Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/64/112. The contractual start date was in July 2014. The final report began editorial review in July 2016 and was accepted for publication in December 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Colin Bicknell has received unrelated fees for consultancy and speaker honoraria from Hansen Medical, Medtronic and Bolton Medical, during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Judah et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Diabetes and its complications
Diabetes is an increasing public health concern worldwide, with an estimated 380 million adults with diabetes diagnosed globally. 1 This corresponds to 8.3% of the world’s adult population, and is expected to rise to over 10% by 2035. Although the rates of other vascular risk factors such as hypertension, smoking and hypercholesterolaemia are falling, the rates of diabetes in the UK are rising.
Diabetes mellitus (hereafter referred to as ‘diabetes’) is a disorder whereby blood glucose levels are elevated over a prolonged period. This is due either to insufficient insulin production or to the lack of proper response to the insulin produced. Type 1 diabetes is an autoimmune condition which results in the pancreas not producing insulin. Treatment requires life-long administration of insulin. Although type 1 diabetes can develop at any age, it commonly occurs in childhood or adolescence. In type 2 diabetes, cells in the body fail to respond to insulin, or there may be insufficient insulin production. Development of type 2 diabetes is strongly associated with obesity. In the UK, approximately 90% of adults with diabetes have type 2 diabetes.
It was estimated that the NHS spends £10B each year to treat diabetes and its complications, representing 10% of the NHS budget for England and Wales at that time. 2 These costs do not include indirect costs to the UK economy of diabetes, including absenteeism, early retirement and benefit payments. The costs of diabetes to the NHS, and wider UK economy, can be expected to rise given the trends of rising diabetes prevalence.
There are a number of health complications associated with all forms of diabetes, many of which involve damage to blood vessels. Diabetes increases the risk of cardiovascular diseases, such as heart attack, stroke and peripheral vascular disease. 3 Diabetes can also lead to damage to the small blood vessels, resulting in the microvascular complications of retinopathy, nephropathy and peripheral neuropathy.
Diabetic retinopathy and screening
People with type 1 or type 2 diabetes are at risk of developing diabetic retinopathy, one of the microvascular complications of diabetes. This condition is caused by damage to small blood vessels at the back of the eyes, which reduces the blood supply, causing ischaemia. This stimulates the growth of fragile, new blood vessels in the eye (neovascularisation), which may bleed (microhaemorrhage) and damage the retina. This leads to sight loss. It is estimated that in England every year 4200 people are at risk of blindness caused by diabetic retinopathy and there are 1280 new cases of blindness caused by diabetic retinopathy. 4 Diabetic retinopathy is one of the leading causes of sight loss in the UK within the working population5 and therefore there is a significant social and financial burden associated with the condition. However, by the time a person is aware that their sight has deteriorated, the damage caused by retinopathy may be largely irreversible, and it significantly harder to treat. Timely diagnosis at an early stage and treatment can significantly reduce the risk of blindness, hence the justification for a screening programme.
In England, all people aged ≥ 12 years with type 1 or type 2 diabetes are offered diabetic retinopathy screening at least annually as part of the diabetic eye screening (DES) programme. The test involves taking a photograph of the retina, which occurs without contact with the eye. Although the DES programme has been considered to be an unqualified success, the effectiveness of any programme depends on its uptake. The rate of uptake for screening is 81%,6 with a range from 7.4% to 91.8% across different primary care trusts7 (when excluding the five primary care trusts with the highest and lowest percentages, the range is 57.7–87.0%). Therefore, a number of people with diabetes are still not being screened. This puts them at risk of developing avoidable sight loss, and is also a waste of resources in the NHS, due to missed appointments, and the cost-effectiveness of the screening programme is reduced.
It is estimated that 0.94% of patients with diabetes are at risk of becoming blind due to diabetic retinopathy, and the rate of blindness within this at-risk group is 30.5%. 8,9 This corresponds to 1280 people becoming blind each year. As the estimated per-person societal cost of blindness is £12,466 every year, the economic, as well as human, implications of suboptimal levels of screening are considerable.
The figures suggest that there are high levels of regional variation in screening uptake. 7 As in other health behaviours, screening rates have been observed to be lower in socially deprived areas. In the UK, diabetes prevalence was found to increase with increasing deprivation, whereas the probability of attending diabetic retinopathy screening decreases and the prevalence of sight-threatening diabetic retinopathy among screened patients increases. 10 This suggests that although screening programmes are beneficial overall, they do have the potential to exacerbate existing inequalities in health outcomes. As the effectiveness of any screening programme is linked to the uptake by the population (and importantly, uptake by those most at risk), simple, inexpensive and cost-effective strategies are required to realise the financial and social benefits of available sight-saving interventions, and to do this in an equitable way.
Behaviour change and financial incentives
The problem of health risks due to lack of engagement with healthy behaviour is not unique to screening or diabetes management. Behaviour change is of major relevance to health, in areas as diverse as medication adherence, smoking cessation, and engagement with hand hygiene in hospitals. Although educational strategies may be used to try and promote healthy behaviours, they are often not effective, as intentions have been shown to correlate poorly with actual behaviour. 11 Therefore, other strategies are necessary in order to achieve desired changes in behaviour, and to help people align their behaviour with their underlying intentions. Of the many available strategies to change behaviour, one that is increasing in prominence is the use of financial incentives. 12
Behavioural economics and the MINDSPACE framework of behaviour change have been considered by the UK government, as ways to encourage preventative health care. 13 Available policy tools to encourage healthy behaviour include legislation, taxes and information campaigns. Incentives in general have been used to encourage people to eat healthier foods, be more physically active, drink less alcohol and give up smoking. 14 Incentives can have a profound effect on individual behaviour at a relatively small cost. The design of incentive schemes is crucial in order to maximise their effectiveness. The interventions proposed in this research use cutting-edge theory from the interdisciplinary field of behavioural economics to design incentives that encourage people to utilise screening opportunities. Therefore, this strategy could be expected to reduce the economic and social costs of unhealthy behaviour, and to reduce inequalities and be cost-effective.
Providing incentives in health care have traditionally been targeted at providers through pay for performance programmes rather than the general public15 (e.g. in increasing cancer screening rates). The substantial increase in the last decade of schemes aimed at changing the health-related behaviour of the public has been accompanied by good evidence that even small incentives can positively influence choices,16,17 although concerns exist about the long-term sustainability of behavioural change when incentives are targeted at the more challenging behaviours such as smoking and obesity. 18,19
Financial incentives can take a range of forms including cash or vouchers. The interest in influencing health behaviour using incentives has increased given the findings that behaviour can be significantly affected by the structure of economic incentives used. 20 Psychological phenomena from behavioural economics have informed the design of incentive-based interventions that are more effective at delivering improved outcomes. Personal incentives have been used to motivate patients and general populations to change their behaviour. 21 Examples of such schemes include the ‘Give It Up For Baby’ programme in Tayside, Scotland, to encourage pregnant smokers to kick the habit, the ‘Pounds for Pounds’ scheme in Kent, England, to influence weight control and the worldwide ‘Food Dudes’ programme to encourage children to eat more fruit and vegetables. 22 The Citizens Counsel convened by the UK National Institute for Health and Care Excellence found that a majority of participants supported the use of incentive schemes.
Financial incentives have been seen to be more effective in increasing performance of infrequent behaviours (e.g. vaccinations) rather than in more sustained behaviours (e.g. smoking). 17,23 However, another review of incentives did not observe this difference in effectiveness between frequent and infrequently performed behaviours. 24 As screening usually requires discrete one-off behaviours, there is the suggestion that incentives may be particularly effective in increasing their uptake. Incentive schemes, using a variety of different payment structures, are increasingly being used to encourage preventative health behaviours, such as chlamydia screening,25 breast cancer screening,26 and health risk assessments through workplace wellness programmes. 27 As financial-based incentives are already being used, we require evidence that they work, and if they do, which method is most cost-effective. This information is vital to avoid wasting public resources in a time when there are significant constraints on the NHS budget.
This study will provide evidence to policy-makers about the role of different incentive schemes in encouraging health promoting behaviours. If evidence is demonstrated of their effectiveness (and cost-effectiveness), their targeted application may be indicated.
Systematic review of literature on incentives in screening
A systematic review was conducted to investigate existing work using financial incentives to promote screening. A search was conducted on PubMed, using the search terms ‘financial incentive(s)’ combined with ‘screening’. The search detected 114 results. A filter of the titles and abstracts, along with further papers identified from these articles, detected 19 relevant articles investigating the impact of financial incentives to patients on screening attendance. A number of the papers from the initial search considered incentives to providers for screening so are not relevant to the research topic under consideration. 28–30 Other studies described incentive programmes, but did not provide a test of the impact of the incentive. 26,31
Of the relevant articles, four considered screening for chlamydia,25,32–34 three investigated breast or cervical screening,35–37 five investigated tuberculosis (TB) screening or returning to collect test results,38–42 two investigated work wellness programmes or private health-care plans providing preventative health-care screening,27,43 and single papers describing incentives in diabetic retinopathy screening,44 and glycated haemoglobin (HbA1c) plus cholesterol screening in patients with diabetes. 45
Three of the four studies investigating chlamydia screening observed a positive impact of incentives,25,32,34 with evidence that vouchers were more effective than prize draws. Two of the three studies of mammograms or cervical screening showed no effect of incentives. 36,37 However, one study in the USA found that offering a voucher for a free mammogram to Hispanic migrants significantly increased uptake (making it 47 times more likely) compared with sending standard clinic instructions. 36 There may be some indication that incentives offered to people who have already made their appointment do not increase actual screening attendance, considering both chlamydia and breast cancer screening. 33,37 The other study of mammograms indicating no impact of incentives may not have offered high enough incentives, as the population was a high-income group and, furthermore, as attendance was ascertained through claims data, the receipt of the incentive was delayed,35 which may have reduced the effectiveness due to temporal discounting.
With the exception of one study looking at incentives for TB screening following release from jail,38 all of the studies on incentives for collection of TB test results found significant effects of the interventions. In both studies of work wellness and private health-care plans, the increase of the incentive offered,27 or the act of signing up to an incentive programme,43 appeared to increase preventative health screening.
Of particular interest are those studies considering the impact of incentives on screening in diabetes. However, only two such studies were identified, and both had methodological limitations. One was a pilot programme in the USA, using petrol station gift cards to encourage those who have not attended for HbA1c or low-density lipoprotein cholesterol screening for a year, to attend screening. 45 Those in the incentive group attended significantly more HbA1c screenings. However, the evidence on the impact on low-density lipoprotein cholesterol screening was mixed. Although those in the intervention group received more tests during the test period, following the pilot programme, this group had fewer screenings than the comparison group. Over the whole monitoring period, the number of screening visits was comparable between the intervention and comparison group. One reason for this may be that the arduous nature of the test (which requires a 12-hour fast) and may discourage test participation. However, the bigger limitations of the study were that participants in the incentive group received a reminder letter, whereas those in the comparison group did not, resulting in uncertainty about whether any effects were due to the reminder or the incentive offer. In addition, the incentive and comparison participants were from different clinics.
The other study of diabetic screening reviewed claims data to investigate the impact of several predictors on diabetic retinopathy screening uptake in the USA, following the institution of a programme to increase completion rates. 44 The incentive within the programme was available only to patients cared for by certain providers, and was set at US$25. However, the use of incentives was associated with significantly lower rates of diabetic retinopathy screening. General limitations of the study include that it was not a trial, but a correlational study of claims data, which may have been incomplete. Data were not available on prior attendance at retinopathy screening. With regard to the incentive, findings from focus groups suggested that an incentive five times higher (so US$125) would be needed to induce the target behaviour, which may indicate that the incentive on offer was not sufficient. Furthermore, the incentive was restricted to only those providers in which Johns Hopkins HealthCare had large patient volumes. Therefore, it is plausible that there were systematic differences between participants who were or were not offered the incentive. In addition, the health-care system in the USA is a very different context from that within the UK. Therefore, there are no rigorous trials of the impact of incentives on screening in diabetes (and, in particular, in DES), and no research in the context of the UK.
Three review papers,24,46,47 which included consideration of financial incentives and screening, were found. One review concluded that patient financial incentives were one of the most effective intervention components to increase uptake of vaccinations and cancer screening;46 however, the incentives considered were mainly about reducing or eliminating co-payments. A review of interventions to increase uptake for screening as primary and secondary prevention showed mixed effects of incentives for patients. 47 Most recently, a review looking at the impact of incentives on a variety of health behaviours found that incentives increased attendance for vaccinations and screening appointments. 24 Interestingly, this review did not find evidence that incentives are more effective for short-term rather than repeated behaviours.
A review was also conducted to determine whether or not relevant studies are currently being conducted, by searching for trials on incentives and screening on ClinicalTrials.gov, and the International Standard Randomised Controlled Trial Number (ISRCTN) website. Seven relevant studies were identified. Four of these are investigating the impact of different levels or types of incentives on colorectal cancer screening (either looking at the return of stool sample test kits, or colonoscopy uptake). Another study is investigating different levels of incentives on human immunodeficiency virus (HIV) test uptake. One study is assessing the acceptability and feasibility of a shopping voucher for use of TB screening. The final study is comparing a fixed gift card and lottery incentive for completion of a health risk assessment.
In summary, the impact of financial incentives on screening uptake is mixed. The available evidence includes many studies that are not randomised trials. There is also heterogeneity in terms of the populations considered, with some studies targeting the whole population, those who had already made an appointment, or only a hard-to-reach population. The evidence considering hard-to-reach populations within the UK is extremely limited. There are no rigorous studies published or being conducted, which test the impact of financial incentives on diabetic retinopathy screening.
Acceptability of incentives
The use of financial incentives to influence behaviour raises ethical questions. Compensating people financially to encourage particular behaviours has the potential to lead to intrinsic motivation being ‘crowded out’ or partially destroyed. 48 In other words, when an activity is associated with an external reward, a person may be less inclined to do the activity in the future without further rewards. Offering incentives may also be perceived as a form of bribery or coercion.
However, an alternative view is that people should be encouraged where possible to perform behaviours that improve health, and that appropriately targeted incentives can reduce inequalities in health outcomes. 17,21,49,50 Marteau et al. 21 suggest a psychological perspective that can help us think about the appropriateness of using incentives. It is known that individuals do not always act in ways that are best for them or that with hindsight they would prefer. Most of us would like to pursue a healthier lifestyle but our behaviours are often in sharp contrast to our intentions. Offering a reward can help people align their actions more closely with their true preferences. From this perspective, ‘incentives operate to enhance rather than restrict autonomy’. 21
However, an important perspective to consider is whether or not patients and the general population feel that financial incentives are acceptable. A recent review of studies on acceptability of financial incentives for health behaviours found that incentives tend to be considered acceptable if they are clinically effective and cost-effective, and if they benefit recipients and the wider society. 51 The review was not able to determine if opinions on acceptability were different for different target behaviours. In addition, incentives for screening were not considered.
The trial team performed some vital work on gaining opinions from service users during the design of this trial. 52 An online questionnaire was issued to 365 of those invited to attend diabetic retinopathy screening in north-west London and those who run the screening service, to ascertain views of diabetic patients and their clinicians on the different ethical aspects of incentives, and different types of incentive. The results revealed that age was an important predictor of the type and strength of concern. Those aged ≥ 65 years took the strongest negative position, whereas those aged between 40 and 64 years were most positive and optimistic about the potential benefits. In addition, those in the most deprived groups found incentives more acceptable than those in the least deprived groups. This study of acceptability has found that, although some ethical concerns are strongly held among certain groups, there is also much support for the principle of incentivising positive behaviours. This highlights the importance of consulting with service users when the incentive offers are designed, and also when the results of this trial become available.
Design of incentive schemes
The field of behavioural economics specifies a number of robust psychological phenomena that help explain the seemingly irrational decisions that people make in a range of settings, including savings, health and education. 13,53 These psychological phenomena are considered in the present study, as they enable the design of more effective incentive-based interventions. Specifically, we will consider the prominent insights of ‘reference points’, ‘overweighting of small probabilities’ and ‘loss aversion’ in the design of our interventions, because those are the three most established phenomena in behavioural economics embodied in the well-known prospect theory, for which Daniel Kahneman was awarded the 2002 Nobel Prize in economics. 54
Reference points can be illustrated in many examples. Offering a very small incentive can get people to pick up their HIV result, but increasing the value of the incentive has little effect. 55 Similarly, in a study in California about the impact of different levels of incentive on encouraging injecting drug users to pick up their TB results showed that although there was a big impact of offering US$5 compared with no incentive (85% compared with 33% return rates, respectively), offering a higher incentive of US$10 had little additional effect (increasing return rates to 90%). 41 A review of the impact of incentives found that even offering small incentives can increase engagement with health behaviours. 17 Therefore, offering large incentives may be unnecessary, as even small incentives may be viewed as gains. This is useful if the aim of an intervention is to be cost-effective as well as effective, as it suggests that offering incentives of large sums are not necessary. Therefore, the present study included an incentive offer of £10 (equivalent to minimal hourly wage for UK workers over the age of 21 years), to cover the opportunity cost to the patient in terms of their time or travel costs.
