Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 14/19/50. The contractual start date was in June 2015. The final report began editorial review in June 2017 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The authors declare no financial support for the submitted work from anyone other than their employer and the National Institute for Health Research (NIHR) as listed above, no spouses, partners or children with relationships with commercial entities that might have an interest in the submitted work and no non-financial interests that may be relevant to the submitted work. Alex Bottle, Paul Aylin and the rest of the Dr Foster Unit at Imperial College London are part funded by Dr Foster®, an independent health-care information company and part of Telstra Health. The Dr Foster Unit is affiliated with the Imperial Centre for Patient Safety and Service Quality, funded by NIHR. Alex Bottle is now a member of the Health Services and Delivery Research panel. The NIHR funded Faiza Chowdhury’s PhD in ‘Common rehabilitation: The overlap between COPD and Heart Failure Rehabilitation’, which feeds into this research alongside her job as a respiratory and general internal medicine doctor.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Bottle et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background and research objectives
Heart failure (HF) and chronic obstructive pulmonary disease (COPD) each affect between 500,000 and 1 million people in the UK, with many more potentially undiagnosed. Inpatient costs are high, and both conditions have high emergency readmission rates. 1,2 The Royal College of Physicians noted that the 2000 National Service Framework was vague about what the interventions for patients with HF should be, and that the quality of HF management has progressed rather more slowly than for other cardiovascular diseases. 3 In international comparisons, England lags behind other countries on HF prevention and treatment. 1 National audits and other studies have documented variations in care processes and outcomes for many patient groups, including those with HF and COPD. 4–6 Although much of the regional variation in emergency admission rates has been found to be related to deprivation, disease prevalence and rurality,7–9 The King’s Fund report on bed use in the elderly10 concluded that ‘variation within each of the six rurality classifications shows that it is possible to achieve significant reductions in bed use even if the major drivers are pushing in the other direction’. To improve patient care, what is needed is an understanding of the drivers of service use and outcomes. This remains limited. Previous work has focused on aggregate emergency admission rates or on patient factors that predict readmission and mortality. Studies that have explored associations between health service factors and outcomes have largely been cross-sectional in approach and from the USA.
In addition, there has been little work done on emergency department (ED) attendances for these conditions: not all (re)admissions come through the ED and not all ED attendances result in admission. Several countries, including the USA and the UK, have financial incentives to reduce readmissions, typically within 30 days. It follows that to understand hospital use – and to be sure that we are using the best-available measures for performance monitoring and improvement – we need to include ED visits. How this should be done is not clear. Both mortality and readmission are frequently used as outcome measures in studies of patients with chronic (and indeed acute) disease as a result of their clear importance for the patient and to the health-care system, and as a result of their established relation with quality of care as assessed by process measures. England is one of a number of countries that publish rates for these outcomes by hospital to inform patient choice and to encourage hospitals to improve performance, with the implication that ‘better’ hospitals will have lower rates of mortality and readmission than average ones. Similarly, it is likely that some ED visits will be preventable with better care, so ED visit rates could potentially be incorporated into a performance measure.
To address these shortcomings, we employ the richness of NHS hospital administrative data and various other types of publicly available information, such as hospital staff numbers and patient experience (PE) surveys.
Overall aims
The study aimed to use mainly existing data to explore determinants of, and variations in, outcomes for patients hospitalised with (1) HF and (2) COPD. The approach could serve as a template to investigate other long-term conditions.
Objectives
The objectives of the project were to answer the following research questions:
-
What are the main patient, primary care and hospital factors associated with variation in readmission and mortality rates?
-
Should accident and emergency (A&E) attendance and reattendance data be considered alongside readmission metrics when measuring hospital performance in terms of unplanned activity? If so, how?
-
How consistently do hospitals perform across different readmission-type metrics?
-
Are the results for COPD similar to those for HF?
The structure of this report is as follows. Chapter 2, Methods, begins by briefly describing the Hospital Episodes Statistics (HES) database as held and processed by the Dr Foster Unit at Imperial College London. There is greater detail given in our previous National Institute for Health Research (NIHR) report, Can valid and practical risk-prediction or casemix adjustment models, including adjustment for comorbidity, be generated from English hospital administrative data (Hospital Episode Statistics)? A national observational study,11 and much of the basic HES processing and description of that database that we give in this report are therefore very similar to what is given in that report. There are subsections on the other data sets used, the outcome measures and the statistical methods, together with their implementation issues. Chapter 3, Results, begins with our analysis of the national PE survey results, as they required preprocessing before entry into our main models and also provided some interesting patterns in their own right. The chapter continues with Objectives 1, 2 and 3; to avoid repetition, we present the results for COPD alongside those for HF for each objective so that we answer the question posed by Objective 4 (are the results for the two conditions similar?) in stages, followed by a brief summary. Chapter 4 gives the discussion and dissemination activity to date and suggests further research and Chapter 5 offers some conclusions.
Chapter 2 Methods
Hospital Episodes Statistics database
The Dr Foster Unit holds annual HES data from 2003/4 to the present, with monthly feeds covering the most recent year. We apply published HES cleaning rules, which may be found on the NHS Digital (formerly the Health and Social Care Information Centre) website under HES. For example, duplicate records and those with unknown age, sex or length of hospital stay (LOS) were excluded (< 0.1% of episodes). Briefly, each record in the inpatient part of the database is a finished consultant episode, representing the continuous period of time during which the patient is under the care of a consultant or allied health professional. We link episodes into ‘spells’ (admissions to one provider) and link spells into ‘superspells’, so that the latter combines any inter-hospital transfers. We will refer to superspells as ‘admissions’ and, in general, use them as the unit of analysis throughout the report.
We have held outpatient HES since it became part of HES in 2003/4, and hold A&E records since they became part of HES in 2007/8. The most recent A&E records we had for the project were for 2013/14; our previous NIHR project report includes an assessment of data quality for these records and found that data from 2009/10 were useable. 11 We nevertheless made an assessment of the consistency of A&E records (for type I units), for instance whether or not the dates of attendance match those of admission for emergencies (to within a day) for the same patient. NHS Digital’s contractors provide a file to enable us to attach the date and cause of death to HES records, with the latest date of death being March 2012. Owing to considerable contractual delays, a more recent update arrived too late to be used for this project.
We derived a number of fields such as various comorbidities, procedure groups and the area-level Carstairs deprivation fifth. 12 Comorbidities are described in Definition of outcome measures and predictors. The Agency for Healthcare Research and Quality’s (AHRQ’s) Clinical Classification software turns the International Classification of Diseases, Tenth Revision (ICD-10)13 codes of the primary diagnosis field into 1 of 259 diagnosis groups designed for health services research [see www.ahrq.org for details (accessed 23 October 2017)]. For procedures, no such system exists for the UK’s Office of Population Censuses and Surveys (OPCS) procedure codes. We have, over several years, in conjunction with clinicians, created a number of procedure groups, with cardiac procedures defined as per our previous work14,15 and lung procedures defined with input from the medical members of the team. The Carstairs fifth was assigned at the super output area geographical level using information from the 2001 census. 12 Although Carstairs is therefore based on older information than the Index of Multiple Deprivation,16 its resolution is greater.
Patient cohorts and definition of index admission
Emergency admissions with a primary diagnosis of I50 for HF and J40–J44 for COPD were extracted for the two financial years 2009/10 and 2010/11 for patients aged ≥ 18 years for HF and ≥ 36 years for COPD. The first of these for each patient was retained. HF in children has different causes from that in adults, and COPD in adults in their twenties and thirties, which is largely a result of alpha-1-antitrypsin deficiency, affects the lungs and liver and is less typical of COPD. We tracked back 3 years from the date of this first admission and excluded anyone with a prior HF or COPD admission during those 3 years; going back more years made little difference. A very small proportion of records (< 0.1%) were dropped because of missing or invalid age, sex, LOS or postcode. Seven COPD and no HF patients were excluded because they had had a heart, lung or heart–lung transplant in the year before their index admission – we considered these patients to be too atypical to include. Records were then excluded from specialist or non-acute hospital trusts (n = 969 for HF and n = 903 for COPD). The remainder comprised our two cohorts, and their admissions will be referred to as ‘index admission’. For the models involving data by general practitioner (GP) practice, records with invalid practice code or practices with missing data were excluded, as described in Chapter 3. Readmission models used records only for those discharged alive from their index admission.
Practice- and hospital-level measures
From the internet we obtained the following information: Quality and Outcomes Framework (QOF) scores relevant to HF and COPD, NHS PE, NHS staff survey, hospital staffing, hospital bed numbers and rehabilitation programme locations and their websites’ patient information. These are summarised below:
-
general practice factors
-
GPs [full-time equivalent (FTE)] per 1000 patients
-
able to make an appointment with a GP within 48 hours (%)
-
ease of seeing a nurse (%)
-
patients know how to contact the out-of-hours (OOH) service (%)
-
able to make an appointment with GP in advance (%)
-
would recommend the surgery (%)
-
did the doctor or nurse ever tell you that you had a care plan?
-
percentage of total clinical points achieved
-
patients with left ventricular disease treated with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (%)
-
prevalence of HF in practice (%)
-
percentage of patients with a chronic disease who smoke and have a record of smoking cessation advice (QOF indicator SM04)
-
COPD patients who have had an influenza immunisation (%) (QOF indicator COPD08)
-
COPD patients who have a record of forced expiratory volume in 1 second (FEV1) in the previous 15 months (%) (QOF indicator COPD10)
-
COPD patients who have had diagnosis confirmed by post bronchodilator spirometry (%) (QOF indicator COPD12)
-
COPD patients who have had a review (%) (QOF indicator COPD13)
-
prevalence of COPD in the practice (%)
-
-
hospital trust factors
-
PE of waiting for a bed after arrival at hospital – score
-
PE of discharge – combined score of four questions – score
-
staff survey – satisfaction with care being given (%)
-
staff survey – recommend to friends and family
-
staff survey – staff rating of effective teamworking
-
doctor rate per 10 beds (mean)
-
mean bed occupancy (%)
-
mean number of beds.
-
We attempted to obtain information on each cardiac and lung rehabilitation programme in England in two ways: an e-mail/internet survey sent to all programme managers and an internet search of programme websites. For the former, we designed a questionnaire, in part based on the existing national audits that ran during 2015, but ensuring that we also asked for information that was not included in those audits. To obtain their co-operation, we waited until after the audit cycles were completed. Our questionnaires and two reminders were then distributed by the national audit co-ordinators to all units registered with them. This asked for the type of patient accepted onto the programme, the length of the programme, how it was staffed and what types of care were offered, for example exercise, education, anxiety and self-management tools. For the latter, we searched for the website of each registered programme to obtain its postcode (to enable calculation of the as-the-crow-flies distance between each patient and the nearest programme) and find out whether or not it provided patient information specific to the programme (rather than simply more generally about the trust, for example), further links for patients, programme acceptance criteria and contact details.
Definition of outcome measures and predictors
Our two primary outcome measures were 1-year total mortality (covering deaths in or out of hospital within 365 days of the index admission date) and 30-day all-cause emergency readmission, which is the most commonly used definition internationally. Unless otherwise stated, ‘readmission’ as an outcome means emergency readmission within 30 days. Readmissions were then split by primary diagnosis into those for HF/COPD and those for any other cause. We did not attempt to split deaths by cause of death, which can be hard to discern in the elderly and in HF. 17
Subsequent ED attendances and outpatient department (OPD) appointments were also captured within 1 year of index discharge. OPD non-attendance was simply defined using the ATENTYPE (attendance type) field; we noted the non-attendance rates in the year after index discharge but also tracked back 1 year before the index and used the number of appointments and the number of non-attendances as predictors, as we have previously found them to be important. 14
When considering ED attendances as a possible adjunct or alternative to readmission, we noted which ones ended in hospitalisation by the disposal field. Only ED attendances following index discharge before another admission of any type occurred were counted; if a patient was discharged, readmitted as either an elective or an emergency and, only after that discharge, attended ED without being hospitalised, then the last attendance was ignored. This is because readmissions are conventionally ‘assigned’ to the index admission that precedes them as they are assumed to be at least as a result of care delivered in that preceding index admission and because of any earlier ones. If ED attendances are to be used as a performance measure, then they must also ‘relate’ to the immediately preceding admission. Table 1 below makes the sequences clear.
| Event | Included in | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Readmission measure and assigned to the index admission? | A&E attendance not ending in admission measure and assigned to the index admission? |
| Index admission | ED attendance, not admitted | ED attendance, admitted | Yes | Yes |
| Index admission | ED attendance, admitted | ED attendance, not admitted | Yes | No |
| Index admission | OPD appointment or other non-ED route, resulting in direct admission | ED attendance, not admitted | Yes | No |
A few patients had two consecutive admissions with overlapping admission and discharge dates. As it was uncertain when the second of these admissions occurred in relation to other hospital contacts, these patients were excluded from analyses concerning ED attendances after the index discharge. A second data inconsistency occurred when the recorded Office for National Statistics (ONS) date of death preceded the hospital discharge date. For administrative reasons, the difference between the two can be 1 day, but if the ONS date was 2 or more days before the hospital discharge date, we assumed that the linkage between HES and ONS mortality had failed (no linkage is perfect) and that the patient left hospital alive. They were, therefore, included in all readmission and ED attendance models.
Other covariates for the risk adjustment models were taken from our national monitoring system, which uses HES18 and previous work on HF:11,15 age, sex, deprivation fifth, year and comorbidities. There is some debate over the terms ‘comorbidity’ and ‘multimorbidity’, but we will refer to any coexisting condition as a comorbidity and not require any causal link between it and the index disease, as is sometimes done. 19 Models also included some cardiac or lung procedures: coronary artery bypass graft, pacing, the insertion of an implantable cardioverter defibrillator and cardiac resynchronisation therapy for HF; and invasive and non-invasive ventilatory support, oxygen therapy and bronchoscopy for COPD. An intensive care unit (ICU) episode during the index stay was used as another binary flag. Further details are given in the relevant later sections.
For objective 2, we derived several measures of the ‘busyness’ of the ED department at which the patient presented, matching on hour and day the number of patients waiting in the department, the number of elderly patients waiting (defined here as aged ≥ 80 years), the number of boarders (those who the hospital had decided to admit but were still in the ED awaiting transfer to an inpatient ward), the number of patients admitted and the proportion waiting more than 4 hours. As we lacked information on the number of ED beds, an overall measure of busyness relative to maximum busyness was derived from all ED data, expressed as a percentage of this maximum; maximum busyness was identified as the 95th percentile of busyness based on all patients attending the ED for each hour of operation. Also included in these models were the day of the week and shift (day, evening and night).
Statistical methods
In this section we give our overall modelling approach first, and follow it with specific details of the analyses run for each objective.
Modelling framework
The terms risk prediction and risk adjustment are closely related despite their differing aims, but a model for predicting mortality, for example, might not include the same set of variables as a risk adjustment model used to compare hospitals’ mortality rates. Risk prediction values parsimony and interpretability, whereas risk adjustment can focus more on confounder control. Risk prediction models could encompass factors such as staffing and bed numbers, or other factors that are (at least partly) under the hospital’s control, whereas this would be wrong for risk adjustment models for comparing providers. When exploring the associations between patient, GP and hospital factors and each outcome, we are in a risk prediction framework; when producing hospital-level measures for performance monitoring, we are in a risk adjustment one.
Prediction of binary outcomes, such as mortality or readmission, is usually done via logistic regression. With multiple health service units, such as GP practices, surgeons or hospitals, the modeller, in principle, needs to account for the ‘clustering’ of patients within these units. The common way of accounting for this is to use multilevel models, particularly with random intercepts for practices and hospitals, and fixed effects for covariates. For this, we used Statistical Analysis Software’s (SAS’s) procedure for binary outcomes, PROC GLIMMIX. From this we derived relative risks (RRs) for each hospital using predicted probabilities from only the fixed effects part of the model. 20,21 We used the noblup ilink options within PROC GLIMMIX to achieve this. These RRs are akin to standardised mortality ratios (SMRs), which represent the ratio of the hospital’s rate to the national average rate. However, as explained below, the hospital-level clustering was found to be minimal, and accounting for clustering by both general practice and hospital was found to be unfeasible, so we also calculated hospital-level RRs using PROC LOGISTIC, which does not account for any clustering.
The Centers for Medicare and Medicaid Services (CMS) in the USA uses, for its publicly reported outcome measures, empirical Bayes ‘shrunken’ estimates of the SMRs22 Confidence intervals (CIs) for the CMS measures are constructed using a complicated bootstrap procedure. Several studies have compared the results from fixed- and random-effects models with regard to provider profiling, in which a key aim is the identification of statistical outliers, especially units with higher than expected mortality. In general, these conclude, as Austin et al. 23 did, that ‘when the distribution of hospital-specific log-odds of death was normal, random-effects models had greater specificity and positive predictive value than fixed-effects models. However, fixed-effects models had greater sensitivity than random-effects models.’ For a full discussion of the evidence around the effect of different adjustments for clustering, including Bayesian methods for provider profiling reviewed by Austin,24 see Chapter 7 of our book, Statistical Methods for Healthcare Performance Monitoring. 25
Multilevel models allow for the estimation of the amount of variation between units at each level, for example, between practices or hospitals. The residual intraclass correlation coefficient (ICC), a measure of clustering used in hierarchical modelling, expresses the proportion of variability explained by the presence of clusters at, for instance, a hospital level. 26 It is computed for logit models as:
where τH is the hospital-level variance and π = 3.14159.
By building up the levels in a hierarchical model, one can assess, for example, how much of the variation in outcomes at hospital level is attributable to the variation between practices or as a result of differences in the distribution of patient factors.
Model fitting
The modelling in this project served two different purposes as explained above, and we therefore took different approaches accordingly. For logistic regression, all candidate covariates were initially retained: we did not use any stepwise methods because of their well-known drawbacks but instead removed non-significant variables (backwards elimination) after checking the impact on the coefficients for the retained covariates. For the most part we did not test for interactions.
Continuous variables were sometimes categorised, depending on their relation with the outcome. Age and LOS were categorised in line with our previous approaches.
In order to explore the relationship between hospital and general practice variables and outcomes, variables were divided into deciles and the percentages of patients who were readmitted/died for each decile were plotted against the mid-value for the explanatory variable deciles. Plots were inspected and, where there was evidence of a clear linear or non-linear pattern, a suitable categorical approach was identified, based on quartiles. Models in which continuous variables were categorised were compared with the main models in which they were treated as continuous. There was no impact on which explanatory variables were retained or on the interpretation of results.
Assessment of model performance
With any risk model comes the need to assess its performance. For binary outcomes, two standard measures for logistic regression are the area under the receiver operating characteristic (ROC) curve, also known as the c-statistic, and the Hosmer–Lemeshow (HL) test output. The former measures discrimination, the ability of the model to predict a higher probability of death for those who died than for those who survived. It is generally considered that values of c above 0.70 represent good and values above 0.80 represent excellent discrimination. The maximum value obtainable is often quoted as 1 but, in fact, varies with the distribution of risk in the population (see Cook27 for a full discussion on this statistic). The HL test describes the model’s calibration and divides the data set into risk deciles. The observed and predicted number of events are compared in each decile, which often shows poor calibration at the extremes, and summarised in a chi-squared statistic. It has been criticised for having high type I and II error rates. 28 Although a simple plot of observed versus predicted rates may be more useful, we will nonetheless report HL test results to be concise. For the HL chi-squared values, we give right-sided p-values. We used 10 bins and 8 degrees of freedom for the HL test, as is standard.
The effect of the semi-competing risk of death on readmission-type measures
As death precludes subsequent readmission, using logistic regression – which ignores any effect of death either during or following the index discharge – may be potentially misleading. In our previous project11,14 we therefore also applied cause-specific proportional hazards modelling and subdistribution proportional hazards modelling. These two survival analysis methods make different assumptions regarding post-discharge deaths. 29,30 Other methods exist, but these are the two most widely used. The PSHREG macro in SAS was run for subdistribution hazards. 31 Our prior work found high agreement between the odds ratios (ORs) and both sets of hazard ratios, so we can be fairly confident that the effect of post-discharge deaths is minor. For this reason, we did not consider the impact of post-discharge death on readmission within 30 days. As is standard, our analyses of readmissions were restricted to patients discharged alive from their index admission. As well as logistic regression we ran standard Cox models.
Methods specific to objective 1
After obtaining the non-hospital data, our first task was to decide what information to take from the patient and staff surveys. Although the inpatient survey is divided into domains, we used the approach of Bos et al. 32 and considered the questions in terms of the pathway a patient takes through hospital. Our two patient representatives highlighted the importance of ‘reassurance when arriving and leaving hospital’ regarding their confidence to manage their disease. We hypothesised that the experience of arrival at hospital as an emergency and the discharge experience, including information about medication and side effects, might affect a patient’s decision to return to the ED to seek help.
We included two indicators from the patient survey: (1) patients’ experience of arrival at the hospital – a single question that asks patients about the waiting time for a bed after arrival at the hospital; and (2) patients’ experience of discharge based on four questions covering discharge delay, information about danger signals and the purpose and side effects of medication.
