Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 13/114/93. The contractual start date was in June 2015. The final report began editorial review in December 2017 and was accepted for publication in May 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rowan Harwood reports that he sat on the National Institute for Health Research Health Technology Assessment Primary Care, Community and Preventative Interventions Topic identification panel from 2014 to 2017. This panel had no relationship to the VOICE study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Harwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
People living with dementia in hospital
Around 850,000 people live with dementia in the UK, and this is projected to rise to 1 million people by 2025. 1 Dementia is common in acute hospitals, with approximately 25% of beds occupied by a person living with dementia. 2,3 Best practice and policy aim to ensure that older people are treated close to home whenever possible, but hospital admission remains necessary for many acute ailments and crises that commonly affect older people, and is likely to remain so. Patients present to hospital with a range of medical emergencies, such as fractures, urinary tract infection, pneumonia or stroke. Such presentations are frequently complicated by falls, immobility, pain, delirium, dehydration or incontinence. 4 During a hospital admission, patients need health care to cure their acute illness, manage exacerbations of chronic conditions, relieve symptoms, restore function and prevent complications. To do this, health-care professionals carry out a range of health-care tasks or activities, such as information gathering, physical assessments, medical investigations, administering medications and physiotherapy. People living with dementia also need support with other aspects of care, such as eating and drinking, washing and dressing, sleeping and safety, known as the ‘fundamentals of care’. 5 Much of the work of hospital health-care professionals involves these tasks;6 effective communication is a prerequisite of effective care.
People living with dementia are vulnerable and need attention to the psychological and emotional aspects of their care as well as the physical, not least to avoid distress and the challenging behaviours that may result. An acute hospital admission can be a frightening experience for those who do not understand it. There is ample evidence that hospital staff feel ill-equipped to care for, and effectively communicate with, people living with dementia. 7,8 The person living with dementia is usually acutely unwell. The complications of delirium or pain may contribute to distress and disorientation, making assessment and interaction more complex than usual. The environment is busy, unfamiliar and often noisy. The thrust of assessment and treatment is towards rapid evaluation, intervention and discharge, leaving little time for rapport building, giving comfort and nuanced communication with those with communication challenges.
Counting the Cost: Caring for Older People with Dementia on Hospital Wards2 reported that nursing staff and nurse managers found caring for people living with dementia to be challenging. Key areas of concern related to managing difficult or challenging behaviours and maintaining safety and communication.
Communication is not solely the responsibility or role of nursing staff. When admitted to hospital, people living with dementia will encounter, and be cared for by, a wide range of health-care professionals, including doctors, nurses, health-care assistants, pharmacists, social workers and allied health professionals (AHPs), such as physiotherapists, speech and language therapists, occupational therapists and dietitians. They also encounter domestic staff, cleaners, porters and hospital volunteers. Some of the key aspirations set out in the Prime Minister’s Challenge on Dementia 20209 are for all hospitals to become dementia-friendly care settings, and for all NHS staff to have training on dementia appropriate to their role.
Outcomes of hospital care for people living with dementia are worse than for people without cognitive impairment. 10,11 People living with dementia have longer lengths of stay, higher readmission rates and a greater likelihood of dying than people without dementia admitted for the same condition. 12 One-quarter of cognitively impaired patients will have died within 3 months of a hospital admission. 6
One possible contributor to this differential is communication difficulties. These are associated with preventable adverse events in the general hospital population,13 length of stay, poorer functional outcome and institutionalisation among stroke patients. 14–16 Studies in residential care have found evidence that poor staff communication, such as use of ‘elderspeak’ (infantilising communication), may exacerbate problem behaviours, such as resistance to care17 and physical and verbal aggression. 18 Both of these increase costs of care. 19 Relatives of people living with dementia report that ineffective communication can result in the exclusion of patients, and care lacking in dignity and respect. 2 Good communication facilitates person-centred care.
Communication problems in dementia
Dementia presents a particular challenge to communication. People living with dementia may experience deterioration in their communication abilities, as well as problems in memory, disorientation, recognition, reasoning and decision-making. 20 People living with dementia often have impaired comprehension and expression, including word-finding difficulties, lack of coherence and repetition of thoughts. As dementia progresses, communication can deteriorate to a state when no intelligible speech is used. 21
The level of communication disability experienced by a person living with dementia will be influenced by contextual factors external to themselves, such as the environment22 and the communication skills of their ‘communication partners’. 23 Hospitals are difficult environments for people living with dementia and rely on an assessment model based on intensive and repeated questioning. People living with dementia may be unable to communicate their needs (such as pain or need for the toilet), and carers may struggle to understand what the person is trying to convey. Such communication breakdown can lead to unmet need, poor care and distress. 24
Data from Counting the Cost: Caring for People with Dementia On Hospital Wards2 indicated that 72% of nursing staff felt that they lacked particular skills to communicate effectively with people living with dementia and wanted additional training. In one acute hospital, staff reported lacking confidence in providing care to people living with dementia, and having received little or no dementia-specific communication skills training. 25 Staff experience stress and reduced job satisfaction arising from challenging interactions with people living with dementia. 7,26
The Equality Act 201027 obliges public services to make ‘reasonable adjustments’ to ensure that services are accessible to all regardless of ‘protected characteristics’ including disability. Such adjustments can be argued to include the communication skills of staff. Reports into poor care for patients within the NHS have highlighted the need for improved communication between hospital staff and patients to reduce errors and improve care. 28 The National Institute for Health and Care Excellence (NICE) guideline29 on care of people with dementia highlights poor communication between the person living with dementia and staff as a factor associated with emotional and behavioural problems. The Building a Safer NHS for Patients report30 recommended communication skills training for health-care professionals.
The importance of nursing staff regularly engaging with their patients in ‘constructive and friendly interactions’ was emphasised by Francis. 28
The government’s position paper Patients First and Foremost: The Initial Government Response to the Report of Mid Staffordshire NHS Foundation Trust Public Inquiry31 advocated improved education and training on dementia, with a commitment to ‘listen most carefully to those whose voices are weakest and find it hardest to speak for themselves’.
Cowdell et al. 32 observed interactions between health-care professionals and people living with dementia in the acute hospital. Almost all communication was related purely to physical care. Many interactions demonstrated elements of ‘malignant social psychology’,33 such as ignoring, infantilisation, disempowerment, stigmatisation, accusation, imposition and disparagement, despite the health-care professional’s belief that they were being kind. 32 The structured non-participant observation method of dementia care mapping has been used to study care delivery for cognitively impaired older adults. Communication by health-care professionals during routine physical care tasks was frequently brief or absent, with a lack of introductions and courtesies, and even ignoring the patient. Patient-initiated interactions were often deflected by health-care professionals, with promises of attention later. Person-centred care, when it was observed, was time-consuming, particularly if the person living with dementia had a communication problem. 6,34
Communication competencies
The ability to communicate sensitively and achieve meaningful interaction is a core competency for supporting people living with dementia. The National Minimum Training Standards for health-care support workers and adult social care workers in England include ‘effective communication’. 35 There is a wealth of advice on communicating with people living with dementia. This includes eliminating distractions, ensuring that hearing aids are working, taking time, positioning oneself in full view and at the same level, speaking clearly and calmly, and using short, simple sentences. 24 There is also a body of practical expertise among mental health professionals. More abstract components, such as the use of body language, making the person living with dementia feel valued or appropriate turn-taking, can be difficult to describe.
Small et al. 36 identified 10 recurrently recommended strategies, of which they found only three had a positive impact on observed communication breakdowns between family caregivers and people living with dementia (eliminating distractions, simple sentences, yes/no questions). One strategy (slow speaking rate) resulted in more breakdowns, a finding confirmed in other studies. 37,38 A slow speaking rate is disliked by older people,39 but is still recommended in a number of current guidelines (e.g. from the Alzheimer’s Society24). The use of closed (‘yes/no’) questions for successful communication is supported,21 but open questions have been found to be useful for facilitating personal conversations about feelings and concerns. 40 Sentence comprehension can be improved by limiting utterances to one proposition,41 paraphrasing and verbatim repetitions. 37 When presented with vignettes, nurses perceived carers who use simplified language as less patronising, and people living with dementia as more competent. 42 Critical communications from caregivers predict negative behaviours43 and, therefore, positive and affirming communications are recommended. 44
Perceptions about communication may differ from objective evidence from recorded interactions. Recommended communication strategies were thought to be helpful by family caregivers and health-care professionals, but both overestimated effectiveness when audio-recordings of interactions were analysed. Despite this, fewer communication breakdowns were observed when recommended communication strategies were used than when they were not. 36
A systematic review45 of the experiences of communication by people living with dementia during interactions with both family caregivers and health-care professionals identified 15 studies. A single study46 explored the views of the person living with dementia, and 14 studies reported the experiences of family caregivers and health-care professionals. Communication difficulty was a common finding. Wang et al. 47 used content analysis of 15 interviews with nurses to explore these difficulties further, and identified two themes. ‘Different language’ referred to the sense that the health-care professional and the patient spoke different languages and so could not understand each other. ‘Blocked messages’ indicated that health-care professionals struggled to interpret patients’ needs and emotions owing to impaired verbal communication and flat affect. In one study,48 nursing staff deconstructed communication with people living with dementia into ‘being in’ communication, whereby they tried to attune themselves to patients’ feelings and attempted to understand the perspective of the person living with dementia, and ‘doing’ communication, which involved using techniques such as active listening, allowing time to talk and asking questions.
The literature does not identify clear communication strategies that can be used for training to overcome communication barriers for health-care professionals and people living with dementia in the acute hospital setting.
Communication skills training
Research suggests that communication skills cannot be improved through experience alone. 49 Skills can be acquired and retained with appropriate teaching, and this leads to greater confidence in communication. 50–52 For training to be effective it needs to be practical, with opportunities to practice and receive feedback. 53,54 Transferring learnt communication skills to clinical practice happens best when courses contain role play with simulated patients (SPs), structured constructive feedback and discussion led by a trained facilitator. 50–52
Reviews of communication skills training interventions for health-care professionals suggest that communication skills can be improved when communicating with a non-communication impaired patient population,55–57 but evidence for their impact on patient health outcomes is uncertain. 53
A systematic review58 of communication skills training in dementia care identified 12 studies, but none was based in acute hospitals or involved the training of doctors. Four interventions were delivered in the patient’s own home, mostly one to one, with a focus on individualised training of the carer, and were not generalisable to hospital staff. The other eight interventions were delivered in care homes, with marked variability in duration (from 3 hours’ training59 to 15 hours’ training plus 2 weeks’ supervised working). 60 Care home studies that used questionnaires and observational measures showed positive effects on the knowledge, skills and attitudes of trained staff, but recommended communication techniques were not always clearly defined and outcome measures were inconsistent.
A systematic review61 of interventions to improve communication between people living with dementia and nursing staff during daily care reported insufficient published evidence to draw firm conclusions. The review included six studies and focused solely on long-term care facilities. Interventions varied in duration, intensity and type, from a single lecture62 to 4 weeks of work-based training. 63 Five out of the six studies showed significant effects on at least one communication outcome, but interpretation of the clinical relevance of these was limited by methodological quality and inconsistency of outcome definition.
Although the literature gives some guidance on communication skills training competencies, minimal evidence comes directly from the general hospital. Most empirical work is based on family and nurses or carers as communication partners, with no studies of doctors or AHPs. To develop an effective communication skills training intervention for interacting with people living with dementia in acute hospitals, we need a better understanding of what works in this setting through basic research to explore the communication problems and how they can be overcome. 54 Recommended attitudes, techniques and approaches cannot simply be assumed to be effective.
Conversation analysis
Conversation analysis (CA) is a well-established sociolinguistic qualitative method for the analysis of social interaction and communication64–66 that has been used to develop successful communication skills training interventions in fields such as stroke,66 psychosis67 and primary care. 68 For example, in stroke care, the recommended ‘supported conversation’ approach to training health-care staff and volunteers to communicate better with people with aphasia69 was based on empirical work using CA to explore the communication of video-recorded volunteers. 70 The skills needed for successfully communicating with people with aphasia were characterised around the concepts of ‘revealing competence’ and ‘acknowledging competence’. The training emerging from this was found to be effective in several trials. 71,72 CA of outpatient consultations between psychiatrists and clients expressing delusional views has demonstrated how the alternative approaches taken by psychiatrists can lead to a change in client responses and thus to more or less constructive consultations73 and this has also been developed into a tested training intervention. 67 CA has also shown that different communication approaches might be more effective at different times. For example, in conversations about advanced decisions and end of life, CA has shown that a direct approach from health-care professionals is harder for the client to deflect and is necessary when an immediate decision is needed, whereas more easily deflected indirect approaches are more appropriate to encourage patient-led decisions when there is more time and a greater priority on avoiding communication breakdown. 74
The existing literature supports the use of fundamental research using CA to collect evidence about communication between health-care professionals and people living with dementia in hospital, and to use this to develop training.
Conclusion
This introduction has outlined that communication problems faced by people living with dementia are common in the acute hospital setting and contribute to problems for staff and poorer experiences and outcomes for patients. Staff feel underqualified to communicate effectively with people living with dementia to deliver satisfactory and fulfilling care. We have identified a lack of evidence to support specific communication training interventions for health-care professionals working with people living with dementia in the acute hospital setting. To improve care, and rise to the challenges set by the public and policy-makers around dementia-friendly hospitals, a deeper understanding is required of how health-care professionals in acute hospitals communicate with people living with dementia, which aspects and techniques are good and which cause communication breakdown.
The specific research questions to be answered in this project were:
-
What should we teach? What constitutes good communication skills, including content, linguistics, context and facilitators that overcome communication challenges experienced between health-care professionals and people living with dementia?
-
How should we teach it? What are the components of an effective communication skills training intervention for health-care professionals caring for people living with dementia and how should this training be delivered?
-
Can we teach it? Is this communication skills training intervention feasible, acceptable and effective?
To answer these questions the following empirical research was undertaken:
-
An update of a systematic review on the content and effectiveness of dementia communication skills training courses. 58
-
Conversation analysis of video-recorded encounters, supplemented by observations, to analyse the structure of communication patterns used by health-care professionals to communicate with people living with dementia.
-
Development of a novel communication skills training intervention based on the findings of the CA, systematic review, expert consensus and service user experience. This included a pilot study to test the training course in real time with selected health-care professionals.
-
An evaluation of the effectiveness of the communication skills training intervention on intermediate outcomes using a before-and-after (B–A) design to assess acceptability of the course and changes in self-assessed competence and confidence, dementia communication knowledge and communication behaviours in health-care professionals who completed the training.
-
An interview study of a sample of the health-care professionals who participated in the training and clinical managers, to examine the acceptability and experience of the training and the importance of this training to the skills of the ward-based clinicians.
Chapter 2 Systematic review
Introduction
The only systematic review58 of communication skills training in dementia care that we found included papers published up to 2010. None of the 12 studies identified was undertaken in a hospital, the training interventions were varied and the methodological quality of the evaluations was generally poor. This review concluded, however, that communication skills training in dementia care led to an increase in positive interactions and improved quality of life for people living with dementia. It also reported a significant impact on the communication skills, knowledge and competencies of both professional and family caregivers.
The aim of the current systematic review was to update the previous review, in order to inform the development of a new communication skills training course and to identify suitable outcome measures for the evaluation. In doing so, we aimed to identify current knowledge on the content, didactic approach and effectiveness of dementia communication skills training courses in various care settings. Specific questions for the review were:
-
What types of communication skills training were evident, taking theory and content into account?
-
What didactic methods were used to deliver the training?
-
What contextual factors (e.g. location, organisation) have been studied, with what results?
-
What is the evidence of the effectiveness of communication skills training, and on what outcomes?
Methods
We developed the search strategy following that described by Eggenberger et al. ,58 in conjunction with a research librarian, and extended it to include online dementia communication skills training. We initially searched for primary research published between January 2010 and August 2015. We updated the review in August 2017 with searches for articles published between August 2015 and August 2017. Electronic bibliographic databases were searched, including MEDLINE, Allied and Complementary Medicine Database (AMED), EMBASE, PsychINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews (CDSR), Web of Science and OpenGrey. Search terms were adapted for use across different databases, including key word and medical subject heading (MeSH) term searches, when appropriate. Box 1 shows the inclusion and exclusion criteria and Figure 1 describes the results of the search and screening process.
-
Title and abstract in English. Translation was sought if a study meeting final criteria had a full text not in English.
-
Evaluation by randomised controlled trials, clinical controlled trials and B–A studies.
-
Trainee population including any health-care professionals, care staff, family caregivers, students or volunteers.
-
Patient population comprised people living with dementia, defined by any criteria and living in any setting.
-
Intervention aimed to improve trainees’ communication with people living with dementia. If the training also incorporated other topics, communication had to form an essential part. Communication skills training could be in a group or one to one, face to face or not. Online learning was included.
-
Use and method of control was recorded.
-
Outcomes included any quantitative outcomes, including at the level of the patient or caregiver.
-
Qualitative or review articles.
-
Intervention studies aimed at training people living with dementia directly, or mixed-patient populations when the training was not specific to the needs of people living with dementia.
-
Communication was not the stated aim, or an essential part of training.
-
Psychosocial interventions aiming to reduce caregiver stress or burden.
-
Cognitive, language or other therapies aimed at changing the person living with dementia’s impairments or functioning.
-
Specifically named approaches with primary non-communication goals including validation, reminiscence, reality orientation, cognitive stimulation and dementia care-mapping.
-
Studies with solely qualitative outcomes.
FIGURE 1.
Communication skills training systematic review 2010–17, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
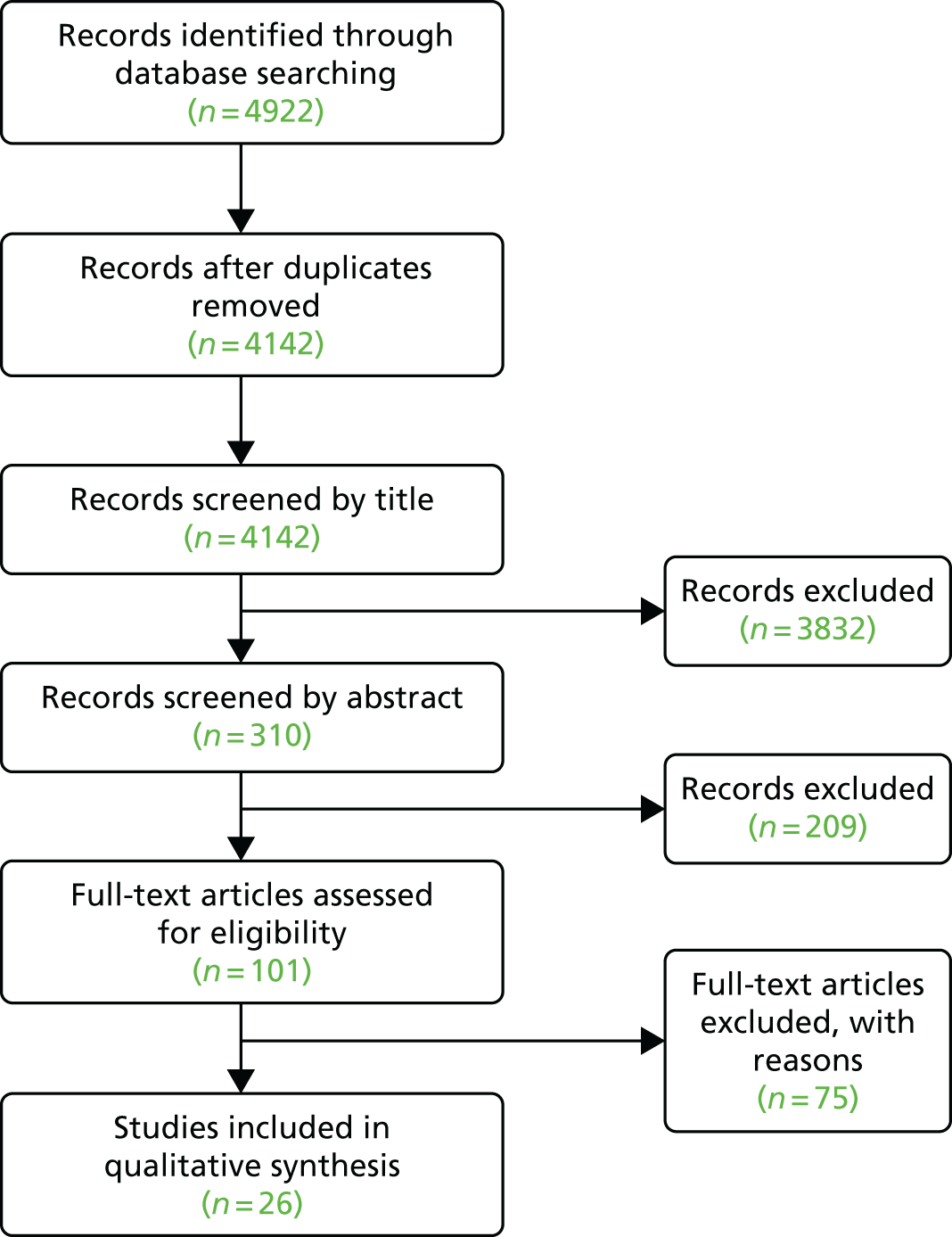
As an example, the search strategy for MEDLINE was a keyword search of:
word group 1 communicat* OR interaction* OR behaviour* OR behaviour* AND
word group 2 train* AND
word group 3 dementia OR Alzheimer* OR “cognitive impairment*” OR “behavioral disturbance*” OR “behavioural disturbance”.
Papers were screened by two researchers (RO’B and RA). Disagreement on whether or not texts met inclusion criteria was resolved by a third reviewer (SG or RH). Methodological quality and risk of bias were assessed using standard criteria, based on the Cochrane Effective Practice and Organisation of Care Review Group Data Collection checklist75 and the Quality Assessment Tool for Before–After Studies with No Control Group. 76 Data were extracted from all studies by two reviewers using standardised forms.
Descriptive data were collected on the:
-
theory or model underpinning the intervention and method of development
-
context for training
-
type of participants
-
duration and model of delivery
-
teaching methods.
The primary outcome data collected were the effectiveness of the training intervention, measured quantitatively, as behavioural changes, or as changes in knowledge, skills, attitudes and well-being, and reported reliability and validity of measures. The systematic review protocol was registered on the PROSPERO database CRD42015023437 (www.crd.york.ac.uk/prospero/display_record.php?RecordID=23437) (accessed 21 August 2018).
Results
We identified 101 studies for full-text review. No full text was identified or accessible for 25 of these. Twenty-one studies were conference abstracts in which no journal paper or report had been published, despite contacting the authors. Two were protocol papers for which the research had not been completed. Two were Doctor of Philosophy (PhD) theses from the USA that could not be obtained and that had not been published. Of the 76 papers with full text available, three required translation into English.
Papers were assessed by two reviewers. Following the full-text review, 49 papers did not meet the inclusion and exclusion criteria. In addition, one study was excluded as it was a duplicate publication under different authorship. This left 26 studies that met the inclusion criteria (see Table 1). Reasons for exclusion included communication training not being the primary aim or a substantial part of the programme, not being specifically aimed at people living with dementia, qualitative studies, protocol papers with no further publications and studies not being training interventions. There was insufficient homogeneity in outcomes for a meta-analysis of the results.
Characteristics of included studies
Table 1 summarises the characteristics of the 26 studies included. Four were randomised controlled trials (RCTs), seven were controlled clinical trials (CCTs), and 15 were B–A study designs. One study was a secondary analysis of one of the RCTs. 101 Duration of direct training varied from one 45-minute workshop to 120-minute workshops fortnightly for 6 months. The modal length of training was 4 hours.
| Study authors and year of publication | Design | Number of participants | Country | Setting | Type of participants | Duration | Mode of delivery |
|---|---|---|---|---|---|---|---|
| Ampe et al., 201777 | CCT | 90 across 18 clusters | Belgium | Care home | Care home staff | Two 4-hour workshops | Group |
| Beer et al., 201278 | RCT | 47 | USA | College | Nursing Aide Students | 45-minute workshop | Group |
| Broughton et al., 201179 | CCT (cluster) | 52 | Australia | Care home | Care home staff | 1.5-hour session (50-minute DVD) | DVD |
| Chao et al., 201680 | B–A | 105 | Taiwan | Long-term care facilities | Nurses | 4-hour lecture and 4-hour workshop plus interenet-based learning activities | Group and online |
| Cockbain et al., 201581 | B–A | 104 | UK | Medical school | Medical students (first year clinical) | 2-hour workshop | Group |
| Conway and Chenery, 201682 | RCT | 34 | Australia | Community care | Care staff | 1-hour training and other activities | Group and 1 : 1 |
| da Silva Serelli et al., 201783 | B–A | 25 | Brazil | Assisted living residences | Nurses and caregivers | 4-hour workshop and other activities | Group and individual |
| DiZazzo-Miller et al., 201484 | B–A | 45 | USA | Not stated | Family caregivers | Three 2-hour workshops | Group |
| Elvish et al., 201485 | B–A | 71 | UK | Hospital | Hospital staff, including doctors | Four 1.5-hour sessions | Group |
| Engel et al., 201686 | CCT | 214 | Germany | Unclear | Family caregivers | 10 2-hour sessions | Group |
| Franzmann et al., 201687 | CCT | 116 | Germany | Nursing home | Nurses/caregivers | 12 2-hour workshops | Group |
| Galvin et al., 201088 | B–A | 540 | USA | Hospital | Hospital staff | One 7-hour session | Group |
| Gitlin et al., 201089 | RCT | 237 | USA | Home | Family caregivers | More than nine 1-hour OT sessions and one 1-hour nurse session and four telephone reviews | 1 : 1 advice to dyad |
| Goyder et al., 201290 | B–A | 25 | UK | Care home | ‘Non-qualified’ care home staff | Two unspecified workshops plus up to 2 hours of additional training delivered in three or four sessions | Mixed: group training and 1 : 1 |
| Haberstroh et al., 201191 | CCT (and time series) | 22 | Germany | Home | Family caregivers | Five 2.5-hour workshops | Group |
| Hobday et al., 201092 | B–A | 40 | USA | Care home | Care home staff – ‘certified nursing assistants’ | Approximately one 1-hour online session | E-learning |
| Irvine et al., 201293 | B–A | 68 | USA | Care home | Care home staff – direct care workers and nurses | Approximately one 2-hour online session | E-learning |
| Karel et al., 201694 | B–A | 38 | USA | Long-term care | Mental health providers and nurses | Approximately 17.5 hours in total | Group |
| Karlin et al., 201495 | B–A | 21 | USA | Care home | Care home staff – ‘mental health providers’ |
Two and a half-day workshops and 25 1.5-hour weekly telephone consultation 17.5-hours direct and 39 hours calls |
Mixed: group workshops and 1 : 1 support |
| Levy-Storms et al., 201696 | B–A | 15 | USA | Nursing home | Certified nursing assistants | 4 hours in total | Group |
| Liddle et al., 201297 | CCT | 29 | Australia | Home | Family caregivers | Two 45-minute DVD sessions | DVD |
| Mason-Baughman and Lander, 201298 | B–A | 14 | USA | Not stated | Family caregivers, friends, others | One workshop, unspecified duration | Group |
| Passalacqua and Harwood, 201299 | B–A | 18 | USA | Care home | Care home staff – care assistants | Four 1-hour weekly workshops | Group |
| Sprangers et al., 201562 | CCT (cluster) | 24 | The Netherlands | Care home | Care home staff – ‘nursing aides’ | One or two 1 : 1 sessions, duration unspecified | 1 : 1 coaching |
| Weitzel et al., 2011100 | B–A | 80 | USA | Hospital | Hospital staff – varied | 12-minute DVD | DVD |
| Williams et al., 2017101 | RCT | 29 staff trained: 42 dyads with29 staff and 27 PLWD analysed | USA | Nursing home | Nursing staff | 3 hours in total | Group and 1 : 1 |
Methodological quality and risk of bias
Two RCTs were assessed as being of high methodological quality with robust allocation methods and measures to prevent cross-contamination of intervention and control groups. 82,102 Blinding of participants to a training intervention was impossible. Many of the outcomes were self-reported by participants, such as ratings of their confidence, attitudes or well-being. This presents a risk of social desirability bias as trainees are likely to rate themselves as better following communication skills training. When studies used more objective measures, such as tests of knowledge or observational measures of behaviour, their psychometric properties were seldom reported.
Review questions
We present findings in relation to each question posed for the review.
What theoretical frameworks or models underpin communication skills training in dementia care?
Thirteen studies referred to a theoretical framework, but there was little consistency between them (Table 2). Five studies supported their training approach using educational theory and three developed their intervention around a communication theory. One intervention used person-centred dementia care as a basis, and one used a clinically derived theory of behavioural techniques. Other theories included caregiver stress and shared decision-making.
| Study authors and year of publication | Framework |
|---|---|
| Educational theory | |
| Beer et al., 201278 | ‘Learning centred classroom’ motivational framework |
| Broughton et al., 201179 | ‘Knowledge translation process’ |
| Chao et al., 201680 | Adult learning theory (see Knowles, 1984,103 1996104) |
| Cockbain et al., 201581 | Seven principles of andragogy and Kirkpatrick’s evaluation levels |
| Liddle et al., 201297 | ‘Knowledge translation process’ |
| Communication theory | |
| Franzman et al., 201687 | TANDEM communication model developed by Haberstroh; stress-strain concept |
| Haberstroh et al., 201191 | Developed TANDEM communication model |
| Sprangers et al., 201562 | Communication enhancement model |
| Other theory | |
| Ampe et al., 201777 | Three-step model of shared decision-making |
| Elvish et al., 201485 | Social cognitive theories |
| Gitlin et al., 201089 | Stress health process model, relating problem behaviours to carer stress |
| Levy-Storms et al., 201696 | Kohler’s105 theory of behavioural techniques to enhance emotional connectedness |
| Passalacqua and Harwood, 201299 | VIPS model106 based on person-centred care for people living with dementia33 |
Twelve studies referenced a theory to underpin the development of their training. One drew on two theoretical frameworks. 87 Several theories were used by more than one study but none was clearly dominant.
Teaching methods used
We examined the pedagogical approaches that the studies used. Table 3 summarises the methods used in the studies. Most of the studies of group communication skills training used a combination of didactic teaching, group discussions, self-reflection, videos and role play, supported by written materials. Seven used ‘homework’, either before training or between sessions. Ten studies used training digital versatile discs (DVDs) or e-learning to give maximum access to a large workforce across care homes and hospitals. Three DVD studies used actors to re-enact narratives illustrating good and bad communication practice. Two studies used real-life clips of interactions. 78,96 Three studies reported online training. A total of 12 studies used videos as part of their training.
| Study authors and year of publication | Method or tool | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Didactic presentation | Written materials | Online or DVD materials | Role play/SPs | Homework | Group discussion, activity or exercises | Video-recordings | Theory | Self-reflection, shared experience | Problem-based learning | Individual advice to dyad | |
| Ampe et al., 201777 | ✓ | ✓ | ✓✓ RP | ✓ | ✓ | ✓ | ✓✓ | ||||
| Beer et al., 201278 | ✓ | ✓ | ✓✓ | ✓✓ | |||||||
| Broughton et al., 201179 | ✓ | ✓✓ DVD | ✓ | ||||||||
| Chao et al., 201680 | ✓✓ | ✓ | ✓✓ internet | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Cockbain et al., 201581 | ✓ | ✓✓ SP | ✓ | ✓ | |||||||
| Conway and Chenery, 201682 | ✓✓ | ✓ | ✓ | ✓ | ✓ | ✓✓ | ✓ | ✓ | |||
| da Silva Serelli et al., 201783 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| DiZazzo-Miller et al., 201484 | ✓ | ✓ RP | ✓ | ||||||||
| Elvish et al., 201485 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Engel et al., 201686 | ✓ | ✓ | ✓ | ||||||||
| Franzmann et al., 201687 | ✓✓ | ✓ | ✓ | ✓ | ✓✓ | ||||||
| Galvin et al., 201088 | ✓✓ | ✓ | ✓ case studies | ||||||||
| Gitlin et al., 201089 | ✓ | ✓✓ | |||||||||
| Goyder et al., 201290 | ✓ DVD | ✓ | ✓ | ✓ | ✓ | ||||||
| Haberstroh et al., 201191 | ✓ | ✓ | ✓ RP | ✓ case studies | ✓ | ✓ | ✓ group | ||||
| Hobday et al., 201092 | ✓ | ✓✓ internet | ✓ | ||||||||
| Irvine et al., 201293 | ✓ | ✓✓ internet | ✓ | ||||||||
| Karel et al., 201694 | ✓✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Karlin et al., 201495 | ✓ | ✓ | ✓ RP | Weekly telephone calls | ✓ | ✓ | |||||
| Levy-Storms et al., 201696 | ✓✓ | ✓ | |||||||||
| Liddle et al., 201297 | ✓ | ✓✓ DVD | ✓ | ||||||||
| Mason-Baughman et al., 201298 | ✓ | ✓ | |||||||||
| Passalaqua and Harwood, 201299 | ✓ | ✓ RP | ✓ | ✓ and guided visualisation | ✓ | ||||||
| Sprangers et al., 201562 | ✓✓ observation with feedback | ✓ | |||||||||
| Weitzel et al., 2014100 | ✓✓ DVD | ||||||||||
| Williams et al., 2017101 | ✓✓ | ✓ | ✓ | ✓ | |||||||
| Totals | 17 | 19 | 10 | 7 | 7 | 15 | 12 | 8 | 8 | 2 | 5 |
The need for practising communication skills and gaining feedback53,55 was supported by the use of role play, simulation or ‘live’ skills practice in seven studies. In one study,81 simulation was the principal training method, with positive effects on confidence, although their measure was not validated.
Context of study as it relates to outcomes
There was huge diversity in the setting and focus of the studies identified (see Table 1). They were conducted in eight different countries, with most from the USA, and comprised a total of 2103 trainees. Settings for the training included 14 care homes, eight private settings (including assisted living residences), three acute hospitals and two higher education institutions. Trainee participants included care and nursing assistants, family caregivers, health-care professionals (including doctors) and students of these professional groups. Control conditions included no intervention, self-help literature and (in a train-the-trainer intervention) training by a different facilitator. Therefore, no general inferences could be drawn concerning the interaction between context and effectiveness of the interventions.
Evidence of effectiveness of communication skills training
We investigated the outcome measures used in each study and whether or not there was any change in these measures that could be attributed to the interventions studied.
Observational checklists
Five studies measured the observed behaviour of trainees. Ampe et al. 77 used the validated OPTION (observing patient involvement in decision-making) scale of shared decision-making107 to measure the degree of involvement of residents and families in discussions and advanced care planning. This comprised a five-point scale to measure the degree to which advanced care planning was discussed, and there was no statistically significant change. Levy-Storms et al. 96 coded specific communication behaviours and residents’ responses in video-recordings using time-sampling methods. The checklist for communication behaviours was based on four therapeutic communication techniques taught in the intervention. Coders were blinded to pre- or post-intervention status and achieved acceptable inter-rater reliability (mean kappa = 0.64). The prevalence of therapeutic communication behaviours increased significantly after training, but the frequency of residents’ refusals of food was unchanged.
Williams et al. 102 used video-recordings to complete staff communication behaviour checklists and residents’ behaviours based on the Resistiveness to Care Scale. 108 Coders were blinded and adequate inter-rater reliability was achieved (90% agreement). Results showed that staff use of ‘elderspeak’ (a communication style characterised by simplistic language, slowed speech, elevated pitch and volume, and inappropriately intimate terms of endearment) reduced significantly after intervention, as did resident resistance to care, and neither persisted at the 3-month follow-up.
