Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 10/1009/09. The contractual start date was in September 2011. The final report began editorial review in September 2017 and was accepted for publication in April 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Stephen Morris and Rachael M Hunter were commissioned by NHS London to conduct an economic evaluation of the London reconfiguration of acute stroke services prior to this study. Anthony G Rudd is National Clinical Director of Stroke, NHS England, and London Clinical Director for Stroke. Pippa J Tyrrell was Clinical Lead for stroke in Greater Manchester (Greater Manchester and Cheshire Cardiac and Stroke Network) (2008–14) and is a Trustee of the Stroke Association. Ruth Boaden is Director of National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Greater Manchester (hosted by Salford Royal NHS Foundation Trust, one of the organisations that has a Hyperacute Stroke Unit in Manchester); she also holds an honorary (unpaid) contract at Salford Royal NHS Foundation Trust as an Associate Director, is a member of the NIHR Dissemination Centre Advisory Group, is Chairperson of the NIHR Knowledge Mobilisation Research Fellowship Panel and is a Health Services and Delivery Research (HSDR) board member. Naomi J Fulop and Stephen Morris were HSDR Board members from 2013 to 2018 and 2014 to 2019, respectively. Angus IG Ramsay and Simon J Turner were associate HSDR Board members from 2015 to 2018 and 2015 to 2017, respectively.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Fulop et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Context
Context and rationale for the research
Policy and research: major system change and innovation
There have been significant changes in the provision of clinical care within the English NHS since the turn of the century, with proposals (some now implemented) to concentrate specialist services, such as major trauma, cardiac surgery, vascular and specialist paediatrics, in fewer centres. 1–4 Major system change (MSC) in health care is seen as having the potential to increase the provision of evidence-based care and improve clinical outcomes at scale. 1 A review of the literature on implementing MSC defines it as ‘interventions aimed at coordinated, system-wide change affecting multiple organisations and care providers, with the goal of significant improvements in the efficiency of healthcare delivery, the quality of patient care, and population-level patient outcomes’. 5 MSC involves the reorganisation of services (sometimes termed ‘reconfiguration’6) at the regional level and may include significant alterations to care pathways. One such change is service centralisation, whereby some aspects of service provision across a given region are concentrated in a reduced number of hospitals. 7–12 It may involve many stakeholders across multiple organisations, and – when implemented successfully – is hypothesised to optimise the balance between quality of care, access, workforce capacity and cost. 1
A useful way to understand these reconfigurations is as processes of innovation. Reviews of the literature on the diffusion of innovations and MSC in health care draw attention to the need for more research on the processes by which such innovations are initiated (e.g. key drivers for change), implemented and sustained (or not), and in what particular contexts. 5,13 A review of evidence on the diffusion of innovations13 suggests that sustainability relates to the nature of the innovation (e.g. the benefits it offers, how complex it is, how it is led, how stakeholders are involved, and the use of evaluation and feedback) and the context into which it is introduced (local staff and organisational structures, interorganisational networks, external pressures). This evaluation aims to contribute to the development of this evidence base by studying in depth the implementation and sustainability of major service reconfiguration, using the example of stroke services.
The impact of centralisation on outcomes has been demonstrated in several specialist health-care settings, including trauma,14–16 cardiac surgery17 and neonatal intensive care. 18 However, when this study was commissioned, there was little evidence on the impact of centralisation in the context of acute stroke care. Furthermore, evidence on how changes of this scale are implemented and the relationship between implementation approaches and the impact of changes on quality of care, outcomes and costs was limited. 1
Policy and research: reconfiguration of acute stroke services
Stroke is a leading cause of mortality and disability worldwide. 19 In England, there are an estimated 125,000 cases of stroke and 40,000 deaths from stroke each year. 20 Organised inpatient Stroke Unit (SU) care is associated with better quality care21 and reduced death and dependency. 22 The case for MSC in acute stroke services was strong, with clear evidence of unacceptable variations in the quality of care, and many patients denied access to evidence-based care. 23 The Department of Health and Social Care’s National Stroke Strategy for England recommended MSC for acute stroke services, identifying that SU care was the single biggest factor that can improve outcomes following stroke. 24 However, evidence on the impact of centralisation of acute stroke services,25,26 and how best to centralise stroke services, was limited.
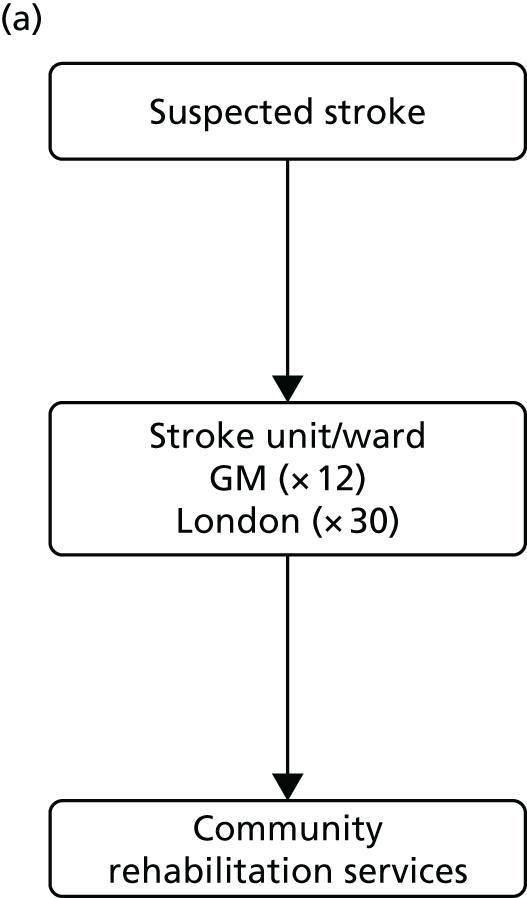
The NHS in London and Greater Manchester (GM) led the way in reconfiguring the acute stroke pathways across their regions. Before reconfigurations, in both London and GM, suspected stroke patients were taken to the nearest A&E department to receive stroke care (Figure 1). Both of the reconfigurations aimed to centralise services into ‘hub and spoke’ models, consisting of a reduced number of services providing acute stroke care up to 72 hours following stroke (hubs), with a larger number of services providing care beyond this acute phase (spokes). However, the two models differed significantly in relation to the degree of centralisation; the model in London could be characterised as a more ‘radical’ change and resulted in five stroke services closing, whereas no services closed in GM.
FIGURE 1.
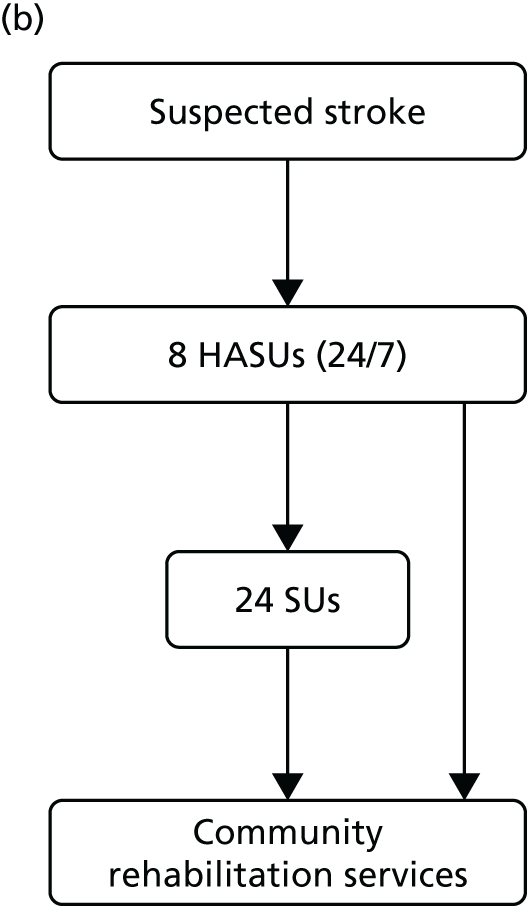
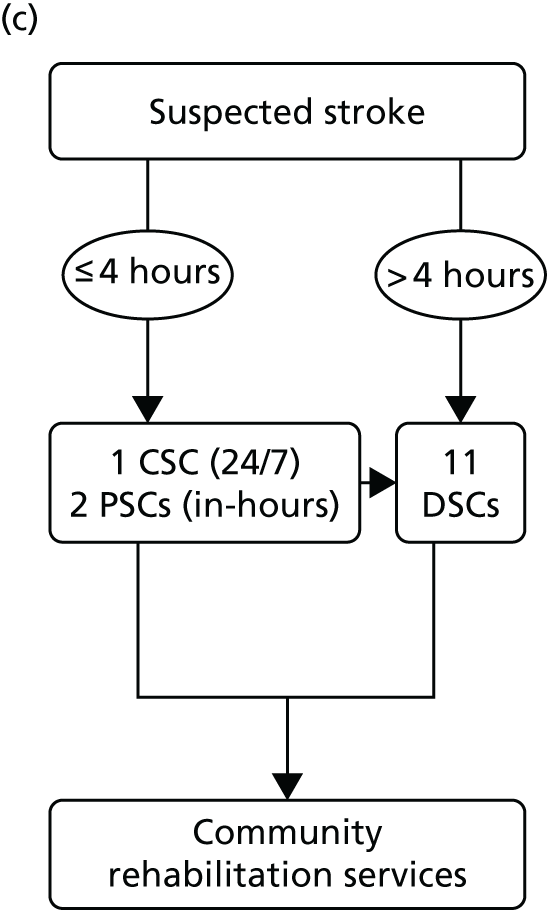
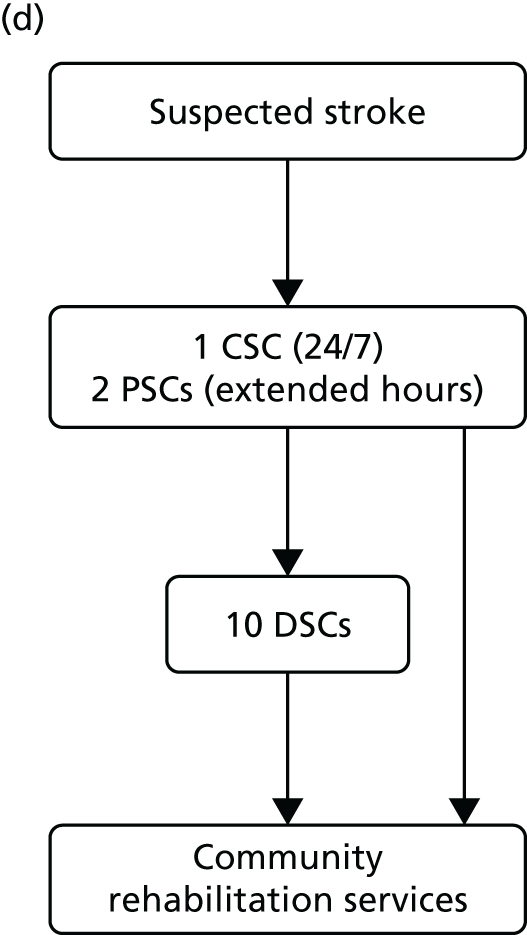
Simplified pre- and post-reconfiguration models in London and GM. (a) Before, London and GM; (b) after, London; (c) after, Greater Manchester A; and (d) after, GMB. CSC, Comprehensive Stroke Centre; DSC, District Stroke Centre. Parts a–c reproduced from Ramsay et al. 26 with permission. Effects of centralising acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46(8):2244–51. Stroke is published on behalf of the American Heart Association, Inc., by Wolters Kluwer. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0) License, which permits use, distribution, and reproduction in any medium, provided that the original work is properly cited, the use is non-commercial, and no modifications or adaptations are made. See https://creativecommons.org/licenses/by-nc-nd/3.0/. Permission to adapt this material has been agreed with Wolters Kluwer.
Reconfiguration of acute stroke services in London
London covers an area of 1570 km2 with a population of 8.17 million people,27 and has approximately 8000 annual hospital admissions following stroke. 25 The London reconfiguration was conducted at the request of the London Strategic Health Authority (SHA). 28,29 Commissioners agreed an additional £20M per annum to be paid through an enhanced tariff, providing that quality standards set by a multidisciplinary steering group were met. The model was developed with the support of a Joint Committee of Primary Care Trusts (PCTs) representing all commissioners in London. 30,31
Hospital trusts participated in a bidding process to host Hyperacute Stroke Units [(HASUs) offering care over the first 72 hours following stroke, including assessment by specialised stroke medical teams, brain imaging and thrombolysis, if appropriate], SUs (offering acute specialist stroke rehabilitation) and transient ischaemic attack (TIA) services.
Following this process, of the stroke services provided by 32 London hospitals pre reconfiguration, eight services were designated as HASUs [admitting suspected stroke patients 24 hours per day, 7 days per week (24/7)] and 24 as SUs and TIA services, and five services were decommissioned. The reconfigured London model was implemented in July 2010; since then, all suspected stroke patients have been eligible for treatment in a HASU, then repatriated to a SU, a nursing home or their own home (see Figure 1b).
Reconfiguration of acute stroke services in Greater Manchester
Greater Manchester covers 1276 km2 with a population of 2.68 million people,27 and has approximately 4000 hospital admissions following stroke per year. 25
In 2007, the Greater Manchester and Cheshire Cardiac and Stroke Network (GMCCSN) was charged by the Greater Manchester Association of PCTs to reconfigure services to allow universal access to hyperacute stroke treatment in the area. 32
In early 2008, local hospital trusts submitted bids to host the new acute stroke services. An External Advisory Group (EAG) composed of local and national stakeholders assessed these bids, and the new acute stroke services were awarded on the basis of the EAG’s recommendations. The reconfiguration was implemented in a number of stages, commencing in December 2008 and ending in April 2010. 32 Three hyperacute services were designated: one Comprehensive Stroke Centre (CSC) and two Primary Stroke Centres (PSCs). A total of 11 District Stroke Centres (DSCs) provided post-4-hour care and ongoing acute rehabilitation services. No stroke services were decommissioned. This service model is referred to as Greater Manchester A (GMA) (see Figure 1c). GMA differed from the London model in two important ways. First, any suspected stroke patient presenting within 4 hours of developing stroke symptoms was transferred to either the CSC or PSC for hyperacute care; once stable, he/she was repatriated to a DSC, a nursing home or their own home. If presenting outside this ‘4-hour window’, stroke patients in GM were taken directly to the nearest DSC, much as they would have been prior to reconfiguration. This ‘4-hour window’ represented a contrast with London, where all suspected stroke patients were eligible for treatment in a HASU. Second, although the CSC admitted patients 24/7, the PSCs admitted stroke patients only between the hours of 07.00 and 19.00, Monday–Friday (see Figure 1c); this contrasted with London, where all HASUs admitted patients 24/7. GM initially chose a service model in which all patients presenting at hospital within 24 hours of onset of stroke symptoms would be treated in a CSC/PSC (i.e. similar to the model implemented in London). However, because some hospitals raised concerns about the impact of centralisation on hospital resources and patient safety, the ‘4-hour’ model was adopted.
Further reconfiguration in Greater Manchester
When the GM reconfiguration was first designed, it was agreed that a formal review of performance should be conducted 12 months post implementation, and, based on this review, the EAG concluded that the GMA model had not fully delivered on its aim to provide local populations with equal access to high-quality acute stroke services. Therefore, further changes were considered, with an agreement to implement a revised model reached in September 2013; in March 2015 a revised model [Greater Manchester B (GMB)] was implemented.
Under the new model (see Figure 1d), any suspected stroke patient was taken directly to either the CSC or a PSC; PSC hours were extended to cover 07.00–23.00, 7 days per week, and DSCs were no longer designated to receive suspected acute stroke patients, meaning that all suspected stroke patients in GM were designated to be treated in a hyperacute unit, similarly to the London model.
Reconfiguration activity in the Midlands and the East of England
The Midlands and East of England covers an area in excess of 48,000 km2 with a population of 15.5 million people. 27 This region has 20,000 hospital admissions following stroke every year. 33 NHS Midlands and East SHA identified variation in acute stroke service performance and outcomes, both across the region and in comparison with other parts of the country. There was interest in the improvements in performance and outcomes achieved in London as a result of its major stroke reconfiguration in 2010.
The SHA recognised substantial differences between the Midlands and the East of England and London, not least in terms of demography and the predominantly rural nature of large parts of the Midlands and the East of England. Consideration was given to how, with differing geography, demography and economic circumstances, the Midlands and the East of England could achieve a step change improvement in stroke outcomes. A review was commissioned to identify the arrangements necessary to achieve this. An important influence on the review (e.g. in terms of timescale) was that it took place during the final year of the SHA, which, along with PCTs and stroke networks, were abolished as part of the NHS reforms brought about by the Health and Social Care Act 2012. 34
An External Expert Advisory Group (EEAG) developed a detailed best-practice specification to guide local service provision, covering the whole care pathway. Within local health systems proposals were developed, co-ordinated by the nine local Stroke Networks, detailing how they would meet this specification. Subsequently, the final recommendations were shared with participating areas in March 2013, shortly before the NHS reforms were implemented (and the SHA, PCTs and stroke networks were abolished). The service changes were anticipated to be implemented by March 2014. However, by December 2015, the reconfigurations either had not commenced or had been halted after making progress in planning and engagement, with several areas opting to carry out improvements within individual hospitals, rather than changing the acute stroke system more radically at a regional level.
Timeline for changes studied
Table 1 presents a timeline of the changes studied in this evaluation, covering changes implemented in GM in 2010 (GMA) and 2015 (GMB), changes implemented in London in 2010 and changes planned in the Midlands and the East of England.
| Area | Date | Event |
|---|---|---|
| GMA | December 2006 | Local clinicians present case for change to commissioners |
| June 2007 | Clinicians present case for change; commissioners and providers approve proposal | |
| September 2007 | Strategic outline case produced; consensus event held | |
| December 2007 | National: launch of National Stroke Strategy | |
| January–April 2008 | Bids for CSC/PSCs; evaluation process | |
| September 2008–February 2009 | DSC bids and evaluation | |
| December 2008–April 2010 | Staged implementation; pathway changes in April, August, September and November 2009, and January and March 2010 | |
| February 2009 | During implementation phase, agreement to shift model to incorporate ‘4-hour window’ | |
| October 2011 | EEAG recommends further centralisation | |
| GMB | July 2012 | Stakeholder workshop to discuss further centralisation |
| November 2012 | Peer reviews of all SUs (CSC/PSCs/DSCs) | |
| April 2013 | National: Health and Social Care Act 201234 comes into force | |
| July 2013 | April 2014 proposed for implementation of fully centralised care model | |
| September 2013 | CCGs formally agree full implementation (removing 4-hour limit) | |
| December 2013 | Gateway Review of process | |
| October 2014 | Clinical Senate review plans for the centralised care pathway | |
| November 2014 | Implementation date moved to end March 2015 (to allow second PSC to be ready) | |
| March 2015 | Fully centralised acute stroke care pathway launched | |
| London | July 2007 | Framework for Action published |
| December 2007 | National: launch of National Stroke Strategy | |
| November 2007–March 2008 | ‘Consulting the Capital’ – Healthcare for London consultation on stroke and trauma35 | |
| October–December 2008 | Bidding and evaluation for HASUs and SUs | |
| January–May 2009 | Public consultation on stroke and trauma reconfiguration | |
| July 2009 | Joint committee of local commissioners approve proposed reconfiguration | |
| October 2009 | Guidance on stroke tariff and service standards published | |
| October 2009–February 2010 | SU implementation | |
| February–July 2010 | HASU implementation | |
| April–October 2011 | Princess Royal University Hospital NHS Trust HASU implementation | |
| December 2011 | Healthcare for London celebration presents cost-effectiveness analysis | |
| April 2013 | National: Health and Social Care Act 201234 comes into force | |
| December 2014 | London stroke tariff and standards reviewed and updated | |
| November 2015–February 2017 | Discussions of alternative repatriation processes | |
| July 2016 | Review of North Central London stroke pathway commenced | |
| September 2016 | Local Stroke Operational Network Leads appointed | |
| Midlands and East of England | November 2011 | NHS Midlands and East SHA sets out ‘ambition’ to improve stroke services |
| April 2012 | Review process launched (including Project Board and EAG) | |
| June 2012 | Whole pathway specification developed | |
| August 2012 | Wave 1 proposals from local networks delivered and reviewed | |
| October 2012 | Wave 2 proposals from local networks delivered and reviewed | |
| February 2013 | Wave 3 proposals from local networks delivered and reviewed | |
| March 2013 | Final recommendations shared with local commissioners | |
| April 2013 | National: Health and Social Care Act 201234 comes into force | |
| December 2013 | Area B: loss of CCG unit shortly before going to consultation – change put on hold | |
| October 2014 | Area A: local CCG concerns regarding capacity – work put on hold | |
| March 2015 | Area A: decision not to conduct change – amended catchment areas, focus on quality improvement |
Aims and research questions
This study aimed to use formative evaluation methods to analyse and inform the reconfiguration of acute stroke services in different regions of England and, in doing so, identify lessons that would help guide future reconfigurations in other services. The primary aims were to:
-
identify the barriers to and facilitators of major system reconfiguration, implementation and sustainability
-
study whether or not the reconfigurations delivered clinical and cost-effective improvements that patients and the public think are worthwhile
-
identify lessons about major service reconfiguration that might be applied in other settings (i.e. other locations and other service domains).
Research questions
-
What are the key processes of and factors influencing the development and implementation of the acute stroke service reconfigurations?
-
To what extent have system changes delivered process and outcome improvements?
-
Have changes delivered improvements that stakeholders (e.g. commissioners, staff, patients and the public, and reconfiguration leads) think are worthwhile?
-
Have changes delivered value for money?
-
How is service reconfiguration influenced by the wider context of major structural change in the NHS?
Overview of the research project
This evaluation was originally funded by the National Institute for Health Research (NIHR) Service Delivery and Organisation, now the NIHR Health Services and Delivery Research (HSDR) programme, from September 2011 to study the reconfiguration of acute stroke services in London and GM, as part of its call on research into promising innovations in health-care delivery. 36 The first extension to the original project (to 31 March 2016) was funded by the NIHR HSDR programme to study the sustainability of the London reconfiguration and the planning, implementation, impact and sustainability of further reconfiguration in GM and reconfigurations across the Midlands and East of England; at this point, we adapted our research questions (RQs) to reflect the changing context of our study, so that we could focus explicitly on the influence of major structural change in the NHS. The study received a second extension from NIHR to the end of June 2017 in order to study the 2015 changes in GM (which had been delayed significantly), and factors influencing the limited progress of planned reconfiguration in the Midlands and the East of England. In addition, because no changes had been implemented across the Midlands and East of England, it was agreed with NIHR that we would not carry out quantitative analyses of the impact on clinical outcomes, clinical interventions and cost-effectiveness.
Structure of the report
This report is structured as follows:
-
Chapter 2 (Research methods) presents the overarching design of the evaluation and provides an overview of the methods employed (detailed information on methods is presented within each findings chapter).
-
Chapters 3–9 (Findings, Part A) presents findings from the reconfigurations implemented in GMA and London in 2010, in relation to the impact on patient mortality and length of stay (LOS) in hospital (see Chapter 3), impact on delivery of clinical interventions (see Chapter 4), cost-effectiveness (see Chapter 5), factors influencing planning and implementation of change (see Chapter 6), relationship between service model, implementation approaches and outcomes of change (see Chapter 7), patient and public involvement (PPI) in planning MSC (see Chapter 8) and impact on patient and carer experience (see Chapter 9).
-
Chapters 10–14 (Findings, Part B) present efforts to reconfigure stroke services across the Midlands and East of England (see Chapter 10), further reconfiguration in GM in terms of impact on clinical outcomes and delivery of clinical interventions (see Chapter 11), cost-effectiveness (see Chapter 12), and planning and implementation (see Chapter 13), and follow-up of the London reconfiguration in terms of the impact on sustainability of clinical outcomes and delivery of clinical interventions (see Chapter 11) and factors influencing sustainability (see Chapter 14).
-
Several of our findings chapters draw on published papers (see Chapters 3, 4, 6 and 7) and manuscripts submitted for publication (see Chapters 5, 8 and 9). Details of the publication status are provided at the beginning of each of these chapters. To ensure coherence across different components of the evaluation, we have provided summary sections on ‘what we already know’ and ‘what this chapter adds’ to each findings chapter.
-
Chapter 15 (Discussion/Conclusions) presents our findings linked to our RQs, the implications for health services and research and the impact of our research to date, on both policy and service reorganisation.
Chapter 2 Research methods
Overview
In this chapter we provide an overview of our study design. We outline the quantitative and qualitative methods used; further details are provided in each findings chapter. We studied these major system innovations in two contrasting but complementary ways (Figure 2). First, we used a traditional health technology assessment approach to address ‘what works and at what cost?’. On its own, however, this approach pays insufficient attention to structural pressures (e.g. professional pressures or processes whereby organisations innovate as a result of economic, regulatory or legal reasons). 37,38 This approach also assumes that innovation is always progressive and poor adopters are conservative, whereas resistance may be a rational response. 39 Therefore, second, to address questions related to ‘understanding development, implementation and sustainability’ we conducted a series of case studies, using qualitative methods and drawing on theories related to the dissemination and sustainability of innovations and of large-scale change. A major review of the evidence on the diffusion of innovations identified characteristics that are more likely to be sustainable. 13 A review of the evidence on large-scale transformation of services identified a number of ‘simple rules’ supporting effective implementation of MSC:5 (1) a combination of designated and distributed leadership, (2) learning from history, (3) improvement through feedback, (4) physician engagement and (5) service user involvement. We used these rules to guide our analysis of how the reconfigurations were planned and implemented. We also drew on theoretical frameworks that analyse the relationships between implementation approaches, implementation outcomes (e.g. fidelity to and sustainability of the intervention) and intervention outcomes (for example, provision of evidence-based care, and clinical outcomes, such as patient mortality). 40–44
FIGURE 2.
Framework for evaluating MSC. HES, Hospital Episode Statistics; ONS, Office for National Statistics; SINAP, Stroke Improvement National Audit Programme; SSNAP, Sentinel Stroke National Audit Programme.

We studied the planning and (where relevant) implementation and outcomes of reconfiguring acute stroke services in a number of settings in England, which allowed us to analyse the relationship between implementation and organisational context. This was a formative evaluation, sharing findings during the course of the study to enable learning by both the systems under study and the wider NHS. Our approach to sharing findings and the impact of our feedback are discussed in Chapter 15, The impact of our study.
Understanding what works and at what cost
This component of the evaluation analysed documentary evidence to establish the models applied in London and GM; it also analysed routinely collected data (e.g. national databases) to determine whether or not these changes were associated with any changes in care provision, clinical outcomes and cost-effectiveness (see Figure 2).
In assessing the nature and results of the reconfigurations, we applied a controlled before-and-after design. 45 This compared the participating regions in terms of the impact they had on delivery of clinical interventions associated with improved clinical outcomes (e.g. SU within 4 hours) (see Chapters 4 and 11), clinical outcomes (see Chapters 3 and 11) and cost-effectiveness of care (see Chapters 5 and 12). In addition to comparing these sites pre and post reconfiguration, we made wider comparisons with the rest of England (RoE); this approach facilitated analysis of these impacts in the context of changes that took place in the RoE over this period.
Understanding development, implementation and sustainability
To develop lessons for future reconfigurations, it is important to establish not just whether or not process and outcome changes took place, but also how and why they occurred; furthermore, it is important to analyse whether or not and how changes and their impact were sustained (defined as ‘the process through which new working methods [e.g. a new referral pathway], performance goals [e.g. improvements in clinical outcomes and delivery of evidence-based clinical interventions] and improvement trajectories are maintained for a period appropriate to a given context’46). 46–49 To study these, we used a range of qualitative methods (documentary analysis, stakeholder interviews and non-participant observation) (see Figure 2).
These data were used to explore themes drawn from the evaluation’s conceptual framework and thus establish the relationships between activities in support of change, the context, the complex interactions between stakeholders, and perceived process and outcome changes. In doing so, we analysed factors influencing the decision to change (or not to change) (see Chapters 6, 8, 10 and 13), the decision on which model to implement (see Chapters 6, 8 and 13), approaches to implementation (see Chapters 6, 7 and 13), and how these factors influenced the patient and carer experience (see Chapter 9) and sustainability of change (see Chapter 14).
Sampling
We studied each case of reconfiguration at both governance and service levels. At governance level (i.e. leadership, oversight and facilitation of the changes), interviewees were purposively sampled to obtain national and pan-regional perspectives on planning and implementation of the centralisations. At the service level, a number of stroke services were purposively sampled to capture the range of experiences of the changes. In GM, we sampled the sole 24/7 CSC, one of the two in-hours PSCs, one of the 11 DSCs and the ambulance service. In London, we sampled two of eight HASUs, on the basis of both performance on the pre-designation service assessment and location (because both were factors considered in the final designation of HASUs), two of the 24 SUs from different areas, the ambulance service and one of the five services that were decommissioned. Interviews were conducted with clinicians and managers within these services (Table 2). 36
| Data source | Data collected (n) |
|---|---|
| National | |
| Stakeholder interviews [participant identifier = Nat01–04] | 4 |
| London: development and implementation (see Chapters 6, 7 and 8), collected April 2012–November 2013 | |
| Documents | 386 |
| Stakeholder interviews | |
| Governance [Lon] | 27 |
| Service A (HASU, North London, high score) [LonA] | 11 |
| Service B (SU, North London) [LonB] | 8 |
| Service C (HASU, South London, low score) [LonC] | 12 |
| Service D (SU, South London) [LonD] | 8 |
| Service E (decommissioned service) [LonE] | 4 |
| Ambulance [LonAmb] | 2 |
| Total interviews | 72 |
| GMA: development and implementation (see Chapters 6, 7 and 8), collected April 2012–December 2013 | |
| Documents | 267 |
| Stakeholder interviews | |
| Governance [GM] | 16 |
| Service F (24/7 CSC) [GMF] | 11 |
| Service G (in-hours PSC) [GMG] | 10 |
| Service H (post-4-hours DSC) [GMH] | 11 |
| Ambulance [GMAmb] | 3 |
| Total interviews | 51 |
| Patient and carer experience (see Chapter 9) collected: February 2013–May 2016 | |
| London [LonA/B/C/D/pat] | 21 |
| GM [GMF/G/H/pat] | 15 |
| Total interviews | 36 |
| Midlands and East of England: development (see Chapter 10), collected May 2013–December 2014 | |
| Documents | 223 |
| Non-participant observations | 12 (≈30 hours) |
| Stakeholder interviews | |
| Service review [ME] | 8 |
| Implementation: Area A [MEA] | 11 |
| Implementation: Area B [MEB] | 2 |
| Implementation: Area C [MEC] | 9 |
| Implementation: Area D [MED] | 3 |
| Total interviews | 33 |
| GMB: development and implementation (see Chapter 13), collected January 2014–March 2017 | |
| Documents | 114 |
| Non-participant observations | 59 (≈120 hours) |
| Stakeholder interviews | |
| Governance | 21 |
| Service F (24/7 CSC) | 19 |
| Service G (in-hours PSC) | 9 |
| Service H (DSC) | 8 |
| Other PSC | 5 |
| Other DSCs | 7 |
| Ambulance | 4 |
| Patient groups | 5 |
| Total interviews | 78 |
| London: sustainability (see Chapter 14), collected December 2013–March 2017 | |
| Documents | 101 |
| Non-participant observations | 21 (≈60 hours) |
| Stakeholder interviews | |
| Governance | 8 |
| Service A (HASU, North London, high score) | 9 |
| Service B (SU, North London) | 11 |
| Service C (HASU, South London, low score) | 8 |
| Service D (SU, South London) | 9 |
| Ambulance | 5 |
| Patient groups | 1 |
| Total interviews | 51 |
| Grand totals | |
| Documents | 1091 |
| Non-participant observations | 92 (≈210 hours) |
| Stakeholder interviews | 325 |
In relation to qualitative data collected (see Table 2), documents included publicly available reports (e.g. consultation documents and minutes of meetings) and internal documents (e.g. progress reports and communications). Stakeholders interviewed included representatives of stroke network boards, pan-regional health authorities, service commissioners, service users and representatives, programme facilitation (including consultancies), clinical leads, provider organisations, stroke service staff, ambulance staff (including managers, trainers and frontline staff) and local and national politicians. Non-participant observations were conducted of meetings and events to plan and oversee implementation of those changes that we were able to study contemporaneously, including engagement events and board meetings (GMB and Midlands and the East of England), and meetings and events related to ongoing sustainability and development of services, including oversight meetings, service reviews and training events (London, GM).
Data collection
Potential interviewees were identified using documentary evidence and ‘snowball’ sampling, and contacted via e-mail or telephone. Interviews were conducted only with fully informed, written consent (the recruitment process for patients and carers is presented in Chapter 9). Interviews lasted approximately 50 minutes and were audio-recorded and professionally transcribed. Non-participant observations were conducted with fully informed consent from the Chairperson and members. All documents analysed were either in the public domain or obtained from local change leaders and service leads.
Presenting qualitative data
When presenting quotations we have used anonymised participant identifiers. The identifiers for each level of our sample are presented in Table 2. For each quotation, we also present a short statement of the individual’s role (e.g. stroke patient, stroke physician, network representative). For quotations from documents we state the document sources. For quotations from non-participant observations we state the event and date on which it took place (e.g. Project Board Meeting, 25/12/2010).
Synthesis of approaches
We used a mixed-method case study approach to draw together the learning from the approaches described above (the cases being each MSC planned and/or implemented, i.e. London, GM and the Midlands and East of England). This facilitated the development and testing of theories on how efforts to bring about change interacted with the context in which they were implemented. 50–52 The qualitative component was designed to allow change to be evaluated, first, in relation to how the reconfigurations of acute stroke services were planned and governed at the regional level and, second, in relation to how services within each studied region experienced the changes. The services we studied were selected to reflect the main forms of change experienced by organisations participating in reconfigurations of this kind, including developing new services and refocusing or decommissioning existing services. We drew together findings from the evaluation’s quantitative and qualitative components to develop and test theories on the relationships between the models selected, implementation approaches applied, the degree to which the model was ‘successfully’ implemented, and how these contributed to the outcomes observed (see Chapter 7) in terms of provision of evidence-based care and clinical outcomes (such as mortality and patient LOS).
Ethics and research governance approvals
We recognised that taking part in the qualitative aspects of this research could potentially cause participants distress, for example patients and carers discussing personal experiences of stroke services, or staff discussing the reconfiguration and/or closure of services. We obtained full ethics approval for this study in September 2011 from the National Research Ethics Service Ethics Committee London-East, which set out how we would minimise this potential distress. Following the November 2012 extension to this project, a substantial amendment to ethics approval was obtained in February 2013, reflecting the additional participating sites and methods. Following the March 2015 extension, a substantial amendment (version 1.9, 23 November 2015) and a non-substantial amendment (version 2.0, 15 January 2016) were obtained from the South East Coast – Surrey NHS Research Ethics Committee. In support of data collection in our studied areas, we obtained local research governance permissions for all relevant organisations (see Appendix 1).
Patient and public involvement
Patient and public involvement informed and enhanced our research throughout the project. We provide a detailed description of our approach to PPI and its impact in Appendix 2.
Findings, Part A: reconfiguration of acute stroke services in Greater Manchester and London
Chapter 3 The impact of the centralisation of acute stroke care on clinical outcomes
Overview
This chapter draws on Morris et al. 25 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.
What was already known?
-
Organised inpatient SU care is associated with higher quality care and reduced death and dependency.
-
Acute stroke services were being centralised in several countries as a means of improving access to organised inpatient SU care, but it was not known if this affected mortality and LOS.
What this chapter adds
-
In London, where all patients were eligible for treatment in a HASU, there was a reduction in mortality and LOS.
-
In GMA, where CSC/PSC care was provided to patients presenting within 4 hours of developing stroke symptoms, there was no impact on mortality but LOS was reduced.
Background
Organised inpatient SU care is associated with better quality care21 and reduced death and dependency. 22 The Department of Health and Social Care’s National Stroke Strategy for England recommended MSC for stroke, identifying that SU care was the single biggest factor that can improve outcomes following stroke. 24 In several countries work has been conducted to centralise acute stroke services as a means of improving access to organised inpatient SU care. Research in the USA,7,8 Canada,9 the Netherlands,10 Denmark11 and Australia12 has suggested that this approach may improve the provision of evidence-based care processes for stroke patients, for example by increasing access to specialist care and thrombolysis. Other evidence has suggested that this approach is highly cost-effective. 53 Although the improved clinical outcomes associated with organised inpatient stroke care were well documented, it was unknown if centralising acute stroke care to a small number of high-volume specialist centres produces better clinical outcomes. 54,55 In addition, the wisdom of focusing on hyperacute stroke care has been questioned. 56
In this chapter we present our analysis of the impact of centralising acute stroke services in London and GMA on mortality and LOS. We used data for all patients in England who had a stroke during a 51-month period and controlled for trends in the RoE during the same period and for other factors that could affect outcomes.
Methods
Data
We obtained patient-level data from the Hospital Episode Statistics (HES) database57 for all patients in England with a primary diagnosis of stroke defined using International Classification of Diseases, 10th Revision (ICD-10)58 codes I61 (intracerebral haemorrhage), I63 (cerebral infarction) or I64 (stroke, not specified as haemorrhage or infarction) between 1 January 2008 and 31 March 2012. We excluded subarachnoid haemorrhage (ICD-10 code I60) because it is managed through a different clinical pathway. 59 The data were linked to mortality data supplied by the Office for National Statistics (ONS)60 using an anonymised unique patient identifier to identify deaths from any cause and at any place of death (hospital or otherwise) at 3, 30 and 90 days after hospital admission. LOS was measured in days as the difference between date of admission and date of discharge, including same-day transfers between hospitals.
January 2008 was defined as the start of our analysis period following the publication of the National Stroke Strategy for the English NHS in December 2007,24 leading to better emergency responses to stroke and acute stroke care around the country. 61 Our data cover a 27-month period before the changes in GM (which occurred in April 2010) and a 24-month period afterwards. In London they cover a 30-month period before the changes (July 2010) and a 21-month period afterwards. In both areas some hospitals began to reconfigure their services before these dates and we controlled for this in our analysis using hospital and time fixed effects.
Our main analysis was confined to patients living in urban areas (defined as ‘urban-less sparse’ using the urban/rural classification for England;62 95% of stroke patients in GM and London lived in these areas compared with 75% in the RoE). We did not restrict the analysis to any type of hospital (we included hospital fixed effects to allow for hospital differences) and we did not impose a minimum number of patients to be treated at each hospital (observations in the hospital-level regressions were weighted by the number of patients). Patients were treated at 11 hospitals in GM, 38 in London and 405 in the RoE over the period. Data were available for 258,915 admissions, of which 17,650 were in GM (9413 prior to reconfiguration, 8237 afterwards) and 33,698 were in London (18,672 prior to reconfiguration, 15,026 afterwards).
Statistical analyses
We evaluated whether or not centralising acute stroke services in GM and London had an impact on mortality and LOS using a between-region difference-in-differences regression analysis,63 comparing the changes over time in GM and London to the change over time in the RoE. The analysis was carried out at hospital level using quarterly observations of risk-adjusted mortality and LOS; the risk adjustment was conducted at the patient level on all patients in the data. The approach was consistent with the Medical Research Council guidelines for using natural experiments to evaluate population health interventions,64 and a similar method was used in an evaluation of the Advancing Quality initiative in the north-west of England. 65
We calculated expected risks of death at 3, 30 and 90 days after admission using patient-level logistic regressions, including binary indicators for sex and age interactions (age measured in 5-year bands), stroke diagnosis using the first four digits of the primary ICD-10 code (19 categories), Charlson Comorbidity Index66 derived from secondary ICD-10 codes, the presence of 16 comorbidities included in the Charlson Comorbidity Index, ethnic group (18 categories), and deprivation quintile67 and urban/rural classification62 (eight categories) of the area in which the patient lived (of 32,482 lower layer super output areas in England). The patient-level regressions were run only on patients who had a stroke before the reorganisations in GM and London so that the risk adjustment was not contaminated by the changes. The regression coefficients (derived from the logistic regressions for the pre-implementation period) were used to predict the probability of mortality for every patient (in both pre- and post-implementation periods). These were aggregated to create a data set of the actual percentage of patients who died and the expected percentage by admitting hospital and quarter. We tested whether or not the reconfigurations had an impact on mortality using least squares regression of the actual minus expected mortality percentage (because we are modelling differences) against interaction terms between GM and the post-reconfiguration period and London and the post-reconfiguration period. We included binary indicators for each of the 454 admitting hospitals (hospital fixed effects) and the 17 quarters (time fixed effects), and each observation was weighted by the number of patients treated at that hospital in that quarter. Standard errors were corrected for heteroscedasticity.