From the 2010 staff survey, we selected three questions a priori that, we hypothesised, may reflect organisational culture and the quality of care patients received: (1) staff rating of effective teamwork based on five questions; (2) staff agreement with the statement: ‘if a friend or relative needed treatment, I would be happy with the standard of care provided by this trust’; and (3) the percentage of staff who are satisfied with the quality of care they give to patients. The responses to each question were aggregated at trust level, and a mean response ranging from one to five or a mean percentage was attributed to each trust.
Questions were selected that ascertain patients’ recall of their experiences of access to primary care, including their knowledge of how to access OOH services. In addition to access, their perceptions of overall care, including whether or not clinical staff took their problems seriously, whether or not they had been told that they had a care plan and whether or not they would recommend the practice, were included.
Descriptive analyses included summarising the outcome rates for each patient-level predictor by chi-squared tests and scatterplots. We investigated the need for hierarchical modelling when assessing the relations between variables at the patient, primary care and hospital level by inspecting the covariance parameter estimates for the random effects. As noted earlier, the data are clearly nested: patients are nested within hospital trusts and separately within general practices. General practices are not generally wholly nested within trusts, that is, their patients do not attend only one trust, although there are exceptions. This is known as a cross-classified design. The large number of general practices and trusts makes it difficult to summarise the level of cross-classification. In addition, it also means that the covariance matrix underlying the multilevel model needed to run a cross-classified model is of size n, in this case over 78,000, but does not have a block diagonal structure that allows efficient algorithms, which is the case in a simple hierarchical model. This resulted in computational challenges when attempting to run a cross-classified analysis and we needed to take an alternative approach. In order to determine the impact of clustering, models were considered that took into account clustering of patients within trusts and, separately, of patients within general practices by including a random effect for trusts and general practices, respectively. The covariance parameters were then compared. This process was repeated for empty models, that is, those with no explanatory models, and models that included all patient-level variables. The covariance parameters were less than 0.05 in all models. The ICC was less than 0.01 in all cases, providing evidence that the amount of variance explained by the trust or practice level was < 1%. As the evidence of clustering affecting model results and interpretation was very weak, the model selected was a simple logistic model, which does not take into account the hierarchical nature of the data.
For readmission as the dependent variable, we first combined all causes, as is generally done, before splitting into two (HF/COPD vs. any other primary diagnosis) and ran separate analyses for each. For mortality as the outcome variable, we ran time-to-event models as outlined above, testing for time-varying effects by including interaction terms with the log of time.
We used logistic regression models to predict having an ED attendance and then to predict admission at that attendance within (1) 7 days and (2) 30 days of index discharge. These models included the inpatient and outpatient survey results and the other predictors for objective 1. We added time (shift) and day of the week of attendance. An interaction term between shift and weekday was tried. As a check to see if any important information was lost by considering these fixed windows of 7 and 30 days, survival analysis was also run, checking for non-proportionality of hazards.
To further explore the ED attendance patterns, we calculated overall outcome rates by time and weekday of attendance, subsequently stratifying by model-predicted risk of death. This showed whether, for example, the sickest patients attended more, attended at different times or were more likely to be admitted than less sick patients. The time of attendance was categorised into three shifts: 00.00–08.00, 08.00–18.00 and 18.00–00.00 hours.
Methods specific to objective 2
The variance of the random effects from the hierarchical models and the quasi-likelihood model dispersion parameter was calculated for the outcomes of 7-day attendance without admission, 30-day attendance without admission, 90-day attendance without admission, 30-day readmission, 7-day attendance without admission or 30-day readmission and 1-year mortality.
The relative contribution of patient and non-patient factors to variation in each outcome was assessed using the omega statistic, which is a ratio. With patient variables in the numerator and community and hospital variables in the denominator, ω = 0 would mean that all the variation in the candidate indicator is predicted by factors other than patient characteristics. Low values of ω are therefore desirable. This statistic cannot be used to judge an individual indicator (administrative data will lack some important patient factors), but it is useful for comparing them. We followed the example of Brown et al. ,33 who compared measures for ICU performance.
Methods specific to objective 3
We calculated RRs for these different measures for each hospital, adjusted for patient factors in logistic regression models. These RRs are akin to SMRs in epidemiology and are the ratio of the observed to the expected deaths or readmissions for each hospital, and so are the hospital’s outcome rate relative to the national average. We compared the sets of RRs using linear and non-linear correlation. As funnel plots are increasingly used to identify providers with unusually ‘good’ or ‘poor’ performance,34,35 we noted that the number and proportion of hospitals with outcome rates beyond 95% and 99.8% control limits. This was done for each outcome and patient group. We noted whether or not the same outliers were consistently identified across the sets.
To illustrate the statistical power to detect performance differences between hospitals, power calculations were carried out to determine the power to detect a change equivalent to 1.5 times the national rate in a small (25th percentile) hospital trust (n = 320 patients). This means that one would have greater power than this to detect larger differences than 1.5 for these hospitals, or to detect the same difference at larger hospitals. Our calculations are clearly not exhaustive but are fairly conservative. Power calculations were carried out using an online calculator provided by the Statistics Department of the University of British Columbia [www.stat.ubc.ca/∼rollin/stats/ssize/b1.html (accessed 23 October 2017)].
There were no methods specific to objective 4: results for HF and COPD were compared in a descriptive manner and are highlighted in each results subsection. Hospital-level RRs for HF and for COPD were compared using simple correlation.
Table 2 summarises the main analyses in this project by objective number.
| Objective | Goal of analysis | Statistical method | Output |
|---|---|---|---|
| 1 | Identify main predictors of mortality and readmission | Logistic regression; survival analysis | Tables 6 and 7 |
| 2 | Identify main predictors of first post-index ED attendance | Logistic regression; survival analysis | Table 9 |
| 2 | Identify main predictors of admission at first post-index ED attendance | Logistic regression | Table 10 |
| 2 | Compare statistical properties of various readmission-type indicators | Quasi-likelihood modelling; multilevel modelling; omega statistics | Tables 11–13 |
| 3 | Determine which hospitals have unusually high or low outcome rates | Funnel plots | Tables 14–16; Figures 2 and 3 |
| 4 | Compare results for HF and COPD | Inspection of model output; funnel plots; correlation of hospital rates | Tables 6, 7, 9–16; Figures 2 and 3; correlation coefficients |
Summary of public and patient involvement in this project
We asked our two patient representatives to go through the NHS Patient Survey questions to pick out those that they thought were particularly relevant either to their own experience of using the NHS or to other patients. They both responded with interesting viewpoints and ideas. They both identified readiness for discharge as problematic, and we specifically tested for the importance of questions related to this in the patient survey. Both representatives were asked to comment on our draft paper on PE score trends, and one of them did. We incorporated her remarks in the manuscript. Both representatives were asked to comment on the lay summary for this report; one of them did and suggested some edits to it.
Chapter 3 Results
Before we present the main modelling results, we first very briefly describe our analysis of the national NHS PE survey data, then we describe which elements were included in the regression.
Pre-regression analysis
Analysis of national NHS patient experience data 2005/6–2014/15
We aimed to determine if:
-
the PE in each setting has changed over time
-
hospital trusts have performed consistently over time
-
there is consistency between hospital settings (ED, inpatient department and OPD).
All 130 acute non-specialist hospital trusts that had inpatient survey results for the 10-year period from 2005/06 to 2014/15 were included. Initially, descriptive analysis of data from the NHS Patient Experience Tool36 was used to determine the patterns in PE scores over time for overall PE, domains and individual questions, inpatients, outpatients and ED scores. Scores in 2005/06 and 2014/15 were also compared. To determine if performance of the highest and lowest scoring trusts was consistent over time, the mean score for each trust for each domain in the first 3 years was calculated, and the 25% highest-scoring and 25% lowest-scoring trusts were identified. The mean scores for these groups of trusts were then calculated and plotted each year.
The consistency of trust performance over time, and trusts’ performances relative to one another, were analysed. The trusts were grouped into four groups using k-means cluster analysis on standardised PE scores. Ward’s minimum variance hierarchical clustering was used to determine the appropriate number of clusters, which ranged from four to nine in different years. Consistent performance was defined as being in the same ranked cluster for more than 5 years.
The results show that overall PE was good during the 10 years, with modest improvements over time across the three hospital settings. Individual questions with the biggest improvement across all three settings were cleanliness (inpatient department: +7.1 points, ED: +6.5, OPD: +4.7) and information about danger signals (inpatient department: +3.8, ED: +3.9, OPD: +4.0). The lowest-scoring questions, regarding information at discharge, were the same in all years and all settings.
The greatest improvement across all three settings was for cleanliness, which has seen national policies and targets. Information about danger signals and medication side effects showed the least consistency across settings and scores remained low over time, despite information about danger signals showing a big increase in score. PE of aspects of access and waiting declined, as has experience of discharge delay, likely reflecting known increases in pressure on England’s NHS.
Consistency over the 10 years was high. A total of 71.5% of trusts were in the same ranked cluster for more than 5 of the 10 years for overall scores. There was also high consistency for individual domains. The gap between the lowest- and highest-performing trusts in the initial period narrowed during the first 3 years, but there was little evidence of the lowest-performing trusts ‘catching up’ after this, except for the ‘Clean, Comfortable, Friendly Place to be’ domain.
Questions regarding waiting, information about medication side effects and danger signals have been consistently low scoring in all three settings since the survey inception. High-scoring questions also show consistency over time and across settings and include being treated with respect and dignity and being given sufficient privacy.
Before the analysis, we shared the patient survey questionnaires with our two public and patient involvement (PPI) representatives and asked them which specific questions or domains were of most importance to them, given their experience of the NHS. They both identified readiness for discharge as problematic. Our findings support this, and we therefore included scores for PE of waiting for a bed after arrival at hospital and PE of discharge in the subsequent regression models.
Other national data
Table 3 describes the variation by general practice or hospital for the non-patient-level data used in the regression modelling. This information came from 7756 general practices and 141 hospital trusts.
| Factor | Variation | |
|---|---|---|
| Median (IQR) | Mean (SD) | |
| General practice factors | ||
| GPs (FTE) per 1000 patients | 0.63 (0.51–0.76) | 0.66 (0.28) |
| Able to make an appointment with a GP within 48 hours (%) | 81.9 (73.3–88.9) | 80.3 (11.5) |
| Ease of seeing a nurse (%) | 91.5 (87.2–94.5) | 90.3 (5.9) |
| Patients know how to contact OOH service (%) | 63.1 (56.7–68.9) | 62.6 (9.4) |
| Able to make an appointment with GP in advance (%) | 72.5 (60.7–82.8) | 71.1 (15.1) |
| Would recommend the surgery (%) | 85.1 (76.6–90.8) | 82.7 (11) |
| Did the doctor or nurse ever tell you you had a care plan? | 10.9 (7.8–14.5) | 11.6 (5.3) |
| Percentage of total clinical points achieved | 98.1 (95.4–99.6) | 96.5 (4.8) |
| Patients with LVD treated with an ACE inhibitor or ARB (%) | 84.4 (78.0–90.9) | 83.9 (10.9) |
| Prevalence of HF in practice (%) | 0.7 (0.5–0.9) | 0.7 (0.3) |
| Percentage of patients with a chronic disease who smoke and have a record of smoking cessation advice (QOF indicator SM04) | 92.4 (90.3–95.1) | 92.1 (5.2) |
| COPD patients who have had an influenza immunisation (%) (QOF indicator COPD08) | 81.7 (77.1–86.0) | 81.3 (7.1) |
| COPD patients who have a record of FEV1 in the previous 15 months (%) (QOF indicator COPD10) | 80.0 (72.2–86.7) | 78.5 (12.1) |
| COPD patients who have had diagnosis confirmed by post bronchodilator spirometry (%) (QOF indicator COPD12) | 77.8 (66.7–87.0) | 75.1 (17.9) |
| COPD patients who have had a review (%) (QOF indicator COPD13) | 82.0 (74.2–87.6) | 79.1 (13.7) |
| Prevalence of COPD in practice (%) | 1.5 (1.1–2.0) | 1.6 (0.8) |
| Trust factors | ||
| PE of waiting for a bed after arrival at hospital – score (possible scores range from 0 to 100, although not a percentage) | 76.3 (73.6–80.5) | 76.8 (5.0) |
| PE of discharge – combined score of four questions (possible scores range from 0 to 100, although not a percentage) | 60.4 (57.7–63.3) | 60.5 (3.9) |
| Staff survey – satisfaction with care being given (%) | 74.4 (71–77.3) | 73.9 (4.8) |
| Staff survey – recommend to friends and family (possible scores range from 1 to 5, with 1 representing that staff would be unlikely to recommend the trust as a place to work or receive treatment, and 5 representing that staff would be likely to recommend the trust as a place to work or receive treatment) | 3.5 (3.4–3.6) | 3.5 (0.2) |
| Staff survey – staff rating of effective teamworking (possible scores range from 1 to 5, with 1 representing ineffective teamwork, and 5 representing effective teamwork) | 3.7 (3.6–3.7) | 3.7 (0.1) |
| Doctor rate per 10 beds (mean)a | 6.8 (5.8 –7.7) | 7.2 (1.9) |
| Mean bed occupancy (%) | 87.5 (83.7–90.9) | 87 (5.4) |
| Mean number of beds | 686.6 (487.7–931.3) | 756.6 (357.1) |
Sources of data for the above table:
-
Number of GPs – Data as at 30 September 2010 37
-
GP Patient Survey 2010/11 38
-
Quality and Outcomes Framework 2010/11 39
-
overall Patient Experience Scores 40
-
Staff Survey 2010 41
-
Monthly NHS Hospital and Community Health Service Workforce Statistics42,43
-
Average Daily Number of Available and Occupied Beds by Sector, NHS Organisations in England, 2009–10. 44
Survey of pulmonary and cardiac rehabilitation programmes
The survey consisted of two parts: postal/e-mail questionnaire and inspection of programme websites. For the survey, despite the support and help from the national rehabilitation audits and two reminders, only 32 responses were received. This was felt to be too few to use, and these are not considered further. Websites were found for all but 2 of the 288 cardiac rehabilitation programmes and all but 6 of the 240 pulmonary rehabilitation programmes listed in the respective national audit programmes. Four were described as joint cardiac and pulmonary.
We assessed each website on four criteria. Of the cardiac rehabilitation sites, 49% were specific to the rehabilitation programme (e.g. rather than to the parent trust), 58% provided contact details and/or the address, 40% gave the acceptance criteria for the programme and only 15% provided links to resources useful to patients, such as further information on heart disease or relevant charities. For the lung rehabilitation sites, these figures were similar at 42%, 50%, 31% and 16%, respectively. We were almost always able to obtain a postcode for the programme and were therefore able to estimate the as-the-crow-flies distance between the patient’s postcode and their nearest rehabilitation centres. Figure 1 shows the services on a map. These four pieces of information were considered as covariates in the mortality and readmission models.
FIGURE 1.
Location of cardiac and pulmonary rehabilitation programmes in England as of 2015.

Patient characteristics
Table 4 describes the two cohorts.
| Factor | Cohort, number of patients (%) | |
|---|---|---|
| HF | COPD | |
| Age group (years) | ||
| 18–44 (36–44 for COPD patients) | 825 (1.1) | 1794 (1.9) |
| 45–64 | 7538 (9.7) | 22,840 (23.8) |
| 65–84 | 42,372 (54.5) | 57,552 (59.9) |
| ≥ 85 | 27,066 (34.8) | 13,867 (14.4) |
| Sex | ||
| Male | 38,695 (49.7) | 46,388 (48.3) |
| Female | 39,106 (50.3) | 49,665 (51.7) |
| Deprivation fifth | ||
| 1 – least deprived | 11,574 (14.9) | 9793 (10.2) |
| 2 | 15,390 (19.8) | 14,707 (15.3) |
| 3 | 16,785 (21.6) | 18,974 (19.8) |
| 4 | 17,516 (22.5) | 24,034 (25.0) |
| 5 – most deprived | 16,536 (21.3) | 28,545 (29.7) |
| Ethnic group | ||
| White British | 68,822 (88.5) | 87,810 (91.4) |
| Mixed | 1015 (1.3) | 820 (0.9) |
| Indian | 2907 (3.7) | 1620 (1.7) |
| Black | 1470 (1.9) | 580 (0.6) |
| Not known | 3587 (4.6) | 5223 (5.4) |
| Living status | ||
| Living alone | 9154 (11.8) | 10,151 (10.5) |
| Comorbidities | ||
| IHD | 37,568 (48.4) | 24,047 (25.0) |
| HF | Not applicable | 9944 (10.4) |
| Stroke | 1725 (2.2) | 996 (1.0) |
| Arrhythmia | 39,092 (50.2) | 19,076 (19.9) |
| Valvular disease | 18,445 (23.7) | 4107 (4.3) |
| Peripheral vascular disease | 7043 (9.1) | 5155 (5.4) |
| Chronic pulmonary disease | 19,579 (25.2) | Not applicable |
| Pneumonia | 10,024 (12.9) | 9720 (10.1) |
| Renal disease | 18,594 (23.9) | 7423 (7.7) |
| Obesity | 3784 (4.9) | 3044 (3.2) |
| Hypertension | 48,952 (62.9) | 40,341 (42.0) |
| Diabetes mellitus | 23,137 (29.7) | 14,946 (15.6) |
| Electrolyte disorders | – | 4415 (4.6) |
| Cancer – with metastases | 3865 (5.0) | 5086 (5.3) |
| Cancer – without metastases | 864 (1.1) | 1158 (1.2) |
| Cognitive impairment (senility and dementia combined) | 7615 (9.8) | 5950 (6.2) |
| Mental health conditions (excluding dementia) | 6779 (8.7) | 12,600 (13.1) |
| Number of comorbidities | ||
| 0 | 3100 (4.0) | 24,564 (25.3) |
| 1 | 10,925 (14.0) | 25,963 (26.8) |
| 2 | 19,246 (24.7) | 20,140 (20.8) |
| ≥ 3 | 44,530 (57.3) | 26,265 (27.0) |
| Experience of hospital within a year prior to or during the index admission | ||
| OPD appointments attended in year before index admission | ||
| 0 | 15,446 (19.9) | 37,936 (39.5) |
| 1 | 10,077 (13.0) | 14,490 (15.1) |
| 2 | 8353 (10.7) | 9964 (10.4) |
| ≥ 3 | 43,925 (56.5) | 33,663 (35.0) |
| OPD appointments missed in year before index admission | ||
| 0 | 56,905 (73.1) | 79,587 (82.9) |
| 1 | 12,372 (15.9) | 10,763 (11.2) |
| 2 | 4464 (5.7) | 3243 (3.4) |
| ≥ 3 | 4060 (5.2) | 2460 (2.6) |
| LOS of index admission (nights) | ||
| 0 | 4529 (5.8) | 10,196 (10.6) |
| 1 | 6989 (9.0) | 13,895 (14.5) |
| 2 | 5378 (6.9) | 10,466 (10.9) |
| ≥ 3 | 60,905 (78.3) | 61,496 (64.0) |
| Inpatient interventions within a year prior to or during the index admissiona | ||
| Echocardiography | 16,406 (21.1) | 4480 (4.7) |
| CABG | 789 (1.0) | 151 (0.2) |
| Narrow definition of CRT | 235 (0.3) | 27 (0.03) |
| Defibrillation implantation (‘ICD’) | 546 (0.7) | 97 (0.1) |
| Other pacing | 2296 (3.0) | 499 (0.5) |
| PTCA | 1686 (2.2) | 549 (0.6) |
| Lung operations including excision | Not applicable | 483 (0.5) |
| Long-term oxygen | Not applicable | 46 (0.1) |
| Bronchoscopy | Not applicable | 1816 (1.9) |
| Interventions during the index admission | ||
| Intensive care | 311 (0.4) | 399 (0.4) |
| Invasive ventilation | Not applicable | 410 (0.4) |
| Non-invasive ventilation | Not applicable | 5912 (6.2) |
| Distance to health care | ||
| Distance from patient residence to admitting hospital (km) | ||
| ≤ 2.5 | 15,791 (20.3) | 20,817 (21.7) |
| 2.5–5.0 | 21,156 (27.2) | 27,485 (28.6) |
| > 5.0 to 7.5 | 12,084 (15.5) | 15,042 (15.7) |
| > 7.5 to 10 | 7252 (9.3) | 8833 (9.2) |
| > 10 | 21,518 (27.7) | 23,876 (24.9) |
| Distance from patient residence to nearest community rehabilitation provision (km) | ||
| ≤ 2.5 | 20,555 (26.4) | 20,459 (21.3) |
| 2.5–5.0 | 22,971 (29.5) | 25,966 (27.0) |
| > 5.0 to 7.5 | 12,306 (15.8) | 15,305 (15.9) |
| > 7.5 to 10 | 7133 (9.2) | 10,015 (10.4) |
| > 10 | 14,836 (19.1) | 24,308 (25.3) |
As expected, the great majority of HF patients were elderly and the COPD population was younger. Comorbidity was very common in both groups, as was OPD contact in the year before index admission. Around half of the patients lived within 5 km of the hospital to which they were admitted or of the nearest community rehabilitation centre.
