Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 13/54/62. The contractual start date was in May 2015. The final report began editorial review in May 2017 and was accepted for publication in November 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Crispin Wickenden, Catharina Koppitz, Gavin Cho, David J Roberts and Gail Miflin are all employees of NHS Blood and Transplant. Richard Grieve is currently a member of the National Institute for Health Research Health Technology Assessment Commissioning Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Grieve et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background, aims and objectives
Whole-blood donors provide around 110 million donations worldwide for transfusions used in the management of general surgery and severe trauma, high-dose chemotherapy regimens, and stem cell and organ transplantation. 1,2 The NHS Blood and Transplant (NHSBT) service is an essential part of the health service in England, and in 2015–16 issued 1.594 million units of red cells at a production cost of approximately £160M. 3 The overall demand for red blood cells for the NHS has fallen by about 17% since 2004–5, due partly to advances in technology and education of health-care professionals, which have reduced red cell use per surgical procedure. 3 The NHSBT has managed the required reduction in the overall supply of whole blood; between 2012 and 2016 the number of registered whole-blood donors fell by around 15%4 (C Wickenden, NHSBT, 2016, personal correspondence). There is interest in identifying cost-effective changes to blood collection services that can be implemented in mobile (temporary) blood collection venues, where around 85% of whole-blood donors currently donate in England, but also at static (permanent) blood collection centres. A future challenge for NHSBT is to maintain the requisite supply of whole blood not just overall, but according to particular donor subgroups.
Demand for some blood types, in particular O negative (O–) (the universal blood type), A negative (A–), B negative (B–) and other rare blood types more common in black and Asian minority ethnic (BAME) donors, is increasing. A major concern is to ensure sufficient supply of those blood types that are in relatively high demand, and there may be times when increased stocks are required to meet higher demand. The O– blood type is essential for those transfusions required by emergency trauma patients if blood types are unmatched, and demand for this blood type accounts for around 13% of all hospital requests. 5 However, only around 7% of the donor population are blood type O–, and so the NHSBT encourages these donors to donate blood more often. A further concern is that patients with genetic blood disorders such as sickle cell disease and thalassaemia disease require multiple transfusions with extensively matched blood to reduce adverse reactions. These transfusions require blood subtypes, such as Ro, which are relatively prevalent in donors from BAME ethnic groups, but BAME donors represent only 5% of the overall donor population. If the required blood types are unavailable, O– blood is used instead, further increasing demand for this blood type. Hence, a key policy objective for the NHSBT is to collect more blood from BAME groups and those with high-demand blood types such as O–. 3
The NHSBT could increase investment in marketing strategies to attract new donors, but finding and retaining donors is costly, and it is more efficient to increase donation frequency among existing donors, in particular those whose blood type is in high demand. 6
A key challenge for the blood service is therefore to develop strategies that can increase the frequency of donation from those donors whose blood type is in relatively high demand, at low additional cost. A central pillar of the 2013–17 Blood Donation Strategy4 is to improve the experience of the 1.3 million registered voluntary blood donors to help encourage existing donors to donate whole blood at the requisite frequency. The NHSBT has invested in strategies to improve the donation experience; for example, donors can book appointments online, the 24 static donor centres across England offer free Wi-Fi, and the NHSBT sends text messages to remind donors about their appointment and then to say when and where the donation has been used. However, it is unclear whether or not these and other future changes to the blood service are clinically effective or cost-effective.
The NHSBT surveys donors to help understand which aspects of the donor experience warrant improvement, but it does not elicit donor preferences using the formal techniques that are required to recognise any trade-offs between the different aspects of the service (e.g. additional travel time to a donor centre vs. improved appointment availability). Hence, these surveys do not provide an adequate basis for predicting the effects of potential service changes on the frequency of whole-blood donation. More generally, there is little evidence on donors’ relative preferences for alternative types of blood donation service. 7–12 The extant literature suggests that donors prefer shorter waiting times before whole-blood donation and convenient locations,13,14 and that non-monetary incentives are more effective than monetary incentives for encouraging blood donation. 15–17 None of these studies have used appropriate formal techniques for preference elicitation, evaluated strategies of direct relevance to the NHSBT or considered subgroups of current policy relevance, such as donors whose blood type is in high demand, BAME donors or those donor subgroups who are less likely to continue donating (younger or less experienced donors).
Economic evaluations of alternative ways of organising the blood service are required, ones that recognise that donation frequency will be driven by donors’ experiences of, and preferences for, alternative features of a blood donation service. Such studies are also required to recognise that donors’ relative preferences for alternative strategies may differ according to the individuals’ characteristics and constraints. There is limited evidence on the costs and cost-effectiveness of alternative ways of organising the blood donation service. 18–28 Previous studies have estimated the effect of previous policy changes on the volume and costs of whole blood collected,18–28 and predicted the efficient location and staffing for static donor centres. 19,29–31 None of these studies estimated the cost-effectiveness of possible changes to the blood donation service that are of direct relevance to future NHSBT strategies, nor have they recognised donors’ relative preferences for alternative changes to the blood service.
A strategy of potential interest to the NHSBT is to invite existing whole-blood donors to donate blood more often, particularly those donors whose blood type is in high demand. In England, the minimum donation interval is currently 12 weeks for men and 16 weeks for women. 32 It is currently unknown if reducing the minimum interdonation interval is clinically effective or cost-effective. 33 Shorter recall intervals may lead to an increased risk of iron deficiency, higher rates of donation deferral (temporary suspension of donors giving blood) and lower health-related quality of life (QoL) for donors. 34–36 Increased rates of deferral may lead to higher costs and encourage donors to leave the register. 37,38 International variations in blood service policy reflect this uncertainty about the optimum minimum interdonation interval. For example, the minimum intervals between blood donations are every 8 weeks (both sexes) in the USA,39 and every 8 (men) and 12 weeks (women) in France and Germany. 40,41 The INTERVAL trial was designed to establish whether or not reducing the minimum recall interval for donors attending static donor centres in England would increase the frequency of whole-blood donation while maintaining donor health. 42 However, the INTERVAL trial alone will not provide sufficient evidence to inform whether or not the NHSBT should reduce the minimum donation interval. In particular, research is also required on the relative costs and cost-effectiveness of reducing the minimum donation interval, in particular for subgroups of prime policy relevance.
Aims and objectives
The study’s aim is to identify cost-effective strategies for maintaining the blood supply. The study estimates the relative cost-effectiveness of alternative minimum interdonation intervals (12 vs. 10 vs. 8 weeks for men; 16 vs. 12 vs. 10 weeks for women). The study adopts formal methods to elicit preferences from donors using stated preference (SP) surveys to estimate the frequency at which they are willing to donate whole blood according to alternative potential changes to the blood donation service. We use these estimates from the surveys along with observed data on deferral rates from the INTERVAL trial to report the relative cost-effectiveness of alternative strategies overall, and particularly for subgroups of prime policy relevance, for example donors whose blood type is in high demand and BAME donors.
Objectives
-
To estimate the cost-effectiveness of alternative minimum donation intervals between whole-blood donations.
-
To investigate the frequency at which donors are willing to donate whole blood according to alternative hypothetical changes to the blood donation service.
-
To estimate the cost-effectiveness of alternative strategies for maintaining the supply of whole blood to the NHS.
Report overview
This report details the three interlinked components of the study. Chapter 2 describes the use of the INTERVAL trial data to report the relative cost-effectiveness of alternative minimum donation intervals over 2 years. Chapter 3 reports the design, and results, of SP surveys that provide estimates of the frequency at which donors are willing to donate whole blood according to alternative future changes to the blood donation service. Chapter 4 estimates the cost-effectiveness of alternative strategies for maintaining the blood supply, drawing on findings from the surveys and the analyses of the INTERVAL trial. The design, analysis and interpretation of each component of the research have been informed by the key service provider (NHSBT), a public representative and current whole-blood donors.
At the design stage, we identified strategies to improve opportunities for existing blood donors to donate. The strategies were identified through a review of NHSBT documents describing future strategies and policies, the results of market research, an informal review of relevant published literature, consultation with NHSBT colleagues and insights from preliminary qualitative research undertaken with INTERVAL donors (see Appendix 1).
The following strategies were chosen for the evaluation presented in this report:
-
provision of a health report, including cholesterol and blood pressure tests, for all donors at each whole-blood donation visit9,43–45
-
extended opening hours (weekend or evening), for both static donor centres and mobile sessions for collecting whole blood
-
increase in the maximum number of whole-blood donations per year for current donors attending static donor centres, as per the INTERVAL trial.
Changes since the proposal
There were three main changes made to the research originally proposed. First, the cost-effectiveness analysis focused on alternative strategies for current whole-blood donors and did not consider alternative strategies for the recruitment of new donors. The NHSBT suggested that an evaluation of the effect of alternative strategies for pre-existing whole-blood donors would provide more relevant evidence to inform future strategy. Second, the approach taken to elicit donor preferences for alternative changes to the blood donation service was to undertake SP surveys rather than a discrete choice experiment (DCE). 46 The SP survey design was chosen as, unlike a DCE, it could capture respondents’ stated intentions regarding frequency of donation. Third, the time horizon chosen for the cost-effectiveness analysis was 1 year, rather than the 10 years originally proposed. This choice of time horizon was in accordance with NHSBT’s requirements, which, given the uncertainties about the future demand for red blood cells in the medium term, were to consider the shorter-term effects of the alternative strategies. Each of these changes was discussed and agreed with the project advisory group.
Chapter 2 Cost-effectiveness analysis of alternative minimum recall intervals between whole-blood donations
Introduction
This chapter will address the question, ‘what is the cost-effectiveness of reducing the minimum interval between whole-blood donations?’. This economic evaluation compares the costs and consequences of the alternative minimum donation intervals considered in the INTERVAL trial. Full details of the INTERVAL trial are provided elsewhere;42,47,48 here we focus on the essential elements of the cost-effectiveness analysis (CEA). The study followed the main principles set out in the INTERVAL trial protocol42 and statistical analysis plan. In particular, the study contrasted the costs and consequences of the alternative randomised arms in INTERVAL according to the intention-to-treat principle. 49 The main time horizon was 2 years, as per the follow-up period of the INTERVAL trial. We estimated the effect of randomisation to alternative minimum recall donation intervals on the mean number of successful whole-blood donations, overall donation deferrals, donation deferrals caused by low haemoglobin (Hb), quality of life (QoL) and cost. The study measured costs from the NHS and Personal Social Services perspectives as recommended by the National Institute for Health and Care Excellence. 50 The costs included were those costs of the blood donation visit that were anticipated to differ over the trial follow-up period and according to strategy, and included the relevant costs of blood collection but excluded processing costs or fixed costs. The cost analysis also included the costs of deferrals and any subsequent health-care costs of those deferrals that were caused by low levels of Hb.
Methods outlines the main features of the INTERVAL trial and the methods used in the CEA. Results summarises the main results, and Discussion discusses the main findings, and outlines how they will inform the subsequent CEA of alternative strategies for maintaining the blood supply beyond reducing the minimum interval between blood donations.
Methods
INTERVAL trial overview
The INTERVAL trial was an open, parallel-group pragmatic randomised controlled trial (RCT) that included a total of 45,263 whole-blood donors (men, n = 22,466; women, n = 22,797) at 25 static donor centres in England. Participants were recruited from June 2012 to June 2014. Male participants were randomly assigned to 12- versus 10- versus 8-week interdonation intervals. Female participants were randomly assigned to 16- versus 14- versus 12-week interdonation intervals. The primary trial end point was the number of whole-blood donations obtained secondary outcomes included measures of donors’ health, including the Short Form questionnaire-36 items (SF-36) health survey and the number of deferrals, both measured over 2 years’ follow-up.
Selection and recruitment of participants, and exclusions from the cost-effectiveness analysis
Donors were eligible for inclusion in the trial if they were aged ≥ 18 years, met the routine criteria for whole-blood donation, were willing to be randomised, had an e-mail address and access to the internet (required to provide baseline and follow-up information), were willing to donate whole blood at a static centre, and returned the baseline questionnaire. Donors already registered at static donor centres, specific subgroups of donors attending mobile sessions who were willing to donate at a static centre for the duration of the trial, and new donors were all considered for inclusion. Those donors who were eligible to take part in the trial, and who consented, were randomised to the three sex-specific intervention groups in a 1 : 1 : 1 ratio. The CEA excluded those donors who withdrew consent for use of their data (n = 221), who died during or after the trial follow-up period (n = 142) until December 2016, when linked NHSBT national blood supply database (PULSE) data were extracted, or who did not have requisite PULSE data available (n = 37), leaving an overall sample for the CEA of 44,863. This is outlined in Figure 1.
FIGURE 1.
The CONSORT flow chart: participation, exclusions and completeness of main CEA (adapted from Di Angelantonio et al. 47).

Baseline measures including variables for subgroup analysis
Information on donors’ baseline characteristics and their donation history for the 2 years prior to randomisation was extracted from the NHSBT national blood supply database, PULSE. The baseline measures included the donors’ sex, age, ethnicity, blood type, whether or not the donor was new, their recruitment source (donor centre, mobile session, no invitation), and their number of donations and deferrals for low levels of Hb in the previous 2 years. At the baseline donation visit, a full blood count was provided the levels of Hb used to define the proportion of low-Hb deferrals who would require additional consultations and tests. Following this visit, participants were asked to complete the online baseline questionnaire, which included the SF-36 questionnaire. At this point, participants provided their weight and were randomised.
Measurement of resource use and consequences
For each randomised donor, the numbers of successful whole-blood donations, deferrals and fainting episodes at a blood donation session over the 2-year follow-up period were extracted from the PULSE database. The volume of blood donated was measured in units of whole blood (each unit is 470 ml). The number of deferrals was recorded, and each deferral was categorised by whether or not it was caused by low levels of Hb, which were anticipated, could differ by randomised arm and have resource consequences (e.g. additional staff time and additional Hb screening tests). The deferral policy used in the trial was the same as in routine practice; for example, donors with Hb levels that were ‘low’, that is < 134 g/l for men and < 124 g/l for women, were deferred for 3 months. The number of deferrals for other reasons (travel, medication, lifestyle restrictions or infection/illness) was also recorded.
Participants were requested by e-mail to complete an online questionnaire, which included the Short Form questionnaire-12 items (SF-12), at the 6-, 12- and 18-month follow-ups, and the SF-36 at the final 2-year follow-up time point. We extracted responses to those questions required to report the Short Form questionnaire-6 Dimensions (SF-6D) utilities, and combined these with the published valuation algorithm51 to report SF-6D utility scores at each time point, anchored on the scale 0 (death) and 1 (perfect health). Data of relevance to resource use were also collected as part of the web-based follow-up questionnaires, including the number of health-care events occurring between donation sessions (doctor or hospital visits required for falls, transport accidents, angina, heart failure, transient ischaemic attack, stroke, myocardial infarction). Although the numbers of these events were reported, they were not anticipated to differ between the randomised arms, and so the ensuing costs were not included in the cost analysis.
Units costs
The unit costs related to blood collection were taken from NHSBT financial records in 2016–17 prices (Laura Hontoria Del Hoyo, NHSBT, June 2016, personal communication; Colin Jackson, NHSBT, April 2017, personal communication). We used expert opinions to estimate the opportunity cost of additional staff time required following a deferred appointment caused by low levels of Hb, and for other reasons. As per NHSBT routine policy, if the donor’s Hb level was ‘very low’, defined as < 125 g/l (men) and < 115 g/l (women), subsequent tests and consultation with a health-care professional were assumed to be required. We used data on participating donors’ Hb levels and ensuing deferrals at their baseline visit to calculate the proportion of low-Hb deferrals where the Hb levels were ‘very low’, and assumed that for this subset of low-Hb deferrals subsequent monitoring would be required. We then applied this proportion (7% of low-Hb deferrals) to calculate the unit costs of all deferrals from low levels of Hb (£10.17, outlined in Table 1).
| Resource-generating event | Unit cost (£) | Main source |
|---|---|---|
| Three-stage reminder for invitation to donate | 2.68 | Expert opinion |
| Low-Hb deferral, additional costs at donor centre | 4.78 | Expert opinion |
| Low-Hb deferral, subsequent health-care costs | 5.39 | Expert opinion |
| Deferral caused by other reasons | 0.97 | Expert opinion |
| Three-stage reminder following non-attendance | 3.10 | Expert opinion |
| Fainting episode at blood donation visit | 20.23 | Expert opinion |
| Variable cost of collecting 1 unit of blood (centre operating with capacity) | 7.62 | NHSBTa |
The frequency of donation visits within the trial follow-up period could reflect trial protocols, including the intensity of donation appointment reminders; we therefore calculated unit costs for issuing study-specific attendance reminders. We used information collated by the trial co-ordinator to calculate the staff time, consumables and, therefore, the unit costs required to issue reminders to donate, according to the three-stage reminder process specified by the INTERVAL trial protocols (see Table 1 and Appendix 2). The number of reminder calls and e-mails made to participants without an appointment was not recorded, and so the requisite unit cost was calculated by dividing the total cost of these reminders by the total number of trial participants. We then applied a constant unit cost of reminders for those without an appointment across all trial arms. The number of times a donor did not attend a scheduled appointment were not measured, and so the ensuing costs were not included in the base-case analysis (see Sensitivity analysis). The unit costs of a fainting episode were calculated according to the additional staff time required at a donor centre to manage a typical fainting episode.
We combined resource use data from each INTERVAL participant with these unit costs to report the costs for each randomised donor over the trial’s 2-year follow-up period. We then calculated the average total costs per donor over 2 years.
Descriptive statistics, reporting of results and analysis
All descriptive statistics and analyses were reported by sex. For each randomised arm, we estimated the average resource use and variable cost per donor over the 2-year follow-up period. The main resource-use measures were the average number of blood donation visits, the average number of deferrals per donor (overall and caused by low levels of Hb) and the average number of faints per donor. We also report the deferral rate per attendance, as required in the subsequent economic evaluation (see Chapter 4). We report the average SF-6D utility score at each time point, according to randomised arm, and the corresponding mean (95% CI) differences between the arms up to 2 years’ follow-up.
We report the incremental cost-effectiveness of the reduced interval strategies, according to the incremental (difference in means) variable cost per additional unit of whole blood donated. We report results overall (by sex), and according to the other prespecified subgroups: high- versus standard-demand blood types, ethnicity, age group, new donor or not and recruitment source (static donor centre vs. mobile session vs. other) (see Appendix 3).
The analysis applied logistic regression models (binary end points), linear regression models (continuous, univariate end points) and seemingly unrelated regressions (SURs) (joint whole-blood donations and cost end point). 52 Rates of deferral were estimated using the data on number of deferrals and attendances, and by applying logistic regression models for grouped data. QoL was estimated using a generalised estimating equation (GEE) model described here. Costs and whole-blood donations were estimated jointly by applying a SUR model. The incremental analysis of economic end points (QoL, costs, whole-blood donations) adjusted for age, ‘high’ versus ‘standard’ demand blood types, ethnicity, new donor or not and recruitment source (static donor centre vs. mobile session vs. other). We estimated subgroup effects by including interaction terms for randomised arm by subgroup. Age was defined as a continuous variable in the model, but predictions were provided according to the requisite categories. We report the results from likelihood ratio tests, to assess whether or not model fit improved with the inclusion of interaction effects for each subgroup by randomised group.
There were missing QoL data for those individuals who did not complete the items required for the SF-6D utility score; the number and percentage of the analysis sample with required responses is reported for each time point (baseline, 6, 12, 18 and 24 months) (see Appendix 4). These missing data were handled by a GEE model that included SF-6D utility score as the dependent variable, with randomised group, time point and the earlier subgroup variables as the fixed effects of interest, together with time point and randomised group as fixed interaction terms. The model included random intercepts for centre and individual to allow for the correlations of measurements within each donor and site. The model reported mean QoL utility scores at each time point including the 2-year follow-up, and the differences in the mean utility scores across the randomised arms. This model assumed that missing QoL data were ‘missing at random’, conditional on the variables included in the model. 53
The SUR model reported incremental (difference in means) costs and volumes, and the incremental cost-effectiveness ratio (ICER), as the incremental cost per additional unit of blood donated from the reduced minimum interval strategies. The CIs around the ICER were constructed by applying a Taylor series expansion on the incremental estimates of cost and volume of blood donated. 54 The accompanying uncertainty around the incremental estimates, allowing for the correlation between cost and the volume of blood donated, was represented on the cost-effectiveness plane.
The base-case analyses assumed that expert opinion provided accurate unit costs for reminders to donate and deferrals, that non-attendances had a zero cost, that there were downstream health-care costs following a deferral caused by Hb below the specified levels, there were costs attributable to fainting episodes and there was capacity at static donor centres to increase donations. The statistical models for blood volume, QoL and cost assume the residuals follow a normal distribution. These assumptions were challenged in the subsequent sensitivity analyses.
Sensitivity analysis
The sensitivity analysis considered whether the conclusions from the base-case analysis were robust to alternative assumptions. Specifically, we estimated a cost for non-attendances by calculating the number of non-attendances as the difference between the observed and maximum number of attendances that could be scheduled over the 2-year follow-up period, allowing for deferrals, and recognising that some donors dropped out. In this sensitivity analysis we assigned a unit cost of reminding donors following a non-attendance as per the trial protocol (see Table 1). The sensitivity analysis also made the alternative assumption that the costs ensuing from a low Hb level, fainting episodes and the invitation to donate were zero, and so excluded them. The sensitivity analysis also considered a scenario in which additional staff costs were required for blood collection donation because the donor centres were at full capacity, and so the unit cost of donation was assumed to be £24.70 versus the base case of £7.62. Finally, we assumed that costs followed a gamma rather than a normal distribution.
Results
For both sexes, the baseline characteristics were similar across the randomised arms, as shown in Table 2. Overall, the mean [standard deviation (SD)] age for men and women was 44.7 years (14.2 years) and 40.9 years (14.0 years), respectively; 13% of men and 14% of women were categorised as having high-demand blood types, and 8% of men and 11% of women were classified as new donors. Over 90% of participants were self-defined as of white ethnic origin, and about 65% of the participants were recruited from static donor centres. The mean number of blood donations in the 2 years preceding the trial was 4.2 for men and 3.4 for women, with mean deferral for a low Hb level of 0.04 for men and 0.12 for women, and mean deferral for other reasons of 0.32 for men and 0.34–0.36 for women. The mean baseline QoL was 0.86 for men and 0.85 for women.
| Characteristic | Sex | |||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| Randomised arm | Randomised arm | |||||
| 8 weeks (N = 7417) | 10 weeks (N = 7413) | 12 weeks (N = 7411) | 12 weeks (N = 7549) | 14 weeks (N = 7545) | 16 weeks (N = 7528) | |
| Mean (SD) age (years) | 44.7 (14.1) | 44.7 (14.2) | 44.7 (14.2) | 40.77 (14.0) | 40.89 (13.9) | 40.94 (14.0) |
| Blood type, n (%) | ||||||
| High demand | 996 (13.43) | 933 (12.59) | 965 (13.02) | 1130 (14.97) | 1062 (14.08) | 1002 (13.31) |
| Standard demand | 6421 (86.57) | 6480 (87.41) | 6446 (86.98) | 6419 (85.03) | 6483 (85.92) | 6526 (86.69) |
| Ethnicity, n (%) | ||||||
| White | 6751 (91.02) | 6752 (91.08) | 6745 (91.01) | 6984 (92.52) | 6992 (92.67) | 6949 (92.31) |
| Black/mixed black | 101 (1.36) | 96 (1.30) | 100 (1.35) | 103 (1.36) | 93 (1.23) | 134 (1.78) |
| Asian/mixed Asian | 255 (3.44) | 271 (3.66) | 258 (3.48) | 171 (2.27) | 177 (2.35) | 154 (2.05) |
| Other or not stated | 310 (4.18) | 294 (3.97) | 308 (4.16) | 291 (3.85) | 283 (3.75) | 291 (3.87) |
| New donor, n (%) | ||||||
| No | 6817 (91.91) | 6818 (91.97) | 6818 (92.00) | 6742 (89.31) | 6744 (89.38) | 6727 (89.36) |
| Yes | 600 (8.09) | 595 (8.03) | 593 (8.00) | 807 (10.69) | 801 (10.62) | 801 (10.64) |
| Recruitment source, n (%) | ||||||
| Centre | 4907 (66.16) | 4840 (65.29) | 4855 (65.51) | 4851 (64.26) | 4921 (65.22) | 4901 (65.10) |
| Mobile | 1437 (19.37) | 1510 (20.37) | 1512 (20.40) | 1545 (20.47) | 1482 (19.64) | 1486 (19.74) |
| No invite | 1073 (14.47) | 1063 (14.34) | 1044 (14.09) | 1153 (15.27) | 1142 (15.14) | 1141 (15.16) |
| Mean (SD) number of deferrals for low levels of Hb in previous 2 years | 0.04 (0.24) | 0.04 (0.23) | 0.04 (0.24) | 0.12 (0.39) | 0.12 (0.38) | 0.12 (0.39) |
| Mean (SD) number of deferrals for other reasons in previous 2 years | 0.32 (0.69) | 0.32 (0.68) | 0.32 (0.69) | 0.36 (0.68) | 0.34 (0.68) | 0.34 (0.68) |
| Mean (SD) number of blood donation visits in previous 2 years | 4.19 (2.40) | 4.22 (2.42) | 4.18 (2.40) | 3.46 (1.91) | 3.45 (1.89) | 3.44 (1.93) |
| Mean (SD) SF-6D score at baseline | 0.86 (0.08) | 0.86 (0.08) | 0.86 (0.09) | 0.85 (0.09) | 0.85 (0.09) | 0.85 (0.09) |
The resource use over 2 years is presented in Table 3. For men, the mean number of blood donation visits was 7.76, 6.60 and 5.68 in the 8-, 10- and 12-week arms, respectively, and for women the corresponding average number of visits was 5.10, 4.60 and 4.01 in the 12-, 14- and 16-week arms, respectively. The average rate of deferral for low levels of Hb, per session attended, was higher in the arms that had shorter minimum donation intervals. For men, this deferral rate was 5.71% in the 8-week arm compared with 3.73% in the 10-week arm and 2.55% in the 12-week arm. For women, this deferral rate increased from 5.05% (16-week arm) to 6.63% (14-week arm) and 7.92% (12-week arm). The corresponding mean numbers of Hb-related deferrals per donor over 2 years were also higher in the randomised arms with reduced donation intervals. The proportion of deferrals caused by other reasons, the average number of fainting episodes (see Table 3) and other donor-reported health-care events (reported in Table 4) were similar across the randomised arms.
| Resource use | Sex | |||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| Randomised arm | Randomised arm | |||||
| 8 weeks (n = 7417) | 10 weeks (n = 7413) | 12 weeks (n = 7411) | 12 weeks (n = 7549) | 14 weeks (n = 7545) | 16 weeks (n = 7528) | |
| Mean blood donation visits | 7.76 | 6.60 | 5.68 | 5.10 | 4.60 | 4.01 |
| Deferrals for low levels of Hb per attendance (%) | 5.71 | 3.73 | 2.55 | 7.92 | 6.63 | 5.05 |
| Deferrals for other reasons per attendance (%) | 4.36 | 4.58 | 4.79 | 6.57 | 6.95 | 7.28 |
| Mean deferrals for low levels of Hb per donor | 0.44 | 0.25 | 0.15 | 0.40 | 0.30 | 0.20 |
| Mean deferrals for other reasons per donor | 0.33 | 0.30 | 0.27 | 0.34 | 0.32 | 0.29 |
| Mean faints per donor | 0.02 | 0.02 | 0.02 | 0.04 | 0.03 | 0.03 |
| Health-care event | Sex | |||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| Randomised arm | Randomised arm | |||||
| 8 weeks (n = 7417) | 10 weeks (n = 7413) | 12 weeks (n = 7411) | 12 weeks (n = 7549) | 14 weeks (n = 7545) | 16 weeks (n = 7528) | |
| Any serious adverse event | 284 (3.83) | 257 (3.47) | 267 (3.60) | 290 (3.84) | 289 (3.83) | 288 (3.83) |
| Doctor-confirmed heart problems | 25 (0.34) | 36 (0.49) | 21 (0.28) | 5 (0.07) | 3 (0.04) | 12 (0.16) |
| Been to hospital after a fall | 172 (2.32) | 130 (1.75) | 149 (2.01) | 230 (3.05) | 232 (3.07) | 226 (3.00) |
| Been to hospital after a transport accident | 104 (1.40) | 110 (1.48) | 115 (1.55) | 66 (0.87) | 63 (0.83) | 61 (0.81) |
The main cost-effectiveness results are presented in Table 5. For both sexes, the average SF-6D utility scores were similar across the randomised arms at the 2-year follow-up and at each of the intervening time points (see Appendix 5). For men, the average number of whole-blood donations over the 2-year follow-up period increased by 1.71 [95% confidence interval (CI) 1.60 to 1.80] for the 8- versus 12-week interval arm, and by 0.79 (95% CI 0.70 to 0.88) for the 10- versus 12-week interval arm. For women, the corresponding increase in the average number of donations was 0.85 (95% CI 0.78 to 0.92) for 12 versus 16 weeks, and 0.46 (95% CI 0.40 to 0.53) for 14 versus 16 weeks. The average costs per donor over 2 years increased for each of the reduced interval strategies. The ICERs were £9.51 (95% CI £9.33 to £9.69) for the 8- versus 12-week interval arm for men, and £10.17 (95% CI £9.80 to £10.54) for the 12- versus 16-week interval arm for women. Figure 2 shows that when the cost-effectiveness results are plotted on the cost-effectiveness plane, the distributions of the mean costs and mean number of donations are centred tightly around the means.
| Resource use | Sex | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||||
| Randomised arm | Mean (95% CI) difference | Randomised arm | Mean (95% CI) difference | |||||||
| 8 weeks (n = 7417) | 10 weeks (n = 7413) | 12 weeks (n = 7411) | 8 vs. 12 weeks | 10 vs. 12 weeks | 12 weeks (n = 7549) | 14 weeks (n = 7545) | 16 weeks (n = 7528) | 12 vs. 16 weeks | 14 vs. 16 weeks | |
| Mean SF-6D score | 0.84 | 0.84 | 0.84 | 0.002 (–0.002 to 0.006) | –0.001 (–0.004 to 0.003) | 0.82 | 0.82 | 0.82 | 0.001 (–0.003 to 0.005) | 0.003 (–0.001 to 0.007) |
| Mean number of whole-blood donationsa | 6.89 | 5.98 | 5.19 | 1.71 (1.60 to 1.80) | 0.79 (0.70 to 0.88) | 4.29 | 3.91 | 3.45 | 0.85 (0.78 to 0.92) | 0.46 (0.40 to 0.53) |
| Mean costs (£)a | 61 | 52 | 45 | 16 (15 to 17) | 7 (6 to 8) | 41 | 37 | 33 | 9 (8 to 9) | 5 (4 to 5) |
| ICERa | 9.51 (9.33 to 9.69) | 9.00 (8.66 to 9.34) | 10.17 (9.80 to 10.54) | 9.98 (9.32 to 10.64) | ||||||
FIGURE 2.
Uncertainty in the incremental costs (£) and number of whole-blood donations, and their joint distribution, for reduced interval strategies vs. standard practice (control arm) over 2 years’ follow-up. (a) Male and (b) female.
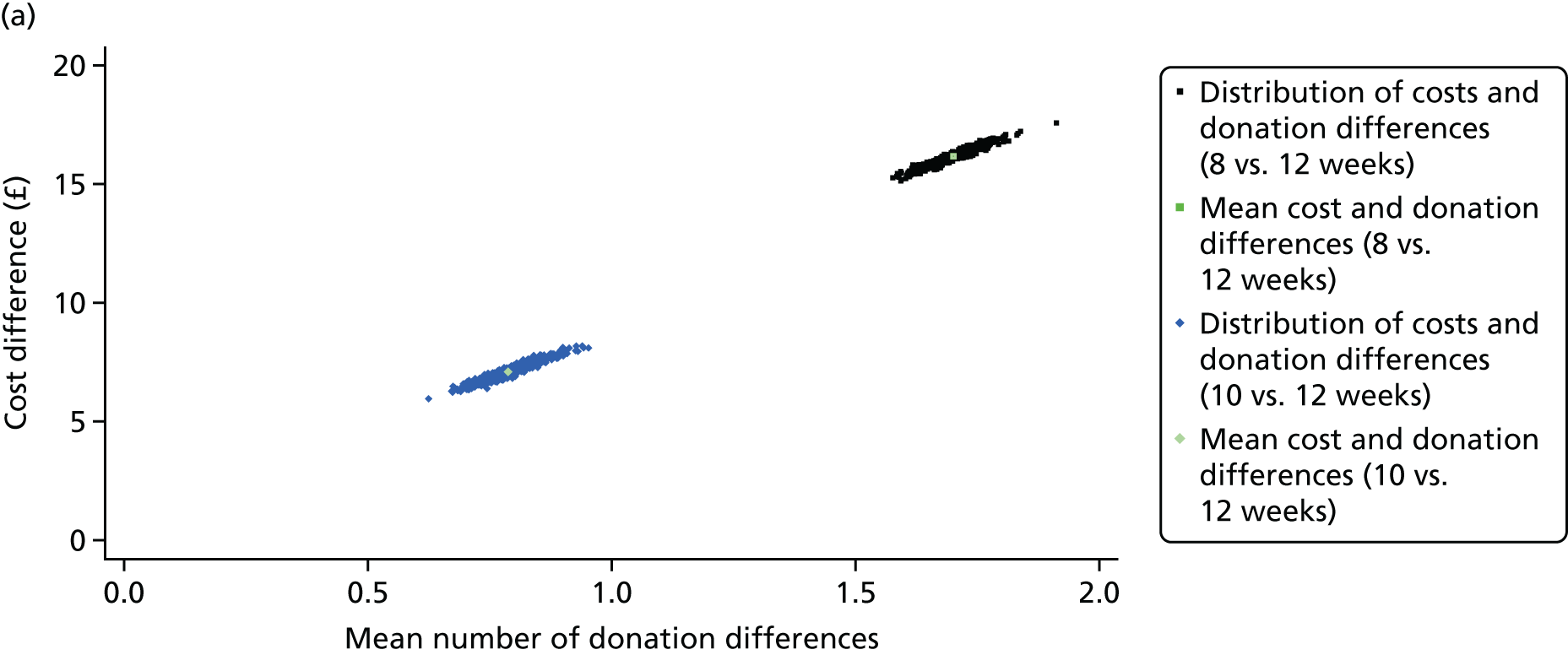

The likelihood ratio test shows that the inclusion of interaction effect for subgroups by randomised group improved model fit (male: χ2 = 79.28, p = 0.0002; female: χ2 = 46.55, p = 0.0153). The subgroup results reported in Table 6 show that the ICERs were similar across almost all subgroups. The main exception was for the 14- versus 16-week contrast for women whose ethnicity was defined as black/mixed black. For this subgroup, the incremental effect of the reduced interval on the number of whole-blood donations was small, and so the accompanying ICER was large (> £200). However, the sample size for this subgroup is low, and the estimates are somewhat unstable (n = 330 across all three arms).
| Characteristic | Sex | |||
|---|---|---|---|---|
| Men | Women | |||
| 8 vs. 12 weeks | 10 vs. 12 weeks | 12 vs. 16 weeks | 14 vs. 16 weeks | |
| Age group (years) | ||||
| 17–30 | 9.23 | 8.54 | 11.78 | 11.03 |
| 31–45 | 9.24 | 8.64 | 10.04 | 9.99 |
| 46–60 | 9.59 | 9.01 | 9.87 | 9.70 |
| ≥ 61 | 10.24 | 10.48 | 9.32 | 9.32 |
| Blood type | ||||
| High demand | 9.48 | 9.42 | 9.40 | 9.23 |
| Standard demand | 9.51 | 8.95 | 10.33 | 10.13 |
| Ethnicity | ||||
| White | 9.47 | 8.93 | 10.10 | 9.89 |
| Black/mixed black | 13.34 | 13.29 | 13.26 | 257.80 |
| Asian/mixed Asian | 10.24 | 10.78 | 18.81 | 4.62 |
| Other or not stated | 9.42 | 9.21 | 10.22 | 10.67 |
| New donor | ||||
| No | 9.52 | 9.02 | 10.16 | 9.85 |
| Yes | 9.32 | 8.8 | 10.36 | 13.11 |
| Recruitment source | ||||
| Donor centre | 9.57 | 9.06 | 10.05 | 10.36 |
| Mobile session | 9.32 | 8.69 | 10.08 | 8.89 |
| No invite | 9.50 | 9.24 | 10.82 | 10.14 |
The sensitivity analysis found that the estimates of incremental cost-effectiveness were generally similar when alternative assumptions were taken to those in the base-case analysis, shown in Table 7. The base-case results were most sensitive to the inclusion of the additional staff costs required if the donor centres had no capacity to collect the additional units of blood. Under this scenario, the ICERs increased to £26.59 (95% CI £26.41 to £26.77) for the 8- versus 12-week interval arm for men, and to £27.25 (95% CI £26.88 to £27.62) for the 12- versus 16-week interval arm for women, compared with the base-case ICERs of £9.51 (95% CI £9.33 to £9.69) and £10.17 (95% CI £9.80 to £10.54), respectively.
| Alternative assumptions | Sex | |||
|---|---|---|---|---|
| Men | Women | |||
| 8 vs. 12 weeks | 10 vs. 12 weeks | 12 vs. 16 weeks | 14 vs. 16 weeks | |
| Base case | 9.51 | 9.00 | 10.17 | 9.98 |
| Including costs of non-attendance | 13.91 | 12.54 | 12.90 | 12.48 |
| Excluding health-care costs caused by Hb deferral | 8.56 | 8.31 | 8.88 | 8.78 |
| Excluding costs of fainting | 9.44 | 8.96 | 10.09 | 9.94 |
| Excluding invitation costs | 9.51 | 9.00 | 10.17 | 9.98 |
| Additional staff costs to collect extra blood | 26.59 | 26.08 | 27.25 | 27.06 |
| Gamma distribution for costs | 9.22 | 8.70 | 7.45 | 8.80 |
Discussion
The main finding from this trial-based CEA undertaken in 25 static donor centres was that, compared with the control arm strategy, which was the current minimum interval specified by the NHSBT, the reduced minimum donation interval strategies increased the average number of donations, at a small additional average variable cost over 2 years. The rate of deferral because of low levels of Hb and the average number of deferrals per donor was higher for the reduced minimum interval strategies. The main finding was that reducing the minimum recall interval yields additional units of blood donated at an additional average variable cost of around £10. This finding was generally similar across the subgroups considered, including those with high-demand blood types, among whom it is particularly important to increase the volume of blood supplied. The time horizon was limited to 2 years in accordance with the follow-up period in the INTERVAL trial. The CEA did not consider the effects that the increased rates of deferrals may have on the rate at which donors leave the donation register, and any additional costs from, for example, recruiting new donors.
The main trial analysis reported that reducing donation intervals resulted in a higher proportion of donors (especially male) reporting symptoms such as fatigue, potentially as a result of iron deficiency or blood donation, but did not find any evidence of an effect on randomised arm according to the physical or mental summary score of the SF-36. 47 The CEA also found that donors’ QoL, measured according to the SF-6D utility score, was similar across arms for all time points concerned. There were no differences in the self-reported fainting episodes or adverse events across the arms. The CEA found that the reduced minimum donation intervals did not lead to an increase in health-care resource use, or in morbidity among donors. The SF-6D is a recommended measurement of health utility, an appropriate approach was taken to handling missing data, and the average utility scores were similar to those for the age- and sex-matched general population (0.81 for men and 0.79 for women). 55 Unlike other generic instruments, such as the EuroQol 5-dimension questionnaire, the SF-6D does include dimensions for vitality and fatigue, but there is still no guarantee that it is sensitive to the small differences in minor symptoms, such as dizziness or restless leg syndrome, reported across randomised arms in the main INTERVAL trial analysis. 47
The INTERVAL trial protocol specified a comprehensive system of reminders to donors to make and keep appointments to donate blood. The trial protocol therefore implied some additional resource use versus routine NHSBT practice, but as this reminder system may have been important in encouraging donors to attend donation sessions according to the frequency observed during the RCT, the costs of reminders for sessions attended were according to the trial protocol. Indeed, a non-randomised comparison of the donation frequency pre versus post randomisation suggests that, for both sexes, the donation frequency for the control arm increased by around 40%, which could reflect a ‘trial effect’ or other temporal differences beyond the INTERVAL trial. As part of an extension to the INTERVAL trial (Phase II), approximately 50% of INTERVAL donors agreed to continue on their previously allocated study donation interval for a further period of 6–24 months, and also to be randomised to receive the enhanced trial protocol reminders or the reminders undertaken as part of routine NHSBT practice. It would be useful to extend the economic analysis undertaken here to examine whether or not the more intensive reminder system undertaken as part of the original INTERVAL trial was in itself cost-effective. 56,57
A strength of the CEA is that it used data from a large, well-conducted RCT with complete follow-up data for the main end points of interest, and included as a control arm the current minimum donation interval in England. The large sample size meant that it was possible to report both the overall effect of alternative minimum donation intervals (on frequency of attendance to donate whole blood and deferrals) and the effect according to subgroups of key policy relevance. The trial provided an excellent vehicle for estimating the rate of deferral per donation on attendance according to alternative donor characteristics. In particular, the INTERVAL trial analysis found that, for 7% of Hb-related deferrals, the level of Hb was sufficiently low to imply additional health-care consultations and costs. These estimates of deferral rates will be used in Chapter 3 in adjusting the estimates from the stated preference survey to predict actual rates of successful donation. The estimated deferral rates will also be used in the subsequent economic evaluation of a wider range of strategies to increase donation frequency (see Chapters 3 and 4).