We used the same approach for LOS, but our risk adjustment equation was estimated using a generalised linear model (GLM) with gamma family and log-link to account for data skewness. 68 We experimented with other GLM specifications and a log-transformation but the selected model gave the best fit in terms of residual plots and Akaike’s information criterion. We added binary indicators for mortality at 3, 30 and 90 days after stroke to the risk equation and used the regression coefficients to predict expected mean LOS.
We undertook pre-trends tests to examine whether or not risk-adjusted mortality and LOS had a different linear trend in GM and London compared with the RoE before the reconfigurations. We reran the models on every quarter prior to the reconfigurations and included linear time trends instead of binary indicators for quarter. We added interaction terms between GM and the linear time trend and London and the linear time trend and tested the individual significance of the interaction terms. In every case they were non-significant (p > 0.05).
Results
Patients in GM and London were slightly younger than those in the RoE, and those in London were less likely to be white British (Table 3). The percentage of strokes that were intracerebral haemorrhage was slightly higher in London, and slightly lower in GM, than in the RoE. Patients in GM and London were less likely to live in deprived areas. Unadjusted outcomes showed a small decline in mortality in London compared with the RoE, and a small decline in LOS in GM and London. There was some evidence of difference-in-differences with respect to age, sex, type of stroke and deprivation in GM and age, ethnic group and stroke type in London.
| Characteristic | Region | Difference-in-differencesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RoE | GM | London | |||||||||
| Before | After | Difference | Before | After | Difference | Before | After | Difference | GM minus RE | London minus RoE | |
| Number of patients | 122,084 | 85,483 | 9413 | 8237 | 18,672 | 15,026 | |||||
| Unadjusted outcomes | |||||||||||
| Unadjusted mortality | |||||||||||
| At 3 days (%) | 6.6 | 5.7 | –0.9 | 6.3 | 5.6 | –0.7 | 5.8 | 4.6 | –1.2 | 0.2 | –0.3 |
| At 30 days (%) | 19.2 | 16.9 | –2.4 | 18.1 | 16.5 | –1.6 | 16.8 | 14.1 | –2.8 | 0.7 | –0.4 |
| At 90 days (%) | 25.8 | 22.7 | –3.1 | 25.2 | 21.9 | –3.3 | 23.0 | 19.4 | –3.6 | –0.2 | –0.4 |
| Unadjusted mean LOS (days) | 21.0 | 18.4 | –2.6 | 21.7 | 17.7 | –4.0 | 20.6 | 17.8 | –2.8 | –1.4 | –0.2 |
| Patient characteristics | |||||||||||
| Mean age (years) | 75.6 | 75.3 | –0.3 | 74.3 | 73.9 | –0.4 | 73.0 | 73.3 | 0.2 | –0.1 | 0.5 |
| ≥ 75 years (%) | 60.6 | 59.3 | –1.3 | 56.0 | 53.6 | –2.4 | 54.3 | 54.4 | 0.1 | –1.1 | 1.4 |
| Female (%) | 53.0 | 52.2 | –0.8 | 52.6 | 50.4 | –2.1 | 51.0 | 49.8 | –1.2 | –1.4 | –0.4 |
| White British ethnic group (%) | 84.3 | 86.4 | 2.1 | 82.9 | 84.2 | 1.2 | 58.5 | 55.0 | –3.5 | –0.9 | –5.6 |
| Intracerebral haemorrhage (%)b | 12.8 | 12.7 | –0.2 | 11.5 | 11.7 | 0.2 | 15.7 | 14.8 | –0.9 | 0.3 | –0.7 |
| Cerebral infarction (%)c | 65.1 | 71.6 | 6.5 | 61.6 | 64.4 | 2.8 | 68.9 | 76.1 | 7.2 | –3.7 | 0.7 |
| Stroke, not specified as haemorrhage or infarction (%)d | 22.1 | 15.7 | –6.3 | 26.9 | 23.9 | –3.0 | 15.4 | 9.1 | –6.3 | 3.3 | 0.0 |
| Charlson Comorbidity Index (mean score) | 1.9 | 1.9 | 0.0 | 2.0 | 2.0 | 0.0 | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 |
| Most deprived quintile (%)e | 17.2 | 17.6 | 0.4 | 8.4 | 10.3 | 1.9 | 12.6 | 13.2 | 0.6 | 1.5 | 0.2 |
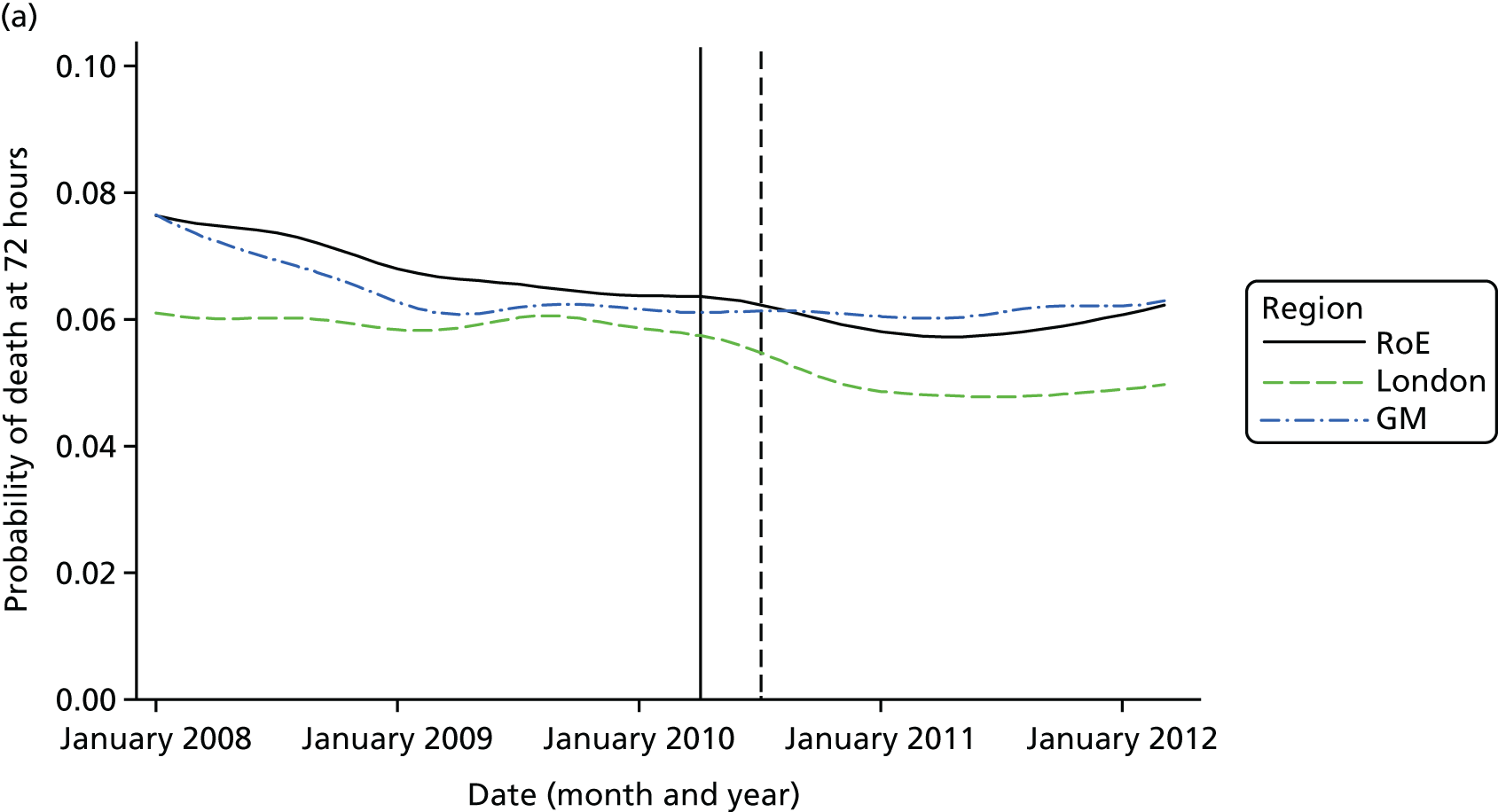
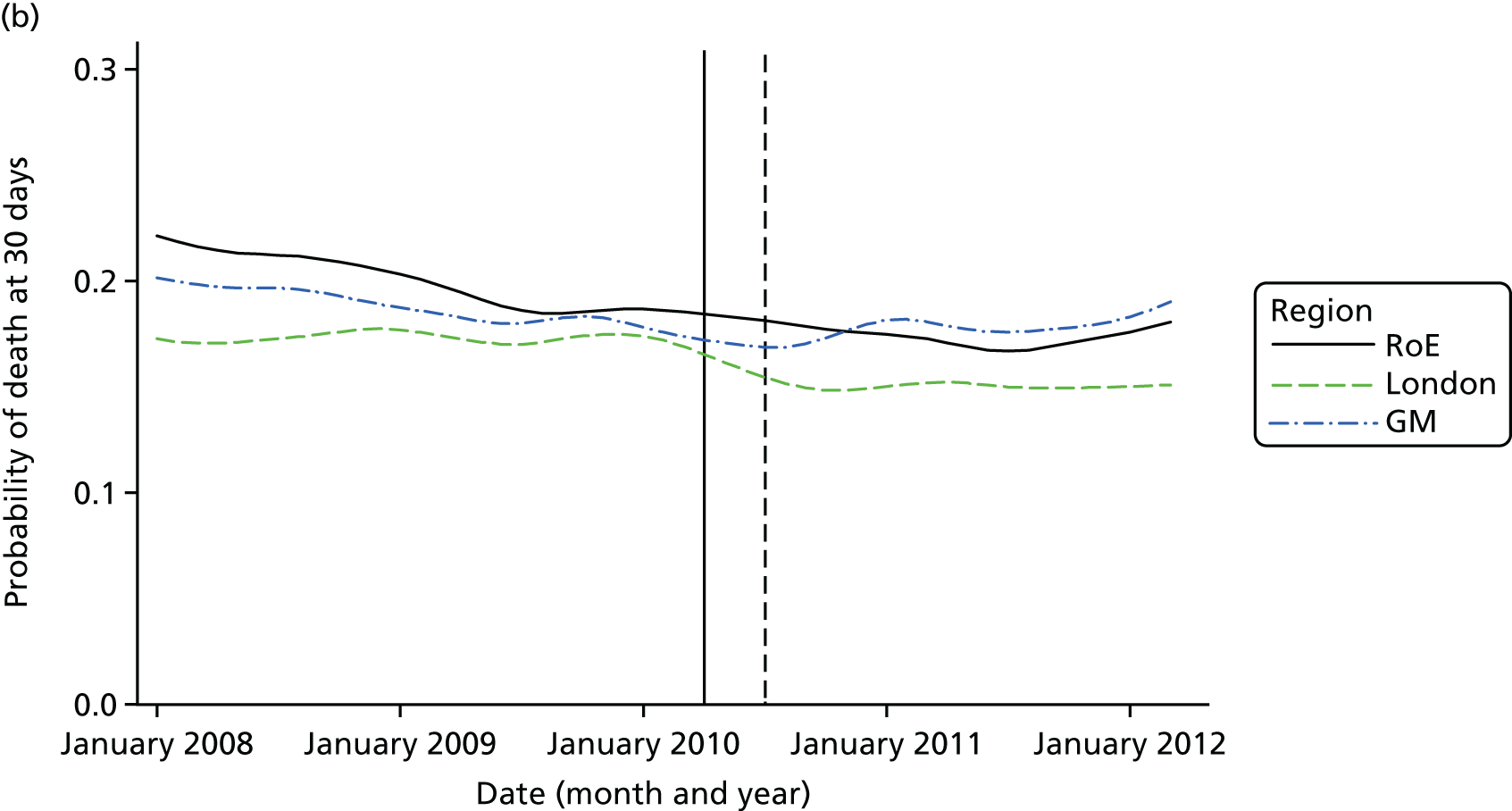
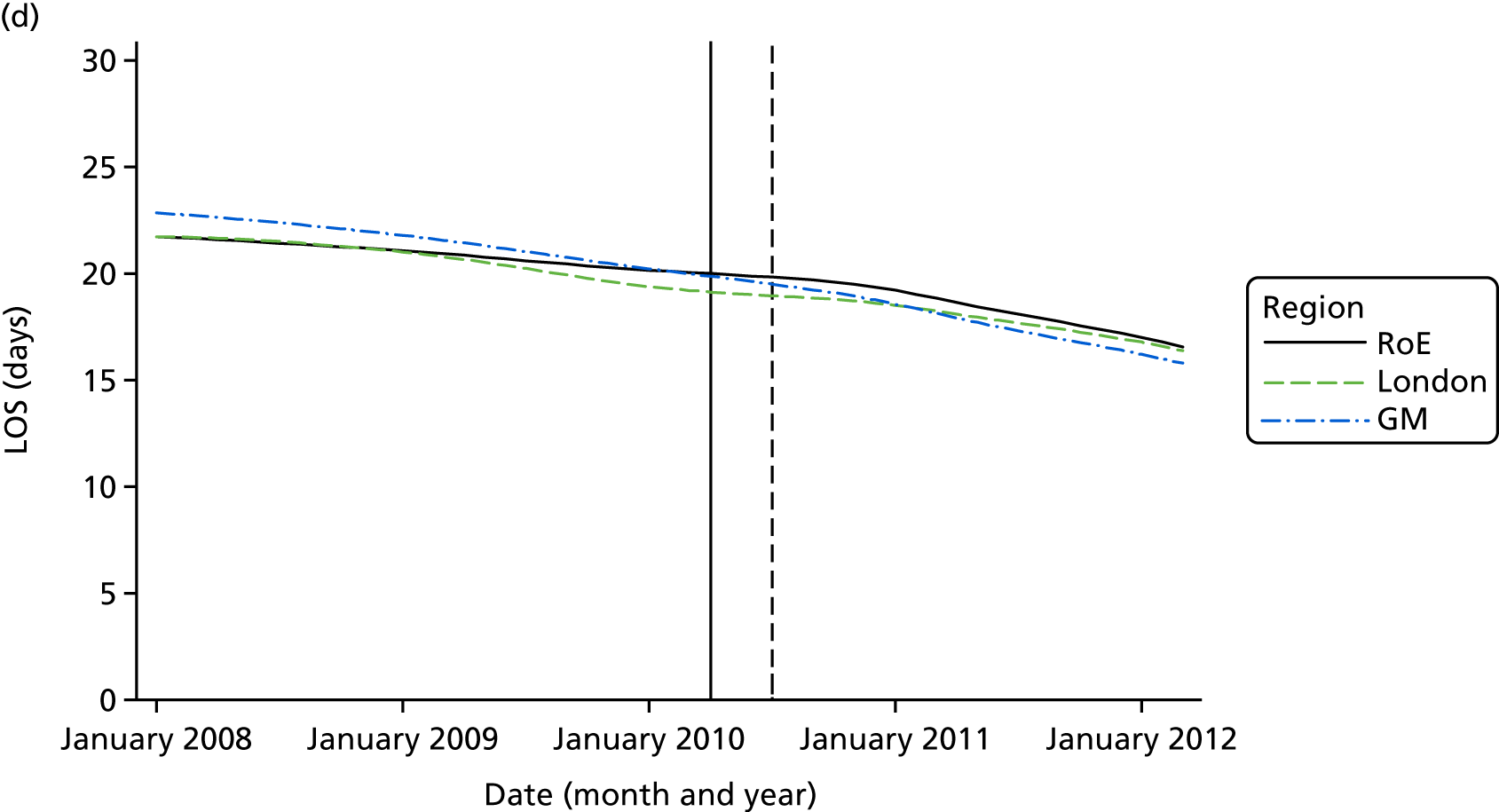
Mortality and LOS fell in GM, London and the RoE during the study period (Figure 3). In London there was a significantly larger absolute reduction in risk-adjusted mortality at 3 days after admission compared with the RoE, by –1.0 percentage points [95% confidence interval (CI) –1.5 to –0.4 percentage points; p < 0.001; Table 4]. There was also a significantly larger absolute reduction in risk-adjusted mortality at 30 days (–1.3%, 95% CI –2.2% to –0.4%; p = 0.005) and 90 days after admission (–1.1%, 95% CI –2.1% to –0.1%; p = 0.03). These absolute differences represent relative reductions in mortality of 17%, 7% and 5%, respectively, which equate to a total reduction of 83 deaths at 3 days (95% CI 38 to 128 deaths), 111 deaths at 30 days (95% CI 34 to 187 deaths) and 96 deaths at 90 days (95% CI 11 to 181 deaths) every year in London. In GM the changes in mortality after the reconfiguration of services were not significantly different from the changes seen in the RoE during the same period.
FIGURE 3.
Probability of mortality and LOS in GM, London and the RoE by month. (a) Mortality at 3 days; (b) mortality at 30 days; (c) mortality at 90 days; and (d) LOS. The solid (dashed) vertical line shows when the reconfiguration occurred in GM (London). Adapted from Morris et al. 25 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/. Figure title and numbering updated for report.

| Outcome measure | Difference-in-differencesa (95% CI); p-value | |
|---|---|---|
| GM minus RoE | London minus RoE | |
| All stroke subtypes combined; patients living in urban areas only | ||
| Risk-adjusted mortality | ||
| At 3 days (%) | –0.04 (–0.7 to 0.6); 0.90 | –1.0 (–1.5 to –0.4); < 0.001 |
| At 30 days (%) | 0.8 (–0.3 to 1.9); 0.15 | –1.3 (–2.2 to –0.4); 0.005 |
| At 90 days (%) | 0.1 (–1.1 to 1.3); 0.89 | –1.1 (–2.1 to –0.1); 0.03 |
| Risk-adjusted LOS (days) | –2.0 (–2.8 to –1.2); < 0.001 | –1.4 (–2.3 to –0.5); 0.002 |
| Intracerebral haemorrhage;b patients living in urban areas only | ||
| Risk-adjusted mortality | ||
| At 3 days (%) | 0.4 (–2.9 to 3.8); 0.80 | –3.3 (–5.7 to –0.9); 0.006 |
| At 30 days (%) | –1.1 (–5.1 to 2.9); 0.60 | –2.0 (–4.8 to 0.8); 0.16 |
| At 90 days (%) | 0.3 (–4.3 to 3.8); 0.90 | –1.1 (–4.0 to 1.7); 0.44 |
| Risk-adjusted LOS (days) | –1.3 (–3.7 to 1.0); 0.27 | –0.7 (–2.4 to 0.9); 0.39 |
| Cerebral infarction;c patients living in urban areas only | ||
| Risk-adjusted mortality | ||
| At 3 days (%) | 0.5 (–0.2 to 1.1); 0.14 | –0.8 (–1.2 to –0.3); 0.001 |
| At 30 days (%) | 1.9 (0.6 to 3.2); 0.004 | –1.3 (–2.2 to –0.3); 0.01 |
| At 90 days (%) | 1.1 (–0.4 to 2.5); 0.14 | –1.1 (–2.2 to –0.03); 0.04 |
| Risk-adjusted LOS (days) | –2.6 (–3.6 to –1.5); < 0.001 | –1.4 (–2.4 to –0.3); 0.009 |
| Stroke, not specified as haemorrhage or infarction;d patients living in urban areas only | ||
| Risk-adjusted mortality | ||
| At 3 days (%) | –0.8 (–2.5 to 0.9); 0.34 | 0.02 (–2.0 to 2.0); 0.98 |
| At 30 days (%) | –0.1 (–2.6 to 2.4); 0.94 | –1.3 (–4.3 to 1.7); 0.40 |
| At 90 days (%) | –1.2 (–3.9 to 1.5); 0.39 | –2.2 (–5.4 to 1.0); 0.18 |
| Risk-adjusted LOS (days) | –0.9 (–2.4 to 0.5); 0.21 | –2.2 (–3.7 to –0.7); 0.004 |
| All stroke subtypes combined; patients living in urban and rural arease | ||
| Risk-adjusted mortality | ||
| At 3 days (%) | –0.1 (–0.7 to 0.6); 0.94 | –1.0 (–1.5 to –0.4); 0.001 |
| At 30 days (%) | 0.8 (–0.2 to 1.9); 0.13 | –1.2 (–2.1 to –0.2); 0.01 |
| At 90 days (%) | 0.1 (–1.2 to 1.4); 0.87 | –1.0 (–2.0 to –0.1); 0.04 |
| Risk-adjusted LOS (days) | –2.1 (–2.9 to –1.3); < 0.001 | –1.4 (–2.3 to –0.5); 0.003 |
In both areas there was a significantly larger decline in risk-adjusted LOS than in the RoE. In GM there was a significant reduction of –2.0 days (95% CI –2.8 to –1.2 days; p < 0.001), and in London of –1.4 days (95% CI –2.3 to –0.5 days; p = 0.002). These represent a 9% reduction in LOS in GM and a 7% reduction in London, and suggest 8842 fewer hospital days each year in GM (95% CI 5359 to 21,326 days), and 12,766 fewer hospital days each year in London (95% CI 4507 to 21,026 days).
We reran our models on patients stratified by type of stroke and found that reductions in mortality and LOS were largely achieved among patients diagnosed with ischaemic stroke, who accounted for the majority of cases (68% of the sample) (see Table 4). Point estimates of the reductions in mortality in London were higher for intracerebral haemorrhage than for ischaemic stroke but the effects for intracerebral haemorrhage were non-significant. In GM there was a significant increase in risk-adjusted mortality at 30 days following cerebral infarction, but there were no significant differences at 3 and 90 days. We reran our models including 73,558 patients who lived in rural areas and this had little impact on the results (see Table 4).
Discussion
Principal findings
Risk-adjusted mortality and LOS fell in GM, London and the RoE during the study period. In London there was a significant reduction in mortality at 3, 30 and 90 days after admission over and above the reduction seen in the RoE; at 90 days the reduction in mortality was 1.1 percentage points. There was also a significant reduction in LOS of 1.4 days over and above the reduction seen in the RoE. In GM there was no impact on mortality over and above the change seen in the RoE but there was a significant reduction in LOS by 2.0 days. Significant reductions in mortality and LOS were largely achieved among patients with ischaemic stroke.
Strengths and weaknesses
The main strengths of the analyses are the large national data set we have used, which contains detailed information on outcomes and patient characteristics, and the robust quasi-experimental framework we have employed, which allowed us to control for trends in the RoE and other factors that could affect outcomes during the same period.
There are several weaknesses, which mean that our findings should be treated with caution – these call into question whether or not the findings of our analyses can be interpreted as causal effects. First, we consider the main limitation of our analysis to be that the HES database does not include information on stroke severity, which has been shown to be an important predictor of mortality. 69 Post-reconfiguration data from the Stroke Improvement National Audit Programme (SINAP)70 show that indicators of stroke severity such as ‘worst level’ of consciousness in the first 24 hours after stroke and neurological deficits on admission varied between GM, London and the RoE (Table 5), but there was no discernible trend over all the indicators. In spite of this, and even though our outcomes were risk-adjusted for a number of patient-level factors and we accounted very flexibly for differences between hospitals and trends over time, we cannot rule out the possibility that the differences in outcomes may be due to variations in stroke severity over time between GM and London and the RoE.
| Indicator | Region | p-value | ||
|---|---|---|---|---|
| RoE | GM | London | ||
| Worst level of consciousness in first 24 hours, % | ||||
| Fully conscious | 76 | 76 | 79 | < 0.001 |
| Drowsy | 15 | 15 | 15 | |
| Semiconscious | 4 | 4 | 3 | |
| Unconscious | 4 | 5 | 3 | |
| Observations | 58,137 | 10,295 | 16,446 | |
| Neurological deficits, % with deficit | ||||
| Face (weakness/sensory loss) | 56 | 52 | 59 | < 0.001 |
| Arm (weakness/sensory loss) | 70 | 69 | 70 | 0.06 |
| Leg (weakness/sensory loss) | 61 | 59 | 62 | < 0.001 |
| Dysphasia | 46 | 42 | 42 | < 0.001 |
| Hemianopia | 18 | 16 | 20 | < 0.001 |
| Inattention/neglect | 18 | 15 | 20 | < 0.001 |
| Brainstem/cerebellar signs | 8 | 7 | 12 | < 0.001 |
| Other neurological deficit | 26 | 22 | 36 | < 0.001 |
| Observations | 56,161 | 9618 | 16,150 | |
Second, we were unable to assess the impact of the reconfigurations on other outcomes, such as quality of life (QoL), disability or neurological and functional impairment, because these measures were not collected in the HES database.
Third, the HES database includes only patients admitted to hospital; it does not include any information about patients who died before they reached hospital, nor does it include information on the time of stroke, and, hence, our analyses of mortality were based on time from admission. If stroke patients in London were more likely to die before reaching the hospital owing to longer distances to HASUs, then the effects of the reconfigurations on mortality would be overestimated. Evidence suggests that this is unlikely, because ambulance journey times for stroke patients did not increase appreciably after the reconfiguration in London, with mean scene-to-hospital times of 14 minutes from January 2005 to March 200821 and of 16 minutes from April 2011 to March 2012. 73 In addition, stroke severity in London post reconfiguration was similar to the severity in the RoE (see Table 5); if more severely ill stroke patients died in London before reaching the hospital, the level of severity in the audit data for London would be lower than elsewhere.
Fourth, LOS was measured as the difference between date of admission and date of discharge. We assumed that when patients were discharged from one hospital and readmitted to another hospital on the same day that it was a transfer related to the original stroke, capturing the movement between components of the stroke care pathway (e.g. between a HASU and a SU in London). Conversely, we assumed that when a subsequent admission occurred ≥ 1 day after discharge that it was a recurrent stroke (there is a risk of stroke recurrence in the first month after discharge of 1.1% to 15%). 74
Fifth, there was a higher than expected number of stroke patients per month in London during the post-reconfiguration period. One possible reason is that after the reconfiguration London treated more patients from surrounding areas who might previously have gone to their local A&E department. It may also have been because greater public awareness of stroke in London meant that more people presented acutely in London rather than staying at home. This could have biased the results in favour of the London reconfiguration if the additional admissions were less severe strokes, but there were no discernible differences in severity between London, GM and the RoE (see Table 5).
Comparison with other studies
Our findings with respect to mortality in London are consistent with a cost-effectiveness analysis of the reconfiguration of acute stroke services in London,53 which also found a significant improvement in survival in London at 90 days after admission. In that study the impact on survival was calculated using survival models estimated before and after the reconfiguration, and an adjustment was applied to the difference in survival to account for national trends in mortality. The present study has a larger sample size, uses national individual-level data and has more patients in the post-reconfiguration period in London, provides a more robust analysis of national trends via the quasi-experimental difference-in-difference design and examines the reconfiguration in GM. Our findings are also consistent with a previous analysis based on national stroke audit data, showing that patients admitted to stroke services with higher levels of organisation were more likely to receive high-quality care and to have a reduced risk of death 30 days after stroke. 21
Implications
Our findings showed that mortality outcomes were different in the two systems. In London, where all patients were eligible for treatment in a HASU, ambulance data indicated that in 2011/12, 98.7% of London stroke patients were transported to the appropriate service: 95.7% were taken appropriately to a HASU, 3% were taken appropriately to an A&E department and only 1.3% were taken to an A&E department when they should have been taken to a HASU. 73 A review of the first year of the new GM model reported that, when only those patients presenting within 4 hours of developing stroke symptoms were eligible to be treated in a CSC/PSC, of the patients who presented with stroke within 4 hours, 36% were not taken to a CSC/PSC. 32 Hence, a higher proportion of patients than planned were admitted to district hospitals where access to specialist expertise and care was more limited. In addition, data on the achievement of processes measuring the quality of care that patients with stroke receive during the first 72 hours of care were collected between April 2011 and December 2012 as part of the SINAP in England. 70 A significantly higher proportion of patients in London received care that was compliant with the care processes than in GM and the RoE (which were broadly similar) (Table 6).
| Compliance with process indicators | Region | p-value | ||
|---|---|---|---|---|
| RoE | GM | London | ||
| Indicator 1: seen by nurse and one therapist within 24 hours and all relevant therapists within 72 hours | ||||
| Per cent compliant | 58 | 53 | 73 | < 0.001 |
| Observations (n) | 36,491 | 4430 | 9543 | |
| Indicator 2: nutrition screening and formal swallow assessment within 72 hours where appropriate | ||||
| Per cent compliant | 85 | 90 | 98 | < 0.001 |
| Observations (n) | 33,627 | 4832 | 11,291 | |
| Indicator 3: patient’s first ward of admission was SU and they arrived there within 4 hours of hospital arrival | ||||
| Per cent compliant | 60 | 60 | 73 | < 0.001 |
| Observations (n) | 39,687 | 4867 | 11,609 | |
| Indicator 4: patient given antiplatelets within 72 hours when appropriate and had adequate fluid and nutrition in all 24-hour periods | ||||
| Per cent compliant | 67 | 67 | 88 | < 0.001 |
| Observations (n) | 35,261 | 4574 | 9909 | |
This suggests that the centralised model of care in London was more closely adhered to and achieved greater compliance with clinical interventions. There is evidence that better compliance with these measures is negatively correlated with mortality. 21 It is also noteworthy that, based on multivariate analyses, these measures independently affect mortality,21 suggesting that different aspects of specialist care provided throughout the HASU can separately affect patient outcomes. The upshot is that differences in mortality may be explained by the lower level of adherence in GM or differences between the two systems in terms of the access to hyperacute care for patients presenting after 4 hours of developing stroke symptoms. This suggests that the type of system redesign and the extent of its implementation can affect patient outcomes and needs to be taken into account by those who are reorganising services. In addition, although there is evidence that organised inpatient stroke care is beneficial to patients with intracerebral haemorrhage as well as ischaemic stroke,55 the improvements in outcomes from centralisation found in this study were largely among patients with ischaemic stroke. On the one hand, this may reflect the poorer prognosis among patients with intracerebral haemorrhage;75 we found some evidence in London that mortality was reduced at 3 days, suggesting that the reconfiguration may have delayed death among these patients but did not change the ultimate outcome. On the other hand, point estimates of the reductions in mortality in London were higher for intracerebral haemorrhage than for ischaemic stroke but the effects were non-significant; the wider CIs may be due to the smaller numbers of patients in the data suffering this type of stroke. On a different point, although the results were consistent when patients living in rural areas were included, they may be less relevant to services operating in rural settings. The greater travel times in rural areas make centralisation challenging and may necessitate other solutions, such as telemedicine, whereby consultation and triage may be conducted remotely by a stroke physician in a specialist SU. 76–78 Finally, our findings may also inform the centralisation of other care services, such as vascular surgery79 and specialised surgery for cancer and other conditions. 80
Chapter 4 The impact of the centralisation of acute stroke care on the delivery of clinical interventions
Overview
This chapter draws on a paper published by Ramsay et al. 26 Effects of centralising acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46(8):2244–51. Stroke is published on behalf of the American Heart Association, Inc., by Wolters Kluwer. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0) License, which permits use, distribution, and reproduction in any medium, provided that the original work is properly cited, the use is non-commercial, and no modifications or adaptations are made. See https://creativecommons.org/licenses/by-nc-nd/3.0/. Permission to adapt this material has been agreed with Wolters Kluwer.
What was already known about this subject?
-
Provision of evidence-based stroke care is associated with better patient outcomes.
-
Centralisation of acute stroke services in London and GM had a significantly different impact on clinical outcomes, with only the London changes associated with significantly greater reductions in mortality (see Chapter 3).
-
There was limited evidence on the impact that centralisation of acute stroke care has on provision of evidence-based clinical interventions, and whether or not this might explain the changes in clinical outcomes observed.
What this chapter adds
-
In London, where almost all patients were treated in a HASU, patients were more likely than elsewhere to receive evidence-based care in the first hours following arrival in hospital.
-
GMA’s CSC/PSCs performed as effectively as HASUs in London, and significantly better than London on several important clinical interventions, but treated only 39% of stroke patients. This difference is explained in part by differing eligibility criteria in GM and London, but also because adherence to the model in GM was lower, with two-thirds of eligible patients treated in hyperacute units.
-
As a result, only patients in London were significantly more likely than patients elsewhere to receive evidence-based care; stroke patients in GM were overall no more likely to receive evidence-based care in the first hours following arrival in hospital than patients in areas where no equivalent centralisation had taken place.
Background
Stroke care based on evidence of clinical effectiveness (e.g. access to stroke specialists, rapid scanning, assessment, treatments and therapies, referred to here as ‘evidence-based clinical interventions’) is associated with better patient outcomes. 21,25,53,81,82 Benefits include reductions in mortality, LOS and disability, and increases in independence and QoL.
Some health systems have centralised their stroke services to create a smaller number of high-volume specialist services, aiming to improve patient access to evidence-based clinical interventions. 7,9,10,83 Recent research indicates that different models of centralisation are associated with different outcomes: although both GM and London centralisations were associated with significantly greater reductions in LOS, only the London centralisation was associated with a significantly greater reduction in stroke patient mortality than in the RoE. 25 This analysis attempts to explain the differences in clinical outcomes by analysing the London and GMA changes in terms of (1) their impact on the provision of evidence-based clinical interventions and/or (2) differences between the GM and London models’ eligibility criteria for admission to a hyperacute unit, and how reliably these criteria were followed.
Method
Design
This study used a controlled before-and-after design. It analysed risk-adjusted likelihood of stroke patients receiving evidence-based clinical interventions in GM and London, pre and post centralisation, compared with urban areas of England where acute stroke services had not been centralised (hereafter referred to as the ‘comparator’).
Data
Patient-level data were drawn from two national audits organised by the Royal College of Physicians: (1) pre centralisation, the National Sentinel Stroke Clinical Audit (Sentinel 2008), conducted from April to August 2008, was used; and (2) post centralisation, the SINAP, which ran from April 2010 to December 2012,72,84 was used. Reflecting the implementation dates for the centralisations, the GM post-centralisation period was April 2010 to December 2012 inclusive, whereas London’s was July 2010 to December 2012 inclusive. Data collected in the two audits differed: Sentinel 2008 collected a ‘snapshot’ of up to 60 patients per participating stroke service, whereas SINAP collected data for all patients receiving stroke care. Consequently, post-centralisation data cover significantly more patients.
The analysis included data submitted by all hospitals providing acute stroke care in GM, London and a comparator area formed of hospitals providing acute stroke care in two parts of England (north-west England, excluding GM, and north-east England), where local documents showed that no equivalent centralisation had occurred. The comparator was limited to hospitals in urban settings equivalent to GM and London (classified as ‘major urban’ by the UK ONS85); it covered 1.8 million people27 and its level of participation in national audits was equivalent to GM and London (details available in Appendix 3). Although Sentinel 2008 had uniformly high participation across England,84 participation in SINAP was variable in several areas of England, with many hospitals submitting few or no data. 72 These differing participation levels meant that the RoE could not act as the comparator. Consequently, data for approximately 56,100 stroke patients (7300 ‘before’, 48,700 ‘after’) were excluded. Data for all patients diagnosed with stroke (intracerebral haemorrhage or cerebral infarction) were included, both those occurring in hospital and those occurring outside hospital. Patients with invalid data were excluded.
Measures
We analysed all evidence-based clinical interventions that had been measured consistently in both audits. 59,86 These measures were calculated from arrival at hospital (or symptom onset if occurring in hospital), and assessed whether or not patients had their first brain scan within 3 hours and 24 hours of arrival (cut-off points were identified in the baseline audit national report reflecting the time to scan to support administration of thrombolysis, and national guidance to scan within 24 hours84); were admitted to a SU within 4 hours; received antiplatelets within 48 hours (if ischaemic); and underwent physiotherapist, nutrition and formal swallow assessments within 72 hours (all if eligible).
Statistical analysis
Descriptive statistics
Descriptive data were calculated at regional level for GM, London and the comparator, pre and post centralisation. Post centralisation, we categorised hospitals based on whether or not they were designated to provide hyperacute care. Consistent data were available on patient characteristics (age, sex, stroke type, worst level of consciousness, and whether the stroke occurred within or outside hospital) and the proportion of patients receiving each clinical intervention analysed.
Hospital-level variation
To understand the impact of the centralisations on patient volume and provision of care at hospital level, the unadjusted proportion of patients receiving evidence-based clinical interventions was calculated at hospital level for each area, both pre and post centralisation. Hospital-level proportions were plotted against the mean number of stroke patients submitted to the audits per day, and categorised by whether or not services were hyperacute.
Risk-adjusted likelihood of receiving evidence-based clinical interventions
Using patient-level data we used logistic regression to analyse whether or not patients received each evidence-based clinical intervention (yes/no) against region (whether or not they were treated in GM or London, with the comparator as the reference category), time period (whether or not they were treated in the ‘after’ period, with being treated in the ‘before’ period as the reference category) and an interaction term between region and time period, controlling for age (in 5-year bands), sex, stroke diagnosis (intracerebral haemorrhage/cerebral infarction), worst level of consciousness (fully conscious/semi-conscious/drowsy/unconscious), and whether stroke occurred within or outside hospital (yes/no). All outcomes were binary (yes/no). We reported marginal effects, showing the adjusted predicted probability of each outcome in each region in each time period. Because the GM and London centralisations had different ‘after’ periods (meaning the comparator data differed slightly), the regression analyses of the two centralisations were conducted separately. We reran our models stratifying by whether or not the patient was treated in a hyperacute or a non-hyperacute stroke service.
Following referral criteria for admission to hyperacute units in Greater Manchester and London
The proportion of patients treated in a HASU was calculated to examine whether or not the models selected in GM and London influenced the likelihood of receiving evidence-based clinical interventions; this was also used to measure how reliably the London hyperacute referral criteria were followed. To examine how reliably GM hyperacute referral criteria were followed, we compared patients’ time of symptom onset with time of arrival at hospital to calculate the proportion of patients who arrived at hospital within 4 hours of symptoms developing (and were thus eligible for hyperacute unit admission), and who were in fact admitted to a hyperacute unit.