Objective 1: what are the main patient, primary care and hospital factors associated with variation in readmission and mortality rates?
Predictors of 1-year mortality
In total, 14.9% of HF patients died during their index admission and another 24.8% died within a year after discharge, resulting in an overall mortality rate within 1 year of index admission of 39.6%. With regard to COPD patients, 5.9% died during their index admission and another 18.2% died within a year after discharge, resulting in an overall 1-year mortality rate of 24.1%. Table 5 gives crude outcome rates for selected patient characteristics.
| Factor | Cohort of patients | |||||||
|---|---|---|---|---|---|---|---|---|
| HF | COPD | |||||||
| Readmissions within 30 days | 1-year mortality | Readmissions within 30 days | 1-year mortality | |||||
| Number of patients | Percentage of patients discharged alive | Number of patients | Percentage of patients admitted | Number of patients | Percentage of patients discharged alive | Number of patients | Percentage of patients admitted | |
| Age group (years) | ||||||||
| 18–44 (HF) or 36–44 (COPD) | 146 | 18.9 | 138 | 16.7 | 208 | 11.6 | 72 | 4.0 |
| 45–64 | 1245 | 17.6 | 1466 | 19.5 | 2809 | 12.6 | 2365 | 10.4 |
| 65–84 | 7281 | 19.7 | 14,787 | 34.9 | 9372 | 17.4 | 14,790 | 25.7 |
| ≥ 85 | 4427 | 20.8 | 14,445 | 53.4 | 2604 | 21.4 | 6126 | 44.2 |
| Sex | ||||||||
| Male | 6612 | 19.9 | 15,218 | 39.3 | 7667 | 17.6 | 12,536 | 27.0 |
| Female | 6487 | 19.6 | 15,618 | 39.9 | 7326 | 15.6 | 10,817 | 21.8 |
| Deprivation fifth | ||||||||
| 1 – least deprived | 1785 | 18.2 | 4769 | 41.2 | 1382 | 15.2 | 2578 | 26.3 |
| 2 | 2427 | 19.1 | 6311 | 41.0 | 2173 | 15.9 | 3850 | 26.2 |
| 3 | 2788 | 19.7 | 6928 | 41.3 | 2980 | 16.8 | 4748 | 25.0 |
| 4 | 3009 | 20.2 | 6947 | 39.7 | 3765 | 16.7 | 5805 | 24.2 |
| 5 – most deprived | 3045 | 21.1 | 5881 | 35.6 | 4693 | 17.3 | 6372 | 22.3 |
| Ethnic group | ||||||||
| White British | 11,661 | 20.0 | 27,971 | 40.6 | 13,953 | 16.9 | 21,714 | 24.7 |
| Mixed | 177 | 19.5 | 320 | 31.5 | 113 | 14.3 | 146 | 17.8 |
| Indian | 526 | 19.6 | 728 | 25.0 | 287 | 18.3 | 288 | 17.8 |
| Black | 298 | 21.8 | 558 | 24.4 | 81 | 14.5 | 104 | 17.9 |
| Not known | 437 | 15.2 | 1456 | 40.7 | 559 | 11.5 | 1101 | 21.1 |
| Living status | ||||||||
| Living alone | 1678 | 21.6 | 3908 | 42.7 | 1901 | 20.2 | 2899 | 28.6 |
| Number of comorbidities | ||||||||
| 0 | 469 | 16.7 | 917 | 29.6 | 2647 | 11.3 | 3131 | 13.0 |
| 1 | 1557 | 16.0 | 3487 | 31.2 | 3578 | 14.6 | 5105 | 19.9 |
| 2 | 2917 | 17.4 | 6670 | 34.7 | 3285 | 17.9 | 5294 | 26.4 |
| ≥ 3 | 8156 | 22.1 | 19,762 | 44.4 | 5483 | 23.2 | 9323 | 374 |
| LOS of index admission (nights) | ||||||||
| 0 | 828 | 21.3 | 1442 | 31.8 | 1484 | 15.0 | 1424 | 14.0 |
| 1 | 1120 | 19.6 | 2575 | 36.8 | 1939 | 14.6 | 2323 | 16.7 |
| 2 | 800 | 18.0 | 1942 | 36.1 | 1404 | 14.2 | 2002 | 19.1 |
| ≥ 3 | 10,351 | 19.8 | 24,877 | 40.9 | 10,166 | 17.8 | 17,604 | 28.6 |
To include information on general practices and rehabilitation programmes, some records had to be excluded from both the mortality and the readmission models: patients with missing distance from nearest rehabilitation programme (seven for HF and five for COPD); patients who are registered at practices with missing QOF data (1368 for HF and 1901 for COPD); patients who are registered at practices with missing GP Patient Survey data (438 for HF and 145 for COPD); and patients who are registered at practices with missing GP supply data (143 for HF and 551 for COPD). These losses totalled 1956 for HF (2.4% of the full cohort) and 2602 for COPD (2.6% of the full cohort).
Table 6 gives the ORs for the final set of predictors for total 1-year mortality (in or out of hospital). For HF, we also included the National Audit hospital-level performance figures; the only measure significant at a p-value of < 0.01 was referral for echocardiography, but the effect size was tiny and it is therefore not shown.
| Factor | Cohort of patients | |||
|---|---|---|---|---|
| HF | COPD | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Patient factors | ||||
| Age group (years) (65–69 is the reference group) | ||||
| 18–44 | 0.61 (0.50 to 0.75) | < 0.001 | 0.26 (0.20 to 0.33) | < 0.001 |
| 45–49 | 0.50 (0.40 to 0.63) | < 0.001 | 0.35 (0.29 to 0.42) | < 0.001 |
| 50–54 | 0.57 (0.48 to 0.67) | < 0.001 | 0.45 (0.39 to 0.51) | < 0.001 |
| 55–59 | 0.62 (0.54 to 0.71) | < 0.001 | 0.57 (0.51 to 0.63) | < 0.001 |
| 60–64 | 0.80 (0.72 to 0.89) | < 0.001 | 0.75 (0.70 to 0.81) | < 0.001 |
| 65–69 | 1 | – | 1 | – |
| 70–74 | 1.11 (1.03 to 1.20) | 0.010 | 1.31 (1.23 to 1.40) | < 0.001 |
| 75–79 | 1.34 (1.24 to 1.44) | < 0.001 | 1.77 (1.67 to 1.89) | < 0.001 |
| 80–84 | 1.81 (1.68 to 1.94) | < 0.001 | 2.34 (2.20 to 2.49) | < 0.001 |
| 85–89 | 2.57 (2.39 to 2.76) | < 0.001 | 3.12 (2.91 to 3.33) | < 0.001 |
| ≥ 90 | 3.80 (3.52 to 4.10) | < 0.001 | 4.50 (4.14 to 4.90) | < 0.001 |
| Sex (male is the reference group) | ||||
| Female | 0.89 (0.86 to 0.92) | < 0.001 | 0.77 (0.74 to 0.79) | < 0.001 |
| Ethnic group (white ethnicity is the reference group) | ||||
| White | 1 | – | 1 | – |
| Indian | 0.73 (0.67 to 0.80) | < 0.001 | 0.74 (0.64 to 0.85) | < 0.001 |
| Black | 0.73 (0.64 to 0.84) | < 0.001 | 0.75 (0.59 to 0.95) | 0.017 |
| Mixed | 0.91 (0.79 to 1.05) | 0.113 | 0.80 (0.66 to 0.98) | 0.027 |
| Not known | 1.09 (1.01 to 1.17) | 0.017 | 1.00 (0.92 to 1.07) | 0.896 |
| Living status | ||||
| Patient coded as living alone | 0.92 (0.88 to 0.96) | 0.001 | – | – |
| Comorbidities | ||||
| IHD | 1.19 (1.15 to 1.23) | < 0.001 | – | – |
| HF | n/a | – | 1.47 (1.40 to 1.55) | < 0.001 |
| Stroke | 1.36 (1.23 to 1.51) | < 0.001 | 1.36 (1.18 to 1.57) | < 0.001 |
| Arrhythmia | – | – | 1.23 (1.18 to 1.29) | < 0.001 |
| Valvular disease | 1.26 (1.22 to 1.31) | < 0.001 | 1.27 (1.18 to 1.37) | < 0.001 |
| Peripheral vascular disease | 1.26 (1.19 to 1.33) | < 0.001 | 1.38 (1.30 to 1.48) | < 0.001 |
| Chronic pulmonary disease | 1.13 (1.09 to 1.17) | < 0.001 | n/a | – |
| Pneumonia | 1.65 (1.58 to 1.73) | < 0.001 | 1.56 (1.52 to 1.68) | < 0.001 |
| Renal disease | 1.89 (1.82 to 1.96) | < 0.001 | 1.42 (1.34 to 1.5) | < 0.001 |
| Hypertension | 0.75 (0.73 to 0.78) | < 0.001 | – | – |
| Electrolyte disorders | n/a | – | 1.85 (1.72 to 1.98) | < 0.001 |
| Cancer – without metastases | 2.04 (1.89 to 2.19) | < 0.001 | 3.38 (3.15 to 3.62) | < 0.001 |
| Cancer – with metastases | 4.48 (3.73 to 5.37) | < 0.001 | 6.36 (5.41 to 7.48) | < 0.001 |
| Cognitive impairment (senility and dementia combined) | 1.73 (1.64 to 1.82) | < 0.001 | 1.73 (1.63 to 1.83) | < 0.001 |
| Mental health (excluding dementia) | 1.10 (1.04 to 1.17) | 0.0006 | 1.26 (1.19 to 1.32) | < 0.001 |
| Experience of hospital | ||||
| Number of outpatient appointments | ||||
| Attended in year prior to admission (per appointment) | – | – | 1.01 (1.00 to 1.01) | < 0.001 |
| Missed in year prior to admission (per appointment) | 1.05 (1.04 to 1.07) | < 0.001 | 1.08 (1.06 to 1.10) | < 0.001 |
| LOS of index admission (nights) (0 nights the reference group) | ||||
| 0 | 1 | – | 1 | – |
| 1 | 1.17 (1.08 to 1.28) | 0.0002 | 1.11 (1.03 to 1.20) | 0.006 |
| 2 | 1.10 (1.01 to 1.20) | 0.0382 | 1.22 (1.13 to 1.32) | < 0.001 |
| ≥ 3 | 1.24 (1.16 to 1.33) | < 0.001 | 1.54 (1.45 to 1.64) | < 0.001 |
| Inpatient interventions | ||||
| CABG | 0.34 (0.28 to 0.42) | < 0.001 | 0.38 (0.23 to 0.63) | < 0.001 |
| Echocardiography | 0.84 (0.81 to 0.88) | < 0.001 | – | – |
| PTCA | 0.65 (0.57 to 0.73) | < 0.001 | 0.57 (0.45 to 0.72) | < 0.001 |
| Defibrillation | 1.31 (1.08 to 1.57) | 0.005 | – | – |
| Lung operations including excision | n/a | – | 1.43 (1.15 to 1.77) | 0.001 |
| Bronchoscopy | n/a | – | 1.65 (1.47 to 1.84) | < 0.001 |
| Interventions during admission | ||||
| Intensive care during index admission | 3.80 (2.99 to 4.83) | 0.002 | 1.50 (1.20 to 1.88) | < 0.001 |
| Invasive ventilation – on admission | n/a | – | 3.53 (2.84 to 4.37) | < 0.001 |
| Non-invasive ventilation – on admission | n/a | – | 2.48 (2.34 to 2.64) | < 0.001 |
| Distance to health care | ||||
| Distance from patient residence to admitting hospital (per km) | 0.999 (0.998 to 1.00) | 0.001 | 0.998 (0.997 to 0.999) | < 0.001 |
| Trust factors | ||||
| Doctor rate per 10 beds (mean) | 0.95 (0.94 to 0.95) | < 0.001 | 0.96 (0.95 to 0.97) | < 0.001 |
| Staff survey – recommend to friends and family (possible scores range from 1 to 5) | – | – | 0.80 (0.73 to 0.87) | < 0.001 |
| GP factors | ||||
| GPs (FTE) per 1000 patients | – | – | 0.89 (0.82 to 0.96) | 0.004 |
| Patients know how to contact OOH service (%) | 1.004 (1.00 to 1.01) | 0.0001 | – | – |
| Model performance | ||||
| Area under ROC curve (c-statistic) | 0.706 | 0.763 | ||
| HL statistic (p-value based on 8 degrees of freedom) | 49.9 (< 0.001) | 190.1 (< 0.001) | ||
| Proportion of standardised residuals outside the range of –1.96 to 1.96 | 0.6% | 3.0% | ||
The two mortality models fitted the data well in terms of residuals but showed some overprediction of low risk (miscalibration). Discrimination (c-statistic) was noticeably higher, at a c-statistic of > 0.7, compared with readmission (see Predictors of 30-day emergency readmission). Older age, male sex, non-white ethnicity and a number of comorbidities such as prior stroke, pneumonia, renal disease, cancer and cognitive impairment were associated with higher odds of mortality within 1 year of the index admission. LOS of ≥ 1 night and missed prior outpatient appointments were strong predictors for both conditions. Intensive care use (both conditions) and the severity proxies for COPD were all associated with higher odds. Hospital and GP factors that we considered were sometimes significant but small in size.
Predictors of 30-day emergency readmission
Approximately one in five (19.8%) HF and one in six (16.5%) COPD patients who were discharged alive from their index admission were readmitted for any cause within 30 days. Table 7 gives the ORs for the final set of predictors for readmission within 30 days of live index discharge. For HF, we also included the National Audit hospital-level performance figures, but none came close to statistical significance and these are not shown.
| Factor | Cohort of patients | |||
|---|---|---|---|---|
| HF | COPD | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Patient factors | ||||
| Age group (years) (65–69 is the reference group) | ||||
| 18–44 | 1.12 (0.92 to 1.36) | 0.276 | 0.81 (0.69 to 0.94) | 0.007 |
| 45–49 | 1.01 (0.82 to 1.24) | 0.922 | 0.83 (0.73 to 0.96) | 0.009 |
| 50–54 | 0.96 (0.81 to 1.13) | 0.603 | 0.90 (0.81 to 1.01) | 0.068 |
| 55–59 | 0.89 (0.77 to 1.02) | 0.101 | 0.85 (0.77 to 0.93) | < 0.001 |
| 60–64 | 0.97 (0.87 to 1.09) | 0.630 | 0.89 (0.83 to 0.97) | 0.005 |
| 65–69 | 1 | – | 1 | – |
| 70–74 | 1.05 (0.96 to 1.15) | 0.294 | 1.14 (1.06 to 1.22) | < 0.001 |
| 75–79 | 1.10 (1.00 to 1.20) | 0.041 | 1.23 (1.15 to 1.31) | < 0.001 |
| 80–84 | 1.14 (1.05 to 1.24) | 0.003 | 1.36 (1.27 to 1.46) | < 0.001 |
| 85–89 | 1.22 (1.12 to 1.33) | < 0.001 | 1.42 (1.32 to 1.53) | < 0.001 |
| ≥ 90 | 1.25 (1.14 to 1.38) | < 0.001 | 1.50 (1.35 to 1.66) | < 0.001 |
| Sex (male is the reference group) | ||||
| Females | – | – | 0.90 (0.87 to 0.93) | < 0.001 |
| Deprivation fifth (1 is the reference group) | ||||
| 1 – least deprived | – | – | 1 | – |
| 2 | – | – | 1.05 (0.98 to 1.13) | 0.183 |
| 3 | – | – | 1.14 (1.06 to 1.22) | < 0.001 |
| 4 | – | – | 1.13 (1.06 to 1.21) | < 0.001 |
| 5 – most deprived | – | – | 1.19 (1.12 to 1.28) | < 0.001 |
| Ethnic group (white ethnicity is the reference group) | ||||
| White | 1 | – | 1 | – |
| Indian | 0.96 (0.87 to 1.07) | 0.464 | 1.04 (0.91 to 1.19) | 0.538 |
| Black | 1.13 (0.99 to 1.30) | 0.076 | 0.80 (0.63 to 1.02) | 0.072 |
| Mixed | 1.00 (0.84 to 1.18) | 0.961 | 0.85 (0.69 to 1.04) | 0.119 |
| Not known | 0.77 (0.70 to 0.86) | < 0.001 | 0.74 (0.67 to 0.81) | < 0.001 |
| Living status | ||||
| Patient coded as living alone | – | – | 1.17 (1.10 to 1.23) | < 0.001 |
| Comorbidities | ||||
| IHD | 1.18 (1.13 to 1.23) | < 0.001 | 1.14 (1.10 to 1.19) | < 0.001 |
| HF | n/a | – | 1.19 (1.12 to 1.26) | < 0.001 |
| Stroke | – | – | 1.31 (1.12 to 1.52) | 0.001 |
| Arrhythmia | – | – | 1.19 (1.14 to 1.25) | < 0.001 |
| Valvular disease | 1.11 (1.06 to 1.16) | < 0.001 | – | – |
| Peripheral vascular disease | 1.17 (1.10 to 1.25) | < 0.001 | 1.12 (1.04 to 1.20) | 0.003 |
| Chronic pulmonary disease | 1.27 (1.22 to 1.33) | < 0.001 | n/a | – |
| Pneumonia | 1.18 (1.11 to 1.25) | < 0.001 | 1.36 (1.29 to 1.44) | < 0.001 |
| Renal disease | 1.25 (1.20 to 1.31) | < 0.001 | 1.21 (1.13 to 1.28) | < 0.001 |
| Diabetes mellitus | 1.08 (1.03 to 1.13) | 0.001 | 1.11 (1.06 to 1.17) | < 0.001 |
| Electrolyte disorders | n/a | – | 1.14 (1.04 to 1.24) | 0.002 |
| Cancer – without metastases | – | – | 1.38 (1.27 to 1.49) | < 0.001 |
| Cancer – with metastases | – | – | 1.45 (1.24 to 1.71) | < 0.001 |
| Cognitive impairment (senility and dementia combined) | 1.30 (1.22 to 1.39) | < 0.001 | 1.28 (1.20 to 1.37) | < 0.001 |
| Mental health (excluding dementia) | 1.19 (1.12 to 1.27) | < 0.001 | 1.43 (1.36 to 1.51) | < 0.001 |
| Experience of hospital | ||||
| Number of OPD appointments | ||||
| Attended (per appointment) | – | – | 1.01 (1.01 to 1.01) | < 0.001 |
| Missed (per appointment) | 1.09 (1.07 to 1.10) | < 0.001 | 1.09 (1.07 to 1.11) | < 0.001 |
| LOS of index admission (nights) (0 nights is the reference group) | ||||
| 0 | 1 | – | 1 | – |
| 1 | 0.88 (0.79 to 0.97) | 0.0113 | 0.93 (0.86 to 1.00) | 0.058 |
| 2 | 0.78 (0.70 to 0.87) | < 0.001 | 0.88 (0.81 to 0.95) | 0.001 |
| ≥ 3 | 0.83 (0.77 to 0.90) | < 0.001 | 1.02 (0.96 to 1.08) | 0.623 |
| Inpatient interventions | ||||
| Echocardiography | – | – | 1.19 (1.10 to 1.29) | < 0.001 |
| Defibrillation | 1.38 (1.12 to 1.69) | 0.002 | – | – |
| Trust factors | ||||
| Hospital size (per 100 beds) | 2.16 (1.34 to 3.48) | 0.002 | 2.27 (1.40 to 3.66) | 0.001 |
| Doctor rate per 10 beds | 0.98 (0.97 to 0.99) | 0.0001 | 0.98 (0.97 to 0.99) | 0.001 |
| Staff rating of effective teamworking (possible scores range from 1 to 5) | – | – | 1.65 (1.21 to 2.24) | 0.001 |
| GP factors | ||||
| None | – | – | – | – |
| Model performance | ||||
| Area under ROC curve (c-statistic) | 0.582 | 0.625 | ||
| HL statistic (p-value based on 8 degrees of freedom) | 12.27 (0.139) | 70.3 (< 0.001) | ||
| Proportion of standardised residuals outside the range of –1.96 to 1.96 (%) | 1.3 | 5.1 | ||
The two models fitted well, however, with low discrimination (c-statistic). Older age and a number of comorbidities such as ischaemic heart disease (IHD), renal disease, cognitive impairment, mental health conditions and pneumonia were associated with higher odds of readmission for both patient groups. LOS was significant for both, but the pattern differed, with same-day discharges for HF and 2-night stays for COPD having the highest odds of readmission. Missed outpatient appointments were a strong predictor for both conditions, with 9% higher odds of readmission per appointment missed in the previous year. Larger hospital size and fewer doctors per bed were both associated with higher odds, though teamworking rating by staff remained in the model for COPD only. No GP factors that we considered were retained.
As well as modelling the standard all-cause 30-day measure, we compared predictors for readmission, split into readmissions for the index condition and those for other causes. Of the 13,099 all-cause 30-day readmissions in the HF cohort, 28.6% had a primary diagnosis of HF, compared with a high of 32.8% at 7 days and only 22.7% at 1 year. For COPD, of the 15,074 all-cause 30-day readmissions, 39.1% had a primary diagnosis of COPD. This, again, was highest at 7 days (43.2%) and fell to 36.2% at 1 year, a much smaller difference than for HF.
