Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 15/77/34. The contractual start date was in July 2016. The final report began editorial review in July 2017 and was accepted for publication in November 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Sheaff et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Origins and nature of multispecialty community providers
Multispecialty community providers (MCPs) have been proposed as a means by which the English NHS can reduce demand pressures on hospitals and general practices while improving the quality, and especially the continuity, of care for people with complex, chronic or multiple health problems, all the while contributing substantial savings to the NHS budget. As with all policies, this policy rests on a complex set of assumptions about what mechanisms will achieve these ambitious and complex policy outcomes, and in what contexts. The explicitly proposed mechanisms include new NHS organisational structures, working practices and interorganisational networks. The purpose of this realist synthesis project is to elicit an initial programme theory (IPT) about MCPs from policy-makers’ assumptions and to use international research evidence to evaluate which of these assumptions are supported by evidence, under which conditions and for which populations. We also identify any assumptions not supported by evidence. From that, we propose possible revisions to the IPT that will yield a more fully evidence-based revised logic model for achieving the policy outcomes that MCPs are intended to achieve.
To what problems are multispecialty community providers a proposed solution?
Multispecialty community providers are a proposed solution for a confluence of epidemiological, managerial and financial problems. The epidemiological aspect is the well-known absolute and relative expansion of the older age strata, people who are living longer (often because of past NHS activity) but also often with chronic and, indeed, multiple chronic conditions. The financial aspect is the restrictive fiscal policies with which UK governments responded to the financial sector market failures of 2008; they have included a policy of reducing the structural budget deficit to 2% of gross domestic product by 2020–21. 1 Given that the NHS accounts for 18.6% of public sector spending2 and hospital spending is some 44% of NHS costs,1 fiscal ‘austerity’ policies were bound to regard the costs of NHS hospitals as a ‘problem’. 2 At the time of the study, the main means of implementing this policy were sustainability and transformation plans. In practice, the term has come to refer both to the plans themselves and to the subregional network of organisations charged with implementing the plan for their area.
During the decade before the idea of MCPs emerged, English health policy had increasingly explicitly assumed that:
-
the apparent demand overload facing NHS hospitals arose largely from increasing numbers of accident and emergency (A&E) attendances
-
these attendances produced increasing numbers of unplanned admissions
-
a substantial proportion of these unplanned admissions were by older people with multiple morbidities
-
a substantial proportion of these unplanned admissions were clinically unnecessary, even iatrogenic (i.e. medical treatment harmful to the patient) and, hence, preventable3
-
once admitted, these patients often remained in hospital for an unnecessarily long time, ‘blocking’ further admissions
-
main obstacles to discharging such patients promptly from hospital were lack of:
-
general practice and/or community health services (CHSs) support necessary for the patient to return home
-
‘integration’ between these services and other frequently necessary services (e.g. therapies, mental health services)
-
residential and/or social care.
-
Therefore, certain themes recur in recent NHS policy and management. One has been that of preventing chronic illness from developing to the point at which hospital admission becomes inevitable. Proposed, and sometimes tested, methods for tertiary secondary prevention have included risk stratification leading to regular general practitioner (GP) or CHS review and, optionally, case management, usually with a nurse practitioner or ‘community matron’ as the case manager. Another method has been to divert unnecessary referrals back into primary care by means of referral-screening mechanisms and to divert unnecessary referrals and self-referrals to emergency services by ‘front door’ triage at A&E departments, diverting patients from A&E departments to on-site GP care and by ambulance paramedics liaising with CHS staff and, in certain cases, treating the patient immediately rather than transporting him/her to the A&E department for treatment there. Ways of partly substituting primary for hospital care have included establishing ‘virtual wards’ (the latest manifestation of ‘hospital at home’), strengthening community hospitals’ capacity and role, out-posting diagnostic services and outpatients clinics, intensifying primary care (in the broadest sense) and concomitantly raising the threshold for hospital admission and discharge, and establishing non-inpatient care pathways, for instance for some musculoskeletal conditions.
As new kinds of services and provider organisations have developed in NHS primary care, and the financial and demand pressures on hospitals and GPs have continued to intensify, the requirement for closer co-ordination of care between these services has become more pressing. At a national level, corresponding initiatives and experiments have included the Evercare Project, leading to the introduction of community matrons, the integrated care pilots4 and the ‘Vanguard’ projects, including, most recently, MCP pilots.
In the meantime, general practices have also independently been under increasing pressure for the same epidemiological reasons as have increased demand on A&E departments. These factors have increased the demand for GP consultations and other general practice-based clinical services (e.g. health checks, disease monitoring). There has also been a gradual but long-term increase in requirements for compliance with national clinical standards [implemented, above all, through the Quality and Outcomes Framework (QOF)]. This has been one source of increased managerial and data collection demands on general practice, but another has been the creation of Clinical Commissioning Groups (CCGs), in which GPs are intended to be the controlling actors. 5 These conditions have made it hard to recruit to the GP workforce, whose age and sex profile and size is changing correspondingly.
Therefore, the past 20 years have seen the following trends in general practice organisation. Mean general practice size has slowly, but continually, increased, with a secular reduction in the proportion of single-handed general practices. There has been a diversification of general practice organisational models, including GP partnerships employing salaried GPs, in effect the nationalisation of those practices that became primary care trust (PCT) administered, the provision of general practice by corporations, proprietary (owner-managed and, often, GP-owned) firms and nurse-led practices, the persistence of some out-of-hours (OOH) co-operatives and the conversion of others into ‘social enterprises’ (often a rather nominal change as the ownership, control and working practices often did not alter much), functional corporatisation (outside firms hired to manage GP-owned practices) and partnership mergers to make ‘super-partnerships’. Networks of general practices have developed. Primary care groups, PCTs, CCGs and GP federations were successively more highly organised examples of such networks attempting to develop joint decision-making, agreed care pathways, the introduction of more clinically specialised forms of general practice with economies of scale and scope in the provision of those services, and economies of administrative scale.
In response to these developments in general practice, and the fiscal and epidemiological pressures noted, NHS England’s Five Year Forward View (5YFV)6 and its successive elaborations adopted six general aims:
-
‘upgrade in prevention and public health’ (p. 3)
-
‘patients will gain greater control of their own care’ (p. 3)
-
‘support people with multiple health conditions, not just single diseases’ (p. 3)
-
‘comprehensive and high quality care’ (p. 5)
-
‘close the £30 billion gap’ in projected NHS funding ‘one third, one half, or all the way’ (p. 5)
-
‘enable new ways of delivering care [to] become the focal point for a far wider range of care’ (p. 20).
NHS England. 4
Five of the seven ‘new ways of delivering care’ were (1) urgent and emergency care networks, (2) ‘viable smaller hospitals’, (3) ‘specialised care’, (4) modern maternity devices and (5) enhanced health in care homes. Sixth was primary and acute care systems (PACSs), in which the essential function is the vertical ‘integration’ of hospital and primary care services for a patient list. Seventh was MCPs, in which the essential function is the horizontal ‘integration’ of primary with CHSs and social care.
What is a multispecialty community provider?
Given the above setting:
The underlying logic of an MCP is that by focusing on prevention and redesigning care, it is possible to improve health and wellbeing, achieve better quality, reduce avoidable hospital admissions and elective activity, and unlock more efficient ways of delivering care.
NHS England. 4
What are the components of this logic, in realist terms?
Multispecialty community provider outcomes
Despite the different approaches to care ‘integration’, the policy outcomes that policy-makers intended MCPs to produce most resemble those of the PACSs and were:
-
the House of Commons Health Committee mentions the Improved Access to Psychological Therapies programme waiting time standards5 in ways that hint that they should apply to mental health services generally
-
‘measurable reduction in age standardised emergency admission rates and emergency inpatient bed-day rates; more significant reductions through the New Care Model programme covering at least 50% of population’8
-
significant measurable progress in health and social care integration, urgent and emergency care (including ensuring a single point of contact), and electronic health record (EHR) sharing, in areas covered by the New Care Model programme7
-
better access to care nearer to home5 (e.g. more convenient care). 8
Multispecialty community provider mechanisms
The 5YFV6 itself describes certain mechanisms that MCPs ‘will’ or ‘would’ use. ‘Expert generalists’ (i.e. GPs) will work more intensively with patients with complex needs (e.g. frail older people; chronic conditions). Nurses, therapists and other CHS professionals will be included in MCP ‘leadership’ (management). There will be a wider range of primary care services. MCPs will draw on the ‘renewable energy’ of carers, volunteers and patients.
Multispecialty community providers ‘may include a number of variants’. 9 A longer list describes mechanisms that MCPs ‘could’ use, hinting that different variants may involve different combinations of the following:
-
fuller use of digital technology
-
fuller use of ‘new skills and roles’ (i.e. new divisions of labour)
-
extended group general practices, ‘either as federations, networks, or single organisations’6
-
general practices employing consultants or making them partners
-
such consultants (and by implication GPs) ‘work[ing] alongside’ CHS staff, pharmacists, psychologists, social workers and others
-
running local community hospitals and perhaps expanding their diagnostic services
-
GP-admitting rights to acute hospitals
-
‘in time’, GPs managing the NHS budget for their patients
-
care hubs, which perhaps may also provide OOH services.
Within MCPs, small independent general practices will continue while GPs wish it, which implies some form of networked rather than line-management relationship between these practices and the rest of the MCP.
A wide range of MCP sizes (the first wave served populations ranging from 63,000 to 330,000) and of possible governance structures is envisaged. Perhaps the most obvious are networks of independent general practices, possibly perhaps with a strong central co-ordinating body (a ‘federation’). MCPs are described as ‘extended group [GP] practices’, which might be ‘federations, networks or single organisations’ (p. 20). 4 The House of Commons Health Committee5 argued that federations allow specialised development of services and care teams while retaining the existing scale of general practices. However, MCPs might also commission specialist providers, implying a potential role for governance and co-ordination through quasi-markets. New hierarchical organisations (e.g. on the lines of NHS foundation trusts) are also foreseen. Potentially, they might also organisationally integrate general practice and CHSs, which the so-called ‘integrated’ care pilots never did. Another expected kind of single organisation is the enlarged professional partnership. The 5YFV comes close to implying that a MCP might also have the structure of a social enterprise or co-operative.
Multispecialty community provider context
Multispecialty community providers’ external relationships with the rest of the NHS will, above all, be through monitoring and a contract. The 5YFV6 expected that standardised data will enable real-time monitoring and evaluation of MCPs’ quality, outcomes, costs and benefits. NHS England is establishing a new operational research and evaluation capability to support this activity.
A ‘new voluntary contract for GPs (Multispecialty Community Provider contract)’ will be MCPs’ main financial link to NHS commissioners. Its three varieties10 are:
-
a ‘partially integrated’ contract [i.e. an additional contract supplementing the General Medical Services (GMS) contract]
-
an integrated single contract based on the GMS contract but excluding the QOF element – a whole population budget for all primary care home (PCH) and CHS services for perhaps 10–15 years
-
‘a virtual, alliance contract’.
The contractor and, by implication, overall co-ordinating body of a MCP might be a community interest company or limited liability company (both wide categories), partnership (including GP federation) or a statutory NHS provider. 4 MCPs will receive capitated payments, but not fees for service (which general practices now do, although it is not usually the main element of their income). 9 The new, longer-term contracts could follow the outcomes-based commissioning approach already being tried elsewhere in the NHS. 11
As usual for NHS organisational innovations, MCPs will be introduced in waves. For the first wave, ‘The purpose of becoming an initial site is not simply to address local needs, but to become a successful prototype that can be adapted elsewhere, designed from the outset to be replicated’. 9
Definition by example
Policy documents and recent plans for the first wave most often characterise MCPs in structural terms (which organisations will participate and collaborate) and, to a lesser extent, in terms of certain cross-organisational care processes. However, these documents expressly leave many possible varieties and options open. 6 Therefore, another way of defining a MCP is ostensibly by considering what common characteristics the first wave of MCPs have12 (see Appendix 1).
Across the 14 first-wave MCPs, the participating organisations (mostly health-care providers) will, in 11 cases, be networks (e.g. federations) of, and individual, general practices (including a super-practice and a proprietary one). Eleven MCPs will also include a NHS hospital trust, nine will include a mental health trust and eight will include a CHS trust. Eight also include one or more CCG. Local authorities, or departments thereof, are included in nine MCPs, in particular, social services (in four). Five MCPs include umbrella organisations for local voluntary organisations and another two MCPs include ‘groups’ of the same. Three MCPs involve urgent-care services (OOH, ambulance). Other, more disparate participants include Healthwatch, one local medical committee, one hospice, commercial pharmacy, NHS England and the Local Government Association (LGA).
As mechanisms, the first-wave MCPs most frequently mention establishing, or strengthening, existing multidisciplinary teams (MDTs) (eight projects). The specific composition varies, but across the projects the team participants include GPs, advanced nurse practitioners, social workers, mental health services, voluntary sector link workers and pharmacists. Next most frequently, five MCPs mention various forms of shared care planning (one of them a GP-led complex-care management service). Another partly overlapping set of five projects plans to create a physical location (‘hub’) in which to combine services and provide a single point of access to them. Three projects mention preventative care, three information technology (IT)-based mechanisms (shared health records, digital access to health care) and three preventative care (including for children and self-care). Two mention care co-ordinators or navigators and two propose to enhance local referral networks and pathways. Various other mechanisms are mentioned by only one prospective MCP (new forms of contract, extended access to GP services, mobile clinics, recruitment of hospital consultants and, contingent on projects outside the NHS, a ‘health and care garden city’).
Working definition of ‘multispecialty community provider’
The foregoing suggests that the essence and function of a MCP is horizontal ‘integration’ among the various primary care providers (general practices, CHSs, mental health, OOH, ambulance, urgent care, etc.) and related non-health services (primarily, social services and residential care). Functional (as opposed to organisational) ‘integration’ will typically mean closer care co-ordination across still-separate provider organisations rather than organisational integration, although even the latter may occur in the future. 6 However, in the meantime, MCPs will be interorganisational networks.
We put the term ‘integration’ within quotation marks because research and policy documents often conflate three distinct concepts:
-
co-ordination – the deliberate combination, connecting and sequencing of separate but interdependent resources,13 above all, individuals’ care activities, into a single care process14
-
continuity – a term covering the cross-sectional, longitudinal, flexible, informational and relational continuities of care;15–17 the common element is the non-interruption of care co-ordination
-
integration – use of a single organisational structure to co-ordinate care. 18
Research and policy documents are especially prone to saying ‘integration’ when referring to (closer) co-ordination.
Namesakes and equivalents in other health systems
Because MCPs are so new, at the start of this project there were no published studies directly concerning them. The initial scoping search of Ovid MEDLINE(R) (1946 to August week 1 2015) for variants of the term ‘multi-specialty community providers’ retrieved zero hits and the same was found when searching EMBASE, PsycINFO, Social Policy and Practice, and PubMed. Therefore, any search for evidence relevant to MCPs must be a search for studies of organisations and/or networks with at least partially similar functions to MCPs, that is, organisations or networks in other health systems or the pre-2016 NHS, which at least partly satisfy the stated definition of the function of a MCP. These MCP-equivalent entities include, but are not limited to, the following:
Gesundes Kinzigtal (Germany)
Gesundes Kinzigtal GmbH, two-thirds owned by a network of local doctors and one-third by a health-care management company, has a shared savings contract between with one large social health insurer [Allgemeine Ortskrankenkasse (AOK)] and one small one [Landwirtschaftliche Krankenkasse (LKK); for farmers]. This contract gives both sides incentives to make and share savings. Some 33,000 people (about half of the area’s population) subscribe to the scheme. Its models of care are based on the collaboration (still unusual in Germany) of doctors, hospitals, social care, nursing staff, therapists and pharmacies. The project offers ‘a set of community initiatives’,19 preventative, patient self-management and health promotion activities. 20 It has been described19 as an accountable care organisation (ACO). It provides individual treatment plans, post-discharge follow-up care and case management. It focuses on removing care pathway bottlenecks (e.g. waits for physiotherapy) and uses a single system-wide EHR.
Buurtzorg (the Netherlands)
Buurtzorg originated as a proprietary CHS nursing and allied health professional (AHP) service provider, but a very mission-led one. It now has 630 work teams whose work includes house cleaning for disabled people (Buurtdiensten), services for young people (Buurtzorg Jong), home-based rehabilitation (Buurtzorgpension) and hospice care (Buurtzorghuis). Buurtzorg charges a flat hourly fee for its work, with self-managed local teams deciding the skill mix ad hoc. The managerial infrastructure is very small. Work co-ordination relies heavily on an IT system based on spreadsheets devised by the teams themselves and a shared EHR. 21 Reflecting practice in the Netherlands generally, the teams do not include doctors (separately organised in small general practices, much as in the NHS).
Swedish vårdcentral (Sweden)
Swedish primary health care clinics (PHCCs) (vårdcentral: ‘polyclinic’) traditionally provided both primary medical care and home nursing care services (i.e. a similar function to NHS community nurses). Some offer OOH emergency services, but not OOH home visits by doctors. For-profit providers have about a 15% market share, as does Praktikertjänst, a medical co-operative. As in the UK, local authorities provide social services, with client copayment. 22 In mental health services, nurses are often the care co-ordinators but, in acute primary care, it is often the GP. Some PHCCs host outreach specialist services (e.g. neurology, geriatrics), therapy services and diagnostics. Multiprofessional teams often operate within each PHCC but generally rely on informal co-ordination. There is no universal EHR, and usually only partial data sharing among health-care providers (among which nursing homes or social services are not included). 23
In Norrtälje, Sweden, the vårdcentral model has been extended. An integrated financial administration (TioHundra Forvaltningen) administers combined (pooled) budgets for all health and social care. They commission a single publicly owned not-for-profit company, TioHundra AB, to provide integrated primary care, hospital and social care services for the whole population. Its PHCC provides medical, nursing and speech therapy services, including post-hospital nursing services for up to 2 weeks after discharge. A separate division within TioHundra AB provides all other community nursing and social care. 24,25
Accountable care organisations (the USA)
The US government’s Centers for Medicare and Medicaid Services (CMMS) defines ACOs as voluntary associations of hospitals, doctors, other health-care providers and professionals who together co-ordinate the care they provide for Medicare patients, in particular chronically ill patients. These ACOs aim to prevent medical errors and service duplication and to make cost savings that the ACO will share with the participating providers. 26
Varieties of ACO programmes have included a Medicare Shared Savings Program (as an alternative to fee-for-service payment), an Advance Payment ACO Model (supplementary incentive programme for selected participants in the Shared Savings Program) and a now discontinued Pioneer ACO Model for early adopters of co-ordinated care.
The NHS now uses the phrase ‘ACO’ to mean the commissioning of a single contract and lead contractor for most of the primary and secondary care health services in a CCG or other wide area. However, in the USA most providers that join an ACO also have non-Medicare (and, in that respect, non-ACO) patients. Provider membership of an ACO is voluntary and, therefore, providers require an incentive to join, usually the financial incentive of sharing the savings.
Awareness of these differences is necessary when interpreting findings about American ACOs for NHS use.
Patient Medical Home (the USA)
In US settings, the term ‘patient-centred medical home’ or ‘primary care medical home’ (PCMH) means something very close to group general medical practices with a stable list of registered patients (as opposed to episodically caring for patients) and providing holistic, co-ordinated, accessible, comprehensive care and also some non-medical services, that is, something similar to the UK model of general practice, with its system of patient lists, since the 1940s. 27 However, recent NHS guidance sees the PCH (a namesake of the US models) as serving a patient list of 30,000–50,000 people, having an integrated workforce, focusing on both population and personalised care, and with ‘alignment of clinical and financial drivers’. 4
Rationale for this study
It is already known that strong continuity of care (often called ‘integrated’ services) assists the delivery of effective, safe and efficient person-centred care for people with multiple morbidities in the community. 28–31 Although there are numerous published studies of care ‘integration’, they tend to focus on what prevents care ‘integration’ or to describe practical models and experiments in working practices and network structures designed to improve ‘integration’ at the disease group level. They less often examine care ‘integration’ at the level of larger populations or of networks of whole organisations, as MCPs are envisaged to be (see Multispecialty community provider mechanisms). Consequently, that body of evidence is disparate and fragmented. Reanalysis of it is needed to draw out the implications for MCPs.
The rationale for establishing MCPs implicitly presupposes that repeated unplanned admissions of older people with multiple morbidities make proportionately heavy use of NHS hospital bed-days. 32,33 Reducing these admissions would substantially reduce cost and access pressures on NHS hospital service. 34 ‘Integrated’ (or, at least, better co-ordinated) care is expected to reduce these admissions by partly replacing hospital care with non-hospital care and, hence, raise the quality and reduce the cost of NHS care. Finally, MCPs will promote such ‘integration’ of care for these patients. To varying extents, the first three of these assumptions have been verified through research (see Namesakes and equivalents in other health systems). The evidential basis of the fourth is more mixed. 35–37 The fifth, about which the present study would synthesise existing evidence, still requires evaluation.
Study aims
Overall, this study therefore aims to appraise and synthesise the diverse sources of knowledge (from the UK and internationally) to understand and test the ‘programme theories’ underpinning the idea of a MCP, elaborating and refining the programme theories to produce more strongly evidence-based logic models. Specifically we aim to:
-
map the current variants of MCPs and their component proposed ‘ways of working’
-
describe the equivalents of MCPs and of the main component mechanisms of MCPs in use internationally
-
identify the ways in which these equivalents are reported to achieve beneficial effects in terms of care integration and the other policy outcomes mentioned in policy related to MCPs, including the 5YFV, local MCP Vanguard ‘logic models’ and other ‘grey’ policy statements
-
describe the causal chains from structural and governance arrangements, through interteam and interprofessional relations and interactions, to practitioner and patient behaviour
-
hypothesise how differences in types of MCP (e.g. networks, confederations) and other external contexts affect how this chain of causation operates
-
reformulate revised logic models for MCP design and implementation.
The rationale for MCPs suggests that, in doing so, we should focus on care for patients with complex needs (i.e. patients who recurrently need services from at least two different provider organisations), for instance, patients with a single long-term condition with complex needs, combined physical and mental health problems or conditions that need both health and social care.
Chapter 2 Research questions and hypotheses
Research questions
Given this background, we addressed the following research questions:
-
How do policy-makers and top NHS managers predict that MCPs will generate the policy outcomes stated in the 5YFV? What variants of MCPs are they creating?
-
Internationally (including in the UK), what equivalents to, or components of, MCPs exist?
-
How do these equivalents and their mechanisms compare with those proposed for MCPs in the NHS?
-
What policy outcomes (comparable with those required of MCPs, rather than clinical outcomes) are these equivalents reported to produce?
-
What is the evidence about the ways in which these mechanisms of action depend on specific contexts (e.g. the presence of non-hospital beds for frail older people), that is, how do the different components of the MCP models of care produce different outcomes in different contexts?
-
What do the answers to the above questions imply for the organisational design (logic models of governance structures, internal management and working practices) of MCPs in the NHS?
As Chapter 3 explains, our method for answering these research questions was a realist synthesis of secondary data.
Chapter 3 Methods
Research design
The overall research design was a realist synthesis. Our rationale for using this method was that we wished to test from secondary evidence, the body of which was likely to be very varied in quality, types and sources, a set of assumptions about how a policy (the creation of MCPs) would produce various outcomes (better care co-ordination, etc.) in NHS contexts. Therefore, we use the terms ‘context’, ‘mechanism’ and ‘outcome’ in their realist senses. By ‘mechanism’, we mean ‘individuals’ reasoning, action and use of resources’. By ‘outcomes’, we mean the empirical, and, indeed, causal, effects of these mechanisms, intended or otherwise (e.g. emergent outcomes). By ‘context’, we mean ‘a moderator, not causally dependent on the mechanism, which is either necessary for the mechanism to produce the outcome or that intensifies the outcome that the mechanism produces’. Thus, contexts do not include intermediate outcomes (mediators). Patient and public involvement (PPI) representatives were consulted in the initial design of the research.
The realist synthesis combined three elements:
Step 1 – eliciting an IPT. Elaboration of NHS policy-makers’ assumptions regarding how MCPs can bring about their intended outcomes, which elicited the ‘initial programme theory’ for MCPs. We elicited policy-makers’ assumptions from the sources (policy documents and stakeholders) reported below (see Step 1: eliciting an initial programme theory for multispecialty community providers).
Step 2 – reviewing the evidence. A systematic search for published evidence relevant to the IPT, formal data extraction of secondary data from included studies, quality assessment of the studies and collation of the extracted data in relation to the IPT.
Step 3 – building a revised logic model. Comparing the IPT with the evidence review findings and reducing, revising and elaborating our programme theory. When programme theory and evidence differed, we removed causal links between components in the IPT for which we did not find evidential support. We then used the evidence review findings to qualify, elaborate and supplement the remaining MCP programme theory for which there was supporting evidence. That ‘logic analysis’38 produced a revised, more strongly evidence-based programme theory of MCPs, that is, an empirically informed revised logic model. 39,40
Accordingly, the project involved two searches of published literature:
-
for policy documents and other materials from which to elicit the IPT in step 1
-
for empirical research (‘evidence’) to provide secondary data for the evidence review in step 2.
The whole study was conducted to Realist And Meta-narrative Evidence Syntheses: Evolving Standards (RAMESES)41 and is reported following those standards, and in conformity with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 42
Step 1: eliciting an initial programme theory for multispecialty community providers
We elicited policy-makers’ assumptions about how MCPs can achieve their outcomes partly from policy documents, supplemented, as explained below (see Stakeholder think tanks), from a ‘think tank’ of MCP ‘stakeholders’.
Identifying core multispecialty community provider policy sources
The original call for proposals for this research, and the research protocol itself, focused on the 5YFV6 as the main policy source about what policy outcomes MCP are intended to produce, and the means by which policy-makers assume MCPs will do so. For this reason, we used the 5YFV as one of our focal documents in step 1.
We conducted a literature search to identify additional English policy statements on care models for people with chronic conditions. The aim of this search was to find a core set of policy documents in order to identify policy-makers’ assumptions about MCPs. This search used the Health Management Information Consortium (HMIC) database (via Ovid), which indexes policy content from the Department of Health and Social Care (DHSC) database (DHSC Data) and The King’s Fund database. HMIC was the only database we searched, as it was found to index all the authoritative policy papers and web pages. Search terms were selected by inspecting the titles and abstracts of the known relevant policy documents mentioned. The search used generic terms describing generic and specific interventions that appeared functionally equivalent to MCPs (e.g. ‘integrated primary and community care’) and particular international examples, such as Buurtzorg and the Wiesbaden Network for Geriatric Rehabilitation. These terms were combined using the Boolean operator ‘AND’ with terms for older people and people with chronic and complex conditions. Both sets of search terms were represented by free-text terms and indexing terms. The search was conducted on 25 August 2016 and date limited to after January 1991 (the foundation of the NHS quasi-market).
The 5YFV used partly different terminology from the other key policy documents identified by web searching. The 5YFV focused on developing ‘sustainable’ ways of organising care to tackle health inequalities, rather than on models of care to tackle chronic conditions. Therefore, we made a supplementary search of HMIC using search terms for ‘inequalities’, ‘health care’ and ‘sustainability’ – a more focused search than the first. Search terms were limited to the notes field of HMIC records, which is used to summarise the contents of a report as a supplement to the abstract that is often not included with policy literature. The search was conducted on 25 August 2016 and no date limit was used.
The results from both searches were exported to an EndNote X7 (Clarivate Analytics, Philadelphia, PA, USA) database. The search strategy and the number of hits for each search are presented in Appendix 2.
Only a handful of policy documents were identified that explained MCPs in much detail (see Chapter 1). The most informative were the ‘logic model descriptions’, which each of the first-wave MCP Vanguard sites prepared and which, as first-hand accounts endorsed by NHS England of what the MCP Vanguards were attempting to do, were especially relevant and important. The focal policy documents used from which to extract policy-makers’ assumptions about MCPs are cited in Chapter 4 and listed in Appendix 3.
Connecting and mapping mechanisms and outcomes
We first elicited as many of the policy-makers’ assumptions about MCPs as we could from the identified sources. In order to elicit the IPT, we framed or reformulated policy-makers’ assumptions in realist terms as context–mechanism–outcome configurations (CMOCs), or parts thereof, with the terms ‘context’, ‘mechanism’ and ‘outcome’ (defined as in Research design).
We articulated these CMOCs in ‘if–then’ statements, that is, statements of the assumed context and mechanism (‘if’) and outcome (‘then’); for example, if MDTs are established in the context that patients want to maintain their own health, then preventative health care will develop. This was a practical necessity as it was rare for the policy statements to specify a context (in the realist sense, i.e. under what conditions the proposed mechanism would or would not work) in addition to mechanism and outcome. Some if–then statements were describing essentially the same CMOC in different words (e.g. about electronically sharing patient information between organisations). We treated these statements as multiple formulations of the same assumption and, in effect, merged them. Many other statements referred (again, sometimes using different words) to essentially the same mechanism (e.g. ‘multi-disciplinary team’, ‘inter-professional team’, ‘cross-professional group’). In some cases, the mechanism of one statement was a subset, component or special case of the mechanism of another (e.g. ‘primary care’ and ‘primary care close to home’). Therefore, we grouped these under the same overarching mechanism. Similarly, many statements referred (again sometimes with different words) to essentially the same outcomes (e.g. ‘patient self-care and activation’ and ‘patient engagement in care and self-care’). We also grouped these accordingly.
This grouping of the if–then statements by mechanism and outcome identified from the policy-makers’ assumptions what the core MCP ‘components’ [mechanism, outcome or context (as the case might be)] were, and the ‘causal links’ between them. We identified 13 components of MCPs and 28 causal links between them, and numbered each linked mechanism and outcome so that the links between components could be traced back to the initial if–then statements.
The MCP components are inter-related in complex ways. Many MCP components were mechanisms for producing several outcomes. Many components were also assumed to be the product of several other components. Often, mechanisms were linked together in chains (‘concatenated’): the outcome of one mechanism was to set up another mechanism producing a further outcome. Producing the second mechanism was, thus, an intermediate outcome.
Next, we mapped what the policy documents assumed the causal links between the MCP components to be, which revealed the assumed chains of MCP components and their complex inter-relationships; in particular, the ways in which some mechanisms were assumed to produce or trigger others. Throughout, and, in the following chapters, we have maintained the same system of numbering for these causal links. For example, ‘(3:10)’ means that component 3 is assumed to be a mechanism that produces component 10. One way of showing the relationships between the mechanisms is by using graphics. In these graphics, we have represented each MCP component by a box containing its constituents and numbered to indicate its source(s). Arrows between components showed the causal links that the policy-makers assumed. Figure A (see Report Supplementary Material 1) shows the first such graphic, based on only the policy documents mentioned previously.
Patient and public involvement
In this study, PPI was through participation in stakeholder ‘think tanks’ (see Stakeholder think tanks). This method of participation was co-designed with PPI during the submission of our research proposal to the National Institute for Health Research (NIHR).
The stakeholder group included four members from the wider Peninsula Collaborations for Leadership in Applied Health Research and Care (CLAHRC) Patient Involvement Group who expressed interest during their involvement in the preparation of the research proposal (see Appendix 4).
Stakeholder think tanks
To check our understanding of the programme theory of MCPs for any missing or misinterpreted elements, we consulted a think tank of patient and NHS ‘stakeholders’. The latter included people who would be implementing MCPs. We used these meetings to:
-
check our interpretation of the initial MCP programme theory
-
resolve ambiguities
-
add any missing components
-
advise as to which MCP components were most important and should, therefore, be prioritised in the evidence review (step 2).
Senior researchers identified stakeholders at the research group meetings. Mark Pearson provided names of service users, Helen Lloyd gave a list of policy-makers and academics and Richard Byng supplied a pool of GPs and managers working in GP surgeries. The final list encompassed stakeholders across England, including senior staff from NHS England.
We held three think tank meetings in October 2016. Participants were general practice members (GP, practice manager), service users, policy-makers (including NHS England) and a minority of academics. The researchers made field notes and (with the participants’ consent) audio-recorded the meetings in order to return to key points, if necessary. After each meeting the if–then statements and map were successively modified.
We held a further meeting with our stakeholders in March 2017 in order to check our understanding of the linkages between MCP components and we will meet the stakeholders again to discuss further how to disseminate our findings.
From the included policy sources and the think tank interpretations we arrived at 242 if–then statements (see Appendix 5).
Deduplicating and consolidating the conceptual map
Given the number of if–then statements, data reduction was necessary. When we had one link A–B–C and one A–C; the first was more informative (about mediating steps) and so we removed the second as a duplicate. We also removed non-redundant, but trivial, links (e.g. ‘if there is scope for local innovation in creating MCPs, then MCPs will be created’).
Even after consulting the stakeholder think tank, many of the if–then statements still explicitly stated only one or two of the context–mechanism–outcome (CMO) trinity, which previous studies43–47 have already shown is often the case with policy sources. In developing the conceptual map, the researchers imputed the missing assumptions from our background knowledge of the English health system and clinical practice within it. In doing so, we:
-
clearly differentiated the imputed assumptions from those explicitly stated in the policy sources
-
selected, when alternative assumptions might be imputed, those that have the strongest evidence base and were most consistent with those explicitly stated in the policy sources, avoiding the construction of a ‘straw man’ or unfairly weak interpretation.
Adding these connections produced a second graphic, Figure B (see Report Supplementary Material 1). The graphic includes the numbered ‘ifs’ and ‘thens’ behind each component in order to illustrate some (but certainly not all) of the complexity of their inter-relationships, the direction of ‘flow’ from input to output (showing which were intermediate and which were final outcomes), but also removing redundant links as explained above. This method ensured that the fully articulated initial MCP programme theory remained as comprehensible as possible while remaining as close as possible to the original policy statements. Chapter 4, The initial programme theory: multispecialty community provider components and the causal links between them formulates the IPT taken forward into step 2. Chapter 4 describes (both verbally and with a graphic), in detail, the mechanisms, intermediate outcomes, final policy outcomes and contexts that, together, comprise the fully articulated IPT for MCPs early in 2017.
Step 2: reviewing the evidence
Exploration and search strategy development
The aim of the realist evidence review was to discover an evidence base against which to ‘test’ IPT (see Step 3: building a revised logic model) and reveal whether or not the IPT omitted any important MCP components or causal links between them. Owing to the size and complexity of the corpus of relevant studies, we were also aware of the necessity for a well-defined and focused search strategy. We focused the search by:
-
Searching for concepts and terminology from the main components of programme theory, starting with the formation of MCPs and its subcomponents (see Chapter 4). This search covered all 13 components of the IPT. The search concentrated on the connections between the 13 components rather than on each component in isolation from its effects and contexts.
-
‘ANDing’ these with names of MCP-equivalent organisations, networks and projects. Chapter 1 defined ‘MCP equivalent’ as performing a similar function of horizontal co-ordination between primary medical care, domiciliary health care, other primary care health services, and social care. Sarah L Brand and Simon Briscoe assembled a list of MCP equivalents [including Chronic Care Models (CCMs)], drawing on the whole research team’s knowledge.
We developed a search in MEDLINE (via Ovid) using the above sets of terms. Search terms were represented by free-text terms and indexing terms. The final search was translated for use in a selection of topically appropriate databases, including MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, PsycINFO (all via Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL; via EBSCOhost) and Applied Social Sciences Index and Abstracts (ASSIA; via ProQuest). The search was conducted on 5 December 2016 and no date limit was used. We exported the search results to EndNote X7 and deduplicated them using automatic and manual checking. The search strategies and number of hits are presented in Appendix 6.
Studies were also identified through opportunistic finds from e-mail updates from relevant journals.
Selection
Five reviewers (RS, MP, SLB, MF and AW) between them screened 1319 titles and abstracts in the EndNote database. There were two rounds of screening. For each round, we developed a screening tool (Appendices 7 and 8), each of which went through two rounds of piloting on 10 studies (20 in total) by all reviewers. Discrepancies in tool use and include/exclude decisions were discussed and resolved after each pilot to achieve consistency in its use.
Screening stage 1
Using screening tool 1 (see Appendix 7), we selected studies about any of the 13 MCP components in the IPT (listed previously). We included only studies with empirical contents, that is, comparative effectiveness studies [randomised controlled trials (RCTs), etc.], process evaluations, reviews of primary research (if the method was stated), qualitative research, surveys, histories, descriptions of models of care, uncontrolled before-and-after comparisons, cohort studies and reanalyses of routine data. We excluded editorial letters, conference abstracts, opinion pieces, audit articles and the numerous a priori, but data-free, ‘models’ of integrated care. Next, we assessed whether or not the selected empirical studies were about horizontal interorganisational linkages in primary care, that is, between any two or more of primary medical care, CHS, ambulance, community health and mental health care, residential care, therapies, primary health care (PHC) dentistry and PHC pharmacy. If not, we excluded them. Hence, we excluded studies purely about hospitals and single-organisation studies. The first stage of screening selected 463 studies.
A second reviewer (SLB or RS) screened 10% of these (n = 99), resulting in eight discrepancies to be resolved by a third reviewer (MP).
Screening stage 2
There were too many studies to review with the time and staff available remained after first screening (n = 463). Therefore, we also excluded pre-2014 studies in order to focus on the most recent data with the assumption that later studies, especially reviews and systematic reviews (SRs), will already refer to the findings from the most important earlier studies. We then carried out a second round of screening on the remaining included studies. Using screening tool 2 (see Appendix 8), we excluded the following studies:
-
Studies that did not concern an Organisation for Economic Co-operation and Development (OECD) country. Realist methodology assumes that similarity of context is a pre-condition for the transferability of mechanisms from one setting to another, and OECD countries’ health systems and wider social contexts were more likely to resemble those of the UK than those of non-OECD countries.
-
Studies that were not specific to horizontal interorganisational co-ordination of primary care, that is, generalities (e.g. training) that may apply, but are not specific, to MCP-equivalent structures; ‘vertical’ (primary–secondary) not ‘horizontal’ service co-ordination; micro-management techniques, health information technologies (HITs) (e.g. medical record design, applications); and studies of purely clinical interventions (e.g. therapy methods or rules for managing polypharmacy).
Ten per cent of round 2 screening was second screened by one reviewer (SLB). Before data extraction, both rounds of screening were checked by Sarah L Brand for any coding mistakes. There were 25 coding errors and missing codes that were identified and rectified. This identified two new studies, giving us 116 included studies.
To automate later data sorting and extraction, the included studies were coded in the EndNote database according to which programme theory component(s) they were relevant to.
Data extraction and quality appraisal
The aim of data extraction was to extract evidence about the 28 causal links in the IPT (see Chapter 4). Four reviewers (RS, MP, SLB and AW) extracted data from the included studies. Each reviewer was allocated 1–4 of the 13 components. The data extraction tool (see Appendix 9) was piloted on two studies by two reviewers (SLB and RS), followed by discussion to resolve any discrepancies or other problems. For each of the 28 causal links, we sought to:
-
extract data tending to corroborate the causal link
-
extract data that were evidence against the causal link
-
extract evidence of new causal links or components not in the IPT
-
specify context(s), that is, evidence specifying the circumstances under which one component would produce another, or fail to
-
note the quantity and strength of evidence about the causal link
-
note any qualifications or limitations to the findings reported in the study from which data were being extracted
-
note which kind(s) of MCP equivalent(s) the study described, in which country and serving which care group(s).
For studies allocated to more than one reviewer (i.e. relevant to more than one component, which was most of the studies), the first reviewer extracted data and saved the data extraction form, the next reviewer extracted data from that study and then checked the first reviewer’s data extraction and added their own data extraction (if any) to the first reviewer’s form, and so on. In this way, 26 out of the 116 included studies were data extracted by two reviewers (22.4%).
Each included study was assessed for methodological quality using the Mixed Methods Appraisal Tool (MMAT). 48 We used the MMAT because, reflecting their complex objects of study, we expected most of the studies to use mixed or qualitative methods, with some quantitative studies. Two reviewers (SLB and RS) piloted MMAT scoring on two studies, then discussed the discrepancies with the wider team to ensure consistency. Any issues arising in quality appraisal assessment were raised and discussed in team meetings during the quality appraisal stage. The data extractor(s) for each study also assessed its MMAT quality score. The MMAT provides a standardised appraisal checklist of four items (hence, scores of 0, 1, 2, 3 or 4) for qualitative studies, and the same for RCTs, non-randomised trials and descriptive quantitative research. For mixed methods, it provides a three-item checklist. Criteria for all the checklists are detailed and well specified. To assess the quality of the included SRs, we used the Assessment of Multiple Systematic Reviews (AMSTAR) quality appraisal tool for SRs. 49 AMSTAR also provides well specified, consistently structured checklists for 11 characteristics indicative of, in this case, the quality of a SR, giving a total score of between 0 and 11 for each SR. MMAT and AMSTAR ratings were conducted by one reviewer. Ten per cent of these were then rated by a second reviewer (SLB, n = 9; RS, n = 3) and one discrepancy was resolved by a third reviewer (HL). The MMAT or AMSTAR rating and a narrative summary of any methodological quality issues with a study were also recorded on its data extraction form.
Collating and coding data
The data extraction tool (see Appendix 9) was structured according to the 28 causal links between MCP components in the IPT, in 11 groups according to which component was the mechanism, as opposed to the outcome, in that causal link. This structure was also the overall coding framework for the data. To automate data sorting and retrieval, we created an NVivo 11 (QSR International, Warrington, UK) database, with a node for each causal link and, therefore, the corresponding section of the data extraction tool. Within each node, subnodes corresponded to the lower-level links between the subcomponents of each MCP component. Data from all the data extraction forms were imported into the corresponding NVivo node(s). When no suitable node existed, we created new nodes, as necessary, during data extraction. These were where additions to and elaborations of the IPT began to emerge.
Step 3: building a revised logic model
Comparing the initial programme theory with the evidence review findings
The 28 causal links between the 13 MCP components were the analytic framework for this comparison. We collated the relevant contents of the completed data thematically into that framework. For the 28 causal links in the IPT, we:
-
assessed the overall evidence for the causal link
-
inducted patterns and subthemes
-
noted strengths of evidence and gaps in the evidence, including any absence of contextual information about each causal link
-
noted new causal links not in the IPT
-
noted any contradictions or ambiguities in the evidence about particular causal links.
Synthesis
For each causal link, we summarised the number and quality of studies supporting, refuting or qualifying it (see Chapter 7). We categorised the strength of each causal link’s evidential support as one of (in descending order):
-
‘substantial’ [i.e. a combination of primary studies and SR(s)]
-
‘supporting’ (i.e. multiple primary studies)
-
‘minimal’ (i.e. a single primary study)
-
‘partial support’ (i.e. some supporting evidence for parts of the underlying programme theory about that causal link – that it only operates in certain conditions, or with certain populations)
-
‘equivocal’ [i.e. evidence both for and against (but we also noted whether or not, in such cases, the evidence was predominantly on one side)]
-
‘none’ (whether evidence to the contrary or just the simple absence of any supporting evidence in the studies available to us).
A single working instance of a causal link between two components (‘minimal’ evidential support) does at least give evidence of the feasibility (‘proof-of-concept’) for that component operating as a mechanism to produce that outcome in another setting provided that the destination context has similar moderating characteristics to the original ‘proof-of-concept’ context. Equivocal evidence is, to the realist mind, a clue to the possible presence of contextual factors, which determine whether or not that mechanism will produce that outcome in different contexts for different populations and what kind or size its impact will be (e.g. the mechanism ‘works’ for one care group or in one kind of health system but not another).
Revising the initial programme theory
To convert the IPT into a revised, more strongly evidence-based logic model, we removed the causal links with no supporting evidence or where evidence existed but was against them. For causal links that had only partial support, we removed the unevidenced elements. These subtractions produced a truncated, but more strongly evidence-based, programme theory.
To that truncated version, we next added:
-
relevant causal links found in the body of evidence but omitted from the IPT
-
contextual statements of the circumstances that qualify the causal link between two MCP components, because certain specific conditions strengthen or weaken the outcome produced.
In places, the IPT was formulated ambiguously (see Chapter 4). To test it as it stood, we left these formulations untouched when comparing it with the secondary evidence. To produce a more coherent, less ambiguous, more evidence-based MCP programme theory, we separated out those concepts (e.g. ‘co-ordination’ and ‘integration’; see Chapters 4 and 6) that the policy sources had conflated.
Adding further contexts and mechanisms made an already complex programme theory more complex. It would be an exaggeration, but one with a grain of truth, to say that the initial MCP programme theory had come close to assuming that, in MCPs, every component helped to produce every other component (see Chapter 4). To differentiate the critical from the non-critical causal links, we used two methods. First, using the categories described above, we also categorised the strength of evidence for each subsequently added causal link, from ‘minimal’ to ‘substantial’ (see Chapter 7). As a graphical representation, we redrew the map of the revised logic model so that the width of each link reflected the ‘strength’ of evidence for it (see Figure 5). Second, to simplify the graphical representations, we eliminated redundant links in the revised logic model, applying the same principle as previously. However, in reviewing the evidence, we included all the links, both direct and indirect.
The product of these subtractions, additions, qualifications and definitions was a revised, more strongly evidence-based programme theory for MCPs, articulated in correspondingly revised verbal, tabular and graphical presentations (see Chapter 6).
Table A (see Report Supplementary Material 2) itemises, in detail, how our methods complied with the RAMESES quality standards. 41
Chapter 4 Step 1 findings: eliciting the initial programme theory from policy sources
Outline of assumptions in policy sources
From the sources and stakeholders mentioned in Chapter 3 (and see Appendices 3 and 4), we obtained and collated 242 statements about what intermediate outcomes and final (policy) outcomes MCPs were designed to attain, by what means and in what contexts (see Appendix 5). Appendix 10 lists, in descending order of frequency, the 20 that were most frequently mentioned in the policy documents that we analysed.
Interpreting the policy sources in realist terms
The policy sources seldom explicitly formulated their assumptions about MCPs as the CMOCs, or parts thereof, which realist synthesis requires.
Underspecified policy-makers’ assumptions
The 5YFV6 and related policy documents stated in general terms that MCPs will promote the ‘integration’ of care for older people with multiple morbidities by partly replacing hospital care with non-hospital care. However, for the most part, MCP policy was unclear about which components might act as mechanisms to produce which specific outcomes. At times, policy statements asserted what should be done without expressly stating how and/or what effects doing this would have. For example, at one Vanguard site there would be ‘more ways for people to digitally access health care (including online directories of local services, and a library of helpful health apps on its website)’,8 but this idea was not explicitly connected to any policy outcomes it would produce or to contextual requirements for it to work. Other statements were so broad as to be difficult to interpret concretely (e.g. that ‘artificial boundaries between hospitals and primary care, health and social care, between generalists and specialists are broken out of’ and ‘long term conditions are better cared for’). 6
Policy-makers may have left these points underspecified so as not to foreclose MCP design options or for other reasons (as with other policies10). Policy documents said that different types of MCP might emerge but not what these variants were or what might differentiate them. They suggested possible MCP contractors,5 but proposed a wide range, including Community Interest Companies, Limited Liability Companies, partnerships (including GP federations) and statutory NHS providers. MCPs were also described as ‘extended group [GP] practices’ that might be ‘federations, networks or single organisations’ (p. 20). 6 Concomitantly, ‘general practice at scale’ might, according to the 5YFV,6 be networks of independent general practices, perhaps with a strong central co-ordinating body (a ‘federation’). The House of Commons Health Committee argued that federations allow specialisation of service and care team development but retain the existing scale of general practices. 5 Although policy documents stated that MCPs will also have an element of vertical integration, or rather co-ordination, of care, short of structurally integrating primary and secondary care, they also usually discussed MCPs separately from PACSs.
Relationships between mechanism and outcome in the policy documents were often underspecified, and there was often ambiguity over whether the terms referred to a mechanism and its context or (without differentiating) both a mechanism and its outcome:
-
‘MCP setup’ – ambiguity between a mechanism (i.e. actions by NHS managers) and a context (favourable or unfavourable background conditions).
-
‘Demand management’ – ambiguity between a mechanism for managing demand (e.g. referral screening, risk stratification) and the outcome of doing so managing demand (fewer referrals and admissions to hospitals).
-
‘Patient diversion’ – ambiguity between mechanisms for diverting patients away from hospital (e.g. providing alternative care outside hospital) and the outcome of doing so (e.g. fewer hospital admissions).
Multiplexity
If–then relationships between MCP components in the policy documents were successively linked (‘concatenated’) and multiplex. They rarely assumed that one mechanism produced just one final outcome, but more often that different mechanisms were concatenated so that the output of one was to create, or to trigger, the next. For example, policy statements expected the creation of MCPs to strengthen the management of provider networks, which would then strengthen referral networks, and then the referral network would lead to patients being diverted from hospital, and so on. The if–then relationships were multiplex in that a single mechanism was sometimes assumed to trigger several others. IT-based care co-ordination would, the policy statements jointly assumed, divert patients away from hospital, strengthen care planning at patient level and make urgent care more responsive. In reverse, the policy statements also assumed that one policy outcome would result from many mechanisms. For example, improved care planning at patient level would be the joint effect of IT-based care co-ordination plus referral networks plus demand management systems (themselves also resulting from care planning at organisational level) plus preventative care.
Translations and nomenclature
The policy documents made little explicit reference to evidence bases beyond some local evaluations. However, they often referred to two main international prototypes for MCPs, the US ACOs and PCMHs.
In NHS policy documents, the term ‘accountable care organisation’ means an ‘overarching organisation that sits above a joined up health and social care system made up of a number of different providers, from health services to the local council’. 50 Such an ACO would be certainly the predominant, perhaps sole, contractor for NHS-funded services with its local commissioner. The US Government’s CMMS defines ACOs as ‘groups of doctors, hospitals, and other health care providers, who come together voluntarily to give co-ordinated high quality care to their Medicare patients’26 (i.e. not for all patients) and provider membership is optional – differences to bear in mind when interpreting the ACO model and research for NHS use. NHS policy statements also borrow the term ‘Patient Centred Medical Home’ which, in the USA, formulates an ambition to construct something similar to what current NHS general practice (usually) is, that is, primary medical care based on the:
underlying principle of a single physician who coordinates the patient’s care and engages a team of health care providers and their patient in an individualized treatment and management plan.
Kash et al. 51
As the Royal College of General Practitioners (RCGP) points out, general practice is (already) ‘the natural medical home for patients’. 52 In the USA, a ‘medical neighbourhood’ is understood as a group of ‘medical homes’. Finally, ‘integration’ in NHS policy documents almost never means ‘organisationally integrated’ (as it would in some countries) but, rather, the closer co-ordination of services provided by separate organisations. Many health systems pursue that aim by creating referral networks53 of providers, with each network having a ‘network administrative organisation’54 doing much of the actual co-ordinating work. Policy documents also implicitly used the term ‘prevention’ in a hitherto non-standard way to mean long-term self-care, ‘activation’ and ‘empowerment’, and patient education, rather than clinical prevention or intersectoral activity, for health promotion.
Apparent omissions
Many policy statements were implicitly in mechanism–outcome configuration, rather than a CMO configuration. From a realist perspective, it was noticeable that policy sources seldom made assumptions (even implicitly) about what contextual factors would moderate the many assumed causal links between MCP components and outcomes. Nevertheless, a few contextual assumptions were stated and are outlined below.
Compared with the policy issues covered in Chapter 1, the policy statements said little about:
-
organisational integration, in the sense of GPs, CHSs and other staff being members of the same organisation
-
lack of residential and social care
-
risk stratification and case management
-
MCPs’ relationships to the other six new models of care.
Imputing the missing causal links and contexts in the policy-makers’ assumptions
To make the policy-makers’ assumptions empirically testable, one has, at times, to impute the necessary missing definitions and terms and operationalise them. As Chapter 3 explained, we did so by:
-
asking our NHS think tank to interpret what, in practical terms, the policy statements appeared to mean to them as NHS clinicians and managers
-
cross-referring between policy statements (at the cost of assuming that the same word means the same thing in different statements)
-
exploiting the textual setting. For example, a statement about information-sharing in the context of hospital referrals and discharges was taken to refer to information sharing between hospitals and GPs, and between hospitals and CHSs
-
referring to the international prototypes that policy documents cite (see Namesakes and equivalents in other health systems), although with due attention given to differences between the original and the NHS settings
-
referring to particular named examples of plans for, or evaluations of, existing proto-MCPs. From these descriptions, the researchers abstracted the more general assumptions about how this MCP would work from its local particularities
-
calling on the researchers’ (who included clinicians) background knowledge of primary care in the NHS and of relevant research to infer what such statements were most likely to allude to, and interpret, such euphemisms as ‘leadership’ for ‘managers’ or ‘expert generalist’ for ‘GP’.
By these means, we, so far as possible, formulated and elaborated the policy statements as ‘if–then’ statements (‘if’ = M–; ‘then’ = –O, provided that C–), which realist synthesis (indeed any empirical test) requires as raw material.
The initial programme theory: multispecialty community provider components and the causal links between them
We grouped by mechanism and by outcome the 242 if–then statements obtained from the policy sources (described in Chapter 3). These 13 groups were named as MCP ‘components’ and were linked by 28 ‘causal links’. Together, these make up the top level of the initial MCP programme theory. Underlying or, rather, composing each causal link are single or multiple if–then statements, making the more detailed content of the IPT. Appendix 11 summarises the 13 MCP components in our interpretation of the policy-makers’ assumptions about MCPs.
Table 1 and Figure 1 illustrate the IPT that we took forward to the evidence review. The IPT is our interpretation of the policy-makers’ assumptions (developed as described in Chapter 3, and glossed at points to explain our reasoning). This IPT is made up of 13 components and 28 causal links between them. As this review is focused on exploring the evidence for how MCPs work and not what MCPs are, it is the causal links between the 13 components and not the 13 components themselves that guide our evidence review (step 2; see Chapter 5 for results of the evidence review). Figure 1 illustrates the 28 causal links. Note that the 13 components (see Appendix 11) include the two main outcomes of MCPs [(1) component 12: patient experience and care will improve; and (2) component 13: NHS costs will reduce). Because these two ‘components’ are the intended end result of MCPs in NHS policy, in the IPT they are not the mechanism for producing any of the other 11 components, and, hence, appear only in the right-hand (‘then’) column in Table 1.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 1. NHS managers establish MCPs | 2. Network management will develop | 1:2 |
| 7. Planned referral networks will develop | 1:7 | |
| 2. Network management develops | 3. MDTs will develop | 2:3 |
| 6. Care co-ordination through IT use will develop | 2:6 | |
| 3. MDTs are established | 7. Planned referral networks will develop | 3:7 |
| 9. Preventative health care will develop | 3:9 | |
| 4. Culture changes occur in the participating organisations | 3. MDTs will develop | 4:3 |
| 8. Demand management systems will develop | 4:8 | |
| 9. Preventative health care will develop | 4:9 | |
| 5. Voluntary sector becomes involved in MCPs | 8. Demand management systems will develop | 5:8 |
| 9. Preventative health care will develop | 5:9 | |
| 6. HITs are used to strengthen informational continuity of care | 7. Planned referral networks will develop | 6:7 |
| 10. Care planning for individual patients will become more prevalent and systematic | 6:10 | |
| 11. More patients will be diverted from inpatient to primary care services | 6:11 | |
| 7. Planned referral networks develop | 8. Demand management systems will develop | 7:8 |
| 10. Care planning for individual patients will become more prevalent and systematic | 7:10 | |
| 11. More patients will be diverted from inpatient to primary care services | 7:11 | |
| 8. Demand management systems develop | 9: Preventative health care will develop | 8:9 |
| 10. Care planning for individual patients will become more prevalent and systematic | 8:10 | |
| 11. More patients will be diverted from inpatient to primary care services | 8:11 | |
| 9. Preventative health care develops | 11. More patients will be diverted from inpatient to primary care services | 9:11 |
| 10. Care planning for individual patients becomes more prevalent and systematic | 9. Preventative health care will develop | 10:9 |
| 11. More patients will be diverted from inpatient to primary care services | 10:11 | |
| 12. Patient experience and care will improve | 10:12 | |
| 11. More patients are diverted from inpatient to primary care services | 12. Patient experience and care will improve | 11:12 |
| 13. NHS costs will reduce | 11:13 | |
| Other. General practice will benefit | 11:other | |
| Other. Care co-ordination and demand management systems develop | Other. Urgent care become more responsive | Other |
FIGURE 1.