Two other studies62,100 used a checklist of positive and negative communication behaviours to rate ‘live’ observations, without rater blinding. Both studies reported statistically significant improvements in specific skills. Sprangers et al. 62 reported acceptable inter-rater reliability on their two checklist measures (75% and 79%), but Weitzel et al. 100 reported no psychometrics.
The results suggest that observing trainee behaviours as an outcome measure is possible, but did not always demonstrate change.
Self-ratings by trainees
Self-ratings of confidence in dementia communication by trainees were used in seven studies (Table 4). All reported significant gains following the communication skills training, although in one case this was on a single subscale. 90 One study that reported psychometric properties found a significant and meaningful difference on their measure. 85 Six studies reported measures related to attitude (Table 5). Of these, only one found a significant effect. 93 Nine studies measured change in knowledge following the training intervention (Table 6). All studies reported gains. Most knowledge tests were developed by individual studies, with a focus on the learning outcomes of their training, and some based on other knowledge tests or translated tests (e.g. Conway et al. ,82 Chao et al. 80). Overall, there was evidence of knowledge gain from training, although the validity of the measures used in the studies was often uncertain.
| Study authors and year of publication | Number of participants | Self-rating measure used | Result reported | Validity |
|---|---|---|---|---|
| Cockbain et al., 201581 | 144 | Single question: rate confidence in communicating | Post > pre | None reported |
| Conway and Chenery, 201682 | 34 | Self-efficacy questionnaire based on inventory of geriatric nursing self-efficacy | TG > CG | Adequate psychometrics reported |
| Elvish et al., 201485 | 71 | CODE | Post > pre | Adequate psychometrics reported |
| Galvin et al., 201088 | 540 | Five confidence items: one communication | Post > pre on each item | None reported |
| Gitlin et al., 201089 | 237 | Five-item caregiver confidence scale using new activities in past month (not communication) | TG > CG | None reported |
| Goyder et al., 201290 | 25 | Sense of competence in dementia scale | Post = pre for whole scale; post > pre for ‘building relationships’ subscale | Adequate psychometrics reported |
| Irvine et al., 201293 | 68 | VST, two items of four scenarios: confidence in knowing what to do next and how to alter the behaviour 20-item self-efficacy measure |
Post > pre Stable on repeated baseline, post > pre |
VST self-efficacy acceptable retest reliability (r = 0.63) Self-efficacy measure acceptable retest reliability (r = 0.76) |
| Study authors and year of publication | Number of participants | Self-rating of attitude | Results | Validity |
|---|---|---|---|---|
| Chao et al., 201680 | 105 | Communication skills attitudes scale translated into Chinese | Post = pre | Adequate psychometrics |
| Conway and Chenery, 201682 | 34 | Approaches to dementia questionnaire | Post = pre | Adequate psychometrics |
| Engel et al., 201686 (in German) | 214 | Family questionnaire | TG > CG | Not reported |
| Goyder et al., 201290 | 25 | Approaches to dementia questionnaire | Post = pre | Not reported |
| Irvine et al., 201293 | 68 | 18-item attitude measure | Stable on repeated baseline post = pre | Previous study reports acceptable retest reliability (r = 0.7) |
| Passalacqua and Harwood 201299 | 26 |
Empathy: interpersonal reactivity index Attitudes to ageing, dementia and person-centred care |
Post = pre Hope item post > pre For the rest, post = pre |
Items taken from longer validated measures |
| Study authors and year of publication | n | Knowledge test | Results | Validity |
|---|---|---|---|---|
| Broughton et al., 201179 | 52 | 17-item open questionnaire on ‘Strategies to support communication & memory’ |
TG showed post > pre; CG did not Overall TG = CG |
Not clear Open questions, blind-rated tests |
| Chao et al., 201680 | 105 | Communication skills knowledge scale (translated into Chinese) | Post > pre at 4 and 16 weeks | Content validity index 0.92; Cronbach’s alpha = 0.94 |
| Conway and Chenery, 201682 | 18 | Communication support strategies in dementia knowledge test | Post > pre | None reported |
| DiZazzo-Miller et al., 201484 | 45 | 18-item ‘Knowing how to assist in five areas of ADLs’ questionnaire (six questions on ‘communication and nutrition’) | TG > CG on each of five modules; biggest effect for ‘communication’ module |
Content validity No other psychometrics reported |
| Elvish et al., 201485 | 71 | 16-item knowledge in dementia scale (two items specifically on communication) | Post > pre |
Psychometrics reported Test published |
| Galvin et al., 201088 | 540 | 9-item ‘Knowledge about dementia’ scale | Post > pre | None reported |
| Hobday et al., 201092 | 40 |
15-item MCQs ‘Dementia Knowledge Test’ Test published |
Post > pre |
Cronbach’s alpha = 0.94 No other psychometrics |
| Irvine et al., 201293 | 68 | Video situation knowledge test, four scenarios: MCQs for each about ‘what to do next’ |
No change on repeated baselines post > pre |
Used previously by same group but no psychometrics reported |
| Liddle et al., 201297 | 29 | Communication and memory support in dementia test |
TG showed post > pre (NS) CG, post = pre |
No psychometrics reported Blinded markers used |
Discussion
This review aimed to identify and evaluate training interventions designed to improve communication in dementia care. Papers published between 2010 and 2017 were evaluated to update the systematic review by Eggenberger et al. ,58 which included papers published to 2010. Communication skills training research for people living with dementia has increased substantially since 2010. Twenty-six studies were identified, mostly B–A designs of variable methodological quality. They used a range of theoretical approaches, and spanned different settings. Few studies were directly applicable to our situation, not being based in acute hospitals or not aiming to improve health-care professionals’ communication with people living with dementia.
Studies demonstrated different teaching approaches, although it was not possible to assess the effectiveness of specific methods. Traditional methods, such as didactic presentations, reading materials and group discussions were popular, as were video-recordings, DVDs and online materials. Role play and simulations were also used. The duration of direct training ranged from a single 45-minute workshop to 120-minute fortnightly workshops for 6 months.
Studies evaluated the effectiveness of the training interventions using a range of outcome measures, including ratings of observed trainee behaviours and subjective ratings of confidence, attitude and knowledge. Several studies developed their own measures or adapted them from previously published measures. Trainees’ communication behaviours showed a variable response to training. Two of the five studies measuring this aspect reported statistically significant improvements in confidence and knowledge after training.
Previous studies indicate that role play and simulations are both viable and acceptable teaching approaches. The review also shows that most interventions used a combination of several approaches to teaching skills. There is evidence that trainee knowledge and confidence improves after training. However, given the heterogeneity of the studies included in this review, it is difficult to draw conclusions about what constitutes optimal communication skills training. The low numbers and poor quality of relevant studies suggest that there is no existing intervention that could be adapted or used in acute care.
Chapter 3 Conversation analytic study
Introduction
Conversation analysis is a sociolinguistic method for studying patterns in real-life communication encounters. It analyses what communication partners actually do, rather than what they think or say they do.
To understand how health-care professionals communicate with people living with dementia, and to what effect, we conducted a study using CA to analyse video-recordings of real ward encounters. Patients, family members and health-care professionals often cannot articulate the tacit knowledge they use when communicating, no matter how expert they are, but video-based research can specify such knowledge and skills. CA is a research method that originated in sociology but draws on insights from other disciplines, such as psychology and linguistics. 109 Its aim is to study the structure and order of naturally occurring talk during interactions. The method has been widely used to study health-care interactions. 64,110–112 We focused on identifying the everyday challenges of communicating with people living with dementia in the acute inpatient setting and, importantly, the communication skills that may overcome these issues.
We harnessed the potential of video-based research by using CA to:
-
classify verbal and non-verbal practices and patterns within health-care interactions involving experienced clinical communicators
-
analyse how the broad recommendations for good practice actually get implemented and operationalised
-
analyse episodes in which there are challenges to the operationalisation of interactions and the ways these challenges are managed.
Methods
The study took place on eight acute geriatric medical (health care of older people) wards in a single large teaching hospital. It was approved by the Yorkshire and The Humber – Bradford Leeds NHS Research Ethics Committee (reference number 15/YH/0184). We adapted protocols for recruitment, consent and data collection used by team members during previous studies of dementia6 and CA studies. 54,66 These protocols were developed with patient and public involvement (PPI) input.
Participation eligibility
We included male and female patient participants who were aged ≥ 65 years and had been admitted to an acute geriatric medical ward. All had a diagnosis of dementia recorded in medical notes, and ward staff reported that they had difficulties communicating. Health-care professional participants were eligible if they were a registered health-care professional (doctor, nurse or AHP). Any relatives or friends of patient participants or other health-care professionals or students present during data collection also participated in the study, subject to consent.
Patient participants were excluded if they did not speak English, were unable to give informed consent and we were unable to obtain consultee agreement, they had a diagnosis of Parkinson’s disease or they were assessed by the clinical team as likely to die within 7 days.
Recruiting and consenting participants
Participant recruitment was carried out by two clinical researchers (RO’B and RA), both Health and Care Professions Council-registered speech and language therapists.
Recruitment of health-care professionals began in August 2015, in advance of the recruitment of people living with dementia. Health-care professionals were recruited by personal approach or via ward managers. We aimed to recruit health-care professionals who were considered by peers to be ‘good communicators’ or ‘good with patients living with dementia’. We aimed to achieve a spread across categories of health-care professionals (doctors, nurses and therapists). We obtained written informed consent from the health-care professional. Table 7 gives details of the health-care professionals recruited and video-recorded. Video-recordings with AHPs comprised five with physiotherapists, three with speech and language therapists and two with occupational therapists. Those with nurses comprised 11 staff nurses, one advanced nurse practitioner and seven mental health nurses. Those with doctors comprised three consultants and eight junior or middle-grade doctors.
| Health-care professional | Number of | ||
|---|---|---|---|
| Health-care professionals recruited | Health-care professionals recruited and then video-recorded | Videos collected | |
| Nurses | 19 | 11 | 19 |
| AHPs | 11 | 6 | 10 |
| Doctors | 11 | 9 | 12 |
| Total | 41 | 26 | 41 |
Patient participants were approached by a clinician working on the ward who introduced the patient to the researchers, if willing. The researcher discussed the study with the patient, and assessed their mental capacity to give or withhold consent to participate. In accordance with the Mental Capacity Act 2005,113 patients were supported in their understanding by the speech and language therapist researcher, for example using a simplified, one-page information sheet, and by showing them the video camera. The two speech and language therapist researchers independently assessed the clinical severity of communication impairment.
No patients in this study had mental capacity to give informed consent, and the requirements of the Mental Capacity Act 2005113 were followed. A family member or informal carer was approached and, following explanation of the study, asked to give consultee agreement. Given the sensitive nature of making video-recordings of patients while in hospital, we did not include participants if they had no family or other personal consultee.
In response to a suggestion arising from pre-study PPI, written informed consent was sought from any relatives or friends who wanted to be included in the video-recording of the interaction between a patient and a health-care professional. This process allowed us to potentially include sensitive conversations between health-care professionals and people living with dementia, in which best practice would be to involve a relative or friend.
After an encounter had been filmed, participants and personal consultees were shown the video on a tablet computer and asked for further consent or agreement for dissemination (e.g. in teaching, at conferences or on material posted to the internet).
Data collection
Data collection was carried out by clinical researchers Rebecca O’Brien and Rebecca Allwood. Interactions on acute geriatric medical wards between health-care professionals and people living with dementia were video-recorded. To identify suitable interactions for recording, researchers talked to ward staff at the beginning of each day about what encounters were expected to occur with consented patients (e.g. an occupational therapist assessment, a consultant doing a ward round). We sought to record routine interactions for staff. We did not film intimate interactions, such as washing, dressing or toileting. All of the video-recorded interactions were initiated by the health-care professional.
The research speech and language therapists set up the equipment to video-record the encounter. A camera with a wide-angle lens was used to maximise capture, connected to a remote microphone worn by the health-care professional, when appropriate. Separate audio-recordings were made using a digital audio-recorder. Cameras, audio-recorders, microphones and the researcher were positioned to be minimally disruptive to the interaction. To maintain confidentiality, any patient name visible to the camera was covered up in advance of recording or edited out afterwards. We recorded the conversations for as long as they lasted.
In total, 41 conversations were video-recorded between September and December 2015. This resulted in a total of 378 minutes of data from 26 patient participants (10 men and 16 women) and 26 health-care professional participants. Eleven (27%) video-recordings included a person with dementia who had mild communication impairment, 22 (54%) who had moderate communication impairment and eight (19%) who had severe communication impairment. Patients could be filmed more than once with a different health-care professional, so some staff and patients appeared up to three times in our data set. The average length of a recording was 9 minutes and 24 seconds, with a range of 2–30 minutes. The video-recordings included thousands of conversational turns, each encapsulating many interactional behaviours. Recordings were digitised and stored in accordance with the University of Nottingham data protection policy. Each encounter was allocated a code to indicate the patient and health-care professional while maintaining anonymity.
Brief observational field notes on the context of each interaction were recorded by the researcher to identify any contextual events that may have influenced the interaction.
Analysis
Data preparation and analysis were carried out by the research speech and language therapists, supported by Suzanne Beeke and Alison Pilnick, the VideOing to Improve dementia Communication Education (VOICE) study’s expert conversation analysts. Recordings were transcribed verbatim using CA notation, by professional transcribers, then anonymised and analysed using CA. The conventions of CA notation are described in Appendix 1.
Conversation analysis was used to reveal the structure of encounters, in terms of interactional phases, and recurrent and systematic interactional features and patterns. This method is well established in the field of doctor–patient interaction. 64,111,112
A core objective of the analysis was to generate recommendations for practice. As a result, we were particularly interested in identifying communication strategies that would be ‘teachable’.
A selection of recordings was viewed by the team, alongside their CA transcriptions, to identify key phases and patterns of interaction. Data were then organised into collections of cases illustrating similar phenomena, which were examined to identify (1) the talk used by health-care professionals when faced with challenges in communicating across different clinical encounters and (2) patients’ interactional responses to health-care professional talk. Within the relevant sequences, close attention was paid to patterns of similarity and difference in the details of talk and body movement, in order to identify those health-care professional practices that appeared most effective.
We drew on relevant evidence generated by other CA studies of health-care talk, as required by the CA approach, as a means of ensuring the robustness, validity and generalisability of findings. 64,68 Procedures to verify and validate findings included group data analysis sessions with experienced CA researchers on the VOICE study team, and sessions at external centres of excellence for CA and health-care research, along with consultation with dementia clinicians and PPI representatives, using raw or disguised data according to the level of consent gained.
All names in our data have been changed to protect anonymity.
Results
Phases of the encounter
In this data set, health-care professionals completed a wide variety of health-care interventions, including medical ward rounds, medication administration, recording of vital signs, leg ulcer dressing, swallow assessments, assistance with eating and drinking, assessment and support with walking, and assessment of activities of daily living.
Our analysis commenced by ascertaining the phases of ward-based hospital encounters. The CA literature highlights the ‘institutional nature’ of health-care interactions. 114 These follow a more predictable structure than ordinary conversation. The phase structure of institutional interactions affects how the health-care encounter is progressed by those involved, because speakers normatively orient to the transitions between phases. Although phases do not follow an exact sequence in all interactions, being described as ‘vague orderly’ by Jefferson,115 trying to identify an overarching structure is important.
There is extensive literature114,116–121 examining the phase structure of a variety of health-care encounters, but we found only a single CA study122 assessing the structure of ward-based, acute hospital encounters that analysed admissions interviews. Consequently, we drew on other contexts for comparison. Research in nursing and medical encounters describes an opening phase, followed by the presenting problem or complaint. 119–121 An information-gathering phase, which may be an examination or assessment, is followed in medical encounters by diagnosis and treatment recommendations,119 whereas in nursing encounters this may be described using terms such as ‘counsel’121 or ‘intervention’. 120 All describe ending with a closing phase.
Despite the diversity of tasks in the current data set, a broad five-phase structure was evident: opening, reason for the visit, information gathering, the ‘business’ phase and closing.
All encounters commenced with an opening phase, which incorporated social greetings and personal identifiers, frequently with the health-care professional using the patient’s name, their own name and their role. Unlike other health-care professionals, nursing staff appeared not to introduce themselves by name or identify their role to patients, perhaps because their consistent presence in a ward bay for a 12-hour shift did not warrant repeated introductions, unlike for staff with a more transient presence.
In the next phase, health-care professionals introduced their reason for the visit, because they were the initiators of these interactions. This represents a fundamental difference between our data set and previous CA findings for medical encounters, in which the patient initiated the interaction or presented a problem to the health-care professional. In most cases in our data set, health-care professionals were explicit about the purpose of their visit. The exception to this was in routine ward rounds, when doctors tended to lead with a typical physicians’ opening question of ‘How are you feeling today?’, presumably as an invitation to encourage patient troubles-telling. 64
In some cases, the next phase was one of information gathering. This phase was highly varied, including history-taking questions about current concerns and symptoms (‘Do you feel sick?’ ‘Any pain anywhere?’), and recent events (‘Did you sleep well?’), as well as attempts to establish patient wishes or concerns (‘What would you like to happen?’). On occasion, tasks were completed without a significant information gathering phase.
Given the heterogeneity of reasons for the health-care encounters, the phase in which these interventions were undertaken was designated the ‘business’ phase. Tasks included recording vital signs; physical examinations; assessment of, and assistance with, physical, cognitive, swallowing and everyday functioning abilities; and completion of care tasks, such as taking medications, feeding and personal care. All included a component of physical action on the part of the health-care professional and patient, working more or less collaboratively.
The closing phase of the encounter was typically initiated by the health-care professional and included planning for future conversations, or the arrangement of care activities or assessments.
Features prioritised for in-depth analysis
The ‘business’ and closing phases were notable because they were frequently associated with interactional trouble around (1) requests and refusals and (2) closings. We focused on these for in-depth analysis.
The ‘business’ phase regularly involved the health-care professional conducting health-care tasks with the person living with dementia, which were achieved through request sequences by the health-care professional. CA research123 suggests that refusals in response to requests are dispreferred (i.e. avoided or less favoured than alternatives), and usually accompanied by extensive explanation or mitigation. However, analysis indicated that in 28 of the 41 recordings (68%), patients responded to a request with some level of reluctance or refusal, often repeated refusal, and with little or no mitigation.
We also identified recurring interactional difficulties in bringing these encounters to a close, along with examples of more successful closing phases.
Requests and refusals
Definitions of requests vary, but typically they are expressions intended by a speaker to ask something of the recipient, such as an action. ‘Directives’ can be distinguished from requests as ‘telling’ people to do something, instead of ‘asking’. 124,125 CA studies of requests,124,126,127 across a range of data sets, have established that they can be analysed in terms of ‘entitlement’ and ‘contingency’. A speaker displays, by the format of their request, how entitled they are to ask the recipient to do something (entitlement), and acknowledges the perceived difficulty of the task and potential barriers to completion for the recipient (contingency).
In this study we designate the term ‘request’ to identify talk in which the health-care professional attempts to get a patient to do an action (such as ‘lift your leg’), and also for utterances that ask permission for the health-care professional to conduct an action involving the patient (e.g. ‘Can I lift your leg?’). Compliance with a request can take the form of an immediately embodied response (e.g. patient lifts leg) completed without comment, a purely verbal response, or both. Rejection may occur, but this contravenes general interactional preferences, and, when refusal occurs, speakers typically carry out interactional work to mitigate rejection, such as hesitations or giving explanations for refusal that clarify their failure to comply. 106
In our data, during each task the health-care professional issued a set of requests for action from the patient, or requested permission to act. For example, when examining a patient’s chest, the health-care professional might request permission to listen to the chest, then ask the patient to adjust their clothing, lean forward and take repeated deep breaths. Each of these individual requests requires a certain degree of physical action or passive co-operation from the patient to be ‘successfully’ completed (from the viewpoint of the health-care professional). The health-care professional interpreted the patient’s response to their requests through the patient’s verbal responses and through their embodied (non-verbal) response, that is, whether or not they completed the action.
Responses from patients could be classified in terms of whether they agreed to the request, refused the request or their response was ambiguous, and also whether responses were exhibited in a verbal or embodied (non-verbal) way (Box 2).
Four of the 28 encounters displaying refusal included separate examples of purely embodied (non-verbal) refusals as well as verbal refusals. Only two comprised non-verbal refusals alone. The refusals were classified as overt refusals (verbal and non-verbal), mitigated refusals and passive non-responses. It was not possible to characterise a small minority of refusals and these cases were excluded from analysis.
Overt refusals
Patients responded with overt verbal refusals in 13 episodes over nine encounters without any mitigation (in the following extracts, HCP stands for health-care professional, and PAT stands for patient):
In the above example, the patient gives no non-verbal indication that she intends to comply following her ‘no’ response. Purely non-verbal overt refusals without any verbal mitigation occurred in six encounters; examples included the patient deliberately turning their head away from an approaching spoon, closing their mouth against a cup and moving their arm from a position needed to take a blood test.
Mitigated refusals
Mitigated refusals were noted in 14 encounters, with 11 of these containing multiple instances. Patients presented three clear accounts to support their reasons for refusal: lack of ability, lack of willingness and lack of perceived need. Some refusals were followed by words that were difficult to interpret, and it was not possible to assess whether or not this constituted a mitigation.
Lack of ability
People living with dementia in hospital are likely to have impairments as a consequence of acute or chronic ill health, making it unsurprising that lack of ability, or lack of confidence in their ability, to do the requested task might explain, in part, refusal or reluctance to comply:
Lack of willingness
On occasion, however, patients explicitly stated that they did not want to carry out the requested action, as the following assertions from different encounters demonstrate:
At times, patients explained their reluctance in terms of contingencies that could be legitimately expected to reduce their engagement, such as pain.
Lack of perceived need
Sometimes patients justified their refusal by clearly stating a lack of perceived need:
Patients questioning the necessity of the requested action indicates a mismatch between their perception of medical or social needs and how these were perceived by the health-care professional. In the following extract, the patient dismissed any problem with her arm, even though it was in plaster (but not easily visible as it was under her cardigan at the time of this encounter):
The patient repeatedly counters the health-care professional’s initial request, and ensuing explanations and requests. The health-care professional is presented with a dilemma of having to address a health-care need in a patient who lacks insight into that need.
Unclear talk
In a number of instances, patients clearly indicated reluctance or refusal, but additional verbal content was ambiguous and may have been an attempt at mitigation. In the context of dementia, in which linguistic and cognitive impairments have an effect on reasoning and language, a patient may struggle to justify their refusal. In such ambiguous circumstances, these patient comments were often treated as mitigated refusals by the health-care professionals, for example:
Passive non-responses
Ten encounters involved health-care professional requests that failed to elicit any obvious verbal or embodied response from the patient. It is possible that non-responses were a deliberate choice to refuse the requested action, a failure to understand the request or to appreciate that a response was required, or an inability to undertake or complete the action requested combined with the inability to convey this. CA does not allow exploration of potential reasons for non-response unless evident in the talk. If a patient’s interactional behaviour lacks any additional relevant information, then the hearer (health-care professional or analyst) may only speculate about reasons for refusal. However, the manner in which the health-care professional reacts to such non-responses indicates their interpretation of the non-response, as they attempt to engage the patient in willing co-operation with their planned intervention.
Health-care professional requests preceding a refusal
In an effort to understand the high rate of refusals in this data set, we analysed the nature of health-care professionals’ requests that preceded overt and mitigated refusals. Alternative request patterns that elicited successful responses were sought in order to pinpoint potentially trainable practices.
Health-care professionals’ requests preceding overt refusals indicated sensitivity to the concepts of entitlement and contingency, both of which can be considered to be ‘high’ or ‘low’. In most cases, health-care professionals displayed low to moderate entitlement to make their request, with high contingency, suggesting a potential lack of ability or willingness to engage on the part of the patient.
Low entitlement, high contingency requesting
In some of the overt refusal sequences, health-care professionals displayed extremely low entitlement to make requests of the people living with dementia. In the most striking case, shown in Extract 6, a nurse uses the ‘I was wondering’ format (lines 5–7), described in calls to out-of-hours general practitioner (GP) services by Curl and Drew. 122 The health-care professional is asking permission to help the patient with the task of ‘relieving some pressure on your bottom’, meaning that the patient needs to stand up. This initial request for permission resulted in a considerably delayed but unmitigated ‘no’ from the person living with dementia in line 9:
By saying ‘just wondering’, the health-care professional clearly exhibits her doubt about whether or not the person living with dementia will comply with the request. The health-care professional does not ‘know’, she can only ‘wonder’ if the proposed course of action will be considered reasonable or acceptable by the patient. The health-care professional’s ‘wondering’ suggests that she anticipates contingencies limiting the patient’s ability or willingness to grant the request. Framing her proposal as an offer to help with an intervention indicates that the health-care professional felt that the patient may be unable to complete the task unaided. We postulate that use of low entitlement and high contingency requesting presents the patient with a clear option to refuse the request.
The nurse demonstrates a positive orientation towards patient choice, empowerment and autonomy, consistent with current ‘best practice’ thinking about person-centred dementia care. 33,128 However, the tendency of health-care professionals to project low entitlement to request actions from patients in these data, although appearing warm and respectful of the patient’s autonomy, presents a clear opportunity for refusal in interactional terms. If a person living with dementia is uncertain about where they are or why they are in hospital, and unclear who the health-care professional is (all of which was evident in our data set), then this low-entitlement request may fail to convey the urgency or importance of an intervention and fail to identify the requester as an expert professional. Therefore, the patient may not appreciate the consequences of a refusal. Our analysis suggests that the unintentional consequence of asking in this low-entitled, apparently ‘person-centred’, way is that a health-care professional may be inadvertently communicating that the interaction or intervention is of low priority, making a refusal seem inconsequential and, therefore, more likely.
Overt refusals in our data were also preceded by very low-entitled ways of requesting, structured with the permission-seeking prefaces ‘Is it alright if I . . .?’ or ‘Is it OK if I . . .?’, as in Extract 7, in which a junior doctor wishes to examine a patient’s chest during a routine encounter:
Here, the health-care professional leads with a permission-seeking question, ‘Is it OK if I have a listen to your chest?’, which demonstrates the conditional ‘if’ and implies the possibility that the request will not be acceptable to the patient.
‘Middle’ levels of entitlement and contingency
Health-care professionals also requested actions, which were subsequently overtly refused, using questioning, modal verb formats, such as ‘would you . . .?’ and ‘can you . . .?’. In the literature,124 these are recognised as having higher entitlement than ‘wondering’ requests. The modal verbs will/would and can/could invoke the patient’s willingness or ability to engage with the request.
Prior to the following exchange, the health-care professional had spent many minutes trying to verbally encourage and physically support a person living with dementia to eat his lunch, as he paced the ward, refusing to sit down. An example of a ‘would you’ request format then follows:
The patient chooses to emphatically decline more food. By posing the question in a ‘would you like?’ format, the option to decline is presented and signals ‘not liking’ as a possible contingency on which basis the patient has the choice to accept or decline.
Health-care professionals also prefaced requests with ‘can you’ prior to a number of overt refusals, with the modal verb here referencing the patient’s ability to agree to the request. In the following extract the doctor is attempting to listen to the patient’s chest with a stethoscope:
As is frequently seen in this data set, the health-care professional’s request for a new action by the patient is formatted as a question of ability, ‘Can you take a deep breath in and out?’. Although this would typically (and normatively) be treated as a request for the patient to start taking deep breaths rather than a query as to whether or not they are able to do deep breathing, in this case the patient’s initial blunt ‘no’ response does not clearly differentiate. If the patient had said ‘no I can’t’ or ‘no I don’t want to’ this would have clarified the basis for the refusal. The health-care professional handles the response as if it was declined because of a lack of perceived ability by encouraging the patient to ‘just try’ (line 205). The patient’s response at line 206 clarifies that her refusal was based on her ‘thinking’ (indicating some uncertainty) that she may be unable to, possibly because of the back pain she had reported. By using the format ‘Can you do . . .?’ the health-care professional has introduced the possibility of a yes or no response, and their use of ‘can’ suggests a potential contingency whereby the patient may be incapable of breathing deeply.
In another example, a health-care professional uses a construction that potentially references both capability and willingness to propose a walk for rehabilitation purposes:
The health-care professional’s format here offers an inbuilt justification for refusing the proposed activity, namely that Margery won’t ‘feel up to it’, which she then confirms as the reason. ‘Feeling up to’ doing something inherently suggests both willingness and ability, and the patient could decline on the basis of either.
It can be argued that the health-care professionals in these cases demonstrated higher levels of entitlement and less orientation to patient contingencies than in the ‘I wonder if?’ and ’Is it alright if?’ prefaced requests, as is argued by Curl and Drew122 in their comparative study of these two types of requesting. In the modal requests, the contingencies of willingness or ability are exhibited but not necessarily presented as problematic, and in this way the health-care professionals may not be projecting a refusal as strongly as in the ‘wondering if’ requests.
High entitlement and low contingency requesting
Most requests prior to an overt refusal were characterised as either low or medium in entitlement. The only exceptions were found during a single encounter in which a health-care professional was attempting to complete a swallow assessment. In an encounter lasting approximately 12 minutes, the patient refused (either verbally or non-verbally) almost all efforts to give him something to either eat or drink, despite the health-care professional employing multiple physical and therapeutic strategies and interactional approaches. The most overt of these refusals occurred some time into the encounter, following requests that combined statements of intent with embodied requests, that is, presenting food or fluid to the patient’s mouth, as in Extract 11:
Although the patient’s speech at line 153 is difficult to decipher, the accompanying embodied refusal (line 154) and the health-care professional’s management of it indicated that it can be analysed as a refusal. The health-care professional’s verbal request, ‘we do it together’, following her indication of topic shift in the prolonged ‘so:::’, is formatted as an announcement of what will happen, without any projection of an option to refuse or accept, and without any overt reference to contingencies that might make the task arduous for the patient. The issuing of a request as a ‘bald imperative’ is a highly entitled way of requesting, and implies low or no contingencies. 129 In this case, the health-care professional also moved the glass towards the patient and thereby embodied a highly entitled directive, which the patient refuted with his emphatic ‘here none of that’ and his movement of the glass away from him. This example demonstrates that highly entitled requesting does not necessarily result in acceptance. However, as will be illustrated shortly, there are some key differences between Extract 11 and the ‘non-refused’ examples in the data.
Requests preceding mitigated refusals
Most requests preceding mitigated refusals were delivered in ways that referenced the patient’s ability or willingness to comply. When health-care professionals referenced ability in their request, patients who used mitigating accounts usually referenced an inability to comply in their responses, as in Extract 12:
Here, the health-care professional asked two consecutive modal questions, one permission seeking question with himself as the agent, ‘Can I have a little look?’, and one request for action, framed as a question about the patient’s ability to act, ‘Can you just march them up and down?’. In this case the patient was able to provide an (almost) fitted response in which she clarified why this suggestion was not feasible (lines 32–34).
There were no low entitlement requests preceding any of the mitigated refusals. There were some highly entitled requests, formatted as imperatives and usually delivered during an ongoing activity when a number of previous refusals had occurred. Most of these were refused by patients on the basis of a lack of willingness, as in Extract 13, taken from a later point in the swallowing assessment in Extract 11:
Request formats preceding acceptance
The analysis of requests that led to overt and mitigated refusals indicated that health-care professionals were mainly formatting these requests in a manner that presented the option of refusal. The hypothesis was thus formed that because higher entitlement requests project acceptance rather than refusal, higher entitlement requests may be more likely to lead to acceptance, all other things being equal. Although refusals did follow a small number of higher entitlement requests, these seemed to occur late on in long sequences of refusal, and verbal requests tended to be accompanied by physically embodied requests, when patients physically rejected an item or activity.
We therefore searched the data for health-care professional requests that were formatted to display higher entitlement. As the overall aim of the project was to identify effective communication strategies that may also be trainable, identifying highly entitled patterns of requesting was important (rather than simply identifying the negative consequences of requesting in warm but low-entitled ways). We found four types of request formats that displayed higher entitlement to ask and that preceded acceptance.
Announcements of future action
Some health-care professionals announced future action and intent through the use of the formats ‘I am/ we are going to’, or ‘I will’, such as ‘I’m just gonna pop this on for you’, ‘We’re going to sit on this chair here’ and ‘I’ll just pop your cardigan off’. Such formats were frequently followed by a checking, permission-seeking question, such as ‘is that OK?’ or ‘alright?’. This type of ‘announcement as request’ was recurrently used by one health-care professional during a swallowing assessment (Extract 14):
At line 266 the health-care professional announced the action that she intended to carry out ‘to’ or ‘for’ the patient, in this case to wipe the patient’s mouth. The manner of this announcement indicated a high entitlement to ask on the part of the health-care professional. She is implying that the action is going to take place and does not present an interactional space in which the patient could decline to engage with the activity.
However, the health-care professional significantly softens this highly entitled request with some important strategies. First, after a 0.6-second pause in which no patient response is forthcoming (line 267), the health-care professional explained why this action needed to be done with her account of the ‘white’ round the patient’s lips, demonstrating sensitivity to the need to qualify such requests because of their dispreferred nature. 123 The health-care professional was also orienting to their epistemic knowledge, which the patient appears to lack, that there is something around her mouth that she has not removed herself. 130 Antaki and Kent129 theorised that some requests in their data (residential care interactions with people with intellectual impairments) might have been completed more efficiently if a rationale had been presented first. Even though the explanation in Extract 14 followed the request, it appeared to come as an immediate response to the pause in the encounter, suggesting that the health-care professional was sympathetic to the patient’s need to have an explanation for the action.
Second, the health-care professional follows her request and explanation with a permission-seeking, or checking, question at line 268, ‘is that OK?’, which she repeated and pursued for a response at line 269. This checking question provided space for the patient to acknowledge that they were ‘not OK’ with the proposed intervention, and thus softens the highly entitled approach, re-establishing the patient’s right to permit or not permit the proposed activity. However, this form of question strongly prefers an affirming response, and this request format using an ‘announcement and checking question’ is followed by assent in every case in our data set.
Third, the health-care professional alluded to contingencies in her downgrading of the task with the minimisers ‘just’ and ‘little’ within her announcement (line 266), ‘I’m just gonna give your mouth a little wipe’. These items work to display the task as less onerous for the patient and, therefore, indicate a lowering of contingencies. Such practices occurred frequently in this data set. This counters Antaki and Kent’s129 notion of ‘bald imperatives’, whereby the speaker takes no account of how engagement with the request may impose on the patient.
In this extract, the context was an encounter in which the patient’s and health-care professional’s goals appeared to be mostly aligned. However, this mode of requesting also occurred in situations in which a patient had previously indicated reluctance to engage with a proposed activity. In Extract 15, the patient had declined to be shaved within the previous hour, when it had been proposed by a nurse in his bay. The video-recorded encounter captured a specialist mental health nurse engaging with the same patient about shaving, and, after their initial discussion, the subsequent conversation occurred as they walk side by side:
Here the health-care professional employed the technique of ‘announcement’ to present the activity as about to happen, with the downgrades ‘just’ (line 20) and ‘quick’ (line 21), and qualified why it might be relevant (‘to get you ready for the day’), before the permission-seeking question ‘is that alright?’. The health-care professional appeared cognisant that the task might appear onerous to the patient, but is ‘selling’ the perspective that this is not the case. Therefore, it would seem that the health-care professional has optimised the chances of assent from the patient, and, in the context of previous refusal, the patient appeared to agree to the activity at this juncture without objection.