Results
Descriptive statistics
Data for 38,623 acute stroke cases submitted to national audit were analysed, covering 51 hospitals pre centralisation (from a total of 189 hospitals participating in the audit across England) and 44 hospitals post centralisation (from a total of 171 hospitals across England). Table 7 presents the unadjusted data for GM and London compared with the comparator. Patient characteristics were similar in GM, London and the comparator in both pre- and post-centralisation time periods, and any potential effects of patient characteristics were controlled for in the regression analyses. Post centralisation, the proportion of patients receiving evidence-based clinical interventions increased in all three areas. It should be noted that denominators for these indicators varied from measure to measure owing to variable eligibility of patients or availability of data. Increases were most pronounced in care provided in the first hours following arrival at hospital (brain scan within 3 hours, admitted to a SU within 4 hours). The proportion of stroke patients receiving these clinical interventions was higher in London than in GM and the comparator, both pre and post centralisation, but the absolute difference was similar. Proportions of patients receiving evidence-based clinical interventions 24–72 hours after admission also increased in all three areas, but in all areas pre-centralisation levels commonly exceeded 80%, and post centralisation all proportions exceeded 90%. Generally, London had higher pre-centralisation levels and post-centralisation levels were similar – approaching the maximum – in each area.
| Patient characteristics/interventions | Region | |||||
|---|---|---|---|---|---|---|
| GM | London | Comparator | ||||
| Before | After | Before | After | Before | After | |
| Total patients (N) | 653 | 10,295 | 1541 | 16,553 | 537 | 9044 |
| Total hospitals | 12 | 11 | 30 | 24 | 9 | 9 |
| Case/day rate | 0.63 | 0.97 | 0.58 | 1.03 | 0.80 | 1.07 |
| HASU case/day rate | 1.37 | 2.17 | ||||
| Non-HASU case/day rate | 0.65 | 0.12 | ||||
| Mean age (years) | 74.5 | 73.2 | 73.3 | 72.7 | 74.6 | 73.6 |
| Proportion > 75 years (%) | 55 | 50 | 51 | 50 | 53 | 51 |
| Proportion female (%) | 52 | 51 | 50 | 49 | 52 | 51 |
| Stroke type (%) | ||||||
| Haemorrhage | 13 | 11 | 14 | 11 | 11 | 11 |
| Infarct | 87 | 89 | 86 | 89 | 89 | 89 |
| Worst consciousness (%) | ||||||
| Fully conscious | 60 | 74 | 67 | 78 | 68 | 75 |
| Semi-conscious | 19 | 15 | 17 | 15 | 11 | 14 |
| Drowsy | 8 | 4 | 7 | 3 | 9 | 5 |
| Unconscious | 13 | 7 | 9 | 4 | 12 | 6 |
| Patients admitted from outside hospital (%) | 96 | 90 | 93 | 95 | 95 | 94 |
| Patients treated in hyperacute unit | – | 4022/10,295 (39%) | – | 15,398/16,533 (93%) | – | – |
| < 4 hours patients treated in hyperacute unit | – | 2170/3259 (66.6%) | – | 5314/5525 (96%) | – | – |
| > 4 hours patients treated in hyperacute unit | – | 869/2154 (40%) | – | 3514/3697 (95%) | – | – |
| Patients where time of onset unknown (n) | – | 4882 | – | 7311 | – | – |
| Interventions | ||||||
| Brain scan 3 hours | 98/461 (21%) | 5785/10,295 (56%) | 496/1353 (37%) | 11,614/16,553 (70%) | 127/461 (28%) | 4902/9044 (54%) |
| SU 4 hours | 93/648 (14%) | 4982/10,295 (48%) | 461/1527 (30%) | 11,360/16,553 (69%) | 121/536 (23%) | 5027/9044 (56%) |
| Brain scan 24 hours | 396/586 (68%) | 8814/9563 (92%) | 1099/1425 (77%) | 14,895/15,679 (95%) | 315/506 (62%) | 8008/8787 (91%) |
| Antiplatelets 48 hours | 452/529 (85%) | 8226/8803 (93%) | 1169/1240 (94%) | 13,062/13,773 (95%) | 375/436 (86%) | 7070/7522 (94%) |
| Physiotherapist 72 hours | 480/556 (86%) | 8030/8867 (91%) | 1206/1337 (90%) | 14,190/14,760 (96%) | 380/440 (86%) | 7703/8155 (94%) |
| Nutrition 72 hours | 504/583 (86%) | 9308/9918 (94%) | 1022/1402 (73%) | 15,745/16,021 (98%) | 398/479 (83%) | 7977/8560 (93%) |
| Swallow 72 hours | 252/304 (83%) | 8521/9388 (91%) | 698/803 (87%) | 15,431/15,684 (98%) | 229/263 (87%) | 7034/7509 (94%) |
Hospital-level variation
Pre centralisation, there was substantial between-hospital variation in the proportion of patients receiving evidence-based clinical interventions in all three areas (see Appendix 3). Post centralisation, hyperacute units in GM (CSC, PSCs) and London (HASUs) treated a higher volume of patients than elsewhere (see Table 7), and provided evidence-based clinical interventions to a higher proportion of their patients (Figure 4 and see Appendix 3, Figure 20).
FIGURE 4.
Between-hospital variations in the proportion of patients admitted to a SU within 4 hours by area, post centralisation. (a) GM; (b) London; and (c) comparator. Adapted from Ramsay et al. 26 Effects of centralising acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46(8):2244–51. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients). Stroke is published on behalf of the American Heart Association, Inc., by Wolters Kluwer. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0) License, which permits use, distribution, and reproduction in any medium, provided that the original work is properly cited, the use is non-commercial, and no modifications or adaptations are made. See https://creativecommons.org/licenses/by-nc-nd/3.0/. Permission to adapt this material has been agreed with Wolters Kluwer. Figure title and numbering updated for report.
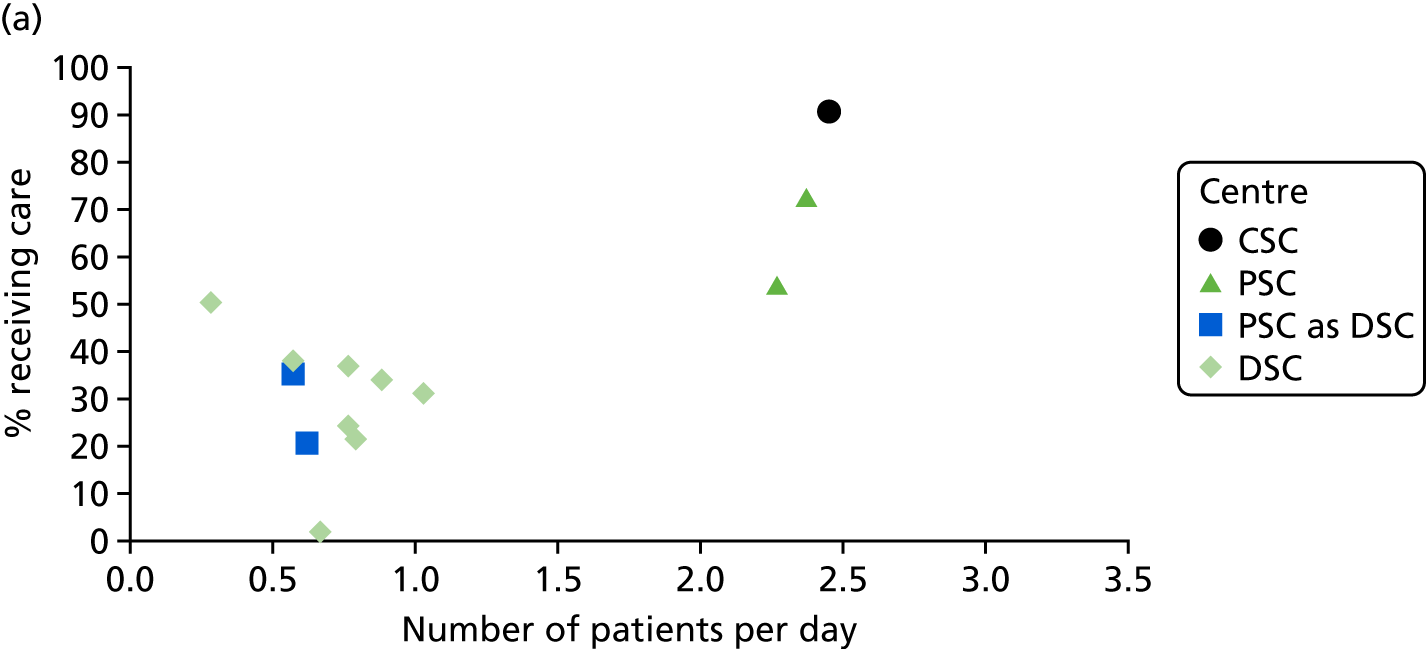
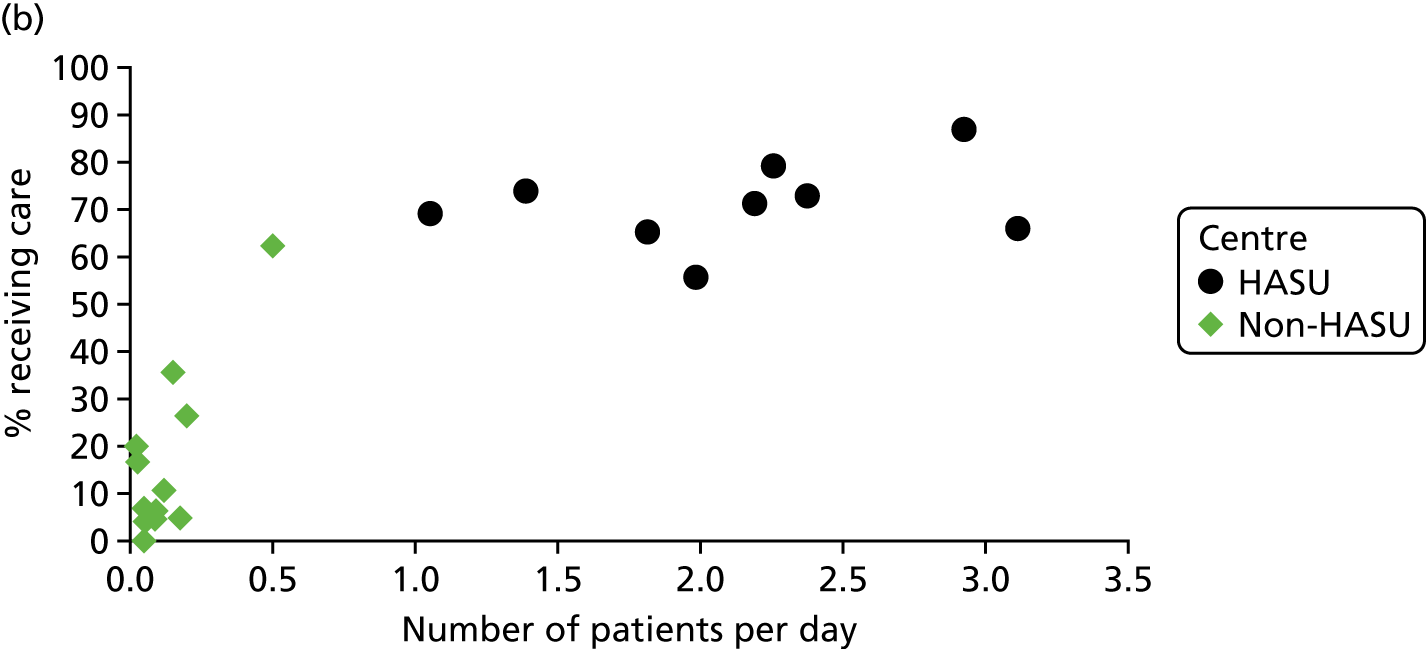

Although the proportion of patients receiving evidence-based clinical interventions increased in GM and London’s non-hyperacute units and in the comparator area overall, patient volume increased less, and the proportion of patients receiving evidence-based clinical interventions tended to be lower and more variable than in the hyperacute units. When GM PSCs operated as DSCs, they performed in line with other DSCs (Figure 4a and see Appendix 3, Figure 20).
Risk-adjusted likelihood of receiving evidence-based clinical interventions
Trends in risk-adjusted proportions of patients receiving evidence-based clinical interventions (Tables 8 and 9) reflected the unadjusted findings (see Table 7). Post centralisation, on all clinical interventions analysed, London patients were overall significantly more likely to receive clinical interventions than comparator patients. GM patients were significantly more likely than comparator patients to receive two interventions (brain scan within 3 hours and 24 hours), significantly less likely to receive three interventions (admission to SU within 4 hours, and physiotherapist and swallow assessments within 72 hours), with no significant difference between GM and comparator patients on the two remaining interventions. London patients were overall significantly more likely than GM patients to receive six of the seven interventions (brain scan within 3 hours and 24 hours, admission to a SU within 4 hours, and physiotherapist, nutrition and swallow assessments within 72 hours), with the magnitude of differences ranging from 1.2% to 10.4% and with no significant difference on antiplatelets within 48 hours. Patients treated in hyperacute units, in both GM and London, were significantly more likely to receive clinical interventions than patients treated either in non-hyperacute units or in the comparator (with one exception, where comparator patients were significantly more likely to receive a physiotherapist assessment than GM hyperacute patients). Patients treated in GM hyperacute units were significantly more likely than London HASU patients to receive four clinical interventions: brain scan within 3 hours, admission to SU within 4 hours, brain scan within 24 hours and antiplatelets within 48 hours (the magnitude of the differences ranged from 2.0% to 13.8%). Patients treated in GM hyperacute units were significantly less likely to receive a physiotherapist assessment within 72 hours than patients treated in London HASUs (magnitude 2.2%). There was no significant difference between GM and London hyperacute units in the number of patients receiving nutrition and formal swallow assessments within 72 hours. To examine whether or not these differences reflected the greater proportion of patients in GM arriving within 4 hours, we reran this analysis focusing only on patients presenting within 4 hours of symptom onset and found that the differences between GM and London hyperacute units reduced substantially (see Appendix 3).
| Intervention | Region, % likelihood (95% CI) | |||||
|---|---|---|---|---|---|---|
| GM | Comparator | |||||
| Overall | CSC/PSCs | DSCs | ||||
| Before | After | After | After | Before | After | |
| Brain scan 3 hours | 25.0 (21.0 to 29.0) | 65.2 (64.3 to 66.2) | 85.5 (84.6 to 86.5) | 52.2 (50.8 to 53.5) | 29.0 (27.1 to 31.0) | 62.3 (61.7 to 63.0) |
| SU 4 hours | 17.8 (14.6 to 21.0) | 55.9 (54.9 to 57.0) | 82.9 (81.8 to 83.9) | 36.7 (35.3 to 38.1) | 25.8 (24.0 to 27.7) | 61.1 (60.5 to 61.7) |
| Brain scan 24 hours | 71.7 (68.2 to 75.1) | 94.0 (93.5 to 94.4) | 98.2 (97.8 to 98.5) | 91.6 (90.9 to 92.2) | 69.4 (67.2 to 71.5) | 92.9 (92.5 to 93.2) |
| Antiplatelets 48 hours | 86.5 (83.8 to 89.2) | 94.2 (93.7 to 94.7) | 97.6 (97.1 to 98.1) | 92.2 (91.5 to 93.0) | 91.3 (90.0 to 92.7) | 94.3 (93.9 to 94.6) |
| Physiotherapist 72 hours | 88.3 (85.7 to 90.8) | 92.1 (91.5 to 92.7) | 93.8 (93.0 to 94.7) | 91.4 (90.7 to 92.2) | 88.2 (86.6 to 89.8) | 95.2 (94.9 to 95.5) |
| Nutrition 72 hours | 89.9 (87.7 to 92.0) | 95.6 (95.3 to 96.0) | 98.5 (98.2 to 98.8) | 94.1 (93.6 to 94.7) | 70.8 (68.6 to 73.0) | 95.8 (95.5 to 96.1) |
| Swallow 72 hours | 87.7 (84.6 to 90.9) | 94.0 (93.6 to 94.4) | 98.6 (98.2 to 98.9) | 91.6 (90.9 to 92.2) | 82.3 (79.7 to 84.9) | 96.0 (95.7 to 96.3) |
| Intervention | Region, % likelihood (95% CI) | |||||
|---|---|---|---|---|---|---|
| London | Comparator | |||||
| Overall | HASUs | SUs | ||||
| Before | After | After | After | Before | After | |
| Brain scan 3 hours | 36.5 (34.0 to 39.1) | 72.1 (71.4 to 72.8) | 74.6 (73.8 to 75.3) | 39.9 (36.9 to 42.9) | 21.5 (18.9 to 24.0) | 55.5 (54.8 to 56.3) |
| SU 4 hours | 29.6 (27.3 to 31.9) | 66.3 (65.6 to 67.1) | 69.1 (68.3 to 69.9) | 28.6 (25.8 to 31.3) | 18.7 (16.4 to 21.0) | 54.4 (53.6 to 55.1) |
| Brain scan 24 hours | 77.9 (75.8 to 80.0) | 95.2 (94.8 to 95.5) | 96.2 (95.9 to 96.5) | 83.4 (81.3 to 85.6) | 62.4 (59.4 to 65.3) | 91.5 (91.1 to 92.0) |
| Antiplatelets 48 hours | 94.1 (92.8 to 95.4) | 94.8 (94.4 to 95.2) | 95.3 (94.9 to 95.7) | 89.6 (87.7 to 91.6) | 85.4 (83.2 to 87.6) | 93.8 (93.4 to 94.2) |
| Physiotherapist 72 hours | 88.9 (87.0 to 90.7) | 95.4 (95.0 to 95.8) | 96.0 (95.6 to 96.4) | 86.3 (83.8 to 88.7) | 87.9 (85.9 to 89.8) | 93.4 (93.0 to 93.8) |
| Nutrition 72 hours | 74.1 (71.7 to 76.4) | 98.3 (98.1 to 98.5) | 98.6 (98.4 to 98.8) | 94.7 (93.4 to 96.0) | 84.2 (81.9 to 86.4) | 93.3 (92.9 to 93.7) |
| Swallow 72 hours | 85.4 (82.8 to 88.0) | 98.2 (97.9 to 98.4) | 99.0 (98.8 to 99.1) | 86.0 (83.6 to 88.5) | 85.4 (82.5 to 88.3) | 92.8 (92.4 to 93.3) |
Access to care in hyperacute units in Greater Manchester and London
Post centralisation, 39% of GM patients were treated in a hyperacute unit, whereas 93% of London patients were (see Table 7). In addition, only 66% of GM stroke patients who presented within 4 hours of symptom onset were admitted to a hyperacute unit (see Table 7), meaning that 34% of patients who were eligible for hyperacute unit care were not admitted to one.
We reran our analyses using all available data for the RoE as the comparator; the results did not change appreciably (see Appendix 3).
Discussion
Principal findings
Post centralisation, the risk-adjusted likelihood of patients receiving evidence-based clinical interventions increased significantly in all areas. London patients were overall significantly more likely than patients elsewhere to receive the interventions. Importantly, hyperacute units in both GM and London were significantly more likely to provide interventions than non-hyperacute units in these areas, and in the comparator area overall.
Fewer than 40% of GM patients were admitted to a hyperacute unit (in line with an analysis conducted by the GMCCSN as part of their 12-month review of the centralisation32) whereas 93% of London patients were admitted to a hyperacute unit; this is likely to explain the overall differences in provision over the two areas and suggests a clear effect of the models selected. This effect partly relates to the different eligibility criteria for admission to a hyperacute unit: in GM, only stroke patients presenting within 4 hours of developing symptoms were eligible for treatment in a hyperacute unit, whereas all London stroke patients were eligible. However, in GM, only two-thirds of patients eligible for care in a hyperacute unit were admitted to one; this suggests that differences between GM and London derived not only from the eligibility criteria, but also from how reliably they were followed, with both contributing to the lower overall likelihood of patients receiving interventions in GM. The fact that GM hyperacute units were significantly more likely than London HASUs to provide four of the interventions analysed may relate to the ways in which London and GM hyperacute units were designed and specified (e.g. in terms of staffing and availability of allied services, GM’s use of the 4-hour eligibility criterion, and the extent to which services had to operate a 24/7 service), and how these specifications were implemented. The contrast in performance by PSCs in-hours and out-of-hours is likely to reflect the differing specifications for hyperacute and non-hyperacute services applied at these times, including staffing levels and access to diagnostic services. In both areas, non-hyperacute units were less likely to provide interventions, reflecting an important consideration when centralising services. In London, only 7% of London patients were treated in SUs; this had a negligible effect, but may reflect issues where the referral protocol was not followed. In GM, DSCs were less likely to provide the interventions analysed; as DSCs treated 61% of GM patients, GM and comparator services performed similarly overall, suggesting that the benefits of this centralisation were limited. Finally, it is unlikely that geography influenced GM patient eligibility significantly, given that the maximum distance from any point in GM to the 24/7 CSC is < 25 miles.
Comparison with other studies
Little is known about how large-scale service centralisation influences provision of evidence-based stroke clinical interventions, but there is substantial evidence that such clinical interventions are associated with better clinical outcomes, for example through dedicated SUs with specialist staff providing rapid assessment, treatment and therapies. 21,59,81,86,87 Recent research indicates that the London centralisation had a significantly greater impact on patient mortality than in the RoE, whereas the GM centralisation did not. 25 The current analysis suggests that this difference might result from better access (on average) to evidence-based clinical interventions on arrival at hospital in London, owing to a higher proportion of patients being admitted to HASUs. This in turn suggests that the model implemented is important: centralising acute stroke services into a small number of hyperacute units can increase access to evidence-based clinical interventions, which may in turn lead to better clinical outcomes. Furthermore, the model selected may influence the extent to which it is followed; the GM model may have been followed less reliably owing to complexity associated with the 4-hour eligibility criterion for admission to hyperacute units and the limited hours of PSCs. This in turn suggests potential advantages of service models that aim for relative simplicity. Therefore, our findings indicate that there may be significant benefits to models in which all stroke patients are eligible for hyperacute unit admission; offering hyperacute unit admission selectively, for example based on time of stroke onset, may limit these benefits. As the centralisations studied were implemented in large metropolitan areas, these findings are of greatest relevance to urban settings.
General increases in the likelihood of patients receiving interventions, reflected in the comparator, should also be noted. These may reflect the influence of national drivers to improve stroke care across England, for example the National Stroke Strategy24 and the ‘Stroke Best Practice Tariff’,88,89 increasing hospitals’ prioritisation of clinical interventions measured in national stroke audits. This may contribute to the overall lower likelihood of GM patients undergoing four of the interventions than comparator patients, as our data indicate comparatively limited improvements in DSC services, whereas general improvements occurred in many of the comparator services.
Strengths and weaknesses
To study the impact of centralising acute stroke services, we have been able to analyse two different models of centralisation implemented within the same national health-care system at approximately the same time, using data from two comprehensive national audits. This allowed us to compare findings between the two centralisations and with a large, urban comparator, thus enhancing external validity. Our study has several limitations. First, because of variable participation in SINAP, it was not appropriate to use the RoE as our comparator; this limits the extent to which we may use these results to explain previous research on the impact of these centralisations on mortality. Second, it is possible that data completeness may have varied across hospitals in the GM, London and comparator areas, thus limiting the confidence with which we interpret the data. Third, owing to data availability, several important clinical interventions could not be analysed. Provision and timing of thrombolysis were excluded because in 2008 thrombolysis rates were not reported with sufficient frequency to permit reliable analysis. However, the stroke services analysed aim to treat all stroke patients, only a proportion of whom (approximately 16%90) are eligible for thrombolysis. Other interventions excluded were anticoagulation of atrial fibrillation (data were not collected in either audit), and occupational therapy assessment and continence assessment (criteria assessed changed over time). Fourth, it was not possible to adjust for stroke severity [e.g. using the National Institutes of Health Stroke Scale (NIHSS)] because these data were not collected in either audit analysed. However, it is recommended that stroke patients receive these interventions regardless of severity (unless contraindicated); therefore, controlling for severity should have little impact on whether or not these interventions are provided. However, the latest version of the audit collects NIHSS for all patients on arrival at hospital, and future research might usefully analyse whether or not stroke severity influences the likelihood of receiving interventions. Fifth, because the areas studied varied in overall performance levels pre centralisation, and several of the selected indicators approached ceiling both pre and post centralisation, our analysis of change over time was limited. Sixth, it was not possible to control for hospital/unit effects because the number and function of stroke services changed significantly over the period studied. Finally, the analysis of patients presenting within 4 hours of their symptoms appearing was derived from SINAP data on time of stroke symptom onset; although we made every effort to exclude unreliable data, it remains that some may have been recorded unreliably.
Implications
Our data indicate that both the centralisation model selected and the degree to which it was followed may have influenced the likelihood of stroke patients receiving evidence-based clinical interventions, and that these factors may be interrelated. Therefore, qualitative analysis of how model selection and implementation processes influenced the development of hyperacute and non-hyperacute services, and the reliability with which patients were transferred to these services, is required to generate valuable lessons for future changes of this kind.
Chapter 5 Cost-effectiveness of centralisations of acute stroke care in London and Greater Manchester A
Overview
This chapter draws on a paper published as Hunter et al. 91 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
What is already known about this subject?
-
A previously published model showed that the reconfigurations in stroke care in London were cost-effective. The model did not take into account changes that occurred in the RoE over the same time period.
-
In London, where hyperacute stroke specialist care was provided to all patients, there was a reduction in mortality and LOS (see Chapter 3).
-
In GM, where specialist hyperacute stroke care was provided only to patients presenting within 4 hours of developing stroke symptoms, there was no impact on mortality but LOS fell (see Chapter 3).
What this chapter adds
-
There was a high probability that the centralised models of stroke care in London and GM were cost-effective. In London this was as a result of improvements in mortality and morbidity and in GM as a result of reduced cost of stroke care due to reduced LOS.
Background
The London reconfigurations occurred at an extra cost of £20M allocated to covering the increased cost per bed-day in a HASU in London, which was given to the health-care organisations that were responsible for commissioning health care at the time, namely PCTs. This additional allocation of funds and increased political interest in evaluating health-care system changes resulted in calls to evaluate whether or not the reconfigurations represent value for money. 26 The London reconfiguration was evaluated as part of a previous cost-effectiveness analysis, which found that it resulted in reduced costs per patient and improved QoL at 90 days and 10 years. 53 The cost-effectiveness model had a number of limitations, including limited data on which to base the analysis before the changes had been implemented and comparative data for what occurred in the RoE over the same time included only as an adjustment, not as a direct comparison. The changes that have occurred in GM were not included. Economic evaluations of MSC in general are rare, with no specific framework existing to guide analysis methods. 92
In this chapter, we aim to address whether or not the reconfigurations in London and GM were cost-effective by calculating their net monetary benefit (NMB). This is calculated using the change in costs and quality-adjusted life-years (QALYs) before and after the reconfigurations compared with changes in the RoE over the same time period, and multiplying the change in QALYs by the NHS’s willingness to pay (WTP) for a QALY gained. 30 The analysis combined the results of the evaluation of mortality and LOS25 and improved access to evidence-based clinical interventions,26 and addresses limitations in Hunter et al. 53 by modelling the costs and impact on mortality at 90 days compared with the RoE. This was supplemented with data from the literature to calculate the cost-effectiveness of the two reconfigurations at 10 years.
Methods
Overview
We estimated difference-in-differences in costs and outcomes to estimate NMBs and to evaluate the effect of the reconfigurations in London and GM on costs, mortality and QALYs compared with metropolitan areas in the RoE. Metropolitan areas were chosen so that population density and blue-light ambulance travel time to hospital were as comparable as possible with London and GM. The analysis was undertaken from the perspective of the English NHS and personal social services. Eight decision–analytic models were developed, each with 1000 hypothetical stroke patients. The time periods were as in Morris et al:25
-
London before the reconfiguration (January 2008 to January 2010)
-
London after the reconfiguration (July 2010 to March 2012)
-
RoE/excluding GM for the same time period as (1)
-
RoE/excluding GM for the same time period as (2)
-
GM before the reconfiguration (January 2008 to November 2008)
-
GM after the reconfiguration (April 2010 to March 2012)
-
RoE/excluding London for the same time period as (5)
-
RoE/excluding London for the same time period as (6).
Total costs and QALYs per 1000 patients were calculated for each of the eight models at 90 days and 10 years after admission, using a discount rate of 3.5% for costs and QALYs. 93 Costs are for the year 2013/14 and are in GB pounds sterling (£). The difference in costs and QALYs for (1) and (2) compared with (3) and (4) was then compared with the difference in costs and QALYs for (5) and (6) compared with (7) and (8).
Data
We used routinely collected hospital data (HES), audit data (Sentinel 200884 and SINAP;72 see Chapter 4) and data from the published literature to construct the model (Table 10). The data were linked to data from the ONS to obtain the date of death. 60 The South London Stroke Register (SLSR), a population-based stroke prospective registry recording all first-ever strokes in patients of all ages living in an area of South London,31 was used to calculate changes in disability before and after the reconfigurations. Further details on the data sets used can be found in Chapter 325 for HES and ONS data, Chapter 426 for SINAP and Sentinel 2008, and Hunter et al. 53 for SLSR.
| Data source | GM | England comparison | London | England comparison | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| HES | 3503 | 7685 | 42,880 | 95,244 | 15,276 | 15,023 | 100,511 | 84,801 |
| Mean age (years) | 74.3 | 73.9 | 75.8 | 75.3 | 73.0 | 73.3 | 75.7 | 75.3 |
| > 75 years (%) | 56.0 | 53.6 | 61.2 | 59.18 | 54.3 | 54.4 | 60.7 | 52.3 |
| Female (%) | 52.6 | 50.4 | 53.0 | 53.2 | 51.0 | 49.8 | 53.0 | 52.2 |
| White British ethnic group (%) | 82.9 | 84.2 | 82.4 | 86.5 | 58.5 | 55.0 | 83.9 | 86.5 |
| Intracerebral haemorrhage (%) | 11.5 | 11.7 | 13.0 | 12.7 | 15.7 | 14.8 | 12.9 | 12.7 |
| Cerebral infarction (%) | 61.6 | 64.4 | 62.7 | 71.3 | 68.9 | 76.1 | 64.4 | 71.2 |
| Stroke not specified (%) | 26.9 | 23.9 | 24.3 | 16.1 | 15.4 | 9.1 | 22.7 | 15.8 |
| Charlson Comorbidity Index (mean score) | 2.0 | 2.0 | 1.9 | 1.9 | 2.0 | 2.0 | 1.9 | 1.9 |
| Most deprived fifth (%) | 8.4 | 10.3 | 17.1 | 17.6 | 12.6 | 13.2 | 17.3 | 17.6 |
| SINAP | 10,295 | 9044 | 16,553 | 9044 | ||||
| Mean age (years) | 73.2 | 73.6 | 72.7 | 73.6 | ||||
| > 75 years (%) | 50 | 51 | 50 | 51 | ||||
| Female (%) | 51 | 51 | 49 | 51 | ||||
| Intracerebral haemorrhage (%) | 11 | 11 | 11 | 11 | ||||
| Cerebral infarction (%) | 89 | 89 | 89 | 89 | ||||
| Sentinel 2008 | 653 | 537 | 1541 | 537 | ||||
| Mean age (years) | 74.5 | 74.6 | 73.3 | 74.6 | ||||
| > 75 years (%) | 55 | 53 | 51 | 53 | ||||
| Female (%) | 52 | 52 | 50 | 52 | ||||
| Intracerebral haemorrhage (%) | 13 | 11 | 14 | 11 | ||||
| Cerebral infarction (%) | 87 | 89 | 86 | 89 | ||||
Statistical analyses
Model structure
Each of the eight models has two components:
-
A 90-day discrete event simulation (DES) of daily acute hospital ward movements, discharge destinations and mortality using data from HES, Sentinel and SINAP (Figure 5).
-
A 10-year Markov model30 with 90-day cycles using information from the 90-day model plus HES, the SLSR and published data to calculate costs and QALYs (see Appendix 4, Figure 27).
All patients entered the 90-day model as an admission to hospital following a stroke. The proportion of patients in each health state in the first cycle of the 10-year model was determined by the final destination of patients in the 90-day model, including still on an acute hospital ward.
FIGURE 5.
Ninety-day DES model structure. ITU, intensive therapy unit; CCU, coronary care unit. Adapted from Hunter et al. 91 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
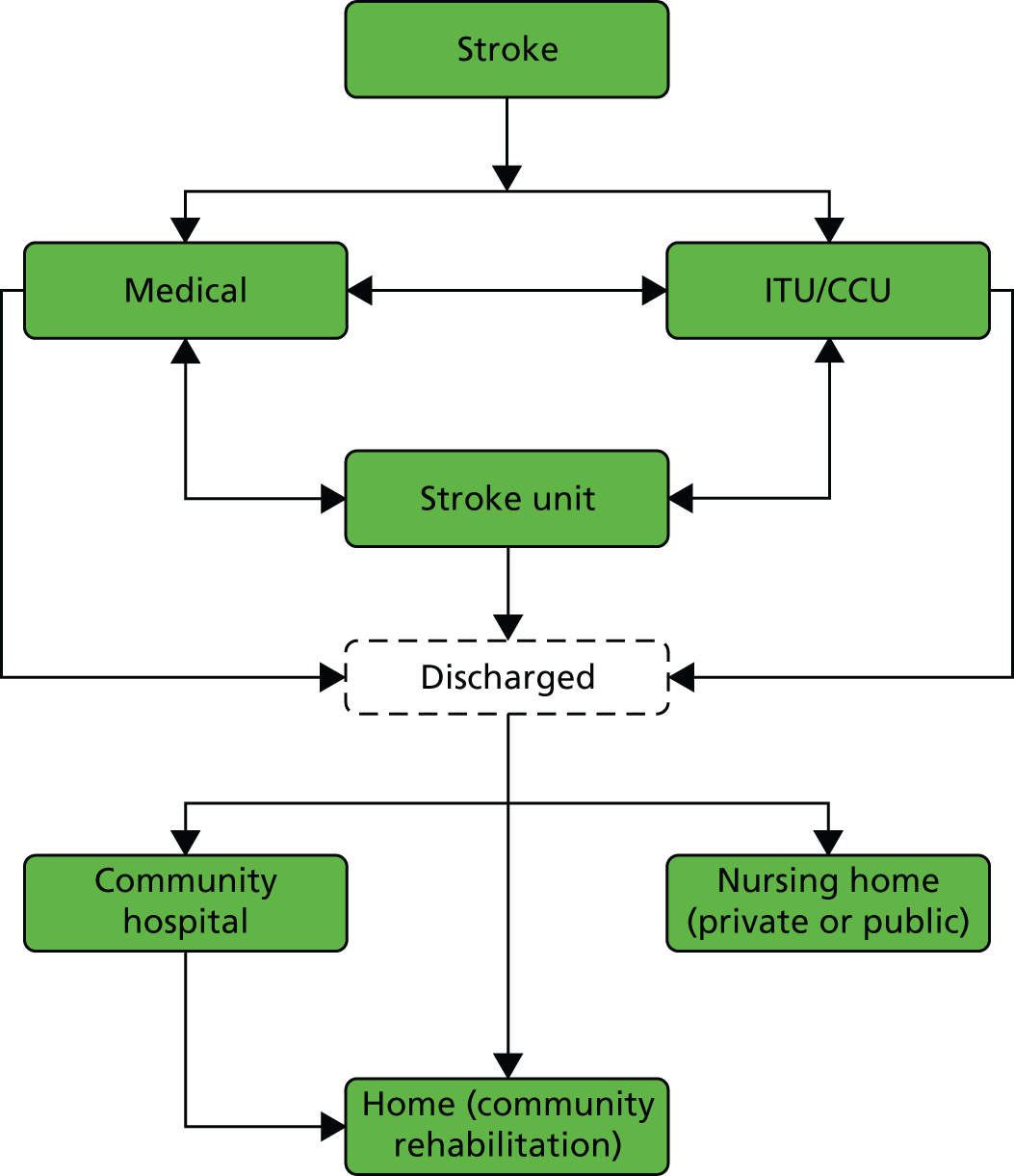
The flow for the 90-day model is illustrated in Figure 5, excluding the state of death, patients had a probability of death every day of the 90-day model regardless of where they were in the model. The same flow was used for all eight models and differed from the stylised diagrams in Figure 1. This is because data from SINAP show only if the first ward of admission was a SU or not, not whether it was a HASU/CSC/PSC or a SU/DSC. 26
Mortality
Mortality at 72 hours, 30 days and 90 days was calculated using the same data and a similar method to Morris et al. ,25 including coefficients to adjust for differences between regions in age, sex, ethnicity, deprivation [Index of Multiple Deprivation (IMD) quintile],67 and comorbidities using the Charlson Comorbidity Index. 66 Random effects for provider were also applied to the model. Two logistic regression models were fitted to the HES data, one for London and the RoE/excluding GM and one for GM and RoE/excluding London. Values for the models are reported in Appendix 4, Table 39. As the 90-day model was made up of 90 1-day transitions, the probability of death at each day was then converted to a 1-day rate using the following formula:
where t is time, αc and βc are the constant and beta coefficient for the effect of centralisation, respectively, and αd and βd are the constant and beta coefficient for discharge destination, respectively. This was carried out for 0–3 days, 3–30 days and 30–90 days. The same daily probability of death was applied to all the patients in hospital, regardless of which ward they were on; only time after admission to hospital determined the probability of death for patients in hospital. This was because HES data do not contain information on which ward a patient is in to allow a calculation of a ward-specific probability of death. Once patients were discharged from hospital, the per day probability of death also included relative probability of death day based on discharge location of the patient: community hospital, residential or nursing home or home (see Appendix 4, Table 39).
Length of stay and discharge destination
The HES data described in the previous sections and in Morris et al. 25 were used to calculate the daily probability of discharge from hospital and discharge destination using a parametric survival model and Weibull distribution. 30 This model was chosen to account for differential time effects so that each day in hospital after stroke has a different probability of discharge. Two analyses were run, both adjusting for age, sex, ethnicity, deprivation (IMD quintile),67 and comorbidities using the Charlson Comorbidity Index66 as above: one for GM and the RoE/excluding London and one for London and the RoE/excluding GM (see Appendix 4, Table 40). It was assumed that the probability of discharge was the same regardless of ward, as there is no information in HES on ward-specific LOS.
Discharge destination was calculated from the percentage of patients discharged to four locations: (1) publicly financed residential care or nursing home, (2) private nursing home, (3) home with community rehabilitation and (4) NHS community hospital. All other patients with other discharge codes were removed from this analysis only. It was assumed that patients were discharged to a community hospital if their discharge destination was ‘Other NHS Hospital’ but they had no subsequent admission for the next day, as community hospitals do not submit data to HES. If the discharge destination was ‘Other NHS hospital’ and a new admission (new episode in HES) occurred within 1 day we assumed that this was a transfer between hospitals. The percentage of patients discharged to each location is reported in Appendix 4, Table 41.
Ward movements
Sentinel 2008 and SINAP included details of the ward to which stroke patients were first admitted and subsequent movements in the first 72 hours, with details collected at each 24-hour follow-up for the following ward categories:
-
SU (including HASU, SU, CSC, PSC and DSC, but not specified)
-
medical assessment unit
-
general ward
-
intensive care or critical care unit
-
other.
These data were then used to calculate the percentage of patients admitted to each ward and daily transition probabilities between wards. The transition probabilities for 48 hours to 72 hours were applied for day 3 to day 90 if patients were still in hospital, as they were considered the most likely to be representative of transition probabilities for 72 hours to discharge (for further details, see Appendix 4, Table 41).
Ten-year model
The model is described in Appendix 4, Figure 27, and model inputs in Appendix 4, Table 42. The possible states in the 10-year model were:
-
home and one of five health states based on the Barthel index,94 a measure of functional independence, where 0 is no functioning and 20 is fully functional (0–4, 5–9, 10–14, 15–19 and 20)
-
residential care or nursing home
-
recurrent stroke and in hospital
-
recurrent stroke and in hospital for > 90 days
-
dead.
The 10-year Markov model was made up of 40 cycles each of 90 days’ duration. This, plus the 90 days from the DES, resulted in a total model duration of 10 years and 1 month. Patient locations at the end of the 90-day DES determined what state patients were in for the first cycle in the 10-year model, including still in hospital (4% of patients have a LOS of > 90 days). The 90-day DES was also used for 90-day costs and outcomes of hospitalisation following recurrent strokes in the 10-year Markov model.
The probability of patients being discharged to a community hospital or being admitted to residential care or a nursing home was taken from the SLSR (see Appendix 4, Table 43).
The probability that patients had a recurrent stroke following discharge was calculated using HES data and parametric survival analysis. A Weibull model30 was used to give differential probabilities over time with probabilities calculated for 90-day cycles. Models were adjusted using the same methodology as above and were calculated for GM versus the RoE/excluding London and London compared with the RoE/excluding GM (see Appendix 4, Table 43).
The probability of recurrent stroke for patients discharged into residential care or a nursing home was calculated from HES data for patients discharged to residential care only.
The 10-year model took into account the health and well-being of patients discharged from acute care using the Barthel index,94 with patients divided into the five Barthel states given above. The proportion of patients in each Barthel state and movements between them was taken from the SLSR with different rates before and after (see Appendix 4, Table 43). No data were available for Barthel indices or any other measure of functioning or QoL for GM or the RoE before the reconfiguration. As a result, it was not possible to evaluate the impact of the reconfigurations on functioning or QoL in GM or compare the changes that occurred in London with what happened over the same period in the RoE. For the purpose of the model and calculating QALYs, the conservative assumption was made that the same improvements were seen in GM, the RoE and London as were seen in the SLSR.
