Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5005/04. The contractual start date was in September 2013. The final report began editorial review in September 2016 and was accepted for publication in February 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David Jayne is a member of the Efficacy and Mechanism Evaluation Strategy Group. There are no competing interests to declare in relation to Intuitive Surgical.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Randell et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Overview
This chapter introduces our study and is organised into three main sections. We begin by explaining what is meant by robot-assisted surgery (RAS). We then provide some background to the study design. This study was a realist process evaluation alongside a randomised controlled trial (RCT) comparing laparoscopic surgery and RAS [the RObotic versus LAparoscopic Resection for Rectal cancer (ROLARR) trial]. We describe the trial and explain what both realist evaluation and process evaluations are. We then define the research aim and objectives. We conclude the chapter by describing the structure of the remainder of the report.
From open to laparoscopic to robot-assisted surgery
In the 1990s, laparoscopic techniques were introduced to surgical practice; these were initially for benign conditions but later extended to the treatment of cancer. Instead of creating large abdominal wounds, the surgeon is able to perform operations using small ‘key-hole’ incisions, through which cameras and instruments are passed. This removes much of the abdominal access trauma. The clinical benefits of laparoscopic surgery were soon realised, including less postoperative pain, shorter hospitalisation, quicker return to normal function and improved cosmetic effect. 1–3 These benefits were outlined in 2007 by Lord Darzi in Saws and Scalpels to Lasers and Robots – Advances in Surgery; he also pointed to how such less invasive techniques allow for the increased use of day surgery, helping to cut waiting times for operations. 4 The use of laparoscopic surgery was also promoted in Delivering Enhanced Recovery – Helping Patients to Get Better Sooner After Surgery, published in 2010. 5 The following year, in Improving Outcomes: A Strategy for Cancer, the Department of Health highlighted encouragement of the uptake of less invasive techniques as an important part of ensuring improved access to high-quality surgery. 6 In addition to patient benefits, laparoscopic surgery is cost-effective for health-care providers,7 the increased operating costs offset by shorter inpatient stays and decreased wound care costs. 3
The restricted abdominal access of laparoscopic surgery comes at a price. Laparoscopic operations are technically more challenging than open surgery, due to the two-dimensional (2D) operative image, instrumentation with limited freedom of movement and reduced tactile feedback. The uptake of laparoscopic surgery has, therefore, been slow; in 2003, the uptake of colorectal laparoscopic surgery was 5%, and this increased to only 40% over the 9 years to 2011,8 despite it being recommended by the National Institute for Health and Care Excellence since 2006. 9
Robot-assisted surgery was developed in an attempt to solve some of the limitations of laparoscopic surgery. The da Vinci® robot (Intuitive Surgical, Sunnyvale, CA, USA) is currently the only commercially available robotic platform for soft tissue surgery (although alternatives to the da Vinci robot, developed by other manufacturers, are expected to come onto the market in 2017). It is a master–slave (or console-manipulator) system, whereby the surgeon sits at a console to control the arms of the robot. Depending on the model, the robot has three or four robotic arms; one arm holds the camera, while the other arms hold a variety of surgical instruments, all of which are inserted into the patient. The robot provides a stable camera image with a three-dimensional (3D) field of view, intuitive instrument handling, tremor elimination, motion scaling and EndoWrist® (Intuitive Surgical, Sunnyvale, CA, USA) instruments, which provide increased freedom of movement. This enables the surgeon to achieve greater precision and control, and reduces some of the technical challenges associated with traditional laparoscopy.
In 2010, the da Vinci robot was reported to cost around £1.3M, with annual maintenance fees of £70,000. 10 As the technology has developed, the price has increased; the latest model of the da Vinci robot costs about £1.7M and there are annual maintenance fees of about £140,000 per robot. 11 Despite the cost, there has been rapid growth in the purchase of da Vinci robots, first in the USA but with Europe quickly following suit. Between 2007 and 2011, the number of da Vinci robots installed in the USA increased from 800 to 1400,12 while in 2011 the number of da Vinci robots installed worldwide reached 2300. 13
In the UK, there has been enthusiasm for RAS among both clinicians and policy-makers, with the Department of Health in 2009 putting RAS forward as an example of new technology supporting the delivery of more effective patient care, helping to meet the goals set out in High Quality Care for All. 14 The first purchase of a da Vinci robot by a NHS hospital occurred in 2001. 15 By 2009, there were nine robots in use in the UK, with three NHS trusts planning to acquire a robot in the following 12 months. 16 By 2012, the number of robots had increased to 27,15 while indications from Intuitive Surgical suggest that by the summer of 2016 there were 61 da Vinci robots in use in the UK. RAS is primarily used in urology; in 2011 over 50% of radical prostatectomies in the UK were carried out using RAS. RAS has been expanding across the surgical disciplines, also being used in gynaecology, ear, nose and throat, colorectal, cardiology and paediatrics.
However, a lack of high-quality evidence concerning the impact of RAS on patient outcomes has led to a more cautious approach among policy-makers in recent years. In February 2014, NHS England announced that it would be reviewing the evidence for RAS to inform future policy and advised that NHS trusts should not purchase further da Vinci robots until that was completed. The first review considered evidence for RAS in the curative treatment of prostate cancer. Two RCTs comparing laparoscopic surgery and RAS found that, although there was no difference in oncological outcomes, RAS offered health-related quality-of-life benefits for patients, in terms of higher rates of continence and sexual function. 17,18 As a consequence of this, in July 2015, NHS England recommended that RAS should be offered as a choice, alongside open and laparoscopic surgery, and when considered clinically appropriate, to all patients with localised prostate cancer. 19
Given the high costs of RAS, with the cost-effectiveness of RAS depending on the number of operations for which the da Vinci robot is used,20 it could be anticipated that NHS trusts that have purchased a da Vinci robot would be seeking to maximise its use. However, the implementation of RAS can be challenging, and there have been reports of da Vinci robots being introduced but then underused. 10 Although accounts of the introduction of RAS suggest a number of factors that are important for successful integration, these accounts come from small case series (descriptive non-randomised studies) undertaken in single institutions, typically by dedicated RAS enthusiasts,3 so little is known about the contextual factors that are necessary for the successful integration of RAS more broadly. Therefore, this study seeks to systematically explore the processes involved in successfully introducing this new technology into the operating theatre (OT).
Robot-assisted surgery is a complex intervention, by which we mean that it is an intervention aimed at producing change in the delivery and organisation of health-care services and that comprises a number of separate components that may act both independently and interdependently. 21,22 These components are not only technological but also organisational and social, and they can all impact on the extent to which RAS is successfully introduced and on subsequent process and patient outcomes. A significant feature of RAS is the way in which it changes the spatial configuration within the OT, with the surgeon at a distance from the patient and the OT team, as shown in Figure 1. While the OT team works with a 2D image of the surgical site, the surgeon’s visual attention is focused on the 3D image provided by the robot, prohibiting face-to-face communication during the operative part of the procedure. More generally, the size of the robot introduces physical space constraints, resulting in a new choreography of movement around the patient. 23 The impact of this change in spatial configuration on communication and teamwork in the OT is not a topic that has been explored in previous evaluations of RAS, which have typically focused on the role of the surgeon. 24 Two small studies have looked specifically at differences in communication between laparoscopic surgery and RAS. One study compared communication in eight operations using laparoscopic surgery (four cholecystectomies and four prostatectomies) and 12 using the da Vinci robot (five cholecystectomies and seven prostatectomies). 25 The other study compared communication in two cholecystectomies, one using laparoscopic surgery and one using endoVia Medical’s (endoVia Medical, Inc., Norwood, MA, USA) Laprotek surgical robot, where it was the first experience for both the surgeon and scrub practitioner of using the robot on a patient. 26,27 Both studies found a significant increase in oral communication between the surgeon and the OT team in RAS, particularly in relation to the orientation and localisation of organs and the manipulation of instruments,25–27 with the effect found to be more pronounced in teams that have less experience of RAS. 25 What these studies do not provide is a consideration of the non-verbal co-ordination that has been shown to be an important aspect of teamwork in the OT or of the strategies the OT team employs to manage the differences in communication and teamwork. 28,29 Neither do they explore the additional contextual factors beyond the technology that affect communication and teamwork.
FIGURE 1.
Spatial configuration in laparoscopic surgery and RAS.

Another significant feature of RAS is the way it changes the information available to the surgeon to inform decision-making. In open surgery, surgeons work primarily with visual and tactile information. In laparoscopic surgery, although tactile information is reduced, experimental studies have revealed that, by touching with the instruments, surgeons are still able to determine features of objects, such as shape, texture and consistency. 30,31 In contrast, in RAS the surgeon receives no tactile information, raising questions about how the surgeon’s decision-making is affected. The nature of the decision-making tasks of the OT team may also be affected by RAS. As the surgeon is no longer in the sterile field, more of the burden falls on the rest of the team to respond in the event of a complication, increasing the importance of the team having a shared awareness of what is happening in the surgical site and how far they are through the procedure. 32 In response to this, interest has emerged in large surgical displays that integrate diverse sources of information,33,34 which could have benefits in the context of RAS. However, this requires an understanding of what information each member of the team needs to work effectively and safely, and how that information can best be communicated. 35
Introducing the study
To explore the issues identified above, regarding how RAS becomes integrated into practice and how it impacts communication, teamwork and decision-making in the OT, we undertook a realist process evaluation alongside a RCT comparing laparoscopic surgery and RAS. Therefore, before presenting the aim and objectives of this study, we introduce the trial and provide a description of process evaluation and realist evaluation.
ROLARR
The current study was conducted alongside an international, multicentre RCT entitled ROLARR, which was funded by the Medical Research Council Efficacy and Mechanism Evaluation programme. 36 The trial sought to establish whether or not RAS improves surgical outcomes in comparison with traditional laparoscopic surgery for the curative treatment of rectal cancer. The assumption underpinning the ROLARR trial was that RAS would facilitate fine tasks, such as dissection and suturing, enabling the surgeon to overcome the challenges experienced in traditional laparoscopic surgery. All of the patients entered into the trial underwent either an anterior resection or an abdominoperineal resection. For patients randomised to the intervention arm of the trial, the surgeon could choose to either undertake the operation totally robot-assisted or undertake a hybrid operation in which the first phase of the operation (mobilisation of the splenic flexure) is undertaken laparoscopically but the rectal mesorectal dissection is undertaken with robot assistance. The primary outcome of interest was conversion to open surgery, which was considered to reflect the ease of surgery; if RAS makes surgery technically easier, there should be fewer conversions to open surgery. The secondary outcomes included the accuracy of the surgery and intraoperative and postoperative complications.
Process evaluations
A process evaluation is ‘a study which aims to understand the functioning of an intervention, by examining implementation, mechanisms of impact, and contextual factors’. 37 Although the RCT design continues to be considered the most reliable method of determining effectiveness,21 process evaluations are now considered to be an essential part of designing and evaluating complex interventions if evaluations are to inform policy and practice. 38 Process evaluations are typically undertaken alongside a trial,39 although there is increasing interest in using process evaluations during the feasibility and piloting phase38 so as to inform the definitive trial, and process evaluations may also be undertaken after a trial. 40 Guidance recommends that process evaluations combine qualitative and quantitative data. 37–39
In examining implementation, process evaluations look at what was delivered, in terms of fidelity (whether or not the intervention was delivered as intended) and dose (how much of the intervention was delivered). 38 This is particularly important in multicentre trials in which the intervention may be implemented in different ways in different sites,39 and so understanding differences in what was delivered can assist in interpreting differences in results. It is also important to look at how the intervention was delivered, in terms of, for example, education and training, support, and communication and management processes. 38 Without this, effective aspects of the intervention may go unmeasured, raising concerns about the validity and reliability of the results of an evaluation41 and preventing replication. 42 For example, an important component of RAS may be the training delivered to the OT team, but if this element of the intervention is not reported and described, health-care organisations may introduce RAS without an equivalent level of training and are unlikely to achieve the same impact.
As well as capturing what and how the intervention is delivered, process evaluations explore the mechanisms through which interventions bring about change,43 which provides important understanding concerning how the impacts of the intervention might be replicated by similar interventions in the future. 38 Alongside this, it is important to capture information about the context in which the intervention is delivered, as an intervention may have different effects in different contexts. 39 Context here is taken to mean anything external to the intervention that may reduce or increase its impact, such as pre-existing circumstances, skills, organisational norms, resources and attitudes. 37
While the objectives of process evaluations have been defined as ‘to assess fidelity and quality of implementation, clarify causal mechanisms and identify contextual factors associated with variation in outcomes’,44 process evaluations do vary in terms of aims. 38 In this study, we wanted to capture what was delivered and how it was delivered within the UK ROLARR sites. All of the centres participating in ROLARR had introduced RAS prior to joining the trial, so there were likely to be variations in how RAS was implemented across the sites. Because ROLARR is an international trial and this study was looking at the UK sites only, it would not be feasible to use the data to understand variations in outcomes across the trial as a whole; in addition, given the relatively small number of operations undertaken by some sites, it is questionable how meaningful it would be to use the data to interpret differences between the UK sites. Nonetheless, given the reported challenges of integrating RAS into routine surgical practice and the fact that this topic has not been explicitly considered by existing studies of RAS, it was considered that an account of the different ways in which RAS was implemented in the UK ROLARR sites, in terms of what was delivered (e.g. totally robot-assisted operations or hybrid operations), how it was delivered (e.g. staff training, organisation of teams) and staff perceptions of the value of those different approaches would provide important information for health-care organisations considering introducing RAS.
In terms of clarifying causal mechanisms, our objective was not primarily to understand how RAS impacts the trial outcome of conversion to open surgery. Complex interventions have the potential to produce unintended consequences, which may be beneficial or harmful, and process evaluations have been identified as providing an opportunity to systematically identify and quantify unexpected unintended outcomes. 45 We wanted to capture impacts outside the scope of the trial, about how RAS, in comparison with laparoscopic surgery, impacts communication, teamwork and decision-making in the OT, and understand how and in what contexts those impacts occurred; thus, to explore this, we carried out a realist evaluation.
Realist evaluation
The evaluation of complex interventions requires a strong theoretical foundation,46 and realist evaluation provides this through a process of eliciting, testing and refining stakeholders’ theories of how an intervention works. Consequently, realist evaluation has been used for studying the implementation of a number of complex interventions in health care. 47 In realist evaluation, interventions in and of themselves are not seen as determining outcomes. Rather, interventions are considered to offer resources to recipients, and outcomes depend on how recipients make use (or not) of those resources, which will vary according to the context. Consequently, patterns in the outcomes of interventions are demi-regularities, the influence of contextual factors making them only semipredictable. 48,49 Realist evaluation seeks to answer not only the question of ‘what works?’ but ‘what works for whom, in what circumstances, and why?’. 50 It seeks to understand not only in what contexts the intended outcomes are achieved, but also unintended outcomes. Further details of how the principles of realist evaluation were applied in this study are provided in Chapter 2.
Aim and objectives
The aim of this study was to understand how and in what circumstances RAS produces both intended and unintended outcomes. The study had the following research objectives:
-
To contribute to the interpretation and reporting of the results of ROLARR by investigating how variations in implementation of RAS, and the context in which RAS is implemented, impact on outcomes such as operation duration, conversion to open surgery and complications.
-
To produce actionable guidance for health-care organisations on factors likely to facilitate the successful implementation and integration of RAS.
-
To produce actionable guidance for OT teams on how to ensure effective communication and teamwork when undertaking RAS.
-
To provide data to inform the development of tools and technologies for RAS to better support teamwork and decision-making.
Structure of the remainder of the report
Chapter 2 provides the details of the study design and research methods used. (Details of the study management, including patient and public involvement in the study, are provided in Appendix 1.) Chapter 3 presents the candidate theories that were developed in preparing the proposal and the literature, regarding both the integration of new technologies in health care and communication, teamwork and decision-making within the OT, that informs them. Chapters 4 and 5 report on phase 1 of the realist evaluation, during which the candidate theories were added to and refined, through the elicitation of stakeholders’ theories about how and in what contexts RAS becomes introduced into routine surgical practice and how and in what contexts RAS impacts communication, teamwork and decision-making in the OT. At the end of phase 1, the theories were prioritised for empirical testing in phase 2, and this process and the resulting decisions are described in Chapter 6. The results of phase 2, during which we sought empirically to test the refined theories, are presented in Chapters 7 and 8. In phase 3, we sought to assess to what extent our findings from colorectal surgery were applicable to other surgical specialties through interviews with surgeons and OT teams in these specialties, and the results of this are described in Chapter 9. Chapter 10 concludes the report by summarising the findings of the study in relation to our original objectives, discusses the strengths and limitations of this research and outlines priorities for future research.
Chapter 2 Design and methods
Realist evaluation
As described in Chapter 1, realist evaluation involves a process of eliciting, testing and refining theories of how an intervention works. 51 Realist evaluation does not employ particular methods of data collection, but a mixture of qualitative and quantitative methods is encouraged. 52 Whereas qualitative methods may be best for gathering data on the processes and contexts of an intervention, quantitative data is desirable for understanding an intervention’s impacts. The stages of a realist evaluation, and how they relate to the phases of the current project, are presented in Figure 2. This report reflects the current guidance for the reporting of realist evaluations. 53
FIGURE 2.
A three-phase realist process evaluation.

A note on theory
Given the focus within realist evaluation on the elicitation, testing and refinement of theories, an explanation of how this term is understood within realist evaluation is necessary. Although theories may sometimes be considered to be abstract and irrelevant, separate from the everyday experience of practitioners, the term can also be used simply to refer to practitioners’ ideas and thoughts about how an intervention works. 54 This is how the term is used in realist evaluation. From a realist standpoint, effective theories typically combine both substantive theory and stakeholders’ theories that are derived from experience. 51,54
Realist theories define the mechanism through which a particular outcome is achieved, whereby the mechanism consists of a resource that the intervention provides and the recipients’ reasoning about and response to that resource, and the context in which that mechanism will be activated. Thus, realist theories are often presented in the format Context + Mechanism = Outcome, or C + M = O, referred to as a context–mechanism–outcome (CMO) configuration. The activation of a mechanism should not be thought of as an on/off switch; rather, there is a continuum of activation, which will affect the outcome. 55 For example, there are varying degrees to which an OT team may feel enthusiastic towards RAS, depending on the context.
Phase 1: theory elicitation and refinement
Phase 1 of the study was concerned with theory elicitation and refinement, the first stage in a realist evaluation. The purpose was to refine and add to the candidate theories developed in the process of preparing the proposal for this study. Theory elicitation can be carried out in a number of ways, such as interviewing stakeholders, reviewing the existing literature on the topic, identifying relevant theories from the literature, or some combination of these approaches. To begin our realist evaluation, we carried out a review of literature related to the use of RAS, focusing on the identification of stakeholders’ theories. This is useful to track the history and adaptation of complex interventions, which can reveal important learning about technology implementation and evaluation. 56 The refined set of theories was then presented to members of the OT teams in interviews during which they were asked to refine, develop and add to the theories based on their direct experience of RAS. Ethics approval for this phase of the study was granted by the School of Healthcare Research Ethics Committee of the University of Leeds (reference number SHREC/RP/339).
Literature review
This review constituted the first ‘theory elicitation’ stage of a realist review. Whereas in a full realist review published evidence is used to test and refine stakeholders’ theories,52 our purpose was to catalogue the theories to be refined and tested in subsequent stages of the study. The details of the methods of this review can be found in Appendix 2.
Teacher–learner cycle interviews
Realist evaluation provides a unique approach to undertaking interviews, referred to as ‘teacher–learner cycle’ interviews. 51 In teacher–learner cycle interviews, the researcher’s theory is the subject matter. An iterative approach is taken, whereby the researcher first teaches the interviewee about the theories they want to explore within the interview. The researcher then invites the interviewee to use their experience of the intervention to reflect on these theories, refining and adding to them, so the interviewee is using their experience to teach the researcher. In interviews with members of OT teams with experience of colorectal RAS, we used this approach to refine and test our literature-based theories.
Settings and participants
The ROLARR team identified that 10 English NHS trusts were using RAS for colorectal surgery at the time of the interviews. Of these 10 trusts, six had met the inclusion criterion for participation in ROLARR, in that surgeons had to have undertaken a minimum of 10 rectal cancer resections using RAS. The other four trusts did not meet this inclusion criterion and thus had not participated in the trial. We invited surgeons and OT teams in all 10 trusts to participate in our phase 1 interviews. By involving both trusts involved in ROLARR and trusts not involved in ROLARR, we ensured that the OT teams involved in the study varied in their level of experience with RAS.
Agreement to participate was obtained and research governance approval was granted at all 10 trusts. However, at one trust, despite numerous attempts at contact by the research team, it was not possible to arrange an interview with the surgeon. Therefore, interviews were conducted across nine sites, including all six of the English ROLARR sites. The staff information sheet for this phase of the study is provided in Appendix 3.
Given the study’s concern with teamwork, it was considered essential that we capture the perspectives of all of the professional groups that make up the OT team. 57 Therefore, a snowball sampling strategy was used. 58 At each trust, one of the colorectal surgeons was interviewed first, and he or she then helped us to identify other members of the OT team to interview. As well as surgeons, interviewees included surgical trainees, theatre nurses, operating department practitioners (ODPs) and anaesthetists. Details of the number of interviews undertaken in each site are provided in Table 1. Although we had initially intended to undertake 10 interviews per trust, recruiting interview participants at some trusts proved challenging (described further below), and therefore there was significant variation in the number of interviews undertaken per trust. In total, 44 interviews were conducted across the nine trusts between November 2013 and August 2014.
| Site | Role | Total | |||||
|---|---|---|---|---|---|---|---|
| Surgeon | Trainee surgeon | Anaesthetist | Nurse | ODP | Other | ||
| 1 | 3 | 3 | 2 | 1 | 3 | 1 | 13 |
| 2 | 1 | 1 | |||||
| 3 | 2 | 1 | 3 | ||||
| 4 | 2 | 1 | 1 | 1 | 5 | ||
| 5 | 1 | 2 | 2 | 5 | |||
| 6 | 1 | 1 | 3 | 1 | 6 | ||
| 7 | 1 | 2 | 1 | 2 | 1 | 7 | |
| 8 | 1 | 1 | 1 | 3 | |||
| 9 | 1 | 1 | |||||
| Total | 12 | 5 | 6 | 12 | 7 | 2 | 44 |
Data collection
The interviews were semistructured. Using an interview topic guide designed by the research team, participants were first asked about when and how RAS was introduced into their hospital. This allowed us to trace the processes of implementation across the nine trusts, helping to identify contextual differences. The participants were then presented with theories taken from the literature and asked to reflect on whether or not, and in what ways, those theories fitted with their own experiences. Open-ended questions were also included to surface new areas for investigation. Thus, the interviews were designed to support both ‘theory gleaning’ and ‘theory refinement’. 59 The questions asked varied according to the participants’ roles, designed to reflect the experience of RAS each participant would have as a result of their role. After each interview, the interview topic guide was reviewed and, when necessary, revisions were made to incorporate new theories and refinements to theories, so that these could be explored in subsequent interviews (see Appendix 4 for the initial interview topic guide).
Interviews in most trusts were undertaken by telephone, although there were exceptions. Interviews were conducted in person at the NHS trust local to the research team. In addition, in three NHS trusts telephone interviews were difficult to organise, as the individual OT team members were in theatre and unavailable when at work. In these three trusts, agreement was obtained for members of the research team to attend on an audit day, when no operations were scheduled, and interview those staff who were available and consented to the interview. All interviews were audio recorded and transcribed verbatim. The interviews ranged from 29 minutes to 1 hour 40 minutes. The average (mean) length of interview was 53 minutes.
Data analysis
An iterative approach to data collection and analysis was taken. As there was often little or no time between interviews, an approach was developed that assisted the team in maintaining an overview of the ways in which the theories were being refined and added to through the interviews, which also supported refinement of the interview topic guide. A working document was maintained that contained the list of theories being explored in the interviews. Following each interview, the researcher would review the list of theories, noting the interviewee’s support for or disagreement with the theories and any refinements to the theories suggested by the interviewee, as well as adding any new theories that emerged in the interview.
In preparation for the Study Steering Committee (SSC) meeting at which theories were to be prioritised for testing (discussed further below), more formal analysis was undertaken using framework analysis, an approach developed for analysing qualitative data for applied policy research. 60 At this point, 23 interviews had been completed. Framework analysis was chosen because it supports systematic and comprehensive data analysis, is well suited to working with large data sets, allows for both inductive and deductive analysis, enables both between- and within-case analysis, is an approach that has previously been used within realist evaluation studies61 and was an approach members of the research team were familiar with. Three members of the research team identified and agreed codes for indexing the data, informed by the interview topic guide and reading of a subset of three of the interview transcripts, as well as familiarity with the interviews through conducting those interviews. These codes focused on capturing how our original theories were expanded, supported and refined, and how different contextual features shaped the mechanisms through which RAS was perceived to become integrated into practice and to impact on communication, teamwork and decision-making. The interview transcripts were entered into NVivo 10 (QSR International, Melbourne, Australia) software for qualitative data analysis. The three members of the research team then indexed four interview transcripts to test the applicability of the codes and assess agreement. When there was variation in the indexing, the codes were refined and definitions were clarified. The refined codes were applied to all transcripts. The indexed data were summarised in a series of matrix displays, created in NVivo 10, to build up a picture of the data as a whole. 62 This involves the abstraction and synthesis of the data while also referencing the original text. There was one matrix display per theme (implementation, teamwork and communication, and decision-making) and, in each matrix display, a row for each interviewee. As data collection continued, new interview transcripts were entered into NVivo 10, indexed and added to the matrix displays.
In the final stage, mapping and interpretation, the matrix displays were used to make both within-case comparisons, exploring intraorganisational and micro aspects of context, such as role, and between-case comparisons, to explore interorganisational aspects of context, returning to the original data when necessary. Narrative summaries of these patterns in the data were written up in a series of working documents, organised by theme. The three members of the research team then went through the narrative summaries, discussing them and comparing the findings with the theories presented in the interviews, in order to develop a refined set of theories.
Phase 2: empirical testing of theories
The purpose of phase 2 of the study was to collect and analyse data to test key theories from phase 1. To do this, we worked with our SSC, patient panel and the clinical members of the research team in order to prioritise the theories. The protocol for this phase of the study was then reviewed and revised, to ensure that we would be gathering the data necessary for testing the selected theories. Data were collected across four case sites, using a combination of methods, including structured observation and video recording of robot-assisted and laparoscopic rectal cancer resections, questionnaires to assess perceived mental and physical workload associated with robot-assisted and laparoscopic operations, and semistructured interviews. Ethics approval for this phase of the study and for phase 3 was granted by the National Research Ethics Service Committee Yorkshire & The Humber – Leeds West (reference 13/YH/0153).
Prioritisation of theories
From a realist perspective, there is an infinite number of potential influences on the interactions between a complex intervention and its intended recipients, and an infinite number of potential impacts resulting from those interactions. 50 It would be impossible to test all of the theories that account for those interactions and, therefore, it is necessary to take some theories on trust, possibly to be tested at some later point, while focusing attention on the testing of other theories. To assist in the process of determining which theories to test, at the SSC meeting and the patient panel meeting, preliminary findings from the interviews were presented and discussion was focused on what outcomes were most important to consider. Once the analysis of the phase 1 interviews was complete, a subset of possible theories to test was identified, based on a consideration of the strength of support offered in the interviews for those theories, the extent to which they concerned outcomes identified by the patient panel and SSC as being important, and the feasibility of testing those theories. This subset was presented to the clinical members of the research team, to capture their perspectives on which theories were most important to test.
Settings and participants
A multisite case study was used63 to generate findings with relevance beyond a single setting. 64 There is no consensus regarding how many case sites to include in a multisite case study. 63 The number of sites depends on the number of aspects of the context anticipated to impact on the phenomenon of interest,63 while also involving a trade-off between breadth and depth of investigation. 65 Four sites were used to enable the identification of organisational-level factors that impacted the deployment of the robot, while providing confidence in the generalisability of findings across sites. This approach has been successfully deployed by members of the research team in a previous multisite case study of the introduction of new technologies. 66 Therefore, it was decided that four case sites would be selected from the nine trusts included in phase 1. Case sites were purposively sampled to ensure variation in the experience of the surgeon and the team with RAS, as this was identified as an important contextual factor in the prioritised theories. In addition, we made sure that the case sites included both large teaching hospitals and district general hospitals, and that three of the case sites were participating in the ROLARR trial and one was not.
Once agreement to participate in phase 2 of the study was obtained from the appropriate colorectal surgeon at each site, visits were made to the sites to explain the study to other surgeons and members of the OT team and to answer any questions or address any concerns (see Appendix 5 for the staff information sheet and Appendix 6 for the staff consent form). Research governance approval was then obtained from each trust for phases 2 and 3.
Once data collection began, it was typically the colorectal surgeon or the research nurses at each site who obtained written consent from patients for us to observe and video record their operations (see Appendix 7 for the patient information sheet and Appendix 8 for the patient consent form). In each of the ROLARR case sites, while recruitment to ROLARR continued, we observed operations involving patients in the trial. Once recruitment to ROLARR ended, and in the case site not participating in ROLARR, we initially used the same inclusion and exclusion criteria as the ROLARR trial, to ensure that the operations observed were comparable (see Appendix 9). Following challenges in recruitment, the inclusion criteria were extended slightly, although these changes were discussed with the clinical members of the research team to ensure that they would not change the nature of the operation observed. Although we observed both laparoscopic and robot-assisted operations in each site, the number of laparoscopic and robot-assisted operations observed in each site depended on the extent to which these two techniques are used within the site and, for case sites in the ROLARR trial, the randomisation of patients to the different arms of the trial.
There is no consensus regarding how many periods of observation are necessary to provide an adequate overview of current practice in a particular setting. 65 We initially proposed to observe 10 rectal cancer resections in each site (n = 40), on the basis that this would be a feasible number to observe within the time frame of the project and would provide over 200 hours of data, which would constitute a substantial corpus. However, the number of suitable operations varied substantially between sites, and data collection at one site ended early due to RAS no longer being used at that trust. In total, between June 2014 and November 2015, we observed 22 rectal cancer resections, 16 robot assisted and six laparoscopic, and we were able to secure 202 hours of data collection, as many of the operations were longer than we had initially anticipated. In addition, the observation of a further 10 colorectal operations was undertaken across three of the sites at different points during data collection, five of which were robot assisted. These provided the opportunity to learn more about colorectal surgery and become familiar with the setting, to trial the structured observation tool OTAS (Observational Teamwork Assessment for Surgery) we would be using and to get to know theatre staff. Details of the number of operations observed in each site are provided in Table 2. In consultation with our SSC, we agreed that we had collected a substantial corpus of data that achieved the anticipated number of hours of observation. Therefore, rather than continue data collection beyond the proposed period, we focused on data analysis.
| Site | Robot assisted | Laparoscopic | Other colorectal robot assisted | Other colorectal open/laparoscopic | Total | ||
|---|---|---|---|---|---|---|---|
| Observation only | Observation and video | Observation only | Observation and video | Informal observation only | Informal observation only | ||
| 1 | 2 | 4 | 2 | 3 | 4 | 15 | |
| 5 | 1 | 1 | 2 | ||||
| 6 | 2 | 2 | 1 | 2 | 7 | ||
| 7 | 3 | 2 | 1 | 1 | 1 | 8 | |
| Total | 8 | 8 | 5 | 1 | 5 | 5 | 32 |
Data collection
Structured observation
Observation is crucially important for understanding teamwork in the OT. 67 Existing studies point to the significance of non-verbal communication in the OT, and the tacit nature of the knowledge that underlies such practices means that it is rarely revealed through interview studies. 29 We selected OTAS, a structured observation tool, for assessing teamwork in the operations we observed. 68 We chose to use OTAS rather than other non-technical evaluation tools related to surgery, such as NOTSS (Non Technical Skills for Surgeons), because, first, it considers the teamwork skills of all professional groups within the OT69 and, second, it considers, and provides separate ratings for, team behaviours that seemed particularly relevant to testing our candidate theories, such as team monitoring and situational awareness. OTAS has been shown to be applicable to various branches of surgery68 and has demonstrated construct validity,70 content validity71 and reliability, minimising error and bias in data collection and therefore increasing confidence in the validity of the findings. 72 OTAS has also demonstrated good inter-rater reliability with short-term training. 32 Further details of OTAS and the training undertaken by members of the research team in preparation for data collection are provided in Appendix 10.
Video recording
To complement the OTAS measures, we also conducted video recordings of teamwork in the OT, adopting the workplace studies approach. 73–75 The value of video recordings is that they capture not only oral communication but also non-verbal communication, such as gesture, gaze and tool manipulation, that has been shown to be important for teamwork in the OT. 29,76 Another benefit of video recordings is that they are permanent, allowing for repeated analysis. They also facilitate collaborative analysis. Additionally, video recording had been used successfully in a number of studies concerned with the impact of teamwork on surgical performance,28,77–87 providing a useful set of background materials with which we could compare and contrast our findings.
For each operation we video recorded, we used two Panasonic high-definition video cameras (model HC-X920; Panasonic UK, Bracknell, UK). These were positioned, on tripods, to capture the surgeon’s perspective on the surgical scene as well as the conduct of the surgical assistant and the scrub practitioner. We used two Sennheiser EK100 wireless lapel microphones (Sennheiser electronic GmBH & Co. KG, Wedemark, Germany), one attached to the surgeon and one attached to the first assistant (which captured the talk of both the first assistant and the scrub practitioner). Video recording began once the patient was draped and stopped before the patient was woken up.
We had initially intended to video record all of the operations that we observed. However, when data collection began, it became apparent that two researchers needed to be present for video recording to be used, so that they could monitor the position of the cameras (although the position of the cameras was fairly static, the movement of the OT team and/or equipment meant that a camera might need to be quickly moved out of the way) and manage the other forms of data collection. Therefore, operations were video recorded only when two researchers could be present. Because there is already a body of video-based studies of laparoscopic surgery, and because most of the robot-assisted operations we observed were hybrid operations, including a laparoscopic phase, we prioritised the video recording of the robot-assisted operations. We collected video data across three sites, video recording eight robot-assisted operations and one laparoscopic operation. This provided us with a total of 52 hours of recordings but, as we used two cameras, this totals 104 hours of video data, which constitutes a substantial corpus. Fifty-two hours of video recording is in line with the number of data collected in other video-based studies of surgical work. 81,85,87
Ethnographic observation
Ethnography, the study of people in their environments where the researcher participates in the setting in order to collect data,65 has been argued as an essential approach for studying the introduction of technology into health-care settings. 88 Ethnographic methods, such as non-participant observation, have also been used in previous realist evaluations as part of the process of theory testing and refinement. 89,90 In addition to recording field notes about the teamwork behaviours relevant for OTAS, the researchers recorded details of behaviours and interactions that fell outside the scope of OTAS. This provided an account of what happened during the operation, and before and after it, which was particularly important for those operations we were not able to video record. The researchers also recorded incidents of observer effects (e.g. participants asking ‘what are you writing?’) to allow an analysis of whether or not participants’ awareness of the researchers’ presence changed over time. 91 Following data collection, field notes were written up. When two researchers observed an operation, their notes were combined to provide a single account of the operation.
SURG-TLX questionnaire
Use of the SURG-TLX questionnaire was not included in our original protocol but was added on completion of phase 1, as a result of theories elicited in phase 1 and taken forward for testing regarding the impact of RAS on mental and physical stress. The SURG-TLX is a multidimensional rating procedure for measuring the subjective workload associated with an operation,92 and is adapted from the NASA Task Load Index (NASA-TLX), the most widely used measure of workload in human factors research. 93 Development of the SURG-TLX was informed by qualitative research that has identified key intraoperative stressors. 92 The subscales included in the SURG-TLX are mental, physical and temporal demands, and task complexity, situational stress and distractions. The use of the six subscales provides valuable information about sources of workload and, relative to unidimensional workload ratings, has been found to reduce variability among participants in the overall workload score. 94 Each subscale is subjectively rated on a 21-point visual analogue scale, from very low to very high.
We developed a questionnaire based on the SURG-TLX, with additional questions about the participant’s role and their levels of experience of RAS and laparoscopic surgery. Although the SURG-TLX was initially designed to be completed by the surgeon, in more recent research it has been provided to all members of the OT team. 95 We gave the questionnaire to staff following each operation we observed, prioritising getting responses from the surgeon, the first assistant and the scrub practitioner. A total of 55 questionnaires were completed. Details of the number of questionnaires collected in each site are provided in Table 3.
| Site | Role | Total | |||
|---|---|---|---|---|---|
| Surgeon | First assistant | Scrub practitioner | Circulating practitioner | ||
| 1 | 7 | 6 | 4 | 4 | 21 |
| 5 | 2 | 1 | 1 | 4 | |
| 6 | 5 | 4 | 3 | 12 | |
| 7 | 6 | 5 | 4 | 3 | 18 |
| Total | 20 | 16 | 12 | 7 | 55 |
Semistructured interviews
At the end of each operation we undertook brief semistructured interviews with some of those who participated in the operation. The researchers endeavoured to undertake interviews with the surgeons whenever possible. In most cases this was unproblematic, but sometimes the surgeons needed to leave the OT immediately. Additional interviews were undertaken with other members of the team when their availability allowed. In the interviews we sought to gather data on those outcomes that cannot be easily gathered by other means, particularly those relating to the perceptions of members of the OT team (e.g. perceptions of the quality of teamwork and others’ engagement during the operation), and perceptions of RAS as an opportunity for training. The interviews also provided an opportunity for the researcher to ask questions about aspects of the operation not immediately intelligible to an observer. As data collection progressed, we also used these interviews as an opportunity to discuss the revisions to our theories, using the teacher–learner cycle described above. A total of 30 postoperation interviews were undertaken. Details of the number of interviews undertaken in each site are provided in Table 4.
| Site | Role | Total | |||
|---|---|---|---|---|---|
| Surgeon | Trainee | Nurse/ODP | Anaesthetist | ||
| 1 | 5 | 4 | 9 | ||
| 5 | 2 | 2 | |||
| 6 | 4 | 2 | 1 | 7 | |
| 7 | 6 | 2 | 3 | 1 | 12 |
| Total | 17 | 4 | 8 | 1 | 30 |
Within realist evaluation, it is recommended not only to undertake interviews in the early phases of the study but also to schedule interviews after observations, when the interviews ‘are guided and informed by incidents arising from the observations’ in order to allow further theory testing and theory consolidation. 59 We undertook a series of longer interviews with surgeons once the observations had been completed and a preliminary analysis of the data was complete, in order to fill in gaps in our understanding regarding why particular events observed during the operations happened. Again, these interviews were semistructured and used the teacher–learner cycle, with the researcher asking questions informed by our revised theories so that participants could support or refine our theories. Although we had hoped to be able to review clips of videos with the surgeons in these interviews, this proved not to be feasible, so instead we produced descriptions of the events we wanted to explore and presented these to our interviewees. All interviews were audio recorded and transcribed verbatim. A total of four such interviews were undertaken.
Data analysis
An iterative approach to data collection and analysis was taken, to enable the ongoing testing and refinement of the theories and the gathering of further data in the light of such revisions. Field notes were entered into NVivo 10 following transcription. As a first step in analysing the data, we produced a series of matrix displays based on the case dynamics matrices described by Miles and Huberman. 62 Although these are normally produced once the data have been indexed, we found it helpful to go straight to producing the matrices, as a way of getting an overview of the data and keeping our analysis focused on the testing of our theories. A matrix display was created for each theory and, with one row for each robot-assisted operation, we summarised the anticipated contextual factors from the theories (whether or not they were present), other contextual factors that appeared to exert influence, anticipated mechanisms from the theories (whether or not they appeared to be at play), other mechanisms that appeared to be at play, and anticipated and unanticipated outcomes. We used data from the field notes, interviews undertaken after the operations, responses to the SURG-TLX questionnaire and the OTAS ratings.
As we began to write up the analysis based on the matrix displays, further questions became apparent, so we returned to the field notes for additional information. This involved indexing the data, using codes relevant to the questions and inductive codes to capture other aspects of the contexts, mechanisms and outcomes relevant to our theories.