Proposals
Health-care professionals also formatted requests as proposals or suggestions for joint activity using ‘let’s’, as in Extract 16:
This extract is taken 4 minutes into an encounter during which a health-care professional has been encouraging a person with dementia to have a drink. ‘Let’s have another go’ alludes to the previous repeated attempts at the activity and presents the activity as shared, one that they will complete together. The health-care professional has been supporting this patient with feeding as he was no longer able to eat or drink independently, using strategies known as a ‘hand over hand’ technique to take the cup to the patient’s mouth (the health-care professional’s hand is placed over the patient’s hand to guide or assist). The process of taking a drink became a combined effort for the health-care professional and patient. The use of ‘let’s’ displays high entitlement to request that the patient participate in the health-care professional’s activity, and uses a persuasive strategy that we might use in everyday talk when trying to recruit someone to do an activity that we want to do ourselves. The option to decline the invitation is not projected, and an ‘OK’ type response is strongly preferred. However, the projection of the activity as a communal one gives the ‘let’s’ format an ‘invitational flavour’, which West131 suggests proposes a more symmetrical relationship between speakers. It fits the search in this data set for more highly entitled ways of requesting that maintain a sense of respect for the patient.
Statements of need
At times, health-care professionals used an announcement of their own needs or the needs of the patient as a form of request. On some occasions, this was difficult to disentangle from statements of need with different functions. In Extract 17, the health-care professional followed up her repeated statements of need (at lines 61 and 66) with a permission-seeking question, ‘is that alright?’, indicating that, on this occasion at least, the statement of the health-care professional’s need was issued as a request for permission to act:
Presenting their own needs as a justification for requesting an action that is in the patient’s interest indicates an extremely high entitlement on the health-care professional’s part. West131 describes (mostly male) GPs frequently instructing patients what they ‘needed to’ (or ‘ought to’) do and characterised this as an ‘aggravated directive’ that was more likely to trigger an ‘aggravated response’. However, in this encounter, the health-care professional characterised or packaged the entire activity as one that she (the health-care professional) needed to carry out and required the patient’s permission to do, and that she would not (and did not) undertake until the patient had agreed. The high entitlement was softened by the health-care professional’s respect for the patient’s autonomy, to allow or not allow the activity to proceed, demonstrated by the checking question ‘is that alright?’.
Direct instructions
Health-care professionals also used direct instructions, or ‘bald imperatives’ as classified by Antaki and Kent,129 when requesting actions of patients. These were constructed with no visible subject, as in ‘have a little drink’ or ‘take a step’, and were used most frequently as part of a sequence of instructions, as demonstrated during an encounter with a physiotherapist:
The health-care professional assisted the patient to walk down the ward and return to her bedside, where she was required to turn around using her frame before seating herself back into her chair, with support and direction as needed. This extract shows a sample from a longer sequence of instructions issued in this manner during this walking activity. Such instructions or ‘commands’ display very high entitlement to ask, and the patient is offered no option to decline. In the literature,129 such instructions are typically considered to lack sensitivity to the recipient’s contingencies, and to express no doubts about the speaker’s entitlement to make the request.
However, in this data set, the health-care professionals use these formats in specific circumstances and in specific ways, which could be considered to ‘soften’ the high entitlement instruction. Many of these instructions were issued during an ongoing ‘agreed to’ activity, for example walking up and down the ward for therapeutic purposes. It appears that once the patient had agreed to ‘try’ walking early on in the encounter (despite some reluctance), the health-care professional could then issue a set of clear and simple instructions to the patient, which she promptly complied with, without any further need for the health-care professional to negotiate each instruction with reference to choice.
Many of the imperatives in the data were issued with reference to contingencies within their construction. In Extract 19, the same health-care professional oriented to the effort involved for a different patient by referencing ‘trying’ in lines 211 and 218:
Indeed, in many encounters, a health-care professional’s only use of the imperative format was in the context of encouraging a patient to ‘try’ something, following some orientation (by either speaker) to difficulty carrying out the task. For example, in the context of persuading a patient to drink more, following a successful sip, the health-care professional asked:
Requesting that a patient ‘tries’ to do something (rather than baldly doing it), displays the health-care professional’s sensitivity to how the patient may experience difficulty completing the task, and orients to the health-care professional not needing success from the patient but, rather, effort.
Health-care professionals also used ‘just’ as part of direct commands, orienting to the requested task as one that might not be as arduous as expected, as demonstrated in these examples:
Managing reluctance: health-care professional responses to patient refusal
Building on our previous analyses, we turned our attention to sequences in which an action is initially refused by a patient, but in which the health-care professional attempted to proceed with the task in the patient’s best interest. We aimed to identify what communication strategies health-care professionals use when they encounter reluctance and refusal from patients. Following the initial request (regardless of how it was formatted), which precipitated a refusal from the patient (mitigated or not), health-care professionals were presented with the dilemma of how to encourage a person living with dementia to do an action (or to allow the health-care professional to do it), while recognising and respecting that individual’s right to choose to accept or decline.
From our analysis, we identified two distinct practices used by health-care professionals that were more likely to precede task achievement:
-
raising the entitlement of the request (e.g. moving from ‘I was wondering if . . .’ to ‘Let’s . . .’)
-
lowering contingency (e.g. specifying the duration or location of an action).
Further analysis of extended sequences in which there was an initial refusal indicated that health-care professionals used both of these practices in an effort to get a more accepting response from the patient. Requests were reformulated in ways that less strongly projected refusal. Contingencies were lowered, sometimes in ways that specifically addressed a patient’s initial refusal (e.g. specifying that standing up would only be brief), and sometimes in more generic ways that downplayed the apparent scale of the task, using minimisers such as ‘just’ and ‘pop’. However, such progressions were gradual and respectful of the accounts given by patients for refusal. Our analysis indicated that, by varying the levels of entitlement and contingency, a negotiation process was facilitated through which it was then possible to achieve task completion. Nonetheless, there were still times when a task could not be completed, which would be in keeping with an environment that was respectful of the personhood of a person living with dementia. Our analysis identified approaches that were more likely to have a successful outcome, and not a means to achieve a task at all cost.
Closings
The second distinctive feature of these encounters focused on the closing phase, when recurring interactional difficulties in bringing encounters to an end were observed, alongside examples of more successful closings.
Existing data from CA studies of closings in face-to-face health-care interactions have mainly come from primary care, for example Heath,132 Robinson133 and West. 134 The nature of primary care interactions means that typically the patient has identified a problem and voluntarily enters into the physician’s space for an appointment. Heath132 talks of the consultation ending as ‘bringing the business to a satisfactory closure’. It is the doctor who signals the closure of the interaction, either with a summation of the problems and arrangement making or through issuing a prescription, but it is the patient who is required to orient to this and to physically leave the doctor’s space. 132 The patient usually responds to the closing signals but may then present unmet needs or residual symptoms, sometimes referred to as the ‘door handle’ or ‘by the way’ phenomenon by doctors. 135
These existing analyses have less relevance in the current setting, because in acute hospital interactions, typically the health-care professional enters the patient’s environment (bedspace), usually without invitation from the patient. The patient may be unclear that there is an issue to be addressed, and this is further intensified for people living with dementia, who often lack insight into where they are and any medical problems they may have. It is also possible that the impaired linguistic ability associated with dementia may lead to missed closing cues or a failure to recognise them. In addition, in a typical acute hospital setting, the encounter ends with the health-care professional physically leaving the patient’s space.
Our analysis of closings has been published136 and is summarised in the following sections.
We identified three phenomena around which there were recurring troubles in the closing phase of our encounters: open-ended pre closings, mixed messages and non-specific language.
Open-ended pre closings
In this setting, open-ended questions seeking to elicit any additional patient concerns (e.g. ‘Can I do anything else for you?’ or ‘Is there anything else you want to ask me while I’m here?’) could extend the closing of the interaction in a problematic way. Patients indicated confusion and sought clarification of the kind of answer that might be expected, or produced non-relevant answers. These sometimes referenced issues that could not be addressed in the health-care context. We acknowledge a tension for health-care professionals in that professional training advocates checking if a patient has any other concerns to be addressed before terminating a consultation. 137 The concept of person-centred dementia care compounds this,33 in that the question potentially orients to patient autonomy and gives the patient an opportunity to influence the agenda. However, in our data, two factors contributed to problems. First, the acute care patient does not initiate the interaction with a health-care professional and is not motivated by a problem they (the patient) wish to discuss; they are routine clinical encounters, carried out in a patient’s best interests, and, perhaps as a consequence, oriented to an imposition on the patient’s time (leading to health-care professionals constructing pre closings such as ‘I’m gonna leave you be’). Second, because of cognitive impairment, people living with dementia in this setting appeared to genuinely lack insight into the purpose and scope of questions, such as ‘Is there anything else you want to ask me?’, in the context of an encounter they had not initiated, when they may not understand that they were unwell, or even that they were in hospital. In this context, we recommend that it is best to avoid such open-ended closing questions probing further patient concerns.
Mixed messages
Mixed messages (e.g. telling a patient you are going to leave, but then having another attempt at an activity, or giving a verbal indication that an encounter has finished but remaining seated) appear to indicate that it can be difficult for a health-care professional to know when to leave a person living with dementia. It is plausible that a health-care professional may wish to try to complete a necessary, but abandoned, health-care task following patient refusal. However, other examples suggested that a protracted closure was linked to a person living with dementia’s lack of orientation to the health-care professional’s attempts at closure. Conversely, some examples indicated that the health-care professional did not quickly progress to a final closure exchange, despite indicators that the patient had oriented to the upcoming closure. This led to continued talk on a patient’s own topic of conversation, which was often beyond the remit of the encounter. The end result could be the health-care professional walking away as the patient continued to talk, or, on occasion, explicit orientation by the patient that the continued talk was unwanted.
Non-specific language
Our analysis also exposed the problematic nature of ambiguous language and vague or indeterminate terms to signal upcoming closure. Pre-closing moves such as ‘I’ll see you soon’, which are common in everyday discourse, can confuse a person living with dementia about the timing of any future encounter. In this context, we propose that concrete arrangement making (e.g. ‘I’ll see you tomorrow’) is preferred. Further investigation could disambiguate whether this is just a concern for people living with dementia or whether it is a wider issue for the acute hospital setting where patients see frequently changing health-care professionals across the time span of an admission.
Successful closing practices
The analysis of closings identified three sources of potential interactional trouble to avoid. We therefore recommend the converse positive practices: using consistent verbal and non-verbal indicators of closing, and concrete arrangement making. In more successful closing encounters, two further positive practices supported closing: making explicit pre closings and using idioms.
Explicit pre closings
When coming towards the end of a health-care task, health-care professionals sometimes gave the patient direct, explicit indications that the interaction was coming to a close. These included explicit notifications ahead of a final task (e.g. Extract 14: I’m just gonna give your mouth a little ↑wipe . . . and then that’ll be us all done) and explicit announcements of completion of the health-care professional’s final activity (e.g. Extract 14: now, that’s us all done).
Idioms
An idiom is a ‘saying’ – a phrase that has a meaning beyond the actual words it contains. Idioms are often used in everyday conversations to end one topic and allow a shift to another. After completion of the health-care professional’s tasks, if the person living with dementia reopened talk, some health-care professionals successfully used idioms to shift the encounter almost immediately to the terminal closure (e.g. 135_208: we’ll keep a close eye on things). These idioms functioned, as in other everyday talk, to acknowledge the person living with dementia’s contribution, briefly leading to affiliation and agreement between the interactants and facilitating mutual termination of the person living with dementia’s topic. 138 Other examples in our data set included ‘all done and dusted,’ ‘never say never,’ and ‘good luck’.
Discussion
The study reported here set out to identify effective communication practices that health-care professionals used when interacting with people living with dementia in an acute hospital setting. From analysis of > 6 hours of data from a range of professional groups interacting with people living with dementia in this situation, a flexible phase structure for the encounters was identified. Two areas of interactional ‘trouble’ were identified for detailed analysis, namely how health-care professionals achieved important health-care tasks, particularly in the face of patient refusal, and how health-care professionals closed encounters.
Requests in the data set could be usefully interpreted in terms of the framework of entitlement and contingency, as developed by Curl and Drew. 124 Higher entitlement ways of requesting, which avoided the projection of a ‘no’ response in their requesting, appeared to support co-operation with the health-care professional’s requests. It is possible that by delivering a request in a manner that communicated a confident expert authority, health-care professionals enhanced the patient’s implicit knowledge of the importance of the request. As well as using higher entitlement, health-care professionals referenced the contingencies (or difficulties) for the patient, but explicitly lowered them. In doing so, the health-care professional oriented to the challenges facing the person living with dementia and demonstrated their intent to make the activity as undemanding and straightforward as possible. Offers to help, framing the action as a joint collaborative endeavour, minimising the task size, duration or frequency, and suggesting that the patient ‘try’ all served to lower the contingency. Health-care professionals did not communicate an absolute right to demand the actions of patients, but clearly indicated in their referencing of contingencies that the patient’s needs, abilities and wishes should be considered.
The prevalence of refusal and reluctance in the data prompted us to consider the ensuing predicament that, it appears, health-care professionals regularly face when caring for people living with dementia in hospital. The health-care professional who aims to provide person-centred dementia care will want to value the individual’s personhood and autonomy and respect their opinions and wishes around their health-care choices. 33,128 However, the health-care professional knows that the individual may lack the necessary information about or understanding of the action, or its consequences on their health or welfare, to make a fully informed decision. In contrast, the health-care professional is aware of how failure to complete the task might affect the person’s well-being. Typically, the health-care professional cannot complete such tasks without the active or passive co-operation of the patient. Therefore, the health-care professional needs to balance how they encourage the patient to comply with a course of action with acknowledging their concerns.
‘Person-centred’ care is often contrasted with ‘task-centred’ care,128 but it is our contention that achieving important health-care tasks and person-centred dementia care are not mutually exclusive. The project’s PPI representatives, having cared for people with dementia, attested to facing similar dilemmas, for example when encouraging their relatives to drink or take medications. When an activity is deemed to be in the person living with dementia’s best interest, the supporting person uses a variety of strategies to motivate and encourage the person living with dementia to comply with the request, with a minimum of distress. Our analysis has sought to explicitly identify what these strategies might be, and their relative effectiveness.
No single way of requesting will always lead to an acceptance or agreement, the patient’s agency being primary. However, in identifying what requesting practices ‘do’ in interactions, we aimed to specify this knowledge so as to better inform health-care professionals of communication practices that could enhance their interactions with people living with dementia.
Analysis of closings revealed a common theme of interactional trouble, with the recurrent use of open-ended pre closings, mixed messages and using non-specific and indeterminate future arrangements. These practices are not necessarily inherently interactionally problematic. In settings where patients do not have cognitive impairment, they may not precipitate trouble and, therefore, our recommendations should be taken in context. Our findings emphasise the importance of context in the analysis of health-care delivery, and the limitations of blanket recommendations. Our findings also identify a need to examine best practice guidance as it is actually produced in interaction, using methods that can unpack the interactional detail involved.
Our analysis also highlights the recurring tension in this setting between seeking to treat people living with dementia as full agents who can collaborate in joint communicative projects, and adapting communicative practices to take impairment into account. People living with dementia demonstrate a wide range of communicative abilities and these abilities can vary with time and context, which introduces another level of complexity to any interaction with them. It is feasible that practice could be improved, for example by helping health-care professionals to develop an awareness of the possible implications of using different closing practices with different patient groups, and by explicitly acknowledging the difficulties that an orientation to more generic person-centred practices can create when communicating with people living with dementia.
Chapter 4 Intervention development
Introduction
Having uncovered new evidence about what communication practices might usefully be changed, we next sought to establish how these practices might be changed through a training intervention. The aim was to develop, through a transparent and robust process, a complex intervention that was ready for feasibility testing. 139
‘Intervention development is seldom a fixed prospective linear process’. 139 In common with other intervention development studies (e.g. French et al. 140), the process described in this chapter was complex, time-consuming and resource intensive. A number of intervention development approaches were used to support development, but in practice the process was iterative, messy and unique.
This chapter describes what actually happened, with accounts given for what was done, by whom and why. The aim is not to publish a description of the intervention that would make the intervention completely replicable, but to make the decision-making processes transparent and to justify the educational approaches that were taken. The output is presented in Findings (what was decided and why), using the structure of the Template for Intervention Description and Replication (TIDieR) checklist for intervention description. 141
The objectives for intervention development were to produce a well-described intervention with learning outcomes based on empirical research. It had to be underpinned by relevant theory, but feasible within the practical constraints of the project. We set out to develop the intervention using an explicit process of expert consensus, and to evaluate it as robustly as was practicable.
Methods
Participants (who made the decisions)
We convened an intervention development team that met for 4 half-days over 4 months. The intervention development team was set up to specify explicit learning objectives, consider evidence on what and how to teach, discuss how to apply this in practice and reach consensus on training intervention components.
All members of the study project management group (PMG) (the coapplicants and collaborators) were invited to join the intervention development team, except for one member who preferred to remain impartial, as she would be carrying out interviews as part of the evaluation of the training. The intervention development team contained health-care professionals, clinical academics, academics, educationalists and carers of people with dementia, with expertise in:
-
medical, nursing, AHP and interdisciplinary clinical education in the NHS
-
dementia and acute hospital care
-
communication skills training
-
CA and the use of ‘real’ video data in training
-
simulation in health-care education
-
electronic (computer-aided) learning.
Two local experts in communication skills training in health care were individually interviewed by the research speech and language therapist. One was a consultant in palliative medicine, who had experience of running a simulation-based interdisciplinary communication skills training course in end-of-life care (Dr Patrick Costello, Nottingham University Hospitals NHS Trust, 2016, personal communication). The other was a lecturer in nursing, who had evaluated a video teaching resource based on CA findings and recordings [also in end-of-life care (Dr Becky Whittaker, University of Nottingham, 2016, personal communication)].
The study steering committee (SSC) was consulted at two points during intervention development to provide an external independent perspective, helping to mitigate against risk of ‘group think’, a potential problem in processes that seek consensus. 139
Processes (how the decisions got made)
The process of intervention design intended to synthesise existing evidence, new evidence, and educational, clinical and experiential expertise to produce a training intervention, illustrated as a four-stage process (Figure 2).
FIGURE 2.
Process of intervention design.

Inputs were findings from the systematic review of communication skills training in dementia care, the CA study and the interviews with local experts.
The systematic review identified a variety of candidate components, including content (what needed to change) and theories, teaching methods and modes of delivery (possible mechanisms for change). However, the quality of the reviewed studies was (at best) moderate and few of these studies were in acute care. Findings could not necessarily be taken at ‘face value’ and used in a new intervention, but needed further consideration and interpretation. 142 This was done by producing tables of components (duration, theoretical underpinnings, and teaching methods and modalities) for critical appraisal and discussion.
The CA findings identified new empirical knowledge about what effective (and less effective) communication looked like. Findings were summarised by the CA analytic team into a list of ‘potential trainables’ (see Chapter 3), but these were not sufficiently refined for training delivery. For example, they had not been considered in detail for relevance, acceptability and intelligibility by an audience unfamiliar with CA. Findings on ‘requests and refusals’ and ‘closings’ were discussed by the intervention development team. Others had previously described the application of CA findings in a group training context. 143
The interviews with local experts in communication skills training complemented the knowledge of the team. Interviewees shared experiences and opinions on questions about intervention design, including benefits of simulation; CA video methods; practical aspects of simulation and video methods (setting up, duration, feeding back, making ‘safe’, use of video and playback within simulation); training group size and composition; trainer and facilitator expertise required; recruiting to, and administering, training courses; methods of evaluation; and promoting the implementation of learning.
The intervention development team was the mechanism through which consensus was sought and intervention decisions were made. Most decisions required further deliberation or work following an intervention development team meeting. A core team undertook this, comprising three clinical academics (RO’B, SG and RH), who had considerable experience across medicine, nursing and AHP roles, working clinically with people living with dementia in acute settings, and in training and education across professional groups.
The work of ‘operationalising’ training included preparing materials and resources. Particular attention was paid to preparing simulation exercises. This was done through six meetings between the lead simulator (MM) and research speech and language therapist (RO’B), with variable involvement of the rest of the core team. The lead simulator produced provisional scenarios for the SPs, which were developed and amended through iteration in collaboration with the core team to ensure their clinical authenticity.
Findings (what was decided and why)
The intervention development team agreed specific learning objectives for the communication skills training:
-
To enable the health-care professional to reflect on, and analyse, his or her own communication, and that of others, when interacting with people living with dementia in the acute health-care setting.
-
To enable the health-care professional to synthesise new and pre-existing knowledge about communication into his or her own clinical and personal context, in order to create new practices.
-
To be able to identify and deploy flexibly a variety of effective communication practices when interacting with people with dementia in the acute health-care setting.
Following the pilot course we derived specific learning outcomes from these objectives:
-
analyse specific aspects of my communication
-
combine what I already know with new knowledge from the course
-
apply this knowledge creatively to me in my clinical interactions
-
be able to flexibly use a variety of effective communication practices.
The TIDieR checklist encourages clear specification of the core components in order to support implementation and replication. As well as reporting what was decided in the development of the intervention, the checklist makes explicit how and why each decision was made.
Name of intervention
The title used during the delivery of training was ‘VOICE for Dementia’. A suitable name was required to support recruitment to the pilot and feasibility studies, and for future implementation of a potentially ‘marketable’ training course. A clear intervention name assists in identifying connected studies, as well as giving an indication of the type of intervention described. 141
Why: the rationale, theory or goals essential to the intervention
The rationale, theory or goals help to identify the ‘active ingredients’ that mediate anticipated changes, clarifying which components are essential. 141
Educational theories, relevant to communication skills training for health-care professionals, that could underpin the intervention were considered.
The systematic review identified a variety of educational and other theories. Two papers gave extensive consideration to educational theories, models and frameworks,78,81 including the ‘learner-centred classroom’, Knowles’ principles of andragogy,103 and reflective practice. 144 Beer et al. 78 supported their description of specific learning activities with references to a variety of educational theorists, including transformative learning theory145 and the motivational framework for culturally responsive teaching. 146
An experiential learning approach was chosen, based on Kolb’s experiential learning cycle147 and the need to support different learning styles. The theories cited in the systematic review (such as andragogy, reflective learning and transformative learning theory) were part of, or derived from, the experiential learning tradition. 148
What: procedures, activities and processes used in the intervention
The ‘activities’ or ‘processes’ comprise the training approaches used.
Content
The content of the training was designed around the new empirical findings from the CA (Table 8). The issues for discussion were how these potentially complex linguistic findings could be distilled and ‘translated’ for a varied health-care professional audience, and how much other content there should be about communication and dementia, based on other research or approaches.
| Sources of evidence | Summary of key considerations for intervention design (content) |
|---|---|
| CA findings |
|
| Practical considerations |
|
| Systematic review |
|
| Expert opinions (Dr Patrick Costello, personal communication and Dr Becky Whittaker, personal communication, 2016) |
|
The CA findings were validated by the clinicians as being highly relevant. PPI members concurred, identifying with the challenges of trying to get important tasks done when a person living with dementia was reluctant, and partings in various contexts. They also agreed that these situations were important to their relatives when unwell in hospital. Clinicians and PPI intervention development team members felt that the findings described communication practices they had not been aware of before and, therefore, regarded them as highly relevant content for the communication skills training.
The intervention development team discussed the number of communication practices that could feasibly be trained in one course. Previous CA-based training has focused on a few practices only. 67,149 We wanted to address two quite independent areas of trouble in the interaction, which involved presenting seven (closings) and 10 (requesting) communication practices to try or avoid. ‘Requesting’ in particular necessitated introducing some complex concepts.
The phase structure of encounters was felt to be helpful for health-care professionals, and there was a desire to present training in a logical ‘openings’ to ‘closings’ structure. Video material on openings was used to orientate trainees to encounters, introduce the experience of learning and encourage self-reflection on communication practices. Simplification was achieved by grouping requesting practices together under the three headings in order to help trainees better identify and remember specific practices: (1) ‘raising entitlement’, (2) ‘lowering contingencies’ and (3) ‘making the task explicit’.
Person-centred care in dementia was presented as an underpinning philosophy,128 but the intervention did not specifically teach on person-centred care. Three openly available e-learning resources relating to dementia,150 person-centred care and communication,151 previously developed by members of the study team, were recommended as pre-training preparation for trainees, who were encouraged to use these resources if they felt it necessary to revise more basic concepts, allowing the intervention to focus on new content. The person-centred care philosophy was emphasised by the facilitators during group discussions and the simulation and video workshops.
Teaching methods
Simulation
Simulated patients are professional actors who represent patients for the training or assessment of health-care staff. This is in contrast to ‘role play’, in which trainees enact roles other than their own. The possibility of professional simulation being used in the new intervention was proposed in the funding application, which included collaborators with expertise in this. The systematic review highlighted simulation and role play as key teaching methods in nine of the 26 studies, including one in which simulation was the sole teaching method,81 with significant gains in the confidence of medical trainees. We decided to use simulation as an experiential learning method, which would give trainees the opportunity to practice skills in ‘real time’ interactions.
The use of simulation in communication skills training has been challenged by a number of authors, however, who have identified ways in which simulated encounters may be ‘inauthentic’ because of systematic differences from naturally occurring interactions. 152–154 Despite potential limitations, simulation was viewed as the best method available for the online practice of new communication skills, particularly for patients with communication and cognitive impairments who could not themselves be easily trained to give feedback. Simulation is reported to be the part of training that trainees remember and value most.
Good-quality simulation has the potential to involve the whole of Kolb’s experiential learning cycle147 (Figure 3). The simulation represents a concrete experience, created as an opportunity from which the trainee can construct their own learning. Opportunities need to be given for the trainee to reflect on their own performance and also to observe and reflect on fellow trainees (reflective observation), which can be particularly valuable for trainees who are reticent or anxious about simulation. Trainees can be given the opportunity to think about and to try to make sense of their experience (abstract conceptualisation), through interpreting their previous knowledge and experience of communication, and through the input from the study findings, in the light of their new experience. Finally, they can be offered an opportunity to actively experiment through rerunning the simulation or discussion with fellow trainees and facilitators, and trying the practices out in their real clinical contexts.
FIGURE 3.
Kolb’s experiential learning cycle.
To address concerns about authenticity, we undertook to develop the simulation in innovative ways using the CA findings and data. 155 The process involved considerable consultation and development work. The research speech and language therapist (RO’B) selected suitable potential participants with dementia from the original video data, and these videos and transcripts were viewed by the researcher and lead simulator. ‘Scenarios’ were developed, the character was given a fictional name and clinical and social history, with descriptions of their retained abilities, appearance, demeanour and manner of speech. Unusually for simulations, we developed additional information for the simulator about the person’s typical interactional patterns, based on close scrutiny of the videos and transcripts and in the light of the CA findings and training content. Some examples are given for the simulation role of ‘Annie’ (Table 9).
| Response | Speech |
|---|---|
| You sometimes produce a lot of speech – which doesn’t make sense | . . . they’ve normally got . . . packages in cardboard on end . . . so they don’t break the points off . . . it was just bare like that . . . and it was about that day, Saturday . . . down there . . . and bent over . . . straightened it out . . . display . . . |
| In response to health-care professional requests: | |
| You question request | What for? |
| You do not agree at first | Hmmm |
| You agree eventually if request is clear and direct | OK |
Once the final scenarios had been agreed as acceptable by the intervention development team, they were sent to the simulators for comment and familiarisation (see Chapter 5).
Simulation has to be properly facilitated, with appropriate support for trainees. Simulation can provoke anxiety, which can inhibit learning. This was operationalised through:
-
building relationships from the beginning of training using participatory exercises (e.g. in pairs, valuing all members and all contributions)
-
building group identity for simulation work, keeping groups constant with the same facilitator
-
allowing trainees control over aspects of their simulation, such as choosing what task to carry out
-
encouraging trainees to pause their simulations (‘time out’) to give control and to allow for advice/ support mid-simulation from the group
-
organising feedback using Pendleton’s model,156 in which the trainee gives positive feedback on self, then facilitators and observers reinforce with further positives, before the trainee, facilitator and then observers make suggestions for change
-
encouraging trainees to replay simulations to experience their ability to control and change their communication behaviours.
Real video data
Use of video in communication skills training is not a teaching ‘method’ in itself, but is commonly used and was reported in 13 out of 26 studies in the systematic review (see Table 3). Some studies described how videos were used in teaching, referring to the demonstration of ‘good’ and ‘bad’ examples, usually staged with actors. Two studies78,96 reported the use of video of real-life encounters, showing ‘good practice’ either from expert trainers78 or from the everyday encounters of nursing aides. 96
As an alternative, conversation analysts have reported ways in which they have used analyses of real encounters as an educational resource (e.g. Kitzinger143) or in evaluations of interventions designed to change practices. 67,149 These methods have been developed into the training approach known as the ‘Conversation Analytic Role-play Method’ or ‘CARM’. 157
This approach involves pausing recordings at a key point in the interaction (e.g. after the patient’s refusal of a request) and asking trainees to consider, usually in small groups, what they might say or do next. After sharing and discussing suggestions, the recording is replayed to show what actually happened. Trainees then discuss the real response and consider how it led to the desired or undesired outcome. The trainee experiences the unfolding of an authentic interaction without knowing the outcome and is given the opportunity to ‘role play’ their responses. By using examples that play out in the direction of conversational travel that is not desired, and contrasting this with more positive examples, the trainee can experience and analyse for themselves what interactional approaches work best.
The CARM training approach can assist the trainee in experiencing the ‘disorienting dilemma’, in which their existing knowledge is challenged by something new. 158 Revealing the negative impacts of interactional practices motivates change, for example in communication partners of people with aphasia. 159,160 The importance of communication partners identifying positive alternative communication practices with which to replace the negative has been highlighted. 159 This process should follow naturally from the CARM approach, in which participants observe and analyse for themselves the interactional consequences of a variety of practices selected by the trainer to meet specific learning objectives.
An awareness of authentic practices that work better than others may not, without practice, be sufficient to change behaviour in real conversation. 161 We therefore decided to combine CARM-inspired techniques with simulation to give trainees the awareness of practices that might benefit from change, followed by skills practice and feedback. Practising communication skills with immediate feedback may work by increasing the facility with which the techniques are used, thus strengthening the trainee’s capability to change, whereas awareness building may be necessary for building motivation to change. 159,160
The research speech and language therapist completed ‘CARM’ training and used this approach to support the planning of the training schedule and resources. Short video extracts were selected to explain and illustrate each key learning point. Shorter clips were animated, with the ‘trainable’ words or phrases shown after the extract. This allowed the facilitator to involve the trainees in identifying the useful practices before confirming their findings with the animations (Figure 4).
FIGURE 4.
Sample slide showing animation of key words. Photographs reproduced with permission of Nottinghamshire Healthcare NHS Foundation Trust.
Three longer sequences (between 2 and 4 minutes) were used for ‘CARM’-style workshops. A sample slide is shown and stopped at the point for discussion (Figure 5).
FIGURE 5.
Sample slide from CARM-type video workshop showing CA transcript. Photographs reproduced with permission of Nottinghamshire Healthcare NHS Foundation Trust.

Reflective learning
Reflection forms part of experiential learning, described in Kolb’s cycle. 147 Simply experiencing something is not enough to learn from it. Reflection and reflective practice, developed from the work of Dewey162 and Schön,163 have been identified as core professional skills for health-care professionals. 164 Reflection is defined as ‘a metacognitive process that creates a greater understanding of both the self and the situation so that future actions can be informed by this understanding’. 165
The process involves ‘noticing’ an event of interest, using a critical reflective stance and applying insights to further situations. A systematic review of reflective learning in the education of health-care professionals found that reflection enabled deeper learning and improved the integration of new learning with existing knowledge and skills. 164
We required health-care professionals to become more aware of their communication practices, critically evaluate where their practices and those of colleagues work well or less well, and then integrate new knowledge in order to develop enhanced communication skills.
Teaching activities that provided opportunities for reflective learning were therefore used. This included the training being split over 2 separate days, with 1 month between sessions. A PPI contributor suggested that a reflective diary might promote the implementation of communication strategies in everyday practice. This was refined into a guided reflection; trainees were asked to reflect on an interaction that had gone well and one that had gone less well. This was thought to be a more realistic request to make of busy health-care professionals than an open-ended ‘diary’ of events. Two reflective models were suggested, a classic descriptive reflection model (what happened, how it felt, what went well and not so well, what else you could have done, and how you might handle it differently next time)166 and a model based on content from the training (‘Did you request any actions of the person living with dementia? Did you try any of the VOICE techniques for requesting? How did they go?’). Trainees were informed that they would be asked to share their reflections when they attended the second day of the course.
The sharing of the reflective diaries took place in groups of up to five trainees, with a facilitator to support, encourage and challenge. Facilitators drew out from trainees’ reflections any learning needs for that individual for the second day of the course, as well as any questions or challenges for the whole group.
Small group discussion
Simulation, annotated video clips and reflection all incorporate elements of small group discussion. All the studies in the systematic review that delivered group-based training mentioned using group ‘discussion’, ‘activities’ or ‘exercises’. Active participation is required for deep learning to take place,167 which may include discussion among peers. The size of the group may support or inhibit involvement; an optimal ‘small group’ is often regarded as between six and eight members. 168
Although early models of experiential learning and adult learning theory emphasised the individual learner,147 social learning theorists highlight the importance of the social context in which learning occurs. 169,170 ‘Supported participation’ and ‘constructive discourse’ are ‘collaborative learning opportunities’ that help learning. 158,171,172
The VOICE training incorporated much small group discussion. Interactive group tasks were designed to encourage active participation in a non-threatening way. Small group facilitators required skills in managing group dynamics and facilitating trainees to participate fully through a combination of listening, questioning and responding. 168
Tasks were also designed as ‘buzz groups’ for pairs of trainees to discuss topics together, with the facilitator’s role being to answer queries and lead feedback in plenary sessions. Discussion activities in pairs involve all trainees and contribution from the whole group, whereas open questions tend to get responses from only a few. Buzz groups use time efficiently, allowing trainees to enter into more detailed talking and thinking together. The energy created by such activities is palpably different from the dynamic that follows the asking of a question to the whole group, but does require skilful management of timing and contributions.
Membership of the small groups for the first simulation was decided by the facilitators, aiming for a variety of perspectives based on factors such as professional group, level of seniority or experience and apparent confidence or anxiety.
E-learning
Internet-based educational approaches in health care have become increasingly popular. 173 A review of internet-based education in health care showed that it was as effective as conventional teaching. 174
Project funding included support for the development of an e-learning resource known as a ‘reusable learning object’ (RLO). A RLO has been defined as ‘an interactive, multimedia web-based resource based on a single learning objective that can be used in multiple contexts’. 175
‘Multimedia’ implies the use of audio, text, images and video in combination, and ‘interactivity’ means the involvement of the learner in exercises related to on-screen content, or interaction with other users or a trainer. Both interactivity and the use of multimedia content improve the effectiveness of online training. 176 Learners accept e-learning technologies best if they are easy to use technically, align with their values and norms, and are perceived to be an advantage over alternatives. The desirability of formative feedback and dialogue with others has been emphasised. 177
Two studies92,93 in the systematic review featured online training only, both describing an interactive, multimedia resource. These two interventions covered broad dementia-related learning objectives (not just communication), and required 2–4 hours’ time investment. They were well received by care home staff trainees, and had positive impacts on measures of knowledge and confidence. Both studies included the use of videos, which trainees evaluated as being helpful in learning new ways to care92 and valuable and ‘believable’. 93 Trainees liked the flexibility of delivery, but in both studies, internet access and information technology caused problems. When asked how to improve the training, some participants suggested that sharing ideas in a group would be more beneficial. 92
The RLO focused on a single learning objective, and aimed to provide around 15 minutes of learning. We intended to use a blended learning approach in which the RLO reinforced learning from the face-to-face training,178 consistent with a behaviourist model of learning, in which repetition and positive reinforcement are key to the retention of new knowledge and behaviours. 179 The opportunity for trainees to review and revise the recommended communication practices was offered through the use of ‘real’ video encounters, which had been consented for potential online use.