The economic evaluation of the INTERVAL strategies has the following limitations. First, although the INTERVAL trial followed donors for 2 years, the higher deferral rates from low levels of Hb reported in the reduced interval arms could lead to a higher rate of donors leaving the blood donation registry in the long run. This is plausible if the levels of Hb, which were on average lower in the reduced interval arms after 2 years, continue to diverge. 47 The Phase II INTERVAL extension study will provide some additional evidence about the relative rate at which donors leave the register, and the potential higher costs of replacing them with new donors. Second, the RCT was undertaken at 25 static donor centres; it is unclear whether or not it would be cost-effective to roll out the reduced interval strategy to mobile sessions. Third, the CEA did not include the full range of costs that may differ according to the minimum recall interval. In particular, data were not available on the number of non-attendances for each individual. In the sensitivity analysis, when we approximated these costs, we found that the ICERs of the reduced interval strategies increased somewhat, but generally remained below an additional variable cost of £30 for an additional unit of blood donated. The results were most sensitive to the assumption that there would be sufficient capacity within the static donor centres to collect the additional units of blood donated. This assumption may not be realistic if this strategy is rolled out to all donors attending static centres. However, if the reduced interval strategies are applied only to those groups whose blood type is in high demand, then current capacity (on average, 75%) may be sufficient to collect the additional units of blood; in which case the base-case ICER is more relevant (around £10 per additional unit of blood collected).
The INTERVAL trial considered only a single set of strategies for maintaining the future blood supply, and yet NHSBT may consider these strategies in conjunction with other changes to the blood service (e.g. extending opening hours at donor centres). Hence, studies that complement the INTERVAL trial in investigating the costs and consequences of other strategies for maintaining the supply of whole blood are required. Chapter 3 reports on surveys of non-INTERVAL but also ex-INTERVAL donors that were undertaken to elicit their preferences for alternative blood donation strategies (including reduced donation intervals). The subsequent economic evaluation then uses estimates from the survey, combined with estimates from INTERVAL for deferral rates, to estimate the cost-effectiveness of a range of strategies for maintaining the blood supply.
Chapter 3 Measuring and analysing donors’ preferences for alternative changes to the blood collection service
Introduction
This chapter reports the design and analysis of the SP surveys. Thus, it is concerned with our second objective: to investigate the frequency with which donors are willing to donate whole blood according to alternative future changes to the blood collection service.
This chapter has the following structure. Development of the stated preference survey describes the development of the SP survey, in particular emphasising the key considerations that drove our design choices. Selection of attributes and levels outlines the process by which the attributes and their levels were selected. Sample size and efficient design details the design of the survey, including the number and allocation of SP questions to participants. Administration of the surveys describes the administration of the surveys, which comprised a pilot survey, a survey of blood donors who had not participated in the INTERVAL trial (non-INTERVAL) and a survey of INTERVAL trial participants (ex-INTERVAL). Analysis of annual frequency of donation from the stated preference surveys describes the analysis of the intended annual frequency of donation. Survey respondents reports response rates to the non-INTERVAL and ex-INTERVAL surveys and describes the respondents in terms of a number of characteristics. Results reports the results of the analysis of the SP surveys and the chapter concludes with Discussion.
Development of the stated preference survey
Five main considerations influenced the design of the SP survey. First, the purpose of the survey was to help inform future changes to the blood collection service. Hence, the attributes chosen were those judged to reflect aspects of the blood collection service that the NHSBT could change in the short term. This has important implications for the selection of attributes, specifically attributes that are under the control of the NHSBT. Second, there were separate male and female questionnaires because of well-established differences between men and women in permitted donation frequency, and our explicit interest in the strategies contrasted in the INTERVAL trial. Third, the donation scenarios presented to donors distinguished between the last place (LP) the respondent donated and a different place (DP). This is necessary because some changes in the opportunity to donate would involve the donor donating in a DP, whereas for others the venue would not change. Fourth, we chose an unlabelled design, which in this context implies that an opportunity to donate was not indicated as being at a static donor centre or a mobile session. Fifth, our interest lay in making predictions regarding frequency of donation by existing blood donors. We were not concerned with the broader question of what factors influence decisions to become a donor or to stop donating.
Donors donate at different annual rates depending on a combination of their personal characteristics and on the opportunities that they have to donate. In effect, we are assuming that donors consider the marginal costs and benefits of donating blood. Changes that increase the cost to the donor of donating (e.g. increased travel time) will tend, other things being equal, to reduce the frequency with which they donate. However, changes that increase the benefits to donors of donating (e.g. provision of a health report) will increase their preferred frequency of donation.
The maximum number of donations allowed annually depends on the donor’s sex. Some donors may be unconstrained in that they are already donating at their preferred frequency, whereas others may be constrained by this maximum in the sense that they would like to donate more frequently. Changes to the maximum frequency with which donors can donate will not change the costs and benefits of donating to an individual donor. However, decreases in the interval required between donations will result in previously constrained donors being able to donate more often. More speculatively, an increased permitted frequency of donation might alter donors’ perceptions regarding a donation norm, which may encourage some donors to donate more frequently.
Selection of attributes and levels
The selection of attributes and levels always involves compromises given limits on the feasible number of scenarios about which to ask respondents. Our choice of attributes to describe the opportunities to donate was informed by a rapid literature review, input from policy-makers at the NHSBT and preliminary findings from qualitative research with blood donors participating in the INTERVAL trial [R Lynch and S Cohn, London School of Hygiene & Tropical Medicine (LSHTM), personal communication, October 2015]. The pilot study explored five attributes identified as pertinent to determining the impact of a number of policy-relevant strategies, namely travel time, opening hours, total donation time, provision of a health report and the maximum number of donations per year. The first three attributes influence the cost to the donor of the blood donation, whereas the provision of a health report potentially increases the benefits to the donor. The INTERVAL trial is investigating the safety of increasing the maximum frequency of donation (three or four times per year for women and 4–6 times for men). This attribute is included to understand how donors might respond if the limits were altered alongside the other possible future changes to the blood service. As noted earlier, this does not affect the costs or benefits of donation but rather the scope for donors to achieve their preferred donation frequency.
Donation venue was not included as an attribute, but the survey had two sections: the first asks the donor to think about donation opportunities at the ‘last place you gave blood’ (LP), and the second asks the donor to ‘imagine you were asked to donate at a different place’ (DP). This was in order to capture more of the context not included in the attributes (e.g. the community aspect of blood donation and familiarity of staff) that had been identified as important to some donors in the qualitative research conducted as part of the INTERVAL trial.
The appropriate levels for each attribute were defined according to summary estimates from the PULSE database, NHSBT market research and consultation with blood donors. Table 8 shows the attributes and levels used in the non-INTERVAL and ex-INTERVAL surveys (see Appendix 6 for those used in the pilot survey).
| Attributes as described in the SP survey | Levels as described in the SP survey |
|---|---|
| 1. Travel time |
10 minutes shorter than your typical travel time Your typical travel time 15 minutes longer than your typical travel time 30 minutes longer than your typical travel time |
| 2. Opening times |
9 a.m.–12 p.m. and 2–5 p.m. 9 a.m.–5 p.m. 9 a.m.–8 p.m. 2–8 p.m. |
| 3. Appointment availability |
Every day: Monday–Sunday Every weekday: Monday–Friday 1 day every 2 months: Monday–Friday 1 day every 2 months: Saturday or Sunday |
| 4. Health report provided |
Health report provided after each blood donation Not provided |
| 5. Maximum number of donations per year | Female
|
The analysis of the pilot survey results revealed a systematic over-prediction of the frequency of blood donation compared with the donation frequency observed in practice. One potential explanation, and a view expressed at our donor workshop, was that some donors might wish to donate more frequently but face additional constraints in practice, which cause observed behaviour to diverge from that predicted. Our analysis of these discrepancies (between the predicted donation frequencies and those observed) showed that these were consistent across various subgroups. 58 For the main survey of non-INTERVAL donors, we replaced the time taken to make the donation with an appointment availability attribute. The appointment availability attribute is a means of ensuring that the SP scenarios explicitly address differences in appointment availability. Two further changes from the pilot survey were made: we reduced the number of levels for the opening times attribute from eight to four and adopted a full factorial design in place of a main effects fractional design. The reduction in opening hours levels was made in order to increase the feasibility of a full factorial design and to facilitate analysis of preference differences between subgroups of donors.
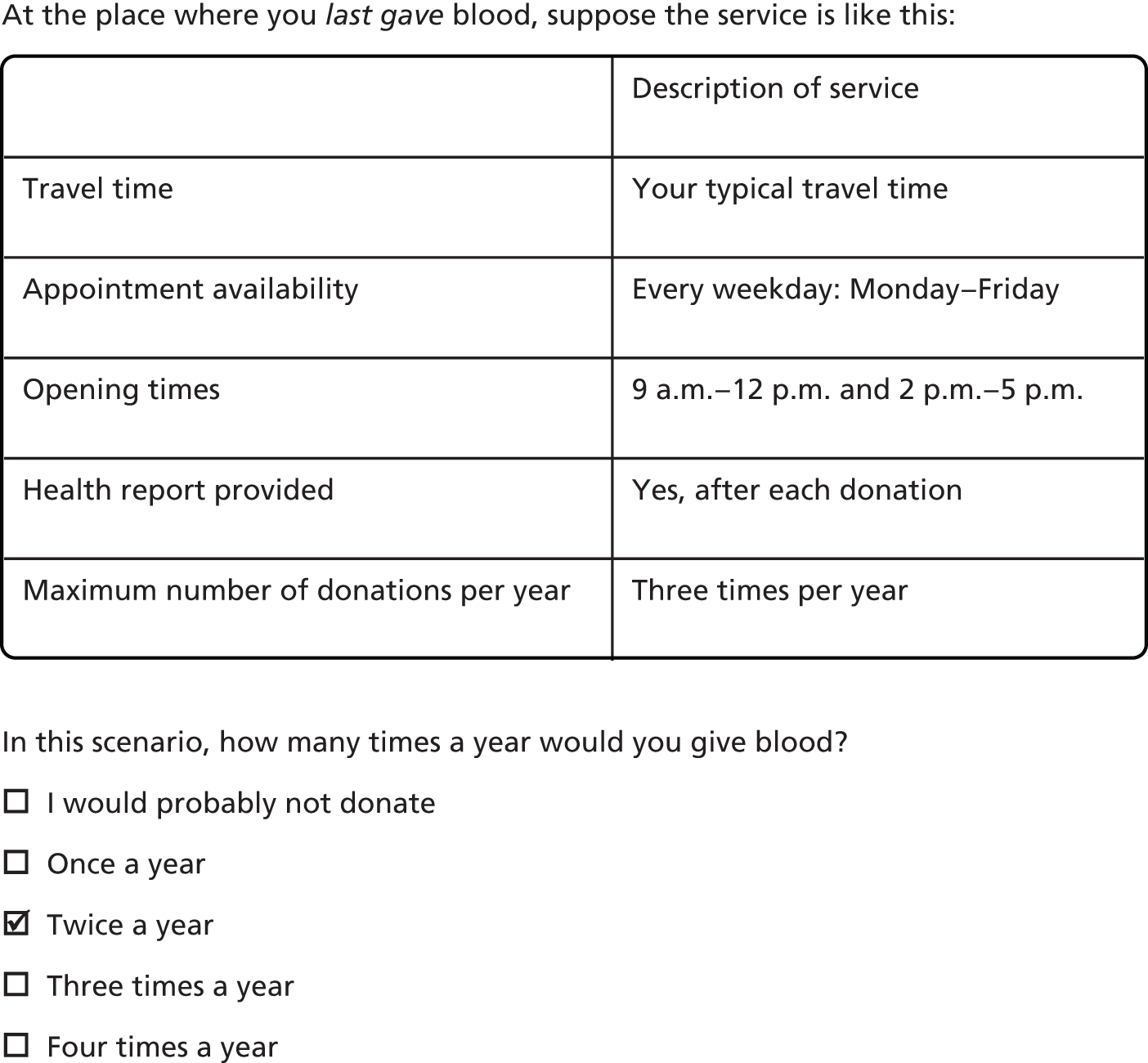
For each set of attribute levels, we asked donors to state the frequency with which they would be willing to donate blood. The survey included the option ‘I would probably not donate’. Figure 3 gives an example of a typical question.
FIGURE 3.
Example of a question from the stated preference survey.
Sample size and efficient design
The purpose of the pilot survey was threefold: (1) to ensure that our overall systems for selecting and inviting donors to participate, and for recording their responses, worked as intended; (2) to obtain reliable information on the likely response rate; and (3) to identify any issues with respect to individual questions and the questionnaire as a whole.
For the pilot study, the NHSBT issued 5016 e-mail invitations to eligible donors. We had anticipated a response rate between 10% and 20% (based on previous NHSBT surveys); however, a response rate of 25% was achieved. The mean time to complete the whole survey was just under 6 minutes (5 minutes and 47 seconds). No calls relating to our pilot survey were logged at NHSBT’s national call centre.
The pilot had a main effects design because to do otherwise was infeasible given the sample size. A full factorial design for men would have involved 96 possible LP (11 × 22 × 31 × 81) and 384 possible DP scenarios (22 × 31 × 41 × 81). For women, 64 possible LP (12 × 23 × 81) and 256 possible DP scenarios (23 × 41 × 81) would have been required. An efficient design was adopted based on the need to estimate the marginal rate of substitution between attributes with reasonable precision (main effects only). NgeneTM 1.1.2 (Choice Metrics Pty Ltd, Sydney, Australia) was used to establish an efficient design by considering each section of the survey as one choice set compared with an ‘opt-out’, resulting in eight LP and 12 DP scenarios for women (12 versions of the survey) and 24 LP and 24 DP scenarios for men (72 versions of the survey). Reflecting the greater number of male scenarios, two men were invited for every women in the pilot survey.
Sample size calculations for SP surveys are not straightforward, and although the response rate in the pilot (given its size) was informative, the likely response rate to the main survey remained uncertain. A sample size of 100,000 was chosen for the main non-INTERVAL survey. This sample size took account of the successful experience from the pilot and the practical challenges associated with inviting larger numbers. The main survey, being much larger, provided an opportunity to adopt a full factorial design. It appeared likely that there would be significant interaction effects; for example, a donor’s willingness to travel might be influenced by the opening hours and appointment availability. Taking into account the changes following the pilot survey with respect to attributes and levels, there are 96 potential LP scenarios for men (42 × 3 × 2) and 384 potential DP scenarios (43 × 3 × 2). The LP scenarios can be divided into 48 blocks of two, and the DP scenarios into 96 blocks of four. For women, the 64 LP scenarios (42 × 22) and 256 DP scenarios (43 × 22) can be divided into 32 blocks of two and 64 blocks of four, respectively. As in the pilot survey, two men were invited for every women in the non-INTERVAL survey, because there were many more possible scenarios in the male survey.
Administration of the surveys
The non-INTERVAL survey received ethics approval from the NHS (reference number 16/YH/0023) and LSHTM (reference number 10384) Research Ethics Committees in November 2015 and January 2016, respectively, and non-substantial amendments were made following the pilot survey in May 2016. For the final protocol for the survey of non-INTERVAL donors, see Report Supplementary Material 1. A total of 100,000 donors were randomly selected from the PULSE database to be invited to participate in the survey of non-INTERVAL donors, according to the following criteria: 17–70 years old, donation of at least one unit of whole blood in the past 12 months, e-mail address held by NHSBT and residence in mainland England. Donors were excluded from the surveys if they were temporarily suspended from giving blood (e.g. donors who had recently had a tattoo), had previously stated that they did not want to participate in surveys or had received a request to participate in a survey or research from the NHSBT (including the INTERVAL trial) in the preceding 6 months. Owing to the recent NHSBT communication policy, women with AB-positive (AB+) blood were also excluded. Selected donors were sent an e-mail invitation from the NHSBT with a link to the online survey built and hosted on FluidSurveysTM (1 January 2015 version; SurveyMonkey®, Palo Alto, CA, USA). For the e-mail invitation sent to non-INTERVAL donors, see Report Supplementary Material 2, and for the consent form and donor information sheet, see Report Supplementary Material 3. Donors who did not complete the survey (excluding those who refused consent) within the first 3 days were sent a reminder e-mail, and the survey closed 3 days after the reminder e-mail. Figure 4 provides a CONSORT-style diagram for the survey.
FIGURE 4.
A CONSORT-style diagram stated preference survey of non-INTERVAL participants. a, Complete PULSE data are not available for 24 of these donors; b, complete PULSE data are not available for 11 of these donors.
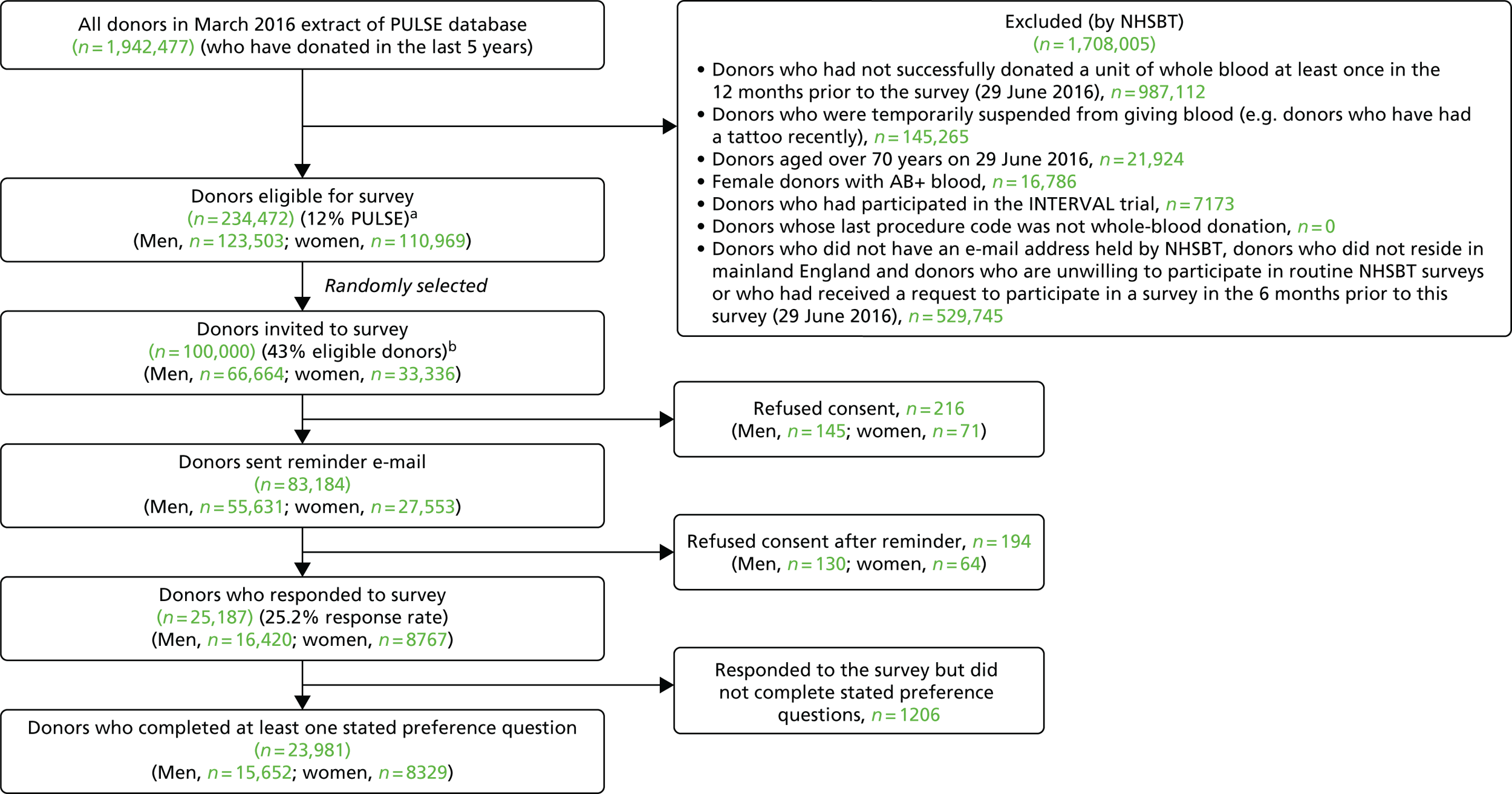
To assess the generalisability of the survey results, the characteristics of the donors who completed the survey were compared with a larger PULSE sample of donors who have donated at least once in the 12 months prior to March 2016.
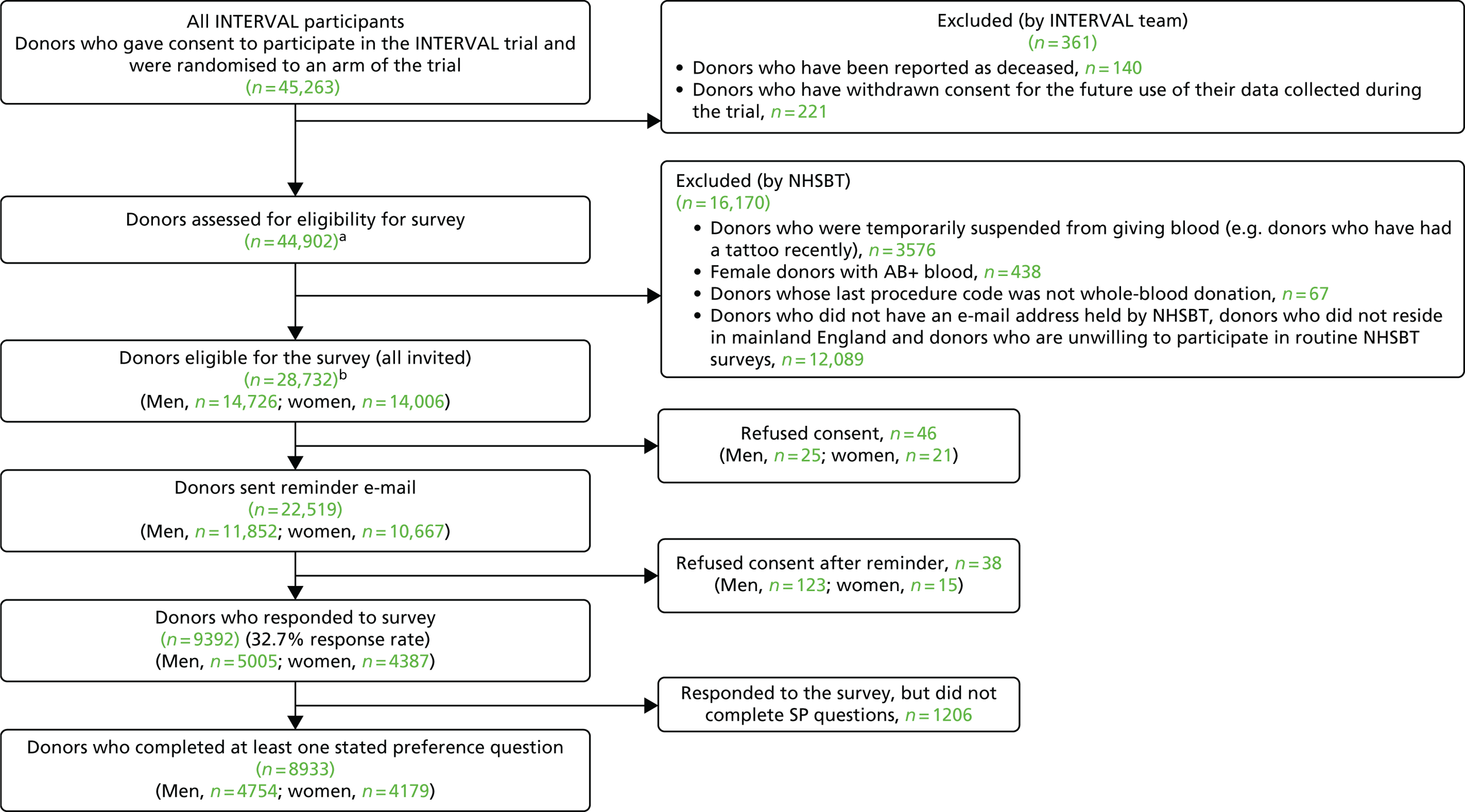
We repeated the same SP survey with the ex-INTERVAL participants using similar exclusions to the previous surveys, but this time did not exclude donors if they had not given blood in the 12 months prior to the survey (in December 2016). For the final protocol of the survey of ex-INTERVAL donors see Report Supplementary Material 4. The ex-INTERVAL participants are of particular interest because many of them have experienced the higher rates of donation featured in some of the SP scenarios. Moreover, all INTERVAL trial donations were made at donor centres and the non-INTERVAL survey had relatively small numbers whose last donation took place in a donor centre (n = 2970), reflecting that most blood in England is collected at mobile sessions. All eligible ex-INTERVAL participants were invited, by e-mail, to participate in the survey. For the ex-INTERVAL donors the invitation e-mail and consent and donor information sheets are available in Report Supplementary Material 5 and 6, respectively. Figure 5 provides a CONSORT-style diagram for the ex-INTERVAL survey.
FIGURE 5.
A CONSORT-style diagram survey of ex-INTERVAL participants. a, Complete PULSE data are not available for 32 of these donors; b, complete PULSE data are not available for one of these donors.
Examples of the non-INTERVAL (which are the same as those administered to the ex-INTERVAL sample) and pilot surveys are reproduced in Appendix 7. In addition to the SP questions, all respondents to the pilot, non-INTERVAL and ex-INTERVAL surveys were asked to complete a series of background questions (see Appendix 8). These included questions about how often the donor recalled giving blood in the past 12 months and how often they wanted to give blood. Regarding the last donation visit, questions related to from where they travelled, how they travelled, how far they travelled, how long it took, how they made the appointment and how easy it was to get a suitable appointment. Finally, they were asked whether their total visit duration was < 1 hour or > 1 hour.
Analysis of annual frequency of donation from the stated preference surveys
The primary purpose of the SP surveys was to predict the average number of whole-blood donations per year given different opportunities to donate, for example with respect to opening hours and travel time. These predictions were central to the assessment of the cost-effectiveness analysis of alternative NHSBT strategies (see Chapter 4).
We chose to use an ordered logit model for the base-case analysis to recognise that the response variable was categorical, but also had a natural ordering (three times per year, two times per year, etc.). The sensitivity analyses examined whether or not the findings were sensitive to the choice of model by considering two alternative models: the two-part model, which recognises that the response ‘I would probably not donate’ is a different kind of response from once, twice or three times per year; and a gamma model, which treats the responses as continuous, but with a lower bound at zero.
The SP surveys for male and female donors had different levels for the minimum donation interval attribute, and so we analysed the responses for each sex separately. We investigated whether or not there was effect modification according to donor subgroup. We estimated two-way interactions of each attribute with the following subgroups: age, blood type, ethnicity and venue for last whole-blood donation (see definitions in Survey respondents). These estimated two-way interactions were used to report results according to donor subgroup.
We used the estimated coefficients from the ordered logit model to predict each individual’s donation frequency according to the alternative levels for each attribute. This was done in two stages. First, a predicted probability for each donor for each category of response variable was calculated from the coefficients of the ordered logit regression model. Second, for each level of each attribute, the expected annual donation frequency for each donor was calculated using the predicted probabilities estimated in the first stage for each response category. The average of these predicted annual donation frequencies according to each attribute level was reported, together with the 95% uncertainty intervals, to reflect the variation in these predictions across the survey sample. We also used the estimated coefficients from the ordered logit model to report the proportion of donors who said that they ‘probably would not donate’ according to each attribute level.
To assess the concern that donors may overstate their donation frequency, we then compared each individual’s predicted annual frequency with their actual donation frequency (recorded in the PULSE database) over the year preceding the survey. Annual donation frequency was predicted by setting attribute levels to represent the donor’s most recent experience, as recorded in PULSE in March 2016.
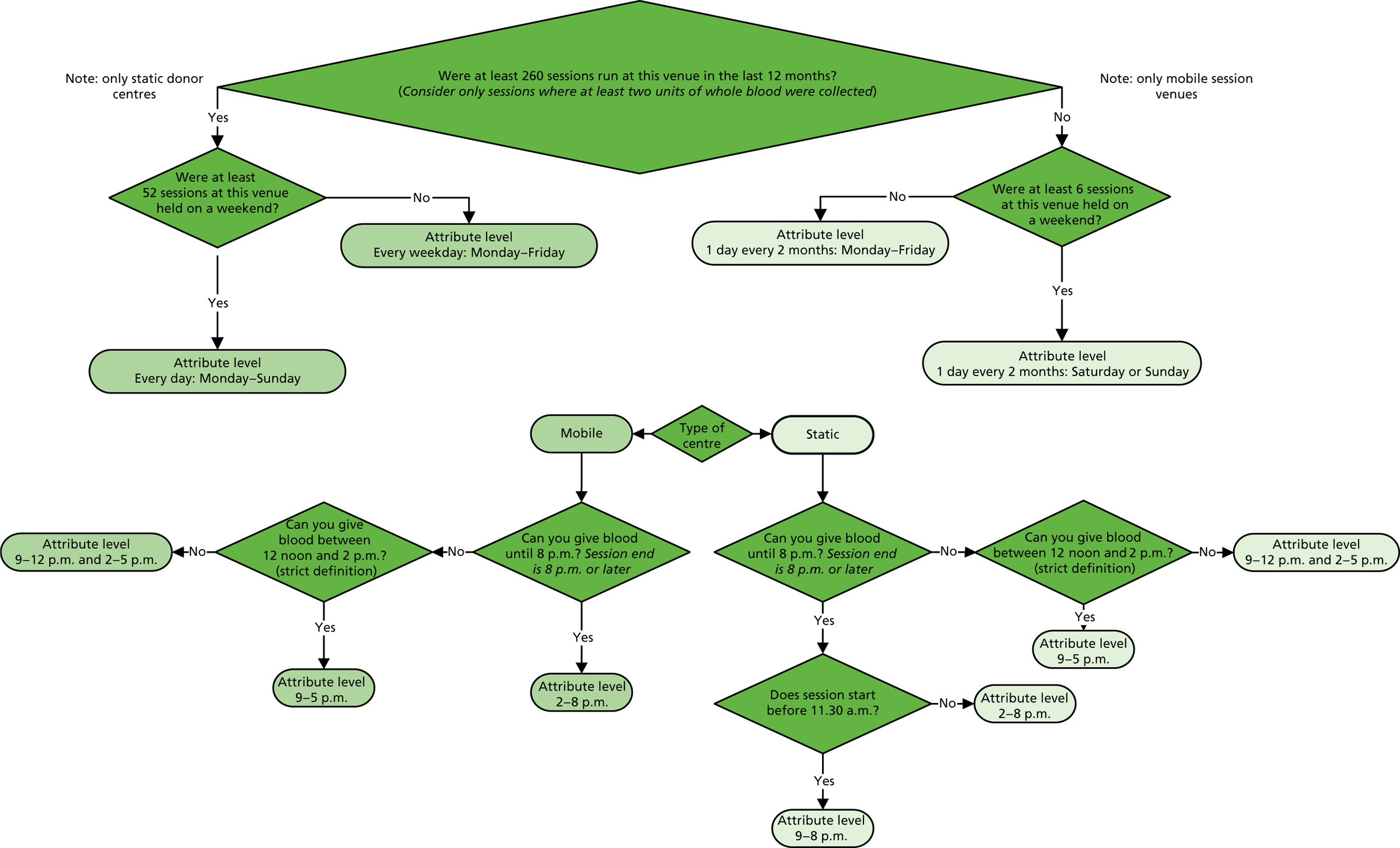
The algorithm used to determine the current service-level characteristics is shown in Figure 6. We then adjusted these predictions using the predicted probability of deferral from the INTERVAL trial arms assigned to the current minimum donation intervals (see Chapter 2). The average discrepancy between the predicted donation frequency and observed donation frequency was reported overall, for men and women, and for each subgroup, with 95% CIs to reflect the uncertainty in the predictions across individuals. However, these CIs do not reflect uncertainty in the estimation of the models used to predict intended donation frequency or deferral rates.
FIGURE 6.
Attributing current service-level characteristics.
Although the surveys were designed to facilitate the prediction of donation frequencies for a range of donation opportunities rather than in order to understand the factors which lead donors to continue or discontinue donating, one of the potential responses was ‘I would probably not donate’. This response can give some insight into, for example, whether increasing travel time might lead more donors to discontinue donation. We report the use of this response category by subgroup.
The non-INTERVAL sample and the eligible ex-INTERVAL participants were invited to complete exactly the same questionnaire. The preferences of the ex-INTERVAL respondents who had been in the intervention arms of INTERVAL may well differ from those of the non-INTERVAL sample, partly because of the former group having experienced shorter donation intervals. However, we would anticipate the preferences of the non-INTERVAL sample whose last donation was at a static donor centre to be more similar to those of ex-INTERVAL control arm participants. They might still differ by virtue of the ex-INTERVAL participants all having expressed a willingness to be randomised to arms with more frequent invitations to donate. If the preferences of the two groups are sufficiently similar, more precise predictions regarding future donation behaviour can be obtained by pooling the two samples.
Survey respondents
The overall response rates were 25.2% for the non-INTERVAL survey and 32.4% for the ex-INTERVAL survey (see Figures 4 and 5). Table 9 compares four groups of male whole-blood donors: respondents to the non-INTERVAL survey, invitees to the non-INTERVAL survey, eligible donors and all donors. These comparisons are made with respect to five donor characteristics: age (17–30, 31–45, 46–60 and ≥ 60 years), blood type (distinguishing high and standard demand), ethnicity [distinguishing white, black/mixed black, Asian/mixed Asian and not stated (for detail on how these are defined, see Appendix 3)], whether or not a nursery donor, and the venue where the donor last gave blood (static donor centre or mobile session). High-demand blood types are O–, A– and B–, and standard-demand blood types are O positive (O+), A positive (A+), B positive (B+), AB+ and AB negative (AB–). A nursery donor is defined as a donor who has given blood 1–4 times in the past 5 years, whereas those donating five times or more in the last 5 years are not nursery donors.
| Characteristic | Donors, n (%) | |||
|---|---|---|---|---|
| Responded to the survey (N = 15,652) | Invited to the survey (N = 66,656) | Eligible for the survey (N = 123,491) | All in March 2016 extract of PULSE database (N = 353,763) who had donated in the past 12 months | |
| Age group (years) | ||||
| 17–30 | 1646 (10.52) | 16,925 (25.39) | 31,307 (25.35) | 73,411 (20.75) |
| 31–45 | 3441 (21.98) | 17,977 (26.97) | 33,211 (26.89) | 86,583 (24.47) |
| 46–60 | 6825 (43.61) | 22,547 (33.83) | 41,982 (34.00) | 131,920 (37.29) |
| ≥ 60 | 3740 (23.89) | 9207 (13.81) | 16,991 (13.76) | 61,849 (17.48) |
| Blood type | ||||
| High demand | 1551 (9.91) | 6273 (9.41) | 11,606 (9.40) | 46,998 (13.29) |
| Standard demand | 14,101 (90.09) | 60,383 (90.59) | 111,885 (90.60) | 306,765 (86.71) |
| Ethnicity | ||||
| White | 14,639 (93.53) | 60,559 (90.85) | 112,270 (90.91) | 323,912 (91.56) |
| Black/mixed black | 98 (0.63) | 738 (1.11) | 1411 (1.14) | 3518 (0.99) |
| Asian/mixed Asian | 367 (2.34) | 2992 (4.49) | 5430 (4.40) | 12,677 (3.58) |
| Other or not stated | 548 (3.50) | 2367 (3.55) | 4380 (3.55) | 13,656 (3.86) |
| Nursery donor | ||||
| Yes | 3542 (22.63) | 26,144 (39.22) | 48,636 (39.38) | 110,279 (31.17) |
| No | 12,110 (77.37) | 40,512 (60.78) | 74,855 (60.62) | 243,484 (68.83) |
| Venue | ||||
| Centre | 1307 (8.35) | 7670 (11.51) | 14,241 (11.53) | 52,808 (14.93) |
| Mobile | 14,345 (91.65) | 58,986 (88.49) | 109,250 (88.47) | 300,955 (85.07) |
| Number of donations in past 12 months | ||||
| 1 | 4054 (25.90) | 28,172 (42.26) | 52,819 (42.77) | 129,404 (36.58) |
| 2 | 5096 (32.56) | 20,247 (30.38) | 37,357 (30.25) | 105,671 (29.87) |
| 3 | 5204 (33.25) | 15,112 (22.67) | 27,582 (22.34) | 93,273 (26.37) |
| 4 | 1250 (7.99) | 3023 (4.54) | 5511 (4.46) | 20,600 (5.82) |
| 5 | 39 (0.25) | 84 (0.13) | 171 (0.14) | 3196 (0.90) |
| 6 | 9 (0.06) | 18 (0.03) | 51 (0.04) | 1619 (0.46) |
As would be expected, the breakdown of eligible male donors differs from all male donors because of the exclusions applied. The distribution of those invited by age, etc., as should be the case, closely follows that of the eligible donors. Comparing the characteristics of the respondents with those of the invited donors, older donors and those indicating white ethnicity are over-represented, and nursery donors, donors making a single donation in the last 12 months and donors who last donated at a static centre are under-represented. The patterns observed for male donors are repeated for female donors, shown in Table 10.
| Characteristic | Donors, n (%) | |||
|---|---|---|---|---|
| Responded to the survey (N = 8329) | Invited to the survey (N = 33,333) | Eligible for the survey (N = 110,957) | All in March 2016 extract of PULSE database (N = 427,265) who had donated in the past 12 months | |
| Age group (years) | ||||
| 17–30 | 1663 (19.97) | 10,983 (32.95) | 36,360 (32.77) | 115,333 (26.99) |
| 31–45 | 2334 (28.02) | 9793 (29.38) | 32,841 (29.60) | 118,922 (27.83) |
| 46–60 | 2999 (36.01) | 9301 (27.9) | 31,001 (27.94) | 135,936 (31.82) |
| ≥ 61 | 1333 (16.00) | 3256 (9.77) | 10,755 (9.69) | 57,074 (13.36) |
| Blood type | ||||
| High demand | 921 (11.06) | 3647 (10.94) | 12,158 (10.96) | 64,950 (15.20) |
| Standard demand | 7408 (88.94) | 29,686 (89.06) | 98,799 (89.04) | 362,315 (84.80) |
| Ethnicity | ||||
| White | 7700 (92.45) | 30,024 (90.07) | 100,233 (90.33) | 400,968 (93.85) |
| Black/mixed black | 103 (1.24) | 656 (1.97) | 2198 (1.98) | 4797 (1.12) |
| Asian/mixed Asian | 195 (2.34) | 1341 (4.0) | 4283 (3.86) | 9,050 (2.12) |
| Other or not stated | 331 (3.97) | 1312 (3.94) | 4243 (3.82) | 12,450 (2.91) |
| Nursery donor | ||||
| Yes | 3024 (36.31) | 16,544 (49.63) | 55,205 (49.75) | 173,223 (40.54) |
| No | 5305 (63.69) | 16,789 (50.37) | 55,752 (50.25) | 254,042 (59.46) |
| Venue | ||||
| Centre | 746 (9.97) | 3748 (11.24) | 12,006 (10.82) | 55,003 (12.87) |
| Mobile | 7583 (91.04) | 29,585 (88.76) | 98,951 (89.18) | 372,262 (87.13) |
| Number of donations in past 12 months | ||||
| 1 | 3094 (37.15) | 16,963 (50.89) | 56,791 (51.18) | 187,862 (43.97) |
| 2 | 2967 (35.62) | 10,393 (31.18) | 34,741 (31.31) | 140,313 (32.84) |
| 3 | 2063 (24.77) | 5516 (16.55) | 18,023 (16.24) | 89,938 (21.05) |
| 4 | 204 (2.45) | 459 (1.38) | 1392 (1.25) | 8860 (2.07) |
| 5 | 1 (0.01) | 1 (0.00) | 6 (0.01) | 254 (0.06) |
| 6 | 0 (0.00) | 1 (0.00) | 4 (0.00) | 38 (0.01) |
Tables 11 and 12, men and women, respectively, provide a comparison of ex-INTERVAL respondents, eligible ex-INTERVAL participants (all of whom were invited) and all those assessed for eligibility, using the same donor characteristics. For both men and women, nursery donors and donors with zero donations in the last 12 months are under-represented in those eligible for the SP survey. As can be seen for both men and women, respondents differ from those invited in that younger (older) donors are under-represented (over-represented) and nursery donors and those who made no or only one donation in the last 12 months are under-represented.
| Characteristic | Donors, n (%) | ||
|---|---|---|---|
| Responded to the survey (N = 4754) | Eligible for the survey (all invited) (N = 14,725) | Assessed for eligibility for the survey (N = 22,249) | |
| Age group (years) | |||
| 17–30 | 253 (5.32) | 1847 (12.54) | 3210 (14.43) |
| 31–45 | 897 (18.87) | 3878 (26.34) | 5798 (26.06) |
| 46–60 | 1971 (41.46) | 5743 (39.00) | 8001 (35.96) |
| ≥ 61 | 1633 (34.35) | 3257 (22.12) | 5240 (23.55) |
| Blood type | |||
| High demand | 643 (13.53) | 1946 (13.22) | 2895 (13.01) |
| Standard demand | 4111 (86.47) | 12,779 (86.78) | 19,354 (86.99) |
| Ethnicity | |||
| White | 4464 (93.90) | 3512 (91.76) | 20,256 (91.04) |
| Black/mixed black | 39 (0.82) | 187 (1.27) | 297 (1.33) |
| Asian/mixed Asian | 106 (2.23) | 494 (3.35) | 784 (3.52) |
| Other or not stated | 145 (3.05) | 532 (3.61) | 912 (4.10) |
| Nursery donor | |||
| Yes | 18 (0.38) | 421 (2.86) | 2207 (9.92) |
| No | 4736 (99.62) | 14,304 (97.14) | 20,042 (90.08) |
| Venue | |||
| Centre | 4132 (86.92) | 13,019 (88.41) | 19,998 (89.88) |
| Mobile | 622 (13.08) | 1706 (11.59) | 2251 (10.12) |
| Number of donations in past 12 months | |||
| 0 | 154 (3.24) | 2164 (14.70) | 6914 (31.08) |
| 1 | 278 (5.85) | 1579 (10.72) | 2239 (10.06) |
| 2 | 552 (11.61) | 2162 (14.68) | 2907 (13.07) |
| 3 | 1073 (22.57) | 3084 (20.94) | 3817 (17.16) |
| 4 | 1699 (35.74) | 3783 (25.69) | 4248 (19.09) |
| 5 | 853 (17.94) | 1639 (11.13) | 1752 (7.87) |
| 6 | 145 (3.05) | 314 (2.13) | 372 (1.67) |
| Characteristic | Donors, n (%) | ||
|---|---|---|---|
| Who responded to the survey (N = 4179) | Eligible for the survey (all invited) (N = 14,006) | Assessed for eligibility for the survey (N = 22,621) | |
| Age group (years) | |||
| 17–30 | 431 (10.31) | 2562 (18.29) | 4761 (21.05) |
| 31–45 | 1043 (24.96) | 4293 (30.65) | 7079 (31.29) |
| 46–60 | 1609 (38.50) | 4828 (34.47) | 7147 (31.59) |
| ≥ 61 | 1096 (26.23) | 2323 (16.59) | 3634 (16.06) |
| Blood type | |||
| High demand | 580 (13.88) | 2028 (14.48) | 3193 (14.12) |
| Standard demand | 3599 (86.12) | 11,978 (85.52) | 19,428 (85.88) |
| Ethnicity | |||
| White | 3925 (93.92) | 13,050 (93.17) | 20,924 (92.50) |
| Black/mixed black | 52 (1.24) | 210 (1.50) | 330 (1.46) |
| Asian/mixed Asian | 76 (1.82) | 286 (2.04) | 502 (2.22) |
| Other or not stated | 126 (3.02) | 460 (3.28) | 865 (3.82) |
| Nursery donor | |||
| Yes | 65 (1.56) | 1067 (7.62) | 4085 (18.06) |
| No | 4114 (98.44) | 12,939 (92.38) | 18,536 (81.94) |
| Venue | |||
| Centre | 3639 (87.08) | 12,387 (88.44) | 20,377 (90.08) |
| Mobile | 540 (12.92) | 1619 (11.56) | 2244 (9.92) |
| Number of donations in past 12 months | |||
| 0 | 201 (4.81) | 2883 (20.58) | 8531 (37.71) |
| 1 | 429 (10.27) | 2203 (15.73) | 3247 (14.35) |
| 2 | 971 (23.24) | 3175 (22.67) | 4272 (18.89) |
| 3 | 1952 (46.71) | 4543 (32.44) | 5238 (23.16) |
| 4 | 617 (14.76) | 1178 (8.41) | 1298 (5.74) |
| 5 | 6 (0.14) | 12 (0.09) | 13 (0.06) |
| 6 | 3 (0.07) | 12 (0.09) | 22 (0.10) |
Results
The full results for the ordered logit models are presented in additional material available on the project website. The incremental effects of changes in the levels of the different attributes were estimated using the separate ordered logit models estimated for non-INTERVAL men and women and ex-INTERVAL men and womem. The predicted changes in the annual frequency of donation are shown in Figures 7–18. Figure 7 reports the results for the full male and full female samples of non-INTERVAL donors. Figures 8–11 report the results for four non-INTERVAL subgroups, namely 17- to 30-year-olds, high-demand blood types, black/mixed black donors and nursery donors. Figure 12 reports the results for the full male and full female samples of ex-INTERVAL donors. Figures 13–16 report the results for four ex-INTERVAL subgroups, namely 17- to 30-year-olds, high-demand blood types, black/mixed black donors and nursery donors.