Analysis of Observational Teamwork Assessment for Surgery
There was substantial variation within robot-assisted operations in terms of how much of the operation was robot-assisted (mean 45%, range 15–64%). Consequently, we combined the data from the robot-assisted and laparoscopic operations and ran a Pearson product-moment correlation to determine the relationship between the percentage of operation that was robot assisted (with laparoscopic operations being 0% robot assisted) and the overall OTAS score. Following this, correlations between the percentage of operation that was robot assisted and OTAS scores for specific subteams and constructs were calculated using the Pearson product-moment correlation (see Chapters 7 and 8). A one-way independent analysis of variance (ANOVA), with the first assistant’s experience of RAS as an independent variable, was performed to determine whether or not there was a relationship between the first assistant’s experience and the surgical subteam co-ordination score.
Analysis of SURG-TLX
A Pearson product-moment correlation was run to determine the relationship between the SURG-TLX overall score and the percentage of operation that was robot assisted. Following this, the analyses focused on specific subscales as they related to the theories being tested (see Chapter 8).
Analysis of video data
For the analysis of the video data, we took several complementary approaches. First, we followed standard methods outlined in the field of workplace studies,96 which draw heavily on ethnomethodology97 and conversation analysis. 98,99 This involves four key stages: (1) a preliminary review of the data to identify short episodes of co-ordination between OT team members, which allows the analyst to begin to identify activities, phenomena or notable extracts that may provide fruitful avenues for further enquiry; (2) detailed transcription of selected extracts using standard orthographies in conversation analysis to detail the temporal organisation of actions and activities – talk, bodily conduct and tool use;100 (3) close consideration of the extracts to unpack the situations and contexts in which these episodes occur and to reveal potential interactional patterns and practices; and (4) a further review of the data to identify and interrogate similar and contrasting examples. The first stage of this analysis was undertaken by multiple members of the research team through a series of ‘video review sessions’. Given the complex and highly specialised character of the setting, a number of video extracts and preliminary analytic observations were discussed with clinical members of the research team and the SSC.
Second, we undertook a quantitative analysis of video data using the video analysis software Transana (University of Wisconsin-Madison Center for Education Research, WI, USA). Although this had not been in our initial protocol, the initial analysis of the OTAS data suggested little variation across operations, while initial analysis of the SURG-TLX data suggested variation that cannot be explained purely by whether or not the operation is robot assisted. Therefore, we felt it necessary to gather additional quantitative data to support our testing of the theories, and the video data provided a good resource for this purpose. The use of these data was also based on an acknowledgement that the impacts we were interested in (e.g. engagement) vary over the course of an operation, while OTAS and SURG-TLX provide data on only the operation as a whole. Through the initial analysis based primarily on the field notes described above, we were able to establish a focus for this quantitative analysis of the video data. Plans for the quantitative analysis of the video data were discussed with the clinical members of our team to ensure that the measures being used were considered to have clinical relevance. Quantitative analysis focused on the time taken for a first assistant to respond to a surgeon’s request. Details of how this analysis was undertaken are provided in Appendix 11.
Phase 3: assessing generalisability of theories
The objectives of phase 3 were (1) to assess the extent to which the theories resulting from phase 2 were generalisable to other surgical specialties and to refine them so that they had wider applicability; and (2) to use the findings of the study to explore ideas for tools and technologies to better support teamwork and decision-making in RAS. The first of these objectives was achieved through undertaking interviews with surgeons and OT teams in other surgical specialties in our phase 2 case sites. The second objective was achieved through a 1-day workshop held at the University of Leeds.
Interviews with other surgical specialties
Settings and participants
Interviews were undertaken at the three phase 2 case sites that were continuing to undertake RAS. Surgeons, theatre nurses, ODPs and trainee surgeons from surgical specialties in which RAS is being used were included. A total of 13 participants were interviewed between May and July 2016. Details of the number of interviews undertaken in each site are provided in Table 5. At site 1, we interviewed two urology surgeons and one urology surgical trainee. The ODPs at site 1 all worked in urology but some had previous experience of working in colorectal surgery. At site 6, because the theatre nurses and ODPs worked across all three specialties in that trust that used the robot (urology, gynaecology and colorectal), all had participated in earlier phases of the study. At site 7, we interviewed one urology surgeon and one upper gastrointestinal (GI) surgeon.
| Site | Role | Total | |||
|---|---|---|---|---|---|
| Surgeon | Trainee surgeon | Nurse | ODP | ||
| 1 | 2 | 1 | 5 | 8 | |
| 6 | 1 | 2 | 3 | ||
| 7 | 2 | 2 | |||
| Total | 4 | 1 | 1 | 7 | 13 |
Data collection
Interviews were conducted face to face at sites 1 and 6 and undertaken by telephone at site 7. At site 1, the interviews with the ODPs were undertaken as a group interview owing to restrictions in the participants’ availability. All other interviews were individual interviews. The interviews were semistructured and conducted using the teacher–learner cycle, as in phase 1. In each interview, participants were first asked about their experience of RAS and how RAS was introduced into their specialty, in order to identify contextual differences across the specialties related to experience and the processes of implementation. The theories that resulted from phase 2 of the research were then described and the participant was invited to comment, expand and discuss the theories based on their experience of RAS. When a theory did not fit with the interviewee’s experience, the researcher probed to identify the contextual factors that limited the applicability of the theory to the interviewee’s specialty. As in phase 1, the interview topic guide was revised as the interviews progressed. All interviews were audio recorded and transcribed verbatim. The interviews ranged in length from 14 minutes to 47 minutes, with the average (mean) length being 30 minutes.
Data analysis
An iterative approach to data collection and analysis was taken as in phase 1, with an initial review of interview transcripts enabling refinements to theories to be explored in subsequent interviews. Once all interviews were completed, a more formal analysis was undertaken using framework analysis. Having read through the interview transcripts, codes for indexing the data were identified, including both codes based on the interview topic guide (largely relating to the theories, as well as codes relating to the process of implementing RAS) and subcodes based on variations to the theories that emerged in the interviews. The interview transcripts were entered into NVivo 10 and indexed. A matrix display was created in NVivo 10 to summarise the indexed data, with a row for each interviewee. For the stage of mapping and interpretation, the matrix displays were used to compare the findings with the theories presented in the interviews, in order to come up with a final set of theories.
A 1-day workshop on designing for robot-assisted surgery
In July 2016 we held a workshop at the University of Leeds entitled ‘Designing for robotic surgery: challenges and opportunities’. The workshop had 25 attendees, made up of a mixture of engineers, computer scientists, psychologists, social scientists, surgeons, theatre nurses, ODPs and members of our patient panel. The findings from the study were presented and then participants were asked to consider the implications of the findings for the design of RAS. When we first envisioned the workshop, before beginning the study, we had anticipated that it would be purely about designing technology, whether designing surgical robots or designing other technologies for the OT that support RAS. However, given the issues that came up in the study around training and the influence of the physical configuration of technology and people in the OT on teamwork, participants were invited to think beyond the design of technology if they so wished.
Chapter 3 An initial theory of robot-assisted surgery
Overview
In preparing the proposal for this study, based on existing knowledge regarding how technology becomes embedded into health-care practice and the nature of communication, teamwork and decision-making in the OT, and consideration of the broader surgical safety literature,101,102 we developed a series of candidate theories for further exploration. In this chapter, we present that literature and the resulting candidate theories with which we started this study.
Integrating robot-assisted surgery into surgical practice
The successful introduction of technology involves interactions between individual clinicians and their work environment until the technology becomes embedded (routinely incorporated into everyday work) and integrated (sustained over time) into routine practice, a process known as ‘normalisation’. 103 Factors impacting the integration of health-care technologies include the skill mix and motivation of users; the acceptability of the technology to clinicians and patients; training; division of labour and workload; organisational culture; and whether or not the introduction of the technology was clinician led. 104,105
When there is a mismatch between the technology and the work practice of the users, users may, both individually and as a group, adapt the technology (system tailoring) and the way they work (task tailoring), both behaviours that have previously been reported in the OT. 106 Such ‘workarounds’ can often lead to a variety of ‘unintended consequences’ that may result in processes and outcomes that are undesirable and/or were unanticipated when the technology was introduced. 107–110
Normalisation process theory suggests that, for successful integration to occur, four key constructs need to be considered: (1) coherence: sense-making – where individuals make sense of the new technology and how it differs from existing practice; (2) cognitive participation – the process of engaging individuals with the introduction of the technology; (3) collective action – how the work processes are adapted and altered to make the intervention happen; and (4) reflexive monitoring – the formal and informal appraisal of the benefits and costs of the intervention. 46,103,111 This suggests that if members of the OT team have been able to ‘make sense’ of RAS, have been engaged in the process of implementation, have been able to adapt their work processes and are able to identify potential benefits of its introduction, it is more likely to become embedded into surgical practice, being used routinely and successfully for surgical operations when it offers benefits to the patient.
Meanwhile, reports of the use of RAS highlight that there is a learning curve for the whole team112 and point to the need for a highly motivated113 and/or dedicated robotic team. 114–116 This suggests that when the OT team is experienced in RAS and members are motivated, RAS is more likely to become embedded into surgical practice.
Communication and teamwork in the operating theatre
The successful performance of a surgical operation is dependent on collaboration among staff from different professional groups. In the UK, the team brought together to perform an operation typically consists of:
-
a consultant surgeon
-
a first assistant – a role performed by a surgical trainee, a theatre nurse or an ODP who has undertaken first assistant training, or occasionally by another consultant surgeon, in which they are working within the sterile field and are responsible for assisting the surgeon in carrying out the operation, particularly assisting with retraction
-
a scrub practitioner – a role performed either by a theatre nurse or by an ODP, working within the sterile field, in which they are responsible for supporting the surgeon in carrying out the operation, particularly ensuring the availability and the sterility of surgical instruments and passing them to the surgeon as they are needed
-
a circulating practitioner – a role performed either by a theatre nurse or by an ODP, working outside the sterile field and responsible for supporting those within the sterile field, particularly the scrub practitioner, by obtaining the necessary equipment and liaising and co-ordinating with others within the OT and in the wider operating department
-
an anaesthetist – working outside the sterile field, responsible for providing anaesthesia and for monitoring and treating, as necessary, the effects of the anaesthetic and the surgery
-
an anaesthetic assistant – a role performed either by a theatre nurse or by an ODP, working outside the sterile field, who assists the anaesthetist in the administration and monitoring of anaesthesia.
This complex division of labour requires team members to use their different skills to collaboratively accomplish a single, principal activity. 28
Communication and teamwork in the OT is a topic that has received much attention over recent years, due to failures in communication and teamwork being identified as key factors in adverse events in the OT. 72 Communication in the OT is defined as ‘the quality and quantity of information exchanged among members of the team’. 117 It has been found to be variable in both quality and quantity, with a lack of formal exchanges between staff about essential information and the completion of basic procedural tasks. 118 There is considerable distraction and interruption in the OT,119 which may negatively impact on communication and teamwork. 120 Communication in the OT may suffer from poor timing, missing or inaccurate information, failure to resolve issues and the exclusion of key individuals. 121 Communication and teamwork failures are a common source of surgical flow disruptions, defined as deviations from the natural progression of an operation, and surgical errors have been found to increase significantly with increases in flow disruptions. 122 Even when communication and teamwork failures do not result in an adverse event, they can limit the surgical team’s ability to compensate for a major event,123 whereas effective teamwork in the OT can reduce the number of small problems and prevent them from escalating to more serious situations. 124 Thus, teamwork and communication are both considered to be markers of surgical excellence. 125
While such work on the relationship between communication and teamwork in the OT and patient safety has been important in highlighting the significance of this area, a limitation of existing work is that too often the emphasis has been on applying the label of failure, rather than seeking to understand and explain, with the terms communication and teamwork often being used interchangeably. 126 The ‘workplace studies’ literature provides an alternative view of communication and teamwork in the OT. Drawing on ethnographic data and naturalistic video recordings, workplace studies are concerned with the interplay of talk, visual conduct and the use of tools and technologies in the achievement of work in complex settings. 73,75 Such studies emphasise the careful collaboration and co-ordination that is an essential part of surgical practice, and illustrate how oral communication is just one strategy used for ensuring smooth co-ordination among team members. 28 This literature draws on the concept of awareness, used to refer to the ways in which members of a team display their activities to, and monitor the activities of, their team members in order to support their collaborative work. 127,128 As such, awareness is seen as essential for effective collaborative work. Such awareness is typically characterised as being ‘effortless’,128 and workplace studies point to the way in which, when co-located, co-operating actors are able to align and integrate their activities through such mutual display and monitoring. 127 For example, there are a number of strategies that scrub practitioners draw on to ensure the smooth passing of instruments in a safe and timely manner. Before the operation, the scrub practitioner will organise potentially relevant instruments, positioning and orientating them so they can be grasped or handed safely. During the operation the scrub practitioner will pay attention to the actions of the surgeon and may reorganise the instruments according to when, based on the sequence of actions observed, the scrub practitioner anticipates they will be required, which also enables the surgeon to take the instrument from the table directly. 28 Through this careful attention to the ongoing work, the scrub practitioner is also able to anticipate when an instrument is required, obviating the need for an oral request from the surgeon. This would be an example of what is referred to as by-product awareness, being generated in the course of activities, in contrast to add-on awareness, whereby team members do additional work either to display to colleagues the status of the work and their activities or to monitor their colleagues’ work. 129 Such studies would imply that the separation of the surgeon from the rest of the OT team in RAS would impact co-ordination because team members are less able to monitor the surgeon’s actions.
Such studies also point to the operation as a moment of training and the embodied conduct used for this purpose; surgeons combine talk and gesture to enable trainees to follow and make sense of a surgical procedure, supported by timely and relevant contributions from other members of the surgical team, and draw on the surgical trainee’s talk and gesture to determine their level of understanding. 87 In laparoscopic surgery, all team members have access to the same view of the surgical site, but talk and gesture are used to ensure that others see what the surgeon sees. 130 This would suggest that the separation of the surgeon and the trainee in RAS and their different views of the surgical site is likely to impact on training.
Decision-making in the operating theatre
Decision-making is an important component of surgical expertise. 131 Despite flexible decision-making strategies being a behavioural marker of surgical excellence,125 there is a paucity of research on decision-making in the OT, and what research there is tends to focus solely on the decision-making of the surgeon. 132,133 Factors that affect the surgeon’s decision-making in the OT include instrument complexity,133 although the decision-making strategy used (rapid, intuitive mode vs. deliberate comparison of alternative courses of action) is not affected by whether the surgery is open or laparoscopic. 134
Situational awareness is defined as the perception of elements in the environment, the comprehension of their meaning and the projection of their status in the near future. 135 The surgeon’s position in the console suggests a reduction in the surgeon’s situational awareness, which would have implications for the surgeon’s decision-making. For example, Klein,136 in his recognition primed decision (RPD) model, highlights the importance of context or situation in ‘triggering’ mental models that guide decision-making in numerous complex decision situations. One model of intraoperative decision-making suggests a continuous cycle where, with the preoperative plan in mind, the surgeon assesses the situation, reconciles new information with existing information and, subsequently, implements a revised course of action. 137 In this cycle, through the use of existing mental models, information may be actively sought or, by remaining observant of what is happening in the OT, perceived without active seeking. Such theories would suggest that a reduction in situational awareness has the potential to negatively impact surgeon decision-making. This is supported by studies that have found that better situational awareness of the surgeon is associated with fewer surgical errors. 138,139
While the decision-making theories described above all focus on individual cognition, the theory of distributed cognition encourages us to think about not only what information the surgeon has access to but also what information other members of the team have access to and how that information is propagated through the system. 140 The spatial configuration of OT teams is not arbitrary but affords particular views of the patient, the rest of the team and different tools and technologies, with the result that different team members have access to different information to inform their decision-making. 141 Consequently, any change to the spatial configuration is likely to have an impact on decision-making. What is key is how that information is shared. For example, Hazlehurst et al. 142 describe how, in cardiac surgery, the surgeon and perfusionist each have only partial access to the information necessary for a successful outcome, with situational awareness for both being achieved through oral exchange.
In RAS, the surgeon is not able to see the patient directly, so to maintain situational awareness he or she is more dependent on the rest of the team communicating the status of the patient. 23,57
Summary
Through consideration of the literature presented above, we developed the following candidate theories for further exploration.
-
Inexperienced team: when the OT team is less experienced in RAS (C), they have more difficulties in setting up and positioning the robot, which can reduce the ease with which OT team members have access to the patient on the operating table (M), resulting in increased operation duration, conversion to open surgery and complications (O).
-
Experienced team: when OT teams are motivated to use RAS and as they become more familiar with the equipment through repeated use (C), they are better able to develop strategies to overcome difficulties created in this reconfigured environment (M), resulting in effective co-ordination, teamwork and communication and reduced operation duration (O).
-
Team involvement: if the whole OT team can feel the advantages of RAS outweigh its disadvantages and are involved in the decision to introduce it (C), they will be more motivated to work together to develop solutions to problems that may arise when they are using it to carry out operations (M), supporting the integration of RAS into routine practice (O).
-
Co-ordination: when the surgeon is separated from the rest of the OT team (C), the team is less aware of the surgeon’s actions, making it more difficult to co-ordinate their actions during the operation (M) and so the operation takes longer (O).
-
Training: when surgeons and trainees have different views of the surgical site (C), it is harder for the surgeon to explain what is happening and monitor the trainee’s understanding (M), resulting in the trainee not learning as much as they would in other forms of surgery (O).
-
Situational awareness: when the team is more experienced in RAS (C), they understand that the surgeon’s situational awareness is dependent on them orally communicating information and they respond by using more oral communication about the patient’s state (M), which in turn improves the surgeon’s situational awareness (O).
Chapter 4 A realist review of stakeholders’ theories
Overview
This chapter presents the first part of phase 1 of our study, a review of the literature undertaken to refine and add to the candidate theories established from the academic literature that were presented in Chapter 3. The review focused on identification of stakeholders’ theories, looking at grey literature, opinion pieces, letters, editorials and the discussion sections of quantitative studies of RAS, which contained ideas and assumptions regarding how RAS might successfully be introduced and how it might impact on communication and decision-making.
Overview of search results
The search retrieved 485 references. Twenty-seven were systematic reviews of studies of colorectal RAS, 159 were systematic reviews of studies of RAS, either of surgical specialties beyond colorectal surgery or not restricted to a particular specialty, 121 were individual studies of colorectal RAS and 178 were editorials or commentaries. These were evaluated together with 188 websites. Two hundred and twenty-eight papers, made up of 22 systematic reviews of colorectal RAS, 94 other systematic reviews of RAS, 37 individual studies of colorectal RAS and 75 editorials or commentaries, were identified as relevant, along with 34 websites. There was considerable repetition of theories across the sources we identified.
Findings
Issues of implementation
The robot-assisted surgery implementation chain
The term ‘implementation chain’ is used to refer to the series of interconnected processes through which an intervention is introduced and delivered to produce immediate and intermediate outcomes and ultimate impacts (both short and long term). The introduction of complex interventions into practice typically involves long implementation chains, influenced by stakeholders at different levels within and beyond the organisation. 50 The way in which an intervention is implemented across different settings is likely to vary, affecting the ultimate impacts of that intervention and often leading to unintended consequences. 50,143 Thus, it is recommended that, both within realist reviews and prior to entering the field as part of a realist evaluation, the implementation chain of an intervention is mapped, identifying which intermediate outcomes need to occur in order to create the context for positive final outcomes, as well as examining the blockages to effective implementation and the contexts that support the flow of implementation. 50,52 Our intention was not to look at the evolution of RAS as a technology over time. Rather, our interest was in the implementation chain from the point at which the purchasing of a surgical robot by a hospital is considered as an option to the point at which RAS is used on a routine basis in that hospital.
The review revealed a number of decision points in the implementation chain of RAS, which are summarised in Figure 3. The first decision is the decision of whether or not to purchase a da Vinci robot, and there appeared to be a number of stakeholders involved in this decision. In most studies that described the introduction of RAS, this had been led by surgeons. However, also involved was whoever would be paying for the initial purchase of the robot (in many NHS trusts, some or all of the funds came from the hospital’s local charity16) and whoever would be paying the ongoing costs associated with RAS.
FIGURE 3.
Decision points on the RAS implementation chain.

For the surgeons, a number of motivations for the introduction of RAS were described. The theory, promoted by Intuitive Surgical, that the increased precision and control RAS offers the surgeon will result in improved patient outcomes was reiterated in many papers. In robot-assisted colorectal surgery, rates of conversion to open surgery are generally found to be low, providing support for this theory144–146 (although, with such studies being non-randomised and authored by RAS enthusiasts, this could be due to case selection). Another motivation was the ergonomic benefits that the robot is anticipated to offer to the surgeon. With the surgeon using natural hand movements147 and sitting at the console,148 the awkward and unnatural positions often required during laparoscopy are removed. This suggests motivation to undertake RAS would be greater among those surgeons who were familiar with Intuitive Surgical’s marketing of the da Vinci robot and/or the published literature on the clinical and ergonomic benefits of RAS. RAS also was seen as a way of providing more patients with the benefits of minimally invasive surgery, being easier to learn than laparoscopy,149,150 suggesting that the motivation to undertake RAS would be greater in those surgical specialties for which laparoscopic surgery was found to be harder and/or among those surgeons who had struggled to adapt to laparoscopic surgery. Surgeons’ natural interest in the development of new tools was also seen as a contextual factor supporting the growth of RAS. 151
At the organisational level, the drivers behind and contextual factors influencing the decision to support the purchase of a robot were less clear, although a couple of benefits for the organisation were identified. For example, RAS was perceived as attractive to surgical trainees, so a hospital may support the decision if ownership of a da Vinci robot was seen as a way to attract the best trainees,15,152 or as a way of attracting highly trained surgeons who already have experience of RAS. 16 The importance of surgeons obtaining the support of the hospital administration and nursing management was emphasised. This support was necessary not only for the initial purchase of the robot but also to ensure the provision of adequate resources while staff are on the learning curve, such as additional OT time. 152,153 How to obtain this support was not explicated, although one report described the need to create a ‘shared vision’ of what the introduction of RAS would enable, starting with the administrators. 154 The underlying theory seems to be that, by being engaged in this process of imagining potential future benefits of RAS, the hospital administration and nursing management will perceive RAS as an innovation that can assist in achieving the organisation’s goals and so will be willing to invest the necessary resources to assist its integration into routine practice.
A contextual factor that appeared to influence the decision-making of both surgeons and administrators was the attitude of patients and the public to RAS. Intuitive Surgical’s marketing of the da Vinci robot has been characterised as aggressive151 and in the USA it has included direct-to-patient advertising on billboards and the internet,155 with the result that RAS has become a symbol of providing enhanced care. 151 In the context of competition among surgeons and hospitals, this perception of RAS among patients and the public is seen as a significant factor in the rapid growth in the purchase of da Vinci robots. 151 This creates a cycle in which, having purchased a robot and wanting to maximise its use, surgeons and hospitals promote RAS to patients, further increasing public enthusiasm for RAS. 150 This promotion of RAS by surgeons and hospitals is not unique to the USA; a number of NHS trust websites were found to promote the benefits of RAS, particularly in relation to prostate cancer, emphasising the advanced, cutting-edge technology and the high cost of the system, with some trust websites describing hospital open days during which members of the public could see and try the robot. Furthermore, the presence of a robot was seen as a means of ‘raising the profile’ of a trust and enabling it to attract patients from ‘elsewhere’ (presumably outside the local catchment area), thus increasing patient volume within the trust. 156
The decision to buy a robot includes a decision about what model of da Vinci robot to purchase, and this appeared to be a decision with implications for the subsequent integration of RAS into practice, including the feasibility of using the robot for particular operations. To date, there have been five different models of the da Vinci robot in use: standard, S, S HD, Si HD and Xi. The standard da Vinci, introduced in 1999, has been discontinued, and technical support in terms of the availability of parts and services stopped in 2014. 157 The larger size of the standard da Vinci compared with later models potentially increases the likelihood of external collision of the robotic arms and also makes it more difficult to manoeuvre. The increased likelihood of collision of the robotic arms and the shorter arms have been cited as reasons why rectal cancer resections must be performed as hybrid operations when using the standard system. 157
Following the decision to purchase a robot, presumably led by a particular surgical specialty, there is then the decision about which (other) surgical specialties the robot should be used for. In many NHS trusts, RAS was initially introduced in urology, for undertaking radical prostatectomy for prostate cancer. 16 The advantages of RAS seem to apply especially to procedures carried out in the pelvis or rectum because surgeons are operating in limited space, where laparoscopic surgery may be difficult. 144,158
Alongside decisions about who will be using RAS are decisions about what training the surgeons and OT teams should receive, although who was involved in such decisions and what influenced the decisions was not reported in the studies. The studies we reviewed described a number of features of RAS surgeons have to become familiar with: the procedure of docking the robot, including the set-up of the robotic arms; how to control the robot and troubleshoot; how to deal with the absence of tactile information; and how to mentally visualise the spatial relationships of the robotic arms, to minimise external clashing of robotic arms and to optimise manoeuvrability and range of motion. 159–162 Training provided for surgeons by Intuitive Surgical was reported to consist of a two-day course with practice in an animal laboratory, but without performance-based end points or verification of skill, leading some to argue that it is an ‘antiquated educational method’ that should be replaced by carefully structured standardised simulator curricula. 163 Intuitive Surgical has since introduced online training modules and there is a simulator curriculum that can be undertaken on the robot itself.
In relation to training for the rest of the team, a well-trained team was described as necessary to ensure the smooth running of a robot-assisted operation. 147 The scrub practitioner and circulating practitioner need to acquire new skills and need to learn their part in draping and docking the robot, how to change instruments, and troubleshooting. 164,165 The first assistant will need to learn all of this, as well as have an understanding of basic laparoscopic surgery. Specific training for first assistants, involving dry-laboratory training, personal instruction sessions, videos and surgery observation, was described. 166 Although studies occasionally talked of theatre nurses participating in training,167 generally there was little mention of training for the wider OT team.
Following on from this is the decision of which operations to use the robot for and on which patients, a decision typically made by the consultant surgeon. This is not a one-off decision but one that is likely to evolve over time as a surgeon’s experience with RAS increases. Some authors recommended starting with more straightforward procedures and then gradually moving to increasingly difficult procedures,167 the underlying theory seeming to be that, by gaining experience with simpler procedures, the surgeon builds up his or her skills, providing him or her with both the skills and the confidence to be able to take on more challenging procedures. A contextual factor that influences the decision of whether or not to undertake an operation with robot assistance is the patient condition, as the longer operation duration of RAS (discussed below) may be prohibitive in certain patients, although it can be difficult to assess who those patients are. 168 Another contextual factor that influences the decision is the anticipated benefit, which is based on the details of the operation to be performed and characteristics of the patient. For example, in the context of rectal cancer surgery, maximum benefit was reported to be expected in cases involving mid to lower rectal cancer, male patients (due to the narrower pelvises), obese patients and patients who have had preoperative chemoradiation therapy. 169,170
Alongside this are decisions about how to undertake a particular operation with the robot, again a decision typically undertaken by the consultant surgeon and again not a one-off decision but one likely to evolve over time. For example, in robot-assisted rectal cancer surgery, there is no standardised technique and the decision has to be made whether to perform it as a hybrid operation or totally robot assisted. 171 As noted above, this decision will be influenced by the model of robot the hospital has acquired. However, there are also other factors that influence the decision. Performing a hybrid operation removes the need to redock the robot, which can add to the duration of the operation,171 and thus the decision may be influenced by concerns about time. This ties in with concerns about the patient condition; if patient factors mean that the operation is likely to be difficult and require considerable time, a hybrid operation is more likely. Experience seems to be an important contextual factor that influences surgeons’ decisions about how to perform an operation. For example, it has been suggested that, for surgeons experienced in laparoscopic surgery, a hybrid approach is preferable, as their increased experience will mean that they feel more comfortable carrying out the first phase of the operation that way,171,172 while a totally robot-assisted procedure may be preferable for surgeons who have less experience of laparoscopic surgery, despite the need to redock. 173 Another decision relates to the positioning of the ports through which the instruments are inserted, and some authors described not wanting to follow the layouts suggested by other surgeons experienced in RAS because they wanted to adapt the positioning they used for laparoscopic operations, again presumably wanting to build on their existing experience. 167
The challenges of robot-assisted surgery
Despite the various motivations for undertaking RAS, it was perceived to introduce its own challenges. For example, surgeons’ reports of the experience of RAS described how the bulk of the robot made it difficult to manoeuvre,152,153 although, as described above, the extent of this challenge will depend on the model of robot. In some review articles, authors argued that the difficulty of moving the robot represents a patient safety issue if prompt conversion to open surgery becomes necessary so that the robot needs to be moved out of the way,158,174 although whether this had caused problems in practice was unclear. This challenge may reduce the willingness of surgeons to undertake RAS, particularly for multiquadrant operations that require the robot to be repositioned during the operation.
Another challenge was that RAS had been found to extend operation duration. Not only does this increase costs by increasing staff and OT time but some authors argued that it can put patients at risk from complications caused by being under anaesthesia for longer. 170 We identified conflicting theories about how RAS increases operation duration. Some authors argued that it is due to the time required to set up and dock the robot. 144,175 Others pointed to the time required to reposition and redock the robot during multiquadrant operations. 171,176 Yet others pointed to a longer operative time, perceived as being due to collisions of the robotic arms, itself a consequence of lack of experience with proper positioning of the robotic ports. 169 It has also been argued that longer operation duration is related to the lack of tactile information, leading surgeons to move more slowly because they have to rely on visual information only. 177 However, one study found no difference in overall duration because, although the set-up time was significantly longer, this was balanced out by a significantly shorter operative time, which the authors argued was due to the technical advantages that the robot provides to the surgeon. 178 Given the high cost of purchasing and maintaining a robotic system, minimising additional costs associated with increased operation duration was perceived by some as essential for ensuring that RAS is integrated into routine practice. 147,179
Overall, there was broad agreement in the perception that operation duration decreases as experience increases, with this often being attributed to a decrease in set-up time. 145,147,180 Thus, the underlying theory is that when the team is experienced and well trained, their knowledge and experience enables them to quickly undertake the tasks required for setting up the robot. With experience, surgeons have reported that they find visual cues sufficient for estimating the tension exerted on the tissue. 172 As familiarity with positioning the robot increases, there should be fewer collisions of the robotic arms, also helping to reduce operation duration. 169
Several strategies were reported that might reduce operation duration by accelerating the acquisition of experience. One strategy was to have a dedicated robotic team114–116,152,154,170,181–183 that can ‘work through the learning curve and, if possible, all robotic cases’. 164 The underlying theory is that by working through all robot-assisted cases, the team more quickly becomes familiar and confident with the equipment and tasks associated with setting up the robot, allowing team members to complete the necessary tasks more quickly, reducing set-up time. Although typically discussed in relation to the surgeon’s learning curve,184 the theory suggests that the number and frequency of robot-assisted operations that take place within the organisation are contextual factors that impact the effectiveness of this strategy. Other contextual factors described were the level of motivation154 and stability of the team. 113 Thus, a team that is not motivated may work through the robot-assisted operations but not engage with them as an opportunity to learn and, consequently, the increased experience of RAS may not translate into increased efficiency in robot set-up.
A number of surgeons also recommended having a dedicated robotic OT,152,185 so that the bulky robot does not need to be moved between OTs, reducing the time spent setting up and putting away the robot and thereby reducing the overall operation duration.
Impact of robot-assisted surgery on communication and teamwork
Reporting on their experience of undertaking RAS, surgeons described how the physical separation of the surgeon from the rest of the team and the lack of visual contact make it harder for the team to hear the surgeon’s oral instructions,152 particularly if the surgeon becomes immersed in the console. 154 Consequently, it has been suggested the team needs to listen more carefully,152 again implying the need for a motivated team. There is a perception that, if the team does not respond in this way, communication is compromised. 152,158 Although the consequences of this were not explicated in the literature we reviewed, one consequence may be a further increase in operation duration, as communication failure is known to be a significant predictor of deviation in expected length of operation. 186 The use of directional cues is considered to be problematic in RAS, potentially resulting in confusion, time-wasting and patient injury. 164 This problem occurs, presumably, because the separation means that team members do not have the same physical context as the surgeon to understand such deictic instructions and, again, the surgeon is unable to support those instructions with gestures.
The impact of RAS on communication between the surgeon and the first assistant was another area in which we found conflicting theories. Some authors argued that communication between the surgeon and first assistant is particularly important in RAS, especially during instrument exchanges, when failure in communication could lead to ‘inadvertent adjustment, movement and complete removal of an instrument that is in use’. 164 However, it has also been argued that less co-ordination and communication may be required between the surgeon and the first assistant because the surgeon controls the camera and, if four robotic arms are used, can do more of the retraction (instead of this being done by the first assistant). 157,187
Strategies for overcoming the communication challenges focused on the use of standardised communication. The use of ‘readback’, in which team members repeat back instructions in a precise, clear, standardised manner, has been advocated, particularly for instrument exchanges and other key transition points. 154 This allows the surgeon to check that his or her instructions have been heard correctly (remembering that, being in the console, they are unable to draw on visual cues to determine this) and, if not, to correct any misunderstandings before they result in actions that could have negative consequences for the patient. The use of agreed terms has been recommended for RAS. 164 The use of anatomic or OT references by the surgeon, rather than directional cues, has been recommended while moving the patient or robot during docking, again to reduce the risk of misunderstandings. 164
The impact of robot-assisted surgery on decision-making
There were competing theories in the literature concerning the impact of RAS on decision-making. Some surgeons reported a ‘tendency for surgeons to bury themselves in the console’, with surgeons ‘block[ing] out the operating room’. 154 This suggests a reduction in the surgeon’s situational awareness. Some surgeons recommended positioning the console so that the surgeon has a clear view of the patient and can immediately see the patient when looking up from the console. 164
There may be beneficial impacts of RAS on surgeon decision-making. It has been argued that, immersed in the console, the surgeon’s ‘distractibility’ is reduced, which could potentially have a positive impact on patient outcomes. 188 This is supported by recent research that reveals that the number of intraoperative interruptions is significantly associated with surgeons’ experienced distraction, and interruptions in the form of case-irrelevant communication in particular are linked to increased surgeon distraction. 189 Others suggest that the 3D image creates a sense of immersion, which presumably contributes to reduced distractibility. 190
Robot-assisted surgery may also impact on surgeon decision-making by reducing the surgeon’s level of stress. Some surgeons have argued that stress arising from a difficult operation may lead a surgeon to decide to convert from laparoscopic surgery to open surgery. 171 By removing the awkward and unnatural movements required during laparoscopy191 and enabling the surgeon to sit comfortably at the console,148 RAS reduces physical discomfort. 192 This leads to the theory that, owing to the ergonomic benefits of RAS, surgeon stress is reduced, which may influence the decision of whether to convert, potentially resulting in a lower rate of conversion. 171
Robot-assisted surgery also impacts on surgeon decision-making by changing the ability of the surgeon to use tactile perception to determine anatomic information. This is considered to be a major limitation of RAS. 193 A significant contextual factor here is the surgeon’s experience, with surgeons finding visual information sufficient for informing their decision-making as their experience of RAS increases. 172
Summary
Our review revealed the series of decisions made in the process of introducing RAS and the series of challenges that need to be overcome if RAS is to be successfully integrated into practice. OT teams have responded by developing creative solutions to mitigate, address or work around the challenges of RAS. Many of the quantitative studies of RAS included in the review were small case series (descriptive non-randomised studies) undertaken within a single institution and thus our review adds to the existing literature by drawing together and finding patterns in the experiences of multiple OT teams.
Table 6 summarises as CMO configurations the theories at the end of the review (to enable the reader to track how the theories develop over the course of the study, the theories are labelled and numbered, with I for theories relating to implementation, T for theories relating to communication and teamwork, and D for theories relating to decision-making). Following Dalkin et al. ,55 we separate out resources and reasoning within the mechanism, drawing attention to the particular resources provided or taken away by RAS.
| Theory | Context | + | Mechanism | = | Outcome | |
|---|---|---|---|---|---|---|
| Resource | Response | |||||
| I1. Organisational support | Hospital administration and nursing management involved in decision to introduce RAS | + | Potential benefits of RAS | Hospital administration and nursing management perceive RAS as assisting in achieving organisation’s goals so invest resources | = | RAS more likely to be integrated into practice |
| I2. Team involvement | Team involved in decision to introduce RAS | + | Potential benefits of RAS | Team more motivated to work together to overcome initial challenges | = | RAS more likely to be integrated into practice |
| I3. Dedicated team | Motivated and stable team High number of frequent robot-assisted operations Support of hospital administration and nursing management |
+ | Dedicated robotic team | Team sees operations as opportunity to learn and more quickly become familiar and confident with equipment and tasks | = | Reduced set-up time |
| I4. Dedicated OT | High number of frequent robot-assisted operations Support of hospital administration and nursing management Availability of suitably sized OT |
+ | Dedicated robotic OT | Team does not need to move robot from/to another location before/after operation | = | Reduced set-up time Quicker turnover to next case |
| T1. Co-ordination | Context unclear | + | Surgeon’s position within console | Team less aware of surgeon’s actions, making it more difficult to co-ordinate their actions | = | Increased operation duration |
| T2. Training | Context unclear | + | Surgeon and trainee have different view of surgical site | Harder for surgeon to explain what is happening and to monitor trainee’s understanding | = | Trainee learns less than in other forms of surgery |
| D1. Situational awareness | Team experienced in RAS | + | Surgeon’s position within console | Team understand surgeon’s awareness dependent on them orally communicating information so respond by orally communicating more information about the patient state | = | Surgeon’s situational awareness is maintained |
| D2. Lack of tactile information | Surgeon inexperienced in RAS | + | Lack of tactile information | Surgeon progresses more slowly | = | Increased operation duration |
| D3. Immersion | Surgeon remains in console | + | Sense of immersion | Surgeon is more focused on the task at hand and is less easily distracted | = | Improved decision-making and patient outcomes |
| D4. Impact of ergonomics | Context unclear | + | Ergonomic console | Surgeon is less stressed and tired | = | Improved decision-making Reduced conversion to open surgery |
In relation to implementation, the review introduced a new theory regarding organisational support. However, with the majority of studies and reviews being authored by surgeons, what was not clear was the extent to which the broader OT team was engaged in the process of implementation and was encouraged to identify benefits of the technology, despite the motivation of the team appearing to be an important contextual factor in strategies designed to support the integration of RAS. Therefore, this candidate theory was taken forward to the interviews for further exploration and refinement.
Theories I3 and I4 describe strategies for overcoming the challenge associated with RAS of increased operation duration, so we present these strategies as the resource. What is clear from considering these theories is that, although discussion of context in realist evaluation is typically concerned with the contexts that determine whether or not a strategy is effective, there are contextual factors that determine if introducing the strategies is feasible. This includes the support of hospital administration and nursing management, to agree to the creation of a dedicated robotic OT and to facilitate the scheduling of rotas to enable a robotic team to develop. Also important are the financial and material resources of the hospital; the feasibility of having a dedicated OT depends on the availability of OTs and the frequency of robot-assisted operations.
A topic relating to implementation raised by the review, but on which there was not enough information contained in the papers retrieved by our search strategies to develop a theory, was that of training in RAS needed by the OT team. Given time constraints, rather than returning to the literature to carry out further searching, we took this forward as an idea to be explored in the interviews.
The review identified less literature concerned with the impact of RAS on communication and decision-making in the OT. However, the findings of the review suggest that RAS can hinder communication. This is due to the physical separation of the surgeon from the rest of the team, which makes it harder for the team to hear the surgeon’s requests. What was not clear were the consequences of this. Therefore, rather than having a specific theory to take forward for refinement in the interviews, we took this forward as an idea for exploration in the interviews, drawing on the substantive theories introduced in Chapter 3 relating to communication and teamwork to provide prompts. Similarly, the findings of the review in relation to co-ordination between the surgeon and the first assistant – whether more or less co-ordination was required – could not be clearly articulated as a realist theory, owing to the absence of information about the contexts in which, and the mechanisms through which, co-ordination would be achieved, so again this was taken forward as a topic for exploration in the interviews. Having not identified information within the review to support their refinement, the candidate theories ‘co-ordination’ and ‘training’ were taken forward to the interviews as they were. Formatting these theories as CMO configurations highlighted gaps in our understanding regarding the contexts in which these challenges would be experienced, gaps that we sought to fill in the interviews.
In relation to decision-making, we started the review with one candidate theory, ‘situational awareness’. The findings of the review supported this theory, but also highlighted variation in the extent to which surgeons remain within the console, which would impact the need for the team to orally communicate information. The review also introduced a number of new theories regarding the impact of RAS on decision-making. What was not clear from the review were the contexts in which the ergonomics of the console could be expected to trigger a decrease in stress and tiredness, a gap in our understanding that we sought to explore during the interviews.