The focus of the first RLO was on ‘requesting in the face of reluctance’, as this was felt to present the greatest conceptual challenge (Figures 6 and 7). Relevant information was presented in text, audio and video (‘talking head’) formats to maximise accessibility. After each slide giving information, an interactive activity was used as reinforcement, using video and transcripts. Trainees were given immediate feedback on correct or incorrect answers and the chance to self-correct. In this way, trainees were given a summary and reminder of previous teaching, allowing for multiple repeats of information delivered in their preferred modality. ‘Testing’ and feedback from the activities gave trainees immediate, private feedback on whether or not they understood key learning points.
FIGURE 6.
Screenshot from Re-useable Learning Object (RLO). Photographs reproduced with permission of Nottinghamshire Healthcare NHS Foundation Trust.
FIGURE 7.
Screenshot from Re-useable Learning Object (RLO). Photographs reproduced with permission of Nottinghamshire Healthcare NHS Foundation Trust.

The process of developing the RLO was supported by the Health E-Learning and Media Team (HELM) at the University of Nottingham. A ’storyboard’ was written, assembling text, video clips, exercises and illustrations. The text was audio and video-recorded, allowing for background commentary and ‘talking head’ sequences.
Summary
Evidence supporting use of each teaching method modality is given in Table 10.
| Teaching method | Sources of evidence | Considerations | |
|---|---|---|---|
| Key for intervention design | Practical | ||
| Simulation | Systematic review |
|
|
| Individual interview (Dr Patrick Costello) |
|
||
| Expert panels (September 2016–February 2017) |
|
||
| Real video data | Systematic review |
|
|
| CA literature |
|
||
| Individual interviews (Ms Becky Whittaker) |
|
||
| Expert panels (September 2016–February 2017) |
|
||
| Small group discussion | Systematic review |
|
|
| Expert panels |
|
||
| E-learning | Systematic review |
|
|
| Expert panels |
|
||
| Reflective practice | Systematic review |
|
|
| Expert panel/s |
|
||
What: materials
Various resources were required to prepare training, but these were the items most often omitted from descriptions in an analysis79 of interventions used in randomised trials. 180
The learning content, including the interactive exercises and presentation of the video materials, was prepared in Microsoft PowerPoint® 2016 (Microsoft Corporation, Redmond, WA, USA) (see Figures 5 and 6). PowerPoint allows video/audio-recordings to be animated with transcript appearing as the words are spoken, thus enabling a CARM-type training approach in video workshops. 152 Online video resources were embedded to illustrate points.
Paper-based resources were also used. A ‘Summary of Recommendations’ card, listing the key training content on a two-sided A4 sheet, to be used as a reference for trainees both in the training and afterwards was produced following experience in the pilot course. One side showed the practices to ‘try’ and to ‘avoid’ in relation to requesting and closings, and the other side showed a diagram of the structure of the encounter, and a summary of person-centred care (Figures 8 and 9). 33,128
FIGURE 8.
Summary of recommendation on requesting and closing. Copyright Nottinghamshire Healthcare NHS Foundation Trust. Reproduced with permission.

FIGURE 9.
Summary of recommendations on structure of the encounter and person-centred dementia care. Copyright Nottinghamshire Healthcare NHS Foundation Trust. Reproduced with permission.
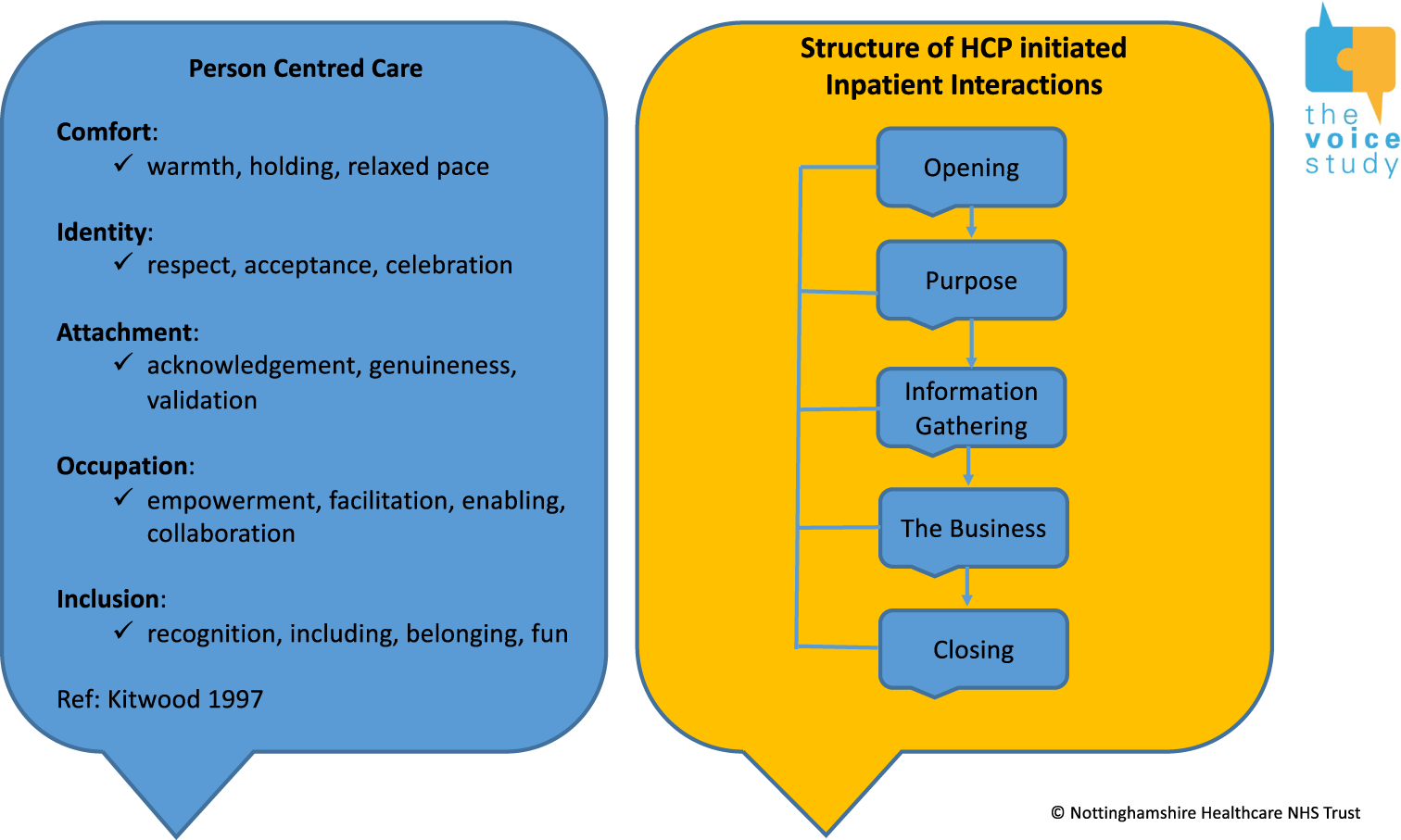
Trainees were provided with paper-based resources to support exercises, including a ‘reflective diary’, a written transcript for a video workshops and a sheet of questions to consider during a final, small-group, ‘implementation’ exercise. Simulation workshops were supported by descriptions of the scenarios, lists of tasks for health-care professionals to choose from, observation feedback sheets to guide trainees in their role as observers of simulation and guidance on simulation for facilitators.
Simulations also required ‘props’ to support authenticity, including hospital beds, chairs, bedside tables, blankets and pillows. Other resources supported the carrying out of selected tasks; these included a choice of drinks and biscuits and wash things, with access to water, cups and bowls. Simulation experts were clear that trainees should not be ‘pretending’ that something was there that was not (i.e. miming), because the health-care professionals should not be seen as ‘acting’ but rather as being themselves in their professional roles; only the simulator is truly ‘acting’.
The e-learning RLO forms part of the ‘materials’ of the course and are available online (lead author Rebecca O’Brien; see www.nottingham.ac.uk/helm/dev-test/dementia_and_request).
Who: provider
The intervention provider should be specified, as they can have an impact on how an intervention is delivered. 181 Educators experienced in simulation highlight the importance of facilitation and facilitator training. Formal and informal training opportunities were sought, including Master’s-level training at the Trent Simulation and Clinical Skills Centre, participating in simulations and receiving feedback from the lead simulator.
Training was led by the research speech and language therapist, an experienced clinician and clinical teacher. She delivered most of the didactic teaching and introduced the training exercises. Two facilitators were required for simulation and small group work; one of the two other members of the core team acted as a second facilitator. All were experienced clinicians and clinical educators. Two simulators were used per training workshop, with the lead simulator present to observe and support for the purposes of intervention development as a result of the innovative nature of using CA-informed simulation.
If the interventions were to be delivered by others, specific consideration should be given to replicating necessary requirements, including experience with the clinical care of people living with dementia and clinical education.
How: modes of delivery
Deciding how large training groups should be was part of the design process. CARM training suggested that a 1-day course can be run for around 30 trainees with small groups of up to seven. 157 Considerably smaller groups, and more time, are required to deliver high-quality simulation. On the ‘Dying to Communicate’ 2-day course, simulations lasted for 45 minutes per trainee, with five or six trainees per group being optimal. This course ran with up to 12 trainees, two simulators and two facilitators.
It was clear that simulation workshops could be allocated only to half days, allowing four trainees to have a 45-minute simulation each. It was estimated for the pilot that a maximum of eight trainees could be invited.
Where
The host hospital had a suitable simulation training centre on site and available. This centre had a ‘suite’ of three clinical simulation and training rooms, with relevant audiovisual equipment for teaching in one room, and two other rooms equipped with a hospital bed, chairs and other necessary props. A second local hospital also had a clinical skills training centre, but less availability, so training was undertaken in two rooms. Such clinical skills centres are commonly found across UK hospitals and nursing and medical schools.
When and how much
Multiple sources of input and competing needs contributed to the discussion on course length. Evidence on effectiveness would ideally inform decisions about duration. However, in both our systematic review (see Chapter 2) and that by Eggenberger et al. ,58 there was great variability in the duration of communication skills training interventions, and neither review had sufficient data to determine how the effectiveness of training might relate to duration. In the absence of definitive evidence, the decision balanced pressures of cost with the need for adequate intensity to be effective. Half-day training was difficult for ward-based nurses.
Training was planned to occur over 2 days, with a 1-month interval in between. This allowed sufficient time to cover multiple ‘trainable’ communication behaviours, video workshops and simulation, and to facilitate reflective activity between the 2 days. Having 2 full days also allowed time for evaluation activities to be completed before and after the training without attrition (health-care professionals had an incentive to attend for the second day, when post-course evaluation took place, as they received further training). The e-learning activity was offered between training days as consolidation.
Tailoring
Tailored interventions are delivered in an individualised way, so that not all recipients receive an identical intervention. 181 The experiential learning approach meant that the course was experienced differently for each trainee. For example, each trainee had different experiences in practice and in simulation to reflect on, and received individualised feedback from the facilitator, their peers and the SP. Each course included participants with a mix of professional skills and experience, so each course represented a different community of learning. 148
Modification
We undertook a pilot study of the training, specifically to allow for modifications before entering the feasibility testing phase.
Fidelity planning
The extent to which an intervention is delivered as planned is referred to as ‘fidelity’. 181 When a complex intervention is delivered by different people in different contexts, the possibility of unintended variation is introduced. The need for a clear process for maintaining and checking fidelity has been recognised, including the potential impact of high or low intervention adherence on outcomes. 182 For the pilot and feasibility testing of the course, delivery was done by the developers and was consistent across courses, so fidelity was not an issue. If others were to deliver the course, quality-control measures would be required, such as ensuring that trainers had undertaken the course first and planning for train-the-trainer activities.
Pilot course
A pilot of the VOICE for Dementia communication skills training course was run with two primary objectives:
-
to optimise the intervention
-
to optimise the evaluation of the intervention.
These aims were consistent with the modelling of process as part of the ‘development’ phase of the Medical Research Council (MRC)’s complex intervention framework. 183 Although definitions of pilot and feasibility studies vary,184 this pilot was designed to be a trial version of the communication skills training course to test whether or not the processes could all work together and allow trainers, simulators and a sample of trainees to experience the intervention and provide feedback on it. 185
Method
The pilot course consisted of running the training programme once prior to the start of the evaluation feasibility study, with research measures taken before day 1 and after day 2, as planned for the evaluation study (see Chapter 5). Trainees for the pilot course were invited by the project team on the basis of the following criteria:
-
considerable experience working with people living with dementia in the acute setting
-
experience in education and the training of health-care professionals
-
confident enough to work as ‘critical friends’ to the research team’s senior clinicians/researchers, giving honest, face-to-face feedback
-
represent a spread of professional groups, including doctors, nurses and therapists.
Trainees were made aware that they would be invited to give verbal feedback as a group at the end of days 1 and 2 of the training. These discussions were facilitated and noted by the research team; completed research measures and post-training evaluation forms were also considered. The core research team that delivered the training, including the lead simulator, also contributed their feedback on the intervention and the evaluation processes. The training took place on site at the acute hospital where the trainees were based. The intervention was delivered by two members of the core research team, with a third observing and participating as a trainee. The lead simulator acted in a supporting and observational role. Two simulators supported the evaluation scenarios and two supported the training scenarios.
Findings
Eighteen health-care professionals were invited to attend the pilot over the 4 months preceding the pilot. On the first day, seven trainees were booked to attend but two were unwell on the day. Five invited trainees attended both days of the training, and one clinician researcher (RH) participated as a trainee to make up numbers.
Intervention optimisation
Trainees evaluated the training extremely positively, commenting that, despite their experience, they valued their learning from the course. They made a number of suggested improvements, including expanding, reducing, simplifying and reordering various exercises, and producing supporting resources. Changes to the simulations emerged from discussions with the lead simulator, trainees and trainers (which included asking for specific, out-of-role feedback from the simulators); providing more props to avoid health-care professionals ‘miming’; increasing the range of simulation tasks; and encouraging the pausing and rerunning simulations. Trainers reflected that the training had run largely according to plan, with minor changes needed to the presentations and resources, and learning gained by them in how best to support simulation exercises in particular.
Evaluation optimisation
Outcome measures were piloted at the beginning of day 1 and at the end of day 2 of the training (see Chapter 5). All five external trainees completed the measures. Trainees gave useful feedback on some of the items in the knowledge test, resulting in some rewording. The confidence scales were completed without problems. Both knowledge and confidence scores showed positive changes from pre to post training, suggesting that they were appropriate measures with the potential to show a training effect. Practicalities before the course commenced proved challenging, including welcoming trainees, the consent process and completing paper-based baseline measures and a video-recorded simulation. An extra research team member therefore attended the feasibility study. After reviewing these video-recorded simulations, changes were made to the way the assessment simulation was introduced and timed. Review of the completed pilot evaluation forms showed extremely encouraging views of the training.
Summary of intervention design
This chapter has presented the ‘who, what, why and when’ of the intervention design processes. The development and evaluation of an evidence-based intervention is a complex, non-linear process. In this chapter, we have described how the VOICE training was coproduced with a wide group of informants. The resulting approach, based on original findings from the CA analysis, has been described in detail. We have presented findings from piloting the training course and its evaluation. Throughout this chapter, attention has been paid to the implications for the delivery of the intervention beyond the context in which it was developed.
Chapter 5 Training of actors
Introduction
This chapter describes the training of actors who play the part of patients for training or assessment purposes (known as SPs). ‘Training’ includes pre-training preparation and resources, the pre-course training day and ongoing feedback and coaching during the courses. We needed SPs for both the training elements and the effectiveness evaluation study. This involved creating scenarios and training a team of SPs to bring scenarios to life.
Six SPs were recruited on the basis of expertise, experience and ‘looking the part’, that is, age and gender were appropriate for the scenarios. Their experience spanned:
-
work in formative and summative settings
-
work in a variety of health-care contexts
-
simulating a range of acute, chronic, mental and physical conditions
-
meeting diverse educational agendas (breaking bad news, end-of-life consultations, exploring patient centredness)
-
being facilitated, self-facilitating and giving feedback
-
knowledge and experience of confidentiality, simulation delivery (consistency, authenticity, adherence to learning outcomes) and teamwork.
Establishing training requirements
The six SPs recruited had to simulate six scenarios. Four scenarios were required for the training sessions (Jack, Maureen, Tom and Alice) and two scenarios for the evaluation assessment (Stan and Annie). Two training scenarios were used on day 1 of the course (Jack and Maureen) and two on day 2 of the course (Tom and Alice). The level of communicative impairment on day 2 was greater than on day 1, in order to present the trainees with more challenge and provide them with more scope to demonstrate their acquisition of communication skills (Table 11).
| SP requirement | Simulations | |
|---|---|---|
| Evaluation assessment | Training | |
| Give a consistent and acceptably authentic portrayal of the patient | ✓ | ✓ |
| Respond to ’trainables’ (requests, closing) to give trainees opportunity to practice communication skills | ||
| Respond appropriately to one set task (refuse request two to seven times) | ✓ | |
| Respond appropriately to a range of tasks (refuse request two to seven times) | ✓ | |
| Prolong the closing | ✓ | ✓ |
| Respond as required to the individual needs of the learners | ||
| Stop and start in response to request to ’time out’ | ✓ | |
| Repeat all/part of the simulation with the same trainee | ✓ | |
| Give feedback out of role | ✓ | |
The training approach
Our approach was based on four out of the five domains from the practice guidelines from the Association of Standardized Patient Educators Standards of Best Practice:186
-
safe work environment
-
scenario development
-
SP training
-
programme management (including communication/feedback processes and channels).
The fifth domain (professional development) was not relevant to course set-up.
Safe work environment
The design of the activity
Simulated patient safety was central in the development of the scenarios in terms of the physical, cognitive and psychological challenges of role portrayal. We moderated our requirements for role delivery in the light of what was feasible and safe. For example, SPs were not required to exhibit physical symptoms that would lead to their discomfort or disbelief on the part of the trainees. The complexity of delivering the simulation, providing opportunities for trainees to demonstrate the ’trainables’ and framing feedback presented a high level of cognitive challenge. We endeavoured to address SP anxiety around meeting this challenge by openly acknowledging that, by its pioneering and unique nature, this simulation work was going to be difficult. We helped SPs gain an understanding of dementia, and provided communication channels through which to share concerns.
Simulated patient debriefing
The VOICE faculty responded to SP requests for feedback on their performance, and time was explicitly allocated for the lead simulator to debrief the SPs. With the evaluation assessment scenarios, this involved eliciting SPs’ thoughts on how the simulations had gone and addressing concerns or questions arising from unexpected events or psychological impact. For instance, one SP needed to explore their response to a trainee who had made a request in a forceful way. With the training simulations, the lead simulator fed back her observations as well as inviting the SPs to share their thoughts on the simulations, facilitation and feedback processes. Further conversations proceeded through e-mail and follow-up telephone calls between the courses.
The simulated patient environment
Simulated patient training and most of the training courses took place in a venue that SPs had worked in previously and was well equipped. There was greater pressure on available space at the second venue, and so it did not offer the same degree of privacy and preparation space for the SPs.
Respect for simulated patients
Respect for the SPs’ personal boundaries was written into the scenarios and then reiterated when suggestions for modifications to the simulation task were mooted. For example, the SPs were never expected to remove all items of clothing even when this was suggested as being necessary for the correct way to listen to the patient’s chest. Implicit in the trainees’ tasks was also a limit on personal touch. No intimate exams were included in the task list.
Scenario development
Scenario preparation
The process included:
-
Identification of the ’trainables’ to determine what the simulations needed to deliver, and the need to ensure that the scenarios gave the trainees opportunities to practise and demonstrate the learning objectives, that is, the SP had to refuse and prolong the closing.
-
Scrutiny of the video-recordings by the lead simulator to determine whether or not simulations could be developed from the patients filmed for the VOICE study. This step involved looking at whether or not the patients’ demeanour and verbal and non-verbal communication could be simulated authentically enough; that is, could behaviours be ’unpicked’ to give the SPs ways to portray that patient?
-
Identification of patients from the video material and CA transcripts on whom the scenarios could be based. These were selected on the basis of the level of communicative impairment.
-
Creating a character for the scenarios based on the video-recordings and CA transcripts while respecting anonymity and privacy.
-
Meetings and conversations were scheduled to draft, review and edit the scenarios prior to the SP pre-course training day.
Scenario components
Once six patients had been identified, scenarios were developed to include:
-
Patient information – social background, insights into character, behaviour, appearance and demeanour. Creation of a ‘backstory’ to provide an underlying logic for the patient’s behaviour.
-
Clinical information – reason for hospital admissions, dementia symptoms, communicative ability, retained abilities, previous medical history and current medication.
-
Information for simulator –
-
A description of what the patient knew and what they could do, for example ‘Your name is Annie. You live with your daughter . . . Generally, you can take turns in a conversation.’
-
A guide to the patient’s manner of speaking taken from interactional patterns identified in the CA transcripts, for example you speak quietly and quickly, you giggle, you say things and smile ‘I’ve made you happy. Hee hee hee.’
-
Responses to the health-care professional at the different phases of the interaction taken from interactional patterns in the CA transcript.
-
The scenarios were sent to two SPs to gain feedback on whether or not the information included was adequate for them to build the simulation. One SP replied in the affirmative, and the other suggested changes.
Simulated patient training
Preparation for the pre-course training day
The training plan embedded the advice of the PPI representatives who, with their first-hand experience of caring for people living with dementia, suggested that the SPs needed to understand the context of the patient experience, the patient’s condition and to have realistic behaviour, both verbal and non-verbal, in order to simulate people living with dementia effectively.
One week prior to the training day SPs received their scenarios and background information and links to online resources to enhance their understanding of the patient experience and condition. 34,150
One day of face-to-face training was arranged for the SPs (Box 3). The plan took into account the order and the way in which the three elements should be addressed on the training day as well as introducing the educational aims. The nature of dementia and the VOICE study and its findings were introduced, Today is Monday (a documentary showing 24 hours in a specialist hospital medical and mental health unit)187 was shown and questions about dementia and watching video-recordings from the VOICE study were shared. Simulated patients worked in ’role groups’ to prepare their scenario.
09.00: introductions and objectives for the day.
09.15: introduction to the VOICE project (RO’B and SG).
10.15: break.
10.30: living with dementia (1): viewing of Today is Monday followed by a question and answer session on symptoms, behaviour and care (RH).
11.30: living with dementia (2): first-hand insights (helping to build authenticity into the roles through watching film footage) (RO’B).
12.30: lunch.
13.15: scenario familiarisation and practice: day 1, training scenarios – Maureen and Jack (RO’B, SG and MM).
14.15: scenario familiarisation and practice: day 2, training scenarios – Alice and Tom (RO’B, SG and MM).
15.15: break.
15.30: scenario familiarisation and practice: assessment scenarios – Annie and Stan (RO’B, SG and MM).
16.00: learning capture and troubleshooting: insights/challenges/what ifs? Practical matters: plans for pilot days 1 and 2.
16.30: finish.
Ongoing training
Ongoing SP training took the form of feedback based on observations of the simulations by the VOICE faculty and the lead simulator. The lead simulator attended the initial courses and attended subsequently in response to the emerging support needs of specific SPs. Further training was tailored around the SPs’ individual ongoing training needs. For example, one SP took the opportunity to shadow a colleague simulating the same role in order to modify her portrayal.
Modifications to the training
Introduction of simulated patient feedback
Simulated patient feedback was not built into the original plan for the training simulations, but this was added after the pilot course. Simulated patients gave feedback ‘out of role’; trainees or facilitators framed specific questions, such as ‘Did you feel I was rushing to get away at the end?’. Simulated patients were advised to frame their feedback around the trainees’ demonstration of person-centred care (see Figure 9).
Simulated patient response to use of touch
It became apparent during the courses that touch was frequently used as a therapeutic tool by health-care professionals. This had been acknowledged, but not explored, at the pre-course training. The VOICE faculty provided insights to SPs on how they should respond to health-care professionals’ touch during simulations.
Programme quality management
Feedback on the quality of the training was requested from trainees at the end of the VOICE in Dementia Courses (see Chapter 6). SPs were asked to complete written feedback and were invited to a face-to-face evaluation session. Key points gathered from SP feedback to be considered in future SP training interventions included the following:
Training resources
-
Simulated patients needed the combination of the written scenario, CA transcript and video-recordings to bring the scenarios to life. One single resource was inadequate.
-
Simulated patients would have preferred more video clips of the patients. With one video clip their response repertoire was limited.
Pre-course training time
-
Simulated patients felt that more time for the scenario familiarisation and practice was needed; half a day was insufficient.
Ongoing training support
-
A feedback session with the faculty 2 weeks into the programme provided opportunities to make amendments to the delivery of the simulations. This also provided an opportunity to validate simulation performance and promote confidence.
Conclusion
Authentically simulating a person living with dementia for the purposes of communication skills training is difficult, but experienced SPs were able to successfully learn and deliver simulations following a careful process of scenario development, training on specific aspects of dementia and the educational objectives of the course, and active feedback and support.
Chapter 6 Can we train? Course evaluation study
Aim
We aimed to evaluate the effectiveness and acceptability of the communication skills training intervention. We used the first three levels of Kirkpatrick’s four-steps evaluation model: reaction, learning and behaviour. 188 The aims of the VOICE communication skills training course were that health-care professionals would increase their confidence in caring for people living with dementia, increase their knowledge of dementia communication and change their communication behaviours. The communication skills training had to be acceptable and useful to the health-care professionals, and feasible to run. Line managers had to be willing to release staff from clinical practice to attend the course and see the benefits of dementia communication skills training.
Study design
Study outline
We evaluated the course using a B–A study design. This was chosen as an efficient research design for detecting changes in communication knowledge, confidence and behaviour. It allows for between-individual variation (prior experience, personality, knowledge, native interpersonal skills, etc.) to be controlled for. B–A study designs can overestimate the benefits of an intervention. 189 B–A designs are commonly used to evaluate dementia training interventions. 190
The study was reviewed and approved by the NHS Health Research Authority Integrated Research Application System number 211817.
Setting
Staff were recruited from wards in two acute hospitals: one a teaching centre, the other a district general hospital. Both hospitals have a specialist dementia and delirium unit, where several of the participants worked.
The training courses and study assessments were held in a suite of two or three clinical skills rooms. The clinical skills rooms were equipped with hospital beds and bedding, tables and chairs and sinks.
Participant identification and recruitment
The health-care professionals all volunteered to take part in the study, following either an approach from their ward or professional manager, or by responding to posters or word of mouth. Health-care professionals interested in the study were referred to the clinical researchers, who answered their questions and sent them a participant information sheet. It was made clear that the study involved a 2-day training course, that the participant had to seek approval from their line manager to attend and that the course was not suitable for them if they were not working with people living with dementia. It was emphasised that participation was dependent on agreement to attend both days and taking part in the evaluation study, including video-recording simulated encounters with a SP. Those who agreed were reminded of their right to withdraw consent without prejudice. If still interested in the training and willing to participate in the study, the health-care professional was booked on to one of the six VOICE communication skills training courses. Written informed consent was taken on the morning of the first day of the course by one of the facilitators. The participant had the opportunity to ask more questions before consenting.
We aimed to recruit a spread of health-care professionals (doctors, nurses and therapists) across the six courses and within each course. We therefore capped numbers of each professional group on each course at half the total number of places available (a maximum of five). Health-care professionals who spoke English as a second language were welcome and encouraged to participate.
Inclusion criteria
-
A registered health-care professional (doctor, nurse or therapist) working with patients with dementia at one of the two participating hospitals.
-
Willing to give informed consent for participation.
-
Male or female, aged ≥ 18 years.
Exclusion criteria
-
Unable or unwilling to attend both days of the course.
-
Unwilling to be video-recorded for the simulated encounter assessment.
Methods
Outcome measures
Prior to the start of the first day of the course, participants were asked to complete the following questionnaires:
-
Demographic information (health-care profession, years of experience working with people living with dementia, ethnicity and gender).
-
The Confidence in Dementia Scale. 85 This is a nine-item scale to assess a person’s confidence in caring for a person living with dementia. A sample question is ‘I feel able to interact with a person with dementia when they cannot communicate well verbally’. Responses were on a Likert scale from one = not able to five = very able.
-
Three additional questions linked to the course ‘trainables’, asking participants to rate their confidence on a scale of 0 = no confidence to 10 = totally confident on ‘ending a conversation when the patient tries to continue it’, ‘achieving a task in the person with dementia’s best interest when their first response is a refusal’ and ‘awareness of the best way to ask someone with dementia to do something’.
-
Dementia Communication Knowledge Test: we developed a 10-item, multiple-choice answer test of general and course-specific knowledge of communication in dementia (see Appendix 2).
At the end of the second day of training, participants were asked to complete the following questionnaires:
-
The Confidence in Dementia Scale. 85
-
Five questions to test confidence in specific areas of dementia communication. These questions asked participants to rate their confidence on a 0–10 scale (0 = no confidence to 10 = total confidence) on awareness of communication skills, use of communication skills and the three questions asked before the course (as 3 above).
-
Dementia Communication Knowledge Test (as 4 above).
-
Evaluation of the training course. We asked participants to rate on a scale of 1–10 if the course was interesting, useful, informative and enjoyable; whether or not they felt respected and safe; and whether or not the course was challenging and relevant to their practice, fulfilled their learning goals and had improved their practice. Participants were asked if the course met their expectations and if they would recommend it to their colleagues. The evaluation was adapted from one used by the ‘Dying to Communicate’ end-of-life communication course, which also used simulation as a teaching method.
Participants were asked at the end of day 2 of the course, and by e-mail 1 month later, if they remembered and were performing the skills they had learnt, and if they considered the skills to be useful in their role.
Space was provided on the evaluation questionnaire for participants to record what they had learnt from the course, what was most helpful about the course, how they thought the course would help with caring for patients and if there was any part of the course to consider changing.
Simulated encounter measure
We evaluated whether or not participants changed their communication behaviours following the VOICE communication skills training. We video-recorded simulated encounters (with SPs) before and after the course.
The simulation assessment involved the participants being given one of two scenarios, containing brief details about the ‘patient’ and the generic health-care task to be completed, which was either to get the SP out of bed or to get the SP to drink some water and eat a biscuit. Participants were asked to treat the encounter as if they were dealing with a real patient in a side room, closing the interaction appropriately. There were two SP roles for the assessments, played by a male and a female SP. To create a clear distinction between the evaluation and teaching, the SPs doing these assessment scenarios were not involved in the simulation workshops during the same training course. Simulated patients were trained to refuse the task several times and to extend the closing of the interaction. In order to keep the course to time, and to orient the health-care professional to some sort of time pressure, they were given an indicator (a knock at the door) after 10 minutes had elapsed, to prompt them to close the encounter and leave as soon as was appropriate. The participant completed the assessment with a different role at baseline and outcome, with half the group doing the baseline assessment with one scenario and the other half with the other scenario in a crossover design. The lead simulator monitored SPs’ performance to ensure consistency (by watching the video-recordings after the courses).
We developed two checklists to rate the participants’ communication behaviour shown on the video-recordings. 191 These checklists identified specific, objectively identifiable communication behaviours that had been identified in the CA and taught on the course (requests and closings). The rating forms are in Appendices 3 and 4.
Ratings were made independently by two trained experienced speech and language therapists, blind to whether or not the video was made before or after the training. Videos were edited to remove time references to morning and afternoon that might have unblinded the raters (blurring clocks, removing greetings that mentioned morning or afternoon). The raters were trained during a 1-day training session. They were introduced to the VOICE study and the communication behaviours taught on the course. They then rated video-recorded simulated assessments from the pilot study and compared their results. Through a discussion of differences, they achieved good reliability by the end of the training. For requests, they agreed on the behaviours being present or absent on 73% of occasions (kappa of 0.42, moderate agreement); for closings, the raters agreed on 89% of occasions (kappa of 0.75, good agreement). 192 Videos were assessed in a random order using a random number sequence. We calculated agreement between the two rates (kappa scores) after the rating exercise.
We also invited PPI representatives to rate the video-recordings. During intervention development meetings PPI representatives raised the possibility that by teaching health-care professionals to make requests in a more entitled way, and to more clearly signal the closing of an interaction, they might appear less person centred. We therefore used a measure of the emotional tone of the communication, the Emotional Tone Rating Scale (ETRS). 193 We sought to check whether or not people living with dementia and their carers would find changes in health-care professionals’ communication behaviours ‘acceptable’ and no less person centred after the training than before. The ETRS is a valid and reliable scale designed to ‘measure the underlying affective qualities of communication with older adults’. 193 Williams et al. 193 reported high inter-rater reliability with an intraclass correlation for agreement of 0.95. The authors describe the scale as requiring minimal training to use. Users rate 12 characteristics on a five-point Likert scale (one = not at all, five = very): ‘The healthcare professional’s communication was [. . .] nurturing, directive, affirming, respectful, patronising, supportive, polite, bossy, caring, dominating, warm, controlling’.
Members of the intervention development group (including three PPI representatives) used the ETRS on a pilot video-recorded simulation assessment. We determined that the scale was easy to use, but agreement on scores between raters was low. We invited PPI representatives from the Alzheimer’s Society Research Network and from the University of Nottingham’s Dementia and Frail Older Person’s PPI group, who had no previous involvement with the VOICE study, to attend at least three of six 4-hour group rating sessions. All the raters either had dementia themselves or cared for a person living with dementia. We trained the PPI raters by asking them to answer a simple question to practice using a Likert scale: ‘how was your journey to the hospital today? Give a score of 1–5 in which 1 is ‘terrible’ and 5 is ‘excellent’’. We introduced the ETRS, gave instructions on completion and asked participants to score a pilot video as practice. We then showed two short clips from the same assessment video. We did not define the ETRS terms, and asked raters to use their own understanding of what they meant. Raters were not told that the videos were from before and after a training course. Videos were presented in a random order, paired for each session, so that individuals rated both the before and after video for each health-care professional. Raters scored each encounter after watching 2 minutes of video: 1 minute starting from the participant’s first request and 1 minute taken from the start of the closing sequence. Video clips were shown twice.
Sample size
We estimated that it was feasible to train 40 health-care professionals over a 6-month period, taking into account staff rotas and release from the wards. Other studies have evaluated dementia communication skills training courses using a B–A design,58,190 with similar sample sizes ranging from 15 to 48 participants. 21,59,194–197 We over-recruited to courses (up to 10 for each course) to allow for health-care professionals cancelling at short notice.
Data analysis
To test inter-rater agreement, kappa scores were calculated for each communication behaviour shown in the assessment simulations that the speech and language therapists and PPI members rated. Participant-related data were summarised using descriptive statistics. Differences in responses before and after training were evaluated using paired t-tests and the Wilcoxon signed-rank with 95% confidence intervals (CIs). Mean changes in ETRS were assessed using paired t-tests.