The probability of dying while in a care home was updated with values obtained from Gordon et al. ,95 as these provide the most recent estimates available in the literature.
Unit costs
Details of unit costs are given in Table 11. The cost of transfer was not included, as transfer costs were included in the additional cost of the HASU. 96
| Cost input | Cost per event/per day (£) | Reference |
|---|---|---|
| HASU London uplift tariff day 1 | 665a | London Stroke Strategy96 |
| HASU London uplift tariff days 2–3 | 399a | London Stroke Strategy96 |
| GM Best Practice tariff per day for first 72 hours if admitted to hyperacute care | 580 | 2014/2015 Department of Health and Social Care’s national payment system;97 GM Guidance32 |
| SU per bed-day cost | 238 | NHS Reference Costs 2013 to 2014 98 |
| Medical assessment ward | 187 | NHS Reference Costs 2013 to 2014 98 |
| General medical ward | 218 | NHS Reference Costs 2013 to 2014 98 |
| Intensive care or critical care unit | 1578 | NHS Reference Costs 2013 to 2014 98 |
| Other ward not otherwise specified | 203 | NHS Reference Costs 2013 to 2014 98 |
| Nursing home | 105 | PSSRU 201499 |
| Private nursing home | 107 | PSSRU 201499 |
| Transfer to other NHS hospital (not acute) | 100 | PSSRU 201499 |
| Thrombolysis | 828 | 2014/15 Department of Health and Social Care’s national payment system97 |
| 90-day costs Barthel score of 20–10 | 459 | Franklin et al. (2014)100 |
| 90-day costs Barthel score of 0–9 | 1926 | Franklin et al. (2014)100 |
| 90-day costs residential care or nursing home | 10,647 | Gordon et al. (2014)95 |
Quality-adjusted life-years
Quality-adjusted life-years were calculated using the Barthel index94 from the SLSR and the methods described in Hunter et al. 53 Utility scores were applied to each ward and discharge location and calculated as a daily rate for the 90-day model and each location for the 10-year model and calculated as a 90-day rate. Details on utility scores can be found in Appendix 4, Table 44.
Cost–utility analysis
A difference-in-differences approach was used to compare cost and mortality at 90 days and costs and QALYs at 10 years; we compared costs, mortality and QALYs in London before and after the reconfiguration outcomes and costs over the same period in the RoE/excluding GM. The same analysis was then conducted for GM/excluding London. Mortality and LOS was adjusted for differences between London, GM and the RoE in age, sex, comorbidities (Charlson Comorbidity Index66), stroke type and level of deprivation. 67
Net monetary benefit is defined as total QALYs multiplied by a WTP for a QALY minus costs. It is commonly reported that the NHS is willing to pay £20,000 to £30,000 per QALY gained, with values greater than this less likely to be considered value for money. 101 NMB was calculated for London before the changes compared with after (models 1 and 2) and the RoE/excluding GM for the same time periods (models 3 and 4) for a range of values of WTP for a QALY gained (£0 to £100,000). If the NMB was higher for London than the RoE then the London reconfiguration was considered to be cost-effective. The same analysis was carried out for GM.
Probabilistic sensitivity analysis
Probabilities were applied to the model using the methodology given in Briggs et al. 30 For costs, a gamma distribution was used with 25% variation around the mean. A total of 5000 iterations of the eight models were run and costs, mortality and QALYs over 10 years and 90 days were captured. The percentage of iterations for which London or GM had a higher NMB than the RoE for a specific WTP for a QALY gained were recorded and shown on a cost-effectiveness acceptability curve. Ninety-five per cent CIs were calculated using the standard deviation of the 5000 iterations of the model.
Other sensitivity analyses
To test assumptions made in the model, one probabilistic sensitivity analysis and four deterministic sensitivity analyses were conducted.
Given that data for changes in the Barthel index before and after reconfigurations were available only for South London from the SLSR, we made the conservative assumption that the same improvements in functioning also occurred in GM and the RoE. A probabilistic sensitivity analysis was run to test the impact of changing the assumption so that the same improvements in functioning that were seen in London also occurred in GM but did not take place in the RoE. Instead, the Barthel index94 breakdown for the RoE remained the same after the reconfiguration as it was before the reconfiguration.
There is limited evidence regarding the probability of patients moving into a care home following stroke. The transition probabilities for moving into a care home in the 10-year model were taken from the SLSR and reflect the probability of moving into a care home in South London only. Other 9-month probabilities of care home admission over 90 days, quoted in the literature for older patients from throughout England, range from 0.9%100 to 0.26%,102 although these do not specifically relate to stroke patients. The impact of using alternative values was tested in a deterministic sensitivity analysis.
The cost of transfers for patients between hospitals was originally included in the London HASU tariff, so was not included as an additional cost. There is the possibility that transfers did result in an additional cost to the health service. The additional cost per transfer was included as a deterministic sensitivity analysis at £43 per transfer. 103 The percentage of patients who transfer between hospitals was calculated from HES.
More detailed evidence from previous analyses found that in London admission to an intensive therapy unit (ITU) was 4% before the reconfigurations compared with 2% after. 53 A deterministic analysis was conducted to determine what impact increasing the percentage of people admitted to ITUs before the reconfiguration in London and GM to 4% would have on the results.
Increasing the cost of a day in the HASU by 50% was also included as a deterministic sensitivity analysis.
Costs of implementation
We undertook reviews of documentary evidence to identify the costs of implementing the reconfigurations in London and GM including one-off financial investments to improve services, research to investigate the optimal configuration of services, and public and staff consultations.
Results
Base case
The characteristics of patients are reported in Table 10 and detailed resource use and costs for the model are shown in Table 11. Compared with the RoE, at 90 days London had a greater improvement in the reduced risk of mortality at 90 days from before to after the reconfiguration. Per 1000 strokes, the adjusted number of deaths in London at 90 days was 100 before the reconfiguration and 75 after, a reduction of 2.7 percentage points in the number of deaths. During the same period in the RoE, there was an adjusted reduction in deaths of 1.8 percentage points (114 deaths before compared with 97 deaths after) (Table 12). This represents a relative reduction of deaths in London compared with the RoE of 0.9%, or nine deaths per 1000 patients. In 90% of iterations of the model, London had a greater reduction in deaths after the reconfigurations than the RoE. There was no reduction in deaths in GM at 90 days (Table 13). Both areas had a reduction in LOS relative to the RoE; 2 days fewer in GM and 0.6 days fewer in London.
| Outcome | London | England | DID | ||||
|---|---|---|---|---|---|---|---|
| Before | After | Difference | Before | After | Difference | ||
| 90-day results | |||||||
| Deaths | 100 (80 to 120) | 75 (56 to 92) | –25 (–41 to –10) | 114 (93 to 133) | 97 (79 to 115) | –17 (–21 to –12) | –9 (–24 to 6) |
| LOS | 19 (18 to 20) | 16 (15 to 17) | –3.1 (–3.6 to –2.5) | 20 (18 to 21) | 17 (16 to 18) | –2.5 (–2.7 to –2.2) | –0.6 (–1.2 to –0.1) |
| QALYs | 102 (96 to 108) | 110 (88 to 132) | 8 (–14 to 29) | 100 (93 to 106) | 104 (98 to 110) | 4 (4 to 5) | 3.6 (–18 to 25) |
| Costs, £ | 5,705,774 (4,598,498 to 6,813,049) | 5,949,155 (5,069,827 to 6,828,484) | 243,381 (–182,658 to 669,421) | 5,492,431 (4,394,421 to 6,590,440) | 4,965,785 (3,956,766 to 5,974,803) | –526,646 (–640,210 to –413,081) | 770,027 (392,152 to 1,147,902) |
| 10-year results | |||||||
| Deaths | 386 (316 to 455) | 360 (289 to 432) | –25 (–38 to –13) | 417 (351 to 483) | 401 (329 to 475) | –15 (–44 to 13) | –10 (–37 to 17) |
| QALYs | 2931 (2499 to 3363) | 3473 (3079 to 3867) | 542 (109 to 975) | 2802 (2391 to 3214) | 3287 (2909 to 3665) | 484 (66 to 903) | 58 (–76 to 193) |
| Costs, £ | 39,459,874 (29,343,049 to 49,576,698) | 38,146,542 (27,941,281 to 48,351,802) | –1,313,332 (–5,315,567 to 2,688,902) | 39,818,504 (29,562,087 to 50,074,921) | 37,490,809 (27,170,660 to 47,810,958) | –2,327,696 (–6,146,669 to 1,491,278) | 1,014,363 (19,462 to 2,009,264) |
| NMB £20,000 per QALY (per patient), £ | 12,163 (5 to 24,321) | 12,015 (338 to 23,692) | 148 (–2208 to 2504) | ||||
| NMB £30,000 per QALY (per patient), £ | 17,588 (581 to 34,566) | 16,859 (488 to 25,599) | 729 (–2766 to 4610) | ||||
| Outcome | GM | England | DID | ||||
|---|---|---|---|---|---|---|---|
| Before | After | Difference | Before | After | Difference | ||
| 90-day results | |||||||
| Deaths | 127 (103 to 151) | 102 (76 to 127) | –25 (–46 to –5) | 130 (109 to 152) | 103 (84 to 121) | –27 (–33 to –22) | 2 (–19 to 23) |
| LOS | 20 (19 to 22) | 16 (14 to 17) | –4.6 (–5.5 to –3.7) | 19 (18 to 21) | 17 (16 to 18) | –2.5 (–2.8 to –2.2) | –2.1 (–3 to –1.2) |
| QALYs | 96 (90 to 102) | 103 (93 to 113) | 7 (–1 to 15) | 98 (92 to 104) | 103 (97 to 110) | 5.5 (4.7 to 6.3) | 1.6 (–6.6 to 9.8) |
| Costs, £ | 5,589,377 (4,441,146 to 6,737,608) | 5,214,732 (4,263,891 to 6,165,572) | –374,645 (–740,668 to –8622) | 5,435,973 (4,339,780 to 6,532,166) | 4,905,210 (3,902,177 to 5,908,243) | –530,764 (–653,699 to –407,828) | 156,119 (–169,632 to 481,869) |
| 10-year results | |||||||
| Deaths | 426 (360 to 492) | 399 (329 to 468) | –27 (–44 to –9) | 429 (364 to 494) | 406 (333 to 479) | –23 (–51 to 6) | –4 (–32 to 24) |
| QALYs | 2751 (2336 to 3167) | 3285 (2906 to 3664) | 534 (113 to 954) | 2747 (2339 to 3156) | 3263 (2882 to 3644) | 516 (103 to 929) | 18 (–122 to 158) |
| Costs, £ | 39,444,918 (34,251,242 to 44,638,594) | 37,087,053 (26,978,822 to 47,195,284) | –2,357,865 (–6,381,868 to 1,666,137) | 39,092,117 (29,011,793 to 49,172,441) | 37,205,099 (26,931,764 to –47,544,760) | –1,887,018 (–5,662,138 to 1,888,103) | –470,848 (–1,882,646 to 940,951) |
| NMB £20,000 per QALY (per patient), £ | 13,033 (1244 to 24,822) | 12,199 (674 to 23,723) | 834 (–1683 to 3350) | ||||
| NMB £30,000 per QALY (per patient), £ | 18,370 (3214 to 34,979) | 17,355 (2829 to 33,643) | 1015 (–2790 to 4821) | ||||
At 10 years, both reconfigurations resulted in more QALYs than their RoE comparator (see Tables 12 and 13). London resulted in 58 more QALYs at 10 years per 1000 patients at an additional cost of £1,014,363. This was equivalent to an incremental cost-effectiveness ratio of £17,452; hence, at a WTP for a QALY gained of £20,000, the London changes had a higher NMB than the RoE changes over the same time period. At 10 years, the reconfigurations that occurred in GM dominated what happened in the RoE over the same time period in that there were more QALYs (18 QALYs per 1000 patients over 10 years compared with the RoE) and less cost (–£470,848 per 1000 patients compared with the RoE over 10 years).
Cost-effectiveness acceptability curve
The probability that the changes that occurred in GM as a result of the reconfigurations had a higher NMB than in the RoE over the same time period and peaked at a WTP for a QALY gained of £7000 and 82% probability (Figure 6).
FIGURE 6.
Cost-effectiveness acceptability curve of the probability that the reconfigurations in London and GM resulted in a higher NMB than in the RoE over the same time period. Adapted from Hunter et al. 91 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
London had a higher probability of being cost-effective than GM at WTP values > £30,000. GM had a higher probability of being cost-effective at lower values of WTP for a QALY; this is shown in Figure 7, where 35% of iterations of the difference in costs and QALYs in GM compared with in the RoE over the same time period fell in the south-west quadrant of the cost-effectiveness plane. The changes in GM cost less than in the RoE but there was only a 60% chance of a reduction in mortality compared with in the RoE. For London, the majority of iterations of the model (80%) fell in the north-east quadrant, whereby London cost more but also had a higher probability that there was an improvement in mortality (81%) (Figure 7).
FIGURE 7.
Difference-in-difference cost-effectiveness plane of the adjusted difference in 10-year costs and QALYs between London before and after reconfigurations minus the difference in the RoE over the same time period and the difference in costs and QALYs in GM compared to the RoE over the same time period. Adapted from Hunter et al. 91 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Sensitivity analyses
The results of the sensitivity analysis are reported in Appendix 4, Tables 45 and 46. None of the sensitivity analyses changed the conclusions drawn as a result of the analysis, other than to say that once the cost of a HASU increased by 50% per night, the London reconfigurations were no longer cost-effective at a WTP of £30,000 per QALY. If it is assumed that improvements in functioning (Barthel index94) occurred only in London and GM as a result of the reconfiguration but not in the RoE, there was a 97% chance in both London and GM that the reconfigurations were cost-effective at 10 years at a WTP of both £20,000 and £30,000 for a QALY (see Appendix 4, Figure 28).
Costs of implementation
In addition to the funding for the increased tariff, additional one-off financial investments were made to bring about the reconfigurations in London and GM. It was estimated that an investment of £9M was made in London to meet the requirements for the new HASUs and SUs, covering capital, equipment and premises refurbishment. 104 In GM it was estimated that an initial investment of £2.79M was required for similar items. 105 Other costs are likely to have been incurred in both London and GM to pay for research to identify the optimal configuration of services and to consult staff and public, but evidence of these costs was not available.
Discussion
Principal findings
Both London and GM models had a higher NMB than changes that occurred over the same time period elsewhere in England at 10 years at a WTP of £20,000 to £30,000 for a QALY. The GM model appeared to be cost saving at 10 years but did not have the same health benefits as the London model and hence had higher values of WTP for a QALY, with London having a higher probability of being cost-effective. The differences between the two regions were small compared with the size of the CIs; therefore, they were not significant.
The results were in line with the findings on the impact of London and GMA on clinical outcomes25 (see Chapter 3) and clinical interventions26 (see Chapter 4) but also provided additional information on the value for money of the reconfigurations in two metropolitan areas in England compared with the rest of the country over the same time period. The London model reiterated findings that the centralisation of stroke care in London corresponded with clinical improvements that did not occur in the RoE over the same time period, but also showed that there was a high probability that the benefits were worth the additional costs. The results of the GMA analysis suggest that MSC of stroke care is possible with the investment of limited additional resources and potentially cost saving, although resulting in fewer clinical improvements.
Comparison with other studies
Evaluations of major system reconfigurations using decision modelling are becoming increasingly common. Although trials can provide information on what system changes should be implemented, decision models and population-level data can provide information on the implications for real clinical settings. 106 Previous evaluations of the centralisation of ovarian cancer treatment have used a combination of decision modelling of clinical trials prior to implementation107 and Dutch registry data following implementation to establish the cost-effectiveness of changes to the patient care pathway. 108 Synthesising audit or routinely collected data from the whole population in addition to data on costs has been used previously in New Zealand109 to show that admission to a SU is more cost-effective than admission to a general ward. In Scotland, population-based data have been used to evaluate the clinical effectiveness of admission to stroke wards,106 and analyses by our own team have used population data to establish the clinical effectiveness of MSC for stroke care in GM and London. 25 This analysis was the first to use whole population data, HES and audit data to evaluate the MSCs that occurred in stroke care in London and GM compared with in the RoE.
Strengths and weaknesses
The key strength of this analysis was that it provided information about the real-world implications of the changes made and hence can inform decisions about MSC in the future.
There are weaknesses to using observational, routinely collected hospital data, most notably coding errors. For example, in one London hospital 3% of 1300 stroke patients had a discharge destination code for discharge to a mental health hospital, compared with 0.13% of patients for hospitals across all of England. It is unlikely that such a large percentage of patients were being discharged to this location; instead, it is more likely that the wrong code had been used and that they were being discharged elsewhere. There is no way to know where and, hence, the only option was to remove the data prior to estimating discharge destination. It is likely that the data set contained other errors in relation to discharge destination. We assumed, however, that those errors would occur equally at random across the three regions. It was also hard to know how ‘stroke mimics’, that is patients presenting with stroke-like symptoms but who do not have a final diagnosis of stroke, appear in the data. They may be screened out of the data sets as not having stroke even though they have a SU admission, or the final diagnosis may potentially be missing from our data set. It was hard to know from the data what proportion were mimics and what impact they had on the cost and outcomes for the care pathway and there were no comparable data from other sources to estimate this.
It is important to note that the values reported in the model for LOS and mortality were values adjusted for differences between patients within the regions in age, ethnicity, sex and comorbidities. As a result they did not represent true values per 1000 patients but adjusted values, to allow for comparison between areas over the same period of time. As the changes occurred in GMA and London at different time points, no direct comparison between GMA, London and the RoE was possible as it would not have taken account of differential changes over time. One of the key weaknesses in the model is that South London was the only area for which there were data available on the impact of the changes on functioning using the Barthel index. 94 Although there were no changes in the proportion of patients discharged home before and after the reconfigurations, after the reconfigurations there may have been some improvements in Barthel scores for people who were discharged home, based on data from the SLSR (see Appendix 4, Table 43). Although GM and London had some information on functioning available for time periods after the reconfigurations, there was no information on functioning available before the reconfigurations with which to compare it, other than in the SLSR. Instead, we had to make the conservative estimate that the same improvement in functioning seen in people discharged home in the SLSR was seen throughout the whole country over the same time period. If we assume that, instead, the improvements were the direct result of the reconfigurations, the sensitivity analysis showed that the London and GMA models had a significantly higher probability of being cost-effective.
In addition, a limited number of data were available on which ward patients were admitted to, how long they spent on each ward and which wards they moved between prior to the reconfigurations. Instead, assumptions were made using the data collected in SINAP after the reconfigurations took place. Previous evaluations in this area suggest that admissions to the ITU reduced in London after the reconfigurations, but as no data were available for GMA or the RoE, no analysis could be done to account for this, only the sensitivity analysis, which also shows that if these improvements can be accounted for there is again a higher probability that the changes were cost-effective.
Implications
Centralised models of stroke care across an entire metropolitan area had a high probability of being a cost-effective way to improve outcomes for stroke patients. In London, this was as a result of improvements in mortality and morbidity and in GM as a result of reduced cost of stroke care attributable to reduced LOS.
Chapter 6 Lessons from implementing major system change in acute stroke services in Greater Manchester and London
Overview
This chapter draws on a paper published by Turner et al. 110 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 3.0 License (www.creativecommons.org/licenses/by-nc/3.0/), which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage).
What was already known about this subject?
-
Evidence on implementing MSC in health care was limited.
-
A realist review of the MSC literature identified five ‘rules’ for enhancing implementation, linking ‘success’ to (1) multiple forms of leadership, (2) learning from history, (3) improvement through feedback, (4) physician engagement and (5) service user involvement.
What this chapter adds
-
By combining qualitative analysis of processes of change and drawing on the quantitative analysis of the impact on clinical outcomes and delivery of interventions, we were able to adapt and extend these lessons for MSC.
-
Both system (top-down) and clinical (bottom-up) leadership were identified as necessary to align multiple stakeholders and thus overcome resistance to change.
-
System leadership can:
-
provide political authority and power to co-ordinate multiple local stakeholders to agree to change services over a wide area
-
capitalise on clinical leadership to develop further support for the goals of change.
-
-
Change in both areas involved clinical leadership, but although system-wide authority was used in London to align stakeholders, it was not applied in GM.
-
Combining feedback with other tools (e.g. use of audit data) was important to build the case for change and to assess its impact.
-
It was necessary to involve a range of stakeholders beyond physicians in planning MSC.
-
Consideration should be given to how system-wide structures can be used to enable the joint planning and implementation of MSC.
Background
Major system change of stroke services
Through the qualitative analysis of stakeholder interviews and documentary evidence, the aim of this chapter is to explain why different models were implemented in London and GM and, in doing so, enhance the Best et al. 5 framework (rules for implementing MSC based on a review of the literature) for future use in planning MSC.
In GM, the case for centralising services was made locally by health professionals to commissioning and provider leads. In London, centralising acute stroke services was recommended as part of a region-wide review of services. 28 In both areas, the process of centralisation was characterised by overlapping phases of planning and development, consultation, and implementation (Figures 8 and 9).
FIGURE 8.
Governance arrangements for centralising acute stroke services in London. HfL, Healthcare for London. Adapted from Turner et al. 110 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 3.0 License (www.creativecommons.org/licenses/by-nc/3.0/), which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage).
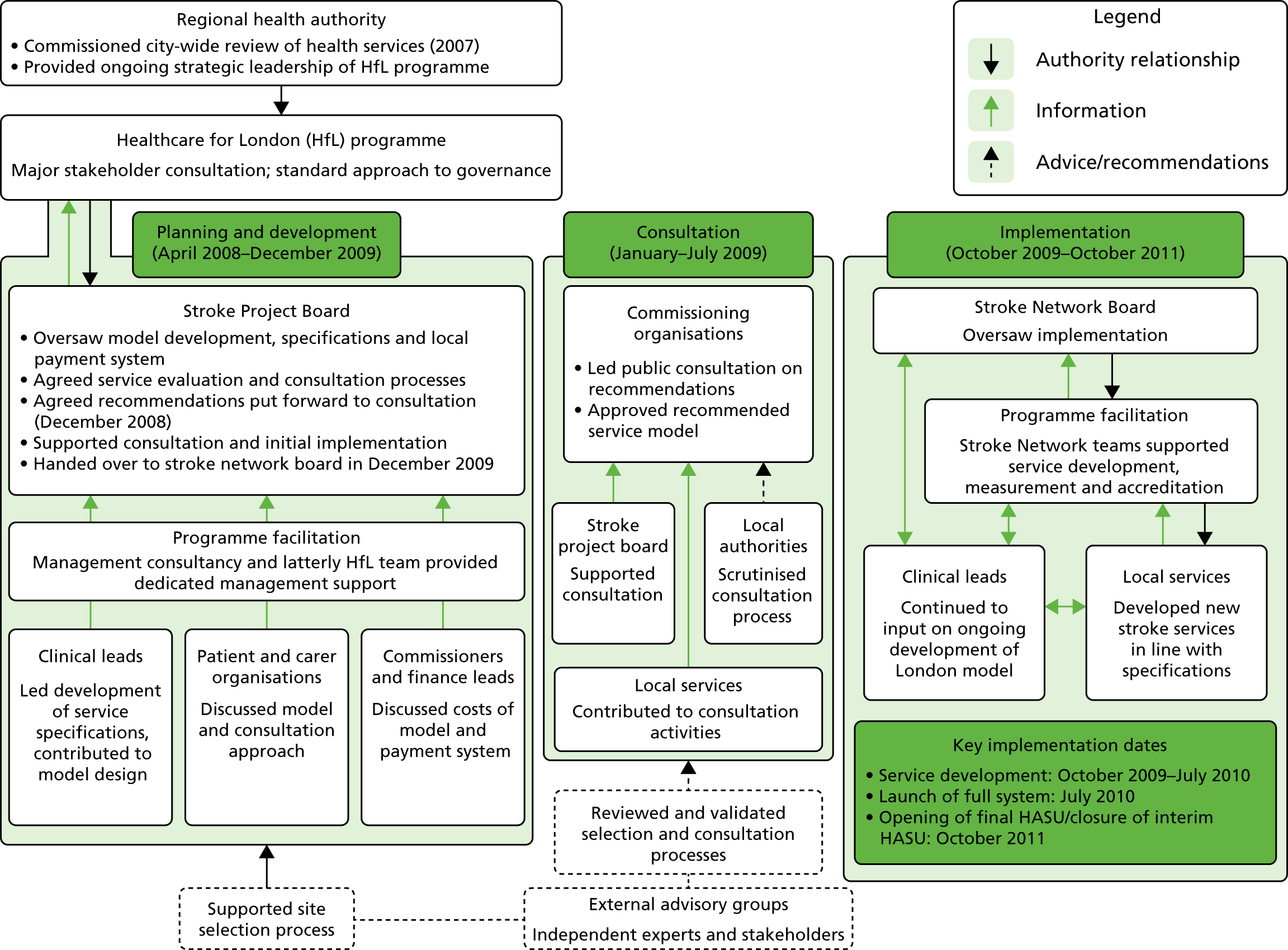
FIGURE 9.
Governance arrangements for centralising acute stroke services in GM. Adapted from Turner et al. 110 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 3.0 License (www.creativecommons.org/licenses/by-nc/3.0/), which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage).
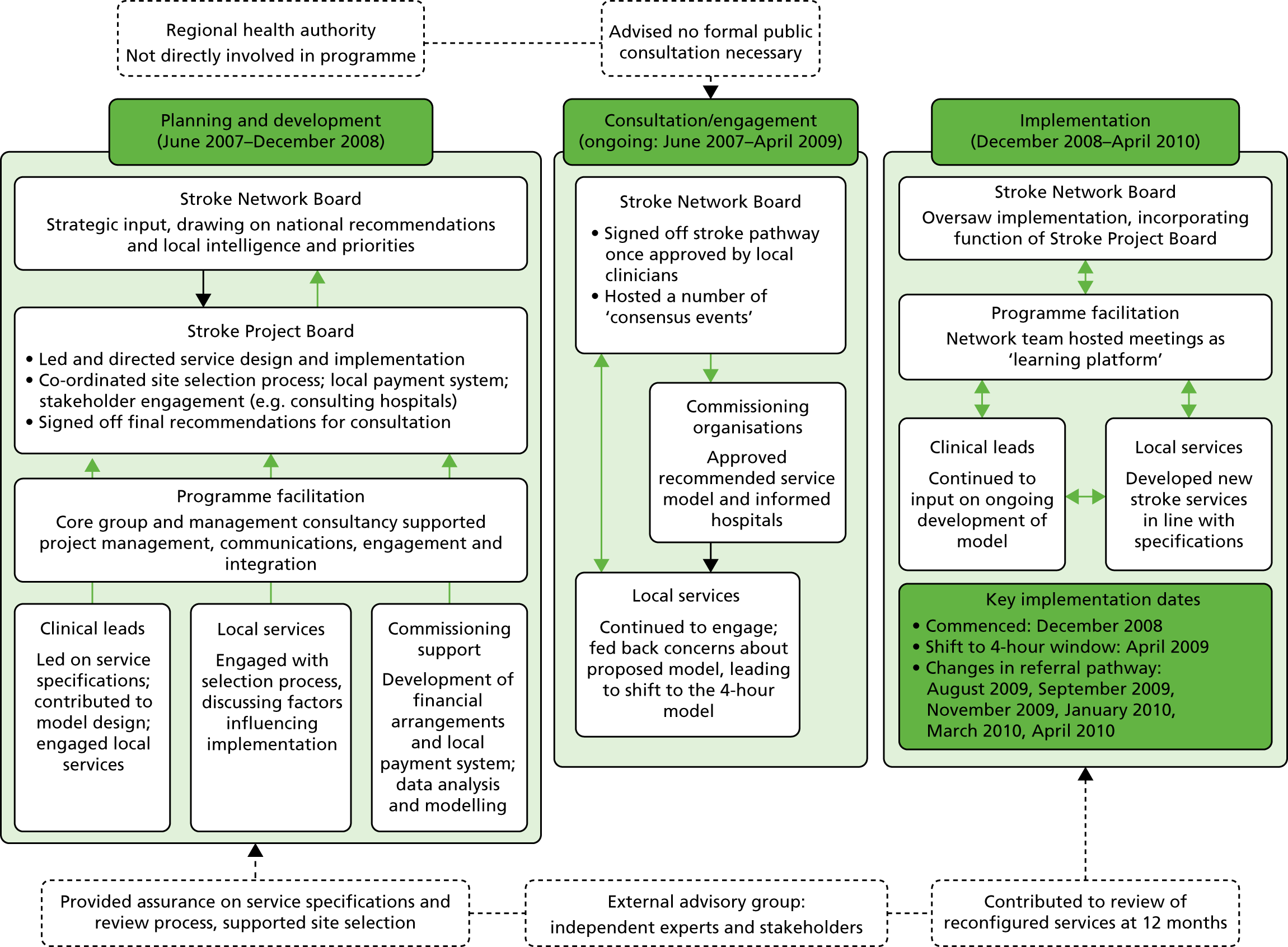
In London, the previous system for delivering acute stroke services involved 32 providers and the local ambulance service. In GM, nine providers and the ambulance service were involved. In both areas, a stroke project board, including providers, commissioners and patient representatives, was established to develop new service models and manage implementation. Change was governed using a ‘top-down’ approach in London, led by the pan-regional health authority (Figure 8), whereas a more ‘bottom-up’ network-based approach was used in GM, led by local providers and commissioners (Figure 9).
Informed by the EAG’s recommendations, commissioners in GM initially chose a service model in which all patients presenting at hospital within 24 hours of onset of stroke symptoms would be treated in one of three HSUs (one 24/7 CSC and two ‘in-hours’ PSCs); post-hyperacute care would be delivered by 11 local DSCs. Shortly before implementation, some hospitals raised concerns about the impact of centralisation on hospital resources and patient safety (e.g. repatriation of frail and elderly patients). Consequently, the model was revised: only patients presenting at hospital within 4 hours of stroke symptoms appearing would be admitted to a CSC/PSC; all others would be taken as before to the nearest DSC. Acute stroke services were not entirely withdrawn from any hospital. In London, the project board’s clinical subgroup recommended 10–12 HASUs, but the project board proposed fewer HASUs (eight), reflecting the earlier pan-London review’s recommendations. Subsequently, 24 providers became SUs and stroke services were withdrawn from five hospitals.
This chapter used qualitative data related to the leadership of change to analyse why different models for centralising services were chosen in London and GM, and to assess how the different approaches to leading change contributed to different centralisation models being implemented.
Method
Data
We conducted 45 semistructured stakeholder interviews and analysed 316 documents (see Table 2 and Chapter 2, Understanding development, implementation and sustainability). Interview topic guides (see Appendix 5), based on MSC literature, covered governance approaches, proposal development, model agreement and implementation. Documentary evidence was used to develop narratives of the transformations (e.g. public consultation arrangements). The interviews represented a retrospective account of the changes and took place when there was awareness of how the services were performing in each area post implementation. To mitigate risk of bias as a result of the length of time since interviews we drew on different types of evidence,111 including interviews with diverse stakeholders and analysis of documentary evidence.
Analysis
Data analysis from interviews and documents was inductive and deductive,112 as coding was informed by themes emerging from the empirical data and a realist review of MSC literature. 5 Data relating to London and GM were initially coded separately to produce narratives of transformation. MSC across the two areas was compared using cross-cutting themes from the narratives, including receptivity to change, stakeholder involvement and experiences of service transformation. Finally, the coded data were analysed using the five rules of Best et al. 5 to compare mechanisms influencing MSC. The research team met regularly during the analysis process to discuss and agree interpretations of the data and to identify questions iteratively for further exploration. To enhance validity, emerging findings were shared with relevant stakeholders from the two study areas, including professionals and service user representatives.
Results
In this section, the approaches to change in London and GM were assessed by applying the five rules from the Best et al. 5 framework, including analysis of where the rules need to be adapted based on our findings, as summarised in Table 14.
| Rule | London | GM | Adapted rule |
|---|---|---|---|
| 1: combine designated and distributed leadership | Centralising services, and an opportunity for investment, endorsed by clinical leaders, despite the risk of losing services. Pan-regional authority oversaw change and helped to align stakeholders | Impetus for centralising services came from senior stroke physicians and public health staff who encouraged others to support change. Change led by local stroke network, which did not have formal authority over providers | Interplay between bottom-up and top-down leadership in achieving MSC; system-wide authority is needed to align multiple stakeholders over a large scale and encourage clinical commitment to system-wide improvement goals |
| 2: feedback loops | Clinicians involved in developing quality standards for new services, as well as commissioners and providers’ finance teams. Providers received financial incentive for meeting standards. Stroke network advised providers on meeting standards | Providers compensated for collecting and sharing national audit data, but no financial incentive for meeting clinical standards. Clinicians and managers from different providers along patient pathway met regularly to review performance | Feedback may need to be combined with other tools to encourage behaviour change (e.g. financial incentives) |
| 3: attend to history | Awareness from previous attempts to transform services across London that implementing change is challenging. Legal firm consulted to avoid subsequent challenges | Drew on experience from members of stroke network involved in an earlier reorganisation of acute cardiac services | Contextual factors can be a barrier to implementing lessons learned; political authority may be needed to challenge the existing context and enable more radical forms of transformation |
| 4: engage physicians | Engaging a variety of health professionals who are important in planning new services, especially ambulance service. Need to engage stakeholders outside the health service; resistance from local politicians to the closure of services | Many stroke physicians supported change; some resistance from providers set to lose services. Need to engage other stakeholders (e.g. hospital managers) as model had to be viable as a ‘business proposal’ | Need to involve a range of stakeholders in planning MSC and have a system-wide governance structure to align their interests |
| 5: involve patients and families | Proposal for centralising stroke care put to public consultation. Quantified support for proposal used to legitimise centralisation of services | Perceived perspective of patients used to steer negotiations among providers and commissioners towards consensus. Some suggested that views of public and patients had limited influence on the model of services | Awareness that the drivers of MSC (e.g. clinical, political, social, financial) influence how different stakeholders’ views come to count during implementation; potential tension between patients’ and others’ perspectives |
Rule 1: combining designated and distributed leadership
The first rule highlights the importance of combining formal or ‘designated’ leadership with shared or ‘distributed’ responsibility for improvement. In GM the leadership of change was mainly ‘distributed’, whereas in London distributed and designated leadership were more fully combined. This meant that when there was resistance to centralising services from some stakeholders in both areas, there was a lack of system-wide leadership in GM to challenge resistance and align stakeholders (as Figure 9 shows, the pan-regional health authority was not directly involved in the programme), resulting in a less radical transformation of services in GM relative to London.
Distributed leadership
In both areas, reorganising stroke services was supported by many clinicians because it was an opportunity for service investment and to gain further recognition as a profession distinct from geriatric medicine:
[clinicians] felt that stroke was a sort of a Cinderella service, that very little investment had been made in stroke. They saw that additional money was coming.
Lon06, Stroke Network Board
Local clinical leaders’ endorsement of centralisation generated wider support. In GM change leaders used ‘bottom-up’ leadership, relying on stroke physicians convincing others that centralisation was necessary:
. . . a lot of it was around peer support and, whether they liked it or not, those antagonists respected some of the lead stroke clinicians that were there around the table.
GM10, Stroke Network Board
Although clinical leadership was visible in both areas, there were differences in system-wide (designated) leadership and how this was used to capitalise on distributed leadership.
Designated leadership
In London, designated leadership was easier to exercise than in GM because programme leaders possessed greater political authority to manage stakeholders’ resistance to change. Programme leaders were members of the pan-regional authority, which oversaw changes to stroke services as part of a wider review of health services (see Figure 8). This system-wide authority structure ensured that the model proposed by the project board for centralising services was implemented:
. . . you are the priesthood if you like of the model, so you keep the fidelity to the model that’s being described and only with your ‘say so’ can people deviate from it.
Lon03, pan-regional health authority
Planning was shaped by the pan-regional health authority because change was informed by a wider pan-London review28 of stroke and other services that had political influence, because it was clinically led and demonstrated public support. Selection criteria for HASUs included ‘strategic coherence’,29 with wider plans to develop major acute hospitals in London with specialist services (e.g. major trauma care) and to ensure patients could reach a HASU by emergency ambulance within 30 minutes. As clinical recommendations concerning designation were considered alongside hospitals’ fit with these broader criteria, some perceived that a ‘top-down’ approach to decision-making was taken:
. . . giving them the model, saying this is what we want to do, and then there was a discussion about it, rather than it coming from the grass roots up.
Lon10, Stroke Network Board
In GM, transformation was led by the stroke network board (see Figure 9). As programme leaders lacked formal authority over providers and commissioners, changes were planned by consensus. As described in Major system change of stroke services above, a late challenge to the 24-hour model came from some hospitals that were set to lose activity in the proposed changes to stroke services. In order to maintain unanimity, programme leaders implemented a 4-hour model:
. . . the minute it felt like unanimity was being compromised on that clinical discussion on the 24- versus the 4-hour pathway I think we were always going to be minded then to tilt towards holding unanimity.
GM04, service commissioner
Interplay between designated and distributed leadership
Designated leadership encouraged further distributed leadership of the changes proposed. In London, designated leadership was used to encourage stakeholders to associate with a wider geography of improvement:
. . . my key mantra at the moment is to remind people constantly that this is the London model. So when an organisation says that they’d like to change something . . . we say you can’t do that without it impacting on the whole of London.
Lon06, Stroke Network Board
Further support for centralisation was garnered through pan-London events during the public consultation, the second phase of the programme shown in Figure 8. Instead of a formal public consultation, GM held two consensus-building events involving providers, commissioners and the public at which proposals for transformation were discussed (see Figure 9).
Reflecting on the comparative difficulty of centralising services in GM, one programme leader suggested that greater authority would have been useful in ensuring different stakeholders prioritised the wider metropolitan area’s interests:
. . . one of the things that we would do now that we didn’t do then would be probably not proceed on the base of unanimity and instead ensure people wear a kind of ‘Greater Manchester population’ hat.
GM04, service commissioner
In London, although change was more ‘top-down’ than in GM, this approach encouraged distributed leadership by engendering recognition that meeting the centralisation programme’s goals, and those of the stroke community, required a pan-London perspective. For instance, two clinical leaders in London performed a visible symbolic role in supporting the proposals, despite this meaning that their own services would not become HASUs. In GM, programme leaders’ weaker authority made encouraging distributed leadership more difficult, despite ‘support from the most senior and most respected clinicians’ (GM06, Stroke Network Board).
Rule 2: establish feedback loops
This rule refers to the importance of measuring outcomes that are trusted by stakeholders and incentivising improvement. Performance data were collected in both areas. In London, the designation process for hospitals was linked to achieving standards (e.g. minimum staff numbers), meaning that providers had to comply in order to receive accreditation. Providers also received a financial incentive for performing well, as ongoing performance data were monitored by the stroke networks and payments were made only if quality standards were met. In GM, a local payment system was required to split the costs of providing services between CSC/PSCs and DSCs, although payments were based on patients treated by each form of provider, not achieving standards. Thus, financial incentives were stronger in London than in GM for improving clinical standards (in GM financial penalties were considered punitive by planners and not used).
Rule 3: attend to history
The Best framework suggests the importance of learning from previous transformation attempts, including ‘failures’. 5 In London, past failures to achieve MSC meant that programme leaders focused on implementing ‘a small number of absolute priorities’ (Lon14, pan-regional health authority). Change leaders were aware that dealing with stakeholders’ differences was critical during planning meetings:
. . . not letting people go out the room if I thought actually they were disagreeing but they weren’t disagreeing in the room.
Lon17, service commissioner
In GM, decision-making based on unanimity was preferred in this case as collective decisions were historically made by consensus among commissioners and providers. One programme leader believed retrospectively that the approach taken ‘introduced an awful lot of risk that we needn’t have played into it’ (GM04, service commissioner).
In both areas, planning utilised stroke network members’ experiences of an earlier reorganisation of acute cardiac services. This highlighted a need to encourage dialogue between the ambulance service and other stakeholders. In London, insistence by the ambulance service informed the decision to take a ‘big bang’ approach to transformation whereby centralised services ‘went live’ on a single date. GM’s ambulance service expressed a similar preference, but a decision was made to implement changes to services in stages instead. A barrier to addressing the ambulance service’s preference was the need to accommodate providers’ concerns about transferring patients in the new system:
The worry was that if you suddenly changed the system, the whole system, you could become completely overwhelmed.