FIGURE 7.
Incremental effect of alternative types of blood service on average number of donations per year for all non-INTERVAL respondents. For men (• n = 15,652) and women (▴ n = 8329). The uncertainty intervals reflect the 5th and 9th percentiles in the predicted donation frequency across the relevant target population.
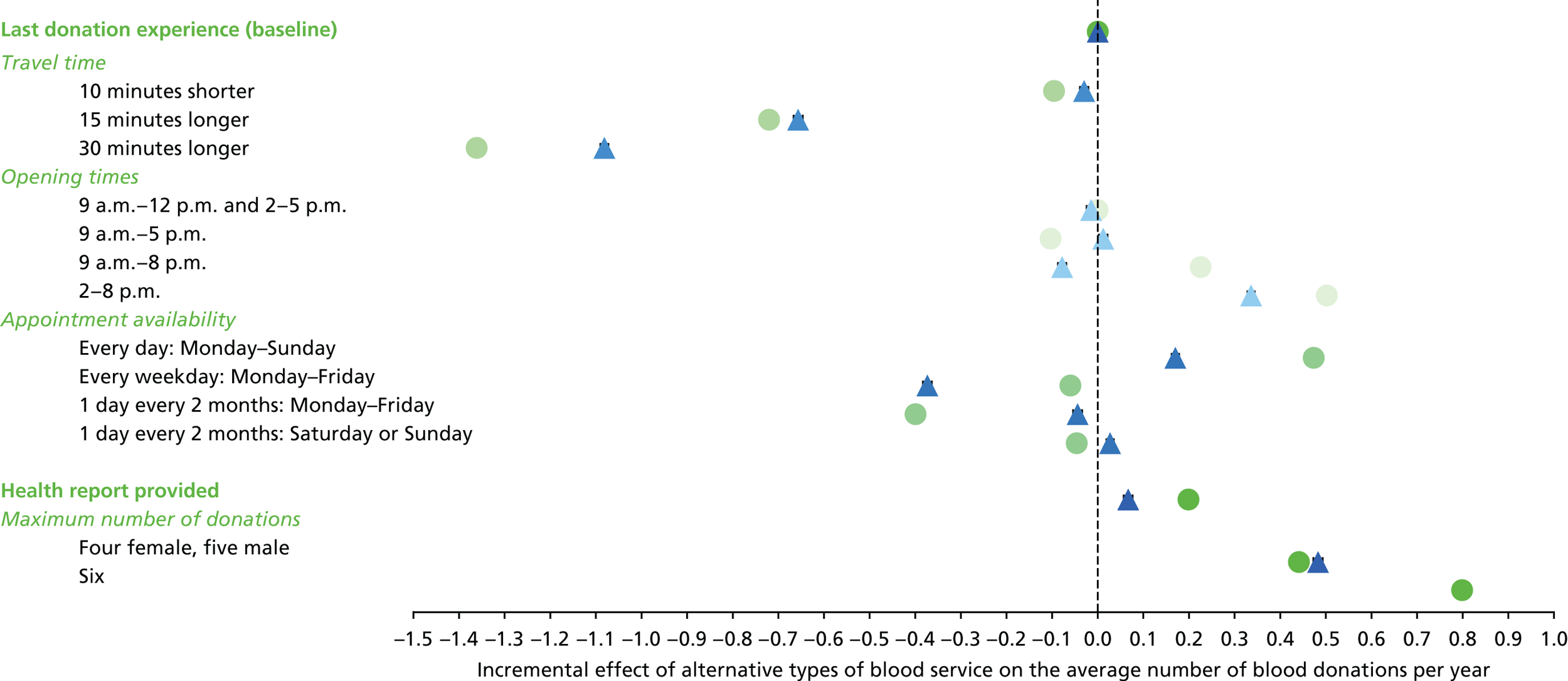
FIGURE 8.
Incremental effect of alternative types of blood service on average number of donations per year for non-INTERVAL respondents aged 17–30 years. For men (• n = 1646) and women (▴ n = 1663). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
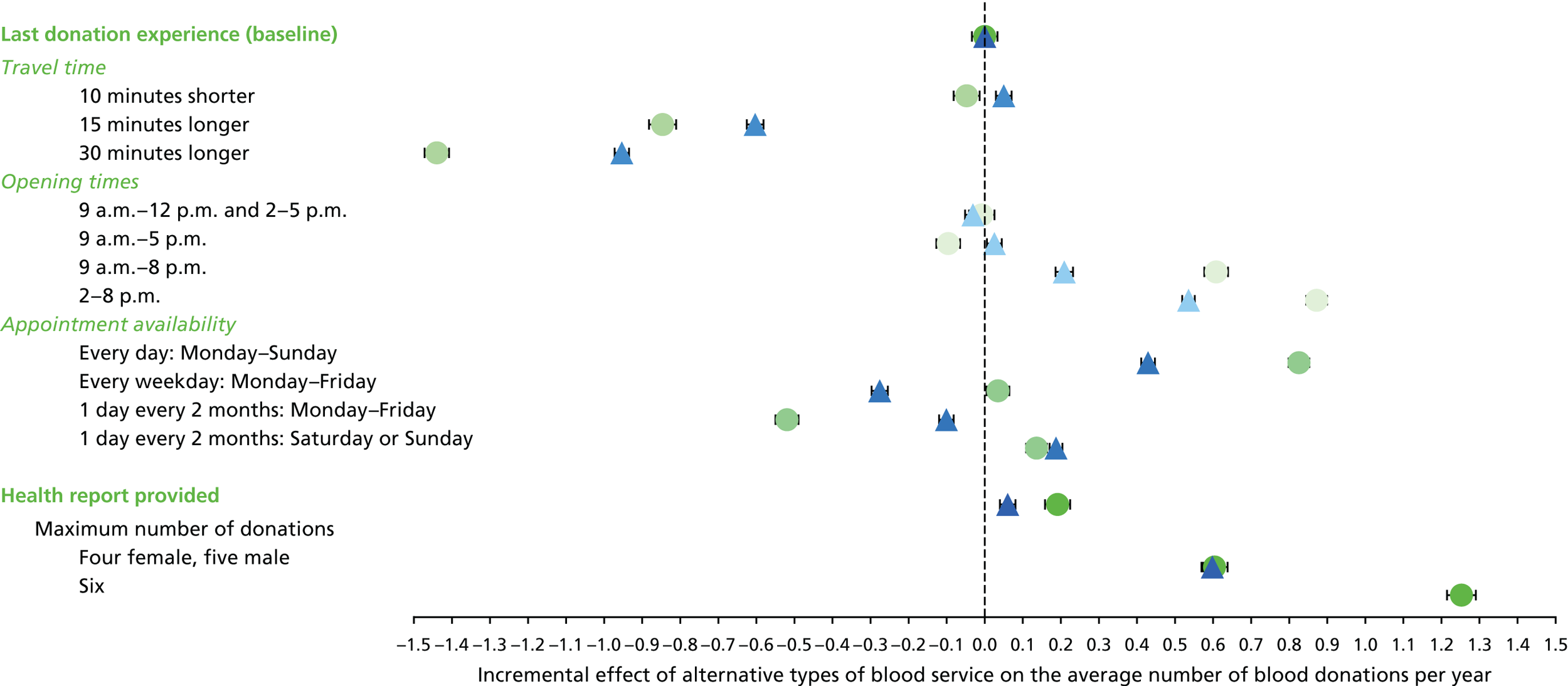
FIGURE 9.
Incremental effect of alternative types of blood service on average number of donations per year for non-INTERVAL high-demand blood type respondent. For men (• n = 1551) and women (▴ n = 921). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.

FIGURE 10.
Incremental effect of alternative types of blood service on average number of donations per year for non-INTERVAL respondents of black/mixed black ethnicity. For men (• n = 98) and women (▴ n = 103). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
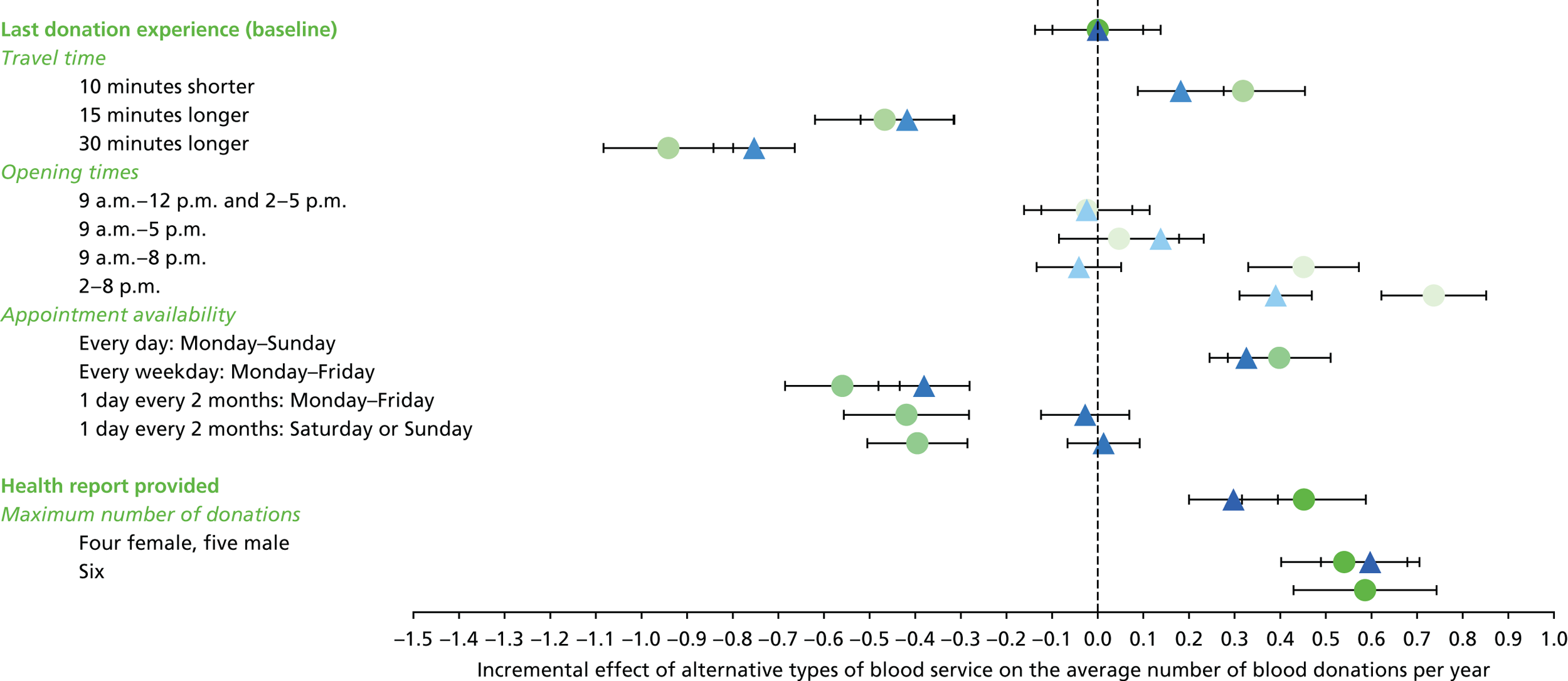
FIGURE 11.
Incremental effect of alternative types of blood service on average number of donations per year for non-INTERVAL nursery respondents. For men (• n = 3542) and women (▴ n = 3024). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
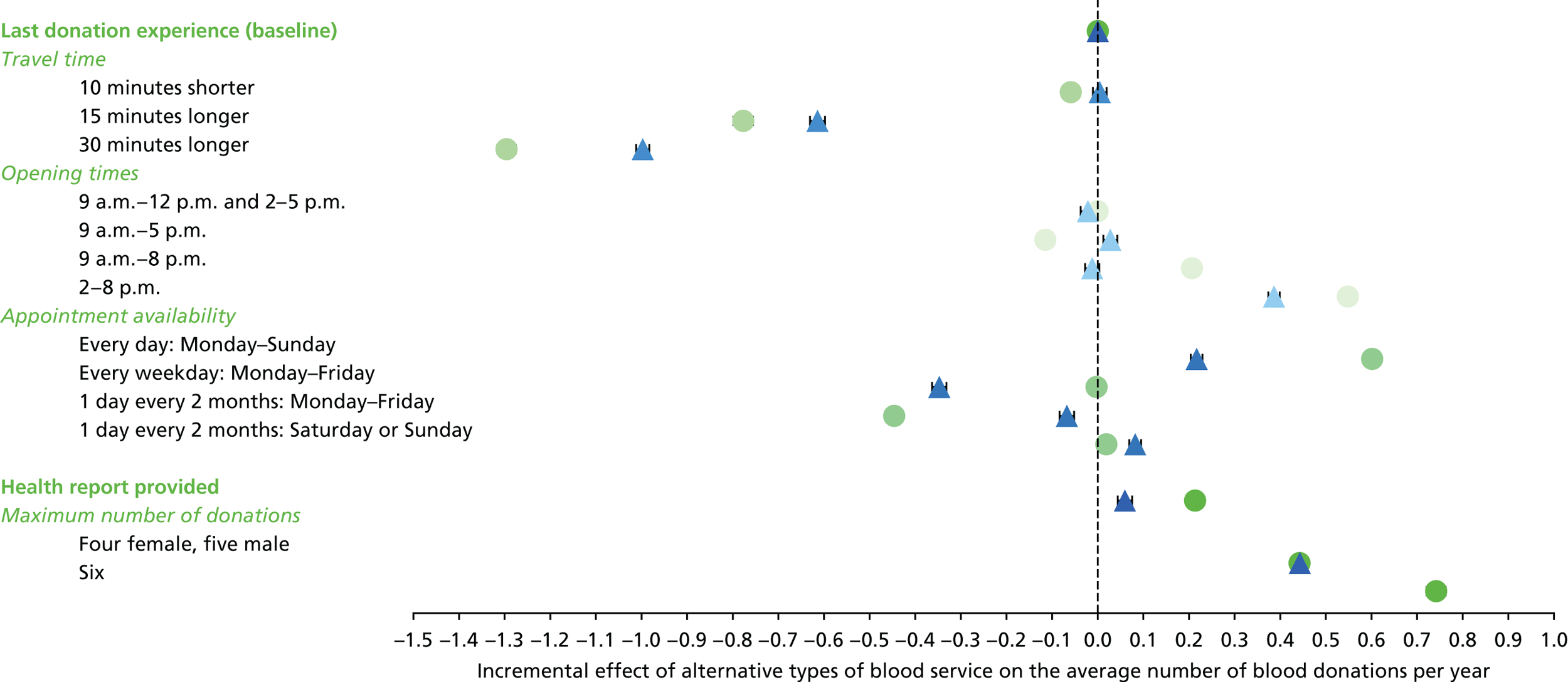
FIGURE 12.
Incremental effect of alternative types of blood service on average number of donations per year for all ex-INTERVAL respondents. For men (• n = 4754) and women (▴ n = 4179). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
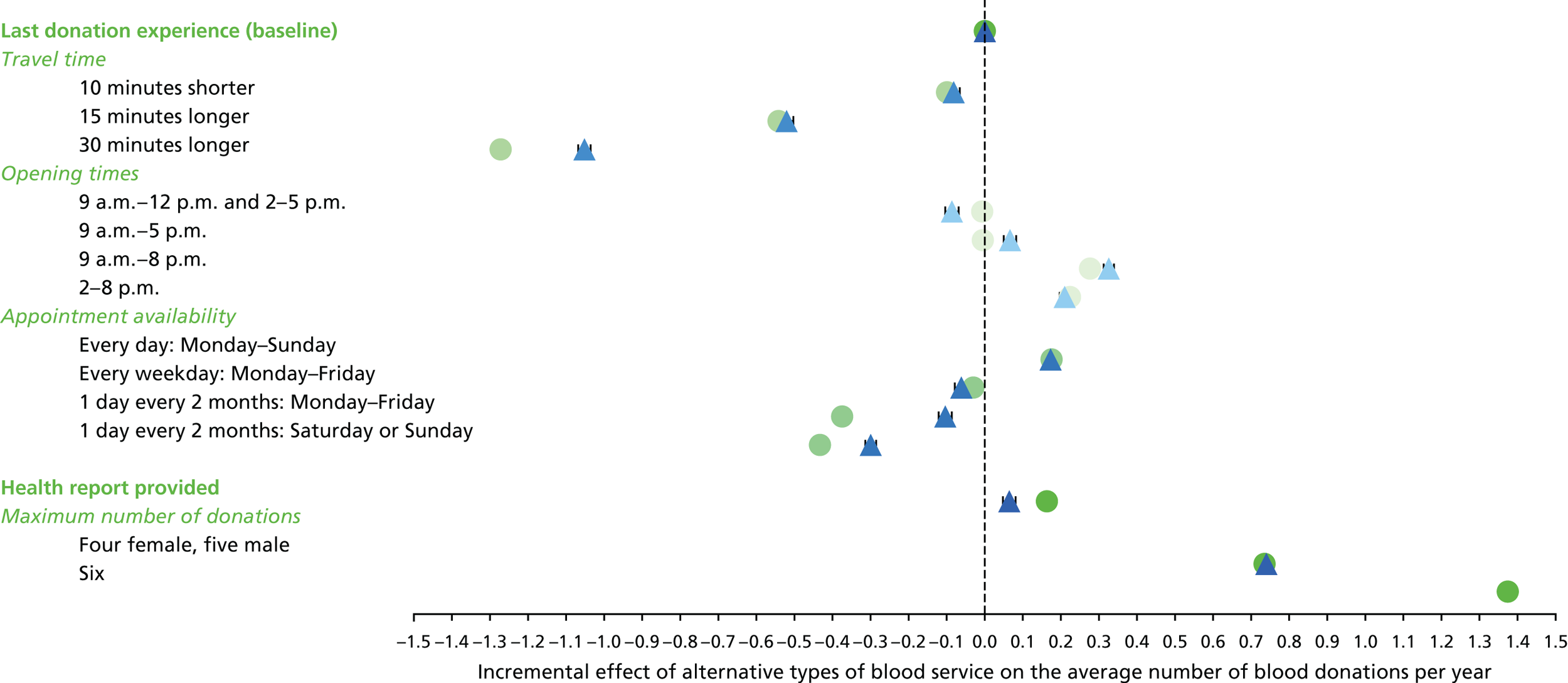
FIGURE 13.
Incremental effect of alternative types of blood service on average number of donations per year for ex-INTERVAL respondents aged 17–30 years. For men (• n = 253) and women (▴ n = 431). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
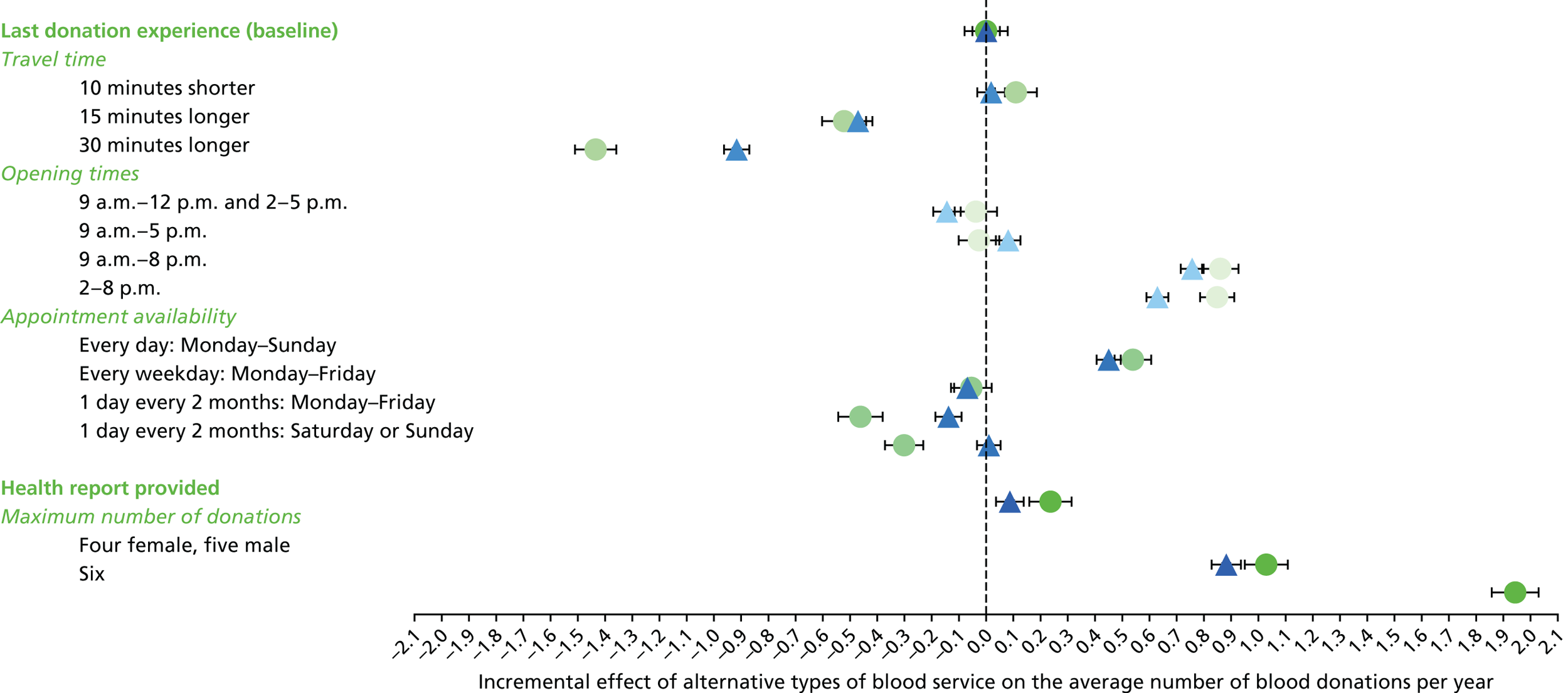
FIGURE 14.
Incremental effect of alternative types of blood service on average number of donations per year for ex-INTERVAL high-demand blood type respondents. For men (• n = 643) and women (▴ n = 580). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
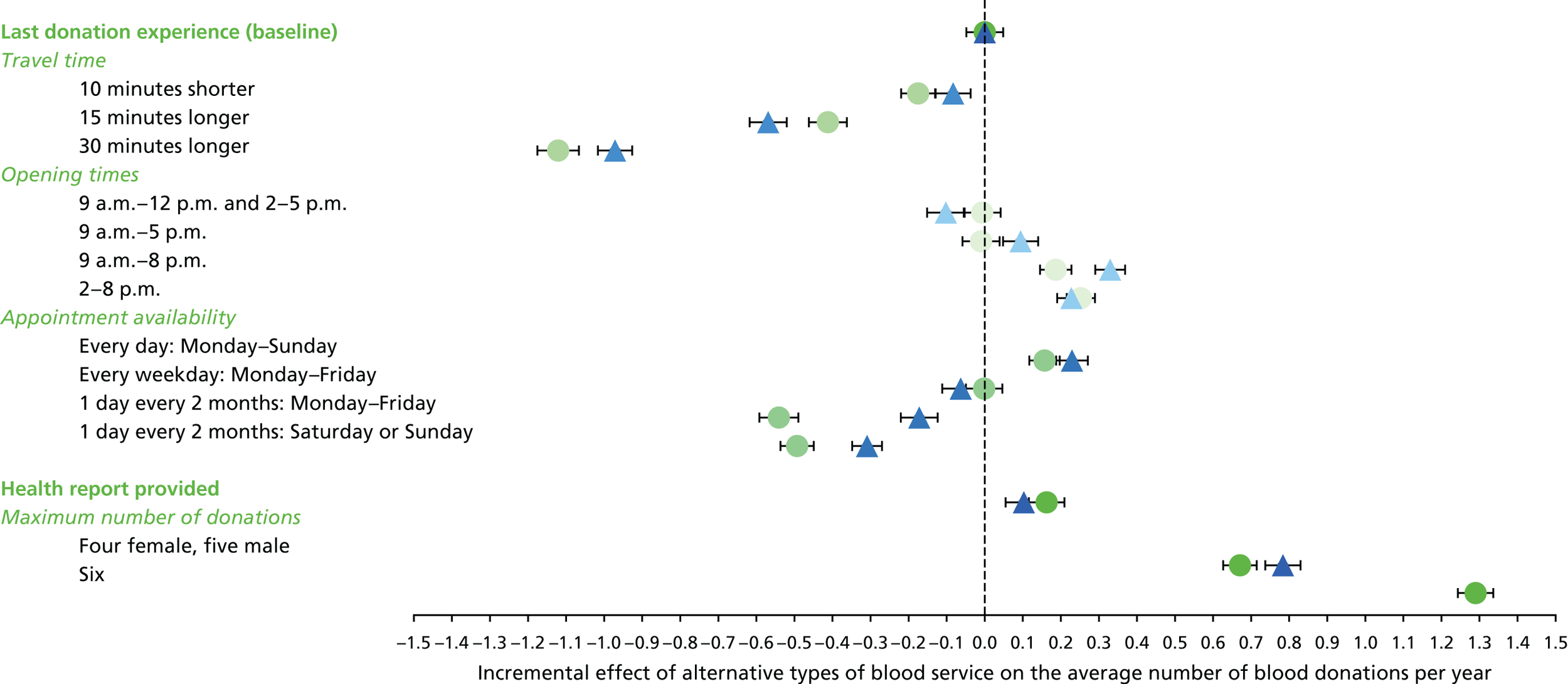
FIGURE 15.
Incremental effect of alternative types of blood service on average number of donations per year for ex-INTERVAL respondents of black/mixed black ethnicity. For men (• n = 39) and women (▴ n = 52). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
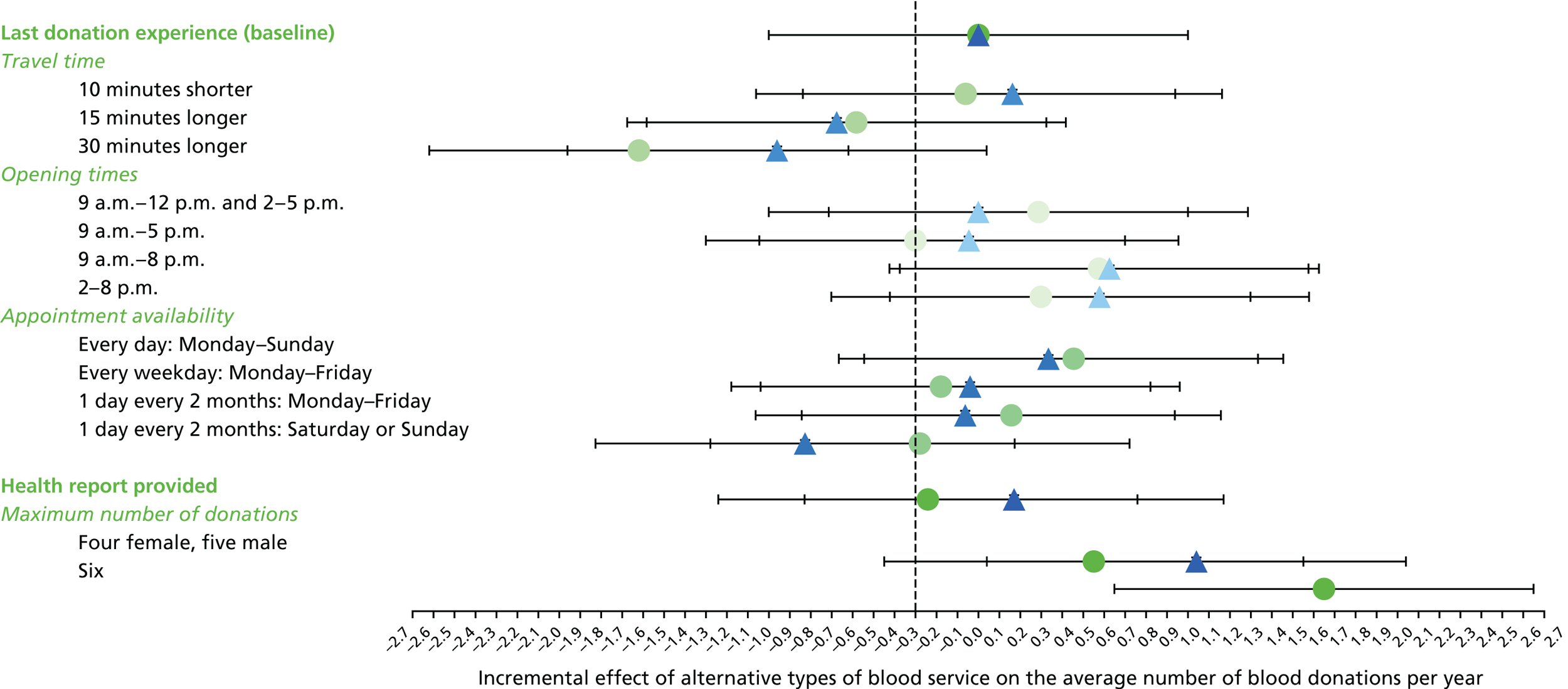
FIGURE 16.
Incremental effect of alternative types of blood service on average number of donations per year for ex-INTERVAL nursery respondents. For men (• n = 18) and women (▴ n = 65). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
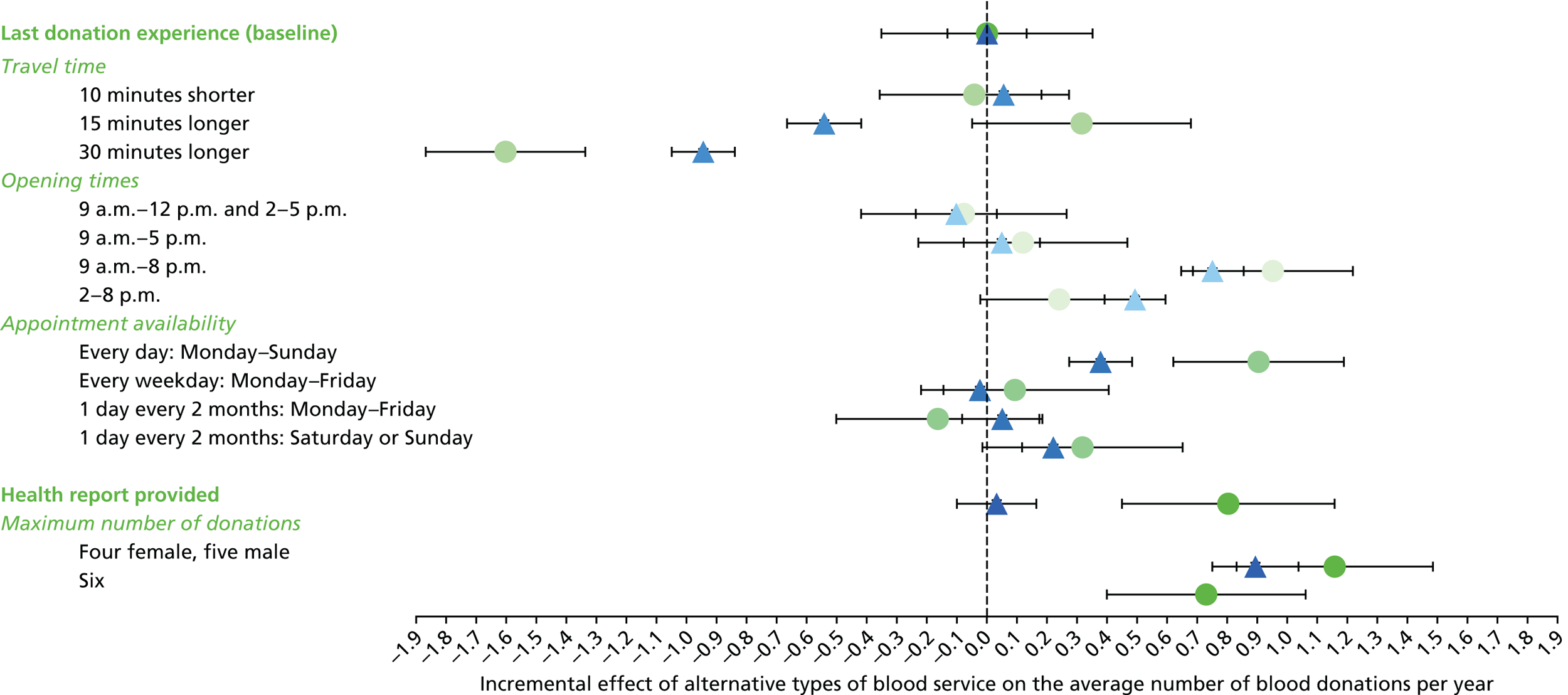
These figures have a common structure. Throughout, • refers to men and ▴ refers to women. Consider, for example, Figure 7. The first row describes the mean (95% uncertainty intervals) under the current service configuration (status quo). All subsequent rows report the average predicted annual donation frequency according to the specific attribute and level in question, and in the absence of any other changes in the opportunities to donate. So in the figures the next three rows indicate the predicted effect of changes in travel time. Thus, for example, if travel time were to increase by 30 minutes, it is predicted that men would, on average, make 1.4 fewer donations per year and women would make 1.1 fewer donations per year. Similarly, if opening hours were to change from the status quo to 2–8 p.m., the model predicts that men would provide 0.5 more donations per year and women 0.3 more donations per year.
There are four important general findings. First, the estimated incremental effects are very much in line with prior expectations. Thus, increases in travel time are predicted to reduce the annual number of donations. Extending evening opening hours is predicted to increase the annual number of donations. Improved availability of appointments is predicted to increase donations, whereas reduced availability of appointments is predicted to decrease the number of donations. Introduction of a health report is predicted to increase the number of donations. Reducing the minimum interval between donations (increasing the maximum number of donations permitted annually) is predicted to increase the number of donations. Second, although there are some subgroup differences, the similarities in the results across subgroups are more striking than the differences. Thus, for example, introduction of a health report is associated with a relatively small increase in frequency of donation for all subgroups. For all subgroups the greatest impact is with respect to increases in travel time. Reducing the interval between donations such that annual opportunities to donate increase by 1 is predicted to increase annual donations on average by about 0.5. Third, although there are differences in incremental effects between men and women, they are generally not large. The greatest differences are with respect to increased travel time and reduced appointment availability. Fourth, these general characterisations of the results apply to both the non-INTERVAL and ex-INTERVAL donors, although, interestingly, the predicted effect of reducing minimum interdonation intervals is about two-thirds greater for ex-INTERVAL donors than for non-INTERVAL donors.