McNemar’s test was used to assess change in the communication behaviours. The McNemar exact test was used when the discordant pairs totalled < 20. The results reported for the speech and language therapist rating are when both raters agreed the communication behaviour was present or absent.
Results
We delivered the course six times between January and May 2017. We recruited 45 health-care professionals who attended one of the courses, and 44 out of 45 participants attended the second day. For many course dates, the course was oversubscribed, although cancellations at late notice meant that numbers attending each course ranged from six to nine participants. Participants comprised a mixture of doctors, nurses and therapists attending each course, with 8 out of 45 (18%) doctors, 19 out of 45 (42%) nurses and 17 out of 45 (38%) AHPs (occupational therapists, physiotherapists, speech and language therapists and one orthotist). One activities co-ordinator also participated. Forty (89%) of the participants were female, 40 (89%) were white, four (9%) were Asian and one identified as mixed race. They had a median 5 years’ experience working with people living with dementia (range 0.3–33 years) (Table 12). Twenty-nine participants (64%; four courses) attended the training at site one, and the rest attended at site two (two courses).
| Variable | Participants |
|---|---|
| Profession, n/N (%) | |
| Doctors | 8/45 (18) |
| Nurses | 19/45 (42) |
| AHPs | 17/45 (38) |
| Other | 1/45 (2) |
| Experience working with patients with dementia | Median 5 years, interquartile range 3–8 years, range 0.3–33 years |
| Gender female, n/N (%) | 40/45 (89) |
| Ethnicity, n/N (%) | |
| White | 40/45 (89) |
| Asian | 4/45 (9) |
| Mixed | 1/45 (2) |
The baseline questionnaires for one participant were not returned, despite repeated requests. One participant did not attend day 2 of the course nor complete outcome questionnaires. Analysis was therefore confined to 43 participants. Five participants missed at least one question on the Dementia Communication Knowledge Test.
Participants increased their confidence in dementia care and knowledge of dementia communication following communication skills training. Confidence improved in all categories, and overall on the Confidence in Dementia Scale (32.8/45 vs. 38.3/45) and course-specific confidence questions (Tables 13 and 14).
| Number | Question: I feel able to . . . | Time point, mean (N = 43), n (95% CI) | Mean difference (N = 43), n (95%CI); p-value | |
|---|---|---|---|---|
| Pre course | Post course | |||
| 1 | . . . understand the needs of a person with dementia when they cannot communicate well verbally | 3.3 (3.1 to 3.5) | 3.9 (3.7 to 4.1) | 0.6 (0.4 to 0.9); p < 0.001 |
| 2 | . . . interact with a person with dementia when they cannot communicate well verbally | 3.5 (3.3 to 3.7) | 4.1 (3.9 to 4.3) | 0.6 (0.4 to 0.9); p < 0.001 |
| 3 | . . . manage situations when a person with dementia becomes agitated | 3.1 (2.9 to 3.4) | 3.9 (3.7 to 4.1) | 0.7 (0.4 to 1.0); p < 0.001 |
| 4 | . . . identify when a person may have a dementia | 3.6 (3.4 to 3.8) | 4.2 (4.0 to 4.4) | 0.6 (0.4 to 0.9); p < 0.001 |
| 5 | . . . gather relevant information to understand the needs of a person with dementia | 3.6 (3.5 to 3.9) | 4.3 (4.1 to 4.9) | 0.6 (0.4 to 0.9); p < 0.001 |
| 6 | . . . help a person with dementia feel safe during their stay in hospital | 3.5 (3.3 to 3.7) | 4.2 (4.0 to 4.4) | 0.7 (0.5 to 0.9); p < 0.001 |
| 7 | . . . work with people who have a diagnosis of dementia | 4.0 (3.8 to 4.2) | 4.6 (4.5 to 4.8) | 0.7 (0.4 to 0.9); p < 0.001 |
| 8 | . . . understand the needs of a person with dementia when they can communicate well verbally | 4.0 (3.8 to 4.2) | 4.5 (4.3 to 4.7) | 0.5 (0.3 to 0.7); p < 0.001 |
| 9 | . . . interact with a person with dementia when they can communicate well verbally | 4.1 (3.9 to 4.3) | 4.5 (4.4 to 4.7) | 0.4 (0.2 to 0.6); p = 0.0002 |
| Total | 32.8 (31.6 to 34.1) | 38.3 (37.2 to 39.5) | 5.5 (4.1 to 6.9); p < 0.001 | |
| Confidence in | Time point, mean, n (95% CI) | Mean difference, n (95% CI); p-value | |
|---|---|---|---|
| Pre course | Post course | ||
| Ending a conversation when the patient tries to continue | 4.5 (3.7 to 5.3) | 7.8 (4 to 10) | 3.3 (2.3 to 4.3); p < 0.001 |
| Achieving a task in the person’s best interest | 4.6 (3.8 to 5.3) | 8.2 (6 to 10) | 3.7 (2.8 to 4.5); p < 0.001 |
| The best way to ask someone to do something | 4.7 (3.9 to 5.4) | 8.7 (6 to 10) | 4.0 (3.1 to 4.9); p < 0.001 |
Participants improved their knowledge on the course-specific Dementia Communication Knowledge Test (mean 7.2/10 vs. 8.8/10), with a mean improvement in total score of 1.5 (95% CI 1.0 to 2.0) (Table 15).
| Number | Question | Time point, answers correct, n/N (%) | Difference in proportion, % (95% CI); p-value | |
|---|---|---|---|---|
| Pre course | Post course | |||
| 1 | Speed of speech | 30/44 (68) | 41/43 (95) | 27 (12% to 42%); p = 0.001 |
| 2 | Introductions | 40/44 (91) | 37/43 (86) | 5 (–18% to 9%); p = 0.48 |
| 3 | Communication strategies | 37/44 (84) | 38/43 (88) | 4 (–10% to 19%); p = 0.56 |
| 4 | Gaining attention | 43/44 (98) | 42/42 (100) | 2 (–2% to 7%); p = 0.33 |
| 5 | Repeating back question when patient says ‘no’ | 37/44 (84) | 36/42 (86) | –2 (–17% to 13%); p = 0.76 |
| 6 | Framing requests when expecting reluctance | 38/44 (86) | 43/43 (100) | 14 (3% to 24%); p = 0.01 |
| 7 | Dealing with a refusal | 25/44 (57) | 39/43 (91) | 34 (17% to 51%); p = 0.0003 |
| 8 | Open-ended pre-closure question (‘anything else?’) when closing | 17/42 (40) | 34/42 (81) | 40 (21% to 59%); p = 0.0001 |
| 9 | Indicating a health-care conversation is about to end | 13/42 (31) | 27/42 (64) | 33 (13% to 53%); p = 0.002 |
| 10 | Non-verbal communication to signal closure | 36/43 (84) | 41/43 (95) | 12 (–1% to 24%); p = 0.08 |
| Total, mean (95% CI) | 7.2 (6.8 to 7.7) | 8.8 (8.4 to 9.1) | 1.5 (1.0 to 2.0); p < 0.001 | |
The course was acceptable to participants, with 95% reporting that the course met their expectations, and 98% reporting that they would recommend it to other health-care professionals. The course was evaluated highly in all the categories investigated. At the end of the course, high scores were given to the question asking the participants if they remembered the skills, were using them in practice, were finding them useful and were confident in awareness and use of communication skills (Table 16).
| Question | Mean score/10 (range); n = 44 |
|---|---|
| Do you remember the skills? | 8.7 (6–10) |
| Are you performing the skills? | 8.2 (6–10) |
| Are the skills helpful? | 9.6 (8–10) |
| The course was: | |
| Interesting | 9.3 (7–10) |
| Useful | 9.4 (7–10) |
| Informative | 9.4 (7–10) |
| Enjoyable | 9.1 (7–10) |
| Challenging | 8.4 (3–10) |
| Relevant to my practice | 9.5 (7–10) |
| I felt respected | 9.7 (8–10) |
| I felt safe | 9.8 (7–10) |
| Fulfilled my learning goals | 9.1 (5–10) |
| Improved my confidence | 9.2 (6–10) |
| Confidence in | |
| Awareness of communication skills | 8.6 (7–10) |
| Use of communication skills | 8.5 (7–10) |
The response rate to the e-mail follow-up 1 month after the second day of the course was 31 out of 44 (70%). Participants gave a mean score of 8.6/10 to the question ‘do you remember the skills you learnt in the training course?’; 8.4/10 for the question ‘are you performing the skills you have learnt in the training course?’ and 9.3/10 for the question ‘are these skills helpful in your role as a healthcare professional?’ There was a small increase in the proportion of participants remembering what was taught (mean 8.2/10 at the end of the course vs. 8.6/10 1 month later; p = 0.02), no change in whether or not the health-care professional was performing the skills learnt (mean 8.7/10 vs. 8.4/10; p = 0.05), and a small reduction in whether or not the health-care professional felt that the skills were helpful (9.7/10 vs. 9.3/10; p = 0.003).
Communication behaviours in the evaluation simulated encounters were considered present or absent only when both speech and language therapist raters agreed. Inter-rater reliability for each communication behaviour was mostly fair or moderate (kappa range 0–0.79; Tables 17 and 18).
| Communication practice when requesting | Example | Inter-rater reliability (kappa) | |
|---|---|---|---|
| First request | Subsequent request | ||
| High entitlement request | |||
| Proposal | Let’s (let’s try a yoghurt) | 0.48 (moderate) | 0.69 (substantial) |
| Announcing future action | Going to/we’ll | 0.22 (fair) | 0.57 (moderate) |
| Statement of need | I need you to; I need to; You need to | 0.59 (moderate) | 0.55 (moderate) |
| Direct instruction | Take a step | 0.32 (fair) | 0.39 (fair) |
| Softened (e.g. with checking/permission-seeking question) | Is that OK? Alright? OK? | 0.43 (moderate) | 0.47 (moderate) |
| Other | Forced alternatives that presume compliance (‘Which finger shall I use?’) | 0.42 (moderate) | 0.24 (fair) |
| Lowering contingencies | |||
| Reduces the size or duration of task | Just, little, pop, quick, for a minute | 0.12 (poor) | 0.55 (moderate) |
| Request includes ‘try’ | Try (shall we give it a try then?) | 0.66 (substantial) | 0.64 (substantial) |
| Explicit offer to help | (What about if I give you a hand?) | 0.31 (fair) | 0.79 (substantial) |
| Frame accurately as collaborative or joint action | We; let’s; for me (shall we go for a walk) | 0.49 (moderate) | 0.17 (slight) |
| State the action explicitly (not just stating the reason for the action) | (What I want to do is give you a shave) | 0.08 (poor) | –0.02 (poor) |
| Communication practice during closing | Examples | Inter-rater reliability (kappa) |
|---|---|---|
| Vague arrangement at closing | (See you soon; see you around) | 0.51 (moderate) |
| Specific closing arrangement | (See you tomorrow; I’ll get that cup of tea now) | 0.25 (fair) |
| Notification ahead of final activity | (Before I go . . .) | 0.31 (fair) |
| Announcing completion of final activity | (That’s us all done) | 0.42 (moderate) |
| Announcing explicit intention to leave | (So I’m gonna go now) | 0.31 (fair) |
| Non-verbal actions supporting verbal closing | Repositioning table; tidying equipment | 0.40 (fair) |
| Closing idiom or saying | (All done and dusted; I’ll leave you be; we’ll keep a close eye on things; you take care) | 0.29 (fair) |
| ‘Is there anything else?’ – type open question during closing | (Anything you want to ask me before I go? Is there anything I can help with?) | 0.37 (fair) |
| Mismatch between non-verbal and verbal actions during closing | Health-care professional gives verbal indications of closing but does not make physical moves to indicate closing/leaving; health-care professional opens new lines of enquiry (verbal) while walking away (non-verbal) | 0.41 (moderate) |
The impact of training on communication behaviours displayed in the evaluation simulations was variable. Results showed that following training, when closing an interaction, participants were less likely to make a vague arrangement (56% before vs. 16% after), more likely to be specific about closing the conversation (51% vs. 79%) and more likely to announce completion of the task (0% vs. 14%) (Table 19).
| Communication practice | Communication technique seen, n/N (%) | McNemar’s test odds ratio (95% CI); p-value | |
|---|---|---|---|
| Before training | After training | ||
| Vague arrangement making | 24/43 (56) | 7/43 (16) | 0 (0 to 0.2); p < 0.001 |
| Specific closings | 22/43 (51) | 34/43 (79) | 4.0 (1.3 to 16); p = 0.007 |
| Notification ahead of closing | 7/43 (16) | 11/43 (26) | 2.0 (0.5 to 9.1); p = 0.4 |
| Announcing completion of task | 0/43 (0) | 6/43 (14) | N/A; p = 0.03 |
| Announcing explicit intention to leave | 22/43 (51) | 23/43 (53) | 1.1 (0.4 to 2.9); p = 0.8 |
| Non-verbal actions supporting verbal actions | 6/43 (14) | 6/43 (14) | 1.1 (0.2 to 4.3); p = 1.0 |
| Closing idiom used | 16/43 (37) | 22/43 (51) | 2.0 (0.7 to 6.5); p = 0.24 |
| ‘Anything else’ question asked | 7/43 (16) | 4/43 (9) | 0.6 (0.1 to 2.2); p = 0.55 |
| Mismatch between verbal and non-verbal communication | 1/43 (2) | 3/43 (7) | 3.0 (0.2 to 158); p = 0.62 |
There were no significant changes in behaviour on the communication techniques related to requests (Table 20). Eighty-six per cent of participants did not make the initial request explicit either before or after training and 79% did not make a subsequent request explicit before or after training. Eighty-eight per cent did not soften the initial request by checking for agreement (‘. . . is that OK?’) before or after training. However, many participants already used some of the recommended communication techniques prior to training. For example, prior to training, 74% of health-care professionals were highly entitled when making a ‘subsequent’ request (i.e. not the first request) before training; 98% of health-care professionals reduced contingencies for subsequent requests.
| Communication practice | Communication technique seen, n/N (%) | McNemar’s test odds ratio (95% CI); p-value | |
|---|---|---|---|
| Before training | After training | ||
| Initial request made in a highly entitled way | 2/43 (5) | 8/43 (19) | 7.0 (0.9 to 315.0); p = 0.07 |
| Subsequent request made in a highly entitled way | 32/43 (74) | 37/43 (86) | 2.2 (0.6 to 10.0); p = 0.27 |
| Initial request softened | 2/43 (5) | 3/43 (7) | 1.5 (0.2 to 18.0); p = 1.0 |
| Subsequent request softened | 8/43 (19) | 11/43 (26) | 1.4 (0.5 to 3.9); p = 0.65 |
| Initial request includes a reduction of contingencies | 13/43 (30) | 9/43 (21) | 0.6 (0.2 to 1.8); p = 0.45 |
| Subsequent requests include reduction of contingencies | 42/43 (98) | 40/43 (93) | 0.3 (0 to 4.2); p = 0.62 |
| Initial request is explicit | 3/43 (7) | 3/43 (7) | 1.0 (0.1 to 7.5); p = 1.0 |
| Subsequent requests are explicit | 2/43 (5) | 8/43 (19) | 7.0 (0.9 to 315); p = 0.07 |
In the evaluation of emotional tone in the evaluation scenarios, the PPI raters showed poor inter-rater reliability on ETRS items (kappa 0.01–0.10). The communication of the health-care professionals was thought to be slightly more controlling (2.2/5 vs. 2.8/5; p = 0.002), bossy (1.9/5 vs. 2.3/5; p = 0.02) and dominating (1.9/5 vs. 2.5/5; p = 0.006). There were no differences in the other categories of emotional tone (warm, nurturing, directive, affirming, respectful, patronising, supportive, polite, and caring).
Free-text feedback identified that the most helpful parts of the course were the simulation workshops, including the immediate feedback, and being able to practice the skills learnt (mentioned by 27 participants):
The simulation exercise. We were able to take part in a small formative groups where we were open and honest with each other. The feedback from the ’patient’ was very helpful.
Participant 17
I wouldn’t say I enjoyed it as such, but the simulation part was really helpful. Being able to stop and replay was particularly good and getting feedback/watching others.
Participant 18
The reflective exercise between the two days was mentioned by five participants:
Reflection of my interactions. Discussion with colleagues, to learn from their experiences and realise that we all feel the same challenges.
Participant 12
Specific techniques/skills learnt were mentioned by eight participants. Being able to watch others undertake communication tasks, interdisciplinary learning and small group sizes were also valued.
The participants were asked how they thought that the course would help them care for patients. A number of participants responded that they felt more confident in their own skills:
Given me the confidence that what I do is correct and works and that I have a high entitlement to do task, and lower the contingency to ensure important aspects of care are achieved.
Participant 25
Much more confidence with persisting with/approaching patients with dementia.
Participant 36
Discussion
We evaluated a dementia communication skills training course using a B–A design. The course was acceptable to participants who reported using the communication techniques taught 1 month after the training. Participants increased their knowledge of dementia communication, were more confident in communicating with people living with dementia and showed some changes in communication behaviours in a simulated encounter. Participants found the simulation workshops, the reflective exercises and the teaching on the specific communication behaviours particularly useful. They felt that increased confidence would improve their care of people living with dementia.
The evaluation of educational interventions is less well developed than for therapeutic interventions in health care. The acquisition of relevant knowledge and skill is generally helpful to health-care professionals, but is cumulative. Individuals will integrate the output of any given teaching intervention with their prior experience, expertise and attitudes. Large-scale randomised controlled trials with ‘hard’ patient-related outcomes (such as mortality) are logistically difficult (or impossible). In any case, there are many more influences on patient-related outcomes than communication alone. Evaluation of education therefore typically focuses on intermediate outcomes, usually self-reported by trainees. We used the long-established theoretic evaluation framework of Kirkpatrick to demonstrate improvement in both confidence and knowledge. 188
A B–A design has disadvantages, not least ‘social desirability bias’; trainees may, subconsciously, report what they think course providers or educational researchers want to hear. If they have enjoyed a course or activity, they are likely to be well disposed towards it, regardless of any real benefit. We undertook a feasibility study to see if we could run a practical course, including innovative use of SPs, within funding, practical and logistical constraints. The evaluation of outcomes was statistically underpowered. Our trainees were volunteers, who by the very act of taking part were displaying enthusiasm for the subject, were well disposed towards learning and almost all had better than average knowledge, skills and confidence before training. We used a mix of established and (unvalidated) bespoke measurement scales that we mostly analysed by item (and which had face validity at minimum).
We attempted to measure changes in communication behaviours, using a video-recorded simulation that we blind-rated according to a checklist of behaviours. We demonstrated some changes in behaviour, especially in relation to closings, but none relating to requests, which perhaps formed the greater part of the training. This was partly because of the high baseline prevalence of some behaviours, and underpowering. We achieved good inter-rater reliability when training our independent speech and language therapist-raters, using video material from the pilot course, but this was not so apparent in rating the evaluation simulations. This will have reduced power to detect real differences, but also illustrates how complex communication behaviours are and how difficult it can be to objectively ascertain them. A communication encounter involves multiple elements: assessing a situation and communication level or ability, creating a rapport with the communication partner, assessing the practical problems and solutions for task completion, and undertaking a negotiation. This is dynamic. For example, a health-care professional may ‘test’ the situation by making a polite, non-threatening, low-entitled, indirect request, maybe with some explanation or rationale (‘I’m wondering if you’ll let me take your blood pressure?’). If the person is reluctant or refuses, different approaches may be tried sequentially, amid possible diversions or distractions, gradually introducing higher-entitled requests and lowered contingencies, until acceptance or abandonment (as part of a ‘leave and return’ strategy). Different techniques may be tried at the next attempt.
We tried to capture this in the communication behaviours checklist, for example by differentiating between first and subsequent requests. Even so, the raters (who were speech and language therapists and specialist health-care communication clinicians, with a grounding in both practical communication problems and linguistics) struggled to reach agreement on whether or not a behaviour was displayed. The task was perhaps easier for closings, when the action was more defined and concrete. The health-care professional trainees themselves considered the course to be successful on their self-assessments of reaction (whether or not the learning was a valuable experience), confidence (whether or not it enabled individuals to know if they were doing the right thing) and learning (whether or not the participant’s knowledge increased after the course). ‘Confidence in competence’ is an important professional attribute. 25 An unmeasured outcome reported by trainees was that we gave them a language to articulate what they already did, helping then to teach or guide members of staff they are managing or mentoring.
Alternatively, some communication behaviours may simply be difficult to change, or our methods were inadequate to do so.
We did not formally measure whether or not the course changed patient outcomes, but health-care professionals reported that they were still using the knowledge and skills 1 month after the course, and had started disseminating it to colleagues.
We concluded that the VOICE communication skills training course was feasible to run, and defined conditions for it to do so successfully, including the use of simulation and video excerpts of real-life communication encounters. The evaluation of educational benefit, based on intermediate outcomes, strongly suggested that it had been successful. However, we studied only a relatively small group of health-care professionals who were experienced and interested and we cannot extrapolate to the general health-care workforce. A cadre of highly trained practitioners might, however, be useful in front-line practice, in role-modelling and in the case-management of difficult cases and teaching.
Chapter 7 Can we train? Interview study
Introduction
We developed and evaluated a communication skills training intervention using a B–A design and quantitative measures of course perception, knowledge, confidence and behaviours. The course included innovative features, and the use of simulation in training was unfamiliar to health-care professionals other than doctors. We wanted to understand and explore these features further, in order to help validate, or refine, the intervention choices made. We were also interested in whether or not, and how, the communication strategies that we taught were useful in practice, and wanted to understand practical and contextual factors in real hospital settings that might enable the use and dissemination of the findings, or provide barriers to implementation. We were aware that hospital clinical settings are busy and hard-pressed, and that resources and time for staff training are limited. We wanted to understand the value placed on communication skills training by clinical managers.
Methods
Participants
Fifteen health-care professionals and clinical managers were interviewed 3–6 months after the communication skills training course.
Interviews were conducted with 10 health-care professionals who attended the training, two ward managers who manage health-care professionals who had attended, and three health-care professionals who both attended the training and had managerial or supervisory roles over other health-care professionals (Table 21).
| Participant code | Job role |
|---|---|
| HCP1 | Junior doctor |
| HCP2 | Physiotherapist |
| HCP3 | Speech and language therapist |
| HCP4 | Middle-grade doctor (registrar) |
| HCP5 | Senior doctor (consultant) |
| HCP6 | Nurse specialist |
| HCP7 | Speech and language therapist |
| HCP8 | Junior doctor |
| HCP9 | Speech and language therapist |
| HCP10 | Middle-grade doctor (registrar) |
| HCP11 | Nurse specialist |
| HCP12 | Nurse |
| HCP13 | Nurse manager |
| M1 | Physiotherapy manager |
| M2 | Nurse manager |
Procedure
All health-care professionals who attended the course were invited to take part. There were challenges in arranging times and suitable locations for interviews, and, on occasions, interviews were postponed at the last minute because of work pressures or changes in shifts. Telephone interviews were offered to participants as an alternative. Five were conducted face to face in the participants’ workplaces, and 10 interviews were conducted over the telephone.
Semi-structured interview schedules were used, with separate schedules for health-care professionals and ward managers (see Appendix 4). Interviews were carried out by an independent occupational psychologist (LT) who was not involved in the development or delivery of the communication skills training. Interviews were audio-recorded and transcribed verbatim.
Analysis
Qualitative data were analysed (LT) using a framework method, drawing out themes concerning the usefulness and effectiveness of the communication skills training and the facilitators of and barriers to transfer of the learning into clinical practice. 198 NVivo 10 (QSR International, Warrington, UK) was used to manage the data and the analysis.
Transcripts and reflective notes were read and the audio-recording were listened to, in order to familiarise the researcher with the content. The first few transcripts were read line by line, and open coding of these transcripts took place. These codes were used to develop an initial analytical framework and a structure of categories and themes under which the codes could be grouped together. Remaining transcripts were then read and coded using the analytical framework. Constant comparison was used to compare codes across the data and to refine the structure of the framework. Coded portions of each transcript were extracted into the framework matrix. Finally, data were interpreted through a process of thematic comparison, in which all items of coded data within the categories were compared with each other for similarity and difference. Themes and subthemes were generated by bringing together items of data that were conceptually similar.
Results
Eighteen themes and 11 subthemes were identified, describing the experience and effectiveness of the communication skills training. These themes and subthemes were organised into categories derived from the study aims (Table 22).
| Framework categories | Themes | Subthemes |
|---|---|---|
| Experience of the programme | ||
| Most useful parts | Learning new techniques | High entitlement |
| Closings | ||
| Openings | ||
| Requesting technique | ||
| Simplifying and breaking down | ||
| Body language | ||
| Terminology for techniques | ||
| Evidence of what works | ||
| Less useful parts | Need for training in communication with aggressive dementia patients | |
| Effectiveness of training | ||
| Training methods | Use of simulation | Convincing as patients |
| Feedback on performance | ||
| Uncomfortable for some | ||
| Watching back simulations | ||
| Use of videos | ||
| Interdisciplinary approach | ||
| Structure of the training | Good organisation | |
| Balance of activities | ||
| Use of a second day | ||
| Effectiveness of approach | Effective training approach | |
| Alternative approaches | ||
| Who should attend | ||
| Transfer into practice | ||
| Facilitators of transfer into practice | Frequent use of skills | |
| Confidence to try | ||
| Cascading learning | ||
| Barriers to transfer into practice | Time with the patient | |
| Need for critical mass | ||
| Low frequency of use | ||
Experience of the training: most useful parts
Learning new techniques
All participants described how they had learnt new techniques for communicating with people living with dementia, and that this learning had been the most useful part of the training. The specific technique identified as most useful varied between individuals, and for some there were multiple techniques.
High entitlement
Many health-care professionals described how this was a new skill that they had learnt through the training and that they had adopted into their usual practice. Even health-care professionals experienced in working with people living with dementia, who reported that some of the other techniques were echoed in their previous practice, reported that ‘high entitlement’ had added a new approach for them:
I’ve changed my behaviour almost certainly because I think I used to address things in a bit more of a lower entitlement kind of fashion which doesn’t always work.
HCP8
I think probably having worked with dementia for a while I think I have always done [some of the other techniques] so I do not think that made any difference but I definitely think the increase in entitlement . . . I have been aware of that, I do not know if I used to do it before or not but I have certainly been more aware of it since the course.
HCP3
It was widely perceived that ‘high entitlement’ ran counter to other communication approaches that health-care professionals receive training in and are encouraged to use. Health-care professionals found it easy to adjust their communication style to a high entitlement approach, but felt that this might be harder for less experienced health-care professionals:
Those juniors just don’t have that [confidence]. You don’t teach that at university, you teach much more of a consent idea, the idea that it’s very much on the person’s terms, and you should give them time, which is absolutely right . . . That is just not going to work with this client group.
HCP2
Closings
Health-care professionals found the techniques for closing an interaction to be useful. This was particularly because health-care professionals found this to be something that had been difficult and often protracted before. Health-care professionals described how this helped them to feel a closure to the task, as well as helping the patient:
I really found it quite interesting about the sort of closing, that can be a challenge for all of us and so that, you know, using those tips like making a specific arrangement, being explicit and sort of non-verbally and sort of indicating that it’s coming to an end . . . so drawing on those skills again, that’s really helpful.
HCP7
Closing a conversation to mark the end of a task was also a clear and definite event, which made it easier to remember to apply it at that specific point in time. The use of a variety of props or actions to mark out a change in the activity was described by health-care professionals:
I used the wrapping-up idioms a lot, and I find that’s a really good way to end a session and notify someone that this is the end of . . . end of the, sort of, task.
HCP2
Using props or . . . using the environmental skills to finish the conversation like by moving the table or putting things down or using terminology that I’m going to finish the conversation.
HCP5
Health-care professionals again noted that this technique of using specific actions to help close down an interaction ran counter to the practice that many had previously used. A number of health-care professionals specifically described their use of open-ended questions at the end of an interaction with a patient, which they had stopped doing after the training:
. . . as a nurse, it has been my practice for 30-odd years that when I leave a patient, I always say, ‘is there anything else I can do for you before I go?’, that is my end line for all patients and I have stopped doing that now.
HCP6
One health-care professional went further to describe why the new techniques for closings ran counter to previous approaches practised, as it could sometimes feel discourteous and that you were not necessarily checking that you were leaving the patient in the best state. They explained how they had justified the change to a more direct closing because of the needs of other patients:
. . . because I think that is something that people really, really, struggle with, is leaving somebody who is still talking to them . . . Because it is just rude isn’t it and it is against everything that we would normally do but [this training] is about being able to give people permission to say, ‘do you know what, sometimes you might just have to do that,’ you know, it is one thing just walking away and ignoring somebody straight off but you know when you have given them 5 minutes, and you have tried your techniques and whatever and they are still going and you know that you have got 10 more patients needing your care, then . . . You just have to say, ‘I did my best’.
HCP6
This particular technique was reported to be useful with a wider group of patients:
. . . the ending the consultation strategy I probably have consciously thought about a lot more than I did previously, not necessarily just with dementia patients but with patients in general.
HCP1
Openings
Two health-care professionals mentioned the opening of a consultation as another communication technique that they found useful. In particular, the need to have a closed and focused opening, rather than an open and vague one, was mentioned by health-care professionals:
And I just tell all my colleagues not to open, like, ’are you OK? Are you well today?’ Or ’are you . . .?’
HCP2
. . . to put the start, to introduce myself and to clarify the purpose of the communication at that time . . .
HCP5
Requesting
A number of health-care professionals spoke about the usefulness of looking at different ways of asking patients to do things. They found a number of techniques to be helpful when requesting patients to co-operate in an activity, such as providing a rationale, or stating an action as a joint act between patient and health-care professional as a way of convincing the patient to join in with the task:
. . . so it has been more successful from the beginning the sessions because I have used the phrases that were suggested in the ways of asking people to do things and so I have probably had less occasions where we have had a difficulty or a challenge in an assessment.
HCP9
Simplifying and breaking down
Three health-care professionals talked about the lessons they had learnt about breaking things down into smaller tasks, and the benefits they had seen in using this with patients:
The breaking it down into the two boxes . . . and the hand-out sheet that she gave us. I found myself constantly talking to my juniors and my other staff members about using those, sort of, tips that they broke them down into different areas. The ’just checking’ question. The ’tries’ and the ’pops’, and like, reducing down your commands into smaller things, and trying to simplify things better.
HCP2
Body language
Learning about the importance of their non-verbal language was reported by two health-care professionals. More specifically, they described how they now understood how their body language indicated certain things to patients, and how it could be used to continue the interaction with a patient:
I’ve started using more non-verbal cues . . . to maintain the contact with the patient.
HCP5
Terminology for techniques
A number of health-care professionals described the value of assigning terminology to the techniques learnt in the training. Having a meaningful label not only helped to describe the technique appropriately in a way that made sense to the health-care professionals but also supported them in explaining the techniques to colleagues:
. . . so the entitlement and the contingency, I have never heard of that before I had never heard it described in that way and although I kind of did it, I did it not knowing what I was doing . . . So actually, but then being able to explain that to other people, is really really helpful.
HCP6
. . . it was quite nice just using that language because it underpinned what we’re doing, you know most of us are delivering this but it’s just giving it a term.
HCP7
However, one health-care professional found the need to use certain terminology to describe the techniques confusing and a bit contrived:
. . . they kind of wanted us to use, I can’t remember the words now, certain words and I think that was quite confusing sometimes for people, that high entitlement . . . they did say that they’d tried all to think of different ways of putting it and that was the easiest way but . . . could get hung up on ‘oh hang on what are we doing now,’ even though we knew what to do, so there was like a label on it which I think was a bit confusing.
HCP1
Evidence of what works
In addition to learning new communication skills and techniques, a number of health-care professionals found it particularly interesting and useful to understand the evidence and research findings that were the basis for the training. They described how this gave them the theoretical and evidence-based knowledge behind the practical skills taught, and this linking of the two elements was particularly valued by some:
I just thought it was so fascinating hearing all the research that had gone on, learning about things that have actually, seen have worked on the wards, it was kind of evidenced in a very useful way that these things had worked with patients, these techniques have worked and there was evidence of that when . . . talking about all the recordings that she has made of certain types of initiating assessment and some of the things that had been used had worked 100% of the time she had observed it and that was really great to hear that sort of evidence.
HCP9
Experience of the training: least useful parts
Most health-care professionals were unable to identify any element of the training that had not been useful in some way.
Need for training on communicating with aggressive dementia patients
One health-care professional felt that a useful addition would have been specific training on managing, and communicating with, patients displaying aggression. This was an expectation that they had before going on the course; it was a challenging area of practice that would be useful to have some practical advice and skills training on:
I guess it would be very difficult for the course to do but actually quite aggressive dementia patients which are the most difficult and wasn’t simulated, yes, wasn’t really part of the course and they’re actually the hardest people to deal . . . because that is a big part of dementia, the really difficult to manage patients.
HCP1
Effectiveness of the training: methods
Use of simulation
Simulation was widely considered the most effective training method used during the course. Health-care professionals felt that the opportunity to role play different health-care activities with SPs reinforced their learning of the skills and techniques taught, and helped them to embed new skills into their practice:
I think role play is definitely a really effective way and it is used in lots of different ways in my medical training as well, although it is difficult to do, it is obviously easier to sit in a lecture, you learn more and remember more from actually having to do it in a simulated environment.
HCP10
I really liked the opportunity to do the role play, that was really important because I think immediately, rather than just being given a piece of paper with this is what you should be taking away, you’re actually embedding it into your practice and trying to change your behaviours.
HCP7
Health-care professionals described how the use of simulation gave them an opportunity to try out different approaches and techniques in a safe, yet realistic, environment, emphasising the need for health-care professionals to adapt and change their approach each time. This was supported by ‘live feedback’ from peers and facilitators during the simulation exercise:
. . . the simulators had so much value as well, that you could challenge yourself, and see whether or not you got better from doing it. Or you could practice some of the techniques, so that was the thing, it’s that we were practising them all the time. I think it’s such a valuable way of learning, and it was completely new to me, and I really felt it was really beneficial really.
HCP2
. . . we got feedback from the people watching, we could stop and start if we needed to and try out different things and see how the patients reacted in different ways.
HCP4
I liked the way that you could try something that you might not actually do in practice and that ability to free you halfway through the scenario was really good. And then just getting feedback from the experts really, on our delivery in those situations.
HCP6
Allowing health-care professionals to choose role-specific tasks to try out with SPs added to the reality of the situation, supporting the embedding of the learning:
. . . being put in that situation where you had a task to do with a person who was acting like they had dementia and then having opportunity to have a go at it and then discuss what went well, what did not go well, discuss other possibilities and then have another go, I thought that was fantastic . . . You did actually feel you were in that situation and you were trying to complete an assessment with somebody with cognitive difficulties. It felt very real. That was absolutely brilliant way to learn strategies that you had been taught at the beginning of the course.
HCP9
. . . they were different scenarios as well, so you could sort of select something that was relevant to your profession like doing a swallow assessment or doing an oromotor assessment but you know, the patients presented very differently and it was interesting seeing how other people work.
HCP7
Being able to practise the techniques in a safe environment through the simulation increased health-care professionals’ confidence in the use of the techniques:
I really enjoyed it . . . having a go at something in a safe environment, where you are not going to be, you can be critiqued but not criticised, I think a lot of people find that very useful.