Chapter 5 Refinement of theories
Overview
This chapter presents the second part of phase 2 of our study, in which we presented our theories to a sample of 44 clinicians with a range of roles in RAS teams in nine sites, through semistructured interviews. The goal was to draw on our interviewees’ experience to both refine our literature-based theories and glean new theories.
Into what contexts was robot-assisted surgery introduced, and how?
We began our interviews by exploring how hospitals had acquired da Vinci robots. Table 7 summarises key findings regarding the process through which sites acquired a robot. In most hospitals, the introduction was led by the urology surgeons. In two sites, the chief executive of the hospital initiated the process. In four of the sites funding came from the hospital’s local charity, and in four other sites funding came from the hospitals’ own funds.
| Site | Type of hospital | Funding | Who led introduction of robot into the hospital | How long robot had been in use by colorectal team at time of interview (how long hospital had had robot) in years | Training |
|---|---|---|---|---|---|
| 1 | Teaching | Hospital charity | A multidisciplinary decision involving urology, paediatric and colorectal surgeons | 4 (11) | The urology team went on a training course together; the colorectal team did not and had to ‘learn on the job’. They received training from an Intuitive Surgical representative and advice from their urology colleagues. Two colorectal surgeons were formally trained and another was trained in-house |
| 2 | District general | Hospital charity | Urology surgeons | No information | Colorectal surgeons went on a formal training course and were mentored by a surgeon from site 1. No information about the rest of the team |
| 3 | District general | Hospital funds | The chief executive | 3 (6) | The team attended a formal training course together and were supported by an Intuitive Surgical representative who attended the first few cases |
| 4 | Teaching | Hospital funds | The chief executive, urology and colorectal surgeons | 1 (1) | The team attended a formal training course together. They hold teaching sessions after work |
| 5 | District general | Hospital charity | Urology and colorectal surgeons | 4 (4) | The team attended a formal training course together and were supported by a company representative. Knowledge has been cascaded down to other staff and a competence package has been created |
| 6 | Cancer centre | Hospital charity | Urology surgeons (one in particular) | 2 (8) | Some of the team attended a formal training course together. Others have learned from their colleagues and an ‘education kit’ has been developed for new starters |
| 7 | District general | Hospital funds | Urology surgeons (one in particular) | 3 (5) | The team have mainly learned ‘in-house’ but have attended workshops together and received a lot of support from the Intuitive Surgical representative. He attended every operation initially |
| 8 | Teaching | Hospital and university funds | Urology, lower GI, gynaecology and colorectal surgeons | 1 (4) | The team attended a formal training course together |
| 9 | District general | No information | No information | 2 (5) | The team were not trained together. The Intuitive Surgical representative provided a lot of training support |
The training received by colorectal teams varied across sites. There was also variation within sites, depending on role and at what point in time the OT personnel joined the hospital. Most, but not all, surgeons reported that they had attended the training provided by Intuitive Surgical. Attitudes varied about the value of this training. For example, one surgeon commented that it was ‘probably enough’ to be able to begin using the robot in practice, whereas another surgeon (from the same trust) said the training was not ‘earth shattering’ and he felt that his main training had come from his colleague, a leader in the field of RAS. One surgeon reported having ‘some experience’ with the robot as a result of a fellowship he had undertaken abroad. However, he stated that one has to use the robot and ‘see how things go’. He explained that he applied the same principles in training to use the robot as he did to his training in laparoscopic surgery: (1) observe cases, (2) assist in the operation and (3) use a preceptor for the first couple of cases. Similar approaches were reported by other surgeons, having visiting surgeons experienced in RAS observe their cases and, following this, the experienced surgeon observing and providing guidance though the first robot-assisted cases.
Approaches to training for the OT team varied significantly between sites. For example, at several sites, selected members of the OT team attended the Intuitive Surgical training with the surgeons, and then had responsibility for cascading that training down to other members of the team. At site 6, there was a structured process for developing the skills of team members who did not attend the Intuitive Surgical training. The team leader would try to allocate one experienced member of staff and two learners, a ‘medium learner’ and a ‘new learner’, to each robot-assisted case. They also tried to ensure that staff new to RAS first worked as a circulating practitioner so that they could watch the scrub practitioner. After this, the team leader would ‘double scrub’ with them for two or three cases and they would work alone only once the team leader had signed them off on a competencies list that had been developed in-house for supporting the training of team members. In contrast, at site 1, the colorectal OT team felt that their training had been inadequate. The urology OT team at this site had attended the training provided by Intuitive Surgical, but the colorectal team was not provided with the same opportunity, saying that they were ‘thrown in at the deep end.’ Some stated that they ‘didn’t have a clue’ what the robot was when it first appeared on their list. They learnt how to use the robot on the day it was first implemented in colorectal surgery. Subsequent support (a few months later) was provided by an Intuitive Surgical representative, but they felt that this came too late. One respondent commented that they had received 2 training hours, which they took little benefit from. It was felt that more support is needed for new staff who will use the robot and that everyone should receive more formal training. Further to this, the team described difficulties with training new staff, as the surgeons like to have experienced practitioners assisting them.
In addition to discussing what training participants had received, we discussed with them what they felt they needed to learn in order to be competent with RAS. Among the OT team, the robot was perceived as ‘such a technical piece of equipment’ that training is required before it can be used. Key pieces of information participants described needing to know were (1) how to prepare for a robot-assisted operation, in terms of setting up the robot and the instruments required; (2) how to dock and undock the robot, including how to manoeuvre the robot into position; (3) how to insert instruments into the robotic arms and how to remove instruments; and (4) how to troubleshoot the robot. Undocking the robot in an emergency situation was described as a huge safety concern; participants felt that this should form part of any training programme and that it would be useful for OT teams to practise this.
Some participants discussed that the whole team should be trained and that this training should be provided or at least facilitated by Intuitive Surgical, as they are the ‘experts’. Those participants who had undertaken training as a team suggested that the important aspect of training was not just about gaining experience of using the robot. Rather, receiving training as a team enabled them to develop trusting relationships with each other, which in turn allowed them to work together as a team to solve problems arising from the implementation of the new technology. For example, teams that had undertaken training together in an Intuitive Surgical training centre said it was ‘inspiring’ and had a ‘bonding’ effect:
[During training together] we learned to trust each other. We came back from Strasbourg with that certain knowledge that between us we knew we would each remember something and we would be able to pull it [RAS] off . . . we seemed to develop a special bond.
Site 5, nurse
The underlying theory seems to be that team training works to support the integration of RAS into practice by establishing trust among the team, giving the surgeon increased confidence in the team’s ability to support him or her during robot-assisted operations. They were able to discuss the resolution of problems together, something that they felt would have been impossible previously. A further benefit of training the team together was the insight it gave team members into the impact of the robot on others’ roles.
Our interviews also identified that team members’ interest in and enthusiasm for RAS was enhanced when they were handpicked to take part in this training. This occurred in four sites; OT personnel were handpicked by the surgeons and/or nursing management to undertake RAS training abroad. These staff were selected for their potential to embrace the new technology and for their ability to train other staff on their return. This was seen as a great privilege, and it motivated staff to overcome the challenges involved:
It was a huge privilege to be invited . . . we’re having this new equipment and this new concept of working and we’re going to be the first people to actually really get trained properly . . . and then we would come back and be able to show all of the others how to do that.
Site 5, nurse
This suggests that team members who were handpicked saw themselves as ‘trail blazers’ who could come back and cascade their expertise to other members of the team. The underlying theory seems to be that when teams are handpicked, this creates a sense of privilege that provides staff with the motivation to overcome the challenges of RAS, increasing the likelihood of RAS becoming embedded into routine practice. However, one participant reported that handpicking staff could have negative consequences, as others resented being overlooked and consequently were not motivated to work with the robot:
The staff that didn’t go and do that training are resentful of [working with] the robot because they don’t feel that they were validated enough to go and do the training abroad so why should they do the work when it’s here.
Site 5, nurse
Participants also highlighted ongoing support from the manufacturer as important in determining their ability to manage the challenges of using the robot following training. Their experience of this ongoing support was variable. Several participants felt that they did not receive adequate support or communication from the manufacturer and that the company seemed uninterested. Some reported that support was good initially but faded away over time, leaving new staff in difficulty:
I think the company are good for establishing it [the robot], embedding it, they are good at the initial phases, and they do encourage you to use it. But compared to other companies that we’ve dealt with . . . there is a lack of support . . . afterwards.
Site 5, surgeon
Others, however, were more satisfied with the support given. One hospital was particularly happy; the team reported that they were shown how to set up and troubleshoot the robot, the surgeons had proctors provided by the company, and they felt that support was available if it was needed:
I think we’ve been lucky with the rep. He seems to have quite a high profile here and I know he’s fully accessible when, you know, the staff are needing support. I see him in the department quite a lot.
Site 9, nurse
Regardless of whether or not support had been experienced, the underlying theory seems to be that RAS is more likely to become embedded into routine practice if manufacturer support is provided, as this gives OT teams confidence that there will be help to overcome problems they might experience when first putting their training into practice.
Gleaning and refining theories about the implementation of robot-assisted surgery
Having gathered information on how RAS was introduced and elicited some new theories in the process, we went on to discuss with participants the theories relating to implementation of RAS that had emerged in our review of the literature. Our review identified a number of ideas regarding what supported and constrained the introduction of RAS, the challenges the introduction of RAS created and how teams addressed these. Key challenges included that acquiring a robot is expensive, that they are bulky to manoeuvre and that they can extend operation duration while teams learn how to set up, dock and work with the robot. Increased duration of operations has potential knock-on effects on the scheduling of operations, for example scheduling fewer operations or cancelling operations. This, in turn, leads to increased waiting times for cancer treatment, an indicator NHS trusts are performance managed on and that, if breached, can lead to fines. Furthermore, in an era of patient choice, higher waiting times could, in theory, lead patients to choose other providers for their treatment. The strategies we identified centred on ways in which the extended duration of the operations using RAS could be reduced, on the assumption that this would support the introduction of RAS within the trust, presumably by mitigating the potential impact on waiting times and thus on trust income. Below we outline our interviewees’ views of these ideas.
Organisational support
One of the theories identified from the review was that, by being engaged in the process of imagining potential future benefits of RAS, the hospital administration and nursing management will perceive RAS as an innovation that can assist in achieving the organisation’s goals and so will be willing to invest the necessary resources to assist its integration into routine practice. Our participants emphasised the vital role of support of the hospital administration in enabling the acquisition of a robot, perceiving the introduction of RAS as impossible without it:
It was paramount that we had the administrators behind the decision and the way things are happening with the NHS at the moment it’s very difficult to introduce anything through a bottom-up scenario and going through your divisional manager or clinical director and then pushing from that point of view. It really has to come from the top, from the board-level administration down.
Site 4, surgeon
Emphasis was placed on financial support, because of the initial cost of the robot and the ongoing costs of maintenance and disposable items:
[Support of the hospital administration] is important because . . . you can’t function in isolation. Obviously it’s expensive . . . so it needs the full input of everybody, and . . . you need the hospital on board with that, and there’s ongoing contracts and warranties.
Site 1, surgeon
It needs a lot of support as in financial support, that is the support that the robot needs. Because everything we use on the robot is expensive and each item that we use is . . . well I say each item, the majority of items that we use are disposable, they only have 10 uses and it won’t let you use it more than 10 times.
Site 1, ODP
It was also felt that the support of the hospital administration was important because of the possible negative consequences of RAS, in terms of the longer operation duration and the impact that this could have, something surgeons in particular were aware of. Consequently, surgeons would not accept responsibility for the implementation of RAS without support from the hospital administration.
Our participants also provided insight into some of the ways in which this support was achieved. Creating a shared vision in some cases literally meant giving the hospital administration the opportunity to see the robot in action:
We had two very important people come and watch an operation and one of the directors of finance and one of the, I can’t remember quite what their titles were, but they came in and one of our surgeons invited them in to come and have a look at the robot. They came and watched a full case and I talked to them afterwards and they said it was very, very informative to actually see what goes on compared to what they hear. And to actually see it they realised how impressive it was and also the benefits to the patient [. . .] I got a lovely e-mail off both of them saying it was very informative and [. . .] when they can go to the board of management [. . .] they can then have a better idea of what they’re talking about to promote robotic surgery.
Site 4, ODP
Whereas this quotation emphasises the perception of patient benefits, other participants emphasised the hospital administration’s awareness of competition, which could outweigh concerns about cost:
Even the chief executive I think has a very sensible approach to it and says, quite frankly, it is expensive, it doesn’t pay for itself, but he sees it as cutting edge, I think as a kudos thing, probably, although he hasn’t quite said that, but I think reading between the lines it’s a kudos thing. And I think he bought robot number 2, I think on the basis that a neighbouring trust was talking buying a robot and he felt that some of our specialities weren’t able to progress because of access to the robot. So he wants to facilitate us being there, you know, in the [region] leading as it were.
Site 4, surgeon
I think the fact that we were the first in this part of the country to have it. I sort of got the impression that there was a little bit of a race on between whether [nearby trust] would have it or [nearby trust] would have it, or we would have it, and just the little snippets that I gleaned from the consultants, you know, that it was clear that it was going to be us. It was considered a very prestigious move, so yes it was considered, you know, to be such a futuristic addition to our theatres that it was very exciting. Everybody was curious.
Site 5, nurse
Functioning in an increasingly competitive situation, RAS was perceived as a mark of prestige or kudos for the trust and enabled it to be viewed as providing cutting-edge services, which in turn enhanced the likelihood that such services would be retained, particularly prostatectomies.
Rather than nursing management, our participants talked about the importance of team leaders in supporting the introduction of RAS, a role taken on by experienced theatre nurses and ODPs. A supportive team leader could facilitate integration of the robot by:
-
gaining access to training for the whole team, which contributed to confidence in using the equipment and to safety
-
co-ordinating the staff rota to ensure that the right skill mix was available to carry out robot-assisted operations
-
ensuring the availability of the robot across specialties in order to co-ordinate use of the robot across the trust
-
managing OT schedules to allow, at least initially, for longer set-up times and for the availability of an OT suitable to accommodate the equipment and personnel safely, without risk of desterilisation or compromising access to the patient.
Although in most sites participants felt that there had been good team leader support, the importance of team leader support was most apparent in those accounts of participants who felt that it had been lacking:
It would have lightened the workload off me and the burden on me to fight all the time to get cases done. etc. . . . Theatre time, theatre space, flexibility when you’re introducing a new technology.
Site 2, surgeon
Finally, support from surgical colleagues was perceived as important in accepting the impact of RAS on operation duration and subsequent impacts on waiting lists. That RAS was more likely to become embedded in practice if surgical colleagues were supportive was not one of our original ideas. Rather, in one of the early interviews, a surgeon stated that he felt it was difficult for RAS to become embedded in the NHS because of what he perceived as a focus on surgeon-specific outcomes. An individual surgeon’s outcomes may worsen during the time they are learning RAS, putting them under increased scrutiny, which may deter some surgeons from adopting RAS:
Everyone’s looking to knock you down, they want the first robotic death, you know, to pooh-pooh the robot. No one looks at it with a positive thing and there’s a real lack of support. So you’re always looking over your shoulder [. . .] the scrutiny as a surgeon is just ridiculous. So you put yourself under more scrutiny and you think, well why would I possibly want to do this, you’ve got to be mad.
Site 2, surgeon
When we asked other surgeons about this in subsequent interviews, they indicated that support from colleagues could mitigate many of the potential threats of RAS on performance indicators (e.g. on extending waiting lists). However, lack of support from colleagues and jealousy from others could limit the use of RAS. As one participant explained:
You need the absolute support of your [surgical] colleagues . . . First of all if you’re going to start spending 10, all day lists on your first 10 cancers then your waiting list increases or the pressure on others increases. If there’s any murmuring from the background . . . you will start to avoid doing this [RAS]. Secondly if colleagues hate the idea of others learning a skill or getting a reputation which they don’t have yet, they could scupper this happening. I’ve been lucky that those things don’t count here and that’s one of the reasons why I can progress. When I speak to colleagues they cite one or all of those, say they not actually allowed to progress.
Site 7, surgeon
This suggests that the introduction of RAS could potentially increase surgical waiting times, because a longer operation duration leads to fewer operations being scheduled or to operations being cancelled. Surgical waiting times for cancer treatment are the focus of government targets; increases in waiting times could attract fines for the trust and possibly lead to patients choosing to have surgery in other trusts and thus to a loss of income for the trust. In this context, a lack of support from surgical colleagues could tip the balance and add to existing constraints to the use of RAS, which in turn could mean that RAS is avoided in order to reduce these risks.
Team involvement
A candidate theory we began the study with was that if the whole OT team can feel that the advantages of RAS outweigh its disadvantages and are involved in the decision to introduce it, they will be more motivated to work together to develop solutions to problems that may arise when using it. The OT team had not been involved in the decision to introduce RAS in any of the nine sites. However, it did not seem that involvement in the decision was essential for the OT team to have the motivation to persist with RAS. In most sites, there was a positive attitude among the OT team towards RAS. For example, one nurse noted that, for them, there was a sense of pride as the robot added ‘another string to their bow’. This view was echoed by a nurse at another site, who described the robot as a ‘good opportunity’ with regard to their CV and professional development, particularly because not all trusts have a robot.
However, attitudes at one site, site 1, were notably different. Whereas the OT team members at the other sites appeared accepting of the fact that they were not involved in the decision, one nurse at site 1 expressed disappointment about this:
I think it’s a nice piece of equipment and I would love to have been asked to be involved in making that decision, not just it being given to me, or handed to me. Because for anyone, it would be nice to have somebody to say, yes I would like to have involvement in that, it seems to be interesting to me, because that would mean they’re curious and they will have that . . . they will be driven to learn more than if they had just been told. They can learn it more intimately than someone who has been given the job. It’s something that the person made the decision to actually get involved with the robot procedures.
Site 1, nurse
What this quotation seems to highlight is a perceived lack of control over aspects of their work; the decision they wanted to be involved in was not the one about whether or not to purchase a robot, but the one about extending the use of that robot to colorectal surgery. An ODP at the same site expressed similar sentiments and felt that having greater staff involvement in the decision would have positively impacted staff engagement:
That element of communication and knowing and agreeing that this is what we’re going to do from the start and this is how we’re going to implement certain areas, and this is what you need, and these are the dangers and these are the benefits, and things like that. I think it’s really important that the team know. And it will make them work better together, you know, you feel more comfortable if you know the bigger picture as opposed to little bits thrown in.
Site 1, ODP
Although theatre nurses and ODPs at this site expressed an appreciation of the potential benefits of RAS for the patient, attitudes to the use of it within their trust were generally negative, and this appeared to be related to a lack of confidence in undertaking the tasks associated with RAS. It was suggested the robot was not very popular because the team were not provided with an opportunity to learn how to use it:
We were actually kind of upset when we were told we were doing it because where was the training. We were all questioning, well I’m not trained, I wasn’t particularly happy with that because I wasn’t trained. I don’t know I’ll be safe, or my patient won’t be safe when I started to do it.
Site 1, nurse
Thus, it seems that motivation to persist with RAS can be achieved without involvement in the initial decision to purchase a robot, but what is essential is training that enables team members to feel confident as they take on the new tasks.
Dedicated team
Participants reported that, in many cases, the people who trained together became a dedicated robotic team, at least initially. Our theory from the literature review was that, by working through all robot-assisted cases, a dedicated team more quickly becomes familiar and confident with the equipment and tasks associated with setting up the robot, allowing members to complete the necessary tasks more quickly, reducing set-up time. Certainly, participants perceived that having a dedicated team could reduce operation duration:
When we had a dedicated team of people who could manoeuvre the robot and position patients . . . to start with you do need a core knowledge . . . it definitely did reduce the time having the same skill set.
Site 4, surgeon
Within most teams, however, it was not always possible to maintain a dedicated team owing to staff changes, holidays and sickness. The tendency for theatre nurses and ODPs to work only within one or two specialties also made a dedicated team hard to achieve, especially where there was a low volume of robot-assisted cases. When a dedicated team was not feasible, a larger pool of people, trained by the experienced staff, was established:
Within our team we have four out of seven people who know how to use the robot . . . So ideally if three or four of those people were at work on any given day and could be put in the same theatre where the robot was, then that would be the ideal. But at worst we should have at least one or two people at work on that day who could be moved in order to do the robot case. But the aim is that over a period of time that everybody within our team should be able to use the robot more efficiently.
Site 1, ODP
At some sites as many as 50% of the staff had been trained, and, at one site, which carried out a large volume of robot-assisted cases, all of the staff could manage the cases.
Dedicated operating theatre
A theory from the review was that having a dedicated OT helps RAS to become embedded in practice because the team does not have to move the robot, and this reduces set-up time and speeds turnover to the next case. Participants agreed with this and, although only three sites had a dedicated OT, participants felt that a dedicated OT would be the ideal situation, to avoid having to move the robot:
I think the biggest issue with it is it’s, from my point of view, it needs to have a specific designated theatre because it’s the time it takes to move it from storage to the theatre it’s going to be used in, what speciality it’s going to be used in and the complexities associated with that.
Site 9, nurse
When there was no dedicated OT, team leaders were perceived to play a vital role in ensuring that a suitably sized OT was available. In fact, an adequately large OT appeared to be a greater concern than necessarily having a dedicated OT. Participants felt that a suitably sized OT would make RAS more efficient because a cramped working environment meant that staff struggled to move around quickly and safely. It could also cause them to break the sterile field:
We always worry about doors opening and bashing the robot . . . or we get desterilised because we’re prepping near the door that leads to the corridor.
Site 1, nurse
The desterilisation of equipment has implications for patient safety and it is then necessary to replace or redrape the equipment, potentially extending the operation duration, with the concomitant risks of having to cancel or reschedule operations planned for later that day. However, OT teams at some sites had developed strategies for reducing the risk of desterilisation:
What we do is we cover the robot and the robot’s arms with sterile drapes, so if anybody pushes against them we don’t desterilise. So we take measures to minimise the chance of contamination of the sterility of the robotic arms anyway.
Site 4, ODP
Gleaning and refining theories about communication and teamwork
The findings from the literature review suggested that communication might be constrained by RAS because of the physical separation of the surgeon and OT team. The interviews were used to expand on these findings by exploring participants’ experiences of how, why and in what circumstances RAS impacts communication and teamwork.
Communication
Some participants suggested that RAS reduced some challenges to communication experienced during laparoscopic surgery. For example, ‘mumbling’ from the surgeon is reduced because they do not wear a surgical mask when seated at the console. However, most participants agreed that RAS resulted in loss of both oral and non-verbal communication, compared with laparoscopic surgery. A theatre nurse explained that during a laparoscopic case:
We’re so close to each other that when you say something, most of the time I would hear it or I would even know what he [the surgeon] intends to do next because I can see it from his movements, his mannerisms.
Site 1, nurse
During laparoscopic surgery, the proximity of the surgeon enables the scrub practitioner and first assistant to hear the surgeon’s requests. However, as suggested, the surgeon’s ‘movements’ and ‘mannerisms’ also convey the message.
Constraints on the use of non-verbal cues were also noted by surgeons, who described that ‘no one notices if they are struggling’ when they are positioned at the console. Non-verbal cues appeared to enable team members to gauge the progress of the operation. These appeared to be particularly important when the operation was at a difficult stage or was not progressing to plan, signalling the need for support from other team members who could step in without the need to be told to do so. However, in RAS, surgeons had to verbalise their concerns to team members, as they could not rely on team members to pick up on their non-verbal cues of stress.
The constraints on the use of non-verbal cues emphasised the importance of oral communication during RAS, but participants discussed how this could be compromised. Although during RAS a microphone is fitted to the console to amplify the surgeon’s voice, team members reported that it was still difficult to hear the surgeon because this microphone did not always work well:
In a robot case, because they [the surgeons] are somewhere in the corner and we hear them through a speaker and sometimes that doesn’t even work very well, and we always have to ask or repeat what he said just to be absolutely sure that whatever we’re going to do is the right thing.
Site 1, nurse
This results in the repetition of instructions from the surgeon and more frequent requests for the surgeon to repeat their instruction. This idea was used to inform a tentative theory that was presented to participants, as follows:
The physical separation [the surgeon from the team] means that it can be difficult for the team to hear what the surgeon says, resulting in increased repetition of instructions, requests to repeat information, reduced co-ordination and longer operation duration.
Subsequent findings reinforced the relevance of this theory. In discussing this theory, participants described strategies (implicit or explicit) that they have used to overcome the separation. Although the literature review suggested that the team might use a number of strategies to overcome the communication challenges of RAS, including the use of standardised communication strategies such as ‘readback’, we did not initially ask participants about the use of such strategies, and so it was interesting to see the similarities in their accounts:
I would always say, scissors coming out, Maryland [dissection instrument] going in, whatever, so they [the surgeon] always knew loud and clear that we’ve [the nurses] not only heard them but we’re actually doing it.
Site 5, nurse
This strategy was perceived to improve information transfer and avoid complications that could extend operation duration.
When presented with the tentative theory, one surgeon commented:
I think that’s possibly true [that it is more difficult to hear the surgeon during RAS], but I think working with two surgeons together makes it a lot easier in that one surgeon can be at the table and the other at the console, and they can communicate very well with each other and then to the theatre staff.
Site 4, surgeon
This quotation reveals another strategy: the use of two surgeons to conduct the operation. At the majority of sites, consultant surgeons initially worked in pairs as they became familiar with the robot. Thus, this strategy seemed to be used more frequently when teams were at the earlier stages of gaining experience in the use of RAS. The participant describes how this strategy enabled effective communication, with one surgeon making ‘many trips’ between the table and the console. The use of two surgeons increases the cost of the intervention, but ensured that the scrub practitioner and the first assistant understood the information communicated by the operating surgeon, again avoiding complications that might extend operation duration.
In an extension of our tentative theory, interviewees suggested that the lack of non-verbal communication and visual contact between the team and the surgeon also resulted in confusion about to whom surgeons’ requests were directed. As one surgeon explained:
If you say, suction and suck the smoke [smoke is sometimes generated when the diathermy is used to cut and cauterise], the nurse might look at the assistant say, who is he talking to? So I think you need clear instructions, who should be doing what. I think that’s probably a skill on its own, sort of to say things a bit more clearly and then in a sort of crisp concise manner I think.
Site 6, surgeon
To counteract this problem, the surgeon explicitly announces to whom he or she is talking and provides ‘clear’ instruction. We refer to this strategy as ‘explicit communication’.
Engagement
As part of the discussion of how and why RAS impacted on communication and teamwork, participants described changes to their duties and responsibilities:
The robot does a lot of the work that the scrub nurse used to do of handing instruments . . . the exchange of instruments is massively reduced because of the dexterity of the instruments we use and the type of instruments we use. And the surgical assistant would . . . you know, if he needs a suture then they just hand it to the surgical assistant and he will put it in and take it out. So it’s basically just the odd handing of instruments.
Site 4, ODP
Consequently, some participants described RAS as ‘monotonous’ and said that ‘there is a lot of standing around’. One surgeon provided some insight into the repercussions of this change:
I’m the only one working and because I’m the only one working, everyone else’s attention gets distracted, including the assistant and they start chitting chatting away and then occasionally I’ll be sitting there with a tied suture waiting for them to cut. I look over and they’re chatting away.
Site 7, surgeon
A preliminary analysis of these accounts was used to develop a new proposition that encapsulated the impact of the reconfigured roles and responsibilities produced by the use of RAS. Owing to this reconfiguration, some team members experienced a reduction in their task load, which might reduce their engagement in the procedure and mean that they stop monitoring the screen and may engage in more case-irrelevant communication than they would during a laparoscopic operation. This affects co-ordination, either because the team do not anticipate what the surgeon wants or because they do not hear him when he asks for something, potentially increasing operation duration. Participants supported this idea.
Teaching and learning were associated with maintaining engagement:
Sometimes I’ve noticed the scrub staff are less engaged because they’re kind of sat back a little bit, [. . .] I suppose a lot of the surgical registrars are still learning, usually they seem totally engaged from my perspective.
Site 4, anaesthetist
A theatre nurse supported this view, reporting that learning had maintained their engagement when RAS was introduced at their trust, and that teaching others prevents boredom now that this is established in practice. However, without these aspects (learning or teaching) they are ‘falling asleep’ during robot-assisted procedures. One surgeon reported using a strategy to maintain engagement that incorporated a teaching element:
The way I deal with my assistant, I say, oh this is the plane you would cut so that they’re not looking away somewhere else chatting or something or someone, and then you say, well this is the normal sort of thing you see, this is the plane you would cut, so I explain the technique and then give them sort of tips on how they can do the operation when it comes to their turn.
Site 6, surgeon
The surgeon provided an educational commentary so that their first assistant would listen to the information and remain focused on the task at hand. This idea was discussed as a potential strategy with remaining participants, who explained that its success was dependent on the recipient:
If you’re interested in the operation yes, that’s [commentary] good, you would take attention and you would concentrate in an operation, if you had not, if the surgeon is still talking to me you wouldn’t be more interested.
Site 7, trainee
Some surgeons felt that they would not be able to provide a commentary during more challenging points of the procedure:
Personally I’m likely to clam up more probably at just the time when you need someone to help you because you’re concentrating more and you don’t want to distract yourself by describing what you’re doing because you’re too busy in yourself doing it.
Site 3, surgeon
Co-ordination
One of our candidate theories, which remained unrevised following the literature review, related to co-ordination between the surgeon and the team:
When the surgeon is separated from the rest of the OT team, the team is less aware of the surgeon’s actions, making it more difficult to co-ordinate their actions during the operation and so the operation takes longer.
Participants refuted this idea, stating that co-ordination is the same in RAS and laparoscopic surgery because in both types of operations the first assistant and the scrub practitioner watch the screen, so that they know what the surgeon is doing and can anticipate what he or she needs:
I don’t really know whether I go along with that [the proposition] because I think if all the theatre team are watching the [. . .] screens, they’re watching exactly what the surgeon does in the same way as they would if it was ordinary laparoscopic surgery.
Site 1, ODP
However, as we explored this idea further, it became clear that this depends on having a team who are familiar with the operation and who do watch the screen, which ties in with the issue of engagement described in the previous section. When this is present, there should be reduced oral communication and improved co-ordination.
Particularly important seemed to be the experience of the first assistant, as this surgeon describes:
If it’s a consultant assisting or very experienced trainee then they will do something, they’ll notice that there’s something they can do to help exposure and they will make the operation easier. And it’s quite difficult to communicate that to a junior or an inexperienced assistant.
Site 3, surgeon
Although in the review there were conflicting ideas about co-ordination between the first assistant and the surgeon, the surgeons we interviewed felt that they were more dependent on the first assistant in a robotic operation because of the physical separation. As one surgeon said, ‘You can’t just grab it [the instrument] yourself’. Surgeons also reported finding it harder to guide the first assistant, because of the communication challenges but also, again, because of the physical separation – ‘you can tell but not show’ – and therefore they felt that it was more important to have an experienced first assistant in a robot-assisted operation. For this reason, some sites were using experienced theatre nurses and ODPs who had done the necessary training as assistants, whereas previously surgical trainees would have assisted.
Training
Among our candidate theories, one was that it is harder for the surgeon to explain what is happening and monitor the trainee’s understanding because of the different views of the surgical site, resulting in the trainee not learning as much as they would in other forms of surgery. However, the findings presented above suggest that the reduced opportunities for learning are due not to the different views of the surgical site but to the reduced role of the first assistant in robot-assisted operations and the greater use of theatre nurses and ODPs as first assistants in place of surgical trainees.
The idea that RAS reduced opportunities for learning was described by the surgical trainees we interviewed:
You really get less opportunity; unless the surgeon lets you into the console you don’t get very much learning experience.
Site 1, trainee
When we discussed this with surgeons, some questioned the appropriateness of involving surgical trainees in robot-assisted operations, on the basis that use of RAS was not widespread enough, an attitude also expressed by some trainees; they were unlikely to need skills in RAS in their first consultant post. However, surgeons did try to find ways to increase the learning opportunities available for surgical trainees within robot-assisted operations. Some surgeons did their robot-assisted operations as hybrid operations, allowing the surgical trainee more involvement in the laparoscopic phase of the operation. As described above, some surgeons would provide an educational commentary, although both surgeons and trainees felt that the surgeon would be less likely to provide a commentary while undertaking a robot-assisted operation:
Because the surgeon is also really by himself concentrating on the operation so it is not as interactive as having each of you side to side or opposite each other.
Site 7, trainee
Gleaning and refining theories about decision-making
We concluded the interviews by exploring four theories about how RAS might influence decision-making within the surgical team, both in terms of individual team members’ decision-making and in terms of its impact on how information is propagated throughout the system.
Situational awareness
Our candidate theory suggested that, when the team is more experienced in RAS, they understand that the surgeon’s situational awareness is dependent on them orally communicating information, and so they respond by using more oral communication about the patient’s state, which in turn improves the surgeon’s situation awareness. The majority of surgeons perceived that their situational awareness is potentially reduced during RAS, stating that they are focused on a small area and therefore are less aware of their environment: they have ‘tunnel vision’. One surgeon provided the example of ‘sucking fluid’; the surgeon can request that their assistant provide suction, but they are unaware if the assistant experiences difficulties fulfilling their request or the reasons why:
Sometimes the assistants struggle to do something and when you’re with them you can see why they’re struggling because something is in the way or whatever. Whereas when you’re away from them in the box you say, can you just suck that fluid out there, and they can’t get the sucker to where you want them to go. And it’s like what’s the matter, what’s the matter? And they’re like, oh I’m . . . and you’re like, come on suck it through there. And it’s like, I can’t, I can’t get to it. And you know, you don’t know why because you’re not there and you can’t see the problem that they’re having.
Site 1, surgeon
Views about the seriousness of this reduced situational awareness varied. One surgeon described being ‘vastly less aware’ of what is going on in the OT but he had not been ‘hindered’ by this and did not think it made any difference to the operation. The strategy described above of two consultant surgeons working together was seen as a way to counteract the problem of reduced situational awareness, with one surgeon stating that he would be very concerned about reduced situational awareness if he operated without a second surgeon present:
Well that’s why with another consultant there who you trust, it’s like a wingman, like a co-driver. I wouldn’t do it without one. That’s why it’s more labour intensive. So in answer to your question, arguably if I was on my own I’d be very concerned about it.
Site 2, surgeon
Only two surgeons felt that their situational awareness was not reduced. One described that he continued to listen to the ‘banter’ among the team in the OT, while the other surgeon made a conscious effort to intermittently ask the team about the patient’s status.
Team members also perceived the surgeon’s situational awareness to be reduced due to their position in the console; for example, the surgeons do not have lateral vision and their sensory feedback, which can indicate problems, is reduced. The consequences of the surgeon’s reduced situational awareness, as described by the team, included the robotic arms impinging on each other, which could damage the robot or prevent the surgeon achieving their aim. Respondents explained the surgeon only realises the robot arms are clashing when he or she is unable to manoeuvre the instruments as desired. On one occasion the robot arms nearly collided with a patient’s head; this problem was averted by the theatre nurse, who intervened.
The overarching strategy described by the surgeons to increase situational awareness was to establish good communication links between the surgeon and the team:
We’re pretty keen that they tell us if they’re concerned. Sometimes when the arm . . . because we dock between the legs, and often one of the joints of the arm is quite close to the thigh, and so often what I will do is say to the nurses, if they think it’s going to close then to let me know and make sure they’re aware that we want to know if there’s an issue like that. And they’re all pretty good at saying that, because we make sure that they’re not nervous about talking or, you know, we don’t have a silent theatre, they can chat and we let them come and talk to us and tell us if they think there’s an issue.
Site 3, surgeon
Good communication was seen as an essential part of RAS. Trust between the surgeon and the team was also emphasised, as the surgeon has to rely on the rest of the team to communicate information outside their field of vision to avoid complications. If the surgeon trusts their team to communicate problems to them, their concern over their reduced situational awareness is lessened. One surgeon commented that a more experienced team might be better able to communicate the necessary information to them.
Communication was also described by the OT teams as the main strategy to increase situational awareness; they saw it as their responsibility to act as the ‘surgeon’s eyes and ears’. In contrast to our tentative theory, the information they described communicating to the surgeon was less about the patient state and more often about the robot, as in the examples described above. Some noted that they just ‘tell the surgeon’ when there are problems and that everyone in their team knows to do this whether it is them or the robot that is struggling. Others described that, because of the physical separation of the surgeon from the team, it is important that team members have voices ‘strong enough for the surgeon to hear’. Good communication was seen as dependent on the relationship between the surgeon and the team. Although this is dependent on individual personalities and approaches, the strategies of training together as a team and having a dedicated team described above were considered by interviewees to increase team members’ confidence to speak up and to transcend existing professional hierarchical boundaries.
Lack of tactile information
One of the theories emerging from the review was that surgeons progress more slowly through a robot-assisted operation because they do not have tactile information to inform their assessment of the situation and to determine whether to persist with or revise their course of action, although this effect becomes less pronounced as their experience with RAS increases. Certainly in the interviews, several surgeons described initial experiences with the robot where, owing to the absence of tactile information, they had not realised how much force they were applying and consequently had, for example, snapped a suture. However, none of the surgeons considered the lack of tactile information a significant problem. Although a couple of the surgeons described being ‘a bit more careful’, ‘a bit more hesitant’, the surgeons we interviewed did not consider that the lack of tactile information led to an increased operation duration. They felt that they had adapted quickly to relying on visual cues, learning to look, instead of feel, for tension. Several surgeons related this to their experience of laparoscopic surgery; with laparoscopic surgery, they had already learnt to work with reduced tactile information. As one surgeon described:
I don’t find that a great problem because you know what . . . from previous experience you know what you’re looking for, so you know the tension that you’re putting on the tissues from what you can actually see. So actually not having the haptic feedback I don’t find that a great problem providing the instruments are in view.
Site 3, surgeon
Interviewees contrasted this with the experience of urology surgeons, who had moved straight from doing open prostatectomies to doing them with robot assistance.
Immersion
Another theory related to decision-making that emerged from the review was that the sense of immersion the robot provides means that the surgeon is more focused, resulting in improved decision-making and patient outcomes. The majority of surgeons we interviewed agreed that the robot produces a sense of immersion. One surgeon described how they can ‘lose themselves’ during the operation, and, referring to the level of concentration, he described this feeling as ‘quite intense’. Other surgeons commented that it is not that the robot creates a sense of immersion but just that they have to concentrate more because they have less experience with RAS than with laparoscopic surgery:
I think the intensity is, your level of concentration because it’s less familiar than say laparoscopic surgery, your level of concentration has to be at a higher level for a longer time, whereas you could probably relax on occasion doing laparoscopic surgery.
Site 2, surgeon
Two surgeons refuted the idea that the robot produces a sense of immersion, commenting that they are immersed in the procedure regardless of whether it is laparoscopic or robot assisted and that technology should not determine whether or not the surgeon is immersed.
A number of theories were suggested by the participants about the contexts in which a sense of immersion occurs. One surgeon anticipated that, although he already experiences a sense of immersion when using the robot, the feeling will probably increase when the ‘mundane’ and routine tasks related to using the robot (e.g. port positioning) have been mastered. In contrast, another surgeon commented that he feels immersed using the console, particularly during complex cases, but that this feeling would probably lessen over time (i.e. it was a feature of his limited experience with the robot). One surgeon described immersion as being dependent on who he has assisting; for example, if he trusts the assistant he can be immersed, as the assistant fulfils requests with ‘silver service’, whereas otherwise he is ‘constantly looking’ as there is anxiety about where the assistant is ‘pointing the instrument’.
Some surgeons described the OT as quiet during RAS, enhancing their concentration, and that there are no distractions. By comparison, in open and laparoscopic cases, the surgeon can chat with the team.
Perceptions of the impact of the sense of immersion varied. Some respondents commented that heightened concentration might lead to better decision-making, but how or why was not articulated. One surgeon described the sense of immersion as making him more focused, which should enable a more precise dissection. Others felt unable to comment on whether or not immersion would be reflected in patient outcomes. One surgeon said that he felt the sense of immersion would not impact his decision-making, except that he may persevere longer with an operation because he is less aware of time. However, this could cause concern if the patient is operated on for an ‘excess amount of time’.