Loss aversion is the phenomenon that people dislike financial losses twice as much as liking financial gains of an equivalent amount. 56 Incentives may be more powerful where they are framed as individuals losing a reward rather than gaining one for attending screening.
Another prominent concept within behavioural economics is the overweighting of small probabilities. People have a tendency to overvalue small probabilities, which explains the popularity of lotteries and insurance. 54 In the review of the effect of incentives on smoking cessation, one reason cited for the lack of effectiveness observed is the low level of incentives on offer in most of the programmes. 18 Therefore, if an incentive programme only has limited resources, it may be more effective to offer a lottery-based programme rather than smaller individual rewards.
A lottery incentive was observed to improve control of warfarin levels in a group of patients who were identified at baseline as being at risk of poor adherence,57 although not for the sample overall. A study of a daily lottery for patients taking warfarin showed a significant improvement in medication adherence and control of warfarin levels seemingly due to the incentive. 58
The impact of a lottery incentive is not always clear. In studies comparing lottery incentives with fixed incentives for increasing chlamydia screening uptake, the fixed incentives were more effective than the lottery incentives at increasing uptake. 25,32 As there appears to be differences in effectiveness of lotteries in different contexts, it is not known whether or not a lottery incentive may be an effective use of limited resources in the context of increasing diabetic retinopathy screening uptake. We proposed a lottery arm where the expected value matches the incentive level offered in the other arm. It may be that overweighting of small probabilities will make a lottery offering (e.g. ‘1% chance for £1000’) more attractive than £10 for certain, so the former should be a more effective incentive for participation in screening.
Aims
The present study applied insights from behavioural economics to study whether or not different schemes may be used to improve attendance at diabetic retinopathy screening for those who are most at risk. Different incentive schemes were tested, to determine:
Are incentives an effective strategy to encourage participation in the screening programme?
Screening for diabetic retinopathy can reduce avoidable blindness, yet attendance at screening is suboptimal. However, to be clinically effective and cost-effective the screening programme requires optimum attendance. This study investigated whether or not targeted financial incentives can increase screening participation.
Does the design of the financial incentive scheme affect its effectiveness in influencing participation in health screening?
There are many ways in which incentives to encourage screening participation could be delivered. Two different types of financial incentive were compared to see which is most effective: a fixed £10 incentive or a prize draw with a 1 in 100 chance of winning £1000.
Does the choice of incentive scheme, if successful, attract patients who have a different demographic or socioeconomic status to those who attend screening regularly?
Those in deprived socioeconomic groups are less likely to attend screening, exacerbating existing inequalities in health. This trial investigated the impact of the two incentive schemes on the demographic profile of those who attend to enable greater understanding about the way in which incentives might be developed to target specific health inequalities. Information was collected on age, gender, postcode and social deprivation status, and distance from screening centre.
Is offering incentives a cost-effective strategy for enhancing participation?
Economic evaluation has been defined as the comparative analysis of alternative courses of action in terms of both their costs and consequences. 59 Cost-effectiveness analysis (CEA) seeks to relate costs to a single, common effect that may differ in magnitude between alternative groups or programmes under study. Few trials of financial incentives have included CEA as part of their evaluation of the incentive. Giles et al. 51 conducted a systematic review of studies of financial incentives for encouraging uptake of health behaviours and concluded that ‘little evidence for cost-effectiveness has been published’. In the current financial environment, it is important to ensure that any interventions are cost-effective. Economic evaluation using well-established economic models were planned to determine value for money of the intervention.
Chapter 2 Methods
Study population and eligibility
Eligible participants were identified from the DES programme before the start of the study by 1st Retinal Screen Ltd (which at the time of the study was providing the DES service in the Kensington, Chelsea and Westminster Clinical Commissioning Groups in West London). Eligible participants were defined as patients who:
-
were in the geographical area due to be invited for screening by 1st Retinal Screen Ltd [defied according to address of the patient’s general practitioner (GP), rather than their own address]
-
were aged ≥ 16 years
-
have been invited to screening in the last 24 months on a yearly basis, but who have failed to attend, and have not contacted the screening service to rearrange an appointment. Any patients who had a manually-added appointment in the past 24 months, regardless of attendance, were therefore excluded.
A minimum 2-month period was left between any appointment invitation letters sent as part of usual care, and enrolment into the trial. This was to ensure that no patient was enrolled who was late to contact the screening service but who still intended to do so. Initially, this period was set as a 3-month minimum; however, it was reduced to ensure the required sample size was achieved. It was agreed by the Trial Management Team (TMT) that leaving a 2-month period between usual care appointment letters and trial letters would leave sufficient time for those who wish to contact the screening services to do so.
In order for 1st Retinal Screen Ltd to comply with contractual requirements, the normal, annual invite process continued for trial participants. Appointment and attendance status was monitored throughout the trial to ensure that identified participants continued to be eligible.
A Structured Query Language Script was produced by 1st Retinal Screen Ltd to identify candidates from the Kensington, Chelsea and Westminster Diabetic Eye Screening Programme managed database. This programme is managed using Digital Healthcare’s OptoMize software (EMIS Health, Leeds, UK; current version V4, Sp2, update2). This script defines a cohort with the following characteristics:
-
Patients aged ≥ 16 years.
-
Not attended a retinal screening or ophthalmology (hospital eye screening) appointment within the last 2 years.
-
Registered with programme for at least 15 months (enough time for two invite cycles).
-
Candidate either waiting for recall when due, or had appointment booked for an appointment in the future. The following states were included in the software:
-
‘StateAwaitingRecallForScreening’: not yet due an appointment
-
‘StateAwaitingClosedScreening’: due, waiting for appointment
-
‘StateAwaitingPreScreening’: appointment made for future
-
‘StateAwaitingFirstStrikeOpenScreening’: did not attend (DNA) appointment 1, waiting for appointment
-
‘StateAwaitingFirstStrikePreScreening’: DNA appointment 1, appointment made for future
-
‘StateAwaitingSecondStrikeOpenScreening’: DNA appointment 2, waiting for appointment
-
‘StateAwaitingSecondStrikePreScreening’: DNA appointment 2, appointment made for future
-
-
Not contacted the service to book an appointment in the last 2 years (regardless of if they attended).
-
Had at least two letters of ‘GP notification screening not taken up’ (OptoMize uses the GP letters to signify the end of the screening cycle).
-
Had not had a letter returned as ‘unknown address’ or ‘not known at this address’ (post office return).
-
Had been registered with the programme for the entire time (has not been marked as ‘moved away’ in last 2 years).
-
Did not have a recorded hospital eye screening encounter.
-
Was not contacted as part of a recent initiative to improve uptake in persistent non-attenders.
The states presented in section 4 followed the patient through the invite process. If they failed to respond or attend, then they would return to ‘StateAwaitingRecallForScreening’. It was anticipated that most of the patients in states b–g would follow this path. However, if a patient attended a screening appointment outside the trial they became ineligible for the trial.
In order to ensure that the study included subjects who were intended to attend screening, the study population was checked against the patient register immediately prior to invitation. This ensured that the most up-to-date address was used and that patients who had left the programme area, had died or who had otherwise become ineligible were not invited. As of 15 April 2015, only patients whose eligibility and address was confirmed by their GP after 1 April 2015 were invited.
Study design and procedure
The study design was a three-arm randomised controlled trial. The methods of the study, along with the justification of the intervention conditions, have also been described in detail in a protocol paper published in BMC Ophthalmology. 60
The impact of two different types of financial incentives was compared with a control group, which received the standard appointment letter with no incentive. Participants were sent a letter by 1st Retinal Screen Ltd inviting them to a fixed appointment. Letters were sent 4 weeks prior to the appointment date. It was decided that participants could reschedule this appointment once (by telephoning a number given on the letter), and still be eligible for the incentive. If participants rescheduled more than once, they would still be able to arrange an appointment, but would be told that they would no longer be eligible to receive the incentive (however, this did not occur in practice within the cohort). The letter sent to participants was determined according to the intervention condition to which participants were assigned (the three conditions are described below).
The study took place at a DES clinic based within St Mary’s Hospital, Imperial College Healthcare NHS Trust, in London, UK. Dedicated clinics were arranged for trial participants and for each of the intervention groups, as it was important to ensure that non-trial patients were not aware of incentives offered to trial patients, and that participants within different groups were not aware of the different incentive conditions. The primary outcome was attendance at the screening appointment. The study procedure is shown in Figure 1.
FIGURE 1.
Flow chart of study procedure.
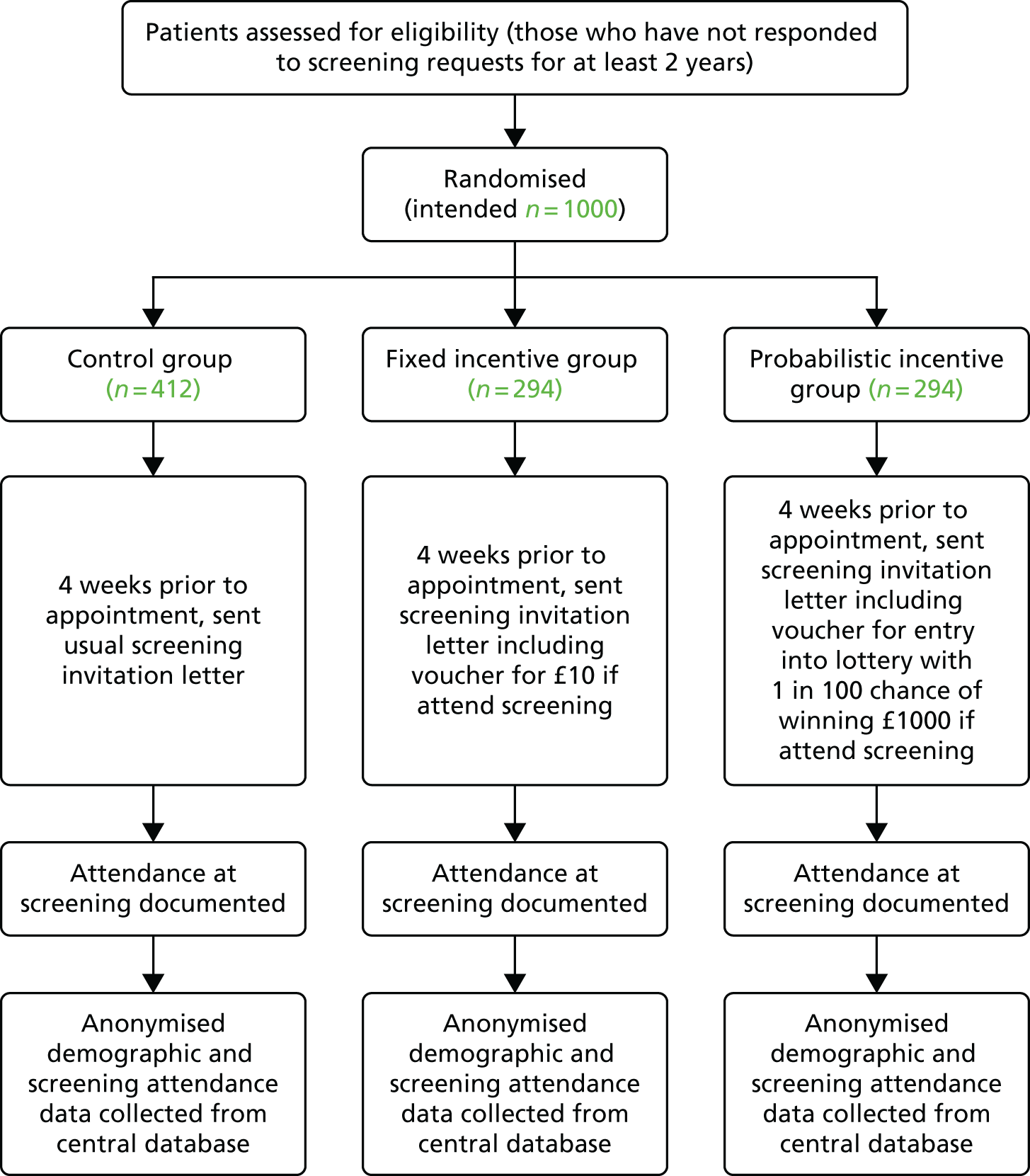
Conditions with justifications
The design of the intervention conditions took into account established psychological phenomenon from prospect theory. 54 In particular, the two intervention conditions were based on the insights of ‘reference points’ and ‘overweighting of small probabilities’. The incentive vouchers also included expiry dates of the date to the screening, to introduce some aspect of ‘loss aversion’, whereby losing a reward is more powerful than gaining a reward. 56
Control group
Participants in this group received the standard invitation letter from the screening service, which invites patients to a fixed appointment at a particular date and time. A telephone number was provided on the letter so patients could contact the screening centre to rearrange their appointment if necessary. The letter was posted 4 weeks before the appointment date.
Fixed incentive group
Participants in this group received the standard invitation letter as in the control group; however, this included additional text and a voucher offering a financial incentive of £10 after screening is completed. The voucher is shown in Figure 2.
FIGURE 2.
Image of voucher added to the invitation letter for the fixed incentive group. The following text was also added to the standard letter: ‘We know that some patients invited for diabetic eye screening do not attend their appointment. Imperial College London is looking to see whether financial incentives help people to attend, and this work is being conducted through our clinics. Once you have been screened, you can exchange this voucher for £10 cash. Please bring this letter with you when you attend your screening appointment. If you have any questions about the financial incentive, please email XXXXXXX‘.

Lottery incentive group
Participants in this group received the standard invitation letter, including additional text and a voucher offering entry into a prize draw for a 1 in 100 chance of winning £1000 following attendance at screening.
This amount of £10 was chosen given the concept of reference points, which can be illustrated by the finding that offering a very small incentive is effective at encouraging people to pick up their HIV test result, but increasing the value of the incentive has little effect. 55 Therefore, the fixed incentive intervention condition included an incentive offer of £10 (equivalent to minimal hourly wage for UK workers aged > 21 years), to cover the opportunity cost to the patient in terms of their time or travel costs.
This condition was designed with an understanding of the phenomenon that people overvalue small probabilities, which explains the popularity of lotteries and insurance. 54 It has been demonstrated that, given fixed resources in an incentive programme, it can be more effective to offer lotteries than smaller individual rewards. 61
To inform the selection of the intervention probability and value, a survey was conducted of 50 patients who attended the vascular and diabetic foot clinics at Imperial College Healthcare NHS Trust. Each was asked which of the following incentives was most attractive for them personally:
-
£5 for sure
-
a 1 in 10 chance of winning £50
-
a 1 in 100 chance of winning £500
-
a 1 in 1000 chance of winning £5000.
All patients either answered (a) or (d); nobody stated that they found the options (b) or (c) to be more attractive. The findings indicated that patients fall into two groups – ‘risk avoiders’ and ‘risk seekers’. Therefore, if even more risky options were available (offering even smaller probability to win even higher payoff), it would be expected that most respondents would still be likely to select either the safest or the riskiest option (see Vlaev et al. 62 for discussion of evidence for such relativity of risk preferences).
Consideration was given to the literature on interpretation of probabilities, in order to select the most appropriate level of incentive for the lottery condition. It is known that people have cognitive difficulties in comprehending, and dealing with, very small probabilities. 54 Therefore, an incentive was chosen with lottery odds 1 in 100 (1%) chance of winning a monetary prize, rather than a smaller probability (e.g. 1 in 1000), as it may be difficult to conceptualise what 1 in 1000 chance means, which may induce further non-linearities in behaviour in addition to probability overweighting (i.e. it may lead individuals to ignore the odds and focus only on the prize).
The magnitude of the offered prize in the lottery incentive group (£1000) was selected so that it is multiple of the £10 payoff offered in the other intervention condition: if there is a 100% chance to get £10 in the fixed incentive group (justified on the grounds of real time and travel costs), then it makes fair sense to offer £1000 when the probability is hundred times smaller (i.e. the ‘expected value’ of the incentive is the same in both conditions). Given the finding that patients can be classified as either ‘risk avoiders’ and ‘risk seekers’, the prize of £1000 is also the highest lottery incentive that could be offered based on the number of people in the trial. The incentive voucher is shown in Figure 3.
FIGURE 3.
Image of voucher added to the invitation letter for the lottery incentive group. The following text was also added to the standard letter: ‘We know that some patients invited for diabetic eye screening do not attend their appointment. Imperial College London is looking to see whether financial incentives help people to attend, and this work is being conducted through our clinics. Once you have been screened, you will be entered into a prize draw where you will have a 1 in 100 chance of winning £1000. Please bring this letter with you when you attend your screening appointment. If you have any questions about the financial incentive, please email XXXXXXX’.

Measures
Following completion of the study, the data set was generated by the data manager at 1st Retinal Screen Ltd, given a database search of their system to extract all relevant attendance and demographic data.
Primary outcome: attendance
The primary end point of the study was the proportion of invitees who attended screening at a designated clinic in each incentive group. This anonymised information was extracted from the screening service database.