Causal relationships between the 13 MCP components in the policy-makers’ IPT.
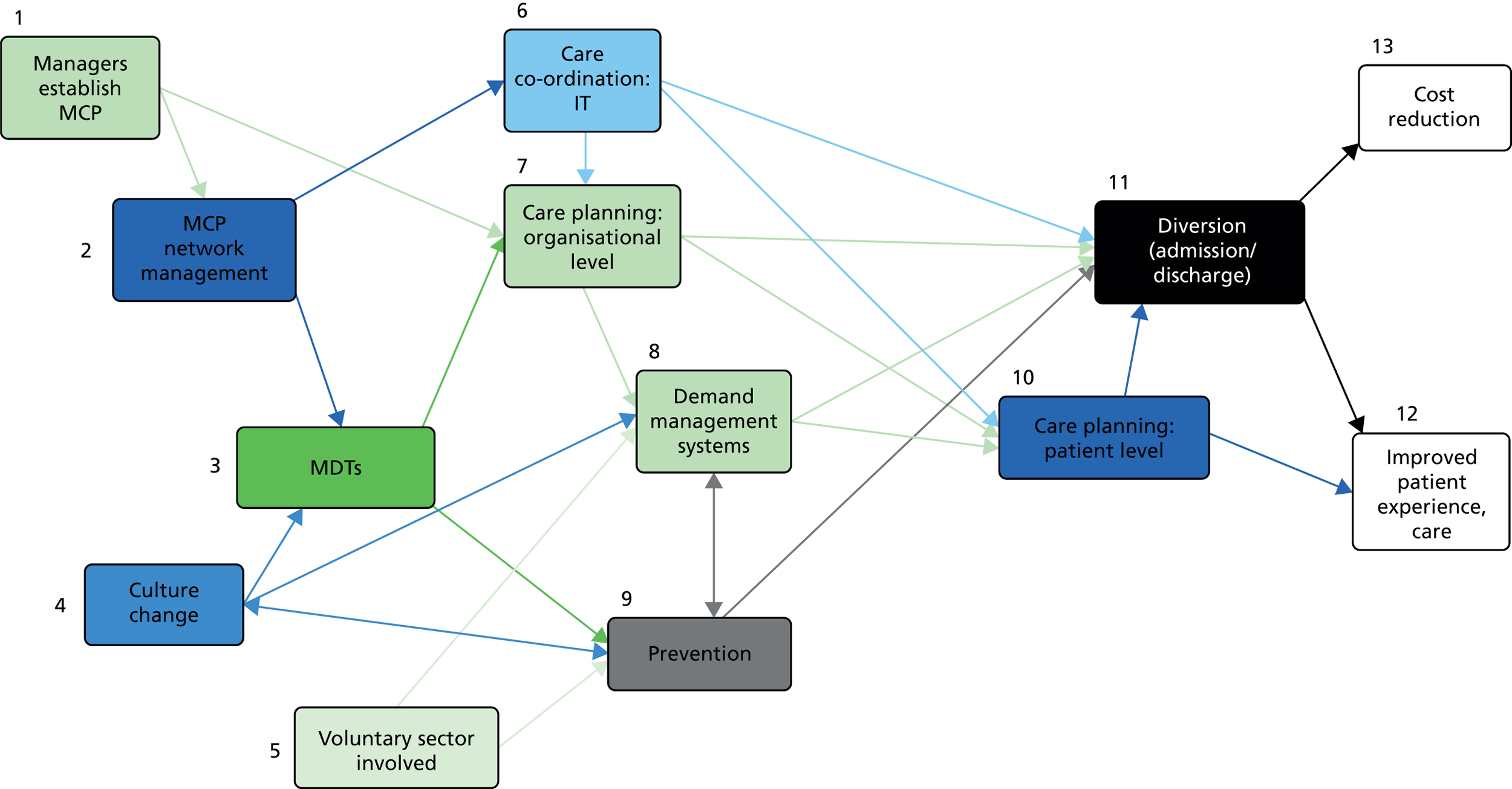
Figure 1 shows the relationships between these overall groups of causal links. Each arrow represents a generalisation from the if–then relationships stated or assumed in the policy documents. In realist terms, each arrow with its left-hand box represents a mechanism and the box at the right-hand (destination) end of the arrow represents its outcome. Table B (see Report Supplementary Material 2) shows the same relationships in tabular form.
Figure 1 shows the ‘flow’ or sequenced linkage (‘concatenation’) of the assumed causal links between components 1 and 11, through to outcomes 12 and 13 (improved care and reduced cost). Each component is assumed to be the mechanism to bring about change in later components, and those components are then assumed to be the mechanism to bring about change in yet later components; these eventually jointly lead to the MCP outcomes (far right).
Contexts
As noted, policy sources contained fuller accounts of assumed mechanisms and outcomes, and some mediating linkages, than of the contexts that might moderate the achievement of those outcomes. However, they did include some detailed assumptions, outlined below, about what initial conditions favour the establishment of MCPs, in particular the first wave (i.e. that concerned only links 1:2 and 1:7). In addition to some managerial mechanisms (a vision of a model of care;9 effective managerial and clinical leadership;9 standardised data to enable real-time monitoring and evaluation of quality outcomes, costs and benefits;9 and planning how to provide care for people with long-term conditions in primary care settings and in their own homes, with a focus on prevention9), the contextual conditions likely to be critical to enable the first wave of MCPs to bring about their intended outcomes were assumed to be:
-
existing progress towards new ways of working9
-
a financial situation that allows start-up money to be found for MCPs – local commissioners support already-agreed funding for the MCP9 existing ‘partners’, such as voluntary and community sector organisations, and ‘communities’9 are supportively engaged with the MCP. Organisations relate to each other in a collaborative, mutually helpful way. Local relationships are good
-
local NHS leadership focus on MCPs and care integration generally
-
the populations served are of a size and type likely to benefit, which we interpret as being large enough to allow economies of scale and scope in collaborative working, and with a health profile and socioeconomic mix that the MCP services can accommodate
-
a population who desire autonomy and control over their health and health care, and are likely to participate (‘engage’) in activities to maintain their own health and to help care informally for those experiencing chronic ill health
-
health professionals and organisations view those whom they care for as people, not patients
-
sufficient staff inputs (time, skill mix)
-
the responsible CCGs show engagement and flexibility, and are not excessively risk averse towards the risks of procuring new organisations and/or networks to operate a MCP
-
a well-functioning GP network (group or federation)
-
the corresponding social services are capable of providing the services needed to sustain and MCP.
Policy statements and informants did foresee certain general contextual problems in establishing interorganisational level care co-ordination, but did not clearly link them to any specific relationships between any of the 13 main components of the policy-makers’ IPT:
-
tension between clinical and financial imperatives
-
the necessity of moving the pressure of demand to new points in the local health system and of removing some roles
-
difficulty in moving beyond information distribution to ensure that organisations within the MCP use that information effectively
-
increased pressure on carers and voluntary sector. If not a context, this might be understood as a side-effect, or perhaps a feedback effect, of links 5:8 and 5:9
-
an initial dip in patients’ experience because some patients would be resistant to the changes in care provision.
However, the fundamental contextual assumption was that a substantial proportion of unplanned admissions of older people with multiple morbidity are clinically unnecessary, even iatrogenic; hence, preventable. 32 This implicitly applies to all the links involving patient diversion (6:11, 7:11, 8:11, 9:11, 10:11, 11:12 and 11:13).
Chapter 5 Step 2 findings: the evidence base
Studies identified, excluded and included in the evidence review
Figure 2 describes the flow of studies through the evidence review. 42 A total of 1319 records were identified (after duplicates were removed). Screening and data extraction resulted in 97 included studies from which data were extracted to provide evidence for step 3 (see Chapter 6).
FIGURE 2.
Flow of included and excluded studies in the realist evidence review.
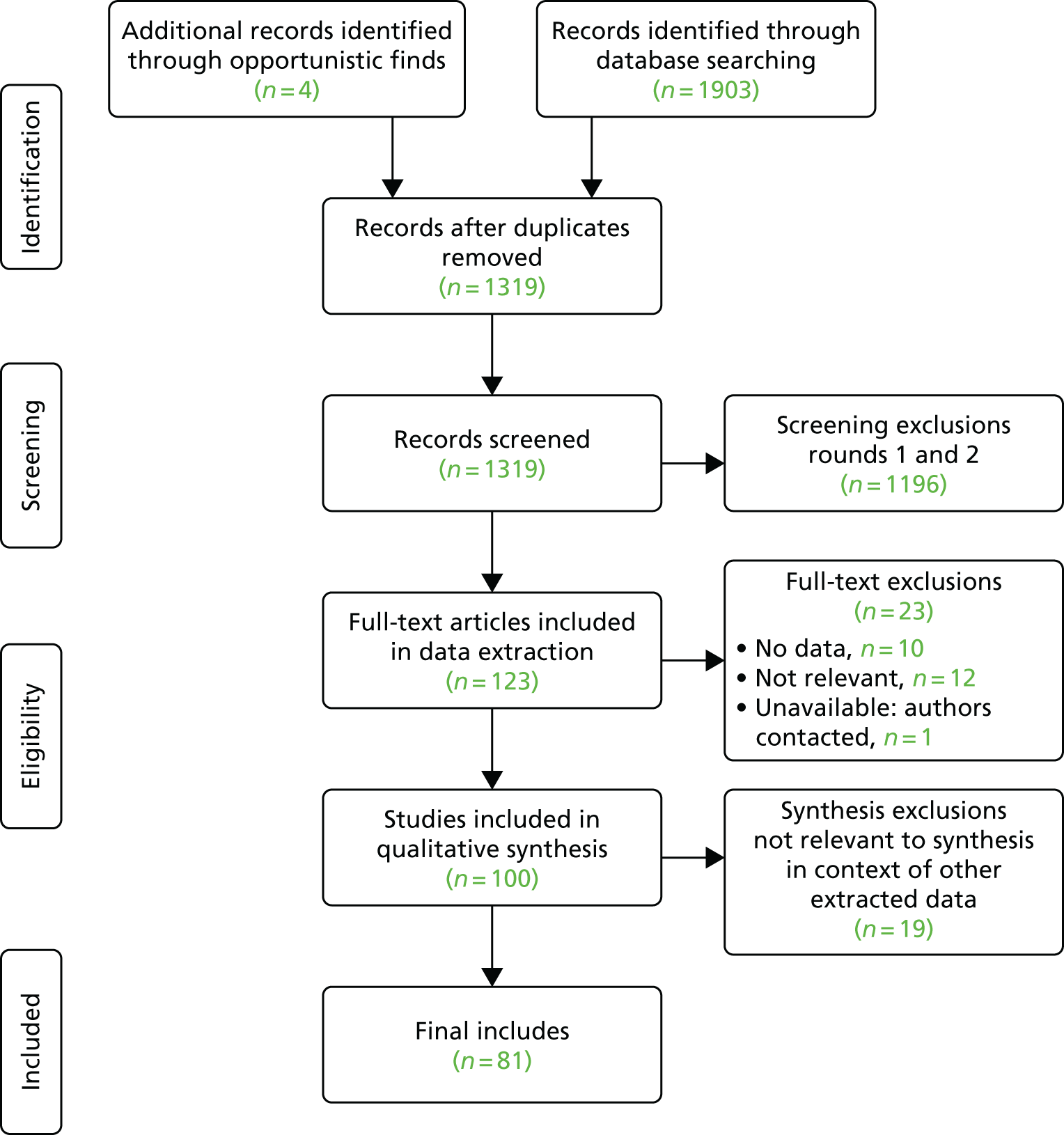
Evidence review: data synthesis
The data we extracted from 19 out of the 97 studies included in data extraction were not included in the synthesis because, once considered in the context of the data from the other included studies, they were not relevant to the synthesis. Appendix 12 shows the details of the studies excluded at the data synthesis stage of the evidence review.
Figure 3 illustrates the number of studies that provided evidence for each of the 28 causal links in the IPT, and the number of studies from which evidence was found for 16 additional causal links not in the IPT. No data were found to extract for causal links 4:8 and 6:8 in the IPT (shown as 0), and some data were found to extract for causal links not appearing in the policy-maker assumptions (shown in bold italicisation). These new causal links most often had components 7 (preventative health care), 12 (improved patient care) and 13 (reduced NHS costs) as the outcome, and components 3 (MDTs), component 5 (culture change) and component 7 (planned referral networks) as the mechanism.
FIGURE 3.
Numbers of studies providing data for each causal link in the IPT. The y-axis is the mechanism ‘if’ and the x-axis is the outcome ‘then’.

Final included studies
Appendix 13 presents details of the 81 studies included in the synthesis.
The most evaluated model of integrated care was patient-centred medical homes (31 studies) and then ACOs (18 studies). The populations under study included physicians, care navigators and patients. The overwhelming majority of studies used qualitative data collection methods and provided evidence for between 1 and 17 causal links between 13 MCP components.
The number of studies providing evidence for each causal link ranged from 1 to 29. The causal links with the most studies from which evidence was extracted were 3:9 (if MDTs are established, then preventative care will develop; 29 studies), 6:10 (if HITs are used, then care planning at the individual level will develop; 27 studies), 3:7 (if MDTs are established, then planned referral networks will develop; 25 studies), 2:7 (if network management develops, then planned referral networks will develop; 25 studies) and 10:12 (if network management develops, then patient care will improve; 21 studies).
We found few studies in which components 12 (improved care) and 13 (reduced NHS costs) were the direct outcome of another component. Two-thirds of the causal links that did have components 12 and 13 as outcomes were not in the IPT but were additional causal links found in the studies reviewed. Evidence for causal links in which 12 and 13 were outcomes usually came from only one or two studies. However, evidence for causal links to 12 and 13 from components 10 (care planning at the patient level) and 11 (diversion from inpatient care) came from a comparatively large number of studies: 21 studies provided evidence for causal link 10:12 (if care planning at the individual level becomes more prevalent, then there will be improved patient care), 16 for causal link 11:13 (if patients are diverted from inpatient care, then NHS costs will reduce) and 10 for causal link 11:12 (if patients are diverted from inpatient care, then there will be improved patient care.
Summary
The evidence review included 79 studies that provided evidence for 44 causal links between 13 MCP components. We found evidence for a new MCP outcome ‘staff health and well-being’ from components 3 (MDT working), 7 (planned referral networks) and 10 (care planning at the patient level), although this outcome was beyond the scope of this review and so we do not report these findings.
Evidence from these 79 studies about the 13 components and 44 (28 IPT and 16 new) causal links provided the analytical framework (see Chapter 3) for reviewing the evidence relevant to the IPT.
Chapter 6 Step 2 findings: comparing identified evidence to the initial programme theory
Setting up multispecialty community provider-like organisations and networks
First, we evaluate causal links 1:2 and 1:7 (Table 2) in which component 1 (the setting up of MCP-like organisations and networks) is the realist mechanism to bring about component 2 (network management) and component 7 (planned referral networks).
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 1. NHS managers establish MCPs | 2. Network management will develop | 1:2 |
| 7. Planned referral networks will develop | 1:7 | |
Evidence about creation of networks of primary care providers mostly, but not entirely, concerned ACOs and PCMHs in the USA. (see Chapter 1, which notes how these terms map onto NHS contexts.) Much more evidence was available about the contexts favouring the formation of MCP-like organisations or networks than about the mechanisms of setting such entities up. Within those limitations, the evidence we found supported the IPT causal links related to establishing MCPs.
Causal link 1:2 – if NHS managers establish multispecialty community providers, then multispecialty community provider-wide management structures will develop
Unless they fail even to get MCPs started, it is a near-tautology to say that if NHS managers establish MCPs, then MCP-wide structures for planning, developing and operating the included services will develop. Nevertheless, the studies we found included some indicating how this mechanism worked and options for the structures to set up. Three main mechanisms contributed to the creation of a MCP-like structure, two of them being motives of the providers joining it.
First, provider organisations wished, but were unable, to provide all the needed health services. The prospect of providing such services on site (e.g. dental and vision care, and specialty medical care) motivated clinics in Boston to form ‘strategic partnerships’ to meet the needs of a complex patient population. 55 Providers with a large share of patients with mental health needs were more motivated than other providers to use an ACO to improve mental health services, both to meet patients’ needs and to reduce the burden on providers themselves. ACOs in regions with a low supply of mental health specialists were also looking for ways to integrate mental health care into other settings, typically primary care, to meet patient demand. 56 That is, the MCP-like structure (for instance, an ACO) appeared to those provider organisations joining it to be relevant to their care group(s) and clinical work. 57 In the USA, the criteria of ‘relevance’ included whether or not the patients would have insurance coverage. 57
Motivation to pursue the member organisations’ interest as organisations was a second mechanism. In another study, the majority of respondents (15/25) gave joining like-minded organisations and minimising future risk (18/25) as ‘important’ or ‘very important’ reasons for forming their ACO. 58 For some, an important consideration was that joining an ACO offered participating organisations the prospect of clinical ‘integration’ without corporate ‘consolidation’ (takeover). Hence, physician practices that participate in ACOs are likely to be large and/or be members of an independent practice association or physician hospital organisation and unlikely to be hospital-owned. 11 Health centres and other ACO members retained their independence but worked together under an ACO contract in new partnerships. 59 Endorsing or improving the member organisations’ internal organisation was also a motive for joining. Some US doctors perceived organising as a patient-centred medical home as key to providing high-quality care and as ‘the right thing to do’. Others described recognition as acknowledgement for how they organised their practice. Physicians described participation in PCMH demonstration projects, quality improvement (QI) initiatives and external support for seeking recognition as key motivating factors. The extent to which substance abuse treatment services’ staff were clinicians with professional degrees predicted these organisations’ likelihood of participating in an ACO. Some also said that participation gave them access to external data sources (such as insurance companies) and to health information exchange, which enhanced their QI strategies and ability to function as PCMHs. 59 Physician groups played a more prominent role than other provider types (including solo-practice physicians) in forming and managing rural ACOs. 58 Organisations’ financial motives for joining ACOs were to increase activity (hence income), in contrast to a NHS context. Preparing for value-based purchasing (14/25 respondents) and getting paid for quality (10/25 respondents) were the most frequently cited ‘very important’ reasons for the ACO formation. 58 Among substance abuse treatment centres specifically, those who reported a greater local competition were more likely to have signed a contract with an ACO. 57
Both of these mechanisms imply that member organisations in a voluntary network are self-selecting, which, a Canadian study suggests, will of itself stimulate evolution towards a more integrated network. Patients may also be self-selecting. Québec clinics’ improvement in ideal type integrated care (ICIT) scores was partly due to a ‘natural selection’ effect of clinics that closed, and the effect was mitigated by clinics that opened after the 2005 survey. Change in ICIT score was associated with both this evolutionary trend and central reform policies. 60
Decisions from higher authority were the third mechanism for the formation of MCP-like entities. Decreed ‘top-down’ reform was instrumental and an obvious prerequisite for initiating change in the Québec health-care system. Coercive and mimetic factors influenced primary care provider organisations’ ICIT score to shift towards greater ‘integration’. These primary care organisations did not regard health and social care centres’ support in creating PCMH-like organisations as very substantial. 60 The Ontario Health Ministry’s use of ‘simple rules’ encouraged change in the desired direction without stifling creativity and innovation. 61 In Australia (a similar primary care system to the UK in many respects), HealthOne Mount Druitt needed sustained support at higher governmental levels (New South Wales Health and the regional Local Health District management), but in a form enabling policy change without attempting to micromanage local developments, which would have ended all chance of GP participation. 62 In general, efforts to improve outcomes by exerting top-down control were often intrusive and futile, slowing down the inherent capacity of the system to adapt and evolve. 62
The studies that we reviewed described different structures that emerged, in different contexts, as the outcome of managers’ attempts to set up MCP-like organisations and networks. In ascending order of common managerial control, the simplest was information managing across the member organisations. Thus, ACOs must report on 33 quality metrics across patient/caregiver experience, care co-ordination/patient safety, preventative care and at-risk population. 11 EHRs are an important means of managing and co-ordinating patient care for effective ACO performance; substance abuse treatment services’ use of EHRs predicted how likely they were to participate in an ACO. 57 Other studies corroborate this pattern63 (see also Chapter 6).
Contracts have also been used to establish MCP-like networks on a quasi-market basis. One example, despite linking only mental health services, are the Integrierte Versorgung networks of primary, secondary and social care services in cities such as Hamburg. In the Netherlands, bundled payments result in a principal contracting entity (provider) being lead contractor for numerous other subcontractors. 64 Billings and de Weger11 describe and distinguish three other contract-based structures: (1) outcomes-based commissioning (an existing NHS approach), (2) the ACO model and (3) an alliance model of a network of providers making a joint contract with a payer (in that respect, closer to the NHS idea of an ACO). However, some ACOs themselves use joint payment contracts. Such ACOs are more likely than others to include community health centres (CHCs; ‘safety-net’ primary care providers), hospitals, medical groups, nursing facilities and specialty groups, but not to have more primary care and specialty clinicians. 59 In passing, Billings and de Weger11 mention four more models: (1) a ‘Partnering Model’, (2) ‘Value-based Health Care’ (which is not specific to MCP-like organisations65), (3) ‘Incomplete Contracting’ and (4) the Alzira Model (which is less relevant here because it focuses on vertical integration).
Managers have often established a central co-ordinating body (‘network administrative organisation’54) to co-ordinate MCP-like networks and quasi-markets. Thus, Intermountain Healthcare in Utah had a central medical and administrative team in which research groups gave economic and organisational support for running clinical programmes. The support elaborated good practice recommendations and the corresponding indicators, which providers followed and measured. 66 In establishing PCMH, one problem for managers, as for researchers and other interventions too, was that different organisations varied considerably in their definitions of a PCMH, so in practice they were not all trying to implement the same intervention. 67
Some health systems have pursued the functions for which MCPs were designed through organisational integration, that is, amalgamating the separate components of primary care (i.e. primary medical care, community nursing, therapies and perhaps mental health services and/or social care) into one organisation, as do the Swedish23,24 and Finnish polyclinic model and Italian Unità Sanitarie Localis (USLs).
We found no evidence either for or against the policy assumptions that population attitudes and beliefs about actively maintaining one’s own health and helping to care informally for people in chronic ill health or about autonomy and control over one’s health and health care were mechanisms that contributed to establishing MCPs. The same applies to whether or not health professionals and organisations view those whom they care for as people not patients. We also found no direct evidence about the requisite state of social services.
Causal link 1:2 – contexts favouring the creation of multispecialty community provider-like networks or organisations
Evidence from several countries suggests that good pre-existing interorganisational relationships facilitate the establishment of interorganisational co-ordination mechanisms, which then reinforce the good relationships in a virtuous circle68 (as has also been reported in studies of hospitals69). This pattern recurred in several studies of ACO formation. Although the joint payment system was new to the ACOs that used it, the organisations that participated had existing informal partnerships. 59 A study of the formation of four rural ACOs also found that prior experience with risk sharing and provider integration facilitated ACO formation. 58 An Australian study corroborated these patterns. Planning for HealthOne Mount Druitt was led by a steering committee with links into the local community through strong representation from local GPs (71% of them were single-handed), community nursing services and the Western Sydney Medicare Local. 62 In Ontario, Canada, organisations with a history of collaboration, pre-existing relationships among partners and a pre-existing focus on integrated care saw the Ontario Health Links model as a natural step forward and found the transition into it easier than for organisations without existing collaborative relationships, knowledge and resources to draw on. 61
Implementing ‘top-down’ decisions to create PCMH-like organisations in Québec also required their internal ‘receptivity’ to joining a network, including a ‘mimetic’ context of other exemplar PHC organisations also participating [but the admired prototypes were family medicine groups (FMGs) and/or network clinics rather than the new health and social services centres], and the presence of ‘local champions’ advocating the new models and demonstrating their feasibility and desirability. 60
A realistic time scale was also required. PCMH programmes typically took a few years to reach maturity and produce measurable effects. 70 In the USA, time was required to obtain ‘bureaucratic’ approvals and check conformity with antitrust regulations. 11 Similarly, in Australia, the planning stage of HealthOne Mount Druitt took 2 years; the greatest challenge was building relationships between the key partners, especially overcoming strong established barriers to trust between GPs and community health. 62
Several studies (e.g. Billings and de Weger11) report substantial ACO start-up costs. Just one ACO in New Jersey (covering 2 million people) required US$2.8M start-up funding, after which it was to become self-sustaining from the savings generated. 71 Patient Care Medical Homes also required start-up funding. For example, participation in a state-funded interpractice collaboration to improve quality helped 20 medical practices to implement a PCMH approach internally. 72 ‘Technical support’ (character unstated) was also required. 72 Conversely, another study70 noted as unusual that the ‘CareFirst’ PCMH project did not require ‘up-front investment’. When that investment has to come from the participating provider organisations themselves, smaller organisations are at a disadvantage. 70 To address that obstacle, the ACO Investment Model programme provided initial investment capital and variable monthly payments to ACO participants in rural and underserved areas that might not otherwise have access to the capital needed for successful ACO formation and operation. CMMS also contracted 32 organisations under a special ‘Pioneer ACOs’ demonstration project. 58
When statute guarantees patients a choice of provider (as in the USA and, in theory, the UK), it will be difficult to steer patients to particular providers,11 weakening the evolutionary pressures towards MCP-like ‘integration’.
We found no direct evidence about maximum or minimum viable size of a MCP, economies of scale or scope, or the demographic or social character of places where it might be easier or harder to establish MCPs. However MCP-like networks appear harder to establish in rural areas, where general practices are small and isolated68 and where providers cannot contribute to MCP start-up costs.
Causal link 1:7 – if NHS managers establish multispecialty community providers, then planned referral networks develop
As regards referral networks specifically, our evidence suggested that some MCP-like networks do indeed develop referral network planning at organisational and/or interorganisation level. Physician practices that participate in ACOs are more likely than non-participating practices to use more care management processes. 11 One study describes a PCMH that negotiated 50 ‘compacts’ (agreed procedures for referring patients between providers) with specialist providers while other nearby PCMHs negotiated few or none, but does not report any contexts explaining why these differences arose. 68 Furthermore, the PCMH is designed to co-ordinate patient care mainly within a primary care team (within a general practice, in NHS terms) rather than across care teams. 68 A limitation to establishing referral networks is that many ACOs do not formally cover post-acute care (the function of CHS in England). A total of 87% of the ACOs that did cover post-acute care included a hospital (compared with 41% of ACOs without post-acute care). CHCs were also more likely to be integrated into ACOs that included post-acute care (58% vs. 49%). 73 Small, isolated rural practices were less likely to establish care compacts. 68
However, all of this does not resolve the issue of whether or not prior collaboration favours the initial formation of a MCP-like organisation or network, or whether or not stronger referral networks result from forming such an organisation or network, or, in a virtuous circle, both. Although it gives proof of concept that it is feasible, the evidence of ACOs also suggests that ‘horizontal’ PHC networks do not automatically develop interorganisational referral networks, in particular between GPs and CHSs (or the local equivalents). This suggests that further specific contexts are required, as yet unidentified in the published research.
Interorganisational network management
The next two causal links in the IPT that we evaluated were those in which component 2 (network management) was the mechanism: if–then statements 2:3 and 2:6 (Table 3).
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 2. Network management develops | 3. MDTs will develop | 2:3 |
| 6. Care co-ordination through IT use will develop | 2:6 | |
Causal link 2:3 – if network management develops, then multidisciplinary teams will develop
The studies also reported many instances of MCP-like networks setting up MDTs. One aim of the Utah Mental Health Integration (MHI) programme was to orient patients towards support by a MDT (general physician, care manager, psychiatrist and psychologist) in ambulatory care, or hospital for the most severe cases. 66 The Versailles geriatrics network brought multidisiciplinary expertise together at the local information and co-ordination centres (Centres Locaux d’Information et de Coordination), Homes for the Autonomy and Integration of Alzheimers’ Patients (Maisons pour l’Autonomie et l’Intégration des malades d’Alzheimer), and the mobile geriatrics teams (Équipes Mobiles Gériatriques), which worked with local hospitals and doctors to avoid hospitalisation. 74 In general, MDTs require clear boundaries, a need to be collectively accountable for patient care, a need to be highly interdependent and a stable membership. 67
Multidisciplinary teams varied in their occupational membership and, therefore, the services that they could provide without external referral. The studies reported MDTs that included:
-
CHSs but not doctors. Buurtzorg nurses worked with community volunteers, social workers, physiotherapists, occupational therapists and community psychiatric nurses21 but, although Buurtzorg also encourage collaboration between their own staff and the patients’ doctors75 (whom they do not employ), their MDTs do not usually include doctors.
-
Doctors but not CHSs, as in a large number of American ACOs (see Causal link 1:7 – if NHS managers establish multispecialty community providers, then planned referral networks develop).
-
Both doctors and CHSs. Swedish and Finnish polyclinics, some primary care providers in Spain and Italian USLs employ doctors, nurses and therapists together within a single organisation. Some NHS Integrated Care pilots relied heavily on MDTs, although the ‘virtual ward’ involved hospital doctors rather than GPs. 76 A survey of ACO-employed social workers found that 65% worked with primary care physicians, 55% with specialty physicians, 74% with nurses or nurse practitioners and 31% with psychologists. A total of 48% had a nurse or nurse practitioner as their immediate supervisor, 25% had a social worker and 4% had a manager. 77 One US variant was a ‘physician-led’ team such as the ‘perioperative surgical home’ (PSH) in which activities included patient ‘rehabilitation’ before surgery and transitions to home or post-acute care designed to reduce complications and readmissions. 51
-
Mental health services. Lewis et al. 56 describe the addition of mental health-workers (e.g. social worker, psychiatrist) to existing primary care teams, so that care management remains with just one provider.
-
The patient and/or informal carer(s). 67
Some MDTs were ‘virtual’ (i.e. co-ordinated by teleconference, video conferences or other HIT systems). 62,78 Between them, the studies that we found reported that MDTs were based (moving from ‘virtual’ to ‘real’) on:
-
The consulting model, in which one clinician consulted another without actually referring (i.e. temporarily transferring) the patient. In some ACOs, the role of consulting mental health specialists included coaching primary care providers in the use of psychiatric medications, assisting with diagnoses, and making appropriate referrals to specialised mental health care services. 56 In the HealthOne Mount Druitt project (Australia), consultations also included ‘more informal exchanges of information.’3
-
A dispersed, partly remote, team linked by IT. Thus, a paediatrics MDT in five English CCGs involved members (including GPs) by teleconference. 76
-
Colocation (e.g. of mental with physical care clinicians). 46,56
-
‘Embedding’ of, for example, mental health-care clinicians within primary care teams;56 in effect, seconding staff from one organisation to another.
-
‘Huddles’, that is, informal, ad hoc, but frequent (e.g. daily) staff meetings, reported in 73% of practices in a survey of 40 small primary care practices (PCPs) in Texas. 79
-
Formally structured cross-organisational teams.
-
Staff all employed by the same organisation.
Care co-ordination and communication sometimes required MDTs to adapt health professionals’ traditional roles. 66
Causal link 2:3 – contexts
Barriers to including pharmacists in MDTs in PHC medical practices in Vermont were the pharmacists being employed by a separate organisation, with pharmacists and physicians being unfamiliar with each other’s scope of practice and roles. 80 Different patients might also have different but often overlapping provider networks, and these overlaps offered the greatest scope for strengthening care co-ordination. 67
Most papers described what care co-ordination activities MCP-like interorganisational networks undertook rather than how these co-ordination arrangements were created or (in the realist sense) their contexts. Many of these arrangements were reported in just one study, but, when there were several reports, they were mutually consistent.
Causal link 2:6 – if network management develops, then care co-ordination supported by health information technology will develop
We found substantial evidence of interorganisational care networks establishing structures and work processes to co-ordinate care across multiple provider organisations, so that clinicians and organisations adapted their work routines and practices to network standards, shared information, created ‘boundary objects’ such as care plans, and standardised organisations’ and clinicians’ roles, and care pathways across organisations. 67
Six out of the 13 sites in the Alidina et al. 68 study reported that implementing co-ordination mechanisms increased communication and trust. Such routines included primary care doctors ‘feeding back’ to specialists. 68 ‘Care compacts’ assisted communication, decision and negotiation between organisations and improved care access and quality. 68 A survey of rural pioneer ACOs found that managing care across the continuum and meeting quality standards were what the respondents most frequently reported as ‘very important’ to the ACO’s success. 68 Initially, maintaining good relationships between the member organisations was important for ACO success, pending the development of more standardised and contractual relationships. 68 Some Ontario Local Health Integration Networks (LHINs) pooled resources across partners and standardised structures and processes related to governance, accountability and administrative functions in an attempt to avoid duplication and waste. 61 Several respondents in those networks suggested that the type of lead organisation mattered less than that organisation’s reputation, existing relationships and partnerships, and leadership style (e.g. having a positive image in the community and among providers and a track record of innovating and following through on commitments, and for tolerating change, risk and ambiguity). 61 The Kinzigtal network (in Germany) jointly developed care pathways across providers and synchronised hospitals’ and ambulatory care providers’ formularies across all care sectors. 81 In the Netherlands, care standards with a modular structure (general and disease-specific elements) were jointly negotiated among providers, an arrangement that routinised collaboration among doctors. 64 The HealthOne Mount Druitt project (in Australia) used case conferences to co-ordinate services at patient level, in particular with non-health-care services, such as social care. 62
Assuming that standardised care pathways and quality standards do, indeed, define the character of patient needs more clearly, these studies tend to support the policy assumption that MCP-like networks will lead to clearer definitions of patient needs and promote evidence-based targets for managing long-term conditions.
Causal link 2:6 – care co-ordinators
The studies that we found neither used the term ‘care navigator’ nor described similar advocates or helpers for individual patients. Instead, many of them reported how MCP-like networks had used care co-ordinators, for example by creating dedicated care co-ordinator positions. 68 Nurses working as care co-ordinators were reported in Texas and Colorado. 82 Community health workers (CHWs) recruited from the local population were used (in Texas) to help bridge gaps between patients and organisations, and between organisations, to enable PHC teams to connect patients with resources that patients need. 83 In New York, social workers were ‘embedded’ in PCPs and included in all practice-based meetings and other aspects of patient care. ACO quality metrics meetings were critical to developing working relationships with PCPs and other members of the care team, and with care co-ordination staff in other programmes. 78 The HealthOne Mount Druitt project (in Australia) also recruited GP liaison nurses for co-ordinating services in ways that the GPs could not because of lack of time or knowledge of the services available (e.g. home care, counselling and other allied health services). 62 These nurses managed communications, case conferencing, case management and overall care co-ordination, and allocated the case management of individual patients to the most appropriate person in the MDT. 62
The foregoing evidence corroborates the policy assumption that primary care provider networks are capable of co-ordinating inputs across multiple services. Contrary to UK policy assumptions, Alidina et al. 68 concluded that the above changes did not require culture changes or payment reform, but Wholey et al. 67 argued (corroborating UK policy assumptions) that they do require large numbers of clients so as to allow economies of scale.
Causal link 2:6 – contracts
Nevertheless, several health systems have attempted to use contractual mechanisms to strength care co-ordination between separate providers. The Kinzigtal network (Germany) co-ordinating body made contracts with the two main social health insurers involved (AOK and LKK) and over 100 local providers to implement various programmes for individual treatment plans, patient self-management, follow-up care and case management. 81 Two complications are the ‘hangover’ of existing contracts and technical difficulties of contract monitoring. In the US ACOs, providers’ decisions whether or not to pursue integrated models depended powerfully on the design of the ACO payment model (implying, at one remove, patients’ insurance status), details of contracts and the quality measures used in contracts. Contract design appeared to influence the extent to which ACOs integrated mental care. 56 In practice, the English NHS has so far had little success in commissioning through outcomes-based incentivised contracts for these purposes because of the difficulty in specifying and measuring the relevant outcomes, and then in knowing whether or not to attribute any changes to the providers, care co-ordination or extraneous confounding factors. 11
Interorganisational co-ordination mechanisms are especially required when patients have highly complex health problems and providers have a low level of knowledge about the patient’s condition,68 as often applies to patients with long-term conditions. In combination with other (unspecified) enabling ‘changes’ within the local health district and the wider New South Wales health sector, the HealthOne Mount Druitt network (Australia) began delivering services through two main streams: (1) chronic aged and complex care and (2) child and family care. 62
Causal link 2:6 – network membership
A limiting factor is what organisations (hence, services) a network contains. In the studies, we found that MCP-like primary care networks varied in whether or not they included:
-
Mental health services – in 2014, 42% of ACOs included mental health-care providers. ACOs with ‘a comprehensive, chronic care management program’ were more likely than others also to have integrated mental and physical care. 56 In Utah, MHI’s co-ordinating approach allowed it to replace the traditional model of partitioned-off, sectorised psychiatry with a co-ordinated combination of ambulatory care, specialist secondary, and first-recourse care, which, in turn, allowed territory-wide, whole-population planning of its services (reducing ‘medical deserts’), organising support networks to promote preventative care, and developing ambulatory services that linked hospital, medicosocial work and social care. 66 Lewis et al. 56 describes two main approaches to overcoming the traditional separation of primary and mental health care –
-
expanding primary care to cover mental health conditions (9 out of 16 PCMHs in that study)
-
integrating primary care providers into existing mental health programmes (2 out of 16 PCMHs).
-
-
Children’s services – for the NHS, Woodman et al. 76 report four ways of bringing paediatric expertise into primary care and/or improving joint working –
-
telephone-based MDTs
-
hospital at home
-
outreach clinics
-
paediatrician advice and guidance to GPs.
These initiatives work by promoting shared responsibility, upskilling GPs, establishing relationships between paediatricians and primary health-care professionals, and by taking specialist care to the patient.
-
-
Community health services (or the equivalent) – 48% of ACOs in the Colla et al. 73 study did not include post-acute care. Those were more likely to be physician-led. ACOs that did include post-acute care were more likely to have programmes to reduce preventable hospital admissions and for end-of-life care. 73
-
‘Safety-net’ services – a substantial number of ACOs included CHCs. A greater proportion of those ACOs with a centre reported experience of public reporting, of having patient-centred medical homes and holding other risk-bearing contracts. ACOs that included at least one federally qualified health centre among their participating provider groups were more likely to report complete integration of services and to offer less common services, such as health coaches and case managers. 56
-
Primary medical care – the studies mentioning general practice engagement in MCP-like networks reported that GPs (or the equivalent) valued the access to additional resources that such networks gave. Versailles doctors (including GPs) participating in a geriatrics network reported being satisfied with the way it provided expert advice and access to hospital-like support for patients at home. 74 Similarly, physicians within integrated health systems in Texas and Colorado frequently discussed the value for care co-ordination purposes of shared resources across sites, for example nurse care co-ordinators, nurses providing advice during and after office hours, enhanced access through expanded office hours, electronic communication, ‘virtual visits’ (to patients), access to hospital records, referral tracking, physical workspaces organised to facilitate team-based care and access to non-physician providers (e.g. dieticians, psychologists). 56 However, it was not always easy for GPs to participate in network activities. In the Versailles study,74 one-third of doctors did not wish to participate in network meetings at patients’ homes, judging them too time-consuming. These studies did not directly report whether or not MCP-like networks reduced general practice overload. Indeed, the Versailles study74 implies the opposite. These studies tend to corroborate the assumption that smaller general practices find it difficult to contribute to networked care co-ordination activities. We found no studies reporting whether or not the creation of MCP-like networks leads to improved infrastructure management in primary/community care.
A network’s membership constrains that of the MDTs within it.
Causal link 2:6 – health information technology adoption
A shared patient record promotes informational connectivity68 and, by implication, informational continuity of care. 15–17,84 We found recurrent accounts of primary care networks attempting to increase staff access information needed for making referral decisions. The Kinzigtal network (Germany) introduced common EHRs across all care sectors. 81 However, initially, many US ACOs did not uniformly have developed, interoperable IT systems. 68 Assuming that shared information will help networks and providers define the character and scale of patient needs more clearly, these studies tend to support the policy assumption that MCP-like networks will lead to clearer definitions of patient needs. Although we found no counter examples, these studies also indicated that such information sharing is not easily achieved.
Causal link 2:6 – contexts
Just as prior collaboration assisted the formation of MCP-like networks, so it facilitated network management. In the 13 ACOs that Alidina et al. 68 studied, more complex co-ordination mechanisms (i.e. communication, decision and negotiation) complemented, rather than replaced, existing ones. 68 Conversely, lack of trust was an initial challenge in setting up the HealthOne Mount Druitt (Australia) system. 62 Irrespective of their profession, uniform training for care co-ordination staff in New York (covering ‘Basics’, Practice, Psychosocial Domains, Disease Conditions, and Medical Services) helped to ensure a consistent approach to care co-ordination. 78 A study of PCPs in Colorado and Texas found that the use of practice facilitators to visit primary care physicians was significantly correlated with the use of sustained chronic care management strategies. Despite external facilitation, it remained difficult for the smaller PCPs practices to implement the CCM. 82 In using contracts to co-ordinate care, pre-existing carve-outs, in which a commercial payer had already contracted mental health care to a separate provider, practically excluded those services from an ACO in the short term. 56
Case-mix was another important context. High patient complexity and low knowledge about the patient’s condition is the situation that, the Alidina et al. 68 study suggests, most requires ‘boundary spanners’ for enabling reciprocal co-ordination between providers.
In New York, preparation for sharing medical records across providers involved extensive training, work-group activity and software development (for reconciling the different PCPs’ discrepant EHR systems). 78 In Virginia, the obstacles appeared to include lack of internet access and computer literacy among the target populations. Even in the Netherlands, where internet usage is extremely high, patients showed a lack of awareness and motivation to hold their own health records, and there were usability problems in the systems for accessing them. 85 There have been similar experiments in Sweden, with mixed success. 23
Multidisciplinary teams
The next causal links in the IPT are that MDTs are a mechanism for bringing about component 7 (planned referral networks) and component 9 (preventative health care). In this section, we first discuss the evidence found in our review in relation to these two causal links (Table 4) and then describe evidence found in this review for additional causal links in which MDTs are the mechanism that were not in IPT (see Table 5).
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 3. MDTs are established | 7. Planned referral networks will develop | 3:7 |
| 9. Preventative health care will develop | 3:9 | |
The research studies that we found provide evidence about causal links 3:7 (MDTs produce planned referral networks, and 3:9 (MDTs improve preventative health care). As additional mechanisms to those in the IPT, we also found secondary evidence that MDTs promote stronger demand management systems, care planning at the patient level, diversion of patients from hospital to primary care and improved patient experience and outcomes. There is also limited evidence suggesting that MDTs support culture change and voluntary sector involvement, and enhance informational continuity of care. A mechanism for many of the above is the development of new or expanded boundary-spanning roles, which expose people working in more traditional roles to new ways of working and encourage engagement, trust and respect for what these new roles (and the corresponding professions) can achieve.
Causal link 3:7 – multidisciplinary team working produces planned referral networks
Multidisciplinary team working produces care network planning at whole-organisational and at interorganisational levels by facilitating co-support and decision-making across disciplinary boundaries. These activities are enabled by:
-
the development of new or expanded boundary-spanning roles that enable fuller formal and informal communication across the MDT, and joint support for decision-making across disciplinary boundaries
-
inclusion of colleagues from a range of disciplines and interprofessional relationship building
-
addressing barriers (e.g. traditional hierarchies, lack of role clarity, divergent expectations) to awareness and understanding of the knowledge, training and benefit of working in an interprofessional way when dealing with complex, multimorbid patients.
Contexts that facilitate this mechanism are reported to be managerial recognition and support of MDT working, and cultivating trust in place of resistance towards other professions.
Causal link 3:7 – co-support and decision-making
Qualitative (five) and mixed-methods (one) studies in the USA (five) and the UK (one) show that, in addition to promoting care planning for individual patients (see Care planning for individual patients), exposure to multidisciplinary working [e.g. through ‘embedding’ (seconding) or colocating staff] creates more opportunities for different professions to improve understanding of each other’s treatment approach. 86–88 It also shifts providers’ expectations for communication and increases their awareness of the importance and benefits of involving other primary care providers in complex cases,89 upskills primary care providers and promotes shared responsibility. 76 A narrative case study of mental health integration in a CCM showed that this co-support across disciplinary boundaries helps members of each profession not to feel alone in the face of complex multimorbidity issues about which they are not specialists and to make shared decisions on complex problems. 66
Causal link 3:7 – boundary-spanning roles
Many studies about how MDTs surmount organisational barriers described new or expanded boundary-spanning roles as a key mechanism. These boundary-spanning roles improved co-ordination and integration of services through improving communication (formal and informal) between the various other care providers, through co-ordinating multiple services, addressing psychosocial as well as physical health issues, providing the conduit for GPs, community health, and other health and social care providers to work together more closely. 90 They also provided support for clinical and administrative staff. 87 MDTs are also part of the health-care delivery system redesign and connection to the community care resources involved in the CCM. 83 A study of focus groups with 387 people from 10 US communities suggested that having non-medical staff in boundary-spanning roles helped to co-ordinate patients’ care and to address barriers to care co-ordination. Patients appreciated having individual care plans with a holistic orientation, including a personal physician providing access to continuous comprehensive care. Two other studies86,91 reported similar findings.
Although MDT members might see these roles as the ‘glue’ holding care co-ordination and care teams together,90 interviews with 25 clinical pharmacists and 17 primary care clinicians found that traditional status hierarchies could be a barrier to effective collaboration and communication in PCMHs, where there were new roles for some or all professionals. 92 An online focus group of people self-identifying as care co-ordinators in PCMHs described primary care doctors as the biggest such barrier. The MDT had to win them over by strong self-promotion if these resistant doctors were to become a resource to the rest of the team. 87 This focus group also indicated the importance of boundary-spanners being embedded within a primary medical care practice. 87 Interviews and a survey of people in different MDT roles in the PCMH (the USA) found that the benefit of these new roles was maximised when there were loosely specified implementation protocols and a vision of the roles’ full potential. 93 Similarly, policy- and decision-makers in a chronic aged and complex care network suggested that boundary-spanners need to have the seniority and expertise to be leaders who earn and maintain the respect of the MDT by initiating culture change, and to have sufficient flexibility in their role to work with GPs to support and add value to the care they provide. 90
Causal link 3:7 – role clarity and expectations
Multidisciplinary teams often involve team members taking on new roles. This creates the potential for lack of clarity about roles and expectations between MDT members and, thus, strained relationships across disciplinary boundaries. In a case study, Matiz et al. 94 observed that responding to PCMH team members’ concerns and clarifying roles by educating teams about each profession’s strengths and limitations proved essential to integrating the MDT. 94 Interviews with primary care providers and clinical pharmacists in PCMHs showed that, despite frustrations between professionals with different opinions about new roles within PCMHs, being exposed to the other professionals’ reasoning improved understanding, respect and communication. 92
Causal link 3:7 – relationship building
Most of the barriers to, and facilitators of, care co-ordination at the organisational level identified in an online focus group of self-identified care co-ordinators in PCMHs by Friedman et al. 87 related directly to relationship building in MDTs, which was facilitated by boundary-spanning roles, enhanced communication (e.g. on-site mental health services), EHRs that interfaced well with outside organisations and training in motivational interviewing. Interviews and a survey with mental and primary care staff in PCMHs showed that mutual familiarity across disciplines through the use of a staff directory (with picture and contact information for each clinician), cross-disciplinary training and a mail server for ongoing, informal, patient non-specific consultation all improved interprofessional relationships. 89 A focus group of 17 primary care clinicians from different ‘integrated’ care models in the USA showed the importance of staff perceptions and knowledge about the training of other disciplines. At first, most doctors seemed reluctant to consider pharmacists as providing patient care but reviewing their training and knowledge led some physicians to value pharmacists’ contribution to patient care. 95
Causal link 3:7 – inclusion of new roles in multidisciplinary teams
Structured team communication in the PCMH in the USA facilitated the inclusion of the new members as part of the MDT and improved recognition of other MDT members’ value. 93 Two studies of the US PCMH model found that facilitators of improved communication are clearly defined expectations with agreed time frames for written updates, judicious use of HIT, electronic information exchange that met confidentiality requirements, jointly determining key information to be shared and frequency of updates89 and using faxes for routine updates so as to reserve the use of telephone calls for urgent matters and pre-planned consultations. 93 Faxable forms worked better if they were concise, easy-to-use, included the desired data and clinical impressions, used tick-boxes to document information and contained a pre-agreed expected minimum level of information to be shared by each discipline. 89 Clinical pharmacists and primary care providers in the PCMH described how delays caused by communicating back and forth electronically, the absence of real-time (or face-to-face) explanations and diverging inferences about each other’s intentions could impede communication within the team. 92
Causal link 3:7 – contexts
Contexts facilitating the operation of the above mechanisms included management, skills development and professional attitudes. One such context was for managers to encourage mutual support between staff of different professions. Interviews with 12 participants in US PCMHs found that giving clinic administrators protected time for interdisciplinary meetings or consultation and allowing for warm handoff in clinicians’ schedules facilitated interdisciplinary working. 88 A qualitative study of US integrated care models found that those integrated care models that were successful on at least one of clinical outcomes, satisfaction and spending, managers had found successful boundary-spanners and facilitated their relationship with other staff (clinical and non-clinical). 96 Conversely, lack of understanding of the integrated care model and the roles of other professionals within it prevented MDTs from surmounting organisational barriers. 88 Training programmes can increase such understanding and address the scope of practice for each profession, liability and confidentiality issues. Interviews with people in ‘successful’ integrated care models in the USA suggested that people in boundary-spanning roles need to be able to be assertive when necessary, to understand practice culture in its setting and to maintain good relationships with everyone caring for the patient. 96 Clinicians needed to be aware of their own limits of expertise and of the skills and limits of each professional, and to consult and refer when a clinical problem was beyond their scope. 88 A mixed-methods study86 of 18 complex care management organisations in the USA found that educating providers about the roles and responsibilities of care managers and providing complementary services that fill patient care gaps helped generate trust and support within MDTs. 86
There was a fragile balance between resistance to including new disciplines (e.g. pharmacists) as MDT members and acknowledging the need for them. In one study,95 some doctors expressed concerns about having pharmacists challenge their prescribing decisions directly or overstepping their professional boundaries, while others valued having pharmacists work with them as team members and saw them as a critical piece of a patient-centred medical team. Single-handed doctors, doctors not affiliated to physician networks or doctors who had never worked with clinically trained pharmacists in primary care had more difficulty envisaging collaborations with pharmacists than doctors in group practices or a hospital–physician network, who had previous working experience with clinical pharmacists. 95 In general, the studies we found suggested that it was necessary to work around or weaken defensive professional perceptions of other professionals;89 and around doctors’ and patients’ resistance to boundary-spanners cultivating cross-professional and cross-organisational relationships. 87
Causal link 3:9 – multidisciplinary teams produce health planning and better preventative care
A narrative case study of an integrated mental health service in the USA66 found that MDTs allow better territorial planning of health as a whole regarding:
-
the health needs of the whole population
-
reducing ‘medical deserts’
-
organising support networks which promote preventative and ambulatory care offering medico-social work, social care and at need hospital care. 66
Coleman and Phillips97 created a ‘teamness’ index based on whether or not non-physicians shared responsibility for managing and co-ordinating care. Practices that scored high in ‘teamness’ were more likely than low-scoring practices to report well-functioning processes to support communication and access to care, and to connect chronically ill patients to self-management programmes. 98 Hong’s mixed-methods study86 of 18 American complex care management organisations found that care co-ordinators negotiating a care plan that reflected the individual patient’s, and their family’s, priorities and preferences facilitated various actions including identifying patients’ behavioural health and social service needs, and using motivational interviewing to encourage patient activation and self-management.
A Canadian survey99 of adult patients and administrators found that MDT working produced better preventative care through better first-contact accessibility (FCA) and accessibility accommodation (AA), which increased equity of access to such services. AA was the way PHC resources were organised to accommodate a wide range of patients’ abilities to contact health-care clinicians and reach health-care services. FCA was the ease with which a person could obtain required care (including advice and support) from the practitioner of choice within a time frame appropriate to the urgency of the problem. Carroll et al. 99 found that FCA was better in clinics with ≤ 10 doctors, a nurse, telephone access 24 hours a day and 7 days a week, and evening walk-in services.
Further US studies corroborated the finding that integrating community and/or mental health professionals into MDTs improved preventative care. Matiz et al. 94 found that doing so made care delivery more comprehensive and identified high-risk populations for care co-ordination. Such organisations had decreased emergency department (ED) utilisation and hospitalisations for asthma, resulting in overall improved outcomes. 94 Briot et al. 66 found that mental health professional integration offered good-quality ambulatory care to more patients at a lower cost and better managed complex family health problems66 than traditional forms of organisation did. Similarly, in a descriptive quantitative study100 including pharmacists in PCMHs allowed screening of diabetes mellitus and hypertension patients, care reviews, inclusion/exclusion decisions and provision of preventative pharmaceuticals.
Causal link 3:9 – patient engagement, patient self-care, activation and empowerment
Evidence from two qualitative studies of PCMHs87,88 suggested that mechanisms by which MDTs improved patient engagement were care co-ordinator roles and capitalising on the primary care relationship. An online focus group of care co-ordinators in PCMHs reported improving engagement of patients by using motivational interviewing, being patient but persistent, keeping promises, listening carefully, using humour, sharing personal anecdotes and earning trust with small gestures so that larger problems could be tackled later. 87 In interviews with five medical and seven mental health clinicians in PCMHs, Rajala88 found that capitalising on a patient’s relationship with their primary care office to connect them with mental health services was one of the largest factors in increasing patient engagement and access to mental health care. 88 By making their health care more coproductive, a US learning network (ImproveCareNow) of patients and health workers increased remission rates from 60% to 79% for children and adolescents with irritable bowel disease. 101
Six qualitative studies (four of PCMHs,87,91,93,94 one of mental health services in the USA66 and one of a geriatric network in France74) evidenced how MDT working produced patient self-care, activation and empowerment through social prescribing, integrating community and mental health into primary care teams and better informed physicians.