Impact of ergonomics
The final theory that came from the review was that the ergonomic benefits of the console mean that the surgeon is less stressed and tired, resulting in better decision-making and reduced conversion to open surgery. Some surgeons discussed that, for them, performing operations using the robot is more stressful than laparoscopic surgery because they are in the early stages of implementation (i.e. they have not used the robot on many occasions). In this context, the surgeons stated that it was sharing the operation with a colleague that reduced their levels of stress, as opposed to the ergonomics of the robot. Other surgeons felt that the robot was an improvement on laparoscopic surgery (ergonomically); how and why it was an improvement were not fully explored, although one surgeon described that they were in a ‘less awkward position’. The surgeons also discussed that using the robot might be physically less tiring than laparoscopic surgery, but it is mentally more so because they have less experience of RAS than laparoscopic surgery. For this reason, they have a higher level of concentration for a longer time using the robot; in comparison, they could relax on occasion during laparoscopic cases.
Two surgeons described how the level of stress is affected by the how the team acts; as one surgeon described it:
I think it probably makes you physically less tired. I think you’re probably mentally more tired [. . .] We’ve all done less robotics than we have laparoscopic, so you’re carrying more of a burden I think robotically. And sometimes you feel like you’re only the person in theatre that knows what’s going on. [. . .] Because you’re there, and you’re the only one there looking.
Site 1, surgeon
The extent to which the robot reduces surgeons’ stress levels was also described as dependent on the stage of the procedure. For example, talking about suturing, one surgeon said:
If I was doing that laparoscopically, it would be a nightmare. It’s just a joy to do it robotically because of the ergonomics.
Site 2, surgeon
In contrast, this surgeon described how with dissection his ‘fear’ of bleeding is increased, because the magnified image means that he notices tiny blood vessels he would not notice otherwise. It was also noted that stress can be dependent on the type of operation performed; for example, a low anterior resection is stressful using both approaches, whereas operations that do not go down to the pelvic floor are less stressful and demanding. Being able to take breaks when using the robot was noted as a benefit.
The extent to which the ergonomics of the robot impacted on decision-making, particularly the decision to convert to open surgery, was difficult to ascertain. It was suggested by some respondents that if the surgeon is more comfortable during surgery he or she might persevere with a difficult operation rather than convert to open surgery. It was also noted that the surgeon can ‘take five minutes’ to consider their decisions during RAS, whereas they might feel more pressure in decision-making during laparoscopic surgery. However, it was also acknowledged that the decision to convert to open surgery is often due to circumstances outside the surgeon’s control; for example, conversion was described as a ‘technical’ matter not linked to ergonomics or how stressed the surgeon was. One surgeon stated that, if anything, he would persevere longer with laparoscopic surgery because that is the technique with which he has more experience and so feels more confident.
How and why the ergonomics of the robot reduced surgeons’ stress levels was also postulated by the wider team. Team members discussed a number of ergonomic benefits of the robot, for example because the surgeon is sitting down and that must mean they are more relaxed, the surgeon can adjust the console’s head piece, the console is padded, and it is easier to have coffee breaks as no scrubbing or descrubbing is required to step away from the console. However, it was also suggested that stress might be dependent on the surgeon’s experience (i.e. those learning how to use the robot do not seem as relaxed using the console). Participants also noted that if a surgeon found a stage of the procedure difficult, this would cause stress regardless of the ergonomics of the robot. The difference between mental and physical tiredness was also highlighted by the team; some described that the surgeon gets tired looking at the 3D image, or that RAS is stressful for their eyes and requires more mental concentration. It was also noted that the surgeon can be hunched in the same position for hours.
Summary
We started the interviews with a series of tentative theories, most of which were drawn from literature authored by surgeons. Through our interviews we were able to refine our theories to reflect the experience of a broader range of OT personnel, as well as identifying new theories. The theories at the end of the interviews are summarised in Table 8, with italics used to show where refinements to the earlier theories have occurred. Our theory regarding organisational support became broken down into several theories about the role of support of different stakeholders within the organisation, with the role of each stakeholder clarified. In relation to teamwork, as occurred with the theories of implementation in the review, we were able to move beyond identifying how and in what contexts RAS impacts co-ordination to also identify strategies teams use to overcome these challenges, developing theories regarding how and in what contexts these strategies would be effective.
| Theory | Context | + | Mechanism | = | Outcome | |
|---|---|---|---|---|---|---|
| Resource | Response | |||||
| I1a. Board-level support | Hospital administration involved in decision to introduce RAS Competitive market |
+ | Potential benefits of RAS | Hospital administration perceive RAS as assisting in achieving organisation’s goal of retaining certain services | = | RAS more likely to be integrated into practice |
| I1b. Team leader support | Team leader involved in RAS implementation | + | Potential benefits of RAS | Team leader assists in creating conditions that accommodate RAS introduction | = | RAS more likely to be integrated into practice |
| I1c. Surgical colleague support | Surgeons perceive introduction of RAS as supported by colleagues | + | Potential benefits of RAS | Surgeons feel more willing to undertake a robot-assisted operation | = | RAS more likely to be integrated into practice |
| I2. Team involvement | Training for team members | + | Need for team members to develop new skills | Team members feel confident to take on new tasks associated with RAS | = | RAS more likely to be integrated into practice |
| I3. Dedicated team | Initial introduction of RAS Motivated and stable team High number of frequent robot-assisted operations Support of hospital administration and nursing management |
+ | Dedicated robotic team | Team sees operations as opportunity to learn and more quickly become familiar and confident with equipment and tasks | = | Reduced set-up time |
| I4. Suitably sized OT | Support of team leader Availability of suitably sized OT |
+ | Suitably sized OT | Staff are able to move around easily and quickly | = | Reduced operation duration Reduced risk of desterilisation |
| I5. Whole-team training | Whole-team training | Need for team members to develop new skills | Staff understand impact of RAS on each other’s roles and feel more confident in each other’s knowledge Surgeon feels more confident in team’s ability to support him/her |
Improved teamwork RAS more likely to be integrated into practice |
||
| I6. Handpicked teams | Handpicked teams | + | Need for team members to develop new skills | Staff feel a sense of privilege, providing them with the motivation to overcome the challenges of RAS | = | RAS more likely to be integrated into practice |
| I7. Manufacturer support | Manufacturer support | + | Need for team members to develop new skills | Team have confidence there will be help to overcome problems | = | RAS more likely to be integrated into practice |
| T1. Co-ordination |
Problems with the microphone
Non-explicit communication |
+ | Surgeon’s position within console | Team have difficulty hearing what surgeon says and knowing who instruction is directed at | = | Increased repetition of instructions and requests to repeat information Reduced co-ordination Increased operation duration |
| T2. Training |
Greater use of theatre nurses and ODPs as assistants
Totally robot-assisted operation |
+ | Reduced role of assistant | Trainee is less involved in operation | = | Trainee learns less than in other forms of surgery |
| Surgeon’s position within console | Surgeon less likely to provide a commentary | |||||
| T3. Readback | Team experienced in RAS | + | Use of readback | Surgeon knows recipient has received the message and is taking action | = | Instrument exchanges completed safely and efficiently |
| T4. Two surgeons | Two consultant surgeons work together | + | Constraints to communication | Liaison surgeon ensures information from console surgeon is received by team | = | Requests completed safely and efficiently |
| T5. Experienced assistant | Assistant has worked with surgeon before Assistant engaged in operation |
+ | Experienced assistant | Assistant is able to anticipate requests and react to events without prompting | = | Improved co-ordination Assistant’s actions performed correctly and in timely manner |
| T6. Explicit communication | Team engaged in operation | + | Explicit communication | Team can hear and understand the message | = | Team complete requests accurately and in timely manner |
| T7. Engagement | RAS in routine use | + | Reduced task load | Assistant and scrub practitioner do not monitor screen and engage in case-irrelevant communication | = | Reduced co-ordination |
| T8. Commentary | Team have limited experience of procedure and are interested in learning about it | + | Surgeon provides a commentary | Team listen to surgeon and remain focused on task at hand | = | Team react to surgeon’s requests in timely manner |
| D1. Situational awareness | Positive relationship between surgeon and team | + | Team orally communicates information about patient and robot | Surgeon adjusts course of action based on information | = | Complications avoided |
| Surgeon feels confident to remain in console | = | Reduced distraction and increased concentration | ||||
| D2. Lack of tactile information | Surgeon experienced in laparoscopic surgery | + | Lack of tactile information | Experience allows surgeon to quickly adapt to relying on visual cues | = | Decision-making unaffected |
| D3. Immersion | Surgeon trusts assistant | + | Sense of immersion | Surgeon is more focused on the task at hand and is less easily distracted | = | Improved decision-making |
| D4. Impact of ergonomics | Surgeon experienced in RAS | + | Ergonomic console | Surgeon feels comfortable to persist longer with operation | = | Reduced levels of stress and tiredness Reduced conversion to open surgery |
Chapter 6 Prioritising theories for testing
Overview
On completing the interviews, we had a total of 21 theories concerning how and in what contexts RAS becomes embedded in routine practice and how and in what contexts it impacts communication, teamwork and decision-making (see Table 8). In this chapter, we describe the process of prioritising the theories.
Prioritisation of impacts
Once the analysis of the phase 1 interviews was complete, a subset of possible theories to test was identified, based on a consideration of the strength of support offered in the interviews for those theories, the extent to which they concerned outcomes identified by the patient panel and SSC as being important, and the feasibility of testing those theories.
Preliminary findings from the interviews were presented and discussed at a patient panel meeting and an SSC meeting, to decide the most important impacts to consider in phase 2. The discussions highlighted three key points:
-
One important impact of RAS, of interest to the ROLARR trial and also in relation to the implementation of RAS, was operation duration. Although the implementation chain of RAS is long and it would only be possible to explore specific points along that chain, operation duration links to patient outcomes and cost-effectiveness further down the chain, outcomes of interest to ROLARR.
-
Many factors can affect operation duration but communication failure is a significant predictor of deviation in expected length of operation duration. 186 Thus, it was agreed that attention should focus on theories concerned with communication and teamwork, rather than on theories of implementation, although it would still be appropriate to consider how those features of implementation, such as team leader support and having a suitably sized OT, played out as contextual factors to influence the processes of communication and teamwork. Although OTAS provides an overall rating of teamwork, specific behaviours rated within OTAS would more closely link to the outcomes in the theories.
-
The primary outcome of ROLARR was conversion to open surgery and therefore it was considered important to include the theory ‘impact of ergonomics’, as this is the theory that directly linked to this outcome. Because conversion to open surgery is a rare occurrence, attention should focus on testing the first part of this theory: for surgeons experienced in RAS, do the ergonomic benefits of RAS result in lower levels of stress and tiredness than laparoscopic surgery? It was agreed that this issue is key for understanding decision-making in RAS.
Given the decision during the patient panel and SSC meetings to focus on theories of communication, teamwork and decision-making, the list of 21 theories was immediately reduced to 12 possible theories for testing. Furthermore, as one of the study objectives was to provide guidance for OT teams on how to ensure effective communication and teamwork when undertaking RAS, it was felt appropriate to focus on those theories that concerned strategies for managing communication and teamwork, rather than on those theories that focused on how and in what contexts RAS impacted communication and teamwork. It was not considered feasible to test the ‘two surgeons’ theory, as this was a strategy only used in some settings.
In relation to decision-making, based on the discussion in the patient panel and SSC meetings, it was considered important to test the theory ‘impact of ergonomics’. The theory ‘lack of tactile information’ was not considered feasible to test within the proposed study design and was also not perceived as an important issue among the surgeons we interviewed. Similarly, the theory ‘immersion’ was not considered feasible to test, because of difficulties of assessing levels of concentration and quality of decision-making within a naturalistic study, and there had been uncertainty among our interviewees about the contexts in which immersion would be experienced and whether or not this would be reflected in patient outcomes. A topic of concern to the surgeons we interviewed was situational awareness within RAS. Our refined theory ‘situational awareness’ explored not only the contexts that impact a surgeon’s situational awareness but also a strategy that can be used by OT team members to increase the surgeon’s situational awareness, potentially providing guidance for OT teams. Monitoring and situational awareness is one of the behaviours rated in OTAS and therefore we would be able to gather quantitative data for testing this theory.
The subset of theories was further discussed with three of the clinical members of our research team (DJ, AK and AG). In relation to communication and teamwork, ‘explicit communication’ was considered the most significant strategy in enabling the first assistant and scrub practitioner to successfully undertake their roles within a robot-assisted operation and therefore it would be important, through testing this theory, to develop a greater understanding of how and in what contexts such a strategy was effective. This was considered of greater relevance than the theory ‘readback’, a topic on which there is already a significant literature in other domains. The theory ‘experienced assistant’ was also considered to be important as it was a theory that, owing to experienced theatre nurses and ODPs acting as first assistants in place of surgical trainees, had implications for the skill mix within robot-assisted operations and for the teaching of surgical trainees, another area of interest to the study. In relation to decision-making, it was agreed that both the theory ‘situational awareness’ and the theory ‘impact of ergonomics’ should be taken forward for testing.
Summary
Through the process of prioritisation with input from clinical stakeholders, we were able to reduce our list theories to take forward for testing from 21 down to four. These theories are presented in Table 9.
| Theory | Context | + | Mechanism | = | Outcome | |
|---|---|---|---|---|---|---|
| Resource | Response | |||||
| T5. Experienced assistant | Assistant has worked with surgeon before Assistant engaged in operation |
+ | Experienced assistant | Assistant is able to anticipate requests and react to events without prompting | = | Improved co-ordination Assistant’s actions performed correctly and in timely manner |
| T6. Explicit communication | Team engaged in operation | + | Explicit communication | Team can hear and understand the message | = | Team complete requests accurately and in timely manner |
| D1. Situational awareness | Positive relationship between surgeon and team | + | Team orally communicates information about patient and robot | Surgeon adjusts course of action based on information | = | Complications avoided |
| Surgeon feels confident to remain in console | = | Reduced distraction and increased concentration | ||||
| D4. Impact of ergonomics | Surgeon experienced in RAS | + | Ergonomic console | Surgeon feels comfortable to persist longer with operation | = | Reduced levels of stress and tiredness Reduced conversion to open surgery |
Chapter 7 Testing theories of communication and teamwork
Overview
Chapter 6 concluded by presenting the list of theories prioritised for testing in phase 2 (see Table 9). In this chapter, we present the findings of phase 2 as they relate to the two theories concerned with communication and teamwork: ‘experienced assistant’ and ‘explicit communication’. The theories related to decision-making are discussed in Chapter 8.
Differences in teamwork between robot-assisted and laparoscopic surgery
We began by exploring differences in the quality of teamwork between laparoscopic surgery and RAS. The mean overall OTAS score for robot-assisted operations was 3.58 [range 3.16 to 4.27, standard deviation (SD) 0.28], while the mean overall OTAS score for laparoscopic operations was 3.57 (range 3.31 to 3.78, SD 0.18). This indicates little difference in the quality of teamwork between the robot-assisted and laparoscopic operations observed, although the quality was more variable within the robot-assisted operations. The mean OTAS scores, broken down by phase of the operation, are shown in Figure 4.
FIGURE 4.
Mean OTAS score by operation phase, with error bars showing 95% confidence intervals.

The OTAS scores were also similar when broken down by subteam, as shown in Figure 5. However, the OTAS scores in the robot-assisted operations were higher for the nursing subteam but lower for the anaesthetic subteam when compared with the laparoscopic operations, where there was little difference between subteams. This suggests that in RAS more behaviours that are supportive of teamwork are demonstrated by the nursing subteam than by the other subteams.
FIGURE 5.
Mean OTAS score by subteam, with error bars showing 95% confidence intervals.
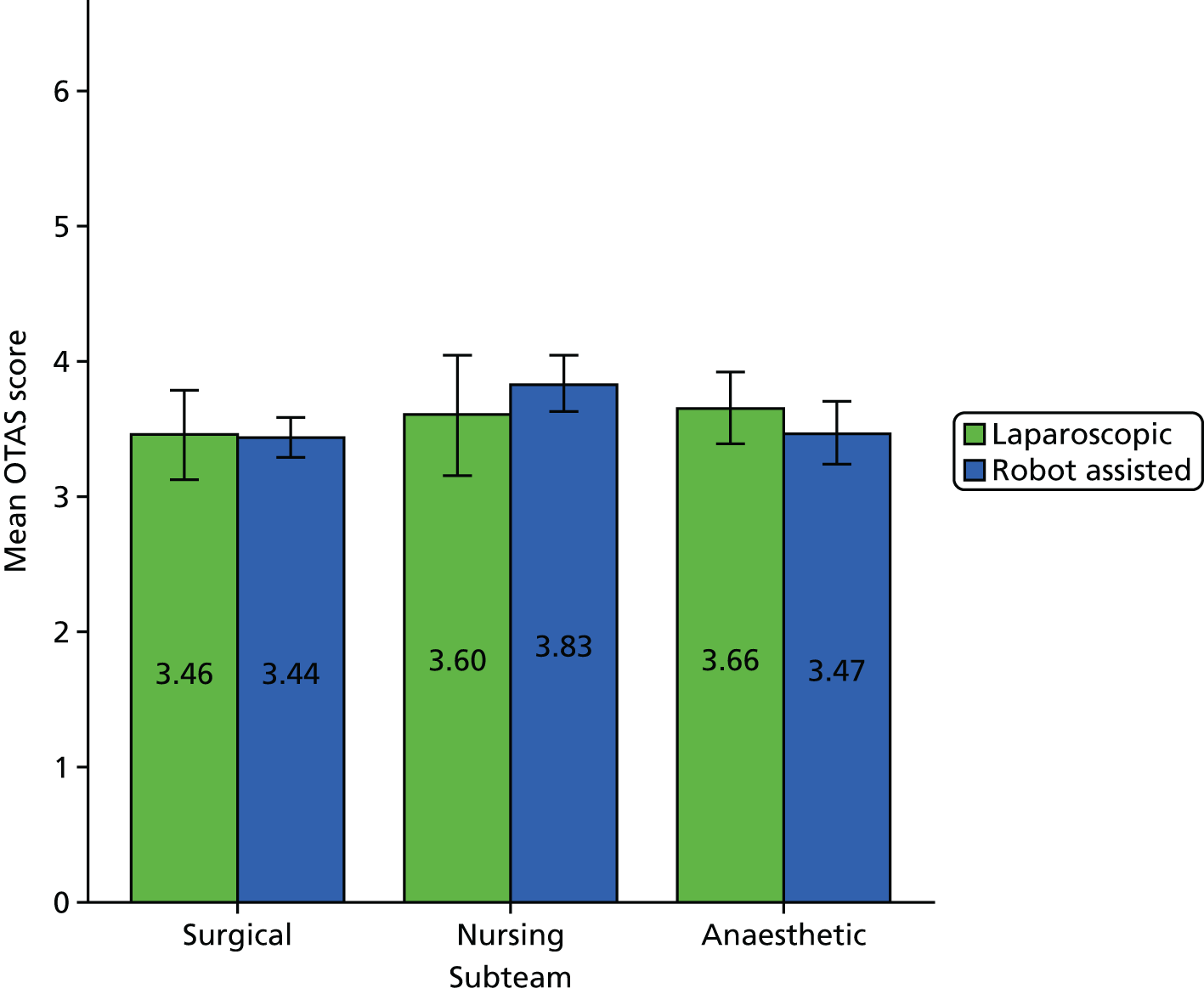
A Pearson product-moment correlation was run to determine the relationship between the percentage of the operation that was robot assisted and the overall OTAS score. There was no significant relationship between the percentage of the operation that was robot-assisted and the overall OTAS score [r = 0.07; p > 0.05 (two-tailed)]. Similarly, there was no significant relationship when looking at the intraoperative score for the surgical subteam [r = 0.03; p > 0.05 (two-tailed)] or the nursing subteam [r = 0.37; p > 0.05 (two-tailed)]. In other words, it seems that the extent to which the operation is conducted with robot assistance does not affect the quality of teamwork.
We now turn to consideration of our two theories regarding communication and co-ordination. To test and refine these theories, we integrate a range of collected data, with qualitative data in the form of field notes, video recording of operations, and interview transcripts and quantitative data in the form of OTAS scores, SURG-TLX ratings and quantitative analysis of the video data. For each theory, we seek to (1) further clarify the distinctions between laparoscopic and RAS, and (2) compare instances within and between sites to explore the various contexts in which the mechanisms of interest operate.
Experienced assistant
Across the 16 robot-assisted operations we observed, first assistants had a range of levels of experience: ODPs and surgical trainees experienced in both colorectal surgery and RAS (experience of RAS ranging from between 11 and 20 operations to between 51 and 100 operations); surgical trainees experienced in colorectal surgery but with limited experience of RAS (having assisted in ≤ 10 robot-assisted operations); surgical trainees experienced in colorectal surgery but with no experience of RAS; foundation year 2 doctors (FY2s) with some experience of surgery generally and RAS; and FY2s with limited experience of surgery generally and no experience of RAS. This enabled us to explore how the different types of experience noted in our theory, experience of RAS and experience of the particular procedure, contribute to ensuring that the first assistant’s actions are performed correctly and in a timely manner. It also enabled us to explore what happens when the first assistant is not experienced and to identify other strategies that support co-ordination in such a context. However, it quickly became clear when undertaking our observations that the tasks a first assistant takes on in a robot-assisted operation are quite different from those he or she would undertake in a laparoscopic operation, so first we describe those differences.
The role of the first assistant in robot-assisted and laparoscopic surgery
Actions first assistants were observed performing in the robot-assisted phases of operations included assisting with retraction, providing suction, inserting and removing swabs (to absorb blood), applying clips to a vessel, changing the robotic instruments and temporarily removing the robotic camera for cleaning. However, the extent of what the first assistant did varied according to their level of experience. For example, in one robot-assisted operation a FY2 took on the role of first assistant and with the guidance of an experienced scrub practitioner, she provided suction, inserted and removed swabs, and applied clips to ligate a vessel, but there were also periods during which she was not holding any instruments, and she did not assist with retraction.
In laparoscopic surgery, generally the first assistant would be holding the laparoscopic camera and a grasper, to assist with retraction. There would be no changes of instruments and the surgeon’s requests would relate to movement of the camera or grasper. The surgeon would be holding in their right hand an instrument for cutting, changing it for another instrument when needing suction or to apply a clip for example, and holding in their left hand a grasper for retraction. However, although this comparison initially suggests that the first assistant does less in a laparoscopic operation, in the laparoscopic operations and the laparoscopic phases of the robot-assisted operations, there was more variation in the extent of the first assistant’s involvement in the operation, depending on his or her level of experience. With more experienced first assistants who were surgical trainees, the operation took on a teaching element and the surgeon and first assistant would frequently swap roles; the first assistant would assist the surgeon for the more difficult parts and then for other parts the first assistant would operate, guided by the surgeon, while the surgeon controlled the laparoscopic camera and assisted with retraction. We observed a laparoscopic case in which two surgical trainees operated without a consultant surgeon present and the laparoscopic phase of a robot-assisted operation in which the two surgical trainees carried out the first part of the operation without the consultant surgeon present. At the other extreme, in several operations across two sites when an inexperienced trainee took on the role of first assistant, the first assistant held the camera but was directed by the surgeon, who would sometimes physically move the first assistant’s hand or move the camera himself and then get the first assistant to hold the camera again.
Does experience of colorectal surgery support co-ordination?
The actions that a first assistant is required or expected to undertake in the robot-assisted phase of an operation are largely tasks that, if they are experienced in laparoscopic surgery in terms of having had the opportunity to operate under the consultant surgeon’s guidance for parts of the operation, they will already be familiar with. The exception to this is changing the robotic instruments. On this basis, we may anticipate that it is experience of the particular procedure that is important, providing the first assistant with knowledge of the steps in the procedure and how to correctly complete them (e.g. when and how to apply clips to a vessel, when suction is likely to be required and how to provide it), enabling them to anticipate the surgeon’s requests and react to events without prompting.
In the laparoscopic operations and the laparoscopic phases of the robot-assisted operations that we observed, the difference in the ability of an experienced first assistant and an inexperienced first assistant to work without prompting was clear, particularly in relation to movement of the camera; whereas an inexperienced first assistant would need to be more explicitly guided by the surgeon, an experienced first assistant would need much less guidance, typically moving the camera without prompting. Similarly, in assisting with retraction, an experienced first assistant would adjust the position of their grasper without prompting. However, while actions were largely performed correctly and in a timely manner in the robot-assisted phases of operations, analysis of the field notes revealed few examples of unprompted action from the first assistant, except for the occasional unprompted provision of suction. It was only when we carefully reviewed the videos looking for such examples that the types of unprompted action performed by the more experienced first assistants became more apparent. These were similar to the unprompted action in the laparoscopic operations, involving small movements, such as using the suction instrument to assist with retraction, pushing back the tissue following the surgeon’s initial request for suction. Additionally, although we saw few examples of unprompted action, with the experienced first assistants there was a sense that they were doing more than just following instructions. For example, although the surgeon may ask the first assistant to provide suction, with an experienced first assistant what then follows is a series of movements by the first assistant as they co-ordinate their movement of the instrument with the movement of the surgeon’s instruments, so as to provide suction without obstructing the movement of the surgeon’s instruments. In contrast, with an inexperienced first assistant, the surgeon would direct the first assistant where to put the suction instrument and then guide them in the movement of that instrument.
Interestingly, in five of the eight video-recorded robot-assisted operations, we identified instances of first assistants offering assistance, for example:
Do you want me to let some of this gas out?
Yes please.
Site 6
Do you want some suction on that left side?
Yeah.
Site 6
Shall I suck that tonsil swab?
Site 7
The surgeon cuts and a pool of blood appears on the screen.
Do you want suction?
Yeah.
Site 7
Most of the offers of assistance related to providing suction or helping with retraction, the two tasks most commonly undertaken by the first assistant in the robot-assisted phase of an operation. We did not identify any instances of offers of assistance in the laparoscopic operations and the laparoscopic phases of robot-assisted operations but, when acting as a first assistant (as opposed to operating under the guidance of the surgeon), the first assistant’s responsibilities are consistent and clearly defined: to move the camera and to assist with retraction. It seems that the first assistant’s experience of colorectal surgery provides them with knowledge of what actions are likely to be required, and they make use of that by making an oral offer of assistance. However, the ability to make these offers of assistance did not appear to require significant experience; the first assistants in these instances varied substantially in their experience of colorectal surgery, with one first assistant being a FY2.
Making an offer of assistance also avoids the situation, observed on a couple of occasions in one operation, of providing assistance unprompted and having that assistance rejected:
As the surgeon cuts, a pool of blood becomes visible on the screen.
Oh lovely.
Suction. Sucker coming in.
I should be able to get it with this, I think [referring to tonsil swab he has].
Site 7
The trainee goes to assist with retraction but the surgeon says ‘I’ve got that, don’t worry’.
Site 7
Again, we did not identify any examples of assistance being rejected in this way in the laparoscopic operations and the laparoscopic phases of the robot-assisted operations, owing to the consistent nature of the tasks the first assistants were undertaking.
In relation to assisting with retraction, it seems that in the robot-assisted phase of an operation it can be challenging for the first assistant to know whether or not the surgeon needs assistance, because of the change in the division of labour RAS introduces, whereby, with a four-arm robot, the surgeon typically uses two of the arms for retraction (with the camera held by one arm and scissors held in the remaining instrument arm). This provides another motivation for offering, rather than providing unprompted, assistance. While some surgeons talked about the first assistants still being on the learning curve, so they were still learning what assistance to provide, it also seemed the surgeons were still learning what they could manage on their own and when they needed assistance, as revealed in the following instances:
Shall I retract up for you, Prof?
Yeah, maybe. Let me just see. I might be able to do something myself. It’s the beauty of having two left hands, yeah? Actually I think it would be helpful if you could just lift this bowel up here.
Site 1
Would you like better traction from me or are you happy?
I would but it seems like we’re making some decent progress so I just want to wait.
Site 6
Do you want me to hold that out the way?
Erm, should be OK [sounding uncertain]. Just trying to work out which plane I’m going to start with.
Site 7
The surgeon in the first example had experience of over 100 colorectal robot-assisted operations, making him one of the surgeons with most experience of RAS in our sample. Such examples suggest that it is only if and when the surgeons establish more routinised ways of working with the robot, in terms of what they undertake alone and where they require assistance, that the first assistant could be expected to have knowledge of what assistance the surgeon is likely to want.
To determine whether or not, despite limited unprompted actions, using an experienced first assistant led to the first assistant’s actions being completed in a timely manner, as hypothesised in the theory, we used the video data to analyse the time taken by first assistants to respond to requests to provide suction and to assist with retraction (see Appendix 11 for further details of the method). A total of 65 instances were identified: 32 requests for suction and 33 requests for retraction. The median time to respond was 11 seconds (range 3 seconds to 69 seconds). In all of the eight responses that took less than 7 seconds, the first assistant had pre-empted the request, either picking up the instrument, and sometimes starting to insert it but outside the field of view of the camera, or asking the scrub practitioner for the instrument. However, again, the ability to pre-empt the request in this way did not seem to require significant experience of colorectal surgery, with the first assistants in these instances varying substantially in their level of experience, one first assistant being a FY2. Although all first assistants showed engagement, in terms of monitoring the screen, there seemed to be an additional factor of keenness to demonstrate to the surgeon their ability to respond quickly.
Equally, the first assistants in the longer responses varied in their level of experience, and some of those first assistants who provided the very quick responses were also those who provided some of the slower responses. In the 11 responses that took more than 20 seconds, there were a variety of causes, including being slowed down by the need to first remove another instrument and the speed of the scrub practitioner’s response. Yet some of the causes did appear to be related to experience. For example, in the longest response, the scrub practitioner had to first show the first assistant how to use that particular grasper, as the first assistant had not used it before:
Do you have a fan retractor?
Yeah.
The scrub practitioner walks to tray [placed a metre or so away from the patient].
Ask him to, can you take the [. . .] suction off, and then put the fan retractor please.
Yeah.
You need to show him how it works, I don’t think he knows how it works.
The scrub practitioner shows the trainee how to open and close the fan retractor. The trainee then takes the retractor and inserts it.
Site 6
There were also several examples, among those responses that lasted 20 seconds or more, in which the first assistant was quick to move the instrument to the port but then struggled to move the instrument into the camera’s field of view. The ability to move an instrument into place could be expected to vary according to the level of experience, and this was a challenge experienced more by inexperienced first assistants, although instances of experienced first assistants undergoing this same challenge were also witnessed.
Does experience of robot-assisted surgery support co-ordination?
As described above, in all operations in which the first assistant had experience of RAS, they also had experience of colorectal surgery. Therefore, we are not able to assess whether or not experience of RAS without experience of the particular procedure is enough to support co-ordination between the surgeon and first assistant. Instead, we compared those cases in which the first assistant had experience of RAS with those in which the first assistant did not have that experience, in order to understand what experience of RAS contributes. The SURG-TLX questionnaire gathered data on participants’ experience of RAS and so this was used to help analyse the OTAS data to determine if the first assistant’s level of experience of RAS impacted the OTAS co-ordination score for the surgical subteam. A one-way independent ANOVA for just the robot-assisted operations, with first assistant’s experience as an independent variable, was performed. There was no significant effect of the first assistant’s experience on the surgical subteam co-ordination score [F(5,15) = 0.68; p > 0.05].
Using the qualitative data, we sought to explore further how and in what contexts the first assistant’s experience of RAS contributed to effective co-ordination. The tasks a first assistant may undertake in RAS that they do not undertake in laparoscopic surgery, and therefore where the impact of experience of RAS could be expected to be noticeable, are changing the robotic instruments and moving the robotic arms externally in order to move the instruments inside the patient, although these tasks did not happen in all of the robot-assisted operations we observed. Certainly, first assistants with experience of RAS were more likely to be asked to change the robotic instruments, whereas the scrub practitioner may be asked to do this if the first assistant does not have experience of RAS (discussed further below).
We might also anticipate that increased experience in RAS means that the first assistant better understands the challenges of communication and co-ordination in RAS and how to manage these challenges. Because in RAS the surgeon rather than the first assistant controls the camera, the first assistant is not able to position the camera in ways that will help them complete their actions. This appears to present a challenge for first assistants and, in response to this, across difference sites and with different first assistants, we observed first assistants asking the surgeon to move the camera:
The ODP, acting as first assistant, is very confident communicating with the surgeon e.g. ‘Move that camera, I can’t see’.
Site 1
The trainee says ‘Can you show me where I am?’ The surgeon draws back the robotic camera so the trainee can see her instruments on the screen.
Site 7
The surgeon tells the trainee to ‘go down a bit’ to find a swab left inside the abdomen . . . The trainee asks the surgeon to move the camera so he can see it.
Site 1
However, asking the surgeon to move the camera in this way does not seem to be the result of experience of RAS but rather just a response to the challenge experienced. For example, whereas two of the field notes extracts above involve first assistants experienced in both colorectal surgery and RAS, the third extract involves a first assistant experienced in colorectal surgery but not in RAS. Although it might be anticipated that first assistants who are more experienced in colorectal surgery would have the confidence to speak up because they would be sure about what they needed, whereas a less experienced first assistant may be hesitant, we also identified instances of first assistants with limited experience of both colorectal surgery and RAS asking the surgeon to move the camera.
What appears to be more significant in encouraging first assistants to change the way they communicate in a robot-assisted operation is how the surgeon encourages them to communicate and, related to this, their relationship with the surgeon. There was variation in this between sites. Surgeons at two of the sites repeatedly encouraged the first assistants to tell them what they were doing and to speak up if they were unsure about something. One surgeon, at site 7, said he did this partly to ensure that the first assistant felt comfortable speaking up when necessary. Another surgeon, at site 5, referring to all staff, said he deliberately engaged in general conversation before an operation to encourage people to talk to him, demonstrating awareness of potential problems arising from staff feeling unable to speak up.
Another strategy one surgeon would use to encourage the first assistant to communicate was to ask the first assistant how they were doing generally, that is, without reference to a particular task. During one operation, for example, there was a pause in the operation while a report was found and the surgeon asked the first assistant, ‘are you OK?’. The surgeon and the first assistant conversed a lot during this operation; afterwards, the surgeon said that the first assistant had limited experience of assisting in RAS but the two of them had worked together often and he perceived this familiarity as enabling the first assistant to feel confident in speaking up.
Does an experienced scrub practitioner support co-ordination?
Comparison of occasions on which there was an inexperienced first assistant also highlighted the role of the scrub practitioner in supporting an inexperienced first assistant. We observed a number of operations with first assistants at different stages of their training and without experience of RAS where, thanks to the scrub nurse’s guidance and assistance, the actions were performed correctly and safely, although not necessarily in a timely manner.
We also observed scrub practitioners assisting first assistants by changing robotic instruments, and sometimes the surgeon explicitly asked the scrub practitioner rather than the first assistant to do this. There was uncertainty among the theatre nurses and ODPs who we spoke to about whether they should be doing this when they had not undertaken training to be a first assistant, with putting instruments inside a patient being perceived as being outside the scope of their role. Despite this, when asked to do it, all scrub practitioners did.
With first assistants inexperienced in colorectal surgery, guidance did not relate only to those aspects that are specific to RAS. Such guidance mainly related to how to use particular laparoscopic instruments, such as how to apply a clip or the fan grasper described above. The scrub practitioner provides this guidance because the surgeon is not there at the patient’s side to be able to demonstrate, and is often asked by the surgeon to help the first assistant in this way, as illustrated by this field note extract:
11.10 a.m. The surgeon says to the scrub practitioner: ‘Have some hem-o-loks ready for me if necessary.’ The camera is dirty – the surgeon asks the first assistant to take it out. He guides her and tells the scrub practitioner to help her – the surgeon moves his head out of the console. They clean the camera and put it back in again but the surgeon says it’s still dirty, so they clean it again . . . The surgeon tells the first assistant to check with him anything she’s unsure about. The scrub practitioner explains to the first assistant about how the suction works. The surgeon asks the scrub practitioner to explain to the first assistant how the hem-o-lok works – she does . . . 11.55 a.m. The surgeon asks for a hem-o-lok for the first assistant to practice. The scrub practitioner gets a hem-o-lok and she and the circulating practitioner guide the first assistant on how to close it.
Site 7
By asking the scrub practitioner to support the first assistant in this way, the surgeon is acknowledging the expertise of the scrub practitioner. Discussing this with a surgeon and theatre nurse after an operation, they described the scrub practitioner as having a more important role and more responsibility during RAS than during laparoscopic surgery. They felt that this flattened the hierarchy between surgeons and the rest of the team.
The comparison of such instances suggests that the skill mix of those at the patient side needs to be considered, rather than just thinking about the experience of the first assistant. In all sites, thought was given to skill mix but there was variability in how this was put into practice. For example, in one operation in site 1, the first assistant was not scheduled to be assisting with that operation, but the surgical trainee who was scheduled to assist (and who had experience with RAS) had just been on a night shift so the surgeon sent him home. A theatre nurse who had experience of RAS was on the team, but it seemed the nurses had decided between themselves that the less experienced nurse would scrub while the other would circulate. Discussing this with clinical members of our team, it was felt that having the more experienced practitioner circulating would be a reasonable strategy if the circulating practitioner took on the role of guiding the scrub practitioner – the circulating practitioner is better placed to be able to take on a facilitative role, whereas the scrub practitioner cannot move outside the sterile field – but the extent to which the circulating practitioner guided the scrub practitioner in this particular operation varied.
The ability of the scrub practitioner to support an inexperienced assistant was dependent on the first assistant’s willingness to acknowledge the need for support and to accept such support. During one operation, the first assistant was experienced in colorectal surgery but it was only his fourth experience of assisting with a robot-assisted operation, and he said to the scrub practitioner:
You’ve done lots of these cases, haven’t you? This is only my fourth . . . I need to hang out with you. I think I’d learn a few things.
Site 6
In another operation, while the surgeon was docking the robot, the first assistant, who had not done a robot-assisted operation before, asked the scrub practitioner what she would be doing:
I’m not really sure what I’ll be doing.
Sorry?
I’m not really sure what I’ll be doing.
You’ll be er . . .
Retracting. I know that.
Retracting. Sucking, suction. Firing hem-o-loks onto vessels.
Nice.
Firing [staple] guns across the bowel potentially.
Cool.
Changing instruments.
She then asks the scrub practitioner to show her the robotic instruments.
Site 1
While the willingness to accept support varied between individuals, it seemed that the surgeon could also influence this willingness by, as described above, asking the scrub practitioner to support the first assistant, highlighting this aspect of the scrub practitioner’s role.
A revised theory
We sought to test the following theory:
Knowledge gained through experience of RAS and/or the particular procedure enables the first assistant to anticipate the surgeon’s requests and react to events without prompting, supporting co-ordination between the surgeon and first assistant and ensuring that the first assistant’s actions are performed correctly and in a timely manner. The success of this strategy may be dependent on their experience of working with the surgeon, as well as how engaged the first assistant is in the procedure (i.e. how carefully they monitor the screen).
On the basis of the analysis presented above, we can make a number of revisions to this theory, more clearly specifying the way in which experience contributes to a timely response and the type of experience that is important:
Knowledge gained through experience of the particular procedure enables the first assistant to work with less guidance, adjusting the position of the grasper and moving the suction instrument without prompting. Their experience also provides them with knowledge of what actions are likely to be required, which they make use of by making oral offers of assistance and/or preparing to act (e.g. by holding the instrument ready). Together, these behaviours support co-ordination between the surgeon and first assistant and increase the likelihood the first assistant’s actions are performed correctly and in a timely manner. The success of this strategy depends not only on engagement in the procedure but also, in preparing to act, the first assistant’s keenness to demonstrate to the surgeon their ability to respond quickly.
Whether or not experience enables first assistants to assist with retraction without prompting if and when surgeons develop more routinised ways of working with the robot remains to be established.
The analysis also suggests that experience of colorectal surgery is not sufficient to engage as a competent practitioner in RAS and, when the first assistant lacks experience of RAS, the experience of the scrub practitioner is an important resource. Thus, we need an additional theory:
Where the first assistant is inexperienced, in either RAS or the particular procedure, but the scrub practitioner is experienced in RAS, knowledge gained through experience enables the scrub practitioner to both guide the first assistant and undertake certain tasks (changing the robotic instruments) on their behalf, ensuring these actions are performed correctly. The success of this strategy depends on the first assistant’s willingness to acknowledge the need for support and to accept such support, which can be increased through the surgeon acknowledging this aspect of the scrub practitioner’s role.