HCP6
I mean there was a lot of time to do role play and practise that and you know, actually I think that was really good because it just made you feel quite comfortable and confident going out as well.
HCP7
. . . it definitely gave me the tools that I needed to go on to use it myself so I feel like it was a lot more natural when I use it on a day-to-day basis but it was nice to be able to practise that in a safe and secure environment where you do get feedback from your colleague before going out to use it.
HCP12
Convincing as patients
Many health-care professionals commented on the quality of acting in the simulation, and found the actors to be very convincing as patients with dementia:
. . . the actors were really good, they were very convincing.
HCP4
How the simulations were used was also described as being very realistic; health-care professionals had a little background information about the patients, but needed to respond to unfolding situations:
The actors and actresses were absolutely amazing and it was real because you got a little bit told what you might be going to do with the actors and actresses, but you did not know a lot, so that was so real that you walked into a situation with somebody who had dementia and I know they were actors and actresses, but you would have thought they were people with dementia and you had to think on your feet.
HCP11
However, one health-care professional felt that they were unable to act in a completely natural and comfortable way because it was a simulated encounter. They found it difficult to ignore the fact that the SPs were acting. Despite this, they felt that actors were good, and that the simulated scenario did not change the way they would have interacted with the patient:
I thought these actors were actually better than I have experienced in the past . . . maybe it was because we . . . knew they were acting, it felt maybe it was as much our feeling that we were not always acting in a completely natural way because it was an artificial situation so maybe it was a bit of both, maybe it was partly the actors but partly us knowing they were acting made it quite difficult but I do not think I particularly, I do not think I responded in an unusual way or a way that I would not have done with a patient, it just felt strange.
HCP3
Uncomfortable for some
Some of the health-care professionals were less comfortable with the simulation exercises than others. In particular, doctors felt that their non-medic colleagues were more nervous because they were less familiar with simulation. In contrast, this was a familiar form of learning for doctors:
I think it was quite apparent that as a medic I probably felt a lot more comfortable doing the simulated scenario because I think we do it a lot more in our training than the other health specialties so that didn’t really faze me doing that, whereas I think quite a few of the nurses were really, really, nervous about that and didn’t particularly seem to like doing it.
HCP1
For some participants, completing simulation exercises in front of others could be intimidating. However, when carried out in a safe and positive setting this initial reaction would dissipate:
I think it can be intimidating for people, myself included, but I feel that for me it’s one of the best ways to learn and I think you’re in a good environment where you shouldn’t feel intimidated, it certainly shouldn’t make you feel that way. It can be quite scary to do it but if you receive the feedback and things and it’s often a good way of learning.
HCP8
Non-doctor participants also noted their lack of familiarity with this learning method but described that their apprehension was quickly overcome during the exercises.
. . . through physiotherapy you’d have no simulated patients whatsoever, well not when I trained . . . I was really apprehensive about the actors – I thought it was going to be a bit weird and a bit fake. In fact I found it really useful, and I found it not weird at all.
HCP2
Feedback on performance
Health-care professionals recalled how the feedback they received during the simulation exercises was particularly useful in developing their learning and skills. The formative nature of the feedback was highlighted as a beneficial tool, allowing health-care professionals to try out different approaches during the exercise. Being able to see and participate in the feedback to other members of the training course was also identified as a good way of learning:
. . . seeing other people mess up as well, that was great. So you really thought, ‘oh, OK, yeah, I’ve done that before, oh I know now what to do instead.’
HCP2
. . . we got feedback from the people watching, we could stop and start if we needed to and try out different things and see how the patients reacted in different ways . . . I think that was really useful.
HCP4
Feedback from the SPs was highlighted as a valuable addition, especially as direct feedback from dementia patients was typically infrequent:
. . . really, really helpful to get their feedback as well, both positive and negative. Because I think at times I was questioning myself . . . ’am I doing this right?’ So to get that feedback to say . . . ‘No, I found it really nice’ . . . But with working with dementia patients, you don’t often get that feedback.
HCP2
Watching back simulations
A number of health-care professionals stated that an additional learning tool would have been to have health-care professionals watch their own video-recorded simulations:
. . . but it almost felt like it would have been useful to see because . . . often we do that on simulation, we have to watch ourselves back and you can learn from that so it felt like almost an opportunity missed.
HCP1
Use of video material
Health-care professionals found the video-recordings of communication interactions with people living with dementia to be a good learning method. It allowed them to see a wide range of examples of the different techniques that were described, which promoted modelling of positive behaviours. Seeing evidence of how different approaches led to more-effective communication with patients reinforced the learning:
. . . because I wasn’t really aware until watching the videos of the impact that it had, but I had found it effective when I’ve changed the way that I, when I’ve used a different way to ask questions at the end of an interview or to close an interview.
HCP10
. . . the videos, where you saw other people go wrong, was really, really helpful, because you watched someone else mess up and then, well, one, it didn’t make you feel so bad when you messed up, which is absolutely fine, and then also you, sort of learnt completely from their mistakes and also saw how they changed that situation as well.
HCP2
Interdisciplinary learning
Health-care professionals responded positively to the interdisciplinary nature of the training course, which included nurses, doctors and AHPs learning together. Participants noted that most of their work was conducted in multidisciplinary teams (MDTs), but the opportunity to attend training with such a mix of professions was rare. They felt that the MDT approach to training was better than running the course with individual professional groups:
I think it was really nice working in a setting with loads of different health professionals which we often don’t really get to do that, we work together with everyone but you don’t actually learn together so I really like that.
HCP1
I think if it had been all speech therapists it would have been not as good.
HCP3
Learning about the perspective and approach of the different disciplines was described as being valuable. Seeing fellow health-care professionals carry out different tasks in the simulations gave insights into other professions’ interactions with patients and some of the challenging aspects of the work they experienced. Having a better appreciation of the constraints on different health-care professionals in terms of the task, and time available to complete it, was an important learning point:
. . . in my group there were a couple of speech therapists so we could see what sort of things they would use and how they could use those techniques as well in their day-to-day role so it was nice.
HCP4
. . . you start to understand the difficulties different people have in their roles but . . . I think it is important to try and understand where they are coming from . . . because the interactions are different aren’t they depending on what your role is . . . if you are a nurse based on a ward and you are doing a 12-hour shift, you have got lots of opportunities to revisit a situation or to change what you are doing, if you found one technique doesn’t work, but if you are a doctor, and you are coming to do a physical examination, or you’re a physio[therapist] and you want somebody to come for a walk with you and those, phlebotomists just want to take a blood sample, they are there for 5 minutes, that interaction is going to be very very different so . . . I think it is good for other people to be able to recognise that as well.
HCP6
This was also thought to generate more understanding and to reduce conflict between professions:
I think it was really beneficial to hear everyone’s different perspective because often we can be quite negative about each other.
HCP1
One manager thought that the transfer of training into a clinical environment was supported by interdisciplinary learning:
I think the increase in education for us as an MDT has been really helpful and as I say, sharing that knowledge with the MDT, particularly junior members of the team.
M1
Effectiveness of the training: structure
Good organisation
Health-care professionals remarked on how well organised the training had been in terms of the booking and administration, the venue and activities on the day:
The whole thing was very well organised to be honest, they sent me stuff in advance, we were sent stuff in between the days, we were sent things after the days to sort of consolidate and remind ourselves to just think about what we’d learnt, so there was plenty of follow-up from that point of view.
HCP4
Balance of activities
Health-care professionals felt that there was a good balance of activities between the theoretical and evidence-based learning and the practical work through simulation. There was a logical progression in the way the course was structured, and that the pace of this progression was right, with the training neither feeling too rushed nor too slow:
I thought the structure of it was really good . . . we were taught different techniques and the theory behind it and then we got to test those out . . . so I thought it was really well organised and very logical steps going through.
HCP4
Health-care professionals liked having time for reflection, discussion and feedback. Having this time built into the programme allowed health-care professionals time to think about the implications of the learning for their practice:
. . . [the trainer] was always asking for our input so that was really good and just being able to hear what other people were feeling and you could get that sense through the day that things were clicking and people were really reflecting on their clinical practice and how they could improve it.
HCP3
Having the variety of activities and teaching methods was also welcomed, as it kept health-care professionals engaged:
It’s a good mix actually, I think if you are sat in one place and being taught for a length of time then you know it can be quite difficult to keep the concentration up but in a . . . if you’re mixing things up and having videos and doing workshops, that I think is a lot more helpful in terms of getting people involved and getting you to actually, it helps the learning experience, so I think it was a good variety of things.
HCP8
Use of the second day
Health-care professionals generally found the 2-day structure to be effective. Returning for a second half-day reinforced learning from the first day. It gave an opportunity for health-care professionals to practise skills and try them out in their usual work before returning for further role play and feedback:
. . . two sessions was good as well because it helps to consolidate that knowledge.
HCP8
One health-care professional felt that the second day was not necessary:
I did think the second day dragged a little bit because it felt very repetitive . . . I know it is for research as well as just being training . . . if it was just training I think you’d have just needed the first day really.
HCP1
Two health-care professionals mentioned the logistical challenge of being able to attend both of the dates for the course, and one health-care professional manager reported that this had prevented some health-care professionals from attending the training:
. . . it was just so difficult to find somebody not having annual leave in one month, but then having it in the other month. That was the only thing.
HCP2
Effectiveness of the training: overall approach
Effective training approach
There was consensus that the approach adopted for the training was effective. There was acknowledgement that the course was labour intensive and, therefore, expensive to run, but this was seen as an inevitable consequence for high-quality training of specialist skills:
I really liked the videos and the audio stuff, I really liked the simulators as well. I almost . . . I just . . . I think that was a really perfect way of doing it, I know it must be really expensive to do it that way, which is difficult.
HCP2
I cannot think of a better way to do it but I am sure it is not a cheap way of training or an easy way of training but it is so much more effective than lots of other things that you do online training and just watching clips, actually getting the opportunity to trial them with an expert there helping you out if needed is great.
HCP9
Alternative approaches
Health-care professionals were asked if there were any alternative training approaches that could be used to teach the communication skills they had learnt. Some suggested that elements of the course could be delivered online, but not all of it. However, others argued that online learning was not an effective approach for some staff and suggested paper-based workbooks instead:
I guess it could be delivered as an online sort of resource but I do not think it would be quite as effective because I think a lot of discussion was quite helpful with people’s own experiences. I think that would be the most effective way but I guess the videos could be shown, I guess some of it could be done prior to a face to face . . .
HCP3
One thing I’m struggling with our core dementia training is getting staff to do the e-learning. Not all staff are very good on computers . . . For some of our staff . . . they might not be able to access that and that would be my worry, so if we do have an e-learning component, we need something that somebody else might access, you know, so work books might be useful.
HCP11
Who should attend?
Health-care professionals commented that the training should be aimed at a wide group of hospital staff who had contact with people living with dementia. It was further suggested that the participants who had attended the training had been people with an interest in and prior experience with dementia, so may already be relatively good at communicating with this group of patients. Other groups of staff may be in greater need of the training:
I think the training would have been as helpful if not more helpful for people who have not had experience of dementia or just starting out.
HCP3
Absolutely everyone [should have the training] . . . Certainly anyone who has patient contact.
HCP11
Some specific groups of health-care staff were mentioned too:
I think it would be beneficial for a wider group of health professionals to do, especially like nursing assistants actually, because I think they’re often with patients that work a lot more intimately with patients so I definitely think they could be a health-care group that were included.
HCP1
. . . [junior doctors] get taught a lot of communication skills throughout our training but we were never ever taught what techniques to use with people with dementia and actually when you’re on a general medical job, very much as I said on my ward probably 30–40% have dementia or delirium or something, then that’s the same very much across the board in most hospitals so I think it would be quite useful if we could try and get that run some similar courses aimed more at the maybe just one and two levels or maybe even going to various hospitals and try and do it as part of their training.
HCP4
Transfer into practice: facilitators
Frequent use of skills
Health-care professionals were asked about any factors that facilitated the transfer of the skills they learnt on the training course into their everyday practice. The most frequently cited factor was the frequency with which the health-care professionals were able to use the techniques they had learnt. Health-care professionals working on wards with a high proportion of patients with delirium or dementia reported that being able to apply the skills on a regular basis helped to reinforce the learning:
. . . so many times a day, if you’re doing a ward round, with certain number of patients and you’re doing it all the time so probably that’s why it’s easier to adopt because actually, and with everyone, because you’re repeating it really.
HCP1
Some health-care professionals described how they were asked to help colleagues with particularly challenging patients, and this allowed them to practise different approaches and techniques:
. . . my colleagues will be like, ‘oh, please can you help with this person, I was just really, really struggling with them’. From this, this and this reason, and it has given me the opportunity to say . . . to approach people in a different way, and to try things in a different sense, and definitely we’ve had different results from me doing it versus them.
HCP2
A few health-care professionals, who were working in an area where there were fewer dementia patients, had used the techniques with other types of patients and found them to be beneficial:
. . . [I have] found them just helpful with everyone, not necessarily with dementia patients.
HCP1
Confidence to try
Health-care professionals also reported increased confidence in trying different communication techniques with their patients:
I mean it is a confidence thing as well because you’ve done the training so you actually feel more confident in the way that you communicate with patients because you feel you’ve had the training, OK, I can do this and you don’t feel necessarily so bad if things don’t go necessarily the way you want them to go.
HCP8
Cascading learning
Health-care professionals reported that they had cascaded the skills learnt to other members of their teams. However, one felt that their colleagues did not use techniques as much as if they had attended the course themselves:
It was a really good experience and something that I am now promoting and telling staff you know we really need to get this embedded in the work that we do.
HCP11
I fed back about the course, and just some of the stuff that we used, and definitely people thought that the leaving idioms were really useful . . . We learn from each other quite a lot, we do a lot of stuff together, but I reiterated some of that, and . . . yeah, I don’t think they probably use it as much as if they had actually been on the training.
HCP2
Transfer into practice: barriers
Time with the patient
A lack of time with each patient was frequently cited as a barrier to using the communication skills in practice. This increases the pressure on health-care professionals, which can mean that they fall back on to previously used communication approaches:
. . . you’re aware in day-to-day life that you’re not necessarily using the best techniques all the time because there’s just not the time to put all that into practice sometimes but that’s not really anybody can do anything about is it, just the nature of the NHS.
HCP4
Interruptions during a patient consultation further reduced time available with that patient, and disrupted the ability to apply the communication skills in practice. The fast pace of activity on the ward was sometimes not conducive to effective communication with a patient:
A lot of it’s a time problem as well, you’re starting a task and you’re interrupted by someone to go and do something else and then you’re going up to do something and then you kind of dealing with some other issue and then, yes, I think it’s probably a bit more like the situation and the time.
HCP8
Need for critical mass
A number of health-care professionals felt that there was a need for a critical mass of staff on a ward to be trained with these communication skills. Only when the majority of staff adopt these communication techniques would there be sufficient consistency in practice for it really to benefit the patients:
. . . unless you get a critical mass, I think that could impact on it because you’ve got some people who are trying hard to do it that way and other people that are not following and that could be confusing to the person with dementia, that there are different approaches, you know consistency when you find the right way, I think is really important.
HCP11
One of the managers interviewed reported that consistency could also be negatively affected by the multidisciplinary nature of teams, which could lead to a variety of approaches being adopted with people living with dementia. This reinforces the benefits of potentially training a group of staff from a MDT:
. . . sometimes the MDT is very beneficial but sometimes the MDT does become a barrier, so we can only educate so much and then a lot of it comes down to staff perceptions of patients with dementia and them being willing to change their approach. So, we do do a lot of teaching but as I say, it does not always fall into practice with the other members of the MDT, so that is sometimes a barrier.
M1
Low frequency of use
Some health-care professionals who attended the training were not working in areas where there were a lot of patients with dementia. This meant that they were not frequently faced with situations that called for them to use the communication skills that they had learnt. Talking about the ‘high entitlement’ technique in particular, one health-care professional said:
I just haven’t had patients that I’ve needed to do that with because we’ve not had anyone feel uncooperative, I would use them and I actually think they’re good but I haven’t needed to.
HCP1
Discussion
The interviews with health-care professionals attending the VOICE communication skills training course and their managers have demonstrated that the participants found the course acceptable and useful, and were remembering and using the skills in practice. The use of simulation was particularly valued as an opportunity to practise skills in real time. Learning from other professional colleagues was also useful and valued. There were challenges to using the skills in practice, and focusing on a critical mass of health-care professionals on a ward attending the course might be beneficial.
Interviews allow an in-depth exploration of issues, and may reveal things that cannot be anticipated in advance. This study provided an independent and overwhelmingly positive description of what trainees thought about, and took from, the course, some months after it had taken place. However, interviews also represent a ‘public voice’, with the risk that participants report what they think they ‘should’ be thinking or doing, or what they think the interviewer wants to hear.
Some of the findings betrayed misunderstanding, for example that requesting in a highly entitled way was a mechanism for making requests, not a separate category from it, or that the communication techniques taught would take more time, rather than to save it, as was intended. However, overall, it appears that course participants had understood and retained what was taught, were using it in practice and were finding it useful.
Chapter 8 Patient and public involvement
We have involved service users and the public in every stage of our research.
The University of Nottingham’s Dementia, Frail Older Person and Palliative Care PPI group was founded in 2012 as a means to involve carers and people with dementia in our research. We felt that this was needed to provide the necessary support and training to members who were potentially experiencing stressful personal circumstances during the course of their involvement in research. The group has 24 members (there is some natural turnover) and meets for 2 hours, 10 times a year, on a set day each month. We provide regular training, both internally and externally. We have facilitators for the group (two research assistants and a research fellow), administrative support (funded from NIHR grants) and senior academics regularly attend. As a thank you to the group for their continued support, we provide a Christmas lunch once a year. As academics, we benefit from the PPI group as their lived experience of dementia contributes ideas and insights to our research.
The group currently supports six active studies and 10 PhD student studies. We pay PPI members an inconvenience allowance for attending study management meetings and steering committee meetings, intervention development group meetings and focus groups, and for time to read and comment on study documentation, in line with INVOLVE recommendations199. We also reimburse travel costs. When funding is available, we encourage PPI members to submit abstracts for conferences and to attend conferences with the research team.
At various stages of this project, from the initial idea to dissemination, we have involved carers, people living with dementia and interested lay people. In total, we have included 16 carers or lay people (mostly carers) and three people living with dementia in this research (13 from our PPI group, six via the Alzheimer’s Society).
Developing the study proposal
Our monthly meetings with the PPI group meant that we already knew that communication between health-care professionals and people living with dementia on hospital wards was an important area that needed improving.
The methods for researching this area were suggested by a speech and language therapist (RA). We felt that we needed to agree the methods, involving video-recording people living with dementia on the ward, as acceptable with the PPI group. Insights from the PPI group were collected through two meetings and a survey. The PPI groups were attended by 12 carers or lay people and one person living with dementia. These identified the importance of the topic of communication between staff and people living with dementia, the need for staff training in appropriate skills and the group provided examples of helpful staff communication behaviours. The group discussed the acceptability of video-recording interactions at length, agreed that video was important to capture non-verbal communication and suggested that mealtimes and discharge discussions were potentially important occasions to video. They also highlighted the possible need for multiple cameras or a wide-angle lens to capture all participants in an interaction (staff, patients and carers).
One of the group, Kate Sartain, agreed to be a coapplicant on the grant. As coapplicant, she attended all the project management group meetings. She has provided detailed feedback on the application and helped to write the lay summary.
Patient and public involvement and governance
We recruited a further two PPI members to attend our PMG meetings. These members both had recent lived experience of close family members with dementia who had experienced hospital care. We recruited another PPI member to the SSC and asked the Alzheimer’s Society to provide a further representative at this meeting. These PPI members at the PMG and SSC meetings support our research with constructive suggestions and challenge our assumptions at times.
Development of study documentation prior to ethics submission
Our PPI coapplicant reviewed all study documents and the lay summary submitted to the NHS Research Ethics Committee, as did a second carer of a person living with dementia. The review ensured that the language used in the participant information sheets was acceptable to someone with dementia or their carer. One of the PPI reviewers, following her review of documentation, asked whether or not family members would be involved in the video-recorded conversations. Her concern was that if the conversation was about a sensitive matter (e.g. a conversation about discharging the patient to a care home), the carer should be present and she would not want the study to interfere with this. This resulted in us changing our procedures slightly to allow an informal carer to be present during the video-recorded conversation if they wished. We also introduced carer participant information sheets and consent forms for them to be included in the video-recordings (two carers were included in the video-recorded conversations).
Intervention development
Three PPI members, including Kate Sartain, were members of the intervention development group (which also included health-care professionals, educational experts and experts in including simulation in training, conversation analysts and academics). The team met four times over a period of 5 months for whole-day meetings. The intervention development group discussed the duration, content and structure of the training, including the SP role profiles and the content of the reusable learning object. The group was shown video-recordings to be used in the training to get their views on the acceptability of them. The PPI members made the following recommendations on the training:
-
The course should be 2 days rather than 1. It was felt that 1 day was insufficient for health-care professionals to grasp the content and change their approach behaviour.
-
There should be a reflective diary between day 1 and 2 of the course. This was an innovative idea that proved very successful on the course and was developed into a reflective workshop on day 2.
-
The PPI members questioned how person-centred some video-recorded health-care professionals were. The videos chosen illustrated well the communication techniques we were to teach on the course. However, these comments changed the focus a little. The focus of the training became how health-care professionals use the taught communication skills techniques alongside person-centred care.
-
The PPI members considered that our initial method for rating communication behaviours shown in the simulation assessments would be too difficult for service users to use (as they would have found the rating form difficult to use themselves). This issue, together with the issues raised by the PPI members that the techniques we were teaching health-care professionals might result in them being less person-centred, resulted in us choosing the ETRS as a tool for service users to rate the simulated encounters before and after training. All members of the intervention development team practised using the ETRS on a pilot video-recorded simulation assessment. It was found to be acceptable by the PPI members, although agreement between the group members on rating scores was low.
Delivering the training intervention
Kate Sartain attended two of the 2-day training courses. Her role was to support the participants (health-care professionals), to help with the administration of consent and evaluation measurement scales, and to report back to the team on the fidelity of the intervention and the acceptability of the training from a service-user perspective. She reported that the course was acceptable, well run and delivered what was planned. Kate Sartain considered that the simulated workshops were done in a very supportive way, but raised a question about whether or not there was more we could do to support participants who are very anxious about simulation. This question was raised after Kate Sartain noticed how one participant appeared ‘out of her depth’ and did not return to day 2 of the course. This situation occurred on the last training course, but we are considering how to make it clear what the course involves and how to provide additional support to health-care professionals who find simulation very challenging when we put on future VOICE training courses. Kate Sartain commented that it was very clear that the interprofessional mixed training groups were obviously of value to the participants and the ambience of the day allowed for supportive conversations. Kate Sartain also raised the issue of health-care professionals who do not have English as their first language. Consideration of further research into this matter is vital if this training is to be of value to the diverse NHS workforce. Kate Sartain also has suggested that further research will be required to design a training package suitable for the workforce in the community, particularly in care homes.
Evaluation of the training
We are aware of recently published guidance from Alzheimer’s Europe that it is no longer acceptable not to include people living with dementia in research. 200 We accept this recommendation, while being aware of the challenges this represents. However, we felt going forward that we need to include people with dementia into our research and did this for the final stage of our research, namely the evaluation of the training. We wanted to know whether or not health-care professionals would remain as person-centred after training as before. To do this, we convened a group of seven service users. These included two people with early dementia and four carers. We organised five half-day sessions (with refreshments and lunch provided) and asked the service users, following training, to rate the B–A evaluation simulations using the ETRS. 193 Feedback from the group at the end was that they found the exercise stimulating and interesting and that they very much enjoyed being included in the research.
Dissemination
Our dissemination plans are ongoing. However, our PPI coapplicant Kate Sartain has presented a poster at the Alzheimer’s Europe conference (Berlin, Germany, October 2017) on service-user involvement in research. Kate Sartain made the opening address at our VOICE dissemination conference (Nottingham, UK, October 2017). She is supporting the work we are doing to develop future VOICE study courses.
Patient and public involvement values highly the positive effect this training will have on the ability of health-care professionals to provide skilled care to people living with dementia in an acute setting, removing much frustration and anxiety. PPI representatives believe that dissemination is essential for the well-being of the patient, their carers and health-care professionals.
Chapter 9 Discussion
Summary of findings
We video-recorded 41 encounters between health-care professionals and people living with dementia in the acute hospital. We used CA to understand what worked and what did not work, in real, practical settings. Encounters followed a recognisable phase structure: opening, purpose, information gathering, business and closing, although not all encounters contained all elements. Most of these phases were trouble-free in interactional terms. Two phases were consistently, and strikingly, associated with problems: requests (and consequent refusals) and closings. The manner in which things were said had a major influence on acceptance or refusal. Unusually for health-care communication, requests were often met with an unmitigated refusal (‘no’). Skilled health-care professionals used several devices in order to gain the agreement of the patients living with dementia: they asked more directly, raised entitlement (authority to ask) and lowered contingency (reduced the difficulty), by making the task sound smaller or shorter, asking the person living with dementia to ‘try’, offering to help or proposing collaborative action (do it together). Closings were often prolonged, with the person living with dementia not recognising the usual verbal or body language cues that the encounter was coming to an end and often reopening the conversation. More satisfactory closings resulted when the end of the task was declared, a specific arrangement was made for what was to happen next, and body language that was congruent with the message or ‘closing idioms’ was used.
These original, and ‘teachable’, findings, together with evidence from a systematic review, were used as the basis for a new communication training course for experienced health-care professionals. An intervention development group was convened that included researchers, clinicians, educationalists (and, in particular, people with expertise in simulation) and PPI. The course comprised 2 days, 1 month apart, and was grounded in experiential learning theory. It used didactic learning, video clips and transcripts from real life, simulation and reflection on practice. We were concerned to draw on, and integrate, health-care professionals’ prior knowledge and experience, and to ensure that the principles of person-centred care were adhered to. As preparation, we asked trainees to complete three brief electronic-learning packages (RLOs) on dementia, basic communication and person-centred care. In addition, we developed a new RLO on requests and refusals that we asked trainees to undertake before the second session as revision. A RLO on closings is in preparation.
A training programme was devised, and manualised, and actors were trained to credibly simulate people living with dementia, with particular regard to refusal and extended closings. Courses comprised six to nine participants, with two trainers/facilitators. Simulations took place in groups of three to five, and allowed for the action to be stopped in order to ask advice or try different strategies or rerun. Peers, facilitators and simulators all fed back on performance. A pilot course was run with experienced health-care professionals, all of whom had an interest in education, and adjustments were made based on the experience.
We ran six courses in two hospitals, involving 45 participants, 44 of whom returned for the second day. Trainees were interdisciplinary, with nurses, doctors and AHPs taking part alongside each other. We undertook a rigorous analysis of the education, including three of Kirkpatrick’s four levels of educational effectiveness. These were feedback on the course, its usefulness and the methods employed; tests of knowledge and confidence in a B–A design; and an assessment of whether or not the course changed communication behaviours. This was done by asking trainees to perform an assessment task with a simulator before and after the training, which was video-recorded, in a crossover design. Videos were blind rated by two independent speech and language therapists against a checklist of behaviours. A panel of PPI representatives, including two people living with dementia, rated the test videos for ‘emotional tone’ as a measure of person centredness. Trainees were asked if they were using the techniques taught and if they were useful in practice 1 month after the course. An independent occupational psychologist interviewed a sample of trainees and managers to investigate facilitators of, barriers to and value placed on the training using a thematic analysis.
The course rated very highly. Knowledge and confidence both improved, statistically significantly, despite fairly high baseline scores. Some aspects of communication behaviour were more commonly observed in the test videos after the training than before. Emotional tone was mostly unchanged (i.e. communication had not become more, or less, person centred), although videos were rated as being slightly more dominating, bossy and controlling after training. Techniques were remembered, used and found useful 1 month after the training. The interviews found that the course was very well received, validated all the decisions made during intervention development, emphasised the value of simulation, interdisciplinary learning, reflection and the 2-day structure. Some participants found simulation uncomfortable, but almost all recognised its educational value. Learning was regularly used in daily practice and was, to an extent, cascaded to other staff, or used as a framework in teaching other staff. Length, cost and lack of consideration of communication during aggressive episodes were considered weaknesses. Wider dissemination was supported.
Strengths and limitations
The teaching on the VOICE training course was grounded in empirical research. In the field of communication training this is, perhaps surprisingly, uncommon. We used a rigorous sociolinguistic method, CA, applied to real encounters between health-care professionals and people living with dementia, to identify the structure of interactions, when problems arose, how skilled practitioners tried to overcome these, what worked in practice and what we considered to be ‘teachable’. The analysis uncovered original and interesting new linguistic findings, but was fundamentally directed at what might be taught to fellow practitioners, and practised both with simulators and in everyday care.
Dementia communication has rarely been studied in the challenging environment of the acute hospital. CA has been increasingly used to understand health-care consultation, but most communication teaching is based on experience, custom and practice. The overriding strength of using CA is that it studies what participants do in practice, not what they think or report that they do. This can also mean identifying and making explicit behaviours that the individual does not necessarily consciously know they are doing. By studying skilled practitioners we could identify both difficulties, and successes, and how difficulties or breakdowns were overcome.
Intervention development was multidisciplinary and interprofessional, including experienced clinical educators, PPI, clinicians and experts in simulation. Simulation has been used in teaching both consultation and practical skills, including scenarios involving people living with dementia,81 and difficult conversations at the end of life. Clinical practice is characterised by the need to ‘think on your feet’, responding in real time to a variety of information of unknown veracity, coloured by emotions and reactions both in the patient and the practitioner. Although some approaches, techniques and ‘tricks of the trade’ can be learnt or refined through experience, the opportunity to practice new skills and gain feedback is reported as invaluable by trainees.
Simulators, actors trained to work in clinical education, can provide consistency and challenge, and feedback either in or out of role. 201,202 In some settings, people living with a condition can take part in education, such as aphasia after stroke. However, this is difficult for people living with dementia, especially those with moderate or severe impairment when communication problems are most troublesome. Portraying a person living with dementia is not easy, with a risk of stereotyping or caricaturing, or simply producing chaotic responses. In this study, we carefully developed training for simulators, based on real cases we had observed, to enable a credible simulation experience.
We used mixed training methods, including didactic information giving (lectures, PowerPoint presentation), and made extensive use of video clips, or transcripts, of real encounters. We also used reflections on practice, and considered how to incorporate previously mastered skills and attributes, especially paying attention to understanding and maintaining person-centredness. We refined the training course based on a pilot course, to which we invited experienced practitioners who themselves had a role or interest in clinical education, allowing an informed educationalist’s view.
Education and training initiatives are often evaluated quite crudely. The opportunity to do true experimental studies is unusual. Training can be evaluated at the levels of reaction, knowledge, behaviour and impact on outcomes. 188 We used questionnaires to study trainees’ perceptions of training methods, the role of simulation and the usefulness of the knowledge gained. We used questionnaires delivered before and after training to assess changes in knowledge and confidence. Innovatively, we used video-recorded simulations before and after the training to assess changes in communication behaviour. Two specialist speech and language therapist raters, blind to whether the simulation was before or after training, used a checklist to identify the use of objectively identifiable communication behaviours, using a crossover design to control for differences in the nature and difficulty of the set task.
The interactions between people living with dementia and health-care professionals that we video-recorded had some limitations, largely determined by the need to gain consent or agreement from participants and to set up recording equipment in advance, but were otherwise unstaged. All were initiated by the health-care professional; we excluded interactions initiated by the patient. Health-care professionals were all willing to be video-recorded, and we targeted health-care professionals whom peers thought were good communicators or ‘good with people living with dementia’. This was appropriate because we were looking to see what worked in real-life practice. Less confident health-care professionals, including some with English as a second language, were reluctant to take part. Interactions were typically brief (2–30 minutes). Analysis could not take into account what previous relationship the health-care professional had with the patient, nor what previous knowledge they were working with. It is possible that health-care professionals changed their behaviour as they were being studied, although it is generally considered impossible to ‘fake’ the sorts of behaviour that are ascertained in CA. Families and other carers are especially important in the support of people living with dementia in hospitals; for the most part we did not include ‘triadic’ conversations, as these had a very different dynamic and were difficult to film in a way suitable for CA (i.e. to include all participants’ body language and expression). Many communication problems arise during personal and intimate care, but we did not film these to respect patients’ privacy and dignity.
Conversation analysis is detailed and time-consuming. Within the resources and time frame available to the study, not all themes or foci of interest could be fully analysed. CA, like any qualitative analysis, is to some extent subjective, or at risk of preconception or bias. To overcome this, regular supervision and group data meetings were held that included experienced conversation analysts. Data and proposed interpretations were also presented and discussed at regional and national data-sharing meetings, a common practice among conversation analysts.
The structure of the training intervention was influenced by our previous experiences of learning and teaching communication skills, especially in aphasia after stroke and end-of-life care. We were also influenced by our experiences researching and teaching person-centred dementia care. 6,34
Use of simulation can be controversial, particularly the issue of authenticity, and relatively expensive. We paid particular attention to authenticity in the training of actors.
The evaluation was based on six repetitions of the course. We invited ‘experienced’ practitioners to take part, and in practice this was self-defined. Participants included senior nurses with leadership roles in dementia education and service development; staff nurses from older persons and surgical wards; and AHPs, including occupational therapists, physiotherapists, speech and language therapists, an orthotist, and junior and more senior doctors from geriatric and general medical specialties. The course was free and, therefore, attractive to staff for whom access to advanced education was limited, but participants were enthusiastic volunteers. These enthusiasts or ‘champions’ are an important training target: they will be role models, will direct or supervise difficult situations in clinical areas, and will teach, informally or formally. We previously found evidence that even experienced practitioners lacked confidence in working with people living with dementia, despite this being an important part of their jobs. 25
Only three participants had English as a second language. Although doctors were well used to simulation as a training medium, this was unfamiliar for other disciplines and was seen as challenging or threatening for some. Participants were told that they were expected to undertake the evaluation tasks (questionnaires and video-recorded simulation) in order to be accepted on to the course, and this may have been off-putting. Almost the first activity undertaken by participants was a fairly challenging video-recorded assessment simulation without feedback. The emotional tone rating of assessment videos was administered by a group of PPI contributors, including two people living with dementia. The scale is simple but used words that are open to interpretation. Inter-rater reliability was poor (there was poor agreement between different raters about whether or not a feature was evident in the interaction).
We have evidence (from the assessment simulations) that the VOICE training changed participants’ strategies for closing encounters with people living with dementia, but not their requesting behaviours. We do not have objective evidence of changed behaviours in real-life clinical practice, nor of any impact on well-being of patients. This requires further research with an implementation focus, involving the systematic observation of trained staff carrying out routine health-care encounters. Our trainees were a self-selecting group, who demonstrated an interest in communication, which may explain why they appeared to have high levels of skill in some domains at the outset. This limits the generalisability of our findings and may explain why requesting behaviours remained unchanged. Trainees were using the strategies of raising entitlement and lowering contingency already, and, although the course gave them a new vocabulary with which to reflect on this, it did not result in significantly changed behaviour as they were intuitively doing it routinely. For trainees finding requesting and subsequent refusals from people living with dementia a challenge, our methods may have resulted in objective change in this regard as well. The interviews also pointed to the fact that some trainees found the concepts of entitlement and contingency confusing, which may have also been a factor in the lack of objective change in this trained behaviour. Direct observation of taught strategies being used in real-life clinical practice will also allow us to contextualise positive reports from trainees when evaluating the course and reflecting on strategy use. We acknowledge that trainees may have been subject to social desirability bias when reporting their views to the team, mitigated to an extent through the use of an independent occupational psychologist in undertaking interviews.