Social prescribing appeared to be more acceptable to patients than other prescriptions in the retrospective observational study of the EPSILON geriatric network in Versailles,74 with compliance rates of 72% for medical prescriptions, 74% for paramedical prescriptions and 100% for social prescribing. Focus groups with 387 people from 10 US communities found that patients appreciated PCMH models that included access to education, social and support resources (e.g. nutritionists, smoking cessation classes, exercise and fitness programmes, weight loss classes, meditation, counselling services, religious groups, peer-support groups) to help patients manage their care better. 91
Four qualitative studies in the USA also provided some evidence that integrating community and mental health workers into primary care teams produces better patient self-care, activation and empowerment. For mental health services, Briot et al. 66 found that integrating mental health professionals into the MDT promoted families’ capacity to mobilise themselves if a family member was in distress. A case study found that integrating CHWs into PCMHs is a means of providing support and education to hundreds of patients. 94 Furthermore, in the USA, Collinsworth et al. 83 found that these workers improved patient knowledge and activation levels, primary care providers’ ability to identify and address specific patient needs, and patient outcomes. Preventative care improved if a MCP-like model of primary care enabled CHWs to undertake disease/illness education, nutritional counselling, patient follow-up; to identify patient barriers to care or self-care, patient activation, social and self-management support (e.g. for diabetes mellitus control); to link patients to community resources; and to co-ordinate care. 83 These boundary-spanning roles directly facilitated patient activation through trust, cultural understanding, common language, manageable goals and a team approach and availability. They did so indirectly by making primary care clinicians more informed about patient goals and barriers and preparing patients more for meeting primary care clinicians. 83 Another study corroborated that MDTs improved patient confidence through making doctors better informed. In interviews with people working in PCMHs, Grace et al. 93 found that routine structured communication facilitated continuity of care and improved co-ordination among team members, which made physicians better informed on the status of shared patients. Well-informed physicians communicated more effectively with patients and increased patient confidence, trust and satisfaction. 93
Causal link 3:9 – contexts for patient engagement through multidisciplinary teams
Studies showed that patients’ own responses were a context determining whether or not MDTs succeed in promoting preventative care. At times, the expectation of greater involvement in their care could be a barrier to patient engagement and could create discomfort for them. The online focus group of care co-ordinators reported by Friedman et al. 87 highlighted patients’ lack of trust, insufficient understanding of the care co-ordinator’s role and inability to take responsibility for self-management of chronic conditions as barriers to improving patient self-care, activation and empowerment. Some patients who agreed to work with care co-ordinators continued to call multiple people in the clinic and attend the ED for needs best treated in the clinic. They ‘technically have a [care co-ordinator] but they continue to have fragmented care’. 87 Patients could feel scared to express their views in front of a MDT and, when asked, might interpret this as an admission from the MDT that they do not know what they are doing: ‘it’s tricky, you know – [clinician] was trying to be patient-centred, but [patient] didn’t have a context for it’. 88 When Rajala88 interviewed five medical and seven mental health clinicians in PCMHs, she found that patients were often surprised when a mental health provider was invited into their appointment, but typically came to appreciate it, for instance when mental health clinicians were introduced in terms of how they could help treat the patient’s particular symptoms. 88 However, some patients experienced integrated care as a loss of control over their information. 88
Our review also discovered evidence for additional causal links (Table 5) to those in the IPT in which MDTs are the mechanism to create change in other MCP components.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 3. MDTs are established | 4. Culture changes occur in the participating organisations | 3:4 |
| 5. Voluntary sector becomes involved in MCPs | 3:5 | |
| 6. Care co-ordination through IT use will develop | 3:6 | |
| 8. Demand management systems will develop | 3:8 | |
| 10. Care planning for individual patients will become more prevalent and systematic | 3:10 | |
| 11. More patients will be diverted from inpatient to primary care services | 3:11 | |
| 12. Patient experience will improve | 3:12 | |
Causal link 3:4 – multidisciplinary team working produces culture change in the health system
One Australian study62 concluded that MDT working has the potential to change the culture of the health-care system. This qualitative study62 of a chronic aged and complex care service model found that the creation or expansion of roles to work across traditional boundaries between other members of the primary care team instigated or enabled system-wide culture change through improving communication (formal and informal) between the various care providers.
Causal link 3:5 – multidisciplinary team supports voluntary involvement
Just one study76 suggested that MDTs encourage family and carer support for patient care. In five instances of MDT initiatives in the UK, enhanced access strategies of telephone MDT, Hospital at Home, and Advice and Guidance services produced better patient experience and less inconvenience and disruption for patients and families, and gave them extra skills and confidence to look after their unwell child without professional support. 76
Causal link 3:6 – multidisciplinary team working produces informational continuity of care
One quantitative descriptive research study100 of pharmacist recommendations and physician responses related to 954 complex patients in a PCMH found that MDT working produced better use of EHRs and electronic communication. However, for remote electronic communication to be successful, face-to-face contact was also needed to build the relationships required. 100
Causal link 3:8 – multidisciplinary teams produce better demand management systems
Three case studies in the UK76 and the USA66,102 provided evidence that MDTs could strengthen demand management systems and redistribute workload pressures across the care system. A multiple case study of five NHS vertical integration projects for paediatric/young persons’ services76 found that MDT working produced better gate-keeping and need- and/or risk-stratification. GPs with access to advice and guidance from a consultant developed specialist expertise and could manage more complex cases without referring to secondary care, thus easing the workload there. 76 A study of the American Veterans Health Administration (VHA)102 found that nurse visits in primary care were associated with a decreased risk of all-cause hospitalisation for veterans > 65 years of age. The Briot et al. 66 case study of MHI in Utah66 found evidence that MDT working redistributed workloads. When consultations were multidisciplinary, health professionals jointly put into effect care strategies individualised and co-ordinated (through a case manager) for the user and their family, using the family’s own health and social networks. That gave users good overall care by a better team at lower cost, reduced GP workload and freed specialists to support more severe cases. 66
Causal link 3:10 – multidisciplinary teams produce care planning at the patient level
Ten studies21,66,68,76,78,83,90,91,93,94 suggested that MDT working facilitated care planning at the patient level through the operation of boundary-spanning roles and by giving greater access to enhanced primary care.
Causal link 3:10 – care planning and boundary spanning
Nine studies21,66,68,78,83,90,91,93,94 provided further evidence that boundary-spanning roles facilitated many forms of care ‘integration’. These roles may be filled by care co-ordinators, nurse practitioners, CHWs and many other occupations. These roles increased awareness and use of care plans in the MDT, organising access to types of care that patients need and desire. 66,78,83,90,91 Alidina et al. 68 found that high performing PCMHs typically had at least one dedicated care co-ordinator position. Lower-performing PCMHs typically had none (care co-ordination responsibilities were shared between staff).
Several studies21,68,78,83,90,93,94 reported how MDT members in boundary-spanning roles helped co-ordinate the MDT to effect individualised care strategies co-ordinated around the patient and her family, make use of a health and social network to provide education for patients and their families, and put their counsellors at their disposal, providing the patient with good overall care, at the right moment, by a better team, at lower cost. Briot et al. 66 describe this in mental health services in Utah. Similarly, for physical health, boundary-spanning MDT members (e.g. embedded CHWs) with close contact with patients found what barriers to treatment patients faced, and communicated these barriers to other MDT members who could then work with patients to overcome them68,78,83,94 and increase the use of care plans (from < 5% to 39%94). Although the GPs in a study in Australia did not have the time or resources to deal with psychosocial aspects of patients’ health, the general practice liaison nurses were able to arrange case conferences between all necessary professionals and develop care plans for patients. 90 A case study of Buurtzorg21 found that, by working in this way, a MDT was able to deliver more person-centred care by allowing staff to organise care that made sense to them and the patient, which made them feel able to deliver good-quality, holistic care and allowed the MDT to organise itself so as to achieve the best possible outcomes for patients.
In the HealthOne Mount Druitt project (Australia), McNab et al. 90 found that primary care providers appreciated the familiar face and voice of the boundary-spanner, with whom they felt they could, over time, build an ongoing relationship of mutual trust. The boundary-spanners’ local knowledge of services and time to liaise with them benefited the GPs because it allowed more efficient and effective liaison than they could themselves provide and made a huge difference to service provision and support for chronically ill patients. 90 Many primary care professionals in a US study acknowledged spending more time co-ordinating care for patients before these roles were implemented, and saw the time savings as allowing them to communicate more effectively with patients. 93
Causal link 3:10 – multidisciplinary team working gives patients access to a wider range of primary care services
In their case studies of five integrated care initiatives, Woodman et al. 76 described how enhanced access strategies used by MDTs improved patient care. If MDTs discussed complex cases at high risk of needing secondary care by telephone each month, GPs became more motivated and confident to manage these patients, gaining skills and access to specialist support to do so, so that patients received higher-quality care from their GP. MDT members better understood their colleagues and service thresholds, established professional relationships and shared norms. Families perceived and patients experienced a more ‘joined-up’ health-care service, trusted the care they received from the GP, felt motivated to seek help from primary care, became confident in managing their own chronic conditions, experienced fewer exacerbations of chronic illness and experienced less inconvenience and disruption. 76 In a qualitative study in Australia, people working in new boundary-spanning roles were found to make a broader range of services available to patients through case conferencing, care planning, liaison and information provision, and being a single point of contact for GPs to access all the other services and professionals in the community. 90
Causal link 3:10 – contexts for multidisciplinary teams facilitating patient-level care planning
In the above studies, the most important context is case-mix. MDTs are particularly necessary for stimulating the use of individual care plans when patients have complex conditions about which clinicians have a lower level of knowledge than for more common conditions and for which reciprocal co-ordination of treatments is necessary. 68
Other contexts were similar to those facilitating MDTs in undertaking network-level care planning. One interview study of embedding CHWs in a CCM in the USA highlighted the importance of other team members’ trust in care co-ordinators as a context supporting better care co-ordination, but also noted that it could take a year of working together to establish this trust. 83 Primary care doctors said that they gained trust in the CHWs as they recognised their many competencies and saw the positive impacts that were had on patients. After recognising their value, these doctors sought to provide the CHWs embedded in a CCM with ‘plenty of support’ to address patients’ clinical needs and helping them to deal with challenging situations. 83 A qualitative study of five PCMH pilot sites93 found that primary care doctors did not always value the boundary-spanning roles. Some reported only ad hoc meetings with the boundary spanners to discuss specific complex cases, ambiguity about the appropriate tasks to delegate to them and indicated that more structured communication was needed. 93 Another study103 provided evidence that trust in the sense of willingness to delegate work within the MDT was another aspect of this context:
When we first started putting care co-ordinators in the offices, we got pushback from the doctors that we were taking away some of the things they do. But after they got familiar with it and realised that these aren’t things that you really need a medical degree for and it actually means that the minutes I’m in the room with the patient I can talk to the patient about their health, they were OK with it.
ACO interview, Shortell et al. 103
Limited evidence from two studies in the USA78,86 suggested that training of staff working in MDTs and care co-ordination roles supported patient-centred care. Hong et al. 86 described how ‘successful’ CCMs offered customised training, including didactic experiences, mentoring and shadowing. A uniform training and education platform for all new and existing care co-ordinators, irrespective of profession, was found in another large ACO to ensure a consistent approach to providing care co-ordination services to patients. Training, together with recruitment difficulties and the retention and cost of care co-ordinators were other barriers to MDTs’ care co-ordination work. 68
Causal link 3:11 – multidisciplinary teams divert patients from hospital to primary care
Two SRs104,105 and one quantitative study106 found that MDT working reduces hospital readmission rates. Two104,106 of these studies described the importance of specialist involvement in the MDT, and two105,106 described that of care co-ordinators. An umbrella review104 (a SR of SRs) of case management found that the CCM, discharge management, complex interventions, patient self-management and MDTs, particularly when they focused on one specific health condition [in particular heart failure and chronic obstructive pulmonary disease (COPD)] and included condition-specific specialists (medical, nursing, pharmacist), together decreased emergency admissions. Half of the SRs quantified the reductions, giving figures ranging from 25% to 43%. 104
In a quantitative study of PACT PCMH implementation by the US VHA, Nelson et al. 106 found that greater continuity of care (i.e. all other providers all working with and communicating with patient’s primary care provider) was associated with lower likelihood of hospitalisation and mortality. Nurse visits in primary care were associated with a decreased risk of all-cause hospitalisation for veterans > 65 years of age. 106 As less direct evidence, a SR105 of RCTs of transitional care interventions that aimed to improve care transitions from hospital to home and to reduce hospital readmissions for chronically ill patients found that a home visit within 3 days, care co-ordination by a nurse and communication between the hospital and the primary care provider were significantly associated with reduced short-term readmission rates. 105 Kinjo et al. 107 describes zaitaku primary care MDTs, as yet on a small scale, replacing hospital end-of-life care in Japan.
Causal link 3:11 – multidisciplinary team working diverts patients from inpatient to primary care services
Three studies21,66,103 found that, where MDTs enabled the flexible mobilisation of a range of professional expertise, training and knowledge, including from community providers, care was more centred around the patient’s goals and needs. In a historical narrative case study of a programme for clinical integration of mental health specialists with community primary care medicine, Briot et al. 66 found that the use of the MDT members adapted according to the severity and complexity of the pathology in order to cosupport in a scalable way. MDT care adapted flexibly to the service users’ mental and physical health, family circumstances, medical and social comorbidities and fed into the provision of specialised care. 66 A similar example was Buurtzorg (the Netherlands), which used self-managed teams to produce and plan patient care, with teams of 8–12 nurses and nurse assistants covering a geographical patch that they themselves choose. 21 A mixed-methods study of 11 purposively sampled ACOs in the USA103 provided another example of how flexible mobilisation of community resources by a MDT supported patient-centred care and, thus, reduced demands on hospitals: a physician-led ACO network in the north east of the USA used an interdisciplinary care team to work with patients with complex needs. One was a patient:
. . . who went 132 times in 12 months to the emergency department. She is . . . in a wheelchair . . . lives in a house with no ramp. She doesn’t have much social support, doesn’t have any food. A diabetic, out of control. She doesn’t have a refrigerator for insulin. From one visit, we engaged our team of care management [who] . . . built her a ramp, donated a refrigerator, and hooked her up to an equivalent of Meals on Wheels so she has food, and arranged for transportation to get her to regular visits to her primary care physician. And in the past ten months . . . she’s not been back [to the ER] one time.
Shortell et al. 103
Causal link 3:12 – multidisciplinary team improves patient experience
Our secondary evidence suggested that MDTs that included pharmacists, nurses and CHWs can improve patient experience, outcomes and continuity of care. We found some evidence that MDT working improves patient experience through boundary-spanning roles (see Causal link 3:7 – boundary-spanning roles), enhanced access to primary care (see Causal link 3:10 – multidisciplinary team working gives patients access to a wider range of primary care services), better communication between providers and, thus, more patient confidence, trust in, and satisfaction with, care. Twenty-eight patients and informal caregivers and 20 health-care providers in community-based PHC in Canada described MDTs as providing a holistic care experience to their patients. 108
A virtual MDT (team members linked remotely by telephone or HIT) and hospital-at-home schemes produced better patient experience with less inconvenience and disruption for the patient and family receiving paediatric health care. 76 In ‘successful’ US primary care–integrated complex care management programmes, the MDTs’ key role was to build trusting relationships between patients and families, and primary care providers and their staff. 86 Routine structured communication in MDTs facilitated continuity of care and co-ordination so that the doctors were better informed on the patients’ status and thus communicated more effectively with patients, which increased patients’ confidence, trust, and satisfaction. 93 In another study, patients indicated that the boundary-spanners were able to bridge the gap between them and the doctors by talking to them on a level they understood, understanding cultural barriers and patiently answering questions. 83 Conversely, a sample of US patients said that a lack of boundary-spanning MDT members tended to leave patients lacking understanding about what was going on with their care, feeling left out of the dialogue and decision-making and feeling vulnerable as a result of their uncertainty. 91 In the Australian HealthOne Mount Druitt project, boundary-spanner care co-ordinators made patients feel more supported and less anxious and thus reduced hospital visits. 90
A reanalysis of administrative data in PCMHs and ACOs that involved pharmacists80 found that pharmacists identified 708 drug therapy problems through direct patient care (336/708; 47.5%), population-based strategies (276/708; 38.9%) and education (96/708; 13.6%). Pharmacists combining academic detailing with direct patient care and population-based medication management probably helped to optimise patient outcomes. 80 Woodman et al. 76 found that UK nurses making home visits in a Hospital at Home team improved child safeguarding and heightened awareness and paediatric referral to all community nursing services. In two cases, informants reported that commissioners and providers had warned of potential harm to children. 76 Similarly, in a study of PCMH implementation by the American VHA, nurse visits in primary care were associated with greater continuity of care and lower mortality rates among a patient cohort. 106 Interviews with CHWs, patients and primary care providers in CCMs found that CHWs facilitate trust, communication, understanding of roles and PCP support, leading to such patient outcomes as improved glycated haemoglobin (HbA1c) control. 83
Culture change
The IPT assumed that culture changes in the participating organisations in a MCP were a mechanism by which to produce MDTs, demand management systems and preventative care (Table 6). We also found evidence for causal links not in the IPT in which culture change was the mechanism (see Table 7). We first describe evidence for the causal links in the IPT and then evidence for the new causal links.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 4. Culture changes occur in the participating organisations | 3. MDTs will develop | 4:3 |
| 8. Demand management systems will develop | 4:8 | |
| 9. Preventative health care will develop | 4:9 | |
Causal link 4:3 – if culture changes occur in the participating organisations, then multidisciplinary teams will develop
The programme theory first assumed that a shift in the culture of care delivery organisations and professions would include shifts in their assumptions about desirable models of care, interorganisational and interprofessional working practices, all of which would produce workforce development and engagement in ways that promoted the development of MDTs.
Causal link 4:3 – workforce development and engagement
Two studies provided evidence that culture change supports different professionals to work together across disciplinary boundaries. Greene et al. 89 conducted qualitative interviews with, and a survey of, providers and staff in mental health and paediatric PCPs in the USA and found that culture change enabled new ways of working and communicating that dismantled a key barrier to collaboration, including improving shared expectations, increasing awareness of what other professionals within the wider care team have to offer and building better understanding of the culture of other professions. 89 Conversely, Bergman et al. 92 interviewed key informants in PCMH and team-based care models and found that those working in more traditional roles can feel defensive around their boundaries and roles and that their expertise or specialism is under threat. 92 Interviews with five medical and seven mental health workers (PCMH, USA) showed that the latter could also be culturally resistant to practising in an integrated model. 88
People working in new boundary-spanning roles may attempt to prevent other team members from feeling threatened by their recommendations or opinions by using indirect, non-threatening forms of communication such as gentle hints, suggestions and questions like ‘are you sure that’s really what you wanted?’. These indirect communications risked important information not being effectively communicated in safety-critical situations. 92 Bergman et al. 92 concluded that reducing these risks and helping the new roles become a driver for culture change could occur by:
-
creating a culture of openness (feeling comfortable speaking up to reduce error when problems are suspected) through training to improve communication across hierarchies, for example the Crew Resource Management training adopted in some US medical and pharmacy training programmes109–111
-
agreeing, at the outset of their collaboration, clear (e.g. written) scopes of practice between different professions, to cultivate awareness and shared expectations of each other’s duties and responsibilities. 92
Two studies found that respect could overcome or bypass the perceived threat of new boundary-spanning roles. McNab et al. 90 found that other members of certain primary care teams came to respect people in boundary-spanning roles when they saw the latter changing culture. That respect enabled further culture change by supporting formal and informal communication between the various clinicians. 90 Bergman et al. 92 provided an example: when primary care doctors working with pharmacists in new expanded roles were exposed to situations in which their opinions conflict, they came to recognise that the pharmacists were ‘usually right’ (about pharmacy-related matters), learned to respect them and see value in their expanded role, which facilitated multidisciplinary working. 92 Producing trusting working relationships between primary health-care doctors and people in boundary-spanning roles has been found to take around 1 year. 83
Causal link 4:3 – ‘joined-up’ working
A web-based survey of ACOs, in the USA, found that shared culture was necessary for their success. 58
Two studies offered evidence about how to create culture change so as to improve primary care teams’ integration. In their qualitative interviews with, and a survey of, staff in mental health and paediatric PCPs in the USA, Greene et al. 89 found that shifting shared expectations, improving awareness of other professionals’ roles in the primary care team and understanding the culture of other professions enabled ways of working and communicating to change, dismantling a key barrier to collaboration. 89 The Weldon et al. 112 study found that sequential sequencing workshops, in which workshop participants’ experienced and then discussed in groups ‘real world’ examples of their role within the health-care system and how what they did impacted on collaborative (person-centred co-ordinated) care, improved staff knowledge and understanding of the impact on collaborative care. In one workshop with GP receptionists in the UK, a new professional structure for GP receptionists appeared to be emerging, with receptionists empowered to see the importance of their role within the wider context of health-care system, as well as how crucial they were for integrated care to work. 112
McNab et al. 90 found that in systems in which there is no system-wide culture change in support of integrated multidisciplinary working across teams embedded in the partner organisations and established throughout the primary health-care sector to support integrated MDT working, there remained a heavy dependence on leadership from the GPs and CHWs on the network steering committee. 90
Causal link 4:3 – contexts for culture change producing multidisciplinary teams
The same study found that one way to support the above culture changes across professions was by creating boundary-spanning roles that themselves instigated or enabled system-wide culture change by improving both formal and informal communication between the various care providers. 90 Wholey et al. 67 argued that tasks are the functions that a team has to perform to achieve its goals (e.g. care co-ordination) and so they, and not culture, are the logical starting point for MDT design. Chapter 7 considers this apparent contradiction more closely.
Interviews and a survey in 13 PCMH practices in the USA found that existing cultures of individual excellence, individual accountability and established practice norms were an obstacle to collaboration. 68
Causal link 4:8 – if culture changes, then demand management systems develop
We found no published research about whether or not or how culture change in an integrated model of care makes demand management systems develop.
Causal link 4:9 – if culture changes, then preventative care develops
We found a little evidence from one study that culture change increased preventative care. In a SR, the creation of a non-intimidating environment/culture was reported to be an enabler of improvements in patient knowledge, self-care behaviour and self-efficacy. Busetto et al. 113 reported that a CHC collaborative could not have led to increased patient self-management without changing the health centre philosophy towards more patient-centredness and empowerment. However, another study suggested that other, less resource-intensive mechanisms for improving prevention may be more acceptable and feasible. 79 None of these studies described what contexts (in the realist sense of the term) were required.
Beyond the IPT, the secondary literature reported further ways in which culture change might be a mechanism for creating change in other MCP components (Table 7), although none of the studies found stated what contexts (in the realist sense) were required.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 4. Culture changes occur in the participating organisations | 7. Planned referral networks will develop | 4:7 |
| 12. Better patient experience, outcomes and staff well-being | 4:12 | |
Causal link 4:7 – if culture changes occur in the participating organisations, then planned referral networks develop
As noted, the Australian study by McNab et al. 90 found that culture change itself resulted in part from introducing boundary-spanning work roles, but also that a wider culture change was needed to ensure that practices and processes are embedded in the member organisations of a MCP-like network, including, by implication, any interorganisational referral networks. Two further studies88,89 reported the particular need, in setting up ACOs, to have a cross-cultural dialogue between medical and mental health providers.
Causal link 4:12 – if culture changes occur in the participating organisations, then there will be better patient experience and outcomes
Demiris and Kneale’s85 narrative literature review found that implementation of patient-centred care depended on culture change in health-care organisations and among health-care consumers. In their SR, Busetto et al. 114 reported a study by Borgermans et al. in which the presence of interdisciplinary diabetes mellitus care teams was associated with significant improvements in HbA1C and low-density lipoproprotein (LDL)-cholesterol levels, and increased statin and anti-platelet therapy use, which were attributed to the quality and task orientation of the teams, shared leadership and shared group norms. Busetto et al. 114 also reported a study in which Yu and Beresford found three critical success factors for their chronic illness model that led to improvements in HbA1c, blood pressure, LDL and urine albumin-to-creatinine ratio, namely (1) leadership commitment to change, (2) increased clinical staff involvement and (3) residents acting as change agents. They also found that the same shift in the culture produced a non-intimidating environment that facilitated better-co-ordinated patient-centred care and improved health workers’ mental health and well-being. 114
Voluntary sector involvement
The IPT assumed that voluntary sector involvement in MCPs (Table 8) would produce better demand management systems (component 8), better preventative health care and improved patient experience of care.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 5. Voluntary sector becomes involved in MCPs | 8. Demand management systems will develop | 5:8 |
| 9. Preventative health care will develop | 5:9 | |
| 12. Improved patient outcomes and experience of care | 5:12 | |
Causal link 5:8 – if there is voluntary sector involvement in multispecialty community providers, then demand management systems will develop
Noël et al. 79 found that community linkages are utilised less often than the other components of the CCM. Bodenheimer et al. (2002; reported in Lafortune et al. 108) described linkages between clinical settings and community health resources as highly important, particularly for health-care professionals who are not operating as part of a large team-based organisation and for those treating patients with chronic illness (and, we add, may, in the USA, have difficulty obtaining health insurance). These apart, we found no studies reporting how, or even if, voluntary sector involvement in MCP-like networks helps them manage the demand either for hospital services or for formal primary care services, carers or voluntary organisations.
Causal link 5:9 – if the voluntary sector becomes involved in multispecialty community providers, then preventative care will develop
Although the MCP programme theory emphasises access to a wide range of resources around a person’s goals, studies reporting whether or not and how voluntary sector involvement strengthens preventative care were sparse. From focus groups with 387 participants in 10 US communities, Mead et al. 91 described the barriers to involving the voluntary sector in the PCMH model. Despite patients reporting a need for community resources, such as education classes, diet and exercise groups, and peer support groups to provide additional support to help them deal with the burdens of managing chronic illness, the PCMH model was limited to formal services within the health-care system and lacked having processes to support, pay for or even refer patients to resources outside the health-care system that could be useful for their health. Hence, this paper suggests that providers who treat disadvantaged populations need training to develop relationships with service providers that will take on low-income, uninsured or underinsured patients, and to be innovative. Understanding each patient’s personal constraints and not just the typical medical history is a critical aspect of patient-centred care but is not highlighted as a key component of the PCMH model. Participants highlighted the importance of religious organisations and community-based organisations and cited several examples of how these resources provided important support in the managing their overall health and well-being. 91 A qualitative study of community-based PHC in Canada found that self-management support groups and resources allowed patients to be more engaged in maintaining their own health and helped to prepare them for discharge or care transitions. 108 Neither study reported what contexts (in realist terms) favour voluntary sector involvement.
Causal link 5:12 – if voluntary sector becomes involved in multispecialty community providers, then patient outcomes improve
We found little evidence consistent with the IPT that involving the voluntary sector in MCP-like networks might improve patient outcomes. In comparative case studies of a German scheme (Kinzigtal), a Netherlands-wide programme (one care group) and 16 English pilot schemes, Busse and Stahl64 report that, in the Kinzigtal care model, multisectoral collaboration had, after 2.5 years, reduced mortality rates by half (from 3.74% to 1.76%) for those enrolled in the programme compared with those who were not. Although the network had voluntary sector input, these results are attributed to the network as a whole, leaving it uncertain whether or not the voluntary sector input contributed to these mortality improvements and, if so, to what extent and through what mechanisms and contexts. 64
Care co-ordination through health information technologies
The IPT assumed that care co-ordination through HITs was a mechanism for producing MCP components 7, 10 and 11 (Table 9).
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 6. HITs are used to strengthen informational continuity of care | 7. Planned referral networks will develop | 6:7 |
| 10. Care planning for individual patients will become more prevalent and systematic | 6:10 | |
| 11. More patients will be diverted from inpatient to primary care services | 6:11 | |
Causal link 6.7 – if health information technology is used to strengthen informational continuity of care, then planned referral networks will develop
Many MCP-like organisations used HIT effectively. A national survey of US physicians found that EHR use in ACO or PCMH settings was associated with increased activity in health management at population level, quality measurement, patient communication and care co-ordination. Two other studies (reported in King et al. 115) found that PCMH doctors who used EHRs had ‘greater quality improvements and changes in utilisation over time on some measures’. One SR (Fontaine et al. , reported by Lafortune108) found evidence that electronic health systems could function as a way to improve patient safety, reduce medical errors, improve access to data and decrease staff time spent on administrative tasks. Through semistructured interviews with physicians in PCMHs, Petersen et al. 82 found that well-designed EHRs allowed them to better co-ordinate care and share information. Links with hospitals were also important. A study of six ACOs found that timely, consistent information about patients’ admissions and discharge enabled the planning of follow-up services that patients might need within 30 days of discharge. 63
Two additional studies described individual projects that effectively used HIT to co-ordinate care in MCP-like contexts. The Gesundes Kinzigtal project reported improved patient and health-worker experience, and reduced costs and mortality. The project relied on sharing an EHR system across providers to co-ordinate care. 64 Buutzorg used a simple, web-based solution designed by nursing assistants, nurses and back-office employees to communicate and share information in real time between locations such as the patient’s home, in the office or on the road. 21
However, in many cases, HIT systems that had not been carefully designed and implemented hindered health professionals in communicating and sharing information. Recurrently reported barriers to effective HIT implementation included lack of interoperability between HIT systems [see Causal link 6.7 – lack of interoperability between health information technology systems (both within and between organisations)], lack of necessary data analysis tools (see Causal link 6.7 – lack of necessary data analysis tools), lack of workflow tools (see Causal link 6.7 – lack of workflow tools) and the limitations of current technology (see Causal link 6.7 – limitations of current technology).
Causal link 6.7 – lack of interoperability between health information technology systems (both within and between organisations)
Almost every study discussed the importance of HIT connectivity both within and between provider organisations. Participants across different studies emphasised the importance of using a common health information system between services or redesigning systems so that they communicated with one another. 88,98 In many cases, HIT systems within an organisation were flawed. Two studies described how care managers needed to use a completely separate system from physicians, resulting in clunky ad hoc systems to collect and manage data. 87,116
Causal link 6.7 – lack of necessary data analysis tools
Research participants across studies lamented the inability of their IT systems to do basic data analyses such as risk-stratifying patients, tracking subpopulations of patients, determining which patients need follow-up, generating relevant reports and tracking hospitalisations. 116
Causal link 6.7 – lack of workflow tools
Many studies78,108,116,117 reported that health workers wanted an IT system that would track patients more effectively. A number of studies mentioned such tools as task management systems, care planning systems, standardised care pathway templates for physicians, notification systems for changes in patient status and individual patient tracking through the health-care system. A recurring frustration was an inability to get the right information at the right time, which resulted in participants assembling ad hoc systems to piece together different software systems to generate needed reports. Richardson et al. 117 describes the ‘shadow system’ of data captured through ‘homegrown’ methods, which was often used when EHRs failed to adequately meet an organisation’s needs. 87
Many organisations reported difficulty in extracting and piecing together data even from EHRs that complied with the continuity of care document standard for interoperability, suggesting that these standards may be insufficient for MCPs’ needs. 117
Causal link 6.7 – limitations of current technology
Two studies discussed the limitations of current HIT for MCP purposes. Bauer et al. 118 noted that traditional HIT tools were not built to monitor populations and subpopulations of patients, actively flag patients for follow-up or to respond to real-time data on patient progress. Rudin and Bates (reported in Richardson et al. 117) concluded that the current HIT marketplace ‘has failed to provide adequate solutions’ for care co-ordination. Another study68 noted that PCMHs tended to use IT systems for more straightforward uses, but more complex patients were dealt with offline owing to underdeveloped technologies. Finally, one study119 found that having an EMR did not automatically improve care co-ordination. These two studies suggested that previous generations of EHRs may not be suitable for new models of care, as the difficulties that many organisations have faced in implementing them symptomatised.
Many studies in our review corroborated that integrated IT systems alone would not lead to co-ordinated care systems. Other mechanisms, such as reworking staff roles and a shared physical space, were also likely to be required.
Several studies specified staff attitudes and skills that were important for a successful EHR. One SR114 found that personal barriers to integrated care interventions included staff reluctance to use HIT, unawareness of system features, unwillingness to share data and lack of IT skills. Another paper92 implied that the structure of the EHR inadvertently made it a battleground between physicians and pharmacists. The same study92 found that 68% of clinical pharmacists who were surveyed in the PCMH context referred to examples of problems with electronic communication in their relationships with primary care physicians.
Many studies emphasised the importance of task delegation, workflows and routines. One study116 found that primary care teams that used EHRs consistently for data entry and agreed on communication methods between staff members were more likely to score high on the National Committee for Quality Assurance (NCQA) 2011 PCMH recognition tool. Best practice in the use of EHRs to facilitate communication between staff members included access to patient information for all staff members, instant messaging, within-chart notes, telephone templates that could be routed to team members’ inboxes, task assignments and ‘huddle sheets’ for the day embedded in the EHR. 116
Finally, care managers emerged as important facilitators of effective HIT use. Morton et al. 120 found that practices with a non-clinician member of staff who was responsible for co-ordinating care were much better at care co-ordinating activities and conducting these activities electronically. Other studies118 also noted that the care manager provided much-needed support to ensure smooth operations.
Causal link 6:7 – contexts
Overall, the evidence suggested that HIT systems can support communication and data sharing between health professionals at MCPs, but only provided that these HIT systems be designed and implemented with care. Otherwise, they risked being a barrier to effective MCP working.
Causal link 6:10 – informational continuity of care produces care planning at the patient level
The evidence from our review supported the assumption that EHR systems, when set up to support co-ordinated care processes, can improve patient outcomes. Two different studies85,114 reported in SRs found that effective EHR use enabled teams to increase quality of care for diabetes mellitus patients. Several studies85,118,121 have also found that electronic patient registries can improve patient quality outcomes.
Across studies, participants agreed on features that increased the effectiveness of EHRs for patient care. A recurring theme was the importance of using the EHR to guide physician practice and workflow, and provide reminders for actions. One practice used 200 different symptom-specific templates. The template system increased productivity and allowed physicians to focus better on patient needs during their appointment. 116 Xenakis’s study78 corroborated this, and participants in other studies108 lamented the lack of a template system in their EHR.
Other EHR features that study participants repeatedly requested included the ability to create care plans (recording goals, barriers and specific steps to that goal) and notification systems to help staff engage patients when the patient’s status changed (e.g. following hospital admission, no-show at follow-up appointments). 116,117 Yet, despite widespread agreement about the characteristics of an ideal EHR, we found only limited evidence of its ability to improve patient care, probably because many provider organisations did not yet have the requisite features for its optimal use.
Many studies described patient-facing electronic tools. One SR85 found very little evidence that an electronic personal health record (accessible by patients) increased care outcomes, care co-ordination or patient engagement. By contrast, another found that patients had very positive responses to a patient portal. 103 These contradictory findings may be explained by the slow uptake of personal health records, and a lack of studies connecting personal health records to patient outcomes. 85 Another study (reported in Bauer et al. 118) described technology-enabled delivery of mental health interventions, such as mobile devices assisting self-management. However, the same study cautioned that patient-facing tools are most effective when combined with a relationship with a health worker, such as a counsellor. Using technology to build a relationship can provide more accountability and support patient engagement, whereas stand-alone interventions require patients to be much more self-motivated. 118
Overall, there is evidence that EHRs can improve patient outcomes, but only when they include robust functionality such as care planning and tracking population- and individual-level data over time. However, HIT use alone does not guarantee improved care. Instead, HIT, whether an EHR or patient-facing tools, must be carefully designed to complement interpersonal relationships.
Causal link 6:11 – informational continuity of care helps divert patients from hospital to primary care
Our review found mixed evidence for the assumption that effective use of data in MCPs can lead to reduced unnecessary A&E admissions. Kaushal et al. 122 found no difference in ED visits and hospital admissions, or hospital readmissions between PCMH and non-PCMH settings over a 3-year study period. However, other studies found mixed or inconclusive evidence. Two studies reported by Demiris and Kneale85 found opposing results for emergency admission rates for home telehealth programmes but not in MCP-like settings. Two additional studies found weak evidence. One survey56 found that ACOs were slightly more likely to track inappropriate ED use than their non-ACO counterparts, whereas another123 concluded that PCMHs with patient registries have the potential to use data to reduce unnecessary ED admissions. The same study recommended network analysis for tracking patients’ movement between providers so that care and resources can be better co-ordinated, possibly leading to reduced admissions.
This review also found evidence for additional outcomes of HIT beyond those in the IPT (Table 10).
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 6. HITs are used to strengthen informational continuity of care | 3. MDTs will develop | 6:3 |
| 8. Demand management systems will develop | 6:8 | |
| 9. Preventative health care will develop | 6:9 | |
| 13. NHS costs will reduce | 6:13 | |
Causal link 6:3 – information continuity of care produces multidisciplinary team working
Although most research on MDT working focused on face-to-face meetings rather than virtual communication,85 several studies noted that well-designed HIT can support effective communication both within organisations and across service providers. 73
We found examples of HIT supporting relationships between physicians and pharmacists, for instance of pharmacists having shared access to the EHR to approve drug requests or, in one case, select patients for further physician screening. 80,100
Many studies noted the importance of creating a shared understanding between staff about routines, roles and processes. Some studies reported confusion as to the proper use of the EHR:
. . . like tasks you put in the EMR [electronic medical record], where do you put it, how do you write it, what do you say, what language do you use, what format, all that stuff.
Rajala88
Other studies85,88,116 reported best practices that worked in particular organisations, such as the ability to send instant messages for informal communication (e.g. for a ‘warm’ hand-off), creating task lists and delegating roles in the EHR, ability for notes to be embedded in a patient chart, telephone templates that could be routed to team members’ inboxes, virtual ‘huddle sheets’ with patients scheduled for the day in the EHR. Systems for accomplishing this shared understanding varied between practices, but all studies emphasised the importance of being able to communicate informally through the EHR and for each staff member to use the EHR consistently.
There were many examples of positive working relationships facilitated through EHRs in the evidence. However, these relationships may be strengthened through opportunities for in-person communication and free-text notes built into the EHR.
Causal link 6:3 – contexts
Using HIT to mediate interprofessional relationships must be done carefully. Bergman et al. 92 describes a particularly complex EHR causing poor relationships between physicians and pharmacists because they negotiated drug approval requests without the support of informal communication (such as free-text explanations for approvals or rejections). Conversely, personal relationships, for example team huddles or informal chats, could make virtual communication more effective. Two studies83,116 emphasised the importance of primary care staff being able to communicate both online and offline.
Causal link 6:8 – informational continuity of care produces demand management systems
One study96 in our review found that one attribute of a successful ACO programme is that it can stratify patients by risk. Many MCP-like networks and organisations did so (although the methods and risk groups differed) but most did not report whether or not this helped providers to manage resources better. 64 However, the Mount Sinai (New York) ACO did report successfully using risk stratification data to guide staff workflow in different ways depending on identified care gaps and whether risk was categorised as high, rising/moderate or low. 78
Causal link 6:9 – informational continuity of care produces preventative care
Care processes in ACOs or PCMHs were more likely than those in their standard counterparts to:
-
create lists of patients due for tests or preventative care, and
-
provide patient reminders for preventative follow-up care.
Accountable care organisations and PCMHs that used EHRs were more likely than providers to carry out these tasks without such records. 115 Xenakis78 described an ACO with workflows in its EMR to support disease prevention, and Johnson et al. 124 described a case in which patients received automated text message reminders about recommended preventative services. Overall, there was a little evidence to support the assumption that HIT and EHRs can assist preventative care, but more research is needed to test these claims.
Causal link 6:13 – informational continuity of care produces cost savings
We found some evidence that HIT can increase organisational efficiencies. Colla et al. 73 concluded that ACOs with HIT investment was likely to save post-acute care costs. Another study108 found that telemedicine-based collaborative care was more cost-effective than a practice-based model in medically underserved areas. Several studies found that HITs could increase administrative productivity, thereby saving costs. A qualitative survey116 of PCMHs found that electronic systems reduced administrative burden and increased data accuracy for physicians when teams had specific role definitions stating who recorded what onto the system and how they recorded it. One prospective cohort study122 described how IT in the PCMH context led to a reduction in specialist visits.
Although these studies describe MCP-like organisations or networks using HIT to reduce costs, few of them clearly explained the links between the two. Overall, they suggested that organisations can reduce costs through using EHRs but only in certain contexts.
Causal link 6:13 – contexts
Colla et al. 73 found that HIT investment probably saved post-acute care costs, but this finding reflects a context of US incentive structures that reward or penalise ACOs according to their costs, and in which private hospitals can make large investments in data analytics. A multisite ethnographic study found that organisations that used a combination of electronic and paper chart systems increased the time demands on staff, suggesting that IT systems need to be fully electronic to be cost-effective (McMurray et al. , reported in Lafortune108) and not duplicated through a shadow paper system of files.
A common context for the above mechanism (HIT) to bring about the other MCP component outcomes described was that HIT must be well designed and mirror the care processes that health workers use in practice. It was consistently reported that technology that was bespoke to the organisation(s) and designed with the users in mind had better outcomes on a variety of measures.
Planned referral networks
Next we consider the causal links in Table 11 in which the mechanism is planned referral networks.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 7. Planned referral networks develop | 8. Demand management systems will be strengthened | 7:8 |
| 9. Preventative health care will develop | 7:9 | |
| 10. Care planning at individual patient level will become more prevalent | 7:10 | |
| 11. More patients will be diverted from inpatient to primary care services | 7:11 | |
Causal link 7:8 – if planned referral networks develop, then demand management systems will develop
We found no evidence to support (or refute) the IPT assumption that referral networks produce better demand management systems, nor did we find any evidence as to whether or not referral networks produce preventative care.
Causal link 7:9 – if planned referral networks develop, then preventative health care will develop
Shortell et al. 103 reported a respondent from an American ACO saying that installing a patient portal had made patients more willing to ‘engage’ with planning their own care. That finding would be relevant to this link only if ‘engaging with care’ included ‘engaging with preventive care’, which the article does not report. Thus, we found no evidence unequivocally corroborating this link.
Causal link 7:10 – if planned referral networks develop, then care planning for individual patients will become more prevalent
We found some evidence that establishing a referral network produces greater use of care plans and more patient-centred care generally.
Colla et al. 73 evaluated the impact that ACOs had on care co-ordination and care management for older populations by exploring the extent to which ACOs incorporated post-acute care into their referral networks. Although the associations were not all statistically significant, Colla et al. 73 concluded that doing so resulted in more comprehensive CCM programmes, and the creation of systems to assure smooth transitions of care across different organisations and settings (ACO, USA). ACO referral networks that included post-acute care services were more likely than those without to have established processes for identifying, counselling and planning for end-of-life care across settings of care. 73
Alidina et al. 68 carried out a mixed-methods study of 13 PCMH ‘medical neighbourhoods’ (local referral and care co-ordination networks of PCMHs) to understand what role co-ordination mechanisms play in them. These networks used communication, negotiation and decision mechanisms through which neighbouring PCMHs agreed how to co-ordinate care and explicitly allocated mutual responsibilities for communication and care co-ordination for shared patients. Such mechanisms included care compacts and agreements negotiated through local Independent Physician Associations. High-performing PCMHs typically had written care compacts with specialists, low-performing PCMHs did not. 68 For care co-ordination at patient level, the most important activities were interorganisationally agreed common working routines, information connectivity and (again) the creation of boundary-spanning roles. A combination of these mechanisms, adjusted to the contextual conditions noted in the following subsection, could improve interorganisational care co-ordination. 68 There was a little evidence from a qualitative study of ACOs and PCMs that care was more patient-centred when referral networks existed. 73
Causal link 7:10 – contexts
As previously noted, Alidina et al. 68 provided important information about what contextual factors call for communication, negotiation and decision mechanisms. These referral network mechanisms are more necessary for patients about whose condition staff have low levels of knowledge, for more complex patients and when reciprocal co-ordination is required (i.e. patients transfer from one organisation to another and back again). Barriers to establishing care compacts were geographical (small or isolated communities), small general practices (small referral base), misaligned payments and time costs (e.g. search costs to find ‘good neighbours’, bargaining and decision-making costs, time to build relationships and costs of internal reorganisation). 68
In a qualitative study of integrating mental health into a primary care setting under the PCMH model, Rajala88 described the operational barriers to care co-ordination through a referral network in the PCMH in the USA: providers having different workflows and expectations, separate medical records or limited access to records and a separate referral process for mental health services. The last barrier resulted in long waiting lists, poor follow-up and less patient centeredness. If mental health services functioned as their own separate subsystem within primary care, there was increased difficulty co-ordinating services. 88
Patients’ own behaviour may be another relevant context. A cross-sectional national survey73 of ACOs defined self-referral as an indicator of ineffective care co-ordination. It found that the trend in the weighted absolute number of self-referred visits among Medicare and private-insurance beneficiaries remained generally stable from 2000 to 2009. Aliu et al. 125 concluded that, whatever attempts ACOs had made at care co-ordination, patients had bypassed them by making self-referrals as well.
Causal link 7:11 – if planned referral networks develop, then more patients will be diverted from inpatient to primary care
Five studies62,73,105,119,126 provided evidence supporting the IPT that referral networks can divert patients from inpatient care. Reassigning care to the PCMH enabled primary care teams to take on additional tasks, reducing specialty visits for low- and, to a limited extent, medium-morbidity patients. 127 In a cross-sectional analysis of the national survey of ACOs, Colla et al. 73 found that ACOs that included post-acute care providers were more likely than those that did not to report a fully developed programme to reduce preventable hospital readmissions. The six components of Wagner’s CCM are (1) community resources and policies, (2) health care organisation, (3) self-management support, (4) delivery system design, (5) decision support and (6) clinical information systems. Five studies28,30,31,49,51 reported in a SR of integrated care models for patients with chronic diseases found that projects that incorporated at least two of the six components had significantly fewer admissions and fewer inpatients days than other integrated care projects. 126
Huber et al. 128 attributed reductions in the likelihood of hospital admission for cardiovascular and COPD, but not respiratory disease, patients to the introduction of care co-ordination and care guidelines in Swiss primary care networks.
A case study of a complex care referral network in Australia62 found that it increased referrals across organisational boundaries and reduced ED use. Referrals to physiotherapy, podiatry, occupational therapy, dietetics and psychosocial services rose and there were fewer referrals to less specialised community home nursing. People enrolled into the programme with chronic and complex conditions had, in the following 12 months, fewer ED presentations and significantly reduced lengths of stay in the ED compared with the 12 months before. Almost 30% of participants had no hospital presentations. 62 In Canada, a cross-sectional patient experience survey99 for ambulatory sensitive conditions found that FCA and AA created a referral network in which care planning process dealt with preventative and acute emergency care in ways that diverted patients from acute secondary services. 99 A meta-analysis of RCTs105 found that transitional care interventions were associated with reduced intermediate-term (31–180 days) and long-term (181–365 days) all-cause hospital readmissions of chronically ill patients.
A set of UK case studies76 highlight reduced tariff income for NHS hospitals when patients were diverted from hospital to primary care as a barrier to patient diversion but, against this, patient diversion was also an opportunity for the hospitals (in any event not lacking work) to free up staff time and beds and meet performance targets. One enhanced access initiative (advice and guidance) created additional capacity in outpatient hospital clinics, which could help hospitals reach performance targets as well as generate additional income by hosting tertiary care clinics. In another example, clinicians spent less time transferring patients to other hospitals after the hospital at home initiative reduced admission and length of stay for other patients. 76
We found only one study104 about whether or not in-reach into hospitals ensures timely discharge of patients, but it was a SR. It found that transition from hospital to home was most effective when interventions to expedite it were initiated during the inpatient phase and continued post discharge.
Causal link 7:11 – contexts
Two studies71,127 stated a specific contextual requirement for referral networks to work as a mechanism for diverting patients from hospital to primary care. The mechanism worked best for high users of acute care and for low- and medium-morbidity patients who could be effectively managed in the community, but high-morbidity patients still required more intensive comanagement by primary care teams and specialists. An analysis71 of routine administrative data from 380,000 records for high users of A&E living at the poverty level found that referral network schemes for diverting patients from hospital had the greatest effect when targeted on the 1% of heavy users (five or more hospitalisations per year). Another study,127 a 48-month interrupted time series from a baseline through PCMH implementation and post-implementation periods for 36,805 hypertension patients, also found reductions in specialist use, but only for low- and medium-morbidity patients. Indeed, high-morbidity patients made significantly increased use of specialist use after PCMH implementation. The study authors127 concluded that referral networks between primary care teams and specialists in the ‘medical neighbourhood’ should cater above all for high morbidity, clinically complex patients. The increased referrals of high-morbidity patients highlighted that primary care teams and specialists also need to sustain effective comanagement of these patients.
Demand management systems
Table 12 lists the causal links in which demand managements systems are the mechanism.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 8. Demand management systems are established | 9. Preventative health care will develop | 8:9 |
| 10. Care planning at individual patient level becomes more prevalent | 8:10 | |
| 11. More patients are diverted from inpatient to primary care services | 8:11 | |
Causal link 8:9 – demand management systems enable preventative care and vice versa
Mead et al. 91 reported evidence from 10 focus groups (n = 387 participants) in purposively sampled US communities that supported the assumption that people with complex health conditions seek ED care when problems with access to preventative services make it difficult for them to manage their health. One apparent solution was to ‘empower’ patients to manage their own condition. However, the few studies that we found were equivocal about what effects that mechanism had.
A German study81 using routine data described how, after 2004, osteoporosis prevalence increased (+ 18%) faster in Kinzigtal than in Baden-Württemberg (+ 6%) as a whole, but the available data were insufficient to determine whether the Kinzigtal increase was an epidemiological trend or resulted from a screening and prevention programme.
Before primary care physician reimbursement was linked to patient quality outcomes, a survey by Hibbard et al. 129 (no sampling strategy reported) in the USA found that only 10% of physicians intended to develop patient self-management as a way of improving incomes. The authors’129 follow-up survey after reimbursement had been linked to patient quality outcomes that used different variables, so an exact comparison was not possible, but it found that 60% of primary care physicians had made little or no increase in their support for patient self-management. Hence, reimbursement changes alone were insufficient to incentivise physicians to develop and support patient self-management.
A non-SR85 of informatics support for patient-centred care identified how communication and information-access portals could facilitate patient-centred care, but were not themselves sufficient to enable it.
Causal link 8:9 – contexts
In the above studies, doctors’ and patients’ characteristics were an obvious context for mechanisms ‘empowering’ patients to manage their own health. The survey by Hibbard et al. 129 in the USA found that some physicians expressed frustration at their inability to change patients’ unhealthy behaviours regarding diet, inactivity, smoking and so on. A total of 70% of the primary care physicians surveyed identified ‘patients’ unwillingness to change behaviours’ as an obstacle to achieving care quality metrics (compared with 65.1% identifying ‘lack of time to spend with patients’, 47.7% identifying ‘lack of high-quality support resources’ and 24.8% identifying ‘not knowing how to support patients in behaviour change’). Among the 15.3% of primary care physicians who, nevertheless, reported that they had increased their support for patient self-management, there was double the number aged < 35 years than there were of an older age. 129
Causal link 8:10 – if demand management systems are established, then care planning for individual patients develops
We did not locate any evidence about whether or not, or how, demand management systems such as risk stratification affected the use of care planning for individual patients.
Causal link 8:11 – if demand management systems are established, then more patients are diverted from inpatient to primary care
Taken together, the two relevant studies130,131 in our review were both equivocal about whether or not demand management systems (as opposed to individual care plans; see Care planning for individual patients) diverted patients away from hospital and into primary care.
One cross-sectional study in the USA,130 which used a convenience sample (n = 150), compared ‘comprehensive care’ (i.e. one physician managing both the primary and tertiary health-care needs of a child: a form of gatekeeping) with usual services. Under comprehensive care there were fewer ED contacts (incidence rate ratio 0.51, 95% confidence interval 0.33 to 0.78) and a lower hospitalisation rate. Without directly comparing it with comprehensive care, this study130 also reported that ‘co-ordinated care’ (defined as a provider sharing information and communicating effectively with child, family and consultants, as well as linking to community resources) did not have either effect.
In a retrospective analysis of longitudinal routine data (2,607,902 patients from 796 clinics), Yoon et al. 131 reported how a PCMH model in the USA increased the use of primary care services. The increase arose from practice reorganisation rather than from patient-facing efforts to increase access to care (e.g. by offering flexible and same-day appointments and non-face-to-face services, such as telecare). However, more granular analysis showed that certain elements of practice organisation, such as team huddles and tracking laboratory tests, were associated with fewer primary care visits per patient, which Yoon et al. 131 characterised as greater ‘efficiency’.
Causal link 8:11 – contexts
Being about the USA, these studies presupposed a particular context. As Chapter 1 explained, the concept of PCMH corresponds to the principles under which NHS general practice has already been organised in principle, and often in practice, since 1948. Therefore, even if the above changes to (the equivalents of) general practice co-ordination did divert patients from hospital to primary care, they may already have been adopted in much of the NHS. So, the scope for marginal gains in patient diversion in the NHS may be less than the findings of Yoon et al. 131 suggest. The practice of the same doctor providing (and co-ordinating) a patient’s primary, secondary and tertiary care occurs only under the ‘admitting rights’ model of hospital medicine that exists in much (although not all of) the US health system but hardly at all in the NHS.
Besser’s132 before-and-after study in the USA found that increased service provision led to increased service use. The introduction of a mental health provider in a PCMH led to an increase in the percentage of visits for depression (2010, 0.86%; 2011, 0.54%; 2012, 1.02%; and 2013, 1.26%) and a significant increase (from 3% to 33%) in the percentage of depression visits seen by mental health specialists. Besser132 concluded that these increases occurred owing to services addressing hitherto unmet needs (and, therefore, also preventing use of other services in the future), but the paper reported no data substantiating that. ‘Roemer’s law’ that ‘a built bed is a filled bed’133 is well established with regard to the USA and, with qualifications, to many European health systems. 134–136 It implies that, even if demand management methods do reduce admissions from existing care groups, other hospital admissions are likely to take their place. In part, this is a consequence of per-patient payment systems used by sick funds, corporate insurers and those public bodies that have copied from them a diagnosis-related group-like payment system.
Preventative care
Just one causal link is at issue here (Table 13).
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 9. Preventative health care develops | 11. More patients will be diverted from inpatient to primary care services | 9:11 |
Causal link 9:11 – preventative care enables referrals to be diverted from inpatient to primary care services
We did not locate any evidence directly reporting whether or not preventative health care enables referrals to be diverted from hospital, or any about whether or not patient self-care activation produces better demand management systems for general practice.
Care planning for individual patients
The IPT’s causal links from component 10 are shown in Table 14.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 10. Care planning at individual patient level becomes more prevalent | 9. Preventative care will improve | 10:9 |
| 11. Patients will more often be diverted from inpatient to primary care | 10:11 | |
| 12. Patient experience/care will improve | 10:12 | |
Causal link 10:9 – care planning at the patient level produces preventative care
The IPT assumed that having a patient care plan builds patient confidence and their capability for making good decisions about their self-care, and so improves preventative care. One US PCMH study91 confirmed that having an embedded case manager using joint care planning and motivational interviewing resulted in more trusting relationships between the patient and doctor, care that is more customised to their patient’s individual needs and in patients making the most of their appointments and taking a more active role in their care. 91 Similarly, a case study in a PCMH found that case managers using motivational interviewing, assessment skills and joint care planning enhanced the value of primary care visits for patients and engaged patients more in their own care. 137
In contrast, a survey of 10,990 adults with asthma, diabetes mellitus or chronic heart disease in a US PCMH138 found that having an individual treatment plan was not associated with patient empowerment. Nevertheless, these adults were more likely to report using preventative and ambulatory care when their care involved at least two of care co-ordination, care continuity and a care plan. In this study,138 unlike the others, the unit of analysis was the patient rather than the provider organisation.
