Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/64/154. The contractual start date was in November 2013. The final report began editorial review in October 2016 and was accepted for publication in March 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Tess Harris is a member of the Health Technology Assessment Primary Care and Community Preventive Interventions Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Carey et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
The World Health Organization defines intellectual disability (ID) as ‘. . . a condition of arrested or incomplete development of the mind, which is especially characterized by impairment of skills manifested during the developmental period, which contribute to the overall level of intelligence, i.e. cognitive, language, motor, and social abilities’. 1 In the UK, ID is commonly referred to as learning disability. 2 This should be viewed distinct from the term ‘learning difficulty’, commonly used across UK education, which can encompass conditions such as dyslexia that do not necessarily imply intellectual impairment and, hence, learning disability. Throughout this report we will refer to learning disability as intellectual disability or ID, except when we are explicitly referring to UK documents or outputs that have used learning disability as their preferred term.
There are three core criteria that must be met for a person to be considered to have an ID:3
-
intellectual impairment (‘a significantly reduced ability to understand new or complex information’3)
-
with social or adaptive dysfunction (‘a reduced ability to cope independently’3)
-
that has started before adulthood (‘with a lasting effect on development’3).
The most common genetic cause of ID is Down syndrome,4 and every child born with Down syndrome will be considered to have some level of ID. Neurological conditions such as cerebral palsy will be strongly associated with ID,5 although they do not necessarily imply low intelligence and, hence, ID. People with other neurodevelopmental disorders such as autism may or may not satisfy all of these criteria depending on where on the autism spectrum they lie. Estimates of the prevalence of ID at all ages vary widely between 1% and 3% of the general population across the UK, the USA and other high-income countries. 6
People with ID have more significant health risks and major health problems than the general population and, as a result, are more likely to die younger. 7 In the NHS, there is evidence that people with ID receive suboptimal care, and this inequity contributes to poor health outcomes, including avoidable mortality. 5 In 2008, an independent inquiry into access to health care for people with learning disability, led by Sir Jonathan Michael, concluded that people with ID receive less effective care, leading to avoidable suffering and death. 8 In addition, the report highlighted the paucity of information on NHS health care for people with ID.
A key focus of national policy has been improving the quality of primary care for people with ID. In 2006, the Disability Rights Commission recommended the introduction of annual health checks,7 which was further supported by Sir Jonathan’s inquiry. 8 Subsequently, in 2009, a national Directed Enhanced Service (DES) was introduced in England, which funds general practices to provide annual health checks to adults with ID and requires that staff receive appropriate training. 9 The health check is intended to identify undetected health problems and improve prescribing and co-ordination with secondary care. 10 Recent systematic reviews have confirmed that health checks are effective in identifying health problems but found a paucity of evidence on their impact on health status and outcomes,11 and have stated the need for an increase in quantity and quality of research on health interventions for people with ID. 12
This study, therefore, aims to fill key knowledge gaps with a large sample evaluation of the effectiveness of annual health checks and a comprehensive study of health and health care in a national sample of adults with ID.
Health of people with intellectual disability
People with ID experience poorer health outcomes than the general population, such as increased emergency admission to hospital13 and mortality. 14 The reasons for this poorer health are complex but are not solely explained by unavoidable biological manifestations of the cause of ID. Local ID register-based studies have identified markedly higher mortality, with estimates in the age-adjusted risk of death ranging between 3 and 18 times higher than those of the general population. 5,15,16 This increased risk of death is seen across a range of conditions and is not limited to causes related to the underlying ID. Studies on disease prevalence and morbidity among people with ID, although limited, provide a similar picture, with an increased risk of epilepsy, diabetes, cardiovascular disease, infections, accidents and sensory impairment. 17–21 For example, it is estimated that about one in four people with ID suffer from epilepsy, compared with < 1% of the general population. 18 The concerns over the health of people with ID have been reinforced by findings from the Confidential Inquiry into Premature Deaths of People with Learning Disability (CIPOLD), which confirmed high premature mortality with a high proportion of unexpected deaths. 22
There is evidence to suggest that the quality of health care received by people with ID contributes to poorer health. This may be due to difficulties in communication that lead to unmet health needs, poorer access to health services and discrimination. 7 Sir Jonathan’s inquiry into access for health care for people with ID concluded that high levels of need were not being met, that people with ID receive less effective care than they are entitled to and that this leads to avoidable suffering and death. 8 The high proportion of unexpected deaths reported by CIPOLD may also indicate that serious health problems are not fully identified in people with ID, leading to poor outcomes. 22
In addition, Sir Jonathan’s inquiry highlighted the paucity of information on NHS health care for people with ID. 8 These data gaps were further summarised and described by the Learning Disabilities Observatory in 2011. 23 Current national systems do not routinely allow a description of primary care use, quality of chronic disease care, hospital utilisation and major health outcomes for people with ID. Specifically, national systems such as cancer registration, Hospital Episode Statistics (HES), mortality registration or general practice data collections (such as the General Practice Extraction Service) either do not systematically record ID or cannot provide analyses separately for people with ID. An initial analysis in 2010 of a primary care database was commissioned as part of the independent inquiry. It reported on a range of measures in people with ID and found evidence for higher rates of obesity, poor seizure control and poorer treatment of urinary tract infections (UTIs). 24 However, this limited analysis was not developed further or submitted for peer-reviewed publication, as far as we are aware. Thus, knowledge of the health of people with ID in the UK up to 2015 has still been primarily based either on selective recording, for example in hospital data, or on selected populations from local ID registers. 25 Similarly, we know very little about the cost implications of providing NHS care for people with ID.
Annual health checks
A key recommendation of Sir Jonathan’s inquiry was the creation of a scheme in primary care to provide annual health checks for people with ID, which was outlined in the 2009 national strategy for learning disability. 26 The primary purpose of annual health checks is to address access barriers experienced by people with ID and to allow the identification of unmet health needs. 9 Health checks also aim to improve prescribing and co-ordination with secondary care and are identified as a reasonable adjustment in accordance with the Disability Discrimination Act 1995. 27
Annual health checks for adults with ID were implemented as a DES for primary care in 2009. 28 This DES funds practices to provide annual health checks to adults with ID, with an emphasis on those who have higher levels of need and who are known to the local authority services. It also requires that senior practice staff attend an approved multiprofessional educational session and that all practice staff receive training to reduce attitudinal barriers and improve communication with this group of patients.
Annual health checks are currently the main NHS intervention to improve the quality of primary care for people with ID. 29 However, estimates from 2011–12 suggested that only 53% of eligible adults with ID had received an annual health check. 30 It may be that more have been invited for a health check, and for a variety of reasons had either refused or missed their arranged appointment, but this is not known. As of 2016, practices participating in the DES are required to invite registered patients on their learning disabilities register, who are aged ≥ 14 years, for an annual health check.
Evidence base for annual health checks
The presumed long-term benefit of health checks assumes that the identification of unmet health needs will lead to appropriate intervention and improvements in well-being and health outcomes. The Learning Disabilities Observatory undertook a systematic review of the evidence base for annual health checks in 2011,11 subsequently updated in 2014,12 which summarised health gains and impacts from similar interventions both in the UK and internationally. The initial review identified 38 studies (45 in the later review) that comprised a total of > 5000 individuals receiving a health check. Most studies were small and the majority were uncontrolled, with only four randomised controlled trials and two controlled studies. The higher-quality studies clearly demonstrated that health checks led to the improved detection of new health problems, with one randomised controlled trial reporting a 60% increase in the diagnosis of new problems and a matched controlled study reporting 2.54 additional health problems identified, on average, in people receiving health checks. 31,32 These studies also reported an increase in the uptake of preventative interventions such as vaccination, cancer screening and sensory testing. These conclusions are also supported by the larger number of uncontrolled studies. 11,12
Evidence on health outcomes relating to health checks is far more limited and of poorer quality. Uncontrolled studies in the UK have reported a variety of benefits of health checks, including improved seizure control and weight management. 33–36 These UK studies were small, with fewer than 100 participants. One larger before-and-after study of a domiciliary preventative intervention in the USA found a reduction in self-reported pain, falls and emergency room visits,37 whereas another larger US study suggested that health screening may help to resolve psychiatric problems by identifying physical problems. 38
The systematic reviews by Robertson et al. 11,12 concluded that there was limited evidence on the effect of health checks on health status and that further work was required to establish the effectiveness of health checks. It is highly plausible that health checks, through identifying unmet health needs and preventative interventions, will lead to an improvement in health outcomes, but evidence to confirm this is important. However, it is also possible that health needs identified in health checks may not be adequately addressed, and that implementation of health checks by non-enthusiasts, outside study settings, will not yield the same benefit in terms of newly identified health needs. For example, health checks may lead to the recording of poor seizure control in epilepsy, but appropriate management may require expertise or specialist input to review anticonvulsant medication, which may not be available.
Aims of the study
The study had two overall aims.
-
Aim 1 was to describe the health, health-care quality, equity of health care, mortality rates and NHS costs for adults with ID in a national sample.
-
Aim 2 was to evaluate the process and outcome effectiveness of annual health checks for adults with ID in primary care.
The original objectives associated with these aims are shown in Table 1.
| Aim | Objective | Location in report |
|---|---|---|
| (1) To describe the health, health care quality, equity of health care and NHS costs for adults with ID in a national sample | Quantify primary and secondary care utilisation by adults with ID, including prescribing | See Chapters 3 and 5 |
| Describe and quantify specific health risks for adults with ID | See Chapters 3 and 4 | |
| Describe the quality of primary care received by adults with ID | See Chapter 3 | |
| Determine whether or not adults with ID experience greater socioeconomic inequities than the general population | See Chapter 3 | |
| Determine annual health service costs for people with ID compared with the general population | See Chapter 3 | |
| (2) To evaluate the process and outcome effectiveness of annual health checks for adults with ID in primary care | Determine whether or not individuals receiving annual health checks experience improvement in health-care process measures and health problem identification | See Chapter 7 |
| Determine whether or not individuals receiving annual health checks experience improvement in health outcomes | See Chapter 6 | |
| Determine whether or not practice participation in the annual health check DES improves outcomes for people with ID | See Chapter 6 | |
| Identify determinants and equity of uptake of annual health checks in practices that participate in the directed enhanced service | See Chapter 7 | |
| Determine the change in health service costs in the year before and the year after an annual health check | See Chapter 7 |
The first aim of our study, to provide a descriptive analysis of health and health-care quality for adults with ID, is explored via two distinct analyses. First, we take a snapshot of the health of the adult population with ID on 1 January 2012, registered in a large primary care database, and describe their chronic disease prevalence compared with an age- and gender-matched control group without ID (from the same general practices). Similarly, we will describe and compare the primary care utilisation of adults with ID in terms of consultations, as well as process measures and prescribing. We will provide a best estimate of annual health-care costs by applying NHS reference costs and drug tariffs for health-care events recorded, including primary care consultations, prescribing, hospital admissions and outpatient consultations.
The second distinct series of analyses encompassing the first aim will follow a group of adults with ID from 2009 to 2013 to describe their secondary care utilisation. Here, we will compare and summarise emergency hospitalisations with an age-, gender- and practice-matched control group of adults without ID. For two indicator conditions [UTIs and lower respiratory tract infections (LRTIs)], which are likely to be common reasons for hospitalisation for adults with ID, we will compare their primary care utilisation in the period before the hospital admission with similarly recorded admissions within the general population. Finally, we will describe mortality patterns between 2009 and 2013 and summarise the key differences between adults with and adults without ID.
The primary outcome for the second aim (evaluation of annual health checks) was identified as emergency hospital admissions. As the evidence base suggests that health checks improve the detection of unmet health needs, the management of chronic disease and the uptake of preventative care,12 the possible longer-term health benefits of health checks may occur across a range of conditions, such as better seizure control in epilepsy, reduced cardiovascular risk and the early treatment or prevention of infection. For all of these conditions, delayed, incomplete or poor management will lead to an emergency hospital admission. Thus, emergency hospital admissions may be an important measure of quality of care for a range of conditions and a common pathway for the benefits of annual health checks. An associated reduction in emergency hospital admissions is likely to be a key measurable and valued benefit from annual health checks, as people with ID experience high levels of emergency admissions. 39 Additionally, unplanned admissions to hospital for patients with ID can be particularly stressful events, and unnecessary delays and omissions in treatment can compromise patient safety. 40
Many unplanned admissions to hospital would be expected to occur even if health checks really were having an underlying beneficial effect. Thus, we will also investigate a subgroup of emergency admissions for ambulatory care-sensitive conditions (ACSCs). 41 These admissions are thought to be potentially preventable with better clinical management in primary care. There is some variation in how ACSCs are explicitly defined,42 particularly as they were originally developed in the USA. 43 However, most definitions will include a combination of conditions for which acute management should prevent an admission (e.g. pyelonephritis) and other chronic conditions, such as chronic obstructive pulmonary disease (COPD), for which effective preventative care may prevent admissions. However, the preventable concept of an ACSC may ultimately depend on the availability of, and referral to, alternative services such as respite care. 44 Some suggested interventions to prevent ACSCs, such as improvements in self-management education and telemedicine,44 may be less effective for patients with ID. Annual health checks may have a role to play here, and although we will have reduced power to investigate this outcome compared with all emergency admissions, emergency admissions for ACSCs may provide a more relevant estimate of effectiveness.
We will also explore a limited economic costing analyses, when our data allow. A more formal cost-effectiveness analysis is not possible using the resources in this study. In addition, a cost-effectiveness analysis would have presumed evidence of effectiveness, and it would have been premature to commit resources to such an analysis before we had determined effectiveness.
Secondary outcomes in relation to health checks included disease-specific and generic process and outcome measures. We will describe what is recorded on a patient’s electronic record at the time of a health check, and then summarise the overall impact that a health check has on a selection of process measures being carried out over time. This will include, for example, the recording of cardiovascular risk factors such as body mass index (BMI), blood pressure and smoking, as well as the recording of the uptake of cervical and breast cancer screening and influenza vaccination. We will also summarise the recording of key health areas for patients with ID, such as incontinence, constipation, mobility, vision and hearing.
Why is the research needed now?
Concerns over the quality and equity of NHS health care received by people with ID are long-standing,7 and the last few years have seen an increase in targeted NHS action to address these concerns. Specifically, in 2009, funding for annual health checks in primary care was introduced in England,30 and since 2016 the NHS has remained committed to the current DES scheme. 29 The rate of uptake of the scheme among eligible adults in 2011–12 was 53%, only a small increase since 2010–11 (48%). 30 For both clinicians and NHS policy-makers, the current economic climate may be a barrier to the wider adoption of annual health checks in primary care, or whether or not the scheme is renewed.
However, the development of Clinical Commissioning Groups may act as a catalyst for the wider implementation of annual health checks, as these groups standardise services offered by primary care in their area. Given this, an evaluation of the outcome effectiveness of annual health checks has the potential to influence policy decisions. If our study can demonstrate a clear benefit from health checks, this will strengthen the case for implementation and for ensuring access for all people with ID. Lack of evidence of any measurable benefit will not invalidate health checks, but it will raise questions over the quality of current implementation and the effectiveness of the service response to identified health needs. Our study should be able to differentiate between these two explanations and guide development of services to maximise health gain from annual health checks.
In summary, our study will evaluate the effectiveness of health checks in improving outcomes as well as processes of care and will also address the paucity of information on the quality of health care for adults with ID.
Chapter 2 Methods
The Clinical Practice Research Datalink
The Clinical Practice Research Datalink (CPRD) is a large, validated primary care database that has been collecting anonymous patient data from participating UK general practices since 1987. 45 It includes a full longitudinal medical record for each registered patient that contains coded information on medical diagnoses, prescribing and tests carried out within the practice. Additionally, referrals to specialists and secondary care settings, and lifestyle information such as smoking and alcohol status, are recorded in the CPRD. By 2015, it had been estimated to include over 4 million active patients, approximately 7% of the UK population. 45
Subject to the practice’s approval, the CPRD patient data are routinely linked to other national administrative databases by a ‘trusted third party’ via their NHS number, gender, date of birth and postcode. These databases include:
-
the Index of Multiple Deprivation (IMD), a small-area measure of deprivation used in England for the allocation of resources46
-
the HES database, which routinely records clinical, patient, administrative and geographical information on all NHS-funded inpatient episodes in the UK
-
Office for National Statistics (ONS) death certification data.
Quality and Outcomes Framework and learning disability
Medical diagnoses on the CPRD are recorded using Read codes. Before we extracted data from the CPRD, we carried out an extensive review of which Read codes we would use to identify patients with ID. The starting point for this was the Quality and Outcomes Framework (QOF). 47 The QOF was introduced in April 2004 as part of a new general medical services contract in the UK, which would remunerate practices based on performance. One key element was the creation of disease registers for many important comorbidities, such as coronary heart disease (CHD) and COPD, using sets of nationally agreed Read codes. This has had a notable impact on the recording of these diseases, such as for CHD,48 with the assumption being that it has led to diagnostic accuracy overall (e.g. for COPD). 49
Intellectual disability, classified as learning disability, has been part of the QOF since 2006. Originally there was only one indicator related to this, LD1 (‘The practice can produce a register of people with learning disability’). Although the rubric for the register suggests that all patients with ID were included, the exact specification of business rules from around this time suggested that only patients aged ≥ 18 years were included. 47 In 2014, the disease register indicator was modified to LD001 (‘The contractor establishes and maintains a register of patients aged 18 or over with learning disabilities’) to make the age criteria more explicit. However, this was changed in 2014–15 to LD003 (‘The contractor establishes and maintains a register of patients with learning disabilities’), and the associated business rules now (from version 30 onwards) allow for patients of any age to be included.
Although published national figures for the QOF learning disability register of patients are available (see Appendix 1), the change in the definition makes it difficult to consistently estimate the prevalence of ID over time. First, published denominators for the first 2 years (2006–7 and 2007–8) appear to be based on all patients, so we have had to estimate the total number of adults to obtain the prevalence of ID within adults only. The addition of non-adults to the QOF learning disability register in 2014–15 meant that no separate adult-only figures were estimated. The fall in the published prevalence from 0.48% in adults in 2013–14 to 0.44% in 2014–15 for all patients suggests that there may be still be a period of catching up for some practices to include all their patients with ID on the register.
It has been argued that the QOF learning disability register provides a poor estimate of the actual number of adults with ID in England. 39,50 This may be because the majority of these patients do not use specialised services for adults with ID and, as a result, are not well known to primary care. The prevalence estimate of 2.17% calculated by Public Health England in 201350 would mean that three out of four patients with ID are not currently on QOF learning disability registers. 51 It seems unlikely that those with a severe or profound ID would not have this recorded on their medical record, so this ‘hidden majority’ would presumably consist of patients with milder disabilities.
Identification of adults with intellectual disability in the Clinical Practice Research Datalink
Rather than rely on the QOF learning disability register to find all patients with ID in CPRD, we electronically searched the full medical record of all adults using an extended range of Read codes. Although there are over 50 Read codes used for QOF definition of learning disability (see Appendix 2), they have been chosen from the main ‘mental retardation’ hierarchical structure and, as a result, are not an exhaustive list in terms of conditions usually associated with ID. For example, a Read code for Down syndrome would not automatically put a patient on the QOF learning disability register. There are also some anomalies (e.g. the code ZS34.11 ‘learning disability’ is not on the QOF list) that we would want to account for.
To create a more extensive list of candidate Read codes for our definition of ID, we manually reviewed Read codes within relevant hierarchies, in addition to performing word searches using key terms on the full set of codes. We included a wide range of chromosomal and metabolic disorders usually associated with ID. Our intention was first to extract a group of patients with these codes, but then to refine the definition, based on all available information in the individual medical record. The key to our approach was ensuring that we were not missing a significant group of people with ID by relying on QOF codes alone.
A Read code list of 232 codes was sent to CPRD in October 2013 to identify all patients who had any of these codes recorded anywhere in their medical record. We also required patients who:
-
were fully registered with an English practice for at least 1 day between 1 April 2007 and 31 March 2013 (we subsequently defined study time from 1 January 2009)
-
were ‘acceptable’ according to CPRD data criteria that identify patients who have been fully registered with their general practitioner (GP) and who have passed CPRD data quality control checks
-
had a birth year of 1995 or earlier.
An initial group of 32,876 patients from a total of 520 English practices (Figure 1) were extracted from the complete version of CPRD. Sixty-nine practices were subsequently excluded from further consideration, as they had stopped providing data to the CPRD by 2009 or did not pass CPRD quality controls for data recording during our study period.
FIGURE 1.
Summary of identification of patients with ID. a, CPRD data criterion for when a practice starts recording data for acceptable quality. PKU, phenylketonuria; UTS, up to speed.
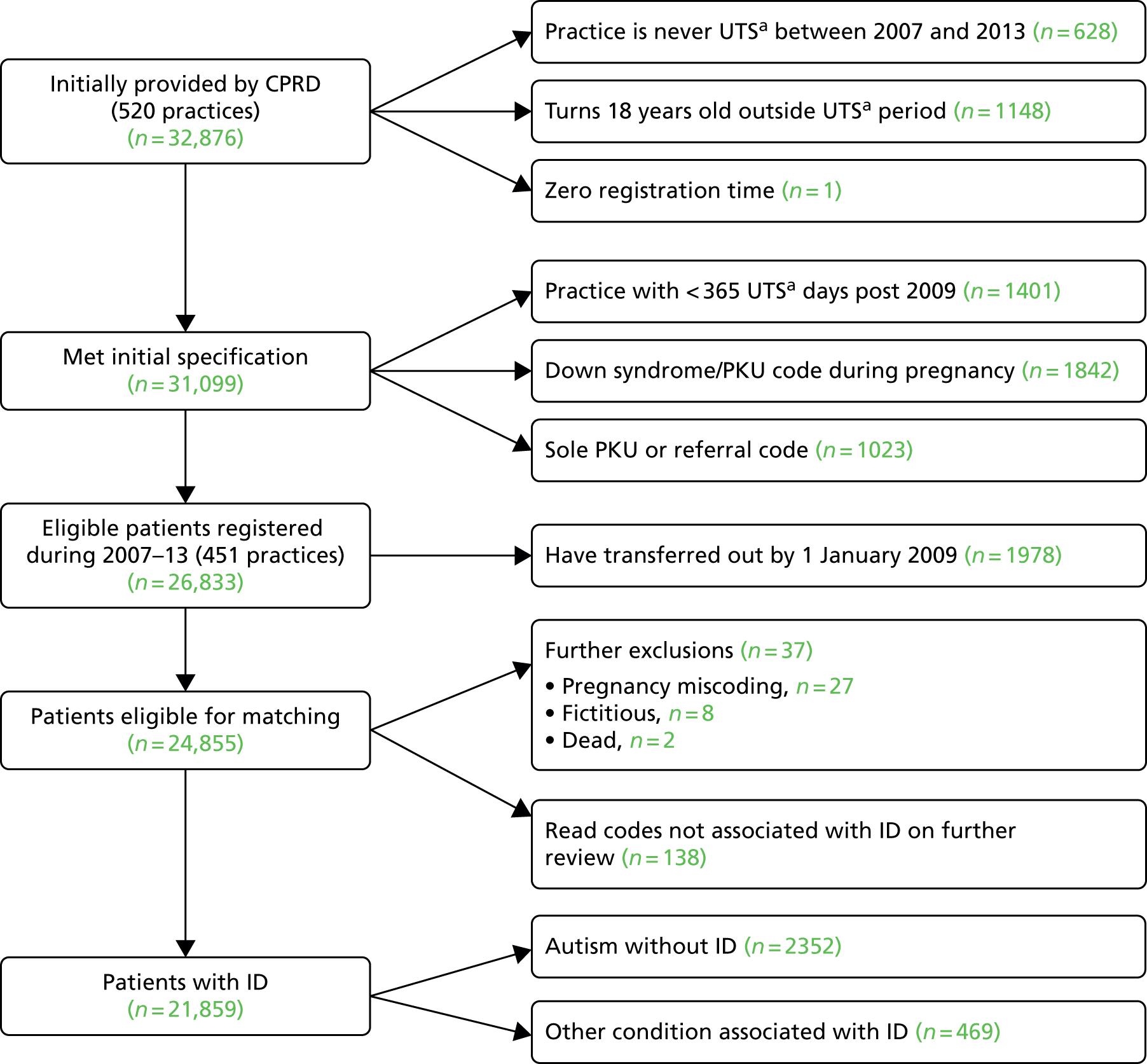
The initial group of 32,876 candidate patients with ID was used to help refine our Read code list. The final list included 186 Read codes (see Appendix 2), 125 of which are not part of the QOF learning disability code set. However, many of these additional codes were infrequently used because they represent very rare conditions. For these additions, we chose to include diagnoses (e.g. Down syndrome, Fragile X syndrome) and observations (e.g. ‘mental handicap problem’, ‘low IQ’), which are strongly related to ID (see Appendix 2 for more examples). We also included administration codes that directly implied that a patient had ID (e.g. ‘learning disability health exam’, ‘learning disabilities annual health assessment’). In theory, practices should be using administration codes for health checks only if a patient is on their learning disability register, but this was not absolute. Adopting the refined Read code list plus a series of exclusions (see Figure 1) allowed us to now identify 24,855 patients with ID, or with conditions associated with ID, for whom we wanted to extract age-, gender- and practice-matched controls.
Exclusions identified after first data extraction
One data issue we identified was with the erroneous historical use of some Read codes for phenylketonuria and Down syndrome in some practices. It appeared that these codes had been used in the past (mainly during 1994–6) to record screening tests for these conditions in pregnancy and infancy, and were applied inappropriately to > 2000 (≈5%) patients who would have been wrongly identified with these conditions based on a simple search for the disease codes. This was one of the main reasons for our two-stage extraction, as clustering of these patients in some practices would compromise matching in these practices.
Phenylketonuria is a cause of ID but it can also be successfully treated. In addition, all newborn babies are screened for phenylketonuria, which may explain the extra codes in the same way as the Down syndrome codes. As the prevalence of phenylketonuria is about 1 in 10,000, it was implausible for a single practice to have ≥ 100 cases (sometimes all born within 2 or 3 years). The clustering of this phenomenon by practice allowed us to quickly identify the problem and create an automated strategy for correcting it. Briefly, using electronic searches of the medical record, we identified calendar years in which a patient was pregnant (or had given birth). If during this year (or an adjacent year) this patient was recorded as having phenylketonuria or Down syndrome without any other evidence of ID in her record, she was excluded from our definition of ID. A total of 1842 patients were excluded in this way (see Figure 1). We also excluded a further 1023 patients who had a sole phenylketonuria Read code during infancy without any further confirmation. Ultimately, we decided not to include phenylketonuria in our definition of ID, so any remaining patients who were solely classified by this Read code were classed among the 469 patients designated as ‘other condition associated with ID’ (see Figure 1).
Matched population controls
A list of 24,855 potential patients with ID (‘cases’) was sent to CPRD in December 2013 (see Figure 1), and corresponding age-, gender- and practice-matched controls were extracted and sent to us in March 2014. The matching was done in house by CPRD following our specification. We required any matched control to be alive and registered on a pre-specified index date. For cases who were actively registered on 1 January 2009, and were at least 18 years old by the end of 2009, we chose 1 January 2009 as the index date. For cases who registered after this date, we chose their registration date if they were aged 18 years in that year. For cases who turned 18 years old after 2009, we chose 1 January of that year as the index date. Our choice of index date ensured that virtually all patients with ID would have a full complement of matched controls at the start of our planned longitudinal analyses. For patients with ID who remained registered from 2009 to 2013, we anticipated losing an average of about one control per patient with ID, owing to deregistration or death.
In total, 173,797 age-, gender- and practice-matched controls were extracted for the initial set of 24,855 patients who had ID or associated conditions, with 99.7% successfully matched to seven controls. Failure to match to seven controls was generally due to a few large clusters of young patients with ID in some practices.
Defining subcohorts for analyses
Further validation work after the extraction of controls identified some further exclusions (see Figure 1): 27 adults with ID who were pregnant and received their only code for ID in the year before pregnancy, eight adults with ID whose medical record appeared fictitious and two adults with ID whose record clearly indicated that they were deceased before 2009. Although we initially planned to include 2352 patients with ‘autism without ID’, as well as a further group with other related conditions (but no evidence of ID), we chose not to use these groups any further in the study. Therefore, the remainder of the report considers only the 21,859 patients with ID (see Figure 1).
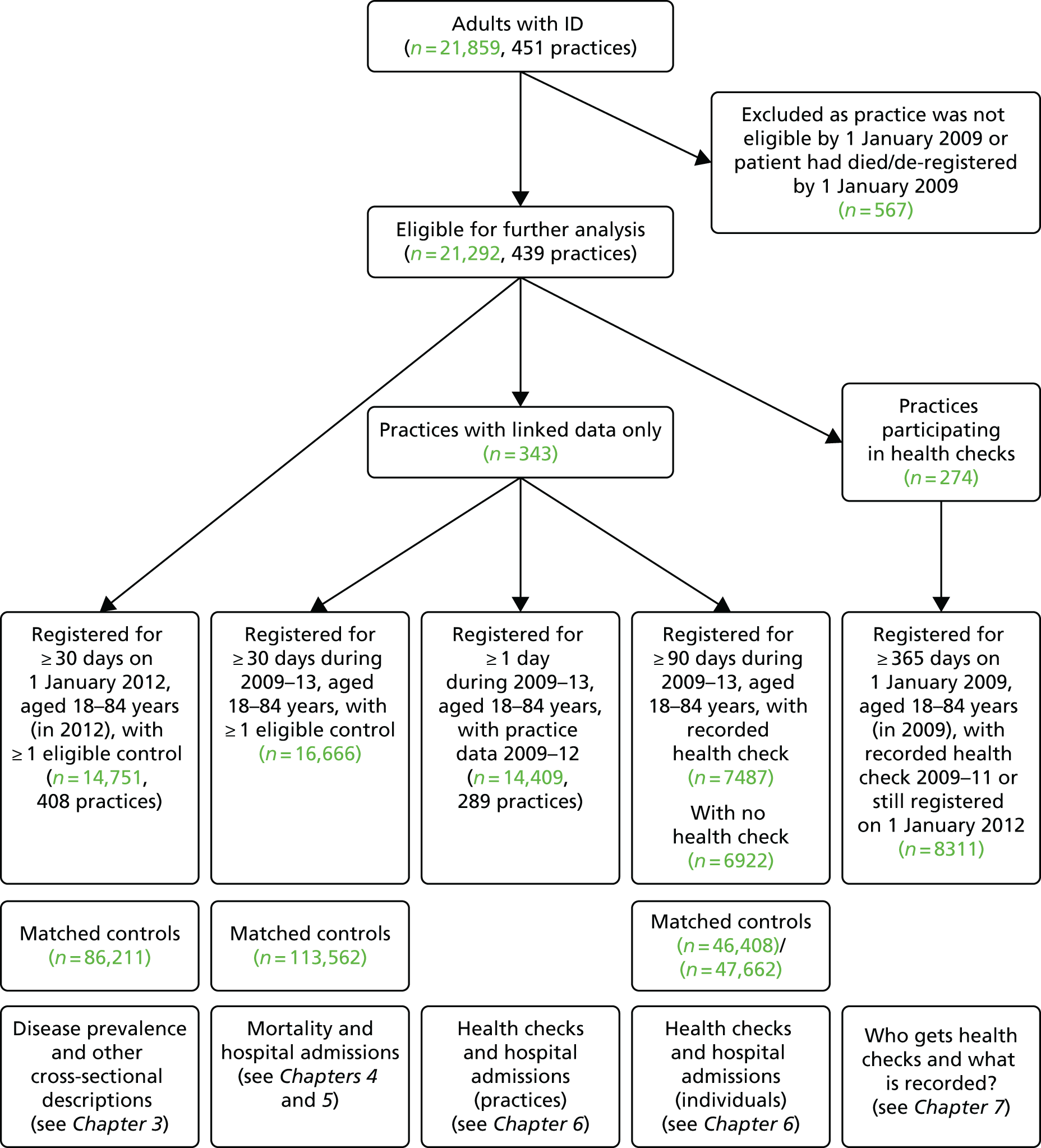
Depending on the specific analysis (e.g. cross-sectional or longitudinal), the number of adults with ID included varied (Figure 2). All analyses of individuals required a minimum registration period of 30 days with their general practice before the patient was eligible to be in our study. As anticipated, very few elderly patients aged > 85 years with ID were identified during the study, and owing to doubts over the validity of the recording of their health status, we made the pragmatic decision to include only patients aged 18–84 years at the beginning of follow-up.
FIGURE 2.
Summary of subcohorts for analyses. Note that subcohorts are overlapping and individuals may appear in multiple cohorts.
-
The cross-sectional descriptions of disease prevalence, health promotion and consultations in primary care (see Chapter 3) were based on 14,751 adults with ID who were alive and still registered on 1 January 2012 (and 86,211 matched controls). Thirty-one practices were no longer providing data to the CPRD by this date, so only 408 practices were included in this analysis.
-
The longitudinal analyses of mortality (see Chapter 4) and hospital admissions (see Chapter 5) were based on 16,666 adults with ID from the 343 practices with linkage to HES or ONS data (and 113,562 matched controls). Study follow-up time for these patients started from 1 January 2009 for those already registered and aged 18 years, or a later date for those registering later or turning 18 years old in a later year.
-
The analyses of health checks and hospital admissions had two distinct components (see Chapter 6). For the analysis carried out at practice level, we restricted to 289 practices with complete recording in CPRD during 2009–12, which identified a total of 14,409 adults with ID. For the analysis specific to individuals, we identified 7487 adults with ID with a first health check during 2009–12 (and 46,408 matched controls). A further 6922 adults with ID without health checks (and 47,662 matched controls) are also included in these analyses.
-
Finally, a further analysis of health checks (see Chapter 7) was based on a subset of 274 practices that had some participation in the DES (20% of eligible adults with ID must have had a health check during 2009–11). This identified a total of 8311 adults with ID who were registered on 1 January 2009 for at least 1 year.
Identification of health checks
Health checks were identified by specific Read codes used by practices to facilitate future payment (69DB., 9HB3., 9HB5.; see Appendix 1). We specifically focused on first health checks carried out from 2009 onwards, as this was the point from which practices in England received remuneration for carrying them out. A small number of patients had checks recorded prior to 2009 and were not included here. Health checks up to the end of the CPRD data collection period (31 March 2013) were included. The numbers of health checks included in the relevant analyses are shown in Figure 3.
FIGURE 3.
Summary of health check analyses.
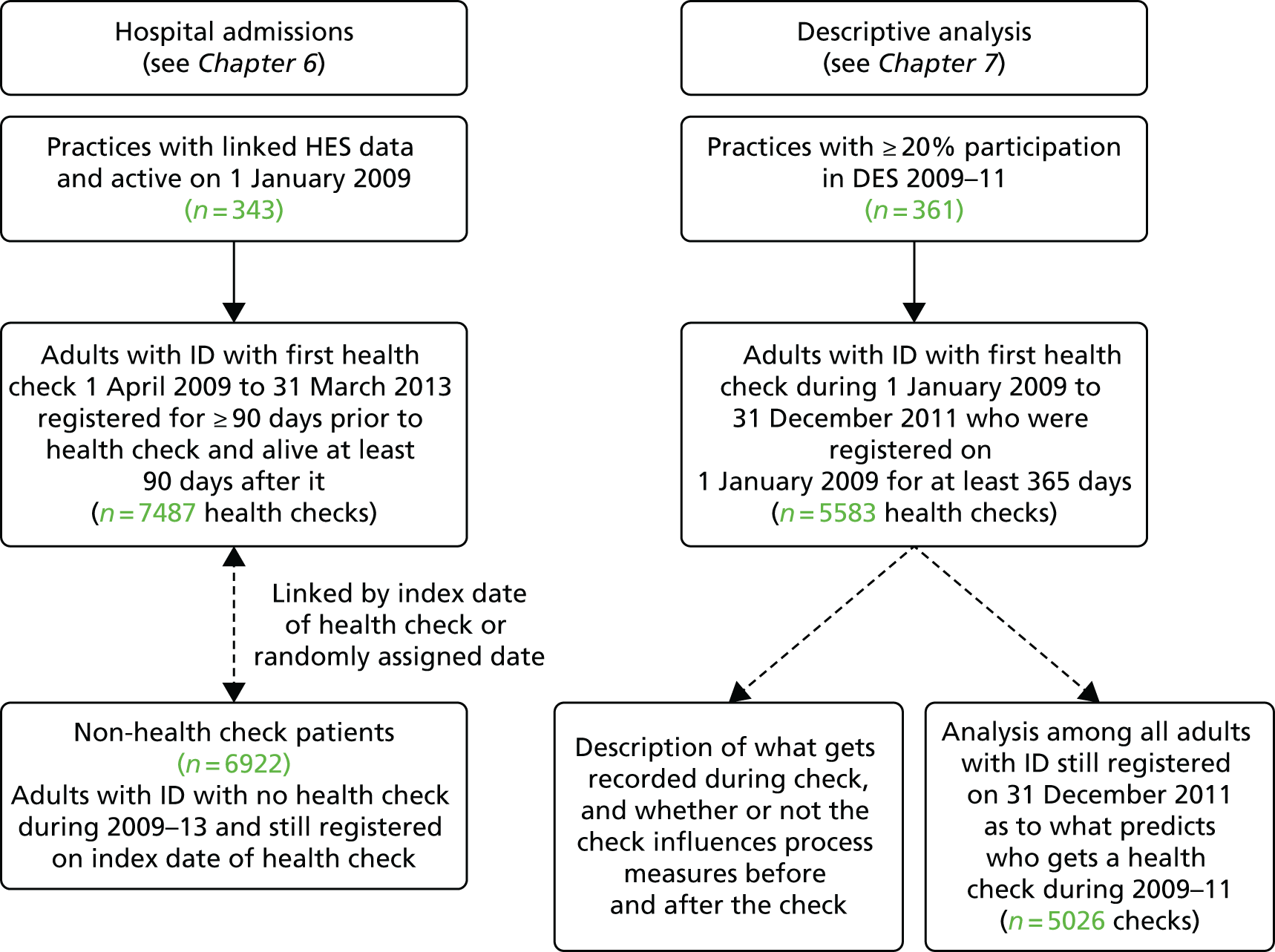
The analyses were divided into two distinct sections: hospital admissions in relation to health checks (see Chapter 6) and a descriptive summary of health checks (see Chapter 7). A total of 8933 first health checks were included across both analyses (with 4137 of the health checks appearing in both).
For the analysis of hospital admissions, we first only included the subset of CPRD practices (n = 343) that were actively recording data on 1 January 2009 and were linked to HES data. All patients were required to be registered with the practice for at least 90 days prior to the health check, and to be alive for 90 days after it. To be included, patients had to be aged 18–84 years at the time of their first health check. In this analysis, all patients were followed to 31 December 2013, or to their death if this was earlier. We were able to retain patients who had deregistered from their practice in the follow-up, as linkage to hospital admissions continued as long they remained resident in England. A total of 7487 adults with ID aged 18–84 years with a first health check between April 2009 and March 2013 were identified.
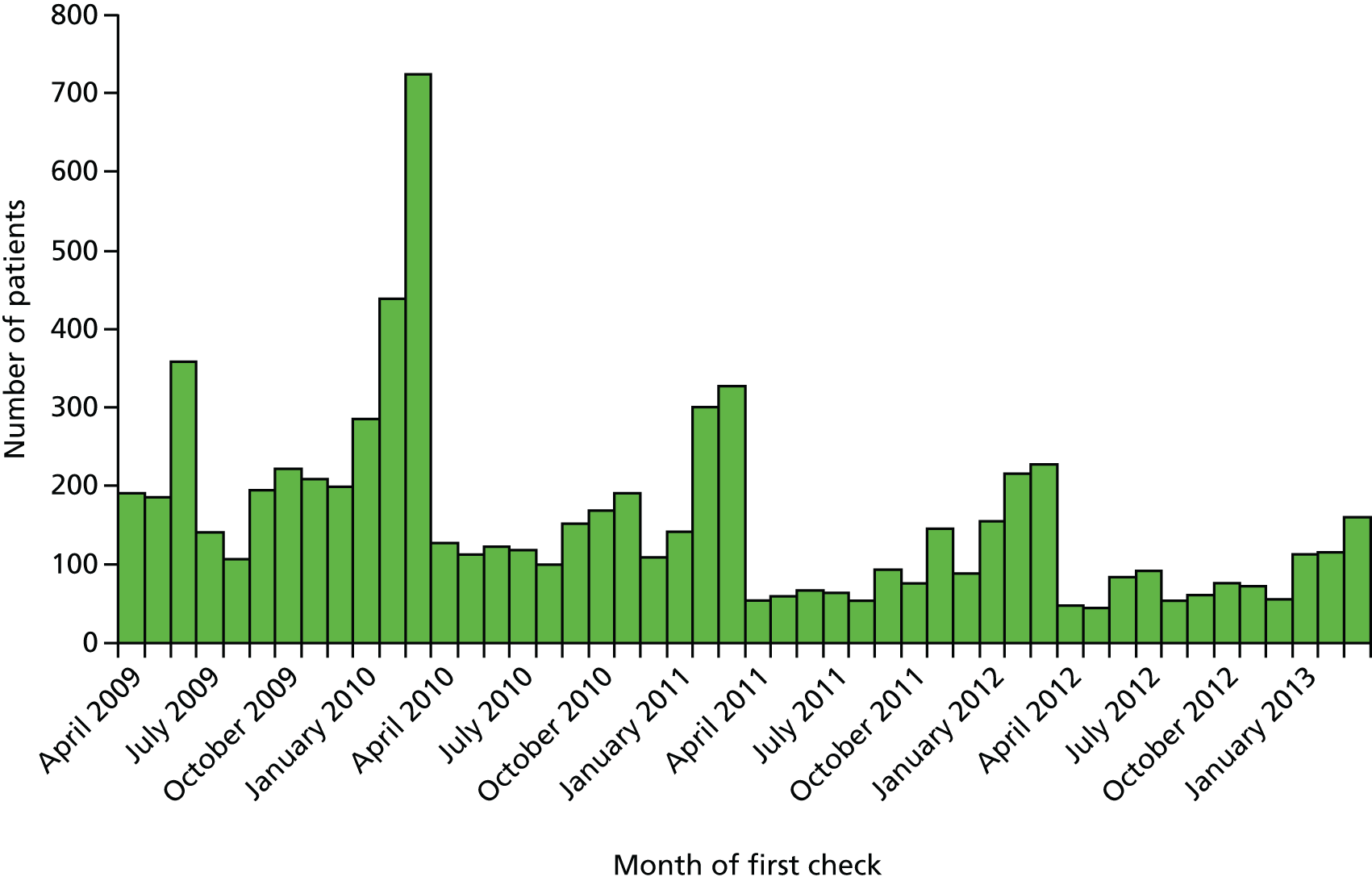
The distribution of month of first health check for the 7487 adults with ID is shown in Figure 4. As the payments for the DES are made at the end of the financial year, there are notable spikes in activity each February and March during the study. The early years (2009–10) were the most common years for a first health check, reflecting that the majority of participating practices joined the scheme during its initial years. The distribution of first health check date was used to assign a random index date to a group of 6922 adults with ID without health checks (see Figure 3). These patients formed a complementary group in our analysis of hospital admissions to check whether or not any observed changes in admissions for adults with ID were specific to those receiving health checks only.
FIGURE 4.
Distribution of month of first health check from April 2009 to March 2013.
For the descriptive analysis of health checks, we included a total of 5583 first health checks made during 2009–11 (see Figure 3). We no longer restricted to practices with linked HES data, so we could include from a wider set. However, we did then restrict to 361 practices with some participation in the DES (at least 20% of adults with ID with health checks) to try to capture regular procedures around the health checks. As some of these analyses would focus on health processes in the year after the health check, we included checks only up to the end of 2011. Finally, we also carried out an analysis that investigated predictors of receiving a health check during 2009–11. We restricted to 7754 adults with ID registered throughout 2009–11, in which 5026 received a first health check during that period.
Definition of severe health needs
Although there are specific Read codes that allow for the severity of a patient’s ID to be classified (e.g. ‘Eu81500 – severe learning disability’), we found that fewer than half of our patients had such a code recorded. For example, among the 14,751 adults with ID alive and registered on 1 January 2012, only 45% had a code indicating the severity of their ID (Table 2). Among those with severity of ID recorded, and using the highest level in their record, 38% had ID classed as mild, 35% had ID classed as moderate, 24% had ID classed as severe and 3% had ID classed as profound.
| Severity of ID | n | % of all adults with ID | % of adults with ID who are men | Mean age in years (SD) |
|---|---|---|---|---|
| Severity recorded | 6565 | 44.5 | 57.2 | 43.5 (15) |
| Severity not recorded | 8186 | 55.5 | 58.4 | 40.9 (16) |
| Severity | ||||
| Mild | 2515 | 38.3 | 56.6 | 43.7 (15) |
| Moderate | 2298 | 35.0 | 58.7 | 43.4 (16) |
| Severe | 1567 | 23.9 | 56.6 | 43.6 (15) |
| Profound | 185 | 2.8 | 53.5 | 40.7 (14) |
With severity missing in over half of the sample, we had to consider two options. The first would be to only look at severity in the subgroup with it recorded. However, this approach is problematic, as the existence of such Read codes probably do not occur at random in our study group, and this group with severity recorded will not be representative of our total group. For example, patients in 2012 with recorded severity were a mean of 2.6 years older than those with no severity recorded (see Table 2).
Therefore, we considered an alternative approach that used Read codes that identify severity when available, and, when these codes were not present, used a selection of other codes in their record that would indicate that the patient had severe or complex health needs. We identified six health areas that encapsulated a wide range of support or severe health needs:
-
epilepsy – Read codes as per QOF definition, but excluding absence seizures
-
mobility – wheelchair use or greater problem; cerebral palsy
-
visual – blind or low vision
-
hearing – deafness, significant impairment, hearing aid use
-
continence – bowel or bladder (recorded after age of 12 years)
-
percutaneous endoscopic gastrostomy feeding.
We refined this list by cross-checking the prevalence of these codes and conditions in the patients with severe or profound ID versus mild or moderate ID (the full list of codes used is provided in Appendix 2). All categories were significantly associated with severe or profound ID, with the exception of hearing impairment. However, we retained this category to enable our definition to be as complete as possible in terms of various health needs. Finally, we improved precision by imposing a restriction that for a patient to have a high level of support or severe health needs, he or she needed to fulfil two or more of these categories (Figure 5). This ensured that we were not just creating, for example, a marker for age-related frailty. The only exception to this rule was that if the patient already had Read codes indicating severe or profound ID.
FIGURE 5.
Definition of severe health needs used as a proxy for severity of ID. PEG, percutaneous endoscopic gastrostomy.

In the cross-sectional analyses (see Chapter 3), this approach identified a total of 3527 patients with ID with severe health needs (23.9% of all patients with ID). This group was made up of 1752 patients with severe or profound ID who are automatically included, plus the inclusion of 686 patients with mild or moderate ID and 1089 patients with no severity of ID recorded on their record. The proportion with severe health needs (13.5%) among those without severity recorded on their GP record was very similar to that estimated from those with mild or moderate ID recorded (14.3%). This suggests that those without severity recorded, as well as being younger, have primarily mild or moderate ID.
Other subgroups of interest
In addition to adults with ID with severe health needs, we identified other ID subgroups of interest: living arrangements, autism spectrum disorder and Down syndrome.
We wanted to describe the living arrangements of our patients with ID, but we were limited by the inconsistent recording of information in relation to this (e.g. carer details, or whether or not they lived with their family). The clearest distinction we could make was to identify patients who were living in dependent settings, such as residential or nursing homes, and to compare these patients with the remainder who were not classified in this way. We could primarily do this by the use of an address flag on the CPRD database, which can identify clusters of patients living at the same address. We have used this flag previously to identify elderly patients in care homes. 52 Here we assumed that the presence of three or more people with ID at the same address indicated communal or shared accommodation. The use of this address flag can vary by practice, so in addition we used some specific Read codes for living arrangements (see Appendix 3) to bolster our definition.
We also stratified analyses, when possible, by whether or not the adult with ID also had a record of autism spectrum disorder and, separately, by whether or not they had Down syndrome. The Read codes for these are provided in Appendix 3.
Definition of a consultation
We defined a consultation as a unique event during which the patient was seen or telephoned by a doctor or nurse. However, identifying patient consultations is not always straightforward in CPRD, as many of the administration entries on the computer system can confusingly resemble a consultation if they are not accounted for. Although there is a specific variable for ‘consultation type’, this is not consistently used across practices, and cannot solely be relied on to identify consultations.
To automate a definition of consultations in CPRD, we restricted it to events on the system for which the consultation type (e.g. surgery consultation) and staff member (e.g. senior partner) met our definition, excluding administrative events and repeat prescribing. For patients with ID, we also excluded consultations on days when a health check was recorded. Within the consultations we identified, we could further subdivide into whether the consultation had been doctor or nurse led, and whether it had been face to face (at the GP surgery or a home visit) or by telephone. Further details of the definition used for consultations are given in Appendix 4.
It is possible to ascertain the length of the patient consultation from within CPRD, using the recorded duration on the system. For face-to-face consultations with a doctor, we classified consultation length into standard (1–10 minutes) and long (> 10 minutes), excluding a small number of zero-length consultations. As each clinician has a unique identifier on the system, we could estimate continuity of care by calculating the highest proportion of doctor consultations with the same doctor. We used a cut-off point of > 50% to summarise continuity, so if a patient had a total of five consultations, they would need at least three with the same doctor to achieve this. Although other indices of continuity have been proposed,53 our summary has the advantage of being largely independent of number of consultations.
Difficulties with Hospital Episodes Statistics linkage
Of the 451 practices initially extracted by the CPRD, 353 (78%) had linkage to HES data. When the linked data set (adults with ID and controls) was provided by the CPRD in March 2014, the HES data were available only to 31 March 2012 as a result of a national postponement in the linkage of all HES data during 2014–15. As our analyses had been powered for follow-up into 2013, the uncertainty over extended linkage presented a dilemma. While waiting for this issue to resolve, we were able to proceed with analyses not involving HES data. When the HES linkage to 31 March 2013 was finally performed and delivered to us in January 2015, we then had a further issue, that patients from practices that dropped out from the CPRD during the linkage postponement could not have their follow-up extended. We made the decision to keep these patients in the analyses, but terminated their follow-up for hospital admissions outcome at 31 March 2012. This affected approximately 2.6% of all of the linked patients in the original extracted data set.
Missing entity data in the Clinical Practice Research Datalink
During the initial data acquisition, we discovered a data extraction error that existed in the complete database held by the CPRD. This had occurred between the extraction of data from the general practices and the building of the CPRD database. Briefly, the Vision system (In Practice Systems Ltd, London, UK) used by the practices allows for more complex data entries, which cannot be conveyed simply by Read codes, to be held in additional data areas called ‘entities’. For example, the diastolic and systolic measurements for blood pressure would be held this way. For three outcome measures we were interested in (medication review, diabetic retinal screening and glomerular filtration rate), we discovered significantly lower than expected recording in the CPRD, owing to an unspecified historical issue with the entity data within some practices. After raising this with the CPRD in the summer of 2014, it took another year for a potential data fix to be provided. However, the fix could be applied to current practices only, which meant that practices no longer contributing to the CPRD were unable to be corrected. Thus, our reporting of these outcomes, particularly medication review, is subject to under-recording. Sensitivity analyses, including only those practices for which a fix was possible, suggested that this under-recording may be around 5–10%. However, even when the fix could be applied, the overall low recording of recent medication reviews left us querying the data integrity for this outcome.
Economic costs
We included a descriptive analysis of NHS costs in our study. The intention was to use the CPRD and HES data to best estimate, when possible, a before-and-after cost comparison to assess the impact of annual health checks on NHS costs, and an estimate of NHS costs for care for adults with ID compared with the general population. We did not, however, commit to a formal cost-effectiveness analysis, as our data do not include some elements of NHS costs or social care costs that would be required for a robust cost-effectiveness analysis.
We identified several sources of external data to guide us in estimating NHS costs. First, the Unit Costs of Health and Social Care, produced by the Personal Social Services Research Unit,54 provided us with many key primary costs, including of consultations. We used the costings produced for 2012, which, for example, produce a guidance cost of £3.70 per minute of patient contact with a GP (including qualification costs and direct care staff costs). Duration of consultation is generally available on the CPRD, and so it is possible to estimate costs using this scaling.
Second, prescribing costs were identified by the Prescription Cost Analysis documents produced by NHS Digital. 55 This allows a net ingredient cost to be identified by drug name, form and strength, which can be linked to prescribing information on the CPRD. Again, we used 2012 costings to estimate prescribing costs.
Finally, for hospital admissions we relied on two sources of data. First, the National Schedule of Reference Costs data for NHS trusts and NHS foundation trusts costings provided costings for all elective and non-elective hospital stays. 56 We generally relied on costings for 2011–12. These costing are coded by Healthcare Resource Groups (HRGs), which are ‘standard groupings of clinically similar treatments which use common levels of healthcare resource’ (contains public sector information licensed under the Open Government Licence v3.0)57 (we used HRG4). We then used the International Classification of Diseases, Tenth Edition (ICD-10)58 and OPCS Classification of Interventions and Procedures version 459 codes on the HES data to translate these into HRGs using the HRG4 2011–12 reference costs Grouper software. 60
A brief summary of the data sets and assumptions used in the economic cost estimation is given in Appendix 5.
Statistical analysis
For the cross-sectional analyses (see Chapter 3), depending on the outcome being studied, we calculated prevalence, odds or relative risk ratios between patients with ID and their matched controls using conditional Poisson and logistic models (Stata Statistical Software: Release 13, 2013; StataCorp LP, College Station, TX, USA). The models were conditioned on the adult with ID–control(s) match-sets; thus, all comparisons are implicitly adjusted for matched factors: age, gender and practice (which will factor in regional and urban–rural variations). For prevalence ratios (PRs), Poisson models were fitted with robust error variances corrections to provide reliable estimates. 61 When the outcome was based on a subgroup defined not solely by age and gender (e.g. influenza vaccination among those with eligible comorbidity; see Table 12), then only match-sets that included an adult with ID and at least one control could be used. An exception to this was when we analysed attainment of QOF indicators (see Table 16), for which this approach was not feasible. As patients could not be matched in this analyses, we fitted a (non-conditional) log-binomial model adjusting for gender and age. Practice was included in the model, assuming an exchangeable correlation structure. When the outcome was number of consultations over the previous year (see Table 17), an offset for number of registered days was added to the Poisson model to allow for patients who had been registered for < 1 year. In the consultation analyses, we further adjusted for comorbidity using a weighted score of QOF conditions. 62 For analyses on consultation length and continuity, we also adjusted for total number of consultations. For cross-sectional analyses with economic cost as the outcome (see Table 20), we fitted (conditional) fixed-effects negative binomial regressions to account for overdispersion, with bias-corrected confidence intervals (CIs) produced from non-parametric bootstrap estimation (1000 simulations).
For the analyses with mortality as the outcome (see Chapter 4), we estimated crude death rates and hazard ratios (HRs) for comparisons between adults with ID and their matched controls. HRs were calculated via Cox regression (SAS version 9.4; SAS Institute Inc., Cary, NC, USA), with further adjustment for a weighted score of QOF conditions, which has been shown to predict mortality in the general population,62 smoking and socioeconomic status using the IMD. 46 For comparisons within subgroups (defined by the adult with ID), we compared the HRs and CIs derived from each adult with ID versus control comparison (e.g. adults with ID with Down syndrome vs. controls) and calculated p-values for these between-group differences. We additionally carried out unmatched analyses focusing only on adults with ID (see Chapter 4, All-cause mortality), fitting models that directly compared each subgroup category (e.g. those with vs. those without Down syndrome), adjusting for age and gender differences, and stratified according to practice.
For the analyses on hospital admissions (see Chapter 5), we estimated crude admission rates for adults with ID and their matched controls. Incidence rate ratios (IRRs) for emergency hospitalisation were calculated using conditional Poisson models described previously, stratifying again on match-sets and similarly adjusting for comorbidities, smoking and deprivation. For the examination of primary care utilisation preceding admission, it was not possible to preserve the matching. Instead, we used logistic regression to estimate an odds ratio (OR) for adults with ID versus controls, adjusting for differences in age and gender.
The analyses that investigated the impact of health checks on hospital admissions (see Chapter 6) primarily used the conditional Poisson model to compare the rate of change over time at a practice or individual level. At practice level, these were conditioned on practice, and all admissions from registered adults with ID in each period were counted, using an offset term to account for the total time registered. The effect of practice participation on hospital admissions was estimated by the interaction between practice participation (fully vs. none) and period (2011–12 vs. 2009–10). At individual level, we conditioned on individual as opposed to match-set, as accounting for the matching variables is not paramount in matched cohort analyses. 63 This model was fitted to adults with ID and controls separately, estimating the individual change in hospital admission rate after compared with before health check, with an offset accounting for the time registered. A combined model of adults with ID and controls with a case–period interaction provides an estimate of the effect of health checks on admission rates among adults with ID, adjusted for temporal trends in admissions. All models used a sandwich estimator to obtain robust standard errors.
The analyses of hospital admissions in individuals with health checks also considered adults with ID without health checks in two sets of sensitivity analyses to check the robustness of our findings. First, using the assigned random index date (see Identification of health checks) instead of the health check date, we simply repeated the analysis on this set of patients and their matched controls to see whether or not any observed changes in the health check patients were also observed here. Second, we also considered a direct comparison of adults with ID with and without health checks using Poisson and negative binomial models, adjusting for age, gender and selected comorbidities (severe health needs, epilepsy, dementia and Down syndrome).
The analyses of health process measures were largely descriptive (see Chapter 7), summarising the recorded information on patient records before and after health checks. We calculated the change in consultation and prescription rates in a period before (2006–8) and during the introduction of health checks (2009–11) using conditional Poisson regression as described previously. We contrasted the change between patients with ID with and without health checks, but did not attempt a formal statistical comparison. Finally, we also carried out an analysis that investigated which factors predicted a health check among a subset of patients with ID registered during 2009–11 in practices that were carrying out health checks. Here a logistic model was fitted, with health check (yes/no) as the outcome and practice included in the model as a random effect.
Patient and public involvement
Throughout the course of the study, a collaborative approach to patient and public involvement was taken,64 and we engaged two groups through regular meetings every 8–12 weeks:
-
ResearchNet – a network of service user and staff members at St George’s, University of London, who collaboratively undertake research to develop services and improve patient experience
-
Carers Support Merton – a local group of family carers of adults with an ID.
The focus of these meetings initially was to identify important outcomes for our study and concerns for patients and carers. This involvement subsequently contributed to changes to the design of the study in terms of choice of outcomes, examination of potential modifying factors, and help in interpreting and disseminating findings.
We have summarised some of the key issues that arose from these initial meetings with ResearchNet (Table 3) and Carers Support Merton (Table 4). We tried, when possible, to explore many of these issues, such as the addition of dysphagia, aspiration pneumonia, constipation and anxiety as potential outcomes in our analysis. The focus on consultation length and continuity of care by health professionals as key measures of health-care effectiveness were important additions to the study that ultimately strengthened some of our published research findings. 66 The groups stressed the importance of living arrangements for adults with ID (e.g. living with their family) and, although the data could not adequately assess this, we were able to identify a subgroup of patients with ID who were recorded as living in shared or communal living arrangements (see Other subgroups of interest). However, not every issue raised by the groups could be adequately explored, owing to limitations with our data.
| Area | Specific details |
|---|---|
| Prominent health issues | Constipation |
| Depression (‘problems with feelings’), anxiety | |
| Diabetes | |
| Epilepsy | |
| Podiatry (‘feet’) | |
| Hearing and vision | |
| Hydrocephalus | |
| Lungs and breathing problems, aspiration pneumonia | |
| Swallowing difficulties, dysphagia | |
| Teeth | |
| Other issues affecting health | Living arrangements (such as whether they lived with their family, independently, in a residential care home or in supported living) were mentioned as an explanation for the variation in how many people had health checks and in accessing primary care generally |
| Health care for patient with ID | Seeing the same doctor, the patient’s regular doctor |
| Having long enough appointments to discuss several things | |
| Hard to make GP appointments for person with ID because they might rely on others to make the appointment or take them to the GP | |
| Health checks | The group identified some checks that they thought could keep someone healthy in future, and that should be part of health checks: BP checks, feet checks, heart checks, kidney/urine checks, blood tests, memory tests, scans and X-rays, weight measurement, smears, advice on self-examination |
| Some mentioned that the following had been particularly helpful to them from their health checks: weight loss advice, help with pain, help with depression, including tablets, regular medications for epilepsy or diabetes, calming tablets, help with addiction | |
| Dislike of health check if it led to blood tests or injections but others recognised that these could be valuable and it was possible to overcome those fears | |
| There was particular interest in the group about being able to talk about mental health issues with your doctor, particularly about being anxious or depressed. Some mentioned that more time was needed to talk about these issues |
| Area | Specific details |
|---|---|
| Diagnosis and management | Epilepsy diagnosis and management and quality of seizure control |
| Identification of depression | |
| Hearing and vision problems | |
| Vitamin D deficiency and osteoporosis diagnoses in older people | |
| Later cancer diagnoses | |
| Gout and osteoarthritis | |
| Monitoring of therapy (e.g. having thyroid function tests if on thyroxine) | |
| Medication | Concern over number of medications prescribed |
| Risks of inappropriate prescribing | |
| Overuse of antipsychotic medications for behavioural problems | |
| Monitoring of epilepsy medication | |
| Preventative care | Importance of overweight and obesity |
| Smoking in those with less severe levels of disability | |
| Screening for hypothyroidism in some conditions (e.g. Down syndrome) | |
| Organisation of care | Impact of place of residence (e.g. with family carer, in supported independent living, in group home) |
| Being able to see the same GP, length of appointments | |
| Organisation of health checks, variation in duration and place of delivery of health checks (e.g. reports of some as short as 10 minutes, some as long as 2 hours, some done over telephone, some as home visits) | |
| What is actually covered in health checks? Content should be according to the Cardiff health check, but is not always so, and there was marked variation in what was covered |
The discussion about health checks with both groups identified varied views on the effectiveness and acceptability of health checks, and differing experiences of the delivery of the health check programme. This highlighted the importance of describing process measures for the health checks, as well our main focus on changes in hospital admissions.
A qualitative research paper65 has been published further detailing the views and experiences of the members of the parent, carer and ResearchNet groups of their involvement in this research. Preliminary findings suggest almost unanimous agreement from both groups that their involvement was meaningful to them and that their participation felt genuine (see Appendix 6).
Chapter 3 Cross-sectional findings
Introduction
In presenting a summary of the health and health care of adults with ID in primary care in England, we chose to carry out a series of cross-sectional analyses on a fixed date (1 January 2012) that would be towards the end of our study period. It also had the benefit of maximising the number of CPRD practices contributing data at that time, as some practices in our study stopped contributing data later in 2012. This date allowed a total of 408 practices to be used in the cross-sectional analyses, from which a total of 14,751 patients with ID who were aged 18–84 years in 2012 were included. These patients were age, gender and practice matched to 86,211 controls without ID (see Figure 2). All patients had been registered with the practice for a minimum of 30 days.
