Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/2004/50. The contractual start date was in March 2013. The final report began editorial review in May 2016 and was accepted for publication in December 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Fiona Burns reports personal fees and other from Gilead Sciences Ltd and personal fees from Janssen HIV, outside the submitted work. Caroline Sabin reports personal fees from Gilead Sciences Ltd, ViiV Healthcare, Janssen-Cilag and Bristol-Myers Squibb, outside the submitted work. Steve Morris is a member of the National Institute for Health Research (NIHR) Health Services and Delivery Research Programme Funding Board, which funded this research. He is also a member of the NIHR Public Health Research Funding Board and the NIHR Programme Grants for Applied Research expert subpanel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Howarth et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
The introduction of combination antiretroviral therapy (ART) has led to a dramatic reduction in human immunodeficiency virus (HIV)-associated morbidity and mortality. 1 The life expectancy for successfully treated people living with human immunodeficiency virus (PLWH) in the UK is now similar to that of the general population2 and ART is also recognised as an effective means of reducing HIV transmission. 3 However, the individual and public health benefits of HIV treatment can be achieved only if PLWH are aware that they are HIV positive, have linked into care and have sustained engagement with care thereafter. Although interest in this area has increased over the past decade and retention in HIV care is now a key measure of quality performance for HIV service providers in the UK,4 it remains a major challenge, with little evidence available on how to optimise engagement in care (EIC). 5 The Retention and Engagement Across Care services for HIV positive patients in the UK (REACH) study set out to explore, describe and understand attendance of PLWH at HIV outpatient services in order to support the development of cost-effective interventions to improve EIC.
The Joint United Nations Programme on HIV and AIDS (UNAIDS)6 set a target that, by 2020, 90% of PLWH should know their HIV status; 90% of those with diagnosed HIV should be on ART; and 90% of people receiving ART should be virally suppressed. The number of PLWH in the UK is estimated to be 107,800 (2.8 per 1000 population aged 15–59 years),7 and only 75% are estimated to be aware of their infection. Once diagnosed, however, year-on-year retention in HIV care is generally good, with only 5% of HIV patients in the UK reported as ‘lost to follow-up’ (LTFU) in any 1 year. On the other hand, cumulative drop over a 10-year period could be as high as one in five patients8 and an analysis of UK cohort data has shown that 17.4% of HIV patients are potentially LTFU. 9 Although the majority of those in HIV care (90%) are on ART and 90% are virally suppressed, we should consider the serious consequences for those who drop out of care. Published studies show that poorer health outcomes, including failure to suppress the viral load, increased drug resistance, reduced cluster of differentiation 4 (CD4) response and acquired immunodeficiency syndrome (AIDS)-defining illness, are consistently reported among HIV patients who engage poorly with care. 5,10,11 Furthermore, poor engagement in HIV care is associated with increased mortality. 12–14
The REACH study is particularly timely in the current context of expanded HIV testing and the development of ‘test and treat’ as a form of secondary HIV prevention. 15 Innovative models of care to improve early diagnosis of HIV must be complemented by strategies to promote long-term integration into care in order to realise the benefits of wider testing and treatment on the future spread of HIV in the UK.
The REACH study set out to examine those who are attending clinics but not at the optimal level, as well as those LTFU. Missed outpatient appointments also have significant resource implications for service providers, with the financial cost of missed appointments to the NHS estimated to be between £585M and £1B. 16–18 National outpatient non-attendance rates, which vary from 5% to 16%, are higher among younger patients and those in deprived areas18 and unpublished data from London HIV clinics indicate that outpatient non-attendance for PLWH can be as high as 25%. Retaining HIV patients in care may also reduce emergency department visits and hospitalisation,19,20 and the resultant financial costs.
In order to explore patterns of retention and engagement in HIV care, it is essential to adopt a valid and reliable measure, and yet there is no gold standard measure of engagement in HIV outpatient care. Researchers have assessed retention in care in a number of different ways. 21–25 These measures have their own strengths and weaknesses,26 but none of them takes into account the fact that frequency of attendance is related to changes in treatment and health status and may also be affected by external forces or changes in clinic policy. In the UK, for example, guidelines at the time the REACH study was conducted indicated that patients should be seen within 2–4 weeks of starting ART and every 3–6 months for routine monitoring on ART if they were considered ‘stable’, with good adherence and an undetectable viral load. 27 Given that frequency of monitoring is dependent on treatment and health status and that gaps between clinic visits may vary quite considerably within the current guidelines, the REACH study aimed to develop a measure of EIC that would be sensitive to changes at an individual or clinic level over time. This measure was then to be applied to an investigation of the health and economic impacts of disengaging from HIV care.
A better understanding of the factors associated with retention in HIV care and a means to predict disengagement is essential for both individual and public health benefit. Previous studies have examined the association between engagement in HIV care, as defined by one of the measures described above, and background characteristics. Although health service provision and populations of PLWH vary from country to country, these studies suggest that PLWH are less likely to disengage from care if they are male,8 older,8,21,28,29 white8,22 and men who have sex with men (MSM)22,23 and have started ART. 8,21 Socioeconomic factors and education have been highlighted in relation to disparities in EIC30,31 and complex patient groups, such as intravenous drug users, migrants and the newly diagnosed, are more likely to disengage from care. 23 Although recent diagnosis is associated with poor retention in care,8 there is also an indication that EIC can diminish over time. 22
Some studies have used qualitative methods and psychometric measures to try and understand why patients do not engage with care. HIV stigma is found to be a significant barrier32–35 and health beliefs may also deter people from attending for care. 36 A qualitative study on non-attendance of HIV clinics in Scotland highlighted issues of mental health, isolation, stigma, poverty and complex social circumstances as contributing to disengagement from care. 37 The REACH study set out to collect extensive quantitative and qualitative data and bring this evidence together to better understand who disengages from HIV care and why.
There have been, to our knowledge, no trials to evaluate interventions to improve engagement in HIV care in the UK. Although a number of interventions have been tested in the USA, a recent systematic review found only 13 published studies on which to base its analysis. 38 The population of PLWH and health-care system are very different in the USA and REACH provides the data to develop interventions for the UK context. Simple changes in the way services are delivered may be effective16 and the REACH study also helps us understand whether or not and how previous interventions to improve EIC in other health-care settings are likely to meet the diverse needs of PLWH in the UK. In this way, REACH provides a crucial bridge from research that purely describes the associations with engagement in HIV care to the development of innovative strategies to maintain patient retention. The data were used, first, to develop a diagnostic retention risk tool to help clinicians identify newly diagnosed patients at risk of disengaging from care and, second, to design and cost behaviour change interventions aimed at improving engagement in HIV care.
Retention in HIV care is vital for treatment success at both individual and population levels. Good engagement is associated with improved adherence, virological and immunological outcomes and survival. 15 It is important to develop NHS services that are flexible and responsive to the needs of the service users and that align to the wider NHS priorities of driving and achieving quality and efficiency within service delivery. The ultimate aim of the REACH study is to ensure the effective use of resources to improve engagement in HIV care and optimise health outcomes.
Aims and objectives
The REACH study aimed to explore, describe and understand HIV outpatient attendance in PLWH, in order to develop cost-effective interventions to optimise their EIC.
Its objectives were to:
-
examine HIV outpatient attendance patterns among PLWH
-
identify predictive factors of disengagement
-
investigate the potential health and financial costs of disengaging from care
-
develop a retention risk assessment tool
-
understand the situational, environmental, behavioural and social factors influencing outpatient attendance
-
develop intervention models to improve EIC, to be tested in future studies.
The full protocol has been published on the National Institute and Health Research Health Service and Delivery Research Programme website. The REACH Management Team met quarterly and benefited from an Advisory Group made up of a clinician from each of the recruitment sites and a patient representative, and a Study Steering Committee made up of four independent experts (see Appendix 1). The Advisory Group met every 6 months and the Study Steering Committee met once a year. In between meetings, communication was electronic, including a quarterly progress update.
Chapter 2 Methods
This chapter describes the methods used to address the aims and objectives of the study. Different methodologies were adopted to address the broad range of objectives that the study sought to address and the following description of them is divided up according to the three key phases of the study:
Phase 1: analysis of the UK Collaborative HIV Cohort database and predictive modelling
During this phase, we explored HIV outpatient attendance (objective 1), identified predictive factors of disengagement (objective 2) and investigated the potential health and financial costs of disengagement (objective 3).
Phase 2: examining patient experience
The second phase of this study comprised quantitative and qualitative methods to understand the factors that influence outpatient attendance (objective 5). Findings contributed to the development of a retention risk assessment tool (objective 4) and informed development of intervention models to improve EIC (objective 6).
Phase 3: key informant study
The final phase of the study aimed to understand the factors that influence outpatient attendance (objective 5) from the service providers’ perspective. It informed development of intervention models to improve EIC (objective 6).
After this, we describe how we calculated the costs for our proposed intervention models and the patient and public involvement in the study.
Phase 1: analysis of the UK Collaborative HIV Cohort data
In this section, we describe phase 1 of the study, which consisted of a detailed analysis of the UK Collaborative HIV Cohort (UK CHIC) database, including an exploration of attendance patterns and factors associated with disengagement from care.
Outpatient attendance patterns among people living with HIV
The UK CHIC collates routine data relating to the clinical care and treatment of PLWH aged ≥ 16 years across many of the UK’s largest HIV services (the data set utilised for the present analyses included data from 15 HIV clinics) since 1 January 1996. The UK CHIC records incorporate additional mortality data provided by Public Health England.
We explored the use of group-based trajectory modelling for identifying clusters of individuals following similar, distinctive progressions of attendance over age or time. 39–41 These methods were applied to the UK CHIC data set in order to distinguish distinct broad groupings of individuals according to their pattern of engagement with the aim of identifying a unique, statistical snapshot of the key characteristics of this complex population. We collaborated with Dr Tracy Glass at the Basel Institute for Clinical Epidemiology and Biostatistics, who has utilised a similar technique to explore and describe patient adherence to ART in the Swiss HIV Cohort Study. 42 Once the trajectories are identified, logistic regression analysis can then be used to predict the probability of an individual being in a particular group according to a particular set of risk factors.
Group-based trajectory analysis is usually applied to a fixed period of time during which individuals are exposed to a condition or data are collected at fixed points in time and a measure of their response can be plotted over time. We therefore developed an EIC algorithm in order to define whether patients were in care or out of care for each month of follow-up.
Development of engagement in care algorithm
As we know that frequency of HIV clinic attendance is related to changes in treatment and health status, our aim was to incorporate clinical factors into our new measure of retention in HIV care.
In the absence of complete data on clinic attendances in the UK CHIC data set, we used CD4 counts, viral loads, haemoglobin measures and ART treatment start or switch dates as markers of clinic attendance. Patients often return for repeat laboratory tests over a short period of time to confirm unexpected findings, resulting in clusters of attendances around a single ‘index’ date. As we did not want to consider each attendance within a cluster as an independent visit and wanted to be able to plot whether patients were in care or out of care for each month of follow-up, we grouped attendances into ‘care episodes’, defined as months (period of 30.4 days since entry into the study) where at least one visit occurred. For each care episode, we established the lowest CD4 count measured in that month (and the change from the previous value), the highest HIV viral load (and the status of this measurement relative to other consecutive values) and the patient’s treatment status.
In order to decide which key clinical factors should be incorporated the EIC algorithm, semistructured, face-to-face interviews were conducted with eight HIV physicians (from August 2013 to February 2014) who had a range of clinical experience and were selected from five of the HIV outpatient clinics where we planned to conduct our primary data collection. We asked physicians to tell us when they planned to see each of their last 10 patients again (number of weeks/months) and why.
A total of 73 patients were discussed in these interviews. One patient was excluded from the analysis because the time of their next appointment was dependent on awaited test results. The time of the next scheduled appointment was missing for another patient and not available for a further five patients who had not attended their last scheduled appointment at the time of the physician interviews. We conducted a content analysis of these qualitative data. For each patient, we noted the time to the next scheduled appointment and the key reason given by their physician. We then identified factors under which to code the key reasons.
The time of the next scheduled appointment in the 66 patients included in the analysis ranged from 1 week to 6 months, with a median of 3 months. Five factors were identified from the content analysis of the interview data as instrumental to the timing of the next scheduled appointment. The first factor can be summarised as ‘routine’ where patients were stable and required routine follow-up. These appointments were mostly arranged for 4–6 months after the previous visit. A routine follow-up appointment was arranged for 3 months’ time for one pregnant woman. Physicians talked about how they extended routine visits to every 6 months when patients were well and stable, both on treatment and in their psychosocial circumstances. The second factor is summarised as ‘virological’ where the next appointment was based on change in viral load (uncontrolled or virological breakthrough). Changes in viral load brought the next scheduled appointment forward to 1–2 months after the last. The third factor, ‘treatment’, described where the next appointment was related to starting ART or changing an existing ART regimen. Patients were given a next appointment date between 2 and 12 weeks later, depending on the treatment start date and virological response or when treatment was planned to start. The fourth and fifth factors were ‘psychosocial’ where mental health or psychosocial issues were identified as instrumental and ‘physical comorbidities’ where a range of physical comorbidities were given as the key reason for the timing of the next appointment. Follow-up appointments of between 1 week and 4 months were arranged depending on psychosocial issues (from specific concerns about mental health to more general needs for social support) and comorbidities: both of which required earlier follow-up even when patients were otherwise stable on treatment.
The data from the physician interviews were used as the basis for developing the EIC algorithm. Although psychosocial well-being and comorbidities were key factors in determining the expected time between patients’ visits, data on these variables are not captured in the UK CHIC data set. We therefore used the clinical data that were available (HIV diagnosis, AIDS diagnosis, treatment start dates, CD4 count and viral load) to determine the patient’s treatment and health status. These data were then used to estimate the expected time to the next scheduled care episode, in accordance with the data collected in the physician interviews. Table 1 shows when the next care episode was expected, according to the specified conditions. Using the EIC algorithm, we cannot consider follow-up after the patient’s last reported care date and it therefore focuses on intermittent periods of disengagement rather than LTFU. It should furthermore be noted that treatment guidelines introduced in the UK in October 2015 (after our physician interviews had been conducted) recommend starting ART irrespective of CD4 count. 43 This should be incorporated into the EIC algorithm when applied to EIC after October 2015.
| Conditions at time of initial care episodea | Next care episode expected within |
|---|---|
| Within 1 month of HIV diagnosis | 2 months |
| AIDS diagnosis | 2 months |
| Started ART | 2 months |
| Started new combination ART regimen | 2 months |
| Not on ART | |
| CD4 ≤ 350 cells/mm3, any drop in CD4 | 2 months |
| CD4 ≤ 350 cells/mm3, no drop in CD4 | 4 months |
| CD4 = 351–499 cells/mm3 | 4 months |
| CD4 ≥ 500 cells/mm3, CD4 drop ≥ 100 cells/mm3 | 4 months |
| CD4 ≥ 500 cells/mm3, CD4 drop < 100 cells/mm3, viral load ≥ 100,000 copies/ml | 4 months |
| CD4 ≥ 500 cells/mm3, CD4 drop < 100 cells/mm3, viral load < 100,000 copies/ml | 6 months |
| Already started ART | |
| Viral load > 200 copies/ml | 2 months |
| Viral load = 51–200 copies/ml, does not appear to be a blipb | 2 months |
| Viral load = 51–200 copies/ml, appears to be a blip | 4 months |
| Viral load ≤ 50 copies/ml, CD4 ≤ 200 cells/mm3 | 4 months |
| Viral load ≤ 50 copies/ml, CD4 > 200 cells/mm3 | 6 months |
The EIC algorithm gives the shortest expected gap between care episodes at 2 months. This is to allow for the fact that clinic visits might occur at any point during the month or care episode into which they are grouped. If the patient was within 1 month of diagnosis, had an AIDS diagnosis, started ART or changed ART at the initial care episode, the next care episode was expected within 2 months. If the patient was not on ART at the initial care episode, the next care episode was expected within 2–6 months, depending mainly on CD4 count. If the patient had started ART, it was expected within 2–6 months, depending on viral load. We used 6 months as the maximum time between visits, as described in the physician interviews. If more than one condition applied at the time of the initial care episode, the next care episode was expected within the least number of months associated with those conditions.
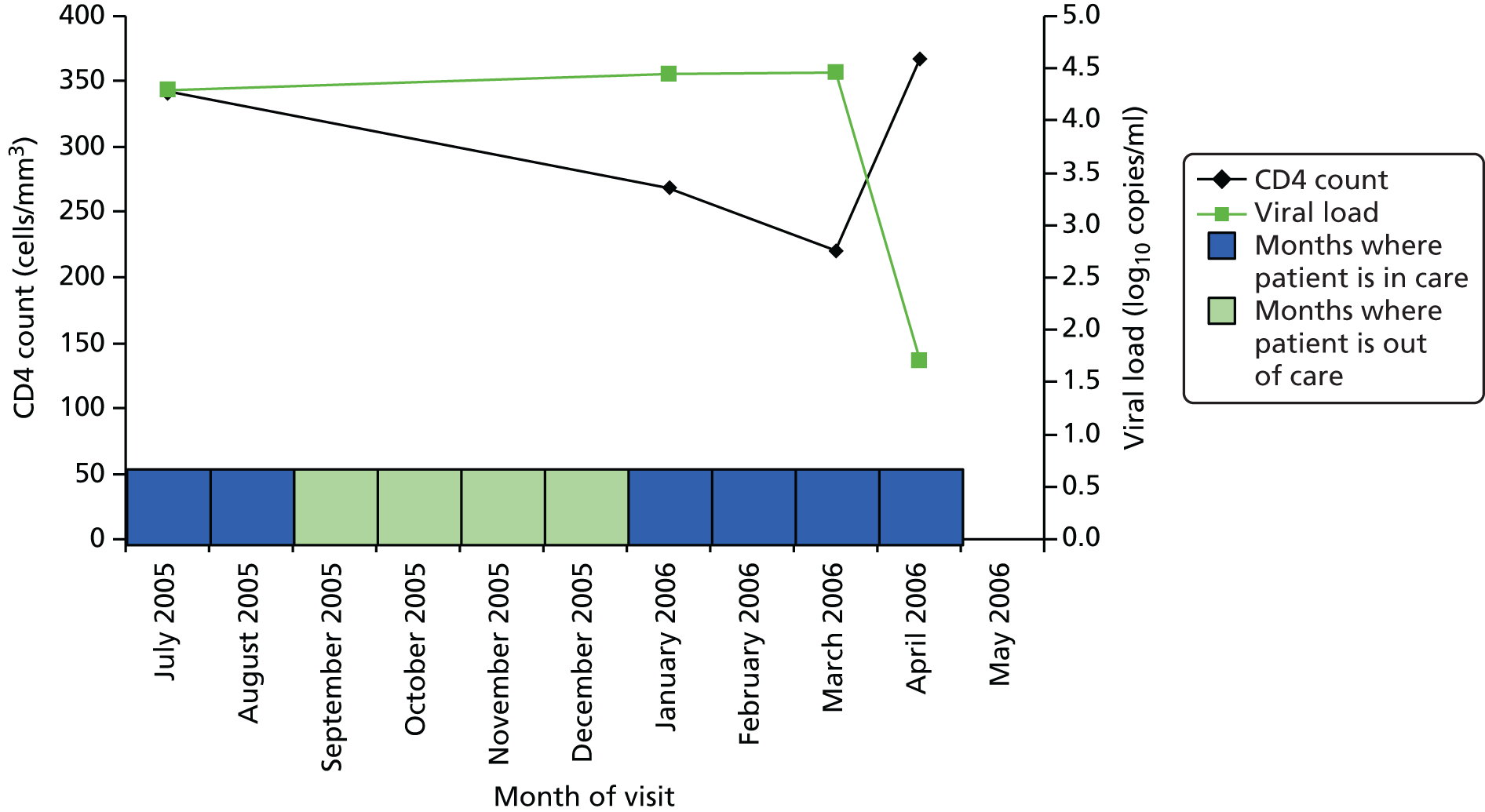
Figure 1 shows an example of how the EIC algorithm is applied to an individual case. The date of the next observed care episode determines whether or not the patient has attended before or after the expected date, and each patient-month is then classified as being in care (where it occurs on or before the time of the next expected care episode) or out of care (where it occurs after the time of the next expected care episode) accordingly. This example begins in July 2005 when the patient was not pregnant, not recently diagnosed, did not have an AIDS diagnosis and was not on ART. Because she was not on ART, we are interested in her CD4 count – which was < 350 cells/mm3 and which had dropped (although that is not shown here), so we expect to see her again within 2 months. However, she did not reattend until 6 months later. Thus, July and August are defined as being in care (blue shading), but September to December are out of care (green shading). At the visit in January 2006, her CD4 count had continued to drop, so we expect to see her again within 2 months. She reattended in March 2006 so January and February are defined as being in care. In March 2006 she started ART, so we expect to see her within 2 months and she comes back within a month, remaining in care.
FIGURE 1.
Measurement of engagement in HIV care applied to an individual care.
Group-based trajectory analysis
We then applied the EIC algorithm to the group-based trajectory analysis. Patients with complete laboratory data who attended a participating UK CHIC clinic on at least two occasions between 1 January 2000 and 31 December 2011 were included in the analysis. Follow-up for each person was considered until the last recorded laboratory marker or clinic visit prior to (or on) 31 December 2011. As we were interested in patterns of attendance from diagnosis onwards, we excluded patients who had been diagnosed before 1 January 2000.
Whereas group-based trajectory analysis is usually applied to a fixed period of time, the exposure period (or time since diagnosis) was highly variable in our analysis and related to the outcome measure of being in care or out of care. It was difficult to apply patterns of engagement to the 12-year period of follow-up for the whole cohort because individuals contributed longer and shorter periods of follow-up, which also varied according to the time that the patient entered or left the cohort. In an attempt to overcome this, we examined patterns of attendance for people diagnosed over three 3-year periods (2000–2, 2003–5 and 2006–8). Although this controls the start point for the analysis to some extent, it does not account for the fact that patient end points will vary over the course of the trajectories. We excluded attendance over the last year of follow-up from the analysis, as this may skew the trajectories.
With group-based trajectory analysis, the researcher determines the number of trajectories each time the analysis is run in order to test which number of trajectories gives the best fit. Models were tested with one to five trajectories for each of the three diagnosis periods and the optimal model (which indicates the optimal number of trajectories) was selected for each of the three diagnosis groups. This was based on statistical fit, by comparing the Bayesian information criterion (BIC), and interpretability. Twice the difference between the BIC for the two models under comparison is used for this purpose. 39 The plots and patterns of attendance from our group-based trajectory analysis are presented and described in Chapter 3. However, this analysis resulted in three different models (one for each of the 3-year diagnosis periods), which we consider to be the minimum number of models to reflect the range of diagnosis dates over a 12-year period. This makes their use in further analysis of associated factors and outcome problematic.
Association with clinical outcomes
In view of the limitations of using the findings from our group-based trajectory analysis in further analysis to examine the associated clinical outcomes, we decided to use the proportion of time in care as a more straightforward and fitting measure of engagement in HIV care for further analysis. The individual case shown in Figure 1, for example, was out of care for 4 of her 10 months’ follow-up and in care for 6 of her 10 months’ follow-up = 60% of months. The EIC measure, furthermore, provides an alternative to the idea that disengagement from care requires an individual to be LTFU for a specified period of time which does not lend itself to quantifying the typical length of disengagement or proportion of patients that re-engage with care, as described in our protocol.
In the following analysis, all patients who attended a participating UK CHIC clinic on two or more occasions between 1 January 2000 and 31 December 2012 were included. The proportion of months in which patients were engaged in HIV care was calculated overall and for patient subgroups defined by gender, age group (< 25, 25–45 and > 45 years), ethnic group (white, black African, other and unknown), mode of HIV acquisition (sex between men, sex between men and women, injection drug use and other/unknown), currently on ART (yes, no), nadir and current CD4 count (both classified as < 200, 200–349 and ≥ 350 cells/mm3), participating clinic, calendar year (2000–3, 2004–7, 2008–12) and time since entry in the study (< 1, 1–5, 5–10 and > 10 years). Each patient-month was then treated as a separate entry in a multivariable logistic regression model with the aim of identifying demographic and clinical factors associated with that month being in care. The analyses were performed using PROC GENMOD in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) with generalised estimating equations used to take account of the repeated entries within each individual patient.
Cox models were used to assess the association between mortality and (i) the cumulative proportion of months a person had been in care; and (ii) the cumulative proportion of months a person was in care prior to ART. For analysis (i), patient follow-up started on the date of entry to the UK CHIC study and ended at the date of death or 6 months after the patient’s last clinic attendance, whichever occurred first. Each person’s total period of follow-up was split into consecutive monthly intervals (as described above) and the cumulative proportion of previous months a patient had been in care at the start of the month was calculated for each month of follow-up. In this way, we were able to include the EIC covariate as a continuous time-updated covariate with each person’s EIC evolving over time in the model. One of the main limitations of analyses that investigate associations between EIC and mortality, particularly where a time-updated covariate is used, is the potential for reverse causality whereby those who are sickest may attend for care more frequently in the months leading up to death. This may make it appear that higher levels of EIC are associated with an increased risk of mortality. In order to reduce the potential for this to bias our analyses, all measures of EIC were lagged by 12 months to separate the assessment of EIC and the mortality outcome by a period of 1 year. At any given time over follow-up (i.e. at the start of each patient-month that is included in the model), therefore, the lagged value of EIC that was entered into the model was the value that was available 12 months earlier. Thus, our estimate of the relative hazard (RH) associated with EIC will provide a description of the ability of our EIC measure to predict mortality events that occur at least 1 year into the future. Note that, by definition, this approach will necessarily restrict analyses to those who had attended clinic for > 1 year, as individuals who die within the first 12 months of follow-up will not contribute any EIC values to the model and will therefore be excluded. In our primary analyses, we adjusted for the demographic factors of age, year, gender, mode of acquisition and ethnic group (all fixed covariates). This was followed by additional adjustment for receipt of ART as a binary time-updated covariate. We next adjusted for the latest CD4 count, as a continuous time-updated covariate and lagged by 12 months, to investigate whether or not any association seen was explained by the fact that those with the lowest EIC values already had lower CD4 counts at the time of measurement of EIC. Finally, we adjusted for the unlagged values of CD4 – this analysis explored whether or not any residual association between EIC and mortality was mediated by lower CD4 counts over the following 12 months.
For analysis (ii), we calculated the EIC prior to ART in the subset of patients who initiated ART and who had been under follow-up at the clinic for at least 1 year prior to ART start (to ensure that the estimate of EIC was based on sufficient patient-months to provide a robust estimate). To explore the potential for confounding, patients were first stratified into six groups based on their pre-ART EIC. The groups (< 50%, 50–69.9%, 70–79.9%, 80–89.9%, 90–99.9% and 100%) were chosen primarily for ease of clinical interpretation and to ensure that each group was of sufficient size to permit robust analyses. Associations between the pre-ART EIC value and the various demographic and clinical factors at ART start were then identified. Next, Cox models considered the association between EIC (as a fixed baseline covariate) and mortality after ART initiation; follow-up started at ART initiation and ended at 6 months after the patient’s last visit or death – whichever of the two occurred first. We first adjusted for age, year, gender, mode of acquisition and ethnic group, then type of ART received [protease inhibitor (PI)-based regimen, non-nucleoside reverse-transcriptase inhibitor (NNRTI)-based regimen, other regimen (including those on both a PI and NNRTI)], then the CD4 count and viral load at ART start (fixed covariates) and then, finally, the latest CD4 count and viral load (as time-updated covariates) measured after ART start. As before, these last analyses investigate whether or not any associations between pre-ART EIC and post-ART mortality can be explained by poorer CD4/viral load responses on ART.
Association with laboratory tests and costs
For our health economic analysis, we planned to measure the impact of disengagement on HIV management costs. As noted in our study protocol, such an analysis was likely to be difficult as it would be limited by the lack of NHS resource utilisation data captured in UK CHIC. Given the limited data for some but not all clinics on outpatient clinic attendances and inpatient stays we therefore limited our study to a descriptive analysis to selected laboratory test costs, which are reliably collected in UK CHIC across participating clinics.
Using the ART data set from analysis (ii), we calculated the total number of laboratory tests performed within each pre-ART EIC group within each 3-month period of follow-up after ART start until the end of the fourth year after starting ART. We included the following tests:
-
CD4
-
HIV viral load
-
liver function tests
-
cholesterol
-
high-density lipoprotein
-
low-density lipoprotein
-
triglycerides
-
full blood count
-
urea
-
creatinine
-
glucose
-
bone health.
Liver function tests included one or more of the following blood tests: alkaline phosphatase, alanine transaminase, aspartate aminotransferase or bilirubin. Full blood counts included haemoglobin and/or platelets. A patient was recorded as having a liver function test or full blood count if they had one or more of the constituent tests on the same day. Bone health profiles were identified when a patient had a phosphate test.
We divided the number of tests by the group size in each 3-month period (which declined over time) to calculate the mean number of tests per patient per quarter, with the group size in each period determined on the basis of the patient’s first and last visit dates, regardless of whether or not she/he had actually attended for care in that period. Unit costs were applied to each laboratory test and summed across all tests, and the mean cost per patient was calculated within each EIC and time stratum. The unit costs were mean costs from two HIV clinics in London, measured in 2016 GBP. The unit costs are summarised in Table 2.
| Test | Test cost (£) |
|---|---|
| CD4 | 22.27 |
| Viral load | 32.91 |
| Liver function tests | 4.68 |
| Cholesterol | 0.75 |
| High-density lipoprotein | 0.94 |
| Low-density lipoprotein | 0.47 |
| Triglycerides | 0.78 |
| Full blood count | 3.65 |
| Urea | 0.59 |
| Creatinine | 0.55 |
| Glucose | 0.76 |
| Bone health | 1.33 |
Mean costs for the six pre-ART EIC groups were then plotted by quarter in the 4 years following ART start. Given the limitations of the data we do not undertake formal tests of differences between groups over time, but focus on descriptive trends. We were particularly interested in investigating whether or not pre-ART EIC affected testing and test costs after starting ART, and in particular whether or not costs became higher in the less engaged groups over time if their health declined, at a more rapid rate than in those who had a higher pre-ART EIC value.
Although we had planned to incorporate the development of a retention risk tool into the first phase of the study, the Management Team agreed that the REACH survey would provide a richer source of data than the UK CHIC data set to identify clinical and non-clinical factors that may predict disengagement. This analysis was therefore moved into phase 2 of the study and is described as follows.
Phase 2: survey, patient interviews, focus groups
Phase 2 of the study consisted of a quantitative survey followed by a nested qualitative substudy. Ethical approval for this phase of study was obtained from the National Research Ethics Service Committee London – City Road & Hampstead (reference 14/LO/0039). NHS permission for research (research and development approval) was granted by the local NHS trusts.
Phase 2a: quantitative component – questionnaire and clinical data collection
Population and setting
A cross-sectional self-completion survey was conducted with adult men and women (aged ≥ 18 years) attending seven London clinics for HIV care between May 2014 and August 2015. Patients were excluded from the study if they had been diagnosed with HIV within the previous 4 months or were unable to provide informed consent. The cohort sizes, composition and models of service delivery varied across the recruitment centres, contributing to the generalisability of the findings:
-
Ambrose King Centre, The Royal London Hospital
-
Bloomsbury Clinic, Mortimer Market Centre
-
Clifden Centre, Homerton University Hospital
-
Greenway Centre, Newham University Hospital
-
Harrison Wing, St Thomas’ Hospital
-
Ian Charleson Day Centre, Royal Free Hospital
-
Kobler Day Care Unit, Chelsea and Westminster Hospital.
Survey sample size
The target sample size was 1000 patients from HIV clinics across London. We aimed to recruit a total of 250 irregularly attending patients and 250 non-attending patients with a comparison group of 500 regularly attending patients (definitions are given in Survey recruitment), providing over 80% power when either disengaged group is compared with the control group to detect a difference in the prevalence of a suspected predictor of disengagement when the population difference is 56% versus 44% or 14% versus 7%, at a 5% significance level.
Survey recruitment
Five of the seven clinics began recruitment in May 2014 and two additional sites began in November and December 2014. Each site recruited patients until they reached their target of 200 patients or 14 August 2015, whichever was the sooner.
Originally it was planned to apply the patterns of attendance identified in phase 1 to our survey sampling. We had envisaged using patient records and electronic/clinical databases to derive an attendance pattern to each patient. However, we decided against this strategy for three reasons. First, we did not find group-based trajectory analysis to be a useful method of identifying patterns of patient attendance that could be used for sampling. Second, applying the EIC algorithm to recruiting patients in each of the clinics would be too complex to undertake. Third, although the UK CHIC analysis explores patterns of attendance over time, we needed to be able to capture current behaviour for the purposes of the survey. We therefore developed a simple algorithm that could be used by research staff in the clinics to recruit patients based on their recent attendance behaviour.
Each clinic aimed to recruit a total of 200 patients who had attended in the following way in the past year:
-
100 regularly attending patients
-
who had attended all intended HIV clinical appointments in the past year
-
-
50 irregularly attending patients
-
who had missed one or more intended HIV clinical appointments (which had not been rescheduled within 1 month) in the past year
-
-
50 non-attending patients
-
who had experienced a period of non-attendance for a year or more at any HIV service that ended within the past year or continued to the present day.
-
Patients were approached by a member of the local research team and asked if they would like to take part in the study. They were given an information sheet summarising the study and its key objectives, and including contact details for the Project Management Team. The local research staff explained the purpose of the study and answered any questions, and patients provided informed consent to participate. Patients were encouraged to complete the questionnaire at the clinic but if they did not have time to do this, they could take the questionnaire with them and return it using a pre-paid envelope. There was also the option to complete the questionnaire online, but very few patients were offered this alternative and only two responses were returned in this way.
Patients who completed a questionnaire at the clinic, handed it back to local research staff in a sealed envelope in order to keep their responses confidential. The completed questionnaires were collected from clinics on a regular basis.
Towards the end of the data collection period, when targeting non-attending patients, clinics were able to send out a letter approved by the Ethics Committee inviting them to participate along with a copy of the questionnaire and return envelope.
Study questionnaire
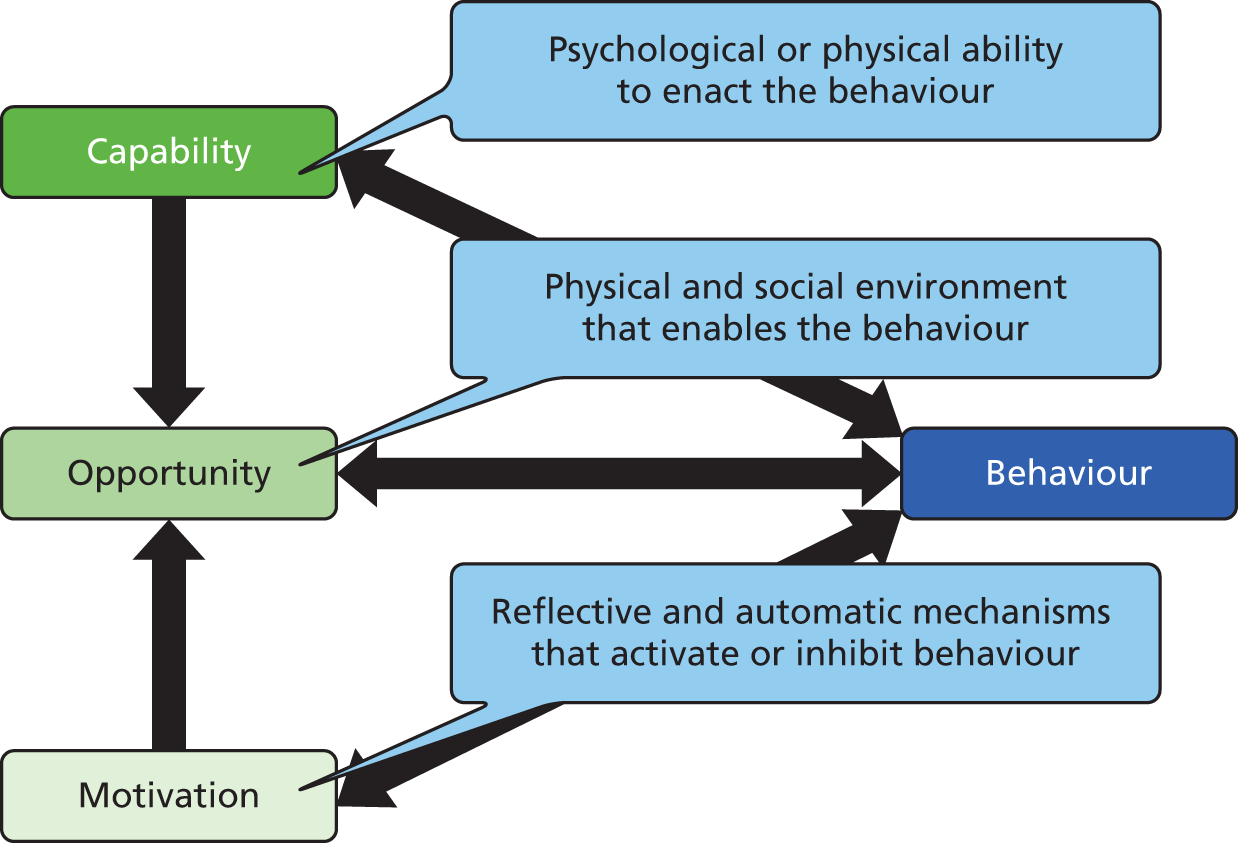
The anonymised pen-and-paper questionnaire was completed in clinics where it was linked to clinical data. It contained 80 questions and took about 20–30 minutes to complete (see Appendix 2). Questions were based on variables from the COM-B (‘capability’, ‘opportunity’, ‘motivation’ and ‘behaviour’) model (Figure 2), which proposes that behaviour occurs as an interaction between three necessary conditions of capability, opportunity and motivation. 45 The COM-B model is at the centre of an integrative framework of behaviour change interventions, the behaviour change wheel comprising nine intervention functions and seven policy categories. It provides a framework for developing, evaluating and synthesising interventions, including selecting behaviour change techniques in the development process.
FIGURE 2.
The COM-B model of behaviour. Adapted from the original figure (see Michie et al. 45) through inclusion of description of the three domains. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
A framework for REACH primary research (see Appendix 3) was created to aid our variable selection. The framework brought together the COM-B model, the associated domains of behavioural influence from the theoretical domains framework46 and related constructs, as well as factors associated with engagement requiring change that were identified in an in-depth report on non-attendance of HIV services in Scotland. 37 Thus, when selecting which items should be included in the questionnaire, we could ensure that we had adequate measures from the different sections of the framework, including the three key domains of capability, opportunity and motivation.
Whenever possible, the questionnaire incorporated validated items used in other large-scale behavioural surveys but new questions were developed where we were unable to find published measures. Items from the following scales were included: three items from Household Food Insecurity Access Scale;47 all seven items from the Strive Internalised Stigma scale;48 three items from the environmental mastery subscale of the Psychological Well-Being Scales (PWB);49,50 five items from the Duke-UNC Functional Social Support Questionnaire (DUFSS);51 all four items from the Patient Health Questionnaire (PHQ4);52 all three items from the European AIDS Clinical Society screening questions for neurocognitive impairment;53 and two items from the Health Information Competence Scale. 54,55 We used the following five items from the 11-item Belief about Medicines Questionnaire (BMQ):56,57
-
my health, at present, depends on these medicines
-
having to take these medicines worries me
-
my health in the future will depend on these medicines
-
I sometimes worry about becoming too dependent on these medicines
-
these medicines give me unpleasant side effects.
We used all five items from the Medication Adherence Report Scale (MARS). 58 We did not use any of the framing statements from the BMQ or MARS due to the limited amount of space in the questionnaire.
The questionnaire content was piloted to test feasibility and acceptability among five PLWH, including a patient representative with extensive experience of working with people who have difficulties engaging in HIV care. The questionnaire was piloted to test keywords and constructs with five PLWH. The content was modified in the light of this feedback.
The final questionnaire was a 24-page printed A5 booklet. In addition, a computer-assisted self-interview (CASI) version was developed, programmed in survey software and tested for the study. However, most of the clinics preferred to use the paper version and the CASI version was only offered to a minority of patients at two of the clinics and electronic responses were returned by two patients. The questionnaire was divided into the following sections:
General information: gender, age, ethnic group, country of birth, immigration status, language, relationship status, children, pregnancy, work status, education, religion, sexual orientation, poverty, accommodation and hunger.
Life with HIV: date of diagnosis, place of diagnosis, activity, HIV disclosure, stigma, environmental mastery, caring responsibilities, domestic abuse, social support, support groups and recreational drug use.
Health and health care: health status, mental health, professional support, neurocognitive impairment, general practitioner (GP) registration, inpatient stays, health information competence.
Human immunodeficiency virus care: reasons for missed appointments, number of clinics attended, frequency of attendance, time at present clinic, travel to clinic, clinic hours, mode of consultations, clinic recommendation, appointment booking, reasons for attending, communication with reception staff, nurse and doctor, and treatment explanation.
Medicines: beliefs about medicines, ART status, home delivery service, medication adherence and reasons for not taking ART.
Linked clinical data
Consent to participate included linking questionnaire responses to routine clinical data. The clinical data consisted of:
Background data: age, gender, transmission group, country of birth, date of diagnosis, ART status. These variables were checked against the questionnaire data and final variables were derived for analysis.
Clinical data: patient complexity according to category 3 criteria of the HIV and AIDS Reporting System (HARS),59 pregnancy at diagnosis, HIV-related inpatient stays, CD4 count at diagnosis or first recorded, CD4 count at ART initiation, most recent CD4 count, viral load at diagnosis or first recorded, most recent viral load, AIDS-defining illness, hepatitis C coinfection, hepatitis B coinfection, mental health and drug/alcohol dependency.
Survey data processing
Clinics were asked to keep a log of all patients who were approached to take part in the study. Each patient was given a unique study number that was written on the front of their questionnaire and entered into a study log. The study log was maintained securely at the clinic. In addition to the unique study number, it contained the date of attendance, clinic number and some basic demographics (age, gender, ethnic group), as well as the outcome of the approach. Local research staff checked the patients’ attendance pattern over the past year to assign them to one of the three attendance groups and this information was added to the study log. Local staff transferred an anonymised version of the clinic log (without clinic number) to the Project Management Team on a monthly basis.
The completed paper questionnaires were entered into IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA) by a data entry clerk and a randomly selected sample of one-third of the questionnaires were checked for accuracy of data inputting.
Survey analysis
Survey data were analysed using IBM SPSS Statistics version 22 and Stata 14 (StataCorp LP, College Station, TX, USA). The following description of the survey analysis begins with the analytical approach used in developing the retention risk tool, followed by the analytical approach used to explore factors associated with poor retention to care.
Analysis for retention risk tool
The retention risk tool is an equation that can be used to calculate the predicted probability that a patient will disengage from care. If the tool or algorithm that we have developed here is externally validated in an independent population, it could be used to help clinicians identify newly diagnosed patients at risk of disengaging from care. It would ultimately be a practical diagnostic tool that could be incorporated into clinical practice for multidisciplinary caregivers of HIV patients. Although we had originally planned to use UK CHIC data to develop the tool, the REACH survey provides a richer data set for this purpose.