Causal link 10:9 – contexts
Taken together, the findings from the three relevant studies91,137,138 on preventative care imply that patient empowerment is sufficient, but not necessary, to stimulate increased use of preventative and ambulatory care.
Causal link 10:11 – care planning diverts patients from inpatient to primary care
Various studies62,87,131,137,139 describe mechanisms by which individual care planning diverts some patients from inpatient to primary care. MDTs’ boundary-spanning roles assist in identifying patients in need of care co-ordination. A UK study76 of paediatric care identified daily specialist community nurses visits to acutely unwell complex patients at home and telephone consultations between the community nurse and hospital duty consultant as means whereby a MDT could support joint care planning so as to avoid emergency hospital admissions and, if they should occur, enable the child to be discharged earlier.
As regards the outcomes so produced, four studies62,131,137,139 found that care planning for individual patients increased patient diversion from hospital to primary care. One study138 did not (described below). Two studies105,140 found that care planning during and after the transition from secondary to primary care reduces readmission rates, and another two86,105 that having operational facilitators to support care planning reduced readmission rates.
In a matched case–control study of American PCMHs, Clarke et al. 139 implemented and evaluated a programme that embedded non-licensed comprehensive care co-ordinators (CCCs) in 14 PCMHs to help primary care doctors execute care plans in order (inter alia) to extend each practice’s ability to support patients before, after and between primary care visits. This intervention reduced ED admissions by 20% annually compared with the control practices. Treadwell and Giardino137 also found that ‘embedding’ a case manager in a PCMH reduced admissions per 1000 patients over the following 18 months. Similarly, the Yoon et al. 131 retrospective longitudinal study of 2,607,902 patients from 796 VHA primary care clinics in the PCMH model found that creating individualised treatment plans, assessing treatment barriers, and better co-ordinating visits, to other physicians decreased the mean number of ED visits by 0.04 visits per patient (p = 0.018). A panel study141 of patients in Philadelphia found that care management by PCMHs (rather than improved access to primary care) reduced ED attendances by between 5.24% and 7.78%, but only for chronically ill patients (especially those with coronary artery disease, hypertension, congestive heart failure, COPD or asthma). A case study62 of a chronic aged and complex care service model in Australia found that the number of ED presentations and length of stay in the ED fell significantly in the 12 months following enrolment compared with the previous 12 months. Almost 30% of participants had no hospital presentations after enrolment. Referrals to non-hospital physiotherapy, podiatry, occupational therapy, dietetics and psychosocial services (but not to community nursing) increased. 62
Against this, Pourat et al. 138 found that the combination of care co-ordination, continuity of care and care plans did not decrease the likelihood of ED use, although it did increase use of preventative and ambulatory care and improved clinicians’ communication with patients. This is an absence of evidence for the outcome that NHS policy-makers had assumed, not evidence of an opposite effect. This study138 also implied that care planning at the patient level is most effective when combined with co-ordination and activities to increase the continuities of care plan.
Other studies argued that individual care planning and co-ordination during and after the transition from hospital to home reduced readmission rates to EDs. One specific mechanism was illness-specific specialised education to support patients in self-management post discharge (i.e. telephone advice on how to monitor one’s weight and look out for warning signs that would prevent an ED visit or hospitalisation). This intervention did indeed make patients more likely to monitor their weight and change their health behaviours, but no less likely to be admitted or re-admitted to hospital. 140 A SR105 of transitional care interventions found that three components of care were associated with reduced short-term admission rates: (1) care co-ordination by a nurse (most frequently a registered nurse or advanced-practice nurse), (2) a home visit within 3 days and (3) communication between the hospital and the primary care provider. Most of the interventions in the review that reduced intermediate- and long-term readmissions involved care co-ordination. 105 A mixed-methods study86 of 18 complex care management organisations suggested that CCM teams that receive timely notifications of their patients’ ED visits could intervene to avoid hospitalisations. Methods for ensuring safe transitions included medication reconciliation and developing contingency plans in case certain trigger events occurred. 86
Causal link 10:11 – contexts
Only two studies about diverting patients from hospital to primary care identified contexts in the realist sense. To minimise re-admissions, CCM teams must help patients find the resources they need in local health systems and communities. 86 Verhaegh et al. 105 observed that developing a valid and reliable method to measure the preventability of a readmission was important to enable clinicians to implement targeted readmission policies and penalties for preventable readmissions. Case management is one form of care planning for individual patients. An umbrella review by Damery et al. 104 found that only one out of eight SRs showed that case management reduced hospital admissions, that is, diverted patients from inpatient to primary care. The exception was a review showing a 49% relative risk reduction of hospital admission for patients with heart failure.
Causal link 10:12 – care planning improves patient experience
The evidence that we found supported the assumption that care plans for individual patients improve patients’ experience of care (10:12), but the studies were few. Just one study137 reported US focus groups’ opinions that joint care planning with patients gained for health-care providers a comprehensive understanding of the client’s or family’s health-care needs, barriers to and potential action regarding a patient’s social support, health literacy, understanding of the care plan, plan adherence, and care preferences. 137
We found limited evidence from three studies91,103,108 that patient involvement in decision-making about (i.e. planning) their own care improved patients’ experience of care. When patients felt they had been left out of decision-making about their own care, they felt vulnerable. 91 A mixed-methods study103 of American ACOs suggested the value of involving in care pathway redesign:
We gave them (patients) an initial care pathway as we saw it and had them fill in what we missed. Every single interview raised using catheters as a point of anxiety for the patient and the urologists didn’t realize that was a point of anxiety.
Shortell et al. 103
In three Canadian focus groups108 with 28 patients and informal caregivers in community-based PHC, patients expressed support for the role of patient advocates who helped them navigate the care system and participate more fully in decision-making. This was particularly important for patients without a family member to bring to appointments. 108
Causal link 10:12 – contexts for improved patient experience
As the context for care planning to improve patient experience, a study of focus groups across 10 communities found that trusting and open relationships between patients and providers created the conditions for care customised to the patients’ specific needs,91 suggesting that there may be a virtuous circle between trusting and open patient–provider relationships and patient-centred care.
Patient diversion
Table 15 shows the causal links in the IPT from patient diversion (component 11). These links are, in a sense, the kernel of the IPT, in the sense of being a key intermediate outcome in the MCP model between (most of) the other components and the final intended outcomes.
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 11. More patients are diverted from inpatient to primary care services | 12. Improved patient outcomes and experience of care | 11:12 |
| 13. NHS costs will reduce | 11:13 | |
Causal link 11:12 – diverting patients from inpatient care improves patients’ experience of care
Three studies66,74,81 published since 2014 reported evidence about what effect diverting patients from secondary care had on their experience of care. A ‘qualitative analysis’ of documentation66 described how a programme that integrated mental health care into ambulatory care enabled practitioners to support those with acute mental health needs and promote a more holistic and empowering approach to self-care that encompassed welfare and healing. The context for these perceived effects is noted in Causal link 3:9 – patient engagement, patient self-care, activation and empowerment. An analysis of patient experience indicators compared the Integrierte Versorgung Gesundes Kinzigtal (IVGK; ‘Healthy Kinzigtal Integrated Care’) programme with ‘usual care’. 81 The adjusted comparison showed that approximately one-third of the indicators were significantly better for people in the IVGK programme. Another third of the indicators changed in the desired direction, but not statistically significantly. The remaining third did not change. 81 In some areas of care, such as osteoporosis treatment, important outcomes such as the number of fractures (closely related to quality of life and of patient experience) were significantly lower for people in the IVGK programme than for those in the control programme. 81
In a descriptively analysed study74 of telephone interviews with patients (convenience sample, n = 15) who received care from the EPSILON geriatrics team network (France), patients reported being satisfied with the way the network enabled access to expert advice and support that would otherwise require hospital admission. ‘Compliance’ with medical and paramedical prescriptions in this small sample was reported as 72% and 74%, respectively, but Canali et al. 74 reported no comparisons with patients outside the network.
Causal link 11:13 – diverting patients from inpatient care reduces costs
We found few post-2014 studies reporting how diverting patients from inpatient care to outpatients departments or nursing homes reduces costs. Regarding the use of ED services, one US case study66 ‘qualitatively analysed’ Institute for Health Improvement documentation and publications to examine the cost impact of integrating mental health care provision into ambulatory care for mental health service users with light, moderate and severe levels of complexity. This integrated approach cost less than a non-integrated approach. 66 Users of the integrated service used urgent care services 54% less than non-integrated service users, although the study66 did not quantify how much money was saved. In primary mental health services the per-patient costs of care increased for both integrated and non-integrated services, but less for integrated care. 66
A matched case–control study in the USA139 evaluated a programme that embedded non-licensed CCCs in 14 PCMHs to help primary care doctors execute care plans so as to extend each practice’s ability to support patients before, after and between primary care visits. This intervention reduced ED admissions by 20% annually compared with the control practices, saving payers approximately US$2000 per ED visit, which implied an estimated total annual cost reduction of US$1.4 million for the whole programme. Salary and benefits costs of the personnel dedicated to the programme (but ignoring the hidden costs of medical directors’ and other support staff time) were approximately US$950,000 annually. 139 Treadwell and Giardino137 also report the equivocal finding that, in two out of five case study sites, PCMH-based care co-ordination by an ‘embedded’ case manager reduced the number of admissions per thousand patients and, therefore, reduced claims costs by US$7 per member per month in one site and US$14 in the other. The range of costs narrowed across all five sites, suggesting stronger managerial control of costs. 137
A German study81 used a difference-in-differences approach to compare the care costs (based on routine data) for people who were in the IVGK programme with those who were not. 81 Care costs for people in the IVGK programme were €322 less per annum than the ‘usual care’ group, with the greatest savings occurring in relation to hospital care (€179 per person), other services (€93 per person) and medicinal products (€37 per person). 81 Huber et al. 128 attributed annual cost savings of CHF440 (cardiovascular patients), CHF780 (diabetes mellitus) or CHF200 (respiratory illnesses) to the introduction of care guidelines in consequence of ‘integrated care’ in Switzerland.
Damery et al. 104 found that, in general, the evidence that ‘integrated care’ (mechanisms not specified) reduced health-care costs was ‘poor and heterogeneous’ and equivocal, with some SRs reporting cost savings, especially for the CCM, and others not.
Causal link 11:13 – contexts
Treadwell and Giardino137 did not report what contexts (in the realist sense) differed between the PCMHs where patients had been diverted away from hospital care (reducing costs) and those where they had not. Briot et al. 66 emphasised the necessity of integrating mental health care provision into (general) ambulatory care. Clarke et al. 139 gave no contextual information (in the realist sense). Huber et al. 128 described capitated (as opposed to activity-based) payments to providers as a favourable context. Kinjo et al. 107 found that replacing hospital with PHC end-of-life care reduced costs only if community care began > 30 days before the patient’s death, although that finding reflected the Japanese structure of GP payments.
Other assumptions
We did not locate any evidence published since the beginning of 2014 relating to the final two causal links in the IPT (Table 16).
| MCP component (1–13) | IPT causal link | |
|---|---|---|
| IF | THEN | |
| 11. When more patients are diverted from inpatient to primary care | General practice will benefit | Other |
| 6, 8. Care co-ordination and demand management systems together occur | More responsive urgent care will develop | Other |
Chapter 7 Step 3: building a revised logic model
Strength of evidence for the initial programme theory
None of the causal links in the IPT had a strong evidence base by the standards of Cochrane reviews or other SRs, although some individual studies were methodologically strong. For each top-level causal link in the IPT of MCPs, Table 17 summarises the extent of evidential support in the studies we found. In Table 17, a combination of primary studies with a SR is categorised as ‘substantial evidence’, multiple primary studies as ‘supporting evidence’, and a single primary study as ‘minimal evidence’. ‘Partial support’ means we found evidence supporting some parts of this causal link but not others (i.e. qualified support). ‘Equivocal evidence’ means that we found evidence both for and against the causal link.
| Number | Causal link | Studies: number (quality appraisal score, %) | Evidential status | |
|---|---|---|---|---|
| 1 | 2 | IF NHS managers will establish MCPs, THEN network management will develop PROVIDED that the specified of contextual conditions apply | 2 (100), 2 (75), 2 (50), 1 (0) | |
| 7 | NHS managers will establish MCPs THEN referral network planning will develop | 1 (100), 2 (50) | ||
| 2 | 3 | IF network management develops, THEN MDTs will be established. | 2 (100), 6 (75), 3 (50), 2 (0) | |
| 6 | IF network management develops, THEN care co-ordination through HIT use will develop. | 1 (100), 6 (75), 5 (50), 2 (25), 1 review (non-SR 0/11) | ||
| 3 | 7 | IF MDTs are established, THEN referral network planning develops | 3 (100), 6 (75), 2 (50), 2 (25) | |
| 9 | IF MDTs are established, THEN preventative health care will develop | 3 (100), 6 (75), 1 (50) | ||
| 4 | 3 | IF culture changes occur in the participating organisations, THEN MDTs develop | 5 [100; including 1 SR (7/10)], 3 (75), 4 (50), 1 (25), 1 (0) | |
| 8 | IF culture changes occur in the participating organisations, THEN that will produce demand management systems | 0 | ||
| 9 | IF culture changes occur in the participating organisations, THEN that will produce preventative care | 2 [100; including 1 SR (7/11)] | ||
| 5 | 8 | IF the voluntary sector becomes involved in MCPs, THEN demand management systems will be strengthened | 0 | |
| 9 | IF the voluntary sector becomes involved in MCPs, THEN preventative health care will develop | 2 (100) | ||
| 12 | If the voluntary sector becomes involved in MCPs, THEN patient outcomes and experience of care will improve | 1 (75) | ||
| 6 | 7 | IF HITs are used to strengthen informational continuity of care, THEN referral networks will develop | 6 (100), 88 (75), 1 (50), 1 [narrative review (1/11)] | |
| 10 | IF HITs are used to strengthen informational continuity of care, THEN care planning at the patient level will become more prevalent and systematic | 3 (100), 2 (75), 1 (50), 2 SRs (7/11 and 0/11), 1 narrative review (1/11) | ||
| 11 | IF HITs are used to strengthen informational continuity of care, THEN patients will be diverted away from hospital services | 1 (100), 1 (75), 1 narrative review (0/11) | ||
| 7 | 8 | IF referral network planning occurs THEN demand management systems will be strengthened | 0 | |
| 10 | IF referral network planning occurs THEN care planning at individual patient level will become more prevalent and systematic | 2 (100), 1 (75), 1 (50) | ||
| 11 | IF referral network planning occurs, THEN more patients will be diverted from inpatient to other services (through admission avoidance, discharge support) | 3 SRs (9/11, 9/11 and 7/11), 2 (100), 2 (75), 2 (50) | ||
| 8 | 9 | IF demand management systems are established, THEN preventative care will become more prevalent and systematic, which will, in turn, strengthen demand management | 1 (100), 1 (75), 1 (0) | |
| 10 | IF demand management systems are established, THEN care planning at individual patient level will become more prevalent and systematic | 0 | ||
| 11 | IF demand management systems are established, THEN more patients will be diverted from inpatient to primary care | 2 (100) | ||
| 9 | 11 | IF preventative health care becomes more prevalent and systematic, THEN more patients will be diverted from inpatient to primary care | 0 | |
| 10 | 9 | IF care planning at individual patient level becomes more prevalent, THEN use of preventative care will increase | 1 (100), 1 (50), 1 (25) | |
| 11 | IF care planning at individual patient level becomes more prevalent, THEN more patients will be diverted from inpatient to primary care | 3 (100), 3 (75), 2 (50), 2 (25), 1 SR (7/11) | ||
| 12 | IF care planning at individual patient level becomes more prevalent, THEN patient experience will improve | 1 (100), 2 (75), 1 (25) | ||
| 11 | 12 | IF patients are diverted from inpatient care, THEN patient experience will improve | 1 (50) | |
| 13 | IF patients are diverted from inpatient care, THEN NHS costs will reduce | 1 (75), 1 (50), 1 (25) | ||
| IF patients are diverted from hospital care, THEN general practice will benefit | 0 | |||
| IF care co-ordination and demand management systems both develop, THEN urgent care will become more responsive | 0 | |||
| Key | No evidence found | |||
| Partial/minimal support | ||||
| Supporting evidence | ||||
| Supporting evidence, with elaborations and additions | ||||
| Equivocal evidence | ||||
| Substantial evidence | ||||
On that basis we removed certain causal links from the IPT, qualified others and elaborated or expanded others again. We continue to flag in bold italics which of the policy-makers’ original causal links each revision applies to.
Causal links removed or qualified
The causal links that lacked evidential support appeared superfluous to an evidence-based logic model. Table C (see Report Supplementary Material 2) lists these superfluous links in full. 20
Therefore, we did not take them forward into the revised logic model. We also removed the unevidenced assumptions about what prior contexts favour the establishment of MCP equivalents (1:2). We found no evidence for whether or not the state of social services, or whether or not health professionals and organisations viewing those whom they care for as people not patients, is relevant to establishing MCP-equivalent networks.
Removing these elements produced a truncated, but more strongly evidence-based, revised logic model.
For some causal links, the evidence conflicted. To the realist mind, this ambivalence is a clue that the outcomes of these mechanisms may depend heavily on contextual factors142 unidentified in the published research we reviewed. One such causal link is that the formation of MCP equivalents necessarily stimulates care planning at an organisational and interorganisational level (causal link 1:7). Although it gives proof of concept that interorganisational care planning can result, the evidence from ACOs also shows that ‘horizontal’ PHC networks do not automatically produce such care planning, in particular between the equivalents of GPs and CHSs. Causal link 6:11, that if HIT is used to strengthen informational continuity of care, then patients will be diverted away from hospital services, was another unresolved case. Another variant, in which different contexts are defined as different stages in a project’s life, might arise with causal link 8:11; demand management schemes may initially increase demand for services (because of more case-finding) before a reduction follows. The evidence was also equivocal about whether or not diverting patients from inpatient to primary care (causal link 11:12) saves money.
Causal link 4:3 was concerned with how, if organisational culture changes in the relevant organisations, MDT working will develop. Some studies identified culture change as a prerequisite, one study produced evidence to the contrary, and another proposed that culture change was a consequence of collaboration. However, causal link 1:2 suggests a possible resolution of this seeming conflict. The formation of a MCP equivalent might, in the right contexts, initiate a virtuous circle: organisations that already have collaborative cultures are more likely to set up interorganisational networks, they provide care in more collaborate ways as a result and this, in turn, reinforces their collaborative culture, and so on.
Contextual qualifications
Our secondary evidence reported certain contexts (moderators of the proposed MCP mechanisms) that the policy-makers’ programme theory omitted and some that qualified the (remaining) MCP mechanisms.
We found considerable additional evidence about favourable contexts for establishing MCP equivalents (causal link 1:2). Organisations are more likely to join them when:
-
joining endorses general practices’ existing activities
-
providers think the MCP equivalent seems relevant to their care group(s) and clinical tasks
-
GPs (or the equivalent) are in partnerships rather than single-handed
-
the MCP equivalent seems to offer its member organisations external resources and/or money
-
similar organisations that they admire join the MCP equivalent
-
external controls are permissive and light, and the MCP equivalent has local champions
-
staff are professionally qualified
-
doing so seems likely to reduce the risks they face, for instance the risks of competition.
However, we found no evidence to support the following assumptions about the context for causal link 1:2.
-
Initial conditions favouring MCP setup include:
-
the populations served are of a size and type likely to benefit
-
the populations served desire autonomy and control over their health and health care, and are likely participate in health-maintaining activities.
-
Secondary evidence about causal link 1:7 (MCP equivalents lead to the development of planned referral networks) included a report68 that one PCMH negotiated 50 ‘compacts’ with specialist providers while other nearby PCMHs negotiated few or none. Contexts that obstructed making care compacts were geographical (small, isolated communities and/or small general practices), financial [misaligned incentives (payments)] and temporal (the time required). 68
Contexts for MCP-equivalent networks to establish HIT-based care co-ordination (causal link 2:6) included the credibility and track record of the lead (network-co-ordinating) organisation, and good relationships between organisations. Payment models can incentivise, and contractual hangover inhibit, interorganisational care co-ordination. The inclusion of health centres (or the equivalents) in MCP equivalents aids provision and co-ordination with less common services. For MCP equivalents to establish virtual MDTs (causal link 2:3) requires HIT infrastructure. HIT training and, above all, system development for sharing EHRs was an indispensable context. ‘Embedding’ or colocating allows informal and meeting-based care co-ordination and improved mutual understanding.
Traditional status and deference hierarchies are a barrier to MDTs developing organisational-level and interorganisational care planning (causal link 3:7). Such planning also requires role clarity, mutual familiarity with other professions’ contributions, and that boundary-spanning staff have sufficient seniority, assertiveness and relational skills. It is necessary that MDT members trust each other and the team co-ordinator, have confidence about their own skills and are clear about not being liable for outcomes beyond their own personal control. Managerial support can help to create these conditions. In particular, it is necessary that doctors do not resist boundary-spanning activities. MDTs require clearly structured communication and common training (e.g. on different professions’ roles and contributions). Shared group goals also help to improve patient outcomes. Other favourable contexts for interorganisational care co-ordination include the credibility and track record of the lead (network-co-ordinating) organisation, and good relationships between organisations. They also include case-mix: high complexity and low knowledge about a patient’s condition increased providers’ dependency on boundary-spanners for making care co-ordination work. Payment models can incentivise, and contractual hangover inhibit, interorganisational care co-ordination. The inclusion of health centres (or the equivalents) in MCP-equivalent networks aids provision and co-ordination with less common services. Employment by same organisation helps MDT working, as does staff familiarity with other professions’ roles and contribution to care, and allowing staff time to participate in collaborative activities.
If changes in organisational culture (causal link 4:3) are to promote the development of MDTs, the main contextual requirements were trust between occupational groups (itself reinforced by experience of working together successfully), mutual respect, shared training and the application of other, unspecified, ‘resources’.
For HIT to be used to co-ordinate care (causal link 6:7), the quality of HIT design is, as noted, all important, but the current IT market is deficient in that respect. HIT systems do not, by themselves, produce care co-ordination, but only in a context of corresponding care management practices. Despite wide agreement about the ideal characteristics of an EHR, there is only limited evidence to confirm its ability to improve patient care (causal link 6:12), and reports of failures, probably because many provider organisations do not yet have such a system and/or exploit in it their everyday working practices. Many reports of successful uses of HIT for MCP-equivalent purposes come from the USA, where corporate hospitals can invest large sums in data analytics.
The effects of planned referral networks on the diversion of patients from inpatient to primary care (causal link 7:11) depend on the case-mix [care group(s)] involved. The outcomes are greatest for low- and medium-morbidity patients, especially the 1% of heavy users (with five or more hospitalisations per year), but the opposite outcome occurs for high-morbidity patients (a finding consistent with studies suggesting that case-management also increases case-finding among patients with complex needs). 34 For care planning at interorganisational level, to stimulate care planning for individual patients (causal link 7:10) and for individual-level care planning (causal link 3:9) and preventative care to develop (causal links 4:5, 5:8, 7:10, 9:11), patients must:
-
trust care co-ordinators and understand that role
-
use the care co-ordinator to co-ordinate their care, rather than the patient spontaneously contacting different providers directly
-
not find MDT care worrying
-
have suitable language skills and acculturation
-
agree to adopt healthier behaviour.
In addition:
-
MDTs have time to discuss the resulting care plans with patients before implementing them
-
younger doctors may be more responsive to incentives for care planning for individual patients (causal link 7:11, 7:10).
For demand management activities to divert patients away from secondary care (causal link 8:11), tariff payments to hospitals (when present) are a perverse incentive. The same applies when providers (e.g. some ACOs in the USA) are paid by volume of activity (e.g. for attracting insurance subscribers as patients) rather than by, say, capitation or according to the character of a resident population served. More generally, we add that any culture change in favour of diverting patients from hospital to primary care has to emerge from, and despite, a context of the medicalisation of ageing, underprovision of social care and under use of hospices.
We found evidence that individual care planning does divert patients from inpatient to primary care (causal link 10:11) but also strong evidence (umbrella review) that case management schemes are an exception to this tendency.
As regards causal links 11:12 and 11:13 (diverting patients from hospital care will reduce costs and improve patient experience), the required context was development of preventative care and making ambulatory medico social work services and social care support routinely available and financially viable. This context also applies to causal links 7:11, 8:11 and 10:11, as they too have patient diversion as an outcome. As our search focused on primary care networks, it is not surprising that we found no studies about factors other than referral patterns reducing hospital costs (causal link 11:13). However, it has long been known that because many hospital services are indivisible, substantial hospital cost reduction happens only if referrals decrease enough for whole clinics or wards to close. Having to provide a wide spectrum of clinical specialties limits how far district general hospitals can do this, and the more immediate effect may be diseconomies of scale (reduced efficiency) rather than lower total costs. Shortening hospital length of stay (e.g. by ‘unblocking’ beds) reduces total cost per episode, but does so by reducing low-cost (recovery and ‘hotel’) rather than the high-cost (initial diagnosis and treatment) bed-days. 34 When the freed bed-days are used for additional patients, the overall effect is, therefore, to replace low-cost with high-cost bed-days. By increasing throughput, this increases hospital productivity and efficiency but also raises (not reduces) total costs. The required context for mechanism (causal link 11:13) to work is, therefore, the opposite of these conditions. Then, under a tariff system, the savings per episode (from reduced hospital income) accrue directly to the primary care provider or payer in the form of reduced tariff claims.
Additional, elaborated or qualified causal links
Chapter 5 noted additional evidence found for causal links that were not in the IPT but are, nevertheless, relevant to MCPs’ intended outcomes. These additions focus, qualify or elaborate the initial MCP programme theory.
On that basis, causal links 1:2 and 2:3 should be qualified by noting that the organisations and professions included (e.g. whether or not mental health professionals are included) when constructing a MCP equivalent define (or, if absent, limit) the services that the MCP equivalent, and the MDTs in it, can co-ordinate. There are also, so to speak, degrees of networking ranging from monitoring information exchange alone to contractual relationships, and to a formalised network with a permanent central co-ordinating body.
The evidence about causal link 2:6 (that network management develops HIT-based care co-ordination) identified specific media and artefacts (‘boundary objects’) through which such co-ordination occurs: care compacts, standardised and agreed care processes and pathways, actively managing across the whole pathway, pooled resources, uniform training across staff groups, case conferences and information feedback between clinicians in separate organisations. Similarly, we found evidence specifying how causal link 3:7 (establishing MDTs leads to care planning at organisational and interorganisational level) works. Studies relating to several causal links (2:7, 2:3, 3:7, 4:3) indicated the necessity for boundary-spanning roles and of health workers mutually supporting and assisting each other across organisational boundaries. Face-to-face communication is quicker, more responsive and less ambiguous than IT-based communication (evidence for preferring colocated to virtual MDTs).
Care plans for individual patient care planning have effects (causal links 10:9, 10:11, 10:12) through the mechanisms of advocacy, care co-ordination by staff in boundary-spanning roles, increasing the continuities of care, making care more person centred and making decision-making a more shared activity.
The largest addition to the IPT concerned MDTs as a care co-ordinating mechanism (causal links 3:7, 3:9). Other than those already listed in the IPT, we found evidence that MDTs are also a mechanism for producing or undertaking:
-
culture change among health professionals (causal link 3:4)
-
voluntary sector involvement (causal link 3:5)
-
informational continuity of care (causal link 3:6)
-
demand management systems, through gate-keeping and need- or risk-stratification (causal link 3:8)
-
care plans for individual patients (causal link 3:10)
-
diversion of patients from unnecessary secondary inpatient to primary care (causal link 3:11)
-
better patient care, in the senses of greater continuity and informal carer involvement (causal link 3:12).
Causal link 4:3 (culture changes in the participating organisations promote MDT working) can be specified more closely: the necessary cultural changes are to strengthen health workers’ knowledge of, and favourable attitude towards, other professions’ contribution to care; a climate of psychological safety; focus on tasks they are practically useful to the MDT members; and the development of shared expectations and values across the MDT, in particular dialogue between medical and mental health providers. An important skill is that of communicating important information clearly in safety-critical situations, but in such ways that maintain good informal relationships. Convergent working practices help to produce cultural convergence across professions, as does cross-professional training. We also found evidence that culture change in a MCP produces patient-level care planning, better patient experience and staff well-being.
For voluntary sector activities to strengthen preventative care (causal link 5:9) they require social prescribing or a similar mechanism for patients to access voluntary sector resources.
In using HIT to co-ordinate care (causal link 6:10), the clearest requirement is high-quality HIT design. Inter alia, different organisations’ HIT systems must be capable of communicating with each other, requiring, in turn, adherence to published common standards and standardised data templates. The systems must be capable of the necessary data analysis (e.g. risk stratification, workflow tracking of patients’ care in real time). EHRs (causal links 6:3, 6:7, 6:9, 6:10, 6:13) have multiple uses (enabling access to patient information for all staff members, instant messaging, within-chart notes, telephone templates that can be routed to team members’ inboxes, task assignments and keeping ‘huddle sheets’) other than storing personal clinical information. Not least was the importance of using the EHR to guide physician workflow and good clinical practice, including action reminders. Non-clinical care co-ordinators may be better than clinicians at co-ordinating care electronically. The HITs that produce informational continuity of care can also, by enabling need and risk stratification, promote demand management systems. Other than those mentioned in the IPT, additional causal links from ‘care co-ordination through HIT’ to other MCP components were:
-
promotes MDT working (causal link 6:3)
-
supports demand management activities (causal link 6:8)
-
promotes preventative care (causal link 6:9)
-
saves cost (causal link 6:13).
Table E (see Report Supplementary Material 2) summarises the evidential status of the additional causal links to those in the IPT.
Therefore, we added these additional causal links between MCP components to the programme theory, except for those producing the outcome of staff well-being, which was not a central aim of the original programme theory and policy.
A revised multispecialty community provider logic model
Adding these additional causal links to the truncated version of the initial MCP programme theory and resolving certain ambiguities produced a revised, more strongly evidence-based logic model. Some concepts in the policy-makers’ causal links, from which we developed the initial MCP programme theory were, in realist terms, ambiguous:
-
‘MCP setup’ – ambiguous between the mechanism of setting up MCPs (i.e. NHS managers’ actions) and the context (favourable or unfavourable initial setting).
-
‘Demand management’ – ambiguous between the mechanisms for managing demand (e.g. referral screening, risk stratification) and their intended outcomes (fewer referrals and admissions to hospitals).
-
‘Patient diversion’ – ambiguous between the mechanisms for diverting patients away from hospital (e.g. providing non-hospital care) and their intended outcomes (fewer hospital admissions, quicker discharge). Furthermore, ‘patient diversion’ means both diversion from hospital and into primary care, extended or enhanced as necessary.
For the purposes of testing the IPT as it stood, we had left these formulations untouched when comparing it with the secondary evidence. But, to produce a more coherent, less ambiguous, more evidence-based revised logic model, we resolved the first ambiguity by defining ‘MCP establishment’ as the mechanism (NHS managerial action) and the other two as the resulting (intermediate) outcomes (e.g. the resulting activities or systems) similar to the other analogous entities in the IPT. At one point in their causal links, the policy-makers’ had skipped a link. Creating a MCP is, of course, a precondition for the subsequent mechanisms that depend on that, but not necessarily the immediate precondition. The mechanism of creating a MCP-equivalent network leads, the secondary evidence suggests, to network management activity in general, and that mechanism (rather than the initial creation of a MCP per se) is what promotes care planning at organisational and interorganisational levels. Therefore, we revised the programme theory accordingly.
These subtractions, additions, qualifications and resolutions of ambiguity together yielded the top-level CMO statements of a revised, more evidence-based, programme theory. Table D (see Report Supplementary Material 2) lists its components and the causal links between them. Appendix 14 shows, in greater detail, the revised logic model and which causal links in the IPT had a least some evidential support (column ‘IPT + E’), which casual links the evidence review added to the IPT (column ‘E’) and which casual links in the IPT we found no supporting evidence for (column ‘IPT’). Chapter 4 sets out the ways in which the IPT operationalised (defined) the causal links it contained. Some of these causal links were carried forward unchanged from the IPT into the revised logic model and so, therefore, were the ways in which they are operationalised (defined).
Dependencies and priorities
Figure 4 shows the revised sequence and dependencies among the set of CMO links that together make the revised logic model for MCPs.
FIGURE 4.
Revised logic model.

In Figure 4, each arrow represents a mechanism. The corresponding outcome is indicated by the box at the right-hand side. All except the first two mechanisms for MCP components (1. NHS managers set up MCP; 4. Culture change) are the outcome(s) of some previous mechanism(s). The realist metaphor of a ‘mechanism’ should not, of course, be misunderstood as implying that each component will act as a mechanism for another component automatically without (in this case) any activity (reasoning, actions, use of resources) by NHS managers, staff and any other relevant agents (not least, patients) or with a guaranteed outcome. Rather, their activities are the mechanisms. Furthermore, each of these mechanisms is able to produce its intended outcome only when the requisite context(s) are present. The final outcomes of cost reduction and improved patient experience depend on all the antecedent mechanisms. This finding is consistent with strong evidence104 that the implementations of the CCM were significantly more effective when multiple components of the CCM were implemented rather than just one.
Figure 4 makes apparent how central two components are in acting as mechanisms to bring about change in other components. One is the operation of MDTs (component 3). MDTs are the mechanism or joint mechanism to produce eight other components on which achievement of MCPs’ two main intended outcomes (improved care and reduced cost) depend. MDTs also contribute directly to improving the quality of patient care and to changing the culture of health-care organisations. Similarly, the second central component is using HIT for care co-ordination (component 6). HIT is the mechanism or joint mechanism for four other components on which the achievement of MCPs’ two main intended outcomes depend, and contributes directly to producing one of these outcomes (cost control). Furthermore, MDTs and the use of HIT for care co-ordination reinforce each other in a virtuous circle. MDT activity and culture change (component 4) reinforce each other in a second virtuous circle. In the more evidence-based revised logic model, preventative health care (component 9) is not on the causal path to reduced NHS costs (component 13) or improving patients’ experience of care (component 12). The justification for it is independent of that (most obviously, that preventative health care is worthwhile in itself). However, the very specific contexts required for cost reduction (see Chapter 5) remind one that, for these causal links to ‘work’, the favourable contexts noted above must either exist or be created.
How well evidenced is the revised logic model?
Despite the above revisions, even the revised logic model did not always have a strong evidence base by the criteria of the hierarchies of evidence used in most non-realist SRs. Even the SRs that support parts of the revised logic model often summarise uncontrolled (non-comparative) or descriptive studies. However, these criteria have to be applied with caution in realist reviews and syntheses and, indeed, to qualitative and mixed-methods studies, which the majority of the studies we selected consisted of. Furthermore, weak evidence is, nevertheless, better than still weaker evidence, or none. Even so, causal links had minimal supporting evidence (just one study). Table 17 categorised each causal link according to its strength of evidence compared with the other causal links that we reviewed. (The categories are defined above; see Strength of evidence for the initial programme theory). Table 18 combines and summarises those categorisations for all the causal links, both inherited and added, in the revised logic model.
| Strength of evidence | Causal links in revised programme theory |
|---|---|
| Substantial | Culture changes in provider organisations help MDTs develop (R4:3) |
| Culture changes in provider organisations help preventative health care develop (R4:9) | |
| Culture changes in provider organisations enable individual care plans to become more widely used (R4:10) | |
| Culture change in health-care providers produces better patient experience (R4:12) | |
| Use of IT to strengthen informational continuity of care enables wider use of individual care plans (R6:10) | |
| Planned referral networks make it more likely that patients will be diverted from unnecessary secondary care to primary care (R7:11) | |
| Individual care plans make it more likely that patients will be diverted from unnecessary secondary care to primary care (R10:11) | |
| Supporting | MCP network management helps MDTs to develop (R2:3) |
| MCP network management helps care co-ordination through IT develop (R2:6) | |
| MCP network management helps planned referral networks develop (R2:7) | |
| MDTs produce culture change in the health system (R3:4) | |
| MDTs help planned referral networks to develop (R3:7) | |
| MDTs produce better demand management systems (R3:8) | |
| IF MDTs are developed, THEN preventative health care develops (R3:9) | |
| MDTs produce care planning at patient level (R3:10) | |
| MDTs produce better patient experience and outcomes (R3:12) | |
| Voluntary sector involvement helps preventative health care develop (R5:9) | |
| Voluntary sector involvement contributes to improved patient outcomes (R5:12) | |
| Informational continuity of care promotes MDT working (R6:3) | |
| Information continuity of care (through IT) helps planned referral networks to develop (R6:7) | |
| Informational continuity of care (through IT) promotes demand management systems (R6:8) | |
| Informational continuity of care (through IT) promotes demand management systems (R6:9) | |
| Planned referral network assist the use of care plans for individual patients (R7:10) | |
| IF care planning at individual patient level becomes more prevalent, THEN use of preventative care will increase (R10:9) | |
| Partial | IF NHS managers set up a MCP, THEN MCP network management develops (R1:2) |
| Demand management systems make it more likely that patients will be diverted from unnecessary secondary care to primary care. (R8:11) | |
| Equivocal | IF care co-ordination through IT develops, THEN Individual care plans are used (R6:10) |
| Care co-ordination through IT makes it more likely that patients will be diverted from unnecessary secondary care to primary care (R6:11) | |
| Planned referral networks make it more likely that patients will be diverted from unnecessary secondary care to primary care (R7:11). | |
| Diverting patients from unnecessary secondary care to primary care will reduce NHS costs (R11:13) | |
| Minimal | MDTs assist culture change in provider organisations (R3:4) |
| MDTs assist voluntary sector involvement (R3:5) | |
| MDT working produces informational continuity of care (R3:6) | |
| MDTs make it more likely that patients will be diverted from unnecessary secondary care to primary care (R3:11) |
The study by Alidina et al. 68 implies that causal link R4:3 (cultural changes lead to MDTs) should be interpreted as meaning that culture change is sufficient to produce MDTs, but not necessary (MDTs can form for other reasons without culture change). The equivocal evidence about causal links R6:7, R6:11, R6:10 and R7:10 was substantial on both sides of the argument. Were it not for a single (small and weak) study that found no effect (rather than a contrary effect), causal link R9:11 (IF care planning for individual patients becomes more prevalent, THEN more patients will be diverted from inpatient care) would have had ‘substantial’ evidence. To the realist mind, equivocal evidence suggests that contextual factors unrecognised in the original studies condition the production of the corresponding outcomes. Figure 5 adapts Figure 4 so that the widths of the arrows reflect which, of the stated categories, evidential support each causal link in the revised logic model has.
FIGURE 5.
Revised logic model of the causal links through which MCPs produce their outcomes: relative strengths of evidence base.
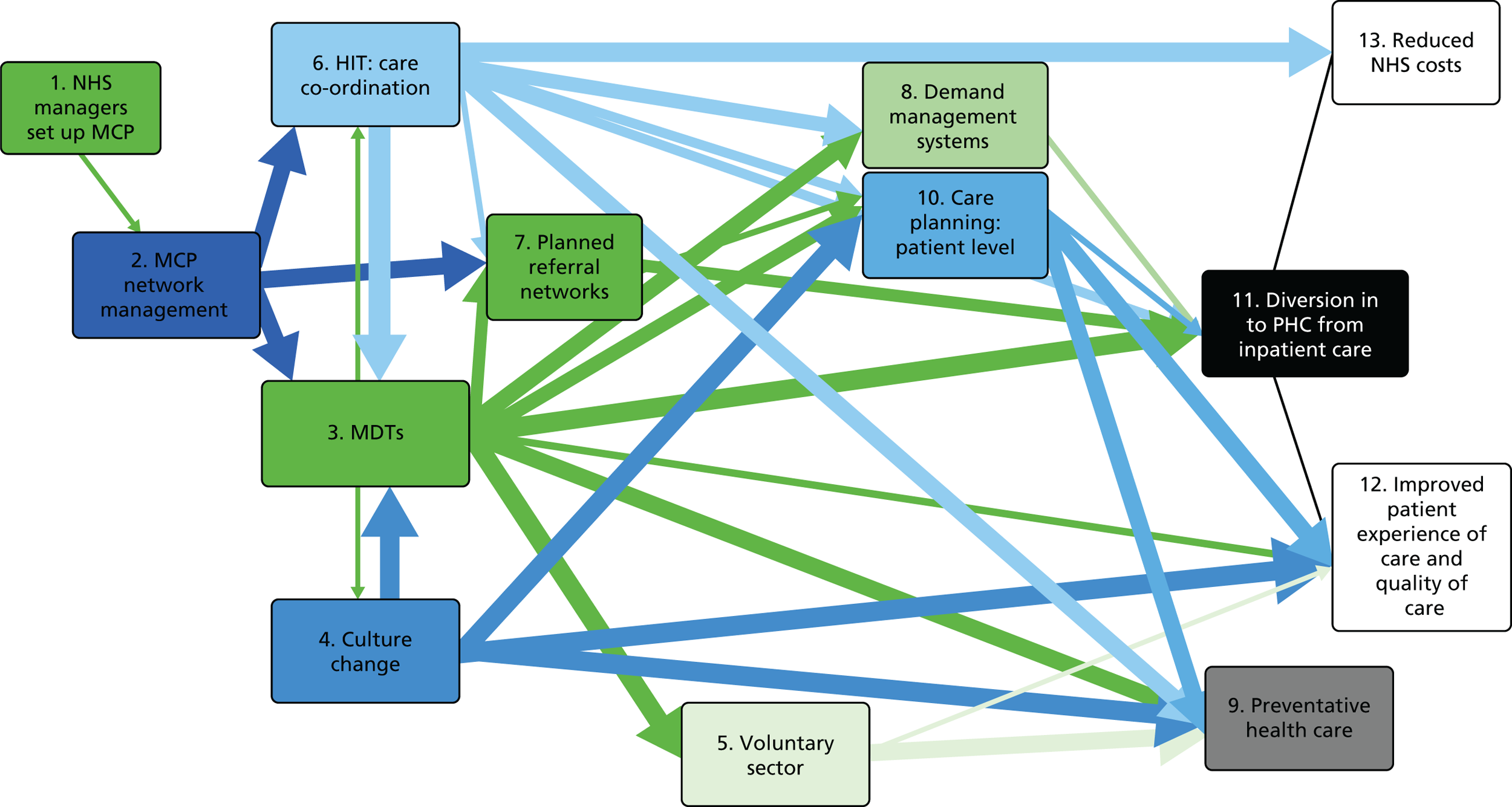
The distribution of evidential support approximately matches the dependencies mapped in Figure 4. The central place of MDTs and HIT in terms of dependencies is largely supported by an evidence base that is strong by the standards of this research literature, if not necessarily strong in Cochrane terms. The same applies to network management and cultural change. Even so, publication bias may still have resulted in successful, rather than failed, attempts to set up such mechanisms being reported in the literature that we reviewed. Because of their lack of attention to context, we also doubt that the studies we reviewed have collectively identified all the feedback loops at work in such large, complex health system changes. Therefore, the above findings are more likely to err towards overestimating than underestimating the likely effects of implementing even the revised logic model.
Similar to its predecessor, the revised logic model contains successively linked (concatenated) mechanisms: the outcome of some mechanisms is to trigger one or more further mechanisms or, indeed, feedback loops – virtuous or vicious, depending on the context. If a later mechanism in the sequence is not in fact triggered, it matters little that it would have been a powerful mechanism if only it had been triggered. Therefore, the evidence for the whole chain of mechanisms (i.e. for the revised logic model as a whole) is only as strong as the evidence for the evidentially weakest mechanism in it. Similarly, the outcomes of the whole chain are constrained by, and depend on, the weakest mechanism(s) and intermediate outcome(s) within it. In the present revised logic model, these considerations apply particularly to the final-step mechanisms. Evidence that diverting patients from hospital into primary care will increase the quality and reduce the costs of patient care was scant among the papers we found, and the contextual requirements quite restrictive.
Chapter 8 Discussion and conclusions
This review was commissioned by the NIHR Health Services and Delivery Research (HSDR) programme as part of a suite of contemporaneous reviews to improve understanding of the new models of care. The other reviews being conducted were:
-
Hanratty et al. ‘Innovation to enhance health in care homes: Rapid evidence synthesis’ (project number 15/77/05)
-
Baxter et al. ‘Understanding new models of care in local contexts: a SR using frameworks to examine pathways of change, applicability, and generalisability of the international research evidence’ (project number 15/77/10)
-
Turner et al. ‘An evidence synthesis of the international knowledge base for new care models to inform and mobilise knowledge for Multispecialty Community Providers (MCPs)’ (project number 15/77/15)
-
Bunn et al. ‘Supporting shared decision-making for older people with multiple health and social care needs: a realist synthesis to inform emerging models of health and social care’ (project number 15/77/25).
Findings from these reviews will, together, probably provide greater insight into the complexities of designing and delivering new models of care in the English NHS. However, because this review was the earliest, it was not possible at the time of writing (July 2017) for us to compare our findings with those from the other reviews. To facilitate use of our findings we have formulated (boxed) ‘prompts for decision-makers’ that provide an action-oriented, condensed set of prompts for decision-makers to consider in the light of their own local and/or regional knowledge.
In this chapter, we report on the strengths and limitations of our review and take the opportunity to critically reflect on how we applied a realist approach to this broad and complex review topic. Our methodological reflections can inform both the design and commissioning of future reviews on complex, system-level health and social care topics, as well as contribute to the development of realist methodology. To prompt methodological development, we also make methodological research recommendations. First, we present and discuss our conclusions about the policy-makers’ original programme theory. Next, we do the same for step 2 (the evidence review) and then the same for step 3 (the synthesis comparing IPT with evidence review findings).
In this review, we followed the RAMESES publication and quality standards (see table A, Report Supplementary Material 2). We used a realist approach in order to gain insight into how MCP-like mechanisms operate in the contexts of different complex systems. Grounded in the knowledge needs that were identified by service leaders, policy-makers, researchers and our stakeholder group, the review provides a decision-relevant, empirically refined understanding of how MCPs might work. We also drew on a broader international literature.
We have documented (see Chapter 3, Appendix 2 and Appendices 6–8) how we applied a realist approach so that the processes for identifying, elaborating and refining programme theories are transparent. Nevertheless, we acknowledge that the complexity of the review topic and the lack of specificity in many included studies about programme components, their implementation and/or the context in which they were delivered limited the extent to which we could contextualise the operation of mechanisms. The vast amount of published research about the policy issues that MCPs address contrasted with the limited time and resources available for completing this project, which is why we limited our selection of studies to those published from the end of 2013 to July 2017. Although some earlier studies are also cited, and although post-2014 SRs should (and often did) include the most important findings from earlier studies, additional earlier studies might also usefully have been included had circumstances allowed. That was the biggest potential empirical limitation in this review. These constraints also limited our opportunity to focus on particular key aspects of the system in depth (as Petticrew et al. 143 recommend). Economic evaluations especially tended to focus was on linear cause–effect relationships with descriptive outcome measures rather than on capturing the CMOCs and their interactions in relation to health and cost outcomes. 144 Our recommendations for further research address some of these issues.
Few reviews endeavour to explain the complex interactions that take place in a health system as a whole. Configurative SRs of social and organisational processes may provide greater insight than inappropriately applying hypothesis-testing SR methods to complex social phenomena. 145 In this way, our review heeded the call146 for research that improves understanding of how events play out within a system and capture the ways in which interactions over time can lead to the conditions that enable or inhibit further interactions. Because of the complexity of the system we were researching, we applied Occam’s Razor (explanations should be only as complex as they need to be, and no more) when constructing the ‘if–then’ statements. Not that we thought of these statements in linear cause–effect terms; rather, we used them to gain insight into the way in which CMOCs emerged over time, interacted and were ‘nested’. Neither do we go beyond the conventional ‘boxes and arrows’ representation to explore less linear ways of graphically representing logic models, so as to encompass such concepts as emergence, feedback loops and tipping points (as Funnell and Rogers147 recommend).
When policy documents were ambiguous or elliptical, focused ‘realist interviews’148 may have helped to elucidate proposed explanatory steps within stakeholders’ programme theory and provided additional insight at this stage. At the theory-refining stage, we ‘populated’ the imputed steps using the located secondary evidence. A further development of our realist approach would be to link its analytic framework more fully to other theories of organisation and health systems so as to facilitate the translation of findings between and across fields of practice, policy, and academic inquiry. 142
The strength of our review is that we have appraised and synthesised evidence about how complex systems of care operate and brought it to bear on policy-makers’ explicit and implicit understandings about how the NHS operates. Although time and resource limitations constrained, in part, the depth and complexity of this review, we believe that it does demonstrate how to conduct collaborative secondary research into complex systems. We believe that our findings, in the form of a revised, evidence-based logic model (and ‘prompts for decision-makers’) are directly relevant to decisions that national policy-makers and regional commissioners will confront in the near future. The way in which we have applied realist synthesis, with both its strengths and limitations, contributes not only to the critical development of research methods into complex systems149 but also to broader debates in public health.
From the foregoing findings, we can summarise answers to our research questions.
The initial programme theory of multispecialty community providers
We begin with the policy-makers’ original assumptions (step 1).
How do policy-makers and top NHS managers predict multispecialty community providers will generate the policy outcomes stated in the Five Year Forward View?
Chapter 4 answers this question more fully, but in brief there was no simple answer to this question. The policy-makers’ programme theories proposed a large number of links, all originating from three starting points (see Figure 1):
-
NHS managers’ action in setting up MCPs as network co-ordination structures for (at least) general practice and CHS, and for a complex of other services that varied between MCPs but usually included social services and urgent care, and (less often) mental health services
-
changes in the culture of these organisations and across the whole MCP
-
voluntary sector willingness to contribute to the activities noted in the following bullet points.
Between them, these three would be the mechanisms producing (as first-wave intermediate outcomes):
-
network management of the above constellation of organisations
-
formation of multidisciplinary care teams
-
referral networks, now planned and managed rather than emergent
-
more active development of preventative care
-
‘demand management’ systems.
Combined with other linkages, these outcomes would then launch a further set of mechanisms, producing as (second-wave) outcomes:
-
care co-ordination by means of HITs
-
further development of ‘demand management’ systems
-
care planning for patients
-
diversion of patients from hospital into primary care.
Finally, the last outcomes would become the mechanisms for reducing the cost of NHS care and improving patients’ experience of care. These effects would result from various parallel, mutually supporting and parallel mechanisms in which main linkages only are summarised above. NHS cost reduction was assumed to be the outcome of (depend on) 39 prior mechanisms in all, improved patient experience on 40.
What variants of multispecialty community provider are policy-makers creating?
So far (spring 2017) neither the policy materials we analysed, including the first-wave MCP logic models, nor professional press rapportage suggest that there are any groups of MCPs in which shared characteristics can be contrasted with those of other groups of MCPs with different shared characteristics. Rather, MCPs at this stage all serve essentially the same function (according to 5YFV6) of horizontally co-ordinating managed referral networks across general practices (and/or general practice ‘at scale’), CHS, social services, mental health, urgent care and (varying by site) miscellaneous other services. Accordingly, MCPs have a similar architecture, with a central body (perhaps one their member organisations) co-ordinating the aforementioned activities and mechanisms across the network as a whole. When MCPs do vary, it is in how each is adapted to its particular local setting and assemblage of member organisations. At most, one might say that the 14 first-wave MCPs represented 14 variants. However, MCPs are still at an early stage of development, so the question of whether or not distinct types (groups) of MCPs will develop remains open. In particular, the relationship of PCH models to MCPs is at present uncertain. In their size, structure, function and governance they are quite different from MCPs. Their policy relationship to MCPs (whether PCHs are an alternative or a part of MCPs) remains, at present, an important undecided aspect of NHS policy. The relationship of MCPs to the English version of ACOs is also unclear at present.
The review of evidence: what equivalents of multispecialty community providers or components of multispecialty community providers exist?
In current NHS policy, the main function of a MCP is to co-ordinate health-care provision ‘horizontally’ across multiple primary care and related (e.g. social care) services. Therefore, international equivalents are the organisations and networks that perform a similar function in other health systems. Chapter 1 briefly described a selection of them and Chapter 6 provided more detail. From a realist viewpoint, similarity of context is the all-important consideration in deciding whether or not a MCP equivalent might be practically transferable into NHS settings. Here, ‘context’ means factors outside the MCP-equivalent mechanisms that moderate their intended outcomes. When considering whether or not MCP equivalents could successfully be replicated in another health system, it is health system, interorganisational and organisational-level contexts that are relevant (rather than contexts operating at individual patient level). Its context determines whether or not a project reported in another health system provides a proof of concept for the NHS or, say, for health systems based on social insurance. Furthermore, MCP-equivalent organisations or networks outside England may have been set up for different purposes than MCPs, that is, to produce different outcomes, for example commercial or insurance outcomes. Therefore, we report next which of the MCP-equivalent entities reported in the literature we found were equivalent to MCPs in terms of:
-
how their client population is defined (a similarity or dissimilarity of context)
-
which services they plan and manage referral networks across (a similarity or dissimilarity of mechanisms)
-
their governance structure (a similarity or dissimilarity partly of context, partly of mechanism).
Clientele
What population is served defines the scale, range and geographical distribution of the services a MCP has to co-ordinate, and what constraints (e.g. extent of patient choice) that apply. Our review showed that MCP-equivalent organisations or networks were designed to cater for either of two kinds of clientele:
-
Individual subscribers to particular social health insurers (‘sick fund’), public (e.g. Medicaid), mutual or corporate insurers. Then, MCP-equivalent entities can plan for only part of the population of a locality, must plan for a clientele that may be widely geographically dispersed and may be unable to decide which providers their clients use (e.g. in Germany). Then, for example, they must recruit patients voluntarily to any programme that selects service providers with a view to reducing referrals and costs. Unless they can informally negotiate other arrangements,46 they are obliged to pay hospitals for each referral the hospital can attract. This applies to the Kinzigtal project (Germany), French and German primary care providers generally, and most American ACOs and PCMHs. In the USA, providers can and do select their clientele by insurance status.
-
Similar to the NHS as a whole, MCPs cater for whole populations defined by place of residence. The same applies to MCP-equivalent bodies in Sweden (e.g. the Norrtälje project24) some Catalan primary care providers, USLs in Italy, LHINs in Canada and the (in that respect atypical) population-based ACO in New Jersey. 71 Planning and referral network management mechanisms developed in this context are more likely to be directly relevant to MCPs.
Within either type of clientele, some MCP equivalents serve everyone, whereas others serve specific care groups defined by morbidity, such as people with multiple long-term conditions, frail older people or people with mental health problems (again, Chapter 6 gives some details). The literature we reviewed focuses less on groups of people with a single major condition (but when they do, coronary heart disease is often studied), but more often on morbidities with functional impact morbidities (e.g. diabetes mellitus) and some forms of high functional impact multimorbidity (e.g. dementia).