Demographic information
Demographic information was collected for all invited participants on gender, age, deprivation score and distance from screening centre. Age in years [on the date of the first Incentives in Diabetic Eye Assessment by Screening (IDEAS) trial clinic: 16 April 2015] is presented in deciles, starting from the lowest eligible age of 16 years (i.e. 16–25 years, 26–35 years, etc.) with those aged ≥ 86 years being in the highest category. Deprivation scores were measured based on the Index of Multiple Deprivation (IMD) score,63 which is based on postcodes, and were calculated based on the address to which the invitation was posted. These data were also presented in deciles to ensure participant confidentiality. Distance to screening site was measured based on the straight-line distance between the address on the day of screening and the screening site, rounded to the nearest half kilometre. Data were also extracted for years registered, up to a maximum of 8 years owing to software changes that mean that data on length of time registered prior to this were not available. Years registered refers to the number of years since the patient was first registered with the programme, so will not account for any periods of absence, such as when a patient may have moved away, and then moved back.
Additional measures
If participants were excluded from the trial after randomisation, but before being invited, the reason for this was recorded. For participants who telephoned the screening centre to opt not to participate in screening, the reason(s) provided were recorded and presented in the data set with all identifying points removed. These reasons were categorised within the final data set to facilitate comparisons.
For those participants who attended their screening appointment, data on their screening outcome score were collected, aggregated by intervention group. These data were presented both according to the retinopathy grading score, and according to the follow-up action (from options of annual recall, digital surveillance, refer to ophthalmology for diabetic retinopathy and refer urgently to ophthalmology for diabetic retinopathy).
When patients attended their appointments, the screener asked them for any reasons why they have not attended their past few appointments, in order to see if there are differences between intervention groups, and to explore common barriers to attendance in this hard-to-reach group. Reasons given were noted in a table containing common reasons for non-attendance, along with the intervention group assignment. The reasons suggested were those given by patients in some informal telephone research conducted by 1st Retinal Screen Ltd. These reasons were later categorised into the four categories of organisational difficulties; practical difficulties; a perception that they did not need to attend; and a definite reason that they not need to attend. The original reasons, clustered by category, are shown in Table 1.
| Suggested reasons for non-attendance | Categorised for reasons for non-attendance |
|---|---|
| Forgot | Organisational difficulties |
| Did not know had an appointment | |
| Did not get round to coming | |
| Was out of the country | Practical difficulties |
| Could not get time off work | |
| Family commitments | |
| Too ill to attend | |
| Problems with transport | |
| Started experiencing problem with eyes | Did not think they needed to attend |
| Did not understand why needed to be screened | |
| Thought optician did it | |
| Did not feel obviously had problems with eyes | |
| Do not consider themselves diabetic | |
| Seen privately | Did not need to attend |
| Under the care of hospital eye services | |
| Other: please specify |
Non-trial population
Demographic details were also collected for the entire patient population who were not invited to the trial, in order to compare the IDEAS trial population with the rest of the DES cohort. As in the IDEAS trial cohort, data were collected on age, gender, IMD deciles and years registered. Information was provided on distance to screening site (measured as the distance between the last appointment location and the patient’s home address on the screening date), to the nearest half kilometre.
Data were also collected on those who were regular attenders, defined as having attended at least twice in the last 3 years (therefore including some people who have been registered for < 3 years, but who have been invited twice and attended twice). Information was recorded on common reasons for exclusion from the IDEAS trial, aside from attendance. These reasons were about whether or not they had attended an ophthalmology appointment in the past, whether or not they had received a letter returned as ‘not at this address’ in the past 2 years, and whether or not they have had a status recorded of ‘moved away’ in the last 2 years.
Patient and public involvement
The trial has been discussed in depth with a number of patients at different stages of the research preparation: in assessing the acceptability of an incentives trial in principle and exploring potential ethical concerns; in determining the incentive schemes offered as part of the intervention; and in assessing the acceptability of the developed invitation letters including the incentive offer. The TMT also included a patient representative.
Before the start of the research, the trial was discussed at a meeting of the Westminster Diabetes User Group (formerly the Westminster Diabetic Patient Forum) to determine acceptability and to highlight any important issues. Although many patients already attend the hospital services without the need for incentives, there was agreement that attendance at screening is vitally important, and anything that can be done to improve rates of attendance should be encouraged. There was not a feeling of resentment in offering monetary incentives, to cover travel and expenses and many in the group thought this was a good idea. When considering the option of offering a lottery-based incentive scheme, there was general acceptance that the use of what equates to the same total amount of money to bring more people to screening would be appropriate use of funds. The importance of information and other incentives apart from financial was raised by many in the group, and so it was acknowledged that if an incentives intervention is implemented, it should occur alongside other strategies to increase awareness of the importance of diabetic retinopathy screening, and other behaviour change techniques.
In designing the interventions for this trial we surveyed over 50 diabetic patients attending hospital within the vascular and diabetic foot clinics at Imperial College Healthcare NHS Trust, in order to get feedback on preferences for different types of incentive schemes. The work conducted and the outcomes from this are described in detail in Conditions with justifications, within the explanation of the lottery incentive group. The feedback from patients was instrumental in determining the details of the incentive offered.
Although the proposal for the trial was under review by the National Institute for Health Research (NIHR), the trial team undertook extensive work assessing opinions of service users about whether or not offering financial incentives to encourage attendance at diabetic retinopathy screening is ethical and acceptable. 52 (The work is discussed in detail in Chapter 1, Acceptability of incentives.) The study indicated that, although some ethical concerns are strongly held among certain groups, there is also much support for the principle of incentivising positive behaviours. The findings highlight the importance of working with service users when designing the invitation letters that are sent as part of the study, and crucially before any potential scale up of an incentives intervention.
The intervention letters were sent to patients at the Tri Borough Diabetes Group, representing Hammersmith and Fulham, Kensington and Chelsea, and Westminster, and the researcher visited a meeting of the group to present the study in more detail, and gather any thoughts and responses to the proposed research materials. This enabled feedback to be obtained on whether or not the letters were easy to understand, and acceptable. Although there was some feedback that the letters were too long, changes could not be made to the standard letter format, as this is centrally determined, and it was important that the control letter (and non-incentive aspects of the intervention letters) comprised the letter that would be received as part of usual care. Both incentive schemes, and the wording and presentation of the incentives within the appointment invitation letters, were found by the group to be acceptable.
The Trial Management Group had on it a patient representative from the Westminster Diabetes User Group. This enabled patient viewpoints to be raised at the trial management meetings, and in e-mail discussion between meetings. The patient representative approved the letter design, and assisted in ensuring that all patient and public facing materials were relevant and would be easily understood by a lay audience.
Sample size calculation
The primary end point was the proportion of each study group who attended their diabetic retinopathy screening appointment. The study cohort comprised patients who had not attended screening for at least 2 years, including some who had never attended, indicating that this is a very hard-to-reach population. This is illustrated by the fact that if participants do not attend an appointment they are sent letters inviting them to a further two appointments, before a letter is sent to their GP to inform them that the patient has not attended their annual diabetic retinopathy appointment. It is then expected that the GP will follow up with the patient, either by letter or by telephone. Throughout the invitation process, if screening staff or people within the central office are able, they also telephone patients to remind them about their appointments. Therefore, those who have not attended an appointment or contacted the screening service for at least 2 years are a very resistant population.
For this reason, attendance in the control group was expected to be extremely low, for example, a nominal 1%. As the eligible study population was also expected to be the sickest, with the greatest risk of having retinopathy, and of losing their sight, even a small increase in attendance would have both clinical and social benefit. An increase in attendance of 10% was deemed clinically significant. A 10% increase was also considered achievable, as a study found that smoking cessation rates among employees of a large company increased from 5% to 14.7% with financial incentives,64 and in another study, warfarin adherence among subjects requiring anticoagulation management improved from 65% to 97.8% with a lottery-based financial incentive. 58
With two intervention groups, each of which is compared with the control group, maximum statistical efficiency is achieved by randomising 1.4 : 1 : 1. Combining this with an assumed increase in attendance of 10%, from 1% in the control arm to 11% in an intervention arm, there would be 95% power if 1000 participants were recruited in total (412 in the control group and 294 in each of the intervention groups). This sample size of 1000 participants in total would also give the study at least 85% power to detect a smaller increase from 1% to 7.5%, which would also be a clinically significant improvement in attendance rates. Should attendance in the control group turn out to be 5%, the study would still have approximately 85% power to detect an increase in attendance of 10% to a level of 15% in an intervention group. 65
Data from 1st Retinal Screen Ltd indicated that the study group would be likely to comprise more than 1000 patients. This would allow oversampling at the randomisation stage, so that the intended sample size will still be reached even if participants become ineligible following randomisation (it was anticipated that the most common reason for this would be likely to be due to participants attending their routine screening appointments).
Randomisation and blinding
The list of eligible participants identified by 1st Retinal Screen Ltd was randomised by the statistician (according to an anonymous identifier provided by the data manager at 1st Retinal Screen Ltd), prior to the start of the study. No personal information about participants was shared by 1st Retinal Screen Ltd. For n eligible participants provided in the list, they were indexed according to numbers generated at random with double precision, to avoid duplicates. Participants were then sorted from smallest to largest according to this random index. Within the sorted list and using the 1.4 : 1 : 1 randomisation ratio, the statistician assigned (1) the lowest n/3.4 participants to the fixed incentive group; (2) the following n/3.4 participants to the lottery incentive group; and (3) the remainder of the participants to the control group. If needed, n/3.4 was rounded to the closest integer.
There were designated clinics for each intervention group so that participants were not aware of the intervention conditions. Therefore, it was not possible for the researcher (who was present at the intervention clinics to administer the incentive) or the screener to be blinded to the group assignment. However, as attendance at the clinic is the primary outcome, the study results could not be biased by the lack of blinding at this stage. For this reason, the lack of blinding was not considered to be problematic in terms of the reliability of the study.
Adverse events and monitoring
Any concern or complaint raised by patients was to be studied by the TMT. If any adverse event occurred during the study, it would be considered by the TMT. In the unlikely event of an adverse event thought to be a result of the study intervention, this was planned to be reported to the Research Ethics Committee (REC) and the sponsor, with amendments to the study protocol made as necessary.
A formal data monitoring committee was not convened for this trial as adverse events specific to the incentives were thought to be unlikely to reported. It was decided that interim safety and efficacy data would not be reviewed unless adverse events secondary to the intervention occur.
The conduct of the trial was managed by the TMT comprising individuals from a broad range of areas of expertise relevant to the study. The TMT met regularly, and as deemed necessary depending on the stage of the study. The TMT meetings were used to agree the final protocol, discuss trial progress, deal with any reported adverse events, agree the statistical analysis plan, and produce outputs for scientific and public dissemination given the results of the study. A subset of the TMT with expertise in data analysis finalised the analysis plan and monitored the data management. TMT members from 1st Retinal Screen Ltd, Imperial College London and the statistician have access to the data set. Investigators have no competing interests in the trial.
Arrangements for payment and lottery
The researcher was present at the screening sessions for the intervention groups, in order to be able to answer any questions about the incentive, and to give the cash incentive to those in the fixed incentive group. Those in the fixed incentive group signed a receipt, so that there was a record of the funds that were paid for the incentive.
The prize draw was conducted using a computer program created on Matlab (version 9.0; Mathworks, Cambridge, UK), which would give each participant an exact 1 in 100 probability of winning. Following every lottery incentive clinic, the researcher entered the participant numbers of those who attended screening into the Matlab program (the participant numbers of attenders were provided by the screener). If a winner of the lottery incentive was identified, their contact details would be given to the researcher by 1st Retinal Screen Ltd and they would be sent a letter to arrange payment of the £1000 prize. To ensure that a prize is awarded, as required for legal reasons, if none of the participants who attended their appointment won the prize following the individual draws by the end of the study, then one participant was planned to be chosen at random as the winner, from all participants who attended from the lottery incentive group.
Analysis plan
Summary statistics
The primary outcome was the count and proportion of attenders (i.e. attendance rate) by treatment group and demographics.
Secondary data were the sight outcome scores from the screening test, aggregated by treatment group. This was presented as a retinopathy score, and according to the recommendation from screening (annual recall, digital surveillance, refer to ophthalmology, refer to ophthalmology – urgent). These scores were also classified as whether or not additional management is required.
Those who became ineligible after randomisation and before receiving the invitation letter were excluded from the analysis due to no longer meeting study inclusion criteria. However, a distribution of numbers becoming ineligible by treatment group, as well as reasons for ineligibility was examined. Although some participants became ineligible after receiving the invitation letter, it can be assumed that the letter itself does not cause ineligibility, and so would occur at random across the experimental conditions. Therefore, participants becoming ineligible after receiving the invitation letter were not excluded from the analysis.
The count and proportion of participants opting not to participate, or rearranging appointments, was planned to be presented by reasons provided, as well as according to treatment group and demographics. If participants rearranged more than one appointment, they become ineligible for the incentive, and would be counted as a non-attender (even those in the control group). The count and proportion of participants who were classified as non-attenders for this reason was to be provided, and they were included in a sensitivity analysis for comparison, where they were classified as attenders.
The impact of incentives on attendance
To address the first research question regarding effectiveness of incentives, a single comparative analysis – the combined fixed and lottery incentive group compared with control – was performed. We include this comparison separately, which effectively combines information contained within two of the following tests.
Pairwise comparisons
Pairwise comparisons of success, or attendance rates (absolute risk differences), were performed between the following groups, adjusting for multiple comparisons using a conservative Bonferroni correction for three independent multiple comparisons, to answer the second research question of identifying whether or not the incentive design affects screening uptake:
-
control versus fixed incentive
-
control versus lottery incentive
-
fixed versus lottery incentive.
Risk differences and risk ratios [i.e. relative risks (RRs)], along with their 95% confidence intervals (CIs), are presented to assess whether or not any significant differences between groups exist. This analysis addressed the first two research questions of whether or not financial incentives, and also the design of the incentive scheme, have an impact on screening attendance.
Further analyses were conducted to explore the third research question about if the incentive schemes attract patients with a different socioeconomic or demographic status. Although groups were checked after randomisation on the basis of demographic factors, an exploratory subgroup analysis was planned to be performed in order to adjust for treatment effect by accounting for the available demographic covariates listed above using a multivariate logistic regression analysis, and performing model selection using a backward stepwise removal process based on a 0.05 significance level. Covariate-adjusted differences in success, or attendance, rates between treatment groups would be computed. Significant associations were identified by those with p-values < 0.05, whereas possible trends towards significance were identified from p-values ≥ 0.05 but < 0.10. With anticipated relatively low success rates globally, resulting estimates from these subgroup analyses could be unstable,66,67 hence affecting possible reliability of the estimates, which is why we emphasise the term exploratory subgroup analysis.
To answer the third research question, comparisons were made to those who are classified as regular ‘current’ attenders (i.e. those who have attended at least two appointments in the past 3 years) to assess possible differences of demographic covariates and secondary outcome data between regular attenders and non-attenders.
Two sensitivity analyses were performed, one to exclude any participants who became ineligible for the study following being sent an invitation letter, and the second to include any trial participants who had to reschedule their appointment to a non-trial clinic, and therefore would no longer count as attenders within the trial, but still attended an appointment.
Cost-effectiveness analysis
In order to investigate the fourth research question about the cost-effectiveness of the interventions, a health economics analysis was planned. The trial results provide data on the differential rate of attendance to screening for each group. To assess the short-term cost-effectiveness of the incentives, the cost per additional screening attendee was planned to be calculated as the cost of screening divided by the observed increase in the number of screening attendees for each pairwise comparison of groups.
A longer-term, 5-year perspective of the cost-effectiveness of the incentives was intended to be assessed using a Markov model. The model would assess the cost-effectiveness from the NHS perspective using the differential rates of screening attendance for each group. The long-term effects of attending screening in terms of the sensitivity of screening, treatment costs and quality of life, as well as the long-term effects of not attending screening, was planned to be determined from the published literature. The costs across the groups will differ only in the monetary incentives provided.
The analysis pertaining to the third research question would indicate if the incentives for attending screening are associated with individual characteristics. If this were the case, the published literature would be consulted to determine if the significant characteristic(s) also impact on the costs and/or quality of life of individuals with diabetes. If so, this was intended to be reflected in the modelling of future costs and quality of life. A sensitivity analysis was planned to be conducted to determine the impact on cost-effectiveness of changes in the key parameters within the model.
Ethical approval and consent
The sponsor for the study was Imperial College London. The study was reviewed by the London Riverside National Health Service REC, from which it received a favourable opinion (reference number 14/LO/1779).
Informed consent was not obtained from research participants. This is similar to other screening trials, as it was not possible to gain informed consent before the screening invitations are sent out. Furthermore, if patients were aware of what is being investigated in the trial, it would jeopardise the reliability of the findings. However, it was planned that following publication of the results, patients will be informed by letter that they were included in a trial, and informed of the findings. This letter is shown in Appendix 4.
The study was registered with ISRCTN, number 14896403.
Summary of changes to the study protocol
A few changes were made to the original study protocol. These were agreed by the TMT, and approved by both the sponsor and the REC.