GM05, stroke physician
In summary, programme leaders attended to history by recognising that the existing system for delivering stroke care was unlikely to be receptive to change. In GM, programme leaders attempted to mitigate potential resistance by making decisions through consensus. However, this approach involved bowing to resistance from some providers and resulted in less radical transformation (i.e. hospital resistance trumped ambulance preferences). Conversely, the political authority with which London’s programme leaders acted was critical in being able to overcome resistance.
Rule 4: engage physicians
This rule highlights the need to engage physicians as actors who have historically had the power to influence MSC, as literature on the sociology of professions shows. 113 Although physician engagement was important in planning transformation, our analysis suggested that the involvement of a range of stakeholders was required. Ambulance services, needed to assess and transport the majority of patients with suspected stroke, were critical:
. . . it wouldn’t have happened if the ambulance service hadn’t been fully on board with it.
Lon06, Stroke Network Board
Obtaining the agreement of hospitals’ senior management was necessary, as changes to stroke provision would affect hospital income and other departments. In GM, one hospital did not bid to become a hyperacute service, despite physician support, on account of senior management concerns about A&E pressures:
. . . senior management had told [the consultant] not to, that the bid shouldn’t go in.
GM05, stroke physician
The wider financial impact of full centralisation was also understood by programme leaders. To meet both physicians’ and hospital managers’ expectations, the model for centralising services needed to achieve ‘clinical consensus’ and be a viable ’business proposal’ (GM01, Stroke Network Board).
As well as affecting stakeholders within health services, MSC in London and GM was affected by local politics. In London, the change programme included establishing a committee of local politicians to scrutinise the proposal’s public interest. The implications of services being discontinued in some areas owing to centralisation caused resistance from local government representatives:
. . . issues which caused the most angst was the removal of facilities from a certain local authority area. Every elected councillor wants to protect their area.
Lon21, local politician
In summary, although stroke physicians were key stakeholders in MSC, the geographic scale and public interest in the changes proposed meant that other stakeholders, both within and outside the health service, needed to be engaged to avoid derailment of change. Champions included senior stroke physicians, who exerted social influence over other clinicians, as the Best framework suggests. Rather than relying on physician engagement, success was enabled through dialogue between, and the alignment of, different stakeholders (e.g. among hospital managers and clinicians and ambulance staff).
Rule 5: involve patients and families
This rule suggests that leaders of MSC should include service users’ perspectives and priorities in the change process. Attempts were made to represent their views in both areas (e.g. patient organisations sat on committees for governing changes). Patients’ perceived priorities informed other stakeholders’ decision-making. In London, the initial pan-London proposal to centralise stroke care underwent public consultation. As the majority of respondents (67% of 3464) agreed with introducing specialist centres,35 this was seen to justify implementing a centralised model:
Our mandate for doing what we were doing came from that public consultation in which ‘about seven’ had been supported, and there were going to be about seven.
Lon03, pan-regional health authority
In GM, patients’ perceived needs were used to reach consensus during decision-making about centralising services. However, some interviewees doubted whether or not service users’ views, despite being sought through consultation on proposed service models, influenced transformation:
I don’t think it really changed anything . . . but at least people felt that they had a voice.
GM13, service user representative
In summary, patients and the public were consulted about, rather than involved in, the decision-making. Furthermore, their perceived views were used instrumentally by leaders to lend support to the implementation of well-defined models of care. In London, as public support for ‘about seven’ specialist centres had been quantified, this aided agreement to implement more fully centralised services. Public involvement had a political dimension as engagement was structured in particular ways (i.e. programme leaders’ framing of the options for consultation and use of the outcomes to legitimise changes to services). In GM, there was no equivalent process to establish service users’ priorities.
Discussion
This chapter analysed two cases of MSC to acute stroke services in large metropolitan areas, which resulted in different outcomes. Although services were more fully centralised in London, a less radical transformation of services took place in GM, as programme leaders did not have the system-wide political authority necessary to overcome resistance from some stakeholders. In GM, the original model was revised to maintain consensus, such that only patients presenting within 4 hours of developing symptoms were eligible for treatment in a CSC/PSC, and no provider closed stroke services. This contrasted with the model in London, in which all patients with suspected stroke were eligible for HASU treatment and stroke services were closed in five providers.
The Best framework was useful for identifying key processes in the transformation of stroke services in London and GM, but it produced an incomplete account of change. Our analysis suggests a need for greater acknowledgement of potential barriers to implementing each rule, potential conflict between rules in service planning and, as others have shown,114,115 the importance of politics in decision-making concerning health-care reorganisation. Our suggested changes to the rules are summarised in Table 14.
Distributed leadership by stroke physicians and other stakeholders was apparent in both transformations, but system-wide leadership was necessary to capitalise on distributed leadership by aligning it with transformation goals. This system-wide designated leadership in London encouraged distributed leadership by aligning actors with a pan-London approach to improvement. This eschewed commitment to sustaining a given hospital’s acute stroke service, even those recognised as providing high-quality care at the time. Managing disparate stakeholder interests was easier in London than in GM because designated leaders exercised greater political authority through pan-regional bodies and committees. Thus, our study suggests that system-wide leadership with authority is necessary to align multiple organisations across a large scale. However, encouraging leadership throughout the system is equally important; evidence from Denmark has shown that implementing stroke service centralisation ‘top-down’ restricted the involvement of front-line staff and undermined ownership. 116
Differences in the use of feedback loops in London and GM highlight the importance of analysing the social and political context in which performance metrics are developed. Variation in responses to feedback can be explained partly by differences in how resources were used to support transformation (e.g. local quality standards were linked to financial incentives to a greater extent in London). As feedback loops include social and financial components, the expectations of policy-makers relying on social influence alone to change behaviour may be blunted in some contexts (e.g. when resources are already strained and subject to competing demands).
In relation to attending to history, Best et al. 5 reviewed examples of MSC that highlighted the importance of learning from experience, but also showed the difficulties associated with implementing those lessons. An analysis of political factors in the current study shows that a potential barrier to applying lessons is the involvement of multiple stakeholder interests in MSC; accommodating these may thwart transformation. In GM, the ambulance service provided advice on the timing of implementation, but a barrier to executing their recommendations was the need to accommodate other stakeholders’ perspectives. Proceeding on the basis of consensus may lessen resistance to transformation, but our study suggests that this approach produced less radical change in this instance. The rule of attending to history, which involves recognising potential barriers to change, is insufficient for improving MSC implementation. As demonstrated in London, system-wide leadership, combined with political authority that includes levers to finance and performance manage change, is needed to challenge the existing context and enable transformation.
The importance of involving a range of stakeholders beyond physicians in MSC was illustrated. MSC necessitated engagement with a range of clinical and managerial groups as planning change involved clinical, financial, logistical and public interest considerations. In this case, privileging the interests of one stakeholder was inappropriate, as other groups could impede MSC, including ambulance services, hospital managers and local authorities.
Patient and public involvement was used instrumentally by programme leaders to demonstrate support for the proposals being developed rather than to fulfil the loftier aspiration of co-designed services, which is often absent from MSC. 5 One reason for this is political; in both areas, a vision for transformed services was already well defined, and programme leaders focused more on gaining public support for the service models stemming from this vision than on obtaining patients’ input into service design (e.g. identifying performance metrics from patients’ perspectives). This resonates with previous research showing that PPI is often guided by health professionals, especially where ‘technical knowledge’ is deemed necessary to participate. 117 Only considering involvement in relation to fulfilling a change programme’s needs neglects the reasons for, and impact of, involvement from the perspective of service users. 118 Furthermore, rules may conflict with one another in this context. Engaging clinicians to pursue a clinical case for change may have implications for involving the public in service design, as their views may not coincide.
An analysis of politics and power explains how different forms of leadership are combined in MSC. In both areas, providers and commissioners were consulted extensively on the new model for stroke care proposed. In London, change leaders had the political authority to maintain their position in response to providers’ concerns. In GM, leaders had less political authority and focused on maintaining consensus among stakeholders, but in so doing implemented a less radical service transformation. The models implemented in London and GM were later found to have significantly different impacts on stroke patient mortality and the provision of evidence-based clinical interventions. 25,26 The more radical transformation of services in London involved system-wide authority structures (the pan-regional health authority) combined with actors (senior clinical leadership) who used persuasive arguments. Although change in GM also involved clinical leadership, weaker authority structures existed for aligning stakeholders.
Implications
The combination of bottom-up leadership and top-down co-ordination of MSC is vital in navigating the complex process of its implementation. Engaging local stakeholders in planning processes is important to ensure that change builds on their experiences, is relevant to their needs, and motivates staff. 119 However, as multiple stakeholders are often involved in MSC, a co-ordinating body with political authority to bring together those different interests is needed. Regional, system-wide leadership – underpinned with the political authority to align stakeholders – should assume a leading role in supporting innovation by co-ordinating processes of MSC.
Since the transformations of stroke care described in this chapter were implemented, pan-regional health authorities have been abolished and many purchasing and performance management duties in England have been transferred to more localised commissioning consortia. 120 Consideration should be given to the collective capacity commissioners and providers have to pursue MSC. Substantial work has been conducted to devolve budgets and decision-making authority back to regions, with GM taking a leading role. 121,122 In relation to these different approaches to decision-making, consideration should be given to how system-wide structures can be used to enable the joint planning and implementation of MSC. Finally, against a background of austerity where many health systems face financial pressure, some ‘rules’ may need to be prioritised over others. Formative research could explore the feasibility of using an explicit framework of rules to inform programme leaders’ decision-making about allocating resources to the activities signified by each ‘rule’ and highlight perceived barriers and enablers to their use in MSC.
Chapter 7 Explaining outcomes of Greater Manchester A and London
Overview
This chapter draws on a paper published by Fulop et al. 123 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
What was already known about this subject?
-
Little was known about the best way to make improvements to health services across a whole geographical area or ‘system’.
-
Research and theory indicated a number of interrelated components of MSC.
-
Following centralisation in GM and London:
-
in both areas, LOS reduced more than in the RoE; only in London did mortality reduce more than in the RoE (see Chapter 3)
-
after centralisation, London stroke patients were significantly more likely to receive evidence-based care than in GM or elsewhere; this was in part because more patients were treated in a HASU in London (93%) than in GM (39%) (see Chapter 4).
-
-
Analysis of planning and implementation of the changes suggested significant contributions of combining top-down, system-wide authority with bottom-up clinical leadership to engage and align multiple stakeholders across the system (see Chapter 6).
What this chapter adds
-
A theory-based framework enabled an analysis of the relationships between planning, implementation and outcomes of MSC by linking quantitative outcomes with qualitative findings on process of change.
-
Referral pathway: in London, where all patients were eligible for HASUs, and all HASUs admitted patients 24/7, the pathway was reported to be more straightforward and inclusive. It was more likely to be understood and followed by both hospital and ambulance staff, maximising the proportion of patients who were treated in a HASU. In GMA, the referral pathway where only a selection of patients were eligible for treatment in a HASU was found to be less inclusive and more complex than in London; this reduced the proportion of patients treated in a HASU, in part through limited adherence to the pathway.
-
Phases of implementation: in London, the single launch date was identified as facilitating clear understanding of and adherence to the pathway. In GMA, phased implementation caused uncertainty among hospital and ambulance staff, both during and post implementation.
-
Use of service standards linked to financial incentives: in London, standards were linked to financial incentives and services could not launch until accredited against the standards. This was reported to have increased the likelihood of services having capacity to provide evidence-based care. In GMA, service standards were not linked to incentives and there was no accreditation process, which may have led to greater variation across stroke services.
-
Facilitation: the local stroke network in London was described as providing substantial co-ordination and hands-on facilitation. In GM, the network facilitated implementation by acting as a platform for sharing learning across sites.
Background
Our analysis of planning and implementation of MSC in GM and London (see Chapter 6) indicated that a number of factors (including leadership approaches, stakeholder engagement and use of measurement) influenced the service model that was selected and how it was implemented. In this chapter, we present an analysis of the relationship between the service model selected and the implementation approaches employed on the impact on clinical interventions (see Chapter 4) and clinical outcomes (see Chapter 3).
Understanding outcomes of implementation of change
The field of implementation science articulates the need for a nuanced approach when evaluating the outcomes of change. An important distinction is drawn between ‘implementation outcomes’ (i.e. the adoption of, fidelity to and sustainability of a given intervention)5,41–44,124–127 and ‘intervention outcomes’, for example changes in the provision of care or patient outcomes. 41 This enables the study of factors that influence implementation (including the nature of the intervention and how its implementation is facilitated), and the potential relationships between these and intervention outcomes,41,42 thereby allowing insights into the ‘black box’ of implementation.
Understanding how evidence-based practice is implemented in complex settings such as health care is enhanced when its various components are considered (decision to change, intervention selection, planning and implementation of change, and outcomes). 40,42,44,125 The value of theory, as represented through conceptual frameworks, is recognised as benefiting the design, application and understanding of implementation approaches. 44,128–130 Such frameworks provide, first, an analysis of how contextual factors, such as national policy or a ‘burning platform’, can influence the decision to change and the type of intervention that is implemented. 5,128,130 Second, they indicate how characteristics of the intervention (e.g. a new service model), such as its complexity or its compatibility with local context, might influence the outcomes of implementation. 40,124,126,127,130 Third, they show how the implementation approaches employed (i.e. how change is facilitated, managed and led) can influence implementation outcomes. 1,40,43,44,128,130
However, research exploring the relationships between implementation approaches, implementation outcomes and intervention outcomes remains limited. 41
Developing a framework to analyse major system change
Drawing on the literature on implementation and MSC described above, we developed a schematic framework that identifies key components of MSC and how they might interact (Figure 10). The framework distinguished between implementation outcomes and intervention outcomes.
FIGURE 10.
Key components of MSC. Adapted from Fulop et al. 123 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
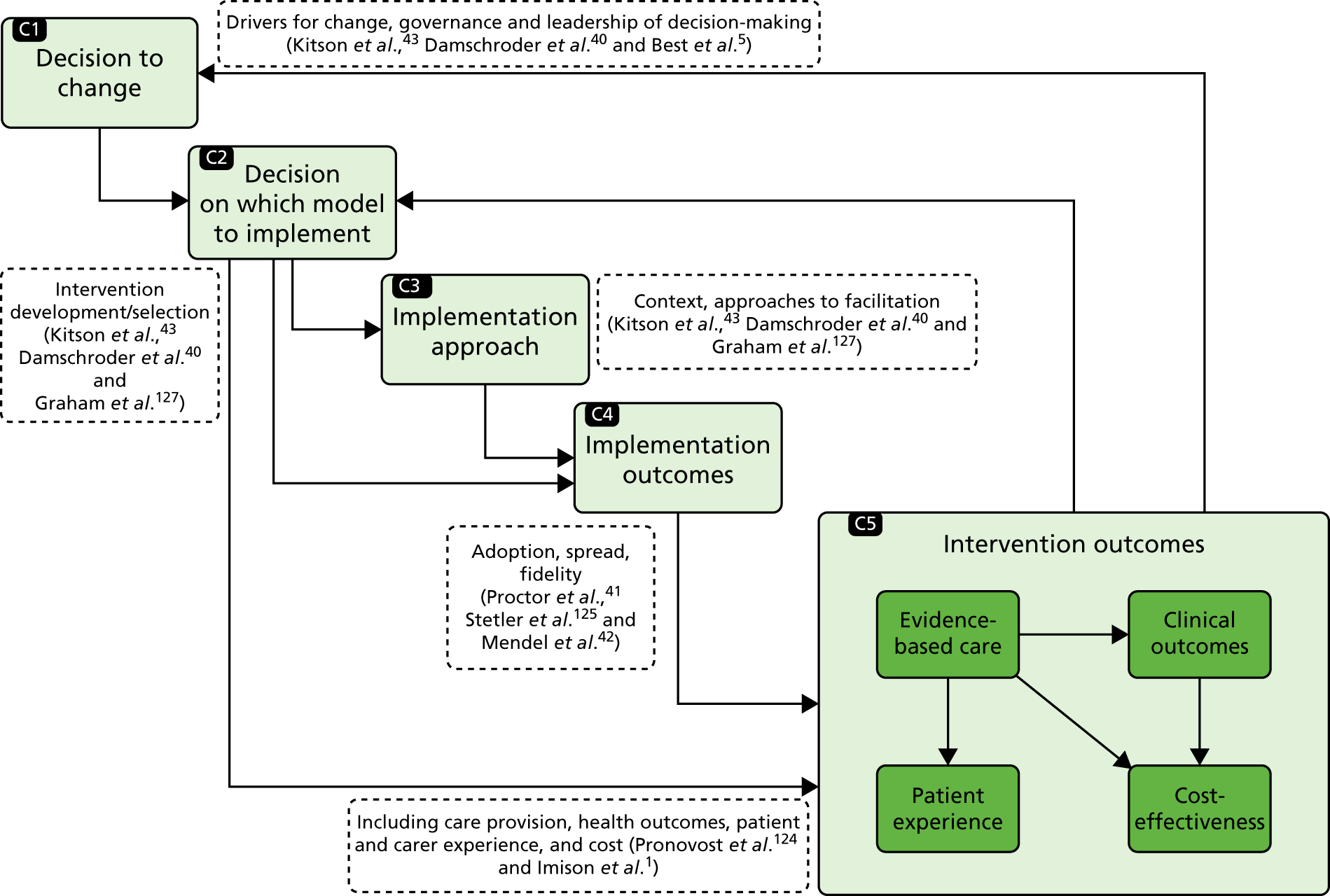
The decision to change [e.g. the drivers for change, governance and leadership of the decision-making process; see Component (C) 1, Figure 10] may influence the nature of the model (i.e. the intervention) that is implemented (C2). 5,40 Through processes of adaptation, both contextual factors (e.g. managerial capacity to lead change) and the model selected (e.g. the scale of change required) may influence the implementation approaches used (e.g. the degree to which local staff may require hands-on support in managing change) (C3). 43,127 Through both its complexity and its compatibility with the context of its introduction, the model selected may also influence implementation outcomes in terms of uptake and fidelity. 41,126 The model may influence intervention outcomes directly, although it is important that the extent to which the effects of the model are mediated through the process of implementation be considered. 41 Implementation approaches, such as how change is facilitated and local staff are supported (C3), have the potential to influence implementation outcomes (C4). 5,43 Implementation outcomes (C4) are likely to influence overall intervention outcomes, including provision of evidence-based care, clinical outcomes, patient and carer experience and cost-effectiveness (C5). 41 Finally, assessment of implementation outcomes may prompt a decision to change again and implement amended or alternative models. 127 The relationships between these components are unlikely to be linear; some (e.g. C1–3) may occur simultaneously and some components may be bypassed [e.g. model characteristics (C2) may influence implementation outcomes (C4) directly].
Major system change in Greater Manchester and London acute stroke services
This analysis was conducted having established the following (Figure 11): the drivers for MSC in both regions included national policy and local awareness of variations in and overall quality of acute stroke care provision;110 there were important differences in how the decision to change was led and governed, how local resistance was managed (see Figure 11, C1)110 and how these influenced the models selected (C2);110 the London and GM changes were associated with different intervention outcomes [London patients were significantly more likely to receive evidence-based care than patients in GM (C5)];26 and only London was associated with significantly greater reduction in stroke patient mortality than other urban regions of England (C5). 25
FIGURE 11.
Previous findings on MSCs in London and GM stroke services. NSD, no significant difference. Adapted from Fulop et al. 123 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
In this chapter, we present a comparative study of these two MSCs, examining the relationships between implementation approaches employed and the implementation outcomes (see Figure 11, C3 and C4). We address how implementation approaches and implementation outcomes were influenced by differences in the model selected (see Figure 11, C2), and how they influenced the differing intervention outcomes (see Figure 11, C5). This analysis contributed to an understanding of the relationships between the service models selected and implementation approaches applied, and how these each influenced implementation outcomes and intervention outcomes (see Figure 11, C3–5). 131
Method
Study design
We focused on GM and London as the only examples of changes of this kind being implemented at such a scale at the time. 36 Qualitative fieldwork, combining documentary analysis and interviews, was undertaken at ‘governance’ and ‘service’ levels to compare the implementation of the changes in the two regions.
Data
We combined analyses of semistructured stakeholder interviews and documents. We conducted 125 semistructured interviews with stakeholders at governance (n= 45) and service (n = 80) levels over the period April 2012 to December 2013 (see Table 2, and Chapter 2, Understanding development, implementation and sustainability). Interviews at governance level covered background to the centralisations (including drivers for change), governance, developing the proposal for change, agreeing the model, implementing changes, the impact of centralisation and reflections on the changes (see Appendix 5). Interviews at service level covered background to changes, processes of service development, the impact of centralisation and reflections on changes (see Appendix 5). In addition, 653 documents were collected from governance and service levels (see Table 2 and Chapter 2, Understanding development, implementation and sustainability).
Analysis
We compared the London and GM changes in terms of the implementation approaches employed and the implementation outcomes. Findings were considered in relation to our previously published findings, that is, the different models implemented110 and their differing impact on intervention outcomes (likelihood of patients receiving evidence-based care26 and patient mortality25).
Data analysis from interviews and documents combined inductive and deductive approaches,112 as themes were drawn from our framework (see Figure 10) and emerged from the empirical data. Documents were analysed to identify various aspects of the changes, including drivers, key events and activities, and overarching chronology. Interviews were analysed to draw out similar information and to understand why and in what ways aspects of implementation were influential, in order to compare the two regions. Analysis took place in two phases, building on the narrative summaries and timelines of the changes developed from documentary analysis used in a previous analysis. 110 In phase one, service-level narrative summaries were developed, using the constant comparative method,132 from documentary evidence and initial readings of interviews. These were developed separately for the changes in London and GM (by the authors AIGR and CP) and covered a number of cross-cutting themes: service-level context, service development processes (including thrombolysis and repatriation protocols, recruiting and training staff), launching new services and perceived impact of changes. In phase two, we used the overall timelines and summaries and service-level summaries to identify key tasks in implementing the models in each region, and contrasts in how these tasks were accomplished. In phase three, a subgroup of the authors (CP, AIGR, SM and NJF) applied the framework (see Figure 10) to a cross-region analysis that sought to test explanations of the differing implementation outcomes identified in previously published quantitative analyses. This phase drew on further thematic analysis of interview and documentary data to identify factors influencing the contrasting implementation approaches, and how the approaches may have influenced the resultant outcomes.
To enhance reliability, emerging findings from each phase were shared and discussed regularly with other co-authors until agreement was reached. To enhance validity, an interim version of this analysis was shared with people who had been involved in the planning and implementation of the changes in London and GM (some of whom we had interviewed for this study).
Results
We present our findings in three sections: factors that influenced implementation approaches, factors that influenced implementation outcomes and understanding outcomes of MSC. The key relationships are summarised in Figure 12.
FIGURE 12.
Findings in relation to MSC in London and GM stroke services. NSD, no significant difference. Adapted from Fulop et al. 123 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
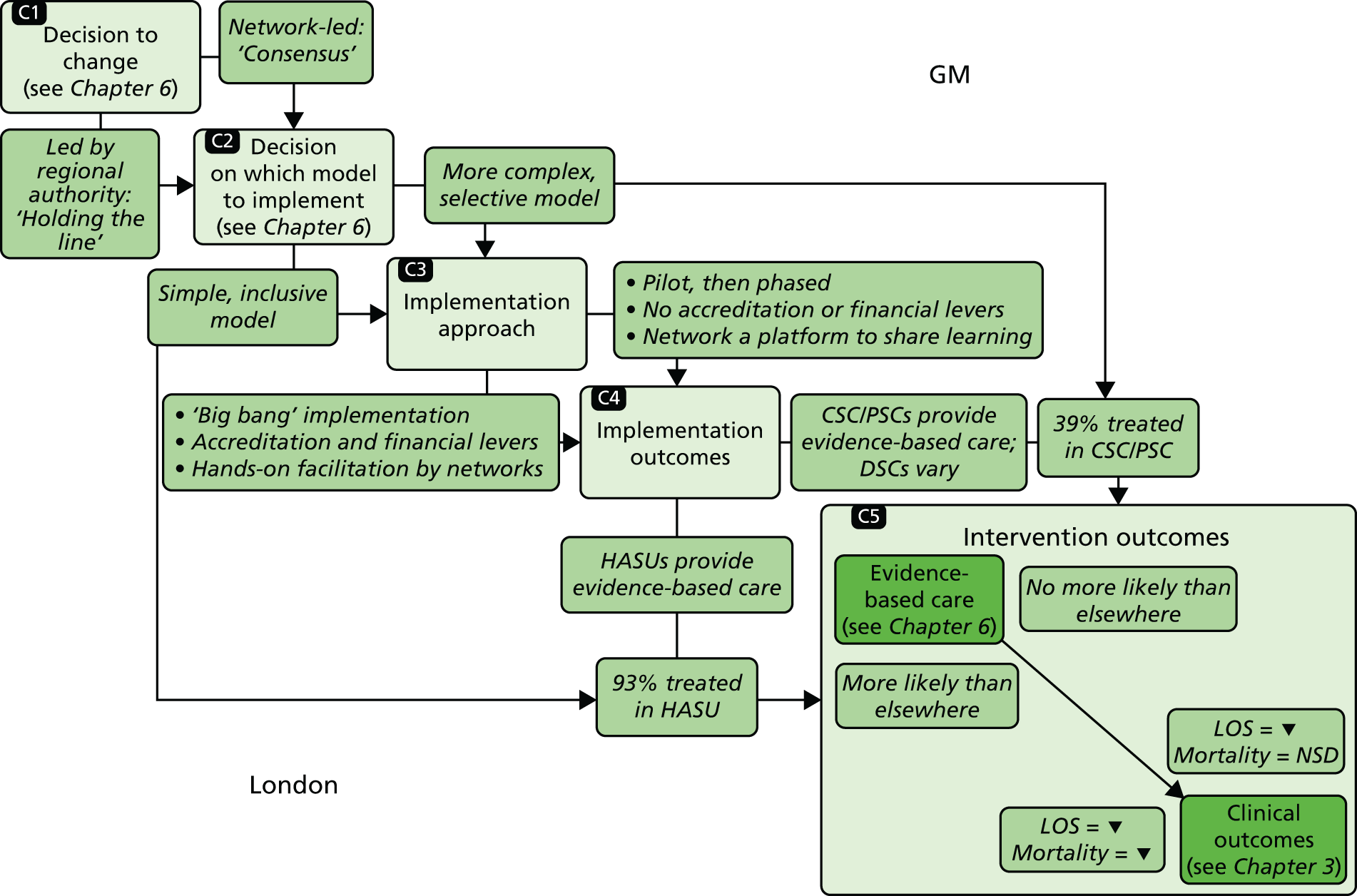
Factors influencing implementation approaches
The approaches to implementation (Figure 12, C3) differed across the two regions on the basis of the degree to which it was phased, the degree to which it was linked to standards set out in service specifications and financial incentives, and the degree to which networks provided hands-on facilitation.
Degree to which implementation was phased (‘big bang’ versus ‘phased’)
London applied a ‘big bang’ approach, with a single ‘launch’ date for the whole system, the time from which all suspected stroke patients were to be transferred to a HASU, regardless of whether or not they were eligible for thrombolysis. 110 The single launch date grew from the view that the changes had to be pan-London in order to ensure system-wide clarity about the model:
. . . if we started having north-east London going off in one direction about some particular aspects of care and south-east doing something a bit different then you very quickly lose the coherence.
Lon05, stroke physician
The launch was postponed by several weeks to ensure that all services developed adequate capacity to launch simultaneously, meaning that all potential stroke patients in London could be taken to HASUs (London Network Board minutes, January–July 2010).
The London Stroke Project Board recognised that the ambulance service was central to agreeing the launch date:
The Chair closed the discussion stating that the timing of the opening of HASUs needs to be agreed with London Ambulance Service.
Minutes, GM extraordinary Stroke Project Board meeting, June 2009
In GM, service development took place over multiple phases. Changes to the referral pathway were piloted around the CSC as it developed, for example in terms of thrombolysis and repatriation processes. The referral pathway then altered several times over the course of implementation, as the remaining PSCs launched and gradually extended their catchment areas (Stroke Project Board minutes, December 2008–April 2010). The network was aware of these changes, but they did not co-ordinate them, instead communicating with ambulance services to ensure their awareness of each change. This phased approach reflected a desire to minimise risk to vulnerable patients by ensuring that the referral pathway worked before scaling up to cover the whole system:
. . . you could become completely overwhelmed and the whole thing might just collapse.
GM05, stroke physician
Use of service specifications and financial incentives
In both regions, service specifications were developed by local clinicians and defined appropriate staffing, infrastructure, education, training and audit processes. However, the London specifications quantified in greater detail how these services should be delivered (e.g. by identifying the number of specialist nursing and therapy staff required at different times of the day). Although standards were used in service selection processes in both regions, only in London did the launch of services depend on these standards being achieved, assessed through a formal accreditation process.
In London, standards were linked to financial incentives, whereby receipt of enhanced funding for stroke services (the ‘stroke tariff’) was conditional on meeting these standards. Following the launch, services were required to meet additional standards reflecting further service developments (achieving a locally defined ‘gold standard’); subsequently, services were reviewed on an annual basis to assess whether or not standards continued to be achieved (if not, provisions were in place for the tariff to be ‘clawed back’). This approach gave change leaders in London a degree of control and assurance that services were likely to provide evidence-based care.
In GM, although commissioners endorsed the changes, payment was not associated with meeting standards on the basis that this might be seen as punitive and inconsistent with the collaborative approach employed. This reduced the need to prioritise standards as a basis for the launch of the new services. 110
Degree of hands-on facilitation by networks
The networks had an important role in facilitating the changes. In both regions, they hosted regular meetings at which staff shared their learning from ongoing service development, for example in relation to developing thrombolysis pathways and managing the transfer of patients from the HASU to their local hospital. The focus on learning derived from the fact that the changes represented an attempt to standardise and integrate stroke care across what were, at the outset, relatively fragmented systems. The benefits of this were described by a member of the GM network:
. . . so much learning came out of it through this . . . informing how the model should look and the paperwork, the communication protocols, the Standard Operating Procedures between, you know, it was all very emergent.
GM07, network representative
Significantly greater ‘hands-on’ facilitation was provided in London. This took the form of network staff project-managing and measuring service development throughout the implementation phase. Network staff engaged actively with senior hospital management and network leadership when implementation was not running to schedule, and in one case a HASU lead was brought in to guide the development of another HASU that was making limited progress. Network staff referred frequently to the pressures of service development and their role in achieving it:
The Programme Board was quite unrelenting really about, ‘these are the targets, we’ve got to hit them’.
Lon17, network representative
We were there to remind them of what they had signed up to, to remind them of what they had committed to do and to remind them of the quality standards that they needed to meet, but always in a supportive manner.
Lon06, network representative
This approach was driven by the tight timeline for a single launch date, linked to achieving service standards: this justified the network providing staff to carry out this intense facilitation approach.
In contrast, the GM changes had no explicit deadline by which all services had to achieve local standards or to launch, and the network did not provide dedicated project management support. Overall, these characteristics indicate that implementation in GM was less actively facilitated by the network, reflecting their view of implementation as a collaborative endeavour, to be led by the services themselves:
I don’t know whether it was an unwritten principle, it probably wasn’t a written principle but actually what we do is hold consensus and try and deliver this through unanimity.
GM04, commissioner representative
Factors influencing implementation outcomes
As previously established (see Figure 11), there were differences, first, in implementation outcomes (i.e. greater fidelity to the referral pathway in London than in GM) and, second, in the greater likelihood of providing evidence-based care in London than in GM (with provision equally high in London HASUs and GM CSC/PSCs, but lower in GM DSCs). 26 We first discuss factors influencing fidelity to the referral pathway (model complexity, ‘big bang’ vs. phased implementation, and the degree of ‘hands-on’ facilitation by networks) (Figure 12, C4). Second, we discuss factors influencing service development (use of service specifications and ‘hands-on’ facilitation). As set out in the preceding section, many of the factors influencing implementation outcomes related to implementation approaches, including the degree to which implementation was phased, the use of standards and financial incentives and the degree of hands-on facilitation.
Fidelity to the referral pathway
Fidelity to the referral pathway was influenced strongly by how consistently it was understood by health-care staff. Understanding of the referral pathway was influenced by the complexity of the models (number of decisions relating to patient transfer) and the number of phases in which these models were implemented.
Influence of model complexity on fidelity to the referral pathway
The models implemented in London and GM differed in complexity. In both regions, the majority of stroke patients were transferred to hospital by ambulance. Ambulance representatives in both regions were consistent in making clear their preference for a simple model:
. . . we cannot give crews fragmented messages, you can’t say that you can get this type of care between 8 and 5 Monday to Friday but not on the second Wednesday of the month because there’s a meeting, crews don’t work that way.
LonAmb01, ambulance service
The difference between the 24-hour pathway in London and the 4-hour pathway in GM resulted in additional decision-making for ambulance crews in GM. As well as deciding on stroke diagnosis, they had to consider time of onset, and whether or not it would be possible to transfer the patient to a CSC/PSC within 4 hours of onset. As a result, a patient’s destination for care depended on potentially uncertain information:
We need to have a definite time of onset . . . or the time when they were last seen well, and if that time exceeds the four hours then we won't be taking them to the Hyper Acute Stroke Unit.
GMAmb02, ambulance service
Although all London HASUs admitted patients 24/7, the two GM PSCs operated an in-hours service only. As noted by a representative of the ambulance services in GM, this may have made it more difficult to know where to take patients:
Time’s always a challenge: between that time and that time they’ll go there, all the rest of the time they’ll go somewhere else. And that’s . . . that’s never, never easy to communicate or for people to remember.
GMAmb01, ambulance service
In addition, some hospital staff indicated uncertainty about the GM referral model overall:
I don’t understand who’s supposed to be going here and who’s supposed to be going there, and if I don’t, I bet other people don’t know.
GMH06, stroke physician
Influence of ‘big bang’ versus phased implementation on fidelity to the referral pathway
Ambulance staff indicated a strong preference for the ‘big bang’ approach employed in London:
The one thing that we really did push for was a ‘go live’ date, not a ‘go live’ date in one area and another in other areas.
LonAmb01, ambulance service
Ambulance staff in GM suggested that the many changes made to the referral pathway over the course of implementation may have contributed to uncertainty and, thus, limited fidelity to the referral pathway:
If you phase it, it does create a degree of confusion. Because you start off with something, and then you change it, and then you change it, and then you may change it again.
GMAmb02, ambulance service
Influence of ‘hands-on’ facilitation by networks on fidelity to the referral pathway
The ‘hands-on’ facilitation provided by networks in London supported fidelity to the referral pathway. A key example was provision of training for ambulance staff to ensure clear understanding of the pathway:
It’s not just the people on the road that need to understand that, it’s people in the control room as well, so they’re familiar . . . So there’s the protocol and then there’s the training to support that.
Lon18, ambulance service
Another task in pursuing fidelity to the referral pathway in London was ensuring that patients were not treated in hospitals that were no longer providing stroke care. A hospital where stroke services had been decommissioned had been continuing to receive stroke patients. To address this, a meeting with staff was organised:
Somebody from the Stroke Network came to speak about ‘. . . we are not meant to be treating any stroke’ . . . So if you are here and you develop a stroke, your thing is to get you to [local HASU], rather than as I said, ‘We’re going to go and scan you first’ . . . As a consequence of that, they’ve all gone . . .
LonE02, senior management, decommissioned service
In GM, audit data indicated that a significant proportion of patients eligible for treatment in a CSC/PSC were not being treated in one, reflecting concerns raised by clinical leads in oversight meetings (meeting minutes, 2009–10). At the time of the GM 12-month review of the centralised system, it was noted that the network was working with both hospital and ambulance staff to corroborate data and identify potential solutions. 32
Service development processes
Reflecting the extent to which implementation was actively managed overall, service development in London and GM was influenced both by the levels to which service specifications and financial incentives were used and by the degree to which facilitation of service development was ‘hands-on’.
Influence of service specifications and financial incentives on service development
The London specifications presented standards that made clear what services had to provide, and the requirement to meet these standards was seen as an important driver for senior management to support these services:
In some respect in terms of staff and sort of thing, it was taken out of our hands because the standards just lay it down, this is what you need for X number of beds.
LonA08, HASU physiotherapist
Having to meet all these standards for assessment, it’s been a real driver for change and improvement. I think the reconfiguration has provided a stick for hospital management to invest in stroke services.
LonD02, SU stroke physician
In GM, standards were not linked to financial incentives, nor were they used as a criterion for the launch of services; this may have contributed to DSCs not providing the planned level of evidence-based care.
Influence of ‘hands-on’ facilitation by networks on service development
As services developed in London, the network’s ‘hands-on’ approach to facilitation was perceived by local staff as valuable in addressing difficulties:
When we had problems, they [the network] wanted us to call them and say, ‘You know what, we’re a bit stuck here, what can you do to help?’ . . . ‘is there experience you have from another site that might be helpful?’. I think we developed a very good relationship with them, and that was obviously key to, you know, opening the HASU.
LonC11, HASU service manager
Furthermore, the ‘hands-on’ approach to facilitation influenced the timing of London’s ‘big bang’ launch. For example, it was only through this ongoing local engagement – and responsiveness to progress that was being reported – that the initial timescale for a co-ordinated launch was altered.
In GM, the networks facilitated learning across services but did not provide staff to support service development, which may also have influenced provision of care in DSCs. This may have derived from the perception of comparatively limited resources dedicated to the GM centralisation:
I heard that they [London] have £2.50 spent for every £1 spent in Manchester. As I say I don’t know if that’s accurate but it would seem that the financial thing wasn’t as such a consideration in London . . . but it was a factor in Manchester.
GM07, network representative
Understanding outcomes of major system change
In this section, we bring together the current findings with those from previous analyses to illustrate how components of MSC (see Figure 10) contributed to the significantly different outcomes associated with the changes to acute stroke services in GM and London. These relationships are summarised in Figure 12, and described below.
The changes in London and GM appeared to be influenced significantly by the degree to which change leaders ‘held the line’ on the models to be implemented (see Figure 12, C1 and C2). 110 The models implemented and the approaches employed played an important role in the outcomes observed.
London’s inclusive 24-hour model (i.e. all suspected stroke patients were eligible for HASU), requiring relatively few referral decisions to be made, increased the likelihood of staff following the referral pathway. In contrast, GM’s 4-hour model was significantly more selective (limiting the number of patients who were transferred to a CSC/PSC) and complex, increasing uncertainty among staff about where suspected stroke patients were to be treated (see Figure 12, C2). Furthermore, these models were implemented differently, reflecting a contrast in the degree to which implementation was actively managed in the two regions (see Figure 12, C3). London adopted a ‘big bang’ approach whereby the new system was launched on a single date, increasing the likelihood of the referral pathway being followed. This launch was dependent on services being accredited against standards linked to financial incentives, increasing the likelihood of services providing evidence-based care. Significant ‘hands-on’ facilitation was provided by the London network to ensure that services met the required standards. In GM, services launched in multiple phases, which limited confidence in the referral pathway, and service specifications were not linked either to service launch or to financial incentives; this may in part have limited development of DSCs.