Some of these results have already appeared in publication in Carey et al. 66 © British Journal of General Practice 2016. This is an Open Access article distributed under the terms of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/), which permits unrestricted reuse, distribution and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Prevalence of intellectual disability among adults in 2012
We were able to estimate the adult prevalence of recorded ID in primary care in 2012 by obtaining denominators by gender and 5-year age groups for all registered patients in CPRD on 1 January 2012. These totalled approximately 2.7 million patients aged 18–84 years from the eligible 408 practices. This allowed us to estimate that the 14,751 adults with ID aged 18–84 years in 2012 represented 0.54% of the total registered population for this age group. For comparative purposes, the published prevalence from QOF for 2011–12 (effectively estimated at 31 March 2012) for all adults aged ≥ 18 years was 0.45% (see Appendix 1), derived from all 8123 practices in England. Thus, our decision to include a wider set of Read codes for ID, and not just those used for the QOF learning disability register (see Appendix 2), increased our cohort of adults with ID by about 20%.
The estimated prevalence in the registered population of adults on 1 January 2012 differed by gender, with a higher rate seen in men (0.63%) than in women (0.45%). When the prevalence was estimated by age (in 2012), there were incremental reductions seen with increasing years of life. For those aged 18–34 years the prevalence was 0.72%, for those aged 35–54 years it was 0.59% and for those aged 55–84 years it fell to 0.34%.
There was considerable variation in the prevalence rate of ID when this was calculated in each of the 408 practices (Figure 6).
FIGURE 6.
Prevalence of ID by practice in adults registered on 1 January 2012.

-
Only 34 practices (8%) reported a prevalence of > 1 in 100 registered patients having ID recorded.
-
There were two notable outliers in terms of prevalence (2.22% with 61 total patients with ID and 2.68% with 114 total patients with ID). More than two in three patients with ID in these practices were estimated to be living in communal or shared accommodation, suggesting that these practices are located near such residences.
-
Although not outliers in terms of prevalence, five practices had > 120 patients with ID registered (n = 173 with prevalence of 1.07%, n = 164 with prevalence of 1.51%, n = 139 with prevalence of 0.93%, n = 124 with prevalence of 1.08% and n = 122 with prevalence of 1.56%).
-
Forty-seven practices (12%) had < 10 registered patients with ID; nine of these practices had fewer than five registered patients with ID.
Overall characteristics of adults with intellectual disability
The distribution of age (calculated in 2012) for the 14,751 adults with ID registered on 1 January 2012 is shown in Figure 7. The resulting distribution is different from the pattern seen in the general UK population,67 which is indicated by the dotted line. There are two peaks (around 18–25 years and 45–50 years) that offset the dearth in the older population with ID seen from the age of about 60 years onwards.
FIGURE 7.
Age distribution of adults with ID registered on 1 January 2012.

Further characteristics of our sample of adults with ID are shown in Table 5. The average age was 42.1 years, and 58% were male. The percentage of men among adults with ID gradually fell with age, from 61% in the youngest group (18–34 years) to 53% in the oldest group (55–84 years). Approximately three in four patients had their ethnicity recorded on their primary care record, with > 90% being recorded as white. Adults with ID with a non-white ethnicity recorded were much younger (mean 34.8 years) but were small in patient numbers, and as a result we did not pursue ethnicity further as a subgroup of interest in this report. Overall, 87% of our sample were on their practices’ QOF registers for learning disability.
| Characteristic | n | % of all adults with ID | % of adults with ID who are men | Mean age in years (SD) |
|---|---|---|---|---|
| All | 14,751 | 100 | 57.9 | 42.1 (16) |
| Gender | ||||
| Women | 6216 | 42.1 | 0 | 43.3 (16) |
| Men | 8535 | 57.9 | 100 | 41.2 (16) |
| Age (years) in 2012 | ||||
| 18–34 | 5365 | 36.3 | 61.2 | 25.3 (5) |
| 35–54 | 6041 | 41.0 | 57.5 | 44.8 (5) |
| 55–84 | 3345 | 22.7 | 53.1 | 64.1 (7) |
| Ethnicity | ||||
| White | 10,192 | 69.1 | 56.7 | 43.1 (16) |
| Other | 921 | 6.2 | 56.0 | 34.8 (13) |
| Not recorded | 3638 | 24.7 | 61.4 | 41.0 (15) |
| ID subgroupa | ||||
| On QOF learning disability register | 12,862 | 87.2 | 58.1 | 42.1 (16) |
| Down syndrome | 1571 | 10.7 | 53.9 | 40.4 (13) |
| Autistic spectrum disorder | 1512 | 10.3 | 76.4 | 32.5 (13) |
| Has severe health needs | 3527 | 23.9 | 52.6 | 44.2 (16) |
| In communal/shared accommodation | 3138 | 21.3 | 55.8 | 49.3 (15) |
| Deprivationb | ||||
| 1 (least deprived fifth) | 1563 | 10.6 | 58.8 | 41.2 (16) |
| 2 | 2000 | 13.6 | 57.7 | 42.9 (16) |
| 3 | 2232 | 15.1 | 59.5 | 41.9 (16) |
| 4 | 2764 | 18.7 | 56.0 | 42.2 (16) |
| 5 (most deprived fifth) | 3056 | 20.7 | 57.8 | 42.4 (16) |
| Not available | 3136 | 21.3 | 57.9 | 41.7 (15) |
| Time with practice (years) | ||||
| < 1 | 1037 | 7.0 | 55.8 | 38.2 (16) |
| 1–5 | 2945 | 20.0 | 56.8 | 40.2 (16) |
| ≥ 5 | 10,769 | 73.0 | 58.3 | 43.0 (16) |
| Annual health check | ||||
| None by 1 January 2012 | 7845 | 53.2 | 58.2 | 40.3 (16) |
| At least one by 1 January 2012 | 6906 | 46.8 | 57.4 | 44.1 (15) |
About 1 in 10 of our adults with ID was recorded as having Down syndrome. Similarly, 1 in 10 had a diagnosis of autistic spectrum disorder in addition to their ID; these patients were markedly younger (mean 32.5 years) and the majority were men (76%). About one-fifth of patients with ID (21%) were identified as living in a communal setting, and this group was notably older (mean 49.3 years).
Socioeconomic status was approximated by IMD quintiles,46 linked at postcode level to the patient’s residence (linked practices only). Although there was a trend towards more adults with ID being found in increasing quintiles of IMD, representing higher deprivation, this mirrors the pattern seen in complete population extracts of CPRD,68 and reflects a small geographical bias within CPRD whereby there are comparatively fewer practices in the north of England. 45 Almost three in four adults with ID (73%) had been registered at their practice for at least 5 years. Just under half (46.8%) had received an annual health check by 1 January 2012.
Disease prevalence among adults with intellectual disability
We chose to describe chronic disease prevalence by focusing on the range of conditions collated by the QOF. 69 For most of these conditions, we used version 26 of the business rules,70 which were in operation circa 2012–13. These identify the set of Read codes used in definitions, and for the most part stay consistent from year to year. For each condition, we searched for the presence of any Read code in the medical record up to 1 January 2012 to allow the description of prevalence. For cancer and depression, we first describe lifetime prevalence, but also include date-specific period prevalence in line with the QOF definition. For asthma, epilepsy and hypothyroidism, in line with the QOF definitions, a recent prescription was also required to give a measure of period prevalence. Severe mental illness was subdivided into schizophrenia and affective disorder. We also included additional conditions of anxiety and dysphagia.
Table 6 summarises the disease prevalences for adults with ID, compared with their controls, using PRs. These were calculated using conditional Poisson models (see Chapter 2, Statistical analysis) that take into account the matched design. Almost one in five adults with ID was recorded with epilepsy that is currently managed (18.5%), compared with < 1 in 100 adults without ID (0.7%). This represents a prevalence 25 times higher than that in controls (PR 25.33, 95% CI 23.29 to 27.57). Other large relative differences in prevalence were seen for severe mental illness (8.6% of adults with ID; PR 9.1, 95% CI 8.3 to 9.9) and dementia (1.1% of adults with ID; PR 7.5, 95% CI 6.0 to 9.5). Adults with ID had a moderately increased risk of dysphagia, hypothyroidism and heart failure (PR of between 2 and 3.5) compared with the general population. In addition, significantly higher in adults with ID (PR of between 1.5 and 2) were osteoporosis, stroke, diabetes and chronic kidney disease.
| Disease | Adults with ID (N = 14,751), n (%) | Controls (N = 86,221), n (%) | Adults with ID vs. controls, PR (95% CI) |
|---|---|---|---|
| Anxiety | 2398 (16.3) | 12,580 (14.6) | 1.13 (1.09 to 1.18) |
| Asthmaa | 1208 (8.2) | 5717 (6.6) | 1.25 (1.18 to 1.33) |
| Atrial fibrillation | 122 (0.8) | 821 (1.0) | 0.91 (0.75 to 1.09) |
| Cancerb | 238 (1.6) | 2090 (2.4) | 0.70 (0.61 to 0.80) |
| Diagnosis since 1 April 2003 | 156 (1.1) | 1490 (1.7) | 0.65 (0.55 to 0.76) |
| Chronic kidney disease | 468 (3.2) | 1746 (2.1) | 1.64 (1.49 to 1.82) |
| COPD | 160 (1.1) | 1184 (1.4) | 0.84 (0.71 to 0.99) |
| Dementia | 160 (1.1) | 134 (0.2) | 7.52 (5.95 to 9.49) |
| Depressionb | 2609 (17.7) | 15,179 (17.6) | 1.03 (0.99 to 1.06) |
| Diagnosis since 1 April 2006 | 1626 (11.0) | 9520 (11.0) | 1.01 (0.96 to 1.06) |
| Diagnosis in last year | 237 (1.6) | 1723 (2.0) | 0.80 (0.70 to 0.92) |
| Diabetes | 1017 (6.9) | 3786 (4.4) | 1.64 (1.53 to 1.75) |
| Dysphagia | 692 (4.7) | 1263 (1.5) | 3.30 (3.01 to 3.61) |
| Epilepsya | 2731 (18.5) | 633 (0.7) | 25.33 (23.29 to 27.57) |
| Heart failure | 121 (0.8) | 324 (0.4) | 2.26 (1.84 to 2.78) |
| Hypertension | 1583 (10.7) | 10,416 (12.1) | 0.93 (0.89 to 0.98) |
| Hypothyroidisma | 1169 (7.9) | 2649 (3.1) | 2.69 (2.52 to 2.87) |
| Ischaemic heart disease | 244 (1.7) | 2316 (2.7) | 0.65 (0.57 to 0.74) |
| Osteoporosis | 246 (1.7) | 822 (1.0) | 1.84 (1.60 to 2.12) |
| Peripheral vascular disease | 61 (0.4) | 423 (0.5) | 0.90 (0.69 to 1.17) |
| Rheumatoid arthritis | 73 (0.5) | 550 (0.6) | 0.82 (0.65 to 1.05) |
| Severe mental illness | 1266 (8.6) | 823 (1.0) | 9.10 (8.34 to 9.92) |
| Schizophrenia | 995 (6.7) | 591 (0.7) | 9.94 (8.99 to 10.99) |
| Affective disorder | 371 (2.5) | 333 (0.4) | 6.66 (5.73 to 7.73) |
| Stroke and TIA | 267 (1.8) | 944 (1.1) | 1.74 (1.52 to 1.98) |
Not all recorded disease prevalence was higher in adults with ID. Recorded lifetime prevalences of both ischaemic heart disease (IHD) (PR 0.65, 95% CI 0.57 to 0.74) and cancer (PR 0.70, 95% CI 0.61 to 0.80) were significantly lower than those seen in the general population. Although a record of depression was equally likely in adults with ID, when only diagnoses in the last year were considered, adults with ID were 20% less likely to have one recorded in their record (PR 0.80, 95% CI 0.70 to 0.92).
Figure 8 displays a mean count of all QOF conditions from Table 6 (excluding anxiety and dysphagia, which are not counted by QOF) in adults with ID and controls. The disparity between the groups is already evident at the age of 18 years, when the mean count is approximately three times higher among adults with ID (0.31 vs. 0.11). The higher burden of comorbidity persists through middle age, but after about 65 years of age the two lines in Figure 8 start to quickly converge. Comorbidity levels are then more similar between adults with ID and matched controls in their seventies. Among the few adults with ID in their eighties in our study (n = 116), levels of comorbidity were lower than among their matched controls.
FIGURE 8.
Mean number of QOF conditions by age in adults with ID and controls.

Disease prevalence in subgroups
When the prevalence comparisons with the general population were made by age group (Table 7), there were some interesting observations. Both cancer and IHD, which were lower overall in adults with ID, were significantly higher (PR 1.98 for cancer and PR 2.68 for IHD) when only the youngest ages (18–34 years) were directly compared. In general, most of the observed differences overall were much greater for the youngest group, with epilepsy 40 times greater (PR 39.99). Heart failure (PR 12.05), osteoporosis (PR 10.07), hypothyroidism (PR 7.56) and chronic kidney disease (PR 5.85) all also showed much greater disparities within this age group. The exception to this trend with age was severe mental illness, for which the disparity between adults with ID and the general population increased with age. Among the oldest age group (55–84 years), only epilepsy (PR 17.97) and severe mental illness (PR 12.37) were more than three times as prevalent in adults with ID as in controls.
| Disease | Age group (years) | |||||
|---|---|---|---|---|---|---|
| 18–34 | 35–54 | 55–84 | ||||
| Adults with ID (%) | PR (95% CI) | Adults with ID (%) | PR (95% CI) | Adults with ID (%) | PR (95% CI) | |
| Anxiety | 12.8 | 1.32 (1.23 to 1.44) | 19.1 | 1.15 (1.09 to 1.21) | 16.8 | 0.95 (0.87 to 1.03) |
| Asthmaa | 8.2 | 1.50 (1.36 to 1.66) | 8.4 | 1.24 (1.13 to 1.36) | 7.8 | 1.00 (0.88 to 1.13) |
| Atrial fibrillation | 0.1 | 3.40 (1.00 to 11.48) | 0.5 | 1.33 (0.89 to 1.99) | 2.7 | 0.80 (0.64 to 0.99) |
| Cancer | 0.5 | 1.98 (1.29 to 3.03) | 1.1 | 0.69 (0.54 to 0.89) | 4.3 | 0.62 (0.53 to 0.74) |
| Chronic kidney disease | 0.3 | 5.85 (2.74 to 12.49) | 2.1 | 3.55 (2.85 to 4.44) | 9.8 | 1.32 (1.18 to 1.49) |
| COPD | 0.02 | 2.61 (0.21 to 33.01) | 0.8 | 1.48 (1.08 to 2.03) | 3.3 | 0.70 (0.58 to 0.85) |
| Depression | 11.6 | 1.05 (0.97 to 1.14) | 20.9 | 1.01 (0.96 to 1.06) | 21.7 | 1.04 (0.97 to 1.12) |
| Depression (last year) | 1.9 | 0.91 (0.73 to 1.12) | 1.6 | 0.73 (0.59 to 0.90) | 1.2 | 0.78 (0.56 to 1.08) |
| Diabetes | 2.1 | 3.26 (2.58 to 4.10) | 6.6 | 1.88 (1.68 to 2.10) | 15.2 | 1.36 (1.24 to 1.48) |
| Dysphagia | 2.8 | 5.85 (4.64 to 7.37) | 4.4 | 3.28 (2.84 to 3.80) | 8.3 | 2.70 (2.36 to 3.10) |
| Epilepsya | 17.2 | 39.99 (33.26 to 48.06) | 19.9 | 24.31 (21.48 to 27.52) | 18.1 | 17.97 (15.44 to 20.92) |
| Heart failure | 0.5 | 12.05 (5.86 to 24.81) | 0.4 | 3.98 (2.38 to 6.65) | 2.2 | 1.60 (1.24 to 2.07) |
| Hypertension | 1.5 | 3.25 (2.46 to 4.29) | 9.1 | 1.11 (1.02 to 1.21) | 28.6 | 0.81 (0.77 to 0.86) |
| Hypothyroidisma | 4.3 | 7.56 (6.18 to 9.25) | 9.2 | 3.15 (2.86 to 3.47) | 11.5 | 1.72 (1.55 to 1.91) |
| IHD | 0.1 | 2.68 (0.91 to 7.89) | 0.8 | 0.74 (0.55 to 0.99) | 5.6 | 0.62 (0.54 to 0.72) |
| Osteoporosis | 0.6 | 10.07 (5.57 to 18.22) | 1.1 | 3.72 (2.77 to 5.01) | 4.3 | 1.29 (1.08 to 1.54) |
| Rheumatoid arthritis | 0.2 | 2.23 (1.02 to 4.89) | 0.5 | 1.02 (0.70 to 1.50) | 1.0 | 0.62 (0.43 to 0.88) |
| Severe mental illness | 4.3 | 7.10 (5.84 to 8.64) | 9.4 | 8.12 (7.18 to 9.19) | 13.9 | 12.37 (10.61 to 14.41) |
| Stroke and TIA | 3.2 | 4.47 (2.33 to 8.53) | 7.6 | 2.42 (1.81 to 3.22) | 10.9 | 1.50 (1.29 to 1.76) |
Within adults with ID, there were some differences in disease prevalence by gender (Figure 9). Generally, women had higher levels of recorded disease than men. For example, there were higher rates in women for hypothyroidism (12.4% vs. 4.7%), chronic kidney disease (4.5% vs. 2.2%), cancer (2.2% vs. 1.2%) and a recording of depression ever (22.0% vs. 14.6%). The only condition with a notably higher rate in men was IHD (1.9% vs. 1.3%).
FIGURE 9.
Prevalence of chronic disease in adults with ID by gender. TIA, transient ischaemic attack.
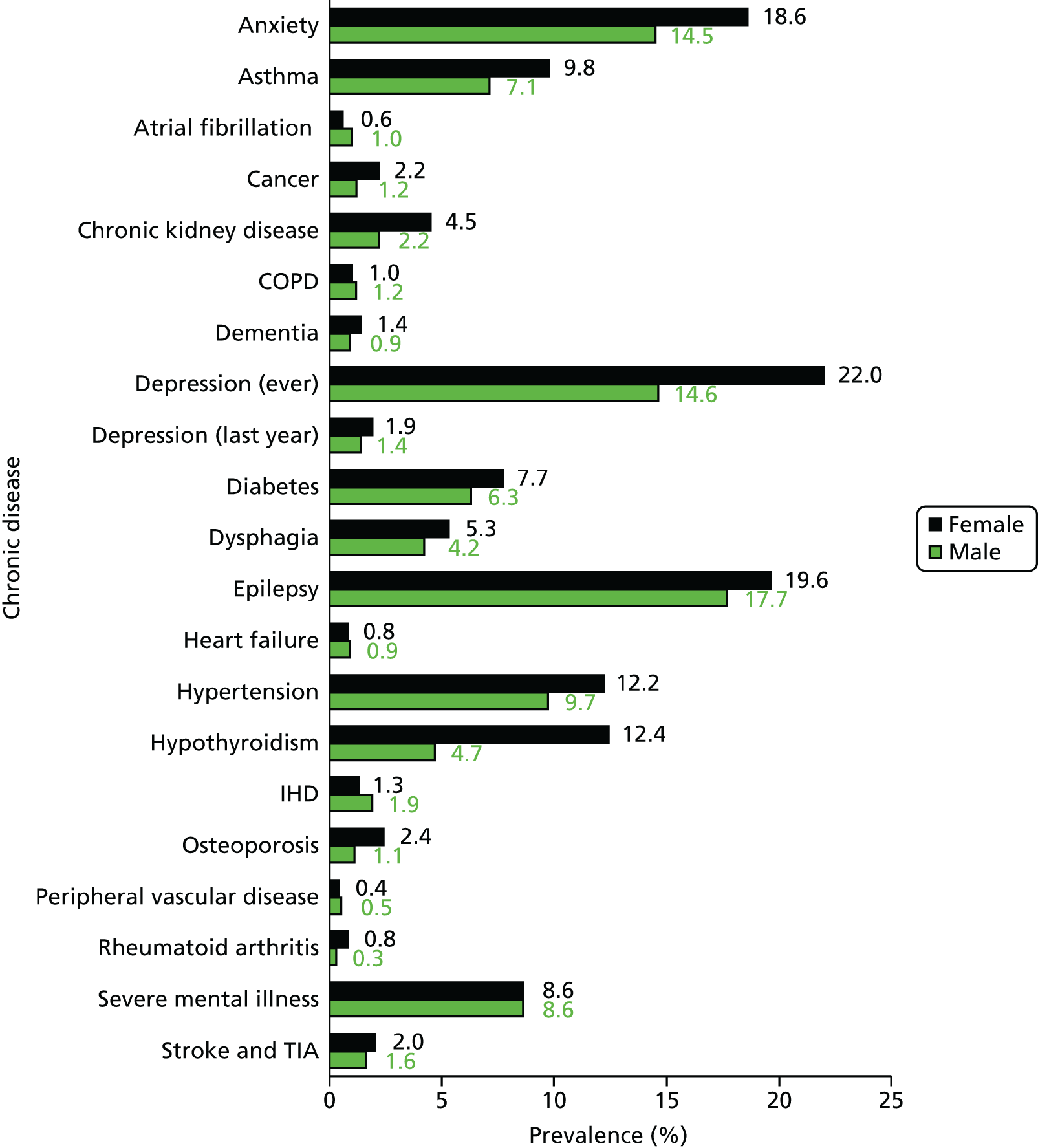
Disease prevalence by severity of ID, when this was recorded, is summarised in Figure 10 (for completeness, patients without severity are also shown in the figures). More than one-third of adults with severe or profound ID (36.2%) had epilepsy, compared with about one in six of adults with mild or moderate ID (16.3%). Compared with their general population controls, adults with severe or profound ID were 50 times more likely to have epilepsy (PR 50.4, 95% CI 39.9 to 63.8). Dysphagia was recorded in about one in nine adults with severe or profound ID (11.0%). However, the prevalence of most other conditions was lower in adults with severe or profound ID, such as anxiety (9.4%), depression (9.6%), diabetes (4.5%), hypertension (6.9%) and severe mental illness (5.9%). Compared with their general population controls, adults with severe or profound ID were four times less likely to have a diagnosis of depression recorded in the last year (PR 0.26, 95% CI 0.14 to 0.49).
FIGURE 10.
Prevalence of chronic disease in adults with ID by recorded severity. TIA, transient ischaemic attack.
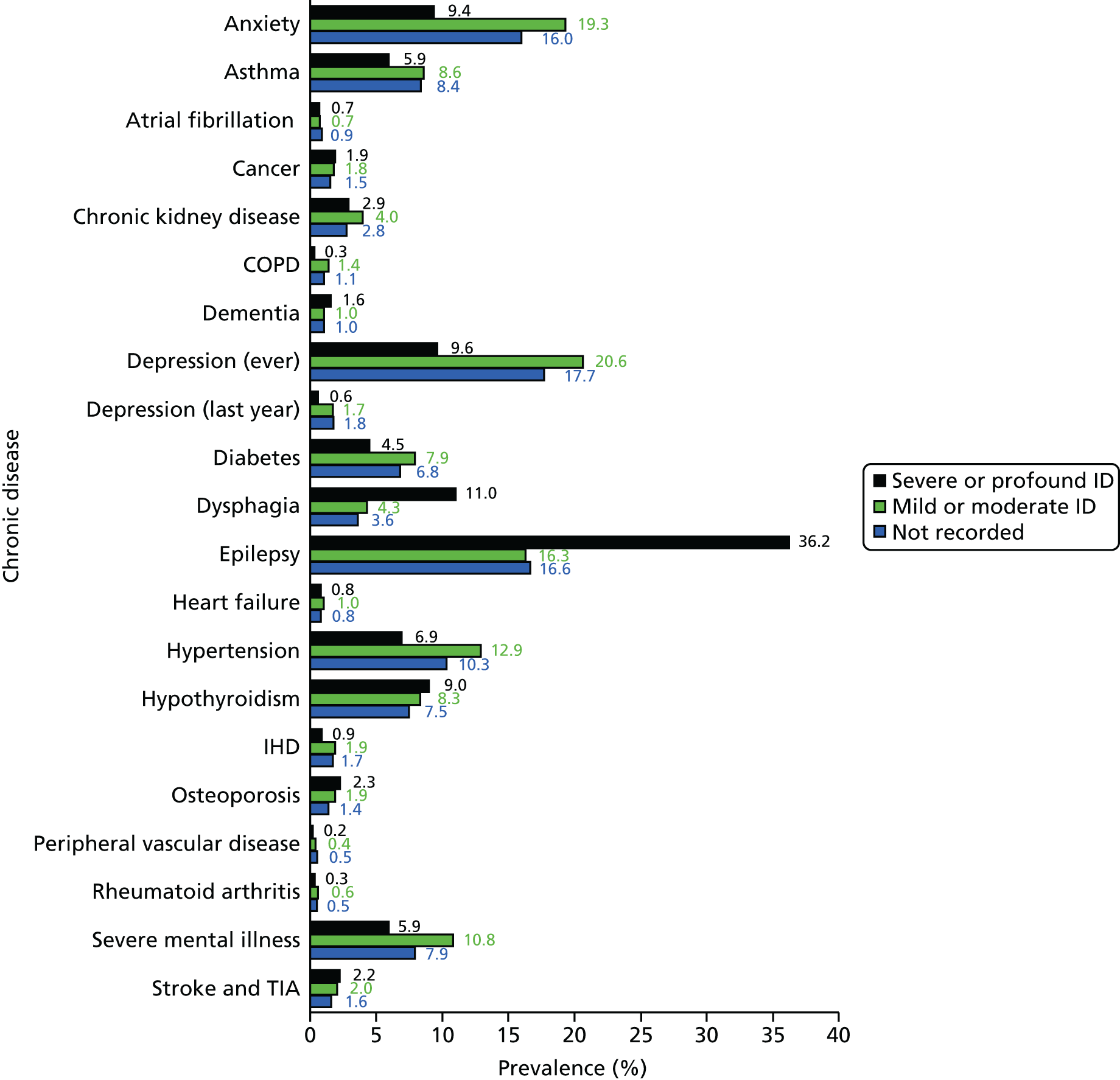
Among adults with ID identified as living in communal settings, there were variations in disease prevalence (Figure 11). Epilepsy (27.8%), severe mental illness (12.6%), hypothyroidism (11.5%), dysphagia (8.4%), dementia (2.9%) and stroke (3.4%) were all higher. However, anxiety (13.2%), currently treated asthma (5.3%) and depression diagnosed in the last year (0.7%) were all lower.
FIGURE 11.
Prevalence of chronic disease in adults with ID by living arrangements. TIA, transient ischaemic attack.
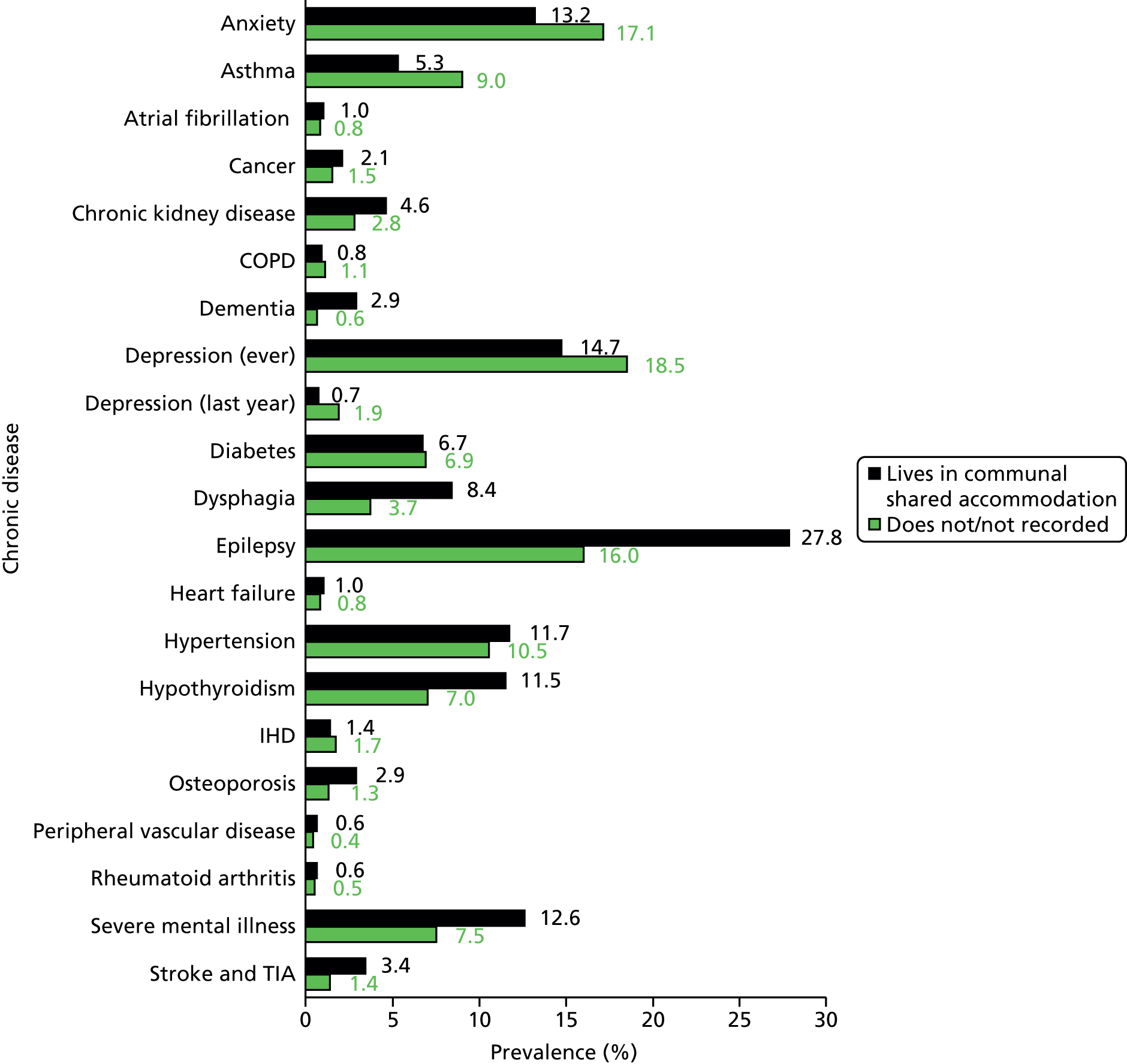
Among adults with ID with Down syndrome (Figure 12), the prevalences of hypothyroidism (31.9%), dysphagia (6.1%), dementia (5.8%) and heart failure (1.6%) were all higher. However, for most recorded chronic diseases the prevelance was lower, for example COPD (0.1%), diabetes (4.8%), epilepsy (6.8%), depression ever (8.7%), hypertension (1.7%) and severe mental illness (1.9%).
FIGURE 12.
Prevalence of chronic disease in adults with ID by presence or absence of Down syndrome. TIA, transient ischaemic attack.
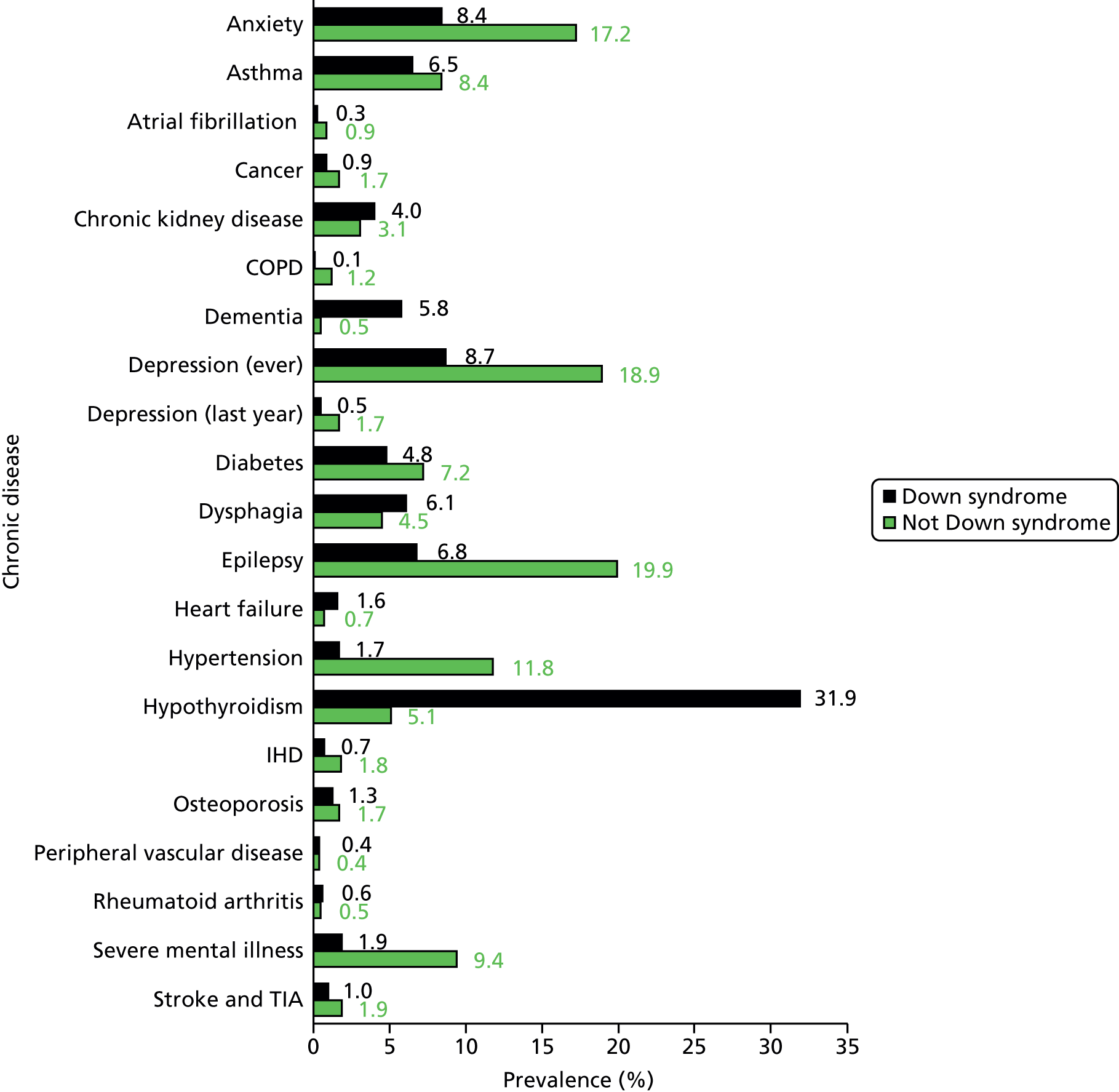
Finally, for disease prevalence among adults with ID only, in Table 8 we present a series of PRs for each condition, mutually adjusted for all the subgroups of interest (gender, severity, communal accommodation, Down syndrome and autism) and age. Many of the patterns observed in Figures 9–12 persist here.
| Disease | Subgroup, PRa (95% CI) | ||||
|---|---|---|---|---|---|
| Female vs. male | Severe/profound ID vs. mild/moderate ID | Communal accommodation vs. not | Down syndrome vs. not | Autism spectrum disorder vs. not | |
| Anxiety | 1.31 (1.22 to 1.40) | 0.51 (0.43 to 0.60) | 0.74 (0.65 to 0.86) | 0.53 (0.44 to 0.62) | 1.39 (1.23 to 1.58) |
| Asthmab | 1.36 (1.22 to 1.52) | 0.78 (0.64 to 0.96) | 0.62 (0.52 to 0.73) | 0.76 (0.62 to 0.93) | 0.64 (0.49 to 0.84) |
| Atrial fibrillation | 0.54 (0.37 to 0.79) | 1.02 (0.51 to 2.07) | 0.71 (0.45 to 1.12) | 0.59 (0.24 to 1.41) | 0.55 (0.19 to 1.60) |
| Cancer | 1.59 (1.24 to 2.04) | 1.11 (0.75 to 1.64) | 0.86 (0.65 to 1.16) | 0.65 (0.38 to 1.11) | 0.94 (0.51 to 1.74) |
| Chronic kidney disease | 1.72 (1.45 to 2.04) | 0.73 (0.53 to 1.00) | 0.91 (0.75 to 1.09) | 1.83 (1.40 to 2.39) | 0.51 (0.26 to 1.00) |
| COPD | 0.73 (0.53 to 1.00) | 0.24 (0.10 to 0.59) | 0.48 (0.33 to 0.70) | 0.18 (0.04 to 0.70) | 0.62 (0.24 to 1.61) |
| Dementia | 1.21 (0.89 to 1.63) | 1.16 (0.68 to 1.98) | 2.10 (1.50 to 2.96) | 19.25 (13.64 to 27.15) | 0.30 (0.04 to 2.13) |
| Depression | 1.49 (1.38 to 1.59) | 0.50 (0.43 to 0.59) | 0.73 (0.64 to 0.83) | 0.49 (0.42 to 0.58) | 1.07 (0.93 to 1.22) |
| Diabetes | 1.09 (0.97 to 1.23) | 0.64 (0.50 to 0.82) | 0.69 (0.59 to 0.81) | 0.81 (0.65 to 1.02) | 0.68 (0.50 to 0.92) |
| Dysphagia | 1.15 (0.98 to 1.34) | 2.32 (1.68 to 3.20) | 1.54 (1.20 to 1.98) | 1.32 (1.06 to 1.64) | 0.79 (0.58 to 1.09) |
| Epilepsyb | 1.09 (1.02 to 1.16) | 2.08 (1.89 to 2.30) | 1.60 (1.46 to 1.75) | 0.32 (0.26 to 0.39) | 0.84 (0.74 to 0.95) |
| Heart failure | 0.78 (0.54 to 1.12) | 0.82 (0.46 to 1.48) | 0.80 (0.53 to 1.21) | 2.87 (1.92 to 4.30) | 0.83 (0.33 to 2.11) |
| Hypertension | 1.10 (1.00 to 1.21) | 0.60 (0.50 to 0.72) | 0.75 (0.66 to 0.84) | 0.19 (0.13 to 0.27) | 0.55 (0.42 to 0.73) |
| Hypothyroidismb | 2.35 (2.10 to 2.62) | 0.93 (0.78 to 1.11) | 1.18 (1.04 to 1.34) | 6.50 (5.81 to 7.25) | 0.81 (0.59 to 1.10) |
| IHD | 0.54 (0.43 to 0.69) | 0.56 (0.33 to 0.97) | 0.44 (0.31 to 0.62) | 0.70 (0.39 to 1.26) | 0.41 (0.15 to 1.11) |
| Osteoporosis | 1.86 (1.44 to 2.39) | 1.22 (0.84 to 1.76) | 1.39 (1.05 to 1.83) | 0.82 (0.53 to 1.26) | 0.46 (0.22 to 0.95) |
| Peripheral vascular disease | 0.79 (0.47 to 1.33) | 0.45 (0.14 to 1.42) | 1.85 (1.06 to 3.24) | 0.85 (0.38 to 1.90) | 0.44 (0.14 to 1.38) |
| Rheumatoid arthritis | 2.79 (1.75 to 4.45) | 0.60 (0.26 to 1.40) | 0.87 (0.55 to 1.39) | 1.31 (0.66 to 2.59) | 0.27 (0.03 to 2.05) |
| Severe mental Illness | 1.02 (0.92 to 1.13) | 0.49 (0.39 to 0.61) | 1.81 (1.56 to 2.10) | 0.19 (0.13 to 0.28) | 1.06 (0.89 to 1.26) |
| Stroke and TIA | 1.15 (0.88 to 1.50) | 0.98 (0.66 to 1.45) | 2.53 (1.98 to 3.24) | 0.47 (0.29 to 0.76) | 0.27 (0.14 to 0.52) |
Women with ID were more likely to have many of these conditions recorded, with the greatest relative disparities observed for rheumatoid arthritis (PR 2.79), hypothyroidism (PR 2.35), osteoporosis (PR 1.86), chronic kidney disease (PR 1.72) and cancer (PR 1.59). Men, on the other hand, were only significantly more likely to have IHD and atrial fibrillation recorded (both PR 0.54).
The prevalence of recording of many conditions was lower for adults with severe or profound ID than for those with mild or moderate ID. For example, both IHD (PR 0.56) and severe mental illness (PR 0.49) were approximately half as likely to be recorded in patients with severe or profound ID. Notable exceptions to this trend were dysphagia (PR 2.32) and epilepsy (PR 2.08), which were much higher in patients with severe or profound ID.
The prevalence of recording of several conditions was much higher for adults with ID living in communal or shared accommodation, even after adjustment for age and severity. These conditions included stroke and transient ischaemic attack (TIA) (PR 2.53), dementia (PR 2.10) and severe mental illness (PR 1.81). However, for some conditions (e.g. IHD) this was, surprisingly, lower (PR 0.44).
As expected, the large disparities seen for dementia (PR 19.25), hypothyroidism (PR 6.50) and heart failure (PR 2.87) for patients with Down syndrome, compared with patients with ID without Down syndrome, remained after adjustment.
For patients with ID and autism spectrum disorder, the rate of recording of all conditions was generally lower than for patients with ID without autism, the lone exception (see Table 8) being anxiety (PR 1.39).
Comorbidity: Quality and Outcomes Framework conditions versus the Charlson index
To further investigate the burden of chronic disease among adults with ID, and to compare this with that of the general population, we compared three different approaches (Table 9). First, we took a frequency count of the conditions from Table 6 that are in the QOF (this excludes anxiety and dysphagia). We compared this with a comorbidity score based on QOF conditions, which was developed using UK primary care data and uses nine conditions in total. 62 Finally, we used the Charlson index, a well-known and widely used predictor of mortality, which was developed in the USA in the 1980s and incorporates 17 common chronic conditions. 71
| Item | Adults with ID (N = 14,751), n (%) | Controls (N = 86,221), n (%) | Adults with ID vs. controls, PR (95% CI) |
|---|---|---|---|
| Number of QOF diseasesa | |||
| 0 | 6320 (42.8) | 53,856 (62.5) | |
| 1 | 5056 (34.3) | 20,901 (24.2) | |
| 2 | 2138 (14.5) | 7174 (8.3) | |
| ≥ 3 | 1237 (8.4) | 4290 (5.0) | |
| ≥ 2 vs. 0–1 | 1.80 (1.74 to 1.86) | ||
| QOF scoreb | |||
| 0 | 9643 (65.4) | 77,050 (89.4) | |
| 1–2 | 4131 (28.0) | 6384 (7.4) | |
| ≥ 3 | 977 (6.6) | 2787 (3.2) | |
| Mean score of ≥ 1 vs. 0 | 3.35 (3.25 to 3.45) | ||
| Charlson indexc | |||
| 0 | 10,323 (70.0) | 63,561 (73.7) | |
| 1–2 | 3803 (25.8) | 20,090 (23.3) | |
| ≥ 3 | 625 (4.2) | 2570 (3.0) | |
| Mean score of ≥ 1 vs. 0 | 1.16 (1.12 to 1.19) | ||
Adults with ID had more multimorbidity (two or more recorded QOF conditions from Table 6), at 22.9%, than the control group, at 13.3% (PR 1.80, 95% CI 1.74 to 1.86). When the Charlson index and the QOF comorbidity score were compared, there was a difference in how the two populations (adults with and adults without ID) were categorised. Adults with ID were more than three times as likely to have a QOF score of ≥ 1 (34.6% vs. 10.6%; PR 3.35, 95% CI 3.25 to 3.45), whereas the proportions with an estimated Charlson index of ≥ 1 were much more similar between the groups (30.0% vs. 26.3%; PR 1.16, 95% 1.12 to 1.19). The difference between the performance of the two scores is primarily due to the inclusion of epilepsy and severe mental illness within the QOF score, but not within the Charlson index. This suggests that the Charlson index may not be a comprehensive summary of comorbidity within the population with ID and, as a result, may be a poorer predictor of mortality for this subgroup.
The mean QOF comorbidity score among adults with ID was 0.76 [standard deviation (SD) 1.18], compared with 0.21 (SD 0.71) for the control group. Figure 13 further summarises the mean QOF comorbidity scores by selected subgroups. The greatest relative disparity between adults with ID and controls was seen among the youngest age group (0.48 vs. 0.04), primarily attributable to epilepsy. Adults with ID with Down syndrome had less comorbidity than adults with ID without Down syndrome, but this may be partly explained by the younger overall age of these adults in our sample (see Table 5). Adults with ID living in communal establishments, or with severe health needs, had mean scores roughly twice as high as adults with ID not designated as such. Although there was a small trend of more comorbidity with deprivation in the control population, no such trend existed in the population of adults with ID. This suggests that our socioeconomic status (the IMD based on residential postcode) behaves differently in the population with ID, and may not predict morbidity and mortality in the same way as it does in the general population.
FIGURE 13.
Mean QOF comorbidity score in adults with ID and controls by subgroup.
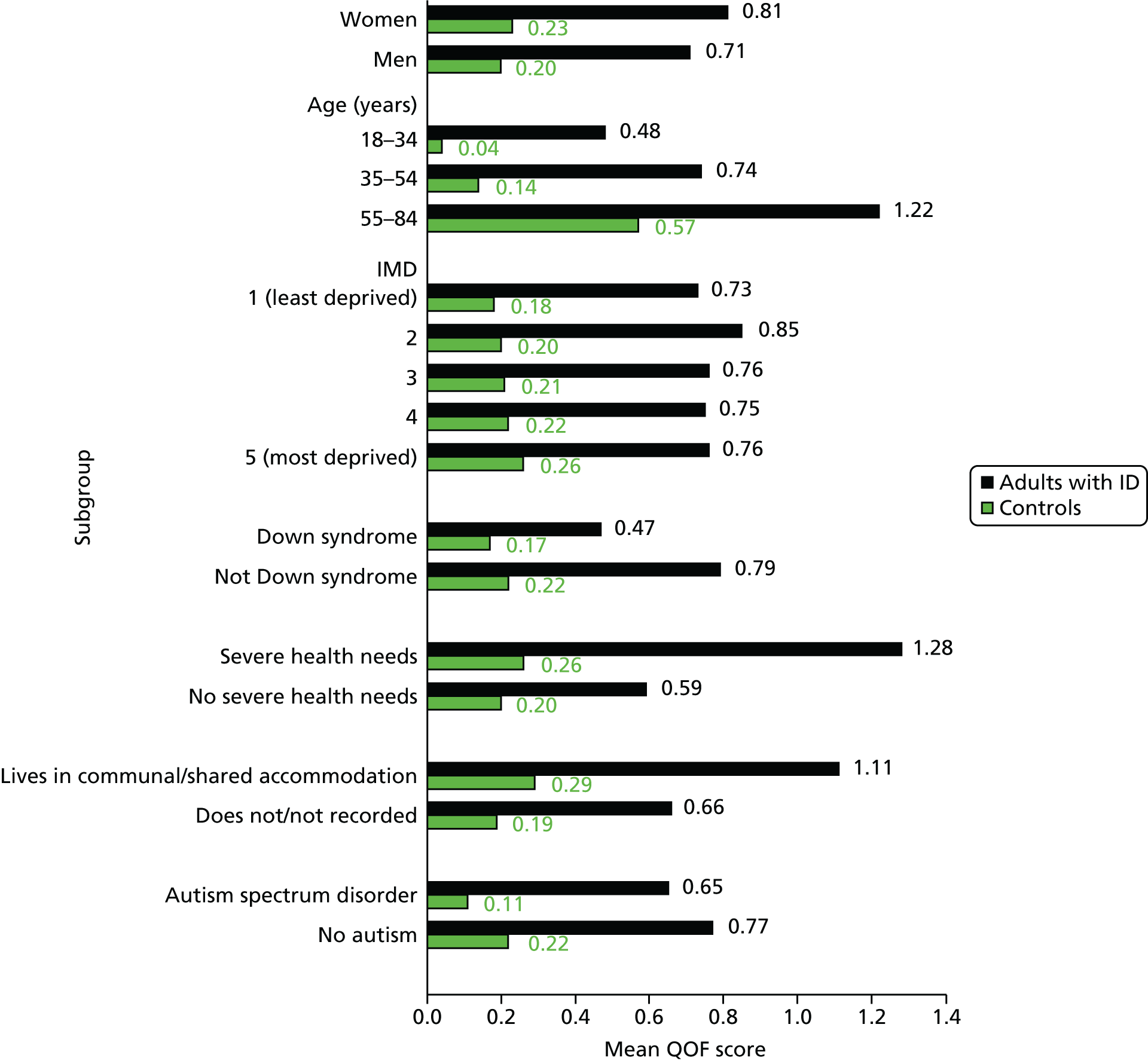
Recording of disability and other problems
We investigated the recording of selected disability (mobility problems, vision loss and hearing impairment) and other problems (continence, constipation and behavioural) in the patient record for adults with ID compared with the control group, summarised by PRs (Table 10). Further adjustment of these ratios for differences in comorbidity between the groups made little difference and did not explain the findings (data not shown), so in Table 10 we present only the unadjusted PRs.
| Recorded disability/problem | Adults with ID (N = 14,751), n (%) | Controls (N = 86,221), n (%) | Adults with ID vs. controls, PR (95% CI) |
|---|---|---|---|
| Mobility | |||
| Recorded ever | 6111 (41.4) | 753 (0.9) | 47.58 (43.63 to 51.88) |
| Some difficulty | 1677 (11.4) | 418 (0.5) | 24.02 (21.53 to 26.79) |
| Vision | |||
| Bilateral visual loss or low vision | 687 (4.7) | 510 (0.6) | 7.86 (7.01 to 8.82) |
| Continence (aged ≥ 12 years) | |||
| Recorded ever | 3017 (20.5) | 3199 (3.7) | 5.68 (5.41 to 5.96) |
| Bowel problem | 579 (3.9) | 240 (0.3) | 14.43 (12.39 to 16.80) |
| Urinary problem | 1755 (11.9) | 2663 (3.1) | 4.00 (3.77 to 4.23) |
| Hearing | |||
| Recorded ever | 7361 (49.9) | 9403 (10.9) | 4.58 (4.47 to 4.71) |
| Impairment | 2752 (18.7) | 7111 (8.3) | 2.28 (2.19 to 2.37) |
| Deaf | 1220 (8.3) | 2784 (3.2) | 2.59 (2.42 to 2.76) |
| Behavioural problems | |||
| Last year | 564 (3.8) | 155 (0.2) | 21.34 (17.86 to 25.50) |
| Last 5 years | 2072 (14.1) | 742 (0.9) | 16.28 (14.97 to 17.71) |
| Constipation | |||
| Ever | 3370 (22.9) | 7135 (8.3) | 2.78 (2.68 to 2.88) |
About 4 in 10 adults with ID (41.4%) had some recording of mobility status in their record, with about 1 in 10 overall (11.4%) reporting some form of difficulty recorded, including the use of an aid or a wheelchair. By comparison, a record of mobility (0.9%) or a mobility problem (0.5%) was rare in the matched control group. Thus, compared with adults of the same age and gender, those with ID were 24 times more likely (PR 24.02, 95% CI 21.53 to 26.79) to have a recorded mobility disability or problem.
A recording of low or loss of vision was found for 1 in 20 adults with ID (4.7%), almost eight times as likely (PR 7.86, 95% CI 7.01 to 8.82) as for those the control group (0.6%). A hearing impairment was recorded for about one in five adults with ID (18.7%), which was twice as likely (PR 2.28, 95% CI 2.19 to 2.37) as for the controls (8.3%).
An incontinence problem (beyond the age of 12 years) was recorded in about one in five adults with ID (20.5%), over five times more often (PR 5.68, 95% CI 5.41 to 5.96) than in controls (3.7%). When the incontinence was specified in the adult with ID, it was more likely to be recorded as a urinary problem (11.9%) than as a bowel problem (3.9%). However, when compared with the control group, bowel problems (PR 14.43, 95% CI 12.39 to 16.80) were relatively more likely than urinary problems to be recorded for adults with ID (PR 4.00, 95% CI 3.77 to 4.23). A record of constipation ever was about three times more likely among adults with ID (22.9% vs. 8.3%; PR 2.78, 95% CI 2.68 to 2.88).
Behavioural problems were far more commonly recorded for adults with ID, with 14.1% having one recorded in the last 5 years and 3.8% having one recorded in the last year. Less than 1% of controls had a behavioural problem recorded in the last 5 years.
There were some differences by gender in the recording of disability and other problems among adults with ID (Figure 14). Mobility problems were more common in women than in men (14.1% vs. 9.4%). Among women, a record of a continence problem (24.7%), particularly urinary (16.1%), was also higher, as was a record of constipation (27.5%). Hearing problems, visual loss and behavioural problems were much more similar between men and women with ID.
FIGURE 14.
Prevalence of disability and other problems in adults with ID by gender.

The recording of disability and other problems was more marked among adults with ID living in communal or shared accommodation (Figure 15). More than one in five adults identified as living communally had a mobility problem recorded (21.4%), whereas approximately one in three adults had a continence problem recorded (31.1%) and, similarly, one in three had a record of constipation ever (34.6%). Behavioural problems were also much more likely to be recorded among this subgroup, with one in four (24.4%) adults with ID having one recorded in the last 5 years.
FIGURE 15.
Prevalence of disability and other problems in adults with ID by living arrangements.

Among adults with Down syndrome, there were fewer differences in the recording of disability and other problems (Figure 16). The main difference was much higher recording of hearing, with more than one in three adults with Down syndrome being recorded as having an impairment (37.4%), and about one in six being recorded as having deafness (16.1%).
FIGURE 16.
Prevalence of disability and other problems in adults with ID by Down syndrome.
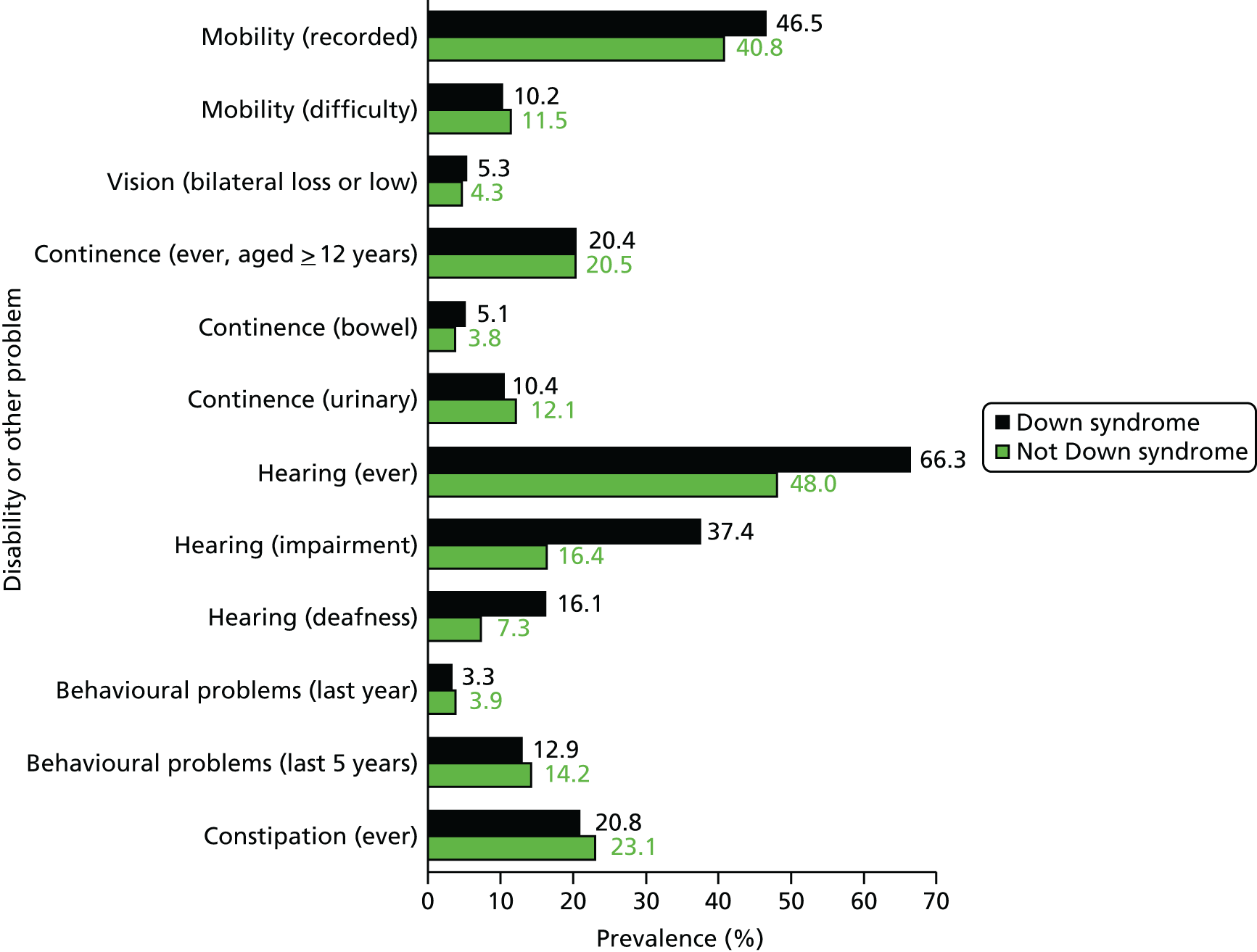
Recording of smoking, body mass index, alcohol consumption and blood pressure
Table 11 summarises the recording of smoking, BMI, alcohol consumption and blood pressure in adults with ID and their matched control group as of 1 January 2012.
| Disease | Adults with ID (N = 14,751), n (%) | Controls (N = 86,221), n (%) | Adults with ID vs. controls PRa (95% CI) |
|---|---|---|---|
| Smoking recorded | |||
| Last 5 years | 13,629 (92.4) | 72,284 (83.8) | 1.10 (1.10 to 1.11) |
| Smoking status (most recent) | |||
| Never smoked | 10,591 (71.8) | 41,512 (48.2) | |
| Current smoker | 2236 (15.2) | 20,411 (23.7) | |
| Ex-smoker | 1648 (11.2) | 20,314 (23.6) | |
| Missing | 276 (1.9) | 3984 (4.6) | |
| Current smoking vs. not | 0.64 (0.61 to 0.66) | ||
| BMI recorded | |||
| Last year | 7771 (52.7) | 21,061 (24.4) | 2.19 (2.14 to 2.23) |
| Last 5 years | 11,352 (77.0) | 49,987 (57.9) | 1.34 (1.32 to 1.35) |
| BMI value (kg/m2) (last 5 years only) | |||
| 10–19.99 | 1083 (9.5) | 3239 (6.5) | |
| 20–24.99 | 2969 (26.2) | 15,518 (31.1) | |
| 25–29.99 | 3170 (27.9) | 16,941 (34.0) | |
| 30–39.99 | 3363 (29.6) | 12,328 (24.7) | |
| ≥ 40 | 767 (6.8) | 1871 (3.8) | |
| Obesity (≥ 30 kg/m2) vs. non-obese | 1.33 (1.29 to 1.37) | ||
| Alcohol status recorded | |||
| Last year | 6903 (46.8) | 13,571 (15.7) | 3.05 (2.97 to 3.12) |
| Last 5 years | 10,925 (74.1) | 39,404 (45.7) | 1.64 (1.62 to 1.66) |
| Alcohol status (last 5 years only) | |||
| Non-drinker | 3980 (36.4) | 4553 (11.6) | |
| Current drinker | 4918 (45.0) | 30,795 (78.2) | |
| Ex-drinker | 1861 (17.0) | 3744 (9.5) | |
| Unknown | 166 (1.5) | 312 (0.8) | |
| Current drinker vs. not | 0.58 (0.57 to 0.59) | ||
| Blood pressure recorded | |||
| Last year | 9073 (61.5) | 33,492 (38.8) | 1.61 (1.58 to 1.63) |
| Last 5 years | 12,473 (84.6) | 62,608 (72.6) | 1.17 (1.16 to 1.18) |
| Blood pressure (last 5 years only) | |||
| < 150/90 mmHg vs. not | 11,196 (89.8) | 54,404 (86.9) | 1.03 (1.02 to 1.04) |
More than 9 in 10 adults with ID (92.4%) had a smoking status recorded in the last 5 years, which was about 10% higher (PR 1.10) than that seen in the control group. Among those with a status recorded, about 7 in 10 adults with ID were recorded as having never smoked (71.8%), compared with approximately half the control group (48.2%). Adults with ID were 36% less likely to be recorded as a current smoker (PR 0.64, 95% CI 0.61 to 0.66).
For BMI, adults with ID were twice as likely as controls to have a valid recording made in the last year (PR 2.19, 95% CI 2.14 to 2.23). Approximately three-quarters of adults with ID (77.0%) had a BMI recorded in the last 5 years. Among those with a BMI recorded in the last 5 years, more than one in three adults (36.4%) with ID were classed as obese (BMI of ≥ 30 kg/m2), and adults with ID were more likely to be obese (PR 1.33, 95% CI 1.29 to 1.37) than the general population. About 1 in 10 adults with ID (9.5%) was classed as being underweight (BMI of < 20 kg/m2), compared with 6.5% of controls (PR 1.48, 95% CI 1.40 to 1.57).
A record of alcohol consumption some time in the last 5 years was found in approximately three-quarters of adults with ID (74.1%), which was much higher than in the control group (45.7%). Among those with a record in the last 5 years, adults with ID were 42% less likely than those in the control group to be reported as a current drinker (PR 0.58, 95% CI 0.57 to 0.59).
Blood pressure was also more likely to be recorded among adults with ID, with 6 in 10 (61.5%) having a measurement during the last year. However, there was little difference in levels between the groups, with 89.8% of adults with ID with a measurement of < 150/90 mmHg compared with 86.9% of all controls.
Among subgroups, some of the biggest disparities were seen for smoking status among adults with ID and severe health needs or Down syndrome (Figure 17). Only 6.7% with severe health needs were classed as current smokers, compared with 17.8% among those not reporting severe health needs. Very few adults with Down syndrome (1.9%) were recorded as current smokers. Among those with a BMI recorded, there were also differences among adults with ID and severe health needs or Down syndrome (data not shown). Adults with ID with Down syndrome were more likely to be classed as obese (46.8%) than those with ID without Down syndrome (35.1%), whereas being underweight (BMI of < 20 kg/m2) was more common among those with severe health needs than among those without (13.6% vs. 9.2%).
FIGURE 17.
Smoking status by severe health needs and Down syndrome.