Finally, RAS requires the first assistant to communicate more explicitly with the surgeon, a topic discussed further below (see Explicit communication), but the ability of the first assistant to do this depends not on their experience but on the encouragement of the surgeon. Thus, we add a second additional theory:
When the surgeon encourages the first assistant to communicate both actions and concerns, the first assistant will feel comfortable to speak up when necessary, supporting co-ordination between the first assistant and surgeon and helping to ensure the first assistant’s actions are performed correctly. The success of this strategy is likely to depend on the first assistant’s relationship with the surgeon.
In summary, we can state that experience of a particular procedure does offer benefits in relation to co-ordination between the first assistant and surgeon in RAS but that this alone is not enough. RAS requires different forms of communication, which the surgeon needs to encourage the first assistant to provide. With the surgeon unscrubbed and at a distance, the first assistant and scrub practitioner work more as a unit than in other forms of surgery, and the skill mix of this unit has important implications for seamless co-ordination and safe working practices in the OT.
Explicit communication
To make an initial assessment of whether or not communication is more explicit in RAS, and therefore better, as some of our phase 1 interviewees anticipated it would be, the correlation between the percentage of the operation that was robot-assisted and the OTAS intraoperative communication scores for the surgical and nursing subteams was calculated using the Pearson product-moment correlation. There was no significant relationship between the percentage of the operation that was robot-assisted and the intraoperative communication score [r = 0.18; p > 0.05 (two-tailed)], failing to provide evidence that communication is better in RAS.
We then used the field notes and video data to identify qualitative differences in the nature of the communication between laparoscopic and robot-assisted phases of the operations observed, with a particular concern for how participants might consider communication to be more explicit during RAS. Although the OTAS data provided an overall assessment of the quality of communication, in line with a realist approach we wanted to identify in what contexts communication is more explicit and in what contexts the strategy of more explicit communication is effective. To do this systematically, we needed to focus the broad concern with communication down to a more narrow range of activities. We selected a significant, pervasive and seemingly simple action of requesting help or assistance. We focused exclusively on the more prevalent range of surgeon-initiated requests, which mainly included requests for suction, retraction and instrument changes.
Existing studies of laparoscopic operations have similarly analysed the organisation of surgeon-initiated requests for immediate action. 85,86 Therefore, we have a comparative analysis on which to explore key points of similarity and difference. Mondada outlines a recurrent sequential format for requests during the intraoperative phase. 86 She suggests that there is often a visible repositioning of the body of the surgeon or the instruments, followed by a terse verbal directive (e.g. ‘clip applier’, ‘OK, hold this’, ‘grasp there’), a silent response from the first assistant (or scrub practitioner), and the possibility of a further acknowledgement or pursuit from the surgeon. Mondada also reveals that the initial repositioning of the surgeon or instruments is often used as a resource by colleagues to anticipate the request and to produce an early response.
Our review of other video-based studies of surgery that include examples of requests,28,80,83,84 as well as our own review of the video from the laparoscopic sections of our recordings, suggest that Mondada’s format is robust. However, when we analysed surgeon-initiated requests in RAS, we identified three main points of contrast, all of which suggest how they could be considered to be more explicit by participants: (1) more explicit initial actions designed to secure the attention of the recipient; (2) the design of longer and more ‘staged’ requests, especially when directing the first assistant on how to move the laparoscopic instruments; and (3) an orientation to the need for more explicit oral responses to requests in key contexts. We now describe each of these features in turn.
More explicit pre-sequences
Whereas Mondada describes quite subtle visible preparation prior to a request, we noticed a great deal of vocal and verbal work that preceded the production of an explicit request. For instance, consider these examples:
.pt (pause) OK and then: (pause) (name) if you can take this now. (pause) just gras:p the artery.
Site 1
Alright, can you clean the camera for me?
Site 7
(Name), can you get a gras:per, and grab where I’m holding please?
Site 1
Notice that often there is vocal or verbal action work prior to the production of the request. In the first instance, there is a lip smack (‘.pt’), a micro-pause, an ‘OK and then’, another micro-pause and the use of the first assistant’s name before the request is produced. In the second instance, the word ‘alright’ is produced prior to the production of the request. In the third instance, the name is used prior to the request being delivered.
So, unlike the laparoscopic sequence format outlined by Mondada, the surgeon seems to do vocal work prior to the delivery of the request. We noticed repeatedly how during these sequences the first assistant (and often the scrub practitioner) would look to the screen or to the surgeon in anticipation of a request. They might have fewer resources to assess how to assist, but these actions were seen as flagging an upcoming request. In one case, the use of the first assistant’s name by the surgeon following a period of silence encouraged the scrub practitioner to stop her conversation with the circulating practitioner and reach out for an instrument, ready to support the first assistant with the request.
It was noticeable that the surgeons’ requests in robot-assisted phases of the operations often included the use of the recipient’s name. In the laparoscopic phases, the surgeon has a range of resources to identify the recipient (volume, gaze orientation, etc.) and they are able to observe whether or not all members of the team are focused on the surgical site. Obviously, such practices for indicating who an instruction is aimed at do not work in the robot-assisted phase of an operation and surgeons are less able to assess what colleagues are doing. The strategy of using the recipient’s name was used even when it was clear from the nature of the request who it was aimed at; so, the use of the recipient’s name may not be necessary to clarify who the intended recipient is but acts as a device to alert the intended recipient’s attention. Indeed, often the surgeon would switch between questions about anatomy (directed to surgical trainees or others in the room) and requests for assistance (directed to the first assistant): these would routinely be marked by the use of a name.
Sometimes, surgeons also used the intended recipient’s name when their initial question did not receive a response, as in these examples:
Is that the artery there? (pause) (Name)?
Don’t know.
Oh, that’s not very helpful. Have a wild guess.
Site 1
Is there anything in that? (pause) Do you think? (pause) What do you think, (Name)?
Site 1
Although taken out of context, these field notes extracts may be interpreted as suggesting that the first assistant did not know or was not sure how to answer the surgeon’s questions; however, this was in fact an experienced first assistant. The first assistant did appear engaged in terms of watching the screen but it seemed it was difficult for the surgeon to gain the first assistant’s attention after there had been a period without communication.
Multipart requests
Whereas Mondada’s account of requests in laparoscopic surgery reveals their terse character (often simply the name of an instrument or a simple deictic), within our corpus from RAS we found that these requests often had multiple components. Aside from the pre-request work discussed above, the requests themselves often had at least two distinct phases. For instance, consider this case from site 1:
(o)k(ay)- (0.5) .hhhhhh (1.3) now you can take it here again er: (.) nhhhh (Name)
(3.2)
.pt (0.5) and kind of- (1.5) pull it to the left.
(.)
Yeah. (0.3) that’s it.
Site 1
These requests would often follow long sequences of little participation from the first assistant. The first assistant might be waiting to assist or they might simply be holding back some part of the internal anatomy. However, when the request comes, they often have to reposition themselves, insert an instrument or switch to a new instrument. The surgeon’s requests are routinely built to allow for that. So, the first part of the request is routinely followed by more specific instructions. In this case the request to ‘take it’ is followed by a 3.2-second pause while the assistant inserts an instrument. As the instrument appears on screen, the request continues (‘and kind of-’) and as it nears the bowel the requests specifies further (‘pull it to the left’).
This reveals the ways in which requests are designed with regard to the very different nature of tasks in the OT. The surgeon has much greater control over the number of instruments and, therefore, can conduct much more of the work in the surgical site him- or herself. When the first assistant is called on, the requests are designed to provide progressively more specific information as they move closer to a position to help.
Oral responses to requests
While our theory discussed in Chapter 5 concerns explicit communication by the surgeon, in looking at these request sequences we also identified more explicit communication practices by other members of the team than occurred in laparoscopic surgery.
Mondada notes that surgeon-initiated requests for immediate action are responded to non-vocally: the action is done and the surgeon can see the first assistant and the scrub practitioner doing it (or moving to do it). 86 In many of our cases, we saw requests carried out with no verbal component. However, in RAS it is necessary to make a distinction between requests that can be monitored by the surgeon through action on screen and requests that have a significant off-screen component that is invisible to the surgeon. The latter requests require verbal acknowledgement or the surgeon cannot tell if the request is being actioned. Consider the following examples from site 1:
(Name), can you get a gras:per, and grab where I’m holding please?
(0.5)
Yeah.
So you can take it here?
(6.5)
(sorry . . . hhhhh (1.0) m’okay.)
In the first case, the first assistant needs to remove his suction tube, take a grasper from the scrub practitioner and insert it before he can complete the request. The ‘yeah’ seems to mark that that work will be done; the surgeon, after all, will not be able to see much of these preparatory actions. In the second case, we see a request for the first assistant to grasp the bowel. The first assistant starts to do this, but struggles with his instrument. The ‘sorry . . . hhhhh (1.0) m’okay’ seems to make available his struggles with his instrument and account for the delay: these struggles are not visible to the surgeon. Thus, at times the first assistant (or, indeed, the scrub practitioner) will use the vocal channel to compensate for the limited visual channel open to the surgeon.
This expectation of verbal acknowledgement at particular times can be seen in this case:
Can I >have an< hem-o-lok, please?
(3.0)
Where is it?
(0.5)
It’s coming.
The surgeon has asked for a new robotic instrument, but after a pause of 3 seconds he asks ‘where is it?’. Interestingly, the scrub practitioner turned to get the instrument on the word ‘hem-o-lok’ and indeed had one loaded with a clip and ready to be inserted. So, in many ways she was fully prepared and acting swiftly. However, none of this embodied work was visible to the surgeon. Indeed, he could not have expected the scrub practitioner to complete the task within 3 seconds. However, he does seem to pursue a response. It is also interesting that, following the pursuit of a response, it is the first assistant and not the scrub practitioner who notes ‘it’s coming’, again showing how they work as a team, a very different working relationship from that in laparoscopic surgery, where each works more directly with the surgeon rather than with each other. Discussing this instance with clinical members of our team, the nature of the task and, associated with it, the surgeon’s sense of time pressure – the surgeon has the vessel displayed and so will want to clip it as quickly as possible – was highlighted as a relevant contextual factor in his desire for a response.
More generally, practices of making the surgeon aware of what is happening were most common in sites 6 and 7, where the surgeons encouraged this from the team. When this does not happen, it can cause problems, in terms of both co-ordination and safety. For example, in one operation, the scrub practitioner was asked to change one of the robotic instruments. She did not tell the surgeon when she was about to reinsert the robotic instrument and, at the time, the surgeon had his head outside the console, so that the instrument was not inserted under the surgeon’s vision. The role of oral responses in supporting the surgeon’s situational awareness is discussed further in Chapter 8.
When explicit communication did not work
In line with our theory, the engagement of team members affected the success of the strategy of using more explicit communication. For example, on one occasion, although the scrub practitioner was generally engaged throughout the operation, at the point that a surgeon made a request for the first assistant to help with retraction, the scrub practitioner was talking to the circulating practitioner and so did not hear the request, leading the first assistant to have to ask the scrub practitioner for a grasper. Because of this, there was a 20-second lag between the surgeon’s request and the grasper being visible on the screen. In the laparoscopic operations and laparoscopic phases we observed, a failure to respond to a request at all only occurred when the request was aimed at the wider team and not to anyone in particular (e.g. a request to turn the lights off).
Even when the intended recipient was engaged, the strategies of explicit communication did not always succeed in supporting co-ordination. Many team members felt that the sound quality of the microphone and speakers made it difficult to hear and understand the surgeon, and this seemed to be affected by the speed or volume with which the surgeon spoke. The surgeon at site 5 chose not to talk through the robot microphone at all and took his head out of the console every time he needed to talk to anyone. We observed many instances in which the first assistant or scrub practitioner had to ask the surgeon to repeat his request several times. Although not unique to RAS, this occurred more frequently in the robot-assisted phases of operations.
Examples of requests that were not explicit
We have been discussing a tendency towards request sequences that are more explicit than those outlined in previous research on laparoscopic surgery. However, this is a tendency or, in realist terms, a demi-regularity. When actions are requested in a series – suction over a period of a minute or so, or a number of clips to be applied to a vein – the first assistant is more able to anticipate what might be required next. The action is available on the shared screen, and the tasks are closely coupled, and thus the practices for co-ordination are much more subtle and delicate.
Another way in which surgeons’ requests were less explicit was in communication with the scrub practitioner. When the surgeon makes a request of the first assistant, such as helping with retraction or providing suction, the scrub practitioner hears this and immediately responds by providing the necessary instrument if the first assistant does not already have it (or, if necessary, asking the circulating practitioner to get it), so that the scrub practitioners across all sites and all operations largely worked without direct prompting. However, exceptions to this were witnessed when the first assistant was inexperienced, so that the surgeon would direct his or her instructions to the scrub practitioner instead, as in the example above when the surgeon asked the scrub practitioner if she had a fan retractor and then asked her to show the first assistant how it worked.
A revised theory
We sought to test the following theory:
By using explicit communication, the team can hear and understand the message, which enables them to act accordingly and complete requests accurately and in a timely manner, supporting effective co-ordination. This strategy will work if the team are engaged and awaiting the surgeon’s requests.
Our analysis revealed some systematic differences in the organisation of surgeon-initiated requests for immediate action during the robot-assisted phase of the operation and, thus, we have unpacked various ways in which communication while the surgeon was working in the console can be seen as more ‘explicit’, as indicated in the theory. In contrast to Mondada’s request format from laparoscopic operations, in robot-assisted phases of surgery:
-
There is a more explicit ‘preparation’ or ‘pre-request’ work.
-
Requests are routinely less terse and are built over a series of utterances.
-
Recipients verbally acknowledge requests for which off-screen work is required before the action is visible on screen. When such acknowledgements are absent, the surgeon may pursue a response.
These communicative practices attend to (and reveal) distinctive qualities of RAS:
-
There are more tasks under the surgeon’s control, with less involvement from others and longer periods in which they are waiting to assist.
-
The roles of the first assistant and scrub practitioner have changed in RAS, with requests now often involving both parties in their successful accomplishment.
-
There are asymmetries in the visibility of conduct – action off-screen is no longer visible to the surgeon.
These differences in the division of labour have implications for the surgeon’s situational awareness, and OT teams are increasingly attentive to these implications in and through their communicative practices, a topic we will return to in Chapter 8.
On the basis of this analysis and the clarification of the ways in which communication is more explicit, it seems that explicit communication is not a single strategy. Thus, we revise our theory into several theories, each addressing the different ways in which the strategies support co-ordination and the contexts in which they do so:
Through oral preparation and/or pre-request work by the surgeon, particularly the use of the intended recipient’s name, the attention of the first assistant and scrub practitioner is secured, enabling them to hear the message, supporting effective co-ordination. The success of this strategy in securing attention depends on the engagement of the first assistant and scrub practitioner.
By using longer oral requests, built over a series of utterances, the surgeon provides the first assistant with time to take any necessary preparation (e.g. reposition themselves, insert an instrument, change an instrument), so they are ready for the more specific instructions that follow. This supports them to complete requests accurately, supporting effective co-ordination. This strategy is more likely to be successful if the first assistant is engaged or the surgeon has already secured the first assistant’s attention. However, the ability of the first assistant to understand the request may be negatively impacted by issues of sound quality and the speed and volume of the surgeon’s voice.
Oral responses to the surgeon’s requests confirm that work will be done and make apparent any challenges in completing the request, supporting effective co-ordination. Use of this strategy by the first assistant and scrub practitioner is more likely when the surgeon encourages the first assistant and scrub practitioner to communicate their actions and its success is dependent on the volume and clarity with which the information is communicated.
Summary
Through the empirical testing of our two theories relating to communication and teamwork in RAS, we have deepened our understanding of how and in what contexts particular strategies work to support communication and teamwork in RAS. This has led to an expansion of our theories, clarifying how and in what contexts they are effective, as well as the identification of additional strategies and theories relating to their use. The refined theories are presented as CMO configurations in Appendix 12.
Chapter 8 Testing theories of decision-making
Overview
Chapter 7 presented the findings of phase 2 in relation to communication and teamwork. In this chapter we present the findings of phase 2 as they relate to the two theories concerned with decision-making: ‘impact of ergonomics’ and ‘situational awareness’. We conclude the chapter by presenting our refined theories in relation to the impact of RAS on decision-making and how and in what contexts particular strategies are effective in supporting decision-making.
Differences in workload between robot-assisted and laparoscopic surgery
We began our consideration of the impact of RAS on decision-making by exploring the differences in workload associated with laparoscopic and RAS. The mean overall SURG-TLX rating for robot-assisted operations was 66.0 (range 21 to 92, SD 16.29), while the mean overall SURG-TLX rating for laparoscopic operations was 71.1 (range 39 to 100, SD 17.57), suggesting that, on average, the workload is lower in robot-assisted operations than it is in laparoscopic operations. Figure 6 shows the mean workload for the surgeon, first assistant and scrub practitioner. This suggests that whereas the workload for the scrub practitioner is reduced in RAS, it is increased for the surgeon and the first assistant. However, there is less variability in workload in RAS for the surgeon and the first assistant than in laparoscopic surgery. This fits with the analysis presented in Chapter 7, concerning the reduced variability of the first assistant’s role in RAS.
FIGURE 6.
Mean total workload by role, with error bars showing 95% confidence intervals.
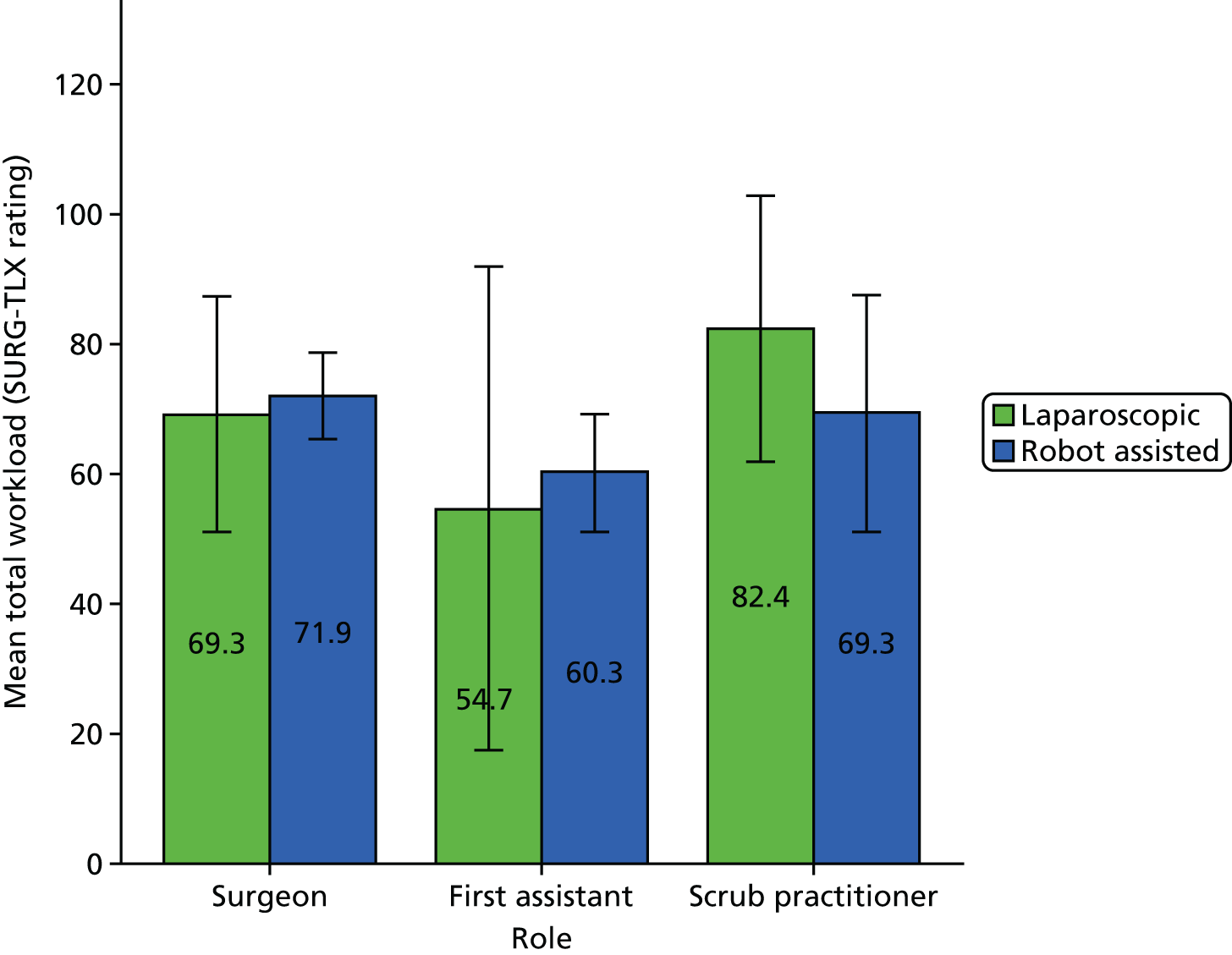
Figures 7 and 8 show the mean mental demands score and the mean physical demands score, broken down by role. This suggests that there is an increase in mental demands for all roles in RAS compared with laparoscopic surgery, while RAS increases the physical demands placed on the first assistant but reduces the physical demands placed on the surgeon and scrub practitioner.
FIGURE 7.
Mean mental demands by role, with error bars showing 95% confidence intervals.
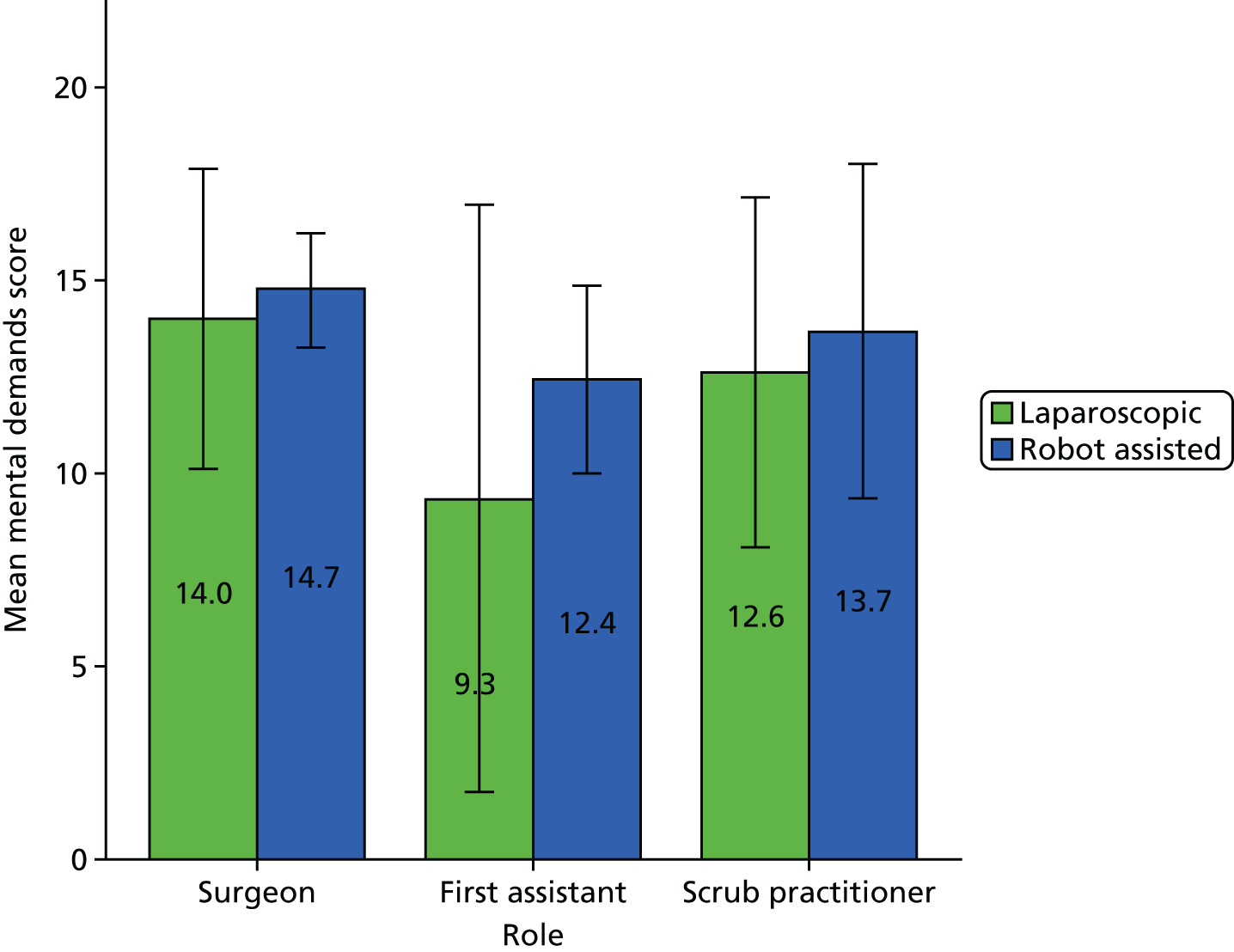
FIGURE 8.
Mean physical demands by role, with error bars showing 95% confidence intervals.
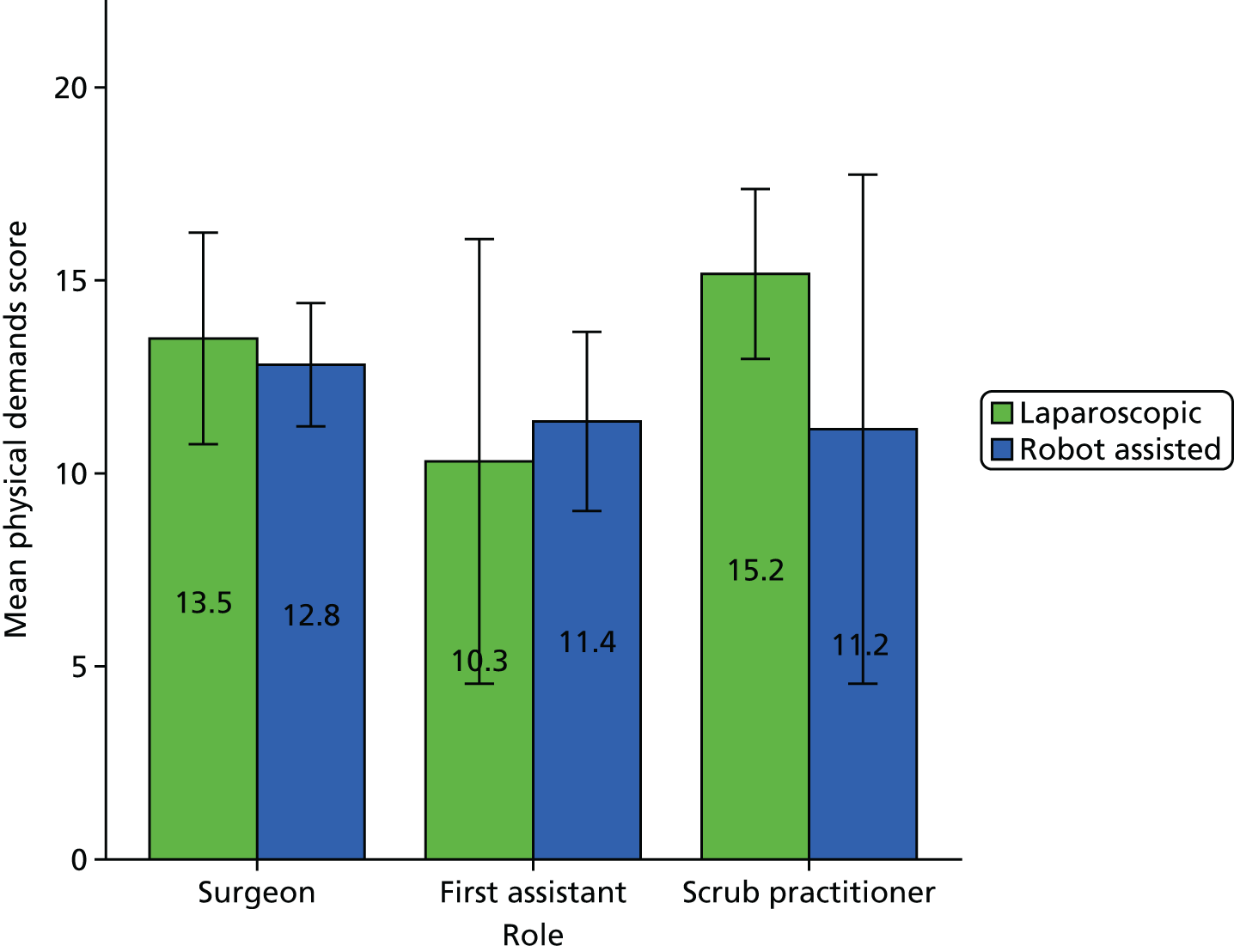
Having undertaken this initial analysis of the impact of RAS on workload, we now turn to the consideration of the theory ‘impact of ergonomics’, which focuses on how RAS impacts the workload of the surgeon and the role that experience of RAS plays as a contextual factor.
Impact of ergonomics
To begin to understand the impact of RAS on the surgeon’s workload, a Pearson product-moment correlation was run to determine the relationship between the percentage of the operation that was robot-assisted and the mental demands, physical demands and situation stress experienced by the surgeon. There was no significant relationship between the percentage of the operation that was robot-assisted and mental demands [r = 0.04; p > 0.05 (two-tailed)], physical demands [r = –0.23; p > 0.05 (two-tailed) or situation stress [r = –0.34; p > 0.05 (two-tailed)].
According to our theory, surgeons would experience reduced levels of stress and tiredness in robot-assisted operations only if they were experienced in RAS. Across the 16 robot-assisted operations observed, there were seven surgeons with a range of levels of experience. Table 10 summarises how many robot-assisted operations were observed according to the surgeon’s level of experience of RAS at that time.
| Level of experience | n (%) |
|---|---|
| 0–10 | 1 (6) |
| 11–20 | 0 (0) |
| 21–30 | 1 (6) |
| 31–40 | 6 (40) |
| 51–100 | 4 (25) |
| > 100 | 4 (25) |
Given the small number of data, it was not feasible to undertake a statistical analysis of the SURG-TLX data to understand the relationship between the percentage of the operation that was robot-assisted, the surgeon’s experience of RAS, and mental demands, physical demands and situation stress. However, Figures 9–11 show the mean mental demand, physical demand and situation stress ratings, broken down by experience, for those experience levels where we had observed surgeons undertaking both RAS and laparoscopic surgery, which provide some support for our theory. They suggest that RAS places greater mental demands on the surgeon than laparoscopic surgery until the surgeon has experience of over 100 robot-assisted operations, at which point they begin to experience less stress than they do with laparoscopic surgery. That surgeons still continue to experience a similar or greater level of physical demand in RAS until they have experience of over 50 operations may be explained by the fact that they are undertaking hybrid operations, so that the laparoscopic phase of the operation contributes to their perception of the physical demands of the operation.
FIGURE 9.
Surgeon mean mental demands by experience.
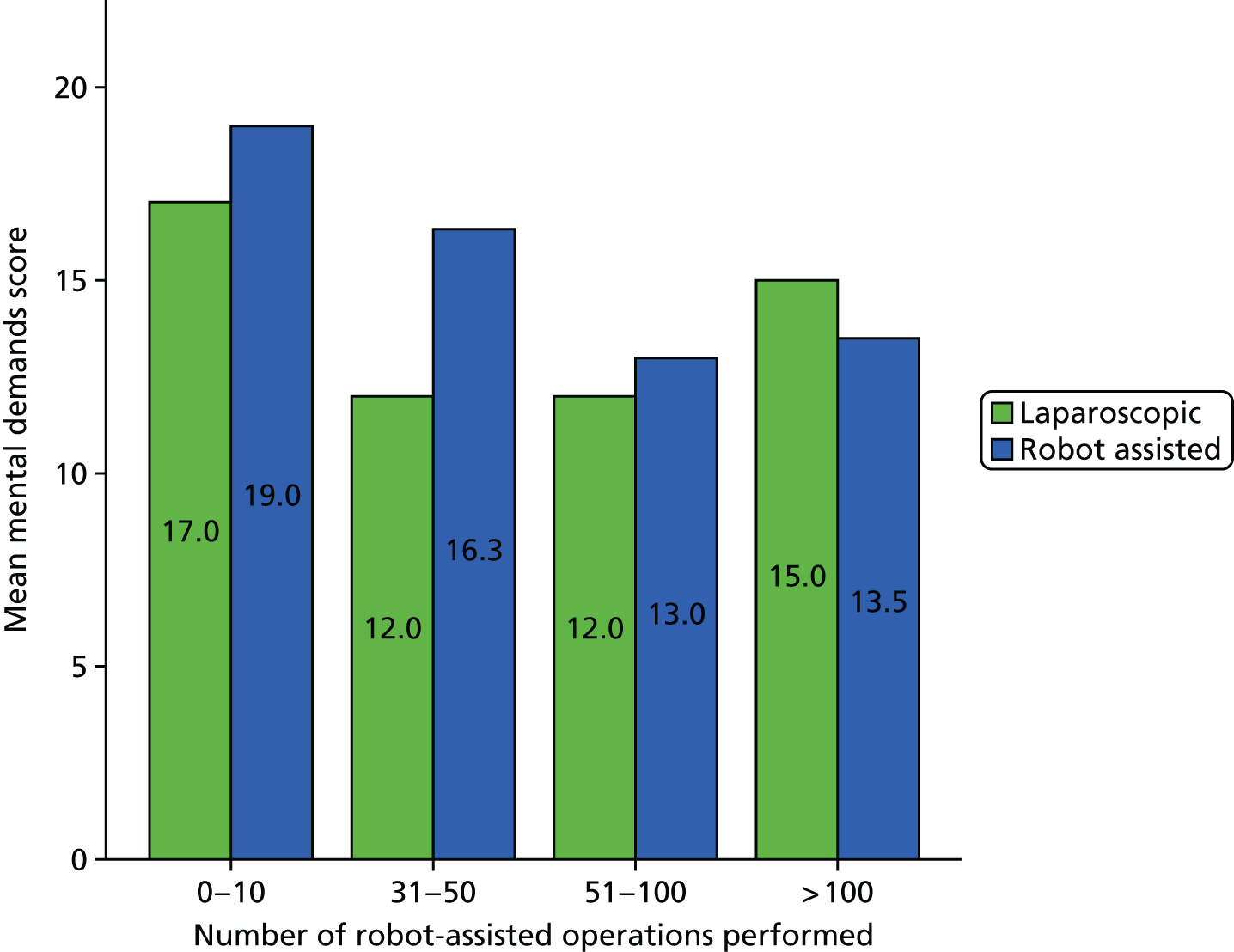
FIGURE 10.
Surgeon mean physical demands by experience.
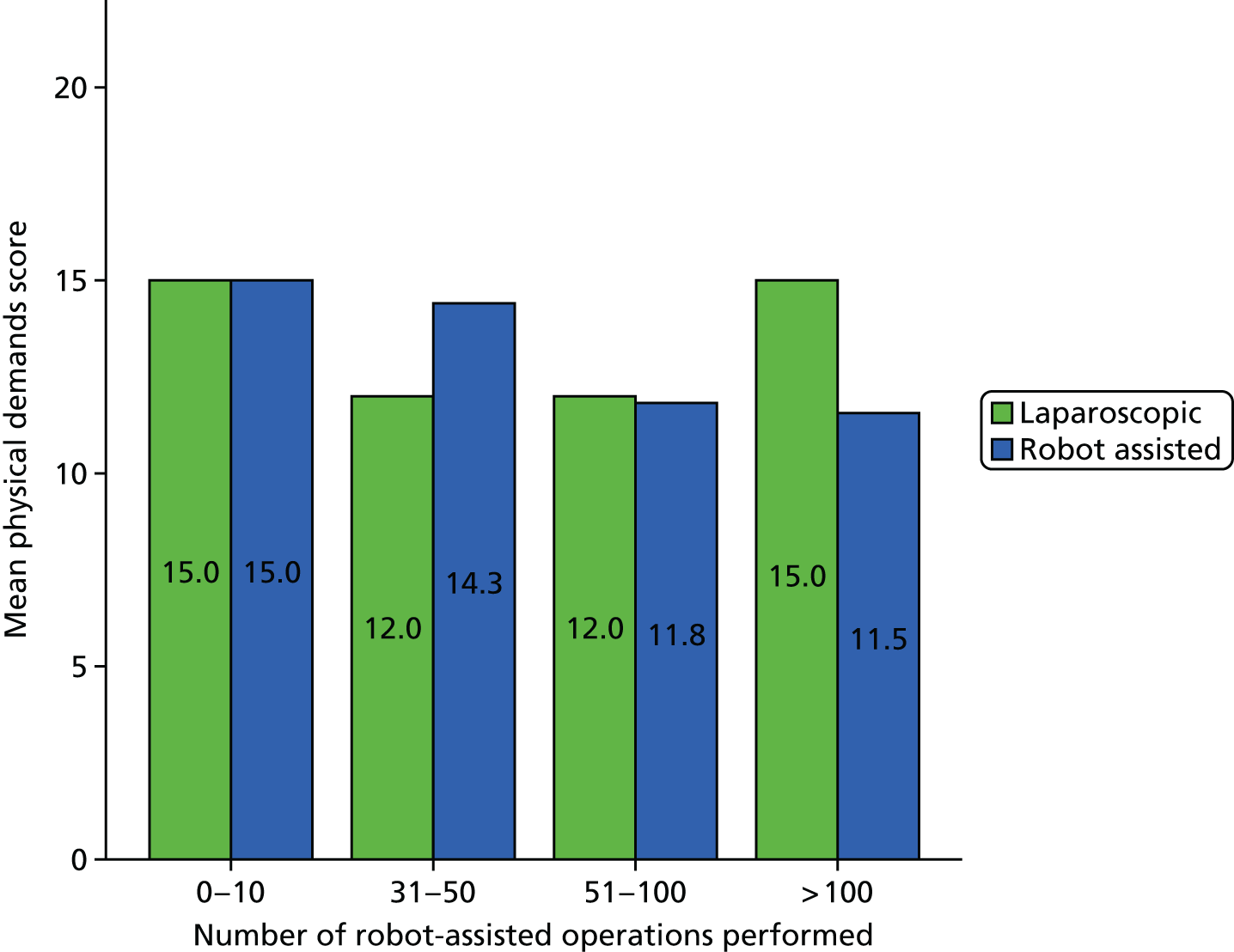
FIGURE 11.
Surgeon mean situation stress by experience.
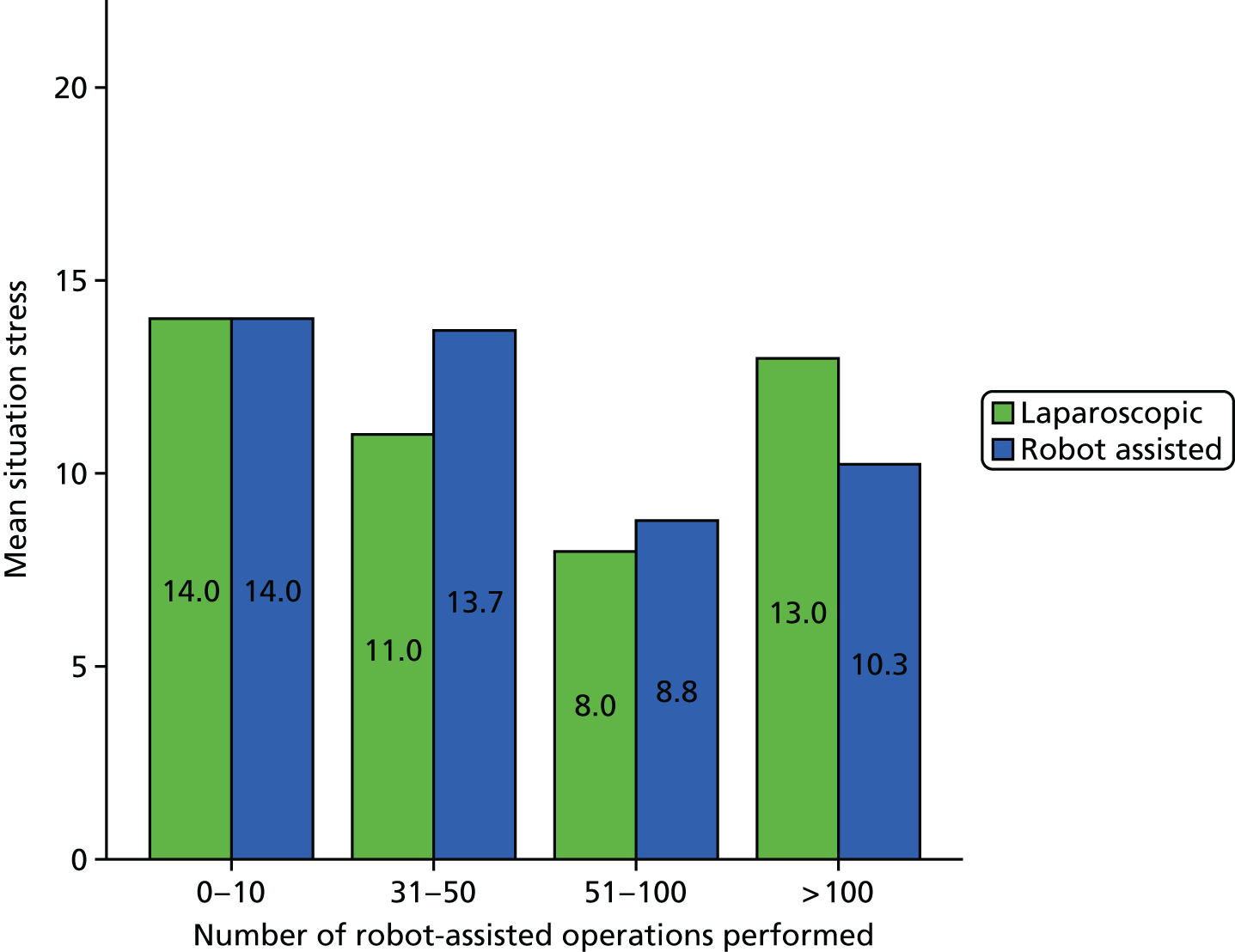
Following this initial analysis, we drew on the field notes, postoperation interviews with surgeons and video data to understand what other contextual factors affect the surgeons’ level of tiredness and stress and how RAS may counteract or enhance these factors.