Tables 13–16 report, for the different attribute levels, the predicted percentage of respondents from the ordered logit model who indicate ‘I would probably not donate’. This is done for all attribute levels and by the levels of donor characteristics. The baseline represents the individual-level service configuration the donor experienced at their last donation. Tables 13–16 present this information for male non-INTERVAL, female non-INTERVAL, male ex-INTERVAL and female ex-INTERVAL respondents, respectively. Increased travel time is associated with markedly higher proportions of ‘stop donating’ responses. Opportunities to donate in the evening and greater availability of appointments are associated with lower proportions of ‘stop donating’ responses. Provision of a health report is associated with a slightly lower proportion of ‘stop donating’ responses. Finally, increases in the maximum number of donations permitted annually are associated with lower proportions of ‘stop donating’ responses.
| Level | All | Age group (years) | Blood type | Ethnicity | Nursery donors | Venue | Number of donations in last 12 months | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17–30 | 31–45 | 46–60 | ≥ 60 | Standard demand | High demand | White | Black/mixed black | Asian/mixed Asian | Other or not stated | Yes | No | Centre | Mobile | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Baseline | 18 | 25 | 21 | 17 | 14 | 19 | 17 | 18 | 24 | 21 | 16 | 22 | 18 | 15 | 19 | 24 | 19 | 15 | 13 | 10 | 7 |
| Travel time | |||||||||||||||||||||
| 10 minutes shorter | 20 | 26 | 23 | 19 | 16 | 20 | 19 | 20 | 19 | 22 | 15 | 23 | 19 | 16 | 20 | 25 | 20 | 17 | 14 | 11 | 7 |
| 15 minutes longer | 31 | 44 | 37 | 30 | 24 | 31 | 32 | 31 | 34 | 35 | 26 | 37 | 30 | 24 | 32 | 40 | 32 | 26 | 21 | 15 | 10 |
| 30 minutes longer | 46 | 60 | 53 | 44 | 36 | 46 | 44 | 46 | 44 | 41 | 41 | 50 | 45 | 37 | 47 | 53 | 47 | 41 | 36 | 29 | 23 |
| Opening times | |||||||||||||||||||||
| 9 a.m.–12 p.m. and 2 p.m.–5 p.m. | 18 | 26 | 21 | 17 | 14 | 19 | 17 | 18 | 25 | 21 | 16 | 22 | 18 | 16 | 19 | 24 | 19 | 15 | 13 | 10 | 7 |
| 9 a.m.–5 p.m. | 20 | 27 | 23 | 19 | 16 | 20 | 19 | 20 | 23 | 24 | 17 | 23 | 19 | 16 | 20 | 25 | 20 | 17 | 14 | 10 | 8 |
| 9 a.m.–8 p.m. | 15 | 16 | 16 | 15 | 15 | 15 | 15 | 15 | 17 | 18 | 16 | 18 | 14 | 10 | 16 | 19 | 16 | 13 | 10 | 7 | 6 |
| 2 p.m.–8 p.m. | 12 | 12 | 12 | 12 | 11 | 12 | 11 | 12 | 13 | 14 | 11 | 14 | 11 | 11 | 12 | 15 | 12 | 10 | 8 | 7 | 5 |
| Appointment availability | |||||||||||||||||||||
| Every day: Monday–Sunday | 12 | 13 | 12 | 12 | 12 | 12 | 12 | 12 | 18 | 16 | 12 | 13 | 12 | 11 | 12 | 15 | 12 | 11 | 9 | 8 | 6 |
| Every weekday: Monday–Friday | 19 | 24 | 21 | 18 | 16 | 19 | 18 | 19 | 35 | 26 | 19 | 21 | 18 | 19 | 19 | 24 | 20 | 16 | 14 | 11 | 10 |
| 1 day every 2 months: Monday–Friday | 25 | 35 | 29 | 23 | 19 | 25 | 24 | 25 | 32 | 31 | 22 | 30 | 23 | 24 | 29 | 32 | 26 | 21 | 17 | 14 | 12 |
| 1 day every 2 months: Saturday or Sunday | 19 | 22 | 20 | 18 | 17 | 19 | 18 | 19 | 31 | 21 | 19 | 21 | 18 | 22 | 18 | 22 | 19 | 17 | 15 | 13 | 12 |
| Health report provided | |||||||||||||||||||||
| Health report | 16 | 22 | 18 | 15 | 12 | 16 | 15 | 16 | 17 | 17 | 13 | 18 | 15 | 13 | 16 | 20 | 16 | 13 | 11 | 8 | 6 |
| Maximum number of donations: five | 13 | 16 | 14 | 12 | 11 | 13 | 12 | 13 | 16 | 17 | 12 | 15 | 12 | 10 | 13 | 18 | 13 | 10 | 7 | 5 | 3 |
| Maximum number of donations: six | 10 | 10 | 9 | 10 | 10 | 10 | 8 | 9 | 16 | 14 | 9 | 12 | 9 | 7 | 10 | 15 | 10 | 6 | 4 | 2 | 1 |
| Level | All | Age group (years) | Blood type | Ethnicity | Nursery donors | Venue | Number of donations in last 12 months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17–30 | 31–45 | 46–60 | ≥ 60 | Standard demand | High demand | White | Black/mixed black | Asian/mixed Asian | Other or not stated | Yes | No | Centre | Mobile | 1 | 2 | 3 | 4 | 5 | ||
| Baseline | 20 | 28 | 22 | 17 | 13 | 20 | 18 | 20 | 25 | 20 | 20 | 24 | 18 | 18 | 20 | 24 | 19 | 16 | 15 | 14 |
| Travel time | ||||||||||||||||||||
| 10 minutes shorter | 20 | 27 | 22 | 18 | 15 | 21 | 19 | 20 | 21 | 19 | 22 | 24 | 19 | 18 | 21 | 23 | 20 | 17 | 17 | 15 |
| 15 minutes longer | 37 | 47 | 40 | 33 | 27 | 37 | 33 | 37 | 37 | 33 | 35 | 41 | 34 | 31 | 37 | 43 | 36 | 30 | 26 | 22 |
| 30 minutes longer | 50 | 59 | 53 | 47 | 41 | 50 | 48 | 50 | 47 | 44 | 50 | 53 | 48 | 43 | 51 | 54 | 50 | 45 | 43 | 35 |
| Opening times | ||||||||||||||||||||
| 9 a.m.–12 p.m. and 2 p.m.–5 p.m. | 20 | 29 | 23 | 17 | 13 | 21 | 18 | 20 | 26 | 22 | 21 | 24 | 18 | 21 | 20 | 24 | 20 | 16 | 15 | 25 |
| 9 a.m.–5 p.m. | 20 | 28 | 22 | 17 | 13 | 20 | 18 | 20 | 22 | 16 | 17 | 23 | 18 | 16 | 20 | 23 | 19 | 16 | 15 | 14 |
| 9 a.m.–8 p.m. | 21 | 24 | 22 | 21 | 19 | 22 | 19 | 21 | 26 | 22 | 22 | 24 | 20 | 10 | 23 | 25 | 21 | 17 | 14 | 9 |
| 2 p.m.–8 p.m. | 13 | 16 | 14 | 12 | 11 | 13 | 12 | 13 | 16 | 14 | 12 | 15 | 12 | 12 | 13 | 16 | 13 | 11 | 9 | 12 |
| Appointment availability | ||||||||||||||||||||
| Every day: Monday–Sunday | 16 | 18 | 17 | 15 | 14 | 16 | 15 | 16 | 18 | 18 | 22 | 19 | 15 | 12 | 17 | 19 | 16 | 13 | 11 | 6 |
| Every weekday: Monday–Friday | 29 | 36 | 31 | 26 | 22 | 29 | 26 | 28 | 36 | 35 | 35 | 33 | 26 | 24 | 29 | 33 | 28 | 24 | 22 | 14 |
| 1 day every 2 months: Monday–Friday | 21 | 31 | 23 | 17 | 13 | 21 | 19 | 21 | 26 | 22 | 22 | 25 | 19 | 28 | 20 | 25 | 20 | 17 | 16 | 13 |
| 1 day every 2 months: Saturday or Sunday | 19 | 23 | 20 | 17 | 15 | 19 | 17 | 19 | 25 | 17 | 22 | 21 | 18 | 24 | 18 | 22 | 19 | 16 | 14 | 9 |
| Health report provided | ||||||||||||||||||||
| Health report | 19 | 27 | 21 | 15 | 12 | 19 | 17 | 19 | 19 | 19 | 18 | 22 | 16 | 17 | 19 | 22 | 18 | 15 | 13 | 13 |
| Maximum number of donations: four | 11 | 16 | 13 | 10 | 8 | 12 | 10 | 11 | 14 | 11 | 13 | 15 | 10 | 8 | 12 | 16 | 10 | 7 | 5 | 3 |
| Level | All | Age group (years) | Blood type | Ethnicity | Nursery donors | Venue | INTERVAL arm = | Number of donations in last 12 months | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17–30 | 31–45 | 46–60 | ≥ 60 | Standard demand | High demand | White | Black/mixed black | Asian/mixed Asian | Other or not stated | Yes | No | Centre | Mobile | Control | Reduced intervals | 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Baseline | 18 | 31 | 24 | 18 | 13 | 18 | 17 | 18 | 18 | 29 | 21 | 44 | 18 | 18 | 21 | 20 | 17 | 28 | 25 | 21 | 19 | 17 | 14 | 13 |
| Travel time | ||||||||||||||||||||||||
| 10 minutes shorter | 19 | 28 | 24 | 19 | 16 | 19 | 19 | 19 | 18 | 28 | 23 | 45 | 19 | 19 | 20 | 21 | 19 | 30 | 26 | 23 | 20 | 18 | 16 | 14 |
| 15 minutes longer | 26 | 41 | 34 | 26 | 21 | 27 | 23 | 26 | 26 | 37 | 23 | 37 | 26 | 26 | 33 | 29 | 25 | 40 | 36 | 32 | 28 | 24 | 21 | 19 |
| 30 minutes longer | 41 | 63 | 53 | 40 | 31 | 41 | 36 | 40 | 48 | 53 | 47 | 86 | 41 | 41 | 42 | 40 | 41 | 58 | 51 | 46 | 42 | 38 | 36 | 34 |
| Opening times | ||||||||||||||||||||||||
| 9 a.m.–12 p.m. and 2 p.m.–5 p.m. | 18 | 31 | 25 | 18 | 13 | 18 | 17 | 18 | 13 | 31 | 20 | 46 | 18 | 18 | 21 | 19 | 18 | 27 | 24 | 21 | 19 | 17 | 15 | 14 |
| 9 a.m.–5 p.m. | 18 | 31 | 25 | 18 | 13 | 18 | 17 | 18 | 22 | 28 | 21 | 41 | 18 | 18 | 22 | 20 | 17 | 30 | 26 | 22 | 19 | 17 | 14 | 12 |
| 9 a.m.–8 p.m. | 14 | 17 | 15 | 14 | 13 | 14 | 14 | 14 | 10 | 14 | 13 | 25 | 14 | 13 | 19 | 15 | 14 | 21 | 19 | 17 | 15 | 13 | 11 | 10 |
| 2 p.m.–8 p.m. | 15 | 17 | 16 | 15 | 14 | 15 | 13 | 14 | 13 | 16 | 19 | 38 | 15 | 15 | 13 | 15 | 14 | 21 | 18 | 16 | 15 | 14 | 13 | 12 |
| Appointment availability | ||||||||||||||||||||||||
| Every day: Monday–Sunday | 15 | 21 | 18 | 15 | 13 | 15 | 15 | 15 | 11 | 21 | 18 | 26 | 15 | 15 | 17 | 16 | 15 | 22 | 20 | 18 | 16 | 14 | 13 | 12 |
| Every weekday: Monday–Friday | 18 | 31 | 25 | 18 | 14 | 19 | 17 | 18 | 20 | 31 | 20 | 42 | 18 | 18 | 22 | 20 | 18 | 29 | 25 | 22 | 19 | 17 | 15 | 14 |
| 1 day every 2 months: Monday–Friday | 24 | 40 | 32 | 23 | 17 | 24 | 25 | 24 | 15 | 38 | 21 | 47 | 24 | 24 | 21 | 24 | 24 | 37 | 31 | 27 | 24 | 22 | 20 | 19 |
| 1 day every 2 months: Saturday or Sunday | 24 | 36 | 30 | 24 | 20 | 24 | 24 | 24 | 21 | 25 | 21 | 37 | 24 | 24 | 23 | 24 | 24 | 34 | 29 | 27 | 25 | 23 | 22 | 21 |
| Health report provided | ||||||||||||||||||||||||
| Health report | 16 | 26 | 21 | 15 | 12 | 16 | 15 | 16 | 21 | 23 | 17 | 28 | 16 | 15 | 19 | 17 | 15 | 26 | 22 | 19 | 17 | 14 | 12 | 11 |
| Maximum number of donations: five | 9 | 15 | 12 | 9 | 7 | 9 | 9 | 9 | 11 | 14 | 12 | 22 | 9 | 9 | 11 | 10 | 9 | 18 | 15 | 12 | 10 | 8 | 7 | 6 |
| Maximum number of donations: six | 5 | 6 | 6 | 5 | 4 | 5 | 5 | 5 | 3 | 5 | 6 | 29 | 5 | 5 | 6 | 5 | 4 | 15 | 10 | 7 | 5 | 3 | 2 | 2 |
| Level | All | Age group (years) | Blood type | Ethnicity | Nursery donors | Venue | INTERVAL arm = | Number of donations in last 12 months | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17–30 | 31–45 | 46–60 | ≥ 60 | Standard demand | High demand | White | Black/mixed black | Asian/mixed Asian | Other or not stated | Yes | No | Centre | Mobile | Control | Reduced intervals | 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Baseline | 19 | 33 | 25 | 17 | 12 | 19 | 21 | 19 | 27 | 19 | 18 | 41 | 19 | 18 | 23 | 19 | 19 | 31 | 27 | 21 | 17 | 14 | 11 | 10 |
| Travel time | ||||||||||||||||||||||||
| 10 minutes shorter | 21 | 33 | 26 | 18 | 14 | 20 | 22 | 21 | 23 | 16 | 19 | 39 | 20 | 20 | 23 | 21 | 20 | 32 | 28 | 22 | 19 | 16 | 11 | 10 |
| 15 minutes longer | 31 | 47 | 38 | 28 | 22 | 31 | 34 | 31 | 45 | 28 | 25 | 58 | 31 | 31 | 30 | 31 | 31 | 48 | 41 | 33 | 28 | 25 | 18 | 14 |
| 30 minutes longer | 46 | 62 | 54 | 44 | 36 | 46 | 46 | 46 | 54 | 41 | 41 | 72 | 46 | 47 | 38 | 45 | 46 | 61 | 55 | 47 | 44 | 41 | 33 | 33 |
| Opening times | ||||||||||||||||||||||||
| 9 a.m.–12 p.m. and 2 p.m.–5 p.m. | 21 | 37 | 28 | 18 | 12 | 21 | 23 | 21 | 27 | 23 | 21 | 44 | 21 | 21 | 23 | 20 | 22 | 35 | 30 | 23 | 19 | 16 | 11 | 10 |
| 9 a.m.–5 p.m. | 18 | 31 | 23 | 15 | 11 | 17 | 18 | 18 | 28 | 17 | 16 | 39 | 17 | 17 | 20 | 18 | 18 | 29 | 25 | 19 | 16 | 13 | 10 | 8 |
| 9 a.m.–8 p.m. | 12 | 16 | 14 | 12 | 11 | 12 | 14 | 12 | 13 | 15 | 11 | 21 | 12 | 12 | 18 | 13 | 12 | 18 | 16 | 14 | 11 | 10 | 8 | 6 |
| 2 p.m.–8 p.m. | 14 | 18 | 16 | 14 | 12 | 14 | 15 | 14 | 14 | 18 | 19 | 27 | 14 | 14 | 14 | 15 | 14 | 22 | 19 | 15 | 13 | 11 | 9 | 9 |
| Appointment availability | ||||||||||||||||||||||||
| Every day: Monday–Sunday | 15 | 22 | 18 | 14 | 12 | 15 | 16 | 15 | 19 | 14 | 15 | 30 | 15 | 14 | 21 | 15 | 16 | 24 | 21 | 17 | 14 | 12 | 12 | 10 |
| Every weekday: Monday–Friday | 20 | 35 | 26 | 18 | 13 | 20 | 22 | 20 | 28 | 21 | 19 | 41 | 20 | 19 | 28 | 20 | 21 | 32 | 29 | 22 | 18 | 15 | 16 | 14 |
| 1 day every 2 months: Monday–Friday | 21 | 37 | 28 | 19 | 13 | 21 | 24 | 21 | 28 | 21 | 21 | 39 | 21 | 21 | 23 | 23 | 20 | 34 | 30 | 23 | 19 | 15 | 15 | 15 |
| 1 day every 2 months: Saturday or Sunday | 25 | 33 | 28 | 24 | 21 | 25 | 27 | 25 | 49 | 19 | 21 | 34 | 25 | 26 | 21 | 26 | 25 | 32 | 30 | 26 | 24 | 22 | 25 | 21 |
| Health report provided | ||||||||||||||||||||||||
| Health report | 18 | 31 | 23 | 15 | 11 | 18 | 18 | 18 | 23 | 16 | 17 | 40 | 17 | 17 | 23 | 18 | 18 | 29 | 25 | 19 | 16 | 13 | 10 | 9 |
| Maximum number of donations: four | 7 | 14 | 10 | 6 | 4 | 7 | 7 | 7 | 8 | 9 | 8 | 19 | 7 | 6 | 11 | 8 | 7 | 17 | 13 | 8 | 5 | 4 | 2 | 1 |
Several patterns by subgroup can be observed in these data. The older the age group, the less frequently they give the ‘I would probably not donate’ response. Generally, men are less likely than women to say that they would stop donating. Although the difference is not marked, high-demand blood type respondents are less likely than standard-demand blood type respondents to say that they will stop donating. For all attribute levels, the proportion of ‘stop donating’ responses by nursery donors exceeds that of non-nursery donors. Among non-INTERVAL donors, those whose last donation was made at a donor centre were less likely to indicate that they would stop donating than those who last donated at a mobile session. Among non-INTERVAL donors, there is a tendency for black/mixed black donors to give a ‘stop donating’ response more often than other ethnic groups. Among ex-INTERVAL donors, there is a tendency for Asian/mixed Asian donors to give a ‘stop donating’ response more often than other ethnic groups. More frequent donation in the past 12 months is associated with lower percentages of ‘stop donating’ responses for men and women in both the non-INTERVAL and ex-INTERVAL surveys.
There is a fairly mixed picture when comparing the percentages of ‘stop donating’ responses across ex-INTERVAL and non-INTERVAL donors, except that ex-INTERVAL nursery donors consistently make fewer ‘stop donating’ responses than non-INTERVAL nursery donors.
Figures 17 (men) and 18 (women) compare the incremental effects on annual donations for the non-INTERVAL donors whose last donation was at a static donor centre, and for ex-INTERVAL donors who were in the control arm (thus not exposed to an increase in the maximum number of donations permitted annually). Figure 17 shows similar patterns for the two groups; however, non-INTERVAL respondents are more responsive to increased travel time, and ex-INTERVAL respondents are more responsive to increases in the maximum permitted number of donations. The results for women follow a similar pattern to the male results but, generally, any differences between the two groups are not statistically significant, possibly reflecting the smaller numbers of respondents.
FIGURE 17.
Incremental effect of alternative types of blood service on average number of donations per year for male, high-demand blood type non-INTERVAL respondents who donated at a static centre and male ex-INTERVAL control arm respondents. For men, high-demand blood type non-INTERVAL respondents who donated at a static centre (• n = 137) and male ex-INTERVAL control arm respondents (• n = 211).
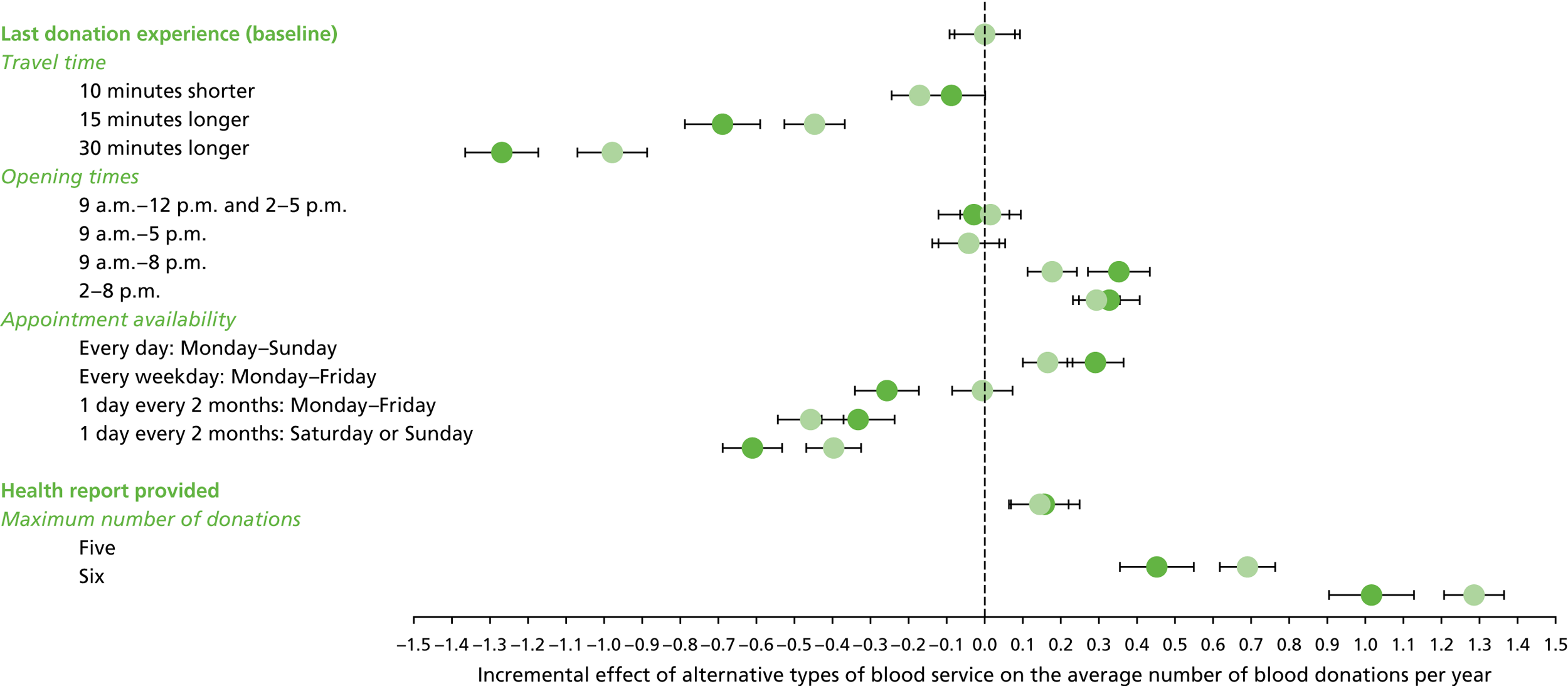
FIGURE 18.
Incremental effect of alternative types of blood service on average number of donations per year for female, high-demand blood type non-INTERVAL respondents who last donated at a static centre and female ex-INTERVAL control arm respondents. For women, high-demand blood type non-INTERVAL respondents who last donated at a static centre (▴ n = 78) and female ex-INTERVAL control arm respondents (▴ n = 78).
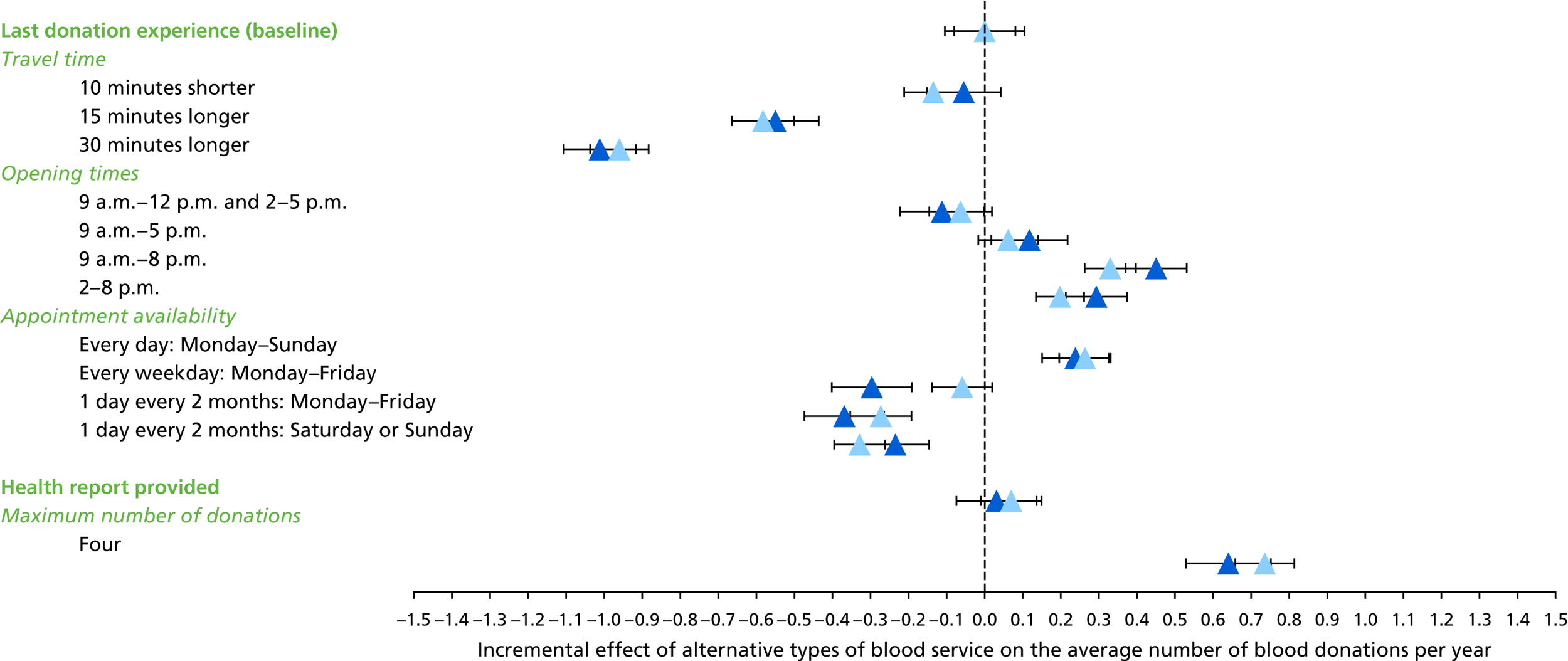
The discrepancy between mean ‘stated’ and mean ‘observed’ donations is shown for non-INTERVAL respondents in Figures 19 (men) and 20 (women). ‘Stated’ consistently exceeds ‘observed’ for men by nearly 20%, whereas the discrepancy for women was negligible. The comparable discrepancies for ex-INTERVAL respondents, shown in Figures 21 and 22, indicate stated donations as less than observed donations for both men (< 10%) and women (about 15%).
FIGURE 19.
Discrepancy between the mean number of ‘observed’ and ‘stated’ donations per year for male non-INTERVAL respondents. The error bars reflect the 95% CI around the average discrepancy in the predicted vs. observed number of donations per year.

FIGURE 20.
Discrepancy between the mean number of ‘observed’ and ‘stated’ donations per year for female non-INTERVAL respondents. The error bars reflect the 95% CI around the average discrepancy in the predicted vs. observed number of donations per year.
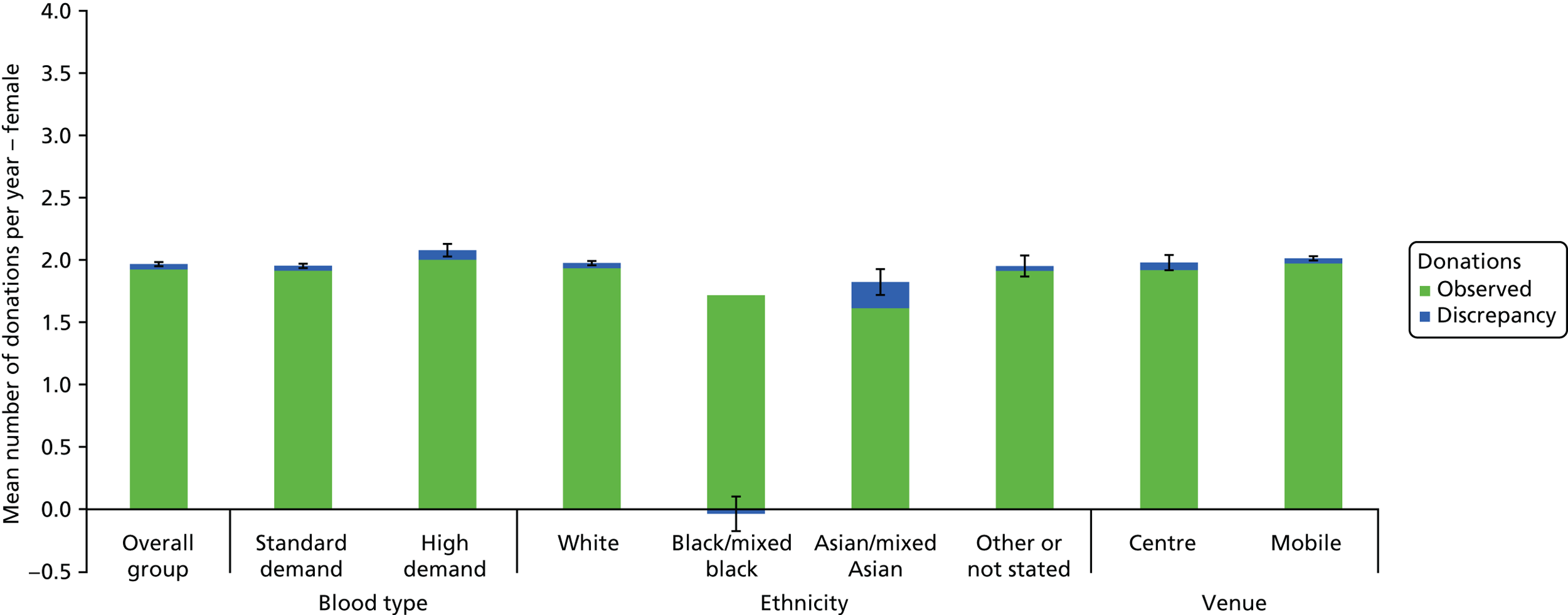
FIGURE 21.
Discrepancy between the mean number of ‘observed’ and ‘stated’ donations per year for male ex-INTERVAL respondents. The error bars reflect the 95% CI around the average discrepancy in the predicted vs. observed number of donations per year.
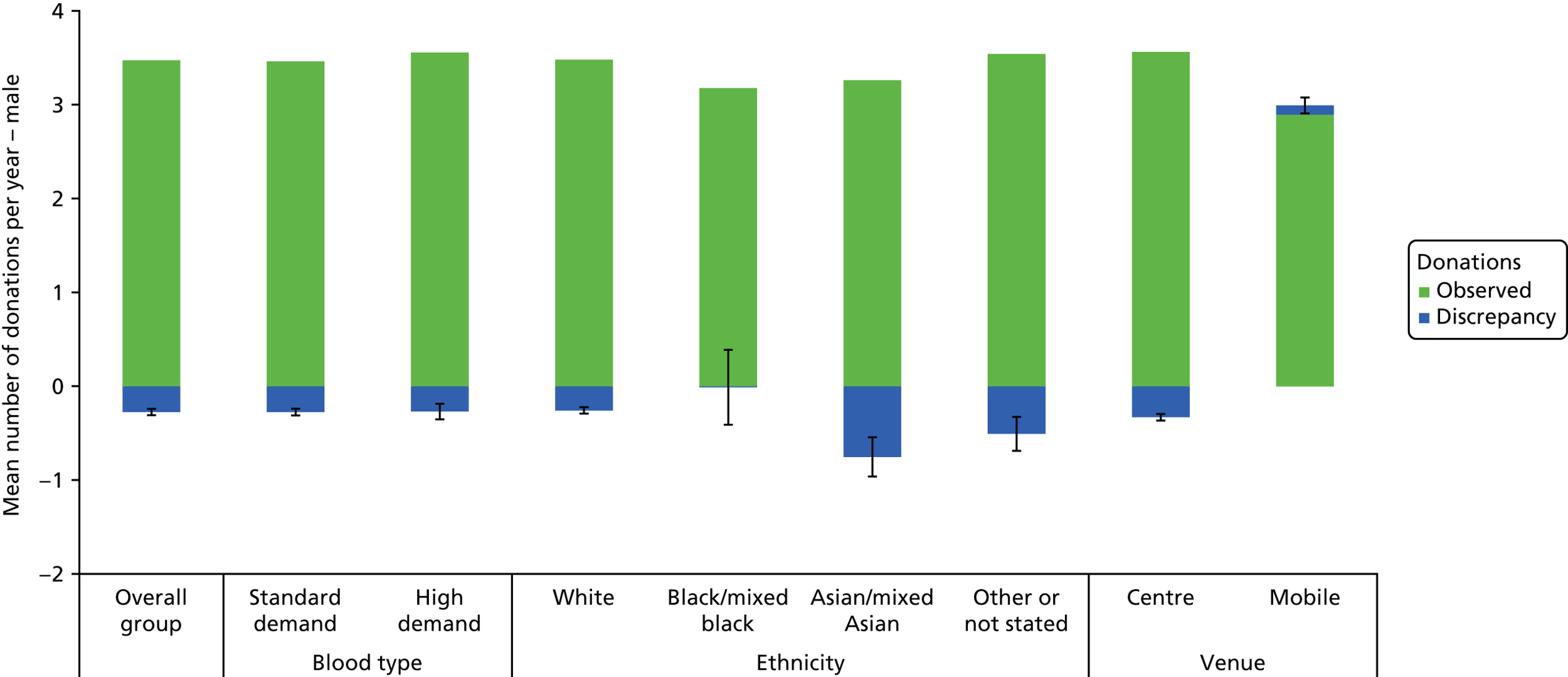
FIGURE 22.
Discrepancy between the mean number of ‘observed’ and ‘stated’ donations per year for female ex-INTERVAL respondents. The error bars reflect the 95% CI around the average discrepancy in the predicted vs. observed number of donations per year.

The incremental effects of changing attribute levels were estimated for all donors, and for high-demand blood type donors, using a gamma model and a two-part model (for both the non-INTERVAL and ex-INTERVAL respondents). The results are shown in Appendices 9–16. The overall pattern of incremental effects is very similar to that reported in the Figures 7, 9, 12 and 14 (based on the ordered logit model), respectively. The only systematic difference is that the estimated response to reductions in the minimum donation interval is slightly reduced when estimated by the gamma and two-part models as compared with the ordered logit model.
Discussion
The SP surveys provide plausible estimates of the effects of alternative future changes to the blood collection service on the stated frequency of donation. We find that reducing the minimum recall period between whole-blood donations, or changing appointment availability to include weekends or evenings, leads to moderately large increases in the stated frequency of blood donation. By contrast, the introduction of a donor health report is predicted to have a relatively small effect on the stated frequency. These findings are in line with our a priori hypotheses, and with feedback from whole-blood donors attending the translation workshops (see Appendix 17). A general concern with the use of SP surveys for service evaluation is that there may be ‘hypothetical bias’; responders tend to overstate their willingness to provide a publicly funded service with altruistic features. 59 However, we found that for men who completed the non-INTERVAL survey, the average annual frequency of donation from the survey responses was only moderately higher than the actual donation frequency, for women the discrepancy was minimal, and for both sexes the discrepancy was similar across subgroups. These findings applied to the current blood service characteristics experienced by the survey responders. This provides a reasonable basis for the subsequent CEA to assume that the relative estimates of service changes predicted from the survey responses predict the effect of future changes to the blood collection service on donation frequency.
A potentially important finding from both surveys is that donors are willing to donate more often if there are more frequent opportunities to donate, in particular if the minimum interval between invitations is reduced. This implies that the overall findings from the INTERVAL trial could apply to donors attending static centres outwith the trial setting. The survey results also extend the trial findings in showing that the reduced interval strategies are predicted to increase donation frequency when considered against, or alongside, alternative changes to the blood service. Of potential policy relevance is the finding that appointment availability at weekends is predicted to increase donation frequency for those subgroups where additional blood donations may be of relatively high value, for example donors with blood types that are of ‘high demand’, donors whose ethnicity was defined as BAME, and nursery donors, who tend to be more difficult to retain. The finding that increasing travel time will reduce donation frequency and increase the rate with which donors leave the register is not surprising, but does have potential policy implications. The NHSBT policy has been to close smaller mobile sessions (i.e. three and six beds), and invest more resources in new static donor centres. Further moves that imply that donors will have to travel longer distances would be predicted to reduce the pool of donors, including those donors whose blood type is in high demand. Future evaluations should recognise the predicted reduction in the donor pool when evaluating the cost-effectiveness of further ‘switching’ of blood collection from mobile sessions to static donor centres.
The SP surveys have several strengths. First, the sample sizes (around 24,000 non-INTERVAL donors; 9000 ex-INTERVAL donors) are large compared with previous surveys, including DCEs in the evaluation of health services,60 and enable the surveys to provide precise estimates, not just overall, but for subgroups of prime interest. Specifically, we provided precise estimates of the relative effects of alternative service changes for subgroups for which it is particularly important to identify strategies that increase the blood supply, namely blood donors with high-demand blood types, BAME donors and nursery donors (all by sex). This will allow the analysis to estimate interaction effects of the inter-related attributes required for the subsequent CEA of alternative strategies. Second, the study administered three surveys in total. The pilot survey was important to check that the survey design provided plausible estimates, and to refine the design by modifying the choice of attributes and levels to try to reflect ‘real-life’ donations. In particular, an additional attribute concerning the availability of blood donation appointments was included in both main surveys. Feedback from the first donor translation workshop was that the definition of the attribute was clear and that the levels specified were relevant both to donors attending donor centres and to those attending mobile sessions (see Appendix 17). The second (non-INTERVAL) and third (ex-INTERVAL) surveys elicit preferences (donation frequencies) from donors attending predominantly mobile sessions and static donor centres, respectively. It was also useful to administer the same SP survey to two groups of donors who had, and had not, experienced the INTERVAL trial protocols. Third, the analytical model (ordered logit) respected the particular form of response data. We also undertook a thorough sensitivity analysis that considered other modelling choices and found that the direction and magnitude of estimated effects were robust to these choices (see Report Supplementary Material 7). Fourth, this study used appropriate methods for eliciting preferences and adopted recommended design principles. 46 The study therefore provides a more robust basis for predicting future donation behaviour than previous surveys of blood donors’ preferences. 7–11
The limitations of the surveys are that, first, it was infeasible to include all the attributes and levels of future policy relevance. Inevitably, choices had to be made according to those aspects of the service most likely to be of future importance for service provision and in line with best practice for survey design; we have reported the rationale for these choices. Second, although the response rates to the surveys were higher than anticipated, around 75% of invited donors did not complete questionnaires. Furthermore, as in the INTERVAL trial, donors who did not have an e-mail address were excluded. Rather than assuming that the requisite survey data were ‘missing completely at random’, we assumed that they were ‘missing at random’, that is, conditional on the variables included in the model. 53 Nonetheless, the estimates could be biased because of unobserved differences between the responders and the target population of prime interest. Third, although the discrepancy analysis found that the donation frequency predicted was similar to that observed, this did not consider the relative effect of service changes. These limitations have to be recognised in interpreting the subsequent findings of the CEAs that use the SP survey results.
The estimates from the SP survey presented in this chapter are not in themselves sufficient to inform the choice of future strategies for the following reasons. First, some of the strategies of interest require that several of the attributes are combined. For example, the strategy that specifies that a static donor centre is open at weekends requires estimates from the interaction of appointment availability and opening times attributes. This can then provide the requisite estimates of the effects of a weekend or evening opening strategy in static donor centres (see Chapter 4). Second, the responders to the survey do not represent the target population of interest, which may differ according to the strategy. However, the estimates presented in this chapter can be used to predict the relative effects of the alternative changes to the blood service for the relevant populations for each of the strategies considered in the CEA of the alternative strategies. Third, the survey results suggest that the introduction of a health report would lead to a relatively small increase in annual donation frequency, whereas strategies that would extend appointment availability would lead to greater increases in annual donation frequency. However, it will also be important to consider the relative costs of the alternative strategies. Chapter 4 will address these issues, using the estimates from the SP surveys to evaluate the cost-effectiveness of alternative strategies for maintaining the supply of whole blood.
Chapter 4 Cost-effectiveness analysis
Introduction
This chapter draws on results of the INTERVAL trial analysis (see Chapter 2), and the findings of two large surveys of donors’ preferences (see Chapter 3), to answer the broader question of which strategies available to the NHSBT are likely to represent value for money for the NHS in England. The NHSBT’s strategic objective is to reduce costs while maintaining the blood supply to the NHS, particularly the supply of blood types in high demand (O–, A– and B–). The research presented in this chapter addresses the third project objective pertaining to the cost-effectiveness of alternative strategies for maintaining the supply of whole blood. We focused on six strategies, which are all designed to improve opportunities for donors to give blood or make blood donation more attractive to donors. These were (1) offering a health report, (2) extending opening hours at static donor centres to weekends, (3) extending opening hours at static donor centres to evenings, (4) shifting opening hours of mobile sessions to weekends, (5) shifting opening hours of mobile sessions to evenings and (6) reducing the minimum interval between donations for donors attending static donor centres. These strategies are not mutually exclusive and may be implemented in combination and in conjunction with other initiatives. All six strategies are likely to increase the total volume of blood collected, which will come at additional cost. Given that the overall demand for whole blood is falling, the NHSBT would need to combine these strategies with other initiatives to release resources, several of which are already in progress, such as the closure of three- and six-bed mobile sessions in favour of more efficient nine-bed sessions. This analysis provides evidence to inform future decisions about the allocation of resources. The NHSBT may be prepared to invest to encourage more frequent donations by particular types of donors, such as those with high-demand blood types. Alternatively, these strategies could be adopted in such a way that the collection of higher demand blood types could be substituted for the collection of other blood types, by offering the service change described in each strategy exclusively to these groups of donors, for example by offering preferential appointments at certain times of the day or week.
