Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/2003/60. The contractual start date was in February 2013. The final report began editorial review in December 2015 and was accepted for publication in June 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David K Raynor received personal payments for his work for Luto Research during the course of the study. David Meads is a member of the HTA Elective and Emergency Specialist Care panel. Claire Hulme is a member of the HTA commissioning board panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Minton et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Outpatient parenteral antimicrobial therapy
The delivery of intravenous (i.v.) antibiotics to patients outside a hospital setting was first described on a small scale in the 1970s in North America. By the end of the 1990s, an estimated quarter of a million patients annually were receiving i.v. antibiotics on an outpatient basis as a result of cost savings, patient preference, better i.v. devices, the introduction of antimicrobial agents that needed administration only once or twice a day and the development of dedicated service providers. A wide variety of infections have been treated through this system, in particular skin and soft tissue infections (SSTIs), but also bone and joint infections, bacteraemia, wound infections, pneumonia, complicated urinary tract infection, intra-abdominal infections, device-related infections, endocarditis and central nervous system infections. Although widely accepted as the standard of care in countries such as the USA and Australia, such services are largely limited to patients with appropriate health insurance cover. 1 Clinical efficacy and safety has been addressed in many different clinical areas and using a variety of models of care, largely using retrospective analysis of single-centre experience. 2–5 Some risks of community-based i.v. therapy have been identified, and projects have been initiated to minimise these. 6 The potential for treatment of micro-organisms resistant to antimicrobials and for limiting the spread of health-care-associated infection has been highlighted as an outpatient parenteral antimicrobial therapy (OPAT) benefit. 7 Newer antimicrobial agents have been assessed for their potential for OPAT use, usually because they have long half-lives with less frequent dosing required and may be effective against resistant micro-organisms such as meticillin-resistant Staphylococcus aureus (MRSA). 8,9 However, such agents are much more expensive, so increase the cost of the service.
Clinical practice in the UK
Over the last 10 years, OPAT services have been developed in more areas of the UK, in both the NHS and private sectors, in response to local pressures in combination with health-care staff initiatives. 7 This has led to many service variations using different heath-care professional groups which can be grouped into four main categories:
-
Outpatient attendance at a health-care facility
-
A variety of NHS hospital departments have set up systems for providing i.v. antibiotics for patients attending on a daily basis, including both specialist and general services. The main disadvantage to this system is the inconvenience to the patient in having to travel, the fact that it is limited to patients who are fit to travel and the cost of transport.
-
-
Self-administration (SA) of i.v. antibiotics
-
Particularly where patients require very long or repeated courses of antibiotics, patients or carers have been taught to self-administer the treatment. This system is likely to be cheaper insofar as less professional time is required once the patient has been trained, but there are potential risks to unsupervised administration, including non-compliance.
-
-
Visiting general nurse (GN) model
-
There are instances of NHS community nurses (e.g. district nurses) administering i.v. antibiotics; this can be efficient as they can perform other tasks such as wound management at the same visit and with minimal travelling as they are based locally. However, they are likely to be less confident and skilled in i.v. antibiotic management, as this makes up a small percentage of their work and they may have insufficient time to add this to their caseload.
-
-
Visiting specialist nurse (SN) model
-
In contrast, specialised visiting nurses have more expertise but may be less efficient as they cover a large geographical area. This is the main model of care in the USA and is generally provided by private specialised companies. In the UK, this model is available through a few providers, both private sector and NHS.
-
Existing evidence on outpatient parenteral antimicrobial therapy service delivery
There have been evaluations of the staff required to provide OPAT services. However, the conclusions of such studies vary, with the benefits of a nurse-led service10 and the need for infection specialists11 both being suggested. There is a striking lack of prospective studies and only one randomised controlled trial (RCT), which was conducted in New Zealand. 12
Although health economics have been addressed in depth overseas (especially in the USA), there is little detailed analysis in the UK. Most economic evidence comes from studies reporting bed-days saved and simple analyses of cost savings, which are reported to be significant. A comprehensive pharmacoeconomic evaluation of OPAT services has yet to be completed, despite the number of published studies. 1 Chapman et al. 13 did complete a cost-effectiveness analysis of OPAT in a UK setting but this included only one centre and was predominantly a comparison of standard hospital inpatient care with daily attendance at a hospital facility. In addition, owing to a lack of appropriate data, the analysis completed was a cost–consequence analysis rather than a cost–utility analysis and, thus, did not adhere to National Institute for Health and Care Excellence (NICE) guidance. 14
Little has been published on patients’ preferences for different services, although reports of patient satisfaction with services have been cited. Only one study was found to evaluate patient preferences directly in this group, finding that 90% of patients preferred treatment at home to treatment in hospital. 15 However, this study was conducted in Canada, had a small sample (n = 71), compared only two fixed-service models (in hospital vs. SA at home with weekly hospital visits) and used willingness-to-pay to measure preferences.
National policy and initiatives
Outpatient parenteral antimicrobial therapy services have the potential to generate significant cost savings for the NHS and to deliver greater patient satisfaction. They may contribute to the delivery of key health-care strategies and directives such as Equity and Excellence: Liberating the NHS,16 Creating a Patient-led NHS,17 Your Health, Your Care, Your Say18 and Start Smart then Focus. 19 As yet, no national policy in this area exists, although we understand that there have been meetings between members of the British Society for Antimicrobial Chemotherapy (BSAC) OPAT steering group and the Department of Health regarding further service development. Health-care providers mainly use the current system of tariffs provided through NHS England Payment by Results system, which can be interpreted in a number of different ways, thus making the commissioning process complex. For more unusual conditions, the provision of OPAT is part of the recommendations of the NHS England Specialised Services Specifications for Infectious Diseases and Bone and Joint Infection Services.
Following a conference on OPAT in 2009 hosted by BSAC, a UK database has been set up in which centres have the option of sharing their data on, for example, service type, patient numbers and outcomes. A voluntary survey of existing services provided by OPAT group members was carried out at the end of 2011 and presented in summary, focusing largely on clinical issues such as types of infection treated. The BSAC-sponsored OPAT project is supporting the development of such services throughout the UK without favouring any particular model of service design. Various resources have been provided to facilitate this, including the development of practice standards, a preceptorship scheme, regional training days, a model business case including a strengths, weaknesses, opportunities, threats (SWOT) analysis of service models, and software to support a virtual ward round (http://e-opat.com/).
Aims and objectives
The full potential of OPAT has not yet been realised in the UK, as there is patchy implementation and significant variation in services geographically. There is a paucity of information upon which the NHS can base decisions regarding the design, supply and commissioning of such services and upon which national guidance developers can base recommendations for best practice. The proposed research would address significant gaps in knowledge about the cost-effectiveness of different i.v. antibiotic services and identify which services patients prefer and which aspects of the services are most important to them. Given that the services available to patients have different costs, effects and risks, it is essential to understand what patients consider most important in the care they receive and what trade-offs they are willing to make. This is especially so assuming that the trend for enhancing patient choice continues in the NHS. The optimal delivery of OPAT may mean offering patients a choice between several services concurrently, which has consequences for future planning and resourcing. The evidence generated by the research would be used to help identify the optimal configuration of services in terms of value for money and patient preference. The research would also help to identify future research priorities and to design clinical studies that would generate the evidence necessary to aid decisions over service provision.
The aims of this research project are to establish the types of i.v. antibiotics services available in England and to identify barriers to the use of each service type; evaluate patients’ preferences for, and the costs and benefits of, delivering i.v. antibiotics in the community; and make recommendations for the optimal delivery of the service and for the design of future research including clinical trials. i.v. antibiotic services have significant potential for cutting NHS costs and for improving patient choice and satisfaction. The research will help to identify which aspects of services and service types are the most preferred and which offer the greatest benefits to patients and the NHS in general.
Structure of the project report
This study was funded by the National Institute for Health Research, Health Services and Delivery Research programme (11/2003/60 CIVAS).
In order to evaluate the existing evidence for different service models for OPAT, we first carried out a systematic review of the existing literature in the field, focusing on efficacy, safety and cost-effectiveness (see Chapter 2).
We carried out an assessment of current OPAT provision by the NHS to establish reasons for current service configuration and to identify barriers to service provision (see Chapter 3). This consisted of an online survey of current service provision and interviews with health-care professionals currently providing services.
We then used a qualitative approach to determine the preferences of service users for different community i.v. antibiotic service attributes (see Chapter 4). We started by holding interviews and focus groups with patients who had received i.v. antibiotics on an outpatient basis to determine the issues or attributes that were most important to them. From this we developed a pilot discrete choice experiment (DCE) whereby we constructed a number of questions to explore patients’ views on key attributes. After testing this with a number of patients, we used this methodology to analyse the preferences of approximately 200 patients, the results of which are presented in Chapter 5.
Our quantitative work (see Chapter 6) uses economic modelling of the clinical effectiveness of different models of service provision using data collected from seven centres in England for both short-term and long-term infection patient groups.
At the end of our project we held an expert panel event to examine the data collected through the different project workstreams, in order make recommendations for future service design and further research. The panel included commissioners with experience in this field, antimicrobial pharmacists, clinicians (medical and nursing) and experts in health economics and clinical trial design. This event is described in Chapter 7.
The report concludes with a synthesis of our main findings (see Chapter 8) and a discussion of the implications for designing and commissioning future NHS OPAT services, with suggestions for future research.
Chapter 2 Systematic review of the efficacy, safety and cost-effectiveness of outpatient parenteral antimicrobial therapy
Background
The provision of i.v. antibiotics on an outpatient basis is accepted practice in most developed countries but has been slow to develop in the UK. In the UK, a number of different models of care are in existence, with variation in the extent of provision geographically.
Research questions
The overall aim of this review was to evaluate the existing evidence in relation to the efficacy, safety and cost-effectiveness of different community-based i.v. antibiotic services, also known as OPAT. Specific research questions were:
-
What is the most clinically effective model of delivering i.v. antibiotics in the community?
-
What is the most cost-effective model of delivering i.v. antibiotics in the community?
-
What is the most appropriate model for delivering i.v. antibiotics in the community in terms of patient safety?
-
Is community delivery of i.v. antibiotics acceptable to patients and health-care providers?
Methodology
Identification of studies
The world literature from 1993 to March 2015 was reviewed to identify existing research related to the safety, efficacy and cost-effectiveness of community i.v. antibiotic delivery services. Separate searches were run to identify (1) studies of i.v. antibiotics and known models of care and (2) reviews of antibiotic use in cellulitis or cystic fibrosis (to allow for the identification of models of care that were unknown to us and subsequently not considered when identifying terms for search 1). We searched MEDLINE via Ovid (1946 to March week 4, 2015), MEDLINE In-Process & Other Non-Indexed Citations (25 March 2015), EMBASE via Ovid (1947 to 25 March 2015), Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost (1981 to March 2015), International Pharmaceutical Abstracts via Ovid (1970 to March 2015), Cochrane Central Register of Controlled Trials, Wiley, Issue 2 of 12 (February 2015), Cochrane Database of Systematic Reviews, Wiley, Issue 3 of 12 (March 2015), Database of Abstracts of Reviews of Effects, Wiley, Issue 1 of 4 (January 2015), NHS Economic Evaluation Database 2015, Issue 1 of 4 (January), Health Technology Assessment Database, Wiley, Issue 1 of 4 (January 2015), Research Papers in Economics (accessed March 2015), Cost-Effectiveness Analysis (CEA) Registry, Tufts (accessed March 2015), and Health Business Elite, Healthcare Database Search (HDAS) NHS Evidence (1922 to 25 March 2015).
Supplementary searches of Web of Science Conference Proceedings Citation Index – Science, Thomson Reuters (1990 to March 2015), the Health Information Management Consortium via Ovid (1983 to 25 March 2015) and the website of the BSAC (accessed March 2015) were conducted to provide relevant unpublished work. In addition, the reference lists of included studies were reviewed for potentially relevant papers. A sample search strategy and databases searched is detailed in Appendix 1.
Selection of studies
Studies were included if the participants were individuals or groups of adult patients or care providers, and (1) they evaluated the clinical effectiveness or cost-effectiveness of an OPAT model, (2) they described or evaluated patient safety issues associated with OPAT or (3) they considered the acceptability of OPAT from the perspective of the patient receiving treatment or the practitioner delivering care. Any form of i.v. antibiotic drug delivery system (e.g. infusion or bolus) was included. No restrictions on language or study design were applied.
Studies were excluded if they considered the costs related to a model of delivery but did not consider patient benefit alongside these, or if they made reference to costs and benefits but did not report specific cost-effectiveness analysis data [e.g. cost per quality-adjusted life-year (QALY)]. Similarly, studies that made reference to clinical effectiveness without reporting specific patient outcomes were also excluded. Studies that included children or that involved multiple routes of antibiotic delivery were reviewed but excluded if they did not differentiate between outcomes for adult patients or for patients receiving i.v. treatment, and those of other participants. Studies that focused only on the method or process of delivery or on the clinical effectiveness of a single treatment or of one class of antibiotic over another were excluded, as were abstracts only, descriptive or commentary pieces and guidance documents.
Titles and abstracts of all identified studies were screened for eligibility, and full-text versions of papers not excluded at this stage were obtained for detailed review. All abstracts were reviewed by one researcher (EDM) with a random selection (20%) independently screened by a second reviewer. Potentially relevant studies were then independently assessed by two reviewers (EDM with JE, DM, CCM or MT) to determine if they met the inclusion criteria. Differences of opinion were discussed until a consensus was reached.
Data extraction
Data extraction was carried out by one reviewer (EDM) using a standardised pro forma. Data for a sample of studies were extracted independently by a second reviewer in order to validate the items being collected. Extracted data included citation details, study purpose, design, location, duration, population details and clinical characteristics (e.g. reason for antibiotic treatment), models of care [hospital outpatient (HO), SA, GN, SN], topic area (clinical effectiveness, cost-effectiveness, safety, acceptability), type of antibiotic, route of delivery, treatment dose, outcome measures, follow-up and key findings. Assessment of bias was carried out as part of this process.
Assessment of bias
Quality assessment was carried out by one reviewer (EDM). Where possible, studies were assessed using previously developed scoring systems. The Cochrane risk-of-bias assessment tool was used for experimental studies (RCTs, clinical trials, controlled before-and-after studies) and the Newcastle–Ottawa scale was used for cohort and case–control studies.
A method of assessing the strength of evidence of observational studies – developed as part of a previous review on the early diagnosis of cancer20 – was modified for this topic area and applied to relevant studies. The main modification to the assessment system was to account for the fact that, although a study in this area might not use a power calculation and might include a relatively small sample size, this is actually the entire population receiving OPAT treatment. As such, it should not be considered automatically to provide weaker evidence. In this system, papers were evaluated on the basis of ‘population’, ‘ascertainment’ and ‘analysis’ (see Appendix 1). Population relates to the method of determining required levels of participation, with use of a sample size calculation or inclusion of all possible patients/providers rated more highly than selective recruitment. Ascertainment relates to methods of obtaining study data, with use of a rigorous method designed to reduce systematic differences between groups (selection, characteristics, treatment, etc.) rated more highly than other methods. Finally, analysis relates to the use of analytic techniques, with reporting of statistically significant differences (or use of appropriate analytic techniques if qualitative) rated more highly than non-statistical comparisons or descriptive data.
Studies at risk of bias were not excluded from the review, but an appraisal of the strength of existing evidence has been reported and findings interpreted in light of this. Many of the papers included in this review used methodologies that did not lend themselves to the scoring systems outlined above. Many studies included all patients in receipt of OPAT since its establishment at a particular institution, or all patients seen over a specified time period, and simply reported conditions treated and therapies used, along with limited outcomes data. Case series such as these, which were to all intents and purposes audits of service provision that included little or no analytic content, were not subject to quality assessment.
Data synthesis
The main characteristics of included studies and findings relating to clinical effectiveness, cost-effectiveness, patient safety and acceptability and study quality have been summarised in narrative and tabular form. The substantial clinical and methodological heterogeneity precluded pooling of data for meta-analysis. The majority of studies included a varied case-mix and did not differentiate their results between conditions treated. In addition, there was variation across studies in relation to what constituted a successful outcome (cure, improvement, deterioration, etc.) as well as a lack of consistent treatment duration. This meant that it was not possible to pool the results of individual studies to provide estimates of true effect size when using OPAT for different patient groups, or even for comparing OPAT as a whole with inpatient treatment. Substantial clinical [condition treated, duration of treatment and definition of a successful outcome (cure, improvement, deterioration, etc.)] and methodological heterogeneity precluded pooling of data for meta-analysis.
Studies included in the review
Overview
The search strategy identified 7214 articles, of which 589 (8.2%) met the inclusion criteria for detailed review (Figure 1). We retrieved the full text of an additional 17 papers identified from the reference lists of previous reviews and included studies, giving a total of 606 potentially relevant papers. In a change to the initial protocol, non-English language papers were not assessed for inclusion owing to the overall volume of literature identified and timescale of the review (n = 69; see Appendix 1), and we were unable to obtain one article from the library document service. A total of 128 papers were included in the final analysis. Reasons for exclusion are shown in Table 1.
FIGURE 1.
Flow of studies into the review.

| Reason for exclusion | n |
|---|---|
| Descriptive or commentary piece | 118 |
| Non-English language (set aside) | 69 |
| Did not consider an OPAT model | 50 |
| Abstract only | 34 |
| Duplicate paper or study | 27 |
| Evidence summarya | 26 |
| Focus on efficacy of a specific antibiotic | 26 |
| Reported non-i.v. antibiotics | 20 |
| No distinction between i.v. antibiotic/other treatments | 19 |
| No distinction between adults/children | 16 |
| Reported costs only | 14 |
| Focus on method or process of delivery | 10 |
| Survey but no acceptability data reported | 10 |
| Unclear if OPAT model involved i.v. | 8 |
| Lacked outcomes data | 6 |
| Studied children only | 6 |
| Studied other antimicrobial | 6 |
| Guidance document | 5 |
| Reported non-specific effectiveness | 5 |
| Focus on adherence to guidelines | 1 |
| Focus on retention of patient training knowledge | 1 |
| Unable to locate article | 1 |
Populations
Three-quarters of studies were carried out in Europe (n = 53; 41%) and North America (n = 45; 35%). Almost two-thirds of the European studies were conducted in the UK. Two studies involved centres in multiple countries,21,22 whereas another was a systematic review of the world literature (Table 2). 23 Most were relatively small in size (mean 476, median 100, range 6–11,427 participants/episodes of care). It was not possible to determine participant numbers in one study24 and in another numbers could be identified only for three of four included groups. 25 The period under study ranged from 6 weeks to 15 years. In general, studies with the largest numbers of subjects (> 1000) either analysed all cases included in an OPAT registry and/or reviewed cases over a more substantial time period. 21,25–34 Most papers were published in the past 10 years (59%).
| Continent/country | n |
|---|---|
| Continent | |
| Africa | 1 |
| Asia | 9 |
| Europe | 53 |
| America | 50 |
| Oceana | 21 |
| Country | |
| Argentina | 2 |
| Australia | 15 |
| Austria | 1 |
| Bahrain | 1 |
| Canada | 11 |
| China | 1 |
| Columbia | 1 |
| France | 4 |
| Greece | 1 |
| Ireland | 2 |
| Israel | 2 |
| Italy | 3 |
| New Zealand | 6 |
| Pakistan | 1 |
| Peru | 1 |
| Puerto Rico | 1 |
| Singapore | 4 |
| South Africa | 1 |
| Spain | 8 |
| Sweden | 1 |
| UK | 33 |
| USA | 34 |
| Worldwide (SR) | 1 |
The most commonly reported reason for treatment was osteomyelitis, followed by endocarditis, SSTI, cellulitis and septic arthritis (Table 3). The majority of studies involved multiple conditions (n = 72, 56%), although six did not specify indications for treatment. These included two qualitative studies and one survey of patient acceptability,35–37 two surveys of practitioner acceptability,38,39 and one secondary analysis of data from an OPAT database. 40
| Reason for i.v. therapy | n (%) |
|---|---|
| Osteomyelitis | 68 (53.1) |
| Endocarditis | 53 (41.4) |
| SSTI | 41 (32.0) |
| Cellulitis | 32 (25.0) |
| Septic arthritis | 29 (22.7) |
| Respiratory infection | 28 (21.9) |
| Bacteraemia | 27 (21.1) |
| Abscess | 27 (21.1) |
| Urinary tract infection | 25 (19.5) |
| Prosthetic joint or metalware infection | 24 (18.8) |
| Pneumonia | 21 (16.4) |
| Wound infection | 19 (14.8) |
| Sepsis | 18 (14.1) |
The total number reported is > 128 studies, as many involved multiple reasons for i.v. therapy.
Outcomes and outpatient parenteral antimicrobial therapy models studied
Of the five areas evaluated in this review, the most commonly considered were patient safety (n = 109; 85%) and clinical effectiveness (n = 89; 70%). Just over one-quarter of studies involved some aspect of patient acceptability (n = 37; 29%), but few determined cost-effectiveness (n = 5) or practitioner acceptability (n = 6). Most reported on multiple areas (70%).
Twenty-two studies (17%) either did not indicate the type of OPAT delivery model used or reported that home treatment was used without providing any additional detail. In the remainder of studies, the most frequently reported method across studies was self- (or carer-) administration (n = 66; 52%), followed by visits from a SN (n = 44; 34%), outpatient attendance (n = 35; 27%) and visits from a GN (n = 14; 11%). Just over half of these studies evaluated a single model of OPAT delivery (n = 59; 55%). Other, less common, delivery methods and locations included infusion centres, home infusion or home care companies, Hospital in the Home Units (HHUs), prison and doctor visits (see Appendix 1, Tables 33–37).
Quality assessment
Few studies employed a controlled trial methodology, with two-thirds being case series or observational in nature (Table 4). Many involved retrospective data collection (n = 57; 45%). Of the 12 included RCTs, five reported on subgroup analyses from the main study, and all failed to provide details of the original trial methodology.
| Study design | n (%) |
|---|---|
| Before and after | 3 (2.3) |
| Case–control | 5 (3.9) |
| Case series | 51 (39.8) |
| Clinical trial (unspecified) | 1 (0.0) |
| Cohort | 4 (3.1) |
| Controlled trial | 1 (0.8) |
| Cross-sectional | 10 (7.8) |
| DCE | 1 (0.8) |
| Decision tree analysis | 3 (2.3) |
| Literature review (not systematic) | 1 (0.8) |
| Observational | 33 (25.8) |
| Qualitative | 3 (2.3) |
| RCT | 12 (9.4) |
Many studies involved a review of medical records and/or the analysis of data from prospectively held OPAT databases (40%); a small number of other studies carried out secondary analysis of national or international OPAT registries. Satisfaction surveys, by either questionnaire or telephone completion, were frequently used, and interviews (face to face, telephone, focus group) and visual analogue scales, although used less frequently, were also well represented. Less common methods of data collection included clinic and ward diaries, direct observation and data from previous studies or from the published literature.
It was often unclear who collected data, and few studies reported on how this was done (e.g. using a standardised pro forma). A significant number provided little or no detail on the methods employed.
Risk of bias within studies
Three of the 14 included trials were assessed as having a low risk of bias,12,41,42 and one was assessed as having a high risk of bias (a pilot in which patients self-selected hospital or home treatment and were recruited consecutively to each arm after this decision was made). 43 In the remaining nine studies, the level of potential bias was unclear. In five cases, it was uncertain whether or not randomisation or controlling had taken place. Four of these studies reported on subgroup analyses from a single open-label trial, and none provided details of the original study methodology. 44–47 The fifth reported on two related trials comparing i.v. with oral treatment for neutropenia in cancer patients, but reported no methodological details of the parent studies. 22,48–51 In the remaining five trials, details of randomisation, allocation concealment and blinding (especially in relation to assessment of outcome measures) were poorly reported. 52
The five case–control studies (three of which were retrospective) rated low for potential bias (median 8/9; range 6–9). Those studies scoring lowest did not provide details on the methods used to determine outcomes. Similarly, four cohort studies (two of which were retrospective) all had low potential for bias (median 8/9; range 7–9).
In the majority of observational studies, the data analysed were derived from reviews of OPAT databases or medical records. Many studies also involved questionnaire surveys. Only five studies included a comparator (inpatient care), with many simply including all OPAT patients over a selected time period. Most studies reported descriptive results only, with no statistical testing of differences.
Twenty of the 128 studies (16%) received full or partial funding from pharmaceutical companies.
Results
Impact on clinical effectiveness
Only 21 of the 89 studies evaluating the clinical effectiveness of OPAT included a comparator, which, with few exceptions, was treatment given on an inpatient basis (see Appendix 1, Tables 32 and 33). Five of the studies did not specify the OPAT model that was being used,29,48,52–54 whereas six others reported combined results for multiple OPAT models. 48,52–55
Synthesis of the findings from these studies indicates that, regardless of the OPAT model used, there is little impact on the duration of i.v. antibiotic treatment in comparison with inpatient treatment12,22,25,41–43,56–58 (see Appendix 1, Table 32). The effect of OPAT on cure rate, however, is less conclusive. When all models are considered, OPAT appears to produce superior results compared with inpatient treatment, a finding that is influenced by the inclusion of positive studies reporting on multiple or unspecified OPAT treatment models. When these studies are removed and specific models are considered individually, outpatient attendance appears to have a lower rate of cure or improvement,22 and SA59 or OPAT by a SN has a higher rate,56,57 whereas OPAT by GN has no impact. 12,41 Results from those studies assessing the impact of treatment via OPAT specifically on lung function in patients with cystic fibrosis were either inconclusive or found no impact. 42,43,58,60
In the remaining studies, which looked at aspects of clinical effectiveness for OPAT only, rates of cure and/or improvement ranged from 61.1% to 100% (mean 89.6%; median 92.4%). When the various OPAT models are considered individually, the highest average cure/improvement rate was seen for the SN model (90.6%), followed by SA (91.3%), the GN model (90.0%) and HO treatment (85.9%). Few studies reported on bacterial eradication, but those that did saw rates of between 57.1% and 100% (mean 86.2%; median 90.0%) (see Appendix 1, Table 33).
Patient safety and adverse events
Only 24 of the 109 studies evaluating OPAT-related safety included a comparator, which in the majority of cases was inpatient treatment (see Appendix 1, Tables 32 and 33). Five studies did not specify the OPAT model that was being used,48,52,55,61,62 and three others reported combined results for multiple models. 29,49,63
Synthesis of the findings from these studies indicates that, regardless of the model used, there is little evidence of impact on either drug-related side effects or number of deaths in OPAT patients in comparison to patients receiving treatment in hospital10,22,25,41–43,56–59,64 (see Appendix 1, Tables 32 and 34). One study looking at outpatient attendance22 did find a higher death rate (1 patient vs. 0 patients), but this was a small study and the overall rate of side effects was lower in OPAT patients (15% vs. 18%). There also appears to be no conclusive evidence of benefit either in relation to hospital readmissions overall, or in relation to those who self-administered therapy,42,59 although there were conflicting results for OPAT provided by nurses, with SNs10,25,57,64 seemingly having superior results to those of GNs. 12,41 Perhaps unsurprisingly, overall there would appear to be more line-related complications in i.v. therapy administered outside hospital.
Across all studies, the most commonly reported adverse events were rash, fever, nausea/vomiting, diarrhoea, allergic reaction or anaphylaxis, phlebitis, leucopenia and line complications (including line infection, occlusion, breakage, and dislodgement).
Cost-effectiveness of outpatient parenteral antimicrobial therapy
Although many of the identified studies reported on the cost of OPAT, only five considered cost-effectiveness (see Appendix 1, Table 35). Three studies applied decision tree models to OPAT provided by SNs, with one also determining the cost-effectiveness of SA. The remaining studies (one literature review, one retrospective observational study) did not specify the OPAT model(s) used.
In two of the three decision tree analyses, i.v. OPAT was found to be more cost-effective than i.v. inpatient therapy. 24,65 In one case, it was also more cost-effective than early discharge with oral therapy and oral outpatient therapy,65 while in the other its dominance was maintained only when the i.v. success rate was > 55%. 24 Conversely, in the third study, i.v. OPAT was found to be less cost-effective than both i.v. to oral switch therapy, and oral treatment both during and after hospitalisation (which was the most cost-effective option). 66 The authors reported the probability estimates used, which were obtained from both published research and institutional data, to be a limitation of their study.
Studies included in the systematic literature review predominantly concluded that home care i.v. antibacterial therapy would lead to significant cost reductions from a societal and third-party payer perspective. 23 In 5 of the 11 studies, inpatient therapy was 2–3 times as expensive as home care therapy. However, there was considerable variation in the ways in which costs were determined and calculated in the individual studies (e.g. incremental costs, costs for selected components only, etc.), and the review itself lacked considerable detail on the methodology used and the criteria for study inclusion and exclusion.
The results of the observational study (based on 435 courses of i.v. antibiotic treatment for respiratory exacerbations in 116 adult patients with cystic fibrosis) indicated that, for both one course and 1 year of treatment, i.v. antibiotic treatment administered mostly in hospital was more effective but more costly than treatment administered mostly at home. 53 This improved clinical effectiveness could be achieved only with the input of considerable additional resources (between £46,000 and £73,000 per patient at 2002 prices). However, when the strictest definition of effectiveness was applied (≤ 0% decline in lung function), hospital treatment was unlikely ever to be cost-effective.
Patient acceptability
Only 4 of the 36 studies considering patients’ acceptability of OPAT involved a comparison of inpatient and outpatient therapy (see Appendix 1, Table 36), two of which involved OPAT delivery by GNs12,41 and two of which involved OPAT delivery by SNs. 25,56 In each case, satisfaction was high, with home treatment seen as being beneficial. One of the two studies involving GN delivery found that only 5% of home group patients would have preferred hospital treatment, compared with 35% of the hospital group who would have preferred home treatment at home (p < 0.001),12 while the other found that patients in the home group were significantly happier with the location of their care than those receiving inpatient therapy (p < 0.001). 41 Similarly, in one study for which patients received home care by SNs, almost all (97%) indicated that they would chose to receive at-home therapy in future and would recommend it to others. The main reasons given for this were quiet and increased home comfort, familiar environment and free choice of activity. However, some patients in the study also reported disadvantages to receiving therapy at home, primarily related to patient and caregiver anxiety. 56
Of the remaining studies that considered acceptability in OPAT patients only, most involved multiple OPAT models (and did not differentiate between them in their findings), or did not specify the model(s) under study. In general, satisfaction with treatment was very high,2,13,27,36,67–83 including when patients had to have frequent attendance at hospital. 5,84 Commonly perceived advantages of OPAT included the ability to resume daily activities,2,27,71,73 feelings of improved self-esteem or greater freedom and control,67,71,73,85,86 and not having to remain in or attend hospital. 2,71,82,85,87 The main disadvantages most commonly related to infusion equipment, and included anxiety about the device and its sterility,35,37,78 the discomfort and limitations imposed by pump devices,36,37 and issues related to storage. 88 Two studies found that younger patients were better able to use infusion devices, and required less support to do so than older patients. 37,89
Two studies determined patients’ willingness to pay to have treatment in their preferred location, and although differences did not reach statistical significance, patients reported that they would pay more for home-based than for hospital-based treatment, including giving up slightly more of their remaining life to ensure this. 15,79
Provider acceptability
Only six studies included some form of assessment of practitioner acceptability, one involving general practitioners (GPs), one involving nurses and four involving infection specialists (see Appendix 1, Table 37). In most cases, professionals saw advantages for patients (or a need for) receiving i.v. antibiotic therapy outside hospital. 39,86,90,91 However, there were also negative perceptions of practitioner involvement. Most GPs saw no advantage to themselves in home treatment, and many thought distance from hospital was an issue for patients. 86 Similarly, nurses perceived that there were challenges in providing this model of care, mainly around the technical nature of the devices used and dealing with patients’ understanding of the technology and its related risks. 39 Finally, many specialists saw logistical and organisational barriers to the use of OPAT relating to a lack of funding, the availability of a dedicated OPAT team, the number of locations involved, leadership, communication and the links between primary and secondary care. 38,90–92 In addition, there were concerns regarding who should assume the cost and/or take clinical responsibility for patients. 90,92
Discussion
This review has provided a comprehensive picture of the evidence surrounding the effectiveness, safety and acceptability of outpatient antibiotic therapy, and, as such, it is a useful addition to the literature in this area. It has established that there are no systematic differences in relation to the impact of OPAT on duration of therapy, or on adverse events associated with i.v. antibiotic treatment, and that, on the whole, OPAT is more cost-effective than inpatient care. However, conclusive evidence of the clinical benefit (or otherwise) of this mode of therapy compared with traditional inpatient i.v. treatment is lacking.
Acceptability of OPAT appears to be high among patients who appreciate the greater freedom that this provides, particularly in relation to being able to resume daily activities (such as going to work or school), having greater control over their illness and not having to attend hospital but being able to stay at home with family. The most commonly identified disadvantages related to the use of infusion equipment. Few studies considered practitioner acceptability, but those that did found some concerns related to the logistics involved in providing an OPAT service, including cost and who would assume clinical responsibility for patients.
Although many studies were identified and included in this review, its conclusions are limited by the lack of studies involving a usual care comparison, or comparison with other models of OPAT delivery. In addition, few studies employed a rigorous study design. Much of the work in this area appears to be based around service evaluation and, as such, many of the studies provided only basic descriptive findings, with no estimates of variance and limited data related to patient outcomes. As a result, it is difficult to grade the quality and robustness of the evidence, even in the few RCTs that have been conducted. Similarly, the heterogeneous nature of studies in terms of their design and the case-mix of included patients meant that it was not possible to pool results to provide estimates of effect size for OPAT use.
It is likely that the increased use of OPAT in the UK over the last decade is based, in no small part, on clinician’s expectations that it should deliver better patient care and better patient experience. However, the evidence for better care is not strong. In addition, few studies reported on the different levels of service required to account for the complexity of patient cases encountered, including those with comorbidity, those requiring ‘one-off’ or longer-term treatment. Many studies provided aggregated results and it was not possible to disentangle results either for individual OPAT models or for the specific conditions treated. This, together with the lack detail on the actual delivery model used, makes it difficult for clinicians and policy-makers to be able to replicate the practice (and, consequently, the potential outcomes) even from positive studies. OPAT services have the potential to deliver significant cost savings and increased patient satisfaction for the NHS, but this information is key and must be reported in future studies if we are to identify best practice and support decision-making at a local level.
Conclusions
This review provides a comprehensive picture of the current evidence surrounding the effectiveness, safety and acceptability of outpatient antibiotic therapy. It found no systematic differences related to the impact of OPAT on duration of therapy or on adverse events associated with i.v. antibiotic treatment. On the whole, OPAT is more cost-effective than inpatient care, and patient acceptability appears to be high. However, conclusive evidence as to the clinical benefit (or otherwise) of this mode of therapy compared with traditional inpatient i.v. treatment is lacking. Few studies considered practitioner acceptability, but those that did found some concerns relating to the logistics involved in providing an OPAT service, including cost and who would assume clinical responsibility for patients.
Few studies involved a comparison with inpatient care (or other models of OPAT). Even fewer employed a rigorous trial design, and much of the work in this area is based around small-scale service evaluations, with limited outcomes data. Given the cost implications and the potential benefits to patients in receiving treatment outside hospital, there is still a need for definitive, large-scale studies in this area.
Chapter 3 Survey and qualitative study examining current outpatient parenteral antimicrobial therapy service provision in England
Introduction
Until recently, OPAT in the UK was limited to a small number of specialist centres but over the last 10 years services have begun to expand in an ad hoc manner as the potential of OPAT is recognised. 93 In contrast, OPAT has been accepted as the standard of care in the USA and Australia for many years, although it has been restricted to those with appropriate insurance. 1 The 2013 BSAC survey of OPAT in the UK94 found that 68% of centres have some form of OPAT service (based on a 63% response rate to an electronic survey). The most commonly reported model of care was a nurse at home, followed by a hospital attendance, but other models are used. Most respondents to the BSAC survey wanted to extend their service (85% of responders), and a range of barriers were identified, including a lack of nursing and clinician resource, lack of buy-in from other departments and the frequency of antibiotics needed. A small-scale study from the Republic of Ireland found that, although 74% of respondents reported sending patients home with i.v. antibiotics, 47% did not describe themselves as having a dedicated OPAT service, but noted that clinicians often simply send patients home on i.v. treatment, sometimes without the appropriate support. 91
In the UK, there are good practice guidelines on the development and delivery of OPAT services93 but anecdotal evidence suggests that implementation is inconsistent. Although the BSAC survey identified significant barriers to service development, much less is known about the drivers to service development or how people have set up services.
The evidence base is poor and unhelpful to commissioners and providers looking for support to develop such a service. This research aims to explore the current picture of services implemented in the UK and to identify the barriers to and facilitators of implementation. It forms one work package in a large programme of exploratory research around outpatient antimicrobial therapies.
Aim
To explore the types of OPAT services that have been commissioned and provided in the UK and to identify perceived barriers to implementing this type of service.
Although surveys of health-care professionals providing OPAT in the UK were carried out by BSAC in 2011 and 2013, these largely focused on clinical matters such as types of infections treated rather than models of service provision. We set out to look at the service models provided and the issues influencing their development.
Objectives
-
To undertake a survey of NHS trusts to identify services in England and their configuration.
-
To sample from these NHS trusts to identify participants to interview to explore the barriers and levers to service provision.
Methods
Health-care professional survey
A brief electronic survey of infectious disease specialists was conducted using the Bristol Online Survey (BOS) to identify services in England and their configuration.
A letter with a link to the BOS survey was sent to all NHS trusts in England. The letter invited the recipient to complete the survey and to pass the link on to relevant colleagues. When a microbiologist, infection specialist or OPAT team could be identified from the NHS trust website, the letter was sent directly to them. When no details could be obtained, the letter was directed to the hospital main administration office. E-mails were also sent to all infection specialists and microbiologists, where addresses could be identified. The survey was distributed in June 2013 and reminders asking people to complete the survey were sent out in July and August 2013.
The survey was designed to enable us to sample a diverse group of respondents for interview. An initial corpus of questions was developed, from which 20 were selected by the clinical team to give a picture of current practice and to enable us to select a diverse sample. The questionnaire was piloted on five local clinicians to ensure that the questions could be easily understood and that the survey links worked.
The survey asked respondents questions relating to the following.
-
Who runs the service and the staffing levels (doctors, nurses, administrators)?
-
The involvement of other clinical specialties (e.g. infection specialists, microbiology, pharmacy).
-
The size of service and numbers of patients seen per month.
-
The involvement of external organisations (e.g. private health-care providers).
-
Any future plans (development of service).
-
Demographics (where is the service based and what is its coverage), respondent details (job title/position, years in post, years running OPAT service).
The findings from this initial survey were then used to construct a purposive sample using the following criteria: NHS trust type [teaching, foundation trust, district general hospital (DGH)]; geographical area (urban and rural); socioeconomic area [low and high socioeconomic status (SES)]; and diverse ethnicity. Some selection criteria were nested (e.g. hospital type, geographical area) and participant selection ensured that a range of view-points were identified.
These results should be considered in the light of the poor response rate, which could be explained by the timing of this survey. BSAC had just undertaken their survey and there may have been an element of ‘fatigue’ among potential respondents or respondents may have thought that it was the same survey.
Health professional interviews
Sampling
Of the 35 people responding to the BOS survey, 25 agreed to be interviewed. Our original protocol provided for a purposive sample of up to 30 service leads to be recruited using the sampling frame below in order to collect a wide range of experiences. It was expected that some selection criteria would be nested (e.g. hospital type, geographical area):
-
NHS trust type (teaching, foundation trust, district general hospital)
-
geographical area (urban and rural)
-
socioeconomic area (low and high SES)
-
diverse ethnicity.
Interview process
Professional leads were identified from the electronic survey and all who gave permission to be contacted were invited to take part in a telephone interview. All agreed to be interviewed, but four interviews were not conducted owing to difficulties organising interviews, and one interview failed to record owing to a technical failure. For those who agreed to participate, written informed consent was requested along with permission to record the interview.
The interviewers used a semistructured topic guide, developed by the study team from the available literature, to explore current service provision and any facilitators of and barriers to implementation. The interviews were audio-recorded and transcribed verbatim where permission was given. Field notes were taken where permission to record was refused.
Procedure
Four researchers [one psychologist, two applied health researchers (one with a nursing background) and one sociologist] carried out the interviews. Recruitment and set up was delayed owing to the limited time that respondents had available. Interviews were conducted by telephone and recorded with the permission of the interviewee. One participant refused consent to record the interview and notes were made during and after the interview and became part of the corpus of data. Interviewees often had limited time, and interviews varied in length from 30 minutes to 90 minutes. In some cases the interview schedule had to be adapted to fit the time available, so there are cases where some information is missing. Unfortunately, owing to changes in personnel at the start of the study, data analysis was not undertaken until all the interviews were completed, so new emerging themes could not be explored as we would have wished.
Data management and analysis
The data were managed based on principles of information governance at the University of Leeds. The data from the interviews were analysed using a framework approach allowing a structured exploration of the participant’s perspectives and a method to compare and contrast different service types. 95 Data analysis comprised five stages: (1) familiarisation with the data; (2) identifying the thematic framework; (3) indexing; (4) charting; and (5) mapping and interpreting. The process of familiarisation enables the researcher to identify emerging themes or issues in the data. Little is known about why NHS trusts choose to deliver specific OPAT models and so the evidence generated from the systematic review and input from our clinical co-applicants was used to help refine the thematic framework (stage 2). All of the data generated from the interviews were indexed numerically according to the particular theme to which they corresponded (stage 3). Data were then lifted from their original text and placed under subheadings derived from the framework (stage 4). A process of constant comparison was used to examine across themes and cases. This approach was employed to ensure the collection of a large amount of detailed information about the range of services, geographical location, barriers and facilitators (personal and attitudinal), resource issues including staff, budgets and processes for managing and monitoring patients. The interview transcripts were used to identify key information for each service and key themes about the development and implementation of OPAT services in the UK. These interview data were considered by the expert panel and informed the modelling and DCE workstreams.
Results of the Bristol Online Survey
A total of 35 responses were received from 120 potential responses. Of the 35 respondents, 17 were in the south of England, nine were in the north of England and six were in the Midlands. A further three were based at tertiary centres covering large parts of England.
Twenty-seven of the 35 respondents (77%) reported that they currently had an outpatient i.v. antibiotic service. Of these, 15 centres covered the entirety of their NHS trust catchment area, and the remainder had a more limited service. An additional three respondents indicated they did not have an OPAT service but offered an ‘ad hoc’ service, delivering home i.v. therapy from one specialty (e.g. renal) or by the district nursing team if there was available capacity.
A broad range of health professionals responded to the survey (Table 5); one-third of respondents were microbiologists and one-quarter were infectious diseases specialists. Five respondents were nurses.
| Job title | n | % |
|---|---|---|
| Consultant in infection/infectious diseases | 6 | 17 |
| Consultant in infectious diseases and microbiology | 2 | 6 |
| Consultant in infectious diseases and general medicine | 1 | 3 |
| Consultant infectious diseases/medical microbiology and virology | 1 | 3 |
| Consultant microbiologist | 12 | 34 |
| Consultant in emergency medicine | 1 | 3 |
| Consultant in respiratory and acute medicine unit | 1 | 3 |
| i.v. therapy team leader | 1 | 3 |
| i.v. nurse/OPAT CNS/i.v. SN | 4 | 11 |
| Advanced nurse practitioner | 1 | 3 |
| Pharmacist | 3 | 9 |
| Specialist registrar microbiology | 1 | 3 |
| Not completed | 1 | 3 |
Service organisation
Outpatient parenteral antimicrobial therapy services were based in a range of departments, with 20% (n = 7) based in acute medicine departments, and 29% (n = 10) based in infectious diseases departments; however, the majority (54%) were spread across a range of departments including microbiology, surgery, orthopaedics, accident and emergency (A&E) and respiratory medicine. Other respondents described their service as ‘informal’ and not located in any specific department. Most services were modest in size, with 48% (n = 13) of services having between one and three whole-time equivalent (WTE) dedicated OPAT nurses, and only 7% (n = 2) having more than five nursing staff. Five services (18%) had nurses who worked on the OPAT service in addition to other roles (e.g. ward nurse). About half of services (48%) had less than one WTE doctor involved in the service, and in most cases the service was consultant led. A small number of services (n = 2, 7%) were led by a microbiologist and a nurse, and one service was covered by a hospital-attached GP.
No one had much administrative support, with 74% (n = 20) reporting less than one WTE administrator working for the service, and 26% reporting that they have no administrative support. Eighteen respondents (two-thirds) had an infection specialist (microbiologist or infectious disease specialist) involved in the service, with another 18% having access to a microbiologist. A total of 15% of respondents had no infection specialist or microbiologist involved.
Eight respondents said that they did not offer an outpatient i.v. service, but went on to provide details of their service. This finding may be explained by the individual completing the survey not having involvement in the services offered by their organisation. Some insights into this have emerged from the qualitative interviews (discussed later). Some of those eight individuals did not class the services provided by their organisation as conforming to an OPAT model. Some described services provided by outlying community hospitals (part of their trust) which were not overseen by the infectious disease specialists. These were not described as official OPAT services by those interviewees.
However, the descriptions provided by those eight respondents appeared broadly similar to those provided by respondents who said that they do offer an OPAT service. Services are based in a range of locations, with two services based in microbiology, two based in acute medicine, one based in infectious diseases, one operating as an ad hoc service with no base and two not running a service. Staffing levels are also similar between services reported by those who said that they ran an outpatient i.v. antibiotic service and those who said that they did not. Half of respondents who did not run an outpatient i.v. antibiotic service had less than one doctor involved in their service; one service had five or more and three said ‘other’. In terms of nurse staffing levels, three respondents had less than one nurse, two had between one and three nurses, one service had five or more nurses and two said ‘other’. A total of 63% (n = 5) of the eight respondents said they had less than one member of administrative staff, with the remaining respondents answering ‘other’ to this question.
Service size
The majority of units that provide an outpatient antibiotic service treated between 1 and 10 short-term and long-term patients per month (44% and 52%, respectively), with only one unit treating 30 or more short-term patients per month. Most units offer more than one model of care (Table 6), with > 80% offering a hospital attendance service and three-quarters offering a district nurse model. Few services use a ‘general nursing’ model for the provision of OPAT.
| i.v. antibiotic outpatient services offered | Number of units offering the service | % |
|---|---|---|
| Patient visits the hospital to get antibiotics | 22 | 81.5 |
| A GN administers antibiotics at patient’s home | 3 | 11.1 |
| A district nurse administers antibiotics at the patient’s home | 20 | 74.1 |
| A SN administers antibiotics at patient’s home | 12 | 44.4 |
| Patient/carer receives training and self-administers antibiotics at home | 14 | 51.9 |
| Other (including GN and SN options) | 12 | 44.4 |
Of the eight respondents who said they did not offer a defined outpatient i.v. service, four services reported that they treated long-term patients (between 1 and 20 patients per month), two services treated between 1 and 30 short-term patients per month and one service stated that they treated 30 or more short-term patients per month. However, half of the services reported that they did not treat long-term patients or did not record figures. Just over half of respondents said that they either did not treat or did not record the number of short-term patients.
Half of the respondents who did not report a specialised outpatient service said that their patients visited the hospital to have treatment. The other half of services offered a district nurse service at home. SNs were used by three of the eight services, and GN and SA models of care were used by two services. Two respondents said that they did not use any of the services.
Rationale for model of care offered
Respondents reported that they offered an OPAT service largely because the lead clinician has been an advocate for change (70%; n = 19). However, there are often several drivers for change, including patient preference (63%), commissioners’ decisions (26%) and management decision (40%). It seems likely that the decision to start or continue a service is multifaceted, involving decision-making at several levels.
Results of health-care professional interviews
Sample characteristics
Of the health professionals interviewed, four stated that they were from a tertiary service that treated patients from a range of counties; two served areas in the north of England, one served an area in the south of England and one service served patients in the north of England and the Midlands.
Some sites serving largely rural populations provided only a service to those living within a few miles of the hospital (although the distances varied), whereas others offered a limited service (generally a nurse at home model) across a larger area. Eleven respondents reported working in a teaching hospital, most of which were also foundation trusts; the remainder were DGHs. Eight respondents worked in areas with high levels of economic deprivation, and these were also areas of high ethnic diversity. Eight respondents reported that they did not run an outpatient i.v. antibiotic service. The majority of those who said that they did not run an OPAT service reported that some clinical specialties had ad hoc services offering some form of community/hospital-at-home service on a case-by-case basis. One respondent did not elaborate on why there was no outpatient i.v. antibiotic service and another respondent stated that there was no service because there was an intention to use a private company to deliver i.v. antibiotics in the community. (A summary of the key characteristics of each service can be found in Appendix 2, Table 38.) The professions of those interviewed included nurse specialists (3), microbiologists (6), pharmacists (2) and infection specialists including joint qualified (9).
This study includes descriptions of 19 OPAT services in the UK (20 interviews), details of which are provided in Appendix 2, Table 39. Service types are summarised below.
Themes from the analysis
The analysis of the data from interviews with professional leads from OPAT services currently being delivered has provided an in-depth analysis across a cross-section of services in England.
A priori assumptions and emerging themes:
-
variations in resources needed to deliver OPAT
-
staffing
-
funding
-
location and geographical issues influencing delivery
-
-
facilitators contributing to service delivery
-
core staff and additional staff needed
-
conditions treated and pathways for doing this
-
monitoring effectiveness
-
evaluation, impact and outcomes
-
-
barriers to delivering a service
-
lack of planning
-
lack of evidence
-
lack of resources or facilitators
-
-
relationships within and between people in organisations (e.g. commissioners, providers, partnerships).
Emerging themes that were uncovered through the analysis have included:
-
the influence of change management in transforming services
-
the role of personal and professionals networks to exchange information
-
risks to patients through management and monitoring of services.
Service models
Service development
The descriptions of service models reported here cover current service models and future aspirations. There was a sense that many services had evolved and that they would continue to do so.
Some described services that lacked formal organisational structure, often summed up by participants as ‘ad hoc’, and many were in the process of trying to develop a more coherent service. This was not always a straightforward process but required the individual clinician to be highly motivated and familiar with commissioning processes:
I’ve been in post which is about 6 years and I’ve been trying for a long time of that to try and get an OPAT team in place.
Interview 10
Other interviewees had gone down a more formal route and approached trust management using business plans to support the case for their OPAT service:
Well we’ve done so many business plans I could talk about them all. For the last [. . .] to keep the service running I think we’ve done about six business plans.
Interview 8
Location and service type
A range of services were described across urban, semirural and rural locations. Services could be delivered in hospital by specialist staff and in the community by either SNs, GNs or a mix of both. The core members of the team were a clinical lead for the service, such as an infectious diseases consultant, a nurse (this was often not a dedicated post and could be added on to other duties), a pharmacist and microbiologist.
There was a range of service model types. For example, in hospital the service required support from at least one nurse and from administration/co-ordination services for its day-to-day running. Sometimes there was a team of people who supported these patients to be discharged from hospital, and in other cases discharge was down to the consultant in charge of a patient’s care.
Most services could discharge patients to their own homes into the care of community or district nurses. This was illustrated by a number of responses:
Community nurses know their communities quite well and many of these patients will have community nurses going in anyway . . . so it’s an efficiency of resources to try and get the same person going.
Interview 12
It made sense using existing district nurses who were around the area anyway rather than a nurse to go from one side of the county to another and spend a lot of time travelling.
Interview 10
The inefficiency of getting a hospital specialist to do it, I think would outweigh any benefits.
Interview 12
In some cases, the delivery of community-based services was commissioned out to a private health-care provider, as this was considered to be more efficient than setting up their own service. One of the issues for providers and commissioners was ensuring that staff had sufficient case-loads to maintain competency in i.v. antibiotic administration:
There was a sense that the ideal model of service would be organised and monitored centrally at the hospital but delivered locally:
What we have been doing . . . is to organise local services to deliver the antibiotics and then trying to keep tabs on them in terms of monitoring bloods, etc.
Interview 5
One respondent described a nurse-led service whereby there was a one-step process to set up OPAT. Someone within the hospital (probably a nurse) would go to assess the patient and that person would also check the regime with an infectious diseases doctor. The respondent stated:
If they want to discharge a patient to OPAT they have a single telephone number that they ring and someone goes and assesses that patient’s suitability.
Interview 10
In this service model, patients could be monitored by their own consultant or by the OPAT team. In other models they would be discharged back to their consultant at the end of the treatment.
Two service leads made it clear that the infectious diseases consultant should be involved to check the appropriateness of the regime:
It has to be at least discussed with the infection consultant to make sure that the antibiotic administration is right and the duration is right.
Interview 12
Only one respondent mentioned telemedicine in relation to providing support for patients in the community:
So we’re just looking into setting up skype™ [Microsoft Corporation, Redmond, WA, USA] and having skype clinics.
Interview 12
Building the case for support and commissioning the service
Business planning
Twelve services had a business plan in place, some of which were more established than others. Some business plans were yet to be approved.
Most respondents emphasised the importance of getting the commissioning right and the need to develop a business case. Demonstrating the need was characterised by one as ‘show me the money’ and another also noted that there was a need to build a financial case. Reference was also made to user satisfaction, which was seen as a priority in all aspects of health care.
Commissioning
Outcomes-based commissioning was alluded to, but examples of this were few. However, other respondents concentrated on the issue of using the promise of decreased bed-days and numbers of beds on wards as the driver for change:
[We have looked at] the length of stay for these patients and then looking at what we could do if we reduce the stay for some of the patients. If we were treating patients, we’d be making more money as we can get patients back in those beds.
Interview 3
The [GP managers] are the people driving it now which is a big plus in facilitating change as it is coming from commissioners now, and they have taken ownership and at the stage of looking at, they have asked us for the financial information whether they need to pump prime for the service, whether they need to get the money from elsewhere generally.
Interview 3
From our point of view, the CCG [clinical commissioning group] do stand to gain quite a lot financially and the trust loses because we lose the excess income from these patients. I think it depends what you put into that bed in its place really whether we then do actually gain or lose.
Interview 10
One respondent said that funding was ‘a bit of a problem’ in relation to getting agreement from the CCGs. Who will pay for the drugs is central: ‘they won’t go home until funding is agreed by the local CCG.’ Most services did appear to have funding arrangements in place with their local CCGs. However, those that had patients from a wide geographical area had more difficulty, with one stating that a national commissioning framework was needed to support future service development and a more consistent approach. It was felt that this would circumvent any problems that could arise from an inexperienced team taking on OPAT delivery. One respondent noted that the CCG had been ‘incredibly supportive’ and that GPs had agreed it was ‘the way forward’. Another said:
Even within our meeting the commissioners do say that quite clearly, even if it costs money, we can’t say it costs money, if it’s in the interests of the patients, we have to do it.
Interview 12
Sometimes, decisions to implement changes were based on current pressures in the system and the availability of short-term funding:
Hospitals are always pushed for bed space in winter [. . .] So the [Trust X] team developed quite a number of pathways for the service, but again, we were still working with commissioners about what we would get paid to provide the service, etc. Eventually what happened was around January they had got some extra funding left over from the end of the financial year that they decided to put forward some money for support.
Interview 13
Patient pathways
There was little reference to service specifications or processes for managing patients, but some areas had looked at this in detail and it is possible that there are other examples that were not discussed in the interviews. A number of services had developed patient pathways or delivery models, with one respondent stating that they had 10 pathways:
We have pathways and we follow all these pathways on a daily basis.
Interview 12
Team members and working relationships
Core and additional team members
The core members of the team were generally described as being a microbiologist, an infectious diseases consultant, a nurse and a pharmacist. There were variations across the services, and the OPAT service formed only part of the roles of these personnel. Some services referred to a support worker or patient liaison service, a pharmacy dispenser, someone from the community team and an i.v. access team. In some cases it was explicit that the hospital team took total responsibility:
The model which we will have is that with a nurse, consultants and pharmacist and administrative staff as part of a team within the hospital and we will find these patients and get them out of hospital, and taking total responsibility for the patients once they have left hospital.
Interview 12
Responsibility and teamwork
However, the placing of responsibility was not clear overall and, for example, included shared responsibility or responsibility lying with the GP in some cases (e.g. for cellulitis) or with the community nurses and the orthopaedic surgeon in others. Responsibility was implicitly mentioned as one of the reasons for developing a formalised service, as noted by one respondent:
All the pieces of the jigsaw are there, what we’re trying to do is bring it all together as one and get a team to take responsibility for this patient.
Interview 3
It was viewed as important that the team should have regular meetings, most likely every week:
We review all the results, prescriptions, side effects and all that side of the stuff for each patient and we do that, so it’s like a virtual ward round.
Interview 5
The expectation will be on a weekly basis, the whole team with the consultant and all the doctors, registrar, the microbiologist, the infectious disease consultant and the infectious disease registrar, a pharmacist, microbiologist, a nurse and admin staff that as necessary and someone from the community team.
Interview 3
One person noted the importance of a good relationship and rapport between team members:
I would say that we have a mutually good relationship, whereas if I rocked up out of the blue and told them to do something with their patients they may not. So I think you need to build rapport and relationship with the clinicians that you’re working with quite closely.
Interview 15
Leadership appeared to be important for some:
You need a champion though, someone who takes it into their daily role to do it and to take it on as their thing.
Interview 15
However, there were examples of services having evolved without a designated clinical lead:
I think the good practice guidelines say we should have a medical lead involved and we don’t at the moment.
Interview 2
The background of the interviewee was not always clear, but leadership roles were undertaken by a nurse, microbiologist and pharmacist in different cases. It was also stated that taking things forward required a team champion:
Unfortunately people don’t think about it so the service is not used, it’s underutilised because you need a team championing it.
Interview 3
A degree of friction between primary and secondary care was apparent in some cases; for example, the district nursing service was described as ‘very patchy’, the practice in primary care was described as ‘quite variable’ and some resistance to sharing data and information was also mentioned.
Self-administration
Respondents were generally positive about SA:
With the right device and training the patient it works just as well.
Interview 3
Getting people back into work or study or family life and the district nursing model can really take away from that from them to the tied into being in the house for 6 to 8 hours a day depending on where they are.
Interview 12
Although in many services SA was offered only in rare or exceptional circumstances, it was something that they would like to increase in the future:
Very occasionally we have [a] patient to self-administer but it’s not the norm.
Interview 10
One respondent noted that if the patient was not able to draw up the antibiotics themselves, prefilled syringes were supplied, although they were not available in all services.
Patients needed to be on the antibiotics for a period longer than 3–7 days for SA to be appropriate:
So the longer they are going to be on it, the more likely we going to try and discuss with them self-admin or family admin.
Interview 12
The nurses watch the patient give their first dose at home:
Once they go home the nurses go round and watch them give the first dose of i.v. antibiotics at home and what they do is they make the environment safe and not every home environment is ideal. They can’t take them [i.v.] and use them before the nurses appear, the nurses bring them to make sure that the home is safe.
Interview 15
Monitoring and review, governance and risk
Monitoring
Monitoring or review of patients was mentioned by most respondents, with 12 services having some kind of formal monitoring system in place. However, seven services had unclear or informal arrangements:
[There isn’t a] single way of capturing all these patients at the moment.
Interview 10
Another respondent notes that:
. . . we found quite a few patients who had literally been on (OPAT) the 6 months, 12 months, longer even with no end in sight and no overview.
Interview 6
The common process for monitoring patients was for patients to come back to the clinic every week, unless this was constrained by distance:
They are coming up every week which is the ideal situation so we assess them and do bloods and make clinical assessments and give them their medication so there is an opportunity for making sure that they are taking their treatments and that everything is right and all engaged.
Interview 12
The same person (interviewee 12) mentioned that a formal database modelled on the BSAC recommendations was planned:
And we set up a database so we can keep track of these patients as well, but only on a day-to-day basis, but in terms of outcomes type basis as well, which we’re designing.
Interview 12
The database used was based on one elsewhere, which was described as:
. . . very good, because it acts as a virtual ward and it is searchable as well, you can query it when you’re auditing for outcomes and side effects and that sort of thing.
Interview 12
It is difficult for some services to know how effective they are being owing to a lack of formal monitoring or evaluation. However, this could have been a result of the lead not choosing to disclose important details in some services. Some of the clinical leads had limited time for the telephone interviews and so may have prioritised other types of information.
Governance
Governance was mentioned by most, with its importance emphasised. One example mentioned clinical governance meetings at which:
We discuss all the adverse outcomes we’ve had, how the service is running; we could have done differently, is there any trends coming out, then we can do better [. . .] I think it is about trying to drive a service that is flexible and meeting the needs of operations with something that can have an appropriate to governance structure.
Interview 13
Some service models were based on monitoring and assessment data routinely collected and governance systems that focused on improving quality and safety for patients by reviewing the length of treatment and issues such as toxicity:
We have a very formal governance structure and a very formal pathway and weekly OPAT patient clinical and governance meeting where we discuss every single patient on OPAT and discuss where the plans are going. We review all the patients who are on OPAT for more than 5 days in our infectious diseases clinics so all patients are followed up quite intensively and again that’s not always (been) the case but we find that this prevents people getting long-term antibiotics that they don’t need.
Interview 12
Recent reports such as the Francis96 and Keogh97 reports do seem to have acted as levers to improve the quality and safety of care in some services.
Risk
There were a small number of services operating with informal monitoring systems and at least two examples of services where patients had been on antibiotics for a prolonged length of time and cases of patients being readmitted for the same problem. This tended to be where services were understaffed or did not have dedicated clinical leads. Services without a clear medical lead had some difficulties and delays in decision-making:
I suppose it’s a bit more difficult trying to get decision-making on a patient, kind of whether you carry on their i.v. treatment or whether you decide to stop them.
Interview 2
There was a perception of an increased risk to patients in the community when services are commissioned out to private companies. Whether or not staff in private companies were skilled in knowing when the patient was at risk or should be referred to a clinician to avoid complications was called into question. For example, one lead stated:
So what we found initially was that they could do OPAT, but it was very much on an ad hoc basis done by the private company just doing whatever they were paid for, and there was no one keeping an overview on the patient, so essentially we found quite a few patients who’d literally been on OPAT for 6 months, 12 months, longer even with no end in sight and no overview.
Interview 6
Evaluation, outcomes and impact
Outcome-based evaluations were few. Around seven services had evaluation plans in place:
Yes we did our last evaluation about 2 years ago and that was a full evaluation looking at patient satisfaction and we also have a look at lines from the point of view of thrombosis and infections and adverse consequences so we have a programme where we review everything on an annual basis so pretty much we take each one of those each month and assess it and basically about 18 months from now we do a full evaluation.
Interview 12
One of the key drivers of implementing this type of service was to save bed-days and to reduce the numbers of patients remaining in hospital for prolonged periods. There are some good cases of services that have saved excess bed-days. At least one service has been able to translate this into changes by reducing the number of beds on wards from 18 to 12. One respondent said that there had been:
an absolutely massive difference on the number of patients that are staying in. So we are reconfiguring wards so there’ll only be 12 beds instead of 18.
Interview 15
In some cases, there was a lack of clarity about how to translate a saving in bed-days into real changes in the system:
I think it is quite complicated so if the patient has now gone home and that bed is empty, the only way you’ll save any money on that bed [is] if you [close] that bed and you no longer staff it. [Closing] random beds scattered throughout the hospital, you’re not really going to make any savings on those, and you’ll only save if you close half a ward or something like that.
Interview 10
There is clearly some knowledge in the system about how to do this and a mechanism to share such learning could be considered.
Most respondents mentioned service evaluation based on feedback from patients, focusing on patient satisfaction and reporting a positive response:
The feedback has been fantastic. Feedback that has helped us to get more funding to be honest, patient satisfaction is extremely high so that’s been good.
Interview 15
It’s a no-brainer for a patient really, they get out of hospital.
Interview 5
Some of the leads alluded to improving outcomes such as returning people back to their usual lives and functioning before they were ill:
The important thing that we found from patients is you know this is not trying to get them out of hospital and save money and although there’s an element to that, the aim is to get them back to the life they had.
Interview 12
Change management
Most services proposed some changes to their current system, although some believed that there was no good reason for change:
It’s primarily configured in the way that it’s configured because no one has decided to configure it in a different way. There just didn’t seem to be the requirement for the sort of configuration that I’ve described that, seemed to work well for the disciplines that were delivering it, so we just left it alone really.
Interview 1
The services with the best levers and agents for change tended to be in urban areas, but there were problems across the board. Many services took an incremental rather than a transformational change approach and did not have a vision of the service for the future. There were often tensions in terms of needing to offer something now (based on a service that evolved over the years) and the need to change based on knowledge of best practice. A few were currently waiting for plans to be approved.
Only one service lead described how they had visited another successful service and adapted the model to suit their own local context:
I spent some time with a service [. . .] when I was trying to set up this service [. . .]. and I tweaked it to kind of suit how we run ours, and that’s how when I set up this service I kind of configured it [based] on theirs.
Interview 2
This indicated some partnership between services. In some areas it could be beneficial for services to work together and share best practice and possibly solve problems, which could occur at a regional level. There is also scope for developing a national commissioning framework that could be adapted at a local level.
There were variations in the perceptions that service leads had about the potential of the organisation to change. In some cases there were reports that senior managers or commissioners were not convinced by the evidence or the scale of the impact and benefit for patients. Some felt that they were deliberately being obstructed.
I don’t really quite understand what all the difficulty is, but there is always historically huge managerial resistance.
Interview 20
Relationships within and between people and organisations
Service leads have developed and evolved services based on their informal and formal networks, which may depend on the size of the trust. Many services developed and evolved based on the motivation and networking abilities of the leads:
It’s a small trust, and we’re pretty well supported by the consultants that use it, so we’re able to contact those fairly easily. I think if it was a bigger trust and you couldn’t get hold of your team then that may be more difficult.
Interview 2
Some service leads did not include a dedicated post such as a pharmacist, but the pharmacy service was still involved in an informal way and may be involved as part of a wider remit:
[. . .] and we are desperately trying to get a nominated pharmacist but that hasn’t happened yet. There is one pharmacist normally in charge of infectious diseases not just OPAT and so if there are problems I ask her but there isn’t regular review or someone to sort of go through the charts and make sure of all the interactions that is really something that we are lacking.
Interview 5
This could indicate a stretched resource or a small number of patients with infections in that area and may be linked to geographical context. Some services such as microbiology and pharmacy are implicit as part of such a service. There were variations in the level of interaction that services had with commissioners, which could be related to the post held by the service lead and their level of seniority. At least two services were jointly commissioned with involvement of partners across CCGs, NHS trusts and community organisations. In some cases, there was tension between providers and commissioners and a lack of trust or engagement between groups.
Discussion
Service models
Muldoon et al. 91 undertook a survey of OPAT in the Republic of Ireland and reported a participation rate of 10.7%. However, their target participants were slightly different from ours in that they had a broader professional base which included consultant physicians who made use of OPAT for their patients. They report similar results to our findings about the most common clinical conditions treated and some of the problems reported by our respondents. Owing to our modest sample size, our survey results are not generalisable but they do enable us to draw up a representative sampling frame to undertake the qualitative interviews.
The OPAT professional leads interviewed delivered services in a range of settings. Most offered some level of hospital provision, administration and delivery as well as a community delivery option. Community services were delivered by district nurses or private companies. Service models varied from being well organised and well planned with regularly updated business plans to those described by leads as being ad hoc. Many services had evolved and changed incrementally, although there were also examples of transformational change.
Lane et al. 38 suggest from their results that routine infectious disease consultations in an OPAT service can potentially improve antimicrobial stewardship, citing Sharma et al. 11 for their conclusions. Sharma et al. 11 found that when the infectious diseases consultant was involved in patient assessment, there was optimisation of the treatment regimen and 27% of patients did not require OPAT. Defining leadership within teams may contribute to improvements in care.
It appears that the further away from the hospital a patient lived, the less likely they were to be offered a service in which a nurse comes to their home. However, there were some exceptions to this. The logistics of delivering the service with populations over wide geographical areas did cause some specific issues for delivering an OPAT service particularly for patients who required more than two i.v. administrations per day. Lane et al. 38 identified the problem of providing care over a large number of locations as a potential barrier to safe care.
Team members and working relationships
Some services had dedicated OPAT posts, whereas others included OPAT as part of a wider remit; this could depend on a range of factors such as the evolutionary organisation and planning of the service. Although good practice recommendations are in place, services did not always manage to meet the criteria on service structure. 93 Responsibility for care was not always explicit and there were examples of clinical leadership in the form of a designated role being lacking. Leadership was often provided by nursing staff in those situations. The lead role was often bestowed on an individual, particularly in the unplanned type of service. This meant that ownership of the service and perceptions of an individual’s ability to improve or transform the service varied. The additional work could be perceived as an additional management burden on top of usual roles and be interpreted as resistance to further change. Again, the OPAT good practice recommendations have specific recommendations regarding leadership of the service, and there were instances in which these were not met. 93
Organisation, funding and commissioning
Business cases were viewed as important to build the case for support based on existing evidence and local insight. This also may reflect the practice of the hospital senior management team and or relationships with commissioning partners. Several services had to provide annual reports to governance committees to maintain funding levels based on the numbers of referrals.
Some services had received short-term funding with no guarantee of continued investment. Services were often limited by their funding, with expansion being difficult. Funding was a primary reason why services might adapt or evolve over time. Muldoon et al. 91 reported problems obtaining funding to cover the costs of OPAT in both the public and private health systems.
Patient management, monitoring and evaluation
A number of services have monitoring systems in place. Some referred to national standards (e.g. the BSAC surveillance system). 93 In many cases, this was linked to a greater level of scrutiny in terms of the clinical governance of the service. Individual patient results were reviewed by multidisciplinary teams (MDTs) via virtual wards and were then passed on to governance committees and intelligence functions, often as formal reports. This level of monitoring is considered best practice in the literature;98 however, the survey work undertaken by Muldoon et al. 91 and Lane et al. 38 report that responsibility for monitoring patients is patchy at best. Lane et al. 38 noted in their US survey that only 22% of respondents reported having a system in place to pick up adverse events or ‘near misses’. They also found that a dedicated OPAT team treating 16 or more patients per month was more likely to have monitoring systems to review and act on patients’ laboratory results. Conversely, the fewer patients treated, the less likely it was that patients would be cared for by a dedicated OPAT team, which the authors conclude is a barrier to providing safe services. 38
Outcomes
For those who were able to discuss evaluation of their service, the outcome on which they tended to focus was reducing bed occupancy, although some did highlight the need to improve patient satisfaction with care received. There is considerable scope to identify relevant patient outcomes and improve care.
Strengths and limitations
A weakness of our study was the poor response rate to our survey. We were limited by sending out our survey shortly after the BSAC sponsored survey, which is likely to have affected response rates. However, our response rate was similar to other surveys91 and did provide us with a wide sample of respondents for our interviews. Owing to a change in personnel during the project, we were unable to analyse the interview data until all the interviews had been completed; therefore, we were unable to explore and expand emerging themes as we had planned. Time was a limiting factor in some of the interviews, with respondents able to give us only 20 to 30 minutes for the interview, which meant that some of the data for some services are missing.
We did not interview anyone from the commissioning side of OPAT and at the expert panel we realised that missing this important stakeholder group out from the health professional interviews may mean that we have missed an opportunity to gain a better understanding of why OPAT is or is not commissioned.
Recommendations emerging from the interviews
Service models
-
A wide range of infections were treated, from cellulitis to bone and joint infections, diabetic foot infections, respiratory, renal and other infections in a broad range of specialties. This means that OPAT services within a number of organisation could be somewhat fragmented. Examples of effective patient pathways could be shared, with the caveat that they be adapted to local circumstances and not just used as an ‘off the shelf’ solution.
-
Self-administration by patients or family members was uncommon among our participants. In some areas it was restricted through lack of resources or a perception that there is no need for it. Shared examples of where this works well and how this has been achieved could support those areas that have not yet succeeded in providing this for their patients.
Working relationships
-
The role of OPAT champions and of leads appointed to transform the service need some consideration. Commissioning guidance could focus on the type of partnership and input that is needed from stakeholders to drive forward and sustain any changes.
-
Some work may be required to establish how formal partnerships succeed or to audit the effectiveness of existing formal and informal networks. It is possible that this work may have to be prioritised at a regional rather than a local level depending on the population served and the size of the trusts involved.
-
One respondent listed five key issues that drive commissioners and providers to develop a more formalised service. These were:
-
clinical governance
-
individualised care that is currently not patient centred
-
decreasing costs and getting people out of hospital and creating beds
-
patients’ preferences to be managed at home
-
meeting national good practice.
-
Some of the leads expressed an interest in the development of a national commissioning framework to benchmark consistency in commissioning and providing OPAT services. This could be helpful particularly if made available from the BSAC website.
-
There are some services that have established evaluation frameworks and it could be helpful if these were shared.
-
Outcomes for services need to be considered and should include improvements in patient care, cost-effectiveness, professional and patient satisfaction with the services provided. This could be informed by national guidance.
Chapter 4 Qualitative study investigating patient perceptions of outpatient parenteral antimicrobial therapy
Context
In this chapter, we present qualitative research that explores patients’ experiences of OPAT services and the subsequent development of the discrete choice questionnaire. This work, along with the findings of the systematic review (see Chapter 2), underpins the development of the DCE, the results of which are presented in Chapter 5.
The systematic review revealed that patients’ acceptability of OPAT is high. The initial review in 2013 identified 34 studies that examined patients’ acceptability of OPAT, but, of these, 21 were quantitative surveys assessing satisfaction with the service and provided little information about patients’ own perceptions of the benefits and disadvantages of OPAT. 5,12,13,15,25,27,36,41,67,68,70,73,75–78,80–82,86,89 Only six interview-based studies were identified by the review. 35,37,79,85,88,99
The literature indicates that patients generally view OPAT very positively. The main benefits relate to the comfort of the home environment56,85,86,99 and increased freedom and autonomy. 2,56,71,73,86 Some patients reported that OPAT provides a sense of ownership over their illness and increased involvement in their treatment. 67 Kieran et al. 2 found that patients trained to self-administer their treatment were happy with this and that over three-quarters would be content with telephone follow-up or less frequent outpatient reviews, providing that the care of their i.v. access could be ensured. Contact with health professionals may also be qualitatively different when patients are cared for via OPAT as opposed to via inpatient care, with Dubois and Santos-Eggimann99 reporting that patients found contacts with health-care professionals delivering OPAT to be less impersonal and more relaxed than contacts with health-care professionals in hospitals.
However, not all patients view OPAT positively. A small UK study found that, although patients felt that they would recover more quickly at home, some expressed concerns about safety and, in particular, about the competence of nursing staff to administer treatment. 35 The information needs of patients are not always sufficiently addressed35 and poor communication between staff and patients may also affect patient satisfaction. 69,99 Some patients and caregivers may find that OPAT causes anxiety,56,99 with concerns about night-time emergencies and a lack of domestic support within the home, as well as concerns about the technology increasing stress levels. 99 Other barriers to OPAT include accessing patient transport69 and the expenses incurred by informal carers. 99
The findings from the systematic review suggest that patients can have some strong views about the characteristics of the services they receive, but the data are often poorly reported, as these findings are often secondary to the main research question. Information on what an attribute should look like is relatively limited, but ‘it should be important to patients/policy makers, “plausible” and capable of being traded’100 and the existing data do not provide sufficient breadth or depth of information upon which to develop the DCE attributes and levels.
Objectives
The aim of this study was to generate an understanding of patients’ experiences of OPAT and to use these to inform the development of the DCE.
Key research questions
Our research questions were:
-
What are patients’ experiences of receiving i.v. antibiotics for infections?
-
What are the benefits of and barriers to OPAT?
-
What aspects of the service are important to patients?
-
How does receiving OPAT impact on everyday life?
-
What would patients change about current OPAT services? What improvements would they make?
Method
This primary qualitative research was part of a mixed-methods approach to developing the DCE alongside the data from the evidence synthesis (see Chapter 2) and health professionals’ interviews (see Chapter 3). Qualitative data collection took place between August and October 2013. Our lay co-applicant service user and patient advisory group (PAG) contributed to the design of the study and data analysis.
Design
Semistructured interviews and focus groups.
Study settings
The study setting for conducting this research was secondary care, and four hospitals were purposively selected because they offered the following care pathways: HO attendance, nurse at home (GN and SN models) and SA; the centres provided more than one model of care. Two were large teaching hospitals and two were DGHs. Each site also offered significant diversity in its sociodemographic characteristics (Table 7).
| Site | Local characteristics |
|---|---|
| 1 | Population: 500,000 |
| Urban and rural | |
| 25% BME population, predominantly of Pakistani heritage (average across England 11%) | |
| In the 10% most deprived local authority areas outside London (IMD score of 26); OPAT services provided: HO attendance, visiting GN, SA (well-established service) | |
| 2 | Population: 798,000 |
| Urban | |
| 19% BME population (90 ethnic groups represented); both very high and very low areas of deprivation (IMD score of 68) | |
| OPAT services provided: HO attendance, SA (both well established) and visiting SN (newly established service) | |
| 3 | Population: 330,000 |
| Urban and rural | |
| Largely white, working-class population (< 4% non-white) | |
| IMD score of 67 | |
| OPAT services provided: visiting SN, HO attendance, SA (well-established services) | |
| 4 | Population: 385,000 |
| Urban (although hospital draws from rural areas outside the town) | |
| 67% white British, 8–12% BME | |
| IMD score of 105 | |
| OPAT services provided: visiting GN, HO attendance (newly established service) |
Participants
A purposive sampling strategy (see Sampling characteristics) was adopted. Two groups of patients were identified: (1) patients requiring short-term i.v. antimicrobials (n = 15); and (2) patients with deep-seated infections requiring longer-term i.v. antimicrobials (n = 25). The estimated sample size was based on previous studies102 and assumes that those on longer-term antimicrobials will be a more diverse population. It was our intention to capture a detailed and comprehensive range of perspectives. The use of qualitative data to develop DCE experiments is relatively new, so the final number of participants was dependent on theoretical saturation. 103,104
Sampling characteristics
-
Short-term and long-term OPAT.
-
OPAT via one of the four care pathways.
-
Sex.
-
Maximum variation in age (≥ 18 years of age).
-
Simple (e.g. cellulitis) and complex infections (e.g. bone infection).
-
SES.
-
Ethnic background.
All participants were invited to take part in a focus group or a qualitative interview (face to face or telephone). Initially, the project intended to conduct five to six focus groups and additional interviews with key informants. However, it proved difficult to recruit participants to focus groups, as working-age patients did not wish to take time off work, so the recruitment plan was revised and only one focus group was completed.
Consent
Participants were approached by NHS research nurses and given a copy of the participant information sheet and given at least 24 hours to decide whether or not to take part. Details of consented participants were then passed to the research team. Ethics approval was sought and obtained from the National Research Ethics Service Committee South West – Frenchay (reference 13/SW/0060).
Procedure
At the start of each session participants were informed of their rights as participants (right to withdraw, confidentiality) and were offered the opportunity to review a copy of the transcript and results. The purpose of the study was explained, and the interview followed the structure below. Interviews took place at the patient’s home or the university. Interviews were audio-recorded, with permission, with one participant refusing to be recorded (notes were taken by the interviewer). Interviews lasted between 30 minutes and 1 hour and 15 minutes.