Univariable associations were explored (using the chi-squared test and the Student’s t-test) between variables derived from responses to the questionnaire and clinical data from patients’ notes and whether or not individuals had recently disengaged from care. For this analysis, we compared regular attenders (RAs) with a combined group of irregular attenders (IAs) and non-attenders (NAs). The decision to combine IAs and NAs was made because the key purpose of the tool in the clinical setting will be to identify someone at risk for any type of disengagement at the point of diagnosis. This should then be followed up with a needs assessment for those identified as being at risk in order to intervene appropriately.
Variables were considered for inclusion in the retention risk tool (and thus included in the univariable analysis) if they were potentially predictive of future disengagement at the time of diagnosis (i.e. variables that pre-exist or may pre-exist diagnosis). As the tool will be tested and used in clinics not included in this study, we excluded the clinic currently attended from the model. We excluded variables that would have occurred after HIV diagnosis, such as HIV service use or HIV disclosure but we included time taken to get to clinic and mode of transport, as these are more likely to remain fixed after HIV diagnosis. We also excluded variables if they were not applicable to all patients or had very high levels of missing data (> 40%). We excluded scales because the incorporation of several items may result in missing data that would be problematic for their use in a practical tool. Responses to psychometric measures, in addition, may change over time. We excluded measures of recent physical or mental health that were current or had reference periods of < 6 months, as these may have been caused by HIV. We excluded variables with very low prevalence (< 5%), as this may not provide sufficient variability to be appropriate for modelling. All items needed to be suitable for collection at the patient’s baseline assessment with a HIV clinician.
Investigation of the 27 candidate variables for inclusion in the model indicated that the mean number of missing data was 2.9% and the maximum number was 7.9%. The majority of variables (n = 21) had < 5% missing data and six had 5–10% missing data. In view of the complicated nature of data imputation and the small number of missing data, we conducted the analysis excluding missing data listwise, whereby all data are removed for cases that have one or more missing values.
Variables that met the above criteria and were significantly associated (p < 0.05) with disengagement from HIV care were included in logistic regression modelling. We used backwards-stepwise binary logistic regression to select the best set of predictive variables associated with disengagement from care. It is important to bear in mind that, when testing models, the general rule of thumb for not over fitting the model is that there should at least 10 cases per covariate in the model. 60 To this end, we fitted the models by adding variables in blocks, which followed a chronological order. First, we fitted the block 1 variables to determine which combination had the best predictive power, then we added the block 2 variables and so on, until all blocks and all 27 variables had been added. The following blocks were applied:
-
block 1: fixed sociodemographic variables
-
block 2: sociodemographic variables that are subject to change over time
-
block 3: variables about circumstances of HIV diagnosis
-
block 4: health, mental health and drug use, ever reported
-
block 5: mental health and drug use, within the past 5 years
-
block 6: variables about potentially transient circumstances
-
block 7: variables about getting to the clinic.
Standard methods for assessing model effectiveness were used. 61–64 The Hosmer–Lemeshow goodness-of-fit test was used to measure model calibration. A p-value that is above the threshold for statistical significance, in this case p > 0.05, indicates that the data predicted by the model are not significantly different from the observed data. Model discrimination was tested using the c-statistic or area under the receiver operating characteristic (AUROC) curve. A value of 0.9–1.0 is considered excellent, 0.8–0.9 is good, 0.7–0.8 is fair, 0.6–0.7 is poor and ≤ 0.6 is worthless. The c-statistic, pseudo-R2 and minus twice the log-likelihood (–2LL) were used to examine effectiveness and compare models. When comparing models, the Cox–Snell pseudo-R2 gives an estimation of the proportion of the outcome variable explained by the predictive variables and a smaller value of –2LL indicates that the model is a better fit. The regression coefficients were used to calculate each person’s probability of disengagement and this was compared with their observed risk in order to examine the sensitivity and specificity of the model.
The face validity of the model was tested by asking clinicians whether or not the predictive variables corresponded to their clinical experience. As the model was developed using the complete REACH survey data set in order to ensure sufficient statistical power, its external validity will need to be tested in a future study by applying the tool to a sample of patients to be followed over a 24-month period to determine if it predicts future disengagement from care.
Analysis of factors associated with engagement in HIV care
We used univariable analysis (chi-squared and the Student’s t-test) to examine associations between variables derived from responses to the questionnaire and clinical data from patients’ notes and membership of attendance groups: RAs, IAs and NAs. Multivariable analysis was used to derive parsimonious sets of variables that are independently associated with engagement in HIV care. As different proportions of RAs, IAs and NAs were recruited from each of the clinics, multivariable analysis also allowed for adjustment by clinic where the respondents were recruited. In addition to clinic, we adjusted for background factors in all models. We used backwards-stepwise multinomial logistic regression to select the best sets of predictive variables associated with irregular and non-attendance, compared with regular attendance. The stepwise method takes standard confounding between variables into account by including and excluding explanatory variables from the model, according to the degree of correlation with the dependent variable. It controls for the effects of the other independent variables as they are entered and can therefore be used to establish the most parsimonious model. Interaction terms were not included in this analysis. We included predictive variables that were significantly associated (p < 0.05) with the attendance groups in the univariable analysis in the models. We collapsed categories where this did not change the statistical association with the dependent variable. We excluded significantly associated variables that did not have any predictive value such as how often respondents expected to have a routine consultation. In order to avoid overfitting the data, we used separate models to explore the association between attendance and background factors, HIV diagnosis and health service use, factors relating to capability, motivation, opportunity (relating to social influences), opportunity (relating to barriers), use of ART and clinical data.
In order to derive two final models for variables associated with irregular and non-attendance, we included variables that the above multinomial logistic regressions indicated were associated with irregular and non-attendance in binary logistic regression. Variables were added to the models in the following order, as described above:
-
block 1: clinic attended and demographic variables
-
block 2: other background variables
-
block 3: mental and physical health in the past year or more
-
block 4: other variables.
The final two models were selected on the basis of having the highest Cox–Snell pseudo-R2 and thereby explaining the highest proportion of the outcome variables.
Phase 2b: qualitative component – individual patient interviews
Exploratory, face-to-face, semistructured interviews were undertaken with a purposively selected sample of men and women who had attended the HIV clinics where survey recruitment was taking place from June 2014 to February 2015. Eligible participants were men and women living with HIV aged ≥ 18 years. Patients were excluded from the individual interviews if they were unable to provide informed consent or if they had acquired HIV through vertical transmission as another study [Adolescents and Adults Living with Perinatal HIV (AALPHI) cohort study of HIV-infected young people]65 was recruiting these patients for individual interviews concurrently.
Patient interview sample size
We planned to recruit a sample of up to 40 men and women. Table 3 shows the quota matrix describing the sampling plan to ensure maximum diversity in terms of attendance pattern and key characteristics. Our primary quotas were attendance pattern and combined gender and sexual orientation. We wanted to oversample IAs and NAs to ensure that a range of their experiences were captured in our interview data. We also wanted to recruit sufficient numbers of gay and bisexual men, heterosexual men and women in order to provide sufficient data to explore their potentially diverse experiences.
| Irregularly attending (n = 20) | Gay male (n = 6–7) | Heterosexual male (n = 6–7) | Female (n = 6–7) |
|---|---|---|---|
| PWID (n = 1–2) | UK born (n = 3–4) | Black African (n = 3–4) | Black African (n = 4–5) |
| Not on treatment (n = 1–2) | Non-UK born (n = 3–4) | Other ethnicity (n = 3–4) | Other ethnicity (n = 2–3) |
| Diagnosed in past 2 years (n = 1–2) | Younger (n = 3–4) | Younger (n = 3–4) | Younger (n = 3–4) |
| Older (n = 2–3) | Older (n = 2–3) | Older (n = 2–3) | |
| Non-attending (n = 10) | Gay male (n = 3–4) | Heterosexual male (n = 3–4) | Female (n = 3–4) |
| PWID (n = 1–2) | UK born (n = 2–3) | Black African (n = 1–2) | Black African (n = 2–3) |
| Not on treatment (n = 1–2) | Non-UK born (n = 1–2) | Other ethnicity (n = 1–2) | Other ethnicity (n = 1–2) |
| Diagnosed in past 2 years (n = 1–2) | Younger (n = 2–3) | Younger (n = 2–3) | Younger (n = 2–3) |
| Older (n = 1–2) | Older (n = 1–2) | Older (n = 1–2) | |
| Regularly attending (n = 10) | Gay male (n = 3–4) | Heterosexual male (n = 3–4) | Female (n = 3–4) |
| PWID (n = 1–2) | UK born (n = 2–3) | Black African (n = 1–2) | Black African (n = 2–3) |
| Not on treatment (n = 1–2) | Non-UK born (n = 1–2) | Other ethnicity (n = 1–2) | Other ethnicity (n = 1–2) |
| Diagnosed in past 2 years (n = 1–2) | Younger (n = 2–3) | Younger (n = 2–3) | Younger (n = 2–3) |
| Older (n = 1–2) | Older (n = 1–2) | Older (n = 1–2) |
Our secondary quotas were age, ethnicity, injecting drug use, treatment status and recent diagnosis. Examination of the literature suggests that age might impact on attendance and we therefore wanted to recruit participants who were both younger and older than 35 years of age. We wanted to ensure adequate representation of black Africans, who are particularly affected by HIV but, as black Africans form a very small minority of gay and bisexual men, we sought diversity in this group by recruiting men born in the UK and those born outside the UK. We also aimed to recruit small numbers of injecting drug users, people who had not started ART and those diagnosed within the past 2 years, to understand their experiences, as the literature indicates that they are at risk of disengaging from care.
Patient interview recruitment
Posters were displayed and fliers were available in the study clinics. Local research staff gave patients an information sheet and explained what the interview entailed to ascertain whether or not they were interested in taking part. If patients agreed, their contact details were passed on to the Project Management Team and AH contacted them to discuss the interview. Some patients contacted AH directly, having seen a flier or poster at their clinic. Two interviewees took part in a follow-up interview after participating in one of the focus groups (FGs) (see Phase 2c: community focus groups). AH checked eligibility according to a set of screening questions (see Appendix 4) and patients were selected to ensure a range of characteristics and experiences. Clinics were kept informed of the patients who had been interviewed; as the cells in the quota matrix were completed, they targeted patients with particular characteristics.
Interviews were arranged at a mutually convenient time. They were conducted face to face and took place on university or NHS trust premises. One patient was interviewed at their work place. At the beginning of each interview, the information sheet was explained, any questions were answered and patients provided informed consent to participate. Interviews took about 60–90 minutes. All participants were given a £20 high street shop voucher at the end of the interview to cover any transport costs and as a small token of thanks. They were offered a copy of the interview transcript and a copy of the findings from the study.
Patient interview content
Interviews were based on a topic guide (see Appendix 5) that was developed with reference to the COM-B model, as described in the questionnaire development. The sections covered:
Human immunodeficiency virus diagnosis and link to services – HIV diagnosis, starting HIV care, use of other HIV services, change over time and frequency of attendance.
Current HIV clinic attendance – expectations, appointment booking, travel to clinic, facilities, communication with reception staff, nurse and doctor, peer support and suggested improvements.
Reasons for attending regularly and irregularly – last appointment missed and suggestions.
Living with HIV – physical and emotional impacts, HIV disclosure, social support, stigma and other barriers.
Taking ART – adherence and home delivery service.
Other NHS services – GP.
Other barriers and facilitators to HIV care – off-putting experiences, agencies and individuals.
Interview data processing
Interviews were digitally recorded (with permission) and transcribed verbatim by a professional transcription service.
Interview analysis
We used framework analysis, a method for analysing qualitative data, developed on the basis of the extensive experience of researchers at the National Centre for Social Research, London. 66 It aims to condense what respondents say in their interviews into a format that facilitates inspection of data across themes and within individuals. At the same time the analyst aims to maintain closeness to the original data, by adopting respondent terminology as far as possible.
Four researchers read through three interview transcripts that were selected to represent different participant characteristics (one RA, one IA and one NA). They marked thoughts and possible themes, and met to discuss these. An index was drawn up covering the themes derived from this discussion and it was circulated for comment and revised in the light of these comments. Themes were categorised according to the COM-B model under the headings identified in the framework for REACH primary research (see Appendix 3).
Two of the above researchers coded the data using computer-assisted qualitative data analysis software, NVivo version 10 (QSR International, Warrington, UK). The use of this software facilitates flexible and effective data management and enables easy retrieval of pieces of text from material that can be voluminous and unstructured. 67
Two researchers coded each transcript individually. During this process, they checked each other’s coding and discussed refinements to the index. 66 Where it became clear that data were not sufficient to support a theme, categories were collapsed or where there were associations between themes, they were combined.
NVivo generates a complete list of quotations for each respondent under each theme heading, providing an overview of each theme while encapsulating the respondent’s contribution to it. Relevant data were retrieved using NVivo and reference was made to the original transcripts where necessary, to ensure that data from each respondent are not taken out of context or misunderstood. The data were then summarised under the theme headings, which included placing data for more complex themes into the framework using a Microsoft Excel spreadsheet (2010; Microsoft Corporation, Redmond, WA, USA), with the themes across the top and respondents listed down the left hand side. The aim of this process was to fill each cell with a summary of the relevant data, keeping as closely as possible to the original data by condensing its meaning and using respondent terminology as far as possible.
The next stage of the analysis was to pull together the key themes in the data to address the objective of this part of the study that is to understand the factors that influence outpatient attendance.
Phase 2c: community focus groups
The individual interviews were supplemented by community FGs. These groups were used to uncover alternative perceptions that may be articulated in a different, non-medicalised setting. Their stated purpose was to explore patients’ experiences, service preferences and perceived barriers to accessing HIV services.
For this part of the study, we considered recruiting up to four FGs of six to eight adults who did not attend the HIV clinic regularly or had stopped going to the clinic (now or in the past). We decided to recruit a total of three groups to represent the two main groups that are affected by HIV (gay men and black African men and women) and a third group to represent other people affected by HIV. These groups were to consist of:
-
gay and bisexual men
-
African men and women
-
non-African men and women.
Focus group recruitment
The FGs were facilitated by a UK Community Advisory Board (UK-CAB) representative and coinvestigator who recruited participants via community contacts (including the UK-CAB forum, YMCA, Positive East, Positively UK, African HIV Policy Network, African Eye Trust, Africa Advocacy Foundation, Terrence Higgins Trust, Body & Soul, NAZ, Organisation of HIV Positive African Men, GMFA and Metro). Posters were sent to community venues and those who were interested in taking part contacted MS directly. MS checked their eligibility according to a set of screening questions. They were eligible to take part if (i) they had not seen a doctor or nurse specialist at a HIV clinic for at least a year; or (ii) if they had ever not seen a doctor about their HIV for a year or more; or (iii) they had missed and not rescheduled at least one appointment at their HIV clinic in the past year. They were asked about their country of birth, ethnic group and sexual orientation but chose to participate in the FG with which they identified. As there were no volunteers for the non-African men and women’s group, this group was cancelled.
The two remaining groups took place in the evening in the Mortimer Market Centre, central London, in January 2015. They were jointly facilitated and refreshments were provided. As with the patient interviews, the information sheet was explained at the beginning of the group and participants had the opportunity to ask questions before signing the consent form. All participants were given £30 at the end of the group to cover expenses and as a token of thanks, and were offered a copy of the findings from the study.
Focus group content, processing and analysis
The FGs were based on a topic guide covering the same key topics as the patient interviews: link to services, experience of using current HIV clinic, reasons for attending regularly and irregularly, reasons for stopping attending, taking ART, stigma, social support, other NHS services. The gay and bisexual men’s group took just over an hour and the African men and women’s group took just over 2 hours. They were digitally recorded and transcribed verbatim.
It was intended that the FGs may serve to access people who fail to engage with medical services but continue to engage with an extended community network. However, all participants were currently engaged in HIV care and, as the data from the FGs were very similar to that from the individual interviews, they were combined for the purposes of analysis. The same index that had been developed for the interviews was applied to their coding and the same analysis procedures were adopted, as described above.
Phase 3: key informant study
We aimed to conduct semistructured interviews with up to 25 service providers and funders to explore ways to optimise patient engagement and potential costs. The sampling frame was defined according to key constituencies in the field of HIV service provision: clinical services, public health, academia, voluntary sector, health promotion and policy.
Key informant recruitment
Organisations and individuals within them were identified for each of the key constituencies. All prospective informants were approached by e-mail and, if they agreed, a 30-minute interview was arranged and conducted by telephone, Skype™ (Microsoft Corporation, Redmond, WA, USA) or face to face. All face-to-face interviews were conducted at mutually convenient locations. Participants were offered a copy of the interview transcript and a copy of the findings from the study. At the time of conducting the interviews, all participants were informed that their contribution would be anonymous. However, it became clear that some participants might prefer for their contribution to be acknowledged. They were therefore asked if they would prefer to be named in any publication of the findings and nine of the participants said that they would like to be identified.
Key informant content, processing and analysis
A topic guide was used to explore why patients miss appointments, what interventions or service improvements would help and what resources were needed (see Appendix 6). The interviews took about 30 minutes. Again, they were digitally recorded and transcribed verbatim.
As the key informant data were much shorter and less in-depth than the patient data – with participants talking about their professional experiences rather than in-depth and more lengthy discussion of their personal experiences – we used a more expedient method of analysis. Rather than coding all the transcripts in NVivo, we moved straight to summarising the data under theme headings that emerged from the data. AH conducted this analysis, reading and rereading the transcripts and organising the emerging data within the context of the COM-B model. In order to validate this analysis, FB and AE each read three transcripts (a total of six between them) and then examined the summary to see whether or not it fitted their understanding of the data.
Intervention costs
Using the methods described above, we put together four interventions that might be tested to improve engagement with care. We then undertook a preliminary evaluation of the costs of the four proposed interventions, after developing detailed descriptors of each. All costs were calculated according to 2015/16 GBP figures. Costs were calculated from a NHS and personal social services perspective. 68 The interventions would each incur a mixture of staff and non-staff costs. The former would include a range of staff types from consultants to unpaid peer support workers. The descriptors for each intervention included estimates of the amount of time each member of staff would spend on that intervention. To these we then applied unit costs per staff type obtained from published sources. 69 We valued the estimated non-staff costs (e.g. transport allowance, food allowance, telephone allowance, room hire, production of posters, leaflets and pocket guides) using market prices. We calculated the total cost of each intervention and then divided this by an estimate of the number of patients likely to receive the intervention to calculate the mean cost per patient. For three of the interventions, we calculated the costs over a 6-month period making assumptions about the number of contacts made by each patient during this time; for the fourth intervention, we calculated mean one-off costs per patient making assumptions about clinic size. The output is a series of indicative costs for each intervention, which can then be evaluated more formally in subsequent prospective studies.
Patient and public involvement
We have engaged with the public and patients at all stages of our project. Our patient and community engagement has been facilitated through the UK-CAB, a network for community HIV treatment advocates across the UK. Since the inception of the project, a representative from the UK-CAB has been involved as a co-applicant and member of our Management Team. She was responsible for recruiting PLWH to participate in our FGs and contributed to the design of the publicity material and the content of the FG topic guide. Our community representative and researcher/project manager jointly facilitated the FGs.
In addition, our Study Steering Committee and Advisory Group have both included community representatives who have contributed to the design and management of the research through these channels. They have provided valuable individual feedback our patient materials, including the content of the questionnaire and topic guides.
The questionnaire was also piloted on five PLWH and considerably revised on the basis of their input. We have interviewed three patient representatives who have contributed their expertise to the study as key informants.
At the time of writing, our findings have only recently been finalised but we will feed back to service user groups from the participating clinics over the coming months and lay summaries of our research findings will be available to patients attending for HIV care at participating sites. We will also host a dissemination and networking event for all key stakeholders in the coming months.
Chapter 3 Findings: patterns and associations with engagement in HIV care
In this chapter, we present findings from our analysis of UK CHIC data to explore patterns of engagement in HIV care, factors associated with poor engagement and the associated health and financial costs. We begin with the findings from the group-based trajectory analysis that we explored as a method for describing attendance patterns of attendance over time. The findings address the following objectives of the study, to:
-
examine HIV outpatient attendance patterns among PLWH
-
identify predictive factors of disengagement
-
investigate the potential health and financial costs of disengaging from care.
Group-based trajectory analysis of patterns of engagement
We examined patterns of attendance for patients who were divided up into three groups: 6110 patients diagnosed between 2000 and 2002; 6747 patients diagnosed between 2003 and 2005; and 5615 patients diagnosed between 2006 and 2008. We compared the fit of the models for plots with one to five trajectories for each of these three diagnosis periods using the BIC and examined the interpretability of the trajectory plots. A total of 15 models of attendance trajectories were examined (five models for each of the three diagnosis groups).
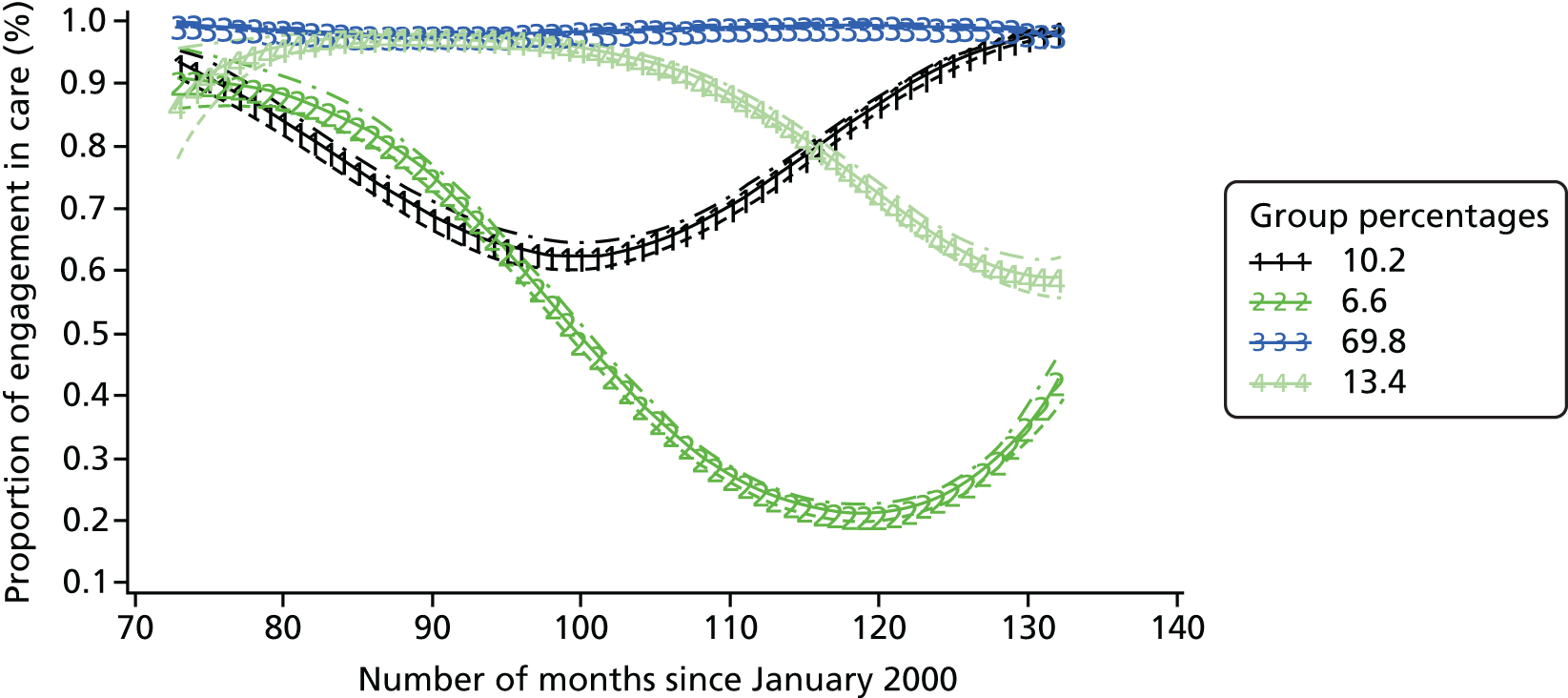
The patterns of trajectories for the five models (with one to five trajectories) were similar across diagnosis groups. The proportions of patients following these trajectories were also similar across the three diagnosis groups. Figures 3–5 illustrate the similarity of the shape of the trajectories and proportion of patients following each of the trajectories when models with four trajectories were specified. This similarity provides a measure of confidence in the robustness of the models. Although the lowest BIC indicates the best model, as the number of trajectories increased, the BIC gradually decreased for all three diagnosis groups, as did twice the difference between the BIC for the alternative (more complex) model and the null (simpler) model (Table 4). This method did not help to determine the best model for this analysis and we therefore selected the final models on the basis on interpretability.
FIGURE 3.
Attendance trajectories for four groups, diagnosed 2000–2.

FIGURE 4.
Attendance trajectories for four groups, diagnosed 2003–5.
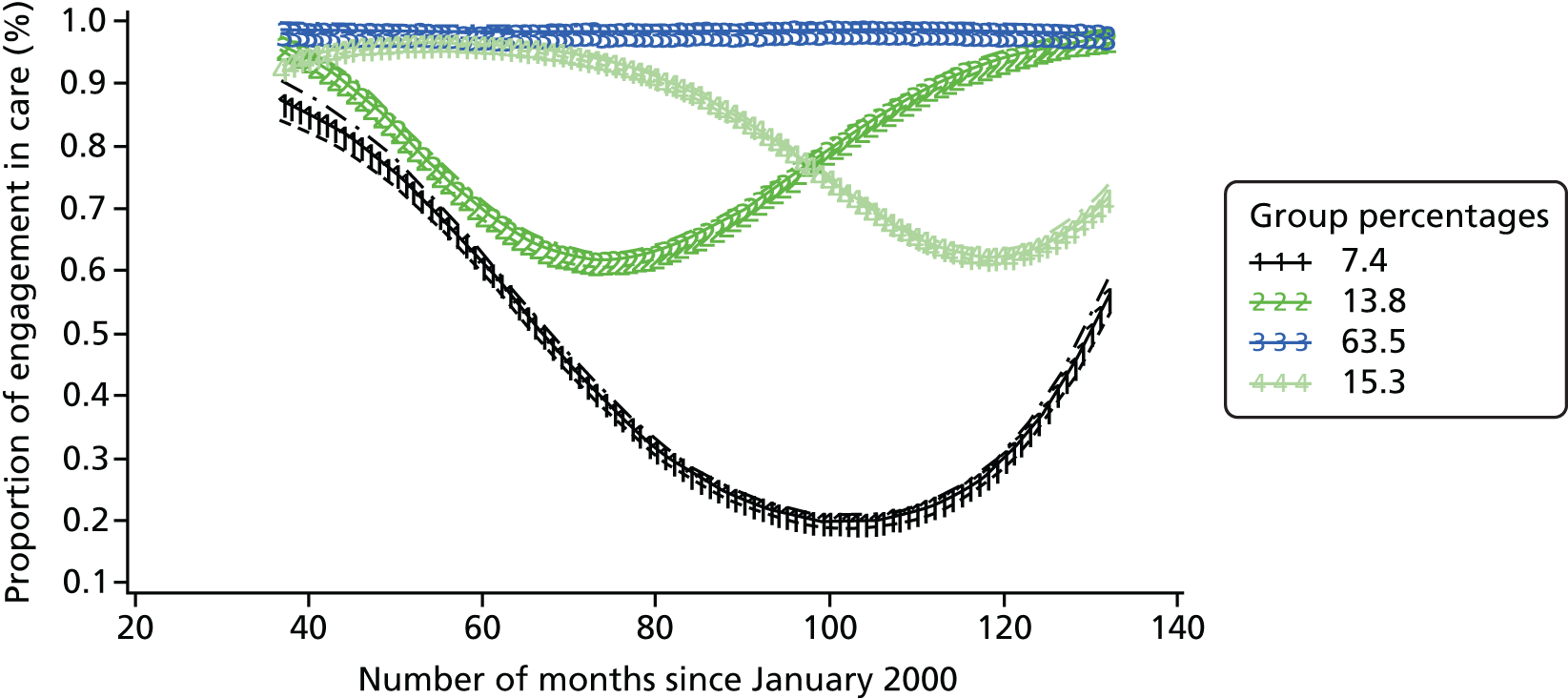
FIGURE 5.
Attendance trajectories for four groups, diagnosed 2006–8.
| Number of trajectories in model | Patients grouped according to time of diagnosis | |||||
|---|---|---|---|---|---|---|
| Diagnosed 2000–2 (n = 6110) | Diagnosed 2003–5 (n = 6747) | Diagnosed 2006–8 (n = 5615) | ||||
| BIC | 2 × diffa | BIC | 2 × diff | BIC | 2 × diff | |
| 1 | –161,085.9 | –128,142.0 | –55,608.4 | |||
| 2 | –122,569.4 | 77,033.0 | –97,366.4 | 61,551.3 | –41,745.1 | 27,726.6 |
| 3 | –114,602.5 | 15,933.8 | –91,340.6 | 12,051.4 | –39,447.8 | 4594.5 |
| 4 | –110,540.6 | 8123.8 | –88,026.2 | 6628.8 | –38,235.3 | 2425.1 |
| 5 | –108,123.9 | 4833.4 | –86,285.8 | 3480.9 | –37,924.3 | 621.9 |
We found that increasing the number of trajectories from four to five did not add to the interpretability and we therefore selected models with four trajectories (see Figures 3–5). The x-axis shows the number of months since the start of the analysis and the number on the y-axis is equivalent to the proportion of patients in care during each month. For all diagnosis periods, the largest group of patients consists of those who are consistently in care over time (62.3–69.8%). There is a second group which starts off in care and whose attendance drops somewhat over time, a third group whose attendance drops off more rapidly before getting better, and a fourth group which represents the smallest proportion of patients (6.6–8.2%) who appear to disengage from care almost completely with a very low proportion in care, before gradually returning to care.
At the end of the period of follow-up, the trajectories suggest that about one-fifth of patients from each of the diagnosis groups are not fully engaged in care and the longer the time since diagnosis, the greater the proportion of time in care at the end of our follow-up period.
Associations with the engagement in care measure
As described above, the group-based trajectory analysis resulted in the three different models shown in Figures 3–5. This makes their use in further analysis of associated factors and outcome problematic. We therefore decided to use the proportion of time in care as a more straightforward and fitting measure of engagement for the following analysis.
A total of 44,432 patients from UK CHIC (2000–12) were included in the initial analysis, as shown in Table 5. Women represented 27.8% of the sample. Half were white (53.3%), one-third were black African (28.9%), 8.7% were of other ethnicity and 9.2% had unknown ethnicity. Around half had acquired HIV through sex between men (50.5%), with 39.1% acquiring HIV through sex between men and women, 3.0% through injection drug use and the remaining 7.4% through other or unknown routes. Their median age at entry into the study was 36 years [interquartile range (IQR) 30–42 years] and the median start date of follow-up was December 2004 (range January 2000–October 2012). The median CD4 count at start of follow-up was 355 (IQR 214–520) cells/mm3; patients were followed for a median of 61 (range 2–156) months with a total follow-up of 3,021,224 patient-months.
| Characteristic | All patients | At ART start |
|---|---|---|
| n | 44,432 | 8730 |
| Gender, % | ||
| Male | 72.2 | 78.2 |
| Female | 27.8 | 21.8 |
| Age (years), median (IQR) | 36 (30–42) | 37 (32–43) |
| Exposure, % | ||
| MSM | 50.5 | 62.3 |
| Heterosexual | 39.1 | 31.1 |
| PWID | 3.0 | 2.9 |
| Other/unknown | 7.4 | 3.7 |
| Ethnic group, % | ||
| White | 53.3 | 63.4 |
| Black African | 28.9 | 20.9 |
| Other | 8.7 | 8.9 |
| Unknown | 9.2 | 6.8 |
| CD4 count (cells/mm3), median (IQR) | 355 (214–520) | 280 (202–368) |
Patients contributed 3,021,224 months of follow-up. Overall, patients were engaged in care for 83.9% of the total follow-up patient-months. Figure 6 shows that this was relatively stable over time and that women had consistently somewhat poorer EIC over time. Table 6 shows the proportion of months that were engaged in care stratified by the various demographic and clinical factors, as well as the results of univariable and multivariable regression models. In univariable analysis, EIC was higher in men, in those aged > 45 years, in those of white ethnicity, in those who acquired HIV through sex between men, in those with higher nadir and current CD4 counts, in later calendar years and in those who had only recently (within the last year) between first seen at the clinic. After adjustment for other factors in the model, most of these associations were unchanged with four main exceptions. First, there was no strong association between gender and EIC after controlling for the other factors. Second, although current use of ART did not appear to be associated with EIC in unadjusted analysis, after adjustment it became apparent that those currently on ART had higher levels of engagement. Third, a lower rate of EIC among those of black African origin was attenuated (and became non-significant) after adjustment, with lower rates of EIC only remaining in those of ‘other’ ethnic origins (which included those of black Caribbean, Asian and mixed race among others). Finally, including adjustment for the nadir CD4 count showed that current CD4 count did not provide any independent association with EIC.
FIGURE 6.
Engagement in care stratified by calendar year.
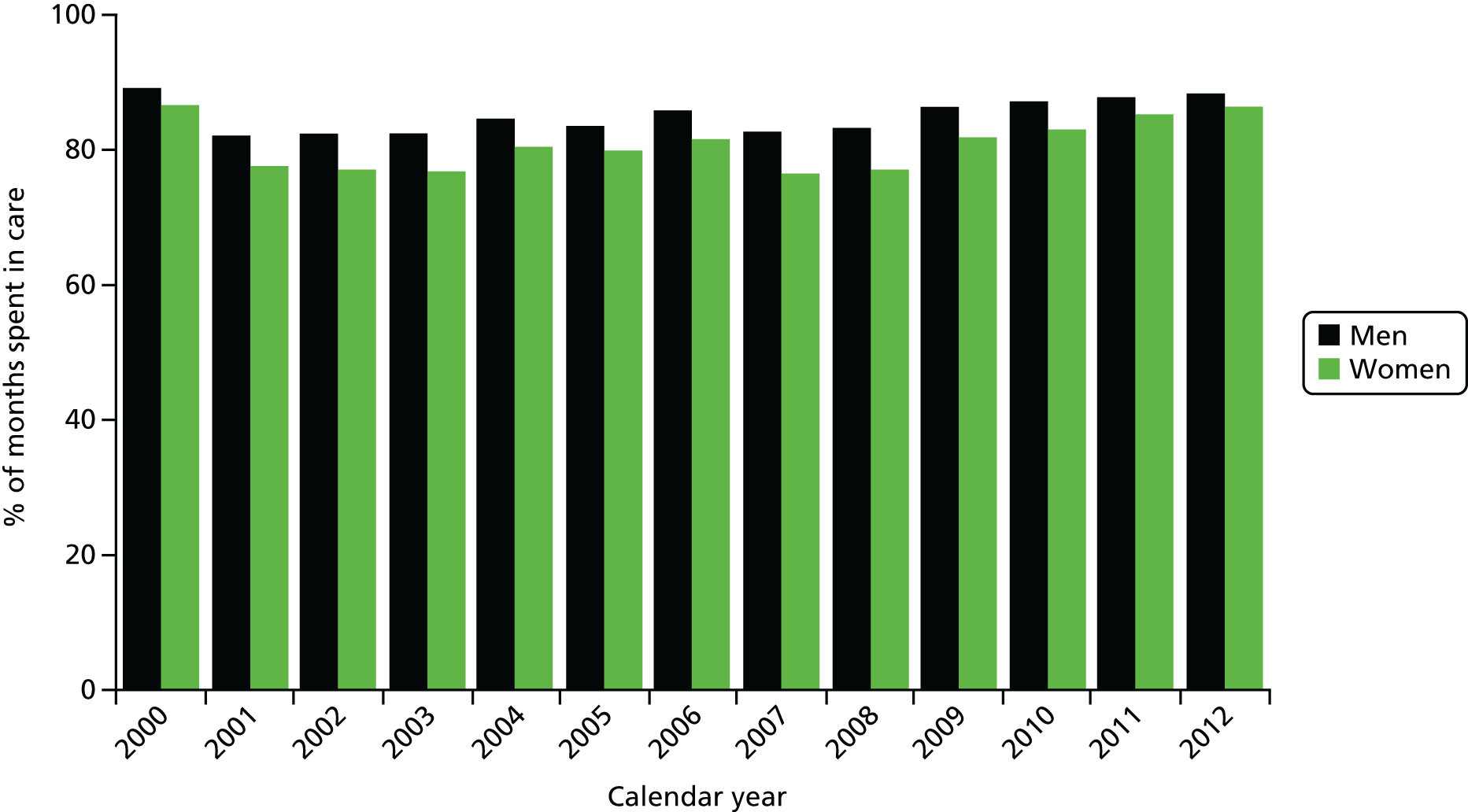
| Factor | Person-months | % EIC | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Gender | ||||||||
| Male | 2,235,135 | 85.1 | 1.36 | 1.31 to 1.40 | 0.0001 | 1.10 | 0.98 to 1.23 | 0.11 |
| Female | 786,089 | 80.7 | 1.00 | – | – | 1.00 | – | – |
| Age group (years) | ||||||||
| < 25 | 83,116 | 77.1 | 0.63 | 0.42 to 0.93 | 0.02 | 0.67 | 0.42 to 1.06 | 0.09 |
| 25–45 | 1,960,061 | 82.5 | 0.66 | 0.53 to 0.81 | 0.0001 | 0.74 | 0.59 to 0.93 | 0.008 |
| > 45 | 978,023 | 87.4 | 1.00 | – | – | 1.00 | – | – |
| Ethnic group | ||||||||
| White | 1,760,442 | 85.5 | 1.00 | – | – | 1.00 | – | – |
| Black African | 802,477 | 81.2 | 0.74 | 0.71 to 0.76 | 0.0001 | 0.96 | 0.83 to 1.11 | 0.55 |
| Other | 239,190 | 81.8 | 0.77 | 0.73 to 0.81 | 0.0001 | 0.79 | 0.68 to 0.92 | 0.002 |
| Unknown | 219,115 | 83.6 | 0.87 | 0.82 to 0.92 | 0.0001 | 0.87 | 0.73 to 1.03 | 0.11 |
| Route of acquisition | ||||||||
| MSM | 1,687,095 | 86.2 | 1.00 | – | – | 1.00 | – | – |
| PWID | 94,014 | 76.3 | 0.52 | 0.48 to 0.56 | 0.0001 | 0.56 | 0.44 to 0.71 | 0.0001 |
| Heterosexual | 1,127,473 | 81.4 | 0.70 | 0.68 to 0.73 | 0.0001 | 0.84 | 0.73 to 0.98 | 0.02 |
| Other/unknown | 112,642 | 82.2 | 0.77 | 0.71 to 0.83 | 0.0001 | 0.80 | 0.67 to 0.95 | 0.01 |
| Currently on ART | ||||||||
| No | 616,201 | 74.6 | 1.00 | – | – | 1.00 | – | – |
| Yes | 2,405,023 | 86.3 | 0.95 | 0.79 to 1.15 | 0.62 | 1.44 | 1.15 to 1.81 | 0.002 |
| Nadir CD4 count (cells/mm3) | ||||||||
| < 200 | 1,528,352 | 87.8 | 0.51 | 0.40 to 0.65 | 0.0001 | 0.55 | 0.41 to 0.74 | 0.0001 |
| 200–349 | 821,951 | 84.3 | 0.33 | 0.25 to 0.45 | 0.0001 | 0.37 | 0.28 to 0.50 | 0.0001 |
| ≥ 350 | 571,445 | 76.7 | 1.00 | – | – | 1.00 | – | – |
| Current CD4 count (cells/mm3) | ||||||||
| < 200 | 256,512 | 80.8 | 0.68 | 0.53 to 0.87 | 0.002 | 0.82 | 0.64 to 1.05 | 0.12 |
| 200–349 | 587,648 | 82.7 | 0.81 | 0.69 to 0.94 | 0.006 | 0.93 | 0.81 to 1.08 | 0.34 |
| ≥ 350 | 2,077,588 | 85.7 | 1.00 | – | – | 1.00 | – | – |
| Calendar year | ||||||||
| 2000–3 | 553,178 | 82.5 | 0.59 | 0.50 to 0.70 | 0.0001 | 0.61 | 0.51 to 0.72 | 0.00011 |
| 2004–7 | 1,500,392 | 85.2 | 1.00 | – | – | 1.00 | – | – |
| 2008–12 | 967,654 | 82.8 | 1.68 | 1.45 to 1.94 | 0.0001 | 1.71 | 1.47 to 1.98 | 0.0001 |
| Time since entry in UK CHIC (years) | ||||||||
| < 1 | 351,190 | 87.4 | 1.80 | 1.34 to 2.40 | 0.0001 | 1.53 | 1.09 to 2.15 | 0.01 |
| 1–5 | 1,137,979 | 82.5 | 1.19 | 0.89 to 1.59 | 0.24 | 1.24 | 0.89 to 1.71 | 0.20 |
| 5–10 | 1,020,656 | 83.4 | 1.22 | 0.92 to 1.61 | 0.16 | 1.13 | 0.85 to 1.51 | 0.41 |
| > 10 | 511,399 | 85.8 | 1.00 | – | – | 1.00 | – | – |
Health outcomes and the engagement in care measure
Table 7 shows the association between EIC and mortality, first without adjustment, then including fixed covariates (age, CD4, year of entry, gender, mode of acquisition and ethnic group), then adding ART status, then the lagged CD4 count and finally the unlagged CD4 count. Table 7 shows the same modelling process with the outcome of AIDS or death. The data with adjustment for fixed covariates and ART status show that higher EIC is associated with improved outcomes (for both the mortality and combined AIDS/mortality outcomes) at least 1 year into the future. Adjustment for the lagged CD4 count resulted in an attenuation of the association between EIC and each outcome, suggesting that a proportion of the association seen can be explained by the fact that those with lower EIC values also have lower CD4 counts. Further adjustment for the unlagged CD4 counts led to further attenuation of the estimate towards 1, suggesting that in addition to poorer CD4 counts at the time of EIC assessment, those with lower EIC values also had lower CD4 counts over the subsequent 12-month period.
| Mortality | AIDS/death | |
|---|---|---|
| Total number (%) of events | 2279 (5.1) | 6685 (15.1) |
| Adjustment for: | ||
| Nonea | 0.91 (0.88 to 0.95) | 0.89 (0.87 to 0.92) |
| Fixed covariatesa | 0.91 (0.88 to 0.95) | 0.89 (0.87 to 0.92) |
| + ART statusa | 0.90 (0.87 to 0.93) | 0.90 (0.87 to 0.92) |
| + latest CD4 count (lagged)a | 0.96 (0.92 to 1.00) | 0.93 (0.91 to 0.96) |
| + latest CD4 count (unlagged)a | 1.00 (0.96 to 1.04) | 0.97 (0.94 to 1.00) |
Next we explored EIC before starting ART among patients who started ART. Table 5 includes the characteristics of these 8730 individuals at the time that they started ART, with Table 8 showing associations between their pre-ART EIC and these characteristics. These data indicate that males are more likely than females to have spent a greater proportion of time in care before starting ART. The same was true for patients who acquired HIV through sex between men, were of white ethnicity, had a higher median CD4 count at start of ART and who started on a NNRTI regimen.
| % months EIC prior to ART | % of group | Male, % | MSM, % | White, % | CD4 (cells/mm3), median | Regimen | |
|---|---|---|---|---|---|---|---|
| PI, % | NNRTI, % | ||||||
| < 50 | 14.7 | 73.1 | 46.2 | 53.5 | 250 | 32.1 | 60.8 |
| 50–69.9 | 14.2 | 76.0 | 59.5 | 60.9 | 259 | 25.3 | 66.4 |
| 70–79.9 | 11.6 | 77.7 | 62.8 | 62.1 | 280 | 25.5 | 67.5 |
| 80–89.9 | 18.2 | 80.1 | 65.6 | 64.9 | 283 | 26.2 | 67.1 |
| 90–99.9 | 24.0 | 79.3 | 66.4 | 65.6 | 290 | 23.0 | 68.6 |
| 100 | 17.3 | 81.0 | 68.6 | 70.3 | 299 | 21.4 | 70.0 |
We examined the association between the proportion of time in care before starting ART and mortality after starting ART. Table 9 shows the association between pre-ART EIC and post-ART mortality, after adjusting for fixed covariates (age, gender, mode of acquisition, ethnic group, calendar year, pre-ART CD4 count and viral load), then adding the latest CD4 count and viral load. As in the previous analyses, the strong association between pre-ART EIC and post-ART mortality that is apparent in unadjusted analyses and analyses that control for the baseline covariates is substantially attenuated towards 1 after further adjustment for the post-ART CD4 counts and viral loads.
| Death | |
|---|---|
| Total number (%) | 237 (2.7) |
| No adjustmenta | 0.29 (0.18 to 0.47) |
| Adjustment for fixed covariatesa | 0.36 (0.21 to 0.61) |
| + latest CD4 count and VLa | 0.74 (0.42 to 1.30) |
Overall, the data from these analyses indicate that higher levels of engagement in HIV care are strongly associated with reduced mortality at all stages of infection.
