time was reported as a barrier in dealing with patients suspected to have heart failure. In addition, elderly people took a long time to get dressed and undressed Diagnostic confusion: difference between respiratory and cardiac origin |
|
| Potential limitations GPs aware that study involved guidelines in management of heart failure; they may have stated socially desirable ideas about heart failure management |
| Citation | Roberts et al. 2011197 |
| Country | UK |
| Setting | Primary care and hospital |
| Dyspnoea/specific condition | Dyspnoea |
| Application of model/pathway | None reported |
| Research question/objectives | Underuse of spirometry in primary and secondary care |
| Research methods | 5 × 1-hour focus groups discussing:
|
| Population/sample | Non-specialised trainee doctors (n = 6); junior doctors (n = 8); senior house officers (n = 3); GPwSIs in education (n = 8); specialist registrar with special interest in respiratory medicine (n = 6) |
| Recruitment | Advertisement on the Student’s Union website |
| Main findings relevant to the research question | General GPs highly aware of the value of spirometry. This may be response to UK NHS GP contract inclusion of accurate COPD diagnosis |
| Specific issues GPs perceived spirometry as a fundamental element of their diagnostic work-up Causes of breathlessness differentiated between urgent and non-urgent Possibility of non-respiratory causes Spirometry cited as an essential diagnostic tool No overt strategy reported for diagnosing COPD More access to spirometry than in the past Importance of bedside teaching and learning within the clinical context Training focused on specific need and retraining was reported as desirable |
|
| Potential limitations GPs may not have been typical of all in primary care, as all were GPwSI in teaching undergraduates recruited while attending an educational session |
| Citation | Tod et al. 2008299 (from reference list) |
| Country | UK |
| Setting | Primary/secondary care interface |
| Dyspnoea/specific condition | Lung cancer |
| Application of model/pathway | None reported |
| Research question/objectives | To identify factors influencing delay in reporting symptoms of lung cancer |
| Research methods | Data collection: interviews |
| Analysis: framework analysis | |
| Population/sample | Patients diagnosed with lung cancer (n = 20) Age range 47–81 years. Gender: 8 female, 12 male |
| Recruitment | Purposive sampling through a respiratory physician and lung cancer nurse specialists |
| Main findings relevant to the research question | General Industrial cultural past encouraged stoicism and independence in the community, adding to delays in help-seeking and inequalities in health-care utilisation |
| Specific issues Tendency, especially for non-smokers, to attribute symptoms to other conditions Poor knowledge and awareness of lung cancer symptoms and treatments (available information focus on other cancers) Most lung cancer information smoking related Fear owing to lack of knowledge of treatment/fatalistic beliefs. Fear of death if cancer diagnosed Belief that smoking cessation reduces lung cancer risk to nil, therefore ignoring symptoms Fear of being perceived as a time waster and memories of bad consultation experiences resulted in further delay Expectation that lung cancer diagnosis would incur stigma and blame Stoicism, putting on a brave face, particularly in older generation and men History of poor health-care utilisation in the community Memories of having to pay for care (pre-NHS) meant that consultations only sought if symptoms perceived to be serious. Media messages reinforced this Families facilitated consultation by noticing symptoms and signs and encouraging help-seeking, possibly making appointment for patient Relatives were advocates for patient if symptoms not investigated |
|
| Potential limitations The sample was limited in terms of ethnicity (not diverse), although this reflects the demographics of the study location |
| Citation | Walters et al. 2008;301 Walters et al. 2005300 (from reference list) |
| Country | Australia |
| Setting | Primary care |
| Dyspnoea/specific condition | COPD |
| Application of model/pathway | None reported |
| Research question/objectives | To investigate attitudes influencing the diagnosis of COPD among doctors and patients with COPD in primary care |
| Research methods | Data collection: interviews and focus groups |
| Population/sample | GPs (n = 19) |
| Patients (n = 14): interviews only | |
| Age: mean = 67 years. Gender: nine female, five male | |