Topic guide
The interviews and focus group discussions were semistructured and explored patient satisfaction, issues and preferences. The topic guide covered four main questions:
-
What has been your experience of receiving i.v. antibiotics for infections? What were the good and bad points of the care/service you received?
-
What are the most important aspects of i.v. antibiotic services for you?
-
If you were designing a service to provide community antibiotic i.v. services what would it look like?
-
How did the i.v. antibiotic treatment course impact on your everyday life (and that of your friends/family)?
These questions had a list of probes related to each question, to prompt further discussion and gain further insight into pertinent issues.
Data analysis
Interviews were conducted by four team members [MT and SM (psychology), JE (sociology) and, following the departure of JE from the study, CCM (nursing)]. MT, CCM and SM contributed to the analysis of the data. The team met regularly throughout the data collection period to review transcripts to ensure that they were covering the same topics and to identify issues to explore in later interviews. Owing to the departure of JE from the project, data collection and analysis became desynchronised, with data analysis starting after 12 interviews had taken place.
The audio-recordings were transcribed verbatim, anonymised and entered into NVivo version 10 software (QSR International, Warrington, UK) to facilitate data organisation, coding and retrieval. Data were analysed thematically for patterns and themes to understand what patients valued about OPAT services and to explore differences in their experiences using constant comparative methods. Data analysis followed the standard methodology for thematic content analysis, with close reading of the data to identify words that capture thoughts or concepts. Labels/codes were attached to these data and became the initial coding frame. Codes were then sorted into categories based on how they relate to one another and grouped into meaningful clusters. We also drew from aspects of framework analysis, developing matrices95 to map the data and aid categorisation.
Initially, two researchers independently read the transcripts and listened to the first three interviews, from which an initial coding frame was developed. Following this, the wider team examined and agreed the preliminary codes, and a coding index was agreed and applied in NVivo to the remaining transcripts. The research team met fortnightly to discuss the analysis and to examine specific cases for disconfirming perspectives. Before the end of coding it became clear that data saturation had been reached, as no new ideas relevant to constructing the DCE were identified from the last five interviews. 103
Three meetings were held between the qualitative team (MT, CCM, SM), chief investigator (JM), modellers (DM, SH) and two of the PAG members (HG, CT) to discuss the relevance and suitability of the themes produced, the ways in which the themes were linked and candidate attributes.
Copies of the interviews were sent to three participants who asked for a copy, but no changes were requested.
Changes from the protocol
We were unable to recruit enough participants to focus groups so we changed our predominant data collection method to interviews. This provided richer data, but required significantly more resources.
Results
A total of 41 patients consented, but four did not attend a focus group session and then declined participation and five subsequently declined to be interviewed. Owing to participants’ preferences and availability, one focus group (four participants) and 28 interviews took place (Table 8). One interview was not used in the final analysis, as the participant did not recall having i.v. antibiotics. The demographic details of participants can be found in Table 8. The results section uses direct quotations from patients to illustrate the analysis. The following code is used: sex, age < 65 years or > 65 years, OPAT service received.
| Demographics | N = 32 |
|---|---|
| Age (years), mean (range) | 53 (21–80) |
| Sex = male, n | 16 |
| Marital status, n | |
| Married | 16 |
| Single | 7 |
| Divorced/separated/widowed | 3 |
| Cohabiting/civil partnership | 6 |
| Ethnicity, n | |
| White British | 29 |
| White European | 2 |
| South Asian | 0 |
| Other (not stated) | 1 |
| Education, n | |
| University | 14 |
| College | 9 |
| Secondary | 7 |
| Primary (did not complete formal schooling) | 2 |
| Employment, n | |
| Full-time (> 30 hours per week) | 12 |
| Part-time (< 30 hours per week) | 4 |
| Unable to work owing to ill health | 5 |
| Retired | 10 |
| Carer | 1 |
| Infection type, n | |
| Short term | 20 |
| Long term | 12 |
| Service received, n | |
| HO attendance | 14 |
| Nurse at home | 13 |
| SA | 5 |
Theme 1: meeting the needs of a diverse population
For many, the benefit of OPAT was not being admitted to hospital. Most did not see their infection as warranting this, and those who had previously been treated as an inpatient saw OPAT as an opportunity to get home earlier. However, being cared for as an outpatient could be challenging:
. . . they [staff] came back and said was I sure this was what I want to do [go home on OPAT], and I was like ‘Yeah, as long as you put in place that I can have someone come out to me, then I’d like to go home.’ I didn’t realise how tiring it would be though. It’s still better than hospital, but I never realised that just making a cuppa could be so tiring!
Female, < 65 years, long term, nurse at home
Older participants, however, often put caveats on when OPAT would be suitable, taking into consideration the severity of infection, the patient’s general health, family circumstances, number of i.v. infusions per day and level of mobility:
It depends on the condition you are in, if you couldn’t move about you would have to wait inside (at home) for somebody to arrive, but only if you have family at home for you, if you could move about, you would be better off coming to hospital.
Male, > 65 years, short term, A&E
The nurse at home model was viewed as more appropriate for the very old or infirm, especially given the challenges of travelling to and parking at hospital. A few participants proposed alternative places where OPAT could be delivered, such as community clinics and GP surgeries and felt that this may be less intimidating for older people than the hospital:
. . . we have a little centre which is mainly all the elders and a lot of them feel, they feel a little bit more scared of the hospitals because some of them are single or widowed so they don’t always have somebody to go with them to the hospital [. . .] a local clinic would be much less stressful for them.
Male, < 65 years, short term, A&E
Many people mentioned that they did not want to be treated in hospital if at all possible, and their reluctance was unrelated to age, length of infection, frequency of infections or type of OPAT received. For some, their fear was linked to the perceived risk of contracting an additional infection, often fuelled by news reports as well as personal experience:
It’s always a worry in hospital, are you going to pick up a staph infection? Things like that.
Female, < 65 years, long term, clinic
Half of participants felt that OPAT services were understaffed, with hospital-based services viewed as particularly affected. None of the patients interviewed felt that this affected the quality of care they received and all were sympathetic to the stress that staff were under, suggesting that more staff and additional facilities were needed to address demand:
I think everyone’s absolutely rushed off their feet in that unit.
Female, < 65 years, short term, A&E
Theme 2: benefits of and barriers to different models of care
A major benefit of OPAT, regardless of the model of care, is the ability to enjoy the comforts of home and not disrupt things such as diet, sleeping patterns and family life, which people felt was hugely important to their recovery and well-being.
Hospital attendance
Younger participants in particular believed that daily attendance at hospital, compared with either inpatient stay or nurse at home, had the potential to cause the least disruption to daily life. Although they had quite serious infections, home care and work responsibilities meant that being an inpatient was an unpalatable option. Indeed, many working-age patients continued to work during their treatment. When HO appointments ran to time, most found once-daily treatment to have a minimal impact on their lives. However, many said that attending hospital more than once a day would be as bad as being an inpatient and none could see any way in which they could attend more than once daily:
I didn’t want to be in hospital at all [. . .] but I really couldn’t imagine having to come back and forth during the day, with the buses, it just wouldn’t work, it’s a non-starter, I’d no sooner get home than turn around again.
Female, < 65 years, short term, clinic
Having an appointment time and being seen on time were of particular importance, and so one group of patients who received OPAT via their A&E department were disappointed to find that all i.v. patients were given the same appointment time of 10.00 or 14.00:
When you’re sat in A&E, and you thought ‘there’s hardly anybody in’, and it took about initially, about an hour and a half for even somebody to acknowledge you being there, that was a disappointment.
Male, < 65 years, long term, A&E
In contrast, a different hospital-based clinic offered appointments that could be fitted around the patient’s lifestyle, and this was valued by patients and improved their overall experience. It allowed them to be able to balance their treatment with work and family life in a way that was as stress-free as possible:
I think it was really important that the staff were so good, if they’d not been as flexible or if they’d not been, if they’d been like the staff in my doctors, it would’ve been a horrific experience.
Female, < 65 years, short term, clinic
One perceived benefit of a hospital-based service was that if there was a problem, patients felt that they were in the best place for prompt access to emergency medical care:
So, personally, for me, I felt like being treated at the hospital was probably the best option because there’d have been people around who could have come and had a look at me if they’d needed to.
Female, < 65 years, short term, clinic
Geographical barriers
For those living further away from hospital, or relying on public transport, the geographical challenges of hospital attendance quickly sank in. For those relying on public transport, there was a range of obstacles, not least having to travel with an i.v. access line, which proved difficult to hide in the height of summer. Another challenge was managing bus and train delays, which were particularly difficult in inclement weather. The patients who relied on public transport often found hospital attendance less convenient than patients with good geographical access or higher social capital:
The distance that I travelled on the bus, you know that’s more inconvenient than sitting and waiting in the hospital really.
Female, < 65 years, short term, A&E
Even when hospital transport was available, this did not attenuate the travel difficulties. For these patients, a 30-minute infusion meant leaving home by 9.00 and returning home in the late afternoon. One patient who had two small children stoically endured this for 8 weeks as the alternative was inpatient care and seeing little of her family.
I have to be ready at 7.30 in the morning, they normally get there about 9, so it takes my whole day to have a half hour drip, but that’s nothing to do with OPAT that’s to do with the way the NHS driving services run.
Female, < 65 years, long term, clinic
Even for those travelling by car, a lack of parking spaces and high car park fees made daily attendance frustrating. Some patients suggested that dedicated short-term parking bays, similar to those used by dialysis patients, would be helpful, as many spent longer finding a parking space than they did receiving their i.v. treatment.
District or specialist nurse at home
Patients who were treated at home by a nurse perceived their treatment regime to be more convenient and less stressful than hospital attendance. Many were retired and a few had complex treatment regimes, often requiring multiple i.v. administrations per day. Even faced with several visits a day, many felt that OPAT allowed them to carry on with everyday life and compared it favourably to being an inpatient:
. . . they used to come about nine, two and six or seven o’clock, so you’d got them in-between bits to do things, go to the shops or whatever, and it didn’t impact on my life at all.
Female, < 65 years, long term, SN
In most cases, the nurse arrived at regular times, but missed appointment times were a source of both frustration and worry, as participants feared they had missed hearing the nurse call. Knowing that the nurse would call at a particular time made them feel safe; when this implicit contract was broken, the relationship could be affected. For some, daily attendance at hospital was inconceivable and the prospect of being discharged home only to come back each day was a worse option than being an inpatient:
. . . for me, I don’t think that [daily attendance at clinic] would have worked, because I was still extremely weak, and we know the . . .one of the main reasons I wanted to come home was for the comfort of my own home, and my own bed, and to be able to rest and to build my strength up. Now to physically have to make a journey each day . . . unnecessarily in my eyes, because if I’d have stayed in hospital I wouldn’t have had to make the journey, so for me having to physically get up, get dressed, go out of the house to go to the hospital to have that done would have been exhausting.
Female, < 65 years, short term, SN
A few participants had a team of nurses visiting them and, although participants worried about the lack of continuity, a greater concern was ensuring that the nurse had been briefed about their infection and treatment plan, and was competent to deliver the treatment. When on one occasion a replacement nurse arrived not knowing that she was supposed to administer an i.v. treatment, the patient started to question the competence of the team:
She had no clue who I was really and arrived not knowing that she was supposed to bring the drugs with her, it did make me wonder about them.
Female, < 65 years, long term, district nurse
Self-administration
Patients who self-administered had recurrent infections and had been administering their own treatment for many years. Some felt that they could fit their treatment around their life, with one patient describing how she had infused in the car on the way to work on occasions. However, two patients felt that they had little time to fit anything else into their day, as planning the next treatment was always at the back of their mind:
There’s no point really going out much or doing much ‘cos you haven’t got much time when you aren’t having to think about getting everything sorted.
Male, < 65 years, long term, SA
Although all were experienced with i.v. administration, only two felt confident with the procedure. Two preferred to have a family member present during their infusion, even if this person was not actively involved, and felt that they would not be willing to self-administer without this support. Another voiced a preference for inpatient care, although he said that this was no longer offered to him and he felt that this was because it was assumed he would self-administer:
If I felt well enough to be at home then I preferred to be at home, because I had lots of back up and support there. [. . .] Living on my own now I would actually prefer to be in hospital I think.
Male, > 65 years, long term, SA
. . . I think because I’ve done them since I was 13 so 20 years I don’t need anybody to do my drips I don’t need anybody to see me, I’m very glad I don’t have to come in to do them ‘cos travelling everyday there and back would be horrendous and very expensive.
Female, < 65 years, long term, SA
Theme 3: effectiveness of treatment
About half of patients believed that i.v. antimicrobials were more effective than oral treatments. Their apparent effectiveness caused patients to question why they had not been offered i.v. earlier, as their infections responded quickly to the change of treatment:
I would have like the full dose, not an escalation policy, i.e. you can only take orally up to a certain level and therefore, when that’s failed, we’ll put the i.v. in. If we know that for many people it’s going to fail then overprescribe. I don’t know what proportion of people that oral antibiotics would work you see.
Male, < 65 years, long term, A&E
Some patients experienced a switch from i.v. to oral treatment and a few worried that their treatment had been changed prematurely, largely because the planned switch was not explained to them. This was noted only for nurse at home and hospital attendance patients. In the case of cellulitis, the time needed to treat the infection is shorter than the time required for the skin to heal, leaving patients uncertain about whether or not the infection was cleared. In these cases, patients commonly said they would have liked to continue i.v. treatment for longer:
He’d said to me that they were going to review after x amount of days, and it was still a little bit red and still swollen. Not as swollen and not as red, and I said ‘look, I’m not happy, I know I’m going to be back here within a fortnight if I don’t have a little bit more’. And he said ‘well go on then!’
Female, < 65 years, short term, clinic
A few patients experienced a subsequent flare-up of their infection, which led them to conclude that their i.v. had ceased prematurely. Unfortunately, as most short-term infection patients were not seen by a health-care professional at the end of treatment, this was picked up only when the patients went back to their GPs, demonstrating an important unmet need of patients:
Well, I went to the GP a week after I finished the i.v. from the hospital, and it (cellulitis) was as bad as ever, so he sends me back to A&E.
Male, > 65 years, short term, clinic
Theme 4: communication
Information provision
Most patients recalled receiving written information about their treatment. This included information about managing with the i.v. device once at home (e.g. how to bathe with it in), how infections are treated using i.v. antimicrobials, description of the OPAT service, risk of infection and what to do/who to contact if the patient had any concerns. Patients appreciated this information and in particular being given a named point of contact, and a few felt empowered to be actively involved in their own care, knowing that support was there:
I think the important issues are being given information, being told clearly what the problem is, what is going to happen and so on, and any data that needs to be given to you. It’s far better to be, to have that clear in your mind, than just let things happen to you, and hope that they’re doing the right thing. I think that’s the most important thing.
Male, > 65 years, short term, A&E
Someone to talk to
The ability to ask questions, even if the patient had none, was viewed as important.
Patients who were cared for at home enjoyed having the nurse to talk to during their appointment and felt that they could ask questions as they were not taking up additional nursing time. Some older patients in particular had concerns about being cared for at home but said that having the nurse to talk to set their mind at ease and gave them the confidence to manage their own care at home. When only one or two nurses covered their care, patients appreciated the continuity, as it meant that they got to know the nurse, which gave them the confidence to ask questions:
. . . I’d got that attention completely for that time, whereas in hospital when the antibiotics are put on, you know, the nurse would come around and say ‘Hi, how are you?’, just generally passing the time of day while they were putting it [drip] all together, and then they’d go. I think being at home, having that person there who’s just, you’ve just got their attention no matter what, then you get to know them. I found them easy then to open up to, to ask questions.
Female, < 65 years, short term
Few of the patients attending hospital recalled being told much about their infection, but most did feel able to ask questions. Although most were not overly concerned by the lack of information given to them, when things went wrong, or recovery was not as they expected, the lack of communication between the health-care professional and the patient became more significant, and patients felt that they had been left in the dark:
. . . the doctor said 4 weeks when I saw her, but I’m more than 4 weeks on from seeing the doctor and it’s still not entirely right so I don’t know, no-one told me anything.
Male, < 65 years, long term, clinic
A lack of communication about treatment became even more significant for patients when accompanied by perceived health-care errors. Patients tended to blame these on the lack of continuity in who treated them and a lack of interstaff communication. For one patient, a lack of detailed information in his notes made it difficult for staff to determine whether or not his infection was responding to treatment, and another described the errors made at his discharge from hospital:
. . . the final one (staff member) discharged me, not really [having] done an examination of me at all, in fact I was sat there, with totally bare arms, with a bruise on this wrist where the cannula had been, and he was saying that he’d have to take the cannula out after they’d given me the shot on Friday, and I said, ‘it was out yesterday’ and he said ‘Oh, why?’ [. . .] I had to tell him [. . .] so I wasn’t too happy about that.
Male, > 65 years, short term, A&E
Not unsurprisingly, patients who self-administered were the most knowledgeable about their condition and most likely to have been told how to manage their i.v. device and how to recognise signs that indicate possible further infection. All patients knew who to contact if they had any worries. However, communication could still break down and damage patient confidence. For one SA patient, the medical equipment that she needed to treat her condition kept breaking down, so she was unable to self-manage effectively. Despite ringing for advice, staff did not answer her questions and did not ring her back to ensure that their proposed solution had worked:
No, I had no phone calls, many from me looking for advice, but none to me.
Female, < 65 years, long term, SA
Theme 5: review and aftercare (follow-up)
Review and follow-up at the end of treatment was important to many but not to all patients and their experiences differed. Most long-term patients were reviewed regularly, and, although some patients felt strongly that this review should be undertaken by a doctor, others were happy for the nurse to do this. What was important was being able to contact the OPAT team between appointments as this provided reassurance that they were being cared for as well as if they were in hospital. Being followed up at the end of treatment was particularly important if the patient had not been reviewed face to face during treatment, and a lack of aftercare was a cause for concern:
I’ve got follow up in a month which is nice so they’re keeping an eye on me, I wouldn’t like it if I hadn’t been.
Female, < 65 years, long term, clinic
However, few short-term patients were followed up at the end of treatment. Some were advised to come back to the clinic if they had concerns. Some were content that this was their responsibility, but others would have preferred an appointment and wanted to know whose responsibility it was to organise this. This was of particular concern to patients who had been cared for by a nurse at home, as they were generally not seen again in person by the doctor after the initial diagnosis:
I suppose I was left in the dark as to know what was after the IV, nothing at all. I’d rather if they said ok, make an appointment to see your doctor.
Male, < 65 years, short term, SN
Self-administration patients were all on longer-term i.v. treatment and valued their mid-term and aftercare appointments, as they saw these as important to their care:
There’s a liaison nurse who comes down on a pre-arranged date and time and they’ll usually do (tests) and chat and make sure everything is going ok, that I haven’t got any issues, see how I’m feeling, see if there’s any improvement or effect.
Male, < 65 years, long term, SA
Theme 6: staff expertise
Patients used the term ‘expertise’ to refer to both a staff member’s level of knowledge of infections, i.v. antimicrobials and treatments and their practical experience of delivering OPAT, emphasising both equally. People valued being dealt with by competent staff, and felt that such expertise was built up over time. Ideally, participants wanted to be cared for by people who understood their infection, were aware of complications that could arise and knew what to do in those circumstances. Patients were impressed with staff who knew about their personal circumstances and liked it when they felt that staff ‘went the extra mile’:
The nurses that come out are specialist nurses who are informed about antibiotics and about lots of illnesses that they are treating, because they obviously need to know [. . .] I felt very comfortable that they were very knowledgeable about what I was experiencing and this reassured me about coping at home alone.
Female, < 65 years, short-term, SN
Some patients wanted their care to be overseen by a doctor, because of their extra training and qualifications. Although these patients were happy to be treated by a nurse, they wanted the reassurance of a doctor overseeing their care:
How would you have felt if it had been, for example, a nurse-led service?
Umm, I would have been worried, I think you need a qualified doctor in charge, to do the examination, and make a decision on what to do next, what to prescribe, to continue the treatment.
Male, > 65 years, short term, A&E
Theme 7: impact on family and friends
Outpatient parenteral antimicrobial therapy treatment often had a significant effect on the patient’s family and friends who provided practical and psychological support to the patient. Patients felt that it was important to consider how their treatment had a wider impact, rather than just how it affected them individually. For those attending hospital on a daily basis, a reliance on family and friends to get them to and from the hospital was important, both in terms of time off work and additional travel costs:
I mean it’s a case of them leaving work to take me there [hospital] on a morning because it was at like 10 o’clock my appointment and then to pick me up afterwards. [. . .] the Wednesday, I had to wait about an hour for treatment, so my dad was hanging around, and he had to leave me because he was in a meeting at work.
Male, < 65 years, short term, A&E
Some patients were more concerned about the impact their infection had on others than their own well-being and recovery. Although being at home was viewed as better than being an inpatient, this often meant that patients tried to carry on as normal, including maintaining family roles, for the sake of their family. Some expressed a sense of guilt about the impact their treatment had on their children or spouse, with participants talking about the treats or visits that were missed because they were unwell, or tasks not completed, as they were their responsibility. Patients expressed guilt that their infection had resulted in them not being able to care for family members as they usually would do:
I got discharged, I am a carer for my mum and at that point I couldn’t take care of myself so what they did was, they had to put her in a residential home for about 2 or 3 weeks so until I picked myself up.
Male, > 65 years, long term (focus group), district nurse
Discussion
This study set out to understand what patients might value about OPAT services, in order to identify potential attributes for a subsequent DCE.
Patient experiences of outpatient parenteral antimicrobial therapy
Overall, patients appreciate the care that they receive and felt that services were generally well run and of high quality. While acutely ill, most patients preferred to be cared for in hospital but, once stabilised, most but not all patients preferred to recover at home, a finding echoed by other studies. 2,15,85,105 Not all patients are suitable for OPAT and are generally selected on the basis of their ability to cope in the community, and the availability of suitable antimicrobials, and these caveats were echoed by patients. 35 In line with other studies, patients felt that they benefited from being in their own homes,56,85,86 but this also required them to be more active in their own care, and the willingness of patients to do this needs to be assessed. 35,99
Patients identified a range of health-care experiences as important. One important concept related to the type of staff involved in the service and the skills that they need to deliver good quality care. For some, this meant the active involvement of doctors within the service, owing to the perception that doctors were more knowledgeable or skilled and that health-care decisions should be made by doctors rather than by nurses or patients. Others focused on the expertise and experience of nursing staff. A good service was one in which staff were perceived to be competent and highly skilled. Studies have found that a minority of patients lack the confidence to manage effectively in the community, so understanding what constitutes good-quality care is important if OPAT provision is to be widened. 73,85
Another important concept relates to interpersonal aspects of care, such as respect and empathy. Most patients were provided with good written information about OPAT, but oral communication between patients and staff was more variable. Relationships with staff can take time to develop and one way in which these were achieved was through staff having time to talk patients about their infection and treatment. Poor communication could leave patients without the knowledge and confidence needed to be a competent collaborator in their own care and could affect their perceptions of the service. These findings resonate with the conclusions of a recent review by Entwistle et al. 106 that looked at the aspects of health-care delivery that are most important to patients. The map described by Entwistle et al. 106 links ‘health care delivery to what people are enabled (or not) to feel, be or do’ and suggests that both the structure of health care and social dynamics are important to the patient experience. Our findings lend support this conclusion.
What are the benefits of and barriers to different service configurations?
Each of the care pathways was viewed as having its own strengths and weaknesses, and the importance people attached to different attributes seemed to be linked to the age and health of the patient.
A nurse at home model was perceived to be particularly well suited to older patients, those needing longer courses of i.v. treatment and those with more complex care needs. For many, the one-to-one time with the nurse was viewed as a key benefit and contrasted strongly with their inpatient experience, a finding also noted by Dubois and Santos-Eggimann. 99 However, this benefit could be quickly eroded if the nursing team is too large and the continuity of the care relationship is broken.
Hospital attendance was considered to be most suitable for those who were fitter, younger and required once-daily, short courses (under 1 week) of i.v. treatment, a view also held by those attending clinic with long-term infections, although several patients were receiving long-term i.v. via the clinic. The availability of a doctor on duty provided the reassurance some needed ‘in case anything went wrong’, and a qualitative study by Bamford et al. 35 suggests that some patients do not feel confident about being treated by a nurse at home. However, the evidence is mixed. A large-scale Health Technology Assessment (HTA)-funded study of doctor-led versus nurse-led care in a bronchiectasis clinic found that patients were happy with a nurse-led clinic because it provided better continuity of care. 107 It is therefore important to understand what patients value about the different aspects of the service that they receive.
A potential benefit of hospital attendance was its convenience, but this required the use of timed appointments, and when appointments were not kept this affected the level of patient satisfaction, a finding reported elsewhere. 69 Characteristics of the service (e.g. if understaffed) and the staff themselves (e.g. not being briefed about the patient) contributed to dissatisfaction. Hospital attendance was the only care pathway in which transport is a significant issue. Poor public transport links, a reliance on hospital transport and poor car-parking facilities at hospitals were also key attributes that affected the acceptability of hospital attendance and have been noted previously. 69 One solution that was proposed by patients as an alternative to hospital-based services was to invest in ‘local clinic services’, perhaps based in local medical centres.
Self-administration was the model of care least well represented in our sample, and all patients undertaking this were chronically ill patients who received regular courses of i.v. treatment and thus do not reflect the views of those who experience a one-off course of antibiotics, for example for a deep-seated infection. Patients who had no personal experience of SA voiced most concerns about the risks of SA. Although those using the service found it convenient, there were residual concerns about the safety of SA, and one patient did not rule out inpatient care as a favoured alternative. A study of cystic fibrosis patients reported similar results and recommended that refresher courses be given. 88 SA is generally offered only to patients who are physically and cognitively able to manage its complexity. The findings of our study and the conclusions of Pilling and Walley88 suggest that when patients are taught to self-administer, treatment choices should be revisited over time to determine whether or not people’s circumstances have changed.
Strengths and limitations
Our data support and develop the earlier scant qualitative research evaluating OPAT services. OPAT services can support patients to self-manage in the community, but when services are not configured in a way that helps patients in this endeavour they can negatively impact on patient satisfaction.
The strengths of this study are that we recruited from four very different sites, including two large teaching hospitals and two DGHs, which between them offered HO attendance, SN and GN at home and SA OPAT services. This enabled us to contrast the views and experiences of those who experienced different models of care and provided contextualised information to unpick the aspects of care that patients valued to construct our attribute shortlist for the DCE. It should be acknowledged that, although we recruited from four very different centres, the experiences described cannot be generalised to all services, and further research is needed to understand patient experiences of OPAT.
We had a broad sampling strategy to obtain views from participants from a diverse range of socioeconomic backgrounds. However, limitations must be acknowledged in our sampling, as we struggled to recruit the very elderly (> 85 years of age) and those from local black and minority ethnic (BME) populations. Feedback from eligible older patients was that they did not feel well enough to be interviewed. We struggled to recruit from the BME community and only three people consented. Of these, two could not be contacted after finishing treatment and one decided not to be interviewed. To improve recruitment, we took advice from researchers with significant experience working with the BME community and from our patient and public involvement (PPI) group but few eligible patients were identified as receiving OPAT during the recruitment period, and response to letters inviting those who had previously received OPAT was poor. We were also able to recruit only five patients with experience of SA, as this was not a model of care offered as widely as the other two services. For that reason, the findings from the interviews with SA patients should be interpreted with caution, and more research is needed to understand the experiences of this population.
We planned to undertake focus groups with relatively few interviews, but recruitment was poor. We therefore introduced the option of an interview, which was taken up by almost all participants. This resulted in much richer data, but did put the study behind time as the analysis took longer than anticipated. This had the knock-on effect of the interviews and analysis becoming desynchronised, so data saturation was reached before we finished interviewing and no new findings were revealed in the final five interviews.
Data collection and analysis were undertaken by three researchers with diverse backgrounds, and the team met regularly to review interview data and to look at the emerging analysis. We view this diversity as a strength of the study, as it added to the richness of our discussions about the data.
Conclusion
Nationally and internationally, health-care organisations have highlighted the importance of patients’ experiences of the services they receive, and, indeed, the NHS Operating Framework for England108 describes each patient’s experience as ‘the final arbiter of everything the NHS does’. In the current drive to have patients cared for in the community, it is important to ensure that services are designed in a way that meet their needs and improves the quality of people’s experiences of health-care delivery. 109 This study shows that satisfaction with OPAT services was a dynamic process, with both the health-care provider and the patient contributing to the subjective experience of OPAT. Therefore, understanding which experiences of health care really matter provides the opportunity to improve services.
The qualitative study identified nine concepts which could be taken forward as possible attributes in the DCE study. However, two of these (in italics below) were excluded as they did not differentiate between services, but were specific to one model of care.
-
Personal effort (travel time): an important difference between services but not included as an attribute in the DCE as relevant only to hospital attendance and as we were not able to vary sufficiently between services.
-
Number of i.v. administrations per day.
-
Accommodating OPAT within patients’ daily lives (appointment times, waiting for nurse).
-
Expertise of staff: dropped as this attribute did not vary between services.
-
Communication with staff.
-
Communication between staff (quality of handover between team members, staff mix).
-
Doctor- versus nurse-led service: who gives the i.v.?
-
Follow-up or ‘aftercare’.
-
Risk of adverse reaction, side effects and complications.
The interview data also identified a range of attitudes towards hospitals, i.v. antibiotic treatment, OPAT services and health care more generally which were used to develop the following points to be included in the DCE.
-
People get better more quickly if they are treated at home.
-
I do not like hospitals.
-
If you are treated in hospital there is an increased risk of contracting a new infection.
-
Giving my own i.v. antibiotics would worry me.
-
Doctors – not patients – are in the best position to decide where patients should be cared for.
-
I would choose to receive i.v. antibiotics in my own home even if this meant waiting several hours for a nurse to visit.
-
I prefer that my recovery is monitored by a doctor rather than by a nurse.
-
I want to be responsible for making decisions about my own treatment.
-
There is a significant health risk if the i.v. treatment is not given properly.
-
Being in hospital would have made things difficult for my family.
-
i.v. antibiotics are more effective than oral antibiotics.
-
I did not know if my illness was cured when my i.v. treatment finished.
Chapter 5 Quantitative analysis of patient preferences
Introduction and background
Ensuring that the views of service users are heard is an important element when designing effective services and is enshrined in government policy, including the 2011 Health and Social Care Bill. 16,108,110,111 This chapter summarises the work carried out to quantitatively understand patient preferences for OPAT services and to examine which service characteristics are important to patients. OPAT services have been growing in the UK for over 10 years, but evidence from the literature112–114 and from our interviews with health professionals (see Chapter 3) suggest that, despite the reported benefits of OPAT, a key inhibitor to the further development of these services is a lack of funding, commissioning gaps and not having the evidence to justify the selection of any particular model of care. Quantifying patient preferences for different types of care can, therefore, inform changes in service provision and provide information to support the development of new services so as to obtain the best outcomes within a given budget. An important component in this work is not just to understand what the overall preferences are (e.g. whether or not hospital treatment is preferred to home treatment), but how the individual characteristics of a given model of care, such as the need to travel, influence that preference. Furthermore, there is interest in understanding how these sensitivities to individual service characteristics and, hence, overall preferences for a given model of care, vary across patient segments.
This chapter describes a DCE which seeks to understand patient preferences for OPAT services. DCEs (also known as stated choice) are being increasingly used in health care to elicit preferences for a range of health-care services,115 for example, smoking cessation in pregnancy116 and communication therapy following stroke. 117 The DCE was developed using the findings from the systematic review and interview data (see Chapter 4).
The survey data were analysed using advanced discrete choice models belonging to the family of random utility structure. 118 Although initial insights into overall preferences can be obtained by direct questioning regarding which service a given patient prefers, such approaches fail to recognise that the actual preferences will be influenced by specific characteristics of the different delivery methods. 118 In particular, they will be the result of trade-offs, whereby a patient selects the option that provides the best overall combination of characteristics, with good performance of some characteristics compensating for poor performance of others. In the context of the present study, such trade-offs may, for example, arise where a patient trades off the fact that i.v. treatment at the hospital gives access to more highly qualified staff than a nurse at home model against the added risk of hospital-acquired infections.
To understand the trade-off behaviour of patients, which reflects the relative importance they attach to individual service characteristics (attributes), we developed a questionnaire with a DCE survey component, the details of which are given below.
The remainder of this chapter is organised as follows. We first discuss the survey design before looking at data collection. We then provide a summary of the modelling approach and model results before providing a discussion and conclusions. Appendix 3 includes the full set of stated choice scenarios, details on the methodology and full results of the modelling work.
Aim and objectives
The aim of the DCE was to determine how patients react to different community i.v. antibiotic service attributes and how this drives their overall preference for a given service.
The objectives were:
-
using qualitative research methods, to identify what aspects of OPAT services are important to patients
-
using qualitative research methods, to identify patients’ attitudes to OPAT services and wider aspects of health-care provision
-
to design, pilot and conduct a DCE and attitudinal survey with OPAT service users
-
to conduct modelling of the survey data to identify patients’ relative strength of preference for different OPAT service attributes
-
to identify heterogeneity across patients in their attitudes and preferences.
Methods
A DCE was designed to assess preferences for OPAT services.
Questionnaire design
Developing the attributes and levels
The DCE provides data on patient preferences by studying choices across a range of hypothetical scenarios in which the characteristics of the different models of care vary. The aim is to understand the influence that these individual characteristics have on the overall preference for a given model of care, where there will also probably be a baseline preference, all else being equal.
The first phase of DCE development used the systematic literature review data (see Chapter 2) and patient interviews (see Chapter 4) to identify possible attributes and levels, whereas phase two refined the wording of items to go into the DCE questionnaire. Throughout this process we were guided by recommendations by Coast et al. 102 on the use of qualitative methods to guide the development of DCEs. Finally, the draft DCE interview was piloted using a think-aloud technique to identify difficulties with question wording and interpretation. 119 Our PAG was closely involved with this work, meeting with the research team to review attributes and levels and to comment on draft versions of the questionnaire.
Attribute development
The systematic review revealed that most patients found OPAT to be acceptable and that they believed that they would recover more quickly at home. 56,71 Others, however, were concerned about receiving treatment in an outpatient setting; particular concerns related to ensuring the sterility of equipment and the risks of being treated at home,35 the quality of communication between staff,35,69 the lack of information provided and costs incurred. 69 However, owing to the quality of reporting in the literature it was often difficult to be certain which OPAT models participants had been exposed to, which limited the usefulness of the existing literature.
To supplement the systematic review findings, qualitative data were collected from 32 patients (see Chapter 4 for sample details). Potential attributes for the DCE were developed on the basis of the findings of the systematic review and the qualitative interviews in which we asked patients what was important to them about the service they received, and what difficulties they had experienced during their OPAT treatment. A list of candidate attributes was constructed and discussed with clinical co-applicants and patient representatives. Some initially plausible attributes were excluded because they did not distinguish between types of service but were more representative of OPAT generally (e.g. wanting to be cared for at home) and some of these reflected attitudes towards health care or OPAT generally; six attributes were retained for the DCE. The draft set of items with potential levels was then shared with PPI members (n = 5) and research nurses (n = 8) at two sites (Leeds and Bradford).
Summary of attributes
Alongside the core type of service (HO, nurse at home, SA), six attributes were chosen to describe the individual models of care.
Attribute 1: number of treatments per day
The literature suggests that patient satisfaction remains high even when the patient requires regular attendance at hospital. 5,84 In contrast, our patient interviews revealed that many were concerned about the practicalities of attending hospital more than once daily for treatment and felt this would impact on their lives significantly. Some younger participants felt that hospital attendance would be more flexible than a nurse at home model, but older people were more varied in their views, perceiving advantages and disadvantages to all services. Continuous infusion pumps reduce the need for multiple visits, but the systematic review suggested that patients may have concerns about the use of pumps, a finding echoed by our study. 37 We therefore chose three levels of treatment (once a day, twice a day and pump to provide continuous infusion).
Attribute 2: appointments
This attribute varied whether or not patients were given an appointment time to receive their treatment. Our interviews revealed that almost half of patients attending hospital or being cared for a by a nurse at home had experienced missed appointment times, a finding echoed by Hitchcock et al. 69 The literature review also revealed that SA is attractive to some as it frees people from appointments. 36 Three levels were selected: daily appointment time given, no appointment time given and no appointment time needed (SA).
Attribute 3: treatment administered by whom?
Participants were split in their opinion about who should deliver i.v. antimicrobials. Some would prefer to be treated by a doctor, but others preferred to see a nurse, a finding echoed in the literature. 35 The patient interviews also identified that some patients were interested in learning how to self-administer their i.v. medications, but were concerned about training and the risks involved, a finding also noted by Lehoux. 37 It is acknowledged that none of the current OPAT services has doctors directly delivering i.v. treatment, but it was decided to include this to determine the strength of this preference. Five levels were identified for this attribute: specialist i.v. nurse, GN, doctor, self with half a day’s training or self with 1-day training.
Attribute 4: communication between patient and health-care professionals
The systematic review and patient interviews show that good communication between patients and staff was vital. When communications between staff break down and information about the patient is not passed on correctly this can worry patients, a finding supported by the literature. 69 Although not picked up in the literature, there was also concern from some patients about who they saw and whether or not the same person cared for them throughout their treatment. Therefore, four levels were selected for this attribute: see a health-care professional who knows you (continuity of care), see a health-care professional who does not know you (whoever is on shift), speak on the telephone with a health-care professional who knows you, speak on the telephone to a health-care professional who does not know you.
Attribute 5: aftercare
The interviews revealed that many patients were concerned about not being followed up at the end of their treatment and, in many cases, were not sure if they would get an appointment, either from their GP or from the hospital or if they should instigate that interaction. Where offered, appointments are currently with a doctor, but as many patients do not receive this, we included an appointment with a nurse or GP as an option to examine patient willingness to be followed up in this way. We selected four levels for this attribute: no appointment, appointment at hospital with a nurse, appointment with your GP, telephone appointment with a nurse.
Attribute 6: risk of adverse reactions
The systematic review undertaken at the start of the study revealed that only 19 out of 91 studies evaluating OPAT-related safety included a comparator. The data analysis suggests that there is little impact on either drug-related side effects or the number of deaths in OPAT compared with hospital treatment. However, there appear to be more line-related complications in i.v. therapy administered outside the hospital, and adverse events such as rash, fever, nausea were reported (see Chapter 2 for details). The following three levels of risk were selected: 1 in 6 chance, 1 in 10 chance, 1 in 25 chance.
Attitude questions
Recent work has shown that when patients are in a situation in which they have to make a decision about treatments, key influences on this behaviour will be the severity of the condition, past experience, etc., but also perceptions and attitudes. Klojgaard and Hess120 argue that when clinical evidence is not clear cut, the perceptions that patients form will play a part in shaping these decisions. Therefore, a measure of patient attitudes was developed for the survey. In the absence of relevant theory or pre-existing literature, the items were developed directly from the qualitative data (see Box 1 for questions used). The attitude questions were phrased as positive or negative statements and scored on a five-point Likert scale. 121
People get better more quickly if they are treated at home.
I do not like hospitals.
If you are treated in hospital there is an increased risk of contracting a new infection.
Giving my own i.v. antibiotics would worry me.
Doctors, not patients, are in the best position to decide where patients should be cared for.
I would choose to have i.v. antibiotics in my own home even if this meant waiting several hours for a nurse to visit.
I prefer that my recovery is monitored by a doctor rather than by a nurse.
Being in hospital would have made things difficult for my family.
I want to be responsible for making decisions about my own treatment.
I did not know if my illness was cured when my i.v. treatment finished.
There is a significant health risk if the i.v. treatment is not given properly.
i.v. antibiotics are more effective than oral antibiotics.
The survey
Patients were given eight hypothetical choice scenarios, each time involving the three models of care: attendance at hospital, nurse at home (which could be further differentiated into GN and SN) and SA. The characteristics of the models of care were described in the form of the six attributes (e.g. number of treatments per day) and levels within attributes (e.g. once daily, twice daily) (see Table 9 for description of attributes and levels). The levels used in these descriptions of services varied across the eight choice tasks, and patients were asked each time to indicate their most preferred option among the three that were presented. This allows us to understand how the preferences for a given model can change as a function of the characteristics of that model of care.
| Attribute | Nurse gives i.v. antibiotics in your home | You have your i.v. antibiotics in hospital | You give i.v. antibiotics to yourself at home |
|---|---|---|---|
| Number of treatments each day | One | One | One |
| Two | |||
| Two | Two | Three | |
| Pump provides continuous treatment | Pump provides continuous treatment | Pump provides continuous treatment | |
| Appointment times given | Daily appointment time given | Daily appointment time given | No appointment needed |
| Daily appointment time not given | Daily appointment time not given | ||
| Who gives the i.v. treatment? | Specialist i.v. antibiotic nurse | Specialist i.v. antibiotic nurse | You give the i.v. treatment yourself after half a day of training |
| GN | |||
| GN | Doctor | You give the i.v. treatment yourself after 1 day of training | |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| See a HCP who does not know you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you | |
| Aftercare from HCPs after the end of treatment | None | None | None |
| Appointment at hospital with nurse | Appointment at hospital with nurse | Appointment at hospital with nurse | |
| Appointment with your GP | Appointment with your GP | Appointment with your GP | |
| Telephone appointment with nurse | Telephone appointment with nurse | Telephone appointment with nurse | |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 6 chance | 1 in 6 chance |
| 1 in 10 chance | 1 in 10 chance | 1 in 10 chance | |
| 1 in 25 chance | 1 in 25 chance | 1 in 25 chance | |
| Please tick which service you would prefer to have | □ | □ | □ |
When presenting respondents with hypothetical choices, the analyst needs to decide the values of the characteristics describing the choices. With the aim of understanding the relative influence that different characteristics (e.g. risk, treatments per day) have on the choice made, it is important to choose combinations that do not lead to an overall dominance for one model of care. The specific combinations of values for the different characteristics to be shown in a given choice task were determined on the basis of an experimental design. We made use of a D-efficient design,122 in which we relied on zero priors in the absence of any meaningful evidence in the literature. We decided against using non-zero priors, because no appropriate values were available from previous studies and because the sample of respondents available to us was too limited to develop priors based on a pilot survey. The full design included 24 rows, and orthogonal blocking was used to split this into three sets of eight choice tasks, with one block used for each respondent. Each respondent in the survey thus received a set of eight choice tasks, and was each time asked to indicate his/her preferred model of care under the specific combination of levels for the six descriptive attributes. A copy of the full set of 24 choices used is included in Appendix 3.
Additional information
In addition to the DCE questions and attitudinal data, we collected information on participants’ educational level, and work history, their infection and its treatment, health-related quality of life, use of health services and satisfaction with services. The last two sets of questions were asked after participants had completed the DCE and attitude questions.
Pre-pilot work
The pre-pilot version of the survey, including the DCE questions, was made available to the research team using an online format provided by ACCENT (a market research company; London). Members of the team, research nurses and PPI members were invited to complete the questionnaire and make comments. The wording of four questions was amended on the basis of feedback from 12 people.
Pilot study
We conducted a pilot study with 30 patients to gather their feedback on the survey.
Research objective
To get participant feedback on the survey and DCE choice sets in terms of whether or not the wording was coherent and made sense to respondents.
Design
Think-aloud study and provisional quantitative analysis of DCE data.
Sample
An opportunity sample of patients aged 18 years and over who were currently or previously on i.v. antimicrobial treatment for an infection (see Table 10 for sample details). Participants were recruited by research nurses based at four acute NHS trusts (see Table 7). Data collection took place between May and June 2014. Thirty people took part, but only 29 sets of data were analysed as one set was not successfully uploaded and the data could not be recovered.
| Sample characteristics | N = 29, n (%) |
|---|---|
| Male | 13 (45) |
| Age (years), mean (range) | 52 (29–84) |
| Ethnicity | |
| White British | 26 (90) |
| Asian or Asian British | 2 (7) |
| Other | 1 (3) |
| Education | |
| University or professional | 13 (45) |
| College (post 16 years) | 4 (14) |
| Secondary | 12 (41) |
| Marital status | |
| Married or cohabiting | 15 (52) |
| Single | 8 (28) |
| Divorced | 2 (7) |
| Widowed | 4 (14) |
| Employment status | |
| Working full-time (> 30 hours per week) | 8 (28) |
| Working part-time (< 30 hours per week) | 3 (10) |
| Retired | 11 (38) |
| Unable to work owing to illness or on long-term sick leave | 7 (24) |
| Duration of treatment | |
| Long term | 22 (76) |
| Short term | 7 (24) |
Procedure
Participants were given an information sheet by research nurses and given at least 24 hours to consider participation. Interviews were then conducted at a mutually convenient time and place (at hospital or the patient’s home). The DCE was presented to participants using a laptop or iPad (Apple Inc., Cupertino, CA, USA). Participants were asked whether or not they understood the information and instructions presented to them, what they thought they had to do next, and their feedback on the wording of questions was sought as they were reading or answering so that detailed feedback could be audio-recorded, and written notes were taken to supplement the analysis.
Main survey
Sampling
Participants were recruited through six NHS acute hospital trusts, representing both teaching hospitals and DGHs. Both retrospective and prospective recruitment was allowed to provide the best opportunity to recruit to our sampling frame. Two groups of patients were identified: patients with short-term infections (largely SSTIs) and patients with deeper infections which generally require longer treatment courses (e.g. joint infections). The modelling work, however, identified only small differences between the two groups in terms of preferences (see Results). We aimed to ensure representation on the basis of age, sex, SES (based on work status), antibiotic experience and ethnicity.
Data collection
Recruitment took place between September 2014 and May 2015. Eligible patients were approached by a research nurse and details of consented participants were passed to the research team. The questionnaire was delivered to patients face to face using a laptop or iPad. Almost all participants completed the questionnaire in their own homes. A total of 512 people were approached, almost half (n = 254) of whom consented, and data were collected from 202 participants (20 participants could not be contacted post recruitment, 15 were too ill to participate, 17 refused post consent). Participants had experience of at least one model of care (Table 11). After asking participants about their preferences we asked about their past experiences with OPAT. A total of 94% (n = 191) of participants reported they were quite satisfied or very satisfied with the service they received.
| Characteristics | n (%) | SD |
|---|---|---|
| Age (years), mean (range) | 56.78 (20–94) | 13.67 |
| Sex (male) | 122 | |
| Children < 18 years | 35 (22) | |
| Ethnicity | ||
| White | 182 (90) | |
| Asian/black British | 16 (8) | |
| Other | 4 (2) | |
| Education | ||
| University or college | 91 (45) | |
| Technical | 28 (14) | |
| Secondary | 78 (39) | |
| Primary | 5 (2) | |
| Working status | ||
| Full-time | 63 (31) | |
| Part-time | 23 (11) | |
| Retired | 75 (37) | |
| Unable to work owing to illness | 31 (15) | |
| Other | 10 (5) | |
| Previous i.v. antibiotic experience | ||
| One current/previous infection | 171 (85) | |
| Two previous infections | 21 (10) | |
| Three previous infections | 6 (3) | |
| Four previous infections | 3 (1) | |
| Model of care experienced (can be more than one model) | ||
| HO attendance | 121 | |
| Nurse at home | 44 | |
| SA | 18 | |
Modelling approach
Pilot data
Frequency data were calculated using Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA) and Statistical Product and Service Solutions (SPSS; SPSS Inc., Chicago, IL, USA) for analysis. Qualitative feedback in form of written notes and audio-recordings were used to inform revisions to the survey and the DCE.
Main study
Our analysis made use of an advanced discrete choice model, which is a mathematical structure used to explain the influence of explanatory variables (e.g. type of treatment, frequency of treatment, risk) on the choices that patients make between the different treatment options presented to them. The model is based on the notion of utility maximisation, with respondents choosing the option that gives them the greatest utility/smallest disutility. For a detailed overview of choice modelling techniques, see Train. 123
The models explain the preference of a patient in a given choice task (i.e. which of the three models of care is chosen) by estimating the sensitivity that the patient has to the different characteristics of each model. With the expectation of major differences across patients in these sensitivities (e.g. one patient cares more about risk than another) and hence in the resulting preferences for a given model of care in a given choice scenario, our work incorporates three levels of heterogeneity in preferences across patients, namely:
-
differences that can be linked to the sociodemographic and infection characteristics of the participant (age, sex, race, education, employment status, past infections and whether they were short-term or long-term patients)
-
idiosyncratic differences across participants in their preferences that cannot be linked to the characteristics of the participant
-
differences in preferences that can be linked to underlying attitudes, namely attitudes towards hospitals and towards health-care responsibility, where we make use of the methods described by (among others) Abou-Zeid and Ben-Akiva,124 Vij and Walker125 and, with an example of a health application, Klojgaard and Hess. 120
Model estimation produces estimates of the sensitivities/preferences of respondents for the different types of treatment as well as treatment characteristics and patient characteristics (e.g. sociodemographics) in explaining these sensitivities and the underlying patient attitudes. These sensitivities in turn drive the preferences that respondents express between the different services presented in a given choice task. For example, the models produce parameters for risk and for waiting time, and their relative values allow us to understand the relative importance of these two characteristics in explaining the choice between the different models of care. These relative sensitivities vary across individual patients. The actual parameter values are determined in a numerical process that produces estimates that allow the model to best explain the choices observed in the data. Full details on the model structure are given in Appendix 3.
Random utility models such as those used in our work recognise the inability of analysts to capture fully the utility of alternatives and thus acknowledge the presence of a remaining random component of utility. Although initial insights into overall preferences can be obtained by direct questioning with regard to which service a given patient prefers, such approaches fail to recognise that the actual preferences will be influenced by the specific characteristics of the different service delivery methods. In particular, they will be the result of trade-offs, whereby a patient selects the option that provides the best overall combination of characteristics, with the good performance of some characteristics compensating for the poor performance of others.
We chose not to segment the analysis by infection type (short term vs. long term) but instead included this variable as a respondent covariate in the modelling, testing for impacts on the preference for treatment types as well as the valuation of treatment characteristics. This provides greater sample sizes and acknowledges that time to infection resolution is on a continuum. In addition, the modelling work showed only small differences between the two groups in terms of their preferences, and these related solely to appointment times, training and seeing and speaking to someone that the patient knows, and not to the type of treatment or key characteristics such as risk.
Full details on the model structure are given in Appendix 3.
Attitudinal data were analysed using principal component analysis with Varimax with Kaiser normalisation to identify underlying structure within the data (SPSS version 20). Factor loadings > 0.7 were accepted and a scree plot was used to determine the number of factors to include in the analysis. 126 The principal component analysis indicated that a two-factor solution provided a good fit to the data and accounted for 32% of the variance in scores: attitude towards hospitals and attitude towards health care being a doctor’s responsibility. In both cases a higher value means stronger disagreement. These factors were then used as latent variables in the analysis, allowing us to understand the role of attitudes in driving preferences (see Appendix 3 for further information).
Results
Pilot data
Minor revisions were made to the survey questions as a result of the qualitative feedback. In addition, further instructions were provided for respondents about the structure of the DCE, as it was found that participants did not understand that the options would change between each choice set. Two attitudinal items were removed, as all participants agreed with these statements so they did not distinguish between patients. Missing data were minimal. The data from the survey were analysed using a multinomial logit model and this yielded significant models whereby the sign and order of the coefficients on service levels were intuitive and in the expected order (i.e. levels that were worse had higher negative model coefficients).
Main study
This section gives a high level overview of the estimation results, with full details provided in Appendix 3.
We first look at the two underlying attitudes retrieved from the data, which are an attitude towards hospitals and an attitude towards health-care responsibility.
-
Participants who present a positive attitude towards hospitals are more likely to be female and non-white, and to live alone, and the impact is stronger the more of these characteristics they have. As this attitude becomes more positive, we also see an increase in the preference for in-hospital treatment in the DCE scenarios.
-
Participants who see health care as the responsibility of the doctor are likely to be > 65 years old and non-white. They are also slightly less likely to have a university degree. As this attitude becomes stronger, these participants have a much greater preference for the attendance at hospital or nurse at home models of care compared with SA in the DCE scenarios.
We next look at the headline results in terms of utility scores for the different treatment types and characteristics. The models explain the preference for a given model of care in a given choice scenario as a function of the sensitivities of the patient and the characteristics of the model of care. Each of these characteristics has an influence on the utility of the model of care, and the model of care with the highest utility obtains the highest probability of being chosen. Here, we work with the mean sensitivities at the sample average in terms of sociodemographic characteristics (which have an influence on preferences both directly and through the underlying attitudes) and the random variation in preferences (both directly and through the underlying attitudes).
The results in terms of the impact on utilities (and hence on preferences) are presented in Figure 2 where, for each attribute, one of the levels is normalised to zero for identification. We observe first of all that treatment type matters the most of any characteristic, followed by treatment frequency and risk. Looking in detail at the findings, we note that:
-
For treatment type, there is a strong overall preference for the nurse at home model, followed by hospital treatment.
-
Respondents prefer a single treatment per day to two treatments, continuous treatment and three treatments per day.
-
Having an appointment time is preferred to not having an appointment time.
-
A SN is preferred to a doctor or a GN.
-
A half-day of training for SA is preferred to a full day of training.
-
Patients prefer seeing someone they know.
-
Patients prefer speaking to someone they know.
-
Patients prefer nurse administered aftercare in hospital to GP aftercare, telephone aftercare and no aftercare.
-
For risk, we obtain clear evidence of non-linearity in sensitivities, leading us away from a continuous treatment of risk (which would have implied, e.g., that a 1 in 25 risk would have been valued 2.5 times as highly as a 1 in 10 risk). With only three levels of risk in the data, we then estimated separate valuations for 1 in 25 and 1 in 10, keeping 1 in 6 as the base. Moving away from a continuous specification was also justified by our analysis showing a lack of differences between valuations for 1 in 6 and in 10 in several groups.
FIGURE 2.
Mean preferences (utility scores) at sample average.

With the different sociodemographic interactions (discussed in Appendix 3) used in the model (e.g. how short-term and long-term patients react differently to appointment times), a total of 48 different sociodemographic groups exist in our model, and we note strong variations in the mean preferences relative to SA across these groups, as highlighted in Figure 3. What these data show is that the sociodemographic variables we measured [age, sex, race, education, employment status, number of past infections, type of current infection (long term vs. short term)] do affect the strength of preferences for a particular service, but these effects are modest. There is an overall preference for nurse at home treatment ahead of hospital treatment and ahead of SA, where this ordering applies to 39 out of 48 groups at the mean sensitivities (i.e. before allowing for random variations in preferences). In addition, nurse at home treatment is always preferred to SA at the mean preferences, and only white males < 50 years of age and living alone marginally prefer hospital treatment to nurse at home treatment.
FIGURE 3.
Mean utilities for models of care (relative to SA) in 48 different sociodemographic groups.
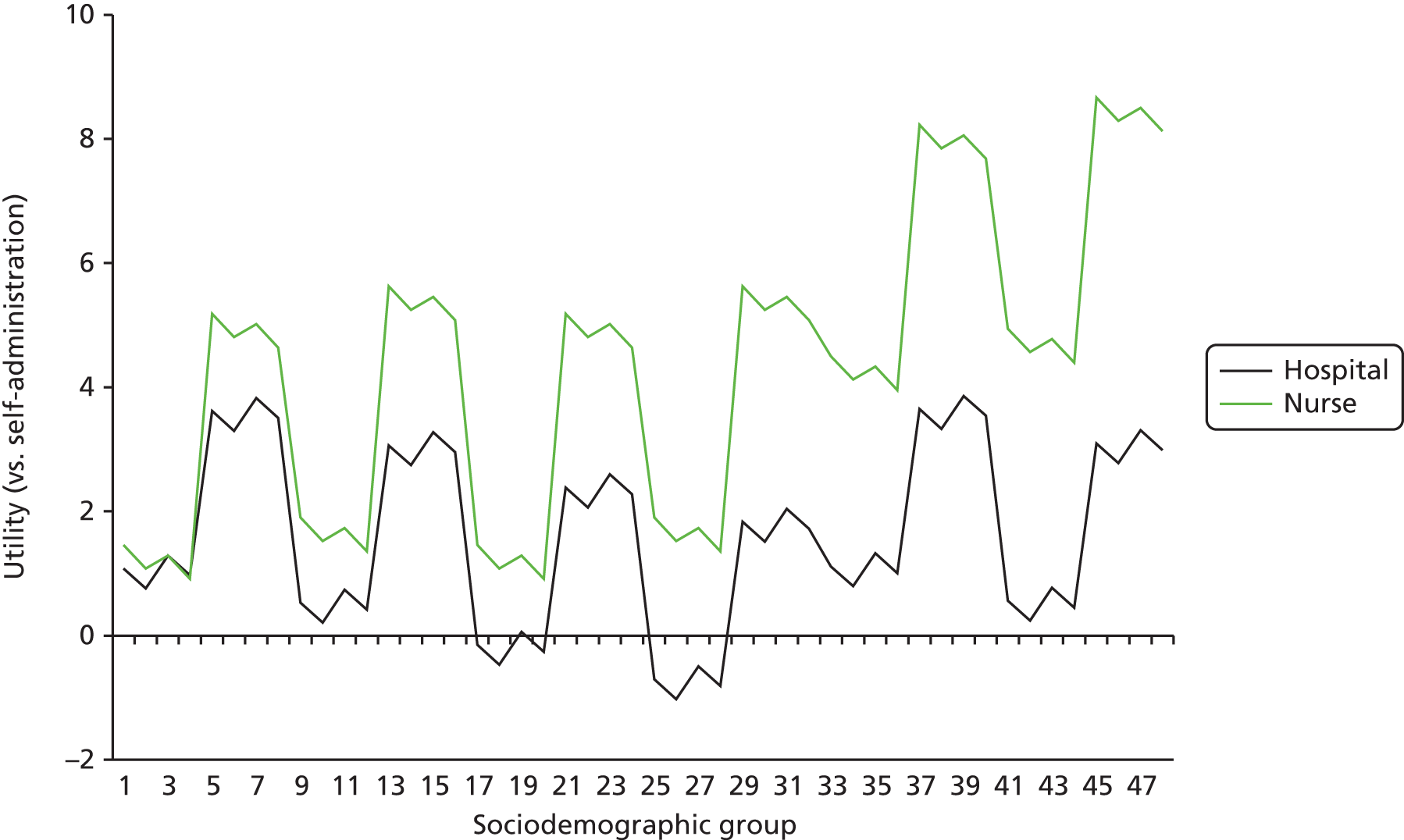
Some groups of patients prefer SA to hospital treatment, namely white respondents aged between 50 and 65 years of age who do not live alone (male or female), or are female and live alone, or are male, live alone and have a university degree. Importantly, the gaps between preferences for the different treatment types differ across groups.
Of course, owing to random heterogeneity, in sensitivities across individual patients, some probability of reversal of preferences exists. Looking at the largest group (age > 65 years, male, white, not living alone, no university degree), the mean utilities for hospital treatment and nurse at home (with SA as the base) are:
-
hospital treatment utility score: 1.11
-
nurse at home utility score: 4.50.
This would mean that the nurse at home model has the highest probability of being chosen, ahead of hospital treatment and SA. However, the large standard deviations caused by random variations in preferences give the following 95% confidence intervals (CIs; as used here referring to 95% of values falling within these intervals, and not suggesting an insignificant estimate, but random variation in preferences):
-
HO treatment: 95% CI –12.17 to 14.40
-
nurse at home treatment: 95% CI –7.48 to 16.48.
We now see that there is clearly a possibility, even with a small probability, of SA being the most preferred option for some patients. An important component in our work is the incorporation of underlying attitudes. As described earlier, participants who present a positive attitude towards hospitals are more likely to choose hospital treatment, whereas those who see health care as the responsibility of the doctor have a much greater preference for attendance at hospital or nurse at home models of care, than for SA. Here, we see that the attitude towards responsibility of health care accounts for 65% of the heterogeneity for the preference for hospital treatment, and 88% for the preference for nurse at home treatment. The attitude towards hospitals, however, accounts for only 6.9% of the heterogeneity for the preference for hospital treatment, and 4.3% for the preference for nurse at home treatment.
For the remaining attributes, we now look only at those in which differences exist across sociodemographic segments (which is not the case for aftercare and frequency of treatment), given that the overall results are included in Figure 2.
Figure 4 shows a very large difference between long-term and short-term patients in their preference for having an appointment time (with no appointment time as the base), with the latter having a much stronger preference for an appointment time.
FIGURE 4.
Appointment time (utility scores; no appointment time as base).
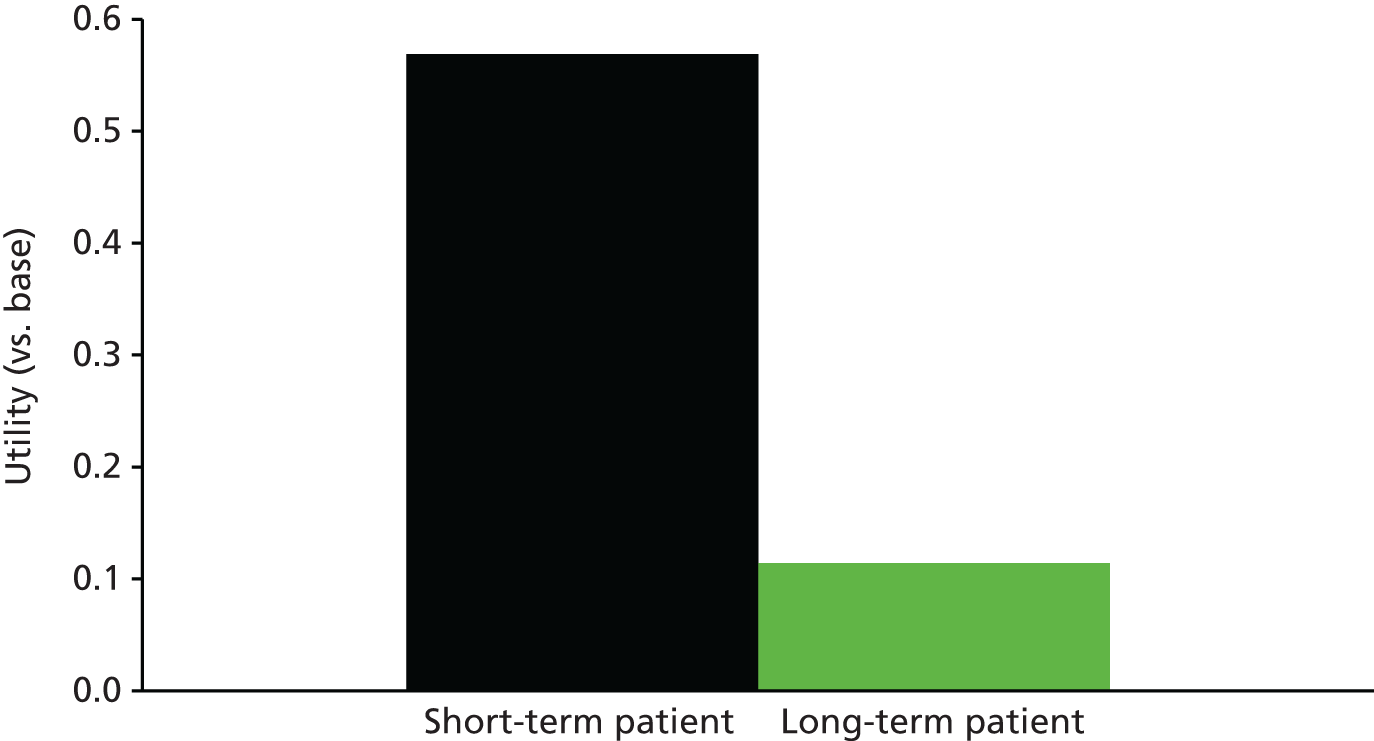
Figure 5 shows that, although patients not living alone have a strong preference for SNs giving the treatment ahead of GNs and doctors, for those living alone, there is a preference for doctors and SNs (where the two are not significantly different) over GNs.
FIGURE 5.
Who gives the treatment? (Utility scores; doctor as base.)
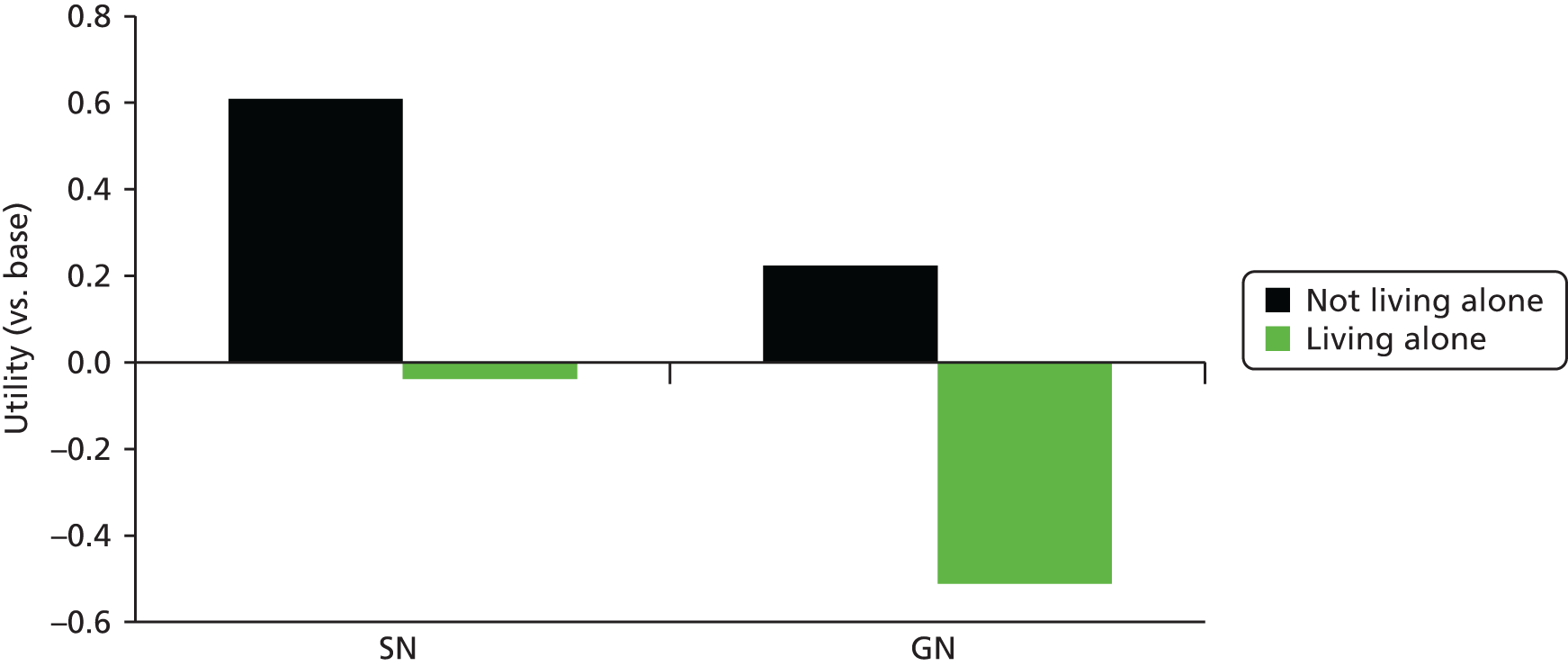
More differences arise across groups in terms of the preferences for a half-day of training for self-administration (with 1 full day as the base). Figure 6 shows that, although long-term patients aged < 50 years who do not live alone have a very strong preference for a half-day of training, short-term patients > 50 years of age and living alone have a strong preference for 1 full day of training. Other groups are in between these two extremes.
FIGURE 6.
Preference for half-day training (utility score; 1 full day as base).

For communication in person, all patient segments prefer seeing someone they know, but Figure 7 shows that this is more important to younger patients and long-term patients. More important differences arise with regard to communication over the telephone, and Figure 8 suggests that, in particular, older patients living alone have a preference for speaking with someone who they do not know.
FIGURE 7.
Communication in person (utility scores; base = unknown person).
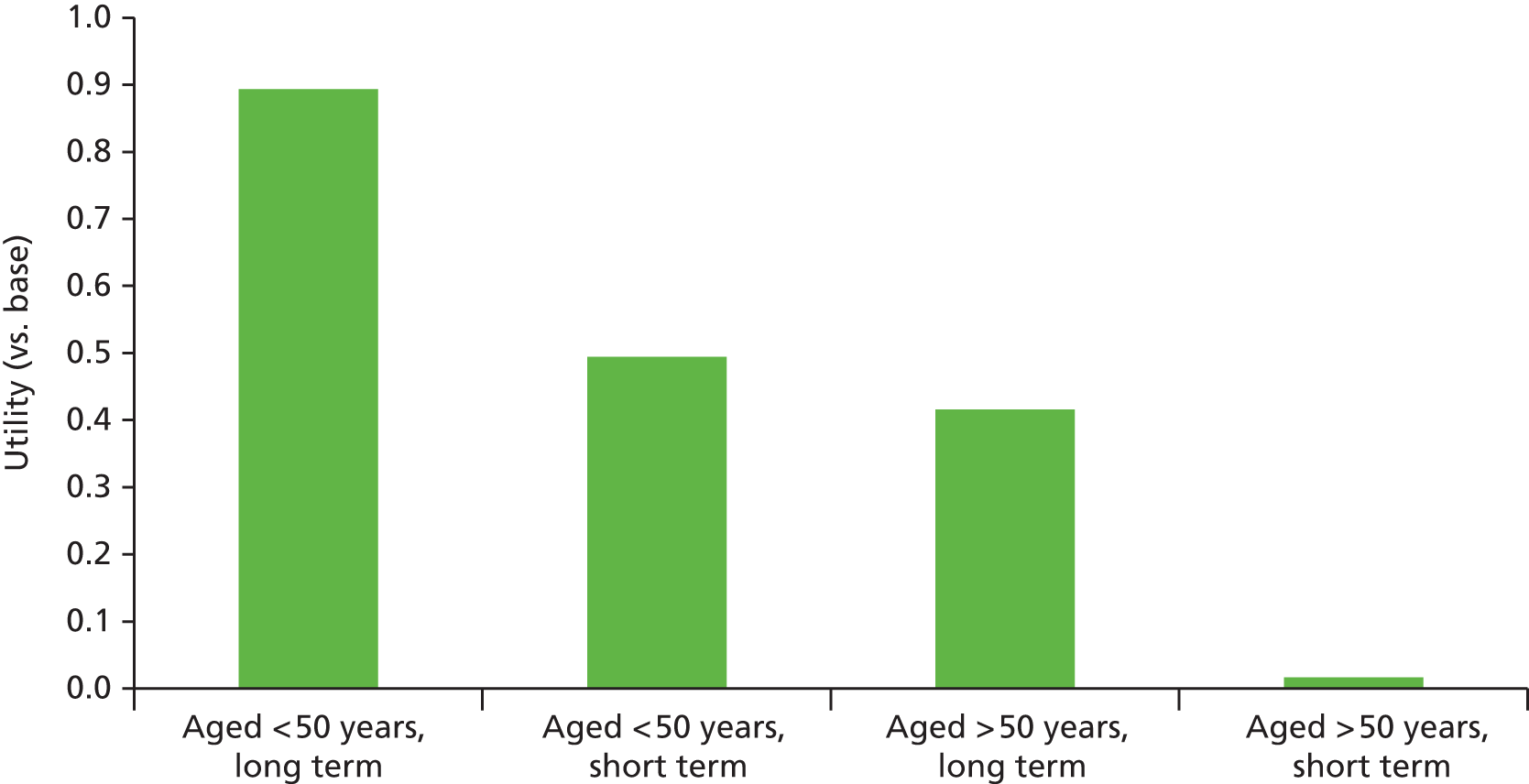
FIGURE 8.
Communication over telephone (utility scores; base = unknown).
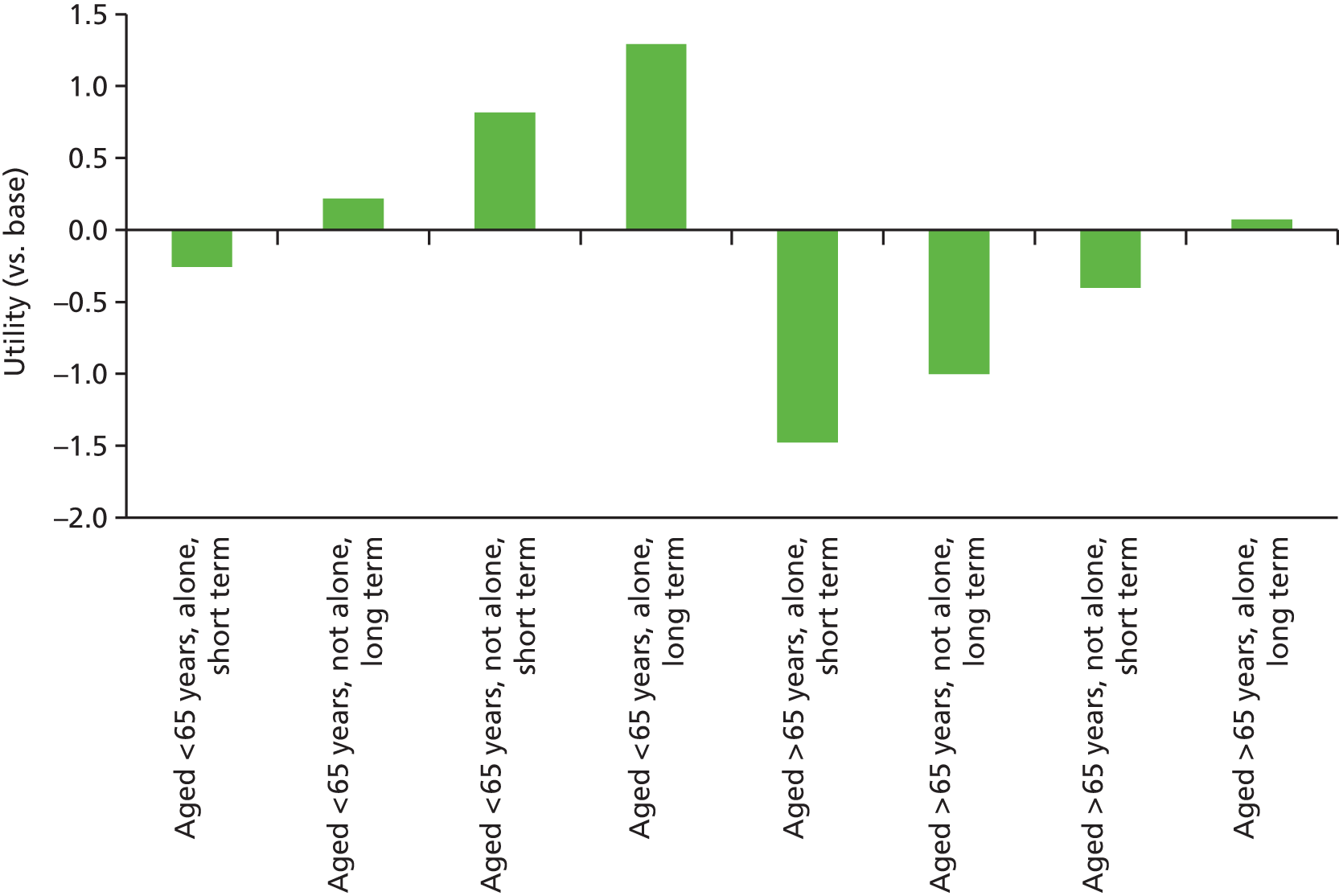
Finally, Figure 9 highlights the sensitivity to risk (e.g. of an adverse event). We see that patients aged < 65 years and not working fail to distinguish between the two higher levels of risk, where the difference in sensitivities between these two levels is stronger for older patients and for those in employment. The difference between the two lowest levels of risk (1 in 10 and 1 in 25) is not significant for those aged > 65 years and in employment, so the positive shift in Figure 9 can be ignored. We chose not to model risk as a continuous variable, as it appeared to be non-linear. Thus, we were not able to identify the marginal rates of substitution between this and other attribute levels.
FIGURE 9.
Risk (utility scores; 1 in 25 as base).
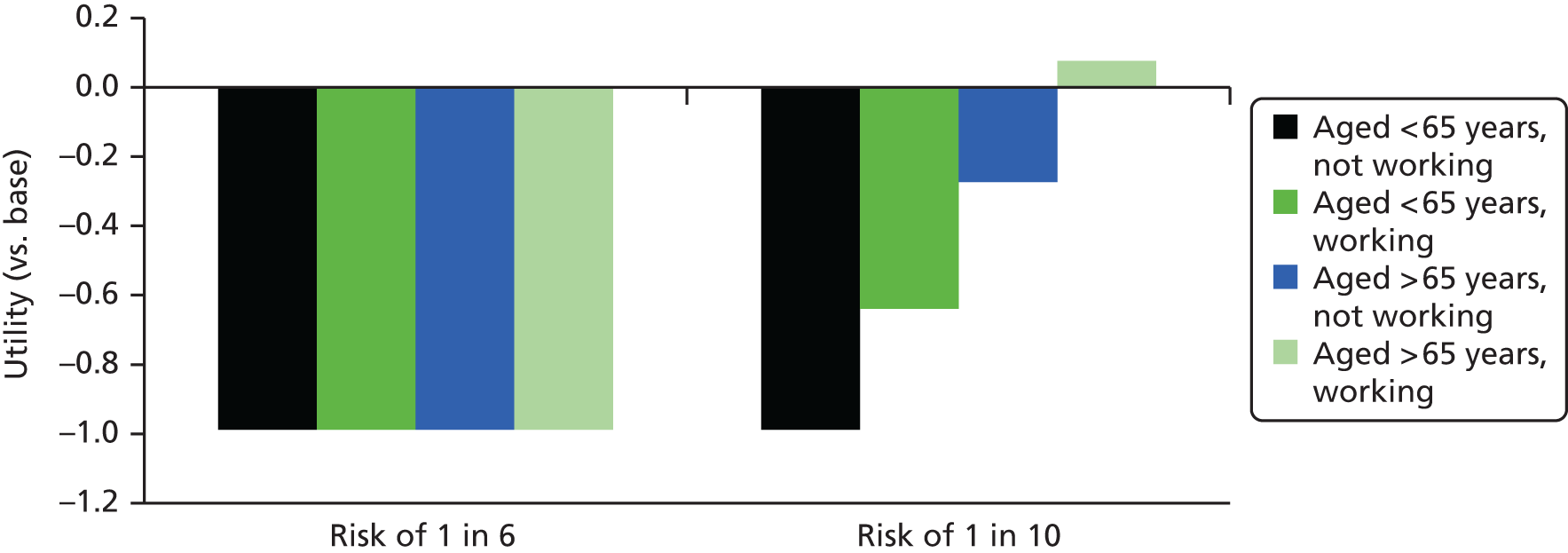
Discussion
Patient choice is becoming increasingly important in the provision of health care in England. Patients are being provided with information relating to health conditions and available treatments and are being empowered to influence the care that they receive. Given this, it is important to factor their preferences and demand for aspects of care in to the design of new services. This chapter described the application of a stated choice technique, the DCE, to understand patient preferences for OPAT services.