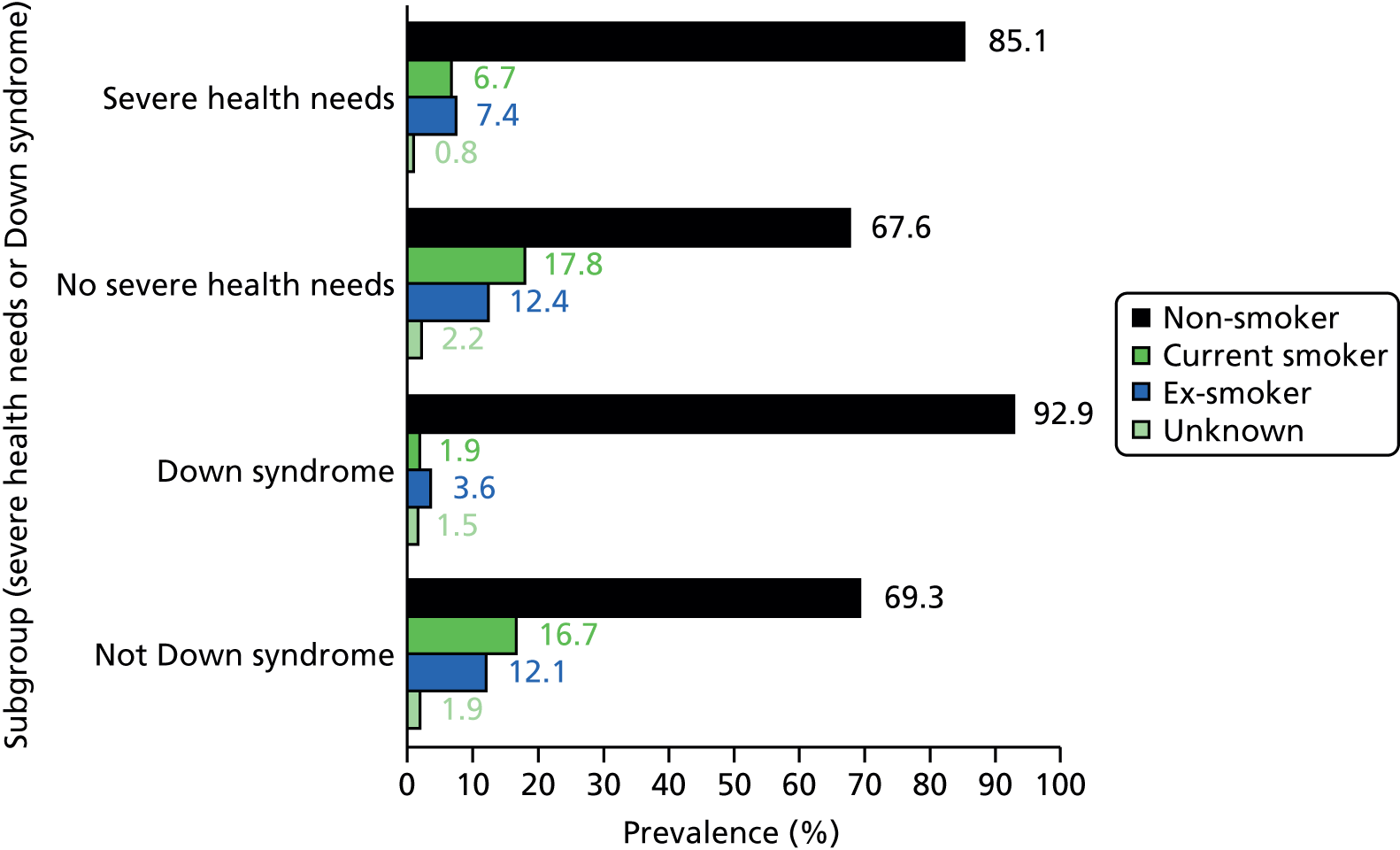
Recording of health promotion
A summary of some health promotion measures, such as vaccination and screening, is shown in Table 12. About 4 in 10 adults had a vaccination for influenza in the last year (41.5%). When restricted to a subgroup with relevant comorbidity (CHD, stroke, diabetes mellitus or COPD), this rose to 76.9% for adults with ID. This was marginally higher than the rate (73.1%) found across all (matched and unmatched) controls with similar comorbidity (CHD, stroke, diabetes mellitus or COPD). A similar difference was observed when the statistical analysis included only controls with at least one of these comorbidities who were matched to these cases (PR 1.03, 95% CI 0.98 to 1.07).
| Health promotion measure | Adults with ID (N = 14,751), n (%) | Controls (N = 86,221), n (%) | Adults with ID vs. controls, PRa (95% CI) |
|---|---|---|---|
| Influenza vaccination | |||
| Last year | 6128 (41.5) | 14,115 (16.4) | 2.61 (2.55 to 2.68) |
| CHD, stroke, diabetes mellitus or COPD only | 1493b | 7039b | |
| Last year | 1148 (76.9) | 5144 (73.1) | 1.03 (0.98 to 1.07) |
| Cervical screening | |||
| Women aged 25–64 years only | 4618b | 27,481b | |
| Smear ever | 2062 (44.7) | 25,088 (91.3) | 0.49 (0.48 to 0.51) |
| Hysterectomy ever | 195 (4.2) | 2218 (8.1) | 0.56 (0.48 to 0.64) |
| Excepted ever | 2206 (47.8) | 2593 (9.4) | 5.06 (4.80 to 5.34) |
| No hysterectomy and not excepted | 2242b | 22,771b | |
| Smear in last 5 years | 1176 (52.5) | 19,304 (84.8) | 0.64 (0.61 to 0.66) |
| Mammogram | |||
| Women aged 50–69 years only | 1846b | 11,709b | |
| Last 3 years | 861 (46.6) | 7310 (62.4) | 0.75 (0.72 to 0.78) |
| Urinalysis | |||
| Last year | 433 (27.6) | 1095 (11.9) | 2.15 (2.09 to 2.22) |
| Thyroid function | |||
| Last year | 4958 (33.6) | 15,765 (18.3) | 1.88 (1.83 to 1.93) |
| Down syndrome match-sets only | 1571b | 9178b | |
| Last year (Down syndrome only) | 974 (62.0) | 1604 (17.5) | 3.64 (3.41 to 3.88) |
| Contraception use/advice | |||
| Women aged 18–54 years only | 4646b | 26,652b | |
| Last year | 1586 (34.1) | 8450 (31.7) | 1.04 (0.99 to 1.08) |
| Medication review | |||
| Last year | 5467 (37.1) | 17,690 (20.5) | 1.84 (1.80 to 1.88) |
| Prescribed medication in 2011 | 12,649b | 57,493b | |
| Last year | 5412 (42.8) | 17,351 (30.2) | 1.46 (1.43 to 1.50) |
Cervical smear coverage in adults with ID was much lower than in controls. Among women with ID aged 25–64 years, fewer than half had a smear ever (44.7%). Almost half (47.8%) had a code in their record of being ‘excepted’ from a smear in the past, a much higher rate than that seen in the controls. These exceptions are based on QOF rules47 that cover Read codes indicating that the screen was ‘not wanted’, ‘refused’ or ‘not indicated’ or that the GP was in receipt of a disclaimer on the patient’s record. When the comparison of cervical smears was restricted to the last 5 years among those with no record of a hysterectomy or an exception ever, adults with ID were still 36% less likely than adults without ID to have had a smear (PR 0.64, 95% CI 0.61 to 0.66) during this period. Severity of ID influenced the likelihood of a recent smear, with women with ID and severe health needs having lower coverage (31.5%) than those without severe health needs (57.1%).
Mammograms were less likely among adults with ID than among the general population, with fewer than half women aged 50–69 years having a record of one during the last 3 years (46.6%). Other investigative tests, however, were more common among adults with ID, with higher recorded rates of urinalysis (27.6%) and thyroid function (33.6%) tests in the last year. Contraceptive advice or recorded use among 18–54 year olds was similar between adults with ID (34.1%) and adults without ID (31.7%).
Medication reviews during the last year were more commonly recorded among adults with ID than among controls, both among all patients (37.1% vs. 20.5%) and among those prescribed medication during the year (42.8% vs. 30.8%). However, these figures are likely to be underestimating the true scale as we have some reservations about the completeness of medications reviews during this period on the CPRD database (see Chapter 2, Missing entity data in the Clinical Practice Research Datalink).
Overall prescribing trends
We first summarised prescribing by collating whether or not each patient had been receiving a prescription in 2011 (Table 13). We further summarised by dividing the drugs into common groupings using British National Formulary (BNF) chapter headings. 72 We then summarised different drug classes by using BNF subchapters to identify and count different drugs. Thus, for example, BNF 2.6.1 (nitrates) is counted as a different drug from BNF 2.6.2 (calcium channel blockers).
| Prescribing group | Adults with ID (N = 14,751), n (%) | Controls (N = 86,221), n (%) | Adults with ID vs. controls, PR (95% CI) |
|---|---|---|---|
| Overall | |||
| Any prescription | 12,649 (85.8) | 57,493 (66.7) | 1.29 (1.28 to 1.30) |
| BNF chapter headings (1 to 13) | |||
| (1) Gastrointestinal system | 5086 (34.5) | 17,347 (20.1) | 1.75 (1.71 to 1.80) |
| (2) Cardiovascular system | 3519 (23.9) | 17,509 (20.3) | 1.23 (1.19 to 1.26) |
| (3) Respiratory system | 3314 (22.5) | 11,810 (13.7) | 1.66 (1.61 to 1.72) |
| (4) Central nervous system | 8847 (60.0) | 24,916 (28.9) | 2.11 (2.07 to 2.14) |
| (5) Infections | 5583 (37.9) | 24,165 (28.0) | 1.36 (1.33 to 1.39) |
| (6) Endocrine system | 2610 (17.7) | 9417 (10.9) | 1.69 (1.62 to 1.75) |
| (7) Obstetrics, gynaecology and urinary tract disorders | 1985 (13.5) | 10,609 (12.3) | 1.06 (1.02 to 1.10) |
| (8) Malignant disease and immunosuppression | 63 (0.4) | 460 (0.5) | 0.81 (0.63 to 1.06) |
| (9) Nutrition and blood | 2721 (18.5) | 5606 (6.5) | 2.88 (2.76 to 3.01) |
| (10) Musculoskeletal and joint diseases | 2388 (16.2) | 10,461 (12.1) | 1.36 (1.31 to 1.42) |
| (11) Eye | 1630 (11.1) | 4944 (5.7) | 1.96 (1.86 to 2.07) |
| (12) Ear, nose and oropharynx | 2285 (15.5) | 7040 (8.2) | 1.92 (1.84 to 2.01) |
| (13) Skin | 5651 (38.3) | 13,950 (16.2) | 2.39 (2.32 to 2.45) |
| Repeat prescribing only | |||
| Any repeat prescription | 10,507 (71.2) | 34,421 (39.9) | 1.82 (1.79 to 1.84) |
| 1–2 drug classes | 3730 (25.3) | 18,404 (21.4) | – |
| 3–5 drug classes | 3758 (25.5) | 9810 (11.4) | – |
| 6–10 drug classes | 2463 (16.7) | 5052 (5.9) | – |
| ≥ 11 drug classes | 556 (3.8) | 1155 (1.3) | – |
Adults with ID were 29% more likely than their matched population controls to have received a prescription during the year, with almost all receiving one (85.8%). When only repeat prescriptions were considered, the disparity increased, and adults with ID were nearly twice as likely (PR 1.8) to be on repeat medication during 2011. Approximately one in five adults with ID (20.5%) was prescribed at least six different drug classes as repeat medication during the year, a much higher rate than seen for controls (7.2%).
When the prescribing was summarised by different BNF chapter headings (1–13 only), some further patterns emerged. Adults with ID were more likely to be prescribed from all drug classes, except the small number of drugs prescribed for malignant disease and immunosuppression. Adults with ID were more than twice as likely to be prescribed drugs from the following groups: nutrition and blood, skin diseases and central nervous system. Six in 10 adults with ID were prescribed a drug from the central nervous system group, with carbamazepine (10%), sodium valproate (9%) and risperidone (7%) being the most frequent drug substances prescribed. Within controls, the pattern in the central nervous system chapter was completely different, with paracetamol or codeine phosphate (17%) and citalopram (10%) being the most prescribed.
An alternative summary measure of prescribing was to calculate the total volume of drugs prescribed in 2011. We calculated the mean number of prescriptions per patient, and then summarised this as a rate per 1000 patients (Figure 18). This revealed larger relative differences for adults with ID, suggesting that they were not only more likely to receive a drug from a particular class, but also more likely to be prescribed more drugs from that class over the year. For example, the prescribing volume of drugs for central nervous system, nutrition and blood, and skin diseases all showed rates five to six times higher for adults with ID than for matched controls. The mean volume of central nervous system drugs (13,387 per 1000 patients) signifies that, on average, an adult with ID was receiving a drug from this class every month during 2011.
FIGURE 18.
Volume of prescribing in 2011 by BNF chapter in adults with ID and controls.
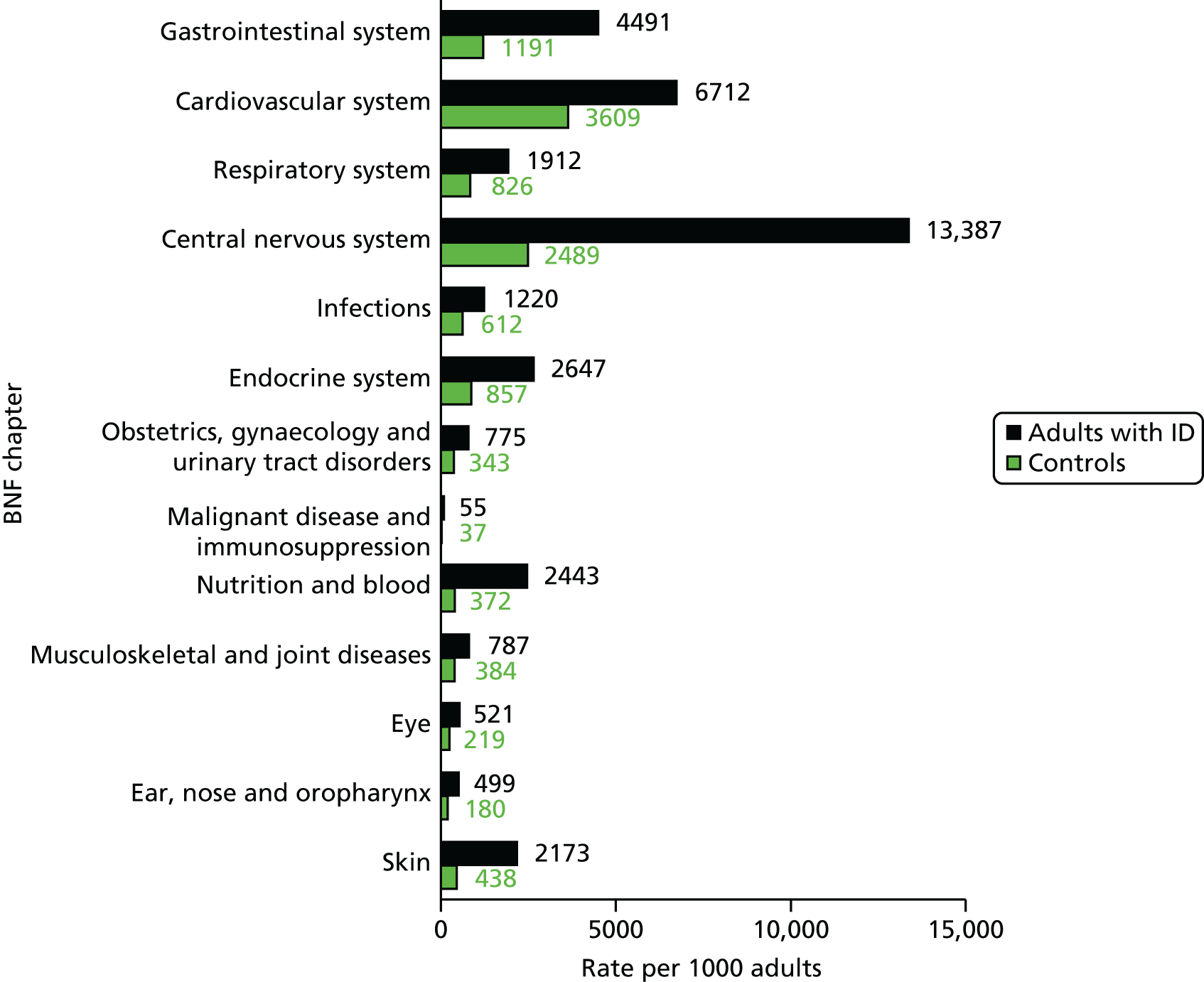
Prescribing of psychotropic drugs
We wanted to further summarise prescribing by analysing patterns of psychotropic medication (BNF chapters 4.1–4.4) between adults with ID and matched controls. Within psychotropic prescribing, we identified the following subgroups of interest: hypnotics/anxiolytics (BNF 4.1.1–4.1.2), antipsychotics (BNF 4.2.1–4.2.2), antimanic drugs (BNF 4.2.3) and antidepressants (BNF 4.3). We excluded from antidepressants any prescriptions for low-dose tricyclic and related antidepressants used at smaller doses than the minimum effective for depression treatment (specifically amitriptyline and nortriptyline at doses of < 50 mg), in line with previous analyses of primary care databases that we have carried out,73 as these doses may be prescribed for reasons other than depression, such as chronic neuropathic pain. We chose not to include the specific chapter of antiepileptic drugs (BNF 4.8) in our definition of psychotropic drugs, but to include a separate category for this instead. We also include a category of drugs classed as benzodiazepines (which are selected hypnotics/anxiolytics and antiepileptic drugs).
Table 14 summarises the pattern of psychotropic prescribing in 2011. Adults with ID were almost three times more likely to be prescribed a psychotropic drug than controls (PR 2.73, 95% CI 2.66 to 2.81), with almost 4 in 10 (38.2%) receiving at least one prescription during the year. Of these, only 51.1% (n = 2874) of adults with ID prescribed a psychotropic drug in 2011 had a recorded medication review during the year.
| Drug class | Adults with ID (N = 14,751), n (%) | Controls (N = 86,221), n (%) | Adults with ID vs. controls, PR (95% CI) |
|---|---|---|---|
| All psychotropic drugs | |||
| Any (BNF 4.1, 4.2, 4.3, 4.4) | 5629 (38.2) | 12,226 (14.2) | 2.73 (2.66 to 2.81) |
| Hypnotics and anxiolytics (BNF 4.1.1, 4.1.2) | 2020 (13.7) | 4457 (5.2) | 2.70 (2.57 to 2.83) |
| Antipsychotics (BNF 4.2.1, 4.2.2) | 2887 (19.6) | 1875 (2.2) | 9.19 (8.69 to 9.73) |
| Antimanic (BNF 4.2.3) | 678 (4.6) | 250 (0.3) | 16.05 (13.89 to 18.55) |
| Antidepressants (BNF 4.3), excluding low-dose amitriptyline | 2905 (19.7) | 8706 (10.1) | 1.99 (1.92 to 2.07) |
| Other selected groupings | |||
| Benzodiazepinesa | 2037 (13.8) | 2998 (3.5) | 4.03 (3.82 to 4.26) |
| Antiepileptic (BNF 4.8) | 3138 (21.3) | 943 (1.1) | 19.60 (18.26 to 21.03) |
| Low-dose amitriptyline (< 50 mg) | 334 (2.3) | 2774 (3.2) | 0.73 (0.65 to 0.82) |
| Among patients with ID without epilepsy onlyb | 12,020 | 69,722 | |
| Any psychotropic drug | 4179 (34.8) | 9698 (13.9) | 2.54 (2.47 to 2.62) |
| Antimanic (BNF 4.2.3) | 245 (2.0) | 123 (0.2) | 11.87 (9.56 to 14.76) |
| Benzodiazepinesa | 1050 (8.7) | 2337 (3.4) | 2.67 (2.48 to 2.86) |
The disparity in psychotropic prescribing was being driven by large differences in antipsychotic prescribing, whereby adults with ID were nine times more likely to receive this class of drug (PR 9.19, 95% CI 8.69 to 9.73), and by antimanic drugs, which were 16 times more likely to be prescribed to adults with ID (PR 16.05, 95% CI 13.89 to 18.55). Smaller differences between adults with ID and controls were seen for hypnotics/anxiolytics (PR 2.70, 95% CI 2.57 to 2.83), and antidepressants (PR 1.99, 95% CI 1.92 to 2.07). Although adults with ID had higher overall prescribing for psychotropic drugs, they were less likely (PR 0.73, 95% CI 0.65 to 0.82) than controls to receive low-dose amitriptyline or nortriptyline (which were excluded from our antidepressants category). The prescribing of benzodiazepines was approximately four times higher among adults with ID than among controls (PR 4.03, 95% CI 3.82 to 4.26).
The higher prevalence of epilepsy in adults with ID compared with controls (25 times higher; see Table 6) is reflected in the similarly higher prescribing of antiepileptic drugs among adults with ID (PR 19.60, 95% CI 18.26 to 21.03). However, the higher prevalence of epilepsy among adults with ID explained only some of the observed difference in psychotropic prescribing in Table 14. Although more than half of adults with ID and epilepsy (n = 2731) were prescribed a psychotropic drug in 2011 (n = 1450, 53.1%), one-third of adults with ID without epilepsy (34.8%) were still being prescribed a psychotropic drug in 2011, which represented a rate two and half times higher (PR 2.54, 95% CI 2.47 to 2.62) than that seen in the matched control group (see Table 14). By contrast, the prescribing of antimanic drugs (BNF 4.2.3) was much more common among adults with ID with epilepsy (15.9% vs. 2.0%). This was primarily due to the prescribing of carbamazepine, which is listed as both an antimanic and an antiepileptic drug (BNF 4.8), and is presumably being prescribed mainly to treat seizures among adults with ID as opposed to bipolar disorder. However, excluding adults with ID with epilepsy from the comparison still resulted a large relative increase compared with the matched controls (PR 11.87, 95% CI 9.56 to 14.76). Benzodiazepine prescribing was also far more common among adults with ID with epilepsy (37.4% vs. 8.7%), and the relative difference between adults with ID and controls fell from a PR of 4.03 to a PR of 2.67 when we excluded adults with ID and epilepsy (and their controls) from the comparison.
Figure 19 displays the top 20 psychotropic drug substances prescribed to adults with ID during 2011, compiled from all prescriptions issued under BNF chapters 4.1–4.4. These are summarised as a rate per 1000 adults (counting a maximum of one prescription per day for each drug class), with the corresponding rates seen in the matched controls also shown in the figure.
FIGURE 19.
Top 20 psychotropic drugs prescribed by volume in 2011 among adults with ID, with rates among controls shown for comparison.
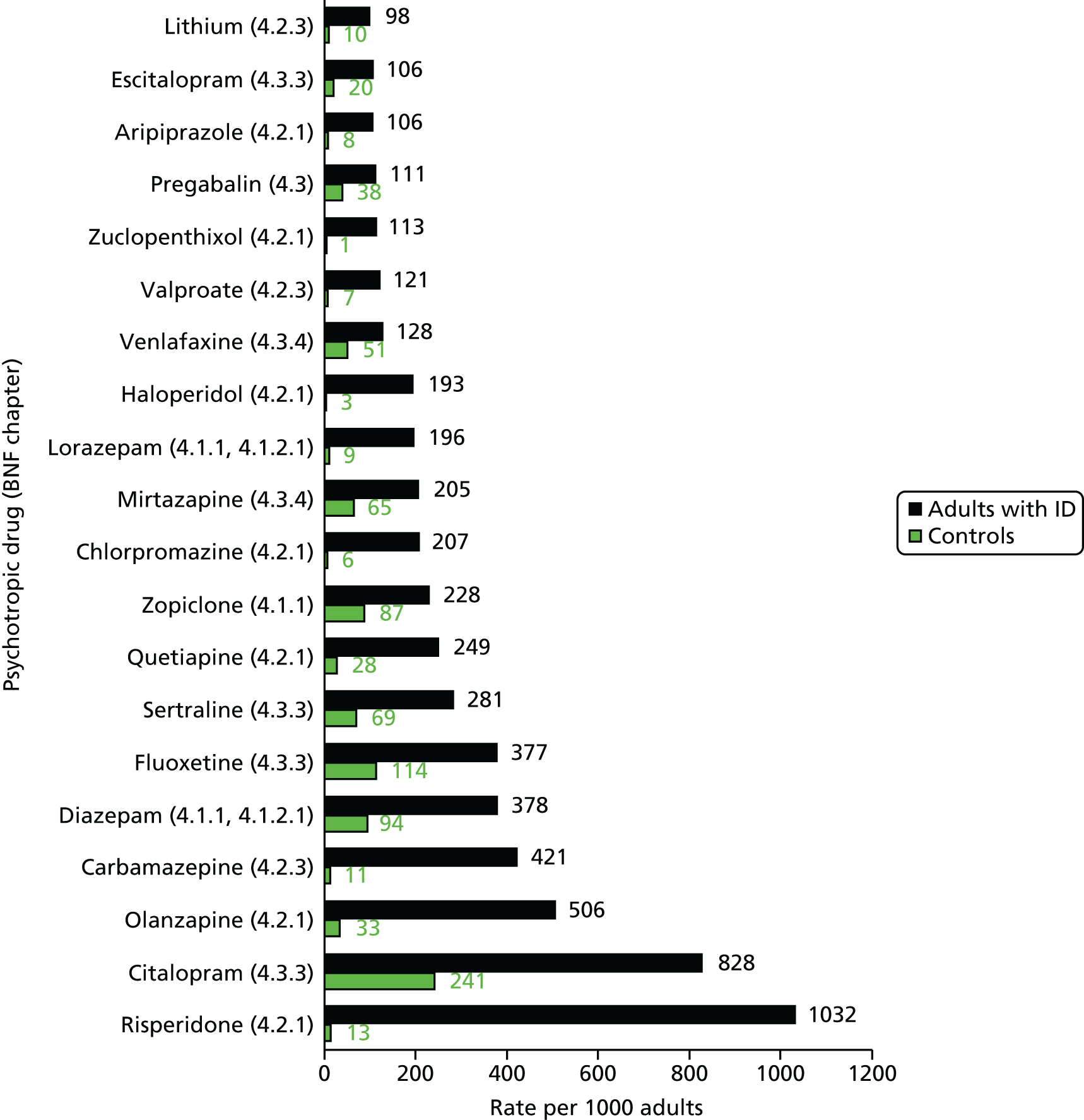
The most commonly prescribed item was the antipsychotic risperidone (1032 prescriptions per 1000 adults), which was rarely prescribed across the control group (13 per 1000). Other large relative disparities were seen for zuclopenthixol (113 per 1000 adults with ID compared with 1.4 per 1000 controls), haloperidol (193 per 1000 adults with ID compared with 3 per 1000 controls) and carbamazepine (421 per 1000 adults with ID compared with 11 per 1000 controls). The most commonly prescribed antidepressants among adults with ID (e.g. citalopram, fluoxetine and sertraline) were prescribed at rates approximately three to four times higher among adults with ID than among controls. Although the most prescribed benzodiazepine among the psychotropic drugs in adults with ID was diazepam (378 per 1000 adults), this was also prescribed frequently among controls (94 per 1000 adults). By contrast, lorazepam, another benzodiazepine, was frequently prescribed among adults with ID (196 per 1000 adults) but rarely prescribed among controls (9 per 1000 adults).
We summarised overall psychotropic prescribing in adults with ID and controls by subgroups of interest (Figure 20). Although women with ID were marginally more likely than men with ID to have received a psychotropic drug in 2011 (41.1% vs. 36.0%), this contrasted with the matched control group, in which women were twice as likely (19.9% vs. 10.0%). Prescribing increased with age, for both adults with ID and controls, but the largest relative disparity was seen among the youngest ages (18–34 years). Approximately 3 in 10 younger adults (28.7%) with ID received a psychotropic drug in 2011, compared with 1 in 10 (9.2%) among the control group.
FIGURE 20.
Psychotropic drug prescribing in 2011 in adults with ID and controls by subgroup.
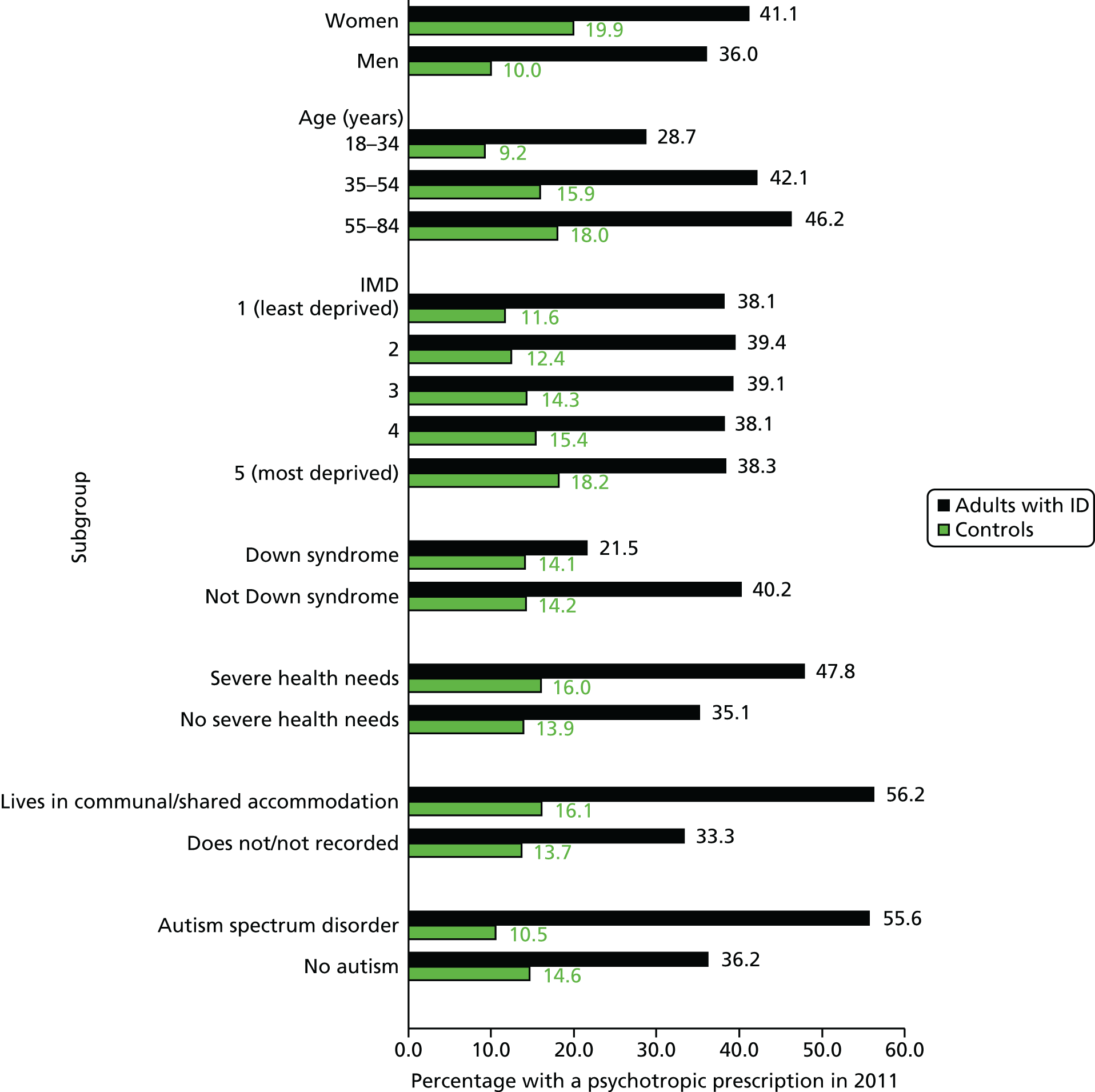
Prescribing of psychotropic drugs by socioeconomic status (using the IMD) showed contrasting patterns between adults with ID and controls. Although controls in more deprived areas were more likely to be prescribed a psychotropic drug during the year (18.2% in most deprived quintile vs. 11.6% in least deprived), no such pattern existed among adults with ID. Those living in the most deprived areas (IMD = 5) had similar psychotropic prescribing levels in 2011 (38.3%) to those in the least deprived areas (38.1%). However, when we restricted the analysis to adults with ID not recorded as living in communal or shared accommodation, there was a weak trend, whereby those living in the most deprived area had higher levels of psychotropic prescribing (34.8%) than those in the living in the least deprived category of IMD (31.7%).
Among subgroups with ID there were some key differences in psychotropic prescribing. Much higher rates were seen among the following: those living in communal or shared accommodation (56.2%), those with autism spectrum disorder (55.6%) and those with severe health needs (47.8%). Adults with ID and Down syndrome, however, were much less likely to be prescribed a psychotropic drug in 2011 (21.5% vs. 40.2%).
Finally, we looked further back in the patient record to summarise longer-term prescribing of psychotropic drugs. Among patients who were continuously registered with their practice for the last 5 years, 36.6% (3940 out of 10,769) of adults with ID averaged more than one prescription per year, compared with 14.4% of controls (10,765 out of 74,784), which compares closely with what we found from the analyses based on a single year (2011). The average number of psychotropic prescriptions per year during the last 5 years was 7.6 for adults with ID, compared with 1.3 per year for controls.
Attainment of Quality and Outcomes Framework indicators
We wanted to compare the achievement for a number of QOF indicators47 between adults with ID and their matched controls. The indicators are generally disease specific and calculated only on patients who are on that particular QOF disease register, making any matched analysis here infeasible.
A summary of the age and gender characteristics of adults with ID and controls on selected QOF disease registers (chronic kidney disease, diabetes, epilepsy, hypertension, hypothyroidism, IHD and stroke) is shown in Table 15. The prevalence of these diseases has previously been described in Table 6.
| QOF register | Adults with ID | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Men (%) | Mean age (SD) | Number excepteda (%) | n | Men (%) | Mean age (SD) | Number excepteda (%) | |
| Chronic kidney disease | 468 | 39.7 | 60.1 (11.9) | 9 (1.9) | 1746 | 44.2 | 67.0 (10.9) | 24 (1.4) |
| Diabetes | 1017 | 52.9 | 53.6 (14.3) | 70 (6.9) | 3786 | 61.5 | 57.5 (13.0) | 187 (4.9) |
| Epilepsy | 2731 | 55.4 | 42.4 (14.7) | 141 (5.2) | 633 | 55.5 | 47.4 (14.7) | 35 (5.5) |
| Hypertension | 1583 | 52.0 | 57.1 (12.7) | 29 (1.8) | 10,416 | 54.8 | 60.4 (11.2) | 150 (1.4) |
| Hypothyroidism | 1169 | 34.3 | 48.3 (14.7) | 8 (0.7) | 2649 | 19.6 | 55.5 (13.2) | 8 (0.3) |
| IHD | 244 | 67.2 | 62.5 (12.0) | 14 (5.7) | 2316 | 69.5 | 64.2 (10.3) | 72 (3.1) |
| Stroke and TIA | 267 | 52.4 | 60.2 (13.6) | 23 (8.6) | 944 | 56.6 | 64.2 (12.0) | 22 (2.3) |
There were some notable differences in the age–gender structure between adults with ID and controls on the QOF disease registers. Generally, adults with ID were about 5 years younger on average. For diabetes, a greater proportion of adults with ID were women (47.1% vs. 38.5%), whereas for hypothyroidism adults with ID were more likely to be men (34.3% vs. 19.6%). Thus, any (unmatched) analysis of QOF indicators must account for age and gender differences.
Table 15 also reports on QOF exception reporting within the selected disease registers. Exception reporting is when GPs are allowed to specifically exclude patients from indicators owing to patient-specific clinical circumstances. 47 For example, this may arise when an indicator includes medication that cannot be prescribed because of a recorded contraindication or side effect. For all selected disease registers, adults with ID were more likely to be excepted from QOF indicators. For example, for stroke (and TIA), 8.6% of adults with ID were excepted, compared with 2.3% of controls.
The selected QOF indicators that we chose to compare from the seven disease registers are shown in Table 16. These were calculated for attainment in the last 12 months on our chosen cross-sectional date (1 January 2012). This differs from QOF, which makes its annual calculations at the end of March each year. 47 We also chose to not to apply the disease exceptions from Table 15 for this comparison. As patients were no longer matched in this analyses, we fitted a log-binomial model here to obtain ratios adjusted for age and gender (see Chapter 2, Statistical analysis).
| QOF indicator | Adults with ID, n (%) | Controls, n (%) | Adults with ID vs. controls PRa (95% CI) |
|---|---|---|---|
| Chronic kidney disease | |||
| Last BP is ≤ 150/90 mmHg (CKD3) | 340 (72.7) | 1151 (65.9) | 1.11 (1.03 to 1.19) |
| Diabetes mellitus | |||
| Last BP is ≤ 150/90 mmHg (DM30) | 861 (84.7) | 3119 (82.4) | 1.03 (1.00 to 1.06) |
| Last cholesterol is ≤ 5 mmol/l (DM17) | 679 (66.8) | 2617 (69.1) | 1.00 (0.95 to 1.04) |
| Last IFCC HbA1c/HbA1c is ≤ 59/7.5% (DM26) | 535 (52.6) | 2011 (53.1) | 1.01 (0.94 to 1.08) |
| Retinal screening (DM21) | 496 (48.8) | 2137 (56.4) | 0.89 (0.84 to 0.95) |
| Foot examination and classification (DM29b) | 658 (65.0) | 2573 (68.1) | 0.97 (0.92 to 1.02) |
| Micro-albuminuria testing (DM13b) | 544 (56.4) | 2145 (60.0) | 0.95 (0.89 to 1.01) |
| Estimated glomerular filtration rate or serum creatinine testing (DM22) | 903 (88.8) | 3409 (90.0) | 0.99 (0.97 to 1.01) |
| Epilepsy | |||
| Record of seizure frequency (EPIL6) | 2202 (80.6) | 501 (79.2) | 1.03 (0.98 to 1.08) |
| Record of seizure free (EPIL8) | 1281 (46.9) | 340 (53.7) | 0.91 (0.83 to 1.00) |
| Hypertension | |||
| Last BP is ≤ 150/90 mmHg (BP5) | 1249 (78.9) | 7927 (76.1) | 1.04 (1.01 to 1.07) |
| Hypothyroidism | |||
| Thyroid function test (THY2) | 1027 (87.9) | 2355 (88.9) | 0.99 (0.97 to 1.02) |
| IHD | |||
| Last BP is ≤ 150/90 mmHg (CHD06) | 211 (86.5) | 1934 (83.5) | 1.02 (0.97 to 1.09) |
| Last cholesterol is ≤ 5 mmol/l (CHD08) | 144 (59.0) | 1508 (65.1) | 0.92 (0.83 to 1.03) |
| Aspirin, an alternative antiplatelet therapy or an anticoagulant (CHD09) | 199 (81.6) | 1917 (82.8) | 0.99 (0.93 to 1.05) |
| Stroke and TIA | |||
| Last BP is ≤ 150/90 mmHg (STR6) | 209 (78.3) | 746 (79.0) | 1.00 (0.92 to 1.07) |
| Last cholesterol is ≤ 5 mmol/l (STR8) | 149 (55.8) | 566 (60.0) | 0.96 (0.96 to 1.08) |
| Aspirin, an alternative antiplatelet therapy or an anticoagulant (STR12c) | 132 (75.0) | 555 (82.8) | 0.96 (0.89 to 1.03) |
Generally, there was little evidence of differences in the attainment of these QOF indicators between adults with ID and controls in our study sample. Indicators for which adults with ID performed relatively poorer were retinal screening among those with diabetes mellitus (48.8% vs. 56.4%; PR 0.89, 95% CI 0.84 to 0.95) and being seizure free for 12 months those with epilepsy (46.9% vs. 53.7%; PR 0.91, 95% CI 0.83 to 1.00).
Primary care consultations in 2011
The total number of primary care doctor and nurse consultations during 2011 was collated for all adults with ID and their matched controls who were registered on 1 January 2012. The resulting distribution is shown in Figure 21; 86.9% of adults with ID consulted at least once in the year, compared with 72.6% of controls. Approximately one in seven adults with ID (14.9%) averaged at least one consultation per month, more than double the rate seen in controls.
FIGURE 21.
Number of primary care consultations in 2011 in adults with ID and controls.
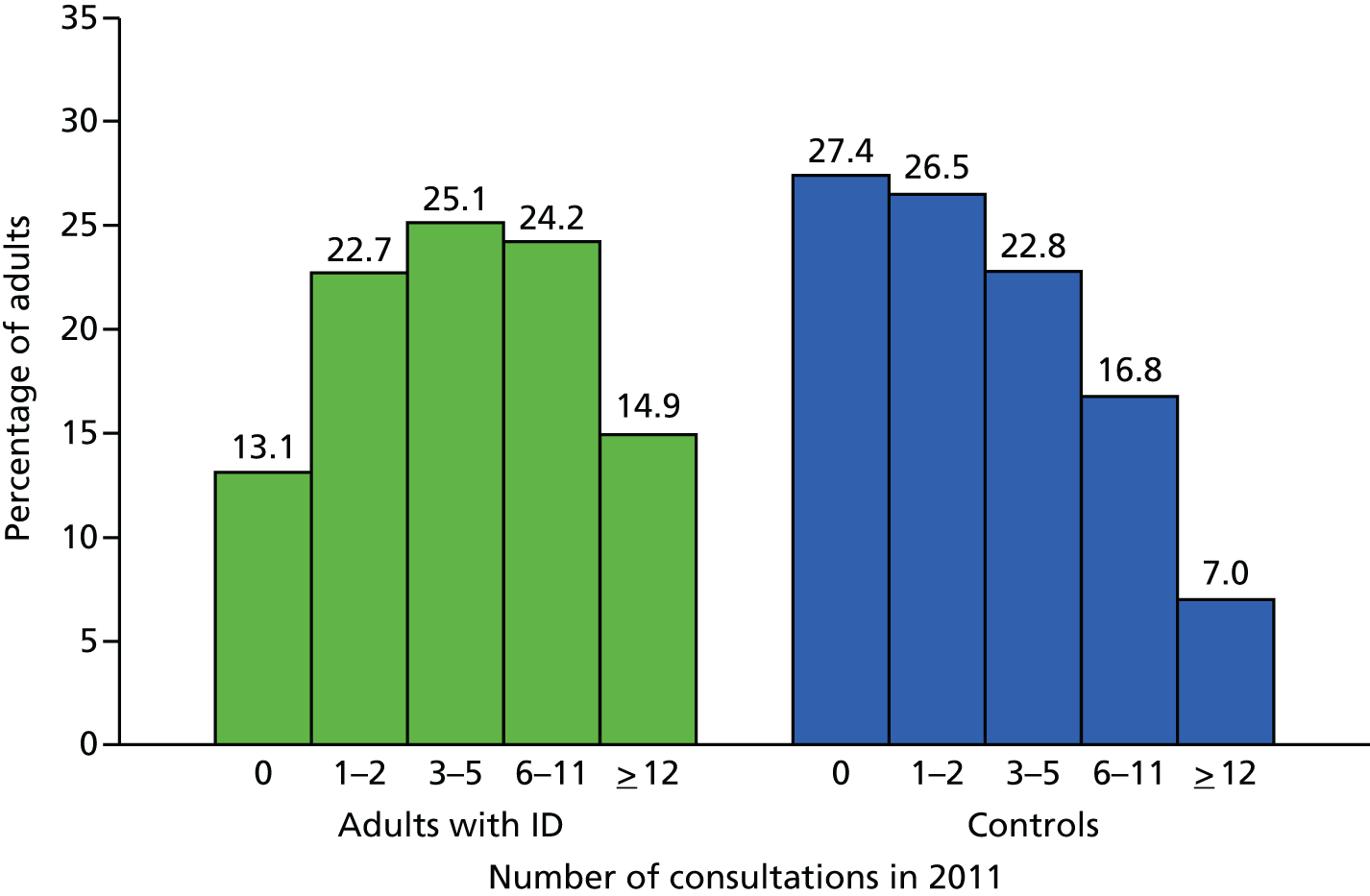
The average number of consultations in 2011 for adults with ID was 6.29 compared with 3.89 in controls (Table 17), an overall rate that was 70% higher. Accounting for greater levels of comorbidity among adults with ID did not explain all of this difference [adjusted rate ratio (RR) 1.49, 95% CI 1.47 to 1.53]. The differences in consultation levels between adults with ID and controls were slightly greater for nurse or telephone consultations and less marked for face-to-face doctor consultations.
| Consultation type | Adults with ID (n = 14,751), mean (SD) | Controls (n = 86,221), mean (SD) | Adults with ID vs. control, RR (95% CI) | |
|---|---|---|---|---|
| RR1a | RR2b | |||
| All consultations | 6.29 (8.33) | 3.89 (5.20) | 1.70 (1.66 to 1.74) | 1.49 (1.47 to 1.53) |
| Telephone | 0.95 (2.56) | 0.44 (1.32) | 2.26 (2.16 to 2.37) | 1.87 (1.78 to 1.97) |
| Doctor | 4.45 (5.81) | 2.88 (3.91) | 1.63 (1.59 to 1.67) | 1.45 (1.41 to 1.48) |
| Doctor (face to face) | 3.65 (4.51) | 2.52 (3.30) | 1.53 (1.50 to 1.56) | 1.37 (1.34 to 1.40) |
| Nurse | 1.84 (4.64) | 1.01 (2.42) | 1.91 (1.83 to 2.00) | 1.64 (1.56 to 1.71) |
The characteristics of all consultations recorded in 2011 are further presented in Figure 22. As a proportion of all consultations, face-to-face consultations were marginally lower among adults with ID (84.9% vs. 88.7%), as telephone consultations were more common (15.1% vs. 11.3%). Similarly, the proportion of all consultations with a doctor was lower in adults with ID (70.8% vs. 73.9%), as nurse consultations were more common (29.2% vs. 26.1%).
FIGURE 22.
Characteristics of primary care consultations in 2011 in adults with ID and controls.

Consultation length was estimated for all consultation during 2011. This was non-zero for approximately 95% of consultations, and was grouped into standard (1–10 minutes) and long length (> 10 minutes). Although adults with ID were more likely to have had a longer doctor consultation at any time during 2011 (51.3% vs. 45.1% for controls; data not shown), the proportion of their consultations that were > 10 minutes was lower (34.7% vs. 42.2%; see Figure 22). Thus, in a logistic regression model (adjusted for comorbidity) that estimates the odds of a long consultation for adults with ID versus controls and takes account of total number of consultations in the year, adults with ID were estimated to be less likely to receive a longer consultation (OR 0.73, 95% CI 0.69 to 0.77).
The mean number of primary care consultations in 2011 was further summarised by subgroup (Figure 23). Women with ID had a greater consultation rate than men with ID, although this trend was similar to that seen in the matched control group. Although consultations increased with deprivation in the general population, this trend was not seen within adults with ID, as those living in the most and least deprived areas had similar consultation rates. Adults with ID living in communal settings had a higher mean level of total consultations during 2011 (7.51), as did those patients with severe health needs (7.46). Lower consultation rates were seen among adults with ID with autism spectrum disorder (4.98) and Down syndrome (5.87).
FIGURE 23.
Mean number of primary care consultations in 2011 in adults with ID and controls by subgroup.

To further assess to what extent the variation in consultations during 2011 by subgroup (see Figure 23) were explained by different underlying characteristics within these groups, a series of Poisson regressions were carried out on adults with ID only (Table 18). These revealed that the higher consultation rate among women was not explained by recorded health needs or other characteristics. However, the higher rate among patients with ID living in communal or shared accommodation was largely attributable to these patients being older and having more severe health needs. The lower consultation rates among patients with ID with autism was explained by them being considerably younger (see Table 5).
| Characteristic of adult with ID | RR1a (95% CI) | RR2b (95% CI) | RR3c (95% CI) |
|---|---|---|---|
| Men vs. women | 0.66 (0.63 to 0.69) | 0.68 (0.66 to 0.71) | 0.69 (0.67 to 0.72) |
| Down syndrome vs. not | 0.92 (0.86 to 0.99) | 0.94 (0.88 to 1.01) | 0.94 (0.87 to 1.01) |
| Severe health needs vs. not | 1.25 (1.19 to 1.32) | 1.17 (1.11 to 1.23) | 1.15 (1.09 to 1.22) |
| Lives in communal accommodation vs. not | 1.26 (1.16 to 1.36) | 1.09 (1.01 to 1.18) | 1.06 (0.97 to 1.14) |
| Autism spectrum disorder vs. not | 0.79 (0.74 to 0.85) | 1.01 (0.94 to 1.09) | 0.98 (0.91 to 1.06) |
Continuity of care among doctor consultations
To assess continuity of care, we restricted analyses to patients who had at least two face-to-face consultations with a doctor during 2011. For each patient we calculated a continuity of care summary measure, defined by whether or not more than half of their consultations had been with the same doctor (see Chapter 2, Definition of a consultation). Table 19 summarises the continuity of care for face-to-face doctor consultations during 2011.
| Consultation type | Adults with ID (N = 14,751), n (%) | Controls (N = 86,221), n (%) | Adults with ID vs. control, OR (95% CI) | |
|---|---|---|---|---|
| OR1a | OR2a | |||
| All adults with ≥ 2 doctor consultations | 9167 | 42,135 | ||
| Number with > 50% with same doctorb | 3962 (43.2) | 20,611 (49.1) | 0.77 (0.73 to 0.81) | 0.77 (0.73 to 0.82) |
| Adults with 2–5 total doctor consultations only | 5906 | 30,332 | ||
| Number with > 50% with same doctorb | 2690 (45.6) | 14,851 (49.0) | 0.87 (0.81 to 0.93) | 0.86 (0.80 to 0.93) |
| Adults with 6–11 total doctor consultations only | 2473 | 9675 | ||
| Number with > 50% with same doctorb | 975 (39.4) | 4713 (48.7) | 0.64 (0.55 to 0.75) | 0.64 (0.54 to 0.75) |
| Adults with ≥ 12 total doctor consultations only | 788 | 2128 | ||
| Number with > 50% with same doctorb | 297 (40.8) | 1109 (52.1) | 0.54 (0.33 to 0.90) | 0.62 (0.36 to 1.06) |
Among the 9167 adults with ID with at least two face-to-face consultations, 43.2% had more than half of their total consultations recorded with the same GP. Although this was higher among the control group (49.1%), the 20,611 controls identified here are strictly no longer a matched set with the 9167 adults with ID. A matched analysis, based on 8677 match-sets in which there was at least one adult with ID and a matched control (n = 27,905) who both had at least two face-to-face doctor consultations, still suggested, however, that adults with ID were less likely to see the same doctor more than half the time in 2011 (adjusted OR 0.77, 95% CI 0.73 to 0.82). This difference was consistent across different total numbers of consultations. For example, among those with at least 12 face-to-face doctor consultations during 2011, 40.8% of adults with ID saw the same doctor for more than half of their consultations compared with 52.1% of controls. This difference was confirmed in adjusted matched regressions, but these were based on very small match-sets as it became increasingly difficult to have the match-sets balanced on total number of consultations.
Economic costings in 2011
Using all available data on the CPRD and HES data sets, we estimated annual NHS costings in 2011 for adults with ID and their matched controls when feasible (see Appendix 5 for more details). As we wanted to factor hospital admissions into the costings, this analysis was based on a subset of the 14,751 adults registered on 1 January 2012 with linked HES data and suitable matched controls. This resulted in a subset of 11,776 adults with ID and 68,428 matched controls.
Table 20 summarises the estimated costs per patient, overall and broken into the individual components in the calculation. An estimated ratio for the costs of adults with ID compared with their matched controls was obtained by conditional negative binomial regressions (see Chapter 2, Statistical analysis). Owing to the non-symmetrical distribution of all of the costing summaries (positively skewed), the model sometimes produced more conservative estimates than the relative mean differences. The estimated mean annual cost for adults with ID in 2011 (£1445.4 per patient) was more than double (RR 2.05, 95% CI 2.01 to 2.10) the estimated costs for the control group (£640.1 per patient). The largest relative discrepancy was seen for primary care prescribing costs (£494.2 per adult with ID vs. £126.6 per control; RR 2.48 95% CI 2.40 to 2.53). Most of the difference in estimated costs for hospital admissions was driven by non-elective (emergency) admissions, for which adults with ID had a more than double estimated cost (£456.4 vs. £186.5 per patient).
| Costed source | Adults with ID (n = 11,776), mean (IQR) | Controls (n = 68,428), mean (IQR) | Adults with ID vs. controls, RRa (95% CI) |
|---|---|---|---|
| GP consultations | 193.0 (37.0–255.3) | 115.2 (0–155.4) | 1.71 (1.67 to 1.75) |
| Nurse consultations | 22.6 (0–26.5) | 10.9 (0–12.4) | 1.95 (1.90 to 2.01) |
| Primary care prescribing | 494.2 (15.8–617.3) | 126.6 (0–79.4) | 2.48 (2.42 to 2.55) |
| Other primary care initiated | 5.7 (0–0) | 3.0 (0–0) | 1.98 (1.84 to 2.13) |
| A&E/casualty | 37.4 (0–0) | 17.6 (0–0) | 1.48 (1.40 to 1.55) |
| Elective hospital admissions | 236.1 (0–0) | 180.4 (0–0) | 1.14 (1.07 to 1.22) |
| Non-elective hospital admissions | 456.4 (0–0) | 186.5 (0–0) | 1.98 (1.86 to 2.10) |
| Total estimated mean cost | 1445.4 (130.0–1360.5) | 640.1 (18.5–418.5) | 2.05 (2.01 to 2.10) |
Annual economic costs were also estimated by subgroup (Figure 24). Although costs were higher for women with ID (£1682.1 vs. £1272.7 per patient), this gender difference was similar in relative terms in the control group. The costs estimated for the youngest (age 18–34 years) group of adults with ID (£1178.8 per patient) still exceeded those estimated for the oldest (age 55–84 years) patients in the control group (£1137.9 per patient). Adults with ID with severe health needs had double the estimated annual costs of those without (£2331.8 vs. £1159.1 per patient).
FIGURE 24.
Mean annual estimated NHS cost per patient in 2011 in adults with ID and controls by subgroup.
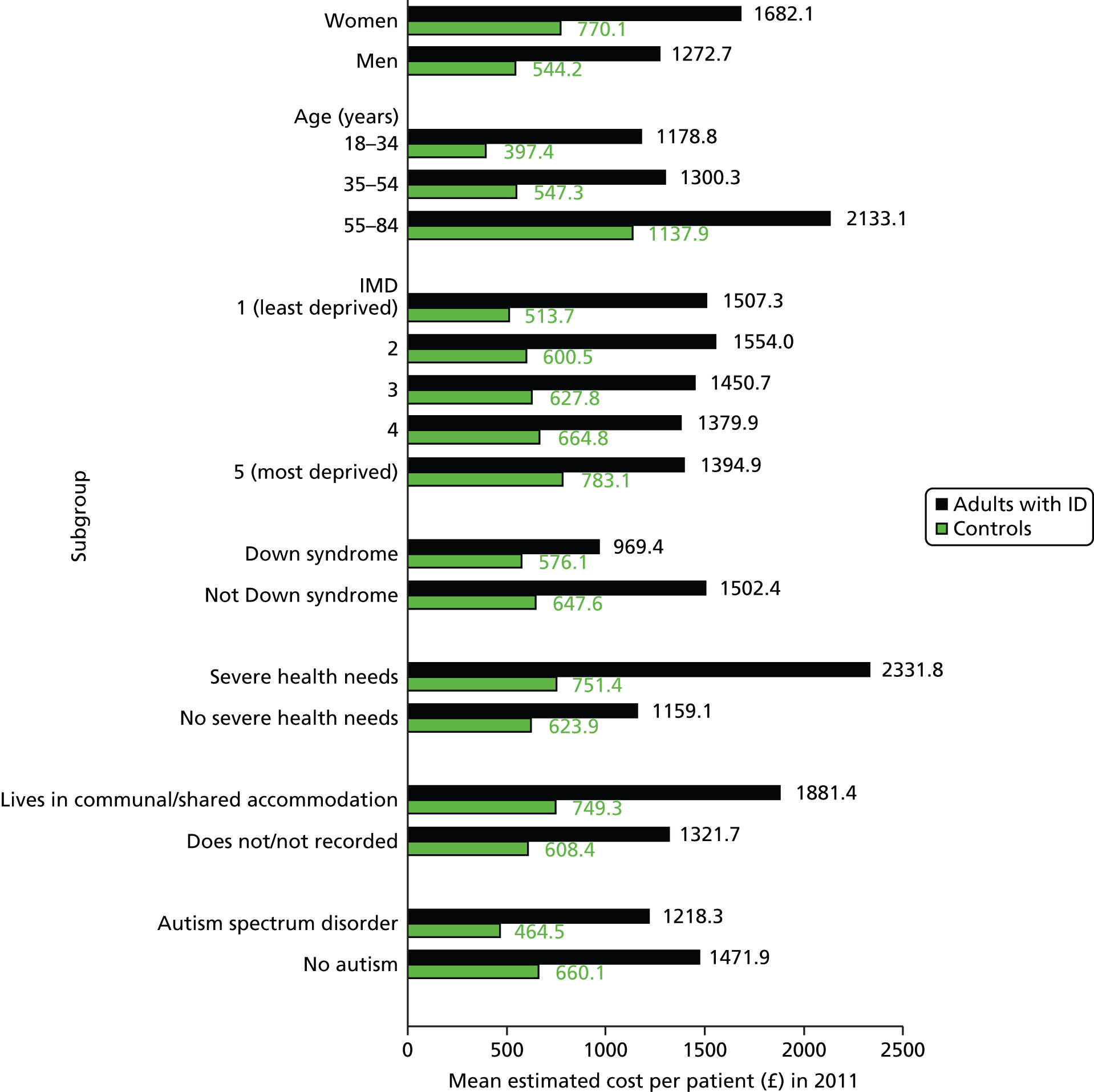
The association between annual NHS costs and deprivation was different between adults with ID and controls. In the general population, costs steadily increased with each quintile of IMD (from £513.7 to £783.1 per patient). However, within adults with ID the trend was not repeated, such that the most deprived group (£1394.9 per patient) had lower costs than the least deprived group (£1507.3 per patient).
The association with deprivation was further explored by stratifying by the accommodation status of the adult with ID (Figure 25). The absence of the trend seen with IMD in the general population was still apparent among adults with ID estimated to be living in the community. However, a much clearer trend towards higher costs with lower levels of deprivation was now seen among adults with ID living in communal accommodation.
FIGURE 25.
Mean annual estimated NHS costs in 2011 in adults with ID and controls by IMD and accommodation status of adult with ID.
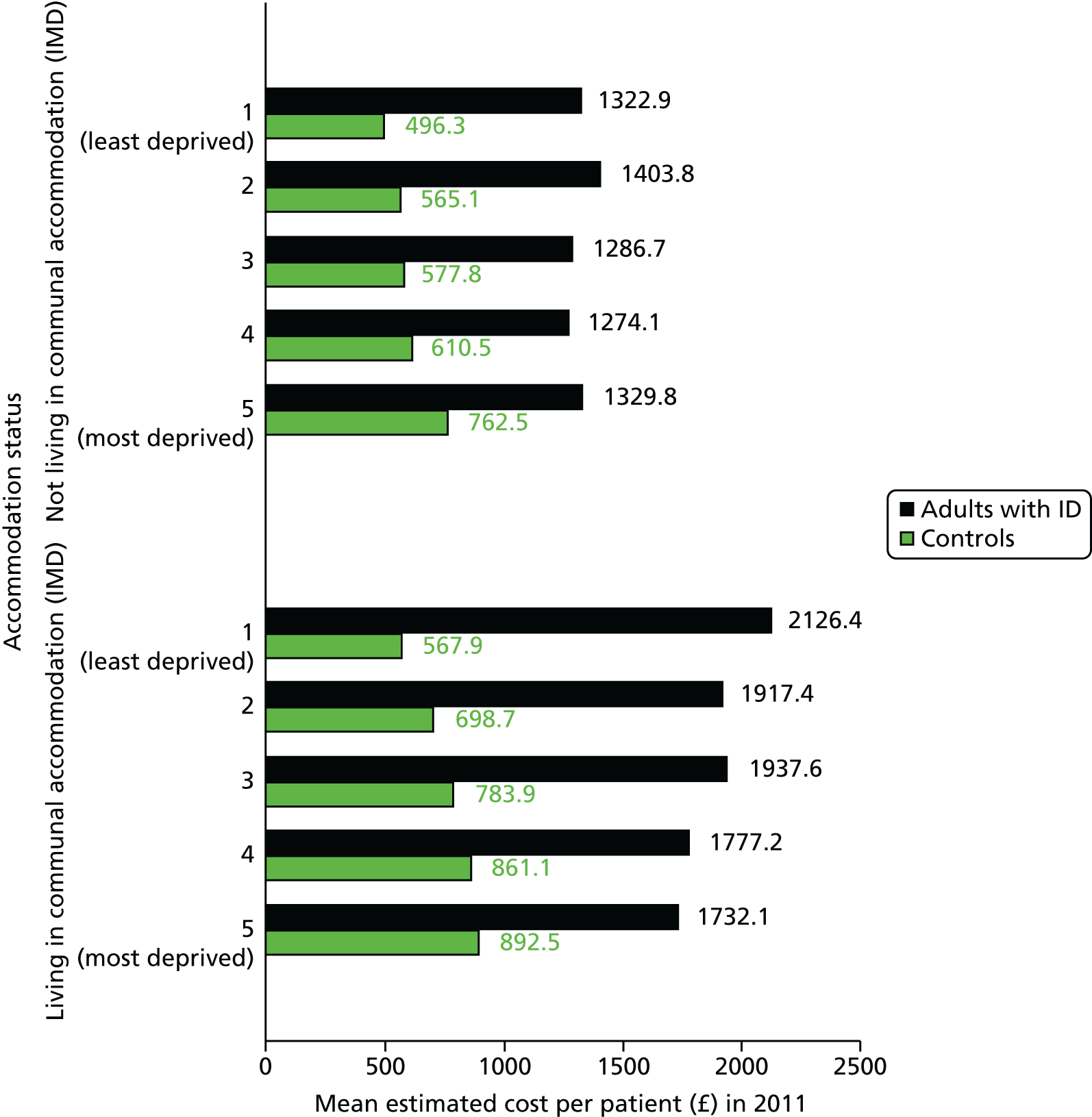
Chapter 4 Mortality
Introduction
As the CPRD data set had been linked to ONS death registration data, it provided an opportunity to describe mortality differences between adults with ID and the age- and gender-matched controls in our study. Although the date of death can be reasonably inferred from CPRD data, cause of death cannot be consistently identified. Therefore, we restricted mortality analyses to the 343 practices with linked data to ONS (see Figure 2). From these practices, a total of 16,666 adults with ID who were aged 18–84 years at the beginning of their follow-up are included (see Figure 2), in addition to the 113,352 age-, gender- and practice-matched controls without ID who were also registered at this point in time.
Some of these results have already appeared in the publication by Hosking et al. ,74 and are reproduced here under the terms of the Open Access licence for non-commercial use with the publisher, the American Public Health Association. Hosking FJ, Carey IM, Shah SM, Harris T, DeWilde S, Beighton C, Cook DG. Mortality among adults with intellectual disability in England: comparisons with the general population. Am J Public Health 2016;106:1483–90. Available at: http://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.2016.303240
Longitudinal design
More details of the longitudinal design for the analysis we devised are shown in Figure 26. All patients had to be registered for at least 30 days before they were eligible for follow-up. We define follow-up to run from 1 January 2009 to 31 March 2013. Of the 16,666 adults with ID included, the majority (n = 11,973) were already registered by 1 January 2009 and were aged ≥ 18 years. To this core group we made two additions to the analysis cohort. First, patients with ID registered by 1 January 2009 but who were not aged 18 years by then (n = 1027) were allowed entry into the cohort on 1 January of the year that they turned 18 (assuming they were still registered at the practice). Second, adults with ID (n = 3666) who were not registered with their practice on 1 January 2009 but subsequently registered some time during the study follow-up (2009–12) were included from the point at which they had been registered with the study practice for 30 days. Matched controls (n = 113,562) were included only if they had been registered at the defined entry point of the cohort for the adult with ID.
FIGURE 26.
Summary of how the longitudinal cohort was constructed.
