Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5002/20. The contractual start date was in September 2013. The final report began editorial review in June 2016 and was accepted for publication in January 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
John Powell works part-time (0.5 whole-time equivalent) for the National Institute for Health and Care Excellence as a Consultant Clinical Adviser in the Centre for Health Technology Evaluation. John Powell is a member of the National Institute for Health Research Health Technology Assessment and Efficacy and Mechanism Evaluation Editorial Board and was previously a member of the NIHR Journals Library Editorial Group. David Sharp was a NHS employee at the beginning of the project, and the NHS is an organisation that funds and uses research. David Sharp currently works at Optum Healthcare, an organisation that sells commercial services to the NHS.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Swan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Relevant literature and research context
In this chapter, we provide an overview of the National Institute for Health Research (NIHR) brief, as well as the literature that can be drawn on to address this brief. We will consider the research needs of the NHS in the light of the knowledge mobilisation literature, which offers a strong theoretical foundation for this type of research. We should note that the aim of this project was not to provide a systematic literature review: several reviews have been conducted previously in this domain. Rather, to identify relevant literature, we began by consulting previous NIHR final reports and scholarly works focused on evidence use in commissioning practice. 1,2 As these were rather few, we then turned to reviews of evidence use in health-care management and, in particular, to recent studies of evidence use in practice. 3–11 We also accessed the literature reviewed, more broadly, by leading scholars in the field in our work in editing our own book on knowledge mobilisation in health care. 12 We combined these sources with other specific works, as relevant to particular topics of interest (e.g. research in clinical commissioning, innovation capabilities and so forth). Given the current gap in knowledge on evidence use in commissioning, we address questions about what counts as evidence, when it counts, how it is used and how it is created in order to develop a conceptual framework for the study.
Introduction to the research
Sustained and successful innovation in health-care services, driven by the best available evidence, is critical to the survival of England’s NHS. 5,13 A major provider of authoritative evidence is the National Institute for Health and Care Excellence (NICE). NICE synthesises and publishes authoritative evidence that is widely considered to be ‘gold standard’. New legislation places NICE at the heart of reforms in health and social care to ensure that patients receive high-quality and cost-effective care. 14 At the same time, more stringent measures to accelerate the uptake and implementation of NICE recommendations are being proposed. Expectations are higher than ever that this evidence will drive innovation as well as lower the costs of service delivery in the NHS.
Much of the responsibility for innovation in the NHS now lies with Clinical Commissioning Groups (CCGs), which are tasked with the design and procurement of health-care services. Multiple evidence-based products are available to commissioners in making informed decisions, including NICE guidance (e.g. guidelines) and quality standards and metrics [e.g. Quality and Outcomes Framework (QOF), CCG Outcomes Indicator Set].
However, fragmentation and variation in the uptake of evidence in health-care decision-making is widely noted. 2,3,15 It is clear that the (abundant) supply of evidence-based products does not always connect to the needs of health-care decision-makers. 1,16 This is especially so when dealing with the kinds of complex, time-dependent and political decision situations faced by health-care managers. 16,17 Walshe and Rundall17 aptly note that the:
constrained, contested, and political nature of many managerial decisions, [make] it difficult for managers to apply research evidence even when it is available.
p. 44517
Thus, a major challenge for NHS commissioning groups is to proactively and strategically consider how they can be better equipped to take hold of, and use, evidence in decisions. A key challenge then, lies in ‘building high learning capacity and appropriate core competencies in NHS organisations, rather than relying on a technological fix’. 5
The imperative for NHS organisations to innovate sits alongside the need to make savings, often by adept commissioning. These objectives can be met only by improving existing services. The need for commissioning managers to use sound evidence is, therefore, greater than ever. 1 In the light of this, organisational capabilities to use evidence in their pursuit of service redesign are especially important and need to be carefully nurtured. Our study, thus, focuses on capabilities to use evidence in service redesign initiatives led by commissioning groups. It seeks to provide an important practical resource for the frontline managers and the clinical leaders of commissioning organisations who will be responsible for making some tough decisions over the coming years. The focus of our work, then, is on improving NHS commissioning organisations’ capabilities to use evidence in decision-making.
Aims and objectives
As stated in our original protocol, the aims of the research were to identify the capabilities (organisational and managerial) that NHS CCGs need to become better users of authoritative evidence. Given our focus on evidence use among social groups in organised settings, we take a knowledge-based view of organisations. 18 As Grant19 comments:
. . . organizational capability, defined as a firm’s ability to perform repeatedly a productive task which relates either directly or indirectly to a firm’s capacity for creating value through effecting the transformation of inputs into outputs.
With this in mind, the objectives of our research were to:
-
Study how authoritative evidence travels into, and is used by, different CCGs (by documenting ‘evidence journeys’).
-
Compare and contrast the travel of evidence across CCGs to understand how and why variations in evidence use occur in practice.
-
Create a toolkit to guide CCGs to better use evidence. Specifically, the tool will help CCGs to assess and improve their capabilities to use evidence. It will be built on process models of how evidence travels in the real world, rather than on abstract accounts.
Our aims and objectives have not changed, but it was apparent from our initial literature search and fieldwork that to understand authoritative evidence use we also needed to consider how commissioners use other forms of evidence too. We had also intended to examine ‘disinvestment’ and ‘investment’ decisions, but it became apparent that, in reality, these are two sides of the same coin. That is, commissioners’ decisions about what not to do occur only when deciding what to do in service design. Hence, the two cannot be practically disentangled when looking at the ways evidence travels in practice, at least in this context.
In the following sections, we outline how our research contributes to the NIHR brief and describe the commissioning landscape as our research context. We then outline our theoretical approach, drawing from ‘knowledge mobilisation’ and evidence-based management (EBMgmt) health-care literatures. 20 Although much can be learned about the ways in which evidence is used in the NHS from these literatures, we conclude that further work is needed to articulate how organisations can improve their capabilities to use evidence more effectively. 1
Response to the National Institute for Health Research Health Services and Delivery Research commissioning brief
This study was funded in response to the NIHR Health Services and Delivery Research (HSDR) programme’s call for ‘research to improve knowledge transfer and innovation in healthcare delivery and organisation’ (HSDR call number 12/5002). The call builds on the Cooksey report21 particularly the observed ‘T2 gap’ between the research output and transfer into practice. The call emphasised research that generates actionable findings to help the NHS make better use of evidence. The three main themes were:
-
research on ‘pull’ by managers (increasing the receptivity and research/innovation capacity of organisations and individual managers)
-
research on ‘push’ by researchers (making the research product/innovation more usable)
-
research on knowledge linkage and exchange (bringing service decision-makers and researchers/industry together in different ways).
The NIHR call focused specifically on the evidence used by organisations and managers to inform decisions about services, rather than on evidence informing clinical interactions, into which there had already been substantial research. 22 Notably, the call sought to build on previous work on ‘knowledge translation’, for example by the Canadian Institute of Health Research, to improve the translational work of NHS management and organisations. The call was premised on the idea that knowledge translation includes a diverse array of activities and should be understood as highly complex, not only in technical, but also in social, organisational and political terms. 6
Research on ‘pull’ by managers
Our study responds directly to the first theme in the NIHR brief for research on evidence ‘pull’. Specifically, we sought to ‘assess the capacity in healthcare organisations to accelerate innovation’ (p. 4 of the brief) by identifying capabilities for evidence use in commissioning decisions. This aim followed directly from earlier NIHR-funded research, including our own, showing how knowledge and evidence was used by commissioning managers in primary care trusts (PCTs) (Service Delivery and Organisation reference 08/1808/244)3 and by NHS chief executives (Service Delivery and Organisation reference 09/1002/36). 5 Findings of those studies indicated the importance of mobilising different kinds of evidence and local knowledge in a timely manner, managing interfaces across project stages, proactively building and managing relationships to create coalitions and building personal knowledge. 2
Informed by previous work, we use the metaphor of the ‘evidence journey’ in our study to capture our emphasis on the flow of evidence into and through practices of commissioning and the capabilities that influence this flow. We intend to:
-
identify key capabilities to use evidence
-
produce a process-based model (roadmap) aimed at improving capabilities by showing the route by which evidence becomes effective in decision-making
-
develop a ‘toolkit’ as a resource for CCGs to assess their evidence-use capabilities.
Research on ‘push’ by knowledge producers
A secondary contribution of our study relates to the theme on evidence ‘push’ by knowledge producers. We also consider the ways in which guidance is produced and communicated, and how this shapes its mobilisation and usage in context. This part of our research begins to explain, albeit in a preliminary way, how the properties of evidence and expectations about the user inscribed in the evidence matter to the way it travels in commissioning organisations.
Research context: the shifting commissioning landscape (2013–16)
Our study began in October 2013, just 6 months after CCGs took over from PCTs. CCGs are part of the government’s attempt to improve health services by bringing the commissioning closer to the community. CCGs differ from PCTs in size and composition. CCGs are much larger and are led by general practitioners (GPs), whereas health-care managers ran PCTs. Commissioning GPs must work alongside managers, consultants, nurses, patients and other experts to manage resources. These groups work together to plan, contract, monitor and transform health care. 23 NHS reforms have sought to create commissioning supported (but not led by) external commercial and not-for-profit providers, changing the relationship between managers and clinicians. Evidence use in commissioning has no doubt been influenced by new modes of working between CCGs and external providers. 1 This shift means there is a need to identify opportunities for learning with regard to developing and sustaining capabilities to use evidence in commissioning.
At last count, 211 CCGs had responsibility for about two-thirds of the NHS budget between them. CCGs can commission any services as long as they meet NHS standards and local needs. Importantly, the Health and Social Care Act 201224 compels CCGs to use evidence to assure the quality of commissioned services. Such evidence is, for the most part, produced by NICE in the form of ‘authoritative guidance, standards, and information’. 25 NICE guidance is considered important so that patient experiences are improved and costs are reduced. 2 However, as above, the uptake of NICE guidance and products has been patchy in commissioning, and recent NIHR research suggests that this shows no sign of changing soon. 1
A key difference around evidence use from these reforms is that some support functions that were formerly ‘internal’ are now ‘external’. 1 Data production, management and analysis is now the remit of commissioning support units (CSUs), whereas PCTs often had dedicated information support managers. These ‘external’ organisations want to support commissioners to make better informed choices. However, a recent qualitative study suggests that this is proving extremely problematic. 1 Wye et al. 1 conclude that:
One consequence of . . . competing organisations . . . was to curtail freely exchanged knowledge transfer . . . organisational boundaries not only frustrated knowledge exchange but also established substantial barriers to the NHS clients’ scope for strengthening commissioning skills . . .
p. xxvii1
This suggests that, although commissioning decisions are now taken by GPs at ‘the coal face’ of service delivery, the ways in which evidence reaches decision-making in commissioning is just as (if not more) varied now as it was in the past.
Theoretical approach: knowledge mobilisation
The so-called ‘gap’ between creating knowledge and using it in practice remains a persistent problem. 23 Policy initiatives aimed at closing the gap include Academic Health Sciences Centres, Biomedical Research Centres and Units, Genetics Knowledge Parks and Collaborations for Leadership in Applied Health Research and Care (CLAHRCs), and new programmes of research (such as this one). NICE was renamed in 2013, following the Health and Social Care Act, in part to emphasis its role in closing the gap. Such initiatives are reinforced by the social and economic costs of not using evidence. 25
However, outcomes have been mixed,5 and it seems that the underpinning assumptions about knowledge may be, in part at least, to blame. 20 Problems still tend to be framed in terms of transferring knowledge in a linear sequence from producer to user. 21 This view treats knowledge as objective facts (e.g. ‘best evidence’) established by scientists and scientific methods, synthesised and captured (e.g. in NICE guidelines) and transferred to users who will pick it up and use it. The limitations of this transfer view as a means of understanding evidence in health care have been well rehearsed (e.g. see Swan et al. 12 for details). While we do not wish to rehash these here, most converge on the observation that health systems often fail to optimally use the substantive body of research evidence available to them. 26
First, linear models depict decision-making as rational process, whereby knowledge travels as objective ‘facts’ from producers to users who rationally decide based on the best information. This objectifies knowledge and divorces it from context and so greatly underestimates its fundamentally social nature and its basis in practice. As critics note, ‘knowing’ is inextricably tied to ‘doing’, and it is divisions of practice (e.g. professional, occupational and organisational) that create divisions of knowledge, and power, in organisations. 15,27,28
Second, health-care settings comprise multiple professional groups, partaking in different practices, with naturally different interests. Experts (e.g. GPs, consultants, nurses and patients) usually seek to use evidence that fits their own ‘epistemic stances’, frames and values. This means that what counts as ‘best’ knowledge and evidence is often contested. 6
Third, the transfer view sees knowledge as an object (e.g. a piece of evidence), divorces it from its context of production and its use (e.g. how things become evidential), and ultimately sees evidence users as merely ‘information sponges’. 29 The knowledge transfer view fails to adequately address the point that mobilising knowledge across boundaries created by different kinds of practice is always a political process. 5 As Crilly et al. 4 note:
The importance of power contests among occupational groups in health systems makes it appropriate to temper positivistic and purely technical approaches to knowledge management with scepticism.
p. 74
Despite these criticisms, the idea of ‘knowledge transfer’ remains pervasive among policy, practitioner and academic groups, with many still attempting to resolve the ‘gap’ between evidence and practice. Indeed, the very notion of a ‘gap’ separates the production and supply of knowledge (or push) from its use (or pull), and places the root of the problem in the hands of ‘users’ who fail to ‘take up’ evidence. The premise is that, if we communicate ‘good’ evidence well, the ‘user problem’ will be resolved. Even the well-informed NIHR programme brief that funded our study called for ‘push’ and ‘pull’ research. This view also predominates the evidence-based medicine (EBM) movement (with its emphasis on ‘the hierarchy of evidence’) and has also been incorporated into streams of research on EBMgmt and implementation science. 1,30 Recently, Greenhalgh et al. 30 argued for a return to ‘real’ evidence-based decision-making, whereby ‘those who produce and summarise research evidence must attend more closely to the needs of those who might use it’. 30
Along with others, we seek to go beyond linear views, which we see as particularly ill-suited to understanding managerial work in health care. 31 When problems are multifaceted, complex and ambiguous, relevant knowledge is not produced in a linear way but, instead, is co-produced through interactions among participating actors. 2 For example, CCGs must account for a multitude of considerations when deciding how to design a service, including quality of care, cost of service delivery and capacity to deliver. Such decisions invoke multiple forms of knowledge and expertise: science alone is not the way to effective decision-making. 31 As Wright et al. 16 put it:
Situated expertise – which is underpinned, in part, by personal experience and judgment – is needed in the handling, adaptation and communication of this evidence . . . evidence does not speak for itself, and neither does it allow decision processes to be enacted without context-sensitive judgement.
p. 17516
We adopt, then, a ‘knowledge mobilisation’ approach, embracing situated and social processes. 20 We pay attention to how evidence is circulated, transformed and arrived at by decision-makers. We define knowledge mobilisation as ‘a proactive process that involves efforts to transform practice through the circulation of knowledge within and across domains’. 12 We share with others the view that evidence is not only passed around as objective facts, but that things become evidential as a result of practice. 9 This approach is underpinned by the following theoretical premises adapted from Swan et al. :12
-
Knowledge is social and context specific; evidence exists in midlines, communities of practice and other collectives that sustain, legitimise and transform it.
-
Knowledge claims depend on people’s epistemic and interpretive frames; what counts as evidence may be contested across professional and occupational groups.
-
At any point certain actors or collectives purposefully pursue knowledge mobilisation; understanding this is, therefore, fundamental to understanding evidence use.
-
Agents produce knowledge across multiple contexts, or ‘domains of action’; how evidence is used in one context (e.g. in the practices of CCGs) is nested within, and shaped by, what happens in another (e.g. in the practices of NICE).
-
Knowledge mobilisation results from complex interactions under specific conditions (often referred to as institutional context); context matters to evidence use.
-
Social relationships and networks (formal and informal) matter to the mobilisation of knowledge; who brokers evidence is important to its use. 32
-
Politics, legitimacy and interests inform knowledge mobilisation; evidence does not speaks for itself, and what counts as evidence may change over time. 3,5
-
Knowledge does not travel untouched through social interactions; evidence is modified at the point of decision-making.
-
Knowledge mobilisation is a transformation process; the same evidence (e.g. NICE guidelines) can be used differently in different organisational contexts. 33
The consequence of adopting this approach goes beyond mere theoretical premises, however; it has concrete implications for the way the ‘gap’ between research and practice is dealt with. In brief, knowledge transfer deals with the robustness and diffusion of evidence, while knowledge mobilisation considers social practice differences that cause different ways of knowing and doing. 12 In knowledge transfer, the gap is closed by creating more research and knowledge in a more timely fashion, upskilling stakeholders to use this evidence and encouraging research cultures in organisations. Conversely, knowledge mobilisation addresses the gap by facilitating and leveraging social processes to circulate knowledge, connecting across boundaries, understanding the role of artefacts and recognising the realities of context.
Knowledge mobilisation and evidence-based management
Evidence-based management literature gained importance in the mid-2000s, having stemmed from the EBM movement. 3 Given its origins, it is unsurprising that the EBMgmt has become particularly salient in health care. Debates about EBMgmt relate to definition and, indeed, whether or not there is good evidence for EBMgmt. 7,34 Some take a rationalistic approach, seeing EBMgmt, similar to its forerunner EBM, as a practice of ‘using scientific knowledge to inform the judgment of managers and the process of decision-making in organisations’. 35 The underlying premises are more akin to a knowledge transfer model, and the key challenge is to ensure the uptake of best scientific knowledge by managers to optimise the rationality of the choices they make. This traditional approach to EBMgmt is criticised for:
-
privileging scientific facts as the objective ‘truth’, which fails to account for the contested nature of knowledge16,31,36
-
underestimating the complexity of management (vis-à-vis professional disciplines such as medicine) and the incompleteness of scientific evidence alone in arriving at management decisions in politically-charged situations17,37
-
lacking support from empirical research on evidence use, which notes the importance of social context in determining what forms of knowledge and information become ‘evidential’, and when, in decision-making2,38
-
losing the importance of situated expertise and judgement of the decision-maker. 10,16,39
Others take a view akin to knowledge mobilisation, recognising that ‘organisational realities seldom map unproblematically onto their idealised “evidence-based” representations’. 33 Furthermore, case study research on evidence use in health-care management suggests that, although practitioners often value scientific evidence [such as NICE guidelines and randomised controlled trial (RCT) data], the way in which they actually use evidence in practice is very different. 32 The complexity of management decisions and organisational context means, also, that the outcomes of evidence use are hard to predict. 40 Walshe and Rundall17 highlight that EBMgmt is challenged by the ‘constrained, contested, and political nature of many managerial decisions, it may be difficult for managers to apply research evidence even when it is available’. 17 These studies reveal that decision-makers, via co-production, mobilise evidence with others. 2 Arndt and Bigelow thus see evidence as ‘an artefact of the social processes that lead to its creation’. 40 That is, things become evidential during, not prior to, decision-making. As Crilly et al. 4 put it:
The original model of strict hierarchies of evidence within clinical practice, transmitted in a linear and rational manner, has been supplanted by a more human and interactive model.
p. 384
This stream of research tells us quite a lot about what types of evidence are likely to be used, and also a bit about when they are likely to be used, in commissioning decisions.
What counts as evidence?
Traditionally, EBM was seen as a means to ensure effective clinical interactions between health-care professionals, patients, families and carers. 41 Decisions were to be based on authoritative, universally applicable evidence from scientific research, including RCTs and evaluation studies. 42 The internal validity associated with scientific research was seen to justify causal links between evidence and clinical behaviour. 43,44 Indeed, evidence champions argued that ‘rational and systematic application of science [brings] about effective, efficient, and accountable practice’. 45 NICE has major responsibility for collating and disseminating this type of evidence in the UK. 13 It is now expected that this type of evidence will be used to ‘organise, structure, deliver, and finance’41 health-care service provision, too.
Although authoritative evidence is still emphasised (e.g. in the ‘hierarchy of evidence’), the role of other evidences is also recognised. In one study, Gabbay and Le May22 showed that primary care clinicians rarely accessed or used explicit research-based evidence but relied on ‘mindlines’, which were informed by colleagues’ experiences and their interactions with each other and with the knowledge, mostly tacit, of opinion leaders, patients and other experts. Indeed, it has been said that there is ‘little compelling support that scientific evidence is treated differently to other types of information’. 43 As Crilly et al. note, ‘even in the medical arena, which draws on experimental, replicable and ostensibly generalisable knowledge, the notion of a hierarchical evidence is contentious’. 4 These findings are also echoed in more recent studies of evidence use commissioning. Wye et al. 1 conducted a study (parallel to ours) of knowledge exchange between commissioners and external providers. They found that commissioners sought information from many sources to build a case for action, defend their position and navigate their proposals through the system. Information was clustered into four primary types:
-
‘people-based’ sources (relationships, ‘whole-picture’ views, experience, contracting and procurement expertise and information on finance, budgets and performance)
-
‘organisation-based’ sources (NICE guidelines, Department of Health information such as National Outcome Frameworks and information from NHS Improving Quality, Public Health, CSUs, ‘think tanks’, such as The King’s Fund, Royal Colleges and other providers)
-
‘tool-based’ sources (electronic software tools, national benchmarking, national and local dashboards, and project management tools)
-
‘research-based’ sources (journals, search engines, universities, Cochrane reviews, regional networks, such as CLAHRCs, Academic Health Science Networks and electronic newsletters).
Wye et al. 1 note, however, that access to data in their study was obtained mainly through external providers; given the timing (in the transition period from PCTs to CCGs), commissioning organisations proved more difficult to enlist. This might have provided a somewhat broader picture of sources of evidence (especially authoritative sources) than those actually used by commissioners. 1 Even so, the study suggested that, for clinical commissioning, local clinical knowledge from GPs about service provision was prioritised: ‘Local information often trumped generalised research-based knowledge or information from other localities’. 1 Most often, this evidence was acquired interpersonally through ‘conversations and stories, as oral methods were fast and flexible, which suited the changing world of commissioning’. 1
Our previous study used a more direct, detailed ethnography of commissioners’ evidence use in redesigning services. 2 Case studies of four PCTs showed the need to source information creatively so that it was fit for purpose. Evidence use entailed a ‘heterarchy’ rather than a hierarchy of evidence, with local and interpersonal practical knowledge and information being coupled with other scientific sources. Evidence did not ‘speak itself’ but needed to be advocated by experts or authorities. Interestingly, a wider sample of commissioning managers suggested that ‘the single most important source of evidence was examples of best practice from other organisations’. 2 Harvey et al. 46 also describe different kinds of evidence, particularly ‘theoretical’ (how and why something works) ‘empirical’ (efficacy) and ‘experiential’ (personal experiences) evidence. They note that the ‘. . . complexity and context-dependent nature of implementation [makes it] impractical to prioritise one type . . . over others’. 46 This work raises questions about what counts as evidence in CCG decision-making, and so this will be an initial focus of our study. 46
This literature suggests that, in addition to NICE, commissioning stakeholders may mobilise a range of other evidences in their decision-making. Thus, we respond to calls to ‘broaden and deepen our understanding of what counts as “evidence” and which types of evidence are best used to inform differing aspects of clinical decision making’. 47
When is evidence used?
To better understand when evidence is used, we draw from studies of innovation processes that recognise complex problems and developing new practical means of dealing with them. 48 Rogers’ very widely cited work refers to three broad stages of innovation: ‘initiation’, ‘adoption/decision’ and ‘implementation’. 49 Responding to criticism that ‘stages’ are an overly linear and rationalised depiction of innovation processes, Clark et al. 50 developed, instead, their ‘Decision Episode Framework’. This suggests that innovation processes unfold in a series of recursive and overlapping ‘decision episodes’. 50,51 These entail the awareness of new ideas to formulate a problem (‘agenda formation’), designing, sorting and selecting potential solutions (‘selection’), introducing these (‘implementation’), and, finally, embedding them into organisation routines (‘routinisation’). It is important to recognise that these are seen as episodes of work, with feedback loops between them. For example, at any point during the selection or even the implementation of a solution, new problems may arise that require the reformulation of the initial problem (agenda formation).
There are different concerns across episodes, from a focus on matters at stake, to practical design of usable solutions, to outcomes and issues of sustainability. Bledow et al. 52 see innovation as resulting from a dissatisfaction with old ideas (‘thesis’), which, in turn, motivates new ideas (‘antithesis’). Synthesis between ideas creates innovation.
Kyratsis et al. 9 usefully applied a process approach, albeit in a different context: a study of the use of evidence in decisions about technology adoption in nine acute care organisations. They found that the practitioners involved (clinicians, nurses and non-clinical managers) held diverse ‘evidence templates’ when seeking and using evidence. These cognitive templates stemmed from the different practical experiences, professional identities and training of these groups, resulting in different ways of defining and making sense of what constituted acceptable and credible evidence in decisions. All groups drew on a ‘practice-based’ template, using experiential practical knowledge as evidential in their decisions. However, whereas clinicians drew mainly from a ‘biomedical-scientific’ template, prioritising science-based, peer-reviewed and published evidence, non-clinical managers drew more from a ‘rational-policy’ template, preferring evidence based on cost, productivity and fit with policy imperatives. Nurses, in contrast, drew from all three templates. Importantly, different evidence was valued at different points of the process, depending on who the key actors were at the time and how they made sense of the problems within their professional domain.
. . . local trials, and the need for ‘pragmatic evidence’, were deemed important by decision-makers in the early stages of innovation adoption. For innovations (and especially those that require changes in practices or processes), creating an evidence base will require agreement about what is regarded as a legitimate epistemological basis for verifying and validating evidence and relevant knowledge.
p. 1369
Evidence use is not, then, a one-off event. It reflects the priorities present at a particular point in time. It is entirely plausible that different evidences will have differing utility and weight across episodes of innovation work. 52 For example, RCTs may be used as evidence when identifying solutions to a problem, whereas financial data may be used to understand feasibility.
Certain types of evidence relevant in one episode may also be questioned or disregarded in another. Recognising this, we use a processual approach in our own methods and analysis,53 tracing evidence use in service redesign over decision episodes. As Wright et al. observe, ‘deeper insights are uncovered when decisions are investigated in ways that “open up” the role of the decision-maker and of the context in the processes leading to the commitment to action’. 10 Keeping with a knowledge mobilisation approach allows us to understand forms of agency that may make decisions evidence based. It can be expected that multiple evidences will be used and that different capabilities may apply across different episodes of commissioning work in service redesign.
How is evidence used: capabilities for evidence use
Although the literature says much about what evidence is used and when it is used, how it is used is less clear. It is important to understand, also, how evidence comes to be used in practice. In other words, what capabilities are required to actually use evidence? Existing research, noted above, points to the fundamental importance of understanding the social and political processes underpinning evidence-based decision-making. Much of the literature from both the UK and Canada points to the social discursive aspects of evidence use. 54
However, questions remain unanswered about ‘the detailed process by which ideas are captured from outside, circulated internally, adapted, reframed, implemented and routinised in a service organisation’. 30 Crilly et al. conclude that ‘NHS Boards should take a clear view on organisational design elements needed to support knowledge mobilisation’. 4 These elements include ensuring that capabilities (including appropriate work practices and enabling organisational conditions) are in place. A few insights can be found in the literature about these capabilities.
First, evidence-based decision-making involves mobilising knowledge across diverse experts, including patients. Recent health-care research shows that stakeholders bring their own values and sense-making to any decision, which informs how evidence is sought out, shared and applied in transforming services. 1 Professional differences influence the use of evidence, too, with McGivern et al. 11 showing clinical consultants as having the ‘power, knowledge, and self-confidence’ to critique NICE guidelines. Other clinicians (e.g. GPs) in this study were shown to ‘not have the time’ and were too ‘overwhelmed’ by the range of guidelines. Instead, these stakeholders worked to balance research findings with local needs. In that study, management saw NICE guidelines as a means for stakeholders to reach consensus and avoid negative consequences from regulatory bodies. 11
Second, just having appropriate experts and evidence present does not guarantee evidence-based decisions. Actors also pursue particular purposes, with varying degrees of influence (they have agency, in other words). The issue of who searches for, synthesises and presents evidence is, therefore, important. 7 When this happens may also be important, as different priorities beckon at different points (ensuring sufficient evidence to solve a problem, identify solutions in our context, etc.). ‘Knowledge brokers’, or individuals who make knowledge embedded in one community available to another, may enable such evidence sharing. 6
Third, research points to the subjective aspects of evidence use and the importance of human judgement. 5 Harvey et al. 46 describe evidence ‘as forms of knowledge seen as “credible”’, while Freeman and Sweeney55 suggest that use relies on perceived ‘relevance’. In a study of hospital organisations, Jones and Exworthy56 describe how different ‘frames of reference’ shape perceived relevance. They show that framing an issue in terms of clinical necessity engages practitioners with change. Frames may be strengthened through co-construction and discussion. Champions or opinion leaders may also communicate certain evidences and push them into dominance. 57
Broader insights can be gained from organisation studies. Organisational structures (e.g. networks) underpinned by trust-based relationships are said to promote the ‘open sharing’ required to innovate. 58 Crilly et al. argue that ‘Relationships trump organisational design . . . Connective ability of individuals is more important than organisational structure when it comes to making organisations effective’. 4 Another influential stream of research is ‘resource-based theory’ (and, its successor, the knowledge-based view), which points to the importance of resources, especially knowledge, in improvement. 59 It is argued that ability to integrate, build and reconfigure internal and external competences to address rapidly changing environments informs competitiveness.
The work outlined to this point sensitises us to the kinds of capabilities NHS organisations and managers may need to use evidence. Indeed, in one of the few studies of capabilities for evidence use in commissioning, Swan et al. 2 show that evidence use in PCTs entails timely evidence use, managing interfaces across the project and proactively building and managing relationships. By better understanding what counts as evidence, when it counts and how it comes to count as evidence, we can begin to develop capabilities to enable evidence use in decision-making. As Ferlie et al. 5 note, the imperative for mobilising knowledge and evidence in NHS organisations lies not in producing ever more information, but in ‘building high learning capacity and appropriate core competencies in NHS organisations’. 5 We seek to understand capabilities for using different types of evidence in the decision-making that occurs across episodes of work involved in CCG innovation.
How is evidence produced?
A knowledge mobilisation approach reminds us that the use of evidence in one domain (e.g. commissioning) is intertwined (or ‘nested’) with the machineries of its production in another. 12 We thus consider, also, the ways in which evidence is produced by considering ‘inscribed meanings’. 60 This research suggests that a ‘programme of action’ or ‘script’ is inscribed in all artefacts. 60–62 Inscribed meanings constitute a sort of instruction manual for the artefact. Designers of artefacts take a view of how it should be used in order to perform its expected function. Akrich et al. describe how the large part of the work of producers involves ‘defining the characteristics of their objects [and] making hypotheses about . . . the world into which the object is inserted’. 60
Inscribed actions exist in everyday objects such as door handles, which carry the programme of action ‘pull the door’. Actions are also inscribed on the internet, where readers of underlined blue text are invited to click and follow related links. In similar ways, evidence is an inscribed artefact. The inscribed use of evidences must be translated into practice through a relational and negotiated process that requires work, time and motivation. 12 Encounters between scripts and users can broadly result in three possible scenarios:
-
Scripts may persuade actors to play the proposed roles through training, coercion or discipline. 63 If scripts differ from established actions and actors subscribe to them, the artefact produces change. For example, a NICE guideline highlighting risks of current methods may change behaviour.
-
More often, users try to descript artefacts and modify actions to fit what they already do. 60 Users try to adapt the artefact to their ‘tastes, competences, motives, aspirations, [and] political prejudices’ through reinvention. 60 It has been suggested that ‘if potential adopters can adapt, refine, or otherwise modify the innovation to suit their own needs, it will be adopted more easily’. 64 For example, NICE guidelines may be disassembled and used selectively when they fit existing priorities and work patterns.
-
Reinvention has limits, however, which may cause users to ignore, disregard or resist the artefact. The third scenario is, therefore, one of non-use. Coercion can be applied, but users may find ways to circumvent the impositions of the artefact. They may, for example, revert to workarounds or utilise artefacts in a ceremonial way only. For example, NICE guidelines may be ignored or their appropriateness for a particular population may be questioned.
Previous studies suggest that scenarios 2 and 3 often apply in health. 63,64 We set out to explore what sort of evidence users are inscribed in evidence production in order to develop further insights into the use of authoritative evidence in commissioning.
Conceptual framework
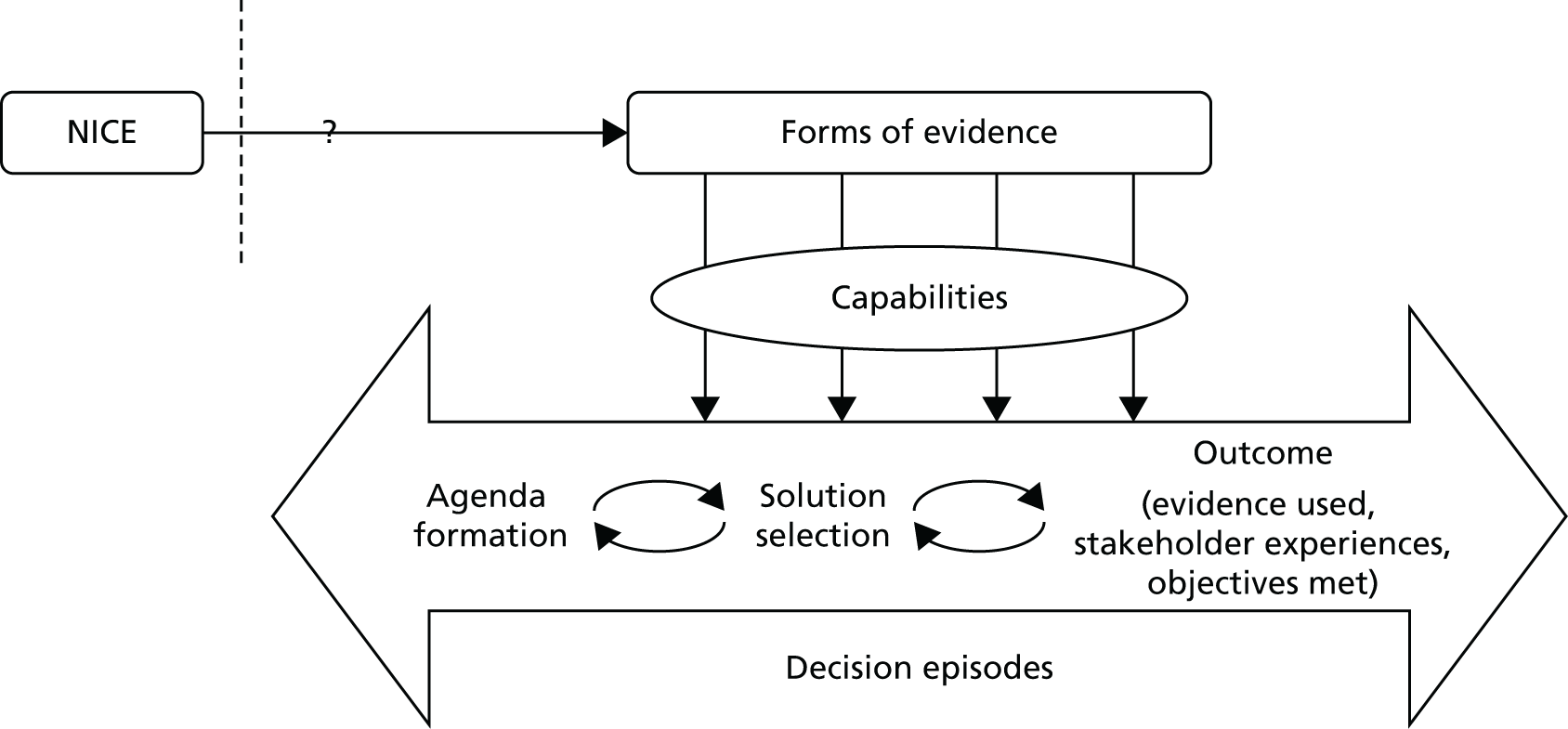
Figure 1 summarises the conceptual framework for our study. It links capabilities to use evidence to evidence production (e.g. NICE), decision episodes and outcomes. The framework provides a holistic approach to explaining evidence use and how this links to variation in process outcomes. It builds on three principles (from the review above).
FIGURE 1.
Conceptual framework for the research.