| Recruitment | By letter: participants from two Tasmanian GP practices |
| Main findings relevant to the research question | General Delay in diagnosis by GPs may be intentional due to poor prognosis and perception that patients are unwilling to receive a diagnosis. This could lead to lack of spirometry use by GPs |
| Specific issues COPD was rarely named; the terms emphysema and asthma were used. Emphysema was regarded by GPs as a term more familiar to patients than COPD Diagnosis rarely given directly, often attached to asthma Diagnosis could vary over short periods of time Patients used terms interchangeably to describe their symptoms GPs also noted changes in language used by respiratory teams over time, which was confusing and difficult to explain to patients COPD was considered in presence of risk factors, especially smoking Reluctance to label the condition given the consequences for the patient (serious, potentially terminal) Delay in diagnosis as no advantage perceived by GP, even after several visits. However, treatment often initiated prior to diagnosis. Patient may come to know the diagnosis from elsewhere (e.g. pharmacist) Patients expressed frustration at not having a diagnosis and may seek information elsewhere (e.g. internet, or remain poorly informed) Delay in diagnosis could result in losing opportunity to discuss smoking cessation. However, GPs were dubious whether or not diagnosis would affect smoking behaviour |
|
| Potential limitations May not have identified the entire COPD population in the practices |
Survey data extractions
| Citation | Boffin et al. 2006303 |
| Country | Belgium |
| Setting | Primary care |
| Dyspnoea/specific condition | COPD/asthma |
| Research question/objectives | To describe the use of spirometers by Flemish GPs, characteristics of their spirometry practice, training needs and preferences, and attitudes towards office spirometry |
| Research methods | Data collection: telephone survey |
| Analysis: SPSS – chi-squared and ANOVA | |
| Population/sample | 197 GPs |
| Recruitment | Random selection from database |
| Main findings relevant to review question | General: response rate was 81%. Just over one-third of GPs had access to a spirometer, and peak flow meters were used to assess lung function |
| Specific issues More than half of GPs had never used a spirometer. < 20% had ever used one Spirometer use had ceased because of the time it takes, which impacts on consultation times, or lack of knowledge and skill Most spirometers were obtained from a pharmaceutical company Peak flow meters were used by over half the GPs GPs agreed that training was needed by GPs ready to perform spirometry A minority agreed that GPs should be able to access centres where they could manage the spirometry test themselves One-third of the GPs and all those using spirometry were guided by an educational session, mainly organised by spirometry providers |
|
| Potential limitations: absence of data on GP referrals for spirometry in specialist settings |
| Citation | Bolton et al. 2005304 |
| Country | UK |
| Setting | Primary care |
| Dyspnoea/specific condition | COPD |
| Research question/objectives | To determine the availability, staff training, use and interpretation of results of spirometry in 72% of general practices in Wales |
| Research methods | Data collection: questionnaire Availability of spirometry; access to local lung function without consultant physician referral; confidence in use (including calibration); interpretation of results; the type and length of training, the number of their registered COPD patients investigated with spirometry Lung function tested using spirometers by experienced users and interpreters |
| Analysis: SPSS – chi-squared test and Mann–Whitney U-test | |
| Population/sample | Practitioners at 227 general practices |
| 125 patients from two practices where diagnosis had been made without spirometry | |
| The response rate was 61.6%, 227 of the 371 practices contacted. Each practice was asked the size of the population they covered. From 214 practices reporting, the estimated population served was 1,415,647 individuals | |
| Recruitment | Randomly selected practices were sent a questionnaire. Patients were drawn from two Cardiff practices |
| Main findings relevant to review question | General Response rate: 61.6%. Increase in access to and use of spirometers is promising and probably reflects a response to guidelines as well as pressure for GPs to undertake a greater role in COPD diagnosis. Most spirometry was performed by practice nurses, which may reflect in the confidence and training levels reported |