Laboratory test costs and the engagement in care measure
The number of patients under follow-up declined over time in each EIC group, mainly due to the censoring date in the analysis of the UK CHIC data (Figure 7), but this may also be due to differential survival between groups (see Health outcomes and the engagement in care measure).
FIGURE 7.
Number of patients under follow-up by pre-ART EIC group and quarter since started ART.
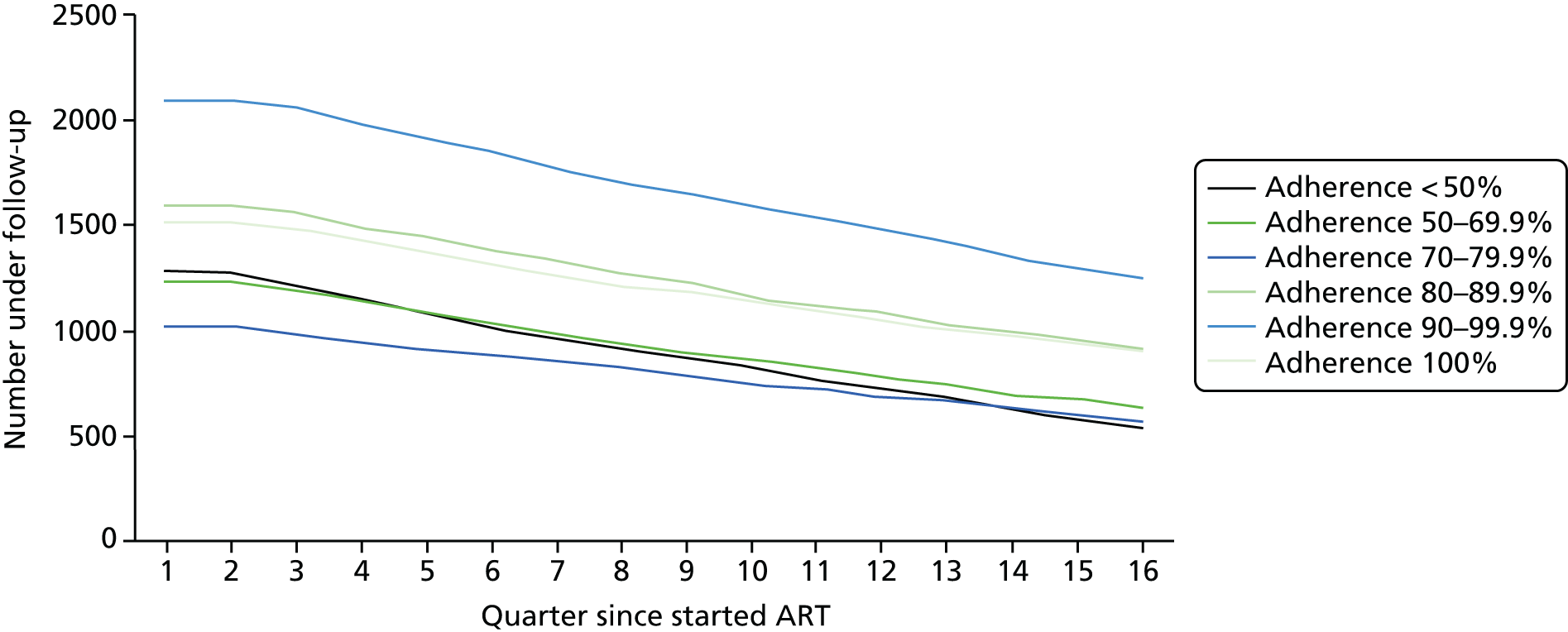
Figures 8 and 9 show the trends over time in mean CD4 count and viral load tests per person per quarter since starting ART, stratified by pre-ART EIC group (< 50%, 50–69.9%, 70–79.9%, 80–89.9%, 90–99.9% and 100%). Figures for the other tests are in Appendix 7. Note that separate unit costs were applied for cholesterol, high-density lipoprotein, low-density lipoprotein and triglycerides, but for simplicity we present a figure for all lipid tests combined (defined as when the patient had one or more of these tests on the same day).
FIGURE 8.
Cluster of differentiation 4 (CD4) count tests per person per quarter since started ART.

FIGURE 9.
Viral load tests per person per quarter since started ART.
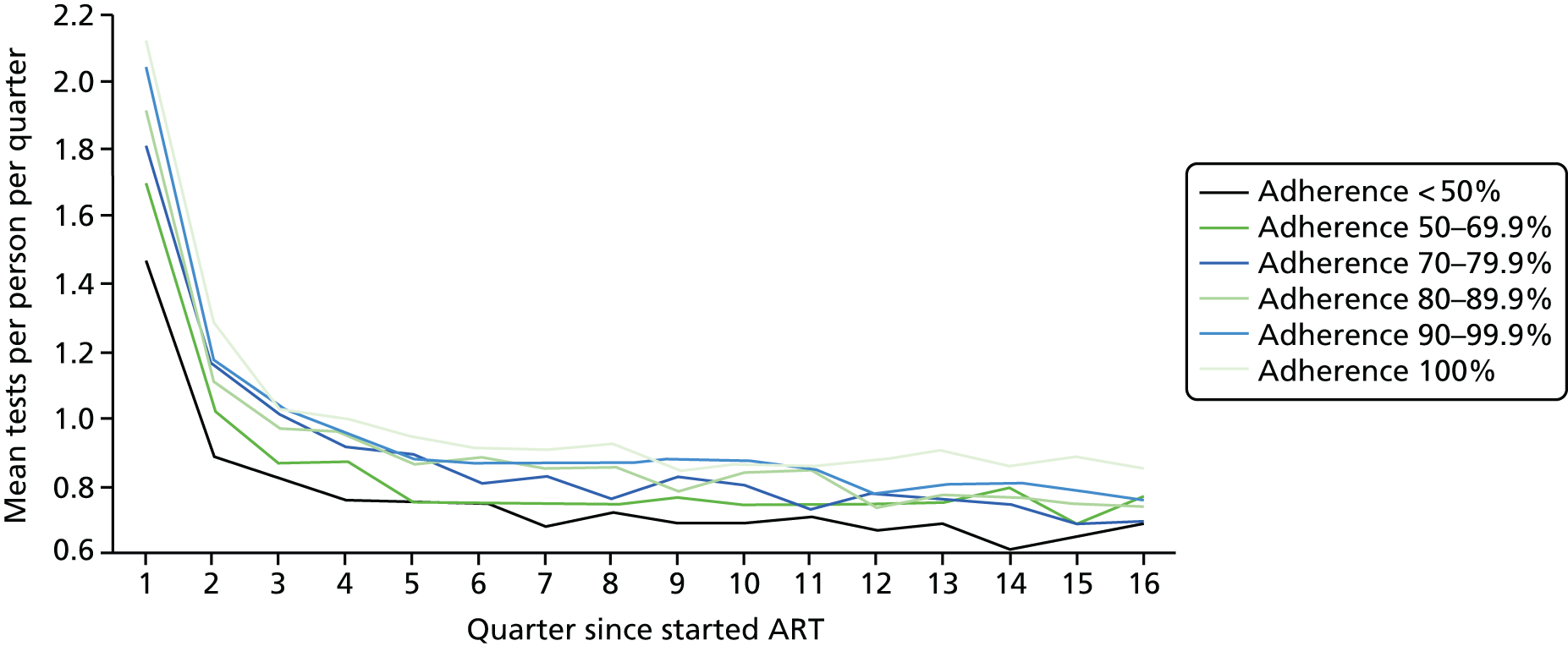
Trends are similar for the other types of test (see Appendix 7). Several themes emerge, and for most types of test the pattern is the same:
-
Across all tests and pre-ART EIC groups the number of tests per patient is highest in the first quarter and then declines thereafter, levelling out by quarters 9 and 10 after starting ART.
-
Generally there does not seem to be much variation in the number of tests per patient by pre-ART EIC group.
-
There is some suggestion that people in the most engaged groups have more tests per patient in each quarter, and those who are least engaged seem to have fewer – but the differences are small.
-
There does not seem to be any appreciable crossover between pre-ART EIC groups in later quarters.
Trends for test costs are in Figure 10; these reflect findings for the individual tests. As with the individual tests, costs are highest in the first quarter and then decline thereafter, levelling out by quarters 9 and 10. Mean costs per person in the 100% EIC group range from £131 in the first quarter after ART start to £50 per quarter in quarter 16. For the < 50% EIC group they range from £93 to £41, a difference of £38 and £9 per quarter compared with the 100% EIC group. There does not seem to be any crossover in test costs between pre-ART EIC groups in later quarters. In all groups and quarters, 80–85% of the test costs is accounted for by the cost of CD4 count and viral load tests.
FIGURE 10.
Mean test costs per person per quarter since started ART.
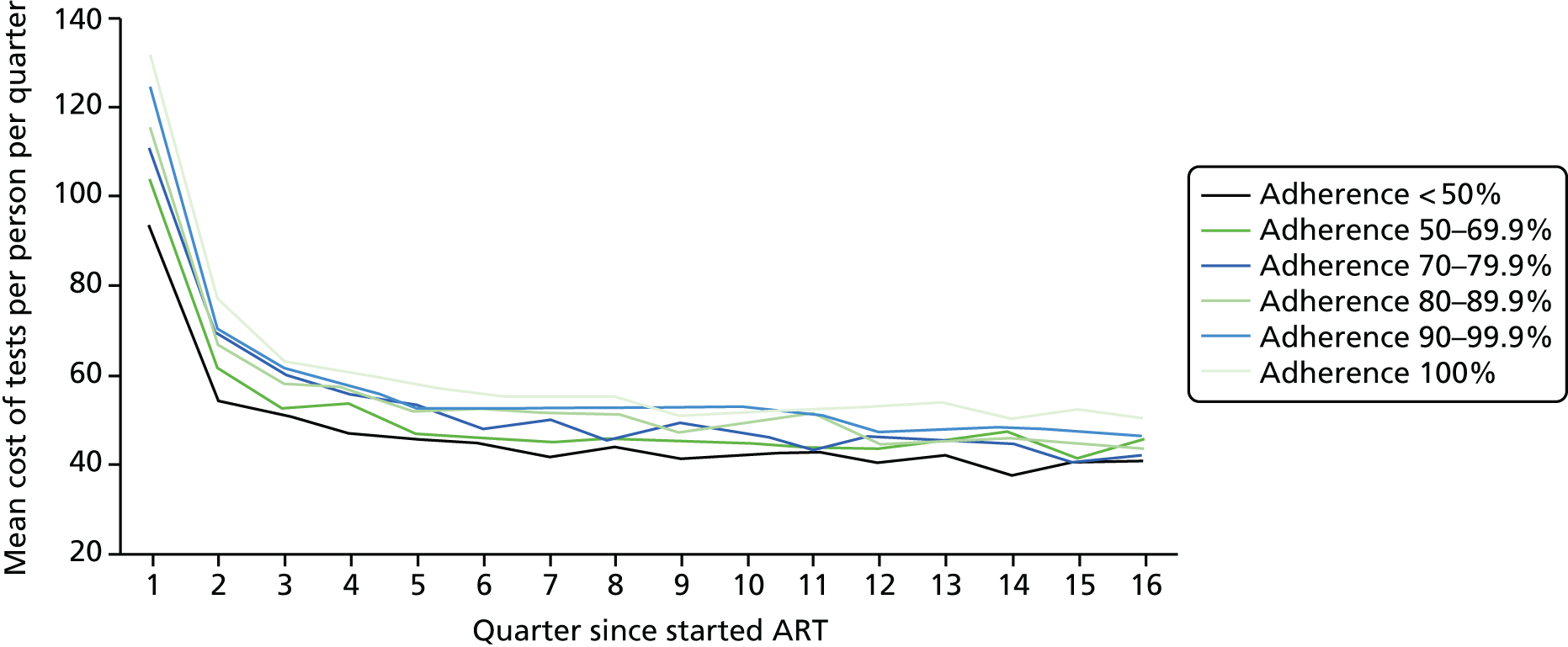
Overall these trends indicate that differences in testing costs between EIC groups are small in absolute terms and reflect pre-ART engagement with no crossover during the 4-year follow-up period under observation.
Summary of findings from Chapter 3
-
Group-based trajectory analysis indicated that four trajectories provide the best fit for the data for each of the three diagnosis groups.
-
Engagement in HIV care is higher in later calendar years, in those aged > 45 years, who acquired HIV through sex between men, with a higher nadir CD4 count, who had recently been first seen at the clinic and were currently on ART; there was some suggestion that EIC was lower in those of ‘other’ ethnicities (i.e. non-white, non-black African).
-
Engagement in HIV care is strongly associated with mortality at all stages of infection.
-
Absolute differences in the cost of lab tests by EIC are small.
Chapter 4 Findings: retention risk tool
In this chapter, we describe how the items for the retention risk tool were selected. The purpose of the tool is to identify newly diagnosed patients who are at risk of disengaging from care. The tool is for use in HIV care services and is one of the key outputs of the study. The chapter will address our fourth objective, to:
-
develop a retention risk assessment tool.
After excluding variables that were not suitable for inclusion in the analysis, 27 candidate variables remained. Table 10 is a list of all the variables that were considered for inclusion in the tool, grouped into blocks, and the proportion of RAs and not regular attenders (NRAs: IAs and NAs) according to each factor. It is possible that some variables, particularly from block 4 onwards, may change as a result of the physical and psychological effects of living with HIV, and it is therefore important that the retention risk tool should be tested in future studies to see how well it predicts future disengagement among newly diagnosed HIV patients.
| Factor | All patients, n | RA, % | NRA, % | p-value |
|---|---|---|---|---|
| Block 1: fixed sociodemographic variables | ||||
| Gender and sexual orientation | ||||
| Female | 271 | 24.8 | 32.4 | 0.027 |
| Heterosexual male | 122 | 12.7 | 12.7 | |
| Gay or bisexual male | 570 | 62.6 | 54.9 | |
| Ethnic group | ||||
| White | 518 | 57.2 | 48.9 | 0.010 |
| Other ethnic group | 449 | 42.8 | 51.1 | |
| Age group at diagnosis (years) | ||||
| ≤ 30 | 458 | 38.5 | 56.8 | < 0.001 |
| 31–45 | 422 | 47.8 | 36.7 | |
| ≥ 46 | 103 | 13.6 | 6.5 | |
| Born in the UK | ||||
| No | 582 | 59.9 | 58.4 | 0.635 |
| Yes | 400 | 40.1 | 41.6 | |
| Block 2: sociodemographic variables that are subject to change over time | ||||
| Education post 18 years old | ||||
| No | 276 | 27.1 | 33.3 | 0.043 |
| Yes | 650 | 72.9 | 66.7 | |
| Religion | ||||
| No | 316 | 31.7 | 33.7 | 0.510 |
| Yes | 653 | 68.3 | 66.3 | |
| Main language | ||||
| Not English | 196 | 21.5 | 18.3 | 0.224 |
| English | 780 | 78.5 | 81.7 | |
| Has children | ||||
| No | 668 | 72.8 | 63.3 | 0.001 |
| Yes | 306 | 27.2 | 36.7 | |
| Block 3: variables about circumstances of HIV diagnosis | ||||
| Where diagnosed | ||||
| GP | 98 | 10.6 | 9.7 | 0.914 |
| Sexual health clinic | 460 | 46.8 | 49.1 | |
| Hospital | 170 | 17.8 | 17.5 | |
| HIV testing service | 110 | 12.1 | 10.6 | |
| Elsewhere | 124 | 12.6 | 13.2 | |
| First recorded CD4 count (cells/mm3) | ||||
| < 200 | 262 | 28.8 | 25.5 | 0.432 |
| 200–349 | 234 | 23.2 | 26.0 | |
| ≥ 350 | 461 | 48.0 | 48.4 | |
| First recorded viral load (log-copies/ml) | ||||
| < 4.00 | 333 | 32.5 | 38.6 | 0.100 |
| 4.00–4.99 | 320 | 34.2 | 33.3 | |
| ≥ 5.00 | 293 | 33.3 | 28.0 | |
| Block 4: health, mental health and drug use, ever reported | ||||
| Long-standing condition affecting access | ||||
| No | 819 | 85.1 | 86.2 | 0.654 |
| Yes | 138 | 14.9 | 13.8 | |
| Ever injected drugs | ||||
| No | 832 | 88.9 | 83.8 | 0.022 |
| Yes | 128 | 11.1 | 16.2 | |
| Ever diagnosed with depression | ||||
| No | 617 | 70.9 | 64.4 | 0.039 |
| Yes | 290 | 29.1 | 35.6 | |
| Ever had inpatient stay | ||||
| No | 284 | 29.9 | 28.5 | 0.655 |
| Yes | 686 | 70.1 | 71.5 | |
| Block 5: mental health and drug use, within the past 5 years | ||||
| Recreational drug use (past 5 years) | ||||
| No | 597 | 66.9 | 56.6 | 0.001 |
| Yes | 360 | 33.1 | 43.4 | |
| Mental health issues (past year) | ||||
| No | 723 | 82.2 | 76.0 | 0.023 |
| Yes | 187 | 17.8 | 24.0 | |
| Drug/alcohol dependency (past year) | ||||
| No | 806 | 94.1 | 82.6 | < 0.001 |
| Yes | 99 | 5.9 | 17.4 | |
| Block 6: variables about potentially transient circumstances | ||||
| Caring responsibilities | ||||
| No | 725 | 77.4 | 72.0 | 0.052 |
| Yes | 241 | 22.6 | 28.0 | |
| Work status | ||||
| Working | 563 | 60.8 | 56.4 | 0.011 |
| Student | 45 | 3.4 | 6.4 | |
| Unemployed | 208 | 19.4 | 24.8 | |
| Other | 140 | 16.4 | 12.4 | |
| Money for basic needs | ||||
| Not all of the time | 553 | 50.5 | 65.1 | < 0.001 |
| All of the time | 419 | 49.5 | 34.9 | |
| Homeowner | ||||
| No | 702 | 66.7 | 79.0 | < 0.001 |
| Yes | 271 | 33.3 | 21.0 | |
| Cohabiting | ||||
| No | 627 | 62.4 | 70.2 | 0.012 |
| Yes | 326 | 37.6 | 29.8 | |
| Immigration status | ||||
| British citizen | 625 | 67.5 | 67.0 | 0.203 |
| EU citizen | 141 | 16.6 | 13.3 | |
| Indefinite leave to remain | 103 | 10.6 | 11.7 | |
| Temporary or no status | 60 | 5.2 | 8.0 | |
| Registered with GP | ||||
| No | 44 | 3.3 | 6.1 | 0.038 |
| Yes | 929 | 96.7 | 93.9 | |
| Block 7: variables about getting to the clinic | ||||
| Travelled to clinic by public transport | ||||
| No | 210 | 26.5 | 18.0 | 0.002 |
| Yes | 708 | 73.5 | 82.0 | |
| Journey > 30 minutes | ||||
| No | 374 | 41.8 | 35.1 | 0.035 |
| Yes | 588 | 58.2 | 64.9 | |
Variables from among the 27 candidates that were significantly associated (p < 0.05) with disengagement from HIV care, as shown in Table 10, were included in logistic regression modelling. We used backwards-stepwise binary logistic regression to select the best set of predictive variables associated with disengagement from care. To summarise, the following variables were considered. The proportion of missing data is shown in (brackets):
-
block 1: fixed sociodemographic variables
-
gender and sexual orientation (3.2%)
-
ethnic group (1.6%)
-
age at diagnosis (0.0%)
-
-
block 2: sociodemographic variables that are subject to change over time
-
education (5.8%)
-
has children (0.9%)
-
-
block 3: variables about circumstances of HIV diagnosis
-
none
-
-
block 4: health, mental health and drug use, ever reported
-
ever injected drugs (2.3%)
-
ever diagnosed with depression (7.7%)
-
-
block 5: mental health and drug use, within the past 5 years
-
recreational drug use (past 5 years) (2.6%)
-
mental health issues (past year) (7.4%)
-
drug/alcohol dependency (past year) (7.9%)
-
-
block 6: variables about potentially transient circumstances
-
work status (2.7%)
-
money for basic needs (1.1%)
-
homeowner (1.0%)
-
cohabiting (3.1%)
-
GP registered (1.0%)
-
-
block 7: variables about getting to the clinic
-
public transport (6.6%)
-
journey > 30 minutes (2.1%).
-
The 17 variables were introduced into the model using the above blocks. Across the 17 variables, missing cases accounted for 0.0–7.9% of the data. Although this is not a large number of missing data for univariable analysis, incorporation of several variables with this level of missing data into multivariable analysis can result in a reduced sample size and unstable models. Therefore, if variables were not significantly associated with disengagement from HIV care when introduced within their block into the model, they were not included as the subsequent block of variables was introduced.
Table 11 shows the changing values of the –2LL, the pseudo-R2 and the c-statistic, as the blocks of variables are added to the model and Table 12 includes the descriptive data and the odds ratios for variables included in each of the models.
| Model | Number of cases | Number of variables | –2LL | Pseudo-R2 | c-statistic | Hosmer–Lemeshow p-value |
|---|---|---|---|---|---|---|
| Block 1 | 983 | 1 | 1295.83 | 0.05 | 0.63 | 0.35 |
| + block 2 | 921 | 3 | 1192.99 | 0.07 | 0.66 | 0.68 |
| + block 3 | – | – | – | – | – | – |
| + block 4 | 952 | 3 | 1230.21 | 0.08 | 0.66 | 0.21 |
| + block 5 | 872 | 4 | 1094.14 | 0.11 | 0.69 | 0.97 |
| + block 6 | 864 | 5 | 1072.66 | 0.12 | 0.70 | 0.83 |
| + block 7 | 813 | 6 | 999.55 | 0.13 | 0.71 | 0.54 |
| Factor | % RA (mean) | % NRA (mean) | aOR | 95% CI | p-value |
|---|---|---|---|---|---|
| Block 1 | |||||
| Age at diagnosis | (34.9) | (30.3) | 0.95 | 0.94 to 0.97 | < 0.001 |
| + block 2 | |||||
| Age at diagnosis (years) | (34.9) | (30.3) | 0.95 | 0.94 to 0.96 | < 0.001 |
| No post-18 years education | 27.1 | 33.3 | 1.36 | 1.01 to 1.83 | .042 |
| Has children | 27.2 | 36.7 | 1.85 | 1.37 to 2.48 | < 0.001 |
| + block 4 | |||||
| Age at diagnosis (years) | (34.9) | (30.3) | 0.95 | 0.94 to 0.96 | < 0.001 |
| Has children | 27.2 | 36.7 | 1.98 | 1.47 to 2.65 | < 0.001 |
| Ever injected drugs | 11.1 | 16.2 | 1.79 | 1.20 to 2.67 | 0.004 |
| + block 5 | |||||
| Age at diagnosis (years) | (34.9) | (30.3) | 0.95 | 0.94 to 0.97 | < 0.001 |
| Has children | 27.2 | 36.7 | 2.37 | 1.71 to 3.28 | < 0.001 |
| Recreational drug use | 33.1 | 43.4 | 1.53 | 1.10 to 2.13 | 0.011 |
| Drug/alcohol dependency | 5.9 | 17.4 | 2.88 | 1.74 to 4.76 | < 0.001 |
| + block 6 | |||||
| Age at diagnosis (years) | (34.9) | (30.3) | 0.95 | 0.94 to 0.97 | < 0.001 |
| Has children | 27.2 | 36.7 | 2.13 | 1.52 to 2.99 | < 0.001 |
| Recreational drug use | 33.1 | 43.4 | 1.65 | 1.18 to 2.30 | 0.004 |
| Drug/alcohol dependency | 5.9 | 17.4 | 2.70 | 1.62 to 4.52 | < 0.001 |
| No money for basic needs | 50.5 | 65.1 | 1.47 | 1.08 to 1.99 | 0.014 |
| + block 7: the final model | |||||
| Age at diagnosis (years) | (34.9) | (30.3) | 0.95 | 0.94 to 0.97 | < 0.001 |
| Has children | 27.2 | 36.7 | 2.20 | 1.55 to 3.13 | < 0.001 |
| Recreational drug use | 33.1 | 43.4 | 1.76 | 1.24 to 2.49 | 0.001 |
| Drug/alcohol dependency | 5.9 | 17.4 | 2.41 | 1.43 to 4.07 | 0.001 |
| No money for basic needs | 50.5 | 65.1 | 1.40 | 1.02 to 1.93 | 0.038 |
| Public transport to clinic | 73.5 | 82.0 | 1.66 | 1.15 to 2.41 | 0.007 |
The final model has a –2LL of 999.55, a pseudo-R2 of 0.13 and the AUROC curve is 0.71, which is considered as fair (Figure 11). All these measures indicate that this was the best model achieved during the testing process. The Hosmer–Lemeshow goodness-of-fit test indicates that the data predicted by the model were not significantly different from the observed data (p = 0.54). The equation for this model, using the beta value from the analysis rather than the odds ratio values that are included in Table 12, is:
FIGURE 11.
Receiver operating curve for the final model.
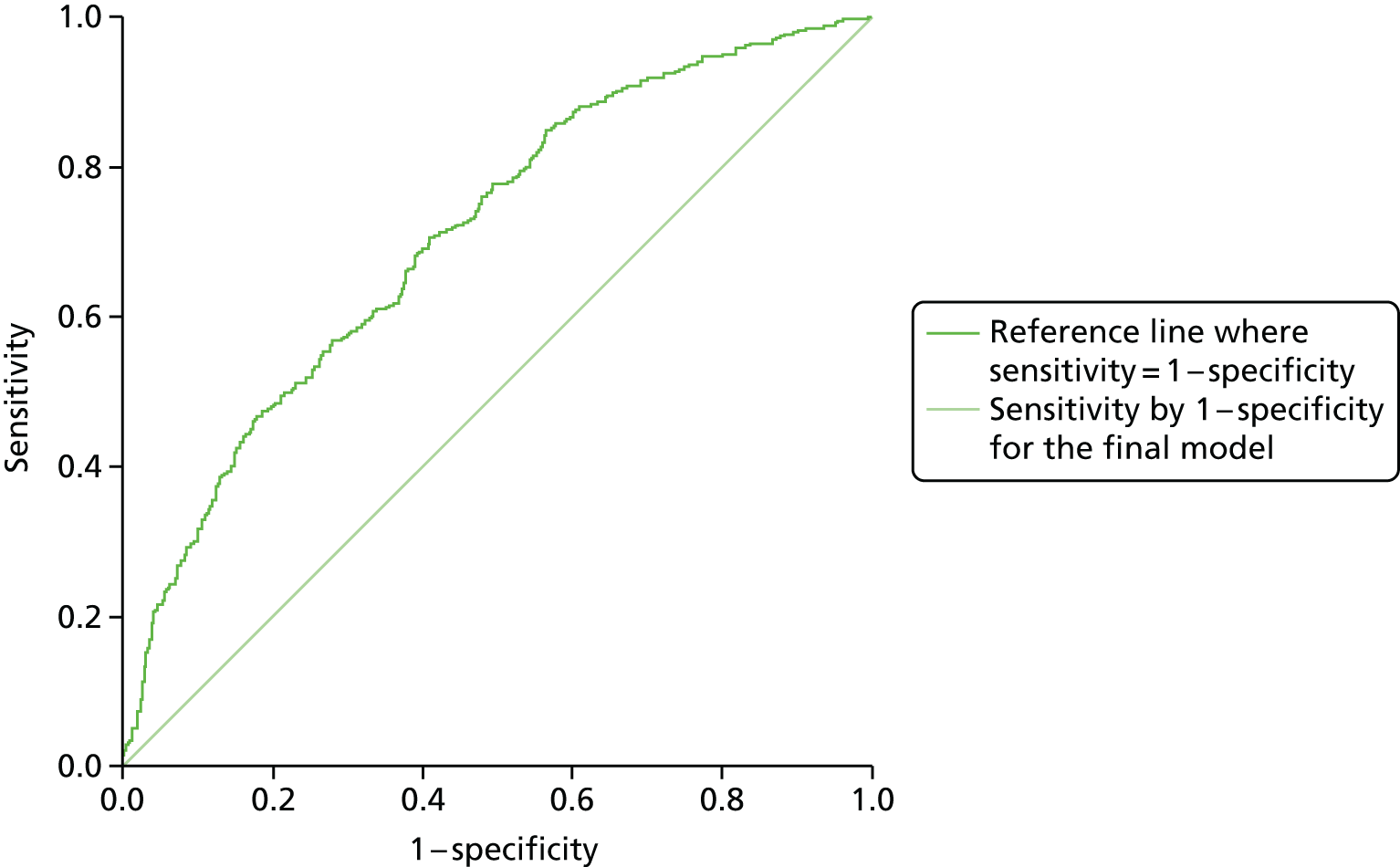
Summary of findings from Chapter 4
-
Our retention risk tool indicates that people who are newly diagnosed with HIV may be at greater risk of disengaging from care if they are younger at the time of diagnosis, have children, have used recreational drugs, have drug/alcohol dependency issues, do not have not enough money for basic needs and use public transport to get to the clinic.
Chapter 5 Findings from survey: factors associated with engagement in HIV care
In this chapter, we examine the data from our survey of HIV patients to explore the factors associated with engagement in HIV care. The findings from this analysis informed the development of intervention models to improve EIC that are described in Chapter 7. The findings address the following objective of the study, to:
-
understand the situational, environmental, behavioural and social factors influencing outpatient attendance.
Respondents
The sample included a total of 983 respondents. It was made up of 550 RAs (56.0%), 269 IAs (27.4%) and 164 NAs (16.7%). Although we succeeded in recruiting our target samples of 500 RAs and 250 IAs, we were unable to reach our target of 250 NAs. Despite including two additional recruitment sites and encouraging clinics to recruit NAs, this group was very hard to reach. NAs form a small minority of the patient population, which makes them statistically hard to recruit, with one of our participating clinics estimating that NAs constituted only 1–2% of the clinic cohort. They are also patients who have experienced difficulties with engaging in HIV care and need to be treated sensitively, which may make them more difficult to recruit into the study. In order to check whether or not a smaller sample would be sufficient, we recalculated the statistical power for a more realistic and achievable sample size, which indicated that there would be a small impact on statistical power: comparing 150 non-attending patients with 500 regularly attending patients would provide over 80% power to detect a difference in the prevalence of a predictor of 57% versus 43% (83% power) or 14% versus 6% (81% power).
The participating clinics had different success rates at achieving a sample of 200 questionnaires completed by 100 RAs (50%), 50 IAs (25%) and 50 NAs (25%), as shown in Table 13. There was a significant difference between the proportion of questionnaires completed by RAs, IAs and NAs at each of the clinics (p < 0.001). Clinics 4 and 5 achieved samples of < 100 respondents who were mainly RAs. Clinics 1, 3 and 7 did not reach their targets of 200 respondents but were more successful at recruiting IAs and NAs. Clinics 2 and 6 reached their targets of 200 respondents, which were divided up according the proportions required. It should be noted that clinic 2 was working towards an earlier target of 40% RAs, 30% IAs and 30% NAs, before this was revised to proportions of 50%, 25% and 25%, respectively.
| Factor | All patients | RA, % | IA, % | NA, % | p-value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Clinic | ||||||
| 1 | 95 | 9.7 | 60.0 | 22.1 | 17.9 | < 0.001 |
| 2 | 207 | 21.1 | 38.6 | 34.3 | 27.1 | |
| 3 | 149 | 15.2 | 66.4 | 25.5 | 8.1 | |
| 4 | 70 | 7.1 | 75.7 | 12.9 | 11.4 | |
| 5 | 83 | 8.4 | 77.1 | 12.0 | 10.8 | |
| 6 | 226 | 23.0 | 48.7 | 27.4 | 23.9 | |
| 7 | 153 | 15.6 | 56.9 | 37.9 | 5.2 | |
Men who identified as gay or bisexual represented 59.2% of the sample; 12.7% were heterosexual men and 28.1% were female. The mean age was 44.5 years [standard deviation (SD) 10.4 years]. Over half of the respondents reported white ethnicity (53.6%), 28.1% were black African and 18.3% were of another ethnicity. The majority were born outside the UK (59.3%) including 30.9% in Africa, 16.3% in another European country and 7.9% in the Americas, but only 20.1% did not speak English as their main language. Among those born outside the UK, two-thirds had arrived in the UK since 1995, with 23.7% having arrived since 2005 and a further 44.1% between 1995 and 2004. Most had completed some full-time education after the age of 18 years (70.2%) and 58.9% reported that they were employed, 21.8% were unemployed and 4.7% were students. We identified the score of the Index of Multiple Deprivation for the neighbourhood in which respondents lived, based on their postcode. Only 56.2% of respondents provided a usable postcode but among them, 43.5% of respondents lived in a neighbourhood that was among the most deprived (quintile 5) in England, and a further 37.9% lived in a neighbourhood that was somewhat less deprived (quintile 4). Two-thirds of respondents (67.4%) reported belonging to a religion, most of whom were Christian (87.0%).
Factors associated with attendance pattern
In the following sections we examine the association between the three attendance patterns used to recruit respondents with the responses that patients reported in our questionnaire and the clinical data that were collected from their notes.
Background factors
The associations between background factors and attendance pattern are shown in Table 14. Just over a quarter of respondents were female (27.6%) and IAs and NAs were more likely than RAs to be female (IA 30.5% and NA 34.1% vs. RA 24.2%; p = 0.020). There was a significant association with age, with RAs more likely to be in the older age group (≥ 45 years, 53.3%) than IAs (40.9%) or NAs (37.8%) (p < 0.001) and IAs and NAs more likely to be younger. There was no significant difference between attendance groups when comparing white ethnicity, black African and other ethnicity, but RAs were more likely to be white than any other ethnicity (RA 57.2% vs. IA 50.0% and NA 47.2%; p = 0.032). NAs were more likely to have left full-time education by the age of 18 years (38.8%) than RAs (27.1%) or IAs (29.9%) (p = 0.021). There were no significant associations between attendance and sexual orientation, region of birth, year of arrival in the UK or religion.
| Factor | All patients | RA, % | IA, % | NA, % | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Gender | ||||||
| Male | 712 | 72.4 | 75.8 | 69.5 | 65.9 | 0.020 |
| Female | 271 | 27.6 | 24.2 | 30.5 | 34.1 | |
| Age group (years) | ||||||
| ≤ 29 | 86 | 8.7 | 6.0 | 13.0 | 11.0 | < 0.001 |
| 30–44 | 432 | 43.9 | 40.7 | 46.1 | 51.2 | |
| ≥ 45 | 465 | 47.3 | 53.3 | 40.9 | 37.8 | |
| Ethnic group | ||||||
| White | 518 | 53.6 | 57.2 | 50.0 | 47.2 | 0.138 |
| Black African | 272 | 28.1 | 25.9 | 29.9 | 32.5 | |
| Other | 177 | 18.3 | 16.9 | 20.1 | 20.2 | |
| Sexual orientation | ||||||
| Heterosexual | 351 | 37.9 | 35.1 | 40.0 | 43.9 | 0.106 |
| Gay or bisexual | 576 | 62.1 | 64.9 | 60.0 | 56.1 | |
| Gender and sexual orientation | ||||||
| Female | 271 | 28.1 | 24.8 | 30.8 | 35.0 | 0.069 |
| Heterosexual male | 122 | 12.7 | 12.7 | 12.0 | 13.8 | |
| Gay or bisexual male | 570 | 59.2 | 62.6 | 57.1 | 51.2 | |
| Region of birth | ||||||
| UK | 400 | 40.9 | 40.1 | 42.7 | 40.7 | 0.503 |
| Europe (non-UK) | 159 | 16.3 | 16.6 | 15.4 | 16.7 | |
| Americas | 77 | 7.9 | 8.6 | 7.9 | 5.6 | |
| Africa | 302 | 30.9 | 29.5 | 31.8 | 34.0 | |
| Western Pacific | 24 | 2.5 | 3.1 | 1.9 | 1.2 | |
| Eastern Mediterranean | 6 | 0.6 | 0.9 | 0.4 | 0.0 | |
| South-east Asia | 10 | 1.0 | 1.3 | 0.0 | 1.9 | |
| Year of arrival in UK | ||||||
| 1984 and before | 37 | 7.4 | 9.6 | 3.7 | 6.2 | 0.216 |
| 1985–94 | 123 | 24.7 | 22.0 | 28.4 | 28.4 | |
| 1995–2004 | 219 | 44.1 | 44.7 | 41.0 | 46.9 | |
| 2005–14 | 118 | 23.7 | 23.8 | 26.9 | 18.5 | |
| Education post 18 years old | ||||||
| No | 276 | 29.8 | 27.1 | 29.9 | 38.8 | 0.021 |
| Yes | 650 | 70.2 | 72.9 | 70.1 | 61.2 | |
| Religion | ||||||
| No | 316 | 32.6 | 31.7 | 35.0 | 31.7 | 0.630 |
| Yes | 653 | 67.4 | 68.3 | 65.0 | 68.3 | |
In multivariable analysis, we used the continuous variable for age, with the adjusted odds ratio (aOR) relating to each 1-year increase in age. Adjusting for the clinic where respondents were recruited, indicated that increasing age was associated with decreasing likelihood of irregular attendance [aOR 0.96, 95% confidence interval (CI) 0.95 to 0.98; p < 0.001] and being female was associated with increased likelihood of irregular attendance (aOR = 1.94, 95% CI 1.32 to 2.87; p = 0.001). Increasing age was also associated with decreasing likelihood of non-attendance (aOR 0.96, 95% CI 0.95 to 0.98; p < 0.001), whereas being female (aOR 2.29, 95% CI 1.46 to 3.61; p < 0.001) and lower education (aOR 1.81, 95% CI 1.21 to 2.71; p = 0.004) were associated with increased likelihood of non-attendance.