Services
Which MCP-equivalent organisation or network is the most relevant proof of concept, even prototype, for a particular MCP will depend on which services it is especially attempting to co-ordinate (i.e. which interorganisational boundaries it is attempting to surmount). Interface by interface, that approach suggests the following international partial equivalents to MCPs:
-
Primary medical care (GP or equivalent) with CHSs – in the existing NHS, these organisations are separate. International equivalents include Kinzigtal (Germany), the Versailles geriatrics network, LHINs in Canada, certain ACOs (e.g. in Texas and Colorado82) and some instances of PCMH (e.g. in Manhattan94). As proof of concept examples of what can be done to integrate these services organisationally, the most relevant international equivalents are Swedish PHCCs (vårdcentral, ‘polyclinic’). Between the two sets of equivalents are the Italian USLs, in which domiciliary nursing care is organisationally integrated with primary medical care, but in a structure of separate subhierarchies (‘silos’) with a common manager only at the most senior level. Catalan primary care centres typically organisationally integrate GPs with CHSs, with specialised services for women’s health and paediatrics and, on occasion, other specialties.
-
Primary medical care (excluding CHS) with mental health services – international equivalents include the Norrtälje project (Sweden24), Inter-Mountain Health, Utah66 and (more narrowly because essentially contract driven) the Integriete Versorgung mental health schemes in Germany. 150–152
-
Primary medical care with social care – for this interface, international equivalents include Local Community Services Centers (Québec), public clinics providing health with social services60 and the Mount Sinai organisation in New York. 78 Italian USLs organisationally integrate primary medical care and social care, but (as noted) in a structure of separate subhierarchies (‘silos’) with a common manager only at the most senior level. Primary care centres in Catalonia offer strong co-ordination by including the provision of social care services, although health and social services remain managed through different hierarchies.
-
Primary medical care with community pharmacy – accounts of this interface were rare. We found just two studies80,100 that described how these two services were co-ordinated, although older studies153,154 about the UK also exist.
-
CHS with social care – for organisational integration between CHS and several forms of social care, some of them (including nursing homes, hospices) residential, an obvious international equivalent to a MCP (including separation of CHS from general medical practice) is Buurtzorg in the Netherlands. 21 In the Italian USLs, social care is organisationally integrated with primary medical care, but in a structure of separate subhierarchies (‘silos’) with a common manager only at the most senior level, much as in Northern Ireland in the past. 155,156
-
CHS with mental health services – again, Inter-Mountain Health instantiates such co-ordination, but Canadian LHINs (for instance in Toronto) developed in a context more similar to that of the NHS and the Norrtälje project in a context more similar still, with a substantial local government role and mental health services previously organisationally separate from other health services.
-
General practice ‘at scale’ – if this means many small, even single-handed, practices co-ordinated through a hub, the HealthOne Mount Druitt project in New South Wales provides the equivalent for those parts of the NHS with many single-handed, general practices dealing with deprived populations. If ‘at scale’ means employing large numbers of PHC doctors in one organisation, more relevant MCP equivalents include some large US providers (e.g. Group Health, Kaiser Permanente). Swedish primary care clinics also tend to be managed in large groups, either by a municipality, a corporation or, in the case of Praktikerjänst, a health worker co-operative that supplies > 15% of primary medical care in Sweden. 23 The PCMH concept derived originally from US perceptions of NHS general practice has now been reimported back to England as a ‘new’ model of care. It is probably intermediate between general practice and a MCP with a larger population and ambitions to incorporate community services and reach across the interface to hospital, but could also be a constituent of a large MCP operating across the catchment of one district general provider hospital.
The above are possible prototypes for interorganisational co-ordination (through referral networks) or organisational integration of primary care. For MDT prototypes and for successful instances of using HIT for care co-ordination, one has to look to the particular studies cited (see Chapter 5).
Governance structures
The types of governance structures within which MCP equivalents are embedded are important contexts defining their relevance (equivalence) to MCPs. Governance structures within them are another point of equivalence (or not) for MCP mechanisms. Modifying Thomson’s157 categories, the three types of governance structures relevant to MCPs are quasi-markets, hierarchy and networks.
-
Networks: the more numerous and varied the member organisations a network contains, the more numerous and varied are the interorganisational boundaries it has to surmount. Similarity in the number and mix of member organisations are, therefore, important criteria of equivalence (relevance) to a given MCP. Hence, two important equivalents (or approximate equivalents) to MCPs are:
-
Networks of doctor-owned practices, including substantial proportions of both single-handed practices and partnerships (see the discussion of ‘general practice at scale’). In these respects, the nearest equivalents to MCPs are Medicare Locals in Australia; in particular, the more ‘integration’ minded ones (e.g. HealthOne Mount Druitt) and local networks (a category partly overlapping with ACOs and the PCMH, but also with Health Maintenance Organizations) in the USA
-
Mixed networks of doctor-owned practices, corporations and voluntary organisations. The relevant examples here are networks such as the Kinzigtal and the Versailles examples, many ACOs in the USA and LHINs in Canada.
-
-
Quasi-markets: for attempts to govern MCP-equivalent groups of organisations by means of contracts (quasi-market governance), the relevant examples are the American ACOs and the Integrierte Versorgung mental health networks in Germany. Although these instances suggest that contracts could finance, even incentivise, organisations to join a network, pursue common goals and MCP-equivalent care co-ordination, US legal studies158,159 also suggest that contracts alone are both too incomplete and too inflexible to establish, by themselves, the mechanisms of action described below. Contracts are between payer and provider organisations, not between clinicians or (even in France and the USA) between clinicians and patients. Other governance and co-ordinating mechanisms are required to supplement them.
-
Hierarchy: as the external context of MCPs, hierarchy would imply either organisational integration into, say, a municipality or, as in the current NHS, a highly centralised and centrally controlled network of formally independent organisations: a ‘quasi-hierarchy’. 160,161 As already noted, the nearest equivalents to a hierarchically structured MCP are the Swedish and Finnish primary care clinics, USLs, some US organisations (Group Health, Kaiser Permanente), and some primary care providers in Catalonia.
The above lists are not exhaustive, even for the MCP-equivalent entities that our secondary data covered. Many studies say only vaguely that a given project ‘brings together’ different providers, omitting the all-important (for present purposes) details of the mechanisms used and whether or not the providers remained organisationally separate. However, the above lists do suggest starting points for developing sampling frames for more detailed research into the mechanisms used.
How do these equivalents and their mechanisms compare to those proposed in the initial programme theory for multispecialty community providers in the NHS?
Does evidence about how multispecialty community provider equivalents ‘work’ support our revised logic model?
The exact mechanisms vary equivalent by MCP equivalent. Chapter 7 itemises the causal links between the components of MCPs in our revised logic model. Nevertheless, certain triggers of care co-ordination mechanisms recurred across many MCP equivalents and contexts.
Tables 17 and 18 in Chapter 7 illustrate the number of studies providing supporting evidence for each mechanism. The six most frequently mentioned mechanisms (each across their different causal links) are what we next report. Five of the six most frequently mentioned mechanisms were also the ones with ‘substantial’ evidential support (i.e. both SRs and additional primary research) according to our realist review (see Tables 17 and 18). The exception was network management, based on ‘supporting’ rather than ‘substantial’ evidence. Most of the studies we reviewed were non-realist and, therefore, present their findings in terms of what we have called the ‘components’ that are antecedents to or triggers of the mechanisms that make up our revised logic model. For short, we use the term ‘MDT based’ to indicate the set of mechanisms for which MDT is the antecedent or trigger (and analogously for the other groups of mechanisms).
Health information technology-based mechanisms
A recurrent theme was that HIT, in particular the EHR, had an effect through the impact that it had on work processes such as task reminders, delegation, workflows and informal communication among staff, instant messaging, within-chart notes and telephone templates that could be routed to team members’ inboxes, task assignments and ‘huddle sheets’ among others, in addition to data retrieval and communication (Box 1). One study116 found that primary care teams that used EHRs consistently for data entry and agreed on communication methods between staff members were more likely to score highly on the NCQA 2011 PCMH recognition tool. This compound mechanism relies heavily on the quality of HIT design in terms of functionality (whether or not a HIT can perform risk stratification, manage workflows, etc.) and interoperability between IT systems, with the prior requirement that such systems are actually available at all.
The right hand cannot work effectively if it doesn’t know what the left hand is doing. How are care plans and work roles being communicated across organisations in your system? What IT systems are available to support this? Importantly, are the IT systems designed so that each health worker can easily and conveniently access and use all the data that he/she needs to read and write, in order to co-ordinate a patient’s care? Is there just one system (and no parallel paper systems)? Can patients access their care plan?
Therefore, we conclude that HIT-based mechanisms will underpin achievement of multiple MCP-like functions and that they can operate whatever the structure of teams and organisations.
Multidisciplinary team-based mechanisms
A similar theme recurred in studies of MDTs (Box 2). MDTs improve patient experience through the impact that it has on many everyday clinical working practices: enhanced patient access to services (e.g. to primary care as an alternative to unnecessary hospital admission); better communication between providers and, thus, more patient confidence in, trust in and satisfaction with care; and a more holistic approach to care. For MDTs to work, it is necessary that they focus on tasks of practical value to their members, include the relevant services, and actually work in a collaborative, interprofessional way within the team itself. An essential component of this mechanism is boundary-spanning roles, such as that of the care co-ordinator whose professional origin appears less important than his/her capacity to support care planning for individual patients (see Care plan-based mechanisms), improve the continuity of care, make care more person centred and promote shared decision-making. Although rare in practice, patient participation in the MDT facilitates all this. When different professions work for different organisations, the boundaries to be spanned are simultaneously interprofessional and interorganisational. Several studies reported the value of face-to-face communication within teams, which implies a practical value for care co-ordination in colocating MDT members.
Multidisciplinary working is central to well-co-ordinated (‘integrated’) care delivery. Individuals are motivated to participate in multidisciplinary teamwork when it improves care and makes their work easier or more productive. Do professionals and patients in your region have a good understanding of how multidisciplinary working can improve care? Do professional and organisational cultures reward or discourage multidisciplinary working? Where are the key points in your system where ‘boundary-spanning’ roles could facilitate multidisciplinary working? Do not underestimate either the importance of patients’ participation in MDT meetings (their ‘seat at the table’ can provide the focus that makes care more patient centred) or of the need to consider power differences between professionals, and between professionals and patients.
Although the formalising of multidisciplinary working into teams, with clarity about roles and boundary-spanning activity, is likely to contribute to MCP objectives, it is unclear in what contexts new teams should form or existing ones be enhanced, and which functions (admission avoidance, proactive care planning or enhancing social connectivity) are particularly supported by MDTs. There is no clear guidance on how much to focus on protocolised role clarity or on flexibility and reducing differentiation between occupational groups.
Care plan-based mechanisms
As a mechanism for diverting patients from hospital to primary care, care plans work by being implemented, above all, by a boundary-spanner (e.g. care co-ordinator). This implies a single care plan (not multiple duplicating plans, as often happens in practice23) for each individual covering all their health-care needs. The care plan can be disease oriented or address an individual’s more social and emotional goals as well as aim to reduce burden of care. As a mechanism for diverting patients from hospital to primary care, care plans work by being implemented above all by a boundary spanner (e.g. care co-ordinator). One component of this mechanism is to develop patients’ self-care and self-management of their condition, which may itself require patient education and indeed patients’ and/or informal carers’ participation in the care planning, shared decision-making and even patient advocacy. Another component is real-time information about what is happening to the patient (see Health information technology-based mechanisms) so that the care co-ordinator can plan and manage the transitions between hospital and home, and other changes in the patient’s condition or circumstances.
The studies available to us contained little evidence about how clinicians or a MDT might use the making of a care plan as a means of deciding with the patient, or at least among themselves, whether or not the patient needs certain kinds of more intense care (e.g. medications, hospital admission). Although enhancing care planning activity (both the interactive decision-making itself and then making shared, comprehensible documentation of the decisions available) appears to be key to generating better outcomes, there is little evidence to guide the level of complexity and multimorbidity that necessitates a shift towards more complex, multidisciplinary plans.
Culture change-based mechanisms
Of all the mechanisms in the IPT, these were the most obscure (Box 3). Many studies examine organisational cultures and cultures of multiprofessionality or collaboration in other health-care settings. Among the studies that we found, many invoked culture change as a mechanism that organisations or networks exploited but few explained how that culture change was produced. Those that did mentioned interprofessional and/or interorganisational training. Some appeared to assume that ‘leadership’ was responsible, perhaps for culture change but certainly for setting up the boundary-spanning mechanisms described above. Two studies62,68 implied that culture change was not the original change-driving force, but perhaps a part of a virtuous circle driven by other causes.
Professional and organisational cultures are important, but we know less about exactly how they impact on achieving change in the delivery of care. This is an area that requires further research. For now, don’t assume that what is accepted in one profession or organisation will necessarily be accepted in the same way by others. We can begin by asking ourselves and our colleagues ‘what are our organisational values?’.
Despite this lack of evidence, we did not interpret culture change as being unimportant; rather that we need more research to define what aspects of culture (e.g. interprofessional equality, person-centredness, positive risk taking) are most important and whether they should be the direct subject of training or seen as indicators of success.
Planned referral networks-based mechanisms
This component was one of designing referral pathways for the main care groups, establishing agreed divisions of labour and working practices across different provider organisations (‘care compacts’), criteria of appropriate referral and, for patients who do not need hospital admission, alternative destinations than hospital, including what in the UK is called ‘social prescribing’ to voluntary sector resources.
Network management-based mechanisms
A network managing (or co-ordinating) body is the mechanism for managing the care ‘continuum’ (i.e. the patient’s experience of care as a whole and over time) (Box 4). Critical components of this mechanism are shared goals and boundary-objects (i.e. objects used in common by all the member organisations at their interfaces), such objects as care compacts, EHR, patient care plans, formularies, agreed care standards and interorganisational care pathways (in addition to any that are used just within a single organisation). Such a network co-ordinating body deliberately supports the production of these goals and objects for the network as a whole, whether by creating them itself from scratch or by adopting and developing any such goals and objects that have already spontaneously emerged ‘bottom up’ from within, and between, the network’s member organisations. Boundary-spanning staff roles are one essential component of this mechanism too. Another is referral network planning (see Diverting patients from secondary to primary care, thereby reducing costs).
Co-ordinating the delivery of complex care across organisations is not easy. The ‘tools’ to enable this co-ordination (such as care plans, EHRs, designated roles) need to be accessible to multiple parties, contain and communicate accurate information, and be perceived as useful and usable. In introducing or revising these tools, a balance needs to be struck between ‘bottom-up development’ and ‘top-down prescription’. How can this be achieved given the particularities of your area?
How do these mechanisms depend on specific contexts
Chapters 6 and 7 itemised which specific contexts each MCP component requires when operating as a mechanism to produce other components. Nevertheless, certain contexts recurred across more than one causal link between the 13 components. Briefly, they were:
-
prior collaboration and mutual trust between provider organisations
-
funding for the start-up costs (network formation, HIT, training) and to establish primary care alternatives to hospital, include payment to enable patients to access voluntary sector support
-
clinician time for setting up and then participating in MDTs
-
status differences between professions and professionals are weak, or deliberately weakened, to facilitate the culture changes mentioned above (see Culture change-based mechanisms)
-
lack of health-worker resistance; GP (or equivalent) participation in particular is indispensable
-
patient’s active participation in the co-ordination of their care and in self-managing their condition, when feasible
-
suitable HIT systems exist (or can be constructed) and are obtainable
-
alternative PHC services to hospitals exist, and are of the necessary types and scale
-
a suitable case-mix of patients, that is, patients who –
-
are heavy users of hospital services (five or more admissions annually)
-
have complex, not well-understood health problems, whose management often requires informal discussions among health workers
-
have chronic single conditions with well-defined treatment plans; hence, are more suitable for HIT-based methods of care co-ordination
-
-
colocated staff, whether out-posted, ‘embedded’ (i.e. seconded) or all employed by the same provider organisation. Colocation requires a suitable clinic (or similar) as the place of colocation or, failing that, organising base for virtual MDTs. 76 It could also be the place for the co-ordinating body of the MCP as a network of provider organisations, and as the central ‘hub’ for a network of general practices. Not least, colocation provides the opportunity for interprofessional working not only in formal meetings but in everyday, informal working practices such as ‘huddles’.
Some evidence about contexts was conflicting; in the realist view, this was a possible marker for as yet undiscovered contextual moderators of the mechanisms mentioned above (see Network management-based mechanisms).
Some of the component mechanisms were mutually reinforcing and had common elements (e.g. boundary-spanning staff); such were MDTs and HIT, and cultural change and MDTs. Notwithstanding the ‘mechanism’ metaphor, all of the above components of MCPs when acting as mechanisms consist (we reiterate) of the individual actions, understanding and resource use (in short, working practices) of the clinicians, managers, other staff and the other agents involved, not least, patients. It should also be noted that the above components act as mechanisms for cross-organisational provision and co-ordination of care, typically for people with chronic, and often multiple, health problems. They are not necessarily needed to provide more casual, non-complex episodic care.
What policy outcomes are these equivalents reported to produce?
Our evidence review provided evidence about MCP equivalents and whether or not, and how, they bring about the two central outcomes of the initial MCP programme theory (i.e. cost reduction and good quality of patient experience of care).
Diverting patients from secondary to primary care, thereby reducing costs
A number of studies reported MCP-equivalent organisations and networks diverting patients from secondary back to enhanced primary care. A few of them suggested what mechanisms and contexts had produced these outcomes. Again, Chapter 6 provides further detail. These studies offer proofs of concept that the mechanisms can produce these intended outcomes, provided the mechanisms are correctly implemented and provided the relevant contextual conditions are present.
Across several countries, the balance of evidence tended to suggest that more active care co-ordination across organisations (and, for emergency admissions, home telehealth programmes)85 can reduce ED use, hospital admissions or readmissions. Studies from Australia,62,90 Canada,99,119 England76 and the USA66,130,131,137,139,162 reported various combinations of such reductions and greater use of (enhanced) primary care services. A SR126 and a meta-analysis of RCTs105 both suggested that transitional care interventions tend to reduce hospital readmissions of chronically ill patients. The three exceptions to this pattern were only partial exceptions. One US study showed no decrease in ED use but did show greater use of preventative and ambulatory care. 138 Two US studies71,127 showed reductions in specialist use for low-and medium-morbidity patients but the opposite for high-morbidity patients. Therefore, the overall pattern suggests that MCP-like interventions can, in favourable contexts, produce the desired outcomes but with two important caveats. First, we have to be aware of publication bias; failed attempts may be less likely to be published. Second, the devil in these studies is in the detail of what specific mechanisms and contexts were necessary.
Supposing that in favourable contexts these mechanisms do reduce unnecessary referrals, we found less evidence for whether or not overall costs of care consequently fall. Several studies (see Chapters 6 and 7) attributed cost reductions through HIT to the partial automation of work, provided the conditions mentioned in Chapter 6 were satisfied. HIT was also an element of the Kinzigtal project, which achieved cost reductions for the social health insurers. 81 However, there was also a little evidence that HIT in the PCMH context reduced specialist visits. 122 However, one study139 did estimate cost savings arising from stronger care co-ordination reducing ED visits (in that study, US$1.4M annually across 14 medical practices serving 25,356 patients). So, although the evidence base is smaller and weaker, this overall pattern also suggests that such MCP-like interventions can, in favourable contexts, reduce the use of hospital services in a suitable context, but the requisite context is, Chapter 6 suggested, narrowly defined unless the savings per episode accrue directly to the primary care provider or payer in the form of reduced tariff bills. Furthermore, this evidence comes from health systems facing less severe budgetary constraints than the current NHS.
Patient experience
For conditions in which the very occurrence or exacerbation is itself an outcome, and for which evidence-based treatments exist, some studies of MCP-like schemes did report improved outcomes, for instance fewer ED and hospital admissions for asthma94 or increased screening of diabetes mellitus and hypertension patients leading to the prescription of preventative pharmaceuticals. 100 Diabetes mellitus is one such condition in which improved outcomes were, according to several studies (including one SR), associated with MDTs, ‘leadership’ (managerial) commitment to changed working practices, shared goals and staff involvement (in designing and implementing the care pathway). The use of EHRs has also been reported to accelerate ‘quality improvements and changes in utilisation over time on some measures’,115 again in several countries: Germany, the Netherlands and the USA. Two other characteristics that are shown in several studies to improve patients’ experience of care in MCP-like organisations and networks are (1) the use of patient panels to strengthen trust in patient–provider relationships91,163 and (2) personalised care and support from people working in boundary-spanning roles. 66,164
However, the studies we reviewed generally lacked evidence about how to evaluate, monitor and adjust the overall flow of patients within a MCP equivalent in order to ensure that it can achieve its aims of improving care within tight resources.
Perverse or unforeseen outcomes
The studies that we reviewed also reported certain perverse outcomes from MCP-like networks and organisations, unforeseen in the UK policy documents:
-
More efficient demand management systems increase case finding, leading (at least initially) to more rather than fewer hospital referrals.
-
Increasing hospital and PHC efficiency increases the total costs of care for the reasons noted in Chapter 6.
-
Roemer’s law133 increased provision (in this case, enhanced primary care and reduced pressure on hospital beds) leads to increased service use,132 whether by lowering referral or treatment thresholds, meeting hitherto unmet needs (see point 1), adding preventative to existing curative services or making it easier for patients to access enhanced primary care. 131
To these outcomes must be added the perennial uncertainties of implementation, especially when changes (such as revising occupational roles) are contentious and may be resisted or renegotiated. 95 As Pineault et al. 60 observed in Québec, modifications of structures and resources come first, with new working practices always lagging behind.
Implications for multispecialty community provider design
The evidence used to answer the above questions, and on which the revised logic model is based, has implications for the organisational design (governance structures, internal management and working practices) of MCPs. These implications become especially clear if, from our earlier revised logic model (see Figure 4), we remove parallel (duplicated) links to leave the graphically simplified, but still multilink version, shown in Figure 6. Nevertheless, the revised logic model is based on evidence about all links, both direct and indirect, between the main components of the programme theory.
FIGURE 6.
Simplified revised logic model: parallel (duplicate) links removed.
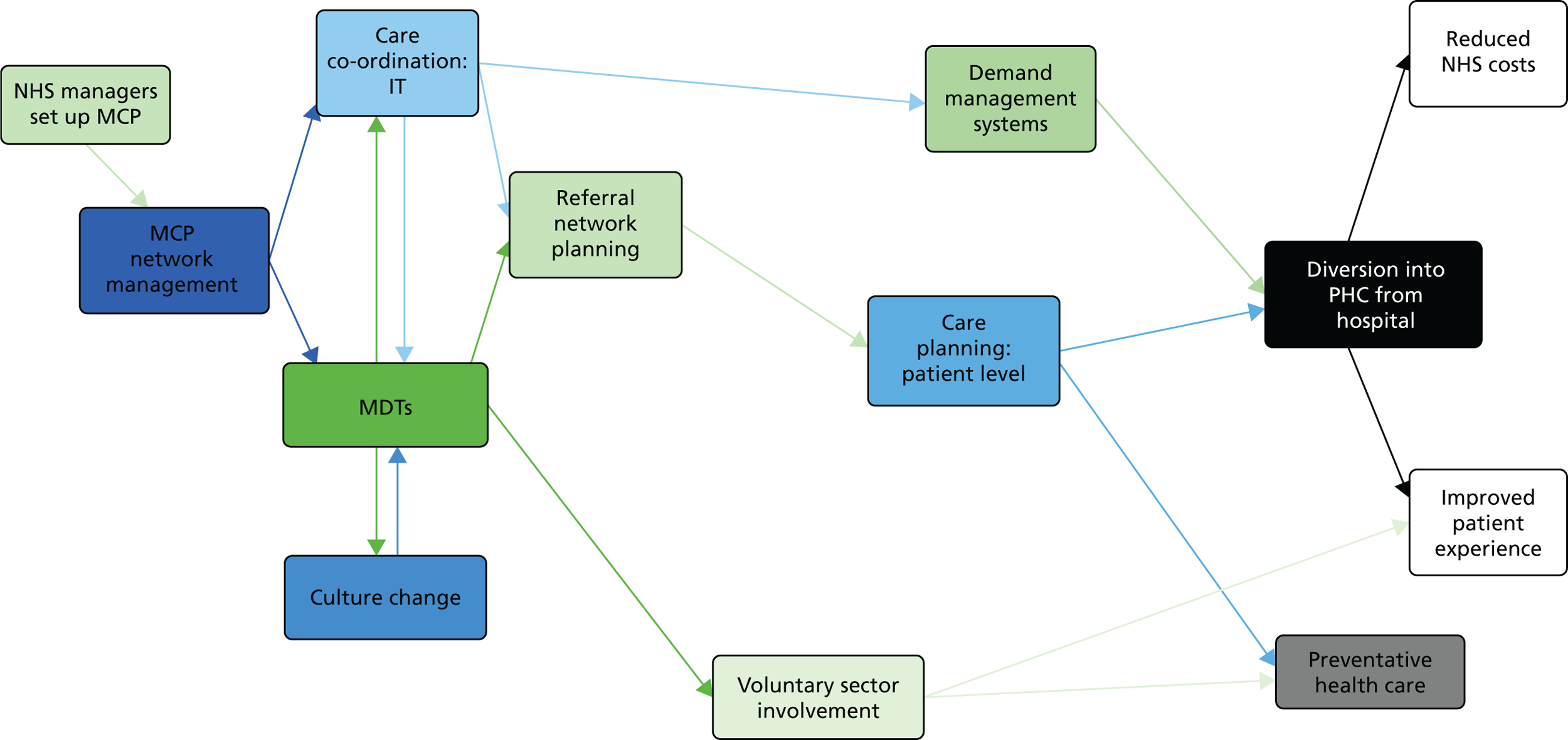
A major implication of our evidence is that MDTs are likely to be the central mechanism by which MCPs work, provided that the MDTs include the relevant professions (hence, organisations) for their care group(s) and indeed, when it comes to care planning, for their individual patients. The foregoing evidence (see Chapter 6) implies that there are three dimensions to this:
-
setting up new MDTs as a core component of a managed referral network, such as the locality teams that many MCP are setting up to manage admission avoidance, for long-term care management and for well-being promotion including social prescribing
-
enhancing existing teams (e.g. in general practices on the PCMH model) that already co-ordinate care for individual patients
-
supporting interprofessional links and collaborative working practices within existing MDTs at both the above levels.
The evidence available to us (see Chapter 6) did not really distinguish sharply between these different functions of MDTs, and the implications for how they might work as mechanisms within MDTs.
For MCPs, and MDTs within them, to function as care co-ordinators and operate the relevant referral networks requires the creation of roles that span the boundaries between organisations and professions. The care co-ordinator is the critical role, but not the only such one. The means of boundary spanning, and for making MDTs have an impact on working practices in ways that are of practical use and value to MDT members, are to create and use boundary objects such as agreed referral criteria, care compacts, shared documentation and agreed standards of care, etc. (see Chapters 6 and 7). The use of HIT, in particular shared EHRs, is an important such boundary object, provided they are designed and implemented as part of clinical working practices, not independently of them. Other critical mechanisms are the interorganisational management of MCPs as a whole referral network, and the use of a shared (not just uni professional23 care plans for each patient with sufficiently complex needs). The most important contexts required appear to be, first, a strong culture of mutual knowledge between professions of what other professions contribute to care, of its value and hence attitudes of mutual respect favouring collaboration. A second main context is the existence of alternative primary care and social services to divert suitable patients into as an alternative to hospital. Colocation and co-employment of MDT members is a third favourable context. However, these contexts facilitate the MDT and its associated mechanisms and are not substitutes for them.
Our findings also suggest that certain general characteristics of governance structures would appear to promote the purposes for which the NHS established MCPs. The governance structures need to enable information sharing between provider organisations, including at clinician-to-clinician level. However, information sharing alone is insufficient. Such governance structures also require the means to promote (to model, incentivise, even coerce) a system-wide division of labour and care co-ordination. They have to include all the relevant providers (see Chapter 6, Setting up multispecialty community provider-like organisations and networks). So far as is possible, the governance structure should be based on (support, strengthen, formalise) existing collaborative and co-ordinational relationships. Specifically, they have to accommodate MDTs, making them collectively accountable for patient care (see Chapter 6, Interorganisational network management), boundary-spanning care co-ordinator roles (see Chapter 6, Interorganisational network management) and rich informal communication (e.g. ‘huddles’) (see Chapter 6, Demand management systems). More perhaps a question of governance style or culture than of governance structure, their managers should resist the temptation to micromanage professional work (see Chapter 6, Setting up multispecialty community provider-like organisations and networks) or to restrict providers’ flexibility to redesign care models and reallocate resources accordingly. Buurtzorg is proof of concept of how a high degree of delegation to MDTs is feasible, with concomitant managerial cost savings. At a minimum, these conditions imply a densely linked care network with a central co-ordinating body, that is, multiple separate providers (general practices, CHSs, third-sector providers, etc.) working together as a single entity with aligned goals and the co-ordinating body instigating collaborative working. The large body of literature on health-care networks and ‘integrated’ care reports many examples.
Such a governance structure might be supplemented with contracts or developed into a single organisation.
Our findings suggest that, although contractual co-ordination can, under favourable conditions (as in the examples of Kinzigtal and certain American ACOs), be used for some MCP-like purposes, it also presents certain difficulties compared with network and hierarchical governance structures. The difficulty of contractual ‘overhangs’ (see Chapter 6, Multidisciplinary teams) or ‘carve-outs’,56 excluding relevant services from a MCP-like entity, is a transitional problem until those contracts are renegotiated. The same applies to converting non-aligned payments and incentives (see Chapter 6, Planned referral networks) into a consistent set of contracts that share cost savings between different providers. A bigger difficulty is that of contracts being at once both too rigid and too incomplete (e.g. regarding practicable monitoring of outcomes) to co-ordinate care at MDT and individual clinician levels. For some self-employed professionals, an attraction of being an independent contractor is explicitly that it appears to limit the state’s or a corporation’s ability to control (including co-ordinate) their work (see Chapter 6, Setting up multispecialty community provider-like organisations and networks).
Alternatively, MCP equivalents can be (and in some countries are) constructed as a single organisation. Our review found numerous structures and contexts that reportedly improve the continuities of care and other aspects of patient experience, and that would appear to be easier to implement within a single organisation. They included:
-
compatible and interoperable IT systems, in particular EHRs (see Chapter 6, Care co-ordination through health information technologies)
-
data sharing (see Chapter 6, Care co-ordination through health information technologies); hence, risk stratification
-
informal contact and familiarisation with other professions’ roles, hence the development of interprofessional trust (see Chapter 6, Interorganisational network management)
-
colocating staff (see Chapter 6, Care co-ordination through health information technologies)
-
mutually consistent working practices and routines such as care compacts, formularies and referral rules (see Chapter 6, Interorganisational network management; Chapter 6, Multidisciplinary teams; Chapter 6, Care co-ordination through health information technologies; and Chapter 6, Planned referral networks).
-
shared standards of care (see Chapter 6, Interorganisational network management), arising partly from shared research and development (see Chapter 6, Interorganisational network management)
-
cross-professional boundary-spanning structures and roles (see Chapter 6, Planned referral networks), including the construction of referral networks (see Chapter 6, Planned referral networks)
-
overcoming past isolation or separation of necessary services (see Chapter 6, Interorganisational network management; and Chapter 6, Planned referral networks) and to that extent removing interorganisational boundaries
-
mutual access to shared resources (see Chapter 6, Interorganisational network management; Chapter 6, Multidisciplinary teams)
-
uniform cross-disciplinary training about IT and care integration (see Chapter 6, Interorganisational network management)
-
planning of care pathways (see Chapter 6, Multidisciplinary teams)
-
shared expectations (see Chapter 6, Multidisciplinary teams) and cultures (see Chapter 6, Culture change)
-
reduced role overlap and ambiguity (see Chapter 6, Multidisciplinary teams)
-
structured communication within MDTs (see Chapter 6, Multidisciplinary teams)
-
whole-population-level service planning (see Chapter 6, Multidisciplinary teams)
-
task delegation, referral and reallocation (see Chapter 6, Multidisciplinary teams; and Chapter 6, Planned referral networks)
-
alignment of payments to different services (see Chapter 6, Planned referral networks).
The case for a single organisation should not be overstated. Some of the above conditions (e.g. shared IT systems) are necessary, but not sufficient, to improve care co-ordination (see Chapter 6, Demand management systems). Some of them (e.g. staff colocation) have also been achieved within networked structures. The above list of conditions also leaves unanswered the question of whether or not a single-organisation (organisationally integrated) MCP would be most likely to serve the purposes described in Chapters 1 and 5 if it were under public, co-operative partnership or corporate or voluntary ownership. Without guaranteeing them, organisational integration would, nevertheless, appear to increase the opportunity for the above conditions to arise, whether emergently or in a deliberately managed way.
Recommendations for research: models of care and methodology
Models of care
Further primary research would be required to test elements of the revised programme theory. In the research that we reviewed, a number of gaps were apparent. They indicate further research needs. We judge them to be in the following descending order of importance. They concerned:
-
How, and what circumstances, MDT-based locality teams and enhanced general practice (the PCMH and general practice ‘at scale’) compare and interact, or can be combined, in managing referral networks so as to reduce workload for other health-care providers.
-
Whether or not and, if so, how and in what circumstances, diverting patients from hospital into enhanced primary care does indeed:
-
reduce the overall cost of health care
-
improve patients’ experience of care.
-
-
How general practices are affected and have to adapt if larger numbers of patients are diverted from hospital to enhanced primary care.
-
How the other new models of care (above all, PACS) being developed concurrently with MCPs interact with MCPs. The work would compare and synthesise the findings from this studies with those from the concurrent studies of the other new models of care.
-
How urgent care services will be affected and have to adapt if more patients are diverted from hospital to enhanced primary care.
-
How care co-ordination through HIT supports (or not) the:
-
– management of interorganisational referral networks
-
– diversion of suitable patients from hospital into enhanced primary care services
-
production and use of care plans for individual patients.
-
-
How the resources and mechanisms deployed in MCPs will contribute to changing care for different groups of people [defined by morbidity, e.g. single major condition (such as cancer), multiple low functional impact morbidities (e.g. diabetes mellitus, HT), high functional impact multimorbidity (e.g. stroke, arthritis, dementia)].
-
How referral networks are established and managed in such a way as to establish referral management systems.
-
How and under what circumstances the management of referral networks promotes (or not) the use of care plans for individual patients.
-
How and under what circumstances the voluntary sector and MCP-like networks and organisations collaborate in pursuit of the ends for which MCPs were set up.
-
How organisational culture is produced and changes in MCP-like contexts (an area lacking research despite the abundance of studies in hospital and non-health-care settings).
As previously noted, equivocal research findings suggest (to realists) areas in which as yet unknown contextual factors might be strongly influencing the effects that component mechanisms of MCPs have. The main ambiguities, requiring further research to resolve them, concerned the contexts in which:
-
‘horizontal’ MCP-equivalent networks develop interorganisational referral networks, in particular between GPs and CHSs (or the local equivalents)
-
care co-ordination through HIT supports (or not) the:
-
management of interorganisational referral networks
-
diversion of suitable patients from hospital into enhanced primary care services
-
production and use of care plans for individual patients
-
-
the management of referral networks promotes (or not) the use of care plans for individual patients.
Methodological development
Our methodological reflections relate to practical (the critical appraisal tool), conceptual (mechanisms and ‘nested’ or ‘ripple’ effects) and translational (practicable outputs for knowledge-users) issues for realist syntheses.
First, the practical issues: our experience of using the MMAT was consistent with the evaluation that demonstrated acceptable inter-rater reliability and timely completion. The MMAT fulfilled its task of structuring critical appraisal of quantitative, qualitative and mixed-method studies, and of the different study designs within each of these paradigms. It also provided criteria for making a judgement about a global quality score for each included study. However, we found it somewhat restrictive in critically appraising the broader aspects of studies that in our view were important for enabling a more nuanced treatment of relevance and rigour in the synthesis. Therefore, we remain unconvinced, for the purposes of realist synthesis, of the benefit of using a mixed-methods critical appraisal tool over using multiple (study type-specific) tools or a generic critical appraisal tool.
Second, the conceptual issues: we adopted an established definition of ‘mechanism’ and used the ‘trick’ of working backwards from an identified outcome to help identify CMOCs. However, we struggled at times to identify mechanisms in the reviewed studies both because some included studies lacked conceptual clarity and because of the slippage we persistently encountered between mechanisms as ‘the thing that causes’ and mechanisms as ‘the thing that is triggered by the circumstances’. This is an important distinction, especially when endeavouring to conduct research that accommodates systems concepts such as emergence, feedback loops and tipping points. Realist thinking endeavours to capture this by allowing consideration of how, over time, mechanisms can lead to the circumstances in which they become contexts that, in turn, potentiate other mechanisms, which may, in turn, transform the context, and so on. 165
This last point leads us to consider the way in which the transformations that are enabled have been termed a ‘ripple effect’. 165 Thinking in terms of a ‘ripple effect’ may indeed be valid for fundamental and wide-ranging mechanisms (such as trust) that have positive effects. However, this risks steering thinking and analysis towards identifying ‘golden mechanisms’ that explain everything at once rather than the somewhat knottier issue in complex systems of identifying multiple mechanisms firing concurrently, possibly in both desired and undesired ways. For example, in our review we identified how the perceived relevance of new structures and ways of working (to managers, practitioners, and service users) pivoted on whether or not they could see how those changes would contribute to meeting patient care needs. Similarly, we identified how practitioners’ engagement was influenced by the value that they placed on the new models as a means of accessing to specialist knowledge or resources. In both of these examples, the mechanism (‘valuing’) could be either positive or negative, enabling or constraining progress towards a desired set of (demi-regular) outcomes and occurring in concert with a range of other CMOCs. In these examples, thinking in terms of a ‘ripple effect’ is too stark and too strongly suggestive of configurations whose outcomes are only positive and synergistic. To accommodate concepts such as emergence, feedback loops and tipping points, and both desired and undesired outcomes, it is better to think about CMOCs being ‘nested’ within each other.
Third, the translational issues: we have endeavoured to show the practical implications of our review. Although we do not have evaluative knowledge about the extent to which knowledge users find such outputs or even how (or whether or not) they use them, such translational outputs are reasonable at face value, and are pitched in similar terms to ours and are consistent with the discussions with our stakeholder group, which emphasised the attractiveness and ease of use of graphical representations, compared with the large amounts of text that NHS staff receive.
Methodological research recommendations
-
Comparative research to establish an optimal (i.e. accurate, usable within a reasonable time frame) critical appraisal tool for the study components necessary for refining programme theory.
-
Exploratory research into ways in which consistent definitions of key realist concepts (in particular, ‘mechanism’) can be applied by those whose experience of applying realist methods ranges from ‘novice’ to ‘expert’.
-
Exploratory research into how researchers and stakeholders apply mutable realist concepts in a way that is consistent with complex systems concepts.
-
Evaluation of complex review knowledge translation strategies (e.g. tailored prompts, infographics, workshops, coaching, and so on) for different groups of knowledge-users.
Acknowledgements
We gratefully acknowledge the help given by our stakeholder group of patients, GPs, NHS managers and representatives of other organisations who, as a condition of participation, must remain anonymous. Furthermore, we are grateful to the anonymous reviewers, whose comments enabled us to strengthen the report, especially the discussion and conclusions.
Contributions of authors
Rod Sheaff (Professor of Health Services Research) co-led the research design and contributed to project management, devising and testing methods of data extraction and analysis, data collection, extraction and interpretation, translation from French and German, evidence synthesis, stakeholder consultations and drafting the report.
Sarah L Brand (Research Fellow) contributed to project management, the day-to-day delivery of the project, all aspects of carrying out the three steps of the realist synthesis project, stakeholder engagement and report writing.
Helen Lloyd [Senior Research Fellow (Qualitative)] contributed to project management, think tank and engagement, data coding and reading and commenting on the draft report.
Amanda Wanner (Information Specialist/Research Fellow) contributed to screening, data extraction, evidence synthesis and drafting the report.
Mauro Fornasiero (Research Assistant) contributed to discourse analysis, evidence synthesis and drafting the report.
Simon Briscoe (Information Specialist) developed and conducted database search strategies and contributed to writing the report.
Jose M Valderas (Professor of Health Services & Policy Research) contributed to the design of the study, translation from Spanish, interpretation and reporting.
Richard Byng (Professor Primary Care) contributed to the project design, interpretation and drafting the report.
Mark Pearson (Senior Research Fellow in Implementation Science) co-led the research design, project management, devising and testing methods of data extraction and analysis, data collection, extraction and interpretation, evidence synthesis, stakeholder consultations and write-up of the report.
Data sharing statement
Most of the data used in this report came from published papers that are, therefore, already available to all, subject to the usual copyright and, in some cases, paywall restrictions. Requests for access to other data (e.g. about the stakeholder meetings) should be addressed to the corresponding author for consideration. Access to available anonymised data may be granted following review and necessary agreements.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Kelly E, Stoye G, Vera-Hernández M. Public Hospital Spending in England: Evidence From National Health Service Administrative Records. London: Institute for Fiscal Studies; 2015.
- Bacchi C. Problematizations in health policy: questioning how ‘problems’ are constituted in policies. SAGE Open 2016;6:1-16. https://doi.org/10.1177/2158244016653986.
- Caminal J, Starfield B, Sánchez E, Casanova C, Morales M. The role of primary care in preventing ambulatory care sensitive conditions. Eur J Public Health 2004;14:246-51. https://doi.org/10.1093/eurpub/14.3.246.
- The Multispecialty Community Provider (MCP) Emerging Care Model and Contract Framework. London: NHS England; 2016.
- Primary Care: Fourth Report of Session 2015–16. London: House of Commons; 2016.
- Stevens S. Five Year Forward View. London: NHS England; 2014.
- The Government’s Mandate to NHS England for 2016–17. London: DHSC; 2017.
- Health and High Quality Care For All, Now and For Future Generations. London: NHS England; 2016.
- The Forward View into Action: Planning for 2015/16. London: NHS England; 2014.
- Roberts N. GPs Can Retain GMS Contract But Drop QOF under MCP Contract, Officials Confirm 2016. www.gponline.com/gps-retain-gms-contract (accessed 31 July 2016).
- Billings J, de Weger E. Contracting for integrated health and social care: a critical review of four models. J Integr Care 2015;23:153-75. https://doi.org/10.1108/JICA-03-2015-0015.
- Multispecialty Community Provider Vanguards. London: NHS England; 2016.
- Okhuysen GA, Bechky BA. Coordination in organizations: an integrative perspective. Acad Manage Ann 2009;3:463-502.
- Van Houdt S, Heyrman J, Vanhaecht K, Sermeus W, De Lepeleire J. An in-depth analysis of theoretical frameworks for the study of care coordination. Int J Integr Care 2013;13.
- Freeman G, Weaver T, Low J, de Jonge E, Crawford M. Promoting Continuity of Care for People with Severe Mental Illness Whose Needs Span Primary, Secondary and Social Care: A Multi-Method Investigation of Relevant Mechanisms and Contexts. London: National Coordinating Centre for the Service Delivery and Organisation; 2002.
- Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ 2003;327:1219-21. https://doi.org/10.1136/bmj.327.7425.1219.
- Reid R, Haggerty J, McKendry R. Defusing the Confusion: Concepts and Measures of Continuity of Healthcare. Ottawa, ON: Canadian Health Services Research Foundation; 2002.
- Ahgren, Axelsson R. Evaluating integrated health care: a model for measurement. Int J Integr Care 2005;5.
- Pimperl A, Schulte T, Mühlbacher A, Rosenmöller M, Busse R, Groene O, et al. Evaluating the impact of an accountable care organization on population health: the quasi-experimental design of the German Gesundes Kinzigtal. Popul Health Manag 2017;20:239-48. https://doi.org/10.1089/pop.2016.0036.
- Hildebrandt H, Hermann C, Knittel R, Richter-Reichhelm M, Siegel A, Witzenrath W. Gesundes Kinzigtal Integrated Care: improving population health by a shared health gain approach and a shared savings contract. Int J Integr Care 2010;10. https://doi.org/10.5334/ijic.539.
- Nandram S, Koster N. Organizational innovation and integrated care: lessons from Buurtzorg. J Integr Care 2014;22:174-84. https://doi.org/10.1108/JICA-06-2014-0024.
- Bihan BL, Martin C. A comparative case study of care systems for frail elderly people: Germany, Spain, France, Italy, United Kingdom and Sweden. Soc Pol Admin 2006;40:26-4. https://doi.org/10.1111/j.1467-9515.2006.00475.x.
- Sheaff R, Halliday J, Øvretveit J, Byng R, Exworthy M, Peckham S, et al. Integration and continuity of primary care: polyclinics and alternatives, an organisational analysis. Health Serv Deliv Res 2015;3.
- Øvretveit J, Hansson J, Brommels M. An integrated health and social care organisation in Sweden: creation and structure of a unique local public health and social care system. Health Policy 2010;97:113-21. https://doi.org/10.1016/j.healthpol.2010.05.012.
- Hansson J, Øvretveit J, Brommels M. Case study of how successful coordination was achieved between a mental health and social care service in Sweden. Int J Health Plann Manage 2012;27:e132-45. https://doi.org/10.1002/hpm.1099.
- Centers for Medicare and Medicaid Services . Accountable Care Organizations (ACO) n.d. www.cms.gov/medicare/medicare-fee-for-service-payment/aco/ (accessed 9 May 2017).
- Patient Centered Medical HomeResource Center . Defining the PCMH n.d. https://pcmh.ahrq.gov/page/defining-pcmh (accessed 22 May 2017).
- Allen SM, Lima JC, Goldscheider FK, Roy J. Primary caregiver characteristics and transitions in community-based care. J Gerontol B Psychol Sci Soc Sci 2012;67:362-71. https://doi.org/10.1093/geronb/gbs032.
- Øvretveit J. Does Care Coordination Improve Quality and Save or Make Money? A Summary of a Review of the Evidence of Costs and Savings of Improvements to Patient Care Coordination. London: The Health Foundation; 2010.
- Priebe S, Watts J, Chase M, Matanov A. Processes of disengagement and engagement in assertive outreach patients: qualitative study. Br J Psychiatry 2005;187:438-43. https://doi.org/10.1192/bjp.187.5.438.
- Sans-Corrales M, Pujol-Ribera E, Gené-Badia J, Pasarín-Rua MI, Iglesias-Pérez B, Casajuana-Brunet J. Family medicine attributes related to satisfaction, health and costs. Fam Pract 2006;23:308-16. https://doi.org/10.1093/fampra/cmi112.
- Lyratzopoulos G, Havely D, Gemmell I, Cook GA. Factors influencing emergency medical readmission risk in a UK district general hospital: a prospective study. BMC Emerg Med 2005;5. https://doi.org/10.1186/1471-227X-5-4.
- Wilson T, Buck D, Ham C. Rising to the challenge: will the NHS support people with long term conditions?. BMJ 2005;330:657-61. https://doi.org/10.1136/bmj.330.7492.657.
- Boaden R, Dusheiko M, Gravelle H, Parker S, Pickard S, Roland M, et al. Evaluation of Evercare: Final Report. Manchester: National Primary Care Research and Development Centre; 2006.
- RAND Europe . National evaluation of the DH integrated care pilots. Rand Health Quarterly 2012;2.
- Gravelle H, Dusheiko M, Sheaff R, Sargent P, Boaden R, Pickard S, et al. Impact of case management (Evercare) on frail elderly patients: controlled before and after analysis of quantitative outcome data. BMJ 2007;334. https://doi.org/10.1136/bmj.39020.413310.55.
- Sheaff R, Boaden R, Sargent P, Pickard S, Gravelle H, Parker S, et al. Impacts of case management for frail elderly people: a qualitative study. J Health Serv Res Policy 2009;14:88-95. https://doi.org/10.1258/jhsrp.2008.007142.
- Rey L, Brousselle A, Dedobbeleer N. Logic analysis: testing program theory to better evaluate complex interventions. Can J Program Eval 2012;26:61-89.
- McLaughlin JA, Jordan GB. Logic models: a tool for telling your programs performance story. Eval Program Plann 1999;22:65-72. https://doi.org/10.1016/S0149-7189(98)00042-1.
- Gugiu PC, Rodríguez-Campos L. Semi-structured interview protocol for constructing logic models. Eval Program Plann 2007;30:339-50. https://doi.org/10.1016/j.evalprogplan.2007.08.004.
- The RAMESES Project . Quality Standards For Realist Synthesis (for Researchers and Peer-Reviewers) 2014. www.ramesesproject.org/media/RS_qual_standards_researchers.pdf (accessed 25 April 2018).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, Research Methods & Reporting. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Barrett DJ. Change communication: using strategic employee communication to facilitate major change. Corporate Comm 2002;7:219-31. https://doi.org/10.1108/13563280210449804.
- Courpasson D. Le changement est un outil politique. Rev Fr Gestio 1998;120:6-16.
- Hewison A. Evidence-based management in the NHS: is it possible?. J Health Organ Manag 2004;18:336-48. https://doi.org/10.1108/14777260410560839.
- Sheaff R, Charles N, Mahon A, Chambers N, Morando V, Exworthy M, et al. NHS commissioning practice and health system governance: a mixed-methods realistic evaluation. Health Serv Deliv Res 2015;3.
- Will CM. Arguing about the evidence: readers, writers and inscription devices in coronary heart disease risk assessment. Sociol Health Illn 2005;27:780-801. https://doi.org/10.1111/j.1467-9566.2005.00474.x.
- Pace R, Pluye P, Bartlett G, Macaulay AC, Salsberg J, Jagosh J, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud 2012;49:47-53. https://doi.org/10.1016/j.ijnurstu.2011.07.002.
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013-20. https://doi.org/10.1016/j.jclinepi.2008.10.009.
- The Vanguard and the People it Serves. London: NHS England; 2016.
- Kash BA, Zhang Y, Cline KM, Menser T, Miller TR. The perioperative surgical home (PSH): a comprehensive review of US and non-US studies shows predominantly positive quality and cost outcomes. Milbank Q 2014;92:796-821. https://doi.org/10.1111/1468-0009.12093.
- A Blueprint For Building the New Deal For General Practice in England. London: RCGP; 2015.
- Southon G, Perkins R, Galler D. Networks: a key to the future of health services. Aust Health Rev 2005;29:317-26. https://doi.org/10.1071/AH050317.
- Provan KG, Kenis P. Modes of network governance: structure, management, and effectiveness. J Publ Adm Res Theor 2008;18:229-52. https://doi.org/10.1093/jopart/mum015.
- Viron M, Zioto K, Schweitzer J, Levine G. Behavioral Health Homes: an opportunity to address healthcare inequities in people with serious mental illness. Asian J Psychiatr 2014;10:10-6. https://doi.org/10.1016/j.ajp.2014.03.009.
- Lewis VA, Colla CH, Tierney K, Van Citters AD, Fisher ES, Meara E. Few ACOs pursue innovative models that integrate care for mental illness and substance abuse with primary care. Health Aff 2014;33:1808-16. https://doi.org/10.1377/hlthaff.2014.0353.
- D’Aunno T, Friedmann PD, Chen Q, Wilson DM. Integration of Substance Abuse Treatment Organizations into Accountable Care Organizations: Results from a National Survey. J Health Polit Policy Law 2015;40:797-819. https://doi.org/10.1215/03616878-3150062.
- Salako A, Zhu X, MacKinney AC, Ullrich F, Mueller K, Rural Health R, et al. Characteristics of rural accountable care organizations (ACOs) – a survey of Medicare ACOs with rural presence. Rural Policy Brief 2015 2015;8:1-4.
- Lewis VA, Colla CH, Schoenherr KE, Shortell SM, Fisher ES. Innovation in the safety net: integrating community health centers through accountable care. J Gen Intern Med 2014;29:1484-90. https://doi.org/10.1007/s11606-014-2911-0.
- Pineault R, Borgès Da Silva R, Prud’homme A, Fournier M, Couture A, Provost S, et al. Impact of Québec’s healthcare reforms on the organization of primary healthcare (PHC): a 2003-2010 follow-up. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-229.
- Evans JM, Grudniewicz A, Wodchis WP, Baker GR. Leading the implementation of health links in Ontario. Healthc Pap 2014;14:21-5. https://doi.org/10.12927/hcpap.2015.24104.
- McNab J, Gillespie JA. Bridging the chronic care gap: HealthOne Mt Druitt, Australia. Int J Integr Care 2015;15. https://doi.org/10.5334/ijic.2243.
- D’Aunno T, Broffman L, Sparer M, Kumar SR. Factors that distinguish high-performing accountable care organizations in the Medicare Shared Savings Program. Health Serv Res 2018;53:120-37. https://doi.org/10.1111/1475-6773.12642.
- Busse R, Stahl J. Integrated care experiences and outcomes in Germany, the Netherlands, and England. Health Aff 2014;33:1549-58. https://doi.org/10.1377/hlthaff.2014.0419.
- Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Brighton, MA: Harvard Business Press; 2006.
- Briot P, Bréchat PH, Reiss-Brennan B, Cannon W, Bréchat N, Teil A. Integrated care delivery system for mental illness: a case study of Intermountain Healthcare (USA). Sante Publique 2015;27:199-208. https://doi.org/10.3917/spub.150.0199.
- Wholey DR, Zhu X, Knoke D, Shah P, White KM, Mick SS, et al. Advances in Health Care Organization Theory. San Francisco, CA: Jossey-Bass; 2014.
- Alidina S, Rosenthal M, Schneider E, Singer S. Coordination within medical neighborhoods: Insights from the early experiences of Colorado patient-centered medical homes. Health Care Manage Rev 2016;41:101-12. https://doi.org/10.1097/HMR.0000000000000063.
- Hoffer Gittell J. Coordinating mechanisms in care provider groups: relational coordination as a mediator and input uncertainty as a moderator of performance effects. Manage Sci 2002;48:1408-26. https://doi.org/10.1287/mnsc.48.11.1408.268.
- Cuellar A, Helmchen LA, Gimm G, Want J, Burla S, Kells BJ, et al. The CareFirst Patient-Centered Medical Home Program: cost and utilization effects in its first three years. J Gen Intern Med 2016;31:1382-8. https://doi.org/10.1007/s11606-016-3814-z.
- Cantor JC, Chakravarty S, Tong J, Yedidia MJ, Lontok O, DeLia D. The New Jersey Medicaid ACO Demonstration Project: seeking opportunities for better care and lower costs among complex low-income patients. J Health Polit Policy Law 2014;39:1185-211. https://doi.org/10.1215/03616878-2822622.
- Bleser WK, Miller-Day M, Naughton D, Bricker PL, Cronholm PF, Gabbay RA. Strategies for achieving whole-practice engagement and buy-in to the patient-centered medical home. Ann Fam Med 2014;12:37-45. https://doi.org/10.1370/afm.1564.
- Colla CH, Lewis VA, Bergquist SL, Shortell SM. Accountability across the continuum: the participation of postacute care providers in accountable care organizations. Health Serv Res 2016;51:1595-611. https://doi.org/10.1111/1475-6773.12442.
- Canali C, De Montgolfier S, Mohebi A, Harboun M. Satisfaction among GPs integrated into a mobile geriatric team: A qualitative study. NPG Neurol Psychiatr Geriatr 2016;16:53-8. https://doi.org/10.1016/j.npg.2015.07.008.