Implementation outcomes (see Figure 12, C4) had a significant influence on intervention outcomes (see Figure 12, C5). Almost all London patients were treated in a HASU, and all HASUs were likely to provide evidence-based care; this meant that London patients were overall more likely to receive evidence-based care and in turn had a larger reduction in mortality than patients in GM and RoE. In contrast, GM patients were far less likely to be treated in a CSC/PSC, with two-thirds treated in DSCs, which were significantly less likely to provide evidence-based care; as a result, GM patients’ likelihood of receiving evidence-based care, and associated mortality, did not differ significantly from in the RoE. 25,26 The 12-month review in GM noted national audit data indicating that DSCs were providing evidence-based care less frequently than CSC/PSCs. 32 Based on this information, and discussion with an EAG, it was agreed that further reconfiguration of acute stroke services should be explored. 110
Discussion
Principal findings
This chapter examined the complex, non-linear relationships between the type of model selected, implementation approaches, implementation outcomes and intervention outcomes. By analysing the centralisation of acute stroke care in two regions, distinguishing between implementation outcomes and intervention outcomes (following Proctor41), we made a significant contribution to the understanding of MSC,5 specifically in terms of the factors influencing outcomes. 1 We demonstrated a number of inter-related factors that potentially influenced such outcomes (as detailed in Chapters 3 and 425,26). Certain characteristics of the intervention and implementation approaches were associated with more positive implementation outcomes and intervention outcomes, and many of these reflected existing implementation and diffusion theories, described in Comparison with other studies below. 5,40–44,126–128 In turn, the service model and implementation approaches were influenced significantly by leadership and engagement factors described in Chapter 6. 110
Strengths and weaknesses
A key strength of this analysis is that it took a theory-driven approach to analyse two cases of MSC or MSC across two geographical regions in order to establish the relationships between the planning, implementation and outcome of MSC. This analysis had a number of limitations. First, because this research was retrospective in nature, interviewees were looking back on the changes, with some awareness (e.g. by monitoring national audit data) of the degree to which changes had succeeded in influencing the provision of evidence-based care. This may have affected the way in which they articulated their views of the implementation of the changes. Future research on changes of this kind would benefit from being carried out contemporaneously, ideally from the pre-implementation stage, and extending over a sufficient time period to allow a formal evaluation of the impact on intervention outcomes, such as patient mortality. Second, although we believe that our data indicate that the identified implementation strategies played a significant part in the implementation outcomes observed, the relative contribution of each component cannot be established. Third, we sampled only a proportion of services in this study, and other factors may have been important to implementation in other services in the reconfigured systems. However, we believe that by conducting interviews at the pan-regional level, and sharing findings with local stakeholders, our findings provide a strong representation of implementation in both regions. Finally, studies of MSC in other acute (and non-acute) care settings would be of value to aid identification of potentially generalisable lessons.
Comparison with other studies
In terms of intervention characteristics, we found that in this case ‘simpler’ and more inclusive referral pathways (such as London’s 24-hour model) were more likely to be understood and followed by both hospital and ambulance staff. This effect might reflect such established concepts as ‘feasibility’,41,128 ‘compatibility’126 and ‘complexity’,40,126 whereby an intervention is more likely to be adopted if it is readily incorporated into existing or standard activities.
The concept of ‘execution’ (i.e. where implementation is achieved as intended40) was highly relevant to this analysis. In terms of timeliness of implementation, the advantages of a ‘big bang’ launch and associated planning, and the disadvantages of phased implementation, were clear: a single launch date gave clear understanding across all stakeholders when implementation was complete. This finding was potentially counter-intuitive, given previous research indicating risks related to ‘big bang’ implementation. 133 However, in changes such as these, when service models have multiple interdependent components, a ‘big bang’ approach appears to be beneficial and represents an example of ‘adaptation’,43,127 whereby an implementation approach is selected to reflect the scale of the task and the complexity of the new system. Further research is required to establish the extent to which this finding applies to similar changes in different health-care settings. Also associated with ‘execution’ was the use of standards: linking the launch of the new model to achieving standards appeared to increase the likelihood of there being uniform capacity to provide evidence-based care and also gave a shared understanding of what had to be delivered in all services. By associating the achievement of standards with financial incentives, the London changes reflected the hypothesised benefits of altering remuneration to encourage adoption of the intervention. 127 The contribution of ‘hands-on’ facilitation, for example by external change agents, to implementation outcomes is acknowledged by various implementation frameworks. 40,43
These differences in relation to a ‘big bang’ versus phased launch, the use of standards and hands-on facilitation reflect an underlying contrast in the implementation approaches adopted in our studied regions, with implementation in London facilitated significantly more actively than in GM.
Implications
This chapter used a framework that draws on key features of change identified in existing implementation theory to analyse two examples of MSC in acute stroke care. We found that the model selected (in terms of simplicity and inclusivity) and implementation approach (launch date, prioritisation of standards, financial incentives and hands-on facilitation) made significant contributions to implementation outcomes observed, and in turn intervention outcomes. 25,26 We explore these factors further in Chapter 10 (in relation to efforts to implement change across the Midlands and East of England), Chapter 13 (in relation to further centralisation in GM) and Chapter 14 (in relation to the sustainability of the London system).
We believe that this analysis has demonstrated the value of considering the interdependencies between intervention, implementation approach and outcomes when planning and evaluating MSC. However, the particular relationships identified in this analysis may vary depending on the nature of the change being implemented. The framework described in this chapter would be strengthened through further use in evaluating MSCs conducted in other health-care settings.
Chapter 8 Patient and public involvement in the planning of major system change
Overview
This chapter draws on a paper published by McKevitt et al. 134 © 2018 The Authors. Health Expectations published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
What was already known about this subject?
-
Patient and public involvement is required where changes to care provided by the UK NHS are proposed. The manner in which involvement should be put into practice is not prescribed, and there is little evidence about which methods should be used in which circumstances. Therefore, involvement is characterised by ambiguity about its rationales, methods and impact.
-
Our analysis of the planning and implementation of the GM and London changes (see Chapter 6) indicated that PPI was used instrumentally by programme leaders to demonstrate support for proposals rather than with the intention of developing co-designed services.
What this chapter adds
-
The study adds new empirical knowledge to the relatively small body of literature on involvement practices in MSC.
-
Lay involvement was enacted through consultation exercises, lay participation in governance structures and the elicitation of patient perspectives.
-
Interviewees’ views of involvement in these MSCs varied, reflecting different views of involvement per se and of implicit quality criteria.
-
The value of involvement was felt to lie not in its contribution to acute service redesign but in how involvement practices facilitated its implementation. Our analysis identified three types of processes – agitation management, verification and substantiation – through which this was achieved.
Background
Internationally, citizens, patients and family carer-givers are increasingly being positioned as collaborators in the labour required to produce and maintain health. 135–139 This includes involvement in individual patient care, research, and planning, development and improvement of health services. 140–142 Carman et al. 143 argue that such involvement challenges a dominant paternalistic approach to health care and systems and has the potential to transform patients, improve outcomes through better systems of care and reduce health-care costs. Yet involvement concepts and practices are characterised by ambiguity in terms of terminology,144,145 rationales,146,147 values148 and the attributes of those who are invited or choose to be ‘involved’. 146,149 The literature also suggests an anxiety that involvement aspirations are not being met, because involvement practices are tokenistic rather than meaningful. 150–153 What makes involvement meaningful or tokenistic has hardly been explained but failure to involve stakeholders in a ‘meaningful’ way has been attributed to the different types of knowledge associated with experts and lay people,154 and the privileging of expert over experiential knowledge. 155 Involvement methods used have been criticised for their failure to adopt democratic models,153 ensuring that traditional models of decision-making are maintained. 152 Evidence on whether or not the anticipated benefits of involvement in research and service development have been realised is limited, raising questions about not just how to evaluate impact but what impacts should be considered. 156–158
Our analysis of the planning and implementation of the changes in London and GM (see Chapter 6) suggested that, overall, PPI was used instrumentally by leaders, in order to demonstrate support for their proposals. In this chapter, we considered the practices and achievements of PPI in relation to the London and GMA centralisations, in order to provide a detailed understanding of how lay involvement was developed and enacted in London and GM changes, and the factors influencing this. We examined how the requirement to involve patients, carers and the public34 was put into practice during implementation, and how involvement, and its value, was represented in interviews with those involved in service redesign. The meanings ascribed to involvement, we shall argue, lay not in the diverse values thought to underpin involvement148 but in the production of value arising from performances of involvement.
Method
In this chapter we focus particularly on interviewees’ accounts of how PPI was enacted during the process of preparing for and implementing the service changes (for details of recruitment and data collection, see Chapter 2, Understanding development, implementation and sustainability). Interviews used a topic guide (see Appendix 5) designed for the study; they were audio-recorded and professionally transcribed in full. Analysis was both inductive and deductive, as themes drew on both the a priori questions from the topic guide and those emerging from the data. Following initial coding and category building to develop themes, findings were refined in group discussions among the authors Angus Ramsay, Catherine Perry, Simon J Turner, Naomi J Fulop, Ruth Boaden and Christopher McKevitt. Initial findings were also shared with the study advisory group to check for accuracy and the face validity of the interpretation being proposed.
Results
Participants
In-depth interviews were conducted with 17 people in GM and 26 in London who were involved in the governance of the changes (see Table 2).
Overall, participants were aware of the need to involve patients and public in the proposed changes, confirming the view that PPI has acquired normative status among professionals in the NHS. Interview data also suggested that there were concerns about the changes from the outset. Hospitals and professionals were concerned about implications of the new models of care in terms of loss of resources and, in London, about the closure of five acute stroke wards. There were concerns about patient safety and acceptability because the new models meant journeys for some patients beyond their locality, as well as transfers from one site to another. The need to engage all types of stakeholder was recognised if the proposed changes were to be implemented.
Involvement practices
At both sites, multiple strategies were used to involve patients at a range of levels. Existing resources were accessed (such as NHS involvement managers and individual voluntary sector organisations); social and other media were used to invite patients to participate. In London, an involvement strategy was developed that outlined plans to access a range of public and patient groups and highlighted the need to access ‘hard to reach’ groups. Interviewees in both areas identified different types of activity through which patients and public were involved, namely stakeholder events and consultations, lay membership of governance structures and the formal elicitation of patient perspectives. No similar single strategy document was developed in GM but project documents, such as that detailing the governance framework, identified the organisational structures through which PPI, and public awareness and education, would be developed and implemented. 159
Information and consultation events
In GM three ‘stakeholder engagement events’ were organised over 8 months between 2007 and 2008, as service development plans were initiated and took shape. The aims of these events reflected the development of plans for service change. Initially the events aimed to get agreement about the need for change, then to inform stakeholders about the planned changes, and then to inform them about progress. Involvement was also described as making use of existing PPI structures, such as Stroke and Cardiac Networks and NHS Patient Advisory Liaison Services, and public health campaigning work into which information about the stroke service changes could be incorporated.
Consultations in London were much more extensive than in GM because these took place under the aegis of the larger region-wide review of health needs across a range of conditions and of current provisions of health care. The well-funded consultation programme, Healthcare for London – Consulting the Capital,35 sought views of members of the public on the range of proposals put forward in the document, Healthcare for London: A Framework for Action. 28 The consultation process was developed, overseen and analysed by Ipsos MORI (London, UK), and used a range of marketing and opinion-seeking activities, including online media, presentations to lay and professional organisations and consultation roadshows at health authority level. 160 The formal consultation received 4734 responses (in online, written, e-mail and other formats) from a wide range of stakeholders that included not only individual members of the public but also local voluntary sector organisations, local council members, health and other professional bodies and local politicians. Comparison between individuals who took part and the London population found some differences with the over-representation of women and older people; however, ‘the ethnic profile of respondents was broadly representative of Londoners’161 (p. 17).
The consultation had a clearly defined scope, concerning ‘Adult services for acute stroke care – explicitly the location and coverage of hyperacute services and acute services in London’. In response to the question about the proposal ‘to create more specialised centres for the treatment of severe injury, stroke and complex emergency surgery needs’ 42% of respondents ‘strongly’ agreed with the proposal to create more stroke specialist centres, and 25% tended to agree. 35
A separate consultation process was subsequently held, which focused on proposed changes for the acute stroke and trauma service, at a cost of £1.2M. Again, this entailed preparation of consultation materials, numerous public events and the formal elicitation of views. It was estimated that participation in the consultation involved 14,000 individual visitors to the website, 13,000 visitors to health fairs and around 14,000 people attending meetings. Responses to the consultation document were received from 8100 individuals and 200 organisations. It was reported that 67% of respondents strongly agreed/tended to agree with the proposed model of acute stroke care that had been developed (i.e. that ‘about seven hospitals’ across London should provide HASU care). 160
Participation in governance structures
Interviewees pointed to lay membership of governance bodies as a way in which involvement policy was implemented. In GM, a local activist (the spouse-carer of someone who had had a stroke in the past), well known to professionals leading the service change, was invited to join a working group and the Stroke Network Board to which it reported. An employee of the Stroke Association and an NHS network PPI manager were also members of this board. Similarly in London, Stroke Association (a charity sector organisation and service provider) employees sat on the project board and Clinical Expert panel, which designed the service specifications to provide patient/carer representation. In addition, a Stroke Patient and Carer subcommittee was established to discuss topics including approaches to PPI, the running of consultation events and the development of the new model of care, and to feed back to the user groups they represented. This subcommittee met four times during the life of the development process.
Eliciting patient perspectives post implementation
Interviewees also identified collecting patient and carer views of the new service as a form of involvement. For example, 12 months into the delivery of the newly reconfigured acute stroke pathway in GM, a review was conducted that included a separate study using a survey and a series of qualitative interviews to elicit patient and carer views of the new pathway. Stroke survivors were invited to take part online and via social media, and through contacts with and visits to support groups across the region. In total 84 people (10% response rate) returned the questionnaire, largely expressing the view that being admitted to a specialist acute centre, rather than the local hospital, was not a concern.
The quality of involvement
Our interviews did not ask participants to comment on whether involvement was meaningful or tokenistic, but many offered their own views of the quality of what was done, with wide variations in their appraisals.
Interviewees suggested that in GM there was little formal consultation of patients and the public. Public events were described as designed to provide information about the planned changes rather than to elicit views. For example, a commissioner said:
I don’t think at any stage we said to the public of Greater Manchester, if there’s such a thing – and there isn’t – we didn’t say, ’Do you want three of these, or five of these?’ We never said that.
GM01, Commissioner, GM
In London, where there were sustained efforts to hold formal consultations, two of three NHS managers interviewed were positive about the consultations because of the efforts made to encourage high levels of participation and to be inclusive. An interviewee from the voluntary sector challenged this view, arguing that consultation was not sufficiently accessible to people with stroke-related aphasia, for example. Others suggested that the consultation was useful because it secured buy-in from the public or ‘political legitimacy’. However, one commissioner, despite conceding that consultation was necessary because of societal expectations of transparency in public services, also expressed concerns about the limits of the public’s knowledge to comment on proposed new plans. Others, including a commissioner and two doctors, either were dubious, expressing doubt about the usefulness or validity of consultation, or considered it ‘a complete waste of time’ (L02) because consulting the public had eclipsed the need to consult more widely with professionals providing long-term services to stroke survivors.
There were also different evaluations of individuals’ contributions to governance structures. Several interviewees from GM spoke about the lone activist who was appointed to the project board because of previous professional political experience, his ability in committee work, his history as a campaigner for stroke service quality and even his willingness to challenge others. Others rehearsed well-known arguments about the limits of individuals’ contributions based on the fact that they were self-selected, or the ‘usual suspects’ and, therefore, unrepresentative, or because they lacked experience in formal committee work.
The limits of how actively involved lay people could be in this involvement process were also recognised. For some, this was related to the nature of MSC itself. It required knowledge of a range of complex problems such as population needs, resource implications and political implications. Because the designs had been worked out by a core group of professionals, there was limited opportunity for lay people to influence the service design. For some interviewees this meant that there was ‘no real involvement’, whereas others conceded that, although this might not be patient-led involvement, consultation processes allowed patients to become ‘advocates for the model’.
The trope of the patient voice also figured in interviewees’ accounts. Involvement was seen as an opportunity for the patient voice to be articulated, represented by interviewees as an important corrective to the dominant perspectives of professionals and organisations. For example, one said:
People (professionals) have to, really have to be brought back to what’s best for the patients and an awful lot of what gets discussed in the NHS is not about that, it’s about what’s best for my organisation.
GM05, stroke physician, GM
However, a minority of interviewees argued that although patient voices may have been heard, they could not be acted on within the scope of the consultation activities, which were specifically limited to the MSC projects’ redesign of acute stroke services. Nevertheless, these interviewees noted that patients took the opportunity to articulate another concern, namely the quality of rehabilitation services. One interviewee clearly made this point:
. . . every single question which was asked in the half hour or so that the meeting was thrown open for questions from the floor was about rehab [rehabilitation] . . . Nobody asked a question or protested about the decisions we were making about the hospital service but all the members of the public and their representative questions were about rehab.
Lon03, project team, London
Patients may have been content to leave acute service design to professionals who carefully made a convincing case that centralisation was able to deliver better quality acute care for all. Yet the question of rehabilitation services was outside the brief of change leads, and even when involvement permitted dialogue between stakeholders, the question of rehabilitation and longer-term care was inadmissible.
Constructing value
It could not, therefore, be argued that involvement in these examples of MSC influenced or improved the design of the acute stroke services. As we have previously argued, involvement was used instrumentally by programme leaders to gain support for change, the case for which had already been made, and for service models already developed. 110 For a minority of interviewees, this indicated a failure to achieve an ideal of patient-led involvement but, for most, even if flawed, the practices of involvement had intrinsic value for the implementation of MSC. We identify three types of value suggested by the interview data; these are summarised in Table 15 and discussed in detail in the following sections.
| Types of value | Perceived effects |
|---|---|
| Managing agitation |
Anticipates organised opposition Pre-empts organised opposition |
| Verification |
Pre-empts patients’ concerns Information reflects patient experience |
| Substantiation |
Patient voice is heard Brings patient into the room Reorients discussion to patient needs |
Managing agitation
First, involvement was represented as a way of managing actual or potential resistance or agitation. 162 Above, we report the significance attributed to a lone activist in GM who had a track record of agitating for improved stroke services after his wife’s stroke. Although interviewees recognised his expertise, he was also described as ‘grit in the system’, that is, an irritant that produces change. In this case, it could be argued that there was an effort to manage an activist’s agitation by incorporating him into official PPI structures.
At a broader level, we have already seen that MSC leads were concerned that patients and families might object to an acute care model that saw the patient being admitted to a non-local hospital and care involving ambulance transfers from one hospital to another. Thus, involvement sought to anticipate and manage any dissent that might arise.
As Martin163 has argued, PPI could be a way of containing and managing citizens’ desires and action, yet PPI itself might give rise to unanticipated forms of agitation, as happened in two localities in London, where local people and politicians objected to the loss of local acute stroke services. Here the MSC leads could point to the consultation work conducted across London as gathering ‘overwhelming support from everywhere else’ (Lon03), thus trumping what could be portrayed as local interests. Indeed, the London model was consistently portrayed as pan-London, rather than locality based.
Verification
Involvement was also described as permitting what could be termed processes of verification, which took place at different levels. Interviewees suggested that involvement permitted the pre-empting of potential concerns that patients and their family members might have about being admitted to a specialist centre that was not their local hospital. This had been perceived as a potential source of disagreement or dissatisfaction among patients. As such, opposition remained limited, and MSC leads were able to verify that their proposed design was acceptable to patients. Moreover, interviewees cited examples of how lay people had been involved in the development of information materials. For example, the work in GM entailed the development of information for ambulance crews instructing them on the new procedures for suspected stroke admissions. Patient input was sought here on the development of scripts to be used by ambulance crew taking patients to a specialist centre, rather than the local hospital. Similarly, interviewees reported that involvement processes enabled them to be reassured that MSC was the right way to proceed. For example, one interviewee reflected that:
. . . it was really important to be able to have their voice, saying, ‘This is a good thing. It should be done’.
Lon03, project team, London
Substantiation
Finally, interviewees evoked an effect that we refer to as substantiation, namely making an idea physically present. Involvement processes enabled the service user simply to be present in the room (as Donaldson164 puts it), or at a public event. By being present, the service user embodied the ‘stroke patient’ as a representative in a symbolic sense rather than a representative in any population/demographic sense, in a way that was useful for a number of reasons. First, the physical presence of the patient relates to the normative status of involvement noted by previous authors; by being present in the room, patients provided physical evidence that involvement policy was being enacted. Thus, presence enabled demonstration of adherence to the NHS vision that health-care development depends not just on ‘technocratic intervention and political whim but also upon social values pertaining to equality, inclusion and social justice’. 165 A stroke physician described the development of a mission statement at the outset of the work, which asserted that the purpose of the MSC was to ensure equality of access to the best possible acute care for every stroke patient:
I thought we should be really clear what it is we were here for and we wrote it on a flip chart and it came out at every meeting and it got stuck up on the wall. Every citizen of Greater Manchester has equal access to high-quality acute stroke care. And actually that was really useful because when the arguments started you could then say, but how does that relate to our vision?
GM05, stroke physician, GM
Second, the patient’s presence in the room was used to manage conflict between professionals faced with decisions that might have consequences for individuals, services or localities. The patient in the room was used to remind stakeholders that the ultimate goal was to improve the quality of patient care:
So . . . we’re bringing the focus back to the patient . . . and what we’re here for. We’re not here to be arguing about politics and you know, who’s the best stroke physician and, you know, people’s ego; it’s about you know what would you want for your grandma or your mum if she had a stroke tomorrow.
GM13, stroke programme board member, GM
Whenever we had stakeholder events, you know, we would always have a speaker who was either a carer or someone from the stoke association or occasionally a patient . . . and again it meant right down at a kind of micro level, people remembered we were doing this to improve patient care, not to protect their institution or their profession.
Lon16, stroke programme board member, London
Despite being seen as effective, it could also be argued that these instances of substantiation had the effect of reasserting the status of both patient and professional. The patient is recast as a beneficiary of the work to improve quality of care, and the professional as an expert provider of benefit. The traditional roles of patient and expert are maintained. In this way, the emancipatory vision of involvement as transforming roles through empowerment does not appear to have been realised.
Discussion
Principal findings
This study drew on project documents and qualitative interview data with a wide range of professionals engaged in complex and protracted processes to redesign acute stroke care in two English cities. The case for MSC was constructed by professionals drawing on clinical and managerial experience and an examination of population-level patient data that demonstrated the need to improve access to best evidence care. This was consistent with NHS strategic guidance that requires service-level changes to be clinically led and underpinned by clinical evidence. 166 This set the parameters of involvement from the outset; the case for change was professionally led but the co-operation and approval of a wide range of stakeholders including clinical staff, NHS managers and local politicians was required. PPI was a tool to facilitate implementation of the changes. In this sense, involvement could be seen as instrumental, achieving the outcomes desired by professionals. 110 However, we would further argue that rather than either ‘tokenistic’, suggesting a cynical position, or ‘meaningful’, implying conformity with some a priori agreed definition of what involvement means, involvement here was enacted in strategic ways.
Interviewees’ accounts varied widely in how they evaluated involvement. Consultations were seen as, at worst, a waste of time to, at best, wide reaching, inclusive events in which the patient voice could be heard and professional transparency be demonstrated. The contribution of individuals taking part in governance structures was also differently viewed. They were variously portrayed as powerful voices reminding professionals – at risk of promoting their own interests – of their true purpose, as making a limited contribution because of their limited competence in meeting behaviour and as self-selected and unrepresentative.
This implied that professionals controlled not only the agenda but also the manner in which involvement was enacted. 117,153 This may be inevitable. The model of involvement that dominates the NHS requires professionals to invite lay people to participate in activities that professionals design, focused on questions that they identify. This differs from PPI’s political antecedents: self-organising patient movements, which drew on principles of social justice and emancipatory practice to challenge biomedical definitions of illness and solutions, counter discrimination and stigma and call for action into emergent health problems. 167 Although involvement may promise a transformation in relations between patients and professionals, as Komporozos-Athanasiou et al. 168 have argued, the ritual nature of PPI activities constitutes ‘a conservative form of engagement in health’ that serves to reinforce existing statuses, neutralising the transformational potential of involvement.
Strengths and weaknesses
Our study provided professionals leading MSC with the opportunity to reflect on involvement practices in two MSC projects in the broad context of service change, rather than focusing on involvement alone. Participants were from a wide range of backgrounds and professions, and so a number of perspectives were captured. This is one of a relatively small number of studies investigating involvement in MSC. The data are limited in that they are retrospective, rather than contemporaneous accounts. The diversity of interviewees and their role in MSC means that each provides something of a partial view of which activities were undertaken and by whom. What emerges is a rather complex picture of diverse activities from information-giving events through to research to collect accounts of patient experience, framed by participants as involvement. The data do not necessarily provide an accurate historical record of PPI in the two MSC projects but they offer a moral account of implementing PPI in the projects.
Implications
Our study offers lessons for thinking about involvement in general and in relation to MSC. In particular, the findings represent a challenge to contemporary concerns that the literature reports processes of involvement but fails to report on impact, and that improved methods to demonstrate impact are required. 137,169 Our interviewees’ accounts did not suggest that it was possible to demonstrate impact of involvement in a linear way, because involvement was designed not to effect but to support change. Involvement here was a strategically symbolic process that served to use the moral authority of the imagined but substantiated patient to support change implementation. Thus, involvement as enacted also reiterated the significance of involvement itself. Conklin et al. 157 and Li et al. 156 have suggested that, rather than focus on impact, we should consider the quality of involvement in processual terms, either as democratic acts in their own right or as strategic acts of informing and legitimising.
Our study also found limitations to involvement as a democratic process. The MSC sought to effect change in acute stroke care and this set the parameters of what was admissible; thus, patients’ concerns about the quality of care needed after discharge from hospital were rendered irrelevant. In London, the need to consider the whole stroke pathway was acknowledged in the Healthcare for London report,160 but this acknowledgement was made after the event and it did not lead to a sustained effort to effect MSC in the priority area identified by patients.
Nevertheless, most participants in the study believed that involvement activities had intrinsic value, facilitating the implementation of MSC. The value attributed to involvement sustained the idea of involvement itself because, as the anthropologist David Graeber has remarked, value can be considered as the way in which specific activities are made meaningful to those involved. 170 Investigating how value is produced – and for whom – through involvement might offer a way of rethinking impact assessment in involvement, which Edelman and Barron158 have faulted for treating as if it were an intervention in its own rather than something integral to a larger process. As these authors suggest, rethinking impact requires revisiting the goals and purpose of involvement. This study further suggests a need to identify those goals and purposes that are shared by different constituencies, because we do not know if patients and the public who were involved in MSC in London and GM would have recognised the value that emerged from our interviewees’ accounts.
Chapter 9 The impact of the centralisation of acute stroke care on patient and carer experience
Overview
This chapter is based on a paper by Perry et al. 171 © 2018 The Authors. Health Expectations published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/.
What was already known about this subject?
-
Patient experience of stroke care has been reported as varied. Patients treated on acute SUs are more satisfied with their care than those on general wards and, more broadly, well-organised stroke care is associated with more positive patient experiences.
-
The impact of centralised acute stroke care pathways on patient experience has not been studied in depth.
What this chapter adds
-
Although the referral pathways differed, similar patient and carer experiences were reported in the two regions.
-
Patients and carers on the centralised acute stroke care pathways reported many positive aspects of care.
-
Participants were impressed with emergency services and the initial reception at hospital; disquiet about travelling further than a local hospital was allayed by clear explanations.
-
Participants described that they knew who was treating them, were involved in decisions and had adequate specialist stroke care.
-
Difficulties for families visiting hospitals a distance from home were raised. Repatriation to local hospitals was not always timely, but no detrimental effects were reported. Discharge to the community was viewed less positively.
Background
Centralised care pathways
Patients treated on acute SUs are more satisfied with their care than those on general wards,172–174 and, more broadly, well-organised stroke care is associated with more positive patient experiences. 175 However, the impact of centralised acute stroke care pathways on patient experience has not been explored in depth. Centralised services may affect patient experience in a number of ways. Services are likely to be relatively high volume, and patient satisfaction with stroke services has been reported to be lower in larger stroke services. 176 Care may be provided in an unfamiliar environment, with travelling distances increased for patients and families. 177 Payne et al. 178 reported that travel for cancer treatment had been described as inconvenient and could be perceived as a barrier to treatment. However, for another clinical issue, Sampson et al. 179 concluded that, although it may be perceived as inconvenient, people would travel further in order to access centralised angioplasty services. One survey study of the experience of patients and carers of the newly centralised stroke care pathways in London reported that the majority of stroke patients and carers were either happy or did not mind being treated in a more distant HASU, and that although some concern was expressed about repatriation, only 6% reported any negative effect of the transfer on recovery or outcome. 177
The importance of patient and carer experience
The definition of quality in health care has expanded to include patient experience,180 and the concept is prominent in the measurement of health service performance. 181 Patient experience is now a recognised component of high-quality care in the English NHS, alongside patient safety and clinical effectiveness. 182 It is mandatory for NHS providers to gather patient experience data. 183
Although there is no single universal definition of patient experience,180 many definitions reflect that of The King’s Fund Point of Care Programme: ‘the totality of events and interactions that occur in the course of episodes of care’. 184 Patient experience is more than ‘patient satisfaction’, and asking patients ‘what happened’ during a specific episode of care is more valid in judging quality of care than just asking about ‘satisfaction’. 175 Good patient experience includes a focus on individualised care, and is tied to patient expectations and whether or not they are positively realised and to the principles of patient- and family-centred care. 180 Positive patient experience is associated with patient safety and clinical effectiveness183 and understanding how patients experience care can highlight substandard care. 185 In 2012, the National Institute for Health and Care Excellence (NICE)186 produced a quality standard to provide the NHS with clear commissioning guidance on the components of a good patient experience: 14 quality statements against which patients’ experience can be measured are presented in Table 16.
| Number | Quality statement |
|---|---|
| 1 | Patients are treated with dignity, kindness, compassion, courtesy, respect, understanding and honesty |
| 2 | Patients experience effective interactions with staff who have demonstrated competency in relevant communication skills |
| 3 | Patients are introduced to all health-care professionals involved in their care, and are made aware of the roles and responsibilities of the members of the health-care team |
| 4 | Patients have opportunities to discuss their health beliefs, concerns and preferences, to inform their individualised care |
| 5 | Patients are supported by health-care professionals to understand relevant treatment options, including benefits, risks and potential consequences |
| 6 | Patients are actively involved in shared decision-making and supported by health-care professionals to make fully informed choices about investigations, treatment and care that reflect what is important to them |
| 7 | Patients are made aware that they have the right to choose, accept or decline treatment and these decisions are respected and supported |
| 8 | Patients are made aware that they can ask for a second opinion |
| 9 | Patients experience care that is tailored to their needs and personal preferences, taking into account their circumstances, their ability to access services and their coexisting conditions |
| 10 | Patients have their physical and psychological needs regularly assessed and addressed, including nutrition, hydration, pain relief, personal hygiene and anxiety |
| 11 | Patients experience continuity of care delivered, where possible, by the same health-care professional team throughout a single episode of care |
| 12 | Patients experience co-ordinated care with clear and accurate information exchange between relevant health and social care professionals |
| 13 | Patients’ preferences for sharing information with their partner, family members and/or carers are established, respected and reviewed throughout their care |
| 14 | Patients are made aware of who to contact, how to contact them and when to make contact about their ongoing health-care needs |
What was already known about patient and carer experiences of acute stroke care pathways?
The literature on patient and carer experience of acute stroke care provides evidence in relation both to the various stages of the acute stroke care pathway, and also to a number of cross-cutting issues that relate to all stages of care.
Initial transfer to hospital
For the majority of stroke patients (70%), the first point of contact with services is through the emergency medical services,187 and research suggests that patients and carers have a generally positive experience with these teams. 188,189 Those calling the emergency services found call handlers to be reassuring and calming,187,189 although not all were clear if an ambulance was on the way or when it might arrive. 187 The importance to patients of ‘holistic care’ from the emergency medical services (defined as handling the whole situation, not just the person with the symptoms) was highlighted,188 as was the speed of arrival of assistance. 189
In-hospital care
Studies of inpatient hospital stroke care indicate that, overall, people had a positive experience. 174,190–194 Often, however, appreciation of a service as a whole was tempered by concerns about service shortfalls,195 particularly in relation to the initial experience of inpatient care, the provision of therapy, and general aspects of care.
In an interview study of people admitted to SUs, although many reported fast access to assessment on admission to A&E, others described delays because of poor availability of staff or beds, and perceived that stroke was not treated as a medical emergency. 189 As patients and carers were generally aware of the importance of time to treatment, these delays caused anxiety and frustration. Those admitted ‘out-of-hours’ reported poor availability of some specialist services such as medical input and imaging, which some perceived as hindering their access to appropriate treatment. 189
Lack of therapy (physiotherapy/speech therapy/occupational therapy) during inpatient care was reported. 195–197 Some stroke survivors associated this with their experience of setbacks in recovery. 195 A lack of help in hospital with emotional problems, such as confusion or depression, has also been reported,174 resulting in a poorer experience of care.
Most stroke patients have reported that they were always treated with respect and dignity,174,193 although other studies have indicated that stroke patients did not always receive the help that they needed with general activities, such as eating or washing. 174,196 Carers felt that they needed to compensate for perceived shortfalls in the care of their relatives on occasion, although the general institutional nature of much hospital care was experienced as preventing family from participating in aspects of care. 192,195
Discharge home
Discharge preparation has been described as lacking, both in the past and more recently. 174,190,191,193,197 Ellis-Hill et al. 198 explored what constituted a ‘good’ or ‘poor’ experience in the transition from hospital to home through interviews with 20 stroke survivors and 13 carers. Discharge was perceived to be successful by stroke survivors if they maintained a sense of momentum about their recovery, felt supported and felt informed about what was happening to them. In the Healthcare Commission survey,174 although 90% of people thought that their general practitioner (GP) had been given sufficient information to care for them once at home and most patients (63%) reported that all the services they needed after leaving hospital were arranged, 15% said that such services were not arranged. Those who had been cared for on a specialist stroke ward were more likely to report that services had been arranged than those who had not. 174
Information provision
Receiving adequate information about care and treatment has been described as contributing to a positive experience of care by stroke patients and carers,172 for example by reducing anxiety. 195 Varied experiences of information provision while in hospital have been described. Some patients considered that they had received enough information, but others felt that they were overloaded, or that they had not received enough,191–193,195,199,200 indicating the need for a service responding to differing patient needs. Payne et al. 200 identified that families of stroke patients found it difficult to get time with staff to find out about a patient’s care. When a lack of information was perceived, this was particularly in relation to treatment and what care to expect after discharge. 195
Personalised care
When asked what constituted good stroke care, stroke survivors articulated that being personally valued and cared about by health-care staff was important. 192 This was echoed by Hewitt et al. 172 in their interviews with 50 patients and 33 carers in acute, inpatient rehabilitation and community phases of care, who reported that being treated with individual care and attention, and having trust and confidence in health-care professionals, led to a positive experience of care. Morris et al. 195 also reported that stroke survivors wanted health-care staff to see them in context as people, not just patients, as this improved their experience.
In this chapter we aimed to explore in depth the impact of the GM and London centralised acute stroke care pathways on the experiences of patients and carers. In the absence of any specific data on patient and carer experience in GM and London prior to the centralisations, the existing literature, summarised above, was used to help frame this analysis.
Method
Sample
Patients were recruited from the research case study sites: three sites in GM (the sole 24/7 CSC, one of two in-hours PSCs and one of 11 local DSCs) and four sites in London (two of eight 24/7 HASUs and two of 24 local SUs). Any patient diagnosed with stroke was eligible for inclusion provided that they had adequate cognitive function. Purposive sampling ensured that patients experiencing different elements of the centralised pathways, such as repatriation to a local SU/DSC or direct discharge home from a HASU, were included.
Participant recruitment and data collection
Recruitment and data collection occurred between April 2013 and May 2016. Potential participants approached shortly before discharge from hospital by a research nurse or clinician were asked if they were willing to speak to a researcher. The researcher explained the study, and if patients were willing to participate, permission to contact them after their discharge from hospital was obtained. Patients were interviewed at home within 3 months of discharge, with fully informed written consent. Carers were included if the patient wished, or if they were incidentally available at the time of the interview and the patient was happy for them to contribute; they were asked about their perceptions of care received by the stroke patient. Most interviews lasted between 45 minutes and 1 hour. A semistructured interview schedule was used, covering experiences from onset of symptoms to experience once home. This interview guide was developed with reference to the literature reviewed, to established recommendations such as the NICE quality standards for patient experience,186 and in relation to the new care pathways. A patient coinvestigator assisted with development of the guide, which was also discussed with the Study Steering Committee (including patient representatives) and a stroke patients’ research group. With the permission of participants, interviews were digitally recorded and then professionally transcribed.
Data analysis
A thematic analysis was undertaken, initially using a deductive approach guided by a baseline framework developed from the literature (see Appendix 6, Table 47). Two of these themes (explanation and information and person-centred approach) were cross-cutting in nature and reflected many of the NICE quality standards for patient experience. 186 Two people (CP and IP) used the baseline framework to analyse early interviews, with some transcripts analysed by both to ensure consistency in data coding. As analysis continued, an inductive approach was used and the final framework was developed to mirror the centralised stroke care pathway (Table 17).
| Main themes | Subthemes |
|---|---|
| Initial transfer to hospital | Initial contact with emergency services |
| Timely response | |
| Information given by emergency services | |
| Concerns with not being taken to local hospital | |
| Reception at hospital | Timely investigations and treatment |
| Stroke treated as a medical emergency | |
| In-hospital care | Clear explanations and shared decision-making |
| Known staff | |
| Adequate therapy provision | |
| Some lack of continuity at weekends | |
| Difficulties for families in travel to a more distant HASU | |
| Repatriation to local hospital | Relatively smooth |
| Staff uncertainties | |
| Delay in obtaining bed at local SU | |
| Transportation to local unit | |
| Transfer of care to local unit | |
| Discharge home | Importance of effective communication with GPs |
| Patient’s need for information about continuation of therapy and follow-up | |
| Difficulties with weekend discharge |
The analysis framework had five main themes: (1) initial transfer to hospital, (2) reception at hospital, (3) in-hospital care, (4) repatriation to local hospital and (5) discharge home. The cross-cutting issues identified in the original framework were evident throughout the stages of the pathway. In order to enhance reliability, the emerging analysis was discussed with a subgroup of the authors (AIGR, NJF, CM and RB). To enhance validity, interim versions of the analysis were presented to stroke patient support groups.
Results
We conducted 36 interviews with stroke patients, and in 17 interviews a partner or carer also participated. A range of experiences were represented in terms of whether people were admitted to a HASU/CSC/PSC or a local SU/DSC, or were repatriated to a local SU/DSC (see Table 2 and Appendix 6, Table 48).
Initial transfer to hospital
Most people who experience stroke are transported to hospital by ambulance. Participants in this study reported that ambulances arrived quickly and ambulance staff gave clear information about likely diagnosis, which served to reduce anxiety. Transferring people to a more distant HASU/CSC/PSC, thus bypassing a local hospital, was voiced as a potential concern by those planning the reconfiguration and concerns were expressed to ambulance staff, particularly by relatives:
We’re going further, that’s going to take longer, what happens if it gets worse on the way there?
GMHp03, family member
However, once explained that transfer was to a specialist unit, fears were usually allayed. One patient described that they were told they would ‘go to the right place that would sort me out’ (LonAp05, patient, London) and another stated that:
They said we’re taking you to [CSC/PSC] because they’ve got a specialist Stroke Unit there, effectively, and I said, ‘well that’s fine’.
GMHp03, patient
The experience with the ambulance service of a woman who took herself to the A&E at a local DSC was less smooth, however. Local DSC staff considered she was eligible for, and would benefit from, CSC/PSC care, and arranged for her transfer by ambulance to a CSC/PSC. The ambulance staff were not clear about what was happening, which was unsettling to the patient who said she had to explain her transfer to them. She commented:
I’d never been in an ambulance before, which was daunting in itself, and then the ambulance man was sort of talking and saying, ‘Well we’ve not had a proper handover, we don’t know what’s going on’.
GMHp07, patient
Transfer from a local SU/DSC to a HASU/CSC/PSC happened relatively infrequently, which may explain the lack of adequate handover to ambulance staff and why they were unclear about the procedure.
Reception at hospital
Transfer from ambulance staff to hospital teams was perceived as smooth. Participants were impressed with the reception they received on arriving at hospital. In the centralised care pathway, HASUs were organised so that stroke teams met the patient on arrival, following a pre-alert from the ambulance service, and this enhanced participants’ experience of care. Participants reported receiving timely investigations and treatment and that the teams who treated them knew what they were doing. Their perception was that stroke was treated as a priority and a medical emergency:
You went in and they were so ready for him, I know they’d radioed through, I know they were prepared for him.