The use of DCEs to inform the design of health services is a well-established approach. 100,127 The models estimated on such data explain the choices that respondents (in this case, patients) make in individual choice scenarios through estimating their sensitivities to the different characteristics that describe the alternative options they can choose from, in this case models of care. Although basic models assume that the sensitivities to individual service characteristics are constant across patients, more advanced models allow for differences across patients, some of which can be linked to sociodemographic characteristics and others of which are a function of attitudes; a remaining layer of heterogeneity is purely random.
This research is the first attempt to understand patients’ preferences for OPAT services and one of the most in-depth explorations of the role of patient attitudes in influencing health-care preferences. The attributes of the service were selected on the basis of the qualitative interviews and systematic review literature which showed which of these are important to patients. The choice survey offered three service options (four including two types of nurse) described by eight attributes (two of these embedded in other attributes). The attributes mainly comprised process aspects of care, including the number of treatments per day, whether or not appointments were given, who delivers the treatment and the level of communication and aftercare provided. However, we did include a health outcome aspect, which was the level of risk of an adverse event.
The results indicate that when we look across respondents and do not take sociodemographic variables into account, the type of service is the most important factor, with the nurse at home being strongly preferred to HO treatment and SA. The next strongest sensitivity relates to treatment frequency, with once per day treatment being strongly preferred to two treatments or continuous treatment. This was closely followed by the preference for the lowest level of adverse event risk. Although other attribute levels were significant in determining respondents’ choices, they were less important. People preferred a SN to a doctor and GN to deliver their intravenous antimicrobials (IVA), preferred an appointment time (to not having one) and preferred to communicate with someone they know regarding their care. These sources of process utility are clearly important to patients and this is a consistent finding across stated preference studies. 128 The order of attribute preference was relatively stable across the short- and long-term infection groups. Indeed, significant differences were not observed for preferences for treatment type or key characteristics such as risk, with differences only for appointment times, training and seeing or speaking to someone the patient knows. Generally, the findings (see also the detailed results in Appendix 3) are in line with the qualitative results, which suggest that no one model is preferred by all patients, with strong heterogeneity across different patient types, albeit with an overall average preference for the nurse at home model. The choices that people make about their health care are influenced by a number of patient characteristics as well as by more general attitudes towards health care.
In this study, younger patients tend to prefer to come to hospital for their care, and older people tend towards a preference for a nurse at home model, compared with the alternative treatments. Previous studies have focused on the acceptability of OPAT more generally,36,84 or have compared acceptability of OPAT with inpatient treatment,12,25,41,56 and have not examined whether or not patients receive a model of care that fits their needs. However, our qualitative work (see Chapter 4) sheds some light onto these findings, as it showed that younger people find hospital attendance to be convenient and older people find it less so, particularly if they do not have their own transport or if their infection leaves them feeling unwell. Given that older people tend to have more comorbidities than younger people, it is not unsurprising that older people prefer to be looked after in their own homes. It is also possible that people who need to travel to work would find it more convenient to visit a hospital for treatment.
Regardless of the model of care preferred, there are some issues that cut across models of care. Overall, there was a preference for once-daily antimicrobials and for follow-up at the end of treatment, which patients would prefer to be face to face rather than by telephone. The qualitative interviews found that many patients did not see a doctor again after they were diagnosed and given their course of i.v. antimicrobials, and this may go some way to explaining the preferences expressed. The interviews with health-care professionals (see Chapter 3) found that patients generally do get reviewed while on their i.v. treatment; however, this is often done as a ‘virtual ward round’, of which the patients are unaware, and, thus, they are left feeling abandoned at the end of their treatment. The results also revealed that many patients prefer to see, and speak to, a health-care professional who is known to them, although this preference did not hold for all patient groups. Similar findings were identified by our systematic review,69 and Talcott et al. 87 reported that some patients felt more isolated at home, than as an inpatient. These feelings may contribute to the desire to be seen at the end of treatment. These perceptions also accord with the notion of continuity of care, which some consider to be a ‘cornerstone of care’. 129,130 Continuity can be defined in terms of longitudinality, but can also be characterised as a structural element of care, largely controlled by the care provider, which means that there are processes and mechanisms in place to ensure that information is passed on efficiently and problems are adequately followed up from one visit to the next. 131 Patients’ wishes to have contact with staff they know and to be followed up after the end of treatment are therefore something that services should consider when reviewing or setting up services.
Although wide levels of preference heterogeneity were observed, certain trends were apparent. For example, age and cohabitation circumstances were consistently important determinants of choices. The finding that older patients who live alone have a strong preference for longer training sessions, face-to-face contact and contact with SNs suggests that this group exhibits greater anxiety about IVA and requires greater support. On average, people preferred less risk, but there were some groups that did not differentiate between the risk levels. We were unable to establish the marginal rates of substitution between risk and other service attributes as risk did not appear to be linear. This may reflect a real non-linear attitude to risk or be a function of the specific survey design employed here. 132 The risks here are higher than those likely to be faced in reality (see the risks used in the economic models used in Chapter 6).
Strengths and limitations
Despite recruiting in NHS trusts with a significant ethnic minority population (10–20%) we were unable to recruit as many people from ethnic minorities as we would have liked. However, the proportion of individuals from ethnic minorities in our sample is in line with the England average. This may be because we did not offer the DCE in languages other than English, but we have no evidence to support this assertion, as only limited data are available on non-participants. This means that issues that may be relevant to the non-white population have perhaps not been sufficiently explored. We are also conscious that all interviewees upon which the DCE is based were from white British ethnic backgrounds, and so we may have missed issues that are of importance to the non-white population; this is a potential source of bias in respect of the attributes themselves. However, the literature that informed the development of the attributes and their levels comes from a range of countries. Although ethnic mix was not reported in any of these studies, literature from the UK,5,35,69 France,84 New Zealand,36 Sweden,71 Ireland2 and Canada37 was used, which does provide some evidence to support the robustness of the attributes.
Some of the options contained in the DCE do not represent current practice. Nurse-led clinics are common in other health-care situations, but much less so in OPAT, and so we wanted to explore patients’ willingness to be followed up in this way, given that, currently, many patients are not seen in clinic at the end of treatment. Likewise, doctors almost never administer i.v. treatment but some patients in our interview study talked in depth about the importance of the doctor, so we included this to test what patients would be willing to trade off to receive care in this way.
Patient preconceptions and past experiences may influence the findings, but evidence suggests that past experience is only a weak predictor of future behaviour,133 and in the present study few patients had significant OPAT experience to draw upon. Only adults who had previously experienced OPAT were included; those who would be eligible for OPAT but were not offered the service were not included, and this population may respond differently. The reason for the sampling used in this study was that the original protocol included the collection of anonymised data from the DCE participants, so that only those who had received OPAT were eligible.
Implications
By quantifying patient preferences for attributes of care, commissioners can use the results of this study to inform changes to service provision so as to obtain the best outcomes within a given budget. The results indicate that where one model of OPAT care is envisaged, a nurse at home model is likely to be preferred by patients. However, where possible, a range of options should be available. The most promising model would be one that offered a SN at home model, utilising one-a-day treatments (where safely available). The service should have a dedicated team of staff caring for patients and have robust governance processes in place to ensure that patients receive continuity of care (i.e. good handover and communication between staff) and are followed up at the end of treatment by a nurse, or where clinically appropriate, a doctor. The qualitative data (see Chapter 4) triangulate the DCE findings and suggest that patients’ attitudes towards health care are important and could form a target for future intervention. The preferences across short- and long-term infection patients were quite stable, suggesting that people in these groups value similar service attributes. Given this, OPAT services may work across infection types.
These findings are likely to be useful in determining future service provision in this area, which takes account of patient preferences. It will help NHS trusts that want to introduce OPAT services by providing a rationale for service configuration.
Caveats are required, however, in translating the results found here into service commissioning. First, we must acknowledge that only services that represent value for money should be offered to patients. The benefits of the services in our DCE were (with exception of risk) based on process utility and not health outcomes. Although convenience is important, and may offer indirect health benefits (e.g. through better adherence), we must be cautious in attributing too great a value to it. Second, the DCE provides information only on stated preferences and may not accurately reflect the choices people would make if faced with the same options in reality. Additional research is required to understand if and how stated preferences in health could be calibrated to better reflect revealed preferences to facilitate service design and planning.
Chapter 6 Cost-effectiveness: report on the economic modelling of Community IntraVenous Antibiotic Study services
Introduction
Although there is encouraging evidence relating to the safety and effectiveness of OPAT from observational and cohort studies, stronger evidence in the form of RCTs is lacking. 114,134 Furthermore, although several observational studies have reported bed-day savings resulting from the introduction of OPAT, robust economic evaluations of OPAT services are few. 134 A recent systematic review of cost-effectiveness analyses of OPAT services found a number of studies presenting cost analyses but none that would meet the technology appraisal reference case criteria set out by NICE. 135 These studies incorporated a number of OPAT service models and antimicrobial (both oral and i.v.) service comparators, including inpatient care and early discharge with oral treatment. The patient populations were varied too, including those with surgical site infections,66 MRSA-complicated SSTIs,136 cystic fibrosis,53 febrile neutropenia65 and prosthetic joint infections. 24 Of the studies identified in the review, only one presented an incremental cost–utility analysis. 65 Using a Canadian health-care provider perspective, the authors in the study found oral antimicrobial treatment at home to be cheaper and less effective than home i.v. treatment and that home i.v. treatment dominated (i.e. were cheaper and more effective than) hospital-based services. 65
It is possible that the evidence gap in this area has stymied investment from decision-makers and service commissioners. In the absence of RCT evidence and robust economic evaluations to commend one OPAT service over another, commissioning in the area is fraught with uncertainty, barriers to the wider adoption of services remain and geographical variation in service provision pervades. It is clear that further research is required to inform decision-making. However, given that research resources are scarce and RCTs often expensive and relatively slow to yield results, it is imperative that they are streamlined to answer the important questions and include the comparators most likely to be cost-effective. This is especially true in OPAT i.v. antimicrobial services in which a number of service configurations are possible. As such, formal characterisations of the value of research137,138 might be beneficial in determining the research agenda in OPAT. The aim of the research described in this chapter was to conduct a decision-modelling-based economic evaluation to estimate the cost-effectiveness of different OPAT services and the value of additional research in order to: (1) provide evidence for decision-making and (2) inform future research by identifying which services should feature in future RCTs and where the greatest uncertainty lay.
Markov models are the most common type of decision-analytic model employed in economic evaluations. Although they offer an efficient method of generating estimates of cost-effectiveness and value of information, there are certain restrictions imposed by their structure. Markov models do not readily provide information on health-care resource capacity, treatment queuing and delays or required staffing levels given a patient population size. A patient simulation approach to modelling is required to overcome these limitations and to provide this information. With this approach it is possible to create mathematical representations of the process and operation of a system, which enables the analyst to experiment and test interventions and scenarios, evaluating their consequences in real time. 139 Two approaches were therefore adopted for the evaluation. One approach used a Markov modelling strategy to generate estimates of cost-effectiveness and value of information and a second approach used patient-level simulation modelling to provide information on service resourcing.
Methods
Decision analysis is an explicit, quantitative and systematic approach to decision-making under conditions of uncertainty. 140 Decision-analytic modelling in health is an analytical approach allowing an economic evaluation of (at least two) alternative courses of action (i.e. treatments or services) that formally characterises the uncertainty in the decision. The decision model is created to reflect the health-care process or pathway, capturing the events that occur to the patient or health system during care and estimating the expected costs and (dis)benefits of the treatment options. Hypothetical patients enter the model and pass through ‘health states’ that represent the health events. Each health state has costs and quality-of-life values associated with it, and the route of the patients through the model is determined by ‘transition probabilities’. Decision-analytic models were developed with best practice in mind141 to estimate the cost-effectiveness of four OPAT service models. The value of information framework142 was employed to estimate the value of, and priorities for, future research. Both Markov and simulation modelling approaches were adopted; these are described separately below.
Markov models
Population, interventions, comparator, outcomes and perspective
The population of interest was broadly considered to belong to one of two groups: those with SSTIs and other short-term infections (short term) and those with longer-term or chronic infections such as joint and bone infections and cystic fibrosis (long term). For the purpose of the analysis we defined short-term infections as any SSTI or infection that, on average, would require, at most, 7 days of IVA or any non-complex SSTI. Infections requiring longer courses of treatment or recurrent infections were considered long term. These two groups posed distinct decision problems and, therefore, estimates of cost-effectiveness were generated separately for short- and long-term infection patients.
After discussions with clinicians it was clear that (inpatient) hospital admission should not be a comparator for the analysis and that the research question should focus on identifying which of the OPAT models was most cost-effective. Although it is acknowledged that many service configurations exist, the four identified as most common in a survey of OPAT units conducted in the programme of research were: outpatient attendance at hospital (HO), treatment provided at home by a GN or district nurse or by a specialist OPAT nurse and SA following training provision (SA). The HO service involves patients attending a HO clinic to receive IVA on a daily basis. In the nurse-led services, nurses administer IVA in the patient’s home on a daily basis. However, the GN administers IVA as part of a range of care provided, whereas the SN is dedicated to delivering OPAT services. Although it is possible that more than one IVA administration is required per day, we assumed that all patients received once-daily treatments, as this is common and increasingly the treatment strategy of choice. Finally, it was assumed that SA patients were given a 2-hour training session and delivered their own IVA on a daily basis but were followed up by nurses through face-to-face visits and telephone calls. Patients in the GN, SN and SA services are required to visit the clinic (one visit assumed in the short term and fortnightly visits in the long term) for a consultation with a doctor and to review test results. In the long term, these groups also visit the clinic for a final review and check-up at the end of IVA treatment. It was assumed that each service used the same i.v. antimicrobial. The service models are outlined in detail in Tables 12 and 13.
| Service | ||
|---|---|---|
| HO | GN | SN |
| Initial consultant-led outpatient clinic visit | Initial home visit by GN (1.5-hour duration) | Initial home visit by SN (1.5-hour duration) |
| Once daily non-consultant-led outpatient clinic visit | Daily home visit by GN (1-hour duration) | Daily home visit by SN (1-hour duration) |
| One consultant-led outpatient clinic review visit | One consultant-led outpatient clinic review visit | |
| Daily IVA delivery | Daily IVA delivery | Daily IVA delivery |
| Service | |||
|---|---|---|---|
| HO | GN | SN | SA |
| Initial consultant-led outpatient clinic visit | Initial home visit by GN (1.5-hour duration) | Initial home visit by SN (1.5-hour duration) | 2-hour training provided by nurse and IVA self-administered |
| Once daily non-consultant-led outpatient clinic visit | Daily home visit by GN (1-hour duration) | Daily home visit by SN (1-hour duration) | Weekly home visit by nurse (1.5-hour duration) |
| One consultant-led outpatient clinic review visit every 2 weeks | One consultant-led outpatient clinic review visit every 2 weeks | One consultant-led outpatient clinic review visit every 2 weeks | |
| One consultant-led outpatient clinic visit at IVA end | One consultant-led outpatient clinic visit at IVA end | One consultant-led outpatient clinic visit at IVA end | |
| Daily IVA delivery | Daily IVA delivery | Daily IVA delivery | Daily IVA delivery |
| Pump/balloon delivery device used | |||
Following expert clinical advice it was decided not to include the SA service in the short-term model, as it was unlikely to be offered to (or demanded by) this patient group. It was also decided to consider HO as ‘standard’ OPAT care in the UK, even though there is geographical variation in service provision. Thus, for the purpose of modelling, the comparisons for the short-term infection group were: HO versus GN versus SN; and for the long-term infection group: HO versus GN versus SN versus SA. The primary outcome for the analysis was cost per QALY. The primary cost perspective chosen was that of the NHS and Health and Personal Social Services (HPSS). We chose this as the primary perspective in order to conform to the NICE technology appraisal guidance reference case. A sensitivity analysis considered a combined HPSS and patient perspective, which incorporated patient out-of-pocket costs.
Model horizon, structure and assumptions
The model structure (Figure 10) was informed by a rapid review of published infection decision models and by discussions with patients and clinicians. Cohort Markov models were constructed with daily cycles until the cohort was healed (or switched to oral antimicrobials) or died. The patient cohort receive one of the OPAT services and every cycle were either healed (or switched to oral treatment), experienced a severe adverse reaction to the treatment (e.g. anaphylactic shock), contracted Clostridium difficile infection (CDI) or contracted a severe secondary-line infection (e.g. Staphylococcus aureus). If patients experienced any of these negative health events then they were subject to a mortality risk. In addition to the more severe negative events, those who were not healed were exposed to a daily risk of a mild adverse event (such as rash, nausea, vomiting, dizziness, fever and minor line events). These events and the associated costs are incorporated within the ‘not healed’ health state and are not associated with a quality-of-life decrement as they are both mild and transient in nature. However, a sensitivity analysis did employ a utility decrement for these events. It was assumed that they do not increase time to heal.
FIGURE 10.
Markov model structure.
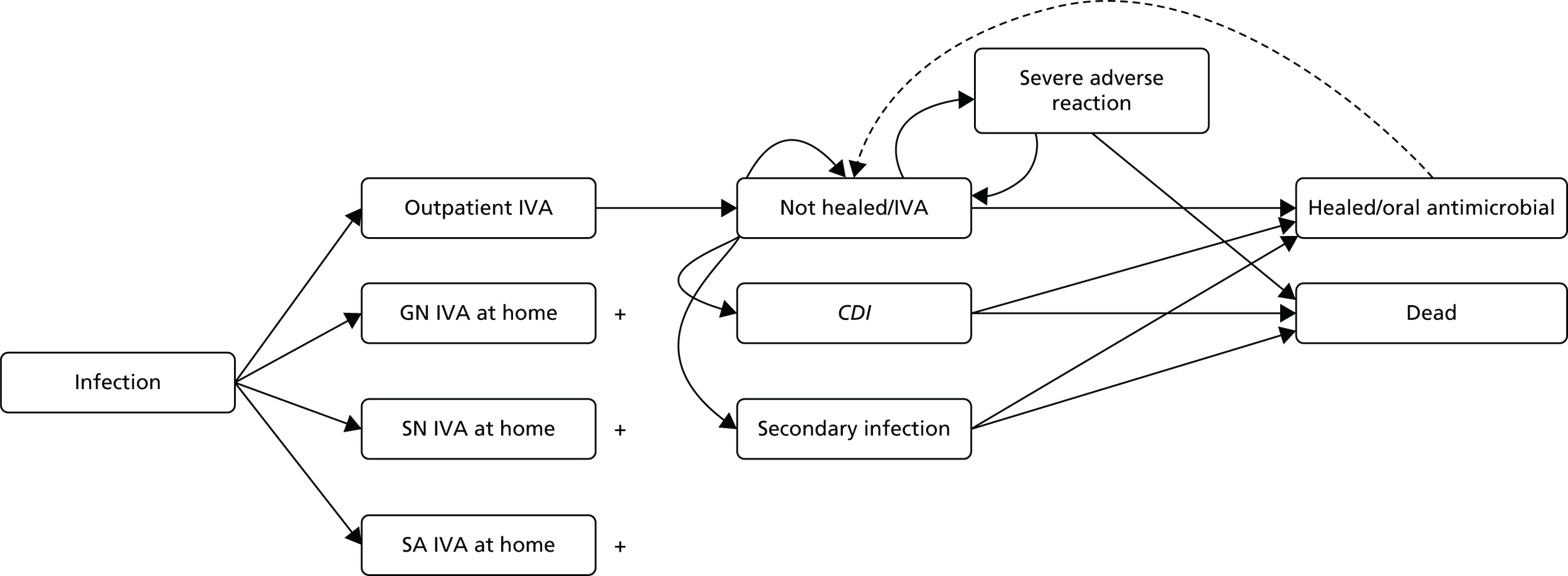
All patients began in the ‘not healed’ state and moved between the health states according to pre-specified probabilities. The cohort of patients flow through the model pathway accruing costs and quality-of-life penalties or ‘decrements’ until they are healed or die or until the model has ended. A sensitivity analysis explored the impact of some patients ‘relapsing’ and needing to receive IVA again after they had been switched to oral antimicrobials. Even though the costs and benefits of IVA treatments may register after the infection period (e.g. those who die as a result of an adverse event will lose future QALYs), we decided to limit the model horizon to the infection period. This was mainly due to the expectation that very few deaths would occur and, hence, the additional complexity and computation time required to model a longer time horizon would not be worthwhile. The time horizons chosen for the short-term (3 months) and long-term (12 months) models reflected the expectation that a vast majority of infections would have resolved within these periods. It was assumed that the period of time spent on oral antimicrobials and their effectiveness was the same across service models and unrelated to their IVA treatment. Given this, it was assumed there were no differential resource use or quality-of-life effects of oral treatment and that modelling these outcomes was not warranted.
The transition probabilities, costs and utility parameter values required for the models were taken from a number of sources including a systematic review134 and expert clinical opinion. In addition, hospital records of a group of patients (n = 503) who had recently received OPAT care were gathered and used to generate parameter values (Table 14 provides sample characteristics).
| Characteristic | Short term (N = 223) | Long term (N = 280) |
|---|---|---|
| Female, n (%) | 90 (40.36) | 133 (47.84) |
| Age (years), mean (SD); range | 52.85 (16.36); 18–89 | 52.59 (18.25); 18–94 |
| Ethnicity, n (%) | ||
| White | 195 (87.44) | 257 (91.79) |
| Asian | 20 (8.97) | 16 (5.71) |
| Black | 2 (0.9) | 3 (1.07) |
| Mixed ethnicity | 6 (2.69) | 4 (1.43) |
| Type of service received, n (%) | ||
| HO | 154 (69.37) | 63 (22.58) |
| GN | 18 (8.11) | 68 (24.37) |
| SN | 36 (16.22) | 34 (12.19) |
| SA | 0 (0.00) | 68 (24.37) |
| Combination | 14 (6.31) | 46 (16.49) |
| Type of infection, n (%) | ||
| Cellulitis/SSTI | 196 (87.89) | 44 (15.71) |
| Cystic fibrosis | 0 (0.00) | 44 (15.71) |
| Respiratory | 8 (3.59) | 37 (13.21) |
| Bone and joint | 0 (0.00) | 73 (26.07) |
| Cardiovascular | 1 (0.45) | 11 (3.93) |
| Urinary tract | 3 (1.35) | 7 (2.5) |
| Intra-abdominal | 2 (0.9) | 7 (2.5) |
| Other | 13 (5.83) | 57 (20.36) |
| Number with infection in past 6 months | 55 (24.66) | 154 (55.0) |
| Number with complex infection | 0 (0.00) | 75 (26.79) |
| C-reactive protein level | 87.28 (93.01) | 80.46 (99.52) |
| White blood cell count | 10.11 (3.55) | 10.84 (5.12) |
| Days to heal, mean (SD); range | 5.77 (5.35);1–55 | 28.54 (20.64); 4–119 |
| Side effects, n (%) | ||
| Rash | 3 (1.35) | 5 (1.79) |
| Nausea/vomiting | 3 (1.35) | 12 (4.29) |
| Dizziness | 1 (0.45) | 2 (0.71) |
| Fever | 2 (0.90) | 1 (0.36) |
| Diarrhoea | 5 (2.24) | 4 (1.43) |
| Infection of i.v. access device | 1 (0.45) | 2 (0.71) |
| Blocked i.v. device | 9 (4.04) | 13 (4.64) |
| Other | 23 (10.31) | 52 (18.57) |
Time to heal was calculated by type of infection and after controlling for a number of factors including markers of infection severity (e.g. C-reactive protein levels and white cell blood count) and patient characteristics (e.g. whether or not they had experienced previous infections in the past 6 months). We applied a negative binomial regression model, as the variable of interest (days to heal) was a non-negative count variable with a wide dispersion (there were several outlier patients with relatively long heal times). We used the margins command in Stata (StataCorp LP, College Station, TX, USA) to produce predictions (and associated standard errors) holding all other covariates at their means. A number of the severity markers were entered into the model as categories: white blood cells as ‘normal’, ‘mild’, ‘severe’ or ‘not known’; and C-reactive protein levels as 0–40 mg/l (normal or mild), 40–200 mg/l (active) and > 200 mg/l (severe).
The final model for the adjustments was:
where Ŷ = time to heal in days; βCR0. . .2 = dummies for C-reactive protein level groups; βWB0. . .2 = dummies for white blood cell groups; βS0. . .4 = dummies for OPAT service type; βC = complex infection and βPr = previous infection in past 6 months.
The adjusted time-to-heal figures for the short and long term are included in Table 15 along with their standard deviations. The cost of resource use was calculated on a daily basis for all services combined using the same variables and the β on days to heal being the adjusted value employed in the model.
| Parameter | Mean | SD | Distribution | Source |
|---|---|---|---|---|
| Short-term model effectiveness (days to heal) | ||||
| HO | 4.73 | 0.24 | Gamma | Adjusted hospital record data |
| GN | 7.36 | 1.00 | Gamma | Adjusted hospital record data |
| SN | 6.33 | 0.65 | Gamma | Adjusted hospital record data |
| Long-term model effectiveness (days to heal) | ||||
| HO | 27.21 | 2.30 | Gamma | Adjusted hospital record data |
| GN | 31.16 | 2.65 | Gamma | Adjusted hospital record data |
| SN | 25.46 | 3.02 | Gamma | Adjusted hospital record data |
| SA | 28.20 | 2.52 | Gamma | Adjusted hospital record data |
| Daily risk of an anaphylactic shock | 0.00005 | 0.00099 | Beta | Matthews et al.29 |
| Anaphylactic shock | ||||
| Anaphylactic shock mortality risk | ||||
| HO | 0.067 | 0.04480 | Beta | Hopf et al.143 |
| GN | 0.13 | 0.04480 | Beta | Assumed double HO risk |
| SN | 0.13 | 0.04480 | Beta | Assumed double HO risk |
| SA | 0.27 | 0.04480 | Beta | Assumed double GN/SN risk |
| Risk of CDI | ||||
| HO | 0.000105 | 0.000023 | Gamma | Hourly risk × 4100 |
| GN | 0.0000087 | 0.000004 | Gamma | Assumed third HO risk and 1-hour contact |
| SN | 0.0000087 | 0.000004 | Gamma | Assumed third HO risk and 1-hour contact |
| Self-administered OPAT | 0.00 | N/A | Fixed | Assumed no risk |
| Time to heal from CDI (days) | 16 | 0.40 | Log normal | Forster et al.144 |
| Daily CDI mortality risk | 0.00040 | 0.00004 | Beta | Wiegand et al.145 |
| Risk of a line infection that leads to a S. aureus infection | 0.00052 | 0.01580 | Gamma | Barr et al.40 |
| Time to heal from S. aureus | 17 | 0.32 | Log normal | Forster et al.144 |
| Daily S. aureus mortality risk | 0.0092 | 0.03346 | Beta | Thwaites et al.146 |
| Short-term model: mild adverse events | ||||
| HO | 0.020 | 0.006 | Gamma | Hospital record data |
| GN | 0.046 | 0.032 | Gamma | Hospital record data |
| SN | 0.054 | 0.025 | Gamma | Hospital record data |
| Long-term model: mild adverse events | ||||
| HO | 0.008 | 0.003 | Gamma | Hospital record data |
| GN | 0.007 | 0.003 | Gamma | Hospital record data |
| SN | 0.009 | 0.004 | Gamma | Hospital record data |
| SA | 0.033 | 0.009 | Gamma | Hospital record data |
| Probability of infection relapse | ||||
| HO | 0.0 | N/A | Beta | Assumed 5% lower than SN |
| GN | 0.0 | N/A | Beta | Assumed same as SN |
| SN | 0.0 | N/A | Beta | Lillie et al.147 |
| SA | 0.0 | N/A | Beta | Assumed same as SN |
Parameter values
Transition probabilities
The systematic review could only identify effectiveness and risk values presented in observational studies. These were of limited value, as there was unlikely to be equipoise in service provision; for example, some departments may have considered only certain patients (e.g. less severe or more independent) for particular OPAT services. Given this, the patient-level data from records was utilised as it allowed the possibility of controlling for severity. In the models, ‘not healed’ patients could travel to the CDI state according to a daily probability based on the time they spent in a hospital environment or in contact with a GN or SN. It was assumed that HO patients had a greater chance of developing CDI than those patients treated at home, and the smallest risk was for those in the SA service. The daily mortality rate (0.0004) and mean time to recover from CDI (16 days) was the same across services. Owing to the constraints of the Markov model, it was assumed that the original infection of patients with CDI or secondary-line infection was healed concurrently with the resolution of the adverse event. Severe adverse reactions were assumed to last for 1 day only and after this the patient returned to the ‘not healed’ state to start their course of i.v. antimicrobials from the beginning. The same values were assumed across services for the risk of a secondary infection and associated recovery time and mortality. Hence, the duration of treatment was considered the main driver for adverse events. The daily risk of a severe adverse reaction was also assumed to be equivalent for each service type, but the mortality risk associated with this for those being treated by nurses at home was assumed to be double that of those in the HO service and for those self-administering was assumed to be double the risk presented by the nurse services.
The ‘effectiveness’ parameter in the model was defined as the number of days of treatment required before the infection was resolved or the individual was moved on to oral antimicrobials. This value is somewhat attenuated in OPAT services because patients are often prescribed a fixed number of days of treatment depending on the underlying infection aetiology; for example, a SSTI may be automatically prescribed 7 days’ worth of IVA. However, the hospital record data appear to suggest that there are differential treatment periods by service. We applied adjusted ‘time-to-heal’ values for the base-case analysis with sensitivity analyses exploring the same heal time across services.
A differential risk of mild adverse events was added for each service based on analysis of the hospital record data. The base-case analysis assumed that the relapse rate was equivalent between services and zero, as it was assumed that the heal time used would capture the additional time for those who had relapsed. However, a scenario analysis was conducted in which heal time was assumed equivalent but a differential relapse rate was adopted. This relapse value was based on a recent UK study147 for SNs and was used for all services except for HO. We reduced the HO relapse rate by 5%, as it was thought that frequent observation of the patient in the hospital setting would allow greater confidence in stopping treatment and better review decisions. Thus, the model is structured such that the risks, costs and quality-of-life impacts are driven by the duration of treatment; services that are slower to resolve the infection expose the patient to greater risks of CDI and secondary infections, incur more visit costs and longer periods with reduced quality of life.
Resource use and costs
The costs for the service models were developed with input from the clinical experts and from the accounts of resource use from the hospital records and are included in Table 16.
| Resource usea | Mean cost (£)b | Source |
|---|---|---|
| HO | ||
| First visit | 237.68 | NHS Reference Costs 2013–14: consultant-led infectious disease outpatient visit, first visit148 |
| Subsequent visit | 145.23 | NHS Reference Costs 2013–14: non-consultant-led infectious disease outpatient follow-up visit148 |
| GN | ||
| GN visit | 33.04 | PSSRU 2014: community nurse (band 6). Band 5 equivalent estimated using mid-range salary (£24,063).c Hourly cost. Each visit = 1 hour except first, which = 1.5 hours149 |
| Paper work per visit | 7.30 | As above. Assume 10 minutes149 |
| SN | ||
| SN visit | 33.04 | PSSRU 2014: community nurse (band 6). Band 5 equivalent estimated using mid-range salary (£24,063).150 Hourly cost. Each visit = 1 hour except first which = 1.5 hours149 |
| Paper work per visit | 7.30 | As above. Assumes 10 minutes149 |
| SA | ||
| Training session cost | 66.08 | £33.04 × 2.149 Assumes delivered by band 5 community nurse over 2 hours |
| Eclipse balloon/pump device | 52.96 | Per-patient: based on expert opinion |
| Check-up nurse visit once a week (daily cost) | 4.72 | PSSRU 2014: community nurse (band 5)149 |
| Two telephone calls per week (daily cost) | 0.94 | PSSRU 2014: Community nurse (band 5).149 Assumes two telephone calls lasting 6 minutes |
| General costs | ||
| Cost for use of health-care services (per day) | 12.81 | Hospital record data |
| Antimicrobial treatment (per day) | 24.59 | Hospital record data |
| Resource use costs | ||
| GP surgery visit | 44.35 | PSSRU 2014149 |
| GP home visit | 113.45 | PSSRU 2014149 |
| District nurse visit | 39.62 | PSSRU 2014149 |
| Inpatient care cost | 208.33 | NHS Reference Costs 2013–14148 |
| Outpatient care costs | 146.45 | NHS Reference Costs 2013–14148 |
| A&E cost | 117.58 | NHS Reference Costs 2013–14148 |
| Cost of adverse events | ||
| Cost of severe line infection treatment | 236.66 | NHS Reference Costs 2013–14: assumed equivalent to kidney or urinary tract infections, with interventions excess bed-day (LA04L)148 |
| Cost of CDI treatment | 289.62 | NHS Reference Costs 2013–14: assumed equivalent to kidney or urinary tract infections, with Interventions excess bed-day (LA04L) and isolation cost148 |
| CDI isolation cost | 52.96 | Updated guidance of the diagnosis and reporting of CDI. Department of Health 2012151 |
| Cost of treating anaphylaxis | 732.34 | NHS Reference Costs 2013–14: shock or anaphylaxis, with CC score of 1 (WA16 W) total HRG148 |
| Patient visit costs | ||
| Patient travel per day (miles) | 6 | Assumption |
| Mileage costs (per mile) | 0.67 | NHS expense reimbursement rate |
| Car parking per visit | 6.30 | £4.20 per hour for 1.5 hours: assumption |
These costs broadly cover: antimicrobial costs, additional expenses required for SA (training and equipment such as specialist delivery devices), nurse (including paperwork and travel) and hospital visit costs for IVA delivery and reviews, additional health-care resources used (e.g. GP visits) by the patient and costs associated with severe adverse events (e.g. hospitalisation following secondary infection). The costs of adverse events were based on published estimates of the length of stay (or time to recover) per event, multiplied by a bed-day cost. The resource use by the patient was based on the hospital record data which reported secondary and primary care health-care usage during their last infection.
Utility values
Targeted searches of published literature were employed in addition to searches of databases152,153 to identify utility values. Utility values were similar for short- and long-term infection patients when healed, but during infection the long-term patients experienced a much larger utility drop, as these infections were believed to be more severe in nature. Those contracting CDI experience a moderate drop (–0.115) in utility, whereas those who experience a secondary-line infection are subject to a more substantive utility decrement associated with being hospitalised (–0.240). No utility loss was assumed following mild events in the main analysis but one was added for a sensitivity analysis. In addition, we collected EQ-5D (three-level) utility data from a group of IVA patients who were participating in the discrete choice aspect of the funded research programme; these base values were included as a sensitivity analysis. Finally, because the mortality risk linked to adverse events presents a risk of reduced length of life, a lifetime QALY loss value (16.6) was estimated. This represented the discounted (at 0.035% per annum) total QALYs lost for individuals who died during the model time horizon using an average starting age of 50 years, survival estimates from life tables and ‘healed’ utility values. This discount rate was used as it conforms to the NICE reference case (Table 17).
| Parameter | Mean | SD | Distribution | Source |
|---|---|---|---|---|
| Utility short term | ||||
| Not healed | 0.4360 | 0.342 | Beta | Mason et al.154 |
| Healed | 0.7395 | 0.280 | Beta | Mason et al.154 |
| Utility long term | ||||
| Not healed | 0.0100 | 0.400 | Normal | Bernard et al.155 |
| Healed | 0.7200 | 0.300 | Beta | Bernard et al.155 |
| Common parameters | ||||
| Hospital-acquired CDI | –0.1150 | Fixed | Beta | Konijeti et al.156 |
| Utility loss per hospital stay owing to line infection/anaphylaxis | –0.2400 | 0.0300 | Beta | Assumed same as for asthma patients157 |
| Death | –16.660 | Fixed | Fixed | Life tables UK based on mean age of 50 years and not healed utility value |
Analyses
The model was run separately for each service and yielded expected costs and effects. In line with the NICE reference case,135 the effects considered were (QALYs and, in the main analysis, a cost–utility analysis presenting incremental cost-effectiveness ratios (ICERs) where appropriate. 158 Services with an ICER < £20,000 in the incremental analysis were considered cost-effective. Net monetary benefit (NMB) was also calculated (QALY × £20,000 – cost) for each analysis and cost-effectiveness was assumed for a service when the incremental NMB over a comparator service was positive (> £0). An analysis of the hospital data suggested there was insufficient heterogeneity in outcomes or risks based on patient characteristics to justify subgroup analyses or a need to control for heterogeneity in analyses. With the exception of the QALY loss estimation following death, all model outcomes were realised by 12 months and, therefore, no discounting was required. All prices are in presented in 2015 UK pounds sterling. A half-cycle correction was not employed as the cycles were daily and, therefore, the timing of health state transitions during the cycle were assumed not to have an impact.
Simulation models
Simulation modelling techniques such as discrete event simulation (DES) afford the opportunity to ‘replicate’ an OPAT service in a community and estimate the number of resources (nurses and rooms/beds) required to cover a specific population. DES is an individual-level modelling technique that originated in the operational research field but is increasingly being used in a health context. The core concepts of a DES model are ‘events’, ‘entities’, ‘attributes’, ‘activities’, ‘queues’ and ‘resources’. The ‘entities’ are a representation of individuals or patients who move through different ‘activities’ in a pre-defined pathway. The entities possess certain ‘attributes’ (or history) such as whether or not they have experienced a particular adverse event which can be modified when performing certain ‘activities’ in combination with different ‘resources’. Those modifications influence the entities’ future movements through the pre-defined pathway. ‘Queues’ can be formed when entities are about to pass through the different activities and this is determined by the number of entities, number of resources and time taken to undertake the activity. In contrast to a Markov model, time is measured as a continuum but evaluated when an events occurs. In the context of an OPAT service, the DES model not only allows patient history to be considered (via attributes), but also the operational aspects of an OPAT service as nurses and/or beds can be represented by ‘resources’ and home or outpatient visits as ‘activities’.
Simulation models have been used previously in health to optimise the use of resources and inform decision-making. The Mayo Clinic’s Centre for Health Care Delivery (Rochester MN, USA) used a DES model to predict the minimum number of beds needed to meet patient demand and the clinic’s quality standard of care. 159 Elsewhere, Troy and Rosenberg160 estimated the required number of beds for an intensive care unit in Canada, and Cipriano et al. 161 evaluated different strategies to reduce waiting time in a total joint replacement unit in Canada. The aim of the current analysis was to replicate the operation of OPAT services to estimate the amount of resources required to run OPAT services in England and Wales taking into account cost-effectiveness and size and type (e.g. rural vs. urban) of population. As the DES technique is an individual-level analysis, instead of a cohort of patients, individuals are followed through a defined pathway in the model. However, the population, model structure and evaluated services are otherwise the same as that used for the Markov modelling. Any divergence in methods is described below.
Model horizon, structure, assumptions and perspective
As with the Markov model, patients enter the simulation after being referred to the OPAT service: HO, GN, SN or SA. Although the simulation model also used daily cycles, it ran for the same period for both short- and long-term infections (12 months). This was longer than the short-term Markov model as the simulation model may run for longer as it is better at capturing the real length of health events. The first step of the simulation assigns patients to one of the four services to commence treatment. The initial treatment for the patients on the GN service would require a home visit of a GN, whereas for the SN and SA services a home visit by a SN to commence treatment or training was necessary. The HO service required a private room in the clinic of the hospital for the service to be delivered.
Although GN and SN service patients ‘wait’ for the nurse visit to deliver their IVA, HO patients wait for an available bed and SA patients administer their own IVA. The duration of the wait is dependent on the availability of resources (nurses and rooms). This availability was given by the number of nurses available, the time required to administer the i.v. service (including travel time) and the working hours (clinic and nurses). As such, if the availability of nurses is limited owing to lack of personnel or high number of patients to be treated, the size of the ‘wait’ will increase. We included a sensitivity analysis in which departments subcontracted a private nurse service to cover any unmet demand (at higher cost) to reduce or prevent delays in care.
As the resource and safety elements are crucial to the functioning and viability of the services, these components were explored further by adding a delayed treatment state to the model. Patients who were not able to be seen within 24 hours for their IVA travelled to this state, as their missed treatment would result in an outpatient visit and a risk of admission to hospital owing to a worsening of their infection. Modelling this eventuality allows us to determine the minimum number of resources needed to avoid delayed cases that can lead to further costs and hospital admissions. Figure 11 shows the structure of the model.
FIGURE 11.
Simulation model structure. Screenshot from Simul8© [Simul8 Corporation (Boston, MA, USA) www.simul8.com (accessed 8 November 2016)].
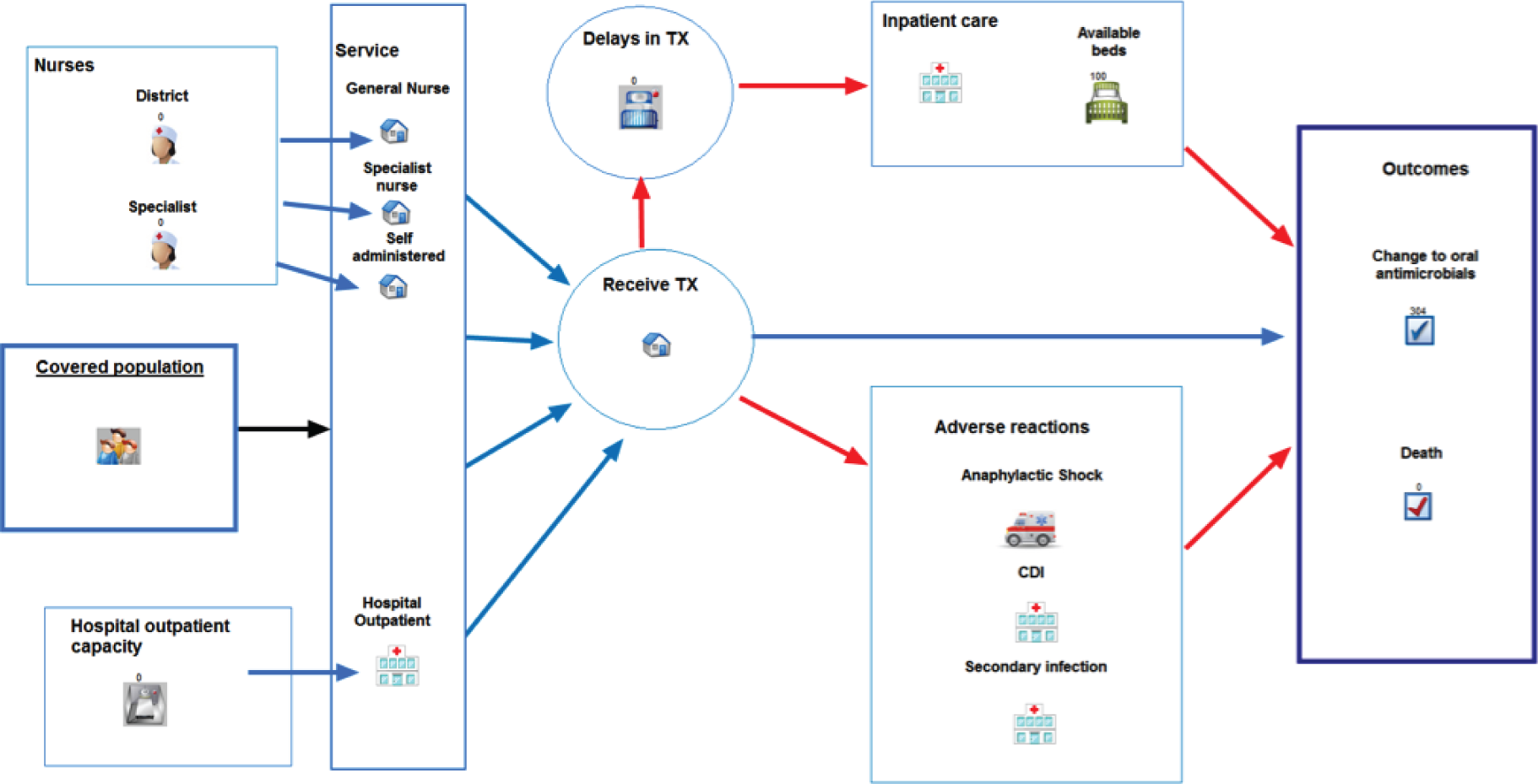
Two different perspectives were used to estimate the costs of the services. The first refers to the HPSS in line with the Markov model analysis. These are based on hourly costs and, therefore, nurse visits (general or specialist) are costed based on the time they spend with the patient. If no patient is seen, the cost of this resource is zero. There is also no limit to the resources available. The second costing perspective is that of the commissioner of services. Here, the cost of a nurse is given by their annual salary irrespective of the number of patients seen in any given time frame. Thus, hiring an additional nurse will represent an increase in the costs equivalent to their annual salary but increase the resources available to treat patients. The rest of the costs, such as hospital beds, outpatient service, etc., are costed from the HPSS.
Additional parameter values
The risk and effectiveness parameters were as represented in the Markov model. However, in contrast with the Markov model, simulation has a ‘memory’; if one of the adverse events occurs, the patients would receive treatment accordingly and when the event has been resolved they would continue with the treatment of their infection from the point they experienced the negative outcome (rather than either starting their IVA from day 1 again or being assumed ‘healed’).
As simulation can capture resources this is where the majority of additional information input to the model was required. Both GN and SN services require a nurse (general or specialist accordingly) to operate. Nurses working hours are divided by shifts (morning, afternoon or night shift) and they tend to operate 7 days a week. According to the Unit Cost of Health and Social Care 2014,149 community nurses work on average 37.5 hours a week and 42 weeks per year. Annual leave was also taken into consideration, with an average of 260 working days assumed per year on the basis of Unit Cost of Health and Social Care 2014. 149 During the first visit it was assumed that they require 1.5 hours to administer the treatment, whereas the subsequent visits were assumed to take 1 hour. Additional costs were added to allow for the completion of all the patient-related paperwork, while a sensitivity analysis explored the cost of private nurse cover. The SA service requires a SN to train and check on the patient. The initial training session takes place in the patient’s home and was assumed to last for 2 hours on average. A check-up session occurs in the patient’s home every week, with an average duration of 1 hour, and patients visit the clinic for a fortnightly review. In the HO service the patient would attend the clinic and be assigned to a private room where the i.v. delivery is administered. It was assumed that the average time for a delivery was 40 minutes and that the service operated for 4 hours per day.
Information from four OPAT services in the UK about the functioning of their service was collected and used to populate the model. The information was used to set the initial conditions of the model. The following variables were collected: number of nurses (district and/or private) and rooms available, shift patterns, clinic working hours, average number of patients seen in a year and average time per treatment. This information was used to estimate the actual capacity of the service. The utility values were the same as those used in the Markov model except for an additional decrement applied when patients experienced a delay in treatment.
Analyses
The simulation model based on the HPSS resource and cost perspective produced the same outcomes (cost per QALY and ICERs) as the Markov model and these were compared. Owing to the different characteristics of the models, they are not directly comparable, but a similar result will provide evidence of the robustness of the results in both models. More interesting was the simulation model analysis using the commissioner perspective. This permitted an estimation of the minimum threshold values such as the minimum number of nurses needed to avoid delays or achieve greatest net benefit. It permitted the exploration of the effects of reducing the number of staff or switching to private nurses and the impact of the changes in operational hours. We also explored the commissioner perspective when responsible for a large or small population (by varying the incidence of infections or patients requiring IVA) and the geographical spread of patients (e.g. rural vs. urban services) by varying the time required for nurses to complete visits. In addition, a series of different combinations in terms of the proportion of patients enrolled to each of the available OPAT services in the community was explored. This aimed to provide information on an optimal combination of service types within an OPAT unit given the amount of resources available.
Uncertainty
To test the sensitivity of the modelling results to the inherent assumptions and parameter values, a number of deterministic one-way and scenario sensitivity analyses were conducted. A probabilistic sensitivity analysis (PSA) was conducted (10,000 Monte Carlo simulation runs) to allow for random changes in all parameter values at the same time based on pre-specified value distributions. These simulated analyses were plotted on a cost-effectiveness plane and the probability that services were cost-effective given a range of QALY willingness-to-pay thresholds was represented on the cost-effectiveness acceptability curve (CEAC). 162 Distribution plots of incremental net monetary benefit (INMB) were also produced.
Value of information analysis
Given that the PSA characterises the level of uncertainty in the analysis, it is possible to cost this formally based on the probability of making a ‘wrong’ decision regarding service provision choice using the value of information framework. 142 We estimated the expected value of perfect information (EVPI) for the population based on the Monte Carlo simulated NMB, identifying in each case the net benefit loss by basing the decision on the average net benefit rather than that based on perfect knowledge (each simulation). This represents the individual value of perfect information and is multiplied by the population of interest over the relevant decision period to gain the population EVPI. The higher the population EVPI, the greater the cost of uncertainty and the greater the value of additional research. Should the EVPI exceed the expected research cost for reducing uncertainty then investment in research is recommended. 163
We assumed that the population that could benefit from the services to be 21,000 patients with short-term infections. This figure is based on the assumption that 70,000 patients per year are hospitalised with SSTIs164 and approximately one-third of these could be eligible for IVA. 137 Although not all of these would be suitable for OPAT (e.g. owing to impaired functioning), this figure is likely to be an underestimate, as it does not include other types of infection such as respiratory and urinary tract infections. To explore this issue we have run the analysis assuming 100% could receive OPAT (n = 21,000) and a scenario in which only 50% are eligible for OPAT (n = 10,500). The long-term infection population group was estimated based on the assumption of 1% prevalence of infections at elective orthopaedic surgical sites. 130 This translates to 2000 joint-related infections in addition to 5000 osteomyelitis-related infections. The total of 7000 was scaled up to include other long-term infections such as endocarditis using the proportions from a UK cohort study. 29
The expected value of partial perfect information (EVPPI) was calculated using a recently developed non-parametric regression method tool. 165 Using the simulated parameter values and associated NMB estimates from the Monte Carlo simulation, the EVPPI represents the monetary value of uncertainty emanating from each of the parameters used. This is particularly useful in identifying which parameters are driving the EVPI and which might be the target of efforts to reduce uncertainty in the decision and future research investments, for example whether it relates to effectiveness, risks or quality-of-life estimates. The expected value of sample information (EVSI) represents the expected value of increased research sample size. EVSI helps determine the optimal sample size for a study based on the marginal benefit from an additional study participant compared with the marginal cost of enrolling them. The optimal point occurs when the marginal benefit is equal to the marginal cost. 12 This analysis was conducted if there was sufficient value of additional research and information was required on the research design.
Results
Markov model: short-term infections
Base case
Table 18 includes the model heal time, adverse event and cost predictions in the short term. The low event rates and costs reflect the low risk rates included in the model and the short period of risk (< 1 week on average during treatment).
| Parameters | Service | ||
|---|---|---|---|
| HO | GN | SN | |
| Time to heal (mean days) | 4.72 | 6.84 | 6.00 |
| CDI person-days (per 1000 people) | 7.80 | 0.94 | 0.83 |
| Secondary infection person-days (per 1000 people) | 35.87 | 51.98 | 45.62 |
| Severe adverse reactions (per 1000 people) | 0.22 | 0.30 | 0.26 |
| Number of relapses (per 1000 initial infections) | 0.00 | 0.00 | 0.00 |
| Days in hospital (per 1000 people) | 43.88 | 53.22 | 46.71 |
| Deaths (per 1000) | 0.35 | 0.52 | 0.46 |
| Costs, £ (per 1000 people) | |||
| CDI | 2258.10 | 272.67 | 239.32 |
| Secondary infections | 8488.15 | 12,300.48 | 10,795.85 |
| Severe adverse reactions | 162.29 | 220.52 | 193.53 |
Table 19 includes the base-case cost-effectiveness results. For an average short-term infection, HO is the most expensive service, at £146 and £219 more expensive than GN and SN services, respectively. However, HO provides (negligible) QALY gains over both of the nurse-led services. Both GN and SN are cost-effective compared with HO. SN is the most cost-effective service overall, yielding a saving of £76,506 for every QALY lost (vs. HO) and a £162 NMB gain over HO.
| Mean | Service | Comparison | ICER | INMB | Interpretation | ||
|---|---|---|---|---|---|---|---|
| HO | GN | SN | |||||
| Costs (£) | 998 | 852 | 779 | HO vs. GN | £31,600 | –£54 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | £76,506 | –£162 | SN cost-effective |
| GN vs. SN | SN dominates | –£108 | SN cost-effective | ||||
Sensitivity analyses: deterministic
For the deterministic sensitivity analyses for short-term infections where risks, effectiveness, cost and utility model parameter inputs are varied and impact on cost-effectiveness observed, see Appendix 4, Table 43. It also includes a number of scenario analyses. In most cases, GN is more cost-effective than HO, and SN is more cost-effective than both HO and GN and, hence, represents the best value for money in the short term. Again, the results are largely driven by costs as the risks of adverse events are low and QALY gains are similar. SN is more cost-effective than HO even if a band 6 nurse is used (instead of a band 5 nurse) or if an additional outpatient review is included at the end of treatment in the nurse services. Changes in utility values do not appear to influence results. HO attendance becomes cost-effective only when scenarios weighted in the service’s favour are employed. Table 20 incorporates threshold analyses where the threshold values are sought, at which point HO become cost-effective (against SN, as this was the most cost-effective service). Holding all else equal, HO would require 2.48 fewer heal days than SN or the cost of nurse visits would have to increase from £33.04 to £58.31 before HO becomes the optimal strategy.
| Values required for HO to be cost-effective vs. SNa | Threshold value |
|---|---|
| HO days to heal | 3.85 (or 2.48 days fewer) |
| Cost of nurse visit (£) | 58.31 (or 25.27 more) |
| Cost of outpatient follow-up visit (£) | 100.93 |
| Relapse rate in SN (%) | 45 |
Sensitivity analyses: probabilistic
Table 21 includes the probabilistic mean estimates from the Monte Carlo simulation. These, and the cost-effectiveness plane which plots the simulated ICERs, corroborate the deterministic results (Figures 12 and 13). These indicate that, even with uncertainty introduced into the results, GN is more cost-effective than HO, and SN is more cost-effective than both. The CEAC (Figure 14) indicates that SN would be the cost-effective option for all values of willingness-to-pay thresholds and at £20,000 has a 78% chance of being cost-effective. The INMB distribution plot shows that, compared with the optimal strategy (SN), there is some overlap in the simulated INMB results with GN, but very few of the HO simulations exceed 0.
| Mean | Service | Comparison | ICER | INMB | Interpretation | ||
|---|---|---|---|---|---|---|---|
| HO | GN | SN | |||||
| Costs (£) | 998 | 851 | 778 | HO vs. GN | £32,634 | –£57 | GN cost-effective |
| QALYs | 0.174 | 0.169 | 0.171 | HO vs. SN | £80,351 | –£166 | SN cost-effective |
| GN vs. SN | SN dominates | –£109 | SN cost-effective | ||||
FIGURE 12.
Cost-effectiveness plane: short-term infections.
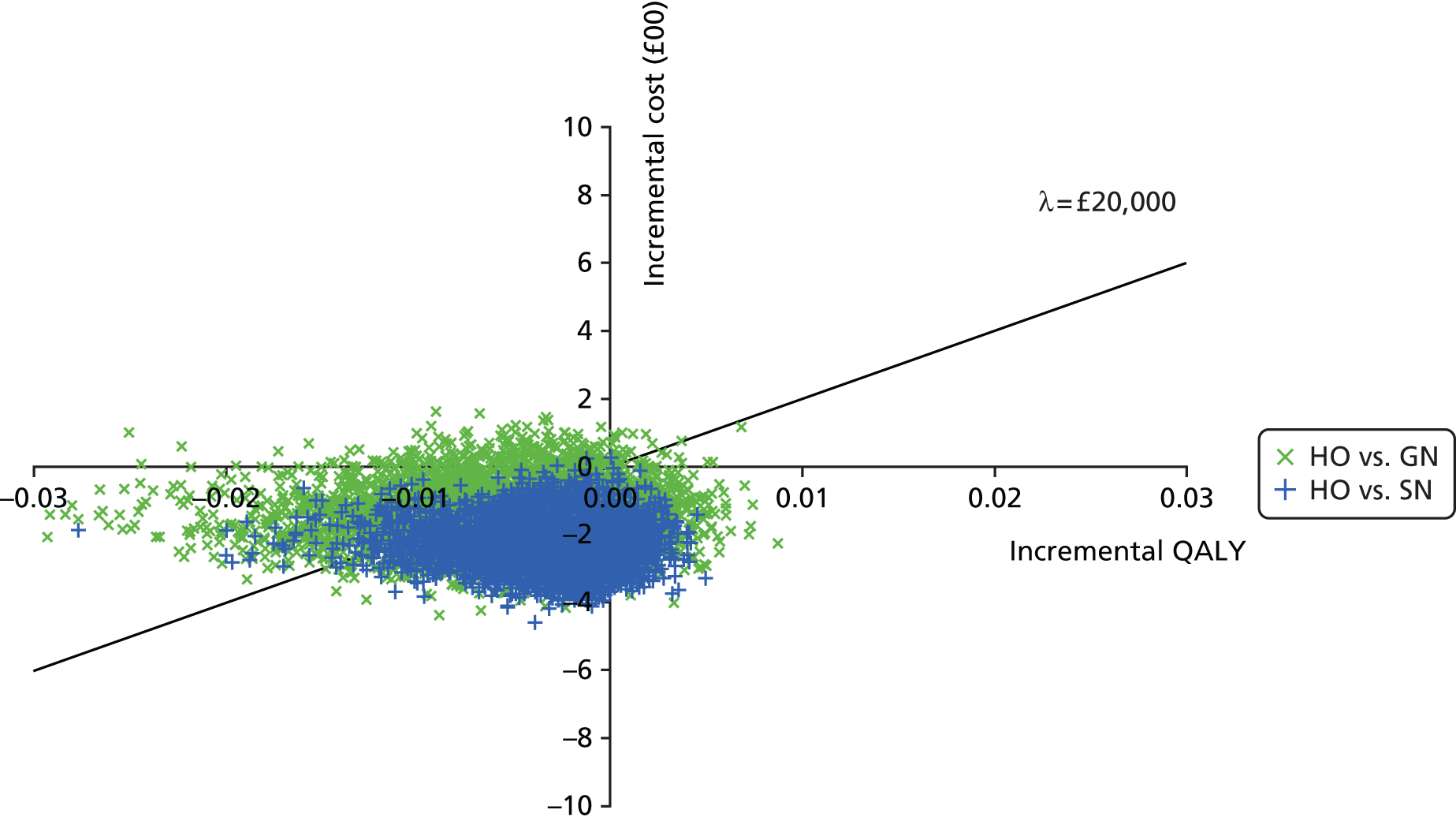
FIGURE 13.
Incremental net monetary benefit distributions vs. SN: short-term infections.
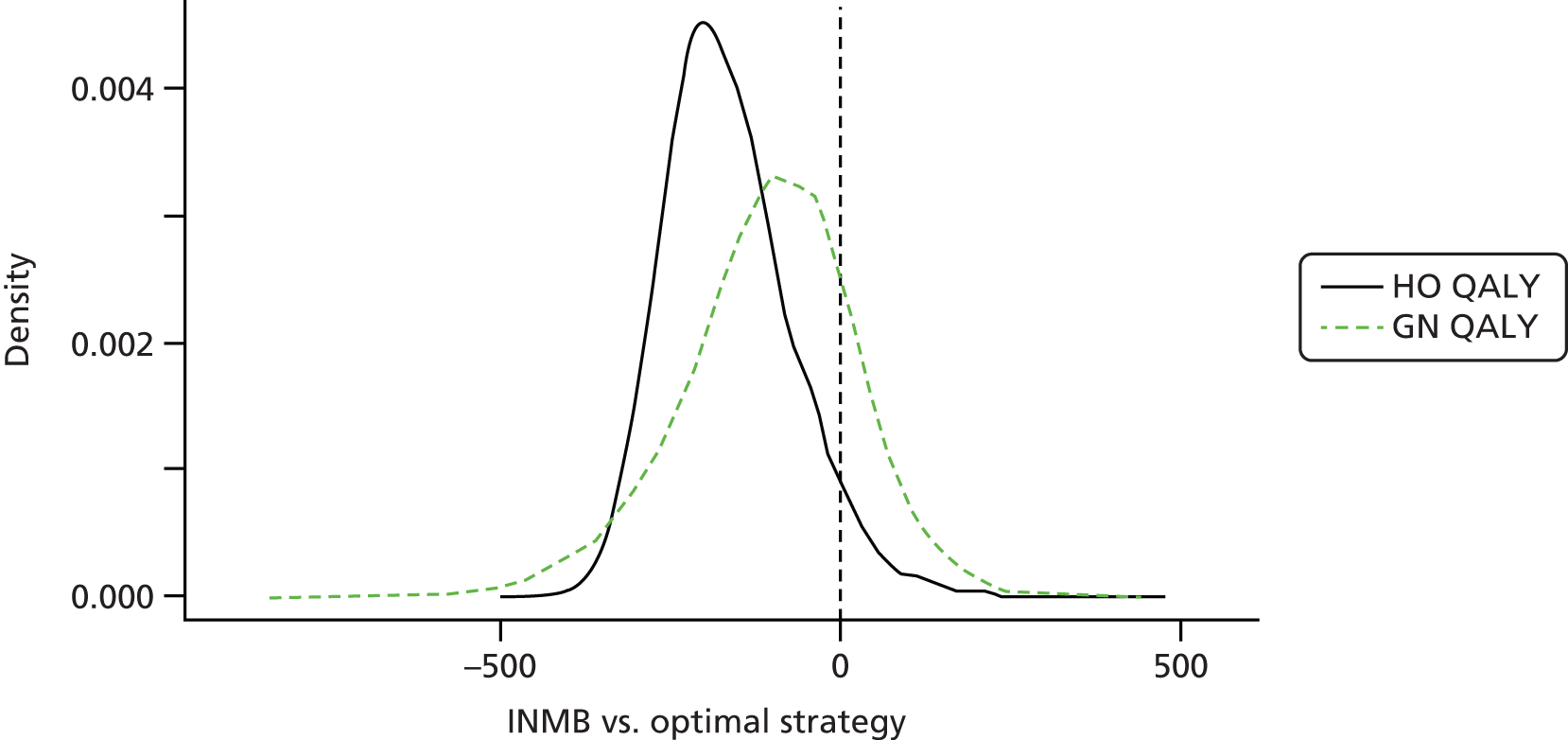
FIGURE 14.
CEAC: short-term infections.

Value of information
The population EVPI is shown in Figure 15. The EVPI is low (£302,353, where λ = £20,000) even with the assumption that 100% of patients requiring IVA would be eligible for OPAT. This reflects the low decision uncertainty in short-term infections given that, in the base-case model, SN clearly offers the best value for money. The EVPPI for individual parameters shown in Figure 16 suggest that the greatest proportion of EVPI is driven by the respective effectiveness parameters (days to heal) in the GN and SN services. Other risks and utility values did register a positive value but their EVPI was small. Over the course of 1 year (and even up to 5 years) it is unlikely that the cost of decision uncertainty characterised here would warrant significant further research investment in the form of a clinical trial. Given the low EVPI values, EVSI was not calculated.
FIGURE 15.
Expected value of perfect information: short-term infections.
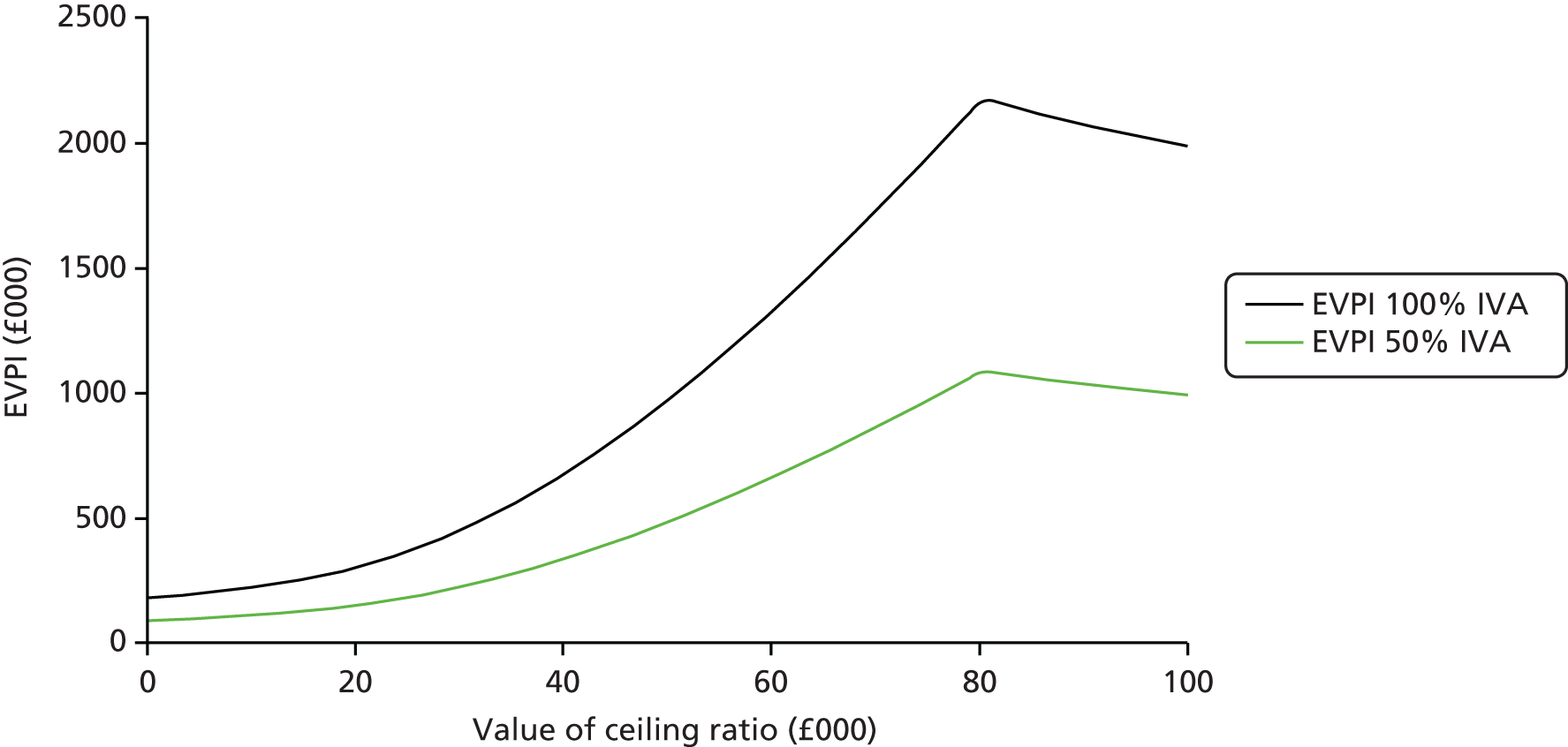
FIGURE 16.
Partial EVPI for single parameters: short-term infection. A. shock, anaphylactic shock; P, probability.
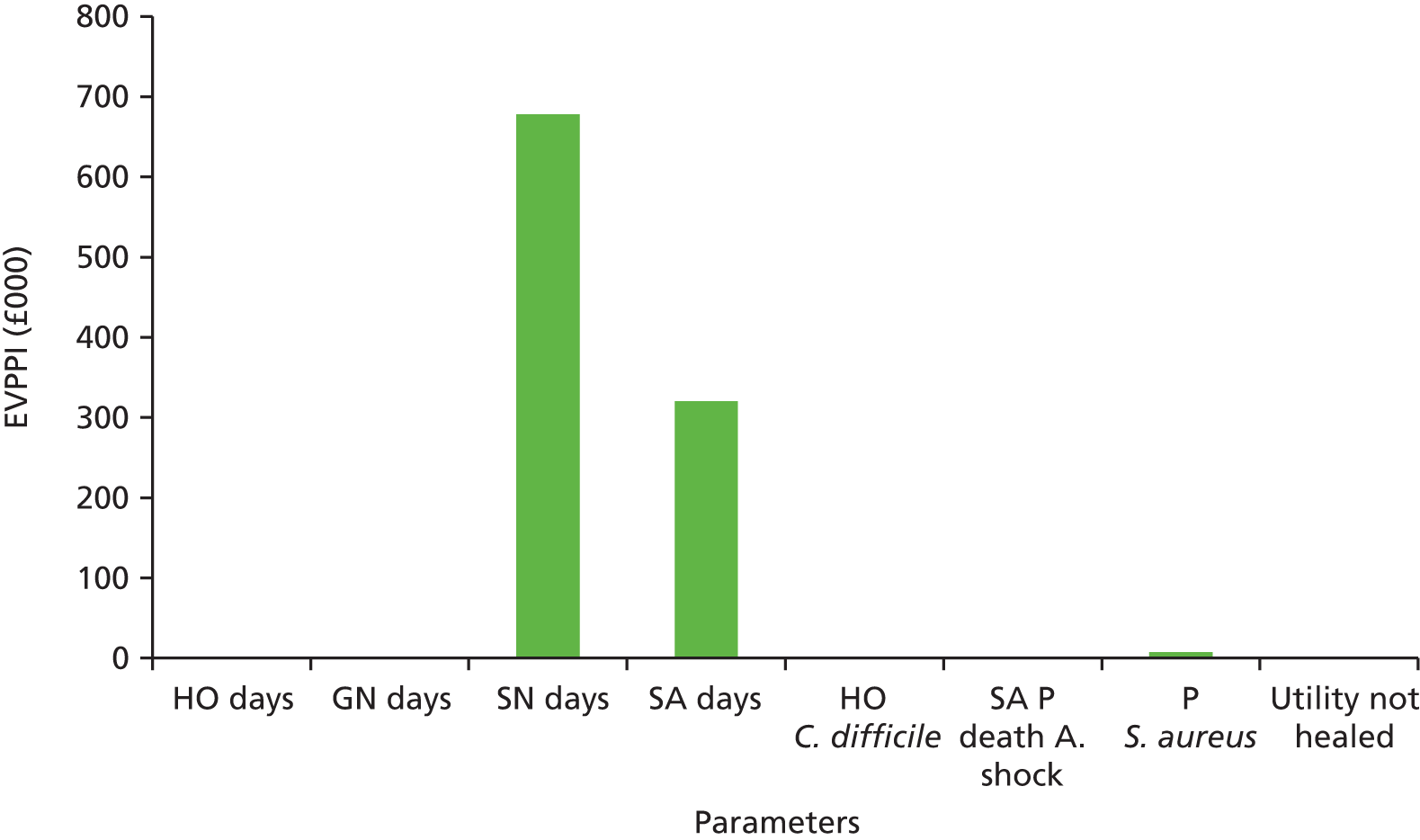
Markov model: long-term infections
Base case
Table 22 includes the model heal time, adverse event and cost predictions for long-term infections. As with the short-term infection model, event rates and per person adverse event costs are low. Table 23 includes the base-case cost-effectiveness results. SA appears to be the cheapest service followed by SN, GN and finally HO. The QALY differential between services was minimal, with SN being the most effective option, followed by HO, SA and GN. Despite providing 0.012 fewer QALYs than HO, GN is still preferred owing to the significant cost savings (£1553). Owing to the relatively large cost-savings and only slightly inferior QALY gain, SA was the most cost-effective service, providing an INMB of £255 per infection over SN.
| Variables | Service | |||
|---|---|---|---|---|
| HO | GN | SN | SA | |
| Time to heal (mean days) | 23.65 | 27.06 | 22.13 | 24.50 |
| CDI person-days (per 1000 people) | 39.19 | 3.74 | 3.06 | 0.00 |
| Secondary infection person-days (per 1000 people) | 180.05 | 206.38 | 168.72 | 186.82 |
| Severe adverse reactions (per 1000 people) | 1.11 | 1.19 | 0.98 | 0.96 |
| Number of relapses (per 100 initial infections) | 0.00 | 0.00 | 0.00 | 0.00 |
| Days in hospital (per 1000 people) | 220.34 | 211.32 | 172.75 | 187.78 |
| Deaths (per 1000) | 1.75 | 2.06 | 1.68 | 1.98 |
| Costs, £ (per 1000 people) | ||||
| CDI | 11,349.43 | 1084.18 | 886.33 | 0.00 |
| Secondary infections | 42,610.03 | 48,842.67 | 39,929.53 | 44,213.75 |
| Severe adverse reactions | 813.00 | 873.68 | 714.24 | 703.00 |
| Mean | Service | Comparison | ICER | INMB | Interpretation | |||
|---|---|---|---|---|---|---|---|---|
| HO | GN | SN | SA | |||||
| Costs (£) | 4631 | 3078 | 2563 | 2114 | HO vs. GN | £128,354 | –£1310 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.638 | HO vs. SN | SN dominates | –£2149 | SN cost-effective |
| HO vs. SA | £448,254 | –£2404 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –£839 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –£1094 | SA cost-effective | |||||
| SN vs. SA | £46,393 | –£255 | SA cost-effective | |||||
Sensitivity analyses: deterministic
For the deterministic sensitivity analyses and scenario analyses for long-term infections. In most cases, SA continues to be the most cost-effective service regardless of parameter value changes and the order of value for money changes rarely with SA > SN > GN > HO, see Appendix 4, Table 44. In many cases SN and SA dominate (are cheaper and more effective than) GN and, in some instances, SN dominates HO, although the incremental QALY remains very small. SA remains cost-effective even when the additional equipment costs required for the service are tripled (from £50 to £150). However, SN does become cost-effective compared with SA when the risks of a severe line infection (S. aureus) are doubled for the latter (making the risk four times that in SN and GN). This is also the case in scenarios in which the heal time for SA is assumed to be 30 days (4.54 days longer than SN) and the SA training costs are doubled (from £66.08 to £132.16). However, in these cases, SA is still preferred to GN and HO. Table 24 incorporates threshold analyses where the threshold values are sought, at which point SN become cost-effective against SA. These services were compared, as they offered the greatest NMB. All else remaining equal, SN would require 4.31 fewer heal days than SA or the cost of nurse visits would have to fall from £33.04 to £17.10 before SN would become the most cost-effective service. The daily probability of anaphylactic shock would have to increase from 0.00005 to 0.00031 (640% increase) before SN becomes better value for money.
| Values required for SN to be cost-effective vs. SAa | Threshold value |
|---|---|
| Days to heal in SN | 23.83 (or 4.31 fewer) |
| Cost of nurse visit (£) | 17.10 |
| Cost of SA training (£) | 305.02 |
| Risk of anaphylactic shock | 0.00031 |
Sensitivity analyses: probabilistic
Table 25 includes the probabilistic mean estimates from the Monte Carlo simulation. This mirrors the deterministic values and decision, with SA being the most cost-effective service. Figures 17 and 18 are the cost-effectiveness planes for all services versus HO and for SA versus SN, respectively. Figure 19 is the CEAC for long-term infections and indicates slightly more decisional uncertainty than the short-term infection model. Where the ceiling ratio is £20,000, SA has a 70% chance of being cost-effective versus a 30% chance for SN. At £30,000 this becomes 62% and 37%, respectively, and when the ceiling ratio increases to £61,000, SN becomes more likely to be cost-effective. GN and HO do not become the optimal strategy at any point across the ceiling ratio range used (up to £100,000). This is further illustrated by the distribution plot of INMB in Figure 20 where only the tail of the distributions for GN and HM cross the INMB = 0 line (vs. SA).
| Mean | Service | Comparison | ICER | INMB | Interpretation | |||
|---|---|---|---|---|---|---|---|---|
| HO | GN | SN | SA | |||||
| Costs (£) | 4635 | 3081 | 2564 | 2112 | HO vs. GN | £130,359 | –£1315 | GN cost-effective |
| QALYs | 0.646 | 0.634 | 0.650 | 0.641 | HO vs. SN | SN dominates | –£2152 | SN cost-effective |
| HO vs. SA | £482,422 | –£2419 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –£836 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –£1103 | SA cost-effective | |||||
| SN vs. SA | £48,845 | –£267 | SA cost-effective | |||||
FIGURE 17.
Cost-effectiveness plane vs. HO: long-term infections.
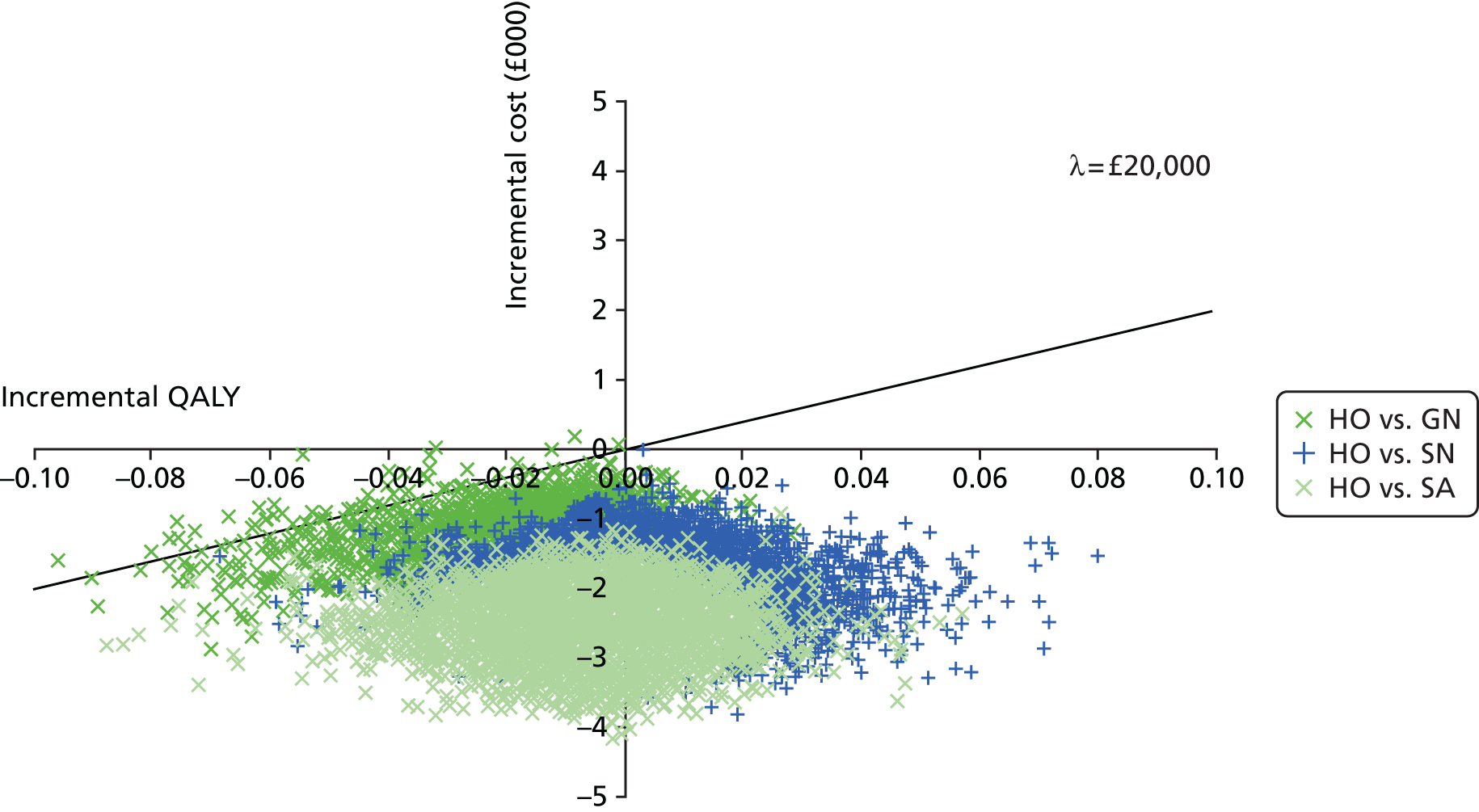
FIGURE 18.