This chapter sets out the methods used to identify the relevant target populations for each strategy (see Methods, Target population), defines the relevant comparator according to service provision recently experienced by donors (the status quo; see Methods, Comparator) and outlines those subgroups relevant to the analysis (see Methods, Subgroups). We describe how the preferences of non-trial participants from the SP survey were modelled to predict the donation frequency of all recent blood donors and how these were combined with deferral rates estimated from the INTERVAL trial (see Chapter 2) to predict the likely effect of each strategy on the volume of blood collected (see Methods, Predicted effect on the volume of blood donated). Relevant costs measured from a NHS and Personal Social Services perspectives over a 1-year time horizon are described (see Costs), with further details provided in Appendices 19 and 20. The results are presented for each target population for all donors, and donors with high-demand blood types (see Results, Base-case analysis), with results for other subgroups provided in additional material available on the project website. Key assumptions made in the analysis are tested with sensitivity analysis (see Methods, Sensitivity analysis and Results, Sensitivity analysis) and are discussed in relation to the main findings (see Discussion).
Strategies of interest
Six strategies of interest were identified through a review of NHSBT strategy documents, market research, an informal review of relevant published literature, consultation with NHSBT colleagues and insights from preliminary qualitative research with INTERVAL donors (R Lynch and S Cohn, LSHTM, personal communication, October 2015). Twelve initiatives were initially considered, of which six were selected for evaluation, details of which are described in Appendix 1. The six chosen strategies were:
-
provision of health report for all whole-blood donors after every donation
-
static donor centres open at weekends in addition to current opening hours
-
static donor centres open on weekday evenings in addition to current opening hours
-
mobile sessions held at weekends (once every 2 months) instead of existing weekday sessions
-
mobile sessions held on weekday evenings (once every 2 months) instead of existing weekday daytime sessions
-
reduced minimum intervals between whole-blood donations for men and women, such that men were able to donate whole blood up to six times a year and women up to four times a year in static donor centres.
Each strategy involved a single change to the blood collection service compared with the current service, as experienced by those donors who would be affected by the service change (described in Methods, Comparator and summarised in Table 17). These changes are not mutually exclusive and are not scalable to the same degree, so we compared each potential change to the relevant status quo for that specific change. A 1-year time horizon was adopted because the longer-term demand for blood is unclear and understanding the costs and benefits of the alternative strategies compared with the status quo over a shorter time horizon was considered by the NHSBT to be more useful for decision-making.
Methods
Target population
The relevant population for the evaluation was whole-blood donors who successfully gave blood at least once in the 12 months prior to March 2016 and who reside in mainland England. This population represents the NHSBT’s core donor base and, therefore, the donors for whom it will be most important to examine the potential effects of each strategy. This population excluded new and lapsed donors, but included some donors who were not eligible for our SP survey (e.g. donors without an e-mail address). In order to evaluate the potential impact of the strategies, we made some assumptions about which donors would be affected by each change to the service.
Conceivably, all donors could be offered a health report after each donation, as specified in our SP survey. The target population for the health report strategy is therefore all donors who met our initial criteria (donors who gave blood in the 12 months and who reside in mainland England).
The donors who would be affected by strategies 2–5 are defined by the donor’s most recent blood donation experience prior to March 2016 (see Chapter 3, Analysis of annual frequency of donation from the stated preference surveys). We assumed that donors who last visited a static donor centre would not be affected by changes to the opening hours of mobile sessions, and similarly that changes to static donor centres would not affect donors who last gave blood at a mobile session. We also assumed that the strategies to enable donors to give blood at weekends and weekday evenings would not affect those donors who had already been able to donate at these times at their most recently attended session and venue. The target population for strategy 2, to open static donor centres at weekends, was therefore all donors who last gave blood at a static donor centre that was not normally open at weekends. The target population for strategy 3, to open static donor centres on weekday evenings, was all donors who last gave blood at a static donor centre in a session that did not permit a blood donation visit at 8 p.m. or later. The target population for strategy 4, to hold mobile sessions on a Saturday or Sunday once every 2 months, was defined by all donors who last gave blood at a mobile session – because no mobile sessions were routinely open at weekends. The target population for strategy 5, to hold mobile sessions on weekday evenings until 8 p.m., was all donors who last gave blood at a mobile session that did not permit a blood donation visit at 8 p.m. or later (see Chapter 3, Analysis of annual frequency of donation from the stated preference surveys).
The strategy of reduced minimum intervals between donations (strategy 6) was judged as unlikely to apply to mobile sessions and so the target population was limited to donors who attended static donor centres at their last visit. The minimum interval was defined according to the lowest investigated in the INTERVAL trial (8 weeks for men; 12 weeks for women). The number of donors in each target population is reported in Table 17, along with a summary of the key methodological standpoints.
| Strategy | Target population of interest | Effectiveness | Costs | Sensitivity analysis | |
|---|---|---|---|---|---|
| Source of effectiveness data | Attribute levels to switch | ||||
| 1. Provision of a health report for all donors |
Donors who gave blood in the 12 months prior to March 2016 n = 781,028 |
Stated preference survey of non-INTERVAL participants Predictions apply ordered logit model estimates to donors who last donated at a mobile session or donor centre Deferral rates: INTERVAL control arm |
Health report not provided → health report provided |
Opportunity cost of measuring blood pressure and a cholesterol test at each donation Communication of abnormal tests Opportunity cost of deferrals Variable cost of blood collection (weighted average of donor centre and mobile session) |
Alternative source of effectiveness data: SP survey of INTERVAL participants in control arm only Alternative variable cost of collecting blood at static donor centres |
| 2. Weekend opening at static donor centres |
Donors who gave blood in the 12 months prior to March 2016 who last donated at a static donor centre not routinely open at weekends n = 60,640 |
Stated preference survey of non-INTERVAL participants Predictions apply ordered logit model estimates to donors who last donated at a donor centre Deferral rates: INTERVAL control arm |
Appointment availability: ‘Every weekday: Monday–Friday’ → ‘Every day: Monday–Sunday’ |
Opportunity cost of deferrals Variable cost of collecting blood (disposables and invitations only) Unsocial hours payment and normal staff costs for additional blood donations |
Alternative source of effectiveness data: SP survey of INTERVAL participants in control arm only |
| 3. Weekday evening opening at static donor centres |
Donors who gave blood in the 12 months prior to March 2016 who last donated at a static donor centre not open until 8 p.m. n = 99,312 |
Stated preference survey of non-INTERVAL participants Predictions apply ordered logit model estimates to donors who last donated at a donor centre Deferral rates: INTERVAL control arm |
Current opening hours → 9 a.m.–8 p.m. |
Opportunity cost of deferrals Variable cost of collecting blood (disposables and invitations only) Unsocial hours payment and normal staff costs for additional blood donations |
Alternative source of effectiveness data: SP survey of INTERVAL participants in control arm only |
| 4. Weekend opening of mobile sessions |
All who gave blood in the 12 months prior to March 2016 who last gave blood at a mobile session which is not routinely open at weekends n = 646,898 |
Stated preference survey of non-INTERVAL participants Predictions apply ordered logit model estimates to donors who last donated at a mobile session Deferral rates: INTERVAL control arm |
Appointment availability ‘1 day every 2 months: Monday–Friday’ → ‘1 day every 2 months: Saturday or Sunday’ |
Opportunity cost of deferrals Variable cost of collecting blood (including cost of disposables and invitations, but excluding cost of staff, travel and venue hire) Unsocial hours payment for additional blood donations |
Alternative source of effectiveness data: SP survey of INTERVAL participants in control arm only |
| 5. Weekday evening opening of mobile sessions |
All donors who gave blood in the 12 months prior to March 2016 who last gave blood at a session in a mobile site which is not routinely open until 8 p.m. on weekday evenings n = 582,910 |
Stated preference survey of non-INTERVAL participants Predictions apply ordered logit model estimates to donors who last donated at a mobile session Deferral rates: INTERVAL control arm |
Current opening hours → 2 p.m.–8 p.m. |
Opportunity cost of deferrals Variable cost of collecting blood (including cost of disposables and invitations, but excluding cost of staff, travel and venue hire) Unsocial hours payment for additional blood donations |
Alternative source of effectiveness data: ex-INTERVAL SP survey, control arm only |
| 6. Shorter minimum interval between donations for both men and women (static centres only) |
All who gave blood in the 12 months prior to March 2016 who last gave blood at a static donor centre n = 107,811 |
Stated preference survey of non-INTERVAL participants Predictions apply ordered logit model estimates to donors who last donated at a mobile session or donor centre. Deferral rates: INTERVAL control arm |
Maximum number of donations per year: Men four → six times per year and women three or four times per year |
Opportunity cost of deferrals Variable cost of collecting blood (for disposables and invitations only) |
Alternative source of effectiveness data: ex-INTERVAL SP survey, control arm only Use observed donation frequency of participants in INTERVAL trial (the average annual donation frequency over 2 years; 8 weeks vs. 12 weeks for men; 12 weeks vs. 16 weeks for women) Alternative variable cost of collecting blood at static centres |
Comparator
For each target population, the status quo or comparator for each strategy was defined by the service-level characteristics at each donor’s most recent blood donation visit using data from PULSE (see Figure 6). Tables 18 and 19 show the numbers and percentages of donors who donated blood in the last 12 months and who reside in mainland England. Fifteen static donor centres were routinely open at weekends (56% of donations at static donor centres); five static donor centres offered sessions until 8 p.m. on weekday evenings (8% of donations at static donor centres). The majority of donors, 86%, last donated at mobile sessions, which are usually held during weekday daytime hours (Table 19).
| Opening hours | Appointment availability, n (%) | ||
|---|---|---|---|
| Weekdays only | Monday–Sunday | Total | |
| 9 a.m.–12 p.m. and 2 p.m.–5 p.m. | 25,009 (23) | 17,615 (16) | 42,624 (40) |
| 9 a.m.–5 p.m. | 17,849 (17) | 38,839 (36) | 56,688 (53) |
| 9 a.m.–8 p.m. | 1 (0) | 27 (0) | 28 (0) |
| 2 p.m.–8 p.m. | 4312 (4) | 4159 (4) | 8471 (8) |
| Total | 47,171 (44) | 60,640 (56) | 107,811 (0) |
| Opening hours | Appointment availability, n (%) | ||
|---|---|---|---|
| Monday–Friday | Saturday or Sunday | Total | |
| 9 a.m.–12 p.m. and 2 p.m.–5 p.m. | 565,276 (84) | 24,482 (4) | 589,758 (88) |
| 9 a.m.–5 p.m. | 17,634 (3) | 1837 (0) | 19,471 (3) |
| 9 a.m.–8 p.m. | 0 (0) | 0 (0) | 0 (0) |
| 2 p.m.–8 p.m. | 63,988 (10) | 0 (0) | 63,988 (10) |
| Total | 646,898 (96) | 26,319 (4) | 673,217 (0) |
These service-level characteristics were used in the SP model to predict donation frequencies under the status quo, according to each individual donor’s personal characteristics.
Subgroups
The main subgroup of interest for the CEA was donors with high-demand blood types. The results of the cost-effectiveness analysis were reported by subgroup. In doing so, we assumed that the strategies would be offered to these groups exclusively, for example, a health report is given only to donors with high-demand blood types and these donors are offered preferential appointments at certain times of the day or week. Other subgroups of interest in the main analysis of SP survey data were also of interest in the CEA: ethnicity, nursery/non-nursery donors, age, sex and number of donations in the 12 months prior to March 2016. Strategies 2 and 3 were assumed to affect only donors who last gave blood at a static donor centre and strategies 4 and 5 were assumed to be of relevance only to donors who last gave blood at a mobile session, so results are not reported by static donor centre/mobile session subgroup. The relevance of other subgroups is likely to vary by strategy, as some subgroup results may be more likely to influence the implementation of that particular strategy. For example, results by sex may be important to understand the effect of reducing the minimum donation interval, but would be unlikely to affect the decision of whether or not to open at weekends.
Predicted effect on the volume of blood donated
The CEA required predictions of the average volume of blood donated per donor under each strategy for the relevant target population of current whole-blood donors, recognising that some appointments may be deferred. We initially calculated the annual frequency of intended blood donation visits before and after the service change. We predicted the intended number of visits according to the status quo by combining the estimated coefficients from the results of the ordered logit models applied to the SP survey data for the non-INTERVAL respondents (see Chapter 3, Analysis of annual frequency of donation from the stated preference surveys) with the relevant PULSE data for the appropriate target population. The same model and target population were then used to predict the number of visits following the introduction of each strategy, with the level of each relevant attribute switched to reflect the service change, for example the introduction of the health report (see Table 17). The models used the estimated interaction effects of venue for last donation (static centre vs. mobile session) with change in the level of each attribute (e.g. appointment availability or not) to provide predictions specific to the strategy in question (e.g. opening static donor centres at the weekend, see Table 17).
We fitted the ordered logit models to the full sample of responders to the non-INTERVAL survey (see Chapter 3), but then limited the target population for the prediction to those donors whose PULSE data showed that they did not have access to the service change in question at their last donation appointment (see Tables 18 and 19). So, for example, the effect of providing weekend appointments in donor centres was predicted only for those donors who did not have the opportunity to donate at the weekend at their last donation visit.
The primary measure of effectiveness was the total volume of blood collected per year, in units of whole blood. We calculated the volume of blood that would be collected under each strategy by subtracting the predicted number of deferrals from the predicted frequency of intended blood donation visits. The predicted deferral rate was taken from the INTERVAL trial data (control arm only for strategies 1–5, relevant trial arm for strategy 6), and subtracted from the predicted number of blood donation visits at the individual level. This adjustment for the likely number of deferrals provided estimates of the expected number of units of blood donated per donor per year, according to donor characteristics (see Chapter 2). The approach to incorporating the deferral rate recognised that the rate would differ according to the absolute frequency of intended blood donation.
The incremental effect of each strategy was calculated as the difference between the predicted mean volumes of blood before and after each potential service change. For example, the estimated effect of the introduction of a health report on the annual frequency of donation, overall and according to donor characteristics, was used to predict the effect of introducing this change for the relevant target population. We multiplied the predicted annual number of units of blood donated per donor by the number of donors in the target population to calculate the annual total volume of blood collected. We also used the estimates from the ordered logit model to report the proportion of donors who stated that they would stop donating before and then after the service change.
This approach is predicated on several assumptions. First, we assumed that the relative effects (on donor donation frequency) observed in the SP survey accurately represent the future donation behaviour in the respondents to the survey. As reported in Chapter 3, we found that for men there was a moderate discrepancy between stated and observed donation frequency, and for women the discrepancy was negligible; for both sexes the magnitude of the discrepancy was constant across subgroups. 58 We considered whether or not the CEA findings were robust to the source of data for predicted donation frequency by using the donation frequencies estimated from the INTERVAL trial as an alternative source of data for the alternative minimum donation interval strategies (see Methods, Sensitivity analysis). Second, we assumed that the predictive model accurately predicted the relative effect of the strategy on the volume of blood donated, which required that (1) the status quo attributes assigned to each donor provided an accurate prediction of the current volume of blood donated and (2) the preferences of responders to the survey represented those of donors in the target population, conditional on the variables in the model. We explore the impact of two alternative predictive models on the cost-effectiveness analysis (see Methods, Sensitivity analysis). Third, we assumed that the deferral rates from the INTERVAL trial applied to the target population of interest, after recognising differences in observed donor characteristics between the INTERVAL trial participants and the target population of interest for the specific strategy concerned.
Costs
The costs captured in the CEA were those relevant to the NHS and Personal Social Services, not just the costs borne by the NHSBT. The costs considered were the variable cost of collecting an additional unit of blood, costs associated with each specific strategy and the cost of deferrals, which have been shown to increase with donation frequency (see Chapter 2). Costs beyond 1 year were not included. Although processing costs are a significant driver of total cost, they were assumed to remain constant across strategies. Unit costs are detailed in full in Appendix 18.
In estimating the costs of the alternative strategies, a key distinction was made between the setting (static donor centre or mobile session) where the changes would be introduced. The changes to opening hours and to appointment availability for donor centres (strategies 2 and 3) were assumed to be an addition to the collection times available under the status quo. The implication for the costing was that these additional sessions would therefore require additional staff time. In contrast, in the case of mobile sessions, it was assumed that evening sessions or weekend availability (strategies 4 and 5) would replace existing sessions. The ensuing assumption for unit costs was that existing sessions at other times of the day or week would close, and the staff redeployed to provide these sessions in the evenings or at weekends.
Variable cost of collecting one unit of blood
The calculation of the unit cost of collecting an additional unit of blood required assumptions to be made about the capacity of mobile sessions and static donor centres to collect additional units of blood within their current resource constraints. Current data reveal that, on average, mobile sessions operate at 95% capacity, whereas sessions at donor centres operate on average at 75% capacity. Owing to the high operating capacity of mobile sessions, in our base-case analysis we assumed that strategies 4 and 5 would require additional resource to collect any additional units of blood. For strategies 1 and 6 it was assumed that there is capacity to collect more blood at donor centres within existing constraints posed by the number of beds and staffing levels.
Strategies 2 and 3 consider weekend and evening opening hours in static donor centres. The NHSBT advises that it would be feasible to consider opening static donor centres for blood collection at these times only as an addition to, rather than as a substitute for, current available appointment times. Hence, the cost analyses recognised that these strategies would require additional staff to collect additional units of blood. In order to meet this requirement, staff costs are captured in the analysis in order to reflect the true variable cost of collecting additional blood. For these two strategies, the cost of collecting an additional unit of blood at donor centres also includes the cost of disposables used (one copper sulphate test, one preoperative skin preparation and one pack per successful donation) and the cost of invitations (5.3 invitations per unit of blood collected at a donor centre) (NHSBT financial data 2015/16) (Laura Hontoria Del Hoyo, NHSBT, June 2016, personal communication; Colin Jackson, NHSBT, April 2017, personal communication). This equates to a cost of £9.41 per additional unit of blood collected.
For all strategies undertaken in mobile sessions, the costing approach recognised that, as the sessions are almost at capacity, more staff time will be required if additional units of blood are to be collected. The variable cost of collecting an additional unit of blood at a mobile session includes staffing, travel and venue costs, in addition to the cost of disposables and the cost of invitations (2.85 invitations per unit of blood collected at a mobile site) (based on NHSBT financial data 2015/16). This equates to a cost of £35.61 per additional unit of blood collected.
We test the main underlying assumption within the sensitivity analysis in Methods, Sensitivity analysis.
Strategy-specific costs
Unsocial hours payments
According to the NHS Agenda for Change61 (the current NHS grading and pay system for NHS staff), unsocial hours payments are owed to staff who work beyond 8 p.m. on weekdays or on Saturdays (any time), and a higher rate on Sundays and public holidays. The rate depends on pay band (donor carers NHS pay band 4, nurses NHS pay band 6). Pay rates were calculated according to standard NHS sources. 62 The cost to collect an extra unit of blood at a mobile session at a weekend was calculated as the weighted average of working on a Saturday or Sunday, instead of during weekday daytime hours, assuming staffing levels and efficiency of a nine-bed session where 3 units of blood can be collected per bed per hour. This reflects the shift from ‘Appointment availability: 1 day every 2 months Monday–Friday’ to ‘Appointment availability: 1 day every 2 months Saturday or Sunday’. The additional cost of shifting a 6-hour mobile session into the evening was calculated by assuming that 2 hours of a 9.35-hour shift would incur the unsocial hours payment.
The cost of collecting blood at the weekend or on weekday evenings at a static donor centre was different because strategies 2 and 3 considered an extension to opening hours, reflecting the shift in attribute levels from ‘Appointment availability: every weekday Monday–Friday’ to ‘Appointment availability: every day Monday–Sunday’. Thus, the cost of extending static donor centre opening hours to the weekend consists of the additional cost of employing staff to work during these hours (assuming session length of 8.5 hours, shift of 9.35 hours). The cost of extending static donor centre opening hours into a weekday evening consists of a 1-hour unsocial hours payment (assuming a session length of 12 hours, shift of 13.1 hours).
Health report
We assumed that the health report would consist of blood pressure and cholesterol readings. For cholesterol readings we assumed that this would be carried out alongside other routine tests taken on a sample of donated blood, at an additional cost of £4.20 per sample. 63 We assumed that measurement of blood pressure would be taken by the donor’s carer during the placement of the cuff prior to donation, but that this would require an additional 1.5 minutes. Owing to the travel required by mobile teams, donor contact time is more expensive in a mobile session than at a static donor centre. Costs for a blood pressure monitor (AND UA-767S Digital Blood Pressure Monitor, Williams Medical Supplies, Rhymney, UK)64 assumed a lifetime of 5000 uses in the base case. We assumed that the health report would be provided in an online format that would incur no additional costs to the NHSBT. Finally, we assumed that 2% of tests would require some form of clinical follow-up that would take the form of a mailed letter (estimated cost £0.55). This estimate reflects the ongoing nature of the tests and that any particularly concerning test results are likely to be detected and resolved at the first screen rather than generate clinical follow-up costs on an ongoing basis. The potential costs and benefits of a health report were limited by the 1-year time horizon.
Cost of deferrals
The cost of deferrals was included for all strategies. Donors can have their donation deferred as a result of low haemoglobin levels or for other reasons (donors who are not eligible to donate blood because of reasons, such as travel, medication, lifestyle or infection/illness).
Owing to low haemoglobin
We assumed that a donor undergoing a deferral for low levels of Hb would first receive a donor screen undertaken by a donor carer. This would be followed by a copper sulphate test for low levels of Hb. Failure of this test would then lead to confirmation of low levels of Hb with a HemoCue® (HemoCue, Radiometer Medical ApS, Denmark). We assumed that each step would be undertaken by a donor carer. The time taken for donor carers to undertake a health screen and fingerprick test and then a HemoCue test was estimated by NHSBT colleagues. The cost of the reagents for the copper sulphate test was estimated from NHSBT financial data, in 2016/17 prices. The cost of a HemoCue® machine and consumables (cuvettes) was taken from an online source (£700 for a machine and £188 for 200 cuvettes). 65 We did not adjust these costs for ‘bulk discounts’ but noted that the costs per test are modest. With regard to HemoCue lifetime we assumed 5000 uses in the base case. We used baseline data from the INTERVAL trial to inform the assumption that 7% of donors who defer because of low levels of Hb would incur downstream health-care costs. This included a general practitioner (GP) appointment, a full blood count test and a serum ferritin test. 62,66,67 We assumed that 50% of these donors would require iron supplements and 10% would be referred for an outpatient appointment. 68,69
Owing to other reasons
We assumed that in the case of deferrals for other reasons, donors would require a health screen and that this would typically take 2 minutes and would be carried out by a donor carer. 62
End points
The following end points will be reported:
-
incremental mean volume of blood collected per donor per year (in units of whole blood)
-
incremental cost per donor per year
-
incremental cost per additional unit of whole blood collected
-
incremental volume of blood collected per year for the target population (in units of whole blood)
-
incremental cost per year for the target population
-
change in percentage of donors who are predicted to stop donating after the service change.
The incremental cost per donor for each strategy compared with the status quo was calculated as the difference in the cost of collecting the mean volume of blood per donor before and the predicted mean volume of blood after the service change, in addition to the strategy-specific costs. Calculations to determine the incremental costs of each strategy are provided in Appendix 19. It is important to note that the incremental costs for an additional unit of blood apply only to the additional units of blood collected beyond the current volume of blood collected by the NHSBT.
Sensitivity analysis
The analysis considered the impact of two forms of uncertainty on the model results – parameter uncertainty and structural uncertainty. To assess the impact of parameter uncertainty we carried out a probabilistic analysis. We resampled estimates of the incremental volume of blood donated from a gamma distribution 10,000 times. This choice of the gamma distribution, which does not allow negative values (in this case for estimates of the incremental volume of blood donated), was informed by the results of the ordered logit model, which reported that the incremental effects of each strategy change were positive. Unit costs were not subject to parameter uncertainty; uncertainty in incremental costs therefore reflects the uncertainty in the volume of blood collected and associated resource use. Neither the uncertainty in the estimation in the model of the SP survey responses nor the uncertainty in the estimation in the model for estimating deferrals from the INTERVAL data is characterised in the probabilistic sensitivity analysis.
The probabilistic sensitivity analysis was rerun to examine the impact of two key structural assumptions of the results. First, we examined the impact of our base-case assumption about current operating capacity. If static donor centres do not have the required capacity, as may occur at the most popular appointment times and/or venues, more blood cannot be collected without incurring the cost of additional staff time. The probabilistic analysis was rerun with a variable cost of collecting a unit of blood that included additional staffing costs for the additional units of blood collected at static donor centres. This cost is not employed for strategies 2 and 3, which already include additional staff costs (as these strategies represent the extension of current opening hours), but did affect strategies 1 and 6. The variable cost of collecting an additional unit of blood collected at a donor centre used in this sensitivity analysis was increased from £9.41 in the base case to £26.49 (see Appendix 18).
Second, we considered alternative predictive models using a two-part model and gamma model rather than the ordered logit model chosen for the base-case analysis (see Chapter 3, Appendices 9–14 and Report Supplementary Material 7). Third, we considered alternative sources of data for predicting the volume of blood donated under the alternative strategies. The average annual frequency of donation observed over the 2-year follow-up in the INTERVAL trial was used to estimate the incremental effect for strategy 6 only (8-week arm vs. control arm, 12 weeks for men and the 12-week arm vs. control arm, 16 weeks for women). The accompanying uncertainty in the trial estimate was propagated through the model. We also used the results from the SP survey of ex-INTERVAL participants who had been randomised to the control arm in the trial. The benefit of using these data is that all INTERVAL participants have experience of donating at a donor centre but have not been exposed to the intervention (in this case, a reduction in the minimum donation interval), which is thought to influence behaviour.
Results
Base-case analysis
For each of the new strategies compared with the status quo, the incremental (differences in means) volumes of blood collected, incremental cost and incremental cost per additional unit of blood are reported for the relevant target population. The incremental results are presented in Tables 20 and 21; absolute values are reported in full in additional material on the project website.
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.113 | 0.519 | 0.455 | 0.070 | 0.484 | 0.678 |
| Incremental cost per donor per year (£) | 15.33 | 15.21 | 10.46 | 3.16 | 18.12 | 6.71 |
| Incremental cost of collection, per donor per year (£) | 3.58 | 14.95 | 10.24 | 3.12 | 17.89 | 6.38 |
| Incremental cost of deferrals, per donor per year (£) | 0.05 | 0.26 | 0.22 | 0.03 | 0.23 | 0.33 |
| Incremental cost of health report, per donor per year (£) | 11.71 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cost per additional unit of blood (£) | 136.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Ranking (1 – most cost-effective strategy; 6 – least cost-effective strategy) | 6 | 3 | 2 | 5 | 4 | 1 |
| Number of donors affected by service change | 781,028 | 60,640 | 99,312 | 646,898 | 582,910 | 107,811 |
| Incremental units of blood collected per year across target population | 88,189 | 31,483 | 45,233 | 45,405 | 282,152 | 73,130 |
| Incremental costs per year across target population (£)a | 11,977,545.00 | 922,051.00 | 1,038,927.00 | 2,042,047.00 | 10,563,294.00 | 723,908.00 |
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.100 | 0.489 | 0.408 | 0.030 | 0.199 | 0.714 |
| Incremental cost per donor per year (£) | 15.27 | 14.29 | 9.34 | 1.35 | 7.46 | 7.02 |
| Incremental cost of collection, per donor per year (£) | 3.17 | 14.09 | 9.17 | 1.34 | 7.37 | 6.72 |
| Incremental cost of deferrals, per donor per year (£) | 0.04 | 0.21 | 0.17 | 0.01 | 0.09 | 0.30 |
| Incremental cost of health report, per donor per year (£) | 12.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cost per additional unit of blood (£) | 152.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Ranking (1 – most cost-effective strategy; 6 – least cost-effective strategy) | 6 | 3 | 2 | 5 | 4 | 1 |
| Number of donors affected by service change | 111,948 | 7965 | 12,874 | 94,258 | 85,075 | 13,884 |
| Incremental units of blood collected per year across target population | 11,214 | 3896 | 5250 | 2829 | 16,970 | 9920 |
| Incremental costs per year across target population (£)a | 1,708,888.00 | 113,819.00 | 120,228.00 | 127,098.00 | 634,620.00 | 97,511.00 |
The results predict that donors would be willing to donate blood more often if changes to the service described in all six strategies were adopted, but that this would incur additional costs. Given the current service and personal characteristics of the target populations, the base-case results predict that donors, on average, successfully give blood between 2.2 and 2.5 times per year (see additional material on the project website; http://hemo.lshtm.ac.uk, accessed November 2018). We now consider each of the strategies versus the status quo, according to the additional units of blood donated per donor. Strategy 6, reducing the minimum donation interval, is predicted to provide the highest gain in the volume of whole blood collected per donor over 1 year, after allowing for the predicted increase in deferrals. The results suggest that, if asked to donate at the shortest minimum interval considered in the INTERVAL trial, donors at static donor centres would, on average, donate 0.68 additional units of blood per year. The strategy that leads to the next largest gain in the volume of whole blood per donor is to extend all donor centres opening times to weekends for all 25 static centres (strategy 2), followed by opening all donor centres into the evening. Opening mobile sessions at weekends (strategy 4) and offering a health report (strategy 1) after each donation are predicted to have the smallest impact, 0.07 and 0.11 additional units per donor, respectively.
The incremental cost per donor per year ranges from £3.16 to £18.12. This represents only the variable cost of collecting the additional blood yield per donor according to the methods set out in the different strategies. The strategy to substitute mobile weekday sessions to sessions held at weekends is the cheapest (£3.16), yet this strategy is associated with the smallest predicted increase in blood donation.
The strategies are ranked in order of their cost-effectiveness in Table 20. At a cost of £136 per additional unit of blood, the health report strategy is not likely to be cost-effective. It is associated with a high cost (£15.34 per donor per year) because all donors donating will receive a health report, yet it results in a small gain in blood volume (0.11 units per donor per year). The cost per additional unit of blood gained with the other strategies ranges from £10 to £45. Offering mobile sessions at weekends is predicted to have the smallest increase in donation frequency because our analysis assumes that weekend sessions will be offered instead of existing weekday sessions. Given that the predicted change in the volume of blood is so small, the incremental cost per donor is also small, resulting in an incremental cost per additional unit of blood of £45. The next best strategy in term of cost-effectiveness is to shift the opening hours of mobile sessions into the evening (strategy 5). This strategy is predicted to result in the third highest donation frequency of donors, but bears the high cost of collecting more blood at a mobile session in addition to a relatively small unsocial hours payment (for 2 hours of the shift).
Proposed changes to static donor centre opening days (strategy 2) and times (strategy 3) are predicted to increase donation frequency by 0.52 and 0.46 additional units per donor, respectively, and can be achieved at a relatively low cost per donor, despite the need to employ staff to work unsocial hours in addition to existing session times. Strategy 3, associated with an incremental cost of £23 per additional unit of blood collected, is more cost-effective than strategy 2, as unsocial hours payments are payable for only 1 hour of these sessions compared with the whole of the weekend session.
At £10, the strategy with the lowest cost per additional unit of blood gained is to reduce the minimum donation frequency (strategy 6). This is the strategy that is predicted to have the biggest increase in donation frequency, at a modest cost, but an outstanding concern about this strategy is the increased rate of deferrals because of low levels of Hb and their associated cost to the service. Although these costs have been included up to 1 year, the longer-term effects of this strategy have not been considered.
Graphs showing the probability that each strategy is cost-effective compared with the current status quo, as willingness to pay for an additional unit of blood increases, can be found in Appendix 20. The cost-effectiveness acceptability curves (CEACs) presented are extremely steep, representing the precision in the estimation of effect, with the model used to analyse the large sample for the SP survey.
A summary of the base-case cost-effectiveness results is presented in Table 20 for all donors (men and women) and then separately in Table 21 for donors with high-demand blood types. For a breakdown of the base-case results, overall and by subgroup, see Report Supplementary Material 8. From a policy perspective, the NHS might be willing to pay more for blood types of high demand or to release resources from elsewhere in order to collect more of these blood types. The cost-effectiveness results show that, if the service changes for each strategy were offered only to high-demand donors, the ranking of the strategies would not change. In fact, only the cost of the health report varied, because this cost accrued for all donations, not just additional donations. Given the responses to the SP survey, donors of black, mixed black, Asian and mixed Asian ethnicities were predicted to donate more frequently than donors of other ethnicities when offered the health report, but not sufficiently to significantly offset the associated costs. The cost per unit of additional blood was on average £69 for black/mixed black donors and £102 for Asian/mixed Asian donors, compared with £136 for all donors (Table 22). Strategy 4, opening mobile sessions at weekends, resulted in fewer donations from donors of black and mixed black ethnicities. The results for other subgroups showed slight changes in the incremental cost per unit of blood but no change in the ranking of strategies. These results can be found in full in the additional material on the project website.
| Target population | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Base case: all donors | 136 | 29 | 23 | 45 | 37 | 10 |
| Base case: all male donors | 109 | 29 | 23 | 44.8 | 37 | 10 |
| Base case: all female donors | 210 | 30 | 23 | 45 | 38 | 10 |
| Base case: all donors with high-demand blood types | 152 | 29 | 23 | 45 | 37 | 10 |
| Base case: all donors with standard-demand blood types | 133 | 29 | 23 | 45 | 38 | 10 |
| Base case: all nursery donors | 132 | 29 | 23 | 43 | 38 | 10 |
| Base case: all non-nursery donors | 138 | 29 | 23 | 45 | 37 | 10 |
| Base case: all white donors | 141 | 29 | 23 | 42 | 37 | 10 |
| Base case: all black/mixed black donors | 69 | 30 | 23 | 49 | 38 | 10 |
| Base case: all Asian/mixed Asian donors | 102 | 30 | 23 | 41 | 38 | 10 |
| Base case: all donors of other ethnicity or not stated | 115 | 29 | 23 | 48 | 37 | 10 |
| Base case: all donors aged 17–30 years | 132 | 29 | 23 | 45 | 38 | 10 |
| Base case: all donors aged 31–45 years | 135 | 29 | 23 | 45 | 38 | 10 |
| Base case: all donors aged 46–59 years | 136 | 29 | 23 | 45 | 37 | 10 |
| Base case: all donors aged ≥ 60 years | 142 | 29 | 23 | 45 | 37 | 10 |
Table 23 shows the percentages of donors who would stop donating in response to the six strategies; all of the strategies result in fewer donors deciding to stop donating than the status quo. The strategies most likely to retain donors were reducing the minimum interval between donations, weekend opening at static donor centres and evening opening of mobile sessions. Donors with high-demand blood types were less likely to decide to stop donating in response to the possible service changes than other donors. Conversely, black and mixed black donors were more likely than donors of other ethnicities to decide to stop donating across all strategies.
| Subgroup | Strategy (%) | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| All donors | 2 | 9 | 8 | 2 | 9 | 9 |
| High-demand blood type donors | 3 | 8 | 6 | 2 | 10 | 8 |
| Standard-demand blood type donors | 1 | 11 | 9 | 3 | 9 | 10 |
| Black/mixed black donors | 2 | 8 | 7 | 1 | 4 | 9 |
| Asian/mixed Asian mixed donors | 2 | 10 | 8 | 2 | 5 | 9 |
| White donors | 2 | 11 | 9 | 4 | 11 | 9 |
| Other ethnicity or not stated | 2 | 8 | 7 | 1 | 8 | 9 |
| Nursery donors | 2 | 9 | 8 | 2 | 9 | 9 |
| Non-nursery donors | 7 | 16 | 8 | 1 | 13 | 10 |
| Donors aged 17–30 years | 3 | 12 | 8 | 4 | 10 | 8 |
| Donors aged 31–45 years | 3 | 10 | 7 | 1 | 9 | 8 |
| Donors aged 46–60 years | 2 | 13 | 11 | 7 | 16 | 11 |
| Donors aged ≥ 60 years | 2 | 10 | 8 | 3 | 11 | 9 |
Tables 20 and 21 also report the predicted number of donors affected by each strategy, as well as the potential yield in terms of the maximum number of units of blood that could be collected via each strategy and the total associated cost. If the NHSBT was willing to pay £10 for an additional unit of whole blood, they could adopt the strategy to reduce the minimum donation interval and collect just over 73,000 units of whole blood (of which 9919 units would be from high-demand blood types). If more blood was required, the next best strategy is keeping donor centres open on weekday evenings, which could yield over 45,000 units of blood at a higher cost of £23 per additional unit of blood.
Sensitivity analysis
Assuming no additional capacity to collect more blood at donor centres
When staff costs are included in the variable cost per unit of blood for strategies 1 and 6, the cost per additional unit of blood for the strategies increases, ranging from £23 to £138, as shown in Table 24 (for sensitivity analysis results presented in full, see Appendix 21). The ranking of strategies in terms of their cost-effectiveness also changes. Now, strategies 2 and 3 are compared with other strategies that also bear the cost of employing additional staff to collect extra units of blood, and are predicted to result in a higher donation frequency. Evening opening hours at donor centres is the most cost-effective strategy, at £23 per additional unit of blood collected, followed by strategy 6 (to reduce the minimum donation interval), at £27 per additional unit of blood, closely followed by strategy 2 (to open static donor centres at weekends). As in the base case, the strategy to offer a health report remains cost-ineffective, at £139 per additional unit of blood. The cost-effectiveness ranking of strategies for high-demand donors is the same, with similar cost per additional unit of blood to all donors (see Table 24).
| Cost per additional unit blood (£) | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval at static donor centres | |
| Base case: all donors | 136.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Sensitivity analysis, higher variable cost of collecting blood at static donor centres to include staff costs: all donors | 138.00 | 29.00 | 23.00 | 45.00 | 37.00 | 27.00 |
| Sensitivity analysis, using two-part model as alternative predictive model: all donors | 129.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Sensitivity analysis, using gamma model as alternative predictive model: all donors | 127.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Sensitivity analysis, using ex-INTERVAL SP survey to predict change in volume of blood: all donors | 114.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Sensitivity analysis, using INTERVAL trial data to predict change in volume of blood: all donors | n/a | n/a | n/a | n/a | n/a | 11.00 |
| Base case: donors with high-demand blood types | 152.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Sensitivity analysis, higher variable cost of collecting blood at static donor centres to include staff costs: donors with high-demand blood types | 155.00 | 29.00 | 23.00 | 45.00 | 37.00 | 27.00 |
| Sensitivity analysis, using two-part model as alternative predictive model: donors with high-demand blood types | 147.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Sensitivity analysis, using gamma model as alternative predictive model: donors with high-demand blood types | 134.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Sensitivity analysis, using ex-INTERVAL SP survey to predict change in volume of blood: donors with high-demand blood types | 116.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Sensitivity analysis, using INTERVAL trial data to predict change in volume of blood: donors with high-demand blood types | n/a | n/a | n/a | n/a | n/a | 11.00 |
Alternative predictive models
Estimates of the predicted change in mean annual donation frequency were carried out using two alternative predictive models (see Appendices 9–16 and Report Supplementary Material 7), the two-part model and the gamma model as described in Chapter 3, Analysis of annual frequency of donation from the stated preference surveys, instead of the ordered logit predictive model used in the base-case analysis. The results showed that both the two-part model and the gamma model predicted slightly higher mean annual donation frequencies according to current service level characteristics than the ordered logit model, and also resulted in bigger increments for several strategies than the base-case model. The ranking of strategies, in terms of the volume of blood collected, differed slightly according to the predictive model employed, in part because of how close some of the predicted incremental volumes of blood estimated (for each strategy) were to each other. Both the two-part model and the gamma model predicted a smaller increment associated with a reduction in the minimum donation interval for donors giving blood at donor centres. Instead, the two-part model predicted the biggest increment in the volume of blood collected for weekday evening openings of static donor centres, whereas the gamma model predicted that the biggest change would be observed in opening donor centres at weekends. Both models predicted that more blood would be donated if mobile sessions were offered on weekday evenings than in the base-case analysis. However, in terms of cost-effectiveness, the ranking of strategies did not change compared with the base case. The incremental cost per additional unit of blood was the same in the base-case analysis, except for very small changes to the health report strategy (see Table 24).
Source of data for prediction of volume of blood donation: estimates from ex-INTERVAL survey data
The sensitivity analysis, using estimates of effect from the stated preference, of ex-INTERVAL participants survey data, again, resulted in predictions of bigger increases in donation frequency for all strategies, except the strategy to open static donor centres at weekends (see Table 24). The prediction for strategy 6 (to reduce the minimum donation interval) resulted in donors giving blood more than one additional time per year (0.36 times per year more than in the base-case analysis). The ranking of strategies in terms of cost-effectiveness did not change from the base-case analysis (see Table 24).