-
It is processual. 53 It takes on board the point that mobilising evidence entails dynamic practices (e.g. sourcing, interpreting and creating evidence) that unfold over time in an iterative, episodic way (hence the double-headed arrows and decision episodes in Figure 1). As per previous work on organisational change, the journey is conceived as an often uncertain process to reflect the ongoing realities of commissioning management decisions, which are far from linear or ‘rational’. 65 It is also more practical because it reflects the situations that managers face, rather than a more idealised version of events. Hence, it helps to provide a direct link from research findings to actionable recommendations for improvement.
-
Our model is underpinned by a knowledge mobilisation approach that sees evidence use as a social and practical accomplishment requiring capabilities: that is, knowing how and when, not just what. Knowledge mobilisation theorising makes us understand evidence use as a skilful performance, situated in context, in which agency, practices and processes matter. 12 We see capabilities as the practices and enabling conditions needed to pursue and accomplish a particular purpose (evidence use in this case), rather than as static variables, or fixed characteristics of individuals and organisations. Capabilities can be applied at the organisational (e.g. resource management) and group level (e.g. role structures).
-
The model will help us to explain variations in evidence use by comparing similar cases, as other authors have done. 66 We will compare and contrast evidence journeys across different CCGs dealing with similar kinds of redesign. We examine, in particular, if (hence the question mark in Figure 1) and how authoritative evidence (e.g. NICE) makes its way into CCGs, along with other forms of evidence. As per our protocol, ‘outcomes’ are to be assessed qualitatively, including evidence use (the extent to which a range of evidences were sourced, interpreted and engaged with), stakeholder experience (the extent to which stakeholders were satisfied or dissatisfied with the process) and objectives met (the extent to which original objectives were met and, if not, whether or not change to them was justified).
Chapter summary
In sum, this research provides an in-depth examination of the use of evidence across NHS organisations (CCGs) in order to identify capabilities for relatively more successful outcomes. As our review of the literature indicates, authoritative evidence never stands alone but must be judiciously combined with other forms of evidence. Viewing evidence use as a process of knowledge mobilisation provides a more realistic (and, therefore, more useful) account. The study develops a process model that articulates the dynamics of evidence use, without losing sight of local work practices, power dynamics and context. This allows us to begin to generate relevant ideas and tools for improvement in practice.
Chapter 2 Methods
Research on evidence ‘pull’
As described in the introduction, our research responds to the NIHR commissioning brief by aiming, primarily, to understand so-called research ‘pull’ by managers by studying evidence journeys in practice (as evidence travels through CCGs). We contribute (as a secondary aim) to the understanding of ‘push’, by considering, also, the start of the journey (i.e. how certain evidence products are made by producers). The following objectives have been operationalised to achieve these aims:
-
to study how authoritative evidence travels in, and is used by, different CCGs, that is, to document ‘evidence journeys’
-
to compare and contrast the travel of evidence across CCGs and analyse comparatively ‘evidence journeys’
-
to create a process model, a roadmap and a toolkit that will guide CCGs in becoming better users of evidence.
Research design
A key parameter case study design was used to compare processes across organisations. Key parameters are similar to inclusion/exclusion criteria. They ensure that a similar process is observed in different but comparable contexts. In our case, redesigns had to be informed by recently released non-mandatory guidance [e.g. on diabetes and on musculoskeletal (MSK) conditions] and had to be considered a CCG priority. As Barley and Kunda note, ‘the design’s forte is that it enables researchers to articulate more clearly how key contingencies differently shape work practices and, hence, engender different patterns’. 67 As per our protocol, data collection combined real-time observations in two CCGs with retrospective inquiry in six further CCGs, based on interviews and available texts. Process analytical methods, as per Langley,53 were used to order to each case from start to finish using temporal bracketing strategies. Innovative service redesign involves iterative, overlapping episodes of work. 51 Process perspectives account for these episodes, changing relationships, and interpretations over time. 68
Patient and public involvement and a Scientific and Stakeholder Advisory Panel (SSAP) also shaped the research. Through UNTRAP (University/User Teaching and Research Action Partnership), which is a partnership between users of health and social care services and carers, and the universities of Warwick and Coventry and the NHS, we engaged service users/carers interested in research and teaching. Two UNTRAP members participated in our SSAP, which also included NHS managers, key opinion leaders and academics. The UNTRAP members provided invaluable feedback throughout the life of the project from the research design stages through to the final report write-up. They were especially helpful with regard to the analysis and reporting, ensuring that the language used was accessible and relevant to service users. They also shared their experiences of health care with us in the light of our findings, which was useful for us in understanding and ensuring the relevance of our research. The SSAP directed us in our research design and interpretation of emerging findings.
Data collection
The first aim of our research was to understand evidence use in the CCG context. In our original project protocol, it was promised that ‘we will select, in close consultation with our Scientific and Stakeholder Advisory Panel, two particular forms of evidence from NICE aimed at innovation in service delivery (not just minor adjustment). We select one piece of evidence recommending pathway changes on the basis of emerging scientific evidence (“Do recommendations”, e.g. NICE Clinical Guidelines on Diabetes in children), and another recommending decommissioning of certain healthcare services or treatments (“Do not do recommendations”, e.g. regarding mucolytic drugs as per NICE guidance CG101 [Clinical Guidelines]).’ We also took into account the date of NICE guideline release, the care setting, the likely cost consequences and the clarity of guidelines for a commissioning audience.
We met our protocol objectives by taking the following actions. We accessed a list of all NICE guidelines released (post 2011). The project team created a shortlist of all those guidelines that were released since January 2012, and that were likely to be picked up by commissioners. We then created a survey targeting senior NHS commissioning managers to identify which NICE guidelines were likely to be important for service redesigns. Thirteen CCG leaders (mostly chief executive officers and associate directors) responded to a list of health needs and were asked: ‘tick if you are confident that your organisations will actually use the following guidelines in commissioning over the next 12 months’. The responses are shown in Table 1. The findings were presented to 15 CCG leaders in November 2013. Feedback was the following: ‘NICE guidelines are not used directly by CCGs’, ‘We are not confident the choices [list of guidelines] offered reflect the priorities of the organisation’, ‘There is no formal process [in our organisation] for using NICE guidelines’, ‘NICE does not drive commissioning . . . it’s clinical outcomes, performance of service (e.g. referral rates, spending)’. Commissioners also told us that decisions on what not to do nearly always result from decisions on how to do things better. In other words, they are two sides of the same coin.
| Clinical area | CCGs, % (n) |
|---|---|
| Hip fracture | 58.30 (7) |
| Diabetic foot problems | 50.00 (7) |
| Common mental health disorders | 50.00 (6) |
| Ovarian cancer | 41.70 (5) |
| Lung cancer | 41.70 (5) |
| Infection control | 41.70 (5) |
| Service users’ experience in mental health | 33.30 (4) |
| Prostate cancer | 33.30 (4) |
| Pressure ulcers | 33.30 (4) |
This was an invaluable exercise for the project team, because it meant that we had to rethink our narrative, which had thus far been focused on ‘how NICE guidelines are used in commissioning’. We quickly realised that we had to be pragmatic for the purposes of this research and select a commissioning process that was of importance to CCGs and in which the use of evidence (the recently produced NICE guidelines identified above) was likely to be relevant and important. In further conversations with CCG leaders, we narrowed down our focus to two core areas of care: MSK services and diabetes services. These were two areas of ‘high importance’ for a large majority of CCGs for service redesign (i.e. CCGs had approved business cases and sponsored related service redesign projects). They were also areas in which key NICE guidelines had been released since January 2012. The implications were that it would be feasible for us to collect meaningful data in accordance with our research protocol, if we focused on relevant redesign projects. We discussed our choices and thinking with our multiexpert SSAP. We explained our rationale for choosing MSK and diabetes services as the focus of our study, and the panel readily endorsed our decision. Retrospectively, it proved a good decision to focus on these two areas, as we were able to recruit a large number of CCGs in our research as a result.
Eight CCGs were used to maximise opportunities to capture variation, while paying attention to the contextual dimensions. We contacted CCGs with varied spend, deprivation and geography through recommendations from Dr David Sharp (then a director at NHS England), internet searches and existing NHS contacts. 69 The CCG names have been changed for anonymity. Four CCGs were working on diabetes services (Seaport, Greenland, Rutterford and Chelsea) and four were working on MSK services (Horsetown, Stopton, Coalfield and Shire). The Yorkshire and Humber Health Intelligence is used to classify CCGs in Table 2. The NHS measures mortality resulting from cardiovascular, respiratory, liver and cancer diseases among those aged < 75 years, as well as emergency room admission for alcohol and death from treatable disease, as CCG performance indicators. Greenland CCG scored in the best 25% for all indicators, while Rutterford CCG scored in the top 25% for some and in the middle for others. Horsetown CCG scored in the middle range on all indicators. Seaport, Chelsea, Coalfield and Shire CCGs scored in the middle range for some indictors and in the bottom 25% for others. Stopton CCG scored in the bottom 25% for all indicators.
| CCG | Young population, above-average black and Asian ethnic groups, moderate deprivation | Older population, rural, low deprivation levels | Average age, deprivation, low population |
|---|---|---|---|
| Seaport | ✓ | ||
| Greenland | ✓ | ||
| Rutterford | ✓ | ||
| Chelsea | ✓ | ||
| Coalfield | ✓ | ||
| Shire | ✓ | ||
| Horsetown | ✓ | ||
| Stopton | ✓ |
We were referred to a ‘key stakeholder’ in the redesign (e.g. a consultant). We explained the purpose of the research and asked them to participate. We also asked them to help us recruit other stakeholders. Open-ended interviews were used,70 with items related to organisational background (e.g. roles and structures), redesign rationale, evidence use, redesign process, supports, outcomes and challenges. This allowed narratives to develop that provide rich, complex accounts. 53,71 When we felt that richer explanations would follow, we interrogated responses with additional probes and follow-up questions.
The participants were informed of the purpose of the study, that participation was voluntary and that data would be anonymised. We use an ‘ethnographic approach’ to understand, in a systematic way, the conduct of people studied from their own perspective. We see the ethnographic approach as a scientific description of peoples and cultures with their customs, habits and mutual difference rather than complete submersion as per ethnography. 68 The interviews were completed in a location of participants’ choice, and they were recorded and transcribed verbatim. The qualitative interviews lasted between 45 and 90 minutes.
We averaged about five stakeholder interviews per CCG (n = 35 stakeholders were interviewed in total), which was fewer than we had expected in our protocol because CCGs were newly established when our project was designed and at that time we did not know the size of redesign groups. During data collection, we soon realised that the number of stakeholders actively involved in each redesign project was lower than we had expected. We countered this shortcoming by conducting follow-up interviews to learn about the outcomes of the redesign work as necessary (n = 77 interviews were completed in total).
In addition to interviews, we observed meetings in two CCGs to gain close familiarity of the groups’ decision processes as they unfolded ‘in vivo’ over time (over approximately 12 months in each case). 72 There were also fewer meetings to observe than we had estimated in our original protocol. Redesign meetings tended to occur once every 2 months and were often disrupted or postponed (other pressures were intense at the time, given that CCGs were still being embedded). This meant that, even if we had been able to start observations from February 2014 (as per protocol), we would only ever have been able to complete an average of 14 observations per CCG. Although we gained access to one CCG (Horsetown) early on in the project, we could not access the second until September 2015. This reduced the number of observations further. These changes reflect the realities of commissioning. Indeed, in many cases, groundwork for decision-making is through conversations over dinner or at coffee, and so we could not always capture this. This groundwork then informs the decisions that go through more formal channels. Meetings lasted, on average, between 2 and 4 hours and were concerned with planning, problem solutions and the evaluation of potential changes. All changes to the protocol are summarised in Table 3.
| Protocol | Final report | Rationale | |
|---|---|---|---|
| Data collection | Select two forms of evidence from NICE related to ‘do’ and ‘do not’ recommendations | Focused on how NICE guidelines are used in commissioning | Commissioners told us decisions not to do nearly always result from decisions on how to do things better. In other words, they are two sides of the same coin |
| Data collection | 40 observations plus 40 interviews at two CCGs, plus 120 ethnographic interviews across six additional CCGs | We averaged about five stakeholders per CCG (77 interviews), plus nine observations | There were fewer stakeholders involved and fewer meetings to observe |
Field notes and memoing (researcher reflections on field notes) were used to capture observations, ensure that research objectives were being met and add trustworthiness to the data. 11 Notes were handwritten and typed up as soon as possible. We also collated documents related to the redesign, including those shared by interview participants and those identified through internet searches. Fieldwork is summarised in Table 4.
| Location | Participants | Documents used | Observations |
|---|---|---|---|
| Seaport | Diabetes consultant (3), diabetes nurse specialist, GP, diabetes specialist nurse with responsibility for education and training | Published papers on the project (4) | |
| Greenland | GP lead on project, GP, CSU project manager, non-executive hospital director, transformation and innovation CSU lead (2) | Presentations (2), HSCIC evaluation | |
| Rutterford | Diabetes nurse consultant (2), transformation manager, chief accountable officer | Board presentation, final report, board papers (2), evaluation paper | |
| Chelsea | Contracting manager, diabetes consultant (1), commissioning manager, patient representative from Diabetes UK | Evaluations (Right Care and HSCIC), service specification, newspaper report | |
| Coalfield | Senior commissioning manager (5), clinical lead for MSK, clinical director, CCG chairperson, support manager for patient engagement | Board papers (2), CCG reports (2) | |
| Shire | Commissioning manager (2), GPwSI (2), GPwSI and consultant, clinical service manager, clinical therapies lead, deputy director of planning and development | Board papers (3), Right Care evaluation | |
| Horsetown | Two GPs, four physiotherapists, CCG director, senior commissioning manager, radiologist, orthopaedic surgeon, commissioning manager | Terms of reference, agenda, reports, e-mails, model of care, provider’s proposal, minutes | Four meetings |
| Stopton | Senior commissioning manager (5), head of therapies, clinical assessment team lead, GP, orthopaedics consultant, rheumatology consultant, physiotherapist, hospital representative | Meeting agendas, minutes, board papers, evaluation presentation | Five meetings |
Ethics approval
Individuals invited to take part in this study were made aware that their participation was voluntary and that their information would be confidential and anonymised. The participants were given the opportunity to ask questions and given time to decide whether or not to take part in the study. Data are stored in locked filing cabinets in locked offices on university premises. Computer files are password protected and stored on the network drives (not local hard disks) of password-protected computers. Detailed plans for handling ethics issues were approved by a NHS Research Ethics Committee.
Data analysis
We developed detailed case descriptions of redesign projects at each CCG. 53 These were assembled from reading and rereading all interview data, observation notes and collated documents for a specific case and written by the team member who had been most closely connected with the data collection. The case descriptions were initially structured chronologically, resulting in lengthy narrative accounts of what happened, when and why, with particular reference to any information that became evidential during the process, and supported by original quotations or excerpts from field notes. However, as the emphasis in our research is on improvement, not just understanding and rich description, we decided to avoid overly lengthy discussions of raw data in order to make our case studies more accessible to practitioners. To achieve this, we rewrote each case according to analytical themes, in which we sought to identify the following.
Evidence use
From the literature, and in consultation with our SSAP, we considered NICE and other evidences. 22,42 Evidence was defined as information with an evidentiary value in decisions. 2 Information is legitimised as evidence not through production but through use. Some information (even NICE guidelines) may be questioned or dismissed as evidence by decision-makers. Therefore, our first step was to identify evidences used. Here, we used deductive and inductive approaches. Deductive template analysis was used here, as we expected evidences already in the literature to appear in our data. 73 We looked at studies of commissioning work, noted the evidences used and searched for these in transcripts. 1,2 We were also open to emergent themes when searching for evidence used in our reading of cases. 74
Capabilities for evidence use
Inductive coding was used to identify evidence-use capabilities. 74 We collated data on evidence use via open coding. 75 Common excerpts were placed in provisional categories (first-order). Categories were then integrated into higher-order researcher-induced themes via axial coding so the team could identify themes from the data. 74 Themes were compared and discussed to decide final classifications. Team members then searched each transcript for any talk on capabilities that appeared to enable evidence use in situ. Themes were also compared against those found in previous research and adjusted when appropriate, thus iterating between data and theory, as suggested by others. 74
Process mapping
Process maps were created to identify patterns of evidence use, capabilities and outcomes within and across cases. 53,76 In these we draw out the chronology of critical events in redesign work. 74 To give structure to our cases, we inductively labelled the episodes of work as they appeared in the raw data. As seen in the literature review, episodes (sometimes labelled ‘phases’) of work are often used in innovation literatures to give structure to process studies. 32,51 We prefer the labelling ‘episode’ to highlight the often non-sequential nature of the work activities entailed.
Cross-case comparisons
Building on within-case analyses, and following Stake,77 we then conducted cross-case comparisons. To do this, team members examined cases to identify ‘prominent themes’ in each and ‘common patterns’, as well as major differences in evidence use. The team then assembled to compare and contrast similarities and differences across cases and to come to tentative assertions about the capabilities that appeared to influence the CCGs’ evidence use, as well as any significant aspects of context. As we were interested in capabilities to use evidence, we also mapped these against process outcomes, identified broadly and indicated qualitatively in terms of evidence use (the extent to which a range of evidences were sourced, interpreted and engaged with), stakeholder experience (the extent to which stakeholders were satisfied with the process) and objectives met (the extent to which the original objectives had been met and, if they had not, whether or not any change to them was justified).
These tentative findings were presented to the SSAP and non-Warwick team members for further discussion and verification. Following this, the findings were presented at our one-day national multistakeholder engagement workshop (n = 46) at which further feedback and verification was elicited. These steps were important in helping to achieve trustworthiness in our analysis. 71 Having identified evidences and capabilities, and compared across cases, we summarised our findings into a toolkit for use by commissioning stakeholders. The questions for this toolkit were also piloted at our national engagement workshop and were modified accordingly.
Research on evidence ‘push’
Our original protocol aimed to trace the evidence journey in CCG commissioning, including the place at which evidence ‘enters’ the journey, as guidelines and other forms of evidence-based products. Here, we conducted a further small-scale exploratory study in one large guidance-producing organisation, in which we considered the ways in which projected users are inscribed in published evidence. Our objectives were to understand:
-
the user image inscribed in published evidence products
-
how an evidence producer believes products will be used in general, and by CCGs.
Over the course of 6 months, during 2015–16, we carried out eight interviews, spoke less formally with other evidence producers and consulted a number of documents, some publicly available and some internal. Informants included project managers and clinical staff involved in the production of the evidence (n = 4); and staff involved in communication, marketing and other customer-facing activities (n = 4). We also observed one visit of an evidence producer to a CCG. We asked questions about the following in semistructured interviews:
-
how the design and production of evidence (e.g. guidance, advice, quality standards) is organised and the nature of the products
-
how products are made available to users and how this has changed over the years
-
a scenario question (according to critical incident interview),78 in which interviewees were asked to focus on a specific product (guidance, quality standard or other) and describe how it is actually used, with examples.
All interviewees gave informed consent. Interviews were recorded and transcribed verbatim. The same inductive coding method was used as described above for identifying capabilities.
Summary of research methods
We conducted two phases of research to understand evidence ‘push and pull’. The findings were shared with case study participants via feedback and with our SSAP by e-mails and meetings. In the next chapters, we present findings from phase 1. We describe what evidences are used (see Chapter 3), and then capabilities for use (see Chapter 4). Building on this in Chapter 5, we show evidences and capabilities in practice through four analytically-structured cases (two on diabetes services and two on MSK services). The remaining cases are shown in Appendices 1–4.
Chapter 3 Clinical Commissioning Group evidence use
We see evidence as information that enables understanding and legitimises judgements. 2 In the first phase of our study, four categories of evidence were identified in our analysis. These were labelled as ‘universal’, ‘local’, ‘expertise-based’ and ‘trans-local’. These labels reflect what kinds of evidence were used in terms of their source. As seen in Chapter 1, universal (i.e. evidence produced through scientific methods that is held to be widely applicable), local and expertise-based have been discussed in the literature to some extent already, while trans-local evidence is more uniquely identified in our study. 1,2
Universal evidence
Information produced by formalised or scientific investigation in institutions or organisations that are deemed to have legitimate authority (e.g. universities, NICE and national bodies) is commonly treated as evidence. 1 This type of evidence is produced systematically through scientific method (e.g. RCTs and NICE guidelines) and/or standardised nationally (e.g. QOF data), so it is considered robust, rigorous, widely applicable and authoritative. Because this type of evidence is expected to be used widely, it has been termed ‘universal’. 2 We think of universal evidence as produced from the ‘top down’. Universal evidence includes NICE guidance, but also NHS reports, public health data, Right Care guidance and academic papers, among others. Previous studies showed the importance of this type of evidence in decision-making in commissioning work. 1,2
Stakeholders also described mobilising universal evidence to understand ‘good practice, best practice’ (Chelsea CCG). They were aware, for example, of ‘what’s being proposed in the Five Year Forward [View]’ (Seaport CCG), the NHS-produced document setting out a vision of ‘GPs, nurses, community health services, and hospital specialists [providing] integrated out-of-hospital care’. 79 A consultant at Seaport CCG described using NICE to inform service design, in this case because it mandates that antenatal diabetes has ‘a midwife, an obstetrician, a consultant, and a scanning machine’. Evidence from academia was also used, including ‘studies about how do you improve service provision, about impact . . . tools’ (Coalfield CCG). Interestingly, some kinds of universal evidence appeared to be more prominent than others. Cochrane reviews were not, for example, referred to in any case (at least directly), whereas NICE guidance was referred to rather more often.
As in other studies, the rigour of universal evidence was understood, but its usability and relevance was questioned. NICE guidelines in particular were problematic, with stakeholders seeing them as ‘very long . . . it’s like reading a book!’ (Horsetown CCG). 1 Furthermore, in order to be widely applicable, this kind of evidence had, by definition, to be abstracted from any particular context, which often left commissioners puzzling over how to apply it in their own setting. At Rutterford CCG, for example, stakeholders described how ‘on top of all of that [NICE] guidance, you have to put in what is going on locally . . . and what is available’.
Local evidence
Information produced and collected locally within CCGs or in their immediate locale was also, and very often, treated as evidence. 1,2 This information is wide ranging, including patient profiles, local activity and performance data, finance data, contracting models and monitoring outcomes. It may also include externalised observations and experiences of stakeholders. Unlike universal evidence, this type of evidence is seen as more readily ‘to hand’ for those involved in commissioning decisions and, therefore, ‘useful’.
Across all cases, local evidence featured heavily in the conversations among commissioning groups. It was used to show problems, for example ‘a 41% increase in hip and knee replacements’ (Horsetown CCG) or ‘you serve only 10% of the population [in the hospital]’ (Seaport CCG). Local evidence was also used to identify solutions to problems, for example ‘The CCG have done a piece [of research] that sort of says . . . 70% of activity can be stripped out [from the hospital]’ (Greenland CCG). Alongside these ‘hard’ numerical data, stakeholders also relied on softer tacit knowledge about the local area: ‘a lot of patients . . . being followed up in secondary care, which is not necessarily where they should be’ (Greenland CCG).
Evidence collected in the local area gives meaning in context, and so it is not surprising that is has been shown as important in the broader literature, too. 1,2 Although readily available at a relatively low cost (in terms of search time and effort, at least), this type of evidence is not subject to the same scrutiny as universal evidence, and so issues around its validity could often arise. 80
Expertise-based evidence
Information based on the experiences and expertise of individuals could also be treated as evidence. Expertise-based evidence can be sourced from health-care providers, colleagues, patients, families, carers and many other groups. 1,2 Stakeholders in our cases used expertise-based evidence to form agendas. At Seaport CCG, for example, the consultant describes how ‘the idea [for the redesign] came from . . . a group of us registrars’ because ‘we always used to talk about it, saying it isn’t quite right the way we’re doing it’. Expertise-based evidence was also used to identify solutions. At Horsetown CCG, for example, commissioners chose community-based solutions because they had ‘set up things in the community before [and seen] the cost reduction . . . the better patient experience’.
Expertise-based evidence is personal, embodied and value-laden. It is often transferred through narratives. It possesses few characteristics of scientific rigour; this evidence is very relevant to context. We noted that individuals with clinical backgrounds who were considered charismatic and were respected tended to be considered holders of ‘expertise’. Although anecdotal, our observation reflects the study by Wright et al. 10 how one individual (‘Dr Clancy’) influenced service redesign in an Australian emergency department.
Trans-local evidence
Examples from elsewhere were also treated as evidence. 1,23 We call this ‘trans-local’ evidence because information is created in one place and used in another. We do not call it ‘best practice’, because evidence can be trans-local without being validated as ‘good’ or ‘best’. In our cases, trans-local evidence was often applied before efficacy evaluations. Rather, the fact that changes were made in other places seemed to have legitimising properties. The GP at Greenland CCG, for example, described choosing to mimic Seaport CCG’s diabetes model. When asked about this decision, the GP at Greenland CCG did not seem entirely certain about efficacy, describing, ‘I suppose we haven’t really got long-term evidence’. The arrival of trans-local evidence in new sites is also far from systematic. Instead, translation through stories or examples, ad hoc gatherings or accidental encounters (e.g. conferences or meetings) seems important. Greenland CCG mimicked Seaport CCG because a GP in the former had heard a talk from a consultant at the latter and ‘felt’ that Seaport’s was the right solution. Chelsea CCG mimicked Seaport CCG because their consultant had seen ‘first hand that it works’. Interestingly, we saw relationships among all categories of evidence. Local and universal evidences were often used together in sense-making activities. Stakeholders understood local evidence (e.g. about patient waiting times) in reference to NHS standards, which are based on universal evidence. In the case of Seaport CCG, for example, local evidence showed that patients were waiting 18 months for an appointment, when the maximum NHS waiting time is 18 weeks. At Greenfield CCG, the transformation manager described how ‘amputation rates were quite high’ (local evidence), especially ‘when compared nationally’ (universal evidence). Stakeholders at Coalfield CCG also described making changes before they had been published (universal evidence) because they knew that certain recommendations were in the pipeline and they felt that those changes were appropriate (expertise-based evidence). It is important, therefore, to recognise that different categories of evidence are relevant to redesign work, but that these are often used, by necessity, in tandem in the practice of decision-making. Hence, in relation to the use of NICE guidelines in commissioning practice, this is not simply a matter of accessing or adopting relevant guidelines in a timely manner, but also requires skilful combinations of different forms of evidence in order to make them actionable in a local context. The four categories of evidence are summarised in Table 5.
| Category | Examples | Properties |
|---|---|---|
| Universal | NHS reports, NICE, Right Care, public health data and academic papers, national frameworks | Legitimated by science or organisational backing |
| Often abstract and taken for granted | ||
| Can be easily ignored | ||
| Local | Patient profile, activity, finance, contracting, outcomes, observations | Highly relevant to context |
| Not legitimised, can be contested | ||
| Difficult to ignore | ||
| Expertise-based | Experiences and expertise of providers, colleagues, patients, families, carers | Highly relevant to context |
| Value laden, biased and subjective | ||
| Can be contested, but difficult to ignore | ||
| Trans-local | Examples from other sites, including new pathways | Relevant if adopted by stakeholders |
| Often narratives, anecdotal | ||
| Can be overlooked |
Chapter summary
In this chapter, we discussed the evidences used by stakeholders, identified as ‘universal’, ‘local’, ‘expertise-based’ and ‘trans-local’ evidences. Evidences were identified deductively from existing literature and also inductively from our raw data. Universal, local and expertise-based evidences have been discussed, albeit under different labels, in previous studies. Trans-local evidence has also been discussed, to an extent, in previous work. Much of the existing literature has described ‘examples from elsewhere’ or ‘best practice examples’ as evidence. Although similar, we use the label ‘trans-local’ to better represent the fact that this type of evidence must be sourced from a locality different from that of its creation. We also avoid ‘best practice’ because, as will be shown in the cases, rigour, evaluation or efficacy were not always considered in using this type of evidence.
Issues around the use of these evidences were clear, especially in the mobilisation of authoritative NICE evidence. In the next chapter we consider capabilities for evidence use that might alleviate these issues.
Chapter 4 Capabilities for Clinical Commissioning Group evidence use
We noted in Chapter 1 that evidence use is often a highly political social process. 3 Drawing on the literature, however, we expect that certain organisational practices and conditions will enable evidence use in redesign work. Because of this, we identified inductively capabilities for evidence use from our cases (see Chapter 2). Our analysis revealed five sets of capabilities that appeared to benefit evidence use in redesign projects. We label these as ‘sourcing and evaluating evidence’, ‘effective framing’, ‘engaging experts’, ‘managing expert collaboration’ and ‘managing roles and expectations’. In the next sections, each capability is defined, its importance is explained and examples from our cases are provided.
Sourcing and evaluating evidence
Clinical Commissioning Groups are expected to use evidence to assure the quality of services. Yet they have received little guidance on how to achieve evidence use. 41,81 The quality and quantity of evidence relevant to health-care contexts is one factor that can undermine its use. It has been estimated that as many as 75 trials and 11 systematic reviews are published daily. 82 Stakeholders in our cases noted that using universal evidence ‘became this minefield because . . . how do you synthesise all this so that you’d end up with something that is succinct so that you can deal with it?’ (Rutterford CCG). Even if evidences can be sourced, application can still be problematic. Stakeholders reported that it was difficult to apply evidence to context, especially ‘NICE guidelines . . . they’re very long . . . it’s like reading a book!’ (Horsetown CCG). The quality of available evidence was also often an issue, as the GP below notes:
[The hospital] thought they had 2000 diabetics [but] it looks like . . . about 500. But they didn’t know, they had no idea at all. If I’d have said to them you’re looking after 8000 they’ll have believed me. Or if I had said it was 150 they would have thought well, we seem very busy but they don’t know. I am astonished, absolutely astonished at the quality of data that comes out of the hospitals . . .
Greenland CCG
Efforts to source and evaluate relevant evidences must be made if CCGs are to conduct evidence-based service design. As noted, ‘evidence does not speak for itself, but needs to be mobilised’. 2 Although mobilising evidence may seem like a fundamental practice, efforts differed across CCGs. In Rutterford CCG, for example, ‘task and finish’ groups were established specifically to achieve evidence use. Subgroups were asked to report if there was:
Any evidence . . . any models of care . . . any best practice . . . [anything] published in the literature that we might want to adopt and change . . .
In other CCGs, efforts towards sourcing and evaluating evidence were weaker and more problematic. At Horsetown CCG, for example, stakeholders reported that they had brought evidence forward but ‘then nothing happened’. At Greenland CCG, the evidence search and evaluation was limited because the outcome had already been decided. Because the solution was predetermined, stakeholders ‘had not considered a lot of options . . . It was just going to go for the community-based team’.
Existing literature (see Chapter 1) also shows that evidence use is time and task dependent. 2 Redesigns involve episodes in which different tasks must be accomplished. During agenda formation episodes, stakeholders may have to scope evidence about demand, while later they may need evidence about efficacy and evaluation. Therefore, it is unlikely that narrowly sourcing evidence early on (as in Greenland CCG) will be sufficient. Instead, stakeholders may need to be responsive to evidence demands across the process. Given the challenges of evidence use, the importance of evidence use in CCG work and the variation shown across our CCGs, our first capability for evidence use is ‘sourcing and evaluating evidence’.
Engaging experts
In addition to sourcing and evaluating, experts must be engaged to effectively apply evidence. By ‘experts’, we mean individuals with relevant education or qualifications (e.g. doctors and consultants) whose expertise is embedded, localised and invested in practice. 27 We also mean non-professionals such as health service users (e.g. patients, families and carers). Indeed, this group is key to the NHS mantra ‘no decision about me, without me’. 83 Both groups of experts can offer expertise-based evidence to redesign projects.
Studies have also shown that different types of evidence are used across professions. 23 Consultants, for example, rely more on universal evidence, whereas nurses and doctors are more familiar with local evidence. 11 It is, therefore, important to have multiple stakeholders engaged who can contribute, discuss, interpret and critique a range of evidences (e.g. local, trans-local and universal). In our cases, engaging experts was also a way to ensure a smooth redesign, because ‘you can’t just simply say well, we’re changing the service as of tomorrow and expect everybody to comply’ (Coalfield CCG).
In the majority of our cases, efforts to engage experts were paramount from the outset of the project. These efforts ranged from ‘sending an e-mail round to everyone’ at Seaport CCG, to contacting individuals in person at Coalfield:
Before every board meeting I spoke to every board member . . . sticking my head round the door while [saying] do you get it? Do you understand? Have you got any questions? Is there anything worrying you? It’s literally simple as that. And they’d go that’s fine, get out, for God’s sake. In the end people get bored, go away . . . we support you, we’re with you . . .
Senior commissioning manager
In some cases, expert engagement among professionals was limited. In most of the cases, patients’ expertise was invoked in a rather ‘afterthought to the main event’ type of fashion. At Greenland CCG, for example, stakeholders described how they did not really feel part of the redesign project. In the next quotation, a stakeholder describes how the project ‘belongs’ to the lead GP:
This is [his] project . . . we’re not really involved – we are in the sense that we’re involved to ask our opinions in terms of information from our diabetes patients but we’re not actively involved with the day-to-day . . . if you think about, if you’ve got only say five or six GPs, you know, that’s a lot of areas that you’re leading on.
Because expert engagement is a means to promote and encourage the use of different types of evidence, and to ensure a smoother redesign processes at CCGs, our second capability for evidence use is ‘engaging experts’ in the redesign.
Effective framing
Effective framing appeared as one means of engaging stakeholders in redesign work. 56 Frames are ‘organising principles that govern the meaning we assign to social events’. 84 Framing allows groups to construct and negotiate meaning. 57 A strong frame resonates with actors and may increase engagement. A weak frame is uncertain and can mean resistance, disinterest and incongruence. 57 It can be expected, then, that stakeholders may be more engaged when a frame resonates with them. In our cases, problems were framed in terms of ‘capacity’, ‘care’ and ‘cost’. Patient care was not good enough, which was often caused by capacity issues, and costs were too high. These issues are all interlinked: capacity issues increase waiting lists, which exacerbates illness so that more complicated care is needed. Care, costs and capacity provide a broad frame that can be understood by different stakeholders. Consultants, for example, may be concerned by capacity, whereas commissioners may be concerned with cost. In many CCGs, this broad framing was applied. The accountable officer at Rutterford CCG, for example, describes how diabetes care was ‘a poor use of resources, not great value for money, and [having] not great outcomes for patients’. In other CCGs, problems were framed more narrowly. In Horsetown CCG, for example, the problem was framed almost entirely in terms of cost: ‘MSK is a high-cost, high-volume activity . . . we were conscious that a lot of people were going into a hospital which is very high cost’.
Framing was also important during solution search. Searching for a solution in a way that was congruent with stakeholders’ expectations was important because ‘you can’t just simply say well, we’re changing the service as of tomorrow and expect everybody to comply’ (Horsetown CCG). A ‘blank sheet’ approach was mentioned across cases, which invited stakeholders to contribute ideas about what could and should be done to improve services prior to any formalisation of a particular model of care. Interestingly, ‘blankness’ occurred on a continuum, ranging from ‘not blank enough’ to ‘too blank’. In Greenland and Chelsea CCGs, the sheet was ‘not blank enough’, because the solution had been pre-selected before stakeholders could contribute to the search, such that they felt that their expertise was being excluded. In other CCGs sheets were ‘too blank’, with experts struggling to understand what kind of evidence they might look at and for what purpose. Some CCGs used, rather, a partially blank approach. In Seaport CCG, for example, the redesign leader knew that community-based care would eventually be a part of the outcome, but asked stakeholders to ‘have a discussion how can we do diabetes care better . . . you tell me what works’. Similarly, stakeholders at Coalfield CCG were asked to give evidence about ‘trying to keep people out of hospital’:
One of them said so what do you want us to do? I said I want you to deliver the best possible care for patients for the limited amount of money you’ve got . . . they said how do you want us to do it? I said I don’t care. I said if you want to get a bag of bones and shake them if that makes a difference that’s fine. I said I just want the outcome . . .
Clinical lead, Coalfield CCG
Our findings suggest that weak frames can mean that stakeholders are less likely to contribute to evidence work, owing to the degree of ambiguity involved and difficulties in reconciling evidence pertaining to multiple concerns (cost, care and capacity). On the other hand, framing problems and solutions too tightly, prior to efforts to engage experts, may seal off the search for evidence too soon and could also create problems of stakeholder engagement with the predefined approach. We suggest, then, that it is important to consider not just what problems and solutions are considered, but how they are put forward, framed and communicated in CCG redesigns if evidence use is to be effective. As a result, we identify ‘effective framing’ as a capability for evidence use.