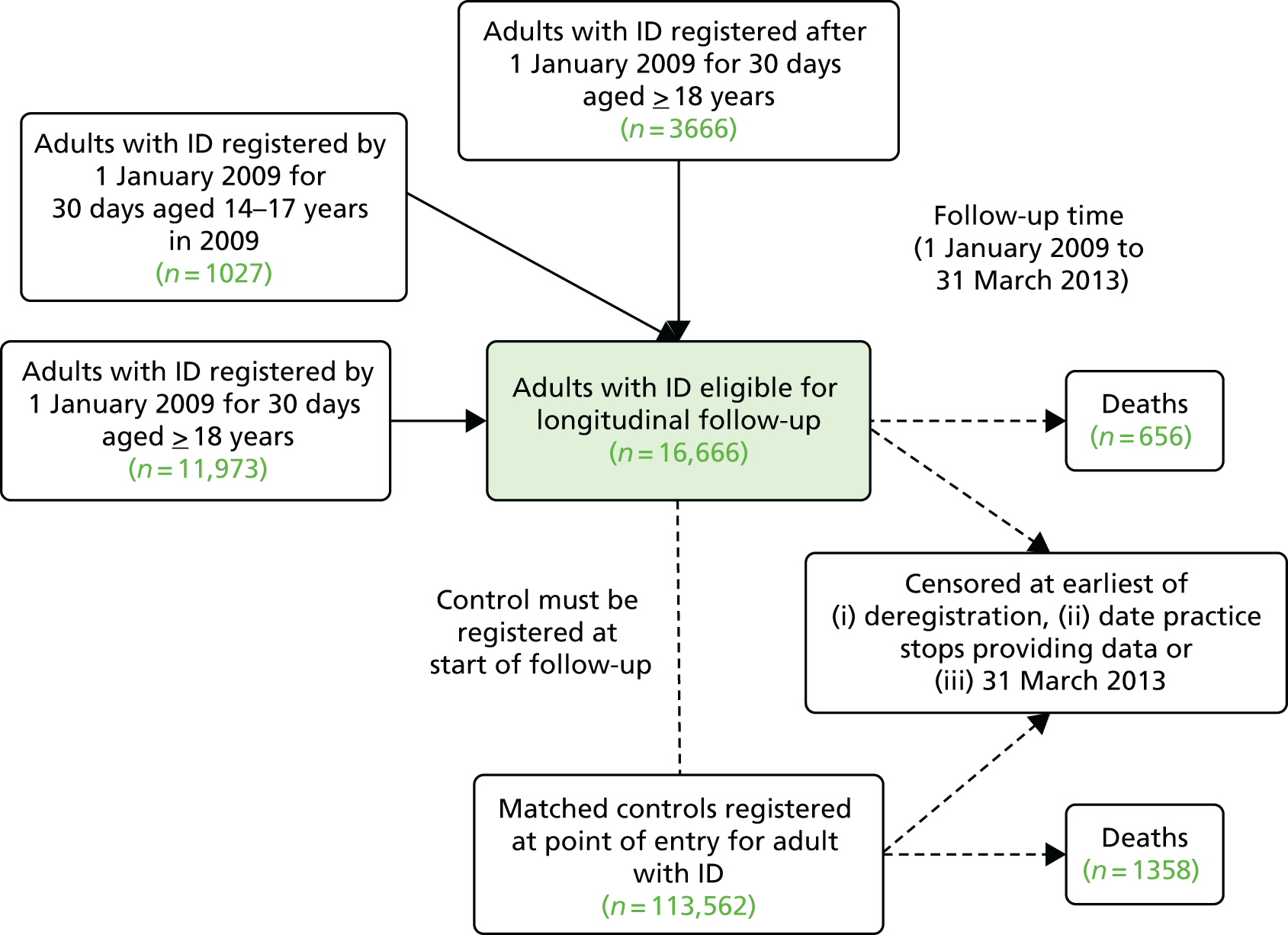
All adults in the longitudinal cohort were followed to the earliest recorded event representing (1) date of death, (2) date of deregistration from the practice, (3) date when their practice stopped providing data to CPRD or (4) 31 March 2013 (see Figure 26). Controls within a match-set were still followed to their end-point date even if their matched adult with ID had exited the cohort earlier. The average length of follow-up for all individuals was approximately 3 years (1097 days).
Primarily, the date of death recorded on the ONS record was used for the majority of deaths. However, we used the date derived from the CPRD record if it was clear that a patient had received no further primary care contact after this date. This inconsistency was often only a matter of a few days, but for a small number of deaths it was approximately 1 year as it appeared that the year of death had been incorrectly recorded on the ONS record and was wrong by one digit (e.g. 2011 rather than 2010).
Cause of death and avoidable mortality
Underlying cause of death was derived from the ONS death registration data for patients who died during the study (656 adults with ID and 1358 controls). For 38 (2%) of these deaths, we were unable to obtain cause of death from the ONS record. A full list of the ICD-10 codes used to group cause of death is in Appendix 7. When examining how often ID is recorded on death certificates, we searched all recorded main and contributory causes of death for ID-associated codes, including an extended range of conditions weakly associated with ID, such as cerebral palsy. 75
Using the recorded cause of death, we further classified deaths as being potentially avoidable. We followed ONS guidelines that have used underlying cause of death to identify where scope exists for intervention to reduce mortality. 76 Potentially avoidable deaths have been further classified as being either (1) amenable to good-quality health care (treatable) or (2) preventable through public health action, or both. These definitions primarily include deaths aged < 75 years except for accidental deaths. For example, deaths due to asthma are identified as amenable to health care through effective long-term treatment, whereas deaths due to lung cancer are identified as preventable through tobacco control. As some causes of death are defined as both amenable and preventable (e.g. IHD), potentially avoidable mortality is smaller than the sum of amenable and preventable mortality.
Characteristics of adults with intellectual disability in longitudinal analyses
Table 21 summarises the characteristics of the 16,666 adults with ID who are included in the longitudinal analyses. Fifty-eight per cent were men, an identical figure to that seen in the cross-sectional analyses (see Table 5). What differed slightly in these analyses was how we defined a patient’s age. We classified age here by the recorded age in the year of entry to the cohort, which was primarily 2009. Therefore, the average age of the longitudinal cohort is summarised as 39.9 years, whereas in the cross-sectional analysis, based on a 1 January 2012 date, it was 42.1 years.
| Characteristic | Adults with ID | Controls | ||
|---|---|---|---|---|
| n | Men (%) | Mean age in years (SD) | n | |
| All | 16,666 | 58.1 | 39.9 (16.2) | 113,562 |
| Gender | ||||
| Women | 6989 | 0 | 41.3 (16.4) | 47,587 |
| Men | 9677 | 100 | 38.8 (15.9) | 65,975 |
| Age (years) (at baseline) | ||||
| 18–34 | 6981 | 61.2 | 24.2 (5.1) | 46,939 |
| 35–54 | 6283 | 57.4 | 44.2 (5.4) | 43,123 |
| 55–84 | 3402 | 52.9 | 64.0 (7.1) | 23,500 |
| Down syndromea | ||||
| Yes | 1793 | 55.0 | 39.1 (14.4) | 12,226 |
| No | 14,873 | 58.4 | 40.0 (16.4) | 101,336 |
| Severe health needsa | ||||
| Yes | 3263 | 54.4 | 41.4 (16.4) | 22,298 |
| No | 13,403 | 59.0 | 39.5 (16.1) | 91,264 |
| Communal accommodationa | ||||
| Yes | 3392 | 57.2 | 47.2 (15.7) | 23,117 |
| No | 13,274 | 58.3 | 38.0 (15.8) | 90,445 |
| Autism spectrum disordera | ||||
| Yes | 1532 | 73.2 | 30.5 (13.3) | 10,387 |
| No | 15,134 | 56.5 | 40.8 (16.1) | 103,188 |
| Epilepsya | ||||
| Yes | 2884 | 55.4 | 41.0 (15.3) | 19,705 |
| No | 13,782 | 58.6 | 39.6 (16.3) | 93,857 |
All-cause mortality
During follow-up from 1 January 2009 to 31 March 2013, a total of 656 (3.94%) adults with ID died compared with 1358 (1.20%) of the matched controls (Table 22). The crude mortality rate was 132.4 per 10,000 persons per year for adults with ID, compared with 39.7 per 10,000 persons per year for controls. Among adults with ID, there were elevated death rates among those with Down syndrome (6.58%, 220.0 per 10,000 persons per year), those with high support needs (5.94%, 190.2 per 10,000 persons per year), those with epilepsy (5.79%, 188.0 per 10,000 persons per year) and those living in communal/shared accommodation (7.8%, 254.7 per 10,000 persons per year). There were fewer deaths among the primarily younger subgroup with autism (0.98%, 36.3 per 10,000 persons per year).
| Characteristic | Adults with ID (N = 16,666) | Controls (N = 113,562) | ||
|---|---|---|---|---|
| n (%) | Rate per 10,000 | n (%) | Rate per 10,000 | |
| All | 656 (3.94) | 132.4 | 1358 (1.20) | 39.7 |
| Gender | ||||
| Women | 291 (4.16) | 139.5 | 538 (1.13) | 37.5 |
| Men | 365 (3.77) | 127.3 | 820 (1.20) | 41.5 |
| Age (years) (at baseline) | ||||
| 18–34 | 48 (0.69) | 25.3 | 69 (0.15) | 5.6 |
| 35–54 | 167 (2.66) | 83.1 | 276 (0.64) | 19.6 |
| 55–84 | 441 (12.69) | 420.0 | 1013 (4.31) | 129.6 |
| Down syndromea | ||||
| Yes | 118 (6.58) | 220.0 | 92 (0.75) | 24.9 |
| No | 538 (3.62) | 121.8 | 1266 (1.25) | 41.6 |
| Severe health needsa | ||||
| Yes | 194 (5.94) | 190.2 | 302 (1.35) | 43.9 |
| No | 462 (3.45) | 117.4 | 1056 (1.16) | 38.7 |
| Communal accommodationa | ||||
| Yes | 265 (7.81) | 254.7 | 416 (1.80) | 56.5 |
| No | 391 (2.90) | 99.9 | 942 (1.04) | 35.1 |
| Autism spectrum disordera | ||||
| Yes | 15 (0.98) | 36.3 | 44 (0.42) | 16.0 |
| No | 641 (4.24) | 141.2 | 1314 (1.27) | 41.8 |
| Epilepsya | ||||
| Yes | 167 (5.79) | 188.0 | 205 (1.04) | 33.7 |
| No | 498 (3.55) | 120.3 | 1153 (1.23) | 41.0 |
Hazard ratios (unadjusted and adjusted for comorbidity, smoking and deprivation) for all-cause mortality are shown in Table 23. The overall HR of 3.62 (95% CI 3.33 to 3.93) for adults with ID versus controls was only partially explained by observed differences in comorbidity between the groups (adjusted HR 3.05, 95% CI 2.73 to 3.41). Although the HR for all-cause mortality was higher for men than for women, this difference was not statistically significant after adjustment (p = 0.07). The higher mortality risk among adults with ID was seen at all ages. Prior to adjustment, the largest disparity between adults with ID and controls was among the youngest ages (18–34 years), but the opposite was true after adjusting for comorbidity and other factors. However, these age differences were not significant in either comparison.
| Characteristic | Base (unadjusted) model | Adjusted modela | ||
|---|---|---|---|---|
| HR (95% CI) | p-valueb | HR (95% CI) | p-valueb | |
| All | 3.62 (3.33 to 3.93) | – | 3.05 (2.73 to 3.41) | – |
| Gender | ||||
| Women | 4.10 (3.61 to 4.66) | 0.01 | 3.50 (2.94 to 4.16) | 0.07 |
| Men | 3.30 (2.96 to 3.68) | 2.81 (2.43 to 3.24) | ||
| Age (years) (at baseline) | ||||
| 18–34 | 4.29 (3.13 to 5.88) | – | 2.43 (1.56 to 3.77) | – |
| 35–54 | 4.17 (3.52 to 4.92) | 0.88 | 3.22 (2.53 to 4.08) | 0.25 |
| 55–84 | 3.39 (3.07 to 3.75) | 0.21 | 3.03 (2.65 to 3.46) | 0.32 |
| Down syndromec | ||||
| Yes | 9.21 (7.22 to 11.76) | < 0.001 | 10.39 (7.13 to 15.13) | < 0.001 |
| No | 3.19 (2.92 to 3.49) | 2.66 (2.36 to 3.00) | ||
| Severe health needsc | ||||
| Yes | 4.77 (4.08 to 5.59) | < 0.001 | 4.95 (4.03 to 6.07) | 0.001 |
| No | 3.28 (2.98 to 3.62) | 3.15 (2.79 to 3.55) | ||
| Communal accommodationc | ||||
| Yes | 4.99 (4.36 to 5.73) | < 0.001 | 4.30 (3.52 to 5.26) | < 0.001 |
| No | 3.05 (2.74 to 3.39) | 2.64 (2.30 to 3.02) | ||
| Autism spectrum disorderc | ||||
| Yes | 2.39 (1.45 to 3.96) | 0.05 | 2.22 (1.01 to 4.86) | 0.40 |
| No | 3.66 (3.37 to 3.98) | 3.07 (2.74 to 3.43) | ||
| Epilepsyc | ||||
| Yes | 6.04 (5.04 to 7.24) | < 0.001 | 7.76 (6.10 to 9.86) | < 0.001 |
| No | 3.18 (2.90 to 3.50) | 2.91 (2.60 to 3.27) | ||
Among adults with ID, those with Down syndrome had a very high relative risk of death compared with controls (HR 9.21, 95% CI 7.22 to 11.76), which was significantly different from the risk of death seen in adults with ID without Down syndrome (p < 0.001) and was not explained by further adjustment. Similarly, adults with ID with severe support needs had a death rate nearly five times higher than that of their controls (HR 4.77, 95% CI 4.08 to 5.59), which was significantly different from that of adults with ID without severe health needs both before and after adjustment (p ≤ 0.001). The same was true for adults with ID recorded living in communal/shared living who had a similarly elevated death rate to their controls (HR 4.99, 95% CI 4.36 to 5.73). Within the population with ID, epilepsy was a strong determinant of mortality risk, relative both to the controls (HR 6.04, 95% CI 5.04 to 7.24) and to other adults with ID without epilepsy (p < 0.001).
The differences in mortality between subgroups was further investigated in additional (unmatched) analyses that directly compared adults with ID in each subgroup (Table 24) and adjusted for age, gender and other confounders. These confirmed the earlier findings in Table 23. For example, an adult with ID with Down syndrome had a risk of death nearly three times as high (HR 2.91, 95% CI 2.31 to 3.66) as that for an adult with ID without Down syndrome. Adults with ID living in communal accommodation, with severe health needs or with epilepsy had risks of death that were, respectively, 44%, 52% and 73% higher than that for adults with ID without each of those criteria. Adults with ID with autism were at lower risk of death (HR 0.56, 95% CI 0.34 to 0.94) than adults with ID without autism.
| Characteristic | Adults with ID, n | Base model,a HR (95% CI) | Adjusted model,b HR (95% CI) |
|---|---|---|---|
| Down syndrome | |||
| Yes | 1793 | 2.92 (2.37 to 3.59) | 2.91 (2.31 to 3.66) |
| No | 14,873 | 1 | 1 |
| Severe health needsc | |||
| Yes | 3263 | 1.48 (1.23 to 1.77) | 1.52 (1.27 to 1.83) |
| No | 13,403 | 1 | 1 |
| Communal accommodationc | |||
| Yes | 3392 | 1.60 (1.33 to 1.92) | 1.44 (1.19 to 1.74) |
| No | 13,274 | 1 | 1 |
| Autism spectrum disorder | |||
| Yes | 1532 | 0.55 (0.34 to 0.90) | 0.56 (0.34 to 0.94) |
| No | 15,134 | 1 | 1 |
| Epilepsy | |||
| Yes | 2884 | 1.64 (1.37 to 1.97) | 1.73 (1.43 to 2.09) |
| No | 13,782 | 1 | 1 |
Cause-specific mortality
The higher mortality risk in adults with ID produced different patterns of cause-specific mortality when compared with the matched controls (Figure 27). In adults with ID, the most common causes of mortality were circulatory diseases (22%), respiratory diseases (19%), neoplasms (15%) and nervous system diseases (12%). This is different from the pattern in controls, in whom neoplasms (37%), circulatory (27%), respiratory (10%) and external causes (8%) were the most common causes.
FIGURE 27.
Recorded causes of death during 2009–13 in (a) adults with ID and (b) controls.
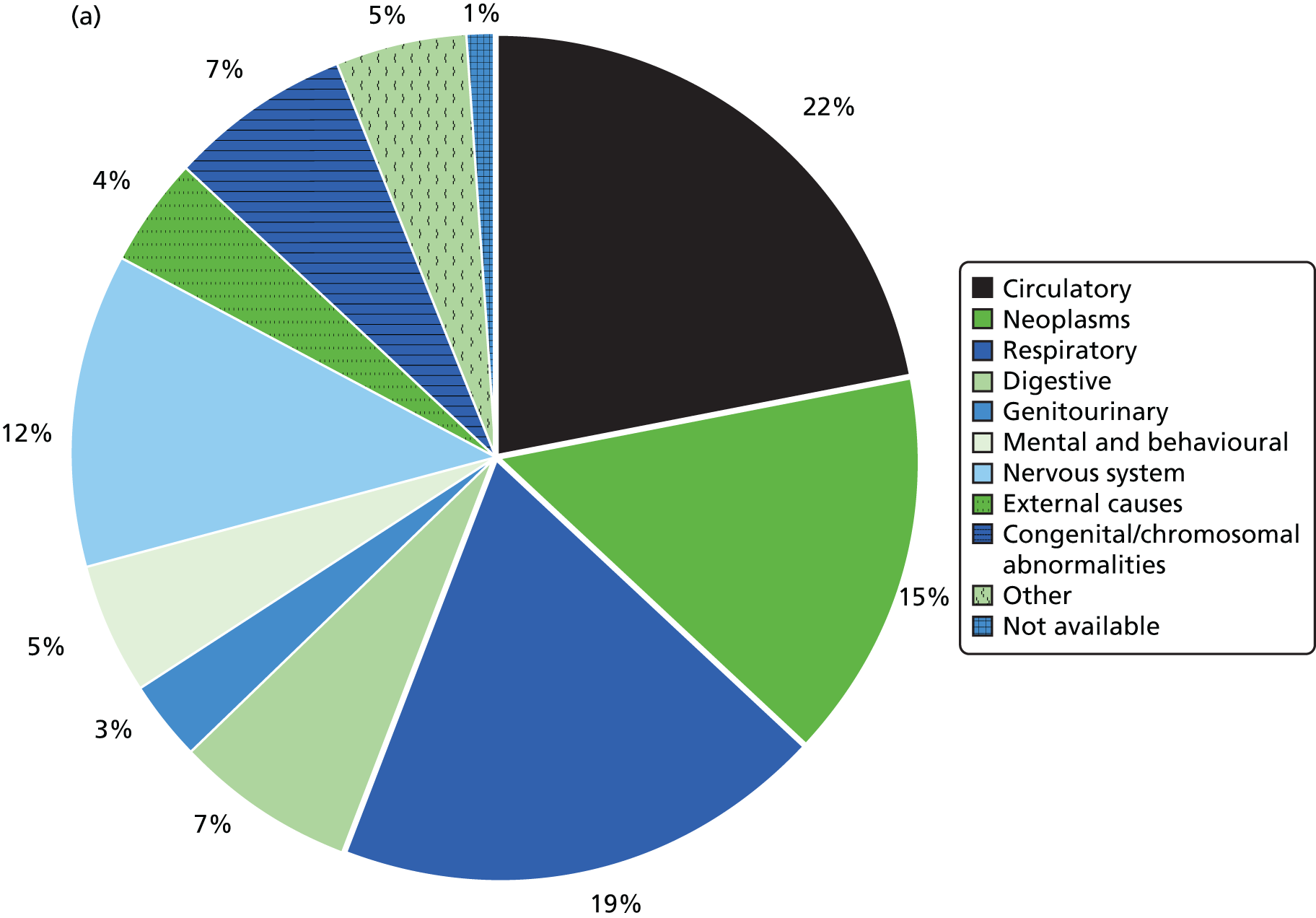
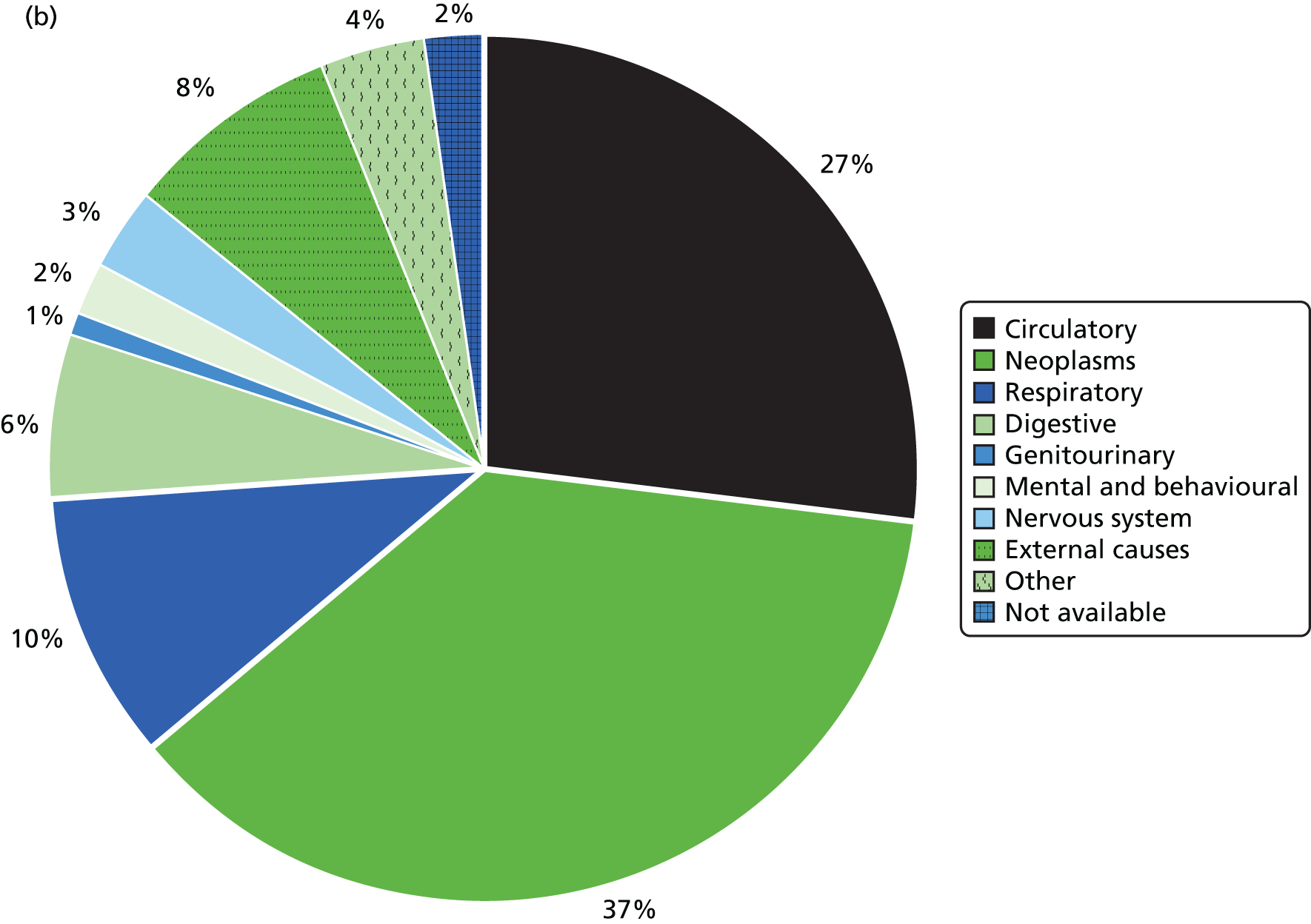
Cause of death is explored in more detail in Table 25, which shows the number and rate (per 10,000 persons per year) of deaths for adults with ID and controls for main causes, and for specific subgroups of these when numbers allow. Notable specific contributions to mortality among people with ID, in comparison with controls, were dementia (n = 27; rate = 5.5 vs. 0.5 per 10,000 persons per year), epilepsy (n = 29; rate = 5.9 vs. 0.1 per 10,000 persons per year), pneumonia (n = 67; rate = 13.5 vs. 1.1 per 10,000 persons per year) and aspiration pneumonitis (n = 21; rate = 4.2 vs. 0.2 per 10,000 persons per year). On the other hand, transport accidents (n = 1) and intentional self-harm (n = 0) were rarely or non-existent recorded causes of death among adults with ID.
| Cause of death | Adults with ID (N = 16,666) | Controls (N = 113,562) | ||
|---|---|---|---|---|
| n | Rate per 10,000 | n | Rate per 10,000 | |
| Infectious and parasitic disorders | 3 | 0.6 | 14 | 0.4 |
| Neoplasms | 98 | 19.8 | 508 | 14.9 |
| Oesophageal | 0 | 0.0 | 16 | 0.5 |
| Colorectal | 17 | 3.4 | 44 | 1.3 |
| Pancreatic | 6 | 1.2 | 22 | 0.6 |
| Lung | 10 | 2.0 | 117 | 3.4 |
| Breast | 7 | 1.4 | 36 | 1.1 |
| Prostate | 2 | 0.4 | 28 | 0.8 |
| Urinary tract | 2 | 0.4 | 30 | 0.9 |
| Lymphoma | 10 | 2.0 | 40 | 1.2 |
| Endocrine, nutritional and metabolic diseases | 13 | 2.6 | 16 | 0.5 |
| Mental and behavioural disorders | 35 | 7.1 | 31 | 0.9 |
| Dementia | 27 | 5.5 | 17 | 0.5 |
| Diseases of the nervous system | 76 | 15.3 | 39 | 1.1 |
| Epilepsy | 29 | 5.9 | 3 | 0.1 |
| Diseases of the circulatory system | 142 | 28.7 | 360 | 10.5 |
| IHD | 62 | 12.5 | 188 | 5.5 |
| Cerebrovascular disease | 34 | 6.9 | 57 | 1.7 |
| Diseases of the respiratory system | 123 | 24.8 | 135 | 3.9 |
| Pneumonia | 67 | 13.5 | 39 | 1.1 |
| COPD | 19 | 3.8 | 59 | 1.7 |
| Aspiration pneumonitis | 21 | 4.2 | 6 | 0.2 |
| Diseases of the digestive system | 46 | 9.3 | 87 | 2.5 |
| Liver disease | 8 | 1.6 | 44 | 1.3 |
| Diseases of the musculoskeletal system | 6 | 1.2 | 8 | 0.2 |
| Diseases of the genitourinary system | 23 | 4.6 | 15 | 0.4 |
| Congenital/chromosomal abnormalities | 45 | 9.1 | 2 | 0.06 |
| External causes of morbidity | 27 | 5.5 | 101 | 3.0 |
| Transport accidents | 1 | 0.2 | 20 | 0.6 |
| Other external causes of accidental injury | 20 | 4.0 | 31 | 0.9 |
| Intentional self-harm | 0 | 0.0 | 35 | 1.0 |
| Other (skin, blood diseases, residual codes) | 10 | 2.0 | 13 | 0.4 |
| Not available | 9 | 1.8 | 29 | 0.8 |
Although cancer (neoplasms) as a cause of death represented a lower proportion of all deaths among adults with ID (see Figure 27), the death rate from cancer overall was marginally higher for adults with ID (19.8 vs. 14.9 per 10,000 per year) (Table 25). There was, however, some variation in types of cancer recorded as the cause of death. Colorectal cancer (n = 17) was the most commonly recorded cause among adults with ID, whereas among the matched controls lung cancer (n = 117) was far more frequent. Urinary tract cancers (n = 2), prostate cancer (n = 2) and oesophageal cancer (n = 0) were rarely recorded causes of death among adults with ID.
The most common underlying cause of death in adults with ID with Down syndrome (n = 118) was respiratory diseases (n = 24, 20%). For an additional 30 adults with ID who died (25%), Down syndrome or other chromosomal abnormalities was given as the underlying cause. Almost all of these adults (n = 26) had respiratory disease listed as a secondary cause of death. If these 26 deaths were assumed to be due to respiratory disease, then the percentage of deaths of adults with Down syndrome caused by respiratory diseases would rise to 42%.
Hazard ratios for selected grouped causes of death are shown in Table 26. These are presented for the unadjusted model only, which accounts for age and gender differences via the matching. These were calculated for both the main groupings (e.g. neoplasms) and, when possible, the subgroups (e.g. colorectal cancer). It was not possible to calculate a HR for deaths from congenital or chromosomal abnormalities owing to the small number of control deaths.
| Cause of death | Base (unadjusted) model, HR (95% CI) |
|---|---|
| Infectious and parasitic disorders | 2.30 (0.70 to 7.48) |
| Neoplasms | 1.44 (1.18 to 1.76) |
| Oesophageal | a |
| Colorectal | 2.82 (1.71 to 4.63) |
| Pancreatic | 1.92 (0.89 to 4.14) |
| Lung | 0.69 (0.37 to 1.28) |
| Breast | 1.42 (0.69 to 2.94) |
| Prostate | 0.54 (0.13 to 2.19) |
| Urinary tract | 0.90 (0.15 to 2.37) |
| Lymphoma | 1.72 (0.91 to 3.26) |
| Endocrine, nutritional and metabolic diseases | 5.38 (2.79 to 10.07) |
| Mental and behavioural disorders | 7.99 (5.19 to 12.31) |
| Dementia | 12.18 (6.84 to 21.69) |
| Diseases of the nervous system | 13.79 (9.70 to 19.62) |
| Epilepsy | 180.6 (24.9 to 1308.2) |
| Diseases of the circulatory system | 3.05 (2.56 to 3.64) |
| IHD | 2.50 (1.93 to 3.23) |
| Cerebrovascular disease | 4.88 (3.34 to 7.12) |
| Diseases of the respiratory system | 6.68 (5.38 to 8.29) |
| Pneumonia | 13.09 (9.09 to 18.87) |
| COPD | 2.43 (1.52 to 3.87) |
| Aspiration pneumonitis | 27.73 (11.48 to 66.95) |
| Diseases of the digestive system | 4.02 (2.92 to 5.54) |
| Liver disease | 1.31 (0.65 to 2.66) |
| Diseases of the musculoskeletal system | 5.50 (2.22 to 13.61) |
| Diseases of the genitourinary system | 10.89 (6.09 to 9.47) |
| Congenital/chromosomal abnormalities | a |
| External causes of morbidity | 1.85 (1.26 to 2.71) |
| Transport accidents | 0.32 (0.05 to 2.26) |
| Other external causes of accidental injury | 4.94 (3.02 to 8.07) |
| Intentional self-harm | a |
| Other (skin, blood diseases, residual codes) | 5.03 (2.40 to 10.54) |
| Not available | 2.27 (1.19 to 4.43) |
The largest (estimable) relative difference in risk of death between adults with ID and adults without ID, for the main groups, was seen for nervous system disorders, primarily epilepsy (HR 13.79, 95% CI 9.70 to 19.62), followed by diseases of the genitourinary system, including UTIs (HR 10.89, 95% CI 6.09 to 19.47). Other notable disparities were seen for diseases of the respiratory system (HR 6.68, 95% CI 5.38 to 8.29), with aspiration pneumonitis (HR 27.73) and pneumonia deaths (HR 13.09) being key contributors, and mental and behaviour disorders (HR 7.99, 95% CI 5.19 to 12.31), which were influenced by the higher risk of dementia-related deaths (HR 12.18).
Although deaths from cancer represented a smaller proportion of deaths among adults with ID than among the general population, the overall risk of death from neoplasms was still marginally higher (HR 1.44, 95% CI 1.18 to 1.76). Cancer-specific estimates were imprecise owing to the small number of deaths with each type, but deaths from colorectal cancer were notably higher for adults with ID (HR 2.82, 95% CI 1.71 to 4.63). Deaths from lung and prostate cancer both produced a HR of < 0.7, but the CIs were wide.
Potentially avoidable mortality
The proportion of all deaths classified as potentially avoidable (amenable and/or preventable) was similar in adults with ID (n = 304, 46.3%) and controls (n = 645, 47.5%). However, individually, the proportion of amenable and preventable deaths differed between the two groups (Figure 28). Within adults with ID, the percentage of amenable deaths (n = 243, 37.0%) was notably higher than that seen in controls (n = 305, 22.5%). This difference is reflected in a large estimated HR (5.86, 95% CI 5.06 to 6.80) for deaths amenable to health care among adults with ID versus controls. This may be an underestimate, as standard ONS definitions do not include a number of causes of deaths in people with ID that may be considered amenable, such as deaths from UTI (n = 12, 1.7%) and aspiration pneumonitis (n = 21, 3.1%).
FIGURE 28.
Amenable and preventable mortality during 2009–13 among adults with ID and controls. Note that total areas inside the squares are proportional to the mortality rates for each subgroup; rates given are per 10,000 people per year. The overall subgroup of avoidable mortality is the total area covered by amenable and/or preventable [ID rate = 61.4 (46.3% of all mortality) and control rate = 18.9 (47.5%); HR 3.44, 95% CI 3.05 to 3.89]. Figure redrawn from Hosking et al. 74 Hosking FJ, Carey IM, Shah SM, Harris T, DeWilde S, Beighton C, Cook DG. Mortality among adults with intellectual disability in England: comparisons with the general population. Am J Public Health 2016;106:1483–90. Available at: http://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.2016.303240
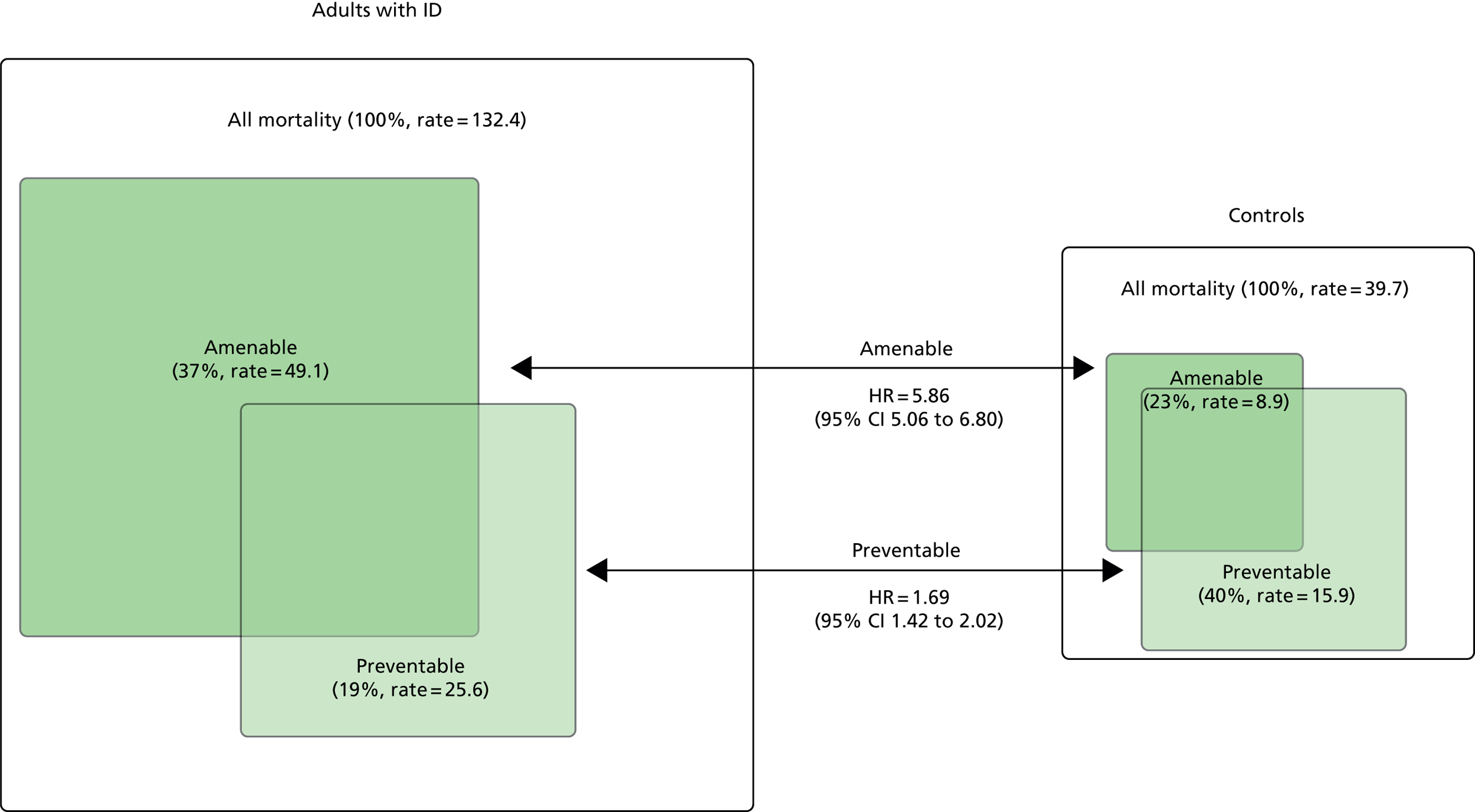
The pattern in preventable deaths was different, with the proportion being smaller among adults with ID (n = 127, 19.4%) than among controls (n = 543, 40.0%). However, preventable deaths were still marginally more likely overall among adults with ID (HR 1.69, 95% CI 1.42 to 2.02).
Recording of intellectual disability on death certificates
Finally, we electronically searched the linked ONS death certification data for any mention of ID or a related condition, as either a main or a contributory cause of death. Only 200 (30.9%) of the linked 647 deaths had any such mention. Therefore, for 7 in 10 deaths among adults with ID there was no mention of their ID on their death certificate. For those with a recorded cause associated with ID, the most commonly listed were Down syndrome (n = 88), cerebral palsy (n = 39) and developmental disorder of scholastic skills (n = 50).
Chapter 5 Hospital admissions
Introduction
In this section, we use the linked hospital admissions data from the HES data set to provide a summary of hospitalisations during our study for adults with ID, and to compare the volume and type of admissions with those of the matched controls. We also take advantage of the linkage by comparing the primary care record prior to admission for two infections, UTIs and LRTIs, which we suspected would be common in both adults with ID and the general population. 41
Analyses are, again, based on 343 practices with linked data (see Figure 2). We used the same longitudinal design that was introduced for mortality analyses in Chapter 4, involving a total of 16,666 adults with ID and 113,562 age-, gender- and practice-matched controls without ID (see Figure 26). Follow-up was from 1 January 2009 to a maximum date of 31 March 2013, with the average length of follow-up for all individuals being approximately 3 years (1097 days). The characteristics of the adults with ID and controls used in the analysis have been described in Table 21.
Some of these results have already appeared in the publication by Hosking et al. 77 Reproduced with permission from Preventable Emergency Hospital Admissions Among Adults with Intellectual Disability in England, September/October 2017, Vol. 15, No. 5, Annals of Family Medicine © 2017 Annals of Family Medicine, Inc.
Categorising admissions
The HES data set contains information on every admission to a NHS hospital in England. 78 This includes information on the date, duration and type (e.g. elective) of admission and the primary reason for admission (coded using ICD-1058). Although multiple episodes can sometimes occur within a continuous period of hospitalisation (such as when a patient is transferred to a different consultant), we decided to focus solely on the initial episode as we were interested in the reason for admission that this represented. 41
We categorised admissions, using the method of admission variable ADMIMETH,79 into the following groups: emergency, elective, maternity and other (such as transfers from other hospital providers). Within emergency admissions, we further identified a subgroup of admissions for ACSCs,41 which represent a group thought to be potentially preventable with better clinical management. We included 20 widely used ACSCs, but also considered an additional five conditions relevant to the population with ID. 13,80 These were constipation, aspiration, gastro-oesophageal reflux disease, osteoporosis and schizophrenia. We chose not to use osteoporosis, as it was rarely recorded as the primary reason for admission, or schizophrenia, owing to the idiosyncratic recording of elective versus emergency for many English psychiatric admissions. 12 This resulted in a total of 23 ACSCs (see Appendix 8).
For elective admissions, a small number of patients were receiving regular elective hospital procedures (e.g. dialysis) during the study and their inclusion was potentially problematic for calculating an overall rate. We made the pragmatic choice to exclude patients in our analyses of elective admission rates who averaged more than six elective admissions per year. This represented about 0.20% of the cohort (adults with ID, n = 32; controls, n = 233).
Summary of overall admissions
Admission rates (per 1000 persons per year) by type are shown in Figure 29. The overall rate for adults with ID was 351.6 per 1000 persons per year, compared with 246.4 per 1000 persons per year for controls. This difference was essentially due to the higher rate among emergency admissions (182.2 vs. 67.7 per 1000 persons per year), as elective rates were similar between groups.
FIGURE 29.
Hospital admissions rates during 2009–13 for adults with ID and controls.
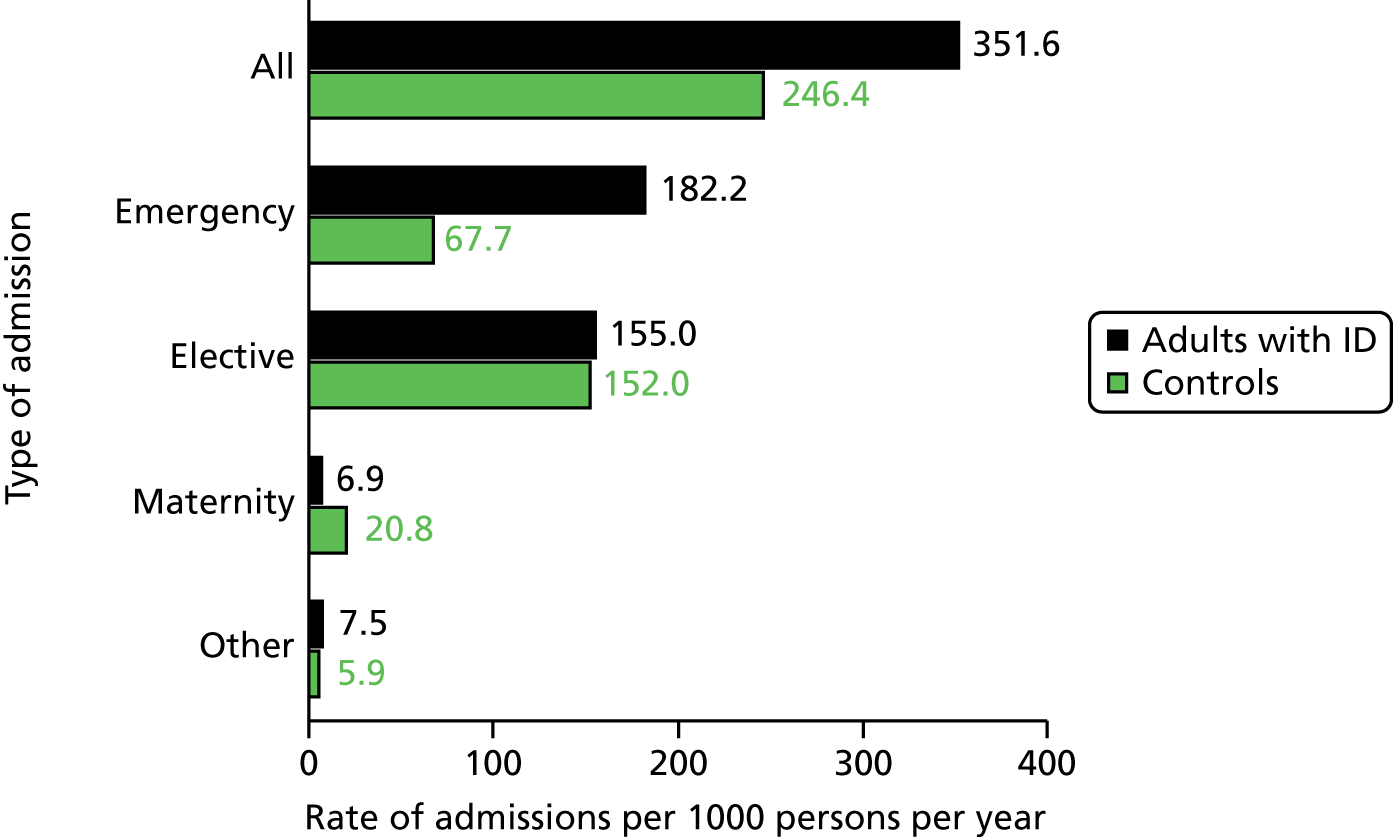
Of the 16,666 adults with ID, 5924 (35.5%) had an emergency or elective admission during follow-up. By comparison, among the age-, gender-, practice-matched controls (n = 113,562), 30,676 (27.0%) had at least one emergency or elective admission during follow-up. For emergency admissions only, 3847 (23.1%) adults with ID had at least one admission, compared with 13,496 (11.9%) of the controls. Only 2525 (66%) of these adults with ID had any corresponding mention of ID on their hospital record. A total of 1809 (10.9%) adults with ID had multiple emergency admissions, compared with 4326 (3.8%) of the controls.
Emergency admissions by subgroups
A summary of emergency hospitalisation rates among subgroups within adults with ID and matched controls is shown in Table 27. A statistical comparison of the rates is shown in Table 28, which estimates the IRRs for hospitalisation for adults with ID versus controls using conditional Poisson regression (see Chapter 2, Statistical analysis). These are presented unadjusted (accounting only for the matching factors) and then adjusted for comorbidities (atrial fibrillation, cancer, COPD, dementia, diabetes mellitus, epilepsy, heart failure, severe mental illness and stroke), smoking and deprivation. Subgroup comparisons used IRRs and CIs derived from ID versus control comparisons to calculate p-values for differences between them.
| Characteristic | Adults with ID (N = 16,666) | Controls (N = 113,562) | ||||
|---|---|---|---|---|---|---|
| Number of people | Admissions | Rate per 1000 | Number of people | Admissions | Rate per 1000 | |
| All | 16,666 | 9026 | 182.2 | 113,562 | 23,148 | 67.7 |
| Gender | ||||||
| Women | 6989 | 4250 | 203.8 | 47,587 | 10,613 | 73.5 |
| Men | 9677 | 4776 | 166.5 | 65,975 | 12,535 | 63.4 |
| Age (years) (at baseline) | ||||||
| 18–34 | 6981 | 2374 | 125.3 | 46,939 | 6217 | 50.5 |
| 35–54 | 6283 | 3201 | 159.3 | 43,123 | 7812 | 55.6 |
| 55–84 | 3402 | 3451 | 328.7 | 23,500 | 9119 | 116.7 |
| Down syndromea | ||||||
| Yes | 1793 | 804 | 150.0 | 12,226 | 2326 | 62.9 |
| No | 14,873 | 8222 | 186.1 | 101,336 | 20,822 | 68.2 |
| Severe health needsa | ||||||
| Yes | 3263 | 2487 | 243.9 | 22,298 | 4826 | 70.2 |
| No | 13,403 | 6539 | 166.2 | 91,264 | 18,322 | 67.1 |
| Communal accommodationa | ||||||
| Yes | 3392 | 2141 | 205.7 | 23,117 | 5523 | 75.0 |
| No | 13,274 | 6885 | 175.9 | 90,445 | 17,625 | 65.7 |
| Autism spectrum disordera | ||||||
| Yes | 1532 | 339 | 82.1 | 10,374 | 1459 | 53.2 |
| No | 15,134 | 8687 | 191.3 | 103,188 | 21,689 | 69.0 |
| Epilepsya | ||||||
| Yes | 2884 | 2725 | 306.8 | 19,705 | 4108 | 67.5 |
| No | 13,782 | 6301 | 155.0 | 93,587 | 19,040 | 67.7 |
| Characteristic | Base (unadjusted) model | Adjusted modela | ||
|---|---|---|---|---|
| IRR (95% CI) | p-valueb | IRR (95% CI) | p-valueb | |
| All | 2.82 (2.66 to 2.98) | – | 2.16 (2.02 to 2.30) | – |
| Gender | ||||
| Women | 2.90 (2.66 to 3.15) | 0.36 | 2.09 (1.89 to 2.30) | 0.45 |
| Men | 2.75 (2.55 to 2.96) | – | 2.20 (2.01 to 2.41) | – |
| Age at baseline (years) | ||||
| 18–34 | 2.54 (2.31 to 2.80) | – | 1.81 (1.61 to 2.04) | – |
| 35–54 | 2.96 (2.69 to 3.25) | 0.03 | 2.10 (1.87 to 2.37) | 0.09 |
| 55–84 | 2.90 (2.63 to 3.19) | 0.06 | 2.43 (2.19 to 2.70) | < 0.001 |
| Down syndromec | ||||
| Yes | 2.61 (2.23 to 3.05) | 0.31 | 2.37 (1.97 to 2.84) | 0.27 |
| No | 2.84 (2.68 to 3.01) | – | 2.11 (1.96 to 2.26) | – |
| Severe health needsc | ||||
| Yes | 3.67 (3.32 to 4.05) | < 0.001 | 3.83 (3.42 to 4.28) | < 0.001 |
| No | 2.59 (2.42 to 2.77) | – | 2.32 (2.16 to 2.49) | – |
| Communal accommodationc | ||||
| Yes | 2.91 (2.63 to 3.22) | 0.50 | 2.15 (1.88 to 2.47) | 0.95 |
| No | 2.79 (2.61 to 2.98) | – | 2.16 (2.00 to 2.33) | – |
| Autism spectrum disorderc | ||||
| Yes | 1.60 (1.32 to 1.94) | < 0.001 | 1.24 (0.98 to 1.57) | < 0.001 |
| No | 2.90 (2.74 to 3.07) | – | 2.21 (2.07 to 2.37) | – |
| Epilepsyc | ||||
| Yes | 4.80 (4.32 to 5.33) | < 0.001 | 4.98 (4.44 to 5.59) | < 0.001 |
| No | 2.39 (2.24 to 2.56) | – | 2.15 (2.00 to 2.30) | – |
The overall rate for emergency hospitalisation in adults with ID (182.2 per 1000 persons per year) represented a nearly three times increase (IRR 2.82, 95% CI 2.66 to 2.98) compared with their matched controls. This remained more than double (HR 2.16, 95% CI 2.02 to 2.30) when adjusting for comorbidities, smoking and deprivation. Although admission rates appeared higher for women with ID than for men with ID (203.8 vs. 166.5 per 1000 persons per year), this difference was not significantly different (p = 0.36). The disparity for emergency admissions between adults with ID and controls was more marked with increasing age.
Higher rates of emergency admission were seen in adults with ID with severe health needs (243.9 per 1000 persons per year) than in adults with ID without severe health needs (166.2 per 1000 persons per year). Compared with their matched controls, adults with ID with severe health needs were at nearly four times the risk of emergency hospitalisation (adjusted IRR 3.83, 95% CI 3.42 to 4.28). This disparity was significantly different from the increased risk seen in adults with ID without severe health needs (p < 0.001).
Rates of emergency admission did not significantly vary by communal accomodation or by Down syndrome when the RR between adults with ID and matched controls was compared (see Table 28). However, there were significant variations in rates of emergency admission by whether the adult with ID had epilepsy or autism. Adults with ID and epilepsy had an emergency hospitalisation rate approximately double that of adults with ID without epilepsy (306.8 vs. 155.0 per 1000 persons per year). Adults with ID and autism had an emergency hospitalisation rate less than half that of adults with ID without autism (82.1 vs. 191.3 per 1000 persons per year).
A direct comparison between subgroups among adults with ID is shown in Table 29, with IRRs adjusted for age, gender and comorbidity. This confirmed the doubling of emergency hospitalisations among those with epilepsy (adjusted HR 2.14), as well as the higher rate among adults with severe health needs (HR 1.54) and lower rates among those with autism (HR 0.61).
| Characteristic | Adults with ID, n | Base model,a IRR (95% CI) | Adjusted model,b IRR (95% CI) |
|---|---|---|---|
| Down syndrome | |||
| Yes | 1793 | 0.86 (0.74 to 1.00) | 1.10 (0.95 to 1.25) |
| No | 14,873 | 1 | 1 |
| Severe health needsc | |||
| Yes | 3263 | 1.40 (1.24 to 1.58) | 1.54 (1.37 to 1.74) |
| No | 13,403 | 1 | 1 |
| Communal accommodationc | |||
| Yes | 3392 | 1.03 (0.89 to 1.20) | 1.00 (0.87 to 1.16) |
| No | 13,274 | 1 | 1 |
| Autism spectrum disorder | |||
| Yes | 1532 | 0.58 (0.47 to 0.71) | 0.61 (0.49 to 0.75) |
| No | 15,134 | 1 | 1 |
| Epilepsy | |||
| Yes | 2884 | 1.95 (1.76 to 2.17) | 2.14 (1.91 to 2.39) |
| No | 13,782 | 1 | 1 |
Emergency admissions for ambulatory care-sensitive conditions
Emergency admissions for ACSCs were much higher among adults with ID than among controls (61.3 vs. 11.7 per 1000 persons per year). Additionally, the proportion of emergency admissions for ACSCs among adults with ID was much higher (33.7% vs. 17.3% for controls). When this relationship with ACSCs was further explored by age (Figure 30), the proportion of emergency admissions that were ACSCs (dark green shading) remained constant across age for adults with ID. Within the controls, however, this proportion increased from 12% in the youngest age group to 24% in the oldest age group.
FIGURE 30.
Emergency admissions, overall and for ACSCs, during 2009–13 by age group in adults with ID and controls.
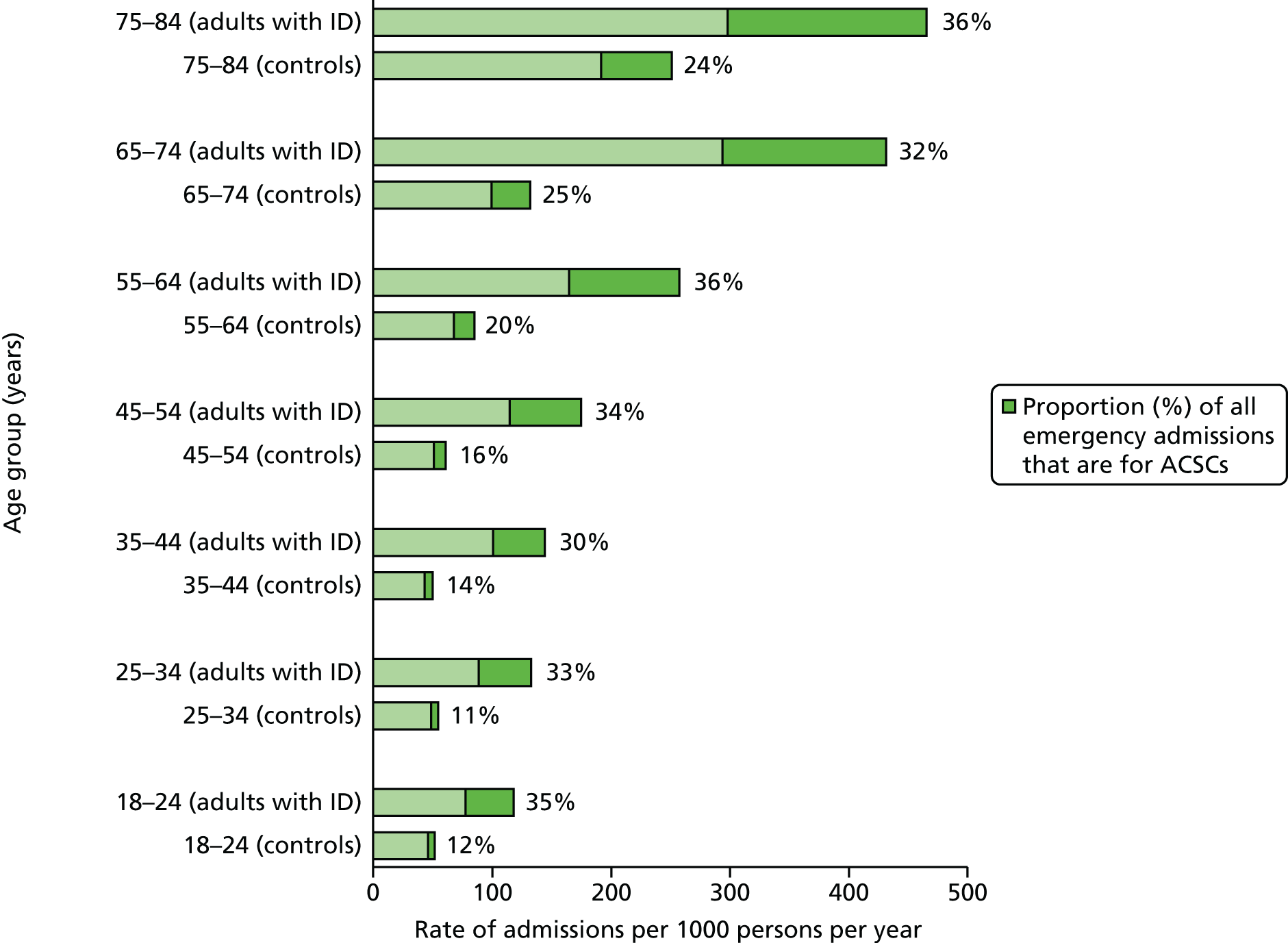
Emergency admissions for ACSCs are summarised in adults with ID and controls by subgroup in Table 30 (rates per 1000 persons per year) and in Table 31 (unadjusted and adjusted IRRs). The relative difference in admission rate was over five times higher for adults with ID (IRR 5.62, 95% CI 5.14 to 6.13). Adjusting for comorbidity explained some of this difference, but adults with ID were still over three times more likely to have an admission for an ACSC (IRR 3.60, 95% CI 3.25 to 3.99).
| Characteristic | Adults with ID (n = 16,666) | Controls (n = 113,562) | ||||
|---|---|---|---|---|---|---|
| Admissions | Rate per 1000 | % of all emergency | Admissions | Rate per 1000 | % of all emergency | |
| All | 3038 | 61.3 | 33.7 | 4008 | 11.7 | 17.3 |
| Gender | ||||||
| Women | 1428 | 68.5 | 33.6 | 1885 | 13.1 | 17.8 |
| Men | 1610 | 56.1 | 33.7 | 2123 | 10.7 | 16.9 |
| Age (years) (at baseline) | ||||||
| 18–34 | 805 | 42.5 | 33.9 | 759 | 6.2 | 12.2 |
| 35–54 | 1041 | 51.8 | 32.5 | 1204 | 8.6 | 15.4 |
| 55–84 | 1192 | 113.5 | 34.5 | 2045 | 26.2 | 22.4 |
| Down syndromea | ||||||
| Yes | 392 | 73.1 | 48.8 | 345 | 9.3 | 14.8 |
| No | 2646 | 59.9 | 32.2 | 3663 | 12.0 | 17.6 |
| Severe health needsa | ||||||
| Yes | 1154 | 113.2 | 46.4 | 830 | 12.1 | 17.2 |
| No | 1884 | 47.9 | 28.8 | 3178 | 11.6 | 17.3 |
| Communal accommodationa | ||||||
| Yes | 915 | 87.9 | 42.7 | 1032 | 14.0 | 18.7 |
| No | 2123 | 54.2 | 30.8 | 2976 | 11.1 | 16.9 |
| Autism spectrum disordera | ||||||
| Yes | 116 | 28.1 | 34.2 | 192 | 7.0 | 13.2 |
| No | 2922 | 64.3 | 33.6 | 3816 | 12.1 | 17.6 |
| Epilepsya | ||||||
| Yes | 1413 | 159.1 | 51.9 | 723 | 11.9 | 17.6 |
| No | 1625 | 40.0 | 28.8 | 3285 | 11.7 | 17.3 |
| Characteristic | Base (unadjusted) model | Adjusted modela | ||
|---|---|---|---|---|
| IRR (95% CI) | p-valueb | IRR (95% CI) | p-valueb | |
| All | 5.62 (5.14 to 6.13) | – | 3.60 (3.25 to 3.99) | – |
| Gender | ||||
| Women | 5.68 (5.03 to 6.42) | 0.81 | 3.35 (2.87 to 3.91) | 0.16 |
| Men | 5.56 (4.91 to 6.30) | – | 3.89 (3.39 to 4.46) | – |
| Age (years) (at baseline) | ||||
| 18–34 | 7.12 (5.96 to 8.51) | – | 3.06 (2.47 to 3.79) | – |
| 35–54 | 6.34 (5.43 to 7.39) | 0.34 | 3.25 (2.74 to 3.87) | 0.67 |
| 55–84 | 4.56 (4.00 to 5.20) | < 0.001 | 4.09 (3.52 to 4.76) | 0.03 |
| Down syndromec | ||||
| Yes | 10.00 (7.54 to 13.28) | 0.001 | 8.28 (5.73 to 11.98) | 0.002 |
| No | 5.26 (4.79 to 5.77) | – | 3.21 (2.88 to 3.58) | – |
| Severe health needsc | ||||
| Yes | 10.31 (8.81 to 12.07) | < 0.001 | 11.78 (9.78 to 14.19) | < 0.001 |
| No | 4.40 (3.95 to 4.90) | – | 4.28 (3.80 to 4.81) | – |
| Communal accommodationc | ||||
| Yes | 6.86 (5.78 to 8.14) | 0.01 | 4.98 (4.01 to 6.20) | 0.006 |
| No | 5.20 (4.70 to 5.76) | – | 3.35 (2.98 to 3.77) | – |
| Autism spectrum disorderc | ||||
| Yes | 4.14 (2.94 to 5.83) | 0.05 | 2.42 (1.54 to 3.81) | 0.04 |
| No | 5.69 (5.20 to 6.23) | – | 3.69 (3.33 to 4.10) | – |
| Epilepsyc | ||||
| Yes | 14.84 (12.59 to 17.49) | < 0.001 | 16.77 (13.83 to 20.34) | < 0.001 |
| No | 3.64 (3.29 to 4.03) | – | 3.46 (3.10 to 3.87) | – |
The relationship of admissions for ACSCs in adults with ID varied by age, with the youngest group (18–34 years) over seven times more likely than their controls to have an admission (IRR 7.12, 95% CI 5.96 to 8.51). However, once comorbidity was adjusted for, the trend by age group reversed and older adults with ID (55–84 years) were now the most likely to have an admission for an ACSC relative to their controls (IRR 4.09, 95% CI 3.52 to 4.76). Even after adjustment for comorbidity, adults with ID with severe health needs were almost 12 times more likely than their controls to have an admission for an ACSC (IRR 11.78, 95% CI 9.78 to 14.19). This difference was significantly different from that estimated between adults with ID without severe health needs and their controls (p < 0.001). A similar observation was seen when the comparison was made between adults with ID with epilepsy and their controls (IRR 16.77) versus adults with ID without epilepsy (IRR 3.46).
For adults with ID with Down syndrome, almost half of emergency admissions were for ACSCs (48.8%). As a result, adults with ID with Down syndrome were estimated to be a higher risk of ACSC admission versus their controls (IRR 8.28) than adults with ID without Down syndrome versus their controls (IRR 3.21), and this was significantly different (p = 0.002). Similarly, adults with ID recorded as living in communal accommodation were at a higher risk of emergency admission for an ACSC than those not recorded as such (p = 0.006).
Among all emergency admissions for ACSCs, the contribution of common conditions within adults with ID and controls separately is summarised in Figure 31. For adults with ID, the most common ACSCs resulting in admission were convulsions/epilepsy (36%), pneumonia/LRTI (19%) and UTI (11%). For matched controls, although pneumonia/LRTI (19%) and UTI (13%) admissions accounted for similar proportions, admissions for convulsions/epilepsy (6%) were much rarer.
FIGURE 31.
Emergency admissions for individual ACSCs during 2009–13 in (a) adults with ID and (b) controls. GE, gastroenteritis.