Cost-effectiveness plane vs. SN: long-term infections.
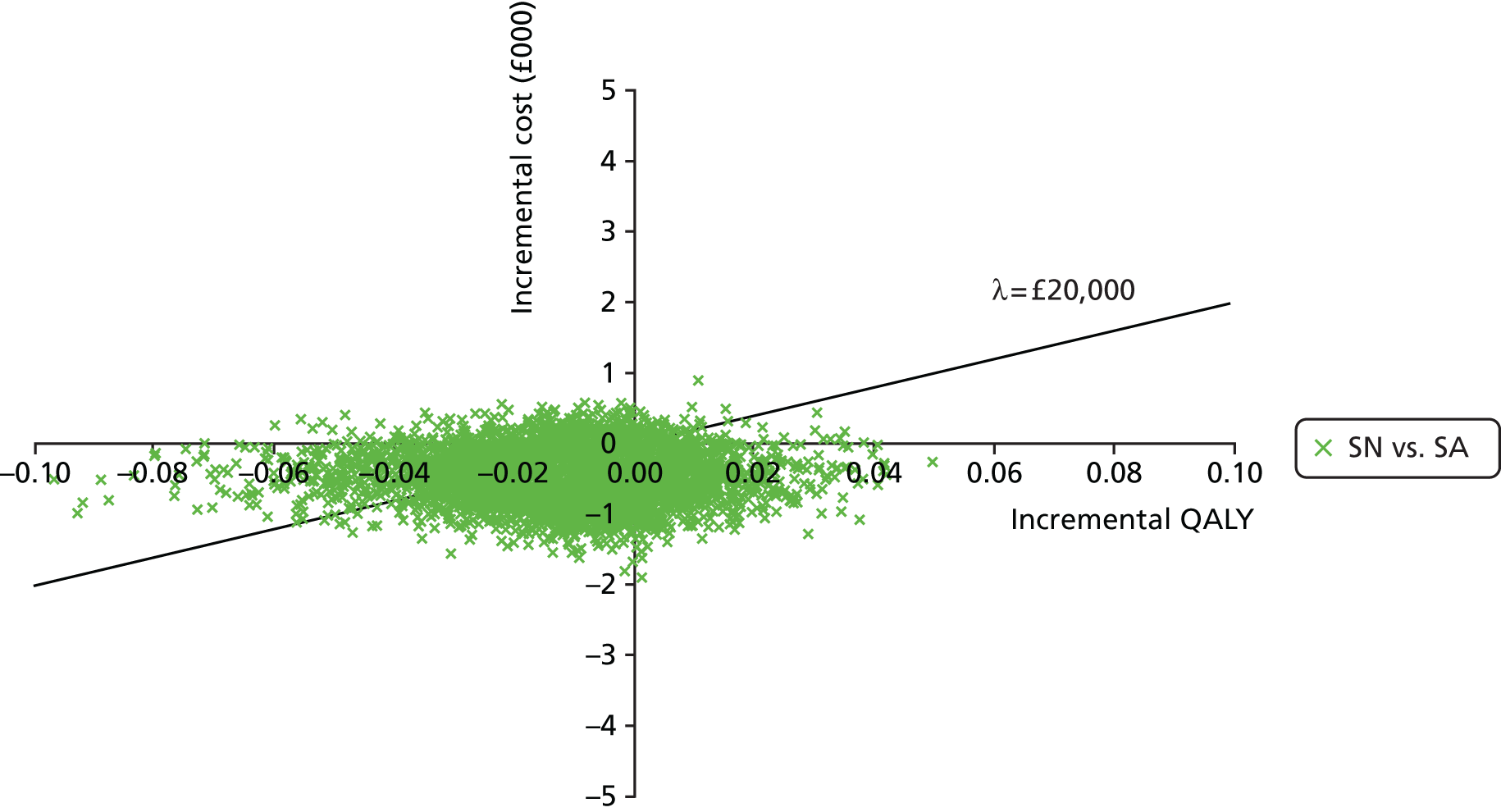
FIGURE 19.
Cost-effectiveness acceptability curve: long-term infections.
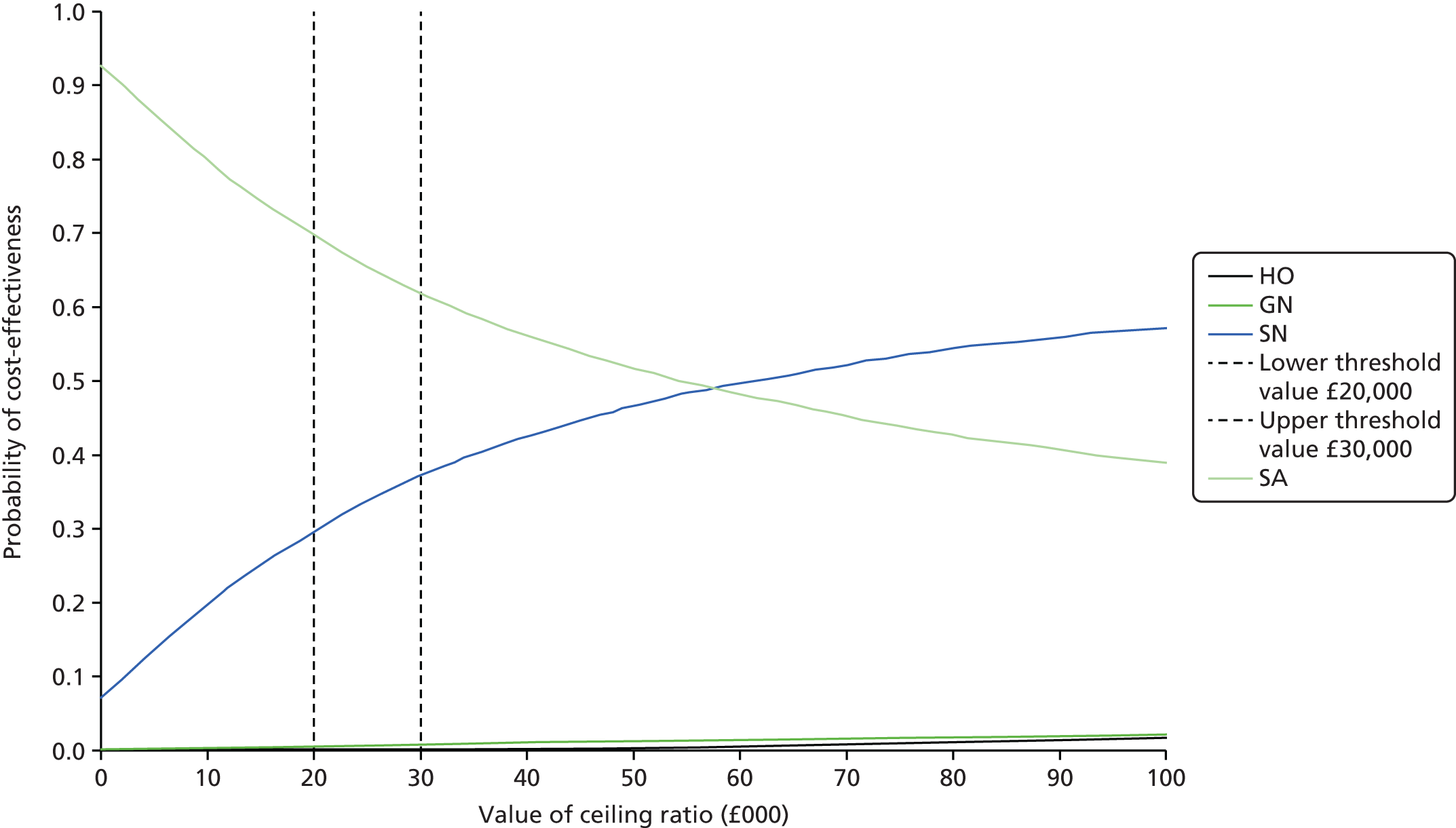
FIGURE 20.
Incremental net monetary benefit distributions vs. SA: long-term infections.
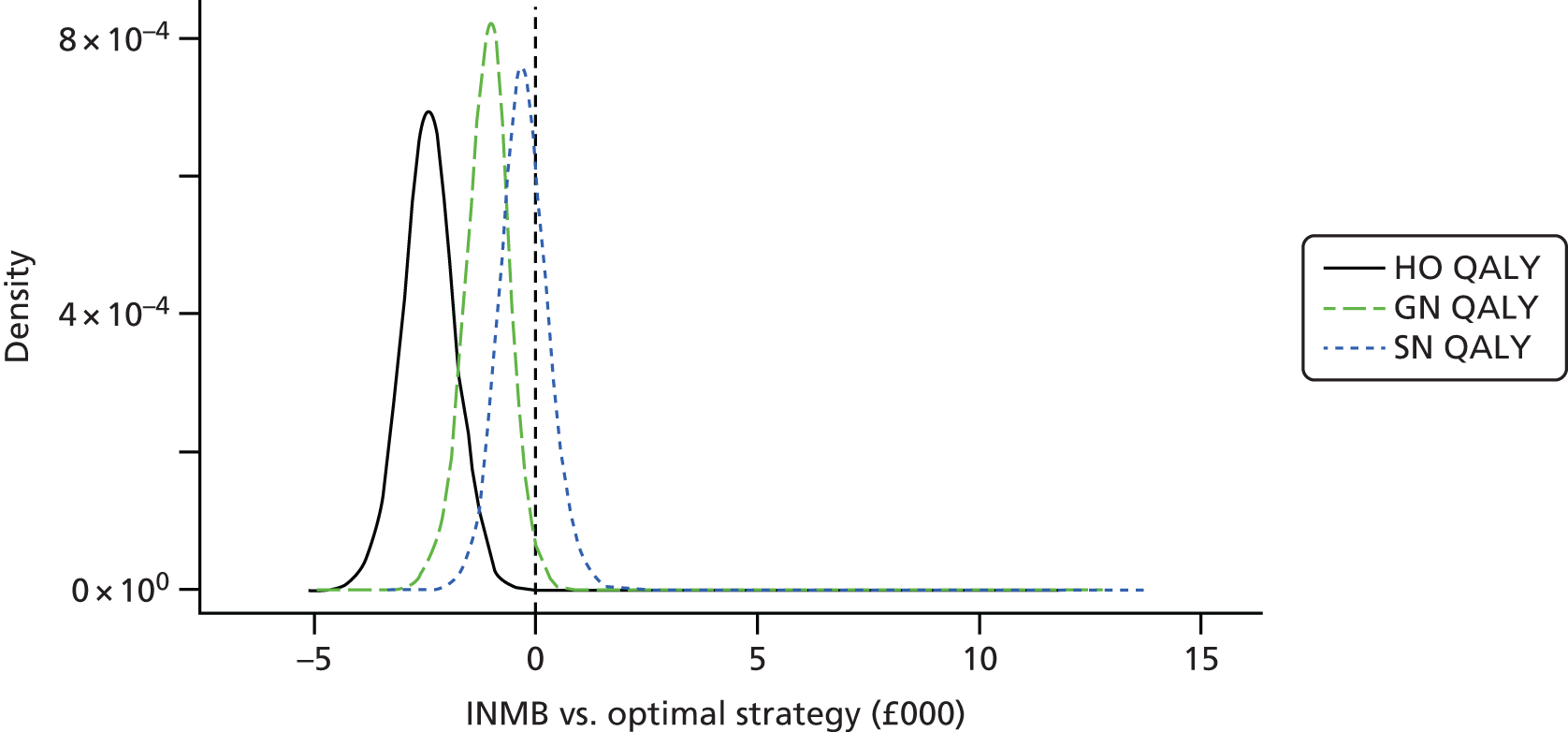
Value of information
The population EVPI is illustrated across the range of ceiling ratios in Figure 21. Over 1 year and where λ = £20,000, the EVPI is £1,078,204. Figure 22 indicates that a large majority of this value is associated with the time to heal values from the SN and SA parameters. There is little value in reducing the uncertainty around the GN effectiveness parameter. The only other factor to register a positive EVPI figure was the probability of S. aureus infection. The value here is higher than that in the short term but still relatively low and, given this, EVSI was not calculated.
FIGURE 21.
Expected value of perfect information: long-term infections.
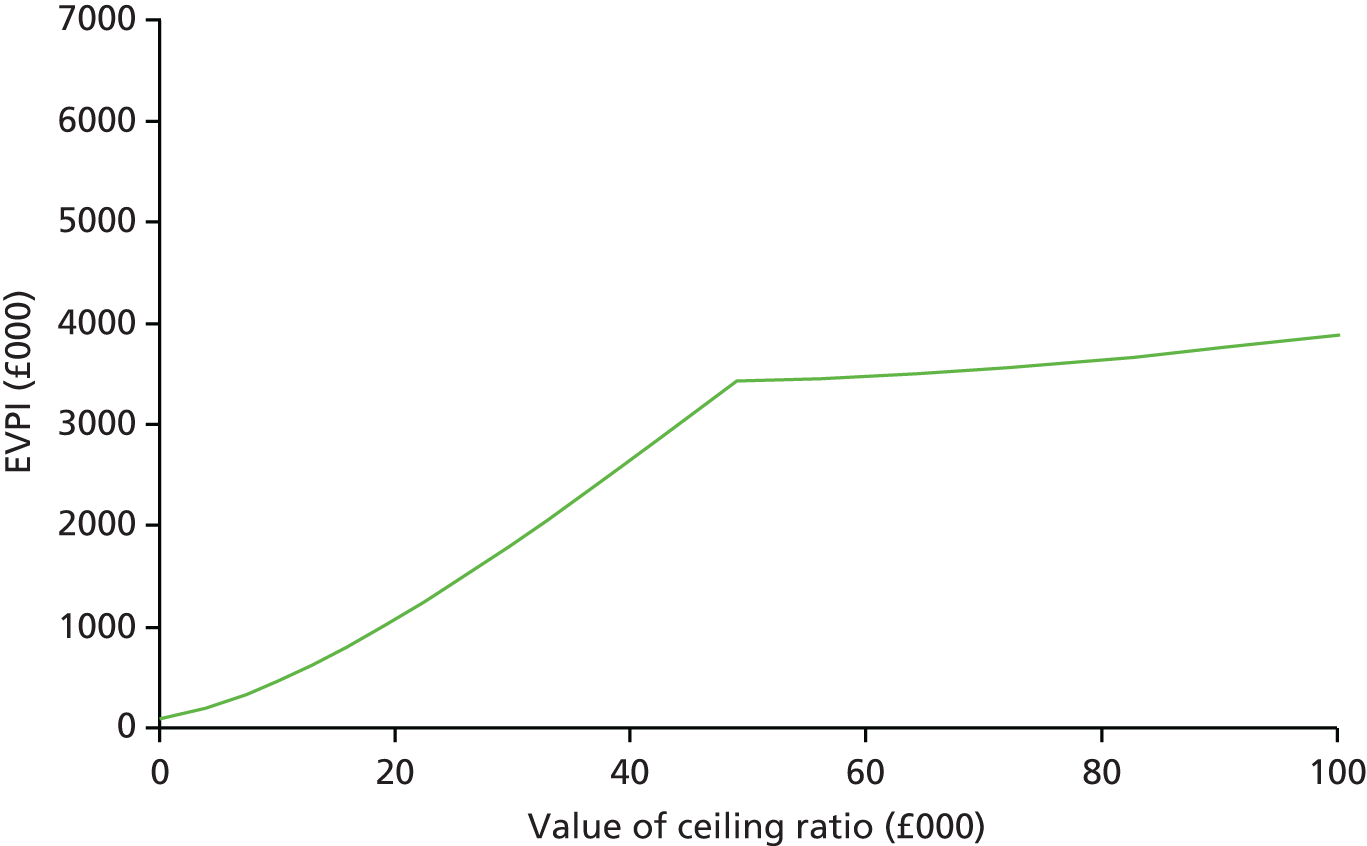
FIGURE 22.
Partial EVPI for single parameters: long-term infections.
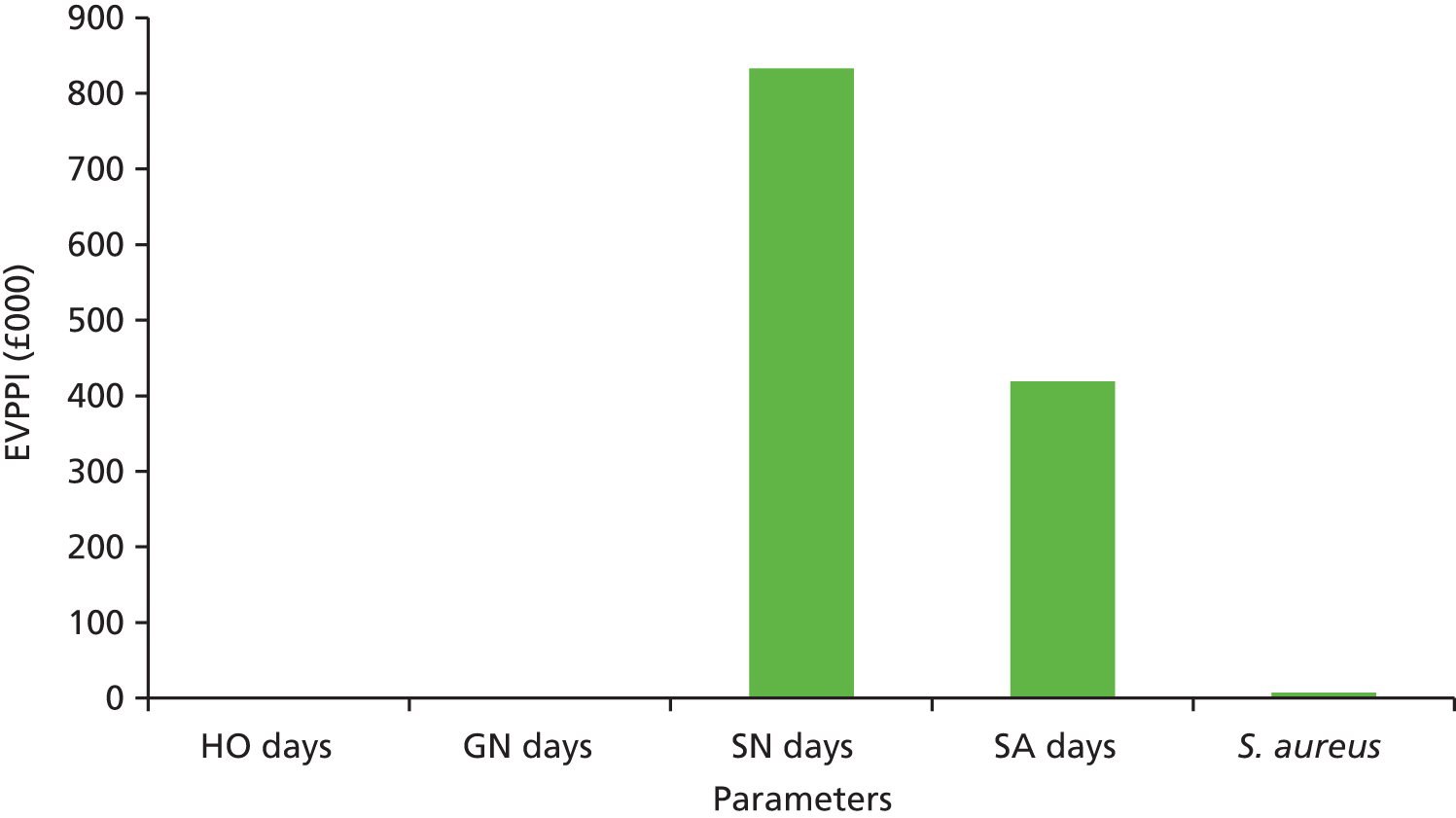
Simulation model
Health and Personal Social Services perspective: comparison with Markov
Tables 26 and 27 include the expected costs and benefits of the OPAT services generated by the simulation model for short- and long-term infections, respectively. These allow a comparison with the Markov model results which are largely in agreement. The decision in the short- and long-term infection groups is unchanged, with SN and SA, respectively, being clearly optimal.
| Interventiona | Costs (£) | QALYs | ICER (vs. SN) | INMB (vs. SN) | Result |
|---|---|---|---|---|---|
| SN | 709.74 | 0.7228 | – | Cost-effective | |
| GN | 787.75 | 0.7193 | Dominated | –£148.25 | Not cost-effective |
| HO | 972.64 | 0.7239 | £233,034 | –£240.30 | Not cost-effective |
| Interventiona | Costs (£) | QALYs | ICER (vs. SA) | INMB (vs. SA) | Result |
|---|---|---|---|---|---|
| SA | 1883.47 | 0.6660 | – | Cost-effective | |
| SN | 2378.92 | 0.6767 | £46,060 | –£280.32 | Not cost-effective |
| GN | 2956.77 | 0.6552 | Dominated | –£1288.99 | Not cost-effective |
| HO | 5135.02 | 0.6670 | Dominated | –£3174.34 | Not cost-effective |
The results of the simulation corroborate what the Markov model estimated, providing certainty to the model results. The CEAC (Figure 23) shows that the probability of SN and SA being cost-effective is > 65%, which is similar to that estimated by the Markov model (> 70%).
FIGURE 23.
Cost-effectiveness acceptability curve: (a) short- and (b) long-term infection simulation model.
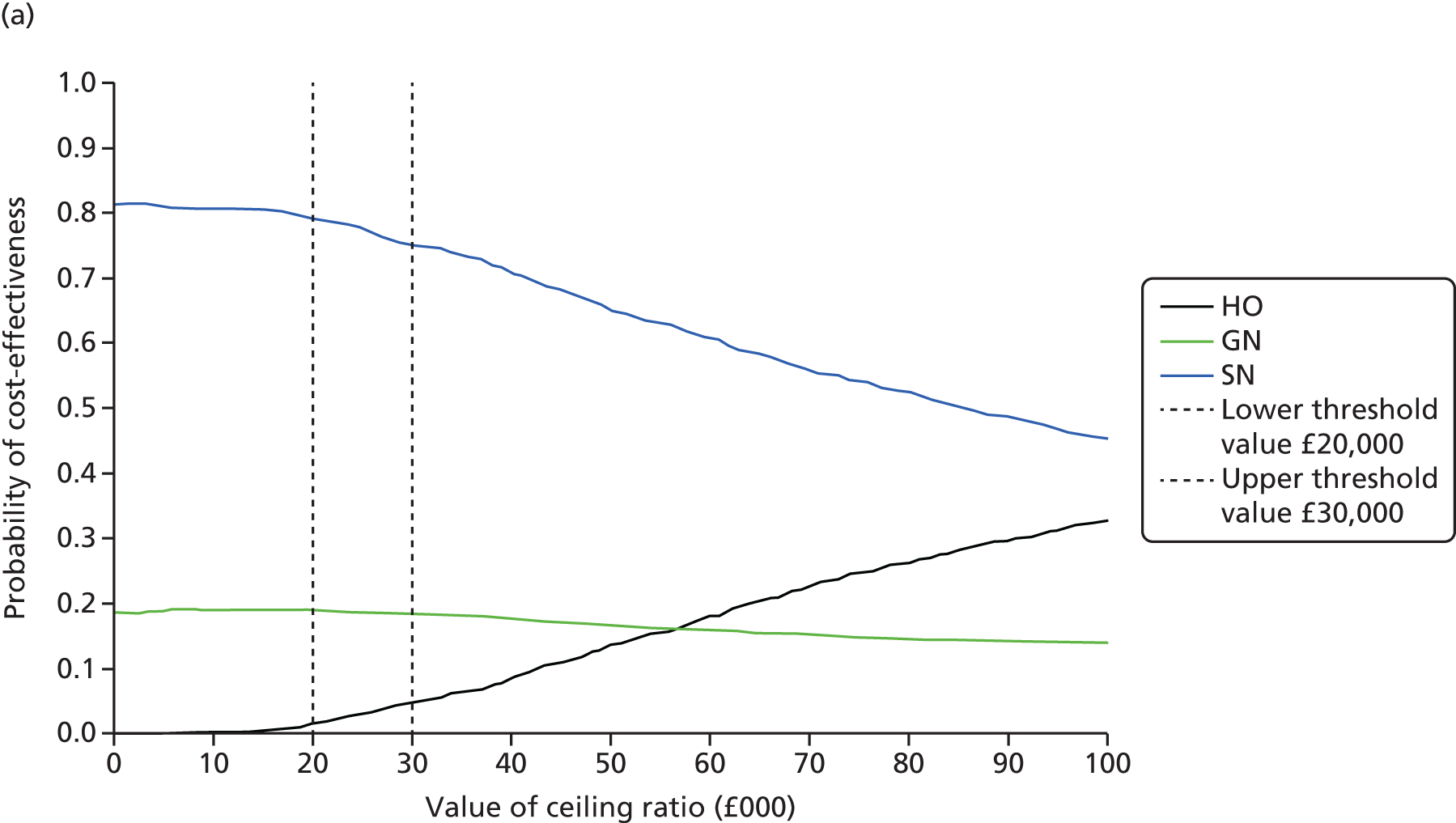
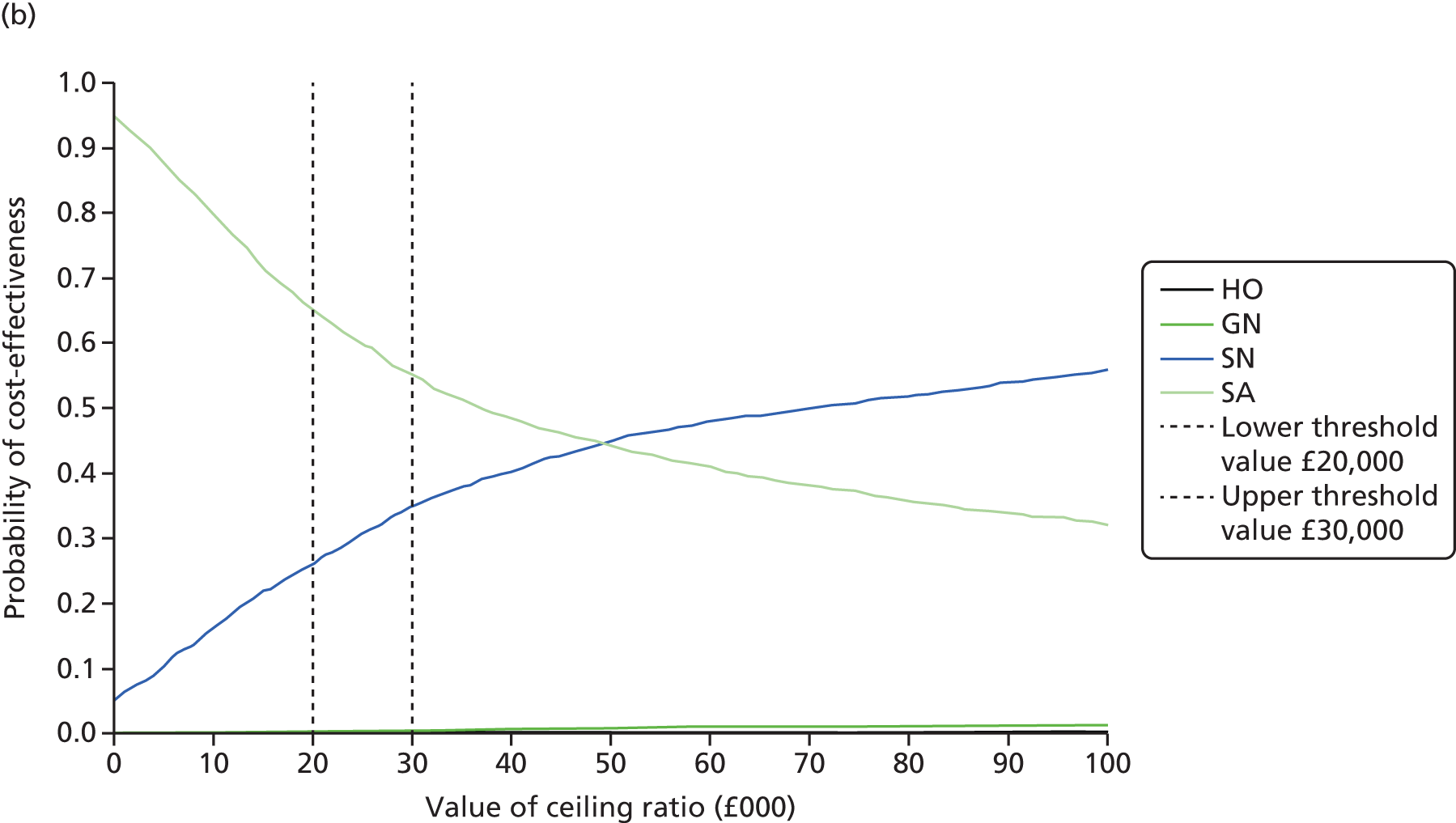
Service combinations (Table 28) introduce combined services into the cost-effectiveness analyses. None of the combined services was cost-effective.
| Interventiona | Costs (£) | QALYs | ICER (vs. SN) | INMB (vs. SN) | Result |
|---|---|---|---|---|---|
| SN | 709.74 | 0.7228 | Cost-effective | ||
| SN 50%, HO 50% | 841.26 | 0.7235 | £182,493 | –£117.10 | Not cost-effective |
| HO | 972.64 | 0.7239 | £233,034 | –£23.24 | Not cost-effective |
| Interventiona | Costs (£) | QALYs | ICER (vs. SA) | INMB (vs. SA) | Interpretation |
|---|---|---|---|---|---|
| SA | 1883.47 | 0.6660 | Cost-effective | ||
| SA 50%, SN 50% | 2127.99 | 0.6721 | £39,819 | –£127.70 | Not cost-effective |
| SN | 2378.92 | 0.6767 | £54,364 | –£158.61 | Not cost-effective |
Commissioning perspective
There was a clear, positive relationship between the number of delays in treatment experienced, costs and time to heal (Figure 24). This is corroborated by Figure 25, which plots the relationship between costs, delays in patient IVA administrations and NMB when different levels of resources (nurse numbers) are available in the long-term SN service. This is presented in two scenarios: (1) an urban setting in which we might expect nurse visit times to be shorter owing to reduced travel times; and (2) a rural setting in which patients may be more dispersed and nurse travel and visit times are increased. For a given patient population (in this case, n = 304), there appears to be a non-trivial number of treatment delays. In the urban setting adding further nurses reduces the number of delays significantly without adding significantly to the costs. However, NMB is relatively unchanged. In the rural setting the delays are much higher and the benefit (reduced costs and increased NMB) of additional nurses is marked.
FIGURE 24.
Impact of delays on time to heal and costs.

FIGURE 25.
Relationship between delays, resources and NMB. (a) Urban setting and (b) rural setting.
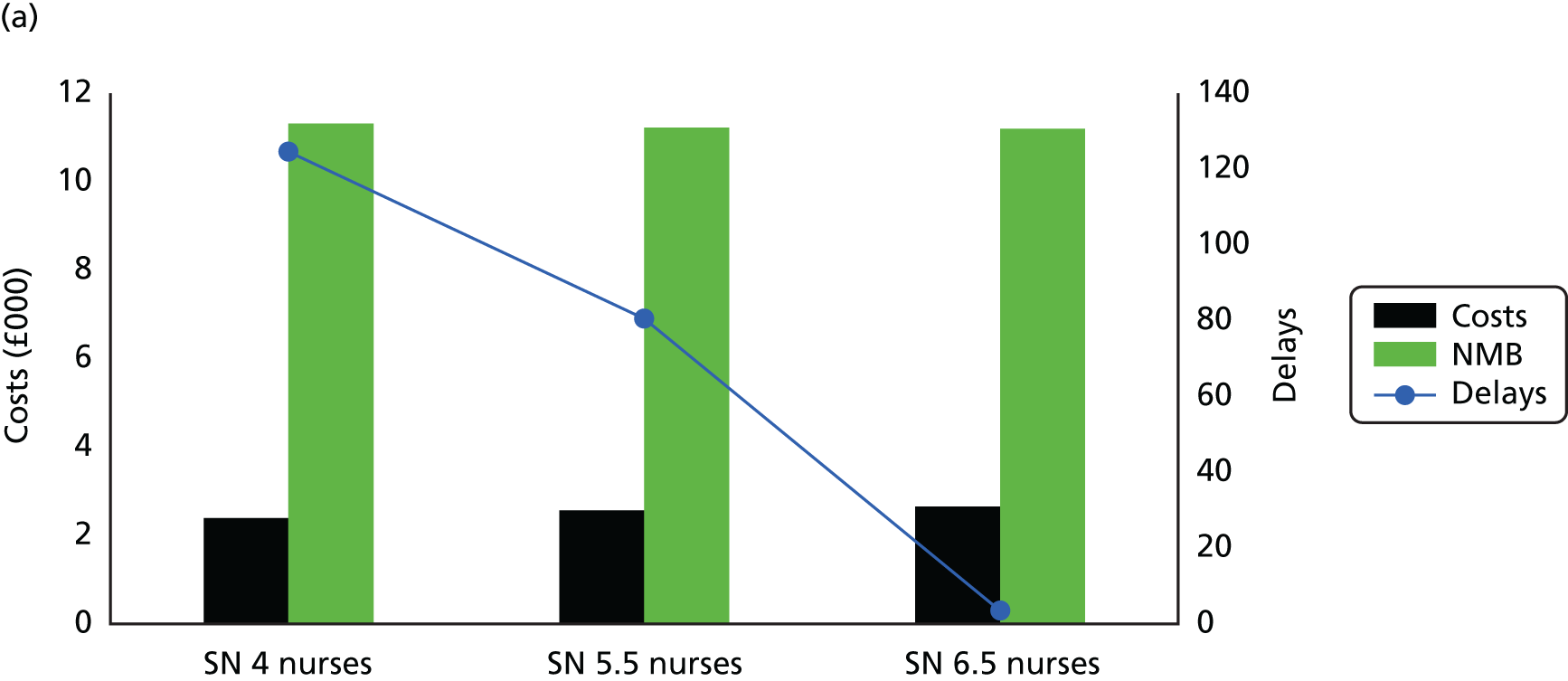

Tables 29 and 30 include the cost-effectiveness results from the commissioning perspective. Here, the costs of resource use are not calculated on an hourly basis but as sunken costs relating to the employment of nurses on a salaried annual basis. In short-term infections, fewer nurses lead to more treatment delays, but this does not translate into greater costs and reduced QALYs. This is the case when the patient population is 304 and 150. In long-term infections the nursing resources do have an impact on costs and QALYs when nurses drop from 4 to 2. Furthermore, when the visits require 2 hours rather than 1 hour, there is a noticeable drop in patient QALYs as a result of treatment delays.
| Intervention | Costs (£) | QALYs | Delays |
|---|---|---|---|
| 304 patients | |||
| SN, four nurses, 2 hours | 1129.69 | 0.7204 | 5.95 |
| SN, two nurses, 1 hour | 1127.10 | 0.7204 | 2.30 |
| 150 patients | |||
| SN, two nurses, 2 hours | 1150.23 | 0.7208 | 12.84 |
| SN, two nurses, 1 hour | 1136.42 | 0.7209 | 4.85 |
| Intervention | Costs (£) | QALYs | Delays |
|---|---|---|---|
| 304 patients | |||
| SN, four nurse, 2 hours | 4336.02 | 0.61556 | 2588 |
| SN, two nurses, 1 hour | 2395.17 | 0.68557 | 125 |
| 150 patients | |||
| SN, two nurses, 2 hours | 4088.57 | 0.62339 | 1109 |
| SN, two nurses, 1 hour | 2536.37 | 0.68604 | 120 |
An estimation of the number of patients that can be seen per nurse was made assuming a long-term infection in an urban setting (1 hour’s treatment including travel time) using the SN service. As shown in Figure 26, 92.50 patients can be treated per nurse in a year before reaching saturation. At this point costs will start to increase, while QALYs will decrease.
FIGURE 26.
Number of patients per nurses before saturation.
Discussion
The aim of the economic evaluation was to produce estimates of the cost-effectiveness of OPAT services. After consultation with clinicians it was decided that two decision problems were pertinent, one for short-term infections (HO vs. GN vs. SN) and one for long-term infections (HO vs. GN vs. SN vs. SA). The models estimated the costs and effects of OPAT services, taking into account treatment time and risks of anaphylactic shock, CDI, severe S. aureus line infections and death.
The estimates of cost-effectiveness were generated using decision-analytic modelling techniques and, where possible, adhered to NICE’s reference case for health technology appraisals. We generated versions of the Markov model for short-term and long-term infections. We also used these to estimate the value of additional research based on the level of uncertainty around the estimates of cost-effectiveness. Although Markov models can provide these estimates relatively easily, there are limits to the level of information they can provide. For example, they cannot readily provide data on required staffing levels, the impact of combinations of treatments or the impact of delays on patients. In view of this, we also chose to conduct DES modelling of the decision problem. These types of models provide information over and above that yielded from Markov models, as well as providing standard estimates of cost-effectiveness.
The systematic review of the literature reported in Chapter 2 identified previous economic evaluations in the area and parameter values to inform the decision models. Only two randomised trials were identified but they were non-UK based and the comparator was inpatient treatment. 12,166 Additional targeted literature searches were conducted for certain key parameters. Comparative data on the safety and effectiveness of the OPAT service types were limited and a majority of studies reporting these were observational cohorts rather than randomised studies and, thus, provided potentially biased values and negated attempts at synthesising results. To illustrate, elderly patients or those with comorbidities may not be asked to attend clinics daily as they may find it difficult to travel. There are also differences in the underlying condition that cohort patients have and variations in the way outcomes are defined (e.g. heal vs. switch to oral). Furthermore, different centres may have alternative protocols relating to switching patients to oral treatment. As a result, for our main effectiveness parameter, we relied on a data set generated from hospital records of previous OPAT patients; this allowed us to control for heterogeneity in the patients receiving each service.
In both short- and long-term infection Markov models, the difference in expected effectiveness (QALYs) across service models was negligible. The explanation for this is that the time horizon employed (3 and 12 months for short-term and long-term infections, respectively) is relatively short and, for many, the health event of interest is transient in nature with a very low risk of mortality. Furthermore, the risk of adverse events was very low across all services. In contrast, there were significant cost differentials between the services that drove the cost-effectiveness results.
The SN service was the optimal service in the short term, being only marginally less effective than HO visits but £220 cheaper and being both more effective and cheaper than GN. These results were relatively insensitive to alternative analyses and did not change with modest changes in the risks, costs or utility parameter values. HO would have to be 2.48 days quicker to ‘heal’ patients than SN or nurse costs almost doubled (to £58.31) before HO became cost-effective. At a willingness-to-pay (per QALY gain) threshold of £20,000, SN had a 78% chance of being cost-effective. For long-term infections, the order of cost-effectiveness was maintained with GN preferred to HO, SN preferred to GN and SA preferred to SN; this also reflected the order of costs, with SA proving to be cheapest. SA was £452, £969 and £2523 cheaper than SN, GN and HO, respectively. The QALY gain differential was largely attributable to the greater time horizon and treatment period but still very small. As in the short-term infection model, it appears that costs drives the results. Again, results were relatively insensitive to parameter changes. The results changed in favour of SN when the risk of S. aureus in SA was doubled. The heal time in SA had to be 4.31 days longer than that in SN or the training cost had to be increased to £305.02 before SN became the optimal strategy. At a threshold of £20,000 SA had a 70% chance of being cost-effective versus a 30% chance for SN.
The results from the simulation modelling corroborated the Markov models results. As in the Markov model, SN and SA were the most cost-effective strategies for the short- and long-term models, respectively. The probability of these strategies being cost-effective was also similar to the Markov model estimations (≥ 65% vs. ≥ 70% in the Markov model).
The simulation yielded several additional useful pieces of information. It showed that NMB was inversely related to the number of treatment delays and that when resources are exhausted, delays and costs increase and QALYs decrease (especially in the long term). The analysis found that for high density population areas where nurse visits might be conducted in 1 hour, each nurse can safely handle just over 90 infections per year. This number clearly would be fewer if travel time or complexity of cases is increased. The results also suggested that long-term infections may best be served by a combination of SA and SN services.
The addition of the simulation model to the evaluation is relatively novel. Many technology appraisals assume that resources (e.g. staff and hospital beds) are infinite and are costed on a per-use basis. However, this clearly neglects key considerations for decision-making in the real world. We conducted a ‘commissioning’ perspective here that also allowed us to specify a set of fixed resources that could be exhausted and to determine the impact on the costs of purchasing additional resources and on patients who may be denied or receive delayed health care. Although not inconsistent with the usual HTA perspective of the health and social care system, it facilitates decision-making at a local level. Here, factors such as the number of patients in a given area, number of available staff and hospital beds, the geography of the local catchment area and patient preferences can be accounted for in the estimates of cost-effectiveness and decision-making.
The results from both Markov and simulation models suggest that SN is the optimal service in the short term. In the long term SA appears optimal, although SN provides slightly higher benefits but at higher cost. The optimal service provision and staffing level strategy from the commissioner’s perspective will be dependent on local factors, local priorities and any external quality standards. For example, providing nursing levels that optimise net benefit (cost-effectiveness) may be less of a priority than avoiding treatments delays.
Patient involvement
The research team met three times with members of the PAG during the development of both the Markov and simulation models. At these meetings we presented the graphics for the preliminary models to the group and provided an oral overview of them and what we were aiming to do. Their input and that of the clinical experts informed the development of the patient pathway described in the models. The patients also provided key insights into the important health events that might occur, on the health-care services that might be used and into the impact on their quality of life that infections had.
Limitations
Decision-analytic modelling is useful when the information required to make a decision is not available or requires synthesis; it can also help to inform research priorities. Notwithstanding this, the usefulness of modelling is a function of the quality of data available to populate the models that are constructed. In this economic evaluation we were constrained to some extent by the available data. There was a paucity of useful comparative UK data on the effectiveness and safety of the OPAT services. Furthermore, the concept of effectiveness in OPAT services is attenuated, as, quite often, fixed treatment courses are prescribed. We used a hospital record data set to derive our measure of ‘effectiveness’ (time to heal). The data set permitted adjustment for patient heterogeneity between services and did indicate differences in time to heal (or switch to oral antimicrobials). The differential between services on this factor may reflect effectiveness or the impact of adverse events and compliance, which may indirectly affect treatment time. Although there are non-trivial chances of experiencing mild adverse events in the course of IVA receipt, these are often self-limiting, transient and have minimal cost implications or impact on patient quality of life. The more severe events that are possible (e.g. severe line infection, anaphylactic shock or CDI) are relatively rare and, in the case of CDI, becoming rarer; thus, their impact at a cohort level is relatively minor. Given these issues, it is debatable whether or not better-quality data would have influenced the results to a great degree.
Antimicrobial stewardship is currently a key concern but we chose not to model antimicrobial resistance. We believe that the differential rate of resistance between the service models would have been negligible and did not warrant the additional modelling resources required or additional layer of complexity in the models. We also acknowledge that other variations on OPAT service models are available, for example delivery by a GP at the local clinic. However, it was not practicable to evaluate all of these. Outcomes data for all possible service configurations would be scarce and we chose to focus on the most commonly used in current practice.
Further research
The value of additional research was formally assessed using the outputs from simulations of the Markov decision models. These values (EVPI) represent in monetary terms the level of uncertainty in the estimates of cost-effectiveness; if these exceed costs we might expect to incur undertaking activities to reduce the uncertainty (e.g. conducting a large RCT) then we may proceed to invest in further research. If they are lower than the research investment then we should make the decision with the current available evidence. In the short-term model the EVPI was relatively small, given the low levels of uncertainty around the decision (SN had a 78% chance of being optimal). The EVPI figure over 1 year is £302,353 and, assuming that a clinical trial to reduce uncertainty would cost in the order of £1M–£3M, is outweighed by the research costs. Given this, additional research of this nature does not appear to be warranted.
The EVPI for long-term infections was higher (£1,078,204), mainly because there was greater uncertainty (present in the decision between SN and SA), and approached the cost of a trial. If we consider that the decision problem may be over the next 5 years then the EVPI increases significantly (although not sufficiently in the short-term group) to a value that exceeds the research costs. However, in reality, further trial-based research may take at least a further 4 or 5 years to report. By this time it is debatable whether or not the research question would still be relevant, as new treatments and modalities become available or current practice is challenged. 167 To illustrate this, the Oral Versus Intravenous Antibiotics for bone and joint infection (OVIVA) trial currently being conducted in Oxford is comparing IVA with oral antimicrobials in bone and joint infections. Should this research find that oral treatments are a suitable alternative to IVA, research investment in establishing evidence for OPAT services in this group will potentially be obsolete.
Should future trials of OPAT be considered, it is likely that they will be based on equivalence and safety. However, it is debatable whether or not the sample sizes sufficient to capture risks of the more severe events would be achievable. This is further complicated by the fact that departments often need to offer more than one type of service because some OPAT services are not suitable for all. Although SA is the most cost-effective option for long-term infections, it would not be possible to provide only this service because it would not be appropriate for those who were not able to self-administer (for cognitive or functional reasons). Furthermore, the expert panel also appeared to agree that a clinical trial may not be the most useful avenue for further research but that the establishment and analysis of large cohort data may be more fruitful. We are aware that BSAC are currently undertaking such an initiative but that, to date, the results have been mixed with variable uptake. Given the results and opinion of the consensus panel, the value of information analysis did not extend to include the EVSI that could inform trial design.
In terms of future methodological pursuits, the current research has highlighted the disconnect between macro- and micro-level decision-making. The real world usefulness of cohort-model-based estimates of cost-effectiveness conducted from the perspective of the health system is questionable when local-level factors are brought to bear. The conclusion we might make from the short-term infection modelling is that SN should be adopted wholesale. However, if combinations of services are required (to include HO), patient preferences are considered important and local resources accounted for, decisions made based on this information may not be optimal. Future research is required to explore the impact of local decision-making factors on estimates of cost-effectiveness and ways in which to consolidate local and wider NHS perspectives. Further analyses might also explore the whole commissioning picture whereby both short- and long-term service decisions are made concurrently.
Changes to protocol
Recruitment to the DCE was initially very slow so rather than risk getting insufficient participants for the health economic evaluation we continued to sample after the initial 200 data sets. As the profile of the DCE patients would then mean oversampling on some models (e.g. hospital attendance), a decision was taken to continue recruiting patients to provide anonymised data. We chose not to conduct the EVSI as the EVPI figures suggested that further research was not warranted.
Chapter 7 Expert panel review of the data
Aims and objectives of the meeting
The role of the expert panel was to consider the evidence generated from the systematic review, qualitative research, economic modelling, DCE and survey of current service provision. The following four questions were constructed to help the expert panel consider the findings of the four workstreams:
-
What is likely to represent an optimal service model for delivery of antimicrobial therapy for the two patient groups (long- and short-term i.v. antibiotic patients)?
-
Where does patient choice fit?
-
Are we ready for a clinical trial?
-
What are the future research priorities?
Method
The expert panel was convened on 28 September 2015 at the Doubletree at Hilton Hotel, Leeds. All panel members were asked to undertake preparatory reading (systematic review and summaries of the patient interviews, health professional interviews, DCE and economic modelling). The reports each contained an executive summary, method section with key results (see Appendix 5). All panel members were advised of the group discussions and provided with the questions prior to the meeting. The meeting was audio-recorded, with consent from attendees (to aid data analysis).
The expert panel meeting was structured as follows: (1) PowerPoint (Microsoft Corporation, Redmond, WA, USA) presentations by the workstream leads; (2) a question-and-answer session to ensure that expert panel members understood the material presented, and to generate discussion about the research findings.
Delegates were provided with a 1-page summary of each workstream to use as an aide-memoire during the subsequent discussions (see Appendix 5). The expert panel was asked to address four questions (detailed above). To facilitate discussions the panel was split into two groups: group 1 discussed questions 1 and 3, and group 2 discussed questions 2 and 4. Each group discussion was facilitated by two members of the research team (JM and MT; CCM and DM).
Panel members were briefed as follows: ‘You should use the data provided to discuss the following questions. You should brainstorm your ideas and nominate a note-taker to record your views on the flipchart provided, and agree a person to feedback to the group’. Facilitators then left the group for 15 minutes to discuss the issues, returning to provide further information and facilitate the discussion.
Panel composition
The panel comprised an independent chair (pharmacist), a CCG representative, an OPAT nurse, a microbiologist, two pharmacists, an infection specialist clinician, a patient, a statistician, a clinical triallist and a health economist. Three members of the panel sit on the BSAC committee. Three patients from our PAG were invited to attend. Two were unable to attend on the day owing to illness and the third attended the first half of the event but was unwell and could not stay for the discussion group. In addition to the independent panel members, six members of the research team were present and were available to answer questions during the group discussions.
Summary of discussion
Question 1: What is likely to represent an optimal service model for delivery of antimicrobial therapy for the two patient groups (long- and short-term i.v. antibiotic patients)?
Members of the expert panel drew on their own clinical expertise and experience as well as the evidence presented from CIVAS when considering these questions. To facilitate their discussion, three inter-related questions were posed.
Are different models needed for long- and short-term infections?
The group discussed what was meant by ‘different models’ and concluded that both long- and short-term infections should be covered by one ‘service’. Expert panel members had experience of different care pathways and this resulted in a long discussion about the merits of these; there was some disagreement between panel members as to the best model of care as there are advantages to all. There was agreement that OPAT services could be located in the NHS trust or be community based and that they should offer different care pathways (e.g. SA, hospital attendance, nurse at home). The group agreed that there needs to be flexibility to accommodate patients with different needs and that within any one OPAT service there should be several care pathways (rather than only one care pathway).
Although the health economics evidence presented suggested that, as costs drive the models, a SN service was optimal for short- and long-term infections, with SA marginally less expensive for long-term infections. However, there was reluctance by the panel to accept these findings.
Should services focus on once-daily antimicrobials (balancing antimicrobial resistance against costs)?
The overwhelming view was that OPAT services should focus on antimicrobials which could be administered once a day, provided that the treatment selected is clinically appropriate. It was felt that once-daily treatment provided the greatest potential for services to treat more patients (freeing up staff who would otherwise be involved in repeat administrations). Twice-daily administrations should be accommodated when the infection dictated (i.e. when no once-daily treatment is available). The group thought that this might be achievable if smaller hospitals that do not currently have infection-specialist input on site could access this input via telemedicine to ensure that the most appropriate antimicrobial was offered. It was agreed that the involvement of an infection specialist/microbiologist at diagnosis and review stages would be appropriate.
How do we ensure equality of access to services and equivalent standards of service across England?
Panel members described situations in which patients could not be sent home because they lived in the ‘wrong’ CCG area, or lived too far from the hospital to receive OPAT. It was thought that in more rural areas a hub and spoke model (network) might be appropriate, similar to the model used in cancer, with tertiary (specialist), DGH and medical centres (CCG level) working together to provide care.
This would require a more ‘joined up’ approach with national-level commissioning and all agreed that a nationally commissioned service is needed, rather than local negotiation with CCGs. There were examples given of how, when one CCG commissions a service and a neighbouring one commissions a different model, secondary care has to try to run two (or more) services, and neither is likely to be cost-effective. National commissioning could also improve the care, as it would make it easier for patients to be seen in tertiary centres and then referred back to local DGHs, which are often out of the area; a nationally commissioned service would be able to accommodate this in a way that locally commissioned services cannot.
Question 2: where does patient choice fit?
How important is patient choice?
The group focussed their discussion on whether or not it is possible to implement patient choice. From a commissioning perspective, patient choice was viewed as essential, although it was acknowledged that this would still be within the confines of what is financially viable. From a clinical perspective patient choice was also viewed as important, but it was acknowledged that the choice may be about whether or not the patient wants to have OPAT or inpatient care, and would not necessarily include a choice about the form of OPAT they receive.
The group discussed the fact that the patient interview data had revealed information which panel members had not previously considered about what people take into account when making choices about their care, such as not wanting to be in hospital because they were worried about the impact on their family. Other challenges such as access to treatment, car parking, etc., were more obvious challenges that would influence the willingness of patients to receive OPAT and their preferences for care.
It was therefore agreed that patient choice is important, but there was the view that any service should also offer good value for money to the NHS. Examples were given about the desire to offer OPAT to those living in rural communities, but the feasibility of offering services in such circumstances is challenging. Although an individual patient may prefer to be cared for at home, patient characteristics (e.g. age, health) and nature of the infection may influence whether or not a model of care is available to the patient (e.g. multiple administrations of i.v. antibiotics in a rural area).
If we think choice is important, how do we translate patient preferences into service commissioning decisions?
It was acknowledged that sometimes the most cost-effective model of care may not be the one which patients would prefer, and the group discussed whether or not the NHS can afford ‘choice’. They concluded that the NHS already takes patient choice into account when commissioning service, via data from patient surveys. However, it was acknowledged that, although there is a desire to ensure that the patients’ voice is heard, final commissioning decisions may not always accord with patient preferences (e.g. decommissioning of services, hospital closures).
It was acknowledge that owing to the geography of the UK it may be difficult in some areas to commission an OPAT service which is sufficiently flexible to include different care pathways and, therefore, meet patient preferences, and any OPAT service must also be able to offer what the NHS acute trust needs to facilitate early discharge (non-admission) of patients requiring i.v. antimicrobials.
There was a desire that patient preferences should be examined at the local health economy level and there were concerns about whether or not the findings of the DCE study, which collected data from across Yorkshire and the Humber, could be translated to other localities, although no definitive reasons why they should differ were identified. There was a discussion about the importance of ensuring that local services support the needs of the local community, for example, through accommodating local geography (rural/metropolitan) or population size. There was strong interest from the commissioner and clinicians to replicate the DCE in other areas and to use these data to make comparisons across the country to test the robustness of the original findings, but to also use the new ‘local’ findings to ensure that local OPAT services meet local needs and preferences, especially in relation to local ethnic diversity. As some CCGs co-commission with other commissioning groups, factors other than patient preferences influence commissioning decisions. Although patient preferences influence what is included in the service tender, the skill set of those tendering to deliver the service may not meet these needs, which may limit the services that can be offered locally. In conclusion, patient preferences were viewed as important but these must be balanced against what is feasible for that community.
How do we balance choice and cost?
It was agreed that different patient characteristics might result in different care preferences, which was borne out (to some extent) by the DCE data, which showed that younger men had a preference for attending hospital, compared with the general population. It was also agreed that scale of the service and demand for treatment may affect the choices available to patients (so their ability to receive the model of care they prefer) and subsequent costs.
There was some debate about whether or not patients are able to make an informed choice about the care they receive. Offering individual patients a choice about the care they receive means educating them to enable them to make an informed/aided decision. It was agreed that patients are unlikely to understand the implications, for example of SA, unless this was clearly explained to them. Therefore, the additional cost of explaining to patients what going home will entail needs consideration.
Attitudes were found by the DCE study to be important predictors of patient preferences, and the group discussed the implications of patient attitudes when considering the push to move to community-based care. It was agreed that the findings of the study indicate that there may be a need for an attitudinal/behavioural shift by both NHS staff and patients to accept these new ways of delivering health care, not just i.v. services. If we can manage this change within OPAT then this could provide a model for other services.
Finally, there was a debate about the nature of choice and preference. It was acknowledged that, although someone may prefer one option, they may choose another owing to other factors. The difference between choice and preference is one that is picked up by the DCE, and the finding that preferences can be influenced by attitudes may also be a good target for intervention. By changing people’s attitudes, might we change their preferences?
Question 3: Is there sufficient evidence upon which to base a clinical trial comparing service models?
Following a long discussion, it was agreed that NIHR would not fund a randomised clinical trial comparing two or more models of care. The rationale for this view was:
-
There is no obvious comparator group.
-
It was difficult to decide what the outcome measure would look like. Quality of life and readmission to hospital seemed the most plausible, but readmission is not common, which would lead to an unfeasibly large trial.
-
The OPAT population is too heterogeneous (e.g. cellulitis vs. bone infection), and a complex cellulitis may also require longer treatment than a simple cellulitis.
-
We do not have a clear picture of what current service provision in England looks like. This makes it impossible to determine whether or not there are sufficient ‘clean’ sites to introduce a new model to test in any future trial. It was suggested that BSAC data should be used to help answer this question. Another source of information would be local commissioners in each CCG.
-
It was agreed that it is unlikely that any CCG would pay for the set up costs of any new services to test the effectiveness of ‘pure’ models (e.g. pure nurse-led community service vs. doctor-run, hospital-led service).
-
There was a discussion about whether or not a hub and spoke model could be used (cluster trial) so that an area could offer both services, but areas would be large and it was agreed that this was unlikely to be feasible.
A final but important stumbling block to any trial was that if the premise from the first discussion holds true, namely that any service should be accessible to as many people as possible, then comparing HO with community-based treatment becomes an invalid comparison. The data presented by the research team indicate that there is heterogeneity in patient preferences, and the group felt that offering choice is important. It was agreed that it is more important to offer forward-thinking models of care that meet the needs of patients, which is why such different models of care have developed across the country.
Finally, there is an acknowledged lack of equipoise in the community (most services are developed and run by enthusiasts, who set up what they believe to be the best model). The group discussed the possibility of other study designs.
What should such a trial (study) look like?
The group concluded that a prospective, observational study might be useful. The likely outcomes to consider would be value for money, safety (patient harm), admission avoidance and early discharge.
How useful are patient preference data (discrete choice experiment): should we be encouraging its use in other health-care settings?
The group found the results of the DCE to be very interesting, and they helped them to understand the impact of attitudes on preferences. The group felt that we should be encouraging the use of DCEs in other areas. The group thought that collecting data on patient attitudes would be of interest for commissioning decisions and commissioners.
Question 4: what should the future research priorities for outpatient parenteral antimicrobial therapy be?
-
It was agreed that replicating the DCE study in other areas would be useful. There were some concerns within the expert panel members around the lack of ethnic diversity in the development of the questionnaire. If the robustness of the measure could be determined by input from further work with patients from a range of ethnic groups, this would be beneficial.
-
The inclusion of attitudinal data questions in the DCE was of particular interest to commissioners and there was a desire to have this included in a commissioning toolkit to inform commissioning decisions and also to use for quality monitoring, along with other measures.
-
It might be useful to consider the cost-effectiveness of OPAT services, but also to consider the costs of ‘injectables’ (e.g. blood products) more widely.
-
One possible area of research would be to consider patients’ willingness to pay. It was proposed that repeating the DCE with a question about willingness to pay would be interesting. However, this may dominate the model, so it would be important to have a split sample to test what impact this has on the data. However, from a commissioning perspective it was viewed as unlikely that patients would be asked to pay for OPAT as it saves the NHS money. We could look at willingness to pay (patient) to travel to a health-care setting to receive care versus care at home. For example, having a service at hospital versus at a general practice/medical centre. Would paying for transport influence patient choices? Would being able to park (e.g. at GP surgery) overcome any reluctance that patients have?
-
Is there a role for a GP-facilitated model of OPAT (not involving GPs, but using their venue with a district nurse/SN model of care)?
-
Is there a role for the third sector (voluntary services) and private companies?
-
Who has overall responsibility for patients’ infections? It was agreed that the governance of OPAT services was an important issue. Although the referring specialist could maintain control, is this clinically effective? Would it be better for the patient to be ‘managed’ by an infection specialist?
Summary and recommendations
Following a long discussion there was general agreement that we do not need different models of care for short- and long-term infections. The health economics evidence presented from this study suggests that a SN at home model was optimal, with SA being marginally less expensive for long-term infections. However, the panel felt that OPAT services (whether based in acute care or in the community) should offer different care pathways depending on local needs (e.g. SA, hospital attendance, nurse at home).
Patient preferences were viewed as having an important role in the commissioning of NHS services, and the data from the current study were welcomed as informing that process. However, panel members were keen to replicate the study in other geographical areas to test the ‘generalisability’ of the model. For example, would the same findings be obtained in very urban areas with good hospital access or in areas with poor access? It was recognised that those commissioning NHS services had to balance the wishes of different patient groups when making commissioning decisions in the current NHS climate. It was also noted that assessing preferences might overstate people’s likelihood of selecting one particular model of care, as there may be other factors that influence behaviour. It is recommended that the DCE study be replicated in other UK sites to test the robustness of the model, preferably with some more qualitative work with ethnic minority groups to ensure the DCE choices meet their needs.
It was agreed that the attitudinal questionnaire developed as part of the DCE would be useful to commissioners for service monitoring within local contracts and would make a welcome addition to their commissioner’s toolkit.
Understanding patient attitudes was also viewed as having an important role in helping the NHS in a practical way. It was felt that if we understand patient attitudes towards different service models then we may be able to use this knowledge to influence behaviour, possibly drawing on ‘nudge theory’ to encourage patients to accept more cost-effective approaches168,169 although this approach has also been criticised. 170 However, it was also acknowledged that we need research into how best to influence health professionals’ behaviour as many are risk averse, so may not discharge patients on OPAT.
It was agreed that introducing patient choice also means ensuring that patients understand that implications of those choices and that there was a need for more research to ensure patients have information to make an informed/aided choice.
Chapter 8 Discussion and conclusions
Introduction
Outpatient parenteral antimicrobial therapy services were initially provided in the UK by a few specialised units with strong leadership to champion such services. 3,13,75 Over the past 10 years, OPAT services have become more common in response to pressure for health care to be delivered in the community rather than hospital, as well as opportunities for cost savings by reducing bed occupancy. 94 However, OPAT coverage has remained patchy, with marked heterogeneity in providers, models of care and clinical services. During the period of this research project, the Five Year Forward View was published171 setting out key challenges and plans for the future NHS, including new care systems relevant to OPAT services. These include closer working between primary and secondary care and the expansion of care provision out of hospital. It is also proposed that patients gain much more control of their own health care, reiterating the longstanding NHS pledge of giving patients choice in how and where they receive care. Better understanding of factors affecting choice and decision-making is needed for the NHS to be able to achieve this.
The aims of this project were to examine current OPAT provision across England and to gain an understanding of patient preferences and cost-effectiveness, which could inform future service provision. Specific objectives were to: carry out a systematic review of the relevant literature; establish the types of OPAT services available in England and identify barriers to the use of each service type; evaluate patients’ preferences for, and the costs and benefits of, delivering i.v. antibiotics in the community; and make recommendations for the optimal delivery of the service and for future research including the design of future clinical trials. The project included an examination of patient preferences and attitudes using a DCE, as well as both traditional health economic and simulation modelling techniques to inform health service planning.
Principal findings
Our systematic review has provided a comprehensive picture of the current evidence surrounding the effectiveness, safety and acceptability of outpatient antibiotic therapy. We concluded that there are no systematic differences in relation to the impact of OPAT on duration of therapy, or on adverse events associated with i.v. antibiotic treatment, and that, on the whole, OPAT is more cost-effective than inpatient care. However, conclusive evidence as to the clinical benefit (or otherwise) of this mode of therapy compared with traditional inpatient i.v. treatment is lacking. Patient acceptability of OPAT appears to be high, with patients particularly appreciating being able to resume their daily activities (such as going to work or school), having greater freedom and control over their illness, and not having to attend hospital but being able to stay at home with family. The disadvantages identified by patients were most commonly related to the use of infusion equipment. Few studies considered practitioner acceptability, but those that did found some concerns related to the logistics involved in providing an OPAT service, including cost and a lack of clarity about who would assume clinical responsibility for patients. Although many studies were identified and included in this review, few involved a comparison with inpatient care (or other models of OPAT), and even fewer employed a rigorous trial design. Much of the work in this area is based around ‘audits’ of services provided with limited data relating to outcomes. In addition, many of the studies involved small numbers of patients.
We sought the views of health-care professionals involved in OPAT through an online survey and in-depth interviews. Unfortunately, the response rate to the survey was low, although a wide range of staff and settings were covered. Much more information was obtained from the interviews with OPAT professional leads who delivered services in a range of settings. Most offered some level of hospital provision, as well as a community delivery option, but SA by patient or carer was relatively rare. They treated a wide range of infections across a broad range of specialties. Service models varied from being well organised with regularly updated business plans to those described by leads as being ad hoc. A number of problems with service delivery were highlighted. Although good practice recommendations are in place,93 services did not always manage to meet these criteria. Responsibility for care was not always explicit and there were examples of clinical leadership being lacking. Some services had received short-term funding with no guarantee of continued investment. Services were often limited by their funding, with expansion being difficult. Interviewees made a number of suggestions on how OPAT service provision might be improved. In particular, there was felt to be a need for guidance on commissioning to ensure consistency of service provision as well as providing information on how to provide appropriate service models according to local population needs, taking into account local resources. Clinical governance was thought to be important and clinical outcomes should be measured as well as cost-effectiveness and professional and patient satisfaction with the services provided. This could be informed by national guidance. Networks at local, regional and national levels could facilitate both governance and service development.
Antimicrobial stewardship is currently a key concern, after the issue of increasing antimicrobial resistance was high-lighted by the Chief Medical Officer and placed on the National Risk Register. 172 The once-daily antibiotics favoured in OPAT may be broader spectrum [ertapenem (Invanz, Merck Sharp & Dohme Limited)8], but some are narrow but of concern for resistance [teicoplanin (Targocid, Sanofi)173] and so research is required on appropriate antibiotic choice by OPAT services and surveillance for the development of antimicrobial resistance following OPAT. 174
Our qualitative study of patients’ perceptions of OPAT was designed to explore the attributes of such services in detail and to compare different models of service delivery. We recruited patients representing a range of socioeconomic backgrounds who between them experienced all four main OPAT service models. Overall, patients appreciated the care they receive and felt that services were generally well run and of high quality. Most acutely ill patients preferred to be cared for in hospital, but once stabilised most preferred to recover at home. Some aspects of the service were identified which were not specific to a particular service model; for example, patients preferred to have a definite appointment for their treatment rather than to be kept waiting. They also were keen to have follow-up after their treatment had finished.
Patients identified a range of health-care experiences as being important. A good service was one in which staff were perceived to be competent and highly skilled. For some this meant the active involvement of doctors within the service but others focused on the expertise and experience of nursing staff. OPAT services can support patients to self-manage in the community but when services are not configured in a way that helps patients, this can negatively impact on patient satisfaction. Poor communication could leave patients without the knowledge and confidence needed to be a competent collaborator in their own care and affect their perceptions of the service. These findings suggest that both the organisation of health care and the personal interactions or social dynamics are important to the patient experience, as noted by Entwistle et al. 106
Each of the care pathways was viewed as having its own strengths and weaknesses, and the importance people attached to different attributes seemed to be linked to the age and health of the patient. A nurse at home model was perceived to be particularly well suited for older patients, those needing longer courses of i.v. treatment and those with more complex care needs. For many, the one-to-one time with the nurse was viewed as a key benefit, but this could be quickly eroded if the nursing team is large and the continuity of the care relationship is broken. Hospital attendance was considered to be most suitable for those who were fitter, younger and who required once-daily, short courses (under 1 week) of i.v. treatment, a view also held by those attending clinic with long-term infections. The availability of a doctor in the clinic provided the reassurance some needed ‘in case anything went wrong’; other qualitative studies such as that by Bamford et al. 35 have suggested that some patients do not feel confident about being treated by a nurse at home.
A potential benefit of hospital attendance was its convenience for hospital staff, but this required the use of timed appointments and when appointments were not kept, this affected the level of patient satisfaction, a finding reported elsewhere. 69 Characteristics of the service (e.g. if understaffed) and the staff themselves (e.g. not briefed about the patient) contributed to dissatisfaction. Hospital attendance was the only care pathway in which transport is a significant issue. Poor public transport links, a reliance on hospital transport and poor car-parking facilities at hospitals were also key attributes that affected the acceptability of hospital attendance and have been noted previously. 69
Self-administration was the model of care least well represented in our sample, and all patients receiving this were chronically ill patients who received regular courses of i.v. treatment, so do not reflect the views of those who experience a one-off course of antibiotics, for example for a deep-seated infection. Patients who had no personal experience of SA voiced most concerns about the risks of this service model. Although those using the service found it convenient, there were residual concerns about the safety of SA, and there was a suggestion that some would prefer inpatient care. SA was offered only to patients who were physically and cognitively able to manage the complexity of delivering the therapy themselves.
Through these interviews and the focus group we were able to identify a number of attributes of such services, which were used to develop our survey of patient preferences through the DCE. In addition, a number of questions to determine patients’ attitudes to OPAT and health care in general were identified.
The DCE was designed to explore the aspects of the different service models that matter most to patients and the aspects that they might be prepared to ‘trade off’. Six key attributes were developed through the systematic review and interviews with health-care professionals and patients:
-
number of treatments per day
-
the importance of a timed appointment
-
who administers the treatment
-
communication with health-care professionals
-
follow-up arrangements
-
risk of adverse reactions.
The DCE was piloted in a small number of patients and after minor adjustments was then administered to just over 200 participants. These were recruited from six centres providing OPAT services across Yorkshire and included those patients with complex infections requiring long-term treatment, as well as those requiring short courses of antibiotics, who between them experienced all the service models. Anonymised clinical and demographic background information was collected, as well as a survey on patients’ attitudes to health care in general and OPAT in particular. In summary, the attitudinal data showed a tendency for non-white females living alone to prefer inpatient treatment. Older, non-white patients were more likely to perceive health care as the responsibility of the doctor.
The DCE showed that, overall, the visiting nurse at home model of care is preferred to attendance at hospital. However, this was influenced by sociodemographic factors; for example, younger males preferred to attend hospital rather than receive treatment at home. The next strongest preference was for once-daily treatment over two or continuous treatments, closely followed by the preference for the lowest level of adverse event risk. Although other attribute levels were significant in determining respondent’s choices, they were less important. People preferred a SN to a doctor and GN to deliver their treatment, preferred having an appointment time (to not having one) and preferred to communicate with someone they have met before regarding their care.
Our health economics workstream examined the value for money of different OPAT service models using two different approaches: a Markov model to generate estimates of cost-effectiveness and a simulation model. In both short- and long-term infection Markov models, the difference in expected effectiveness (QALYs) across service models was negligible. The explanation for this is that the period of observation (3 and 12 months for short-term and long-term infections, respectively) is relatively short and, for many, the infection being treated is transient in nature with a very low risk of mortality. Furthermore, the risk of adverse events was very low across all services. In contrast, there were significant cost differentials between the services which drove the cost-effectiveness results.
The SN service was the optimal service for short-term infections, being only marginally less effective than HO visits and being more cost-effective than a GN. SA was very rare for the treatment of short-term infections, so we did not include this model in the analysis. These results were relatively insensitive to alternative analyses and did not change with modest changes in the risks, costs or utility parameter values. For long-term infections, the order of cost-effectiveness was maintained, with GN preferred to HO, SN preferred to GN and SA preferred to SN; this also reflected the order of costs, with SA proving cheapest. The QALY gain differential was largely attributable to the greater time horizon and treatment period but was still very small. As in the short-term infection model, it appears that costs drives the results. Again, results were relatively insensitive to parameter changes; for example, the results changed in favour of SN only when the risk of line infection in SA was doubled. The results from the simulation modelling largely corroborated the Markov models results, although SN was found to be optimal for long-term treatment, in contrast to SA which was optimal in the Markov model. However, the ordering in terms of costs and QALYs across treatments was the same and the actual difference in NMB between the models was minimal. The results from both Markov and simulation models suggest that SN is the optimal service in the short term. In the long term, SA appears optimal, although SN provides slightly higher benefits but at higher cost. Not all patients will be able to self-administer their treatment, so an alternative model of care needs to be available.
The simulation yielded several additional useful pieces of information. It showed that net benefit was inversely related to the number of treatment delays and that when resources (e.g. availability of nurses to provide treatment) are exhausted, delays and costs increase and QALYs decrease (especially in the long term). The analysis found that for high-density population areas where nurse visits might be conducted in 1 hour, each nurse can safely handle just over 90 infections per year. This clearly would be fewer if travel time or the complexity of cases is increased. The results also suggested that long-term infections may best be served by a combination of SA and SN services.
Once we had completed the project workstreams described above, we presented the results to a panel of expert researchers and health-care professionals in the field. We asked them to review the findings and give their opinions on a number of questions.
There was broad agreement that OPAT services could be based either in an acute hospital trust or in the community. The panel agreed that there needs to be flexibility to accommodate patients with different needs and, therefore, that within any one OPAT service there should be more than one care pathway (e.g. SA, HO attendance, nurse at home).
The health economics evidence suggested that a SN service was the most cost-effective for short- and long-term infections, with SA being less expensive for long-term infections. However, there was some reluctance to accept these findings by some panel members who favoured their current care pathways for their patient populations. However, there was agreement that OPAT services should focus on antimicrobials which could be administered once a day, provided that the treatment selected is clinically appropriate, as this provided the greatest potential for services to treat more patients (by freeing up staff who would otherwise be involved in repeat administrations).
The expert panel agreed that a nationally commissioned service is needed, rather than local negotiation with CCGs. It was thought that in more rural areas a hub and spoke model (network) might be appropriate, similar to the model used in cancer, with tertiary (specialist), DGH and medical centres (CCG level) working together to provide care. Technological advances such as telemedicine could be employed in more remote areas.
It was agreed that patient choice is important, but that any service should also offer good value for money to the NHS and be appropriate for the individual patient’s clinical needs. Service users need to be provided with appropriate information to inform their decision-making. Local services must both support the needs of the whole community and take into account the skill set of the available providers. The group discussed the implications of patient attitudes when considering the push to move to community-based care. It was agreed that the findings of the study indicate that there may be a need for an attitudinal/behavioural shift by both NHS staff and patients to accept these new ways of delivering health care in the community in general, not just i.v. services. It was suggested that the attitudinal questionnaire developed as part of the DCE would be useful to commissioners for service monitoring within local contracts and would make a welcome component of a commissioner’s toolkit.
Strengths and limitations
Our systematic review, revised just before submission of this report, provides the most comprehensive review of the research published on OPAT in recent years. This helped us to plan our subsequent research focusing on areas in which little was known, such as patient preferences. Our interviews with health-care professionals across England working in a range of services provide insight into the challenges of setting up and running such services.
Our data support and develop the earlier limited qualitative research evaluating OPAT services. The strengths of this part of the study are that we recruited from four sites, including two large teaching hospitals and two DGHs, which between them offered HO attendance, SN and GN nurse at home and SA OPAT services. We had a broad sampling strategy to obtain views from participants from a diverse range of socioeconomic backgrounds. This enabled us to contrast the views and experiences of those who experienced different models of care and provided relevant information to examine the aspects of care that patients valued to construct our attribute shortlist for the DCE.
The use of DCEs to inform the design of health services is a well-established approach. 100,127 This research is the first attempt to understand patients’ preferences for OPAT services and one of the most in-depth explorations of the role of patient attitudes in influencing health-care preferences. The DCE provided useful information on not only the type of services that individuals prefer but also on which aspects of those services were important to them. This is crucial for designing new services and, should more patient choice be offered, understanding demand. The DCE also highlighted the high degree of heterogeneity in preferences which would be useful for tailoring services.
The cost-effectiveness modelling and extensive sensitivity analyses represent the most in-depth economic evaluation of OPAT services to date. The addition of the simulation model to the evaluation is relatively novel. Many technology appraisals assume that resources (i.e. staff and hospital beds) are infinite and are costed on a per use basis. However, this clearly neglects key considerations for decision-making in the real world. We conducted a ‘commissioning’ perspective here that also allowed us to specify a set of fixed resources that could be exhausted and determine the impact on the costs of purchasing additional resources and on patients who may be denied or receive delayed health care. Although not inconsistent with the usual HTA perspective of the health and social care system, it facilitates decision-making at a local level. Here, factors such as the number of patients in a given area, number of available staff and hospital beds, the geography of the local catchment area and patient preferences can be accounted for in the estimates of cost-effectiveness and decision-making.
The expert panel event allowed us to obtain feedback on our findings and to formulate further research questions from specialists in the field who were independent of the project. This included individuals involved in commissioning and formulating policy for NHS England on antimicrobial stewardship and representatives of BSAC, the professional society championing OPAT.
Our survey of health-care providers was hampered by a low response rate, which was probably due to ‘survey fatigue’ and the pressure of work – such problems have been noted elsewhere. 91 Owing to delays in gaining ethics approval, we had only 3 months in which to recruit to the interview study. We planned to undertake focus groups with relatively few interviews, but recruitment was poor. We therefore switched to interviews only, which resulted in much richer data, but did put the study behind time as the analysis took longer than anticipated. This had the knock-on effect of the interviews and analysis becoming desynchronised, so some data saturation was reached before we finished interviewing and no new findings were revealed in the final five interviews.
Overall recruitment of patients to the study was initially behind target, so we increased our number of sites to include two more sites. However, we still lacked patients with experience of SA for single infections rather than recurrent infections, so we then opened a further site where this model of care is commonly used. We struggled to recruit the very elderly (> 85 years of age) and those from local BME populations. Feedback from eligible older patients was that they did not feel well enough to be interviewed. We struggled to recruit from the BME community despite advice from researchers with significant experience working with the BME community and our PPI group. We may therefore have missed issues that are of importance to the non-white population and this is a potential source of bias in respect of the DCE attributes themselves. However, the systematic review evidence that informed the development of the attributes and their levels comes from a range of countries.
Caveats are required in translating the results found here into service commissioning. First, we must acknowledge that only services that represent value for money should be offered to patients. 175 The benefits of the services in our DCE were (with the exception of risk) based on process utility and not health outcomes. Although convenience is important and may offer indirect health benefits (e.g. through better adherence), we must be cautious in attributing too great a value to it. Second, the DCE provides information only on stated preferences and may not accurately reflect the choices that people would make if faced with the same options in reality.
Some of the options contained in the DCE do not represent current practice. For example, doctors rarely administer i.v. treatment but some patients in our interview study talked in depth about the importance of the doctor, so we included this to test what patients would be willing to trade to receive this level of care.
Patient preconceptions and past experiences may influence the findings. Only adults who had previously experienced OPAT were included; those who would be eligible for OPAT but were not offered the service were not included, and this population may respond differently. The reason for the sampling used in this study was that the original protocol included the collection of anonymised data from the DCE participants, so only those who had received OPAT were eligible.