Does robot-assisted surgery lead to less tiredness for the surgeon?
Beyond the self-reports provided via the SURG-TLX, it was difficult from the observations and video data of the robot-assisted phases of operations to assess the surgeons’ levels of tiredness, particularly given their position within the console. However, noticeable when observing the laparoscopic operations and the laparoscopic phases of robot-assisted operations was that the surgeons do not exhibit many obvious signs of physical fatigue. They stand squarely and on two feet, and operate for hours without a break or a drink. During the laparoscopic phase of one operation, a surgeon instructed a relatively inexperienced trainee in how to place his arms to minimise physical fatigue:
Put your elbows in next to your sides or you will get too tired . . . use both hands so you don’t have to bend.
Site 7
This indicates that the surgeon is aware of strategies to overcome fatigue. The first assistants and scrub practitioners, however, often show signs of physical fatigue in laparoscopic operations and the laparoscopic phases of operations, such as moving their weight from one foot to the other and bending and stretching their backs. The first assistants also got into more awkward positions than the surgeons. When we interviewed the surgeons about this, they agreed that they had learned to work in ways that were less tiring. This suggests that the ergonomic benefit of being able to sit down that RAS provides offers less benefit to experienced surgeons, in terms of levels of tiredness, than might be initially expected, because they have already learnt to manage this challenge of laparoscopic surgery. However, in the interviews, the surgeons also pointed to the fact that the spatial configuration in laparoscopic surgery is designed to support the consultant surgeon, which is another reason why first assistants might find themselves in more awkward positions:
The primary surgeon usually has the best position because he needs to have it. So it’s often that the assistants around this person have to adapt because obviously the person who is operating needs to have the best kind of comfortable position, and also usually the position looking straight forward onto the operating field, whereas the assistants are all coming from the side.
Site 1, surgeon
Although it did not make a significant difference, the surgeons still perceived that working at the console was more comfortable and could be less tiring:
You know, once you’re used to laparoscopic surgery, once you’re used to standing and operating, which is what we are used to, then to do it sitting down and then operating yes it helps, but I don’t think that it is a big enough . . . it is difficult to quantify but it is certainly advantage is what I would say.
Site 6, surgeon
It’s not just the fact of sitting down, but also the fact that you can relax in between you can . . . because you’re not scrubbed you can walk away and have a drink and all these kind of things, look at your phone [laughs].
Site 1, surgeon
Stress can also impact on the perceived physical and mental demands of an activity, which we consider in the following section.
Does robot-assisted surgery lead to less stress for the surgeon?
The analysis of the field notes and video data indicated a number of factors that could affect the surgeon’s level of stress, which could be both positively and negatively impacted by the use of the robot, and in some cases strategies were established for reducing the negative impact. For example, time pressures can be a source of stress. ROLARR found that the mean operative time (defined as time from first incision to time of wound closure completion) for robot-assisted operations was 4 hours and 59 minutes, while for laparoscopic operations it was 4 hours and 21 minutes. 194 The mean theatre time (defined as time between the patient first entering the anaesthetic room and the patient leaving theatre) for robot-assisted operations was 6 hours and 31 minutes, while for laparoscopic operations it was 5 hours and 53 minutes. Similarly, we found that the mean operative time (using the same definitions as ROLARR) for the robot-assisted operations we observed (6 hours and 32 minutes, range 4 hours and 55 minutes to 8 hours and 50 minutes) was longer than for the laparoscopic operations (5 hours and 29 minutes, range 3 hours and 42 minutes to 6 hours and 25 minutes). OT teams in our sites recognised this fact and, therefore, if at all possible, would put a single robot-assisted case on a list, to reduce the time pressure. If more than one operation was scheduled, the robot-assisted case would ideally be placed at the beginning so that the set-up could be completed in good time. In many other cases the initial lack of a bed caused delays to the start of robot-assisted operations. This sometimes appeared to cause stress to the team, and to the surgeons, as they were left in a state of uncertainty. Some team members became annoyed about the possibility of the list over-running. Although such delays occurred for both laparoscopic and robot-assisted operations, there was particular concern when it happened for a robot-assisted operation because of the longer duration of robot-assisted operations. It was not clear how the team’s feelings about this situation affected the stress levels of the surgeon during the operation. Some concerns were vocalised in the surgeon’s hearing, however, and may have had an adverse effect on some occasions. In one operation at site 7, the robot was required by another surgeon at a specified time, and this may also have caused some stress.
Unfamiliar equipment can also be a source of stress, and some of the surgeons were relatively inexperienced with the robot. The stereo viewer and the touchpad in the armrest can display more than 300 different messages and icons for the surgeon to interpret and act on. During several operations, the surgeon did not know what the messages meant. In one operation at site 6, for example, a message came up on the screen saying ‘move master grip to match the instruments’ but the surgeon did not know what this meant, first asking the scrub practitioner what it meant and then telling the first assistant to ‘make a note of that and I will ask [the representative]’. However, even for more experienced surgeons, the robot could be a source of confusion. During an operation at site 1, a message appeared on the screen that the surgeon did not seem to understand, and two yellow warning lights appeared on the arm. He remarked ‘it has a life of its own’, suggesting that he was not sure what was going on at that particular moment, despite having experience of over 100 robot-assisted operations.
A frequent problem concerning robot-assisted equipment was the arms clashing, and this happened at least once in the majority of the operations we observed. Ideally, the ports should be positioned in such a way as to prevent this problem but that was not always possible and clashes often occurred. When this happens the surgeon cannot move his instruments. If this happens at a crucial point it could be dangerous and so may cause the surgeon stress.
The complexity of a procedure and patient factors will also affect the level of stress, and the robot can interact with this in multiple ways. For example, if the case is complex, a surgeon may choose to use the robot to take advantage of the precise and dexterous EndoWrist instruments and enhanced vision. In one operation, the tumour was low in the rectum and the surgeon warned that an abdominoperineal resection may have to be performed. The surgeon remarked to the researchers that ‘things would be tense’ in the OT because of this, indicating that he may have been feeling some stress. Another surgeon observing told the researchers that the tumour was ‘as low as a rectal tumour can get’ and that an anterior resection might be easier to achieve using the robot. However, patient factors can compound problems related to lack of confidence with RAS. In one operation at site 7, the patient was unusually tall. Despite a relatively high level of experience with RAS, having completed over 100 robot-assisted colorectal operations by the end of the period of data collection, the surgeon admitted having difficulty remembering where to place the ports for the robotic instruments and always used a leaflet supplied by the manufacturer to guide him. In this case the length of the patient’s abdomen was longer than normal and the usual positioning of the ports did not work as well. This may have caused stress during the operation, as the surgeon remarked that the instruments could not be placed in ‘ideal positions’ during the surgery. Similarly, in an operation at site 1, the patient had an unusually narrow abdomen, which meant that, despite the surgeon’s experience with RAS, it was difficult to avoid the robot arms clashing.
Issues related to teamwork could also be a source of stress and, as described in the previous chapter, RAS introduces a number of challenges for teamwork and communication. For example, having to repeat requests can be a source of frustration for the surgeon. Whereas tiredness was difficult to judge, frustration was often easy to detect in the surgeon’s voice and, for those operations we video recorded, the microphone the surgeon wore allowed us to capture their deep sighs and cursing. Related to this is the experience of the team, and it seemed that working with inexperienced staff can be stressful for the surgeon. This was apparent during an operation at site 6, where the first assistant was inexperienced and the surgeon’s words and tone of voice revealed that he was stressed on several occasions. At one point, for example, when the first assistant was failing to adequately help the surgeon with retraction, the surgeon said, ‘stop poking around there . . . If you can’t see, I can’t see’. Later on, when there was a possibility that the first assistant would do harm to the patient, the surgeon said loudly, ‘hold this – don’t pull until I have let go of it or it will tear. Jesus Christ man, don’t scrape the sacrum or you will cause a sacral bleed!’. In an operation at site 1, both the first assistant and the scrub nurse were inexperienced with the robot and problems occurred, which appeared to cause stress for the surgeon.
The surgeon’s position in the console could have both positive and negative impacts on their level of stress. It has the potential to aid the focus of the surgeon, decreasing the surgeon’s stress by reducing distractions, although the extent to which surgeons stayed in the console varied considerably, again influenced by the experience of the team (discussed further below). The fact that the surgeon was obliged to have their head in the console could also be a cause of stress if they did not trust the team to let them know what was happening.
Noise within the OT can also be a cause of stress, particularly in RAS. In part, it is because of the speakers within the console, which increase the surgeon’s awareness of noise within the OT. As a result, whereas music was typically played in laparoscopic operations, music was not played in any of the robot-assisted phases we observed. Additionally, the difficulties in communication between the surgeon and the scrub practitioner and first assistant could be exacerbated if there was additional talking by circulating, anaesthetic or other staff. Surgeons at all sites recognised this problem. During an operation at site 7, for example, the surgeon asked the staff to be quiet at one point so he could hear what the first assistant was saying. In another example, during an operation at site 6, the anaesthetist was speaking loudly on his mobile phone. This obviously distracted the surgeon at the console, and he politely asked the anaesthetist to leave the OT. The surgeon at site 5 agreed that if staff talked too loudly it could be difficult for the first assistant to hear the surgeon on the console. One of the surgeons at site 1 explained that, if extra staff came into the OT and wanted to chat before he had finished his case, he asked the scrub practitioner to send them out as he found it distracting.
Decisions related to the implementation of RAS could also impact on the level of stress in a robot-assisted operation. For example, there was general agreement among the staff we observed that a large enough OT was essential to the smooth running of a robot-assisted procedure. The surgeon at site 7 felt very strongly about this matter, as it could compromise patient safety:
This theatre is too small for a robotic case.
Just chill.
I can’t just chill, I want to do a good operation and the circumstances are not good. It won’t be your name that goes on the list, it will be mine.
The surgeon at site 6 was also unhappy if a large OT was not available for his robot-assisted cases and after one operation said the operation was difficult to perform because of the small OT. At this site a number of large OTs were available but were sought after by several surgeons. At site 7 a large, dedicated robotic OT had been established, while at site 5 the OTs were not very large but there was no choice about using them.
The consideration of the above issues suggests the robot does not, necessarily, lead to less stress for the surgeon. If the surgeon was able to use the robot with an experienced assistant and an experienced team, and in a large enough, quiet OT, the potential stress-reducing benefits of the system (good vision, focus, control of the camera and precise and ergonomic instruments) might be realised.
A revised theory
We started with the following theory:
When a surgeon is experienced in undertaking RAS, the ergonomic benefits of RAS reduce the surgeon’s levels of stress and tiredness, enabling them to persist longer with the operation and thereby reducing the rate of conversion to open surgery.
We sought to test the first part of this theory, that for surgeons experienced in RAS, the ergonomic benefits of RAS result in lower levels of stress and tiredness than in laparoscopic surgery. Our analysis suggests that, although surgeons find working at the console more comfortable, this does not significantly impact their level of tiredness because they have developed strategies to manage the physical demands of laparoscopic surgery. A number of contextual factors can affect a surgeon’s stress levels in an operation, and the resources introduced and taken away by RAS interact with these factors in both positive and negative ways. The most significant of these factors appeared to be the experience of the team, particularly the first assistant and the scrub practitioner, tying in with the theories ‘experienced assistant’ and ‘scrub practitioner support’ presented in Chapter 7. Therefore, it is appropriate to revise these theories to include reduced levels of stress for the surgeon as an additional impact of these strategies:
Knowledge gained through experience of the particular procedure enables the first assistant to work with less guidance, adjusting the position of the grasper and moving the suction instrument without prompting. Their experience also provides them with knowledge of what actions are likely to be required, which they make use of by making oral offers of assistance and/or preparing to act (e.g. by holding the instrument ready). Together, these behaviours support coordination between the surgeon and first assistant and increase the likelihood that the first assistant’s actions are performed correctly and in a timely manner. This in turn reduces the surgeon’s level of stress. The success of this strategy depends not only on engagement in the procedure but also, in preparing to act, the first assistant’s keenness to demonstrate to the surgeon their ability to respond quickly.
Where the first assistant is inexperienced, in either RAS or the particular procedure, but the scrub practitioner is experienced in RAS, knowledge gained through experience enables the scrub practitioner to both guide the first assistant and undertake certain tasks (changing the robotic instruments) on their behalf, ensuring these actions are performed correctly and reducing the surgeon’s level of stress. The success of this strategy depends on the first assistant’s willingness to acknowledge the need for support and to accept such support, which can be increased through the surgeon acknowledging this aspect of the scrub practitioner’s role.
Situational awareness
To test the theory ‘situational awareness’, we sought to understand how and in what contexts RAS impacts the surgeon’s situational awareness and the consequences of this, with particular attention to the role the team plays in maintaining the surgeon’s situational awareness.
Does robot-assisted surgery reduce the surgeon’s situational awareness?
The mean surgical subteam intraoperative monitoring score across all operations observed was 3.68 (range 2–5, SD 0.72), suggesting that, in most cases, the situational awareness of the surgeon and first assistant either moderately enhanced team functioning or had neither a positive nor negative impact. To make an initial assessment of the impact of RAS on the surgeon’s situational awareness, the correlation between the percentage of the operation that was robot-assisted and the OTAS intraoperative monitoring score for the surgical subteam was calculated using the Pearson product-moment correlation. There was no significant relationship between the percentage of the operation that was robot-assisted and the intraoperative monitoring score for the surgical subteam [r = 0.21; p > 0.05 (two-tailed)].
This was reflected in our observations, where we found that, in general, the surgeons did seem to be aware of what was going on within the OT, despite their position in the console. This was demonstrated, for example, by joining in with discussions with the rest of the OT team. However, this did vary over the course of the operation, with the surgeon becoming more focused on what was happening in the surgical site and less likely to join in conversations or to communicate with the rest of team as the operation progressed. For example, on one occasion there were two surgical trainees assisting with the operation, one observing and the other acting as first assistant. At one point in the operation, the trainees swapped roles and did not inform the surgeon. He became aware of this change only when he directed a question at one of the surgical trainees and was told that the other surgical trainee was now acting as first assistant. Although such examples serve to illustrate the way in which the surgeon’s awareness of the broader OT varies over the course of the operation, we did not observe any problems resulting from the surgeon lacking awareness of what was happening outside the console.
It is also important to note that, while the theory assumes that the surgeon’s situational awareness is reduced because of their position in the console, there was significant variation in the extent to which the surgeon stayed in the console. For example, in one totally robot-assisted operation, the surgeon brought his head out of the console only twice, both times when a member of staff approached him to ask him a question. When the camera was being cleaned, meaning that surgeon would not be able to see or do anything, he kept his head inside the console, appearing to use this as an opportunity to rest. Resonating with our theory, the extent to which the surgeon came out of the console appeared to vary according to the experience of the first assistant, suggesting that when the surgeon trusts the first assistant to carry out the requests successfully they feel more comfortable remaining in the console. Related to this may be the ability of a more experienced first assistant to tell the surgeon what is happening, as suggested by this quotation from a surgeon:
If there is anything which doesn’t look right, I just need to see what is happening, and that is only possible by taking your eyes off the console and seeing what is happening. [. . .] If you have an experienced assistant then at least okay you don’t need to take your eyes off but at least you can ask them, you can talk to them and they will communicate things back to you.
Site 6, surgeon
The extent of the trust a surgeon has in the team that are supporting him or her can change over the course of an operation. For example, following an incident involving a first assistant and scrub practitioner with limited experience of RAS where a robotic instrument was incorrectly inserted, the surgeon then regularly brought his head out of the console to see what the first assistant and scrub practitioner were doing, and on numerous occasions left the console in order to help them. This also suggests that the level of distraction experienced by the surgeon will vary according to the experience of the first assistant and the scrub practitioner.
There was variation across the sites in terms of where the robotic console was positioned, which impacted the ability of the surgeon to be able to observe what the first assistant and the scrub practitioner were doing. In site 1, the console was placed parallel to the patient and the team, enabling the surgeon to see the first assistant and scrub practitioner simply by moving his head out of the console. In the other sites, the console was placed further away, making it harder for the surgeon to see the first assistant and the scrub practitioner without leaving the console.
We also observed instances in which, drawing on cues provided through the robotic console, the surgeon’s situational awareness appeared to be better than the team’s. Some support for this is provided by the OTAS data, in which the mean surgical subteam intraoperative monitoring score in robot-assisted operations was 3.69 (range 2–5, SD 0.7), slightly higher than the mean nursing subteam intraoperative monitoring score of 3.62 (range 2–5, SD 0.81). For example, the surgeon might experience difficulties in moving the robotic instruments and ask the team if the robotic arms are clashing, if the team has not already noticed it and alerted the surgeon. There were multiple instances in which the surgeon asked if the gas that was inflating the patient’s abdomen was leaking, leading the team to check and correct the situation. OT staff we spoke to thought the surgeon might be more aware of that because of the magnified 3D image that the robot provides, but the surgeons understood the situation differently. Potentially, RAS makes it harder to perceive this, because they can only draw on cues from within the surgical site:
I think laparoscopic you see it easier because you are standing there, you know. You’re standing there, you know that the tube is kinked or the anaesthetic muscle relaxant has worn off so the patient is coughing, so that it is pressing, or the suction is being operated, so you probably pick it up earlier with laparoscopic surgery.
Site 6, surgeon
The reason that the surgeons notice the gas is leaking is because of their role, because it affects the ease with which they can operate:
I mean the surgeon is acutely aware that there’s a loss of volume and that everything gets more difficult, so the surgeon is certainly aware that things are becoming difficult and the reason is there’s no gas. But he’s always going to be the first one who is aware of it because he gets the feedback that things have suddenly become more difficult.
Site 1, surgeon
This again points to the implications of a changed division of labour; because the first assistant is doing less within the surgical site, they may be less likely to notice such changes.
Where the surgeons did seem to lack situational awareness related to the positioning of instruments within the surgical site, in terms of both instruments held by the robotic arms and laparoscopic instruments inserted by the first assistant. There were a number of occasions when surgeons would say that they had ‘lost’ an instrument. Sometimes this was in reference to the robotic arms not moving in the way the surgeon wanted, usually because the robotic arms were clashing. More often, such statements were made when the surgeon had positioned one instrument to assist with retraction, moved the camera away, and then was struggling to find it again. This was not a significant problem because if they pull back the camera they can find the instrument, but it was an issue that appeared to disrupt the flow of the operation, and there are safety risks if the surgeon tries to move the instrument when it is not under vision.
This problem did not appear to be due to lack of experience, as it was a challenge encountered both by surgeons experienced in RAS and by those who had less experience. That this problem occurred frequently led us to consider whether the changed division of labour, with the surgeon now controlling the camera and up to three instruments, increased the cognitive load for the surgeon. Some support for this was provided by the analysis of the SURG-TLX mental demand data presented above. However, the surgeons did not perceive the situation in that way, emphasising that the cognitive demands of monitoring and co-ordinating with the first assistant were reduced:
I don’t think it impacts my cognitive load because when I’m operating laparoscopic, if I have anyone junior to middle grade inclusive, junior to middle grade I’m forever having to correct them on their assistant positioning and camera holding. So actually it’s allowed me to concentrate more on what I’m operating on.
Site 7, surgeon
Another common occurrence relating to the positioning of instruments within the surgical site, and one that appeared to cause significant frustration for both the surgeon and the first assistant, was when the surgeon asked the first assistant to insert an instrument and the first assistant did it, but the surgeon did not realise. This results in delays to the procedure as the surgeon has to ask what is happening. We did not witness such instances in the laparoscopic operations or in the laparoscopic phases of the robot-assisted operations that we observed. In a laparoscopic operation, the surgeon is standing by the first assistant and, even if they cannot see the instrument on the screen (the intracorporeal view), they can observe that the first assistant has inserted the instrument (the extracorporeal actions) and would be able to determine from how the assistant is holding the instrument where the end of that instrument is. But in RAS, the surgeon does not have these cues.
As described in Chapter 7, some surgeons were aware of this problem and overcame it by encouraging their first assistants to tell them what they were doing as they were doing it, leading to the use of more explicit communication than observed in the laparoscopic phases. For example, in one operation, the two first assistants were both inexperienced and the surgeon frequently asked them to tell him what they were doing. Waiting for one of them to insert an instrument, he said, ‘tell me when you are coming in’. However, this surgeon was also observed asking similar questions of very experienced assistants. In a postoperation interview this surgeon explained that he insisted on this frequent communication because it reduced his stress and frustration, and said that ‘a second can seem like a minute’ when he is at the console. He also said that he wanted to make sure the assistants were happy to speak to him.
This problem was also prevented by the first assistant asking the surgeon to move the camera so that they could see the instrument being inserted, so whether such a breakdown occurs will be affected by whether or not the first assistant is willing to direct the surgeon’s movement of the camera.
Related to this, while surgeons may have lacked confidence in the ability of an inexperienced first assistant to tell them what was happening with the patient, as described above, they did use the strategy of asking questions to maintain their awareness of what the first assistant was doing:
OK, are you putting traction with your left hand?
Yes, the left side.
OK, are you sucking, yeah?
Site 6
So where’s your grasper?
Site 6
And while you’re in there, suck, OK?
I am, yep, I’m sucking. Constantly.
Are you sucking?
Yep.
Site 7
That this was only observed when working with an inexperienced first assistant suggests the issue is not only that the surgeon cannot see what the first assistant is doing but also that they do not necessarily have confidence that the first assistant is completing their requests correctly.
Does the team contribute to the surgeon’s situational awareness?
In line with our theory, we observed several instances of members of the team, most often the scrub practitioner, alerting the surgeon to the robotic arms clashing:
The scrub nurse and first assistant tell the surgeon that the scissors and prograsp are clashing. The surgeon briefly pulls his head out of the console to look over to the robotic arms.
Site 1
You’re clashing.
Which way is arm 2 pointing?
They then move the robotic arms to different ports to overcome this problem.
Site 7
We also observed an occasion of the first assistant warning the surgeon that the gas was leaking and the scrub practitioner alerting the surgeon to an error message on the screen.
Beyond this, we did not observe the communication of other information to the surgeon, apart from relaying messages from staff and visitors outside the OT. This may be because there was no other information that it was necessary for them to communicate. However, more generally, we found that very little communication was initiated by the team, with nearly all communication with the surgeon being initiated by the surgeon. This was despite good relationships between the surgeons and the teams we observed. Generally, the surgeons were friendly and appreciative of the team’s help. Across the sites, staff seemed to appreciate that the surgeons might be stressed prior to an operation and that this might lead to a level of abruptness. Only at one site was a comment made by an ODP that she would be nervous about speaking up in front of one of the surgeons and that others felt the same, which she felt could affect patient safety if staff were reluctant to point out things that were going wrong.
The exception was site 7, where team members would initiate communication with the surgeon, for example asking him questions. Although this surgeon would express his frustration with the staff at times, it was clear that team members were not upset by this and often smoothed such incidents over with jokes. In the phase 1 interviews at this site, and in the brief interviews following the operations observed there, the surgeon and other members of the team talked about the need for a different type of communication within RAS, without the hierarchy, again pointing to differences of culture between sites.
The ability of the OT team to communicate information to the surgeon is also dependent on the team’s situational awareness. The mean nursing subteam intraoperative monitoring score across all operations observed was 3.64 (range 2–5, SD 0.79), suggesting that in most cases the monitoring behaviours of the scrub practitioner and circulating practitioner either moderately enhanced team functioning or had neither a positive nor a negative impact. The correlation between the percentage of the operation that was robot-assisted and the nursing subteam intraoperative monitoring score was calculated using the Pearson product-moment correlation. There was no significant relationship between the percentage of the operation that was robot-assisted and the nursing subteam intraoperative monitoring score [r = 0.01; p > 0.05 (two-tailed)]. However, as noted above, the leaking of gas from the abdomen was something the team did fail to perceive on repeated occasions. If such problems are not identified and resolved quickly, this does have the potential to negatively impact the surgeon’s decision-making because his or her view of the surgical site is hindered. If the surgeon does not immediately realise what is happening, he or she may base the decision on a restricted view or may simply be distracted by it.
There was also one occasion when the robotic arms were clashing but the first assistant and scrub practitioner did not appear to notice, becoming aware of it only when the surgeon asked them if the arms were clashing. This was not necessarily because they were disengaged but because they were looking elsewhere, the first assistant watching the screen and the scrub practitioner looking at the instrument trolley. This suggests a possible conflict between being engaged in the operation in terms of watching the screen and maintaining awareness of what else is happening. In other operations, we observed the scrub practitioner repeatedly moving their attention between the robotic arms and the screen, not only to see if the robotic arms were clashing but also to see if the arms were coming too close to the patient. This was a behaviour witnessed among those scrub practitioners who had greater experience of RAS.
The spatial configuration also appeared to impact the ability of the scrub practitioner to monitor both the screen and the robotic arms. For example, in one operation the scrub practitioner was seated with her back to the patient, facing the trolley and turning her head to the left to see the screen. In other operations, the scrub practitioner would sit facing the screen, able to look left to see the patient and the robotic arms and able to look right to see the instruments. However, such positioning is under the control of the scrub practitioner, and it was among the scrub practitioners more experienced in RAS that we observed them positioning themselves so as to be able to easily move their attention between the screen and the robotic arms.
A revised theory
We sought to test the following theory:
When the OT team communicate information to the surgeon to make them aware of problems with the patient or the robot, the surgeon’s situational awareness is maintained, enabling them to adjust their decision/course of action based on this information, avoiding complications during the procedure. Where the surgeon has trust in his team to communicate problems in this way, the surgeon will feel confident to remain within the console, resulting in reduced distraction and increased concentration, which in turn can positively impact decision-making. This strategy will be successful where the team has a positive relationship with the surgeon.
On the basis of the analysis presented above, we can make a number of revisions to this theory. Whereas the theory suggests the extent to which the surgeon stays in the console is based on the extent to which they trust the OT team to communicate problems with the patient or the robot, our observations suggest it is their trust in the first assistant’s ability to carry out requests successfully that impacts the extent to which they stay in the console, and that this trust in turn depends on the first assistant’s level of experience:
Where the surgeon trusts the first assistant to successfully carry out requests and to communicate any problems, the surgeon will feel more confident to remain within the console. This trust is likely to be present when the first assistant is experienced or where they are being supported by an experienced scrub practitioner. In this context, RAS results in reduced distraction and increased concentration for the surgeon.
A significant difference from the theory was that the surgeon’s overall situational awareness did not appear to be reduced. Where their situational awareness was reduced was in relation to whether or not the robotic arms were clashing, the position of the robotic instruments and whether or not a request had been actioned. The analysis provides insight into strategies used by scrub practitioners to maintain their awareness of the position of the robotic arms so as to be able to communicate this information to the surgeon:
Experience of RAS makes the scrub practitioner aware of the need to move their attention between the screen and the robotic arms and to position themselves in such a way that facilitates this. This enables them to notice when the robotic arms are clashing or about to clash and to alert the surgeon to this, maintaining the surgeon’s situational awareness and enabling them to adjust the positioning of their instruments.
In relation to the surgeon’s awareness of the position of the robotic instruments, we can add the following theory:
When the surgeon positions a robotic instrument to assist with retraction and then moves the camera to another area of the surgical site, they may lose awareness of the position of the instrument. This can disrupt the flow of the operation, potentially affecting the operation duration, and there are patient safety risks if the instrument is moved while not under vision.
Whether or not a request has been actioned is covered by the ‘oral response’ theory presented at the end of Chapter 7 and, therefore, we can revise this theory to reflect its contribution not only to co-ordination but also to the surgeon’s situational awareness:
Oral responses to the surgeon’s requests confirm that work will be done and make apparent any challenges in completing the request, supporting effective co-ordination and increasing the surgeon’s situational awareness. The use of this strategy by the first assistant and scrub practitioner is more likely when the surgeon encourages the first assistant and scrub practitioner to communicate their actions and its success is dependent on the volume and clarity with which the information is communicated.
Whether or not the team communicate information to the surgeon depends not only on the team’s situational awareness. Although the theory proposed that the team would communicate information when they have a positive relationship with the surgeon, our analysis suggests that this is not enough. Rather, the team need to be encouraged to communicate. This fits with the ‘license to speak’ theory presented at the end of Chapter 7. Although that theory focuses on communication by the first assistant, the analysis presented here suggests that this is a more general phenomenon and, therefore, we will revise that theory to reflect this:
When the surgeon encourages the OT team to communicate both actions and concerns, team members will feel comfortable to speak up when necessary. This supports co-ordination and helps to ensure tasks are completed correctly, as well as increasing the surgeon’s situational awareness. The success of this strategy is likely to depend on the team’s relationship with the surgeon.
Summary
Through empirical testing of our two theories relating to decision-making in RAS, we have increased our understanding of how RAS impacts the surgeon’s level of stress and situational awareness, and strategies that can reduce these impacts. Communication, teamwork and decision-making are closely interlinked, with practices of communication and teamwork affecting the propagation within the OT of information used for decision-making. Consequently, the analysis presented here has led to the revision of a number of the theories presented in Chapter 7. The refined theories at the end of phase 2 are summarised in Appendix 13.
Chapter 9 Widening the applicability of the theories
Overview
Chapter 8 concluded by presenting the refined list of theories generated through empirical data collection within the context of colorectal surgery in phase 2 of the study. In phase 3, we sought to assess the generalisability of these theories through interviewing surgeons and OT teams in other surgical specialties that undertake RAS. In this chapter, we present the findings of these interviews and the resulting refinements to the theories.
In phase 3, we also organised a ‘design workshop’ with a range of relevant stakeholders in order to identify and explore potential practical responses to the findings of the study. This chapter draws together some of the key outcomes from that workshop.
Interview findings
Across the three sites, the robot was used for a range of operations. At site 1, the robot was used in urology for prostatectomies and they were planning to start using it for cystectomies. At site 6, in urology the robot was used for prostatectomies and nephrectomies and in gynaecology it was used for hysterectomies. At site 7, the robot was used in urology for pyeloplasty and adrenal surgery and in upper GI for Heller myotomy, gastric pacing, oesophagectomies and gastrectomies.
In general, our interviewees reported that the theories presented to them fitted with their experiences. However, in relation to certain theories, the relevance of particular contextual factors became apparent. We discuss these theories in turn below. The surgeons we interviewed also had a different perspective on the impact of RAS on their situational awareness, so we also present these ideas, before considering other differences between specialties in relation to the introduction of RAS.
Experienced assistant
Our interviewees agreed with the importance of having an experienced first assistant in RAS, and all emphasised the importance of having an experienced scrub practitioner. However, it seemed that in other types of robot-assisted operations an experienced first assistant would be more likely to act without prompting, rather than making an oral offer of assistance. It was felt there were several contextual factors that affected this. The first was the extent to which the steps of the operation were routinised. For example, at site 1, all interviewees agreed that robot-assisted prostatectomies were carried out in a very routinised way, making it easy for the first assistant to know what assistance was required:
Because we’ve always done prostatectomies, and both our surgeons are quite methodical, aren’t they, that it will be the same thing every single time. So that’s why we [the scrub practitioners] know exactly what we’re doing, and they [the surgical trainees] know exactly what they’re doing. It’s only when we have turnaround of [surgical trainees], say like in October, they might struggle again.
Site 1, urology ODP
I suppose we’re a bit further down the line, and it’s almost protocol, it’s almost automatic what we do.
Site 1, urology surgeon
Related to this was the extent to which patient factors would affect the assistance required. For example, in colorectal surgery, variation in patient anatomy significantly impacts the extent of assistance needed for retraction. In our phase 2 observations, surgeons and first assistants would talk about the difficulty of achieving adequate retraction if a patient had a ‘floppy’ colon. In contrast, it was felt that the amount of retraction needed when undertaking a prostatectomy was more consistent across patients:
So maybe it works a bit different in prostates [. . .] compared to colorectal where there’s a lot of bowel and things like that hanging around.
Site 1, urology trainee
Another contextual factor was the frequency with which first assistants participate in robot-assisted operations and the variation in the operations that they do. At site 1, although the robot was used for a number of different colorectal operations, urology surgical trainees would participate in four robot-assisted prostatectomies a week, enabling them to quickly build up the experience to know what assistance was needed and to have confidence in providing that assistance without prompting.
Discussing this theory with the urology ODPs and the surgical trainee also revealed that, in relation to the changing of robotic instruments and monitoring the movements of the robotic arms to see if they are clashing, the responsibility was more shared, in part due to the spatial configuration in the OT. Whereas in most colorectal operations the robot would be brought in from the side, with the first assistant and the scrub practitioner standing on the other side of the patient, in prostatectomies the robot is brought in between the legs (see Figure 1), with the first assistant standing on one side and the scrub practitioner standing on the other side. The first assistant would take responsibility for the robotic arms on their side of the patient and the scrub practitioner would take responsibility for the robotic arms on the other side. Thus, there was less confusion about who was responsible for what.
Awareness of instruments
Our interviewees perceived that a surgeon ‘losing’ a robotic instrument, in terms of losing awareness of robotic instruments outside the camera’s field of view, was less likely to happen in other types of surgery because in those other types of surgery the surgeons are working in a smaller space:
More often than not all my instruments are in the field of view and I don’t need to change that field of view, I don’t need to pan in or out because I’m working in the pelvis.
Site 1, urology surgeon
I think this is mainly colorectal isn’t it because they have so much more than we would.
Site 7, upper GI surgeon
Situational awareness
In discussing the impact of RAS on situational awareness, a theme that came up among the comments of the surgeons we interviewed was that, although they cannot see what is happening in the OT, they hear it more clearly. Some said that this was because of the speakers inside the console: that although problems with the microphone in the console may mean that the OT team cannot hear the surgeon well, the speakers inside the console work well. However, others felt that they became more alert to noise because their ability to draw on their other senses was reduced:
But my theory is that you lose tactile feedback, so I think your other senses become enhanced and definitely sense of sound. And I’m very conscious that I can hear every single sound in the room, so if there’s something slightly different I pick it up.
Site 1, urology surgeon
I would also say I think I have a more heightened sense of awareness with my hearing in doing robotic case because you can’t see what’s going on in the room.
Site 7, urology surgeon
This could be problematic, as case-irrelevant discussions in the OT could be a distraction, a sentiment expressed by colorectal surgeons in phase 2.
The interviews also gave insight into the possible negative consequences of the surgeon not being able to see what is happening in the OT. Having participated in a greater number of robot-assisted operations, our interviewees were able to draw on that experience to provide examples of the rare occurrences we were unlikely to have witnessed in the sample of robot-assisted operations we observed and that the colorectal surgeons and OT teams may not have experienced. One urology surgeon described a robot-assisted operation in which the patient had a cardiac arrest. The anaesthetist was not part of the normal team and did not communicate to the surgeon what was happening. Although this is only one instance, we felt that its significance meant that it was important to include the description here, and it suggests that this is a topic on which further research is needed:
Back 2 or 3 years ago we had a death on table and it was a patient who had had previous cardiac surgery but had been passed fit, and we had a locum anaesthetist. And the case from a surgical perspective was absolutely fine; I was looking at the console, we were winding to a finish, I’d taken the prostate out and I was just starting the anastomosis. And the staff were getting worried in theatre, restless. And I could hear them having a conversation with the anaesthetist, and the anaesthetist wasn’t really . . . didn’t say anything at all. And then next I got one of my senior sisters who was not scrubbed telling me, we’re going to have to disconnect the robot, this man’s not well. And we disconnected and left him very rapidly and he arrested on the table with the cardiac arrest team and what have you. And he presumably had an MI, and I think, you know, it’s unfamiliarity with the stresses of the robotic surgery for an anaesthetist who is completely new to it, and for somebody who is not interacting with the team because they’re not a normal member of it. And it’s a lesson in communications. I mean probably there would have been a point earlier in the operation where the anaesthetist, if they’d been part of the team normally would have said, I’m not happy with this chap, is this normal. Or got somebody else who was more familiar with it. But there was absolutely nothing, zero, you know, from that. And if I was at the table and there were problems with the monitors I’d see it, and because my heads in the box, my back to everything, I just thought things were going swimmingly, absolutely no problem at all. And you know . . . but it’s a real lesson.
Site 1, urology surgeon
Implementation of robot-assisted surgery
During the interviews, we also asked about how RAS was introduced within the specialty, in order to understand if this was a relevant contextual factor. The interviewees’ comments reiterated many of the ideas about implementation reported in the phase 1 interviews. In relation to training, the importance of whole-team training was reiterated, as was the importance of understanding the impact of RAS on each other’s roles. Consequently, the two urology surgeons at site 1 felt that a surgeon should not operate at the console without previously having had experience of acting as a first assistant in a robot-assisted operation.
The interviewees also revealed differences within sites in the extent to which the OT team had been involved in the introduction of RAS and had been able to adapt their work processes around this new technology. For example, at site 1, where the colorectal OT team felt that they had little or no input into the introduction of RAS within their specialty, the urology OT team described the way in which they reviewed the instrument trays, cutting down the number of instruments in order to make their work easier:
So then we didn’t have lots of stuff on your trolley because you’ve got your lens, you know, the big lens, and that’s quite a big bit of kit, you know, you don’t want to be knocking stuff on the floor, getting yourself flustered. So we just said, right, let’s just . . . do we need this, do you think we need this . . .?
Site 1, urology ODP
Design workshop
As noted in Chapter 2, during phase 3 of the project, we held a workshop at the University of Leeds entitled ‘Designing for robotic surgery: challenges and opportunities’. The 25 participants included engineers, computer scientists, psychologists, social scientists, surgeons, theatre nurses, ODPs and members of our patient panel.
The day consisted of a series of presentations and workshop activities. Presentations included a broad introduction to the project, a more in-depth analysis concerned with the theory ‘explicit communication’, an analysis of the key issues relevant to situational awareness in RAS and an introduction to a parallel study concerned with simulation training in RAS.
There was also an opportunity for small groups of participants to analyse one incident in some detail. Participants were shown the audio-visual recording of the incident to (1) learn more about the nature of teamwork in RAS, (2) generate observations and ideas that could feed into later discussions about technology design and deployment and (3) illustrate the relevance of audio-visual data for studies of this kind.
The day culminated in a design discussion, during which all of the materials presented and encountered throughout the day were drawn together to identify and explore their implications for the design and implementation of RAS. Although a number of ideas were generated regarding the design of surgical robots, much of the discussion centred on issues of training. Key ideas and issues raised included:
-
the potential value and design of whole-team training
-
the potential for simulation training to support the implementation of RAS, and preparation for emergency situations
-
the opportunities to build in ‘awareness’ tools to the robot to support the work of the teams (using both enhanced sounds and visuals)
-
a range of communicative practices that could be used to support the engagement of teams and teamwork more generally
-
the changes to surgical ‘culture’ needed to encourage more junior members of the team to notify the surgeon of potential problems
-
the need to ensure appropriate sets of skills and experience (with the robot) in OT teams
-
the organisational ‘levers’, and additional forms of evidence, that might be required to ensure that some changes are embedded in practice.