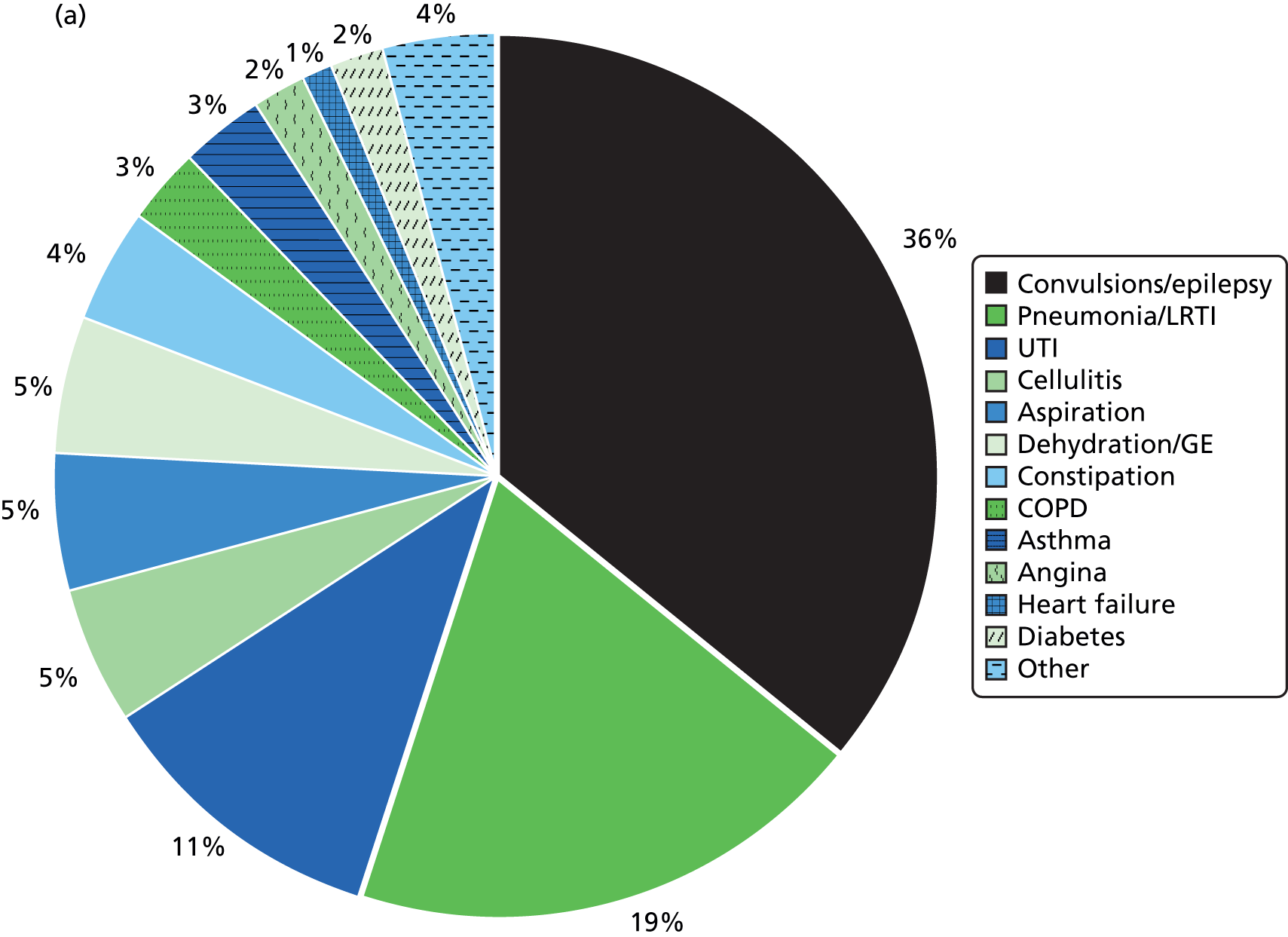
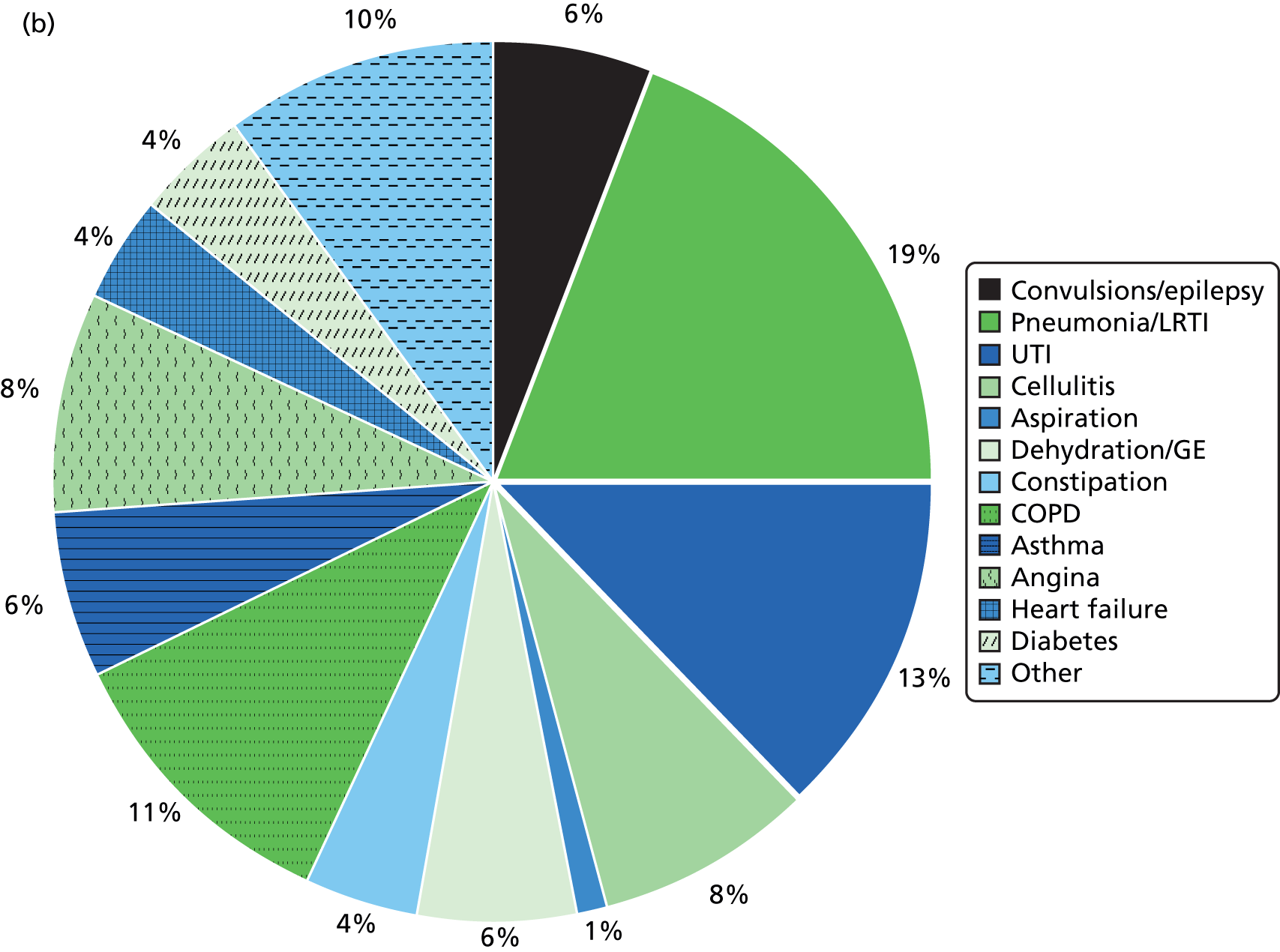
The rates of emergency admissions for each of the 23 ACSCs, and the associated IRRs for adults with ID versus controls (when estimable), are shown in Table 32. The largest relative disparities between adults with ID and controls were seen for aspiration (IRR 85.9, 95% CI 45.3 to 162.9) and convulsions/epilepsy (IRR 31.2, 95% CI 24.6 to 39.5). Among emergency admissions with sufficient occurrence in both groups, only angina did not show any evidence of a higher admission rate among adults with ID (IRR 1.00, 95% CI 0.60 to 1.68).
| ACSC | Adults with ID (N = 16,666) | Controls (N = 113,562) | Base (unadjusted) model, IRR (95% CI) | ||
|---|---|---|---|---|---|
| n | Rate per 1000 | n | Rate per 1000 | ||
| Angina | 47 | 1.0 | 329 | 1.0 | 1.00 (0.60 to 1.68) |
| Aspiration | 152 | 3.1 | 25 | 0.07 | 85.9 (45.3 to 162.9) |
| Asthma | 91 | 1.8 | 233 | 0.7 | 2.84 (1.99 to 4.06) |
| Cellulitis | 156 | 3.1 | 331 | 1.0 | 3.31 (2.56 to 4.28) |
| COPD | 105 | 2.1 | 454 | 1.3 | 1.68 (1.04 to 2.70) |
| Congestive heart failure | 44 | 0.9 | 156 | 0.5 | 2.21 (1.44 to 3.38) |
| Constipation | 128 | 2.6 | 142 | 0.4 | 6.79 (5.17 to 8.91) |
| Convulsions/epilepsy | 1081 | 21.8 | 256 | 0.8 | 31.2 (24.6 to 39.5) |
| Dehydration and gastroenteritis | 141 | 2.9 | 224 | 0.7 | 4.71 (3.60 to 6.17) |
| Dental conditions | 22 | 0.4 | 52 | 0.2 | 2.80 (1.67 to 4.71) |
| Diabetes complications | 61 | 1.2 | 140 | 0.4 | 3.26 (1.90 to 5.58) |
| Ear, nose and throat | 28 | 0.6 | 132 | 0.4 | 1.42 (0.93 to 2.17) |
| Gangrene | 1 | 0.02 | 10 | 0.03 | a |
| Gastro-oesophageal reflux disease | 22 | 0.4 | 74 | 0.2 | 2.22 (1.35 to 3.67) |
| Hypertension | 3 | 0.06 | 32 | 0.1 | a |
| Influenza | 8 | 0.2 | 18 | 0.05 | a |
| Iron deficiency anaemia | 21 | 0.4 | 40 | 0.1 | 3.97 (2.18 to 7.20) |
| Nutritional deficiencies | 0 | 0 | 2 | 0.01 | a |
| Pelvic inflammatory disease | 5 | 0.1 | 26 | 0.08 | a |
| Perforated/bleeding ulcer | 10 | 0.2 | 20 | 0.06 | 3.78 (1.63 to 8.75) |
| Pneumonia and other LRTIs | 566 | 11.4 | 772 | 2.3 | 5.59 (4.85 to 6.45) |
| Tuberculosis and other vaccine preventable | 1 | 0.02 | 11 | 0.03 | a |
| UTIs | 345 | 7.0 | 528 | 1.5 | 4.76 (3.99 to 5.68) |
Primary care utilisation before admission
We sought to use the linked CPRD and HES databases to describe the primary care utilisation and management prior to admission for ACSCs. We decided to choose two infections (UTIs and LRTIs) as exemplar ACSCs as they are common in both adults with ID and adults without ID. Although epilepsy is a much larger contributor to ACSC admissions in adults with ID owing to its high prevalence (see Table 6), the corresponding low prevalence in adults without ID makes any comparison potentially difficult.
We identified all recorded UTI and LRTI admissions during our study follow-up (see Table 31), and then included the first admission when there was no evidence of a prior admission for UTI or LRTI at any time previously in the patient’s record. This resulted in 727 UTI admissions and 1128 LRTI admissions. For each of these we electronically searched in the primary care record 2 weeks before admission to investigate whether or not there were any differences in primary care utilisation between adults with ID and adults without ID. Specifically, we sought whether or not these patients had consulted their GP during normal operating hours or if they had an emergency encounter during this time. We included all Read codes that indicated that the patient had been seen in the following locations: walk-in centre, out-of-hours service and accident and emergency department. For those who consulted their GP during the 2-week period, we then searched for the following: (1) any relevant diagnosis or suspected diagnosis, (2) an antibiotic prescription (first-line antibiotics for UTI were defined as nitrofurantoin or trimethoprim, and for LRTI were amoxicillin, clarithromycin, doxycycline or erythromycin) and (3) whether or not a urine test had been performed (for UTI admissions only).
It was no longer possible to preserve any age, gender or practice matching in the comparison between adults and controls with UTI (Table 33) and LRTI (Table 34) admissions. Therefore, in the logistic regressions, which estimated separate ORs for consultation, diagnosis or antibiotic prior to admission, we directly adjusted for age and gender differences between the two groups.
| Characteristics | Adults with ID (N = 276), n (%) | Controls (N = 451), n (%) |
|---|---|---|
| Age (years) | ||
| 18–34 | 43 (15.6) | 123 (27.3) |
| 35–54 | 77 (27.9) | 115 (25.5) |
| 55–84 | 156 (55.6) | 213 (47.2) |
| Gender | ||
| Men | 134 (48.6) | 150 (33.3) |
| At high risk of UTIa | ||
| Yes | 139 (50.4) | 117 (25.9) |
| Category of health-care use | ||
| Consulted at general practice | 156 (56.5) | 251 (55.7) |
| Had emergency encounterb | 19 (6.9) | 32 (7.1) |
| Other recordc | 70 (25.4) | 85 (18.8) |
| No record | 31 (11.2) | 83 (18.4) |
| Details of GP consultation | ||
| All | 156 | 251 |
| Diagnosis recorded | 22 (14.1) | 45 (17.9) |
| Urine testedd | 44 (28.2) | 75 (29.9) |
| Antibiotics prescribed | 62 (39.7) | 115 (45.8) |
| None of the above | 76 (48.7) | 118 (47.0) |
| Type of antibiotics | ||
| All | 62 | 115 |
| First linee only | 29 (46.8) | 57 (49.6) |
| Other only | 28 (45.2) | 52 (45.2) |
| Front linee and other | 5 (8.1) | 6 (5.2) |
| Number of antibiotics | ||
| One antibiotic | 55 (88.7) | 94 (81.7) |
| More than one | 7 (11.3) | 21 (18.3) |
| Characteristics | Adults with ID (N = 457), n (%) | Controls (N = 671), n (%) |
|---|---|---|
| Age (years) | ||
| 18–34 | 84 (18.4) | 81 (12.1) |
| 35–54 | 145 (31.7) | 194 (28.9) |
| 55–84 | 228 (49.9) | 396 (59.0) |
| Gender | ||
| Men | 260 (56.9) | 384 (57.2) |
| At high risk of admissiona | ||
| Yes | 108 (23.6) | 23 (3.4) |
| Category of health-care use | ||
| Consulted at general practice | 277 (60.6) | 368 (54.8) |
| Had emergency encounterb | 27 (5.9) | 39 (5.8) |
| Other recordc | 97 (21.2) | 131 (19.5) |
| No record | 56 (12.3) | 133 (19.8) |
| Details of GP consultation | ||
| All | 277 | 368 |
| Diagnosis recorded | 60 (21.7) | 80 (21.7) |
| Antibiotics prescribed | 111 (40.1) | 163 (44.3) |
| None of the above | 151 (54.5) | 187 (50.8) |
| Type of antibiotics | 0.0 | 0.0 |
| All | 111 | 163 |
| First lined only | 65 (58.6) | 113 (69.3) |
| Other only | 32 (28.8) | 34 (20.9) |
| First lined and other | 14 (12.6) | 16 (9.8) |
| Number of antibiotics | ||
| One antibiotic | 88 (79.3) | 130 (79.8) |
| More than one | 23 (20.7) | 33 (20.2) |
The pattern of primary care utilisation in the 2 weeks before a UTI admission is shown for 276 adults with ID and 451 adults without ID (see Table 33). Adults with ID were more likely to be men (48.6% vs. 33.3%), older (55.6% vs. 47.2% aged > 55.6 years) and at a high risk of a UTI (50.4% vs. 25.9%). However, both groups had a similar proportion with a primary care consultation (about 56%) or an emergency encounter (about 7%) in the 2-week period. The adjusted odds of a primary care consultation for adults with ID were not significantly different (OR 1.04, 95% CI 0.77 to 1.40). For patients who did consult with their GP, adults with ID were slightly less likely to receive a UTI diagnosis (14.1% vs. 17.9%), although this was not statistically significant (OR 0.78, 95% CI 0.52 to 1.17). Similarly, adults with ID were less likely to be prescribed an antibiotic (39.7% vs. 45.8%), but a statistical comparison of this difference was imprecise (OR 0.75, 95% CI 0.43 to 1.31).
For LRTI, 457 adults with ID with an admission were compared with 671 adults without ID (see Table 34). Although both groups had a similar proportion of men, adults with ID were more likely to be younger (18.4% vs. 12.1% aged 18–34 years) and far more likely to be at a high risk for a LRTI (23.6% vs. 3.4%). The percentage of adults with ID consulting with their GP in the 2 weeks before admission was marginally higher than among adults without ID (60.6% vs. 54.8%), although this difference was not formally statistically significant (OR 1.26, 95% CI 0.99 to 1.60). Both groups had a similar proportion (about 6%) with an emergency consultation in the 2-week period. Among patients with a consultation, an associated LRTI diagnosis during this period was similar between the groups (both 21.7%; OR 0.99, 95% CI 0.68 to 1.45). Prescribing of an antibiotic was marginally lower for adults with ID (40.1% vs. 44.3%), but not significantly different from that for controls (OR 0.84, 95% CI 0.61 to 1.15).
Chapter 6 Health checks and hospital admissions
Introduction
In this chapter we present a robust observational methodology, using practice- and individual-level designs, to assess whether or not the introduction of health checks in 2009 reduced emergency hospitalisation for adults with ID. First, we compare practices with high participation in the DES with practices with low participation in the DES, evaluating change in admission rates for all adults with ID, controlling for underlying differences between practices. However, the possibility remains that practices participating in the DES improved the care of their patients with ID independent of introducing the health checks. Therefore, we also present a matched cohort study (Figure 32) comparing the change in admission rates of 7487 individual adults with ID who had health checks with the change seen in the matched population controls without ID. This will account for any secular trends in practice care or hospital admissions that may have taken place.
FIGURE 32.
Matched cohort design for individual health check analyses.
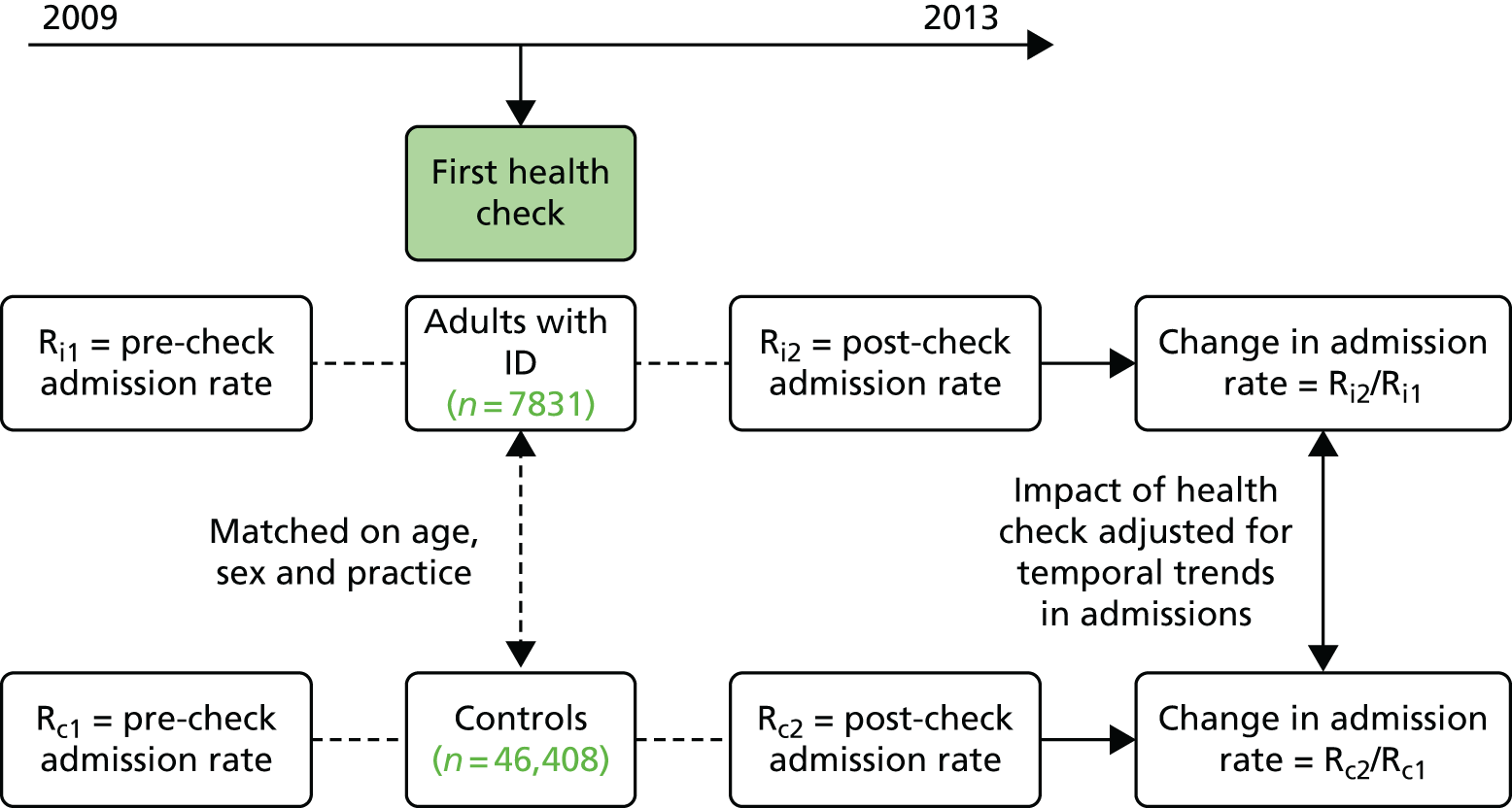
However, there is also a possibility that during our study there might have been underlying trends in admissions specific to all patients with ID in England. Therefore, a second matched cohort study for adults with ID not receiving health checks is used to confirm the specificity of findings to those having a health check only. In Figure 32 the date of health check is replaced with a random index date based on the known distribution of health check dates (see Figure 4).
Some of these results have already appeared in Carey et al. 81 Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC-BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Classification of practices
For the analysis carried out at practice level, we restricted to 289 practices with complete data from 1 January 2009 to 31 December 2012 (Figure 33). We then classified practice participation in the DES by calculating the percentage of patients registered on 1 January 2009 on the QOF learning disability register who subsequently received a health check by the end of 2010 or 2012. We defined full practice participation as practices with ≥ 50% of their adults with ID having a health check by the end of 2010. A total of 126 out of 289 (43.6%) practices were classed as fully participating. Non-participating practices were defined as practices with < 25% of their adults with ID having a health check by 2012, and 68 (23.5%) practices satisfied this criterion. Finally, 95 practices satisfied neither criterion and were classed as partially participating, having participation rates of ≥ 25% and < 50%. Of the 289 practices, 72 had zero participation by 2010, which fell to 35 by 2012.
FIGURE 33.
Summary of health check analyses. a, 14,080 total adults with ID with ≥ 1 registered day in these practices during 2009–12; b, practices with ≥ 25% and < 50% of adults with ID with health check by end of 2010, or only achieves ≥ 50% during 2011–12; c, adults with ID must have been registered for 90 days prior to the health check and have been alive from at least 90 days after it; d, controls subject to same criteria as above using their case’s health check date as the index date; e, adults with ID without health checks were assigned an index date using the distribution of known health check dates.
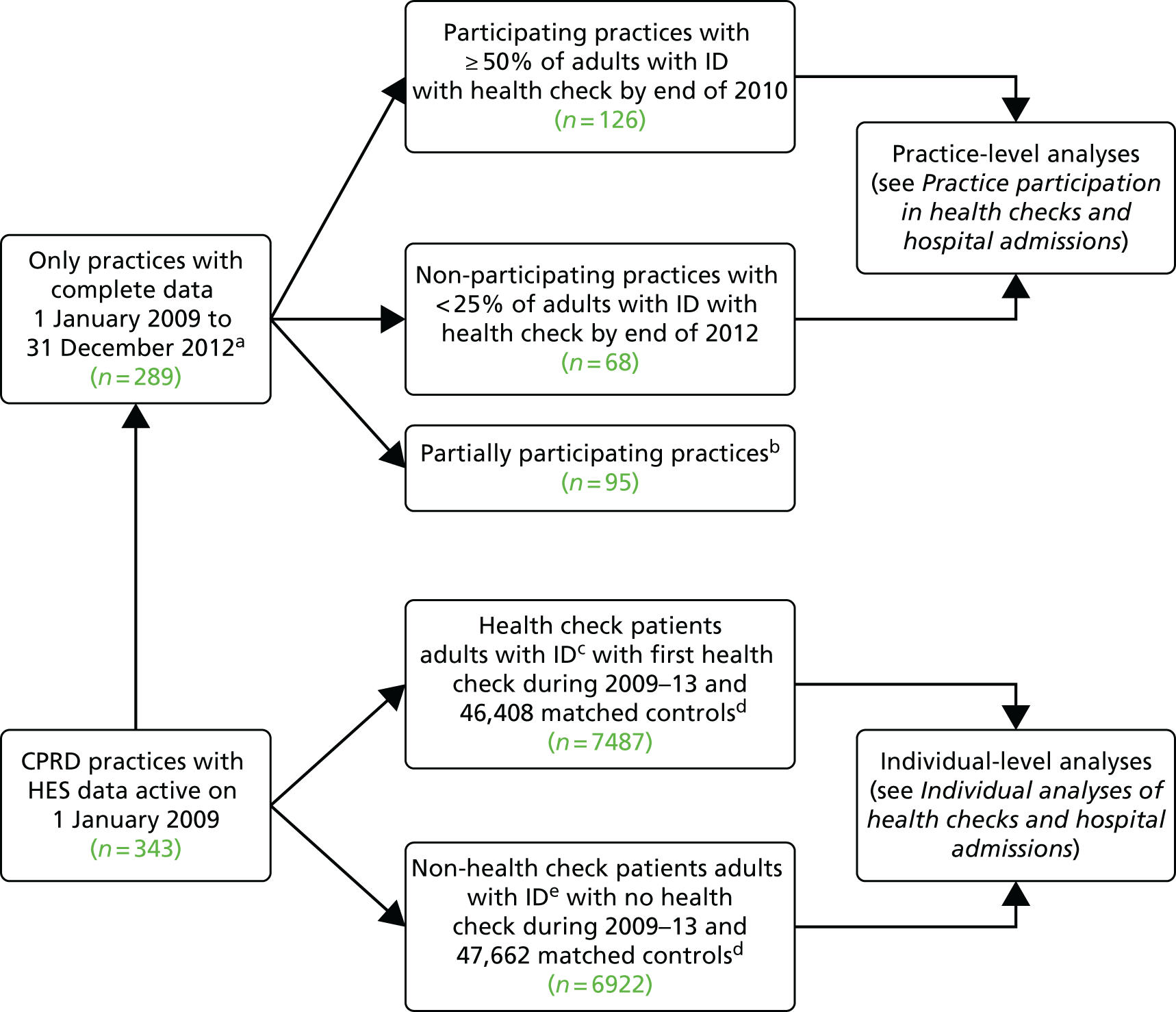
We were able to compare some practice characteristics of fully participating practices with those of non- or partially participating ones. Practices located in the north or midlands of England were marginally more likely to be classified as fully participating in health checks (48/102, 47.1%) than those located in the south (78/187, 41.7%). Practices located in the most deprived fifth of IMD were similarly more likely to be fully participating (25/60, 41.7%) than practices located in the least deprived fifth (15/44, 34.1%).
We then compared the patient characteristics of practices fully participating in health checks with the patient characteristics of those not participating, by first calculating the mean for a summary measure in each practice, and then calculating the median value across all practices in each participation group (Table 35). For example, for mean percentage of adults with ID registered on 1 January 2009 who had a health check by the end of 2010, among the median practice in the fully participating group, 69.5% of adults with ID had a health check by that point. This compared with 0.0% of adults with ID in the median practice for non-participating practices and 22.2% of adults with ID in in the median practice among partially participating practices. As the percentage rose to 58.6% for the median practice among partially participating practices for health checks by the end of 2012, we chose to keep these practices separate from the fully participating ones, as we wanted to assess any effect from the early adoption of the scheme.
| Characteristics of adults with ID summarised at practice levela | Level of practice participation, median (IQR) | |||
|---|---|---|---|---|
| All (n = 289) | Non-participatingb (n = 68) | Partially participatingb (n = 95) | Fully participatingb (n = 126) | |
| Total patients registered at any time during 2009–12,c n | 43.0 (25.0–64.0) | 36.0 (16.0–50.0) | 46.0 (31.0–64.0) | 45.0 (24.0–79.0) |
| Number of patients registered on 1 January 2009 only, n | 34.0 (19.0–52.0) | 26.5 (12.5–39.5) | 34.0 (31.0–64.0) | 38.0 (19.0–61.0) |
| With health check by end of 2010 (%) | 43.1 (1.6–65.8) | 0.0 (0.0–0.0) | 22.2 (4.3–41.7) | 69.5 (60.0–80.0) |
| With health check by end of 2012 (%) | 66.7 (28.6–81.8) | 0.0 (0.0–0.0) | 58.6 (41.0–68.8) | 81.8 (74.2–87.9) |
| Men (%) | 57.6 (50.0–64.3) | 55.6 (50.0–64.5) | 58.3 (50.0–63.2) | 57.5 (50.0–65.0) |
| Age (in 2009), mean | 41.6 (38.7–44.8) | 41.9 (38.9–45.8) | 40.5 (37.5–43.8) | 42.6 (39.4–45.0) |
| With severe health needsd (%) | 18.8 (10.5–27.0) | 15.2 (8.2–21.6) | 17.4 (10.2–27.8) | 22.2 (14.0–30.0) |
| Living in communal establishment residenced (%) | 9.7 (0.0–26.4) | 5.9 (0.0–23.1) | 8.6 (0.0–21.4) | 15.8 (2.3–34.2) |
| With epilepsy (%) | 17.1 (12.2–22.1) | 16.3 (9.4–24.4) | 16.7 (11.1–21.1) | 18.3 (13.5–22.2) |
The median of the mean number of adults with ID registered on 1 January 2009 was higher among all participating practices (38.0 patients) than among non-participating ones (26.5 patients). This may be attributed to the former having a higher mean percentage of patients recorded living in shared or communal establishments (median 15.8 vs. 5.9%). Practices fully participating in health checks tended to have more patients with ID with severe health needs than those non-participating (median 22.2 vs. 15.2%). However, it may be that each of these measures reflects higher recording levels on the general practice systems by more engaged staff in these participating practices.
Practice participation in health checks and hospital admissions
A summary of hospital admissions (all emergency, emergency ACSCs and elective) among adults with ID during 2009–12 is shown in Figure 34. In each plot the admission rate per quarter has been calculated by dividing the total admissions during that quarter by the total registration time from those patients. Unlike analyses presented elsewhere in this report, these plots include patients with no minimum registration period, and include a total of 14,080 adults with ID who were registered at any time during 2009–12 irrespective of whether or not they received a health check. For elective admissions, we excluded the small number of patients who had abnormally high elective admissions rates in any period (see Chapter 5, Categorising admissions). The data are then analysed in Table 36, in which two periods are now considered, 2009–10 and 2011–12, and annual rates have been calculated. The effect of practice participation on hospital admissions has been estimated by the interaction IRR between practice participation (fully vs. none) and period (2011–12 vs. 2009–10) in a conditional Poisson model (see Chapter 2, Statistical analysis).
FIGURE 34.
Hospital admissions in each quarter during 2009–12 by practice level of participation in health checks. (a) All emergency admissions; (b) emergency ACSCs admissions only; and (c) elective admissions.
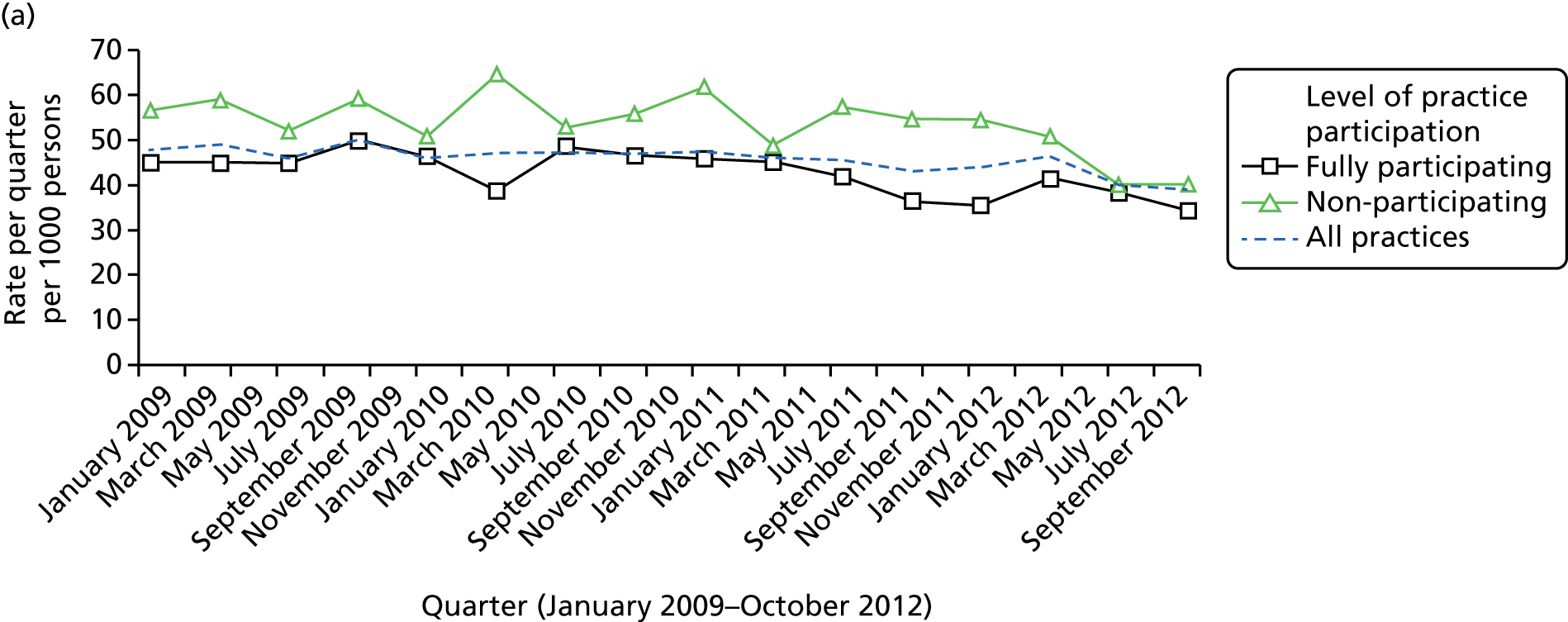
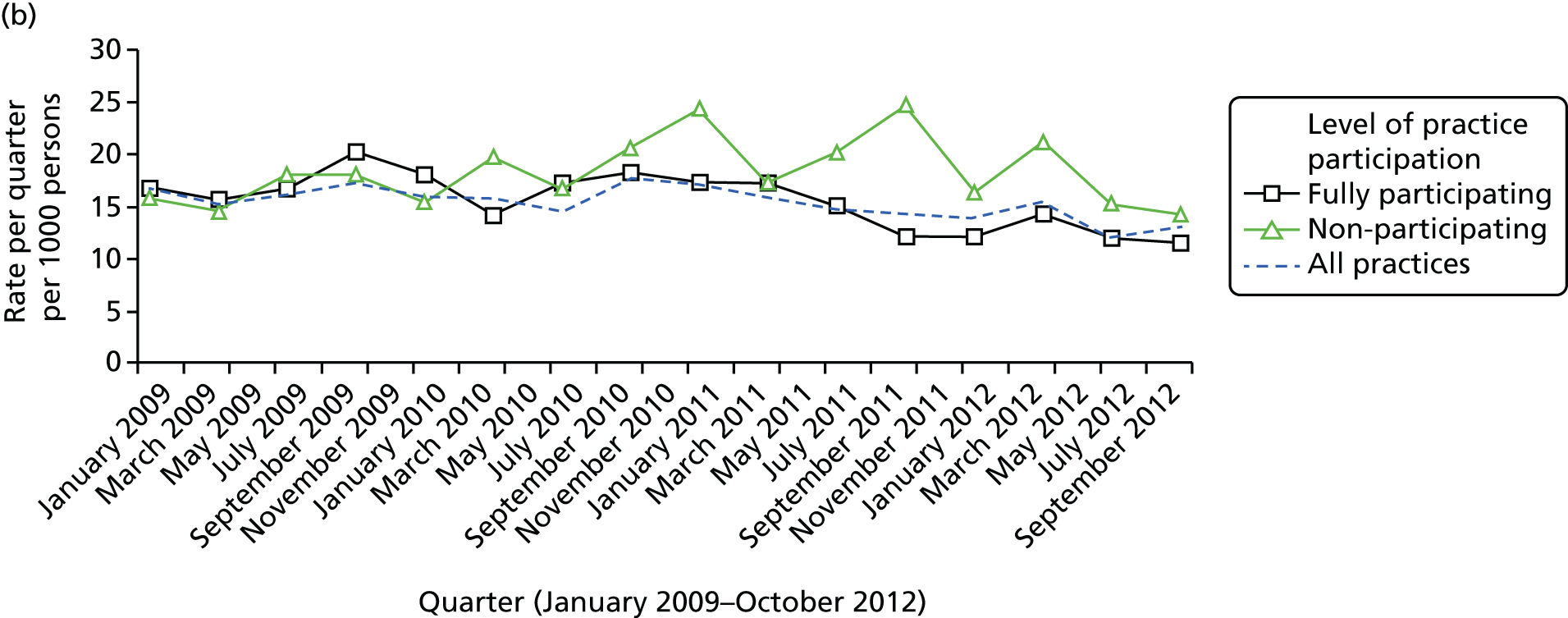
| Level of practice participation | 2009–10 admissions, rate per 1000 person-years | 2011–12 admissions, rate per 1000 person-years | Period change, IRRa (95% CI) | Fully vs. non-participating period change, IRRb (95% CI) |
|---|---|---|---|---|
| All (n = 289) | ||||
| All emergency admissions | 191.1 | 176.7 | 0.92 (0.86 to 0.99) | – |
| Emergency ACSCs only | 64.9 | 58.6 | 0.91 (0.82 to 1.00) | – |
| All elective admissionsc | 117.1 | 119.2 | 1.02 (0.95 to 1.09) | – |
| Fully participating (n = 126) | ||||
| All emergency admissions | 183.6 | 160.6 | 0.88 (0.80 to 0.96) | 0.97 (0.78 to 1.19) |
| Emergency ACSCs only | 69.2 | 56.3 | 0.82 (0.72 to 0.92) | 0.74 (0.58 to 0.95) |
| All elective admissionsc | 112.4 | 114.0 | 1.02 (0.92 to 1.14) | 1.02 (0.84 to 1.25) |
| Non-participating (n = 68) | ||||
| All emergency admissions | 226.9 | 205.3 | 0.90 (0.75 to 1.09) | 1.00 (Baseline) |
| Emergency ACSCs only | 70.1 | 77.1 | 1.10 (0.89 to 1.36) | 1.00 (Baseline) |
| All elective admissionsc | 125.9 | 127.3 | 1.00 (0.85 to 1.19) | 1.00 (Baseline) |
Emergency admission rates calculated in each quarter (see Figure 34) tended to fall over time in all practice participation categories. This is summarised annually in Table 36 as a fall from 191.1 per 1000 adults per year in 2009–10 to 176.7 in 2011–12. Non-participating health-check practices had consistently higher emergency admission rates throughout than practices that were fully participating (see Figure 34), with both groups of practices experiencing a similar fall over time (IRR 0.97, 95% CI 0.78 to 1.19).
When emergency admissions for only ACSCs were considered, the pattern was different (see Figure 34 and Table 36). Although these admissions had fallen among those practices fully participating in health checks (69.2 in 2009–10 to 56.3 in 2011–12 per 1000 adults), this was not replicated in practices not participating in health checks (70.1 in 2009–10 to 77.1 in 2011–12 per 1000 adults). A statistical comparison of the difference in this change showed an overall benefit of greater practice participation (IRR 0.74, 95% CI 0.58 to 0.95). There was no evidence of any difference in the change over time in elective admissions between fully and non-participating practices (IRR 1.02, 95% CI 0.84 to 1.25).
Alternative modelling approaches provided similar findings. For example, a fixed-effects (conditional) negative binomial showed no trend with all emergency ACSCs (IRR 0.98, 95% CI 0.82 to 1.18), but reduced change with emergency ACSCs (IRR 0.76, 95% CI 0.59 to 0.98).
Assigning an index date to adults with intellectual disability without health checks
We now consider analyses based on 7487 individuals with a first health check between 1 April 2009 and 31 March 2013. As explained previously in Chapter 2 (see Identification of health checks), we also include in our analyses 6922 adults with ID who did not receive a health check during this period but were assigned a random index date. We could then analyse this group in a complementary analysis to ensure that any findings from our study are specific to adults with ID with health checks and not due to underlying trends in hospital admissions in the population of adults with ID that might have taken place during our study period.
Briefly, this matching involved assigning a random date based on the known distribution of health checks between 1 April 2009 and 31 March 2013 in our data (Figure 35). For this, we used the dates from 7831 individuals with health checks we originally identified (344 of these individuals had subsequently been excluded owing to age, registration or data criteria). These dates were then randomly assigned to the 7751 adults without health checks, who we had identified as being potentially eligible for our analyses. This was achieved by iteratively sampling (without replacement) from the pool of 7831 dates. For a date match to be successful, the adult without the health check had to be alive and registered for at least 90 days on the potential index date. Unsuccessful date matches were returned to the pool of matching dates, until no more matches were possible.
FIGURE 35.
Summary of date matching between adults with ID with and without health checks.
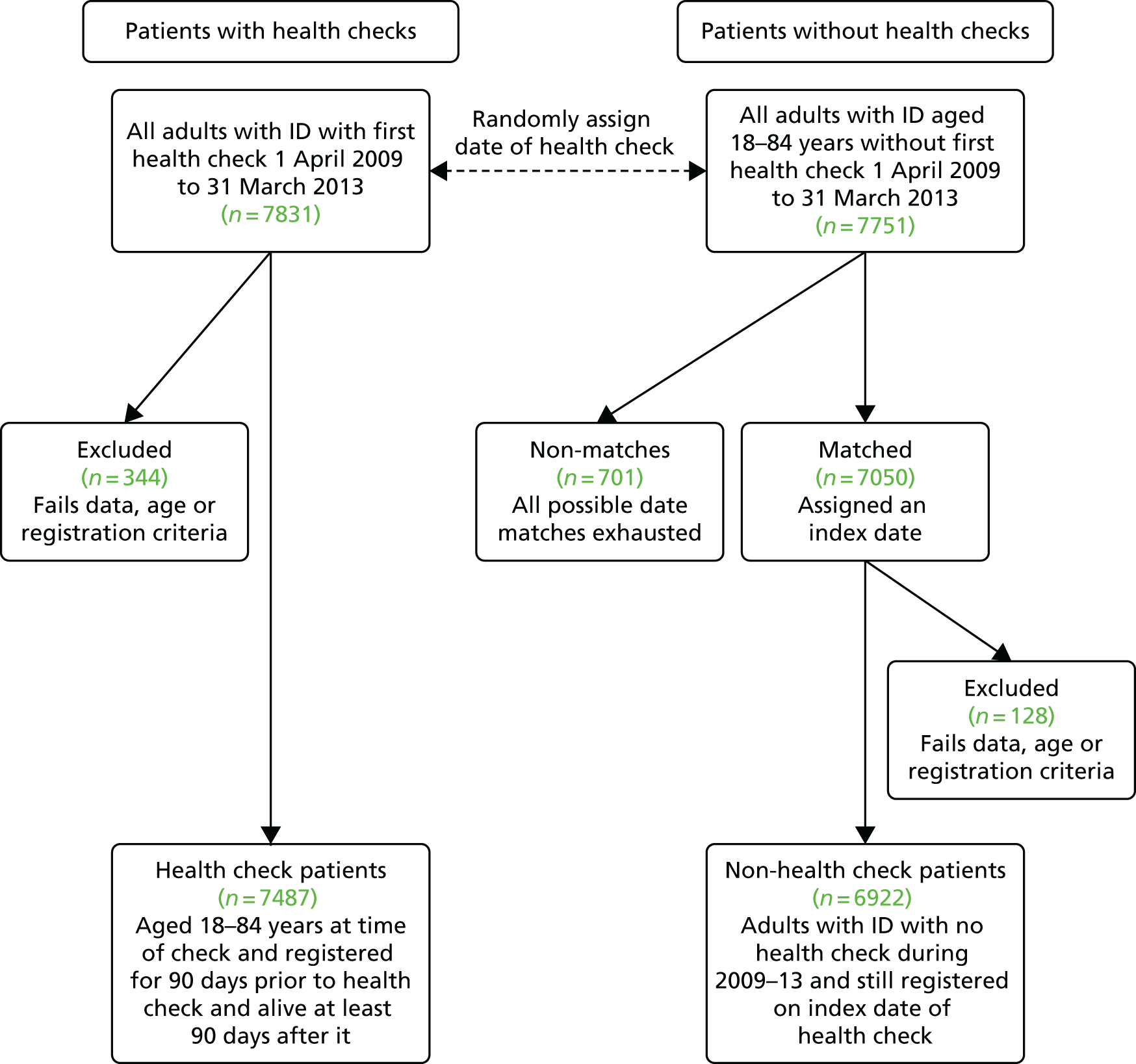
At the end of this process, 7050 (91%) successful date matches were assigned. Among this group, 58% of index dates were in 2009 or 2010, compared with 59% among the 7487 individuals with health checks. Most rejections were due to the patient only being registered for a short period, or only being age eligible (i.e. 18 years old) in 2013. A further 128 patients were rejected after the date assignment, mainly as a result of insufficient follow-up of 90 days that we required. This left 6922 adults without a health check with an assigned index date that we could use in the complementary analyses of health checks, which also used their 47,622 matched population controls.
Individual analyses of health checks and hospital admissions
A comparison of adults with ID with (n = 7487) and without (n = 6922) health checks is summarised in Table 37. Although the two groups had a similar gender distribution (58% men), adults with ID with health checks were notably older (mean age 42.6 vs. 39.0 years). More than one in four adults with ID with a health check were classed as having severe health needs (27.2%) or living in a communal establishment (25.6%). These proportions were much higher than those recorded in those without health checks (12.9% and 11.7%, respectively). The mean follow-up time was similar in both groups [adults with ID with health check, 560 days (pre) and 1081 days (post); adults with ID without health check, 521 days (pre) and 1059 days (post)].
| Characteristic | Adults with ID with health check (N = 7487), n (%) | Adults with ID without health check (N = 6922), n (%) |
|---|---|---|
| Gender | ||
| Women | 3183 (42.5) | 2889 (41.7) |
| Men | 4304 (57.5) | 4033 (58.3) |
| Age (years) (at health check) | ||
| 18–34 | 2579 (34.5) | 3159 (45.6) |
| 35–54 | 3136 (41.9) | 2432 (35.1) |
| 55–84 | 1772 (23.7) | 1331 (19.2) |
| Down syndrome | ||
| Yes | 914 (12.2) | 639 (9.2) |
| No | 6573 (87.8) | 6283 (90.8) |
| Severe health needsa | ||
| Yes | 2035 (27.2) | 891 (12.9) |
| No | 5452 (72.8) | 6031 (87.1) |
| Communal accommodationa | ||
| Yes | 1913 (25.6) | 811 (11.7) |
| No | 5574 (74.5) | 6111 (88.3) |
| Autism spectrum disorder | ||
| Yes | 743 (9.9) | 499 (7.2) |
| No | 6744 (90.1) | 6423 (92.8) |
| Epilepsy | ||
| Yes | 1552 (20.7) | 975 (14.1) |
| No | 5935 (79.3) | 5947 (85.9) |
Hospital admission rates (all emergency, emergency ACSCs and elective) before and after the health check are summarised in Table 38. Four groups are shown: adults with ID with and without health checks (using their random index date), and the matched controls for each of these two groups. Conditional Poisson models were used to estimate the IRR for period and interaction effects (see Chapter 2, Statistical analysis). This model was first fitted to adults with ID with a health check and their controls separately, estimating the individual change in hospital admission rate after as compared with before health check (or index date). A combined model of adults with ID and controls with a case–period interaction then provided an estimate for the effect of health checks (or index dates) on admission rates among adults with ID, adjusted for any temporal trends in admissions. The process was then repeated using the adults with ID without health checks and their controls.
| Patient group and health check status | Pre health check, rate per 1000 person-years | Post health check, rate per 1000 person-years | Period change, IRRa (95% CI) | Fully vs. non-participating period change, IRRb (95% CI) |
|---|---|---|---|---|
| Adults with ID with health check (n = 7487) | ||||
| All emergency admissions | 145.7 | 173.2 | 1.22 (1.11 to 1.34) | 0.96 (0.87 to 1.07) |
| Emergency ACSCs only | 52.4 | 59.3 | 1.11 (0.95 to 1.29) | 0.82 (0.69 to 0.99) |
| All elective admissionsc | 115.9 | 122.4 | 1.11 (1.01 to 1.21) | 0.96 (0.87 to 1.06) |
| Adults with ID without health check (n = 6922) | ||||
| All emergency admissions | 186.0 | 212.2 | 1.20 (1.09 to 1.32) | 1.05 (0.94 to 1.17) |
| Emergency ACSCs only | 52.7 | 66.7 | 1.35 (1.14 to 1.60) | 1.11 (0.92 to 1.36) |
| All elective admissionsc | 119.1 | 128.4 | 1.02 (0.93 to 1.12) | 0.90 (0.81 to 1.00) |
| Controls for ID with health check (n = 46,408) | ||||
| All emergency admissions | 58.6 | 70.1 | 1.27 (1.20 to 1.34) | – |
| Emergency ACSCs only | 9.5 | 12.9 | 1.40 (1.24 to 1.58) | – |
| All elective admissionsc | 102.4 | 121.3 | 1.15 (1.11 to 1.20) | – |
| Controls for ID without health check (n = 47,662) | ||||
| All emergency admissions | 56.9 | 66.1 | 1.15 (1.09 to 1.21) | – |
| Emergency ACSCs only | 8.5 | 11.0 | 1.28 (1.14 to 1.44) | – |
| All elective admissionsc | 88.4 | 106.2 | 1.13 (1.09 to 1.18) | – |
For the 7487 adults with a health check, all emergency admissions rose by 22% from 145.7 to 173.2 annually per 1000 persons (IRR 1.22, 95% CI 1.11 to 1.34). By contrast, in their 46,408 matched controls the rate for all emergency admissions increased by 27%, from 58.6 to 70.1 (IRR 1.27, 95% CI 1.20 to 1.34). Therefore, in the combined Poisson model, the interaction for the impact of health checks on adults with ID is estimated to be < 1 (IRR 0.96, 95% CI 0.87 to 1.07). Adults with ID without health checks had higher overall admission rates for emergency admission (e.g. 186.0 vs. 145.7 annually per 1000 persons pre index date) and a slight subsequent increase in admission rate post index date relative to their controls (IRR 1.05, 95% CI 0.94 to 1.17).
Although emergency admissions for ACSCs among adults with ID with health checks also showed a rise post health check (52.4 to 59.3 per 1000 persons per year), this change was smaller than that seen in the control group (11% vs. 35%). The combined Poisson model produced a statistically significant interaction (IRR 0.82, 95% CI 0.69 to 0.99), which represents the change in admission rate post health check compared with controls. This interaction effect and trend was not replicated in adults with ID without a health check (IRR 1.11, 95% CI 0.92 to 1.36).
For elective hospital admissions, the estimated post health check was similar between adults with ID with health checks and controls (IRR 0.96, 95% CI 0.87 to 1.06). There was some evidence that elective admissions among adults with ID without health check had shown a reduced change compared with their controls (IRR 0.90, 95% CI 0.81 to 1.00) after their assigned index date.
We carried out sensitivity analyses using a different statistical modelling approach that directly compared the change in admissions between adults with ID with health checks and those without health checks (see Chapter 2, Statistical analysis). The models accounted for underlying differences between the two unmatched groups by adjusting for age, gender and comorbidity. The Poisson and negative binomial models produced similar findings to our previous approach. For example, for the negative binomial models the interaction IRRs were all emergency admissions (IRR 1.04, 95% CI 0.90 to 1.19), emergency ACSCs (IRR 0.80, 95% CI 0.66 to 0.99) and elective admissions (IRR 1.03, 95% CI 0.90 to 1.17).
Table 39 summarises the estimate of the impact of health checks on emergency hospital admissions, stratified by individual characteristics for both adults with ID with and adults with ID without health checks. These are the case–period interaction IRRs from the conditional Poisson models fitted to each group separately. A significant rise in admissions was seen among adults with Down syndrome with health checks compared with their population controls (IRR 1.55, 95% CI 1.15 to 2.08). However, this increase was replicated among adults with Down syndrome without health checks (IRR 1.55) compared with their controls, suggesting a trend specific to adults with Down syndrome. By contrast, although health checks were associated with a smaller change in emergency admissions among adults with ID with severe health needs compared with their controls (IRR 0.80, 95% CI 0.67 to 0.95), this trend was not replicated in adults with ID without health checks with severe health needs compared with their controls (IRR 1.07, 95% CI 0.85 to 1.35). A further analysis of adults with ID with severe health needs receiving health checks also suggested a decrease in their emergency admissions for ACSCs compared with controls (IRR 0.76, 95% CI 0.56 to 1.01).
| Characteristic | Adults with ID with health check (n = 7487), IRR (95% CI) | Adults with ID without health check (n = 6922), IRR (95% CI) |
|---|---|---|
| Gender | ||
| Women | 1.07 (0.92 to 1.25) | 1.13 (0.95 to 1.34) |
| Men | 0.88 (0.76 to 1.01) | 0.98 (0.85 to 1.13) |
| Age (years) (at health check) | ||
| 18–34 | 1.01 (0.81 to 1.25) | 0.97 (0.80 to 1.16) |
| 35–54 | 0.95 (0.80 to 1.13) | 1.12 (0.92 to 1.34) |
| 55–84 | 0.96 (0.81 to 1.14) | 0.96 (0.78 to 1.18) |
| Down syndrome | ||
| Yes | 1.55 (1.15 to 2.08) | 1.55 (1.08 to 2.22) |
| No | 0.91 (0.82 to 1.02) | 1.01 (0.90 to 1.14) |
| Severe health needsa | ||
| Yes | 0.80 (0.67 to 0.95) | 1.07 (0.85 to 1.35) |
| No | 1.06 (0.93 to 1.22) | 1.03 (0.90 to 1.17) |
| Communal accommodationa | ||
| Yes | 1.13 (0.92 to 1.38) | 1.22 (0.92 to 1.62) |
| No | 0.91 (0.80 to 1.03) | 1.02 (0.90 to 1.15) |
| Autism spectrum disorder | ||
| Yes | 1.18 (0.76 to 1.82) | 1.25 (0.75 to 2.08) |
| No | 0.95 (0.85 to 1.05) | 1.04 (0.93 to 1.16) |
| Epilepsy | ||
| Yes | 0.88 (0.73 to 1.07) | 1.17 (0.91 to 1.49) |
| No | 1.03 (0.90 to 1.17) | 1.01 (0.89 to 1.15) |
Chapter 7 Who gets health checks and what is recorded?
Introduction
The final part of the analysis in the report considers two further questions: (1) what gets recorded on a patient’s electronic record during a health check and (2) what predicts who gets a health check?
To answer these questions, we focused on health checks that took place during 2009–11, only including 274 practices that had a minimum involvement (≥ 20% of registered patients with ID with a health check) in the DES (see Figure 3). We also required patients to be registered at the beginning of follow-up (1 January 2009) for at least 1 year, thereby ensuring that these health checks were not being performed on recently registered patients. This identified 5583 first health checks on established patients with ID, from which we summarised what was being electronically recorded on their record around the time of the check (Figure 36).
FIGURE 36.
Summary of cohort design for analyses investigating impact of health checks on recording of health measures.
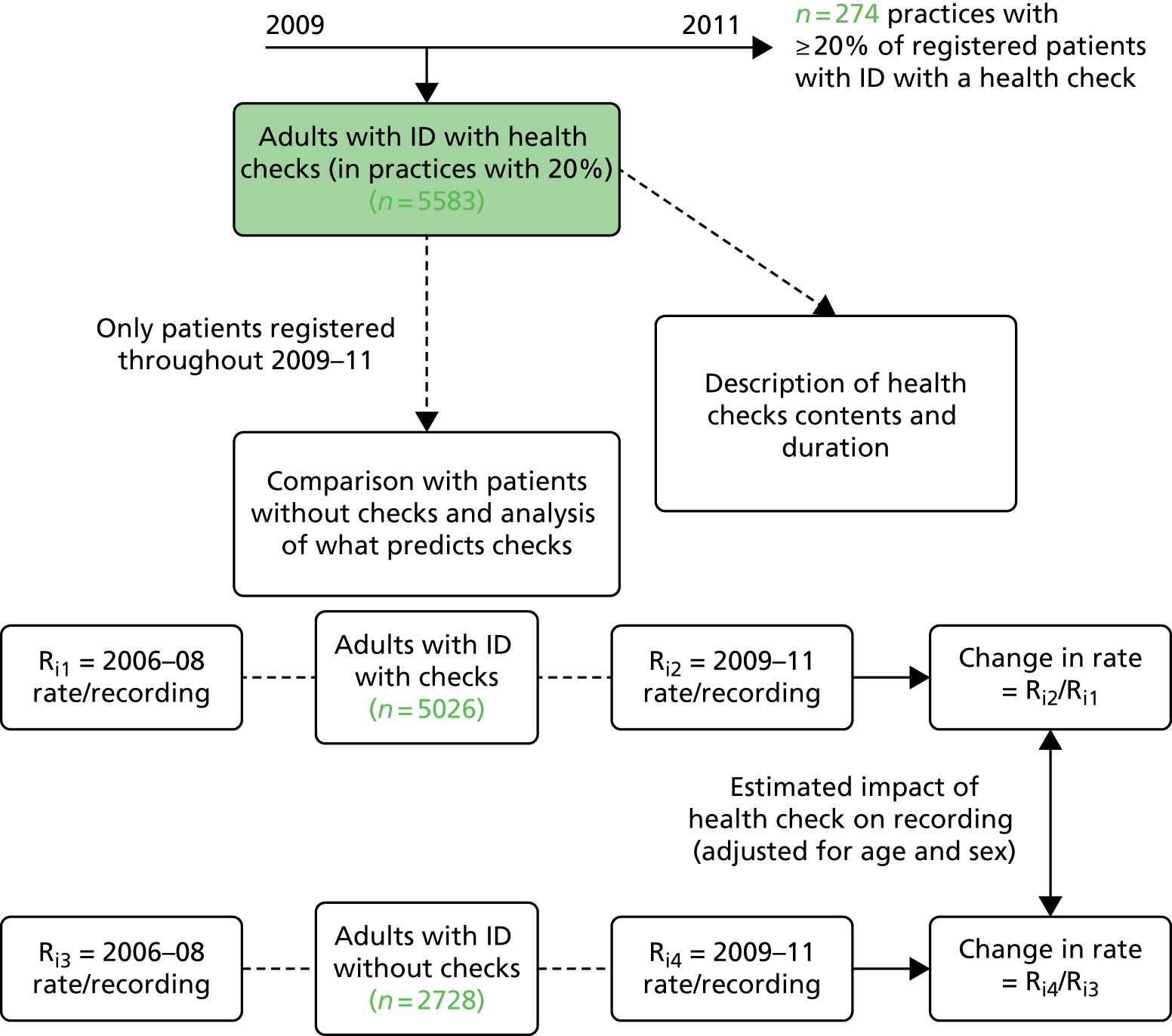
We then estimated what difference the health check had made to the overall recording of some selected process measures by further restricting to the 5026 patients with health checks who were still registered at 31 December 2011. This allowed a comparison of two distinct periods (see Figure 36): one before health checks were introduced (2006–8) and one during the period when the check took place (2009–11). The change in their records between these two periods was then contrasted with the records of 2728 adults with ID from the same practices who did not get a health check during 2009–11. Finally, we present an analysis that investigates which factors, if any, predict who from this combined group of 7754 adults with ID received a health check during 2009–11.
The characteristics of the three groups of adults with ID used in the analyses described above are given in Table 40. As described previously (see Table 37), patients with and without health checks differ significantly with respect to severe health needs, epilepsy and living arrangements.
| Characteristic | First health check during 2009–11, n (%) | No health check during 2009–11, n (%) | |
|---|---|---|---|
| All patients | Registered 2009–11 | Registered 2009–11 | |
| All | 5583 (100) | 5026 (100) | 2783 (100) |
| Gender | |||
| Women | 2404 (43.1) | 2153 (42.8) | 1116 (40.9) |
| Men | 3179 (56.9) | 2873 (57.2) | 1612 (59.1) |
| Age (years) (at health check) | |||
| 18–34 | 1578 (28.3) | 1489 (29.6) | 1053 (38.6) |
| 35–54 | 2555 (45.8) | 2351 (45.8) | 1127 (41.3) |
| 55–84 | 1450 (26.0) | 1186 (23.6) | 548 (20.1) |
| Down syndrome | |||
| Yes | 725 (13.0) | 644 (12.8) | 219 (8.0) |
| No | 4858 (87.0) | 4382 (87.2) | 2509 (92.0) |
| Severe health needsa | |||
| Yes | 1485 (26.6) | 1336 (26.6) | 388 (14.2) |
| No | 4098 (73.4) | 3690 (73.4) | 2340 (85.8) |
| Communal accommodationa | |||
| Yes | 1766 (31.6) | 1551 (30.9) | 245 (9.0) |
| No | 3817 (68.4) | 3475 (69.1) | 2483 (91.0) |
| Autism spectrum disorder | |||
| Yes | 457 (8.2) | 401 (8.0) | 127 (4.7) |
| No | 5126 (91.8) | 4625 (92.0) | 2601 (9.5) |
| Epilepsy | |||
| Yes | 1201 (21.5) | 1080 (21.5) | 372 (13.6) |
| No | 4382 (78.5) | 3946 (78.5) | 3946 (86.4) |
What is recorded during a health check?
To investigate what was being recorded during the 5583 first health checks carried out between 2009 and 2011, we extracted all information 14 days either side of the recorded date of the health check. Although the majority of information was being recorded on the date of the health check, by allowing 2 weeks either side of this date we were able to account for (1) health checks that took place across multiple days and (2) results of tests that were apparent on the system only after the check had taken place.