GMGp02, family member
In GM, patients admitted to a local DSC rather than a CSC/PSC also reported timely care, stating that admission to hospital was ‘very expeditious’ (GMHp01, patient) and that there was ‘no waiting . . . attention was very quick’ (GMHp04, patient).
In-hospital care
Generally, patients were happy with in-hospital care, with most indicating that they knew who was treating them, they received clear explanations and they were involved in decisions about their care. In addition, patients reported receiving adequate therapy, which they perceived had aided their recovery. There was some lack of continuity reported in the evenings and at weekends. For example, participants commented that staff in the evenings and at weekends were unfamiliar to them, and that some were not so clear about their/their family member’s care, which caused some anxiety.
Although patients did not mind being admitted to a more distant CSC/PSC, visiting for families and friends was raised as an issue which worried them:
It was a bit awkward being so far away.
GMHp07, patient
I can imagine it would affect people if they were in Kent or something.
LonAp04, patient
Carers recounted difficulties in visiting a more distant hospital. One said that ‘it was so expensive . . . well I were [sic.] there twice a day’ and also explained the impact that the distance to travel had on her:
Back home again, you have no time. I think I’d get home, took the dog out, come back and go again. There was just no time and, you know, you couldn’t just not go.
GMGp02, family member
Repatriation to local hospital
The repatriation of patients from a HASU/CSC/PSC to local SUs/DSCs was something about which concern was expressed by those planning the reconfigurations. As part of the centralised stroke pathway, patients who were admitted to a unit that was not their local hospital were returned to their local SU/DSC after they had received their acute care, if they were not well enough for discharge home. For participants in this study, repatriation happened relatively smoothly and patients were mostly happy about the way in which they were transferred from the HASU/CSC/PSC to their local SU/DSC:
Once they told me yes there’s a bed available they then came and said we’ve ordered an ambulance and it will take between 1 and 4 hours to come, I remember them saying that. But it came well within 4 hours, under 4 hours.
GMHp04, patient
Being kept informed about what was happening contributed to a smooth transfer:
They kept me informed of what was going on, that was . . . I think is the most important thing. Rather than just leaving you laying there or sitting there as the case may be, not knowing whether it’s daytime or night time.
LonCp01, patient
Most people perceived that their care was continued smoothly once they were transferred and that staff were aware of what had happened to them, and that repatriation did not have any impact on the trajectory of their recovery. Some commented favourably on the increase in therapy input once they had been transferred (an increase which would be expected as local units were focused on rehabilitation).
However, some difficulties were described with repatriation. It was reported that hospital staff were not always sure which hospital a patient should be repatriated to; this may reflect the fact that staff were learning to work with a new care pathway and had initial uncertainties. Some people also described delays in obtaining a bed at a local SU/DSC. Although this could be frustrating, patients generally accepted this situation if it was explained to them that they were waiting for a bed to become available, as long as they were kept informed about what was happening. This emphasises the importance of clear information. A family member explained that:
We were waiting for a bed to become available at [local unit], that was the reason he was in [CSC/PSC] a bit longer.
GMHp02, family member
This can be contrasted with the experience of another family member who did not understand what was happening:
Somebody told us she would definitely be going at one time, then she didn’t go and then somebody else said no . . . you know it was a little bit confusing.
LonDp02, family member
Once it was decided that a patient was to be repatriated and a time for transfer was given, some delays in transport to a local SU were described. This kind of delay was not tolerated well by patients and their families:
We weren’t very happy if you recall at the time with transfer from HASU to local Stroke Unit, because it took 6 hours, which left both of us in a very het up and upset state.
LonDp01, family member
For some people, delay in transportation to a local unit resulted in transfer happening later in the evening, which was another situation patients were unhappy about. One patient described his experience:
The next day they said they wanted to send me to [hospital], which was the nearest hospital to home. I set out, I didn’t set out, they said you'll be going later on in the day and an ambulance would come for me. Well I sat around all day and nothing happened and by half past nine at night no ambulance had arrived so I said well I'm not going, I'm not going to be carted in the middle of the night through a big city and you know it was snowing.
GMHp09, patient
This person also described being transferred to a bed on another ward for one night because of the pressure on CSC/PSC beds.
Discharge home
With centralised acute stroke care pathways some HASU/CSC/PSC patients would be discharged home to a different area from where they had received their acute care. This potentially posed difficulties to hospital teams that did not know the local processes of care, or the teams to which they were discharging people. In terms of transfer between hospital and community, most participants thought that communication between hospitals and GPs happened effectively and that their GP was aware of their stroke and the care they had received. However, some people were not clear about their follow-up once home and were unsure about when, whether or how this was to happen, or experienced some delay. For example:
It’s unclear even to me today what’s going to happen with physiotherapy in the future because apparently there is . . . a waiting list and I’ve not heard much from them.
GMHp03, patient, GM
Being discharged at weekends was also problematic in terms of arranging adequate care once home, and in practice rarely happened. This meant that some people stayed in hospital for longer than necessary, or that they had to wait to receive ongoing care once home, which was perceived to slow down recovery.
Discussion
Principal findings
This chapter has explored patient and carer experience of centralised acute stroke care pathways in two metropolitan areas. Similar experiences were reported by participants from the two different geographical regions, which is perhaps unsurprising: although the care pathways differed in terms of who was eligible for HASU/CSC/PSC care (those presenting within 4 hours of symptom onset in GM and all patients in London), patients went through similar stages of care in both locations following stroke. The findings contribute to knowledge about patient experiences of acute stroke care services and also to the wider body of knowledge relating to the centralisation of services in general. The data also demonstrate how patient experience can provide valuable information about how a service is operating, what is working well and what is not. 185 For example, one patient’s observation that ambulance staff were not sure why they were transferring her to a different hospital indicates that the pathway was not operating optimally.
Strengths and weaknesses
Strengths include that this was a rare opportunity to compare patient and carer experiences of two centralisations of acute stroke care, which differed in terms of service model implemented and impacts on care provision and clinical outcomes. A range of experiences were represented in terms of whether people were admitted to a HASU/CSC/PSC or a local SU/DSC, were repatriated to a local SU/DSC or discharged straight home. Interviews took place within 3 months of discharge from hospital to enhance recall of events. In addition, interim findings of the analysis were presented to stroke patient support groups for their comment.
In terms of weaknesses, only stroke patients who were cognitively able to participate in an interview were recruited into the study, and it is possible that the experience of those who had a less positive outcome after their stroke was different. The study was of centralisation of stroke care pathways in two metropolitan areas of England; centralised services in more rural areas may well be experienced differently by patients and carers. Finally, some patients taken onto the centralised acute stroke care pathways in GM and London were ultimately not diagnosed with stroke. These so called ‘stroke mimics’ were thus transferred to a hospital more distant from their homes with no particular benefit for themselves and were not part of this study. It is important that the experience of this group of patients is analysed in any overall evaluation of centralised acute stroke care pathways.
Comparison with other studies
Participants in this study were impressed with both their contact with the emergency services and their initial reception at hospital. Their experience of timely investigations and initial treatment suggested that stroke was treated as a priority and a medical emergency. This was in line with the National Stroke Strategy24 and in contrast to some earlier studies. 189 Once admitted to hospital, patients described that they knew who was treating them, they received clear explanations about their care, were involved in decisions about care and had adequate access to therapy. This was again in contrast to much published literature on patient experience,178,192,195 but reflected what is known about the relationship between well-organised stroke care and more positive patient experience. 175 The extent to which timely investigation and treatments, and the availability of therapy, can be attributed solely to the centralised acute stroke care pathways is difficult to discern, as national initiatives such as the National Stroke Strategy24 were current at the time of the centralisations in GM and London and would have driven such improvements in care. However, the centralisations introduced HASUs/CSCs/PSCs, which are associated with a greater likelihood of receiving evidence-based care interventions. 26
Other findings from this study of stroke care pathway centralisation are relevant to the centralisation of any services where patients are taken to more distant care settings and discharged to the community from there, or repatriated back to a local hospital. There was some evidence in our data of these processes of care impacting upon patient experience. Patients, and particularly family members, expressed some disquiet on being informed that they were going further than their local hospital, but were generally reassured once an explanation was given. This emphasised the importance of clear explanations by the paramedic team on initial contact with stroke patients, and reflects NICE quality standards regarding communication. 186 The importance of effective and timely information provision, as shown in the literature, was clear at this stage of the pathway. Difficulties for families visiting hospitals a distance from their homes were discussed, in terms of time and financial costs, but patients and carers broadly prioritised quality of care and outcomes over the issues presented by being cared for at a more distant site. This was similar to the survey findings of Moynihan et al. 177
Repatriation was not always smooth in terms of happening in a timely manner (within 72 hours), but on the whole worked well from the perspective of patients. When there were issues, the importance of communication with patients and carers was emphasised. If patients were informed about what was happening, knew where they were being repatriated to and when, and had the reason for any delays explained, they were more likely to report a good experience. This reflected the NICE quality standards for patient experience186 and the stroke-specific literature in which the importance of clear information, communication and explanation about care are emphasised. In both study areas a lot of effort was put into ensuring that patients understood the care pathway from initial admission to a HASU/CSC/PSC and knew about the possibility of moving to a more local SU for ongoing care.
Repatriation also involved the transition of care from a HASU/CSC/PSC to a local SU. The NICE quality standards186 suggest that care should be well co-ordinated between different health-care professionals. The experience of patients in this study was that care was handed over smoothly, and nobody perceived that the transfer had any adverse effect on the trajectory of their recovery, similar to the findings of Moynihan et al. 177 The most difficult transition for patients was discharge to care in the community, for example patients’ reports of not being clear about follow-up care. Clarity about addressing ongoing care needs is one of the NICE patient quality standards. 186 Although in general people being discharged from a specialist stroke ward are more likely to have adequate follow-up care arranged than those from a general ward,174 patients in this study, who were all discharged from a specialist ward either at a HASU/CSC/PSC or local SU/DSC, experienced some difficulties. This may reflect the focus of the stroke care pathway centralisations on hyperacute care, and known variations in early supported discharge (ESD) and community therapy services across GM and London, as well as the difficulties experienced by hospital staff in trying to arrange care for patients to be discharged to different geographical areas.
Implications
Patients and carers on the centralised acute stroke care pathways in GM and London reported many positive aspects of care and it is evident that they often experienced standards of care in line with the NICE quality standards for patient experience. 186 Taking a broader perspective, the findings from this study suggest that the centralisation of care pathways in general can offer patients and carers good experiences of care. The disadvantages of travelling further were perceived to be outweighed by the opportunity to receive the best-quality care. This chapter has demonstrated the need for the delivery of clear and understandable information to patients and their families about every stage of the care pathway, to maximise their experience of care, and highlighted the importance of wide stakeholder engagement in the provision of centralised services (e.g. the importance of the role of the ambulance service in explaining the centralised pathway on initial contact with stroke patients).
Findings, Part B: reconfiguration of acute stroke services in the Midlands and East of England, further reconfiguration in Greater Manchester and sustainability in London
Chapter 10 Lessons from efforts to implement major system change across the Midlands and East of England
Overview
What was already known about this subject?
-
Several factors, including approaches to leadership, stakeholder involvement, use of measurement and data, and learning from previous experiences of change, play a significant part in the planning and implementation of MSC in acute stroke services (see Chapters 6 and 7). However, these lessons were generated in relation to changes conducted in urban areas and there may be value in studying similar changes conducted in more rural contexts.
-
Stroke care across the Midlands and East of England was reviewed as part of a programme led by the SHA (a source of system-wide leadership) over the period 2012–13. This led to recommendations of MSC in acute stroke services across the nine network regions covered by the SHA, representing an opportunity to study MSC in less urban contexts.
-
However, following these recommendations, no MSC to acute services was implemented.
What this chapter adds
-
Several factors known to be associated with the successful implementation of MSC were either absent or severely hampered; this was perceived to have influenced progress of this programme.
-
Although system-wide leadership of the programme was evident in the beginning, support from senior leaders in the SHA reduced over time and following the NHS reforms implemented in April 2013, the complete loss of this top-down leadership was reported to have made it easier for local commissioners to withdraw than if the SHA had remained.
-
Data were used to build the case for reconfigurations and proposals for MSC. However, local stakeholders did not feel sufficiently engaged in the process, resulting in limited local ownership of recommendations, prompting local areas to repeat similar modelling exercises.
-
The programme sought to engage local networks and stakeholders throughout the review process, but the impending reforms of NHS commissioning made this challenging.
-
The programme used lessons from previous MSC to make their case. However, local stakeholders did not engage with these lessons because they related to work conducted in what were perceived to be very different contexts (i.e. in terms of rurality).
-
Underlying these issues, the NHS reforms implemented in 2013 had a significant influence on the progress of this programme. Key examples of this included (1) disrupting system commissioning and governance across the English NHS, (2) introducing significant distraction throughout the system and (3) limited time to develop reconfiguration proposals because recommendations had to be delivered before the SHA was abolished.
Background
Obstacles to implementation of major system change
As discussed in Chapters 1, 6 and 7, MSC may be seen as particularly complex, interacting across multiple levels – the macro (policy), meso (organisation) and micro (team/individual) – and multiple boundaries (sectoral, organisational, professional). 5,6,110,201 Given its scale and complexity, there are many factors that might influence the way in which MSC is developed and implemented, and indeed whether or not it is implemented.
Evidence suggests that a purely rational approach, based on resource use or potential outcomes, is unlikely to be sufficient to build an effective case for MSC. 6,202 Instead, active engagement of a range of stakeholders, using system-wide leadership to align these stakeholders, must be sustained throughout and beyond the change process. 6,110,202,203
Integral to these complex and dynamic relationships is the context in which the change is to be implemented. Context, for example in terms of national policy or local organisational, financial, political or resource factors, plays a significant part in how MSC develops and is implemented. 6,110,203 The process of implementation is likely to reflect an ongoing interplay between these intervention, implementation and contextual factors. 6,201 Therefore, there are many ways in which MSC might be adapted,110 delayed204 or halted over the course of planning and implementation.
The NHS Midlands and East review of stroke care
The review of stroke services across the Midlands and East of England (serving a population of 16 million people), conducted over the period 2012–13, recommended MSC of acute stroke services across the participating regions. Despite a year of detailed planning and engagement to agree the recommendations, and significant local expenditure of time and effort, MSC was not implemented in any of these areas.
Elsewhere in this report we have analysed examples of MSC in acute stroke services that were implemented and that illustrated the influence of these complex dynamics (see Chapters 6 and 7). The focus of this chapter was a case in which MSC was planned but not implemented. In this chapter, we have addressed the following RQ: which factors influenced progress of implementation of MSC in acute stroke services across the Midlands and East of England? Based on our findings, which we organised with reference to lessons for ‘successful’ implementation of MSC (in general,5 and adapted in relation to acute stroke services110), we aimed to illustrate how plans for MSC were developed in a context of significant contextual disruption (i.e. the reforms to the NHS in England implemented in 2013).
Method
Design
This was a case study analysis, focusing on (1) the overarching service review and (2) local programmes to implement the recommendations resulting from the review (including implementation of MSC); the four local case studies were categorised in terms of their degree of rurality, with one area classified as ‘urban’ [a large conurbation (Area A)], two as ‘rural’ [predominantly rural areas (Areas C and D)] and one as ‘mixed’ [i.e. encompassing both urban centres and rural areas (Area B)] (Table 18).
| Area | Proposal | Outcome/progress |
|---|---|---|
| A (urban) | Reduce from six to three HASUs |
|
| B (mixed) | Reduce from five to three HASUs |
|
| C (rural) | Reduce from two to one HASUs |
|
| D (rural) | No reduction proposed |
|
Data
For this analysis, we conducted 33 interviews and 12 non-participation observations (covering approximately 30 hours) and collected > 200 documents over the period May 2013–December 2014 (see Table 2). We interviewed stakeholders involved in the service review (including members of the Programme Board and Advisory Group); we also interviewed representatives of the teams that led local implementation, including representatives of the project teams, the local network, hospital, ambulance and commissioning organisations, and patient representatives. We observed meetings related to planning the changes in Area A, including the Programme Board and the Clinical Advisory Group. We analysed documents relating to the review and local implementation programmes, in each case covering project plans, meeting minutes, supporting documents and modelling reports.
Analysis
This was a single case study analysis guided by the adapted rules for implementing MSC in acute stroke services (see Chapter 7). 110 Throughout, we considered factors that the literature suggests might work against implementation, with a particular focus on the influence of context at macro and meso levels.
Results
Our findings are structured as follows: first, we briefly summarise the review process; second, we present the progress of local implementation of the review recommendations; and, third, we discuss factors that might explain why MSC was not implemented across the Midlands and East of England.
The NHS Midlands and East review
Drivers and context of the review
The NHS Midlands and East review ran from April 2012 to the end of March 2013, with the aim of ‘step change improvement in quality of stroke and TIA services and outcomes’. 205 This was launched by SHA leadership in response to the reported improvements achieved following centralisation in London:
A conversation had taken place at NHS Midlands and East at their board, and that was looking at the outcomes that’d been achieved in London following the review of stroke services, and there was an aspiration to see whether something similar could be achieved across Midlands and East.
ME08, Programme Board member
As discussed in Chapter 1, this review was conducted in the 12 months directly preceding implementation of major reforms of the NHS in England, including the abolition of SHAs, PCTs and stroke clinical networks.
Governance of the review
The review was led by a Programme Board, which was chaired by the National Clinical Director for Stroke and included senior representatives of the SHA, local stroke networks, service users, experts on various aspects of stroke care and a member of a shadow Clinical Commissioning Group (CCG), which was in preparation at the time of the review. Subgroups of the board were set up to focus on (1) tariff development, (2) data and modelling (including patient numbers, travel times, workforce capacity and cost), (3) education and workforce and (4) rehabilitation. An EEAG provided independent expert, for recommendations on the development and findings of the review, and a management consultancy were commissioned to support aspects of modelling (including patient numbers, travel times, workforce capacity and cost). 205
The review process
The Programme Board met approximately bimonthly over the review period. Key tasks included (1) the development of a service specification covering the whole stroke pathway and (2) three waves of consultation and engagement with networks across the Midlands and East of England to discuss how localities would meet the specifications.
The service specification covered prevention, through early detection, acute hospital care (including HASU, SU and TIA services), community care (including ESD), secondary prevention and end-of-life care. The specification went through several iterations, reviewed by the Programme Board, EEAG and Stroke Network leads, and was finalised at the end of June 2012.
A template was shared with local networks, asking that they describe current activity and propose how localities would meet these standards; the three waves of network proposals were fed back in August 2012, October 2012, and February 2013 and each wave was reviewed by the Programme Board and EEAG, who then provided feedback to the localities. The review’s final recommendations were sent to local networks in late March 2013, with proposed changes to organisation of acute stroke services highlighted.
Progress of local implementation
Table 18 summarises how implementation of MSC progressed in the four areas we studied.
It is noteworthy that in two areas that aimed to implement MSC (Areas A and B) the review recommendations were viewed as a starting point for planning local changes to services, rather than a model to be implemented. In Areas A and B, local project boards were set up to develop local implementation plans, drawing together a range of stakeholders. Much work was conducted to model local capacity, transport and finances, building towards new recommendations for MSC. These local programmes did not progress from planning to public consultation owing to a range of factors, including CCG concerns about changes in activity (whether in terms of losing services or in meeting increased demands). As a result, CCGs were unable to sustain a shared direction, and decisions to implement MSC were put on hold.
In Area C, the recommendations for MSC were seen as not appropriate until significant improvements had been made in the service felt most appropriate to be a HASU.
In Area D, during the three waves of proposals there was debate between the local network and the Programme Board over the need for further centralisation (services had already been reorganised in 2009). Following a site visit by members of the EEAG, it was agreed that no further reorganisation of acute services should take place, which permitted greater prioritisation of other parts of the stroke pathway, including development of ESD services:
We’ve not changed our reconfiguration, we fought quite hard not to, and we were very much, ‘We’re not, we’ve done that piece of work’. We gave quite strong justifications for why we didn’t want to change our reconfiguration, but it has pushed in terms of looking at quality again and giving a big re-impetus to stroke.
MED02, network representative, Area D
In the sections that follow, we present our findings to reflect the adapted lessons for MSC in acute stroke services, covering leadership, use of feedback loops, stakeholder engagement, and learning from history. 110 We summarise these findings in Table 19.
| Factor | Influence in the Midlands and East of England | Perceived impact |
|---|---|---|
| Leadership: system-wide authority important in sustaining alignment of stakeholders and managing resistance |
|
|
| Feedback loops: measuring performance (care provision, outcomes, capacity, travel times, finance) can facilitate agreement to change, focus and priorities of change, and assessing progress of implementation |
|
|
| Stakeholder engagement: engaging relevant stakeholders helps with development of viable proposals that are understood and supported by key groups |
|
|
| Learning from history: applying lessons from previous change can influence approaches (e.g. in addressing established local barriers to change) |
|
|
Leadership
As noted in Chapter 6, combining top-down (system-wide) and bottom-up leadership can help to ensure that plans for change are agreed and maintained through to implementation. However, system-wide authority may be especially important in sustaining alignment of stakeholders and managing resistance to proposals for change.
The Programme Board, in combination with the EEAG, represented a wide range of experts, many with national and regional remits (including the National Clinical Director for Stroke), and senior representatives of the SHA. The approach to developing recommendations was consensual, based on a dialogue of local proposals and central responses. This approach was questioned by some members of the project team, who suggested a stronger steer may have been valued locally:
I think people struggled, they almost wanted direction, they almost wanted to say, no, there was only going to be one hyperacute centre in this geography.
ME03, project team
Although senior leaders of the SHA launched the programme, there was a view that SHA ownership of the programme reduced over the course of the review, as a result of its impending abolition and the departure of some senior staff:
Public health was changing, the network was changing, the SHA was changing, and so that change [of SHA leadership] . . . was a real problem to the project. I felt that at the end the SHA were thinking, ‘Oh goodness, there was that stroke review by that guy: I suppose we ought to finish that off, really’. So I don’t think they had any ownership of what they were doing.
ME08, Programme Board
The SHA’s reduced ownership of the programme over time may have limited the influence of the final recommendations at the time of delivery. However, shortly after the recommendations were delivered the SHA was abolished, resulting in a more pronounced loss of system leadership.
From April 2013, in the absence of the SHA, and the associated loss of system leadership, local implementation faced a number of obstacles. Change was to be led by the newly formed CCGs, which were still establishing their role. There was significantly reduced support for work of this kind; for example, although some stroke network representatives were retained as part of the new NHS England Strategic Clinical Networks (SCNs), they no longer had an operational support role and their capacity to engage and facilitate leadership of change had been much reduced. As discussed above, it is likely that changes in leadership and loss of capacity to support change limited sustained local ownership of proposals; this in turn is likely to have contributed to local implementation either not commencing or halting when challenging decisions required a united response from leaders.
Feedback loops
Measuring system performance on various levels (including care provision and outcomes, service capacity, travel times and finance) and communicating it effectively to stakeholders can be an important facilitator of MSC, including agreeing to change, agreeing the focus and priorities of change, assessing progress and sustainability of changes.
A number of data were used in support of the review process. This included available evidence, for example citing local service performance on the national stroke audit. The programme also generated evidence that would help with developing viable proposals by conducting a series of modelling exercises to analyse current activity and capacity in services, and travel times between services. In support of this work, a subgroup (working with a commissioned management consultancy) developed a number of processes, including a tool for local systems to model activity levels.
However, despite substantial work, some of the modelling results were not seen as convincing locally. Local staff suggested that greater involvement in modelling would have permitted more meaningful discussion of local variations in performance of ambulance services and transport infrastructure:
It was only when they really got the ambulance service modelling, the remodelling our region where anybody in the region would . . . could put their name to understanding the geography, the travel times and the potential consequences of reconfiguration. I didn’t see a single piece of [Review Programme] modelling that looked like it was realistic or sensible . . . they’re paying this company a lot of money to do this and we’re not getting information on which we could base decisions.
MEC04, stroke physician, Area C
However, the team leading the modelling process suggested that this was in part a result of how localities engaged with it:
I thought the networks would lead this and really help it, all they did was they cut and pasted it like a jigsaw, they asked the different providers to fill it in, and they didn’t even review what had been filled in. So, when we had one where their costs were double their income, and we said, ‘Are you sure this is right?’ they were like, ‘Oh, we didn’t even look at the piece of paper, we just cut and pasted the thing together and sent it off to you’.
ME03, project team
These views suggest that there were underlying difficulties in engaging with local stakeholders in relation to the purpose and the process of the review. The outcome of gaps in engaging stakeholders is likely to have contributed to local decisions to repeat modelling exercises and, furthermore, decreased local ownership of the review recommendations.
Stakeholder engagement
Engaging relevant stakeholders (including service providers, commissioners, stroke patients and carers, members of the public and local politicians) helps to ensure the development of viable proposals that are understood and supported by these key groups.
In the review, the Programme Board included a wide range of experts in stroke care, and the process had several points at which localities had the opportunity to feed into the process. However, there was a view that the review could have been strengthened by engaging more or differently with certain stakeholder groups. For example, some CCG representatives indicated that the stroke service specification was too ‘input-focused’, that is, prioritising what services should look like rather than the outcomes they achieved, and that greater involvement of commissioners in its development could have prevented this:
These are people you know with huge expertise, massive national reputations, you know brilliant guys, but they’ve never commissioned . . . When you’re making service change and service planning decisions like this, you know you need the right people in the room.
MEC08, commissioner, Area C
This may in turn have led to the programme focusing too strongly on MSC as the solution:
Rather than focusing only on ‘What can we achieve in terms of a better outcome?’, the conversation already got right into how can you change the configuration.
ME08, Programme Board member
However, other areas saw significant engagement from CCGs in the process; for example, shadow CCGs from Area B presented the third and final set of proposals for their area to the EEAG towards the end of the review process.
One important issue raised in relation to the overall engagement dynamic was that building relationships and negotiating how stakeholders participate in them can take substantial investment of time and effort. The impending NHS reforms meant that there existed a challenging ‘hard deadline’ of March 2012, by which time recommendations had to be delivered. Furthermore, the fact that the reforms would involve closure of many of the participating organisations meant that many people were distracted, whereas others did not see this period as appropriate for planning a change of this kind and indeed felt little ownership of its objectives:
The big headline for me is, don’t try and do a review on the whole of the NHS – and it was the biggest restructuring I’ve ever encountered – don’t do it in that year. Don’t restrict yourself to 12 months: it’s better to leave it slightly longer – and do it in more detail with more ownership.
ME08, Programme Board member
Learning from history
Applying lessons from previous change can be an important factor in how change develops and is implemented, for example in terms of addressing established contextual barriers in a given locality.
The Midlands and East of England review was driven and informed by lessons from previous implementation of MSC in acute stroke services. Key examples of this included the goal of achieving improvements in outcomes previously achieved in London and the approach to engagement (e.g. working with local networks, shadow CCGs and ambulance services). However, as discussed above, the NHS reforms meant that leaders were aware that the task of engaging key stakeholders was inherently more complex, especially in terms of ensuring sustained ownership of proposals beyond April 2013. Therefore, the lessons in relation to engagement may have required greater adaptation to this challenging context.
Furthermore, lessons from past changes were used as a means of engaging stakeholders across the Midlands and East of England in developing the proposals for change in these areas. Documents and summaries of how change was carried out in London and GM were used throughout developing the service specification, and examples of engagement strategies and care protocols were shared with local networks and on the programme website (which is no longer online). However, local stakeholders noted that many of the key lessons were drawn from experiences of implementing change in highly urban areas, such as London; furthermore, many people referred to the changed financial context in which changes were to be carried out:
I think that was one of the constant bits of feedback about you know well it’s all very well to say use the London model but London had however many millions of pounds to implement this with the additional nurses and now London is seeing the costs savings and probably cut back on some of the things but they had that initial amount of money to be able to do it. And yeah, I think that’s . . . that was really hard for those organisations.
ME01, Programme Board member
As a result, evidence from previous changes achieved limited purchase with local stakeholders. Indeed, it may have compounded for some a view that leaders of the review did not appreciate the context in which the proposed changes were to be implemented, which may in turn have contributed to the difficulties in engaging stakeholders, described above.
Discussion
Principal findings
The review of stroke services in the Midlands and East of England between April 2012 and March 2013 entailed substantial activity, including stakeholder engagement, specification development and service/system modelling. However, although local systems did work to improve their services in line with some aspects of the service specification, the MSCs recommended were not implemented in any of the areas we studied.
System leadership – how it was used during the review and its absence during local implementation – had a significant role throughout. During the review, some questioned whether or not there was a need for a more top-down approach, noting that support from senior representatives of the SHA may have been important; during implementation the loss of system-wide leadership made it harder for local commissioners to sustain a united position when challenging decisions relating to service reorganisation. Programme leaders used national audit and locally collected data to build the case for change and develop proposals for how services might best be organised. However, there were challenges in engaging local stakeholders in the process, and there was limited local ownership of the findings and associated recommendations, prompting local areas to repeat similar exercises before considering implementing and changes. The programme sought to engage local networks and stakeholders throughout the review process, but because of the impending reorganisation of NHS commissioning it proved difficult to involve shadow CCGs sufficiently in order to gain their input on and ownership of the programme recommendations. Finally, although the programme was guided in its approach by lessons from previous changes, and indeed made use of these lessons as a means of strengthening the case for change, the fact that these lessons related to changes conducted in significantly different political and financial contexts may have reduced local stakeholders’ engagement with the process.
Underlying these issues, the NHS reforms implemented in 2013 had a significant influence on the progress of this programme. Key examples of this include (1) disrupting system commissioning and governance across the English NHS (thus removing established sources of system leadership through SHAs and operational support for MSC through clinical networks, (2) introducing significant distraction throughout the system (reducing the extent to which stakeholders could engage) and (3) setting a ‘hard deadline’ to deliver recommendations (which limited time to conduct the review process and engage these distracted stakeholders).
Strengths and weaknesses
A strength of this analysis was that we had the opportunity to study contemporaneously both the review and local efforts to implement its recommendations. This gave us access to data on implementation as it took place, rather than relying on retrospective accounts of the programme.
A potential weakness of this analysis is that it focused on only four of the nine areas covered by the Midlands and East review. Other factors may have played a part in changes not being influenced elsewhere in the Midlands and East of England. However, the area studied still represented a substantial proportion of the changes (covering a population of approximately 4.8 million people); furthermore, the study team engaged regularly with local network representatives to establish progress across the whole of the Midlands and East of England, these discussions confirmed that there was similarly slow progress across these areas, and suggested that many of the same factors had a significant role.
Comparison with other studies
This chapter has demonstrated that factors identified as important to planning and implementing MSC successfully [described by Best et al. 5 and modified by our evaluation (see Chapter 6)110] can also be used to explain how and why MSCs are not implemented. Our findings on the impact of both the 2013 NHS reforms and the wider financial issues faced by the NHS at the time of this analysis reflected past evidence on how financial and political context can influence efforts to implement changes to how care is organised and provided. 6,110,202,206 The case of Area D, where it was agreed over the course of the review process negotiations that no MSC would be recommended, may be seen as an example of data being used to guide decision-making in relation to whether or not and how services should develop.
Implications
The absence of system-wide leadership made it difficult to sustain stakeholder unity over the course of MSC. Effective communication of system and service data can support and guide change, but only if the relevant stakeholders have ownership of the evidence. Stakeholder engagement requires an understanding of which groups are likely to influence both implementation and sustainability of the intended changes, and ensuring that these stakeholders have sufficient time and capacity to participate effectively in planning and implementation processes. Finally, contextual factors will influence change throughout and beyond the implementation phases. This suggests that planners should attend not just to current local and national issues (including political and financial issues), but also consider the likely impact of factors that are still on the horizon.
Chapter 11 The impact of Greater Manchester B and the sustainability of London changes in terms of clinical outcomes and clinical interventions
This chapter draws on Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Overview
What was already known about this subject?
-
The initial reconfiguration of acute stroke services in GMA covering the period up to March 2012 was associated with no impact on mortality but LOS fell, whereas in London there was a significant reduction in both mortality and LOS (see Chapter 3).
-
Post-reconfiguration patients treated at HASUs in GM and London were significantly more likely to receive evidence-based interventions than those treated at non-hyperacute units in these areas and in the RoE. However, a far higher proportion of patients were treated in a HASU in London than in a CSC/PSC in GM. This might explain the difference in mortality outcomes between the two areas (see Chapter 4).
What this chapter adds
-
Following implementation of MSC in GMB, there was a reduction in mortality in GM of approximately 189 deaths per year.
-
However, there were also reductions in mortality in the RoE, and most of the differences in mortality between GMB and the RoE were not statistically significant.
-
In GMB there was a reduction in mortality over and above that seen in the RoE for people treated in CSC/PSCs (there were approximately 69 fewer deaths per year).
-
The proportion of patients treated in CSC/PSCs in GMB increased to 86% in 2015/16; this was a higher proportion than in GMA (39%), but still lower than in London (93%).
-
LOS fell in GMB, resulting in around 6750 fewer bed-days per year.
-
There was no significant variation in mortality or LOS over time since the reconfiguration in London, indicating that the reductions in mortality and LOS following centralisation in London were sustained.
-
These patterns were reflected by the analyses of clinical interventions in both areas. Following the implementation of MSC in GMB, the delivery of clinical interventions improved significantly, while also improving (although generally not to the same degree) in the RoE; in London the delivery of clinical interventions either improved or was sustained.
Background
Our chapters studying the impact of the reconfigurations in London and GMA on clinical outcomes (i.e. stroke patient mortality and LOS; see Chapter 3) and evidence-based clinical interventions (see Chapter 4) found the following:
-
In GM, where hyperacute stroke care was provided to patients presenting within 4 hours of developing stroke symptoms, there was no impact on mortality but LOS fell significantly.
-
In London, where hyperacute stroke care was provided to all patients, there was a significant reduction in mortality and LOS.
-
We calculated that if the 1.1-percentage-point reduction in 90-day mortality in London was achieved in GM then it would lead to 50 fewer deaths per year.
-
Post reconfiguration, patients treated at HASUs in GM and London were significantly more likely to receive evidence-based interventions than those treated at non-hyperacute units in these areas and in the RoE.
-
In GM 39% of stroke patients were admitted to a HASU, whereas 93% of London patients were, possibly explaining the difference in mortality outcomes between the two areas.
Since these findings were published (in 201425 and 201526) there has been a growing interest and activity in the impact of MSC in specialist health-care services. This has been reflected in national policy80,208 and in guidelines on good practice. 209 Further evidence in relation to MSC has also emerged in relation to its impact on acute stroke services,210–212 but little has been added in relation to the impact of different models of centralised acute stroke care on patient outcomes, and little is known about the sustainability of the impact of centralised acute stroke care.
In GM, further reconfiguration of the acute stroke system was implemented from April 2015. The main changes brought about by the GMB reconfiguration (Figure 13) were:
-
All patients were eligible for treatment in a CSC/PSC, rather than just those arriving at hospital within 4 hours (therefore, more in line with the London model).
-
PSCs extended the hours in which they would admit stroke patients (07.00–23.00, Monday to Sunday).
FIGURE 13.
Comparison of the service models implemented through GMA and GMB. (a) GMA model; and (b) GMB model. Figure 13a adapted with permission from Ramsay et al. (2015). 26 Effects of centralising acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46(8):2244–51. Stroke is published on behalf of the American Heart Association, Inc., by Wolters Kluwer. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0) License, which permits use, distribution, and reproduction in any medium, provided that the original work is properly cited, the use is non-commercial, and no modifications or adaptations are made. See https://creativecommons.org/licenses/by-nc-nd/3.0/. Permission to adapt this material has been agreed with Wolters Kluwer.
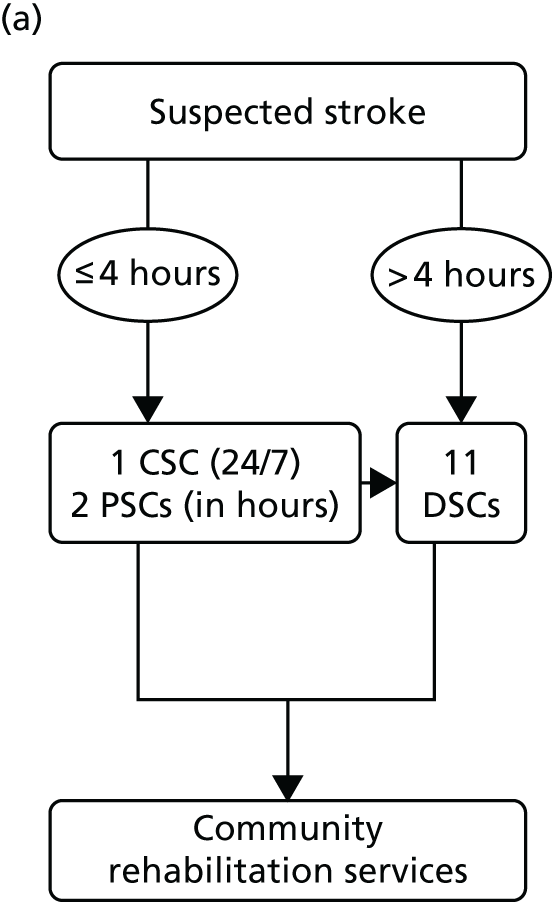
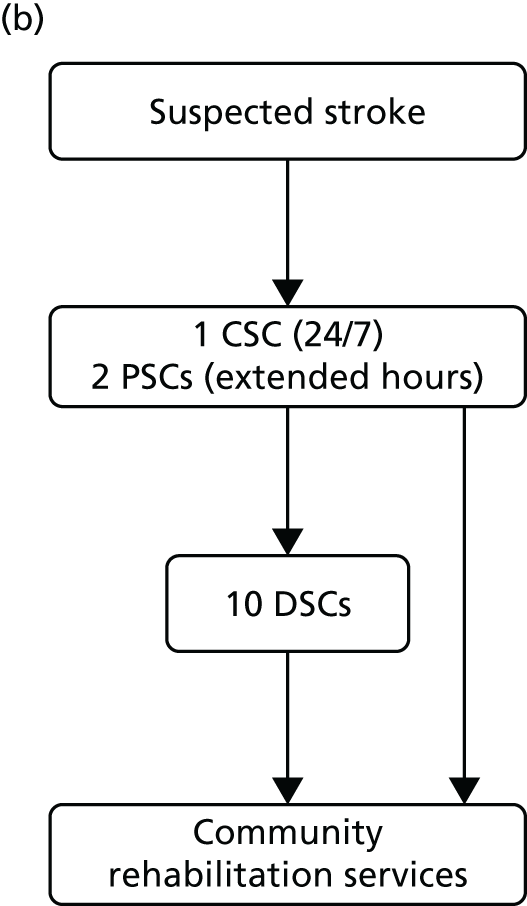
Given the growing interest in the reconfiguration of services, plus the further reconfiguration in GM in April 2015, in this chapter we extended the original analyses with the addition of new data covering the period up to March 2016. This allowed us to investigate two issues. First, whether or not the further reconfiguration of acute stroke services that occurred in GM in April 2015 (GMB) had an impact on clinical outcomes and interventions and, second, whether or not the impact of the reconfiguration of acute stroke services in London that occurred in July 2010 on clinical outcomes and interventions was sustained (i.e. did they remain consistent or improve) beyond March 2012. The RQs we addressed in this analysis were:
-
In GM, what was the impact of further reconfiguration on mortality, LOS and clinical indicators?
-
In London, was the impact of centralisation on mortality, LOS and clinical indicators sustained over the 4-year period since our previous analysis (6 years since centralisation)?
-
In GM and London, what proportions of acute stroke patients were treated in a HASU?