Rather than present the two very large tables, we will now summarise the differences in the predictors for readmissions with the same primary diagnosis as the index admission (i.e. HF or COPD) and predictors for any other readmission diagnosis. For HF patients, the statistically significant (p < 0.01) effects of older age, comorbidities (such as IHD, peripheral vascular disease, pneumonia, COPD, diabetes mellitus, renal disease) and prior missed outpatient appointments were similar; no significant associations for any readmission diagnosis were seen for sex, trust factors, GP factors or other community factors. A few variables showed significant associations with readmissions for HF only: black ethnicity (OR 1.44, 95% CI 1.16 to 1.79; p = 0.001), valvular disease (OR 1.26, 95% CI 1.17 to 1.36; p < 0.00010), defibrillation (OR 1.61, 95% CI 1.18 to 2.20; p = 0.0024) and same-day index discharge. Compared with an index LOS of zero, an index LOS of 1 night had an OR of 0.77 (95% CI 0.65 to 0.90) and p-value of 0.0011, a 2-night index stay had an OR of 0.64 (95% CI 0.53 to 0.76) and a p-value of < 0.0001, and index stays of ≥ 3 nights had an OR of 0.64 (95% CI 0.56 to 0.72) and a p-value of < 0.0001. On the other hand, a few variables showed significant associations with readmissions for non-HF diagnoses only: deprivation (p = 0.009), cancer with metastases (OR 1.38, 95% CI 1.09 to 1.73; p = 0.0063), cognitive impairment [senility and dementia (OR 1.37, 95% CI 1.27 to 1.47; p < 0.0001) and mental health conditions excluding dementia (OR 1.21, 95% CI 1.12 to 1.30; p < 0.0001] and living alone (OR 1.11, 95% CI 1.04 to 1.19; p = 0.0017).
For COPD, the main similarities were for sex (females had lower odds of readmission), pneumonia, mental health conditions except dementia, referral for echocardiography (15% higher odds if recorded), prior missed OPD appointments (stronger effect seen for non-COPD readmissions) and, as with HF, the lack of any significant associations with any of the hospital, GP or community factors that we tried. Age relations differed considerably by readmission diagnosis. Compared with patients aged 60–69 years, those aged under 55 years had lower odds of COPD readmissions but similar odds of other readmissions. Those aged ≥ 70 years had only slightly higher odds of COPD readmission (and not statistically significantly higher for ages of ≥ 85 years), but much higher odds of non-COPD readmission, rising to a peak OR of 1.75 (95% CI 1.55 to 1.97) and a p-value of < 0.0001 for those aged ≥ 90 years. Just two variables showed significant associations with readmissions for COPD only: deprivation and non-invasive ventilation on admission (OR 1.29, 95% CI 1.15 to 1.45; p < 0.0001). A much larger number of variables showed significant associations with non-COPD readmissions only: almost all comorbidities, living alone (OR 1.21, 95% CI 1.13 to 1.29; p < 0.0001) and index LOS [the lowest odds were for 2-night stays (an OR compared with same-day discharges of 0.88, 95% CI 0.79 to 0.98; p = 0.0165) and the highest odds were for stays of ≥ 3 nights (OR 1.10, 95% CI 1.02 to 1.19; p = 0.0168]. The direction of association for hypertension differed by readmission diagnosis, with lower odds if readmitted for COPD (OR 0.91, 95% CI 0.86 to 0.96; p = 0.0013) but higher odds if readmitted for any other diagnosis (OR 1.07, 95% CI 1.02 to 1.12; p = 0.0032).
In summary, for both cohorts the set of predictors of readmission for the index condition showed several differences from the set for readmission for other conditions. There were fewer predictors, perhaps because of the smaller sample size. However, there were a few predictors of readmissions for the index condition that did not significantly predict readmissions for other conditions; for example, index LOS and defibrillation were significant predictors of readmission for HF only. There were very few predictors for non-COPD readmissions that differed from the list of predictors for all-cause readmissions for the COPD cohort.
Attendance at accident and emergency following the index stay and likelihood of admission during that attendance
Many of the patients with either of our two chronic diseases were regular visitors to each hospital setting. We began by counting the number of ED attendances, OPD appointments, elective admissions and emergency admissions per patient in the year following index discharge (survivors of the index only). For HF these are summarised in our British Medical Journal Open article (see Chapter 4, Dissemination activity), together with the proportions of patients for whom the National Institute for Health and Care Excellence guideline number 187,45 for cardiologist follow-up within a fortnight of inpatient discharge, was met (just 7% of patients overall, with large differences by age and comorbidity). To better understand the drivers of readmission, and as preparation for objective 2, we ran some further analyses on the use of ED after discharge from the index stay. There are two parts: (1) regression modelling to determine which factors are associated with (a) ED attendance and (b) admission to hospital at that ED attendance; and (2) an assessment of the statistical properties of different indicators covering ED attendance and readmission.
During our 2 index study years, 66,219 HF patients were discharged alive from their index HF admission, of whom 11,513 (17.4%) attended the ED within 30 days with no intervening elective or emergency admission (as described in Chapter 2). Of the 90,351 COPD patients discharged alive from their index COPD admission, 14,039 (15.5%) attended the ED within 30 days using the same definition. Of these ED attendances, 76.9% for HF and 74.0% for COPD resulted in admission. There were some variations in the proportion of attendances that resulted in admission by day of the week, time of day, the number of elderly patients waiting and the number of patients admitted during the hour of arrival. Table 8 gives the 7- and 30-day attendance rates and proportion who were subsequently admitted for each cohort.
| Feature of ED attendance | Cohort of patients, attendance (% admitted) | |||
|---|---|---|---|---|
| HF | COPD | |||
| ED7 | ED30 | ED7 | ED30 | |
| Timing | ||||
| Day of week | ||||
| Monday | 563 (78.7) | 1695 (78.5) | 674 (76.4) | 2131 (74.7) |
| Tuesday | 548 (78.3) | 1636 (75.7) | 704 (73.7) | 1990 (73.1) |
| Wednesday | 551 (80.4) | 1616 (77.5) | 662 (75.2) | 1914 (74.0) |
| Thursday | 567 (78.3) | 1624 (77.0) | 632 (77.7) | 1897 (74.2) |
| Friday | 570 (78.6) | 1725 (77.8) | 688 (76.3) | 2095 (74.4) |
| Saturday | 608 (76.6) | 1645 (76.7) | 778 (77.6) | 2056 (74.7) |
| Sunday | 644 (74.8) | 1572 (74.2) | 679 (72.3) | 1956 (72.4) |
| Period of week | ||||
| Weekday | 2799 (78.9) | 8296 (77.3) | 3360 (75.8) | 10,027 (74.1) |
| Weekend | 1252 (75.7) | 3217 (75.5) | 1457 (75.2) | 4012 (73.6) |
| Time | ||||
| 00.00–08.00 hours | 840 (79.8) | 2145 (79.3) | 922 (75.4) | 2519 (75.6) |
| 08.00–18.00 hours | 2147 (76.7) | 6463 (75.1) | 2595 (75.6) | 7843 (72.9) |
| 18.00–00.00 hours | 1064 (78.9) | 2905 (78.7) | 1300 (75.9) | 3677 (75.1) |
| Patients seen within 4 hours in the hour when HF/COPD patient arrives | ||||
| ≥ 98% | 3658 (77.7) | 10,369 (76.5) | 4399 (75.4) | 12,703 (73.8) |
| ≥ 95% | 3887 (78.1) | 11,098 (76.8) | 4682 (75.5) | 13,556 (73.9) |
| Number of boarders during hour of arrival | ||||
| 0 | 3644 (77.8) | 10,448 (76.6) | 4325 (75.8) | 12,625 (74.0) |
| 1 | 84 (81.0) | 226 (81.0) | 103 (71.8) | 308 (75.7) |
| 2 | 56 (73.2) | 139 (79.9) | 78 (79.5) | 219 (74.4) |
| 3 | 36 (86.1) | 106 (82.1) | 52 (80.8) | 156 (76.9) |
| 4 | 21 (85.7) | 76 (88.2) | 39 (82.1) | 113 (72.6) |
| ≥ 5 | 210 (77.1) | 518 (75.9) | 220 (70.0) | 618 (70.7) |
| Busyness of ED as % of maximum busyness | ||||
| ≤ 75 | 2745 (78.2) | 7659 (76.9) | 3184 (75.2) | 9126 (73.5) |
| > 75 to ≤ 80 | 274 (75.6) | 763 (78.6) | 297 (81.1) | 941 (76.1) |
| > 80 to ≤ 85 | 208 (76.0) | 677 (74.9) | 329 (76.3) | 868 (75.6) |
| > 85 to ≤ 90 | 219 (76.7) | 597 (74.5) | 245 (74.7) | 717 (73.5) |
| > 90 to ≤ 95 | 150 (80.0) | 462 (77.9) | 200 (80.0) | 627 (75.8) |
| > 95 to < 100 | 109 (79.8) | 324 (78.4) | 141 (70.9) | 423 (70.5) |
| At maximum busyness | 346 (77.5) | 1031 (76.4) | 421 (74.6) | 1337 (74.6) |
| Number of very elderly patients waiting during hour of arrival | ||||
| 0 or 1 | 366 (74.3) | 902 (74.3) | 537 (72.6) | 1549 (68.4) |
| 2–4 | 775 (75.4) | 2230 (75.5) | 910 (75.8) | 2577 (73.7) |
| 4–6 | 958 (79.3) | 2690 (77.7) | 1141 (74.7) | 3307 (74.2) |
| 6–8 | 1337 (77.5) | 3787 (76.4) | 1435 (76.1) | 4341 (75.0) |
| 8–10 | 282 (80.9) | 881 (79.7) | 354 (76.6) | 1025 (73.3) |
| ≥ 11 | 333 (82.6) | 1023 (78.5) | 440 (79.1) | 1240 (77.5) |
| Seen in 4 hours (%) | ||||
| ≥ 95 | 3887 (78.1) | 11,098 (76.8) | 4682 (75.5) | 13,556 (73.9) |
| < 95 | 164 (76.7) | 415 (79.1) | 135 (79.2) | 483 (77.8) |
| ≥ 98 | 3658 (77.7) | 10,369 (76.5) | 4399 (75.4) | 12,703 (73.8) |
| < 98 | 393 (80.8) | 1144 (80.4) | 415 (78.5) | 1336 (75.5) |
| Number of patients admitted during hour of arrival | ||||
| 0 | 474 (73.0) | 1425 (68.8) | 598 (67.6) | 1698 (63.3) |
| 1 | 711 (74.1) | 2055 (74.6) | 784 (75.6) | 2314 (73.6) |
| 2 | 743 (75.6) | 2066 (76.6) | 818 (76.5) | 2483 (73.5) |
| 3 | 628 (80.7) | 1749 (78.7) | 745 (74.9) | 2215 (75.4) |
| 4 | 493 (77.9) | 1310 (77.5) | 584 (79.6) | 1612 (76.5) |
| 5 | 356 (80.9) | 1027 (80.0) | 437 (79.2) | 1231 (78.5) |
| ≥ 6 | 646 (83.8) | 1881 (81.5) | 851 (76.5) | 2486 (76.7) |
In Table 8 the proportion admitted is slightly lower at the weekend, especially on Sunday, and lower during the day shift. In contrast, admission appeared more likely with an increasing number of elderly patients waiting when the HF or COPD patient arrived and also with an increasing number of patients admitted that hour. There was a large jump in the proportion of HF or COPD patients admitted, from no patients admitted (irrespective of presenting complaint) to one patient admitted. This was most noticeable for COPD patients, as if they arrived at the ED (within 30 days of their index discharge) in an hour when no one was admitted, their own chance of admission was 63%, and this rose to nearly 74% if one person was admitted. The proportion admitted was also higher if the patient arrived at a time when waits were longer, as measured by breach of the 4-hour target at either the old strict 98% or the less stringent 95% rate threshold. There were no clear associations with the number of boarders or with relative busyness of the department, although one might say that a few boarders – two or three – were associated with higher odds than having either no or many boarders.
Table 9 gives the significant predictors from the regression model of ED attendance within 30 days of index discharge. These included older age, particularly for COPD, male sex (COPD only), deprivation, living alone, prior missed OPD appointments, same-day index discharge (HF only), defibrillation implantation (HF only), referral for echocardiography (COPD only) and most of the comorbidities on our list. In contrast with 30-day readmissions in objective 1, a number of the hospital and practice factors that we included showed significant associations with ED attendance for COPD. The biggest effects were for staff survey – would recommend to friends and family (OR per point increase 0.66; p < 0.0001) and number of GPs (FTE) per 1000 patients in the practice (OR 0.87; p = 0.002) and, for HF, QOF-recorded prevalence in 2009 (OR per percentage point prevalence increase 0.84; p < 0.0001). These associations are in the expected direction: ED attendance was more likely in COPD patients when the hospital scored worse on the Friends and Family Test, when there was lower GP supply and, for HF, lower HF prevalence at the practice.
| Explanatory variable | Cohort of patients | |||
|---|---|---|---|---|
| HF | COPD | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Patient factors | ||||
| Age group (years) (65–69 is the reference group) | ||||
| 18–44 | 1.03 (0.84 to 1.27) | 0.763 | 0.69 (0.60 to 0.79) | < 0.0001 |
| 45–49 | 1.01 (0.82 to 1.26) | 0.894 | 0.96 (0.84 to 1.10) | 0.578 |
| 50–54 | 0.89 (0.74 to 1.07) | 0.206 | 0.98 (0.88 to 1.09) | 0.728 |
| 55–59 | 0.91 (0.79 to 1.06) | 0.233 | 0.88 (0.80 to 0.96) | 0.007 |
| 60–64 | 0.96 (0.85 to 1.09) | 0.567 | 0.93 (0.86 to 1.01) | 0.088 |
| 65–69 | 1 | 1 | ||
| 70–74 | 1.01 (0.92 to 1.12) | 0.793 | 1.09 (1.02 to 1.17) | 0.016 |
| 75–79 | 1.08 (0.98 to 1.18) | 0.116 | 1.21 (1.13 to 1.30) | < 0.0001 |
| 80–84 | 1.12 (1.02 to 1.23) | 0.015 | 1.31 (1.22 to 1.41) | < 0.0001 |
| 85–89 | 1.18 (1.07 to 1.29) | 0.0005 | 1.38 (1.27 to 1.49) | < 0.0001 |
| ≥ 90 | 1.23 (1.12 to 1.36) | < 0.0001 | 1.52 (1.37 to 1.68) | < 0.0001 |
| Sex (male is the reference group) | ||||
| Females | – | – | 0.89 (0.86 to 0.92) | < 0.0001 |
| Deprivation fifth (1 is the reference group) | ||||
| 1 – least deprived | 1 | – | 1 | – |
| 2 | 1.02 (0.94 to 1.09) | 0.677 | 1.05 (0.97 to 1.13) | 0.266 |
| 3 | 1.04 (0.97 to 1.12) | 0.258 | 1.15 (1.06 to 1.23) | 0.0003 |
| 4 | 1.12 (1.05 to 1.21) | 0.001 | 1.16 (1.08 to 1.25) | < 0.0001 |
| 5 – most deprived | 1.15 (1.07 to 1.23) | 0.0002 | 1.25 (1.17 to 1.35) | < 0.0001 |
| Ethnic group (white ethnicity is the reference group) | ||||
| White | 1 | – | 1 | – |
| Indian | 1.00 (0.90 to 1.11) | 0.953 | 1.10 (0.97 to 1.25) | 0.146 |
| Black | 1.12 (0.98 to 1.28) | 0.099 | 1.06 (0.86 to 1.32) | 0.578 |
| Mixed | 1.07 (0.90 to 1.26) | 0.438 | 0.92 (0.76 to 1.12) | 0.397 |
| Not known | 0.77 (0.69 to 0.86) | < 0.0001 | 0.74 (0.68 to 0.82) | < 0.0001 |
| Living status | ||||
| Patient coded as living alone | 1.11 (1.05 to 1.19) | 0.001 | 1.24 (1.17 to 1.31) | < 0.0001 |
| Comorbidities | ||||
| IHD | 1.23 (1.18 to 1.28) | < 0.0001 | 1.17 (1.12 to 1.22) | < 0.0001 |
| Elixhauser HF | n/a | – | 1.15 (1.08 to 1.22) | < 0.0001 |
| Elixhauser arrhythmia | – | – | 1.19 (1.14 to 1.25) | < 0.0001 |
| Elixhauser chronic pulmonary disease | 1.29 (1.24 to 1.35) | < 0.0001 | n/a | – |
| Pneumonia | 1.20 (1.13 to 1.28) | < 0.0001 | 1.31 (1.24 to 1.39) | < 0.0001 |
| Elixhauser renal disease | 1.15 (1.10 to 1.21) | < 0.0001 | – | – |
| Elixhauser obesity | 0.86 (0.78 to 0.95) | 0.003 | – | – |
| Diabetes | 1.08 (1.03 to 1.13) | 0.001 | – | – |
| Cancer – without metastases | – | – | 1.22 (1.13 to 1.32) | < 0.0001 |
| Cognitive impairment (senility and dementia combined) | 1.39 (1.30 to 1.49) | < 0.0001 | 1.30 (1.21 to 1.4) | < 0.0001 |
| Mental health conditions (not dementia) | 1.20 (1.12 to 1.29) | < 0.0001 | 1.48 (1.41 to 1.56) | < 0.0001 |
| Experience of hospital | ||||
| Number of outpatient appointments | ||||
| Attended in year prior to admission (per appointment) | – | – | 1.01 (1.00 to 1.01) | < 0.0001 |
| Missed in year prior to admission (per appointment) | 1.09 (1.07 to 1.11) | < 0.0001 | 1.10 (1.08 to 1.12) | < 0.0001 |
| Inpatient interventions | ||||
| Echocardiographya | – | – | 1.24 (1.15 to 1.35) | < 0.0001 |
| Defibrillationa | 1.33 (1.07 to 1.65) | 0.010 | – | – |
| Distance to health care | ||||
| Distance from patient residence to nearest community rehabilitation unit (per km) | 0.99 (0.99 to 1.00) | < 0.0001 | 0.99 (0.99 to 1.00) | < 0.0001 |
| Trust factors | ||||
| PE of waiting for a bed after arrival at hospital – score | 0.99 (0.99 to 1.00) | 0.0001 | 0.99 (0.99 to 1.00) | 0.0003 |
| Staff survey – satisfaction with care being given | 1.01 (1.01 to 1.02) | < 0.0001 | 1.02 (1.01 to 1.02) | < 0.0001 |
| Staff survey – recommend to friends and family | – | – | 0.66 (0.57 to 0.77) | < 0.0001 |
| GP factors | ||||
| GPs (FTE) per 1000 patients | – | – | 0.87 (0.79 to 0.95) | 0.002 |
| Patients know how to contact OOH service (%) | – | – | 0.99 (0.99 to 0.99) | < 0.0001 |
| HF prevalence (per cent point increase) | 0.84 (0.78 to 0.90) | < 0.0001 | n/a | – |
Once the patient had attended the ED, we assessed their odds of being hospitalised at that visit. Table 10 gives the ORs for variables with a p-value of < 0.01.
| Explanatory variable | Cohort of patients | |||
|---|---|---|---|---|
| HF | COPD | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Patient characteristics | ||||
| Age group (years) (65–69 is the reference group) | ||||
| 18–44 | 0.98 (0.64 to 1.50) | 0.916 | 0.37 (0.28 to 0.49) | < 0.0001 |
| 45–49 | 0.83 (0.54 to 1.28) | 0.404 | 0.48 (0.37 to 0.62) | < 0.0001 |
| 50–54 | 0.97 (0.67 to 1.41) | 0.859 | 0.69 (0.56 to 0.86) | 0.001 |
| 55–59 | 1.05 (0.77 to 1.43) | 0.745 | 0.74 (0.61 to 0.90) | 0.002 |
| 60–64 | 1.14 (0.88 to 1.48) | 0.317 | 0.84 (0.71 to 0.99) | 0.044 |
| 65–69 | 1 | – | 1 | – |
| 70–74 | 1.23 (1.00 to 1.52) | 0.050 | 1.13 (0.97 to 1.32) | 0.123 |
| 75–79 | 1.34 (1.10 to 1.63) | 0.003 | 1.00 (0.87 to 1.17) | 0.954 |
| 80–84 | 1.34 (1.11 to 1.62) | 0.003 | 1.21 (1.03 to 1.41) | 0.017 |
| 85–89 | 1.54 (1.27 to 1.86) | < 0.0001 | 1.18 (1.00 to 1.39) | 0.054 |
| ≥ 90 | 1.57 (1.28 to 1.94) | < 0.0001 | 1.13 (0.91 to 1.41) | 0.280 |
| Comorbidities | ||||
| Elixhauser HF | – | – | 1.23 (1.08 to 1.40) | 0.002 |
| Pneumonia | – | – | 1.28 (1.13 to 1.45) | 0.0001 |
| Elixhauser obesity | – | – | 1.49 (1.18 to 1.89) | 0.001 |
| Cancer – without metastases | – | – | 1.45 (1.21 to 1.74) | 0.0001 |
| Mental health conditions (excluding dementia) | – | – | 0.87 (0.78 to 0.96) | 0.009 |
| LOS of index admission (nights) (0 nights is the reference group) | ||||
| 0 | 1 | 1 | ||
| 1 | 1.18 (0.94 to 1.47) | 0.147 | 1.08 (0.93 to 1.25) | 0.3158 |
| 2 | 1.21 (0.95 to 1.53) | 0.123 | 1.23 (1.04 to 1.44) | 0.015 |
| ≥ 3 | 1.49 (1.25 to 1.77) | < 0.0001 | 1.73 (1.52 to 1.96) | < 0.0001 |
| Inpatient interventions | ||||
| Non-invasive ventilation – on admission | – | – | 1.37 (1.12 to 1.68) | 0.003 |
| A&E characteristics | ||||
| Shift of attendance [day shift (08.00–18.00 hours) is the reference group] | ||||
| 08.00–18.00 | 1 | – | 1 | – |
| 18:.00–00.00 | 1.20 (1.07 to 1.34) | 0.0015 | 1.16 (1.06 to 1.28) | 0.002 |
| 00.00–08.00 | 1.35 (1.19 to 1.53) | < 0.0001 | 1.23 (1.10 to 1.37) | 0.0002 |
| Number of patients admitted during hour of arrival | 1.05 (1.03 to 1.07) | < 0.0001 | 1.02 (1.01 to 1.04) | 0.001 |
| Proportion who were seen within 4 hours fell below 98% (≥ 98% is the reference group) | 1.40 (1.18 to 1.64) | 0.0001 | – | – |
The main predictors were:
-
older age (for HF, with weaker evidence for COPD) but also age < 60 years (COPD only)
-
index LOS of ≥ 3 nights
-
non-invasive ventilation during the index COPD admission
-
evening or night attendance (both conditions)
-
comorbidities of HF, pneumonia, obesity or cancer (all COPD only – having a coded mental health condition was associated with 13% lower odds of admission)
-
two hospital-level variables:
-
for each patient admitted for any condition from the ED during the hour of arrival, the odds of the HF or COPD patient being admitted rose by 5% for HF and 2% for COPD
-
the odds of HF patients being admitted from the ED were 40% higher if the overall proportion of waiting patients seen within 4 hours was < 98% than if it was ≥ 98%.