Decision-analytic modelling is useful when the information required to make a decision is not available or requires synthesis; it can also help to inform research priorities. 127 Notwithstanding this, the usefulness of modelling is a function of the quality of data available to populate the models that are constructed. In this economic evaluation we were constrained to some extent by the available data. There was a paucity of useful comparative UK data on the effectiveness and safety of the OPAT services. The more severe events that are possible (e.g. severe line infection, anaphylactic shock or CDI) are relatively rare and, in the case of CDI, becoming rarer;176 thus, their impact at a cohort level is relatively minor. Given these issues, it is debatable if better quality data would have influenced the results to a great degree. We also acknowledge that other variations on OPAT service models are available, for example, delivery by a GP at the local clinic. However, it was not practical to evaluate all of these. Outcomes data for all possible service configurations would be scarce and we chose to focus on the most commonly used in current practice. Although topical,172 we chose not to model the development of antimicrobial resistance. We believe the differential rate of resistance between the service models would have been negligible and did not warrant the additional modelling resources required or the additional layer of complexity in the models. However, with antimicrobial stewardship being a key concern in light of increasing antimicrobial resistance,172 some of the once-daily antibiotics favoured in OPAT (e.g. teicoplanin173) may be of concern for resistance; thus, research is required on appropriate antibiotic choice by OPAT services and surveillance for the development of antimicrobial resistance following OPAT. 174
Conclusions
It has been long established in the UK and many other countries that OPAT is generally preferred by patients, is safe and is cost-effective in comparison to inpatient admission for i.v. antibiotic treatment. Through the systematic review, this project has highlighted the lack of previous research into the cost-effectiveness of different models of service provision and patient choice in this area. In addition, conclusive evidence as to the clinical benefit (or otherwise) of this mode of therapy compared with traditional inpatient i.v. treatment is lacking. Because OPAT services are now established in many areas of the UK, in response to general policy initiatives to treat patients in the community rather than in hospital, and to save on hospital admission costs it would be now very difficult to conduct such a study.
Our survey of OPAT provision and interviews with health-care providers in England makes it clear that there is a great variation both in the extent of services provided and models of care in existence. In addition, some respondents were struggling to maintain or even set up OPAT services because of the lack of clear commissioning directives and/or engagement by senior managers.
Our qualitative studies, although confirming that most patients prefer to receive treatment through OPAT services, did highlight some organisational shortcomings, for example where patients were kept waiting for long periods to receive treatments or where aftercare expectations were not met. This suggests that the governance of such services needs to be improved to meet both specific and general clinical standards.
Our DCE modelling data collected on patient preferences showed that most patients preferred to be treated at home, although certain sociodemographic groups would rather attend a hospital clinic. The health economics workstream similarly showed that visiting nurse model was the most cost-effective overall. NMB values for HO, GN and SN services were £2493, £2547 and £2655, respectively, for short-term services. SA is also cost-effective for patients willing and competent to be trained to do this; in practice this is useful only for those patients requiring longer or repeated courses of treatment. NMB values for HO, GN, SN and SA services were £8240, £9550, £10,388 and £10,644, respectively. The simulation model provides a useful method of calculating the capacity of services according to the number of staff employed.
Implications for future service planning and commissioning
Commissioning of health-care services in England is increasingly complex and has been subject to frequent change in recent years,177 including through the introduction of commercial and non-NHS providers of services. The Five Year Forward View171 accepts that one size does not fit all and recommends an emphasis on ‘diverse solutions and local leadership’ supported by ‘meaningful local flexibility in the way payment rules, regulatory requirements and other mechanisms are applied’. Currently, most common conditions are commissioned locally by CCGs, whereas rarer diseases are commissioned at national level by NHS England. At present there is no clear commissioning mechanism for OPAT services. There is much variation in how the cost of treating cellulitis, the most common condition requiring OPAT, is reimbursed to providers (see Jones et al. 178). The NHS England service specification requires that infectious diseases services include a requirement to provide OPAT services; however, infectious diseases units are largely based in tertiary referral hospitals so areas of the country remote from large centres of population do not have such services. In general, commissioning decisions appear to be based on a variety of factors including local knowledge, advice from clinicians, informal contacts and, less frequently, traditionally published academic research. It has been proposed that research evidence should be communicated in ways that are more accessible to commissioners. 177
Patient preferences
Patient choice has become an important factor in decision-making on health-care services, but formal research into how those views can be quantified is limited. It is known that direct questioning may provide information on which type of service is preferred overall but does not give information on the relative importance of the multiple attributes patients consider when making their decision. Commissioners are increasingly sensitive to patient views in supporting service developments, particularly qualitative research. 109,179 Therefore, our study of patient preferences for particular services models will help to inform decision-making on provision of OPAT services. Commissioners can also use the results of this study to influence service provision so as to obtain the best outcomes within a given budget. The results indicate that where one model of OPAT care is envisaged, a visiting nurse at home model is likely to be preferred by patients. However, where possible, a range of options should be available. One solution that was proposed by patients as an alternative to hospital-based services was to invest in ‘local clinic services’, perhaps based in local medical centres. This might fit within the ‘Multi-Specialty Community Provider’ model proposed in the Five Year Forward View by NHS England. 171 Patients valued good communication between staff members, and robust governance processes should be in place to ensure patients receive continuity of care. Aftercare, such as follow-up by a nurse at the end of treatment for short-term infections such as cellulitis,10 is expected by patients. The qualitative data (see Chapter 4) support the DCE findings and suggest that patients’ attitudes towards health care are important and could form a target for future intervention. The preferences across short- and long-term infection patients were quite stable, suggesting that people in these groups value similar service attributes. These findings are likely to be useful in determining future service provision in this area, which takes account of patient preferences. It will help NHS trusts who want to introduce OPAT services by providing a rationale for service configuration.
Recommendations for future research
Together with the expert panel we concluded that a RCT of different models of care is not feasible because of the problems of centres being not able to provide all the necessary comparator services for randomisation, difficulties in deciding on the outcome measure and the heterogeneous nature of the patient population. However, other, more pragmatic, study designs could be considered, such as prospective observational multicentre studies to collect information of outcomes, adverse events, and costs. For example, the use of the proposed multi-specialty community provider facilities for OPAT could be evaluated in this way. Other specific research topics include the development of a patient-reported outcome measure which could be used to standardise research in the OPAT, and the evaluation of mixed models of care, including provision of other i.v. therapies (such as diuretics for patients with heart failure).
The expert panel suggested that replicating the DCE study in other areas of the country would be useful to further validate our findings. There were some concerns among the expert panel members around the lack of ethnic diversity in the development of the questionnaire. If the robustness of the measure could be determined by input from further work with patients from a range of ethnic groups, this would be beneficial.
There are a number of potential topics for research which could develop our patient preference evaluation. Additional research is required to understand if and how stated preferences in health could be calibrated to better reflect revealed preferences to facilitate service design and planning. One possible area of research would be to consider patients’ willingness to pay for aspects of care using the DCE, for example the extent of the influence of costs of transport to or parking at a health-care setting on patient choices.
Our methodology is transferable to other health service evaluations. The economic models are tools that could be used to evaluate other (related) interventions, including oral therapy as an alternative to i.v. antibiotics. 66 The simulation model could be used to aid commissioning decisions. For example, with knowledge of patient numbers and local geography, the models can inform the optimal configuration of services and staffing levels. The DCE methods could be applied to inform the development of other local services to ensure that they meet local preferences.
In summary, our specific recommendations for further research in order of priority are:
-
prospective observational multicentre studies to collect information of outcomes, adverse events and costs of OPAT, including mixed models of care
-
the development of a patient-reported outcome measure which could be used to standardise research in OPAT
-
an assessment of how stated preferences in health could be calibrated to better reflect revealed preferences to facilitate service design and planning, in particular, direct costs to the patient
-
an evaluation of simulation model as a tool to plan OPAT services in different areas.
Acknowledgements
Contributions of authors
Jane Minton (Consultant in Infectious Diseases and Honorary Clinical Associate Professor) contributed clinical expertise and wrote the introduction and discussion chapters of the report.
Carolyn Czoski Murray (Senior Research Fellow in Applied Health) led the survey and interviews with health-care professionals and wrote Chapter 3 of the report. She also contributed to the systematic review and analysis of patient interviews.
David Meads (Associate Professor of Health Economics) led the economic modelling work and prepared the results for publication. He also contributed to the development of the DCE survey and the interpretation of the health economics literature.
Stephane Hess (Professor of Choice Modelling) led the choice modelling workstream and analysed the DCE.
Armando Vargas-Palacios (Research Fellow) conducted the health economics analysis, in particular the simulation modelling, and prepared the results for publication.
Elizabeth Mitchell (Senior Research Fellow) carried out the systematic review and wrote Chapter 2 of the report.
Judy Wright (Senior Information Specialist) carried out the literature searches for the systematic review.
Claire Hulme (Professor of Health Economics) advised on the health economic work and the preparation of the final report.
David K Raynor (Professor of Pharmacy Practice) advised on community pharmacy aspects of the project, contributed to the analysis of the health professional interviews and the expert panel review.
Angela Gregson (Clinical Pathway Lead for Community i.v. Antibiotics Service) advised on community nursing aspects of the project, including costs and patient pathways for the economic evaluation.
Philip Stanley (Consultant in Infectious Diseases) contributed clinical expertise in OPAT, in particular to the economic modelling and development of the DCE and contributed to Chapters 1 and 8 of the report.
Kate McLintock (Clinical Lecturer in Primary Care) contributed clinical expertise from a community perspective and took part in the Expert panel review.
Rachel Vincent (Lead Pharmacist in Infectious Diseases, OPAT and HIV) contributed expertise on antimicrobials and devices used for OPAT and costs for the economic evaluation.
Maureen Twiddy (Senior Research Fellow in Applied Health) led the qualitative workstream (patient interviews) and wrote up Chapter 4 for publication. She contributed to the development of the DCE survey and DCE choice modelling report, wrote the expert panel chapter and acted as PPI lead and project manager.
Other key contributors to the research
Miss Samantha Mason and Mrs Janine Heeley (DCE data collection).
Dr Jill Edwards and Dr Janine Bestall (health professionals study).
Research nurses at all seven sites.
Patient advisory group
Mrs Heather Gent, Mrs Andrea McGowan, Mr Paul Parmenter and Mr Christopher Townsley.
Independent Steering Committee Members
Professor Jenny Hewison (chairperson), Dr Ann Chapman, Dr Philip Howard, Dr Claire McKenna, Dr Sue Pavitt, Dr Jonathan Sandoe and Dr Barbara Summers.
Publications
Czoski Murray CJ, Mitchell ED, Twiddy M, Wright J, Meads D, Minton J. Outpatient Parenteral Antimicrobial Therapy (OPAT): A Systematic Review of Models of Care. Paper presented at the Federation of Infection Societies 2014, Harrogate, 24–26 November 2014.
Czoski-Murray CJ, Mitchell ED, Twiddy M, Wright J, Meads D, Minton J. Outpatient Parenteral Antimicrobial Therapy (OPAT): A Systematic Review of Models of Care. Poster presented at the OPAT 2015 National Conference, London, 13 April 2015.
Czoski Murray C, Twiddy M, Meads D, Hess S, Wright J, Mitchell ED, et al. Community intravenous Antibiotic Study (CIVAS): a protocol for an evaluation of patient preferences for and cost-effectiveness of community intravenous antibiotic services. BMJ Open 2015;5:e008965.
Data sharing statement
All data can be obtained from Dr Maureen Twiddy. Data will be archived at the University of Leeds for 15 years.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis 2010;51:198-20. http://dx.doi.org/10.1086/653520.
- Kieran J, O’Reilly A, Parker J, Clarke S, Bergin C. Self-administered outpatient parenteral antimicrobial therapy: a report of three years experience in the Irish healthcare setting. Eur J Clin Microbiol Infect Dis 2009;28:1369-74. http://dx.doi.org/10.1007/s10096-009-0794-5.
- Mackintosh C, White H, Seaton R. Outpatient parenteral antibiotic therapy (OPAT) for bone and joint infections: experience from a UK teaching hospital-based service. J Antimicrob Chemother 2011;66:408-15. http://dx.doi.org/10.1093/jac/dkq445.
- Nazarko L. Providing outpatient antibiotic therapy for cellulitis in primary care. Br J Community Nurs 2008;13:520-4. http://dx.doi.org/10.12968/bjcn.2008.13.11.31524.
- Yan YM, Singh M, Tonks K, Kavi J, Langford NJ. Delivering outpatient antibiotic therapy (OPAT) in an Acute Medical Unit. Acute Med 2011;10:22-5.
- Gilchrist M, Franklin BD, Patel JP. An outpatient parenteral antibiotic therapy (OPAT) map to identify risks associated with an OPAT service. J Antimicrob Chemother 2008;62:177-83. http://dx.doi.org/10.1093/jac/dkn152.
- Török ME, Chapman AL, Lessing MP, Sanderson F, Seaton RA. Outpatient parenteral antimicrobial therapy: recent developments and future prospects. Curr Opin Investig Drugs 2010;11:929-39.
- Bazaz R, Chapman A, Winstanley T. Ertapenem administered as outpatient parenteral antibiotic therapy for urinary tract infections caused by extended-spectrum-beta-lactamase-producing Gram-negative organisms. J Antimicrob Chemother 2010;65:1510-13. http://dx.doi.org/10.1093/jac/dkq152.
- Tice AD, Rehm SJ. Meeting the challenges of methicillin-resistant Staphylococcus aureus with outpatient parenteral antimicrobial therapy. Clin Infect Dis 2010;51:171-5. http://dx.doi.org/10.1086/653517.
- Seaton RA, Bell E, Gourlay Y, Semple L. Nurse-led management of uncomplicated cellulitis in the community: evaluation of a protocol incorporating intravenous ceftriaxone. J Antimicrob Chemother 2005;55:764-7. http://dx.doi.org/10.1093/jac/dki092.
- Sharma R, Loomis W, Brown RB. Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy. Am J Med Sci 2005;330:60-4. http://dx.doi.org/10.1097/00000441-200508000-00002.
- Corwin P, Toop L, McGeoch G, Than M, Wynn-Thomas S, Wells JE, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38309.447975.EB.
- Chapman AL, Dixon S, Andrews D, Lillie PJ, Bazaz R, Patchett JD. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother 2009;64:1316-24. http://dx.doi.org/10.1093/jac/dkp343.
- Guide to the Methods of Health Technology Appraisal. London: NICE; 2013.
- Marra CA, Frighetto L, Goodfellow AF, Wai AO, Chase ML, Nicol RE, et al. Willingness to pay to assess patient preferences for therapy in a Canadian setting. BMC Health Serv Res 2005;5. http://dx.doi.org/10.1186/1472-6963-5-43.
- Equity and Excellence: Liberating the NHS. London: DH; 2010.
- Creating a Patient-Led NHS. London: DH; 2005.
- Your Health, Your Care, Your Say. London: DH; 2006.
- Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J. ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart – Then Focus. J Antimicrob Chemother 2012;67:i51-63. http://dx.doi.org/10.1093/jac/dks202.
- Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer 2008;98:60-7. http://dx.doi.org/10.1038/sj.bjc.6604096.
- Esposito S, Noviello S, Leone S, Tice A, Seibold G, Nathwani D, et al. Registry. Outpatient parenteral antibiotic therapy (OPAT) in different countries: a comparison. Int J Antimicrob Agents 2004;24:473-8. http://dx.doi.org/10.1016/j.ijantimicag.2004.06.004.
- Rapoport BL, Sussmann O, Herrera MV, Schlaeffer F, Otero JC, Pavlovsky S, et al. Ceftriaxone plus once daily aminoglycoside with filgrastim for treatment of febrile neutropenia: early hospital discharge vs. Standard In-patient care. Chemotherapy 1999;45:466-76. http://dx.doi.org/10.1159/000007240.
- Krauth C, Jalilvand N, Welte T, Busse R. Cystic fibrosis: cost of illness and considerations for the economic evaluation of potential therapies. PharmacoEconomics 2003;21:1001-24. http://dx.doi.org/10.2165/00019053-200321140-00002.
- You JH, Lee GC, So RK, Cheung KW, Hui M. Linezolid versus vancomycin for prosthetic joint infections: a cost analysis. Infection 2007;35:265-70. http://dx.doi.org/10.1007/s15010-007-6304-8.
- Dall L, Peddicord T, Peterson S, Simmons T, Dall T. Hospitalist treatment of CAP and cellulitis using objective criteria to select patients. Infect Med 2003;20:379-99.
- Duncan CJ, Barr DA, Seaton RA. Outpatient parenteral antimicrobial therapy with ceftriaxone, a review. Int J Clin Pharm 2012;34:410-7. http://dx.doi.org/10.1007/s11096-012-9637-z.
- Goodwin DD, Hanson JC, Berry CP. The changing face of Canadian home parenteral therapy. J Infus Nurs 2002;25:372-8. http://dx.doi.org/10.1097/00129804-200211000-00005.
- Laupland KB, Gill MJ, Schenk L, Goodwin D, Davies HD. Outpatient parenteral antibiotic therapy: evolution of the Calgary adult home parenteral therapy program. Clin Invest Med 2002;25:185-90.
- Matthews P, Conlon C, Berendt A, Kayley J, Jefferies L, Atkins B, et al. Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother 2007;60:356-62. http://dx.doi.org/10.1093/jac/dkm210.
- Montalto M, Lui B, Mullins A, Woodmason K. Medically-managed Hospital in the Home: 7 year study of mortality and unplanned interruption. Aust Health Rev 2010;34:269-75. http://dx.doi.org/10.1071/AH09771.
- Williams DN. Home intravenous antibiotic therapy (HIVAT): indications, patients and antimicrobial agents. Int J Antimicrob Agents 1995;5:3-8. http://dx.doi.org/10.1016/0924-8579(94)00046-W.
- Wynn M, Dalovisio JR, Tice AD, Jiang X. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin-sensitive Staphylococcus aureus. South Med J 2005;98:590-5. http://dx.doi.org/10.1097/01.SMJ.0000145300.28736.BB.
- Seetoh T, Lye DC, Cook AR, Archuleta S, Chan M, Sulaiman Z, et al. An outcomes analysis of outpatient parenteral antibiotic therapy (OPAT) in a large Asian cohort. Int J Antimicrob Ag 2013;41:569-73. http://dx.doi.org/10.1016/j.ijantimicag.2013.01.015.
- Barr DA, Semple L, Seaton RA. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicrob Agents 2012;39:407-13. http://dx.doi.org/10.1016/j.ijantimicag.2012.01.016.
- Bamford KB, Desai M, Aruede MJ, Lawson W, Jacklin A, Franklin BD. Patients’ views and experience of intravenous and oral antimicrobial therapy: room for change. Injury 2011;42:24-7. http://dx.doi.org/10.1016/S0020-1383(11)70129-2.
- Chambers S, Gallagher K, Pithie A. Patient acceptability of home intravenous antibiotic therapy. N Z Med J 2004;117.
- Lehoux P. Patients’ perspectives on high-tech home care: a qualitative inquiry into the user-friendliness of four technologies. BMC Health Serv Res 2004;4. http://dx.doi.org/10.1186/1472-6963-4-28.
- Lane MA, Marschall J, Beekmann SE, Polgreen PM, Banerjee R, Hersh AL, et al. Outpatient parenteral antimicrobial therapy practices among adult infectious disease physicians. Infect Control Hosp Epidemiol 2014;35:839-44. http://dx.doi.org/10.1086/676859.
- Lehoux P, Richard L, Pineault R, Saint-Arnaud J. Delivery of high-tech home care by hospital-based nursing units in Quebec: clinical and technical challenges. Nurs Leadersh (Tor Ont) 2006;19:44-55. http://dx.doi.org/10.12927/cjnl.2006.18048.
- Barr DA, Semple L, Seaton RA. Self-administration of outpatient parenteral antibiotic therapy and risk of catheter-related adverse events: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2012;31:2611-19. http://dx.doi.org/10.1007/s10096-012-1604-z.
- Richards DA, Toop LJ, Epton MJ, McGeoch GR, Town GI, Wynn-Thomas SM, et al. Home management of mild to moderately severe community-acquired pneumonia: a randomised controlled trial. Med J Aust 2005;183:235-8.
- Wolter JM, Bowler SD, Nolan PJ, McCormack JG. Home intravenous therapy in cystic fibrosis: a prospective randomized trial examining clinical, quality of life and cost aspects. Eur Respir J 1997;10:896-900.
- Esmond G, Butler M, McCormack AM. Comparison of hospital and home intravenous antibiotic therapy in adults with cystic fibrosis. J Clin Nurs 2006;15:52-60. http://dx.doi.org/10.1111/j.1365-2702.2005.01236.x.
- Angel JV. Outpatient antibiotic therapy for elderly patients. HIAT Study Group. Am J Med 1994;97:43-9.
- Mauceri AA. Treatment of bone and joint infections utilizing a third-generation cephalosporin with an outpatient drug delivery device. HIAT Study Group. Am J Med 1994;97:14-22.
- Poretz DM. Treatment of serious infections with cefotaxime utilizing an outpatient drug delivery device: global analysis of a large-scale, multicenter trial. HIAT Study Group. Am J Med 1994;97:34-42. http://dx.doi.org/10.1016/0002-9343(94)90286-0.
- Poretz DM. Treatment of skin and soft-tissue infections utilizing an outpatient parenteral drug delivery device: a multicenter trial. HIAT Study Group. Am J Med 1994;97:23-7. http://dx.doi.org/10.1016/0002-9343(94)90284-4.
- Wolter JM, Cagney RA, McCormack JG. A randomized trial of home vs hospital intravenous antibiotic therapy in adults with infectious diseases. J Infect 2004;48:263-8. http://dx.doi.org/10.1016/S0163-4453(03)00135-X.
- Rehm S, Campion M, Katz DE, Russo R, Boucher HW. Community-based outpatient parenteral antimicrobial therapy (CoPAT) for Staphylococcus aureus bacteraemia with or without infective endocarditis: analysis of the randomized trial comparing daptomycin with standard therapy. J Antimicrob Chemother 2009;63:1034-42. http://dx.doi.org/10.1093/jac/dkp051.
- Sebban C, Dussart S, Fuhrmann C, Ghesquieres H, Rodrigues I, Geoffrois L, et al. Oral moxifloxacin or intravenous ceftriaxone for the treatment of low-risk neutropenic fever in cancer patients suitable for early hospital discharge. Support Care Cancer 2008;16:1017-23.
- Stein GE, Schooley SL, Havlichek DH, Nix DE. Outpatient intravenous antibiotic therapy compared with oral linezolid in patients with skin and soft tissue infections: a pharmacoeconomic analysis. Infect Dis Clin Pract 2008;16:235-39.
- Escalante CP, Rubenstein EB, Rolston KV. Outpatient antibiotic therapy for febrile episodes in low-risk neutropenic patients with cancer. Cancer Invest 1997;15:237-42. http://dx.doi.org/10.3109/07357909709039721.
- Thornton J, Elliott RA, Tully MP, Dodd M, Webb AK. Clinical and economic choices in the treatment of respiratory infections in cystic fibrosis: comparing hospital and home care. J Cyst Fibros 2005;4:239-47. http://dx.doi.org/10.1016/j.jcf.2005.08.003.
- Duncan CJ, Barr DA, Ho A, Sharp E, Semple L, Seaton RA. Risk factors for failure of outpatient parenteral antibiotic therapy (OPAT) in infective endocarditis. J Antimicrob Chemother 2013;68:1650-4. http://dx.doi.org/10.1093/jac/dkt046.
- Martone WJ, Lindfield KC, Katz DE. Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry. Int J Clin Pract 2008;62:1183-7. http://dx.doi.org/10.1111/j.1742-1241.2008.01824.x.
- Fernández-Avilés F, Carreras E, Urbano-Ispizua A, Rovira M, Martínez C, Gaya A, et al. Case-control comparison of at-home to total hospital care for autologous stem-cell transplantation for hematologic malignancies. J Clin Oncol 2006;24:4855-61. http://dx.doi.org/10.1200/JCO.2006.06.4238.
- Montalto M, Dunt D. Home and hospital intravenous therapy for two acute infections: an early study. Aust N Z J Med 1997;27:19-23. http://dx.doi.org/10.1111/j.1445-5994.1997.tb00908.x.
- Pond MN, Newport M, Joanes D, Conway SP. Home versus hospital intravenous antibiotic therapy in the treatment of young adults with cystic fibrosis. Eur Respir J 1994;7:1640-4. http://dx.doi.org/10.1183/09031936.94.07091640.
- Bedi P, Sidhu MK, Donaldson LS, Chalmers JD, Smith MP, Turnbull K, et al. A prospective cohort study of the use of domiciliary intravenous antibiotics in bronchiectasis. NPJ Prim Care Respir Med 2014;24. http://dx.doi.org/10.1038/npjpcrm.2014.90.
- Bradley JM, Wallace ES, Elborn JS, Howard JL, McCoy MP. An audit of the effect of intravenous antibiotic treatment on spirometric measures of pulmonary function in cystic fibrosis. Ir J Med Sci 1999;168:25-8. http://dx.doi.org/10.1007/BF02939576.
- Yang A, Fung R, Brunton J, Dresser L. Outpatient parenteral antimicrobial therapy for surgery patients: A comparison with previous standard of care. Can J Infect Dis Med Microbiol 2013;24:74-8.
- Lacroix A, Revest M, Patrat-Delon S, Lemaître F, Donal E, Lorléac’h A, et al. Outpatient parenteral antimicrobial therapy for infective endocarditis: a cost-effective strategy. Med Mal Infect 2014;44:327-30. http://dx.doi.org/10.1016/j.medmal.2014.05.001.
- Yong C, Fisher DA, Sklar GE, Li SC. A cost analysis of Outpatient Parenteral Antibiotic Therapy (OPAT): an Asian perspective. Int J Antimicrob Agents 2009;33:46-51. http://dx.doi.org/10.1016/j.ijantimicag.2008.07.016.
- Mazo S, Emparan C, Vallejo M, Soriano P. Hospital-in-the-home treatment of surgical infectious diseases: an economic analysis. Surg Infect 2007;8:567-74. http://dx.doi.org/10.1089/sur.2006.047.
- Teuffel O, Amir E, Alibhai S, Beyene J, Sung L. Cost effectiveness of outpatient treatment for febrile neutropaenia in adult cancer patients. Br J Cancer 2011;104:1377-83. http://dx.doi.org/10.1038/bjc.2011.101.
- Patanwala AE, Erstad BL, Nix DE. Cost-effectiveness of linezolid and vancomycin in the treatment of surgical site infections. Curr Med Res Opin 2007;23:185-93. http://dx.doi.org/10.1185/030079906X162700.
- Grayson ML, Silvers J, Turnidge J. Home intravenous antibiotic therapy. A safe and effective alternative to inpatient care. Med J Aust 1995;162:249-53.
- Hindes R, Winkler C, Kane P, Kunkel M, Poretz DM. Outpatient intravenous antibiotic therapy in Medicare patients: Cost-savings analysis. Infec Dis Clin Pract 1995;4:211-8. http://dx.doi.org/10.1097/00019048-199505000-00016.
- Hitchcock J, Jepson AP, Main J, Wickens HJ. Establishment of an outpatient and home parenteral antimicrobial therapy service at a London teaching hospital: a case series. J Antimicrob Chemother 2009;64:630-4. http://dx.doi.org/10.1093/jac/dkp212.
- Huminer D, Bishara J, Pitlik S. Home intravenous antibiotic therapy for patients with infective endocarditis. Eur J Clin Microbiol 1999;18:330-4. http://dx.doi.org/10.1007/PL00015014.
- Johansson E, Bjorkholm M, Wredling R, Kalin M, Engervall P. Outpatient parenteral antibiotic therapy in patients with haematological malignancies. A pilot study of an early discharge strategy. Support Care Cancer 2001;9:619-24. http://dx.doi.org/10.1007/s005200100247.
- Kayley J, Berendt AR, Snelling MJ, Moore H, Hamilton HC, Peto TE, et al. Safe intravenous antibiotic therapy at home: experience of a UK based programme. J Antimicrob Chemother 1996;37:1023-9. http://dx.doi.org/10.1093/jac/37.5.1023.
- Martel A. Home intravenous self-injection of antibiotic therapy. Can J Infect Dis Med Microbiol 1994;5:51C-55C. http://dx.doi.org/10.1155/1994/673183.
- Nathwani D. The management of skin and soft tissue infections: outpatient parenteral antibiotic therapy in the United Kingdom. Chemotherapy 2001;47:17-23. http://dx.doi.org/10.1159/000048564.
- Nathwani D, Morrison J, Seaton RA, France AJ, Davey P, Gray K. Out-patient and home-parenteral antibiotic therapy (OHPAT): evaluation of the impact of one year’s experience in Tayside. Health Bull 1999;57:332-7.
- Rodríguez-Cerrillo M, Poza-Montoro A, Fernandez-Diaz E, Romero AI. Patients with uncomplicated diverticulitis and comorbidity can be treated at home. Eur J Intern Med 2010;21:553-4. http://dx.doi.org/10.1016/j.ejim.2010.09.002.
- Rodríguez-Cerrillo M, Poza-Montoro A, Fernandez-Diaz E, Iñurrieta-Romero A, Matesanz-David M. Home treatment of patients with acute cholecystitis. Eur J Intern Med 2012;23:e10-13. http://dx.doi.org/10.1016/j.ejim.2011.07.012.
- Seaton RA, Nathwani D, Williams FL, Boyter AC. Feasibility of an outpatient and home parenteral antibiotic therapy (OHPAT) programme in Tayside, Scotland. J Infect 1999;39:129-33. http://dx.doi.org/10.1016/S0163-4453(99)90004-X.
- Teuffel O, Cheng S, Ethier MC, Diorio C, Martino J, Mayo C, et al. Health-related quality of life anticipated with different management strategies for febrile neutropenia in adult cancer patients. Support Care Cancer 2012;20:2755-64. http://dx.doi.org/10.1007/s00520-012-1397-8.
- Tice A. The use of outpatient parenteral antimicrobial therapy in the management of osteomyelitis: data from the Outpatient Parenteral Antimicrobial Therapy Outcomes Registries. Chemotherapy 2001;47:5-16. http://dx.doi.org/10.1159/000048563.
- Tice AD. Experience with a physician-directed, clinic-based program for outpatient parenteral antibiotic therapy in the USA. Eur J Clin Microbiol Infect Dis 1995;14:655-61. http://dx.doi.org/10.1007/BF01690748.
- Al Ansari A, Al Alawi S, Al Qahtani M, Darwish A. Outpatient parenteral antimicrobial therapy (OPAT) in the Kingdom of Bahrain: efficacy, patient satisfaction and cost effectiveness. Open Infect Dis J 2013;7:90-5. http://dx.doi.org/10.2174/1874279301307010090.
- Esposito S, Leone S, Noviello S, Ianniello F, Fiore M, Russo M, et al. Outpatient parenteral antibiotic therapy for bone and joint infections: an Italian multicenter study. J Chemother 2007;19:417-22. http://dx.doi.org/10.1179/joc.2007.19.4.417.
- Bernard L, El-Hajj, Pron B, Lotthé A, Gleizes V, Signoret F, et al. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: evaluation of efficacy, tolerance and cost. J Clin Pharm Ther 2001;26:445-51. http://dx.doi.org/10.1046/j.1365-2710.2001.00380.x.
- Montalto M. Patients’ and carers’ satisfaction with hospital-in-the-home care. Int J Qual Health Care 1996;8:243-51. http://dx.doi.org/10.1016/1353-4505(96)00029-4.
- Parker SE, Nathwani D, O’Reilly D, Parkinson S, Davey PG. Evaluation of the impact of non-inpatient i.v. antibiotic treatment for acute infections on the hospital, primary care services and the patient. J Antimicrob Chemother 1998;42:373-80. http://dx.doi.org/10.1093/jac/42.3.373.
- Talcott JA, Whalen A, Clark J, Rieker PP, Finberg R. Home antibiotic therapy for low-risk cancer patients with fever and neutropenia: a pilot study of 30 patients based on a validated prediction rule. J Clin Oncol 1994;12:107-14.
- Pilling M, Walley T. Parenteral antibiotics at home in cystic fibrosis: experiences and attitudes of recipients. Health Soc Care Community 1997;5:209-12.
- Cox AM, Malani PN, Wiseman SW, Kauffman CA. Home intravenous antimicrobial infusion therapy: a viable option in older adults. J Am Geriatr Soc 2007;55:645-50. http://dx.doi.org/10.1111/j.1532-5415.2007.01133.x.
- Seaton RA, Nathwani D. Outpatient and home parenteral antibiotic therapy (OHPAT) in the UK: survey of infection specialists’ experience and views. Clin Microbiol Infect 2000;6:387-90. http://dx.doi.org/10.1046/j.1469-0691.2000.00112.x.
- Muldoon EG, Allison GM, Gallagher D, Snydman DR, Bergin C. Outpatient parenteral antimicrobial therapy (OPAT) in the Republic of Ireland: results of a national survey. Eur J Clin Microbiol Infect Dis 2013;32:1465-70. http://dx.doi.org/10.1007/s10096-013-1899-4.
- Muldoon EG, Switkowski K, Tice A, Snydman DR, Allison GM. A national survey of infectious disease practitioners on their use of outpatient parenteral antimicrobial therapy (OPAT). Infect Dis 2015;47:39-45. http://dx.doi.org/10.3109/00365548.2014.967290.
- Chapman AL, Seaton RA, Cooper MA, Hedderwick S, Goodall V, Reed C, et al. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statement. J Antimicrob Chemother 2012;67:1053-62. http://dx.doi.org/10.1093/jac/dks003.
- Seaton A. The UK OPAT Initiative – Then, Now & In the Future, Overview of 2013 Audit 2013. https://s3-eu-west-1.amazonaws.com/opat-national-conference-2013/Dr-Andrew-Seaton-The-UK-OPAT-Initiative.pdf (accessed 14 September 2016).
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Francis R. Report of the Mid Staffordshire NHS Foundation Trust Public Enquiry. London: The Stationery Office; 2013.
- Keogh B. Review into the Quality of Care and Treatment Provided by 14 Hospital Trusts in England: Overview Report. London: NHS England; 2013.
- Halilovic J, Christensen CL, Nguyen HH. Managing an outpatient parenteral antibiotic therapy team: challenges and solutions. Ther Clin Risk Manag 2014;10:459-65. http://dx.doi.org/10.2147/TCRM.S48906.
- Dubois A, Santos-Eggimann B. Evaluation of patients’ satisfaction with hospital-at-home care. Eval Health Prof 2001;24:84-98. http://dx.doi.org/10.1177/01632780122034812.
- Ryan M. Using Consumer Preferencs in Health Care Decision Making. The Application of Conjoint Analysis. London: Office of Health Economics; 1996.
- Department for Communities and Local Government . Index of Multiple Deprivation 2010, Local Authority District Rank of Average Rank n.d. http://opendatacommunities.org/data/societal-wellbeing/deprivation/imd-rank-la-2010 (accessed 19 October 2015).
- Coast J, Al-Janabi H, Sutton E, Horrocks S, Vosper A, Swancutt D, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ 2011;6:730-41.
- Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. London: Sage; 1990.
- Green J, Thorogood N. Qualitative Methods for Health Research. London: Sage; 2013.
- Baldie DJ, Entwistle VA, Davey PG. The information and support needs of patients discharged after a short hospital stay for treatment of low-risk community acquired pneumonia: implications for treatment without admission. BMC Pulm Med 2008;8. http://dx.doi.org/10.1186/1471-2466-8-11.
- Entwistle V, Firnigl D, Ryan M, Francis J, Kinghorn P. Which experiences of health care delivery matter to service users and why? A critical interpretive synthesis and conceptual map. J Health Serv Res Policy 2012;17:70-8. http://dx.doi.org/10.1258/jhsrp.2011.011029.
- Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al. A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic. Health Technol Assess 2002;6. http://dx.doi.org/10.3310/hta6270.
- Operational Guidance to the NHS – Extending Patient Choice of Provider. London: DH; 2011.
- Ziebland S, Hunt K. Using secondary analysis of qualitative data of patient experiences of health care to inform health services research and policy. J Health Serv Res Policy 2014;19:177-82. http://dx.doi.org/10.1177/1355819614524187.
- Patient and Public Engagement in the New Commissioning System: Discussion Paper. London: NHS Confederation; 2011.
- Health and Social Care Bill. London: The Stationery Office; 2011.
- Jones G, Cumming D, Gilchrist M, Seaton R. Outpatient antimicrobial therapy (response). BMJ 2013;346.
- Davey P, Wilcox M, Irving W, Thwaites G. Antimicrobial Chemotherapy. Oxford: Oxford University Press; 2005.
- Chapman AL. Outpatient parenteral antimicrobial therapy. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1585.
- de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145-72. http://dx.doi.org/10.1002/hec.1697.
- Morgan H, Hoddinott P, Thomson G, Crossland N, Farrar S, Yi D, et al. Benefits of Incentives for Breastfeeding and Smoking cessation in pregnancy (BIBS): a mixed-methods study to inform trial design. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19300.
- Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, et al. Clinical effectiveness, cost-effectiveness and service users’ perceptions of early, well-resourced communication therapy following a stroke: a randomised controlled trial (the ACT NoW Study). Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16260.
- Lanscar E, Burge P, Hess S, Daly A. Handbook of Choice Modelling. Cheltenham: Edward Elgar Publishing; 2014.
- Willis G. Cognitive Interviewing: A Tool for Improving Questionnaire Design. London: Sage; 2005.
- Kløjgaard ME, Hess S. Understanding the formation and influence of attitudes in patients’ treatment choices for lower back pain: testing the benefits of a hybrid choice model approach. Soc Sci Med 2014;114:138-50. http://dx.doi.org/10.1016/j.socscimed.2014.05.058.
- Edwards AL, Kenney KC. A comparison of the Thurstone and Likert techniques of attitude scale construction. J Appl Psychol 1946;30:72-83. http://dx.doi.org/10.1037/h0062418.
- Rose JM, Bliemer MCJ, Hess S, Daly A. Handbook of Choice Modelling. Cheltenham: Edward Elgar Publishing; 2014.
- Train KE. Discrete Choice Methods in Simulation. Cambridge, MA: Cambridge University Press; 2009.
- Abou-Zeid M, Ben-Akiva M, Hess S, Daly A. Handbook of Choice Modelling. Cheltenham: Edward Elgar Publishing; 2014.
- Vij A, Walker J, Hess S, Daly A. Handbook of Choice Modelling. Cheltenham: Edward Elgar Publishing; 2014.
- Field A. Discovering Statistics Using IBM SPSS Statistics. London: Sage; 2013.
- Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics 2014;32:883-902. http://dx.doi.org/10.1007/s40273-014-0170-x.
- Higgins A, Barnett J, Meads C, Singh J, Longworth L. Does convenience matter in health care delivery? A systematic review of convenience-based aspects of process utility. Value Health 2014;17:877-87. http://dx.doi.org/10.1016/j.jval.2014.08.2670.
- Freeman GK, Olesen F, Hjortdahl P. Continuity of care: an essential element of modern general practice?. Fam Pract 2003;20:623-7. http://dx.doi.org/10.1093/fampra/cmg601.
- Cabana MD, Jee SH. Does continuity of care improve patient outcomes?. J Fam Pract 2004;53:974-80.
- Hill KM. Understanding and Measuring Continuity of Care in Stroke. Leeds: University of Leeds; 2008.
- Harrison M, Rigby D, Vass C, Flynn T, Louviere J, Payne K. Risk as an attribute in discrete choice experiments: a systematic review of the literature. Patient 2014;7:151-70. http://dx.doi.org/10.1007/s40271-014-0048-1.
- Ajzen I. Residual effects of past on later behaviour. Habituation and reasoned action perspectives. Pers Soc Psychol Rev 2002;6:107-22. http://dx.doi.org/10.1207/S15327957PSPR0602_02.
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376-80. http://dx.doi.org/10.1136/bmj.311.7001.376.
- Lees T. The National Vascular Database Report 2009. London: The Vascular Society of Great Britain and Ireland; 2009.
- Seaton RA, Johal S, Coia JE, Reid N, Cooper S, Jones BL. Economic evaluation of treatment for MRSA complicated skin and soft tissue infections in Glasgow hospitals. Eur J Clin Microbiol Infect Dis 2014;33:305-11. http://dx.doi.org/10.1007/s10096-013-1956-z.
- Claxton KP, Sculpher MJ. Using value of information analysis to prioritise health research: some lessons from recent UK experience. PharmacoEconomics 2006;24:1055-68. http://dx.doi.org/10.2165/00019053-200624110-00003.
- Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P. The role of modelling in prioritising and planning clinical trials. Health Technol Assess 2003;7. http://dx.doi.org/10.3310/hta7230.
- Marshall DA, Burgos-Liz L, IJzerman MJ, Osgood ND, Padula WV, Higashi MK, et al. Applying dynamic simulation modeling methods in health care delivery research-the SIMULATE checklist: report of the ISPOR simulation modeling emerging good practices task force. Value Health 2015;18:5-16. http://dx.doi.org/10.1016/j.jval.2014.12.001.
- Goldie SJ, Corso PS, Haddix AC, Teutsch SM, Corso PS. Prevention Effectiveness. A Guide to Decision Analysis and Economic Evaluation. New York, NY: Oxford University Press; 2003.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices – overview a report of the ISPOR-SMDM modeling good research practices task force – 1. Med Decis Making 2012;32:667-77. http://dx.doi.org/10.1177/0272989X12454577.
- Claxton K, Neumann PJ, Araki S, Weinstein MC. Bayesian value-of-information analysis. An application to a policy model of Alzheimer’s disease. Int J Technol Assess Health Care 2001;17:38-55. http://dx.doi.org/10.1017/S0266462301104058.
- Hopf Y, Watson M, Williams D. Adverse-drug-reaction related admissions to a hospital in Scotland. Pharm World Sci 2008;30:854-62. http://dx.doi.org/10.1007/s11096-008-9240-5.
- Forster AJ, Taljaard M, Oake N, Wilson K, Roth V, van Walraven C. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. Can Med Assoc J 2012;184:37-42. http://dx.doi.org/10.1503/cmaj.110543.
- Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 2012;81:1-14. http://dx.doi.org/10.1016/j.jhin.2012.02.004.
- Thwaites GE. United Kingdom Clinical Infection Research Group (UKCIRG) . The management of Staphylococcus aureus bacteremia in the United Kingdom and Vietnam: a multi-centre evaluation. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0014170.
- Lillie PJ, Andrews D, Eaves K, Darton TC, Chapman AL. Baseline factors predicting the duration of intravenous antibiotic therapy for cellulitis in an outpatient setting. Eur J Clin Microbiol Infect Dis 2010;29:347-9. http://dx.doi.org/10.1007/s10096-009-0855-9.
- NHS Reference Costs 2013–14 England. London: DH; 2014.
- Curtis LA. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Royal College of Nursing . NHS Payscales for NHS Nursing Staff in England, Wales, Scotland and Northern Ireland from 1 April 2015 n.d. www.rcn.org.uk/support/pay_and_conditions/pay-rates-2015-16 (accessed 15 October 2015).
- Updated Guidance of the Diagnosis and Reporting of CDI. Best Practice Guidance. London: DH; 2012.
- Boyd O, Jackson N. How is risk defined in high-risk surgical patient management?. Crit Care 2005;9:390-6. http://dx.doi.org/10.1186/cc3057.
- Prytherch DR, Sutton GL, Boyle JR. Portsmouth POSSUM models for abdominal aortic aneurysm surgery. Br J Surg 2001;88:958-63. http://dx.doi.org/10.1046/j.0007-1323.2001.01820.x.
- Mason JM, Thomas KS, Crook AM, Foster KA, Chalmers JR, Nunn AJ, et al. Prophylactic antibiotics to prevent cellulitis of the leg: economic analysis of the PATCH I & II trials. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0082694.
- Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385:875-82. http://dx.doi.org/10.1016/S0140-6736(14)61233-2.
- Konijeti GG, Sauk J, Shrime MG, Gupta M, Ananthakrishnan AN. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis 2014;58:1507-14. http://dx.doi.org/10.1093/cid/ciu128.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7. http://dx.doi.org/10.3132/pcrj.2007.00002.
- Drummond MF, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Marmor YN, Rohleder TR, Cook DJ, Huschka TR, Thompson JE. Recovery bed planning in cardiovascular surgery: a simulation case study. Health Care Manag Sci 2013;16:314-27. http://dx.doi.org/10.1007/s10729-013-9231-5.
- Troy PM, Rosenberg L. Using simulation to determine the need for ICU beds for surgery patients. Surgery 2009;146:608-17. http://dx.doi.org/10.1016/j.surg.2009.05.021.
- Cipriano LE, Chesworth BM, Anderson CK, Zaric GS. An evaluation of strategies to reduce waiting times for total joint replacement in Ontario. Med Care 2008;46:1177-83. http://dx.doi.org/10.1097/MLR.0b013e31817925e8.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. http://dx.doi.org/10.1002/hec.903.
- Wilson EC. A practical guide to value of information analysis. PharmacoEconomics 2015;33:105-21. http://dx.doi.org/10.1007/s40273-014-0219-x.
- Barr D, Seaton R. Outpatient parenteral antimicrobial therapy (OPAT) and the general physician. Clin Med 2013;13:495-9. http://dx.doi.org/10.7861/clinmedicine.13-5-495.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. http://dx.doi.org/10.1177/0272989X13505910.
- Caplan GA, Ward JA, Brennan NJ, Coconis J, Board N, Brown A. Hospital in the home: a randomised controlled trial. Med J Aust 1999;170:156-60.
- Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLOS Med 2015;12. http://dx.doi.org/10.1371/journal.pmed.1001825.
- Thaler R, Sustein C. Nudge: Improving Decisions about Health, Wealth and Happiness. New York, NY: Penguin; 2008.
- Hawkes N. Finding the techniques to nudge the population to better health. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d389.
- Bonell C, McKee M, Fletcher A, Wilkinson P, Haines A. One nudge forward, two steps back. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d401.
- NHS Trust Development Authority . Five Year Forward View 2014. www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 20 October 2015).
- Davies SC. Annual Report of the Chief Medical Officer, Volume 2, 2011: Infections and the Rise of Antimicrobial Resistance. London: Department of Health; 2011.
- Graninger W, Wenisch C, Wiesinger E, Menschik M, Karimi J, Presterl E. Experience with outpatient intravenous teicoplanin therapy for chronic osteomyelitis. Eur J Clin Microbiol Infect Dis 1995;14:643-7. http://dx.doi.org/10.1007/BF01690746.
- Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother 2015;70:965-70. http://dx.doi.org/10.1093/jac/dku517.
- Closing the NHS Funding Gap: How to get Better Value Health Care for Patients. London: Monitor; 2013.
- Quarterly Analyses: Mandatory MRSA, MSSA and E. coli bacteraemia and C. difficile in England (up to July-September 2015). London: Public Health England; 2015.
- Wye L, Brangan E, Cameron A, Gabbay J, Klein J, Pope C. Knowledge Exchange in Health-Care Commissioning: Case Studies of the Use of Commercial, Not-For-Profit and Public Sector Agencies, 2011–14. Southampton: Health Services and Delivery Research; 2015.
- Jones GR, Cumming DVE, Honeywell G, Ball R, Sanderson F, Seaton RA, et al. How is income generated by outpatient parenteral antibiotic treatment (OPAT) in the UK? Analysis of payment tariffs for cellulitis. J Antimicrob Chemother 2015;70:1236-40. http://dx.doi.org/10.1093/jac/dku541.
- Barker KL, Reid M, Minns Lowe CJ. What does the language we use about arthritis mean to people who have osteoarthritis? A qualitative study. Disabil Rehabil 2014;36:367-72. http://dx.doi.org/10.3109/09638288.2013.793409.
- Anand V, Levine H, Friedman M, Krespi Y, Panje W, Schettino R, et al. Intravenous antibiotics for refractory rhinosinusitis in nonsurgical patients: preliminary findings of a prospective study. Am J Rhinol 2003;17:363-8.
- Berman SJ, Johnson EW. Out-patient parenteral antibiotic therapy (OPAT): clinical outcomes and adverse events. Hawaii Med J 2001;60:31-3.
- Chan DSG, Archuleta S, Llorin RM, Lye DC, Fisher D. Standardized outpatient management of Klebsiella pneumoniae liver abscesses. Int J Infect Dis 2013;17:e185-8.
- Dalovisio JR, Juneau J, Baumgarten K, Kateiva J. Financial impact of a home intravenous antibiotic program on a medicare managed care program. Clin Infect Dis 2000;30:639-42.
- Dargan S, Zvonar RK, Saginur R. A review of outpatient parenteral antimicrobial therapy practices and experience at the Ottawa Hospital. Can J Hosp Pharm 2007;60:177-83.
- Donald M, Marlow N, Swinburn E, Wu M. Emergency department management of home intravenous antibiotic therapy for cellulitis. Emerg Med J 2005;22:715-7.
- Esposito S, Leone S, Noviello S, Ianniello F, Russo M, Foti G, et al. Outpatient parenteral antibiotic therapy in the elderly: an Italian observational multicenter study. J Chemother 2009;21:193-8. http://dx.doi.org/10.1179/joc.2009.21.2.193.
- W, Presterl E, Wenisch C, Schwameis E, Breyer S, Vukovich T. Management of serious staphylococcal infections in the outpatient setting. Drugs 1997;54:21-8. http://dx.doi.org/10.2165/00003495-199700546-00006.
- Gross R, Graziani AL, Laufer D, Turner JL, Ondercin JP, Macgregor RR. Adverse effects of the use of intravenous pentamidine in the home. Infect Dis Clin Pract 1996;5:456-58.
- Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother 2010;65:2641-4. http://dx.doi.org/10.1093/jac/dkq355.
- Larioza J, Girard A, Brown RB. Clinical experience with daptomycin for outpatient parenteral antibiotic therapy. Am J Med Sci 2011;342:486-8.
- Larioza J, Heung L, Girard A, Brown RB. Management of infective endocarditis in outpatients: clinical experience with outpatient parenteral antibiotic therapy. Southern Med J 2009;102:575-9.
- Lopardo G. Management of endocarditis: outpatient parenteral antibiotic treatment in Argentina. Chemotherapy 2001;47:24-32.
- McMahon JH, O’Keeffe JM, Grayson ML. Victorian Hith Outcomes Study Group . Is hospital-in-the-home (HITH) treatment of bacterial endocarditis safe and effective?. Scand J Infect Dis 2008;40:40-3.
- Montalto M. An audit of patients admitted for home intravenous therapy directly from the emergency department. Int J Clin Pract 1997;51:433-7.
- Morales JO, Von Behren L. Secondary bacterial infections in HIV-infected patients: an alternative ambulatory outpatient treatment utilizing intravenous cefotaxime. Am J Med 1994;97:9-13.
- Murray H, Stiell I, Wells G. Treatment failure in emergency department patients with cellulitis. CJEM 2005;7:228-34.
- Nathwani D, Barlow GD, Ajdukiewicz K, Gray K, Morrison J, Clift B, et al. Cost-minimization analysis and audit of antibiotic management of one and joint infections with ambulatory teicoplanin, in-patient care or outpatient oral linezolid therapy. J Antimicrob Chemother 2003;51:391-6. http://dx.doi.org/10.1093/jac/dkg061.
- Partridge DG, O’Brien E, Chapman ALN. Outpatient parenteral antibiotic therapy for infective endocarditis: a review of 4 years’ experience at a UK centre. Postgrad Med J 2012;88:377-81.
- Pérez-López J, San José Laporte A, Pardos-Gea J, Tapia Melenchón E, Lozano Ortín E, Barrio Guirado A, et al. Safety and efficacy of home intravenous antimicrobial infusion therapy in older patients: a comparative study with younger patients. Int J Clin Pract 2008;62:1188-92. http://dx.doi.org/10.1111/j.1742-1241.2008.01747.x.
- Seaton RA, MacConnachie AA. Experience with daptomycin in an infectious diseases service over 1 year: utility in an outpatient parenteral antibiotic programme. Int J Antimicrob Agents 2008;31:492-7.
- Seaton RA, Sharp E, Bezlyak, Weir CJ. Factors associated with outcome and duration of therapy in outpatient parenteral antibiotic therapy (OPAT) patients with skin and soft-tissue infections. Int J Antimicrob Agents 2011;38:243-8. http://dx.doi.org/10.1016/j.ijantimicag.2011.05.008.
- South R. Retrospective study of teicoplanin as home continuation of hospital-initiated therapy. Int J Antimicrob Agents 1998;9:219-25.
- Theocharis G, Rafailidis PI, Rodis D, Kontopidis I, Barbas SG, Falagas ME. Outpatient parenteral antibiotic therapy (OPAT) at home in Attica, Greece. Eur J Clin Microbiol Infect Dis 2012;31:2957-61. http://dx.doi.org/10.1007/s10096-012-1647-1.
- Upton A, Ellis-Pegler RB, Woodhouse A. Outpatient Parenteral Antimicrobial Therapy (OPAT): a review of experience at Auckland Hospital. N Z Med J 2004;117.
- Walton AL, Howden BP, Grayson LM, Korman TM. Continuous-infusion penicillin home-based therapy for serious infections due to penicillin-susceptible pathogens. Int J Antimicrob Agents 2007;29:544-8. http://dx.doi.org/10.1016/j.ijantimicag.2006.10.018.
- White HA, Davis JS, Kittler P, Currie BJ. Outpatient parenteral antimicrobial therapy-treated bone and joint infections in a tropical setting. Intern Med J 2011;41:668-73. http://dx.doi.org/10.1111/j.1445-5994.2009.02136.x.
- White B, Seaton RA, Evans TJ. Management of suspected Lyme borreliosis: experience from an outpatient parenteral antibiotic therapy service. QJM 2013;106:133-8. http://dx.doi.org/10.1093/qjmed/hcs189.
- Yadlapalli NG, Vaishnav A, Sheehan P. Conservative management of diabetic foot ulcers complicated by osteomyelitis. Wounds 2002;14:31-5.
- Htin AK, Friedman ND, Hughes A, O’Brien DP, Huffam S, Redden AM, et al. Outpatient parenteral antimicrobial therapy is safe and effective for the treatment of infective endocarditis: a retrospective cohort study. Intern Med J 2013;43:700-5. http://dx.doi.org/10.1111/imj.12081.
- Lai A, Tran T, Nguyen HM, Fleischmann J, Beenhouwer DO, Graber CJ. Outpatient parenteral antimicrobial therapy at large Veterans Administration medical center. Am J Manag Care 2013;19:e317-24.
- Mohammadi S, MacKay K, Ward TT, Forrest GN. Clinical outcomes of a veterans affairs outpatient antimicrobial treatment program. South Med J 2013;106:345-9. http://dx.doi.org/10.1097/SMJ.0b013e3182967e8f.
- Sims AL, Baker P, Bellamy R, McMurtry IA. Outpatient parenteral antibiotic therapy in primary hip and knee arthroplasty infection managed with debridement and retention of prosthesis: a retrospective cohort study. Surg Infect 2013;14:293-6. http://dx.doi.org/10.1089/sur.2012.078.
- Subedi S, Looke DF, McDougall DA, Sehu MM, Playford EG. Supervised self-administration of outpatient parenteral antibiotic therapy: a report from a large tertiary hospital in Australia. Int J Infect Dis 2015;30:161-5. http://dx.doi.org/10.1016/j.ijid.2014.11.021.
- Keller SC, Ciuffetelli D, Bilker W, Norris A, Timko D, Rosen A, et al. The Impact of an Infectious Diseases Transition Service on the Care of Outpatients on Parenteral Antimicrobial Therapy. J Pharm Technol 2013;29:205-14. http://dx.doi.org/10.1177/8755122513500922.
- Amodeo MR, Clulow T, Lainchbury J, Murdoch DR, Gallagher K, Dyer A, et al. Outpatient intravenous treatment for infective endocarditis: safety, effectiveness and one-year outcomes. J Infect 2009;59:387-93. http://dx.doi.org/10.1016/j.jinf.2009.09.009.
- Cervera C, Del Rio A, Garcia L, Sala M, Almela M, Moreno A, et al. Efficacy and safety of outpatient parenteral antibiotic therapy for infective endocarditis: a ten-year prospective study. Enferm Infec Microbiol Clin 2011;29:587-92. http://dx.doi.org/10.1016/j.eimc.2011.05.007.
- Chambers S, Gallagher K, Pithie A. Home intravenous antimicrobial service – twelve months experience in Christchurch. N Z Med J 2002;115:216-18.
- Cheong EA, Katelaris CH, Sisson CM, Anderson EA, Byth K. Adverse drug reactions associated with home parenteral therapy. J Pharm Pract Res 2008;38:267-70. http://dx.doi.org/10.1002/j.2055-2335.2008.tb00386.x.
- Dobson PM, Boyle M, Loewenthal M. Home intravenous antibiotic therapy and allergic drug reactions: is there a case for routine supply of anaphylaxis kits?. J Infus Nurs 2004;27:425-30. http://dx.doi.org/10.1097/00129804-200411000-00008.
- Gourdeau M, Deschênes L, Caron M, Desmarais M. Home iv antibiotic therapy through a medical day care unit. Can J Infect Dis 1993;4:158-62. http://dx.doi.org/10.1155/1993/412737.
- Heintz BH, Halilovic J, Christensen CL. Impact of a multidisciplinary team review of potential outpatient parenteral antimicrobial therapy prior to discharge from an academic medical center. Ann Pharmacother 2011;45:1329-37. http://dx.doi.org/10.1345/aph.1Q240.
- Lin JW, Kacker A, Anand VK, Levine H. Catheter- and antibiotic-related complications of ambulatory intravenous antibiotic therapy for chronic refractory rhinosinusitis. Am J Rhinol 2005;19:365-9.
- Smego RA, Khan MA, Khowaja K, Rafique R, Datoo F. A university-sponsored Home Health Nursing Program in Karachi, Pakistan. Home Healthc Nurse 2005;23:710-16. http://dx.doi.org/10.1097/00004045-200511000-00007.
- Tice AD, Hoaglund PA, Nolet B, McKinnon PS, Mozaffari E. Cost perspectives for outpatient intravenous antimicrobial therapy. Pharmacotherapy 2002;22:63S-70S. http://dx.doi.org/10.1592/phco.22.4.63S.33653.
- Allison GM, Muldoon EG, Kent DM, Paulus JK, Ruthazer R, Ren A, et al. Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014;58:812-19. http://dx.doi.org/10.1093/cid/cit920.
- Barr DA, Irvine S, Ritchie ND, McCutcheon J, Seaton RA. Risk of venous thromboembolism in patients treated for bacterial infection in the community with outpatient parenteral antimicrobial therapy. QJM 2014;107:207-11. http://dx.doi.org/10.1093/qjmed/hct239.
- Pajarón M, Fernández-Miera MF, Allende I, Arnaiz AM, Gutiérrez-Cuadra M, Cobo-Belaustegui M, et al. Self-administered outpatient parenteral antimicrobial therapy (S-OPAT) for infective endocarditis: a safe and effective model. Eur J Intern Med 2015;26:131-6. http://dx.doi.org/10.1016/j.ejim.2015.01.001.
- Hess S, Train KE, Polak JW. On the use of a Modified Latin Hypercube Sampling (MLHS) approach in the estimation of a Mixed Logit model for vehicle choice. Trans Res B 2006;40:147-63. http://dx.doi.org/10.1016/j.trb.2004.10.005.
- Huber PJ. The Behavior of Maximum Likelihood Estimates under Nonstandard Conditions. Proc Fifth Berkeley Symp Math Stat Prob 1967;1:221-33.
- Health and Social Care Act 2012. London: The Stationery Office; 2012.
- Briefing Notes for Researchers: Involving the Public in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2012.
- Popay J, Collins M. The Public Involvement Impact Assessment Framework Guidance. Universities of Lancaster, Liverpool and Exeter; 2014.
Appendix 1 Community IntraVenous Antibiotic Study systematic review
| Database | Dates |
|---|---|
| BSAC | Accessed March 2015 (http://bsac.org.uk/) |
| CINAHL (via EBSCOhost) | 1981 to March 2015 |
| The Cochrane Library (via Wiley Online Library) | Accessed 25 March 2015 |
| EMBASE Classic + EMBASE (via Ovid) | 1947 to 25 March 2015 |
| Health Business Elite (via HDAS, NHS Evidence) | 1922 to 25 March 2015 |
| Health Management Information Consortium (via Ovid) | 1983 to 25 March 2015 |
| International Pharmaceutical Abstracts (via Ovid) | 1970 to March 2015 |
| Ovid MEDLINE(R) | 1946 to March Week 4, 2015 |
| Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations | 25 March 2015 |
| Research Papers in Economics | Accessed March 2015 (http://ideas.repec.org/) |
| CEA Registry | Accessed March 2015 (https://research.tufts-nemc.org/cear4/Default.aspx) |
| Web of Science Conference Proceedings Citation Index – Science (via Thomson Reuters) | 1990 to March 2015 |
Sample search A: studies of intravenous antibiotics and known models of care
Ovid MEDLINE(R)
Date range searched: 1946 to March week 4, 2015.
-
exp Anti-Bacterial Agents/ (497,300)
-
Anti-Infective Agents/ (36,221)
-
exp Anti-infective agents, Urinary/ (28,162)
-
exp antifungal agents/ (126,501)
-
or/1–4 (612,854)
-
Administration, Intravenous/ (151)
-
infusions, intravenous/ (45,941)
-
injections, intravenous/ (75,646)
-
Home infusion therapy/ (579)
-
or/6–9 (120,138)
-
((parenteral or intravenous or IV or inject* or infusion*) adj5 (antibiotic* or anti-biotic* or antimicrobial* or anti-microbial* or antifungal* or anti-fungal* or anti-infective* or antiinfective* or antibiotherap*or anti-biotherap*)).tw. (10,477)
-
(5 and 10) or 11 (20,711)
-
exp Ambulatory Care Facilities/ (41,613)
-
exp Delivery of healthcare/ (739,029)
-
Critical Pathways/ (4202)
-
Ambulatory Care/ (34,170)
-
Emergency Service, Hospital/ (39,312)
-
outpatients/ (7955)
-
inpatients/ (11,488)
-
day care/ (4526)
-
inpatients/ (11,488)
-
hospitalization/ (66,091)
-
community health services/ (25,779)
-
community health nursing/ (17,982)
-
home care services/ (26,544)
-
home care services, hospital-based/ (1552)
-
home nursing/ (7802)
-
home infusion therapy/ (579)
-
or/13–28 (936,219)
-
12 and 29 (1550)
-
(‘emergency ward*’ or ‘emergency room*’ or ‘accident and emergency’ or ED or ‘emergency department*’ or A&E).tw. (77,291)
-
outpatient*.tw. (95,608)
-
(home or homes).tw. (141,950)
-
(self adj3 (treat* or care or regime*)).tw. (14,552)
-
((clinic or clinics) adj3 (treat* or care or regime*)).tw. (11,443)
-
(community adj3 (treat* or care or regime*)).tw. (15,994)
-
(ambulatory adj3 (treat* or care or regime*)).tw. (11,257)
-
‘district nurs*’.tw. (1351)
-
(‘specialist nurs*’ or ‘nurse specialist*’).tw. (3498)
-
‘community nurs*’.tw. (2437)
-
((hospital* or ward or clinic) adj2 (patient or patients)).tw. (58,637)
-
inpatient*.tw. (54,156)
-
(‘care pathway*’ or ‘care model*’ or ‘model* of care’).tw. (6275)
-
or/31–43 (438,938)
-
44 and 12 (2102)
-
‘outpatient parenteral antibiotic* therapy’.tw. (103)
-
‘outpatient antibiotic* therapy’.tw. (64)
-
(ohpat or opat).tw. (116)
-
or/45–48 (2140)
-
49 or 30 (2678)
Sample search B: reviews of intravenous antibiotic use in cellulitis or cystic fibrosis
Ovid MEDLINE(R)
Date range searched: 1946 to March week 4, 2015.