The use of simulation in evaluation and testing has been criticised (e.g. in employment procedures, assessing competencies and examinations). 152,203 The main problem is the tendency to perform to the teaching, learning goals or expectations in a way that would not happen in real clinical practice; this is analogous to exaggerated looking in the mirror during a driving test. For example, simulators will be given brief background information and limited key information. The assessment ‘game’ becomes for the trainee to ‘extract’ this information, and verbal devices for enabling this soon become common knowledge, thereby diminishing the validity of the assessment. In a learning situation this is not necessarily a problem; a skill is practised with a reactive human partner, enabling the interaction to be experienced, rather than just contemplated or imagined, and feedback given. In our assessment of behaviour this could have occurred: before the training; the task was undertaken without knowing specifically what we were looking for; after the training; the behaviours we were teaching had been made clear. In educational practice, the ideal assessment is clearly mapped to learning goals, making the argument somewhat tautologous. To overcome this we prompted an overt link to what health-care professionals did in clinical practice, and asked for reflection on this in writing, then in a discussion group.
We did not study the influence of communication training on health outcomes for patients. This would require a very large-scale trial, possibly cluster randomised, with large-scale training of involved staff members. This would be very difficult to do logistically, and is rarely reported for communication skills training. Instead, we interviewed trainees and managers about the usefulness of what they had learnt, whether or not they had used it, the barriers to and facilitators of use in practice, and the priority given to training in communication with people living with dementia by service leaders.
Context
In 2011 Tadd et al. 204 published their Dignity in Practice report and stated that: ‘a key message echoed by staff at all levels in the organisations involved in this study was that the acute hospital is not the “right place” for older people. The prevalence of this view has resulted in the physical environment, staff skills and education and organisational processes acting as barriers to delivering dignified care to older people’.
People living with dementia, and other vulnerable frail older people, comprise core NHS acute and general hospital users. Two-thirds of hospital users are aged > 70 years. Half of emergency admissions of people aged > 70 years have cognitive impairment (dementia, delirium or, most commonly, delirium complicating dementia), and 40% of them have dementia. 10,205 Almost half of people who break their hips have dementia, whereas others have delirium, or develop it postoperatively. 206 People living with dementia are complex and are disproportionately represented among those with very prolonged hospital stays. Health policy rightly promotes ongoing attempts to minimise the need for hospital admission, and to expedite discharge for those who are admitted. However, most admissions are for legitimate medical conditions or injuries, and the delivery of necessary assessment, treatment or future care planning. 4 Caring for people living with dementia is, and will remain, an important part of what acute general hospitals do.
Hospitals are well known to be difficult and challenging environments for people living with dementia. This is partly because of the need to focus on the efficient and safe delivery of effective physical health care, but also reflects a failure to make the ‘reasonable adjustments’ to environment, staffing, training and processes required to make services as good as they can be for people living with dementia. 25,204 Staff often recognise, and are frustrated by, a lack of appropriate knowledge and skills, and identify communication as a key topic requiring further training. 2,25
Communication difficulties are well recognised as a problem for people living with dementia. This includes the specific language skills of understanding and expression, which are compounded by poor memory, impaired mental processing or reasoning, and problems in recognition, planning, initiation and social control. In addition, comorbid problems with hearing or vision, mouth or teeth problems, delirium, insomnia and pain also make communication more difficult. A noisy and busy environment can be overstimulating, and assessment processes involving multiple new and unfamiliar faces and locations, and repeated questioning, can be overwhelming.
Attempts to improve staff training and hospital experience for people living with dementia emphasise communication. Individualising care, seeing the perspective of the person living with dementia, building relationships, promoting inclusion and providing purposeful activities are key components of person-centred dementia care, and implicitly require good communication. Misunderstanding or misinterpretation of instructions or actions, especially when delivering personal or intimate care, or ensuring safety, are important drivers of distress and behaviours indicating distress. Some advice is uncontentious: optimising hearing (e.g. by ensuring that hearing aids are working), introducing yourself and saying what you are doing. Skilled practitioners, especially from mental health and palliative care professional backgrounds, have developed considerable expertise, although they sometimes struggle to articulate exactly what they are doing, making teaching or sharing skills difficult. Little of what is promoted has derived from research using rigorous methods, although much clearly ‘works’. 190
Most published evaluations of communication skills training for people living with dementia have taken place in care homes and have targeted nurses and unregistered care workers. Brief medical student teaching, using simulation, has been reported and was successful, although in some cases it only made students more aware of their limited skills. 81 Attitudes towards communication with people living with dementia have been studied, and a framework for communication has been published based on empirical research,207 but the effectiveness of implementation has not been reported.
Interpretation
Our results draw on CA findings from other settings; the structure of encounters was similar to that previously reported,114 and the roles of increasing ‘entitlement’ and reducing ‘contingencies’ to gain agreement have been described before. 127 The value of ‘direct imperatives’ has also been reported. 208 The findings ‘made sense’ to experienced practitioners, who had not previously had the concepts or language to describe what they were doing.
Conversation analysis is strictly an empirical methodology: it describes and makes explicit what was done and what the response was, and avoids speculating about motivation, or mechanisms of action. Interpretation therefore necessarily goes beyond CA.
Dementia (and its related complication, delirium) causes cognitive (or neuropsychological) impairments, including language, information processing and reasoning. Health-care professionals strive to be empathetic and polite. Many are aware of the power imbalance between patient and professional, and the disempowering effect of the unfamiliar hospital environment, care being delivered by strangers and the unusual or threatening nature of many health-care assessments and procedures (including personal and intimate care). Staff adapt their language to mitigate this, often becoming deferential (showing ‘low entitlement’) and offering choices that imply the possibility of refusal. This is also common in everyday English language and culture. In closing an interaction we routinely rely on the giving and registration of cues that the conversation is coming to an end, in order to try not to give offence, but these can be subtle.
In order to decipher the ‘message’ from among the social and cultural etiquette requires understanding, processing, perception and insight, processes with which a person living with dementia is likely to struggle. Person-centred dementia care philosophy holds that people living with dementia require an ‘enriched’ or ‘enhanced’ social environment, in which the health-care professional takes greater responsibility for making the relationship, even in the face of reluctance or resistance. Adapting communication to make it easier for the person living with dementia can be seen as a central part of this. The person living with dementia does not benefit from social etiquette if meaning is unclear, ambiguous, open to misinterpretation, invites refusal or results in a necessary medical or personal care task being neglected or argued over. The person living with dementia needs to feel satisfied with communication, avoiding, when possible, contradiction or argument. Reaching swift and unambiguous agreement is a virtue.
The risk is that language can become unduly coercive, or can fail to respect the identity or vulnerability of the person living with dementia. In many ways all language carries this risk; the lines between agreement, persuasion and deception are subtle. Rhetoric, marketing, propaganda and political messaging all deliberately attempt to persuade or change opinion. 209 CA has an overtly ethical dimension: findings can be used to promote good, or can be misused and result in harm. 143 The importance of professional, ethical and person-centred practice is undiminished by learning what language can be used to gain agreement or end an encounter.
We were aware of this potential problem, emphasised it in teaching and used an independent rating of ‘emotional tone’ of assessment videos before and after training (as a proxy for person-centeredness). This revealed some tension between effective communication and person-centredness, in that independent ratings suggested that communication was slightly more controlling, bossy and dominating after training. However, we do not believe that our findings reflect an incompatibility between person-centred care and effective communication. Several caveats are worth noting in this regard. How a conversation ‘sounds’ (the basis for rating emotional tone) is not necessarily a reflection of its person centredness. For example, a highly entitled direct request may promote inclusion and occupation, and does not necessarily diminish identity. Second, brief video clips offer little information about the context or necessity of a request, nor what occurred before or after. Thirdly, the inter-relater reliability of assessments was poor, suggesting that different people see different things in an interaction.
We undertook this CA-based study of dementia care communication precisely because most best practice guidelines in this area are not underpinned by objective research. Furthermore, it is unclear how person-centred care is operationalised in terms of communication behaviours during health encounters with a person living with dementia (or during health-care encounters generally). We have found evidence that some of the more common strategies for enacting person-centred care (like asking ‘is there anything else I can help you with?’) may be inappropriate for people living with dementia as a result of their communicative and cognitive difficulties.
We (and our trainees) concluded that our training changed knowledge, skills and behaviour and was useful to staff in diverse roles in everyday front-line clinical practice (and indeed may be useful for patients who are cognitively intact). Health-care professionals already have considerable knowledge and skills, technical and discipline specific, but also generic and interpersonal. Many experienced in working with people living with dementia are at least familiar with the ideas behind good communication and person-centred care. The key elements of our educational endeavour were the provision of new knowledge, a framework for understanding why communication can break down, the integration of prior skills and attributes, the opportunity to rehearse, practice and have feedback on communication behaviours, the opportunity reflect on communication encounters between the 2 days of the course and progression to more challenging simulation on the second day.
One feature that was commented on by trainees was the value of interdisciplinary learning. Different disciplines may not regularly observe how others communicate; watching peers and colleagues communicate in simulations proved as valuable as direct experiential learning. Another particular feature valued by trainees was the use of real-life video clips or transcripts illustrating learning points, both positive and negative. These carried especial validity (they were, after all, real) and were often memorable, but also illustrated real-life complexity, difficulties, failures and the sense of negotiation often required to gain agreement, which is difficult to encapsulate in writing.
A further effect of making explicit good communication practice is the engendering of ‘confidence in competence’. Griffiths et al. 25 identified that even when health-care professionals were doing their best, and delivering care well in difficult circumstances, they were often unsure, or frustrated that they were not doing well enough: they lacked ‘confidence in competence’. Health-care professionals’ knowledge that they are doing the right thing is important for job satisfaction and the avoidance of stress and burnout.
The VOICE course was mapped onto the Skills for Health – Dementia Core Skills Education and Training Framework Tier 3 (expert level) for communication, interaction and behaviour in dementia care and for person-centred dementia care. 35 This level defines the expectations of expert practitioners. There is little current provision in the UK for this level of training, and identifying such training is a current Health Education England priority (C Surr, Leeds Beckett University, 2018, personal communication). We have assessed the course using the Dementia Training Design and Delivery Audit Tool (DeTDAT), which assesses how well dementia training and education packages for hospital staff meet evidence-based good practice criteria. The VOICE course met all the requirements of this tool. 190,210
Implications
Hospitals and other care settings should make further ‘reasonable adjustments’ to ensure that staff are prepared to look after people living with dementia. 27 Many factors influence quality of care: the UK Care Quality Commission has characterised this as requiring leadership, attitudes, skills and resources. 12 Staff skills alone are not enough if staff numbers and time, the physical (and auditory) environment, processes and priorities represent barriers to dignified and person-centred care. Leadership requires both a commitment to training and the application of skills and knowledge in practice. Training can influence attitudes: showing that things can be done, and done well, helps to avoid a tendency to nihilism. Good communication helps to support identity, inclusion and occupation, which is more satisfying and defends against objectification and infantilisation.
Teaching adequate staff skills, however, remains central to the provision of good care. Care of people with dementia is complex and can be difficult. Poor communication results in missed therapeutic opportunities, mistakes, distress, denial of choice or autonomy and poor decisions. Distress, or unexpressed need, can result in difficult behaviours. Creating comforting relationships is the key to enhancing well-being and improving satisfaction. Unless senior staff understand and can model best practice, less experienced staff and students will not be adequately supported.
The main barriers to widespread implementation are expense and the need to train actors. The cost is modest in commercial training terms (full economic cost of about £300–350 per person, 2017 value), but health-care professionals often have little or no access to funds for training. Incorporation into undergraduate or postgraduate training structures (such as Foundation Programme or Higher Specialist Medical Training for doctors, or Learning Beyond Registration for other health-care professionals) would provide another avenue. The most likely niche will be as a fairly centralised resource for specialist practitioners. Given the importance of older people with dementia in hospitals, however, the numbers of people requiring the skills that our course teaches is very considerable.
In order to train actors in credible and effective simulation of people living with dementia we are preparing a manual and supporting materials (including video clips and the 22-minute documentary Today is Monday about people living with dementia and the staff who care for them in an acute hospital). 187
In addition, we have developed two brief electronic-learning multimedia packages (RLOs)175 that support the training but cannot replace the face-to-face content.
We are also exploring shorter packages, to minimise cost.
Further research can be done from within the corpus of video data collected for this study, and to explore related questions (including the enactment of person-centred care) and settings (such as care homes). The CA-based methodology is powerful and generates highly applicable practical output, but is labour-intensive and requires careful consideration of consent, data security and reuse of material. 211 However, the methodology provides great opportunities for further understanding communication in health care.
We undertook a feasibility or proof-of-concept study. In the face of known and acknowledged problems, it is likely a priori that teaching communication will be worthwhile for this patient group in this setting. A large RCT to demonstrate benefit in terms of patient-level health-care outcomes would be unfeasibly large and expensive, and would be unprecedented in the field of communication training. Our evaluation study may be considered sufficiently ‘positive’ to support implementation and roll-out without further large-scale evaluation. However, further research should be done to adapt or develop training for a wider body of staff, and to evaluate its effectiveness. This might include unregistered practitioners (such as health-care, therapy or nursing assistants), all registered staff who work with people living with dementia (who may be less skilled initially, more unwilling to learn and engage less well in training than the volunteers we studied) or staff with English as a second or additional language.
The development of communication training and its evaluation is also required for staff who work in care homes, domiciliary care staff and for family carers. The interplay between communication skills and person-centred care requires further exploration. A wider range of communication encounters (beyond health-care practitioner-initiated requests), and how they are managed, might be studied, although the practicalities of CA might make this difficult (e.g. gaining agreement in advance and setting up a camera).
The ultimate goal of staff training is to improve the quality or efficacy of care. Research methods to determine the impact of communication practices on patient outcomes, such as health status, well-being or distress, or health-care-related metrics, such as safety, discharge destination or length of stay, are poorly developed and require attention. Non-participant observation may be required. Similarly, from an organisational perspective, enablers of and barriers to implementation require investigation, including features such as ‘critical mass’ of trained staff, leadership and culture, and competing priorities, and how such conflicts or trade-offs are best managed.
Traditional methods of teaching communication skills for people living with dementia in hospital have been inadequate. We have drawn on multiple different pedagogic approaches to develop an innovative and effective training course, teaching evidence-based key practical knowledge.
Acknowledgements
We thank patients, family members and carers, and health-care professional staff who agreed to be video-recorded, and staff and managers who took part in the communication skills training courses, evaluation and interviews. Patrick Costello and Becky Whittaker gave expert advice on the use of simulation in health-care communication training. Giulia Miles helped to arrange simulators and made arrangements for the training courses and contributed to intervention development. Gillian Knight, Judith Booth, Carolyn Oldershaw, Terry Stevenson, Kevin Hayes and Martin Slipp were expert simulators, who enthusiastically and creatively engaged with learning how to simulate people living with dementia. Heather Whitehouse and Talie Smith (speech and language therapists) did the ratings of communication behaviours. Liz Stokoe chaired the external study steering group, which comprised Zoe Wryko, Simon Thacker, Danielle Hindle, Mary Heritage, Margaret Kerr, Eleanor Ford and Justine Tomlinson.
Contributions of authors
Professor Rowan H Harwood contributed to the study conception, supervised pilot work, wrote the funding application, supervised ethics and governance application, designed the communication skills training intervention, delivered the communication skills training course, designed the evaluation, drafted the discussion chapter and edited the complete text, had general oversight and leadership, sat on the management group, approved the final report and undertook dissemination.
Mrs Rebecca O’Brien contributed to the study conception, undertook pilot work, wrote the funding application, undertook the systematic review, undertook the ethics application, recruited participants, collected and analysed the video data, designed the communication skills training intervention, designed the simulations and trained the simulators, delivered the communication skills training course, designed the evaluation (including the rating of simulation video data), drafted the interventions development chapter, drafted the CA chapter, sat on the management group, approved the final report and undertook dissemination.
Dr Sarah E Goldberg contributed to the study conception, supervised pilot work, wrote the funding application, undertook the systematic review, supervised the ethics and governance application, undertook the project management, designed the communication skills training intervention, designed the simulations and trained the simulators, delivered the communication skills training course, designed the evaluation (including the rating of simulation video data), analysed training evaluation findings, drafted the evaluation chapter and PPI chapter, edited the final report, chaired the management group, approved the final report and undertook dissemination.
Mrs Rebecca Allwood contributed to the study conception, undertook pilot work, wrote the funding application, undertook the ethics application, recruited participants, collected and analysed the video data, designed the communication skills training intervention, drafted the CA chapter, sat on the management group, approved the final report and undertook dissemination.
Professor Alison Pilnick supervised the CA, advised on the use of CA findings in professional training, designed the communication skills training intervention, drafted the CA chapter, sat on the management group, approved the final report, undertook dissemination and led the linked Economic and Social Research Council impact accelerator award.
Dr Suzanne Beeke wrote the funding application, supervised the CA, advised on use of CA findings in professional training, designed the communication skills training intervention, designed the evaluation (including the rating of simulation video data), drafted the CA chapter, sat on the management group, approved the final report and undertook dissemination.
Dr Louise Thomson wrote the funding application, designed the evaluation, undertook and analysed the interviews, drafted the evaluation chapter, sat on the management group and approved the final report.
Ms Megan Murray designed the communication skills training intervention, designed the simulations and trained the simulators, delivered the communication skills training course, drafted the chapter on training simulators, sat on the management group and approved the final report.
Dr Ruth Parry wrote the funding application, advised on video-recording methods and ethics, advised on the use of CA findings in professional training, sat on the management group and approved the final report.
Dr Fiona Kearney drafted the introduction, checked and edited the references, checked and edited the complete text and approved the final report.
Professor Bryn Baxendale contributed to the study design, designed the communication skills training intervention, facilitated the delivery of training courses, advised on interpretation and dissemination, sat on the management group, approved the final report and undertook dissemination.
Mrs Kate Sartain advised on the study design, video-recording methods and ethics, designed the communication skills training intervention, delivered the communication skills training course, advised in interpretation, was a PPI representative on the management group, approved the final report and undertook dissemination.
Professor Justine Schneider designed the communication skills training intervention, edited references and the final report, compiled the references, sat on the management group, approved the final report and undertook dissemination.
Publications
Allwood R, Pilnick A, O’Brien R, Goldberg S, Harwood RH, Beeke S. Should I stay or should I go? How healthcare professionals close encounters with people with dementia in the acute hospital setting. Soc Sci Med 2017;191:212–25.
O’Brien R, Goldberg SE, Pilnick A, Beeke A, Schneider J, Sartain K, et al. The VOICE study – a before and after study of a dementia communication skills training course. PLOS ONE 2018;13:e0198567.
Data-sharing statement
Owing to the nature of the data collected and conditions attached to ethics approval, there are limited data available for wider use. All queries should be submitted to the corresponding author in the first instance.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK: Second Edition – Overview. London: Alzheimer’s Society; 2014.
- Lakey L. Counting the Cost: Caring for People with Dementia on Hospital Wards. London: Alzheimer’s Society; 2009.
- National Audit of Dementia Care in General Hospitals 2012–13: Second Round Audit Report and Update. London: Healthcare Quality Improvement Partnership; 2013.
- Glover A, Bradshaw LE, Watson N, Laithwaite E, Goldberg SE, Whittamore KH, et al. Medical Crises in Older People Study Group . Diagnoses, problems and healthcare interventions amongst older people with an unscheduled hospital admission who have concurrent mental health problems: a prevalence study. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-43.
- Kitson A, Conroy T, Wengstrom Y, Profetto-McGrath J, Robertson-Malt S. Defining the fundamentals of care. Int J Nurs Pract 2010;16:423-34. https://doi.org/10.1111/j.1440-172X.2010.01861.x.
- Goldberg SE, Whittamore KH, Pollock K, Harwood RH, Gladman JR. Caring for cognitively impaired older patients in the general hospital: a qualitative analysis of similarities and differences between a specialist Medical and Mental Health Unit and standard care wards. Int J Nurs Stud 2014;51:1332-43. https://doi.org/10.1016/j.ijnurstu.2014.02.002.
- Edberg AK, Bird M, Richards DA, Woods R, Keeley P, Davis-Quarrell V. Strain in nursing care of people with dementia: nurses’ experience in Australia, Sweden and United Kingdom. Aging Ment Health 2008;12:236-43. https://doi.org/10.1080/13607860701616374.
- Gladman J, Porock D, Griffiths A, Clissett P, Harwood RH, Knight A, et al. Care of Older People with Cognitive Impairment in General Hospitals. Final Report NIHR Service Delivery and Organisation Programme. Southampton: National Institute for Health Research; 2012.
- Prime Minister’s Challenge on Dementia 2020. London: Department of Health and Social Care; 2015.
- Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009;195:61-6. https://doi.org/10.1192/bjp.bp.108.055335.
- National Audit of Dementia Care in General Hospitals, 2016–2017: Third Round of Audit Report. London: Royal College of Psychiatrists; 2017.
- Dignity and Nutrition Inspection Programme National Overview. Newcastle upon Tyne: CQC; 2011.
- Bartlett G, Blais R, Tamblyn R, Clermont RJ, MacGibbon B. Impact of patient communication problems on the risk of preventable adverse events in acute care settings. CMAJ 2008;178:1555-62. https://doi.org/10.1503/cmaj.070690.
- Dickey L, Kagan A, Lindsay MP, Fang J, Rowland A, Black S. Incidence and profile of inpatient stroke-induced aphasia in Ontario, Canada. Arch Phys Med Rehabil 2010;91:196-202. https://doi.org/10.1016/j.apmr.2009.09.020.
- Wee JY, Hopman WM. Stroke impairment predictors of discharge function, length of stay, and discharge destination in stroke rehabilitation. Am J Phys Med Rehabil 2005;84:604-12. https://doi.org/10.1097/01.phm.0000171005.08744.ab.
- Gialanella B. Aphasia assessment and functional outcome prediction in patients with aphasia after stroke. J Neurol 2011;258:343-9. https://doi.org/10.1007/s00415-010-5868-x.
- Williams KN, Herman R, Gajewski B, Wilson K. Elderspeak communication: impact on dementia care. Am J Alzheimers Dis Other Demen 2009;24:11-20. https://doi.org/10.1177/1533317508318472.
- Talerico KA, Evans LK, Strumpf NE. Mental health correlates of aggression in nursing home residents with dementia. Gerontologist 2002;42:169-77. https://doi.org/10.1093/geront/42.2.169.
- Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry 2002;17:403-8. https://doi.org/10.1002/gps.490.
- Dooley J, Bailey C, McCabe R. Communication in healthcare interactions in dementia: a systematic review of observational studies. Int Psychogeriatr 2015;27:1277-300. https://doi.org/10.1017/S1041610214002890.
- Ripich DN, Wykle M, Niles S. Alzheimers-disease caregivers: the focused program. A communication skills training program helps nursing assistants to give better care to patients with disease. Geriatr Nurs 1995;16:15-9. https://doi.org/10.1016/S0197-4572(05)80073-4.
- International Classification of Functioning, Disability and Health (ICF). 2001.
- Kagan A, Simmons-Mackie N, Rowland A, Huijbregts M, Shumway E, McEwen S, et al. Counting what counts: a framework for capturing real-life outcomes of aphasia intervention. Aphasiology 2008;22:258-80. https://doi.org/10.1080/02687030701282595.
- Communicating. London: Alzheimer’s Society; 2016.
- Griffiths A, Knight A, Harwood R, Gladman JR. Preparation to care for confused older patients in general hospitals: a study of UK health professionals. Age Ageing 2014;43:521-7. https://doi.org/10.1093/ageing/aft171.
- Ekman SL, Norberg A, Wahlin TB, Winblad B. Dimensions and progression in the interaction between bilingual/monolingual caregivers and bilingual demented immigrants: analysis of video-recorded morning care sessions in institutions coded by means of the Erikson theory of ‘eight stages of man’. Int J Aging Hum Dev 1995;41:29-45. https://doi.org/10.2190/3TJC-32DN-3QF2-38MH.
- Equality Act 2010. London: The Stationery Office; 2010.
- Francis R. Report of the Mid Staffordshire NHS Foundation Trust Public Inquiry Executive Summary. London: The Stationery Office; 2013.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care. Clinical Guideline CG42. London: NICE; 2006.
- Building a Safer NHS for Patients: Implementing an Organisation with a Memory. London: DHSC; 2001.
- Patients First and Foremost: The Initial Government Response to the Report of Mid Staffordshire NHS Foundation Trust Public Inquiry. London: DHSC; 2013.
- Cowdell F. The care of older people with dementia in acute hospitals. Int J Older People Nurs 2010;5:83-92. https://doi.org/10.1111/j.1748-3743.2010.00208.x.
- Kitwood T. Dementia Reconsidered: The Person Comes First. Oxford: Open University Press; 1997.
- Goldberg SE, Bradshaw LE, Kearney FC, Russell C, Whittamore KH, Foster PER, et al. Care in a specialist medical and mental health unit with standard care for older people with cognitive impairment admitted to a general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347. https://doi.org/10.1136/bmj.f4132.
- National Minimum Training Standards for Healthcare Support Workers and Adult Social Care Workers in England. Leeds and Bristol: Skills for Care, Skills for Health; 2013.
- Small JA, Gutman G, Makela S, Hillhouse B. Effectiveness of communication strategies used by caregivers of persons with Alzheimer’s disease during activities of daily living. J Speech Lang Hear Res 2003;46:353-67. https://doi.org/10.1044/1092-4388(2003/028).
- Small JA, Kemper S, Lyons K. Sentence comprehension in Alzheimer’s disease: effects of grammatical complexity, speech rate, and repetition. Psychol Aging 1997;12:3-11. https://doi.org/10.1037/0882-7974.12.1.3.
- Tomoeda CK, Bayles KA, Boone DR, Kaszniak AW, Slauson TJ. Speech rate and syntactic complexity effects on the auditory comprehension of Alzheimer patients. J Commun Disord 1990;23:151-61. https://doi.org/10.1016/0021-9924(90)90019-U.
- Kemper S, Harden T. Experimentally disentangling what’s beneficial about elderspeak from what’s not. Psychol Aging 1999;14:656-70. https://doi.org/10.1037/0882-7974.14.4.656.
- Tappen RM, Williams-Burgess C, Edelstein J, Touhy T, Fishman S. Communicating with individuals with Alzheimer’s disease: examination of recommended strategies. Arch Psychiatr Nurs 1997;11:249-56. https://doi.org/10.1016/S0883-9417(97)80015-5.
- Rochon E, Waters GS, Caplan D. The relationship between measures of working memory and sentence comprehension in patients with Alzheimer’s disease. J Speech Lang Hear Res 2000;43:395-413. https://doi.org/10.1044/jslhr.4302.395.
- Savundranayagam MY, Ryan EB, Anas AP, Orange JB. Communication and dementia: staff perceptions of conversational strategies. Clin Gerontol 2008;31:47-63. https://doi.org/10.1300/J018v31n02_04.
- Vitaliano PP, Young HM, Russo J, Romano J, Magana-Amato A. Does expressed emotion in spouses predict subsequent problems among care recipients with Alzheimer’s disease?. J Gerontol 1993;48:P202-9. https://doi.org/10.1093/geronj/48.4.P202.
- Communicating with Older Adults: An Evidence-Based Review of What Really Works. Washington, DC: GSA; 2012.
- Alsawy S, Mansell W, McEvoy P, Tai S. What is good communication for people living with dementia? A mixed-methods systematic review. Int Psychogeriatr 2017;29:1785-800. https://doi.org/10.1017/S1041610217001429.
- Day AM, James IA, Meyer TD, Lee DR. Do people with dementia find lies and deception in dementia care acceptable?. Aging Ment Health 2011;15:822-9. https://doi.org/10.1080/13607863.2011.569489.
- Wang JJ, Hsieh PF, Wang CJ. Long-term care nurses’ communication difficulties with people living with dementia in Taiwan. Asian Nurs Res 2013;7:99-103. https://doi.org/10.1016/j.anr.2013.06.001.
- Eggers T, Ekman SL, Norberg A. Nursing staff’s understanding expressions of people with advanced dementia disease. Res Theory Nurs Pract 2013;27:19-34. https://doi.org/10.1891/1541-6577.27.1.19.
- Fellowes D, Wilkinson S, Moore P. Communication skills training for health care professionals working with cancer patients, their families and/or carers. Cochrane Database Syst Rev 2004;2. https://doi.org/10.1002/14651858.CD003751.pub2.
- Fallowfield LJ, Jenkins VA, Beveridge HA. Truth may hurt but deceit hurts more: communication in palliative care. Palliat Med 2002;16:297-303. https://doi.org/10.1191/0269216302pm575oa.
- Wilkinson S, Perry R, Blanchard K, Linsell L. Effectiveness of a three-day communication skills course in changing nurses’ communication skills with cancer/palliative care patients: a randomised controlled trial. Palliat Med 2008;22:365-75. https://doi.org/10.1177/0269216308090770.
- Maguire P, Booth K, Elliott C, Jones B. Helping health professionals involved in cancer care acquire key interviewing skills – the impact of workshops. Eur J Cancer 1996;32A:1486-9. https://doi.org/10.1016/0959-8049(96)00059-7.
- Moore PM, Rivera Mercado S, Grez Artigues M, Lawrie TA. Communication skills training for healthcare professionals working with people who have cancer. Cochrane Database Syst Rev 2013;3. https://doi.org/10.1002/14651858.CD003751.pub3.
- Parry R. Are interventions to enhance communication performance in allied health professionals effective, and how should they be delivered? Direct and indirect evidence. Patient Educ Couns 2008;73:186-95. https://doi.org/10.1016/j.pec.2008.05.029.
- Berkhof M, van Rijssen HJ, Schellart AJ, Anema JR, van der Beek AJ. Effective training strategies for teaching communication skills to physicians: an overview of systematic reviews. Patient Educ Couns 2011;84:152-62. https://doi.org/10.1016/j.pec.2010.06.010.
- Chant S, Tim, Randle J, Russell G, Webb C. Communication skills training in healthcare: a review of the literature. Nurse Educ Today 2002;22:189-202. https://doi.org/10.1054/nedt.2001.0690.
- Lewin LO, Cole-Kelly K, Greenfield M. A year-long course for third-year students on ethics, professionalism, and communication. Acad Med 2001;76. https://doi.org/10.1097/00001888-200105000-00041.
- Eggenberger E, Heimerl K, Bennett MI. Communication skills training in dementia care: a systematic review of effectiveness, training content, and didactic methods in different care settings. Int Psychogeriatr 2013;25:345-58. https://doi.org/10.1017/S1041610212001664.
- Williams KN. Improving outcomes of nursing home interactions. Res Nurs Health 2006;29:121-33. https://doi.org/10.1002/nur.20117.
- Burgio LD, Stevens A, Burgio KL, Roth DL, Paul P, Gerstle J. Teaching and maintaining behavior management skills in the nursing home. Gerontologist 2002;42:487-96. https://doi.org/10.1093/geront/42.4.487.
- Machiels M, Metzelthin SF, Hamers JP, Zwakhalen SM. Interventions to improve communication between people with dementia and nursing staff during daily nursing care: A systematic review. Int J Nurs Stud 2017;66:37-46. https://doi.org/10.1016/j.ijnurstu.2016.11.017.
- Sprangers S, Dijkstra K, Romijn-Luijten A. Communication skills training in a nursing home: effects of a brief intervention on residents and nursing aides. Clin Interv Aging 2015;10:311-19. https://doi.org/10.2147/CIA.S73053.
- Dijkstra K, Bourgeois M, Burgio L, Allen RS. Effects of a communication intervention on the discourse of nursing home residents with dementia and their nursing assistants. J Med Speech Lang Pathol 2002;10:143-57.
- Heritage J, Robinson JD. The structure of patients’ presenting concerns: physicians’ opening questions. Health Commun 2006;19:89-102. https://doi.org/10.1207/s15327027hc1902_1.
- Sidnell J. Conversation Analysis: An Introduction. London: Wiley-Blackwell; 2010.
- Beeke S, Maxim J, Wilkinson R. Using conversation analysis to assess and treat people with aphasia. Semin Speech Lang 2007;28:136-47. https://doi.org/10.1055/s-2007-970571.
- McCabe R, John P, Dooley J, Healey P, Cushing A, Kingdon D, et al. Training to enhance psychiatrist communication with patients with psychosis (TEMPO): cluster randomised controlled trial. Br J Psychiatry 2016;209:517-24. https://doi.org/10.1192/bjp.bp.115.179499.
- Heritage J, Robinson JD, Elliott MN, Beckett M, Wilkes M. Reducing patients’ unmet concerns in primary care: the difference one word can make. J Gen Intern Med 2007;22:1429-33. https://doi.org/10.1007/s11606-007-0279-0.
- National Clinical Guidelines for Stroke. London: Royal College of Physicians; 2008.
- Simmons-Mackie N, Kagan A. Communication strategies used by ‘good’ versus ‘poor’ speaking partners of individuals with aphasia. Aphasiology 1999;13:807-20. https://doi.org/10.1080/026870399401894.
- Kagan A, Black SE, Duchan FJ, Simmons-Mackie N, Square P. Training volunteers as conversation partners using ‘Supported Conversation for Adults with Aphasia’ (SCA): a controlled trial. J Speech Lang Hear Res 2001;44:624-38. https://doi.org/10.1044/1092-4388(2001/051).
- Legg C, Young L, Bryer A. Training sixth-year medical students in obtaining case-history information from adults with aphasia. Aphasiology 2005;19:559-75. https://doi.org/10.1080/02687030544000029.
- McCabe L, Greasley-Adams C, Goodson K. ‘What I want to do is get half a dozen of them and go and see Simon Cowell’: reflecting on participation and outcomes for people with dementia taking part in a creative musical project. Dementia 2015;14:734-50. https://doi.org/10.1177/1471301213508128.
- Parry R. Giving reasons for doing something now or at some other time. Res Lang Soc Interact 2013;46:105-24. https://doi.org/10.1080/08351813.2012.754653.
- Cochrane Effective Practice and Organisation of Care Review Group . Data Collection Checklist n.d. https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/datacollectionchecklist.pdf (accessed August 2018).
- National Institute for Health Research . Quality Assessment Tool for Before–After (Pre-Post) Studies With No Control Group 2014. www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after.
- Ampe S, Sevenants A, Smets T, Declercq A, Van Audenhove C. Advance care planning for nursing home residents with dementia: influence of ‘we DECide’ on policy and practice. Patient Educ Couns 2017;100:139-46. https://doi.org/10.1016/j.pec.2016.08.010.
- Beer LE, Hutchinson SR, Skala-Cordes KK. Communicating with patients who have advanced dementia: training nurse aide students. Gerontol Geriatr Educ 2012;33:402-20. https://doi.org/10.1080/02701960.2012.702165.
- Broughton M, Smith ER, Baker R, Angwin AJ, Pachana NA, Copland DA, et al. Evaluation of a caregiver education program to support memory and communication in dementia: a controlled pretest-posttest study with nursing home staff. Int J Nurs Stud 2011;48:1436-44. https://doi.org/10.1016/j.ijnurstu.2011.05.007.