- Buurtzorg . Welcome to Buurtzorg. Humanity over Bureaucracy n.d. www.buurtzorgusa.org/medical-practitioners/ (accessed 13 May 2017).
- Woodman J, Lewis H, Cheung R, Gilbert R, Wijlaars LP. Integrating primary and secondary care for children and young people: sharing practice. Arch Dis Child 2016;101:792-7. https://doi.org/10.1136/archdischild-2015-308558.
- Gehlert S, Collins S, Golden R, Horn P. Social work participation in accountable care organizations under the Patient Protection and Affordable Care Act. Health Soc Work 2015;40:e142-7. https://doi.org/10.1093/hsw/hlv054.
- Xenakis N. The Role of Social Work Leadership: Mount Sinai Care, the Accountable Care Organization, and Population Health Management. Soc Work Health Care 2015;54:782-809. https://doi.org/10.1080/00981389.2015.1059399.
- Noël PH, Romero RL, Robertson M, Parchman ML. Key activities used by community based primary care practices to improve the quality of diabetes care in response to practice facilitation. Qual Prim Care 2014;22:211-19.
- Kennedy AG, Chen H, Corriveau M, MacLean CD. Improving population management through pharmacist-primary care integration: a pilot study. Popul Health Manag 2015;18:23-9. https://doi.org/10.1089/pop.2014.0043.
- Hildebrandt H, Pimperl A, Schulte T, Hermann C, Riedel H, Schubert I, et al. Pursuing the triple aim: evaluation of the integrated care system Gesundes Kinzigtal: population health, patient experience and cost-effectiveness. Bundesgesundheitsblatt Gesundheitsforschung, Gesundheitsschutz 2015;58:383-92. https://doi.org/10.1007/s00103-015-2120-y.
- Petersen DM, Zickafoose J, Hossain M, Ireys H. Physician perspectives on medical home recognition for practice transformation for children. Acad Pediatr 2016;16:373-80. https://doi.org/10.1016/j.acap.2015.12.001.
- Collinsworth A, Vulimiri M, Snead C, Walton J. Community health workers in primary care practice: redesigning health care delivery systems to extend and improve diabetes care in underserved populations. Health Promot Pract 2014;15:51-6. https://doi.org/10.1177/1524839914539961.
- Baker R, Boulton M, Windridge K, Tarrant C, Bankart J, Freeman GK. Interpersonal continuity of care: a cross-sectional survey of primary care patients’ preferences and their experiences. Br J Gen Pract 2007;57:283-9.
- Demiris G, Kneale L. Informatics systems and tools to facilitate patient-centered care coordination. Yearb Med Inform 2015;10:15-21. https://doi.org/10.15265/IY-2015-003.
- Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program?. Issue Brief 2014;19:1-19. https://doi.org/10.15868/socialsector.25007.
- Friedman A, Howard J, Shaw EK, Cohen DJ, Shahidi L, Ferrante JM. Facilitators and barriers to care coordination in patient-centered medical homes (PCMHs) from coordinators’ perspectives. J Am Board Fam Med 2016;29:90-101. https://doi.org/10.3122/jabfm.2016.01.150175.
- Rajala K. Exploring the provider experience of primary care behavioral health integration in health centers transitioning to the patient-centered medical home model. Diss Abs Int Sci Eng 2015;76.
- Greene CA, Ford JD, Ward-Zimmerman B, Honigfeld L, Pidano AE. Strengthening the coordination of pediatric mental health and medical care: piloting a collaborative model for freestanding practices. Child Youth Care Forum 2016;45:729-44. https://doi.org/10.1007/s10566-016-9354-1.
- McNab J, Paterson J, Fernyhough J, Hughes R. Role of the GP liaison nurse in a community health program to improve integration and coordination of services for the chronically ill. Aust J Prim Health 2016;22:123-7. https://doi.org/10.1071/PY14089.
- Mead H, Andres E, Regenstein M. Underserved patients’ perspectives on patient-centered primary care: does the patient-centered medical home model meet their needs?. Med Care Res Rev 2014;71:61-84. https://doi.org/10.1177/1077558713509890.
- Bergman AA, Jaynes HA, Gonzalvo JD, Hudmon KS, Frankel RM, Kobylinski AL, et al. Pharmaceutical role expansion and developments in pharmacist–physician communication. Health Commun 2016;31:161-70. https://doi.org/10.1080/10410236.2014.940672.
- Grace SM, Rich J, Chin W, Rodriguez HP. Flexible implementation and integration of new team members to support patient-centered care. Healthc 2014;2:145-51. https://doi.org/10.1016/j.hjdsi.2014.02.003.
- Matiz LA, Peretz PJ, Jacotin PG, Cruz C, Ramirez-Diaz E, Nieto AR. The impact of integrating community health workers into the patient-centered medical home. J Prim Care Community Health 2014;5:271-4. https://doi.org/10.1177/2150131914540694.
- Smith M, Cannon-Breland ML, Spiggle S. Consumer, physician, and payer perspectives on primary care medication management services with a shared resource pharmacists network. Res Social Adm Pharm 2014;10:539-53. https://doi.org/10.1016/j.sapharm.2013.08.003.
- Anderson GF, Ballreich J, Bleich S, Boyd C, DuGoff E, Leff B, et al. Attributes common to programs that successfully treat high-need, high-cost individuals. Am J Manag Care 2015;21:e597-600.
- Coleman K, Phillips K. Providing underserved patients with medical homes: assessing the readiness of safety-net health centers. Issue Brief 2010;85:1-14.
- Annis AM, Harris M, Robinson CH, Krein SL. Do patient-centered medical home access and care coordination measures reflect the contribution of all team members? A systematic review. J Nurs Care Qual 2016;31:357-66. https://doi.org/10.1097/NCQ.0000000000000192.
- Carroll JC, Talbot Y, Permaul J, Tobin A, Moineddin R, Blaine S, et al. Academic family health teams: part 2: patient perceptions of access. Can Fam Physician 2016;62:e31-9.
- McConaha JL, Tedesco GW, Civitarese L, Hebda MF. A pharmacist’s contribution within a patient-centered medical home. J Am Pharm Assoc 2015;55:302-6. https://doi.org/10.1331/JAPhA.2015.14119.
- Batalden M, Batalden P, Margolis P, Seid M, Armstrong G, Opipari-Arrigan L, et al. Coproduction of healthcare service. BMJ Qual Saf 2015;25:509-17. https://doi.org/10.1136/bmjqs-2015-004315.
- Nelson KM, Helfrich C, Sun H, Hebert PL, Liu CF, Dolan E, et al. Implementation of the patient-centered medical home in the Veterans Health Administration: associations with patient satisfaction, quality of care, staff burnout, and hospital and emergency department use. JAMA Intern Med 2014;174:1350-8. https://doi.org/10.1001/jamainternmed.2014.2488.
- Shortell SM, Sehgal NJ, Bibi S, Ramsay PP, Neuhauser L, Colla CH, et al. An Early Assessment of Accountable Care Organizations’ Efforts to Engage Patients and Their Families. Med Care Res Rev 2015;72:580-604. https://doi.org/10.1177/1077558715588874.
- Damery S, Flanagan S, Combes G. Does integrated care reduce hospital activity for patients with chronic diseases? An umbrella review of systematic reviews. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011952.
- Verhaegh KJ, MacNeil-Vroomen JL, Eslami S, Geerlings SE, de Rooij SE, Buurman BM. Transitional care interventions prevent hospital readmissions for adults with chronic illnesses. Health Aff 2014;33:1531-9. https://doi.org/10.1377/hlthaff.2014.0160.
- Nelson K, Sun H, Dolan E, Maynard C, Beste L, Bryson C, et al. Elements of the patient-centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage 2014;37:331-8. https://doi.org/10.1097/JAC.0000000000000032.
- Kinjo K, Sairenji T, Koga H, Osugi Y, Yoshida S, Ichinose H, et al. Cost of physician-led home visit care (Zaitaku care) compared with hospital care at the end of life in Japan. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-016-1961-x.
- Lafortune C, Huson K, Santi S, Stolee P. Community-based primary health care for older adults: a qualitative study of the perceptions of clients, caregivers and health care providers. BMC Geriatr 2015;15. https://doi.org/10.1186/s12877-015-0052-x.
- Dunn EJ, Mills PD, Neily J, Crittenden MD, Carmack AL, Bagian JP. Medical team training: applying crew resource management in the Veterans Health Administration. Jt Comm J Qual Patient Saf 2007;33:317-25. https://doi.org/10.1016/S1553-7250(07)33036-5.
- Helmreich RL. On error management: lessons from aviation. BMJ 2000;320:781-5. https://doi.org/10.1136/bmj.320.7237.781.
- West P, Sculli G, Fore A, Okam N, Dunlap C, Neily J, et al. Improving patient safety and optimizing nursing teamwork using crew resource management techniques. J Nurs Adm 2012;42:15-20. https://doi.org/10.1097/NNA.0b013e31823c17c7.
- Weldon SM, Ralhan S, Paice E, Kneebone R, Bello F. Sequential Simulation (SqS): an innovative approach to educating GP receptionists about integrated care via a patient journey – a mixed methods approach. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0327-5.
- Busetto L, Luijkx KG, Elissen AM, Vrijhoef HJ. Intervention types and outcomes of integrated care for diabetes mellitus type 2: a systematic review. J Eval Clin Pract 2016;22:299-310. https://doi.org/10.1111/jep.12478.
- Busetto L, Luijkx KG, Elissen AMJ, Vrijhoef HJM. Context, mechanisms and outcomes of integrated care for diabetes mellitus type 2: a systematic review. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-015-1231-3.
- King J, Patel V, Jamoom E, DesRoches C. The role of health IT and delivery system reform in facilitating advanced care delivery. Am J Manag Care 2016;22:258-65.
- O’Malley AS, Draper K, Gourevitch R, Cross DA, Scholle SH. Electronic health records and support for primary care teamwork. J Am Med Inform Assoc 2015;22:426-34. https://doi.org/10.1093/jamia/ocu029.
- Richardson JE, Vest JR, Green CM, Kern LM, Kaushal R. HITEC Investigators . A needs assessment of health information technology for improving care coordination in three leading patient-centered medical homes. J Am Med Inform Assoc 2015;22:815-20. https://doi.org/10.1093/jamia/ocu039.
- Bauer AM, Thielke SM, Katon W, Unützer J, Areán P. Aligning health information technologies with effective service delivery models to improve chronic disease care. Prev Med 2014;66:167-72. https://doi.org/10.1016/j.ypmed.2014.06.017.
- Carroll JC, Talbot Y, Permaul J, Tobin A, Moineddin R, Blaine S, et al. Academic family health teams: Part 1: patient perceptions of core primary care domains. Can Fam Physician 2016;62:e23-30.
- Morton S, Shih SC, Winther CH, Tinoco A, Kessler RS, Scholle SH. Health IT-enabled care coordination: a national survey of patient-centered medical home clinicians. Ann Fam Med 2015;13:250-6. https://doi.org/10.1370/afm.1797.
- McGough PM, Bauer AM, Collins L, Dugdale DC. Integrating behavioral health into primary care. Popul Health Manag 2016;19:81-7. https://doi.org/10.1089/pop.2015.0039.
- Kaushal R, Edwards A, Kern LM. Association between the patient-centered medical home and healthcare utilization. Am J Manag Care 2015;21:378-86.
- Merrill JA, Sheehan BM, Carley KM, Stetson PD. Transition networks in a cohort of patients with congestive heart failure: a novel application of informatics methods to inform care coordination. Appl Clin Inform 2015;6:548-64. https://doi.org/10.4338/ACI-2015-02-RA-0021.
- Johnson TL, Brewer D, Estacio R, Vlasimsky T, Durfee MJ, Thompson KR, et al. Augmenting predictive modeling tools with clinical insights for care coordination program design and implementation. EGEMS 2015;3. https://doi.org/10.13063/2327-9214.1181.
- Aliu O, Sun G, Burke J, Chung KC, Davis MM. Specialist participation in healthcare delivery transformation: influence of patient self-referral. Am J Manag Care 2014;20:e22-6.
- Desmedt M, Vertriest S, Hellings J, Bergs J, Dessers E, Vankrunkelsven P, et al. Economic impact of integrated care models for patients with chronic diseases: a systematic review. Value Health 2016;19:892-90. https://doi.org/10.1016/j.jval.2016.05.001.
- Liss DT, Fishman PA, Rutter CM, Grembowski D, Ross TR, Reid RJ. Specialty use among patients with treated hypertension in a patient-centered medical home. J Gen Internal Med 2014;29:732-40. https://doi.org/10.1007/s11606-014-2776-2.
- Huber CA, Reich O, Früh M, Rosemann T. Effects of integrated care on disease-related hospitalisation and healthcare costs in patients with diabetes, cardiovascular diseases and respiratory illnesses: a propensity-matched cohort study in Switzerland. Int J Integr Care 2016;16. https://doi.org/10.5334/ijic.2455.
- Hibbard JH, Greene J, Sacks R, Overton V. Does compensating primary care providers to produce higher quality make them more or less patient centric?. Med Care Res Rev 2015;72:481-95. https://doi.org/10.1177/1077558715586291.
- Raphael JL, Rattler TL, Kowalkowski MA, Brousseau DC, Mueller BU, Giordano TP. Association of care in a medical home and health care utilization among children with sickle cell disease. J Natl Med Assoc 2015;107:42-9. https://doi.org/10.1016/S0027-9684(15)30008-0.
- Yoon J, Liu CF, Lo J, Schectman G, Stark R, Rubenstein LV, et al. Early changes in VA medical home components and utilization. Am J Manag Care 2015;21:197-204.
- Besser CS. An impact assessment of including a behavioral health provider within the structure of the Army Patient Centered Medical Home Model: a longitudinal study. Diss Abs Int Sci Eng 2016;76.
- Roemer MI. Bed supply and hospital utilization: a natural experiment. Hospitals 1961;35:36-42.
- Kopetsch T. Gilt Roemer’s Law auch in Deutschland?/Does Roemer’s Law apply in Germany?. Jahrb Natl Stat 2006;226:646-69. https://doi.org/10.1515/jbnst-2006-0606.
- Kroneman M, Siegers JJ. The effect of hospital bed reduction on the use of beds: a comparative study of 10 European countries. Soc Sci Med 2004;59:1731-40. https://doi.org/10.1016/j.socscimed.2004.01.036.
- van Noordt M, van der Zee J, Groenewegen PP. Regional variation in hospital admission rates in the Netherlands, Belgium, northern France, Nordrhein-Westfalen. Gesundheitswesen 1992;54:173-8.
- Treadwell J, Giardino A. Collaborating for care: initial experience of embedded case managers across five medical homes. Prof Case Manag 2014;19:86-92. https://doi.org/10.1097/NCM.0000000000000017.
- Pourat N, Charles SA, Snyder S. Availability of care concordant with patient-centered medical home principles among those with chronic conditions: measuring care outcomes. Med Care 2016;54:262-8. https://doi.org/10.1097/MLR.0000000000000498.
- Clarke R, Bharmal N, Di Capua P, Tseng CH, Mangione CM, Mittman B, et al. Innovative approach to patient-centered care coordination in primary care practices. Am J Manag Care 2015;21:623-30.
- Shaw JD, O’Neal DJ, Siddharthan K, Neugaard BI. Pilot program to improve self-management of patients with heart failure by redesigning care coordination. Nurs Res Pract 2014;2014. https://doi.org/10.1155/2014/836921.
- David G, Gunnarsson C, Saynisch PA, Chawla R, Nigam S. Do patient-centered medical homes reduce emergency department visits?. Health Serv Res 2015;50:418-39. https://doi.org/10.1111/1475-6773.12218.
- Westhorp G. Developing complexity-consistent theory in a realist investigation. Evaluation 2013;19:364-82. https://doi.org/10.1177/1356389013505042.
- Petticrew M, Anderson L, Elder R, Grimshaw J, Hopkins D, Hahn R, et al. Complex interventions and their implications for systematic reviews: a pragmatic approach. J Clin Epidemiol 2013;66:1209-14. https://doi.org/10.1016/j.jclinepi.2013.06.004.
- Anderson R, Hardwick R. Realism and resources: towards more explanatory economic evaluation. Evaluation 2016;22:323-41. https://doi.org/10.1177/1356389016652742.
- Petticrew M. Time to rethink the systematic review catechism? Moving from ‘what works’ to ‘what happens’. Syst Rev 2015;4. https://doi.org/10.1186/s13643-015-0027-1.
- Hawe P, Shiell A, Riley T. Theorising interventions as events in systems. Am J Community Psychol 2009;43:267-76. https://doi.org/10.1007/s10464-009-9229-9.
- Funnell SC, Rogers PJ. Purposeful Program Theory: Effective Use of Theories of Change and Logic Models. San Francisco, CA: Jossey-Bass/Wiley; 2011.
- Manzano A. The craft of interviewing in realist evaluation. Evaluation 2016;22:342-60. https://doi.org/10.1177/1356389016638615.
- Hawe P. Lessons from complex interventions to improve health. Annu Rev Public Health 2015;36:307-23. https://doi.org/10.1146/annurev-publhealth-031912-114421.
- Amelung V, Hildebrandt H, Wolf S. Integrated care in Germany – a stony but necessary road!. Int J Integr Care 2012;12. https://doi.org/10.5334/ijic.853.
- Kunze H, Priebe S. Integrierte versorgung-perspektiven für die psychiatrie und psychotherapie. Psychiat Prax 2006;33:53-5. https://doi.org/10.1055/s-2005-915398.
- Schlette S, Lisac M, Blum K. Integrated primary care in Germany: the road ahead. Int J Integr Care 2009;9. https://doi.org/10.5334/ijic.311.
- Hassell K, Whittington Z, Cantrill J, Bates F, Rogers A, Noyce P. Managing demand: transfer of management of self limiting conditions from general practice to community pharmacies. BMJ 2001;323:146-7. https://doi.org/10.1136/bmj.323.7305.146.
- Hassell K, Noyce PR, Rogers A, Harris J, Wilkinson J. A pathway to the GP: the pharmaceutical ‘consultation’ as a first port of call in primary health care. Fam Pract 1997;14:498-502. https://doi.org/10.1093/fampra/14.6.498.
- Heenan D, Birrell D. The integration of health and social care: the lessons from Northern Ireland. Soc Pol Admin 2006;40:47-66. https://doi.org/10.1111/j.1467-9515.2006.00476.x.
- Reilly S, Challis D, Burns A, Hughes J. Does integration really make a difference? A comparison of old age psychiatry services in England and Northern Ireland. Int J Geriatr Psychiatry 2003;18:887-93. https://doi.org/10.1002/gps.942.
- Thomson G, Frances J, Levacic R, Mitchell J. Markets, Hierarchies and Networks. The Coordination of Social Life. London: Sage; 1991.
- Korobkin R. The efficiency of managed care ‘patient protection’ laws: incomplete contracts, bounded rationality, and market failure. Cornell Law Rev 1999;85:1-88.
- Macneil IR. Contracts: adjustment of long-term economic relations under classical, neoclassical, and relational contract law. Nw UL Rev 1977;72.
- Exworthy M, Powell M, Mohan J. The NHS: quasi-market, quasi-hierarchy and quasi-network?. Public Money Manage 1999;19:15-22. https://doi.org/10.1111/1467-9302.00184.
- Sheaff R, Schofield J, Ferlie E, Montgomery K, Reff Pedersen A. The Oxford Handbook of Health Care Management. Oxford: Oxford University Press; 2016.
- Every Child Matters. London: HMSO; 2003.
- Fix GM, Asch SM, Saifu HN, Fletcher MD, Gifford AL, Bokhour BG. Delivering PACT-principled care: are specialty care patients being left behind?. J Gen Intern Med 2014;29:695-702. https://doi.org/10.1007/s11606-013-2677-9.
- Janiszewski D, O’Brian CA, Lipman RD. Patient experience in a coordinated care model featuring diabetes self-management education integrated into the patient-centered medical home. Diabetes Educ 2015;41:466-71. https://doi.org/10.1177/0145721715586577.
- Jagosh J, Bush PL, Salsberg J, Macaulay AC, Greenhalgh T, Wong G, et al. A realist evaluation of community-based participatory research: partnership synergy, trust building and related ripple effects. BMC Public Health 2015;15. https://doi.org/10.1186/s12889-015-1949-1.
- Multispecialty Community Providers Vanguard Sites. London: NHS England; n.d.
- Biernacki PJ, Champagne MT, Peng S, Maizel DR, Turner BS. Transformation of care: integrating the registered nurse care coordinator into the patient-centered medical home. Popul Health Manag 2015;18:330-6. https://doi.org/10.1089/pop.2014.0131.
- Broffman L, Brown K, Bayley BK, Savitz L, Rissi J. Funding accountable care in Oregon: financial models in two coordinated care organizations. J Healthc Manag 2016;61:291-302. https://doi.org/10.1097/00115514-201607000-00009.
- Cook N, Hollar L, Isaac E, Paul L, Amofah A, Shi L. Patient experience in health center medical homes. J Community Health 2015;40:1155-64. https://doi.org/10.1007/s10900-015-0042-0.
- Cook N, Hollar TL, Zunker C, Peterson M, Phillips T, De Lucca M. Supporting medical home transformation through evaluation of patient experience in a large culturally diverse primary care safety net. J Public Health Manag Pract 2016;22:265-74. https://doi.org/10.1097/PHH.0000000000000263.
- Farrell TW, Tomoaia-Cotisel A, Scammon DL, Brunisholz K, Kim J, Day J, et al. Impact of an integrated transition management program in primary care on hospital readmissions. J Healthc Qual 2015;37:81-92. https://doi.org/10.1097/01.JHQ.0000460119.68190.98.
- Geltman PL, Fried LE, Arsenault LN, Knowles AM, Link DA, Goldstein JN, et al. A planned care approach and patient registry to improve adherence to clinical guidelines for the diagnosis and management of attention-deficit/hyperactivity disorder. Acad Pediatr 2015;15:289-96. https://doi.org/10.1016/j.acap.2014.12.002.
- Knapp C, Chakravorty S, Madden V, Baron-Lee J, Gubernick R, Kairys S, et al. Association between medical home characteristics and staff professional experiences in pediatric practices. Arch Public Health 2014;72. https://doi.org/10.1186/2049-3258-72-36.
- Lemmens LC, Molema CC, Versnel N, Baan CA, de Bruin SR. Integrated care programs for patients with psychological comorbidity: A systematic review and meta-analysis. J Psychosom Res 2015;79:580-94. https://doi.org/10.1016/j.jpsychores.2015.07.013.
- Lewin A, Mitchell S, Beers L, Schmitz K, Boudreaux M. Improved contraceptive use among teen mothers in a patient-centered medical home. J Adolesc Health 2016;59:171-6. https://doi.org/10.1016/j.jadohealth.2016.04.007.
- Liem RI, O’Suoji C, Kingsberry PS, Pelligra SA, Kwon S, Mason M, et al. Access to patient-centered medical homes in children with sickle cell disease. Matern Child Health J 2014;18:1854-62. https://doi.org/10.1007/s10995-013-1429-0.
- Lubetkin EI, Zabor EC, Brennessel D, Kemeny MM, Hay JL. Beyond demographics: differences in patient activation across new immigrant, diverse language subgroups. J Community Health 2014;39:40-9. https://doi.org/10.1007/s10900-013-9738-1.
- Miller-Matero LR, Dykuis KE, Albujoq K, Martens K, Fuller BS, Robinson V, et al. Benefits of integrated behavioral health services: The physician perspective. Fam Syst Health 2016;34:51-5. https://doi.org/10.1037/fsh0000182.
- Philpot LM, Stockbridge EL, Padrón NA, Pagán JA. Patient-centered medical home features and health care expenditures of medicare beneficiaries with chronic disease dyads. Popul Health Manag 2016;19:206-11. https://doi.org/10.1089/pop.2015.0077.
- Rosenthal MB, Alidina S, Friedberg MW, Singer SJ, Eastman D, Li Z, et al. Impact of the Cincinnati aligning forces for quality multi-payer patient centered medical home pilot on health care quality, utilization, and costs. Med Care Res Rev 2016;73:532-45. https://doi.org/10.1177/1077558715618566.
- Stock R, Hall J, Chang AM, Cohen D. Physicians’ early perspectives on Oregon’s Coordinated Care Organizations. Healthc 2016;4:92-7. https://doi.org/10.1016/j.hjdsi.2015.07.003.
- van der Kluit MJ, Ros WJ, Schrijvers AJ. Nurse-led clinics for patients with chronic diseases in hospital and transmural care organizations. Clin Nurse Spec 2014;28:332-42. https://doi.org/10.1097/NUR.0000000000000079.
- van Leeuwen KM, Bosmans JE, Jansen AP, Hoogendijk EO, Muntinga ME, van Hout HP, et al. Cost-effectiveness of a chronic care model for frail older adults in primary care: economic evaluation alongside a stepped-wedge cluster-randomized trial. J Am Geriatr Soc 2015;63:2494-504. https://doi.org/10.1111/jgs.13834.
- Pyne JM, Fortney JC, Mouden S, Lu L, Hudson TJ, Mittal D. Cost-effectiveness of on-site versus off-site collaborative care for depression in rural FQHCs. Psychiatr Serv 2015;66:491-9. https://doi.org/10.1176/appi.ps.201400186.
Appendix 1 First-wave multispecialty community providers
| Site | Mechanism (work process) | Organisations |
|---|---|---|
| Principia Partners in Health (southern Nottinghamshire) |
|
Community interest company of GP practices (126,000 patient list), CHSs, CCGs and ‘social care partners’ |
| All Together Better Sunderland |
|
Two GP federations, CHS foundation trust, City Hospitals Sunderland Foundation Trust, mental health foundation trust, care and support services (former local authority direct care for adults), Healthwatch, local medical committee, Cumbria and North East Area Team, and Voluntary and Community Action Sunderland |
| Wellbeing Erewash |
|
Derbyshire Community Health Services NHS Foundation Trust, Derbyshire Healthcare NHS Foundation Trust, Erewash GP Provider Company, Derbyshire Health United (OOH service and NHS 111) and NHS Erewash CCG |
| West Wakefield Health and Wellbeing Ltd |
|
Federated network of GP practices, Wakefield CCG, Wakefield Council, Wakefield District Housing, South West Yorkshire Partnership NHS Foundation Trust, Healthwatch Wakefield, Mid-Yorkshire Hospitals NHS Foundation Trust, Nova (voluntary community sector representative body), Yorkshire Ambulance Service and Local Care Direct |
| Modality Birmingham and Sandwell |
|
One GP partnership that operates from 15 practice sites (70,000 patient list) |
| Encompass (Whitstable, Faversham and Canterbury) | Extended primary care and community services through the expansion of community health and social care teams; we will reduce hospital admissions and length of stay | 16 GP practices, CCG, East Kent Hospitals University NHS Foundation Trust, CHS foundation trust, NHS and Social Care Partnership Trust, Coast Ambulance Service Foundation Trust, Wellbeing Board, County Council, Pilgrims Hospices, voluntary and community organisations |
| Dudley Multispecialty Community Provider |
|
Metropolitan Borough Council, Black Country Partnership NHS Foundation Trust, Dudley Group NHS Foundation Trust, Dudley and Walsall Mental Health Partnership NHS Trust, Dudley Council for Voluntary Services and Future Proof Health Ltd |
| Tower Hamlets Integrated Provider Partnership | Single shared assessment and plan for patients | GP Community Interest Company, Barts Health NHS Trust, East London NHS Foundation [mental health] Trust Borough of Tower Hamlets (social care), voluntary and community organisations, and user groups |
| Better Local Care (southern Hampshire) |
|
27 GP practices, Southern Health NHS Foundation Trust, 16 other local NHS, local government and voluntary sector organisations |
| Fylde Coast Local Health Economy |
|
Fylde and Wyre CCG, Blackpool CCG, Blackpool Teaching Hospital NHS Foundation Trust, Lancashire CC, Lancashire Care NHS Foundation Trust, Blackpool Council and ‘services provided by the voluntary sector’ |
| Calderdale Health and Social Care Economy |
|
Network: Pennine GP Alliance (23/26 Calderdale practices), Calderdale and Huddersfield Foundation Trust, Calderdale CCG, MBC, South West Yorkshire Partnership Foundation Trust, local community partnerships (NHS) and Voluntary Action Calderdale (128 health-related organisations) |
| West Cheshire Way |
|
NHS West Cheshire CCG and Primary Care Cheshire (a single entity), Partnership Foundation Trust, Countess of Chester [hospital NHS Foundation Trust] Cheshire West and Chester Council |
| Stockport Together |
|
MBC, NHS Stockport Foundation Trust, community and mental health foundation trust, CCG |
| Lakeside Healthcare (Northamptonshire) |
|
GP super-practice (300,000 patient list), Kettering General Hospital, Peterborough and Stanford Hospital, University Hospitals Leicester, Northampton General Hospital and Northamptonshire Healthcare Trust, Northamptonshire Healthcare Trust, Northamptonshire CC; Corby Town Council, Celesio (Lloyds Pharmacy), local social service providers and voluntary and community sector |
| New cities of Ebbsfleet and Bicester | Health and care garden city, rethinking physical design of the infrastructure, new technologies, ‘deep integration of health and care with supported housing and other public services’9 | NHS England, LGA |
Appendix 2 Scoping search strategy and hits
Scoping searches
Integrated care and chronic conditions
Database: HMIC.
Host: Ovid.
Data parameters: 1979 to July 2016.
Date searched: 25 August 2016.
Searcher: Sarah L Brand.
Hits: 3667.
Search strategy
“ageing population*”.tw,nt.
((older or geriatric or frail or vulnerable) adj2 (person* or people or patient* or population* or “local resident*”)).tw,nt.
older people/
((“long term” or chronic* or complex* or multidimensional or “multi dimensional” or multiple) adj4 (need* or condition* or problem* or healthcare or care or patient* or disease*)).tw,nt.
Long term care/
chronic disease/
or/1-6
((integrat* or continuity or continuous or “co ordinat*” or coordinat* or collaborative* or “multi disciplinary” or multidisciplinary or “culturally appropriate” or transition* or transmural or seamless or comprehensive) adj2 (health or healthcare or service* or care or “social care” or “personal commissioning”)).tw,nt.
integrated care/
collaborative care/
((community or outreach or “out reach”) adj1 (health or healthcare or service* or care or hospital*)).tw,nt.
((personali?ed or “person centred” or “person centered” or “patient centred” or “patient centered” or holistic* or tailor*) adj3 (health or healthcare or service* or care)).tw,nt.
patient centred care/
(network* adj2 (care or healthcare or service* or provider* or provision)).tw,nt
((continuity or continuous) adj2 (provider* or provision)).tw,nt.
(“primary and acute care system*” or PACS or polyclinic* or polysystem*).tw,nt.
((GP or “general practice*” or “general practitioner*” or “family physician*” or “family doctor*” or “family medicine” or “family practice*”) adj6 (“health centre*” or “health center*” or “co-operative*” or cooperative* or collaborative* or “community health”)).tw,nt.
(“allied health professional*” adj2 (“general practice*” or gp)).tw,nt.
(“multispecialty community provider*” or “multi specialty community provider*” or MCP* or MSCP*).tw,nt.
(virtual adj2 (ward* or provider*)).tw,nt.
((“co located” or colocated or collocated) adj2 service*).tw,nt.
(hospital adj2 (outreach or “follow up”)).tw,nt.
((vertical* or horizontal*) adj2 integrat*).tw,nt.
((shared or sharing) adj3 (“patient* record*” or “patient* data” or “patient* information” or “patient* assessment*” or “information technology”)).tw,nt.
((ambulatory or “out of hours”) adj1 care).tw,nt.
(“medical home*” or “primary care hub*” or “care home liaison*” or “self management plan*”).tw,nt.
(“single assessment process*” or “single access point*” or “multi dimensional assessment plan*” or “multidimensional assessment plan*”).tw,nt.
or/8-27
(buurt?org or “one window model*” or “hospital at home” or “community assessment and rehabilitation team*” or “working unit for continuous care” or “multidimensional assessment district unit*” or “multi dimensional assessment district unit*” or “wiesbaden geriatric rehabilitation network*” or “wiesbaden network for geriatric rehabilitation” or “wiesbaden geriatric network*” or “information system for all activities carried out in the territory” or “rapid response team*”).tw,nt.
(7 and 28) or 29
limit 30 to yr=“1991 –Current”
| Database | Records (n) |
|---|---|
| HMIC | 3667 |
| Total number of records | 3667 |
| Duplicate records | 201 |
| Unique records | 3466 |
Health-care divisions and strategies
Database: HMIC.
Host: Ovid.
Data parameters: 1979 to July 2016.
Date searched: 25 August 2016.
Searcher: Sarah L Brand.
Hits: 357.
Search strategy
(gap or gaps or inequalit* or division* or divide*).nt.
(health or care or service* or healthcare or hospital*).nt.
(change* or need* or sustain* or financ* or save* or saving* or strateg* or policy).nt.
1 and 2 and 3
| Database | Records (n) |
|---|---|
| HMIC | 357 |
| Total number of records | 357 |
| Duplicate records | 3 |
| Unique records | 354 |
Appendix 3 Policy sources
Core policy documents analysed
Department of Health and Social Care (DHSC). Equity and Excellence: Liberating The NHS. London: DHSC; 2011.
Department of Health and Social Care (DHSC). The Government’s Mandate to NHS England for 2016–17. London: DHSC; 2017.
Grant S. Multispecialty Community Providers development (MCP) – Vanguard South Hampshire. London: Southern Health; 2016.
House of Commons Health Committee. Primary Care: Fourth Report of Session 2015–16. London: House of Commons; 2016.
NHS Confederation, the Local Government Association, NHS Clinical Commissioners and NHS Providers. Factsheets: Understanding the Vanguards. London: NHS Confederation; 2016.
NHS England. Multispecialty Community Providers Vanguard Sites. London: NHS England; 2016. URL: www.england.nhs.uk/ourwork/futurenhs (accessed 25 April 2018).
NHS England. Multispecialty Community Provider Vanguards. London: NHS England; 2016. URL: www.england.nhs.uk/ourwork/new-care-models/vanguards/care-models/community-sites/ (accessed 11 October 2017).
NHS England. The Forward View Into Action: Planning For 2015/16. London: NHS England; 2014.
NHS England. The Multispecialty Community Provider (MCP) Emerging Care Model and Contract Framework. London: NHS England; 2016.
NHS England. The Multi-Speciality Community Provider (MCP) Emerging Care Model and Contract Framework. NHS England Board paper PB 28.07.2016/04. London: NHS England; 2016.
NHS England. What Makes a good Multispecialty Community Provider? London: NHS England; 2016.
Stevens S. Five Year Forward View. London: NHS England; 2014.
Appendix 4 Stakeholder group members
| Member | Role |
|---|---|
| Patient 1 | PPI |
| Patient 2 | PPI |
| Patient 3 | PPI |
| Patient 4 | PPI |
| Social care lead | CLAHRC |
| Director of Integration | Academic Health Science Network |
| Manager 1 | CCG |
| Director | NHS England |
| Manager 2 | Community Services Provide |
| Social work manager | Children and Family Court Advisory and Support Service |
| Lead author Sheffield MCP review | Midlands & Lancashire Commissioning Support Unit |
| Head of research and clinical effectiveness | Partnership NHS Foundation Trust |
| Assistant director | Strategy and improvement, CCG |
| GP 1 | MCP in formation |
| GP 2 | MCP in formation |
| GP 3 | MCP in formation |
| GP 4 | MCP in formation |
| GP 5 | MCP in formation |
| Researcher in residence | NHS Foundation Trust |
| Evaluator | NHS England |
| Director | MCP in formation |
| Business analyst | NHS Trust |
| Director of intelligence | Academic Health Science Network |
| Project manager | Academic Health Science Network |
| Manager 3 | NHS |
| Manager 4 | CCG |
| Advisor | Health foundation |
Appendix 5 If–then statements from policy sources and stakeholders
| IPT ID | Source | If (C–M) | Then (O) | Whose CMO |
|---|---|---|---|---|
| 1 | MCP#1 FYFV, NHS England, 2015, p. 6 and p. 166 | IF artificial boundaries between hospitals and primary care, health and social care, and generalists and specialists are ‘broken out of’ | THEN care will be genuinely co-ordinated and personalised around what people need and want, and long-term conditions better cared for | NHS policy-makers |
| 2 | MCP#1 FYFV, NHS England, 2015, p. 166 | IF there is a partnership with patients over the long term rather than a single unconnected episode of care | THEN long-term conditions are better cared for | NHS policy-makers |
| 3 | MCP#1 FYFV, NHS, 2015, p. 166 | IF the NHS manages systems (networks of care) not just organisations | THEN long-term conditions are better cared for | NHS policy-makers |
| 4 | MCP#1 FYFV, NHS England, 2015, p. 166 | IF out-of-hospital care becomes a much larger part of what the NHS does | THEN long-term conditions are better cared for | NHS policy-makers |
| 5 | MCP#1 FYFV, NHS England, 2015, p. 166 | IF services are integrated around the patient | THEN long-term conditions are better cared for | NHS policy-makers |
| 6 | MCP#1 FYFV, NHS England, 2015, p. 166 | IF general practice operates at scale, such that 20 GPs and 150 staff operate from three modern sites providing many of the tests, investigations, minor injuries and minor surgery usually provided in hospital (e.g. Kent) | THEN there are better results, better care, better experience for patients and significant savings | NHS policy-makers |
| 7 | MCP#1 FYFV, NHS England, 2015, p. 176 | IF nursing and residential homes are linked by secure video to the hospital allowing consultations with nurses and consultants in and out of normal hours (from cuts and bumps to diabetes mellitus and the management of the onset of confusion) (e.g. Airedale) | THEN emergency admissions and A&E attendances from nursing and residential homes are reduced (Airedale: by 35% and 53%) and residents rate service highly | NHS policy-makers |
| 8 | MCP#1 FYFV, NHS England, 2015, p. 176 | IF trained volunteers and health and social care professionals work side by side (e.g. Cornwall) | THEN this supports patients with long-term conditions to meet their own health and life goals | NHS policy-makers |
| 9 | MCP#1 FYFV, NHS England, 2015, p. 176 | IF GPs and community matrons work with advisors who know what voluntary services are available for patients with long term-conditions (social prescribing service, e.g. Rotherham) | THEN the need for visits to A&E, outpatient appointments and hospital admissions is cut | NHS policy-makers |
| 10 | MCP#1 FYFV, NHS England, 2015, p. 176 | IF integrated care pioneers that combine NHS, GP and social care services are set up (e.g. London) | THEN fewer people move permanently in to nursing care homes and emergency admissions are reduced and economic savings are made (e.g. Greenwich saved nearly £1M and > 5% of community health expenditure) | NHS policy-makers |
| 11 | MCP#1 FYFV, NHS England, 2015, p. 196 | IF extended group practices form as federations, networks or single organisations | THEN primary care can build on the traditional strengths of ‘expert generalists’, proactively target services at registered patients with complex needs (e.g. frail elderly or chronic conditions) and work more intensively with these patients, expand the leadership of primary care to include nurses, therapists and other community-based professionals, make fuller use of digital technologies and offer greater convenience for patients | NHS policy-makers |
| 12 | MCP#1 FYFV, NHS England, 2015, p. 196 | IF MCPs shift the majority of outpatient consultations and ambulatory care out-of-hospital settings | THEN MCPs will become the focal point for a far wider range of care needed by registered patients | NHS policy-makers |
| 13 | MCP#1 FYFV, NHS England, 2015, p. 196 | IF a MCP is a larger group practice | THEN the MCP can employ consultants or take them on as partners, bring in senior nurses, consultant physicians, geriatricians, paediatricians and psychiatrists to work alongside community nurses, therapists, pharmacists, psychologists, social workers and other staff | NHS policy-makers |
| 14 | MCP#1 FYFV, NHS England, 2015, p. 196 | IF MCPs take over the running of local community hospitals | THEN they can substantially expand their diagnostic services as well as other services, such as dialysis and chemotherapy | NHS policy-makers |
| 15 | MCP#1 FYFV, NHS England, 2015, p. 196 | IF GPs and specialists in the MCP are credentialed in some cases to directly admit patients to acute hospitals, with OOH inpatient care being supervised by a new cadre of ‘hospitalists’ (e.g. other countries) | THEN MCPs will become the focal point for a far wider range of care needed by registered patients | NHS policy-makers |
| 16 | MCP#1 FYFV, NHS England, 2015, p. 206 | IF MCPs take on the delegated responsibility for managing the health service budget for their registered patients, or where funding is pooled with local authorities, a combined health and social care budget could be delegated to MCPs | THEN MCPs will become the focal point for a far wider range of care needed by registered patients | NHS policy-makers |
| 17 | MCP#1 FYFV, NHS England, 2015, p. 206 | . . . | THEN MCPs will draw on the ‘renewable energy’ of carers, volunteers and patients themselves, accessing hard-to-reach groups and taking new approaches to changing health behaviours | NHS policy-makers |
| 18 | E-mail, Helen Lloyd, 19 July 2016 | IF MCPs are created | THEN some of the IT and administrative barriers to integration and primary care care co-ordination will be overcome | Commissioner |
| 19 | E-mail, Helen Lloyd, 19 July 2016 | IF nurses are integrated with GPs in MCP groups | THEN teams can streamline QOF reporting and, therefore, cut back on administrative burden associated with completion of single practices/organisations | Commissioner |
| 20 | MCP#2 MCP care model, NHS England, 2016, p. 412 | IF a MCP offers integrated care by dissolving the divides between primary, community, mental health and social care and acute services and involves redesigning care around the health of the population irrespective of existing institutional boundaries | THEN care will be joined up, preventative, high quality and efficient | NHS policy-makers |
| 21 | MCP#2 MCP care model, NHS England, 2016, p. 412 | IF MCPs focus on prevention and redesigning care | THEN it is possible to improve health and well-being, achieve better quality, reduce hospital admissions and elective activity, and unlock more efficient ways of delivering care | NHS policy-makers |
| 22 | MCP#2 MCP care model, NHS England, 2016, p. 412 | IF a MCP builds a community network, connects with the voluntary sector and supports patient activation and self-care | THEN managing demand on general practice will be improved | NHS policy-makers |
| 23 | MCP#2 MCP care model, NHS England, 2016, p. 412 | IF federations and super-practices combine with community services | THEN a broader, more holistic and resilient form of general practice will be created | NHS policy-makers |
| 24 | MCP#2 MCP care model, NHS England, 2016, p. 412 | IF a MCP supports practices to work at scale | THEN the practices will benefit from working with larger community-based teams | NHS policy-makers |
| 25 | MCP#2 MCP care model, NHS England, 2016, p. 512 | (When at its most integrated form, a MCP holds a single, whole population budget for all the services it provides, including primary medical services.) IF a MCP has sufficient decision-making rights to deploy that budget flexibly | THEN the MCP can reshape the local care delivery system around what really works best for different groups of patients | NHS policy-makers |
| 26 | MCP#2 MCP care model, NHS England, 2016, p. 612 | IF institutional forms, contracts and financial flows are merely rewired | THEN there will not be any change | NHS policy-makers |
| 27 | MCP#2 MCP care model, NHS England, 2016, p. 1012 | IF a MCP engages and activates patients, their carers, families and communities | THEN patients will be able to effectively take control of their own care | NHS policy-makers |
| 28 | MCP#2 MCP care model, NHS England, 2016, p. 1012 | IF a MCP harnesses digital technology | THEN it can provide fully interoperable electronic records and real time data and redesign the process of care delivery, including telephone and Skype™ (Microsoft Corporation, Redmond, WA, USA) consultations, diagnostics, the use of applications and early adoption of innovative drugs and devices | NHS policy-makers |
| 29 | MCP#2 MCP care model, NHS England, 2016, p. 1012 | IF a MCP creates new MDTs, redesigns jobs so that they are more rewarding, sustainable and efficient, and implements newer professional roles | THEN a MCP will empower and engage staff to work in different ways | NHS policy-makers |
| 30 | MCP#2 MCP care model, NHS England, 2016, p. 1012 | IF time and effort is put in to developing a new workforce culture, building skills, and developing roles | THEN multidisciplinary working between health and social teams is supported | NHS policy-makers |
| 31 | MCP#2 MCP care model, NHS England, 2016, p. 1012 | IF there are joined up care records across primary, community and social care and acute services (MCP proposals are extending use of GP record in to community services), real-time data, business and intelligence systems and access to significant analytical capability; and if differential needs, activity and spend are mapped; and if analytical models are used to predict the health interventions that will be required by subpopulations and individual patients; and if it is identified where quality and efficiency improvements can be made to tackle unwarranted variation; and if a whole-population provider budget is held | THEN a MCP can stratify risk (p. 11 four levels of MCP care model pyramid) and segment its population and manage care accordingly and far better align resources to needs | NHS policy-makers |
| 32 | MCP#2 MCP care model, NHS England, 2016, p. 1112 | IF a MCP uses high-quality business intelligence systems with data that are real time | THEN core aspects of what is currently ‘commissioning support’, such as business intelligence, will increasingly become ‘population health management support’ | NHS policy-makers |
| 33 | MCP#2 MCP care model, NHS England, 2016, p. 1112 | IF a MCP adapts or adopts the NHS Rightcare method (www.rightcare.nhs.uk) | THEN it will be supported to understand and tackle unwarranted variation in the health outcomes and costs of their population | NHS policy-makers |
| 34 | MCP#2 MCP care model, NHS England, 2016, p. 1112 | IF a MCP uses the four levels of the MCP care model (highest need < ongoing care needs < urgent care needs < whole population; diagram p. 11)12 | THEN it can stratify risk and segment the population | NHS policy-makers |
| 35 | MCP#2 MCP care model, NHS England, 2016, p. 1112 | IF MCP works with voluntary sector and social care | THEN it can reach out to vulnerable people who find it difficult to access traditional services | NHS policy-makers |
| 36 | MCP#2 MCP care model, NHS England, 2016, p. 1112 | IF a MCP stratifies and identifies risk (using trigger tools and case finding) and segments the population | THEN it can provide an extensivist service for the small group of patients with high needs and high costs, a broader range of integrated services in the community for people with ongoing care needs, a more coherent and effective local network of urgent care using enhanced primary care as the core model, and support for the population to stay well, change unhealthy behaviours and manage own health | NHS policy-makers |
| 37 | MCP#2 MCP care model, NHS England, 2016, p. 1112 | IF care is taken to understand specific subgroups of the population with the greatest needs (e.g. particular housing estates, care homes, remote rural neighbourhoods, toddlers, frail elderly, people who are homeless or in the lowest quintile of population deprivation) | THEN . . . | NHS policy-makers |
| 38 | MCP#2 MCP care model, NHS England, 2016, p. 1212 | IF (the six principles of engagement) care and support is person-centred (i.e. personalised, co-ordinated and empowering), services are created in partnership with citizens and communities, focus is on equality and narrowing inequality, carers are identified, supported and involved, voluntary community and social enterprises, and housing sectors are involved as key partners and enablers, and volunteering and social action are key enablers | THEN local people and communities are engaged with a MCP | NHS policy-makers |
| 39 | MCP#2 MCP care model, NHS England, 2016, p. 1212 | IF volunteers are engaged as community health champions (e.g. All Together Better Sunderland), large-scale social prescribing schemes are developed and tailored to particular patient groups (e.g. Better Local Care, southern Hampshire), MCPs look beyond integration with social care and public health to how they can work with schools, housing associations, job centres and youth justice and probation services | THEN social capital and community resilience are nurtured | NHS policy-makers |
| 40 | MCP#2 MCP care model, NHS England, 2016, p. 1312 | IF (the eight commissioning standards in local system MCPs will operate as part of) patients can make a single call to get an appointment OOH, data can be sent between providers, the capacity for NHS 111 and OOH is jointly planned, the summary care record is available in the clinical hub and elsewhere, care plans and patient notes are shared between providers, the system can make appointments to in-hours general practice, there is joint governance across local urgent and emergency care providers, there is a clinical hub containing (physically or virtually) GPs and other health-care professionals | THEN urgent care is responsive and accessible | NHS policy-makers |
| 41 | MCP#2 MCP care model, NHS England, 2016, p. 1312 | IF more patients are signposted by care navigators [e.g. West Wakefield Health and Wellbeing Ltd MCP: a care navigation framework (a directory of services) is embedded across practices and receptionists use it to signpost patients to cost-effective and appropriate services to meet their needs in a timely manner] | THEN GP time is released | NHS policy-makers |
| 42 | MCP#2 MCP care model, NHS England, 2016, p. 1312 | IF health applications and telecare are used | THEN self-care is supported | NHS policy-makers |
| 43 | MCP#2 MCP care model, NHS England, 2016, p. 1412 | IF alternatives to face-to-face appointments are provided, including video calls, e-mail and telephone consultations (e.g. Modality MCP, Birmingham and Sandwell developed an app that allows people to book appointments, send messages to clinicians and receive real-time feedback) | THEN the need for surgery visits is reduced, did-not-attends are reduced and patient experience is improved | NHS policy-makers |
| 44 | MCP#2 MCP care model, NHS England, 2016, p. 1412 | IF there is a fully interoperable clinical record system where all points of care access (e.g. OOH GP, walk-in centre, A&E, ambulance) have access to view the 10 key fields from the GP record, (e.g. Principia Partners in Health, southern Nottinghamshire), or ambulances can access feedback from their control via these records while at patients’ homes (e.g. East Midlands Ambulance Service) | THEN the admitting clinician has information at the point of access to support management plans or avoid admission and reduce need for conveying patients to hospital | NHS policy-makers |
| 45 | MCP#2 MCP care model, NHS England, 2016, p. 1412 | IF practices work at scale and pool together their urgent workload into a single service that is operated from a central location and resourced by the practices (e.g. ‘same day access’ at Better Local Care, southern Hampshire) | THEN demand for face-to-face appointments is reduced (two-thirds of people accessing this service had their needs met over the telephone) | NHS policy-makers |
| 46 | MCP#2 MCP care model, NHS England, 2016, p. 1412 | IF paramedics are attached to general practices to act as the first responder to urgent patient calls so that if a home visit is required, the paramedic attends and assesses the patient and has access to the full patient record and to the duty GP for advice (Encompass, Whitstable, Faversham and Canterbury) | THEN there is a reduction in conveyancing (e.g. 15%), response times are increased and patient satisfaction is improved | NHS policy-makers |
| 47 | MCP#2 MCP care model, NHS England, 2016, p. 1512 | IF a wide range of diagnostic tests (such as blood tests, blood gases, urine analyses, pregnancy tests, radiography, ultrasounds, bladder scans, electrocardiograms) are delivered in the MCP’s community-based facilities (e.g. some clinical monitoring regimes have moved in their entirety from hospital to community settings under the supervision of the GP; context: with appropriate software support and rapid direct access to specialist advice where required) | THEN urgent and routine care are supported and fewer patients are required to attend hospital | NHS policy-makers |
| 48 | MCP#2 MCP care model, NHS England, 2016, p. 1512 | IF diagnostic tests in community-based facilities are coupled with an observations unit so that clinicians can observe the patients for up to 12 hours | THEN a more complete treatment plan can be developed and implemented that can obviate the need for hospital admission | NHS policy-makers |
| 49 | MCP#2 MCP care model, NHS England, 2016, p. 1512 | IF MCPs follow standardised protocols and integrate primary, community, mental health, social and urgent care | THEN the breadth of primary care services delivered is increased | NHS policy-makers |
| 50 | MCP#2 MCP care model, NHS England, 2016, p. 1512 | IF MCPs increasingly provide services that traditionally have been delivered within outpatient settings | THEN the depth of intervention delivered within outpatient services is increased | NHS policy-makers |
| 51 | MCP#2 MCP care model, NHS England, 2016, p. 1512 | IF the core component of each hub within a MCP is the integrated community MDT and MDTs are supported by colleagues from other sectors and by care co-ordinators who provide dedicated support to patients and carers who have multiple interactions with different care settings | THEN the MDT provides support to patients at high predicted risk of unplanned hospitalisation and also ensures that responsive care is offered to all individuals who need it | NHS policy-makers |
| 52 | MCP#2 MCP care model, NHS England, 2016, p. 1512 | IF the MDT provides in-reach into hospitals | THEN this ensures timely discharge of patients | NHS policy-makers |
| 53 | MCP#2 MCP care model, NHS England, 2016, p. 1612 | IF a series of standardised tools in the EMIS clinical system such as comprehensive health checks for people presenting with a new comorbidity and tools that help clinicians to consider the patient’s needs as a whole rather than focusing on an individual long-term condition are available | THEN the patient consultation is improved (54% of participating practices rating) | NHS policy-makers |
| 54 | MCP#2 MCP care model, NHS England, 2016, p. 1712 | IF community services are ultimately fully integrated with primary care, including for example, core community care which focuses on the maintenance of health (e.g. falls prevention, administration of medication, monitoring for deterioration), rehabilitation and reablement which focuses on recovery after a period of ill health and supporting independent living for as long as possible, and specialist care which focuses on a specific aspect of a patient’s condition in the community (e.g. wound care, Encompass, Whitstable, Faversham and Canterbury MCP) | THEN . . . | NHS policy-makers |
| 55 | MCP#2 MCP care model, NHS England, 2016, p. 1712 | IF MCP focuses on rehabilitation and reablement in the community after a period of ill health | THEN independent living is supported for as long as possible | NHS policy-makers |
| 56 | MCP#2 MCP care model, NHS England, 2016, p. 1712 | IF a recovery-at-home service has a single point of access to crisis support and intermediate care and reablement services (e.g. All Together Better, Sunderland MCP) | THEN this brings together a wide range of health and social care professionals and other local support organisations so that people who need short-term, intensive care at home have a service wrapped around them | NHS policy-makers |
| 57 | MCP#2 MCP care model, NHS England, 2016, p. 1712 | IF enhanced health in care homes becomes a core part of all MCPs and PACs | THEN ambulance responses to care homes are reduced (e.g. Principia Partners in Health, southern Nottinghamshire MCP 55/100 beds vs. south Nottinghamshire 108/100), hospital conveyances are reduced (e.g. 29 vs. 64), there are fewer community-acquired pressure sores in older people resident in care homes (e.g. none in last two quarters of 2015/16), and reduced risk of falls and hip fractures with a nurse led community approach gives financial savings (e.g. of £73,000 a year, a return on investment of 52%) | NHS policy-makers |
| 58 | MCP#2 MCP care model, NHS England, 2016, p. 1812 | IF personal health budgets are provided to a small but growing proportion of a MCPs population (e.g. those with complex long-term needs) | THEN the influence of personal health budgets’ collective decision-making is likely to help improve the quality of mainstream care, and people opting for personalised care tends to reduce total cost of care to public services | NHS policy-makers |
| 59 | MCP#2 MCP care model, NHS England, 2016, p. 1812 | IF people opt for more personalised care | THEN there tends to be a reduction in the total cost of care to public services | NHS policy-makers |
| 60 | MCP#2 MCP care model, NHS England, 2016, p. 1812 | IF GPs can easily get immediate expert advice from hospital consultants about a patient who has visited their surgery (e.g. Consultant Connect Service, Stockport Together MCP) 24 hours a day, 7 days a week | THEN this prevents the need for patients to be referred for an outpatient appointment (e.g. in Stockport reduction by 70% of hospital referrals) | NHS policy-makers |
| 61 | MCP#2 MCP care model, NHS England, 2016, p. 1812 | IF an e-referral service is provided for patients with renal problems | THEN the number of people who need to attend an outpatient appointment is drastically cut (e.g. Tower Hamlets Together MCP: 50% referrals dealt with without need for hospital visit and advice given in average of 5 days vs. 64 days for patients attending hospital) | NHS policy-makers |
| 62 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 612 | IF alternative and sustainable models of care are developed alongside interventions and pathways (MCP vanguard: West Wakefield) | THEN ongoing demand in the future is modified | NHS policy-makers |
| 63 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 712 | IF the care navigation system is improved, with > 100 care navigators (mostly administration staff who generally have first contact with patients) working in practices and trained to direct patients to the most appropriate care (MCP vanguard: West Wakefield) | THEN patients are directed to the care they need faster | NHS policy-makers |
| 64 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 712 | IF there is a mobile clinic (MCP vanguard: West Wakefield) | THEN engagement with hard-to-reach groups improved (such as the gypsy/traveller population) | NHS policy-makers |
| 65 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 712 | IF there is continued development of integrated teams (MCP vanguard: West Wakefield) | THEN the combined skills of different professionals including physical health, mental health and social care will redesign the way in which the most vulnerable are cared for in the community | NHS policy-makers |
| 66 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 712 | IF there is 24/7 technological connectivity (MCP vanguard: West Wakefield) and the integrated community teams are all co-ordinated through a command and control centre approach that can deploy tactical teams (MCP vanguard: West Wakefield) | THEN those at risk feel more secure and receive early proactive management and proactive assistance to people to prevent hospital admission and to support earlier discharge from hospital following admission | NHS policy-makers |
| 67 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 712 | IF there are more ways for people to digitally access health care (including online directories of local services, and a library of helpful health applications on its website) (MCP vanguard: West Wakefield) | THEN | NHS policy-makers |
| 68 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 812 | IF pupils in primary school are entered in to a competition to design health applications that will be developed and launched (MCP vanguard: West Wakefield) | THEN primary school children are engaged in health care | NHS policy-makers |
| 69 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 812 | IF patients have access to self-service kiosks in practices (MCP vanguard: West Wakefield) | THEN patients can be pointed to appropriate care before they enter a clinic room | NHS policy-makers |
| 70 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 1412 | IF there is integrated care (MCP Better Local Care) | THEN patients will not have to remember and repeat their medical history and staff will understand their needs wherever they go for help | NHS policy-makers |
| 71 | MCP#3 MCP vanguard descriptions, NHS England, 2016, p. 2012 | IF there is a proactive care plan that is in place and discussed with their local health and care team on a regular basis (MCP Principia Partners in Health) | THEN this will build patient confidence and capability for them to make good decisions about what they do to keep themselves fit and well and when they need to escalate the level of support they need irrespective of the time of day or week | NHS policy-makers |
| 72 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 312 | IF a MCP commissions services differently, moving away from current item-of-service payment mechanisms to commissioning best practice pathways of care and this forms part of a gain sharing agreement between the CCG and the MCP in the future | THEN the MCP takes on the demand management of value added treatment services | MCP |
| 73 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 312 | IF ‘generic’ worker use is increased within MDTs | THEN links are enhanced to voluntary sector services | MCP |