-
exp *Anti-Bacterial Agents/or *Anti-Infective Agents/or exp *antifungal agents/or exp *Anti-infective agents, Urinary/ (380,181)
-
*Administration, Intravenous/or *infusions, intravenous/or *injections, intravenous/or *Home infusion therapy/ (5005)
-
1 and 2 (324)
-
((parenteral or intravenous or IV or inject* or infusion*) adj5 (antibiotic* or anti-biotic* or antimicrobial* or anti-microbial* or antifungal* or anti-fungal* or anti-infective* or antiinfective* or antibiotherap*or anti-biotherap*)).ti. (1464)
-
3 or 4 (1649)
-
exp Anti-Bacterial Agents/ (498,780)
-
Anti-Infective Agents/ (36,330)
-
exp Anti-infective agents, Urinary/ (28,211)
-
exp antifungal agents/ (126,949)
-
or/6–9 (614,703)
-
Administration, Intravenous/ (177)
-
infusions, intravenous/ (46,068)
-
injections, intravenous/ (75,786)
-
Home infusion therapy/ (580)
-
or/11–14 (120,429)
-
((parenteral or intravenous or IV or inject* or infusion*) adj5 (antibiotic* or anti-biotic* or antimicrobial* or anti-microbial* or antifungal* or anti-fungal* or anti-infective* or antiinfective* or antibiotherap*or anti-biotherap*)).tw. (10,513)
-
(10 and 15) or 16 (20,758)
-
‘outpatient* parenteral’.tw. [finds more] (189)
-
‘outpatient parenteral antibiotic* therapy’.tw. (103)
-
‘outpatient antibiotic* therapy’.tw. (64)
-
(ohpat or opat).tw. (116)
-
Cellulitis/ (6046)
-
cellulitis.tw. (5439)
-
Cystic Fibrosis/ (27,005)
-
(cystic adj fibrosis).tw. (29,491)
-
‘fibrocystic disease*’.tw. (807)
-
17 or 19 or 20 or 21 (20,796)
-
or/22–26 (43,980)
-
(5 or (18 or 19 or 20 or 21)) and 28 [major headings or title occurrence of IV Antibiotic terms AND MINOR Cell/CF terms] (125)
-
17 and 28 [most sensitive search] (1037)
-
limit 30 to ‘review articles’ (150)
-
29 or 31 (254)
Community IntraVenous Antibiotic Study systematic review: quality assessment – observational studies
| OPAT model(s) | Inferior | Superior | No impact | Inconclusive |
|---|---|---|---|---|
| Duration of treatment (all models) | ◉ | |||
| Outpatient attendance vs. inpatient treatment22 | ● | |||
| SA vs. inpatient treatment42,43,58 | ● | |||
| GN vs. inpatient treatment12,41 | ● | |||
| SN vs. inpatient treatment25,56,57 | ◉ | |||
| Rate of cure or improvement (all models) | ◉ | |||
| Outpatient attendance vs. inpatient treatment22 | ● | |||
| SA vs. inpatient treatment59 | ● | |||
| GN vs. inpatient treatment12,41 | ● | |||
| SN vs. inpatient treatment56,57 | ◉ | |||
| Improved lung function in CF (all models) | ○ | |||
| SA vs. inpatient treatment for FEV142,43,58,60 | ● | |||
| SA vs. inpatient treatment for FVC42,43,58,60 | ○ | |||
| SA vs. inpatient treatment for PEFR58,60 | ○ | |||
| Drug-related side effects (all models) | ◉ | |||
| Outpatient attendance vs. inpatient treatment22 | ● | |||
| SA vs. inpatient treatment42,43,58,59 | ● | |||
| GN vs. inpatient treatment41 | ● | |||
| SN vs. inpatient treatment10,56,57,65 | ○ | |||
| Venous access complications (all models) | ◉ | |||
| SA vs. inpatient treatment42,43,59 | ◉ | |||
| GN vs. inpatient treatment41 | ● | |||
| Hospital admission (all models) | ○ | |||
| SA vs. inpatient treatment42,49 | ● | |||
| GN vs. inpatient treatment12,41 | ● | |||
| SN vs. inpatient treatment10,25,57,65 | ◉ | |||
| Deaths (all models) | ● | |||
| Outpatient attendance vs. inpatient treatment22 | ● | |||
| SA vs. inpatient treatment42,59 | ● | |||
| GN vs. inpatient treatment41 | ● | |||
| SN vs. inpatient treatment25,56 | ● |
| Author (country) | Study design | OPAT model(s) | OPAT group(s)a | Comparator(s)a | Findings |
|---|---|---|---|---|---|
| Comparator studies | |||||
| Bradley et al.60 (UK) | Observational (retrospective) | SA | Home therapy: 8 patients (mean age 26 years); 50% male | Combined therapy: 14 patients (mean age 24 years); 57% male Inpatient therapy: 29 patients (mean age 24 years); 83% male |
On completion of therapy, there was no significant difference between the groups in FEV1 (51 vs. 66 vs. 60), FVC (63 vs. 73 vs. 71) or FEF25–75 (32 vs. 43 vs. 29) PEFR was significantly greater in the OPAT and combined groups than in the hospital group (84 vs. 73 vs. 67; p < 0.05) When change was expressed as a percentage of the baseline value, FEF25–75 showed a large improvement in each group (approximately 15–48%). There was a significantly greater improvement in FVC, FEV1 and FEF25–75 in the hospital group than in the other two groups |
| Corwin et al.12 (New Zealand) | RCT | GN | Home therapy: 98 patients (mean age 55 years); 62% male | Inpatient therapy: 96 patients (mean age 48 years); 73% male | There was no significant difference in mean days to no advancement of cellulitis between home and hospital groups (1.50 days vs. 1.49 days; 95% CI –0.3 to 0.28) This was also the case for days on i.v. antibiotics (HR 0.84, 95% CI 0.63 to 1.12; p = 0.23) and days to discharge (HR 0.93, 95% CI 0.70 to 1.23; p = 0.60) |
| Dall et al.25 (USA) | Before and after | SN | Home therapy: 92 pneumonia patients (97% aged > 50 years); 44% male/64 cellulitis patients (mean age 51 years); 70% male | Inpatient therapy: 10728 pneumonia patients (83% aged > 50 years); 51% male/cellulitis patients (no numbers provided) | Most OPAT cellulitis patients (45/64; 70%) required only 2 day’s treatment (range 2–6 days) Among pneumonia patients, 58% (53/92) required ≤ 3 days of treatment (range 2–9 days) Median length of stay was shorter for OPAT pneumonia patients than for historical comparators (0.07–1.07 days vs. 2–4 days vs. 6–9 days; p < 0.05). This was also the case for SSTI patients (0.1 day vs. 1.3 days; p < .001) |
| Escalante et al.52 (USA) | Clinical trial (unspecified) | Not specified | i.v. therapy: 43 patients (median age 39 years); 42% male | Oral therapy: 40 patients (median age 52 years); 50% male | There was a response in 41/43 (95%) patients receiving i.v. therapy compared with 35/40 (88%) patients receiving oral treatment (p = 0.19) Preliminary results of a second trial (altered oral regime) showed a response rate of 87% (78/90 patients) in the i.v. arm and 90% (80/89 patients) in the oral arm |
| Esmond et al.43 (UK) | Controlled trial | SA | Home therapy: 15 patients (mean age 27); 40% male | Inpatient therapy: 15 patients (mean age 23 years); 60% male | There was no significant difference between groups in mean duration of treatment: home group 14 days (range 10–18 days), hospital group 15 days (range 10–25 days) Improved lung function was significantly greater in the hospital group than in the home group (FEV1 2.0 vs. 5.2; p = 0.05 and FVC 2.1 vs. 12.3; p = 0.01). When change in the groups was directly compared, only the difference in FVC was significant (p = 0.01) |
| Fernández-Avilés et al. (Spain)56 | Case–control | SN | Home therapy: 50 patients (mean age 47 years); 62% male | Inpatient therapy: 50 patients (median age 50 years); 54% male | There was no significant difference between home and hospital patients in relation to median duration of treatment (8 days vs. 9 days) Median duration of fever was significantly shorter in home patients (2 days vs. 5 days; p = 0.00003) |
| Martone et al.55 (USA) | Observational (retrospective) | Not specified | OPAT: 539 patients (57% aged > 50 years); 56% male | Inpatient therapy: 410 patients (63% aged > 50 years); 45% male | Median duration of OPAT only treatment was 17 days (range 3–144 days). Median duration of hospital treatment was 7 days (range 1–153 days) Success rates were higher in OPAT (510/539) than in hospital (354/41) patients (94.6% vs. 86.3%; p < 0.001) |
| Montalto and Dunt57 (Australia) | Observational (retrospective) | SN (HHU) | HHU therapy: 55 cellulitis patients (mean age 52 years); 60% male 14 pyelonephritis patients (mean age 30 years); 0% male |
Inpatient therapy: 22 cellulitis patients (mean age 51 years); 73% male 10 pyelonephritis patients (mean age 35 years); 30% male |
Mean duration of stay was lower for HHU cellulitis patients (6.00 days vs. 8.55 days; 95% CI 0.24 to 4.85 days) but not for pyelonephritis patients (4.57 days vs. 4.00 days; 95% CI –1.86 to 0.72 days) Full recovery was expected in 51/55 HHU cellulitis patients (93%); the remaining four showed recovery back to a stable pre-existing condition. Recovery was expected in all 22 hospital patients Recovery was expected in 13/14 HHU pyelonephritis patients (93%), and in 9/10 hospital patients (90%). The remaining patient in each group was expected to return to a stable pre-existing condition Mean time to febrifuge was lower for HHU cellulitis (1.96 days vs. 2.00 days) and pyelonephritis patients (1.79 days vs. 2.40 days), although the difference was not statistically significant |
| Pond et al.58 (UK) | Case–control (retrospective) | SA | Home therapy: 25 patients (mean age 21 years); 56% male | Inpatient therapy: 25 patients (mean age 22 years); 56% male | Mean duration of treatment was similar in the two groups (OPAT 14.1 days vs. hospital 16.7 days) There was no difference between the OPAT and hospital groups in relation to adjusted mean improvement in FVC (0.567 vs. 0.644), FEV1 (0.456 vs. 0.403), PEFR (66.1 vs. 57.5) or other outcome variables. The only variable to show a significant difference was total white cell count (–3.64 vs. –4.72; p < 0.05) |
| Rapoport et al.22 (multinational) | RCT | OP | Outpatient therapy: 40 patients (median age 45 years); 52% male | Inpatient therapy: 44 patients (median age 48 years); 32% male | Median duration of treatment was similar for both OPAT and inpatients groups (6.0 days vs. 6.3 days) Treatment was successful in 34/38 OPAT patients (89%) and 40/42 inpatients (95%) |
| Rehm et al.49 (USA) | RCT | SA; SN; (also skilled nursing facility; assisted-living; nursing home; rehabilitation centre) | Home therapy: 103 patients (median age 50 years); 58% male | Inpatient therapy: 97 patients (median age 54 years); 61% male | OPAT patients received longer courses of antibiotics than hospital patients (mean 25.4 days vs. 13.5 days; p < 0.001). OPAT patients received a mean of 14.9 days of therapy outside the hospital setting There was a higher rate of clinical success among OPAT patients (89/103; 86.4%) than hospital patients (54/97; 55.7%; p < 0.001) |
| Richards et al.41 (New Zealand) | RCT | GN | Home therapy: 24 patients (mean age 50 years); 54% male | Inpatient therapy: 25 patients (mean age 50 years); 52% male | Median time to discharge was 4 days (range 1–14 days) in the home group and 2 days (range 0–10 days) in the hospital group (p = 0.004) There was no difference in the number of days on i.v. antibiotics (3 days vs. 2 days; p = 0.22) At 2 weeks, there was no difference in patient-rated symptoms. There was a significant difference in sleep disturbance (home median ‘occasional’; hospital median ‘never’; p < 0.01), but this did not persist at 6 weeks. There was no difference in time to resolution of fever, tachycardia or tachypnoea |
| Seaton et al.10 (UK) | Before and after | SN | Standard OPAT: 230 patients (median age 49 years); 52% male | Nurse-led OPAT: 112 patients (median age 50 years); 61% male | Total median duration of i.v. therapy was reduced from 5 days (range 1–37 days) to 4 days (range 1–23 days) (p = 0.01) Median duration of outpatient therapy was reduced from 4 days (range 1–37 days) to 3 days (range 1–22 days) (p = 0.02) Cure or improvement was similar for pre- (225/228) and post-protocol (106/108) patients (99% vs. 97%) |
| Sebban et al.50 (France) | RCT | OP | i.v. therapy: 47 patients (mean age 52 days); 40% male | Oral therapy: 49 patients (mean age 52 days); 31% male | Success was observed for 34/46 (73.9%) patients in the i.v. group and 38/48 (79.2%) patients in the Oral group (calculated risk difference was 5.3%; 95% CI 11.9 to 22.4%) |
| Stein et al.51 (USA) | RCT | OP | i.v. therapy: 10 patients (mean age 54 years); 60% male | Oral therapy: 10 patients (mean age 53 years); 40% male | Average number of OPAT doses was eight (range 1–30). Average number of oral doses was 19 (range 9–40) Cure and improvement rates were higher for oral group patients (10/10; 100%) than for OPAT patients (6/10; 60%), although this was not statistically significant (p = 0.09) |
| Thornton et al.53 (UK) | Observational (retrospective) | Not specified | Home therapy: 47 patients (mean age 26 years); 36% male | Combined therapy: 18 patients (mean age 25 years); 61% male Inpatient therapy: 51 patients (mean age 26 years); 59% male |
Mean treatment duration was 63 days for home patients 54 days for hospital patients and 66 days for combined patients Mean FEV1 improvement from baseline ‘best’ was significantly higher for hospital patients (mean diff. 4.6%, 95% CI 1.8–7.4; p = 0.001) More hospital (41/236; 17%) than home courses (18/199; 9%) were classified as effective (p = 0.001) At the ≤ 0% level, there was a mean decline in FEV1 for home patients after 1 year, but an improvement for hospital patients (mean difference 10.1%, 95% CI 2.9 to 17.2; p = 0.003). The number of patients in whom treatment was classified as effective was higher in the hospital (n = 30; 59%) than in the home group (n = 20; 43%). When effectiveness was defined as an FEV1 decline of ≤ 2%, the difference became statistically significant (43% vs. 63%; p = 0.045) |
| Wolter et al.48 (Australia) | RCT | Not specified | Home care: 44 admissions (median patient age 43 years); 45% male | Inpatient therapy: 38 admissions (median patient age 49 years); 34% male | Median duration of treatment was similar between the groups (home 11.5 days, range 3–57 days; hospital 11.0 days, range 4–126 days; p = 0.002) Median number of investigations was significantly lower in the home group (12.5 vs. 19.0; p < 0.001) There was no difference in numbers with an ‘Improved’ outcome following treatment (home 84.1% vs. hospital 92.1%; p = 0.32) Average time to the next admission was longer in the hospital group (12 vs. 32 days; p = 0.006) |
| Wolter et al.42 (Australia) | RCT | SA | Home care: 13 admissions (median age 22 years); 28% male | Inpatient therapy: 18 admissions (median age 22 years); 39% male | There were no significant differences in the duration of treatment or use of antibiotics. Median duration of treatment was 12 days (range 10–24 days) in the home group and 11 days (range 7–26 days) in the hospital group (p = 0.2) Home patients had fewer investigations performed than inpatients (p = 0.002) There was no significant difference between groups in improved lung function (FEV1 p = 0.27; FVC p = 0.30) There was no significant difference in time to next admission between the groups (p = 0.68) |
| Yong et al.63 (Singapore) | Case–control (retrospective) | OP; SA | OPAT: 69 patients (mean age 53 years); 53% male | Inpatient therapy: 93 patients (mean age 56 years); 51% male | Average length of OPAT-only care was 24.3 days (range 4–93 days). Mean duration of treatment for OPAT patients was 42.5 days (range 4–145 days), significantly longer than for hospital patients (mean 19 days; range 4–183 days) (p < 0.001) At the end of the 4-month follow-up period, 59/72 OPAT episodes (81.9%) were considered cured compared with 75/93 hospital patients (80.6%) |
| Bedi et al.59 (UK) | Cohort | SA | Home therapy (domiciliary i.v.): 52 patients (median age 61 years); 58% male Home therapy (ESD); 23 patients (median age 65 years); 35% male |
Inpatient therapy: 36 patients (median age 71 years); 36% male | There was resolution of infection in 76% of the inpatient group, 80% of the ESD group and 80% of the domiciliary i.v. group Within-group comparisons showed significant (p < 0.05) improvements in FEV1, FVC, incremental shuttle walking test, 24-hour sputum volume, sputum bacterial clearance and parameters of inflammation in all groups |
| Yang et al.61 (Canada) | Case–control | Not specified | OPAT: 21 patients (mean age 59 years); 57% male | Previous standard care: 21 patients (mean age 59 years); 57% male | There was a better overall cure rate in the OPAT group (61.9% vs. 57.1%, p = 0.1) For a composite end point of clinical success (i.e. combined cures and controlled/improved cases) the results were 95% in the OPAT group and 86% in the previous care group (p = 0.62) There were three (14.3%) treatment failures in the control group and one (4.8%) in the OPAT group |
| OPAT-only studies | |||||
| Anand et al.180 (USA) | Case series | Home i.v. therapy (unspecified) | 52 patients | – | Patients showed a significant improvement in all symptoms post treatment (p < 0.001) |
| Angel44 (USA) | RCT | SA | 62 patients (mean age 71 years); 53% male | – | Mean length of inpatient treatment was 3.6 days fewer than expected from DRGs Documented cure was seen in 35/50 evaluable patients (70%), while 14 (28%) had improved. There was one clinical failure Bacteriologic eradication was seen in 16/28 (57%) evaluable patients; 10 others (36%) were clinically improved (no culture could be obtained). Two patients had persistent pathogens |
| Barr et al.34 (UK) | Observational (retrospective) | OP; SA; SN; GN | 2233 patients (median age 51 years); 58% male | – | Median duration of treatment was 5 days (range < 1–328 days) Most patients were cured (1,501/2233; 67%) or improved (n = 562; 25%). Small numbers showed no change (n = 52; 2%) or deteriorated (n = 91; 4%) |
| Berman and Johnson181 (USA) | Case series (retrospective) | SA | 221 patients (median age 41 years); 66% male | – | Median duration of treatment was 18 days (range 3–307 days) OPAT was successful in 283/302 episodes (94%). Treatment failed in 19 episodes (6%), of which 16 (84%) required hospitalisation |
| Bernard et al.84 (France) | Observational (?) | SA; SN | 39 patients (mean age 44 years); 64% male | – | Mean duration of treatment was 4 months (range 1.5–12 months) Twenty-eight of 30 patients (93%) available for follow-up were considered cured with a mean delay of 24 months after completion of therapy. There was one clinical failure and one relapse within 1 month of treatment |
| Chan et al.182 (Singapore) | Observational | Not specified | 109 patients (mean age 57 years); 66% male | – | Average duration of i.v. therapy was 32 days (range 11–98 days). Mean length of OPAT was 16 days (range 2–54 days) Eighty of 109 patients (74%) achieved a clinical response at four weeks. All patients achieved cure |
| Chapman et al.13 (UK) | Observational (retrospective) | SA; Infusion centre | 334 treatment courses (mean patient age 46 years); 59% male | – | Mean duration of treatment for SSTI (n = 198) was 4.9 days (range 1–46 days). Mean duration of treatment for other infections (n = 136) was 22.5 days (range 1–163 days) A total of 291/334 patients (87%) were cured or improved on completion of therapy; 11 were recorded as ‘no change’ Sixty-two records of SSTI patients were selected at random for follow-up data. Mean duration of follow-up was 27.8 days (range 4–122 days); 61 (98.4%) were cured or improved |
| Cox et al.89 (USA) | Observational (retrospective) | SA | 205 patients (mean age 59 years); 99% male | – | Median duration of treatment was 22 days (range 2–105 days) for older adults (≥ 60 years) and 29.5 days (range 4–450 days) for younger adults A total of 54/231 (23%) courses resulted in cure at the end of home i.v. treatment, while 159 courses (69%) were deemed to have stable or improved infection. In 16 courses (7%) treatment was considered a failure (in all cases attributed to a difficult-to-treat infection rather than adverse effects) |
| Dalovisio et al.183 (USA) | Observational (retrospective) | SA; SN | 62 patients (mean age 69 years) | – | Average duration of treatment was 23.4 days Average number of home care visits per course was 18.8 The majority of treatment courses (60/66; 91%) met the goals of therapy |
| Dargan et al.184 (Canada) | Case series (retrospective) | OP; Home therapy (unspecified) | 66 patients (mean age 57 years); 50% male | – | Mean total duration of therapy was 32.8 days (range 3–169 days) Mean duration of OPAT treatment was 27.3 days (range 2–169 days) Most patients (56/66; 85%) had a successful outcome; 10 patients experienced treatment failure |
| Donald et al.185 (Australia) | Case series (retrospective) | SN | 124 patients (aged 16–97 years); 60% male | – | Mean duration of treatment was 6.24 days The majority of patients were treated successfully (105/124; 85%) |
| Esposito et al.186 (Italy) | Observational | OP; SA; visit by doctor or nurse (also care facility) | 176 patients (aged > 65 years); 47% male | – | At the end of therapy, almost 90% of cases were recorded as cured or improved (156/176) Seventy-one of 176 patients were available for follow-up (30 days after the end of therapy); cure or improvement was recorded in 81% (56/71) of patients |
| Esposito et al.83 (Italy) | Observational (retrospective) | OP; SA; visit by doctor or nurse (also care facility) | 239 patients (aged 11–80+ years); 62% male | – | Mean duration of treatment was 71.2 days (SD 39.3 days) Clinical results were satisfactory, with only 20 failures observed at the end of therapy (8.4%) Of 120 patients available for follow-up (30 days after end of therapy), 72 (60%) were cured and 35 (29%) were improved |
| Esposito et al.21 (multinational) | Observational (retrospective) | OP; SA; SN; GN; emergency room; infusion centre | 9826 US registry patients (aged 1–80+ years); 57% male 981 UK registry patients (aged 11–80+ years); 63% male 620 Italian registry patients (aged 1–80+ years); 57% male |
– | Mean duration of treatment was longer in Italy (56.0 days) than in the USA (22.5 days) and UK (19.9 days) Most patients (10629/11427; 93%) achieved clinical cure or improvement (USA 93%; UK 97%; Italy 95%) |
| Goodwin et al.27 (Canada) | Case series | SN; GN | 2405 patients (mean age 46 years); 55% male | – | Duration of treatment ranged from 1 to > 42 days (longest was 209 days) Five patients had a resolution of their infection earlier than expected |
| Graninger et al.187 (Austria) | Case series | OP | 54 patients | – | Mean duration of treatment for osteomyelitis was 62 days (range 28–150 days) Cure was achieved in 18 (41%) and improvement in 19 (43%) of 44 patients. Seven patients failed to respond even after the minimum treatment period of 1 month (range 25–84 days) Average duration of treatment for endocarditis was 49 days (range 28–88 days) Eight of 10 patients were cured (80%), and there were two treatment failures |
| Grayson et al.67 (Australia) | Case series | GN | 20 patients (mean age 58 years) | – | Mean duration of treatment was 26 days (range 11–44 days) Eighteen of 20 patients (90%) were clinically and microbiologically cured after therapy |
| Gross et al.188 (USA) | Case series (retrospective) | Home i.v. therapy (unspecified) | 13 patients (mean age 38 years); 100% male | – | There were no treatment failures |
| Hindes et al.68 (USA) | Case series | SA | 48 patients (mean age 65 years) | – | Mean duration of therapy was 22 days All patients (48/48) had eradication or arrest of their infection |
| Hitchcock et al.69 (UK) | Case series | OP; SA; GN; private home health-care company | 273 patients (mean age 60 years); 54% male | ― | Mean length of treatment was 24 days (range 1–165 days) In 278/303 episodes (92%), patients were successfully treated as per plan |
| Ho et al.189 (Singapore) | Case series | OP | 29 patients (median age 41 years); 90% male | – | Median length of hospital stay prior to OPAT was 15 days (range 2–48 days) Median duration of treatment was 18 days (range 1–85 days) All but one patient (28/29; 96.6%) completed the intended duration of OPAT therapy |
| Huminer et al.70 (Israel) | Case series | SA; SN (also nursing home; kibbutz) | 37 patients (mean age 64 years); 57% male | – | Mean duration of treatment was 26.2 days (range 14–49 days) Cure after a first course of treatment was documented in 34/37 patients (92%) |
| Johansson et al.71 (Sweden) | Case series | SA | 11 patients (mean age 51 years); 73% male | – | Median duration of OPAT was 4 days (range 1–12 days). Patients received therapy during 16 episodes of neutropenic fever None of the patients developed recurrent fever |
| Kieran et al.2 (Ireland) | Case series (retrospective) | SA; nurses attached to commercial company | 56 patients (median age 50 years); 57% male | – | Median duration of treatment was 16 days (range 2–84 days) Clinical cure was documented in 52/56 patients (93%) There was no significant difference in outcomes between self-administered courses (n = 48) and those given by a nurse (n = 12); (p = 0.61) |
| Krauth et al.23 (worldwide) | Literature review | Not specified | 11 studies | – | Only one of seven studies evaluating efficacy of home i.v. therapy for CF found significant differences between home and inpatient care with regard to short-term outcomes such as FVC% and FEV1%, etc. The only randomised study supported the (short-term) equivalence of home care and inpatient therapy. One trial performed under everyday conditions found that home care therapy produced significantly poorer FVC and FEV1 results |
| Larioza et al.190 (USA) | Case series (retrospective) | Home infusion company | 33 patients (76% aged ≥ 50 years); 61% male | – | Mean duration of treatment was 29 days Thirty-one of 33 patients (94%) were successfully treated |
| Larioza et al.191 (USA) | Case series (retrospective) | Home infusion company | 43 patients (56% aged > 50 years); 67% male | – | Patients were treated for 4–8 weeks; duration of OPAT was 70% for patients with surgery and 80% for patients without (p = 0.0561) All patients survived for 1 year following therapy without infective endocarditis relapse (43/43) |
| Lillie et al.147 (UK) | Case series | SN | 98 patients (mean age 55 years); 61% male | – | Average duration of treatment was 6.3 days Elevated baseline CRP (p = 0.002), male sex (p = 0.004), prolonged duration of symptoms prior to OPAT (p = 0.001) and higher antibiotic dose (p = 0.006) were associated with prolonged treatment via OPAT |
| Lopardo192 (Argentina) | Case series (retrospective) | OP; SA; SN | 48 patients (median age 55 years); 62% male | – | Clinical and microbiologic cure was achieved in 100% of cases (48/48) |
| Mackintosh et al.3 (UK) | Case series (retrospective) | OP; SA; SN | 198 patients (aged < 40–89 years); 64% male | – | Success rate following initial OPAT was 86.4% (171/198), ranging from 71.8% for diabetic foot or stump infection to 100% for metalwork-related infection Median duration of follow-up was 60 weeks (range 6–104 weeks). A total of 59/198 patients (29.8%) failed initial treatment, presented with a recurrence or relapse of their infection or died during the follow-up period. Of those who failed, 55 (93%) did so within 12 months |
| Mauceri45 (USA) | RCT | SA | 27 patients | – | Mean duration of OPAT treatment was 30.50 days. Mean duration of prior hospitalisation was 12.17 days (1.77 days longer than allotted by DRGs) Cure was achieved in 11/18 evaluable patients (61%) and improvement in four patients (22%) Bacteriologic success rate was 79% (11/14 patients) |
| McMahon et al.193 (Australia) | Observational | SN | 40 patients [mean age 59 years (male), 49 years (female)]; 75% male | – | Mean duration of treatment was 47.7 days, with the mean inpatient treatment duration being 23.5 days (range 8–75 days), and the mean HITH treatment duration being 24.2 days (range 2–63 days) Cure was achieved in 37/40 (93%) patients, with the three remaining patients considered failures |
| Montalto194 (Australia) | Case series (retrospective) | SN (HHU) | 133 patients (mean age 46 years); 52% male | – | Mean duration of HHU stay was 4.8 days (range 1–28 days) Full recovery was expected in 132/133 patients (99%) |
| Morales and Von Behren195 (Puerto Rico) | Case series | SA | 22 patients (mean age 33–41 years); 82% male | – | Mean length of inpatient treatment was 4.5–6.5 days shorter than allotted by DRGs On completion of treatment, 19/20 evaluable patients (95%) were cured or improved Bacteriologic eradication was seen in 15/17 (88%) evaluable patients. There were no super infections or reinfections |
| Murray et al.196 (Canada) | Observational | Emergency department | 75 patients (mean age 48 years); 57% male | – | Two of 29 patients in the oral group failed treatment (6.8%), compared with 12/46 patients in the i.v. group (26%) |
| Nathwani74 (UK) | Case series | OP; SA; SN | 125 patients | – | Mean duration of treatment was 5.32 days The majority of patients (123/125; 98%) were cured or improved. Two patients worsened and required surgery |
| Nathwani et al.197 (UK) | Observational (retrospective) | OP; SA | 50 patients | – | Mean duration treatment was 37.7 days All patients (50/50) were deemed to have improved or be cured at the end of treatment |
| Nathwani et al.75 (UK) | Case series | OP; SA | 101 patients | – | Most patients were cured or improved (95/101; 94%), although two patients were unchanged (4%) and the remaining four were worse (4%) |
| Parker et al.86 (UK) | Observational | OP; SA | 29 patients; 38 general practices | – | Of the 29 enrolled patients, 23 (79%) had complete resolution of their symptoms. The remaining six were indeterminate |
| Partridge et al.198 (UK) | Case series (retrospective) | OP; SA | 34 patients (mean age 55 years); 79% male | – | Median duration of treatment was 27 days (range 7–65 days) Most episodes (34/36; 94%) were treated successfully, with no evidence of recurrence at a median of 30 months following completion of OPAT (range 6–57 months) |
| Patanwala et al.66 (USA) | Decision tree analysis | SN | 41 patients (mean age 53 years); 44% male | – | Cure was documented in 18/41 patients (44%) and presumed in 11/41 patients (27%). Twelve patients were treatment failures |
| Pérez-López et al.199 (Spain) | Observational | SN | 145 patients (mean age 68 years); 48% male | – | Mean duration of treatment was 8.8 days (range 2–60 days) Definitive discharge was given to 83/90 (92%) elderly group patients (≥ 70 years) owing to satisfactory clinical evolution (no data reported for younger patients) |
| Poretz46 (USA) | RCT | SA | 238 patients (mean age 45–52 years); 57% male | – | Of 211 evaluable patients, 158 (75%) were cured and 43 (20%) were improvement. Response was unsatisfactory in the remaining 10 patients (5%) Bacteriologic eradication was seen in 125/134 (93%) evaluable patients. There was persistence in eight patients (6%) |
| Poretz47 (USA) | RCT | SA | 130 patients (mean age 45 years); 59% malea | – | For patients with initial inpatient treatment, length of stay was 2.4 days shorter than allotted by DRGs. Mean duration of treatment completed entirely at home was 10.4 days Clinical cure or improvement was seen in 115/118 (98%) evaluable patients Bacteriologic eradication was noted in 78 (94%) of the 83 evaluable patients |
| Rodríguez-Cerrillo et al.76 (Spain) | Case series | SN (HHU) | 24 patients (mean age 73 years) | – | Mean duration of HHU stay was 9 days All patients had a favourable clinical outcome (24/24) |
| Rodríguez-Cerrillo et al.77 (Spain) | Case series | SN (HHU) | 25 patients (mean age 59 years); 48% male | – | Mean duration of HHU stay was 11 days All patients (25/25) had a favourable clinical outcome |
| Seaton and MacConnachie200 (UK) | Case series (retrospective) | SA; other (unspecified) | 19 patients (mean age 55 years); 53% male | – | Average duration of OPAT was 34 days (range 3–128 days) Twelve of 19 patients (63%) were cured, while four (21%) were improved) |
| Seaton et al.201 (UK) | Observational (retrospective) | OP | 963 patients (median age 48 years); 59% male | – | Median duration of treatment was 3 days (IQR 2–5 days) The success rate (no progression of infection, no readmission and no significant adverse events) was 87.1% (839/963 patients) Progression of infection was seen in 27/963 patients (2.8%) |
| South202 (UK) | Case series (retrospective) | OP; SA; nurse (unspecified) | 57 patients (mean age 41 years); 77% male | – | Median duration of non-inpatient treatment was 5 days (range 1–14 days) for haematological malignancies and 24 days (range 9–78 days) for bone and joint infections Cure or improvement was achieved in 60/64 evaluable episodes (94.8%; 95% CI 88.2% to 99.8%). There were two clinical failures (both Hickman line infections) Bacteriological success was achieved in 33/40 evaluable episodes (82.5%; 95% CI 71.4% to 94.6%) |
| Talcott et al.87 (USA) | Observational (pilot) | SA; SN | 30 patients (median age 38 years); 43% male | – | Median duration of treatment was 3.5 days (range 1–24 days) Twenty-one of 30 patients (70%) completed home therapy without complication or readmission Medically eligible patients who did not enrol in the trial had a shorter duration of neutropenia (median 4 days vs. 6 days; p < 0.005) than enrolled patients |
| Theocharis et al.203 (Greece) | Case series (retrospective) | SN | 91 patients (mean age 85 years); 31% male | – | Cure rate was 72.5% (66/91) Mean duration of treatment was 4.7 days (range 1–18 days) |
| Tice80 (USA) | Observational (retrospective) | OP; SA; SN; Infusion centre | 500 patients | – | Most patients (269/424; 56%) were treated for at least 4 weeks, although treatment duration varied from < 1 week to 11 weeks Most patients assessed for clinical outcome were improved (259/266; 97%). There were failures in three patients and no change in four others. Most failures occurred in the first few months (65% in ≤ 3 months); the majority (95%) in the first year |
| Tice81 (USA) | Case series | OP; SA; SN | 538 patients (mean age 45 years); 52% male | – | Clinical improvement was recorded in 484/491 patients who could be evaluated (98.6%); failure was recorded in seven patients Eradication was reported in 244/265 cases (92%) for which bacteriological outcome could be assessed. There was persistence in 17 cases (6.4%) and superinfection in four (1.5%) |
| Upton et al.204 (New Zealand) | Case series | SA | 100 patients (mean age 51 years); 59% male | – | Median duration of treatment was 22 days (range 3–160 days) Cure was achieved in 94/107 treatment courses in 88 patients (88%). Ten patients relapsed, one did not respond and one died |
| Walton et al.205 (Australia) | Case series | SN | 35 patients (mean age 50 years); 71% male | – | Mean duration of treatment was 24 days (range 6–47 days) Of 31 patients who were assessable after treatment completion, all were clinically and bacteriologically cured (31/31) Twenty of 31 patients (65%) were reviewed ≥ 2 months after completion of treatment (median 6.5 months; range 2–21 months), and all remained free of relapse for the duration of follow-up |
| White et al.206 (Australia) | Case series (retrospective) | OP; SN; GN | 55 patients (mean age 50 years); 65% male | – | Median duration of i.v. therapy, including time spent as an inpatient, was 42 days (range 4–79 days) Median duration of treatment was 22 days (range 3–56 days) Forty-six of 55 patients (84%) were considered to have had a successful outcome at completion of i.v. OPAT |
| White et al.207 (UK) | Case series (retrospective) | SA | 72 patients (mean age 42 years); 42% male | – | Median duration of treatment was 21 days (range 1–43 days) Definite improvement was seen in 20/72 patients (28%), modest/slight/transient improvement was seen in 24 patients (33%) and no improvement was seen in 26 patients (36%) |
| Wynn et al.32 (USA) | Observational (retrospective) | Physician-based programme; home health organisation; hospital-based programme (no other details) | 1252 patients (mean age 52 years); 65% male | – | A total of 1202/1252 patients (96%) were treated successfully for their condition |
| Yadlapalli et al.208 (USA) | Case series (retrospective) | Not specified | 58 patients (mean age 60 years); 83% male | – | Mean duration of antibiotic therapy was 40.3 days (range 19–90 days) At the end of 12-months’ follow-up, 46/58 patients (79.3%) were cured (mean healing time 15.4 weeks). Three of the 12 patients (5.2%) who failed to heal had an amputation and nine (15.5%) had persistence of ulceration after 1 year |
| Yan et al.5 (UK) | Case series (retrospective) | OP; GN | 140 patients (aged 17–89 years); 64% male | – | Mean duration of treatment for patients with cellulitis (n = 128) was 4.4 days Duration of treatment ranged from 0 to > 15 days. Around 60% of patients received i.v. antibiotics for ≤ 3 days The failure/complication rate among patients was low (8/140; 5.7%) |
| Al Ansari et al.82 (Bahrain) | Case series (retrospective) | OP | 101 patients; 57% male | – | Average duration of treatment ranged from three to 10 days Total cure was achieved in 97 (96%) treated patients. Two patients were lost to follow-up with the OPAT clinic |
| Duncan et al.54 (UK) | Observational (retrospective) | Not specified | 55 completed OPAT patients (median age 59 years); 80% male/25 failed OPAT patients (median age 67 years); 60% male | – | Median duration of OPAT was 28 days (IQR 20–38 days); all patients had a prior period of inpatient management (median 22 days; IQR 14–30 days) OPAT failure occurred in 25/80 (31.3%) episodes. Increased probability of failure was associated with coexisting cardiac or renal failure (OR 7.29, 95% CI 1.84 to 29.66; p = 0.005), specialist referral (OR 0.25, 95% CI 2.01 to 37.47; p = 0.00), and use of teicoplanin (OR 8.69, 95% CI 0.06 to 1.11; p = 0.068) |
| Htin et al.209 (Australia) | Case series (retrospective) | SN | 68 patients (median age 68 years); 87% male | – | Median duration of antimicrobial therapy was 24 days (range 4–42 days) Treatment success rate was 94% (64/68). There were two relapses |
| Lai et al.210 (USA) | Case series (retrospective) | SA; SN | 333 patients (mean age 62 years); 98% male | – | Mean duration of treatment was 21.1 days (range 0–88 days; IQR 9–30 days). Overall completion rate was 79.6% (313/393 courses) Factors associated with non-completion were bacteraemia (OR 1.82; p = 0.040), concomitant CHF (OR 1.64; p = 0.051), and concomitant ESRD (OR 2.59; p = 0.015). Only ESRD remained significant when the three variables were combined in a multivariable model (OR 2.20; p = 0.047) |
| Mohammadi et al.211 (USA) | Case series (retrospective) | SA; SN | 190 patients (mean age 63 years); 98% male | – | Median duration of OPAT was 30 days (range 5–56 days) The overall cure rate for all infections treated was 78% at end of treatment, decreasing to 58% at 90 days post OPAT (p < 0.001) |
| Seetoh et al.33 (Singapore) | Cohort | OP; SA; SN | 2229 patients (median age 56 years); 64% male | – | Treatment was completed as planned in 1874 (84.1%) of 2229 episodes Median time to treatment completion was 16 days (IQR 8–27 days) |
| Sims et al.212 (UK) | Case series (retrospective) | OP | 14 patients (mean age 63 years); 71% male | – | Average duration of OPAT treatment was 58 days Overall, 11/14 joints (79%) were salvaged. Of the three failures, one patient improved initially but had a recurrence 5 months later (different organism identified recurrence), one had an excellent initial response but infection recurred 18 months later, and one developed infection with an atypical organism 3 years after primary surgery |
| Subedi et al.213 (Australia) | Case series | SA; SN | 144 patients (median age 55 years); 74% male | – | Median duration of OPAT treatment was 22 days (range 4–106 days) Overall cure rate was 93% (140 episodes) |
| Author (country) | Study design | OPAT model(s) | OPAT group(s)a | Comparator(s)a | Findings | Notes |
|---|---|---|---|---|---|---|
| Comparator studies | ||||||
| Corwin et al.12 (New Zealand) | RCT | GN | Home therapy: 98 patients (mean age 55); 62% male | Inpatient therapy: 96 patients (mean age 48 years); 73% male | Eleven of 98 patients in the home group (12%) were admitted to hospital Three of 96 hospital patients (3%) required readmission within 1 month |
Reasons for admission were: unsatisfactory clinical improvement (4), surgical drainage (1), PICC line insertion (2), ischaemic toe (1), rash (1), nausea/vomiting on starting post-discharge oral antibiotics (1), and not coping at home (1) |
| Dall et al.25 (USA) | Before and after | SN | Home therapy: 92 pneumonia patients (97% aged > 50 years), 44% male; 64 cellulitis patients (mean age 51 years); 70% male | Inpatient therapy: 10,728 pneumonia patients (83% aged > 50 years), 51% male; cellulitis patients (no numbers provided) | Two of 92 patients with pneumonia were readmitted (rate over 30-day period, 2%). The readmission rate for comparator patients was 7.4% (69/933 patients) Six cellulitis patients were hospitalised briefly (9.4%). One treatment failure resulted in readmission (for MRSA) There were no deaths among OPAT patients |
|
| Escalante et al.52 (USA) | Clinical trial (unspecified) | Not specified | i.v. therapy: 43 patients (median age 39 years); 42% male | Oral therapy: 40 patients (median age 52 years); 50% male | Three of 40 patients receiving oral treatment had an adverse drug reaction Six patients receiving oral treatment (15%) were admitted to hospital; there were no admissions in patients receiving i.v. treatment There were no infection-related complications or deaths |
Adverse drug reactions were: renal toxicity (3) Reasons for admission were: renal toxicity (3), and persistent fever (3) |
| Esmond et al.43 (UK) | Controlled trial | SA | Home therapy: 15 patients (mean age 27 years); 40% male | Inpatient therapy: 15 patients (mean age 23 years); 60% male | There were no drug reactions, i.v. line problems or sepsis reported in either group | |
| Fernández-Avilés et al.56 (Spain) | Case–control | SN | Home therapy: 50 patients (mean age 47 years); 62% male | Inpatient therapy: 50 patients (median age 50 years); 54% male | There was no significant difference in mucositis (home 24% vs. 34%), diarrhoea (35% vs. 39%) or bacteraemia between the two groups In 40% of home patients’ day unit consultations, physician intervention was required Four home patients (8%) were readmitted. The median number of admitted days was 8 days for home patients and 25 days for hospital patients (p < 0.00001) No patients in either group died |
Reasons for intervention were: fever (75%), catheter-related complication (10%), and mucositis, bleeding, diarrhoea, or chemotherapy-related exanthema (15%) Reasons for readmission were: fever with hemodynamic instability (3) and pneumonia (1) |
| Martone et al.55 (USA) | Observational (retrospective) | Not specified | OPAT: 539 patients (57% aged > 50 years); 56% male | Inpatient therapy: 410 patients (63% aged > 50 years); 45% male | A total of 216 adverse events were reported in 50/539 OPAT patients and 81/410 hospital patients (9.3% vs. 19.8%; p < 0.0001) A total of 89 adverse events possibly related to therapy were experienced by 31 OPAT and 34 hospital patients (5.8% vs. 8.3%; p = 0.12) Significantly more hospital patients had diarrhoea (2.4% vs. 0.2%; p = 0.001) |
|
| Matthews et al.29 (UK) | Cohort (retrospective) | SA; GN; community hospital staff | Self-administered OPAT: 513 episodes (mean patient age 46 years) | Professionally administered OPAT: 1621 episodes (mean patient age 61 years) | Drug-associated complications were experienced in 189/1536 hospital OPAT episodes (12%) and 59/473 SA OPAT episodes (12%) Complications related to vascular access affected eight (0.5%) H-OPAT patients and five (1%) S-OPAT patients (p = 0.2) The overall complication rate was 23% (353/1536 episodes) for H-OPAT patients and 24% (112/473 episodes) for S-OPAT (p = 0.7) Re-admission occurred for 193 H-OPAT and 50 S-OPAT patients (rates of 12.6% and 10.5%, respectively; p = 0.3) Two H-OPAT patients died (unrelated cardiac causes) |
Drug-related complications included: GI side effects (58), drug rash (52), drug fever (51), unclassified drug reaction (45), neutropenia (41), anaphylaxis (4), renal impairment (1), and C. difficile diarrhoea (1) Access-related complications were: line infection (3), leaking line (3), mechanical phlebitis (2) and thrombosis (6) |
| Mazo et al.64 (Spain) | Case–control (retrospective) | HHU (unspecified) | HHU therapy: 150 patients (mean age 67 years); 47% male | Inpatient therapy: 150 patients (mean age 63 years); 51% male | HHU decreased the number of complications or readmissions to the surgical or emergency unit at 45 days (HHU rate 0.67% vs. hospital rate 3.5%) | |
| Montalto and Dunt57 (Australia) | Observational (retrospective) | SN (HHU) | HHU therapy: 55 cellulitis patients (mean age 52 years), 60% male; 14 pyelonephritis patients (mean age 30 years), 0% male | Inpatient therapy: 22 cellulitis patients (mean age 51 years), 73% male; 10 pyelonephritis patients (mean age 35 years), 30% male | Incidents were recorded for 3/69 HHU patients (4%) and 10/32 hospital patients (31%) Four hospital patients (three with cellulitis, one with pyelonephritis) were readmitted within 4 weeks |
HHU incidents were: drainage of abscess (2), and vasovagal episode at administration of medication (1) Hospital group incidents were: unexpected non-urgent operation (2), drug reaction (1), unexpected returned to hospital within four weeks (4), anaphylaxis (1), readmission for related condition (1), and unexpected gastroscopy (1) |
| Pond et al.58 (UK) | Case–control (retrospective) | SA | Home therapy: 25 patients (mean age 21 years); 56% male | Inpatient therapy: 25 patients (mean age 22 years); 56% male | There were 2/25 (8%) adverse reactions in each group. All consisted of mild skin rashes that subsided with withdrawal of the relevant antibiotic | |
| Rapoport et al.22 (multinational) | RCT | OP | Outpatient therapy: 40 patients (median age 45 years); 52% male | Inpatient therapy: 44 patients (median age 48); 32% male | Incidence of adverse events was similar for OPAT patients (6/40; 15%) and inpatients (8/44; 18%) Three events (one OPAT, two inpatient) were considered to be potentially drug related; two events were severe (one in each group) There was one death in the OPAT group |
|
| Rehm et al.49 (USA) | RCT | SA; SN (also skilled nursing facility; assisted-living; nursing home; rehabilitation centre) | Home therapy: 103 patients (median age 50 years); 58% male | Inpatient therapy: 97 patients (median age 54 years); 61% male | Fewer patients in the OPAT group (48/103; 46.6%) experienced a serious adverse event compared with hospital patients (52/97; 53.6%) A total of 53 hospital patients (54.6%) discontinued therapy compared with 10 OPAT patients (9.7%). This was most commonly related to adverse events (80% of OPAT; 47% of hospital) Eighteen of 103 OPAT patients (17.5%) required readmission Fewer deaths occurred in the OPAT group (n = 4; 3.9%) than in the hospital group (n = 18; 18.6%) (p = 0.001) |
Reasons for OPAT discontinuation were: skin problems (4), renal failure (2), diabetic gastroparesis (1), and blood creatine phosphokinase increased (1) Reasons for inpatient discontinuation were: cardiovascular problem (4), skin problem (4), infection (3), renal failure (3), blood creatine phosphokinase increased (2), fever (2), sepsis (2), anaphylactic reaction (1), hypoxia (1), red man syndrome (1), thrombocytopenia (1) and vomiting (1) Reasons for OPAT readmission were: other medical condition (11), related to initial infection (4) and problems related to treatment provision in post-acute care setting (3) |
| Richards et al.41 (New Zealand) | RCT | GN | Home therapy: 24 patients (mean age 50 years); 54% male | Inpatient therapy: 25 patients (mean age 50 years); 52% male | Two patients in each group reported nausea and candidiasis There were five extra-pulmonary infections in home patients (21%) and four in hospital patients (16%), and two pulmonary complications in the home group (8.3%) and one (4%) in the hospital group Two of 24 patients were transferred from the home group to hospital (one with pulmonary infection). One hospital patient was readmitted (clinical deterioration) There were no deaths in either group |
Reasons for home to hospital transfer were: development of empyema in legionella infection (1); development of bullous myringitis (1) Extra-pulmonary infection in the home group included three i.v. site infections |
| Seaton et al.10 (UK) | Before and after | SN | Standard OPAT: 230 patients (median age 49 years); 52% male | Nurse-led OPAT: 112 patients (median age 50 years); 61% male | Medical review was required for 21/112 patients (19%) post introduction of a nurse-led protocol Re-admission (6% vs. 7%), drug reaction (4% vs. 7%) and change in therapy rates (5% vs. 4%) were similar for pre- and post-protocol patients |
Reasons for review were: other medical problem (5), rash (8), incision of abscess required (3), and therapy change or admission for lack of improvement (5) |
| Sebban et al.50 (France) | RCT | OP | i.v. therapy: 47 patients (mean age 52 years); 40% male | Oral therapy: 49 patients (mean age 52 years); 31% male | There were five adverse events in the i.v. group (5/46) and three in the oral group (3/48). There were no statistical differences between the two groups for toxicity Nine i.v. patients (19.6%) and five oral patients (10.4%) were readmitted. There were no significant differences between groups in all-cause mortality, clinical deterioration, relapse of infection, new infection, modification of therapy or readmission |
Reasons for readmission were: persistent temperature (9), chose to be readmitted (2), severe dental infection (1), positive initial blood cultures (1) and allergic reaction to ceftriaxone (1) |
| Stein et al.51 (USA) | RCT | OP | i.v. therapy: 10 patients (mean age 54 years); 60% male | Oral therapy: 10 patients (mean age 53 years); 40% male | There were no side effects in the OPAT group. Two of 10 patients in the oral group (20%) experienced adverse events (one discontinued treatment) Four of 10 OPAT patients (40%) were hospitalised owing to lack of improvement |
Adverse events were: skin and tongue pruritus (1) and diarrhoea (1) |
| Wolter et al.48 (Australia) | RCT | Not specified | Home care: 44 admissions (median patient age 43 years); 45% male | Inpatient therapy: 38 admissions (median patient age 49 years); 34% male | There were 23 adverse events reported in the home group (52%) and 31 in the hospital group (82%). Five were considered serious Five hospital and three home group events were considered to be directly/possibly related to place of therapy There were seven readmissions in the home group and four in the hospital group 30 days after discharge. Three patients receiving home therapy had unplanned readmission during treatment |
Serious events were: CVA (1), pneumothorax (1), line-related sepsis (1) and PICC vein thrombosis (2) Location-related events were: hospital patient with vancomycin-resistant enterococci (4) or MRSA (1), home patient with peripheral i.v. infection (1) or presented to another hospital for readmission (1) or PICC line fracture (1) |
| Wolter et al.42 (Australia) | RCT | SA | Home care: 13 admissions (median age 22 years); 28% male | Inpatient therapy: 18 admissions (median age 22 years); 39% male | There were no adverse drug reactions, short-term readmissions, events or deaths attributable to the drugs used One patient had a pneumothorax associated with central line insertion There were no differences between groups in i.v. complication rates (p = 0.57) or numbers of line changes required (p = 0.5) |
There was no significant difference in time to next admission between the two groups (p = 0.68) |
| Yong et al.63 (Singapore) | Case–control (retrospective) | OP; SA | OPAT: 69 patients (mean age 53 years); 53% male | Inpatient therapy: 93 patients (mean age 56 years ); 51% male | Complications occurred in 18/72 OPAT episodes (25%), eight of which required readmission At the end of 4-months’ follow-up, there was no difference in the number of OPAT (13/72; 18.1%) and hospital patients (18/93; 19.4%) readmitted owing to relapse (p = 0.991) There was one patient death in each group |
Complications were: PICC line-related (8), adverse drug reaction (2), admission for underlying medical condition (7) and admission for adverse drug reaction (1) |
| Bedi et al.59 (UK) | Cohort | SA | Home therapy (domiciliary i.v.): 52 patients (median age 61 years); 58% male Home therapy (early supported discharge): 23 patients (median age 65 years); 35% male |
Inpatient therapy: 36 patients (median age 71 years); 36% male | Thirty-day readmission rates were similar across groups (inpatient 13.8%; ESD 12.5%; domiciliary i.v. 14.2%) Antibiotic side effects developed in four inpatients (5%), two ESD patients (6.3%) and four domiciliary i.v. patients (4.7%) There were no i.v. access-related complications in the inpatient group, two (6.3%) in the ESD group, and three (3.6%) in the domiciliary i.v. group No deaths were recorded in any group |
Reasons for admission were: exacerbation of bronchiectasis (all) Access-related complications were: line blockage (3), line falling out (2) and line sepsis (1) |
| Keller et al.214 (USA) | Before and after | Home agency; skilled nursing facility; other (not specified) | Pre-infectious disease transition service: 215 patients (mean age 56 years), 60% male Standard OPAT (pre): 70 patients (mean age 58 years); 69% male |
Post-IDTS: 147 patients (mean age 57 years), 54% male Standard OPAT (post): 56 patients (mean age 55 years), 68% male |
Overall, 199 patients (40.8%) experienced either a readmission and/or ED visit within 60 days of discharge (IDTS arm: pre n = 82, 38.1%; post n = 41, 27.9%; standard OPAT arm: pre n = 35, 50.0%; post n = 16, 28.6%) There was no relationship between the presence of the IDTS and readmission and/or ED visit at 60 days (adjusted OR 0.48; 95% CI 0.13 to 1.79). Similar results were observed for 7-day and 30-day readmissions and/or ED visits The IDTS was associated with decreased antimicrobial prescribing errors (OR 0.062; 95% CI 0.015 to 0.262), increased receipt of laboratory test results (OR 27.85; 95% CI 12.93 to 59.99), and increased follow-up (OR 2.44; 95% CI 1.50 to 3.97) During the 60-day period after discharge, drug-related adverse events, catheter complications, and infection relapse were common |
|
| Lacroix et al.62 (France) | Observational (retrospective) | Not specified | OPAT: 18 patients (mean age 60 years), 61% male | Inpatient therapy: 21 patients (mean age 68 years), 67% male | During OPAT, three patients (16.7%) presented with severe adverse events requiring rehospitalisation; none was attributed to OPAT Following OPAT, three patients (16.7%) presented with severe adverse events, including one death. This compares with seven readmitted patients (33.3%) and one death (4.8%) in the hospital group |
Reasons for readmission were: haemorrhagic stroke with subdural haematoma (1); fever attributable to beta-lactams requiring rehospitalisation for a switch to vancomycin (1) and cardiac failure (1) Adverse events were: death from pneumonia with severe hypoxemia (1), relapse of IE (1) and progressive mitral regurgitation requiring mitral surgery after IE cure (1) |
| Yang et al.61 (Canada) | Case–control | Not specified | OPAT: 21 patients (mean age 59 years), 57% male | Previous standard care: 21 patients (mean age 59 years); 57% male | Incidence of 30-day readmission was lower in the OPAT group than in the previous care group (14.3% vs. 28.6%, p = 0.51), and mean length of hospital stay was shorter by 3.2 days (10.7 vs. 13.9, p = 0.36) The total incidence of adverse drug reactions (n = 5, 23.8%) and VAD-related complications (n = 2, 9.5%) was the same for each group. Three patients in the previous care group (60%) required a change or discontinuation of therapy compared with one (20%) in the OPAT group (p = 0.62) There were no readmissions for adverse events in the OPAT group, and two in the previous care group (9.5%) |
Adverse drug events included: allergy (3), haematological (1), renal/liver toxicity (2), GI-related (1), neurological (1) and abnormal drug level (2) |
| OPAT-only studies | ||||||
| Amodeo et al.215 (New Zealand) | Observational (retrospective) | SA; GN | 100 treatment courses (mean patient age 65 years), 75% male | – | Adverse events occurred in 27/100 episodes; 17 minor events managed in clinic and 10 readmissions There were two deaths in the 12-month follow-up period Five patients were readmitted with further episodes of IE; mean number of days between completion of OPAT and readmission was 220 (range 109–344 days) |
Minor adverse events were: ototoxicity (1), drug-induced hepatitis (1), itch (1), PICC line occlusion (6), line migration (2), phlebitis (2), cellulitis (1) and high residual infusate unrelated to PICC line patency (3) Reasons for readmission were fever and rash (3), drug fever (2), diarrhoea and vomiting (1), hepatitis (1), worsening congestive heart failure (1), flank pain secondary to renal sub-capsular bleed (1) and recurrence of angina (1) |
| Anand et al.180 (USA) | Case series | Home i.v. therapy (unspecified) | 52 patients | – | Seven of 52 patients (13%) had minor complications requiring a change of antibiotic or therapy duration | Complications included: rash (1), increased LFTs (1), transient neutropenia/septicaemia (1) and bleeding at PICC site (1) |
| Angel44 (USA) | RCT | SA | 62 patients (mean age 71 years), 53% male | – | Of 14 reported events, seven were judged unrelated to therapy There were no deaths during the study period |
Related adverse events were: phlebitis (2), rash (2), diarrhoea (1), nausea (1) and fever (1) |
| Barr et al.40 (UK) | Observational (retrospective) | OP; SA | 854 treatment courses, 55% male | – | Twenty of 854 patients had diagnosed line infections, 2.3% of all indwelling line episodes, and an incidence of 0.79 per 1000 line use days (95% CI 0.68 to 0.91 days) Incidence of other line events was 125/854 (14.6%), 4.9 per 1000 indwelling line use days (95% CI 4.68 to 5.21 days) |
There was a lower rate of line infection in SA than in clinic OPAT, but this was not statistically significance (OR 0.6846; 95% CI 0.2805 to 1.671; p = 0.362) There was a higher risk of other line events in SA than in clinic OPAT (OR 1.62; 95% CI 1.06 to 2.46; p = 0.032). This was no longer significant in multivariate analysis (p = 0.22) |
| Barr et al.34 (UK) | Observational (retrospective) | OP; SA; SN; GN | 2233 patients (median age 51 years), 58% male | – | Crude admission rate from OPAT was 11.7% (262/2233 patients). Unplanned readmission was recorded for 204 patients (9.1%) (CR of 6.3 events per 1000 OPAT days) A total of 219/2233 episodes (9.8%) were associated with adverse drug reaction (CR 6.7 events per 1000 days) Line infections occurred in 14/2233 patients (0.6%) (CR 0.4 events per 1000 days). Other line events occurred in 92 patients (4.1%) (CR 2.8 events per 1000 days) Eight patients died (0.4%) |
Unplanned admission included: deterioration in infection (76), new medical event not infection related (63), adverse drug reaction (28), surgery (12), line complication (7) and logistic reasons (7) Drug reactions were: rash (89), severe GI upset (38), chills or fever (28), leucopenia (22), thrombocytopenia or anaemia (21), nephrotoxicity (13) and hepatotoxicity (6) Other line events included: line obstructed or leaking, ‘chemical’ or ‘mechanical’ phlebitis, patient default of follow-up with line in situ and line fall out |
| Berman and Johnson181 (USA) | Case series (retrospective) | SA | 221 patients (median age 41 years), 66% male | – | Side effects were documented in 74/302 episodes (25%). In addition, 18% of patients (55 episodes) complained of constitutional symptoms Three patients (1.4%) were hospitalised for side effects |
Side effects included: renal dysfunction (21), drug rash (18), anaemia (13), diarrhoea (13), vestibular dysfunction (11), fever (9) thrombocytopenia (8), neutropenia (7) and hepatitis (3) Constitutional symptoms were: fatigue (20), headache (12), nausea (19), anorexia (5), weakness (2), palpitations (1), sleepiness (1) and insomnia (1) |
| Bernard et al.84 (France) | Observational (?) | SA; SN | 39 patients (mean age 44 years), 64% male | – | Four of 39 patients reported adverse events (10%) Four of 39 patients were readmitted for medical reasons (10%) There were no haematological or renal complications |
Adverse events were: rash (2) and nausea and vomiting (2) Reasons for readmission were: deep-venous thrombophlebitis (3) and allergic reaction (1) |
| Cervera et al.216 (Spain) | Observational | SA; SN | 73 patients (mean age 60 years), 75% male | – | Twelve of 73 patients (16.4%) had complications requiring readmission Three patients had fatal complications |
Non-fatal events were: heart failure (2), catheter-related sepsis (1), variceal haemorrhage (1), abdominal pain (1), dizziness (1), lower-back pain (1), fever (1) and hypersensitivity reaction (1) Fatal complications were: health-care-related pneumonia (1), cerebral haemorrhage (1) and pulmonary oedema (1) |
| Chambers et al.217 (New Zealand) | Case series | SA; SN | 153 patients (median age 55 years); 58% male | – | Complications of treatment developed in 31/153 (20%) patients All catheter related infections (n = 3) resolved with removal of the devices Fifteen patients (10%) were readmitted within 1 month of discharge from hospital |
Complications were: phlebitis (9), line occlusion (12), line leakage or breakage (6), infection (3), thrombosis (1) and pump failure (4) Reasons for readmission were: washout of infected prosthesis (2), slow resolution of cellulitis (3), jugular vein thrombosis (1) line sepsis (1), uncontrolled pain (3), discharged to inappropriate level of care (1) and unrelated condition (4) |
| Chan et al.182 (Singapore) | Observational | Not specified | 109 patients (mean age 57 years), 66% male | – | Nine patients were readmitted from OPAT There were no deaths or relapses at 30 days post cessation of antibiotics |
Reasons for readmission were: surgical drainage of abscess after initial drainage failure (3), worsening of underlying non-infectious comorbidity (5) and infectious condition unrelated to KLA (1) |
| Chapman et al.13 (UK) | Observational (retrospective) | SA; infusion centre | 334 treatment courses (mean patient age 46 years), 59% male | – | Twenty-one of 334 treatment episodes (6.3%) ended in readmission In 11 episodes OPAT was terminated because of a change of plan Two patients developed C. difficile diarrhoea during i.v. antibiotic therapy; a further patient had diarrhoea on starting OPAT, and was found to have C. difficile toxin |
Reasons for readmission were: unrelated to OPAT (6), inappropriate referral (6), non-resolving soft tissue sepsis (3), deep sepsis with inadequate clinical response (1), symptom control (1), alternative i.v. therapy following antibiotic reaction (1), line infection (1), fractured humerus after fall travelling to OPAT unit (1), cut off end of PICC self-administering antibiotics (1) |
| Cheong et al.218 (Australia) | Case series | GN | 714 treatment courses (mean patient age 53 years), 42% male | – | Adverse drug reactions occurred in 38/714 courses of treatment (5.3%) Drug reactions in five patients were ranked as serious or life-threatening (all were readmitted) |
Adverse events included: skin reaction (18), abdominal symptoms (8), vascular symptoms (5), renal function changes (4), hypotension (2), shortness of breath (1) and severe cutaneous symptoms (1) |
| Cox et al.89 (USA) | Observational (retrospective) | SA | 205 patients (mean age 59 years), 99% male | – | Adverse events occurred in 99/231 (43%) home i.v. courses Venous access complications were frequent (n = 57; 25%) but rarely serious. Catheter or infusion problems resulted in admission in five treatment courses All other events related to adverse drug reactions (n = 42; 18%) |
Venous access complications were: catheter pulled out (22), occluded catheter (18), irritation from dressing (6), site infection (4), leakage (3), bacteraemia (3) and phlebitis (1) Adverse drug reactions were: nephrotoxicity (10); eosinophilia (9), rash (8), leucopenia (7), anaemia (5) and thrombocytopenia (3) |
| Dalovisio et al.183 (USA) | Observational (retrospective) | SA; SN | 62 patients (mean age 69 years) | – | Five of 66 treatment courses (7.6%) resulted in readmission within 30 days; none was directly related to the infectious diagnosis Twelve of 62 patients (19.4%) experienced line complications No patients died while receiving i.v. treatment |
Reasons for readmission included: pneumonia, chest pain, surgical wound infection, hepatic failure and tumour fever Line complications included: line breakage, leakage, infection and infiltration |
| Dargan et al.184 (Canada) | Case series (retrospective) | OP; home therapy (unspecified) | 66 patients (mean age 57 years); 50% male | – | Nineteen of 66 patients (29%) experienced a total of 25 complications. Most (n = 15) were attributed to i.v. access, and the remainder to adverse drug reactions Five patients (all treatment failures) were readmitted to hospital for reasons attributable to the infection |
Access related events were: interstitial of i.v. line (6), occluded line (3), phlebitis (3), bleeding (1), leakage of PICC line (1) and thrombosis (1) Drug related events were: nausea (3), acute renal failure (1), allergic reaction (1), hypokalaemia (1), nephrotoxicity (1), neutropenia (1), vomiting (1) and yeast infection (1) |
| Dobson et al.219 (Australia) | Case series | SA; SN; GN | 770 patients (mean age 48 years), 66% male | – | Allergic reactions were experienced by 28/770 patients (3.6%). No patient had anaphylaxis Mean time from treatment start to reaction onset was 19.6 days (range 1–39 days) |
Allergic reactions included: rash (13), rash with pruritus (4), fever (3) and perioral angioedema (3) No reactions were experienced by patients who received drugs via bolus or intermittent administration alone [although this did not reach significance (OR 0; 95% CI 0 to 1.7)] |
| Donald et al.185 (Australia) | Case series (retrospective) | SN | 124 patients (aged 16–97 years), 60% male | – | One patient developed self-limiting diarrhoea. There were no other complications attributable to therapy Nineteen of 124 patients (15%) were readmitted |
Reasons for readmission were: failure of cellulitis to resolve (13), abscess requiring surgery (4), analgesia needed (1) and admission for central venous access (1) |
| Duncan et al.26 (UK) | Observational (retrospective) | Not specified | 1377 treatment courses | – | A total of 51 adverse reactions were observed in 1377 episodes (3.7%), a prevalence rate of 37.0 per 1000 new patient episodes | Adverse reactions were: rash (20), hepatic enzyme abnormality (10), leucopenia, thrombocytopenia or anaemia (6), GI symptoms (5); anaphylaxis (4), chills or fever (3) and acute kidney injury (3) |
| Esposito et al.186 (Italy) | Observational | OP; SA; visit by doctor or nurse (also care facility) | 176 patients (aged > 65 years); 47% male | – | Adverse drug reactions, usually mild, were detected in 22/176 patients (13%) | Adverse reactions were: rash (7), fever (3), itching (3), renal impairment (2), hepatic impairment (1), diarrhoea (1), anaphylactic reaction (1), eosinophilia (1), dizziness (1), urticaria (1) and dyspnoea and palpitations (1) |
| Esposito et al.83 (Italy) | Observational (retrospective) | OP; SA; visit by doctor or nurse (also care facility) | 239 patients (aged 11–80+ years), 62% male | – | Adverse drug reactions, usually mild, were detected in 27/239 patients (11%), and were more frequently seen in patients who received combination therapy (74.1% vs. 25.9%) | Adverse reactions were: rash (12), fever (3), hepatic impairment (3), nausea/vomiting (2) renal impairment (2), anaphylactic reaction (1), diarrhoea (1), itching (1), leucopenia (1) and urticaria (1) |
| Goodwin et al.27 (Canada) | Case series | SN; GN | 2405 patients (mean age 46 years), 55% male | – | Thirty-three of 1642 patients (2%) discontinued therapy before prescribed completion (treatment related in 25 cases) Fifty-two of 1642 patients (3.2%) were admitted to hospital Fifty-four of 1642 patients (3.3%) had unplanned interruptions to therapy; only two were unrelated to treatment (comorbidity) |
Reasons for discontinuation were: adverse effect of therapy (9), inadequate venous access (5), unscheduled admission owing to worsening infection (4), early resolution of infection (5), change of therapy (4), change to oral therapy (9), comorbidity (5) and patient decision (3) Reasons for admission were: comorbidity (36), treatment failure (12) and inability to manage self-therapy (4) Reasons for interruption were: venous access complication (15), temporary loss of venous access (26), device problem (n = 18) |
| Gourdeau et al.220 (Canada) | Case series (retrospective) | OP; SA | 124 patients (mean age 41 years), 56% male | – | Two of 124 patients (1.6%) experienced adverse reactions requiring treatment modification There were no cases of catheter-related infection One patient was readmitted (owing to lack of improvement) |
Reasons for treatment modification were: rash (1) and recurrent phlebitis (1) |
| Graninger et al.187 (Austria) | Case series | OP | 54 patients | – | Adverse events were seen in nine of 54 patients (17%) | Adverse events were: thrombocytopenia (3), rash (3), nausea (1), C. difficile colitis (1), fever (1), transient hearing impairment (1) and leucopenia (1) |
| Grayson et al.67 (Australia) | Case series | GN | 20 patients (mean age 58 years) | – | No major infective complications related to i.v. antibiotic administration were noted Two complications were seen (2/20; 10%) |
Complications were: localised soft-tissue infection at catheter exit site (1) and progressive renal dysfunction (1) |
| Gross et al.188 (USA) | Case series (retrospective) | Home i.v. therapy (unspecified) | 13 patients (mean age 38 years), 100% male | – | Eleven patients (85%) experienced 22 adverse events (one patient had four, two patients had three, four patients had two and four patients had one) There were no deaths |
Adverse events were hypoglycaemia (8), nephrotoxicity (6), hypotension (5), hyperkalaemia (1), hypercalcaemia (1) and elevated pancreatic enzymes |
| Heintz et al.221 (USA) | Observational | OP; SA; SN; infusion centre (also long-term care facility; interim-care facility; prison) | 494 treatment courses | – | Infection and/or OPAT-related complications occurred in 35/494 cases (7.1%) Intervention by the infectious disease pharmacist post discharge occurred in 26/494 cases (5.3%) |
Complications included: readmission and/or emergency department visit within 30 days of discharge (28), 30-day infection-related mortality (2) and drug-induced toxicity (11) Drug-induced adverse effects were: rash/hives (5), nephrotoxicity (3), bone-marrow suppression (2) and intractable nausea and vomiting (1) |
| Hindes et al.68 (USA) | Case series | SA | 48 patients (mean age 65 years) | – | Complications were seen in four of 48 patients (8%) Three patients required readmission (6%) |
Complications were: serum sickness reaction (3) and subclavian vein thrombosis (1) Reasons for readmission were: serum sickness (1), new site infection (1) and seizure unrelated to therapy (1) |
| Hitchcock et al.69 (UK) | Case series | OP; SA; GN; private home health-care company | 273 patients (mean age 60 years), 54% male | – | Twenty three of 303 courses (8%) were followed by readmission during therapy or within 28 days of completion Thirteen admissions were related to the original diagnosis or to OPAT drug treatment; 10 were unrelated |
Reasons for OPAT readmission were: surgery after treatment failure (3), line problem (2), adverse drug effect (2), treatment failure at end of original course (3), super infection (1) and worsening clinical condition (2) |
| Ho et al.189 (Singapore) | Case series | OP | 29 patients (median age 41 years), 90% male | – | Six of 29 patients (21%) required readmission, five during OPAT and one during the 30-day follow-up period There were no deaths or significant misadventures |
Reasons for hospital readmissions were: PICC infections (2), complications related to endocarditis (2), bacteraemia caused by a different organism (2) Incidence of readmission and PICC infection was similar to that found in non-IVDU endocarditis patients treated in OPAT |
| Huminer et al.70 (Israel) | Case series | SA; SN (also nursing home; kibbutz) | 37 patients (mean age 64 years), 57% male | – | Eight of 37 patients (22%) experienced adverse drug reactions Ten of 37 patients had local complications (27%) Six patients were readmitted before completing OPAT |
Adverse drug reactions were: fever (5), rash (2), and syncope (1) Local complications were: occluded line (10), thrombophlebitis (6) and intravascular infection (1) Reasons for admission were: fever (5) and PSV tachycardia (1) |
| Johansson et al.71 (Sweden) | Case series | SA | 11 patients (mean age 51 years), 73% male | – | One patient telephoned the hospital on one occasion for help with CVAD handling One patient developed a CVAD exit-site infection All other patients recovered without readmission and without antibiotic changed |
|
| Kayley et al.72 (UK) | Case series | SA; GN; doctor administered | 67 patients | – | Complications of home therapy were minimal (17/67; 25%) Line infections (n = 3) were all seen in HIV-positive patients who were treated for > 6 months continuously |
Complications were: line infection (3), broken line clamp (5), blocked line (2), dislodged line (3), drug allergy (2) and C. difficile diarrhoea (2) |
| Kieran et al.2 (Ireland) | Case series (retrospective) | SA; nurses attached to commercial company | 56 patients (median age 50 years), 57% male | – | Adverse events occurred in 12/60 episodes (20%) Five of 56 patients (7%) had a line-related complication (rate of 3.9 per 1000 outpatient central line days) Drug-related adverse events occurred in 4/60 episodes (6.7%) In seven of 60 episodes (12%), patients required readmission related to an adverse event; in five cases (8.3%) this was therapy related |
Line complications were: line infection (2), line blockage (2) and dislodged line (1) Drug-related events were: rash (2), acute hepatitis (1) and neutropenia (1) Reasons for readmission were: acute hepatitis (1), neutropenia (1), failure to manage OPAT (1), anxiety (1) and line infection (1) |
| Larioza et al.190 (USA) | Case series (retrospective) | Home infusion company | 33 patients (76% aged ≥ 50 years), 61% male | – | Four of 33 patients (12.1%) developed complications There were no deaths |
Complications were: line infection (2), deep-vein thrombophlebitis (1) and C. difficile diarrhoea (1) |
| Larioza et al.191 (USA) | Case series (retrospective) | Home infusion company | 43 patients (56% aged > 50 years), 67% male | – | Ten of 43 patients (23%) were readmitted to hospital during antibiotic treatment | Reasons for readmission were: antibiotic-associated diarrhoea (1), PICC-related issues (6), interstitial nephritis (1) and drug rash (2) |
| Laupland et al.28 (Canada) | Observational | OP; SA; SN | 3145 patients (mean age 46 years), 55% male | – | Overall, 139 of 3145 patients (4%) were admitted to hospital within seven days of starting home therapy Another 82 patients (3%) had interruptions to therapy |
Reasons for interruption were: venous access related (45), infusion delivery system related (30), comorbidity (3) and unspecified (4) |
| Lillie et al.147 (UK) | Case series | SN | 98 patients (mean age 55 years), 61% male | – | Of 64 patients attending 4-week follow-up, two (3.1%) had a relapse of infection; one patient required admission for treatment | |
| Lin et al.222 (USA) | Case series (retrospective) | Not specified | 177 patients | – | Antibiotic complications occurred at a rate of 16% (28/177). Most were minor in nature PICC line-related complications occurred in four patients (2%) There were no permanent complications or deaths |
Antibiotic complications were: rash (11), diarrhoea (6), transient neutropenia (3), itchiness (2), fatigue (2), flushing (1), fever (1), black tongue (1) and elevated LFTs (1) PICC-related complications were: line thrombosis (3) and septicaemia (1) |
| Lopardo192 (Argentina) | Case series (retrospective) | OP; SA; SN | 48 patients (median age 55 years), 62% male | – | Complications occurred in 7/48 patients (15%; only one occurred while the patient was still receiving antibiotic treatment (heart failure) | Complications were: heart failure (3) and clinically significant emboli (4) |
| Mackintosh et al.3 (UK) | Case series (retrospective) | OP; SA; SN | 198 patients (aged < 40–89 years), 64% male | – | Twenty of 198 patients (10.1%) suffered an adverse reaction to initial i.v. therapy | Adverse reactions were: rash (9), leucopenia or thrombocytopenia (4), vomiting (3), fever (2), tinnitus (1) and acute kidney injury (1) |
| Mauceri45 (USA) | RCT | SA | 27 patients | – | Ten treatment-related adverse effects were reported in 27 patients, (two were considered severe) Three patients were withdrawn from the study owing to adverse events |
Adverse events were: rash (3), gastrointestinal symptoms (3), growth-resistant organisms (3) and fever (1) |
| McMahon et al.193 (Australia) | Observational | SN | 40 patients [mean age 59 years (male), 49 years (female)], 75% male | – | Nine of 40 patients (23%) experienced a total of 11 adverse events. All were considered to be related to the antibiotic or to complications of venous access Three patients required hospital readmission Three patients who were treatment failures were readmitted |
Adverse events were: rash (2), neutropenia (2), catheter-related (2), pseudomonas bacteraemia (1), fever (1), diarrhoea (1), nephrotoxicity (1) and chest pain during infusion (1) Reasons for treatment failure readmission were: dyspnoea and pericardial effusion (2) and cellulitis around a cardiac surgery site (1) |
| Montalto et al.30 (Australia) | Case series (retrospective) | SN (HHU) | 3423 treatment courses (patient age 0–80+ years), 55% male | – | Unexpected telephone calls were made by 607/3423 patients (18%) There were 177 unexpected staff callouts (5%). Patients receiving antibiotics were more likely to have a staff call out than patients receiving non-antibiotic therapy (p < 0.001) A total of 143 episodes of care (4%) involved an unplanned return to hospital. Patients who received antibiotic therapy were more likely to require an unplanned return to hospital (p = 0.001) There were five patient deaths; two during HHU stay and three after return to hospital |
Reasons for callout included: pump malfunction (46), venous access problems (30), pain (19), vomiting (17), fever (14) and anxiety (13). Patients who called were more likely to return to hospital than those who did not (14.5% vs. 2.2%, p < 0.001) Reasons for readmission included: lack of improvement (40), cardiac problem (22) and fever (19). No patient had an anaphylactoid drug reaction; four patients were returned for other drug reactions |
| Montalto194 (Australia) | Case series (retrospective) | SN (HHU) | 133 patients (mean age 46 years), 52% male | – | Twenty-eight telephone calls were received from 22/133 patients (17%; range 1–3 calls). Thirteen were initiated by the patient or carer either anxious or asking about the condition or its management. Most required reassurance and follow-up Thirteen calls resulted in unscheduled staff callouts. One patient was readmitted Five of 133 patients (3.8%) were returned to hospital during their HHU admission |
Reasons for calls were: nausea or vomiting (6), pain (3), allergic reaction (1), diarrhoea (1), fever (1), problems with venous cannula (2) and perception of worsening condition (2) Results of callouts were: i.v. maxolon given (5), management of anaphylaxis (1), vasovagal episode during drug administration (1), discussion with GP/unit director and action planned (5) and readmission (1) Reasons for readmission were: carer withdrawal (1), exploration of possible abscess (1), exploration of possible palmar space collection (1), worsening respiratory state in COPD (1) and non-resolving pneumonia (1) |
| Morales and Von Behren195 (Puerto Rico) | Case series | SA | 22 patients (mean age 33–41 years); 82% male | – | Six of 22 patients (27%) reported nine adverse events (all mild or moderate) Four patients were withdrawn owing to adverse events There were no deaths during the study period |
Unrelated adverse events were: headache (1), urethral disorder (1) and diarrhoea (1) Reasons for withdrawal were: urticaria (1), probable allergic reaction (1), flushing (1), and fever, rash, diarrhoea and urethral disorder (1) |
| Nathwani74 (UK) | Case series | OP; SA; SN | 125 patients | – | There were no complications associated with vascular devices One patient was readmitted for OPAT failure (as a result of various logistical reasons) |
|
| Nathwani et al.75 (UK) | Case series | OP; SA | 101 patients | – | Six of 101 patients experienced an adverse drug reaction (6%) Eight patients had an unscheduled readmission (8%) Twelve patients (12%) had PICC line complications |
|
| Parker et al.86 (UK) | Observational | OP; SA | 29 patients; 38 general practices | – | Eight of 29 patients (28%) reported a problem | Adverse events were due to speed of the injection or discomfort caused by i.v. access (6), and hypersensitivity-type reactions (2) |
| Partridge et al.198 (UK) | Case series (retrospective) | OP; SA | 34 patients (mean age 55 years), 79% male | – | Adverse events occurred in 12/36 episodes (33%), including one patient death (unrelated to OPAT) Two patients with line infection required readmission Three patients were readmitted for non-OPAT-associated medical problems |
Adverse events included: line infection (4), split Hickman line (1), PICC line dressing allergy (1), pain in PICC arm (1), ototoxicity (1), renal toxicity (1), diarrhoea (1), and mycotic aneurysm of the spleen (1) |
| Patanwala et al.66 (USA) | Decision tree analysis | SN | 41 patients (mean age 53 years), 44% male | – | Nine of 41 patients (22%; all treatment failures) were readmitted | |
| Pérez-López et al.199 (Spain) | Observational | SN | 145 patients (mean age 68 years), 48% male | – | There were 23 adverse events; most common was phlebitis (n = 21; 15%) A total of 11/145 patients (8%) required hospitalisation No patients died A further 22 patients were readmitted during the 3-month follow-up period (four died while in hospital) |
There were no significant differences in the number of adverse events between older (n = 14; 15%) and younger (n = 9; 16%) patients (p = 0.65) |
| Poretz46 (USA) | RCT | SA | 238 patients (mean age 45–52 years); 57% malea | – | Seventy-two of 238 patients (30%) reported adverse experiences considered related to therapy (seven classified as severe, 25 moderate, 65 mild) | Adverse events included: phlebitis (13), rash (12), diarrhoea (8), maculopapular rash (4), hypoxia (3), allergic reaction (3), fever (3), headache (3) and nausea (3) |
| Poretz47 (USA) | RCT | SA | 130 patients (mean age 45 years), 59% malea | – | Thirty-one of 130 patients (24%), reported adverse events (27 events were related to the study drug and four to the i.v. line) | Adverse events included: rash (7), diarrhoea (4), phlebitis (4), allergic reaction (3) and maculopapular rash (3) |
| Rodríguez-Cerrillo et al.76 (Spain) | Case series | SN (HHU) | 24 patients (mean age 73 years) | – | Three of 24 patients contacted the HHU for minor symptoms or doubts about treatment One patient required an unscheduled visit by HHU staff (vomiting) No patient had an unexpected return to hospital |
|
| Rodríguez-Cerrillo et al.77 (Spain) | Case series | SN (HHU) | 25 patients (mean age 59 years), 48% male | – | One of 25 patients (4%) made an unexpected telephone call for diarrhoea (after starting oral antibiotics) There were no urgent visits by HHU staff, hospital readmissions during HHU treatment, or admissions in the month following discharge |
|
| Seaton and MacConnachie200 (UK) | Case series (retrospective) | SA; other (unspecified) | 19 patients (mean age 55 years), 53% male | – | Eighteen patients (95%) survived > 6 months after hospital discharge There were two adverse events and one patient death. No further events were noted in the OPAT setting with therapy up to 128 days There were no unplanned readmissions |
Adverse events were: above knee amputation for osteomyelitis/septic arthritis (1) and reversible myotoxicity (1) |
| Seaton et al.201 (UK) | Observational (retrospective) | OP | 963 patients (median age 48 years), 59% male | – | Significant adverse events were observed in 68/963 patients (7.1%) Admission or readmission occurred in 58/963 cases (6%) |
Adverse events included: drug-related rash requiring a change in therapy (25), GI side effects, particularly diarrhoea (16) and abnormal liver function (11). Three patients had severe allergy or anaphylaxis Reasons for admission included: treatment failure with progression of infection or lack of response (19) and other medical complication (15) |
| Smego et al.223 (Pakistan) | Case series | SA | 316 patients (mean age 45 years), 53% male | – | Medication-related adverse effects were few (14/316), generally mild, and resolved with drug dosage adjustment or discontinuation of use No unscheduled clinic or emergency room visits |
Side effects were: renal impairment (4), skin rash (3), diarrhoea (3), leucopenia (2), hepatitis (1) and drug fever (1) Minor thrombophlebitis was the only catheter-related effect (3) |
| South202 (UK) | Case series (retrospective) | OP; SA; nurse (unspecified) | 57 patients (mean age 41 years), 77% male | – | There were two adverse events among the 69 episodes treated (2.9%) | Adverse events were: shaking following i.v. bolus administration (1) and truncal rash (1) |
| Talcott et al.87 (USA) | Observational (pilot) | SA; SN | 30 patients (median age 38 years), 43% male | – | Five patients were readmitted for recurrent or prolonged fever (17%). Four patients were readmitted for medical complications (13%) There were no lasting complications or deaths |
None of the 27 medically eligible patients who did not enter the study had complications. The complication rate for all medically eligible patients was 4/57 (7%) |
| Theocharis et al.203 (Greece) | Case series (retrospective) | SN | 91 patients (mean age 85 years), 31% male | – | Mortality was 27.5% (25/91; (those who were not cured) Thirteen patients were admitted to hospital (14%); three died |