Factors relating to HIV diagnosis and health service use
The associations between factors relating to HIV diagnosis and health service use are shown in Table 15. Three-quarters of the respondents had been diagnosed with HIV for > 5 years, with over half diagnosed for ≥ 10 years (53.2%) and a quarter (23.7%) diagnosed for 5–10 years. There was a significant association between time since diagnosis and attendance. IAs and NAs were more likely to have been diagnosed for ≥ 10 years than RAs (IA 59.5% and NA 55.5% vs. RA 49.5%; p = 0.002). One-quarter of women had been diagnosed with HIV during pregnancy (25.3%), but this was not significantly associated with attendance. Neither was place of diagnosis related to attendance, with about half of respondents diagnosed at a sexual health clinic (47.8%), 17.7% in hospital, 11.4% at a HIV testing service and 10.2% at the GP.
| Factor | All patients | RA, % | IA, % | NA, % | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Time since diagnosis (years) | ||||||
| < 1 | 37 | 3.8 | 5.6 | 2.2 | 0.0 | 0.002 |
| 1–5 | 190 | 19.3 | 20.4 | 19.0 | 16.5 | |
| 5–10 | 233 | 23.7 | 24.5 | 19.3 | 28.0 | |
| > 10 | 523 | 53.2 | 49.5 | 59.5 | 55.5 | |
| Diagnosis in pregnancy | ||||||
| No | 201 | 74.7 | 76.3 | 78.0 | 66.1 | 0.237 |
| Yes | 68 | 25.3 | 23.7 | 22.0 | 33.9 | |
| Where diagnosed | ||||||
| GP | 98 | 10.2 | 10.6 | 10.7 | 8.0 | 0.857 |
| Sexual health clinic | 460 | 47.8 | 46.8 | 50.0 | 47.5 | |
| Hospital | 170 | 17.7 | 17.8 | 16.0 | 19.8 | |
| HIV testing service | 110 | 11.4 | 12.1 | 11.5 | 9.3 | |
| Elsewhere | 124 | 12.9 | 12.6 | 11.8 | 15.4 | |
| Attended one or more HIV clinics | ||||||
| No | 671 | 69.9 | 72.6 | 66.4 | 66.5 | 0.118 |
| Yes | 289 | 30.1 | 27.4 | 33.6 | 33.5 | |
| Years at current clinic | ||||||
| < 1 | 63 | 7.0 | 8.6 | 3.6 | 7.2 | 0.241 |
| 1 to < 5 | 241 | 26.8 | 27.3 | 27.0 | 24.5 | |
| 5 to < 10 | 226 | 25.1 | 24.6 | 24.6 | 28.1 | |
| ≥ 10 | 369 | 41.0 | 39.5 | 44.8 | 40.3 | |
| How often expects routine consultations | ||||||
| Every month | 50 | 5.3 | 3.6 | 7.7 | 7.0 | < 0.001 |
| Every 3 months | 257 | 27.2 | 22.3 | 37.5 | 26.6 | |
| Every 4 months | 121 | 12.8 | 13.8 | 13.5 | 8.2 | |
| Every 6 months | 456 | 48.2 | 53.7 | 38.6 | 45.6 | |
| Every year | 62 | 6.6 | 6.6 | 2.7 | 12.7 | |
| Recommendation of clinic | ||||||
| Extremely likely | 753 | 80.6 | 80.8 | 80.2 | 80.5 | 0.854 |
| Likely | 143 | 15.3 | 15.6 | 14.2 | 16.2 | |
| Neither | 21 | 2.2 | 2.1 | 3.2 | 1.3 | |
| Unlikely | 17 | 1.8 | 1.5 | 2.4 | 1.9 | |
| Registered with GP | ||||||
| No | 44 | 4.5 | 3.3 | 3.0 | 11.0 | < 0.001 |
| Yes | 929 | 95.5 | 96.7 | 97.0 | 89.0 | |
| Last attended GP | ||||||
| In past year | 739 | 79.5 | 80.5 | 81.7 | 72.7 | 0.020 |
| 1–5 years ago | 141 | 15.2 | 14.8 | 14.7 | 17.3 | |
| > 5 years ago | 29 | 3.1 | 2.1 | 2.8 | 7.3 | |
| Never | 20 | 2.2 | 2.7 | 0.8 | 2.7 | |
| At last visit | ||||||
| Booked appointment | ||||||
| No | 93 | 10.3 | 9.7 | 8.9 | 14.5 | 0.162 |
| Yes | 806 | 89.7 | 90.3 | 91.1 | 85.5 | |
| Routine consultation | ||||||
| No | 102 | 12.2 | 10.0 | 11.9 | 21.3 | 0.003 |
| Yes | 732 | 87.8 | 90.0 | 88.1 | 78.7 | |
| Felt unwell | ||||||
| No | 642 | 84.7 | 86.4 | 83.8 | 80.3 | 0.226 |
| Yes | 116 | 15.3 | 13.6 | 16.2 | 19.7 | |
| Advice about HIV | ||||||
| Very important | 566 | 64.6 | 66.5 | 60.1 | 65.4 | 0.319 |
| Somewhat important | 173 | 19.7 | 18.0 | 21.9 | 22.2 | |
| Not important | 137 | 15.6 | 15.5 | 18.0 | 12.4 | |
| Other advice | ||||||
| Very important | 322 | 38.7 | 38.4 | 42.5 | 33.6 | 0.373 |
| Somewhat important | 252 | 30.3 | 29.2 | 29.2 | 35.7 | |
| Not important | 258 | 31.0 | 32.4 | 28.3 | 30.7 | |
| Practical support | ||||||
| Very important | 170 | 21.4 | 19.9 | 24.2 | 21.7 | 0.687 |
| Somewhat important | 115 | 14.4 | 13.9 | 14.4 | 16.3 | |
| Not important | 511 | 64.2 | 66.2 | 61.4 | 62.0 | |
| More HIV medication | ||||||
| Very important | 645 | 73.0 | 73.8 | 79.6 | 59.0 | < 0.001 |
| Somewhat important | 92 | 10.4 | 9.0 | 10.4 | 15.3 | |
| Not important | 147 | 16.6 | 17.2 | 10.4 | 25.7 | |
The majority of respondents had only ever attended one HIV clinic (69.9%), with two-thirds having attended their current clinic for at least 5 years, including 41.0% who had attended the same clinic for ≥ 10 years. There was no significant association between number of clinics attended or number of years at current clinic and attendance pattern. Respondents were asked how often they expected to have a routine consultation at their HIV clinic. Just over half the RAs expected 6-monthly visits (RA 53.7% vs. IA 38.6% and NA 45.6%), whereas half of the IAs expected visits every 3–4 months (IA 50.1% vs. RA 36.1% and NA 34.8%); NAs were the most likely to expect yearly visits (NA 12.7% vs. RA 6.6% and IA 2.7%; p < 0.001). Most respondents were either extremely likely (80.6%) or likely (15.3%) to recommend their clinic and this did not vary significantly between attendance patterns.
Although the majority of respondents were registered with a GP (95.5%), NAs were less likely to be registered (NA 89.0% vs. RA 96.7% and IA 97.0%; p < 0.001). Similarly, RAs and IAs were more likely to have attended their GP in the last year (RA 80.5% and IA 81.7%) than NAs (72.7%; p = 0.020).
We were interested in what brought the respondents into the clinic and we asked them about the circumstances and purpose of their last appointment. The majority had a booked appointment (89.7%) and reported that they did not feel unwell (84.7%). These circumstances were not significantly associated with attendance pattern. However, RAs and IAs were more likely than NAs to be attending a routine consultation at last visit than NAs (RA 90.0% and IA 88.1% vs. NA 78.7%; p = 0.003). Needing more HIV medication was reported as a very important reason for attending (73.0% of respondents) compared with wanting advice about HIV (64.6%), wanting other advice (38.7%) or needing practical support (21.4%). Reasons for attending did not vary significantly by attendance pattern except for medication requirement, with RAs and IAs more likely to need medication than NAs (RA 73.8% and IA 79.6% vs. NA 59.0%; p < 0.001).
The multivariable analysis, using the continuous variable for number of years since diagnosis and adjusting for the clinic, age, gender and education, indicated that increasing number of years since HIV diagnosis was associated with irregular attendance (aOR 1.05, 95% CI 1.02 to 1.07; p < 0.001). It indicated that increasing number of years since HIV diagnosis was associated with increasing likelihood of non-attendance (aOR 1.05, 95% CI 1.02 to 1.08; p = 0.001), and not being registered with a GP was associated with non-attendance (aOR 3.92, 95% CI 1.79 to 8.58; p = 0.001).
Factors relating to capability
In this section we look at factors relating to respondents’ capability of attending the clinic (Table 16). Only one-fifth of respondents did not speak English as their main language (20.1%) and this was not significantly associated with attendance pattern. Symptoms of neurocognitive impairment (memory loss, feeling mentally slower, difficulty paying attention) were reported by 41.3% of respondents and this was more likely among IAs (52.5%) than RAs (37.3%) or NAs (36.9%; p < 0.001). IAs were also less likely than IAs or NAs to report excellent or very good health in the past 4 weeks (IA 35.2% vs. RA 50.6% and NA 42.2%) and more likely to report fair or poor health (IA 38.3% vs. RA 24.5% and NA 24.2%; p < 0.001). More than two-thirds of respondents had ever had an inpatient stay (70.7%), one-fifth had been hospitalised in the past year (19.7%), nearly half reported that HIV affected their day-to-day activity to some extent (46.3%), and 14.4% reported a long-standing condition that caused difficulty with accessing buildings, streets or vehicles. None of these factors was significantly associated with attendance.
| Factor | All patients | RA, % | IA, % | NA, % | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Main language | ||||||
| Not English | 196 | 20.1 | 21.5 | 17.5 | 19.8 | 0.406 |
| English | 780 | 79.9 | 78.5 | 82.5 | 80.2 | |
| Neurocognitive impairment | ||||||
| No | 569 | 58.7 | 62.7 | 47.5 | 63.1 | < 0.001 |
| Yes | 401 | 41.3 | 37.3 | 52.5 | 36.9 | |
| Health (past 4 weeks) | ||||||
| Excellent, very good | 434 | 45.0 | 50.6 | 35.2 | 42.2 | < 0.001 |
| Good | 258 | 26.8 | 24.9 | 26.4 | 33.5 | |
| Fair or poor | 272 | 28.2 | 24.5 | 38.3 | 24.2 | |
| Ever had inpatient stay | ||||||
| No | 284 | 29.3 | 29.9 | 25.6 | 33.3 | 0.211 |
| Yes | 686 | 70.7 | 70.1 | 74.4 | 66.7 | |
| Last inpatient stay | ||||||
| In past year | 124 | 19.7 | 17.9 | 24.6 | 17.3 | .390 |
| 1–5 years ago | 253 | 40.1 | 41.9 | 36.0 | 40.8 | |
| > 5 years ago | 254 | 40.3 | 40.2 | 39.4 | 41.8 | |
| HIV affects activity | ||||||
| No | 522 | 53.7 | 55.1 | 46.6 | 60.5 | 0.062 |
| Yes, a little | 311 | 32.0 | 30.7 | 37.2 | 27.8 | |
| Yes, a lot | 139 | 14.3 | 14.2 | 16.2 | 11.7 | |
| Long-standing condition affecting access | ||||||
| No | 819 | 85.6 | 85.1 | 84.2 | 89.3 | 0.323 |
| Yes | 138 | 14.4 | 14.9 | 15.8 | 10.7 | |
| Last time injected drugs | ||||||
| < 1 year | 74 | 7.8 | 5.8 | 10.9 | 9.6 | 0.107 |
| 1–5 years ago | 20 | 2.1 | 1.7 | 3.1 | 1.9 | |
| > 5 years ago | 30 | 3.2 | 3.2 | 2.3 | 4.5 | |
| Never | 827 | 87.0 | 89.4 | 83.7 | 84.1 | |
| Recreational drug use (past 5 years) | ||||||
| No | 597 | 62.4 | 66.9 | 58.3 | 53.8 | 0.003 |
| Yes | 360 | 37.6 | 33.1 | 41.7 | 46.2 | |
| Rating of health information competence (two items) | ||||||
| Understanding of where to get information | ||||||
| Disagree | 105 | 11.0 | 10.9 | 10.6 | 11.9 | 0.940 |
| Uncertain | 118 | 12.4 | 11.7 | 13.3 | 13.2 | |
| Agree | 731 | 76.6 | 77.4 | 76.1 | 74.8 | |
| Feeling in control about getting information | ||||||
| Disagree | 116 | 12.2 | 12.2 | 13.6 | 10.0 | 0.670 |
| Uncertain | 118 | 12.4 | 11.4 | 13.2 | 14.4 | |
| Agree | 717 | 75.4 | 76.4 | 73.2 | 75.6 | |
The majority of respondents had never injected drugs (87.0%) and this was not significantly associated with attendance pattern, but recreational drug use in the past 5 years was more common (37.6%) and more likely to be reported by IAs (41.7%) and NAs (46.2%) than by RAs (33.1%; p = 0.003).
We included two items measuring health information competence and three-quarters of respondents both understood where to get health information (76.6%) and felt in control of health information (75.4%), which did not vary significantly by attendance.
The multivariable analysis, adjusting for the clinic and background factors, indicated that respondents who reported symptoms of neurocognitive impairment were more likely to be IAs (aOR 1.79, 95% CI 1.26 to 2.55; p = 0.001), as were those who reported poorer health compared with very good or excellent health (aOR 1.89, 95% CI 1.25 to 2.85; p = 0.002). Non-attendance was associated with reporting recreational drug use in the past 5 years (aOR 2.03, 95% CI 1.27 to 3.25; p = 0.003) and weakly associated with reporting good health compared with very good or excellent health (aOR 1.57, 95% CI 98 to 2.50; p = 0.059).
Factors relating to motivation
Motivation describes the mechanisms that activate or inhibit a behaviour and, in this section, we explore how items relating to motivation are associated with attendance, as shown in Table 17. One-third of respondents said that religion was very important in their life (30.3%), but this was not significantly associated with attendance pattern. We asked patients if they had experienced any of a list of seven feelings in the past 12 months because of their HIV status. At least one of the seven items relating to internalised HIV stigma had been experienced by 43.8% of participants; the most likely to be reported was low self-esteem (34.1%), followed by self-blame (28.8%) and shame (28.7%); only 4.9% of respondents ticked, ‘I feel I should be punished’. IAs were more likely than RAs or NAs to report low self-esteem (IA 40.0% vs. RA 32.6% and NA 29.4%; p = 0.046) and feeling suicidal (IA 16.5% vs. RA 9.4% and NA 11.9%; p = 0.013), but there was no significant difference between the attendance patterns on an overall internalised stigma summative score.
| Factor | All patients | RA, % or mean (SD) | IA, % or mean (SD) | NA, % or mean (SD) | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % or mean (SD) | |||||
| Importance of religion | ||||||
| Very | 229 | 30.3 | 30.2 | 29.6 | 31.7 | 0.975 |
| Somewhat | 95 | 12.6 | 12.9 | 11.7 | 13.0 | |
| Not very | 116 | 15.3 | 16.2 | 15.0 | 13.0 | |
| No religion | 316 | 41.8 | 40.7 | 43.7 | 42.3 | |
| Internalised stigma (seven items) | ||||||
| I feel ashamed | ||||||
| No | 687 | 71.3 | 71.6 | 71.2 | 70.6 | 0.966 |
| Yes | 276 | 28.7 | 28.4 | 28.8 | 29.4 | |
| I blame myself | ||||||
| No | 686 | 71.2 | 72.0 | 70.0 | 70.6 | 0.827 |
| Yes | 277 | 28.8 | 28.0 | 30.0 | 29.4 | |
| I have low self-esteem | ||||||
| No | 635 | 65.9 | 67.4 | 60.0 | 70.6 | 0.046 |
| Yes | 328 | 34.1 | 32.6 | 40.0 | 29.4 | |
| I feel suicidal | ||||||
| No | 850 | 88.3 | 90.6 | 83.5 | 88.1 | 0.013 |
| Yes | 113 | 11.7 | 9.4 | 16.5 | 11.9 | |
| I feel guilty | ||||||
| No | 770 | 80.0 | 80.8 | 76.9 | 81.9 | 0.345 |
| Yes | 193 | 20.0 | 19.2 | 23.1 | 18.1 | |
| I blame others | ||||||
| No | 903 | 93.8 | 94.5 | 94.6 | 90.0 | 0.097 |
| Yes | 60 | 6.2 | 5.5 | 5.4 | 10.0 | |
| I should be punished | ||||||
| No | 916 | 95.1 | 95.6 | 93.5 | 96.3 | 0.328 |
| Yes | 47 | 4.9 | 4.4 | 6.5 | 3.8 | |
| No internalised stigma | ||||||
| No | 541 | 56.2 | 54.7 | 58.5 | 57.5 | 0.563 |
| Yes | 422 | 43.8 | 45.3 | 41.5 | 42.5 | |
| Internalised stigma summative score | 963 | 1.3 (1.6) | 1.3 (1.6) | 1.5 (1.8) | 1.3 (1.5) | 0.166 |
| Environmental mastery (three items) | ||||||
| Life gets me down | ||||||
| Disagree | 431 | 45.2 | 48.6 | 39.2 | 43.3 | 0.076 |
| Uncertain | 142 | 14.9 | 15.3 | 14.6 | 14.0 | |
| Agree | 381 | 39.9 | 36.1 | 46.2 | 42.7 | |
| Good at managing life | ||||||
| Disagree | 148 | 15.5 | 15.4 | 15.0 | 16.5 | 0.322 |
| Uncertain | 135 | 14.1 | 12.1 | 17.3 | 15.8 | |
| Agree | 673 | 70.4 | 72.5 | 67.7 | 67.7 | |
| In charge of life | ||||||
| Disagree | 175 | 18.4 | 17.8 | 17.6 | 21.7 | 0.044 |
| Uncertain | 171 | 18.0 | 15.5 | 23.8 | 16.6 | |
| Agree | 606 | 63.7 | 66.7 | 58.6 | 61.8 | |
| Environmental mastery summative score | 936 | 10.5 (2.8) | 10.7 (2.8) | 10.1 (2.7) | 10.3 (2.7) | 0.027 |
| PHQ4: anxiety and depression (past 2 weeks) | ||||||
| Normal | 699 | 76.8 | 79.5 | 70.1 | 79.1 | 0.057 |
| Mild | 113 | 12.4 | 10.6 | 16.7 | 11.5 | |
| Moderate | 98 | 10.8 | 10.0 | 13.1 | 9.5 | |
| Ever diagnosed with depression | ||||||
| No | 617 | 68.0 | 70.9 | 61.8 | 68.6 | 0.043 |
| Yes | 290 | 32.0 | 29.1 | 38.2 | 31.4 | |
We asked respondents about symptoms of anxiety and depression using the PHQ4 scale. 52 None of our respondents reported severe levels of anxiety and depression, although it would not have been appropriate for clinics to approach patients with severe symptoms about participation. There was an indication that IAs were less likely than RAs or NAs to report normal levels (IA 70.1% vs. RA 79.5% and NA 79.1%; p = 0.057); they were also more likely to report that they had ever been diagnosed with depression (IA 38.2% vs. RA 29.1% and NA 31.4%; p = 0.043).
We included three items from a scale measuring environmental mastery in our questionnaire. Although findings from this a subscale of the PWB49,50 are reported here under motivation, environmental mastery is also likely to affect capability. IAs scored lower on a summative score of environmental mastery than RAs or NAs (IA 10.1 vs. RA 10.7 and NA 10.3; p = 0.027); they were more likely to report that the demands of life often got them down (IA 46.2% vs. RA 36.1% and NA 42.7%) and less likely to feel in charge of the situation in which they lived (RA 58.6% vs. RA 66.7% and NA 61.8%, p = 0.027).
In multivariable analysis, irregular attendance was associated with ever being diagnosed with depression (aOR 1.61, 95% CI 1.11 to 2.33; p = 0.012) and with being uncertain rather than agreeing about being in charge of life (aOR 1.75, 95% CI 1.13 to 2.72; p = 0.013).
Factors relating to opportunity: social influences
Social influences shape an individual’s opportunity to enact behaviours. In this section, we explore respondents’ social environment in terms of the people they interact with both within and outside the HIV clinic, as shown in Table 18.
| Factor | All patients | RA, % | IA, % | NA, % | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Length of relationship | ||||||
| Not in relationship | 486 | 51.3 | 49.3 | 52.9 | 55.6 | 0.895 |
| < 1 year | 46 | 4.9 | 4.5 | 5.7 | 4.6 | |
| 1–5 years | 121 | 12.8 | 13.0 | 12.5 | 12.4 | |
| 5–10 years | 90 | 9.5 | 10.4 | 8.4 | 8.5 | |
| > 10 years | 204 | 21.5 | 22.8 | 20.5 | 19.0 | |
| Cohabiting | ||||||
| No | 627 | 65.8 | 62.4 | 69.8 | 70.8 | 0.041 |
| Yes | 326 | 34.2 | 37.6 | 30.2 | 29.2 | |
| Partner has HIV | ||||||
| Yes | 210 | 45.2 | 44.1 | 46.7 | 46.5 | 0.835 |
| No | 231 | 49.7 | 51.5 | 47.5 | 46.5 | |
| Do not know | 24 | 5.2 | 4.4 | 5.7 | 7.0 | |
| Abused by or afraid of partner (past year) | ||||||
| No | 578 | 79.4 | 81.3 | 76.5 | 77.6 | 0.336 |
| Yes | 150 | 20.6 | 18.7 | 23.5 | 22.4 | |
| Told anyone about HIV | ||||||
| No | 127 | 13.1 | 11.6 | 14.7 | 15.4 | 0.304 |
| Yes | 843 | 86.9 | 88.4 | 85.3 | 84.6 | |
| Told partner about HIV | ||||||
| No | 62 | 11.3 | 10.1 | 11.3 | 15.6 | 0.351 |
| Yes | 486 | 88.7 | 89.9 | 88.7 | 84.4 | |
| Told family about HIV | ||||||
| Some or all | 494 | 58.0 | 54.2 | 62.9 | 63.2 | 0.035 |
| None | 357 | 42.0 | 45.8 | 37.1 | 36.8 | |
| Told friends about HIV | ||||||
| Some or all | 622 | 70.0 | 70.7 | 71.4 | 65.5 | 0.421 |
| None | 266 | 30.0 | 29.3 | 28.6 | 34.5 | |
| Told GP about HIV | ||||||
| No | 82 | 10.7 | 11.3 | 8.9 | 11.4 | 0.634 |
| Yes | 685 | 89.3 | 88.7 | 91.1 | 88.6 | |
| Rating of social support (five items) | ||||||
| People who care | ||||||
| Not enough | 252 | 26.8 | 25.0 | 30.4 | 26.9 | 0.270 |
| Enough | 689 | 73.2 | 75.0 | 69.6 | 73.1 | |
| Love and affection | ||||||
| Not enough | 311 | 33.3 | 32.0 | 34.4 | 36.1 | 0.579 |
| Enough | 622 | 66.7 | 68.0 | 65.6 | 63.9 | |
| Chances to talk | ||||||
| Not enough | 337 | 35.9 | 33.0 | 38.9 | 40.9 | 0.101 |
| Enough | 601 | 64.1 | 67.0 | 61.1 | 59.1 | |
| Invitations to go out | ||||||
| Not enough | 314 | 33.7 | 31.7 | 36.7 | 35.3 | 0.347 |
| Enough | 618 | 66.3 | 68.3 | 63.3 | 64.7 | |
| Help when sick | ||||||
| Not enough | 340 | 37.2 | 33.7 | 41.8 | 41.6 | 0.046 |
| Enough | 574 | 62.8 | 66.3 | 58.2 | 58.4 | |
| Social support score | ||||||
| Low (≤ 12) | 147 | 16.5 | 15.2 | 18.9 | 17.1 | 0.435 |
| Higher (12+) | 744 | 83.5 | 84.8 | 81.1 | 82.9 | |
| Received support group information from clinic | ||||||
| Yes | 660 | 67.9 | 71.6 | 62.9 | 63.8 | 0.084 |
| No | 182 | 18.7 | 16.9 | 22.0 | 19.6 | |
| Cannot remember | 130 | 13.4 | 11.6 | 15.2 | 16.6 | |
| Used support group | ||||||
| Yes | 298 | 30.9 | 31.8 | 32.6 | 25.3 | 0.056 |
| No, would like to | 159 | 16.5 | 14.3 | 20.7 | 17.1 | |
| No, do not want to | 422 | 43.8 | 46.1 | 38.3 | 44.9 | |
| Not aware of groups | 84 | 8.7 | 7.7 | 8.4 | 12.7 | |
| Found group helpful | ||||||
| Yes, definitely | 147 | 53.1 | 57.9 | 44.3 | 51.3 | 0.327 |
| Yes, to some extent | 98 | 35.4 | 32.7 | 41.8 | 33.3 | |
| No | 32 | 11.6 | 9.4 | 13.9 | 15.4 | |
| At last visit | ||||||
| Friendly reception staff | ||||||
| Yes, definitely | 795 | 82.1 | 83.1 | 81.3 | 80.4 | 0.308 |
| Yes, to some extent | 158 | 16.3 | 15.1 | 16.8 | 19.6 | |
| No | 15 | 1.5 | 1.8 | 1.9 | 0.0 | |
| Understood explanation of treatment | ||||||
| Yes, completely | 828 | 90.1 | 91.7 | 87.4 | 89.0 | 0.351 |
| Yes, to some extent | 75 | 8.2 | 6.6 | 10.5 | 9.7 | |
| No | 16 | 1.7 | 1.7 | 2.0 | 1.3 | |
| Doctor was friendly | ||||||
| Yes, definitely | 913 | 95.3 | 95.5 | 94.7 | 95.7 | 0.840 |
| Not definitely | 45 | 4.7 | 4.5 | 5.3 | 4.3 | |
| Doctor listened | ||||||
| Yes, definitely | 902 | 94.0 | 93.8 | 94.3 | 93.9 | 0.969 |
| Not definitely | 58 | 6.0 | 6.2 | 5.7 | 6.1 | |
| Confidence in doctor | ||||||
| Yes, definitely | 881 | 91.9 | 93.3 | 90.8 | 88.9 | 0.157 |
| Not definitely | 78 | 8.1 | 6.7 | 9.2 | 11.1 | |
| Nurse was friendly | ||||||
| Yes, definitely | 892 | 93.9 | 94.9 | 94.6 | 89.5 | 0.037 |
| Not definitely | 58 | 6.1 | 5.1 | 5.4 | 10.5 | |
| Nurse listened | ||||||
| Yes, definitely | 875 | 92.4 | 93.2 | 94.6 | 86.4 | 0.006 |
| Not definitely | 72 | 7.6 | 6.8 | 5.4 | 13.6 | |
| Confidence in nurse | ||||||
| Yes, definitely | 855 | 90.7 | 90.9 | 92.6 | 86.9 | 0.143 |
| Not definitely | 88 | 9.3 | 9.1 | 7.4 | 13.1 | |
Half of the respondents were not currently in a relationship (51.3%), whereas 31.0% had been in a relationship for at least 5 years. Although this was not significantly associated with attendance pattern, RAs were more likely to be cohabiting (37.6%) than IAs (30.2%) or NAs (29.2%; p = 0.041). Among respondents with a partner, almost half had a partner who was also HIV positive (45.2%) and most had told their partners about their HIV status (88.7%), neither of which varied significantly by attendance pattern. One-fifth of respondents had been abused by, or afraid of, a partner or ex-partner in the past year (20.6%) but this was not significantly associated with attendance.
Although most respondents had told someone about their HIV status (86.9%), 42.0% had not told any family members and 30.0% had told none of their friends. RAs were more likely to have told none of their family members about their HIV status (RA 45.8% vs. IA 37.1% and NA 36.8%; p = 0.035) but there was no significant difference between groups on telling friends about their HIV status. Most respondents had told their GP (89.3%) and this did not vary significantly by attendance.
We included five items from the DUFSS. 51 Across the attendance patterns, the majority of respondents reported that they had enough people who cared about what happened to them (73.2%). Somewhat fewer felt that they got enough love and affection (66.7%), enough invitations to go out (66.3%) and enough chances to talk (64.1%). IAs and NAs were less likely than RAs to report getting enough help when they were sick in bed (IA 58.2% and NA 58.4% vs. RA 66.3%; p = 0.046), although there was no significant difference between the attendance groups on an overall score for level of social support. Two-thirds of respondents overall had received information from their clinic about HIV support groups (67.9%), but only 30.9% had used a support group in their local area and 16.5% had not used a group but would like to. Among those who had used a group, 53.1% definitely found it helpful compared with 11.6% who did not find it helpful. None of these variables was significantly associated with attendance pattern.
We asked respondents a range of questions about their interactions with clinic staff on the last occasion that they came to the clinic. Most reported that the reception staff were definitely friendly and approachable (82.1%), that they completely understood explanations for any treatment or action (90.1%), that the doctor was definitely friendly and approachable (95.3%), the doctor definitely listened to them (94.0%) and that they definitely had confidence and trust in the doctor (91.9%). This did not vary significantly between attendance groups. All respondents were similarly likely to report that they had confidence and trust in the nurse (90.7%), but NAs were less likely than RAs or IAs to report that the nurse was friendly (NA 89.5% vs. RA 94.9% and NA 94.6%; p = 0.037) or listened to them (NA 86.4% vs. RA 93.2% and IA 94.6%; p = 0.006).
In multivariable analysis, irregular attendance was associated with having told family members about their HIV status (aOR 1.52, 95% CI 1.06 to 2.20; p = 0.025) and with not having enough help when sick in bed (aOR 1.64, 95% CI 1.14 to 2.37; p = 0.008). Non-attendance was associated with having told family members about their HIV status (aOR 1.63, 95% CI 1.02 to 2.60; p = 0.043), not having enough help when sick in bed (aOR 1.76, 95% CI 1.12 to 2.79; p = 0.015) and not being listened to by the nurse (aOR 2.17, 95% CI 1.06 to 4.42; p = 0.034).
Factors relating to opportunity: barriers
In the same way that social influences shape an individual’s behaviour, structural factors can also enable or hinder attendance at the HIV clinic. In this section, we explore these barriers and facilitators of HIV care, as shown in Table 19.
| Factor | All patients | RA, % | IA, % | NA, % | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Number of children | ||||||
| None | 668 | 70.8 | 74.6 | 68.2 | 62.3 | 0.003 |
| One | 100 | 10.6 | 7.9 | 14.0 | 14.3 | |
| Two | 91 | 9.7 | 10.5 | 6.6 | 11.7 | |
| Three or more | 84 | 8.9 | 7.0 | 11.2 | 11.7 | |
| Caring responsibilities | ||||||
| No | 725 | 75.1 | 77.4 | 73.3 | 69.8 | 0.109 |
| Yes | 241 | 24.9 | 22.6 | 26.7 | 30.2 | |
| Immigration status | ||||||
| British citizen | 625 | 67.3 | 67.5 | 68.0 | 65.4 | 0.458 |
| EU citizen | 141 | 15.2 | 16.6 | 12.5 | 14.7 | |
| Indefinite leave to remain | 103 | 11.1 | 10.6 | 10.9 | 12.8 | |
| Temporary or no status | 60 | 6.5 | 5.2 | 8.6 | 7.1 | |
| Work status | ||||||
| Working | 563 | 58.9 | 60.8 | 56.5 | 56.3 | 0.056 |
| Student | 45 | 4.7 | 3.4 | 5.8 | 7.5 | |
| Unemployed | 208 | 21.8 | 19.4 | 25.8 | 23.1 | |
| Other | 140 | 14.6 | 16.4 | 11.9 | 13.1 | |
| Money for basic needs | ||||||
| Not all of the time | 553 | 56.9 | 50.5 | 64.7 | 65.8 | < 0.001 |
| All of the time | 419 | 43.1 | 49.5 | 35.3 | 34.2 | |
| Hunger scale | ||||||
| Little or none | 734 | 80.7 | 86.4 | 71.5 | 76.2 | < 0.001 |
| Moderate or severe | 176 | 19.3 | 13.6 | 28.5 | 23.8 | |
| Homeowner | ||||||
| No | 702 | 72.1 | 66.7 | 77.9 | 80.9 | < 0.001 |
| Yes | 271 | 27.9 | 33.3 | 22.1 | 19.1 | |
| Time in accommodation | ||||||
| < 6 months | 88 | 10.1 | 9.7 | 10.7 | 11.0 | 0.090 |
| 6 months–1 year | 72 | 8.3 | 8.0 | 9.0 | 8.2 | |
| 1–5 years | 263 | 30.3 | 26.5 | 36.3 | 33.6 | |
| > 5 years | 444 | 51.2 | 55.9 | 44.0 | 47.3 | |
| Deprivation quintile | ||||||
| 1: least deprived | 14 | 2.5 | 2.5 | 1.4 | 4.7 | 0.425 |
| 2 | 26 | 4.7 | 5.2 | 5.6 | 1.2 | |
| 3 | 63 | 11.4 | 12.7 | 9.9 | 9.3 | |
| 4 | 209 | 37.9 | 36.1 | 37.3 | 45.3 | |
| 5: most deprived | 240 | 43.5 | 43.5 | 45.8 | 39.5 | |
| Mode of transport to clinic | ||||||
| Public transport | 723 | 78.8 | 75.2 | 82.0 | 86.0 | 0.022 |
| Car or motorbike | 96 | 10.5 | 11.1 | 11.5 | 6.7 | |
| On foot or bicycle | 90 | 9.8 | 12.6 | 5.7 | 6.7 | |
| Other | 9 | 1.0 | 1.1 | 0.8 | 0.7 | |
| Time to get to clinic | ||||||
| < 30 minutes | 374 | 38.9 | 41.8 | 36.0 | 33.7 | 0.363 |
| 30–60 minutes | 412 | 42.8 | 41.6 | 43.7 | 45.4 | |
| 1–2 hours | 150 | 15.6 | 14.3 | 16.5 | 18.4 | |
| > 2 hours | 26 | 2.7 | 2.2 | 3.8 | 2.5 | |
| Clinic opening hours | ||||||
| Very convenient | 603 | 62.7 | 64.6 | 61.7 | 58.0 | 0.218 |
| Fairly convenient | 311 | 32.3 | 31.7 | 31.4 | 35.8 | |
| Not convenient | 48 | 5.0 | 3.7 | 6.9 | 6.2 | |
| Convenient additional clinic opening hours | ||||||
| Early morning (Monday–Friday) | ||||||
| No | 759 | 77.2 | 76.0 | 79.2 | 78.0 | 0.572 |
| Yes | 224 | 22.8 | 24.0 | 20.8 | 22.0 | |
| Evening (Monday–Friday) | ||||||
| No | 654 | 66.5 | 70.0 | 61.7 | 62.8 | 0.033 |
| Yes | 329 | 33.5 | 30.0 | 38.3 | 37.2 | |
| Saturday | ||||||
| No | 649 | 66.0 | 68.0 | 65.4 | 60.4 | 0.188 |
| Yes | 334 | 34.0 | 32.0 | 34.6 | 39.6 | |
| Sunday | ||||||
| No | 836 | 85.0 | 84.9 | 85.9 | 84.1 | 0.879 |
| Yes | 147 | 15.0 | 15.1 | 14.1 | 15.9 | |
| Experience of consultations with doctor or nurse (in the past) | ||||||
| Face to face | ||||||
| No | 59 | 6.4 | 5.4 | 7.2 | 8.4 | 0.331 |
| Yes | 862 | 93.6 | 94.6 | 92.8 | 91.6 | |
| Telephone | ||||||
| No | 580 | 63.0 | 62.4 | 63.9 | 63.6 | 0.906 |
| Yes | 341 | 37.0 | 37.6 | 36.1 | 36.4 | |
| Text messaging | ||||||
| No | 773 | 83.9 | 81.7 | 85.9 | 88.3 | 0.085 |
| Yes | 148 | 16.1 | 18.3 | 14.1 | 11.7 | |
| No | 748 | 81.2 | 80.9 | 77.9 | 87.7 | 0.049 |
| Yes | 173 | 18.8 | 19.1 | 22.1 | 12.3 | |
| Skype or similar | ||||||
| No | 918 | 99.7 | 99.6 | 100.0 | 99.4 | 0.504 |
| Yes | 3 | 0.3 | 0.4 | 0.0 | 0.6 | |
| Preference for consultations with doctor or nurse (in the future) | ||||||
| Face to face | ||||||
| No | 140 | 15.0 | 12.7 | 17.4 | 19.0 | 0.072 |
| Yes | 791 | 85.0 | 87.3 | 82.6 | 81.0 | |
| Telephone | ||||||
| No | 478 | 51.3 | 50.8 | 52.2 | 51.9 | 0.924 |
| Yes | 453 | 48.7 | 49.2 | 47.8 | 48.1 | |
| Text messaging | ||||||
| No | 688 | 73.9 | 75.0 | 73.1 | 71.5 | 0.647 |
| Yes | 243 | 26.1 | 25.0 | 26.9 | 28.5 | |
| No | 564 | 60.6 | 60.0 | 57.7 | 67.1 | 0.153 |
| Yes | 367 | 39.4 | 40.0 | 42.3 | 32.9 | |
| Skype or similar | ||||||
| No | 715 | 76.8 | 78.3 | 73.1 | 77.8 | 0.266 |
| Yes | 216 | 23.2 | 21.7 | 26.9 | 22.2 | |
| Last appointment booked | ||||||
| In person | 641 | 68.0 | 75.7 | 63.5 | 49.1 | < 0.001 |
| By telephone | 289 | 30.6 | 23.4 | 36.1 | 46.5 | |
| By e-mail or text | 13 | 1.4 | 0.9 | 0.4 | 4.4 | |
| Ease of booking last appointment | ||||||
| Very easy | 618 | 72.5 | 74.7 | 70.4 | 68.6 | 0.197 |
| Fairly easy | 192 | 22.5 | 21.8 | 22.6 | 25.0 | |
| Not easy | 42 | 4.9 | 3.5 | 7.1 | 6.4 | |
Regular attenders were less likely to have children (25.4%) than IAs (31.8%) or NAs (37.7%; p = 0.003). However, there was no significant difference between attendance groups on responsibility for looking after children, family members or others that was reported by 24.9% of respondents. Two-thirds of respondents were British citizens (67.3%), 15.2% were European Union citizens, 11.1% had indefinite leave to remain and 6.5% had temporary or no official immigration status. This was not significantly associated with attendance pattern. The majority of respondents were working (58.9%) and there was some indication that RAs were less likely than IAs or NAs to be students (RA 3.4% vs. IA 5.8% and NA 7.5%) and less likely to be unemployed (RA 19.4% vs. IA 25.8% and NA 23.1%; p = 0.056).
Several indicators of economic status were included in the questionnaire, which showed that respondents were generally financially disadvantaged but that this was more likely among IAs and NAs. Less than half of the respondents had enough money for their basic needs all the time (43.1%) and this was less likely among IA (35.3%) and NAs (34.2%) than among RAs (49.5%; p < 0.001). Moderate or severe hunger was reported by 13.6% of RAs, compared with 28.5% of IAs and 23.8% of NAs (p < 0.001). One-third of RAs (33.3%) were homeowners, compared with 22.1% of IAs and 19.1% of NAs (p < 0.001). There were a lot of missing postcode data to create and Index of Multiple Deprivation score for respondents’ neighbourhoods, but 43.5% lived in a neighbourhood that was among the most deprived in England. Time in accommodation was not significantly associated with attendance group and 51.2% had lived in their current accommodation for > 5 years.
We asked respondents several questions relating to the convenience of the clinical service provision. RAs were less likely than IAs and NAs to travel to the clinic by public transport (RA 75.2% vs. IA 82.0% and NA 86.0%) and were more likely to go on foot or by bicycle (RA 12.6% vs. IA 5.7% and NA 6.7%), whereas NAs were less likely to use a car or motorbike than RAs or IAs (NA 6.7% vs. RA 11.1% and IA 11.5%; p = 0.022). There were no significant differences by attendance on the time it took to get to the clinic or convenience of the opening hours. The majority took < 1 hour to get to the clinic (81.7%), with 38.9% taking < 30 minutes, but 15.6% took 1–2 hours and 2.7% took > 2 hours. Most respondents found the opening hours very or fairly convenient (95.0%). A minority of all respondents wanted Sunday opening (15.0%) and somewhat more wanted weekday early morning opening (22.8%). One-third of all respondents wanted Saturday opening (34.0%) and mid-week evening opening (33.5%), with the latter more popular among IAs and NAs than RAs (IA 38.3% and NA 37.2% vs. RA 30.0%; p = 0.033).
In terms of previous and desired mode of communication for consultations, the only significant difference was that NAs were less likely to have used e-mail than RAs or IAs for this purpose (NA 12.3% vs. RA 19.1% and IA 22.1%; p = 0.049). Comparison of previous and desired mode of communication indicates that 93.6% of respondents have had face-to-face consultations and 85.0% would like face-to-face consultations in the future; 37.0% have had telephone consultations and 48.7% would like telephone consultations; 16.1% have had consultations by text and 26.1% would like this in the future; 18.8% have had e-mail consultations and 39.4% would like consultations by e-mail in the future; and only three respondents reported consultations by Skype or similar and 23.2% would like this type of consultation in the future.
Three-quarters of RAs booked their last appointment in person (75.7%), compared with 63.5% of IAs and 49.1% of NAs, whereas NAs were more likely than RAs and IAs to book by telephone (NA 46.5% vs. RA 23.4% and IA 36.1%) or by e-mail/text (NA 4.4% vs. RA 0.9% and IA 0.4%; p < 0.001). However, there were no significant differences between attendance groups on ease of booking, which was mostly reported as being very easy (72.5%).
In multivariable analysis, irregular attendance was associated with having children (aOR 2.11, 95% CI 1.39 to 3.20; p < 0.001) and not having enough money for basic needs (aOR 1.74, 95% CI 1.19 to 2.56; p = 0.005). Non-attendance was associated with having children (aOR 2.76, 95% CI 1.69 to 4.50; p < 0.001), not having enough money for basic needs (aOR 1.63, 95% CI 1.02 to 2.61; p = 0.042) and not owning one’s own home (aOR 1.87, 95% CI 1.09 to 3.20; p = 0.023).