| Specific issues The majority of practices (187) owned a spirometer and most of these (160) used one Of the remaining 27 practices, 11 had never used a spirometer, 12 had recently acquired one (four no response) Over half of practices carrying out spirometry were confident and one-third reported satisfaction with their result interpretations Nearly half of practices reported limited/no confidence Over one-third of practices used a spirometer to diagnose COPD in all suspected cases Where spirometry was carried out, the practice nurse either did so alone or shared with the GP in the majority of practices (145/160) Use of spirometry was associated with confidence in its use (p < 0.001) Use of spirometer and confidence in its use was positively associated with higher median time in training (range 1 hour to 6 hours; p < 0.001). Confidence in interpretation of results was also associated with higher median hours of training (range 0.6–4 hours; p < 0.001). No details available on training content or access Provision of spirometry training varied, including specific spirometry courses, hospital based, pharmaceutical company based, and one-to-one tuition A higher median confirmation of COPD was evident in practices reporting greater confidence in use and interpretation (p = 0.155) than in those reporting lower confidence Three practices without a spirometer had access to hospital facilities to assess lung function |
|
| Potential limitations None reported |
| Citation | Caramori et al. 2005305 (from reference lists) |
| Country | Italy |
| Setting | Primary care |
| Dyspnoea/specific condition | COPD |
| Research question/objectives | To investigate the degree of use of spirometry to establish the diagnosis of COPD in Italy |
| Research methods | Data collection: questionnaire |
| Analysis: statistical package (Qubisoft) | |
| Population/sample | General practices |
| Recruitment | A total of 2424 questionnaires were analysed after eliminating one questionnaire that was incomplete |
| Main findings relevant to review question | General Response rate: 2474/2475 (almost 100%). Only a small minority of COPD cases are diagnosed by GPs in Italy. There may be need for specific educational programmes targeted at GPs in Italy |
| Specific issues Most GPs felt that COPD prevalence in Italy had increased over 10 years Most GPs reported that COPD symptoms are better controlled owing to early diagnosis and availability of effective treatments Main symptoms assessed for diagnosis of COPD were persistent cough, expectoration and exercise dyspnoea Spirometry was used to confirm the diagnosis by 69.8% of GPs Just fewer than half of GPs reported to be able to diagnose COPD independently Reasons for underuse of spirometry include lack of access to lung function laboratories, or the perception that spirometry is unnecessary |
|
| Potential limitations: retrospective epidemiological study |
| Citation | Halpin et al. 2007306 |
| Country | UK |
| Setting | Primary care |
| Dyspnoea/specific condition | COPD |
| Research question/objectives | To assess the confidence of HCPs in diagnosing and managing COPD |
| Research methods | Data collection: telephone interview (questionnaire); case scenarios |
| Analysis: Mann–Whitney U-test, Kruskall–Wallis test and chi-squared test | |
| Population/sample | 2001: 60 practice nurses; 46 GPs |
| 2005: 61 practice nurses; 39 GPs | |
| All nurses ran respiratory clinics | |
| Recruitment | Practices telephoned at random |
| Main findings relevant to review question | General Response rate not reported. The majority of GPs and practice nurses were aware of guidelines which may be reflected in the increase in GP confidence in COPD diagnosis. No comparable increase was evident for practice nurses |
| Specific issues Between 2001 and 2005, there were no significant differences reported in number of COPD patients seen per week or frequency of respiratory clinics, nurse qualifications or experience Most nurses were aware of national guidelines by 2005, especially those provided by NICE. Awareness of guidelines was associated with greater confidence in diagnosing COPD Confidence of GPS in diagnosing COPD was higher in 2005 than in 2001, although practice nurses expressed less confidence and no change between 2001 and 2005 80% of GPs and 70% of practice nurses reported being confident in differentiating between COPD and asthma in 2005 Most participants reported that spirometry was an essential tool in diagnosis and less than one-third reported that referral was essential, although this had risen by 10% from 2001 Spirometer access increased from 78% of practices in 2001 to 95% in 2005 GPs reported higher confidence than practice nurses in interpreting spirometry results, having increased significantly from 2005, although no similar increase was evident for practice nurses |