Managing roles and expectations
In engaging experts, it is also important that their roles and expectations are recognised. Certain CCG roles are mandated (e.g. CCG chairperson and accountable officer), but others are more ambiguous. Stakeholders are often brought in to participate in projects without clear governance on what their role is or what they expect from the role. Although it may not be feasible or practical to detail the role of every stakeholder prior to project commencement, this is not to say that roles should not be a considered. In some of our cases, stakeholder roles were very clear. In relation to evidence use, the diabetes consultant at Chelsea CCG had a clear role in bringing trans-local evidence forward because he had worked in a hospital in which the ‘model had already been developed, delivered, evaluated’ and had ‘seen first hand how it works’. Similarly, at Greenland CCG, the CSU project manager was tasked with bringing different evidences forward. As the GP at Greenland CCG describes:
Without [her] we wouldn’t have been able to do this . . . we are doing our day jobs . . . she . . . gets the information on the table . . . on different models.
Roles were not limited to evidence use. At Seaport CCG, for example, one of the GPs described how his role was to act as ‘similar other’ who could justify and legitimise the redesign work to other GPs. He described, for example, how he would say to other GPs:
This affects me [too] . . . I work in the practice . . . I understand . . . I am going to have to do more than I have traditionally done but . . . I can do that because I am supported and we’ve got the right package of support that is available in the community . . .
Roles, however, were not clearly defined and managed in all CCGs. At Shire CCG, it was felt that the ‘wrong’ people were sometimes involved, which was detrimental because ‘unless everybody understands why they’re there and understands what their role is, then potentially you’re not going to get further forward’. In this case, some stakeholders were not ‘engaged in doing that piece of work . . . and just turning up and being there because they’ve been asked to be’. Similarly, at Horsetown CCG, stakeholders had certain expectations of their role but these were not met. One of the academic GPs described how ‘nobody looked at the evidence . . . in the meetings . . . I was hoping . . . because I’m there and I work in research . . . maybe they will ask me . . . they didn’t do that’. If stakeholder roles and expectations are not considered, incongruence and conflict arising from ambiguity of roles may occur. Therefore, considering what stakeholders expect, and what they are expected to do, is important, and we identify ‘managing roles and expectations’ as a fourth capability.
Managing expert collaboration
Our analysis suggests that while engaging experts it is also necessary to manage ongoing collaborations between them as the service redesign process unfolds. This is because interprofessional health-care work is high-stakes and ‘fraught with tension and anxiety’. 85 In commissioning, individual jobs, contracts, issues of governance, compliance and patient care are simultaneously in question. The transformation manager at Rutterford CCG describes: ‘challenges . . . disagreements . . . debates . . . change is frightening . . . it can make you feel a bit insecure’. Stakeholders were well aware of the challenges inherit in this type of work, describing how vested and competing interests mean that having everyone ‘around the table had got that sort of political aspect to it’ (Horsetown CCG). These concerns could prevent stakeholders from ‘properly discussing’, interpreting and critiquing different forms of evidence, Moreover, during the lifetime of these redesign efforts, experts came and went. This meant that ongoing attention to managing collaborations appeared to be very critical. In some CCGs, expert collaboration was managed well. Stakeholders worked to have teams focus on a common ground, in many cases centred on patient care. In Seaport CCG, for example, a nurse described how the consultant leading the redesign work had:
A way of getting people to recognise . . . we’re trying to achieve the same thing . . . good-quality care for patients . . .
Collaboration was managed less successfully in other CCGs. In Greenland CCG, for example, the lead GP made efforts, but he was unsuccessful in managing these collaborations. He was aware that there was ‘anxiety about levels of knowledge and the increasing workload’ and felt that he had done ‘a huge amount of work’ to engage stakeholders and address these issues. He acknowledged, however, that evidence for the redesign effort was still being ignored ‘there are still some practices who say that they don’t know anything about it’. Concerns about collaboration persisted at Horsetown CCG:
[Y]ou suddenly think ‘oh, what are they going to change?’ There was quite a lot of concern over what was it going to mean . . . Technically we’re in competition . . .
Because collaboration between stakeholders can be problematic and can limit their ability to share ideas and discuss evidence openly, we identify the following ‘managing expert collaboration’ as our fifth, and final, capability for evidence use.
Chapter summary
In this chapter, we identified capabilities for evidence use: ‘sourcing and evaluating evidence’, ‘engaging experts’, ‘effective framing’, ‘managing roles and expectations’ and ‘managing expert collaboration’. Figure 2 shows capabilities for evidence use (dark green box), why they are important (light green box) and an example from the data (white box).
FIGURE 2.
Capabilities for evidence use.
Chapter 5 Selected cases
Introduction to case studies
In what follows, four case studies – Seaport, Greenland, Horsetown and Stopton CCGs – are presented. These four cases were selected as exemplars of our finding across cases. Owing to constraints on space in the main body of this report, the remaining four case studies – Rutterford, Chelsea, Coalfield and Shire CCGs – are presented in appendices. Developing these case studies addresses our first objective of studying how evidence is used in CCGs. These case studies also lay the groundwork for comparing evidence use across cases (objective 2) and creating a process model (objective 3).
Each case study begins with an overview of the redesign project and of the study participants. This overview is followed by a chronology of the main events. Decision episodes are applied to structure our cases. 32,51 Stakeholders across cases described how redesigns usually began when a problem was recognised. Akin to Robertson et al. ,86 we label this episode ‘agenda formation’. After this, work to generate a solution tended to commence. Again, we replicate Robertson et al. 86 and label this episode ‘solution selection’. Next, stakeholders described how (and if) they made changes to practice. 86 Other outputs included reports and future plans. We label this episode ‘process outcomes’. These episodes structure the cases described throughout this report. We identify in the cases key themes related to (1) evidence use and (2) capabilities for use so that these can be drawn out in cross-case comparisons. For ease of reference, we indicate evidences used with bold text, and indicate capabilities with bold italic text.
We also summarise whether or not redesign projects were ‘successful’ at the end of the case study. As seen, we adopt three broad criteria to assess this: (1) evidence used effectively, (2) satisfied stakeholders and (3) objectives met. Process maps of the case are also drawn53 and will be presented at the end of each individual case study.
The findings from Seaport CCG are presented first in detail to familiarise readers with our approach to data analysis.
Seaport Clinical Commissioning Group
Case study overview
When we first visited Seaport CCG, we spoke with a diabetes consultant, a senior diabetes nurse specialist, a GP lead for long-term conditions and a diabetes specialist nurse. At the time of our visit, Seaport’s diabetes services had been redesigned and changes had been implemented for around 18 months. In their interviews, stakeholders described how they had wanted to reduce diabetes care provided in the hospital because of ‘capacity’ issues. They also saw reducing hospital-based diabetes care as a means to reducing ‘complications’ and ‘costs’. The diabetes consultant was described as the project lead and was responsible for driving change forward with the support of other stakeholders. Although the consultant faced initial lack of interest in change, they managed to engage multiple experts by inviting them to contribute ideas about how diabetes care could be done better. After several meetings and discussions, stakeholders identified aspects of care that could be removed from the hospital. With structural, educational and financial supports, changes were made to diabetes care practice. At our last meeting, stakeholders were evaluating the impacts of these changes, with a view to further improvements. The redesign process is described in detail next.
Agenda formation
Experts at Seaport picked up multiple evidences showing problems with diabetes care. There was the diabetes consultant, for example, who described using his expertise-based evidence, describing how ‘the idea [for the redesign] came from . . . a group of us registrars . . . we always used to talk about it, saying it isn’t quite right [how we provide care] the way we’re doing it’. He described supporting his expertise-based evidence with local and universal evidence, too. These evidences showed issues with hospital capacity and the provision of diabetes care. Specifically, patients were waiting 18 months to be seen in the hospital (routine local evidence), which is well beyond the NHS maximum recommended wait of 18 weeks for ‘good’ care (universal evidence). The consultant describes the problem as follows:
You serve only 10% of the [diabetes] population [in the hospital] . . . 90% is out in primary care . . . if anybody got in trouble you’d send him to the hospital [but] the hospital never had the capacity to deal with that . . . you build up waiting lists . . . have people waiting to be seen for 18 months . . . [that] is a lifetime. It’s not good care . . .
Consultant
The community diabetes team, which was established in 2007 to ‘support and educate nurses, dieticians, administration staff, and consultants about improving care’ (report), also used local evidence to show issues with care caused by a ‘huge range of standards and quality of care’ (diabetes nurse) across GP practices.
As described above, multiple evidences (expertise based, local and universal) were used to show care and capacity issues. Two capabilities for evidence use are shown: effective framing and expert engagement. During the agenda formation episode, the problem with diabetes care is framed in terms of care and capacity issues. Specifically, care is not good enough because the hospital does not have the capacity. These issues are likely to speak to a range of stakeholders because they are about improving service efficacy and patient experience. Perhaps because of this, multiple experts (consultant, nurses, GPs) were engaged in the redesign from the outset. As noted in Chapter 4, expert engagement enables evidence to be contributed and evaluated from multiple sources. In this case, the diabetes consultant contributed expertise-based, universal and local evidences, with the last of these being echoed by the diabetes team.
Selecting a solution
Having framed the problem in terms of care and capacity, the consultant described seeking a better approach to service provision. He described the first step he took was sending ‘an e-mail round to everybody’ (the GPs), in which he suggested that changes to diabetes care services were necessary. This action shows efforts to further engage experts in the redesign effort. In this case, however, GPs, who are known to have access to expertise-based and local evidence11 were slow to respond. In the consultant’s words, responses were a bit ‘hit and miss’. He attributed this initial resistance to the fact that secondary care was ‘reluctant to make any changes’, while primary care was reluctant and had a sense of being underqualified. This resistance was informed by ‘historical rhetoric’, wherein consultants are capable of treating diabetes, but GPs are not (diabetes consultant). This type of historical rhetoric was seen as a barrier to actors sharing their expertise with each other.
To overcome this, the consultant ‘got a handful of GPs’ that he knew to ‘start the ball rolling’. He asked them ‘why don’t we have a discussion how can we do diabetes care better . . . you tell me what works’. This framed the solution search in a way that made stakeholders feel the ‘ball is in their court and provides an ‘open canvas’ for discussion’. Doing so also drew out expertise-based evidence and was a way to avoid getting:
. . . people’s backs up, because [they don’t] think hang on a second, you just made an independent decision . . . I don’t want that . . .
Consultant
Framing the solution search in this way further engaged experts and gave them clear role expectations. The GP, for example, described how his role was, at least in part, to act as a ‘similar other’ who could justify and legitimise redesign work to GP stakeholders. He described, for example, how he would say to other GPs:
. . . this affects me just as much as it affects you . . . I work in the practice, I look after diabetes patients, I understand that . . . I am going to have to do more than I have traditionally done but . . . I can do that because I am supported and we’ve got the right package of support that is available in the community . . .
GP
Stakeholders were also involved in sourcing and evaluating universal, expertise-based and local evidence. The consultant, for example, seemed to be more familiar with universal evidence. He described in his interview how the national situation emphasises community-based care. For example, he referred to ‘the Five Year Forward View’, in which it is noted that services can ‘close health, quality, and spending gaps by . . . creat[ing] integrated out-of-hospital care’. He also referred to other universal evidence (NICE) to understand how to achieve this community-based care. He noted that NICE says that antenatal diabetes requires ‘a midwife, an obstetrician, a consultant, and a scanning machine’, which means that secondary care is better equipped to provide this particular aspect of care. GPs also had a role in contributing expertise-based evidence. GPs used their ‘common sense’ about insulin pumps being too ‘high tech’. This knowledge meant that they wanted to avoid ‘having a crack at [that because] . . . that’s something that needs specific training’ (consultant).
The consultant also commented on the role of external individuals. He emphasised project managers, who were at the ‘core of trying to make things improve, they’re always there . . . their role was to steer the clinical view through’. Project managers provided finance data showing that the hospital may lose income initially because they would no longer be paid for care that was moved to the community, but that they could break even by making ‘a similar reduction in the workforce’ (diabetes consultant). The project managers were also necessary because clinicians come with their own values and perspectives on an issue and the evidence associated with it. Professional backgrounds can skew the interpretation and use of evidence, and so having a ‘neutral outsider’ was an important means to ensure that the redesign stayed on track.
After several months of discussions, stakeholders at this CCG managed to develop and agree a new pathway. Based on local, universal and expertise-based evidence, and aided by framing and role management, they identified that the majority of diabetes care could be moved to the community. They also agreed that a small amount of diabetes care needed to remain in the hospital (e.g. antenatal care).
Process outcomes
Having agreed the new pathway, stakeholders worked to implement changes in practice. Managing roles and expectations was particularly important in this episode of work and was achieved through education, training, support and financial incentives. Education and training, for example, was used to ‘upskill’ the primary care workforce so that they could provide the diabetes care that was normally offered in the hospital. This training enabled GPs to understand their role. Structural rearrangements were also made so that consultants assumed a new role in which they were deployed to community-based clinics. This rearrangement ensured the presence of expertise-based evidence while changes were being made practice. In some instances, they had a physical presence in the practice, with the consultant describing how he would say to the GPs, ‘why don’t you see me as your employee for 3 or 4 hours for the patients in your surgery’. In other situations, consultants were also available virtually:
When we have said that we [would be at the end of the phone] we meant it . . . we deliver it at the end of the day so it didn’t conflict with existing duties, we deliver till 7 in the evening . . .
Consultant
Financial incentives were also offered during this episode of work to encourage changes in practice. Incentives meant that stakeholders understood what they should be providing, and were not fearful of losing money as roles were changed to reflect the new pathway. The diabetes nurse describes how:
. . . each surgery could have one GP and one practice nurse undergone training to initiate insulin, so patients who previously have been referred to the hospital [could be seen in the community] . . . and they would get paid more . . .
Diabetes nurse
The project documents that were reviewed as part of this case study indicate that the new pathway was largely successful. By 2011, contracts were signed and the pathway was rolled out to the majority of surgeries. By 2012, a number of inpatient diabetics had been discharged and considerable savings were reported. Documents also note that ‘amputation rates [are also] improving’. Stakeholders commented during face-to-face interviews that the change to roles meant everyone was ‘doing what [they were] paid for’, including answering ‘e-mails and phone [calls].’ The diabetes nurse describes starting to see ‘that people weren’t falling through the net, there wasn’t this great big gaping hole that all these patients were sitting in and nobody was looking after them’. When probed about any initial doubts she might have experienced about the redesign work, the same nurse commented how, if there had been evidence:
. . . I guess I would have felt a bit more reassured but . . . it’s the people that put those models into practice . . . you can have the best model in the world but if the team don’t work together, then it won’t work . . . people make it happen . . .
Diabetes nurse
The nurse emphasised how the consultant managed expert collaborations because he was someone who ‘has this gift [laughs] with people . . . can build relationships, build bridges, get people thinking outside the box’.
Other influences included time to build the relationships (diabetes nurse). Being early adopters was also described as enabling change. The consultant described how there is currently a ‘huge move to move everything into primary care [which means] you’re overburdening primary care’. Picking up on this, the GP commented:
Some of the things that we were able to do . . . might not be so easy or so possible at the moment . . . we’ve got an overworked primary care workforce . . .
GP
The nature of the NHS was also a feature in stakeholder reflections. The consultant described the organisation being ‘risk averse’, which he saw as a barrier to redesign work:
I think the other thing is that in the NHS I think we’re too risk averse. That stops things from happening. I think clinicians, and I include nurses and doctors and any clinicians really, we are our worst enemies . . . because sometimes we think too much, we mull too much, we want to get the perfect system . . .
Consultant
At the same time, the NHS was seen as poor to measure and understand changes, which seems contradictory in ‘a risk averse organisation’. Yet, as shown in the following quotation, the GP sees the NHS as emphasising change without properly measuring impact:
One of the biggest challenges for the NHS is that there are so many changes . . . nobody ever gives one change a chance to work before they decide I’m making another . . . it would just be nice to let this bed and see over a little bit . . .
GP
Overall, the redesign at Seaport CCG was very successful. It used a mix of evidences without any major hurdles, it managed to implement changes in practice, and stakeholders seemed largely satisfied with the process. This last point was shown throughout the case study, but also by the fact that stakeholders were still engaged long after implementation. The GP described, for example, the new pathway as still being subject to interpretation and debate. The GP commented that they are still ‘learning and adapting’ to make it ‘fit for purpose as we move forward’, summarising the new pathway as having:
. . . the right principles, but actually we’re still not getting the outcomes that we would want and desire there so it needs some fine-tuning . . .
GP
Seaport summary of findings
Local, universal and expertise-based evidence was used to frame care and capacity issues related to diabetes services at Seaport CCG. A number of experts engaged with these evidences (e.g. GP, diabetes nurses and consultant). Led by the clinical consultant, these stakeholders began to work to select a solution. Although not everyone was enthusiastic to begin with, the consultant framed the solution search in terms of a blank sheet, which meant the multiple stakeholders selected and evaluated universal, local and expertise-based evidences. From this, stakeholders agreed a new pathway in which the majority of diabetes care would be moved to the community setting. They also agreed a small amount of diabetes care that would remain in the hospital.
Process mapping: explanation
Process maps are split into two levels, with the top level containing evidence (shaded in white) and the bottom level containing capabilities (shaded grey). Evidences are shown in differently bordered boxes as follows: local evidence is in a box with a solid line and represented by (L); universal evidence is in a box with a dotted line and represented by (U); expertise-based evidence is in a box with double lines and represented by (E); and trans-local evidence is in a box with dashed lines and represented by (TL). When more than one evidence is used, a double box is presented with both evidence letters included.
The capabilities are shown in circles and labelled as follows: engaging experts (EE), managing expert collaboration (MEC), effective framing (EF), sourcing and evaluating evidence (SEE) and managing role expectations (MRE). If outcomes were achieved (effective evidence use, changes in practice and satisfied stakeholders), these are indicated in hexagonal shapes.
Process maps should be read left to right, following the arrows. Agenda formation, solution selection and process outcome episodes are indicated along the bottom. The rotated triangles indicate barriers to progress. The process map symbols are summarised in a legend in Figure 3 and the redesign process at Seaport CCG is shown in Figure 4.
FIGURE 3.
Process legend.
FIGURE 4.
Evidence use process at Seaport CCG.
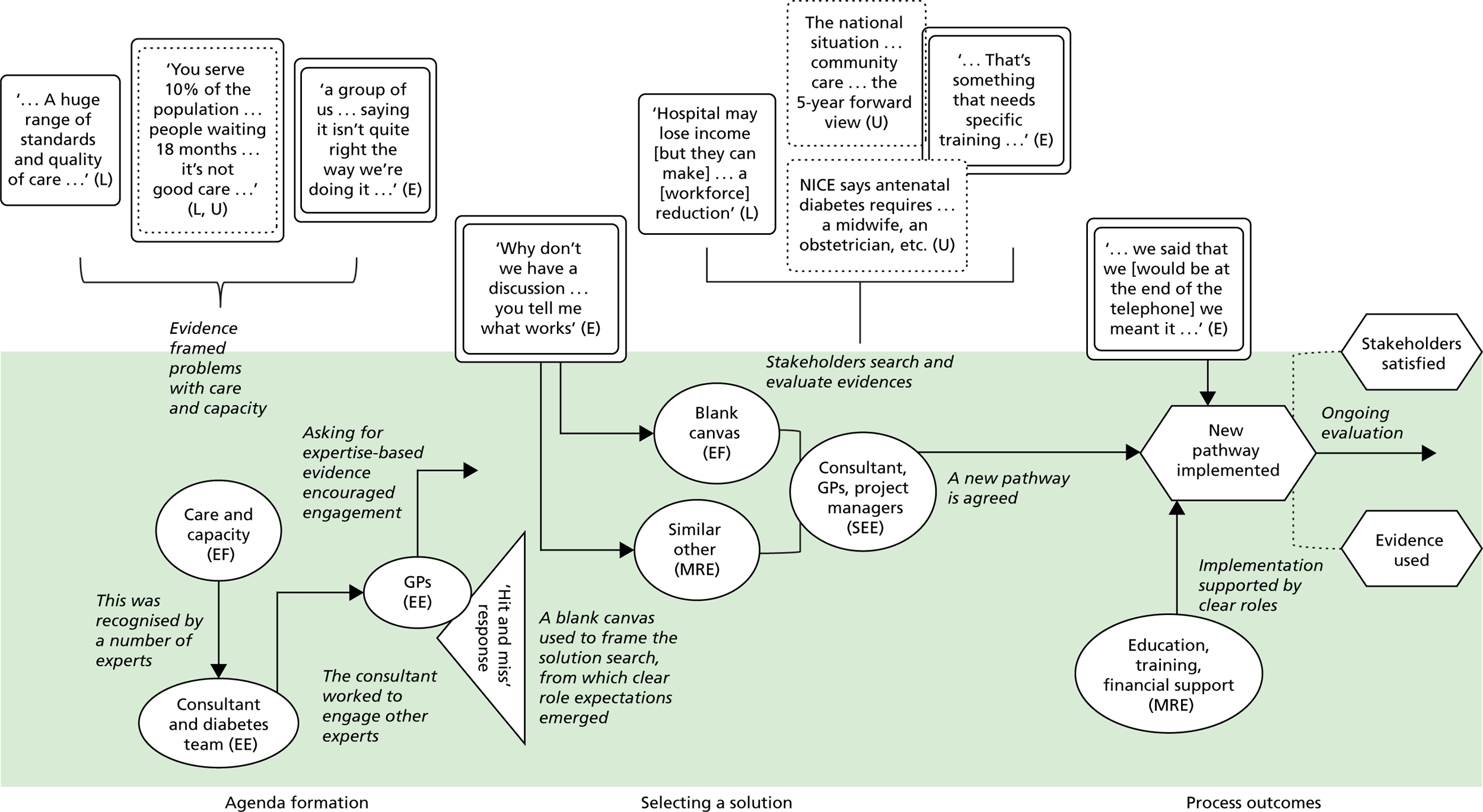
Greenland Clinical Commissioning Group
Case study overview
When we visited Greenland CCG, we spoke with the GP leading the project, another GP lead for mental health, maternity, and patient engagement, a project manager from the CSU and a non-executive hospital director. At the time of our visit, they had been working to redesign diabetes services for around 6 months. They described concerns over the quality of ‘care’ and ‘duplication of work’ in diabetes care. The GP lead and the CSU project manager were particularly involved in the redesign efforts. They were keen to mimic changes that had occurred in Seaport CCG because they felt that this was the best way to address their problems. Although efforts were made to push this new practice forward, they were met with several challenges. Almost 1 year after our first contact, we managed to follow up with a transformation and innovation lead at the same CSU. She had replaced the original CSU manager and informed us that the CCG had not yet managed to overcome the challenges it faced. The redesign project had been put on hold and the CCG was re-evaluating evidence to understand the need (if any) for the redesign.
Agenda formation
The GP lead and the CSU project manager picked up several types of evidences which showed a problem with diabetes care at Greenland. Local evidence, specifically information sourced from ‘primary care discharge letters’ and ‘feedback from patients during a consultation in practice’ (GP), was used to show that there was a problem with care. Other local evidence included routine data, which in this case showed ‘a greater number of complications . . . [and] amputation rates [that] are quite high’ (GP). These local evidences were given meaning by reference to universal evidence, with the GP describing how complications and amputations were high when ‘compared nationally’. They also used local and universal evidences to understand problems related to fragmentation and duplication of work. The GP described how ‘[the patient] goes to the hospital, [but] the consultant may not have the up-to-date bloods, and they may not have the up-to-date investigation’. National benchmarking standards and key performance indicators caused work to be repeated unnecessarily. The GP describes how the QOF (universal evidence) means that patients have the same tests multiple times:
To hit our QOF targets patients still have to go through a diabetes check at the local surgeries . . . patients find that frustrating because they’ll say I’ve been to the diabetes clinic at the hospital. Yet the hospital may not provide us enough clinical information for us to put that into our database . . . when I send a patient into A&E [accident and emergency] and I’ve recorded their blood pressure do they really need to have [it] repeated?
GP
The GP and the CSU project manager used local and universal evidences to show problems in terms of care and capacity. The problem was framed only in terms of ‘improving outcomes . . . [and the] patient journey’ (GP), which is narrower than in the previous CCG.
Selecting a solution
Having decided they wanted to change diabetes care, the GP and the CSU project manager led the work at Greenland. They felt that the problems were caused by ‘a lot of patients . . . being followed up in secondary care, which is not necessarily where they should be’ (local evidence; GP lead). At around the time, the GP attended a talk by the consultant from Seaport. He described how:
. . . it just struck me as a very good idea . . . the most innovative . . . there were others there, which we’d already dealt with, you know, GPs with special interest or practices who were looking after patients for neighbouring practices and that sort of thing, and none of them seemed very original. And we had already considered many of those in the past . . .
Seeing the work that had been completed Seaport as ‘particularly interesting’, the GP decided to bring it ‘to the board . . . as an idea and they were enthused by . . . and so we decided to develop it’. In doing so, the search for a solution is framed in a closed and predefined manner. That is, rather than using a blank sheet approach as in Seaport, stakeholders (lead GP and CSU manager) decided explicitly what the solution would be. Because this solution was transformed from Seaport for use at Greenland, it acts as trans-local evidence. The non-executive hospital director describes the value of mimicking Seaport CCG because ‘all the indications were it [Seaport’s model] seemed to work . . . there’s a better outcome’. He also felt that it would make their work less risky:
If it hadn’t been de-risked, and somebody came up with it as a concept it would be very much more difficult to get it implemented. The NHS is risk averse and people generally manager included will not do anything, which exposes them . . .
The GP made efforts to also engage other stakeholder experts (GPs, nurses). He described asking them to ‘read material, and try to evaluate the information’ about what had been achieved at Seaport. Meetings were held so that stakeholders could evaluate evidence. They were asked to contribute expertise-based evidence, with the GP lead describing efforts to involve ‘various stakeholders and trying to [get their] advice’. Patients were also asked ‘how we can try and improve patient care and patient ownership of their condition too?’ (GP lead). Local evidence was also drawn on in these meetings, with one of the GPs describing how they contacted ‘the hospital . . . to look at anonymised patient data . . . in terms of how appropriate those patients were to be seen at the hospital’ (GP). Last, they considered universal evidences at the meeting. They used NICE evidence, for example, to understand ‘appropriate management of patients [with comorbidities]’ (GP).
The CSU project manager had a clear role in making information accessible to clinicians who were too busy to search for it alongside their day jobs:
Without [her] we wouldn’t have been able to do this . . . we are doing our day jobs . . . she ultimately writes the projects . . . She’ll organise the stakeholder events, gets the information on the table . . .
GP
In addition to discussing evidences, meetings were about managing role expectations. The GP lead described ‘anxiety about levels of knowledge and the increasing workload’ among stakeholders. He reflected that he did ‘a huge amount of work [around] patient . . . and GP involvement’ to address this. He felt he had ‘been quite good’ at engagement, but did acknowledge, however, that ‘there are still some practices who say that they don’t know anything about it’. Other stakeholders we spoke with were not entirely convinced by the evidence for the redesign, questioning how it had been framed, with one of the GPs describing that:
. . . the CCG have done a piece [of research saying] 70% of the activity can be stripped out . . . we’re challenging that . . . I’ve spoken to a GP colleague [who had] one patient that she felt could be managed in their practice . . .
Perhaps because of this, there were issues with project ownership. The other GP felt that they lacked expert engagement because the project ‘belonged’ to the GP lead:
This is [his] project . . . we’re not really involved – we are in the sense that we’re involved to ask our opinions in terms of information from our diabetes patients but we’re not actively involved with the day-to-day . . . if you think about, if you’ve got only say five or six GPs, you know, that’s a lot of areas that you’re leading on.
GP
The GP lead also felt a lack of collaboration among stakeholders and felt that they did not want to make changes even though the case was ‘self-evident’. He described them as wanting to ‘deliver as they’ve always delivered it’. He also commented on resistance at the patient level because patients ‘like to be seen at the hospitals . . . to be seen by the specialist’.
Process outcomes
After the initial interviews, we struggled to engage stakeholders in follow-up interviews. Almost 1 year later, we managed to secure a follow-up interview with a CSU project manager who had taken over from the original manager. The new manager told us that the redesign project had been put on hold owing to a requestioning of the evidence and limited expert engagement. The lead GP had resigned in order to set up his own company. The original CSU project manager had left with the GP to work in the new company. This staffing change had created conflicts of interest because the GP’s new company offered:
. . . management support and solutions to GPs . . . It became a massive conflict of interest . . . he was the clinical lead for the work, they were writing a business case for it for investment for a community based team and all of a sudden he was going to go and start his own. So the project was stopped immediately . . .
CSU project manager
The second reason the redesign was put on hold was related to the framing of the solution. The CSU manager described that the initial framing was too narrow and that alternative approaches (other than community-based care) had not been widely considered:
It seemed [the commissioners] weren’t happy with the way the project had gone . . . There was a lot of flux . . . which muddies the waters as to who was at fault . . . their business case had not considered a lot of options . . . just the community team . . .
CSU project manager
Other challenges included a continued lack of interest and lack of engagement from clinicians. The fact that the project was led by a GP, meaning that the link to secondary care was not in place as it had been in Seaport CCG, was also seen as problematic. The new CSU manager reflected in her interview that ‘unless you have an acute diabetician who is very keen I think you are pretty much scuppered’. She also noted ambiguity around framing the case for need for change and uncertainty about the evidence, expecting that ‘[the CCG] won’t be high [on a list of underperforming CCGs]’. Access to secondary care evidence also problematised the redesign. Uncertainty numbers muddied the clinical need. The new CSU manager described difficulty in sourcing and evaluating the data:
It depends how it is coded though. So every intervention in a hospital is recorded and coded . . . it is just a matter of deciphering what it is that is relevant and what is not. So someone might come in with a stroke and it might not necessarily say they have got diabetes but it could have been the diabetes that caused the stroke . . .
CSU project manager
At our last meeting, the new CSU manager described how she was trying to identify what outcomes the CCG would want from the redesigning of diabetes services. She told us that there was no timeline for a redesign to happen in the area, and there was a general uncertainty about one happening at all. Overall, it can be said that the redesign at Greenland CCG was not very successful. There were several barriers to evidence use in this context. They did not manage to implement changes in practice, and stakeholders were largely dissatisfied with the process.
Greenland summary of findings
Local and universal evidence was used to frame issues with patient care. The GP lead and the CSU manager decided to mimic Seaport CCG’s redesign (trans-local evidence). Efforts were made to engage experts in the solution search. The CSU manager had a role in selecting the evidence. There were efforts to manage fears and anxieties, but not everyone felt included. After nearly a year, we managed to conduct follow-up interviews in which we learned that the project had been ‘put on hold’. This resulted from the narrow stakeholder engagement and difficulties in searching and evaluating evidence. There were also questions about the evidence used to frame the problem. The redesign process for this CCG is summarised in Figure 5. Guidance for reading process maps is shown in Figure 3.
FIGURE 5.
Redesign process at Greenland CCG.
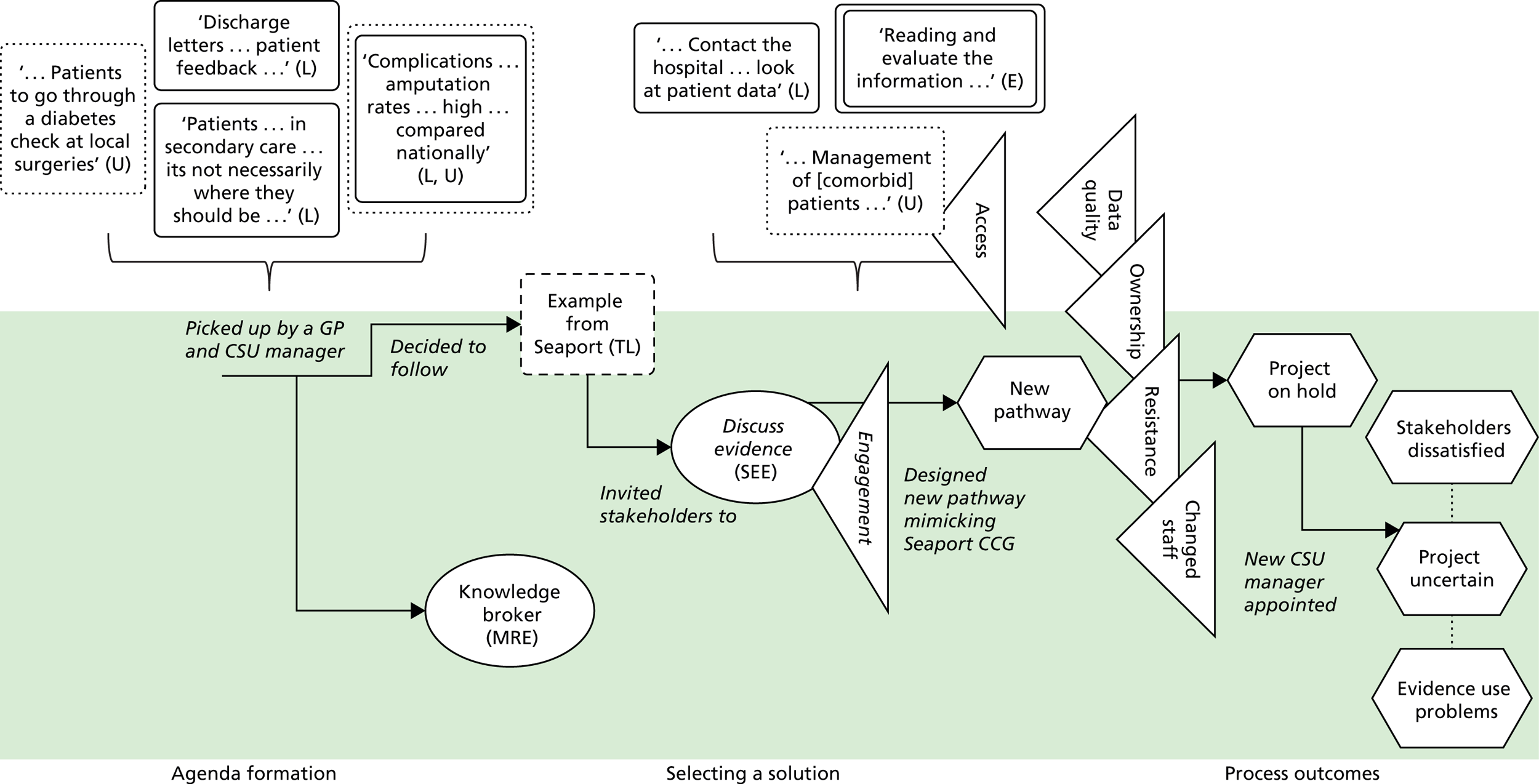
Horsetown Clinical Commissioning Group
Case study overview
We conducted interviews and observations at Horsetown CCG. The participants included three GPs (3), the CCG director, a senior commissioning manager, a commissioning manager, a radiologist, four physiotherapists and an orthopaedic surgeon. They were asked to source evidence and ‘provide clinical advice . . . and produce options for a simplified clinical model that improves patient outcomes, service quality and patient experience’ (report). Simply, they were instructed to produce a report about how MSK services could be redesigned to shift care closer to the patient’s home (terms of reference, January 2014; emphasis added by authors).
It should also be noted that Horsetown had already commissioned a MSK community service in which a ‘physio and a GP [were] in the room to triage the patient’ (CCG manager). This had been unsuccessful, and its failure seen to result in the hospitals not being ‘represented’ in design, GPs being unhappy about being ‘told what to do’ (GP), and expense (CCG manager). Referral between GPs had also been an issue, as was communication between primary and secondary care (GP). Thus, part of the current redesign was to ‘decommission that pilot community service’.
The CCG framed the redesign as an effort to reduce cost and improve care. The CCG developed a ‘clinical reference group’ (CRG) to advise on the redesign. The group was instructed to use multiple evidences to identify a solution. Several challenges were faced, however, and many stakeholders were dissatisfied with the process. The commissioner produced a new pathway that was heavily criticised by stakeholders. At our last meeting, a second pathway had been produced but stakeholders were uncertain that it would be put into practice.