Source of data for prediction of volume of blood donation: estimates from the INTERVAL trial
The sensitivity analysis, using estimates of effect from the INTERVAL trial to predict the impact of each strategy on donation frequency and cost, revealed very similar estimates of cost-effectiveness as the base-case analysis, using estimates from the stated preference survey of non-INTERVAL donors (see Table 24). Owing to the sample size of the INTERVAL trial, compared with the stated preference survey, the estimates of effect were slightly less precise (see Appendix 20), which reveals a slightly less steep CEAC.
Discussion
This analysis has estimated the relative cost-effectiveness of offering whole-blood donors a health report at each donation (strategy 1), extending static donor centre opening hours to weekends (strategy 2) and weekday evenings (strategy 3), shifting mobile sessions to weekends (strategy 4) and weekday evenings (strategy 5), and reducing the minimum interval between donations (strategy 6) – all compared with the status quo (current practice). The results showed that the most cost-effective strategies are those that would be implemented solely in donor centres, rather than those that would be implemented at mobile sessions or at both types of venue (strategy 1). The findings were similar across all the subgroups of donors considered, including those donors whose blood types are in relatively high demand. For BAME subgroups, the incremental cost per additional unit of blood for the health report was £69 (compared with £135 for the overall sample), which implies that the health report was still relatively cost-ineffective, although it should be recognised that the sample size of BAME donors was relatively small for both surveys.
Strategy 6, reducing the minimum interval between donations to the shortest minimum interval for donors of both sexes at donor centres, is predicted to have a relatively low additional variable cost of £10 per additional unit of whole blood collected. The strategies to extend opening times in static donor centres to weekday evenings and weekends can also provide additional units of whole blood at moderate additional costs, an extra £23 and £29 per additional unit of blood collected, respectively. In other words, if the NHSBT were to adopt these strategies, it would cost an extra £10 to £29 in variable costs for each unit of blood collected beyond the current volume.
The NHSBT currently collects almost 1.6 million units of whole blood at a cost to the NHS of £120 per unit. This cost covers the whole supply chain (collections, processing, transport, testing, donor marketing, hospital services, storage and donor records), so is not directly comparable to the variable costs applied in this analysis. The proportion of the £120 charge per unit that is attributable to blood collection is around 40%, and, hence, the comparable amount paid for a unit of blood, which includes both variable and fixed costs, is around £48. The NHSBT has recently closed small mobile sessions as the costs per unit of blood collected were relatively high (> £50 per unit). The incremental cost for an additional unit of blood for all strategies except the health report, therefore, seems to be within an acceptable range, although the cost-effectiveness threshold remains unknown. This is particularly the case for approaches to providing more units of high-demand blood types: the NHSBT might be prepared to release more resources to pay for an additional unit of these blood types or substitute the collection of other blood types. In contrast, we find that the introduction of the health report is likely to be cost-ineffective.
The results were generally robust to the base-case assumptions concerning the source of data, as well as the choice of model used to predict donation frequencies from the survey data. The results were somewhat sensitive to the assumption that there is sufficient capacity at static donor centres to collect extra blood with existing levels of staff. When the costs of additional static donor centre staff were included, the strategy of reducing the minimum interval between donations became slightly less cost-effective compared with the status quo. The results suggest that up to 73,130 units of blood could be collected by reducing the minimum interval between donations for donors who give blood at static donor centres; this would be a 29% increase in the volume of blood collected at donor centres in 2015/16. Current staffing constraints would not be sufficient to support the collection of additional blood in this quantity. However, if the extra collection of blood was limited to high-demand blood types (up to 9900 units), it is feasible that this could be accommodated at times and locations sufficiently convenient for donors to give blood at the frequency predicted by their survey responses. Alternative approaches to providing more whole blood from the high-demand subgroup of donors would be to extend the opening times for static donor centres into weekday evenings or weekends. These strategies would provide additional capacity for whole-blood collection in static centres at a reasonable incremental cost per additional unit.
This analysis adds to the limited literature on the cost-effectiveness of alternative changes to the blood collection service. 18–28 Strengths of this analysis include the consideration of strategies of live policy relevance, the results of which provide timely evidence to inform decision-making. The analysis was based on the results of two large SP surveys, which had a combined sample size of > 30,000 whole-blood donors in England. Responders had given blood within the past 12 months and donors attending mobile sessions and static donor centres were well represented across the two surveys. The SP surveys were developed to offer flexibility in assessing the potential impact of changing different aspects of the blood service for two very different types of blood donation experience (static donor centres and mobile sessions), including aspects that do not currently exist (provision of a health report). Predictions from the SP survey model were similar to the observed donation frequency of female donors, differing moderately from the observed donation frequencies of male donors, and were similar across donor subgroups. The analysis draws on the deferral rates observed in the INTERVAL trial over 2 years and provides predictions about the population of prime relevance – the donors who live in mainland England and who gave blood in the past 12 months.
An important limitation of the analysis was that the 1-year time horizon did not allow for the costs and consequences of the strategies to be fully considered. In particular, although the increased rate of deferrals was captured in the analysis, the long-term effects of deferrals were not. The INTERVAL trial analysis found that, after 2 years, those in the reduced interval arms had lower haemoglobin and more haemoglobin-related deferrals. Beyond the health-care costs associated with treating donors with very low haemoglobin levels, there may well be longer-term costs and consequences, such as a higher proportion of donors leaving the donor register in response to repeated deferrals. Replacing donors is costly in terms of recruiting new donors and the opportunity costs of replacing a regular donor with another who may prefer to donate less frequently. The ensuing reduction in blood volume collected and the associated increase in costs could render this strategy less cost-effective than our analysis suggests. Further research is required on deferral rates over time. The INTERVAL extension study may provide opportunity to undertake such research, given that it will collect data on participants for up to 3 years.
The additional unit costs included in the model may not correspond to the full variable cost of collecting blood. Although the costs of deferrals to the service were included, we did not account for costs associated with donors who did not attend (DNA) their appointment, although there is no evidence to suggest that the rate of DNAs increases with higher donation frequency. Processing costs were excluded from the analysis, but in the likely event that the NHSBT does not collect more blood, but different blood types, these costs will not change. There may also be cost savings to the service not considered in the analysis. We did not consider efficiency savings of relying on a smaller pool of donors who give blood more frequently, which may be considerable, given the high cost of recruiting new donors and keeping them engaged in the process of regular blood donation. In addition, we did not incorporate the effects of patients switching from donation at mobile sessions to static donor centres (and vice versa). If opening hours increase at static donor centres, it is likely that some donors will opt to give blood at static donor centres instead of at their usual mobile session, an approach that may become more common if mobile sessions are closed. Savings made from the closure of less efficient mobile sessions might therefore improve the cost-effectiveness of strategies to improve opportunities to donate at static donor centres. Any cost savings would be specific to the particular session, but could potentially offset the additional costs required to implement these strategies.
Direct costs to donors were not considered. Opportunity costs faced by donors in donating whole blood more frequently may have been captured in their responses to the SP surveys, in terms of how often they would donate. Costs such as travel expenses may affect donation behaviour, although, because they are accrued in the pursuit of an altruistic act, including these costs in an analysis should be accompanied by the incorporation of benefits to donors in terms of increased utility from blood donation itself.
Uncertainty in the model predictions on donation frequency and associated resource use was incorporated in the probabilistic sensitivity analysis. Unit costs were assumed to be fixed; the largest were set by standard NHS sources and contracts, so are unlikely to vary. Other sources of uncertainty, most importantly structural uncertainty in the costing model and uncertainty in the extent to which SP surveys predict actual donor behaviour in response to service changes, have not so far been explored.
In conclusion, this analysis found that, for whole-blood donors attending static donor centres, reducing the minimum intervals between donations or extending the opening times for blood collection to include weekday evenings or weekends would provide additional units of whole blood at moderate additional variable costs compared with current practice. In contrast, although the introduction of a donor health report or extending opening times for mobile sessions are also predicted to increase the volume of whole blood donated, the additional costs are somewhat higher. The results of the analysis were consistent across a large number of prespecified subgroups, including those of prime policy relevance: donors with high-demand blood types, BAME donors, young donors and nursery donors. Chapter 5 will consider the overall conclusions from all aspects of this research and the general strengths, limitations and overall implications for the blood service and for further research.
Chapter 5 Discussion and conclusions
The HEMO study consisted of three interlinked components: SP surveys to elicit donor preferences, a CEA of reduced interdonation intervals from the INTERVAL trial, and a CEA of alternative strategies for maintaining the supply of whole blood to the NHS. An important finding from the SP surveys is that if donors attending static centres are offered further opportunities to donate at weekends or on weekday evenings, or if the minimum interval between blood donations is reduced, then they would be willing to donate whole blood more frequently. The INTERVAL CEA found that, although the reduced interval strategy might be a relatively cost-effective way of increasing donation frequency, it does increase the rate of deferrals because of low levels of Hb. Hence, a concern about extending this strategy to routine practice is that it may lead to reduced donor retention and higher costs in the long run.
The CEA of alternative strategies reports that further opportunities to donate at donor centres, such as offering appointments at weekends or during weekday evenings, are relatively cost-effective ways of increasing whole-blood donation, with low additional variable costs per unit of whole blood donated of around £29 and £23, respectively. As the NHSBT has a fixed budget, and any additional costs will have to be funded by releasing resources elsewhere, these further opportunities to donate could be limited to those donor subgroups about which there is concern that future demand will exceed supply, in particular donors whose blood type is in high demand. The required resources could be released, by for example, providing fewer opportunities to donate for other donor subgroups, or closing those mobile sessions that have relatively high costs per additional unit of blood collected (> £50 per additional unit of whole blood collected).
The surveys found that, on average, the introduction of a donor health report or moving mobile sessions from weekdays to weekends leads to small increases in stated donation frequency, and so these strategies are predicted to have, on average, relatively high costs per additional unit of blood collected. The survey findings also suggest that if there are changes to the blood collection service that increase donors’ travel time, for example closure of mobile sessions, will increase the proportion of donors who say they probably would not donate. The survey results show that some of the subgroups of donors who are particularly important to retain (BAME and younger donors) are relatively likely to say that they probably would not donate if travel time is increased by 15 or 30 minutes.
Patient and public involvement
The views of blood donors and a public representative informed the design of the research and the interpretation and implications of the findings. The choice of strategies for evaluation was informed by discussions with blood donors and suggestions from a public representative. Blood donors guided the design of the SP surveys by suggesting aspects of the service that might be important to change, including extending donation opportunities to weekday evenings and weekends. Blood donors also identified attributes that should not be included, for example financial payment for donating blood, and provided insights on what the questionnaire recipient might be thinking when attempting to answer the questions. The questionnaire design was also informed by findings from qualitative research on blood donors’ views, which suggested that it was important to recognise that donors may prefer to continue to donate at the same venue rather than a different setting, irrespective of other features of an opportunity to donate (R Lynch and S Cohn, LSHTM, personal communication, October 2015). The design of the SP survey was informed by a large pilot study of blood donors; donors and the public representative reviewed the donor information and consent forms prior to submission to the NHS Research Ethics Committee. The eventual CEA was driven by the responses to the surveys administered to large numbers of blood donors, both those who were previously participants in the INTERVAL trial (n = 9318) and those who were not (n = 25,187).
The translation workshops with donors were an important element of the research. The first translation workshop was undertaken following the preliminary analysis of the first SP survey, and it offered insight into the early interpretation of the preference results. It also endorsed the changes to the survey design, made in response to the results of the pilot study. The second translation workshop allowed the research team to gather views on the overall cost-effectiveness findings from the project and provided insights as to how they should best be communicated to donors and what, from the donors’ perspective, should be the priorities for future research.
Strengths
The study provided estimates of the relative cost-effectiveness of alternative strategies of direct policy relevance for donor subgroups of prime interest. The results presented extend the limited extant literature on the cost-effectiveness of alternative strategies for maintaining the blood supply. In particular, the direct input of the NHSBT during the design stage ensured that the study considered those strategies of prime relevance for key subgroups of interest. This required the use of evidence from three complementary sources: a large well-conducted RCT, SP surveys and the NHSBT donor registry. The INTERVAL trial provided estimates of the relative effect of reduced donation intervals on donor health and on a key concern – relative rates of deferral for donors attending static donor centres. The surveys investigated a key concern set out in the NHSBT blood 2020 strategy document (pillar 1),4 regarding improving the donors’ experience, informed by the understanding of their relative preferences for alternative changes to the blood service, including those such as the introduction of a donor health report that are not currently available.
The study also extended previous research on blood donation by following choice-based principles. A particular strength of the study was that it was replicated in two contrasting settings. Among ex-INTERVAL participants, all of whom had experience of donating at a static donor centre, and among non-INTERVAL participants, the majority of whom had donated at mobile settings. The main findings concerning donor preferences were similar across the surveys of non-INTERVAL and ex-INTERVAL donors. The large sample sizes in the INTERVAL trial and in both stated preference surveys enabled results to be reported across many different subgroups, including those of prime interest to the NHSBT. The PULSE data set provided a sampling frame for the study and allowed the representativeness of the study populations to be defined. It was also used to define the target population for the CEA and the current practice experienced by blood donors. This was important to ensure that the CEA results were provided for the population of prime interest.
The study provided a framework that could be followed in future evaluations, not just of alternative changes to the blood service but also of health services more widely. In particular, the research found that it was feasible to quickly elicit preferences from large samples of the public. The research also found that the discrepancy between stated and actual behaviour was moderate (men) or small (women); this finding from large survey and corresponding registry data extends the limited methodological literature in health care on stated versus revealed preferences. Finally, the CEA was accompanied by extensive sensitivity analyses, which found that the findings were robust to alternative standpoints, notably the source of data for the estimates of relative donation frequency, and the assumptions underlying the unit cost estimates.
Limitations
The main time horizon for the cost-effectiveness analysis was 1 year, and so the long-term effects of the alternative strategies on, for example, the rate and costs of donors leaving the register, or any effects of the choice of strategies on fixed costs, were not considered. In particular, the INTERVAL trial found that the reduced donation intervals did lead to increased rates of Hb-related deferrals and, if, in the long term, this does encourage donors to leave the donation register, this will make this strategy less cost-effective. Costs were measured from the perspective of the NHS, so any additional costs to donors, for example from time off work or increased travel time, were not considered. This was consistent with the measurement of consequences, which excluded any change in donor’s well-being from donating blood more often beyond that which would be detected by the generic measure of health-related QoL (SF-6D utility score). The research did not evaluate all the strategies of potential interest to the NHSBT, such as expanding the number of static donor collection centres or closing and/or merging of mobile sessions. The cost-effectiveness results relied on estimates from the 25.2% (non-INTERVAL) and 32.4% (ex-INTERVAL) responders to the SP surveys. The analysis adjusted for differences between the survey responders and the target population (e.g. according to donation history), but does not allow for those differences that remain unobserved (e.g. intrinsic motivation).
Conclusions and implications for the blood collection service
-
Donors whose last donation was at a static donor centre would strongly prefer more opportunities to donate during weekday evenings or at weekends. For donors with blood types that are in high demand, providing improved opportunities to donate by extending opening hours to the evenings or weekends in all donor centres would be relatively cost-effective (£23 and £29 per additional unit donated, respectively).
-
A strategy of reducing minimum intervals between donations from 12 to 10 or 8 weeks (men), or from 16 to 14 or 12 weeks (women), is preferred by donors, and can provide additional whole blood at £10 per additional unit. In the short run, this strategy might help maintain the required levels of red cells for blood types that are in high demand, such as O–, but will increase rates of Hb-related deferrals and so may lead to donors leaving the register and increase costs in the longer term.
-
Moving mobile sessions to the weekends or providing a donor health report at each donation visit would not, on average, lead to sufficient increases in the frequency of whole-blood donations to justify the additional costs.
-
Requiring donors to travel further to donate whole blood will encourage donors to leave the donation register; subgroups of younger or less experienced donors and some BAME (ex-INTERVAL) donors are among those most likely to stop donating. As it is important to retain these donor subgroups, if the NHSBT continues to close mobile sessions and increase travel time for donors, it may be important to adopt strategies targeted at retaining these particular subgroups of donors.
Implications for further research
-
If any of these strategies are implemented, it will be important to monitor the preferences and donation frequency for different donor subgroups, and in alternative settings. Such an evaluation could build on the research framework presented here and consider the costs and consequences of scaling up the strategy, in real time, by using large-scale surveys of preferences calibrated to actual donation behaviour as recorded in the PULSE registry.
-
Further research is required to investigate the effectiveness and cost-effectiveness of tailoring opportunities to donate blood according to the preferences of particular donor subgroups. There is a particular requirement to understand the preferences of BAME donors, as their blood is in high demand, and only a relatively small sample of these donors were surveyed in the HEMO study.
-
Improved understanding of how to predict which donors are likely to have Hb-related deferrals would allow stratification of the donor population by likelihood of deferral. This could allow increased blood collection using shorter interdonation intervals for a defined subpopulation of donors whose blood type is in high demand.
-
Further research is warranted on evaluating novel marketing strategies to encourage the recruitment of particular subgroups of new donors (e.g. those with O– blood type, BAME donors, younger donors), to ensure that there is the required mix of blood donors to sustain the future blood supply.
Acknowledgements
We are grateful to the National Institute for Health Research (NIHR) Health Services Delivery Research Programme for funding this project.
We wish to acknowledge the contribution of NHSBT staff Dr Naim Akhtar, Andy Byrne, Donna Cullen, Laura Hontoria Del Hoyo, Yasmine Hugill, Gareth Humphries, Jon Latham, Varshini Parayoganathan, Hannah Perry, Mike Stredder and Nick Watkins to the design, conduct, analysis and interpretation of the study.
We wish to acknowledge Dr Stephen Kaptoge, Professor Simon Thompson and Dr Matthew Walker for the INTERVAL trial analysis, as well as other members of the INTERVAL trial steering committee, and Professor John Danesh (joint chief investigator).
We wish to acknowledge the contribution of the qualitative researchers on the INTERVAL trial, Dr Rebecca Lynch and Dr Simon Cohn, whose preliminary findings informed the design and interpretation of the stated preference surveys.
We wish to thank all the blood donors who participated in the surveys and workshops and the staff from NHSBT who supported the conduct of the study. We also wish to thank Peter Zollman (public representative) for advising on the design of the study, interpretation and communication of the results, the design of the study website and the workshops with blood donors.
Health Economics Modelling study management group
Professor Richard Grieve (chief investigator), Professor John Cairns, Kaat De Corte, Professor Neil Hawkins, Dr Mark Pennington, Dr Silvia Perra, Dr Zia Sadique and Sarah Willis.
Health Economics Modelling study advisory group
Professor Janet T Powell (independent chairperson), Dr Gavin Cho, Laura Hontoria Del Hoyo, Dr Gail Miflin, Dr Carmel Moore, Professor David J Roberts, Crispin Wickenden and Peter Zollman.
The INTERVAL study
Participants in the INTERVAL RCT were recruited with the active collaboration of NHSBT, which has supported field work and other elements of the trial. DNA extraction and genotyping was funded by the NIHR, the NIHR BioResource (http://bioresource.nihr.ac.uk/) and the NIHR Cambridge Biomedical Research Centre (www.cambridge-brc.org.uk). The academic co-ordinating centre for INTERVAL was supported by core funding from the NIHR Blood and Transplant Research Unit in Donor Health and Genomics, the UK Medical Research Council (G0800270), the British Heart Foundation (SP/09/002) and the NIHR Cambridge Biomedical Research Centre. A complete list of the investigators and contributors to the INTERVAL trial is provided in Moore et al. 42
Contributions of authors
Richard Grieve (Professor, Health Economics Methodology) conceived and designed the overall study, directed the acquisition, analysis and interpretation of the results, and drafted and critically revised the manuscript.
Sarah Willis (Research Fellow, Health Economics) contributed to the design of the study, the delivery of the preference surveys and the acquisition of survey, PULSE and cost data; conducted the CEA and interpreted the results, and drafted and critically revised the manuscript.
Kaat De Corte (Research Degree Student, Health Economics) contributed to the design of the study, contributed to the acquisition, analysis and interpretation of the survey, PULSE and INTERVAL data; conducted the discrepancy analysis, and drafted and critically revised the manuscript.
M Zia Sadique (Assistant Professor, Health Economics) conducted the analysis and interpretation of the INTERVAL trial data, the SP survey data and predictions used in the CEA, and drafted and critically revised the manuscript.
Neil Hawkins (Professor, Health Economics and Health Technology Assessment) contributed to the design of the CEA, the analysis and interpretation of the data, and the drafting and critical review of the manuscript.
Silvia Perra (Research Fellow, Statistics) contributed to the design of the study, conducted the analysis of the pilot SP survey, contributed to the acquisition of the PULSE data, the analysis of the preference survey data, and the drafting and critical review of the manuscript.
Mark Pennington (Senior Lecturer, Health Economics) contributed to the design of the study, the acquisition, analysis and interpretation of the cost data, and the drafting and critical review of the manuscript.
Jenny Turner (Research Manager) co-ordinated the HEMO study, organised the workshops with blood donors, designed and created the study website, and contributed to the drafting and critical review of the manuscript.
Carmel Moore (Senior Scientific Research Co-ordinator, Trials) conducted the scientific co-ordination of the INTERVAL trial, contributed to the interpretation of the results pertaining to the INTERVAL trial and the drafting and critical review of the manuscript.
Crispin Wickenden (Head of Donor Management, NHSBT) contributed to the design and delivery of the preference surveys and use of PULSE data, the interpretation of the results, and the drafting and critical review of the manuscript.
Catharina Koppitz (Market Research Analyst, NHSBT) contributed to the design and administration of the preference surveys and use of PULSE data, the interpretation of the results, and the drafting and critical review of the manuscript.
Gavin Cho (Consultant Haematologist, NHSBT) contributed to the interpretation of the results and the drafting and critical review of the manuscript.
David J Roberts (Professor and Consultant Physician, Haematology) was joint chief investigator for the INTERVAL trial, and contributed to the interpretation of results and the drafting and critical review of the manuscript.
Gail Miflin (Medical and Research Director, NHSBT and Consultant Haematologist) contributed to the design of the study, interpretation of the results, and the drafting and critical review of the manuscript.
John A Cairns (Professor, Health Economics) helped design the overall study, directed the design and analysis of the preference surveys and the overall results, and drafted and critically revised the manuscript.
Publication
Willis S, De Corte K, Cairns JA, Sadique MZ, Hawkins N, Pennington M, et al. Cost-effectiveness of alternative changes to a national blood collection service [published online ahead of print 16 May 2018]. Transfus Med 2018.
Data-sharing statement
Owing to the nature of this study and the conditions attached to original data agreements, there are no data available for wider use. All queries should be submitted to the corresponding author in the first instance.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Blood Safety and Availability. Geneva: WHO; 2016.
- Seifried E, Klueter H, Weidmann C, Staudenmaier T, Schrezenmeier H, Henschler R, et al. How much blood is needed?. Vox Sang 2011;100:10-21. https://doi.org/10.1111/j.1423-0410.2010.01446.x.
- NHSBT . NHS Blood and Transplant Annual Report and Accounts 2015 to 2016 2016. www.gov.uk/government/publications/nhs-blood-and-transplant-annual-report-and-accounts-2015-to-2016 (accessed 6 April 2017).
- Strategic Plan 2015-20: Improving Outcomes for Patients and Donors. 2015.
- NHSBT . Group O n.d. www.blood.co.uk/why-give-blood/the-need-for-blood/group-o/ (accessed 4 May 2016).
- Devine D, Goldman M, Engelfriet CP, Reesink HW, Hetherington C, Hall S, et al. Donor recruitment research. Vox Sang 2007;93:250-9. https://doi.org/10.1111/j.1423-0410.2007.00962.x.
- Boenigk S, Leipnitz S, Scherhag C. Altruistic values, satisfaction and loyalty among first-time blood donors. Int J Nonprofit Volunt Sect Mark 2011;16:356-70. https://doi.org/10.1002/nvsm.433.
- Veldhuizen IJT. ‘Thank you! Please visit us again’. Reflecting on the donor retention literature – implications for retention practices. ISBT Sci Series 2010;5:196-200. https://doi.org/10.1111/j.1751-2824.2010.01372.x.
- Mews M. To pay or not to pay – the role of monetary incentives in an optimal blood donation service bundle. Int J Nonprofit Volunt Sect Mark 2013;18:192-20. https://doi.org/10.1002/nvsm.1465.
- Schlumpf KS, Glynn SA, Schreiber GB, Wright DJ, Randolph Steele W, Tu Y, et al. Factors influencing donor return. Transfusion 2008;48:264-72.
- Schreiber GB, Schlumpf KS, Glynn SA, Wright DJ, Tu Y, King MR, et al. Convenience, the bane of our existence, and other barriers to donating. Transfusion 2006;46:545-53. https://doi.org/10.1111/j.1537-2995.2006.00757.x.
- McKeever T, Sweeney MR, Staines A. An investigation of the impact of prolonged waiting times on blood donors in Ireland. Vox Sang 2006;90:113-18. https://doi.org/10.1111/j.1423-0410.2006.00734.x.
- Germain M, Glynn SA, Schreiber GB, Gélinas S, King M, Jones M, et al. Determinants of return behavior: a comparison of current and lapsed donors. Transfusion 2007;47:1862-70. https://doi.org/10.1111/j.1537-2995.2007.01409.x.
- Jovanović R, Radlovački V, Pečujlija M, Kamberović B, Delić M, Grujić J. Assessment of blood donors’ satisfaction and measures to be taken to improve quality in transfusion service establishments. Med Glas 2012;9:231-8.
- Lacetera N, Macis M, Slonim R. Will there be blood? Incentives and displacement effects in pro-social behavior. Am Econ J-Econ Polic 2012;41:186-223. https://doi.org/10.1257/pol.4.1.186.
- Costa-Font J, Jofre-Bonet M, Yen ST. Not all incentives wash out the warm glow: the case of blood donation revisited. Kyklos 2013;66:529-51. https://doi.org/10.1111/kykl.12034.
- Niza C, Tung B, Marteau TM. Incentivizing blood donation: systematic review and meta-analysis to test Titmuss’ hypotheses. Health Psychol 2013;32:941-9. https://doi.org/10.1037/a0032740.
- Williamson LM, Devine DV. Challenges in the management of the blood supply. Lancet 2013;381:1866-75. https://doi.org/10.1016/S0140-6736(13)60631-5.
- Beliën J, Forcé H. Supply chain management of blood products: a literature review. Eur J Oper Res 2012;217:1-16. https://doi.org/10.1016/j.ejor.2011.05.026.
- Katsaliaki K. Cost-effective practices in the blood service sector. Health Policy 2008;86:276-87. https://doi.org/10.1016/j.healthpol.2007.11.004.
- Pereira A. Economies of scale in blood banking: a study based on data envelopment analysis. Vox Sang 2006;90:308-15. https://doi.org/10.1111/j.1423-0410.2006.00757.x.
- van der Pol MM, Cairns JA. The efficient organization of blood donation. Health Econ 1998;7:455-63. https://doi.org/10.1002/(SICI)1099-1050(199808)7:5<455::AID-HEC356>3.0.CO;2-8.
- van der Pol MM, Cairns JA, Galea G. The efficient organisation of blood donation: what determines the number of donors and donations?. Transfusion Med 2000;10:5-11. https://doi.org/10.1046/j.1365-3148.2000.00226.x.
- Abraham I, Sun D. The cost of blood transfusion in Western Europe as estimated from six studies. Transfusion 2012;52:1983-8. https://doi.org/10.1111/j.1537-2995.2011.03532.x.
- Lowalekar H, Ravichandran N. Model for blood collections management. Transfusion 2010;50:2778-84. https://doi.org/10.1111/j.1537-2995.2010.02944.x.
- Rautonen J. Redesigning supply chain management together with the hospitals. Transfusion 2007;47:197-200. https://doi.org/10.1111/j.1537-2995.2007.01385.x.
- Varney SJ, Guest JF. The annual cost of blood transfusions in the UK. Transfus Med 2003;13:205-18. https://doi.org/10.1046/j.1365-3148.2003.00443.x.
- Dixon S, James V, Hind D, Currie CJ. Economic analysis of the implementation of autologous transfusion technologies throughout England. Int J Technol Assess Health Care 2005;21:234-9.
- Alfonso E, Xie X, Augusto V, Garraud O. Modeling and simulation of blood collection systems. Health Care Manag Sci 2012;15:63-78. https://doi.org/10.1007/s10729-011-9181-8.
- Nagurney A, Masoumi A, Boone T, Jayaraman V, Ganeshan R. Sustainable Supply Chains. New York, NY: Springer; 2012.
- De Angelis V, Felici G, Impelluso P. Integrating simulation and optimisation in health care centre management. Eur J Oper Res 2003;150:101-14. https://doi.org/10.1016/S0377-2217(02)00791-9.
- Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee (JPAC) . Guidelines for the Blood Transfusion Services n.d. www.transfusionguidelines.org/dsg (accessed 2 February 2017).
- Sayers M, Centilli J. Contemplating the effect on blood availability if the interdonation interval of 56 days is prolonged. Transfusion 2013;53:1132-6. https://doi.org/10.1111/j.1537-2995.2012.03900.x.
- Spencer BR, Johnson B, Wright DJ, Kleinman S, Glynn SA, Cable RG. REDS-II RISE Analysis Group . Potential impact on blood availability and donor iron status of changes to donor hemoglobin cutoff and interdonation intervals. Transfusion 2016;56:1994-200. https://doi.org/10.1111/trf.13663.
- Gandhi MJ, Duffy K, Benike M, Jenkins S, Stubbs JR. Effect of increasing hemoglobin cutoff in male donors and increasing interdonation interval in whole blood donors at a hospital-based blood donor center. Transfusion 2012;52:1880-8. https://doi.org/10.1111/j.1537-2995.2011.03533.x.
- Baart AM, van den Hurk K, de Kort WL. Minimum donation intervals should be reconsidered to decrease low hemoglobin deferral in whole blood donors: an observational study. Transfusion 2015;55:2641-4. https://doi.org/10.1111/trf.13195.
- Custer B, Chinn A, Hirschler NV, Busch MP, Murphy EL. The consequences of temporary deferral on future whole blood donation. Transfusion 2007;47:1514-23. https://doi.org/10.1111/j.1537-2995.2007.01292.x.
- Hillgrove T, Moore V, Doherty K, Ryan P. The impact of temporary deferral due to low hemoglobin: future return, time to return, and frequency of subsequent donation. Transfusion 2011;51:539-47. https://doi.org/10.1111/j.1537-2995.2010.02881.x.
- Blood Products Advisory Committee . Hemoglobin/Hematocrit/Acceptance/Standards/and/Interdonation/Interval/in/Blood/Donors n.d. https://wayback.archive-it.org/7993/20170113014841/http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/ucm221857.htm (accessed November 2018).
- Goldman M, Magnussen K, Gorlin J, Lozano M, Speedy J, Keller A, et al. International Forum regarding practices related to donor haemoglobin and iron. Vox Sang 2016;111:449-55. https://doi.org/10.1111/vox.12431.
- Karp JK, King KE. International variation in volunteer whole blood donor eligibility criteria. Transfusion 2010;50:507-13. https://doi.org/10.1111/j.1537-2995.2009.02392.x.
- Moore C, Sambrook J, Walker M, Tolkien Z, Kaptoge S, Allen D, et al. The INTERVAL trial to determine whether intervals between blood donations can be safely and acceptably decreased to optimise blood supply: study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-363.
- Goette L, Stutzer A, Yavuzcan G, Frey BM. Free cholesterol testing as a motivation device in blood donations: evidence from field experiments. Transfusion 2009;49:524-31. https://doi.org/10.1111/j.1537-2995.2008.02007.x.
- Mortimer D, Ghijben P, Harris A, Hollingsworth B. Incentive-based and non-incentive-based interventions for increasing blood donation. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD010295.
- Ringwald J. Established ways to keep donor’s interest alive. ISBT Sci Series 2010;5:17-23. https://doi.org/10.1111/j.1751-2824.2010.01389.x.
- Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice to Value Health and Health Care. Dordrecht: Springer; 2008.
- Di Angelantonio EA, Thompson SG, Kaptoge S, Moore C, Walker M, Armitage J, et al. Efficiency and safety of varying the frequency of whole blood donation: randomised trial of 45,000 donors. Lancet 2017;390:2360-71. https://doi.org/10.1016/S0140-6736(17)31928-1.
- Moore C, Bolton T, Walker M, Kaptoge S, Allen D, Daynes M, et al. Recruitment and representativeness of blood donors in the INTERVAL randomised trial assessing varying inter-donation intervals. Trials 2016;17. https://doi.org/10.1186/s13063-016-1579-7.
- Thompson S. INTERVAL Trial Steering Committee . INTERVAL Trial – Statistical Analysis Plan for Principal Paper 2016. www.intervalstudy.org.uk/files/2016/01/SAP_v5_final_08Jan16-2.pdf (accessed 2 November 2018).
- Guide to the Methods of Technology Appraisals. London: NICE; 2013.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Greene WH. Econometric Analysis. Upper Saddle River, NJ: Prentice Hall; 2003.
- Rubin D. Inference and missing data. Biometrika 1976;63:581-92. https://doi.org/10.1093/biomet/63.3.581.
- Willan AR, Briggs AH. Statistical Analysis of Cost-Effectiveness Data. Chichester: John Wiley & Sons; 2006.
- van den Berg B. Sf-6d population norms. Health Econ 2012;21:1508-12. https://doi.org/10.1002/hec.1823.
- Masser B, France CR, Foot J, Rozsa A, Hayman J, Waller D, et al. Improving first-time donor attendance rates through the use of enhanced donor preparation materials. Transfusion 2016;56:1628-35. https://doi.org/10.1111/trf.13496.
- Poto-Ferreira FA, de Almeida-Neto C, Murphy EL, Montebello SC, Nogueira FA, Koga da Silva EM, et al. A randomized trial to evaluate the use of text messaging, letter, and telephone call reminders to improve return of blood donors with reactive serological tests. Transfusion 2016;57:102-7.
- De Corte K, Willis S, Perra S, Pennington M, Hawkins N, Cairns J, et al. Harnessing a Large Observational Database to Interpret the Results of a Stated Preference Survey: The Case of Blood Donation n.d.
- Fifer S, Rose J, Greaves S. Hypothetical bias in stated choice experiments: Is it a problem? And if so, how do we deal with it?. Transportation Res A-Pol 2014;61:164-77. https://doi.org/10.1016/j.tra.2013.12.010.
- de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient 2015;8:373-84. https://doi.org/10.1007/s40271-015-0118-z.
- The NHS Staff Council . NHS Terms and Conditions of Service Handbook n.d. www.nhsemployers.org/your-workforce/pay-and-reward/agenda-for-change/nhs-terms-and-conditions-of-service-handbook (accessed May 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Department of Health and Social Care (DHSC) . Economic Modelling for Vascular Checks 2008. www.healthcheck.nhs.uk/document.php?o=225 (accessed 21 July 2016).
- Williams Medical . AND UA-767S Digital Blood Pressure Monitor n.d. www.wms.co.uk/Blood_Pressure_and_ABPM/ (accessed March 2017).
- Health-Care Equipment Medical Group . HemoCue 201+ Haemoglobin Analyser for Blood Haemoglobin Determinations n.d. www.hce-uk.com/HemoCue-201-Haemoglobin-Analyser-for-blood-Heamoglobin-determinations (accessed March 2017).
- Czoski-Murray C, Lloyd Jones M, McCabe C, Claxton K, Oluboyede Y, Roberts J, et al. What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16500.
- Costing Statement NG8. London: NICE; 2015.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 28 March 2017).
- Reference Costs 2015–16. London: DHSC; 2016.
Appendix 1 Choice of strategies to evaluate
Initially, the strategic objective for NHSBT was to reduce costs while maintaining the blood supply. Strategic initiatives that could help the organisation meet this objective were identified through a review of NSHBT documents that describe future strategies and policies, market research, an informal review of relevant published literature, consultation with NHSBT colleagues and insights from preliminary qualitative research undertaken with INTERVAL donors. Twelve initiatives were identified as being of potential interest for the evaluation. Of those, the strategies for evaluation were selected according to the following criteria:
-
The strategy can be defined as a distinct series of service changes, with attributable costs and consequences that can be estimated.
-
The strategy is anticipated to have an effect on important attributes of the donor experience.
-
Decisions by NHSBT on whether or not to adopt a particular strategy could be informed by evidence from the study. Any strategies partially or fully adopted by NHSBT during the timeframe of this study were excluded on the basis they were no longer relevant.
-
Timing will allow for using the results of the INTERVAL trial.