Use of and beliefs about antiretroviral therapy
Table 20 shows the respondents’ use of, and beliefs about, ART. There were no significant differences between attendance groups on their scores relating to their belief about medicines, either for the general benefit subscale or for the general distrust subscale. RAs were more likely to be taking ART than IAs or NAs (RA 94.2% vs. IA 90.7% and NA 64.6%), whereas NAs were more likely than RAs or IAs to have taken ART in the past (NA 15.9% vs. RA 0.9% and IA 4.5%) or not at all (NA 19.5% vs. RA 4.9% and IA 4.8%; p < 0.001). Multivariable analysis, adjusting for the clinic, age, gender and education, indicated that IAs were more likely to have been on ART previously than those who were currently on ART (aOR 3.83, 95% CI 1.29 to 11.40; p = 0.016), but they were not more likely to have never been on ART. NAs were more likely to have been on ART previously (aOR 18.17, 95% CI 6.44 to 51.28; p < 0.001) and were more likely to have never been on ART (aOR 5.35, 95% CI 2.89 to 9.90; p < 0.001).
| Factor | All patients | RA, % or mean (SD) | IA, % or mean (SD) | NA, % or mean (SD) | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % or mean (SD) | |||||
| Belief about medicines | ||||||
| General benefit score | 940 | 8.8 (2.2) | 8.8 (2.2) | 8.9 (2.1) | 8.6 (2.3) | 0.422 |
| General distrust score | 874 | 10.1 (3.3) | 9.9 (3.1) | 10.2 (3.7) | 10.4 (3.2) | 0.206 |
| Currently taking ART | ||||||
| Yes | 868 | 88.3 | 94.2 | 90.7 | 64.6 | < 0.001 |
| No: but have done | 43 | 4.4 | 0.9 | 4.5 | 15.9 | |
| No: never have | 72 | 7.3 | 4.9 | 4.8 | 19.5 | |
Table 21 shows that, among those on ART, IAs and NAs were more likely than RAs to have been taking it for longer: 5–10 years (IA 31.7% and NA 32.0% vs. RA 24.6%) or > 10 years (IA 39.4% and NA 45.9% vs. RA 35.9%; p = 0.005). RAs on ART were more likely to be using the home delivery service (59.5%) than IAs (46.6%) or NAs (38.7%) on ART (p < 0.001). IAs and NAs reported poorer adherence to ART with lower scores on the MARS than RAs (IA 22.6 and NA 22.3 vs. RA 24.0; p < 0.001) and greater likelihood of missing a dose on 1 or more days in the past week (IA 35.5% and NA 38.5% vs. RA 12.8%; p < 0.001). They reported lower scores on the ART necessity subscale (IA 17.4 and NA 17.2 vs. RA 18.0; p = 0.040) and higher scores on the ART concerns subscale (IA 10.0 and NA 9.8 vs. RA 9.1; p = 0.005). 56,57
| Factor | All patients | RA, % or mean (SD) | IA, % or mean (SD) | NA, % or mean (SD) | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % or mean (SD) | |||||
| Years since starting ART | ||||||
| < 1 year | 84 | 9.7 | 11.6 | 8.1 | 4.9 | 0.005 |
| 1–5 years | 212 | 24.4 | 27.9 | 20.7 | 17.2 | |
| 5–10 years | 240 | 27.6 | 24.6 | 31.7 | 32.0 | |
| > 10 years | 333 | 38.3 | 35.9 | 39.4 | 45.9 | |
| Whether or not clinic has ART delivery service | ||||||
| Yes: using it | 444 | 53.2 | 59.5 | 46.6 | 38.7 | < 0.001 |
| Yes: used before | 86 | 10.3 | 8.3 | 14.2 | 11.3 | |
| Yes: have not used | 171 | 20.5 | 19.6 | 21.1 | 23.6 | |
| No | 51 | 6.1 | 5.2 | 5.6 | 11.3 | |
| Do not know | 82 | 9.8 | 7.5 | 12.5 | 15.1 | |
| MARS | 784 | 23.4 (2.5) | 24.0 (1.8) | 22.6 (3.2) | 22.3 (3.0) | < 0.001 |
| Missed dose (past week) | ||||||
| No | 642 | 77.6 | 87.2 | 64.5 | 61.5 | < 0.001 |
| On 1+ days | 185 | 22.4 | 12.8 | 35.5 | 38.5 | |
| Belief about medicines | ||||||
| ART necessity score | 820 | 17.7 (3.5) | 18.0 (3.2) | 17.4 (3.7) | 17.2 (3.8) | 0.040 |
| ART concerns score | 805 | 9.4 (3.8) | 9.1 (3.5) | 10.0 (4.1) | 9.8 (4.0) | 0.005 |
Among the minority of respondents (n = 102) who were not taking ART and responded to the follow-up questions, 46.1% said that they had been advised to take ART. NAs made up the largest proportion of respondents who were not taking ART against the doctor’s advice (NA 60.0% vs. RA 13.3% and IA 26.7%). Table 22 shows the reasons that these respondents gave for not taking ART against advice at this time. As the numbers are small, they are not divided up by attendance group. The reason that was most likely to be reported as very important was the side effects of medication (61.7%), followed by feeling depressed or overwhelmed (54.3%), not wanting to think about being HIV positive (32.6%) and not wanting anyone to see the medication (29.8%). Reasons that were very important for a smaller proportion of respondents were homelessness (11.4%), harmfulness of medication (9.1%), use of alcohol or drugs (8.9%), information from the media (8.7%), information from friends (8.5%), complication of taking medication (6.8%) and, finally, use of alternative therapy (2.3%).
| Factor | n | % |
|---|---|---|
| Advised to take ART | ||
| No | 55 | 53.9 |
| Yes | 47 | 46.1 |
| Reasons for not taking ART against advice (11 items) | ||
| Side effects | ||
| Very important | 29 | 61.7 |
| Somewhat important | 9 | 19.1 |
| Not important | 9 | 19.1 |
| Feeling depressed | ||
| Very important | 25 | 54.3 |
| Somewhat important | 15 | 32.6 |
| Not important | 6 | 13.0 |
| Not wanting to think about HIV | ||
| Very important | 15 | 32.6 |
| Somewhat important | 9 | 19.6 |
| Not important | 22 | 47.8 |
| Alcohol or drugs | ||
| Very important | 4 | 8.9 |
| Somewhat important | 12 | 26.7 |
| Not important | 29 | 64.4 |
| Not wanting medication to be seen | ||
| Very important | 14 | 29.8 |
| Somewhat important | 10 | 21.3 |
| Not important | 23 | 48.9 |
| Homelessness | ||
| Very important | 5 | 11.4 |
| Somewhat important | 3 | 6.8 |
| Not important | 36 | 81.8 |
| Using alternative therapy | ||
| Very important | 1 | 2.3 |
| Somewhat important | 5 | 11.6 |
| Not important | 37 | 86.0 |
| Too complicated to take | ||
| Very important | 3 | 6.8 |
| Somewhat important | 6 | 13.6 |
| Not important | 35 | 79.5 |
| Harmful | ||
| Very important | 4 | 9.1 |
| Somewhat important | 9 | 20.5 |
| Not important | 31 | 70.5 |
| Information from friends | ||
| Very important | 4 | 8.5 |
| Somewhat important | 16 | 34.0 |
| Not important | 27 | 57.4 |
| Information from media | ||
| Very important | 4 | 8.7 |
| Somewhat important | 8 | 17.4 |
| Not important | 34 | 73.9 |
Clinical data
In addition to fielding our questionnaire, we collected clinical data from patients’ notes. The item above on whether or not patients were currently taking ART is an amalgamation of patient-reported and clinical data, and the item on years since starting ART is from the clinical data set. Both items are included in the above description of the data, as they fit into the section on use of and beliefs about ART. All other data taken from patients’ notes are reported below and shown in Table 23.
| Factor | All patients | RA, % | IA, % | NA, % | Overall p-value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| HARS 3: complex patient criteria (10 items) | ||||||
| Current tuberculosis | 6 | 0.6 | 0.4 | 1.1 | 0.6 | 0.431 |
| Chronic viral liver disease | 35 | 3.6 | 3.6 | 3.3 | 3.7 | 0.975 |
| Oncological treatment | 5 | 0.5 | 0.5 | 0.7 | 0.0 | 0.564 |
| Active AIDS diagnosis | 10 | 1.0 | 0.7 | 1.1 | 1.8 | 0.459 |
| Advanced end-organ disease | 11 | 1.1 | 0.9 | 1.5 | 1.2 | 0.755 |
| Current pregnancy | 13 | 1.3 | 0.5 | 0.4 | 5.5 | < 0.001 |
| Under psychiatric care | 54 | 5.5 | 5.1 | 7.4 | 3.7 | 0.203 |
| Persistent viraemia | 59 | 6.0 | 4.0 | 8.6 | 8.5 | 0.012 |
| Under care of social worker | 9 | 0.9 | 0.5 | 1.9 | 0.6 | 0.162 |
| None of these | 791 | 80.5 | 84.0 | 75.5 | 76.8 | 0.007 |
| Ever had HIV-related inpatient stay | ||||||
| No | 638 | 75.1 | 78.6 | 69.1 | 73.7 | 0.022 |
| Yes | 211 | 24.9 | 21.4 | 30.9 | 26.3 | |
| CD4 count at diagnosis (cells/mm3) | ||||||
| < 200 | 187 | 33.0 | 35.7 | 31.8 | 25.5 | 0.300 |
| 200–349 | 131 | 23.1 | 23.7 | 22.3 | 22.3 | |
| ≥ 350 | 249 | 43.9 | 40.6 | 45.9 | 52.1 | |
| First recorded CD4 count (cells/mm3) | ||||||
| < 200 | 75 | 19.2 | 18.3 | 19.8 | 21.3 | 0.253 |
| 200–349 | 103 | 26.4 | 22.5 | 32.8 | 27.9 | |
| ≥ 350 | 212 | 54.4 | 59.2 | 47.4 | 50.8 | |
| CD4 count (cells/mm3) at diagnosis or first recorded CD4 count | ||||||
| < 200 | 262 | 27.4 | 28.8 | 26.5 | 23.9 | 0.615 |
| 200–349 | 234 | 24.5 | 23.2 | 26.9 | 24.5 | |
| ≥ 350 | 461 | 48.2 | 48.0 | 46.6 | 51.6 | |
| CD4 count at ART initiation (cells/mm3) | ||||||
| < 200 | 279 | 39.9 | 40.7 | 35.6 | 45.1 | 0.489 |
| 200–349 | 222 | 31.7 | 32.1 | 33.7 | 26.5 | |
| ≥ 350 | 199 | 28.4 | 27.2 | 30.7 | 28.4 | |
| Current CD4 count (cells/mm3) | ||||||
| < 200 | 67 | 7.0 | 4.8 | 7.6 | 13.0 | < 0.001 |
| 200–349 | 98 | 10.2 | 8.5 | 7.6 | 19.8 | |
| ≥ 350 | 798 | 82.9 | 88.6 | 84.7 | 67.3 | |
| Viral load at diagnosis (log-copies/ml) | ||||||
| < 4.00 | 133 | 25.0 | 18.8 | 28.2 | 42.2 | < 0.001 |
| 4.00–4.99 | 197 | 37.0 | 38.6 | 31.0 | 41.0 | |
| ≥ 5.00 | 203 | 38.1 | 42.5 | 40.8 | 16.9 | |
| First recorded viral load (log-copies/ml) | ||||||
| < 4.00 | 200 | 48.4 | 51.3 | 48.3 | 39.1 | 0.283 |
| 4.00–4.99 | 123 | 29.8 | 28.1 | 32.5 | 30.4 | |
| ≥ 5.00 | 90 | 21.8 | 20.5 | 19.2 | 30.4 | |
| Viral load (log-copies/ml) at diagnosis or first recorded viral load | ||||||
| < 4.00 | 333 | 35.2 | 32.5 | 37.4 | 40.8 | 0.115 |
| 4.00–4.99 | 320 | 33.8 | 34.2 | 31.7 | 36.2 | |
| ≥ 5.00 | 293 | 31.0 | 33.3 | 30.9 | 23.0 | |
| Current viral load | ||||||
| Undetectable | 741 | 76.7 | 86.2 | 76.9 | 45.1 | < 0.001 |
| Detectable | 225 | 23.3 | 13.8 | 23.1 | 54.9 | |
| Ever had AIDS-defining illness | ||||||
| No | 690 | 77.1 | 78.7 | 74.8 | 75.6 | 0.447 |
| Yes | 205 | 22.9 | 21.3 | 25.2 | 24.4 | |
| AIDS-defining illnesses (20+ patients) | ||||||
| Herpes simplex virus | 20 | 2.0 | 1.8 | 1.9 | 3.0 | 0.601 |
| Oesophageal candidiasis | 27 | 2.7 | 3.3 | 1.5 | 3.0 | 0.329 |
| Kaposi’s sarcoma | 30 | 3.1 | 2.4 | 4.5 | 3.0 | 0.261 |
| Pulmonary tuberculosis | 45 | 4.6 | 4.4 | 4.8 | 4.9 | 0.936 |
| Pneumocystis carinii pneumonia | 65 | 6.6 | 6.9 | 6.7 | 5.5 | 0.812 |
| Hepatitis C coinfection | ||||||
| No | 917 | 94.2 | 95.4 | 92.9 | 92.7 | 0.222 |
| Yes | 56 | 5.8 | 4.6 | 7.1 | 7.3 | |
| Hepatitis B coinfection | ||||||
| No | 938 | 96.4 | 96.9 | 96.6 | 94.5 | 0.357 |
| Yes | 35 | 3.6 | 3.1 | 3.4 | 5.5 | |
| Mental health issues (past year) | ||||||
| No | 723 | 79.5 | 82.2 | 75.9 | 76.1 | 0.074 |
| Yes | 187 | 20.5 | 17.8 | 24.1 | 23.9 | |
| Drug/alcohol dependency (past year) | ||||||
| No | 806 | 89.1 | 94.1 | 83.3 | 81.5 | < 0.001 |
| Yes | 99 | 10.9 | 5.9 | 16.7 | 18.5 | |
Although the majority of patients (80.5%) did not meet any of the HARS 3 categories for being classified as complex patients, almost one in five did. RAs were less likely than IAs or NAs to be reported to meet any of the HARS 3 complex patient criteria (RA 16.0% vs. IA 24.5% and NA 23.2%; p = 0.007). NAs were more likely than RAs or IAs to be pregnant (NA 5.5% vs. RA 0.5% and IA 0.4%; p < 0.001), suggesting that pregnancy had brought them back into care; and IAs and NAs were more likely than RAs to have persistent viraemia after 6 months on ART (IA 8.6% and NA 8.5% vs. RA 4.0%; p = 0.012). IAs were more likely than RAs or NAs to have ever had a HIV-related inpatient stay (IA 30.9% vs. RA 21.4% and NA 26.3%; p = 0.022).
There were no significant differences between attendance groups in CD4 count at diagnosis or the first recorded CD4 count (where the CD4 count at diagnosis was not available), or on CD4 count at ART initiation. One-third of patients had a CD4 count of < 200 cells/mm3 at diagnosis (33.0%), one-fifth had a first recorded CD4 count of < 200 cells/mm3 (19.2%), and 39.9% had a CD4 count of < 200 cells/mm3 at ART initiation. RAs and IAs were more likely to have a current CD4 count of > 350 cells/mm3 than NAs (RA 88.6% and IA 84.7% vs. NA 67.3%; p < 0.001). NAs were less likely than RAs or IAs to have a viral load of > 5.0 log-copies/ml at diagnosis (NA 16.9% vs. RA 42.5% and IA 40.8%; p < 0.001). There was no significant difference between groups for first recorded viral load. RAs were more likely to have a current viral load that was undetectable (RA 86.2% vs. IA 76.9% vs. NA 45.1%; p < 0.001).
About one-fifth of patients had ever had an AIDS-defining illness (22.9%) and this did not vary significantly according to attendance group. The most commonly reported AIDS-defining illness was Pneumocystis carinii pneumonia in 6.6% of patients, followed by pulmonary tuberculosis in 4.6%, Kaposi’s sarcoma in 3.1%, oesophageal candidiasis in 2.7% and herpes simplex virus in 2.0%, none of which varied significantly by attendance pattern. A minority of patients had hepatitis C current coinfection (5.8%) or hepatitis B coinfection (3.6%), but neither was significantly associated with attendance. One-fifth of patients had experienced mental health issues in the past year (20.5%) and this was not statistically associated with attendance. Whereas 5.9% of RAs had issues of drug or alcohol dependency in the past year, this was true of 16.7% of IAs and 18.5% of NAs (p < 0.001).
In multivariable analysis, irregular attendance was associated with being a complex patient according to the HARS 3 criteria (aOR 1.76, 95% CI 1.16 to 2.67; p = 0.008), having a HIV-related inpatient stay (aOR 1.51, 95% CI 1.01 to 2.27; p = 0.045) and drug/alcohol dependency issues (aOR 2.90, 95% CI 1.67 to 5.05; p < 0.001). Non-attendance was only significantly associated with drug/alcohol dependency issues (aOR 3.95, 95% CI 2.17 to 7.21; p < 0.001).
Factors associated with irregular and non-attendance
Variables that were significantly associated with irregular attendance in the multinomial logistic regression models were included in binary logistic regression models with backwards-stepwise selection models in the following order:
Block 1: clinic attended and demographic variables –
-
clinic currently attended
-
gender
-
current age.
Block 2: other background variables –
-
number of years since diagnosis
-
has children.
Block 3: mental and physical health in the past year or more –
-
neurocognitive impairment
-
ever diagnosed with depression
-
HARS 3 (complex patient)
-
ever had HIV-related inpatient stay
-
drug/alcohol dependency, past year.
Block 4: other variables –
-
health, past 4 weeks
-
told family about HIV
-
get help when sick in bed
-
enough money for basic needs, currently
-
in charge of life.
The final model is shown in Table 24 (adjusting for clinic attended) where the Cox–Snell R2 = 0.165.
| Factor | aOR | 95% CI | p-value |
|---|---|---|---|
| Age | 0.94 | 0.92 to 0.96 | < 0.001 |
| Years since diagnosis | 1.04 | 1.02 to 1.07 | 0.002 |
| Has children | 2.53 | 1.69 to 3.80 | < 0.001 |
| Neurocognitive impairment | 1.54 | 1.08 to 2.19 | 0.016 |
| Drug/alcohol dependency | 2.44 | 1.37 to 4.34 | 0.002 |
| Health | |||
| Excellent | 1.00 | ||
| Good | 1.44 | 0.95 to 2.19 | 0.086 |
| Poor | 1.96 | 1.30 to 2.96 | 0.001 |
Variables that were significantly associated with non-attendance were included in binary logistic regression models with backwards-stepwise selection models in the following order:
Block 1: clinic attended and demographic variables –
-
clinic
-
gender
-
age
-
education.
Block 2: other background variables –
-
number of years since diagnosis
-
has children
-
not registered with GP
-
not homeowner.
Block 3: mental and physical health in the past year or more –
-
recreational drug use, past 5 years
-
drug/alcohol dependency, past year.
Block 4: other variables –
-
told family about HIV
-
get help when sick in bed
-
enough money for basic needs, currently
-
nurse did not listen.
The final model is shown in Table 25 (adjusting for clinic attended) where the Cox–Snell R2 = 0.215.
| Factor | aOR | 95% CI | p-value |
|---|---|---|---|
| Age | 0.95 | 0.93 to 0.98 | < 0.001 |
| Years since diagnosis | 1.05 | 1.02 to 1.09 | 0.002 |
| Has children | 4.37 | 2.65 to 7.20 | < 0.001 |
| Not registered with GP | 4.85 | 2.13 to 11.06 | < 0.001 |
| Not homeowner | 2.18 | 1.31 to 3.65 | 0.003 |
| Drug/alcohol dependency | 3.36 | 1.74 to 6.50 | < 0.001 |
| Nurse did not listen | 2.88 | 1.47 to 5.65 | 0.002 |
Reasons given for missed appointments
Respondents were asked to report how often they had missed appointments since their HIV diagnosis for 14 given reasons. In addition, we asked respondents whether or not they had missed appointments because of drinking alcohol or taking recreational drugs. All 16 reasons for missed appointments are shown in Table 26, with the ‘no’ and ‘yes’ responses to the items about alcohol and drugs incorporated into the table under the ‘never’ and ‘often’ columns. The reasons are organised according to the COM-B model. Only 60.7% of patients had never forgotten an appointment. The key reasons that respondents gave for missed appointments (sometimes or often) were feeling depressed (21.2%), forgetting (20.5%), not wanting to think about being HIV positive (19.9%) or feeling too tired (19.0%).
| Reason | All patients (%) | IA, % | NA, % | IA vs. NA, p-value | |||
|---|---|---|---|---|---|---|---|
| Never (no) | Rarely | Sometimes | Often (yes) | Sometimes or often (yes) | Sometimes or often (yes) | ||
| Capability | |||||||
| Forgot | 60.7 | 18.8 | 16.2 | 4.3 | 33.1 | 31.1 | 0.668 |
| Depressed | 70.8 | 7.9 | 14.8 | 6.4 | 29.4 | 25.6 | 0.398 |
| Too sick | 71.1 | 12.7 | 14.1 | 2.1 | 24.5 | 16.5 | 0.047 |
| Too tired | 72.6 | 8.4 | 15.3 | 3.7 | 24.9 | 23.8 | 0.791 |
| Alcohol | 96.0 | 4.0 | 6.4 | 6.4 | 0.978 | ||
| Drugs | 93.2 | 6.8 | 13.2 | 12.5 | 0.841 | ||
| Motivation | |||||||
| Not wanting to think about HIV | 75.4 | 4.7 | 11.0 | 8.9 | 19.3 | 28.7 | 0.025 |
| Afraid to be seen at clinic | 84.7 | 3.7 | 7.4 | 4.2 | 10.0 | 18.9 | 0.009 |
| Felt well | 81.0 | 4.1 | 7.9 | 7.0 | 13.0 | 20.7 | 0.033 |
| Had enough medication | 80.5 | 4.9 | 8.0 | 6.6 | 13.8 | 24.4 | 0.005 |
| Doctor could not help | 89.9 | 3.8 | 4.5 | 1.8 | 6.3 | 10.4 | 0.129 |
| Opportunity: social influences | |||||||
| Not followed doctor’s advice | 86.2 | 5.0 | 6.2 | 2.6 | 10.0 | 14.6 | 0.150 |
| Opportunity: barriers | |||||||
| No money | 77.6 | 7.5 | 11.1 | 3.9 | 17.8 | 19.5 | 0.664 |
| No transport | 81.1 | 6.9 | 8.1 | 4.0 | 14.5 | 17.1 | 0.472 |
| No time off work | 75.1 | 10.3 | 9.2 | 5.4 | 16.0 | 23.2 | 0.063 |
| Caring responsibilities | 88.0 | 4.0 | 5.8 | 2.2 | 11.2 | 17.1 | 0.079 |
As RAs, by definition, were less likely to have missed appointments, we compared reasons for missed appointments (sometime or often) given by IAs and NAs. These are also shown in Table 26.
Irregular attenders were more likely than NAs to miss appointments because they felt too sick (24.5% vs. 16.5%; p = 0.047). NAs were more likely to give reasons related to motivation. They were more likely than IAs to not want to think about being HIV positive (28.7% vs. 19.3%; p = 0.025), to be afraid to be seen at the clinic (18.9% vs. 10.0%; p = 0.009), to feel well (20.7% vs. 13.0%; p = 0.033) and to have enough medication (24.4% vs. 13.8%; p = 0.005). There were no significant differences between IAs and NAs on reasons relating to opportunity.
Summary of findings from Chapter 5
-
Irregular attendance is independently associated with being younger, diagnosed with HIV for longer, having children, symptoms of neurocognitive impairment, drug/alcohol dependency and poorer recent health. Irregular attendance is also associated with being female, depression, having complex needs (according to HARS 3), HIV-related hospitalisation, uncertainty about being in charge of life, telling family about HIV status, not getting help when sick in bed and not having money for basic needs.
-
Non-attendance is independently associated with being younger, diagnosed with HIV for longer, having children, not being registered with a GP, not being a homeowner, drug/alcohol dependency and not feeling listened to by the nurse. Non-attendance is also associated with being female, less educated, recreational drug use, telling family about HIV status, not getting help when sick in bed and not having money for basic needs.
-
IAs are more likely than NAs to miss appointments when they feel unwell whereas NAs are more likely miss appointments because of reasons relating to HIV stigma or when they had enough medication.
Chapter 6 Findings from qualitative research: factors associated with engagement in HIV care
In this chapter, we bring together the findings from our qualitative research – individual patient interviews, FGs and key informant interviews – to explore the factors associated with engagement in HIV care. We continue to address the fifth objective of the study, to:
-
understand the situational, environmental, behavioural and social factors influencing outpatient attendance.
Participants
Individual patient interviews
We undertook a total of 33 interviews with PLWH. They were made up of 10 RAs, 13 IAs and 10 NAs. We had originally planned to recruit up to 20 IAs (making an overall total of 40 interviews), but after recruiting 13 IAs, we were hearing similar stories from the participants and decided that we had reached sample saturation. Our NAs included people who had ever experienced a 1-year period of non-attendance in comparison with our survey participants who had experienced a recent period of non-attendance. When we began interviewing people, we found that they were able to talk in detail about their experiences of non-attendance, even if it had occurred several years before.
In addition to attendance pattern, our other primary sampling criterion was combined gender and sexual orientation. We aimed to recruit roughly equal proportions of gay or bisexual men and heterosexual men and women. However, women were more willing to participate and our sample consists of nine gay men, eight heterosexual men and 16 heterosexual women. No men who identified as bisexual took part in the interviews and, with regard to ethnicity or country of birth and age, the subsamples were made up as follows:
Gay men:
-
seven UK born; two non-UK born
-
two aged ≤ 35 years; seven aged > 35 years.
Heterosexual men:
-
four black African; four other ethnicity
-
three aged ≤ 35 years; five aged > 35 years.
Women:
-
10 black African; six other ethnicity
-
five aged ≤ 35 years; 11 aged > 35 years.
Among these participants, we aimed to recruit between three and six people who had ever injected drugs, between three and six patients who were not on ART and between three and six who were diagnosed within the past 2 years. We succeeded in recruiting three people who had injected drugs, two patients who were not on ART (a long-term non-progressor and a woman who had been diagnosed 3 years before during pregnancy) and four who had been diagnosed in the past 2 years.
Participants had been diagnosed for a median of 7 years (ranging from 4 months to 30 years). Their median age was 40 years (ranging from 25 to 56 years). Almost half were of white ethnicity (45.5%), 39.4% were black African, 9.1% were black Caribbean and 6.1% were of mixed ethnicity. The majority were born outside the UK (72.7%). Over half were not in a relationship (57.6%), 21.2% were in a relationship but not living with a partner and 21.1% were living with a partner. Two-thirds had some further or higher education (66.7%) and the majority were not working (72.7%).
Our participants are reasonably representative of an ageing HIV positive population. We did not manage to recruit as many young people as we would have liked, but this may be partly explained because we excluded patients who had acquired HIV through vertical transmission as the AALPHI study was recruiting these patients at the same time. It should also be noted that almost three-quarters of our participants were not working. This is not surprising, given that those who are unemployed are more likely to have the time to take part in an interview, but it means that our qualitative data include fewer perspectives from working people who may also have difficulties with attendance for this reason.
Focus groups
We conducted two FGs – the first (FG1) consisted of four gay men and the second (FG2) of six black African men and women (including two men and four women). Although we encouraged discussion among FG participants, most of their comments were addressed directly to the FG facilitators and the data were very similar in format to the interview data. The data were therefore incorporated into the interview analysis, although some different stories may have been elicited by recruiting these participants through community outreach rather than at their HIV clinics. Participants had been diagnosed for a median of 14 years (ranging from 6 to 27 years). Their median age was 46 years (ranging from 35 to 55 years). All had experienced periods of a year or more when they had not seen a doctor about their HIV and had missed and not rescheduled at least one appointment at the HIV clinic in the past year.
The names used below for both individual interview and FG participants are pseudonyms. In order to provide more context for the data, we will describe whether they were interviewed for the study as a RA, IA, NA or FG participant (FG1 or FG2); and include the following shorthand to describe them, followed by their age (no men who identified as gay or bisexual black Africans took part in this part of the study):
-
female, black African: Fem-BA
-
female, other ethnic group: Fem-Other
-
heterosexual male, black African: HetMale-BA
-
heterosexual male, other ethnic group: HetMale-Other
-
gay male, UK born: GayMale-UK
-
gay male, non-UK born: GayMale-NonUK.
Key informant interviews
We conducted a total of 19 semistructured interviews with service providers and funders. The key constituency of ‘voluntary sector’ was revised to ‘community support’ to reflect the fact that some patient reps are employed by the NHS to work in HIV clinics. The key informant representation from across the key constituencies in the field of HIV service provision was as follows:
Clinical:
-
clinical nurse specialist, NHS
-
senior sexual health adviser (Ceri Evans), Chelsea and Westminster Hospital NHS Foundation Trust
-
HIV nurse specialist, NHS
-
HIV consultant, NHS
-
receptionist, NHS
-
consultant in genitourinary medicine (GUM) and HIV (Vanessa Apea), Barts Health NHS Trust.
Public health:
-
public health expert 1, public sector
-
public health expert 2 (Gillian Holdsworth), SH:24.
Academia (health service researchers):
-
Professor of Medicine (Michael Mugavero), University of Alabama at Birmingham, USA
-
clinical research fellow/Consultant in HIV (Shema Tariq), University College London
-
academic research nurse, university.
Community support/health promotion:
-
patient representative 1
-
patient representative 2
-
chief executive (Juliet Reid), Centre for All Families Positive Health
-
health services manager (Mesfin Ali), Embrace UK
-
patient representative 3 (Chris Sandford), Mortimer Market Centre
-
peer caseworker (Sophie Strachan), Positively UK.
Policy:
-
director of policy, non-governmental organisation
-
NHS manager, NHS.
Key informants are identified by their job title. Their initials are also included if they have agreed to be identified. In the following description of the findings, we refer to ‘key informants’ who took part in this part and we refer to ‘patients’ who took part in the individual interviews and FGs. As previously described, all the people who took part in the individual interviews and FGs were currently engaged in care, and the word ‘patients’ will therefore be used to distinguish their contribution to the study from that of the ‘key informants’.
Factors associated with HIV clinic attendance
This section explores factors associated with HIV clinic attendance and disengagement from care. In the same way as we recruited RAs, IAs and NAs for the survey, we recruited RAs for our qualitative study as well as patients who had difficulties with keeping appointments and those who had disengaged from care completely. Although it was straightforward to distinguish between attendance patterns over the previous year for survey recruitment, it became clear during the qualitative data collection that there can be an overlap between missing appointments and disengaging completely. Some patients had ongoing issues whereby they both missed appointments and stopped attending altogether, whereas others attributed a period of disengagement to particular circumstances and did not usually miss appointments. Furthermore, patients’ behaviour often changed over time, with some participants having disengaged completely in the past but now fully engaged in care, and others having recently returned to care after a period of disengagement. Among RAs, eight reported good EIC after diagnosis and one reported poor early retention; among IAs, eight engaged with care after diagnosis, four had poor early retention and one disengaged post partum; and among NAs, 10 reported good early engagement, four had poor early engagement and one disengaged post partum.
The following analysis will explore the key factors associated with poor EIC generally, considering all the data provided in the individual patient interviews, FGs and key informant interviews. Given the overlap in attendance behaviour described above, we incorporate data provided by all patient participants into this exploration of factors associated with clinic attendance. At the end of the chapter, we summarise the data on why NAs stop going to the HIV clinic and why they return, drawing on data from patients who had experienced a period of 1 year or more when they had not attended for care.
The data are again presented in the context of the COM-B model, which provided the framework for this analysis. Figure 12 summarises the key influences on attendance. It indicates where factors have a positive (+ve), negative (–ve) and mixed (+ve and –ve) impact on attendance. The findings from the key informant interviews were found to echo the findings from the patient interviews and FGs. In addition, key informants described how those who disengaged from care often led complicated lives and particular groups of vulnerable individuals were identified, including people who inject drugs, young people, those who had acquired HIV through vertical transmission, people experiencing abuse, people coming out of detention and those with mental health issues.
FIGURE 12.
Key influences on HIV clinic attendance.

Physical capability
Feeling unwell (+ve and –ve)
In thinking about physical capability, we could consider two conditions: physical disability and physical health. Only two of the patients had visible physical disabilities, and neither of them considered this to be a barrier to attending for care, although one of them appreciated a recent clinic refurbishment that had made the clinic more accessible. Physical health or feeling unwell could be a barrier to attending and patients reported this as one of the reasons for missed appointments. Being diagnosed with hepatitis C and dealing with the extreme mental and physical effects of its treatment turned Matt (IA, GayMale-UK, 44 years), who had been diagnosed with HIV many years previously, from a RA to somebody who often found it difficult to turn up for scheduled appointments. On the other hand, feeling unwell could also provide the motivation to take ART and attend more regularly:
At the moment, I feel that ill that I feel no, this is stupid, it’s not achieving anything so no matter how down you feel, it’s not going to help feeling ill as well.
Bill (IA, HetMale-Other, 52 years)
Key informants also said how people sometimes felt unwell and not up to attending their appointments.
When patients felt well, this could prevent them from attending their appointments, as described below (see Motivation). Thus, the physical capacity to attend was not sufficient in itself to engage with care.
Psychological capability
Forgetting (–ve)
One aspect of psychological capability is memory and patients reported missing appointments because they forgot about them. As described earlier, the conditions of the COM-B model interact and stigma may also be implicated in not prioritising HIV care and therefore forgetting to attend, as Veronica said:
I miss it because I forgot but the reason why I forgot, because I don’t want to have HIV.
Veronica (IA, Fem-Other, 54 years)
Depression was also implicated in forgetting to attend. Key informants attributed forgetting appointments to various causes from infrequent appointments to substance abuse, in the form of alcohol abuse, injecting drug use and chemsex, or neurocognitive impairment.
All the patients who mentioned text reminders thought they were an excellent idea but they did not all receive them. Patients who had disengaged from care could be highly responsive to a personal telephone call from the clinic when it occurred at a time when they were ready to be approached although they had often ignored calls before this time. Some patients felt the clinic could be more proactive in reaching out to them:
Yes, I need to accept responsibility but I also need them to help me do that, as it were, perhaps, so them being more active and chasing a bit was perhaps needed.
Dean (NA, GayMale-UK, 41 years)
Drug and alcohol use (–ve)
Capabilities can also be affected by alcohol and drug use. Only a small proportion of those who took part in the study had experienced problems with alcohol or drugs, but this could also affect attendance:
So the only thing which would stop me, sometimes stop me coming in for appointments, is if I’d had drug use and I just wasn’t up to it, and drug use was usually because I was lonely, isolated, wanted company.
Matt (IA, GayMale-UK, 44 years)
As Matt describes, he had mostly managed to keep up regular attendance until his hepatitis C diagnosis, apart from when he had taken drugs, which, in itself, was a symptom of other psychological issues.
Empowerment through knowledge (+ve)
Another element of psychological capability is the knowledge or awareness of the existence of something that enables the enactment of the behaviour. Those who had been diagnosed for many years described the lack of information in the early days, but the underlying theme of these data was how poor understanding of HIV still shaped patients’ fear and self-stigma and how empowering knowledge could be. Kelly and Jade both talked about their lack of knowledge about HIV transmission before they were diagnosed:
And they used to be racist with it as well, like if you was from Africa, and you just . . . you know those sort of . . . yes. And that’s why as well, I’m not going to lie, I didn’t think I could get it but that’s how ignorant I was. But only because I had picked that up from school.
Kelly (IA, Fem-Other, 32 years)
I thought HIV was only in Africa. It was a little bit naive of me, but it was the last thing I expected.
Jade (FG2, Fem-BA, 37 years)
Patients learnt about HIV in various ways. Patients often did their own personal research into HIV on the internet, Googling or checking specific websites for information about services, HIV or ART. As Bill describes, however, this was not a replacement for receiving timely information from the clinic:
Then a friend of mine brought a laptop in and there’s wireless network and I googled PCP pneumonia, only to find out not only did I have HIV, I was in final stage which was AIDS.
Bill (IA, HetMale-Other, 52 years)
In addition, although the clinic provided an important source of information, patients did not always get what they wanted:
So I never, I felt I wasn’t getting answers and I went elsewhere to get some information and came back here, and I don’t think I should have had to do that, really.
Stephen (NA, GayMale-NonUK, 48 years)
Most of the patients had been directed to support groups that provided invaluable information. Some felt that peer support should be available within the clinic or that a more proactive approach from the clinic would help them engage with support groups, whereas others gave various reasons for not attending. They thought the groups were not for them, they did not have the time or the money, or they were not ready to meet other HIV-positive people. Adele (NA, Fem-BA, 27 years) described how she was not originally bold enough to attend a group and Mary (RA, Fem-BA, 54 years) could not understand the flyers. Josephine had been too fearful to attend any groups but was really happy with the information that she received when she returned to care:
Knowing there is a day where I can go and meet other people who are in the same boat as me, I think is marvellous. But when you are out of this routine, you don’t know whether there is. You don’t even know where to start . . . yes, they’ve laid everything on the table, which is good for me. I’m so happy.
Josephine (NA, Fem-BA, 42 years)
Those who had attended support groups praised what they got out of them. In addition to information provided by professionals, they learnt from their peers about their experiences of using ART, for example, and had the opportunity to talk about HIV. Seeing people who had lived with HIV for a long time also made them rethink their understanding and realise it was possible to live well with HIV:
And then I got educated and said no, these people came late for medication. You’ve got chance, you can prevent that, you don’t have to go that far. Taking your medication protects you from getting other illnesses.
Amy (FG2, Fem-BA, 55 years)
Patients specifically related the knowledge that they gained from engaging with peer support to the empowerment that helped them engage with care:
So that kind of gave me the empowerment and the knowledge that I needed to take my life back on track.
Adele (NA, Fem-BA, 27 years)
You gain more knowledge, you become more knowledgeable, you become wise and you become mentally strong.
Vincent (FG2, Male-BA, 47 years)
Motivation
Denial (–ve)
In the same way that stigma contributed to patients stopping attending for care altogether, it also contributed to their ‘forgetting’ about appointments, as Veronica described, because she did not want to have HIV. There was a strong theme relating blocking out HIV to disengagement from care that was expressed by several patients who struggled with attendance, as illustrated by the following patients:
When I was at school, [HIV] was like, it was a really bad, seen as a really bad illness, it was dirty, that’s why when I had it I just wanted to forget it and block it out.
Kelly (IA, Fem-Other, 32 years)
I don’t know. Like, I just feel, why me? Like, I’m young. I’m young, and that. I don’t need this and that. Sometimes I think to myself – sometimes I think I don’t have it but I do have it. That’s how I feel.
Julie (NA, Fem-BA, 25 years)
I just thought, oh we’re just going to forget about it, and it’s not real, and it’s not happening, and I don’t need to . . . you know, I’m not sick, and yes, so I just put it to the back of my mind, and got on with it, really.
Ben (NA, GayMale-UK, 33 years)
As Ben explains, feeling well, which is described below, could give people a reason for not thinking about HIV and not engaging with care.
Key informants also described how coming to the clinic could remind people of their HIV status. The stigma associated with HIV was a key issue:
I think that focusing on or recognising, doing something about stigma, is just, I feel, the real crux of it.
Consultant in GUM and HIV (VA)
They said how patients could be overwhelmed by HIV and in denial, particularly soon after diagnosis.
Feeling well (–ve)
When patients felt well, this could provide a reason for not attending the HIV clinic at all, particularly before starting ART. Kelly also explained how she only took ART when she felt ill and did not want to attend when she was healthy:
I stopped coming to clinics because I thought I was all right.
Kelly (IA, Fem-Other, 32 years)
Key informants agreed that feeling well could stop patients from attending. It could also seem like a waste of time getting to the clinic and waiting for a short appointment and was particularly disruptive for stable patients to attend clinic appointments only to be told that everything was fine:
If someone travels for one and a half hours or more and sits in the reception for 20 minutes, 30 minutes and they’re seeing a consultant for 10 minutes, it’s – to them, it doesn’t feel productive. It doesn’t feel satisfying.
Patient representative 2
Similarly, when patients did not need to take ART after giving birth, with the implication that they were well, this could also contribute to disengagement from care. It should be noted that ART is now recommended for all HIV patients regardless of CD4 count, and is unlikely to be offered as a temporary intervention during pregnancy. 43
Depression (–ve)
Depression was a key theme across the participants and many of the patients talked about the depression that they experienced. It was particularly prominent among IAs and NAs who often related their disengagement to depression and an inability to do anything when they felt particularly depressed. It made patients feel that they could not leave the house. It led to feelings of lack of self-worth whereby they did not see the point in looking after their health, as Bill explains:
I’d fall into these depressions and why would you take life-saving medicine when you’re depressed about everything, do you know what I mean?
Bill (IA, HetMale-Other, 52 years)
Although patients who were engaged in care also expressed feelings of depression, they were more likely to find the motivation to get themselves to the clinic:
Sometime you lose confidence, sometimes you feel like you don’t want to talk to nobody but you drag yourself to come to the clinic because you have to come to the clinic.
Miriam (NA, Fem-BA, 39 years)
Some patients were concerned about the mental health provision within the NHS and how long it could take to be referred for therapy.
Key informants said that poor mental health was very common and could underpin engagement in HIV care, although it was often unclear whether it was a cause or a result of HIV. People suffering from depression could give up hope or sometimes people were just having a bad day and could not face attending. There was a subpopulation of patients with severe mental illness who had ongoing issues with EIC.
Low self-esteem (–ve)
A HIV diagnosis is also likely to affect patients’ self-esteem. Patients described low self-esteem as a powerful cause of disengagement from care that could stop them from attending appointments and/or taking ART. For example, Kelly (IA, Fem-Other, 32 years) said that she did not value herself and only took ART during pregnancy for her baby; and Rick (NA, GayMale-UK, 44 years) said that he stopped attending because he did not want to look after himself and did not think he was worth it.
Self-efficacy and control (+ve)
There was a keen sense of self-efficacy among some of the RAs. Jackie (RA, Fem-Other, 44 years) expressed strong motivation and desire to take complete responsibility for her health and Peter (RA, GayMale-NonUK, 40 years) said he felt in control of his health and empowered. A sense of self-belief could help to live with HIV and engage in care:
I choose to fight, you know, and I live with HIV.
Gabriela (RA, Fem-Other, 49 years)
I must make sure that every time I know I have to attend and I have to make sure I’m well.
Daniel (RA, HetMale-BA, 32 years)
If I want to get well, I have to keep up my appointment. I don’t see any big deal in it.
Patricia (RA, Fem-BA, 34 years)
By comparison, there were indications that lack of engagement in HIV care was associated with feelings of a lack of control. Stephen felt that he was in a different space now to when he was having difficulties with engaging and that helped him to attend:
I’m more confident in stating what my needs are. I mean, I know I’m not a sheep being, you know, herded into one place or another. I can speak up now.
Stephen (NA, GayMale-NonUK, 48 years)
Some key informants described how some people lacked the motivation to take responsibility for their health care.
Adherence to antiretroviral therapy (–ve)
There was a strong association between non-adherence to ART and missed appointments – some patients clearly avoided attending when they had not taken their medication. They knew that not taking their tablets would show up in their laboratory results and might avoid attending if they felt embarrassed or did not want to be told off:
Sometimes, when I don’t take my medication, and I know that the results might possibly not be so good, then I will decide not to go because I don’t want to be stressed twice by the doctor asking me what’s happening, why haven’t you been taking your medicine . . . once I start feeling a bit better, then they will find me three months later, you know, with my CD4 count back on track.
Jade (FG2, Fem-BA, 37 years)
I haven’t taken my meds for about a month I can’t go. It’s like going into school on Monday morning when you haven’t done your homework, just like, ah no I’ll phone in sick I won’t go to school on Monday and then I’ll do my homework and it’ll all be fine by the time I go back in on Tuesday.
Rick (NA, GayMale-UK, 44 years)
Familiarity with clinic (+ve)
The vast majority of the RAs talked very warmly about how comfortable they felt at the HIV clinic and the friendliness of the staff, and patients, in general, valued getting to know staff and feeling familiar with the clinic. On the whole, patients tended to stick to the clinic with which they were familiar:
. . . there is a sense almost of community there, I guess, because you are familiar with some other patients and doctors, I guess, so that helps to engage, I guess, with the clinic.
Leonard (FG1, GayMale, 45 years)
Many patients expressed loyalty towards their clinic. Gabriela felt that her clinic had supported her very well and she felt an obligation to return this support:
So that pushed me and helped me to be very regular and don’t miss any appointments and to be very loyal to the clinic, you know, because they showed to me that they was very loyal to me. So it’s the least I can do, you know.
Gabriela (RA, Fem-Other, 49 years)
Some patients also travelled long distances in order to attend the clinic with which they were familiar. Jose (IA, HetMale-Other, 50 years) said that he would walk for 2 hours to get to the clinic if he did not have the money for transport. Clinic appointments provided a rare opportunity for some patients to talk openly to trusted individuals about their HIV and feel supported. Veronica explained how she was not able to do this under other circumstances:
You know, it’s strange but it’s like you saying, can you leave your leg at home, you know, part of you must be cancelled.
Veronica (IA, Fem-Other, 54 years)
Faith (+ve and –ve)
Faith played a supportive role in patients’ lives although they mostly did not discuss their HIV in this context. Key informants were more likely to point out the possible negative impacts of stigma within religious communities. Although certain religious beliefs and a belief that they had been healed might prevent people coming to the clinic, the HIV nurse specialist that we interviewed said that the spate of religious ‘cures’ had disappeared.
Opportunity: social influences
People finding out you have HIV (–ve)
Social influences played an important role in shaping patients’ engagement in HIV care. Many patients were not open about their HIV status, choosing to tell very few members of their circles of friends and family. Attending the HIV clinic could be a cause for concern because you might bump into someone who would find out that you had HIV. Opinions were divided over whether the HIV clinic should be incorporated into sexual health clinics so that HIV patients could not be distinguished from other patients, or whether or not providing separate space for HIV patients made it feel more secure. Some patients continued to feel the discomfort of going to the clinic they had been diagnosed for several years. Kelly had been living with HIV for 6 years:
. . . obviously you don’t want . . . anyone to know, the way it is, is just, you cringe when you’re coming [in], because you don’t know who’s going to be sitting there. That’s the only thing, I think. And that is every single time I come, I have that same feeling, even today.
Kelly (IA, Fem-Other, 32 years)
Being part of a black African community gave people particular cause for concern that their HIV status might be discovered and become the subject of gossip. Sara (IA, Fem-BA, 44 years) changed clinics because the HIV clinic closest to her home was used by a lot of people from her home country.