-
There was only one screening centre for the trial rather than two. For logistical reasons, all screening took place at St Mary’s Hospital, and so Chelsea and Westminster Hospital was not a screening centre for the trial.
-
Following publication of the study results, all invited participants were planned to be sent a letter informing them that they had taken part in a research study (see Appendix 4), and explaining what this involved. This amendment was requested in the REC approval letter, and has the purpose of informing participants that they have taken part in the study, and to avoid the participants expecting a financial reward the next time they are invited for a screening appointment.
-
To ensure fairness, and timeliness, of the lottery incentive, slight changes were made to how this was conducted. In the original protocol it was stated that 1% of the screened patients will be selected at random at the end of the study. However, this is not optimal, as the number of attending participants was not likely to be a multiple of 100; therefore, it would not have been possible to conduct the draw according to the stated probability of a 1 in 100 chance of winning. In addition, if the draw were conducted only at the end of the study, this would not have been fair to participants screened at the beginning of the study, who would have needed to wait a long time for their opportunity to win the lottery. Therefore, it was decided that a fairer and more honest way of conducting the prize draw was to enter the participant numbers for all those who attended screening into a computer program, which gives each person an exact 1 in 100 probability of being selected as a winner. This process means that all participants can be entered into the lottery just a short time after they attend screening, and that regardless of the number of people attending, each person still has a 1 in 100 chance of winning, as stated on the invitation letters. In order to comply with laws governing prize draws, if there was no winner from the individual draws, then a winner would be picked at random from all attending participants.
-
The protocol was amended to clarify that participants could reschedule their appointment once, and still be eligible for the incentive. If they rescheduled again, they would be booked onto a non-trial screening appointment, and would not be eligible to receive the incentive. To clarify this, if participants rescheduled they would receive a different version of the invitation letter, which states that if they reschedule they cannot receive the incentive. The implication of this is that patients attending who rescheduled once would be treated the same way as those who attended the appointment they were initially sent, and patients who reschedule more than once would be counted as DNA.
-
The trial team decided to capitalise on the opportunities afforded by the study to gather some more exploratory information about the reasons why people may not attend their appointments. Therefore, the protocol was amended so that when participants attend, they were asked by the screener if there is any reason why they have not attended in the past. The response given was recorded into a spreadsheet with a list of example responses. In addition, if patients telephoned to say that they are not attending their appointment, any reasons given were stored. These were categorised to ensure that no personal identifying information was shared. These changes enable collection of data on some barriers to attendance for those who rarely attend their appointments.
-
In order to assess whether or not the different interventions tested have an impact on different patient groups, it was decided that the differences in sight outcome data between participants who attended in the different groups would be investigated. The sight outcome scores were aggregated by intervention group, so the total number of participants with each sight outcome category was provided within each group. In that way, no health outcome data can be linked to any individual participant within the data set.
Chapter 3 Results
Participant invitation and eligibility
Among the 1274 patients who were deemed eligible and randomised, 524 were randomised to the control group, and 375 patients each were randomised to the fixed and lottery incentive groups, respectively. Between randomisation (12 March 2015) and the time at which invitation letters were mailed to patients (which began on 19 March 2015 and continued through to 20 August 2015 for appointments on 17 September 2015), 223 individuals (17.5% of those randomised) became ineligible and therefore were not mailed an invitation letter or included in the analyses. Therefore, a total of 1051 participants were sent an invitation letter and included in the trial. The numbers assessed for eligibility in the trial and included at each stage are shown in Table 2. Reasons for ineligibility are provided in Table 3. The most common reason for patients becoming ineligible before being invited for screening was attending their annual DES appointment (44.4% of those who became ineligible). The next most common reason was participants moving out of the area covered by the screening service (22.4% of those who became ineligible).
| Stage | Number of patients |
|---|---|
| Assessed for eligibility (number of patients on 1st Retinal database at point of assessment 12 March 2015) | n = 13,947 |
| Eligible and randomised | Total n = 1274 |
| Control n = 524 | |
| Fixed n = 375 | |
| Lottery n = 375 | |
| Patient sent letter (i.e. still eligible at point of invitation) These patients will all be included in the ITT analysis |
Total n = 1051 |
| Control n = 435 | |
| Fixed n = 312 | |
| Lottery n = 304 | |
| Sensitivity analysis 1: excluding all participants with any reason for ineligibility following the invitation letter being sent (even if they actually attended screening) | Excluded: |
| Total n = 68 | |
| Control n = 27 | |
| Fixed n = 25 | |
| Lottery n = 16 | |
| Total included: | |
| Total n = 983 | |
| Control n = 408 | |
| Fixed n = 287 | |
| Lottery n = 288 | |
| Sensitivity analysis 2: include as ‘attenders’ those who rebooked their trial appointment but were placed in normal screening as long as they attended that appointment | Total n = 1051 |
| Control n = 435 | |
| Fixed n = 312 | |
| Lottery n = 304 |
| Reason for ineligibility | Randomised but not invited due to ineligibility (excluded from all analyses) | Invited and became ineligible (included in ITT analysis; excluded in sensitivity analysis 1) |
|---|---|---|
| Patient stated (s)he no longer had diabetes | 12 (5.38%) | 2 (2.94%) |
| Patient stated (s)he moved out of the area | 50 (22.42%) | 37 (54.41%) |
| No recent confirmation from GP | 25 (11.21%) | 0 (0.00%) |
| Deceased | 13 (5.83%) | 16 (23.53%) |
| No invite window | 10 (4.48%) | 0 (0.00%) |
| Patient stated to opt out of the trial | 9 (4.04%)a | 9 (13.24%) |
| Screening was postponed | 4 (1.79%) | 1 (1.47%) |
| Patient manually booked an appointment, but DNA | 1 (0.45%) | 0 (0.00%) |
| Attended their screening appointment prior to the invitation | 99 (44.39%) | 0 (0.00%) |
| Medically unfit | 0 (0.00%) | 2 (2.94%) |
| Seen by another programme | 0 (0.00%) | 1 (1.47%) |
| Total | 223b | 68c |
Among the 1051 eligible patients who were invited to participate in the trial, 435 participants belonged to the control arm, 312 and 304 belonged to the fixed and lottery incentive arms, respectively, thus achieving the 1.4 : 1 : 1 sampling ratio determined in the protocol. The loss of ineligible patients did not affect the required sample size (1000 overall, or 412 controls and 294 in each of the incentive groups) due to oversampling. The 1051 eligible, invited patients comprise the intention-to-treat (ITT) analysis.
Table 4 further shows the ineligibility reasons by treatment group following the invitation letters being sent. Nine patients (0.9% of eligible participants, and 13.24% of those who became ineligible after invitation) chose to opt out of the trial for the following reasons: seen privately (five patients); had a scheduled appointment with a regular consultant (one patient); and three patients did not provide a reason for opting out. Most of those who opted out were male (77.8%), aged > 55 years (88.9%) and in an incentive group (55.6% overall or 44.4% lottery and 11.2% fixed). Eight of the nine (88.9%) who opted out of the trial belonged to the second most deprived IMD decile, and one patient belonged to the third IMD decile. Although it appears that there are no significant differences between groups in the reasons for ineligibility (p = 0.736), with such small counts, we cannot confidently rely on results of a chi-squared analysis.
| Reason for ineligibility | Control, n (%) (N = 27) | Fixed, n (%) (N = 25) | Lottery, n (%) (N = 16) | Total, n (%) (N = 68) |
|---|---|---|---|---|
| Medically unfit | 1 (3.7) | 1 (4.0) | 0 (0.0) | 2 (2.9) |
| Deceased | 6 (22.2) | 7 (28.0) | 3 (18.75) | 16 (23.5) |
| Patient stated (s)he no longer had diabetes | 1 (3.7) | 1 (4.0) | 0 (0.0) | 2 (2.9) |
| Patient stated (s)he moved out of the area | 13 (48.2) | 15 (60.0) | 9 (56.25) | 37 (54.4) |
| Patient stated to opt out of the trial | 4 (14.8) | 1 (4.0) | 4 (25.0) | 9 (13.3) |
| Screening was postponed | 1 (3.7) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| Seen by another programme | 1 (3.7) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
Description of participants
Descriptive statistics of participant sociodemographic factors are presented in Table 5. Chi-squared tests (for categorical factors) and tests for mean differences (MDs) (for continuous factors) were performed to ascertain balance among sociodemographic factors by treatment group as a result of randomisation. No significant differences were found in sociodemographic factors by treatment group.
| Sociodemographic factor | Overall invited IDEAS trial participants (n = 1051) | Control (n = 435) | Fixed (n = 312) | Lottery (n = 304) |
|---|---|---|---|---|
| Gender | ||||
| Male | 609 (57.94%) | 263 (60.46%) | 170 (54.49%) | 176 (57.89%) |
| Female | 442 (42.06%) | 172 (39.54%) | 142 (45.51%) | 128 (42.11%) |
| Comparison across treatment groups | p = 0.264 | |||
| Age (years) | ||||
| 16–25 | 12 (1.14%) | 4 (0.92%) | 2 (0.64%) | 6 (1.97%) |
| 26–35 | 37 (3.52%) | 16 (3.68%) | 10 (3.21%) | 11 (3.62%) |
| 36–45 | 113 (10.75%) | 50 (11.49%) | 27 (8.65%) | 36 (11.84%) |
| 46–55 | 181 (17.22%) | 72 (16.55%) | 53 (16.99%) | 56 (18.42%) |
| 56–65 | 235 (22.36%) | 87 (20%) | 81 (25.96%) | 67 (22.04%) |
| 66–75 | 237 (22.55%) | 106 (24.37%) | 68 (21.79%) | 63 (20.72%) |
| 76–85 | 173 (16.46%) | 72 (16.55%) | 54 (17.31%) | 47 (15.46%) |
| ≥ 86 | 63 (5.99%) | 28 (6.44%) | 17 (5.45%) | 18 (5.92%) |
| Comparison across treatment groups | p = 0.780 | |||
| IMD decile | ||||
| 0–9 (most deprived) | 29 (2.76%) | 7 (1.61%) | 9 (2.88%) | 13 (4.28%) |
| 10–19 | 275 (26.17%) | 127 (29.20%) | 65 (20.83%) | 83 (27.30%) |
| 20–29 | 252 (23.98%) | 100 (22.99%) | 85 (27.24%) | 67 (22.04%) |
| 30–39 | 216 (20.55%) | 87 (20.00%) | 68 (21.79%) | 61 (20.07%) |
| 40–49 | 158 (15.03%) | 66 (15.17%) | 51 (16.35%) | 41 (13.49%) |
| 50–59 | 107 (10.18%) | 43 (9.89%) | 28 (8.97%) | 36 (11.84%) |
| 60–69 | 14 (1.33%) | 5 (1.15%) | 6 (1.92%) | 3 (0.99%) |
| Comparison across treatment groups | p = 0.219 | |||
| Distance from clinic (in km), mean (SD) | 2.7 (1.81) | 2.69 (1.82) | 2.81 (1.84) | 2.59 (1.76) |
| Comparison across treatment groups | p = 0.305 | |||
| Years registered (in km) mean (SD) | 6.0 (2.17) | 6.0 (2.12) | 5.8 (2.23) | 6.0 (2.20) |
| Comparison across treatment groups | p = 0.480 | |||
Overall, there were more men (57.94%) in the study than women (42.06%), although the proportion of men compared with women is not significantly different across treatment groups (p = 0.264). Over two-thirds (67.36%) of the participants were aged > 55 years. Across treatment groups, there were no significant differences in participants’ age (p = 0.780). Over half (52.90%) of participants were in the bottom three IMD deciles (0–20th), and 88.49% were in the fifth decile or lower (0–40th), confirming that study participants are among the most deprived and hard-to-reach populations. No significant differences in IMD deciles were found across treatment groups (p = 0.219). The mean distance from clinic, based on participant address on the day of screening measured to the nearest kilometre, was 2.7 km [standard deviation (SD) 1.81 km]. Across all treatment groups, the median distance from clinic on screening day was 2.5 km. There were no significant differences in distance by treatment groups (p = 0.305). Over 60% of trial participants were registered for at least 6 years in the Kensington, Chelsea and Westminster Diabetic Eye Screening Programme, which has been in place for 8 years. There were no significant differences in years registered by treatment group (p = 0.480).
Comparison of trial participants to the general screening population
This trial provides the opportunity to compare trial participants (who by definition, have not attended their appointments recently) with other patients who are invited for diabetic retinopathy screening. Therefore, the trial participants were compared in terms of sociodemographic variables with the general 1st Retinal Screening Ltd population, which was further subdivided according to regular attenders (defined as those who have attended at least two appointments in the past 3 years) and non-regular attenders (those who have attended fewer than two appointments in the past 3 years). The non-regular attenders may differ from the trial participants according to factors such as not having been registered with 1st Retinal Screening Ltd for long enough to be eligible for the trial, having been seen by hospital eye services, or having moved out of the area for a period of time or generated a post office return notice.
The comparison of trial participants with the general 1st Retinal Screening Ltd population according to sociodemographic variables can be seen in Table 6. Trial participants appear to be significantly different from regular and non-regular attenders from the 1st Retinal Screening Ltd general population with regard to age (p < 0.0001 and p = 0.026, respectively) and distance from clinic (p < 0.0001 for both comparisons). A smaller proportion of trial participants (45.0%) were aged > 65 years than regular attenders (51.1%), and a larger proportion than non-regular attenders (40.9%).
| Sociodemographic factor | Invited IDEAS trial participants (n = 1051) | General population (n = 9964) | Regular attenders (n = 7821) | Non-regular attenders (n = 2143) |
|---|---|---|---|---|
| Gender | ||||
| Male | 609 (57.94%) | 5572 (55.9%) | 4373 (55.9%) | 1199 (55.9%) |
| Female | 442 (42.06%) | 4365 (43.8%) | 3433 (43.9%) | 932 (43.5%) |
| Not specified | 0 (0.0%) | 14 (0.1%) | 8 (0.1%) | 6 (0.3%) |
| Missinga | 0 (0.0%) | 13 (0.1%) | 7 (0.1%) | 6 (0.3%) |
| Comparison with IDEAS trial participants | p = 0.245b | p = 0.238b | p = 0.368b | |
| Comparison between regular and non-regular attenders in the general population | p = 0.841b | |||
| Age (years) | ||||
| 16–25 | 12 (1.14%) | 86 (0.9%) | 48 (0.6%) | 38 (1.8%) |
| 26–35 | 37 (3.52%) | 200 (2.0%) | 109 (1.4%) | 91 (4.2%) |
| 36–45 | 113 (10.75%) | 574 (5.8%) | 359 (4.6%) | 215 (10.0%) |
| 46–55 | 181 (17.22%) | 1662 (16.7%) | 1210 (15.5%) | 452 (21.1%) |
| 56–65 | 235 (22.36%) | 2567 (25.8%) | 2096 (26.8%) | 471 (22.0%) |
| 66–75 | 237 (22.55%) | 2668 (26.8%) | 2215 (28.3%) | 453 (21.1%) |
| 76–85 | 173 (16.46%) | 1777 (17.8%) | 1455 (18.6%) | 322 (15.0%) |
| ≥ 86 | 63 (5.99%) | 430 (4.3%) | 329 (4.2%) | 101 (4.7%) |
| Comparison with IDEAS trial participants | p < 0.0001 | p < 0.0001 | p = 0.091 | |
| Comparison between regular and non-regular attenders in the general population | p < 0.0001 | |||
| Aged ≤ 65 years | 578 (55%) | 5089 (51.1%) | 3822 (48.9%) | 1267 (59.1%) |
| Aged > 65 years | 473 (45%) | 4875 (48.9%) | 3999 (51.1%) | 876 (40.9%) |
| Comparison with IDEAS trial participants | p = 0.016 | p < 0.0001 | p = 0.026 | |
| Comparison between regular and non-regular attenders in the general population | p < 0.0001 | |||
| IMD decile | ||||
| 0–9 (most deprived) | 29 (2.76%) | 269 (2.7%) | 211 (2.7%) | 58 (2.7%) |
| 10–19 | 275 (26.17%) | 2425 (24.3%) | 1934 (24.7%) | 491 (22.9%) |
| 20–29 | 252 (23.98%) | 2394 (24.0%) | 1872 (23.9%) | 522 (24.4%) |
| 30–39 | 216 (20.55%) | 1930 (19.4%) | 1491 (19.1%) | 439 (20.5%) |
| 40–49 | 158 (15.03%) | 1605 (16.1%) | 1258 (16.1%) | 347 (16.2%) |
| 50–59 | 107 (10.18%) | 1200 (12.0%) | 945 (12.1%) | 255 (11.9%) |
| 60–69 | 14 (1.33%) | 141 (1.4%) | 110 (1.4%) | 31 (1.4%) |
| Comparison with IDEAS trial participants | p = 0.481 | p = 0.499 | p = 0.462 | |
| Comparison between regular and non-regular attenders in the general population | p = 0.644 | |||
| IMD 0–20 | 556 (52.9%) | 5088 (51.1%) | 4017 (51.4%) | 1071 (50.0%) |
| IMD 30–60 | 495 (47.1%) | 4876 (48.9%) | 3804 (48.6%) | 1072 (50.0%) |
| Comparison with IDEAS trial participants | p = 0.257 | p = 0.348 | p = 0.120 | |
| Comparison between regular and non-regular attenders in the general population | p = 0.256 | |||
| Distance from clinic (km) | ||||
| Mean (SD) | 2.7 (1.81) | 1.67 (2.08) | 1.64 (2.13) | 1.78 (1.84) |
| Median (range) | 2.5 (< 0.5–17.5) | 1.0 (< 0.5–93.5) | 1.0 (< 0.5–93.5) | 1.0 (< 0.5–19.5) |
| Missinga | 0 | 536 | 46 | 490 |
| Comparison with IDEAS trial participants | p < 0.0001; MD = –1.03, 95% CI –1.16 to –0.90 | p < 0.0001; MD = –1.05, 95% CI –1.17 to –0.93 | p < 0.0001; MD = –0.92, 95% CI –1.06 to –0.77 | |
| Comparison between regular and non-regular attenders in the general population | p = 0.016;c MD = –0.14, 95% CI –1.06 to –0.77 | |||
| Years registered | ||||
| Mean (SD) | 5.96 (2.17) | 5.95 (2.21) | 5.98 (2.20) | 5.86 (2.22) |
| Median (range) | 7.0 (2–8) | 7.0 (2–8) | 7.0 (2–8) | 6.0 (2–8) |
| Comparison with IDEAS trial participants | p = 0.945; MD –0.01, 95% CI –0.15 to 0.14 | p = 0.788; MD 0.02, 95% CI –0.12 to 0.16 | p = 0.260; MD –0.09, 95% CI –0.26 to 0.07 | |
| Comparison between regular and non-regular attenders in the general population | p = 0.035; MD = –0.11, 95% CI –0.22 to –0.01 | |||
Trial participants had a significantly larger distance from home to clinic than regular attenders (MD –1.05 km, 95% CI –1.17 to –0.93 km) and non-attenders (MD –0.92 km, 95% CI –1.06 to –0.77 km). However, this is to be expected due to the design of the study – trial participants were invited to a single screening clinic but, in the usual annual screening invitations, participants are invited to a screening centre that they have most recently attended or which is geographically closer to them. Therefore, the distance to the screening centre is likely to be smaller in the general population than for those invited to the current trial.