We then attempted to summarise the total information recorded by identifying common categories that were being used (Table 41). These categories were defined to be as broad as possible to try to capture whether a specific health area or concern had been addressed during the check. So, for example, the category ‘alcohol’ would count Read codes estimating alcohol consumption as well as any codes around lifestyle advice in relation to alcohol. ‘Ears’ would cover hearing tests and assessments, examination or symptoms of the ears and whether or not they had been seen by an audiologist. In the end we identified 22 common categories (see Table 41) that we thought were applicable to all adults with ID. A further five categories (medication review, breast examination, cervical smear, epilepsy and influenza vaccination) were summarised for specific subgroups only. A list of the Read codes used is given in Appendix 9.
| Category identified | Details | n (%) |
|---|---|---|
| Top 10 categories | ||
| Weight/BMI | Measured, gain/loss, BMI measured, health education/weight management/advice | 4323 (77.4) |
| Blood pressure | Measured | 4279 (76.6) |
| Alcohol | Consumption, advice/counselling, screen, intervention | 3952 (70.8) |
| Smoking | Tobacco consumption, health education/advice | 3334 (59.7) |
| Mobility | How mobile, assessment, walking aid | 3099 (55.5) |
| Ears | Hearing, blocked/waxy ears, seen by audiologist | 3060 (54.8) |
| Eyes | Visual symptoms, wears glasses, examination, ophthalmological monitoring, normal vision | 2949 (52.8) |
| Carer | Details, paid/voluntary, does not have carer | 2535 (45.4) |
| Pulse | Measured/examined | 2396 (42.9) |
| Height | Measured | 2385 (42.7) |
| Other common categories | ||
| Health action plan | Offered, declined, reviewed or completed | 2269 (40.6) |
| Behaviour | Problems, change, assessment | 2056 (36.8) |
| Dental | Dental examination, advice, seen by dentist | 2027 (36.3) |
| Communication | Speech, writing, responding | 1733 (31.0) |
| Exercise | How much, able to exercise, health education/advice | 1522 (27.3) |
| Diet | Diet, allergies, appetite, advice/health education | 1512 (27.1) |
| Blood test | Taken, requested or results recorded | 1503 (26.9) |
| Urine test | Obtained, sent to laboratory, dipstick, results recorded | 1393 (25.0) |
| Mental health | Symptoms/none, mood, depression screening, mental health review | 772 (13.8) |
| Bowels and bladder | Health education, continence, catheter, assessment | 739 (13.2) |
| Respiratory | Examination, rate of respiration, breath sounds, respiratory flow rates | 664 (11.9) |
| Sexual related | Sexually active, contraception, health education | 587 (10.5) |
| Specific subgroupsa | ||
| Medication review (on repeat medication) | Medication monitoring, medication review, epilepsy (and others) medication | 1123 (26.1) |
| Breast examination (women) | Examination/self-examination, mammography | 493 (20.5) |
| Cervical smear (women) | Given, offered, refused, not indicated | 404 (16.8) |
| Epilepsy (epilepsy prior to 2009) | Monitoring, fit frequency, last fit, seizure free | 537 (44.7) |
| Influenza vaccination (health check September–January only) | Given | 387 (19.1) |
We also observed a pattern associated with health checks in some practices where there was consistently little or no recorded information on the electronic patient record around the time of the check. We think that these checks are probably being performed away from the GP surgery, as this absence of informative recording was more common in practices with large clusters of adults with ID living in communal or shared accommodation. We do not necessarily believe that no tests or examinations are being carried out in these checks, but can only summarise them as being ‘non-informative’ based on what was recorded in the patient electronic record. We automated identification of these as those in which none of the top 10 categories listed in Table 41 were being recorded. A total of 458 (8.2%) checks were identified as ‘non-informative’.
The most common category of recorded information during the health check was weight or BMI related, for which 4323 health checks had related information (see Table 41). This represented 77.4% of all 2009–11 health checks, or 84.4% of the 5125 ‘informative’ health checks only. This was followed by blood pressure, alcohol, smoking and mobility, for all of which related information was given in more than half of the health checks. Only 4 in 10 health checks (40.6%) had a record of a health action plan being offered, declined, reviewed or completed. Only a small proportion (< 15%) of health checks had recorded information relating to mental health and bowels or bladder.
Across practices, there was considerable variation in the volume of recorded information around the time of the health checks. Among the 22 common categories identified from Table 41, 49 (18%) of the 274 practices had health checks that averaged fewer than six categories. By contrast, 53 (19%) had health checks that averaged more than 12 different categories being recorded.
Recorded length and general practitioner involvement in health checks
We sought to determine the length of the health check and summarise who was involved in carrying it out. To do this, we first excluded the 458 non-informative health checks, as our assumption was that the lack of electronic information on the system reflected that these checks that were primarily taking place outside the GP surgery. From the remaining checks, we further excluded 179 with missing or zero duration length, which resulted in 4946 health checks. We then identified the singular day on which the majority of the top 10 categories listed in Table 41 appeared. In the rare event of a tie, we used the date on which the Read code for the health check appeared.
Of the 4946 health checks recorded during 2009–11 containing informative electronic information on duration, approximately half (n = 2464, 49.8%) appeared to be conducted solely by the GP. A further 686 (13.9%) had information indicating both GP and nurse involvement, whereas 1287 checks (26.1%) had only nurse involvement indicated. For about 1 in 10 checks (n = 509, 10.3%) neither a GP nor a nurse was directly recorded, with ‘administrator’ being the most common role indicated. Across subgroups (Figure 37), the percentage with GP involvement in the health check remained around 6 in 10 for most categories.
FIGURE 37.
Percentage of first health checks during 2009–11 that involved a GP and were > 30 minutes’ duration by subgroups.
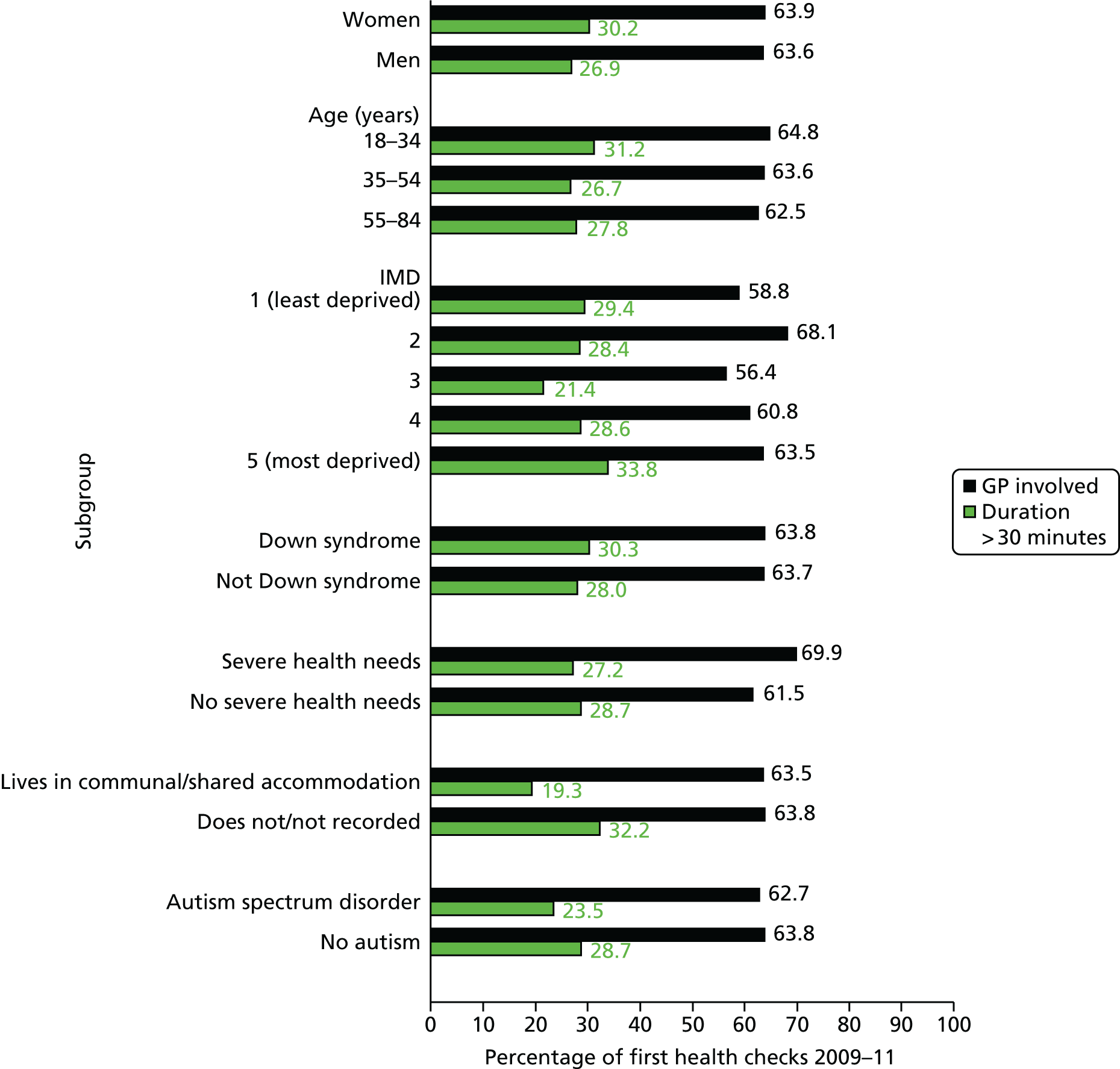
Of the 4946 health checks with duration recorded, about 3 in 10 (n = 1399, 28.3%) were estimated to be > 30 minutes in duration for the singular day that contained the most information recorded. Across subgroups (see Figure 37), the largest variation in duration was by living arrangement. Here, adults with ID living in communal or shared accommodation were recorded as less likely to have a check lasting 30 minutes (19.3%) than those not recorded as such (32.2%).
Process measures before and after health checks
Using the identified categories from Table 41, we now wished to summarise the added benefit of health checks in reference to how the information was recorded prior to the introduction of health checks. To do this, we compared the recording of these categories during 2006–8 versus 2009–11 for the 5026 adults with ID who received a health check during 2009–11 in practices that had a minimum level (20%) of participation in the DES. We contrasted the absolute change in recording with the corresponding one seen in the 2728 adults with ID from the same set of practices who did not receive a check during this time (see Figure 36). This is summarised in Table 42.
| Category identified | Adults with ID with health check 2009–11 (n = 5026) | Adults with ID without health check 2009–11 (n = 2728) | ||||
|---|---|---|---|---|---|---|
| % 2006–8 | % 2009–11 | ± change | % 2006–8 | % 2009–11 | ± change | |
| Top 10 categories | ||||||
| Weight/BMI | 59.9 | 95.3 | +35.4 | 50.7 | 54.8 | +4.1 |
| Blood pressure | 69.8 | 95.3 | +25.6 | 60.3 | 64.4 | +4.1 |
| Alcohol | 38.8 | 89.9 | +51.1 | 34.3 | 40.2 | +5.9 |
| Smoking | 73.8 | 92.4 | +18.5 | 69.9 | 72.4 | +2.5 |
| Mobility | 4.8 | 72.1 | +67.3 | 3.3 | 12.2 | +8.9 |
| Ears | 17.3 | 75.6 | +58.3 | 11.1 | 20.7 | +9.6 |
| Eyes | 14.4 | 74.6 | +60.1 | 11.1 | 21.0 | +9.9 |
| Carer | 3.4 | 63.2 | +59.8 | 2.6 | 11.6 | +9.0 |
| Pulse | 16.1 | 67.4 | +51.3 | 14.0 | 25.6 | +11.7 |
| Height | 35.4 | 65.4 | +30.0 | 30.6 | 27.6 | –3.1 |
| Other common categories | ||||||
| Health action plan | 1.8 | 60.0 | +58.2 | 1.5 | 13.5 | +12.0 |
| Behaviour | 4.6 | 53.5 | +48.9 | 2.3 | 8.9 | +6.6 |
| Dental | 1.6 | 53.6 | +52.0 | 0.8 | 8.7 | +7.9 |
| Communication | 0.9 | 44.5 | +43.6 | 0.5 | 5.3 | +4.8 |
| Exercise | 21.9 | 46.4 | +24.6 | 20.2 | 20.7 | +0.5 |
| Diet | 24.2 | 47.1 | +22.9 | 19.0 | 21.5 | +2.5 |
| Blood test | 62.3 | 77.6 | +15.4 | 51.8 | 58.7 | +6.9 |
| Urine test | 39.0 | 58.7 | +19.6 | 30.8 | 32.4 | +1.6 |
| Mental health | 29.2 | 35.7 | +6.6 | 22.5 | 26.3 | +3.8 |
| Bowels and bladder | 15.3 | 30.2 | +14.9 | 11.7 | 13.4 | +1.7 |
| Respiratory | 11.6 | 25.0 | +13.4 | 12.9 | 15.0 | +2.1 |
| Sexual related | 7.9 | 21.0 | +13.1 | 8.8 | 10.7 | +1.9 |
| Specific subgroupsa | ||||||
| Medication review | 60.7 | 65.1 | +4.4 | 46.6 | 50.8 | +4.2 |
| Breast examination | 8.6 | 41.8 | +33.2 | 9.1 | 14.0 | +4.9 |
| Cervical smear | 52.7 | 65.5 | +12.8 | 50.0 | 54.7 | +4.7 |
| Epilepsy | 96.9 | 98.6 | +1.7 | 97.3 | 96.8 | –0.5 |
| Influenza vaccination | 49.7 | 60.6 | +10.9 | 30.5 | 37.7 | +7.2 |
The biggest impact that health checks had was on the recording of health issues regarding mobility (+67.3% difference), eyes (+60.1%), carer details (+59.8%) and ears (58.3%). Prior to health checks there had been minimal information on mobility or carer details, with < 5% of patients having any associated information for these categories. Although adult patients who did not receive a health check up to the end of 2011 had significant increases in all these categories differences ranging from +9% to +10%, the level of change was much smaller than for patients with health checks. Other categories for which the observed change differed notably between these groups of patients were alcohol, pulse, dental, behaviour and communication.
Categories for which the health check appeared to have minimal impact on recording over time were mental health and medication review. During 2009–11, only one in three (35.7%) adults with ID who received a health check had any recording concerning mental health. Although we have identified data issues regarding the completeness of medication reviews on the system (see Chapter 2, Missing entity data in the Clinical Practice Research Datalink), the observed change in recording was similar (+4%) between patients with and patients without health checks.
Vaccination rates for influenza among adults with ID with health checks improved from 49.7% to 60.6%, an increase (+10.9%) that was not notably different from that in those without checks (+7.2%). However, overall coverage was much higher among those with health checks (60.6% vs. 37.7% in 2009–11), due in part to greater health needs among those with checks (e.g. 27% vs. 14% for severe health needs; see Table 40).
Diagnoses, consultations and prescribing before and after health checks
We now investigated whether or not the introduction of health checks had an impact on the diagnosing of common QOF conditions over time. This was done by comparing the change in prevalence rates for selected QOF conditions from 2006–8 to 2009–11 for the 5026 adults with ID who received a health check during 2009–11 (which, by definition, has to be positive) with the change in prevalence in the 2728 adults with ID without a health check during this time (see Figure 36). There was no consistent pattern in the increase in prevalence between the groups, with both groups showing an absolute increase of 1–2% for most conditions (Table 43). The most notable disparity was for a diagnosis of depression, for which patients with ID without health checks had a greater increase (+2.41%) than patients with ID with health checks (+1.59%).
| Category identified | Adults with ID with health check 2009–11 (n = 5026) | Adults with ID without health check 2009–11 (n = 2728) | ||||
|---|---|---|---|---|---|---|
| % 2006–8 | % 2009–11 | ± change | % 2006–8 | % 2009–11 | ± change | |
| Diabetes | 6.03 | 7.54 | +1.51 | 5.61 | 7.29 | +1.68 |
| Hypertension | 10.07 | 12.14 | +2.07 | 11.07 | 12.83 | +1.76 |
| Chronic kidney disease | 2.43 | 3.94 | +1.51 | 2.27 | 3.48 | +1.21 |
| Hyperthyroidism | 8.81 | 10.27 | +1.46 | 5.50 | 6.67 | +1.17 |
| IHD | 0.99 | 1.49 | +0.50 | 2.02 | 2.46 | +0.44 |
| Osteoporosis | 1.37 | 2.03 | +0.66 | 1.25 | 1.72 | +0.47 |
| Depression | 15.10 | 16.69 | +1.59 | 17.16 | 19.57 | +2.41 |
| Severe mental illness | 7.86 | 8.50 | +0.64 | 6.23 | 6.78 | +0.55 |
| Epilepsy | 26.34 | 27.12 | +0.78 | 18.15 | 18.73 | +0.58 |
| COPD | 0.44 | 0.80 | +0.36 | 1.32 | 1.80 | +0.48 |
Figure 38 shows the percentage of patients in 2008 and 2011 with a consultation, a prescription (any, repeats only or psychotropic only) and any referrals made in primary care in 2008 and 2011, by whether or not they received a health check during 2009–11.
FIGURE 38.
Percentage of patients with consultations, prescriptions and referrals in 2008 and 2011 in adults with ID with and adults with ID without health checks 2009–11.
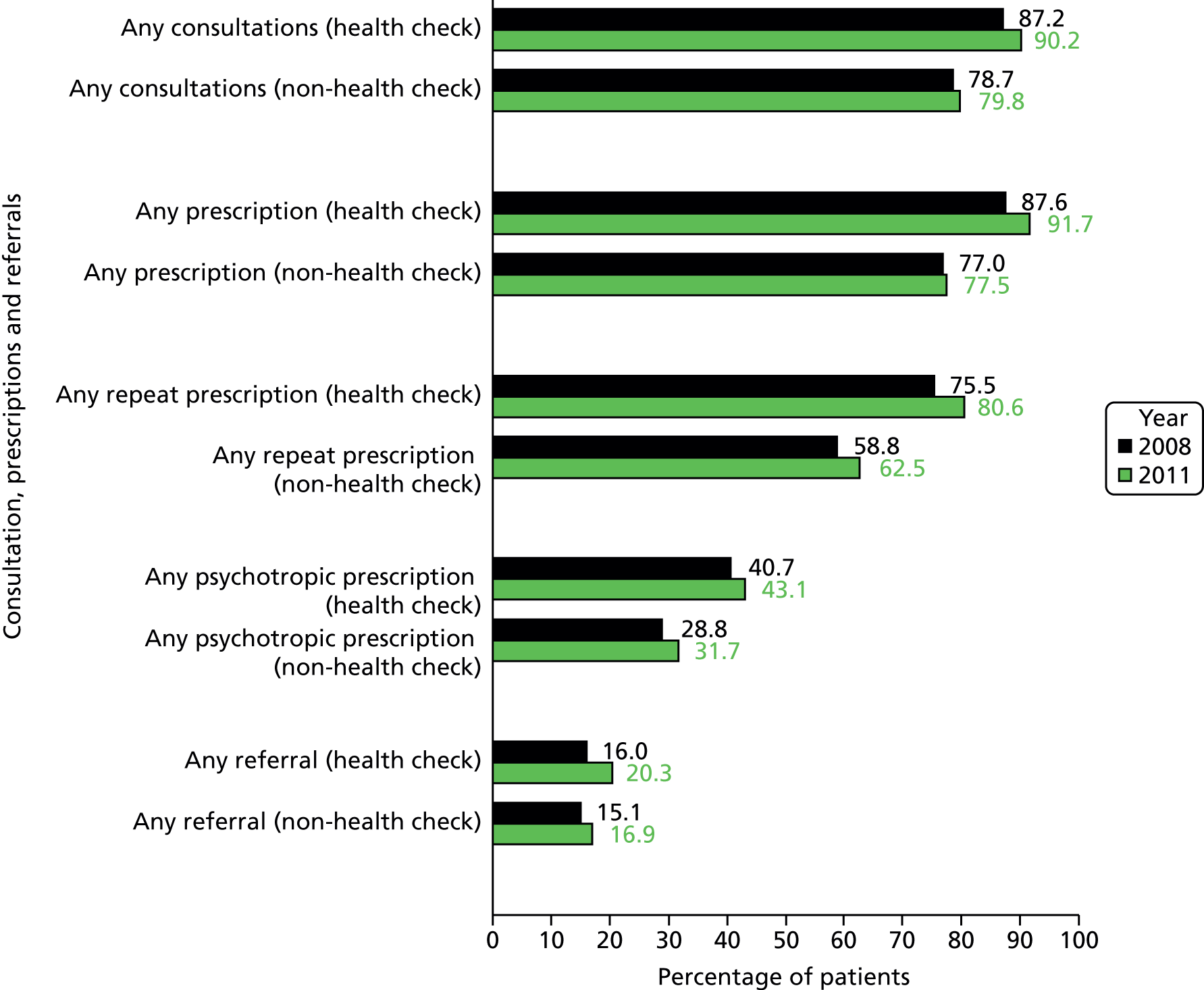
There were clear baseline differences between the two groups in 2008, whereby adults with ID who would go on to receive a health check were already more likely to consult in the year (87.2% vs. 78.7%) or receive any prescription (87.6% vs. 77.0%). By 2011, both groups showed small increases over time, which were generally higher in the health checks group. For example, the percentage of patients with a consultation (not counting the health check itself) increased from 87.2% to 90.2% in the health checks group, compared with 78.7% to 79.8% in the non-health checks group. The percentage of patients with a referral rose from 16.0% to 20.3% for those with health checks, compared with an increase from 15.1% to 16.9% for those without health checks.
We also compared the recording of being seizure free for patients with ID with epilepsy before and after health checks. During 2006–8, 632 of 1080 (58.5%) were seizure free, a figure that rose to 694 of 1080 (64.3%) during 2009–11. This is an absolute increase of 5.8% compared with a 2.7% increase in 372 epilepsy patients without health checks over the same period (which rose from 55.9% to 58.6%).
Finally, we compared the mean level of consultations, prescribing and referrals (made within primary care) in 2008 and 2011, and the associated absolute change, for adults with ID with and adults with ID without health checks (Table 44). To assess if the change in mean level of each outcome differed between groups, we carried out a conservative test based on the change in outcome for each individual. The changes were ranked, and a Wilcoxon rank-sum test was carried out to see if they differed between groups.
| Category identified | Adults with ID with health check 2009–11 (n = 5026) | Adults with ID without health check 2009–11 (n = 2728) | Difference in change,a p-value | ||||
|---|---|---|---|---|---|---|---|
| 2008 | 2011 | ± change | 2008 | 2011 | ± change | ||
| Consultations | 5.38 | 5.93 | +0.55 | 4.64 | 5.38 | +0.74 | 0.71 |
| Drug classes | 5.09 | 5.90 | +0.81 | 4.04 | 4.54 | +0.50 | < 0.001 |
| Drug classes (repeats only) | 3.02 | 3.62 | +0.60 | 2.23 | 2.66 | +0.43 | < 0.001 |
| Psychotropic prescriptions | 0.65 | 0.69 | +0.04 | 0.45 | 0.50 | +0.05 | 0.44 |
| Referrals (made in primary care) | 0.23 | 0.30 | +0.07 | 0.21 | 0.25 | +0.04 | 0.08 |
Although there was no evidence that health checks had led to any significant change in the mean level of consultations over time (p = 0.71), there was some evidence that the change in the overall mean level of prescribing was greater among patients with health checks (p < 0.001), although not for psychotropic prescribing.
Change in estimated economic costs before and during health checks
We also revisited our estimates of annual NHS costs in relation to health checks. Here, we use the costings identified for 2011 (see Appendix 5) and apply these to both 2008 and 2011 for the groups of adults with ID with and adults with ID without health checks. To assess if the change in costs differs between groups, we again ranked the changes for each individual, and carried out a Wilcoxon rank-sum test to see if they differed between groups (Table 45).
| Category identified | Adults with ID with health check 2009–11 (n = 5026) | Adults with ID without health check 2009–11 (n = 2728) | Difference in change,a p-value | ||||
|---|---|---|---|---|---|---|---|
| 2008 | 2011 | ± change | 2008 | 2011 | ± change | ||
| Primary care costs | |||||||
| Mean consultations | 159.4 | 216.7 | +57.3 | 146.1 | 180.4 | +34.3 | < 0.001 |
| Mean prescribing | 455.3 | 559.7 | +104.4 | 310.2 | 399.5 | +89.3 | < 0.001 |
| Secondary care costsb | |||||||
| Elective admissions | 204.0 | 194.8 | –9.2 | 197.9 | 196.1 | –1.8 | 0.80 |
| Non-elective admissions | 292.7 | 429.6 | +136.9 | 311.2 | 472.4 | +161.2 | 0.90 |
Primary care costs for consultations and prescribing rose for both groups, but the mean change within individual patients was greater for adults with ID with health checks (p < 0.001). However, this difference was not replicated when we looked at secondary care costs among patients with linkage to the HES data. Although the cost of elective admissions (based on 2011 costings) remained flat over time for the two groups, there were large increases of approximately 50% for non-elective admissions. Although the overall mean increase was higher for adults with ID without health checks (+£161.2 vs. £136.9 per patient), there was no statistical difference of the comparison of the within-individual change using the Wilcoxon rank-sum test (p = 0.90).
Predictors of first health check during 2009–11
We now investigate what factors were predictors of receiving a first health check during 2009–11 among 7754 adults with ID registered throughout practices with a minimum level (20%) of participation in the DES. A logistic model with practice fitted as a random effect (see Chapter 2, Statistical analysis) was used to produce mutually adjusted ORs for all factors investigated. We carried out sensitivity analyses excluding patients from practices with exceptionally high participation in the DES (> 90%), but this made no material difference to our conclusions.
Table 46 summarises the baseline factors that were important in predicting the receipt of a first health check between 2009 and 2011. Middle-aged and older patients (aged ≥ 35 years at the beginning of follow-up) were more likely to get a health check than younger patients (68.0% vs. 58.7%). The strongest associations were seen among patients with pre-existing epilepsy (87.6%) and those living in communal or shared accommodation (86.4%). Patients who were already being seen in primary care frequently prior to the introduction of health checks (≥ 6 consultations in 2008) were subsequently more likely to get a health check during 2009–11 (69.3%). There was no evidence of a trend with level of area deprivation (p = 0.85).
| Characteristic | Total | With a health check | % | Adjusted ORa (95% CI) |
|---|---|---|---|---|
| All | 7754 | 5026 | 64.8 | – |
| Gender | ||||
| Women | 3269 | 2153 | 65.9 | – |
| Men | 4485 | 2873 | 64.1 | 1.01 (0.90 to 1.13) |
| Age (years) (in 2009) | ||||
| 18–34 | 2669 | 1567 | 58.7 | – |
| 35–54 | 3483 | 2370 | 68.0 | 1.33 (1.17 to 1.51) |
| 55–84 | 1602 | 1089 | 68.0 | 1.19 (1.01 to 1.39) |
| Down syndrome | ||||
| Yes | 863 | 644 | 74.6 | 2.11 (1.75 to 2.55) |
| No | 6891 | 4382 | 63.6 | – |
| Severe health needsb | ||||
| Yes | 1338 | 1117 | 83.5 | 2.39 (2.00 to 2.86) |
| No | 6416 | 3909 | 60.9 | – |
| Communal accommodationb | ||||
| Yes | 1796 | 1551 | 86.4 | 4.35 (3.61 to 5.23) |
| No | 5958 | 3475 | 58.3 | – |
| Autism spectrum disorder | ||||
| Yes | 528 | 401 | 76.0 | 1.63 (1.28 to 2.09) |
| No | 7226 | 4625 | 64.0 | – |
| Epilepsy | ||||
| Yes | 1052 | 921 | 87.6 | 3.46 (2.79 to 4.28) |
| No | 6702 | 4105 | 61.3 | – |
| Deprivationb | ||||
| 1 (least deprived fifth) | 802 | 483 | 60.2 | – |
| 2 | 1126 | 790 | 70.2 | 1.33 (1.04 to 1.69) |
| 3 | 1240 | 848 | 68.4 | 1.22 (0.96 to 1.56) |
| 4 | 1519 | 993 | 65.4 | 1.07 (0.84 to 1.36) |
| 5 (most deprived fifth) | 1661 | 1073 | 64.6 | 1.12 (0.88 to 1.43) |
| Test for trend | p = 0.85 | |||
| Consultations (during 2008) | ||||
| 0–1 | 2219 | 1284 | 57.9 | – |
| 2–5 | 2958 | 1955 | 66.1 | 1.17 (1.03 to 1.34) |
| ≥ 6 | 2577 | 1787 | 69.3 | 1.30 (1.12 to 1.51) |
Predictors of repeated health check during 2010–11
Finally, we investigated the influence of baseline factors on a repeated health check. To do this, we focused on the 3995 patients who received a first health check during 2009 or 2010 from Table 46. For patients with a health check during 2009 (n = 1900), we searched to see if they received another one during 2010. For patients with a health check during 2010 (n = 2095), a subsequent one during 2011 was searched for. Overall, 2425 patients (60.7%) with a first health check during 2009 or 2010 received a second health check during the following calendar year.
Table 47 summarises the baseline factors that were important in predicting a repeated health check between 2010 and 2011. The factors that predicted a first health check showed smaller associations here, with communal living (68.6%) and epilepsy (64.6%) again showing higher attainment. This time, there was a significant trend with deprivation (p < 0.001), with patients living in more deprived areas being less likely to get a repeated check (54.2%).
| Characteristic | Total | With a repeated health check | % | Adjusted ORa (95% CI) |
|---|---|---|---|---|
| All | 3995 | 2425 | 60.7 | – |
| Gender | ||||
| Women | 1729 | 1063 | 61.5 | – |
| Men | 2266 | 1362 | 60.1 | 0.94 (0.81 to 1.09) |
| Age (years) (in 2009) | ||||
| 18–34 | 1207 | 681 | 56.4 | – |
| 35–54 | 1910 | 1186 | 62.1 | 1.30 (1.09 to 1.54) |
| 55–84 | 878 | 558 | 63.6 | 1.41 (1.13 to 1.76) |
| Down syndrome | ||||
| Yes | 511 | 325 | 63.6 | 1.24 (0.99 to 1.56) |
| No | 3484 | 2100 | 60.3 | – |
| Severe health needsb | ||||
| Yes | 593 | 325 | 64.6 | 1.03 (0.86 to 1.24) |
| No | 3077 | 1832 | 59.5 | – |
| Communal accommodationb | ||||
| Yes | 1368 | 938 | 68.6 | 1.60 (1.32 to 1.94) |
| No | 2627 | 1487 | 56.6 | – |
| Autism spectrum disorder | ||||
| Yes | 329 | 205 | 62.3 | 1.20 (0.91 to 1.58) |
| No | 3666 | 2220 | 60.6 | – |
| Epilepsy | ||||
| Yes | 748 | 483 | 64.6 | 1.19 (0.98 to 1.45) |
| No | 3247 | 1942 | 59.8 | – |
| Deprivationb | ||||
| 1 (least deprived fifth) | 336 | 203 | 60.4 | – |
| 2 | 649 | 446 | 68.7 | 1.36 (0.97 to 1.90) |
| 3 | 720 | 482 | 66.9 | 1.08 (0.77 to 1.51) |
| 4 | 803 | 443 | 55.2 | 0.79 (0.57 to 1.09) |
| 5 (most deprived fifth) | 840 | 455 | 54.2 | 0.71 (0.50 to 1.00) |
| Test for trend | p < 0.001 | |||
| Consultations (during 2008) | ||||
| 0–1 | 984 | 560 | 56.9 | – |
| 2–5 | 1566 | 950 | 60.7 | 1.12 (0.93 to 1.36) |
| ≥ 6 | 1445 | 915 | 63.3 | 1.23 (1.01 to 1.51) |
Chapter 8 Discussion
Introduction
In this final section, we now summarise the results from the study (see Chapters 3–7) and discuss them further, including strengths and limitations, placing them in context with the existing literature. Finally, we highlight implications that we have identified. To recap, the study originally had two overall aims (see Table 1):
-
aim 1 – to describe the health, health-care quality, equity of health care, mortality rates and NHS costs for adults with ID in a national sample
-
aim 2 – to evaluate the process and outcome effectiveness of annual health checks for adults with ID in primary care.
For each aim, we now discuss in turn a summary of the findings from the study, its strengths and limitations, how the results compare with other literature and, finally, implications arising from the study.
Aim 1: health, health-care quality, mortality and NHS costs
Summary of findings
We used data from 408 English general practices to show that, compared with an age-, gender- and practice-matched group of patients without ID, adults with ID:
-
had higher overall levels of most chronic diseases and multimorbidity, although recording was lower for CHD and cancer
-
had greater overall primary and secondary care utilisation and costs, particularly prescribing
-
had higher levels of psychotropic prescribing, particularly antipsychotics and benzodiazepines
-
were less likely to have longer doctor consultations and had lower continuity of care with the same doctor
-
were estimated to contribute approximately double the amount of NHS costs across primary and secondary care
-
did not demonstrate the same pattern of greater disease prevalence and prescribing with increases in area deprivation.
We then used data from national hospital admissions and mortality data sets linked to primary care records in 343 practices to create a retrospective longitudinal study between 2009 and 2013, and show that, compared with an age-, gender- and practice-matched group of patients without ID, adults with ID:
-
had a risk of death more than three times higher, even after adjusting for differences in comorbidity
-
had more than one-third of their deaths classed as potentially amenable to health-care interventions
-
were three times as likely to be admitted to hospital for an emergency admission, five times as likely for admissions classed as potentially preventable (ACSCs)
-
had one-third of their emergency admissions classed as potentially preventable
-
did not appear to differ in the primary care utilisation and management before admissions for two common ACSCs (UTIs and LRTIs), despite being at an increased risk of complications.
Strengths and limitations
We have provided a systematic description of the health needs and consultation patterns of adults with ID in English primary care, which has addressed a variety of data gaps that have been highlighted for this group, including chronic disease prevalence. 82 By primary care, we specifically mean health care delivered through the general practice and, thus, other types of primary care (e.g. dentistry and optometry) will not be covered in our summary analyses. The inclusion of controls without ID, or conditions related to ID such as autism, enabled direct age and gender comparisons within the same English population, which is an advantage over approaches that have relied on whole external populations for comparable estimates of chronic disease in the general population. 83 By matching on general practice, we were able to overcome potential variations in the practice recording of health promotion and chronic conditions that are likely to exist in our data, in addition to dissimilarities in consultation access between different practices.
Another potential strength of our approach was the inclusion of a large unselected group of patients with ID identified as such in primary care. As ID (as ‘learning disability’) has been included in QOF since 2006, and the associated prevalence has stabilised (see Appendix 1), it seems reasonable to presume that we have included most adults with severe ID in our study. However, our reliance on primary care data to identify ID could also be viewed as a limitation, as there are noted concerns about the under-recording of ID on primary care systems (see Chapter 2, Quality and Outcomes Framework and learning disability). 39,50 Thus, our results must be viewed in the context of ID identified and recorded by GPs, which will represent the most important group of adults with ID. However, we think it is unlikely that any under-recording of ID could explain away any of the key differences in health-care utilisation that we have observed and detailed here.
There are other limitations that relate to the under-recording or incomplete recording of other characteristics in primary care that we sought to measure in our study. We detailed issues regarding the recording of medication reviews in CPRD (see Chapter 2, Missing entity data in the Clinical Practice Research Datalink), which led to the suggestion that we may be underestimating these, but this would not invalidate comparisons between adults with ID and adults without ID. Key characteristics such as living arrangements or severity of ID were not routinely recorded, so we had use additional information, when available, to bolster these measures. For example, for severity of ID we created a proxy measure of severe health needs that would capture severity through a combination of other recorded health needs (see Figure 5). However, the evidence from the systematic review of health checks for people with ID in 201412 suggested that the identification of some chronic conditions and health needs is incomplete in adults with ID, and so our results should be interpreted as conservative estimates of the true extent of need. For living arrangements, we were restricted to identifying only patients who were recorded as living in shared or communal accommodation by either a specific Read code or clustering of address flag. This approach, although crude, nevertheless allowed us to identify large differences between patients with ID classified this way and those not classified this way. Patients who were not classified this way, however, will have heterogeneous living arrangements, in terms of carers or family support.
Our study attempted to summarise consultation length by using the recorded duration on the underlying computer system that the CPRD practices use (Vision). This, however, must be viewed as an approximation, as the system may also be counting periods when the GP views the electronic record before and after the relevant face-to-face consultation with the patient. We also observed that some duration entries were implausibly zero or overlong, presumably as a result of user error. We attempted to mitigate this by summarising length into binary categories (1–10 vs. > 10 minutes). Despite some uncertainty over consultation length, we do not believe that the aforementioned errors would be disproportionate between adults with ID and controls, and thus our relative comparisons and observed differences are valid.
We also estimated continuity of care by anonymously identifying the GP or nurse during the recorded consultation from their unique system identification on the Vision system. Although this simplistic approach addresses continuity of care with the same clinician, known as relational continuity, it does not address measures of management continuity. These would include the consistency of clinical management or co-ordination of care, which will also make a significant contribution to a patient’s experience of care over time. 84
We also presented a comparison of estimated NHS costs between adults with ID and adults without ID during a single calendar year (2011) using published costings to allocate costs to recorded events. Although events taking place at the GP surgery such as consultations and prescribing are, on the whole, clearly identified on the patient record and could be costed accordingly, events outside the practice, such as outpatient attendance or visits to accident and emergency, were inconsistently recorded, and as a result could not always be identified. Furthermore, we were unable to ascertain the costs of other primary care activities such as laboratory tests. Thus, our estimates of cost must be acknowledged as a significant underestimate, although we do not believe that the under-recording of events would differ disproportionally between patients with ID and patients without ID. For this reason, we chose to compare relative differences in costs as opposed to absolute differences. The doubling of estimated costs compared with the general population appeared to be primarily driven by a similar relative difference in the underlying admission rate. Despite our caveat about our NHS costs estimates, we were still able to highlight an association of falling costs with increasing levels of area deprivation among adults with ID living in shared or communal accommodation, which is the inverse of what is observed in the general population.
We also provided a comprehensive description of the patterns in mortality and emergency hospital admissions for a large cohort of adults with ID in England between 2009 and 2013. The linkage of primary care data to routine data sources of mortality and secondary care use directly addresses a key data gap that has been recently highlighted in a 2015 review of mortality for people with ID in England,25 and featured as a recommendation (number 16) in the CIPOLD. 22 Our detailed comparison of emergency hospitalisation rate for adults with ID with the general population extends an area of limited research. 85 Our work makes a significant contribution by quantifying mortality and hospitalisation disparities for adults with ID compared with the general population, an area in which accurate and detailed information is essential for future planning and policy-making. 86
This study’s utilisation of linked primary care data allows for better ascertainment of adults with ID, which in the UK has been historically been poor in mortality data25 and thought to be low among hospital admissions data. 13 In our study, we found a low proportion (31%) with a recording of ID or associated condition as a secondary cause on their death certificates, similar to that found by others. 25 Likewise, only 66% of adults with ID with a hospital admission in our study had ID recorded on their record, emphasising the limitation of studies based on hospitalisation records or death certificates alone. The linked primary care records in our study also allows for control and stratification by factors not routinely available in hospital or mortality data, such as comorbidity and smoking.
For the mortality analyses, one of the main limitations of our study is the potentially incomplete and inaccurate recording in death certification data. For example, in our study many patients with Down syndrome had this condition recorded as their underlying cause of death and respiratory diseases given as a secondary cause, when the latter was probably the more appropriate underlying cause. This miscoding would have had no impact in our analyses of avoidable mortality, as either condition would still have been classified as an amenable, and hence avoidable, death. However, it could also be argued that some deaths among adults with ID are ultimately less avoidable owing to the conditions associated with ID. For example, immune defects common in adults with Down syndrome may make such adults more prone to infection87 and, subsequently, less amenable to treatment.
In our analysis of hospital admissions, a small number had an uninformative primary diagnosis of ID, so we were unable to determine a more specific reason for their admission. In our comparison of primary care utilisation prior to hospitalisation for two common infections, we suspected that urine dipstick tests were poorly recorded across both groups and likely to be underestimated. This analysis was unmatched and, although we adjusted for age and gender differences between patients with ID and controls in those presenting, we cannot be sure how comparable the scenarios are for the two groups. Similarly, although epilepsy was a common reason for admission, we chose not to compare epilepsy admissions between adults with and adults without ID, as we had reservations about how comparable the severity of the condition would be between groups. In addition, epilepsy management, such as drug and dose changes, are mostly initiated and managed by non-primary care specialists.
Comparison with other studies
Disease prevalence
A number of studies in the UK and internationally have described the prevalence of health problems in people with ID. 19,20,83,88–92 These have shown high levels of comorbidity, although direct comparisons of estimated prevalence with the general population has generally been difficult owing to population selection and disease definition. Only a recent Scottish study in primary care of 8014 adults with ID has been able to provide comprehensive standardised prevalence rates by age groups,92 and produced findings for 2007 similar to our own published findings for 2012. 66
In addition, the recent studies in Scotland,92 Ireland83 and the Netherlands91 have all considered multimorbidity in adults with ID. These studies considered a wider range of conditions than our study and, as a result, reported higher levels of multimorbidity than we did. This makes any direct comparison difficult; however, the relative doubling of multimorbidity (defined as two or more conditions) between adults with and adults without ID in the Scottish study92 were similar to our findings, in which adults with ID were 1.8 times more likely to have multiple QOF conditions. The Dutch study finding of greater multimorbidity among adults with Down syndrome91 was the opposite of what we found, presumably owing to this study involving older adults only (≥ 50 years), whereas our patients with Down syndrome were primarily younger (73% were < 50 years old).
Looking at individual conditions, our estimate of the prevalence of epilepsy in adults with ID (18.5%) compared favourably with an estimate of 18.8% found in the recent Scottish primary care study. 92 Both are lower than an estimate of 26% found in Leicestershire from a regionally based register in 2006,18 but this may reflect regional and methodological differences. There has also been a concern that epilepsy has been historically overdiagnosed in people with ID, estimated at around 3 in 10 from a review in 2011,93 and so our more recent findings may represent an improvement in diagnosis.
We also demonstrated an excess of recorded mental health problems among patients with ID, which require good access to specialist services and present a challenge to primary care in managing such patients, for which GPs may lack sufficient support. 94 Our high prevalence of recorded mental health problems such as schizophrenia (6.8%) is similar to that found in the Scottish primary care study (5.6%),92 and consistent with an earlier population-based survey95 undertaken in Glasgow in the early 2000s, which found that 4.4% of 1023 adults with ID received a clinical diagnosis of a psychotic disorder, including schizophrenia. Although the recording of depression ever in the patient record for adults with ID (18%) was similar to that reported in the Scottish primary care study (16%),92 we found no difference when compared with our matched controls, whereas in Scotland adults with ID were significantly more likely to have a diagnosis than population controls. 92 When we restricted to diagnoses made in the last year, we actually found that adults with ID were less likely to receive a depression diagnosis. This may have reflected the reluctance of some GPs to make a diagnosis, which during 2011 would have required the further use of assessment tools in QOF,47 which may not be appropriate for some patients with ID (and would not have been the case for the Scottish study reported in 2007). 92
There has been limited information on the physical and sensory disability prevalence among adults with ID from the UK. The Scottish study of primary care data estimated hearing loss at 8.2% and visual impairment at 3.2%,92 which compares favourably with our estimates of deafness (8.3%) and bilateral visual loss or low vision (4.7%). Internationally, our estimates of severe visual problems was close to the prevalence of blindness (5.0%) reported in a detailed Dutch study of visual impairment among adults with ID. 20 Similarly, our recorded prevalence of behavioural problems was similar to the prevalances reported in earlier regional studies in England96 and Norway. 97
The lower recording of cancer, IHD and COPD in adults with ID was surprising, especially given the high prevalence of comorbid risk factors for IHD, such as diabetes, obesity, hypothyroidism, chronic kidney disease and stroke. However, any apparent higher risk may be offset by the much lower recorded rates of smoking and alcohol use among adults with ID. The lower prevalence of these conditions was also observed in comparisons with the general population in Scotland with age- and sex-standardised ORs of 0.69 for cancer, 0.43 for CHD and 0.84 for COPD,92 which compare with our PRs of 0.65 (cancer and IHD) and 0.84 for COPD. Internationally, a recent Dutch longitudinal study of older adults with IHD estimated the incidence of CHD to be 6.5 per 1000 person-years, compared with 7.3 from general population estimates. 98 In addition to the noted difference in lifestyle factors, there are two other possible explanations for the lower prevalence of these conditions. One would be that the data reflect inadequate identification among adults with ID,99 and the recorded prevalence is a poor estimate of the true underlying prevalence. For cancer, for example, a diagnosis may be delayed through communication difficulties regarding symptoms with their carers or family members. 100 Alternatively, the data correctly reflect reality, but owing to the premature mortality among adults with ID there is a survivor-type effect within the population with ID. If a significant proportion of younger adults with ID who would have gone on to develop cancer or IHD in later life never reach the advanced age at which these diseases are typically diagnosed within the general population, then the prevalence of these conditions in later life would be lower. This argument is given some credence by the observation that a higher prevalence of both cancer and ID was seen when the comparison was restricted to younger adults only, although numbers with the conditions were small (see Table 7).
We also showed that, compared with the general population, adult patients with ID were more likely to be recorded both as obese (BMI of > 30 kg/m2) and as underweight (BMI of < 20 kg/m2). Our estimate that 36.4% of adults with ID measured were obese is similar to other UK findings,101,102 but far exceeds a pooled prevalence estimate of 15% among adolescents with ID from several countries. 103 Although the association between ID and being underweight in adulthood is generally accepted owing to poor feeding and swallowing,17 we were not aware of any population estimates of its prevalence. Older patients with ID are known to suffer an earlier onset of frailty than the general population,104 and our higher prevalence of recorded osteoporosis reflects the high prevalence of low bone quality that has been measured among older patients with ID. 105 A recent Dutch study98 showed that a low BMI among older patients with ID was predictive of 3-year mortality.
Consultations
Our overall estimate, of a 70% higher rate in GP consultations between adults with and adults without ID of the same age and gender, matched that found in a Dutch study19 of 71 general practices during 2001. We were able to further demonstrate that this higher consultation rate was not explained by the higher prevalence of conditions included in the QOF.
This finding of higher consultations contrasted with two small earlier UK studies, one in London106 that sampled 187 adults with ID from 40 practices and another based on 142 adults in the east of England. 107 Neither found an increase in consultation among their adults with ID when the authors compared their study results with expected consultation levels estimated using national data. 108 Our study has the advantage of directly comparing consultation behaviour within practices, accounting for any practice variations or trends. In addition to the methodological differences, these older studies may also reflect temporal changes in consultation behaviour for adults for ID that may have taken place in the UK.
Our analysis of recorded consultation length showed that although adults with ID had more consultations of a long duration (> 10 minutes) overall with a GP or nurse during the year than their matched controls, they were less likely to have a longer consultation when their higher overall consultation level was taken into account. In other words, any given consultation with a GP or nurse is likely to be shorter on average for an adult patient with ID. For continuity of care, patients with ID were consistently less likely to see the same doctor, no matter how many consultations they had during the year. This may partly reflect a greater propensity for these patients to consult for acute problems for which an urgent appointment is more important than continuity per se. Although this may be true, the ability to see their regular GP was highlighted by our patient group in the study as an important factor in their health care (see Table 3). Discussions with the patient group also found that allotted appointment times were not always adequate for discussing their health issues. Both increased consultation times through double appointments and enhanced continuity of care have been highlighted as reasonable adjustments that general practices could be expected to make in improving the access of health care for people with ID. 109
Prescribing
The prescribing of psychotropic medication for challenging behaviour in adults with ID is much discussed and controversial in nature, with concerns of overprescribing within this group. 110 Additionally, there has been observed a low level of recorded ancillary information in the electronic GP records of patients with ID to justify the level of prescribing observed. 111 In the UK, the scale of the prescribing of psychotropic drugs to patients with ID nationally has been previously described in the CPRD data between 2009 and 2012,112 and more recently in another primary care database (THIN) from 1999 to 2013. 111 The study based on CPRD data found that, among adults with ID over a 4-year period, 41.3% of follow-up time was exposed to at least one psychotropic drug (including antiepileptic drugs). We provided an alternative summary (and did not count antiepileptic drugs), describing instead the proportion of adults with ID who received a psychotropic drug at any time during single year (2011), and found a similar 4 in 10 proportion. This was lower than that reported in Scotland during 2002–4 (49.5%),95 but more similar to other international cross-sectional findings from the Netherlands (32%),113 Norway (37%)114 and Australia (35%). 115 Although these studies generally showed that antipsychotics were the most frequent type of psychotropic medication being prescribed to this group, in our study antipsychotics and antidepressants were equally likely to be prescribed.
The most comprehensive comparison of prescribing trends between adults with and adults without ID in a primary care setting that we are aware of is a 2001 Dutch primary care study. 19 This study of 868 patients with ID found that 82% received any prescription during the year, compared with 69% of age-, gender- and practice-matched controls. By contrast, we found 86% and 67%, respectively, and similarly found antipsychotic drugs to be the most common class of drug prescribed to this group.
Among antipsychotics, the most common drugs prescribed to adult patients with ID in 2011 were the atypical/second generation antipsychotics risperidone and olanzapine, which are effective in reducing aggressive behaviour in patients with ID in comparison with typical/first-generation drugs. 116 However, typical/first-generation antipsychotics such as chlorpromazine and haloperidol were still widely prescribed to adults with ID, although these were almost non-existent in the general population. Many patients with ID are treated long term with antipsychotics for many years,117 and the prevalence of adverse effects resulting from such drugs is thought to be high. A recent Dutch study118 reported associations between psychotropic drugs and quality of life, with a large majority of patients with ID (> 90%) on psychotropic drugs experiencing an adverse event during a 2-year follow up.
The greater prescribing of benzodiazepines among adults with ID will be partly attributable to the higher prevalence of epilepsy in this group, as benzodiazepines such as clobazam are licensed for the prevention and treatment of seizures in epilepsy. 119 Although we found the rate of antidepressant prescribing to be double that for adults with ID compared with the general population, the prescribing of low-dose amitriptyline was an exception, being lower in adults with ID. As amitriptyline is often prescribed for neuropathic pain,120 our finding may indicate that patients without ID are more often prescribed amitriptyline for this important indication.
Mortality
Our finding of an increased overall risk of death associated with ID is consistent with numerous contemporary findings, both in the UK and internationally, that show premature mortality for this group. 14 In the UK, studies of mortality among people with ID have used a number of data sources, including local registers, death certification data alone and national registers. 25 The largest existing UK study to date121 was based on the follow-up of a regional disease in Leicestershire between 1993 and 2006, identifying 503 deaths among adults with ID, and found an increased risk of death of just under three [standardised mortality ratio (SMR) 2.77] compared with the general population. 122 This was slightly lower than our age- and gender-adjusted HR of 3.62, which may be attributable to regional as well as period and other methodological differences. Internationally, a recent large retrospective longitudinal study123 in New South Wales, Australia, used linked health data for 817 deaths among people with ID aged 5–69 years to produce a SMR of 3.15.
Gender differences that may have an impact on mortality within the population with ID are not well understood. 124 In our study we observed higher age- and gender-adjusted mortality rates for women (139.5 per 10,000 persons per year) than for men (127.3), although no statistical difference remained when we adjusted for differences in comorbidity between the genders. This was similar to a recent US study124 utilising information from four state level-disability service systems, which found higher mortality rates for women with ID than for men with ID (18.9 vs. 16.2 per 1000). However, simply comparing overall mortality rates could hide any potential gender disparity, as men of a similar age in the general population may have a higher underlying mortality rate than women from being more likely to engage in higher-risk lifestyles or behaviours, a difference that may not exist within the population with ID. 124
Therefore, although more deaths are observed among adult men with ID in many studies,82,122,123,125 when the authors’ analyses compare observed mortality with expected deaths in their control populations, using SMRs, they observe much higher expected mortality for women with ID. For example, in the New South Wales study,123 the authors reported a SMR of 4.26 for women versus a SMR of 2.52 for men, whereas the Leicestershire study122 produced a similarly higher SMR for women (3.24) compared with men (2.28). A comparable gender disparity was also seen for SMRs in all ages in a recent study in Ireland126 using national databases of people with ID and census data. In our study, we also observed more deaths among adult men with ID than among women with ID (365 vs. 291; see Table 22), but a greater relative mortality risk for women (HR 4.10; see Table 23) relative to their general population controls than the corresponding estimate for men (HR 3.30). Although our analysis seemingly has the advantage of directly comparing adults with ID with age-, gender- and practice-matched controls, rather than to a larger reference population, a potential drawback is that it is then based on a smaller number of deaths within its control population as we only have a sample of all adults without ID. This may account for differences in the estimated mortality in the general population, especially at younger ages, and why our gender difference was not as notable as that found previously in the Leicestershire study. 122 Regardless of these methodological differences, the gender relationship between ID and mortality is complex and warrants further investigation. 123
We found an elevated risk of mortality in adults with Down syndrome, which was approximately three times higher than that in adults with ID without Down syndrome. Mortality in people with Down syndrome has been widely studied. 125,127–130 A large Danish study129 of 3530 persons with Down syndrome found a HR of 8.94 for standard trisomy 21 versus the general population for mortality between 1968 and 2009, which compares closely with the HR of 9.21 (see Table 23) that we found before any adjustment for comorbidity. A smaller American study130 of 169 adults with Down syndrome residing in the community found an adjusted risk of death almost four times as high (3.77) as that for other adults with ID without Down syndrome. A recent study in Ontario, Canada,125 of 172 deaths among people of all ages with ID also found an elevated risk for Down syndrome, but only among those aged > 60 years.
Among patients with ID with autism spectrum disorder, we found some evidence that their risk of mortality was lower than that for patients with ID without autism (HR 0.56; see Table 24), even after adjusting for the age differences between the groups. However, we are cautious about overinterpreting this finding, as very few of this younger subgroup died during our study (n = 15, 1.0%). Their risk of death was still estimated to be twice that of their matched controls without ID (HR 2.2; see Table 23). A doubling of mortality risk with autism spectrum disorder compared with the general population has been shown in several population cohorts worldwide;131 however, this risk increases in studies that were able to further restrict the comparison to subjects with a co-existing ID131 or neurological disorders. 132 Although a recent large Swedish case–control study131 reported an OR of 5.8, the median age of death for the group with co-existing ID (40 years) suggests that insufficient follow-up in our study (3 years) may account for our imprecise findings among the younger subgroup of adults with ID with autism, who had an average age of only 30.5 years at the beginning of follow-up.
We also estimated a higher risk of mortality for adults with ID with epilepsy than for adults with ID without epilepsy. There is established concern about epilepsy as a condition more commonly associated with death for people with ID,75 particularly the contribution of sudden unexpected death associated with epilepsy (SUDEP). 133,134 A Swedish study135 of 1478 people with ID found associations between epilepsy and mortality between 1987 and 1992, with an estimated SMR of 5.0 for those with epilepsy compared with 1.6 for those without epilepsy. This compares with the HRs we found of 6.0 and 3.2 before adjusting for mortality (see Table 23). In the Leicestershire study,133 elevated SMRs for adults with ID with epilepsy were seen in both men (SMR 3.2) and women (SMR 5.6), with both rising dramatically when the outcome was restricted to SUDEP, identified from case notes and post-mortem reports. In Ontario, elevated mortality with epilepsy for people with ID compared with that for those without epilepsy was about 1.8 times higher for ages 20–60 years,125 compared with our estimate of 1.6–1.7 (see Table 24).
Our description of cause-specific mortality by comparison of ICD-10 categories is broadly similar to findings from the Leicestershire study,122 with the smaller number of deaths within some categories accounting for some variation. No association with cancer was found in the earlier studies in Lecistershire,122 nor was it found a large 35-year follow-up study in Finland. 15 Although we found a small excess of mortality from cancer in adults with ID in our study, it varied by type, and was notably smaller for lung and prostate cancer. Cancer is thought to be a less prominent cause of death for people with ID, perhaps owing to the premature mortality within this group. 75 However, we still demonstrated increased associations with some cancers (particularly colorectal; see Table 25), which suggests that the associations with different neoplasms are more nuanced. Our findings may also highlight an important change resulting from an ageing population of people with ID due to increases in life expectancy. 136
A high proportion of deaths amenable to health-care intervention was described in CIPOLD. 137 However, the inquiry was only able to compare this proportion with the national UK average, and could not quantify either the absolute or the relative risk. Our study extends this work, and provides quantitative estimates of this risk for adults with ID (see Figure 28), with the rate of such deaths being almost six times higher among adults with ID than they were for adults of the same age and gender within the general population without ID. However, existing definitions of amenable mortality do not include some important treatable causes of deaths among people with ID, including UTIs and aspiration, and so are likely to underestimate the true burden of amenable mortality. However, at the same time it may be that some causes of death are less preventable or amenable in adults with ID owing to the underlying cause of the ID itself. For example, the immune defects observed in people with Down syndrome may lead to infections being more common, more severe and less amenable to treatment. 130
The difference in the relative contribution of preventable and amenable deaths to avoidable mortality compared with the general population may be partly explained by differences in lifestyle exposures. For example, we found that adults with recorded ID in primary care were also far less likely to be recorded as smokers or consumers of alcohol on their electronic patient record. Adherence to current medical guidelines may also differ owing to communication difficulties with patients with ID. 99 However, the high absolute risk of deaths amenable to health-care intervention reflects established concerns over difficulties accessing health care, delays in diagnosis and poorer management experienced by people with ID. 8,22
Hospital admissions
There are few recent studies about emergency hospital usage by adults with ID. 138 In England, the only previous national study, by Glover and Evison,13 used earlier hospital data from 2005–9 and, although large, it relied solely on the identification of ID from hospital data. Using the linked data sets in our study, we estimated that approximately one in three adults with ID who has an emergency admission in England does not have ID recorded anywhere on his or her hospital record. This may explain the small difference in crude admission rates for emergency ACSCs between our study (61 per 1000 per year) and that found in the earlier 2005–9 study by Glover and Evison13 (76 per 1000 per year), as less severe cases of ID are presumably less likely to be recorded in hospital data. However, when Glover and Evison13 compared admission rates for ACSCs with those for the general population, they also found a similar five times relative difference to what we found (see Table 31).
In terms of different ACSCs involved, the findings in Glover and Evison13 were broadly similar to those that we observed, with emergency admissions for epilepsy and convulsions accounting for 41% of ACSCs, compared with 36% in our study. Both studies found much higher emergency admissions for constipation and pneumonia, but we did not observe the same rates of admission seen for complications of diabetes, although they were still higher for adults with ID than for the general population.
There are three other large-scale studies139–141 on hospitalisations of adults with ID that we are aware of, but none differentiated between emergency and planned admissions. Our focus on preventable emergency admissions means that any comparison is difficult, as we would not expect good primary care management to decrease planned admissions for ACSCs. However, the large Canadian study142 from Manitoba found elevated hospitalisation rates during 1999–2003 for both epilepsy (RR 54) and constipation (RR 7.9) compared with the general population, both of which will be dominated by emergency admissions, and as a result gave a similar picture to the pattern of emergency admissions in our study.
Costs
We are not aware of any other studies that have compared NHS costs between age- and gender-matched patients with ID and age- and gender-matched patients without ID.
Implications
We have identified the following implications from our cross-sectional analysis of disease prevalence, consultations and prescribing, and NHS costs.
-
Our findings on prevalence of chronic disease raise concerns about the inadequate identification of some conditions such as cancer and IHD. The lower prevalence of cancer in particular needs further exploration, as this may indicate late diagnosis or poorer survival. A particular focus could be on colorectal cancer, for which higher mortality rates were observed.
-
The main burden of excess chronic disease for adult patients with ID is provided by epilepsy and severe mental illness such as schizophrenia. Ways to address these challenges for primary care and to improve access to specialist services need consideration.
-
Although psychotropic prescribing was much higher for adults with ID, the prescribing of low-dose amitriptyline was lower. As the latter drug is often prescribed for neuropathic pain, one interpretation might be that diagnoses of pain in patients with ID are missed, and that these patients are less likely to communicate their symptoms well.
-
The high burden of obesity among adults with ID is a concern, but it also presents an ongoing opportunity to build on weight loss interventions for patients with ID. 143 Additionally, adults with ID are more likely to be underweight, which also needs recognition and action.
-
The higher level of chronic disease in adults with ID than in the general population is not adequately captured by the Charlson index, emphasising this may not be the most appropriate measure of comorbidity and mortality risk for this group.
-
As higher consultation levels for adults with ID were not explained by comorbidity, this implies that the resource implications of caring for adult patients with ID are unlikely to be addressed through the present remuneration systems developed for QOF. Additionally, the high levels of need and utilisation by patients in communal establishments will lead to variable demands on practices, depending on local variations in the density of communal establishments.
-
Practices could take steps to improve access to longer consultations and continuity of care for patients with ID, as part of a reasonable adjustment. 109 This may be achieved by simple flags on computerised primary care records that prompt receptionists to offer double appointments when possible and bypass on-call doctor arrangements for specific patients.
-
The higher levels of prescribing and prescribing costs in primary care for adults with ID, combined with the low levels of recorded medication reviews for this group, suggest that there is potential for changes to practice that could improve quality of care and potentially reduce NHS prescribing costs. In particular, the higher prescribing of psychotropic drugs among adults with ID is a concern and warrants further investigation.
-
The high excess costs for adults with ID for emergency hospital admissions confirm the importance of examining emergency hospital admissions as an outcome for the effectiveness of health checks.
-
The inverse association of NHS costs with increasing deprivation among adults with ID living in communal or shared accommodation needs further explanation, as it may represent inequitable health care of patients from this subgroup who live in poorer areas.
-
The lack of comparable data in the literature on NHS costs for adults with ID suggests that more research is needed in this important area, which is vital for planning services and resources.
We have identified the following implications from our longitudinal results of mortality and hospital admissions.
-
The consistently higher mortality risk for adults with ID seen at all ages reiterates the overall greater health-care need of people with ID. Consistent guidance on the recording of ID as a contributory, but not underlying, cause on death certificates would be helpful for the ongoing surveillance of the health of people with ID in all countries. 144
-
The higher burden of respiratory deaths among adults with ID is important to highlight, as national strategies in developed countries often give a lower priority to respiratory health. The large contribution of pneumonia and aspiration represents a potential focus for improving health care for people with ID.
-
The much greater risk of death from urinary and neurological causes among adults with ID highlights further potential opportunities to improve care for this population through better management of UTIs and by optimising seizure control in people with ID.
-
Our finding that more than one-third of deaths among adults with ID were amenable to health care emphasises that strategies for improving health among people with ID need to prioritise access to and quality of health care as well as preventative interventions. Existing population-wide strategies for working-age adults in high-income countries focus on cardiovascular risk and lifestyle factors, which, although important for people with ID, do not address their different health-care needs. Addressing the health and mortality disparities experienced by adults with ID is a key challenge for health-care systems and a potentially important indicator of health-care system equity and effectiveness.
-
The higher emergency admission rate for adults with ID, which is even more marked for preventable admissions, highlights a specific area in which improvements could be made. As the life expectancy of adults with ID increases,136 it is essential that preventable admissions are fully described, so that appropriate interventions, specific to adults with ID, can be developed.