-
The strategy ensures that the survey has a manageable number of attributes.
| Potential strategy | Included in evaluation? | Reason for exclusion |
|---|---|---|
| Provision of health reports for all whole-blood donors | Yes | |
| Closure of all three- and six-bed sites for whole-blood collection | No | No longer relevant – almost all three- and six-bed mobile sites have been closed |
| Extension of opening times for both permanent and temporary sites collecting whole blood | Yes | |
| Increase in the maximum number of whole-blood donations per year, pending the results of the INTERVAL trial | Yes | |
| Reduction in the time taken for booked appointments to 1 hour (‘1 hour pledge’) | No | Not feasible to define the service changes required for NHSBT to meet this pledge. The importance of total donation time to donors is recognised and was included as an attribute in the pilot survey |
| Reimbursement of parking charges | No | No longer relevant – has already been introduced in some sites (both mobile sessions and static donor centres) |
| Extension of the provision of Wi-Fi | No | Alternative forms of mobile internet access are likely to become increasingly available and the cost of Wi-Fi provision is likely to decline |
| Refurbishment of donation venues | No | It was not possible to describe the scope and outcomes of these refurbishments for inclusion in either the decision model or the SP survey |
| Elimination of walk-in appointments | No | Evidence from the study would not be anticipated to inform a future decision |
| Provision of online check-in and completion of pre-donation questionnaire | No | No longer relevant as these strategies are already planned |
| Provision of a non-invasive Hb test | No | Unlikely to have a substantial impact on costs or donation frequency |
Appendix 2 Unit costs and assumptions (trial-based analysis)
| Resource-generating event | Unit cost (£) | Main source | Costing assumptions made, items included |
|---|---|---|---|
| ISAT three-stage reminder (first appointment, no appointment, last appointment) | 2.68 | ISAT recorded time, at NHS band 4 costs62 | |
| Low-Hb deferral | 5.98 | Expert opinion47 | Includes cost of copper sulphate test (NHSBT financial data 2016/17a), HemoCue® machine hire,65 consumables, and staff time at NHS band 4 costs62 |
| Health-care costs for low-Hb deferral | 5.39 | Expert opinionb | Includes downstream health-care costs for 7% of low-Hb deferrals who have a Hb of < 12.5 g/dl for men or < 11.5 g/dl for women. GP appointment (PSSRU),62 full blood count test,66 ferritin test,67 cost of iron supplements (expert opinion 50% compliance, standard adult dose),68 10% attend outpatient appointment69 |
| Deferral because of other reasons | 0.97 | Expert opinionb | Based on NHS band 4 costs62 |
| DNA three-stage reminders | 3.10 | Based on ISAT recorded time at NHS band 4 costs62 | |
| Faints on session | 20.23 | Expert opinion47 | Based on moderate symptoms at NHS band 4 costs62 |
| Variable cost of collecting 1 unit of blood (operating with capacity) | 7.62 | NHSBT | Based on disposables used [1 × copper sulphate test (£0.02) including 1 × ChloraPrep™ (Becton Dickinson and Company, Franklin Lakes, NJ, USA) (£0.74 including VAT), 1 × pack (£6.85 including VAT) (NHSBT financial data, 2016/17 prices)] |
Appendix 3 Definition of subgroups
| Subgroup | Definition |
|---|---|
| Male | Donors coded on the PULSE database as male |
| Female | Donors coded on the PULSE database as female |
| 17- to 30-years age group | Donors aged between 17 and 30 years inclusive at the time the PULSE data were extracted |
| 31- to 45-years age group | Donors aged between 31 and 45 years inclusive at the time the PULSE data were extracted |
| 46- to 60-years age group | Donors aged between 46 and 60 years inclusive at the time the PULSE data were extracted |
| > 61-years age group | Donors aged ≥ 61 years at the time the PULSE data were extracted |
| Standard demand blood types | Donors with blood types O–, A– and B– |
| High-demand blood types | Donors with blood types O+, A+, B+, AB+ and AB– |
| White ethnicity | Donors coded as any of the following on the PULSE database: English, Welsh, Scottish, Northern Irish, British, white Irish, other white background |
| Black/mixed black ethnicity | Donors coded as any of the following on the PULSE database: black Caribbean, black African, any other black African/Caribbean background, mixed white and black Caribbean, mixed white and black African |
| Asian/mixed Asian ethnicity | Donors coded as any of the following on the PULSE database: Asian-Indian, Asian-Pakistani, Asian-Bangladeshi, other Asian Background, Chinese, mixed white and Asian |
| Other ethnicity or not stated | Donors coded as any of the following on the PULSE database: any other mixed, multiple ethnic background, Gypsy or Irish Traveller, Arab, unknown, not disclosed, any other group |
| ‘Nursery’ donors | Donors who have given blood between one and four times in the past 5 years, at the time the PULSE data were extracted |
| Not ‘nursery’ donors | Donors who have given blood between five or more times in the past 5 years, at the time the PULSE data were extracted |
| Static donor centre donors (sometimes referred to as ‘Centre’ donors) | Donors who last gave blood at a static donor centre, at the time the PULSE data were extracted |
| Mobile session donors (sometimes referred to as ‘Mobile’ donors | Donors who last gave blood at a mobile session, at the time the PULSE data were extracted |
| INTERVAL arm: control | Ex-INTERVAL participants who were randomised to the control arm of the INTERVAL trial (12-week intervals for men; 16-week intervals for women) |
| INTERVAL arm: control | Ex-INTERVAL participants who were randomised to an intervention arm of the INTERVAL trial which involved donating at shorter intervals than the control arm (8- and 10-week intervals for men; 12- and 14-week intervals for women) |
| Recruitment source: centre | Ex-INTERVAL participants who were recruited to the INTERVAL trial through their donations at a static donor centre |
| Recruitment source: mobile | Ex-INTERVAL participants who were recruited to the INTERVAL trial through their donations at a mobile session |
| Recruitment source: no invite | Ex-INTERVAL participants who were not invited to participate but who volunteered to the INTERVAL trial |
| Number of donations in the last 12 months: no/one/two/three/four/five/six | Donors with no/one/two/three/four/five/six whole-blood donations recorded on the PULSE database in the last 12 months, at the time the PULSE data were extracted |
Appendix 4 Number (%) of responses to the Short Form questionnaire-36 items/Short Form questionnaire-12 items at each time point
Results are presented for the SF-36/SF-12 questionnaires with complete information to calculate the SF-6D score.
| Level | Sex, n (%) | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| 8 weeks (N = 7417) | 10 weeks (N = 7413) | 12 weeks (N = 7411) | 12 weeks (N = 7549) | 14 weeks (N = 7545) | 16 weeks (N = 7528) | |
| Baseline | 6766 (91) | 6765 (91) | 6778 (91) | 6776 (90) | 6724 (89) | 6746 (90) |
| 6 months | 5714 (77) | 5644 (76) | 5527 (75) | 5673 (75) | 5760 (76) | 5652 (75) |
| 12 months | 5197 (70) | 5183 (70) | 5162 (70) | 5148 (68) | 5210 (69) | 5133 (68) |
| 18 months | 4633 (62) | 4659 (63) | 4679 (63) | 4515 (60) | 4624 (61) | 4533 (60) |
| 24 months | 4701 (63) | 4747 (64) | 4746 (64) | 4506 (60) | 4486 (59) | 4444 (59) |
Appendix 5 Mean Short Form 6D score at each time point, by randomised arm and sex
| Level | Sex, n (%) | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| 8 weeks (N = 7417) | 10 weeks (N = 7413) | 12 weeks (N = 7411) | 12 weeks (N = 7549) | 14 weeks (N = 7545) | 16 weeks (N = 7528) | |
| Baseline | 0.86 | 0.86 | 0.86 | 0.85 | 0.85 | 0.85 |
| 6 months | 0.85 | 0.85 | 0.85 | 0.83 | 0.83 | 0.83 |
| 12 months | 0.85 | 0.85 | 0.85 | 0.82 | 0.82 | 0.82 |
| 18 months | 0.85 | 0.85 | 0.85 | 0.82 | 0.82 | 0.82 |
| 24 months | 0.84 | 0.84 | 0.84 | 0.82 | 0.82 | 0.82 |
Appendix 6 Summary of relevant donor experience attributes and associated levels from the pilot survey
| Relevant donor experience attribute | Attribute levels |
|---|---|
| 1. Invitation to donate at usual blood donation venue |
|
| 2. Donors’ travel time to blood donation venue |
|
| 3. Opening times at blood donation venue |
|
| 4. Total time to donate blood (from time of arrival at blood donation venue to time of departure) |
|
| 5. Availability of health report |
|
| 6. Maximum number of donations per year | Women
|
Men
|
Appendix 7 Example survey questions
Non-INTERVAL donors
Appendix 8 Summary of the responses to the background questions in the survey of non-INTERVAL and ex-INTERVAL donors
Question 1: how many times did you give blood in the last 12 months?
If you cannot remember, please give your best guess.
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| I did not give blood in the past 12 months | 96 (1) | 69 (1) | 123 (3) | 144 (3) |
| Once | 2728 (17) | 2283 (27) | 187 (4) | 300 (7) |
| Twice | 5064 (32) | 3179 (38) | 487 (10) | 816 (19) |
| Three times | 5852 (37) | 2470 (30) | 1221 (25) | 2171 (50) |
| Four times | 1860 (12) | 316 (4) | 2232 (45) | 864 (20) |
| More than four times (please specify) | 50 (0) | 8 (0) | 657 (13) | 32 (1) |
| Respondent specified (times) | ||||
| 3 | 1 (0) | – (–) | – (–) | – (–) |
| 4 | 1 (0) | – (–) | – (–) | – (–) |
| 5 | 28 (0) | 5 (0) | 473 (10) | 21 (0) |
| 6 | 9 (0) | 1 (0) | 153 (3) | 4 (0) |
| 7 | 3 (0) | – (–) | 11 (0) | 1 (0) |
| 8 | – (–) | – (–) | 5 (0) | 3 (0) |
| 9 | 1 (0) | – (–) | 2 (0) | 1 (0) |
| 10 | – (–) | – (–) | 2 (0) | 2 (0) |
| 11 | – (–) | – (–) | 2 (0) | – (–) |
| 12 | 1 (0) | – (–) | 9 (0) | – (–) |
| Total number of respondents | 15,650 | 8325 | 4907 | 4327 |
Question 2: how many times did you want to give blood in the last 12 months?
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| I did not give blood in the past 12 months | 73 (0) | 69 (1) | 42 (1) | 37 (1) |
| Once | 607 (4) | 590 (7) | 37 (1) | 53 (1) |
| Twice | 1949 (12) | 1725 (21) | 122 (3) | 273 (6) |
| Three times | 5315 (34) | 3546 (43) | 648 (13) | 1747 (40) |
| Four times | 6748 (43) | 2165 (26) | 2646 (54) | 1952 (45) |
| More than four times (please specify) | 958 (6) | 230 (3) | 1374 (28) | 254 (6) |
| Respondent specified (times) | ||||
| 0 | 1 (0) | – (–) | – (–) | – (–) |
| 3 | 1 (0) | – (–) | – (–) | – (–) |
| 4 | 2 (0) | – (–) | 1 (0) | – (–) |
| 5 | 176 (1) | 47 (1) | 570 (12) | 89 (2) |
| 6 | 453 (3) | 91 (1) | 614 (13) | 126 (3) |
| 7 | 13 (0) | 2 (0) | 38 (1) | 2 (0) |
| 8 | 25 (0) | 4 (0) | 44 (1) | 7 (0) |
| 9 | 2 (0) | 2 (0) | 4 (0) | – (–) |
| 10 | 14 (0) | 3 (0) | 14 (0) | 7 (0) |
| 11 | – (–) | – (–) | 1 (0) | – (–) |
| 12 | 135 (1) | 31 (0) | 62 (1) | 21 (0) |
| 13 | – (–) | – (–) | 2 (0) | – (–) |
| 14 | – (–) | – (–) | 1 (0) | – (–) |
| 16 | – (–) | 1 (0) | 1 (0) | – (–) |
| 17 | 1 (0) | – (–) | – (–) | – (–) |
| 18 | – (–) | – (–) | 1 (0) | – (–) |
| 20 | 2 (0) | – (–) | 3 (0) | – (–) |
| 24 | 4 (0) | – (–) | 1 (0) | – (–) |
| 26 | 3 (0) | – (–) | 1 (0) | – (–) |
| 30 | 1 (0) | – (–) | – (–) | – (–) |
| 50 | – (–) | 1 (0) | – (–) | 1 (0) |
| 52 | 5 (0) | – (–) | 3 (0) | – (–) |
| 55 | 1 (0) | – (–) | 1 (0) | – (–) |
| 100 | – (–) | 2 (0) | – (–) | – (–) |
| 365 | 20 (0) | 3 (0) | 12 (0) | 1 (0) |
| Total number of respondents | 15,650 | 8325 | 4869 | 4316 |
Question 3: when you last gave blood, where did you travel from?
If you cannot remember, please give your best guess.
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| Your home | 10,564 (68) | 5887 (71) | 3164 (65) | 2796 (65) |
| Your workplace | 4683 (30) | 2218 (27) | 1565 (32) | 1402 (33) |
| Somewhere else | 403 (3) | 220 (3) | 113 (2) | 89 (2) |
| Total number of respondents | 15,650 | 8325 | 4842 | 4287 |
Question 4: roughly how far did you travel to the place where you last gave blood?
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| < 2 miles | 8114 (52) | 4499 (54) | 1366 (28) | 1407 (33) |
| 2–4 miles | 4765 (30) | 2622 (31) | 1495 (31) | 1532 (36) |
| > 4 miles (please tell us to the nearest mile) | 2771 (18) | 1204 (14) | 1933 (40) | 1290 (31) |
| Respondent specified (miles) | ||||
| 5 | 405 (3) | 194 (2) | 204 (4) | 187 (4) |
| 6 | 492 (3) | 228 (3) | 287 (6) | 198 (5) |
| 7 | 249 (2) | 123 (1) | 170 (4) | 132 (3) |
| 8 | 285 (2) | 124 (1) | 171 (4) | 105 (2) |
| 9 | 116 (1) | 44 (1) | 74 (2) | 66 (2) |
| 10 | 247 (2) | 122 (1) | 227 (5) | 138 (3) |
| 11 | 44 (0) | 31 (0) | 47 (1) | 32 (1) |
| 12 | 141 (1) | 59 (1) | 128 (3) | 78 (2) |
| 13 | 31 (0) | 14 (0) | 34 (1) | 24 (1) |
| 14 | 25 (0) | 19 (0) | 36 (1) | 30 (1) |
| 15 | 105 (1) | 49 (1) | 104 (2) | 71 (2) |
| 16 | 34 (0) | 18 (0) | 33 (1) | 29 (1) |
| 17 | 35 (0) | 12 (0) | 26 (1) | 14 (0) |
| 18 | 31 (0) | 9 (0) | 37 (1) | 23 (1) |
| 19 | 13 (0) | 2 (0) | 10 (0) | 4 (0) |
| 20 | 70 (0) | 23 (0) | 84 (2) | 38 (1) |
| 21 | 7 (0) | 4 (0) | 8 (0) | 2 (0) |
| 22 | 18 (0) | 4 (0) | 14 (0) | 5 (0) |
| 23 | 11 (0) | 2 (0) | 11 (0) | 5 (0) |
| 24 | 12 (0) | 2 (0) | 5 (0) | 6 (0) |
| 25 | 58 (0) | 14 (0) | 47 (1) | 25 (1) |
| 26 | 5 (0) | 4 (0) | 11 (0) | 3 (0) |
| 27 | 7(0) | 1 (0) | 4 (0) | 3 (0) |
| 28 | 4 (0) | 1 (0) | 4 (0) | 3 (0) |
| 29 | – (–) | 1 (0) | 1 (0) | 2 (0) |
| 30 | 47 (0) | 14 (0) | 33 (1) | 12 (0) |
| 31 | 4 (0) | 1 (0) | 1 (0)0 | – (–) |
| 32 | 3 (0) | 3 (0) | 6 (0) | 1 (0) |
| 33 | 3 (0) | – (–) | 2 (0) | – (–) |
| 34 | 2 (0) | – (–) | 4 (0) | 4 (0) |
| 35 | 25 (0) | 2 (0) | 19 (0) | 8 (0) |
| 36 | 5 (0) | 1 (0) | 2 (0) | 6 (0) |
| 37 | 3 (0) | 1 (0) | 2 (0) | 2 (0) |
| 38 | 2 (0) | 3 (0) | 2 (0) | 0 (0) |
| 39 | – (–) | – (–) | 3 (0) | – (–) |
| 40 | 35 (0) | 7 (0) | 10 (0) | 11 (0) |
| 41 | 1 (0) | – (–) | 1 (0) | 1 (0) |
| 42 | – (–) | – (–) | 1 (0) | – (–) |
| 43 | 2 (0) | – (–) | 1 (0) | – (–) |
| 44 | – (–) | 1 (0) | – (–) | – (–) |
| 45 | 17 (0) | 3 (0) | 3 (0) | 1 (0) |
| 48 | 1 (0) | – (–) | 1 (0) | – (–) |
| 50 | 15 (0) | 1 (0) | 10 (0) | 4 (0) |
| 51 | 2 (0) | – (–) | 2 (0) | – (–) |
| 52 | 2 (0) | – (–) | 2 (0) | – (–) |
| 55 | 3 (0) | – (–) | 1 (0) | 1 (0) |
| 56 | 1 (0) | – (–) | 1 (0) | – (–) |
| 58 | 1 (0) | – (–) | 1 (0) | – (–) |
| 60 | 7 (0) | 2 (0) | 4 (0) | 4 (0) |
| 62 | – (–) | – (–) | 1 (0) | – (–) |
| 63 | 1 (0) | – (–) | – (–) | – (–) |
| 64 | – (–) | – (–) | 1 (0) | – (–) |
| 65 | 1 (0) | – (–) | 3 (0) | 1 (0) |
| 68 | – (–) | – (–) | – (–) | 1 (0) |
| 69 | – (–) | – (–) | 1 (0) | 0 (0) |
| 70 | 4 (0) | 1 (0) | 4 (0) | 1 (0) |
| 75 | 2 (0) | – (–) | 2 (0) | 0 (0) |
| 78 | – (–) | – (–) | – (–) | 1 (0) |
| 80 | 3 (0) | – (–) | 5 (0) | 0 (0) |
| 82 | – (–) | – (–) | – (–) | 1 (0) |
| 83 | 1 (0) | – (–) | 1 (0) | – (–) |
| 92 | 1 (0) | – (–) | – (–) | – (–) |
| 95 | – (–) | – (–) | 1 (0) | – (–) |
| 96 | – (–) | – (–) | 1 (0) | – (–) |
| 100 | 2 (0) | – (–) | 2 (0) | – (–) |
| 110 | 1 (0) | – (–) | 1 (0) | – (–) |
| 111 | – (–) | – (–) | 1 (0) | – (–) |
| 120 | 2 (0) | – (–) | – (–) | 1 (0) |
| 124 | 1 (0) | – (–) | – (–) | – (–) |
| 130 | – (–) | – (–) | – (–) | 1 (0) |
| 145 | – (–) | – (–) | – (–) | 1 (0) |
| 170 | 1 (0) | – (–) | 1 (0) | – (–) |
| 190 | – (–) | – (–) | – (–) | 1 (0) |
| 200 | 1 (0) | – (–) | 1 (0) | – (–) |
| 213 | 1 (0) | – (–) | – (–) | – (–) |
| 250 | 1 (0) | – (–) | – (–) | – (–) |
| 800 | – (–) | – (–) | – (–) | 1 (0) |
| 999 | – (–) | – (–) | 1 (0) | – (–) |
| Total number of respondents | 15,650 | 8325 | 4794 | 4229 |
Question 5: how did you travel to the place where you last gave blood?
Please choose the answer that applies to the longest part of your journey.
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| Walk | 3909 (25) | 1868 (22) | 866 (18) | 827 (19) |
| Cycle | 734 (5) | 182 (2) | 302 (6) | 166 (4) |
| Car | 10,132 (65) | 5629 (68) | 2362 (49) | 2013 (47) |
| Public transport (e.g. bus, tube, train or tram) | 875 (6) | 646 (8) | 1312 (27) | 1281 (30) |
| Total number of respondents | 15,650 | 8325 | 4842 | 4287 |
Question 6: how long did it take you to travel to the place where you last gave blood?
If you cannot remember, please give your best guess.
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| < 10 minutes | 7661 (49) | 4176 (50) | 892 (18) | 858 (20) |
| 10–30 minutes | 6803 (43) | 3607 (43) | 2511 (52) | 2223 (52) |
| 30–60 minutes | 1077 (7) | 508 (6) | 1272 (26) | 1089 (25) |
| > 60 minutes (please tell us how long in minutes) | 109 (1) | 34 (0) | 164 (3) | 112 (3) |
| Respondent specified (minutes) | ||||
| 10 | 1 (0) | – (–) | – (–) | – (–) |
| 12 | – (–) | – (–) | 1 (0) | – (–) |
| 50 | – (–) | – (–) | 1 (0) | – (–) |
| 61 | – (–) | 1 (0) | 1 (0) | – (–) |
| 62 | – (–) | – (–) | 1 (0) | – (–) |
| 65 | 3 (0) | 2 (0) | 12 (0) | 7 (0) |
| 67 | – (–) | 1 (0) | 1 (0) | – (–) |
| 70 | 7 (0) | 6 (0) | 21 (0) | 16 (0) |
| 74 | – (–) | 1 (0) | – (–) | – (–) |
| 75 | 13 (0) | 5 (0) | 32 (1) | 24 (1) |
| 76 | – (–) | – (–) | 2 (0) | – (–) |
| 80 | 10 (0) | 5 (0) | 16 (0) | 14 (0) |
| 85 | 1 (0) | – (–) | 3 (0) | – (–) |
| 90 | 41 (0) | 9 (0) | 40 (1) | 36 (1) |
| 95 | 2 (0) | – (–) | 1 (0) | 1 (0) |
| 100 | 5 (0) | 2 (0) | 7 (0) | – (–) |
| 105 | 3 (0) | – (–) | 1 (0) | – (–) |
| 110 | 1 (0) | – (–) | 2 (0) | 2 (0) |
| 120 | 8 (0) | 1 (0) | 13 (0) | 5 (0) |
| 135 | – (–) | – (–) | 1 (0) | 1 (0) |
| 140 | 1 (0) | – (–) | 1 (0) | 1 (0) |
| 150 | 2 (0) | – (–) | 3 (0) | 1 (0) |
| 160 | – (–) | – (–) | – (–) | 1 (0) |
| 170 | 1 (0) | – (–) | 1 (0) | 1 (0) |
| 180 | 1 (0) | 1 (0) | – (–) | – (–) |
| 200 | 0 (0) | – (–) | 2 (0) | 1 (0) |
| 240 | 2 (0) | – (–) | – (–) | – (–) |
| 450 | – (–) | – (–) | 1 (0) | – (–) |
| 600 | – (–) | – (–) | – (–) | 1 (0) |
| Total number of respondents | 15,650 | 8325 | 4839 | 4282 |
Question 7: roughly how long did your last blood donation visit take?
From your scheduled appointment time to the time you arrived at the tea table and were free to leave (including waiting time).
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| ≤ 1 hour | 11,673 (75) | 5947 (38) | 4228 (87) | 3659 (85) |
| > 1 hour | 3977 (25) | 2378 (15) | 614 (13) | 628 (15) |
| Total number of respondents | 15,650 | 8325 | 4842 | 4287 |
Question 8: what prompted you to make your last appointment to give blood?
Please choose the answer that best applies to you.
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| I booked at a previous blood donation visit | 8424 (54) | 4308 (52) | 3220 (67) | 2711 (64) |
| I received an invitation letter | 1470 (9) | 814 (10) | 416 (9) | 531 (13) |
| I received an e-mail, phone call or text message | 1395 (9) | 712 (9) | 369 (8) | 337 (8) |
| I saw an advert, publicity or campaign | 219 (1) | 266 (3) | 3 (0) | 8 (0) |
| I booked without being prompted | 3408 (22) | 1870 (22) | 644 (13) | 528 (13) |
| I did not make an appointment | 250 (2) | 97 (1) | 54 (1) | 31 (1) |
| Other | 484 (3) | 258 (3) | 77 (2) | 74 (2) |
| Total number of respondents | 15,650 | 8325 | 4783 | 4220 |
Question 9: are you able to make appointments to give blood as often as you would like, at a day and time that suits you?
| Response | Sex, n (%) | |||
|---|---|---|---|---|
| Non-INTERVAL donors | Ex-INTERVAL participants | |||
| Male | Female | Male | Female | |
| Yes, easily | 9154 (58) | 4806 (58) | 3788 (73) | 3193 (70) |
| Yes, but with some difficulty or delay | 4047 (26) | 2293 (28) | 686 (13) | 734 (16) |
| No, it is very difficult | 2449 (16) | 1226 (15) | 309 (6) | 293 (6) |
| Total number of respondents | 15,650 | 8325 | 4783 | 4220 |
Appendix 9 Incremental effect of alternative types of blood service on average number of donations per year for all non-INTERVAL respondents (two-part model)
FIGURE 23.
Incremental effect of alternative types of blood service on average number of donations per year for all non-INTERVAL respondents (two-part model). For men (• n = 15,652) and women (▴ n = 8329). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
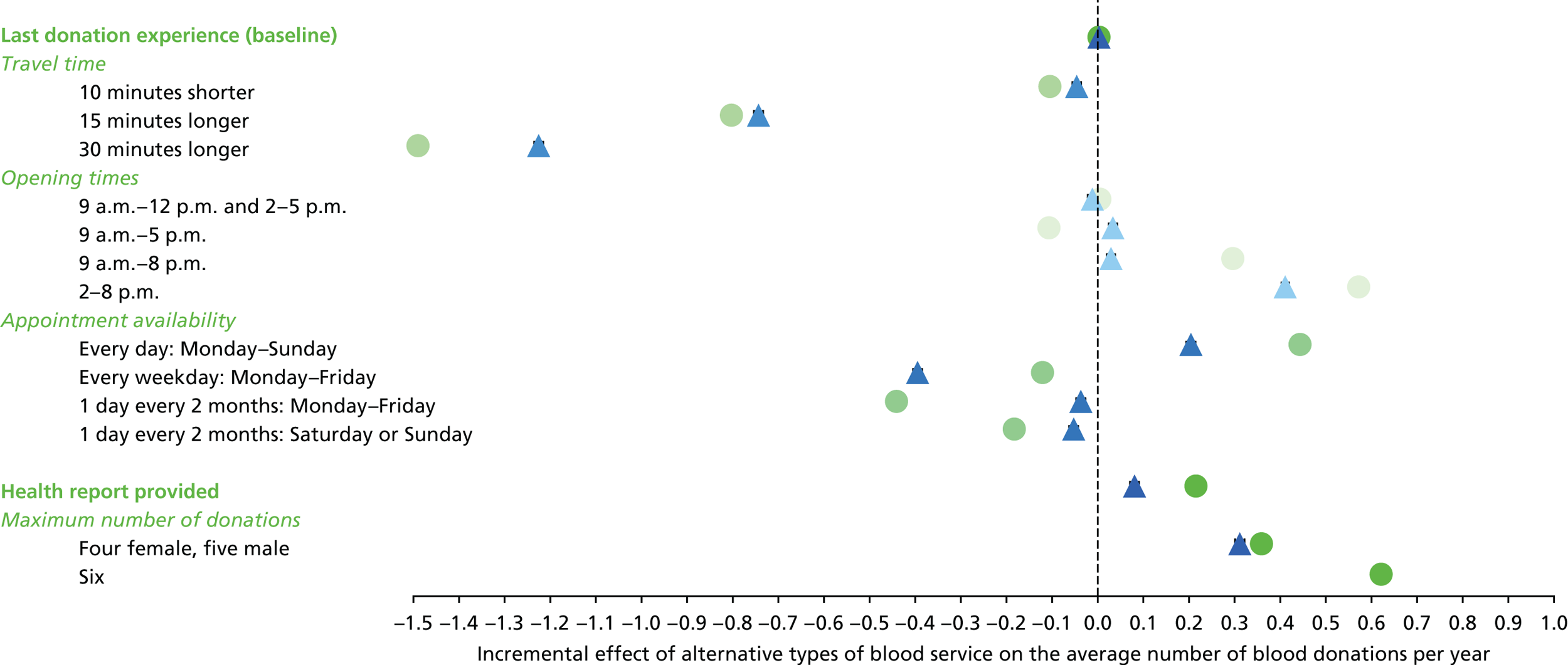
Appendix 10 Incremental effect of alternative types of blood service on average number of donations per year for high-demand non-INTERVAL respondents (two-part model)
FIGURE 24.
Incremental effect of alternative types of blood service on average number of donations per year for high-demand non-INTERVAL respondents (two-part model). For men (• n = 1551) and women (▴ n = 921). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.

Appendix 11 Incremental effect of alternative types of blood service on average number of donations per year for all non-INTERVAL respondents (gamma model)
FIGURE 25.
Incremental effect of alternative types of blood service on average number of donations per year for all non-INTERVAL respondents (gamma model). For men (• n = 15,652) and women (▴ n = 8329). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
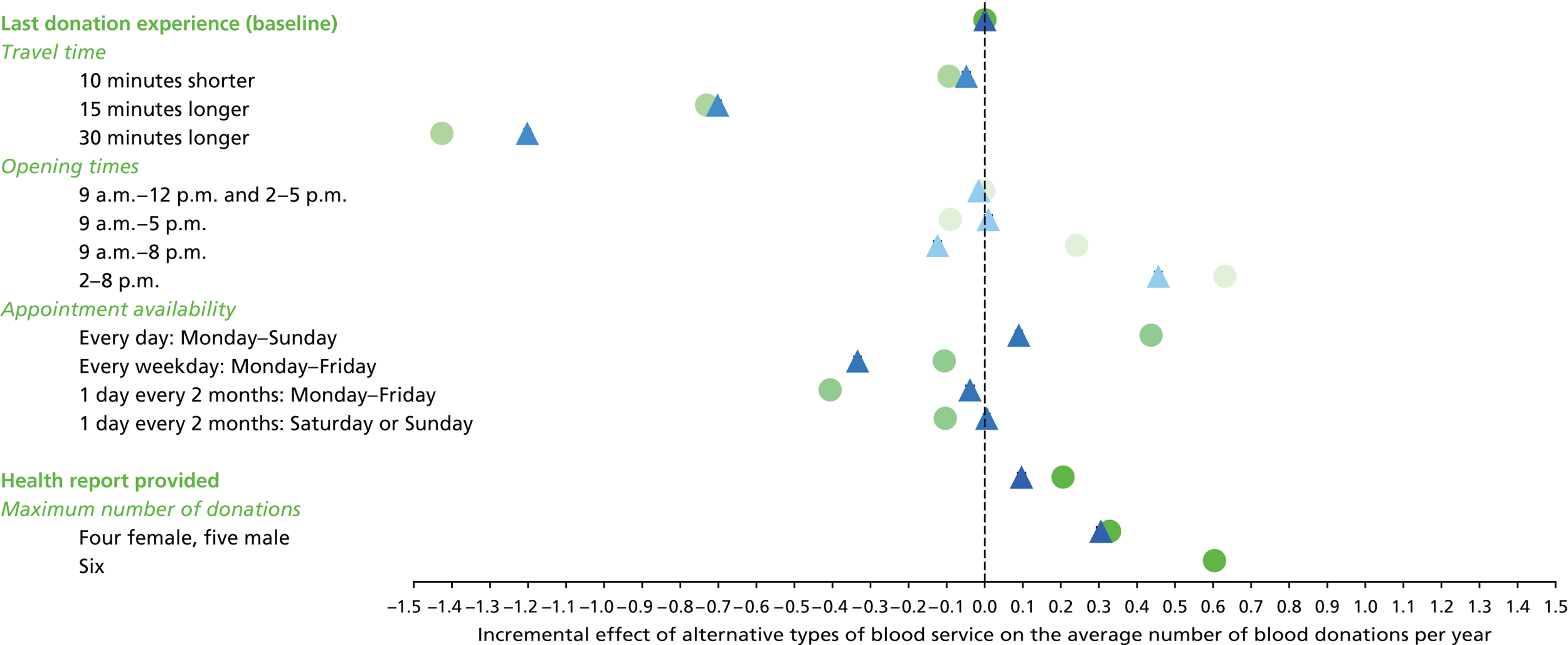
Appendix 12 Incremental effect of alternative types of blood service on average number of donations per year for high-demand non-INTERVAL respondents (gamma model)
FIGURE 26.
Incremental effect of alternative types of blood service on average number of donations per year for high-demand non-INTERVAL respondents (gamma model). For men (• n = 1551) and women (▴ n = 921). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
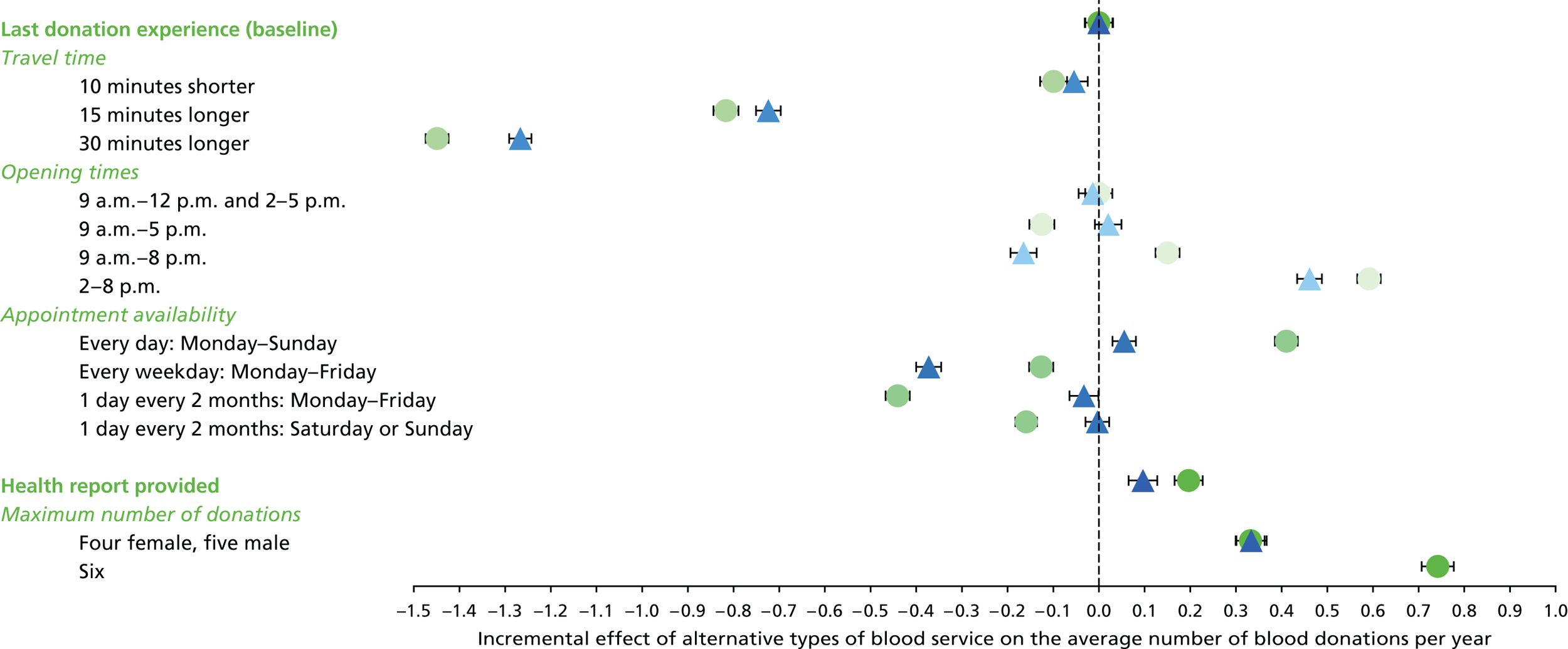
Appendix 13 Incremental effect of alternative types of blood service on average number of donations per year for all ex-INTERVAL respondents (two-part model)
FIGURE 27.
Incremental effect of alternative types of blood service on average number of donations per year for all ex-INTERVAL respondents (two-part model). For men (• n = 4754) and women (▴ n = 4179). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
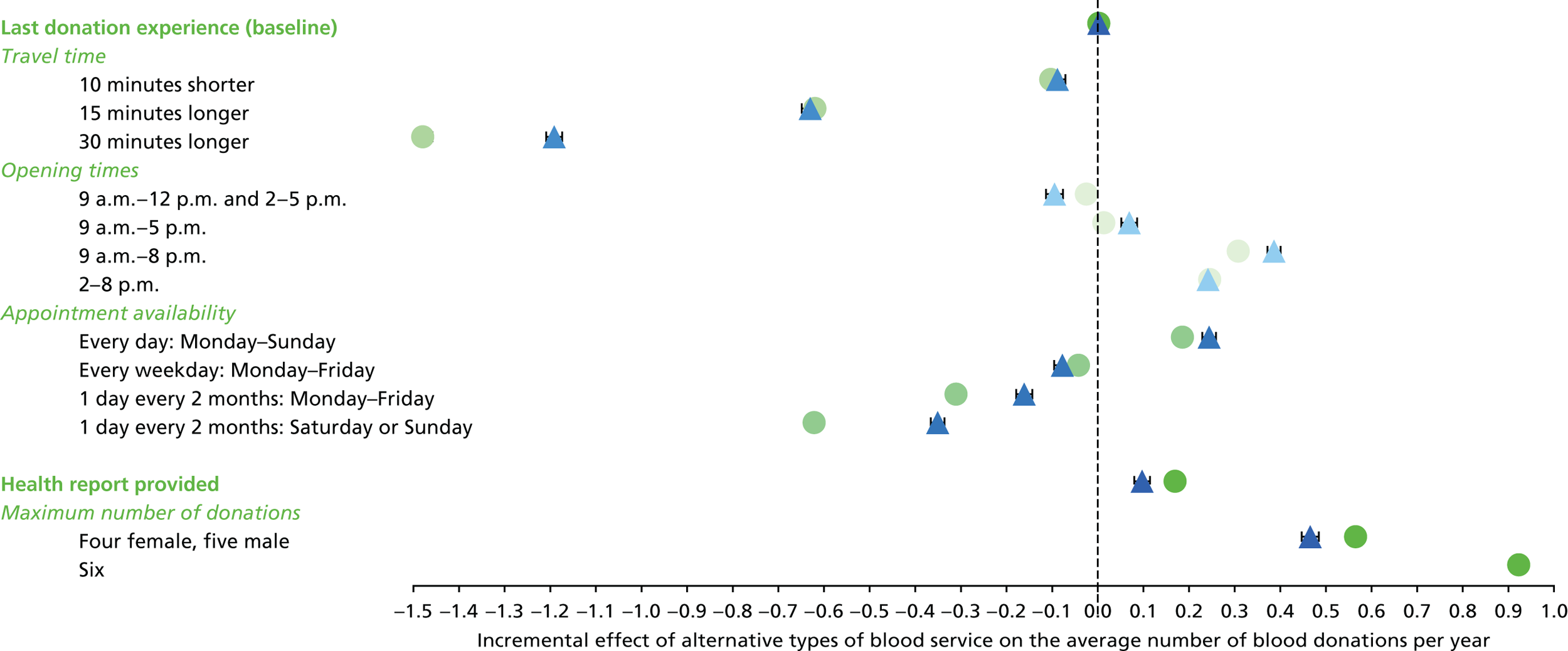
Appendix 14 Incremental effect of alternative types of blood service on average number of donations per year for high-demand ex-INTERVAL respondents (two-part model)
FIGURE 28.
Incremental effect of alternative types of blood service on average number of donations per year for high-demand ex-INTERVAL respondents (two-part model). For men (• n = 643) and women (▴ n = 580).
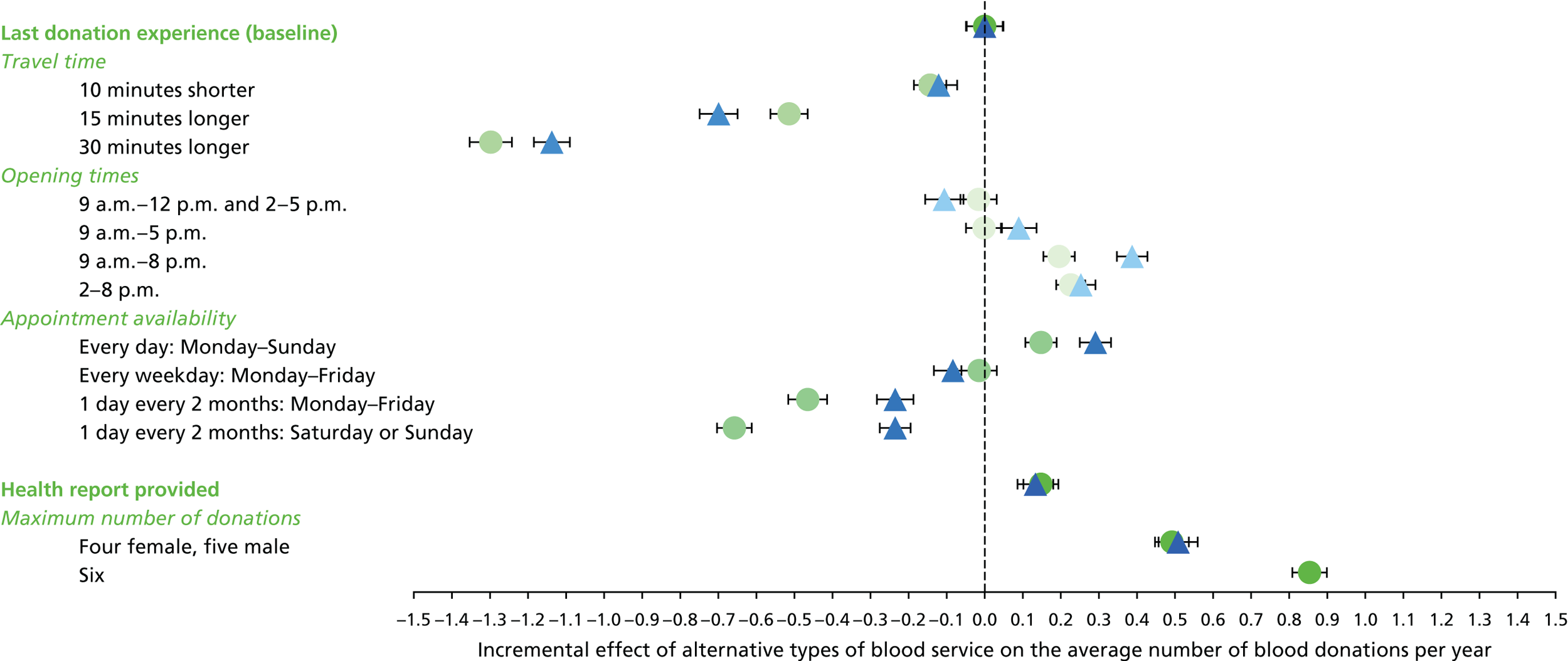
Appendix 15 Incremental effect of alternative types of blood service on average number of donations per year for all ex-INTERVAL respondents (gamma model)
FIGURE 29.
Incremental effect of alternative types of blood service on average number of donations per year for all ex-INTERVAL respondents (gamma model). For men (• n = 4754) and women (▴ n = 4179).