-
In other words, admission from the ED became more likely if our HF patients arrived during the evening or at night, if they arrived when other patients were being admitted (both also true for our COPD patients) or if they arrived when other patients were waiting a long time. In contrast to the crude cross-tabulations in Table 8, the regression model found no association by day or time of arrival or with the number of elderly patients waiting.
Although the majority of 30-day readmissions were via the ED, around one in five was not. We might therefore expect some predictors of readmission to differ from the set of predictors for admission following an ED attendance, and this was true. Age patterns were similar, though the ORs for admission from the ED in the COPD cohort were much lower than the ORs for any readmission. Most of the comorbidities were statistically significant predictors of readmission, but only a few remained in the model for admission from the ED. The number of prior OPD appointments missed was a strong predictor of readmission but not associated with admission from the ED. Index LOS showed different patterns, with same-day discharges having higher odds of any readmission but much lower odds of admission from the ED. Hospital size and doctors per bed were significantly associated with readmission via any route but not with admission from the ED.
Objective 2: should accident and emergency attendance and reattendance data be considered alongside readmission metrics when measuring hospital performance in terms of unplanned activity? If so, how?
For objectives 2 and 3, we did not use the GP or other community factors and, therefore, did not need to exclude records with missing GP practice information. Our sample size was therefore 66,219 HF and 90,351 COPD patients.
Some of the features of an ideal indicator of hospital performance are statistical. One is the discrimination between levels of performance, which can be assessed simply by the amount of variation between hospitals. A second is the amount of signal compared with the amount of noise. If the observed variation can all be ascribed to randomness and/or differences in case mix (patient factors) between hospitals, then the indicator is useless. A better indicator has a strong hospital ‘footprint’. 46 The first step was to summarise the variation in crude rates by hospital (Table 11).
| Hospital-level outcome | Cohort of patients | |||
|---|---|---|---|---|
| HF | COPD | |||
| Median (IQR) | National rate (%) | Median (IQR) | National rate (%) | |
| Number of discharged patients | 428.5 (320–582.5) | – | 626.5 (435–829.5) | – |
| Readmission | ||||
| 7 days (n) | 28 (21–40.5) | – | 34.5 (22–52) | – |
| Rate (%) | 6.6 (5.7–7.8) | 6.8 | 5.6 (4.8–6.2) | 5.7 |
| 30 days (n) | 85 (63.5–121.5) | – | 99 (68.5–142) | – |
| Rate (%) | 19.6 (18.1–21.4) | 19.8 | 16.5 (14.8–17.9) | 16.5 |
| 90 days (n) | 145.5 (109.5–212.5) | – | 173 (119.5–238.5) | – |
| Rate (%) | 33.8 (32.2–35.7) | 34.1 | 28.5 (26.6–30.3) | 28.6 |
| 365 days (n) | 225 (170.5–320.5) | – | 297 (198.5–382) | – |
| Rate (%) | 52.5 (49.8–54.1) | 52.2 | 47.2 (44.6–49.1) | 47.4 |
| A&E attendance | ||||
| 7 days (n) | 26.5 (19–36) | – | 31 (20–47) | – |
| Rate (%) | 6.4 (4.7–7.6) | 6.2 | 5.2 (4.0–6.5) | 5.3 |
| 30 days (n) | 76.5 (55–103) | – | 95 (63.5–138) | – |
| Rate (%) | 17.6 (14.6–21.7) | 17.4 | 15.5 (12.2–18.7) | 15.5 |
| 90 days (n) | 130.5 (97–167) | – | 161 (111.5–226) | – |
| Rate (%) | 29.9 (25.6–36.3) | 29.9 | 27.8 (22.5–31.9) | 27.0 |
| 365 days (n) | 199.5 (147.5–264.5) | – | 274 (189.5–372) | – |
| Rate (%) | 46.2 (40.3–54.3) | 45.8 | 46.2 (38.7–52.7) | 45.0 |
| A&E attendance or readmission | ||||
| 7 days (n) | 33 (25–49.5) | – | 39.5 (28–61.5) | – |
| Rate (%) | 8.0 (6.8–9.2) | 8.0 | 6.7 (5.7–7.7) | 6.0 |
| 30 days (n) | 93.5 (71–136.5) | – | 114.5 (82.5–167.5) | – |
| Rate (%) | 22.9 (20.5–24.5) | 22.7 | 19.2 (17.4–21.3) | 19.6 |
| 90 days (n) | 167 (126–236) | – | 206.5 (142.5–281) | – |
| Rate (%) | 38.9 (36.2–40.7) | 38.6 | 33.6 (31.2–36.5) | 33.8 |
| 365 days (n) | 255 (194–360) | – | 340 (237–446) | – |
| Rate (%) | 58.2 (55.4–60.8) | 58.3 | 55.4 (52.2–58.2) | 55.5 |
| A&E 7 days or readmission 30 days (n) | 87 (65.5–127) | – | 103 (74–149.5) | – |
| Rate (%) | 20.8 (18.7–22.2) | 20.7 | 17.2 (15.4–18.9) | 17.4 |
The most common outcome in Table 10 was ED attendance or readmission within a year at > 55%, with around half of the patients in either cohort being readmitted within a year of index discharge. The rate of readmission is higher than ED attendance at all time periods. HF patients have a higher rate of both readmission and ED attendance. The interquartile ranges for hospital rates for all of these measures were quite narrow.
We next adjusted the rates for patient factors. Table 12 gives the amount of variation in the resulting risk-adjusted hospital rates (as estimated by the dispersion parameter) and the size of the hospital ‘footprint’ (as estimated by the omega statistic).
| Outcome | Dispersion parameter | Covariance parameter estimate | SE | ICC (%) | Covariance parameter estimate | SE | ICC (%) | |
|---|---|---|---|---|---|---|---|---|
| HF | COPD | HF | COPD | |||||
| Readmission | ||||||||
| 7 days | 1.23 | 1.39 | 0.011 | 0.005 | 0.35 | 0.012 | 0.005 | 0.38 |
| 30 days | 1.12 | 1.41 | 0.005 | 0.002 | 0.16 | 0.008 | 0.002 | 0.24 |
| 90 days | 0.84 | 1.28 | 0.002 | 0.001 | 0.08 | 0.007 | 0.002 | 0.20 |
| 365 days | 0.68 | 0.95 | 0.004 | 0.001 | 0.13 | 0.006 | 0.001 | 0.18 |
| A&E attendance | ||||||||
| 7 days | 2.53 | 3.51 | 0.074 | 0.015 | 2.22 | 0.103 | 0.018 | 3.06 |
| 30 days | 5.08 | 6.28 | 0.113 | 0.017 | 3.33 | 0.134 | 0.020 | 3.90 |
| 90 days | 7.01 | 8.45 | 0.142 | 0.020 | 4.16 | 0.142 | 0.019 | 4.13 |
| 365 days | 8.18 | 10.67 | 0.168 | 0.022 | 4.85 | 0.170 | 0.022 | 4.93 |
| A&E attendance or readmission | ||||||||
| 7 days | 1.29 | 1.46 | 0.011 | 0.004 | 0.33 | 0.013 | 0.005 | 0.41 |
| 30 days | 1.17 | 1.59 | 0.006 | 0.002 | 0.19 | 0.010 | 0.002 | 0.31 |
| 90 days | 0.91 | 1.42 | 0.005 | 0.001 | 0.15 | 0.009 | 0.002 | 0.28 |
| 365 days | 0.74 | 1.04 | 0.008 | 0.002 | 0.23 | 0.010 | 0.002 | 0.29 |
| A&E 7 days or readmission 30 days | 1.13 | 1.39 | 0.005 | 0.002 | 0.17 | 0.008 | 0.002 | 0.24 |
As described in Chapter 2, the dispersion parameter was estimated by modelling the number of outcomes for each trust against the expected number of outcomes after adjusting for patient factors. If there is purely random variation so that the number of outcomes followed a purely Poisson distribution, the resulting dispersion parameter estimate would be one. If the estimate is > 1, then there is more than just random variation and, therefore, patient and/or system factors are also contributing. Dispersion was lowest for readmission-based metrics and appeared to fall with time since index discharge, but was considerably > 1 for ED attendance, indicating a high degree of unexplained (non-random) variability. Estimates of dispersion were generally slightly higher for COPD than HF. Combining ED attendance with readmission only increased the dispersion parameter estimates a little compared with those for readmission alone.
The covariance parameter estimates were significantly greater than zero, which indicates that there is more than just random variation between hospital-level outcome rates. However, the ICCs – the variation between hospitals compared with the total variation in the model – were very low, particularly for outcomes that included readmissions. The most variation between hospitals was seen for ED attendance up to a year after index discharge, though the ICC reached only 5%. This means that hospital factors explain only a small proportion of the total variation in outcome rates.
Next, we calculated the omega statistics (Table 13). Omega statistics compare the relative contribution of patient and hospital characteristics in models of hospital outcomes. Values of < 1 mean that the contribution of patient factors is less than that of non-patient ones (i.e. any community and hospital factors in the model), and so low values are desirable.
| Outcomes | Omega (95% CI): hospital variables included in the model | Omega (95% CI): hospitals included as fixed effects | ||
|---|---|---|---|---|
| HF | COPD | HF | COPD | |
| Readmission | ||||
| 7 days | 18.2 (7.5 to 44.4) | 48.3 (16.6 to 140.7) | 1.8 (1.3 to 2.6) | 3.0 (2.2 to 4.2) |
| 30 days | 32.7 (15.4 to 69.7) | 57.2 (30.6 to 107.1) | 4.4 (3.2 to 6.0) | 9.2 (7.1 to 12.0) |
| 90 days | 89.7 (37.6 to 214.0) | 103.0 (56.1 to 189.1) | 10.2 (7.6 to 13.8) | 16.1 (12.5 to 20.6) |
| 365 days | 141.4 (55.5 to 324.7) | 136.4 (75.9 to 245.6) | 15.5 (11.7 to 20.6) | 23.1 (18.2 to 29.5) |
| ED attendance | ||||
| 7 days | 22.8 (8.8 to 59.3) | 11.2 (6.2 to 23.9) | 0.6 (0.4 to 0.9) | 0.8 (0.6 to 1.1) |
| 30 days | 14.3 (8.5 to 23.7) | 16.9 (11.2 to 25.4) | 0.5 (0.4 to 0.7) | 0.8 (0.7 to 1.0) |
| 90 days | 12.9 (8.9 to 18.6) | 22.3 (15.7 to 31.6) | 0.8 (0.6 to 0.9) | 1.2 (1.0 to 1.3) |
| 365 days | 11.8 (8.9 to 15.8) | 14.2 (10.8 to 18.7) | 0.9 (0.8 to 1.1) | 1.0 (0.9 to 1.1) |
| ED attendance or readmission | ||||
| 7 days | 31.5 (10.7 to 92.5) | 37.9 (15.0 to 96.2) | 2.0 (1.4 to 2.8) | 3.2 (2.3 to 4.3) |
| 30 days | 44.9 (19.4 to 104.0) | 49.4 (27.5 to 89.3) | 4.3 (3.2 to 5.9) | 7.5 (5.9 to 9.7) |
| 90 days | 89.7 (37.7 to 213.5) | 83.7 (47.5 to 147.9) | 8.6 (6.4 to 11.4) | 12.4 (9.9 to 15.6) |
| 365 days | 219.6 (76.6 to 629.5) | 122.8 (65.3 to 230.7) | 11.4 (8.8 to 14.7) | 14.2 (11.4 to 17.7) |
| ED attendance within 7 days or readmission within 30 days | 36.0 (16.3 to 79.4) | 55.1 (29.5 to 102.8) | 4.4 (3.2 to 5.9) | 8.9 (6.9 to 11.7) |
The omega statistics in the left-hand part of Table 13 were much greater than 1 and were highest for measures involving readmissions. The omega statistics in the right-hand part of the table are derived from models in which each hospital was added as a fixed effect in order to account for hospital factors that we had not been able to include in the other models. As expected, these were much lower than the first set, though the patterns were generally similar with the exception that omega statistics were consistently lower for HF than for COPD, rather than mixed. These figures with hospital fixed effects suggest that patient factors became more important as follow-up increased after the index discharge (the CIs were narrower than those for the first set and often do not overlap for readmissions). Both sets of omega statistics suggest that patient factors were of the least importance for ED attendance, although many of the CIs did overlap, so we cannot be certain.
Objective 3: how consistently do hospitals perform across different readmission-type metrics?
Pearson correlations between crude hospital-level rates varied from just 0.22 to 0.98, although most were highly significant (p < 0.001). The correlations were lowest between ED attendance and readmission and strongest between the combined measure and readmissions when both used the same time frame. They were similar for COPD and HF. Correlations between adjacent time frames, for example 7 days and 30 days, were stronger than between time frames further apart in time, for example 7 days and 90 days.
In a small hospital trust (n = 320), the power to detect a change of 1.5 times the national rate was greater than 90% for all outcomes for ≥ 30 days since discharge for either patient cohort. However, for 7 days since discharge the power was reduced to between 40% and 52% for ED attendances or readmissions.
The numbers of hospitals that were outside the 95% and 99.8% funnel plot control limits are shown in Table 14.
| Outcomes | Number of trusts | |||||||
|---|---|---|---|---|---|---|---|---|
| Above control limit | Below control limit | |||||||
| 99.8% | 95% | 99.8% | 95% | |||||
| HF | COPD | HF | COPD | HF | COPD | HF | COPD | |
| Readmission | ||||||||
| 7 days | 0 | 1 | 1 | 5 | 1 | 1 | 7 | 11 |
| 30 days | 0 | 0 | 4 | 6 | 1 | 0 | 8 | 8 |
| 90 days | 0 | 0 | 5 | 6 | 0 | 1 | 7 | 6 |
| 365 days | 0 | 0 | 5 | 3 | 0 | 1 | 4 | 3 |
| ED attendance | ||||||||
| 7 days | 0 | 4 | 9 | 13 | 5 | 12 | 15 | 23 |
| 30 days | 5 | 6 | 21 | 23 | 12 | 18 | 24 | 27 |
| 90 days | 9 | 8 | 23 | 32 | 13 | 15 | 24 | 29 |
| 365 days | 5 | 5 | 21 | 27 | 10 | 13 | 20 | 24 |
| ED attendance or readmission | ||||||||
| 7 days | 0 | 2 | 3 | 5 | 1 | 1 | 7 | 3 |
| 30 days | 0 | 1 | 3 | 6 | 0 | 0 | 9 | 9 |
| 90 days | 0 | 1 | 4 | 3 | 1 | 2 | 7 | 10 |
| 365 days | 0 | 0 | 2 | 3 | 1 | 0 | 6 | 5 |
| ED attendance within 7 days or readmission within 30 days | 1 | 1 | 4 | 3 | 0 | 0 | 8 | 9 |
As expected from the dispersion parameters in the previous section, ED attendance had the most outliers by far; for this outcome there were more outliers for the COPD patient cohort than for the HF cohort (it was less clear for the other outcomes). Figure 2 shows the variation in standardised 30-day readmissions ratios by hospital, with randomly generated two-character labels for the outliers; Figure 3 shows the same but for 30-day ED attendances. These ratios are akin to SMRs and equal the ratio of the observed to the expected number of attendances, multiplied by 100.
FIGURE 2.
Funnel plot of hospital-level 30-day ED attendance rates for HF. SD, standard deviation. The letters in the plot are randomly generated hospital identifiers, to maintain the anonymity of the hospitals. Reproduced with permission from Honeyford et al. 47 Honeyford K, Aylin P, Bottle A. Should emergency department attendances be used with or instead of readmission rates as a performance metric?: comparison of statistical properties using national data [Epub ahead of print 29 March 2018]. Medical Care 2018. https://journals.lww.com/lww-medicalcare/pages/default.aspx (accessed April 2018).
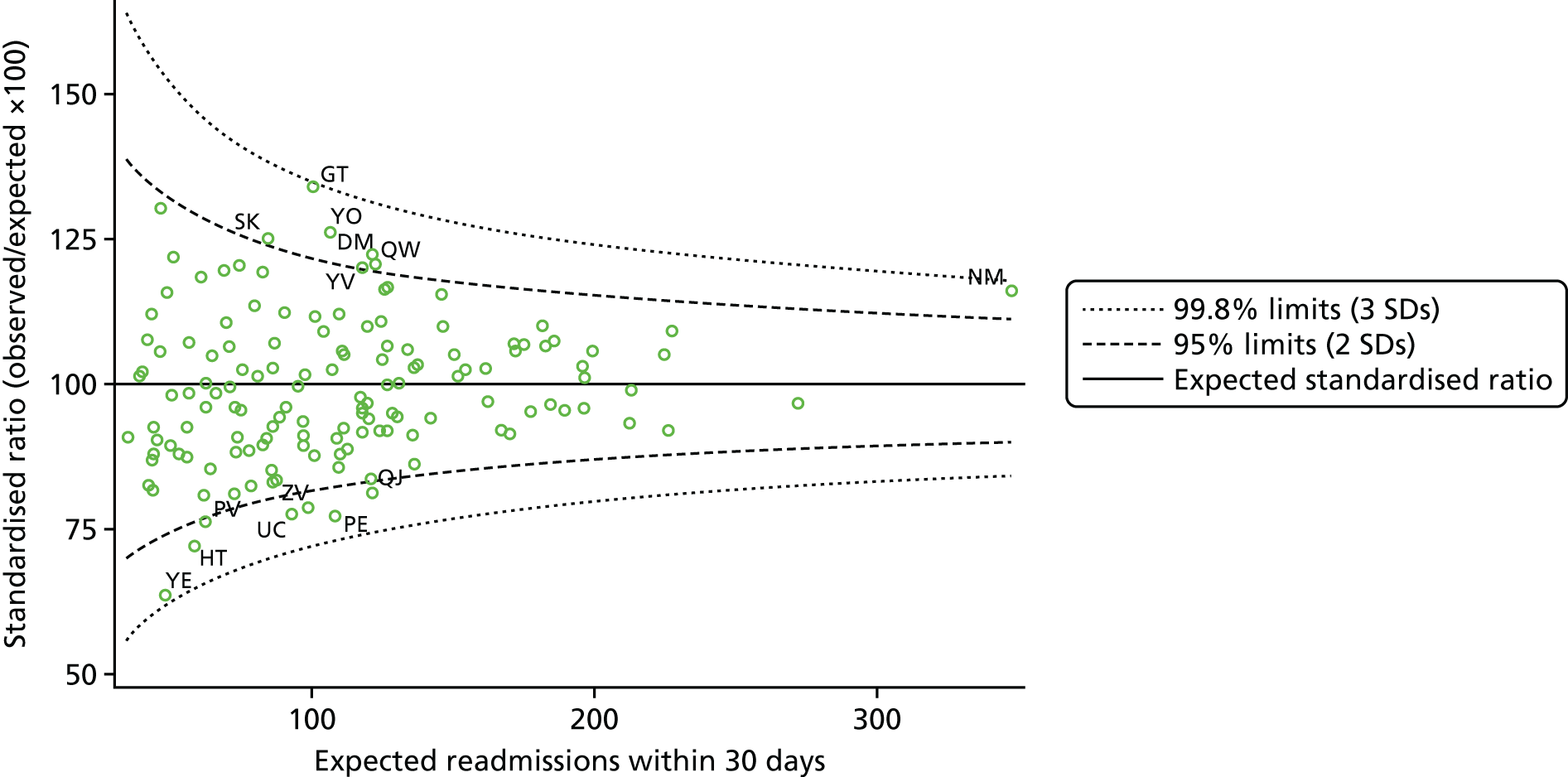
FIGURE 3.