-
We observed that one in three adults with a diagnosis of ID from primary care had no mention of ID on his or her hospital record. The inadequate flagging of these patients is seen as barrier to effective and safe hospital care. 40 Improving the sharing of information about diagnoses of ID across NHS services, particularly from GP systems, should continue to be part of a reasonable adjustment to improve the health-care needs for these patients. 40
-
Although the primary care utilisation and management prior to an admission for a UTI or LRTI for an adult patient with ID was not noticeably different from that for patients without ID, the primary care records for the former group did identify them as being at higher risk of UTI or LRTI. As integrated risk stratification software is increasingly available in primary care,145 this could be reasonably extended to better incorporate patients with ID, thereby facilitating the most appropriate initial management and follow-up monitoring. 146
Aim 2: health checks and effectiveness of health checks
Summary of findings
We used several methodological approaches to investigate the impact of health checks for adults with ID and found:
-
there was no evidence that the introduction of health checks was associated with a fall in overall emergency hospitalisation, except for adults with severe health needs
-
the change in the rate of potentially preventable emergency admissions was lower than expected after health checks, both within individuals and at a practice level
-
there were large variations in recorded information on the patient record around the time of the health check, both between individuals and between practices
-
adults with ID who would go on to receive health checks were already consulting more and had higher prescribing levels and NHS costs than other adults with ID who did not go on to have health checks
-
adults with ID who received health checks had larger increases in prescribing levels and costs than adults with ID without health checks, but patterns with consultation levels were less clear
-
among practices carrying out health checks, adults with ID who had more severe health needs or who were living in communal establishments were more likely to receive a health check
-
practices in the most deprived areas were more likely to offer health checks during 2009–12 than those in the least deprived areas; however, among patients who received a health check during 2009–10, those living in more deprived areas were less likely to receive a follow-up health check in 2010–11.
Strengths and limitations
We believe that our study is the first to report on the health outcome benefits of health checks for adults with ID rather than just on process measures. 90 Although the systematic reviews by Robertson et al. 12,147 showed the effectiveness of health checks in detecting unrecognised health needs in people with ID, they highlighted the lack of evidence regarding whether or not the provision of health checks translated into important longer-term benefits, such as a reduction in avoidable hospitalisations or mortality. For health checks among the general population (for 40- to 74-year-olds), a recent study148 using CPRD data showed that their introduction increased the identification of cardiovascular risk factors, but an earlier Cochrane systematic review149 for similar general health checks failed to find evidence that they reduced mortality, hospitalisation or disability.
A strength of our analysis of health checks and hospital admissions was that we reached a similar conclusion from two different approaches, one based on practice-level comparisons and the other based on individuals. As these two strategies used slightly different patient groups and definitions of time, the same conclusion would not necessarily be expected. An example of how the different groups behaved in the analyses could be seen in the trends in emergency hospital admissions over time. In the analyses of individual patients with ID, emergency hospital admissions were rising post health check for those with checks, or post index date for those without health checks (see Table 38). On the other hand, the practice-level analyses showed an apparent fall in admissions during 2011–12 (see Table 36). The observed rise in admissions in the same individuals is partly explained by their ageing over time, plus the fundamental requirement for them to be alive at the time of health check (or on the index date). This means that any deaths during the study for this group of patients can occur post health check only, and these would probably be associated with a rise in admissions beforehand. By contrast, the observed practice trends were based on an open cohort of all patients with ID aged 18–84 years in each calendar year, keeping average age effectively constant and allowing mortality within patients during each year.
Our analysis of health checks and hospital admissions has some limitations. The analysis at practice level was unmatched, and would probably be subject to residual confounding from unmeasured factors and characteristics at both practice and individual level. We observed that practices that regularly performed health checks were more likely to have adults with ID recorded with severe health needs, or who were recorded as living in communal establishments, than practices who did not participate (see Table 35). However, this may reflect different levels of recording in these practices, as the group of practices that went on to regularly carry out health checks in our study already had lower emergency hospital admissions rates among their patients with ID at the outset in 2009 (see Table 36). These practices might have further reduced admissions anyway, and the subsequent adoption of health checks may simply be a marker of other improvements in their care over the study period.
In order to control for any practice-level changes over time, we matched individual adults with ID receiving health checks with population controls in the same practice. This analysis now adjusts for any temporal change, be it artefact or real, across practices or hospitals that might have taken place during the study. However, this adjustment would still fail to account for any changes specific to people with ID that might have happened. These could feasibly have occurred in the UK as a result of two high-profile independent inquiries that have taken place during the last decade. 8,22 Therefore, our analysis also crucially included patients with ID without health checks as a second control group not exposed to health checks. Instead, we assigned them a random health check date based on the distribution of observed dates for health checks. As this group of patients showed no similar reduction in ACSCs compared with their matched controls, it provided additional evidence for the effectiveness of health checks. On the other hand, as our finding that adults with Down syndrome increased emergency admissions by 55% post health check was also replicated in adults with Down syndrome without health checks, we concluded that this trend was specific to patients with Down syndrome and not to health checks. This increase in emergency admissions for patients with Down syndrome may reflect premature ageing associated with the condition, such as early-onset Alzheimer’s disease,150 combined with better survival into middle age, in part due to advances in childhood cardiac surgery. 128
Although we have provided a description of the information recorded on the electronic patient record at the time of the health check, this may not represent all of the important events that actually took place. It also cannot be assumed that the amount of information recorded directly correlates with the overall quality of the health check. There may be reasons specific to certain practices why some features of the health check are not regularly recorded electronically. For example, we observed that a cluster of practices that featured a high proportion of patients living in communal establishments recorded zero information besides the system flag to facilitate payment. We do not believe that these health checks were truly empty in their content. Therefore, our findings need to be seen in the context of the limitations of recorded electronic information.
Our analysis comparing changes in specific recorded process measures between adults with ID with and adults with ID without health checks was unmatched, and has limitations as a result of the potential non-comparability of the two groups. Before health checks were introduced, patients who would go on to receive health checks in our study already had higher levels of recording for many process measures, as well as higher levels of prescribing. Additionally, they were more likely to have severe health needs or to be resident in communal accommodation. This makes any comparison between the two groups of patients with ID difficult to interpret. As a result, we kept the statistical approach austere, focusing on change within individuals, and using non-parametric tests to compare the change between the groups. Sensitivity analyses, investigating the change in consultation and prescribing levels comparing with the matched population controls, in the same manner as the analysis of hospital admissions in Chapter 6, produced similar findings to those of the unmatched analyses.
Although we did not attempt a formal economic costing of the effectiveness of the health check scheme, we estimated annual NHS costs before and after health checks. As already noted, there were already cost disparities before the scheme began, with patients who would go on to receive health checks already having higher primary care costs. Our comparison of within-person changes in costs showed higher increases for both primary care consultation and prescribing costs for patients with health checks. Although the mean overall costs for non-elective hospital admissions appeared to have increased less for health check patients, our statistical comparison of within-patient cost showed no evidence of a difference, owing to the majority of patients having zero costs in both periods.
Comparison with the literature
Health checks and hospital admissions
Reducing emergency hospital admissions to contain health-care costs is a major international concern, but evidence for successful community interventions has been limited. 151 Although our primary outcome of overall emergency hospital admission showed no change after the introduction of health checks for adults with ID, the evidence for a reduction in potentially preventable admissions was more consistent, and plausible. Given that admissions for ACSCs represent less than one in five emergency admissions in the UK,41 it is perhaps not surprising that we failed to detect a change among the broader group of all emergency admissions.
Within the general population, there has been a lack of evidence to support case management as an effective intervention for reducing emergency admissions. 151 Similar to the DES for annual health checks, GPs in England have been recently incentivised to case manage patients identified as high risk (approximately 2%) as part of UK policy to reduce emergency admissions. 29 Despite this, it has been argued that the focus should move towards admissions for conditions that are more amenable to prevention in the community,151 such as ACSCs. Although we were not able to determine the proportion of adults with ID who were being classified as high risk by GPs, we have confirmed their higher overall emergency admission rates to hospital, and estimated that about one in three of these was for an ACSC. Admissions for epilepsy contributed about 4 in 10 emergency admissions for ACSCs for adults with ID, so one possible explanation is that health checks are facilitating better overall management of epilepsy and seizures among patients with ID. Similar to earlier findings from CPRD data from 2007,24 our cross-sectional analysis during 2011 showed that adults with ID had lower recorded rates of being recorded as seizure free during the year than adults with epilepsy from the general population. This difference may be attributable to differences in disease severity and seizure types that are harder to manage. 24 Our longitudinal analysis suggested minor improvements in seizure-free recording since health checks had been introduced. However, any such benefit would be important, as the improved service provision of patients with ID with epilepsy has been identified as a mechanism for reducing excess mortality among all people with ID. 152
It has been argued that regular health checks for adults with ID are an efficient way of closing the health inequality gap that this group may experience; however, this may also be widened if more easily managed patients are more likely to get health checks. 153 It is, therefore, reassuring that we found that those with more complex health needs were more likely to receive a health check. In our study, the decrease in emergency admission rates for ACSCs was more marked (27%) when we directly compared participating with non-participating practices, which suggests that there may be a ‘practice-level benefit’ of health checks, whereby changes in care have benefited all patients with ID within the practice irrespective of whether or not they have the health check. However, this may be an oversimplification, as a recent serious case review in the UK into the deaths of two adults with ID found that they had been invited to a health check but had failed to attend. 154 Interestingly, our analysis of individuals suggested that health checks produced the greatest benefit in reducing emergency admission to hospital in those with more severe and complex needs.
Health checks and process measures
The systematic review by Robertson et al. 12 identified many worldwide studies showing that similar health checks for adults with ID have had meaningful impacts on health promotion and screening activity in primary care. In the UK, for example, a small Scottish trial of an annual intervention for adults with ID32 reported large increases in the performance of vision and hearing tests,155 similar to our findings of increased recording in these areas for patients with health checks compared with those without. However, many of the studies in the review are now 10–20 years old, and the additional beneficial gains seen historically may not necessarily apply to English primary care, in which the recording of such conditions is now incentivised.
Post introduction of the DES for annual health checks in England (2009), two large studies further investigated the effect of health checks on process measures. The study by Chauhan et al. 156 used data from 171 practices in six primary care trusts to identify approximately 4000 adults with ID in both 2010 and 2011. The study by Buszewicz et al. 90 used English data from the THIN database to compare recording during 2009–11 among 4645 patients with ID with health checks from 222 incentivised practices with 611 patients with ID from 48 non-incentivised practices. Both studies90,156 found increased recording of a wide range of health assessments, such as sight and hearing.
We found that although health checks appeared to have increased prescribing levels among adults with ID over time, there was little impact on medication reviews over time. This contrasted with the study by Buszewicz et al. ,90 which found more reviews among patients with health checks. We acknowledge that the recording of medication reviews on CPRD may not be complete (see Chapter 2, Missing entity data in the Clinical Practice Research Datalink), and this may explain the discrepancy seen in reviews recorded during 2009–11 in our study (65%) and that seen in Buszewicz et al. 90 (84%) over the same period. The 3-year recording of any medication review in our study was much higher than what we observed recorded during the checks themselves (26% for patients on repeat medication). As medication reviews are incentivised elsewhere in QOF,47 it may be that many patients have already had a relevant review by the time they receive the check.
The systematic review by Robertson et al. 12 also concluded that health checks had been effective in detecting a range of previously undetected conditions such as cancer and heart disease. Although Chauhan et al. 156 found that health checks were associated with an increased identification of conditions incentivised by QOF, such as diabetes, Buszewicz et al. 90 found increases in post-2009 diagnoses only for conditions likely to be a focus of health checks for patients with ID, such as constipation or gastrointestinal disorders. We found little evidence to suggest that health checks were associated with increased diagnoses during 2009–11 for a range of QOF conditions. The lower prevalence of recorded cancer in adults with ID in our study suggests that improvements in timely diagnoses of cancer in people with ID may still be possible. 157
Our finding of increased prescribing levels and associated costs in adults with ID who had health checks compared with those who did not have health checks is novel, and further investigation is needed to confirm whether or not the checks are driving this increase. The pattern with consultations in primary care was less clear. The suggestion was that the checks had led to greater costs associated with consultations, with no change in the number of consultations themselves.
Although we estimated annual NHS costs from available data, we did not attempt to estimate the costs of health checks themselves, and thus assess the cost-effectiveness of the health check scheme. The large variation in recording procedures across practices for health checks needs to be better understood to enable better cost estimates of health checks on a large scale. Both in the UK158,159 and internationally,160 small trials of health check intervention have suggested that there were no associated higher costs in terms of service use compared with standard care. 158,159 However, these studies may not have fully accounted for longer-term hospitalisation costs, which in turn could have led to an underestimation of any potential economic savings. 159 Therefore, costs implications and benefits of health checks remain unclear and require further evaluation.
A few studies have recently investigated factors influencing uptake and attendance of health checks. A 3-year study explored variations in uptake in Northern Ireland,161 where overall uptake of their DES of health checks has been higher than in England (64% of eligible patients had received a check by 2013–14). Similar to our findings, they found higher uptake with age, and that patients living in nursing or residential homes (82%) were significantly more likely to have a health check than those living independently (63%). They also found that patients living in more deprived areas were less likely to have had a check, whereas we found a relationship with deprivation only when we focused on repeated checks over time.
Attendance at health checks, once a check has been offered, was investigated in a recent Australian meta-analysis of three community trials,162 and showed that Down syndrome was the only consistent characteristic associated with health check attendance. By comparison, the recent study of English primary care data found that non-attendance was associated with being younger and living in more deprived areas. 90 Our analysis of repeated health checks could be thought of as a proxy attendance measure, and similarly found that repeated checks were less likely with younger age and deprivation.
Implications
We have identified the following implications from our analysis of health checks and hospital admissions.
-
Annual health checks for adults with ID can improve access to care and may be influential in reducing preventable admissions to hospital, which make up one-third of all emergency hospitalisations for adults with ID. Although the evidence has been weak for community-initiated case management interventions in reducing preventable admissions in the general population, our results argue for the continued implementation of annual health checks for all patients with ID. As we did not undertake a formal cost analysis in this study, future research could helpfully estimate whether or not the cost of health checks is offset by savings from fewer emergency hospitalisations.
-
Ensuring that all eligible adults, especially those with the most severe or complex needs, receive an annual health check will continue to address key issues of health inequality and discrimination for adults with ID. This can be achieved both within practices already participating in the DES, and by encouraging wider practice uptake of the health check DES towards a suggested and necessary target of 90%. 153
We have identified the following implications from our analysis of health checks and process measures.
-
Although there is published guidance on what the GP should cover during a health check,10 our study has shown that there is substantial variation in the information recorded. This suggests that the experience of a health check may differ across practices, and our discussions with patient and user groups consistently reinforced this view (see Chapter 2, Patient and public involvement). So, although the patient view of health checks has been shown to be mainly positive,163 better standardisation through reinforcing guidance and practice may lead to improvements in the overall patient experience of the health check, and possibly in health outcomes.
-
The low levels of recording with regard to mental health during health checks contrasts with its importance in terms of burden of disease for adults with ID from our cross-sectional analyses and from our patient and carer group discussions (see Chapter 2, Patient and public involvement). Improved access from primary care to specialist mental health services for patients with ID would encourage greater detection and recording of mental health problems as part of health checks.
-
Despite aspiration being a frequent cause of emergency admission to hospital, as well as a cause of death, among adults with ID, it was not clear that annual health checks were specifically recording any issues around eating, drinking and swallowing. We estimated that 1 in 20 adults with ID had dysphagia recorded, lower than some estimates,164 so the recent call for dysphagia-related questions to be included in the annual health check has merit. 107
Overall study limitations
We have described in detail the limitations of the study in relation to its two original aims: (1) health, health-care quality, mortality and NHS costs and (2) health checks and effectiveness of health checks. We summarise the key limitations again here.
-
Our study population of adults with ID is based on patients with ID who are known to their GP, and so may be missing patients with milder forms of ID who are not in regular contact with primary care. Additionally, our description of primary care does not include other non-GP-led services, such as optometry and dentistry, which will be important for adults with ID.
-
Our description of many outcomes, such as disease prevalence or content of health check, is based entirely on recorded information from the GP electronic patient record. Although this may not capture everything that is occurring for these patients, the lower recording of some outcomes is still of importance (e.g. delayed cancer diagnosis).
-
The recording of key characteristics for this group, such as the severity of their ID and their living arrangements, was incomplete, and we had to rely on proxies (severe health needs, communal accommodation) to try to describe these. For ethnicity, one in four adults with ID had no recording, and we chose not to investigate further by ethnic group.
-
For patients not recorded as living in shared or communal accommodation, we were unable to further determine the level of independence of their living arrangements, such as living with a family carer.
-
Our estimates of NHS costs must be viewed as conservative and an underestimate of the true cost.
-
Our headline finding of reduced emergency admissions for ACSCs associated with the introduction of health checks is derived from observational data and, although we have tried to adjust for confounding and temporal factors, we cannot replicate the conditions of a randomised trial to test their effectiveness.
Research recommendations
Overall, we wanted to emphasise the following recommendations for research that this study identified.
-
We think that further research regarding health checks should focus on two important observations from our study. The first would be in relation to practices that are participating in the DES but are unable to get the majority of their patients with ID to attend an annual health check. Ensuring that all eligible patients are being appropriately invited, and determining reasons for non-attendance, could be investigated. Second, it is necessary to understand the recording variations in the patients’ medical records around the time of health checks. This could confirm our findings of low recordings of key areas such as mental health and medication reviews. If confirmed, further research could also identify barriers to carrying out standardised health checks, and suggest recommendations for improvement.
-
We would also suggest that the lower prevalence of cancer and IHD in adults with ID compared with the general population requires further investigation. It would be important to determine whether or not patients are being diagnosed later, as well as assessing if survival time from diagnosis differs between patients with and patients without ID.
-
The potential factors contributing to the observed lower continuity of care and shorter appointment times with their GP for adults with ID could be explored by further surveys of all key parties involved. What are the common barriers for patients and carers, and what steps can practices make as reasonable adjustments?
-
The high levels of psychotropic prescribing among adults with ID, particularly among patients whose medical records have no recent indication or medication review, is a concern. Health checks may have been expected to address this, but further understanding is needed, particularly in relation to a reliance on some first-generation antipsychotics.
-
The high rate of emergency hospital admissions that are potentially preventable for adults with ID suggests that a continued targeted approach, such as annual health checks, for this group of patients may be effective in reducing admissions. Further research could helpfully focus on conditions with high admission rates such as epilepsy and UTI, identifying possible interventions.
-
The significant contribution of respiratory causes, such as pneumonia and aspiration, to emergency admissions and mortality makes improved access to staff with dysphagia training desirable.
-
Further detailed research relating to NHS costs for adults with ID could be carried out. The inverse association with deprivation among patients living in communal living needs explanation. As this study suggested that preventable emergency hospitalisations may reduce as a result of health checks, a formal cost–benefit analysis would be appropriate.
Conclusions
In summary, our study has addressed the paucity of information on the quality of health care for adults with ID, and has also evaluated the effectiveness of annual health checks in improving outcomes as well as processes of care. Compared with the general population, adults with ID have more chronic diseases, greater utilisation of both primary and secondary care and associated costs, and higher rates of mortality. However, the lower recorded rates of cancer and CHD in primary care are of potential concern as they may represent missed early diagnoses, and this finding requires further investigation. With more than one-third of deaths potentially amenable to health-care interventions, continued improvements in access to, and quality of, health care are urgently required. In primary care, better continuity of care and longer appointment times are important examples that we identified.
We found evidence that the introduction of health checks for adults with ID may have been influential in reducing preventable emergency admissions to hospital during the study. However, we failed to find any evidence of a wider reduction across all emergency admissions. Although health checks were introduced to reduce health inequalities, the current incentivised scheme means that not every eligible adult with ID receives one. Furthermore, the recording of health measures associated with the health check varies considerably by practice, with low recording of medication reviews and mental health, and may reflect differences in patient experience. Future research is needed to confirm this finding. Improvements in the standardisation of health checks, and encouraging wider practice uptake of the health check scheme, will continue to address health inequalities and possibly improve health outcomes.
Dissemination
The analyses and results from this study have already been actively disseminated in multiple ways, including the following:
-
January 2016: The Society for Academic Primary Care, London Annual Scientific Meeting – an oral presentation of ‘Do health checks for adults with intellectual disability reduce emergency hospital admissions? Evaluation of a natural experiment’ was given by Iain Carey.
-
January 2016: The Society for Academic Primary Care, London Annual Scientific Meeting – an oral presentation of ‘Disparities in mortality and deaths amenable to healthcare intervention in adults with intellectual disability’ was given by Fay Hosking.
-
April 2016: the paper ‘Health characteristics and consultation patterns of people with intellectual disability: a cross-sectional database study in English general practice’66 was published by the British Journal of General Practice.
-
June 2016: the paper ‘Do health checks for adults with intellectual disability reduce emergency hospital admissions? Evaluation of a natural experiment’81 was published online by the Journal of Epidemiology and Community Health.
-
June 2016: Mencap Local Adults First, Merton – an oral presentation of ‘St George’s Learning Disability Study’ was given by Iain Carey.
-
July 2016: Skills for Life conference, St George’s Hospital – an oral presentation of ‘St George’s Learning Disability Study’ was given by Carole Beighton with assistance from ResearchNet.
-
August 2016: the paper ‘Mortality among adults with intellectual disability in England: comparisons with the general population’74 was published by the American Journal of Public Health.
-
September 2016: Society for Social Medicine 60th Annual Scientific Meeting, University of York, UK – an oral presentation of ‘Do health checks for adults with intellectual disability reduce emergency hospital admissions? Evaluation of a natural experiment’ was given by Iain Carey.
-
September 2016: Society for Social Medicine 60th Annual Scientific Meeting, University of York, UK – an oral presentation of ‘Disparities in mortality and deaths amenable to healthcare intervention in adults with intellectual disability’ was given by Fay Hosking.
-
September 2017: ‘Preventable emergency hospital admissions among adults with intellectual disability: comparisons with the general population in England’ was published by Annals of Family Medicine.
Acknowledgements
We would like to pay tribute to our former colleague Dr Sunil Shah, who conceived the idea for the study, and led it during its initial stages before his death in September 2015.
Contributions of authors
Dr Iain M Carey (Lecturer, Epidemiology and Medical Statistics) contributed to the original conception and design of the study, oversaw the initial data extraction, helped design the analyses, carried out the statistical analyses in Chapters 3, 6 and 7, and wrote the majority of the report.
Dr Fay J Hosking (Research Fellow in Statistics) performed the statistical analyses in Chapters 4, 5 and 7 and helped draft the report.
Dr Tess Harris (GP and Reader in Primary Care) contributed to the original conception and design of the study, co-led the patient and public involvement component of the study and helped draft the report.
Dr Stephen DeWilde (GP and Senior Lecturer in Primary Care Epidemiology) contributed to the original conception and design of the study and helped draft the report.
Ms Carole Beighton (Senior Research Fellow) co-led the patient and public involvement component of the study and helped draft the report.
Professor Derek G Cook (Professor of Epidemiology) contributed to the original conception and design of the study and helped draft the report.
Publications
Carey IM, Hosking FJ, DeWilde S, Harris T, Beighton C, Cook DG. Learning disability registers in primary care. Br J Gen Pract 2016;66:351–52.
Carey IM, Shah SM, Hosking FJ, DeWilde S, Harris T, Beighton C, Cook DG. Health characteristics and consultation patterns of people with intellectual disability: a cross-sectional database study in English general practice. Br J Gen Pract 2016;66:E264–70.
Hosking FJ, Carey IM, Shah SM, Harris T, DeWilde S, Beighton C, Cook DG. Mortality among adults with intellectual disability in England: comparisons with the general population. Am J Public Health 2016;106:1483–90.
Carey IM, Hosking FJ, Harris T, DeWilde S, Beighton C, Shah SM, Cook DG. Do health checks for adults with intellectual disability reduce emergency hospital admissions? Evaluation of a natural experiment. J Epidemiol Community Health 2017;71:52–8.
Hosking FJ, Carey IM, DeWilde S, Harris T, Beighton C, Cook DG. Preventable emergency hospital admissions among adults with intellectual disability in England. Ann Fam Med 2017;5:462–70.
Beighton C, Victor C, Carey IM, Hosking FJ, DeWilde S, Cook DG, et al. ‘I’m sure we made it a better study . . .’: Experiences of adults with intellectual disabilities and parent carers of patient and public involvement in a health research study [published online ahead of print August 16 2017]. J Intellect Disabil 2017. https://doi.org/10.1177/1744629517723485
Data sharing statement
Owing to the CPRD licence of use, there are no data that can be directly shared from the project. However, anyone wishing to access CPRD data can do so at a cost. See www.cprd.com for more details.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- World Health Organization . ICD-10 Guide for Mental Retardation 1996. www.who.int/mental_health/media/en/69.pdf (accessed 4 August 2016).
- Emerson E, Heslop P. A Working Definition of Learning Disabilities 2010. http://webarchive.nationalarchives.gov.uk/20160704162453/http://www.improvinghealthandlives.org.uk/uploads/doc/vid_7446_2010-01WorkingDefinition.pdf (accessed 14 August 2017).
- Department of Health . Valuing People: A New Strategy for Learning Disability for the 21st Century 2001. www.gov.uk/government/uploads/system/uploads/attachment_data/file/250877/5086.pdf (accessed 4 August 2016).
- Bittles AH, Bower C, Hussain R, Glasson EJ. The four ages of Down syndrome. Eur J Public Health 2007;17:221-5. https://doi.org/10.1093/eurpub/ckl103.
- Hollins S, Attard MT, von Fraunhofer N, McGuigan S, Sedgwick P. Mortality in people with learning disability: risks, causes, and death certification findings in London. Dev Med Child Neurol 1998;40:50-6.
- Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil 2011;32:419-36. https://doi.org/10.1016/j.ridd.2010.12.018.
- Equal Treatment: Closing the Gap. Health Formal Investigation Report. London: Disability Rights Commission; 2006.
- Michael J. Healthcare for All. Report of the Independent Inquiry into Access to Healthcare for People with Learning Disabilities. London: Department of Health; 2008.
- Hoghton M, Martin G, Chauhan U. Annual health checks for people with intellectual disabilities. BMJ 2012;345. https://doi.org/10.1136/bmj.e7589.
- Hoghton M. A Step by Step Guide for GP Practices: Annual Health Checks for People With a Learning Disability 2010. www.rcgp.org.uk/clinical-and-research/clinical-resources/∼/media/Files/CIRC/CIRC-76-80/CIRCA%20StepbyStepGuideforPracticesOctober%2010.ashx (accessed 16 September 2016).
- Robertson J, Roberts H, Emerson E, Turner S, Greig R. The impact of health checks for people with intellectual disabilities: a systematic review of evidence. J Intellect Disabil Res 2011;55:1009-19. https://doi.org/10.1111/j.1365-2788.2011.01436.x.
- Robertson J, Hatton C, Emerson E, Baines S. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil 2014;35:2450-62. https://doi.org/10.1016/j.ridd.2014.06.007.
- Glover G, Evison F. Cambridge: Improving Health and Lives, Learning Disabilities Observatory. Hospital Admissions That Should Not Happen 2013. www.ndti.org.uk/uploads/files/IHAL-2013-02_Hospital_admissions_that_should_not_happen_ii.pdf (accessed 3 November 2015).
- Heslop P, Lauer E, Hoghton M. Mortality in people with intellectual disabilities. J Appl Res Intellect Disabil 2015;28:367-72. https://doi.org/10.1111/jar.12196.
- Patja K, Molsa P, Iivanainen M. Cause-specific mortality of people with intellectual disability in a population-based, 35-year follow-up study. J Intellect Disabil Res 2001;45:30-4. https://doi.org/10.1046/j.1365-2788.2001.00290.x.
- Lavin KE, McGuire BE, Hogan MJ. Age at death of people with an intellectual disability in Ireland. J Intellect Disabil 2006;10:155-64. https://doi.org/10.1177/1744629506064011.
- Emerson E, Baines S. Health Inequalities &Amp; People With Learning Disabilities in the UK: 2010 n.d. http://webarchive.nationalarchives.gov.uk/20160704153130/http://www.improvinghealthandlives.org.uk/uploads/doc/vid_7479_IHaL2010-3HealthInequality2010.pdf (accessed 14 August 2017).
- McGrother CW, Bhaumik S, Thorp CF, Hauck A, Branford D, Watson JM. Epilepsy in adults with intellectual disabilities: prevalence, associations and service implications. Seizure 2006;15:376-86. https://doi.org/10.1016/j.seizure.2006.04.002.
- Straetmans JM, van Schrojenstein Lantman-de Valk HM, Schellevis FG, Dinant GJ. Health problems of people with intellectual disabilities: the impact for general practice. Br J Gen Pract 2007;57:64-6.
- van Splunder J, Stilma JS, Bernsen RM, Evenhuis HM. Prevalence of visual impairment in adults with intellectual disabilities in the Netherlands: cross-sectional study. Eye 2006;20:1004-10. https://doi.org/10.1038/sj.eye.6702059.
- Sherrard J, Tonge BJ, Ozanne-Smith J. Injury risk in young people with intellectual disability. J Intellect Disabil Res 2002;46:6-16. https://doi.org/10.1046/j.1365-2788.2002.00346.x.
- Heslop P, Blair PS, Fleming P, Hoghton M, Marriott A, Russ L. The Confidential Inquiry into premature deaths of people with intellectual disabilities in the UK: a population-based study. Lancet 2014;383:889-95. https://doi.org/10.1016/S0140-6736(13)62026-7.
- Glover G, Emerson E, Baines S. NHS Data Gaps for Learning Disabilities 2011. http://webarchive.nationalarchives.gov.uk/20160704155744/http://www.improvinghealthandlives.org.uk/uploads/doc/vid_11422_IHAL2011-06-NHSDataGaps.pdf (accessed 14 August 2017).
- NHS Digital . Access to Healthcare for People With Learning Disabilities 2010. www.hscic.gov.uk/catalogue/PUB08591/acc-heal-care-peop-lear-disa-rep.pdf (accessed 11 July 2016).
- Heslop P, Glover G. Mortality of people with intellectual disabilities in England: a comparison of data from existing sources. J Appl Res Intellect Disabil 2015;28:414-22. https://doi.org/10.1111/jar.12192.
- Valuing People Now: Summary Report March 2009 – September 2010. London: Department of Health; 2010.
- Disability Discrimination Act. London: Her Majesty’s Stationery Office; 1995.
- NHS England . NHS Enhanced Services 2008 09. 2008. www.nhsemployers.org/your-workforce/primary-care-contacts/general-medical-services/enhanced-services/enhanced-services-200809 (accessed 29 February 2016).
- NHS England . NHS Enhanced Services 2015. www.nhsemployers.org/your-workforce/primary-care-contacts/general-medical-services/enhanced-services (accessed 23 December 2015).
- Glover G, Emerson E, Evison F. The Uptake of Health Checks for Adults With Learning Disabilities: 2008 9 to 2011 12 2012. http://webarchive.nationalarchives.gov.uk/20160704164709/https://www.improvinghealthandlives.org.uk/uploads/doc/vid_16402_IHAL2012-07%20Health%20Checks%20for%20People%20with%20Learning%20Disabilities%202008-9%20to%202011-12v3.pdf (accessed 14 August 2017).
- Lennox N, Bain C, Rey-Conde T, Purdie D, Bush R, Pandeya N. Effects of a comprehensive health assessment programme for Australian adults with intellectual disability: a cluster randomized trial. Int J Epidemiol 2007;36:139-46. https://doi.org/10.1093/ije/dyl254.
- Cooper S, Morrison J, Melville C, Finlayson J, Allan L, Martin G, et al. Improving the health of people with intellectual disabilities: outcomes of a health screening programme after 1 year. J Intellect Disabil Res 2006;50:667-77. https://doi.org/10.1111/j.1365-2788.2006.00824.x.
- Bollard M. Improving primary health care for people with learning disabilities. Br J Nurs 1999;8:1216-21. https://doi.org/10.12968/bjon.1999.8.18.6484.
- Hunt C, Wakefield S, Hunt G. Community nurse learning disabilities: a case study of the use of an evidence-based screening tool to identify and meet the health needs of people with learning disabilities. J Intellect Disabil 2001;5:9-18. https://doi.org/10.1177/146900470100500102.
- Martin DM, Roy A, Wells MB. Health gain through health checks: improving access to primary health care for people with intellectual disability. J Intellect Disabil Res 1997;41:401-8. https://doi.org/10.1111/j.1365-2788.1997.tb00727.x.
- Wells MB, Turner S, Martin DM, Roy A. Health gain through screening – coronary heart disease and stroke: developing primary health care services for people with intellectual disability. J Intellect Dev Disab 1997;22:251-63. https://doi.org/10.1080/13668259700033471.
- Aronow H, Hahn J. Stay well and healthy! Pilot study findings from an inhome preventive healthcare programme for persons ageing with intellectual and/or developmental disabilities. J Appl Res Intellect Disabil 2005;18:163-73. https://doi.org/10.1111/j.1468-3148.2005.00245.x.
- Ryan R, Sunada K. Medical evaluation of persons with mental retardation referred for psychiatric assessment. Gen Hosp Psychiatry 1997;19:274-80. https://doi.org/10.1016/S0163-8343(97)00023-6.
- Emerson E, Hatton C, Robertson J, Roberts H, Baines S, Evison F, et al. People With Learning Disabilities in England 2011 2012. http://webarchive.nationalarchives.gov.uk/20160704171409/http://www.improvinghealthandlives.org.uk/publications/1063/People_with_Learning_Disabilities_in_England_2011 (accessed 14 August 2017).
- Tuffrey-Wijne I, Giatras N, Goulding L, Abraham E, Fenwick L, Edwards C, et al. Identifying the factors affecting the implementation of strategies to promote a safer environment for patients with learning disabilities in NHS hospitals: a mixed-methods study. Health Serv Deliv Res 2013;1.
- Bardsley M, Blunt I, Davies S, Dixon J. Is secondary preventive care improving? Observational study of 10-year trends in emergency admissions for conditions amenable to ambulatory care. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002007.
- Purdy S, Griffin T, Salisbury C, Sharp D. Ambulatory care sensitive conditions: terminology and disease coding need to be more specific to aid policy makers and clinicians. Public Health 2009;123:169-73. https://doi.org/10.1016/j.puhe.2008.11.001.
- Billings J, Zeitel L, Lukomnik J, Carey TS, Blank AE, Newman L. Impact of socioeconomic status on hospital use in New York City. Health Aff 1993;12:162-73.
- Purdy S. Avoiding Hospital Admissions. What Does the Research Evidence Say? 2010. www.kingsfund.org.uk/publications/avoiding_hospital.html (accessed 26 January 2017).
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- English Indices of Deprivation 2010. London: Department for Communities and Local Government; 2011.
- NHS England . General Medical Services Contract. 2015. www.nhsemployers.org/your-workforce/primary-care-contacts/general-medical-services/quality-and-outcomes-framework (accessed 16 July 2015).
- Carey IM, Dewilde S, Harris T, Whincup PH, Cook DG. Spurious trends in coronary heart disease incidence: unintended consequences of the new GP contract?. Br J Gen Pract 2007;57:486-9.
- Quint JK, Muellerova H, DiSantostefano RL, Forbes H, Eaton S, Hurst JR, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005540.
- People with Learning Disabilities in England 2013. London: Public Health England; 2014.
- Carey IM, Hosking FJ, DeWilde S, Harris T, Beighton C, Cook DG. Learning disability registers in primary care. Br J Gen Pract 2016;66:351-2. https://doi.org/10.3399/bjgp16X685861.
- Shah SM, Carey IM, Harris T, DeWilde S, Hubbard R, Lewis S, et al. Identifying the clinical characteristics of older people living in care homes using a novel approach in a primary care database. Age Ageing 2010;39:617-23. https://doi.org/10.1093/ageing/afq086.
- Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev 2006;63:158-88. https://doi.org/10.1177/1077558705285294.
- Curtis L. Unit Costs of Health and Social Care 2012 2012. www.pssru.ac.uk/project-pages/unit-costs/2012/index.php (accessed 8 June 2017).
- NHS Digital . Prescription Cost Analysis – England, 2012 2013. http://content.digital.nhs.uk/article/2021/Website-Search?productid=11412 (accessed 19 August 2016).
- Department of Health . NHS Reference Costs: Financial Year 2011 to 2012 2012. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed 8 June 2017).
- NHS Digital . Introduction to Healthcare Resource Groups 2016 n.d. http://content.digital.nhs.uk/hrg (accessed 8 June 2017).
- International Classification of Diseases. Geneva: World Health Organization; 2010.
- NHS . OPCE Classification of Interventions and Procedures. n.d. www.datadictionary.nhs.uk/web_site_content/supporting_information/clinical_coding/opcs_classification_of_interventions_and_procedures.asp (accessed 8 June 2017).
- NHS Digital . HRG4+ 2015 16 Reference Costs Grouper 2016. http://content.digital.nhs.uk/casemix/costing (accessed 8 June 2017).
- Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6. https://doi.org/10.1093/aje/kwh090.
- Carey IM, Shah SM, Harris T, DeWilde S, Cook DG. A new simple primary care morbidity score predicted mortality and better explains between practice variations than the Charlson index. J Clin Epidemiol 2013;66:436-44. https://doi.org/10.1016/j.jclinepi.2012.10.012.
- Sjölander A, Greenland S. Ignoring the matching variables in cohort studies – when is it valid and why?. Stat Med 2013;32:4696-708. https://doi.org/10.1002/sim.5879.
- National Institute for Health Research . Public Involvement in Research: Values and Principles Framework 2015. www.invo.org.uk/wp-content/uploads/2015/11/Values-and-Principles-framework-final-October-2015.pdf (accessed 5 October 2016).
- Beighton C, Victor C, Carey IM, Hosking F, DeWilde S, Cook DG, et al. ‘I’m sure we made it a better study. ’: experiences of adults with intellectual disabilities and parent carers of patient and public involvement in a health research study [published online ahead of print August 16 2017]. J Intellect Disabil 2017. https://doi.org/10.1177/1744629517723485.
- Carey IM, Shah SM, Hosking FJ, DeWilde S, Harris T, Beighton C, et al. Health characteristics and consultation patterns of people with intellectual disability: a cross-sectional database study in English general practice. Br J Gen Pract 2016;66:e264-70. https://doi.org/10.3399/bjgp16X684301.
- ONS . Age Structure of England and Wales, 1961–2085 2016. www.neighbourhood.statistics.gov.uk/HTMLDocs/dvc2/EWPyramid.html (accessed 27 May 2016).
- Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med 2013;187:1226-33. https://doi.org/10.1164/rccm.201210-1758OC.
- NHS Digital . Quality and Outcomes Framework 2016. http://content.digital.nhs.uk/qof (accessed 8 June 2017).
- NHS Digital . Quality and Outcomes Framework – 2012–13 2013. http://content.digital.nhs.uk/catalogue/PUB12262 (accessed 8 June 2017).
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Harris T, Carey IM, Shah SM, DeWilde S, Cook DG. Antidepressant prescribing in older primary care patients in community and care home settings in England and Wales. J Am Med Dir Assoc 2012;13:41-7. https://doi.org/10.1016/j.jamda.2010.09.005.
- Hosking FJ, Carey IM, Shah SM, Harris T, DeWilde S, Beighton C, et al. Mortality among adults with intellectual disability in England: comparisons with the general population. Am J Public Health 2016;106:1483-90. https://doi.org/10.2105/AJPH.2016.303240.
- Glover G, Ayub M. How People With Learning Disabilities Die 2010. http://webarchive.nationalarchives.gov.uk/20160704181356/https://www.improvinghealthandlives.org.uk/uploads/doc/vid_9033_IHAL2010-06%20Mortality.pdf (accessed 14 August 2017).
- Avoidable Mortality in England and Wales, 2012. London: ONS; 2014.
- Hosking FJ, Carey IM, DeWilde S, Harris T, Beighton C, Cook DG. Preventable emergency hospital admissions among adults with intellectual disability in England. Ann Fam Med 2017;5:462-70. https://doi.org/10.1370/afm.2104.
- NHS Digital . Hospital Episode Statistics (HES) Analysis Guide 2015 2014. http://content.digital.nhs.uk/media/1592/HES-analysis-guide/pdf/HES_Analysis_Guide_March_2015.pdf (accessed 8 June 2017).
- NHS Digital . HES Data Dictionary: Admitted Patient Care 2015 2016. http://content.digital.nhs.uk/media/19425/APC-DD-Final-Doc2/pdf/DD-APC-V7.pdf (accessed 19 August 2016).
- Balogh RS, Ouellette-Kuntz H, Brownell M, Colantonio A. Factors associated with hospitalisations for ambulatory care-sensitive conditions among persons with an intellectual disability – a publicly insured population perspective. J Intellect Disabil Res 2013;57:226-39. https://doi.org/10.1111/j.1365-2788.2011.01528.x.
- Carey IM, Hosking FJ, Harris T, DeWilde S, Beighton C, Shah SM, et al. Do health checks for adults with intellectual disability reduce emergency hospital admissions? Evaluation of a natural experiment. J Epidemiol Community Health 2017;71:52-8. https://doi.org/10.1136/jech-2016-207557.
- McCallion P, McCarron M. Deaths of people with intellectual disabilities in the UK. Lancet 2014;383:853-5. https://doi.org/10.1016/S0140-6736(13)62190-X.
- McCarron M, Swinburne J, Burke E, McGlinchey E, Carroll R, McCallion P. Patterns of multimorbidity in an older population of persons with an intellectual disability: results from the intellectual disability supplement to the Irish longitudinal study on aging (IDS-TILDA). Res Dev Disabil 2013;34:521-7. https://doi.org/10.1016/j.ridd.2012.07.029.
- Freeman G, Hughes J. Continuity of Care and the Patient Experience 2010. www.kingsfund.org.uk/sites/files/kf/field/field_document/continuity-care-patient-experience-gp-inquiry-research-paper-mar11.pdf (accessed 2 August 2016).
- Williamson T, Flowers J, Cooke M. Quantifying emergency department admission rates for people with a learning disability. Emerg Med J 2012;29:771-2. https://doi.org/10.1136/emj.2010.107318.
- Glover G. Numbers and policy in care for people with intellectual disability in the United Kingdom. J Appl Res Intellect Disabil 2015;28:12-21. https://doi.org/10.1111/jar.12131.
- Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin Exp Immunol 2011;164:9-16. https://doi.org/10.1111/j.1365-2249.2011.04335.x.
- van Schrojenstein Lantman-De Valk HM, Metsemakers JF, Haveman MJ, Crebolder HF. Health problems in people with intellectual disability in general practice: a comparative study. Fam Pract 2000;17:405-7. https://doi.org/10.1093/fampra/17.5.405.
- Reichard A, Stolzle H. Diabetes among adults with cognitive limitations compared to individuals with no cognitive disabilities. Intellect Dev Disabil 2011;49:141-54. https://doi.org/10.1352/1934-9556-49.2.141.
- Buszewicz M, Welch C, Horsfall L, Nazareth I, Osborn D, Hassiotis A, et al. Assessment of an incentivised scheme to provide annual health checks in primary care for adults with intellectual disability: a longitudinal cohort study. Lancet Psychiatry 2014;1:522-30. https://doi.org/10.1016/S2215-0366(14)00079-0.
- Hermans H, Evenhuis HM. Multimorbidity in older adults with intellectual disabilities. Res Dev Disabil 2014;35:776-83. https://doi.org/10.1016/j.ridd.2014.01.022.
- Cooper SA, McLean G, Guthrie B, McConnachie A, Mercer S, Sullivan F, et al. Multiple physical and mental health comorbidity in adults with intellectual disabilities: population-based cross-sectional analysis. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0329-3.
- Chapman M, Iddon P, Atkinson K, Brodie C, Mitchell D, Parvin G, et al. The misdiagnosis of epilepsy in people with intellectual disabilities: a systematic review. Seizure 2011;20:101-6. https://doi.org/10.1016/j.seizure.2010.10.030.
- Fredheim T, Haavet OR, Danbolt LJ, Kjønsberg K, Lien L. Intellectual disability and mental health problems: a qualitative study of general practitioners’ views. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002283.
- Cooper SA, Smiley E, Morrison J, Williamson A, Allan L. Mental ill-health in adults with intellectual disabilities: prevalence and associated factors. Br J Psychiatry 2007;190:27-35. https://doi.org/10.1192/bjp.bp.106.022483.
- Emerson E, Kiernan C, Alborz A, Reeves D, Mason H, Swarbrick R, et al. The prevalence of challenging behaviors: a total population study. Res Dev Disabil 2001;22:77-93. https://doi.org/10.1016/S0891-4222(00)00061-5.
- Holden B, Gitlesen JP. A total population study of challenging behaviour in the county of Hedmark, Norway: prevalence, and risk markers. Res Dev Disabil 2006;27:456-65. https://doi.org/10.1016/j.ridd.2005.06.001.
- de Winter CF, van den Berge AP, Schoufour JD, Oppewal A, Evenhuis HM. A 3-year follow-up study on cardiovascular disease and mortality in older people with intellectual disabilities. Res Dev Disabil 2016;53–54:115-26. https://doi.org/10.1016/j.ridd.2016.01.020.
- Baxter H, Lowe K, Houston H, Jones G, Felce D, Kerr M. Previously unidentified morbidity in patients with intellectual disability. Br J Gen Pract 2006;56:93-8.
- Tuffrey-Wijne I, Bernal J, Hubert J, Butler G, Hollins S. People with learning disabilities who have cancer: an ethnographic study. Br J Gen Pract 2009;59:503-9. https://doi.org/10.3399/bjgp09X453413.
- Melville CA, Cooper SA, Morrison J, Allan L, Smiley E, Williamson A. The prevalence and determinants of obesity in adults with intellectual disabilities. J Appl Res Intellect Disabil 2008;21:425-37. https://doi.org/10.1111/j.1468-3148.2007.00412.x.
- Emerson E, Hatton C, Baines S, Robertson J. The physical health of British adults with intellectual disability: cross sectional study. Int J Equity Health 2016;15. https://doi.org/10.1186/s12939-016-0296-x.
- Maïano C, Hue O, Morin AJ, Moullec G. Prevalence of overweight and obesity among children and adolescents with intellectual disabilities: a systematic review and meta-analysis. Obes Rev 2016;17:599-611. https://doi.org/10.1111/obr.12408.
- Schoufour JD, Mitnitski A, Rockwood K, Evenhuis HM, Echteld MA. Development of a frailty index for older people with intellectual disabilities: results from the HA-ID study. Res Dev Disabil 2013;34:1541-55. https://doi.org/10.1016/j.ridd.2013.01.029.
- Bastiaanse LP, Mergler S, Evenhuis HM, Echteld MA. Bone quality in older adults with intellectual disabilities. Res Dev Disabil 2014;35:1927-33. https://doi.org/10.1016/j.ridd.2014.04.018.
- Turk V, Kerry S, Corney R, Rowlands G, Khattran S. Why some adults with intellectual disability consult their general practitioner more than others. J Intellect Disabil Res 2010;54:833-42. https://doi.org/10.1111/j.1365-2788.2010.01312.x.
- Perez CM, Ball SL, Wagner AP, Clare IC, Holland AJ, Redley M. The incidence of healthcare use, ill health and mortality in adults with intellectual disabilities and mealtime support needs. J Intellect Disabil Res 2015;59:638-52. https://doi.org/10.1111/jir.12167.
- Hippisley-Cox J, Stables D, Pringle M. QRESEARCH – a new general practice database for research. J Inform Prim Care 2004;12:49-50. http://dx.doi.org/10.14236/jhi.v12i1.108.
- Turner S, Robinson C. Reasonable Adjustments for People With Learning Disabilities – Implications and Actions for Commissioners and Providers of Healthcare 2011. http://webarchive.nationalarchives.gov.uk/20160704152144/http://www.improvinghealthandlives.org.uk/uploads/doc/vid_11084_IHAL%202011%20-01%20Reasonable%20adjustments%20guidance.pdf (accessed 14 August 2017).
- Matson JL, Neal D. Psychotropic medication use for challenging behaviors in persons with intellectual disabilities: an overview. Res Dev Disabil 2009;30:572-86. https://doi.org/10.1016/j.ridd.2008.08.007.
- Sheehan R, Hassiotis A, Walters K, Osborn D, Strydom A, Horsfall L. Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population based cohort study. BMJ 2015;351. https://doi.org/10.1136/bmj.h4326.
- Prescribing of Psychotropic Drugs to People with Learning Disabilities and/or Autism by General Practitioners in England. London: Public Health England; 2015.
- de Kuijper G, Hoekstra P, Visser F, Scholte FA, Penning C, Evenhuis H. Use of antipsychotic drugs in individuals with intellectual disability (ID) in the Netherlands: prevalence and reasons for prescription. J Intellect Disabil Res 2010;54:659-67. https://doi.org/10.1111/j.1365-2788.2010.01275.x.
- Holden B, Gitlesen JP. Psychotropic medication in adults with mental retardation: prevalence, and prescription practices. Res Dev Disabil 2004;25:509-21. https://doi.org/10.1016/j.ridd.2004.03.004.
- Doan TN, Lennox NG, Taylor-Gomez M, Ware RS. Medication use among Australian adults with intellectual disability in primary healthcare settings: a cross-sectional study. J Intellect Dev Disabil 2013;38:177-81. https://doi.org/10.3109/13668250.2013.778968.
- Amore M, Bertelli M, Villani D, Tamborini S, Rossi M. Olanzapine vs. risperidone in treating aggressive behaviours in adults with intellectual disability: a single blind study. J Intellect Disabil Res 2011;55:210-18. https://doi.org/10.1111/j.1365-2788.2010.01352.x.
- de Kuijper G, Mulder H, Evenhuis H, Scholte F, Visser F, Hoekstra PJ. Determinants of physical health parameters in individuals with intellectual disability who use long-term antipsychotics. Res Dev Disabil 2013;34:2799-809. https://doi.org/10.1016/j.ridd.2013.05.016.
- Scheifes A, Walraven S, Stolker JJ, Nijman HL, Egberts TC, Heerdink ER. Adverse events and the relation with quality of life in adults with intellectual disability and challenging behaviour using psychotropic drugs. Res Dev Disabil 2016;49–50:13-21. https://doi.org/10.1016/j.ridd.2015.11.017.
- Pernea M, Sutcliffe AG. Clobazam and its use in epilepsy. Pediatr Rep 2016;8. https://doi.org/10.4081/pr.2016.6516.
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD005454.pub2.
- Tyrer F, Smith LK, McGrother CW. Mortality in adults with moderate to profound intellectual disability: a population-based study. J Intellect Disabil Res 2007;51:520-7. https://doi.org/10.1111/j.1365-2788.2006.00918.x.
- Tyrer F, McGrother C. Cause-specific mortality and death certificate reporting in adults with moderate to profound intellectual disability. J Intellect Disabil Res 2009;53:898-904. https://doi.org/10.1111/j.1365-2788.2009.01201.x.
- Florio T, Trollor J. Mortality among a cohort of persons with an intellectual disability in New South Wales, Australia. J Appl Res Intellect Disabil 2015;28:383-93. https://doi.org/10.1111/jar.12190.
- Lauer E, McCallion P. Mortality of people with intellectual and developmental disabilities from select US State disability service systems and medical claims data. J Appl Res Intellect Disabil 2015;28:394-405. https://doi.org/10.1111/jar.12191.
- Ouellette-Kuntz H, Shooshtari S, Balogh R, Martens P. Understanding information about mortality among people with intellectual and developmental disabilities in Canada. J Appl Res Intellect Disabil 2015;28:423-35. https://doi.org/10.1111/jar.12195.
- McCarron M, Carroll R, Kelly C, McCallion P. Mortality rates in the general Irish population compared to those with an intellectual disability from 2003 to 2012. J Appl Res Intellect Disabil 2015;28:406-13. https://doi.org/10.1111/jar.12194.
- Hill DA, Gridley G, Cnattingius S, Mellemkjaer L, Linet M, Adami HO, et al. Mortality and cancer incidence among individuals with Down syndrome. Arch Intern Med 2003;163:705-11. https://doi.org/10.1001/archinte.163.6.705.
- Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet 2002;359:1019-25. https://doi.org/10.1016/S0140-6736(02)08092-3.
- Zhu JL, Hasle H, Correa A, Schendel D, Friedman JM, Olsen J, et al. Survival among people with Down syndrome: a nationwide population-based study in Denmark. Genet Med 2013;15:64-9. https://doi.org/10.1038/gim.2012.93.
- Esbensen AJ, Seltzer MM, Greenberg JS. Factors predicting mortality in midlife adults with and without Down syndrome living with family. J Intellect Disabil Res 2007;51:1039-50. https://doi.org/10.1111/j.1365-2788.2007.01006.x.
- Hirvikoski T, Mittendorfer-Rutz E, Boman M, Larsson H, Lichtenstein P, Bölte S. Premature mortality in autism spectrum disorder. Br J Psychiatry 2016;208:232-8. https://doi.org/10.1192/bjp.bp.114.160192.
- Schendel DE, Overgaard M, Christensen J, Hjort L, Jørgensen M, Vestergaard M, et al. Association of psychiatric and neurologic comorbidity with mortality among persons with autism spectrum disorder in a Danish population. JAMA Pediatr 2016;170:243-50. https://doi.org/10.1001/jamapediatrics.2015.3935.
- Kiani R, Tyrer F, Jesu A, Bhaumik S, Gangavati S, Walker G, et al. Mortality from sudden unexpected death in epilepsy (SUDEP) in a cohort of adults with intellectual disability. J Intellect Disabil Res 2014;58:508-20. https://doi.org/10.1111/jir.12047.
- Young C, Shankar R, Palmer J, Craig J, Hargreaves C, McLean B, et al. Does intellectual disability increase sudden unexpected death in epilepsy (SUDEP) risk?. Seizure 2015;25:112-16. https://doi.org/10.1016/j.seizure.2014.10.001.
- Forsgren L, Edvinsson SO, Nyström L, Blomquist HK. Influence of epilepsy on mortality in mental retardation: an epidemiologic study. Epilepsia 1996;37:956-63. https://doi.org/10.1111/j.1528-1157.1996.tb00533.x.
- Patja K, Iivanainen M, Vesala H, Oksanen H, Ruoppila I. Life expectancy of people with intellectual disability: a 35-year follow-up study. J Intellect Disabil Res 2000;44:591-9. https://doi.org/10.1046/j.1365-2788.2000.00280.x.
- Heslop P, Marriott A. Making a difference – the impact of the Confidential Inquiry into premature deaths of people with learning disabilities. Br J Learn Disabil 2015;43:142-9. https://doi.org/10.1111/bld.12114.
- Morgan CL, Ahmed Z, Kerr MP. Health care provision for people with a learning disability. Record-linkage study of epidemiology and factors contributing to hospital care uptake. Br J Psychiatry 2000;176:37-41. https://doi.org/10.1192/bjp.176.1.37.
- Balogh RS, Hunter D, Ouellette-Kuntz H. Hospital utilization among persons with an intellectual disability, Ontario, Canada, 1995–2001. J Appl Res Intellect Disabil 2005;18:181-90. https://doi.org/10.1111/j.1468-3148.2005.00247.x.
- Ailey SH, Johnson T, Fogg L, Friese TR. Hospitalizations of adults with intellectual disability in academic medical centers. Intellect Dev Disabil 2014;52:187-92. https://doi.org/10.1352/1934-9556-52.3.187.
- Skorpen S, Nicolaisen M, Langballe EM. Hospitalisation in adults with intellectual disabilities compared with the general population in Norway. J Intellect Disabil Res 2016;60:365-77. https://doi.org/10.1111/jir.12255.
- Balogh RS, Brownell M, Ouellette-Kuntz H, Colantonio A. Preventable hospitalization rates for people with ID: a population perspective. J Appl Res Intellect Disabil 2010;23.
- Jinks A, Cotton A, Rylance R. Obesity interventions for people with a learning disability: an integrative literature review. J Adv Nurs 2011;67:460-71. https://doi.org/10.1111/j.1365-2648.2010.05508.x.
- Landes SD, Peek CW. Death by mental retardation? The influence of ambiguity on death certificate coding error for adults with intellectual disability. J Intellect Disabil Res 2013;57:1183-90. https://doi.org/10.1111/j.1365-2788.2012.01614.x.
- Hippisley-Cox J, Coupland C. Predicting risk of emergency admission to hospital using primary care data: derivation and validation of QAdmissions score. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003482.
- Freund T, Campbell SM, Geissler S, Kunz CU, Mahler C, Peters-Klimm F, et al. Strategies for reducing potentially avoidable hospitalizations for ambulatory care-sensitive conditions. Ann Family Med 2013;11:363-70. https://doi.org/10.1370/afm.1498.
- Robertson J, Roberts H, Emerson E. Health Checks for People With Learning Disabilities: A Systematic Review of Evidence 2010. http://webarchive.nationalarchives.gov.uk/20160704155636/http://www.improvinghealthandlives.org.uk/uploads/doc/vid_7646_IHAL2010-04HealthChecksSystemticReview.pdf (accessed 14 August 2017).
- Forster AS, Dodhia H, Booth H, Dregan A, Fuller F, Miller J, et al. Estimating the yield of NHS Health Checks in England: a population-based cohort study. J Public Health 2015;37:234-40. https://doi.org/10.1093/pubmed/fdu079.
- Krogsbøll LT, Jørgensen KJ, Grønhøj Larsen C, Gøtzsche PC. General health checks in adults for reducing morbidity and mortality from disease: Cochrane systematic review and meta-analysis. BMJ 2012;345. https://doi.org/10.1136/bmj.e7191.
- Roizen NJ, Patterson D. Down’s syndrome. Lancet 2003;361:1281-9. https://doi.org/10.1016/S0140-6736(03)12987-X.
- Wallace E, Smith SM, Fahey T, Roland M. Reducing emergency admissions through community based interventions. BMJ 2016;352. https://doi.org/10.1136/bmj.h6817.
- Robertson J, Hatton C, Baines S, Emerson E. Systematic reviews of the health or health care of people with intellectual disabilities: a systematic review to identify gaps in the evidence base. J Appl Res Intellect Disabil 2015;28:455-523. https://doi.org/10.1111/jar.12149.
- Slowie D, Martin G. Narrowing the health inequality gap by annual health checks for patients with intellectual disability. Br J Gen Pract 2014;64:101-2. https://doi.org/10.3399/bjgp14X677293.
- Suffolk Safeguarding Adults Board . Safeguarding Adults Reviews 2015. www.suffolkas.org/safeguarding-adults-reviews/ (accessed 6 November 2015).
- Lennox N, Ware R, Bain C, Taylor Gomez M, Cooper SA. Effects of health screening for adults with intellectual disability: a pooled analysis. Br J Gen Pract 2011;61:193-6. https://doi.org/10.3399/bjgp11X561186.
- Chauhan U, Reeve J, Kontopantelis E, Hinder S, Nelson P, Doran T. Impact of the English Directly Enhanced Service (DES) for Learning Disability. Manchester: University of Manchester; 2012.
- Hogg J, Tuffrey-Wijne I. Cancer and intellectual disability: a review of some key contextual issues. J Appl Res Intellect Disabil 2008;21:509-18. https://doi.org/10.1111/j.1468-3148.2008.00422.x.
- Romeo R, Knapp M, Morrison J, Melville C, Allan L, Finlayson J, et al. Cost estimation of a health-check intervention for adults with intellectual disabilities in the UK. J Intellect Disabil Res 2009;53:426-39. https://doi.org/10.1111/j.1365-2788.2009.01159.x.
- Cooper SA, Morrison J, Allan LM, McConnachie A, Greenlaw N, Melville CA, et al. Practice nurse health checks for adults with intellectual disabilities: a cluster-design, randomised controlled trial. Lancet Psychiatry 2014;1:511-21. https://doi.org/10.1016/S2215-0366(14)00078-9.
- Gordon LG, Holden L, Ware RS, Taylor MT, Lennox N. Comprehensive health assessments for adults with intellectual disability living in the community – weighing up the costs and benefits. Aust Fam Physician 2012;41:969-72.
- McConkey R, Taggart L, Kane M. Optimizing the uptake of health checks for people with intellectual disabilities. J Intellect Disabil 2015;19:205-14. https://doi.org/10.1177/1744629514568437.
- Ware RS, Lennox NG. Characteristics influencing attendance at a primary care health check for people with intellectual disability: an individual participant data meta-analysis. Res Dev Disabil 2016;55:235-41. https://doi.org/10.1016/j.ridd.2016.04.012.
- Perry J, Felce D, Kerr M, Bartley S, Tomlinson J, Felce J. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil 2014;27:200-11. https://doi.org/10.1111/jar.12072.
- Chadwick DD, Jolliffe J. A descriptive investigation of dysphagia in adults with intellectual disabilities. J Intellect Disabil Res 2009;53:29-43. https://doi.org/10.1111/j.1365-2788.2008.01115.x.