Comparisons between regular and non-regular attenders in the 1st Retinal Screening Ltd general population revealed significant differences with respect to the following factors: age (p < 0.0001); distance from clinic (p = 0.016 removing missing values, or p = 0.008 including missing values); and years registered (p = 0.035). There was no significant difference between regular and non-regular attenders by IMD decile (p = 0.644) and gender (p = 0.841) in the general population. Although within the group of regular attenders there was a relatively even distribution of those over and under the age of 65 years (51.1% vs. 48.9%, respectively), among non-regular attenders 40.9% were aged > 65 years. Non-regular attenders were significantly further from screening location than regular attenders (MD –0.14 km, 95% CI –1.06 to –0.77). Non-regular attenders were also registered for a significantly smaller time, on average (MD –0.11 km, 95% CI –0.22 to –0.01).
The impact of incentives on attendance
The primary outcome was screening attendance rate (Table 7). Sixty-one trial participants attended screening (5.8% of those invited). Thirty-four participants (7.82% of those invited) attended from the control arm, and 27 participants (4.38% of those invited) attended from the incentive arms combined. Seventeen participants (5.45%) attended from the fixed incentive arm and 10 participants (3.29%) attended from the lottery incentive arm. Therefore, a greater proportion of participants invited attended in the control group than did in the incentive groups. (It is interesting to note that the 89 participants who were randomised but become ineligible prior to being invited as they attended their appointment, corresponds to 6.99% of those randomised, which is relatively comparable with the attendance rate within the control group of the trial.)
| Treatment group | Number who attended screening | Attendance rate per treatment group (95% CI) |
|---|---|---|
| Control (n = 435) | 34 | 7.82% (5.29% to 10.34%) |
| Fixed incentive (n = 312) | 17 | 5.45% (2.93% to 7.97%) |
| Lottery incentive (n = 304) | 10 | 3.29% (1.28% to 5.29%) |
| Combined incentive (n = 616) | 27 | 4.38% (2.77% to 6.00%) |
| Total | 61 | 5.80% (4.39% to 7.22%) |
To address the first research question regarding effectiveness of incentives, a single comparative analysis – the combined fixed and lottery incentive group compared with control – was performed. We include this comparison separately, which effectively combines information contained within two of the following tests. Pairwise comparisons, adjusting for multiple treatment comparisons, were conducted to answer the second research question of identifying whether or not the incentive design affects screening uptake. A conservative Bonferroni correction for three independent multiple comparisons was performed corresponding to the comparisons outlined in the analysis plan. Risk differences and RRs, with corresponding 95% CIs are presented in Table 8. Two significant results were found. Those in an incentive group were 44% less likely to attend screening than controls (RR 0.56, 95% CI 0.34 to 0.92), and those in the lottery arm were 58% less likely to attend screening than controls (RR 0.42, 95% CI 0.18 to 0.98). As the upper limit of the CI is near 1, then it is important to note that evidence merely shows a weak association, and may be due to chance. No significant differences in attendance rate were found between the following comparisons: fixed incentive versus control (RR 0.70, 95% CI 0.35 to 1.39); and fixed incentive versus lottery incentive (RR 1.66, 95% CI 0.65 to 4.21).
| Pairwise comparison | Risk difference (95% CI) | RR (95% CI) |
|---|---|---|
| Combined incentive vs. control | –0.03 (–0.06 to –0.01)a | 0.56 (0.34 to 0.92)a |
| Fixed incentive vs. control | –0.02 (–0.07 to 0.02) | 0.70 (0.35 to 1.39) |
| Lottery incentive vs. control | –0.05 (–0.09 to –0.003)b | 0.42 (0.18 to 0.98)b |
| Fixed incentive vs. lottery incentive | 0.02 (–0.02 to 0.06) | 1.66 (0.65 to 4.21) |
Therefore, not only were incentives not an effective strategy to increase participation in retinopathy screening but also the offer of financial incentives was associated with lower rates of screening uptake. The second research question concerned the design of the incentive scheme, and how that may impact on effectiveness. When considering the two incentive groups separately, the fixed incentive group did not significantly differ in screening uptake rates from the control group. However, the group who were offered the lottery incentives were significantly less likely to attend screening than those in the control group. Although the fixed and incentive groups did not significantly differ from each other, these findings suggest that the use of a lottery incentive may be the approach, which is least likely to promote screening uptake.
Secondary analyses
To detect if the incentive schemes attracted people with different sociodemographic characteristics, the factors presented in Table 5 were explored, comparing trial participants who attended and DNA, and further subdividing this according to those in the control compared with incentive groups. These comparisons are shown in Table 9. Owing to small counts among attenders within the original age and IMD factors, binary categorisations were used for age (> 65 years) and IMD (0–20 vs. 30–60) when assessing differences by treatment group among trial attenders. There were no significant differences between attenders and non-attenders among trial participants with regard to any of the sociodemographic factors. Additionally, no significant differences in sociodemographic factors are seen among trial attenders when comparing control and incentive participants.
| Sociodemographic factor | IDEAS trial participants (invited: n = 1051) | Trial non-attenders (n = 990) | Trial attenders (n = 61) | ||||
|---|---|---|---|---|---|---|---|
| Total (n = 61) | Control (n = 34) | Incentive (n = 27) | |||||
| Total (n = 27) | Fixed (n = 17) | Lottery (n = 10) | |||||
| Gender | |||||||
| Male | 609 (57.9%) | 577 (58.3%) | 32 (52.5%) | 20 (58.8%) | 12 (44.4%) | 9 (52.9%) | 3 (30.0%) |
| Female | 442 (42.1%) | 413 (41.7%) | 29 (47.5%) | 14 (41.2%) | 15 (55.6%) | 8 (47.1%) | 7 (70.0%) |
| p = 0.371 (non-attenders vs. attenders) | p = 0.264 (incentive vs. control) | ||||||
| Age (years) | |||||||
| ≤ 65 | 578 (55.0%) | 541 (54.6%) | 37 (60.7%) | 19 (55.9%) | 18 (66.7%) | 10 (58.8%) | 8 (80.0%) |
| > 65 | 473 (45.0%) | 449 (45.4%) | 24 (39.3%) | 15 (44.1%) | 9 (33.3%) | 7 (41.2%) | 2 (20.0%) |
| p = 0.360 (non-attenders vs. attenders) | p = 0.392 (incentive vs. control) | ||||||
| IMD decile | |||||||
| 0–20 | 556 (52.9%) | 522 (52.7%) | 34 (55.7%) | 19 (55.9%) | 15 (55.5%) | 10 (58.8%) | 5 (50.0%) |
| 30–60 | 495 (47.1%) | 468 (47.3%) | 27 (44.3%) | 15 (44.1%) | 12 (44.4%) | 7 (41.2%) | 5 (50.0%) |
| p = 0.648 (non-attenders vs. attenders) | p = 0.980 (incentive vs. control) | ||||||
| Distance from clinic (km) | |||||||
| Mean (SD) | 2.7 (1.81) | 2.71 (1.80) | 2.53 (1.94) | 2.94 (2.25) | 2.0 (1.32) | 2.09 (1.47) | 1.85 (1.06) |
| Median (range) | 2.5 (0.0–17.5) | 2.5 (0.0–17.5) | 2.0 (0.5–10.0) | 2.5 (0.5–10.0) | 2.0 (0.5–6.5) | 1.5 (0.5–6.5) | 2.0 (0.5–3.5) |
| p = 0.447; MD –0.18, 95% CI –0.65 to 0.29 (non-attenders vs. attenders) | p = 0.059; MD 0.94, 95% CI –0.04 to 1.92 (incentive vs. control) | ||||||
| Years registered | |||||||
| Mean (SD) | 5.96 (2.17) | 5.96 (2.19) | 5.84 (1.95) | 5.79 (1.92) | 5.89 (2.03) | 5.65 (2.18) | 6.30 (1.77) |
| Median (range) | 7.0 (2–8) | 7.0 (2–8) | 6.0 (2–8) | 6.0 (2–8) | 6.0 (2–8) | 5.0 (2–8) | 6.5 (3–8) |
| p = 0.654; MD –0.13, 95% CI –0.69 to 0.43 (non-attenders vs. attenders) | p = 0.852; MD –0.95, 95% CI –1.11 to 0.92 (incentive vs. control) | ||||||
Attendance by sociodemographic factors shows that 5.25% of the total males invited attended, and 6.56% of the total females invited attended. Of those who attended their appointment, 52.46% were male. This difference was non-significant (p = 0.371); thus, there was no significant association between gender and attendance. Approximately 85% of attenders were aged > 45 years, about two-thirds were aged > 55 years, and roughly 40% were aged > 65 years. A subgroup analysis assessing the association of age (≤ 65 years vs. > 65 years) on attendance showed no significant association (p = 0.360). Over half (55.74%) of attenders were in the lowest three IMD deciles; 78.69% were in the bottom four IMD deciles. However, there was no significant association between attendance and deprivation status when comparing the lowest three deciles (0–20th) with those above it (30–60th) (p = 0.648). Also similar to all trial participants (who were invited to attend an appointment), approximately 90% of those who actually attended were among the most deprived half (i.e. below the 5th decile, or 50th percentile) of the population. Over half (54.1%) of the attenders were within 2 km of the clinic location on the day of screening, with nearly 95% within 5 km. There was no significant difference in average distance from screening between attending and non-attending participants (MD –0.18 km; p = 0.447). Similarly, there was no significant difference in the average years registered between attenders and non-attenders (MD –0.13; p = 0.654). Therefore, it does not appear that attending the trial appointment was associated with any sociodemographic characteristics. Furthermore, there were no significant differences in sociodemographic factors between attenders in the control and the incentive groups.
The impact of sociodemographic factors on attendance was further investigated, as an exploratory analysis, using a backward stepwise logistic regression analysis. This revealed that only the lottery group (p = 0.013) was significantly associated with attendance (with belonging to the lottery group associated with lower probability of attendance). The order in which other sociodemographic factors were removed is years registered (p = 0.752); the binary categorisation of IMD (p = 0.536); distance from screening location (p = 0.397); gender (p = 0.267); age > 65 years (0.303); and fixed incentive group (p = 0.195). Results were similar when combining the fixed and lottery groups into an incentive group compared with control (p = 0.021). Again, all other factors were non-significantly associated with attendance.
The third research question sought to address whether or not the choice of incentives scheme (if successful) attracted patients with different sociodemographic characteristics from those who attend screening regularly. However, the incentive schemes were not found to be effective and, overall, there were no differences between trial participants who did attend or DNA.
Sight outcome scores by treatment groups
Sight outcome among trial attenders was a secondary outcome of the study, which was aggregated by treatment group (Tables 10–13). Two types of outcome data are presented. The first is the degree of retinopathy observed (see Table 10), and the second is the recommendation for follow-up requirements following screening (see Table 11). To detect whether or not there are any significant differences between groups in the proportion of patients attending who require additional management, the outcome scores were converted into a binary score. The retinopathy classifications of R0M0 and R0M1, and the follow-up recommendation of ‘annual recall’, were classified as ‘no additional management required’, whereas all other levels were classified as ‘additional management required’. This binary categorisation of the screening outcome is shown in Table 12. A chi-squared analysis comparing treatment group (incentive vs. control) with outcome recommendation shows no significant association (p = 0.387). Additional pairwise comparisons of sight outcome by each treatment group were conducted (see Table 13), which further demonstrate the lack of significance between the outcome recommendation and treatment group, indicating that the groups did not differ according to whether or not attendees require additional management following screening.
| Sight outcome | Control (n = 34) | Fixed (n = 16) | Lottery (n = 10) |
|---|---|---|---|
| R0M0 | 14 (41.2%) | 6 (37.5%) | 6 (60%) |
| R1M0 | 14 (41.2%) | 5 (31.3%) | 2 (20%) |
| R1M1 | 3 (8.8%) | 2 (12.5%) | 2 (20%) |
| R2M1 | 1 (2.9%) | 1 (6.3%) | 0 (0%) |
| R3AM0 | 1 (2.9%) | 1 (6.3%) | 0 (0%) |
| R3SM1 | 1 (2.9%) | 0 (0%) | 0 (0%) |
| R3AM1 | 0 (0%) | 1 (6.3%) | 0 (0%) |
| Follow-up recommendation | Overall IDEAS trial participants attending (n = 60) | Control (n = 34) | Fixed (n = 16) | Lottery (n = 10) |
|---|---|---|---|---|
| Annual recall | 47 (78.3%) | 28 (82.4%) | 11 (68.8%) | 8 (80.0%) |
| Digital surveillance | 6 (10.0%) | 3 (8.8%) | 1 (6.2%) | 2 (20.0%) |
| Refer to ophthalmology for diabetic retinopathy | 4 (6.7%) | 2 (5.9%) | 2 (12.5%) | 0 (0.0%) |
| Refer to ophthalmology urgently for diabetic retinopathy | 3 (5.0%) | 1 (2.9%) | 2 (12.5%) | 0 (0.0%) |
| Recommendation | Overall IDEAS trial attenders (n = 60) | Control (n = 34) | Incentive (n = 26) | Fixed (n = 16) | Lottery (n = 10) |
|---|---|---|---|---|---|
| No additional management required | 47 (78.3%) | 28 (82.4%) | 19 (73.08%) | 11 (68.8%) | 8 (80.0%) |
| Additional management required | 13 (21.7%) | 6 (17.6%) | 7 (26.92%) | 5 (31.2%) | 2 (20.0%) |
| Pairwise comparison | Risk difference (95% CI) | RR (95% CI) |
|---|---|---|
| Combined incentive vs. control | –0.09 (–0.30 to 0.12) | 0.89 (0.67 to 1.17) |
| Fixed incentive vs. control | –0.14 (–0.44 to 0.16) | 0.83 (0.53 to 1.30) |
| Lottery incentive vs. control | –0.02 (–0.36 to 0.31) | 0.97 (0.64 to 1.48) |
| Fixed incentive vs. lottery incentive | –0.11 (–0.54 to 0.31) | 0.86 (0.49 to 1.49) |
Reasons for past non-attendance by treatment group
Attending participants were asked if there were any reasons why they had not attended their eye screening appointments for a while. The free responses given were categorised according to a pre-specified list of potential reasons (see Chapter 2, Table 1). The reasons given for past non-attendance by trial attenders, categorised by treatment group are shown in Table 14. Fifty-six attending patients (91.8%) provided responses, including one patient who provided two reasons, resulting in 57 responses. The most common response (‘did not get round to coming’) was provided by 22.81% of attending participants. Of the nine participants who selected a reason of ‘other’, which was recorded as free text, four were then placed into relevant categories in Table 14, and a further five in the higher-level categories, as shown in Table 15.