Funnel plot of hospital-level 30-day ED attendance rates for COPD. SD, standard deviation. The letters in the plot are randomly generated hospital identifiers, to maintain the anonymity of the hospitals. Reproduced with permission from Honeyford et al. 47 Honeyford K, Aylin P, Bottle A. Should emergency department attendances be used with or instead of readmission rates as a performance metric?: comparison of statistical properties using national data [Epub ahead of print 29 March 2018]. Medical Care 2018. https://journals.lww.com/lww-medicalcare/pages/default.aspx (accessed April 2018).
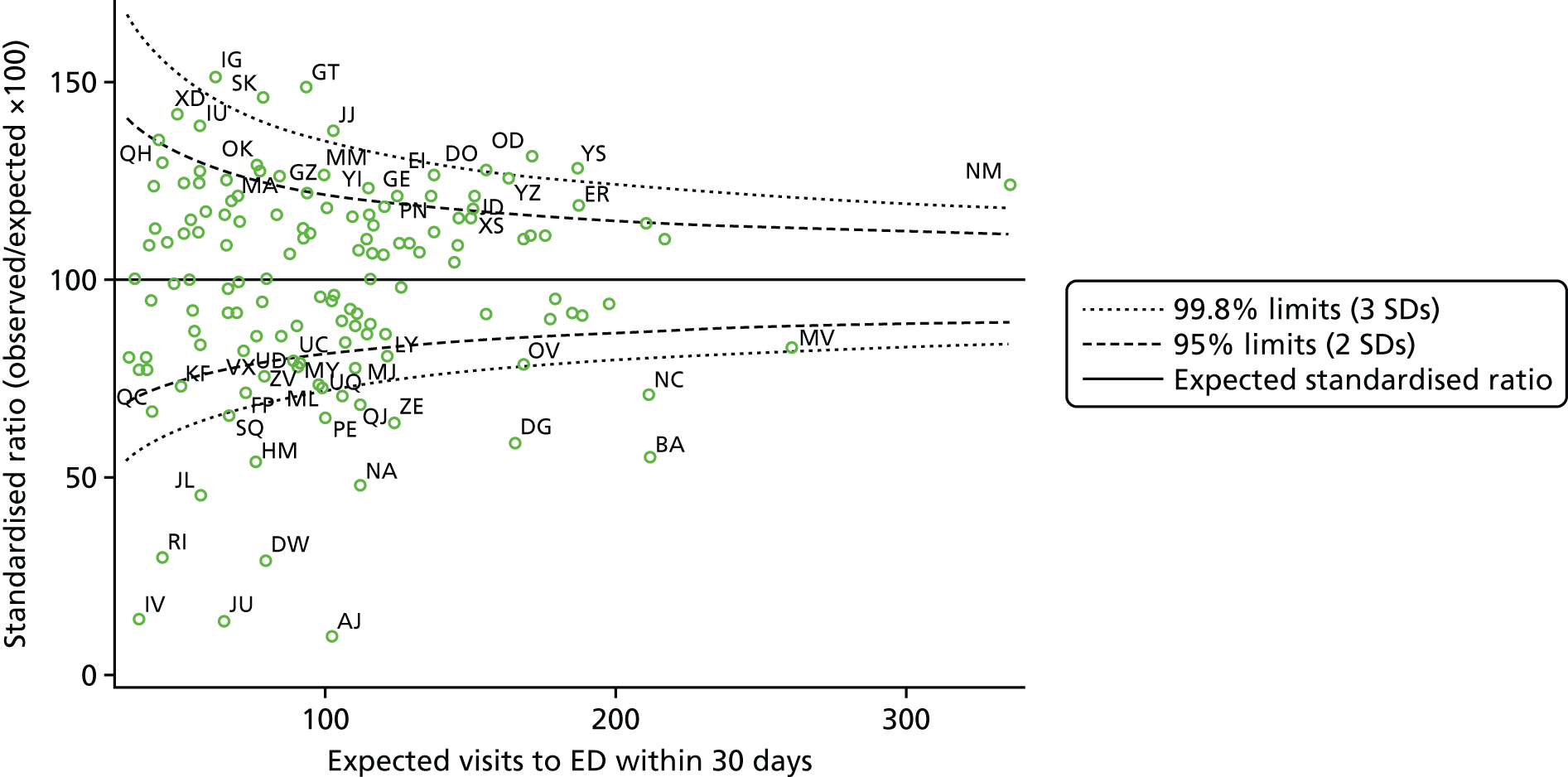
As 30-day ED attendance performed best, according to the results of the previous section, we compared the number of hospital outliers for it against the number for the most established readmission-type measure, 30-day all-cause readmission. The two funnel plots in Figures 2 and 3 show the whole distribution of standardised ratios. Tables 15 and 16 compare the outliers on two measures: ED attendance within 30 days and readmission within 30 days.
| A&E attendance within 30 days | Readmission within 30 days, n (%) | |||||
|---|---|---|---|---|---|---|
| Low | Not outlying | High | Totals | |||
| 3 SDs | 2 SDs | 2 SDs | 3 SDs | |||
| Low | ||||||
| 3 SDs | 0 | 3 (2.1) | 9 (6.4) | 0 | 0 | 12 (8.6) |
| 2 SDs | 1 (0.7) | 2 (1.4) | 7 (5.0) | 1 (0.7) | 0 | 11 (7.9) |
| Not outlying | 0 | 2 (1.4) | 93 (66.4) | 1 (0.7) | 0 | 96 (68.5) |
| High | ||||||
| 2 SDs | 0 | 0 | 16 (11.4) | 0 | 0 | 16 (11.4) |
| 3 SDs | 0 | 0 | 3 (2.1) | 2 (1.4) | 0 | 5 (3.6) |
| Totals | 1 (0.7) | 7 (5.0) | 128 (91.4) | 4 (2.9) | 0 | 140 (100) |
| A&E attendance within 30 days | Readmission within 30 days, n (%) | |||||
|---|---|---|---|---|---|---|
| Low | Not outlying | High | Totals | |||
| 3 SDs | 2 SDs | 2 SDs | 3 SDs | |||
| Low | ||||||
| 3 SDs | 0 | 2 (1.4) | 14 (10.0) | 0 | 0 | 16 (11.4) |
| 2 SDs | 0 | 2 (1.4) | 11 (7.9) | 0 | 0 | 13 (9.3) |
| Not outlying | 0 | 3 (2.1) | 81 (57.9) | 4 (2.9) | 0 | 88 (62.9) |
| High | ||||||
| 2 SDs | 0 | 0 | 15 (10.7) | 0 | 0 | 15 (10.7) |
| 3 SDs | 0 | 0 | 5 (3.6) | 3 (2.1) | 0 | 8 (5.7) |
| Totals | 0 | 7 (5.0) | 126 (90.0) | 7 (5.0) | 0 | 140 (100) |
For HF, two-thirds of hospitals were within the 95% control limits for both measures. No hospital was a 99.8% outlier on both. A total of 35 hospitals were outside the 95% or 99.8% limits on ED attendances but not on readmissions, whereas only three hospitals were outside the 95% or 99.8% limits on readmissions but not on ED attendances.
For COPD, just over half of the 140 hospitals were within the 95% control limits for both measures. As with HF, no hospital was a 99.8% outlier on both. A total of 45 hospitals were outside the 95% or 99.8% limits on ED attendances but not on readmissions, whereas only seven hospitals were outside the 95% or 99.8% limits on readmissions but not on ED attendances.
Objective 4: comparison of results for heart failure and for chronic obstructive pulmonary disease
This has, for the most part, already been covered in previous sections. Patients hospitalised for HF were clearly younger, on average, than those hospitalised for COPD, although the clear majority of both were aged 65 years or over. The COPD group lived in more deprived areas. Comorbidity, particularly hypertension, IHD, arrhythmias and diabetes, was common in both groups; COPD was recorded in one-quarter of HF patients, and HF was recorded in one-tenth of COPD patients. Index inpatient stays were longer for the HF cohort.
One-year mortality and post-index ED attendance and readmission rates were high in both cohorts of patients. In general, we can say that the statistically significant patient predictors of readmission and mortality (which included age, index LOS and comorbidity) and the predictors of hospital-level ED attendance and readmission rates were largely similar. The relation between doctors per bed and both 1-year mortality and 30-day readmission was significant and similar for the two groups, as was the relation between hospital bed numbers and readmission, but the other hospital, community and GP factors that we tried were either not significant for either group or inconsistent. Model discrimination (c-statistic) was higher for the COPD cohort for both main outcomes. We will return to the specific differences in Chapter 4.
Variations in ED and readmission rates by hospital were similar for the two conditions, as were the omega statistics and funnel plot analyses.
One new comparison that we made, specific to this objective, was for hospital-level RRs for the HF and COPD cohorts, which were compared using simple correlation. The Spearman coefficient was 0.30 (p < 0.0001) for 30-day readmission, 0.81 (p < 0.0001) for ED visits within 30 days and 0.58 (p < 0.0001) for 1-year mortality. In contrast, when we considered diagnosis-specific readmissions for each cohort, dividing them into those for the index condition and those for any other primary diagnosis, the correlations were much smaller and not statistically significant: 0.11 (p = 0.20) for HF versus non-HF readmissions and 0.03 (p = 0.75) for COPD versus non-COPD readmissions.
Chapter 4 Discussion of methods and findings
We set out to better understand the drivers of readmissions and mortality following an index hospital admission for each of two common, serious and costly chronic diseases; this index admission is not merely a convenient starting point for follow-up but represents an important milestone in the disease history. We combined patient-level administrative databases with practice- and hospital-level information and explored patient post-index use of ED departments. In this chapter, we first discuss the results of each of our objectives in turn in the context of what is already known in the literature. We then note the main limitations to our analyses and suggest further work.
Comparison with previous work
Objective 1: what are the main patient, primary care and hospital factors associated with variation in readmission and mortality rates?
In these two elderly, multimorbid cohorts, hospital use and 1-year mortality rates were high. A number of studies reported survival from diagnosis, but we were interested in survival since first admission. One-year mortality following HF admission has been estimated at between 20% and 40% in the review by Cowie et al. ;48 the most comparable figure in terms of population and methodology, although not time period, is 45% from Scotland in the decade to 1995. 49 Rates have fallen since, and our figure was 40%. In-hospital mortality for COPD varies from between < 10% and 60%, based on the severity level of the population studied50 and the 1-year rate is about one in four (28% for Canadian discharge data between 2001 and 2004;51 and 25% within 1 year of admission for the first acute exacerbation between 2005 and 2007 in Taiwan). 52 Our mortality rate within 1 year of an index COPD admission was 24%.
We found the main predictors for 1-year mortality for both conditions to be similar, and they included older age, male sex, non-white ethnicity, severity proxies, prior missed OPD appointments, LOS of ≥ 1 night and a number of comorbidities, such as prior stroke, pneumonia, renal disease, cancer and cognitive impairment. Hospital and GP factors that we considered were sometimes significant but small in size. Other studies have also found higher odds for older age,51,53,54 severity,51,55 comorbidities such as HF (in COPD patients), cancer, renal failure,55 depression56 and diabetes. 53 For COPD, Fidahussein et al. 54 also found an association for LOS. Not all studies reported sex differences, although some agreed with our finding of higher odds for males for both conditions. 51,55
We found that one in five HF and one in six COPD patients were readmitted for any cause within 30 days of live index discharge. These rates are slightly lower than those reported for the US Medicare population (25% for HF57 and 20% for COPD58) or 21% from the wider US population using the Nationwide Inpatient Sample,59 although much lower rates have been reported for a private insurance plan in the USA (8%). 60 Some studies also reported disease-specific readmission rates (e.g. 7% for acute lower respiratory infection or acute exacerbation for COPD in New Zealand61 and also 7% for COPD or bronchiectasis in the USA),59 showing that readmissions within 30 days for the index condition comprise about one-third of the total, as in our study.
In our analysis, readmission was more likely for both index conditions with older age, missed outpatient appointments and a number of comorbidities such as IHD, renal disease, cognitive impairment, mental health conditions and pneumonia. LOS was significant for both, but the pattern differed, with same-day discharges for HF and 2-night stays for COPD having the highest odds of readmission. Males had significantly higher odds for COPD. Larger hospital size and fewer doctors per bed were both associated with higher odds, although teamworking rating by staff remained in the model for COPD only. No GP factors that we considered were retained. Other studies had also noted the association for older age and comorbidities,54,60,62 although the much-cited systematic review of risk adjustment and risk prediction models for readmission in HF patients by Ross et al. 62 found much inconsistency in the selection of variables across models, with the exceptions of age, sex and race/ethnicity. We did not find any statistically significant difference in odds by sex for HF or by ethnicity for either cohort, except lower odds for the ‘not known’ group, which we commonly find in our models,11,18 and may relate to this group consisting more of overseas patients and others with lower follow-up rates. For the COPD cohort, we found higher odds of readmission in deprived areas, and in US studies, race/ethnicity often captures the deprivation aspect. Sharif et al. 60 found an association for COPD patients with LOS (higher odds if < 2 or > 5 days, whereas for us it was 2 days) and several quality-of-care aspects, such as prescribing and follow-up.
Objective 2: should accident and emergency attendance and reattendance data be considered alongside readmission metrics when measuring hospital performance in terms of unplanned activity? If so, how?
Much less attention in the literature has been paid to ED attendances after inpatient discharge than to rehospitalisation. This gap was recognised by Brennan et al. ,63 who, like us, examined 30-day ED utilisation and all-cause readmissions following a hospital admission, albeit for all patients combined rather than for any given index condition. With their national US data, they found that nearly one in five patients presented to the ED within 30 days of an inpatient hospitalisation and over half of these patients were readmitted. They concluded that ‘readmission measures that incorporate ED visits following an inpatient stay might better inform interventions to reduce avoidable readmissions.’ Vashi et al.,64 in the USA, looked at ED visits within 30 days after discharge and also a combined measure of ED visits (not resulting in admission, or ‘treat and release’) or readmission within the same time frame, a rare example of combining the two as we have done. They reported figures overall and for some high-volume conditions. For HF and shock, their 2008–9 rates were as follows: ED treat and release, 9.6%; readmission, 27.7%; and combined (i.e. ED visit or readmission), 37.4% within 30 days. Our rates for HF were all lower: 4.0% for ED treat and release, 19.8% for readmission and 22.7% for combined within 30 days.
In our HF cohort, 6.2% visited the ED within 7 days and 17.4% with 30 days; for the COPD cohort these figures were 5.3% and 15.5%, respectively. Most figures in the literature are from the USA. A total of 22.5% of Medicare beneficiaries came to the ED within 30 days after hospitalisation for HF, with congestive HF the most common reason for attendance, although accounting for only 11% of these visits. 65 The same study noted wide hospital-level variation in post-discharge ED visit rates for each condition: acute myocardial infarction (AMI) (median 8.3%, 5th to 95th percentile 2.8% to 14.3%), HF (median 7.3%, 5th to 95th percentile 3.0% to 13.3%) and pneumonia (median 7.1%, 5th to 95th percentile 2.4% to 13.2%). The authors concluded that ‘policymakers and researchers should further study post-discharge ED visits as measures of health-care access and care transitions in the vulnerable Medicare population.’ Another US study found that 84% of patients who present to the ED with acute HF are admitted, with some regional variation but huge differences by insurance status. 66 With HES A&E data, we were unable to identify whether the ED visit was for HF or COPD.
Studies on COPD focused on ED presentations for acute exacerbations rather than all ED visits as with our study. In 16 EDs across Canada, 49% of 501 patients with acute exacerbations of COPD who were interviewed had been admitted. 67 In a Spanish cohort also covering 16 EDs, 62% of patients were admitted on arrival for a COPD exacerbation. 68 For our two cohorts, approximately three in four ED visits for any reason within 30 days resulted in admission. These conversion rates were higher than for all ED visits in England during the same period according to published figures from HES: for those aged < 65 years attending between 2009/10 and 2012/13, only 18% resulted in admission, whereas for people aged 65–84 years the figure was 50% and for people aged ≥ 85 years it was 63%. 69
Regarding the factors associated with admission at an ED visit, there have been a few studies covering all ED attendances. Using Healthcare Cost and Utilisation Project data, Pines et al. 70 found higher ED admission rates to be associated with a range of factors such as more inpatient beds, higher hospital occupancy rates and, at county level, more primary care physicians per capital.
An analysis of the 2010 National Hospital Ambulatory Care Survey ED data of hospitals with admission rates from the ED of between 5% and 50% did not report the proportion of ED visits resulting in admission, but focused on the variation in ED admission rates by hospital after accounting for patient and hospital factors: ‘even after accounting for hospital teaching status, ownership, urban/rural location, and geographical location, 7.0% of the variation in risk-standardized hospital admission rates from the ED was still attributable to an institution-specific effect.’71 Another US study, from three hospitals, assessed the variation in rates of admission from the ED by individual physician. Among the 89 attending emergency physicians, admission rates varied from 21% to 49%, similar to previous Canadian studies. 72 Taken together, these US studies show appreciable variation in ED admission rates by hospital, some of which can be explained by specific hospital factors but some that cannot, with individual physician decision-making being one likely contributing factor to the variation.
There have also been some studies specific to HF and COPD patients. These have found higher odds of ED admission for age,66,67 various markers of COPD severity and respiratory distress,67,68 comorbidity and lower levels of social support, but not sex, although Vidal et al. 68 did not retain comorbidity or social support in their final model. We also found effects of older age (although Vidal et al. 68 did not, perhaps because of their detailed physiological variables) and comorbidity for the COPD group. Unlike Pines et al. ,70 we found no association between higher odds of ED admission and hospital size or quarterly occupancy levels, but instead found that the number of patients admitted during the hour of arrival – a likely proxy for bed availability and hence lower occupancy at that time – to be positively associated with the odds of admission. For HF patients, admission was more likely if they arrived during a period of long ED waiting time, as measured by the proportion seen within 4 hours. We are not sure why this would be the case, other than a chance finding. We have not seen other studies that have assessed this effect in this way.
In summary for this objective, previous studies have highlighted patient factors such as age and disease severity, and the role of the hospital and attending physicians in predicting the odds of admission from the ED. Our findings also show the importance of those patient factors plus the hour of attendance and the number of patients admitted at the same hospital during the hour of arrival. Some commentators have advocated the use of ED visits, perhaps in combination with readmissions, as a performance metric63,64 but without giving clear guidance on how this should be done. We took a statistical approach to comparing the different options and also found considerable variation in hospital-level rates, particularly for ED visits. Variations in readmission rates were consistent with random (binomial) variation as suggested by the dispersion parameter of near one, the small number of funnel plot outliers and low ICCs. In contrast, the ED visit rates showed a lot more than purely random variation – they were very overdispersed, generating many more funnel plot outliers. Before an indicator that shows such overdispersion can be used in practice, the reasons for the extra variation should be sought. Spiegelhalter35 warns against using indicators where the reasons are largely related to data quality flaws or inadequate risk adjustment. The ICC and omega statistics are useful here, and both showed bigger hospital ‘footprints’ for ED visits than for readmissions. Our a priori suggestion of combining visits within 7 days with readmission within 30 days performed no better than the standard readmission within 30 days, and the other combinations were also inferior to the straightforward ED visits. After including hospitals as fixed effects in the model, as per Brown et al. ,33 in order to capture remaining hospital factors for which we did not have data, only ED visits had omega statistics of < 1, with 95% CIs that excluded one for HF. This means that the influence of the hospital was greater than that of the patient in explaining hospital-level rates, a desirable property.
Objective 3: how consistently do hospitals perform across different readmission-type metrics?
We assessed this using simple correlation and by counting funnel plot outliers with 95% and 99.8% control limits. Correlations varied greatly. There was moderate correlation between 30-day ED visit rates and 30-day readmission rates (ρ just above 0.5); adding ED visits to readmissions made little difference to the relations between ED visits alone and readmissions alone. We have found before that even very high correlations, where ρ exceeds 0.9, the effect on which hospitals are labelled as funnel plot outliers is unpredictable: up to 10% of hospital trusts changed outlier status when different risk adjustment models were used in deriving hospital mortality rates. 73 With much lower correlations in this project, we found that many trusts had different outlier status depending on the measure used. ED visits generated more outliers than readmissions, although when hospitals were flagged as outliers on both measures, it was usually the same set of hospitals for both measures.