Appendix 1 Adult prevalence of intellectual disability estimated using Quality and Outcomes Framework learning disability register data
Prevalence of intellectual disability estimated using the Quality and Outcomes Framework in England from 2006–7 to 2014–15
| Year | Number of practices | Total list size | Number of adultsa | Register count | Prevalence of ID (%) | QOF indicator |
|---|---|---|---|---|---|---|
| 2014–15 | 7779 | 56,817,654 | NA | 252,446 | 0.44b | LD003: the contractor establishes and maintains a register of patients with learning disabilities |
| 2013–14 | 7921 | 56,324,887 | 44,667,478 | 214,352 | 0.48 | LD001: the contractor establishes and maintains a register of patients aged ≥ 18 years with learning disabilities |
| 2012–13 | 8020 | 56,012,096 | 44,238,483 | 206,132 | 0.47 | LD1: the practice can produce a register of patients with learning disabilities |
| 2011–12 | 8123 | 55,525,732 | 43,855,136 | 198,877 | 0.45 | LD1: the practice can produce a register of patients with learning disabilities |
| 2010–11 | 8245 | 55,169,643 | 43,578,391 | 188,819 | 0.43 | LD1: the practice can produce a register of patients with learning disabilities |
| 2009–10 | 8305 | 54,836,561 | 42,613,280 | 179,064 | 0.42 | LD1: the practice can produce a register of patients with learning disabilities |
| 2008–9 | 8229 | 54,310,660 | 40,041,250 | 160,165 | 0.40 | LD1: the practice can produce a register of patients with learning disabilities |
| 2007–8 | 8294 | 54,009,831 | NA | 144,909 | 0.36c | LD1: the practice can produce a register of patients with learning disabilities |
| 2006–7 | 8372 | 53,681,098 | NA | 139,321 | 0.35c | LD1: the practice can produce a register of patients with learning disabilities |
Appendix 2 Read codes used in the definition of intellectual disability
Listing of all Read codes used in the definition of intellectual disability
| Read code | Description | QOF LDa |
|---|---|---|
| 13Z3.00 | Low I.Q. | |
| 6664.00 | Mental handicap problem | |
| 69DB.00 | Learning disability health exam | |
| 918e.00 | On learning disability register | Y |
| 9HB..00 | Learning disabilities administration status | |
| 9HB0.00 | Learning disabilities health action plan declined | |
| 9HB1.00 | Learning disabilities health action plan offered | |
| 9HB2.00 | Learning disabilities health action plan reviewed | |
| 9HB3.00 | Learning disabilities health assessment | |
| 9HB4.00 | Learning disabilities health action plan completed | |
| 9HB5.00 | Learning disabilities annual health assessment | |
| 9HB6.00 | Learning disabilities annual health assessment declined | |
| 9HB6.11 | Learning disabilities annual health check declined | |
| 9HB7.00 | Did not attend learning disabilities annual health assessment | |
| 9HB7.11 | Did not attend learning disabilities annual health check | |
| 9hL..00 | Exception reporting: learning disability quality indicators | |
| 9hL0.00 | Exc learn disability quality indicators: informed dissent | |
| 9hL1.00 | Exc learn disability quality indicators: patient unsuitable | |
| 9mA..00 | Learning disability annual health check invitation | |
| 9mA0.00 | Learning disability annual health check verbal invitation | |
| 9mA1.00 | Learning disability annual health check telephone invitation | |
| 9mA2.00 | Learning disability annual health check letter invitation | |
| 9mA2000 | Learning disability annual health check invitation 1st letter | |
| 9mA2100 | Learning disability annual health check invitation 2nd letter | |
| 9mA2200 | Learning disability annual health check invitation 3rd letter | |
| C03..11 | Cretinism | |
| C031.00 | Goitrous cretin | |
| C03z.12 | Cretinism | |
| C372.11 | Lesch – Nyhan syndrome | |
| C372000 | Hypoxanthine-guanine-phosphoribosyltransferase deficiency | |
| C372011 | Lesch – Nyhan syndrome | |
| C372300 | Lesch-Nyhan syndrome | |
| C372z00 | Other disorder of purine or pyrimidine metabolism NOS | |
| E141.00 | Disintegrative psychosis | |
| E141.11 | Heller’s syndrome | |
| E141000 | Active disintegrative psychoses | |
| E141100 | Residual disintegrative psychoses | |
| E141z00 | Disintegrative psychosis NOS | |
| E3..00 | Mental retardation | Y |
| E30..00 | Mild mental retardation, IQ in range 50–70 | Y |
| E30..11 | Educationally subnormal | Y |
| E30..12 | Feeble-minded | Y |
| E30..13 | Moron | Y |
| E31..00 | Other specified mental retardation | Y |
| E310.00 | Moderate mental retardation, IQ in range 35–49 | Y |
| E310.11 | Imbecile | Y |
| E311.00 | Severe mental retardation, IQ in range 20–34 | Y |
| E312.00 | Profound mental retardation with IQ less than 20 | Y |
| E312.11 | Idiocy | Y |
| E31z.00 | Other specified mental retardation NOS | Y |
| E3y..00 | Other specified mental retardation | Y |
| E3z..00 | Mental retardation NOS | Y |
| Eu7..00 | [X]Mental retardation | Y |
| Eu70.00 | [X]Mild mental retardation | Y |
| Eu70.11 | [X]Feeble-mindedness | Y |
| Eu70.12 | [X]Mild mental subnormality | Y |
| Eu70000 | [X]Mld mental retard with statement no or min impairm behav | Y |
| Eu70100 | [X]Mld mental retard sig impairment behav req attent/treatmt | Y |
| Eu70y00 | [X]Mild mental retardation, other impairments of behaviour | Y |
| Eu70z00 | [X]Mild mental retardation without mention impairment behav | Y |
| Eu71.00 | [X]Moderate mental retardation | Y |
| Eu71.11 | [X]Moderate mental subnormality | Y |
| Eu71000 | [X]Mod mental retard with statement no or min impairm behav | Y |
| Eu71100 | [X]Mod mental retard sig impairment behav req attent/treatmt | Y |
| Eu71y00 | [X]Mod retard oth behav impair | Y |
| Eu71z00 | [X]Mod mental retardation without mention impairment behav | Y |
| Eu72.00 | [X]Severe mental retardation | Y |
| Eu72.11 | [X]Severe mental subnormality | Y |
| Eu72000 | [X]Sev mental retard with statement no or min impairm behav | Y |
| Eu72100 | [X]Sev mental retard sig impairment behav req attent/treatmt | Y |
| Eu72y00 | [X]Severe mental retardation, other impairments of behaviour | Y |
| Eu72z00 | [X]Sev mental retardation without mention impairment behav | Y |
| Eu73.00 | [X]Profound mental retardation | Y |
| Eu73.11 | [X]Profound mental subnormality | Y |
| Eu73000 | [X]Profound ment retrd wth statement no or min impairm behav | Y |
| Eu73100 | [X]Profound ment retard sig impairmnt behav req attent/treat | Y |
| Eu73y00 | [X]Profound mental retardation, other impairments of behavr | Y |
| Eu73z00 | [X]Prfnd mental retardation without mention impairment behav | Y |
| Eu7y.00 | [X]Other mental retardation | Y |
| Eu7y000 | [X]Oth mental retard with statement no or min impairm behav | Y |
| Eu7y100 | [X]Oth mental retard sig impairment behav req attent/treatmt | Y |
| Eu7yy00 | [X]Other mental retardation, other impairments of behaviour | Y |
| Eu7yz00 | [X]Other mental retardation without mention impairment behav | Y |
| Eu7z.00 | [X]Unspecified mental retardation | Y |
| Eu7z.11 | [X]Mental deficiency NOS | Y |
| Eu7z.12 | [X]Mental subnormality NOS | Y |
| Eu7z000 | [X]Unsp mental retard with statement no or min impairm behav | Y |
| Eu7z100 | [X]Unsp mentl retard sig impairment behav req attent/treatmt | Y |
| Eu7zy00 | [X]Unspecified mental retardatn, other impairments of behav | Y |
| Eu7zz00 | [X]Unsp mental retardation without mention impairment behav | Y |
| Eu81400 | [X]Moderate learning disability | Y |
| Eu81500 | [X]Severe learning disability | Y |
| Eu81600 | [X]Mild learning disability | Y |
| Eu81700 | [X]Profound learning disability | Y |
| Eu81z00 | [X]Developmental disorder of scholastic skills, unspecified | Y |
| Eu81z11 | [X]Learning disability NOS | Y |
| Eu81z12 | [X]Learning disorder NOS | Y |
| Eu81z13 | [X]Learn acquisition disab NOS | Y |
| Eu84112 | [X]Mental retardation with autistic features | |
| Eu84200 | [X]Rett’s syndrome | |
| Eu84300 | [X]Other childhood disintegrative disorder | |
| Eu84311 | [X]Dementia infantalis | |
| Eu84312 | [X]Disintegrative psychosis | |
| Eu84313 | [X]Heller’s syndrome | |
| Eu84400 | [X]Overactive disorder assoc mental retard/stereotype movts | |
| PJ0..00 | Down’s syndrome – trisomy 21 | |
| PJ0..11 | Mongolism | |
| PJ0..12 | Trisomy 21 | |
| PJ0..13 | Trisomy 22 | |
| PJ00.00 | Trisomy 21, meiotic nondisjunction | |
| PJ01.11 | Trisomy 21, mitotic nondisjunction | |
| PJ02.00 | Trisomy 21, translocation | |
| PJ02.11 | Partial trisomy 21 in Down’s syndrome | |
| PJ0z.00 | Down’s syndrome NOS | |
| PJ0z.11 | Trisomy 21 NOS | |
| PJ1..00 | Patau’s syndrome – trisomy 13 | |
| PJ10.00 | Trisomy 13, meiotic nondisjunction | |
| PJ11.00 | Trisomy 13, mosaicism | |
| PJ11.11 | Trisomy 13, mitotic nondisjunction | |
| PJ12.00 | Trisomy 13, translocation | |
| PJ12.11 | Partial trisomy 13 in Patau’s syndrome | |
| PJ1z.00 | Patau’s syndrome NOS | |
| PJ1z.11 | Trisomy 13 NOS | |
| PJ2..00 | Edward’s syndrome – trisomy 18 | |
| PJ20.00 | Trisomy 18, meiotic nondisjunction | |
| PJ21.00 | Trisomy 18, mosaicism | |
| PJ21.11 | Trisomy 18, mitotic nondisjunction | |
| PJ22.00 | Trisomy 18, translocation | |
| PJ22.11 | Partial trisomy 18 in Edward’s syndrome | |
| PJ2z.00 | Edward’s syndrome NOS | |
| PJ2z.11 | TRISOMY 18 NOS | |
| PJ30.00 | Antimongolism syndrome | |
| PJ30.11 | Deletion of long arm of chromosome 21 | |
| PJ31.00 | Cri-du-chat syndrome | |
| PJ31.11 | Deletion of short arm of chromosome 5 | |
| PJ32.00 | Deletion of short arm of chromosome 4 | |
| PJ32.11 | Wolff – Hirschorn syndrome | |
| PJ33100 | Deletion of long arm of chromosome 18 | |
| PJ33111 | 18p- syndrome | |
| PJ33200 | Deletion of short arm of chromosome 18 | |
| PJ33211 | 18q- syndrome | |
| PJ33300 | Smith-Magenis syndrome | |
| PJ33400 | Jacobsen syndrome | |
| PJ33500 | Greig cephalopolysyndactyly syndrome | |
| PJ33700 | 3p deletion syndrome | |
| PJ33800 | Chromosome 4q deletion syndrome | |
| PJ33900 | Langer-Giedion syndrome | |
| PJ33A00 | Kleefstra syndrome | |
| PJ3z.00 | Monosomies and deletions from the autosomes NOS | |
| PJ50.00 | Whole chromosome trisomy syndromes | |
| PJ50000 | Trisomy 6 | |
| PJ50100 | Trisomy 7 | |
| PJ50200 | Trisomy 8 | |
| PJ50300 | Trisomy 9 | |
| PJ50400 | Trisomy 10 | |
| PJ50500 | Trisomy 11 | |
| PJ50600 | Trisomy 12 | |
| PJ50700 | Other trisomy C syndromes | |
| PJ50800 | Trisomy 22 | |
| PJ50w00 | Whole chromosome trisomy, meitotic nondisjunction | |
| PJ50x00 | Whole chromosome trisomy, mosaicism | |
| PJ50x11 | Whole chromosome trisomy, mitotic nondisjunction | |
| PJ50y00 | Other specified whole chromosome trisomy syndrome | |
| PJ50z00 | Whole chromosome trisomy syndrome NOS | |
| PJ51.00 | Partial trisomy syndromes | |
| PJ51000 | Major partial trisomy | |
| PJ51100 | Minor partial trisomy | |
| PJ51200 | 10q partial trisomy syndrome | |
| PJ51300 | Trisomy 4p syndrome | |
| PJ51400 | Trisomy 9p syndrome | |
| PJ51500 | 15q partial trisomy syndrome | |
| PJ51z00 | Partial trisomy syndrome NOS | |
| PJ52.00 | Trisomies of autosomes NEC | |
| PJ52z00 | Trisomy of autosomes NEC NOS | |
| PJ9..00 | Mowat-Wilson syndrome | |
| PJyy200 | Fragile X chromosome | |
| PJyy400 | Fragile X syndrome | |
| PKy0.11 | Prader-Willi Syndrome | |
| PKy0.12 | Prader-Willi syndrome | |
| PKy4.00 | William syndrome | |
| PKy9300 | Prader – Willi syndrome | |
| Pyu0200 | [X]Other reduction deformities of brain | |
| PyuA000 | [X]Oth specif trisomies & partial trisomies of autosomes | |
| R034y11 | [D]Global retardation | |
| ZS34.00 | Developmental disorder of scholastic skill | |
| ZS34.11 | Learning disability |
Top 20 occurring non-administration Read codes that were used to define intellectual disability that did not appear in the Quality and Outcomes Framework definition of learning disability
| Read code | Read rubric | Total patients in initial extraction | % who appear on QOF learning disability register |
|---|---|---|---|
| PJ0..00 | Down’s syndrome – trisomy 21 | 1824a | 81 |
| ZS34.11 | Learning disability | 1527 | 66 |
| 6664.00 | Mental handicap problem | 837 | 73 |
| PJ0z.00 | Down’s syndrome NOS | 329a | 81 |
| 13Z3.00 | Low I.Q. | 204 | 32 |
| ZS34.00 | Developmental disorder of scholastic skill | 156 | 68 |
| PJyy200 | Fragile X chromosome | 87 | 34 |
| PJyy400 | Fragile X syndrome | 69 | 49 |
| PKy4.00 | William syndrome | 57 | 59 |
| PJ0..11 | Mongolism | 50 | 78 |
| Eu84200 | [X]Rett’s syndrome | 47 | 68 |
| PKy9300 | Prader – Willi syndrome | 40 | 53 |
| Eu84112 | [X]Mental retardation with autistic features | 38 | 81 |
| PJ0..12 | Trisomy 21 | 33 | 79 |
| R034y11 | [D]Global retardation | 26 | 49 |
| PJ33300 | Smith-Magenis syndrome | 16 | 70 |
| PKy0.11 | Prader-Willi Syndrome | 11 | 61 |
| PJ31.00 | Cri-du-chat syndrome | 10 | 71 |
| Eu84400 | [X]Overactive disorder assoc mental retard/stereotype movts | 6 | 60 |
| C03z.12 | Cretinism | 6 | 17 |
Appendix 3 Read codes used to define intellectual disability subgroups
Read codes used for subgroups which identify a range of severe health needs for patients with intellectual disability
| Read code | Description | Subgroup |
|---|---|---|
| 13C5.00 | Confined to chair | Severe mobility |
| 13C5.11 | Chairbound | Severe mobility |
| 13C6.00 | Bed-ridden | Severe mobility |
| 13C6.11 | Bedbound | Severe mobility |
| 13CC.00 | Immobile | Severe mobility |
| 13CD.00 | Mobility very poor | Severe mobility |
| 13CE.00 | Mobility poor | Severe mobility |
| 14U5.00 | H/O: gastrostomy | PEG feeding |
| 1593.00 | H/O: stress incontinence | Continence |
| 16F..00 | Double incontinence | Continence |
| 19E2.00 | Soiling – encopresis | Continence |
| 19E2.11 | Encopresis symptom | Continence |
| 19E2.12 | Soiling symptom | Continence |
| 19E3.00 | Incontinent of faeces | Continence |
| 19E3.11 | Incontinent of faeces symptom | Continence |
| 1A22.00 | Enuresis | Continence |
| 1A22000 | Nocturnal enuresis | Continence |
| 1A22011 | Bedwetting | Continence |
| 1A22100 | Daytime enuresis | Continence |
| 1A23.00 | Incontinence of urine | Continence |
| 1A24.00 | Stress incontinence | Continence |
| 1A24.11 | Stress incontinence – symptom | Continence |
| 1A26.00 | Urge incontinence of urine | Continence |
| 1B75.00 | Loss of vision | Severe visual loss |
| 1B77.00 | Deteriorating vision | Severe visual loss |
| 1C13.00 | Deafness | Severe hearing impairment |
| 1C13300 | Bilateral deafness | Severe hearing impairment |
| 1C17.00 | Hearing aid problem | Severe hearing impairment |
| 2836.00 | O/E – quadriplegia | Severe mobility |
| 2BL..11 | O/E – deaf | Severe hearing impairment |
| 2BL3.00 | O/E – significantly deaf | Severe hearing impairment |
| 2BL4.00 | O/E – very deaf | Severe hearing impairment |
| 2BL5.00 | O/E – completely deaf | Severe hearing impairment |
| 2DG..00 | Hearing aid worn | Severe hearing impairment |
| 2DH0.00 | Uses hearing loop | Severe hearing impairment |
| 3930.00 | Bowels: incontinent | Continence |
| 3931.00 | Bowels: occasional accident | Continence |
| 3940.00 | Bladder: incontinent | Continence |
| 3941.00 | Bladder: occasional accident | Continence |
| 3960.00 | Dependent: chair/bed transfer | Severe mobility |
| 3980.00 | Immobile | Severe mobility |
| 3981.00 | Independent in wheelchair | Severe mobility |
| 3982.00 | Minimal help in wheelchair | Severe mobility |
| 398A.00 | Dependent on helper pushing wheelchair | Severe mobility |
| 6688.00 | Registered partially sighted | Severe visual loss |
| 6688.11 | Registered partially blind | Severe visual loss |
| 6689.00 | Registered blind | Severe visual loss |
| 6689.11 | Registered severely sight impaired | Severe visual loss |
| 668C.00 | Certificate of vision impairment | Severe visual loss |
| 668D.00 | Registered sight impaired | Severe visual loss |
| 7007300 | Insertion of auditory implant to brainstem | Severe hearing impairment |
| 7308400 | Placement of hearing implant in external ear | Severe hearing impairment |
| 7308500 | Attention to hearing implant in external ear | Severe hearing impairment |
| 7308600 | Removal of hearing implant from external ear | Severe hearing impairment |
| 7311A00 | Insertn bone anchors subcutaneous bone anchored hearing aid | Severe hearing impairment |
| 7317C00 | Placement of hearing implant in middle ear | Severe hearing impairment |
| 7317D00 | Attention to hearing implant in middle ear | Severe hearing impairment |
| 7317E00 | Removal of hearing implant from middle ear | Severe hearing impairment |
| 7319.00 | Attachment of bone anchored hearing prosthesis | Severe hearing impairment |
| 7319000 | Insertion fixtures bone anchored hearing prosthesis Stage 1 | Severe hearing impairment |
| 7319100 | Insertion fixtures bone anchored hearing prosthesis Stage 2 | Severe hearing impairment |
| 7319200 | Reduction soft tissue for bone anchored hearing prosthesis | Severe hearing impairment |
| 7319300 | Attention to fixtures for bone anchored hearing prosthesis | Severe hearing impairment |
| 7319400 | One stage insert fixtures bone anchored hearing prosthesis | Severe hearing impairment |
| 7319500 | Fitting external hearing prosthesis bone anchored fixtures | Severe hearing impairment |
| 7319y00 | Other specified attachment bone anchored hearing prosthesis | Severe hearing impairment |
| 7319z00 | Attachment of bone anchored hearing prosthesis NOS | Severe hearing impairment |
| 7617.00 | Gastrostomy operations | PEG feeding |
| 7617.12 | Creation of gastrostomy | PEG feeding |
| 7617000 | Creation of permanent gastrostomy | PEG feeding |
| 7617100 | Creation of temporary gastrostomy | PEG feeding |
| 7617400 | Attention to gastrostomy tube | PEG feeding |
| 7617500 | Removal of gastrostomy tube | PEG feeding |
| 7617600 | Change of gastrostomy tube | PEG feeding |
| 7617700 | Maintenance of percutaneous endoscopic gastrostomy tube | PEG feeding |
| 7617z00 | Gastrostomy operation NOS | PEG feeding |
| 7619.11 | Gastrotomy NEC | PEG feeding |
| 761E300 | Temporary percutaneous endoscopic gastrostomy | PEG feeding |
| 761E400 | Permanent percutaneous endoscopic gastrostomy | PEG feeding |
| 761E600 | Fibreoptic endoscopic percutaneous insert gastrostomy (PEG) | PEG feeding |
| 761E900 | Fibreoptic endoscopic removal of gastrostomy tube | PEG feeding |
| 761EA00 | Fibreoptic endoscopic percutaneous insertion of gastrostomy | PEG feeding |
| 8CJ2.00 | Percutaneous endoscopic gastrostomy feeding | PEG feeding |
| 8D2..00 | Auditory aid | Severe hearing impairment |
| 8D2..11 | Auditory aid provision | Severe hearing impairment |
| 8D2..12 | Hearing aid provision | Severe hearing impairment |
| 8D21.00 | Provide head worn hearing aid | Severe hearing impairment |
| 8D22.00 | Provide body worn hearing aid | Severe hearing impairment |
| 8D23.00 | Ear fitting hearing aid | Severe hearing impairment |
| 8D24.00 | Replace hearing aid battery | Severe hearing impairment |
| 8D25.00 | Physiolog. hearing assistance | Severe hearing impairment |
| 8D2Z.00 | Auditory aid NOS | Severe hearing impairment |
| 8D3..00 | Visual aid | Severe visual loss |
| 8D3..13 | Visual aid provision | Severe visual loss |
| 8D31.00 | Physiolog. visual assistance | Severe visual loss |
| 8D3Z.00 | Visual aid NOS | Severe visual loss |
| 8D73.00 | Nocturnal bladder warning syst | Continence |
| 8D73.11 | Enuretic alarm | Continence |
| 8D73.12 | Enuresis alarm | Continence |
| 8D9..13 | Wheel chair | Severe mobility |
| 8D92.00 | Self propelled wheel chair | Severe mobility |
| 8D93.00 | Pedal powered wheel chair | Severe mobility |
| 8D94.00 | Powered wheel chair | Severe mobility |
| 8D95.00 | Wheel chair unspecified | Severe mobility |
| 8D9A.00 | Attendant powered wheel chair | Severe mobility |
| 8D9B.00 | Wheelchair seating | Severe mobility |
| 8E3..00 | Deafness remedial therapy | Severe hearing impairment |
| 8E3Z.00 | Deafness remedial therapy NOS | Severe hearing impairment |
| 8F6..11 | Blind rehabilitation | Severe visual loss |
| 8F61.00 | Blind rehabilitation | Severe visual loss |
| 8F62.00 | Blind lead dog rehabilitation | Severe visual loss |
| 8HHC.00 | Referred for wheelchair assessment | Severe mobility |
| 8HlE.00 | Referral to visual impairment multidisciplinary team | Severe visual loss |
| 8M41.00 | Hearing aid requested | Severe hearing impairment |
| 9m08.00 | Excluded from diabetic retinopathy screening as blind | Severe visual loss |
| 9N0b.00 | Seen in hearing aid clinic | Severe hearing impairment |
| 9NfB.00 | Requires deafblind communicator guide | Severe hearing impairment |
| 9NfB.00 | Requires deafblind communicator guide | Severe visual loss |
| 9NlD.00 | Seen by visual impairment teacher | Severe visual loss |
| 9R43.00 | Wheelchair in need of repair | Severe mobility |
| 9R44.00 | Wheelchair in good repair | Severe mobility |
| 9RA..00 | Wheelchair applied for | Severe mobility |
| A560200 | Rubella deafness | Severe hearing impairment |
| E276.00 | Non-organic enuresis | Continence |
| E276000 | Non-organic primary enuresis | Continence |
| E276100 | Non-organic secondary enuresis | Continence |
| E276z00 | Non-organic enuresis NOS | Continence |
| E277.00 | Non-organic encopresis | Continence |
| E277000 | Non-organic continuous encopresis | Continence |
| E277100 | Non-organic discontinuous encopresis | Continence |
| E277z00 | Non-organic encopresis NOS | Continence |
| E311.00 | Severe mental retardation, IQ in range 20–34 | Severe/profound |
| E312.00 | Profound mental retardation with IQ less than 20 | Severe/profound |
| E312.11 | Idiocy | Severe/profound |
| Eu72.00 | [X]Severe mental retardation | Severe/profound |
| Eu72.11 | [X]Severe mental subnormality | Severe/profound |
| Eu72000 | [X]Sev mental retard with statement no or min impairm behav | Severe/profound |
| Eu72100 | [X]Sev mental retard sig impairment behav req attent/treatmt | Severe/profound |
| Eu72y00 | [X]Severe mental retardation, other impairments of behaviour | Severe/profound |
| Eu72z00 | [X]Sev mental retardation without mention impairment behav | Severe/profound |
| Eu73.00 | [X]Profound mental retardation | Severe/profound |
| Eu73.11 | [X]Profound mental subnormality | Severe/profound |
| Eu73000 | [X]Profound ment retrd wth statement no or min impairm behav | Severe/profound |
| Eu73100 | [X]Profound ment retard sig impairmnt behav req attent/treat | Severe/profound |
| Eu73y00 | [X]Profound mental retardation, other impairments of behavr | Severe/profound |
| Eu73z00 | [X]Prfnd mental retardation without mention impairment behav | Severe/profound |
| Eu81500 | [X]Severe learning disability | Severe/profound |
| Eu81700 | [X]Profound learning disability | Severe/profound |
| Eu9y000 | [X]Nonorganic enuresis | Continence |
| Eu9y100 | [X]Nonorganic encopresis | Continence |
| F132100 | Progressive myoclonic epilepsy | Epilepsy |
| F132111 | Unverricht – Lundborg disease | Epilepsy |
| F137.00 | Symptomatic torsion dystonia | Cerebral palsy |
| F137.11 | Athetoid cerebral palsy | Cerebral palsy |
| F137.12 | Athetosis – congenital | Cerebral palsy |
| F137.13 | Vogt’s disease | Cerebral palsy |
| F137000 | Athetoid cerebral palsy | Cerebral palsy |
| F137011 | Vogt’s disease | Cerebral palsy |
| F137100 | Double athetosis | Cerebral palsy |
| F137111 | Congenital athetosis | Cerebral palsy |
| F137y00 | Other specified symptomatic torsion dystonia | Cerebral palsy |
| F137z00 | Symptomatic torsion dystonia NOS | Cerebral palsy |
| F23..00 | Congenital cerebral palsy | Cerebral palsy |
| F23..11 | Congenital spastic cerebral palsy | Cerebral palsy |
| F23..12 | Infantile cerebral palsy | Cerebral palsy |
| F23..13 | Littles disease | Cerebral palsy |
| F23..14 | Cerebral atonia | Cerebral palsy |
| F230.00 | Congenital diplegia | Cerebral palsy |
| F230.11 | Paraplegia – congenital | Cerebral palsy |
| F230000 | Congenital paraplegia | Cerebral palsy |
| F230100 | Cerebral palsy with spastic diplegia | Cerebral palsy |
| F230z00 | Congenital diplegia NOS | Cerebral palsy |
| F231.00 | Congenital hemiplegia | Cerebral palsy |
| F232.00 | Congenital quadriplegia | Cerebral palsy |
| F232.11 | Tetraplegia – congenital | Cerebral palsy |
| F233.00 | Congenital monoplegia | Cerebral palsy |
| F233.11 | Congenital spastic foot | Cerebral palsy |
| F234.00 | Infantile hemiplegia NOS | Cerebral palsy |
| F23y.00 | Other congenital cerebral palsy | Cerebral palsy |
| F23y000 | Ataxic infantile cerebral palsy | Cerebral palsy |
| F23y100 | Flaccid infantile cerebral palsy | Cerebral palsy |
| F23y200 | Spastic cerebral palsy | Cerebral palsy |
| F23y300 | Dyskinetic cerebral palsy | Cerebral palsy |
| F23y400 | Ataxic diplegic cerebral palsy | Cerebral palsy |
| F23y500 | Worster-Drought syndrome | Cerebral palsy |
| F23y511 | Congenital suprabulbar paresis | Cerebral palsy |
| F23yz00 | Other infantile cerebral palsy NOS | Cerebral palsy |
| F23z.00 | Congenital cerebral palsy NOS | Cerebral palsy |
| F240.00 | Quadriplegia | Severe mobility |
| F240.11 | Tetraplegia | Severe mobility |
| F240100 | Spastic tetraplegia | Severe mobility |
| F241.00 | Paraplegia | Severe mobility |
| F241100 | Spastic paraplegia | Severe mobility |
| F242.00 | Diplegia of upper limbs | Severe mobility |
| F243.00 | Monoplegia of lower limb | Severe mobility |
| F244.00 | Monoplegia of upper limb | Severe mobility |
| F25..00 | Epilepsy | Epilepsy |
| F250.00 | Generalised nonconvulsive epilepsy | Epilepsy |
| F250200 | Epileptic seizures – atonic | Epilepsy |
| F250300 | Epileptic seizures – akinetic | Epilepsy |
| F250500 | Lennox-Gastaut syndrome | Epilepsy |
| F250y00 | Other specified generalised nonconvulsive epilepsy | Epilepsy |
| F250z00 | Generalised nonconvulsive epilepsy NOS | Epilepsy |
| F251.00 | Generalised convulsive epilepsy | Epilepsy |
| F251000 | Grand mal (major) epilepsy | Epilepsy |
| F251011 | Tonic–clonic epilepsy | Epilepsy |
| F251200 | Epileptic seizures – clonic | Epilepsy |
| F251300 | Epileptic seizures – myoclonic | Epilepsy |
| F251400 | Epileptic seizures – tonic | Epilepsy |
| F251500 | Tonic–clonic epilepsy | Epilepsy |
| F251y00 | Other specified generalised convulsive epilepsy | Epilepsy |
| F251z00 | Generalised convulsive epilepsy NOS | Epilepsy |
| F253.00 | Grand mal status | Epilepsy |
| F253.11 | Status epilepticus | Epilepsy |
| F254.00 | Partial epilepsy with impairment of consciousness | Epilepsy |
| F254000 | Temporal lobe epilepsy | Epilepsy |
| F254100 | Psychomotor epilepsy | Epilepsy |
| F254200 | Psychosensory epilepsy | Epilepsy |
| F254300 | Limbic system epilepsy | Epilepsy |
| F254400 | Epileptic automatism | Epilepsy |
| F254500 | Complex partial epileptic seizure | Epilepsy |
| F254z00 | Partial epilepsy with impairment of consciousness NOS | Epilepsy |
| F255.00 | Partial epilepsy without impairment of consciousness | Epilepsy |
| F255000 | Jacksonian, focal or motor epilepsy | Epilepsy |
| F255011 | Focal epilepsy | Epilepsy |
| F255012 | Motor epilepsy | Epilepsy |
| F255100 | Sensory induced epilepsy | Epilepsy |
| F255200 | Somatosensory epilepsy | Epilepsy |
| F255300 | Visceral reflex epilepsy | Epilepsy |
| F255311 | Partial epilepsy with autonomic symptoms | Epilepsy |
| F255400 | Visual reflex epilepsy | Epilepsy |
| F255500 | Unilateral epilepsy | Epilepsy |
| F255600 | Simple partial epileptic seizure | Epilepsy |
| F255y00 | Partial epilepsy without impairment of consciousness OS | Epilepsy |
| F255z00 | Partial epilepsy without impairment of consciousness NOS | Epilepsy |
| F257.00 | Kojevnikov’s epilepsy | Epilepsy |
| F25B.00 | Alcohol-induced epilepsy | Epilepsy |
| F25C.00 | Drug-induced epilepsy | Epilepsy |
| F25D.00 | Menstrual epilepsy | Epilepsy |
| F25E.00 | Stress-induced epilepsy | Epilepsy |
| F25F.00 | Photosensitive epilepsy | Epilepsy |
| F25X.00 | Status epilepticus, unspecified | Epilepsy |
| F25y.00 | Other forms of epilepsy | Epilepsy |
| F25y000 | Cursive (running) epilepsy | Epilepsy |
| F25y100 | Gelastic epilepsy | Epilepsy |
| F25y200 | Locl-rlt(foc)(part)idiop epilep&epilptic syn seiz locl onset | Epilepsy |
| F25y300 | Complex partial status epilepticus | Epilepsy |
| F25y500 | Panayiotopoulos syndrome | Epilepsy |
| F25yz00 | Other forms of epilepsy NOS | Epilepsy |
| F25z.00 | Epilepsy NOS | Epilepsy |
| F25z.11 | Fit (in known epileptic) NOS | Epilepsy |
| F2B..00 | Cerebral palsy | Cerebral palsy |
| F2B0.00 | Spastic quadriplegic cerebral palsy | Cerebral palsy |
| F2B1.00 | Spastic hemiplegic cerebral palsy | Cerebral palsy |
| F2By.00 | Other cerebral palsy | Cerebral palsy |
| F2Bz.00 | Cerebral palsy NOS | Cerebral palsy |
| F49..00 | Blindness and low vision | Severe visual loss |
| F49..11 | Impaired vision | Severe visual loss |
| F49..12 | Low vision | Severe visual loss |
| F49..13 | Partial sight | Severe visual loss |
| F49..14 | Sight impaired | Severe visual loss |
| F490.00 | Blindness, both eyes | Severe visual loss |
| F490000 | Unspecified blindness both eyes | Severe visual loss |
| F490100 | Both eyes total visual impairment | Severe visual loss |
| F490400 | Better eye: near total VI, Lesser eye: near total VI | Severe visual loss |
| F490600 | Better eye: profound VI, Lesser eye: total VI | Severe visual loss |
| F490900 | Acquired blindness, both eyes | Severe visual loss |
| F490z00 | Blindness both eyes NOS | Severe visual loss |
| F491.00 | Better eye: low vision, Lesser eye: profound VI | Severe visual loss |
| F491000 | One eye blind, one eye low vision | Severe visual loss |
| F491100 | Better eye: severe VI, Lesser eye: blind, unspecified | Severe visual loss |
| F491300 | Better eye: severe VI, Lesser eye: near total VI | Severe visual loss |
| F491400 | Better eye: severe VI, Lesser eye: profound VI | Severe visual loss |
| F491500 | Better eye: moderate VI, Lesser eye: blind, unspecified | Severe visual loss |
| F491700 | Better eye: moderate VI, Lesser eye: near total VI | Severe visual loss |
| F491z00 | One eye blind, one eye low vision NOS | Severe visual loss |
| F492.00 | Low vision, both eyes | Severe visual loss |
| F492000 | Low vision, both eyes unspecified | Severe visual loss |
| F492200 | Better eye: severe VI, Lesser eye: severe VI | Severe visual loss |
| F492300 | Better eye: moderate VI, Lesser eye: low vision unspecified | Severe visual loss |
| F492400 | Better eye: moderate VI, Lesser eye: severe VI | Severe visual loss |
| F492500 | Better eye: moderate VI, Lesser eye: moderate VI | Severe visual loss |
| F492z00 | Low vision, both eyes NOS | Severe visual loss |
| F493.00 | Visual loss, both eyes unqualified | Severe visual loss |
| F494.00 | Legal blindness USA | Severe visual loss |
| F497.00 | Severe visual impairment, binocular | Severe visual loss |
| F498.00 | Moderate visual impairment, binocular | Severe visual loss |
| F49z.00 | Visual loss NOS | Severe visual loss |
| F49z.11 | Acquired blindness | Severe visual loss |
| F4H7300 | Cortical blindness | Severe visual loss |
| F581211 | Noise induced deafness | Severe hearing impairment |
| F59..11 | Deafness | Severe hearing impairment |
| F590.11 | Conductive deafness | Severe hearing impairment |
| F591.13 | Perceptive deafness | Severe hearing impairment |
| F591211 | Nerve deafness | Severe hearing impairment |
| F591400 | Congenital sensorineural deafness | Severe hearing impairment |
| F591500 | Ototoxicity – deafness | Severe hearing impairment |
| F591511 | Drug ototoxicity – deafness | Severe hearing impairment |
| F591800 | Congenital prelingual deafness | Severe hearing impairment |
| F592.00 | Mixed conductive and sensorineural deafness | Severe hearing impairment |
| F593.00 | Deaf mutism, NEC | Severe hearing impairment |
| F594.00 | High frequency deafness | Severe hearing impairment |
| F595.00 | Low frequency deafness | Severe hearing impairment |
| F596.00 | Maternally inherited deafness | Severe hearing impairment |
| F598.00 | Moderate acquired hearing loss | Severe hearing impairment |
| F599.00 | Severe acquired hearing loss | Severe hearing impairment |
| F59A.00 | Profound acquired hearing loss | Severe hearing impairment |
| F59A.11 | Deafened | Severe hearing impairment |
| F59z.00 | Deafness NOS | Severe hearing impairment |
| F59z.11 | Chronic deafness | Severe hearing impairment |
| Fyu9.00 | [X]Cerebral palsy and other paralytic syndromes | Cerebral palsy |
| Fyu9000 | [X]Other infantile cerebral palsy | Cerebral palsy |
| Fyu9100 | [X]Other specified paralytic syndromes | Cerebral palsy |
| FyuU000 | [X]Deaf mutism, not elsewhere classified | Severe hearing impairment |
| K198.00 | Stress incontinence | Continence |
| K586.00 | Stress incontinence – female | Continence |
| Kyu5A00 | [X]Other specified urinary incontinence | Continence |
| P40z.11 | Deafness due to congenital anomaly NEC | Severe hearing impairment |
| R00A.00 | [D]Poor mobility | Severe mobility |
| R00C.00 | [D]Immobility | Severe mobility |
| R076.00 | [D]Incontinence of faeces | Continence |
| R076000 | [D]Encopresis NOS | Continence |
| R076100 | [D]Sphincter ani incontinence | Continence |
| R076z00 | [D]Incontinence of faeces NOS | Continence |
| R083.00 | [D]Incontinence of urine | Continence |
| R083000 | [D]Enuresis NOS | Continence |
| R083100 | [D]Urethral sphincter incontinence | Continence |
| R083200 | [D]Urge incontinence | Continence |
| R083z00 | [D]Incontinence of urine NOS | Continence |
| SJ15.12 | Deafness – traumatic – NOS | Severe hearing impairment |
| Z1J..00 | Procedures to aid continence | Continence |
| Z6R3.00 | Wheelchair dancing therapy | Severe mobility |
| Z6R8100 | Wheelchair sport | Severe mobility |
| Z6X1.00 | Wheelchair transfer practice | Severe mobility |
| Z6Z..00 | Wheelchair education | Severe mobility |
| Z6Z1.00 | Wheelchair use training | Severe mobility |
| Z6Z1200 | Propelling wheelchair training | Severe mobility |
| Z6Z1300 | Controlling electric wheelchair training | Severe mobility |
| Z8B5.00 | Ability to use hearing aid | Severe hearing impairment |
| Z8B5100 | Able to use hearing aid | Severe hearing impairment |
| Z8B5200 | Unable to use hearing aid | Severe hearing impairment |
| Z8B5300 | Does use hearing aid | Severe hearing impairment |
| Z8B5311 | Uses hearing aid | Severe hearing impairment |
| Z8B5400 | Does not use hearing aid | Severe hearing impairment |
| Z8B5500 | Difficulty using hearing aid | Severe hearing impairment |
| Z911.00 | Hearing aid procedure | Severe hearing impairment |
| Z911100 | Fit hearing aid | Severe hearing impairment |
| Z911300 | Adjust hearing aid settings | Severe hearing impairment |
| Z911400 | Changing hearing aid battery | Severe hearing impairment |
| Z911500 | Checking hearing aid | Severe hearing impairment |
| Z911700 | Switching on hearing aid | Severe hearing impairment |
| Z911800 | Turning off hearing aid | Severe hearing impairment |
| Z911900 | Putting on hearing aid | Severe hearing impairment |
| Z911A00 | Listening for feedback whistle of hearing aid | Severe hearing impairment |
| Z911B00 | Attention to hearing aid | Severe hearing impairment |
| Z911E00 | Fit ear mould for existing hearing aid | Severe hearing impairment |
| Z96..00 | Provision for visual and hearing impairment | Severe visual loss |
| Z961.00 | Provision of guide help for visual and hearing impairment | Severe visual loss |
| Z9E2.00 | Optical low vision aid provision | Severe visual loss |
| Z9E3.00 | Provision of optical low vision aid – near | Severe visual loss |
| Z9E3100 | Provision of magnifier low vision aid – near | Severe visual loss |
| Z9E3200 | Provision of low vision hand magnifier | Severe visual loss |
| Z9E3300 | Provision of low vision stand magnifier | Severe visual loss |
| Z9E3500 | Provision of spectacle low vision aid – near | Severe visual loss |
| Z9E3600 | Provision of telescopic spectacles | Severe visual loss |
| Z9E3700 | Provision of spectacle magnifier | Severe visual loss |
| Z9E3900 | Near low vision aid – clip-on spectacle magnifier | Severe visual loss |
| Z9E3A00 | Provision of spectacle telescope | Severe visual loss |
| Z9E3B00 | Near low vision aid – integral spectacle telescope | Severe visual loss |
| Z9E3C00 | Near low vision aid – clip-on spectacle telescope | Severe visual loss |
| Z9E3D00 | Near low vision aid – extra cap for telescope | Severe visual loss |
| Z9E3E00 | Provision of headband telescope | Severe visual loss |
| Z9E4.00 | Provision of optical low vision aid – distance | Severe visual loss |
| Z9E5.00 | Provision of non-optical low vision aid | Severe visual loss |
| Z9E5200 | Provision of closed circuit television | Severe visual loss |
| Z9E5300 | Provision of image intensifier | Severe visual loss |
| Z9E5400 | Provision of ancillary low vision aid | Severe visual loss |
| Z9E5700 | Provision of work board | Severe visual loss |
| Z9E6.00 | Provision of visual appliance | Severe visual loss |
| Z9E6500 | Provision of audiotaped services | Severe visual loss |
| Z9E6600 | Provision of talking book | Severe visual loss |
| Z9E8100 | Hearing aid provision | Severe hearing impairment |
| Z9E8111 | Auditory aid provision | Severe hearing impairment |
| Z9EA.00 | Provision of incontinence appliance | Continence |
| Z9EA100 | Provision of nocturnal bladder warning system | Continence |
| Z9EA111 | Provision of enuresis alarm | Continence |
| Z9EA112 | Provision of enuretic alarm | Continence |
| Z9EH400 | Provision of wheelchair | Severe mobility |
| Z9MO.00 | Enuresis support | Continence |
| ZC65200 | Gastrostomy feeding | PEG feeding |
| ZC65300 | Percutaneous endoscopic gastrostomy feeding | PEG feeding |
| ZC65311 | PEG – Percutaneous endoscopic gastrostomy feeding | PEG feeding |
| ZC65400 | Button gastrostomy feeding | PEG feeding |
| ZC65500 | Jejunostomy feeding | PEG feeding |
| ZE83200 | Hearing for loud voice impaired | Severe hearing impairment |
| ZE84200 | Hearing for voice impaired | Severe hearing impairment |
| ZE87.00 | Hearing loss | Severe hearing impairment |
| ZE87.11 | Deafness | Severe hearing impairment |
| ZE87.13 | Hard of hearing | Severe hearing impairment |
| ZE87.16 | HL – Hearing loss | Severe hearing impairment |
| ZE87.17 | HOH – Hard of hearing | Severe hearing impairment |
| ZL22400 | Under care of continence nurse | Continence |
| ZN56800 | Blind telephone user | Severe visual loss |
| ZN56900 | Deaf telephone user | Severe hearing impairment |
| ZO2..00 | Unable to mobilise | Severe mobility |
| ZO4..00 | Does not mobilise | Severe mobility |
| ZO72.00 | Unable to mobilise indoors | Severe mobility |
| ZO74.00 | Does not mobilise indoors | Severe mobility |
| ZO75.00 | Difficulty mobilising indoors | Severe mobility |
| ZO92.00 | Unable to mobilise using mobility aids | Severe mobility |
| ZO93.00 | Does mobilise using aids | Severe mobility |
| ZO94.00 | Does not mobilise using mobility aids | Severe mobility |
| ZO96.00 | Ability to mobilise using wheelchair | Severe mobility |
| ZO96.11 | Wheelchair mobility | Severe mobility |
| ZO96100 | Able to mobilise using wheelchair | Severe mobility |
| ZO96200 | Unable to mobilise using wheelchair | Severe mobility |
| ZO96300 | Does mobilise using wheelchair | Severe mobility |
| ZO96311 | Mobilises using wheelchair | Severe mobility |
| ZO96400 | Does not mobilise using wheelchair | Severe mobility |
| ZO96500 | Difficulty mobilising using wheelchair | Severe mobility |
| ZOC6200 | Unable to get in and out of a chair | Severe mobility |
| ZOC6400 | Does not get in and out of a chair | Severe mobility |
| ZOC8200 | Unable to get out of a chair | Severe mobility |
| ZOC8400 | Does not get out of a chair | Severe mobility |
| ZOC9200 | Unable to get on and off a bed | Severe mobility |
| ZOC9400 | Does not get on and off a bed | Severe mobility |
| ZOCA200 | Unable to get on a bed | Severe mobility |
| ZOCB200 | Unable to get off a bed | Severe mobility |
| ZOCB400 | Does not get off a bed | Severe mobility |
| ZOD2.00 | Unable to move in bed | Severe mobility |
| ZOD4.00 | Does not move in bed | Severe mobility |
| ZOD6200 | Unable to roll over in bed | Severe mobility |
| ZOD6211 | Unable to turn over in bed | Severe mobility |
| ZOD7500 | Difficulty turning onto side in bed | Severe mobility |
| ZOD8200 | Unable to move up and down bed | Severe mobility |
| ZT12711 | Voice associated with hearing loss | Severe hearing impairment |
| ZV44100 | [V]Has gastrostomy | PEG feeding |
| ZV45G00 | [V]Presence of external hearing-aid | Severe hearing impairment |
| ZV45N00 | [V]Bone anchored hearing aid in situ | Severe hearing impairment |
| ZV46200 | [V]Dependence on wheelchair | Severe mobility |
| ZV4L011 | [V] Poor mobility | Severe mobility |
| ZV53200 | [V]Fitting or adjustment of hearing aid | Severe hearing impairment |
| ZV53800 | [V]Fitting or adjustment of wheelchair | Severe mobility |
| ZV53D00 | [V]Adjustment and management of implanted hearing device | Severe hearing impairment |
| ZV55100 | [V]Attention to gastrostomy | PEG feeding |
Read codes used to identify living arrangements that were deemed to be communal or shared
| Read code | Description |
|---|---|
| 13F4.00 | Warden attended |
| 13F4.11 | Lives in warden-controlled accommodation |
| 13F4000 | Resident in sheltered accommodation |
| 13F5.00 | Part III accommodation |
| 13F5.11 | Part 3 accommodation |
| 13F5100 | Part III accommodation arranged |
| 13F5111 | Part 3 accommodation arranged |
| 13F5200 | Resident in part III accommodation |
| 13F6.00 | Nursing/other home |
| 13F6100 | Lives in a nursing home |
| 13F7.00 | Residential institution |
| 13F7100 | Lives in a welfare home |
| 13F7200 | Lives in an old peoples home |
| 13F7300 | Lives in a childrens home |
| 13F7400 | Admitted to a children’s home |
| 13F8100 | Long-stay hospital inpatient |
| 13F9.00 | Living in hostel |
| 13F9.11 | Living in sheltered accomodatn |
| 13FK.00 | Lives in a residential home |
| 13FS.00 | Long stay hospital inpatient |
| 13FT.00 | Lives in an old peoples home |
| 13FV.00 | Lives in a welfare home |
| 13FX.00 | Lives in care home |
| 13FY.00 | Lives in a children’s unit |
| Z177100 | 24-hour care |
| Z177500 | Custodial care |
| Z177C00 | Residential care |
| Z177D00 | Local authority residential care |
| Z177D11 | LA – local authority residential care |
| ZU37.00 | Lives in a community |
| ZU37100 | Lives in a school community |
| ZU37200 | Lives in boarding school |
| ZV60600 | [V]Institution resident |
| ZV60611 | [V]Boarding school resident |
| ZV60700 | [V]Sheltered housing |
| ZU37100 | Lives in a school community |
Read codes used to identify autism
| Read code | Description |
|---|---|
| E140.00 | Infantile autism |
| E140.11 | Kanner’s syndrome |
| E140.12 | Autism |
| E140.13 | Childhood autism |
| E140000 | Active infantile autism |
| E140100 | Residual infantile autism |
| E140z00 | Infantile autism NOS |
| E2F5.00 | Mixed development disorder |
| Eu83.00 | [X]Mixed specific developmental disorders |
| Eu84.00 | [X]Pervasive developmental disorders |
| Eu84000 | [X]Childhood autism |
| Eu84011 | [X]Autistic disorder |
| Eu84012 | [X]Infantile autism |
| Eu84013 | [X]Infantile psychosis |
| Eu84014 | [X]Kanner’s syndrome |
| Eu84100 | [X]Atypical autism |
| Eu84111 | [X]Atypical childhood psychosis |
| Eu84511 | [X]Autistic psychopathy |
| Eu84y00 | [X]Other pervasive developmental disorders |
| Eu84z00 | [X]Pervasive developmental disorder, unspecified |
| Eu84z11 | [X]Autistic spectrum disorder |
Read codes used to identify Down syndrome
| Read code | Description |
|---|---|
| PJ0..00 | Down’s syndrome – trisomy 21 |
| PJ0..11 | Mongolism |
| PJ0..12 | Trisomy 21 |
| PJ0..13 | Trisomy 22 |
| PJ00.00 | Trisomy 21, meiotic nondisjunction |
| PJ01.00 | Trisomy 21, mosaicism |
| PJ01.11 | Trisomy 21, mitotic nondisjunction |
| PJ02.00 | Trisomy 21, translocation |
| PJ02.11 | Partial trisomy 21 in Down’s syndrome |
| PJ0z.00 | Down’s syndrome NOS |
| PJ0z.11 | Trisomy 21 NOS |
Appendix 4 Definition of a consultation in Clinical Practice Research Datalink
FIGURE 39.
Summary of how consultations were identified in CPRD.
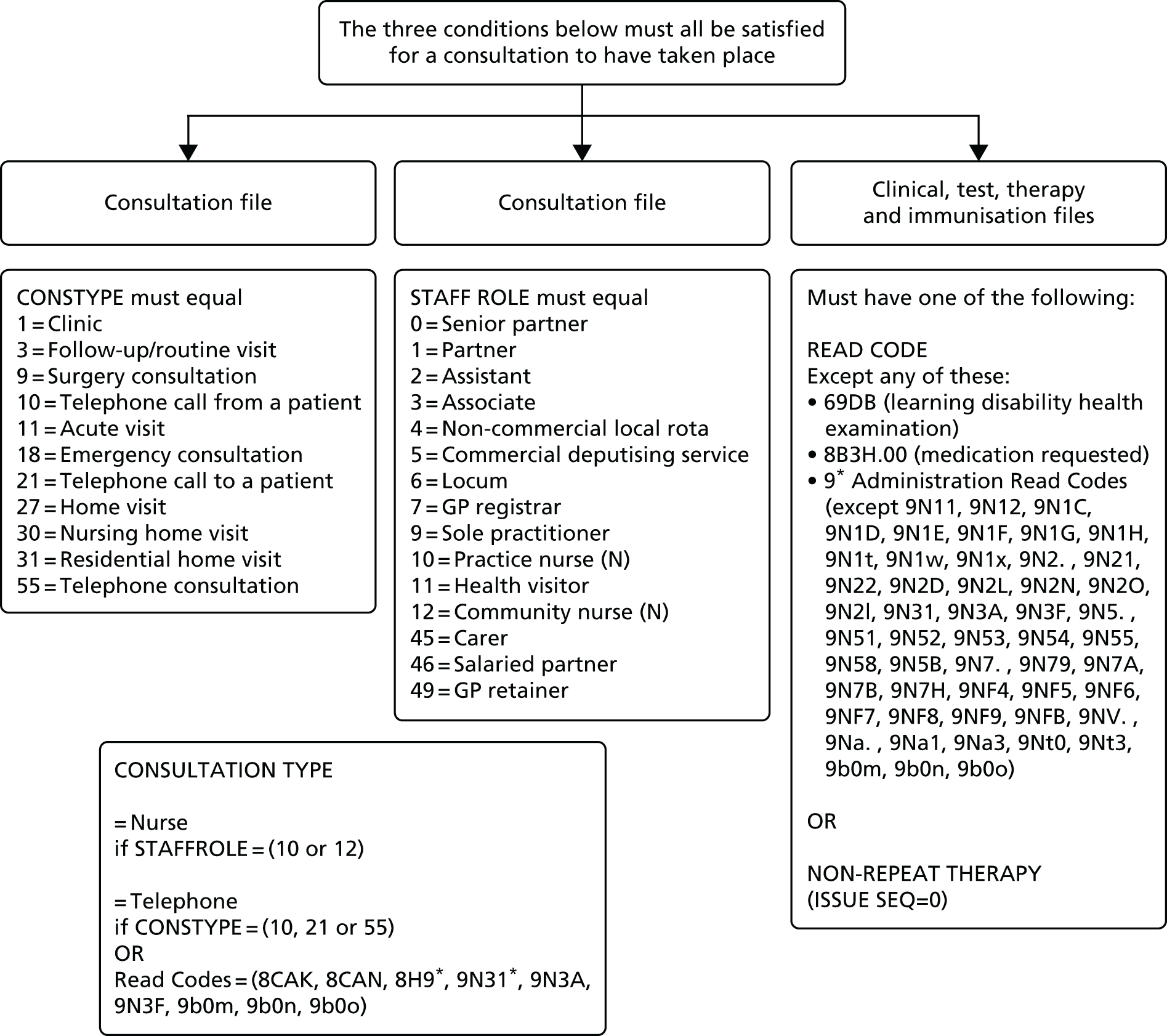
Appendix 5 Economic costs
Summary of calculation estimates for costing analysis
| Area | Calculation details |
|---|---|
| Primary care consultations | GP consultations: £3.70 per minute (maximum length 60 minutes). If ≤ 5 minutes, or not recorded, assume £43.00 per consultation |
| Nurse consultations: £0.88 per minute (maximum length 60 minutes). If ≤ 5 minutes, or not recorded, assume £10.34 per consultation | |
| GP home visits £110.00 per visit | |
| Prescribing (primary care) | Use net ingredient cost per quantity when a quantity tablets or capsules are issued |
| Use net ingredient cost per item for other drug formulations | |
| Use a default average cost of £9.85 per item where it was not possible to easily merge CPRD and prescription cost analysis data | |
| Other primary care-led activity | Referrals (community services only) costed at £33 each (maximum of one per day) |
| Outpatients (evidence of attendance) were costed at £139 each (maximum of one per day) | |
| A&E or casualty attendance | £112 each (maximum of one per day) |
| Hospital admissions | Use NHS Reference Costs for 2011–12 classified by HRG4, calculated from ICD-1058 and OPCS-4 codes.59 When a hospitalisation has multiple episode, use the episode with the maximum cost |
| Some exceptions failed to merge and were coded differently: cystic fibrosis (2009–10 costings used), dialysis (2009–10 costings used), non-specialist mental health service provider (2012–13 costings used) | |
| Admissions that could not be assigned by the above were costed by defaults estimated by PSSRU:54 elective impatient stays = £3191; non-elective inpatient long stay (≥ 2 days) = £2461; non-elective inpatient short stay (0–1 day) = £586; elective day cases = £680 |
Appendix 6 Patient and public involvement quotations
Quotations from ResearchNet and Carers Support Merton regarding patient and public involvement
| Patient and public involvement group | Quotation |
|---|---|
| Carers Support Merton | I genuinely felt, and I’ve said this to various people, but this wasn’t just a tick box exercise, ooh yes, I’ve consulted carers, it was a genuine . . . let’s see how you can get involved and I’d like to incorporate your ideas in it, so it did feel like genuine involvement which was greatCP3. . . it was a very positive experience all around and umm . . . I’m absolutely delighted that both parents and people with a learning disability viewpoints actually were taken in to the study and I’m sure we made it a better study as a result. I think that should be an exemplar for all LD studies as you feel you’re being listened to and helping shape what’s important rather than having it come from top down what people think is bestCP1Definitely. I would definitely work with this team from St George’s again as I know that they are serious about what they are doing. You know that they are serious about involving parents and they have listened to us. I just hope the research makes an impactCP2To actually involve the carers and the people themselves. If there was a way of flagging that up and making that best practice, that would be fantasticCP4 |
| ResearchNet | What did you feel about helping to guide this research project using your expertise?IDP1:Loved every minuteIDP3:Loved itIDP5:Loved everything about itIDP2:50/50What was/what’s 50/50 [name], what didn’t you like?IDP2:Umm . . . I think . . . something . . . something what . . .Was it because we were asking you to share things, your personal story?IDP2:I don’t know, maybe yesDid you feel it was a waste of time?IDP2:NoIDP1:No, far from itIDP4:Not at allIDP3:We . . . we are actually being listened to and taken note ofIDP2:It’s important to get our views across and we’re not just numbers on someone’s spreadsheetIDP5:Exactly. Well said . . . |
Appendix 7 Cause of death groupings
Listing of International Classification of Diseases, Tenth Edition codes used to identify and group causes of death
| ICD-10 code | Main grouping | ICD-10 code | Secondary group of interest |
|---|---|---|---|
| A00–B99 | Infectious and parasitic disorders | ||
| C00–D48 | Neoplasms | C16 | Oesophageal cancer |
| C17 | Stomach cancer | ||
| C18–21 | Colorectal cancer | ||
| C25 | Pancreatic cancer | ||
| C33–34 | Lung cancer | ||
| C43–44 | Skin cancers | ||
| C50 | Breast cancer | ||
| C53 | Cervical cancer | ||
| C61 | Prostate cancer | ||
| C64–68 | Urinary tract cancers | ||
| C81–96 | Lymphoma | ||
| E00–90 | Endocrine, nutritional and metabolic diseases | ||
| F00–99 | Mental and behavioural disorders | F00–03 | Dementia |
| G00–99 | Diseases of the nervous system | G40–41 | Epilepsy |
| I00–99 | Diseases of the circulatory system | I20–25 | Ischaemic heart disease |
| I60–69 | Cerebrovascular disease | ||
| I61, I63–64 | Stroke | ||
| J00–99 | Diseases of the respiratory system | J09–11 | Influenza |
| J40–47 | Chronic lower respiratory disease | ||
| J41–44, J47 | COPD | ||
| J69 | Pneumonitis due to solids and liquids | ||
| K00–93 | Diseases of the digestive system | K70–77 | Diseases of liver |
| M00–99 | Diseases of the musculoskeletal system and connective tissue | ||
| N00–99 | Diseases of the genitourinary system | ||
| Q00–99 | Congenital malformations, deformations and chromosomal abnormalities | ||
| V01–Y98 | External causes of morbidity and mortality | V01–99 | Transport accidents |
| W00–X59 | Other external causes of accidental injury | ||
| X60–84 | Intentional self-harm | ||
| All other | Other (skin, blood, residual codes) | ||
Appendix 8 Ambulatory care-sensitive conditions for emergency hospital admission
Listing of International Classification of Diseases, Tenth Edition codes used to identify and group ambulatory care-sensitive conditions
| Condition | ICD-10 code |
|---|---|
| Angina | I20, I24.0, I24.8–24.9 |
| Aspiration | J69.0, J69.8 |
| Asthma | J45–46 |
| Cellulitis | L03–04, L08, L88, L98.0, L98.3 |
| Congestive heart failure | I11.0, I50, J81 |
| Constipation | K59.0 |
| Convulsions/epilepsy | G40–41, R56, O15 |
| COPD | J41–44, J47 |
| Dehydration and gastroenteritis | E86, K52.2, K52.8, K52.9 |
| Dental conditions | A69.0, K02–06, K08, K09.8, K09.9, K12–13 |
| Diabetes complications | E10.0–10.8, E11.0–11.8, E12.0–12.8, E13.0–13.8, E14.0–14.8 |
| Ear, nose and throat infections | H66–67, J02–03, J06, J31.2 |
| Gangrene | R02 |
| Gastro-oesophageal reflux disease | K21 |
| Hypertension | I10, I11.9 |
| Iron deficiency anaemia | D50.1, D50.8–50.9 |
| Influenza | J10–11 |
| Nutritional deficiencies | E40–43, E55, E64.3 |
| Pelvic inflammatory disease | N70, N73–74 |
| Perforated/bleeding ulcers | K25.0–25.2, K25.4–25.6, K26.0–26.2, K26.4–26.6, K27.0–27.2, K27.4–27.6, K28.0–28.2, K28.4–28.6 |
| Pneumonia and other acute LRTI | J13–14, J15.3–15.4, J15.7, J15.9, J16.8, J18.1, J18.8, J20–20.2, J20.8, J20.9, J22 |
| Tuberculosis and other vaccine preventable | A15–16, A19, A35–37, A80, B05–06, B16.1, B16.9, B18.0–18.1, B26, G00.0, M01.4 |
| UTI/pyelonephritis | N10–12, N13.6, N39.0 |
Appendix 9 Read codes used to define categories summarising content of health checks
Read code listing of health check content categories
| Category identified | Read codes (* indicates all codes in hierarchy) |
|---|---|
| Weight/BMI | 162*, 22A*, 66C*, 679P.00, 67I9.00 |
| Blood pressure | 246* |
| Alcohol | 136*, 388u.00, 6792.00, 67H0.00, 8CAM.00, 9k1* |
| Smoking | 137*, 6791*, 67H1.00, 67H6.00, 8CAL.00 |
| Mobility | 13C*, 398*, 399*, 39A*, 39B*, 68O*, ZO* |
| Ears | 1C1*, 1C2*, 1C3*, 1C4*, 1CD..00, 1CE..00, 2BL*, 2BM*, 2D. . .11, 2D13.00, 2D16.00 2D5*, 2D6*, 2D7*, 2D8*, 2D9*, 2DG..00, 2DH*, 2DZ..00, 313*, 7P12*, 9N2T.00, Z174500, ZE*, ZF*, ZV41200, ZV41300 |
| Eyes | 1B7*, 1B8*, 22E*, 2B6*, 2B7*, 2B8*, 2B9*, 2BA*, 2BB*, 2BC*, 2BD*, 2BE*, 2BF*, 2BG*, 2BH*, 2BI*, 2BJ*, 2BT*, 312*, 668*, 9N2U.00, 9N2V.00, Z174300, ZL47*, ZV41* |
| Carer | 8O7..00, 9180*, 918F*, 918J*, 918K.00, 918L.00, 918V.00 |
| Pulse | 24* except 246* |
| Height | 229* |
| Health action plan | 9HB0.00–9HB4.00 |
| Behaviour | 1B1X.00, 1P*, 3AB*, Z15*, ZV40.11, ZV40300 |
| Dental | 254*, 3165.00, 67IG.00, 9N2C.00, Z174600, Z174700, Z174800, ZL9G500 |
| Communication | 13o*, 1B9*, 8E2*, ZT4* |
| Exercise | 138*, 6798.00, 67H2.00, 8CA5* |
| Diet | 13A*, 13B*, 161*, 1F*, 6799.00, 67H7.00, 8CA4* |
| Blood test | 4131.00, 41D0.00, 4142.00 – 4145.00, 42*, 44*, 7L17* |
| Urine test | 41D1.00, 4146.00, 46*, 4JJ*, 68K* |
| Mental health | 1B1*, 1BD*, 1BE*, 1BF*, 1BG*, 1BH*, 1BI..00, 1BJ..00, 1BK..00, 1BL..00, 1BM..00, 1BN*, 1BO..00, 1BP..00, 1BP0.00, 1BQ..00, 1BR*, 1BS*, 1BT*, 1BU..00, 225*, 6891*, 6896.00, 6A6*, 8CM2.00, 8CR7.00, ZQ3E.00 |
| Bowels and bladder | 16F..00, 19E*, 19F*, 1A.*, 1A.*, 1A1*, 1A2*, 1A3*, 1A4*, 26. . .00, 26. . .12, 393*, 394*, 39H*, 679H*, 8C14*, 8D7*, ZQ3B.00, ZQ3C.00 |
| Respiratory | 23*, 339* |
| Sexual related | 1AB*, 61*, 6777.00, 679K.00, 679S.00, 67IJ*, 8CAw.00 |
| Medication review | 66c*, 8B31400, 8B3S*, 8B3V.00, 8B3h.00, 8B3j.00, 8B3k.00, 8B3l.00, 8B3x.00, 8B3y.00, 8BI*, 8BM*, 9N73.00 |
| Breast examination | 1A8*, 26. . .11, 26B*, 6795.00, 6862*, 8CAz.00, 9OH*, Z1P1400 |
| Cervical smear | 4149.00, 4JRL.00, 4K2*, 4K3*, 4K4*, 4K55.00, 6793.00, 685*, 8I6K.00, 9O8*, ZG52100, ZV762* |
| Epilepsy | 667* |
| Influenza vaccination | 65E*, ZV048* |
List of abbreviations
- ACSC
- ambulatory care-sensitive condition
- BMI
- body mass index
- BNF
- British National Formulary
- CHD
- coronary heart disease
- CI
- confidence interval
- CIPOLD
- Confidential Inquiry into Premature Deaths of People with Learning Disability
- COPD
- chronic obstructive pulmonary disease
- CPRD
- Clinical Practice Research Datalink
- DES
- Directed Enhanced Service
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICD-10
- International Classification of Diseases, Tenth Edition
- ID
- intellectual disability
- IHD
- ischaemic heart disease
- IMD
- Index of Multiple Deprivation
- IRR
- incidence rate ratio
- LRTI
- lower respiratory tract infection
- ONS
- Office for National Statistics
- OR
- odds ratio
- PR
- prevalence ratio
- QOF
- Quality and Outcomes Framework
- RR
- rate ratio
- SD
- standard deviation
- SMR
- standardised mortality ratio
- SUDEP
- sudden unexpected death associated with epilepsy
- TIA
- transient ischaemic attack
- UTI
- urinary tract infection