Some of these issues are discussed further in Chapter 10.
Summary
Through conducting the phase 3 interviews, we were able to determine that our theories have relevance beyond colorectal surgery, reflecting the experience of surgeons and OT staff in other surgical specialties. We also gained insight into other contextual factors that have relevance to our theories, and we have revised the theories to incorporate these factors. Indeed, the interviews revealed how contextual factors (especially regarding the extent to which the procedures are routine and predictable, and furthermore the extent to which the surgeon adopts a constrained surgical field of view) can mitigate certain mechanisms and, therefore, reduce their significance.
Meanwhile, the phase 3 design workshop allowed us to explore the significance of our findings for a wide group of stakeholders and to work with them to explore the potential practical implications of the study.
The final list of theories is presented in Table 11.
| Theory | Context | + | Mechanism | = | Outcome | |
|---|---|---|---|---|---|---|
| Resource | Response | |||||
| T5a. Experienced assistant | Assistant engaged in operation Operation is routinised Assistant keen to demonstrate ability to respond quickly |
+ | Knowledge gained through experience of procedure | Assistant is able to work with less guidance and to anticipate what is required, so they act without prompting, offer assistance and/or prepare to act | = | Improved co-ordination Assistant’s actions performed correctly and in timely manner Reduced surgeon stress |
| T5b. Scrub practitioner support | Assistant is willing to acknowledge need for and accept support Surgeon acknowledges scrub practitioner’s role in supporting assistant |
+ | Experienced scrub practitioner | Scrub practitioner guides assistant and undertakes tasks on their behalf | = | Actions are performed correctly |
| T5c. Licence to speak | Positive relationship between team and surgeon | + | Surgeon encourages team to communicate actions and concerns | Team members feel comfortable to speak up | = | Improved co-ordination Actions are performed correctly Increased surgeon situational awareness |
| T6a. Request preparation | Assistant and scrub practitioner engaged in operation | + | Surgeon undertakes preparation and/or pre-request work | Attention of assistant and scrub practitioner is secured, enabling them to hear the message | = | Improved co-ordination |
| T6b. Long requests | Assistant engaged in operation Microphone working |
+ | Surgeon uses longer requests | Assistant has time to prepare so they are ready for more specific instructions | = | Improved co-ordination Assistant’s actions are performed correctly |
| T6c. Oral response | Assistant and scrub practitioner communicate loudly and clearly | + | Assistant and scrub practitioner respond orally to surgeon’s requests | Surgeon knows request will be completed and is made aware of any challenges in doing this | = | Improved co-ordination |
| D1a. Reduced distraction | Surgeon trusts assistant to successfully carry out requests and communicate problems | + | Surgeon’s position within console | Surgeon feels confident to remain in console | = | Reduced distraction Increased concentration |
| D1b. Experienced scrub practitioner | Experienced scrub practitioner | + | Surgeon’s position within console | Scrub practitioner aware of need to move attention between screen and robotic arms, enabling them to notice when arms are clashing and alert surgeon | = | Surgeon’s situational awareness is maintained |
| D1c. Awareness of instruments | Multiquadrant operation | + | Increased control of retraction | Surgeon moves camera away from retraction instrument, losing awareness of position of instrument | = | Disruption to flow of operation Increased operation duration Increased patient safety risk |
Chapter 10 Discussion and conclusion
Revisiting the research objectives
The study had four objectives:
-
To contribute to the interpretation and reporting of the trial results by investigating how variations in implementation of RAS, and the context in which it is implemented, impact on outcomes such as operation duration, conversion to open surgery and complications.
-
To produce actionable guidance for health-care organisations on factors likely to facilitate the successful implementation and integration of RAS.
-
To produce actionable guidance for OT teams on how to ensure effective communication and teamwork when undertaking RAS.
-
To provide data to inform the development of tools and technologies for RAS to better support teamwork and decision-making.
We discuss each of these objectives below, and the extent to which they have been met.
Implications for the interpretation and reporting of ROLARR
The ROLARR trial found no significant difference between RAS and laparoscopic surgery in the primary outcome of conversion to open surgery, while the economic analysis found RAS to be more expensive than laparoscopic surgery due to the cost of consumables. 194 At the time of writing, an analysis of longer-term patient outcomes is not yet available. The phase 1 interviews were conducted at all six of the NHS ROLARR sites, enabling us to provide an account of the different ways in which RAS was implemented in these sites, in terms of components of the intervention (e.g. the amount and nature of training provided), and stakeholders’ perspectives of the value of those different approaches. The publication of these findings alongside the reporting of the ROLARR trial provides important information for health-care organisations that are considering introducing RAS, acting as a response to the call for better description of surgical interventions,195 while acknowledging that mandating the details of an intervention may increase surgeon reluctance to take up such interventions if they disagree with such details. 196 Below we have summarised the findings in the form of guidance for health-care organisations. We chose not to use variation in the components of the intervention to interpret differences between sites because, owing to the small number of operations undertaken by some sites, it is questionable how meaningful this would be.
We were also able to capture unanticipated consequences of RAS in terms of impacts on communication, teamwork and decision-making, along with the strategies used to counteract such unanticipated consequences. This has implications for the conduct of RAS, which we summarise below in the form of guidance for OT teams. These findings also act as an important reminder that surgical interventions are not static, suggesting that evaluations of surgical interventions need to track how the intervention changes over time as stakeholders respond to, and find ways to overcome, the challenges the intervention presents.
While a strength of the study is the methods of data collection, which allowed us to develop a detailed understanding of how and in what contexts RAS impacts communication, teamwork and decision-making, and how and in what contexts particular strategies work to support communication, teamwork and decision-making in RAS, the small number of operations observed could be perceived as a limitation. The account provided by the urology surgeon presented in Chapter 9, in which the surgeon was unaware of the patient’s deterioration, suggests that there may be negative impacts of RAS on the surgeon’s situational awareness that we have not captured. However, a predominantly qualitative study is not the appropriate design for capturing such rare adverse events. The IDEAL framework for surgical innovation recommends long-term surveillance through registries to capture rare end points relating to patient safety,197 and RAS has been presented as an example of why such systems of surveillance are needed. 151,198 In the USA, research has been undertaken into adverse events associated with RAS using the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database,199 but there is known under-reporting of adverse events associated with RAS12 and it is questionable whether less indirect consequences of RAS, such as the example described above, which do not represent device malfunctions, would be reported. Thus, how to capture such impacts is a methodological question that remains to be explored.
This study started after the trial, which limited the impact it could have on the analysis of the trial. For example, in the discussion about emerging findings with the trial management group, there was interest in the impact of the skill mix of the first assistant and the scrub practitioner. However, although the trial case report form captured quantitative information about the experience of the surgeon, information about the experience of the OT team was captured as free text, making it difficult to use those data to support the testing and refinement of our theories. Therefore, there is a methodological issue here. Our experience suggests that, although realist evaluation can play a valuable role alongside RCTs, the elicitation of theories would ideally happen before the trial to ensure that relevant data are secured to support the testing of identified theories. This fits with recent calls for realist feasibility and pilot studies. 200
Implications for the implementation of robot-assisted surgery
Whereas phase 2 focused on the testing of theories related to communication, teamwork and decision-making, phase 1 captured a significant number of data regarding the different ways in which RAS was introduced across nine NHS hospitals, and stakeholders’ perspectives on the value of those approaches and the contexts in which they are effective. While acknowledging that the theories regarding implementation remain to be empirically tested, our findings suggest that, for health-care organisations seeking to introduce RAS or to increase the use of an already purchased da Vinci robot, the following strategies may be beneficial:
-
Engagement of staff at different levels of the organisation. Although board-level support is likely to be essential for the introduction of RAS, it is also important to engage team leaders, as they can assist in creating conditions that accommodate the introduction of RAS, such as organising training and ensuring that the right skill mix is available. Engagement of those surgeons who will not be using the robot is also important; if surgeons perceive that the introduction of RAS is supported by their colleagues, they are likely to be more willing to undertake an operation with robot-assistance despite the initial longer operation duration.
-
Handpicked dedicated robotic team. While unlikely to be feasible as a long-term strategy, a handpicked dedicated team can increase the speed with which experience is built up, increasing confidence and efficiency. However, care should be taken not to alienate those who are not part of that initial team.
-
Whole-team training. Ideally, the whole team should train together. This is beneficial in terms of understanding the impact of RAS on each other’s roles, supporting teamwork. It can work to increase trust in each other’s knowledge so the surgeon feels more confident in the team’s ability to support him or her.
-
A suitably sized OT. By having a suitably sized OT, operation duration is reduced, as staff are able to move quickly and the risk of desterilisation is reduced.
A key issue that arose throughout the study related to point 3: whole-team training for RAS. This was perceived as an important issue by the OT teams we interviewed and our study revealed substantial variation in the training received by OT teams. Our phase 1 interviews revealed that simply bringing together interdisciplinary teams of professionals can be useful to support team bonding and enhance trust across the team. However, our findings also suggest that the nature of the training can be further enhanced. Currently, training focuses on technical skills related to the manipulation and use of the robot: setting up the robot; docking and undocking the robot; inserting, using and removing instruments; troubleshooting; and so forth. Phase 2 revealed differences in the organisation of roles and issues for communication between RAS and laparoscopic surgery. Therefore, it makes sense for training to enable participants to learn more explicitly about the implications of the division of labour in RAS; the limitations and constraints on the surgeon’s field of view; and differences in the organisation of teamwork and communication in RAS. In sum, our findings suggest that there is value in providing whole-team training to reflect overtly on communication and other differences in teamwork in RAS.
Indeed, the project has already inspired preliminary work to consider the value of new forms of training for RAS. Following discussions of research within the project, one of the project team (AG) developed a pilot training simulation for whole-team training in RAS. The simulation enabled participants to experience and reflect upon a one-off robot-assisted emergency situation, and, in particular, to work together as a whole team and explore their respective roles and responsibilities. The findings from this study were presented at the phase 3 workshop in Leeds and point towards possibilities for further research on training. These issues and opportunities for training are highly relevant to organisations that are introducing RAS. They suggest the need not only for whole-team training, but also for training that moves beyond the technical through to training in teamwork and role distribution. This is an area in which further research is needed to develop training for the OT team and evaluate its impact.
Related to this is the issue of skill mix. Both organisations looking to introduce RAS and those already undertaking RAS should look not just at the training and experience of the surgeon but also at the training and experience of the first assistant and the scrub practitioner. The findings from phase 2 suggest that experience of assisting or undertaking a particular procedure openly or laparoscopically is not sufficient to assist competently when that procedure is being undertaken with robotic assistance. When the first assistant does not have the necessary experience, they should be supported by an experienced scrub practitioner.
A more general issue relates to the process by which RAS is introduced into an organisation. The implementation of RAS within NHS trusts has largely been surgeon led. This reflects what has been described as a more general pattern, whereby innovations are introduced into surgical practice through informal processes with an absence of quality control efforts, and some have argued that these informal processes put patients at greater risk of adverse events. 201 In none of the nine phase 1 sites did OT team members perceive themselves to have been involved in the introduction of RAS. When this is combined with a lack of training, it can create the sense that RAS is something that has been thrust upon the OT team, leading to feelings of resentment regarding this new technology. Although our phase 1 interviewees emphasised the importance of team leader support, it does not appear that those team leaders were involved in discussions prior to the introduction of RAS. Rather, it was only once the robot had been introduced that the support required became apparent. Our findings would suggest that there is potential benefit to be gained through involving team leaders earlier in the process, so issues of training for the OT team and skill mix can be properly addressed before the robot is introduced into practice.
Implications for communication and teamwork in robot-assisted surgery
Our findings reveal a number of strategies, implicit and explicit, used by surgeons and OT teams to encourage effective communication and teamwork in RAS. These are strategies that they have developed through experience, in response to the challenges RAS can present for teamwork and communication, and that could be employed by surgeons and OT teams elsewhere. As a consequence of the relationship between communication and teamwork in the OT and patient safety, there are already a number of interventions designed to improve communication and teamwork in the OT, such as the World Health Organization’s Surgical Safety Checklist, the use of which has been mandatory in NHS OTs since February 2010. 202 Evaluations of interventions that seek to make communication in the OT more explicit highlight the need to be sensitive to the cultural context, such as an emphasis on autonomy, a belief in individual excellence, or time pressures, which could lead to resistance,203 and the possibility of negative unanticipated effects, such as disrupting the dynamic flow of conversation, reinforcing professional divisions and/or creating tension. 204 Therefore, our intention is not to provide a rigid set of guidelines but instead to suggest strategies surgeons and OT teams can try, acknowledging the realist principle that not all strategies will work in all contexts and that use of one strategy may change the context, impacting on whether or not other mechanisms continue to fire.
For surgeons, our findings suggest the importance of:
-
Encouraging the OT team to communicate both their actions and concerns. This helps OT team members feel comfortable to speak up, leading to improved co-ordination and increased situational awareness for the surgeon. This strategy is more likely to be effective when there is a positive relationship between the OT team and the surgeon.
-
Alerting the attention of the first assistant before issuing a request, particularly after a period without communication. This supports the first assistant to hear the message, leading to improved co-ordination. This strategy is more likely to be effective if the first assistant is already engaged in the operation.
-
Acknowledge the role of the scrub practitioner in supporting an inexperienced first assistant, so as to increase the first assistant’s willingness to accept that support. When the scrub practitioner is experienced, this increases the likelihood that actions are performed correctly.
For the OT team, our findings suggest the importance of:
-
Providing an oral response to the surgeon’s requests. This reassures the surgeon that the request will be completed and makes them aware of any challenges in doing this. This strategy requires that team members communicate loudly and clearly.
-
The scrub practitioner positioning themselves so they can easily move their attention between the screen and the robotic arms. This enables the scrub practitioner to notice when the robotic arms are clashing and alert the surgeon, increasing the surgeon’s situational awareness. More experienced scrub practitioners will be aware of the need to do this.
This guidance, and our theories relating to communication and teamwork, may have relevance for other areas of surgery in which technology leads to the separation of the surgeon from the rest of the team. For example, numerous surgical specialties use surgical microscopes. Although when using these the surgeon is not physically separated from the rest of the team, there are similarities in terms of the surgeon being focused on a different view of the surgical site and no longer able to see the wider OT, requiring a move from byproduct awareness to add-on awareness.
Implications for the design of technologies for robot-assisted surgery
In the phase 3 workshop, a number of suggestions for revisions to the design of the robot were made, largely focused on increasing the surgeon’s awareness of what is happening both in the surgical site and in the OT. To increase the surgeon’s awareness of the position of robotic instruments outside his field of view, a display on the console could indicate this. This could be extended to also show the position of laparoscopic instruments held by the first assistant. Related to this, a safety mechanism could be introduced that prevents the surgeon from moving a robotic instrument when it is outside their field of view. Although the surgeons in our phase 1 interviews did not see the lack of tactile information as a significant issue, workshop participants suggested that tactile information may enable the surgeon to better co-ordinate their movements with the first assistant, as they would be able to feel when their instruments were clashing.
To increase the surgeon’s awareness of the wider OT, the suggestion was made that the console could incorporate a video stream that provided a ‘window’ onto the OT, which could be turned on and off. Workshop participants also discussed issues related to the spatial configuration of team members and technology in the OT. Above we described differences in spatial configuration across our sites, which affected the ease with which the surgeon could see the patient and have face-to-face communication with the first assistant and the scrub practitioner. However, having discussed this with staff at the sites, it appears that this was not intentional but rather the result of aspects of the layout of the OT, with the console positioned to avoid doorways and to limit the number of cables across the floor. This is an area where in situ experimental studies could provide insight into the impact of different configurations. One suggestion was to have the console on a raised platform, allowing the surgeon to have a better view of the OT when they brought their head out of the console.
More generally, it appears that the design of RAS has focused on the needs of the surgeon. For example, although the control of the robotic instruments is generally perceived to be easy to learn, our observations suggest the colour-coding used to indicate when it is safe to insert a robotic instrument is not experienced as intuitive by first assistants and scrub practitioners. Although this may be overcome by better training, recognition that successful use of RAS depends not just on the surgeon but on the whole team suggests that there is value in considering the wider team in the design of the robot.
Implications for future research
On the basis of our findings, we suggest that the following topics would be fruitful areas for future research.
-
Exploration of other areas of surgery in which technology leads to the separation of the surgeon from the rest of the team, either physically or perceptually, and the transferability of guidance for effective communication and teamwork to those settings. Possible areas are the use of microscopes in plastic and reconstructive surgery, ophthalmic surgery, and neurosurgery.
-
Investigation of the potential for realist evaluation to contribute to the design of RCTs and associated process evaluations through the inclusion of realist methods in feasibility and pilot studies.
-
Assessment of the feasibility of using routinely collected data, such as those contained within the NHS National Reporting and Learning System and national registries, to understand the impact of RAS on rare end points associated with patient safety.
-
Development and evaluation of methods for whole-team training.
-
Experimental evaluation, in situ, of the impact of different physical configurations of the robotic console and team members on communication and teamwork in the OT, with quantitative and qualitative data collection and analysis.
Acknowledgements
We would like to thank all of the OT teams who participated in this research, for generously giving their time to be interviewed and for their willingness to have their work practice observed and video recorded.
We are very grateful to the members of our patient panel: Peter Allen, Jean Gallagher and Gill Stone.
We thank our Study Steering Committee for the support and guidance given over the course of the project: Professor Jane Blazeby, Professor Julia Brown, Tracy Coates, Dr Ana Manzano, Professor Kenton O’Hara, Professor Ray Pawson, Professor Nick Sevdalis, Gill Stone and Professor Ian Watt.
Contributions of authors
Dr Rebecca Randell (Lecturer, Evaluation of Healthcare Technology) was the principal investigator, led the design of the study, contributed to the data collection and analysis and is the lead author of this report.
Dr Stephanie Honey (Research Fellow, Qualitative Research) was responsible for the day-to-day management of the project, undertook data collection and was involved in the analysis and preparation of this report.
Professor Jon Hindmarsh (Professor of Work and Interaction, Workplace Studies) was coapplicant, was involved in study design (particularly in relation to the collection of video data), led the qualitative analysis of the video data and contributed to the preparation of this report.
Dr Natasha Alvarado (Research Associate, Realist Evaluation) undertook data collection and was involved in analysis and preparation of this report.
Dr Joanne Greenhalgh (Associate Professor, Realist Evaluation) was coapplicant and was involved in study design (particularly in relation to realist evaluation), analysis and preparation of this report.
Professor Alan Pearman (Professor of Management Decision Analysis, Decision-making) was coapplicant and was involved in study design, analysis (particularly in relation to decision-making) and preparation of this report.
Professor Andrew Long (Professor of Health Systems Research, Health Services Research) was coapplicant and was involved in study design, analysis and preparation of this report.
Dr Alexandra Cope (NIHR Academic Clinical Lecturer, Surgical Workplace Studies) was involved in study design, data collection, analysis (particularly of the video data) and preparation of this report. She contributed a surgical perspective.
Arron Gill (Lecturer, Peri-operative Practice) was involved in study design, analysis and preparation of this report. He brought an ODP perspective to the project.
Dr Peter Gardner (Senior Lecturer, Patient Safety) was coapplicant and was involved in study design and analysis (particularly the analysis of the OTAS and SURG-TLX data) and preparation of this report.
Dr Alwyn Kotze (Consultant Anaesthetist, Implementation Science) was coapplicant and was involved in study design, analysis and preparation of this report. He contributed a clinical perspective.
David Wilkinson (lay member) was coapplicant and was involved in study design (particularly the design of the patient information sheet and consent form), analysis and preparation of this report. He contributed a patient perspective.
Professor David Jayne (Professor of Surgery, Robot-assisted Surgery) was coapplicant and was involved in study design, analysis and preparation of this report. He contributed a surgical perspective.
Julie Croft (Senior Trial Manager, Clinical Trials) was coapplicant and was involved in study design and set-up (particularly in establishing links with UK ROLARR sites), analysis and preparation of this report.
Professor Dawn Dowding (Visiting Nurse Service of New York Professor of Nursing, Decision-making) was coapplicant and was involved in study design, analysis (particularly in relation to decision-making) and preparation of this report.
Publications
Randell R, Greenhalgh J, Hindmarsh J, Dowding D, Jayne D, Pearman A, et al. Integration of robotic surgery into routine practice and impacts on communication, collaboration and decision making: a realist process evaluation protocol. Implement Sci 2014;9:52.
Randell R, Alvarado N, Honey S, Greenhalgh J, Gardner P, Gill A, et al. Impact of robotic surgery on decision making: perspectives of surgical teams. AMIA Annu Symp Proc 2015;2015:1057–66.
Gill A, Randell R. Robotic surgery and its impact on teamwork in the operating theatre. J Perioper Pract 2016;26:42–5.
Randell R, Honey S, Alvarado N, Pearman A, Greenhalgh J, Long A, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cogn Tech Work 2016;18:423–37.
Data sharing statement
The data will be kept until August 2026 (10 years from the completion of the study) and can be accessed by other researchers during this time, subject to the necessary ethical approvals being obtained. Requests for access to these data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Bann S, Khan M, Hernandez J, Munz Y, Moorthy K, Datta V, et al. Robotics in surgery. J Am Coll Surg 2003;196:784-95. https://doi.org/10.1016/S1072-7515(02)01750-7.
- Dobson MW, Geisler D, Fazio V, Remzi F, Hull T, Vogel J. Minimally invasive surgical wound infections: laparoscopic surgery decreases morbidity of surgical site infections and decreases the cost of wound care. Colorectal Dis 2011;13:811-15. http://dx.doi.org/10.1111/j.1463-1318.2010.02302.x.
- Smith A, Smith J, Jayne DG. Telerobotics: surgery for the 21st century. Surgery (Oxford) 2006;24:74-8. http://dx.doi.org/10.1383/surg.2006.24.2.74.
- Darzi A. Saws and Scalpels to Lasers and Robots – Advances in Surgery. London: Department of Health; 2007.
- Delivering Enhanced Recovery – Helping Patients to Get Better Sooner After Surgery. London: Enhanced Recovery Partnership Programme; 2010.
- Improving Outcomes. London: Department of Health; 2011.
- Franks PJ, Bosanquet N, Thorpe H, Brown JM, Copeland J, Smith AM, et al. Short-term costs of conventional vs laparoscopic assisted surgery in patients with colorectal cancer (MRC CLASICC trial). Br J Cancer 2006;95:6-12. https://doi.org/10.1038/sj.bjc.6603203.
- LAPCO National Training Programme for Laparoscopic Colorectal Surgery n.d. www.lapco.nhs.uk (accessed 8 December 2016).
- Laparoscopic Surgery for Colorectal Cancer (Review). London: National Institute for Health and Care Excellence; 2006.
- Jones A, Sethia K. Robotic surgery. Ann R Coll Surg Engl 2010;92:5-8. http://dx.doi.org/10.1308/003588410X12518836439362.
- Trehan A, Dunn TJ. The robotic surgery monopoly is a poor deal. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f7470.
- Cooper MA, Ibrahim A, Lyu H, Makary MA. Underreporting of robotic surgery complications. J Healthc Qual 2015;37:133-8. http://dx.doi.org/10.1111/jhq.12036.
- Abrishami P, Boer A, Horstman K. Understanding the adoption dynamics of medical innovations: affordances of the da Vinci robot in the Netherlands. Soc Sci Med 2014;117:125-33. http://dx.doi.org/10.1016/j.socscimed.2014.07.046.
- High Quality Care for All: Our Journey So Far. London: Department of Health; 2009.
- Bennett K. Robotic surgery: da Vinci and beyond. Bull R Coll Surg Engl 2012;94:8-9. http://dx.doi.org/10.1308/147363512x13189526438431.
- Murphy D, Dasgupta P, Haig I. Can the NHS afford robotic surgery?. Clin Serv J 2009:37-9.
- Porpiglia F, Morra I, Lucci Chiarissi M, Manfredi M, Mele F, Grande S, et al. Randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol 2013;63:606-14. http://dx.doi.org/10.1016/j.eururo.2012.07.007.
- Asimakopoulos AD, Pereira Fraga CT, Annino F, Pasqualetti P, Calado AA, Mugnier C. Randomized comparison between laparoscopic and robot-assisted nerve-sparing radical prostatectomy. J Sex Med 2011;8:1503-12. http://dx.doi.org/10.1111/j.1743-6109.2011.02215.x.
- NHS England Specialised Services Clinical Reference Group for Specialised Urology . Clinical Commissioning Policy: Robotic-Assisted Surgical Procedures for Prostate Cancer 2015.
- Scales CD, Jones PJ, Eisenstein EL, Preminger GM, Albala DM. Local cost structures and the economics of robot assisted radical prostatectomy. J Urol 2005;174:2323-9. https://doi.org/10.1097/01.ju.0000181830.43340.e7.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: Medical Research Council; 2000.
- Lai F, Entin E. Robotic surgery and the operating room team. Proc Hum Factors Ergon Soc Annu Meeting 2005;49:1070-3. http://dx.doi.org/10.1177/154193120504901115.
- Sgarbura O, Vasilescu C. The decisive role of the patient-side surgeon in robotic surgery. Surg Endosc 2010;24:3149-55. http://dx.doi.org/10.1007/s00464-010-1108-9.
- Nyssen A-S, Blavier A. Verbal Communication As a Sign of Adaptation in Socio-Technical Systems: The Case of Robotic Surgery n.d.:267-72.
- Cao CGL, Taylor H, Khalid HM, Helander MG, Yeo AW. Work with Computing Systems. n.d.
- Webster JL, Cao CG. Lowering communication barriers in operating room technology. Hum Factors 2006;48:747-58. http://dx.doi.org/10.1518/001872006779166271.
- Svensson MS, Heath C, Luff P, Bannon L, Wagner I, Gutwin C, et al. ECSCW’07: Proceedings of the Tenth European Conference on Computer Supported Cooperative Work; 24–28 September 2007, Limerick, Ireland. Springer; 2007.
- Weldon SM, Korkiakangas T, Bezemer J, Kneebone R. Communication in the operating theatre. Br J Surg 2013;100:1677-88. http://dx.doi.org/10.1002/bjs.9332.
- Bholat OS, Haluck RS, Kutz RH, Gorman PJ, Krummel TM. Defining the role of haptic feedback in minimally invasive surgery. Stud Health Technol Inform 1999;62:62-6.
- Bholat OS, Haluck RS, Murray WB, Gorman PJ, Krummel TM. Tactile feedback is present during minimally invasive surgery. J Am Coll Surg 1999;189:349-55. https://doi.org/10.1016/S1072-7515(99)00184-2.
- Russ S, Hull L, Rout S, Vincent C, Darzi A, Sevdalis N. Observational teamwork assessment for surgery: feasibility of clinical and nonclinical assessor calibration with short-term training. Ann Surg 2012;255:804-9. http://dx.doi.org/10.1097/SLA.0b013e31824a9a02.
- Egan M. Clinical dashboards: impact on workflow, care quality, and patient safety. Crit Care Nurs Q 2006;29:354-61. https://doi.org/10.1097/00002727-200610000-00008.
- Parush A, Kramer C, Foster-Hunt T, Momtahan K, Hunter A, Sohmer B. Communication and team situation awareness in the OR: implications for augmentative information display. J Biomed Inform 2011;44:477-85. http://dx.doi.org/10.1016/j.jbi.2010.04.002.
- Healey A, Nagpal K, Moorthy K, Vincent C. Engineering the system of communication for safer surgery. Cogn Tech Work 2011;13:1-10. http://dx.doi.org/10.1007/s10111-010-0152-5.
- Collinson F, Jayne D, Pigazzi A, Tsang C, Barrie J, Edlin R, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis 2012;27:233-41. http://dx.doi.org/10.1007/s00384-011-1313-6.
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process Evaluation of Complex Interventions: UK Medical Research Council (MRC) Guidance 2015.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h1258.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. https://doi.org/10.1136/bmj.332.7538.413.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009. http://dx.doi.org/10.1136/bmj.b3496.
- Wolff N. Randomised trials of socially complex interventions: promise or peril?. J Health Serv Res Policy 2001;6:123-6. https://doi.org/10.1258/1355819011927224.
- Lindsay B. Randomized controlled trials of socially complex nursing interventions: creating bias and unreliability?. J Adv Nurs 2004;45:84-9. https://doi.org/10.1046/j.1365-2648.2003.02864.x.
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455-9. https://doi.org/10.1136/bmj.39108.379965.BE.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster-randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14:1-10. http://dx.doi.org/10.1186/1745-6215-14-15.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. http://dx.doi.org/10.1186/1741-7015-8-63.
- Marchal B, van Belle S, van Olmen J, Hoerée T, Kegels G. Is realist evaluation keeping its promise? A review of published empirical studies in the field of health systems research. Evaluation 2012;18:192-21. http://dx.doi.org/10.1177/1356389012442444.
- Jagosh J, Macaulay AC, Pluye P, Salsberg J, Bush PL, Henderson J, et al. Uncovering the benefits of participatory research: implications of a realist review for health research and practice. Milbank Q 2012;90:311-46. http://dx.doi.org/10.1111/j.1468-0009.2012.00665.x.
- Pawson R. Evidence-based Policy: A Realist Perspective. London: Sage; 2006.
- Pawson R. The Science of Evaluation: A Realist Manifesto. London: Sage; 2013.
- Pawson R, Tilley N. Realistic Evaluation. London: Sage Publications; 1997.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review – a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34. http://dx.doi.org/10.1258/1355819054308530.
- Wong G, Westhorp G, Manzano A, Greenhalgh J, Jagosh J, Greenhalgh T. RAMESES II reporting standards for realist evaluations. BMC Med 2016;14. http://dx.doi.org/10.1186/s12916-016-0643-1.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Qual Saf 2015;24:228-38. http://dx.doi.org/10.1136/bmjqs-2014-003627.
- Dalkin SM, Greenhalgh J, Jones D, Cunningham B, Lhussier M. What’s in a mechanism? Development of a key concept in realist evaluation. Implement Sci 2015;10. http://dx.doi.org/10.1186/s13012-015-0237-x.
- Pawson R, Greenhalgh J, Brennan C, Glidewell E. Do reviews of healthcare interventions teach us how to improve healthcare systems?. Soc Sci Med 2014;114:129-37. http://dx.doi.org/10.1016/j.socscimed.2014.05.032.
- Healey A, Benn J. Teamwork enables remote surgical control and a new model for a surgical system emerges. Cogn Tech Work 2009;11:255-65. http://dx.doi.org/10.1007/s10111-008-0125-0.
- Emmel N. Sampling and Choosing Cases in Qualitative Research: A Realist Approach. Sage; 2013.
- Manzano A. The craft of interviewing in realist evaluation. Evaluation 2016;22:342-60. http://dx.doi.org/10.1177/1356389016638615.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- Cheyne H, Abhyankar P, McCourt C. Empowering change: realist evaluation of a Scottish Government programme to support normal birth. Midwifery 2013;29:1110-21. http://dx.doi.org/10.1016/j.midw.2013.07.018.
- Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA: Sage; 1994.
- Yin RK. Case Study Research: Design and Methods. Thousand Oaks, CA: Sage; 2003.
- Randell R, Wilson S, Woodward P. Variations and commonalities in processes of collaboration: the need for multi-site workplace studies. CSCW 2011;20:37-59. https://doi.org/10.1007/s10606-010-9127-6.
- Hammersley M, Atkinson P. Ethnography: Principles in Practice. London: Routledge; 1995.
- Dowding D, Mitchell N, Randell R, Foster R, Lattimer V, Thompson C. Nurses’ use of computerised clinical decision support systems: a case site analysis. J Clin Nurs 2009;18:1159-67. http://dx.doi.org/10.1111/j.1365-2702.2008.02607.x.
- Catchpole K, Wiegmann D. Understanding safety and performance in the cardiac operating room: from ‘sharp end’ to ‘blunt end’. BMJ Qual Saf 2012;21:807-9. https://doi.org/10.1136/bmjqs-2012-001135.
- Undre S, Sevdalis N, Healey AN, Darzi A, Vincent CA. Observational teamwork assessment for surgery (OTAS): refinement and application in urological surgery. World J Surg 2007;31:1373-81. http://dx.doi.org/10.1007/s00268-007-9053-z.
- Sharma B, Mishra A, Aggarwal R, Grantcharov TP. Non-technical skills assessment in surgery. Surg Oncol 2011;20:169-77. http://dx.doi.org/10.1016/j.suronc.2010.10.001.
- Sevdalis N, Lyons M, Healey AN, Undre S, Darzi A, Vincent CA. Observational teamwork assessment for surgery: construct validation with expert versus novice raters. Ann Surg 2009;249:1047-51. http://dx.doi.org/10.1097/SLA.0b013e3181a50220.
- Hull L, Arora S, Kassab E, Kneebone R, Sevdalis N. Observational teamwork assessment for surgery: content validation and tool refinement. J Am Coll Surg 2011;212:234-43.e1. http://dx.doi.org/10.1016/j.jamcollsurg.2010.11.001.
- Hull L, Arora S, Aggarwal R, Darzi A, Vincent C, Sevdalis N. The impact of nontechnical skills on technical performance in surgery: a systematic review. J Am Coll Surg 2012;214:214-30. http://dx.doi.org/10.1016/j.jamcollsurg.2011.10.016.
- Luff P, Hindmarsh J, Heath C. Workplace Studies: Recovering Work Practice and Informing System Design. Cambridge: Cambridge University Press; 2000.
- Syzmanski MH, Whalen J. Making Work Visible: Ethnographically Grounded Case Studies of Work Practice. Cambridge: Cambridge University Press; 2011.
- Heath C, Luff P. Technology in Action. Cambridge: Cambridge University Press; 2000.
- Grimshaw AD. Sound-image data records for research on social interaction. Sociol Methods Res 1982;11:121-44. https://doi.org/10.1177/0049124182011002002.
- Catchpole KR. Task, team and technology integration in the paediatric cardiac operating room. Prog Pediatr Cardiol 2011;32:85-8. http://dx.doi.org/10.1016/j.ppedcard.2011.10.005.
- Catchpole KR, Giddings AE, de Leval MR, Peek GJ, Godden PJ, Utley M, et al. Identification of systems failures in successful paediatric cardiac surgery. Ergonomics 2006;49:567-88. https://doi.org/10.1080/00140130600568865.
- Schraagen JM, Schouten T, Smit M, Haas F, van der Beek D, van de Ven J, et al. Assessing and improving teamwork in cardiac surgery. Qual Saf Health Care 2010;19:1-6. http://dx.doi.org/10.1136/qshc.2009.040105.
- Bezemer J, Murtagh G, Cope A, Kress G, Kneebone R. ‘Scissors, please’: the practical accomplishment of surgical work in the operating theater. Symbol Interact 2011;34:398-414. http://dx.doi.org/10.1525/si.2011.34.3.398.
- Korkiakangas T, Weldon SM, Bezemer J, Kneebone R. Nurse-surgeon object transfer: video analysis of communication and situation awareness in the operating theatre. Int J Nurs Stud 2014;51:1195-206. http://dx.doi.org/10.1016/j.ijnurstu.2014.01.007.
- Korkiakangas T, Weldon S-M, Bezemer J, Kneebone R, White S, Cartmill J. Communication in Surgical. Practice. Sheffield: Equinox; 2016.
- Koschmann T, LeBaron C, Goodwin C, Feltovich P. ‘Can you see the cystic artery yet?’ A simple matter of trust. J Pragmatics 2011;43:521-41. http://dx.doi.org/10.1016/j.pragma.2009.09.009.
- Koschmann T, LeBaron C, Goodwin C, Zemel A, Dunnington G. Formulating the triangle of doom. Gesture 2007;7:97-118. https://doi.org/10.1075/gest.7.1.06kos.
- Mondada L. Instructions in the operating room: how the surgeon directs their assistant’s hands. Discourse Stud 2014;16:131-61. http://dx.doi.org/10.1177/1461445613515325.
- Mondada L, Drew P, Couper-Kuhlen E. Requesting in Social Interaction. Amsterdam: John Benjamins Publishing Company; 2014.
- Svensson MS, Luff P, Heath C. Embedding instruction in practice: contingency and collaboration during surgical training. Sociol Health Illn 2009;31:889-906. http://dx.doi.org/10.1111/j.1467-9566.2009.01195.x.
- Greenhalgh T, Swinglehurst D. Studying technology use as social practice: the untapped potential of ethnography. BMC Med 2011;9. http://dx.doi.org/10.1186/1741-7015-9-45.
- Greenhalgh T, Humphrey C, Hughes J, Macfarlane F, Butler C, Pawson R. How do you modernize a health service? A realist evaluation of whole-scale transformation in London. Milbank Q 2009;87:391-416. http://dx.doi.org/10.1111/j.1468-0009.2009.00562.x.
- Rycroft-Malone J, Fontenla M, Bick D, Seers K. A realistic evaluation: the case of protocol-based care. Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-38.
- McDonald S. Studying actions in context: a qualitative shadowing method for organizational research. Qual Res 2005;5:455-73. http://dx.doi.org/10.1177/1468794105056923.
- Wilson MR, Poolton JM, Malhotra N, Ngo K, Bright E, Masters RS. Development and validation of a surgical workload measure: the surgery task load index (SURG-TLX). World J Surg 2011;35:1961-9. http://dx.doi.org/10.1007/s00268-011-1141-4.
- Hart SG, Staveland LE. Development of NASA-TLX (Task Load Index): results of empirical and theoretical research. Adv Psychol 1988;52:139-83. https://doi.org/10.1016/S0166-4115(08)62386-9.
- Cao A, Chintamani KK, Pandya AK, Ellis RD. NASA TLX: software for assessing subjective mental workload. Behav Res Methods 2009;41:113-17. http://dx.doi.org/10.3758/BRM.41.1.113.
- Hallbeck S, Lowndes B, Bingener J. Laparoscopic surgical team stress measures during randomized controlled trials of 4-port vs. single incision cholecystecomies: a pilot study. Proc Hum Factors Ergon Soc Annu Meeting 2013;57:654-7. http://dx.doi.org/10.1177/1541931213571141.
- Heath C, Hindmarsh J, Luff P. Video in Qualitative Research: Analysing Social Interaction in Everyday Life. London: Sage; 2010.
- Garfinkel H. Studies in Ethnomethodology. Cambridge: Polity Press; 1967.
- Sacks H. Lectures on Conversation: Volumes I & II. Oxford: Blackwell; 1995.
- Maynard DW, Heritage J. Conversation analysis, doctor-patient interaction and medical communication. Med Educ 2005;39:428-35. https://doi.org/10.1111/j.1365-2929.2005.02111.x.
- Laurier E, Philo C, Knoblauch H, Raab J, Soeffner H-G, Schnettler B. Video Analysis: Methodology and Methods, Qualitative Audiovisual Data Analysis in Sociology. Oxford: Peter Lang; 2006.
- Gurses AP, Kim G, Martinez EA, Marsteller J, Bauer L, Lubomski LH, et al. Identifying and categorising patient safety hazards in cardiovascular operating rooms using an interdisciplinary approach: a multisite study. BMJ Quality &Amp; Safety 2012;21:810-18. http://dx.doi.org/10.1136/bmjqs-2011-000625.
- Vincent C, Moorthy K, Sarker SK, Chang A, Darzi AW. Systems approaches to surgical quality and safety: from concept to measurement. Ann Surg 2004;239:475-82. https://doi.org/10.1097/01.sla.0000118753.22830.41.
- Finch T, Mair F, O’Donnell C, Murray E, May C. From theory to ‘measurement’ in complex interventions: methodological lessons from the development of an e-health normalisation instrument. BMC Med Res Methodol 2012;12. https://doi.org/10.1016/j.jneb.2013.11.017.
- Randell R, Wilson S, Fitzpatrick G. Evaluating new interactions in healthcare: challenges and approaches. Int J Hum Comput Interact 2010;26:407-13. https://doi.org/10.1080/10447311003719847.
- Mair FS, May C, O’Donnell C, Finch T, Sullivan F, Murray E. Factors that promote or inhibit the implementation of e-health systems: an explanatory systematic review. Bull World Health Organ 2012;90:357-64. http://dx.doi.org/10.2471/BLT.11.099424.
- Cook RI, Woods DD. Adapting to new technology in the operating room. Hum Factors 1996;38:593-61. http://dx.doi.org/10.1518/001872096778827224.