Agenda formation
Commissioning managers at Horsetown CCG used local, universal and expertise-based evidences to frame problems with MSK services in terms of costs. They suggested that moving care into the community would reduce these costs. Local evidence showed that MSK services was a high-cost activity, but the CCG director was keen to emphasise that ‘new clinical solutions should precede financial calculations’ in meetings with stakeholders. A senior commissioning manager describes his framing of the redesign project as follows:
MSK is a high-cost, high-volume activity . . . as people age, they’ve got more problems . . . we were conscious that a lot of people were going into a hospital which is very high cost when we could find some other community based services . . . Because people have to travel [to the hospital] . . . If we can find some way of providing a service that’s closer to where they live then that has to be better for them in they’ll actually appreciate the service more . . .
Senior commissioning manager
Local and universal evidences included analyses of hospital admission and referral rates. Local evidence showed a ‘41% increase in elective hip and knee replacements . . . [meaning] referrals into orthopaedics . . . are increasing’. Universal evidence was also used, showing ‘the rate at which we intervene in orthopaedics in an acute setting is far higher than other CCGs’. Other universal evidence was shared, including the Right Care document,79 which showed MSK services as a source of potential savings. Drawing on their expertise-based evidence, commissioners justified a focus on community-based solutions:
We’ve set up things in the community before we can see the cost reduction, we can see the better patient experience. You know, we run things. I can’t give you specific examples but . . . it’s things that people like and it saves us money . . .
Commissioners established a CRG. The CRG engaged experts, including ‘GPs, specialists, radiologists, physiotherapists’. Role expectations were clearly outlined: the CRG was to advise on community-based solutions to current problems with MSK services in the region [general practitioner with a special interest (GPwSI)]. Evidences were communicated to the CRG prior to the first meeting to highlight the need to create community-based care.
Selecting a solution
The commissioning manager framed the solution search by asking experts to identify ‘how to shift hospital-based care to community . . . we want to use your expertise’. He described:
. . . engaging with people . . . Even if it’s not a significant change, you still need to take the hearts and minds of people . . . some of our contracting colleagues don’t quite appreciate [that] you can’t just simply say well, we’re changing the service as of tomorrow and expect everybody to comply. You can’t.
Commissioning manager
However, there was uncertainty in the CRG about the search and evaluation of evidence used to frame the problem. Clinician questions about the ‘validity of this [analysis]’ were a barrier to progression, with stakeholders re-evaluating the evidence and questioning ‘the actual proof of where it wasn’t working’ (private physiotherapist):
We’ve never had any numbers on referrals to secondary . . . what is the extent of the problem? Show me the extent of the problem and I’ll see if we can help . . . They [CCG] did a whole load of figures but it didn’t break it down . . . it just showed that there were a lot of referrals. It didn’t say that they were inappropriate . . .
A NHS physiotherapist contrasted her experience with a meeting at another CCG, where:
. . . they’d done a whole piece of work on ‘this is the demographic . . . the costs [and] it felt more robust . . . The consultants got a chance to sort of say ‘I don’t agree with that statistic and you know, can you tell us where you got that from?’. . . So I think in terms of getting over some of the political stuff, the use, the facts, the figures, the data . . .
Physiotherapist
The GPwSI summarised how the CCG wanted ‘us to find out how to improve the service but they didn’t do a lot of work on giving us information on what is actually happening’. The exception to this was one NHS physiotherapist, who was aware of the issues but explained how she had ‘the advantage of being the staff governor [and going] to the board meetings . . . so I understand the finances from the trust perspective’.
Despite the challenges and barriers, the CRG was tasked with sourcing and evaluating evidence about community-based care. Physiotherapists were tasked to look specifically at local evidence and ‘consider local evidence for self-referrals and telephone triage for patients’. Physiotherapists were also asked to consider universal evidence, specifically to ‘review published research . . . the START back pathways’. In both instances physiotherapists reported that the evidence was ‘difficult to evaluate’. Commissioners also relied on clinicians to use their expertise to translate other universal evidence from NICE:
There are NICE guidelines around orthopaedics . . . they’re very long . . . it’s like reading a book! If we’re going to . . . put in place something that works it’s got to be simple . . . [we] are the ones going to take on board all the information . . . and distil it into something, which is sensible and simple for people to follow . . .
Although commissioners were relying on clinicians to interpret universal evidence, this message did not seem to be widespread. When asked about using NICE evidence in a later interview, a GPwSI commented:
Nobody looked at the evidence, specifically, properly, in the meetings . . . I was hoping . . . because I’m there and I work in research and have a research background maybe they will ask me: ‘could you tell us the evidence about something, could you go and summarise the evidence and tell us what works for shoulder pain? Do injections work? Does physiotherapy work?’ . . . They didn’t do that.
GPwSI
Even if this evidence was to be explicitly used, the GPwSI commented that it was very hard to evaluate and so there were problems related to interpretation and contextualisation:
There are guidelines on management of knee pain . . . But they are national . . . [you have to] summarise them to help you locally which might be slightly different [across contexts] because the services are different . . .
GPwSI
Indeed, talk between physiotherapists at meetings showed variation in the evaluation of universal evidence. In one case, acupuncture for lower-back pain had been completely decommissioned from NHS providers, but private providers continued to offer the service (still paid by the NHS). Private providers commented that this was to be in ‘line with NICE’, but the NHS physiotherapist counterargued that ‘we got the stance that the NICE evidence is not very strong’. The private physiotherapist said: ‘I told the commissioners . . . we wouldn’t be stopping [acupuncture] because . . . it was in the NICE guidelines’. One NHS provider said: ‘It is frustrating that you do acupuncture. There is inequity here!’.
Alongside problems with interpreting and evaluating the available evidence, there was uncertainty about role expectations among stakeholders. Many were unclear about the purpose of their task. When asked what the main objective of the project was, for example, the GPwSI responded: ‘I don’t know! If you ask me now what is the objective I really don’t know. I can tell you a broad thing, which is improving MSK service but if you tell me what does that mean . . . I don’t know’. One of the physiotherapists commented that ‘there should be a little bit more of a framework . . . for us to work with because you know, we could have made suggestions that were completely random‘ (private physiotherapist). A NHS physiotherapist echoed similar sentiments, describing contrasting work done in another CCG that had:
. . . a completely different approach, they brought in a company that presented a lot of analysis . . . then they sort of presented a model they want to work towards . . . they’re being very overt in their intention about the lead provider model. So that seems to be moving apace. I mean, that’s really moving and very different feel about it.
Others felt that expert engagement was lacking and that their contribution to interpreting the evidence was tokenistic. The GPwSI said in an interview:
The whole system needs to be aware of it [the STAR Tool] because it all works on GP, sick patient, and applying the tool and decide this patient need to go to have this, or this patient need to go to have that, but then you’ll need to let the physiotherapist know about this . . . But then nothing happened.
Finally, political issues around managing expert collaboration were noted. One physiotherapist described ‘so many people in the room, with so many different ideas . . . we were floundering a bit’. She noted how ‘There was quite a lot of concern over what was it going to mean . . . what would be the effect on my business?’. Another physiotherapist said she felt ‘a little bit wary about what information to share and what to hold back’. Another physiotherapist summarised: ‘Technically we’re in competition . . . I’m not sure the commissioners fully appreciated that putting that group around the table had got that sort of political aspect to it’. Whereas the physiotherapists might have felt that politics were not acknowledged, a commissioning manager later noted:
We recognised that everybody has got a vested interest . . . we tried to make sure we got all the vested interest in the room . . . So rather than have no agendas in the room, if you don’t have anybody with an agenda in the room, you don’t have any expertise in the room, so better to get them all.
However, difficulties in managing collaboration once experts were ‘in the room’ were also quite evident.
Professional boundaries also caused issues around ‘what conditions ought to be included in the MSK pathway?’ and, therefore, what evidence should be sourced. A physiotherapist reflected how they all
Realised we were talking at cross-purposes because what us physios were talking about as MSK triage was totally different to what the commissioners understood to be MSK triage . . . because to them MSK triage was a service . . . To us triage is what we do day in, day out.
Even with these challenges, several meetings were held so that each group could present and discuss its evidence search. One NHS physiotherapist described this evidence sharing as necessary because ‘there was a wealth of data there . . . And [the] presentation about ‘this is the challenge’, ‘what can we do about it’. Some discussion, some feedback’.
Process outcomes
Quite suddenly, and in the face of ongoing hurdles around the evaluation and interpretation of evidence, task purpose and politics, the commissioning manager attempted to reframe the problem by sharing a drawing of a new pathway with the group. No other member of the group (apart from another commissioning manager) had contributed to this new pathway, meaning that expert engagement was lacking from this. In presenting the pathway, the manager suggested: ‘This is what the pathway might look like . . . no way final’. Multiple reactions from providers resulted (based on their particular expertise). Comments included: ‘there is nothing about diagnostics’, ‘this is clearly about cost, I am not clear what happens’, ‘How do you oversee the appropriateness of this new pathway?’ and ‘I don’t think GPs will be interested’. The proposed pathway also reinforced stakeholder views that their engagement was tokenistic and that their contribution was not taken seriously:
It felt like . . . we did the work and [commissioners] ticked a box by asking us . . . but . . . in one of the last meetings . . . it was kind of ‘oh, and by the way we’ve introduced this model with the GP provider in the city and this is what’s happening’ . . . it was kind of well, why did you ask us to do that then?
NHS physiotherapist
We started off with thinking . . . it’s great, they’re listening . . . the last meeting they went ‘oh, by the way, here’s the model’. And you went: ‘actually that doesn’t bear resemblance to the work or what you asked us to do . . .’
Private physiotherapist
The map was redrawn and shared again. The commissioning manager explained that they had looked at another CCG (trans-local evidence) that had ‘reduced their costs since introducing the new [pathway]’. When the pathway was recirculated, replies included: ‘your diagram looks very simple . . . I [think it is] important [to] include patient support groups, education for GPs, decision-making tools for patients and clinicians’ (hospital manager). At our last contact, the senior commissioning manager described the ‘next phase would be looking at how [to] implement it and . . . scope what is included in the MSK . . . And then we need to work out how we’re going to actually get those services in the . . . local area’. Others were less clear, with the GPwSI commenting that ‘it became . . . like loose end. It’s not stopped, no conclusion, we were not told what will happen next’. A NHS physiotherapist also mentioned how she was ‘a little bit disappointed with . . . where we’ve got to . . . because it feels like everything is suspended at the moment’.
Overall, it can be said that the redesign at Horsetown CCG was not very successful. There were several barriers to evidence use in this context. The CCG did not manage to implement changes in practice, and stakeholders were largely dissatisfied with the process.
Horsetown summary of findings
Horsetown CCG sought to identify how community-based services could be provided, framing this in terms of local (e.g. increases in procedures) and universal (e.g. more surgery than other places) evidence. Work was done to engage experts in CRG, who, in turn, questioned the evidence validity. CRG members were tasked with evaluating a variety of local (e.g. referrals to triage) and universal (e.g. START back pathways). This evidence search was narrowly focused on community-based options. The CRG reported that the evidence was difficult to interpret. Issues with expert collaboration and role expectations also problematised their work. A commissioner suddenly developed a new pathway, which was met with several criticisms. The pathway was redrawn (using trans-local evidence). At our last contact we were told that more work would be needed (e.g. defining MSK) to take the pathway forward. The redesign process for this CCG is summarised in Figure 6. Guidance for reading process maps is shown in Figure 3.
FIGURE 6.
Redesign process at Horsetown CCG.
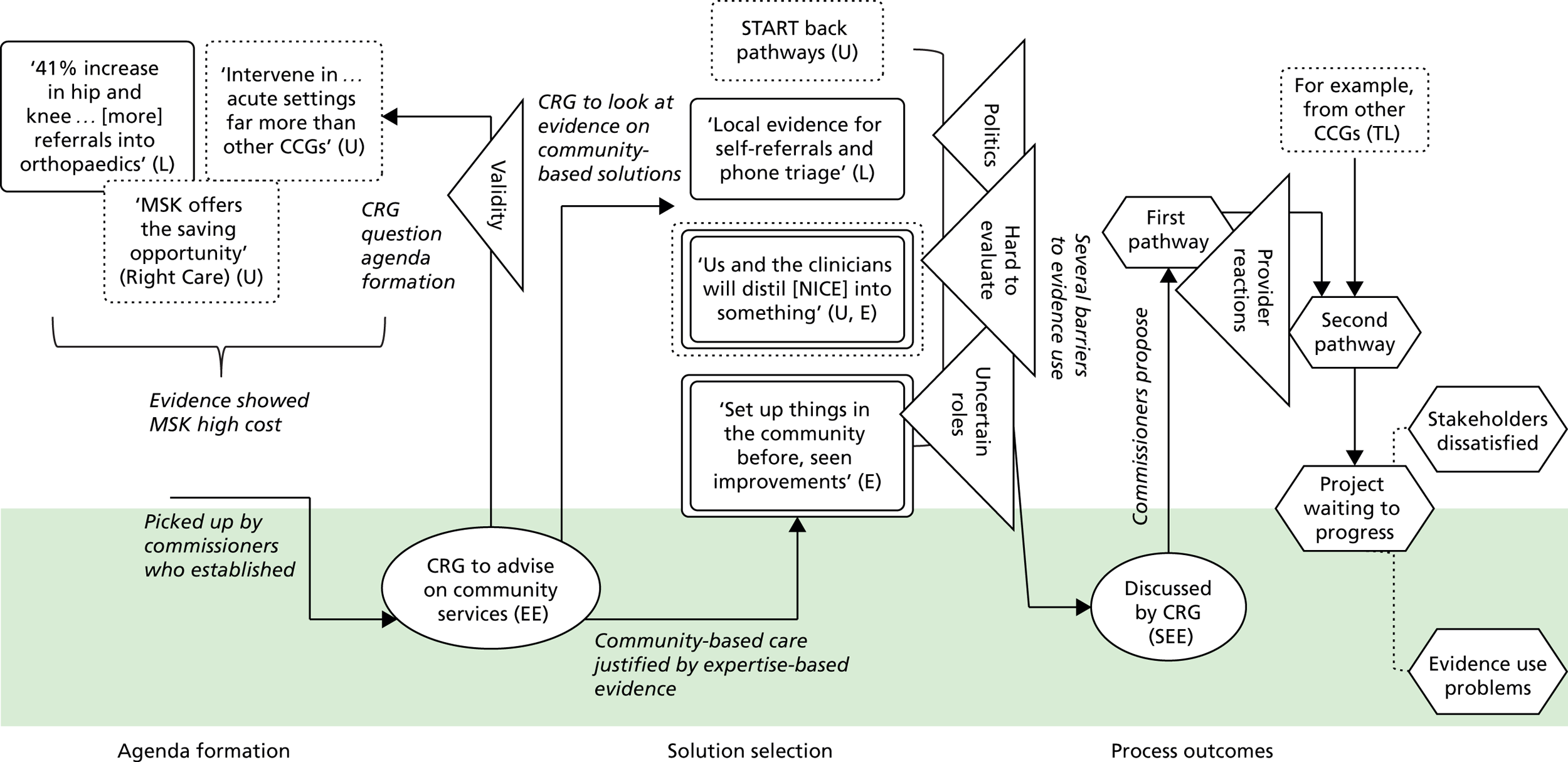
Stopton Clinical Commissioning Group
Case study overview
We conducted interviews and observations at Stopton CCG with participants: a senior commissioning manager, the head of therapies, a clinical assessment team lead, a GP, an orthopedics consultant, a rheumatology consultant, a physiotherapist and a hospital representative. Stopton had been working on the lower limb MSK pathway and wanted to create ‘a gold standard [with] outcomes [built] into contract’ (report). Once we arranged to do fieldwork at the CCG, however, it had decided that it needed to address issues with its triage service before tackling the whole pathway. As such, the triage is the focus of the case study. The team had been working together for about 15 months, believing that ‘the patients might not be going through the right service’ (senior commissioning manager). In terms of the pilot, there was already a triage in place but the team felt that it was necessary to redesign it.
A transformation group was established once issues with care and capacity were recognised. We followed the team for just under 1 year. During this time, they discussed a mix of evidences, many of which were provided by the team lead. They were hampered by a lack of funding but still managed to design and implement a new pilot. There were several challenges to evaluating the effectiveness of the pilot. At our last point of contact, the team were conducting further evaluation and waiting to present it to the next board.
Agenda formation
A triage service had been established in Stopton in 2009 in which patients could refer directly (or be referred by a GP) to a MSK service (e.g. physiotherapy). This type of triage is intended to stop patients going to the hospital unnecessarily and so improve their care experiences. At the same time, it prevents unnecessary hospital care and so, as a by-product, reduces costs and addresses capacity issues. At our first meeting, the transformation manager at Stopton explained that the existing MSK triage service had ‘played its part when it was created’ but there were some problems with it. Issues were framed in terms of care and capacity and based on local evidence, which suggested that more patients than anticipated were using the self-referral option and that the GPs were still doing a lot of work:
Physio was completely over the planned demand so they started to struggle. The GPs were still being inundated by MSK . . . they would just be referred on, sometimes appropriately, sometimes inappropriately . . .
Transformation manager
The clinical assessment team lead described how local evidence was gathered from ‘a couple of engagement sessions’ in which clinicians mapped out the current pathway, looked at ‘where the bottlenecks were’ and considered ‘how we could actually change’. At meetings, stakeholders seemed to agree that there was a problem that needed to be addressed (i.e. the frame was strong).
Because the contract for the current triage service was ‘running out’, a group of providers was engaged in discussions. From this group, a ‘transformation team’ was established with the aim of trialling a new triage ‘this side of Christmas’ (transformation manager). This team engaged experts who contributed to designing a better triage service (transformation manager). Although the commissioners initiated the triage, it was hoped that the transformation team would be the ones to manage the ongoing collaborations deemed necessary to ‘drive it forward, steer it’ (transformation manager). In a later interview, the clinical assessment team lead commented that this engagement and ongoing collaboration with GPs, patients and providers meant that:
. . . there was common ground . . . here I think it’s been more like an open discussion of actually how we can all move forward together. And I think that’s why it’s been so successful in actually being able to do the change because it, you know, nothing has been imposed, it has been consultation . . .
Selecting a solution
Recognising issues with the MSK triage, the team set about discussing a new pilot. The clinical assessment team lead had clear role in sourcing evidence for evaluation, including ‘article[s] in physio journal[s]’ as well as challenges set by the NHS and other bodies (e.g. the Prime Minister’s Challenge). The local evidence showed ‘some GPs have MSK expertise, some don’t’, meaning variation in care (head of therapies). The team decided to move away from GPs being the first contact; instead, patients would go straight to ‘an MSK centre, [and they] would receive the same input, same level of expertise and . . . the same outputs’ (head of therapies).
Local evidence suggested that there would be no objections ‘because in this area . . . we actually haven’t got any . . . GP with special interest in musculoskeletal’ (clinical assessment team lead). Trans-local evidence about another CCG that had trained receptionists to triage patients was also used. The script contained ‘red flags’ that sent patients to an urgent clinic, a general MSK clinic or a clinician (clinical assessment team lead). Other trans-local evidence was also discussed, with stakeholders describing how similar services had been implemented: ‘in Berkshire . . . Pennine Trust do something . . . trying to think, was it Surrey? No, no, Lake District . . . in Cumbria’ (therapies lead). Taking these evidences together, stakeholders concluded that the proposed ‘type of model will work quite well . . . you’ve got to look at your demographics of your patients, also your GPs and everything and try and get the best fit of what actually happens’ (therapies lead).
The team talked about running the pilot at meeting, including discussions of shared time frames. They agreed ‘6 to 8 weeks in one practice’ (transformation manager). Some felt that this was insufficient, commenting that ‘if it’s too complicated to do it in more than one [practice] it might be too complicated anyway’ (hospital representative). This was countered with ‘it’s not that it’s too complicated, it’s a resource issue’ (clinical assessment team lead). Resources were limited at Stopton because they had applied for money to support the redesign but had been unsuccessful. This meant that ‘there was no extra funding to actually do this, we were actually doing it on existing staff and whatever’. Thus, they agreed to ‘scale back’ some of their plans (transformation manager).
They also talked about reactions. The GP drew on expertise-based evidence to say that it would be ‘good for patients’ and so would be welcomed by his GP peers. He expected some contention, but felt that this would be minimal because ‘it’s not about replacing GPs, it’s about modern medicine’. The team agreed that GPs would ‘probably be OK’ with self-referred patients. The transformation manager later noted how important the GP was in engaging ‘his colleagues . . . making sure they are happy’. The GP also allowed the pilot to run in his practice, thereby allowing further local evidence to be sourced and evaluated.
Last, the team discussed how they would evaluate the pilot to understand its efficacy. They wanted to get both qualitative patient feedback and comparisons against local data, thus creating evidence as they went. The former (patient feedback) was viewed as ‘difficult to capture’, but they felt that they were fortunate with the latter because:
. . . compared to others we get quite a lot of really good data so we get really detailed referral data [on] which elements of the service have they [the patients] gone into. So have they gone into triage? Have they gone into physio?
Transformation manager
The transformation manager explained that much of the evidence would be collected by the clinical assessment team lead because she was ‘really good at keeping her own track of patients’. As such, this individual had a clear role in bringing evidence to the table. The same individual benefited the team in terms of managing collaboration among stakeholders too, particularly because ‘she is that kind of link between primary care and secondary care’ (transformation manager).
Process outcomes
Overall, the pilot progressed as planned. The transformation manager attributed this to a ‘subset of the community contract already [being in place]’. She commented that it was not ‘a big bang change’ that involved ‘moving care from the hospital’ because this type of change had already been done in original triage and so stakeholders were not worried about risk. Stakeholders described how they conducted ‘an assessment, and then tried to actually start [patients] off on self-management, and then signpost them to the most appropriate management option’ (clinical assessment team lead). They also collected local evidence to help them evaluate the pilot:
We kept a record of where we sent patients to . . . Then we entered it into a database after we’d done the 3 months’ trial . . . They also sent out patient satisfaction questionnaires . . . on the day of the assessment . . .
Clinical assessment team lead
The evidence showed that pilot was not as successful as expected. Patients were still going ‘to the hospital because they know the hospital’ (therapies lead). The transformation manager explained this pattern with a shopping metaphor, where people think ‘I always shop at [supermarket] but now there’s a corner shop . . . I might try it but actually I’m going to go back to what I know’. They also had issues interpreting the data because of ‘a lot of hidden activity’. Broadly, their local evidence showed that a lot of patients referred into the ‘self-management’, which could mean anything from ‘advice and exercises, advice on diets, advice on what to do with their condition . . . let’s see how you go’.
They presented their results to the ‘Service Development Committee’ (SDC), which has responsibility for deciding next steps. The transformation manager described how the committee would have:
[T]he chance to question [their data] . . . And then . . . decide what they want to do . . . they could turn around and say actually . . . we’ve looked at this and we don’t want to do it. And then it’s back to the drawing board. And they’ll probably say we want you to come back with some other options or we want you to work with the existing model . . .
The SDC’s judgement was based on expertise, especially ‘in terms of what’s bothering them, does this address it?’ (transformation manager). In this instance, its judgement was that the team should ‘do a supplementary, week-long perspective audit to find out what this hidden demand is so that we can accurately cost up the service’ (transformation manager). Essentially, it was not convinced of the pilot’s efficacy. The team planned to source more evidence, suggesting that with ‘a bit more data we can make a robust, cost-effective case for the model that they are happy with’ (transformation manager).
Overall, the project at Stopton CCG was largely successful. Although it had issues interpreting evidence, this related to evidence collected during the pilot. It achieved its objectives, and stakeholders were largely satisfied with the process, which is indicated by the fact that they were willing to persist beyond the SDC’s rejection.
Stopton summary of findings
Stopton CCG sought to pilot a new MSK triage. It used local and universal evidence to frame this as a care issue and did a lot of work to engage experts and manage collaboration. There were also clear roles, especially with the clinical assessment team lead sourcing and evaluating the evidences. The team used trans-local evidence to identify how the new pilot would run, and, bar some minor challenges, they seemed to be on board with the pilot. The transformation manager saw this as resulting from the fact that there was no real risk involved. The team ran the pilot and collected evaluation data. At our last contact, they had presented this to the SDC, which had requested an additional audit.
The redesign process for this CCG is summarised in Figure 7. Guidance for reading process maps is shown in Figure 3.
FIGURE 7.
Redesign process at Stopton CCG.
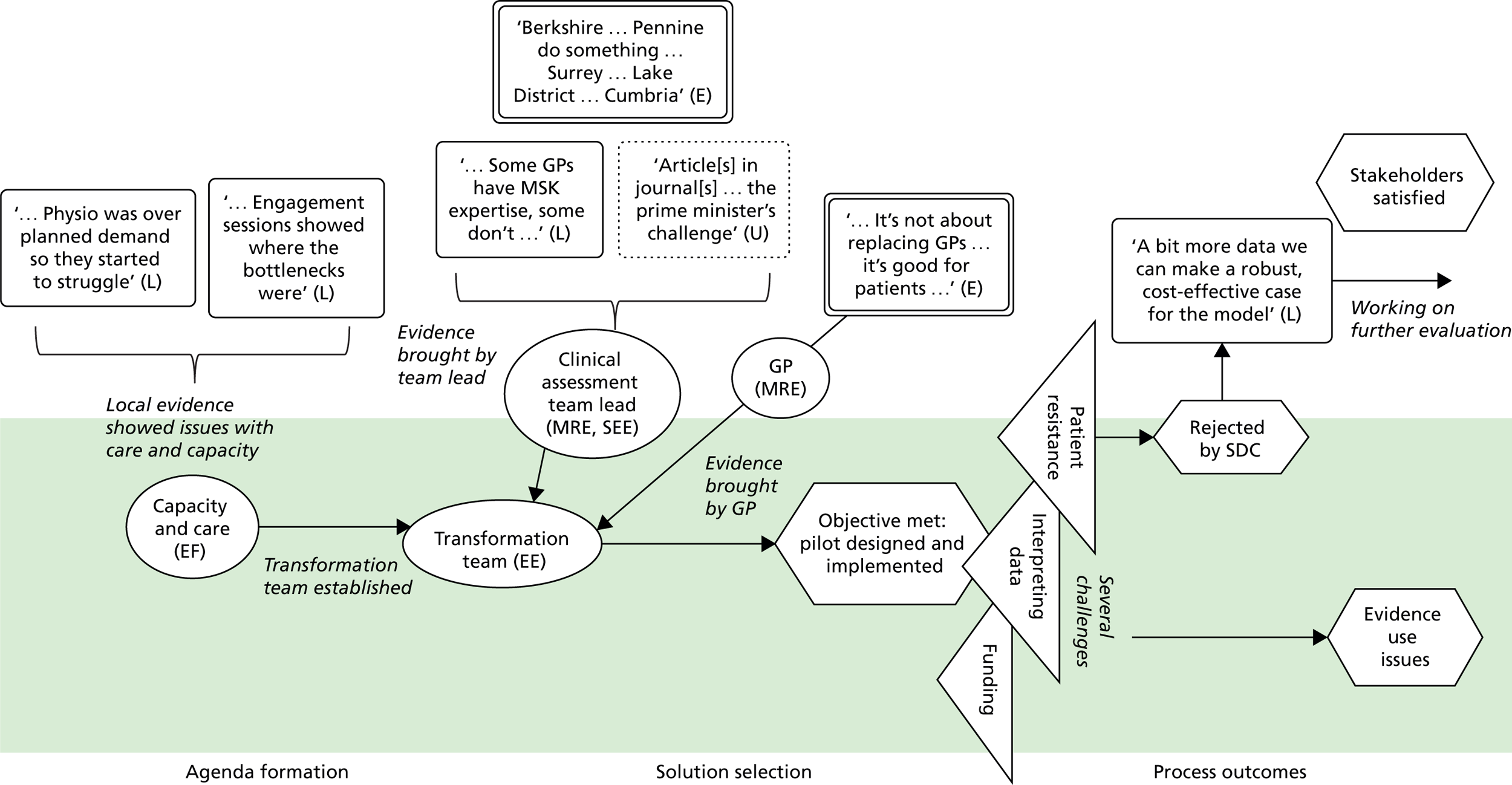
Chapter 6 Cross-case comparisons
Overview
The second objective of our research was to compare and contrast across cases in order to gain insights into capabilities for evidence use by commissioning organisations. In doing so, we endeavoured, also, to understand qualitatively how and why variations in evidence use occur in practice. We began this with our coding exercise (see Chapter 2), which used the whole data set to identify what forms of evidence were being used by CCGs and which kinds of capabilities actually mattered to innovation (the service redesign) process. We have presented these for each of our cases above (and in Appendices 1–4). In this chapter, we extend this analysis by directly comparing patterns of evidence use across the eight cases. We then consider the presence and absence of capabilities across cases, before summarising the outcomes. Cross-case comparisons are summarised in Table 6.
Evidence use
As shown, local, universal, trans-local and expertise-based evidences are used together in redesign work. Evidence use is shown in the process maps. Rather than one type of evidence being more ‘authoritative’, with regard to carrying more weight and legitimacy, different evidences are used, often in tandem, in decisions. During the agenda formation and solution selection episodes, multiple forms of evidence were apparent in every case. Local evidence was often judged against universal evidence. At Greenland CCG, for example, local and universal evidences to show ‘amputation rates were quite high . . . when compared nationally’ (transformation manager). In some cases, expertise-based and trans-local evidences were also used. When describing process outcomes episodes, stakeholders made rather fewer references to evidence use. This finding should be considered with caution because only just over half of our CCGs (n = 5) actually began to implement changes. Many stakeholders also described plans to implement or pilot new models and then evaluate efficacy, so that process outcome episodes become more about creating new evidence than about implementation per se. This reflects the open-ended nature of the decision processes in this context; projects to improve services were rarely ‘finished’ but were ongoing endeavours in which work generated new forms of evidence and judgements that changed, or sometimes halted temporarily, redesign efforts.
This open-endedness of management decisions raises challenging questions about how to assess and understand variation ‘decision outcomes’, in our case in terms of whether or not they are evidence based. In other words, if ‘outcomes’ are continuously emerging, then decision-making is never quite complete, which makes it difficult to judge the efficacy of evidence use. We can, however, compare cases in terms of the processes themselves and see patterns in the extent to which (in the time frame observed) these are relatively smooth (or not) in so far as they allow actors to take action and proceed to the next step. Table 6 provides a summary of evidence use across cases and across episodes. In sum, the patterns of evidence use are as follows:
-
Because it was used during agenda formation and solution selection in all eight CCGs, it can be said that local evidence plays an important role during initial redesign episodes. Of the five CCGs that managed to actually implement their service redesign, local evidence was mentioned in three. This was largely in relation to conducting evaluation (e.g. Chelsea CCG) or understanding local needs in more detail (e.g. Shire CCG, Stopton CCG).
-
Universal evidence was used during agenda formation in seven CCGs. Stopton CCG was the only CCG not to explicitly use universal evidence in this episode. Universal evidence was also used during solution selection in all eight CCGs. Therefore, universal evidence is equally important during initial redesign episodes. Chelsea CCG was the only one to refer to universal evidence during process outcome episodes.
-
In all CCGs, bar Shire, expertise-based evidence was explicitly used in solution selection episodes. Interestingly, expertise-based evidence was relied on in just two CCGs in agenda formation and process outcome episodes. This evidence was used to identify the problem (Seaport CCG) and to push a better way of doing things forward (Chelsea CCG). Expertise was then used to guide practice changes at both CCGs. Both Seaport and Chelsea CCGs were implementing community-based diabetes care (with Chelsea explicitly mimicking Seaport). Both were also led by a diabetes consultant, which may explain the emphasis on expertise-based evidence in these contexts.
-
Last, trans-local evidence was used during agenda formation at Chelsea CCG, during solution selection at Greenland and Chelsea CCGs, and during process outcomes Coalfield and Horsetown CCGs. The reasons for using trans-local evidence varied, however. At Coalfield CCG trans-local evidence was used in terms of evaluating outcomes using examples from other countries, whereas at Horsetown this evidence was used to justify the new model.
Local and universal evidences are most commonly used, perhaps because CCGs are under pressure to implement evidence based (usually meaning universal evidence) in context. Whereas previous research suggests that local ‘trumps’ universal evidence, our findings suggest that the two operate together. We saw many instances in which local evidence is judged against a backdrop of universal evidence (albeit not always formally presented) and becomes ‘evidential’ because discrepancies are seen that indicate there is a problem. Thus, our findings suggest that local evidence needs universal evidence in order to be interpreted, and, on the flipside, universal evidence needs local evidence in order to be made to fit the context. Without local evidence and expertise, universal evidence lacks precision and applicability.
Expertise-based evidence was used across episodes but appeared to become particularly important in solution selection. Here, individuals’ experience of ‘what actually works’ played a particularly important role in concretising designs ‘on paper’ into designs ‘in use’. Hence, in those cases where we saw relatively more positive outcomes, we also saw the design being worked through, and championed, by individuals with significant experience and legitimate authority among other groups that were to be relied on to implement (or pilot) the redesign. Trans-local evidence was used across multiple episodes, acting as useful heuristics or short-cuts to redesign efforts. However, although it was often assumed that these were based on ‘good evidence’ (hence the label ‘best practice’), this was not always the case. This suggests that trans-local evidence, although helpful, needs to be interrogated and unpacked, not simply mimicked, or its ‘evidential’ qualities taken at face value, especially as the local conditions (e.g. population demographics, public health economics) vary significantly.
Capabilities for evidence use
Capabilities that enable evidence use in redesign work included ‘sourcing and evaluating evidence’, ‘effective framing’, ‘engaging experts’, ‘managing expert collaboration’ and ‘managing roles and expectations’. We distinguish between the presence of a capability, the failure to apply a capability and the absence of a capability in the data in Table 6. When a capability was present in the data, a tick is used (✓). A dash (–) indicates that the capability was not shown in the data, while a cross (✗) indicates that a capability was not applied. We see the distinction between failure to apply a capability and the absence of it in the data as important, because all capabilities may not be needed for evidence use in certain contexts.
| CCG | ||||||||
|---|---|---|---|---|---|---|---|---|
| Seaport | Greenland | Rutterford | Chelsea | Coalfield | Shire | Horsetown | Stopton | |
| Evidence use | ||||||||
| Agenda formation | L, U, E | L, U | L, U | L, U, E, TL | L, U | L, U | L, U | L |
| Solution selection | L, U, E | L, U, E, TL | L, U, E | U, E, TL | L, U, E | L, U | L, U, E | L, U, E |
| Process outcomes | – | – | – | L, U, E | TL | L | TL | L |
| Capabilities | ||||||||
| Engaging experts | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Managing expert collaboration | – | ✗ | ✓ | – | ✓ | ✗ | ✗ | – |
| Effective framing | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Managing roles and expectations | ✓ | ✓ | ✓ | ✓ | – | ✗ | ✗ | ✓ |
| Selecting and evaluating evidence | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Outcomes | ||||||||
| Evidence use | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ |
| Objectives met | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Satisfied stakeholders | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ |
In sum:
-
Rutterford was the only CCG in which all five capabilities were shown.
-
Seaport and Stopton CCGs showed four of the five capabilities. Here, ‘managing expert collaborations’ was not prevalent in the data. It may not be necessary to manage collaborations if issues do not arise, which would explain the absence of this capability in CCGs where multiple other capabilities were recorded.
-
Coalfield CCG also showed four capabilities, bar ‘managing roles and expectations’, which was not recorded in the data, perhaps because the group leading the redesign had been working together over a long period of time. Because of this, roles and expectations may have been established, which would remove the need for them to be managed.
-
Chelsea CCG showed three of the five capabilities. ‘Managing expert collaboration’ did not appear in the data, perhaps because the consultant led the project almost single-handedly. As mentioned, our study missed prior discussions, and perhaps disagreements, about community-based care. During our fieldwork such agreements had already been reached. ‘Sourcing and evaluating evidence’ was also not achieved at this CCG, but this may have been less of a concern because they had decided to mimic Seaport CCG.
-
Shire CCG also showed three of the five capabilities, but in this case efforts to manage expert collaboration and manage roles and expectations were not successful.
-
Both Greenland and Horsetown CCGs showed two of the five capabilities. Both failed to ‘manage collaborations’ or apply ‘effective framing’. Greenland CCGs also failed to engage experts’, while Horsetown CCG failed to ‘manage roles and expectations’.