| Reason for past non-attendance among trial attenders | Overall (n = 57) | Control (n = 30) | Fixed (n = 17) | Lottery (n = 10) |
|---|---|---|---|---|
| Forgot | 4 (7.02%) | 4 (13.33%) | 0 (0.00%) | 0 (0.00%) |
| Did not know I had an appointment | 6 (10.53%) | 4 (13.33%) | 0 (0.00%) | 2 (20.00%) |
| Did not get round to coming | 13 (22.81%) | 7 (23.33%) | 3 (17.65%) | 3 (30.00%) |
| Was out of the country | 4 (7.02%) | 2 (6.67%) | 1 (5.88%) | 1 (10.00%) |
| Have started experiencing problem with eyes | 3 (5.26%) | 1 (3.33%) | 2 (11.76%) | 0 (0.00%) |
| Did not feel they obviously had problems with eyes | 1 (1.75%) | 0 (0.00%) | 1 (5.88%) | 0 (0.00%) |
| Could not get time off work | 1 (1.75%) | 0 (0.00%) | 1 (5.88%) | 0 (0.00%) |
| Did not understand why needed to be screened | 3 (5.26%) | 1 (3.33%) | 1 (5.88%) | 1 (10.00%) |
| Thought optician did it | 4 (7.02%) | 3 (10.00%) | 0 (0.00%) | 1 (10.00%) |
| Family commitments | 2 (3.51%) | 1 (3.33%) | 1 (5.88%) | 0 (0.00%) |
| Too ill to attend | 3 (5.26%) | 0 (0.00%) | 3 (17.65%) | 0 (0.00%) |
| Seen privately | 2 (3.51%) | 2 (6.67%) | 0 (0.00%) | 0 (0.00%) |
| Problems with transport | 1 (1.75%) | 1 (3.33%) | 0 (0.00%) | 0 (0.00%) |
| Not consider themselves diabetic | 1 (1.75%) | 0 (0.00%) | 1 (5.88%) | 0 (0.00%) |
| Under the care of hospital eye services | 4 (7.02%) | 2 (6.67%) | 2 (11.76%) | 0 (0.00%) |
| Other | 5 (8.77%) | 2 (6.67%) | 1 (5.88%) | 2 (20.00%) |
| Reason | Overall (n = 55) | Control (n = 30) | Incentive (n = 25) | Fixed (n = 15) | Lottery (n = 10) |
|---|---|---|---|---|---|
| Organisational | 24 (43.64%) | 16 (53.33%) | 8 (32.0%) | 2 (13.33%) | 6 (60.0%) |
| Practical/logistic | 12 (21.82%) | 4 (13.33%) | 8 (32.0%) | 6 (40.0%) | 2 (20.0%) |
| Did not think need to attend | 12 (21.82%) | 5 (16.67%) | 7 (28.0%) | 5 (33.34%) | 2 (20.0%) |
| Did not need screening (seen privately, under hospital eye services care, or pregnant) | 7 (12.72%) | 5 (16.67%) | 2 (8.0%) | 2 (13.33%) | 0 (0.0%) |
The reasons for past non-attendance were grouped into three main categories (see Table 15): organisational problems (‘forgot’, ‘did not know I had an appointment’ and ‘did not get round to coming’); practical/logistic problems (‘out of the country’, ‘could not get time off work’, ‘family commitments’, ‘too ill to attend’ and ‘problems with transport’); and did not think needed to attend (‘seeking treatment elsewhere’, ‘did not feel they obviously had problems with eyes’, ‘didn’t understand why needed to be screened’, ‘believed optician did it’ and ‘did not consider themselves diabetic’). In the grouping process, it was determined that seven attending participants had valid reasons for not attending appointments in the past, as they were seen privately (n = 2) or under the care of hospital eye services (n = 4) or were pregnant (n = 1); in addition, one participant selected two reasons for non-attendance: ‘was out of the country’ and ‘have started experiencing problem with eyes’. These eight participants were not included in the chi-squared analysis to identify any differences in past non-attendance by treatment group. Among the 48 attending participants included, there was no significant association between reason for past non-attendance and treatment group as incentive (fixed and lottery combined) compared with control (p = 0.119). Among the 48 attending participants who needed screening, half stated they DNA past appointments due to organisational reasons, whereas one-quarter each selected practical/logistic problems and that they did not think they needed to attend.
We further explored whether or not any sociodemographic factors were associated with reasons for past non-attendance and found gender (p = 0.046) to be significantly associated. In particular, males are 91% more likely than females to have organisational reasons for past non-attendance compared with practical reasons (RR 1.91, 95% CI 1.01 to 3.61). Age > 65 years (p = 0.957), IMD (0–20th vs. 30–60th; p = 0.920), years registered (p = 0.713) and distance from screening (p = 0.158) were not associated with reasons for past non-attendance.
Sensitivity analyses
Following the primary ITT analysis, two sensitivity analyses were performed. In the first sensitivity analysis, all participants who had any reason for exclusion following the sending of the invitation letter (see Table 4) were removed from the analysis. In the second sensitivity analysis, any patients who could not attend their trial appointment and were booked into (and attended) a normal screening clinic, were included in the analysis as attenders. The attendance rates for the ITT analysis and according to each sensitivity analysis are shown in Table 16.
| Treatment group | Attenders in ITT group | ITT attendance rate | IDEAS trial attenders: sensitivity analysis 1a | Attendance rate per treatment group: sensitivity analysis 1 | IDEAS trial attenders: sensitivity analysis 2b | Attendance rate per treatment group: sensitivity analysis 2 |
|---|---|---|---|---|---|---|
| Control | 34 | 7.82% | 34 | 9.09% | 36 | 8.28% |
| Fixed | 17 | 5.45% | 13 | 4.74% | 20 | 6.41% |
| Lottery | 10 | 3.29% | 10 | 3.60% | 12 | 3.95% |
| Total | 61 | 5.80% | 57 | 5.80% | 68 | 6.47% |
Sensitivity analysis 1: excluding participants with any reason for ineligibility following the invitation letter
The modification in the first sensitivity analysis yielded no significant changes when assessing sociodemographic factors by treatment group from the ITT analysis. However, when considering those who attended their appointment, and comparing the different intervention groups, control participants who attended were significantly further from screening location than incentive participants (fixed and lottery combined) who attended their appointment (p = 0.023). This might suggest that the control letter attracted people living at a greater distance from the screening centre than did the incentive letter.
Among pairwise comparisons in sensitivity analysis 1 (Table 17), those in an incentive group were 52% less likely to attend screening than control participants (RR 0.48, 95% CI 0.29 to 0.80). When compared by incentive groups separately, the lottery incentive group were about 58% less likely to attend screening than control participants (RR 0.42, 95% CI 0.18 to 0.97). These results were similar to the primary ITT analysis.
| Pairwise comparison | Risk difference (95% CI) | RR (95% CI) |
|---|---|---|
| Combined incentive vs. control | –0.04 (–0.07 to –0.01)a | 0.48 (0.29 to 0.80)a |
| Fixed incentive vs. control | –0.04 (–0.08 to 0.01) | 0.54 (0.25 to 1.16) |
| Lottery incentive vs. control | –0.05 (–0.09 to –0.004)b | 0.42 (0.18 to 0.97)b |
| Fixed incentive vs. lottery incentive | 0.01 (–0.03 to 0.05) | 1.30 (0.49 to 3.49) |
As in the primary analysis, any differences in the sight outcome from screening between the attenders in the different groups (incentive vs. control) was assessed using a chi-squared analysis (Table 18). Similar to the ITT analysis, no significant association (p = 0.443) was found in whether or not additional management was recommended following screening between the combined incentive group and the control group. Pairwise comparisons of sight outcome by treatment group further demonstrate the lack of significance between the outcome recommendation and treatment group (Table 19).
| Recommendation | Overall IDEAS trial attenders (n = 57) | Control (n = 34) | Incentive (n = 26) | Fixed (n = 16) | Lottery (n = 10) |
|---|---|---|---|---|---|
| No additional management required | 45 (78.9%) | 28 (82.4%) | 17 (73.90%) | 9 (69.2%) | 8 (80.0%) |
| Additional management required | 12 (21.1%) | 6 (17.6%) | 6 (26.10%) | 4 (30.8%) | 2 (20.0%) |
| Pairwise comparison | Risk difference (95% CI) | RR (95% CI) |
|---|---|---|
| Combined incentive vs. control | –0.08 (–0.30 to 0.13) | 0.90 (0.67 to 1.20) |
| Fixed incentive vs. control | –0.13 (–0.45 to 0.19) | 0.84 (0.52 to 1.36) |
| Lottery incentive vs. control | –0.02 (–0.36 to 0.31) | 0.97 (0.64 to 1.48) |
| Fixed incentive vs. lottery incentive | –0.11 (–0.55 to 0.33) | 0.87 (0.48 to 1.55) |
An exploratory analysis showed, using a backward stepwise logistic regression analysis, only the lottery incentive group (p = 0.012) was significantly associated with lower attendance, as observed in the ITT analysis. The order in which other sociodemographic factors were removed was binary categorisation of IMD (p = 0.648); years registered (p = 0.542); distance from screening location (p = 0.290); age > 65 years (p = 0.274); gender (p = 0.323); and fixed incentive group (p = 0.519). Results were similar when combining the fixed incentive and lottery incentive groups into a combined incentive group compared with control (p = 0.005). Again, all other factors were non-significantly associated with attendance.
A limitation of sensitivity analysis 1 is that the group sizes (control, n = 408; fixed, n = 287; lottery, n = 288) no longer meet those required (control, n = 412; fixed, n = 294; lottery, n = 294) based on our power analysis, although there may not be enough divergence to yield substantial differences.
Sensitivity analysis 2: including trial participants who rearranged their appointments to a non-trial clinic
The second sensitivity analysis considers the ITT list but now includes among attenders those who rebooked their appointment and were placed in normal screening, as long as they attended that rebooked appointment (or a subsequent rearranged appointment, without being classified as DNA, and within 4 months of the original trial appointment). This occurred when participants tried to rebook their appointment, but were not able to attend another designated trial clinic. They were therefore booked onto normal screening, but were no longer eligible for the incentive. This sensitivity analysis added an additional seven participants, as attenders, to the primary ITT analysis (see Table 16). Similar to both the ITT and first sensitivity analyses, there were no significant differences between sociodemographic factors and treatment group. The second sensitivity analysis did not show that the lottery incentive group had a significant effect on attendance compared with the control group (RR 0.48, 95% CI 0.22 to 1.04); and the comparison of the combined incentive group to control participants showed only a weak association (RR 0.63, 95% CI 0.40 to 0.99). Incentive participants were 37% less likely to attend screening than control participants. There was also not a significant effect of the fixed incentive on attendance (Table 20).
| Pairwise comparison | Risk difference (95% CI) | RR (95% CI) |
|---|---|---|
| Combined incentive vs. control | –0.03 (–0.06 to –0.001)a | 0.63 (0.40 to 0.99)a |
| Fixed incentive vs. control | –0.02 (–0.07 to 0.03) | 0.77 (0.41 to 1.47) |
| Lottery incentive vs. control | –0.04 (–0.09 to 0.001) | 0.48 (0.22 to 1.04) |
| Fixed incentive vs. lottery incentive | 0.02 (–0.02 to 0.07) | 1.62 (0.69 to 3.80) |
The difference in findings, where the lottery incentive group was no longer significantly different from the control group, can be attributed to the information provided by the additional seven attenders included in sensitivity analysis 2. With overall low attending numbers in general, the inclusion of an additional few participants changes the result of the analysis for the lottery group versus controls.
Screening outcome was not available for the seven participants who were classified as attenders in the second sensitivity analysis. Therefore, we cannot assess sight outcome results for this analysis.
Using a backward stepwise logistic regression analysis, none of the factors was significantly associated with attendance, in contrast with the ITT and first sensitivity analysis, which showed a difference by lottery incentive group. The order in which other sociodemographic factors were removed was years registered (p = 0.979), binary categorisation of IMD (p = 0.691), distance from screening location (p = 0.406), gender (p = 0.293), age > 65 years (p = 0.347) and treatment group (p = 0.228). When combining the fixed incentive and lottery incentive groups into an combined incentive group compared with control, the order in which factors were removed was the same (and those factors are also non-significant); however, the combined incentive group was now significantly, although weakly, associated with attendance (p = 0.047).
Adverse events
One adverse event was reported throughout the trial. A bed-bound patient in the fixed incentive arm was not suitable for normal retinal screening and had to be turned away. This participant was considered as an attender, although no sight outcome data were obtained. This patient was included in the ITT analysis and sensitivity analysis 2, but was excluded from sensitivity analysis 1.
Cost-effectiveness analysis
This trial sought to use CEA to address its fourth research question – is offering incentives a cost-effective strategy for enhancing participation? The costs of providing the incentive would be assessed relative to the primary outcome: attendance at screening.
The costs associated with the intervention in incentive trials are somewhat different from those incurred in other health-care intervention trials. In most health-care trials, every individual randomised to the treatment arm(s) of the study will receive a treatment for which there is an associated cost (e.g. a course of medication or therapy). In an incentive trial, typically the differential costs are incurred only if the preferred outcome – in the case of the IDEAS trial, attendance at screening – occurs. We assume that the costs of sending out the invitation letter are minimal and neither the costs of sending the letter, nor the cost of screening, differ between the control and incentive groups. In the IDEAS trial, the average cost per attendee, for the fixed incentive group was £10 (the fixed incentive). For the lottery incentive group, given that 10 participants attended, the average cost per attendee was £100 (although this would be lower had larger numbers been invited and therefore attended, tending to a minimum possible cost per attendee of £10). The average cost per attendee was of course minimal for the control group.
As reported in the effectiveness analysis results, the RR of attending screening was significantly lower for the lottery incentive group than the control group and there was no difference in the RR of attending screening between the fixed incentive and control groups, although the risk difference suggested a trend towards lower attendance in the fixed incentive group. Owing to the lack of a positive effect, which would mean that the intervention would not be implemented, a Markov model was not conducted, as originally planned.
In CEAs, the term ‘dominance’ is used to describe situations in which an option is both more costly and less effective than an alternative. 68 For obvious reasons, such an option would not be considered for funding. Thus, in the current trial, the control option dominates the lottery incentive. It is also unlikely that the fixed incentive would be funded given its greater costs and no improvement in attendance at screening.
Chapter 4 Discussion
Overview of findings
The study found that incentives were not an effective way to improve uptake of diabetic retinopathy screening. Numbers of attendees were low overall, with rates of attendance at 7.8% in the control group, 5.5% in the fixed incentive group (£10) and 3.3% in the lottery incentive group (1 in 100 chance of winning £1000). In contrast with expectations, those who received an invitation letter containing an incentive offer were actually significantly less likely to attend screening than those who received a standard appointment invitation only.
Considering each incentive scheme separately, although there was no significant difference between the control group and the £10 group, the lottery group were significantly less likely to attend than those in the control group. This suggests that the lottery incentive in particular was detrimental to screening attendance.
When excluding patients who had a reason for ineligibility after they were sent the invitation letter, there was still a significant difference between those in the control condition and the two incentive conditions combined; in particular, the lottery incentive group were still significantly less likely to attend screening than controls, supporting the finding that the lottery incentive had a negative impact on screening attendance. As the incentive did not promote retinopathy screening, and may even have been harmful, the use of incentives cannot be said to be a cost-effective way to improve screening uptake.
The sociodemographic characteristics (age, gender, deprivation, distance from screening centre, or years registered) of those attending the trial appointment were not significantly different from the remainder of the invited group. There were also no sociodemographic differences between attenders from the control compared with the intervention groups.
Comparisons between the participants in the trial (who had not attended an appointment in at least 2 years) and regular attenders at diabetic retinopathy appointments revealed that trial participants were significantly younger, the distance to travel to screening centre was greater than regular attenders, but there was no difference in IMD or gender. Regular attenders in the general screening population were significantly older than non-regular attenders, suggesting that increasing age is associated with regular attendance, perhaps as there is availability in time to attend appointments. The increased distance from home to the screening centre can be explained by the trial methodology.
Interpretation of findings
The findings of a null effect, and more so a negative effect of incentives, was unexpected. In general, the proportion of invited participants who attended screening was low. However, this is to be expected given that the study was targeting a very hard-to-reach population, who had not attended screening for 2 years or more, including some who had never attended screening. A potential reason for the low retinopathy screening rates in the target population could be that people with diabetes often feel well, and may only start attending all of their health appointments following a major event, such as an ulcer or heart problem. It is therefore possible that the trial population have not yet had a major event that has prompted them to recognise the importance of their health checks, and therefore to be more likely to attend their diabetic retinopathy appointment. In support, it was observed that regular attenders are significantly older than abstainers from screening, so as the likelihood of having a major health event increases with the length of time since being diagnosed with diabetes, the likelihood of attendance increases.