It was not always just other patients that they were concerned about. Adele (NA, Fem-BA, 27 years) disengaged from HIV care completely when she felt threatened by a member of the hospital staff from her home country. Jose (IA, HetMale-Other, 50 years) used to live close to the hospital and expressed his early concerns that somebody working at the clinic might turn out to be a neighbour.
Many key informants talked about patients’ fears of being recognised in the clinic either by a member of your community or by a former partner. Also, people often did not want friends and family who they had not told about their HIV to be curious about the appointments that they were attending.
Health-care professionals (+ve and –ve)
Various members of the clinic team provided emotional support that could be a strong motivation for attending the clinic:
They really, really cared about me, you know, and they really care about my health, more than I do, you know.
Gabriela (RA, Fem-Other, 49 years)
Most patients wanted to see the same doctor and some concern was expressed about non-continuity of care and a lack of opportunity to build rapport or the mixed messages that could result. Some patients said that they would attend as frequently as their doctor asked, whereas others wanted a more balanced relationship and talked about making informed decisions as the outcome of teamwork. Time invested in the doctor–patient relationship was a good thing although some patients did not feel that they got enough time with their consultants. Stephen (NA, GayMale-NonUK, 48 years) had ongoing issues with feeling that he was not being listened to until he started seeing a specialist registrar who had more time for him. Nurses were also very highly regarded. Patients appreciated the practical support they got from clinic staff and peer workers – although they felt more support of this nature would be helpful.
On the negative side, both Dean (NA, GayMale-UK, 41 years) and Stephen (NA, GayMale-NonUK, 48 years) had problems with communication with health-care professionals, which contributed to their disengagement:
There was no negotiation, there was no way out of that, and I thought, you know, it was just another problem that I then had to deal with . . . I disengaged somewhat, actually, at that point.
Stephen (NA, GayMale-NonUK, 48 years)
Key informants talked about how bad experiences with clinic staff could put patients off attending. They said some patients felt that doctors just did not understand them and this could engender distrust. The doctor–patient relationship might also make it difficult for patients to explain their circumstances or difficulties with attending. Key informants also found that some patients could feel ashamed when they had missed an appointment and some felt that they would be ‘told off’ at the clinic for not coming in:
And once people haven’t come once, one thing that we do find is that when they decide to come back they often feel very ashamed about having been away so they put off coming back because they’re really worried about what’s going to happen when they come back.
HIV consultant
Key informants highlighted the stigma that people experienced in other health-care settings and a clinical research fellow/consultant in HIV (ST) described the findings from her research on the widespread occurrence of negative experiences among women in maternity care at the time of delivery.
Partner (+ve and –ve)
There was a clear impact of partners who could have a protective or destructive effect. Meeting a new partner after diagnosis could raise self-esteem and provide the motivation and support to attend for care and take ART. Patients’ experiences with the partners that they already had when they were diagnosed varied widely from the very positive to the very negative. Four women had experienced abuse from their partners that they associated directly with their HIV diagnosis. Gabriela (RA, Fem-Other, 49 years) talked about how her husband used her HIV against her and Lena (RA, Fem-Other, 26 years) was now living in a refuge after her husband started abusing her verbally and physically – he also threw away her medication. Sara’s (IA, Fem-BA, 44 years) husband had told people about her HIV which contributed to her depression and Josephine’s reaction to her HIV diagnosis contributed to her disengagement from care – he refused to tell her his HIV status, and became abusive and a burden:
The only stigma I’ve faced is from my husband, all the time, all the time, yes. The rejection is only from him, but outside people, no.
Josephine (NA, Fem-BA, 42 years)
Several patients talked about how having HIV prevented relationships – both from their experience in the past and looking forward to the future.
Peer and community support (+ve)
Peer support could be very powerful in helping patients on a number of levels. Meeting someone who was HIV positive could provide hope that you would also live well with HIV, as Michelle explains:
I said, you don’t look HIV, and she said, what do you think the HIV people look like, you know? I said, well, not you. She said, well, you’ll be like me one day, you know, people will walk past you and won’t know that you’re HIV. And she kind of gave me inspiration.
Michelle (IA, Fem-Other, 42 years)
Talking to other people who were HIV positive provided the opportunity to talk about living with HIV and exchange information that could help guide thoughts and decisions about their health care. It provided the warmth of emotional support and helped people understand that they were not alone. Patients said how invaluable peer support had been, particularly soon after they were diagnosed, and the courses for newly diagnosed patients run by community organisations and clinics were universally praised by those who had attended and their absence was noted by those who did not:
I think the only sad thing for me was, my early days of diagnosis, I came to the clinics and did what I had to do and then I was on my own, really. I had to go through all that on my own.
Rob (FG1, GayMale, 45 years)
Peer support continued to provide an important role for many over the years, although some commented on how provision had been cut. Some patients did not think of themselves as the sort of people who went to support groups or did not have time to go and it could be daunting to take the first step.
Key informants also highlighted the importance of peer support – which will be discussed further in Chapter 7.
Opportunity: barriers
Although specific barriers may affect only small numbers of the patients who took part in our study, they could have a dramatic effect on attendance and were often associated with socioeconomic status. The barriers to attending were frequently cited by key informants as reasons for disengaging from care. Many key informants described how attending the clinic was not always a priority when people were struggling with the pressures of daily life and juggling priorities – their basic needs took precedence:
I think there are structural factors that if folks don’t have a safe place to live, a stable source of transportation, and a stable source of food, that a lot of times those structural things can take precedence over coming to the clinic.
Professor of Medicine (MM)
I think in terms of your hierarchy of needs, coming to HIV appointments falls lower and lower when you’re actually sort of scrabbling to survive.
Clinical Research Fellow/Consultant in HIV (ST)
Sometimes an escalation of difficulties or crisis could prompt disengagement.
Financial difficulties (–ve)
Three-quarters of the interview patients were unemployed (we did not collect these data for FG patients), many were living on benefits and some talked about their financial difficulties. Daniel (RA, HetMale-BA, 32 years) and Sophie (IA, Fem-BA, 38 years) both said that they sometimes had to cancel appointments when they did not have money for transport, even though Daniel knew he would get reimbursed. Julie (NA, Fem-BA, 25 years) said that she did not attend her appointments when she did not have enough money for transport.
Key informants frequently highlighted financial constraints. Sometimes people could not afford the fare and they did not always go to their closest clinic so transport could cost them more:
It could be just to do with poverty; they can’t just afford to go for their appointment. Even if the money is going to be refunded . . . but if they haven’t got the money to go to their appointment, they can’t go.
Chief Executive (JR)
In addition to the cost of getting to the clinic, the overall time needed for an appointment could be a deterrent, especially when patients were well and stable and everything was fine.
Work responsibilities (–ve)
For some patients, a work commitment was one of the key reasons for not attending HIV clinic appointments. A number of the patients had put work responsibilities before clinic appointments and Jade (FG2, Fem-BA, 37 years) explained how her schedule changed often and she could not attend if she was busy at work. For Josephine (NA, Fem-BA, 42 years) and Joyce (NA, Fem-BA, 36 years), a combination of work and caring responsibilities had contributed to their disengagement from care. Sometimes patients missed appointments not because they were busy at work but because they did not want to lose their jobs and Alex (IA, HetMale, 34 years) felt that he could not miss work regularly to come to the clinic for this reason.
Key informants talked about logistical issues such as child care and work. Some people were busy at work and others were concerned about losing their jobs if they took time off. It could be difficult to squeeze in appointments after work and not easy to tell an employer about being HIV positive in order that appointments could be accommodated around work schedules.
Caring and child care (–ve)
New mothers could find that they had little time to think about themselves and prioritise their HIV care and, as described by Jade (FG2, Fem-BA, 37 years), the repeat HIV testing of a newborn baby could put some women off attending for care. The combination of work and child care responsibilities could also contribute to disengagement from care, as illustrated above. Child care responsibilities could also result in missed appointments and Brenda (IA, Fem-BA, 39 years) sometimes missed appointments because she could not afford child care but did not want to bring her children to the HIV clinic and expose them to the information that was displayed there.
Of the women diagnosed during pregnancy, Patricia (RA, Fem-BA, 34 years) had always been a RA and appreciated the excellent and continuous care she received from the same doctor during and after her two pregnancies. Adele (NA, Fem-BA, 27 years) and Josephine (NA, Fem-BA, 42 years) disengaged after their babies were born, Joyce (NA, Fem-BA, 36 years) disengaged many years later and Kelly (IA, Fem-Other, 32 years) had ongoing problems with engagement. She described taking ART religiously during pregnancy for the baby – like folic acid. None of these women related their disengagement to the quality of HIV care they received during and after pregnancy.
Homelessness (–ve)
Seven patients talked about experiencing homelessness, from street homelessness to the insecurity of staying in temporary accommodation. Two more patients had also lost their homes. There was a clear link between Charles (NA, HetMale-BA, 47 years) becoming homeless and stopping taking his ART (because it needed refrigeration) and attending the HIV clinic. Patients who had stayed in hostels had experienced stigma because of their HIV and receiving letters from the clinic was difficult in temporary accommodation. At the same time, the HIV clinic could provide some support and facilities when patients were homeless.
Immigration issues (–ve)
Some patients talked about difficulties over their immigration status but only Samuel (IA, HetMale-Other, 56 years) related it to his EIC because he became depressed when he was unable to work and stopped attending. In other patients, immigration issues were among the personal problems that they faced and needed to prioritise.
Key informants discussed how issues with immigration status could mean that HIV care was not a priority. Although none of the patients linked fear of deportation to fear of attending the HIV clinic, key informants had found that immigration status could also stop people from attending because they wanted to avoid institutions or were concerned that they may be charged for health care:
It isn’t always easy to disentangle the HIV care from other bits of care . . . and the threat then of charges and intrusive questioning as to their status can deter people also from the HIV Service even though it’s free.
Director of Policy
Clinic facilities
Patients had specific suggestions for service improvements and interventions to improve engagement in HIV care that are summarised below. Although services could be improved to address the factors described above, the experiences that patients described indicated that facilities were not a key factor in disengagement from care. Refurbishments were generally well received although there were some concerns about spending money and lack of thought in new design. Key informants said that at some clinics, patients might not feel that they fit in with the other people in the clinic and a feeling of belonging might help them to feel more connected to the clinic.
Service provision
Patients generally lived fairly close to their clinic and/or found their journey by public transport convenient. Those who lived further away chose to stay with the clinic with which they were familiar although some missed appointments were attributed to moving out of the area. Patients’ negative experiences of other health-care provision generally contrasted with the positive experiences at the HIV clinic.
Patients wanted their care to be integrated. Those with other conditions had experienced differences of opinion between the HIV service and other NHS services. It could be difficult to understand how services fitted together and, generally, patients would prefer everything to be in one place:
It would be great if it was, you know, all under one roof.
Bill (IA, HetMale-Other, 52 years)
Key informants also felt that the health-care system could be difficult to negotiate or not easily accessible with too many appointments to attend and too many clinics.
Why do non-attenders stop going to the HIV clinic?
We have described the factors associated with HIV clinic attendance, including factors relating to missed appointments and complete disengagement from care. In the following section we disentangle these two types of disengagement to explore why NAs said that they stopped going to the clinic altogether and what brought them back in to the clinic, focusing on the data provided by the subsample of patients who had experienced a period of disengagement for 1 year or more.
Patients often attributed their non-attendance to a range of causes and were able to pinpoint various triggers for disengagement. With reference to the COM-B model, the key conditions associated with dropping out of care are related to motivation and opportunity to attend, whereas lack of capability did not appear to play a key role in non-attendance.
Motivation
Although not everyone articulated a direct cause and effect, there was a clear and pervasive relationship between the stigma associated with being HIV positive and disengagement from care. This self-stigma was associated with negative thoughts such as not being able to tell anybody about being HIV positive and not being able to live the life one wanted to lead. Stigma affected both those who engaged regularly with care and those who did not. Stigma was a barrier to people accessing support when it was more comfortable not to think about HIV or when they worried about been seen at the HIV clinic. Patients described how the associated depression and low self-esteem could make taking care of oneself seem pointless. Although patients appeared to be particularly likely to engage with this type of thinking and dropping out of care when they were newly diagnosed, it could continue to affect engagement for people like Julie who had been diagnosed for 4 years:
I just think, why should I bother because even though I go there, this thing what I have, is not going to disappear. It’s not going to disappear. I’ve still got it, so I don’t know. I don’t . . . I just, sometimes I don’t, like, I don’t put it in my head that I’ve got HIV.
Julie (NA, Fem-BA, 25 years)
The possibility of passing on HIV to a child was also found to be difficult to deal with. For example, Jade (FG2, Fem-BA, 37 years) did not attend for 18 months after she had her second baby, because she had found the frequent HIV testing and possible HIV diagnosis of her first baby very stressful.
These feelings of self-stigma were especially strong among the black African patients and could be exacerbated by stigma experienced or imagined from other community members. Adele (NA, Fem-BA, 27 years) felt threatened by a member of the hospital staff from her home country that she confided in when she was having her baby and subsequently withdrew from care for 2 years.
Self-stigma was also found to be implicated with not attending the clinic when patients felt well, particularly before starting ART, even though they knew that they were expected to attend the HIV clinic for routine check-ups:
I was down, I was still in the process of denial . . . so I completely thought to myself, you know, what’s the point? I just need to, sort of like, stay strong for the kids as well as myself, and being that I kept well, I’ve never felt unwell and all that. So I kept thinking, oh, I’m fine. I’ll go next month. I’ll go a month after, and month turned into months turned into . . . and yes.
Josephine (NA, Fem-BA, 42 years)
However, Charles (NA, Male-BA, 47 years) understood that it was not necessary to attend because he was well and only attended twice in the 4 years after his diagnosis. Similarly, Miriam explains how the message she took away when she did not start ART at diagnosis was that she did not need to engage with care:
I just completely forgot, not forgot, just said, OK fine, I’m OK, because if they think if I’m not ready for medication it means I’m OK, you know, it means I’m fine.
Miriam (NA, Fem-BA, 39 years)
In relation to this, Rick (NA, GayMale-UK, 44 years) said that 6-monthly appointments were the ‘worst thing that could have happened to me’ because it seemed like he was doing well and could stop taking his medication.
Once ART was prescribed, patients’ experiences illustrate how their motivation to attend could be shaped by ART. Cynthia (FG2, Fem-BA, 35 years) stopped attending for a year when she was advised to start taking ART and was fearful of doing so. Among people who had started ART, the stress of taking it could result in a conscious decision to stop taking it and stop attending:
Along the way I think emotionally I got fed up with the drugs and stopped taking it without telling them.
Joyce (NA, Fem-BA, 36 years)
Jade (FG2, Fem-BA, 37 years) also stopped taking ART because it was ‘permanent persecution every day’, reminding her about HIV. Difficulties with ART could result in more gradual changes to adherence that could equally result in disengaging from care:
So I kind of drifted off that. I stopped going into the clinic because I didn’t want to admit to them that I drifted off that.
Rick (NA, GayMale-UK, 44 years)
As Rick describes, the relationship between non-adherence and non-attendance may be mediated by the desire to avoid the perceived censure of health-care professionals when not taking medication. The hierarchical relationship between doctor and patient was apparent across the diverse cultural backgrounds of the patients, with references to being a good patient who attends regularly and takes their medication and not wanting to be told off for not doing so. The association between adherence, attendance and the doctor–patient relationship also illustrates the interaction between the elements of the COM-B model.
Opportunity: social influences
Although patients generally thought well of clinic staff, two of them described how periods of disengagement resulted from a breakdown in their relationship with health-care professionals at their HIV clinic. Both felt that they were not listened to and therefore withdrew from care.
Opportunity: barriers
Various barriers were implicated in stopping attending that were often particular to individuals but all resulted in complete disengagement from care. Charles (NA, HetMale-BA, 47 years) became homeless, which made it difficult to keep medication that required refrigeration and to keep up attendance. Although Joyce (NA, Fem-BA, 36 years) made a conscious decision to stop attending when she stopped taking her ART, this was preceded by changes in her child care arrangements which impacted her working hours. Some patients, such as Rick (NA, GayMale-UK, 44 years), describe a range of causes, including self-esteem, adherence and working in a responsible job with erratic hours, which made it difficult to adhere to complicated medical regimes and attend for HIV care. Barriers to attendance are interwoven with motivational issues, social influences and not prioritising HIV care. For example, Josephine (NA, Fem-BA, 42 years) was diagnosed during pregnancy – she was doing two jobs, her husband was not working and abusive, she had four children including the baby and one with a serious disorder that needed a lot of care:
So whenever I had a free time, I was too exhausted even to think of coming to the clinic . . . I was always tired and the thing overwhelmed me. My mind was just concentrating at the immediate basic things in the house. My children, mortgage, work.
Josephine (NA, Fem-BA, 42 years)
Why do non-attenders come back to the HIV clinic?
Patients recognised that when they became disengaged from care, it could be difficult to return to the clinic:
Just life got in the way, and then, for me, it’s like really hard to make those steps, kind of, back . . . and the longer you leave it, the harder it gets.
Ben (NA, GayMale-UK, 33 years)
Some patients had been concerned that their doctors might react badly when they returned, but they reported receiving a positive response. Dean (NA, GayMale-UK, 41 years) described how he actively sought the help of a community organisation to contact the HIV clinic on his behalf in order to re-establish communication after disengagement.
It often took a particular trigger to bring them back into clinic, although Julie (NA, Fem-BA, 25 years) explains how she came after she ran out of ART:
I know I done bad, but them times I was kind of worried as well so I should just go, and check, like, for my health and that, if I’m all right. I came back 2 weeks ago.
Julie (NA, Fem-BA, 25 years)
Becoming ill was one of the main reasons why patients returned to care, and social influence also played a key role. Several patients talked about a nurse or doctor at the clinic who persuaded them to come in. When this came at a time when they were ready to come back to the clinic, it could provide a welcome bridge back to HIV care:
I just welcomed the phone call, I felt there was a bit of interest in my situation, so I feel OK about coming here.
Stephen (NA, GayMale-NonUK, 48 years)
Other patients were supported and advised by trusted professionals from outside HIV to return to care and starting a new relationship could also give patients additional motivation to make this change in their lives.
Summary of findings from Chapter 6
-
Physical capability to attend the HIV clinic is affected if patients feel unwell and psychological capability is affected when patients forget their appointments or when they have used alcohol or recreational drugs. Knowledge about HIV can empower patients, enabling better EIC.
-
Motivation to attend is affected by not wanting to think about HIV, feeling well, being depressed, low self-esteem, poor self-efficacy and control, and poor adherence to ART. Familiarity with the clinic and the staff can improve motivation to attend.
-
Opportunity incorporates social influences and some people are afraid that people will find out about their HIV status when they attend the clinic. Health-care professionals and partners can have positive or negative influences; peer support is very powerful.
-
Barriers such as financial difficulties, work and child care responsibilities, homelessness and immigration issues can limit opportunity.
Chapter 7 Findings: improving engagement in HIV care
This chapter begins with a brief summary of the findings from the previous chapters on the factors associated with engagement in HIV care, with a focus on where potential interventions should be targeted.
We will then summarise the data collected from PLWH and key informants on how to improve engagement in HIV care. We were primarily interested in asking patients about their lived experiences, whereas service improvements and interventions formed the main focus of the key informant interviews. The short summary of patient suggestions is followed by the more detailed data provided by the key informants.
Last, we will present our suggestions for interventions to improve engagement in HIV care based on this accumulation of evidence, including a shortlist with preliminary costing. This chapter addresses the final objective of our study, to:
-
develop intervention models to improve EIC, to be tested in future studies.
Summary of evidence from REACH on where to target interventions
The data from the three phases of REACH provide consistent evidence on where to target interventions to improve engagement in HIV care.
Background
The evidence from our survey complemented the evidence from secondary analysis of UK CHIC data on the background factors associated with regular clinic attendance. In both cases, the analysis suggested that the association with gender could be explained by other factors, whereas there was a clear indication that younger patients were more likely to disengage from care. On the other hand, survey respondents were also more likely to disengage the longer they had been diagnosed whereas higher engagement among UK CHIC participants was found only in the first year after being initially seen at a clinic. Both analyses found an association between acquisition of HIV through sex between men and being on ART with better EIC. The survey found better engagement among white participants and UK CHIC found that those of ‘other ethnicity’ had poorer engagement. In addition, the survey indicated that lower education and not being registered with a GP were associated with a period of disengagement. The data suggest that interventions might be usefully targeted at these patient groups.
Physical capability
The findings from the survey were supported and enhanced by the findings from the qualitative component of the study. The data indicated that, when patients felt unwell, they are more likely to miss appointments, but ill health could also be the trigger for people to re-engage with care. Poorer health was reported among IAs and feeling unwell was given as a reason for not attending appointments by survey participants and those taking part in qualitative research. The data from clinic notes also showed that irregular attendance was more likely among patients who had had stayed in hospital because of their HIV. The study suggests that patients may need extra help – both psychological and practical – to attend the HIV service when they are not well.
Psychological capability
The quantitative and qualitative data indicated that forgetting to attend was a key reason for missed appointments. The survey data furthermore suggested that this may be associated with neurocognitive impairment. The evidence indicates that appointment reminders could be usefully employed to reduce missed appointments.
Data from the questionnaire and clinic notes indicated that alcohol and recreational drugs use were associated with disengagement from care. Although only one interviewee discussed recreational drug use, he gave this as a key reason for missed appointments, and interventions targeting drug and alcohol use should be considered.
Patients who took part in interviews and FGs showed the importance of information in tackling self-stigma and becoming empowered to take care of one’s health. Education can provide patients with the knowledge to engage with care.
Motivation
Depression emerged as a key reason for irregular attendance from both the quantitative and qualitative data sets. Although depression is more common among PLWH,70 the data from REACH suggested that people who disengage from care may be less well equipped to manage this and take charge of their health care. Mental health services have a key role to play in maintaining EIC.
Interview and FG participants expressed a clear association between not adhering to ART and not attending HIV services. Interventions tackling poor adherence to ART might help some people engage with HIV care more generally, as well as interventions targeting the language used by clinicians when addressing non-adherence with their patients.
Narratives of stigma were widespread among those participating in the qualitative research and self-stigma could contribute to disengagement from care. Items from the quantitative measure of internalised stigma indicated high prevalence of low self-esteem (34.1%), self-blame (28.8%) and shame (28.7%), but the summative score did not distinguish between groups. NAs were, however, more likely to give issues relating to stigma, such as not wanting to think about HIV or not wanting to be seen at the clinic, as reasons for not attending. This suggests that interventions that deal with stigma could help people to engage in HIV care.
Opportunity: social influences
Although many patients felt that the dedicated HIV clinic created a safe space, as described above, fear of involuntary disclosure of HIV status could stop people attending the clinic. Holding clinics in premises not associated with HIV might encourage some people to attend. Although there was little in the survey about peer support, its potential to address stigma in addition to other important benefits was consistently highlighted in the qualitative component of the study.
The quantitative and qualitative data generally suggested that HIV staff created a positive environment for patients. However, the relationship with health-care professionals could break down and interventions targeting staff interactions with patients might help some patients attend more regularly.
Opportunity: barriers
The quantitative and qualitative research emphasised the negative impact of poverty on EIC. Patients dealing with social issues like homelessness or immigration problems did not always prioritise their health care. Although we cannot tackle the overall problem of economic inequality, free transport to the clinic might reduce missed appointments. In addition, multidisciplinary, holistic support including social workers, psychologists and peer workers might provide advocacy and support to help patients engage in care.
Other barriers, including lack of child care and work responsibilities, were raised in both the survey and interviews. More flexible services, which offer services outside usual hours or in different locations, might help to address these obstacles.
Patients’ suggestions to improve engagement in HIV care
Suggestions that address capability
Appointments
Text reminders were seen as helpful, as were online appointment systems. Text reminders might include the options: press 1 – attend, press 2 – not attend, press 3 – telephone consultation. Clinics should always call patients when they missed their appointments.
Information
A booklet for new patients that helped to signpost facilities and an information pack about HIV for patients who were admitted to hospital at the point of diagnosis and had no access to information.
Adherence
Dosette boxes should be issued as standard.
Suggestions that address motivation
Education of others
A strong public message was needed to destigmatise HIV because people were still thinking about the ‘coffin adverts’. Health-care professionals from outside the field of sexual health and HIV need more education about HIV.
Clinics
Clinics aimed at particular groups (women, gay men and so on) might make coming to clinic more comfortable for some people.
Suggestions that address opportunity: social influences
Interaction with staff
Receptionists should not make patients feel like they are being told off and consultants should ask patients directly what they should do if they do not attend their appointments.
Peer support
Patients wanted peer support based in the HIV service. They wanted an opportunity to get together informally to talk about issues. Peer support at the point of starting medication was particularly useful.
Suggestions that address opportunity: barriers
Advice and support
Patients wanted advisers who could give practical social support in the HIV service.
Opening hours
Flexible opening hours including evening and weekends would be helpful.
Transport
Transport should be paid in advance and hospital transport made more available for people with disabilities.
Consultations
Home visits and Skype consultations would be useful.
Facilities
Patients wanted a crèche for children, somewhere to leave them during an appointment that was away from HIV information.
Key informant suggestions to improve engagement in HIV care
There was no one-size-fits-all solution to improving engagement in HIV care and a range of tools should be developed based on patient need, with case conferences helping to devise tailored support. It might also help to look at other conditions for interventions or service improvements that had been successfully implemented.
Key informants raised the importance of systematically identifying NAs as well as redirecting resources to them, and we begin this section with a summary of these two issues. This is followed by a description of interventions based on the key informant interviews according to the three elements of the COM-B model.
Identifying non-attenders
Key informants raised the issue of pre-empting non-attendance by identifying those at risk of disengaging and keeping an eye on them, as well as considering that risk may change over time. They highlighted the importance of taking a systematic approach to tracing people who had disengaged from care by drawing up an explicit, consistent follow-up policy involving a service with accountable staff who had time dedicated to this work. A service manager with clinical support or a lead clinician with multidisciplinary support could lead this.
The key informants talked about the sort of systems that might work and who should be involved. For example, there could be an electronic solution whereby a member of staff was responsible for an active process of flagging and tracking NAs. This might require integrating multiple data systems and improving data sharing between clinics. Technology might also be used to help keep patients contact details up to date, asking them to text their new address or telephone number.
Regular clinics or meetings could be held to identify NAs who should then be contacted by telephone, e-mail and/or letter. All attempts to contact patients should be documented. There were different suggestions about who should make the contact with patients. There was some benefit in the consultant who knew the patient making the approach, whereas in other services a nurse took responsibility for tracking people down. Patient representatives could also take on the role or health advisors had the skills to make an initial approach and then make an appointment to see the patient when they came into clinic. GPs could also be involved in tracing patients.
Redistribution of resources
Another central topic of discussion was the redistribution of resources. Reconfiguring services to reduce the cost of stable patients might free resources to be available for the most vulnerable patients who had engagement issues.
Interventions that address capability
One of the factors associated with disengagement is forgetting. This is one of the possible outcomes of substance abuse that could be tackled at the clinic by, for example, introducing a drug and alcohol worker into the clinic.
Although text messages can remind patients of their appointments, a personal reminder might be more powerful. An intervention was described whereby patients received a personal reminder call from someone that they knew in advance of their appointment and a personal call from the same person within 24–48 hours of missing an appointment. Others reported that an informal personal reminder has been found to be effective:
So some people tell me personally, oh, can you remind when it’s getting to, so maybe, a next day or a week to the appointment. I just give them a call and said, oh, you have appointment, so you need to come. And they will come.
Receptionist
The qualitative data described earlier indicated that knowledge could empower patients to take control of their health and engage with care. Peer support was one of the key means of imparting knowledge and we will come on to look at that under social influences. Areas of learning which might help patients to engage with the HIV service included education about the implications of disengagement, about going to clinic even if you are well, about the confidentiality of the clinic and around self-stigma.
Reinforcing an expectation of attendance might also encourage engagement. Professor of Medicine (MM) described creating a clinic culture that reinforced this expectation through the use of posters and staff messages and public health expert 1 talked about a proactive telephone call on one missed appointment to suggest that attending is the norm, with staff reinforcing this message.
Interventions that address motivation
Various ways of addressing stigma were suggested. These included raising public awareness through national campaigns or role models with the aim of normalising HIV and showing how it is now a treatable condition. The Time to Change campaign against mental health discrimination was given as an example. Media campaigns could also be used to engage different groups. Changes might also be made at the local level to destigmatise the clinic and to reassure patients that they are safe when they come to clinic.
Awareness should be raised among other health-care providers who needed updating on HIV. They could sit in at HIV clinics or attend specially designed workshops. Teaching to medical students could be reconfigured to include HIV stigma.
One key informant talked about providing HIV services in a way that was more similar to other long-term conditions might also have the effect of ‘normalising’ HIV by demything the condition and making care more accessible, although it may still need special provision:
There’s always this battle in one’s own head about shall we normalise HIV and, you know, do we need all this kind of special treatment? And then, every week you come across appalling instances of how people with HIV are treated, it makes you realise it sadly isn’t normal yet.
Patient representative 1
Appropriate and improved mental health provision was widely recognised as being needed for PLWH, reflecting the impact on mental health from moment of diagnosis and an elevated need for psychology in the population as a whole. Although mental health professionals were the key providers, additional support from a member of staff who was trained in psychological well-being could provide back-up to this service.
Self-efficacy was also seen as an important element of motivation, incorporating the idea of patients as their own caseworker. Clinic staff could be trained to raise health literacy and promote self-management. Specific interventions aimed at empowering patients included educational programmes and techniques such as the Expert Patient Programme, the Living Well Programme and Cornerstone Conversations. Technological solutions, such as hand-held health records, could engender patient activation by helping them to monitor and self-manage their health. Non-technical solutions included a written record of lab tests and a written dedicated action plan. Small changes might also help empower people. Examples included asking the patient to write down the date of their next appointment or providing the facility for them to book their own appointment.
Interventions that address opportunity: social influences
Key informants emphasised the importance of continuity of care, of health-care professionals building a relationship with patients and making them feel welcome and valued. There should be an ongoing dialogue between consultants and their patients. However, developing and maintaining this relationship could take time.
In-house training could make staff more welcoming and engender a clinic culture of valuing the patient being there. When patients came back to the clinic, it was particularly important that staff should be trained to respond to them in an appropriate and sensitive way.
The importance of peer support in building confidence and understanding was highlighted and different ways of providing peer support were discussed. Peer support could involve patient representatives who were part of the service or peer mentors who were either employed by or affiliated to community organisations. There were different ideas on whether peer mentors should be volunteers or paid members of staff. Being a paid member of staff could raise the issue of confidentiality and discussing sensitive issues about the clinic and the staff with another member of the staff. On the other hand, paid staff could work with other professionals and provide an effective way of facilitating valuable discussion. Payment also implied a professional identity and certain expectations of performance. Professor of Medicine (MM) talked about the high turnover in peer support and how stressful the work could be. He raised the importance of training, roles and responsibilities, and supervision.
When peer support was integrated into the HIV service, it could be used to change the dynamics and start conversations. Peer mentors had time to talk to patients and gave patients a sense of hope:
It’s very powerful, I can say to a patient, shake their hand, say, I’m Chris, I’ve been living with HIV for over thirty years, so any idea of you dying – forget. And they can see that . . . it’s a visual form of education.
Patient representative 3 (CS)
In addition to one-to-one discussions, peer support groups held in the clinic or elsewhere could also enable people to learn from each other. It was suggested that peer mentors could also visit community clinics once a month to provide information.
It was crucial to try and engage with people as soon as possible after diagnosis both through one-to-one peer support and courses for the newly diagnosed. Newly diagnosed people should be routinely directed towards this support and, when peer mentors were available in the clinic, they could be introduced to them right away.
Interventions that address opportunity: barriers
Key informants indicated that multidisciplinary, holistic support helping patients to prioritise their health care should be funded and consistently available across the country. HIV clinics should work towards providing an overarching supportive environment and not just a clinical service, with social support incorporated through the clinic or in collaboration with external agencies, such as community organisations and Citizens Advice Bureaux.
Making changes to patient pathways could mean that patients would not always need to come into the HIV clinic for bloods or to see the doctor; services could be taken to them. The community nurse specialist role needed definition but might deliver such a service, providing continuity of care by seeing patients in the clinic, on the ward and at home. Such changes to service delivery might also include greater involvement of GP practices. Key informants suggested different configurations, including HIV clinicians or nurse specialists doing clinics at the GP practice, bloods being done at the GP practice or at home and skilling up GPs for shared care. Some patients, however, found it easier to have all their care in one service:
You go to your GP and say to your GP, my HIV clinician said I must come to see you about this, and the GP says, I’m sorry, I can’t treat you for that, you need to go back to your clinic . . . we’ve got to get rid of this ping-pong.
Patient representative 1
In addition, key informants suggested that some consultations for some patients could be conducted using various technological and innovative solutions including a virtual HIV clinic; consultations or results by telephone, e-mail, WhatsApp (WhatsApp Inc., Mountain View, CA, USA) or Skype; and electronic access to results.
Patient-friendly facilities and services that were flexible and accessible might facilitate attendance. Key informants talked about organising facilities so that the waiting area was out of earshot of reception, seats were not facing the door, and there was a place for prams and child care provision. A comfortable environment could make a difference and calm the patients. Other suggestions included evening and weekend clinics; a more flexible walk-in system; fewer appointments if patients were stable on treatment; co-ordinated appointments so that patients could see all health-care professionals on the same day; and one telephone number to call. Volunteers from community organisations could also help with logistical issues including babysitting or driving someone to clinic.
Interventions for addressing financial issues might enable patients to stay engaged. Suggestions included giving supermarket vouchers to patients who were badly in need. The problem of getting to the clinic could be addressed by pre-paid Oyster cards, an emergency travel fund or guaranteed travel reimbursement. It was important, however, to explain what was available to patients and facilitate travel reimbursement because it would be embarrassing and complicated to claim. People who were very difficult to engage in care might be provided with a cheap mobile phone to call or text clinic and which the clinic could also use to contact them.
Selected interventions
The key informant interviews did not provide sufficient detail to be able to put together complex interventions so the interventions that we have developed are based on the factors identified as important from across all the components of the study. The above evidence indicates that there is no one-size-fits-all approach to improving engagement in HIV care. Clinics should consider incorporating suggestions made by patients and key informants into their care provision, such as text reminders and standardised systems for identifying and following up NAs. The prevalence and impact of poverty suggests that financial incentives such as supermarket voucher schemes and travel reimbursement might also help people to attend the HIV clinic. Our findings suggest that child-friendly clinics could be beneficial, but such broad-brush changes to service provision are difficult to cost.
We have identified four interventions based on our findings and put together a preliminary analysis of their costs. The interventions are listed in Table 27, together with the setting and target population for each. Each intervention addresses one or more of the key barriers to attendance described above, in line with the COM-B model, which proposes that behaviour occurs as an interaction between the three elements and suggests that more complex interventions will necessarily draw on each of the elements to a greater or lesser extent.
| Intervention | Setting | Target patient population |
|---|---|---|
| Structured peer involvement | HIV clinic | Disengaged |
| ‘One-stop-shop’ MDT clinic | HIV clinic | Disengaged |
| Clinic in alternative setting | Not HIV clinic | Disengaged |
| Low-cost clinic-wide intervention | HIV clinic | All |
Our research indicated that peer support has an important role to play in EIC. It helps PLWH cope with self-stigma and provides role models, emotional support and an opportunity to talk about HIV care and living with HIV. Our first intervention describes a structure within which to implement one-to-one peer support. This individualised support is aimed at patients who are at risk of disengaging from care. It enables identification of needs so that they can be directed to the level of care that they require, including signposting to other services such as psychology and social care that our research has identified as important, as well as provision of information and support to deal with the stigma that is a key barrier to attendance. Patients are offered up to eight sessions with a peer worker but some may only need two or three sessions, so costs will be variable. The intervention offers advice and support within the clinic, as recommended by patients and key informants.
Our second intervention brings together a multidisciplinary team (MDT), including a consultant, specialist nurse, psychologist, social worker and peer caseworker, into one clinic for patients who have difficulty engaging with care. It provides holistic care and the opportunity to address the range of health, psychological, social and economic needs identified above at the same time. There is no need for patients to incur the costs of returning to the clinic on several occasions.
The above interventions address stigma through peer support but some PLWH do not attend the HIV clinic because they are afraid to be seen there. In our third intervention, the clinic is held in an alternative setting, providing the opportunity to reach these patients and a possible bridge into regular care at the HIV clinic. Key informants, in particular, recommended the idea of taking services to patients.
Our fourth intervention has been proven to be effective in the USA through the use of co-ordinated messaging to encourage attendance. 71 Staff are trained to create a welcoming environment and deliver messages that clarify the importance of keeping appointments and practical steps to achieving this. The intervention showed a relative overall improvement in keeping two consecutive outpatient HIV appointments in the USA from pre-intervention to intervention of 7.0%. However, the effects were much greater among groups who are at particular risk for disengaging from care. For example, the relative improvement was 28.2% for new or re-engaging patients compared with 5.3% for active patients; and 19.9% for patients aged 16–29 years compared with 6.1% for patients aged 50–85 years. This intervention aims to reinforce an expectation of attendance, as identified by our key informants, and to address issues of communication between patients and staff that have been implicated in disengagement from care.
Intervention costs
A description of each of the four identified interventions, and a preliminary analysis of their costs, is provided below.
Intervention 1: structured peer involvement
Description of intervention
This intervention comprises a collaborative model of care between the NHS and a third sector agency, with the latter providing support for patients using peer support workers. The peer support workers have individual sessions in a variety of formats with patients over a 6-month period, providing the support for the following:
-
signposting and supportive conversations about living with HIV and seeking treatment for it
-
providing practical support about living with HIV and seeking treatment for it
-
helping patients to build relationships with clinical staff.
The peer support worker may provide support for the patient via face-to-face meetings in non-threatening locations and at flexible times, and also via telephone, online and social media.
The intervention is likely to comprise the following activities:
-
identifying patients who are eligible for peer support
-
booking patients into the peer support programme and making appointments to see the peer support worker
-
devising an individual peer support care plan designed to support engagement with care
-
up to eight sessions between the patient and peer support worker, with the latter providing support of various kinds as described above
-
a monthly supervision session with a psychologist for each peer support worker
-
allowances for both patients and peer support workers to facilitate interaction.
Cost analysis
The costs of this intervention are summarised in Table 28. They comprise each of the activities described above. Patient identification is assumed to be undertaken by a senior clinic worker [Agenda for Change (AfC) band 7] and takes 15 minutes per patient. The patient is assumed to be booked into the programme by a receptionist (AfC band 2), taking 15 minutes in total. For each patient in the programme an individualised care plan is devised, which is assumed to take 1 hour of time by an AfC band 5 worker. The main element of the programme consists of up to eight individual sessions each of 1 hour’s duration with a peer support worker. The peer support worker might range from being an unpaid helper to a paid peer caseworker at AfC band 5, with a range of costs. We calculated the costs for up to eight sessions with each type of peer support worker and then used the average. Patients are expected to receive a transport allowance and a food allowance for meetings with the peer support worker, valued at £10 per session and £5 per session, respectively. Peer support workers are also expected to receive the same allowances, and in addition receive a telephone allowance of £50 a month, a proportion of which is used to contact each patient. We assume that on average each patient may receive peer support from a range of peer support worker types, and taking the average cost across all types, the expected cost of this intervention per patient over a 6-month period is £538 (see Table 28). The actual provision of this and the other interventions considered in this section may vary from one location to another. If only, for example, three peer support sessions were needed instead of eight then the total costs would be £433. If all of the peer support sessions were provided by an unpaid peer helper the total costs would be £370; if half were provided by an unpaid peer helper and half by a paid peer helper at AfC band 2 the cost would be £416; and if they were all provided by a paid peer caseworker at AfC band 5 the total costs would be £658. If there was only one supervision session with a psychologist during the 6-month period the cost would be £501; and if there were three sessions the cost would be £516.
| Cost component | Cost (£) |
|---|---|
| Patient identified as eligible by clinical team member | |
| 15 minutes of one AfC band 7 worker at £54 per hour | 14 |
| Patient booked into peer assessment appointment with clinician/third sector | |
| 15 minutes of one AfC band 2 worker at £23 per hour | 6 |
| Individualised peer care plan devised to support engagement with care | |
| 1 hour of one AfC band 5 worker at £36 per hour | 36 |
| Eight sessions (1 hour each) with a designated level of peer worker | |
| Eight sessions with an unpaid peer helper | 0 |
| Eight sessions with a paid peer helper (AfC band 2) at £23 per hour | 184 |
| Eight sessions with a paid peer mentor (AfC band 3) at £25 per hour | 200 |
| Eight sessions with a paid peer caseworker (AfC band 5) at £36 per hour | 288 |
| Average | 168 |
| Monthly peer worker supervision with psychologist (1 hour) | |
| Six sessions with a psychologist at £54 per hour | 324 |
| Six sessions with support workers at an average of £21 hour | 126 |
| Subtotal | 450 |
| Mean cost per patient (estimated 10 patients seen each month) | 45 |
| Allowances for patients | |
| Transport allowance (eight sessions at £10 per session) | 80 |
| Food allowance (eight sessions at £5 per session) | 40 |
| Allowances for peer workers | |
| Transport allowance (eight sessions at £10 per session) | 80 |
| Food allowance (eight sessions at £5 per session) | 40 |
| Telephone allowance (£50 per month for 6 months per peer worker, assume 10% per patient) | 30 |
| Total (mean cost per patient over a 6-month period) | 538 |
Intervention 2: ‘one-stop-shop’ multidisciplinary team clinic
Description of intervention
This intervention comprises a weekly MDT clinic, a 4-hour session involving a range of health and social care workers providing care for patients. The clinic is designed to provide a ‘one-stop-shop’ for patients, reducing the number of appointments needed to meet with each member of the team. The MDT is like to comprise the following workers:
-
consultant
-
specialist nurse
-
administrator
-
clinical psychologist
-
social worker
-
peer caseworker.