Certain capabilities also appeared to be particularly important in certain episodes of work. Effective framing, for example, was noted in both agenda formation and solution selection episodes in those cases that had relatively positive outcomes. Expert engagement, where observed, also occurred on early in the process in relatively more successful cases. In most instances, a problem was identified and then redesigns leaders worked to engage other relevant stakeholders in fine-tuning their understanding of the central issues. Sourcing and evaluating evidence, managing expert collaborations, and managing roles and expectations all tended to be important when stakeholders were working to select a solution.
It is important to note that the capabilities we observed, while distinguished analytically, were also linked. For example, the way in which problems and solutions were framed also had implications for engaging experts, managing collaboration and sourcing evidence. Here, a ‘blank sheet’ approach helped to engage active contributions of experts and also helped to widen the search for information, because it did not pre-prescribe a particular course of action. However, using a completely ‘blank sheet’ also had drawbacks, leaving experts puzzled as to quite what their roles were, opening up room for conflict and ongoing disagreement around what actually comprised evidence, and generating inertia. Effective framing (blank but not too blank) appeared, in contrast, to let stakeholders set aside their own interests and values, which are at least partly responsible for failed collaborations, but also to contribute expertise and information focused on a shared goal (e.g. the patient). Our findings suggest, then, that it is not just the presence or absence of a particular capability that matters, but, rather, the ways in which capabilities are used together in particular contexts. As seen, lack of capability in one area (e.g. managing roles and expectations at Coalfield CCG) may not preclude positive outcomes where contextual conditions substitute for it in some way (e.g. in that case by having a long-established group that already had clear role expectations).
Outcomes
As discussed, commissioning decisions are characteristically open-ended and iterative (as, we would argue, are most complex management decisions). Therefore, there is often no clear end (or start) point. Here, a process approach is useful as it reminds us that ‘outcomes’ are always in flux (and can be ‘inputs’, if the focus is to explain the next step). This means that the success or otherwise of ‘outcomes’ is always subjective, being judged relative to where one is (or hoped to be) at that particular point in the process. Nonetheless, we can use qualitative indicators to compare cases in terms of whether or not process outcomes were relatively successful, when judged against actors’ own expectations. We thus assessed outcomes qualitatively in terms of evidence use, objectives met and stakeholder experiences, as shown in Table 6.
Evidence use
To evaluate process outcomes, we consider, first, the extent to which the project team managed to identify, select, evaluate/interrogate and use different kinds of evidence, as relevant to their redesign work. We also consider whether or not major barriers to evidence use, identified in the case studies above (e.g. access, political stand-offs or difficulties of evaluation), were effectively overcome. Evidence use is judged as effective if a CCG redesign team used a range of evidences to inform their decisions. It would not be effective, however, if a team focused narrowly on certain pieces of evidence and/or were inflexible about, or ignored, additional sources of information.
In four cases (Seaport, Chelsea, Shire and Coalfield CCGs), barriers to evidence use were relatively few. In all of these cases, we identified effective framing of the problems too. Effective framing of the search for solutions was also noted at both Seaport and Coalfield CCGs, where a partially ‘blank sheet’ was used to guide the search for information. Although the blank sheet was not used at Shire CCG, evidence use was proactively managed via the creation of subgroups (e.g. a working group and a GP group). While Chelsea CCG showed no explicit efforts to manage evidence use, this redesign occurred against the backdrop of a trust-wide agreement to address diabetes care by reducing demands on hospital capacity. Because this agreement had already been reached when we commenced data collection, it is possible that disagreements and issues around evidence use were just not captured by our research.
Key actors in Horsetown, Greenland, Stopton and Rutterford CCGs all reported significant issues with evidence use. In Horsetown CCG, for example, actors reported issues with the applicability and usability of universal evidence. For example: ‘There are NICE guidelines around orthopaedics . . . they’re very long . . . it’s like reading a book!’. And:
There are guidelines on management of knee pain . . . they are national . . . [you have to] summarise them to help you locally which might be slightly different [across contexts] because the services are different . . .
Unique problems were reported at Greenland and Stopton CCGs. Evidence use at Greenland CCG became a problem after a solution was selected and the project had been put on hold. The CSU manager described evaluating the evidence that had been considered in the first iteration of the project with a view to understanding if there was a clinical need for changes. In Stopton CCG, issues arose with evaluating the evidence that had been collected from its own pilot project, rather than external evidence.
Interestingly, we noted effective framing (both problem and solution search) at Rutterford CCG. Stakeholders at this CCG also used a blank sheet approach and created subgroups. Yet issues with evidence use were still reported. In this case, actors described problems with data quality, and having too much information, which meant that they failed to reach an agreement.
Meeting objectives
In addition to evidence use, we considered whether or not objectives of redesign projects were met (to date, at least) against actors’ own expectations. These objectives varied: two CCGs had sought to create a report about how changes could be made (Rutterford and Horsetown CCG), and the remaining six CCGs (Seaport, Chelsea, Shire, Coalfield, Stopton and Greenland) had sought to implement actual changes in service delivery.
Project objectives were clearly met in four of our CCGs (Seaport, Chelsea, Shire and Coalfield). We also noted fewer issues with evidence use in these cases. Objectives were also met in Rutterford CCG, however, even though evidence use had seemed quite problematic. Perhaps proactively managing these issues allowed Rutterford CCG to meet its primary objective (to create a report to guide change) and to begin making changes in practice.
Objectives were only partially met in Horsetown CCG. In this case, a report was produced as per the original plan, but commissioners developed it ‘quite suddenly’ and in the midst of ongoing stakeholder disagreements. Thus, here there remained doubts among some team members about whether or not objectives had actually been met as expected.
Stopton CCG had met its objective of implementing a pilot triage. However, the board decided to block the wider implementation of the triage, pending further data collection, leading to quite some frustration within the team. Stakeholders were actively engaged with this data collection and so it is possible that broader practice changes would have been witnessed if our study had been over a longer time frame.
Last, Greenland CCG’s project was stalled when the GP lead and CSU manager left the CCG, and so objectives were not met at the time of our fieldwork. The CCG was, however, considering the clinical need for changing pathways, which the original redesign team had felt to be well established.
Stakeholder experiences
The last outcome indicator relates to stakeholders’ experiences, specifically the extent to which key actors felt satisfied or dissatisfied with redesign projects. Of course, in the following, we must bear in mind that this may link to a number of things others than whether or not evidence was used in their decision-making (e.g. to unexpected shifts in priorities at board level). Across the majority of CCGs (Seaport, Coalfield, Chelsea, Stopton and Rutterford), most stakeholders we spoke to during interviews and in follow-up told us that they were generally satisfied and had been able to overcome major challenges faced during redesign work. At Seaport CCG, for example, the diabetes nurse described quite succinctly that, after the redesign, everyone was ‘doing what [they were] paid for . . . [and] people weren’t falling through the net’. Similarly, at Stopton CCG, the clinical assessment team lead commented that they ‘move[d] forward together as a group . . . it’s been more like an open discussion . . . And I think that’s why it’s been so successful . . . because . . . nothing has been imposed, it has been consultation’.
In other CCGs, however, stakeholders were very openly dissatisfied with the redesign process. Horsetown commissioners produced a report, and stakeholders reported that the project was left at a ‘like loose end . . . no conclusion, we were not told what will happen next’ (GPwSI). A NHS physiotherapist also mentioned how she was ‘a little bit disappointed with . . . because it feels like everything is suspended at the moment’. Interestingly, stakeholders at Shire CCG were not at all satisfied with the redesign process, especially as the project had been put out to tender. They described how, in implementing the new pathway, they were ‘firefighting and ironing out the crinkles’ (clinical therapies lead). They did say, however, say that this was ‘less and less every day’, and that they remained optimistic with ‘lots of great ideas about what we’ll want to do and how we want to deliver it’ (clinical service manager).
Comparing across cases
Objectives were broadly met in Seaport, Chelsea, Coalfield, Rutterford, Stopton and Shire CCGs. Of these, Seaport, Chelsea and Coalfield CCGs appeared to have the best outcomes. They met their objectives, we found few issues with evidence use, and there were broadly satisfied stakeholders. These outcomes occurred even when certain capabilities were not recorded [Seaport CCG (managing expert collaboration) and Coalfield CCG (managing roles and expectations)], suggesting that capabilities may not always be necessary.
Rutterford CCG demonstrated all five capabilities. Here, issues with evidence use were reported but overcome. Similarly, Stopton CCG showed four capabilities, but there were issues with evidence use. This case is slightly different from the others because the issues with evidence use arose from data it had collected to evaluate its own triage. In both of these CCGs, however, objectives were met and stakeholders were satisfied. Shire CCG showed only three capabilities, but still achieved its objectives and used evidence effectively. Stakeholders were largely dissatisfied, however, especially as the project was put to external tender.
Greenland and Horsetown CCGs experienced the worst outcomes; both failed to meet objectives, use evidence or satisfy stakeholders. Both lacked framing and managing collaboration capabilities. Greenland CCG failed to engage experts, while Horsetown CCG failed to search for and evaluate evidence. Thus, although redesign projects may certainly fail for all sorts of reasons, our data indicate that CCGs with fewer capabilities also experienced worse outcomes with regard to the redesign process overall. These data must be treated with caution, of course, as larger studies would be needed to evaluate the strength of any links between the two factors. Two main propositions can be drawn from these findings:
-
There is an inverse relationship between applying evidence use capabilities and redesign outcomes. That is, CCGs with fewer capabilities report poorer outcomes.
-
Context matters. The absence of some capabilities to use evidence did not guarantee poor service redesign outcomes. Rather, using capabilities as appropriate to the particular context seemed more important with regard to outcomes.
We conclude, then, that a tightly prescriptive ‘one-size-fits-all’ approach to capabilities for evidence use in redesign work is not appropriate. Instead, consideration is needed of what capabilities are required in given particular contextual contingencies.
Chapter 7 Toolkit development
Introduction
In this chapter, we report work on our development of a toolkit aimed at helping commissioning organisations improve capabilities to use evidence. To this point our research has identified four categories of evidence and particular capabilities to use evidence (Table 7).
| Simple definition | |
|---|---|
| Evidence | |
| Universal | Information produced through formalised or scientific investigations in institutions and organisations deemed to have legitimate authority |
| Local | Information produced and collected locally within CCGs or their immediate locale was also, and very often, treated as evidence |
| Trans-local | Information produced in other local areas and taken up as evidence in the current locale, e.g. examples of ‘best’ or ‘good’ practice from elsewhere |
| Expertise based | Information based on the experiences and expertise of individuals could also be treated as evidence |
| Capabilities | |
| Sourcing and evaluating evidence | Making efforts to source and evaluate evidences relevant across episodes of redesign work |
| Engaging experts | Ensuring individuals with relevant education, qualifications, and experiences are involved in the redesign |
| Effective framing | Constructing and negotiating shared meanings |
| Managing roles and expectations | Considering what stakeholders expect, and are expected to do, in redesigns in order to avoid incongruence, conflict or ambiguity |
| Managing expert collaboration | Efforts to overcome boundaries, politics, and anxiety associated with different professional groups working together |
We see these capabilities as being able to be nurtured, both at the level of the commissioning teams working on redesign projects and at the level of the organisation. Accordingly, the design of the toolkit takes two target audiences into account: (1) those responsible for the overall functioning and management of commissioning organisations (usually director level staff), and (2) those managing/leading local redesign projects (usually middle-level commissioning leads and the teams they assemble). We develop in our toolkit a structured set of questions aimed at each of these groups. The intention is not to prescribe a particular course of action, but to allow each of these groups to (1) reflect on how well they use evidence and (2) identify opportunities to improve their own capabilities to use evidence.
The toolkit may also allow practitioners to track the development and maturity of their own capabilities over time (e.g. during different stages of a project). Owing to its interactive features, the toolkit will prompt practitioners to think critically about the role of evidence in redesign projects. Our toolkit is centred on the five capabilities identified from the CCG data (see Chapter 4): sourcing and evaluating evidence, engaging experts, effective framing, managing roles and expectations, and managing expert collaboration.
Designing the toolkit
We developed our toolkit using feedback from stakeholders and examples in the existing data. In terms of stakeholders, we held two workshops to elicit feedback from individuals external to the research team. The first workshop took place on 1 March 2016, in which a small group of SSAP, including patient representatives, participated. We made a short presentation outlining the aims and objectives of the toolkit, including a set of questions targeted at different toolkit users. Stakeholders suggested we should make the following changes:
-
remove the word ‘diagnostic’ from the toolkit, as this may have misleading connotations
-
target different types of users within the toolkit (e.g. director-level CCG managers and redesign project leads) because these individuals will have different interactions and understandings of evidence and capabilities
-
simplify and reword the language used in some questions to increase usability
-
include the criterion ‘assessing local applicability and relevance of evidence’ to make clear the purpose of the toolkit.
On the basis of this feedback, other offline comments and emergent findings regarding the capabilities, we revamped the toolkit questionnaire. In addition to using stakeholder feedback, we looked at examples of toolkits developed by academics for practitioner audiences, including the normalisation process toolkit (http://normalizationprocess.org). This toolkit includes a number of user-friendly features, such as a radar plot, which visualises answers and enables users to understand potential strengths and weaknesses (in that context, implementation of new technology). The radar plot can also be used to visualise ‘performance’ against specific capabilities (e.g. showing high ‘expert engagement’ vs. low ‘effective framing’). We decided to include a radar plot in our toolkit to enable users to get a sense of how well prepared their organisations and teams are to use evidence. A consequence of this decision is that item wording needs to elicit answers that are not simply ‘yes’/’no’ or open-ended, but reflect different levels of capability (e.g. Likert-scale types of questions). We therefore took particular care about how items were phrased to make the production of a radar plot possible.
Against this background, we presented an updated toolkit at our second national workshop (18 May 2016). This was a much larger workshop, comprising 46 participants with backgrounds in a number of NHS organisations, including CLAHRCs, Academic Health Science Networks, NHS commissioning policy units, CSUs and foundation trusts. We presented the findings of our research and then used interactive exercises to elicit participants’ feedback about the toolkit. After we made a short presentation describing the toolkit, the workshop participants were split into five groups. Each group was asked to examine three or four questions from the toolkit for approximately 20 minutes and offer their feedback on these. We also supplied each group with sticky notes and asked them to give comments on question sets. For example, one group was asked to comment on the set of questions concerning ‘managing expert collaboration’, while another was asked to debate and discuss the appropriateness and practical usefulness of the set of questions concerning ‘engaging the expert’.
Owing to the interactive nature of this workshop, the feedback we gathered was very rich and immensely helpful. The workshop participants made specific and constructive comments on all aspects of the toolkit. The participant comments and suggested questions per capability are summarised in Table 8 and were used to inform the final toolkit development. Feedback informed significant changes, and the resulting toolkit is presented in Appendix 5. This toolkit would be accompanied by a summary table that defines different types of evidence and different capabilities with concrete examples.
| Capability | General comments/considerations | Question selection |
|---|---|---|
| Sourcing and evaluating evidence | Make explicit reference to NHS Academic Health Science Network, library and knowledge services | Do you have access to evidence? Are there explicit criteria to assess evidence? |
| Engaging experts | Consider:
|
Include questions about identifying, not only engaging, the right experts |
| Effective framing | Is it about framing or scoping or defining? | Is the problem a symptom of another, deeper problem? Can we solve the problem by adapting what we do now, copying what others do, or starting from scratch? |
| Managing roles and expectations | Some projects may not have clearly defined roles so a flexible approach is adopted Think about project life cycle Too advanced for commissioners! |
– |
| Managing expert collaboration | What does ‘balancing’ and ‘preparing’ for divergent interests mean? Is it managing or facilitating/accommodating? How do you measure divergence of interests? |
– |
Chapter summary
In this chapter, we described our toolkit for improving evidence use in commissioning. The development of this toolkit was shaped by our research findings, by the literature and by feedback from our SSAP and workshop participants. We have described the importance of appraising capabilities for evidence use, both within the team and at the organisational level. We have selected a radar plot tool to enable easy interpretation among users. It is important to note that this is just an overview of our toolkit. We also secured an Economic and Social Research Council Impact Acceleration Grant to refine the design and develop this toolkit (e.g. wording) into a user-friendly online learning resource. Work (6 months) began on this resource immediately after the completion of the current NIHR project. The resource ensures that our ‘actionable findings’ have the best chance of having an impact on practice. The work was being carried out in collaboration with Health Education England, the West Midlands CLAHRC and local AHNs. The resource is available at www2.warwick.ac.uk/fac/soc/wbs/research/ikon/commissioning/.
Chapter 8 Exploratory study of evidence production
In the previous chapters of this report, evidence use by managers was considered as our main response to the commissioning brief (i.e. research on ‘pull’). A secondary aim was to consider the ways in which the production of evidence shapes its use in health-care organisations (research on ‘push’). In this chapter, we consider the design and production of evidence-based products in one large guidance-producing organisation.
We use the lens of ‘inscribed meanings’ to conduct this part of the analysis. 60 Inscribed meanings constitute, in effect, the ‘instruction manual’ for evidence, whereby the view that designers take of how target audiences are meant to use evidence become incorporated into the design of their products. 63,64 Accordingly, we set out to explore what sort of imagined and projected users are inscribed in evidence products aimed at improving design and commissioning of health-care services. From our interviews and visits, we saw three main discourses, which we label as ‘the discourse of production’, ‘the discourse of audience feedback’ and ‘the discourse of implementation consultancy and direct marketing’. These are described next.
Discourses of use and users
During our interviews and visits it became increasingly clear that the meaning behind key terms used – such as ‘adoption’, ‘implementation’ and ‘impact’ – and the ways in which these were being operationalised varied across the producer organisation. These observations are important because previous research suggests that complex organisations are unlikely to constitute totally coherent entities. More likely, they may host a number of slightly different organisational ‘logics’ or subcultures. The result is a situation of institutional pluralism and culture complexity, which generates specific challenges for management. 87,88
From our initial interviews and observations, it appeared that different parts of the organisation operated slightly different visions and projections on how their products would be used in practice, and on what concerned commissioning groups. Most of the time, such visions were not openly discussed (although there were notable exceptions). They were, however, reflected in the different ways members of the organisation talked about how they could sustain the take-up and utilisation of evidence products and other forms of guidance. At least three of these local discourses of use and users were encountered in our study. We call them here the discourse of production, the discourse of audience feedback and the discourse of implementation consultancy and direct marketing. We use the term ‘discourse’ to reflect the fact that these broad views defined different ways of talking, choices of words and ideas, but also practical beliefs, predispositions and specific practices around how you deal with use and users. 89 It is important to reiterate that these three discourses emerged from a limited sample of interviewees (and observations), and all findings from this exploratory study would need further work to be developed, corroborated and possibly refined.
The discourse of production
A first discourse we heard during our exploratory study stemmed from the concerns associated with the production of core evidence products (guidelines, health technology assessment). Developing such materials, quite naturally, is the largest, most traditional activity in organisations that publish evidence. It is also the task that many of our informants indicated as ‘core’. The work of developing evidence products in this organisation was carefully structured, highly regulated by standards and operating procedures, and closely monitored. It was also highly sensitive and exposed to close external accountability and scrutiny. According to our conversations, these activities were driven by an operational culture that is (appropriately) strongly outcome focused and quality driven. Our informants indicated that most of their corporate key performance indicators were focused on internal processes: ‘quality is controlled at each step’. Great effort and focus are also placed on ensuring that the processes are robust and ‘very much evidence driven’. Quality assurance and several layers of internal checks were often mentioned and described to us.
The relationship between this core production and the end-users, although present and considered by all to be very important, was highly mediated by the processes in place, which were often document based. Many of the informants reported that ‘we look at consultation documents . . . report from stakeholder consultation’. Externally facing parts of the organisation did report back to this production core on how their products were perceived in the users’ world, but for efficiency reasons this was often done in a highly structured way through succinct summaries, slides packs and brief structured interactions. Informants were aware of the existing constraining conditions:
One of the issues for us is about the cycle of evaluating and of course implementation doesn’t happen just like that so it is about well, what can we do in our resource levels that allows us to have another go at it or evaluate . . . pretty much internal process stuff.
As this quotation suggests, the attention to implementation was tempered by a healthy dose of realism and by the daily work pressure – fuelled in part by the fact that one of the traditional criticisms of guidance production is that products often take too long to surface and are not available when needed (this was also mentioned by the same interviewee).
The image of the user inscribed by the existing processes and procedures was, in turn, a rather rational and traditional one, based on the idea that the quality of the product will determine its fate and, in the end, evidence will speak for itself:
The key point is it’s established from best evidence and the assumption is that when you use best evidence to inform your practice that you will then achieve excellence.
In this discourse of production, the burden of use is mainly (albeit not exclusively) on the side of users, and the main duty of the producer is to deliver high-quality and timely products. This is illustrated by a focus on improving the usability of the products. This included, among other things, a focus on timeliness, ‘how guidelines are presented’, and how people access the information. Most interviewees gave us a sense that they believed the organisation was taking important strides to grapple with such issues.
In general, and somewhat as expected, members who were associated with a discourse of production admitted limited direct knowledge of CCGs, with one respondent candidly affirming that ‘I have never been to a CCG and I do not have an idea of how they look like’. Reasons included lack of opportunities, their recent establishment and the type of task they were engaged in (‘my work is very similar to project management’), but also difficulties in engaging with these organisations. The projected image of CCGs as users was also slightly vague and generally rather normatively constituted. Consider the following response to our scenario question (‘Can you describe in detail how will specific guideline XYZ will be used in a specific CCG?’):
I don’t know how much the commissioning groups themselves per se will be looking at it. I think their expectation maybe may be that others will be looking at it. I think they would be interested in particular aspects of care within the guideline which may sort of, you know, make a big improvement to practice but will be saving money I suppose is, would be a key component. In terms of who are the people who will be actually looking at it . . . Some of them, I think some of them will be support teams. I think some of them will be, there will be clinical roles within that so it could be a clinical lead for patient safety for example.
In the extract, there is an open admission of scarce familiarity with this particular type of organisation (respondents seemed much more confident when it came to describing activities in acute trusts). This model of use is based on the idea that the burden of evidence uptake is on the side of the receivers, the expectation being the ‘that commissioners would, I think ‘pay regard’ or ‘take account’ of [the organisation]’. Although these results are not totally surprising – one would not expect all members of such an organisation to be closely acquainted with all of its multiple stakeholders – they indicate that the user inscribed in the product may result from the coming together of a number of very practical concerns and processes that may result in both intended and unintended consequences. Critically interrogating these processes to ensure that they align with the espoused mission of the organisation is an important effort to ensure the success of the product and of the organisation.
The discourse of audience feedback
A second discourse that we encountered in our exploratory investigation was much more openly oriented towards the needs of the end-users, the sense being that all efforts needed to be made to make evidence-based products as accessible as possible, in terms of dissemination, retrievability and usability. This second discourse was informed by a communicative view of innovation diffusion. 49 The related assumption was that providing the right information at the right time to the right stakeholders, using the appropriate channels, is critical for innovations to be adopted. This discourse, we suggest, could be recognised both in how people talked about use and users and in the institutional initiatives they described. It was informed by an attention to better outward communication (e.g. through the use of the web and social media) and better use of the feedback from the users. Two examples are as follows:
There is a dissemination strategy which will target who it should be sent to, the format usually being electronic, possibly supported by bulletins and articles in professional journals which will not only tell people that the guidance is out there but perhaps will focus on key areas of potential confusion or controversy.
There is a need to understand how people actually are using it, how people find out about the guidance and through that searching journey and then once they find out information is that what they are looking for. And if it is how they use it, why they use it and if there are any challenges . . . And then when we look at the user we break out as different audience groups as like GPs, commissioners, doctors, nurses, managers, commissioners because each one they look at evidence and the search for evidence, how they use evidence may be different and to then get their feedback.
These two extracts suggest that the discourse of audience feedback focuses especially on the communicative affordances of the products, the communication channels used to make them available, the information behaviour of potential users and the reinstating of a feedback loop that can be used to improve the existing products accordingly.
We propose that this discourse of audience attention and feedback co-exists with the discourse of production discussed above (the same respondent would use both). Indeed, at times, both discourses seemed to interact. The discourses of production, for example, seemed to inflect (or infect) the discourse of audience attention with a positive orientation. One of our respondents, for example, talked about the current effort to incorporate users’ feedback and users’ needs in the production process itself:
My understanding of where we are trying to move to is more, not so much producing a piece of guidance and then implementing it but actually bringing, going much further upstream and actually bringing the implementation process into the development of guidance itself and having it evolved right from the early stages of guideline development rather than trying to do it later.
This quotation also suggests that injecting the traditional production-centred discourse with an attention to the need of the customers is (still) a work in progress (although, again, the size of our sample may have been a factor here). This was attributed sometimes to the fact that, historically, the organisation did not have a remit for implementation and sometimes (in part) to the current division of labour within the organisation.
At least in our interviews, we found that this discourse was carried and actively promoted by staff who worked in the teams faced with new and non-clinical stakeholders as well as by staff belonging to new internal services and staff functions. Carriers of the discourse of audience feedback tended to have a much more defined and less abstract view of the projected users as demonstrated by the following quotation from an interview:
Whether or not they use . . . products is based on their awareness level whether they are aware of . . . products or not. However, awareness doesn’t . . . is one of the factors whether they use it or not but it’s not the only essential thing because that is also influenced by whether it’s suitable or not, the content, how it is presented, and also impacted by their national priorities and whether is there any resources impact . . . we don’t have any statistical data in terms of how it impacts on each other but we have, you know, informal model about how they are related together.
The quotation is interesting because it indicates the existence of a rather well-defined projected user. This user invoked in the extract is much more clearly, and, we would argue, realistically depicted than in the previous discourse. Awareness is central, but recognition is given also to other factors. Pragmatically, the focus is on improving dissemination. In fact, several informants told us of the importance of ‘using a number of routes’ and different forms and ‘modes of presentation’ of the products (how the guidance is written, packaged and presented so that it fits the needs of the users) to reach different audiences. There is also a more clearly articulated sense that evidence does not implement itself and competes with other priorities. Moreover, unlike in the previous discourse, there is a recognition that evidence tools can be designed around the need of the users so that the latter are not required to do all the work of translating the former in practice.
The traditional idea that one form of evidence fits all, which seemed to inform the production discourse, is replaced here with a more pluralistic view; one that accepts that form, if not substance, needs to be profoundly changed when talking to different publics. Finally, some of the interviewees displayed an acute sense that the organisation needs to accompany their products with tools that facilitate such translation. One of the middle managers we interviewed, for example, spoke of the importance of producing costing tools that provided an assessment of the financial impact or the resource impact estimated at both the national and the local level. In her words, ‘[we are] trying to bring cost impact alongside the cost effectiveness analysis which obviously goes into the production of the guidelines themselves’. In short, the respondents taking this discourse saw the organisation itself as needing to shoulder some of burden of translation of the evidence in practice by helping to marry universal and local forms of evidence. The image of CCGs as clients, or customers, also seemed to be more concrete and realistic than in the production discourse, although direct information was still in short supply.
[Our product] goes out as a link, a hyperlink, and then whoever in the Clinical Commissioning Group receives that in theory but I am not so up to speed with how the CCGs work should have some kind of governance process in place that allows them to disseminate that appropriately.
This lack of direct empirical experience of CCGs may, of course, reflect the limited number of participants in our exploratory study. However, in one case at least, this was attributed to the difficulty of finding points of entry in CCGs: ‘[it has been difficult to engage with CCGs although] we’ve made an effort to include them as stakeholders, to invite them and that means they get lots of information if they’re not sure what’. It should be added that this comment was accompanied by the recognition that not all CCGs are the same and, just as we need to differentiate between types of organisation, we need to distinguish between ‘good and bad CCGs’.
Our interview indicated, however, that the lack of direct understanding of how CCGs actually work tended to go hand in hand with a view of the take-up of evidence that was much more structured and procedure-based than in the actual cases we observed of CCGs working with evidence (described in Chapter 5). From a distance, and in the absence of direct experience with commissioning work, designers were often projecting images of users borrowed from acute trusts: ‘In the trusts the idea was that the manager would receive that so they may well be a band 5 clinical audit facilitator but in the end it was their role to look at guidance and disseminate it onwards’. The assumption is that in CCGs it will work the same way.
The discourse of implementation consultancy and direct marketing
A third discourse that we encountered was one of implementation consultancy and direct marketing. This discourse was recognisable mainly from what people did and the terminology used (‘we do campaigns’; ‘we are consultants’; ‘we deal with consumers’). Our hypothesis is that this discourse is rather different from the other two, which assume that good and rigorous evidence products will eventually be adopted by virtue of their inner quality. Indeed, the reverse is true in this case: evidence products need to be accompanied to the clients, presented to them and, if necessary, ‘sold’ in terms of the benefits they will produce:
I have always thought of it as being a sales role. Some of my team find that a little bit unseemly. But I look upon myself as a salesman . . . The great thing is that there are no price fees in our products other than the opportunity cost at the time.
This third discourse manifested itself in a small number of tasks, especially in the work of a number of implementation consultants whose activities loosely followed the model of a medical sales representative (but without some of the difficulties associated with this). Work included meeting customers, carrying product folders, sharing promotional materials, informing them of new products, promoting new tools and products and collecting feedback that is constantly fed back, also, to the production core of the organisation. The vignette reconstructed from field notes, next, provides an example of this particular organisational discourse and set of practices (names have been changed for anonymity):
Mr Smith, the implementation consultant, starts his ‘pitch’ in a way that is similar to how he introduced his work to me: ‘what [the organisation] is trying to do . . . improve the offer to GPs . . . improve the take up of guidelines’. He explains he ‘wants to know where the CCGs are . . . how guidelines are used . . . locally on [a named product] and how they can be improved’. Second, he is interested in knowing whether there has been any progress with the inclusion of certain products in the general practice records system [Egton Medical Infomation Systems (EMIS), EMIS Health, Leeds, UK). He mentions that he has met the EMIS developer but that this ‘did not lead to any concrete follow up’. Later, Mr Smith produces a folder with a number of documents. He shows Mr Green, the CCG client, the new content of the document and the menu of indicators, explaining them and giving one example. He talks Mr Green through the document, explains the benefits and gives the document to him so he can handle it – not unlike what a medical sales representative would do . . .
Here, Mr Smith is actively trying to persuade the client of the relevance of the product, and illustrates both the potential uses and the added value that this could have for the CCG. He is, in effect, translating the product so that it fits the local situation of the CCG. Through his mediating function he localises evidence-based products in conversation with the client. This contrasts with what we have seen in the two previous discourses of use. Whereas in the two previous discourses most of the translation work was on the part of the users, the reverse is true here. The implementation consultant is now taking up a significant part of the burden of translation by trying actively to find out a correspondence between local needs and available solutions.
Although this particular way of talking and dealing with user and customers was limited to the activity of a very small team in the producer organisation, we mention it here for two reasons. First, the work of this team was often referred to in our conversations with others and, therefore, was considered particularly relevant. Second, it carries the seed of rather different and more service-oriented way of conceiving the work of an evidence-producing organisation. For example, the self-image projected by this activity is significantly different from the one embodied by the dominant production paradigm.
Because they interact with CCGs on a regular basis, the implementation staff seemed to have very grounded view of CCGs as users of evidence products. One aspect that is often emphasised is the high variability of CCGs and their differential processes and capabilities with relation to the adoption and assimilation of evidence. In the words of one implementation consultant, ‘The main thing within commissioning which is what we’re talking about now as opposed to say a provider organisation is one of variability’. Implementation consultants have learned, in particular, to distinguish between ‘good’ and ‘not so good’ CCGs in terms of process and capabilities:
If it was a good CCG which there are some examples, they would have somebody whose job is to receive a monthly newsletter . . . and to scan it to see what guidance has come out which is likely to be relevant for their services and then they will pick that up and they will make sure that somebody knows about it. But more importantly they will make sure that that person knows what it is they are meant to do with it.
These, and other carriers of this discourse, are also more aware, or more verbal, about the situated and therefore unpredictable ways in which evidence products enter organisations. In CCGs people may come across evidence products ‘by accident probably because it gets on the front page of Pulse’; with ‘a bit of luck’, they will look at them and say ‘hmm, I know a lot of my colleagues still prescribe aspirin for people who come in with atrial fibrillation, we need to do something to get them to move them on to anti-coagulation’, and, ‘if they remember . . .’, may do something. In short, and in line with our study, implementation consultants seemed to believe that ‘implementation’ depends on the nature of the evidence products as much as on the capabilities of the organisations that are supposed to adopt them.
What real users look like
Before concluding, it is worth reporting a brief extract of our observation in the field, this time turning our attention to the actual conduct of the users. This is because, in spite of our extremely limited sample, we encountered a user whose orientation was very different from that mentioned during our conversation. The extract is from a visit to a CCG by one of the implementation consultants who we shadowed in the field (names have been changed for anonymity):
Mrs Shore arrives with Mr Rainer, a junior person from the governance unit. Mr Rainer will remain rather silent during the meeting while Mrs Shore unremittingly tells the implementation consultant all the amazing things they have done. Mrs Shore starts the conversation explaining that she is moving to larger practice and leaving the CCG. She will work on medicine management but also do some practice development. After a short attempt by the implementation consultant to present his organisation’s offering, Mrs Shore launches into what a long praise of the work of her CCG in relation to the use of technology to support evidence based change of service provision. She speaks using highly technical language, mentioning acronyms, showing thorough knowledge of evidence-based products and guidelines. For long stretches the implementation consultant listens to Mrs Shore’s enthusiastic account. Mrs Shore starts by explaining the work they have done to use the EMIS [Egton Medical Infomation Systems] system to identify patients at risk. She explains that they modify the EMIS so that when certain input suggests people at risk, this is flagged automatically to the GP. The implementation consultant is able to chip in briefly and add that ‘we have a guideline for this . . . it includes a scenario . . . I did a session with another CCG nearby’. Unperturbed, Mrs Shore explains how recent guidance on drug safety was also included in the EMIS system (in the process she ‘recites’ the content of the guidance). Mrs Shore continues by affirming that she is a big fan of systematisation: ‘we have an automatic audit . . . we have a programme . . . we do it already’. She explains that whenever they extract data they compare with ‘previous studies’ and then put in place actions to educate their clients. She gives an example of how they worked with some of the care homes in the area where certain indicators were not as good. She states that ‘our data are brilliant’. All the implementation consultant can do is to nod and say ‘good stuff’.
Mrs Shore appears to be quite a unique user who does not fit with the implementation consultant’s a priori expectations. She is not resisting the use of evidence; quite the contrary, she endorses it to point of being able to recite pieces of guidance. Yet she is keen to make evidence-based guidance ‘disappear’ for frontline clinicians by embedding it in the local general practice records system. Although her interaction with the implementation consultant is not as one would have expected (she is telling things to the consultant, rather than the other way around), this is motivated by a desire to demonstrate (and showcase) her CCG’s capacity and skill. The implementation consultant is granted the type of reception one would give to a highly ranked authority.
We mention this encounter because Mrs Shore was a surprise to us and, more importantly, because people like Mrs Shore were never even remotely imagined for us during our conversations with members of the evidence-producing organisation. Our hypothesis, which would, of course, require further work to be corroborated, is that a user of this type could hardly have been inscribed in any evidence product, as she did not enter the collective imagination of the evidence producers. Mrs Shore reminds us that the only way to design products around real end-users is to go out there and see what they do (something that the producer organisation is starting to do).
Chapter summary
We set out to understand how teams at one organisation that produces evidence-based products think their products will be used in general, and by NHS commissioning organisations in particular. Owing to the very small sample and the limited duration of our engagement, we are in a position to offer only a few brief considerations to inform future research.
First, different (in this case, three) discourses of use and users were detected between teams in the same evidence-producer. As we suggested above, this can be expected given the type of organisation, and the presence of complexity and pluralism is neither good nor bad. Pluralism can be a source of innovation or internal conflict. Diverse discourses and subcultures can co-exist as long as they are brought to the surface and managed at a strategic level.
Second, our exploratory study suggests possible historical parallels with other organisations in the private sector that, over the years, have transitioned from seeing themselves (and acting) as makers of products to providers of services. A notable example is IBM, an organisation that over two decades went through a remarkable transformation from a struggling seller of hardware to a successful broad-range provider of solutions. 90 Our study raises the question of to what extent producers of evidence-based products in health care are going through the same process, and whether or not what we observed was the first trace of this process happening. The industrial context is quite different, of course, but comparisons with historic cases may be a useful means by which to reflect on one’s own identity and try to manage such processes.