|
| Tice et al.224 (USA) | Observational (retrospective) | OP; SA; office based; pharmacy based | 971 patients | – | One hundred and nineteen of 1053 (11%) episodes were complicated by adverse events Early discontinuation of therapy occurred in 6.4% of episodes (most commonly for rash and fever) One hundred and forty-six venous access device-related adverse events were observed |
Adverse events were: rash, renal toxic reaction, fever, nausea and vomiting, urticaria, diarrhoea, anaphylactic reaction, leucopenia, and vestibular toxic reaction. Rash was common (3%) as were renal toxicity (1.5%) and fever (1.3%) Access events included: phlebitis (42), thrombosis (21), local infection (17), leakage (12) and bacteraemia (9) |
| Tice80 (USA) | Observational (retrospective) | OP; SA; SN; infusion centre | 500 patients | – | A minority of patients (12%) discontinued treatment prematurely, most commonly for adverse drug reaction (5.2%). Discontinuation related to clinical failure in around 1% of cases, and to death in 0.4% | |
| Tice81 (USA) | Case series | OP; SA; SN | 538 patients (mean age 45 years); 52% male | – | Medication was changed owing to an adverse effect in 16 cases Forty-two of 538 patients (7.8%) were hospitalised after starting OPAT. Only one patient (drug rash) was hospitalised as a result of the OPAT programme or medications One death occurred during treatment (patient with advanced AIDS) Three patients were removed from the programme because of failure to comply |
Adverse events were: rash (11), leucopenia (1), neuromuscular disturbance (1), renal toxicity (1), laryngeal oedema (1) and intractable ‘red man’ syndrome (1) Reasons for admission were: surgery unable to take place at outpatient centre (20), poor clinical response (5), inadequate home care (3), neurological problem (3), cardiac disease (2), bleeding (2), chest disease (2), vomiting (2), leucopenia and fever (1), reimbursement problem (1) and drug rash (1) |
| Upton et al.204 (New Zealand) | Case series | SA | 100 patients (mean age 51 years), 59% male | – | Thirty-five of 100 patients (35%) experienced complications during treatment Twenty-two patients had central line complications (incidence of 5.3 per 1000 central line days). Twelve occurred after discharge and five resulted in readmission Twenty-two patients had adverse drug reactions; three resulted in readmission One patient died 2 days after completing treatment (ruptured iliac aneurysm) |
Line complications were: line blockage (8), infection (5), DVT (4), phlebitis (3), failed line insertion (1) and line intolerance (1) Adverse drug reactions were: rash (8), GI symptoms (7), chest discomfort (6), abnormal LFTs (5), leucopenia (4) and nephrotoxicity (3) Reasons for readmission: PICC line infection (3), PICC line reinsertion (2), anxiety (1), nausea (1) and allergic reaction (1) |
| Walton et al.205 (Australia) | Case series | SN | 35 patients (mean age 50 years), 71% male | – | Four patients stopped treatment prematurely No patients developed penicillin associated neutropenia Four patients had problems with PICC venous access; three required replacement |
Reasons for discontinuation included: probable drug-related febrile hypersensitivity reaction (1) PICC-associated septicaemic shock (1) and weakness and focal seizures (1) Reasons for PICC replacement were: catheter blockage (1) and exit site infection (2) |
| White et al.206 (Australia) | Case series (retrospective) | OP; SN; GN | 55 patients (mean age 50 years); 65% male | – | Seven of 55 patients (13%) had adverse drug events, with i.v. antibiotics responsible for events in three patients There were no complications related to i.v. access |
Antibiotic events were: rash (1), thrombocytopenia (1) and deranged liver enzymes (1) |
| White et al.207 (UK) | Case series (retrospective) | SA | 72 patients (mean age 42 years); 42% male | – | A total of 39 drug reactions were documented in 29/72 patients (40%). Therapy was discontinued in 10 patients (14%) One patient was admitted to hospital (line infection with sepsis) |
Drug reactions were: neutropenia (13), mild liver function derangement (8), line infection (3), rash (3), allergic reaction (2), diarrhoea (4), headache (1), nausea (1), fatigue (1), oral thrush (1), shingles (1) and dyspepsia (1) Reasons for discontinuation were: neutropenia (4), drug rash (3), allergic reaction (1) and line infection with severe sepsis (1) |
| Williams31 (USA) | Observational (retrospective) | SA | 1045 patients (mean age 38 years); 58% male | – | Drug related side effects were relatively infrequent (2–5%) Venous access issues were common (375/1500; 25%) Rate of readmission was 5–6% (75–90 patients) No deaths were directly attributable to home therapy and overall morbidity was low |
Drug side effects were: nephrotoxicity (75), skin rash (75) and leucopenia (30) Access issues included: infiltration of i.v. and phlebitis of a peripheral cannula Reasons for readmission included: antibiotic failure, therapy change and surgery |
| Wynn et al.32 (USA) | Observational (retrospective) | Physician-based programme; home health organisation; hospital-based programme (no other details) | 1252 patients (mean age 52 years), 65% male | – | A total of 66 adverse drug reactions were reported in 1515 patients (4%) There were 106 vascular access complications in 1232 patients (9%) |
Adverse drug reactions were: rash (26), diarrhoea (8), anaphylaxis (6), leucopenia (6), renal toxicity (6), fever (5), nausea/vomiting (5) and unspecified (4) Access complications included: phlebitis (31), leakage (19), thrombosis (10), local reaction (9) and bacteraemia (4) |
| Yadlapalli et al.208 (USA) | Case series (retrospective) | Not specified | 58 patients (mean age 60 years), 83% male | – | During 12-months’ follow-up, 10 patients (17.2%) required readmission for further treatment | |
| Yan et al.5 (UK) | Case series (retrospective) | OP; GN | 140 patients (aged 17–89 years), 64% male | – | Five of 140 patients (3.6%) required hospital admission owing to an inadequate response Three patients (2.1%) developed a rash There were no reported complications related to use of i.v. catheters (some patients inadvertently removed their cannula at home) |
Three of the patients admitted to hospital required referral to the orthopaedic/surgical team owing to abscess formation and tracking of cellulitis |
| Al Ansari et al.82 (Bahrain) | Case series (retrospective) | OP | 101 patients; 57% male | – | Two patients (2%) were readmitted to hospital | |
| Allison et al.225 (USA) | Cohort (retrospective) | SN | 782 patients (mean age 58 years), 57% male | – | Twenty-six per cent (207/782) of patients were readmitted within 30 days Nearly half of 16 patients prescribed aminoglycosides at discharge; 6 were readmitted for medication side effects Regression analysis showed that risk of readmission was related to age (OR 1.09 per decade, 95% CI 0.99 to 1.21; p = 0.10), aminoglycoside use (OR 2.33, 95% CI 1.17 to 4.57; p = 0.01), drug resistant organisms (OR 1.57, 95% CI 1.03 to 2.36; p = 0.03) and number of prior hospital discharges without i.v. antibiotics in the past 12 months (OR 1.20 per prior admission, 95% CI 1.09 to 1.32; p < 0.001) |
Reasons for readmission were non-infectious disease related (63), worsening infection (62), new infection (48), adverse drug reaction (30), line complication (20) and diarrhoea (2) Medication side effects were: acute renal insufficiency (4), rash (1) and rash and neutropenia (1) |
| Barr et al.226 (UK) | Observational (retrospective) | Not specified | 780 patients (median age 52 years); 58% male | – | During or up to 90 days following OPAT, 34 patients (4.4%) were investigated for suspected symptomatic VTE; two DVTs were diagnosed [incidence 2/780, 0.26% (95% CI 0.03 to 0.92%)] Up to 1 year following OPAT, there were a total of five VTEs (0.64%). The rate of VTE appeared constant in the year following OPAT |
|
| Duncan et al.54 (UK) | Observational (retrospective) | Not specified | 55 completed OPAT patients (median age 59 years), 80% male; 25 failed OPAT patients (median age 67 years), 60% male | – | Twenty-one episodes resulted in readmission (26.3%), seven in an adverse drug event (8.8%), three in line complications (3.8%) and two in drug resistance (2.5%) | Reasons for readmission included: suspected endocarditis decompensation (7) and non-endocarditis related (4) Reasons for OPAT failure included: hyperkalaemia (1), vomiting (1), acute renal dysfunction (1), presyncope during administration (1) and resistance to oral component of the OPAT regimen (1) |
| Htin et al.209 (Australia) | Case series (retrospective) | SN | 68 patients (median age 68 years), 87% male | – | Three patients (4%) developed complications and required readmission There were two deaths, both unrelated to OPAT. Of the remaining 66 patients, 65 were still alive and 1 patient was lost to follow-up [1-year survival 96% (65/68)] |
Reasons for readmission were: line-related bloodstream infection (1), fever attributable to flare of gouty arthritis (1), worsening anaemia in IHD and Crohn’s disease (1) |
| Lai et al.210 (USA) | Case series (retrospective) | SA; SN | 333 patients (mean age 62 years), 98% male | – | Complications occurred in 96/393 OPAT courses (24.4%). The most common was readmission (n = 49, 12.5%) Adverse drug events were present in 40 of the 393 courses (10.2%). Shortening of OPAT (including switches to oral antimicrobial therapy) occurred in 29 of the 40 cases (72.5%; 7.4% of all) The overall line-related complication rate was 6.4% (25/393) over the period (PICC line, n = 22; peripheral i.v. line, n = 3) Rates of PICC complications decreased across the period (8.4% to 4.5%), although this was not statistically significant |
Reasons for readmission included: failure to improve on OPAT (15), reason unrelated to OPAT (13), PICC-related (9) and adverse drug event (8) Adverse drug events were: acute kidney injury (11), pruritus/rash (10), leucopenia (7), GI problems, including diarrhoea (6) and C. difficile-associated diarrhoea (2) Access-related complications included: occlusion/accidental displacement (9), erythema or tenderness (6) and bloodstream infection (6) |
| Lane et al.38 (USA) | Cross-sectional | OP; SA | 555 adult infectious diseases physicians | – | OPAT-associated complications were perceived as being rare (≤ 5% of patients) The most commonly reported in greater numbers than this were line occlusion/(80% of respondents), rash (39%), and nephrotoxicity (39%) Patients commonly required line exchange or removal or change in antibiotic therapy because of complications; hospitalisation was less common |
|
| Mohammadi et al.211 (USA) | Case series (retrospective) | SA; SN | 190 patients (mean age 63 years), 98% male | – | Adverse events occurred in 12/90 patients (6.3%) during the review period. No emergence of a drug-resistant organism or C. difficile was observed There were five peripherally inserted central catheter line adverse events, including three admissions (1.6%) Eight deaths during OPAT were related to malignancies |
Adverse drug events included: neutropenia, diarrhoea, drug allergy rash, and nephrotoxicity Access-related events included line infection (4), readmission (3) and ED visits for line-related events (2) |
| Muldoon et al.91 (Ireland) | Cross-sectional | Not specified | 55 consultant physicians (15% clinical microbiologists) | – | 61% of those reporting on readmission estimated the 30-day readmission rate for OPAT patients as being < 10%, while the remainder estimated it at between 10% and 25% | |
| Muldoon et al.92 (USA) | Cross-sectional | Not specified | 316 adult and paediatric infectious diseases physicians | – | Most respondents had witnessed at least one complication in the last year (243/274, 89%) Adverse drug reaction was the most commonly reported (69%), followed by thrombosis (58%), C. difficile-related disease (53%), line-related bacteraemia/exit site infection (51%), readmission (43%), phlebitis (40%) and death (2%) |
|
| Pajarón et al.227 (Spain) | Case series (retrospective) | SA | 45 patients (mean age 63 years), 76% male | – | Eight patients returned to hospital with complications and six were admitted (12.5%) An additional 11 patients had less serious complications (24.4%) There were three recurrences in 2/45 patients during the year following the initial episode (one patient had two recurrences) No patients died in the course of treatment at home. Five patients (13%) died during the first year following discharge from hospital (two related to endocarditis and three to pre-existing advanced cancer) |
Reasons for readmission were: cardiac insufficiency (2), acute renal insufficiency with hypokalaemia (1), sepsis (1), medication-related anaphylactic reaction to allopurinol and acute secondary renal insufficiency (1), and hepatic-renal syndrome secondary to hepatocarcinoma (1) Adverse events were: congestive cardiac insufficiency (4), angina (1), acute episode of previous chronic renal insufficiency (1), drug side effect (4) and catheter-related infection (3) |
| Seetoh et al.33 (Singapore) | Cohort | OP; SA; SN | 2229 patients (median age 56 years); 64% male | – | Re-admission was needed in 281 episodes (12.6%), and 74 patients (3.3%) ended treatment early Re-admission rate as a result of clinical deterioration was 9.0%, and occurred a median of 11 days (IQR 5–23 days) after beginning treatment SN OPAT episodes were more than twice as likely to deteriorate as OP episodes (20% vs. 7.9%; aHR 2.5, 95% CI 1.7 to 3.8; p < 0.001). There was no significant difference compared with SA (20% vs. 9.6% aHR 0.9, 95% CI 0.6 to 1.3; p = 0.572) Of those who did not complete treatment as planned (i.e. those readmitted or ceasing early without readmission): 60 (2.7%) had adverse drug reactions, 16 (0.7%) had PICC line complications, four (0.2%) absconded, and two (0.1%) died suddenly |
Reasons for readmission included: elective procedures (42) and clinical deterioration (201) |
| Sims et al.212 (UK) | Case series (retrospective) | OP | 14 patients (mean age 63 years), 71% male | – | Four patients (29%) required readmission, two for medical conditions unrelated to OPAT (UTI and haematemesis), and two for deterioration in the clinical status of their infected arthroplasty There were no line complications There was one unrelated patient death |
|
| Subedi et al.213 (Australia) | Case series | SA; SN | 144 patients (median age 55 years), 74% male | – | There were 11 (7%) drug-related adverse events, one resulting in readmission (6.9%). The rest were managed with symptomatic treatment, earlier cessation or a change of antibiotic Line-related events occurred in five patients (3%) or 1.4/1000 catheter-days OPAT-related readmission occurred in nine patients (6%) within 28 days of cessation of i.v. antimicrobials |
Reasons for readmission were: drug fever (1), reinsertion of PICC line (1), clinical deterioration requiring source control or surgical debridement (6) and change of antimicrobial therapy (1) Adverse drug events included: rash (6), biliary lithiasis (1), drug fever (1) and acute kidney injury (2) Access-related events were: infection (2), lymphatic leakage (2), and line falling out (1) |
| Author (country) | Study design | OPAT model(s) | OPAT group(s)a | Comparator(s)a | Findings |
|---|---|---|---|---|---|
| Krauth et al.23 (worldwide) | Literature review | Not specified | 11 studies | Inpatient therapy | Studies predominantly concluded that home i.v. therapy would lead to significant cost reductions from the societal perspective as well as from the third-party payer perspective. In five studies, inpatient therapy was 1.8–3.3 times as expensive as home care; three studies that considered incremental costs revealed potentially significant cost savings with home therapy. Two of the remaining three studies reported divergent results, primarily related to pharmaceutical pricing and duration of therapy Only one study included loss of working days and its monetary valuation and found that the indirect costs per course of inpatient treatment totalled DM868 compared with DM476 for home treatment |
| Patanwala et al.66 (USA) | Decision tree analysis | SN | 41 patients (mean age 53 years), 44% male | Switch therapy (i.v.-oral) Oral therapy |
Oral treatment during hospitalisation and after discharge had superior effectiveness and lower cost than the switch and OPAT scenarios in the base-case incremental cost-effectiveness analysis (US$8923, 0.87 vs. US$11,479, 0.78 vs. US$12,481, 0.71, respectively). The cost variable that was expected to have the largest impact on the model was cost per day in hospital. Varying the baseline cost per day in hospital (range US$463–2500) did not affect the dominance of the oral option The switch option would be the cost-effective choice if the length of hospitalisation was < 6 days or if the probability of cure with the oral option was ≤ 0.72 |
| Teuffel et al.65 (Canada) | Decision tree analysis | SN | 77 patients | Early discharge with oral therapy Inpatient therapy Oral outpatient therapy |
Oral outpatient treatment yielded an average QAFNE of 0.65, inferior to OPAT (0.72) and early discharge (0.66), but superior to hospital treatment (0.62). Oral outpatient treatment was cost saving, but less effective than outpatient i.v. (ICER of CAD10,186 per QAFNE); early discharge and Hospital treatment were dominated strategies Early discharge (CAD9265) and inpatient therapy (CAD21866) were less cost-effective than either oral or i.v. outpatient therapies (OPAT, CAD5810; oral, CAD5338). At a WTP threshold of CAD4000 per QAFNE, oral treatment was cost-effective in 54% of the simulations and OPAT was effective in 38%. Early discharge was cost-effective in 8% and traditional hospital management in < 1%. Beyond certain cost thresholds, dominance changed from the oral to OPAT strategy, but there was no constellation when early discharge or inpatient treatment became superior |
| Thornton et al.53 (UK) | Observational (retrospective) | Not specified | Home therapy, 47 patients (mean age 26 years), 36% male | Combined therapy: 18 patients (mean age 25 years), 61% male Inpatient therapy: 51 patients (mean age 26 years), 59% male |
When effective treatment was classed as ≤ 0% decline in FEV1, mean ICER was £46,098 (–£374,044 to 362,472); when ≤ 2% was used, mean ICER was £73,885 (2.5th percentile £1236, 97.5th percentile £269,023). These are the amounts that must be spent to obtain one more year of effective treatment with hospital-based care for one patient. The cost-effectiveness planes indicated increased effectiveness and increased cost for hospital treatment compared with home treatment. There was a slight possibility that hospital treatment may be less effective and more expensive than home treatment at a ≤ 0% decline; when a ≤ 2% decline is used, this was not the case The CEAC showed that if a decision-maker was willing to pay up to £262,500 for one extra patient with an FEV1 decline of ≤ 2% over 1 year, there was a 95% probability that hospital care would be cost-effective. If a decline of ≤ 0% was used, the probability that hospital care would be cost-effective never reached 95% (even if the decision-maker was willing to pay £10M for one extra patient) |
| You et al.24 (China) | Decision tree analysis | SA; SN | All patients (no numbers provided) | Oral therapy (early discharge) Inpatient therapy |
The base-case analysis showed that OPAT (US$14,470 per patient) was the least costly alternative, followed by oral (US$17,877) and inpatient treatment (US$19,980). Results were sensitive to variation of the success rates of the i.v. and oral drugs: (1) if the i.v. success rate was < 55%, oral treatment would become the least costly option; if the success rate was > 80%, both OPAT and hospital i.v. treatment would be less costly; (2) if the oral treatment success rate was > 80%, this would be the least costly option; if the success rate was < 5%, this would be the most costly. Throughout the ranges of all variables, OPAT remained less costly than hospital treatment OPAT was less costly than oral and hospital treatment in 64% and 100% of the simulations, with mean savings of US$2313 (95% CI US$2188 to US$2438) and US$4881 (95% CI US$4869 to US$4893) per patient, respectively |
| Author (country) | Method | OPAT model(s) | OPAT group(s) | Comparator(s) | Findings |
|---|---|---|---|---|---|
| Comparator studies | |||||
| Corwin et al.12 (New Zealand) | Questionnaire survey | GN | Home therapy: 98 patients (mean age 55 years), 62% male | Inpatient therapy: 96 patients (mean age 48 years), 73% male | Most patients in both treatment groups were satisfied with the care received (home 96%; hospital 96%). Only 5% of home patients would prefer hospital treatment, whereas 35% of hospital patients would prefer home treatment (p < 0.001). Nine per cent of the home group reported no preference for location compared with 34% of the hospital group |
| Dall et al.25 (USA) | Telephone survey | SN | Home therapy: 92 pneumonia patients (97% aged > 50 years), 44% male; 64 cellulitis patients (mean age 51 years), 70% male | Inpatient therapy: 10,728 pneumonia patients (83% aged > 50 years), 51% male; cellulitis patients (no numbers provided) | Overall satisfaction with the program was extremely high, with > 90% of patients responding positively to the telephone survey. The ability to have medical care at home was consistently cited as a benefit |
| Fernández-Avilés et al.56 (Spain) | Questionnaire survey | SN | Home therapy: 50 patients (mean age 47 years), 62% male | Inpatient therapy: 50 patients (median age 50 years), 54% male | All 30 of the patients and caregivers surveyed felt safe at home. Most patients (97%) indicated that they would choose to receive treatment at home again, and that they would recommend the procedure to other patients. The main advantages reported were quiet and increased home comfort (67%), familiar environment (27%), free choice of activity (27%), free choice of food (20%) and increased privacy (13%). Reported disadvantages (10 patients) including anxiety and fatigue (20%), cost of local hotel or apartment (7%) and caregiver anxiety (7%) |
| Richards et al.41 (New Zealand) | Questionnaire survey | GN | Home therapy: 24 patients (mean age 50 years), 54% male | Inpatient therapy: 25 patients (mean age 50 years), 52% male | All home group patients were very happy with their care, compared with 60% of hospital patients (p = 0.001). Similarly, most patients were happy with the location of their care, but the home group were happier; 92% of patients in the home group were very happy with the location, compared with 32% in the hospital group (p< 0.001) |
| OPAT-only studies | |||||
| Bamford et al.35 (UK) | Interviews | Not specified | 12 interviewees (mean age 62–63 years), 58% male | – | Most interviewees initially preferred oral administration. The most frequently cited disadvantages of i.v. administration were pain or discomfort and the inconvenience related to movement, followed by problems finding or maintaining the cannula and fear of needles Most thought the main advantage was that it would work quicker; other advantages mentioned were that it would be ‘more effective’, ‘less hassle’, ‘it’s correct and you don’t forget’ and ‘it’s probably gentler on the side effects on the stomach’ When specifically asked about i.v. antibiotics at home, four interviewees were clear that they would not be happy with the idea, three because of delivery issues, ‘I just don’t believe a district nurse could do it’, and one preferred oral therapy. Four others were happy with the idea or had concerns about equipment/sterility but would be happy if management of the i.v. could be delivered at home, ‘if there was no fear and it could be done sterilely’. When asked about management of future infections, 75% of those who gave an opinion preferred oral antibiotics. Of the two patients who preferred the i.v. route, one could not swallow tablets and one thought that i.v. antibiotics were better |
| Bernard et al.84 (France) | Not specified | SA; SN | 39 patients (mean age 44 years), 64% male | – | None of the patients complained about having to return frequently for consultation with their attending physician and they accepted this inconvenience in order to remain outside the hospital |
| Chambers et al.36 (New Zealand) | Questionnaire survey | SA | 100 eligible patients (mean age 63 years) | – | Home i.v. therapy was rated as good or very good by 97% of respondents. Two patients (2%) reported that they would not have it again. When patients were asked to comment freely, 91% of the replies were extremely positive (the most common reason being a preference for home over hospital) Most patients rated the experience of having a PICC or midline inserted as very good (60%) or good (35%); 28% reported some problem with the i.v. catheter (leakage, blockage, discomfort, inconvenience, arm swelling, skin reaction to dressing, connector becoming undone). Most respondents with a pump device rated this as good or very good (88%); 8% reported discomfort or restricted movement, and 3% reported kinking. Twelve patients (14%) self-administered antibiotics and all would do so again. Of those who did not, 27% would have liked to have done so, the main reason given being freedom from appointments with nurses |
| Chapman et al.13 (UK) | Questionnaire survey | SA; infusion centre | 334 treatment courses (mean patient age 46 years), 59% male | – | Of 276 patients completing questionnaires since the OPAT service was established, 98.6% rated the service as very good or excellent; 99.6% reported that they would choose the OPAT service again |
| Cox et al.89 (USA) | Unscheduled calls or visits | SA | 205 patients (mean age 59 years), 99% male | – | The younger group was significantly more likely than the older group to perform infusions unaided (41% vs. 20%; p < 0.001) Older patients were significantly more likely than younger patients to have an urgent care visit with a problem or question about the catheter or infusion (31.4 visits/1000 home i.v. days vs. 14.3 visits/1000 home i.v. days; p < 0.001). Similarly, there were more telephone calls to the infectious disease pharmacist from the older group (14.3/1000 home i.v. days vs. 8.57/1000 home i.v. days; p = 0.04) |
| Esposito et al.83 (Italy) | Not specified | OP; SA; visit by doctor or nurse (also care facility) | 239 patients (aged 11–80+ years), 62% male | – | Satisfaction with the OPAT regimen was high, with 91% of patients giving favourable feedback |
| Goodwin et al.27 (Canada) | Questionnaire survey | SN; GN | 2405 patients (mean age 46 years), 55% male | – | Most of the 424 responding patients were very satisfied or completely satisfied with home therapy (61%); 6% were not very satisfied and 5% were totally dissatisfied. Most of the satisfaction related to resuming daily activities such as work or school and most dissatisfaction to costs incurred |
| Grayson et al.67 (Australia) | Not specified | GN | 20 patients (mean age 58 years) | – | All patients reported a strong preference for home i.v. treatment over inpatient therapy, with many describing a sense of improved self-esteem, ‘ownership’ of their illness and involvement on therapy |
| Hindes et al.68 (USA) | Questionnaire survey; personal communication | SA | 48 patients (mean age 65 years) | – | Surveys and personal communications demonstrated a high degree of satisfaction with the care provided, and strong endorsement of the home therapy provided as an alternative to inpatient care (no other details provided) |
| Hitchcock et al.69 (UK) | Questionnaire survey | OP; SA; GN; private home health-care company | 273 patients (mean age 60 years), 54% male | – | Most of the 84 survey respondents felt that the service met their expectations and would be happy to receive this form of treatment again should the situation arise (96%). All were happy with the support they received from the OPAT team during treatment; 95% felt that their quality of life during the period of infection had been improved by outpatient management (two of the patients who did not think this suffered an adverse drug event) Most respondents (83%) thought that the service could not be improved. The most frequent comments from those who felt improvements could be made related to patient transport problems transport (6/14); two patients thought that communication between consultants, other staff and patients could be improved, three that they had waited too long and one patient suggested referrals could be made directly by their GP |
| Huminer et al.70 (Israel) | Not specified | SA; SN (also nursing home; kibbutz) | 37 patients (mean age 64 years), 57% male | – | Most patients (95%) reported a high level of satisfaction with this type of therapy (no other details provided) |
| Johansson et al.71 (Sweden) | Questionnaire survey; VAS | SA | 11 patients (mean age 51), 73% male | – | All patients reported that OPAT was of great value (median VAS rating of 97 mm). Prior expectations of OPAT included peace and quiet, greater freedom and more time to do other things, the home environment being important for well-being and being an everyday part of the family. The 10 patients who responded after OPAT said that their expectations had been met All patients said they would prefer to have OPAT again during any subsequent i.v. antibiotic therapy, and all agreed there were many advantages to having treatment at home. Nine out of 10 patients never felt worried during administration of the drug (although they sometimes worried about the direction of their underlying condition). Nine out of 10 patients were fully satisfied with the education (including potential complications) Specific advantages reported by patients included not having to go to hospital every day for therapy, more freedom, more time to do household duties and for leisure, the feeling of having control, freedom and flexibility, making it possible to be a part of daily issues at home, and being at home with your family. No examples of disadvantages were given |
| Kayley et al.72 (UK) | Not specified | SA; GN; doctor administered | 67 patients | – | Patients said they were happy to receive their treatment at home (anecdotal only – no formal survey) |
| Kieran et al.2 (Ireland) | Telephone survey | SA; nurses attached to commercial company | 56 patients (median age 50 years), 57% male | – | All 12 patients surveyed reported that they were very happy to complete their course of antibiotics at home rather than remaining in hospital. Reasons given were preference of home to hospital (44%), the ability to resume activities of daily living (11%) or both (44%) All patients were satisfied with the service received, none felt that they had inadequate training and none felt that their infection took longer to be treated because they received OPAT. All stated that they would prefer to be treated at home if a similar situation arose in the future. Almost three-quarters (70%) would be happy to be followed up by telephone or internet with less frequent outpatient reviews, providing that care of the i.v. access device could be done in the community |
| Lehoux37 (Canada) | Interviews | SA | 6 interviewees (mean age 64 years), 50% male | – | User acceptance of home i.v. treatment was shaped by different forms of anxiety, such as protecting the catheter site to avoid potential infection or the possibility of the catheter becoming dislodged. The pump alarm system could go off too easily and false alarms frequently disturbed sleep and were initially perceived as very stressful (over time they became a ‘normal’ disturbance) Professional and social life was slightly limited, and patients were generally passive or submissive about this, ‘you’re always a slave to it, having to carry it everywhere’. Carers sometimes curtailed social activities because they felt needed by the patient User-acceptance was closely linked to competence. Older patients did not feel comfortable with the electronic components of the pump, and the manual dexterity required to properly manipulate it could be problematic for older people, ‘if my eyes were OK, I’d have been able to do it. But I was frightened of not doing it properly, of not seeing the needle, which is so tiny’ (i.v. carer). Direct observation showed patients who were unable to read messages on the digital screen owing to poor eyesight, limited English or illiteracy. They relied on memory or made informed guesses |
| Marra et al.15 (Canada) | Questionnaire survey | SA; SN | 91 patients (mean age 56 years), 69% male | – | The majority of the 91 enrolled patients (96%) indicated a treatment location preference; 89% preferred home, while the remainder preferred hospital. Most patients (82%) gave an interpretable response regarding WTP for treatment in their preferred location. Of these patients, 90% preferred home with a median WTP of CAD490, and the remainder preferred hospital with a median WTP of CAD500 (not statistically significant). Total WTP for the patients who preferred hospital treatment was CAD7859 versus CAD60,712 for the patients who preferred home treatment. Those who stated preferences were willing to pay more than those who did not (p < 0.0001) |
| Martel73 (France) | Questionnaire survey | SA | 116 patients [mean age 36 (male), 41 (female)], 76% male | – | Patients who received home treatment (n = 33) had a significantly higher internal locus of control, whereas patients who preferred to continue their treatment in hospital (n = 17) had a higher external locus of control. The main reasons for accepting home treatment were sociofamilial, wanting to carry on normal activities and to have higher autonomy or freedom. The hospital group had low confidence in their own ability The majority of home group patients (89%) would use home treatment again if the cost was paid by a third party; 32% agreed to participate again even if they were responsible for the cost of antibiotics |
| Montalto85 (Australia) | Telephone interviews | SN (HHU) | 67 patients (aged 16–70+ years), 49% male; 65 carers (aged 16–70+ years), 29% male | – | Preference for the convenience and comfort of home was the most commonly cited reason for agreeing to enter the HHU (67%). More than one-quarter of patients (28%) mentioned avoidance of hospitalisation and a smaller number (13%) felt that it saved a bed for another person; three patients believed that they were not ill enough to warrant a bed. Seven patients (10%) felt that their choice was constrained as no inpatient beds were available at the time. Almost all (97%) patients would use the service again if the opportunity arose Benefits of a home environment were the most commonly perceived advantages of HHU care (62%), with patients reporting feeling happier, more comfortable at home, greater personal freedom, companionship and less disruption for themselves and their family. Positive characteristics of the HHU were mentioned by 60% of patients; only two mentioned disadvantages of HHU care Seven patients recalled feeling worried during their stay, but when asked whether or not they felt confident during their time in the HHU, all 67 patients, including those who felt worried, responded positively. The most common reason given for this was the 24-hour contact numbers for emergency backup |
| Nathwani74 (UK) | Not specified | OP; SA; SN | 125 patients | – | Patient satisfaction with OPAT was very high (no other details provided |
| Nathwani et al.75 (UK) | Questionnaire survey | OP; SA | 101 patients | – | Most patients (79%) were happy with all aspects of the care received; 96% reported that the service improved their quality of life, and 89% that this form of treatment met or exceeded their expectations. For 93% of patients it was preferable to inpatient care, and 93% would prefer this model in the future if the need arose. Most patients’ family/carers were satisfied with the service (93%) |
| Parker et al.86 (UK) | Questionnaire survey; focus group; telephone interviews with practices | OP; SA | 29 patients; 38 general practices | – | Most patients (79%) reported the freedom of being at home as the main reason for taking part. Other reasons included less family disruption (31%) and increased social contact (21%). Of 26 patients who completed the end of study questionnaire, 92% were very much in favour of non-inpatient treatment and would repeat this form of therapy Questionnaire results were supported by the focus group; all patients would repeat this form of therapy and felt that treatment outside hospital improved their quality of life |
| Pilling and Walley88 (UK) | Interviews | SA; commercial home care organisation | 11 CF patients (aged 17–30 years); 14 parents (of children aged 6–16 years) | – | Most of the respondents (92%) had considerable experience of home therapy, having received home i.v. antibiotics for ≥ 1 year. All preferred to be treatment at home rather than in hospital provided that they were reasonably well. Most patients (96%) were satisfied with the quality and comprehensiveness of training, although one respondent felt that more information should have been provided on potential adverse reactions, and that the quality of homecare could be improved by the provision of refresher courses on the management of infusion devices. Support arrangements were thought to be good, and all knew how to obtain advice if needed Fifteen respondents (60%) had experienced one or more problems with their therapy (drug effervescence, painful administration, line blockage, failure of ambulatory infusion device, storage of drugs or equipment, getting rid of used equipment, etc.). Most were minor, but some, such as line blockages, required a visit to hospital or a home visit from the nurse. Patients who self-constituted their antibiotics reported difficulties in obtaining adequate supplies of ancillary items (arbitrary quantities were provided and further supplies were difficult to obtain). They also reported problems with storage of drugs and equipment, and disposal of used equipment, particularly needles. With this exception however, all respondents were satisfied with the support they received from hospital and had no suggestions for improvement |
| Rodríguez-Cerrillo et al.76 (Spain) | Questionnaire survey | SN (HHU) | 24 patients (mean age 73 years) | – | 95% of patients treated expressed satisfaction with this type of treatment |
| Rodríguez-Cerrillo et al.77 (Spain) | Questionnaire survey | SN (HHU) | 25 patients (mean age 59 years), 48% male | – | All patients expressed their satisfaction with treatment at home (no other details provided) |
| Seaton et al.78 (UK) | Questionnaire survey | Not specified | 205 eligible patients (mean age 49 years), 51% malea | – | Most of the 183 responding patients (84%) thought that OPAT would be an acceptable alternative to inpatient therapy. Only three of the 29 patients who disagreed gave explanations (severe arthritis, fear of the i.v. device and disagreement in principle). This group was significantly older than those who were willing to have i.v. therapy at home (mean 64 years vs. 46 years; p < 0.001). Acceptability was not influenced by sex All groups of patients (by condition) were equally agreeable to the prospect of outpatient therapy. Of the 95 patients who received i.v. antibiotic therapy, 87% thought that OPAT was an acceptable alternative to inpatient therapy, and 72% had a caregiver who would be willing to administer the antibiotic at home |
| Talcott et al.87 (USA) | Likert scales | SA; SN | 30 patients (median age 38 years), 43% male | – | Before home therapy, all 30 patients overwhelmingly preferred home to hospital therapy and had little fear of isolation from their physician if a serious problem occurred. These convictions were unchanged after completion of home therapy The report of family comfort with home care increased after treatment: support for the statement, ‘My family would rather have me near them than be in the hospital when something happens like this (developing fever and neutropenia)’ increased from 42% agreement to 70% agreement There was some evidence that patients felt slightly more isolated at home than in the hospital: agreement with the statement ‘I feel uneasy about calling my doctor from home with a complaint that worries me a little, for fear that I will be disturbing him or her with such a minor problem’ increased from 10% to 24% |
| Teuffel et al.79 (Canada) | Interviews; VAS | Not specified | 78 participants (mean age 54 years), 41% male | – | Most respondents (75%) preferred some form of outpatient management. Home oral treatment was most commonly ranked first (36%), but 21% of respondents preferred home i.v. and 18% preferred early discharge. All three outpatient strategies were associated with higher mean VAS scores than inpatient care (5.3 vs. 5.7 for early discharge, 6.1 for home oral, and 6.2 for home i.v.) There was no significant difference between the outpatient strategies in relation to how much remaining life patients would be willing to give up to avoid inpatient care; x¯ 9.1 weeks for early discharge, 9.6 weeks for home i.v., and 9.3 weeks for home oral. On average, patients would give up < 1% of their remaining lifetime to avoid inpatient care Findings were similar for willingness to pay (mean CAD282 for early discharge, CAD327 for home i.v., and CAD255 for home oral). The maximum WTP to receive i.v. treatment at home instead of in hospital was CAD4500 (maximum CAD2000 for early discharge and CAD1500 for home oral) |
| Tice80 (USA) | Questionnaire survey | OP; SA; SN; infusion centre | 500 patients | – | Almost all of the respondents (99%) reported that, should they require parenteral antibiotic therapy again, they would definitely or probably choose to be treated at home; < 1% said they would prefer hospitalisation |
| Tice81 (USA) | Questionnaire survey | OP; SA; SN | 538 patients (mean age 45 years), 52% male | – | Most of the 294 responders, 89% reported that they definitely would have outpatient therapy instead of being hospitalised if the need arose again and 10% said they probably would have outpatient therapy again. Of the four remaining patients, two said they probably would not and two said they definitely would not |
| Yan et al.5 (UK) | Questionnaire survey | OP; GN | 140 patients (aged 17–89 years), 64% male | – | The survey was completed following an OPAT pilot scheme; 88% of patients (no numbers given) were pleased with the service as it enabled earlier discharge, even if this meant daily trips back to hospital |
| Al Ansari et al.82 (Bahrain) | Questionnaire survey | OP | 101 patients; 57% male | – | Survey response was high (96.9%). Patients were highly satisfied with the OPAT service (mean rating 4.41, SD 0.31) |
| Sims et al.212 (UK) | Telephone survey | OP | 14 patients (mean age 63 years), 71% male | – | One patient died of unrelated causes prior to questionnaire completion. All of the 13 patients contacted were satisfied with the OPAT service, thought that it was more convenient than an inpatient stay and, should the same problem recur, would undergo OPAT again. All reported that they would recommend the service to a friend under similar circumstances |
| Author (country) | Method | OPAT model(s) | OPAT group(s) | Comparator(s) | Findings |
|---|---|---|---|---|---|
| Lehoux et al.39 (Canada) | Questionnaire survey | SA | 51 nurses | – | Respondents strongly agreed that home i.v. therapy gave patients greater autonomy (mean score 5.2; SD 1.0) and made it easier to pursue a normal life (mean 4.4; SD 1.0). They viewed the technology as simple to use (mean 4.5; SD 1.1) but believed it triggered some patient anxiety (mean 3.3; SD 1.3) Nursing teams perceived that i.v. therapy provided some challenges for providers in relation to the technical complexity of devices (mean 3.5; SD 1.3), number of devices used by their hospital (mean 3.3; SD 1.4), pace of product renewal (mean 3.2; SD 1.4) and fit with the home setting (mean 3.1; SD 1.4) Dealing with patients’ psychological (mean 3.0; SD 1.0) and cognitive limitations (mean 3.0; SD 1.1) was viewed as slightly more demanding than dealing with physical limitations (mean 2.9; SD 1.1). There was a perceived increase in effort related to teaching patients how to use the technology (mean 3.1; SD 1.1), about associated risks (mean 3.0; SD 1.1), how to manage their anxiety (mean 3.0; SD 1.1) and how to manage impact on their life (mean 2.8; SD 1.0) The two features reported as being most important for improvement, were ease of use for patients (mean 3.9; SD 1.2) and integrating the technology into the patient’s life (mean 4.0; SD 1.3) |
| Parker et al.86 (UK) | Questionnaire survey; focus group; call to practices | OP; SA | 29 patients; 38 general practices | – | Most general practices (76%) saw no advantage for themselves in home i.v. treatment. Those that did mentioned that admissions saved and freed up hospital beds, that the GP maintained responsibility for the patient and that the patient could be treated at home. Most practices (70%) saw a substantial disadvantage in increased workload, and a small number were concerned about the safety of i.v. access (18%). However, almost all (94%) thought that patients would benefit from being in their own environment, although many saw distance from hospital and lack of support and nursing care as a disadvantage (68%) |
| Seaton and Nathwani90 (UK) | Questionnaire survey | Not specified | 157 infection specialists (92% clinical microbiologists) | – | Most of the 157 respondents had experience of OPAT, but only 21% had an established programme within their institution. Of those with no programme, 61% thought there was a definite need, while 14% thought there was no need for a formal programme. Only 2% thought that i.v. therapy should always be given in hospital Of the 124 specialists without a programme, perceived barriers to development of OPAT were mainly organisational, including source of funding (n = 43), lack of leadership (n = 42), links between hospital and community (n = 37), and identification and training of staff (n = 21). Small numbers of patients or fragmentation of patient distribution within trusts was also a concern (n = 34). Concerns over lack of experience (n = 17) or patient safety and acceptability (particularly line care and antibiotic administration), were expressed by a few respondents (n = 12) There was no consensus regarding funding and clinical responsibility for patients; 40% thought that secondary care should pay and that specialists should take day-to-day clinical responsibility, whereas 50% thought that care should be shared between hospital and community practitioners |
| Lane et al.38 (USA) | Questionnaire survey | OP; SA | 555 adult infectious diseases physicians | – | Lack of a dedicated OPAT team was reported as the most common barrier to providing safe OPAT services (median rank 2), followed by the large number of locations at which patients received OPAT, communication issues, and volume of laboratory results (median rank 3) |
| Muldoon et al.91 (Ireland) | Questionnaire survey | Not specified | 55 consultant physicians (15% clinical microbiologists) | – | Forty-one respondents (74%) had discharged patients with i.v. antibiotics, but almost half (n = 26, 47%) did not have a designated OPAT service available to them; all felt that there was a need for a local OPAT service. Of respondents answering the question, 63% (12/19) reported that they had experienced difficulty obtaining funding for OPAT Forty-five per cent (13/18) reported that they thought that > 75% of their patients were satisfied with OPAT services; only one consultant (6%) felt that < 50% of patients were satisfied |
| Muldoon et al.92 (USA) | Questionnaire survey | Not specified | 316 adult and paediatric infectious diseases physicians | – | Thirty-four respondents rarely or never participated in OPAT: 48% cited logistic reasons or lack of an OPAT programme, 15% that another practitioner had responsibility for OPAT, 15% reported lack of control of patient management, 12% lack of reimbursement, and 10% low patient volume In the ‘Comments’ section, many respondents reported frustration about a lack of institutional and financial support for OPAT, while also expressing a significant liability burden |
Included studies
Amodeo MR, Clulow T, Lainchbury J, Murdoch DR, Gallagher K, Dyer A, et al. Outpatient intravenous treatment for infective endocarditis: safety, effectiveness and one-year outcomes. J Infect 2009;59:387–93. http://dx.doi.org/10.1016/j.jinf.2009.09.009
Anand V, Levine H, Friedman M, Krespi Y, Panje W, Schettino R, et al. Intravenous antibiotics for refractory rhinosinusitis in nonsurgical patients: preliminary findings of a prospective study. Am J Rhinol 2003;17:363–8.
Angel JV. Outpatient antibiotic therapy for elderly patients. HIAT Study Group. Am J Med 1994;97:43–9.
Bamford KB, Desai M, Aruede MJ, Lawson W, Jacklin A, Franklin BD. Patients’ views and experience of intravenous and oral antimicrobial therapy: room for change. Injury 2011;42(Suppl. 5):24–7. http://dx.doi.org/10.1016/S0020-1383(11)70129-2
Barr DA, Semple L, Seaton RA. Self-administration of outpatient parenteral antibiotic therapy and risk of catheter-related adverse events: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2012;31:2611–19.
Barr DA, Semple L, Seaton RA. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicrob Agents 2012;39:407–13.
Berman SJ, Johnson EW. Out-patient parenteral antibiotic therapy (OPAT): clinical outcomes and adverse events. Hawaii Med J 2001;60:31–3.
Bernard L, El Hajj, Pron B, Lotthe A, Gleizes V, Signoret F, et al. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: evaluation of efficacy, tolerance and cost. J Clin Pharm Thers 2001;26:445-51.
Bradley JM, Wallace ES, Elborn JS, Howard JL, McCoy MP. An audit of the effect of intravenous antibiotic treatment on spirometric measures of pulmonary function in cystic fibrosis. Ir J Med Sci 1999;168:25–8.
Cervera C, Del Rio A, Garcia L, Sala M, Almela M, Moreno A, et al. Efficacy and safety of outpatient parenteral antibiotic therapy for infective endocarditis: a ten-year prospective study. Enferm Infec Microbiol Clin 2011;29:587–92.
Chambers S, Gallagher K, Pithie A. Patient acceptability of home intravenous antibiotic therapy. N Z Med J 2004;117:U865.
Chambers S, Gallagher K, Metcalf S, Pithie A. Home intravenous antimicrobial service – twelve months experience in Christchurch. N Z Med J 2002;115:216–18.
Chan DSG, Archuleta S, Llorin RM, Lye DC, Fisher D. Standardized outpatient management of Klebsiella pneumoniae liver abscesses. Int J Infect Dis 2013;17:e185–8.
Chapman AL, Dixon S, Andrews D, Lillie PJ, Bazaz R, Patchett JD. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother 2009;64:1316–24. http://dx.doi.org/10.1093/jac/dkp343
Cheong EA, Katelaris CH, Sisson CM, Anderson EA, Byth K. Adverse drug reactions associated with home parenteral therapy. J Pharm Pract Res 2008;38:267–70.
Corwin P, Toop L, McGeoch G, Than M, Wynn-Thomas S, Wells JE, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ 2005;330:129.
Cox AM, Malani PN, Wiseman SW, Kauffman CA. Home intravenous antimicrobial infusion therapy: a viable option in older adults. J Am Geriatr Soc 2007;55:645–50.
Dall L, Peddicord T, Peterson S, Simmons T, Dall T. Hospitalist treatment of CAP and cellulitis using objective criteria to select patients. Infect Med 2003;20:379–90.
Dalovisio JR, Juneau J, Baumgarten K, Kateiva J. Financial impact of a home intravenous antibiotic program on a medicare managed care program. Clin Infect Dis 2000;30:639–42.
Dargan S, Zvonar RK, Saginur R. A review of outpatient parenteral antimicrobial therapy practices and experience at the Ottawa Hospital. Can J Hosp Pharm 2007;60:177–83.
Dobson PM, Boyle M, Loewenthal M. Home intravenous antibiotic therapy and allergic drug reactions: is there a case for routine supply of anaphylaxis kits? J Infus Nurs 2004;27:425–30.
Donald M, Marlow N, Swinburn E, Wu M. Emergency department management of home intravenous antibiotic therapy for cellulitis. Emerg Med J 2005;22:715–7.
Duncan CJ, Barr DA, Seaton RA. Outpatient parenteral antimicrobial therapy with ceftriaxone, a review. Int J Clin Pharm 2012;34:410–17. http://dx.doi.org/10.1007/s11096-012-9637-z
Escalante CP, Rubenstein EB, Rolston KV. Outpatient antibiotic therapy for febrile episodes in low-risk neutropenic patients with cancer. Cancer Invest 1997;15:237–42.
Esmond G, Butler M, McCormack AM. Comparison of hospital and home intravenous antibiotic therapy in adults with cystic fibrosis. J Clin Nurs 2006;15:52–60.
Esposito S, Leone S, Noviello S, Ianniello F, Russo M, Foti G, et al. Outpatient parenteral antibiotic therapy in the elderly: an Italian observational multicenter study. J Chemother 2009;21:193–8.
Esposito S, Leone S, Noviello S, Ianniello F, Fiore M, Russo M, et al. Outpatient parenteral antibiotic therapy for bone and joint infections: an Italian multicenter study. J Chemother 2007;19:417–22.
Esposito S, Noviello S, Leone S, Tice A, Seibold G, Nathwani D, Scaglione F, International OPAT Registry. Outpatient parenteral antibiotic therapy (OPAT) in different countries: a comparison. Int J Antimicrob Agents 2004;24:473–8.
Fernández-Avilés F, Carreras E, Urbano-Ispizua A, Rovira M, Martínez C, Gaya A, et al. Case-control comparison of at-home to total hospital care for autologous stem-cell transplantation for hematologic malignancies. J Clin Oncol 2006;24:4855–61.
Goodwin DD, Hanson JC, Berry CP. The changing face of Canadian home parenteral therapy. J Infus Nurs 2002;25:372–8.
Gourdeau M, Deschênes L, Caron M, Desmarais M. Home iv antibiotic therapy through a medical day care unit. Can J Infect Dis 1993;4:158–62.
Graninger W, Presterl E, Wenisch C, Schwameis E, Breyer S, Vukovich T. Management of serious staphylococcal infections in the outpatient setting. Drugs 1997;54(Suppl. 6):21–8.
Grayson ML, Silvers J, Turnidge J. Home intravenous antibiotic therapy. A safe and effective alternative to inpatient care. Med J Aust 1995;162:249–53.
Gross R, Graziani AL, Laufer D, Turner JL, Ondercin JP, Macgregor RR. Adverse effects of the use of intravenous pentamidine in the home. Infect Dis Clin Pract 1996;5:456–58.
Heintz BH, Halilovic J, Christensen CL. Impact of a multidisciplinary team review of potential outpatient parenteral antimicrobial therapy prior to discharge from an academic medical center. Ann Pharmacother 2011;45:1329–37. http://dx.doi.org/10.1345/aph.1Q240
Hindes R, Winkler C, Kane P, Kunkel M, Poretz DM. Outpatient intravenous antibiotic therapy in Medicare patients: Cost-savings analysis. Infect Dis Clin Pract 1995;4:211–18.
Hitchcock J, Jepson AP, Main J, Wickens HJ. Establishment of an outpatient and home parenteral antimicrobial therapy service at a London teaching hospital: a case series. J Antimicrob Chemother 2009;64:630–4. http://dx.doi.org/10.1093/jac/dkp212
Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother 2010;65:2641–4. http://dx.doi.org/10.1093/jac/dkq355
Huminer D, Bishara J, Pitlik S. Home intravenous antibiotic therapy for patients with infective endocarditis. Eur J Clin Microbiol Infect Dis 1999;18:330–4.
Johansson E, Björkholm M, Wredling R, Kalin M, Engervall P. Outpatient parenteral antibiotic therapy in patients with haematological malignancies. A pilot study of an early discharge strategy. Support Care Cancer 2001;9:619–24.
Kayley J, Berendt AR, Snelling MJ, Moore H, Hamilton HC, Peto TE, et al. Safe intravenous antibiotic therapy at home: experience of a UK based programme. J Antimicrob Chemother 1996;37:1023–9.
Kieran J, O’Reilly A, Parker J, Clarke S, Bergin C. Self-administered outpatient parenteral antimicrobial therapy: a report of three years experience in the Irish healthcare setting. Eur J Clin Microbiol Infect Dis 2009;28:1369–74. http://dx.doi.org/10.1007/s10096-009-0794-5
Krauth C, Jalilvand N, Welte T, Busse R. Cystic fibrosis: cost of illness and considerations for the economic evaluation of potential therapies. PharmacoEconomics 2003;21:1001–24.
Larioza J, Girard A, Brown RB. Clinical experience with daptomycin for outpatient parenteral antibiotic therapy. Am J Med Sci 2011;342:486–8. http://dx.doi.org/10.1097/MAJ.0b013e31821e1e6b
Larioza J, Heung L, Girard A, Brown RB. Management of infective endocarditis in outpatients: clinical experience with outpatient parenteral antibiotic therapy. South Med J 2009;102:575–9. http://dx.doi.org/10.1097/SMJ.0b013e3181a4eef2
Laupland KB, Gill MJ, Schenk L, Goodwin D, Davies HD. Outpatient parenteral antibiotic therapy: evolution of the Calgary adult home parenteral therapy program. Clin Invest Med 2002;25:185–90.
Lehoux P, Richard L, Pineault R, Saint-Arnaud J. Delivery of high-tech home care by hospital-based nursing units in Quebec: clinical and technical challenges. Nurs Leadersh 2006;19:44–55.
Lehoux P. Patients’ perspectives on high-tech home care: a qualitative inquiry into the user-friendliness of four technologies. BMC Health Serv Res 2004;4:28.
Lillie PJ, Andrews D, Eaves K, Darton TC, Chapman AL. Baseline factors predicting the duration of intravenous antibiotic therapy for cellulitis in an outpatient setting. Eur J Clin Microbiol Infect Dis 2010;29:347–9. http://dx.doi.org/10.1007/s10096-009-0855-9
Lin JW, Kacker A, Anand VK, Levine H. Catheter- and antibiotic-related complications of ambulatory intravenous antibiotic therapy for chronic refractory rhinosinusitis. Am J Rhinol 2005;19:365–9.
Lopardo G. Management of endocarditis: outpatient parenteral antibiotic treatment in Argentina. Chemotherapy 2001;47(Suppl. 1):24–32.
Mackintosh CL, White HA, Seaton RA. Outpatient parenteral antibiotic therapy (OPAT) for bone and joint infections: experience from a UK teaching hospital-based service. J Antimicrob Chemother 2011;66:408–15. http://dx.doi.org/10.1093/jac/dkq445
Marra CA, Frighetto L, Goodfellow AF, Wai AO, Chase ML, Nicol RE, et al. Willingness to pay to assess patient preferences for therapy in a Canadian setting. BMC Health Serv Res 2005;5:43.
Martel AY. Home intravenous self-injection of antibiotic therapy. Can J Infect Dis 1994;5(Suppl. C):51–5.
Martone WJ, Lindfield KC, Katz DE. Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry. Int J Clin Pract 2008;62:1183–7. http://dx.doi.org/10.1111/j.1742-1241.2008.01824.x
Matthews PC, Conlon CP, Berendt AR, Kayley J, Jefferies L, Atkins BL, Byren I. Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother 2007;60:356–62.
Mauceri AA. Treatment of bone and joint infections utilizing a third-generation cephalosporin with an outpatient drug delivery device. HIAT Study Group. Am J Med 1994;97:14–22.
Mazo S, Emparan C, Vallejo M, Soriano P. Hospital-in-the-home treatment of surgical infectious diseases: an economic analysis. Surg Infect 2007;8:567–74. http://dx.doi.org/10.1089/sur.2006.047
McMahon JH, O’Keeffe JM, Grayson ML, Victorian Hith Outcomes Study Group. Is hospital-in-the-home (HITH) treatment of bacterial endocarditis safe and effective? Scand J Infect Dis 2008;40:40–3.
Montalto M, Lui B, Mullins A, Woodmason K. Medically-managed Hospital in the Home: 7 year study of mortality and unplanned interruption. Aust Health Rev 2010;34:269–75. http://dx.doi.org/10.1071/AH09771
Montalto M. An audit of patients admitted for home intravenous therapy directly from the emergency department. Int J Clin Pract 1997;51:433–7.
Montalto M, Dunt D. Home and hospital intravenous therapy for two acute infections: an early study. Aust N Z J Med 1997;27:19–23.
Montalto M. Patients’ and carers’ satisfaction with hospital-in-the-home care. Int J Qual Health Care 1996;8:243–51.
Morales JO, Von Behren L. Secondary bacterial infections in HIV-infected patients: an alternative ambulatory outpatient treatment utilizing intravenous cefotaxime. Am J Med 1994;97:9–13.
Murray H, Stiell I, Wells G. Treatment failure in emergency department patients with cellulitis. CJEM 2005;7:228–34.
Nathwani D. The management of skin and soft tissue infections: outpatient parenteral antibiotic therapy in the United Kingdom. Chemotherapy 2001;47(Suppl. 1):17–23.
Nathwani D, Barlow GD, Ajdukiewicz K, Gray K, Morrison J, Clift B, et al. Cost-minimization analysis and audit of antibiotic management of bone and joint infections with ambulatory teicoplanin, in-patient care or outpatient oral linezolid therapy. J Antimicrob Chemother 2003;51:391–6.
Nathwani D, Morrison J, Seaton RA, France AJ, Davey P, Gray K. Out-patient and home-parenteral antibiotic therapy (OHPAT): evaluation of the impact of one year’s experience in Tayside. Health Bull 1999;57:332–7.
Parker SE, Nathwani D, O’Reilly D, Parkinson S, Davey PG. Evaluation of the impact of non-inpatient i.v. antibiotic treatment for acute infections on the hospital, primary care services and the patient. J Antimicrob Chemother 1998;42:373–80.
Partridge DG, O’Brien E, Chapman ALN. Outpatient parenteral antibiotic therapy for infective endocarditis: a review of 4 years’ experience at a UK centre. Postgrad Med J 2012;88:377–81.
Patanwala AE, Erstad BL, Nix DE. Cost-effectiveness of linezolid and vancomycin in the treatment of surgical site infections. Curr Med Res Opin 2007;23:185–93.
Pérez-López J, San José Laporte A, Pardos-Gea J, Tapia Melenchón E, Lozano Ortín E, Barrio Guirado A, Vilardell Tarrés M. Safety and efficacy of home intravenous antimicrobial infusion therapy in older patients: a comparative study with younger patients. Int J Clin Pract 2008;62:1188–92. http://dx.doi.org/10.1111/j.1742-1241.2008.01747.x
Pilling M, Walley T. Parenteral antibiotics at home in cystic fibrosis: experiences and attitudes of recipients. Health Soc Care Commun 1997;5:209–12.
Pond MN, Newport M, Joanes D, Conway SP. Home versus hospital intravenous antibiotic therapy in the treatment of young adults with cystic fibrosis. Eur Respir J 1994;7:1640–4.
Poretz DM. Treatment of serious infections with cefotaxime utilizing an outpatient drug delivery device: global analysis of a large-scale, multicenter trial. HIAT Study Group. Am J Med 1994;97:34–42.
Poretz DM. Treatment of skin and soft-tissue infections utilizing an outpatient parenteral drug delivery device: a multicenter trial. HIAT Study Group. Am J Med 1994;97:23–7.
Rapoport BL, Sussmann O, Herrera MV, Schlaeffer F, Otero JC, Pavlovsky S, et al. Ceftriaxone plus once daily aminoglycoside with filgrastim for treatment of febrile neutropenia: early hospital discharge vs. Standard In-patient care. Chemotherapy 1999;45:466–76.
Rehm S, Campion M, Katz DE, Russo R, Boucher HW. Community-based outpatient parenteral antimicrobial therapy (CoPAT) for Staphylococcus aureus bacteraemia with or without infective endocarditis: analysis of the randomized trial comparing daptomycin with standard therapy. J Antimicrob Chemother 2009;63:1034–42. http://dx.doi.org/10.1093/jac/dkp051
Richards DA, Toop LJ, Epton MJ, McGeoch GR, Town GI, Wynn-Thomas SM, et al. Home management of mild to moderately severe community-acquired pneumonia: a randomised controlled trial. Med J Aust 2005;183:235–8.
Rodríguez-Cerrillo M, Poza-Montoro A, Fernandez-Diaz E, Romero AI. Patients with uncomplicated diverticulitis and comorbidity can be treated at home. Eur J Intern Med 2010;21:553–4. http://dx.doi.org/10.1016/j.ejim.2010.09.002
Rodríguez-Cerrillo M, Poza-Montoro A, Fernandez-Diaz E, Iñurrieta-Romero A, Matesanz-David M. Home treatment of patients with acute cholecystitis. Eur J Intern Med 2012;23:e10–3. http://dx.doi.org/10.1016/j.ejim.2011.07.012
Seaton RA, MacConnachie AA. Experience with daptomycin in an infectious diseases service over 1 year: utility in an outpatient parenteral antibiotic programme. Int J Antimicrob Agents 2008;31:492–7.
Seaton RA, Sharp E, Bezlyak V, Weir CJ. Factors associated with outcome and duration of therapy in outpatient parenteral antibiotic therapy (OPAT) patients with skin and soft-tissue infections. Int J Antimicrob Agents 2011;38:243–8. http://dx.doi.org/10.1016/j.ijantimicag.2011.05.008
Seaton RA, Bell E, Gourlay Y, Semple L. Nurse-led management of uncomplicated cellulitis in the community: evaluation of a protocol incorporating intravenous ceftriaxone. J Antimicrob Chemother 2005;55:764–7.
Seaton RA, Nathwani D. Outpatient and home parenteral antibiotic therapy (OHPAT) in the UK: survey of infection specialists’ experience and views. Clin Microbiol Infect 2000;6:387–90.
Seaton RA, Nathwani D, Williams FL, Boyter AC. Feasibility of an outpatient and home parenteral antibiotic therapy (OHPAT) programme in Tayside, Scotland. J Infect 1999;39:129–33.
Sebban C, Dussart S, Fuhrmann C, Ghesquieres H, Rodrigues I, Geoffrois L, et al. Oral moxifloxacin or intravenous ceftriaxone for the treatment of low-risk neutropenic fever in cancer patients suitable for early hospital discharge. Support Care Cancer 2008;16:1017–23. http://dx.doi.org/10.1007/s00520-007-0383-z
Smego RA, Khan MA, Khowaja K, Rafique R, Datoo F. A university-sponsored home health nursing program in Karachi, Pakistan. Home Healthc Nurse 2005;23:710–16.
South R. Retrospective study of teicoplanin as home continuation of hospital-initiated therapy. Int J Antimicrob Agents 1998;9:219–25.
Stein GE, Schooley SL, Havlichek DH, Nix DE. Outpatient intravenous antibiotic therapy compared with oral linezolid in patients with skin and soft tissue infections: A pharmacoeconomic analysis. InfectDis Clin Pract 2008;16:235–39.
Talcott JA, Whalen A, Clark J, Rieker PP, Finberg R. Home antibiotic therapy for low-risk cancer patients with fever and neutropenia: a pilot study of 30 patients based on a validated prediction rule. J Clin Oncol 1994;12:107–14.
Teuffel O, Amir E, Alibhai S, Beyene J, Sung L. Cost effectiveness of outpatient treatment for febrile neutropaenia in adult cancer patients. Br J Cancer 2011;104:1377–83. http://dx.doi.org/10.1038/bjc.2011.101
Teuffel O, Cheng S, Ethier MC, Diorio C, Martino J, Mayo C, et al. Health-related quality of life anticipated with different management strategies for febrile neutropenia in adult cancer patients. Support Care Cancer 2012;20:2755–64.
Theocharis G, Rafailidis PI, Rodis D, Kontopidis I, Barbas SG, Falagas ME. Outpatient parenteral antibiotic therapy (OPAT) at home in Attica, Greece. Eur J Clin Microbiol Infect Dis 2012;31:2957–61. http://dx.doi.org/10.1007/s10096-012-1647-1
Thornton J, Elliott RA, Tully MP, Dodd M, Webb AK. Clinical and economic choices in the treatment of respiratory infections in cystic fibrosis: comparing hospital and home care. J Cyst Fibros 2005;4:239–47.
Tice AD, Hoaglund PA, Nolet B, McKinnon PS, Mozaffari E. Cost perspectives for outpatient intravenous antimicrobial therapy. Pharmacotherapy 2002;22:63S–70S.
Tice A. The use of outpatient parenteral antimicrobial therapy in the management of osteomyelitis: data from the Outpatient Parenteral Antimicrobial Therapy Outcomes Registries. Chemotherapy 2001;47(Suppl 1):5–16.
Tice AD. Experience with a physician-directed, clinic-based program for outpatient parenteral antibiotic therapy in the USA. Eur J Clin Microbiol Infect Dis 1995;14:655–61.
Upton A, Ellis-Pegler RB, Woodhouse A. Outpatient Parenteral Antimicrobial Therapy (OPAT): a review of experience at Auckland Hospital. N Z Med J 2004;117:U1020.
Walton AL, Howden BP, Grayson LM, Korman TM. Continuous-infusion penicillin home-based therapy for serious infections due to penicillin-susceptible pathogens. Int J Antimicrob Agents 2007;29:544–8.
White HA, Davis JS, Kittler P, Currie BJ. Outpatient parenteral antimicrobial therapy-treated bone and joint infections in a tropical setting. Intern Med J 2011;41:668–73. http://dx.doi.org/10.1111/j.1445-5994.2009.02136.x
White B, Seaton RA, Evans TJ. Management of suspected Lyme borreliosis: experience from an outpatient parenteral antibiotic therapy service. QJM 2013;106:133–8. http://dx.doi.org/10.1093/qjmed/hcs189
Williams DN. Home intravenous antibiotic therapy (HIVAT): Indications, patients and antimicrobial agents. Int J Antimicrob Agents 1995;5:3–8.
Wolter JM, Cagney RA, McCormack JG. A randomized trial of home vs hospital intravenous antibiotic therapy in adults with infectious diseases. J Infect 2004;48:263–8.
Wolter JM, Bowler SD, Nolan PJ, McCormack JG. Home intravenous therapy in cystic fibrosis: a prospective randomized trial examining clinical, quality of life and cost aspects. Eur Respir J 1997;10:896–900.
Wynn M, Dalovisio JR, Tice AD, Jiang X. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin-sensitive Staphylococcus aureus. South Med J 2005;98:590–5.
Yadlapalli NG, Vaishnav A, Sheehan P. Conservative management of diabetic foot ulcers complicated by osteomyelitis. Wounds 2002;14:31–5.
Yan YM, Singh M, Tonks K, Kavi J, Langford NJ. Delivering outpatient antibiotic therapy (OPAT) in an Acute Medical Unit. Acute Med 2011;10:22–5.
Yong C, Fisher DA, Sklar GE, Li SC. A cost analysis of Outpatient Parenteral Antibiotic Therapy (OPAT): an Asian perspective. Int J Antimicrob Agents 2009;33:46–51. http://dx.doi.org/10.1016/j.ijantimicag.2008.07.016
You JH, Lee GC, So RK, Cheung KW, Hui M. Linezolid versus vancomycin for prosthetic joint infections: a cost analysis. Infection 2007;35:265–70.
Al Ansari A, Al Alawi S, Al Qahtani M, Darwish A. Outpatient Parenteral Antimicrobial Therapy (OPAT) in the Kingdom of Bahrain: efficacy, patient satisfaction and cost effectiveness. Open Infect Dis J 2013;7:90–5.
Allison GM, Muldoon EG, Kent DM, Paulus JK, Ruthazer R, Ren A, Snydman DR. Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014;58:812–19. http://dx.doi.org/10.1093/cid/cit920
Barr DA, Irvine S, Ritchie ND, McCutcheon J, Seaton RA. Risk of venous thromboembolism in patients treated for bacterial infection in the community with outpatient parenteral antimicrobial therapy. QJM 2014;107:207–11. http://dx.doi.org/10.1093/qjmed/hct239
Bedi P, Sidhu MK, Donaldson LS, Chalmers JD, Smith MP, Turnbull K, et al. A prospective cohort study of the use of domiciliary intravenous antibiotics in bronchiectasis. NPJ Prim Care Respir Med 2014;24:14090. http://dx.doi.org/10.1038/npjpcrm.2014.90
Duncan CJ, Barr DA, Ho A, Sharp E, Semple L, Seaton RA. Risk factors for failure of outpatient parenteral antibiotic therapy (OPAT) in infective endocarditis. J Antimicrob Chemother 2013;68:1650–4. http://dx.doi.org/10.1093/jac/dkt046
Htin AK, Friedman ND, Hughes A, O’Brien DP, Huffam S, Redden AM, Athan E. Outpatient parenteral antimicrobial therapy is safe and effective for the treatment of infective endocarditis: a retrospective cohort study. Intern Med J 2013;43:700–5. http://dx.doi.org/10.1111/imj.12081
Keller SC, Ciuffetelli D, Bilker W, Norris A, Timko D, Rosen A, et al. The impact of an infectious diseases transition service on the care of outpatients on parenteral antimicrobial therapy. J Pharm Technol 2013;29:205–14.
Lacroix A, Revest M, Patrat-Delon S, Lemaître F, Donal E, Lorléac’h A, et al. Outpatient parenteral antimicrobial therapy for infective endocarditis: a cost-effective strategy. Med Mal Infect 2014;44:327–30. http://dx.doi.org/10.1016/j.medmal.2014.05.001
Lai A, Tran T, Nguyen HM, Fleischmann J, Beenhouwer DO, Graber CJ. Outpatient parenteral antimicrobial therapy at large Veterans Administration medical center. Am J Manag Care 2013;19:e317–24.
Lane MA, Marschall J, Beekmann SE, Polgreen PM, Banerjee R, Hersh AL, Babcock HM. Outpatient parenteral antimicrobial therapy practices among adult infectious disease physicians. Infect Control Hosp Epidemiol 2014;35:839–44. http://dx.doi.org/10.1086/676859
Mohammadi S, MacKay K, Ward TT, Forrest GN. Clinical outcomes of a veterans affairs outpatient antimicrobial treatment program. South Med J 2013;106:345–9. http://dx.doi.org/10.1097/SMJ.0b013e3182967e8f
Muldoon EG, Allison GM, Gallagher D, Snydman DR, Bergin C. Outpatient parenteral antimicrobial therapy (OPAT) in the Republic of Ireland: results of a national survey. Eur J Clin Microbiol Infect Dis 2013;32:1465–70. http://dx.doi.org/10.1007/s10096-013-1899-4
Muldoon EG, Switkowski K, Tice A, Snydman DR, Allison GM. A national survey of infectious disease practitioners on their use of outpatient parenteral antimicrobial therapy (OPAT). Infect Dis 2015;47:39–45. http://dx.doi.org/10.3109/00365548.2014.967290
Pajarón M, Fernández-Miera MF, Allende I, Arnaiz AM, Gutiérrez-Cuadra M, Cobo-Belaustegui M, et al. Self-administered outpatient parenteral antimicrobial therapy (S-OPAT) for infective endocarditis: a safe and effective model. Eur J Intern Med 2015;26:131–6. http://dx.doi.org/10.1016/j.ejim.2015.01.001
Seetoh T, Lye DC, Cook AR, Archuleta S, Chan M, Sulaiman Z, et al. An outcomes analysis of outpatient parenteral antibiotic therapy (OPAT) in a large Asian cohort. Int J Antimicrob Agents 2013;41:569–73. http://dx.doi.org/10.1016/j.ijantimicag.2013.01.015
Sims AL, Baker P, Bellamy R, McMurtry IA. Outpatient parenteral antibiotic therapy in primary hip and knee arthroplasty infection managed with debridement and retention of prosthesis: a retrospective cohort study. Surg Infect 2013;14:293–6. http://dx.doi.org/10.1089/sur.2012.078
Subedi S, Looke DF, McDougall DA, Sehu MM, Playford EG. Supervised self-administration of outpatient parenteral antibiotic therapy: a report from a large tertiary hospital in Australia. Int J Infect Dis 2015;30:161–5.
Yang A, Fung R, Brunton J, Dresser L. Outpatient parenteral antimicrobial therapy for surgery patients: a comparison with previous standard of care. Can J Infect Dis Med Microbiol 2013;24:74–8.
Community IntraVenous Antibiotic Study systematic review: non-English language studies
Ambulatory treatment of lower respiratory tract infections in the aged [French]. Rev Prat Med Gen 1993;7:31–4.
Abbes S, Camara B, Murris-Espin M. [Respiratory infections and cystic fibrosis]. Rev Malad Respir 2009;1(Suppl. 3):S80–91.
Bakker W, Vinks AA, Mouton JW, de Jonge P, Verzijl JG, Heijerman HG. [Continuous intravenous home treatment of airway infections using ceftazidime administration via portable pump in patients with cystic fibrosis; a multicenter study.] Ned Tijdschr Geneeskd 1993;137:2486–91.
Batlle M, Lloveras N. [Management of low-risk febrile patients with neutropenia.] Enferm Infecc Microbiol Clin 2005;23(Suppl. 5):30–4.
Betegnie AL, Cracowski C, Bedouch P, Segond C, Robein-Dobremez MJ, Pin I, Allenet B. [Peripherally inserted central catheter antibiotic therapy for cystic fibrosis patients.] Rev Mal Respir 2014;31:822–30. http://dx.doi.org/10.1016/j.rmr.2013.11.002
Boumis E, Cicalini S, Petrosillo N. [New advances in antibiotic therapy of infective endocarditis.] Trends Med 2005;5:5–17.
Chen YC, Liu SQ. [The evaluation of intravenous antibiotic therapy of outpatients.] Pharm Care Res 2005;5:132–3.
Di Salvo L, Pardo F, Iapichino L, Mantione L, Marrone P, et al. [Home therapy with elastomeric infusors.] G Ital Farma Clin 1994;8:64–70.
Esposito S. [Outpatient parenteral antibiotic treatment: the Italian model.] Infez Med 2001;9:7–12.
Esposito S, Ianniello F, Noviello S, Leone S, Ascione T, Tice A, et al. [Outpatient Parenteral Antibiotic Therapy (OPAT): the Italian registry.] Infez Med 2002;10:169–75.
Estrada Cuxart O, Cuxart Mèlich A, Bonet Papell G, Riera Riezu C. [Home intravenous antibiotic treatment and home hospitalization.] Med Clin 2007;128:798.
Faure K. [Assessment, triage, and follow-up of a patient with: acute CAP COPD.] Med Mal Infect 2006;36:734–83.
Ferrere F, Perez Molina JA. [General and ambulatory management of patient with suspicion of viral infection.] Medicine 2010;10:3990–6.
Feuerwerker LC, Merhy EE. [Home care’s contribution to alternative health care networks: deinstitutionalization and transformation of practices.] Rev Panam Salud Publica 2008;24:180–8.
Francioli P. [Outpatient antimicrobial drug therapy.] Med Hygiene 1999;57:385–87.
Galpérine T, Ader F, Piriou P, Judet T, Perronne C, Bernard L. [Outpatient parenteral antimicrobial therapy (OPAT) in bone and joint infections.] Med Mal Infect 2006;36:132–7.
Garde C, Goenaga MA. [Outpatient intravenous antibiotic treatment.] Enferm Infecc Microbiol Clin 2005;23:393–5.
Garde C, Millet M, Goenaga M, Arzelus E, Cuende A, Sarasqueta C, et al. [Treatment of respiratory infection by Pseudomonas aeruginosa in adult patients within a hospital at home service: clinical characteristics and analysis of prognostic factors for relapse.] Enferm Infecc Microbiol Clin 2009;27:257–62.
Girón RM, Cisneros C, Nakeeb ZA, Hoyos N, Martínez C, Ancochea J. [Efficiency of the home intravenous antibiotics treatment in cystic fibrosis.] Med Clin 2006;127:567–71.
Girón RM, Martínez A, Máiz L, Salcedo A, Beltrán B, Martínez MT, et al. [Home intravenous antibiotic treatments in cystic fibrosis units of Madrid.] Med Clin 2004;122:648–52.
Goenaga MA, Carrera JA, Garde C, Millet M. [Parenteral antibiotics at home.] Enferm Infecc Microbiol Clin 1999;17:369–70.
Goenaga Sanchez MA, Garde Orbaiz C, Millet Sampedro M, Carrera Macazaga JA. [Ambulatory parenteral antimicrobial therapies. Five-year experience.] Rev Clin Espan 2002;202:142–7.
Goenaga MA, Garde C. [Domiciliary parenteral antibiotic therapy.] Sem Fund Espan Reumatol 2006;7:177–82.
Goenaga Sánchez MA, Garde Orbaíz C, Millet Sampedro M, Arzelus Aramendi E. [Readmissions among patients receiving parenteral antibiotic therapy at home.] Med Clin 2003;121:595–6.
Gómez J, Muñoz R, Baños V, Gómez G. [Treatment of community-acquired urinary infections: current perspectives and patient clinical approach.] Rev Esp Quimioter 2005;18:319–27.
Graf von der Schulenburg JM, Greiner W, Klettke U, Wahn U. [Economic aspects in treatment of cystic fibrosis with chronic pulmonary pseudomonas infection. Ambulatory intravenous therapy in comparison with inpatient treatment.] Med Klin 1997;92:626–9.
Gross H. [Ambulatory pneumonia: Intravenous antibiotic therapy without advantage.] Deutsch Med Wochenschr 2007;132:8.
Hase R, Hosokawa N, Uno S, Mikawa T, Uwamino Y, Muranaka K. [The first trial of OPAT (outpatient parenteral antimicrobial therapy) with continuous infusions in Japan.] Kansenshogaku Zasshi 2014;88:269–74.
Hazas J, Fernández-Miera MF, Sampedro I, Fariñas MC, García de la Paz AM, Sanroma P. [Domiciliary intravenous antibiotic therapy.] Enferm Infecc Microbiol Clin 1998;16:465–70.
Hazas J, Sampedro I, Fernández-Miera MF, García de la Paz AM, Sanroma P. [A program of intravenous antibiotic therapy at home.] Enferm Infecc Microbiol Clin 1999;17:463–9.
Henn-Menetre S, May I, Derelle J, Vidailhet M. [Cystic fibrosis and intravenous antibiotics. Costs of three mode of administration (hospital versus home).] J Pharm Clin 2002;21:64–70.
Horcajada JP, García L, Benito N, Cervera C, Sala M, Olivera A, et al. [Specialized home care for infectious disease. Experience from 1995 to 2002.] Enferm Infecc Microbiol Clin 2007;25:429–36.
Karthaus M, Cornely OA, Südhoff T, Meran J. [Antimicrobial therapy of febrile neutropenia – current developments.] Wien Med Wochenschr 2001;151:66–72.
Karthaus M, Meran JG, Geissler RG, Böhme A, Jürgens H, Ganser A. [Possibilities and limits of ambulatory supportive measures in oncology exemplified by antibiotic therapy of febrile neutropenia.] Wien Med Wochenschr 1998;148:427–32.
Klettke U, Magdorf K, Staab D, Bisson S, Paul K, Wahn U. [Ambulatory vs. inpatient intravenous antibiotic therapy in mucoviscidosis patients – a controlled study.] Pneumologie 1999;53:31–6.
Krauth C, Busse R, Smaczny C, Ullrich G, Wagner TO, Weber J, Welte T. [Cost comparison of hospital and ambulatory i.v. therapy in adult cystic fibrosis patients. Results of a controlled prospective study.] Med Klin 1999;94:541–8.
Lamy O, Zanetti G, Bille J, Aubert JD, Berger JP, Malinverni R, et al. [Ambulatory and hospital management of home-acquired pneumonia. Summary of the recommendations for the clinical practice of the Department of Medicine of the University Hospital Center of Vaud.] Med Hygiene 2004;62:1573–8.
Lopez Hernandez MA, Herrera Alvarez W, Sibaja Nieto L, Alvarez Vera JL. [Low-risk febrile neutropenia in patients with lymphoblastic acute leukemia. Amikacin-ceftriaxone or oral fluoroquinolones.] Med Intern Mex 2010;26:219–25.
Magdorf K, Klettke U, Staab D, Paul K, Wahn U. [Home intravenous antibiotic therapy vs. hospital therapy – an alternative for patients with cystic fibrosis?] Monat Kinderheilkunde 1996;144:1047.
Marvaso A, Esposito S, Noviello S, Ianniello F, Leone S, Maiello A, Petronella P. [Outpatient parenteral antibiotic therapy (OPAT) of diabetic foot infections with piperacillin/tazobactam.] Infez Med 2002;10:230–5.
Meani E, Romanõ C. [Treatment of osteomyelitis by local antibiotics using a portable electronic micropump.] Rev Chir Orthop Reparatrice Appar Mot 1994;80:285–90.
Mendoza-Ruiz de Zuazu H, Casas-Arrate J, Martínez-Martínez C, de la Maza I, Regalado de los Cobos J, Cía-Ruiz JM. [Home intravenous antibiotic treatment: a study in 515 patients.] Enferm Infecc Microbiol Clin 2005;23:396–401.
Nigro FS, Buonopane G, Iandoli M, Matarazzo M, Maio P, Siano F, et al. [Preliminary experience with O.P.A.T. (Outpatient parenteral antimicrobial-drug therapy) in infective endocarditis.] Infez Med 2001;9:108–10.
Ortiz Rodriguez M, Mauri Plana M, Capdevila Morell JA. [General and ambulatory hospital management of patients with comorbidity and suspicion of an infectious disease. Evaluation of the febrile patient and comorbidity.] Medicine 2010;10:3373–80.
Pardos-Gea J, Maza Ú, Pérez-López J, San José Laporte A. [Home intravenous antibiotic therapy of empyema and lung abscess: safety and efficacy.] Enferm Infecc Microbiol Clin 2011;29:237–9. http://dx.doi.org/10.1016/j.eimc.2010.09.011
Pardos-Gea J, Pérez-López J, San José Laporte A, Vilardell Tarrés M. [Home intravenous antibiotic therapy of hepatic abscess: Safety, efficacy and predictive factor of hospital readmission.] Med Clin 2010;134:473–6. http://dx.doi.org/10.1016/j.medcli.2009.10.050
Pea F. [Oral or injectable? Choice criteria for home antibiotic therapy.] G Ital Malat Torace 2004;58:261–5.
Pensotti C, Nacinovich F, Vidiella G, Carbone E, Marin M, Di Stefano C, et al. [Teicoplanin in the treatment of bone and joint infections due to methicillin resistant staphylococci. Experience in adult patients.] Medicina 2002;62(Suppl. 2): 40–7.
Pérez López J, San José Laporte A, Alemán Mansó C, Pardos-Gea J, Vilardell Tarrés M. [Intravenous antibiotic treatment in a hospital based home care unit. Predictors of hospital readmission.] Med Clin 2008;131:290–2.
Pérez-López J, Pardos-Gea J, San José Laporte A, Almirante Gragera B, Marian Oltean D, Vilardell Tarrés M. [Home intravenous antimicrobial therapy in multi-drug resistant microorganism infections.] Med Clin 2012;138:557–61. http://dx.doi.org/10.1016/j.medcli.2011.03.028
Regalado de Los Cobos J, Cía Ruiz JM. [Treatment of liver abscesses: new evidence of the usefulness of hospital at home.] Med Clin 2010;134:486–8. http://dx.doi.org/10.1016/j.medcli.2009.12.006
Regalado J, Mendoza H, Aizpuru F, Altuna E, Gómez M, Cía JM. [Acute pyelonephritis treated under ‘home hospitalization.’ Ten years’ experience.] Enferm Infecc Microbiol Clin 2006;24:629–33.
Riethmuller J, Busch A, Ziebach R, Stern M. [Intravenous antibiotic therapy in cystic fibrosis - Hospitalization or at home?] Monat Kinderheilkunde 2000;148:850–4.
Ritter M, Alter P, Maisch B. [Possibilities and limits of outpatient antibiotic therapy of infective endocarditis.] Herz 2001;26:418–23.
Rutschmann O, Sierro MC. [Outpatient parenteral antibiotic therapy.] Med Hygiene 2004;62:845–9.
San Jose Laporte A, Perez Lopez J, Aleman Llanso C, Rodriguez Gonzalez E, Chicharro Serrano L, Jimenez Moreno FX, et al. [Specialized home care of medical diseases in an urban tertiary university hospital. Coordination between the medical services of the hospital and the primary health care.] Rev Clin Esp 2008;208:182–6.
Sebban C, Fuhrmann C, Perol D, Devaux Y, Ghesquiere H, Galand-Desme S, et al. [Home health care and short febrile neutropenia after chemotherapy: Analysis of a two years experimental study made in a cancer centre.] Oncologie 2006;8(Suppl. 1):HS29–34.
Serrais J, Lacasa C, Vila A, Ortega L, Martinez J, et al. [Home administration of vancomycin: two patient study.] Farm Clin 1996;13:636–9.
Sopena N, Benitez R, Molinos S, Cuxart A, Equipo Médico de la Unidad de Hospitalización Domiciliaria. [Outpatient parenteral antimicrobial therapy for Staphylococcus aureus bacteremia.] Med Clin 2011;137:663–4. http://dx.doi.org/10.1016/j.medcli.2011.03.005
Springer G. [Cost reduction by means of ambulatory therapy with antibiotics.] Krankenhaus Arzt 1995;68:294–5.
Spuch JA, Mirón M, Florit L, Escuder J, Castellote M, Zornoza A. [Home treatment of patients with pancreatic fistula.] Cir Esp 2008;83:129–33.
Steinmetz D, Edelstein H, Berkovits E, Raz R. [Home intravenous antibiotic therapy for osteomyelitis patients.] Harefuah 2002;141:439–41,498.
Tremblay S. [Intravenous antibiotics therapy at home. Quality, efficiency, economy.] Infirm Que 1996;3:23.
Trzeciak P, Foremny J, Szygula-Jurkiewicz B, Zembala M. [Heart Valve Disease Outpatient Clinic – Outpatient care in persons after mechanical heart valve prosthesis grafting. Infective endocarditis, haemorrhagic and thromboembolic complications prophylaxis.] Fam Med Prim Care Rev 2006;8:1328–34.
Ullrich G, Smaczny C, Steinkamp G, Weber J, Welte T, Busse R, Wagner TO. [Why do adults with mucoviscidosis refuse a medically recommended course of intravenous antibiotic therapy?] Pneumologie 1997;51:822–7.
Ullrich G, Weber J, Smaczny C, Busse R, Welte T, Wagner TO. [How convenient is home intravenous antibiotic therapy for adult cystic fibrosis patients?] Prav Rehabil 1998;10:89–96.
van Aalderen WM, Mannes GP, van Bommel G, Voorthuis I, Bosma E, Heymans HS. [Continuous intravenous antibiotic home treatment in 11 patients with cystic fibrosis in The Northern Netherlands.] Ned Tijdschr Geneeskd 1993;137:2482–6.
van den Broek PJ, Haerkens HM, van Weert NJ, Vermeij P. [Favorable results with intravenous antimicrobial therapy outside the hospital.] Ned Tijdschr Geneeskd 1997;141:2297–301.
van Haaren CPLC, Wijlhuizen TJ, Van Den Broek PJ, Vermeij P. [Home intravenous treatment of Lyme disease.] Pharm Week 1994;129:680–3.