|
| Potential limitations Possible selection bias (all practices confirmed that they saw patients with COPD). However, practices were chosen at random. Using case scenarios as a way of assessing diagnostic strategies |
| Citation | Kaminsky et al. 2005191 (from reference list) |
| Country | USA |
| Setting | Primary care |
| Dyspnoea/specific condition | COPD |
| Research question/objectives | To assess primary care physicians’ knowledge and use of office spirometry for the detection of COPD |
| Research methods | Data collection: mailed questionnaire to assess the size and location of the practice as well as characteristics of the practice and prevalence/management of patients with COPD Workshop: slide presentation followed by 30 minutes’ hands-on use of a spirometer. Daily log of spirometry use and costs |
| Analysis: descriptive statistics; Fisher’s exact test for associations | |
| Population/sample | General practices n = 29 |
| Recruitment | Contact with all primary care medical groups associated with the local Area Health Education Centre |
| Main findings relevant to review question | General Response rate: 51%. Confidence in interpreting spirometry results increased following workshop |
| Specific issues Main reasons for not performing spirometry following the workshops:
|
|
| Potential limitations Only half of the target population responded to the survey, despite attempts to maximise return. The population may not be representative of the primary care community. Main data collection tool had not undergone validation with the same population. Primary outcome measure of success was number of spirometry tests performed in a relatively short time frame. Sources for data were subject to recording and classification error. Tests were not stratified by underlying diagnosis, indication or repeated tests on the same subjects |
List of abbreviations
- 2D
- two-dimensional
- AF
- atrial fibrillation
- AOM
- acute otitis media
- BAAP
- British Association of Audiological Physicians
- BNP
- B-type natriuretic peptide
- BP
- blood pressure
- BSCMR
- British Society for Cardiovascular Magnetic Resonance
- BSE
- British Society of Echocardiography
- BSG
- British Society for Gastroenterology
- CCG
- Clinical Commissioning Group
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COPD
- chronic obstructive pulmonary disease
- COSHH
- Control of Substances Hazardous to Health
- CPD
- continuing professional development
- CRP
- C-reactive protein
- CT
- computed tomography
- DARE
- Database of Abstracts of Reviews of Effects
- DEC
- Diagnostic Evidence Co-operative
- DNA
- deoxyribonucleic acid
- DRS
- diabetic retinopathy screening
- ECG
- electrocardiogram
- ENT
- ear, nose and throat
- FEV
- forced expiratory volume
- FVC
- forced vital capacity
- GI
- gastrointestinal
- GMC
- General Medical Council
- GP
- general practitioner
- GPwSI
- general practitioner with a special interest
- HbA1c
- glycated haemoglobin
- HCP
- health-care professional
- HTA
- health technology assessment
- INR
- international normalised ratio
- ISAS
- Imaging Services Accreditation Standard
- ISO
- International Standards Organisation
- IT
- information technology
- JAG
- Joint Advisory Group on Gastroenterology
- LVSD
- left ventricular systolic dysfunction
- MaDOx
- Monitoring and Diagnosis in Oxford
- MeSH
- medical subject heading
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MRI
- magnetic resonance imaging
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NT-proBNP
- N-terminal pro B-type natriuretic peptide
- OME
- otitis media with effusion
- PCT
- primary care trust
- POC
- point of care
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies
- RCGP
- Royal College of General Practitioners
- RCR
- Royal College of Radiologists
- RCT
- randomised controlled trial
- SCoR
- Society of Radiographers
- STEPPED-UP
- Skills, Training, Equipment, Premises, Public perspectives, Economics, Drivers, User perspective and Primary–secondary interface
- STEP-UP
- Skills, Training, Equipment, Premises, User perspective and Primary–secondary interface
- VAT
- value-added tax