Third, and finally, our exploratory study raises questions about the potential benefits of a more explicit and broad engagement of evidence producers with what is loosely described as ‘customer-centric design’ or ‘design thinking’. Brown and Wyatt91 refer to design thinking as an approach to innovation that draws from the designer’s toolkit to integrate the needs of people, the possibilities of technology and the requirements for business success. The approach is supported by a growing set of techniques and engagement modes, some of which we recognised to an extent within this evidence producer. Design thinking puts understanding of what users do, where they are and their active, direct involvement at the core of the development of new product and services. In this way, implementation issues can be designed out rather than grappled with at the ‘end of the journey’ of evidence, and users such as Mrs Shore can become assets to the circulation of products rather than outliers.
Chapter 9 Discussion and concluding remarks
In this chapter, we conclude with a discussion of the ways in which our findings inform evidence-based practice and service redesign work. In brief, we have shown the need for multiple ‘evidences’ to be skilfully combined and have highlighted sets of capabilities can enable this to happen in specific contexts. In doing so, we contribute to recent research on the ways in which different forms of knowledge and information come to be mobilised as ‘evidence’ in health-care management and shed light on practices and conditions that enable this. We have shown, as do others, that evidence-based redesign work is a socially complex activity, shaped by the application of certain capabilities, not just the type of information being used. We discuss these points further in the following paragraphs, drawing in theoretical and applied implications from our findings, as well as considerations for this project and future research on the topic.
Mobilising evidence
The use of ‘authoritative’ evidence (from NICE) is a central consideration of our study, but it was clear that this needed to be coupled with other forms of evidence, identified as local, expertise-based and trans-local. Although different labels can be applied, the categories of universal, local and expertise-based evidence mirror those found in previous work. 23 Rigorous scientific evidence (which we term ‘universal’), evidence from within the local area (which we term ‘local’) and evidence from knowledgeable stakeholders (which we term ‘expertise based’) have been repeatedly shown as important in the literature. The category of trans-local evidence (i.e. evidence created in one place but used in another) is new. Although this resembles what other studies have labelled ‘best practice’ examples,1 our cases indicate that this evidence had often not been actually demonstrated as ‘best practice’ in its original setting before it was adopted and applied in our cases. Hence we think that ‘trans-local’ is a better label, one that also acts as a caution to the assumption that examples that have been tried elsewhere are actually ‘tried and tested’.
Although there were clear differences between the four categories of evidence, in reality they were used in tandem. We showed, for example, that stakeholders do rely on universal evidence (e.g. guidelines on amputations) to understand local evidence and performance data. Conversely, universal evidence needed local evidence to make them applicable to the context of adoption. Evidences are also coupled in more indirect ways. In terms of expertise-based evidence, for example, stakeholders may be asked directly for their opinions. If this is not the case, their interpretations of, and reactions to, other evidences (e.g. local and universal) are likely to be informed by their own expertise anyway. In some cases, experts questioned even NICE guidelines with legitimate authority among their peers. Thus, categories of evidence need to be understood, not as mutually exclusive (e.g. with one ‘trumping’ the other)1 but as tightly coupled, being used interdependently for sense-making.
In addition to understanding what evidence is used, our qualitative research design provided rich insights about these evidences. We know, for example, that commissioning stakeholders, in many instances, were less concerned with the properties of evidence and more concerned with accessibility, relevance and applicability to context. Trans-local evidence, which served as a shortcut to solutions in many instances, may in itself be testament to the reality of evidence use in this context. Indeed, stakeholders who opted to mobilise this type of evidence were often swayed more by the fact that certain CCGs had managed, in fact, to implement a change, than by the efficacy or outcomes of change. Indeed, there was rarely ‘any long-term evidence’ available (e.g. Greenland CCG). Choosing a solution because it has been applied elsewhere, without clear knowledge of efficacy, is likely symptomatic of the highly politicised, time-pressured environments in which health-care managers work.
It is interesting that these evidences have been shown as important in many studies, but issues with mobilisation still persist. 3,5,12 The inherent challenges of mobilising NICE guidelines, for example, have been described by our stakeholders and in the wider literature. 92 Whereas some kinds of universal were mobilised (e.g. NICE evidence, Five Year Forward View, QOF data), it was apparent that other kinds of universal evidence – Cochrane reviews, for example – were never referred to in our data.
Our case studies also offered insights into the challenges of mobilising evidence in other forms. Local evidence, for example, was often difficult to access and interpret. Routes for accessing this kind of evidence were often quite ad hoc and interpersonal, and so depended on the right experts being involved at the right time. Variations in recording and coding data (e.g. between primary and acute care) also posed problems for using local evidence in several CCGs (e.g. Greenland CCG). Structural issues related to the use of different systems to store local evidence also problematised the use of this evidence (e.g. Chelsea CCG). Similarly, the mobilisation of trans-local evidence seemed to be somewhat of a lottery. Although there are centralised sources of ‘best practice’ examples (e.g. Right Care Casebooks), our stakeholders did not describe using them. Instead, stakeholders were informed through more random avenues, including attendance at presentations (e.g. Greenland CCG) and experiences in other settings (e.g. Chelsea CCG).
These prolonged issues with evidence mobilisation may lead to variation and fragmentation in evidence-based decisions. 93,94 One answer is to add to existing calls for CCGs to be supported to use evidence. We know, however, that commissioning stakeholders are not always keen to use supports (e.g. CSUs), because they are costly, and also because they often lack local knowledge and relationships (relative to practising clinicians). 95 By identifying capabilities, in contrast, we show how evidence use can be enhanced from within the redesign group and the commissioning organisation itself. Engaging the right experts (including patient groups) in agenda formation, for example, is seen to ensure access to a wider range of evidences. 11,23 It helps, also, to ensure that stakeholders who will be affected by the change are represented in commissioning design work, thereby increasing the likelihood that a change will be accepted and actually implemented. Collaborations among experts need to be actively managed, however, and the evidence that they ‘bring to the table’ needs to be judiciously evaluated.
Before considering implications for improving capabilities, there is one caveat to our findings. Our SSAP has, quite rightly, advised that we consider how our research design may have influenced our findings. We chose to study how CCGs redesign diabetes or MSK services. This design meant that our research captured large-scale/-scope processes of change, where clinical need, cost, patient satisfaction and staff satisfaction, as well as a host of other factors, have to be considered. With projects of this scope, a multitude of evidences become relevant. If we had, instead, focused more narrowly (e.g. on the redesign of a particular part of the patient pathway), a different pattern of evidence use may have been observed. In this case, our SSAP suggested that universal evidence related to clinical guidance or best practice (e.g. NICE) may have been at the forefront of decision-making. With this in mind, we situate our findings as an overview of evidence use in redesign work, and encourage further, perhaps more targeted, investigations of the topic in future research.
Capabilities to use evidence
The main purpose of our study was to understand capabilities that enable evidence use, that is, practices and conditions that allowed decision-makers to have a solid evidence base for their decisions. Note that, here, our focus was on capabilities to use evidence, in particular, not on capabilities to manage change processes, in general. Although much is known about managing change in health care, including the challenges of dealing with complex, so-called ‘wicked problems’, there has been little focus on specific capabilities to use evidence. 96 Evidence use is only one aspect of any change process, however, so links between process outcomes and capabilities to use evidence are not direct and need to be treated with caution.
This said, we identified five capabilities that appeared in our cases to encourage the use of different types of evidence in decision-making: ‘sourcing and evaluating evidence’, ‘effective framing’, ‘engaging experts’, ‘managing expert collaboration’ and ‘managing roles and expectations’. We have discussed the relevance of each of these capabilities to evidence use, and also the interdependencies between them, in the previous sections (e.g. see Chapter 6). To our knowledge, identifying and collating capabilities for evidence use by commissioning groups conducting redesign work is a unique contribution of our work.
Interestingly, these capabilities reflect broader health-care management literature. Scholars have long argued that evidence is contested, negotiated and socially legitimated. 17 Far from being objective, evidence use is a highly political, tense and anxious process. 85 Reflecting this, we showed that managing expert collaboration and expectations is an important capability for evidence use. Similarly, the literature suggests that who presents evidence and how evidence is presented informs use. 7,46 The perceived relevance of evidence is also important. 46 Our cases showed the importance of framing evidence to resonate with stakeholders (e.g. the blank sheet approach). Overlap between our findings and the literature suggests that these capabilities are an avenue through which more general improvements in health-care management can be made, including enabling evidence-based decision-making.
Although our five capabilities were identified across cases, we do not claim to have created an exhaustive list. It is likely that other capabilities for evidence use in CCGs exist, and we encourage further, larger-scale studies to explore these. In addition to identifying new capabilities, more research to examine whether or not those shown in the present study are generalisable to other situations of health-care management would be beneficial, as would closer examination of the different ways in which capabilities can be developed in practice. We showed, for example, that engaging experts is a capability for evidence use. While commissioners made reference to the often informal ways in which engagement can be achieved (e.g. ‘sticking your head round the door before every meeting’), going forward, researchers may want to consider exactly how these capabilities can be developed.
Finally, research might also consider how capabilities vary across project contexts. We have already seen that not all capabilities are always relevant, and we are conscious that our design will have influenced the capabilities identified. CCG redesign work requires individuals to work across boundaries, and so it is not surprising that capabilities related to engagement and collaboration management were so frequently noted in our data. Had we chosen a less complex management situation (e.g. evidence use within a homogenous professional group,97 it is possible that other capabilities would have been as important. That said, existing literature tells us that health-care management problems are typically very complex, so our choice of empirical setting should mean that the findings have broad relevance.
The cross-case comparisons allowed indicated that there does seem to be some relationship between capabilities to use evidence and process outcomes, albeit indirect (as noted above). Put simply, we saw that CCGs with the narrowest range of capabilities also had the worst outcomes. Importantly, however, our data suggest that the frequency of capabilities was not directly proportional to successful outcomes. Rather, applying capabilities as appropriate in context may have a more positive impact. Just as evidence use may vary across CCG contexts, so too might conditions and practices that enable evidence use. This suggests that, instead of a broad-brush, prescriptive approach to improving capabilities, it is more fruitful to support commissioning groups in understanding their own capabilities for evidence use so that they can consider whether or not the range of capabilities they are deploying is appropriate for their context, or whether or not there are gaps. The ability, at the meta-level, to identify and apply capabilities when needed is an important practical application of our findings. The toolkit we have begun to develop from the findings is just one step in supporting CCGs to do just that.
A roadmap of capabilities for evidence use in Clinical Commissioning Groups
Our research findings to this point attest to the highly iterative nature of the evidence journey in NHS organisations and to the messy and fluid ways in which various forms of information come to be used as evidence. We have shown how multiple kinds of evidence are coupled together across different episodes of the innovation process. This means that improving the ability of authoritative evidence (e.g. NICE guidelines) to actually change practice necessarily means improving its use alongside other forms of evidence in local contexts. These different forms of evidence are shown in our ‘roadmap’ for evidence use in Figure 8. Although these evidences have definitive characteristics, they contribute to redesign decisions when used in tandem.
FIGURE 8.
Capabilities roadmap.
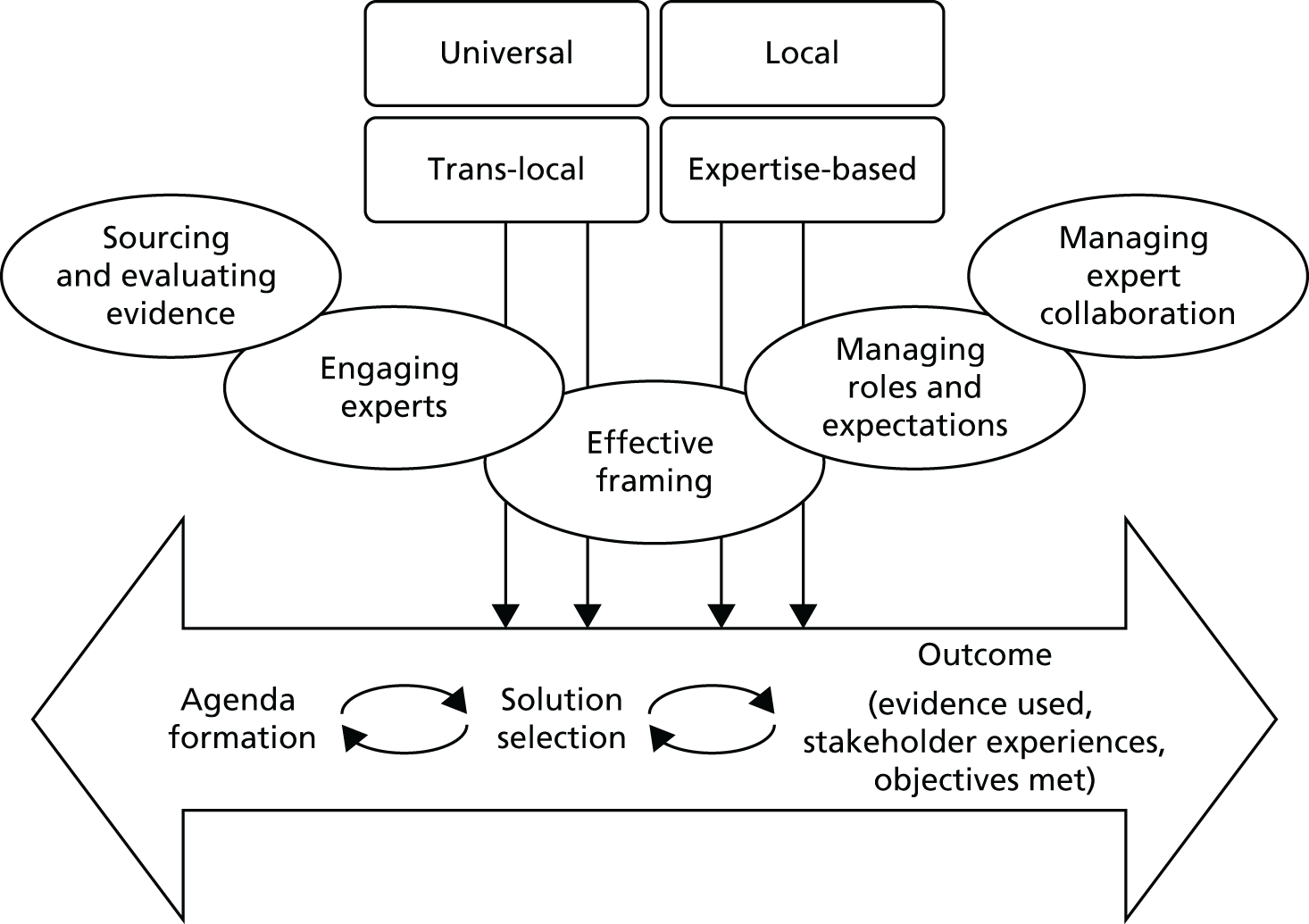
We also sought to understand the capabilities (practices and organisational conditions) that enable evidence to be deployed in context. Just like the evidence discussed in the previous section, capabilities also come in many shapes and forms, and were often about managing and understanding social relationships and politics among redesign stakeholders, reflecting the highly politicised nature of EBMgmt in health care. We also observed interesting patterns in terms of the way in which these capabilities to use evidence mediated outcomes of redesign work. At CCGs where fewer capabilities were recorded, worse outcomes were also shown. However, it is not simply the case that more is always better. Rather, the importance of each capability to successful outcomes depends on the context. In some of our cases redesigns were quite successful, even though certain capabilities were not present. It can also be assumed there is a hierarchy, or, at the very least, an interdependence, across capabilities. Framing, for example, would probably have little impact on evidence-based redesign work without engaging experts.
We conclude that, although there is no ‘silver bullet’ for evidence-based commissioning, support to improve awareness of different kinds of evidence and capabilities would be beneficial. In addition, we have shown varied understandings of evidence use and users among those involved in producing and publishing evidence-based products. These understandings are inscribed in the authoritative evidence produced by these organisations and may also need fine-tuning in order to support the translation of evidence in practice.
Figure 8 draws these findings together as a ‘roadmap for evidence use’. We construct this from our original conceptual framework (drawn from existing literature) in order to show how our findings build on the existing corpus of knowledge and how they developed through our research project. This roadmap shows that:
-
Multiple evidences are used alongside each other throughout the redesign process. No one evidence is given precedence over another, but used together they aid commissioning groups in decision-making.
-
There are challenges and barriers to evidence use in health care, but certain conditions and practices across the organisation and team can enable commissioning organisations to make more evidence-based decisions. Observations can also be made about the timing and relationships between capabilities. Engaging experts and effective framing, for example, are particularly important during agenda formation work. These capabilities have a mutual dependence, too, with expert engagement itself often increased through effective framing, while framing efforts may be redundant if experts are not present.
-
Importantly, the presence of these capabilities to use evidence appeared to mediate project outcomes. Specifically, in commissioning groups where fewer capabilities were shown overall, worse outcomes were reported.
To stay true to the nature of our findings, our roadmap, drawn schematically in Figure 8, is non-linear in nature. Metaphorically, it is perhaps best understood not as a single road, but as a ‘spaghetti junction’, where dynamic capabilities for evidence use are combined in practical settings.
Toolkit: improving capabilities to use evidence
We encourage the application of our findings in practice through the creation of a toolkit (see Chapter 7) and will develop this into a user-friendly and accessible format after the completion of the current project. Specifically, we have secured an Economic and Social Research Council Impact Acceleration Grant that will enable us to create a user-friendly, online learning resource that incorporates the main findings, as well as illustrative case stories, examples, links to other resources and the toolkit. We will consult further with key stakeholders on this impact work, initially through our collaboration in the project with Health Education England, and the West Midlands CLAHRC that is supporting us in this work. We will also be sure to avoid the duplication of existing supports and networks, instead aiming to link to these to ensure that the toolkit is as widely accessed as possible. The Economic and Social Research Council Impact Acceleration Grant is an important outcome of this project, as it will help to ensure that our findings have the best chance of actually impacting on practice.
Considerations and future directions
Although several important insights into EBMgmt in CCGs have been gained, there are of course further considerations to be taken into account. We have already discussed some of these, including the need for further research on the contextual factors shaping evidence use and evidence use in other health-care management settings. It is important, also, to consider how we conceptualise ‘authoritative’ evidence. At the outset of this project we defined guidance produced by NICE as a highly important, albeit not the only, source of authoritative evidence for CCGs. Although NICE guidance was certainly used in our cases, it was rarely given special acknowledgements or consideration. Instead, it was used in tandem with other, usually considered less ‘authoritative’, kinds of evidence.
Whereas health-care research tends to assign authoritativeness according to the attributes of evidence sources, the Oxford English Dictionary defines authoritative as ‘able to be trusted or reliable’. 98 This latter definition seems closer to the realities of health-care management, where authoritativeness is assigned not through modes of creation, but through an ability to mobilise evidence in making judicious decisions at the right time. Although we are not the first to notice, our observations reiterate the importance of reconsidering our conceptualisation of ‘authoritative’ evidence so that the realities of evidence-based health-care management are seriously noted. 2,22
Some other considerations arising from our findings relate to temporal aspects and generalisability of findings. Throughout this report we have discussed innovation with regard to service redesign, in which we expected to follow the use of evidence from inception through to changes in practice. Redesigns were, however, often very slow, iterative and interrupted. Some cases did not make changes within the time frame of our study, as they had anticipated. Other cases did implement changes, but re-evaluation for future work was almost instantaneous. Rather than following a process with distinct starting and finishing points, our research steps into an ongoing stream of redesign work. Unfortunately, the time required to implement change and our study time frame did not coincide so certain episodes of work (e.g. ‘routinisation’) were not captured. A longer study period may have enabled this, although our time frame of 30 months is already quite considerable. Instead, future research might target certain episodes of work to understand evidence use within them.
Another point to consider is the generalisability of our findings. We chose to sample eight CCGs in order to maximise opportunities to explain variation, while attending to the contextual and processual dimensions of redesign work. Doing so allowed us to achieve rich, qualitative insights into evidence-based redesign work. Although we sampled CCGs operating in different parts of the country and serving different populations (see Chapter 2), the generalisability of our findings is, of course, limited by our empirical setting. In future work, generalisability could potentially be achieved by using a bigger sample and a more quantitative approach. Doing so might enable us to understand statistically how outcomes vary according to evidence use and capabilities.
Finally, fieldwork was conducted at a time of significant reorganisation of NHS commissioning. Given the political context, more reorganisations are likely to occur in the future. This, of course, may raise questions about whether or not, and to what extent, our findings will still be relevant. To answer these concerns, we have focused on the process of commissioning, not on the specific organisation and governance of CCGs per se. Given that evidence-based commissioning processes will always be required in the NHS, we believe that our findings will be sustainable, even if further structural change takes place.
Summary of findings
We studied redesign processes in eight CCGs where four categories of evidence – local, universal, expertise-based and trans-local – were used, often in tandem, to make sense of tasks. Evidence use was often challenging, but certain practices and conditions were shown to be enabling, including sourcing and evaluating evidence, effective framing, engaging experts, managing expert collaboration and managing roles and expectations.
Implications for practice
Our findings showed clear implications for commissioning in practice, especially with regard to the use of evidence, the importance of social capabilities and support for decision-makers. These can be summarised as follows:
-
Findings show that evidence is not just accepted as relevant or reliable. Various pieces of information are brought forward and contested or applied. There were issues of perceived relevance (universal evidence), quality (local evidence), quantification and evaluation (expertise-based evidence) and access (trans-local evidence). Support to understand and address these issues might enable better evidence use in practice, which is important given the interdependence across the four categories that was shown in our findings.
-
Although redesign projects are complex, certain social conditions and practices were shown to enable evidence use. Although some capabilities identified in our study relate explicitly to evidence (e.g. sourcing and evaluating evidence), many related much more to managing social interests, conflicts and interactions (e.g. engaging experts and managing expert collaborations). Rather than technical capabilities (e.g. data searching and quality appraisals), our findings suggest that developing and supporting these social capabilities is key to evidence-based CCG redesign work in practice.
-
Cross-case comparisons suggest that successful redesign projects – in terms of evidence use, stakeholder satisfaction and meeting objectives – are informed by capabilities being in place as needed. Quantity, however, is not a measure of quality, and not all capabilities are necessary in all contexts. Managing collaborations may be less necessary, for example, when project teams have worked together and already have established norms and role expectations. It is important, then, not only to support the development of social capabilities but also to empower stakeholders to recognise when and where these are necessary.
-
Supporting health-care managers and commissioning staff to recognise, access and evaluate evidence drawing on their own capabilities relevant to the particular task and context might be an effective way of enabling evidence-based decisions. As NHS organisations are frequently restructured, this may be more sustainable approach than one that prescribes that certain evidences or certain sets of capabilities need to be utilised.
-
Much is to be gained if evidence providers consistently question themselves with regard to which model of user they inscribe, intentionally or otherwise, in their guidelines and other products. The exploratory study also suggests that potential benefits might be derived from a more explicit and broad engagement of evidence producers with the practices and techniques of ‘customer-centric design’ and ‘design thinking’.
Research recommendations
-
First and foremost, our research ‘begins with the needs of knowledge users’ as recommended by Jacobson et al. 99 We documented journeys of evidence through realistic accounts of redesign work to provide a detailed account that has been missing from the literature to this point. Although we see these insights as critical to achieving evidence-based health care, they are not likely to be universal across contexts. Further research is, therefore, needed to understand the nuances and caveats in difference contexts so that commonalities and differences can be identified.
-
We expected universal evidence to be an antecedent of redesign work, given its place in the evidence hierarchy and in studies of evidence-based health care to date. 29 We found, however, that universal evidence often set parameters around projects and is used in tandem with other types of evidence. This finding demonstrates how evidence is actually treated in practice. 2,22 It also builds on existing knowledge by showing that certain types of evidence do not ‘trump’ others;1 instead, they are often used in tandem in sense-making.
-
Much of the research on evidence-based health care has suggested structural or technological improvements (e.g. knowledge management systems). 26 We respond to work by scholars such as Ferlie et al. ,100 who called for a move, instead, towards ‘core competences’. We identified five capabilities that seemed to support better evidence use. Future research might build on this to consider, for example, how exactly capabilities come into existence and are maintained in commissioning settings. Future research might also more systematically measure the extent to which they are present and the impact of these on commissioning effectiveness. Our research provides a snapshot of this impact and should be used as a foundation for more in-depth analysis.
-
Cross-case comparisons suggest that certain capabilities impact on evidence use, stakeholder satisfaction and meeting objectives. Going forward, more nuanced insights might be gained from looking exactly at what parts of the process impacted stakeholder appraisals, or if capabilities had more or less impact on the ability of teams to meet certain objectives. Other outcomes could also be considered (e.g. timeliness and patient experience).
Acknowledgements
We are grateful to NIHR HSDR for funding this project, and to the participants who gave up their time and welcomed us into their organisations. We thank all of our participants and hope that they find the final report both useful and interesting.
We would like to acknowledge the valuable advice received on all aspects of the project from members of our SSAP: Bill Fulford (Honorary Consultant Psychiatrist, University of Oxford; Emeritus Professor of Philosophy and Mental Health, University of Warwick Medical School); Karen Keates (UNTRAP patient representative); Sue Lacey Bryant (Senior Advisor, Knowledge for Healthcare, Health Education England); Val Moore (Former Director at NICE; Chair of Healthwatch Cambridgeshire); Jane Whitehurst (UNTRAP patient representative); and Claudia Roginski (Head of Information at NHS Coventry).
We would also like to thank all of those who gave their time to attend our national workshop for sharing their valuable feedback on findings and the draft toolkit.
We would like to give special thanks to our project co-ordinator, Dawn Coton, for providing the clerical and administrative support throughout our project.
Contributions of authors
Jacqueline Swan (Professor, Organisational Behaviour) acted as the Business School Lead and was responsible for the overall project co-ordination and management, project design, and supervision of research fellows and administrative/clerical staff. She was also involved in data collection, analysis and dissemination events, co-ordinating the SSAP, report writing and publications.
Emmanouil Gkeredakis (Assistant Professor of Information Systems) was the Lead Research Fellow. He led on the qualitative research design, data collection and analysis. Emmanouil left the project to take up his Professorship at the Business School but continued to allocate 10% of his time to the project.
Rachel M Manning (Research Fellow, Warwick Business School) was the Second Research Fellow, replacing Emmanouil Gkeredakis on the project. She led on data collection, analysis, dissemination activities, report writing, workshop organisation and publications.
Davide Nicolini (Professor, Organisation Studies) led on ethics approval for overall project and was involved in project design, data collection, analysis and write-up.
David Sharp (Senior Vice President Growth Strategy, Optum International) began the project as a Director of NHS England and provided advice on project design, case selection, dissemination and analysis.
John Powell (Professor, Public Health Medicine) was involved in project design, and contributed to the interpretation of data and the final report.
Data sharing statement
Data that have been anonymised for confidentiality can be obtained from the corresponding author on request.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Wye L, Brangan E, Cameron A, Gabbay J, Klein JH, Pope C. Knowledge exchange in health-care commissioning: case studies of the use of commercial, not-for-profit and public sector agencies, 2011–14. Health Serv Deliv Res 2015;3.
- Swan J, Clarke A, Nicolini D, Powell J, Scarborough H, Roginski C, et al. Evidence in Management Decisions (EMD) – Advancing Knowledge Utilization in Healthcare Management 2012.
- Crilly T, Jashapara A, Ferlie E. Research Utilisation and Knowledge Mobilisation: A Scoping Review of the Literature 2010.
- Crilly T, Jashapara A, Trenholm S, Peckham A, Currie G, Ferlie E. Knowledge Mobilisation in Healthcare Organisations: Synthesising Evidence and Theory Using Perspectives of Organisational Form, Resource Based View of the Firm and Critical Theory 2013.
- Ferlie E, Crilly T, Jashapara A, Peckham A. Knowledge mobilisation in healthcare: a critical review of health sector and generic management literature. Soc Sci Med 2012;74:1297-304. https://doi.org/10.1016/j.socscimed.2011.11.042.
- Oborn E, Barrett M, Racko G. Knowledge Translation in Healthcare: A Review of the Literature. Cambridge: Cambridge Judge Business School; 2010.
- Briner RB, Denyer D, Rousseau DM. Evidence-based management: concept cleanup time?. Acad Manage Perspect 2009;23:19-32. https://doi.org/10.5465/AMP.2009.45590138.
- Rousseau DM. The Oxford Handbook of Evidence-based Management. Oxford: Oxford University Press; 2012.
- Kyratsis Y, Ahmad R, Holmes A. Making sense of evidence in management decisions: the role of research-based knowledge on innovation adoption and implementation in healthcare. Health Serv Deliv Res 2014;2.
- Wright AL, Zammuto RF, Liesch PW, Middleton S, Hibbert P, Burke J, et al. Evidence-based management in practice: opening up the decision process, decision-maker and context. Br J Manage 2016;27:161-78. https://doi.org/10.1111/1467-8551.12123.
- McGivern G, Lambrianou A, Ferlie E, Cowie M. Enacting evidence into clinical practice: the case of coronary heart disease. Public Money Manage 2009;29:307-12. https://doi.org/10.1080/09540960903205956.
- Swan J, Newell S, Nicolini D. Mobilizing Knowledge in Health Care: Challenges for Management and Organization. Oxford: Oxford University Press; 2016.
- NHS Constitution. London: DH; 2009.
- Gerry P, Wyatt S. NHS Chief Executive’s Review of Innovation in the NHS Summary of the Responses to the Call for Evidence and Ideas 2011.
- Dopson S, FitzGerald L, Ferlie E, Gabbay J, Locock L. No magic targets! Changing clinical practice to become more evidence based. Health Care Manage Rev 2002;27:35-47. https://doi.org/10.1097/00004010-200207000-00005.
- Wright AL, Middleton S, Greenfield G, Williams J, Brazil V. Strategies for teaching evidence-based management what management educators can learn from medicine. J Manage Educ 2016;40:194-219. https://doi.org/10.1177/1052562915624123.
- Walshe K, Rundall TG. Evidence-based management: from theory to practice in health care. Milbank Q 2001;79:429-57. https://doi.org/10.1111/1468-0009.00214.
- Zander U, Kogut B. Knowledge and the speed of the transfer and imitation of organizational capabilities: an empirical test. Organ Sci 1995;6:76-92. https://doi.org/10.1287/orsc.6.1.76.
- Grant RM. Prospering in dynamically-competitive environments: organizational capability as knowledge integration. Organ Sci 1996;7:375-87. https://doi.org/10.1287/orsc.7.4.375.
- Fitzgerald L, Harvey G. Translational networks in healthcare? Evidence on the design and initiation of organizational networks for knowledge mobilization. Soc Sci Med 2015;138:192-200. https://doi.org/10.1016/j.socscimed.2015.06.015.
- Cooksey D. A Review of UK Health Research Funding. 2006.
- Gabbay J, Le May A. Evidence based guidelines or collectively constructed ‘mindlines?’ Ethnographic study of knowledge management in primary care. BMJ 2004;329. https://doi.org/10.1136/bmj.329.7473.1013.
- Gkeredakis E, Nicolini D, Swan J. Objects of Evidence in Organisations: Insights from Studying Healthcare Funding Decision Making n.d.
- Health and Social Care Act 2012: Chapter 7, Explanatory Notes. London: The Stationery Office; 2012.
- Department of Health . Challenging the NHS to Innovate 2015. www.gov.uk/government/speeches/challenging-the-nhs-to-innovate (accessed 20 March 2017).
- Straus SE, Tetroe J, Graham I. Defining knowledge translation. CMAJ 2009;181:165-8. http://dx.doi.org/10.1503/cmaj.081229.
- Carlile PR. A pragmatic view of knowledge and boundaries: boundary objects in new product development. Organ Sci 2002;13:442-55. https://doi.org/10.1287/orsc.13.4.442.2953.
- Dopson S, Fitzgerald L. Knowledge to Action? Evidence-based Health Care in Context. Oxford: Oxford University Press; 2005.
- Estabrooks CA. The conceptual structure of research utilization. Res Nurs Health 1999;22:203-16. https://doi.org/10.1002/(SICI)1098-240X(199906)22:3<203::AID-NUR3>3.0.CO;2-9.
- Greenhalgh T, Howick J, Maskrey N. Evidence Based Medicine Renaissance Group . Evidence based medicine: a movement in crisis?. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g3725.
- Learmonth M. Speaking out: evidence-based management: a backlash against pluralism in organizational studies?. Organization 2008;15:283-91. https://doi.org/10.1177/1350508407087763.
- Kyratsis Y, Ahmad R, Holmes A. Making sense of evidence in management decisions: the role of research-based knowledge on innovation adoption and implementation in healthcare. Study protocol. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-22.
- Nicolini D, Powell J, Korica M. Keeping knowledgeable: how NHS chief executive officers mobilise knowledge and information in their daily work. Health Serv Deliv Res 2014;2.
- Pfeffer J, Sutton RI. Hard Facts, Dangerous Half-Truths, and Total Nonsense: Profiting from Evidence-Based Management. Harvard Business Press; 2006.
- Rousseau DM. Is there such a thing as ‘evidence-based management’?. Acad Manage Rev 2006;31:256-69. https://doi.org/10.5465/AMR.2006.20208679.
- Tourish D. ‘Evidence based management’, or ‘evidence oriented organizing’? A critical realist perspective. Organization 2013;20:173-92. https://doi.org/10.1177/1350508411435281.
- Tranfield D, Denyer D, Smart P. Towards a methodology for developing evidence informed management knowledge by means of systematic review. Br J Manage 2003;14:207-22. https://doi.org/10.1111/1467-8551.00375.
- Reay T, Berta W, Kohn MK. What’s the evidence on evidence-based management?. Acad Manag Perspect 2009;23:5-18. https://doi.org/10.5465/AMP.2009.45590137.
- Morrell K. The narrative of ‘evidence based’ management: a polemic. J Manage Stud 2008;45:613-35. https://doi.org/10.1111/j.1467-6486.2007.00755.x.
- Arndt M, Bigelow B. Evidence-based management in health care organizations: a cautionary note. Health Care Manage Rev 2009;34:206-13. https://doi.org/10.1097/HMR.0b013e3181a94288.
- Hewison A. Evidence-based management in the NHS: is it possible?. J Health Organ Manag 2004;18:336-48. http://dx.doi.org/10.1108/14777260410560839.
- Rycroft-Malone J, Seers K, Titchen A, Harvey G, Kitson A, McCormack B. What counts as evidence in evidence-based practice?. J Adv Nurs 2004;47:81-90. http://dx.doi.org/10.1111/j.1365-2648.2004.03068.x.
- Contandriopoulos D, Lemire M, Denis JL, Tremblay E. Knowledge exchange processes in organizations and policy arenas: a narrative systematic review of the literature. Milbank Q 2010;88:444-83. http://dx.doi.org/10.1111/j.1468-0009.2010.00608.x.
- Dobrow MJ, Goel V, Upshur RE. Evidence-based health policy: context and utilisation. Soc Sci Med 2004;58:207-17. https://doi.org/10.1016/S0277-9536(03)00166-7.
- Hewison A. Evidence-based management in the NHS: is it possible?. J Health Organ Manage 2004;18:336-48. https://doi.org/10.1108/14777260410560839.
- Harvey G, Fitzgerald L, Fielden S, McBride A, Waterman H, Bamford D, et al. The NIHR collaboration for leadership in applied health research and care (CLAHRC) for Greater Manchester: combining empirical, theoretical and experiential evidence to design and evaluate a large-scale implementation strategy. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-96.
- Jones L, King L, Wilson C. A literature review: factors that impact on nurses’ effective use of the Medical Emergency Team (MET). J Clin Nurs 2009;18:3379-90. http://dx.doi.org/10.1111/j.1365-2702.2009.02944.x.
- Swan J, Scarbrough H, Robertson M. The construction of ‘Communities of Practice’ in the management of innovation. Manag Learn 2002;33:477-96. https://doi.org/10.1177/1350507602334005.
- Rogers EM. Diffusion of Innovations. New York, NY: Free Press; 1983.
- Clark P, Bennett D, Burcher P, Newell S, Swan J, Sharifi S. The decision-episode framework and computer-aided production management (CAPM). Int Stud Manag Organ 1992;22:69-80. https://doi.org/10.1080/00208825.1992.11656593.
- Swan J, Newell S. Linking knowledge management and innovation. ECIS 2000 Proceedings 2000.
- Bledow R, Frese M, Anderson N, Erez M, Farr J. A dialectic perspective on innovation: conflicting demands, multiple pathways, and ambidexterity. Ind Organ Psychol 2009;2:305-37. https://doi.org/10.1111/j.1754-9434.2009.01154.x.