The suggested reasons above for low attendance among trial participants may explain a difficulty in finding a significant effect. However, they do not help to explain why incentives were found to be associated with significantly lower attendance rates than an invitation letter alone. One common criticism of extrinsic rewards such as financial incentives is that they can crowd out intrinsic motivation to perform a behaviour. 69,70 The crowding out of intrinsic motivation does not appear to be a relevant concern related to the target population, as they had not attended screening for at least 2 years, so were unlikely to have strong motivation that would be harmed through the offer of an incentive.
One possible reason for the negative effect found is that the offering of an incentive elicits feelings of dread. It may be that if people see they are being offered an incentive to attend a health check, it may reinforce a view that the screening is unpleasant, and therefore make people dread the appointment and result in them being less likely to come. This suggestion is supported by the evidence that when considering the incentives individually, it was the lottery offer that was associated with significantly lower attendance; the lottery for a very large incentive of £1000 to attend screening may have been more likely to reinforce the view that screening is unpleasant than the modest offer of £10.
Evidence from behavioural economics suggests that preference is constructed during the judgement and choice process. 62 In other words, we construct our inner life and our actions from (limited) information that is currently accessible from the environment and from memory. For example, a classic study71 asked people to do a boring task and then paid them either US$1 or US$20 to persuade others to do the task. People paid more found the task more aversive and were less likely to repeat it. When subjects asked themselves ‘why did I do this?’, the intuitive answer was ‘if I was only paid US$1, it can’t have been too bad’, and the opposite inference was made for the US$20 payment. A more recent study has shown that in some cases people do not even have a pre-existing sense of whether or not an experience is good or bad (even when they have experienced a sample of it); and the valence of the experience is a matter of whether the individual is paying for the experience (leading to positive valuation, ‘it must be good’) or being paid for the experience (leading to negative valuation, ‘if I had to pay it must be unpleasant’). 72
There is conflicting evidence when considering this question; financial incentives may be effective at promoting uptake of a cervical smear test,46 which could also be expected to evoke feelings of dread. There is a trial registered on ClinicalTrials.gov (URL: https://clinicaltrials.gov/ct2/show/NCT02660671, accessed 26 April 2016), which is offering a US$100 financial incentive for attending a screening colonoscopy. If that trial finds a negative effect of incentives, it may support this hypothesis about the incentives evoking dread and reducing participation. Care should be taken with generalisation, as the study is taking place in the USA, it is possible that people are more comfortable with health care having more of a financial and transactional nature, so the offer of an incentive may not be as surprising.
Another potential explanation as to why the incentive condition may have been less effective is that it is possible that the presence of the incentive voucher on the invitation letter may have given some people the impression that it was junk mail, and so they may not have read the letter properly.
When considering these findings in light of other literature on screening and incentives, the reviews commonly, but not exclusively, support the effectiveness of incentives. 24,46,47 Notable studies that show null effects of incentives include one considering different types of incentives for chlamydia screening;33 however, this was looking at people who had already requested a test kit, and another considering fixed and lottery incentives to promote mammogram uptake. 35 The present study observed a significant negative effect of incentives on screening, rather than just no effect.
Another study assessing health care claims to establish predictors of rates of diabetic retinopathy screening found that the offering of incentives (both provider and patient incentives of US$25) was associated with lower odds of being screened. 44 Although this study was only assessing correlational data from potentially incomplete claim records, and took place in the USA, which has a very different health-care context, the consistency of finding a negative effect of incentives on retinopathy screening rates lends support to the reliability of the results of the present trial.
Other potentially negative effects of financial incentives have been observed, although not always concerning the primary outcomes as in the present study. For example, a US study assessed the impact of a pilot programme involving a reminder letter and offer of a US$6 voucher at a local petrol station for attendance at blood glucose and cholesterol screenings for diabetic patients who had missed those screenings in the previous year. Although those in the pilot incentive scheme attended more blood glucose and cholesterol screenings than patients who did not, following the end of the pilot scheme, the incentive patients attended fewer screening appointments than those in the control group. It is unclear whether the main effect of the pilot programme was due to the small incentive, or the receipt of a reminder letter. The lack of sustained effect of the incentives is consistent with the observation that the effects of incentives on repeated behaviours are not sustained following withdrawal of the incentive. 23
Another study assessed the impact of incentives on a peer referral scheme for mammogram screening for uninsured women in the USA, comparing a control group with a group offered US$5 each time they nominated someone for screening, and a group offered US$20 if their referral ended with a completed mammogram. Although there were more nominations from the US$5 group than from the US$20 group and control group, those in the control group achieved as many referred mammograms as those in the US$20 per completed mammogram group, and significantly more than the US$5 per referred name group. This is therefore further evidence where a control group performed significantly better than an incentive group. An interpretation of this study could be that the payment is perceived as recompense for a bad experience.
Generalisability of findings
It may be hard to generalise the findings from this study to screening studies more widely, due to existing differences in findings even within the same screening behaviour,32,33 which may depend on the country and health-care context, the population being studied and details of the incentive scheme.
As the results are consistent with the other study looking at the impact of screening on diabetic retinopathy,44 the findings may be generalisable to diabetic retinopathy screening more widely in patients who are consistent non-attenders. The question remains about whether or not these findings can be assumed to generalise to other types of screening appointments for people with diabetes and more regular attenders. Although it was found that small incentives increase blood glucose and cholesterol screening for the duration of a pilot programme, it is not clear if this was due to the sending of reminder letters, so further work would be required to reliably establish the impact of incentives in other types of screening in diabetes.
It is unlikely that the findings could be applied to day-to-day management of diabetes, such as regarding diet or medication adherence, as there is evidence that regularly performed behaviours are distinct from infrequent behaviours in terms of how they are affected by incentives. 17,23 It was proposed that frequent behaviours are harder to influence with incentives than infrequent behaviours, which may suggest that day-to-day diabetes management is even less likely to be positively affected by incentives. However, it may be the case that there were other reasons why the incentives were ineffective or less effective in the present study, such as exacerbating any feelings of dread about screening.
The lack of an effect is consistent with a study of similar incentive schemes for attendance at mammogram, which was also targeting a hard-to-reach group who had not been screened for over 2.5 years. 35 It may be the case that for patients who have not engaged with a health check for a prolonged period of time, there are additional barriers to attendance (attitudinal or other), which are not overcome through offering a financial incentive.
Strengths of study
The study was a randomised controlled trial, comparing the screening invitation letter received as part of usual care to two types of financial incentive interventions. The interventions were designed so as to be as well matched to the usual care control group, with only the addition of the incentive offer, in the form of a visual voucher printed on the letter to attract attention, and a short passage within the text. The fixed and lottery incentive conditions were designed based on principles in behavioural economics, in order that they should be as effective as possible. Furthermore, the incentive was designed given patient involvement at different stages, in order to ensure that the intervention should be acceptable and effective. Therefore, the TMT have confidence that the incentive was designed as well as possible in order to be able to detect any effect of financial incentives on retinopathy screening uptake.
Furthermore, numerous checks were conducted to ensure that the cohort included were appropriate. For example, patients were not eligible if a letter had been returned stating unknown address or not known at that address, if the GP had not recently verified that they were registered at the practice, or if they had been contacted as part of a recent initiative by the screening service to improve uptake in persistent non-attenders. These checks all enhance the reliability of the study and its findings.
Limitations of study
Although checks were conducted to ensure that all participants had correct addresses, there are high rates of population turnover in the study area (estimated to be 35% annually in Westminster). A total of 18,100 people moved in and 21,300 moved out in the year to June 2012 according to the Westminster Joint Strategic Needs Assessment. 73 This may have resulted in the inclusion of participants who were no longer at their addresses, and therefore who would not have received the invitation letter. The fact patients had to have not attended for at least 2 years to be eligible means that there would have been multiple opportunities for a post office return to have been received for patients who have moved. However, this would not have detected patients who have moved more recently than their last invited appointment date. Yet, the fact that participants were randomised should mean that the groups would be expected to be balanced according to the proportion of participants who may have no longer been at their addresses.
The area in which the study took place is ethnically diverse, with many different languages spoken. Thirty-one per cent of the population in Westminster has a main language that is not English and, of these, one in eight states that they are not able to speak English well – almost 4% of the borough’s population. Arabic is the most common language after English, followed by French, Spanish and Italian. 74 This is of importance, as the diverse population in this area may mean that the results of this study are not applicable throughout the country. Of people who attended screening, many came with younger family members who served as translators. We were unable to record the ethnicity of non-attenders in this cohort as this information is not available. It is possible, however, that rather than a lack of incentives, the language barrier may have been a bigger barrier to attendance in this population. This could be due to not understanding the letter or how to request a translator from the screening service, or not having a family member who can attend as a translator on the day of the appointment.
In terms of the population studied in Kensington, Chelsea and Westminster, overall deprivation is higher than the England average, whereas obesity, rates of diabetes and overall life expectancy are significantly better than the England average. The health of people in these areas is certainly varied. Life expectancy is 11.3 years lower for men and 7.9 years lower for women in the most deprived areas of Westminster than in the least deprived areas. Life expectancy is 14.3 years lower for men and 4.3 years lower for women in the most deprived areas of Kensington and Chelsea than in the least deprived areas. 73 It may therefore be important to test whether or not the results can be generalised to a different population profile in England. However, due to the lack of any support for the incentive scheme, it may be unlikely that such research would be conducted.
The distance to the screening centre was significantly greater for trial participants than in the general screening population. This is almost certainly because, attempts are generally made to ensure screening is close to patients, by inviting them to an appointment at the location of their past screening appointment, or if they have not previously attended, patients will be invited to the closest screening centre to their home address. As trial participants were all invited to a single screening centre, this would explain why the distance to screening was greater. This may have made it harder for participants to attend their appointments compared with regular non-trial appointments. However, as the groups were randomised, there were no group differences in distance to the screening centre, so this should not have had an impact on the group comparisons.
This trial has found that financial incentives were not effective on average. We have attempted to understand if patients attending screening in the trial were of a different age or socioeconomic background in incentive compared with control groups. However, this does not mean that there were certain segments of the population that would be encouraged to attend that has gone unrecognised or due to sample selection bias. As the numbers attending the screening were small in each group no further analysis was deemed suitable to further investigate this point.
The study was powered based on an anticipated 1% attendance in the control group, but still with 85% power to detect an increase from 5% in the control group to 15% in the incentive group. In actuality the attendance rate was nearly 8% in the control group, which was much higher than anticipated. This may suggest that the study was not appropriately powered to detect a result. However, the actual levels of attendance in the incentive groups were lower, and significantly lower than those in the control group, suggesting that lack of power was not a reason for a positive effect of incentives not being detected.
Impact of patient and public involvement
The patient and public involvement conducted at all stages of the study was useful in terms of the study design and progressing, and in ensuring acceptability. The meetings with the Westminster Diabetes User Group to discuss incentives and the intervention generally, and later to discuss the specific intervention materials were useful. However, in a later meeting there was substantial feedback on the aspects of the invitation letter sent as usual care, which are centrally determined and could not change, resulting in feedback that could not be implemented as part of the trial. The extensive feedback on the standard incentive letter, along with the strong desire that it should be shorter and clearer may indicate that greater collaboration with patients and the public could be valuable to the screening service, and health services more broadly.
Despite the lottery incentive being designed given patient feedback, this was found to be significantly less effective than the control of usual care. It may therefore be the case that self-reports from people about what they think they would like to receive does not necessarily correspond to what actually drives their behaviour. This has been observed in other interventions designed given extensive public involvement, such as using the stairs rather than an elevator.
Having a patient representative on the TMT was very helpful in terms of providing useful insights and feedback during and between meetings, and on any trial materials. However, our intended trial patient representative, identified at the planning stage, was taken ill – and other suitable representatives needed to be approached. This meant there was unfortunately not a patient representative on the TMT for the early meetings – and therefore not available for final trial design stages. The design of the letter was therefore discussed with relevant diabetic groups. In future, we propose that efforts should be made early in the setup phase of the trial to find multiple representatives in this role to increase the likelihood that there would be a patient representative at every meeting, in case one person is not able to attend for any reason.
Chapter 5 Conclusions
Implications for health care
This study did not find any evidence to support the use of incentives to increase uptake at DES in a group of patients consistently not attending appointments. On the contrary, the use of incentives, and particularly the use of a lottery-based incentive, may actually decrease rates of attendance at DES. There was no evidence that the incentives attracted patients with different sociodemographic characteristics, or patients who were more likely to require further management of retinopathy, compared with those who received the usual invitation letter.
As a relatively costly intervention, this would therefore be both detrimental to screening rates, and a waste of resources. Although the fixed incentive condition was not significantly worse than the control condition, it would still be a costly method to use given no evidence that it could be expected to increase screening attendance rates.
Recommendations for future research
It is perhaps most crucial to conduct further work on barriers to attendance and reasons for non-attendance. Simply asking people who attended their trial appointment indicated that across all groups, approximately half the attenders DNA in the past due to organisational reasons (such as forgetting), one-quarter due to practical or logistical reasons (such as illness, or not being able to take time off work), and one-quarter due to not thinking that they needed to be screened (such as not thinking they have problems with their eyes). These barriers may not be effectively overcome through provision of incentives. Future research could further investigate these potential barriers to study more effective ways to promote improved attendance. This group, however, is difficult to sample adequately by the very nature of their failure to attend health checks.
Second, it may be worthwhile to pursue different methods for increasing screening attendance. Incentives could be studied again, but using food vouchers or cinema tickets rather than financial incentives (but one may fear the same outcome as this trial). It is established that text messaging can increase appointment attendance. 75 However, it may be interesting to test whether or not text messages addressing a particular barrier to attendance may be more effective than a simple reminder text message. This is a cheap, widely applicable intervention that could be instituted, and may be especially effective in young patients (who seem to attend less often than the older age group).
Given that patients from the work conducted to inform the design of the incentive trial could be classified as either ‘risk avoiders’ or ‘risk seekers’, it may have been helpful to include a condition where participants could select when they attend whether or not to receive £10 or a 1 in 100 chance of winning £1000. A choice condition like this was included in a study of mammogram attendance,35 and although this study did not find any significant impact of incentives on screening uptake, this condition did lead to greater attendance in an exploratory analysis of a subgroup who had attended screening most recently. Although it may be unlikely that this would make the incentive significantly better as opposed to significantly worse than a control condition, given the evidence for differences in risk preferences, it may be a valuable option to test in future research on incentives.
Finally, analysis of the demographics of participants revealed that patients that were in our trial population (who are by definition non-attenders) were significantly younger than patients who regularly attend. This may mean that there is merit in work to target younger patients in general to attend screening.
Overall conclusions
In this trial, offering a financial incentive was not effective at promoting attendance at DES in a group of patients who have consistently failed to attend screening over 2 years. Participants receiving an incentive were less likely than control participants to attend screening, especially for the lottery-based incentive group.
Despite being designed using insights from behavioural economics, the incentive interventions were detrimental to screening attendance rates. This suggests that there are many factors that can predict attendance behaviour, which incentives may not address and, furthermore, that incentives may have unexpected effects. The results of the study highlight the importance of testing interventions in context even if they appear to be supported by theory.
Acknowledgements
We would like to thank the NIHR Health Services and Delivery Research programme for funding the IDEAS trial and Imperial College London for supporting the trial. Many thanks go to all the staff from 1st Retinal Screen Ltd for their help in setting up and running the trial, and for managing the database. Of course, we would also like to thank all the IDEAS trial participants.
Contributions of authors
Gaby Judah was responsible for the design of the study, the acquisition of data, analysis of data, interpretation of the data, manuscript writing and approved the final version of the manuscript to be published.
Ara Darzi was joint chief investigator of the study, a grant applicant, responsible for the design of the study and approved the final version of the manuscript to be published.
Ivo Vlaev was a grant applicant, responsible for the design of the study, interpretation of the data, manuscript writing and approved the final version of the manuscript to be published.
Laura Gunn was a grant applicant, performed the statistical analysis, was responsible for the design of the study, the design of the statistical plan, analysis of data, interpretation of the data, manuscript editing and approved the final version of the manuscript to be published.
Derek King was a grant applicant, responsible for the design of the study, the design of the health economics analysis, analysis of data, interpretation of the data, manuscript editing and approved the final version of the manuscript to be published.
Dominic King was a grant applicant, was responsible for the design of the study, interpretation of the data, manuscript editing and approved the final version of the manuscript to be published.
Jonathan Valabhji was a grant applicant, was responsible for the design of the study, interpretation of the data, manuscript editing and approved the final version of the manuscript to be published.
Lisa Bishop was a grant applicant, was responsible for the design of the study and approved the final version of the manuscript to be published.
Adrian Brown was a grant applicant, was responsible for the design of the study and approved the final version of the manuscript to be published.
Grant Duncan was responsible for the design of the study and approved the final version of the manuscript to be published.
Anna Fogg was responsible for the design of the study and approved the final version of the manuscript to be published.
Gemma Harris was a grant applicant, was responsible for the design of the study and approved the final version of the manuscript to be published.
Peter Tyacke was responsible for the design of the study, the acquisition of data, analysis of data, manuscript editing and approved the final version of the manuscript to be published.
Colin Bicknell was joint chief investigator of the study, a grant applicant, was responsible for the design of the study, the acquisition of data, analysis of data, interpretation of the data, manuscript editing and approved the final version of the manuscript to be published.