Appendix 2 Community IntraVenous Antibiotic Study health-care provider survey and interviews
| Number | Location | SA | Business plan | Monitoring | Evaluation | Patient pathway | Clinical governance |
|---|---|---|---|---|---|---|---|
| 001 | Urban | ✓ | ✗ | ✓ | – | ✗ | ✓ |
| 002 | Semi-rural | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 003 | Urban | ✓ | ✓ | ✗ | ✗ | ✓ | In dev |
| 004 | Urban and semi-rural | ✗ | In dev | ✗ | ✗ | ✓ | ✓ |
| 005 | Rural | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ |
| 006 | Urban | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| 007 | Urban and semi-rural | ? | In dev | ✓ | – | ? | ✓ |
| 008 | Urban | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 009 | Semi-rural | ? | ✓ | ✓ | In dev | ✓ | ✓ |
| 010 | Urban and semi-rural | ✓ | In dev | ✗ | ✗ | ✗ | ✓ |
| 011 | Urban | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 012 | Urban and semi-rural | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ |
| 013 | Rural and semi-rural | ✗ | In dev | ✗ | ✗ | ✗ | ✗ |
| 014 | Urban | ✓ | ✓ | ✓ | ✓ | ? | ✓ |
| 015 | Semi-rural | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| 016 | Urban | ✓ | ✗ | ✓ | ✗ | ✗ | ✓ |
| 017 | Urban and semi-rural | ✗ | ✓ | ✓ | ? | ✓ | ✓ |
| 018 | Rural and semi-rural | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| 019 | Urban | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Service/setting | Description | Level of planning | Conditions | Effectiveness | Capacity for change | Relationships in organisation |
|---|---|---|---|---|---|---|
| 001, urban | Service is provided by MAU and outpatients with responsibility of patients with specialties (e.g. renal/respiratory). A community arm supports patients to administer at home. SA is unusual | Fragmented services based on outdated audit. No reference to business plan | Cellulitis, renal, respiratory, diabetes mellitus (foot), endocarditis, bone or joint infection, prosthetic joint infection | No evaluation. Respiratory service has evaluated the clinical effectiveness of service for patients with bronchiectasis No reference to cost-effectiveness |
Perceived to be no reason to change based on audit from 10 years ago | Services work in isolation from one another No clinical governance issues (reactive) No information about commissioning |
| 002, semi-rural | In-hospital service with patients referred from ward and outpatient clinics, patients are administered antibiotics in the community by district nurse and can sometimes self-administer | Annual business case prepared each year Service adapted based on best practice and observation at another trust |
Bone and joint infections, otitis externa, endocarditis, osteomyelitis, diabetic foot infection, staphylocooccus bacteremias | Monitoring: 100 patients referred and 90 accepted in the service 11/12. 159 referred and 140 accepted into the service 12/13 Service is monitored and reported to BI |
There are some levers for change and quality improvement in terms of monitoring and calculation of bed-days saved | Informal network in small trust |
| 003, urban | GPs, MAU or consultants refer patients to community team for administration of antibiotics. Sometimes patients administer their own antibiotics | Service has a business plan | Cellulitis, bone and joint infections, septic arthritis. Expect that an extended service will see more cellulitis, and bone/joint infections who are discharged and multiresistant gram-negative infections, endocarditis, meningitis | No formal evaluation or monitoring No cost-effectiveness |
There are plans to extend the service. They aim to develop a team to support patients to be discharged from hospital and to set up MDT for patient review | There do not appear to be established relationships (e.g. between commissioners and providers) |
| 004, urban and semi-rural | Limited service, referral from hospital, clinicians send patients to i.v. infusion service run by nurses. No SA | Currently unplanned but a business case has been developed to extend future service | Orthopaedic, cellulitis, respiratory (refers to diversity of patients but does not list all conditions) | No formal evaluation or monitoring No cost-effectiveness data |
Future service aims to include pharmacist support, to help with assessment and evaluations and to select appropriate antibiotics. Also aim to use electronic prescriptions to aid monitoring | Some willingness to change and future plans developed |
| 005, rural | Service facilitates early discharge from hospital as part of admission avoidance pathway. Patients can self-administer antibiotics. Some patients are attended by a district nurse or private health-care provider | Service was set up based on a business plan 3 years ago attached to infectious diseases short-stay ward | Bone and joint infections, cellulitis | No formal evaluation. Patients are monitored via MDT Service is seen as clinically effective with good outcomes No cost-effectiveness data |
Changes to service are not discussed | There is some tension about the use of the private company and whether or not they review patients appropriately |
| 006, urban/city | Patients reviewed at hospital (a specialist centre). Antibiotics are administered by local teams via local protocol [e.g. district nurses or private company (MediHome)]. Patients from other areas seen. Aim to increase the number of patients self-administering | Based on a business plan | Bone and joint infections, osteomyelitis, endocarditis, meningitis, pylonephritis, intra-abdominal infections, vascular patients, vascular graft infections, diabetic feet, prosthetic joint | No formal evaluation but patient satisfaction assessed. Database of patients to track patient process and outcomes to prevent anyone being on antibiotics too long and based on BSAC version supports auditing | Co-ordinator is developing an integrated care booklet, patient information sheets and integrated care pathway booklet and is reviewing drug delivery mechanisms | Some liaison with commissioners about drug tariffs |
| 007, urban/semi-rural | Jointly commissioned service. Referrals from acute hospital. Patients treated and reviewed at hospital Discharged to community team (district nurses). i.v. outreach | No reference to business plan | Cellulitis, bacteraemia, nephritis, endocarditis, orthopaedic joint infection | Annual report to board. Data monitored on a monthly basis, outcomes are monitored for all patients. Patient satisfaction is assessed routinely | Changing service from 5-day service with on call system to a 7-day service | Service is commissioned by CCG |
| 008, urban | Virtual ward service, in hospital treatment and review and community administration by district nurses and GPs. Patients are referred from A&E or hospital ward. No SA | Business plans have been developed | Cellulitis, neurosurgery patients, orthopaedic, diabetic foot, spinal osteomyelitis, endocarditis, ENT referrals, vascular graft, herpes, cryptospirosis, lyme disease, pulmonary infections, liver abscess, multiresistant TB and UTI | Evaluation plan based on BSAC surveillance form. Patients stated the service had exceeded their expectations Over 3 years they saved 6000 bed-days |
Need more microbiology time. Savings in bed-days were not translated into more services. It would be good if OPAT services were networked in related areas | Informal networks and good relationships between staff keep service performing well Relationship between commissioner and provider is not clear |
| 009, semi-rural | Three arms to service. Admission avoidance, children’s OPAT and adult OPAT. A private healthcare provider administer i.v. antibiotics in the community. Patients managed via virtual ward | There is reference to a plan | Cellulitis, bone and joint infections, endocarditis, respiratory infections | They do have a method of monitoring patients and readmissions | In the process of setting up the BSAC OPAT database to enable routine capture of service information | Informal networks support the development of the service. A formal plan is in development |
| 010, urban/semi-rural | Patients are treated and reviewed in hospital but discharged into the community with antibiotics. Only occasional patient/carer SA programme | In transitional state, not a formal service until business plan is approved | Cellulitis, diabetic foot, vascular graft infection, endocarditis, orthopaedics, bronchitis | No evaluation plan | Some plans to extend the service and to look at excess bed-days saved. Some uncertainty about what happens once bed-days are saved | Commissioners say that there is not enough evidence to develop a new service |
| 011, urban/city | In-hospital service and specialist team to support community nurses and provide education. District nurses support patients or family members to self-administer. Short stay ward via A&E with day wards for 12-hour follow-up | Planned service evolved over 10 years with business case and patient pathways | Bone and joint infections, diabetic foot infections, respiratory patients, cellulitis | Last full evaluation was 2 years ago including patient satisfaction and any complications. This occurs approximately every 18 months. Presented to OPAT network | Potential to change. Service has evolved over 10 years with specific planning processes, protocols and pathways being developed | Referred to commissioners but at arm’s length; discusses need for national commissioning framework to ensure consistency in all areas |
| 012, urban and semi-rural | District nurses work as specialist i.v. team as part of district nursing. Doctors discharge patients into community care for i.v. antibiotic administration. Antibiotic pharmacists. 12 administrations a day across two trusts. Patients are treated in hospital via MAU and antibiotics are administered in the community | Business plan and service specification | Cellulitis, respiratory (bronciectais), bone and joint infections | Monitoring database has been set up and patients are reviewed as part of pathways | Working with commissioners to assess payment for the service. Current service based on end of year money so unclear about future of service | Service is a pilot but is jointly commissioned. There is a partnership between the commissioners and the providers |
| 013, rural/semi-rural | Hospital at home service owing to the closure of existing hospital services. At hospital there is a medical admissions unit and a medical day unit for patients to receive i.v. antibiotics | A business plan has been developed for the future service Previously clinical directors rejected the proposal for a formal OPAT service |
Cellulitis, orthopaedic – bone and joint infections | No formal monitoring or evaluation | A business plan has been developed for a future service. Projected savings may not make it a priority | There have been discussions with CCGs |
| 014, urban | Hospital service via MAU and infectious diseases ward. District nurses support administration of i.v. antibiotics at home. Patients self-administer, nurses supervise and perform risk assessments | Formal service with business plan | Bone and joint infections, orthopaedic and diabetic foot infections | 175 patients episodes with 148 patients Monitoring database Saved 5020 bed-days. A massive difference so wards are being reconfigured from 18 beds to 12 beds. New tariff charges to see infectious diseases consultant (new appointment, £271; follow-up, £209; community supervision of patient taking antibiotics, £180; nurse visits, £100 a visit) |
Continual improvement cycle via clinical governance meetings | CCG and hospital board work together to achieve outcomes |
| 015, semi-rural over wide area | Hospital service but no engagement with community services. Patients come up to the hospital on a daily basis at acute investigation or medical wards | Ad hoc/informal service, no business plan | Bone and joint infection (longer term) not cellulitis and endocarditis | No formal evaluation and clinical lead knows about numbers of patients seen | There are barriers to change within the organisation | There is a lack of joined up working across organisations |
| 016, urban | Hospital clinic service and patients can self-administer in the community or a district nurse can administer (patients are given a choice where possible) | Improved planning in last 18 months but no business plan in place | Referrals from infectious diseases team, respiratory disease (bronchiectasis), cellulitis, UTI resistant to antibiotics, orthopaedic bone and joint infections, cancer and haematology, MRSA and Lyme disease could extend to cardiology and diabetic foot | Database to monitor patients and governance arrangements are in place. Patient satisfaction was measured. Service numbers are monitored: 71 patients discharged and accepted and 20 declined this year from March 2014 | Improvement culture within the organisation | Relationships within and across units and community seem to be good No discussion of commissioners |
| 017, urban/semi-rural | Patients are administered i.v. antibiotics by district nurses and some attend the hospital for 12 administrations on either site per day; not more than three times a day | Commissioned service | Cellulitis, diabetic foot infection, endocarditis, bone and joint infections, bronchiectasis, intra-abdominal and intrapelvic infections, TB, antibiotic-resistant UTIs | Database to monitor patients has been set up. 203 referrals with 190 accepted into the service. Patient satisfaction is measured. Clinical effectiveness lies with individual teams | Improvement culture across the organisations | Evidence base seen as being too weak to convince commissioners in committing to a formal service as here there is a difference as there are two NHS trusts and two CCGs in the area |
| 018, urban | Patients are referred from hospital and treated by CIT, SA is unusual | Unplanned no business plan | Bone and joint infections, renal patients, pilot in one site for cellulitis | Service is not formally monitored and previous audit was some time ago | Some plans for change were discussed | Discusses hospital service and discharge to community. No reference to commissioners |
| 019, urban | In-hospital and community service. Referrals from hospital and patients in MAU/A&E. Patients are reviewed at weekly MDT including a focus on non-responders | Informally planned it was set up on back of previous flu epidemic | Cellulitis, otitis externa, joint infections, septic arthritis or prosthetic joint infection, spinal infection, streptococcus infection, infected grafts (e.g. vascular grafts, diabetic foot) | Service is not formally evaluated and monitoring figures are available but were not described | Change is perceived as occurring despite management | Not great. Virtually everything achieved has been despite managerial input |
| Service number | Leada | Delivered by | Pharmacist | Microbiologist | Community administration | Patient pathways | Clinical governance |
|---|---|---|---|---|---|---|---|
| 01 | Consultant microbiologist | Nurses | Yes | Yes (lead for service) | District nurses | Not described | Overseen by senior committee with competing priorities |
| 02 | No strategic or clinical lead formally appointed | Nurse | Yes | Yes | District nurses | They refer to inclusion criteria and have a process of monitoring and review | Patients are reviewed in clinic but processes for review could be improved to assess length of time antibiotics are received and for readmissions |
| 03 | Nurse led from community-based service Individual consultants liaise with community team |
Nurses | No dedicated post described | Yes | District nurses | Pathway for patients with cellulitis | Aim to set up review MDT |
| 04 | Consultant links with nurse-led i.v. infusion service No SA yet |
One dedicated nurse | No dedicated post described | Not described | District nurses | Patient pathway for home i.v. antibiotics is in place | Unclear |
| 05 | Consultant and registrar in infectious diseases | Six senior nurses rotate in and out of service | No dedicated post but arranged informally | Yes | Private company called MediHome administer antibiotics | Not described | MDT meeting reviews patients and length of treatment |
| 06 | Consultant in infectious diseases | OPAT co-ordinator | Antimicrobial pharmacist | Yes | District nurses or MediHome | Integrated care pathway booklet | MDT meeting to review patients and length of treatment |
| 07 | Nurse | Nurses Medical physicians |
No | Four microbiologists rotate in service | District nurses | Nurse writes all protocols and guidance to guide practice | MDT review patients as a virtual ward (e.g. via electronic records). Some patients are supported to self-administer. Follow BSAC guidelines |
| 08 | Consultant lead (hands-off approach) | Three nurses trained in midline insertion | No formal pharmacist for the service; they make use of antimicrobial pharmacist | Two microbiology consultants | District nurses | No reference to patient pathways | Patients are reviewed in clinic by consultants |
| 09 | Consultant | Nurses | No | Yes | Healthcare at Home | Unclear | Weekly review of patients looking for evidence of benefit and sign of toxicity |
| 10 | Consultant | Nurse | Yes | Yes | District nurses | No reference to patient pathway | MDT including consultants (microbiology and diabetes mellitus), podiatrist, nurses |
| 12 | Consultant | Clinical nurse specialists in hospital and to provide education about hygiene techniques | Yes | Infectious diseases team | District nurses support patient/carer SA | Patient pathway in place | Formal governance structure and pathways for patients. Patients are reviewed at a weekly multidisciplinary clinical and governance meeting on a weekly basis. Patients on OPAT > 5 days are reviewed in infectious diseases clinics |
| 14 | No clear clinical lead for OPAT | MAU and day ward provide facilities for patients to receive i.v. treatment and they are looked after by the consultant from the specialty they came from | Unclear | Unclear | SNs in community attend the patient at home | Unclear | Unclear as no review or monitoring process is discussed |
| 15 | Infectious diseases consultant with dedicated post in this area | One band 7 nurse, two band 6 nurses and one band 3 nurse and a trainee support worker to liaise with patients and get them ready for home, infection specialist working with individual teams (e.g. orthopaedic surgeons). Looking to expand into cardiovascular area (endocarditis) | Pharmacist and technician | Two microbiologists | District nurses support patients at home | Patients are reviewed weekly on day ward or OP clinic. Patients are discussed at clinical governance meetings [e.g. adverse outcomes or problems (continual improvement cycle)]. BUPA (The British United Provident Association Ltd, London) provide equipment (prefilled devices) | |
| 16 | No dedicated clinical lead | Microbiologist works with i.v. access team that work with teams and patients on wards | No dedicated post | Yes | No community-based service | No patient pathways described | Patients are reviewed by microbiology consultant as case numbers are small |
| 17 | Clinical nurse specialist | Infection and microbiology consultants, clinical nurse specialist, district nurses Rapid access infection clinic |
Not identified in interview | Yes | District nurses, community nurses and matrons | No patient pathways described | Governance arrangements are in place but no detail. Online referral, nurse liaises with hospital and community staff. Data collection for any adverse events experienced by patients |
| 18 | Microbiologist | Nurse consultant, virtual hub co-ordinators | Yes | Yes | District nurses, nurse manager and community manager | Patient pathways developed | Patients are reviewed on a weekly basis at MAU/clinic. Clinical effectiveness lies with individual teams |
| 19 | Consultant | Consultant, nurse-led community team and link to consultants on wards | No dedicated post described | Yes | District nurses and CIT | None referred to | Patients are reviewed in hospital and when stable discharged to CIT |
| 20 | Infectious diseases consultant | Two SNs | Yes | Yes | Community nurses | Patient pathways are referred to | Patients are reviewed by consultants |
Appendix 3 Detailed modelling methodology
The analysis of the data made use of mathematical structures known as discrete choice models. These models explain the choices/preferences of respondents between the three alternatives on the basis of the presented characteristics. This is done through formulating a utility function for each of the three alternatives, expressing the gain the respondent would get from choosing that alternative. The assumption of rational decision-making then postulates that a respondent chooses the alternative that maximises his/her utility (or minimises his/her disutility).
The first steps in the development of the model structure focus on the role of the explanatory variables alone, where the utility of alternative i (with i = 1,. . .,3) for respondent n in choice task t is given by:
The estimation of a discrete choice model is reliant on appropriate normalisation, as only differences in utilities across alternatives (rather than absolute levels) can influences choices. With this in mind, base levels were chosen for each of the attributes, and the associated coefficients were fixed to zero, explaining, for example, the absence of the self-administering treatment from the above equation (with the two remaining β parameters giving the estimated difference in utility for the other two types of treatments, i.e. hospital and nurse at home). The δi term captures ordering effects given concerns about an underlying propensity for respondents to choose options on the left, where, for normalisation, we set δ3 = 0.
Random utility models such as used in our work recognise the inability by analysts to fully capture the utility of alternative and thus acknowledge the presence of a remaining random component of utility, with Ui,n,t = Vi,n,t + εi,n,t, where εi,n,t follows a type I extreme value distribution.
With the above specification, the probability of respondent n choosing alternative i in choice task t is now given by:
where, with the properties of the type I extreme value distribution, the error term εi,n,t drops out.
Sociodemographic interactions
The above specification is making a major assumption in that the preferences are constant across individual respondents, as represented by the generic model parameters. As a first step, we now interact the coefficients with sociodemographic characteristics of the respondent. Using sex as an example, and focusing on the parameter associated with hospital treatment, we would have, for respondent n:
where δfemale,n = 1 if respondent n is female, and 0 otherwise. The additional parameter βfemale,hospital then estimates the difference between men and women in the preference for hospital treatment. We tested for such sociodemographic interactions for all model parameters and for the following covariates:
-
age (< 50 years, 50–65 years, > 65 years)
-
sex
-
race (white vs. non-white)
-
living status (alone vs. not alone)
-
education (university vs. not university educated)
-
employment status (working vs. not working)
-
number of past infections
-
long-term vs. short-term infection.
Random heterogeneity and attitudes
Although the above effects allow us to capture a certain degree of heterogeneity across respondents, we additionally allow for unexplained random differences in preference across respondents for the type of treatment. In particular, we allow the βhospital and βnurse home to follow a normal distribution across respondents.
Finally, we allow for an influence of underlying attitudes on decision-making. We formulate two latent attitudes, which are informed by earlier factor analysis work carried out on the same data. In particular, we have the latent variables αl, with l = 1,. . .,2, hereafter referred to as:
-
attitude towards hospitals
-
attitude towards health care being a doctor’s responsibility.
Each of these latent attitudes is defined to have a deterministic and a random component, with latent attitude l for person n being:
where the estimates of γl capture the impact of a range of sociodemographic characteristics of person n (zn) on the latent attitude, and where ξl, is a standard normal variate (mean 0, standard deviation 1), distributed across respondents, capturing the random element of the latent attitude. The sociodemographic terms tested for effects on the latent attitudes were the same as those used in the above direct interactions with coefficients, allowing for a direct and indirect impact on preferences.
The latent variables are then interacted with the preference for the type of treatment, where we now have:
In the above specification, βhospital is an estimated mean preference, γhospital relates to sociodemographic impacts on this preference (where we earlier showed the example of sex) and σhospital is an estimated standard deviation for the random variation across respondents, ξhospital,n is a standard normal variate (mean 0, standard deviation 1) distributed across respondents and τhospital,l measures the impact of the latent attitude αl,n on the preference by respondent n for hospital treatment. A corresponding specification is used for βnurse home.
An important point needs to be made here. Indeed, all respondent characteristics included in the deterministic component of the latent attitude have also been included in the covariate effects for βhospital and βnurse home, thus avoiding a situation in which a sociodemographic effect is erroneously captured as relating to attitudes when it may just relate to underlying modality preferences, or vice versa. In very much the same way, the modality parameters now include a random component that relates to the latent attitudes (through the inclusion of ∑l=1Lτhospital,lαl,n above in the example of hospital treatment), while a separate random component (σhospitalξhospital,n) relates to random variations in preferences for treatment type which cannot be linked to latent attitudes.
This brings us to an additional important point. The specification of the two modality terms (βhospital and βnurse home) now includes two separate random terms, both following a normal distribution, and both with deterministic interactions on the mean, some of which relate to the same underlying sociodemographic variables. In the current form, this would be an overspecification, with two parameters capturing the same effect. What allows us to separately identify the two components is that one of them, namely the latent variable component, is also used in a separate measurement model, to which we shall turn our attention next.
Measurement model
We have two latent attitudes in our model and these are used in the measurement model component of our overall framework to explain the answers that respondents give to a number of attitudinal questions.
In particular, the first (hospital attitude) latent variable is used to explain the answers to the following statements:
-
people get better more quickly if treated at home
-
I do not like hospitals
-
hospitals increased the risk of contracting a new infection
-
I would have i.v. at home even if waiting hours for nurse
-
being in hospital would make things difficult for family.
The latent attitude towards health-care responsibility is used to explain the answers to the following statements:
-
giving myself i.v. would worry me
-
doctors not patients are best placed to decide where patients should be cared for
-
I would prefer monitoring by a doctor than by a nurse
-
I want to be responsible for my own treatment.
All questions use a 5-level Likert scale, with higher values meaning stronger disagreement. We now use the two latent attitudes to explain the answers to the attitudinal questions. With Is used to refer to a given attitudinal question, and letting αl be the associated latent attitude, we use an ordered logit model to explain the likelihood of the actual observed value of Is for respondent n as:
where Xls,n,p=1 if and only if respondent n chooses answer p for question s. The tls,p parameters are thresholds that are to be estimated, with the normalisation that tls,0=−∞ and tls,5=+∞. The estimated parameter ζl,s measures the impact of the latent variable αl on Is. A significant estimate for ζl,s thus shows us that the latent attitude αl has a statistically significant impact on the answers provided to the attitudinal question Is.
Joint specification
With in,t being the alternative chosen by respondent n in task t (out of T = 8), we have that the likelihood of the eight observed choices and nine answers to attitudinal questions for respondent n is given by:
where we use a logit kernel for the choice model component, and where LIs,n is defined as above. Both the component relating to the choices (i.e. the logit kernel) and the component relating to the attitudinal questions are a function of the vector of latent variables α, while the choice model component is also a function of the random components used in the marginal utility coefficients (β). This is why the entire likelihood function is integrated over the distribution of α and β. For estimation, we work with the log-likelihood function (the logarithm of the above equation) and approximate this using numerical simulation (i.e. maximising the simulated log-likelihood). In this process, we need to take draws (where we rely on 200 modified Latin hypercube sampling draws per person, see Hess et al. ,228) for four normally distributed random terms. All models were coded in Ox version 6.2 (Timberlake Consultants Press, London, UK) and the standard errors reported in the results are obtained with the sandwich method (Huber229).
Detailed model results
The results are presented in Table 41.
In summary, we observe the following for the baseline effects:
-
ASC_A and ASC_B show that there is a slight preference for left most alternative, but this is not significant, while the middle alternative is chosen least often, but this is not highly significant
-
hospital and nurse_home show that, for a respondent in the base sociodemographic category, there is no statistically significant difference in preference between HO attendance and SA, with a preference, albeit with low statistical significance, for nurse treatment at home
-
treatm_one, treatm_two and treatm_three show that, with continuous treatment as the base, there is a strong preference for one treatment only. The differences between two or three treatments and continuous treatment are not statistically significant, although we see some trend
-
appt_time_no shows a strong preference for having an appointment
-
who_special and who_general show that, with doctor being the base, the base respondent type has a preference for a SN over hospital treatment, with no significant difference between doctor and GN
-
who_self_half shows that for the base respondent, there is no significant difference between a half-day and full-day training programme
-
comm_see_known shows no difference between seeing someone the patient knows or does not know
-
comm_speak_known shows a preference, albeit not highly significant, for speaking to someone the patient knows
-
after_none, after_hosp_nurse and after_GP show that having no after treatment is worse than a telephone or GP appointment, while a hospital nurse is preferred
-
risk_6_or_10 combines the effects for 1 in 6 and 1 in 10 risk levels, which were not significantly different for the base respondent, but were perceived as significantly worse than a 1 in 25 risk.
Turning to the sociodemographic interactions, we see that:
-
younger patients prefer a half-day of training, have a preference for seeing someone they know and also have a stronger preference for in-hospital treatment
-
older patients have a preference for nurse treatment at home, the difference between speaking to someone they know or does not know disappears (when summing up comm_see_known and age_over_65_comm_speak_known) and they evaluate a risk of 1 in 10 as being less bad than 1 in 6 (summing up risk_6_or_10 and age_over_65_risk_10)
-
those living alone make no distinction between doctors and special nurses but prefer them to GNs (summing who_special and alone_who_special, and who_general and alone_who_general). They dislike half-day training and the preference for speaking to someone they know cancels out (comm_speak_known and alone_comm_speak_known)
-
those working understand the distinction between 1 in 6 and 1 in 10 risks
-
those on longer treatment care less about having an appointment and have a stronger preference for seeing someone they know (and speaking to someone they know, although this is not significant). Summing up who_self_half and long_who_self_half also shows that these respondents have a slight preference for a half-day training programme, but this is again not statistically significant at high levels.
Turning to the random heterogeneity parameters (i.e. allowing for differences in preferences across participants who cannot be linked to sociodemographics), the estimates for hospital_sig and nurse_sig show a significant level of random variation across respondents in their preferences for different treatment modalities. This heterogeneity is stronger for hospital treatment (i.e. setting it apart from the two at home treatments).
We next look at the impact of the latent variables on the attitudinal questions. We see that:
-
the signs of zeta_lv_1_I_1, zeta_lv_1_I_2, zeta_lv_1_I_3, zeta_lv_1_I_6 and zeta_lv_1_I_10 mean that the first latent variable is a pro-hospital attitude, as people with a more positive latent variable disagree more with statements such as ‘People get better more quickly treated at home’
-
these respondents are less likely to be female (female_lv_1) or non-white (nonwhite_lv_1) and more likely to live at home (alone_lv_1), although this last effect is not significant at high levels
-
respondents with a more positive pro-hospital attitude (i.e. more positive latent variable) have a stronger preference for in-hospital treatment in the choice model (tau_lv1_hospital is positive), while the baseline preference for nurse treatment at home is reduced (tau_lv1_nurse is negative)
-
the signs of zeta_lv_2_I_4, zeta_lv_2_I_5, zeta_lv_2_I_7 and zeta_lv_2_I_8 mean that the second latent variable relates to people seeing health care as a doctor’s responsibility, with those with a more positive latent variable agreeing, for example, with ‘Giving own i.v. would worry me’. The sociodemographic influences suggest that people > 65 years see health care more as a doctor’s responsibility, as do non-white respondents, although this is reduced (although not statistically significant) for people with a university degree
-
respondents who are more of the view that health care is a doctor’s responsibility have a stronger preference for in-hospital treatment in the choice model (tau_lv2_hospital is positive) but the preference for nurse treatment at home is increased even further (tau_lv2_nurse is positive).
The remaining set of 36 threshold parameters simply capture the distribution of answers to attitudinal questions in the data.
| Parameter | Estimation | rob t-rat | Meaning of parameter |
|---|---|---|---|
| ASC_A | 0.2129 | 0.88 | Constant for left alternative (base = right) |
| ASC_B | –0.4938 | –1.47 | Constant for middle alternative (base = right) |
| hospital | –0.1508 | –0.23 | Main effect for hospital treatment (base = self) |
| nurse_home | 1.4585 | 1.57 | Main effect for nurse at home treatment (base = self) |
| treatm_one | 0.7074 | 5.14 | Main effect for one treatment (base = continuous) |
| treatm_two | 0.1819 | 0.70 | Main effect for two treatments (base = continuous) |
| treatm_three | –0.2956 | –1.04 | Main effect for three treatments (base = continuous) |
| appt_time_no | –0.5688 | –3.51 | Main effect for no appointment (base = appointment) |
| who_special | 0.6091 | 1.91 | Main effect for SN in hospital (base = doctor) |
| who_general | 0.2237 | 0.59 | Main effect for GN in hospital (base = doctor) |
| who_self_half | –0.3578 | –0.72 | Main effect for SA after half day training (base = full day) |
| comm_see_known | 0.0166 | 0.12 | Main effect for see someone you know (base = unknown) |
| comm_speak_known | 0.8155 | 1.47 | Main effect for speak to someone you know (base = unknown) |
| after_none | –0.1562 | –1.13 | Main effect for no after consultation (base = telephone) |
| after_hosp_nurse | 0.1578 | 1.05 | Main effect for after consultation with hospital nurse (base = telephone) |
| after_GP | 0.0153 | 0.09 | Main effect for after consultation with GP (base = telephone) |
| risk_6_or_10 | –0.9880 | –6.22 | Main effect for 1 in 6 or 1 in 10 relapse (base = 1 in 25) |
| age_under_50_hospital | 1.2314 | 1.72 | Shifts for < 50 years of age (base = 50–65) |
| age_under_50_who_self_half | 1.1833 | 2.47 | |
| age_under_50_comm_see_known | 0.4776 | 2.33 | |
| age_over_65_nurse_home | 1.5504 | 2.48 | Shifts for ≥ 65 years of age (base = 50–65) |
| age_over_65_comm_speak_known | –1.2197 | –3.00 | |
| age_over_65_risk_10 | 0.7145 | 2.85 | |
| alone_who_special | –0.6479 | –1.54 | Shift for living alone (base = not alone) |
| alone_who_general | –0.7355 | –1.53 | |
| alone_who_self_half | –1.0338 | –1.85 | |
| alone_comm_speak_known | –1.0737 | –2.04 | |
| working_risk_10 | 0.3493 | 1.98 | Shifts for working (base = not working) |
| long_appt_time_no | 0.4548 | 2.09 | Shifts for long vs. short (base = short) |
| long_who_self_half | 0.7676 | 1.58 | |
| long_comm_see_known | 0.3991 | 2.34 | |
| long_comm_speak_known | 0.4771 | 1.03 | |
| hospital_sig | 2.2462 | 4.10 | Pure random heterogeneity in modality preferences |
| nurse_sig | –1.1869 | –1.74 | |
| zeta_lv_1_I_1 | 1.2866 | 4.17 | Impact of first latent on ‘People get better more quickly treated at home’ (positive means disagree) |
| zeta_lv_1_I_2 | 0.8945 | 4.28 | Impact of first latent on ‘I do not like hospitals’ (positive means disagree) |
| zeta_lv_1_I_3 | 0.8606 | 3.21 | Impact of first latent on ‘Hospitals increased risk contracting new infection’ (positive means disagree) |
| zeta_lv_1_I_6 | 1.8487 | 3.89 | Impact of first latent on ‘Would have i.v. at home even if waiting hours for nurse’ (positive means disagree) |
| zeta_lv_1_I_10 | 1.1457 | 4.19 | Impact of first latent on ‘Being in hospital would make things difficult for family’ (positive means disagree) |
| female_lv_1 | –0.4954 | –2.31 | Sociodemographic impacts on first latent variable |
| nonwhite_lv_1 | –0.3323 | –1.42 | |
| alone_lv_1 | 0.1887 | 0.90 | |
| tau_lv1_hospital | 1.1159 | 3.16 | Impact of first latent attitude on hospital |
| tau_lv1_nurse | –0.8918 | –1.87 | Impact of first latent attitude on nurse at home |
| zeta_lv_2_I_4 | –2.2614 | –4.91 | Impact of second latent on ‘Giving own i.v. would worry me’ (positive means disagree) |
| zeta_lv_2_I_5 | –0.4332 | –2.43 | Impact of second latent on ‘Doctors not patients best to decide where patients cared for’ (positive means disagree) |
| zeta_lv_2_I_7 | –0.5798 | –2.82 | Impact of second latent on ‘Would prefer monitoring by doctor than by nurse’ (positive means disagree) |
| zeta_lv_2_I_8 | 0.1699 | 0.99 | Impact of second latent on ‘I want to be responsible for own treatment’ (positive means disagree) |
| age_over_65_lv_2 | 0.3700 | 1.44 | Sociodemographic impacts on second latent variable |
| nonwhite_lv_2 | 0.8505 | 4.51 | |
| university_lv_2 | –0.0933 | –0.70 | |
| tau_lv2_hospital | 3.4171 | 5.12 | Impact of second latent attitude on hospital |
| tau_lv2_nurse | 4.0320 | 9.25 | Impact of second latent attitude on nurse at home |
| t1_I1 | –1.1770 | –3.77 | Threshold parameters for ‘People get better more quickly treated at home’ |
| t2_I1 | 0.3912 | 1.67 | |
| t3_I1 | 2.0141 | 5.55 | |
| t4_I1 | 4.1935 | 6.69 | |
| t1_I2 | –1.6936 | –7.92 | Threshold parameters for ‘I do not like hospitals’ |
| t2_I2 | –0.2661 | –1.44 | |
| t3_I2 | 0.6429 | 3.17 | |
| t4_I2 | 2.7464 | 8.35 | |
| t1_I3 | –0.8715 | –4.25 | Threshold parameters for ‘Hospitals increased risk contracting new infection’ |
| t2_I3 | 0.9017 | 4.19 | |
| t3_I3 | 1.6472 | 6.91 | |
| t4_I3 | 3.4597 | 8.22 | |
| t1_I4 | –1.6471 | –0.75 | Threshold parameters for ‘Giving own i.v. would worry me’ |
| t2_I4 | –0.3395 | –0.17 | |
| t3_I4 | 0.0544 | 0.03 | |
| t4_I4 | 1.9610 | 1.18 | |
| t1_I5 | –1.8055 | –4.31 | Threshold parameters for ‘Doctors not patients best to decide where patients cared for’ |
| t2_I5 | –0.1547 | –0.41 | |
| t3_I5 | 0.7880 | 2.04 | |
| t4_I5 | 2.2158 | 4.99 | |
| t1_I6 | –2.0871 | –3.64 | Threshold parameters for ‘Would have i.v. at home even if waiting hours for nurse’ |
| t2_I6 | 0.6702 | 2.32 | |
| t3_I6 | 1.0196 | 3.42 | |
| t4_I6 | 3.2735 | 5.58 | |
| t1_I7 | –2.6319 | –4.81 | Threshold parameters for ‘Would prefer monitoring by doctor than by nurse’ |
| t2_I7 | –1.4832 | –2.84 | |
| t3_I7 | 0.4947 | 0.97 | |
| t4_I7 | 2.3679 | 4.59 | |
| t1_I8 | –0.9482 | –4.34 | Threshold parameters for ‘I want to be responsible for own treatment’ |
| t2_I8 | 0.4399 | 1.97 | |
| t3_I8 | 1.1794 | 4.90 | |
| t4_I8 | 2.7860 | 7.80 | |
| t1_I10 | –0.6502 | –2.64 | Threshold parameters for ‘Being in hospital would make things difficult for family’ |
| t2_I10 | 0.6962 | 2.88 | |
| t3_I10 | 1.5152 | 4.58 | |
| t4_I10 | 4.3082 | 5.80 |
Discrete choice (stated choice) scenarios
Table 42 contains a list of the 24 choice scenarios used in the survey. The actual ordering of the three treatment types was randomised across respondents, but kept constant across the eight choices for a given respondent. In addition, the order of the eight choices within each block was randomised across respondents.
| Nurse gives IVA in your home | You have your IVA in hospital | You give IVA to yourself at home | |
|---|---|---|---|
| Block 1, choice task 1 | |||
| Number of treatments each day | One | Pump provides continuous treatment | Three |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | Doctor | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Appointment at hospital with nurse | Telephone appointment with nurse | None |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 25 chance | 1 in 10 chance |
| Block 1, choice task 2 | |||
| Number of treatments each day | Two | Two | Pump provides continuous treatment |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | GN | GN | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | None | Appointment at hospital with nurse | Telephone appointment with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 25 chance | 1 in 10 chance |
| Block 1, choice task 3 | |||
| Number of treatments each day | Two | Two | Pump provides continuous treatment |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | GN | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Telephone appointment with nurse | Appointment with your GP | None |
| Risk of a problem such as another infection or having to go into hospital | 1 in 10 chance | 1 in 6 chance | 1 in 25 chance |
| Block 1, choice task 4 | |||
| Number of treatments each day | Two | Two | Two |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | GN | GN | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | Telephone appointment with nurse | Appointment with your GP | None |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 25 chance | 1 in 10 chance |
| Block 1, choice task 5 | |||
| Number of treatments each day | One | Pump provides continuous treatment | One |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | Doctor | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | Telephone appointment with nurse | None | Appointment with your GP |
| Risk of a problem such as another infection or having to go into hospital | 1 in 25 chance | 1 in 6 chance | 1 in 10 chance |
| Block 1, choice task 6 | |||
| Number of treatments each day | One | Pump provides continuous treatment | Two |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | GN | Specialist i.v. antibiotic nurse | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | None | Telephone appointment with nurse | Appointment with your GP |
| Risk of a problem such as another infection or having to go into hospital | 1 in 25 chance | 1 in 6 chance | 1 in 10 chance |
| Block 1, choice task 7 | |||
| Number of treatments each day | Pump provides continuous treatment | One | One |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | GN | Specialist i.v. antibiotic nurse | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Telephone appointment with nurse | Appointment at hospital with nurse | Appointment at hospital with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 10 chance | 1 in 25 chance |
| Block 1, choice task 8 | |||
| Number of treatments each day | Pump provides continuous treatment | One | Pump provides continuous treatment |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | Doctor | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | Appointment with your GP | None | Telephone appointment with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 25 chance | 1 in 10 chance | 1 in 6 chance |
| Block 2, choice task 1 | |||
| Number of treatments each day | One | Pump provides continuous treatment | Three |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | GN | Specialist i.v. antibiotic nurse | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Appointment with your GP | None | Appointment with your GP |
| Risk of a problem such as another infection or having to go into hospital | 1 in 10 chance | 1 in 25 chance | 1 in 6 chance |
| Block 2, choice task 2 | |||
| Number of treatments each day | Pump provides continuous treatment | One | Three |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | GN | Specialist i.v. antibiotic nurse | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Telephone appointment with nurse | None | Appointment at hospital with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 25 chance | 1 in 6 chance | 1 in 6 chance |
| Block 2, choice task 3 | |||
| Number of treatments each day | Pump provides continuous treatment | One | One |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | GN | GN | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | None | Appointment at hospital with nurse | Telephone appointment with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 10 chance | 1 in 25 chance | 1 in 6 chance |
| Block 2, choice task 4 | |||
| Number of treatments each day | One | Pump provides continuous treatment | Pump provides continuous treatment |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | GN | Specialist i.v. antibiotic nurse | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | Appointment at hospital with nurse | Telephone appointment with nurse | None |
| Risk of a problem such as another infection or having to go into hospital | 1 in 25 chance | 1 in 10 chance | 1 in 6 chance |
| Block 2, choice task 5 | |||
| Number of treatments each day | Pump provides continuous treatment | One | One |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | GN | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | Appointment at hospital with nurse | Telephone appointment with nurse | Appointment at hospital with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 25 chance | 1 in 10 chance | 1 in 6 chance |
| Block 2, choice task 6 | |||
| Number of treatments each day | One | Pump provides continuous treatment | Two |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | Doctor | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | None | Appointment with your GP | Appointment with your GP |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 10 chance | 1 in 25 chance |
| Block 2, choice task 7 | |||
| Number of treatments each day | Pump provides continuous treatment | One | Two |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | GN | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | None | Telephone appointment with nurse | Appointment at hospital with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 10 chance | 1 in 25 chance | 1 in 6 chance |
| Block 2, choice task 8 | |||
| Number of treatments each day | Two | Two | Two |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | GN | GN | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Appointment at hospital with nurse | None | Telephone appointment with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 10 chance | 1 in 6 chance | 1 in 25 chance |
| Block 3, choice task 1 | |||
| Number of treatments each day | Two | Two | Three |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | Doctor | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Appointment with your GP | Appointment with your GP | None |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 10 chance | 1 in 25 chance |
| Block 3, choice task 2 | |||
| Number of treatments each day | Two | Two | One |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | GN | Specialist i.v. antibiotic nurse | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Appointment at hospital with nurse | Appointment at hospital with nurse | Telephone appointment with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 25 chance | 1 in 10 chance |
| Block 3, choice task 3 | |||
| Number of treatments each day | Pump provides continuous treatment | One | Pump provides continuous treatment |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | GN | Specialist i.v. antibiotic nurse | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | None | Appointment with your GP | Telephone appointment with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 10 chance | 1 in 6 chance | 1 in 25 chance |
| Block 3, choice task 4 | |||
| Number of treatments each day | Pump provides continuous treatment | One | One |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | Doctor | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | Appointment with your GP | Telephone appointment with nurse | None |
| Risk of a problem such as another infection or having to go into hospital | 1 in 6 chance | 1 in 10 chance | 1 in 25 chance |
| Block 3, choice task 5 | |||
| Number of treatments each day | One | Pump provides continuous treatment | Pump provides continuous treatment |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | GN | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | Appointment at hospital with nurse | Appointment with your GP | Appointment at hospital with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 25 chance | 1 in 6 chance | 1 in 25 chance |
| Block 3, choice task 6 | |||
| Number of treatments each day | Two | Two | Three |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | Doctor | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Appointment with your GP | Appointment at hospital with nurse | Appointment with your GP |
| Risk of a problem such as another infection or having to go into hospital | 1 in 25 chance | 1 in 6 chance | 1 in 10 chance |
| Block 3, choice task 7 | |||
| Number of treatments each day | One | Pump provides continuous treatment | Three |
| Appointment times given | Daily appointment time given | Daily appointment time not given | No appointment needed |
| Who gives the IVA? | GN | Specialist i.v. antibiotic nurse | You give the IVA yourself after 1 day of training |
| Communication between you and HCPs | See a HCP who does not know you | See a HCP who knows you | Speak on the telephone with a HCP who knows you |
| Aftercare from HCPs after the end of treatment | Telephone appointment with nurse | None | Appointment at hospital with nurse |
| Risk of a problem such as another infection or having to go into hospital | 1 in 10 chance | 1 in 10 chance | 1 in 10 chance |
| Block 3, choice task 8 | |||
| Number of treatments each day | Two | Two | Two |
| Appointment times given | Daily appointment time not given | Daily appointment time given | No appointment needed |
| Who gives the IVA? | Specialist i.v. antibiotic nurse | Doctor | You give the IVA yourself after half a day of training |
| Communication between you and HCPs | See a HCP who knows you | See a HCP who does not know you | Speak on the telephone with a HCP who does not know you |
| Aftercare from HCPs after the end of treatment | Appointment with your GP | Appointment at hospital with nurse | Appointment with your GP |
| Risk of a problem such as another infection or having to go into hospital | 1 in 10 chance | 1 in 25 chance | 1 in 6 chance |
Appendix 4 Economic modelling
| Outcomes | Service | Comparison | ICER (£) | INMB (£) | Interpretation | ||
|---|---|---|---|---|---|---|---|
| HO | GN | SN | |||||
| Effectiveness and risk | |||||||
| Assume same heal time (average 6.14 days) | |||||||
| Costs (£) | 1215 | 765 | 765 | HO vs. GN | 2,208,170 | –445 | GN cost-effective |
| QALYs | 0.172 | 0.172 | 0.172 | HO vs. SN | 2,208,176 | –445 | SN cost-effective |
| GN vs. SN | N/A | 0 | Equivalent | ||||
| Double the CDI risk for all services | |||||||
| Costs (£) | 1000 | 852 | 779 | HO vs. GN | 32,343 | –57 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 78,570 | –165 | SN cost-effective |
| GN vs. SN | SN dominates | –109 | SN cost-effective | ||||
| Double line infection risk for all services | |||||||
| Costs (£) | 1006 | 863 | 789 | HO vs. GN | 20,007 | 0 | GN cost-effective |
| QALYs | 0.169 | 0.162 | 0.165 | HO vs. SN | 49,539 | –129 | SN cost-effective |
| GN vs. SN | SN dominates | –129 | SN cost-effective | ||||
| Half time to resolve CDI and line infection | |||||||
| Costs (£) | 993 | 846 | 773 | HO vs. GN | 42,052 | –77 | GN cost-effective |
| QALYs | 0.177 | 0.174 | 0.175 | HO vs. SN | 100,083 | –176 | SN cost-effective |
| GN vs. SN | SN dominates | –99 | SN cost-effective | ||||
| Assume HO i.v. treatment three times per week | |||||||
| Costs (£) | 605 | 852 | 779 | HO vs. GN | HO dominates | 339 | HO cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | HO dominates | 231 | HO cost-effective |
| GN vs. SN | SN dominates | –108 | SN cost-effective | ||||
| Assume same CDI risk for all (HO risk) | |||||||
| Costs (£) | 998 | 855 | 781 | HO vs. GN | 30,493 | –49 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 73,904 | –158 | SN cost-effective |
| GN vs. SN | SN dominates | –109 | SN cost-effective | ||||
| Costs | |||||||
| Initial consultant-led review in all services | |||||||
| Costs (£) | 998 | 1089 | 1016 | HO vs. GN | HO dominates | 184 | HO cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | HO dominates | 75 | HO cost-effective |
| GN vs. SN | SN dominates | –108 | SN cost-effective | ||||
| +20% to costs of hospital visit | |||||||
| Costs (£) | 1153 | 899 | 826 | HO vs. GN | 54,679 | –161 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 113,786 | –269 | SN cost-effective |
| GN vs. SN | SN dominates | –108 | SN cost-effective | ||||
| +20% to costs of nurse visit | |||||||
| Costs (£) | 998 | 900 | 821 | HO vs. GN | 21,213 | –6 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 61,641 | –120 | SN cost-effective |
| GN vs. SN | SN dominates | –114 | SN cost-effective | ||||
| Half cost of treating C. difficile | |||||||
| Costs (£) | 997 | 852 | 778 | HO vs. GN | 31,386 | –53 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 76,155 | –161 | SN cost-effective |
| GN vs. SN | SN dominates | –108 | SN cost-effective | ||||
| Band 6 nurse for SN visits | |||||||
| Costs (£) | 998 | 1035 | 941 | HO vs. GN | HO dominates | 129 | HO cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 20,042 | 0 | SN cost-effective |
| GN vs. SN | SN dominates | –129 | SN cost-effective | ||||
| Wider cost perspective (including patient travel and parking costs and expenses) | |||||||
| Costs (£) | 1037 | 860 | 787 | HO vs. GN | 38,131 | –84 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 87,056 | –193 | SN cost-effective |
| GN vs. SN | SN dominates | –108 | SN cost-effective | ||||
| Utility | |||||||
| EQ-5D values from discrete choice survey (no infection = 0.683; infection = 0.532) | |||||||
| Costs (£) | 998 | 852 | 779 | HO vs. GN | 39,056 | –72 | GN cost-effective |
| QALYs | 0.162 | 0.159 | 0.160 | HO vs. SN | 94,019 | –173 | SN cost-effective |
| GN vs. SN | SN dominates | –101 | SN cost-effective | ||||
| +50% to utility decrements for all AEs for all services | |||||||
| Costs (£) | 998 | 852 | 779 | HO vs. GN | 31,571 | –54 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 76,450 | –162 | SN cost-effective |
| GN vs. SN | SN dominates | –109 | SN cost-effective | ||||
| Adding a quality-of-life decrement (0.2) for mild AEs | |||||||
| Costs (£) | 998 | 852 | 779 | HO vs. GN | 31,727 | –54 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 77,176 | –163 | SN cost-effective |
| GN vs. SN | SN dominates | –109 | SN cost-effective | ||||
| No QALY loss for death | |||||||
| Costs (£) | 998 | 852 | 779 | HO vs. GN | 81,792 | –111 | GN cost-effective |
| QALYs | 0.180 | 0.179 | 0.179 | HO vs. SN | 202,991 | –198 | SN cost-effective |
| GN vs. SN | SN dominates | –87 | SN cost-effective | ||||
| Scenarios | |||||||
| Assume 1 day fewer heal time for HO and 5% relapse rates for nurse services | |||||||
| Costs (£) | 847 | 860 | 787 | HO vs. GN | HO dominates | 143 | HO cost-effective |
| QALYs | 0.176 | 0.170 | 0.171 | HO vs. SN | 12,727 | 34 | HO cost-effective |
| GN vs. SN | SN dominates | –109 | SN cost-effective | ||||
| Double risks and mortality rates for all AEs for all services | |||||||
| Costs (£) | 1006 | 860 | 786 | HO vs. GN | 12,207 | 93 | HO cost-effective |
| QALYs | 0.160 | 0.148 | 0.152 | HO vs. SN | 29,535 | –71 | SN cost-effective |
| GN vs. SN | SN dominates | –164 | SN cost-effective | ||||
| Half C. difficile rate for HO; 5% relapse rate and 20% increase in nurse visit costs GN and SN | |||||||
| Costs (£) | 997 | 909 | 830 | HO vs. GN | 18,117 | 9 | HO cost-effective |
| QALYs | 0.175 | 0.170 | 0.171 | HO vs. SN | 54,034 | –105 | SN cost-effective |
| GN vs. SN | SN dominates | –115 | SN cost-effective | ||||
| Utility decrement for mild adverse event (0.2) and cost (daily resource use cost) | |||||||
| Costs (£) | 1000 | 858 | 785 | HO vs. GN | 30,520 | –49 | GN cost-effective |
| QALYs | 0.175 | 0.170 | 0.172 | HO vs. SN | 74,177 | –157 | SN cost-effective |
| GN vs. SN | SN dominates | –108 | SN cost-effective | ||||
| Service | Comparison | ICER (£) | INMB (£) | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| HO | GN | SN | SA | |||||
| Effectiveness and risk | ||||||||
| Assume same heal time (average 28.01 days) | ||||||||
| Costs (£) | 4763 | 2793 | 2793 | 2102 | HO vs. GN | 2,171,523 | –1952 | GN cost-effective |
| QALYs | 0.641 | 0.640 | 0.640 | 0.638 | HO vs. SN | 2,171,523 | –1952 | SN cost-effective |
| HO vs. SA | 946,897 | –2605 | SA cost-effective | |||||
| GN vs. SN | N/A | 0 | Equivalent | |||||
| GN vs. SA | SA dominates | –653 | SA cost-effective | |||||
| SN vs. SA | 363,117 | –653 | SA cost-effective | |||||
| Double the CDI risk for all services | ||||||||
| Costs (£) | 4636 | 3096 | 2578 | 2133 | HO vs. GN | 124,483 | –1293 | GN cost-effective |
| QALYs | 0.643 | 0.631 | 0.647 | 0.637 | HO vs. SN | SN dominates | –2135 | SN cost-effective |
| HO vs. SA | 425,431 | –2386 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –842 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1093 | SA cost-effective | |||||
| SN vs. SA | 45,769 | –251 | SA cost-effective | |||||
| Double line infection risk for SA | ||||||||
| Costs (£) | 4631 | 3078 | 2563 | 2147 | HO vs. GN | 128,354 | –1310 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.608 | HO vs. SN | SN dominates | –2149 | SN cost-effective |
| HO vs. SA | 69,942 | –1773 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –839 | SN cost-effective | |||||
| GN vs. SA | 39,778 | –463 | SA cost-effective | |||||
| SN vs. SA | 10,514 | 375 | SN cost-effective | |||||
| Triple risk of anaphylactic shock | ||||||||
| Costs (£) | 4632 | 3080 | 2564 | 2115 | HO vs. GN | 103,227 | –1251 | GN cost-effective |
| QALYs | 0.641 | 0.626 | 0.643 | 0.629 | HO vs. SN | SN dominates | –2110 | SN cost-effective |
| HO vs. SA | £211,256 | –2278 | SA cost-effective | |||||
| GN vs. SN | SN dominates | -£859 | SN cost-effective | |||||
| GN vs. SA | SA dominates | -£1027 | SA cost-effective | |||||
| SN vs. SA | £31,998 | -£168 | SA cost-effective | |||||
| Assume HO i.v. treatment three times per week | ||||||||
| Costs (£) | 2770 | 3078 | 2563 | 2114 | HO vs. GN | HO dominates | 550 | HO cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.638 | HO vs. SN | SN dominates dominates | –288 | SN cost-effective |
| HO vs. SA | 116,845 | –544 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –839 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1094 | SA cost-effective | |||||
| SN vs. SA | 46,393 | –255 | SA cost-effective | |||||
| Assume same CDI risk for all (HO risk) | ||||||||
| Costs (£) | 4631 | 3087 | 2570 | 2124 | HO vs. GN | 124,952 | –1297 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.647 | 0.638 | HO vs. SN | SN dominates | –2137 | SN cost-effective |
| HO vs. SA | SA dominates | –2389 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –840 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1093 | SA cost-effective | |||||
| SN vs. SA | 45,995 | –253 | SA cost-effective | |||||
| Double anaphylaxis mortality risk for none HO | ||||||||
| Costs (£) | 4631 | 3078 | 2563 | 2114 | HO vs. GN | 109,062 | –1268 | GN cost-effective |
| QALYs | 0.644 | 0.629 | 0.646 | 0.635 | HO vs. SN | SN dominates | –2114 | SN cost-effective |
| HO vs. SA | 298,396 | –2348 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –846 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1080 | SA cost-effective | |||||
| SN vs. SA | 41,788 | –234 | SA cost-effective | |||||
| Costs | ||||||||
| Initial consultant-led review all services | ||||||||
| Costs (£) | 4631 | 3316 | 2801 | 2352 | HO vs. GN | 108,699 | –1073 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.638 | HO vs. SN | SN dominates | –1911 | SN cost-effective |
| HO vs. SA | 405,918 | –2167 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –839 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1094 | SA cost-effective | |||||
| SN vs. SA | 46,393 | –255 | SA cost-effective | |||||
| –20% nurse costs | ||||||||
| Costs (£) | 4631 | 2897 | 2414 | 2068 | HO vs. GN | 143,332 | –1491 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.638 | HO vs. SN | SN dominates | –2297 | SN cost-effective |
| HO vs. SA | 456,500 | –2451 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –806 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –959 | SA cost-effective | |||||
| SN vs. SA | 35,815 | –153 | SA cost-effective | |||||
| Triple SA equipment costs | ||||||||
| Costs (£) | 4631 | 3078 | 2563 | 2214 | HO vs. GN | 128,354 | –1310 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.638 | HO vs. SN | SN dominates | –2149 | SN cost-effective |
| HO vs. SA | 430,442 | –2304 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –839 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –994 | SA cost-effective | |||||
| SN vs. SA | 36,061 | –155 | SA cost-effective | |||||
| Half outpatient consultant visit cost | ||||||||
| Costs (£) | 4512 | 2731 | 2258 | 1788 | HO vs. GN | 147,244 | –1539 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.638 | HO vs. SN | SN dominates | –2336 | SN cost-effective |
| HO vs. SA | 485,086 | –2611 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –797 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1072 | SA cost-effective | |||||
| SN vs. SA | 48,463 | –275 | SA cost-effective | |||||
| Utility | ||||||||
| EQ-5D values from discrete choice survey (no infection = 0.683; infection = 0.532) | ||||||||
| Costs (£) | 4631 | 3078 | 2563 | 2114 | HO vs. GN | 227,021 | –1415 | GN cost-effective |
| QALYs | 0.643 | 0.636 | 0.645 | 0.639 | HO vs. SN | SN dominates | –2102 | SN cost-effective |
| HO vs. SA | 584,864 | –2430 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –687 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1015 | SA cost-effective | |||||
| SN vs. SA | 74,454 | –328 | SA cost-effective | |||||
| +50% to utility decrements for all AEs for all services | ||||||||
| Costs (£) | 4631 | 3078 | 2563 | 2114 | HO vs. GN | 128,321 | –1310 | GN cost-effective |
| QALYs | 0.6434 | 0.6313 | 0.6475 | 0.6378 | HO vs. SN | SN dominates | –2149 | SN cost-effective |
| HO vs. SA | 448,574 | –2404 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –839 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1094 | SA cost-effective | |||||
| SN vs. SA | 46,367 | –255 | SA cost-effective | |||||
| No QALY loss for death | ||||||||
| Costs (£) | 4631 | 3078 | 2563 | 2114 | HO vs. GN | 225,852 | –1415 | GN cost-effective |
| QALYs | 0.673 | 0.666 | 0.676 | 0.671 | HO vs. SN | SN dominates | –2128 | SN cost-effective |
| HO vs. SA | 1,395,644 | –2480 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –713 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –1066 | SA cost-effective | |||||
| SN vs. SA | 93,062 | –353 | SA cost-effective | |||||
| Scenarios | ||||||||
| Assume utility decrement for mild AEs (–0.01), double SA rate of line infection and SA equipment cost | ||||||||
| Costs (£) | 4631 | 3078 | 2563 | 2197 | HO vs. GN | 128,354 | –1310 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.608 | HO vs. SN | SN dominates | –2149 | SN cost-effective |
| HO vs. SA | 69,165 | –1730 | SA cost-effective | |||||
| GN vs. SN | –31,899 | –839 | SN cost-effective | |||||
| GN vs. SA | 38,172 | –420 | SA cost-effective | |||||
| SN vs. SA | 9329 | 419 | SN cost-effective | |||||
| Assume 30 days to heal in SA and double SA training costs | ||||||||
| Costs (£) | 4631 | 3078 | 2563 | 2293 | HO vs. GN | 128,354 | –1310 | GN cost-effective |
| QALYs | 0.644 | 0.631 | 0.648 | 0.633 | HO vs. SN | –508,664 | –2149 | SN cost-effective |
| HO vs. SA | 215,743 | –2121 | SA cost-effective | |||||
| GN vs. SN | SN dominates | –839 | SN cost-effective | |||||
| GN vs. SA | SA dominates | –811 | SA cost-effective | |||||
| SN vs. SA | 18,146 | 28 | SN cost-effective | |||||
Appendix 5 Expert panel summaries
Summary of the systematic review
Aim
This review was undertaken in 2013/14 with an update in May 2015 specifically to identify and evaluate the existing evidence in the published literature.
We were specifically looking for evidence of:
-
efficacy of treatment within the four potential service models
-
patient safety issues
-
effectiveness of care in the service models
-
cost-effectiveness.
Methods
Databases searched included MEDLINE, EMBASE and Cochrane from 1993 to April 2013 (now May 2015). Our protocol included all i.v. antibiotic types and method of administration. We excluded children and non-i.v. antibiotics, for example, oral administration antibiotics. Titles and abstracts were screened and checked for quality by at least two reviewers. Potential papers were obtained in full text for review.
Results
The number of papers that matched our inclusion and exclusion criteria was 110. The majority were from the UK, with the remainder from Europe and North America and Australia. There were 12 RCTs. Synthesised data showed mixed results for cure or improvement in the patient. When OPAT models were considered individually, HO attendance appears least effective and GN appears most effective. However, these results need to be considered in relation to the quality of the evidence. Twelve RCTs met our selection criteria; however, none of these provided a robust description of their methods.
The results from a large number of studies were aggregated, which made it difficult to unpick who got what and when. Only 19 studies included a usual care comparator (hospital inpatient). Most importantly, there was often no definition of the kind of OPAT service being reported. For example, the paper reported that the patients received care from an ‘OPAT’ service with no further explanation.
This systematic review demonstrates that the existing evidence base has been quite poor.
We have been able to describe the literature and present our results, but they could not be used on their own to inform patients, clinicians and commissioners about which model or models of OPAT are most effective.
Summary of health professional work package
Aim
To explore the types of OPAT services that have been commissioned and provided in England and to identify any perceived barriers to implementing this type of service and to establish reasons for current service configuration.
Method
We carried out a brief electronic survey and contacted 120 individuals/organisations. We had 35 responses, which give a snapshot of some of the services offered.
The majority of respondents reported that they offered more than one model of care (i.e. daily hospital visits or district nurses at home). The survey was also used to inform the selection of professionals who had agreed to a follow-up telephone interview. We selected those for interview who represented a range of services provided, geographical location and diversity of populations served.
We completed 20 interviews with a range of relevant staff.
Results
Most services included some hospital provision with community options supplied by GNs/SNs or private health-care providers. There were differences in the prominence given to the service within organisations. Some described well-developed administrative systems with designated staff, whereas others described their service as ad hoc and seemingly reliant on good working relationships with colleagues who could help when needed. The list of types of infections treated was fairly extensive and included cellulitis, bone and joint infections, diabetic foot infections, renal infections and respiratory infections.
Patient SA was the norm in one service (with back up if the patient was not able to do this), but other interviewees were less enthusiastic about this option. Geographical location presented a problem for some organisations if the patients were some distance from the hospital base. This was particularly problematic if the patient needed more than two dose administrations per day. This resulted in restricted services for patients.
Leadership and working relationships
There were notable differences in how this was described by interviewees. Those who had come to the role as champions of OPAT were perhaps more able to influence development of the service. Others had been given the role with limited opportunity to take ownership of the service and develop it. Some organisations had little clinical leadership provision and others had stepped in to take the role to ensure that the service continued. In the well-developed service model, teams have core medical staff with expertise in infectious diseases, microbiology, pharmacy and nursing, with input from a wide range of other relevant professionals. These services had well defined communication and input from the i.v. access teams and community nurses in primary care.
Finances and resources
Business cases were a feature of most of those with responsibility for OPAT services. Some reported that their resources were precarious and were dependent on the number of referrals into the service annually. Financial instability is a common feature and this means in reality that, although services may be delivered there is limited scope for development and expansion.
One interviewee reported that they had their business case turned down because there was insufficient reduction in bed occupancy to justify the service. This demonstrated a lack of perception that patient preference could be a motivator for the development of services, although some reported using patient satisfaction surveys to support the continuation of the service.
Governance and patient safety issues
All of the interviewees who had input into OPAT services reported some form of monitoring. How this worked depended on how the service was configured. Those who described their service as using BSAC surveillance methods tended to have well-defined structures in place to follow up patients and audit their outcomes.
However, several interviewees reported problems in the past, whereby patients had not received optimal care, for example, patients had been on i.v. antimicrobials for unjustified lengths of time, patients had to be retreated and one patient had a severe reaction to the agent, which may have been avoided.
Less well-developed and resourced services might be perceived as a potential risk to patients.
Cost-effectiveness summary
Aim
To estimate costs and benefits of four OPAT services for two types of patient (short-term and longer-term infections) using two modelling methods: a Markov model and a Simulation model.
Markov model results
-
Short-term infections
-
HO is the most expensive but most effective service
-
SN is cheapest and more effective than GN, and the costs of GN and SN were very similar
-
these results were robust to changes in risks, costs and quality of life values
-
the results only changed in favour of HO when it was assumed that HO required fewer treatment days than the alternatives and had lower risks.
-
-
Long-term infections
-
HO is the most expensive but least effective alternative
-
the costs and benefits of GN and SN are similar, although the latter is more often cheaper and more effective than the former
-
SA is cheaper and more effective than SN making it the preferred service
-
these results are robust to a number of sensitivity analyses.
-
Simulation model results
These confirm the results from the Markov model for both short- and long-term infections:
-
Short-term infections
-
SN alone is the most cost-effective strategy for both costing perspectives
-
combining SN with HO (80 : 20% ratio) is not a cost-effective strategy compared with SN alone but better than GN alone service (cost per hour)
-
QALYs affected only when the number of staff is not able to cope with the demand of patients
-
reducing the number of staff reduces service costs (commissioning)
-
A service with two nurses is more cost-effective than one with four nurses when covering 300 patients a year (commissioning).
-
-
Long-term infections
-
SA or SN alone are the most cost-effective strategies for per-hour and commissioning perspectives, respectively
-
combining SN with SA was better (in terms of costs and QALYs) than combining SN with HO (per hour perspective)
-
costs and QALYs only affected when staffing is insufficient
-
a SN service can function with only one nurse
-
the second best alternative is combining SN with SA. However, the higher the proportion of SA, the more costly the strategy
-
QALYs are reduced when the number of staff available is unable to cope with demand making the service more expensive and less effective.
-
Value of information: not very informative owing to lack of decision uncertainty in the models as they are currently formulated.
Executive summary for discrete choice experiment development (qualitative work)
Aims
-
To generate data to ascertain service issues that were important to patients, to inform the development of the DCE.
-
To identify potential attributes and levels, informed by the literature review and qualitative interviews.
-
To pilot the draft DCE.
Method
Semistructured interviews and focus group with n = 32 patients.
Eligibility
Adults aged > 18 years who had an infection in the last 3 years that was treated via OPAT.
Data analysis
Thematic analysis.
Key results
Meeting the needs of patients
-
Patients want to avoid hospital (fear of infection, and the impact on family and work).
-
Past experience, age and health affect views about optimal OPAT model.
Benefits of and barriers to different models of care
-
Older people seem to prefer home care, but this is also likely to be influenced by the severity of infection and/or comorbidities.
-
Older participants do not feel daily attendance at hospital is appropriate for the infirm or very old.
-
Nurse at home model means patients get the undivided attention of a nurse.
-
Attendance at hospital more than once a day was viewed as unacceptable by most people.
-
Self-administration was potentially acceptable, especially by those who had repeated infections.
-
Geography is important – public transport routes and car parking affect acceptability.
Effectiveness of treatment
-
Most patients believe that i.v. antimicrobials are more effective than oral alternatives.
-
Patients are concerned if a switch is made from i.v. to oral antimicrobials without their infection being assessed by a doctor.
-
Short-term i.v. patients were often unsure if their infection had cleared by the time their treatment course had finished.
Communication
-
Most patients want to be followed up at the end of their i.v./oral treatment.
-
Few participants felt that they knew who they should contact for follow-up.
-
Almost all patients received written information about OPAT and/or their infection, but not everyone was given a contact number in case of emergencies.
-
For some patients it was important to have a named person to contact, but others just wanted to know they could contact staff if they needed to.
Review and follow-up
-
Some patients wanted their treatment to be managed (seen by) a doctor. Others were happy to be managed by a nurse, provided competent and qualified to provide i.v. care.
-
Patients want staff to be aware of their infection/treatment plan (continuity of care).
-
Patients expressed frustration if appointment times were missed.
Executive summary of the results of the discrete choice survey and modelling work
Patients recruited for the study took part in a stated choice survey, where they faced eight hypothetical choices between three models of care (HO attendance, nurse at home, SA), with the characteristics of each varying across the eight choice tasks. The three treatments were described in terms of:
-
the type of treatment (hospital, nurse at home, SA)
-
the frequency of treatment (once, twice, three times, continuous)
-
whether or not an appointment time is given (not relevant for SA)
-
who administers the treatment (doctor, SN or GN; not relevant for SA)
-
whether a full or half day of training is given for SA
-
whether or not the patient sees a health-care professional she or he knows (not relevant for SA)
-
whether or not, if self-administering, the patient speaks (on the telephone) to a health-care professional that she or he knows
-
what type of aftercare is given (none, appointment at hospital with nurse, appointment with GP, telephone appointment with nurse)
-
the risk of a problem (1 in 6, 1 in 10 or 1 in 25).
Our analysis made use of an advanced discrete choice model, which is a mathematical structure used to explain the influence of explanatory variables (e.g. type of treatment) on choices. The model is based on the notion of utility maximisation, with respondents choosing the option that gives them the greatest utility/smallest disutility. With the expectation of major differences in preferences across patients, our work incorporates three levels of heterogeneity in preferences across patients, namely:
-
heterogeneity linked to sociodemographics
-
heterogeneity as a function of underlying attitudes (attitude towards hospitals and attitude towards whose responsibility health care is)
-
unexplained ‘random’ differences.
All three are found to have a major role in explaining choices, whereby the level of heterogeneity attributable to underlying attitudes and other unexplained ‘random’ differences outweighs those variations that can be linked to observed characteristics. We find that, overall:
-
There is a strong preference for the nurse at home model, with the lowest ranked treatment being SA. However, there is very extensive variation across respondents in these preferences.
-
The type of treatment is far more important to respondents than the characteristics of that treatment (e.g. in terms of aftercare, where the number of treatments and the level of risk of problems are the next most important factors in explaining choices).
-
There is a preference for a single dose of antimicrobials per day, ahead of two doses, continuous treatment and three doses.
-
Patients prefer to have an appointment time (for hospital or nurse at home treatment); however, the importance of this is much reduced for long-term patients.
-
Those patients not living alone have a preference for being treated by SNs, ahead of GNs and then doctors, whereas those living alone marginally prefer doctors to SNs, far ahead of GNs.
-
Most patients prefer a half-day of training for SA, except those aged < 50 years who live alone and are short-term patients, those aged > 50 years who live alone and are long-term patients and short-term patients aged < 50 years.
-
Patients prefer seeing someone they know, and this preference is stronger for those aged < 50 years; those > 50 years of age on short-term treatment place little importance on this.
-
When communicating over the telephone, younger patients generally prefer speaking to someone they know, with the opposite applying for some older patients.
-
For aftercare, nurse in hospital is preferred to GP aftercare, which is ranked higher than telephone aftercare and then no aftercare.
-
Patients < 65 years of age who are not working do not distinguish between a risk of 1 in 6 and 1 in 10 of problems.
Appendix 6 Patient and public involvement
The NIHR and other major funding bodies actively support public involvement throughout the research cycle, and this has become enshrined as good research practice (Health and Social Care Act,230 INVOLVE231). To support meaningful PPI, bodies such as INVOLVE have devised good practice guidelines and information. There is, however, no one way in which to involve people, and here we discusses our approach to involvement and the strategies we used to try to make this meaningful to both patients and the research team.
Patient and public involvement group
The CIVAS project worked to develop a specially constituted PPI group set up to advise on this project. Unlike many clinical conditions (e.g. diabetes mellitus or heart disease), there was no existing patient group with which we could work. Many patients will receive only one course of i.v. antibiotics in their lives and so the topic is not of great importance to them. Others, such as those with cystic fibrosis and other chronic conditions, will have repeated courses and, thus, the topic is of greater importance, but their experiences and priorities may be quite different from those ‘infrequent recipients’. Our aim was to foster a collaborative approach from the outset and to ensure that the voices of both groups were heard. Therefore, this appendix describes how the group was set up, the challenges of maintaining such as group, how roles were negotiated over time and the input provided by our patient group.
Two researchers (MT and CCM) have a long track record of involving patients and the public in research. In keeping with our previous engagement and involvement activities, a flexible approach was adopted to enable our members to come into and out of the group as their health and work/care commitments required, and to allow people to draw on their existing skills to support the research endeavour.
Early days
It is recognised that meaningful PPI starts at the earliest stages of the research process. 231 Our involvement activities during the design of the study included meeting with individual patients to introduce the project, during which we discussed what taking part in the study would mean for patients (time commitment, sensitivity of topics discussed, timing of interviews) and we asked them how they would like the PPI group to run. The patients we met with were supportive of the aims of the study and did not suggest any changes. One patient (HG) agreed to become co-applicant on the grant.
Before the study started we contacted the patients involved pre award to inform them of the success of the grant application. This was our first challenge. Although patients were supportive of the study, most did not feel that they could continue to be involved. OPAT treatment is for many people a transitory experience and by the time the project was funded two patients no longer felt that the study was of relevance to them, and one felt that they could not be involved owing to their health. Only one patient (HG) was in a position to remain involved in the study. We therefore set about constituting a new PPI group.
Setting up a new patient advisory group
We advertised for new members via the clinicians involved in the study, and through the distribution of an information sheet to patients in OPAT services in Bradford and Leeds. We received five responses to our mailings. A PAG was formed, comprising short- (n = 2) and long-term (n = 3) OPAT patients. However, both of the short-term infection patients dropped out of the group during 2013, as they were unable to get time off work to attend meetings. We therefore continued to recruit patients to the PAG throughout the project, and two new PPI members joined the group in early 2014 (one long-term and one short-term i.v. patient). During 2014 our lay co-applicant’s health deteriorated and she was no longer able to attend meetings in person. She and another chronically ill patient therefore became ‘virtual’ members and contributed as and when they could by e-mail.
Negotiating meeting frequency and types of involvement
The PAG met at key points throughout the project. We wanted members to have as much, or as little, involvement as they felt was appropriate to them. This approach was taken for two reasons. First, we wanted to maintain the involvement by those of working age and the chronically ill and felt that the best way to do this was to allow people to miss meetings without feeling that they were in any way ‘letting the group down’ by not attending. Second, we wanted patients to be involved at a number of key points and this could result in patients feeling overburdened, so by taking a ‘menu’ approach to involvement, we hoped to allow patients to play to their strengths, and not feel that they had to contribute to everything.
During our first meeting we presented an overview of the research project and each of the workstreams and discussed with members what opportunities for involvement there would be during the project (i.e. steering group committee membership, reviewing documentation for readability, reviewing interview findings, contributing to the selection of DCE attributes, commenting on readability of DCE items, testing our DCE, providing a patient perspective on the health economic modelling). PAG members were briefed about forthcoming involvement opportunities and told that they would be invited to participate, if they expressed an interest in being involved.
We also discussed their training needs. We had planned to send PAG members on the Macmillan Involving People course, but none wished to attend and preferred that we brief them at the start of any activity about what the task would entail, so this is what we did. In consultation with our lay members it was agreed that rather than the PAG meet as a standalone group, PAG members would attend relevant research meetings and engage directly with the researchers engaged on that workstream (with MT or CCM facilitating the discussion). Between meetings, updates on the project were provided to the group via e-mail. The lay co-applicant (HG) was invited to quarterly co-applicant meetings and attended steering committee meetings as a member of the research team. All lay members were reimbursed for their time and travel expenses. E-mails were used between meetings to update lay members of progress and to advise them about future meetings.
How the patient advisory group influenced the research
Ethics application and participant materials
Our lay co-applicant had significant input into writing and commenting on the study documentation prior to ethics review. She helped us to rephrase some of the wording on several sections of the information sheets, both to improve readability and acceptability. At this point she was the only PPI member directly involved in the study, so input was sought from an existing PPI generic group within the Leeds NHS Trust, which also provided feedback on our documentation prior to ethics review.
Piloting topic guides
We piloted our patient interview topic guides on three PAG members. This involved lay members participating in an interview and providing informal feedback about the phrasing and order of questions. Feedback from PAG members was that patients would appreciate an opportunity to ‘tell their story’, so the topic guide was adapted to allow for a more narrative approach. These pilot data were not included in the corpus of data collected for the study, as informed consent was not taken, and interviews were not recorded, as we wanted to ensure that there were clear boundaries between PPI and participation in the study.
The patient interviews
Two PAG members met with the research group to discuss the interpretation of the patient interview data. They provided feedback on how well the draft DCE items were drawn from the corpus of interview data and how well they reflected their own experiences as patients. They worked with the research team to redraft wording for both the DCE items and attitudinal questions based on initial drafts constructed by the research team. The research team sought the advice of the PAG on improving recruitment of patients from ethnic minority groups to the interview study. The lay members felt that they were of limited help, as the group did not include any ethnic minority members.
The discrete choice experiment
Prior to formal piloting of the DCE a paper copy was circulated to six of our lay members via e-mail for comments. Changes were made in light of their feedback.
-
PAG members found it confusing to have eight sets of very similar questions presented to them. To address this, clear instructions were incorporated into the DCE, and also at their suggestion an example DCE was presented to participants before the choice options to ensure the participant understood the procedure.
-
Participants also asked for definitions of each service model to be included (e.g. GN vs. SN), and this suggestion was incorporated.
-
Participants noted that there was no option for self-employment, so this was included as an option for ‘employment’.
-
The wording of two attitude questions was revised to improve readability (e.g. ‘administering my own intravenous antibiotics would worry me’ was changed to ‘giving my own i.v. antibiotics would worry me’).
-
The wording of some factors in the DCE caused some confusion. The term ‘aftercare’, by which researchers meant ‘care after the infection episode was complete’, was interpreted as care once the infection was being treated. The wording was clarified in the later version.
-
One PAG member was concerned that some patients may become anxious because their specific circumstances are not covered by the options provided by the questionnaire and so may disengage with the questionnaire. It was agreed to test this in the pilot study (feedback was very positive on the measure and no changes were made).
Health economic modelling
Patient advisory group members were invited, along with clinical experts, to three meetings to discuss the development of the economic model. Two lay members attended the meetings and contributed to a wide-ranging discussion about the use of health economic modelling. Feedback indicated that both members found the session interesting and illuminating. At these meetings the health economists presented the graphics for the preliminary models and provided an oral overview of them. Lay members took the opportunity to gain insight into the way such models are constructed. The research team sought guidance from the patients and clinical experts about whether or not the model as presented fitted with their experience of receiving/giving i.v. antibiotics, and significant health events that may occur. This led to changes to the preliminary models. At the subsequent meetings later versions of the Markov model were presented along with the simulation model. PAG members felt that the data generated would be a useful lever for getting OPAT adopted more widely.
Expert panel
All PAG members were invited to the expert panel which was held in Leeds in September 2015. Three people agreed to attend. Unfortunately, two lay members had to drop out prior to the meeting owing to ill health. A third lay member (CT) attended but became unwell and had to leave so was not able to contribute fully to the discussions. Although we had translated the technical research language into lay terms for this event, we do think that the amount of reading required prior to the event put some PAG members off attending (a 10-page report for each workstream). The PAG members were fully involved in the development and running of the dissemination event which took place in May 2016 and which was open to patients as well as professionals.
Discussion
Studies suggest that patient participation in research can occur at three levels: consultation, collaboration and user involvement. In the development of our project involvement was largely consultative, with some collaboration, However, during the delivery of the CIVAS project involvement has been collaborative, with meetings or virtual interactions in which participants contributed to the project, for example in terms of the development of the DCE.
On reflection, our flexible approach to patient involvement was essential and resulted in support, both from the research team and PAG. Our collaborative approach was acceptable to members of the patient group and informal feedback has been very positive, although formal feedback was not sought. Working with our patient group in this way has facilitated substantive contributions to decision-making at each stage of the project, in particular to ensure that the DCE was acceptable and understandable to patients. Evidence of the impact that our PAG made to this work can be seen in particular from the piloting of the DCE, which patients found to be very easy to use. Both lay members and professionals valued the interaction and exposure to others’ views, as evidenced by our research team actively requesting meetings with the PAG members. Involving our PAG in the way we did made a difference to the quality of the research project.
The project itself has not been without its challenges, and this has meant that there have often been large gaps between meetings, which possibly contributed to the loss of members from the group, although the reasons given related to work or health issues. The turnover of attendees has, however, also resulted in bringing fresh eyes to the project and a more dynamic group. We would have missed out on involving some eloquent and informed patients had our group been more stable, as we may not have sought additional participants.
The difficulties maintaining the patient advisory group
We were careful not to cross the line between our PAG members being our collaborators and our study participants. A strength of the approach we took is that engagement between the lay members was usually with multiple members of the research team. This served to build a good rapport between the research team and the lay members, as some members of the research team were involved in several work strands so got to know the lay members very well and built up trust with the group.
Despite attempts to recruit members from ethnic minorities to the PAG (e.g. direct contact with patients from ethnic minorities at OPAT clinics, asking community groups catering for ethnic minority groups), no one came forward. None of the research group had existing links with ethnic minority groups so we used links via university colleagues to gain access to local community groups but did not identify anyone who had experience of OPAT who could join the group.
Owing to the ill health of our lay co-applicant, she has not been able to be actively involved in the project since 2014. Initially she was able to maintain her involvement via e-mail, commenting on documents and responding to e-mail requests, but her health has meant that this could not be maintained.
Evaluation of involvement
Given the size of the group, and the way in which we involved people (integrating them into our research meetings), we did not undertake any formal evaluation of the effectiveness of our involvement strategies, but instead ensured that at the end of every meeting we had an informal discussion with the lay members at the meeting to find out how they felt it had gone. This approach to involvement does not perhaps lend itself as well to evaluation as formal workshops or meetings, and we did not put in place a formal framework to evaluate its effectiveness, as advocated by Popay’s recent guidance. 232 Had this guidance been in place at the start of our project (early in 2013), we might perhaps have benefited from evaluating our own perceptions of the purpose of involvement and considering the role our biases play in how involvement activities were presented to our lay members. Having used the approach taken in this study in previous research projects, we perhaps presented the opportunities in such a way that members felt this was the ‘right’ approach. However, the passage of time means this cannot be sensibly tested.
What worked well
-
Inviting lay members to research meetings.
-
Using a facilitator in meetings (who, although not independent, was not an expert in the topic).
-
Support from the research team.
-
Agreeing roles at the start of the project.
-
Offering a menu of involvement opportunities.
-
Providing an induction pack.
What we would do differently
-
Advertise more widely.
-
Explore the expectations and needs of PAG members more deeply.
-
Plan evaluation activities into the design.
-
Plan meetings later in the day (evening) to accommodate ‘working’ members, and also be flexible to accommodate shift workers.
Glossary
- Discrete choice experiment
- A survey that presents respondents with multiple hypothetical choice tasks and collects data on their choices. These data are then analysed using choice models.
List of abbreviations
- A&E
- accident and emergency
- BME
- black and minority ethnic
- BOS
- Bristol Online Survey
- BSAC
- British Society for Antimicrobial Chemotherapy
- CCG
- clinical commissioning group
- CDI
- Clostridium difficile infection
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CIVAS
- Community Intravenous Antibiotic Study
- DCE
- discrete choice experiment
- DES
- discrete event simulation
- DGH
- district general hospital
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- EVSI
- expected value of sample information
- GN
- general nurse
- GP
- general practitioner
- HDAS
- Healthcare Database Search
- HHU
- Hospital in the Home Unit
- HO
- hospital outpatient
- HPSS
- Health and Personal Social Services
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- INMB
- incremental net monetary benefit
- i.v.
- intravenous
- IVA
- intravenous antimicrobials
- MDT
- multidisciplinary team
- MRSA
- meticillin-resistant Staphylococcus aureus
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OPAT
- outpatient parenteral antimicrobial therapy
- PAG
- patient advisory group
- PPI
- patient and public involvement
- PSA
- probabalistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SA
- self-administration
- SES
- socioeconomic status
- SN
- specialist nurse
- SPSS
- Statistical Product and Service Solutions
- SSTI
- skin and soft tissue infection
- WTE
- whole-time equivalent