Objective 4: are the results for chronic obstructive pulmonary disease similar to those for heart failure?
The two cohorts had several similarities, such as having the clear majority aged ≥ 65 years with, often, a number of comorbidities – including the other condition – plus high use of the ED and inpatient wards after their index discharge and high mortality at 1 year. Many of the patient factors that we included, such as age, index LOS and comorbidity, were predictive of readmissions or mortality for both conditions, although the patterns for the index LOS differed. Likewise, the relations between more doctors per bed and lower odds of both 1-year mortality and 30-day readmission were significant and similar for the two groups, as was the relation between more hospital bed numbers and lower odds of readmission, but the other hospital, community and GP factors that we tried were usually not significant for either group or sometimes inconsistent.
Patterns of hospital-level rates of ED visits and/or readmissions were very similar for the two cohorts. Furthermore, there was significant but modest correlation between the rates for HF patients and the rates for COPD patients for readmissions and strong correlation between the rates for ED visits within 30 days. Correlation for 1-year mortality was moderate (ρ = 0.58). In contrast, we found no relation between readmission rates for the index condition (HF or COPD) and rates for all other conditions combined. Although the literature is large on the relations between performance on different measures or process versus outcome rates by health-care unit, there have been few such comparisons of rates for different index conditions, especially for readmissions. One example is the study by Horwitz et al. 74 The authors compared publicly reported 30-day risk-standardised mortality and readmission rates for Medicare patients admitted with AMI, HF and pneumonia across the USA. Every mortality measure was significantly, but modestly, correlated with every other mortality measure (range of correlation coefficients, 0.27–0.41; p < 0.0001). Every readmission measure was significantly but also modestly correlated with every other readmission measure (range of correlation coefficients, 0.32–0.47; p < 0.0001, similar to what we found with HF vs. COPD). Coefficients were higher among larger hospitals, presumably at least in part because large hospitals’ rates are estimated more precisely. The authors concluded that ‘that there may be common hospital-wide factors affecting hospital outcomes’. If this is the case, then improvement efforts might be better focused on hospital-wide, rather than simply disease-specific, activities. The authors also suggested that correlation could vary according to hospital characteristics; for example, smaller or non-teaching hospitals might be more homogeneous in their care than larger ones. Horwitz et al. 74 had over 4000 hospitals on which to run such subanalyses, but we would have much less statistical power with HES to do this in England.
Finally, for each cohort we considered whether or not a hospital with high (or low) readmission rates for the index condition also had high (or low) readmission rates for any other primary diagnosis in the same set of patients. We found no such correlation for either HF or COPD patients. We are not aware of other literature on this, apart from our earlier HF analysis. 15
Study strengths and limitations
This project has benefited from the use of comprehensive, national data by a team experienced with using them, a range of other publicly available information on GP and hospital quality and activity and an objective, statistical approach to assess how one might go beyond the standard 30-day all-cause readmission to explore other indicators of unplanned hospital activity. We focused on two common patient groups and outcomes that are of interest to patients, the NHS and policy-makers.
The principal limitations concern the data and, in particular, the quality and scope of some of the fields and sources. Although the high accuracy of the primary diagnosis and the primary procedure fields are well established for administrative data,75 the sensitivity of the recording of comorbidities is known to be modest and variable by hospital, imparting an unknown amount of bias. Despite this, studies comparing the electronic data with case note reviews have shown consistent results in terms of ORs for the outcomes modelled, which we review elsewhere. 25 Administrative data, such as HES, lack a number of important case mix details regarding disease or symptom severity. For our two conditions, these include ejection fraction and FEV1, and markers, such as brain natriuretic peptide, that are associated with poorer outcomes. 76 Although some were significantly associated with the outcomes, our proxies of intensive therapy unit bed use (variably recorded in HES), bronchoscopy, oxygen support and ventilation are not likely to capture severity very well. This will have contributed to the modest discrimination of our models and could have led to some of the unexplained variation between hospitals in their outcome rates. Other studies already mentioned have shown notable influences of hospital factors on ED use and readmission rates,71–73 so residual confounding is unlikely to explain all the between-hospital differences in our study.
The HES A&E records have now improved in coverage since our first NIHR-funded study and are useful in terms of the fact, date, hour and outcome of attendance, but still not for the reason for the attendance as a result of both high levels of missing values and the restrictive coding system used. We were therefore unable to distinguish visits for HF or COPD from visits for other reasons, whereas some US-based studies have been able to focus on the odds of admission for specific conditions. 64,77
The HES OPD records also still lack usable diagnosis information, so we were unable to tell what the reasons for appointments were. We found both attendance and non-attendance at appointments prior to the index admission to be strong predictors of adverse outcomes but could not distinguish between those for HF or COPD and those for other conditions. This would be interesting to explore if data quality improves in the future.
Except for the QOF data, the non-patient-level information from surveys was at hospital level and not specific to either of our two conditions. This may be why we found few significant associations with these variables, even though our other results suggest hospital-level influences on ED use and outcomes. Our measures of ED ‘busyness’ were calculated at hospital level for all patients, which, for this aspect of care, is the most relevant level. Although we found some significant associations, we did not have information on ED beds or patients needing one-to-one care, both of which are elements of the National ED Overcrowding Study index. 78
Despite the support of existing national audit organisations, the response rate to our questionnaire was too low to allow it to be used; the survey of websites did not yield any statistically significant predictors of the two main outcomes, although it did provide some interesting variations in themselves. We do not think that this has lost any important insights. A more fruitful, but logistically difficult, approach would be to link records at an individual patient level (our survey was, in any case, only at the level of the rehabilitation programme) from the audits to HES and ONS death files. This has been done in the past for the Myocardial Ischaemia National Audit Project and is currently being explored for the national HF and COPD audits.
With the changing NHS landscape, it would be interesting to repeat some of this work, on ED attendances in particular, with more up-to-date data. Delays in obtaining linkage to the ONS mortality file restricted us to using records up to March 2012, as we received more recent death information too late in the project to use.
We also recognise some limitations to our analysis. We used two primary outcome measures (30-day readmissions and 1-year mortality), both of which use common, but arbitrary, time frames. For readmissions and ED visits, we also tried models for a 7-day window but at the cost of an appreciable loss in statistical power. It was possible that covariate effects differed in the early post-discharge period from later, partly in view of the competing risk of death when modelling non-fatal outcomes, and we therefore applied two different survival analysis methods. The resulting HRs were similar to the ORs from logistic regression, which gives us confidence in the latter’s coefficients and that our choice of time frames did not affect the findings.
We had to decide which of the many PE survey variables to include. Although we did a lot of testing and asked our PPI representatives for variables of a priori interest, it is nonetheless possible, but perhaps unlikely, that other variables could have shown relations with the outcomes.
We fitted a number of models and, therefore, used a lower p-value threshold (p < 0.01). Although many covariates had p-values of < 0.001, there could still have been some false positives results near our chosen 0.01 threshold.
Recommendations for future research
We used the concept of an index admission to ‘start the clock’ and follow patients up, as this represents an important milestone in the progression of disease and simplifies the modelling. For readmissions, more sophisticated approaches, such as multistate models to deal with multiple hospital contacts or cluster analysis to look for patterns of activity, could be usefully explored. DeLia et al. 79 used multinomial probit regression to look at three outcomes at once: no ED attendance, ED attendance and readmission.
Time-to-event methods could be applied to determine the extent to which variation in hospital-level performance is explained by differences in the timing of outcomes. For example, when do the ‘excess’ deaths or readmissions occur at hospitals with high rates compared with those with low rates? Some recent evidence for HF patients suggests that the timing of readmissions within the standard 30-day follow-up period does not vary in Medicare patients,57 but this is not well established with other populations, patient groups or outcomes.
We had access to only aggregated primary care information. For HF, we have begun work using the Clinical Practice Research Datalink (CPRD) to explore the use of primary care consultations and management on patient outcomes and post-discharge follow-up. CPRD is linked to HES and the ONS death registry. We are currently exploring the use of local or regional databases that include linkage to social care activity.
There is surprisingly little work done on variation by hospital of ED visits, especially after an index discharge or in the UK. The diagnostic coding in HES A&E data limits what one can do to explore the reasons for the visits, but broad categories could be used if the proportion with missing values falls in the future. The relation between OPD follow-up and subsequent outcomes could be assessed using HES, although the non-random character of such follow-ups would need to be considered in the analysis, for example if a suitable instrument could be found for an instrumental variable analysis. Hernandez et al. 80 addressed this ‘endogeneity’ (hospitals will ask to see higher-risk patients in OPD, who are also at the highest readmission risk) by analysing the association between hospital-level rates of follow-up visits within 14 days of discharge and patient-level likelihood of readmission. They found that HF patients who were discharged from hospitals with higher follow-up visit rates were less likely to experience readmission within 30 days.
Dissemination activity
Up until May 2017, we have disseminated various findings from this project as follows:
-
Oral presentation of Use of Hospital Services by Age and Comorbidity After an Index HF Admission in England. Health Services Research UK, Nottingham, 6–7 July 2016.
-
Bottle A, Goudie R, Bell D, Aylin P, Cowie MR. Use of hospital services by age and comorbidity after an index heart failure admission in England: an observational study. BMJ Open 2016;6(Suppl. 6):e010669.
-
Oral presentation accepted on patient journeys through A&E at the International Health Conference, Oxford, 29 June–1 July 2017.
-
Poster accepted on The Relation Between Length of Stay, A&E Attendance and Readmission for Heart Failure Patients. British Cardiovascular Society Annual Conference, Manchester, 5–7 June 2017.
-
Oral presentation accepted on How Many Hospital Websites Provide Information to Attract Patients to Attend Cardiac/Pulmonary Rehabilitation Across England? The International Society for Quality in Healthcare (ISQua), London, 2–4 October 2017.
-
Poster accepted on How Many Pulmonary/Cardiac Rehabilitation Services are Available per CCG in Relation to Prevalence of COPD & Heart Failure? The International Society for Quality in Healthcare (ISQua) in London, 2–4 October 2017.
-
Poster accepted on What Happens to Heart Failure and COPD Patients After Discharge from Hospital? Predictors of Emergency Department Activity. The International Society for Quality in Healthcare (ISQua) in London, 2–4 October 2017.
-
Oral presentation accepted on Comparison of Hospitalisation and Mortality for Patients with Heart Failure in England and Lombardy Region. The International Society for Quality in Healthcare (ISQua) in London, October 2017. This was not a specific objective of the project but an interesting additional use of the project’s data extract. A paper on this has now been published online:
-
Bottle A, Ventura CM, Dharmarajan K, Aylin P, Ieva F, Paganoni AM. Regional variation in hospitalisation and mortality in heart failure: comparison of England and Lombardy using multistate modelling [published online ahead of print 28 July 2017]. Health Care Manag Sci 2017. https://doi.org//10.1007/s10729-017-9410-x.
-
Two more papers have been submitted to journals and are under review, with others in preparation. We are working with relevant charities to decide on the best way to publicise our work relating to HF. With the help of Pumping Marvellous [http://pumpingmarvellous.org/ (accessed 26 October 2017)], the HF charity, we submitted a grant application for follow-on funding and await the decision.
Chapter 5 Conclusions
This project followed on from our previous NIHR-funded project that produced a risk adjustment methodology for use with HES data and outcome measures, such as readmission. We also showed, in our previous project, the importance in patients with HF of considering readmissions for HF separately from readmissions for other conditions, in addition to looking at all-cause readmissions. The project also revealed the strong association between missed OPD appointments and readmissions. We brought this learning into the current project to investigate the influences of a range of factors on patient outcomes for HF but also for COPD.
Patient factors for 30-day readmission and 1-year mortality following a first emergency admission for HF or COPD included older age, comorbidities, prior missed OPD appointments, index LOS and COPD severity proxies. These largely agree with previous studies, with two novelties. Although we have found an association with prior missed OPD appointments for HF patients before, we are not aware of this having been shown for COPD patients. We suspect that missing appointments indicates some mix of disease severity, life circumstances, personal priorities and health system organisation and administration, none of which could be captured very well with our data. Index LOS has been found to predict readmission before, but we found different patterns from those in the literature (highest odds for readmission were seen with same-day HF discharges and 2-night stays for COPD) and also a positive association with 1-year mortality. The only consistent associations with our two outcomes for the hospital, community and GP factors were doctors per hospital bed (both outcomes) and hospital size (readmissions only). Some predictors differed when we considered diagnosis-specific readmissions, but age, comorbidities and prior missed appointments were always important.
The use of the ED after the index admission ends is common in both patient groups, with around one-quarter of the first visits not ending in admission. We found that the odds of admission from the ED were lower in young COPD patients and higher in elderly HF patients compared with people in their late sixties. Admission was least likely during the day shift and more likely if other patients were being admitted that hour, although no weekday effects or ED busyness effects were retained in the final model.
Although one in four first ED visits and one in three or more subsequent ED visits did not end in admission, only about three-quarters of 30-day readmissions came through the ED. This reduces the overlap between readmissions and ED visits, reflected in there being less than complete overlap between the predictors of each and the big differences in the distribution of hospital-level rates for each. Although readmission rates were fairly consistent with the binomial distribution, ED rates showed a lot of overdispersion. However, the omega statistics suggest that it is readmissions that are more affected by patient factors than ED visits; this suggests that the hospital ‘footprint’ is larger for ED visits and that they would have greater use as a performance measure than the commonly used readmission rate. There has been much less attention in the literature on ED visits than on readmissions.
Lastly, we found that the results for our two chronic conditions had a lot in common with each other. Hospital use before and after the index admission was high. The associations between the outcomes and factors such as age, comorbidity, index LOS and prior missed OPD appointments were consistent, as were the absence of associations for aggregate-level variables, particularly those relating to primary care. Hospital-level ED use and readmission rates had similar distributions for each, and there was moderate correlation between readmission rates for the HF patients and for the COPD patients. In contrast, in the HF group, readmission rates for HF showed no relation with rates for non-HF diagnoses, and the same was true for the COPD group when readmissions were dichotomised into those for COPD versus those for other conditions. These findings have several implications for practice and policy, which we now consider.
Implications for practice and translation of findings
Several messages from this project are of relevance to policy regarding patient management and performance indicators. The first is the importance, for both HF and COPD, of comorbidity and missed outpatient appointments. As we found, both patient groups are known to have high levels of comorbidity, including the other condition, and this increases the odds of poor outcomes. 56,62,81 Disease-specific readmission rates showed that a hospital’s readmission rate for the index condition bore no relation to its readmission rates for all the non-index conditions. One possible explanation for this would be if the medical team focused only on stabilising the HF or COPD during the index admission, and left the comorbidities for another time. New HF guidelines recommend greater emphasis on comorbidities. 48 We, and others, had previously found that patients who miss their appointment go on to have poorer outcomes 1 year later, an association that was unlikely to be explained wholly by disease status or comorbidity. 11 Our current project found strong relations with both outcomes for both conditions. This suggests that repeat non-attendance should be considered a warning sign that closer monitoring is needed. The proportion of readmissions that could be prevented with better care depends largely on the definition of ‘preventable’ and the population in question, but it is considerable; in their June 2007 report to the US Congress, the Medical Payment Advisory Commission estimated that 75% of Medicare readmissions are potentially preventable. 82 Much has been written about strategies for reducing readmissions in patients with chronic conditions such as HF and COPD, such as continuity of care and better co-operation with community teams,83,84 medication reconciliation,84 self-management,85 health education, telemonitoring,83 pulmonary rehabilitation,86,87 and care bundles,88 although the evidence base in terms of randomised controlled trials for reducing COPD readmissions is disputed89 and a multifactorial approach is needed. 84 We also note that our analysis found that mortality and readmission were more likely at hospitals with fewer doctors per bed and that readmission was also more likely at smaller hospitals. This suggests that some service design elements contribute to outcomes, although this volume–outcome relationship, commonly seen in surgery, is not direct evidence in favour of centralisation as it will be a proxy for other hospital characteristics. It should also be considered in the context of the modest variation in mortality and readmission rates that we found between hospitals. Despite such modest inter-hospital variation, the monitoring of these rates over time at a given hospital is still important as part of its quality improvement efforts.
A second possible explanation concerns the length of the index stay. The odds of readmission were highest for stays of zero nights for HF and 2 nights for COPD, whereas the odds of mortality rose linearly with LOS. For mild exacerbations of HF requiring only a short stay, a few hours might not be long enough; COPD stabilisation takes longer, but it could be that, for some patients, 2 nights was not long enough. Imperfect risk estimation by the medical team and pressure on inpatient wards at high occupancy could lead to premature discharges. In addition to the growing numbers of delayed transfers of care in recent years since data collection began in 2010,90 we note from our analysis that the lowest-scoring questions in the national PE surveys were the same in all years and all settings, and concerned information at discharge.
A third point to note is that post-index ED attendances were common, and three in four resulted in hospitalisation. We derived several proxies for ED and ward busyness from HES A&E records to explore the factors influencing the odds of admission at these visits. Although we found several crude associations, such as admission being least likely on a Sunday or during the day shift and more likely with the number of boarders, the number of very elderly patients waiting, busyness of the ED relative to its own maximum and the 4-hour waiting target being breached during the hour of arrival, most of these effects disappeared in the regression models. The two remaining factors were time of day of arrival, with lowest odds of admission still during the day, and the number of patients being admitted that hour, whereby the odds went up the more patients were admitted, probably indicating spare inpatient capacity. We found no weekend effect. Daytime arrival might indicate lesser symptom severity than evening or night-time arrival that our data were unable to capture. Alternatively, it could reflect temporarily lower inpatient availability, as many hospitals struggle to discharge their patients before noon, or differences in staff decision-making. Obermeyer et al. 91 looked at deaths in people sent home from the ED, a patient safety issue, and found a relation at hospital level between conversion rates and post-discharge death rates within 7 days. Decision-making in the ED must consider the risk of short-term outcomes, and risk stratification for conditions such as HF and COPD is challenging. Collins and Storrow92 concluded, in 2009, that no ‘simple, validated methods to assess low-risk acute HF in the ED exist,’ although by 2017 two Canadian studies reviewed by Miro et al. 93 had set out with that aim. Shortness of breath is a frequent complaint in patients presenting to EDs, but only a small proportion of them will have HF or COPD. Making the diagnosis, characterising the disease, particularly for HF, and deciding on the need for an inpatient stay is difficult given the multiple aetiologies for HF, the frequent presence of comorbidity for both conditions and psychosocial and non-medical factors. Furthermore, for many patients, the response to treatment for their acute HF treatment is not immediate, requiring an observation period ranging from a few hours to 48 hours. However, not all EDs worldwide can provide this observation time frame, and patients may end up being admitted to the hospital. 94
Finally, ED visits following an index inpatient spell are increasingly recognised as an important issue, irrespective of whether or not they result in hospitalisation as an inpatient. 64,65 They can be for another deterioration of the HF or COPD or for one or more of the patient’s various comorbidities, and can of course be for a totally new medical or surgical problem unrelated to either. They can also occur for a wide range of other reasons, for example problems with discharge medication, patients’ lack of confidence in their ability to self-manage, health-care access and fragmented care transitions. Other commentators have suggested that ED visits be used as a performance measure and that we could learn from EDs with low admission rates but good outcomes. 94 However, there is limited information on variation in visit rates by hospital, especially outside North America, and even less on the statistical properties of such measures. We have shown how an objective statistical approach can shed light on the debate over the most appropriate indicators. Before measures such as 30-day ED visits are adopted, one must also take into account stakeholder views and submit the measures to field testing.
Acknowledgements
We thank Sherry Morris for her key contribution to the smooth administration of the project and for creating and revising the project website. We are, of course, indebted to our database manager, Hima Daby, for maintaining our HES databases.
We thank our two project PPI representatives, Jill Wakefield and Peter Owen, for sharing their experiences of the NHS as patients with heart conditions and for their input in selecting PE variables for objective 1 modelling and commenting on lay summaries of outputs. We thank Jill for reviewing our manuscript on PE surveys and the lay summary for this report.
Contributions of authors
Alex Bottle (Reader in Medical Statistics) managed the project, prepared the HES data extracts, oversaw and contributed to the analysis and drafted the report.
Kate Honeyford (Research Associate) obtained the non-HES data, performed all statistical analyses and contributed to writing of the report.
Faiza Chowdhury (Medical Registrar) provided clinical input into some of the manuscripts and contributed to writing the report.
Derek Bell (Professor of Acute Medicine) provided clinical and policy input into the project and associated manuscripts.
Paul Aylin (Clinical Professor in Epidemiology and Public Health) contributed to the management of the project and to writing of the report.
Publications
Bottle A, Goudie R, Bell D, Aylin P, Cowie MR. Use of hospital services by age and comorbidity after an index heart failure admission in England: an observational study. BMJ Open 2016;6(Suppl. 6):e010669.