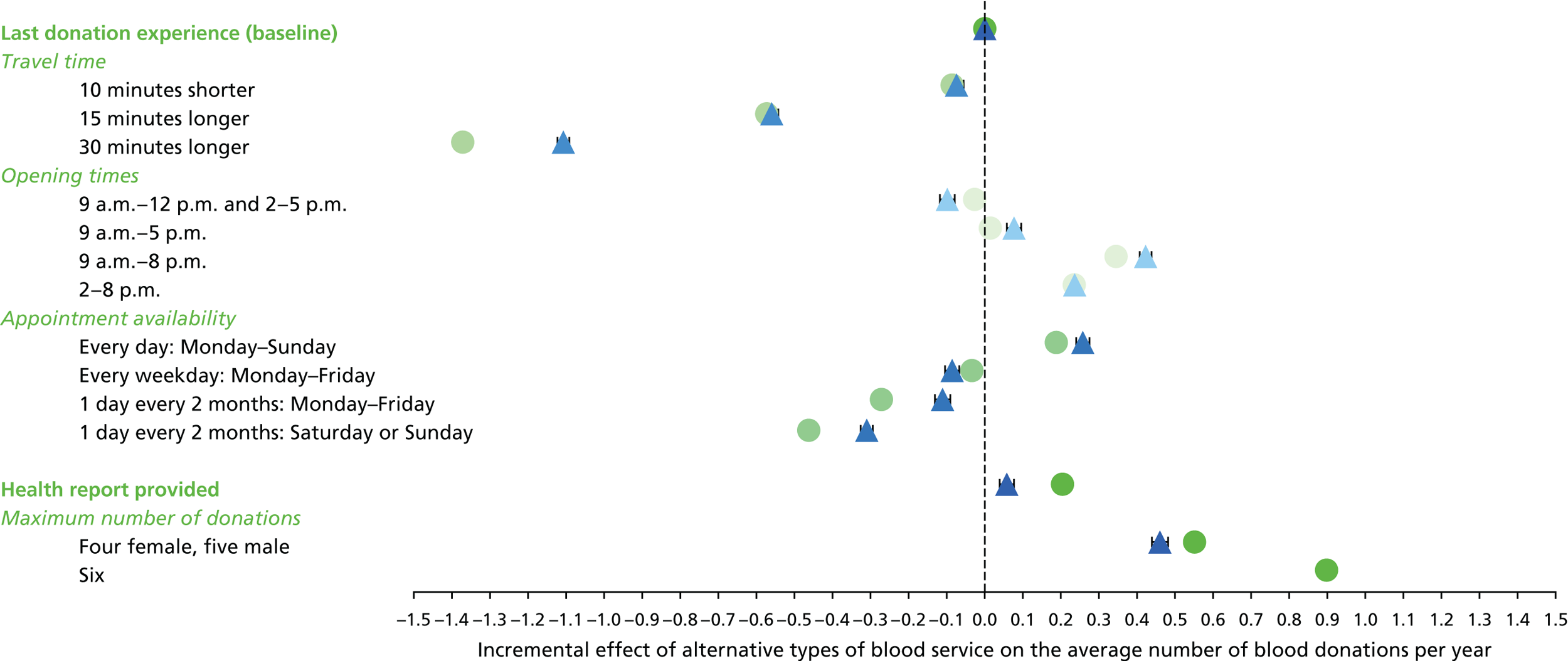
Appendix 16 Incremental effect of alternative types of blood service on average number of donations per year for high-demand ex-INTERVAL respondents (gamma model)
FIGURE 30.
Incremental effect of alternative types of blood service on average number of donations per year for high-demand ex-INTERVAL respondents (gamma model). For men (• n = 643) and women (▴ n = 580). The uncertainty intervals reflect the 5th and 95th percentiles in the predicted donation frequency across the relevant target population.
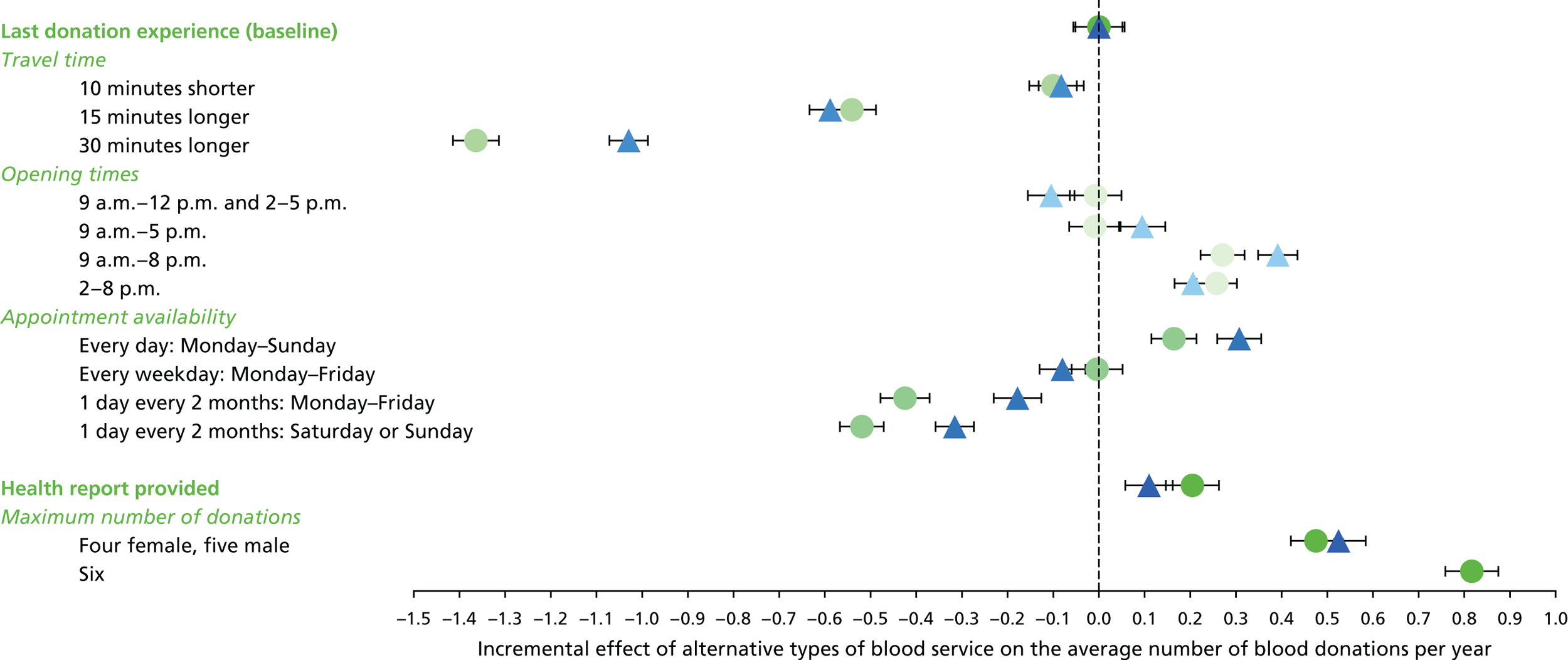
Appendix 17 Summary notes from translation workshops
Translation workshop I: key themes from the discussion
Monday 21 November 2016, 11.15–15.15.
At LSHTM, London.
Attended by seven donors (all of whom were non-INTERVAL donors) and a public representative. Attendees were split into three groups for the key discussions, joined by colleagues from NHSBT, and each group was facilitated by a member of the research team (RG, SW and KDC).
This is a summary of the general feedback and discussion that followed each break out session at the workshop, focusing on the main themes.
Discussion I: the strategies
The questions asked:
-
How do you decide how often to give blood?
-
Which of the six possible changes (reducing intervals, static donor centres on weekday evenings, static donor centres at weekends, mobile sessions on weekday evenings, mobile sessions at weekends and provision of a health report) would be important to you?
-
How do you think other donors would react to these changes? Can you rank our suggestions from best to worst?
-
What else might encourage donors to give blood more often?
Summary of responses:
-
Some donors rephrased the question to ‘would donors plan to donate less than they are able? What would affect this?’
-
Response from two groups that appointment availability is more limiting than the strategies listed (Donor: ‘How often I give blood is not decided by me’). Noted that this will differ between static donor centres and mobile sessions.
-
Appointment reminders were also mentioned by two groups as having a big influence.
-
Weekday evenings opening supported by two groups.
-
A reflection from one group that reducing intervals would offer more choice to donors, but only if it will affect appointment availability.
-
Health report – a mixed response. One group positive/could be seen as a health promotion activity (but felt it should be optional – select beforehand on the appointment booking), one ambivalent and one felt it would deter people from donating.
Other comments and ideas mentioned:
-
Suggested marketing/operating the blood service in shopping centres, etc.
-
Could people donate more than 1 unit at a time?
-
Make donation venues nicer places to be.
-
Provide better, more dynamic, information (e.g. in an app) on ‘your nearest site with appointment availability’ – would work for London-based donors in particular.
-
Discussion about what an appointment means to a donor. Related to waiting time as the idea of being seen within 10 minutes of your booked time slot was thought important.
Discussion II: the survey and the discrepancy between observed and predicted number of donations
The questions asked:
-
What do you think about the way the questions are asked? Are there features missing?
-
Do you feel confident answering a survey question?
-
Why is there a difference between predicted and observed number of donations?
Summary of responses:
-
Appointment availability was mentioned again as an important and limiting factor to the number of donations. For example, if the donor has to cancel an appointment, another one will not be available and so they will have a long interval between donations. Especially so at a mobile session (could they be offered an alternative – static – venue at the time of cancellation?). Discussed strategies such as allowing donors to book ‘back-up’ appointments that would then ‘auto cancel’ if the first appointment was utilised.
-
Felt there were important interactions between appointment availability, location, and maximum number of donations allowed.
-
Optimism about how healthy/free you may be.
-
‘Waiting time’ on the day also important but people have different views – some would rather travel further/wait for less time, others would rather wait but donate locally.
Some questions for future exploration were raised:
-
Could a future survey provide live feedback inside the survey to the donor about the difference between their observed data and their survey responses? This might nudge them to be more realistic and also provide an opportunity to ask them what is limiting their observed pattern of donations.
-
Ask people about the probability of their needing to cancel a session.
-
Some regular donors think more about ‘donation intervals’ rather than ‘number of donations per year’.
-
Feedback that the survey may have forced unnatural choices – open questions would provide more nuance – but accept they do not really work for this type of survey.
-
Distinction between mobile sessions and static centres may not be clear; some donors did not know static centres existed.
-
Suggested question for future surveys: would you travel to your nearest static centre if your local appointment is cancelled?
Discussion III: exploring a more personalised donation service
The questions asked:
-
What are your views about tailoring the donation service more according to the particular donor’s circumstances and donation history?
-
What do you see as the ‘pros’ and ‘cons’?
-
What further research would be useful?
Summary of responses:
-
One group felt that making the donation service personal to the donor was acceptable if it is based on patient need (i.e. for certain blood types) but less acceptable if it is based on donor behaviours. Basing it on patient need would be understandable and motivating to donors if it was very clearly communicated.
-
Use personalised ‘feedback’ to individual donors to help people understand their own behaviours/intentions and limitations better → potentially more accurate survey responses and more reflection from donors – especially motivating if their blood is understood to be in particular demand.
-
A comment that personalising by subgroup is actually only a step towards personalisation.
-
Personalisation of communications would be helpful – for example, regular, committed donors do not always feel the marketing is suitable for them.
-
Have opportunistic appointments available for some donors?
-
Could not understand the reasons for the sex difference in the impact of travel time on donations.
-
Some uneasiness if the health report was only available for some people.
Other comments/ideas for future exploration raised:
-
Survey people on an iPad/their own device while they are waiting for their appointment at the centre/session? Ask them about what matters to them/what influences their own donation patterns.
-
Share live waiting times on the website/app.
-
Encourage those with a high-demand blood type to encourage their family members to donate (NHSBT confirmed that they already do this).
-
Noted that deferrals do put people off.
-
Suggested future question: do you know if your blood type is high demand?
Translation workshop II: key themes from the discussion
Monday 24 April 2017, 5.30 p.m.–8.30 p.m.
At LSHTM, London.
Attended by 13 donors (a mix of ex-INTERVAL participants and non-INTERVAL donors, nursery and more experienced donors). Attendees were split into three groups for the three discussions, joined by colleagues from NHSBT and each group facilitated by a member of the research team (RG, SW and KDC).
This is a summary of the general feedback and discussion that followed each break out session at the workshop, focusing on the main themes.
Discussion I: donor preferences
The results of the SP survey for the overall group and the high-demand subgroup were shared with donors. The questions we asked were:
-
What in these results surprises you?
-
What in these results is as you expected?
Summary of responses:
-
If a health report was to be implemented, additional support to donors would be required, specifically, to help communicate and interpret the results of any such report.
-
Evening opening was welcomed enthusiastically (although acknowledging that this group of donors had chosen to attend a weekday evening workshop), weekend opening seen as less so.
-
However, noted that there might be logistical issues – can NHSBT process blood late in the evening and in what volumes/locations?
-
Increasing travel time, which might happen if a donor can no longer donate locally but needs to go to a static donor centre, was clearly noted as an issue for donors.
-
One donor who had completed the survey said that they made an assumption, when the travel time was set to 10 minutes shorter, that other aspects of the service might be less convenient to them than their current, very convenient, blood service. It was also acknowledged that donors like to go where they ‘know how things work’.
-
Poor appointment availability can put donors off.
-
Some ex-INTERVAL participants who had donated at reduced intervals during the trial expressed frustration at having to return to longer intervals after the trial finished.
-
Several donors said they always book at the maximum number of donations allowed – or enabled – by the appointment availability of their mobile session (where applicable). Some donors book their next appointment as they leave the last one.
-
One group suggested that if a donor has ‘made the commitment’ to donate then they would be unlikely to be affected by the practical issues considered by the survey.
-
Acknowledged that a range of individual circumstances would affect donors’ preferences.
-
Donors welcomed the information about different blood types being in different demand, and understanding that NHSBT needed to strive to not overcollect blood. Felt that there are opportunities to educate more donors about these things and others (e.g. the tests that NHSBT carries out on blood collected, at the ‘tea table’ after a donation).
Other comments and ideas mentioned:
-
It was asked whether or not the INTERVAL trial had led to overcollection; this was not considered likely, given the relative number of donors who participated in the trial.
-
Observed that the survey does not tell us anything about the attributes of a blood donation service to attract new donors.
-
Consider a wider range of venues for mobile sessions that might attract particular subgroups of donors. An example given was a Buddhist temple – although it was recognised that this might not be economically viable.
-
Some donors had received text messages after their donation to say at which hospital their blood had been used. This was welcomed by donors, but some unease about the implication if they do not receive a text that their blood has not been used (especially if two family members donate together and only one receives the text).
-
Donors enjoy being able to walk in to appointments.
-
Some comments about the donation restrictions, for example following certain foreign travel.
Discussion II: making choices
The results of the CEA for the overall group and the high-demand subgroup were shared with donors. The questions asked were:
-
What in these results surprises you?
-
What in these results is as you expected?
-
What choice would you make (if you were NHSBT)?
Summary of responses:
-
Reducing intervals, if safe to do so, was considered the strongest choice if more blood is needed. Accepted that there would be operational issues for NHSBT to work through.
-
Weekday evenings. It was surprising that extending collection opening times into the evening was not more popular.
-
Targeting specific donor subgroups, based on patient need, was suggested, and a number of approaches to this were raised in different groups (e.g. targeted publicity, proactively asking people to donate via their other interactions with health or other public services). It was noted that it would be interesting to consider how subgroups behave in relation to the service attributes over long periods of time.
-
Suggested employing some of the strategies at different times in response to stocks; if more/less blood, or blood of a particular type, is needed temporarily, then particular strategies could be turned on or off in sequence.
-
The cost of service changes to increase donation frequency should be compared with the cost of attracting new donors (it was expected that service changes would be more costly) – although attrition and retention would also need to be considered. Noted that some of the strategies being discussed might well attract new donors.
-
Interest in seeing results for more subgroups, for example nursery donors, different ethnicities.
-
Could the choice NHSBT makes be to do nothing? Considered that this might be the most cost-effective choice, but need to consider what will happen to the blood supply in the future.
Other comments and ideas mentioned:
-
Suggested asking ‘how can we reduce the constraints on the donor?’, for example better digital services.
Discussion III: potential future work
The question asked:
-
From today, what would your key piece of advice be for NHSBT?
Summary of responses:
-
Reduce intervals, if safe to do so.
-
Look creatively at recruiting new donors (social media, university recruitment, etc.) and encouraging them to form the donation ‘habit’; for example, tell donors more about the importance of their blood at their first donation.
-
Related to this – it was felt that there are opportunities to help donors understand more about the donation process, the testing that is done to donated blood and the reasons that donors may sometimes be suspended. More knowledge would help donors stay more engaged.
The question asked:
-
What questions could be asked by potential future research?
Summary of responses:
-
Interested for research to consider travel time in more depth, including understanding how donors’ responses to that attribute were influenced by their current travel time and donation experience, and the interaction between travel time and switching venues.
-
Talk to lapsed donors. Find out more about why they lapsed and what constrains them. Acknowledged that this is a hard-to-reach group. Related to this – when does someone consider themselves a ‘donor’? How recently do they have to have donated? How can ‘nursery’ donors be converted to ‘experienced’ donors?
Appendix 18 Costing assumptions (cost-effectiveness analysis)
| Strategy | Cost (£) | Assumptions and sources |
|---|---|---|
| Health report (at a mobile session) | 4.82 | Donor–carer time to measure blood pressure 1.5 minutes, costed according to NHS band 4.62 Multiplier of 1.875 applied to mobile staff to reflect time spent travelling from the mobile base to mobile venue. Blood pressure monitor per use £0.006.63 Cholesterol test cost £4.20.66 Clinical follow-up £0.01 (2% donors assumed require clinical follow-up, a mailed letter at £0.55) |
| Health report (at a static donor centre) | 5.34 | |
| Static donor centre: session held on a weekend | 19.39 | Weighted average of additional staff costs to shift opening hours to a Saturday or a Sunday.61 Assumed 8.5-hour session, 9.35-hour shift according to standard staffing levels for an average nine-bed donor centre where 3 units of blood are collected per hour per bed. NHS band 4, £24 per working hour; band 5, £29.00; band 6, £36.4662 |
| Static donor centre: session held on a weekend until 8 p.m. | 13.07 | Staff costs to run an additional session from 9 a.m.–8 p.m. Assumed 12-hour session, 13.1-hour shift to include 1 hour, which attracts the unsocial hours payments after 8 p.m. Staffing levels according to an average nine-bed donor centre where 3 units of blood are collected per hour per bed |
| Cost to shift mobile session from weekday to a weekend | 9.35 | Weighted average of additional staff costs to shift opening hours to a Saturday or a Sunday.61 Assumed 6-hour session, 9.35-hour shift according to standard staffing levels for a nine-bed session where 3 units of blood are collected per hour per bed |
| Cost to shift mobile session from a weekday daytime to weekday 2–8 p.m. | 1.43 | Additional staff costs to shift opening hours to include 2 hours which attract the unsocial hours payments after 8 p.m.61 NHSBT data on standard staffing levels for a nine-bed session where 3 units of blood are collected per hour per bed |
| Cost of a deferral because of low levels of Hb | 9.21 | Expert opinion (7 minutes of donor–carer time). Includes cost of copper sulphate test (NHSBT prices 2016/17), HemoCue® machine hire, consumables and staff time at NHS band 4 costs.62,65 Includes downstream health-care costs for 7% of low-Hb deferrals who have a Hb of < 12.5 g/dl for men or < 11.5 g/dl for women (baseline data from INTERVAL trial); GP appointment, full blood count test, Ferritin test, iron supplements (expert opinion, 50% compliance), 10% assumed to attend outpatient appointment62,66–69 |
| Cost of a deferral for other reasons (not low levels of Hb) | 0.97 | Expert opinion (2 minutes of donor–carer time). NHS band 4 costs62 |
| Variable cost per unit (at a mobile session) | 8.59 | Includes cost of invitations (2.85 invites per unit of blood collected at 34p each) and the cost of disposables used (1 × copper sulphate test, 1 × preoperative skin preparation, 1 × pack per successful donation = £7.62) |
| Variable cost per unit (at a static donor centre) | 9.41 | Includes cost of invitations (5.3 invites per unit of blood collected at 34p each) and the cost of disposables used as above |
| Variable cost per unit including staff costs (at a mobile session) | 35.61 | Average variable cost per unit of blood collected by mobile team, according to NHSBT financial data 2015/16. Includes cost of travel and venue hire |
| Variable cost per unit including staff costs (at a static donor centre) | 26.49 | Used in sensitivity analysis only. Based on the West End donor centre staff costs, according to NHSBT financial data 2015/16. Assumes a zero venue cost |
Appendix 19 Calculating incremental costs
where:
nD = total number of donors affected by service change,
nUnitsCurrent = number of units of blood collected under status quo,
nUnitsNew = number of units of blood collected under new strategy,
nDeferralOtherCurrent = number of deferrals collected under status quo,
nDeferralOtherNew = number of deferrals collected under new strategy,
nLowHbDeferralCurrent = number of deferrals collected under status quo,
nLowHbDeferralNew = number of deferrals collected under new strategy,
A = variable cost health report,
B = variable cost of collecting blood,
C = variable staff costs at unsocial hours payment rate,
D = variable cost of deferrals because of low levels of Hb, and
E = variable cost of deferrals because of other reasons.
| Search | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval at static donor centres | |
| nD (n) | 781,028 | 60,640 | 99,312 | 646,898 | 582,910 | 107,811 |
| nUnitsCurrent (n) | 1,779,986 | 139,037 | 245,086 | 1,449,946 | 1,279,563 | 268,715 |
| nUnitsNew (n) | 1,866,917 | 170,386 | 290,358 | 1,495,514 | 1,561,653 | 341,860 |
| nLowHbDeferralsCurrent (n) | 80,043 | 6419 | 11,287 | 64,810 | 57,178 | 12,459 |
| nLowHbDeferralsNew (n) | 83,947 | 7890 | 13,434 | 66,940 | 69,641 | 15,859 |
| nDeferralOtherCurrent (n) | 116,864 | 9112 | 16,043 | 95,332 | 83,943 | 17,596 |
| nDeferralOtherNew (n) | 122,628 | 11,158 | 18,938 | 98,325 | 102,532 | 22,288 |
| A (£) | 4.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| B (base case) (£) | 31.68 | 9.41 | 9.41 | 35.61 | 35.61 | 9.41 |
| B (sensitivity analysis) (£) | 34.24 | 9.41 | 9.41 | 35.61 | 35.61 | 26.49 |
| C (£) | 0.00 | 19.39 | 13.07 | 9.35 | 1.43 | 0.00 |
| D (£) | 9.21 | 9.21 | 9.21 | 9.21 | 9.21 | 9.21 |
Base-case results
| Search | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval at static donor centres | |
| IncrCostBlood (£) | 2,753,974.00 | 294,988.00 | 426,007.00 | 1,622,656.00 | 10,045,248.00 | 688,300.00 |
| IncrCostDeferrals (£) | 41,544.00 | 15,53.007 | 22,583.00 | 22,527.00 | 132,817.00 | 35,869.00 |
| IncrStrategyCost (£) | 9,144,160.00 | 607,844.00 | 591,701.00 | 426,055.00 | 403,390.00 | 0.00 |
| Incremental cost per donor (£) | 15.29 | 15.14 | 10.47 | 3.20 | 18.15 | 6.72 |
Appendix 20 Cost-effectiveness acceptability curves comparing each strategy with the relevant status quo comparator
FIGURE 31.
Cost-effectiveness acceptability curves for each strategy vs. status quo, for all donors.
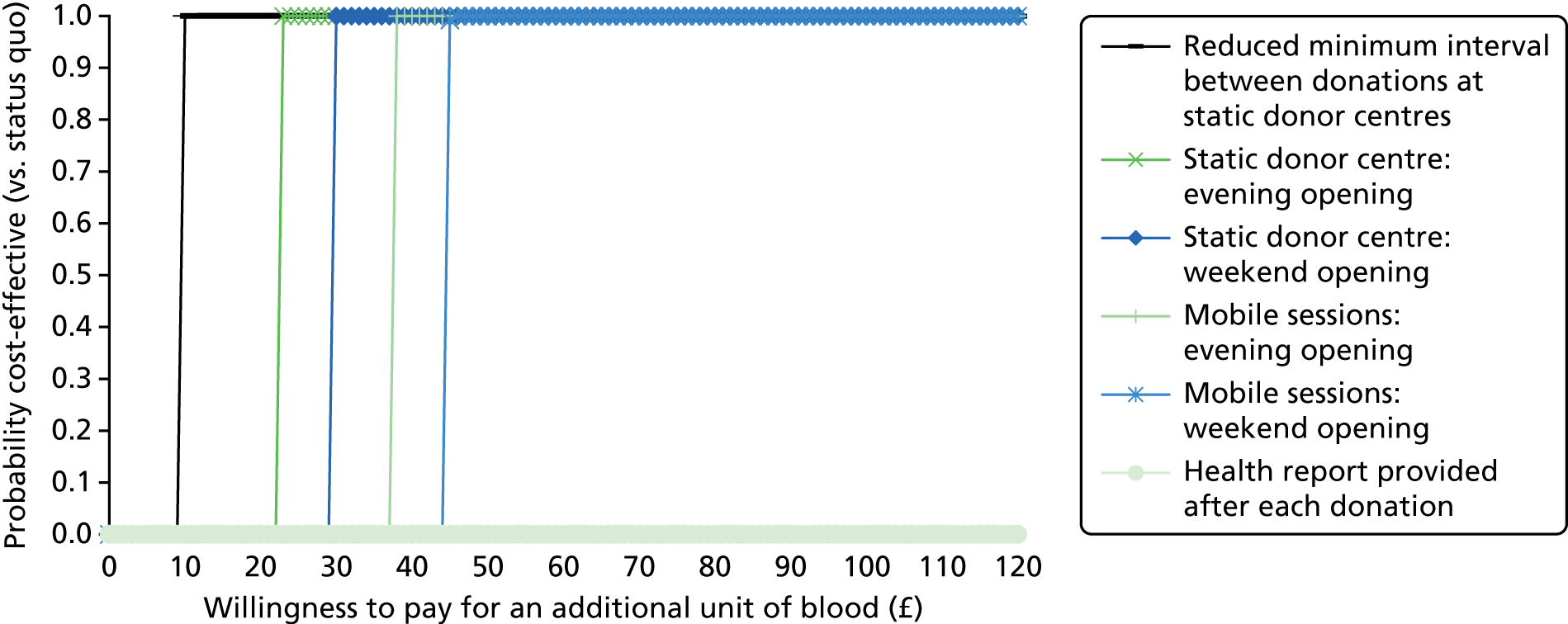
FIGURE 32.
Cost-effectiveness acceptability curves for each strategy vs. status quo, for black and mixed black donors.
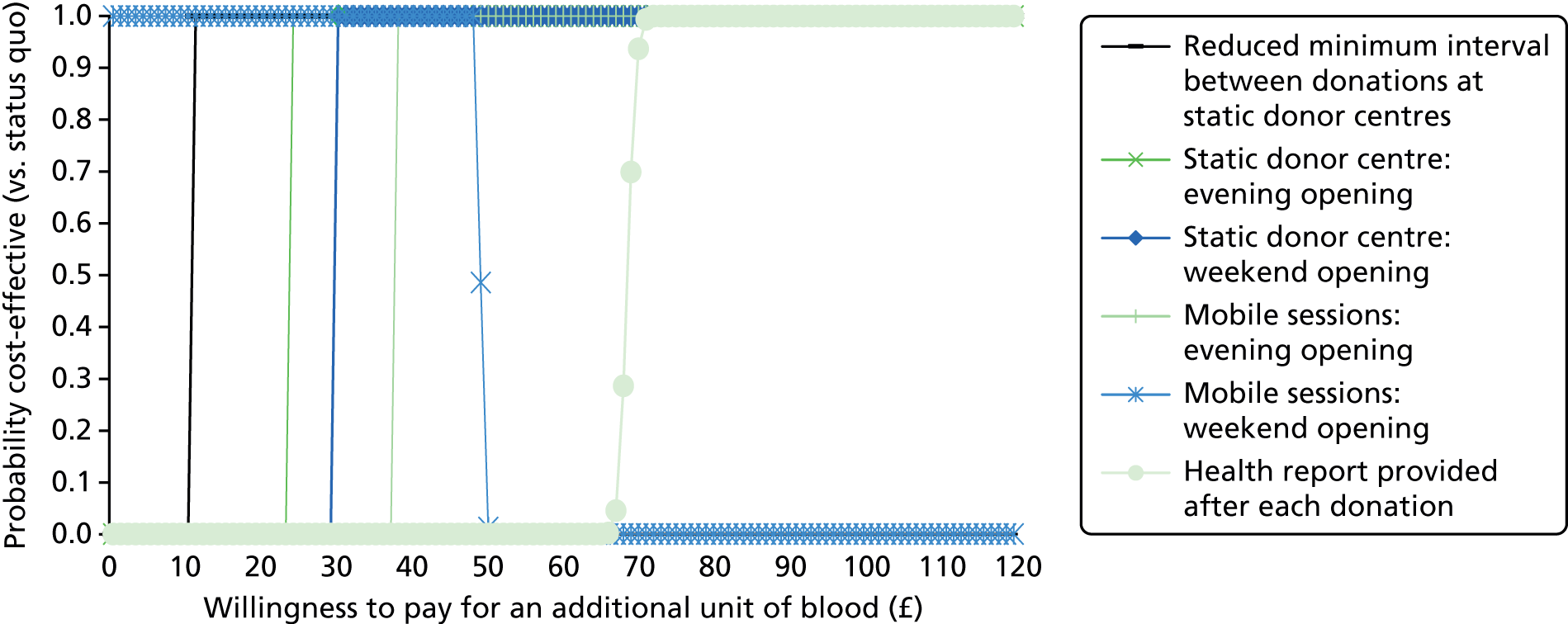
FIGURE 33.
Cost-effectiveness acceptability curves for strategy 6 vs. status quo, using different sources of data to predict volume of blood collected.
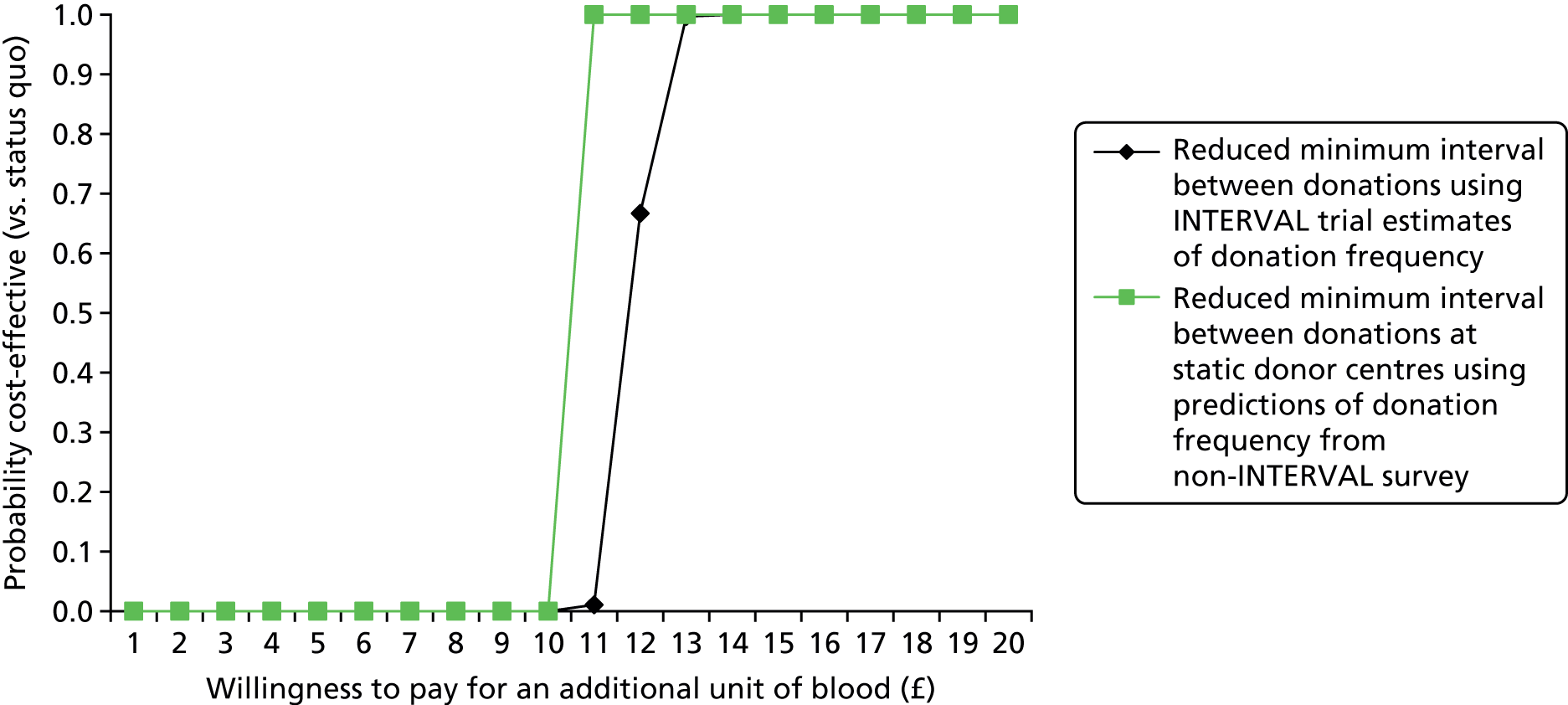
Appendix 21 Results of the sensitivity analysis for the cost-effectiveness analysis
Sensitivity analysis: assuming no additional capacity to collect more blood at donor centres, for all donors
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.113 | 0.519 | 0.455 | 0.070 | 0.484 | 0.678 |
| Incremental cost per donor per year (£) | 15.62 | 15.21 | 10.46 | 3.16 | 18.12 | 18.30 |
| Cost per additional unit of blood (£) | 138.00 | 29.00 | 23.00 | 45.00 | 37.00 | 27.00 |
| Ranking | 6 | 3 | 1 | 5 | 4 | 2 |
| Number of donors affected by service change | 781,028 | 60,640 | 99,312 | 646,898 | 582,910 | 107,811 |
| Incremental units of blood collected per year across target population | 88,173 | 31,482 | 45,233 | 45,402 | 282,159 | 73,121 |
| Incremental costs per year across target population (£) | 12,202,925.00 | 922,033.00 | 1,038,921.00 | 2,041,947.00 | 10,563,571.00 | 1,972,727.00 |
Sensitivity analysis: assuming no additional capacity to collect more blood at donor centres, for donors with high-demand blood types
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.100 | 0.489 | 0.408 | 0.030 | 0.200 | 0.715 |
| Incremental cost per donor per year (£) | 15.52 | 14.29 | 9.34 | 1.35 | 7.46 | 19.23 |
| Cost per additional unit of blood (£) | 155.00 | 29.00 | 23.00 | 45.00 | 37.00 | 27.00 |
| Ranking | 6 | 3 | 1 | 5 | 4 | 2 |
| Number of donors affected by service change | 111,948 | 7965 | 12,874 | 94,258 | 85,075 | 13,884 |
| Incremental units of blood collected per year across target population | 11,216 | 3895 | 5250 | 2825 | 16,973 | 9920 |
| Incremental costs per year across target population (£) | 1,737,712.00 | 113,794.00 | 120,229.00 | 126,920.00 | 634,714.00 | 266,955.00 |
Sensitivity analysis: using a two-part model to predict effect of strategies on the volume of blood collected, for all donors
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.123 | 0.589 | 0.543 | –0.044 | 0.543 | 0.500 |
| Incremental cost per donor per year (£) | 15.93 | 17.25 | 12.48 | –1.96 | 20.34 | 4.95 |
| Cost per additional unit of blood (£) | 129.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Ranking | 6 | 3 | 2 | 5 | 4 | 1 |
| Number of donors affected by service change | 781,028 | 60,640 | 99,312 | 646,898 | 582,910 | 107,811 |
| Incremental units of blood collected per year across target population | 96,237 | 35,708 | 53,952 | –28,265 | 316,751 | 53,920 |
| Incremental costs per year across target population (£) | 12,439,540.00 | 1,045,77.009 | 1,239,11.009 | –1,270,531.00 | 11,858,679.00 | 533,669.00 |
Sensitivity analysis: using a two-part model to predict effect of strategies on the volume of blood collected, for donors with high-demand blood types
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.107 | 0.579 | 0.506 | –0.085 | 0.508 | 0.546 |
| Incremental cost per donor per year (£) | 15.73 | 16.91 | 11.58 | –3.83 | 18.99 | 5.36 |
| Cost per additional unit of blood (£) | 147.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Ranking | 6 | 3 | 2 | 5 | 4 | 1 |
| Number of donors affected by service change | 111,948 | 7965 | 12,874 | 94,258 | 85,075 | 13,884 |
| Incremental units of blood collected per year across target population | 12,017 | 4611 | 6508 | –8036 | 43,220 | 7577 |
| Incremental costs per year across target population (£) | 1,760,503.00 | 134,724.00 | 149,024.00 | –360,833.00 | 1,615,659.00 | 74,475.00 |
Sensitivity analysis: using a gamma model to predict effect of strategies on the volume of blood collected, for all donors
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.126 | 0.515 | 0.591 | 0.025 | 0.584 | 0.511 |
| Incremental cost per donor per year (£) | 15.97 | 15.10 | 13.58 | 1.13 | 21.88 | 5.06 |
| Cost per additional unit of blood (£) | 127.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Ranking | 6 | 3 | 2 | 5 | 4 | 1 |
| Number of donors affected by service change | 781,028 | 60,640 | 99,312 | 646,898 | 582,910 | 107,811 |
| Incremental units of blood collected per year across target population | 98,562 | 31,257 | 58,725 | 16,193 | 340,711 | 55,114 |
| Incremental costs per year across target population (£) | 12,469,393.00 | 915,414.00 | 1,348,863.00 | 728,565.00 | 12,755,962.00 | 545,484.00 |
Sensitivity analysis: using a gamma model to predict effect of strategies on the volume of blood collected, for donors with high-demand blood types
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.120 | 0.510 | 0.537 | –0.004 | 0.571 | 0.578 |
| Incremental cost per donor per year (£) | 16.08 | 14.90 | 12.30 | –0.19 | 21.36 | 5.68 |
| Cost per additional unit of blood (£) | 134.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Ranking | 6 | 3 | 2 | 5 | 4 | 1 |
| Number of donors affected by service change | 111,948 | 7965 | 12,874 | 94,258 | 85,075 | 13,884 |
| Incremental units of blood collected per year across target population | 13,431 | 4061 | 6916 | –392 | 48,606 | 8031 |
| Incremental costs per year across target population (£) | 1,800,080.00 | 118,655.00 | 158,364.00 | –17,553.00 | 1,817,062.00 | 78,929.00 |
Sensitivity analysis: using data from the ex-INTERVAL SP survey, for all donors
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.129 | 0.427 | 0.584 | 0.131 | 0.572 | 1.036 |
| Incremental cost per donor per year (£) | 14.70 | 12.52 | 13.41 | 5.89 | 21.42 | 10.25 |
| Cost per additional unit of blood (£) | 114.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Ranking | 6 | 3 | 2 | 5 | 4 | 1 |
| Number of donors affected by service change | 781,028 | 60,640 | 99,312 | 646,898 | 582,910 | 107,811 |
| Incremental units of blood collected per year across target population | 101,046 | 25,919 | 57,990 | 84,663 | 333,469 | 111,665 |
| Incremental costs per year across target population (£) | 11,483,870.00 | 759,044.00 | 1,331,942.00 | 3,808,253.00 | 12,484,405.00 | 1,105,397.00 |
Sensitivity analysis: using data from the ex-INTERVAL SP survey, for donors with high-demand blood types
| End point | Strategy | |||||
|---|---|---|---|---|---|---|
| 1: health report | 2: weekend opening of static donor centres | 3: evening opening of static donor centres | 4: weekend opening of mobile sessions | 5: evening opening of mobile sessions | 6: reduce minimum donation interval for donors at static donor centres | |
| Incremental units of blood collected per donor per year | 0.126 | 0.394 | 0.487 | 0.173 | 0.558 | 0.989 |
| Incremental cost per donor per year (£) | 14.53 | 11.51 | 11.15 | 7.76 | 20.85 | 9.72 |
| Cost per additional unit of blood (£) | 116.00 | 29.00 | 23.00 | 45.00 | 37.00 | 10.00 |
| Ranking | 6 | 3 | 2 | 5 | 4 | 1 |
| Number of donors affected by service change | 111,948 | 7965 | 12,874 | 94,258 | 85,075 | 13,884 |
| Incremental units of blood collected per year across target population | 14,059 | 3137 | 6269 | 16,293 | 47,450 | 13,729 |
| Incremental costs per year across target population (£) | 1,626,856.00 | 91,661.00 | 143,570.00 | 731,828.00 | 1,773,758.00 | 134,969.00 |
Sensitivity analysis: using estimate of effect of reducing the minimum interval between donations from the INTERVAL trial
| End point | Strategy 6: reduce minimum donation interval for donors at static donor centres |
|---|---|
| Incremental units of blood collected per donor per year | 0.62 |
| Incremental cost per donor per year (£) | 6.71 |
| Cost per additional unit of blood (£) | 11.00 |
| Number of donors affected by service change | 107,811 |
| Incremental units of blood collected per year across target population | 66,789 |
| Incremental costs per year across target population (£) | 723,827.00 |
List of abbreviations
- A–
- A negative
- A+
- A positive
- AB–
- AB negative
- AB+
- AB positive
- B–
- B negative
- B+
- B positive
- BAME
- black and Asian minority ethnic
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- DCE
- discrete choice experiment
- DNA
- did not attend
- DP
- different place (of blood donation)
- GEE
- generalised estimating equation
- GP
- general practitioner
- Hb
- haemoglobin
- HEMO
- Health Economics MOdelling of blood donation
- ICER
- incremental cost-effectiveness ratio
- ISAT
- INTERVAL study administration team
- LP
- last place (of blood donation)
- LSHTM
- London School of Hygiene & Tropical Medicine
- NHSBT
- NHS Blood and Transplant
- NIHR
- National Institute for Health Research
- O–
- O negative
- O+
- O positive
- PULSE
- the NHSBT national blood supply database
- QoL
- quality of life
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SP
- stated preference
- SUR
- seemingly unrelated regression