| 74 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 512 | IF there are ongoing public consultations (e.g. on primary care estate), website and literature explaining the MCP, participatory budgeting, staff and patient engagement in pathway design | THEN there is a move away from consumerism and towards mutualism with shared ownership and shared responsibility | MCP |
| 75 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 512 | IF there are more integrated IT supports, such as mobile IT solution holding patient records for community-based staff and MDTs, development of interoperable system across all MCP services | THEN this supports more integrated services (with improved information sharing) increased efficiency, and safer services | MCP |
| 76 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 512 | IF there is close and collaborative working within the system, nationally and with expert partners | THEN a new form of contract can be developed to commission the MCP, this needs to balance capitated budgets, throughput and outcome measures, gain sharing and risk management | MCP |
| 77 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 512 | IF appropriate governance arrangements are designed, including development of specific workstream drawing on organisations across the system and external experts and implementation of preferred option through procurement of MCP | THEN the change in institutional infrastructure needed in order to deliver the MCP contract is supported | MCP |
| 78 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 612 | IF a MCP provides an enhanced range of services in primary and community settings | THEN it can improve patient experience and outcomes at the same time as reducing costs | MCP |
| 79 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 712 | IF there is improved access to care; improved systems and skills in primary care, reduction in back office costs (more efficient use of resources); improved estates in primary/community care; more proactive, targeted diagnosis and management of higher risk patients; better medicines management | THEN there is increased capacity and capability in primary and community care; more services are provided out of hospital (associated savings) | MCP |
| 80 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 712 | IF there is improved access to care; improved systems and skills in primary care, reduction in back office costs (more efficient use of resources); improved estates in primary/community care; improved and quicker access to information, advice and guidance (patients and staff); they find it easier to do the right thing; reduced unwarranted variation in pathways and more appropriate referrals; and better care planning, increased patient knowledge of condition(s), increased ability to self-manage | THEN there is reduced (and more appropriate) use of secondary care and improved discharge (associated savings) | MCP |
| 81 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 712 | IF there is improved access to care; reduced unwarranted variation in pathways and more appropriate referrals; better care planning, increased patient knowledge of condition(s), increased ability to self-manage; and improved patient access to holistic support services (e.g. voluntary sector) | THEN there are improved outcomes for higher risk patients, they are more activated, in control of their care and self-managing, reduction in inequalities (associated savings) | MCP |
| 82 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 712 | IF there is improved access to care; reduced unwarranted variation in pathways and more appropriate referrals; better care planning, increased patient knowledge of condition(s), increased ability to self-manage; improved and quicker access to information, advice and guidance (patients and staff); improved patient access to holistic support services (e.g. voluntary sector); new ‘generalist’ roles, the workforce is better matched to need | THEN there is improved patient experience of care, reduced patient social isolation, better quality of life – including at the end of life (associated savings) | MCP |
| 83 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 712 | IF there is improved and quicker access to information, advice and guidance (patients and staff); and new ‘generalist’ roles, the workforce is better matched to need | THEN there is increased staff empowerment/engagement (associated savings) | MCP |
| 84 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 712 | IF there is greater insight, more clearly defined needs and better designed services; improved information sharing, increased efficiency; useable and replicable contractual model for MCPs, better system incentives; robust system of governance, best possible option in development of MCP organisation(s); better evidence on outcomes, greater insight | THEN the MCP intended outcomes are enabled | MCP |
| 85 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1012 | IF there is engagement with GPs to stimulate demand for advice and guidance (e.g. through training/monitoring non-advice and guidance referrals) and work is done with consultants/Dudley group to stimulate supply of advice and guidance (e.g. use of CQUINS) | THEN there is improved communication, better GP access to consultant advice, and increased use of A&E AND THEN increased capacity and capability in primary and community care, more services provided out of hospital/faster referral back to primary care; AND reduced (and more appropriate) use of secondary care, improved use of consultant time and system resources | MCP |
| 86 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1012 | IF work is done with consultants/Dudley group to stimulate supply of advice and guidance (e.g. use of CQUINS) | THEN GPs feel empowered/feel that they have sufficient knowledge to manage more cases in primary care, AND THEN increased capacity and capability in primary and community care, more services provided out of hospital/faster referral back to primary care; AND reduced (and more appropriate) use of secondary care, improved use of consultant time and system resources; AND improved patient experience | MCP |
| 87 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1012 | IF there is engagement with GPs to stimulate demand for advice and guidance (e.g. through training/monitoring non-advice and guidance referrals) AND work is done with consultants/Dudley group to stimulate supply of advice and guidance (e.g. use of CQUINS) AND clinical groups are used to develop general service specification (for tailoring) to formalise, for example, expectations on/payment for follow-ups | THEN there is reduction in unnecessary referrals to secondary care and reduction in unnecessary follow-up appointments, AND THEN reduced (and more appropriate) use of secondary care, improved use of consultant time and system resources; AND improved patient experience; AND more optimal and effective pathways, reduced unexplained/unwarranted variation in care | MCP |
| 88 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1012 | IF clinical groups are used to develop general service specification (for tailoring) to formalise, for example, expectations on/payment for follow-ups AND scale opportunity for reducing variation (e.g. by reviewing use of follow-up appointment) | THEN there is increased knowledge of current practice, clearer (contractual) expectations for pathways and associated payments; AND THEN improved patient experience AND more optimal AND effective pathways, reduced unexplained/unwarranted variation in care | MCP |
| 89 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1212 | IF outcome targets are reduced in current QOF and a focus put on evidence-based targets for managing long-term conditions | THEN there is an increased focus on patients with long-term conditions AND THEN reductions in administration and changes in skill mix, increased productivity and more efficient use of resources in practices (including change in GP inputs) AND improved outcomes for patients with long-term conditions: they are more activated, in control of their care and self-managing and there is a reduction in inequalities (associated savings) | MCP |
| 90 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1212 | IF contracts are simplified, bringing in Department of Education and Science/Local Improvement Scheme schemes into a single pot AND outcome targets are reduced in current QOF and a focus put on evidence-based targets for managing long-term conditions AND EMIS templates are simplified to support more holistic assessments, standard advice and better care plans | THEN there is increased flexibility for GP practices to manage higher risk patients more proactively AND THEN reductions in administration and changes in skill mix, increased productivity and more efficient use of resources in practices (including change in GP inputs) | MCP |
| 91 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1212 | IF EMIS templates are simplified to support more holistic assessments, standard advice and better care plans AND practices are trained, schemes piloted and refined and formative evaluation of roll out is used | THEN there is reduced variation in advice given to support self-management and increased patient knowledge of condition(s) AND more consistent care planning and joint goal-setting with patients AND THEN there are improved outcomes for patients with long-term conditions: they are more activated, in control of their care and self-managing and there is a reduction in inequalities (associated savings) and improved patient experience of care, reduced patient social isolation, better quality of life – including at the end of life (associated savings) | MCP |
| 92 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1412 | IF MDT structure is devised (mental health, social care, VCS, community nursing, pharmacy, etc.) and MDT established in every practice and every locality, and services mapped and joined up | THEN there is increased knowledge of services available for patients AND THEN there is improved patient experience of care (they receive more co-ordinated care), reduced social isolation and better quality of life (including at the end of life) | MCP |
| 93 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1412 | IF MDT structure is devised (mental health, social care, VCS, community nursing, pharmacy, etc.) and MDT established in every practice and every locality, and services mapped and joined up AND risk stratification is used to identify those most at risk of emergency admission (minimum top 2% of other cases added in by staff) AND there are MDT meetings and follow-up actions to co-ordinate care | THEN there is more proactive identification and management of most at risk in primary care AND THEN reduced use of non-elective secondary care AND improved patient experience of care (they receive more co-ordinated care), reduced social isolation and better quality of life (including at the end of life) | MCP |
| 94 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1412 | IF risk stratification is used to identify most at risk of emergency admission (minimum top 2% of other cases added in by staff) AND there are MDT meetings and follow-up actions to co-ordinate care | THEN duplication of service inputs in reduced, care is more co-ordinated and teams are working to shared outcomes AND THEN there is more efficient use of system resource, reduced duplication/increased co-ordination of service inputs AND increased staff empowerment/engagement | MCP |
| 95 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1412 | IF there are MDT meetings and follow up actions to co-ordinate care | THEN there are increased referrals to community services and activities (VCS) AND THEN there is improved patient experience of care (they receive more co-ordinated care), reduced social isolation and better quality of life (including at the end of life) AND there is more efficient use of system resource, reduced duplication/increased co-ordination of service inputs AND increased staff empowerment/engagement | MCP |
| 96 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1412 | IF there are MDT meetings and follow-up actions to co-ordinate care AND organisational development programmes to support continuous improvement and evolution of MDT model AND formative evaluation of model | THEN there is increased knowledge of effective MDT working AND THEN increased staff empowerment/engagement | MCP |
| 97 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1612 | IF there is increased patient activation and self-care | THEN there will be reduced use of services | MCP |
| 98 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1612 | IF there is empowerment of frontline staff | THEN they are able to resolve patient needs sooner | MCP |
| 99 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1612 | IF there is increased upstream and proactive intervention | THEN services used are less expensive/reactive and restorative | MCP |
| 100 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1612 | IF there is improved communication, advice and guidance | THEN staff have access to the right information at the right time to make the right decision | MCP |
| 101 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1612 | IF there is insight from multiple sources of evidence | THEN services are better designed and adapted to meet evolving needs | MCP |
| 102 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1612 | IF there is reduced duplication, waste and failure demand | THEN multiple services will better coordinate inputs, increasing efficiency and resolving needs sooner | MCP |
| 103 | MCP#6 Dudley MCP description and logic models, Dudley MCP, 2016, p. 1612 | IF there is greater consistency | THEN staff and patients know what to do/what to expect | MCP |
| 104 | MCP#5 NHSE vanguard logic models, 2016, modality (Birmingham and Sandwell)12 | IF the interface between the MCP and secondary care is managed explicitly | THEN this will reduce inappropriate hospital utilisation (e.g. diverting admissions, supporting early discharge and preventing readmissions) | MCP |
| 105 | MCP#5 NHSE vanguard logic models, 2016, West Wakefield Health & Wellbeing Ltd12 | IF there are integrated teams and call centre access from home | THEN admissions avoidance | MCP |
| 106 | MCP#5 NHSE vanguard logic models, 2016, West Wakefield Health & Wellbeing Ltd12 | IF there are integrated teams and assistive technology | THEN early supported discharge | MCP |
| 107 | MCP#5 NHSE vanguard logic models, 2016, Tower Hamlets Together12 | IF a MCP has a good culture [Sarah L Brand note: or is this a description of what they mean by a good culture?] | THEN staff will be polite and respectful to patients, will respect their confidentiality, will let them know who the MCP is and what the MCP does, will communicate clearly and openly with patients in the way that the patients need them to, will respond to telephone calls, e-mails and letters quickly, will ensure that patients only need to tell their story when they choose, will take in to account patients’ mental, physical and social needs, will be informed and prepared for appointments with patients and have read patients notes, will work with patients as an equal partner, jointly agreeing care plans and including patient personal wishes and goals, will support patients to support themselves where possible, will involve and listen to carers involved in a patient’s care AND services will provide good value and high-quality care and support, be locally based and accessible, be sensitive to the needs of the diverse community they serve | MCP |
| 108 | MCP#5 NHSE vanguard logic models, 2016, Tower Hamlets Together12 | IF there is frailty assessment | THEN this supports care co-ordination | MCP |
| 109 | NHS managers think tank October 2016 if–thens | IF there is a high level of ownership of the budget | THEN the buy-in of partners will be higher | NHS managers |
| 110 | NHS managers think tank October 2016 if–thens | IF MCPs are effective in bringing about systemic change | THEN they should result in GPs, health and social services having a shared budget and long-term contracts | NHS managers |
| THEN GPs will be integrated with community services and providing for one population including prevention work | ||||
| 111 | NHS managers think tank October 2016 if–thens | IF organisational forms are changed AND there are operational changes | THEN care will be taken closer to home | NHS managers |
| 112 | NHS managers think tank October 2016 if–thens | IF there is more joined up working, with people talking more to each other in joined up way with positive relationships AND there is a supporting system | THEN there will be more co-ordinated care AND reduced inefficiencies AND this will be better for the patient because everyone involved in their care will be ‘on message’ | NHS managers |
| 113 | NHS managers think tank October 2016 if–thens | IF there is patient activation AND communication between engaged health and social care providers who take a holistic view using a more social model | THEN there will be better support for patients to take responsibility for their own health AND there will be more health behaviour change in community AND THEN there will be less demand on health services | NHS managers |
| 114 | NHS managers think tank October 2016 if–thens | IF there is empowerment, shared decision-making, planning, an emphasis on what matters to patients | THEN . . . | NHS managers |
| 115 | NHS managers think tank October 2016 if–thens | IF there is education and staff | THEN this helps to overcome the fact that some patients do not want change in the way they interact with their health services | NHS managers |
| 116 | NHS managers think tank October 2016 if–thens | IF patients do not know about something (e.g. community staff visits to home) | THEN they will not engage with it | NHS managers |
| 117 | NHS managers think tank October 2016 if–thens | IF there is a move from a model of illness to a model of well-being AND patient empowerment | THEN responsibility for health moves to the patient AND supports culture change in the way the population understand and use health services | NHS managers |
| 118 | NHS managers think tank October 2016 if–thens | IF physical health services learn from mental health services in terms of patient-centred care and a holistic philosophy | THEN physical health services can be improved | NHS managers |
| 119 | NHS managers think tank October 2016 if–thens | IF there is culture change such that a strengths-based approach is used to look at a person in a positive way in terms of their goals and community involvement, etc. AND staff are also treated in this way | THEN this is a starting point for (improved?) care planning | NHS managers |
| 120 | NHS managers think tank October 2016 if–thens | IF GPs become more involved in managing risk in the community by being more involved in complex cases in the community | THEN complex cases are cheaper to manage in the community (reduced cost of care for complex cases) BUT GPs may not want to take on that risk if things can go wrong AND IF GPs are not aware of the rest of the pathway THEN it is difficult for them to take on risk [minute 25:50 in first policy think tank recording] | NHS managers |
| 121 | NHS managers think tank October 2016 if–thens | IF more people are supported not to be admitted or to be discharged from the hospital | THEN there will be added pressure in the community for services and carers and the voluntary sector | NHS managers |
| 122 | NHS managers think tank October 2016 if–thens | IF carers are not supported | THEN the carer could also become ill and then there would be two rather than one patient in need of health services | NHS managers |
| 123 | NHS managers think tank October 2016 if–thens | IF there is a shift in the model and culture | THEN the full workforce can be skilled and working in a different way (including health, social and voluntary) | NHS managers |
| 124 | NHS managers think tank October 2016 if–thens | IF there is the capacity and skills in the voluntary sector | THEN the ‘logic model’ of MCPs can be brought to life | NHS managers |
| 125 | NHS managers think tank October 2016 if–thens | IF services are tight/protective/inflexible about their role boundaries THEN there will not be joined up care BUT IF the boundaries are merged or blurred too much | THEN there is the risk that roles will not be delivered and responsibility for care diffused (tension between interdisciplinary working and flexible roles) | NHS managers |
| 126 | NHS managers think tank October 2016 if–thens | IF staff work across disciplinary boundaries | THEN they will pick up new skills | NHS managers |
| 127 | NHS managers think tank October 2016 if–thens | IF GPs feel challenged by a lack of boundaries around roles | THEN . . . | NHS managers |
| 128 | NHS managers think tank October 2016 if–thens | IF GPs see that they are losing administration jobs because of lack of boundaries around roles and are more able to use their key skills | THEN . . . (more likely to engage with new ways of working?) | NHS managers |
| 129 | NHS managers think tank October 2016 if–thens | IF MCPs are starting from a different place | THEN they will take different length of time and different route on the pathway to their outcomes (e.g. come may start from a not working well place, others may start from a place in which many MCP-type things are in place and can be rebranded) | NHS managers |
| 130 | NHS managers think tank October 2016 if–thens | IF there is engagement | THEN this will drive down system costs | NHS managers |
| 131 | NHS managers think tank October 2016 if–thens | IF the workers believe that the only way that system costs can be reduced is by losing people | THEN they will be mistrustful of any new model of care or way of working coming from above, especially if it involves merging of roles, as they will expect that it is a hidden way of reducing costs (workers may believe that role change is about cost cutting, not about quality improvement) | NHS managers |
| 132 | NHS managers think tank October 2016 if–thens | IF staff and patients believe that change is about bringing in something cheaper and less good | THEN they will be cynical about change AND it will be difficult to convince them to do something better from both a clinical and a financial angle | NHS managers |
| 133 | NHS managers think tank October 2016 if–thens | IF the view is taken (by change agents) that cost savings will be made simply by fewer people being in hospitals | THEN this cost is just transferred elsewhere in the system | NHS managers |
| 134 | NHS managers think tank October 2016 if–thens | IF the view is taken (by change agents) that it is about doing more across the system with what we have got | THEN there may be efficiency savings rather than simply moving cost from one part of system to another | NHS managers |
| 136 | NHS managers think tank October 2016 if–thens | IF trust and supportive relationships between providers take up to 10 years to build | THEN outcomes in MCPs will take many years to show as this is the foundation of the type of change the system is trying to make | NHS managers |
| 137 | NHS managers think tank October 2016 if–thens | IF deficits are not simply shifted around the system AND there is the financial mechanism of fixed price contracts AND people’s minds and cultures are supported to change in the right direction | THEN this supports boundaries between organisations to be informally reduced | NHS managers |
| 138 | NHS managers think tank October 2016 if–thens | IF there is the financial mechanism of fixed price contracts | THEN this avoids the perverse incentives of the payment by results system | NHS managers |
| 139 | NHS managers think tank October 2016 if–thens | IF there is a strong focus on outcomes (in distal sense) | THEN this can distract from more important outcomes in the model (e.g. intermediate outcomes) | NHS managers |
| 140 | NHS managers think tank October 2016 if–thens | IF a MCP is in a rural location with a limited service provision | THEN changes might be easier and more acceptable than in a large urban area (e.g. London) | NHS managers |
| 141 | NHS managers think tank October 2016 if–thens | Incentives/payments: IF change is asked for and proof given for change before payment structure to support it is changed | THEN it will be difficult to get change financed BUT IF they are going to put savings back in to primary care THEN this would be more acceptable and engaging | NHS managers |
| 142 | NHS managers think tank October 2016 if–thens | IF awareness of voluntary sector is raised so that GPs have improved knowledge of the voluntary sector and what is available locally and how to engage with them | THEN they will use these resources more | NHS managers |
| 143 | NHS managers think tank October 2016 if–thens | IF voluntary sector organisation engagement with MCPs is formal AND voluntary sector workers incentives and motivations come from working for voluntary sector | THEN voluntary sector workers may feel that they are becoming too incorporated in to ‘the system’ AND energy and resource of voluntary sector may be reduced | NHS managers |
| 144 | NHS managers think tank October 2016 if–thens | IF resource is put in to galvanising the voluntary sector AND GPs know what the state and structure of the voluntary sector is locally | THEN this is a cheap but effective way of building resource locally for patients in community | NHS managers |
| 145 | NHS managers think tank October 2016 if–thens | IF the social services are locally not in a good state because of lack of funding | THEN the voluntary sector tends to pick up the slack | NHS managers |
| 146 | NHS managers think tank October 2016 if–thens | IF there is data sharing and information governance between health, social and voluntary sector | THEN MCP is supported BUT different teams will interpret things in different ways | NHS managers |
| 147 | NHS managers think tank October 2016 if–thens | IF the voluntary sector is focusing on different things to the NHS local need identification results AND there is the assumption that the voluntary sector is available | THEN this may reduce MCP chances of engaging them | NHS managers |
| 148 | NHS managers think tank October 2016 if–thens | Important local contexts for MCPs discussed (but not linked explicitly to M or O):
|
THEN . . . | NHS managers |
| 149 | NHS managers think tank October 2016 if–thens | IF MCPs start with a GP-centric focus | THEN over time relationships can be built between community organisations AND the central focus of MCPs on GPs can change over time | NHS managers |
| 150 | NHS managers think tank October 2016 if–thens | IF MCPs form AND more patients are taken off the GP AND/OR more services are pulled in to GPs to support them (the shift of focus and power here will be different in different localities) | THEN there will be a tension between what is the best model clinically and what is the best model financially | NHS managers |
| 151 | NHS managers think tank October 2016 if–thens | IF outcomes include patient experience such as social inclusion | THEN this can look ‘small’ in metrics | NHS managers |
| 152 | NHS managers think tank October 2016 if–thens | IF MCPs change GP usage, delivery and model | THEN there is an implementation challenge in terms of the moving of the pressure on the system to other parts of the system and getting rid of some roles, etc. | NHS managers |
| 153 | NHS managers think tank October 2016 if–thens | IF people do not want change in health service provision or change is experienced as challenging by population or do not want to take more responsibility for their own health | THEN patient experience may dip initially for a few years AND improve | NHS managers |
| 154 | NHS managers think tank October 2016 if–thens | IF MCPs make people more attuned to what is available | THEN demand on the system may increase (initially: how long is this and is there a payback down the line?) | NHS managers |
| 155 | NHS managers think tank October 2016 if–thens | IF there is the assumption that information that is collected will magically filter in to effective action and be used effectively by system | THEN knowledge will not be (effectively/appropriately?) used within the system | NHS managers |
| 156 | NHS managers think tank October 2016 if–thens | IF there is a change in culture to be more analytical and use data to feed its working AND increased skills in system to analyse data collected | THEN this supports the shift towards prevention and identifying users and forecasting local needs, etc. | NHS managers |
| 157 | NHS managers think tank October 2016 if–thens | IF an integrated IT system is not simply seen as an easy solution to integration of organisations AND it is seen that MCPs can be robust without integrated IT system | THEN this supports other types of integration to be actioned locally (i.e. resources put in to other mechanisms to increase integration – like roles/interaction/space/organised/managed – without assuming IT will do the work for them) | NHS managers |
| 158 | NHS managers think tank October 2016 if–thens | IF health service staff feel like they have seen schemes come and go and that MCPs are just another way for them to tick boxes to get money | THEN there will be complacency, lack of signing up to vision, and lack of engagement AND money will be got and then syphoned off locally to elsewhere in local system that is seen as a local priority | NHS managers |
| 159 | GP think tank October 2016 if–thens | IF building a MCP involves local context-driven innovation rather than top-down imposition of a strict framework of how to do it | THEN local resources can be creatively adapted to local context and local need AND staff well-being is supported (because staff are able to get rid of barriers to working in the ways they want to work and this reduces their frustration and stress) | GPs |
| 160 | GP think tank October 2016 if–thens | IF the CCG in the local area that the MCP is commissioned by is effective and open to being creative and not risk-averse | THEN the MCP is more likely to be able work in the way it wants to | GPs |
| 161 | GP think tank October 2016 if–thens | IF local GPs or other staff are willing to put in extra effort and time and thinking space outside their own hours | THEN there will be more innovation and creativity locally AND the local MCP will be more likely to work | GPs |
| 162 | GP think tank October 2016 if–thens | IF the ability of a MCP to get started relies on the will to push at the local individual level (i.e. GPs putting in large amount of effort and time unpaid) | THEN this will/resource is not sustainable BUT IF this time and money were actually funded through the CCG commissioning for the MCP, THEN the MCP outcome of cost reduction would be undermined (because it would take a huge amount of resource if these hours were actually paid for) | GPs |
| 163 | GP think tank October 2016 if–thens | IF there are commissioning barriers to innovation (i.e. CCG risk averse) | THEN there will be no change | GPs |
| 164 | GP think tank October 2016 if–thens | IF there is top-down policy informed change, and bottom-up clinician led change | THEN the barrier is at the middle management level, where they have to abide by organisational rules and cannot be creative and flexible | GPs |
| 165 | GP think tank October 2016 if–thens | IF a MCP starts collaborating first and begins to get results from their own creativity | THEN commissioners find it easier to fund the innovation (can see it in action already, less risk if already shown to be operating) | GPs |
| 166 | GP think tank October 2016 if–thens | IF a MCP sets up as a community interest group | THEN all the partner services will be more committed to and engaged with the MCPs ongoing development and plans | GPs |
| 167 | GP think tank October 2016 if–thens | IF the risk of procurement were removed (i.e. have to start procurement process and begin it without knowing whether or not will actually get the money) | THEN more GP practices would be likely to procure for MCP | GPs |
| 168 | GP think tank October 2016 if–thens | IF a GP practice or group of GPs is too small (i.e. serve too small a population) and, therefore, cannot be individually commissioned as a MCP | THEN they can become a group or Federation of GPs to be commissioned to be a MCP, BUT IF they need to spread across an area that spans more than one CCG to serve a large enough population, THEN they will not be able to be commissioned as a MCP together or alone | GPs |
| 169 | GP think tank October 2016 if–thens | IF a MCP supports staff well-being | THEN the MCP will get more from its resources | GPs |
| 170 | GP think tank October 2016 if–thens | IF a MCP supports staff to overcome organisational and other barriers to working in the way that they believe would be sensible to work | THEN staff frustration will decrease | GPs |
| 171 | GP think tank October 2016 if–thens | IF financial constraints are increased on a CCG | THEN barriers to GPs working in the way they would like to are increased | GPs |
| 172 | GP think tank October 2016 if–thens | IF a MCP supports staff to overcome organisational and other barriers to working in the way that they believe would be sensible to work | THEN staff well-being and productivity will increase | GPs |
| 173 | GP think tank October 2016 if–thens | IF a MCP supports staff to focus care around patients, rather than on process | THEN staff can work in ways that align with their own intrinsic motivation to look after patients, AND THEN staff well-being and productivity is supported | GPs |
| 174 | GP think tank October 2016 if–thens | IF barriers between services are opened up and worked across in a MCP | THEN there is improved patient access to services they need/want | GPs |
| 175 | GP think tank October 2016 if–thens | IF there is joined up IT and shared records | THEN a MCP is possible (not possible to work in this way without these things) | GPs |
| 176 | GP think tank October 2016 if–thens | IF data collection and tools are used to understand your local population and the spread of their needs | THEN you can better manage demand, prevent need for care, and more effectively use your resources | GPs |
| 177 | GP think tank October 2016 if–thens | IF a MCP can shift the default position of patients and the system (biomedical model) of going to the GP in the first instance | THEN the full range of resources will be better spread across the system and diverted away from primary care AND the dependency on GPs will be cut | GPs |
| 178 | GP think tank October 2016 if–thens | IF GPs will not or cannot take responsibility for financial risk | THEN this financial risk needs to be held higher up in the system (as part of a joint venture?), for example at the level of network management (commissioning at this level) | GPs |
| 179 | GP think tank October 2016 if–thens | IF a MCP is small enough | THEN it is more able to explore, understand and respond to local need with local resource (reason to keep MCPs small enough to relate to a local context), BUT if a MCP is large enough, THEN it will have enough patients to be able to fight more effectively for funds from commissioners | GPs |
| 180 | GP think tank October 2016 if–thens | IF there is targeting of services to patients at all levels of need (not just complex needs) | THEN there is better use of local resources and better local demand management | GPs |
| 181 | GP think tank October 2016 if–thens | IF a MCP thinks of partners in terms of how they can help the MCP to do what | THEN this focuses the MCP on collaboration as opposed to ‘bringing in’ | GPs |
| 182 | GP think tank October 2016 if–thens | IF a MCP gets stuck in a transactional reactive loop | THEN staff will be stressed, BUT IF a MCP can be more proactive THEN the MCP will not spend all of its time fighting fires, but preventing them | GPs |
| 183 | GP think tank October 2016 if–thens | IF MCPs are small enough to be responsive to local needs (and collect data on local need across the spectrum of high to low need) | THEN they can respond proactively to these local needs | GPs |
| 184 | GP think tank October 2016 if–thens | IF local social services are not sufficient to support people locally to be cared for at home or in the community | THEN a MCP will not be able to reduce readmission to hospital | GPs |
| 185 | GP think tank October 2016 if–thens | IF a MCP cannot affect the quality of social care and cannot affect social care commissioning locally | THEN a MCP will not be able to reduce hospital readmission by having patients cared for by social services in the community | GPs |
| 186 | GP think tank October 2016 if–thens | IF adult social care is not a part of a MCP | THEN the MCP will not be able to remove a major barrier to improving hospital readmission. [Rod Sheaff: context] | GPs |
| 187 | GP think tank October 2016 if–thens | IF a local group of practices are preparing to become a MCP | THEN the groundwork they are doing will prepare them for other future eventualities also (e.g. if NHS fails and go private then will need to have larger patient lists to compete for funding on open market and be able to show data collection and local need) | GPs |
| 188 | GP think tank October 2016 if–thens | IF practitioners from other services that are part of the MDT are referred to throughout a patients care pathway as part of the same team or closely related to the GP | THEN the patient will not see them as a substitute for the GP or feel shunted off, but will be happier with perceived expertise of their care (e.g. from the US model) | GPs |
| 189 | GP think tank October 2016 if–thens | IF a MCP operates at scale | THEN it is more likely that partners and people lower in the decision-making hierarchy will not be interested in understanding the decision-making process and may be less engaged | GPs |
| 190 | GP think tank October 2016 if–thens | IF a MCP uses ‘admission avoidance’ as a way to engage, sell to and interest commissioners | THEN this will also deliver them the ability to offer person-centred care to a person in the way and place that they need it AND to ‘do the right thing’ (which is what clinicians want to do) with resources available | GPs |
| 191 | GP think tank October 2016 if–thens | IF a MCP focuses on workload, satisfaction and sustainability | THEN partner services will be more engaged | GPs |
| 192 | PPI think tank October 2016 if–thens | IF new model of care needs patients to change how they interact with their GP and/or other practitioners | THEN there are some sections of the community that will be adverse to these changes (especially elderly who do not deal well with change, or who are not good at using modern technology) | PPI |
| 193 | PPI think tank October 2016 if–thens | IF care for vulnerable elderly is integrated in to their lives AND is user friendly | THEN this can help them to work with services in new ways even though change is difficult for them | PPI |
| 194 | PPI think tank October 2016 if–thens | IF MCPs want to increase access to the right point in the health-care system | THEN they need to offer a variety of opportunities to engage in new ways that suit all generations, especially the elderly and the young | PPI |
| 195 | PPI think tank October 2016 if–thens | IF a patient has complex needs but wants to stay in the community and not be in a care home BUT the community care available does not offer the level of intensity of care required | THEN the patient will have to go to a care home/secondary care | PPI |
| 196 | PPI think tank October 2016 if–thens | IF there is not any social care support for the elderly with complex needs | THEN they cannot leave their beds when they are hospitalised | PPI |
| 197 | PPI think tank October 2016 if–thens | IF a care co-ordinator or GP acts as a gate keeper | THEN some patients will feel that they cannot get past the gate keeper to get the care that they would like (e.g. if GP or care co-ordinator has a different opinion to patient in regards to the best or most appropriate care) | PPI |
| 198 | PPI think tank October 2016 if–thens | IF there is a multidisciplinary health team in a MCP | THEN the GP is not the only gate keeper | PPI |
| 199 | PPI think tank October 2016 if–thens | IF patients have complex health needs | THEN their care should be co-ordinated by a ‘community matron’ figure | PPI |
| 200 | PPI think tank October 2016 if–thens | IF a patient is allocated a health and social carer | THEN patients will feel more comfortable | PPI |
| 201 | PPI think tank October 2016 if–thens | IF a patient is allocated a health and social carer | THEN this health professional can be present at GPs appointment and advocate for the patient | PPI |
| 202 | PPI think tank October 2016 if–thens | IF MCPs include new job roles, for example care co-ordinators | THEN . . . | PPI |
| 203 | PPI think tank October 2016 if–thens | IF MCPs include new job roles, for example care co-ordinators | THEN these people should be trained in a holistic approach, which encompasses mental health | PPI |
| 204 | PPI think tank October 2016 if–thens | IF it is not clear who takes responsibility for a patients care (e.g. GP) | THEN . . . | PPI |
| 205 | PPI think tank October 2016 if–thens | IF a care co-ordinator or advocate can support a patient to meet their immediate needs | THEN hospital admission can be avoided | PPI |
| 206 | PPI think tank October 2016 if–thens | IF multidisciplinary health teams in MCPs can deal with complex health needs | THEN patient waiting lists get shorter | PPI |
| 207 | PPI think tank October 2016 if–thens | IF support for management of long-term illnesses in responsive, flexible and available at all hours (e.g. for COPD) | THEN patients are supported to self-care, take control of management of their own illness (e.g. monitor own symptoms and respond with antibiotics immediately without waiting for a GP prescription, which might be too late and, therefore, result in hospital admission), AND to avoid being admitted to secondary care (e.g. hospital) | PPI |
| 208 | PPI think tank October 2016 if–thens | IF knowledge and information and education around illnesses and illness management and self-care and about the treatments/services/support that are available locally AND patients know how to find this information | THEN patients can access appropriate services themselves in a timely fashion AND demand for primary care is reduced AND cost savings made AND patient experience of health care improved | PPI |
| 209 | PPI think tank October 2016 if–thens | IF there is an information hub as part of a MCP | THEN patient self-care is supported and enabled THEN demand for primary and/or secondary care is reduced and managed in community services instead | PPI |
| 210 | PPI think tank October 2016 if–thens | IF patients know what is available locally and how to refer themselves to these services | THEN the patient will not go to the GP AND the demand for primary care is reduced AND patients are supported to have control over their own care AND will have better-quality experiences of health services | PPI |
| 211 | PPI think tank October 2016 if–thens | IF MCPs become social hubs where patients can informally discuss their health issues | THEN patients will feel more comfortable to see their GPs | PPI |
| 212 | PPI think tank October 2016 if–thens | IF MCPs become social hubs | THEN they will speed up the recovery of patients with complex needs | PPI |
| 213 | PPI think tank October 2016 if–thens | IF the voluntary sector gets involved | THEN MCP can become social hubs | PPI |
| 214 | PPI think tank October 2016 if–thens | IF MCPs become social hubs | THEN patients have to be prepared to contribute financially | PPI |
| 215 | PPI think tank October 2016 if–thens | IF care has to change | THEN it has to be user friendly | PPI |
| 216 | PPI think tank October 2016 if–thens | IF hospitals are integrated in the community | THEN they maximise their resources | PPI |
| 217 | PPI think tank October 2016 if–thens | IF local hospitals are used to their full potential | THEN they can stay open | PPI |
| 218 | PPI think tank October 2016 if–thens | IF there are hubs that offer services specialised around particular illnesses (such as multiple sclerosis) | THEN there will be better patient experience of care | PPI |
| 219 | PPI think tank October 2016 if–thens | IF there are crisis centres that patients know about | THEN the demand for A&E will be reduced | PPI |
| 220 | PPI think tank October 2016 if–thens | IF care is proactive and takes primary care services to communities that are at risk | THEN this will support prevention and admission avoidance | PPI |
| 221 | PPI think tank October 2016 if–thens | IF care is proactive and takes a mobile service to the community which makes the service more accessible locally (such as the See Hear bus in north Devon provided by Living Options) | THEN this will improve patient experience of care AND will support prevention and reduce demand for primary care | PPI |
| 222 | PPI think tank October 2016 if–thens | IF vulnerable or isolated communities have mobile services visit them and provide basic health care (such as farming or rural communities) | THEN community illness prevention is supported | PPI |
| 223 | PPI think tank October 2016 if–thens | IF elderly patients are given education around the benefits of changes to health care provision | THEN this helps to overcome their fears about and resistance to change and to using health services in a different way | PPI |
| 224 | PPI think tank October 2016 if–thens | IF MCPs focus on prevention | THEN this will reduce costs to NHS of illnesses such as diabetes mellitus | PPI |
| 225 | PPI think tank October 2016 if–thens | IF MCPs offer teleconferences for house-bound people to talk to each other or to health-care professionals | THEN this is a cheap way to deliver emotional support that can greatly improve quality of life and mental health AND improve health outcomes AND experience of services | PPI |
| 226 | PPI think tank October 2016 if–thens | IF MCPs enable/support people to take control of their own health | THEN patient experience will improved | PPI |
| IF MCPs provide an advocate or person who can guide you through your care decisions and support you to navigate the health system |
THEN patient experience is improved (These two IF–THENS are opposites and reflect that different people are on different points in terms of wanting autonomy or control over their health care and wanting to be supported and guided through the health system – both of these need to be available to patients depending on their individual needs.) |
|||
| 227 | PPI think tank October 2016 if–thens | IF MCPs include knowledge for patients about illnesses and services, education around public health issues (weight and diet/exercise) and work to overcome the default of the GP as the point of contact for any medical issue for a patient | THEN patients are enabled to take control of their own health (‘integrated self-care’) | PPI |
| 228 | PPI think tank October 2016 if–thens | IF MCPs provide flexible and responsive access to ‘your’ health-care professional (whether this is a GP or care co-ordinator), such as by text, telephone, appointment, video consultation, AND/OR this person acts as an advocate for you (e.g. with GP who does not want to listen to your needs) | THEN patient experience will be improved (except for older people who want a face-to-face consultation only and do not use digital technology – so the choice of either is important to cater for all generations) | PPI |
| 229 | PPI think tank October 2016 if–thens | IF there is responsive e-mail or online support available 24 hours for all levels of issues | THEN needs that can be met elsewhere are diverted away from primary care and GP time demand reduced | PPI |
| 230 | PPI think tank October 2016 if–thens | IF there is a ‘virtual doctor’ | THEN . . . | PPI |
| 231 | PPI think tank October 2016 if–thens | IF people with complex needs have access to people with specialised knowledge without having to find these people themselves (e.g. specialist MS physiotherapist) | THEN patient experience of care will be improved | PPI |
| 232 | PPI think tank October 2016 if–thens | IF a patient being treated for a complex condition that is known to be comorbid with other conditions AND treatment for these other potential conditions or prevention of them (e.g. depression with MS) is included in the care plan or discussion of care options with patient | THEN patient access to appropriate care will be improved AND patient experience of care will be improved | PPI |
| 233 | PPI think tank October 2016 if–thens | IF knowledge and access to services in community is improved for an individual patient, THEN their experience of health-care system will be better | THEN that patient is supported to take self-care and take control of their own care | PPI |
| 234 | PPI think tank October 2016 if–thens | IF a GP listens to a patient | THEN experience of health services is improved | PPI |
| 235 | PPI think tank October 2016 if–thens | IF GPs all work in a way in which they make shared decisions with the patient | THEN patient experience of care is improved | PPI |
| 236 | PPI think tank October 2016 if–thens | IF a patient feels that they have an advocate in the health-care system who is on their side and can support them to make choices related to their medical/social care | THEN the patient will have a better experience of the health system | PPI |
| 237 | PPI think tank October 2016 if–thens | IF a GP considers the whole range of services as opposed to just medical services and can refer or inform patients about these (e.g. non-medical and green prescriptions and osteopaths) | THEN patients will have access to a wider range of potentially useful services for social/medical problems AND patient experience of care will be improved | PPI |
| 238 | PPI think tank October 2016 if–thens | IF patients had access to a community information hub | THEN they would go there first to find out available services or solutions to an issue they are not sure is appropriate to take to the GP; IF this hub is not available, THEN the patient feels they have no choice but to go to GP | PPI |
| 239 | PPI think tank October 2016 if–thens | IF a MCP includes education (e.g. obesity in schools, or for parents of kids at risk of diabetes mellitus) | THEN prevention is supported | PPI |
| 240 | PPI think tank October 2016 if–thens | IF a MCP supports patients to get better in the way that they want to (e.g. swimming lessons rather than antidepressants) | THEN patient experience of care is improved | PPI |
| 241 | PPI think tank October 2016 if–thens | IF you can persuade GPs to stay in their practices | THEN GP practices can be kept | PPI |
| 242 | PPI think tank October 2016 if–thens | IF GPs listen to their patients | THEN patients will not book GP appointment so often | PPI |
Appendix 6 Search strategy and hits
Search strategies for identifying evidence
Database: MEDLINE
Host: Ovid.
Data parameters: 1946 to November week 4 2016.
Date searched: 5 December 2016.
Searcher: Sarah L Brand.
Hits: 676.
Search strategy
(“Australian Better Health Initiative” or “Enhanced Primary Care” or “More Allied Health Services” or “National Primary Care Collaborative*” or “Team Care Arrangement” or “Patient cent* medical home*”).tw.
((SIPA or PRISMA) and australia*).tw.
(“Health and Social Services Cent*” or “Program of Research to Integrate the Services for the Maintenance of Autonomy” or “System of Integrated Care for Older Persons” or “Family Health Team*” or “Health and Social Services Cent*” or “Local Health Integration Network*”).tw.
(“acute room*” or “geriatric team*” or medcom).tw.
“Municipal health cent*”.tw.
(“health network*” and (france or french)).tw.
(“reseau* de sante” or “Quality and Coordination of Care Fund*”).tw.
“Alzira model”.tw.
(“Kinzigtal care network*” or “Gesundes Kinzigtal” or “Wiesbaden Geriatric Rehabilitation Network*” or “Medizinisches Versorgungszentrum” or polikum).tw.
“Working Unit for Continuous Care”.tw.
(Buurt?org or “One Window Model” or “shared care arrangement*” or “Transmural Care”).tw.
HealthOne.tw.
(canterbury adj2 “health board”).tw.
AFAIR.tw.
“System of Integrated Services for the Frail Elderly”.tw.
((“Primary Health Care Cent*” or “chains of care” or SIPA) and (sweden* or swedish)).tw.
(“Primary Care Medical home” or “Accountable Care Organi?ation*” or “Program of All-inclusive Care for the Elderly”).tw.
(PACE adj5 (US or USA or “united states” or medicare or medicaid)).tw.
*Accountable Care Organizations/
(“Symphony South Somerset Program Somerset” or “Long Term Conditions Shared Management Project” or “Community Assessment and Rehabilitation Team*” or “The Chronic Care Model” or “Rapid Response Team*” or “Hospital at Home” or “Single Assessment Process*” or “primary care hub*” or “Patient medical home” or “Sustainability and Transformation fund*”).tw.
(“multispecialty community provider*” or “multi specialty community provider*”).tw.
((MCP or MSCP or PACS) and (NHS or “national health service*” or UK or “united kingdom*” or england* or wales* or scotland* or ireland*)).tw.
“primary and acute care system*”.tw.
polyclinic*.tw.
(“Integrated Service Improvement Programme*” or “Realising the Value Programme*” or “House of Care” or “Better Care Fund*” or “Year of Care” or “integrated personal commissioning programme*” or “Integrated care pioneer*”).tw.
(“Delivering Quality in Primary Care” or “Living Well in Communities” or “Long Term Conditions Collaborative” or “Managed Clinical Network*” or “Prescription for Excellence” or “Integrated Care Fund”).tw.
((“Reshaping Care for Older People” or RCOP) adj1 Change Fund).tw.
(“Better Health” adj2 “Better Care”).tw.
(“National vision for chronic disease control” or “Rainbow Model of Integrated Care”).tw.
(vanguard and (“integrated primary and acute care” or “enhanced health in care homes” or “urgent and emergency care” or “acute care collaboration*”)).tw.
or/1-30
((“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) adj5 (“at scale” or extension* or extend* or expand* or integrat* or network* or combin* or “multi disciplin*” or multidisciplin*)).tw.
((“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) adj8 (“group practice*” or “community team*” or “community health” or “community based”)).tw.
((“gp surger*” or “gp service*” or “gp practice*”) adj5 (“at scale” or extension* or extend* or expand* or integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*)).tw.
((“gp surger*” or “gp service*” or “gp practice*”) adj8 (“group practice*” or “community team*” or “community health” or “community based”)).tw.
((“health budget*” or “health service* budget*”) and (ownership or delegate* or responsib* or shared)).tw.
(care adj1 (coordinat* or integrat* or continuity or navigat*)).tw.
((collaborat* or “bring* in” or employ* or recruit* or commit* or engag* or “work* alongside”) adj3 (consultant* or nurse* or physician* or geriatrician* or p?ediatrician* or psychiatrist* or therapist* or pharmacist* or psychologist* or “social worker*” or partner*)).tw.
((integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*) and ((manag* or reduce or control* or inappropriate or avoid*) adj3 (refer* or transfer* or admission* or admit*))).tw.
((substitut* or replac* or transfer*) adj4 (hospital* or “secondary care” or inpatient*)).tw.
or/32-40
*“Delivery of Health Care, Integrated”/
(“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*” or “gp surger*” or “gp service*” or “gp practice*”).tw.
(“group practice*” or “community team*” or “community health” or “community based”).tw.
42 and (43 or 44)
31 and (41 or 45)
Database: MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 2 December 2016.
Date searched: 5 December 2016.
Searcher: Sarah L Brand.
Hits: 162.
Search strategy
(“Australian Better Health Initiative” or “Enhanced Primary Care” or “More Allied Health Services” or “National Primary Care Collaborative*” or “Team Care Arrangement” or “Patient cent* medical home*”).tw.
((SIPA or PRISMA) and australia*).tw.
(“Health and Social Services Cent*” or “Program of Research to Integrate the Services for the Maintenance of Autonomy” or “System of Integrated Care for Older Persons” or “Family Health Team*” or “Health and Social Services Cent*” or “Local Health Integration Network*”).tw.
(“acute room*” or “geriatric team*” or medcom).tw.
“Municipal health cent*”.tw.
(“health network*” and (france or french)).tw.
(“reseau* de sante” or “Quality and Coordination of Care Fund*”).tw.
“Alzira model”.tw.
(“Kinzigtal care network*” or “Gesundes Kinzigtal” or “Wiesbaden Geriatric Rehabilitation Network*” or “Medizinisches Versorgungszentrum” or polikum).tw.
“Working Unit for Continuous Care”.tw.
(Buurt?org or “One Window Model” or “shared care arrangement*” or “Transmural Care”).tw.
HealthOne.tw.
(canterbury adj2 “health board”).tw.
AFAIR.tw.
“System of Integrated Services for the Frail Elderly”.tw.
((“Primary Health Care Cent*” or “chains of care” or SIPA) and (sweden* or swedish)).tw.
(“Primary Care Medical home” or “Accountable Care Organi?ation*” or “Program of All-inclusive Care for the Elderly”).tw.
(PACE adj5 (US or USA or “united states” or medicare or medicaid)).tw.
(“Symphony South Somerset Program Somerset” or “Long Term Conditions Shared Management Project” or “Community Assessment and Rehabilitation Team*” or “The Chronic Care Model” or “Rapid Response Team*” or “Hospital at Home” or “Single Assessment Process*” or “primary care hub*” or “Patient medical home” or “Sustainability and Transformation fund*”).tw.
(“multispecialty community provider*” or “multi specialty community provider*”).tw.
((MCP or MSCP or PACS) and (NHS or “national health service*” or UK or “united kingdom*” or england* or wales* or scotland* or ireland*)).tw.
“primary and acute care system*”.tw.
polyclinic*.tw.
(“Integrated Service Improvement Programme*” or “Realising the Value Programme*” or “House of Care” or “Better Care Fund*” or “Year of Care” or “integrated personal commissioning programme*” or “Integrated care pioneer*”).tw.
(“Delivering Quality in Primary Care” or “Living Well in Communities” or “Long Term Conditions Collaborative” or “Managed Clinical Network*” or “Prescription for Excellence” or “Integrated Care Fund”).tw.
((“Reshaping Care for Older People” or RCOP) adj1 Change Fund).tw.
(“Better Health” adj2 “Better Care”).tw.
(“National vision for chronic disease control” or “Rainbow Model of Integrated Care”).tw.
(vanguard and (“integrated primary and acute care” or “enhanced health in care homes” or “urgent and emergency care” or “acute care collaboration*”)).tw.
or/1-29
((“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) adj5 (“at scale” or extension* or extend* or expand* or integrat* or network* or combin* or “multi disciplin*” or multidisciplin*)).tw.
((“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) adj8 (“group practice*” or “community team*” or “community health” or “community based”)).tw.
((“gp surger*” or “gp service*” or “gp practice*”) adj5 (“at scale” or extension* or extend* or expand* or integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*)).tw.
((“gp surger*” or “gp service*” or “gp practice*”) adj8 (“group practice*” or “community team*” or “community health” or “community based”)).tw.
((“health budget*” or “health service* budget*”) and (ownership or delegate* or responsib* or shared)).tw.
(care adj1 (coordinat* or integrat* or continuity or navigat*)).tw.
((collaborat* or “bring* in” or employ* or recruit* or commit* or engag* or “work* alongside”) adj3 (consultant* or nurse* or physician* or geriatrician* or p?ediatrician* or psychiatrist* or therapist* or pharmacist* or psychologist* or “social worker*” or partner*)).tw.
((integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*) and ((manag* or reduce or control* or inappropriate or avoid*) adj3 (refer* or transfer* or admission* or admit*))).tw.
((substitut* or replac* or transfer*) adj4 (hospital* or “secondary care” or inpatient*)).tw.
or/31-39
30 and 40
Database: PsycINFO
Host: Ovid.
Data parameters: 1806 to November week 4 2016.
Date searched: 5 December 2016.
Searcher: Sarah L Brand.
Hits: 265.
Strategy: see MEDLINE in-process search strategy
Database: Cumulative Index to Nursing and Allied Health Literature
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 5 December 2016.
Searcher: Sarah L Brand.
Hits: 756.