- Langley A. Strategies for theorizing from process data. Acad Manage Rev 1999;24:691-710.
- Elliott H, Popay J. How are policy makers using evidence? Models of research utilisation and local NHS policy making. J Epidemiol Community Health 2000;54:461-8. https://doi.org/10.1136/jech.54.6.461.
- Freeman AC, Sweeney K. Why general practitioners do not implement evidence: qualitative study. BMJ 2001;323:1100-2. https://doi.org/10.1136/bmj.323.7321.1100.
- Jones L, Exworthy M. Framing in policy processes: a case study from hospital planning in the National Health Service in England. Soc Sci Med 2015;124:196-204. http://dx.doi.org/10.1016/j.socscimed.2014.11.046.
- Cornelissen JP, Werner MD. Putting framing in perspective: a review of framing and frame analysis across the management and organizational literature. Acad Manag Ann 2014;8:181-235. https://doi.org/10.1080/19416520.2014.875669.
- Hansen MT. Knowledge networks: explaining effective knowledge sharing in multiunit companies. Organ Sci 2002;13:232-48. https://doi.org/10.1287/orsc.13.3.232.2771.
- Alvarez SA, Barney JB, Hitt MA, Ireland RD, Camp SM, Sexton D. Strategic Entrepreneurship: Creating a New Mindset. Wiley-Blackwell; 2002.
- Akrich M, Callon M, Latour B, Monaghan A. The key to success in innovation part I: the art of interessement. Int J Innov Manage 2002;6:187-206. https://doi.org/10.1142/S1363919602000550.
- Gibson JJ, Shaw R, Shaw R, Bransford JD. Perceiving, Acting, and Knowing: Toward an Ecological Psychology. Hoboken, NJ: John Wiley & Sons; 1977.
- Norman DA. The Psychology of Everyday Things. New York, NY: Basic Books; 1988.
- Nicolini D. The work to make telemedicine work: a social and articulative view. Soc Sci Med 2006;62:2754-67. https://doi.org/10.1016/j.socscimed.2005.11.001.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. https://doi.org/10.1111/j.0887-378X.2004.00325.x.
- Van de Ven AH, Poole MS. Explaining development and change in organizations. Acad Manage Rev 1995;20:510-40. https://doi.org/10.2307/258786.
- Pisano GP, Bohmer RM, Edmondson AC. Organizational differences in rates of learning: evidence from the adoption of minimally invasive cardiac surgery. Manage Sci 2001;47:752-68. https://doi.org/10.1287/mnsc.47.6.752.9811.
- Barley SR, Kunda G. Bringing work back in. Organ Sci 2001;12:76-95. https://doi.org/10.1287/orsc.12.1.76.10122.
- Van de Ven AH, Huber GP. Longitudinal field research methods for studying processes of organizational change. Organ Sci 1990;1:213-19. https://doi.org/10.1287/orsc.1.3.213.
- Ragin CC, Brady HE, Collier D. Rethinking Social Inquiry: Diverse Tools, Shared Standards. Lanham, MD: Rowman & Littlefield; 2004.
- Spradley JP. The Ethnographic Interview. Belmont, CA: Wadsworth; 1979.
- Guba EG, Lincoln YS, Denzin NK, Lincoln YS. Handbook of Qualitative Research. Thousand Oaks, CA: Sage Publications; 1994.
- Spradley JP. Participant Observation. Belmont, CA: Wadsworth; 1980.
- King N, Symon G, Cassell C. Qualitative Organizational Research: Core Methods and Current Challenges. London: Sage Publications Ltd; 2012.
- Gioia DA, Price KN, Hamilton AL, Thomas JB. Forging an identity: an insider-outsider study of processes involved in the formation of organizational identity. Admin Sci Q 2010;55:1-46. https://doi.org/10.2189/asqu.2010.55.1.1.
- Locke KD. Grounded Theory in Management Research. Sage Publications Ltd; 2001.
- Huberman AM, Miles MB, Denzin NK, Lincoln YS. Handbook of Qualitative Research. Sage Publications: Thousand Oaks, CA; 1994.
- Stake RE, Madaus GF, Scriven MS, Stufflebeam DL. Evaluation Models: Viewpoints on Educational and Human Services Evaluation. Dordrecht: Springer Netherlands; 1983.
- Bitner MJ, Booms BH, Tetreault MS. The service encounter: diagnosing favorable and unfavorable incidents. J Marketing 1990:71-84. https://doi.org/10.2307/1252174.
- Five Year Forward View. London: NHS England; 2014.
- Powell AE, Davies HT, Thomson RG. Using routine comparative data to assess the quality of health care: understanding and avoiding common pitfalls. Qual Saf Health Care 2003;12:122-8. https://doi.org/10.1136/qhc.12.2.122.
- Naylor C, Curry N, Holder H, Ross S, Marshall L, Tait E. Clinical Commissioning Groups: Supporting Improvement in General Practice?. London: The King’s Fund, Nuffield Trust; 2013.
- Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up?. PLOS Med 2010;7. http://dx.doi.org/10.1371/journal.pmed.1000326.
- Coulter A, Collins A. Making Shared Decision-Making a Reality: No Decision About Me, Without Me. London: The King’s Fund; 2011.
- Goffman E. Framing Analysis. An Essay on the Organization of Experience. New York, NY: Harper & Row; 1974.
- Öberg G. Facilitating interdisciplinary work: using quality assessment to create common ground. Higher Education 2009;57:405-15. https://doi.org/10.1007/s10734-008-9147-z.
- Robertson M, Swan J, Newell S. The role of networks in the diffusion of technological innovation. J Manage Stud 1996;33:333-59. https://doi.org/10.1111/j.1467-6486.1996.tb00805.x.
- Kraatz MS, Block ES, Greenwood R, Oliver C, Suddaby R, Sahlin-Andersson K. The Sage Handbook of Organizational Institutionalism. London: Sage Publications Ltd; 2008.
- Sackmann SA. Cultural Complexity in Organizations: Inherent Contrasts and Contradictions. Sage Publications; 1997.
- Foucault M, Nazzaro AM. History, discourse and discontinuity. Salmagundi n.d.:225-48.
- Harreld JB, O’Reilly CA, Tushman ML. Dynamic capabilities at IBM: driving strategy into action. Calif Manage Rev 2007;49:21-43. https://doi.org/10.2307/41166404.
- Brown T, Wyatt J. Design thinking for social innovation. SSIR 2010;12:31-5. https://doi.org/10.1596/1020-797X_12_1_29.
- Kelly M, Morgan A, Ellis S, Younger T, Huntley J, Swann C. Evidence based public health: a review of the experience of the National Institute of Health and Clinical Excellence (NICE) of developing public health guidance in England. Soc Sci Med 2010;71:1056-62. https://doi.org/10.1016/j.socscimed.2010.06.032.
- Russell L, Tzortziou Wardell. A strategic approach to promote research among CCGs. Health Serv J 2013.
- Holder H, Robertson R, Ross S, Bennett L, Gosling J, Curry N. Risk or Reward? The Changing Role of CCGs in General Practice. London: Nuffield Trust, The King’s Fund; 2015.
- Petsoulas C, Allen P, Checkland K, Coleman A, Segar J, Peckham S, et al. Views of NHS commissioners on commissioning support provision. Evidence from a qualitative study examining the early development of clinical commissioning groups in England. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005970.
- Ferlie E, McGivern G, Bennett C. Making Wicked Problems Governable?: The Case of Managed Networks in Health Care. Oxford: Oxford University Press; 2013.
- Newhouse RP. Creating infrastructure supportive of evidence-based nursing practice: leadership strategies. Worldviews Evid Based Nurs 2007;4:21-9. https://doi.org/10.1111/j.1741-6787.2007.00075.x.
- Oxford English Dictionary. n.d.
- Jacobson N, Butterill D, Goering P. Consulting as a strategy for knowledge transfer. Milbank Q 2005;83:299-321. https://doi.org/10.1111/j.1468-0009.2005.00348.x.
- Ferlie E, Crilly T, Jashapara A, Trenholm S, Peckham A, Currie G. Knowledge mobilization in healthcare organizations: a view from the resource-based view of the firm. Int J Health Policy Manage 2015;4:127-30. https://doi.org/10.15171/ijhpm.2015.35.
- Garud R, Gehman J, Kumaraswamy A. Complexity arrangements for sustained innovation: lessons from 3M corporation. Organ Stud 2011;32:737-67. https://doi.org/10.1177/0170840611410810.
- Hargadon A, Sutton RI. Technology brokering and innovation in a product development firm. Admin Sci Q 1997;42:716-49. https://doi.org/10.2307/2393655.
- Ansari SM, Fiss PC, Zajac EJ. Made to fit: how practices vary as they diffuse. Acad Manage Rev 2010;35:67-92. https://doi.org/10.5465/AMR.2010.45577876.
Appendix 1 Rutterford Clinical Commissioning Group
Case study overview
We first contacted a GP professor in diabetes at Rutterford CCG. Through him, we met stakeholders who had been involved in the diabetes redesign. We spoke with a diabetes nurse consultant and a CCG transformation manager. We later followed up with the diabetes nurse consultant and interviewed the chief accountable officer at the CCG. At the time of our visit, the CCG had spent the previous year developing recommendations on ‘delivering the best possible care’. Increased numbers of patients with diabetes, increased complications and increased spend were primary concerns in the CCG. The CCG had secured some funding that could be used to buffer any upfront losses from changes to the diabetes pathway. They also had a diabetes education programme in place for upskilling community staff. With these resources in place, a transformation team was put in place. They faced some challenges, which were mostly overcome by the creation of subgroups. These groups collected, evaluated and reported on evidences related to their specific disciplines. Each group made recommendations about the pathway that were then collated into one overall report. The team decided to improve care ‘downstream’, that is, at the beginning of the pathway, in order to prevent complications, improve care and, ultimately, save money. At our last point of contact, which was approximately 2 years after the first interviews, around one-third of practices had attended the education programme, a specification for new services had been written, and they were in the process of evaluating referral data to understand the impact of their redesign work.
Agenda formation
Stakeholders at Rutterford CCG broadly framed problems with care, capacity and cost using local and universal evidences. The diabetes nurse consultant described how there had been a ‘spurt in chronic diabetes’ that meant ‘to even offer basic care service in the future’ changes in the pathway were necessary. Current services were seen as ‘very acute centric’, with the transformation manager describing how they had ‘seven diabetology consultants and even if we appointed another 70 we still wouldn’t have enough to . . . see absolutely everybody’. As a result, ‘11–12% of the NHS budget [was being spent] on complications’ (report, 2013).
Duplication of work was also problematic because it contradicted the universal evidence. This violation was understood in terms of local evidence that showed that they had ‘two foot clinics [that] both happen on the same day’, which violates NICE guidance that patients should have ‘access to a multidisciplinary team within 24 hours’. Local evidence also showed that there was variation in care across clinics, with one operating in line with national guidance (around one patient in every six was seen again), while the other had a ‘follow-up ratio of 1 to 10’ (transformation manager). Overall, these evidences suggested that current services were a ‘poor use of resources, not great value for money and [having] not great outcomes for patients’ (accountable officer).
Recognising these issues, the diabetes nurse described how they ‘formulat[ed] a transformation steering group to understand actually who is likely to be able to make decisions to mobilise this plan [to address the issues]’. She wanted this group to engage experts and offer them an ‘opportunity to . . . get together and really think about what diabetes care in [the area] should look like’ (diabetes nurse). The transformation manager saw the group as ensuring that ‘everybody had a voice . . . was listened to’ and that ‘all the right . . . all of the stakeholders’ were involved. There was broad involvement in the group (there were upwards of 35 in one meeting alone; diabetes nurse).
Selecting a solution
Having established the groups, the transformation manager endeavoured to develop a shared frame on the search for a solution. The nurse commented that they wanted to bring ‘about change that people agreed to and that actually happen, rather than people coming up with ideas but it never going anywhere’. Stakeholders were asked to use expertise-based evidence take a ‘step back [and think] . . . what would [the service] look like if we had a blank piece of paper’:
. . . we tried not to look at this is what we’ve got, this is how we need to change it because that keeps you a bit stuck . . . so we did very much a ‘if we could do anything what would it look like?’ Obviously you can’t do anything, there are restrictions in terms of finance and people and bodies. But in the beginning, we did a lot of visioning and a lot of blue sky thinking and a lot of ‘what would we really want it to look like?’
Diabetes nurse
The diabetes nurse commented that these discussions took place at a time when there was a lot of ‘talk around the ‘left shift’, that is . . . moving non-acute services from the acute trusts back to primary care’. She referred to the universal evidence, including NHS ‘Better Care Together’ (www.bettercaretogether.co.uk) and Monitor’s ‘Care Closer To Home’ (www.gov.uk/guidance/moving-healthcare-closer-to-home). Each of these documents focused on ‘the repatriation of people from secondary care into primary care, reducing hospital admissions, length of stay’ (diabetes nurse). Because of this, the solution search used a blank sheet, but it was not completely blank.
Two factors in the broader landscape were noted as critical to moving beyond agenda formation: funding and education. A transformation fund to support service redesign planning was won from ‘a joint bid between the CCGs and the [hospital]’ (report). They were awarded £1M to ‘do a full pathway review of diabetes care in the region’ (transformation manager), which protected them against financial penalties. Because of this, the work was ‘de-risked’:
The acute trust potentially [could] lose out on income which means that we had to be able to financially compensate them for at least for year 1 . . . if they’re going to lose a couple of million pounds it has a significant impact . . .
Transformation manager
The transformation fund was described as giving ‘extra impetus’ to an education programme for primary care that had already been created in the CCG (transformation manager). Educating primary care stakeholders was seen as vital to avoid putting patients in ‘a risk situation’ (transformation manager). Education was spread ‘right from health-care assistants, right the way up to practice’ so that ‘they feel valued’ (diabetes nurse). These supports meant the group was able to think about ‘how that change might be managed’ (diabetes nurse). The diabetes nurse saw education as a way to strengthen the redesign because:
. . . if people don’t feel competent and skilled to look after [a new way of providing care] it fails and it doesn’t work . . . the people that are doing the caring have the skills and the expertise and competency to do that. If you’ve got that it’s much more likely to succeed . . .
Three main ‘challenges . . . disagreements . . . debates’ arose. First, ‘change is frightening . . . it can make you feel a bit insecure’ and so some stakeholders were hesitant to participate (diabetes nurse). Second, stakeholders ‘at the beginning . . . [had] very fixed ideas about what they wanted to see at the end of the project’. This limits the scope of the evidence search and precludes them from contributing fully to the blank sheet (transformation manager). Last, professional boundaries were a challenge, with secondary care not convinced that primary care could take care of patients (diabetes nurse). Conversely, primary care saw secondary care as ‘holding on’ to patients longer than necessary:
[Secondary care] feel that they are probably the only people who can look after anybody with a foot ulcer. So they will hang on to that person . . . until such time as that ulcer is completely cured and then … maybe we could just hang onto you a bit longer . . . patients essentially went into a foot clinic and stayed there . . .
Diabetes nurse
Despite these challenges, the transformation manager described how she continued to push for the use of a ‘blank sheet’ because she felt that ‘unless we understand the whole way across the pathway then you can’t deal with a particular part of the pathway unless you can see the whole picture’. A variety of approaches was taken to manage expert collaboration and overcome challenges. Among these, informal approaches such as ‘having a chat, taking them out for a meal, having a walk with them’ (accountable officer) were noted as useful. Another approach was to frame the problem so that ‘everybody start[ed] from the point of view of the person with diabetes and what they needed and what would be best for them and how that might be best provided’ (transformation manager).
The most impactful approach to managing expectations, however, was the formation of ‘task and finish’ subgroups. These subgroups were tasked with identifying ‘gaps in provision and making recommendations’ for improvement (report, 2013). They had to ‘map out exactly what everyone provided [bringing] . . . everything onto the table’ (diabetes nurse). Each subgroup was to consider ‘what are we good at, what do we do well, what aren’t we good at, what might be the reasons for that’. They had to look at local evidence (e.g. patient numbers) and utilise expertise-based evidence to identify potential areas of improvement. The groups were also asked ‘is there any evidence, is there any models of care elsewhere, are there any best practice . . . that’s been published in the literature that we might want to adopt and change’ (i.e. universal evidence; transformation manager). The following quotation illustrates an example of the work one subgroup:
We took antenatal care we looked at, you know, clinical outcomes, you know, what sort of levels of care people had when they came into antenatal, were they referred early, were they referred late, you know, were they planned pregnancies, unplanned pregnancies, what were their care during pregnancy, what was their outcome.
Diabetes nurse
The creation of subgroups was a considerable step in managing expert collaboration because stakeholders were asked to identify, evaluate and present evidence from within their field and alongside their peers. Thus, issues around power and interprofessional working are removed so that work can be completed.
Although evidence use seemed to benefit from subgroups, challenges remained. Subgroups had problems with evidence quality. The diabetes nurse described that while ‘there has always been . . . a culture of looking at our data and . . . auditing the service . . . local evidence . . . [was] the one thing that was actually really, really difficult to get’.
Even when evidence could be accessed it was somewhat ambiguous and even outdated:
In secondary care diabetes was either [labelled] under diabetes, integrated medicine, specialty medicine or diabetes and endocrinology. So the whole idea of untangling some of that was incredibly difficult . . .
Diabetes nurse
Working with data that was more than a year old so it wasn’t particularly helpful . . . we tried to do was understand . . . what was the current activity, and then secondly what was our current spend. But diabetes gets wrapped into other conditions. So even if we’re doing something like ‘what’s our drugs spend?’ it became a best guess. Because . . . if the patient had diabetes they were likely to be on the following combinations of therapies so do you count them or do you just count the diabetes ones? Diabetes in the secondary care setting is, it’s just so tied up with loads of other things . . .
Transformation manager
Stakeholders also noted that there was ‘too much’ universal evidence, which often ‘became this minefield because . . . how do you synthesise all this so that you’d end up with something that is succinct so that you can deal with it?’ (transformation manager). Contextualising universal evidence was also problematic, with the diabetes nurse describing it as ‘useful where it exists’, but:
. . . sometimes you get some clinical disagreement with the decisions that NICE has made and you get some local needs that are very specific. So you need to provide something different . . . some areas of the city have got a very young population so there are issues around type 1 diabetes . . . we’ve got lots of Asian people where English isn’t the first language. So you have to, on top of all of that guidance, you have to put in what is going on locally and what isn’t and you know, and what is available. Because, sadly, the same resource isn’t available everywhere . . . so the model has to be realistic into what is available.
Diabetes nurse
Despite these barriers, the transformation manager described how there was good engagement among stakeholders. There was a kind of common-sense ‘buy in’ that meant that stakeholders persisted with their work, even when there were difficulties. The transformation manager reflected that stakeholders really wanted:
. . . to buy into this . . . they saw the vision we want to get to a foot clinic every single day of the week, Monday to Friday . . . they knew fundamentally that was going to offer a better service . . .
Process outcomes
Each subgroup delivered a pathway, and these pathways were then merged into a larger pathway. This new, larger pathway was presented to the overall transformation group. In the larger pathway, stakeholders agreed on the aspects of diabetes care that would remain in the hospital and those that would be moved to the community. The team released a report documenting the new pathway. They also wrote a service specification and attained a firm commitment to a diabetes education programme. As of December 2015, the diabetes nurse reported that the target set for attendance at the education programme had been met with sustained interest and increasing numbers of practices in attendance. It was felt that this programme had caused changes to how people worked by giving them more confidence and knowledge. They had also identified ‘key performance indicators and objectives for the project’. They had also got ‘a number of practices . . . up to enhanced level . . . we’ve got 23 of the 63 practices at enhanced level’ (diabetes nurse), meaning that they would be competent to provide community-based diabetes care. At this point statisticians at the CLAHRC were looking into referral data to establish the impact of the redesign work.
Stakeholders also made reference to the issues of doing and continuing innovative redesign work in the NHS more generally. One questioned the need to have separate diabetes projects across CCGs, suggesting that ‘the NHS is very bad at leveraging best practice and taking what aids that and implement it. They’re more likely to say actually this is my patch and I’ll do it my way, go away type stuff’ (accountable officer), In terms of sustaining innovation, the need for ongoing evaluation was highlighted:
Just because you’ve transformed a service doesn’t mean it’s OK . . . there should be a rolling program of review . . . what we’re going to change this year, what is the long term planning in terms of year 1 . . . 2 . . . 3 . . . I don’t think that has been built in . . . And then things get stale don’t they, and then you have to start again. Whereas I think it would be much better to . . . continually looking and evaluating. But we have, you know, we look for other funding for things that happen.
Diabetes nurse
Summary of findings
Local and universal evidences were used to broadly frame problems with care, capacity and costs. Efforts were made to engage multiple experts during the entire process and involvement in the group was broad. Redesign leaders managed to develop a shared frame on the solution search by using a blank sheet approach. It is important to note that this sheet was not completely blank because stakeholders had an idea that they wanted to move care to the community based on universal evidence talk around the ‘left shift’. A variety of approaches was taken to manage expert collaboration; the most impactful approach to tackling the barriers was the formation of ‘task and finish’ subgroups. These groups looked at local evidence (e.g. patient numbers) and used expertise-based evidence to identify potential areas of improvement. The groups were also asked to look at universal evidence. Although they faced several barriers during the redesign, stakeholders managed to produce a report as per their brief. Attendance at the education programme was sustained, meaning that many practices were competent to provide community-based diabetes care. Statisticians are currently looking into referral data to establish the impact of the redesign work. Overall, evidence use was problematic at Rutterford CCG, but it overcame this to achieve its objectives. Stakeholders also seemed to be satisfied with the process. The redesign process for this CCG is summarised in Figure 9. Guidance for reading process maps is shown in Figure 3.
FIGURE 9.
Redesign process at Rutterford CCG.
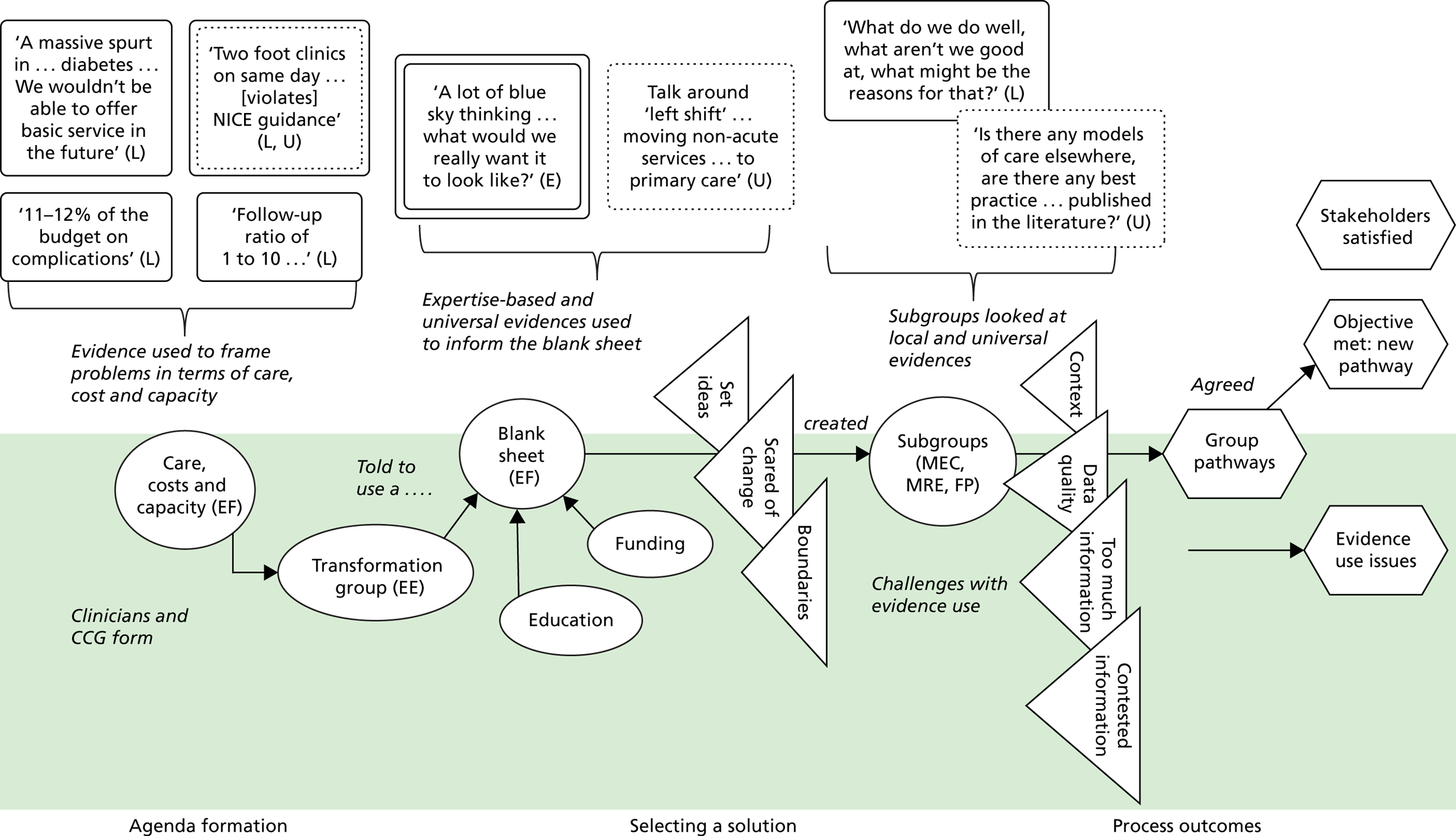
Appendix 2 Chelsea Clinical Commissioning Group
Case study overview
Chelsea was one of four CCGs in a trust serving ‘a population of nearly a million’ (report). The trust was concerned with diabetes care and decided that all four CCGs should improve their diabetes care. Participants at Chelsea included a contracting manager, a diabetes consultant, a commissioning manager and a patient representative from a leading charity. The consultant described how each CCG had ‘differing ideas, differing needs. Population profile is entirely different in all four’ (diabetes consultant), but the trust had embraced a model in which patients ‘who can be managed in the community will be sent off to the community to be looked after by the community nurse and the GPs for whom support in various different forms will be provided’ (diabetes consultant). Chelsea was the first of the four to have to ‘a reasonable extent . . . done . . . gone to the first phase where we’re actually discharging people now’ (diabetes consultant). During interviews, stakeholders described how knowing what the new pathway would look like from the outset and so their work was about making evidence from elsewhere relevant to context. The diabetes consultant and performance team were largely responsible for presenting evidence to a steering group. Although it had some faced barriers around communication and finance, the CCG managed to implement changes. At our last contact, the CCG planned to evaluate the impact of the redesign. The remaining three CCGs were still in pre-contemplation stages of change.
Agenda formation
The trust in which Chelsea belongs chose to adopt a model that involved moving patients to the community. This model had been developed at Seaport CCG and was seen as a means to improve care provision by reducing demands on hospital capacity. The diabetes consultant described how ‘philosophy as just the same [as Seaport CCG’s model]’ meaning that trans-local evidence was fundamental in agenda formation. The consultant also had a role in bringing expertise-based evidence because he had worked in a hospital where the ‘model been developed, delivered, evaluated’ and ‘seen first-hand how it works’. He also brought universal evidence, describing, for example, to ‘a paper, I can’t remember where, but there is a paper’ showing model efficacy. He also cited public health data showing ‘diabetes prevalence is slightly higher than the England average’.
The performance team also had a role in bridging ‘the gap between information and the team, to allow [them] to get the information . . . so that we’ve got the data that we can do things’. The performance team was largely responsible for providing local evidence around ‘activity and finance . . . waiting list data’, which was used to show how in ‘the existing system . . . patients are due an appointment in 9 months . . . were just seen just last month’ (diabetes consultant). This delay was caused by long waiting lists, which, in turn, left complex patients needing emergency care. Their emergency care often overlapped with scheduled visits, causing a duplication of work. In the new model, however, it was expected that ‘the complex needs patients who actually need the most will get it on time’ (consultant).
Selecting a solution
Although a solution had been identified from the outset, experts were engaged in a steering group with ‘representation from CCGs, from the trust, from the intermediate care services’. The steering group met ‘every six weeks [to discuss] how we are going to deliver this, what is the next step and how will the patients be discharged’. Over a relatively short period (18 months) the group agreed which diabetes services would be left in the hospital and which would be moved to the community. The services that were left in the hospital were the same as those that were left in the hospital at Seaport, meaning that trans-local evidence played a major role in this episode of work. Expertise-based and universal evidences were also used in tandem during this episode. The contracting manager, for example, described how there was an ‘assumption that the NICE guidelines and those sorts of things are being met and that it will be the responsibility of the lead consultant to ensure that everything is being [met]’ (contracting manager).
Although trans-local evidence meant that stakeholders at this CCG were largely mimicking Seaport, structural conditions acted as barriers to their progress. Communication was the first major barrier mentioned in talk. The diabetes consultant described how the steering group had wanted to develop a computer-based referral system so that he did not have to ‘free up an hour of [his] time for GPs to call . . . [to talk about] discharged patients’. The computer-based communication was not feasible, however, because ‘half of [the practices] are still on a system called System One and half of them are on IBIS and they don’t talk to each other’. Other communication issues came to light when the team were trying to get the trans-local evidence contracted into practice. The contracting manager described how she often had: ‘no idea who I need to speak to, you know . . . it makes life very difficult because every trust is set out differently as well so you never know who’s best to ring’.
Other barriers to implementing the trans-local evidence were financial in nature. Although the trust had been provided ‘about four diabetes nurses’, the CCG still did not have the money to backfill them so that they could go on upskilling days (as had been done in Seaport). Because of this, Chelsea was yet to develop ‘locally enhanced services’ (as Seaport and Rutterford CCGs had). Instead, much of the responsibility for supporting community-based diabetes services was left with the consultant:
The CCG is funding the practice nurses who want to do it to do [education] modules . . . it’s all fully funded [but] there has not been takers for those practices . . . Because they can’t release the people. Because there is, yeah, somebody has to backfill them . . .
Diabetes consultant
Finance was also a challenge because consultants were ‘paid on the number of patients’ seen. This arrangement meant that once the ‘numbers go down’ (as patients are referred to the community), consultants would potentially be at a loss. At our meeting there had been no reassurances put in place, but the diabetes consultant said that there was ‘plenty for him [and other consultants] to do’ and so he was certain that the CCG would find the money to keep him employed. Despite this uncertainty, the consultant had decided to take a risk and let support for the community-based model come ‘out of [his] own time, I’ve just done it’.
Process outcomes
Relative to other CCGs, the redesign work at Chelsea was quite straightforward. It decided to move care into the community, mimicking, almost exactly, the pathway that had been developed in Seaport. Chelsea of course faced some challenges, particularly around finance and communication. Overall, however, Chelsea had agreed a new pathway and had ‘gone to the first phase where we’re actually discharging people’ (diabetes consultant). The consultant described how the steering group continued to hold a discharge meeting in which he used expertise-based evidence while going through a ‘list to identify say 30 patients to be discharged . . . the panel will agree and then the patients will be discharged back to the community with a letter to the GP, with a letter to the patient to say your care is going’.
An evaluation of the changed services was also planned and would be completed by ‘a separate quality team [who have] have a quality review meeting on a quarterly basis with the commissioners . . . and the support unit’ (contracting manager). The evaluation would be based largely on local evidence about ‘follow-ups . . . making sure that they are seen on time, time to first wait’. These evaluations would then be contrasted to outcome markers produced by creators of universal evidence (e.g. the Public Health Observatory) using universal tools (e.g. the Dove Tool).
Overall, Chelsea CCG had met its objectives and stakeholders were largely satisfied. Perhaps because it had decided to mimic Seaport CCG prior to our fieldwork, no major issues with evidence use were recorded in our data either.
Chelsea summary of findings
Chelsea CCG was one of four CCGs in a trust that had been instructed to consider diabetes service provision. Diabetes had been a concern for over 3 years in the area. Once it was given the go ahead, it took just 18 months to implement a new model of diabetes care in practice. This model was based, almost exclusively, on trans-local evidence from Seaport CCG. This meant that the problem and solution search were framed quite narrowly, although role expectations about the redesign were quite clear from the offset. There was a lot of work around expert engagement, especially with the steering group. The group used local, universal and expertise-based evidence to frame issues around capacity for diabetes care during agenda formation episodes. Indeed, the diabetes consultant and the performance team had very clear role structures as actors who brought relevant evidence forward. Although they had faced barriers around communication and finance, they pressed forward with the redesign. At our last point of contact, the CCG had plans to evaluate the impact of its redesign using universal-evidence based tools alongside local evidence about patient experiences of care (e.g. follow-ups, time to first wait). The redesign process for this CCG is summarised in Figure 10. Guidance for reading process maps is shown in Figure 3.
FIGURE 10.
Redesign process at Chelsea CCG.
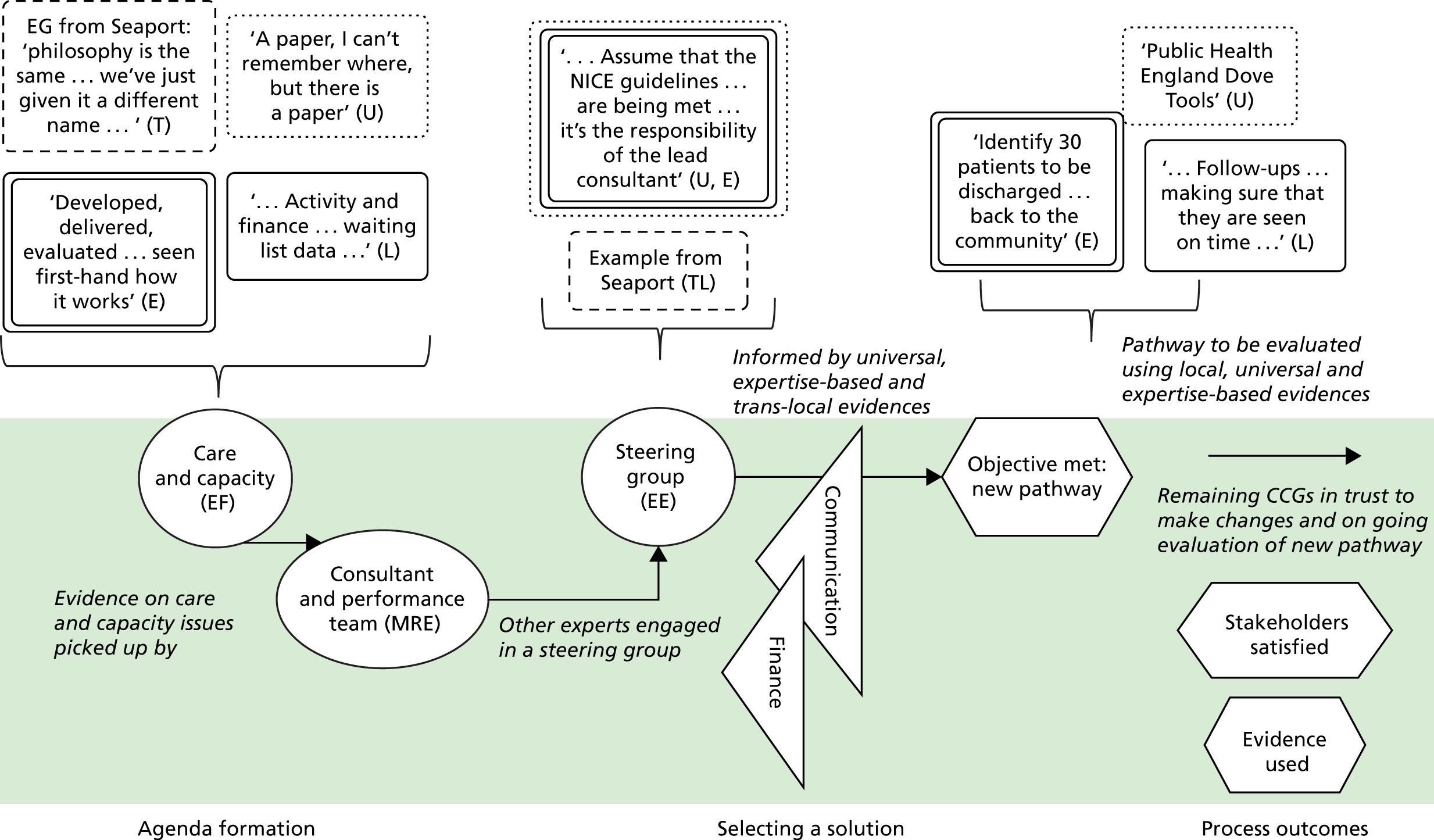
Appendix 3 Coalfield Clinical Commissioning Group
Case study overview
We first contacted a senior commissioning manager at Coalfield CCG. This individual had played a significant role in the redesign work. He also referred us to other stakeholders who had been involved, including the clinical lead for MSK, the clinical director, the CCG chairperson and a support manager patient engagement. At the time of our visit, this core group had been working together for several years to incrementally change different parts of the MSK pathway (e.g. respite services). They had reached a point, however, at which they felt that the only way ‘to move forward was through whole-scale integration’ of services (senior commissioning manager), and so this is the focus of our case study. At the time of our first meeting, Coalfield had designed and agreed a more integrated MSK pathway and it was about to ‘go live’ (i.e. be implemented in practice). We followed up with the senior commissioning manager on three additional occasions after these first interviews to capture implementation progress. Although there were some challenges, the new pathway was implemented and, at our last contact, stakeholders were working to evaluate its efficacy.