Bottle A, Ventura CM, Dharmarajan K, Aylin P, Ieva F, Paganoni AM. Regional variation in hospitalisation and mortality in heart failure: comparison of England and Lombardy using multistate modelling [published online ahead of print 28 July 2017]. Health Care Manag Sci 2017. https://doi.org/10.1007/s10729-017-9410-x
Honeyford K, Greaves F, Aylin P, Bottle A. Secondary analysis of hospital patient experience scores across England’s National Health Service – how much has improved since 2005? PLOS ONE 2017;12:e0187012.
Honeyford K, Aylin P, Bottle A. Should emergency department attendances be used with or instead of readmission rates as a performance metric?: comparison of statistical properties using national data [Epub ahead of print 29 March 2018]. Medical Care 2018.
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Sutherland K. Bridging the Quality Gap: Heart Failure. London: The Health Foundation; 2010.
- Invisible Lives. Chronic Obstructive Pulmonary Disease (COPD) – Finding the Missing Millions. London: British Lung Foundation; 2007.
- Managing Chronic Heart Failure: Learning From Best Practice. London: Clinical Effectiveness and Evaluation Unit, Royal College of Physicians; 2005.
- Cleland J, Dargie H, Hardman S, McDonagh T, Mitchell P. National Heart Failure Audit 2011/12. London: National Institute for Cardiovascular Outcomes Research; 2013.
- Heidenreich PA, Hernandez AF, Yancy CW, Liang L, Peterson ED, Fonarow GC. Get With The Guidelines program participation, process of care, and outcome for Medicare patients hospitalized with heart failure. Circ Cardiovasc Qual Outcomes 2012;5:37-43. https://doi.org/10.1161/CIRCOUTCOMES.110.959122.
- López-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM. European COPD Audit team . Variability of hospital resources for acute care of COPD patients: the European COPD Audit. Eur Respir J 2014;43:754-62. https://doi.org/10.1183/09031936.00074413.
- O’Cathain A, Knowles E, Maheswaran R, Pearson T, Turner J, Hirst E, et al. A system-wide approach to explaining variation in potentially avoidable emergency admissions: national ecological study. BMJ Qual Saf 2014;23:47-55. https://doi.org/10.1136/bmjqs-2013-002003.
- Purdy S. Avoiding Hospital Admissions. What Does the Research Evidence Say?. London: The King’s Fund; 2010.
- Soljak M, Brettell R, Cowie M, Cecil E, Tuppin P, Majeed A. Reducing heart failure admission rates in England 2004–11 are not related to changes in primary care quality: national observational study. Eur Heart J 2013;34. https://doi.org/10.1093/eurheartj/eht309.P3357.
- Imison C, Thompson J, Poteliakhoff E. Older People and Emergency Bed Use: Exploring Variation. London: The King’s Fund; 2012.
- Bottle A, Gaudoin R, Goudie R, Jones S, Aylin P. Can valid and practical risk-prediction or casemix adjustment models, including adjustment for comorbidity, be generated from English hospital administrative data (Hospital Episode Statistics)? A national observational study. Health Serv Deliv Res 2014;2.
- Carstairs V, Morris R. Deprivation and Health in Scotland. Aberdeen: Aberdeen University Press; 1991.
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems 1990.
- Bottle A, Aylin P, Bell D. Effect of the readmission primary diagnosis and time interval in heart failure patients: analysis of English administrative data. Eur J Heart Fail 2014;16:846-53. https://doi.org/10.1002/ejhf.129.
- Bottle A, Goudie R, Cowie MR, Bell D, Aylin P. Relation between process measures and diagnosis-specific readmission rates in patients with heart failure. Heart 2015;101:1704-10. https://doi.org/10.1136/heartjnl-2014-307328.
- Ministry of Housing, Communities & Local Government . English Indices of Deprivation 2015. www.gov.uk/government/statistics/english-indices-of-deprivation-2015 (accessed 31 January 2018).
- Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646-59. https://doi.org/10.1161/CIRCRESAHA.113.300268.
- Bottle A, Aylin P. Intelligent information: a national system for monitoring clinical performance. Health Serv Res 2008;43:10-31. https://doi.org/10.1111/j.1475-6773.2007.00742.x.
- Huber MB, Wacker ME, Vogelmeier CF, Leidl R. Excess costs of comorbidities in chronic obstructive pulmonary disease: a systematic review. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0123292.
- Cohen ME, Dimick JB, Bilimoria KY, Ko CY, Richards K, Hall BL. Risk adjustment in the American College of Surgeons National Surgical Quality Improvement Program: a comparison of logistic versus hierarchical modeling. J Am Coll Surg 2009;209:687-93. https://doi.org/10.1016/j.jamcollsurg.2009.08.020.
- Glance LG, Dick A, Osler TM, Li Y, Mukamel DB. Impact of changing the statistical methodology on hospital and surgeon ranking: the case of the New York State cardiac surgery report card. Med Care 2006;44:311-19. https://doi.org/10.1097/01.mlr.0000204106.64619.2a.
- The Centre for Medicare and Medicaid Services . Statistical Issues in Assessing Hospital Performance n.d. www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf (accessed 26 October 2017).
- Austin PC, Alter DA, Tu JV. The use of fixed- and random-effects models for classifying hospitals as mortality outliers: a Monte Carlo Assessment. Med Decis Making 2003;23:526-39. https://doi.org/10.1177/0272989X03258443.
- Austin PC. A comparison of Bayesian methods for profiling hospital performance. Med Decis Making 2002;22:163-72. https://doi.org/10.1177/0272989X0202200213.
- Bottle A, Aylin P. Statistical Methods for Healthcare Performance Monitoring. Boca Raton, FL: CRC Press; 2016.
- Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: Sage Publications; 1999.
- Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928-35. https://doi.org/10.1161/CIRCULATIONAHA.106.672402.
- Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997;16:965-80. https://doi.org/10.1002/(SICI)1097-0258(19970515)16:9<965::AID-SIM509>3.0.CO;2-O.
- Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244-56. https://doi.org/10.1093/aje/kwp107.
- Haller B, Schmidt G, Ulm K. Applying competing risks regression models: an overview. Lifetime Data Anal 2013;19:33-58. https://doi.org/10.1007/s10985-012-9230-8.
- Kohl M, Heinze G. PSHREG: A SAS Macro for Proportional and Nonproportional Subdistribution Hazards Regression with Competing Risk Data. Vienna: Medical University of Vienna; 2012.
- Bos N, Sizmur S, Graham C, van Stel HF. The accident and emergency department questionnaire: a measure for patients’ experiences in the accident and emergency department. BMJ Qual Saf 2013;22:139-46. https://doi.org/10.1136/bmjqs-2012-001072.
- Brown SE, Ratcliffe SJ, Halpern SD. An empirical comparison of key statistical attributes among potential ICU quality indicators. Crit Care Med 2014;42:1821-31. https://doi.org/10.1097/CCM.0000000000000334.
- Mayer EK, Bottle A, Rao C, Darzi AW, Athanasiou T. Funnel plots and their emerging application in surgery. Ann Surg 2009;249:376-83. https://doi.org/10.1097/SLA.0b013e31819a47b1.
- Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med 2005;24:1185-202. https://doi.org/10.1002/sim.1970.
- NHS England . NHS Patient Experience Tool n.d. www.england.nhs.uk/statistics/statistical-work-areas/pat-exp/sup-info/ (accessed 8 May 2017).
- NHS Digital . Number of GPs – Data As at 30 September 2010 n.d. https://indicators.hscic.gov.uk/webview/#38;node=0&mode=documentation&submode=ddi&v=2&top=yes&language=en (accessed 31 January 2018).
- NHS England . GP Patient Survey 2010 11 n.d. https://gp-patient.co.uk/surveys-and-reports#december-2010 (accessed 10 March 2016).
- NHS Digital . Quality and Outcomes Framework 2010 11 n.d. www.hscic.gov.uk/catalogue/PUB05756 (accessed 10 March 2016).
- NHS England . Overall Patient Experience Scores: Supporting Information – Diagnostic Tool in Comma-Separated Value Format for Acute and Specialist Trusts n.d. www.england.nhs.uk/statistics/statistical-work-areas/pat-exp/sup-info/ (accessed 30 June 2015).
- NHS England . Staff Survey 2010 – Detailed Spreadsheets n.d. www.nhsstaffsurveys.com/Page/1023/Past-Results/Staff-Survey-2010-Detailed-Spreadsheets/Picker Institute Europe/NHS England (accessed 10 March 2016).
- NHS Digital . Monthly NHS Hospital and Community Health Service (HCHS) Workforce Statistics – England, Quarterly Supplemental Information, January 2011, Provisional Experimental Statistics (and March, July and September) n.d. www.hscic.gov.uk/article/2021/Website-Search?productid=7099&q=nusrsing+data&topics=13209&infotype=13367&sort=Relevance&size=10&page=10&area=both#top HSCIC (accessed 10 March 2016).
- NHS Digital . Monthly NHS Hospital and Community Health Service (HCHS) Workforce Statistics in England – July 2013, Provisional Statistics n.d. www.hscic.gov.uk/article/2021/Website-Search?productid=12545&q=Monthly+NHS+Hospital+and+Community+Health+Service+(HCHS)+Workforce+Statistics+in+England+%E2%80%93+July+2013%2c+Provisional+statistics&sort=Relevance&size=10&page=3&area=both#top (accessed 10 March 2016).
- NHS England . Bed Availability and Occupancy Data – Overnight. Average Daily Number of Available and Occupied Beds by Sector, NHS Organisations in England, 2009–10 n.d. www.england.nhs.uk/statistics/statistical-work-areas/bed-availability-and-occupancy/bed-data-overnight/ (accessed 10 March 2016).
- National Institute for Health and Care Excellence . Acute Heart Failure: Diagnosing and Managing Acute Heart Failure in Adults 2014.
- Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, Manning WG. The unreliability of individual physician ‘report cards’ for assessing the costs and quality of care of a chronic disease. JAMA 1999;281:2098-105. https://doi.org/10.1001/jama.281.22.2098.
- Honeyford K, Aylin P, Bottle A. Medical Care. 2018.
- Cowie MR, Anker SD, Cleland JGF, Felker GM, Filippatos G, Jaarsma T, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Fail 2014;1:110-45. https://doi.org/10.1002/ehf2.12021.
- MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, et al. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 2000;102:1126-31. https://doi.org/10.1161/01.CIR.102.10.1126.
- Johnston AK, Mannino DM, Wedzicha JA, Martinez F. Exacerbations of Chronic Obstructive Pulmonary Disease (COPD). New York, NY: Informa Healthcare; 2008.
- Nie JX, Wang L, Upshur REG. Mortality of elderly patients in Ontario after hospital admission for chronic obstructive pulmonary disease. Can Respir J 2007;14:485-9. https://doi.org/10.1155/2007/425248.
- Ho T-W, Tsai Y-J, Ruan S-Y, Huang C-T, Lai F, Yu C-J. The HINT Study Group . In-hospital and one-year mortality and their predictors in patients hospitalized for first-ever chronic obstructive pulmonary disease exacerbations: a nationwide population-based study. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0114866.
- Santibáñez M, Garrastazu R, Ruiz-Nuñez M, Helguera JM, Arenal S, Bonnardeux C, et al. Predictors of hospitalized exacerbations and mortality in chronic obstructive pulmonary disease. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0158727.
- Fidahussein SS, Croghan IT, Cha SS, Klocke DL. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy 2014;7:105-12.
- Hasegawa W, Yamauchi Y, Yasunaga H, Sunohara M, Jo T, Matsui H, et al. Factors affecting mortality following emergency admission for chronic obstructive pulmonary disease. BMC Pulm Med 2014;14. https://doi.org/10.1186/1471-2466-14-151.
- Salte K, Titlestad I, Halling A. Depression is associated with poor prognosis in patients with chronic obstructive pulmonary disease – a systematic review. Dan Med J 2015;62.
- Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Hospital readmission performance and patterns of readmission: retrospective cohort study of Medicare admissions. BMJ 2013;347. https://doi.org/10.1136/bmj.f6571.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418-28. https://doi.org/10.1056/NEJMsa0803563.
- Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001–12 and Nationwide Emergency Department Sample 2006–11. Chest 2015;147:989-98. https://doi.org/10.1378/chest.14-2146.
- Sharif R, Parekh TM, Pierson KS, Kuo Y-F, Sharma G. Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014;11:685-94. https://doi.org/10.1513/AnnalsATS.201310-358OC.
- Milne RJ, Beasley R. Hospital admissions for chronic obstructive pulmonary disease in New Zealand. N Z Med J 2015;128:23-35.
- Ross JS, Mulvey GK, Stauffer B, Patlolla V, Bernheim SM, Keenan PS, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med 2008;168:1371-86. https://doi.org/10.1001/archinte.168.13.1371.
- Brennan JJ, Chan TC, Killeen JP, Castillo EM. Inpatient readmissions and emergency department visits within 30 days of a hospital admission. Western J Emerg Med 2015;16:1025-9. https://doi.org/10.5811/westjem.2015.8.26157.
- Vashi AA, Fox JP, Carr BG, D’Onofrio G, Pines JM, Ross JS, et al. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA 2013;309:364-71. https://doi.org/10.1001/jama.2012.216219.
- Venkatesh AK, Wang C, Ross JS, Suter LG, Vellanky S, Grady J, et al. Characterizing hospital-level variation in emergency department visitation after hospital discharge for Medicare beneficiaries. Ann Emerg Med 2015;66:S57-8. https://doi.org/10.1016/j.annemergmed.2015.07.194.
- Storrow AB, Jenkins CA, Self WH, Alexander PT, Barrett TW, Han JH, et al. The burden of acute heart failure on U.S. emergency departments. JACC Heart Fail 2014;2:269-77. https://doi.org/10.1016/j.jchf.2014.01.006.
- Rowe BH, Villa-Roel C, Guttman A, Ross S, Mackey D, Sivilotti ML, et al. Predictors of hospital admission for chronic obstructive pulmonary disease exacerbations in Canadian emergency departments. Acad Emerg Med 2009;16:316-24. https://doi.org/10.1111/j.1553-2712.2009.00366.x.
- Vidal S, González N, Barrio I, Rivas-Ruiz F, Baré M, Blasco JA, et al. Predictors of hospital admission in exacerbations of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2013;17:1632-7. https://doi.org/10.5588/ijtld.13.0177.
- Wittenberg R, Sharpin L, McCormick B, Hurst J. Understanding Emergency Hospital Admissions of Older People. Oxford: Centre for Health Service Economics & Organisation; 2014.
- Pines JM, Mutter RL, Zocchi MS. Variation in emergency department admission rates across the United States. Med Care Res Rev 2013;70:218-31. https://doi.org/10.1177/1077558712470565.
- Capp R, Ross JS, Fox JP, Wang Y, Desai MM, Venkatesh AK, et al. Hospital variation in risk-standardized hospital admission rates from US EDs among adults. Am J Emerg Med 2014;32:837-43. https://doi.org/10.1016/j.ajem.2014.03.033.
- Abualenain J, Frohna WJ, Shesser R, Ding R, Smith M, Pines JM. Emergency department physician-level and hospital-level variation in admission rates. Ann Emerg Med 2013;61:638-43. https://doi.org/10.1016/j.annemergmed.2013.01.016.
- Bottle A, Jarman B, Aylin P. Hospital standardized mortality ratios: sensitivity analyses on the impact of coding. Health Serv Res 2011;46:1741-61. https://doi.org/10.1111/j.1475-6773.2011.01295.x.
- Horwitz LI, Wang Y, Desai MM, Curry LA, Bradley EH, Drye EE, et al. Correlations among risk-standardized mortality rates and among risk-standardized readmission rates within hospitals. J Hosp Med 2012;7:690-6. https://doi.org/10.1002/jhm.1965.
- Burns EM, Rigby E, Mamidanna R, Bottle A, Aylin P, Ziprin P, et al. Systematic review of discharge coding accuracy. J Public Health 2012;34:138-48. https://doi.org/10.1093/pubmed/fdr054.
- Pavasini R, Tavazzi G, Biscaglia S, Guerra F, Pecoraro A, Zaraket F, et al. Amino terminal pro brain natriuretic peptide predicts all-cause mortality in patients with chronic obstructive pulmonary disease: Systematic review and meta-analysis. Chron Respir Dis 2017;14:117-26. https://doi.org/10.1177/1479972316674393.
- Venkatesh AK, Dai Y, Ross JS, Schuur JD, Capp R, Krumholz HM. Variation in US hospital emergency department admission rates by clinical condition. Med Care 2015;53:237-44. https://doi.org/10.1097/MLR.0000000000000261.
- Weiss SJ, Derlet R, Arndahl J, Ernst AA, Richards J, Fernández-Frackelton M, et al. Estimating the degree of emergency department overcrowding in academic medical centers. Results from the National ED Crowding Study (NEDOCS). Acad Emerg Med 2004;11:38-50. https://doi.org/10.1197/j.aem.2003.07.017.
- DeLia D, Tong J, Gaboda D, Casalino LP. Post-discharge follow-up visits and hospital utilization by Medicare patients, 2007–10. Medicare Medicaid Res Rev 2014;4. https://doi.org/10.5600/mmrr.004.02.a01.
- Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010;303:1716-22. https://doi.org/10.1001/jama.2010.533.
- Jhund PS, Tavazzi L. Has the ‘epidemic’ of heart failure been replaced by a tsunami of co-morbidities?. Eur J Heart Fail 2016;18:500-2. https://doi.org/10.1002/ejhf.529.
- Hackbarth G, Reischauer RD, Miller ME. Report to the Congress: Promoting Greater Efficiency in Medicare. Washington, DC: MedPAC, The Medicare Payment Advisory Commission; 2007.
- Yang F, Xiong ZF, Yang C, Li L, Qiao G, Wang Y, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD 2017;14:251-61. https://doi.org/10.1080/15412555.2016.1256384.
- Bradley EH, Curry L, Horwitz LI, Sipsma H, Wang Y, Walsh MN, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes 2013;6:444-50. https://doi.org/10.1161/CIRCOUTCOMES.111.000101.
- Harrison SL, Janaudis-Ferreira T, Brooks D, Desveaux L, Goldstein RS. Self-management following an acute exacerbation of COPD: a systematic review. Chest 2015;147:646-61. https://doi.org/10.1378/chest.14-1658.
- Hakamy A, Bolton CE, McKeever TM. The effect of pulmonary rehabilitation on mortality, balance, and risk of fall in stable patients with chronic obstructive pulmonary disease. Chron Respir Dis 2017;14:54-62. https://doi.org/10.1177/1479972316661925.
- Neves LF, Reis MH, Gonçalves TR. Home or community-based pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Cad Saude Publica 2016;32:S0102-311X2016000602001. https://doi.org/10.1590/0102-311X00085915.
- Ospina MB, Mrklas K, Deuchar L, Rowe BH, Leigh R, Bhutani M, et al. A systematic review of the effectiveness of discharge care bundles for patients with COPD. Thorax 2017;72:31-9. https://doi.org/10.1136/thoraxjnl-2016-208820.
- Prieto-Centurion V, Markos MA, Ramey NI, Gussin HA, Nyenhuis SM, Joo MJ, et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc 2014;11:417-24. https://doi.org/10.1513/AnnalsATS.201308-254OC.
- Edwards N. What’s Behind Delayed Transfers of Care? Briefing. London: The Nuffield Trust; n.d.
- Obermeyer Z, Cohn B, Wilson M, Jena AB, Cutler DM. Early death after discharge from emergency departments: analysis of national US insurance claims data. BMJ 2017;356. https://doi.org/10.1136/bmj.j239.
- Collins SP, Storrow AB. Acute heart failure risk stratification: can we define low risk?. Heart Fail Clin 2009;5:75-83. https://doi.org/10.1016/j.hfc.2008.08.010.
- Miro O, Levy PD, Mockel M, Pang PS, Lambrinou E, Bueno H, et al. Disposition of emergency department patients diagnosed with acute heart failure: an international emergency medicine perspective. Eur J Emerg Med 2017;24:2-12. https://doi.org/10.1097/MEJ.0000000000000411.
- Pang PS, Schuur JD. Emergency departments, acute heart failure, and admissions: one size does not fit all. JACC Heart Fail 2014;2:278-80. https://doi.org/10.1016/j.jchf.2014.03.003.
List of abbreviations
- A&E
- accident and emergency
- AMI
- acute myocardial infarction
- CI
- confidence interval
- CMS
- Centers for Medicare and Medicaid Services
- COPD
- chronic obstructive pulmonary disease
- CPRD
- Clinical Practice Research Datalink
- ED
- emergency department
- FEV1
- forced expiratory volume in 1 second
- FTE
- full-time equivalent
- GP
- general practitioner
- HES
- Hospital Episodes Statistics
- HF
- heart failure
- HL
- Hosmer–Lemeshow
- ICC
- intraclass correlation coefficient
- ICU
- intensive care unit
- IHD
- ischaemic heart disease
- LOS
- length of hospital stay
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- OOH
- out of hours
- OPD
- outpatient department
- OR
- odds ratio
- PE
- patient experience
- PPI
- public and patient involvement
- QOF
- Quality and Outcomes Framework
- RR
- relative risk
- SAS
- Statistical Analysis Software
- SMR
- standardised mortality ratio