Search strategy
TI ( “Australian Better Health Initiative” or “Enhanced Primary Care” or “More Allied Health Services” or “National Primary Care Collaborative*” or “Team Care Arrangement” or “Patient cent* medical home*” ) OR AB ( “Australian Better Health Initiative” or “Enhanced Primary Care” or “More Allied Health Services” or “National Primary Care Collaborative*” or “Team Care Arrangement” or “Patient cent* medical home*” )
TI ( (SIPA or PRISMA) and australia* ) OR AB ( (SIPA or PRISMA) and australia* )
TI ( “Health and Social Services Cent*” or “Program of Research to Integrate the Services for the Maintenance of Autonomy” or “System of Integrated Care for Older Persons” or “Family Health Team*” or “Health and Social Services Cent*” or “Local Health Integration Network*” ) OR AB ( “Health and Social Services Cent*” or “Program of Research to Integrate the Services for the Maintenance of Autonomy” or “System of Integrated Care for Older Persons” or “Family Health Team*” or “Health and Social Services Cent*” or “Local Health Integration Network*” )
TI ( “acute room*” or “geriatric team*” or medcom ) OR AB ( “acute room*” or “geriatric team*” or medcom )
TI “Municipal health cent*” OR AB “Municipal health cent*"
TI ( “health network*” and (france or french) ) OR AB ( “health network*” and (france or french) )
TI ( “reseau* de sante” or “Quality and Coordination of Care Fund*” ) OR AB ( “reseau* de sante” or “Quality and Coordination of Care Fund*” )
TI “Alzira model” OR AB “Alzira model"
TI ( “Kinzigtal care network*” or “Gesundes Kinzigtal” or “Wiesbaden Geriatric Rehabilitation Network*” or “Medizinisches Versorgungszentrum” or polikum ) OR AB ( “Kinzigtal care network*” or “Gesundes Kinzigtal” or “Wiesbaden Geriatric Rehabilitation Network*” or “Medizinisches Versorgungszentrum” or polikum )
TI “Working Unit for Continuous Care” OR AB “Working Unit for Continuous Care"
TI ( Buurt?org or “One Window Model” or “shared care arrangement*” or “Transmural Care” ) OR AB ( Buurt?org or “One Window Model” or “shared care arrangement*” or “Transmural Care” )
TI HealthOne OR AB HealthOne
TI (canterbury N1 “health board”) OR AB (canterbury N1 “health board”)
TI AFAIR OR AB AFAIR
TI “System of Integrated Services for the Frail Elderly” OR AB “System of Integrated Services for the Frail Elderly"
TI ( (“Primary Health Care Cent*” or “chains of care” or SIPA) and (sweden* or swedish) ) OR AB ( (“Primary Health Care Cent*” or “chains of care” or SIPA) and (sweden* or swedish) )
TI (“Primary Care Medical home” or “Accountable Care Organi?ation*” or “Program of All-inclusive Care for the Elderly” ) OR AB ( “Primary Care Medical home” or “Accountable Care Organi?ation*” or “Program of All-inclusive Care for the Elderly”)
TI ( PACE N4 (US or USA or “united states” or medicare or medicaid) ) OR AB ( PACE N4 (US or USA or “united states” or medicare or medicaid) )
(MM “Accountable Care Organizations”)
TI ( “Symphony South Somerset Program Somerset” or “Long Term Conditions Shared Management Project” or “Community Assessment and Rehabilitation Team*” or “The Chronic Care Model” or “Rapid Response Team*” or “Hospital at Home” or “Single Assessment Process*” or “primary care hub*” or “Patient medical home” or “Sustainability and Transformation fund*” ) OR AB ( “Symphony South Somerset Program Somerset” or “Long Term Conditions Shared Management Project” or “Community Assessment and Rehabilitation Team*” or “The Chronic Care Model” or “Rapid Response Team*” or “Hospital at Home” or “Single Assessment Process*” or “primary care hub*” or “Patient medical home” or “Sustainability and Transformation fund*” )
TI ( “multispecialty community provider*” or “multi specialty community provider*” ) OR AB ( “multispecialty community provider*” or “multi specialty community provider*” )
TI ( (MCP or MSCP or PACS) and (NHS or “national health service*” or UK or “united kingdom*” or england* or wales* or scotland* or ireland*) ) OR AB ( (MCP or MSCP or PACS) and (NHS or “national health service*” or UK or “united kingdom*” or england* or wales* or scotland* or ireland*) )
TI ( “primary and acute care system*” ) OR AB ( “primary and acute care system*” )
TI polyclinic* OR AB polyclinic*
TI ( “Integrated Service Improvement Programme*” or “Realising the Value Programme*” or “House of Care” or “Better Care Fund*” or “Year of Care” or “integrated personal commissioning programme*” or “Integrated care pioneer*” ) OR AB ( “Integrated Service Improvement Programme*” or “Realising the Value Programme*” or “House of Care” or “Better Care Fund*” or “Year of Care” or “integrated personal commissioning programme*” or “Integrated care pioneer*” )
TI ( “Delivering Quality in Primary Care” or “Living Well in Communities” or “Long Term Conditions Collaborative” or “Managed Clinical Network*” or “Prescription for Excellence” or “Integrated Care Fund” ) OR AB ( “Delivering Quality in Primary Care” or “Living Well in Communities” or “Long Term Conditions Collaborative” or “Managed Clinical Network*” or “Prescription for Excellence” or “Integrated Care Fund” )
TI ( (“Reshaping Care for Older People” or RCOP) N0 “Change Fund” ) OR AB ( (“Reshaping Care for Older People” or RCOP) adj1 “C(“Reshaping Care for Older People” or RCOP) N0 “Change Fund”hange Fund” )
TI “Better Health” N1 “Better Care” OR AB “Better Health” N1 “Better Care"
TI ( “National vision for chronic disease control” or “Rainbow Model of Integrated Care” ) OR AB ( “National vision for chronic disease control” or “Rainbow Model of Integrated Care” )
TI ( vanguard and (“integrated primary and acute care” or “enhanced health in care homes” or “urgent and emergency care” or “acute care collaboration*”) ) OR AB ( vanguard and (“integrated primary and acute care” or “enhanced health in care homes” or “urgent and emergency care” or “acute care collaboration*”) )
S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30
TI ( (“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) N4 (“at scale” or extension* or extend* or expand* or integrat* or network* or combin* or “multi disciplin*” or multidisciplin*) ) OR AB ( (“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) N4 (“at scale” or extension* or extend* or expand* or integrat* or network* or combin* or “multi disciplin*” or multidisciplin*) )
TI ( (“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) N7 (“group practice*” or “community team*” or “community health” or “community based”) ) OR AB ( (“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) N7 (“group practice*” or “community team*” or “community health” or “community based”) )
TI ( (“gp surger*” or “gp service*” or “gp practice*”) N4 (“at scale” or extension* or extend* or expand* or integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*) ) OR AB ( (“gp surger*” or “gp service*” or “gp practice*”) N4 (“at scale” or extension* or extend* or expand* or integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*) )
TI ( (“gp surger*” or “gp service*” or “gp practice*”) N7 (“group practice*” or “community team*” or “community health” or “community based”) ) OR AB ( (“gp surger*” or “gp service*” or “gp practice*”) N7 (“group practice*” or “community team*” or “community health” or “community based”) )
TI ( (“health budget*” or “health service* budget*”) and (ownership or delegate* or responsib* or shared) ) OR AB ( (“health budget*” or “health service* budget*”) and (ownership or delegate* or responsib* or shared) )
TI ( care N0 (coordinat* or integrat* or continuity or navigat*) ) OR AB ( care N0 (coordinat* or integrat* or continuity or navigat*) )
TI ( (“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) N7 (“group practice*” or “community team*” or “community health” or “community based”) ) OR AB ( (“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) N7 (“group practice*” or “community team*” or “community health” or “community based”) )
TI ( (integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*) and ((manag* or reduce or control* or inappropriate or avoid*) N2 (refer* or transfer* or admission* or admit*)) ) OR AB ( (integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*) and ((manag* or reduce or control* or inappropriate or avoid*) N2 (refer* or transfer* or admission* or admit*)) )
TI ( (substitut* or replac* or transfer*) N3 (hospital* or “secondary care” or inpatient*) ) OR AB ( (substitut* or replac* or transfer*) N3 (hospital* or “secondary care” or inpatient*)) )
S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40
(MM “Health Care Delivery, Integrated”)
TI ( “general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*” or “gp surger*” or “gp service*” or “gp practice*” ) OR AB ( “general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*” or “gp surger*” or “gp service*” or “gp practice*” )
TI ( “group practice*” or “community team*” or “community health” or “community based” ) OR AB ( “group practice*” or “community team*” or “community health” or “community based” )
S42 AND (S43 OR S44)
S31 AND (S41 OR S45)
Database: Applied Social Sciences Index and Abstracts
Host: ProQuest.
Data parameters: not applicable.
Date searched: 5 December 2016.
Searcher: Sarah L Brand.
Hits: 44.
Search strategy
(TI,AB(“Australian Better Health Initiative” or “Enhanced Primary Care” or “More Allied Health Services” or “National Primary Care Collaborative*” or “Team Care Arrangement” or “Patient cent* medical home*” or ((SIPA or PRISMA) and australia*) or “Health and Social Services Cent*” or “Program of Research to Integrate the Services for the Maintenance of Autonomy” or “System of Integrated Care for Older Persons” or “Family Health Team*” or “Health and Social Services Cent*” or “Local Health Integration Network*” or “acute room*” or “geriatric team*” or medcom or “Municipal health cent*” or (“health network*” and (france or french)) or “reseau* de sante” or “Quality and Coordination of Care Fund*” or “Alzira model” or “Kinzigtal care network*” or “Gesundes Kinzigtal” or “Wiesbaden Geriatric Rehabilitation Network*” or “Medizinisches Versorgungszentrum” or polikum or “Working Unit for Continuous Care” or Buurt?org or “One Window Model” or “shared care arrangement*” or “Transmural Care” or HealthOne or (canterbury n/1 “health board”) or AFAIR or “System of Integrated Services for the Frail Elderly” or ((“Primary Health Care Cent*” or “chains of care” or SIPA) and (sweden* or swedish)) or “Primary Care Medical home” or “Accountable Care Organi?ation*” or “Program of All-inclusive Care for the Elderly” or (PACE n/4 (US or USA or “united states” or medicare or medicaid)) or “Symphony South Somerset Program Somerset” or “Long Term Conditions Shared Management Project” or “Community Assessment and Rehabilitation Team*” or “The Chronic Care Model” or “Rapid Response Team*” or “Hospital at Home” or “Single Assessment Process*” or “primary care hub*” or “Patient medical home” or “Sustainability and Transformation fund*” or “multispecialty community provider*” or “multi specialty community provider*” or ((MCP or MSCP or PACS) and (NHS or “national health service*” or UK or “united kingdom*” or england* or wales* or scotland* or ireland*)) or “primary and acute care system*” or polyclinic* or “Integrated Service Improvement Programme*” or “Realising the Value Programme*” or “House of Care” or “Better Care Fund*” or “Year of Care” or “integrated personal commissioning programme*” or “Integrated care pioneer*” or “Delivering Quality in Primary Care” or “Living Well in Communities” or “Long Term Conditions Collaborative” or “Managed Clinical Network*” or “Prescription for Excellence” or “Integrated Care Fund” or ((“Reshaping Care for Older People” or RCOP) n/0 “Change Fund”) or (“Better Health” n/1 “Better Care”) or (“National vision for chronic disease control” or “Rainbow Model of Integrated Care”) or (vanguard and (“integrated primary and acute care” or “enhanced health in care homes” or “urgent and emergency care” or “acute care collaboration*”)))) AND (TI,AB(((“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) n/4 (“at scale” or extension* or extend* or expand* or integrat* or network* or combin* or “multi disciplin*” or multidisciplin*)) or ((“general practi*” or “general physician*” or “general doctor*” or “general medicine” or “family practi*” or “family physician*” or “family doctor*” or “family medicine” or “primary care” or “primary healthcare” or “primary service*” or “primary physician*”) n/7 (“group practice*” or “community team*” or “community health” or “community based”)) or ((“gp surger*” or “gp service*” or “gp practice*”) n/4 (“at scale” or extension* or extend* or expand* or integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*)) or ((“gp surger*” or “gp service*” or “gp practice*”) n/7 (“group practice*” or “community team*” or “community health” or “community based”))) OR TI,AB((“health budget*” or “health service* budget*”) and (ownership or delegate* or responsib* or shared)) OR TI,AB(care n/0 (coordinat* or integrat* or continuity or navigat*)) OR TI,AB((collaborat* or “bring* in” or employ* or recruit* or commit* or engag* or “work* alongside”) n/2 (consultant* or nurse* or physician* or geriatrician* or p?ediatrician* or psychiatrist* or therapist* or pharmacist* or psychologist* or “social worker*” or partner*)) OR TI,AB((integrat* or federat* or network* or combin* or “multi disciplin*” or multidisciplin*) and ((manag* or reduce or control* or inappropriate or avoid*) n/2 (refer* or transfer* or admission* or admit*))) OR TI,AB((substitut* or replac* or transfer*) n/3 (hospital* or “secondary care” or inpatient*)))
| Database | Records (n) |
|---|---|
| MEDLINE | 676 |
| MEDLINE In-Process & Other Non-Indexed Citations | 162 |
| PsycINFO | 265 |
| CINAHL | 756 |
| ASSIA | 44 |
| Total number of records | 1903 |
| Duplicate records | 584 |
| Unique records | 1319 |
Appendix 7 Screening tool 1
| EndNote: database name/article ID number | |||
|---|---|---|---|
| Activity | Subsection | Description | Code |
| Reviewer ID | Who is screening? | AbSLB | |
| AbMF | |||
| AbRS | |||
| AbMP | |||
| AbAW | |||
| AbHL | |||
| How source was located | Stakeholders | AbSH | |
| Hand searching | AbHS | ||
| Website | AbWeb | ||
| Citation chasing | AbCC | ||
| Table of contents alerts | AbToC | ||
| Browsing | AbBrws | ||
| Database search | AbDS | ||
| On basis of abstracts | |||
| Does the source contain or test programme theories about any of the components in the initial theoretical MCP model? | |||
| Inclusion/exclusion criteria | Empirical? | Include: comparative effectiveness study (RCT, etc.), process evaluation, review of primary research (if method is stated), qualitative research, surveys, history, descriptions of models of care, uncontrolled before and after, cohort and reanalysis of routine data |
AbEmpYes AbEmpNo |
| Exclude: editorials, opinion pieces and advertorials | |||
| Relevant? (to horizontal interorganisational linkages in primary care) | Include: interorganisational links in any combination of – primary medical care, CHS, ambulance, community mental health, residential care, therapies, PHC dentistry and PHC pharmacy |
AbRelYes AbRelNo |
|
| Exclude: purely hospital studies, single-organisation studies | |||
| Classification | 1. Field of practice to which source predominantly refers (code all that apply) | MCP context | AbContext |
| MCP created | AbCreated | ||
| Network management | AbNW | ||
| MDT | AbMDT | ||
| Culture change | AbCulture | ||
| Third sector | Ab3S | ||
| Care co-ordination: IT | AbCCIT | ||
| Care planning: organisational level | AbCarePlanOrg | ||
| Demand management | AbDmgt | ||
| Prevention | AbPrev | ||
| Diversion patient level | AbDivPt | ||
| Care planning: patient level | AbPtCarePlan | ||
| Cost | AbCost | ||
| Patient experience/care | AbCare | ||
| 2. Type of source (code one only) | Policy document | AbPD | |
| Viewpoint/editorial | AbVE | ||
| Grey documents (from MCP sites) | AbLM | ||
| Primary research | AbPR | ||
| Rapportage | AbRap | ||
| Decision | |||
| Include/exclude decision | Include | IncAb | |
| Exclude | ExcAb | ||
Appendix 8 Screening tool 2
| EndNote: database name/article ID number | |||
|---|---|---|---|
| Activity | Subsection | Description | Code |
| Reviewer ID | Who is screening? | Ab2SLB | |
| Ab2MF | |||
| Ab2RS | |||
| Ab2MP | |||
| Ab2AW | |||
| Ab2HL | |||
| Screening round | Which round of screening? | First round (empirical and relevance to 14 components) | |
| Second round (major or minor decision on papers < 3 years old) | Screen#2 | ||
| Decision | |||
| Inclusion/exclusion criteria | Major relevance? | Study mostly reports the working of established MCP-like structures in an OECDa country | AbMajor |
| Minor relevance? |
|
AbMinor | |
Appendix 9 Data extraction tool
| MCP-like model of care | |
|---|---|
| Name of MCP-like model of care | |
| Country/area | |
| Study | |
| Authors (year) | |
| Study type | |
| Aim of study | |
| Quality appraisal MMAT scoring metric or AMSTAR score | MMAT scoring metrics: For each retained study, an overall quality score may be not informative (in comparison to a descriptive summary using MMAT criteria), but might be calculated using the MMAT. As there are only a few criteria for each domain, the score can be presented using descriptors such as *, **, *** and ****. For qualitative and quantitative studies, this score can be the number of criteria met divided by four [scores varying from 25% (*; one criterion met) to 100% (****; all criteria met)]. For mixed-methods research studies, the premise is that the overall quality of a combination cannot exceed the quality of its weakest component. Thus, the overall quality score is the lowest score of the study components. The score is 25% (*) when QUAL = 1 or QUAN = 1 or MM = 0; it is 50% (**) when QUAL = 2 or QUAN = 2 or MM = 1; it is 75% (***) when QUAL = 3 or QUAN = 3 or MM = 2; and it is 100% (****) when QUAL = 4 and QUAN = 4 and MM = 3 (QUAL being the score of the qualitative component; QUAN the score of the quantitative component; and MM the score of the mixed-methods component) |
| AMSTAR rating (/11) where each tick box counts for 1 | |
| Quality appraisal narrative summary | |
| Year(s) MCP-like model of care operating | |
| Year(s) study carried out | |
| Research methods | |
| Theoretical approach (if stated) | |
| Sample method | |
| Participants (characteristics/number) | |
| Data collection (include number of patients per data collection method, if appropriate) | |
| Analysis | |
| Time of follow-up | |
| Evidence about assumption# | For each assumption below think about:
|
| 1 MCP context | (AbContext RS) |
| 1a | Context produces interorganisational network management |
| 1b | Context produces organisational-level care planning |
| 2 Interorganisational network management | (AbNW and AbCreated RS) |
| 2a | Interorganisational network management produces care co-ordination |
| 2b | Interorganisational network management produces MDT working |
| 3 MDT working | (AbMDT SLB) |
| 3a | MDT working produces organisational-level care planning |
| 3b | MDT working produces preventative care |
| 4 Culture change | (AbCulture SLB) |
| 4a | Culture change produces MDT working |
| 4b | Culture change produces demand management |
| 4c | Culture change produces preventative care |
| 5 Third sector | (Ab3S SLB) |
| 5a | Third-sector involvement produces demand management |
| 5b | Third-sector involvement produces preventative care |
| 6 Care coordination via IT – informational continuity of care | (AbCCIT AW) |
| 6a | Care co-ordination is produced by informational continuity of care |
| 6b | Informational continuity of care supports diversion at the patient level |
| 6c | Informational continuity of care supports care planning at the patient level |
| 7 Care planning at organisational level | (AbCarePlanOrg SLB) |
| 7a | Care planning at organisational level produces patient diversion |
| 7b | Care planning at organisational level produces care planning at patient level |
| 7c | Care planning at organisational level produces demand management |
| 8 Demand management | (AbDMgt MP) |
| 8a | Demand management produces patient diversion |
| 8b | Demand management produces care planning at patient level |
| 8c | Demand management produces preventative care and vice-versa |
| 9 Preventative care | (AbPrev MP) |
| 9a | Preventative care produces patient diversion |
| 10 Care planning at patient level | (AbPtCarePlan HL) |
| 10a | Care planning at patient level produces patient diversion |
| 10b | Care planning at patient level improves patient experience |
| 11 Patient Diversion | (AbDivPt MP) |
| 11a | Patient diversion reduces costs |
| 11b | Patient diversion improves patient experience |
| 12 Other minor connections | (AbDMgt MP, AbDivPt MP) |
| 12a | General practice will benefit from patient diversion |
| 12b | Care co-ordination and demand management will together produce more responsive urgent care |
| Additional notes | |
Appendix 10 Main topics in multispecialty community provider policy documents analysed
| Topic | Frequency of mention (n) |
|---|---|
| Better patient experience | 27 |
| Workforce engagement, training (in context of MDT) | 26 |
| Exploiting data access and use through IT | 23 |
| Cost/’efficiency’ savings | 19 |
| Reduce A&E admissions | 18 |
| Better care for long-term conditions | 17 |
| Single point of access to services | 14 |
| Patient self-activation | 14 |
| (Better) care co-ordination | 13 |
| Managing networks (‘systems’) of organisations | 12 |
| MDTs care for patients | 12 |
| Patient education/information | 12 |
| General practice demand management systems | 11 |
| Wider range of services (than existing general practice) | 10 |
| Preventative care | 10 |
| Standardised protocols, models of care | 10 |
| Involve volunteers, voluntary organisations | 10 |
| Surmount organisational boundaries | 9 |
| Patients’ knowledge, attitudes, beliefs | 9 |
| Gate-keeping on basis of need, risk | 9 |
Appendix 11 The 13 components of the initial programme theory of multispecialty community providers
| MCP component | Description |
|---|---|
| 1. NHS managers set up MCPs | Implicitly, MCPs are set up by NHS managers using existing organisational and network structures, budgets, contractual rights and existing relationships with non-NHS bodies |
| 2. Network management | A MCP would be, above all, the co-ordinating body of a network of (at least) general practices and CHSs, often also including social services and mental health services. MCPs will co-ordinate a wide range of health professions and take responsibility for managing budgets across this ensemble of services. This activity takes place at the level of whole care groups and at interorganisational level, not patient by individual patient (on that, see below). A MCP will:
|
| 3. MDTs | What MDTs are is already widely, and comparatively clearly, defined and understood in clinical and managerial practice, policy statements and research studies. The policy statements emphasised that MDTs will:
|
| 4. Culture change | Implicitly, the relevant culture change was in the organisations, professions and care teams involved in MCPs. The most explicitly defined ‘culture change in health service understanding and use’, and ‘shifts in the models of care and culture of care delivery’ in the policy statements that we analysed was a ‘strengths-based approach’, which we interpret as identifying, promulgating and elaborating existing successes in care co-ordination |
| 5. Voluntary sector involvement | Voluntary sector involvement in MCPs meant involving both individuals (carers, volunteers) and whole organisations in MCP activities, harnessing the volunteers’ special capacities and skills such as knowledge of gaps in the local health care and engaging volunteers and voluntary organisations as a ‘resource’ |
| 6. Care co-ordination through HIT | Care co-ordination through HIT was assumed to involve greater informational continuity of care (i.e. that a patient’s care plan be decided on the basis of all the available relevant information about her history, current condition, circumstances and care needs).43–46 That, policy documents assumed, requires patient records to be directly accessible by all the health professionals seeing patients registered with any practice within a GP federation, network or OOH service,39 and more skilled, intelligent data analysis and use |
| 7. Planned referral networks | Once established, MCPs will use their networks of local health-care providers to co-ordinate patient flows between the different services, often in separate organisations, relevant to each care group. That is, as referral networks.41 Through its referral network a MCP would:
|
| 8. Demand management systems | Demand management systems were assumed, including gate keeping, need- and risk-stratification, targeting services on patients with complex needs, having a single point of access for all services in a locality and being information hubs |
| 9. Preventative health care | ‘Prevention’ meant (in the policy documents we examined, but not necessarily more widely) long-term patient self-care, ‘activation’ and ‘empowerment’, engagement in caring for others, giving patients access to knowledge access to information provided about their own health problems (e.g. on possible comorbidities with new diagnosis), and patient education to address ‘barriers to patients engaging with change in health services delivery’. Intersectoral activity for illness prevention and health equalisation was mentioned but less prominent |
| 10. Care planning at the patient level | Care planning at patient level was assumed to involve a personal care plan and care co-ordination (hence, a care co-ordinator) for each patient with complex care needs (‘care designed around diagnosis’). Patient-level care planning also involved care closer to home, advocacy for patients and ‘patient-centred care’ oriented towards patients’ personal goals’ through shared decision-making |
| 11. Diversion | Patient diversion was assumed to mean hospital admission avoidance and/or support for timely discharge, covering both planned admissions (from outpatient departments) and unplanned (from A&E), and from any source including admissions from and discharges to nursing homes and residential care. Concomitantly, GPs would increasingly manage (clinical) risks in the community, outpatient department care would become more intense, primary would substitute increasingly for hospital care, diagnostic services and observation units would be combined, and MCPs would provide a wider range of services than existing general practices, combining NHS, GP and social care services. In short, MCPs would divert patients from inpatient care to enhanced primary care services |
| 12. Improved patient care | Better patient experience and care (especially for long-term conditions) was taken to mean personalised care (see above) with older patients being less isolated, better quality of life, living independently, having recovery and/or rehabilitation and/or emotional and mental health support ‘in the community’ (i.e. at the patient’s own home or care home) |
| 13. Reduced NHS cost | ‘Efficiency savings’ meant cost reduction not cost shifting between providers |
Appendix 12 Studies excluded at synthesis: details
| Authors (date) | Population | Model of care | Data collection | Participants | Evidence for causal link(s) |
|---|---|---|---|---|---|
| Biernacki et al. (2015)167 | Diabetic patients enrolled in a PCMH | PCMH | Pre–post design including EMR data and satisfaction surveys | 937 |
10:11 10:12 |
| Broffman et al. (2016)168 | Regional collaboratives: ‘co-ordinated care organisations’, Oregon | Global budget for organisations within regional collaboratives | Case studies: interviews and grey documentation | Two collaborative care organisations | 1:2 |
| Cook et al. (2015)169 | Patients from five health centre PCMHs in south Florida | Health centre PCMH | Face-to-face survey | 488 | 10:12 |
| Cook et al. (2016)170 | Racially and ethnically diverse patients of four primary care safety net organisations | PCMH | Survey | 351 | 10:12 |
| Farrell et al. (2015)171 | University of Utah’s community care patients (excluding ED, paediatric, psychiatric, labour and delivery, neonatal intensive care unit, newborn nursery, maternal newborn care) who had been admitted to University Hospital from June 2010 to May 2011, who had a subsequent admission to that hospital from June 2011 to September 2013, and who came under Primary Care and Transition Management programme | Care by Design (University of Utah’s Community Clinic’s version of PCMH) | Routine data | 118 | 2:7 |
| Geltman et al. (2015)172 | Paediatric patients with attention-deficit/hyperactivity disorder | Planned Care System | Pilot project data | 321 (250 pre-existing diagnoses, 71 newly diagnosed) | 6:7 |
| King et al. (2016)115 | Non-federal office-based physicians | PCMHs/ACOs | Survey | 8198 | 6:10 |
| Knapp et al. (2014)173 | Paediatric practice staff | PCMH | Survey compared against practice data and data from the core project | 20 practice and 170 staff | 3:7 |
| Lemmens et al. (2015)174 | Literature reporting interventions that used at least two of the six CCM components and concerned psychological comorbidity | Integrated care programmes for patients with psychological comorbidity with somatic morbidity | Literature review | 15 includes |
8:10 10:11 10:12 11:12 11:13 |
| Lewin et al. (2016)175 | Teenage mothers and their children | PCMH (‘Generations’) at Academic Medical Centre | Structured interview | 150 mother/child pairs |
2:8 11:12 |
| Liem et al. (2014)176 | Parents/guardians of children with sickle cell disease | PCMH | Survey | 200 | 10:12 |
| Lubetkin et al. (2014)177 | English/Spanish/Haitian-Creole speaking patients at one inner city hospital ambulatory care practice | PCMH | Survey | 461 | 4:9 |
| Miller-Metero et al. (2016)178 | Senior staff physicians. Residents in PCMH | PCMH with psychologist addition | Survey | 19 staff and 91 residents | 3:9 |
| Philpot et al. (2016)179 | Medicare enrolees aged ≥ 65 years, with a usual source of care other than ED and with one of the five most prevalent chronic conditions within Medicare population | PCMH | Survey | 2153 patients | 8:13 |
| Rosenthal et al. (2016)180 | Practices piloting the PCMH programme; patients with multiple or complex needs | PCMH | Census of data on quality of care | 30,000 patients (11 practices, 37 physicians) | 11:13 |
| Stock et al. (2016)181 | Physicians with previous experience caring for Medicaid patients | Co-ordinated care organisation/ACO | Semistructured interviews | 22 | 3:7 |
| van der Kluit et al. (2014)182 | Nurses working in nurse-led clinics transmural clinics for heart failure, rheumatoid arthritis, Parkinson’s disease and multiple sclerosis; patients who had received a consultation | Transmural care organisation for specialised nurses | Interviews, patient records | 218 patients; 7 nurses | 10:12 |
| van Leeuwen et al. (2015)183 | ‘Frail’ community-dwelling older adults | Geriatric care model based on CCM | Questionnaires, interviews, carer diaries, surveys, and physical and mental health data | 1147 |
3:9 11:13 |
Appendix 13 Included studies: details
| Authors (date) | Population | Model of care | Data collection | Participants, n | Evidence for causal link(s) |
|---|---|---|---|---|---|
| Alidina et al. (2016)68 | Physicians from 13 PCMH ‘practices’ (practice lead and co-ordinated care lead) | PCMH: ‘medical neighbourhoods’ | Interviews and survey | 40 |
1:2 1:7 2:7 4:3 6:7 7:10 |
| Aliu et al. (2014)125 | All new visits to specialists (non-federal, employed, office-based physicians engaged in direct patient care) from 2000 to 2009 in neurology, otolaryngology, dermatology, orthopaedics, urology, general surgery, ophthalmology, cardiology, obstetrics/gynaecology, psychiatry) | ACO | Survey | 32,784 patient visits to physicians (generalist and specialist) | 7:10 |
| Anderson et al. (2015)96 | Programmes that a literature review identified as being successful on at least one of their triple aims (spending, satisfaction, clinical outcomes) in treating adults with high costs or high needs in the USA | Types of programs included in review: ACOs, readmission initiatives, special needs plans, care transition programs, and PCMHs | Semistructured interviews | 45 |
3:7 3:10 6:7 10:12 |
| Annis et al. (2016)98 | Research studies (not policy or opinion) published between 2007 and August 2014. Studies that were within the USA, were about PCMH and had outcome measures of access to care and/or care co-ordination. | PCMH | SR | 42 includes |
3:9 7:8 7:9 |
| Batalden et al. (2015)101 | Patients and health workers participating in self-management schemes in Scotland and the USA | Self-management initiative, NHS Scotland with shared medical appointments | Participant observation |
NHS Scotland: 600 patients, 900 health professionals USA: network of 71 organisations |
3:9 |
| Bauer et al. (2014)118 | Papers on how HIT and collaborative care can support one another | Collaborative care | Literature review | N/A |
6:7 6:10 6:11 |
| Bergman et al. (2016)92 | Clinical pharmacists and primary care physicians from seven Midwestern federally funded medical centres and associated primary care clinics | PCMH and team-based care models | Semistructured interviews | 42 |
3:9 3:10 4:3 4:9 4:10 6:7 6:10 |
| Besser (2016)132 | Adolescents aged 13–18 years seen in an army health-care facility and who were examined for depression | Army PCMH | Census data | 196,536 unique individuals, of which 11,704 seen for depression |
8:10 8:11 |
| Billings and de Weger (2015)11 | N/A | Four models of contracting for integrated care:
|
Literature review | N/A |
1:2 1:7 2:7 11:12 11:13 |
| Bleser et al. (2014)72 | Small- to mid-sized medical practices in Pennsylvania during the first regional rollout of a state-wide PCMH initiative | PCMH | Semistructured interviews, focus groups | 20 small/medium medical practices, 136 persons, 7 focus groups |
1:2 1:7 |
| Briot et al. (2015)66 | A health-care system in Utah that integrated mental health specialists into PCPs | Integrated care delivery system | Literature review and analysis of reports, communications, and published literature about the health-care system being studied | N/A |
1:2 2:7 3:7 3:9 5:9 6:10 7:11 7:13 10:11 10:12 11:13 |
| Busetto et al. (2016)114 | Integrated care interventions for type 2 diabetes mellitus that include at least two of the four CCM components | Integrated care | SR | 32 includes |
3:9 4:3 4:5 4:9 4:12 6:7 6:10 7:11 11:13 |
| Busetto et al. (2016)113 | Integrated care interventions for type 2 diabetes mellitus that include at least two of the four CCM components | Integrated care | SR | 42 includes |
3:9 4:3 4:5 4:9 6:7 6:10 7:11 11:13 |
| Busse and Stahl (2014)64 | Purpose sample of most-developed projects in three countries | Gesundes Kinzigtal, English integrated care pilots, the Netherlands bundled payment model | Routine administrative data and surveys [and interviews (the Netherlands only)] | One local German scheme, Netherlands-wide programme (one care group) and 16 English pilot schemes |
1:2 1:7 2:7 5:9 6:7 7:10 8:11 |
| Canali et al. (2016)74 | GPs near Grand Versailles, participating in EPSILON during 2013 | EPSILON geriatrics network | Medical records of patients aged > 75 years in one health system and questionnaires (given to GPs) | 9 GPs and 15 monitored patients |
2:7 3:9 4:5 5:9 6:7 10:12 11:12 |
| Cantor et al. (2014)71 | Purposive selection of ACOs in New Jersey | ACO | Patient records | 380,000 patient records |
1:2 7:8 7:11 8:11 11:13 |
| Carroll et al. (part 1, 2016)119 | Patients, administrators, and Interprofessional family health teams or academic family health teams across six sites in Toronto | ACO | Questionnaire | 1200 patient and 6 administrators |
3:9 6:10 7:11 |
| Carroll et al. (part 2, 2016)99 | Patients, administrators, and Interprofessional family health teams or academic family health teams across six sites in Toronto | ACO | Questionnaire | 1200 patients and 6 administrators |
10:12 11:13 |
| Clarke et al. (2015)139 | Embedded one CCC per practice in 14 of the 28 primary care sites within University of California, Los Angeles Health. The control sites were the remaining 14 practices that did not receive a CCC | PCMH | Administrative data analysis | 14 CCCs |
10:11 10:12 |
| Colla et al. (2016)73 | ACO organisations | ACO | Questionnaire | 269 |
1:2 1:7 2:7 2:8 3:7 4:3 4:5 4:9 5:9 6:3 6:7 6:10 6:11 7:10 10:12 11:12 11:13 |
| Collinsworth et al. (2014)83 | Patients, CHWs and primary care providers, five community clinics. Focus on Hispanic patients | CCM | Structured interview and administrative data | 12 patients, 6 physicians, 1 nurse practitioner and 5 CHWs |
2:7 3:7 3:9 3:10 4:3 4:9 6:7 10:12 |
| Cueller et al. (2016)70 | Adults aged 18–64 years, residing in Maryland, VA, and the District of Columbia, and insured by CareFirst for at least 3 consecutive months between 2010 and 2013 | PCMH | Administrative data | 1,433,297 |
1:2 1:7 2:7 10:11 11:13 |
| Damery et al. (2016)104 | Adult patients with one or more chronic condition, except those receiving palliative, complementary and alternative, and ‘purely psychosocial’ interventions | ‘Integrated care’ [i.e. at least two of primary care, community care (taken to include social care), secondary care] | Earlier SRs | 50 includes |
1:2 3:9 7:8 11:13 |
| D’Aunno et al. (2015)57 | Substance abuse treatment organisations’ directors and clinicians | Substance abuse treatment organisations and ACOs | Census and telephone interviews | 635 |
1:2 2:7 |
| David et al. (2015)141 | Patients from 280 PCMH practices | PCMH | Census | 460,000 | 2:7 |
| Demiris and Kneale (2015)85 | Literature on IT patient-centred medical homes/co-ordinated care contexts | PCMH | Literature review | 50 includes |
1:7 2:7 4:9 4:10 6:7 6:10 6:11 8:10 |
| Desmedt et al. (2016)126 | Literature on integrated care models for chronic diseases | Integrated care | SR | 26 includes |
7:11 10:12 11:12 11:13 |
| Evans et al. (2014)61 | Leaders and providers from Health Links and LHINs | Health Links: the Health Links bring together multiple clinical and social service providers on a voluntary basis, including a minimum of 65% of primary care providers in each region | Interviews | No number stated |
1:2 1:7 2:7 6:10 |
| Fix et al. (2014)163 | HIV providers (clinicians and other staff) and patients | Patient-aligned care teams (PCMH principles) | Interviews | 41 HIV providers and 20 patients |
4:3 10:12 |
| Friedman et al. (2016)87 | Those identifying as performing care co-ordination in primary care (regardless of job title) | PCMH | Private online discussion forum used to gather perceptions and experiences | 25 (17 of whom completed full study) |
3:7 3:9 6:7 6:10 |
| Gehlert et al. (2015)77 | Social workers employed by ACOs | ACO | Survey | 395 |
3:7 3:9 |
| Grace et al. (2014)93 | Primary care personnel | PCMH | Semistructured interviews and survey | Interviews: 22; physician survey: 71; and staff survey: 329 | 3:7 |
| Greene et al. (2016)89 | Mental health providers, primary care providers and staff | Patient-centred medical home – neighbourhood | Qualitative surveys and interviews | Surveys: 6 mental health care providers and 7 primary care providers. Interviews: 12 mental health care providers and 10 primary care providers and staff |
3:7 4:3 4:7 6:10 |
| Hibbard et al. (2015)129 | Primary care providers | ACO | Two surveys and interviews | Survey 1: 157; survey 2: 150; Interviews: pre implementation: 48; 6-month follow-up: 18; and 1-year follow-up: 30 | 8:10 |
| Hildebrandt et al. (2015)81 | AOK and LKK (sick funds) subscribers in the Kingzigtal region | IVGK: combined care | Census from relevant databases | 33,000 |
1:2 1:7 5:8 6:7 6:10 7:10 8:9 11:12 11:13 |
| Noël et al. (2014)79 | Autonomous primary care clinics in south Texas | Practice facilitation. External facilitators guide clinical audit in PHC general practices and activities corresponding for four CCM components | Practice environment checklist. Data collection during facilitation fieldwork: baseline, 12- and 24-month follow-up. Semistructured interviews at baseline | 40 |
1:7 2:7 7:10 7:12 10:12 |
| Hong et al. (2014)16 | ACO sites and staff delivering successful complex care management systems | ACOs | Semistructured interviews. Review of manuscripts and programme materials. Measurements of outcomes from each site | 18 sites: three key informants per site for interviews |
3:7 3:9 3:10 6:10 10:11 10:12 |
| Huber et al. (2016)128 | Patients registered with Helsana (health insurer), Switzerland | Network of GPs, with structured care guidelines and referral network to other clinicians | Analysis of routine administrative data | 12,526 patients with diabetes mellitus, 71,778 with cardiovascular diseases and 17,498 with respiratory illnesses |
7:11 11:13 |
| Janiszewski et al. (2015)164 | Diabetes mellitus patients | Diabetes mellitus self-management education delivered in a PCMH | Focus groups | 37. Six groups: 4–10 participants per group | 10:12 |
| Johnson et al. (2015)124 | Low-income and poverty-level patients, Denver, CO | PCMH with CCM. Network of health centres, school clinics, outpatients, hospital and substance abuse services | Participant observation | Health professionals (number unstated) producing risk stratification system | 6:9 |
| Kash et al. (2014)51 | Literature on the evolution and implementation of perioperative systems | PSH | Literature review | 152 includes |
2:7 3:7 3:9 6:10 7:11 8:9 8:10 10:11 10:12 11:12 11:13 |
| Kaushal et al. (2015)122 | Patient records in PCPs with > 200 patients | PCMH | Census | Patients: 230,593; PCPs: 275 | 6:7 |
| Kennedy et al. (2015)80 | PHC practices | PCMHs and ACOs which involve pharmacists | Unclear | 7 practices and 8 pharmacists |
3:7 3:9 6:10 6:11 10:12 11:12 11:13 |
| Kinjo et al. (2017)107 | Terminally ill patients in Japan | Zaitaku model: end-of-life care at home | Cross-sectional survey, analysis of routine administrative data | 106 terminal care patients | 3:11 |
| Lafortune et al. (2015)108 | Clients, informal care givers, and health-care providers | Community-based PHC | Focus groups | 28 clients and informal care givers, and 20 health-care providers |
3:9 5:8 5:9 6:7 6:10 10:12 11:12 11:13 |
| Lewis et al. (2014)59 | ACOs | ACO with ‘safety net’ CHC | Survey: census of ACOs. Oversample of ACOs containing a CHC | 156 ACOs and 36 interviews |
1:2 1:7 2:7 3:7 3:9 4:3 6:11 |
| Lewis et al. (2014)56 | ACOs | ACO: Medicare’s Shared Savings Program, Pioneers ACOs, Medicaid ACOs and commercial-payer ACOs | Survey: census of ACOs. Interviews | 156 ACOS and 16 interviews |
1:2 1:7 2:7 3:7 3:9 4:5 10:11 |
| Liss et al. (2014)127 | Adults with hypertension | PCMH | Census of data on patient observation | 36,805 |
7:11 7:12 |
| Matiz et al. (2014)94 | Providers in five PCMHs | PCMH | Survey and review of referral numbers | Unknown |
3:7 3:9 |
| McConaha et al. (2015)100 | Patients with concomitant diabetes mellitus and hypertension not currently treated with angiotensin-converting-enzyme inhibitor or angiotensin-II receptor blocker attending 16 out of the 19 PCP offices in one PHC practice | PHC medical practice | Census of patient data | 954 |
3:7 3:9 4:3 6:7 |
| McGough et al. (2016)121 | Patients with moderate to serious mental health diagnoses and needs, 70% of which with insurance | Neighbourhood Clinic Network | Census of relevant patients on registry | 1256 |
1:2 1:7 3:7 3:9 6:7 6:10 6:11 10:12 11:12 11:13 |
| McNab and Gillespie (2015)62 | Older Aboriginal people with chronic complex illness | Community based colocation of services with virtual hub | Patient survey and census of health-provider data | 125 |
1:2 1:7 2:7 3:7 3:9 4:5 6:7 6:10 6:11 7:11 7:10 10:11 |
| McNab et al. (2016)90 | Members of the HealthOne Mount Druitt care model steering committee (policy and decision-makers, GPs, carers and patients) | Chronic aged and complex care service model | Semistructured interviews and focus group | 32 interviewed and one focus group with 9 members |
3:7 3:9 4:3 4:7 |
| Mead et al. (2014)91 |
(1) Patients who have used safety net health services (2) Patients who have suffered with heart failure or acute myocardial infarction |
PCMH | Focus groups | 387 in 33 focus groups of 8–12 |
3:9 3:10 5:8 8:9 8:11 9:11 10:12 |
| Merrill et al. (2015)123 | Adults with an International Classification of Diseases, Ninth Revision diagnosis code 428.0–428.9 (heart failure/disease) who had a least one outpatient visit between July 2011 and 2012 | PCMH, ACOs, patient-centred specialty programme | Census of routine data | 4803 | 6:7 |
| Morton et al. (2015)120 | Clinicians | PCMH | Questionnaires | 275 CHCs, 284 health system-owned practices, 247 small physician-owned practices and 191 large physician-owned practices | 6:10 |
| Nandram and Koster (2014)21 | Staff, founder, cofounders, coaches, nurses, clients and trainer at the Buurtzorg | Buurtzorg | Interviews | 38 |
2:7 3:7 3:9 4:3 4:9 6:7 6:10 |
| Nelson et al. (2014)106 | VHA patients with more than two primary care visits | PCMH | Census | 2,630,171 patients | 3:9 |
| Nelson et al. (2014)102 | All VHA patients and all VHA primary care staff | PCMH | Census | 5,653,616 patients and 5404 primary care staff | 3:9 |
| O’Malley et al. (2015)116 | Physicians/practice team members at PCMHs. National experts on primary care teamwork | PCMH | Interviews | 60 physicians/practice team members and 3 experts | 6:10 |
| Peterson et al. (2016)82 | Medicaid-covered child special care need practice before 2011 | PCMH for children with special care needs | Semistructured interviews | 11 paediatricians and 9 family physicians |
1:2 2:7 4:3 6:7 |
| Pineault et al. (2014)60 | Administrators of FMG study organisations | PCMH-like FMGs | Survey | 376 organisations |
1:2 1:7 2:7 |
| Pourat et al. (2016)138 | Adults aged > 17 years who received usual care and had been diagnosed with asthma, diabetes mellitus or chronic heart disease | Usual care that has three of the PCMH characteristics | Survey | 10,990 |
10:9 10:11 10:12 |
| Pyne et al. (2015)184 | Middle-aged, low-income, Caucasian women with moderate depression who are unemployed and uninsured | Collaborative care | Census of depression-free days data | 364 patients in five federally qualified health centers | 6:10 |
| Rajala (2015)88 | Medical and behavioural health providers | PCMH | Semistructured interviews | 12 |
3:7 3:10 4:9 4:7 6:10 |
| Raphael et al. (2015)130 | Parents of children with a diagnosis of either haemoglobin-S sickle cell disease or sickle beta zero thalassemia | PCMH | Questionnaires | 150 | 8:11 |
| Richardson et al. (2015)117 | PCMH representatives, EHR vendors and associated stakeholders | PCMH | Semistructured telephone interviews | 28 | 6:10 |
| Salako et al. (2015)58 | Rural ACOs | ACOs | Census | 118 |
1:2 1:7 2:7 4:3 |
| Shaw et al. (2014)140 | Patients with a diagnosis of heart failure | Two components of the CCM | Questionnaire at discharge | 40 |
9:11 10:9 10:11 |
| Shortell et al. (2015)103 | ACOs | ACOs | Survey, interviews and data from site visits | Survey: 101 ACOs; interviews: 11 ACOs; and site visits: 2 ACOs |
3:7 3:9 3:11 6:9 6:10 7:10 9:12 9:13 10:12 |
| Smith et al. (2014)95 | Primary care physicians and consumers (focus groups), and public and private payers (semistructured discussions) | Medical homes, health homes, community-based care transition teams, medical neighbourhoods, ACOs | Focus groups; semistructured discussions | Four focus groups of 17; three discussions |
3:7 3:9 |
| Treadwell and Giardino (2014)137 | Staff at five medical home practices | PCMH | Survey | Not stated |
10:11 10:12 |
| Verhaegh et al. (2014)105 | RCTs of interventions aiming to improve transitions from hospital to home and reduce readmissions for chronically ill patients | Transitional care interventions | Literature review | 26 includes |
3:8 7:8 10:11 |
| Viron et al. (2014)55 | Massachusetts Mental Health Center patients who lacked primary care or were interested in switching providers | Behavioural health homes | Census of patient data | Not stated |
2:7 3:4 6:10 |
| Weldon et al. (2015)112 | GP receptionists, nurses, integrated care pioneer members, psychiatrists, pharmacists, lay partners, patients and carers (60–68% receptionists) | North West London Whole Systems Integrated Care programme | Questionnaires, field notes, video recordings of events and workshops | Not detailed. Each workshop of 40–47 participants | 4:3 |
| Wholey et al. (2014)67 | N/A | Care management teams | Secondary research texts | N/A |
1:2 2:7 3:7 4:3 6:7 |
| Woodman et al. (2016)76 | Clinicians involved in joint working initiatives | Four different services designed to bring paediatric expertise into primary care and/or improve joint working | Presentation/meetings, and interviews and e-mail follow-up | Five paediatricians, one community matron and one GP |
1:2 2:7 3:7 3:9 7:11 11:13 |
| Xenakis (2015)78 | Mount Sinai Health System | ACO/Medicare Shared Savings Programme | Participant observation | 280 doctors, 26 PHC practices and one hospital |
2:7 3:7 6:7 6:10 7:10 11:12 |
| Yoon et al. (2015)131 | Patients with at least two primary care visits in financial year 2009 and used outpatient care in financial year 2011 | PCMH: patient-aligned care teams | Pre-existing survey data | 2,607,902 patients from 796 VA primary care clinics | 3:11 |
Appendix 14 Full table of causal linkages (initial programme theory and revised logic model)
| MCP component (1–13) | Contexts in the CONTEXT that | MCP component (1–13) | Causal link | Programme theory from: | Strength of evidence | |||
|---|---|---|---|---|---|---|---|---|
| IF | THEN | IPT | IPT + E | E | ||||
| 1. NHS managers establish MCPs |
The member organisations have already made progress towards new ways of working Local commissioners’ have already agreed funding for the MCP Existing ‘partners’ such as voluntary and community sector organisations, and ‘communities’ are supportively engaged with the MCP Joining endorses general practices’ existing activities (e.g. in care co-ordination) The network seems relevant to the providers’ care group(s) and clinical tasks GPs (or the equivalent) are in partnerships rather than single-handed The network seems to offer its member organisations external resources and/or money Similar organisations which they admire as prototypes join the network External controls are permissive and light, and the network has local champion Staff are professionally qualified Joining the network seems likely to reduce risks for its member organisations, for instance the risks of competition The referral network includes all services required to maintain patients out of hospital The population are in large, non-isolated communities Payment systems are aligned (do not penalise collaboration) The time that network participation requires of general practices is not prohibitive First-cohort MCPs have: |
2. Network management will develop | 1:2 | |||||
| 7. Planned referral networks will develop | 1:7 | |||||||
2. Network management activities developed by:
|
The lead (network-co-ordinating) organisation has credibility and a good ‘track record’ There are good relationships between the member organisations It bears repeating that when different professions work for different organisations, MDTs are also interorganisational teams. |
3. MDTs will develop | 2:3 | |||||
| 6. Care co-ordination through IT use will develop | 2:6 | |||||||
| 7. Care planning at organisational and interorganisational level develops | 2:7 | |||||||
| 3. MCPs establish MDTs, in particular by giving their members boundary-spanning roles |
Status differences and deference between professions are weak or absent MDT roles are clearly defined MDT members are familiar with other professions’ contributions Boundary-spanning roles develop, especially when patients are of high complexity and staff have low knowledge about these individual patients Boundary-spanning staff have seniority, assertiveness and relational skills Doctors do not resist the boundary-spanning activities MDT members trust each other, and the team co-ordinator, and have confidence about their own skills MDT members do not feel liable for outcomes beyond their personal control The MDT has clearly structured communication and common training MDT members have shared group goals Staff are employment by same organisation Staff are familiar with other professions’ roles and contribution to care Staff have time to participate. Staff communicate face to face as well as by HIT Patients: |
4. Culture change will be promoted in the participating organisations | 3:4 | |||||
| 5. Voluntary sector involvement will increase | 3:5 | |||||||
| 6. Informational continuity of care and care co-ordination using HIT will develop | 3:6 | |||||||
| 7. Planned referral networks will develop | 3:7 | |||||||
| 8. Demand management systems will develop | 3:8 | |||||||
| 9. Preventative health care will develop | 3:9 | |||||||
| 10. Care planning at the patient level will develop | 3:10 | |||||||
| 11. Patients will more often be diverted from hospital | 3:11 | |||||||
| 12. Patient experience of care will improve | 3:12 | |||||||
|
4. Culture changes occur in the participating organisations that increase health workers’ knowledge of, and favourable attitude towards, other professions’ contribution to care A climate of psychological safety Focus on tasks of practical use to MDT members Shared expectations and values develop in the participating organisations Staff learn to communicate safety-critical information in ways that cannot be ignored but still maintain good informal relationships |
Different professions trust and respect each other There is common training across organisations and professions Other ‘resources’ for culture change are brought to bear Patients: |
3. MDTs will develop | 4:3 | |||||
| 7. Planned referral networks will develop | 4:7 | |||||||
| 8. Demand management systems will develop | 4:8 | |||||||
| 9. Preventative health care will develop | 4:9 | |||||||
| 10. Care planning at the patient level will become more prevalent | 4:10 | |||||||
| 12. Patient experience of care will improve | 4:12 | |||||||
| 5. Voluntary sector becomes involved in MCPs | Patients:
|
8. Demand management systems will develop | 5:8 | |||||
| 9. Preventative health care will develop | 5:9 | |||||||
| 12. Improved patient outcomes and experience of care | 5:12 | |||||||
| 6. HITs are used to strengthen informational continuity of care |
Such HITs exist at all The HITs are well designed for their uses and users HITs are implemented in tandem with the corresponding care management practices including elimination of parallel (e.g. paper-based) systems Health organisations can invest large sums in data analytics |
3. MDTs will develop | 6:3 | |||||
| 7. Planned referral networks will develop | 6:7 | |||||||
| 8. Demand management systems will develop | 6:8 | |||||||
| 9. Preventative care will develop | 6:9 | |||||||
| 10. Care planning for individual patients will become more prevalent and systematic | 6:10 | |||||||
| 11. More patients will be diverted from inpatient to primary care services | 6:11 | |||||||
| 13. NHS cost saving | 6:13 | |||||||
| 7. Care planning occurs at organisational and interorganisational level, is applied to a suitable case-mix of patients [i.e. high users of acute care (e.g. those patients with 5 or more hospitalisations per year), low and medium morbidity patients] |
Payment models do not penalise interorganisational care coordination MCP-like networks include health centres (or the equivalents); hence, less commonly used services No contractual hangover prevents collaboration Doctors are responsive to incentives to implement the resulting care plans The necessary preventative care, primary care, social work services and social care support services are available; hence, financially viable Patients: |
8. Demand management systems will develop | 7:8 | |||||
| 10. Care planning for individual patients will become more prevalent and systematic | 7:10 | |||||||
| 11. More patients will be diverted from inpatient to primary care services | 7:11 | |||||||
| 8. Demand management systems are used to screen referrals |
The necessary preventative care, primary care, social work services and social care support services are available, hence financially viable Hospitals do not face contrary (‘perverse’) incentives such as tariff payments |
9. Preventative health care will develop | 8:9 | |||||
| 10. Care planning for individual patients will become more prevalent and systematic | 8:10 | |||||||
| 11. More patients will be diverted from inpatient to primary care services (through admission avoidance/discharge support) | 8:11 | |||||||
| 9. Preventative health care develops | 11. More patients will be diverted from inpatient to primary care services | 9:11 | ||||||
10. Care plans for individual patients are more widely used, and apply the mechanisms of:
|
The necessary preventative care, primary care, social work services and social care support services are available; hence, financially viable MDTs have the time to discuss the care plan with patients before implementing it |
9. Preventative health care will develop | 10:9 | |||||
| 11. More patients will be diverted from inpatient to primary care services | 10: 11 | |||||||
| 12. Improved patient outcomes and experience of care | 10:12 | |||||||
| 11. More patients are diverted from inpatient to primary care services |
The necessary preventative care, primary care, social work services and social care support services are available; hence, financially viable Hospital care remains available for the most complex cases Referrals decrease so much that in hospitals whole clinics or wards can close Unblocking beds does not increase the average intensity (hence, cost) of inpatient care |
12. Improved patient outcomes and experience of care | 11:12 | |||||
| 13. NHS costs will reduce | 11:13 | |||||||
| General practice will benefit | 11:Other | |||||||
| Other. Care co-ordination and demand management systems develop | Urgent care becomes more responsive | Other | ||||||
| KEY | No evidence found | |||||||
| Partial/minimal support | ||||||||
| Supporting evidence | ||||||||
| Supporting evidence, with elaborations and additions | ||||||||
| Equivocal evidence | ||||||||
| Substantial evidence | ||||||||
List of abbreviations
- 5YFV
- Five Year Forward View
- A&E
- accident and emergency
- AA
- accessibility accommodation
- ACO
- accountable care organisation
- AHP
- allied health professional
- AMSTAR
- Assessment of Multiple Systematic Reviews
- AOK
- Allgemeine Ortskrankenkasse
- ASSIA
- Applied Social Sciences Index and Abstracts
- CCC
- comprehensive care co-ordinator
- CCG
- Clinical Commissioning Group
- CCM
- Chronic Care Model
- CHC
- community health centre
- CHS
- community health service
- CHW
- community health worker
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CLAHRC
- Collaborations for Leadership in Applied Health Research and Care
- CMMS
- Centers for Medicare and Medicaid Services
- CMO
- context–mechanism–outcome
- CMOC
- context–mechanism–outcome configuration
- COPD
- chronic obstructive pulmonary disease
- DHSC
- Department of Health and Social Care
- ED
- emergency department
- EHR
- electronic health record
- FCA
- first-contact accessibility
- FMG
- family medicine group
- GMS
- General Medical Services
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HIT
- health information technology
- HMIC
- Health Management Information Consortium
- HSDR
- Health Services and Delivery Research
- ICIT
- ideal type integrated care
- IPT
- initial programme theory
- IT
- information technology
- IVGK
- Integrierte Versorgung Gesundes Kinzigtal
- LDL
- low-density lipoproprotein
- LGA
- Local Government Association
- LHIN
- Local Health Integration Network
- LKK
- Landwirtschaftliche Krankenkasse
- MCP
- multispecialty community provider
- MDT
- multidisciplinary team
- MHI
- Mental Health Integration
- MMAT
- Mixed Methods Appraisal Tool
- NCQA
- National Committee for Quality Assurance
- NIHR
- National Institute for Health Research
- OECD
- Organisation for Economic Co-operation and Development
- OOH
- out of hours
- PACS
- primary and acute care system
- PCH
- primary care home
- PCMH
- primary care medical home
- PCP
- primary care practice
- PCT
- primary care trust
- PHC
- primary health care
- PHCC
- primary health care clinic
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSH
- perioperative surgical home
- QI
- quality improvement
- QOF
- Quality and Outcomes Framework
- RAMESES
- Realist And Meta-narrative Evidence Syntheses: Evolving Standards
- RCGP
- Royal College of General Practitioners
- RCT
- randomised controlled trial
- SR
- systematic review
- USL
- Unità Sanitarie Locali
- VHA
- Veterans Health Administration