- Chao HC, Kaas M, Su YH, Lin MF, Huang MC, Wang JJ. Effects of the advanced innovative internet-based communication education program on promoting communication between nurses and patients with dementia. J Nurs Res 2016;24:163-72. https://doi.org/10.1097/jnr.0000000000000109.
- Cockbain BC, Thompson S, Salisbury H, Mitter P, Martos L. A collaborative strategy to improve geriatric medical education. Age Ageing 2015;44:1036-9. https://doi.org/10.1093/ageing/afv100.
- Conway ER, Chenery HJ. Evaluating the MESSAGE Communication Strategies in Dementia training for use with community-based aged care staff working with people with dementia: a controlled pretest-post-test study. J Clin Nurs 2016;25:1145-55. https://doi.org/10.1111/jocn.13134.
- da Silva Serelli L, Reis RC, Laks J, de Pádua AC, Bottino CM, Caramelli P. Effects of the Staff Training for Assisted Living Residences protocol for caregivers of older adults with dementia: a pilot study in the Brazilian population. Geriatr Gerontol Int 2017;17:449-55. https://doi.org/10.1111/ggi.12742.
- DiZazzo-Miller R, Samuel PS, Barnas JM, Welker KM. Addressing everyday challenges: feasibility of a family caregiver training program for people with dementia. Am J Occup Ther 2014;68:212-20. https://doi.org/10.5014/ajot.2014.009829.
- Elvish R, Burrow S, Cawley R, Harney K, Graham P, Pilling M, et al. ‘Getting to Know Me’: the development and evaluation of a training programme for enhancing skills in the care of people with dementia in general hospital settings. Aging Ment Health 2014;18:481-8. https://doi.org/10.1080/13607863.2013.856860.
- Engel S, Reiter-Jäschke A, Hoftner B. ‘Edukation demenz’ psychoedukatives schulungsprogramm für angehörige von menschen mit demenz. Z Gerontol Geriatr 2016;49:187-95. https://doi.org/10.1007/s00391-016-1034-0.
- Franzmann J, Haberstroh J, Pantel J. Train the trainer in dementia care. A program to foster communication skills in nursing home staff caring for dementia patients. Z Gerontol Geriatr 2016;49:209-15. https://doi.org/10.1007/s00391-016-1041-1.
- Galvin JE, Kuntemeier B, Al-Hammadi N, Germino J, Murphy-White M, McGillick J. ‘Dementia-friendly hospitals: care not crisis’ an educational program designed to improve the care of the hospitalized patient with dementia. Alzheimer Dis Assoc Disord 2010;24:372-9. https://doi.org/10.1097/WAD.0b013e3181e9f829.
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA 2010;304:983-91. https://doi.org/10.1001/jama.2010.1253.
- Goyder J, Orrell M, Wenborn J, Spector A. Staff training using STAR: a pilot study in UK care homes. Int Psychogeriatr 2012;24:911-20. https://doi.org/10.1017/S1041610211002559.
- Haberstroh J, Neumeyer K, Krause K, Franzmann J, Pantel J. TANDEM: Communication training for informal caregivers of people with dementia. Aging Ment Health 2011;15:405-13. https://doi.org/10.1080/13607863.2010.536135.
- Hobday JV, Savik K, Smith S, Gaugler JE. Feasibility of Internet training for care staff of residents with dementia: the CARES program. J Gerontol Nurs 2010;36:13-21. https://doi.org/10.3928/00989134-20100302-01.
- Irvine AB, Billow MB, Bourgeois M, Seeley JR. Mental illness training for long term care staff. J Am Med Dir Assoc 2012;13. https://doi.org/10.1016/j.jamda.2011.01.015.
- Karel MJ, Teri L, McConnell E, Visnic S, Karlin BE. Effectiveness of expanded implementation of STAR-VA for managing dementia-related behaviors among veterans. Gerontologist 2016;56:126-34. https://doi.org/10.1093/geront/gnv068.
- Karlin BE, Visnic S, McGee JS, Teri L. Results from the multisite implementation of STAR-VA: a multicomponent psychosocial intervention for managing challenging dementia-related behaviors of veterans. Psychol Serv 2014;11:200-8. https://doi.org/10.1037/a0033683.
- Levy-Storms L, Harris LM, Chen X. A video-based intervention on and evaluation of nursing aides’ therapeutic communication and residents’ agitation during mealtime in a dementia care unit. J Nutr Gerontol Geriatr 2016;35:267-81. https://doi.org/10.1080/21551197.2016.1238430.
- Liddle J, Smith-Conway ER, Baker R, Angwin AJ, Gallois C, Copland DA, et al. Memory and communication support strategies in dementia: effect of a training program for informal caregivers. Int Psychogeriatr 2012;24:1927-42. https://doi.org/10.1017/S1041610212001366.
- Mason-Baughman MB, Lander A. Communication strategy training for caregivers of individuals with dementia. Perspect Gerontol 2012;17:78-83. https://doi.org/10.1044/gero17.3.78.
- Passalacqua SA, Harwood J. VIPS communication skills training for paraprofessional dementia caregivers: an intervention to increase person-centered dementia care. Clin Gerontol 2012;35:425-45. https://doi.org/10.1080/07317115.2012.702655.
- Weitzel T, Robinson S, Mercer S, Berry T, Barnes M, Plunkett D, et al. Pilot testing an educational intervention to improve communication with patients with dementia. J Nurses Staff Dev 2011;27:220-6. https://doi.org/10.1097/NND.0b013e31822e0738.
- Williams KN, Perkhounkova Y, Jao YL, Bossen A, Hein M, Chung S, et al. Person-centered communication for nursing home residents with dementia [published online ahead of print March 1 2017]. West J Nurs Res 2017. https://doi.org/10.1177/0193945917697226.
- Williams KN, Perkhounkova Y, Herman R, Bossen A. A communication intervention to reduce resistiveness in dementia care: a cluster randomized controlled trial. Gerontologist 2017;57:707-18. https://doi.org/10.1093/geront/gnw047.
- Knowles MS. Andragogy in Action: Applying Modern Principles of Adult Learning. San Francisco, CA: Jossey-Bass; 1984.
- Knowles M, Edwards R, Hansom A, Raggatt P. Boundaries of Adult Learning. London: Routledge; 1996.
- Kohler S. How to Communicate with Alzheimer’s: A Practical Guide and Workbook for Families. Venice, CA: Granny’s Rocker Publishing; 2004.
- Pillet-Shore D, Nussbaum J. Oxford Research Encyclopedia of Communication. New York: Oxford University Press; 2017.
- Elwyn G, Hutchings H, Edwards A, Rapport F, Wensing M, Cheung WY, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect 2005;8:34-42. https://doi.org/10.1111/j.1369-7625.2004.00311.x.
- Mahoney EK, Hurley AC, Volicer L, Bell M, Gianotis P, Hartshorn M, et al. Development and testing of the Resistiveness to Care Scale. Res Nurs Health 1999;22:27-38. https://doi.org/10.1002/(SICI)1098-240X(199902)22:1<27::AID-NUR4>3.0.CO;2-T.
- ten Have P. Doing Conversation Analysis: A Practical Guide. Thousand Oaks, CA: Sage Publications Ltd; 2007.
- Pilnick A, Hindmarsh J, Teas Gill V. Communication in Healthcare Settings: Policy, Participation and New Technologies. London: Wiley-Blackwell; 2010.
- Reuber M, Toerien M, Shaw R, Duncan R. Delivering patient choice in clinical practice: a conversation analytic study of communication practices used in neurology clinics to involve patients in decision-making. Health Serv Deliv Res 2015;3.
- Reuber M, Monzoni C, Sharrack B, Plug L. Using interactional and linguistic analysis to distinguish between epileptic and psychogenic nonepileptic seizures: a prospective, blinded multirater study. Epilepsy Behav 2009;16:139-44. https://doi.org/10.1016/j.yebeh.2009.07.018.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Heritage J, Silverman D. Qualitative Research: Theory, Method and Practice. London: Sage Publications Ltd; 1997.
- Jefferson G. On the sequential organization of troubles-talk in ordinary conversation. Social Problems 1988;35:418-41. https://doi.org/10.2307/800595.
- Byrne P, Long B. Doctors Talking to Patients: A Study of the Verbal Behaviour of General Practitioners Consulting in their Surgeries. London: Her Majesty’s Stationery Office; 1976.
- Dingwall R, Robinson K, Gubrium J, Sankar A. The Home Care Experience: Ethnography and Policy. Newbury Park, CA: Sage Publications; 1990.
- Pilnick A. The interactional organization of pharmacist consultations in a hospital setting: a putative structure. J Pragmat 2001;33:1927-45. https://doi.org/10.1016/S0378-2166(00)00079-5.
- Robinson JD, Heritage J. The structure of patients’ presenting concerns: the completion relevance of current symptoms. Soc Sci Med 2005;61:481-93. https://doi.org/10.1016/j.socscimed.2004.12.004.
- Yi M. Analysis of conversation between elderly patients with dementia and nurses: focusing on structure and sequential patterns. J Korean Acad Nurs 2009;39:166-76. https://doi.org/10.4040/jkan.2009.39.2.166.
- Staples S. The Discourse of Nurse-Patient Interactions: Contrasting the Communicative Styles of US and International Nurses. Amsterdam: John Benjamins Publishing Company; 2015.
- Jones A. Admitting hospital patients: a qualitative study of an everyday nursing task. Nurs Inq 2007;14:212-23. https://doi.org/10.1111/j.1440-1800.2007.00370.x.
- Schegloff EA. Sequence Organization in Interaction: A Primer in Conversation Analysis, Volume 1. Cambridge: Cambridge University Press; 2007.
- Curl TS, Drew P. Contingency and action: a comparison of two forms of requesting. Res Lang Soc Interact 2008;41:129-53. https://doi.org/10.1080/08351810802028613.
- Craven A, Potter J. Directives: entitlement and contingency in action. Discourse Stud 2010;12:419-42. https://doi.org/10.1177/1461445610370126.
- Lindström A, Hakulinen A, Selting M. Syntax and Lexis in Conversation. Amsterdam: John Benjamins Publishing Company; 2005.
- Heinemann T. ‘Will you or can’t you?’: Displaying entitlement in interrogative requests. J Pragmat 2006;38:1081-104. https://doi.org/10.1016/j.pragma.2005.09.013.
- Brooker D. Person-Centred Dementia Care: Making Services Better. London: Jessica Kingsley Publishers; 2007.
- Antaki C, Kent A. Telling people what to do (and, sometimes, why): contingency, entitlement and explanation in staff requests to adults with intellectual impairments. J Pragmat 2012;44:876-89. https://doi.org/10.1016/j.pragma.2012.03.014.
- Stevanovic M, Perakyla A. Deontic authority in interaction: the right to announce, propose, and decide. Res Lang Soc Interact 2012;45:297-321. https://doi.org/10.1080/08351813.2012.699260.
- West C. Not just ‘doctors’ orders’: directive-response sequences in patients’ visits to women and men physicians. Discourse Soc 1990;1:85-112. https://doi.org/10.1177/0957926590001001005.
- Heath C, Heath C. Body Movement and Speech in Medical Interactions. Cambridge: Cambridge University Press; 1986.
- Robinson JD. Closing medical encounters: two physician practices and their implications for the expression of patients’ unstated concerns. Soc Sci Med 2001;53:639-56. https://doi.org/10.1016/S0277-9536(00)00366-X.
- West C, Heritage J, Maynard DW. Communication in Medical Care: Interaction between Primary Care Physicians and Patients. Cambridge: Cambridge University Press; 2006.
- White J, Levinson W, Roter D. ‘Oh, by the way . . . : the closing moments of the medical visit. J Gen Intern Med 1994;9:24-8. https://doi.org/10.1007/BF02599139.
- Allwood R, Pilnick A, O’Brien R, Goldberg S, Harwood RH, Beeke S. Should I stay or should I go? How healthcare professionals close encounters with people with dementia in the acute hospital setting. Soc Sci Med 2017;191:212-25. https://doi.org/10.1016/j.socscimed.2017.09.014.
- Walker HK, Hall WD, Hurst JW. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston, MA: Butterworths; 1990.
- Drew P, Holt E. Figures of Speech: Figurative expressions and the management of topic transition in conversation. Lang Soc 1998;27:495-522. https://doi.org/10.1017/S0047404500020200.
- Hoddinott P. A new era for intervention development studies. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0032-0.
- French SD, Green SE, O’Connor DA, McKenzie JE, Francis JJ, Michie S, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-38.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Lovell K, Bower P, Richards D, Barkham M, Sibbald B, Roberts C, et al. Developing guided self-help for depression using the Medical Research Council complex interventions framework: a description of the modelling phase and results of an exploratory randomised controlled trial. BMC Psychiatry 2008;8. https://doi.org/10.1186/1471-244X-8-91.
- Kitzinger C, Antaki C. Applied Conversation Analysis: Intervention and Change in Institutional Talk. London: Palgrave Macmillan UK; 2011.
- Schön DA. Educating the Reflective Practitioner: Toward a New Design for Teaching and Learning in the Professions. San Francisco, CA: Jossey-Bass; 1987.
- Cranton P. New directions for adult and continuing education. Teach Transform 2002;2002:63-72.
- Ginsberg MB, Wlodkowski JR. CAEL Forum News. 2009.
- Kolb DA. Experiential Learning: Experience as the Source of Learning and Development. Upper Saddle River, NJ: Prentice-Hall; 1984.
- Yardley S, Teunissen PW, Dornan T. Experiential learning: AMEE Guide No. 63. Med Teach 2012;34:e102-15. https://doi.org/10.3109/0142159X.2012.650741.
- Jenkins L, Reuber M. A conversation analytic intervention to help neurologists identify diagnostically relevant linguistic features in seizure patients’ talk. Res Lang Soc Interact 2014;47:266-79. https://doi.org/10.1080/08351813.2014.925664.
- Goldberg SE, Windle R, Harwood RH, Dowling G, Taylor M, . Caring for People With Dementia in the General Hospital – Person Centred Dementia Care 2014. http://sonet.nottingham.ac.uk/rlos/mentalhealth/dementia_care/ (accessed 8 December 2017).
- Goldberg SE, Windle R, Harwood TH, Schneider J, Dowling G, Taylor M, et al. Caring for People with Dementia in the General Hospital: Communication. Nottingham: University of Nottingham, HELM; 2014.
- Stokoe E. The (in)authenticity of simulated talk: comparing role-played and actual interaction and the implications for communication training. Res Lang Soc Interact 2013;46:165-85. https://doi.org/10.1080/08351813.2013.780341.
- White SJ, Casey M. Understanding differences between actual and simulated surgical consultations: a scoping study. Aust J Linguistics 2016;36:257-72. https://doi.org/10.1080/07268602.2015.1121534.
- de la Croix A, Skelton J. The reality of role-play: interruptions and amount of talk in simulated consultations. Med Educ 2009;43:695-703. https://doi.org/10.1111/j.1365-2923.2009.03392.x.
- Murtagh GM, Nestel D, Bearman M. Simulated Patient Methodology: Theory, Evidence And Practice. London: John Wiley & Sons, Inc.; 2015.
- Pendleton D. The Consultation: An Approach to Learning and Teaching. Oxford: Oxford University Press; 1984.
- Stokoe E. The conversation analytic role-play method (CARM): a method for training communication skills as an alternative to simulated role-play. Res Lang Soc Interact 2014;47:255-65. https://doi.org/10.1080/08351813.2014.925663.
- Mezirow J. Learning as Transformation. Critical Perspectives on a Theory in Progress. San Francisco, CA: Jossey-Bass; 2000.
- Johnson FM, Best W, Beckley FC, Maxim J, Beeke S. Identifying mechanisms of change in a conversation therapy for aphasia using behaviour change theory and qualitative methods. Int J Lang Commun Disord 2017;52:374-87. https://doi.org/10.1111/1460-6984.12279.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Beckley F, Best W, Johnson F, Edwards S, Maxim J, Beeke S. Conversation therapy for agrammatism: exploring the therapeutic process of engagement and learning by a person with aphasia. Int J Lang Commun Disord 2013;48:220-39. https://doi.org/10.1111/j.1460-6984.2012.00204.x.
- Dewey J. How We Think. A Restatement of the Relation of Reflective Thinking to the Educative Process. Boston, MA.: Health and Company; 1933.
- Schön D. The Reflective Practitioner: How Professionals Think in Action. New York, NY: Basic Books; 1983.
- Mann K, Gordon J, MacLeod A. Reflection and reflective practice in health professions education: a systematic review. Adv Health Sci Educ Theory Pract 2009;14:595-621. https://doi.org/10.1007/s10459-007-9090-2.
- Sandars J. The use of reflection in medical education: AMEE Guide No. 44. Med Teach 2009;31:685-95. https://doi.org/10.1080/01421590903050374.
- Gibbs G. Learning By Doing: A Guide to Teaching and Learning Methods. Oxford: Oxford Brookes University; 1988.
- Marton F, Saljo R. Qualitative differences in learning: I. Outcome and process. Br J Educ Psychol 1976;46:4-11. https://doi.org/10.1111/j.2044-8279.1976.tb02980.x.
- Edmunds S, Brown G. Effective small group learning: AMEE Guide No. 48. Med Teach 2010;32:715-26. https://doi.org/10.3109/0142159X.2010.505454.
- Vygotsky LS, Cole M. Mind in Society: Development of Higher Psychological Processes. Cambridge, MA: Harvard University Press; 1978.
- Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice Hall; 1977.
- Billett S. Toward a workplace pedagogy: guidance, participation, and engagement. Adult Educ Q 2002;53:27-43. https://doi.org/10.1177/074171302237202.
- Bennett C. ASTER Project: investigating the use of electronic resources. Interactions J 2000;3. https://warwick.ac.uk/services/ldc/resource/interactions (accessed 29 October 2018).
- Gordon M, Chandratilake M, Baker P. Low fidelity, high quality: a model for e-learning. Clin Teach 2013;10:258-63. https://doi.org/10.1111/tct.12008.
- Cook DA, Levinson AJ, Garside S, Dupras DM, Erwin PJ, Montori VM. Instructional design variations in internet-based learning for health professions education: a systematic review and meta-analysis. Acad Med 2010;85:909-22. https://doi.org/10.1097/ACM.0b013e3181d6c319.
- Bath-Hextall F, Wharrad H, Leonardi-Bee J. Teaching tools in evidence based practice: evaluation of reusable learning objects (RLOs) for learning about meta-analysis. BMC Med Educ 2011;11. https://doi.org/10.1186/1472-6920-11-18.
- Marinopoulos SS, Dorman T, Ratanawongsa N, Wilson LM, Ashar BH, Magaziner JL, et al. Effectiveness of continuing medical education. Evid Rep Technol Assess 2007;149:1-69.
- Wong G, Greenhalgh T, Pawson R. Internet-based medical education: a realist review of what works, for whom and in what circumstances. BMC Med Educ 2010;10. https://doi.org/10.1186/1472-6920-10-12.
- Masters K, Ellaway R. e-Learning in medical education Guide 32 Part 2: Technology, management and design. Med Teach 2008;30:474-89. https://doi.org/10.1080/01421590802108349.
- Walsh D. The Nurse Mentor’s Handbook: Supporting Students in Clinical Practice. New York, NY: McGraw-Hill Education; 2014.
- Hoffman A, Bateman DR, Bartels S, Blandin K, Santulli RB, Lee H, et al. Patient and caregiver needs and preferences for decision support interventions in alzheimer’s disease. Am J Geriatr Psychiatry 2013;21:98-9. https://doi.org/10.1016/j.jagp.2012.12.130.
- Hoffman A, Bateman D, Bartels SJ. Systematic review of interventions to support well-informed person-centered decision making for dementia care. Am J Geriatr Psychiatry 2014;22. https://doi.org/10.1016/j.jagp.2013.12.097.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. https://doi.org/10.1186/1748-5908-2-40.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. PAFS consensus group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355. https://doi.org/10.1136/bmj.i5239.
- Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-67.
- Lewis KL, Bohnert CA, Gammon WL, Hölzer H, Lyman L, Smith C, et al. The Association of Standardized Patient Educators (ASPE) Standards of Best Practice (SOBP). Adv Simul 2017;2. https://doi.org/10.1186/s41077-017-0043-4.
- Davies O, Foster P. Today Is Monday 2012. https://vimeo.com/93365033 (accessed 8 December 2017).
- Kirkpatrick DL, Kirkpatrick JD. Evaluating Training Programs: The Four Levels. San Francisco, CA: Berrett-Koehle Publishers; 1994.
- Sedgwick P. Before and after study designs. BMJ 2014;349. https://doi.org/10.1136/bmj.g5074.
- Surr CA, Gates C. What works in delivering dementia education or training to hospital staff? A critical synthesis of the evidence. Int J Nurs Stud 2017;75:172-88. https://doi.org/10.1016/j.ijnurstu.2017.08.002.
- Best W, Maxim J, Heilemann C, Beckley F, Johnson F, Edwards SI, et al. Conversation therapy with people with aphasia and conversation partners using video feedback: a group and case series investigation of changes in interaction. Front Hum Neurosci 2016;10. https://doi.org/10.3389/fnhum.2016.00562.
- Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall/CRC; 1990.
- Williams KN, Boyle DK, Herman RE, Coleman CK, Hummert ML. Psychometric analysis of the emotional tone rating scale: a measure of person-centered communication. Clin Gerontol 2012;35:376-89. https://doi.org/10.1080/07317115.2012.702648.
- Teodorczuk A, Mukaetova-Ladinska E, Corbett S, Welfare M. Learning about the patient: an innovative interprofessional dementia and delirium education programme. Clin Teach 2014;11:497-502. https://doi.org/10.1111/tct.12203.
- Waugh A, Marland G, Henderson J, Robertson J, Wilson A. Improving the care of people with dementia in hospital. Nurs Stand 2011;25:44-9. https://doi.org/10.7748/ns2011.04.25.32.44.c8452.
- Allen-Burge R, Burgio LD, Bourgeois MS, Sims R, Nunnikhoven J. Increasing communication among nursing home residents. J Clin Geropsychol 2001;7:213-30. https://doi.org/10.1023/A:1011343212424.
- Surr CA, Smith SJ, Crossland J, Robins J. Impact of a person-centred dementia care training programme on hospital staff attitudes, role efficacy and perceptions of caring for people with dementia: a repeated measures study. Int J Nurs Stud 2016;53:144-51. https://doi.org/10.1016/j.ijnurstu.2015.09.009.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- National Institute for Health Research . INVOLVE n.d. www.invo.org.uk (accessed 29 October 2018).
- Gove D, Diaz-Ponce A, Georges J, Moniz-Cook E, Mountain G, Chattat R, et al. Alzheimer Europe’s position on involving people with dementia in research through PPI (patient and public involvement). Aging Mental Health 2018;22:723-9.
- Nestel D, Bearman M. Simulated Patient Methodology: Theory, Evidence and Practice. London: Wiley-Blackwell; 2014.
- Cleland JA, Abe K, Rethans JJ. The use of simulated patients in medical education: AMEE Guide No. 42. Med Teach 2009;31:477-86. https://doi.org/10.1080/01421590903002821.
- Atkins S, Roberts C, Hawthorne K, Greenhalgh T. Simulated consultations: a sociolinguistic perspective. BMC Med Educ 2016;16. https://doi.org/10.1186/s12909-016-0535-2.
- Tadd W, Hillman A, Calnan S, Calnan M, Bayer T, Read S. Dignity in Practice: An Exploration of the Care of Older Adults in Acute NHS Trusts. Southampton: NIHR Service Delivery and Organisation Programme; 2011.
- Whittamore KH, Goldberg SE, Gladman JR, Bradshaw LE, Jones RG, Harwood RH. The diagnosis, prevalence and outcome of delirium in a cohort of older people with mental health problems on general hospital wards. Int J Geriatr Psychiatry 2014;29:32-40. https://doi.org/10.1002/gps.3961.
- Holmes J, House A. Psychiatric illness predicts poor outcome after surgery for hip fracture: a prospective cohort study. Psychol Med 2000;30:921-9. https://doi.org/10.1017/S0033291799002548.
- Young T, Manthorp C, Howells D, Tullo E. Developing a carer communication intervention to support personhood and quality of life in dementia. Ageing and Society 2011;31:1003-25. https://doi.org/10.1017/S0144686X10001182.
- Stanyon MR, Griffiths A, Thomas SA, Gordon AL. The facilitators of communication with people with dementia in a care setting: an interview study with healthcare workers. Age Ageing 2016;45:164-70. https://doi.org/10.1093/ageing/afv161.
- Thompson M. What’s Gone Wrong with the Language of Politics?. London: Bodley Head; 2016.
- Surr CA, Gates C, Irving D, Oyebode J, Smith SJ, Parveen S, et al. Effective dementia education and training for the health and social care workforce: a systematic review of the literature. Rev Educ Res 2017;87:966-1002. https://doi.org/10.3102/0034654317723305.
- Parry R, Pino M, Faull C, Feathers L. Acceptability and design of video-based research on healthcare communication: evidence and recommendations. Patient Educ Couns 2016;99:1271-84. https://doi.org/10.1016/j.pec.2016.03.013.
Appendix 1 Conversation analysis transcription notation
Appendix 2 Dementia Communication Knowledge Test
| Number | Question |
|---|---|
| 1 | When communicating with people with dementia it’s best to speak: |
| (a) fast and clearly | |
| (b) slowly and clearly | |
| (c) at a normal rate and clearly | |
| 2 | When approaching a patient with dementia to carry out a health-care task the best introduction would be: |
| (a) Hello Margaret. Do you remember me? | |
| (b) Hello Margaret. I’m Diane, one of the doctors here. I’ve come to see if you’re getting better | |
| (c) Hello Margaret. Can I check your blood pressure? | |
| 3 | Which of these communication strategies might help when communicating with someone with dementia: |
| (A) Using gestures, objects or pictures to show what you mean | |
| (B) Using metaphors to explain things | |
| (C) Touching the part of the body you are talking about | |
| (D) Using short sentences | |
| (E) Using one step instructions | |
| (a) A, B, C, D, E | |
| (b) A, C, D, E | |
| (c) A, B, D, E | |
| 4 | If a patient with dementia is distracted, what is the best way to get their attention so you can talk with them? |
| (a) Use their name | |
| (b) Speak loudly | |
| (c) Ask the relative rather than the patient | |
| 5 | Repeating back what you understand of what a patient just said to you, when you don’t completely understand them, is likely to be: |
| (a) A useful way of indicating you are listening and trying to understand | |
| (b) Confusing for someone with dementia | |
| (c) Annoying for someone with dementia | |
| 6 | When requesting a particular patient with dementia takes an important medication, which you know they are often reluctant to do, it may help to: |
| (a) Frame the request as a question about their willingness to do it, such as ‘Joan, do you want to take your tablet now?’ | |
| (b) Frame the request as a very polite question, such as ‘Joan, I was wondering if you might possibly want to take your tablets now?’ | |
| (c) Frame the request as a statement of what you are proposing will happen, with a checking question at the end, such as ‘Joan, I’ve brought your tablets for you to take now. Is that OK?’ | |
| 7 | When a patients says or communicates ‘no’ to doing something you have asked (and which the team and family thinks is important and in their best interest), which of the following approaches would be unhelpful? |
| (a) Keep repeating the request in the same way, slowly and clearly, until they agree | |
| (b) Make the task sound less demanding, by reducing the size or duration of the task, for example ‘just for a minute’ | |
| (c) Say that you need them to do it, for example ‘I need you to take these, for your diabetes’ | |
| 8 | Towards the end of your session, if you ask the patient an open question like ‘Is there anything else you want to ask me?’ this is likely to lead to the patient with dementia: |
| (a) being silent | |
| (b) being confused about what they are expected to say and not reporting any health-care concerns | |
| (c) making some attempt to share their health-care concerns or questions with you | |
| 9 | To indicate to the patient that the session is about to finish, in a way that feels respectful, which of the following strategies/statements would work best? |
| (a) I’ll see you soon | |
| (b) I’ll see you tomorrow morning | |
| (c) You’re doing really well, and there’s nothing to worry about | |
| 10 | As you are ending a session with a patient on the ward, if you stand up, clear away your equipment and pull the curtains back, this is likely to: |
| (a) appear rude to the patient with dementia | |
| (b) make no difference to the patient with dementia as they won’t notice or understand these signals | |
| (c) help the patient with dementia understand that you are about to leave |
Appendix 3 Communication behaviour rating forms: requests
| VOICE communication practices checklist: for health-care professional requesting in the face of patient reluctance | ||||
|---|---|---|---|---|
|
Rating started at (time code): Rating finished at (time code): |
||||
| Communication practice | Exemplars | Time code of initial request | Time code of further requests | Quotations/queries/comments |
| High entitlement request: proposal | Let’s: so let’s have another go; let’s try a yoghurt | |||
| High entitlement request: announcing future action | Going to/gonna/we’ll: we’re just gonna use this bathroom here; I’m just gonna pop this on; we’ll give you a quick shave | |||
| High entitlement request: statement of need | I need you to; I need to; you need to; I need to put a bandage on your leg; you need to wake up a minute; you need to bring that forward | |||
| High entitlement request: direct instruction | Take a step; have a little drink | |||
| High entitlement request softened, for example with checking/permission-seeking question | Is that OK? Alright? OK?; then we’ll give your mouth a little wipe – is that OK?; we’re going in this bathroom here – alright? | Note: ‘please’ may act in this way | ||
| High entitlement: other (please give quotation) | Forced alternatives that presume compliance – ‘Which finger shall I use?’; format ‘I think it would.’ | |||
| Lowering contingencies: reduces the size or duration of task | Just, little, pop, quick, for a minute: just a little bit; I need to pop this on your finger; if you let me have a quick listen; it’ll just be here for a minute | |||
| Lowering contingencies: request includes ‘try’ | Try: shall we give it a try then?; let’s try a drink | |||
| Lowering contingencies: explicit offer to help | Can I help?; what about if I give you a hand? | |||
| Lowering contingencies: frame accurately as collaborative or joint action | We, let’s, for me: we’re going in this way; shall we go for a walk; let’s try a yoghurt; have a drink for me | |||
| State the action explicitly (not just stating the reason for the action) | Can we try and have a stand up then; what I want to do is give you a shave | |||
| Action required of patient is not stated explicitly | I was just wondering if we could relieve the pressure on your bottom?; can I take your blood pressure? | |||
Appendix 4 Communication behaviour rating forms: closings
| VOICE communication practices checklist: for closing of encounter | ||||
|---|---|---|---|---|
|
Rating started at (time code): Rating finished at (time code): Total time coded: |
||||
| Communication practice during closing phase | Exemplars | Tick if present | Time code | Quotations/queries/comments |
| Vague arrangement at closing | See you soon; see you around; some people will be around (without specific arrangement first) | |||
| Specific closing arrangement | See you tomorrow; the nurse will be here in 5 minutes; I’ll go and get that cup of tea now | |||
| Notification ahead of final activity | Before I go (then announces a final task or action or question) | |||
| Announcing completion of final activity | That’s us all done; that’s it, got what we needed | |||
| Announcing explicit intention to leave | So I’m gonna go now | |||
| Non-verbal actions supporting verbal closing (body position, furniture, equipment) | Repositioning table, doll, blankets; tidying equipment; breaking eye contact | |||
| Closing idiom or saying | All done and dusted; I’ll leave you be; we’ll keep a close eye on things; you take care | |||
| ‘Is there anything else?’ type open question during closing | Anything you want to ask me before I go?; do you want a hand with anything before I go?; is there anything I can help with? | |||
| Mismatch between non-verbal and verbal actions during closing | Health-care professional gives verbal indications of closing but does not make physical moves to indicate closing/leaving; health-care professional opens new lines of enquiry (verbal) while walking away (non-verbal) | Do not include here activities that happen after a ‘before I go . . .’ announcement, as this was a trainable | ||
| Closing ‘other’: state whether facilitator of or barrier to closing; give quotation | No data classed here as ‘other’ will be counted | |||
Appendix 5 Interview schedule
Work package 3 intervention testing: interview guide
Introduction
-
Introduce interviewer.
-
Explain the aims and purpose of the study and give a brief description of the interview structure.
-
Ensure that participants have read the information sheet and understand that participation is voluntary and they are free to withdraw at any time.
-
Discuss digital recording of the interview and confidentiality.
-
Opportunity for participant to ask any question.
-
Complete the consent form and give a copy to participant, or obtain verbal consent and record it.
Topics, questions and prompts: health-care professionals
Background details
-
What is your current job role?
-
What type of ward do you work on?
-
How long have you worked in that role/that setting?
Enrolment on the course
-
How did you first hear about the training?
-
Could you immediately see how it could be of use in your work?
Experience of the programme
-
How did you find the training generally, for example the venue, organisation, pace, balance of learning activities?
-
What were the most useful parts of the training? Why?
-
What were the least useful? Why?
Overall perceptions of effectiveness
-
Do you think this training is an effective way to teach these specialist communication skills to health-care professionals?
-
What other approaches to training do you think could be used?
Transferring learning into practice
-
Since the training, which of the techniques/lessons from the training have you most easily adopted into your everyday job role?
-
What factors have facilitated this?
-
Are there any techniques/lessons that you’ve not been able to use in your job?
-
What factors have prevented this?
Topics, questions and prompts: line/ward managers
Background details
-
What is your current job role?
-
What type of ward do you work on?
-
How long have you worked in that role/that setting?
Enrolment on the course
-
How did health professionals that you manage/on your ward become enrolled on the programme?
-
How many health-care professionals that you manage/on your ward attended the training?
Perceived impact on health-care professional practice
-
What have you heard about the contents of the training and what health-care professionals learnt?
-
Have you noticed any changes in how health-care professionals communicate with patients as a result of attending the course?
-
Have you noticed any changes in patient experience as a result?
The barriers to, and facilitators of, successful implementation
-
Are there any factors that have facilitated health-care professionals in changing how they communicate with people living with dementia on their job?
-
Are there any factors that have prevented health-care professionals changing practice?
In case of distress
If the participant becomes distressed during the interview, ask the participant if they would like to stop the interview and offer the participant the contact number for the staff counselling service for their organisation.
If a participant reveals information that is of concern and may need reporting, that is, potential risks to another person or to themselves, or criminal behaviour, you should discuss this with the principal investigator at the earliest opportunity and when appropriate report accordingly.
Short debrief
The interviewer will now explain that the interview is now officially over and there are no more questions. They will state when the project will be ending and that if, after this date, it gets published, we will let them know. The volunteers will be thanked for their participation and asked if they would like to have a more in-depth debrief, for example if what has been discussed has made them feel particularly emotional. Even if they decline the debrief at the time, it will be reinforced that we can arrange for one if, on reflection, they feel that they would like to talk to someone. The interviewer will ensure that participants are not left distressed, and we can signpost them to individuals with expertise in this topic area if they require extra support.
List of abbreviations
- AHP
- allied health professional
- B–A
- before and after
- CA
- conversation analysis
- CARM
- conversation analysis role-play method
- CCT
- controlled clinical trial
- CI
- confidence interval
- DVD
- digital versatile disc
- ETRS
- Emotional Tone Rating Scale
- GP
- general practitioner
- MDT
- multidisciplinary team
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- OPTION
- observing patient involvement in decision-making
- PMG
- project management group
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- RLO
- reusable learning object
- SP
- simulated patient
- SSC
- study steering committee
- TIDieR
- Template for Intervention Description and Replication
- VOICE
- VideOing to Improve dementia Communication Education