Publications
Wadge H, Bicknell C, Vlaev I. Perceived ethical acceptability of financial incentives to improve diabetic eye screening attendance. BMJ Open Diab Res Care 2015;3:e000118.
Judah G, Vlaev I, Gunn L, King D, King D, Valabhji J, et al. Incentives in Diabetic Eye Assessment by Screening (IDEAS): study protocol of a three-arm randomised controlled trial using financial incentives to increase screening uptake in London. BMC Ophthalmol 2016;16:28.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137-49. http://dx.doi.org/10.1016/j.diabres.2013.11.002.
- Diabetes.co.uk . Cost of Diabetes 2016. www.diabetes.co.uk/cost-of-diabetes.html (accessed 7 April 2016).
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-22. http://dx.doi.org/10.1016/S0140-6736(10)60484-9.
- Diabetes.co.uk . Signs of Diabetic Retinopathy Found in One in Five Patients 2014. www.diabetes.co.uk/news/2014/nov/signs-of-diabetic-retinopathy-found-in-one-in-five-patients-91423005.html (accessed 7 July 2016).
- Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004015.
- Harris M. The NHS Diabetic Eye Screening Programme: New Common Pathway 2012. www.rcophth.ac.uk/wp-content/uploads/2014/08/Focus-Winter-2012.pdf (accessed 7 July 2016).
- NHS RightCare . NHS Atlas of Variation, Map 21: Percentage of the Diabetic Population Receiving Screening for Diabetic Retinopathy by PCT 2011. http://fingertips.phe.org.uk/profile/atlas-of-variation (accessed 7 July 2016).
- Economics A. Future Sight Loss UK 1: The Economic Impact of Partial Sight and Blindness in the UK Adult Population 2009. www.rnib.org.uk/knowledge-and-research-hub/research-reports/general-research/future-sight-loss-uk-1 (accessed 7 July 2016).
- NHS Choices . Diabetic Eye Screening 2013. http://diabeticeye.screening.nhs.uk/statistics (accessed 7 July 2016).
- Scanlon PH, Carter SC, Foy C, Husband RF, Abbas J, Bachmann MO. Diabetic retinopathy and socioeconomic deprivation in Gloucestershire. J Med Screen 2008;15:118-21. http://dx.doi.org/10.1258/jms.2008.008013.
- Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull 2006;132:249-68. https://doi.org/10.1037/0033-2909.132.2.249.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-42.
- Dolan P, Hallsworth M, Halpern D, King D, Metcalfe R, Vlaev I. Influencing behaviour: the mindspace way. J Econ Psychol 2012;33:264-77. https://doi.org/10.1016/j.joep.2011.10.009.
- Oliver A. Can financial incentives improve health equity?. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3847.
- Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care?. Ann Intern Med 2006;145:265-72. https://doi.org/10.7326/0003-4819-145-4-200608150-00006.
- Giuffrida A, Torgerson DJ. Should we pay the patient? Review of financial incentives to enhance patient compliance. BMJ 1997;315:703-7. https://doi.org/10.1136/bmj.315.7110.703.
- Sutherland K, Christianson JB, Leatherman S. Impact of targeted financial incentives on personal health behavior: a review of the literature. Med Care Res Rev 2008;65:36-78. http://dx.doi.org/10.1177/1077558708324235.
- Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.CD004307.pub3.
- Paul-Ebhohimhen V, Avenell A. Systematic review of the use of financial incentives in treatments for obesity and overweight. Obes Rev 2008;9:355-67. https://doi.org/10.1111/j.1467-789X.2007.00409.x.
- Sindelar JL. Paying for performance: the power of incentives over habits. Health Econ 2008;17:449-51. http://dx.doi.org/10.1002/hec.1350.
- Marteau TM, Ashcroft RE, Oliver A. Using financial incentives to achieve healthy behaviour. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b1415.
- Citizens Council Meeting: The Use of Incentives to Improve Health. NICE; 2010.
- Mantzari E, Vogt F, Shemilt I, Wei Y, Higgins JP, Marteau TM. Personal financial incentives for changing habitual health-related behaviors: a systematic review and meta-analysis. Prev Med 2015;75:75-8. http://dx.doi.org/10.1016/j.ypmed.2015.03.001.
- Giles EL, Robalino S, McColl E, Sniehotta FF, Adams J. The effectiveness of financial incentives for health behaviour change: systematic review and meta-analysis. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0090347.
- Zenner D, Molinar D, Nichols T, Riha J, Macintosh M, Nardone A. Should young people be paid for getting tested? A national comparative study to evaluate patient financial incentives for chlamydia screening. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-261.
- Dietrich AJ, Tobin JN, Cassells A, Robinson CM, Reh M, Romero KA, et al. Translation of an efficacious cancer-screening intervention to women enrolled in a Medicaid managed care organization. Ann Fam Med 2007;5:320-7. https://doi.org/10.1370/afm.701.
- Fronstin P, Roebuck MC. Financial incentives, workplace wellness program participation, and utilization of health care services and spending. EBRI Issue Brief 2015;417:1-23.
- Glidewell L, West R, Hackett JE, Carder P, Doran T, Foy R. Does a local financial incentive scheme reduce inequalities in the delivery of clinical care in a socially deprived community? A longitudinal data analysis. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0279-9.
- Honein-AbouHaidar GN, Rabeneck L, Paszat LF, Sutradhar R, Tinmouth J, Baxter NN. Evaluating the impact of public health initiatives on trends in fecal occult blood test participation in Ontario. BMC Cancer 2014;14. http://dx.doi.org/10.1186/1471-2407-14-537.
- Scott A, Sivey P, Ait Ouakrim D, Willenberg L, Naccarella L, Furler J, et al. The effect of financial incentives on the quality of health care provided by primary care physicians. Cochrane Database Syst Rev 2011;9. http://dx.doi.org/10.1002/14651858.CD008451.pub2.
- Claxton G, Rae M, Panchal N, Whitmore H, Damico A, Kenward K, et al. Health benefits In 2015: stable trends in the employer market. Health Aff 2015;34:1779-88. http://dx.doi.org/10.1377/hlthaff.2015.0885.
- Niza C, Rudisill C, Dolan P. Vouchers versus lotteries: what works best in promoting chlamydia screening? A cluster randomised controlled trial. Appl Econ Perspect Policy 2014;36:109-24. http://dx.doi.org/10.1093/aepp/ppt033.
- Dolan P, Rudisill C. The effect of financial incentives on chlamydia testing rates: evidence from a randomized experiment. Soc Sci Med 2014;105:140-8. http://dx.doi.org/10.1016/j.socscimed.2013.11.018.
- Currie MJ, Schmidt M, Davis BK, Baynes AM, O’Keefe EJ, Bavinton TP, et al. ‘Show me the money’: financial incentives increase chlamydia screening rates among tertiary students: a pilot study. Sex Health 2010;7:60-5. http://dx.doi.org/10.1071/SH08091.
- Merrick EL, Hodgkin D, Horgan CM, Lorenz LS, Panas L, Ritter GA, et al. Testing novel patient financial incentives to increase breast cancer screening. Am J Manag Care 2015;21:771-9.
- Skaer TL, Robison LM, Sclar DA, Harding GH. Financial incentive and the use of mammography among Hispanic migrants to the United States. Health Care Women Int 1996;17:281-91. http://dx.doi.org/10.1080/07399339609516245.
- Debari R, Servodidio C, Palomares M, Rodriguez-Furlow M, Staff I. Patient compliance/incentive study. Oncol Nurs Forum n.d.
- White MC, Tulsky JP, Reilly P, McIntosh HW, Hoynes TM, Goldenson J. A clinical trial of a financial incentive to go to the tuberculosis clinic for isoniazid after release from jail. Int J Tuberc Lung Dis 1998;2:506-12.
- Brassard P, Bruneau J, Schwartzman K, Sénécal M, Menzies D. Yield of tuberculin screening among injection drug users. Int J Tuberc Lung Dis 2004;8:988-93.
- FitzGerald JM, Patrick DM, Strathdee S, Rekart M, Elwood RK, Schecter MT, et al. Use of incentives to increase compliance for TB screening in a population of intravenous drug users. Vancouver Injection Drug Use Study Group. Int J Tuberc Lung Dis 1999;3:153-5.
- Malotte CK, Rhodes F, Mais KE. Tuberculosis screening and compliance with return for skin test reading among active drug users. Am J Public Health 1998;88:792-6. https://doi.org/10.2105/AJPH.88.5.792.
- Malotte CK, Hollingshead JR, Rhodes F. Monetary versus nonmonetary incentives for TB skin test reading among drug users. Am J Prev Med 1999;16:182-8. https://doi.org/10.1016/S0749-3797(98)00093-2.
- Mehrotra A, An R, Patel DN, Sturm R. Impact of a patient incentive program on receipt of preventive care. Am J Manag Care 2014;20:494-501.
- Hatef E, Vanderver BG, Fagan P, Albert M, Alexander M. Annual diabetic eye examinations in a managed care Medicaid population. Am J Manag Care 2015;21:e297-302.
- Austin S, Wolfe BL. The effect of patient reminders and gas station gift cards on patient adherence to testing guidelines for diabetes. WMJ 2011;110:132-7.
- Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, Grimshaw JM, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med 2002;136:641-51. https://doi.org/10.7326/0003-4819-136-9-200205070-00006.
- Walter U, Krauth C, Wienold M, Dreier M, Bantel S, Droste S. Interventions for increasing uptake in screening programmes. GMS Health Technol Assess 2006;2.
- Frey BS, Oberholzer-Gee F. The cost of price incentives: an empirical analysis of motivation crowding-out. Am Econ Rev 1997;87:746-55.
- Forde I, Zeuner D. Financial incentives to promote social mobility. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3219.
- Cookson R. Should disadvantaged people be paid to take care of their health? Yes. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a589.
- Giles EL, Robalino S, Sniehotta FF, Adams J, McColl E. Acceptability of financial incentives for encouraging uptake of healthy behaviours: A critical review using systematic methods. Prev Med 2015;73:145-58. http://dx.doi.org/10.1016/j.ypmed.2014.12.029.
- Wadge H, Bicknell C, Vlaev I. Perceived ethical acceptability of financial incentives to improve diabetic eye screening attendance. BMJ Open Diabetes Res Care 2015;3. http://dx.doi.org/10.1136/bmjdrc-2015-000118.
- Sunstein C, Thaler R. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2012.
- Kahneman D, Tversky A. Choices, Values, and Frames. Cambridge: Cambridge University Press; 2000.
- Thornton RL. The demand for, and impact of, learning HIV status. Am Econ Rev 2008;98:1829-63. http://dx.doi.org/10.1257/aer.98.5.1829.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica 1979;47:263-91. https://doi.org/10.2307/1914185.
- Kimmel SE, Troxel AB, Loewenstein G, Brensinger CM, Jaskowiak J, Doshi JA, et al. Randomized trial of lottery-based incentives to improve warfarin adherence. Am Heart J 2012;164:268-74. http://dx.doi.org/10.1016/j.ahj.2012.05.005.
- Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA 2008;300:2631-7. http://dx.doi.org/10.1001/jama.2008.804.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Judah G, Vlaev I, Gunn L, King D, King D, Valabhji J, et al. Incentives in Diabetic Eye Assessment by Screening (IDEAS): study protocol of a three-arm randomized controlled trial using financial incentives to increase screening uptake in London. BMC Ophthalmol 2016;16. http://dx.doi.org/10.1186/s12886-016-0206-4.
- Volpp KG, Asch DA, Galvin R, Loewenstein G. Redesigning employee health incentives – lessons from behavioral economics. N Engl J Med 2011;365:388-90. http://dx.doi.org/10.1056/NEJMp1105966.
- Vlaev I, Chater N, Stewart N, Brown GD. Does the brain calculate value?. Trends Cogn Sci 2011;15:546-54. http://dx.doi.org/10.1016/j.tics.2011.09.008.
- Measuring Multiple Deprivation at the Small Area Level: The Indices of Deprivation 2000. London: DETR; 2000.
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med 2009;360:699-70. http://dx.doi.org/10.1056/NEJMsa0806819.
- Machin D, Campbell MJ, Tan S-B, Tan S-H. Sample Size Tables for Clinical Studies. Chichester: John Wiley & Sons; 2011.
- Wittes J, Balakrishnan N. Methods and Applications of Statistics in Clinical Trials, Volume 1. Hoboken, NJ: Wiley; 2014.
- King G, Zeng L. Logistic regression in rare events data. Polit Anal 2001;9:137-63. https://doi.org/10.1093/oxfordjournals.pan.a004868.
- Drummond MF, McGuire A. Economic Evaluation in Health Care: Merging Theory with Practice. USA: Oxford University Press; 2001.
- Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull 1999;125:627-68. https://doi.org/10.1037/0033-2909.125.6.627.
- Gneezy U, Meier S, Rey-Biel P. When and why incentives (don’t) work to modify behavior. The Journal Econ Perspect 2011;25:191-209. https://doi.org/10.1257/jep.25.4.191.
- Festinger L, Carlsmith JM. Cognitive consequences of forced compliance. J Abnorm Psychol 1959;58:203-10. https://doi.org/10.1037/h0041593.
- Ariely D, Loewenstein G, Prelec D. Tom Sawyer and the construction of value. Journal Econ Behav Organ 2006;60:1-10. https://doi.org/10.1016/j.jebo.2004.10.003.
- Joint Strategic Needs Assessment . JSNAs for Westminster, Kensington & Chelsea and Hammersmith &Amp; Fulham 2015. www.jsna.info/local-health-profiles (accessed 26 April 2016).
- Office for National Statistics . Census 2011 2001. www.ons.gov.uk/census/2011census (accessed 7 July 2016).
- Hallsworth M, Berry D, Sanders M, Sallis A, King D, Vlaev I, et al. Stating appointment costs in SMS reminders reduces missed hospital appointments: findings from two randomised controlled trials. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0137306.
Appendix 1 Letter to control participants
Appendix 2 Letter to fixed incentive participants
Appendix 3 Letter to lottery incentive participants
Appendix 4 Proposed text of letter to trial participants sent to coincide with publication of the study
Dear Eye Screening patients,
We are writing to inform you that you were included in some research into ways to increase attendance at Diabetic Eye Screening appointments. This project was called IDEAS: Incentives in Diabetic Eye Assessment by Screening. You were automatically included as a patient under the care of 1st Retinal Screen Ltd.
Why was the research done?
Everyone with diabetes is at risk of damage to his or her sight, due to a condition called diabetic retinopathy. If retinopathy is found early, it can be treated more easily, therefore, people with diabetes are invited every year to diabetic eye screening. However, only 81% of people who are invited actually attend, which puts some people at risk of unnecessary sight loss. Financial incentives have been shown to support people in taking on other healthy behaviours. Therefore, this research was testing whether financial incentives would be an effective way to encourage more people to attend their diabetic eye screening.
How was the research conducted?
People who have not attended their eye screening for two years or more were automatically included in the research. The research was testing the effect of two different types of financial incentives. Some people were offered £10 if they attended their screening appointment, and some people were offered a 1 in 100 chance of winning £1000. There was also a comparison group, who were sent the normal appointment invitation letter. The research was comparing the numbers of people in each group who came to their screening appointment.
What did the research show?
We found that 8% of people who received the normal letter came to their screening appointment. However, only 5% people attended from the £10 group, and just 3% of people from the £1000 lottery group.
As fewer people came to screening if they were offered an incentive compared to people who just received the normal letter, this shows that incentives did not improve screening attendance, and that incentives may actually make people less likely to come to their appointment.
Who conducted the research?
The study was conducted by researchers at Imperial College London.
The IDEAS project was funded by the National Institute for Health Research’s Health Services and Delivery Research Programme.
The research and you
As a participant in the research, you would have been randomly assigned to one of the three groups (offered £10, offered 1 in 100 chance of winning £1000, or sent the normal invitation letter). If you were offered an incentive, it is important to know that as this was just research, you will not be offered the incentive again in future. It is still important that you go to your eye screening appointment when invited.
No personal information was shared with anyone outside the screening service (1st Retinal Screen Ltd). All data that were analysed were completely confidential, and securely stored. It would not have been possible to identify you from any of the data.
If you have any questions about the research, please contact Colin.Bicknell@imperial.ac.uk.
Kind regards
1st Retinal Screen Ltd
This project, the Incentives in Diabetic Eye Assessment by Screening (IDEAS) Trial was funded by the National Institute for Health Research’s Health Services and Delivery Research Programme
List of abbreviations
- CEA
- cost-effectiveness analysis
- CI
- confidence interval
- DES
- diabetic eye screening
- DNA
- did not attend
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HIV
- human immunodeficiency virus
- IDEAS
- Incentives in Diabetic Eye Assessment by Screening
- IMD
- Index of Multiple Deprivation
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- MD
- mean difference
- NIHR
- National Institute for Health Research
- REC
- Research Ethics Committee
- RR
- relative risk
- SD
- standard deviation
- TB
- tuberculosis
- TMT
- Trial Management Team