Cost analysis
The costs of this intervention are summarised in Table 29. The MDT meeting is assumed to be a weekly 4-hour meeting involving all the team members described above. In addition to the 4 hours of clinical time, additional administrative time is allocated to each meeting. As well as these staff costs, patients are expected to receive a transport allowance and a food allowance for the MDT meetings, valued at £10 per session and £5 per session, respectively. Peer case support workers are also expected to receive the same allowances for participating in the meetings. The cost of the weekly MDT meeting is estimated to be £1472. To calculate the cost per patient, we assume that, on average, each MDT will see eight patients each week, and that eligible patients will be seen once every 3 months. On this basis, the expected cost of this intervention per patient over a 6-month period is £398 (see Table 29). If each eligible patient was seen more or less frequently, for example every month, 2 months or 6 months, the estimated costs would be £1194, £597 or £199 per patient, respectively.
| Cost component | Cost (£) |
|---|---|
| Weekly 4-hour MDT (including clinical and administration time) | |
| 5 hours (4 hours clinical, 1 hour administration) of one consultant at £105 per hour | 525 |
| 5 hours (4 hours clinical, 1 hour administration) of one specialist nurse (AfC band 7) at £54 per hour | 270 |
| 1 hour of one administrator (AfC band 4) at £28 per hour | 28 |
| 5 hours (4 hours clinical, 1 hour administration) of one clinical psychologist (AfC band 7) at £54 per hour | 270 |
| 4 hours (clinical) of one social worker at £55 per hour | 220 |
| 4 hours of one peer caseworker (AfC band 5) at £36 per hour | 144 |
| Allowances for peer caseworkers | |
| Transport allowance at £10 per session | 10 |
| Food allowance at £5 per session | 5 |
| Subtotal | 1472 |
| Number of patients per weekly MDT | 8 |
| Allowances for patients | |
| Transport allowance at £10 per session | 10 |
| Food allowance at £5 per session | 5 |
| Mean cost per patient per weekly MDT | 199 |
| Number of times patient seen over a 6-month period | 2 |
| Total (mean cost per patient over a 6-month period) | 398 |
Intervention 3: running a clinic in a non-NHS/alternative NHS setting
Description of intervention
This intervention comprises a weekly specialist clinic, which, rather than being held on the usual NHS site, is instead held off-site in an easy-to-access non-intimidating setting providing anonymity for patients (e.g. a library, general practice or pharmacy). The intention is that this may improve engagement by making it less obvious that patients are visiting the clinic (i.e. preserving anonymity), and also making the clinic easier to get to. If the clinic takes place in a general practice setting it may also facilitate management of comorbidities requiring GP involvement.
Cost analysis
The costs of this intervention are summarised in Table 30. The clinic is assumed to be a weekly 4-hour session involving a consultant and specialist HIV nurse and administrator. The calculation allows for clinical and administration time.
| Cost component | Cost (£) |
|---|---|
| 4-hour clinical session per week in a non-intimidating space (including clinical and administration time) | |
| 5 hours (4 hours clinical, 1 hour administration) of one consultant at £105 per hour | 525 |
| 5 hours (4 hours clinical, 1 hour administration) of one specialist nurse (AfC band 7) at £54 per hour | 270 |
| 4 hours of one administrator (AfC band 4) at £28 per hour | 112 |
| Room hire (at £800/month + VAT) | 222 |
| Staff transport (at £10 per staff member for three staff) | 30 |
| Subtotal | 1129 |
| Number of patients per clinic | 8 |
| Allowances for patients | |
| Transport allowance at £10 per session | 10 |
| Mean cost per patient per weekly clinic | 151 |
| Number of times patient seen over a 6-month period | 2 |
| Mean cost per patient over a 6-month period | 302 |
As well as these staff costs, costs will be incurred for staff transport to the venue, and patients are expected to receive a transport allowance of £10 per session. The main potential non-staff cost is room hire, which we assume in a non-NHS setting will cost £800 per month. The cost of the weekly clinic is estimated to be £1129. To calculate the cost per patient we assume that, on average, each clinic will see eight patients each week, and a patient will attend the clinic once every 3 months. On this basis, the expected cost of this intervention per patient over a 6-month period is £302 (see Table 30). If the clinic was held at a zero-costed venue the estimated costs would be £247 per patient. If only 1 hour of dedicated administrator time is needed (e.g. if the venue is a GP surgery with reception staff), then the cost would be £281 per patient. If each eligible patient was seen more or less frequently, for example every month, 2 months or 6 months, the estimated costs would be £906, £453 or £151 per patient, respectively.
Intervention 4: low-cost clinic-wide intervention
Description of intervention
This intervention comprises brief verbal messages to be used by clinic staff, encouraging patients to adhere to treatment and engage with care. It also includes a set of printed materials including a leaflet, posters and staff pocket guides.
Cost analysis
The costs of this intervention are summarised in Table 31. The leaflets, posters and staff pocket guides have been costed based on design and printing costs of £300, which would produce sufficient volume of materials for a total of 2800 patients in a single clinic. Staff training is required to instruct staff in the use of brief verbal messages to encourage engagement with care. This includes the costs of a professional trainer and the cost of clinic staff attending the training sessions provided by the trainer. It is assumed that each staff member will attend one 2-hour group training session and that to train 27 staff in one clinic will require one 2-hour session of professional trainer time plus preparatory work. The total cost of the printed materials and training is £4787 in a clinic with 2800 patients. Therefore, the cost per patient is £2 (see Table 31). This estimate assumes the intervention affects only current patients. For example, if twice as many patients were affected if the intervention effects persist into the future then the cost per patient would be £1.
| Cost component | Cost (£) |
|---|---|
| Posters/leaflets/staff pocket guides (design and printing costs per clinic) | 300 |
| Professional trainer (per clinic) | |
| Preparatory work | 75 |
| One 2-hour session at £900 + VAT | 1080 |
| Subtotal | 1155 |
| Staff attendance at training (per clinic, each staff member to attend one 2-hour session) | |
| Three AfC band 2 workers at £23 per hour | 138 |
| Three AfC band 3 workers at £25 per hour | 150 |
| Four AfC band 6 workers at £45 per hour | 360 |
| One AfC band 7 worker at £54 per hour | 108 |
| Seven specialist registrars at £49 per hour | 686 |
| Nine consultants at £105 per hour | 1890 |
| Subtotal | 3332 |
| Total | 4787 |
| Number of patients | 2800 |
| Mean one-off cost per patient | 2 |
Summary of findings from Chapter 7
-
Interventions are proposed that support a holistic approach to care including peer support; address stigma by holding clinics in alternative locations; and encourage patients to attend through training staff to deliver co-ordinated messages.
-
The costs of the interventions range from £2 to £538 (Table 32).
| Intervention | Cost (£) |
|---|---|
| Structured peer involvement | 538 |
| ‘One-stop-shop’ MDT clinic | 398 |
| Running a clinic in a non-NHS/alternative NHS setting | 302 |
| Low-cost clinic-wide intervention | 2 |
Chapter 8 Discussion and conclusions
This chapter summarises the findings of the study and how they address the objectives that we set out to achieve. We will discuss the limitations of our findings in relation to each of the objectives.
Objective 1: outpatient attendance patterns among people living with HIV
In addition to our original objectives we have developed the EIC algorithm, which provides a flexible new approach to measuring engagement in outpatient HIV care. It adapts to the changing treatment and health status of the patient, which reflects the reality described to us by clinicians. It provides a binary measure of whether patients are in care or out of care for each month of follow-up which can be used to analyse patterns of engagement over time42 and to examine associations between predictive variables and the proportion of months that patients are in care following diagnosis.
The EIC algorithm was developed from interviews with clinicians, who indicated that the timing between appointments is dependent on a range of factors. We used 6 months as the maximum time between routine visits in the algorithm, as described by physicians and in accordance with the UK guidelines for routine monitoring at the time. 27 However, the EIC algorithm can be adapted to changing guidelines and to local clinic policies on how often to see patients. For example, guidelines in the UK now recommend starting ART irrespective of CD4 count,43 and this can be incorporated into the EIC algorithm when applied to future cohort data.
Any algorithm is clearly only an approximation to a far more complex clinical process and it is difficult in an observational cohort setting to incorporate other factors, such as social factors, that may lead to more regular scheduled visits. Although such data are not currently collected for UK CHIC, they could be incorporated into an algorithm if it were available. For example, the HARS 3 measure of patient complexity that we used in our survey could be incorporated into future iterations of the EIC algorithm. As it stands, the algorithm may provide an underestimation of engagement in HIV care as it does not account for patients whom clinicians may wish to see earlier for treatment of comorbidities and psychosocial issues associated with HIV. In addition, it measures EIC up to the point of last visit and does not therefore include time when patients are LTFU after this.
In common with other analyses of EIC using HIV cohort data, we have used laboratory data and ART start dates as surrogate markers of clinic visits. We may therefore have missed some visits at which no laboratory test was performed. On the other hand, visits were grouped into care episodes to negate the effect of repeated laboratory measures within short time intervals.
As analysis of our survey data has shown, different factors are associated with irregular attendance and with stopping clinic attendance altogether. Although this is a limitation of measuring a cumulative effect of EIC, the EIC algorithm allows for the fact that patients may move between attendance patterns over time, as described in our qualitative data. We have developed a new concept of how to measure EIC by incorporating a time-updated measure of patients’ treatment and health status that adds to the options available for measuring this key performance indicator.
We used the EIC algorithm in group-based trajectory analysis to explore patterns of engagement in HIV care over time. This method of analysis is applied to cohort data to identify groups of individuals following a similar progression in a behaviour or outcome of interest over age or time. Since its early use for monitoring antisocial behaviour in boys as they grew older,39 its applications have included patterns of sexual risk behaviour among participants in the National Longitudinal Survey of Youth,72 diaphragm use among South African and Zimbabwean women taking part in a clinical trial73 and adherence to ART among patients in the Swiss HIV Cohort Study. 42 The last, for example, identified four groups whose adherence could be characterised as good (51.8%), worsening (17.4%), improving (17.6%) or poor (13.2%). Our analysis identified four attendance patterns whereby the first consisted of the majority of patients who were consistently engaged in care, then two groups who experienced a decline in attendance at different points on the trajectories and a fourth group who appeared to drop out before gradually returning to care. These groups were relatively consistent for patients who had been diagnosed in each of the three diagnosis periods that we examined (2000–2, 2003–5 and 2006–8).
Whereas the Swiss HIV Cohort Study42 limited its analysis to the 4.5-year period when adherence data were collected from questionnaires, there was no set time delineation for our analysis of UK CHIC data. Assuming that patients were vulnerable to dropping out of care soon after diagnosis8 and more likely to disengage from care the longer they had been diagnosed,22 we grouped patients into three 3-year diagnosis periods. Grouping around 6000 patients into four trajectories is a necessarily broad-brush attempt to characterise how HIV patients engage in care over time. However, the consistency between the trajectories for the three different diagnosis groups lends credibility to the findings.
We selected a four-trajectory solution, based on the interpretability of the plots. The first and largest group engages consistently in care, whereas the last group appears to drop out of care and then return after about 4–6 years. It is more difficult to describe and understand what is happening to the remaining two groups. Furthermore, it would be useful to know what factors are associated with the points at which the trajectories change direction but difficult to conceptualise such an analysis. Given this degree of uncertainty about the meaning of the findings from the group-based trajectory analysis, we considered the proportion of time that patients were engaged in care to be a better and more flexible measure for exploring predictive factors for disengagement.
Objective 2: predictive factors of disengagement
Using the EIC algorithm showed that patients were engaged in care for 83.9% of months over the follow-up period of up to 13 years. Our findings are consistent with previous studies among UK populations in showing that disengagement from HIV care is more likely among women and less likely among MSM. 8,22 A study of gender disparities concluded that poorer engagement in HIV care by women was underpinned by a complex array of factors,74 and findings from both our survey and analysis of UK CHIC data suggested that the association between gender and disengagement could be explained by other factors.
There was strong evidence from both of our data sources that younger patients were more likely to disengage from care and other studies have also found an association between younger age and disengagement from care. 8,21,28 Patients who were not currently on ART were more likely to disengage according to both survey and cohort data and in keeping with other research. 21 Although our survey suggested that PLWH were increasingly likely to disengage from care the longer they had been diagnosed, this pattern was not found in the analysis of UK CHIC data.
There are many advantages to using cohort data,25 such as the associated years of follow-up, statistical power and representative patient populations. We have included a description of the limitations of using cohort data above. In addition, our analysis of predictive factors was restricted to the limited number of background factors available in the UK CHIC data set to predict disengagement from care. Our survey provided a much richer source of data for this purpose and its advantages and limitations will be discussed below under Objective 4: retention risk tool and Objective 5: factors influencing outpatient attendance. Our study furthermore benefits from using multiple data sources and the triangulation of our findings.
Objective 3: health and financial costs of disengaging from care
Health costs of disengagement
We examined the association between EIC and mortality, adjusting for CD4 count to see if differences were being mediated by differences in CD4 count profiles. There were 2279 deaths in the cohort and the analysis showed that each 10% increment in EIC was associated with a 9% reduction in the risk of mortality. This remained fairly constant when we adjusted for fixed covariates but, importantly, when we adjusted for the CD4 count at the time and over follow-up, the association was attenuated completely to 1. The results were consistent when we included AIDS or death as the outcome measure, suggesting that patients with poor EIC tended to experience poorer outcomes because their CD4 counts were generally lower.
Next we restricted the analysis to patients who had started ART. The analysis showed that around 40% were engaged in care for 90–100% of the time. As the proportion of EIC increased, more MSM and people of white ethnicity were represented among these groups. As we would expect, patients with higher engagement had a higher CD4 count on average and, as a reflection, were more likely to start a non-nucleoside as opposed to a PI.
The association with mortality was equally strong for this group, even though they were on treatment, with a very strong association between EIC before starting treatment and mortality after starting treatment. This was affected only minimally by adjustment for fixed covariates, but when we controlled for the CD4 count and viral load, the effect moved towards 1 and became non-significant. This confirmed that people with poor EIC generally not only have lower CD4 counts at the time of starting treatment but have poorer CD4 count and viral load responses after starting treatment.
Our analysis indicated that higher EIC is associated with improved clinical outcomes, even when looking at least 1 year into the future and when we consider a subgroup of patients who are already in care and who start treatment, a group that we would think would be at relatively low risk of mortality. On the whole, clinical outcomes were largely explained by poorer CD4 count profiles in those with suboptimal EIC.
Although this analysis is subject to the limitations of the EIC algorithm, as described earlier, it benefits from the advantages of using the extensive UK CHIC data set to explore the health costs of disengaging from HIV care. Although death and AIDS diagnoses occur infrequently in the population, we have found a strong association with disengaging from care. The analysis is also complicated by the fact that these negative outcomes are associated with high levels of engagement in the period leading up to the event when patients attend services because they are unwell. We have accounted for this by looking at EIC before starting ART and death after starting ART in order to avoid contamination of this ‘reverse causality’ effect.
Association between test costs and disengagement from care
Our analysis of financial costs was limited by available data in UK CHIC on NHS resource use, and hence we focused on laboratory test costs. Given the limitations of the data, our analysis was descriptive. There was some suggestion that people in the most engaged groups had more tests per patient in each quarter, and therefore higher test costs and that those who are least engaged seem to have fewer tests and lower test costs but the differences are small. There does not seem to be any appreciable crossover in test costs between pre-ART EIC groups in later quarters as their health declines.
Further research focusing on a wider range of NHS resource use would be beneficial, including clinical appointment costs, medication costs and costs of hospital stays. This requires a data set with detailed information on NHS resource use associated with each of these items plus measures of EIC. A more sophisticated analysis would take a wider costing perspective, such as a societal perspective, which would also consider costs of disengaging from care on patients and families, business and costs to social services and other parts of the public sector. When considering impacts on patients and families, this should account for the possibility that, on the one hand, engaging in care might increase time and travel costs to patients and families (e.g. taking time off work to attend clinics, travelling to and from clinics), but, on the other hand, may also reduce costs to patients and families by avoiding deteriorations in health.
As well as evaluating the costs of disengaging with care, further research would also be beneficial to evaluate the cost-effectiveness of interventions to improve EIC, such as the interventions described in Chapter 7. Economic evaluation of interventions such as this should account for:
-
the cost of the interventions
-
the impact of the interventions on EIC
-
the impact of engaging in care on health outcomes (both in terms of mortality and health-related quality of life)
-
the impact of engaging in care on costs associated with illness, from both NHS and wider societal perspectives.
As noted above, evaluation of the interventions on mortality, health-related quality of life and NHS and other costs should appropriately account for biases caused by ‘reverse causality’.
Objective 4: retention risk tool
The retention risk tool was developed to help clinicians identify newly diagnosed patients who are at risk of disengaging from care. From the outset of the study, it was agreed that this part of the study was not funded to be a tested electronic tool but would take the form of an algorithm or equation, summarising the key variables and their contribution to risk retention. We found that a combination of six variables from the REACH survey and clinical data set provided the best set of predictive variables. These were age at diagnosis, having children, recreational drug use in the past 5 years, drug/alcohol dependency in the past year, money for basic needs and use of public transport to get to the clinic. The AUROC curve for this model was 0.707, which is fair. Other tools developed for use in sexual health have achieved similar levels of performance in, for example, identifying patients at risk for chlamydia and/or gonorrhoea (AUROC curve = 0.74)64 and identifying MSM at risk for HIV acquisition (AUROC curve = 0.67). 75
It was suggested that having children may be more likely to affect attendance among women than men and, indeed, the gender variable was excluded from the model when having children was introduced. However, univariable analysis indicated that there was a significant effect for being a mother or a father compared with having no children and this variable did not provide any additional explanatory value when incorporated into the model instead of the binary variable.
The original plan had been to use UK CHIC data to develop this tool. The size of the data set would have enabled the use of cross-validation methods to generate estimates of the AUROC curve and pseudo-R2 and to provide a measure of internal validity. Although the UK CHIC data set is rich in longitudinal clinical data from the point of diagnosis, other variables for potential use in the tool are limited to age, gender, ethnic group, route of acquisition, country of birth and laboratory data at the point of diagnosis. The survey provides an extensive data set of additional explanatory variables for identifying patients at risk of disengaging from care. It was not, however, powered to be divided for comparison of subgroups in order to validate the tool internally. Although it is also possible that different sets of predictive variables may apply to different groups of individuals, such as men and women, the survey was similarly not powered for such analysis.
Our survey was neither longitudinal nor prospective and this is a limitation to using these data for developing a retention risk tool for newly diagnosed patients. In order to develop our tool, it was necessary to exclude variables from the analysis that would have occurred after or as a result of HIV diagnosis. As the survey respondents had already disengaged from care when they took part in the study, it remains possible that the tool may not be predictive of disengagement at the point of diagnosis. Although we have been able to test a broad set of factors associated with disengagement from HIV care for use in our tool, it will be important to test its external validity among newly diagnosed patients in order to examine its predictive value.
It should also be noted that our retention tool was developed using data from patients attending HIV clinics in London. It is possible that barriers to engagement in HIV care may differ across the UK. Although the qualitative study on non-attendance of HIV clinics in Scotland highlighted similar key factors to those that we found among patients attending HIV clinics in London,37 use of public transport and issues of getting to the clinic may be different outside the capital and require testing outside London.
Clinicians have expressed great interest in the tool. If successful, it would be a very valuable way of identifying people who may be at risk of disengaging from care, thereby triggering further action within the clinic.
Objective 5: factors influencing outpatient attendance
Before looking at the factors associated with outpatient attendance, it is important to consider the experiences and circumstances across the population of HIV patients who took part in our survey. The data indicate that, in addition to managing their physical health, many HIV patients are faced with social and psychological issues that may, at some point, overwhelm them and/or take priority over their health care. Over a half of our sample did not always have enough money for basic needs (57%), one-fifth reported moderate or severe hunger (19.3%) and one-fifth were currently unemployed (22%). Two-fifths reported feelings of self-stigma (44%) and one-third had been diagnosed with depression (32%). One-fifth had been recently abused by or afraid of a partner (21%) and nearly one-fifth reported low social support (17%). This indicates high support needs across the population and the importance of constant vigilance to ensure that patients do not fall out of care with changing needs and circumstances.
The data from the phases 2 and 3 of the study, which incorporated the quantitative and qualitative examination of patient experience and the key informant study, were highly consistent in their identification of the factors that influence outpatient attendance. This triangulation of the findings from our survey of almost 1000 HIV patients, interviews with 33 patients, two FGs and 19 key informant interviews provides a strong measure of validation. Questions included in our investigation of patient experience and key informant interviews were based on the COM-B model, which proposes that behaviour is the outcome of an interaction between an individual’s capability, opportunity and motivation. 45 Our primary data collection aimed to understand the factors associated with capability, opportunity and motivation to engage in HIV care in order to develop interventions to address these issues and improve outpatient HIV attendance.
Capability to attend was more often associated with irregular attendance and missed appointments rather than dropping out of care altogether. Feeling or being unwell could stop patients from attending their appointments. On the other hand, when patients were very unwell, this could bring them back into care after a period of disengagement. Patients often forgot their appointments and this may be associated with neurocognitive impairment. It may also be associated with stigma and wanting to put HIV to the back of their minds, illustrating how the components of the COM-B model work together to influence outpatient attendance. Engagement in HIV care and adherence to ART have been associated with alcohol and drug use,21,31,76,77 and our findings also indicated that use of alcohol and recreational drugs were associated with disengagement from care. We also found that knowledge about HIV was very powerful in giving patients the capability to tackle self-stigma and prioritise their health.
Patients needed to have the motivation to attend clinic and depression consistently came up as a factor that stopped people from feeling able to come to the clinic. This is supported by our findings that poorer engagement was associated with low self-efficacy and feelings of not being in control of life. Previous research has found that real and perceived stigma can seriously undermine access to HIV care and other support services. 32,34,35 We found that stigma had a strong impact on attendance and the findings suggest that the necessary conditions for dropping out of care were underpinned by self-stigma. Symptoms of psychological distress are highly prevalent among PLWH and have also been associated with poorer adherence to ART. 78 Although the scores from the MARS may not be directly comparable to those from studies using the complete validated scale with framing statements, our data indicated a clear association between not adhering to ART and not coming to the clinic. Some patients did not want their clinician to know that they had been having difficulties with adherence that could delay their clinic attendance and drift into long periods of disengagement. Similarly, a paternalistic approach to patients was found to be a barrier to care. 79
Opportunity to enact behaviour is shaped by the social environment, and the actual or expected interaction with clinic staff had an impact on attendance, as described above in relation to adherence to ART. Patients want to feel that staff are listening to them80 and our findings reflect previous research indicating that HIV patients were less likely to miss appointments when care was patient centred and they were known as individuals. 81 Many of the patients who took part in the REACH study reported excellent relationships with their clinicians and a sense of belonging at their clinic, which could provide strong motivation to attend. However, the fear of being seen at the clinic and people finding out that you have HIV remained a powerful disincentive for attending among some patients.
Peer support could have a very positive effect on EIC, helping people to address stigma and providing role models, emotional support and the opportunity to exchange ideas with other PLWH. Many people praised the courses for newly diagnosed patients that they had attended. Although partners could also provide a strong source of social support, we found that partners could also have a negative effect on attendance by undermining patients’ confidence. Previous research has found that intimate partner violence is associated with missed HIV appointments. 82
Financial difficulties were highlighted as one of the key barriers to attendance. Other barriers, including lack of child care and work responsibilities, were often raised and, although the effects of homelessness or immigration issues were less prevalent, they had a clear and direct impact on EIC for some people. In common with other work, service providers in our study were concerned that immigration issues could adversely impact attendance. 83 Our findings support the proposition that PLWH who are burdened with a range of socioeconomic problems are less likely to prioritise their HIV care. 84
Our qualitative research suggested an overlap between missing appointments and dropping out of care that bears some similarity to a cyclical process of attendance and non-attendance found in other qualitative research. 85 However, our survey found some differences between people who had missed appointments in the past year and those who had recently dropped out of care altogether. The survey indicated that younger patients, those who had been diagnosed for longer, those with children and with drug and/or alcohol issues were likely to both miss appointments and disengage from HIV care altogether. IAs were also more likely to report symptoms of neurocognitive impairment and ill health which may be associated with missing appointments, whereas NAs were less likely to be registered with a GP, to be a homeowner or to feel that the nurse listened to them at their last clinic visit. Furthermore, NAs were more likely to cite reasons associated with motivation for missing appointments. It is possible that some of the reasons provided are capturing the same underlying causes and a multivariate analysis might identify underlying dimensions. In terms of improving EIC, our qualitative data indicate that these differences should be interpreted cautiously, and the support needs of patients at risk of disengagement should be assessed individually.
The same factors were consistently highlighted as associated with EIC across our different data sources. These factors were similar to the emerging findings from the Antiretrovirals, Sexual Transmission Risk and Attitudes (ASTRA) study (presented at the Conference on Retroviruses and Opportunistic Infections 2015), which showed that virological rebound was associated with increasing financial hardship, non-employment, non-homeownership, non-university education and low social support. This suggests that our difficulties with recruiting NAs did not adversely affect our results. It should be noted, however, that our final survey sample of NAs (n = 164) was two-thirds of the size originally planned, which may have limited our ability to detect statistical differences between RAs and NAs. In addition, our sample of NAs had all returned to care, which may impact on their responses to our survey and colour their perceptions and reflections when talking about their experiences of disengaging from care. Our finding that 45% of NAs had an undetectable viral load suggests that they do not form a cohesive group, but one that is made up of a variety of patients including stable patients and elite controllers (who may only be expected to attend clinic once a year); patients who have been back in care after a period of LTFU but are now on ART and undetectable; as well as those who have very recently returned to care and are not virologically suppressed. It was always understood, however, that recruiting this group of patients would be challenging and, despite this, we managed to achieve a sample that was large enough to be used in analysis.
In addition, the FG for non-African men and women had to be cancelled because no one volunteered to take part. However, experiences of heterosexual non-African men and women were collected from 10 individuals who took part in in-depth interviews. We also found that the FGs did not lend themselves to discussion between the participants because participants were keen to take turns to relate their individual stories and experiences rather than discuss issues among themselves. The data were therefore more similar to interview data and integrated well into the qualitative data set. Framework was a useful analytical approach for organising and reducing our extensive data set, while retaining a close link to the original data and the capacity to consider the relationship between both themes and participants. 66 We changed our sampling strategy to ensure that patients’ attendance patterns were categorised according to current or recent behaviour. As described earlier, the fact that our data were not collected prospectively may bias our survey results to the extent that patients respond differently in the light of their previous experiences of irregular or non-attendance. The findings from our qualitative research may also reflect the fact that most of the participants were not currently working and may represent a certain subselection of the patient population. Faith is furthermore an important part of life for many black Africans,86 which did not come up a great deal in our interviews but might bear further examination.
It is also important to note that our quantitative and qualitative data were wholly collected in London. Although it is possible that the factors that we have identified as driving disengagement from HIV care may be different outside London, it is reassuring that the findings from the analysis of our primary data are similar to those from our analysis of UK CHIC data, and are also congruent with the findings from the ASTRA study, which included study sites across the UK.
Our findings have shaped the development of the intervention models that follow. They can also help clinicians to define roles within the service and identify where they need to target their attention in order to improve engagement in outpatient HIV services and meet the quality performance targets set by the British HIV Association standards.
Objective 6: intervention models to improve engagement in care
Supported by data from REACH, we put together four interventions that aim to address the needs of people who have disengaged from care or who are at risk of doing so. It will be important to formally test the interventions in clinics based both in London and outside London to determine their acceptability and feasibility, and assess their ability to improve engagement in HIV care in different contexts.
The evidence from our qualitative research highlighted the importance of peer support. Our first intervention comprises structured peer involvement that is targeted at PLWH who are at high risk of disengaging from care. This is the most expensive intervention although costs will vary depending on need. In addition to the peer element of the intervention that is vital, the individual nature of the intervention reflects the no one-size-fits-all finding of our study while providing a clear structure to enable the effects of the intervention to be evaluated. A number of studies in other HIV settings have found that patients benefit from peer support,87–89 and the evidence in the UK so far has been promising but limited in scale. 90
Our second intervention addresses the diverse needs of patients by providing a ‘one-stop-shop’ where they can see different members of the MDT at the same visit. The evidence from REACH indicates that patients have high needs for social and psychological support. Poverty and poor mental health were prevalent among those that took part in our study, and even more so among those who disengaged from care. Putting the various elements of support together in one clinic enables patients who may not prioritise their health care to benefit from a holistic approach to their overall well-being and facilitates access to social, psychological and peer support.
One-fifth of NAs in our survey said that they sometimes or often missed appointments because they were afraid to be seen at the clinic and the qualitative research found a connection between wanting to block out HIV to disengaging from care. As our survey was conducted among people who are currently attending the clinic, this suggests that the problem could be even more severe among the population of PLWH as a whole. Our third intervention was aimed at this group of people who may prefer to attend a clinic at an alternative venue, including those who would prefer not to take their children into a sexual health or HIV clinic. Although such clinics are aimed at circumventing the self-stigma that patients currently experience, they also provide a link back to the HIV clinic. They do, however, raise the issue of whether their implementation will lead to more or less stigma in the long run.
The fourth intervention that we have costed is based on successful intervention that was implemented in the USA. 71 It is the only intervention that is not targeted at people who have disengaged from care or are at risk of doing so. Although the US study found that the benefits across HIV patient population were modest, there was a much greater effect among new or re-engaging patients than among active patients, and among younger compared with older patients. The cost of the intervention makes it an attractive option should it be similarly successful in the UK. Our findings have indicated that there is room for improvement in communication between health-care staff and patients that this intervention would aim to address.
The costs of the four interventions range from £2 to £538 per patient included in the intervention. There are several caveats that should be borne in mind when considering these estimated figures. First, there is considerable uncertainty with these estimates as they have not yet been subject to empirical testing; further work is needed to evaluate the actual costs under both research and real-world conditions. Second, caution should be taken when comparing between the interventions based on their costs alone, as such a comparison does not take into account potential differential effects between the interventions. For example, it may be the case that more expensive interventions are also more effective at improving engagement. Third, our analysis considers the potential intervention costs for a range of possible interventions for improving EIC. A full cost analysis would include not just the costs of the interventions but also their impact on subsequent health and social care costs; for example, impact on treatment costs due to better engagement with care. These costs should be included in a full economic evaluation of the interventions.
In addition to the above selection, our data suggest other interventions that would benefit from detailed economic evaluation. For example, the use of various technological and innovative solutions may not only reduce missed appointments but, if adopted among more stable patients, could make resources available to redistribute for the support of patients who require more intensive care.
Practice implications
Findings from this research indicate that the HIV specialist outpatient clinic provides valued patient-centred care for the majority of service users. Despite this, however, significant barriers to optimal engagement and outcomes exist for a high proportion of clinic attendees. Clinics linked with mental health services, drug and alcohol support, social and outreach services and peer support will be best placed to address these diverse challenges.
In keeping with other studies, health outcomes were poorer in IAs, as well as those experiencing a period of disengagement. Clinical practice would benefit from information technology systems to monitor engagement in real time, enabling timely intervention where needed.
Patient and public involvement
The REACH project has fundamentally been about listening to PLWH with the aim of adapting services to better meet their needs. At the same time, we have found the additional perspectives of community representation to be invaluable in the design and implementation of our research. The project has also benefited from the inclusion of participants who were recruited via community contacts: Memory Sachikonye, a collaborator on this study, is co-ordinator at the UK-CAB, a network for community HIV treatment advocates across the UK. She was responsible for recruiting FG participants and the discussion that occurred in our FGs suggested that recruiting people in this way may have enabled PLWH to talk more openly about their experiences with clinic staff.
Our findings will be fed back to service user groups and patients at participating clinics over the coming months and a summary of the findings has been published on www.aidsmap.com an online information resource which creates and disseminates information rooted in the experience of those most affected by HIV and AIDS.
Summary of limitations
Our analyses using UK CHIC were limited by the data available within the data set. The EIC algorithm is, thereby, an approximation of a complex clinical decision that does not account for time LTFU after a patient’s last visit. Our examination of the financial costs of disengagement necessarily focused on the costs of laboratory tests.
Developing the retention risk tool for newly diagnosed patients using the rich REACH survey data set instead of UK CHIC data meant that we needed to make certain assumptions about which variables to include in the analysis and were unable to test its external validity.
Primary quantitative and qualitative data were collected from patients using HIV clinics in London and from London-based providers. Although the findings are consistent with those from UK-wide studies, the factors and interventions that we have identified may not equally apply to clinic populations outside London.
The costs that we have calculated for our proposed interventions have not been tested and all models require a full economic evaluation to incorporate their potential impacts into the costs.
Conclusions
The REACH study has shown the adverse health impacts of disengaging from HIV care. Our findings have indicated that particular factors are associated with disengaging from care and we have developed a retention risk tool that could help clinicians to identify those most at risk of suboptimal EIC and to intervene accordingly. We recommend that the predictive power of this tool is tested across different clinic populations and settings. We believe this to be of particular importance given that there is presently no proven method for clinicians to identify which newly diagnosed PLWH are most at risk of disengaging from care and thereby take preventative measures. Our findings have demonstrated the importance of the wider health and social context in being able to manage HIV effectively and suggest that interventions need to address broader issues that impact on health-care utilisation. We have developed two complex interventions that seek to do this under the current financial constraints by providing holistic approaches that benefit from the strengths of peer support and aim to manage the multiple psychological, social and economic issues which deter optimal engagement in HIV care. We have developed a third intervention which provides an alternative clinic setting for people who are particularly concerned about disclosure of their HIV status and our fourth intervention uses co-ordinated messaging to encourage attendance and improve communication with patients. We recommend conducting robust clinical trials of these interventions that incorporate full health and economic evaluation.
Acknowledgements
We are immensely grateful to all the patients, community participants and key informants who contributed to the development of the EIC algorithm and to phase 3 of the study. We are also very grateful to the services that supported our study, with particular thanks to the principal investigators, research nurses and research assistants at each of the sites.
We would like to thank the UK-CAB and the members of our Advisory Group and Study Steering Committee (listed in Appendix 1) for their invaluable contribution to our work.
We are very grateful to Dr Rebecca O’Connell for her work on the analysis of the qualitative interviews, to Ms Sophie Jose and Dr Teresa Hill for their advice on UK CHIC during phase 1 of the study, to Dr Tracy Glass for her advice on the use of group-based trajectory analysis and to Mr Roger Pebody for his help with our plain English summary.
Contributions of authors
All authors contributed to the design of the study and have approved the final report.
Dr Alison Howarth (Senior Research Associate) was responsible for project management and data collection. She conducted all interviews and jointly facilitated the FGs. She conducted data analysis in all phases of the study and led on the authorship of the report.
Dr Vanessa Apea (Consultant in GUM/HIV medicine) was lead investigator on phase 2 of the study. She contributed expert knowledge on the clinical management of HIV.
Professor Susan Michie (Professor of Health Psychology) contributed expert knowledge on the theory of behaviour change in relation to health, and its application to intervention development. She advised on the content of questionnaires and topic guides.
Professor Steve Morris (Professor of Health Economics) was lead investigator on the two health economic components of the study. He conducted the analysis of laboratory tests costs and intervention costs and was lead author on these sections of the report.
Ms Memory Sachikonye (Co-ordinator, UK-CAB) was the lead for patient and public involvement. She was responsible for recruiting FG participants. She jointly facilitated the FGs and contributed to qualitative data analysis.
Dr Catherine Mercer (Reader in Applied Statistics) was the lead investigator on the development of the retention risk tool.
Ms Amanda Evans (Chartered Psychologist) contributed to the analysis of qualitative data. She contributed expert knowledge on psychological impacts of living with HIV.
Dr Valerie Delpech (Head of HIV Surveillance, Public Health England) contributed expert knowledge on the epidemiology of HIV and public health interventions.
Professor Caroline Sabin (Professor of Medical Statistics and Epidemiology) was co-principal investigator on the study, with responsibility for phase 1 of the study. She contributed expert epidemiological and statistical knowledge and advice. She conducted the analysis of association with clinical outcomes.
Dr Fiona Burns (Clinical Senior Lecturer and Honorary Consultant Physician) was principal investigator with overall responsibility for the study. She advised on all elements of data collection and analysis and contributed to the analysis of qualitative data. She contributed expert knowledge on the clinical management of HIV.
UK Collaborative HIV Cohort study
UK Collaborative HIV Cohort Steering Committee: Jonathan Ainsworth, Sris Allan, Jane Anderson, Abdel Babiker, David Chadwick, Valerie Delpech, David Dunn, Martin Fisher, Brian Gazzard, Richard Gilson, Mark Gompels, Phillip Hay, Teresa Hill, Margaret Johnson, Sophie Jose, Stephen Kegg, Clifford Leen, Fabiola Martin, Mark Nelson, Chloe Orkin, Adrian Palfreeman, Andrew Phillips, Deenan Pillay, Frank Post, Jillian Pritchard, Caroline Sabin, Memory Sachikonye, Achim Schwenk, Anjum Tariq, Roy Trevelion, John Walsh.
UK Collaborative HIV Cohort Central Co-ordination: University College London (Teresa Hill, Sophie Jose, Andrew Phillips, Caroline Sabin, Alicia Thornton, Susie Huntington); Medical Research Council Clinical Trials Unit at University College London (David Dunn, Adam Glabay).
UK Collaborative HIV Cohort Participating Centres: Brighton and Sussex University Hospitals NHS Trust (M Fisher, N Perry, S Tilbury, E Youssef, D Churchill); Chelsea and Westminster Hospital NHS Foundation Trust (B Gazzard, M Nelson, R Everett, D Asboe, S Mandalia); King’s College Hospital NHS Foundation Trust (F Post, H Korat, C Taylor, Z Gleisner, F Ibrahim, L Campbell); Mortimer Market Centre, University College London (R Gilson, N Brima, I Williams); Royal Free NHS Foundation Trust/University College London (M Johnson, M Youle, F Lampe, C Smith, R Tsintas, C Chaloner, S Hutchinson, C Sabin, A Phillips, T Hill, S Jose, A Thornton, S Huntington); Imperial College Healthcare NHS Trust (J Walsh, N Mackie, A Winston, J Weber, F Ramzan, M Carder); Barts and The London NHS Trust (C Orkin, J Lynch, J Hand, C de Souza); Homerton University Hospital NHS Trust (J Anderson, S Munshi); North Middlesex University Hospital NHS Trust (J Ainsworth, A Schwenk, S Miller, C Wood); The Lothian University Hospitals NHS Trust (C Leen, A Wilson, S Morris); North Bristol NHS Trust (M Gompels, S Allan); Leicester, University Hospitals of Leicester NHS Trust (A Palfreeman, K Memon, A Lewszuk); Middlesbrough, South Tees Hospitals NHS Foundation Trust (D Chadwick, E Cope, J Gibson); Woolwich, Lewisham and Greenwich NHS Trust (S Kegg, P Main, S Mitchell, M Hunter), St George’s Healthcare NHS Trust (P Hay, M Dhillon); York Teaching Hospital NHS Foundation Trust (F Martin, S Russell-Sharpe); University Hospitals Coventry and Warwickshire NHS Trust (S Allan, A Harte, S Clay); The Royal Wolverhampton Hospitals NHS Trust (A Tariq, H Spencer, R Jones); Ashford and St Peter’s Hospitals NHS Foundation Trust (J Pritchard, S Cumming, C Atkinson); Public Health England (V Delpech); UK-CAB (M Sachikonye, R Trevelion).
Publications
Howarth A, Burns F, Apea V, Jose S, Hill T, Delpech V, et al. Development and application of a new measure of engagement in out-patient HIV care. HIV Med 2016;18:267–74.
Sabin CA, Howarth A, Jose S, Hill T, Apea V, Morris S, et al. Association between engagement in-care and mortality in HIV-positive persons. AIDS 2017;31:653–60.
Data sharing statement
Proposals for research studies requiring access to UK CHIC data from existing and potential new collaborators are subject to review and approval by the UK CHIC Steering Committee; a proposal template is available to submit details of proposals at the UK CHIC website: www.ctu.mrc.ac.uk/UKCHIC; support for statistical analyses may be available from the study team.
Researchers who are interested in accessing the REACH survey data should contact the corresponding author with a description of their proposal.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Mayer KH. Introduction: linkage, engagement, and retention in HIV care: essential for optimal individual- and community-level outcomes in the era of highly active antiretroviral therapy. Clin Infect Dis 2011;52:205-7. http://dx.doi.org/10.1093/cid/ciq043.
- May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014;28:1193-202. http://dx.doi.org/10.1097/QAD.0000000000000243.
- Metsch LR, Pereyra M, Messinger S, Del Rio C, Strathdee SA, Anderson-Mahoney P, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clin Infect Dis 2008;47:577-84. http://dx.doi.org/10.1086/590153.
- Standards of Care for People Living with HIV. London: BHIVA; 2013.
- Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: where are we? Where do we go from here?. Clin Infect Dis 2010;50:752-61. http://dx.doi.org/10.1086/649933.
- 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva: UNAIDS; 2014.
- HIV in the United Kingdom: 2014 Report. London: Public Health England; 2014.
- Rice BD, Delpech VC, Chadborn TR, Elford J. Loss to follow-up among adults attending human immunodeficiency virus services in England, Wales, and Northern Ireland. Sex Transm Dis 2011;38:685-90. http://dx.doi.org/10.1097/OLQ.0b013e318214b92e.
- Hill T, Bansi L, Sabin C, Phillips A, Dunn D, Anderson J, et al. Data linkage reduces loss to follow-up in an observational HIV cohort study. J Clin Epidemiol 2010;63:1101-9. http://dx.doi.org/10.1016/j.jclinepi.2009.12.007.
- Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr 2012;59:86-93. http://dx.doi.org/10.1097/QAI.0b013e318236f7d2.
- Kerr JC, Stephens TG, Gibson JJ, Duffus WA. Risk factors associated with inpatient hospital utilization in HIV-positive individuals and relationship to HIV care engagement. J Acquir Immune Defic Syndr 2012;60:173-82. http://dx.doi.org/10.1097/QAI.0b013e31824bd55d.
- Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis 2009;48:248-56. http://dx.doi.org/10.1086/595705.
- Helleberg M, Engsig FN, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Retention in a public healthcare system with free access to treatment: a Danish nationwide HIV cohort study. AIDS 2012;26:741-8. http://dx.doi.org/10.1097/QAD.0b013e32834fa15e.
- Ndiaye B, Ould-Kaci K, Salleron J, Bataille P, Bonnevie F, Cochonat K, et al. Characteristics of and outcomes in HIV-infected patients who return to care after loss to follow-up. AIDS 2009;23:1786-9. http://dx.doi.org/10.1097/QAD.0b013e32832e3469.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793-800. http://dx.doi.org/10.1093/cid/ciq243.
- Martin SJ, Bassi S, Dunbar-Rees R. Commitments, norms and custard creams – a social influence approach to reducing did not attends (DNAs). J R Soc Med 2012;105:101-4. http://dx.doi.org/10.1258/jrsm.2011.110250.
- BBC . NHS to Reveal Cost of Missed Appointments to Patients 2015. www.bbc.co.uk/news/uk-33375976 (accessed 1 May 2016).
- Fit For The Future? Dr Foster Hospital Guide. London: Dr Foster Ltd; 2012.
- Cree M, Bell NR, Johnson D, Carriere KC. Increased continuity of care associated with decreased hospital care and emergency department visits for patients with asthma. Dis Manag 2006;9:63-71. http://dx.doi.org/10.1089/dis.2006.9.63.
- Gill JM, Mainous AG, Nsereko M. The effect of continuity of care on emergency department use. Arch Fam Med 2000;9:333-8. https://doi.org/10.1001/archfami.9.4.333.
- Mocroft A, Kirk O, Aldins P, Chies A, Blaxhult A, Chentsova N, et al. Loss to follow-up in an international, multicentre observational study. HIV Med 2008;9:261-9. http://dx.doi.org/10.1111/j.1468-1293.2008.00557.x.
- Gerver SM, Chadborn TR, Ibrahim F, Vatsa B, Delpech VC, Easterbrook PJ. High rate of loss to clinical follow up among African HIV-infected patients attending a London clinic: a retrospective analysis of a clinical cohort. J Int AIDS Soc 2010;13. http://dx.doi.org/10.1186/1758-2652-13-29.