Agenda formation
The senior commissioning manager, the clinical lead for MSK and the clinical director drove the redesign project at Coalfield. These stakeholders effectively framed a more integrated MSK pathway as a means to improve patient care. The need for the new pathway was based on local evidence, with the senior commissioning manager describing how patients were ‘going to the wrong ology at the wrong time’, which meant that they were not getting the right care. He also provided a narrative example, describing one patient who was employed as a steel-grinder, but was approaching retirement. This patient was suffering joint pain and, in the current pathway, would be offered an operation. However, the operation would mean a significant period of recovery that would ultimately jeopardise the patient’s employment. In the new pathway, however, patient need would be the main concern and so, in the case of the steel-grinder, services to maintain his condition (e.g. physiotherapy) would be offered first. Once the patient reached retirement age, other treatment options could be considered, if necessary.
Local evidence pertaining to contracting arrangements was also used to frame the problem. The group felt that the current ‘payment by results system’ was not good for ‘the outcomes of the patients’ (clinical director). They wanted to adopt a form of contracting that recommended by the NHS (universal evidence) known as the capitated outcomes-based incentivised care (COBIC) contract (www.cobic.co.uk). COBIC is a block contract based on patient outcomes, rather than on the number of patients seen (clinical lead). The group decided to pursue this contracting model because it means that ‘behaviour is focused on what’s best for the patients’ (support manager).
The commissioning manager described ‘just trying to keep people out of hospital, lowest intervention, less intensive interventions’ as a means to achieve better patient care. As mentioned in the previous cases, community-based care is informed largely by universal evidence.
Selecting a solution
Work began on the redesign when the group ‘sat down and chewed it over . . . spoke to a few people and mulled it about’ (commissioning manager). As in other CCGs, the group decided to frame the solution search in terms of a ‘blank sheet’ in order to engage stakeholders. Again, however, this sheet was only partly blank given the focus on reducing hospital-based care. The clinical lead saw the blank sheet as important because it engages stakeholders, allowing them to think that ‘actually I’m a bit more in control of my own destiny here’.
Engagement was not straightforward, however, and the first meeting was described as ‘hilarious’ because ‘orthopods hated podiatrists, who hated the physios and they all sat with their back to each other . . . in silence for about 5 minutes’. There was resistance between groups until:
. . . one of them said so what do you want us to do? I said I want you to deliver the best possible care for my patients for the limited amount of money you’ve got. And they said how do you want us to do it? I said I don’t care. I said if you want to get a bag of bones and shake them if that makes a difference that’s fine. I said I just want the outcome . . .
Clinical lead
The commissioning manager was understanding of these issues, however, describing how stakeholders ‘didn’t know each other, they’d never met before . . . didn’t know what clinical skills and options the others could provide’. He described efforts to manage engagement to ensure that they ‘came round and started to have conversations’. First, he felt that because they had done ‘lots and lots of MSK commissioning’ they would have ‘a lot of credibility [because stakeholders] knew [they had] done some big things . . . knew [they’d] done commissioning that worked well’. Other efforts to manage engagement included creating a project website, which the clinical lead saw as ‘something . . . that really pulls people together’. Ultimately, expert engagement was seen as:
. . . the stuff that means your project survives when it gets to a rocky bit . . . a bit cold and a bit difficult . . .
Clinical lead
Efforts to engage patient experts were also notable. Meetings were held with patients, carers and the public so that pathways could be ‘coproduced’. The commissioning manager described how not having ‘local people who completely believe in what you’re trying to do [means] you can’t get anywhere’ The clinical director described how patients can be used as experts, and also be used to manage expert collaboration. As in other CCGs, patients can force stakeholders with divergent ideas or competing interests to reach agreement:
The value of . . . involving the patients . . . right from the word go because they bring a sort of sense of credibility is not the right word but it’s a sense of realism into it right from the outset that you can’t argue with . . . Sometimes you get into little sort of power plays between specialists in the area but actually if you have the view of the patients involved they cut through all that crap . . .
Commissioning manager
During this episode stakeholders sourced and evaluated a mix of universal and expertise-based evidence. The commissioning manager described using universal evidence, including ‘national evidence . . . international evidence’, especially NICE, in relation to ‘good practice, best practice’. This evidence gave ‘a bit of assurance that [stakeholders] are not going to do something whacky’ (clinical lead). Stakeholders also described having to use expertise-base evidence to understand and evaluate universal evidence:
We’re working with expert clinicians . . . many of which have actually written the papers. So they really can understand and make very clear to each other . . . and also argue the case with each other . . .
Senior commissioning manager
Expertise-based evidence was not only important to interpret universal evidence, but also critical to making changes. The clinical director described the MSK screening tool that they wanted to use. It had been produced by academics, but had not been widely trialled before being selected for use at Coalfield. As the clinical director described:
We actually started the changes before the evidence was published so we knew the evidence was coming and you know, we did . . . so we were quite brave really. We sort of thought it’s the right thing to do, let’s do it . . .
They also drew on local evidence to identify a solution. They discussed, for example, how they had a number of facilities in the community (e.g. gyms and sports halls) that were currently underused. They agreed that these spaces could be used for prevention services. So, for example, in these spaces ‘if you need to have your hip replaced would you . . . do your pre-physio [and] your physio afterwards [to] get yourself fit before you have the operation’.
Process outcomes
After several meetings, a new pathway was agreed among stakeholders. The senior commissioning manager described how secondary care stakeholders were ‘saying this [the pathway] fantastic because this is what we’re trained to do . . . We trained in medical school to look after, to help and support people. They were all incredibly frustrated by the systems they currently work in, the silos, the fragmentation’. He also described how the ‘finance director . . . the chief exec . . . the chairman . . . the medical director’ being on board. Although stakeholders were happy with the pathway, the senior commissioning manager still did a ‘lot of leg work’ to ensure expert engagement so that it would be actually implemented in practice:
Before every board meeting I spoke to every board member . . . sticking my head round the door while [saying] do you get it? Do you understand? Have you got any questions? Is there anything worrying you? It’s literally simple as that. And they’d go that’s fine, get out, for God’s sake. In the end people get bored, go away . . . we support you, we’re with you . . .
There were some barriers in implementing the pathway, especially resistance to change. The clinical lead described how ‘the NHS doesn’t have the appetite for innovation that exists in the private sector because the private sector have to make a profit’. Because of this, there was resistance to changing the current pathway, even though it was not working well:
No one has bankrupted each other to be totally blunt. The system has managed. It’s been a complicated way of doing it but . . . the health system as an economy has survived. So . . . to unpick something they don’t like but on the other hand which is relatively low risk and they’ve learned to manage . . . was hugely challenging.
Commissioning manager
To overcome resistance, the clinical lead described how they ‘threatened to stick [the new pathway] out to tender to get [resistant parties] to play ball’. Forcing co-operation in this manner was complicated by governance because it meant that they had:
. . . NHS England on [our] necks because we were ‘privatising’ . . . but once we said we want to work with [local trusts] we had Monitor on our backs saying you’ve not put it out to tender . . . we had to jump through lots of hoops to demonstrate why we weren’t putting out to tender . . . So it’s just a system that doesn’t join up . . .
Clinical lead
Despite challenges, the pathway went live several months after our initial interviews. During follow-ups, stakeholders had a strong interest in evaluating the pathway with methods from other countries (trans-local evidence). They were particularly interested in ‘clinical quality, safety . . . patient gain and patient experience’ (clinical lead). The commissioning manager noted that:
The thing I think the NHS what I’ve seen does badly usually is we do the stakeholder bit really well, we do the implementation planning, we kick off the implementation and then we just pull back. Do you know what I mean? And we don’t do the crucial bit then of studying what happens and then making changes . . . we’re always looking to sort of improve things and sort of make changes. But then as soon as we made a change we’re onto the next thing . . .
Overall, the redesign work at Coalfield CCG was successful. It met its objectives and used a variety of evidences, and stakeholders were satisfied.
Coalfield summary of findings
Stakeholders used local and universal evidences to frame problems with MSK services. They were largely concerned with improving patient care experiences, but did also acknowledge costs. Experts were engaged with the redesign from the offset (senior commissioning manager, clinical lead for MSK, clinical director, CCG chairperson and support manager for patient engagement) but they did a lot of work to build and manage further expert collaboration. In the search for a solution, for example, they adopted the blank sheet approach to frame the search for a solution. Again, this sheet was not completely blank because they had an idea that they wanted to move care to the community based on universal evidence. A variety of approaches was taken to manage expert collaboration, including relying on the group’s credibility, creating a website and focusing on the patients. Universal evidence informed many of their decisions in terms of the selected solution, but this was usually understood in the light of local and expertise-based evidences. Although there were some barriers (e.g. resistance to change), the stakeholders worked to continue to engage experts at the implementation episode. When they were not successful, they used the threat of external tender. The new pathway went live several months after our initial interviews, and stakeholders showed a strong interest in evaluating its impact. The redesign process for this CCG is summarised in Figure 11. Guidance for reading process maps is shown in Figure 3.
FIGURE 11.
Redesign process at Coalfield CCG.
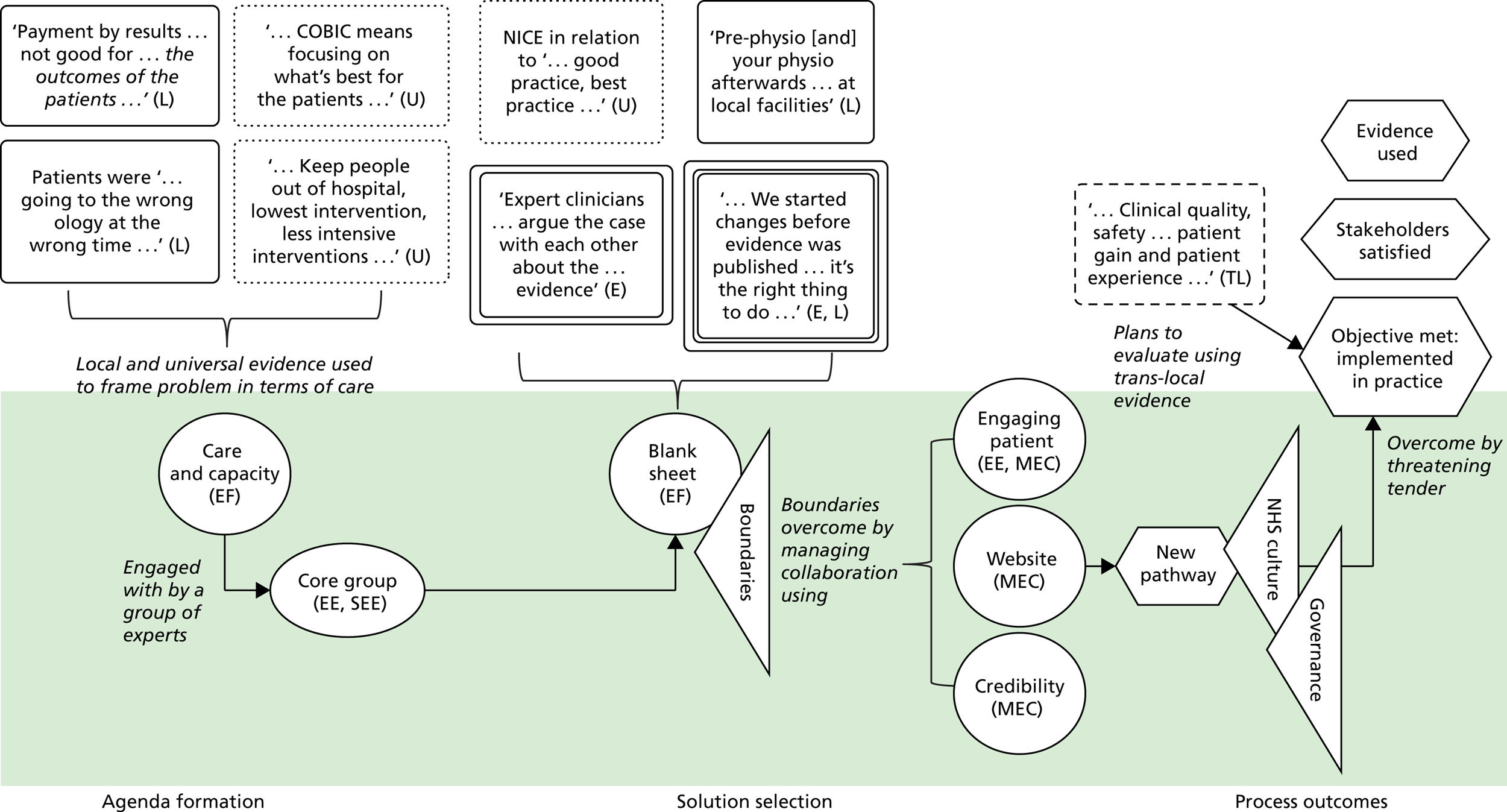
Appendix 4 Shire Clinical Commissioning Group
Case study overview
We first contacted the commissioning manager and a GPwSI, who described how the MSK services redesign had originated (approximately 10 years ago) with a GP who ‘a didn’t feel as if some of the services that we were providing locally fitted [the] need’ (GPwSI). The original GP had since retired and the GPwSI interviewed for our study essentially had taken over his role. She shared his feeling that they ‘could do it so much better’. At our first meeting, the GPwSI and the commissioning manager described how they began by inviting a small group of stakeholders. This group later developed into bigger working groups. Between them they had designed a new pathway. Because of several challenges, they were forced to put the pathway out to tender. Stakeholders who had been involved in the design of the pathway came together in a consortium and bid for the tender so that external, private providers would not be able to take their contracts. They were successful and, at our last interviews, had just implemented changes in practice. Other stakeholders interviewed at Coalfield CCG included an external consultant GP, a service manager, a clinical therapies lead and the deputy director of planning.
Agenda formation
The commissioning manager and the GPwSI drew on local and universal evidences to frame problems with MSK services in terms of costs, care and capacity. The commissioning manager drew on local evidence to describe how they did not have ‘the physical space . . . the staff and [were unable] to turn [patients] around quickly enough’. She felt that patients were ‘floating around [secondary care] having treatment which they could have had in the community’. Universal evidences from ‘Atlas of Variation’, which is produced by the Department of Health, showed that Shire CCG was ‘outliers in terms of . . . waiting lists [and] spent too much money’ (GPwSI). Both the commissioning manager and the GPwSI felt that ‘a more integrated pathway’ was necessary that would mean that patients were not ‘bounced from provider to provider’ (GPwSI). Addressing capacity issues was expected to improve patient experiences of care.
Selecting a solution
The GPwSI and the commissioning manager began by engaging ‘probably about six or seven’ stakeholders in a small group (commissioning manager). They decided to engage ‘about 20’ additional experts in ‘MSK working groups’. Groups were tasked with sourcing and evaluating local and universal evidence on ‘the activity, the benchmarking’ (commissioning manager). A GP group was also engaged during this episode of the redesign. The GP group was asked to look at local evidence on ‘the primary care pathway side of things’ (commissioning manager). The GPs also used universal evidence from the ‘Map of Medicine’ (commissioning manager). The Map of Medicine is a resource ‘of national pathways that can then be adapted to local needs’ (GPwSI). The commissioning manager described having good engagement in these groups, including ‘patient representatives . . . the acute trust . . . the community trust . . . physios . . . consultants [and] commissioners’. As in other CCGs, however, there were the barriers that come with multiple professionals working together. The commissioning manager describes how these groups were when:
. . . the conversations got slightly, I wouldn’t say more tense, the conversations were never tense just more difficult because we involved more people who all had a slightly different view of what we were trying to do . . .
In addition to the groups, the GPwSI was working with consultants ‘adapting each pathway [in the Map of Medicine] to [the] local needs’. This adaption was informed by local evidence related to ‘what services you had available locally’. Using local evidence they ‘just tweaked the pathway to say actually don’t send it there, send it here instead, and things like that’. They designed a pathway in which more care would be provided in the community setting, with only patients who needed secondary care getting to the consultants. Universal evidence is also likely to have influenced the wish to have ‘secondary care consultants [to] work in the community service and provide mentorship to the clinicians there and also to do sort of sessions every so often’ (commissioning manager).
The GPwSI produced a new pathway and a service specification. The commissioning manager thought that everybody agreed, noting that an onlooker would have ‘gone away going well, they’re all on board with this. They all seem to get it’ She was ‘adamant that this was the right thing to do . . . and . . . to push it forward’.
Despite her optimism, the commissioning manager described how they ‘just could not get all the different parties to come together to do the one pathway’. In our interview she said she was not able to ‘quite put [her] finger on why’ they could not move towards implementation. Throughout our conversation, however, talk indicated several potential barriers. These were later reinforced in interviews with other stakeholders:
First, the GPwSI described risk averseness and unwillingness to change, noting stakeholders as ‘a bit resistant and set in their ways’. She described how the proposed changes would have been ‘the first major service redesign in the local health economy . . . it’s millions and millions and millions of pounds’. She felt that the risks associated with such a big change were ‘at the back of people’s minds’, causing them to have cold feet. However, in our interviews with clinicians (service manager and clinical therapies lead), a different opinion was shared. These stakeholders described how the service specification that they had been given was ‘very brief . . . with minimal information’. They found it very difficult to ‘understand the commissioners’ wants and desires . . . from the information that was there’.
Second, they both commented that implementation may have been stalled by unclear roles. They said that sometimes the ‘wrong’ people were around the table and ‘unless everybody understands why they’re there and understands what their role is, then potentially you’re not going to get further forward’ (commissioning manager). Some stakeholders involved in the solution selection were seen as not particularly ‘engaged in doing that piece of work . . . and just turning up and being there because they’ve been asked to be’ (GPwSI).
Last, they listed finance as a barrier. In the new pathway, patients would be released to community services and ‘people were scared of losing patients’ (commissioning manager). The acute trust were ‘seeing some of their physio coming out, some of their pain coming out, some of their anaesthetics coming out . . . and not getting over that’ (commissioning manager). Although the commissioning manager tried to manage discussions during expert collaboration, she felt stakeholders could not see ‘that there is a different way of maintaining their income’. She described trying to make them understand the new way would be more effective because specialists would not be ‘seeing so many people who . . . have got a sore toe, [they would be] seeing people who need a hip replacement’.
Contrasting this, clinicians commented that the service specification showed an ‘extreme lack of knowledge about what was happening . . . in the current services and . . . going forward . . . [It didn’t have] an activity schedule and . . . a financial schedule’ (service manager).
Several meetings and discussions were held ‘once every 4–6 weeks for easily 2 years, probably longer’ to try to reach agreement about these barriers (GPwSI). They were unsuccessful, however, and the commissioning manager described how she felt ‘enough is enough’ and ‘made the decision that they were going to go to tender for part of the pathway’ that they could not agree on. Tendering was not seen as an ideal for two reasons. First, it ‘costs both providers and commissioners a lot of time and resource . . . the amount of hours that have been put into the tender is ridiculous, absolutely ridiculous’ (commissioning manager). Second, it is not always reliable and ‘in other places [private providers] had a big contract and they failed to provide on that’ (GPwSI).
After ‘to-ing and fro-ing [about] the wording of [tendering] documents . . . it eventually went out and [there were] . . . three main bidders’ (commissioning manager). Two were private and one was a consortium of health providers that had been involved in the redesign process. The consortium recognised that ‘no one current organisation could actually bid on their own’ (clinical service manager). The consortium agreed ‘no one trust would disadvantage another trust’, which made the tender ‘incredibly difficult to deliver . . . [because] If you want to do a true service redesign . . . organisations have to be disadvantaged’ (clinical service manager). They used local evidence to inform agreement, describing for example that the contract was ‘worth roundabout 1.4 million pounds’ to them (deputy director). They also felt that local providers offer the service would ‘improve patient care . . . people [will be] seen in the right way and having a better journey’ (deputy director). The consortium was the successful bidder for the contract.
Process outcomes
Although they reached agreement and won the bid for the new service, the new group still faced barriers. Recruitment of staff to provide the new pathway was difficult because it is NHS policy not to recruit ‘at risk’. This meant that they were not allowed to recruit new staff until the service was up and running. Because of this they ‘had to start by begging and borrowing people from lots of other services’ (clinical therapies lead). Both the clinical therapies lead and the clinical service manager noted that although, on paper, the NHS encourages competiveness, issues like this undermine progress in ways that you would not find ‘in the private sector at all’.
Despite the hurdles, the objectives were met and a new service was opened. In some cases, however, stakeholders were not satisfied. The clinical service manager described how they ‘ended up in a situation where we all still deliver what we were delivering with bits added on but through different contracting arrangements’. At the time of our meeting the service had been live for just 2 weeks, which meant that they were ‘fire fighting and ironing out the crinkles’. They did say that this was ‘less and less every day’ and that they were ‘getting there’. They also seemed optimistic, with ‘lots of great ideas about what we’ll want to do and how we want to deliver it’ (clinical service manager).
Shire summary of findings
Shire CCG’s redesign was against years of work trying to improve MSK services. Problems were framed in terms of costs, care and capacity. Universal and local evidence were used with the understanding that too much care was in the hospital. The commissioning manager and the GPwSI recruited small groups, which were later expanded to working expert groups. These groups were tasked with looking at various evidences (universal, local) about a solution. The GPwSI was also working up a new pathway, external to, but informed by, the groups. Several challenges were presented and discussed at meetings, until they to went for tender. Providers formed a consortium, which eventually won the tender. After the tender was won, several additional barriers were faced, including recruitment and their inability to ‘disadvantage’ each other. The redesign process for this CCG is shown in Figure 12. Guidance for reading process maps is shown in Figure 3.
FIGURE 12.
Redesign process at Shire CCG.
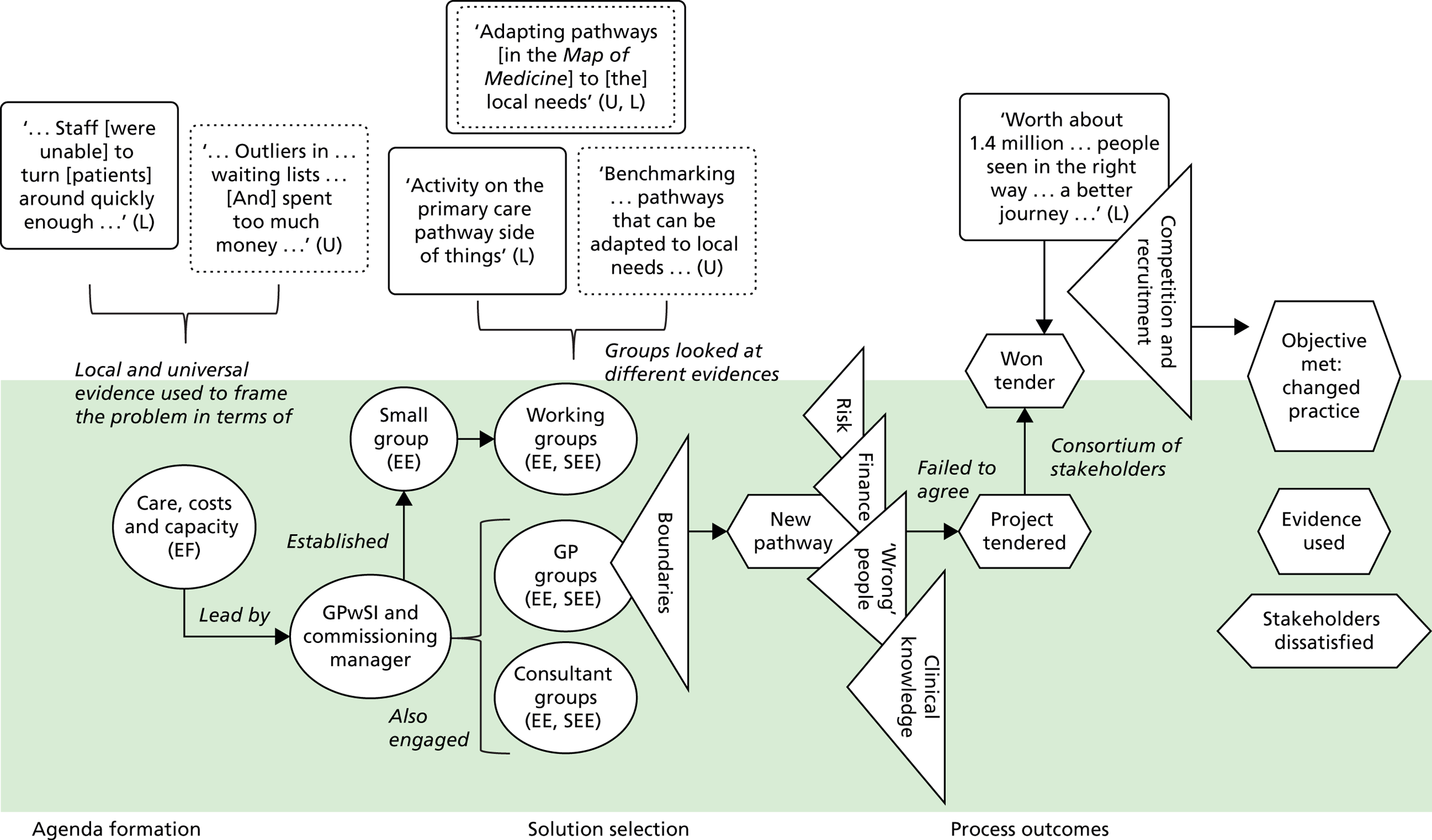
Appendix 5 Toolkit
Work practices
This section of the toolkit is aimed at understanding and improving work practices around commissioning teams’ use of evidence.
Understanding how ready your project team is to use evidence
(Target user: lead/members of redesign projects.)
Preamble Successful service redesign projects are those that develop effective, realistic and well-thought proposals/solutions for changing the commissioning of health-care services at a local level. Service redesign teams are more likely to develop such solutions when their decision making processes are well managed and well informed, that is, backed up with evidence, local knowledge and insights of various experts. In our research, we found that decisions need to be based on different kinds of evidence (universal, trans-local, local, expertise-based). We also identified specific capabilities that can contribute to improving the use of evidence in service redesign decision processes. These are summarised in the attached sheet. The following questionnaire aims to help you assess these capabilities in your own setting so that you can identify areas where your project may be more, or less, ready to use evidence to inform effective redesign solutions.
Area 1: sourcing and evaluating evidence
This set of questions is about how the project team gathers and uses different types of evidence in decision-making.
1a. We have thought about the different types of evidence (local, trans-local, universal and expertise-based) that might be relevant, given the objectives and scope of this project.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
1b. The project team knows how to identify and gather new evidence to inform its decision-making process.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
1c. The project team has explicit criteria (e.g. relevance, applicability) to assess evidence.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
1d. The project team gets together to discuss, debate and assess available evidence.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
Area 2: engaging experts
This set of questions is about the makeup of project teams.
2a. Given the aims and objective of our redesign project, we have carefully considered all possible stakeholders that may be affected by project outcomes.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
2b. Given the aims and objective of this project, we have identified all individuals who might have relevant insights, information and expertise to inform our decisions.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
2c. We have carefully considered when and how individuals with relevant insights, information and expertise will be asked to offer their input and feedback.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
Area 3: effective framing
This set of questions is about the HOW the project team makes sense of, or ‘frames’, their problem and search for solutions.
3a. The aims and objectives of this project reflect a balanced mix of different priorities (e.g. to improve quality of patient care, cost-effectiveness, capacity to delivery health care).
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
3b. Given the project objectives, we have gathered evidence on the benefits and drawbacks of different solutions (e.g. adapting what we do now, copying what others do or starting from scratch).
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
3c. Available resources for this project might affect our problem-solving efforts.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
Area 4: managing roles and expectations
This set of questions is about roles and role expectations within the project team.
4a. Given the project objectives, it is critical that everyone knows what is expected of them.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
4b. Every member of the project team understands that bringing new evidence and information to the team’s attention is part of their role.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
4c. We have carefully considered a range of different roles for participants; for example, some may offer expertise, others may offer real-world experience.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
4d. Every member of the project team received feedback on the new evidence and information they present that seems relevant to the project.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
Area 5: managing expert collaboration
This set of questions is about how well different experts may work together to achieve the project’s objectives.
5a. Project members have different goals and objectives for this project.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
5b. It is important for all members to declare any conflicts of interest.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
5c. We have considered how we can get key experts (including patients) to share relevant information with one another, even if it might be politically sensitive.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
5d. We can recognise and handle a situation in which a project team member seeks to influence the decision-making process to serve their specific vested interest.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
Participant answers to the above questions can then be used to generate a radar plot, an example of which is shown in Figure 13.
FIGURE 13.
Example of radar plot for team capabilities to use evidence.
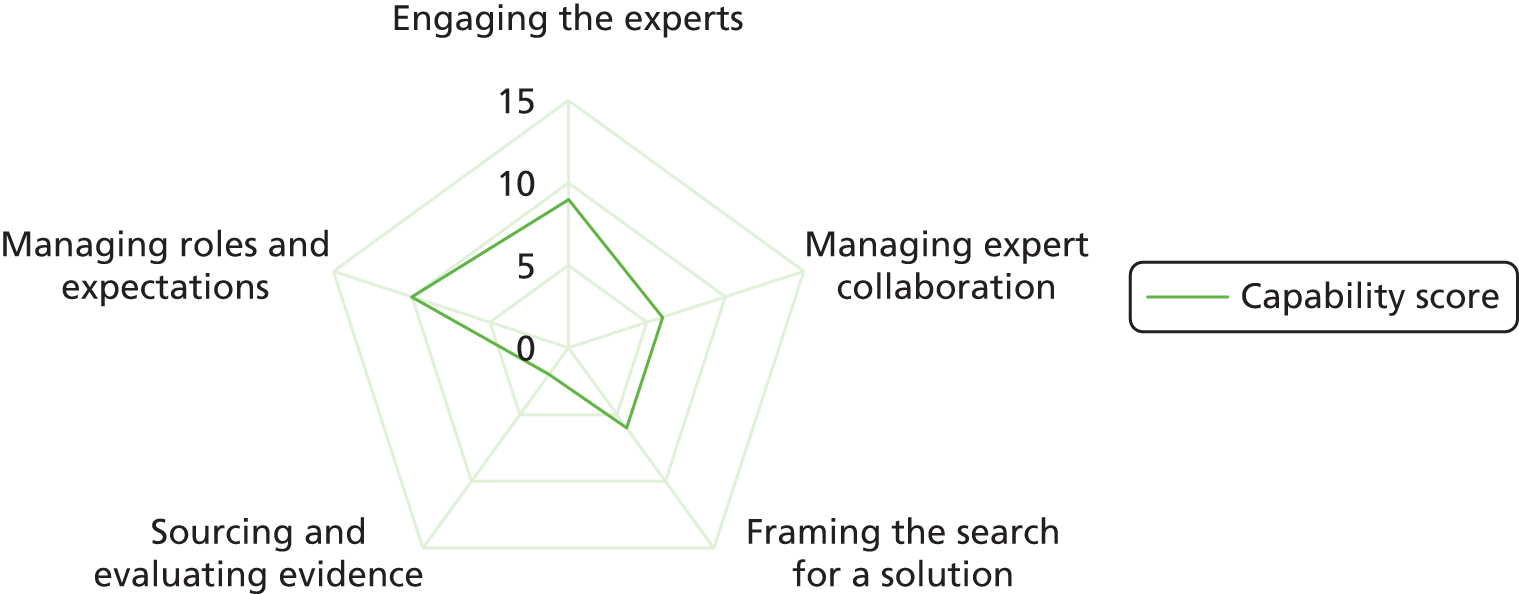
Organisational conditions
The management literature, as well as feedback from our national workshop, emphasised the importance of noting that redesign projects do not take place in a vacuum. 101,102 Organisational context (e.g. resources available, organisational culture) may also influence evidence use. Redesign projects are often initiated as a result of boards appraising and approving a business case because it meets organisational objectives (e.g. savings). Organisational context might also impact on sourcing and evaluating evidence. There are often numerous attempts to improve the organisation of health services. Organisations (CCGs) often have a weak memory for these so that learning is not often transferred from old projects. That is, evidence of ‘what worked’ and ‘what didn’t’ at a local level may not be mobilised as a result of poor organisational memory. Such evidence might be important given the local nature of projects. In addition to organisational memory, organisational culture may play an important role in deciding what and how evidence is used. Prior research suggests that organisations are ‘cultural universes’, which place emphasis on, for example, certain values and philosophies. 103 Some organisations value evidence-based decision-making more than others, and so it can be assumed that employees in this type of organisation will be more likely to pay attention to evidence. It is important, then, to also consider the readiness of the organisation for evidence use. We have developed the following items for our toolkit.
Assessing how favourable the organisational context is for using evidence
(Target user: director/associate director of commissioning organisation.)
Preamble Using evidence effectively to inform decisions is a very localised skill. Middle-level managers, project teams and other stakeholders involved in commissioning need to take specific actions to find and use evidence in decision-making. In our research, we found that specific practices (e.g. identifying and engaging the right experts) enhance the use of evidence in the design and commissioning of health-care services. At the same time, we found that the organisational environment influences commissioning groups’ efforts to use evidence. The following questionnaire aims to help senior managers, such as you, identify areas in which your organisation may provide a more or less favourable context for the use of evidence in commissioning decision-making.
Area 1: central support for using evidence
1a. My organisation has clear processes in place to meet its various needs for information and evidence throughout the commissioning cycle.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
1b. My organisation has produced a structured framework (e.g., internal guidance or policy), which specifies how it should meet its various needs for information and evidence throughout the commissioning cycle.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
1c. My organisation has allocated a budget to serve its needs for information and evidence through the provision of specialised services and/or roles (e.g. library services, chief knowledge officer).
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
Area 2: culture for using evidence
2a. In my organisation, before making important decisions, we value spending a lot of time gathering relevant data, information and evidence.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
2b. In my organisation, we believe that it is crucial that we understand and evaluate the evidence behind the problems we face.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
2c. In my organisation, before making important decisions, we believe that it is crucial that we understand and evaluate the evidence when considering alternative solutions.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
2d. In my organisation, we value the input of different internal and external experts (e.g. patients, researchers, clinicians) when making important commissioning decisions.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
Area 3: organisational memory
3a. My organisation has clear processes in place to capture evidence and learning from past projects.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
3b. In my organisation, before making important decisions, we almost always check how related or similar decisions in the past were taken here and/or check how other organisations have tackled the problem.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
3c. My organisation maintains repositories, which include information about past projects, project outputs and project participants and these are easily accessible.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Strongly disagree | ⦿ | ⦿ | ⦿ | ⦿ | ⦿ | Strongly agree |
Participant answers to the above questions can then be used to generate a radar plot, an example of which is shown in Figure 14.
FIGURE 14.
Example of radar plot for organisational preparedness to use evidence.
List of abbreviations
- CCG
- Clinical Commissioning Group
- CLAHRC
- Collaboration for Leadership in Applied Health Research and Care
- CRG
- clinical reference group
- CSU
- commissioning support unit
- EBM
- evidence-based medicine
- EBMgmt
- evidence-based management
- GP
- general practitioner
- GPwSI
- general practitioner with a special interest
- HSDR
- Health Services and Delivery Research
- MSK
- musculoskeletal
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PCT
- primary care trust
- QOF
- Quality and Outcomes Framework
- RCT
- randomised controlled trial
- SDC
- Service Development Committee
- SSAP
- Scientific and Stakeholder Advisory Panel
- UNTRAP
- University/User Teaching and Research Action Partnership