- Ash JS, Sittig DF, Dykstra R, Campbell E, Guappone K. The unintended consequences of computerized provider order entry: findings from a mixed methods exploration. Int J Med Inform 2009;78:69-76. http://dx.doi.org/10.1016/j.ijmedinf.2008.07.015.
- Ash JS, Sittig DF, Dykstra RH, Guappone K, Carpenter JD, Seshadri V. Categorizing the unintended sociotechnical consequences of computerized provider order entry. Int J Med Inform 2007;76:21-7. http://dx.doi.org/10.1016/j.ijmedinf.2006.05.017.
- Browne JA, Braden CJ. Definition and relational specification of work-around. NI 2012 2012;2012.
- Campbell EM, Sittig DF, Ash JS, Guappone KP, Dykstra RH. Types of unintended consequences related to computerized provider order entry. J Am Med Inform Assoc 2006;13:547-56. https://doi.org/10.1197/jamia.M2042.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. http://dx.doi.org/10.1177/0038038509103208.
- Jayaraman S, Davies W, Schlachta CM. Getting started with robotics in general surgery with cholecystectomy: the Canadian experience. Can J Surg 2009;52:374-8.
- Goldstraw MA, Patil K, Anderson C, Dasgupta P, Kirby RS. A selected review and personal experience with robotic prostatectomy: implications for adoption of this new technology in the United Kingdom. Prostate Cancer Prostatic Dis 2007;10:242-9. https://doi.org/10.1038/sj.pcan.4500968.
- D’Annibale A, Morpurgo E, Fiscon V, Trevisan P, Sovernigo G, Orsini C, et al. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum 2004;47:2162-8. http://dx.doi.org/10.1007/s10350-004-0711-z.
- Meehan JJ, Sandler A. Pediatric robotic surgery: a single-institutional review of the first 100 consecutive cases. Surg Endosc 2008;22:177-82. http://dx.doi.org/10.1007/s00464-007-9418-2.
- Patel VR. Essential elements to the establishment and design of a successful robotic surgery programme. Int J Med Robot 2006;2:28-35. http://dx.doi.org/10.1002/rcs.77.
- Healey AN, Undre S, Vincent CA. Developing observational measures of performance in surgical teams. Qual Saf Health Care 2004;13:33-40. https://doi.org/10.1136/qshc.2004.009936.
- Vincent C. How to improve patient safety in surgery. J Health Serv Res Policy 2010;15:40-3. http://dx.doi.org/10.1258/jhsrp.2009.09s103.
- Healey AN, Sevdalis N, Vincent CA. Measuring intra-operative interference from distraction and interruption observed in the operating theatre. Ergonomics 2006;49:589-604. http://dx.doi.org/10.1080/00140130600568899.
- Carthey J, de Leval MR, Reason JT. The human factor in cardiac surgery: errors and near misses in a high technology medical domain. Ann Thorac Surg 2001;72:300-5. https://doi.org/10.1016/S0003-4975(00)02592-3.
- Lingard L, Espin S, Whyte S, Regehr G, Baker GR, Reznick R, et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care 2004;13:330-4. https://doi.org/10.1136/qshc.2003.008425.
- Wiegmann DA, ElBardissi AW, Dearani JA, Daly RC, Sundt TM. Disruptions in surgical flow and their relationship to surgical errors: an exploratory investigation. Surgery 2007;142:658-65. https://doi.org/10.1016/j.surg.2007.07.034.
- de Leval MR, Carthey J, Wright DJ, Farewell VT, Reason JT. Human factors and cardiac surgery: a multicenter study. J Thorac Cardiovasc Surg 2000;119:661-72. http://dx.doi.org/10.1016/s0022-5223(00)70006-7.
- Catchpole KR, Giddings AE, Wilkinson M, Hirst G, Dale T, de Leval MR. Improving patient safety by identifying latent failures in successful operations. Surgery 2007;142:102-10. https://doi.org/10.1016/j.surg.2007.01.033.
- Carthey J, de Leval MR, Wright DJ, Farewell VT, Reason JT. Behavioural markers of surgical excellence. Saf Sci 2003;41:409-25. http://dx.doi.org/10.1016/s0925-7535(01)00076-5.
- Patterson ES, Wears RL. Beyond ‘communication failure’. Ann Emerg Med 2009;53:711-12. http://dx.doi.org/10.1016/j.annemergmed.2008.07.014.
- Heath C, Svensson MS, Hindmarsh J, Luff P, vom Lehn D. Configuring awareness. CSCW 2002;11:317-47.
- Schmidt K. The problem with ‘awareness’: introductory remarks on ‘awareness in CSCW’. CSCW 2002;11:285-98. http://dx.doi.org/10.1023/a:1021272909573.
- Simone C, Bandini S. Integrating awareness in cooperative applications through the reaction-diffusion metaphor. CSCW 2002;11:495-530. http://dx.doi.org/10.1023/a:1021213119071.
- Koschmann T, Curtis L, Goodwin C, Feltovich P. The Mystery of the Missing Referent: Objects, Procedures, and the Problem of the Instruction Follower n.d. http://dx.doi.org/10.1145/1180875.1180932.
- Jacklin R, Sevdalis N, Darzi A, Vincent C. Mapping surgical practice decision making: an interview study to evaluate decisions in surgical care. Am J Surg 2008;195:689-96. http://dx.doi.org/10.1016/j.amjsurg.2007.02.016.
- Flin R, Youngson G, Yule S. How do surgeons make intraoperative decisions?. Qual Saf Health Care 2007;16:235-9. https://doi.org/10.1136/qshc.2006.020743.
- Pugh CM, Santacaterina S, DaRosa DA, Clark RE. Intra-operative decision making: more than meets the eye. J Biomed Inform 2011;44:486-96. http://dx.doi.org/10.1016/j.jbi.2010.01.001.
- Pauley K, Flin R, Yule S, Youngson G. Surgeons’ intraoperative decision making and risk management. Am J Surg 2011;202:375-81. http://dx.doi.org/10.1016/j.amjsurg.2010.11.009.
- Endsley MR. Measurement of situation awareness in dynamic systems. Hum Factors 1995;37:65-84. http://dx.doi.org/10.1518/001872095779049499.
- Klein G. Naturalistic decision making. Hum Factors 2008;50:456-60. http://dx.doi.org/10.1518/001872008x288385.
- Cristancho SM, Vanstone M, Lingard L, LeBel M-E, Ott M. When surgeons face intraoperative challenges: a naturalistic model of surgical decision making. Am J Surg 2013;205:156-62. http://dx.doi.org/10.1016/j.amjsurg.2012.10.005.
- Catchpole K, Mishra A, Handa A, McCulloch P. Teamwork and error in the operating room: analysis of skills and roles. Ann Surg 2008;247:699-706. http://dx.doi.org/10.1097/SLA.0b013e3181642ec8.
- Mishra A, Catchpole K, Dale T, McCulloch P. The influence of non-technical performance on technical outcome in laparoscopic cholecystectomy. Surg Endosc 2008;22:68-73. http://dx.doi.org/10.1007/s00464-007-9346-1.
- Hutchins E. Cognition in the Wild. Cambridge, MA: MIT Press; 1995.
- Goodwin D. Upsetting the order of teamwork: is ‘the same way every time’ a good aspiration?. Sociology 2007;41:259-75. http://dx.doi.org/10.1177/0038038507074973.
- Hazlehurst B, McMullen CK, Gorman PN. Distributed cognition in the heart room: how situation awareness arises from coordinated communications during cardiac surgery. J Biomed Inform 2007;40:539-51. http://dx.doi.org/10.1016/j.jbi.2007.02.001.
- Pawson R. Evidence and policy and naming and shaming. Policy Stud 2002;23:211-30. http://dx.doi.org/10.1080/0144287022000045993.
- Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Robot-assisted laparoscopic surgery of the colon and rectum. Surg Endosc 2012;26:1-11. http://dx.doi.org/10.1007/s00464-011-1867-y.
- Lin S, Jiang HG, Chen ZH, Zhou SY, Liu XS, Yu JR. Meta-analysis of robotic and laparoscopic surgery for treatment of rectal cancer. World J Gastroenterol 2011;17:5214-20. http://dx.doi.org/10.3748/wjg.v17.i47.5214.
- Memon S, Heriot AG, Murphy DG, Bressel M, Lynch AC. Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol 2012;19:2095-101. http://dx.doi.org/10.1245/s10434-012-2270-1.
- Alasari S, Min BS. Robotic colorectal surgery: a systematic review. ISRN Surg 2012;2012. http://dx.doi.org/10.5402/2012/293894.
- Kanji A, Gill RS, Shi X, Birch DW, Karmali S. Robotic-assisted colon and rectal surgery: a systematic review. Int J Med Robot 2011;7:401-7. http://dx.doi.org/10.1002/rcs.432.
- Menon M. Robot-assisted surgery: searching for the pony. J Endourol 2012;26:1540-1. http://dx.doi.org/10.1089/end.2012.1554.
- Weissman JS, Zinner M. Comparative effectiveness research on robotic surgery. JAMA 2013;309:721-2. http://dx.doi.org/10.1001/jama.2013.1107.
- Paul S, McCulloch P, Sedrakyan A. Robotic surgery: revisiting ‘no innovation without evaluation’. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1573.
- Huettner F, Dynda D, Ryan M, Doubet J, Crawford DL. Robotic-assisted minimally invasive surgery; a useful tool in resident training – the Peoria experience, 2002–9. Int J Med Robot 2010;6:386-93. http://dx.doi.org/10.1002/rcs.342.
- Toro JP, Lin E, Patel AD. Review of robotics in foregut and bariatric surgery. Surg Endosc 2015;29:1-8. http://dx.doi.org/10.1007/s00464-014-3646-z.
- Payne TN, Pitter MC. Robotic-assisted surgery for the community gynecologist: can it be adopted?. Clin Obstet Gynecol 2011;54:391-41. http://dx.doi.org/10.1097/GRF.0b013e31822b4998.
- Nelson B. Wrestling over robotic surgery. Cancer Cytopathol 2011;119. http://dx.doi.org/10.1002/cncy.20138.
- Murphy D, Dasgupta P, Haig I. Can the NHS afford robotic surgery?. Clin Serv J 2009;18:37-9.
- Koh DC-S, Tsang CB-S, Kim S-H. A new application of the four-arm standard da Vinci surgical system: totally robotic-assisted left-sided colon or rectal resection. Surg Endosc 2011;25:1945-52. http://dx.doi.org/10.1007/s00464-010-1492-1.
- Fung AK, Aly EH. Robotic colonic surgery: is it advisable to commence a new learning curve?. Dis Colon Rectum 2013;56:786-96. http://dx.doi.org/10.1097/DCR.0b013e318285b810.
- Iranmanesh P, More IP, Wagner O, Inan I, Pugin F, Hagen M. Set-up and docking of the da Vinci surgical system: prospective analysis of initial experience. Int J Med Robot 2010;6:57-60.
- Marecik SJ, Prasad LM, Park JJ, Pearl RK, Evenhouse RJ, Shah A, et al. A lifelike patient simulator for teaching robotic colorectal surgery: how to acquire skills for robotic rectal dissection. Surg Endosc 2008;22:1876-81. https://doi.org/10.1007/s00464-007-9736-4.
- Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc 2011;25:240-8. http://dx.doi.org/10.1007/s00464-010-1166-z.
- Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 2011;25:855-60. http://dx.doi.org/10.1007/s00464-010-1281-x.
- Stefanidis D, Wang F, Korndorffer J, Dunne JB, Scott D. Robotic assistance improves intracorporeal suturing performance and safety in the operating room while decreasing operator workload. Surg Endosc 2010;24:377-82. http://dx.doi.org/10.1007/s00464-009-0578-0.
- Higuchi T, Gettman T. Atlas of Robotic Urologic Surgery. New York, NY: Humana Press; 2011.
- Maan ZN, Gibbins N, Al-Jabri T, D’Souza AR. The use of robotics in otolaryngology-head and neck surgery: a systematic review. Am J Otolaryngol 2012;33:137-46. http://dx.doi.org/10.1016/j.amjoto.2011.04.003.
- Pigazzi A, Luca F, Patriti A, Valvo M, Ceccarelli G, Casciola L, et al. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol 2010;17:1614-20. http://dx.doi.org/10.1245/s10434-010-0909-3.
- Spinoglio G, Summa M, Priora F, Quarati R, Testa S. Robotic colorectal surgery: first 50 cases experience. Dis Colon Rectum 2008;51:1627-32. http://dx.doi.org/10.1007/s10350-008-9334-0.
- Wisselink W, . ‘Is robotic surgery right for vascular procedures? Report of 100 aortoiliac cases’ by Petr Stadler. Eur J Vasc Endovasc Surg 2008;36:405-6. http://dx.doi.org/10.1016/j.ejvs.2008.07.001.
- Kwak JM, Kim SH, Kim J, Son DN, Baek SJ, Cho JS. Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum 2011;54:151-6. http://dx.doi.org/10.1007/DCR.0b013e3181fec4fd.
- Parra-Davila E, Ramamoorthy S. Lap colectomy and robotics for colon cancer. Surg Oncol Clin N Am 2013;22:143-51. http://dx.doi.org/10.1016/j.soc.2012.08.007.
- Luca F, Cenciarelli S, Valvo M, Pozzi S, Faso FL, Ravizza D, et al. Full robotic left colon and rectal cancer resection: technique and early outcome. Ann Surg Oncol 2009;16:1274-8. http://dx.doi.org/10.1245/s10434-009-0366-z.
- Du XH, Shen D, Li R, Li SY, Ning N, Zhao YS, et al. Robotic anterior resection of rectal cancer: technique and early outcome. Chin Med J 2013;126:51-4.
- Stănciulea O, Eftimie M, David L, Tomulescu V, Vasilescu C, Popescu I. Robotic surgery for rectal cancer: a single center experience of 100 consecutive cases. Chirurgia 2013;108:143-51.
- Baik SH. Robotic colorectal surgery. Yonsei Med J 2008;49:891-6. http://dx.doi.org/10.3349/ymj.2008.49.6.891.
- Bencini L, Annecchiarico M, Di Marino M, Moraldi L, Perna F, Coratti A. Gastrointestinal robotic surgery: challenges and developments. Robot Surg Res Rev 2015;2:11-27. https://doi.org/10.2147/RSRR.S50266.
- Hance J, Rockall T, Darzi A. Robotics in colorectal surgery. Dig Surg 2004;21:339-43. https://doi.org/10.1159/000081350.
- Lim DR, Min BS, Kim MS, Alasari S, Kim G, Hur H, et al. Robotic versus laparoscopic anterior resection of sigmoid colon cancer: comparative study of long-term oncologic outcomes. Surg Endosc 2013;27:1379-85. http://dx.doi.org/10.1007/s00464-012-2619-3.
- Helvind NM, Eriksen JR, Mogensen A, Tas B, Olsen J, Bundgaard M, et al. No differences in short-term morbidity and mortality after robot-assisted laparoscopic versus laparoscopic resection for colonic cancer: a case-control study of 263 patients. Surg Endosc 2013;27:2575-80. http://dx.doi.org/10.1007/s00464-013-2792-z.
- Averbach M, Popoutchi P, Marques OW, Abdalla RZ, Podgaec S, Abrao MS. Robotic rectosigmoidectomy – pioneer case report in Brazil. Current scene in colorectal robotic surgery. Arq Gastroenterol 2010;47:116-18. https://doi.org/10.1590/S0004-28032010000100018.
- Turchetti G, Palla I, Pierotti F, Cuschieri A. Economic evaluation of da Vinci-assisted robotic surgery: a systematic review. Surg Endosc 2012;26:598-606. http://dx.doi.org/10.1007/s00464-011-1936-2.
- Guru K, Menon M. How do we improve techniques in robotic surgery?. J Urol 2011;185:1186-7. http://dx.doi.org/10.1016/j.juro.2011.01.040.
- Ho CM, Wakabayashi G, Nitta H, Ito N, Hasegawa Y, Takahara T. Systematic review of robotic liver resection. Surg Endosc 2013;27:732-9. http://dx.doi.org/10.1007/s00464-012-2547-2.
- Ramirez PT, Adams S, Boggess JF, Burke WM, Frumovitz MM, Gardner GJ, et al. Robotic-assisted surgery in gynecologic oncology: a Society of Gynecologic Oncology consensus statement. Developed by the Society of Gynecologic Oncology’s Clinical Practice Robotics Task Force. Gynecol Oncol 2012;124:180-4. http://dx.doi.org/10.1016/j.ygyno.2011.11.006.
- Whiteside JL. Robotic gynecologic surgery: a brave new world?. Obstet Gynecol 2008;112:1198-200. http://dx.doi.org/10.1097/AOG.0b013e3181904919.
- Kariv Y, Delaney CP. Robotics in colorectal surgery. Minerva Chir 2005;60:401-16.
- Gillespie BM, Chaboyer W, Fairweather N. Factors that influence the expected length of operation: results of a prospective study. BMJ Qual Saf 2012;21:3-12. http://dx.doi.org/10.1136/bmjqs-2011-000169.
- Ng KH, Lim YK, Ho KS, Ooi BS, Eu KW. Robotic-assisted surgery for low rectal dissection: from better views to better outcome. Singapore Med J 2009;50:763-7.
- Deutsch GB, Sathyanarayana SA, Gunabushanam V, Mishra N, Rubach E, Zemon H, et al. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc 2012;26:956-63. http://dx.doi.org/10.1007/s00464-011-1977-6.
- Weigl M, Antoniadis S, Chiapponi C, Bruns C, Sevdalis N. The impact of intra-operative interruptions on surgeons’ perceived workload: an observational study in elective general and orthopedic surgery. Surg Endosc 2015;29:145-53. http://dx.doi.org/10.1007/s00464-014-3668-6.
- Spitz S. Canada lags in using robotic surgery. CMAJ 2013;185:E305-6. http://dx.doi.org/10.1503/cmaj.109-4429.
- Lee EC, Rafiq A, Merrell R, Ackerman R, Dennerlein JT. Ergonomics and human factors in endoscopic surgery: a comparison of manual vs telerobotic simulation systems. Surg Endosc 2005;19:1064-70. http://dx.doi.org/10.1007/s00464-004-8213-6.
- Kim JC, Yang SS, Jang TY, Kwak JY, Yun MJ, Lim SB. Open versus robot-assisted sphincter-saving operations in rectal cancer patients: techniques and comparison of outcomes between groups of 100 matched patients. Int J Med Robot 2012;8:468-75. http://dx.doi.org/10.1002/rcs.1452.
- Simorov A, Otte RS, Kopietz CM, Oleynikov D. Review of surgical robotics user interface: what is the best way to control robotic surgery?. Surg Endosc 2012;26:2117-25. http://dx.doi.org/10.1007/s00464-012-2182-y.
- Jayne D. MRC/EME/ROLARR/Trial:/The/First/Results n.d.
- Cook A, Douet L, Boutron I. Descriptions of non-pharmacological interventions in clinical trials. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f5212.
- Blencowe N, Mills N, Whiting P, Blazeby J. Providing adequate and practical descriptions in surgical trials. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f6143.
- Cook JA, McCulloch P, Blazeby JM, Beard DJ, Marinac-Dabic D, Sedrakyan A. IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2820.
- Dahm P, Sedrakyan A, McCulloch P. Application of the IDEAL framework to robotic urologic surgery. Eur Urol 2014;65:849-51. http://dx.doi.org/10.1016/j.eururo.2013.11.003.
- Andonian S, Okeke Z, Okeke DA, Rastinehad A, Vanderbrink BA, Richstone L, et al. Device failures associated with patient injuries during robot-assisted laparoscopic surgeries: a comprehensive review of FDA MAUDE database. Can J Urol 2008;15:3912-6.
- Fletcher A, Jamal F, Moore G, Evans RE, Murphy S, Bonell C. Realist complex intervention science: Applying realist principles across all phases of the Medical Research Council framework for developing and evaluating complex interventions. Evaluation 2016;22:286-303. http://dx.doi.org/10.1177/1356389016652743.
- Kellogg Parsons J, Messer K, Palazzi K, Stroup SP, Chang D. Diffusion of surgical innovations, patient safety, and minimally invasive radical prostatectomy. JAMA Surg 2014;149:845-51. http://dx.doi.org/10.1001/jamasurg.2014.31.
- Vats A, Vincent CA, Nagpal K, Davies RW, Darzi A, Moorthy K. Practical challenges of introducing WHO surgical checklist: UK pilot experience. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.b5433.
- Lingard L, Regehr G, Orser B, Reznick R, Baker GR, Doran D, et al. Evaluation of a preoperative checklist and team briefing among surgeons, nurses, and anesthesiologists to reduce failures in communication. Arch Surg 2008;143:12-7. http://dx.doi.org/10.1001/archsurg.2007.21.
- Whyte S, Lingard L, Espin S, Baker G, Bohnen J, Orser B, et al. Paradoxical effects of interprofessional briefings on OR team performance. Cogn Tech Work 2008;10:287-94. http://dx.doi.org/10.1007/s10111-007-0086-8.
Appendix 1 Study management
The study was undertaken by a multidisciplinary Project Management Group made up of academics, health-care professionals representing different members of the OT team, a lay member who provided a patient perspective, and the researchers. A SSC was convened, which met with members of the Project Management Group at three points over the course of the project. The SSC provided advice on the design and conduct of the study and contributed to the selection of key theories for testing in phase 2.
Patient and public involvement
The lay member of the Project Management Group was involved throughout the study, contributing to the design and management of the study and providing a patient perspective on the analysis and interpretation of the data. During preparation of the proposal and the final report he provided feedback on the plain English summary and during the setting up of the study he provided feedback on the design of the patient information sheets and consent forms.
A patient panel was established with four members and chaired by the lay member of the Project Management Group. The panel nominated one member to sit on the SSC. The patient panel met four times over the course of the project, with three of those meetings timed to take place immediately before the SSC meeting so that feedback from the patient panel could be fed into the SSC meeting. The panel provided advice on the selection of key theories for testing in phase 2 and on appropriate strategies for disseminating the research findings to relevant interest groups. Two members of the panel participated in a design workshop where the implications of the study findings for the design of RAS were discussed.
Appendix 2 Searching process for the review of stakeholders’ theories
We aimed to identify papers that described practitioners’ theories of how and in what circumstances RAS can be integrated into clinical practice and how it may affect communication and decision-making in the OT. As such theories are likely to be found in editorials, comments, letters and news articles, we searched MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations, limiting our search to these publication types (see Appendix 1). The websites of relevant professional organisations (e.g. Royal College of Surgeons) and professional journals (e.g. the Annals of the Royal College of Surgeons of England, the Nursing Times, and the Health Service Journal) were searched, and a number of searches were run on Google (GoogleTM, Mountain View, CA, USA). In reviewing the literature when preparing the proposal for this study, we discovered that discussion sections of quantitative studies of RAS also sometimes contain such theories. Therefore, we searched MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations to identify systematic reviews and individual studies of colorectal RAS and systematic reviews of general RAS (see Appendix 14). Reference lists were used to identify further relevant individual studies.
Selection and appraisal of documents
The purpose of the review was to identify and catalogue the range of theories regarding how RAS becomes integrated into practice and how it impacts communication, teamwork and decision-making, rather than to assess the validity of those theories. Therefore, the selection and appraisal of identified papers was based on relevance to the review question, rather than on rigour, as is the case in the theory elicitation phase of a realist review. All of the retrieved records were screened based on title and abstract. Reviewers asked (1) is this about RAS using the da Vinci robot (as distinct from other uses of robots in the surgical context) and (2) does it potentially contain ideas about how RAS works, for whom and in what circumstances? Full-text copies of all potentially relevant papers were retrieved. Reviewers read the papers to determine whether or not they contained ideas about how RAS is introduced into practice and affects communication and decision-making in the OT (the mechanisms), the contexts in which this happens and/or the consequences of this (the outcomes).
Data extraction, analysis and synthesis
Two reviewers extracted the authors’ theories about the mechanisms through which (1) RAS gets integrated into practice; and (2), once adopted in practice, how RAS affects communication, teamwork and decision-making in the OT. When available, information on the contexts that trigger these mechanisms and the subsequent outcomes was also extracted. All of this formed the data for the review and was recorded in a working document with links to the original source. The reviewers discussed the extracted data, drawing together data from multiple studies to develop tentative theories, which were added to and refined as further papers were identified. In developing the theories, it was often necessary to return to the papers for further detail. During this process, we encountered conflicting theories. This is not surprising and reflects the contrasting views of multiple, different stakeholders regarding how RAS is best introduced or impacts on teamwork, as well as the cumulative knowledge that comes from successive revisions to ways in which RAS has been implemented and the small and large adaptions to work routines made by clinicians on the ground to support its use in practice. Therefore, we did not remove or ignore such conflicting theories, but instead took them forward for further exploration in our interviews with OT teams. Although, as noted above, the intention was to catalogue the theories identified, rather than to assess their validity, we sought to indicate where there was evidence to support the theories and which theories were presented without supporting evidence.
Appendix 3 Phase 1 staff information sheet
Appendix 4 Initial phase 1 interview topic guide
Appendix 5 Phase 2 staff information sheet
Appendix 6 Phase 2 staff consent form
Appendix 7 Phase 2 patient information sheet
Appendix 8 Phase 2 patient consent form
Appendix 9 ROLARR inclusion and exclusion criteria
Inclusion criteria
-
Aged ≥ 18 years.
-
Able to provide written informed consent.
-
Diagnosis of rectal cancer amenable to curative surgery either by anterior resection or abdominoperineal resection (i.e. stages T13, N02, M0 by CT and MRI or transrectal ultrasound).
-
Rectal cancer suitable for resection by either standard or robot-assisted laparoscopic procedure.
-
Fit for standard or robot-assisted laparoscopic rectal resection.
-
American Society of Anesthesiologists score of ≤ 3.
Exclusion criteria
-
Benign lesions of the rectum.
-
Cancers of the anal canal.
-
Locally advanced cancers not amenable to curative surgery.
-
Locally advanced cancers requiring en bloc multivisceral resection.
-
Synchronous colorectal tumours requiring multisegment surgical resection.
-
Coexistent inflammatory bowel disease.
-
Clinical or radiological evidence of metastatic spread.
-
Concurrent or previous diagnosis of invasive cancer that could confuse diagnosis.
-
History of psychiatric or addictive disorder or other medical condition would preclude the patient from meeting the study requirements.
-
Pregnancy.
-
Participation in another rectal cancer clinical trial relating to surgical technique.
Appendix 10 Description of Observational Teamwork Assessment for Surgery, training on Observational Teamwork Assessment for Surgery and assessment of inter-rater reliability
Observational Teamwork Assessment for Surgery comprises ratings on five team behaviour constructs:
-
communication – quality and quantity of information exchanged among members of the team
-
co-ordination – management and timing of activities and tasks
-
co-operation and back-up behaviour – assistance provided among members of the team, supporting others and correcting errors
-
leadership – provision of directions, assertiveness and support among members of the team
-
team monitoring and situational awareness – team observation and awareness of ongoing processes.
Observational Teamwork Assessment for Surgery distinguishes between different subteams in the OT (surgeons, anaesthetists and nurses) and different phases of a procedure (pre-, intra- and postoperative). For one operation a total of 45 behavioural ratings are generated (five behaviour constructs × three sub-teams × three phases). During observation of the surgery, field notes are made about the actions of members of the team, informed by the exemplar behaviours that OTAS provides for each combination of construct/subteam/phase. These field notes are then used to decide what the score should be for each behaviour, for each subteam and each phase, on a 7-point scale from 0 to 6, where 0 signifies problematic behaviour that severely hinders team function and 6 signifies exemplary behaviour that is very highly effective in enhancing team function.
In preparation for data collection, four members of the research team undertook training in OTAS at Imperial College London. The training was delivered by two OTAS experts who had been involved in its development and refinement and had significant experience of using it in practice. During training, video clips of operations were watched and scored using OTAS. The researchers were able to discuss these scores with the experts and come to a shared understanding of how to apply the scoring system. Following this initial training, further video clips were provided to the research team. These were viewed and scored, and a detailed discussion followed of how the scores were allocated. The team then scored clips individually. Inter-rater reliability was assessed, using intraclass correlation coefficients. When using OTAS for research purposes, a minimum intraclass correlation coefficient of 0.71 is recommended. Agreement between the ratings of the experts and the ratings of the research team members ranged between 0.75 and 0.89, indicating very high agreement for all research team members.
The three members of the research team who were to be undertaking the observations then observed several colorectal operations in the field to trial OTAS. The researchers worked in pairs, with both making notes to inform the OTAS ratings. Afterwards, the researchers wrote up their notes and separately used OTAS to score the operation observed, before coming together to discuss how they had decided on the scores. Once the observation of the rectal cancer resections began, two researchers observed each operation, with both using OTAS to score the teamwork observed. Inter-rater reliability was assessed using intraclass correlation coefficients. Agreement between the researchers ranged between 0.72 and 0.82, indicating a suitably high level of agreement when using OTAS for research purposes.
Appendix 11 Description of quantitative analysis of video data
We looked at two particular types of request: for the first assistant to provide suction to remove blood when the first assistant is not currently holding the suction instrument; and for the first assistant to assist with retraction when the assistant is not currently holding a grasper. We chose these requests on the basis that these requests occurred across all of the videos of robotic operations. In deciding how to code the data, we contacted researchers who had done similar work, regarding the time taken for a scrub practitioner to respond to a surgeon’s request. In that work, timing was from the end of a request to the onset of passing the instrument. However, whereas in that work requests were typically brief, requests within our data set were longer (e.g. ‘Can you maybe get a grasper [name] in there and just er gently get on that and see if you can gently give me some traction’), so the scrub practitioner and first assistant would know what was being requested before the surgeon finished making the request. Given the limited number of tasks the first assistant was asked to do during the robotic phase of an operation, it was also likely that the first assistant would be able to anticipate what the request would be as soon as the surgeon began to speak. Therefore, we timed from the beginning of the surgeon’s request to when the instrument is first visible on the screen. Once all instances had been identified, the video extracts were reviewed to determine the mechanisms and contextual factors that contributed to whether or not the task was completed in a timely manner.
Appendix 12 Refined theories of teamwork and communication
| Theory | Context | + | Mechanism | = | Outcome | |
|---|---|---|---|---|---|---|
| Resource | Response | |||||
| T5a. Experienced assistant | Assistant engaged in operation Assistant keen to demonstrate ability to respond quickly |
+ | Knowledge gained through experience of procedure | Assistant is able to work with less guidance and to anticipate what is required, so they offer assistance and/or prepare to act | = | Improved co-ordination Assistant’s actions performed correctly and in timely manner |
| T5b. Scrub practitioner support | Assistant is willing to acknowledge need for and accept support Surgeon acknowledges scrub practitioner’s role in supporting assistant |
+ | Experienced scrub practitioner | Scrub practitioner guides assistant and undertakes tasks on their behalf | = | Actions are performed correctly |
| T5c. Licence to speak | Positive relationship between assistant and surgeon | + | Surgeon encourages assistant to communicate actions and concerns | Assistant feels comfortable to speak up | = | Improved co-ordination Actions are performed correctly |
| T6a. Request preparation | Assistant and scrub practitioner engaged in operation | + | Surgeon undertakes preparation and/or pre-request work | Attention of assistant and scrub practitioner is secured, enabling them to hear the message | = | Improved co-ordination |
| T6b. Long requests | Assistant engaged in operation Microphone working |
+ | Surgeon uses longer requests | Assistant has time to prepare so they are ready for more specific instructions | = | Improved co-ordination Assistant’s actions are performed correctly |
| T6c. Oral response | Assistant and scrub practitioner communicate loudly and clearly | + | Assistant and scrub practitioner respond orally to surgeon’s requests | Surgeon knows request will be completed and is made aware of any challenges in doing this | = | Improved co-ordination |
Appendix 13 Refined theories of communication, teamwork and decision-making
| Theory | Context | + | Mechanism | = | Outcome | |
|---|---|---|---|---|---|---|
| Resource | Response | |||||
| T5a. Experienced assistant | Assistant engaged in operation Assistant keen to demonstrate ability to respond quickly |
+ | Knowledge gained through experience of procedure | Assistant is able to work with less guidance and to anticipate what is required, so they offer assistance and/or prepare to act | = | Improved co-ordination Assistant’s actions performed correctly and in timely manner Reduced surgeon stress |
| T5b. Scrub practitioner support | Assistant is willing to acknowledge need for and accept support Surgeon acknowledges scrub practitioner’s role in supporting assistant |
+ | Experienced scrub practitioner | Scrub practitioner guides assistant and undertakes tasks on their behalf | = | Actions are performed correctly |
| T5c. Licence to speak | Positive relationship between team and surgeon | + | Surgeon encourages team to communicate actions and concerns | Team members feel comfortable to speak up | = | Improved co-ordination Actions are performed correctly Increased surgeon situational awareness |
| T6a. Request preparation | Assistant and scrub practitioner engaged in operation | + | Surgeon undertakes preparation and/or pre-request work | Attention of assistant and scrub practitioner is secured, enabling them to hear the message | = | Improved co-ordination |
| T6b. Long requests | Assistant engaged in operation Microphone working |
+ | Surgeon uses longer requests | Assistant has time to prepare so they are ready for more specific instructions | = | Improved co-ordination Assistant’s actions are performed correctly |
| T6c. Oral response | Assistant and scrub practitioner communicate loudly and clearly | + | Assistant and scrub practitioner respond orally to surgeon’s requests | Surgeon knows request will be completed and is made aware of any challenges in doing this | = | Improved co-ordination |
| D1a. Reduced distraction | Surgeon trusts assistant to successfully carry out requests and communicate problems | + | Surgeon’s position within console | Surgeon feels confident to remain in console | = | Reduced distraction Increased concentration |
| D1b. Experienced scrub practitioner | Experienced scrub practitioner | + | Surgeon’s position within console | Scrub practitioner aware of need to move attention between screen and robotic arms and positions themselves so as to facilitate this, enabling them to notice when arms are clashing and alert surgeon | = | Surgeon’s situational awareness is maintained |
| D1c. Awareness of instruments | Surgeon moves camera away from retraction instrument | + | Increased control of retraction | Surgeon loses awareness of position of instrument | = | Disruption to flow of operation Increased operation duration Increased patient safety risk |
Appendix 14 Search strategies for MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations
Search 1 (grey literature)
-
robot*.ti.
-
surg*.ti,ab.
-
1 and 2
-
Comment/
-
Letter/
-
Editorial/
-
news/ or newspaper article/
-
“Comment on”.ti.
-
(letter* adj3 editor*).ti.
-
opinion*.ti.
-
(view or views).ti.
-
or/4-11
-
3 and 12
-
limit 13 to (english language and yr=“2000 -Current”)
Search 2 (systematic reviews of colorectal robot-assisted surgery)
-
robot*.ti.
-
surg*.ti,ab.
-
1 and 2
-
colorectal.ti.
-
colon*.ti.
-
rectal.ti.
-
4 or 5 or 6
-
3 and 7
-
meta-analysis.mp.
-
meta-analysis.pt.
-
review.pt.
-
search:.tw.
-
9 or 10 or 11 or 12
-
8 and 13
-
limit 14 to (english language and yr=“2000 -Current”)
Search 3 (individual studies of colorectal robot-assisted surgery)
-
robot*.ti.
-
surg*.ti,ab.
-
1 and 2
-
colorectal.ti.
-
colon*.ti.
-
rectal.ti.
-
4 or 5 or 6
-
3 and 7
-
limit 8 to (english language and yr=“2000 -Current”)
Search 4 (systematic reviews of robot-assisted surgery)
-
robot*.ti.
-
surg*.ti,ab.
-
1 and 2
-
Cochrane database of systematic reviews.jn.
-
search.tw.
-
meta-analysis.pt.
-
Medline.tw.
-
systematic review.tw.
-
4 or 5 or 6 or 7 or 8
-
3 and 9
-
limit 10 to (english language and yr=“2000 -Current”)
Glossary
- Abdominoperineal resection
- An operation to remove the rectum in its entirety as well as a variable amount of the sigmoid colon and surrounding lymph nodes in the mesorectal fat and sigmoid mesentery. As the entire rectum and anus is removed, a permanent colostomy is made as part of this operation. This operation is usually performed for the curative treatment of rectal cancer.
- Anaesthetic assistant
- A role performed either by a theatre nurse or by an operating department practitioner, working outside the sterile field, who assists the anaesthetist in the administration and monitoring of anaesthesia.
- Anterior resection
- An operation to remove the rectum and a variable amount of the sigmoid colon as well as the surrounding lymph nodes situated in the mesorectal fat and in the sigmoid colon mesentery. This operation is usually performed for the curative treatment of rectal cancer.
- Circulating practitioner
- A role performed either by a theatre nurse or by an operating department practitioner, working outside the sterile field and responsible for supporting those within the sterile field, particularly the scrub practitioner, by obtaining the necessary equipment and liaising and co-ordinating with others within the operating theatre and in the wider operating department.
- Conversation analysis
- This is an approach to ethnomethodological work that focuses on the production of social order in interaction, both in everyday conversations and in institutional encounters. It relies on audio/video recordings to explore how contributions to interaction (vocal and non-vocal) are organised and how this interactional organisation helps to accomplish aspects of work for the participants, such as giving bad news and managing instructions. The approach was developed by Harvey Sacks, Emmanuel Schegloff and Gail Jefferson.
- Demi-regularity
- A pattern in the outcomes of an intervention that is only semipredictable, due partly to the impact of contextual factors.
- Docking
- The action of pushing the robot towards the patient, correctly positioning it, connecting the robotic arms to the laparoscopic ports, and inserting the camera and robotic instruments.
- Ethnomethodology
- This is a specific perspective within the broad field of sociology which was developed by Harold Garfinkel. It is concerned with the production of social order and, in particular, the methods and practices that people use in making sense of the conduct of others and in ordering and organising their everyday affairs. It is often characterised by ethnographic studies or analyses of audio or video recordings.
- First assistant
- A role that may be undertaken by a surgical trainee, a theatre nurse or an operating department practitioner who has undertaken first assistant training, or occasionally by another consultant surgeon, in which they are working within the sterile field and are responsible for assisting the surgeon in carrying out the operation, particularly assisting with retraction.
- Hybrid operation
- An operation of which part is undertaken laparoscopically and part is undertaken using robot-assisted surgery.
- Implementation chain
- The series of interconnected processes through which an intervention is introduced and delivered to produce immediate and intermediate outcomes and ultimate impacts (both short and long term).
- Laparoscopic surgery
- Surgery that is performed by inserting a camera and instruments into small incisions in the patient’s abdomen, in contrast to open surgery in which large abdominal wounds are created in order to access the surgical site.
- Mechanism
- Used in realist evaluation to refer to a resource that an intervention provides and recipients’ reasoning about and response to that resource, leading to a particular outcome.
- Operating department practitioner
- An allied health professional who has undertaken training to be able to provide a high standard of skilled support during perioperative care. They may take on the role of a scrub practitioner, a circulating practitioner or an anaesthetic assistant.
- Retraction
- Using an instrument to pull or push tissue away from the surgical site, improving the surgeon’s view of and access to the surgical site.
- Scrub practitioner
- A role performed either by a theatre nurse or by an operating department practitioner, working within the sterile field, in which they are responsible for supporting the surgeon in carrying out the operation, particularly ensuring the availability and the sterility of surgical instruments and passing them to the surgeon as they are needed.
- Suction
- Removing either blood or surgical smoke produced through the use of diathermy, by inserting a suction tube, thereby improving the surgeon’s view of the surgical site.
- Totally robot-assisted operation
- An operation that is undertaken using robot-assisted surgery with no part of the operation being undertaken laparoscopically (in contrast to a hybrid operation).
- Workplace studies
- Studies that adopt a broadly ethnographic approach to the analysis of technology in use in a range of organisational and everyday settings. The studies are principally concerned with understanding the situated, tacit and contingent social and collaborative practices that underpin the use of technologies.
List of abbreviations
- 2D
- two-dimensional
- 3D
- three-dimensional
- ANOVA
- analysis of variance
- CMO
- context–mechanism–outcome
- FY2
- foundation year 2 doctor
- GI
- gastrointestinal
- NASA-TLX
- NASA Task Load Index
- ODP
- operating department practitioner
- OT
- operating theatre
- OTAS
- Observational Teamwork Assessment for Surgery
- RAS
- robot-assisted surgery
- RCT
- randomised controlled trial
- ROLARR
- RObotic versus LAparoscopic Resection for Rectal cancer
- SD
- standard deviation
- SSC
- Study Steering Committee
- SURG-TLX
- Surgery Task Load Index