- Lanoy E, Mary-Krause M, Tattevin P, Dray-Spira R, Duvivier C, Fischer P, et al. Predictors identified for losses to follow-up among HIV-seropositive patients. J Clin Epidemiol 2006;59:829-35. https://doi.org/10.1016/j.jclinepi.2005.11.024.
- Lanoy E, Lewden C, Lièvre L, Tattevin P, Boileau J, Aouba A, et al. How does loss to follow-up influence cohort findings on HIV infection? A joint analysis of the French hospital database on HIV, Mortalité 2000 survey and death certificates. HIV Med 2009;10:236-45. http://dx.doi.org/10.1111/j.1468-1293.2008.00678.x.
- Sabin CA. Cohort studies: to what extent can they inform treatment guidelines?. Curr Opin Infect Dis 2010;23:15-20. http://dx.doi.org/10.1097/QCO.0b013e32833521b4.
- Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS 2010;24:607-13. http://dx.doi.org/10.1089/apc.2010.0086.
- Asboe D, Aitken C, Boffito M, Booth C, Cane P, Fakoya A, et al. British HIV Association guidelines for the routine investigation and monitoring of adult HIV-1-infected individuals 2011. HIV Med 2012;13:1-44. http://dx.doi.org/10.1111/j.1468-1293.2011.00971.x.
- Craw J, Wilson T, Giordano T, Mugavero M, Keruly J, Drainoni M-L, et al. Baseline correlates of retention in HIV primary care at 6 HIV clinics in the United States. J Int Assoc Physicians AIDS Care 2012;11.
- McGettrick P, Ghavami-Kia B, O’Halloran J, Sheehan G, Lambert J, Mallon P, et al. The HIV Care Cascade: ‘Gap’ analysis of those linked to, but not retained in care. HIV Med 2015;16:12-76.
- Muthulingam D, Chin J, Hsu L, Scheer S, Schwarcz S. Disparities in engagement in care and viral suppression among persons with HIV. J Acquir Immune Defic Syndr 2013;63:112-19. http://dx.doi.org/10.1097/QAI.0b013e3182894555.
- Anthony MN, Gardner L, Marks G, Anderson-Mahoney P, Metsch LR, Valverde EE, et al. Factors associated with use of HIV primary care among persons recently diagnosed with HIV: examination of variables from the behavioural model of health-care utilization. AIDS Care 2007;19:195-202. https://doi.org/10.1080/09540120600966182.
- Anderson J, Doyal L. Women from Africa living with HIV in London: a descriptive study. AIDS Care 2004;16:95-105. http://dx.doi.org/10.1080/09540120310001634001.
- Baran R, Mulcahy F, Krznaric I, Monforte Ad Samarina A, Xi H, . Reduced HIV symptoms and improved health-related quality of life correlate with better access to care for HIV-1 infected women: the ELLA study. J Int AIDS Soc 2014;17. http://dx.doi.org/10.7448/IAS.17.4.19616.
- Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med 2009;24:1101-8. http://dx.doi.org/10.1007/s11606-009-1068-8.
- Konkle-Parker DJ, Amico KR, Henderson HM. Barriers and facilitators to engagement in HIV clinical care in the Deep South: results from semi-structured patient interviews. J Assoc Nurses AIDS Care 2011;22:90-9. http://dx.doi.org/10.1016/j.jana.2010.06.002.
- Beer L, Fagan JL, Valverde E, Bertolli J. Never in Care Project. Health-related beliefs and decisions about accessing HIV medical care among HIV-infected persons who are not receiving care. AIDS Patient Care STDS 2009;23:785-92. http://dx.doi.org/10.1089/apc.2009.0032.
- Morrison C, Betney K, McCulloch C. A Report on a Study Exploring Non-Attendance at Specialist Clinical HIV Services in Scotland. Edinburgh: NHS Health Scotland; 2011.
- Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV AIDS Rep 2012;9:313-25. http://dx.doi.org/10.1007/s11904-012-0136-6.
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001;29:374-93. https://doi.org/10.1177/0049124101029003005.
- Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res 2007;35:542-71. https://doi.org/10.1177/0049124106292364.
- Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109-38. http://dx.doi.org/10.1146/annurev.clinpsy.121208.131413.
- Glass TR, Battegay M, Cavassini M, De Geest S, Furrer H, Vernazza PL, et al. Longitudinal analysis of patterns and predictors of changes in self-reported adherence to antiretroviral therapy: Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2010;54:197-203. http://dx.doi.org/10.1097/QAI.0b013e3181ca48bf.
- British HIV Association Guidelines for the Treatment of HIV-1-Positive Adults with Antiretroviral Therapy 2015. London: BHIVA; 2015.
- Howarth A, Burns F, Apea V, Jose S, Hill T, Delpech V, et al. Development and application of a new measure of engagement in out-patient HIV care. HIV Med 2016;18:267-74. http://dx.doi.org/10.1111/hiv.12427.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-42.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. ‘Psychological Theory’ Group . Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. https://doi.org/10.1136/qshc.2004.011155.
- Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide. Washington, DC: USAID; 2007.
- Stangl AL, Brady L, Fritz K. Measuring HIV Stigma and Discrimination: Technical Brief. Washington, DC: Strive; 2012.
- Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological wellbeing. J Pers Soc Psychol 1989;57:1069-81. https://doi.org/10.1037/0022-3514.57.6.1069.
- Ryff CD, Keyes CL. The structure of psychological well-being revisited. J Pers Soc Psychol 1995;69:719-27. https://doi.org/10.1037/0022-3514.69.4.719.
- Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Med Care 1988;26:709-23. https://doi.org/10.1097/00005650-198807000-00006.
- Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50:613-21. http://dx.doi.org/10.1176/appi.psy.50.6.613.
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010;24:1243-50. http://dx.doi.org/10.1097/QAD.0b013e3283354a7b.
- Gustafson DH, Hawkins R, Pingree S, McTavish F, Arora NK, Mendenhall J, et al. Effect of computer support on younger women with breast cancer. J Gen Intern Med 2001;16:435-45. https://doi.org/10.1046/j.1525-1497.2001.016007435.x.
- Gustafson DH, McTavish FM, Stengle W, Ballard D, Hawkins R, Shaw BR, et al. Use and impact of eHealth system by low-income women with breast cancer. J Health Commun 2005;10:195-218. https://doi.org/10.1080/10810730500263257.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1-24. https://doi.org/10.1080/08870449908407311.
- Gonzalez JS, Penedo FJ, Llabre MM, Durán RE, Antoni MH, Schneiderman N, et al. Physical symptoms, beliefs about medications, negative mood, and long-term HIV medication adherence. Ann Behav Med 2007;34:46-55. http://dx.doi.org/10.1080/08836610701495565.
- Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health 2002;17:17-32. https://doi.org/10.1080/08870440290001502.
- Public Health England . HIV Surveillance Systems n.d. www.gov.uk/guidance/hiv-surveillance-systems (accessed 1 May 2016).
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-18. https://doi.org/10.1093/aje/kwk052.
- Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol 2006;194:888-94. https://doi.org/10.1016/j.ajog.2005.09.002.
- Adams ST, Leveson SH. Clinical prediction rules. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.d8312.
- Falasinnu T, Gustafson P, Gilbert M, Shoveller J. Risk prediction in sexual health contexts: protocol. JMIR Res Protoc 2013;2. http://dx.doi.org/10.2196/resprot.2971.
- Falasinnu T, Gustafson P, Hottes TS, Gilbert M, Ogilvie G, Shoveller J. A critical appraisal of risk models for predicting sexually transmitted infections. Sex Transm Dis 2014;41:321-30. http://dx.doi.org/10.1097/OLQ.0000000000000120.
- Medical Research Council Clinical Trials Unit . Adolescents and Adults Living With Perinatal HIV n.d. www.ctu.mrc.ac.uk/our_research/research_areas/hiv/studies/aalphi/ (accessed 1 May 2016).
- Ritchie J, Lewis J. Qualitative Research Practice. London: Sage; 2003.
- Fielding NG, Lee RM. Computer Analysis and Qualitative Research. London: Sage; 1998.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/article/pmg9/chapter/foreword (accessed 1 May 2016).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit; 2015.
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001;158:725-30. http://dx.doi.org/10.1176/appi.ajp.158.5.725.
- Gardner LI, Marks G, Craw JA, Wilson TE, Drainoni ML, Moore RD, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis 2012;55:1124-34. https://doi.org/10.1093/cid/cis623.
- Murphy DA, Brecht ML, Herbeck DM, Huang D. Trajectories of HIV risk behavior from age 15 to 25 in the national longitudinal survey of youth sample. J Youth Adolesc 2009;38:1226-39. http://dx.doi.org/10.1007/s10964-008-9323-6.
- van der Straten A, Shiboski S, Montgomery ET, Moore J, De Bruyn G, Ramjee G, et al. Patterns and predictors of adherence to diaphragm use in a phase III trial in sub-Saharan Africa: a trajectory analysis. J Acquir Immune Defic Syndr 2009;50:419-26. http://dx.doi.org/10.1097/QAI.0b013e3181958511.
- Sohler NL, Li X, Cunningham CO. Gender disparities in HIV health care utilization among the severely disadvantaged: can we determine the reasons?. AIDS Patient Care STDS 2009;23:775-83. http://dx.doi.org/10.1089/apc.2009.0041.
- Menza TW, Hughes JP, Celum CL, Golden MR. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis 2009;36:547-55. http://dx.doi.org/10.1097/OLQ.0b013e3181a9cc41.
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care 2000;12:255-66. http://dx.doi.org/10.1080/09540120050042891.
- Glass TR, De Geest S, Weber R, Vernazza PL, Rickenbach M, Furrer H, et al. Correlates of self-reported nonadherence to antiretroviral therapy in HIV-infected patients: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2006;41:385-92. http://dx.doi.org/10.1097/01.qai.0000186371.95301.52.
- Harding R, Lampe FC, Norwood S, Date HL, Clucas C, Fisher M, et al. Symptoms are highly prevalent among HIV outpatients and associated with poor adherence and unprotected sexual intercourse. Sex Transm Infect 2010;86:520-4. http://dx.doi.org/10.1136/sti.2009.038505.
- Mallinson RK, Rajabiun S, Coleman S. The provider role in client engagement in HIV care. AIDS Patient Care STDS 2007;21:77-84. http://dx.doi.org/10.1089/apc.2007.9984.
- Robert GR, Cornwall J. What Matters to Patients? Policy Recommendations. Coventry: NHS Institute for Innovation and Improvement; 2011.
- Flickinger TE, Saha S, Moore RD, Beach MC. Higher quality communication and relationships are associated with improved patient engagement in HIV care. J Acquir Immune Defic Syndr 2013;63:362-6. http://dx.doi.org/10.1097/QAI.0b013e318295b86a.
- Schafer KR, Brant J, Gupta S, Thorpe J, Winstead-Derlega C, Pinkerton R, et al. Intimate partner violence: a predictor of worse HIV outcomes and engagement in care. AIDS Patient Care STDS 2012;26:356-65. http://dx.doi.org/10.1089/apc.2011.0409.
- Francis K, Tookey PA, Peters H, Tariq S. The impact of immigration issues on the care of women living with HIV during pregnancy: a survey of healthcare providers in UK & Ireland maternity units. HIV Med 2016;17:14-71.
- Cavaleri MA, Kalogerogiannis K, McKay MM, Vitale L, Levi E, Jones S, et al. Barriers to HIV care: an exploration of the complexities that influence engagement in and utilization of treatment. Soc Work Health Care 2010;49:934-45. http://dx.doi.org/10.1080/00981389.2010.514563.
- Rajabiun S, Mallinson RK, McCoy K, Coleman S, Drainoni ML, Rebholz C, et al. ‘Getting me back on track’: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDS 2007;21:20-9. http://dx.doi.org/10.1089/apc.2007.9990.
- Ridge D, Williams I, Anderson J, Elford J. Like a prayer: the role of spirituality and religion for people living with HIV in the UK. Sociol Health Illn 2008;30:413-28. http://dx.doi.org/10.1111/j.1467-9566.2007.01062.x.
- Horvath KJ, Oakes JM, Rosser BR, Danilenko G, Vezina H, Amico KR, et al. Feasibility, acceptability and preliminary efficacy of an online peer-to-peer social support ART adherence intervention. AIDS Behav 2013;17:2031-44. http://dx.doi.org/10.1007/s10461-013-0469-1.
- Richter L, Rotheram-Borus MJ, Van Heerden A, Stein A, Tomlinson M, Harwood JM, et al. Pregnant women living with HIV (WLH) supported at clinics by peer WLH: a cluster randomized controlled trial. AIDS Behav 2014;18:706-15. http://dx.doi.org/10.1007/s10461-014-0694-2.
- Van Tam V, Larsson M, Pharris A, Diedrichs B, Nguyen HP, Nguyen CT, et al. Peer support and improved quality of life among persons living with HIV on antiretroviral treatment: a randomised controlled trial from north-eastern Vietnam. Health Qual Life Outcomes 2012;10. http://dx.doi.org/10.1186/1477-7525-10-53.
- Anderson A, Anderson J, Fadojutimi M, Reeves I, Wills M. Peer navigators – can patient-led support contribute to clinical and well-being outcomes?. HIV Med 2015;16.
Appendix 1 Advisory Group and Study Steering Committee
Advisory Group membership
Dr Chloe Orkin: Ambrose King Centre, Royal London Hospital.
Dr Paul Benn, Dr Simon Edwards: Bloomsbury Clinic, Mortimer Market Centre.
Professor Jane Anderson, Dr Iain Reeves: Clifden Centre, Homerton University Hospital.
Dr Rebecca O’Connell: Greenway Centre, Newham University Hospital.
Dr Julie Fox: Harrison Wing, St Thomas’ Hospital.
Dr Leena Sathia Ian Charleson Day Centre, Royal Free Hospital.
Dr Ann Sullivan, Dr Tristan Barber: Kobler Day Care Unit, Chelsea and Westminster Hospital.
Ms Sophie Strachan: Positively UK.
Study Steering Committee
Mr Paul Clift: King’s College Hospital NHS Foundation Trust/UK-CAB.
Professor Paul Flowers: Glasgow Caledonian University.
Professor Jonathan Sterne: University of Bristol.
Dr Ann Sullivan: Chelsea and Westminster Hospital NHS Foundation Trust.
Appendix 2 REACH questionnaire
Appendix 3 Framework for REACH primary research
| COM-B system: sources of behaviour | Theoretical domains framework | Constructs | Factors related to engagement in HIV services requiring change |
|---|---|---|---|
| Opportunity: social | Social influences: interpersonal processes that can change thoughts, feelings, behaviours | Social norms, social support, alienation, modelling, power, group identity, social pressure, group conformity, social comparisons, group norms, intergroup conflict |
|
| Opportunity: physical | Environmental context and resources: circumstances that discourage or encourage development of skills and abilities, independence, social competence, adaptive behaviour | Barriers and facilitators, environmental stressors, resources, organisational culture/climate, salient events/critical incidents, person/environment interaction |
|
| Capability: physical | Physical skills: ability acquired through practice | Skills, skills development, competence, ability, practice, skill assessment | |
| Capability: psychological | Knowledge: awareness of existence of something | Knowledge, procedural knowledge, knowledge of task environment |
|
| Cognitive and interpersonal skills: ability acquired through practice | Skills, competence, interpersonal skills, skills development, ability, practice, skill assessment | ||
| Memory, attention, decision processes: ability to retain info, focus selectively and choose between alternatives | Decision-making, cognitive overload/tiredness, memory, attention, attention control | ||
| Behavioural regulation: management or change of objectively observed or measured actions | Self-monitoring, breaking habit, action planning |
|
|
| Motivation: automatic | Reinforcement: increasing probability of response through a given stimulus | Incentives, consequences, reinforcement, rewards, punishment, contingencies, sanctions | |
| Emotion: complex reaction pattern to deal with personally significant matter or event | Fear, anxiety, affect, stress, depression, positive/negative affect, burn-out |
|
|
| Motivation: reflective | Social/professional role and identity: coherent set of behaviours and displayed personal qualities in social or work setting | Social identity, group identity, leadership, professional identity, professional role, identity, professional boundaries, professional confidence, organisational commitment | |
| Beliefs about capabilities: acceptance of truth, reality or validity about ability, talent or facility | Self-confidence, perceived competence, self-efficacy, perceived behavioural control, beliefs, self-esteem, empowerment, professional confidence | ||
| Optimism: confidence things will happen for the best or desired goals will be attained | Optimism, pessimism, unrealistic optimism, identity | ||
| Intentions: conscious decision to perform a behaviour/resolve act in a certain way | Stability of intentions, stages of change model, transtheoretical model/stages of change | ||
| Goals: mental representations of desired outcomes/end states | Goal priority, goals (distal/proximal), goal priority, goal/target setting, goals (autonomous/controlled), action planning, implementation intention | ||
| Beliefs about consequences: acceptance of truth, reality, validity of behaviour outcomes in given situation | Consequences, beliefs, outcome expectancies, characteristics of outcome expectancies, anticipated regret, consequences |
Appendix 4 Patient interview screening questions
-
What is your gender? Are you male, female, transgender?
-
What is your date of birth?
-
Were you born with HIV?
-
When did you first find out you were HIV positive?
-
Are you currently taking HIV medicine (HIV treatment/ART/highly active ART)?
-
What is your ethnic group?
-
If black, are you black African, black Caribbean or any other black background?
-
Which country were you born in?
-
(Men only) I’m going to read out a list of terms people sometimes use to describe how they think of themselves:
-
heterosexual or straight
-
gay or lesbian
-
bisexual
-
other.
-
-
As I read the list again please say ‘yes’ when you hear the option that best describes how you think of yourself.
-
Have you ever injected yourself with non-prescribed drugs or other substances?
-
Since your HIV diagnosis, have there been any periods of a year or more when you have not seen a doctor about your HIV?
If yes, when was the last time that this happened?
-
In the last 12 months, how many appointments with a doctor or nurse specialist have you missed and not rescheduled at your HIV clinic?
Appendix 5 Topic guide: in-depth interviews
Appendix 6 Topic guide: key informant interviews
Appendix 7 Tests per person per quarter by starter since started antiretroviral therapy
FIGURE 13.
Liver function tests per person per quarter by starter since started ART.
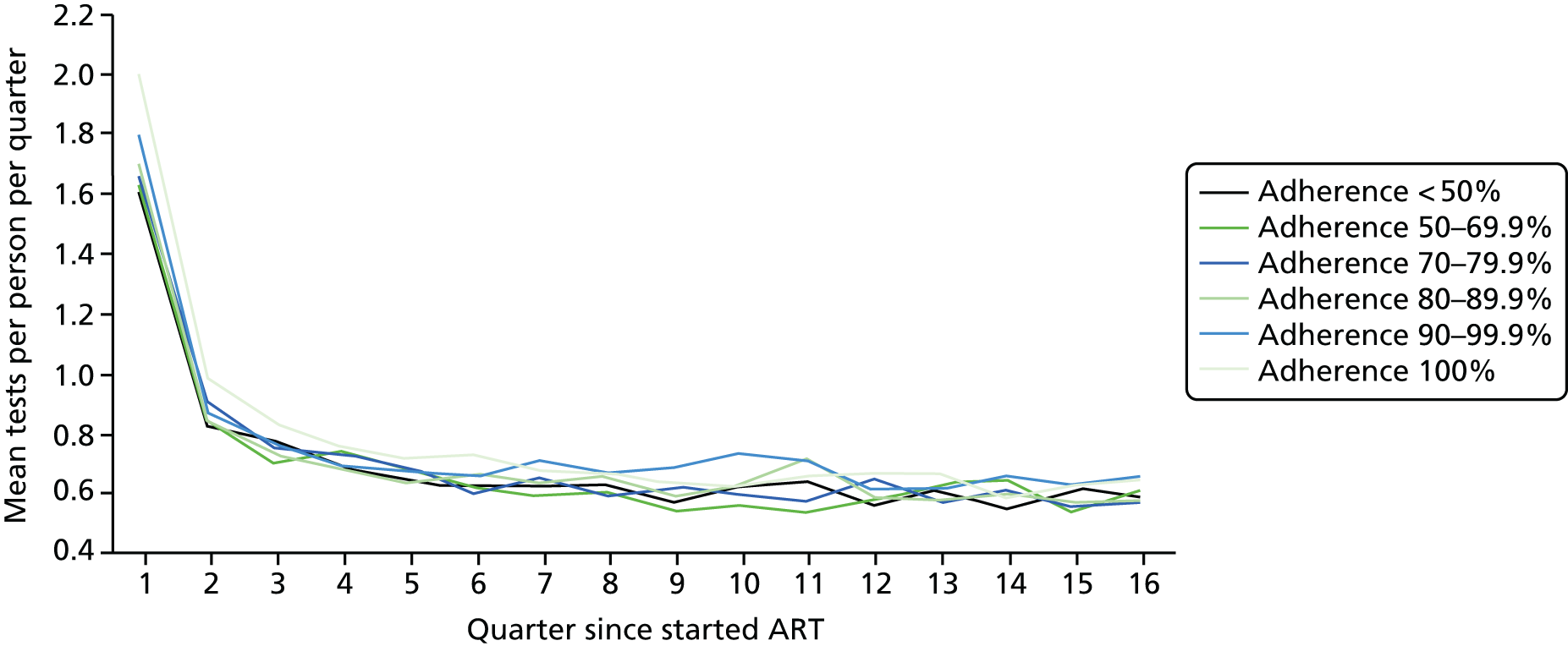
FIGURE 14.
Lipid tests per person per quarter by starter since started ART.

FIGURE 15.
Full blood count tests per person per quarter by starter since started ART.
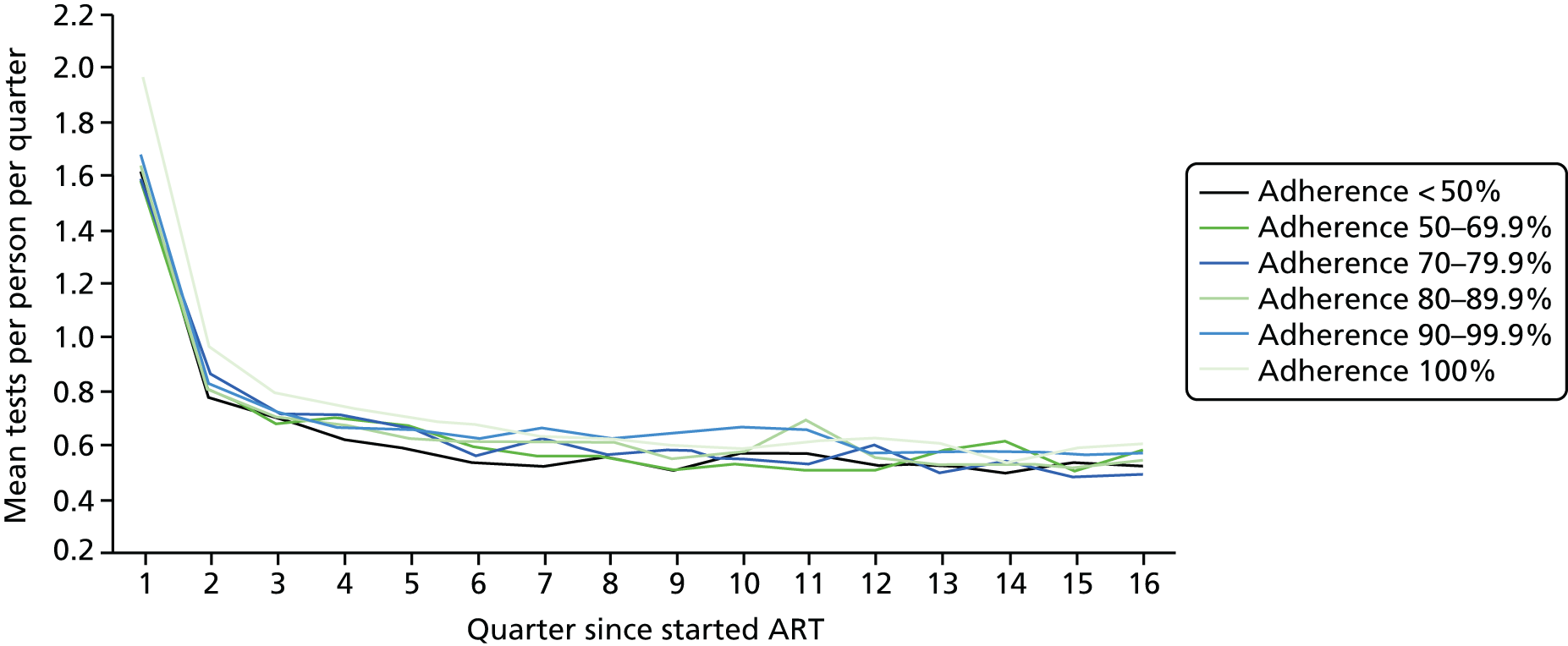
FIGURE 16.
Urea tests per person per quarter by starter since started ART.
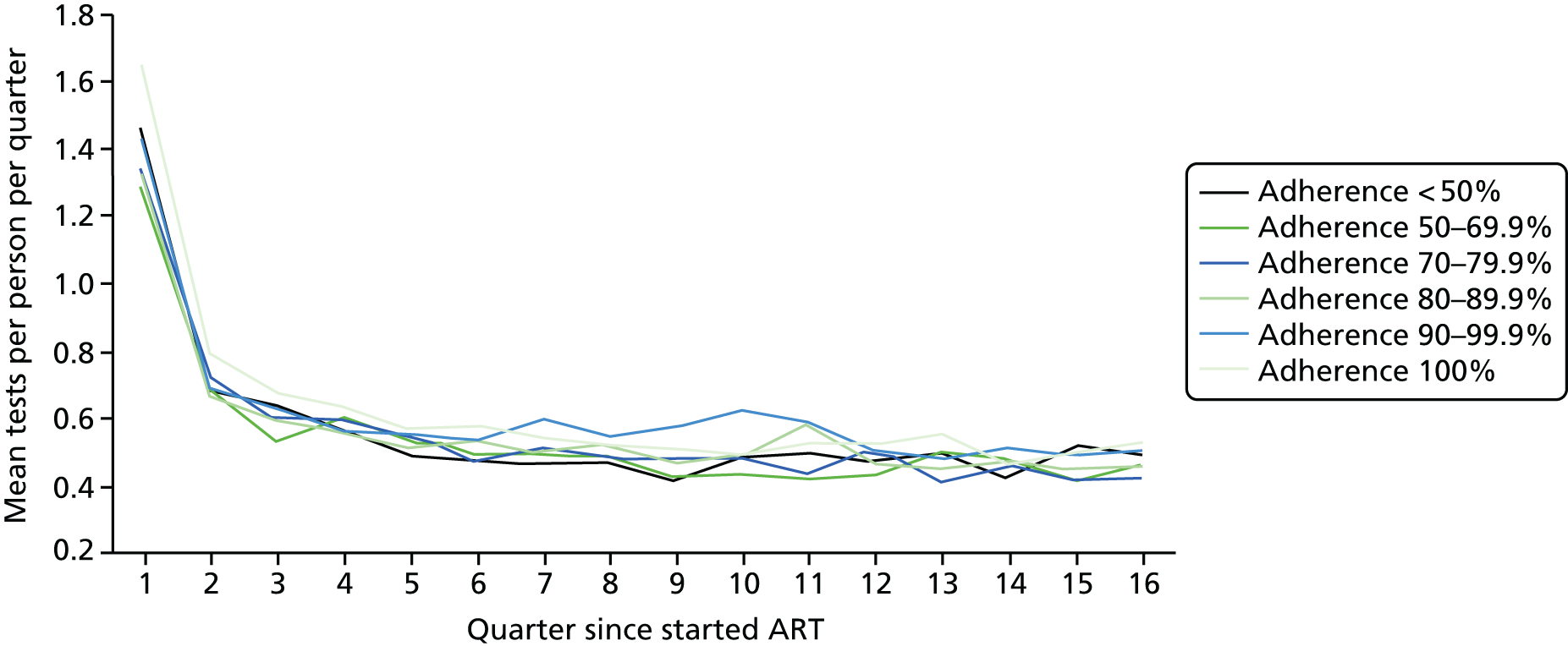
FIGURE 17.
Creatinine tests per person per quarter by starter since started ART.
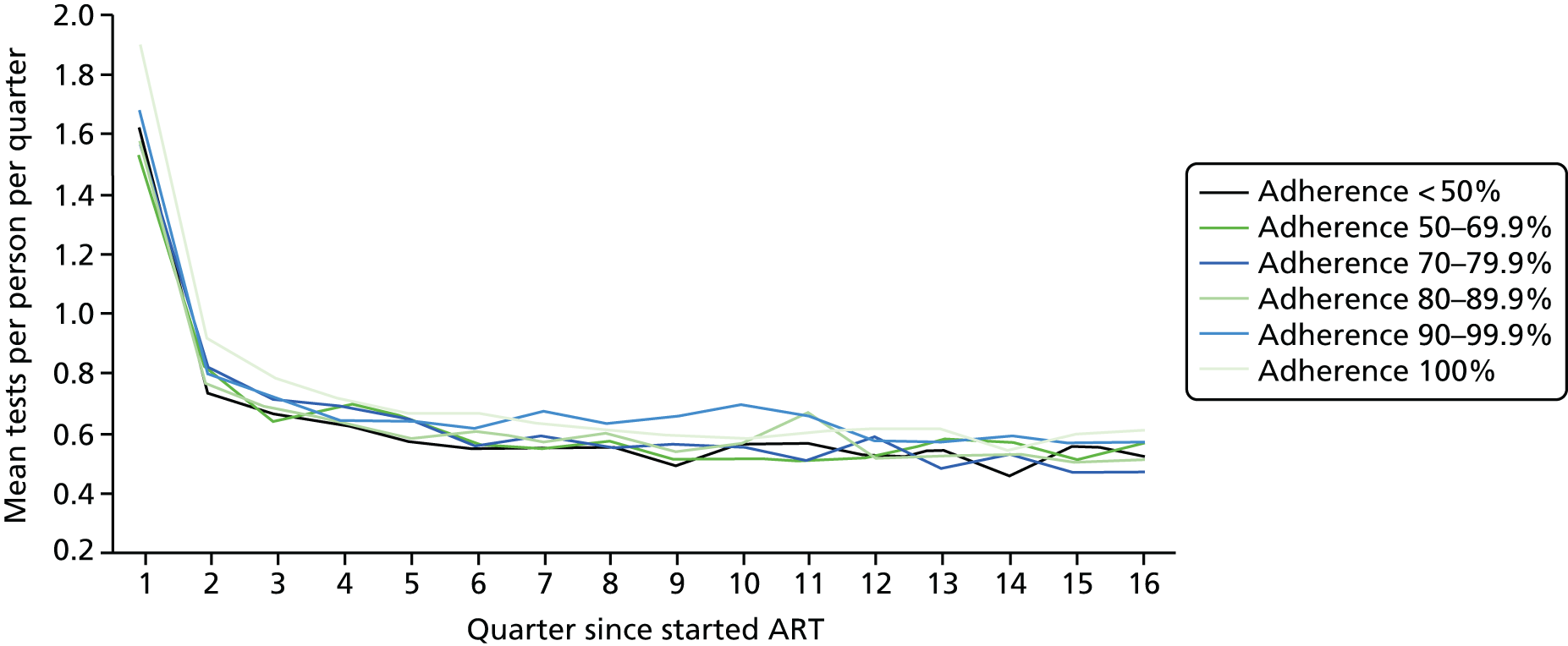
FIGURE 18.
Glucose tests per person per quarter by starter since started ART.
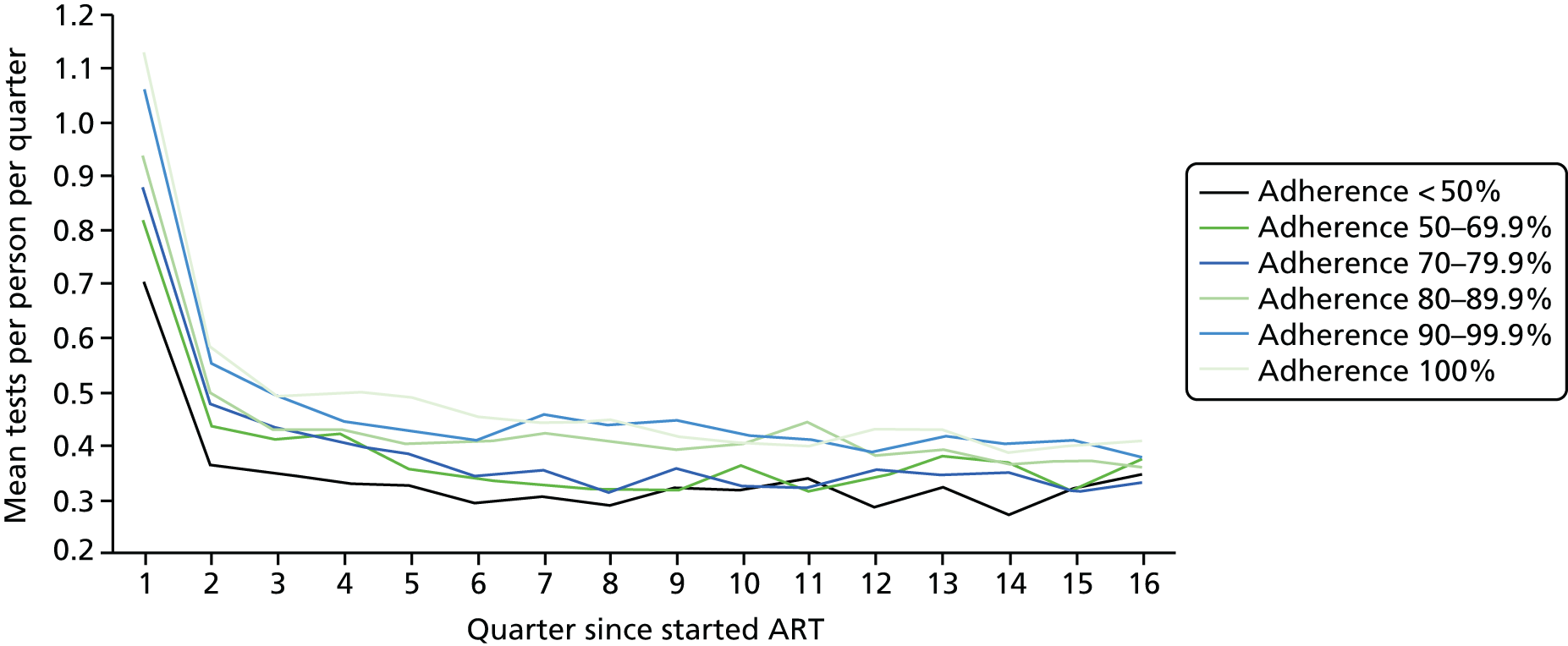
FIGURE 19.
Bone health (phosphate) tests per person per quarter by starter since started ART.
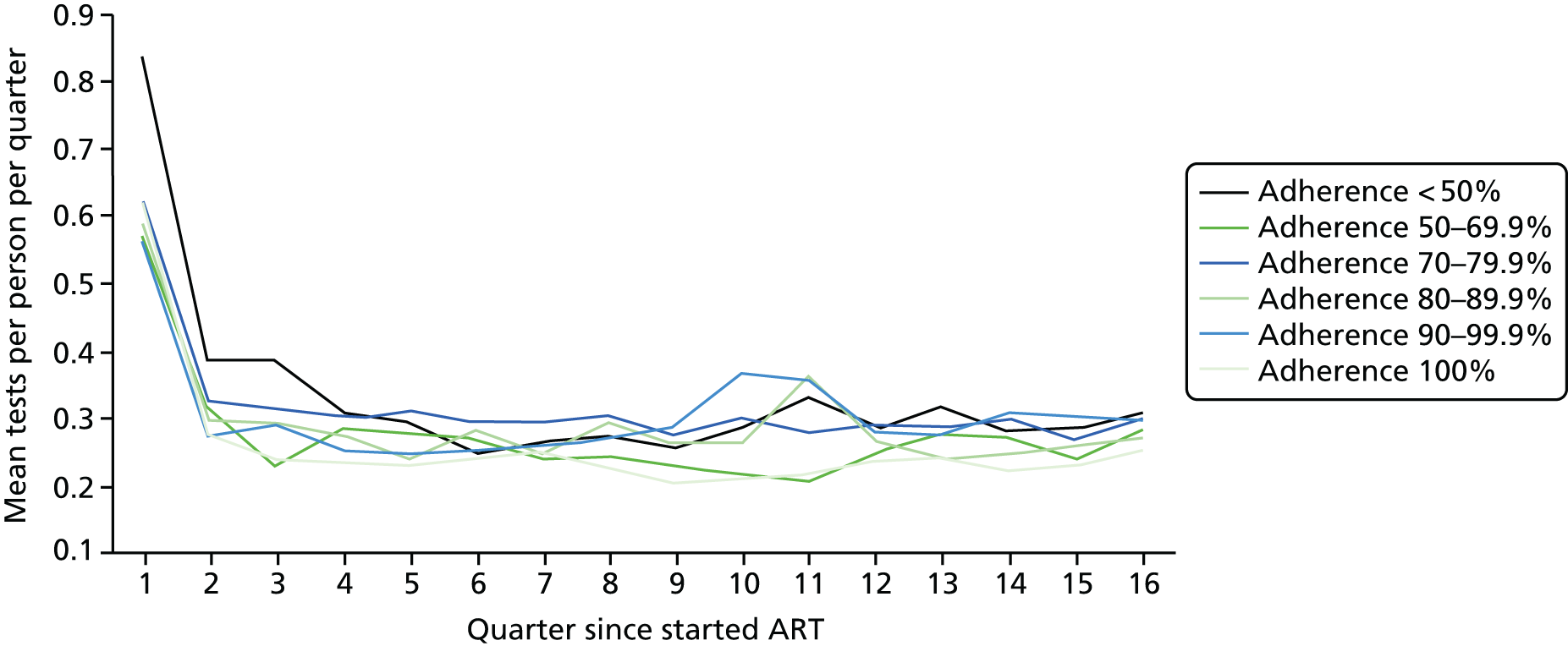
Glossary
- Adolescents and Adults Living with Perinatal HIV
- A prospective cohort study of two groups of young people: perinatally human immunodeficiency virus-infected individuals and human immunodeficiency virus-negative controls.
- Agenda for Change
- The NHS grading and pay system for all staff, except apprentices, doctors, dentists and some senior managers.
- Antiretroviral therapy
- Treatment for people with human immunodeficiency virus; the standard treatment is a combination of three or more drugs that suppress human immunodeficiency virus replication.
- Area under the receiver operating characteristic curve
- A graphical plot illustrating the performance of a binary classifier system as its discrimination threshold is varied.
- Cluster of differentiation 4
- Cluster of differentiation 4 cells are white blood cells which play an important role in the immune system; the cluster of differentiation 4 count indicates how strong the immune system is.
- COM-B model
- ‘Behaviour system’ proposing that behaviour (B) occurs as an interaction between three necessary conditions of capability (C), opportunity (O) and motivation (M).
- Computer-assisted self-interview
- A research technique in which the respondent uses a computer to answer the questions.
- Duke-UNC Functional Social Support Questionnaire
- An eight-item survey instrument to measure the strength of an individual’s social support network.
- HIV and AIDS Reporting System
- A data set of information on patients diagnosed with human immunodeficiency virus and attending human immunodeficiency virus outpatient care in England, Wales and Northern Ireland.
- Household Food Insecurity Access Scale
- A nine-item survey instrument that is used to distinguish food-insecure from food-secure households across different cultural contexts.
- Indefinite leave to remain
- The right to stay in the UK without any time restrictions.
- Irregular attender
- A person living with human immunodeficiency virus who has missed one or more of their human immunodeficiency virus outpatient clinic appointments within the past year and not rebooked within 4 weeks.
- Men who have sex with men
- Any male who engages in sexual activity with members of the same sex, irrespective of sexual identity.
- Non-attender
- A person living with human immunodeficiency virus who has experienced a period of non-attendance for a year or more at any human immunodeficiency virus outpatient service that ended within the past year.
- Non-nucleoside reverse transcriptase inhibitor
- Antiretroviral therapy that is used to treat the human immunodeficiency virus.
- Patient Health Questionnaire
- A four-item survey instrument used for brief and accurate measurement of depression and anxiety.
- People who inject drugs
- People who inject drugs, often in the context of substance dependence and/or recreational drug use.
- Protease inhibitor
- A class of antiretroviral therapy that is widely used to treat the human immunodeficiency virus/acquired immunodeficiency syndrome.
- Psychological Well-Being Scales
- A survey instrument that measures the multiple facets of psychological well-being.
- Regular attender
- A human immunodeficiency virus patient who has attended all human immunodeficiency virus clinical appointments in the past year.
- UK Collaborative HIV Cohort
- A database of routinely collected clinical information on human immunodeficiency virus-positive adults who have attended one of the collaborating centres for care since in 1996.
- Value added tax
- An indirect tax on the domestic consumption of goods and services.
- Viraemia
- The presence of detectable virus in the blood.
- Viral load
- The amount of human immunodeficiency virus in the blood expressed in copies/ml.
List of abbreviations
- –2LL
- minus twice the log-likelihood
- AALPHI
- Adolescents and Adults Living with Perinatal HIV
- AfC
- Agenda for Change
- AIDS
- acquired immunodeficiency syndrome
- aOR
- adjusted odds ratio
- ART
- antiretroviral therapy
- ASTRA
- Antiretrovirals, Sexual Transmission Risk and Attitudes
- AUROC
- area under the receiver operating characteristic
- BIC
- Bayesian information criterion
- BMQ
- Belief about Medicines Questionnaire
- CASI
- computer-assisted self-interview
- CD4
- cluster of differentiation 4
- CI
- confidence interval
- COM-B
- ‘capability’, ‘opportunity’, ‘motivation’ and ‘behaviour’
- DUFSS
- Duke-UNC Functional Social Support Questionnaire
- EIC
- engagement in care
- FG
- focus group
- GP
- general practitioner
- GUM
- genitourinary medicine
- HARS
- HIV and AIDS Reporting System
- HIV
- human immunodeficiency virus
- IA
- irregular attender
- IQR
- interquartile range
- LTFU
- lost to follow-up
- MARS
- Medication Adherence Report Scale
- MDT
- multidisciplinary team
- MSM
- men who have sex with men
- NA
- non-attender
- NNRTI
- non-nucleoside reverse transcriptase inhibitor
- NRA
- not regular attender
- PHQ4
- Patient Health Questionnaire
- PI
- protease inhibitor
- PLWH
- people living with human immunodeficiency virus
- PWB
- Psychological Well-Being Scales
- RA
- regular attender
- REACH
- Retention and Engagement Across Care services for HIV positive patients in the UK
- RH
- relative hazard
- SD
- standard deviation
- UK-CAB
- UK Community Advisory Board
- UK CHIC
- UK Collaborative HIV Cohort