Method
Analyses of clinical outcomes
Data
The analysis in Chapter 3 was based on patient-level data from the HES database from 1 January 2008 to 31 March 2012. For this chapter we used new data from the same database from 1 April 2012 to 31 March 2016, and have merged these two data sets to produce a combined data set with data from 1 January 2008 to 31 March 2016 (i.e. 33 quarters/99 months). As with the previous analysis, we have included only patients admitted with a primary diagnosis of stroke defined using ICD-10 codes I61 (intracerebral haemorrhage), I63 (cerebral infarction) or I64 (stroke, not specified as haemorrhage or infarction). The analysis was confined to patients living in urban areas, and the HES data were linked to mortality data supplied by the ONS using an anonymised unique patient identifier to identify deaths from any cause and at any place of death (hospital or otherwise) at 3, 30 and 90 days after hospital admission.
The data set was cleaned in the same way as the previous analysis in Chapter 3. As before, we created regional variables to identify whether or not the hospital trust to which the patient was admitted was in London, GM or the RoE.
Statistical analyses
The reconfigurations in GM took place in April 2010 (GMA) and April 2015 (GMB). The reconfiguration in London took place in July 2010.
In the analysis of clinical outcomes for GM we ran similar analyses to before, to answer the RQs about the impact of the GMB reconfiguration. Our analyses for London were specified differently because the RQs were concerned with whether or not the effects in London had been sustained over time.
In the mortality analysis for GM we did the following. First, we plotted the unadjusted proportions of patients who died by region and by month for deaths within 3 days, 30 days and 90 days of admission in GM and the RoE/excluding London.
Second, using patient-level data for GM only we regressed mortality at each time point against age, sex, ethnic group, deprivation, type of stroke, number and type of comorbidities, hospital and calendar month, plus indicator variables for whether or not the patient was admitted during the GMA or GMB periods (vs. the ‘Before’ period, i.e. prior to GMA). We reran all models including an indicator variable for whether or not the patient was admitted during the GMB period (vs. the ‘Before’ period or GMA period).
Third, we calculated unadjusted between-region difference-in-differences comparing GM to the RoE/excluding London, based on the proportion of patients who died in each region in each time period.
Fourth, following our original analysis we ran an adjusted between-region difference-in-differences analysis using a two-stage estimation procedure involving a case-mix adjustment at the patient level and a difference-in-differences regression analysis at the provider level. We ran the second stage using both quarters and months as the unit of analysis; the results are qualitatively the same and we report here the analysis by month. The model was set up so that it was possible to compare GMB with the period before the first reconfiguration in GM and also to compare GMB with the period before this reconfiguration (i.e. also including GMA).
Fifth, to explore the reasons for our findings we reran the difference-in-differences analysis only for the GM CSC/PSCs. Note that the PSCs were not 24/7 HASUs, but data on time of arrival at the hospital were not available and so the analysis included all patients treated at these hospitals irrespective of when they arrived (i.e. we included people who were treated at the PSCs even when they were not operating as HASUs).
We ran all of the above separately for stroke diagnoses (ICD-10 codes I61, I63 and I64). When analysing mortality, given that our previous analysis showed no impact of the GMA reconfiguration, our preferred comparison is between GMB versus the ‘Before’ period plus the GMA period.
In the mortality analysis for London, using patient-level data for London only, we regressed mortality at each time point against age, sex, ethnic group, deprivation, type of stroke, number and type of comorbidities, hospital and month. We used logistic regression analysis. We then calculated the marginal effects of month on mortality and plotted these in a graph. We tested for significant variations in adjusted mortality since the reconfiguration using Wald tests, and present the results as p-values under the null hypothesis that the regression coefficient for every month after the reconfiguration (which occurred in July 2010) was the same as the regression coefficient for July 2010. We made these calculations including and excluding controls for hospital and the results were not qualitatively different; results are presented including hospital indicators.
In the analysis of LOS in GM, first we calculated the unadjusted mean LOS in GM and the RoE/excluding London during the ‘Before’, GMA and GMB periods. We then used the same two-stage adjusted between-region difference-in-differences approach as for the mortality analysis except that we used GLM with gamma family and log-link to account for data skewness. We also added binary indicators for mortality at 3, 30 and 90 days as control variables in the regression model. Given that our previous analysis showed a significant impact of the GMA reconfiguration on LOS, our preferred comparison was between GMB versus the GMA period. We computed the impact of the GMB reconfiguration on hospital bed-days.
In the analysis of LOS in London we used a similar approach as for the mortality analysis except that we used the GLM as above and controlled for mortality at 3, 30 and 90 days.
We computed the impact of the analyses on deaths averted (and bed-days) in GM assuming an estimated 4500 strokes in GM each year.
Analyses of clinical interventions
Data analysed
We used patient-level data collected for the clinical audit component of the Sentinel Stroke National Audit Programme (SSNAP),213 organised by the Royal College of Physicians. This programme replaced SINAP, which we used in our previous analysis of clinical interventions. Our data covered the period 1 April 2013 to 31 March 2016 and included data submitted by every SU in England. Consequently, our comparator area in this analysis covers all stroke patients reported in England aside from those treated in London and GM.
Measures
As with our previous analysis, we requested data relating to a range of clinical interventions. However, because we were analysing data from a single audit, it was possible to analyse significantly more clinical interventions than we were able to in the analysis reported in Chapter 4 (Table 20).
| Type of measure | Indicator/intervention |
|---|---|
| Where patient was treated | Whether the patient was treated in a HASU/CSC/PSC |
| ‘Front door’ processes | Whether the patient underwent a brain scan within 60 minutes/180 minutes/24 hours |
| Whether or not the patient was given thrombolysis (tPA) if eligible and whether or not it was given within 60 minutes | |
| Whether or not the patient underwent a swallow screen within 4 hours | |
| Whether or not the patient was admitted to a SU within 4 hours | |
| Assessments over the first 72 hours | Whether or not the patient underwent assessment by stroke consultant physician within 14/24 hours |
| Whether or not the patient underwent assessment by stroke nurse within 12/24 hours | |
| Whether or not the patient underwent assessment by specialist physiotherapist within 24/72 hours | |
| Whether or not the patient underwent assessment by occupational therapist within 24/72 hours | |
| Whether or not the patient underwent formal swallow assessment by SaLT within 24/72 hours | |
| Whether or not the patient underwent communication assessment by SaLT within 24/72 hours |
Statistical analyses
For the analysis of the evidence-based clinical interventions we undertook the following. First, using logistic regression analysis applied to the patient-level SSNAP data we regressed whether or not each of the clinical interventions had been achieved against the time period [year 1 (1 April 2013 to 31 March 2014), year 2 (1 April 2014 to 31 March 2015), year 3 (1 April 2015 to 31 March 2016)] controlling for age (in 5-year bands), sex (male/female), stroke diagnosis (infarct/haemorrhage), whether stroke happened in hospital or outside hospital and worst level of consciousness (alert/verbally arousable/not alert/totally unresponsive). 214 We ran these models separately, first for patients treated at hospitals in GM.
We used data for all regions combined (GM, London and RoE) and regressed the outcomes against all covariates and interactions between region and year; this means that when we analysed the effects of time period in each region, we controlled for differences in the covariates between regions and over time. Second, for each region we then calculated the marginal effects for each 1-year time period on achievement of the clinical indicators conditional on the covariates. In GM we reran our analyses including only patients treated in a CSC/PSC.
Given the GMB reconfiguration occurred in April 2015, we were interested in whether or not achievement of the clinical indicators improved in year 3 of our data beyond the changes seen in the RoE comparator. In London we were interested to see whether or not the achievement of the clinical indicators was sustained (i.e. was constant) over the 3-year period. For comparison we also present the findings alongside the results of our original analyses, which included fewer clinical indicators.
Results
Analyses of clinical outcomes
In the analyses for GM the findings are summarised as follows. First, across all stroke types combined, mortality at 3, 30 and 90 days declined over time in GM, although the decline was not smooth (see Appendix 7, Figure 29). Note that only 1 year’s worth of data were available for the GMB period.
Across all stroke types combined, comparing GMB with ‘Before’ plus GMA, adjusted mortality at 90 days in GM fell by 4.2 percentage points (see Appendix 7, Table 49). If there are an estimated 4500 strokes in GM each year, then this represents 189 fewer deaths per year (i.e. 4500 × –4.2/100). Running the same adjusted before-and-after analyses for the RoE gives a reduction of –2.6% (see Appendix 7, Table 49). The unadjusted between-region differences are broadly in line with these findings, showing an additional 1.1-percentage-point reduction in 90-day mortality in GMB compared with ‘Before’ plus GMA over and above the reduction seen in the RoE over the same time period (see Appendix 7, Table 51).
The adjusted between-region difference-in-differences across all types of stroke combined were negative and significant for mortality at 3 days, non-significant for mortality at 30 days and borderline significant (95% CI –2.7 to 0.01; p = 0.05) for mortality at 90 days (Table 21). The point estimates broadly reflect the results of the above analyses. When we reran the above analysis for all stroke types only for patients treated at the GM CSC/PSCs, there was a 1.8-percentage-point reduction in 90-day mortality (95% CI –3.4 to –0.2; p = 0.03; see Table 21) – the coefficient was larger (more negative) than the all-hospital model and significantly different from zero. Assuming that 86% of the 4500 strokes in GMB went to CSC/PSC (see Analyses of clinical interventions), then if this reduction was applied to the estimated 4500 strokes in GM each year this indicates 69 fewer deaths per year (i.e. 4500 × 0.86 × –1.8/100).
| Outcome measure by stroke type | GM vs. RoE | CSC/PSCs in GM vs. RoE | ||||
|---|---|---|---|---|---|---|
| GMB vs. ‘Before’ + GMA | GMB vs. ‘Before’ + GMA | |||||
| Difference-in-differences | 95% CI | p-value | Difference-in-differences | 95% CI | p-value | |
| All stroke types | ||||||
| Mortality at 3 days | –0.9 | –1.6 to –0.2 | 0.02 | –0.7 | –1.6 to 0.1 | 0.10 |
| Mortality at 30 days | –0.8 | –2.0 to 0.3 | 0.15 | –0.9 | –2.3 to 0.5 | 0.22 |
| Mortality at 90 days | –1.3 | –2.7 to 0.01 | 0.05 | –1.8 | –3.4 to –0.2 | 0.03 |
| Intracerebral haemorrhage (I61) | ||||||
| Mortality at 3 days | –3.1 | –6.7 to 0.5 | 0.09 | –2.5 | –6.9 to 2.0 | 0.28 |
| Mortality at 30 days | –3.1 | –7.4 to 1.1 | 0.15 | –4.1 | –9.4 to 1.1 | 0.12 |
| Mortality at 90 days | –4.1 | –8.4 to 0.2 | 0.06 | –5.0 | –10.3 to 0.4 | 0.07 |
| Cerebral infarction (I63) | ||||||
| Mortality at 3 days | –0.6 | –1.2 to 0.04 | 0.07 | –0.7 | –1.5 to –0.1 | 0.04 |
| Mortality at 30 days | –0.7 | –1.9 to 0.6 | 0.29 | –1.1 | –2.5 to 0.4 | 0.15 |
| Mortality at 90 days | –1.2 | –2.6 to 0.2 | 0.10 | –1.9 | –3.6 to –0.3 | 0.02 |
| Stroke, not specified as haemorrhage or infarction (I64) | ||||||
| Mortality at 3 days | 0.04 | –3.1 to 3.1 | 0.98 | 4.7 | –0.7 to 10.1 | 0.09 |
| Mortality at 30 days | –1.4 | –5.9 to 3.0 | 0.53 | 5.1 | –2.7 to 12.9 | 0.20 |
| Mortality at 90 days | –1.0 | –5.8 to 3.7 | 0.67 | 4.6 | –3.8 to 12.9 | 0.28 |
In the analysis of mortality in London there was little variation over time since the reconfiguration in terms of mortality at 3 days (see Appendix 7, Figure 32), 30 days (see Appendix 7, Figure 33) and 90 days (Figure 14). For example, in the analysis of mortality at 90 days, the p-value under the null hypothesis that the regression coefficient for every month after the reconfiguration is the same as the regression coefficient for July 2010 (when the reconfiguration occurred) was 0.09. This indicates that the reduction in mortality following centralisation has been sustained.
FIGURE 14.
Adjusted trends in mortality at 90 days in London. The dashed vertical line indicates when the reconfiguration in London occurred (July 2010). Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
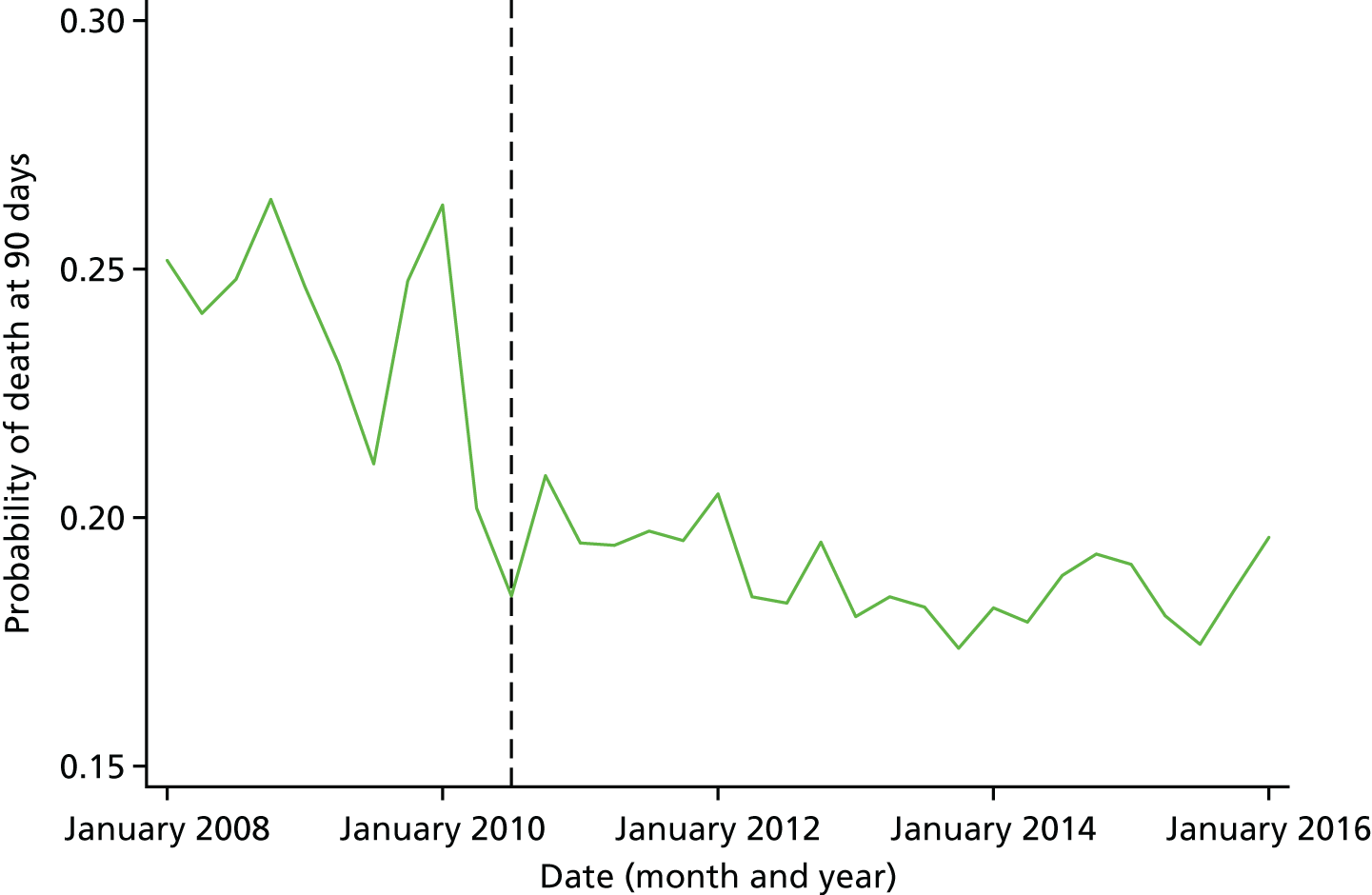
In terms of LOS, mean unadjusted LOS was 14.4 days in GMB and 17.4 days in GMA. Figures for the same time periods in the RoE were 15.7 days and 18.1 days, respectively (see Appendix 7, Table 54). Across all stroke types there was a significantly larger decline in risk-adjusted LOS compared with the RoE, by –1.5 days (95% CI –2.5 to –0.4; p < 0.01; Table 22). If this reduction was applied to the estimated 4500 patients with a stroke in GM each year this would result in 6750 fewer bed-days a year.
| Stroke type | After GMB vs. after GMA | ||
|---|---|---|---|
| Difference-in-differences | 95% CI | p-value | |
| All stroke subtypes | –1.5 | –2.5 to –0.4 | < 0.01 |
| Intracerebral haemorrhage (I61) | –1.2 | –3.3 to 1.0 | 0.29 |
| Cerebral infarction (I63) | –1.3 | –2.4 to –0.2 | 0.02 |
| Stroke, not specified as haemorrhage or infarction (I64) | –4.4 | –7.0 to –1.8 | < 0.01 |
In London, LOS since the reconfiguration declined (Figure 15). The p-value under the null hypothesis that the regression coefficient for every month after the reconfiguration is the same as the regression coefficient for July 2010 was < 0.01; this appears to be due to a further decline in LOS since 2015.
FIGURE 15.
Adjusted trends in LOS in London. The dashed vertical line indicates when the reconfiguration in London occurred (July 2010). The p-value, under the null hypothesis that the regression coefficient for every month after the reconfiguration (which occurred in July 2010), is the same as the regression coefficient for July 2010, at a p-value of < 0.01. Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
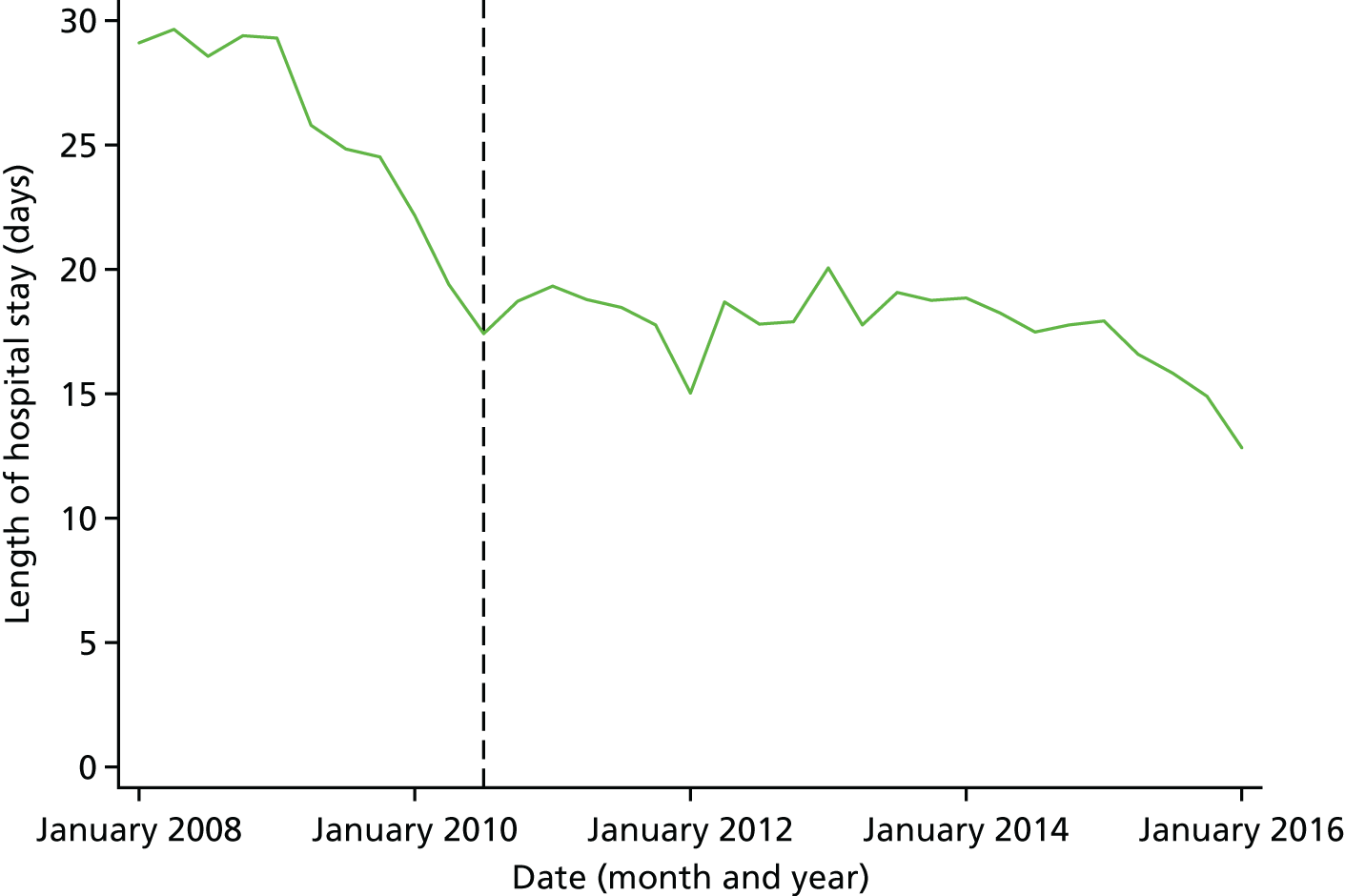
Analyses of clinical interventions
Treatment in a Comprehensive Stroke Centre/Primary Stroke Centre
In GM the proportion of patients treated in a CSC/PSC increased from 39% in 2010–12, to 57% in 2013/14, to 64% in 2014/15 and to 86% in 2015/16 (see Appendix 7, Table 56; the last time period corresponds to the GMB time period). Equivalent figures for London were ≥ 93% for every period.
Clinical interventions
In GMB, achievement of evidence-based clinical indicators in year 3 either stayed the same or improved compared with years 1 and 2 (Table 23; see Appendix 7, Figure 34). In year 3 there were improvements of > 10 percentage points for several ‘front door’ processes (brain scan within 60 minutes, arrival in SU within 4 hours), and assessments over the first 72 hours (consultant assessment within 14 hours, physiotherapist assessment, occupational therapist assessment and speech and language therapist within 24 hours). Improvements and levels of achievement in the RoE comparator during the same time period were either similar or lower than those seen in GM. In London the ‘front door’ processes, use of thrombolysis and assessments during the first 72 hours all stayed high and constant over the 3 years, with no more than a ± 5 percentage point fluctuation each year (Figure 16; see Appendix 7, Table 61).
| Intervention | Risk-adjusted proportions (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| GM | Comparator | ||||||
| Ramsay et al. (2015)26 | 2013/14 | 2014/15 | 2015/16 | 2013/14 | 2014/15 | 2015/16 | |
| Scan 60 minutes | 40.7 (39.1 to 42.2) | 49.2 (47.6 to 50.8) | 60.8 (59.4 to 62.2) | 43.0 (42.6 to 43.4) | 44.8 (44.4 to 45.2) | 47.6 (47.3 to 48.0) | |
| Scan 180 minutes | 65.2 (64.3 to 66.2) | 74.1 (72.7 to 75.4) | 81.7 (80.5 to 82.9) | 86.4 (85.4 to 87.3) | 70.9 (70.6 to 71.3) | 74.0 (73.7 to 74.3) | 77.0 (76.7 to 77.3) |
| Scan 24 hours | 94.0 (93.5 to 94.4) | 95.1 (94.4 to 95.8) | 97.1 (96.5 to 97.6) | 97.4 (96.9 to 97.9) | 94.9 (94.8 to 95.1) | 96.0 (95.8 to 96.1) | 96.7 (96.6 to 96.8) |
| tPA to eligible patients | 67.9 (63.6 to 72.1) | 82.3 (78.1 to 86.5) | 88.6 (85.5 to 91.7) | 80.6 (79.8 to 81.3) | 89.1 (88.5 to 89.7) | 91.8 (91.3 to 92.4) | |
| tPA within 60 minutes | 74.4 (69.6 to 79.2) | 70.5 (65.1 to 75.8) | 74.6 (70.2 to 78.9) | 53.1 (52.0 to 54.2) | 57.1 (56.0 to 58.1) | 59.7 (58.6 to 60.7) | |
| Swallow screen in 4 hours | 58.7 (57.1 to 60.3) | 63.4 (61.8 to 65.0) | 69.2 (67.8 to 70.6) | 59.7 (59.3 to 60.1) | 63.9 (63.5 to 64.2) | 67.7 (67.3 to 68.0) | |
| SU within 4 hours | 55.9 (54.9 to 57.0) | 57.4 (55.8 to 59.1) | 58.8 (57.1 to 60.5) | 79.1 (77.9 to 80.4) | 51.3 (50.9 to 51.7) | 52.8 (52.4 to 53.2) | 53.4 (53.0 to 53.7) |
| Consultant assessment within 14 hours | 57.6 (55.9 to 59.2) | 58.9 (57.2 to 60.6) | 79.2 (78.0 to 80.4) | 50.6 (50.2 to 51.0) | 52.1 (51.7 to 52.5) | 52.6 (52.2 to 53.0) | |
| Consultant assessment within 24 hours | 78.2 (76.9 to 79.6) | 80.2 (78.9 to 81.5) | 93.0 (92.3 to 93.8) | 81.1 (80.8 to 81.4) | 82.8 (82.5 to 83.1) | 84.3 (84.1 to 84.6) | |
| Nurse assessment within 12 hours | 86.4 (85.3 to 87.4) | 86.6 (85.5 to 87.7) | 91.2 (90.4 to 92.0) | 86.5 (86.2 to 86.7) | 88.1 (87.8 to 88.3) | 89.3 (89.0 to 89.5) | |
| Nurse assessment within 24 hours | 93.4 (92.7 to 94.2) | 92.8 (92.0 to 93.6) | 94.6 (93.9 to 95.2) | 93.2 (93.0 to 93.4) | 93.9 (93.7 to 94.0) | 94.5 (94.4 to 94.7) | |
| Physiotherapist assessment within 24 hours | 55.4 (53.7 to 57.0) | 63.2 (61.6 to 64.8) | 80.7 (79.5 to 81.9) | 55.6 (55.2 to 56.0) | 57.1 (56.7 to 57.4) | 59.7 (59.3 to 60.1) | |
| Physiotherapist assessment within 72 hours | 92.1 (91.5 to 92.7) | 95.1 (94.3 to 95.8) | 97.0 (96.4 to 97.6) | 97.5 (97.1 to 98.0) | 93.6 (93.4 to 93.8) | 93.4 (93.2 to 93.6) | 93.7 (93.5 to 93.9) |
| Occupational therapist within 24 hours | 50.5 (48.8 to 52.2) | 61.2 (59.6 to 62.8) | 79.4 (78.1 to 80.6) | 45.2 (44.7 to 45.6) | 47.5 (47.1 to 47.9) | 52.3 (51.9 to 52.7) | |
| Occupational therapist within 72 hours | 94.4 (93.6 to 95.1) | 96.4 (95.8 to 97.0) | 96.9 (96.4 to 97.4) | 87.3 (87.1 to 87.6) | 88.5 (88.2 to 88.7) | 89.7 (89.4 to 89.9) | |
| SaLT swallow within 24 hours | 53.2 (50.8 to 55.6) | 56.9 (54.4 to 59.4) | 55.7 (53.1 to 58.2) | 48.4 (47.8 to 49.0) | 49.9 (49.3 to 50.5) | 54.0 (53.4 to 54.6) | |
| SaLT swallow within 72 hours | 94.0 (93.6 to 94.4) | 85.5 (83.9 to 87.2) | 91.3 (89.9 to 92.7) | 91.9 (90.6 to 93.3) | 78.8 (78.3 to 79.3) | 82.2 (81.7 to 82.6) | 84.9 (84.5 to 85.3) |
| SaLT communication within 24 hours | 39.7 (37.3 to 42.2) | 53.8 (51.5 to 56.2) | 69.9 (68.0 to 71.7) | 35.5 (34.9 to 36.1) | 38.0 (37.5 to 38.6) | 42.0 (41.5 to 42.6) | |
| SaLT communication within 72 hours | 86.7 (85.1 to 88.3) | 93.4 (92.2 to 94.5) | 96.6 (95.9 to 97.3) | 78.3 (77.8 to 78.7) | 81.6 (81.2 to 82.0) | 85.1 (84.7 to 85.5) | |
FIGURE 16.
Risk-adjusted likelihood of patients receiving clinical interventions in London. Error bars, 95% CI; SaLT, Speech and Language Therapist; tPA, tissue plasminogen activator. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients). Scan 60, brain scan within 60 minutes of clock start; Scan 180, brain scan within 180 minutes of clock start; Scan 24 hours, brain scan within 24 hours of clock start. Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
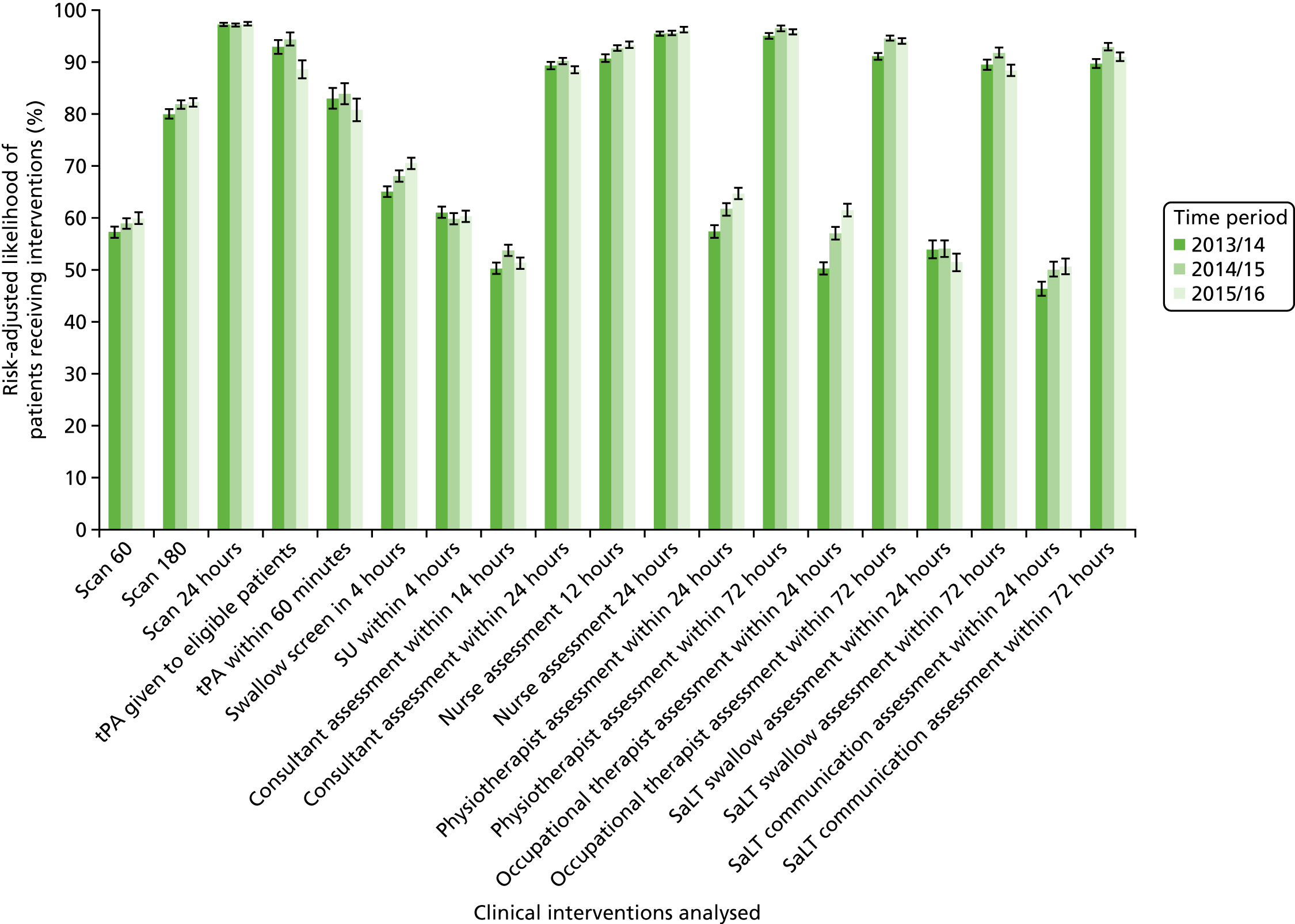
Discussion
Principal findings
The principal findings of this analysis are as follows.
-
There was a decline in stroke mortality following the GMB reconfiguration, but reductions in mortality were also seen elsewhere in the RoE over the period; these national reductions appear to have been slightly smaller than the reductions in GMB, although most of the changes over time were not statistically significantly different between the two areas. We interpret this to mean that across all hospitals in GM there was not a significant impact on mortality over and above the changes seen in the RoE. There was, however, a reduction in all-cause mortality in any location (not just the hospital) seen in the GM CSC and PSCs during the GMB period that was statistically significantly different and larger than the reductions seen elsewhere in the RoE, and would amount to approximately 69 fewer deaths per year.
-
There was a significant reduction in LOS in GMB of 1.5 days per patient over and above the reduction seen in the RoE, which would amount to approximately 6750 fewer bed-days a year.
-
In GM a larger proportion of patients were treated in CSC/PSC following the GMB reconfiguration. Achievement of evidence-based clinical indicators either stayed the same or improved compared with the RoE comparator.
-
In the analysis for London there appears to be little variation in mortality, LOS and evidence-based clinical indicators over time since the reconfiguration in July 2010. This indicates that the improvements following the centralisation in London have been sustained.
Strengths and weaknesses
The strengths and weaknesses are similar to the previous analyses (see Chapters 3 and 4). The strengths related to the large national data sets that we used and the robust, quasi-experiment analytical framework we employed. Limitations included a lack of data on stroke severity in the HES database, a lack of data on QoL, a lack of data on pre-hospital processes, outcomes and limitations in measurement of LOS when patients may be repatriated between SU. Our analysis used only data for patients treated up to 1 year after the GMB reconfiguration, which may have limited the likelihood of demonstrating a significant impact of the further reconfiguration.
Implications
The increased number of patients treated in CSC/PSC in GM following further reconfiguration led to improvements in LOS and in clinical indicators on average across GM as a whole. There were reductions in mortality among patients treated at a CSC/PSC, but there was no overall reduction in mortality on all admitted stroke patients (i.e. including the proportion who were not treated at a CSC/PSC). The reduction in mortality seen in the GM CSC/PSCs resulted in 69 fewer deaths per year. This exceeded the ‘50 fewer deaths per year’ figure that we calculated based on the results of our previous analysis (see Chapter 3), if the 1.1-percentage-point reduction in 90-day mortality in London was achieved in GM. Although this reduction in mortality in the CSC/PSCs saved lives given that in London > 90% patients are treated in a HASU, this suggests that even more lives might be saved if a higher proportion of GM stroke patients were treated in CSC/PSC. We were able to analyse only data for patients treated up to 1 year after the GMB reconfiguration, and SSNAP data indicate that the proportion of patients treated in a CSC/PSC and receiving clinical interventions has continued to increase in GM. 213 It would, therefore, be beneficial for further research on and monitoring of the relationship between the proportion of patients treated at a CSC/PSC, delivery of clinical interventions, and reductions in mortality and LOS over a longer period. The impact of the London reconfiguration in July 2010 on mortality, LOS and evidence-based clinical indicators was sustained up until March 2016, which suggests that changes of this kind are sustainable.
Chapter 12 Cost-effectiveness of further reconfiguration of acute stroke care in Greater Manchester B
Overview
What was already known about this subject?
-
The reconfiguration of acute stroke services in GMA covering the period up to March 2012 was found to be cost-effective compared with changes that happened elsewhere in England. This was primarily as a result of cost-savings per patient attributable to reductions in LOS in GM compared with in the RoE (see Chapter 5).
-
The GMB model was associated with significant improvements in care provision and LOS overall, and significant reductions in patient mortality for patients treated in CSC/PSCs (see Chapter 11).
What this chapter adds
-
Over 90 days, GMB cost £932 less per stroke patient than GMA. This is compared with a cost saving of £635 in the RoE over the same time period.
-
GMB also resulted in 4 additional QALYs per 1000 patients over 90 days compared with GMA. In the RoE there were 3 additional QALYs per 1000 patients over the same time period.
-
At 90 days there was an 88% probability that GMB was cost-effective compared with changes that occurred in the RoE over the same time period at a WTP for a QALY of £20,000 to £30,000.
-
At 10 years there was a 31% probability that GMB was cost-effective compared with changes that occurred in the RoE over the same time period at a WTP for a QALY of £20,000 (39% for a £30,000 WTP for a QALY). This was driven primarily by an apparent 3% increase in discharge to non-acute NHS inpatient providers, according to HES data. Further work is required to validate this and refine other values in the model, potentially with richer data sets such as SSNAP.
Background
In Chapter 5 we presented our analysis of the cost-effectiveness of the reconfigurations in London and GMA. The analysis was based on a 10-year decision model and the same data used in the analysis of clinical outcomes and clinical interventions (see Chapters 3 and 4). The results of this analysis can be summarised as follows:
-
The reconfigurations in London and GM were cost-effective compared with changes that occurred over the same time periods in the RoE at a cost per QALY threshold of £20,000 to £30,000 per QALY gained.
-
In London this was primarily due to reductions in mortality that resulted in additional QALYs for patients.
-
In GM this was a result of per patient cost-savings from reduced LOS.
Our analysis of clinical outcomes of GMB found no significant reduction in mortality compared with the RoE, except in CSC/PSCs, but found a significant reduction in LOS (see Chapter 11). This significant reduction in LOS in particular warranted an evaluation of the cost-effectiveness of GMB. Given that the primary reconfiguration (GMA) was found to be cost-effective, the appropriate analysis was to compare the cost-effectiveness of GMB compared with that of GMA. As improvements in mortality and LOS have also been seen in the RoE, we used a difference-in-differences analysis63 to evaluate if GMB resulted in a higher NMB (either more QALYs or cost-savings) than in the RoE over the same time period.
The improvements in mortality and LOS were sustained in London (see Chapter 11). It was, therefore, reasonable to assume that the reconfiguration in London continued to be cost-effective and that an additional evaluation of the cost-effectiveness of the London reconfiguration was unnecessary.
The RQ we addressed in this chapter was:
-
In GM, was further centralisation cost-effective compared with changes that occurred in the RoE at the same time period at 90 days and 10 years?
Method
Data
We used the same data as the analysis of clinical outcomes and clinical interventions (see Chapter 11, Analyses of clinical outcomes and Analyses of clinical interventions for variables and data used).
Statistical analyses
The decision model described in Chapter 5 was updated with data for the reconfigurations in GM that took place in April 2010 (GMA) and April 2015 (GMB). Data for the same time period for the RoE (urban areas only and excluding London) were also used to update the model.
The cost of implementation was from a provider perspective only. An extra £2.4M in investment was provided to assist with the additional cost of the CSC/PSCs. This was given as an uplift to the tariff and included in the analysis as an additional cost of a CSC/PSC admission. All other costs remained the same as those reported in Table 11.
We report the NMB of the additional costs and QALYs of GMB compared with those of GMA, and compare that to the additional costs and QALYs in the RoE over the same time period. We report the results at 90 days and 10 years. Time to recurrent stroke and time to death were also updated in the 10-year model based on HES data. Costs and QALYs beyond 12 months were discounted at a rate of 3.5%.
We conducted sensitivity analyses to test the impact of different assumptions made in the model. The results for only those sensitivity analyses that had a significant impact on the results are reported in this chapter.
Results
Analyses of clinical outcomes
The results of the 90-day and 10-year cost-effectiveness analysis are reported in Table 24. At 90 days GMB compared with GMA resulted in lower costs and more QALYs than in the RoE. There was an 88% chance, however, that the changes were cost-effective at a WTP for a QALY gain of £20,000 and £30,000. At 10 years there was a 31% probability that GMB is cost-effective compared with changes that happened in the RoE at a WTP of £20,000 per QALY (39% at a WTP of £30,000; Figure 17). Although GMB resulted in more QALYs than the RoE over the same time period (an additional 6 QALYs) this was also for a greater cost (£719,948), resulting in an incremental cost-effectiveness ratio of £130,667 per QALY gained.
| Outcomes | Region | DID | |||||
|---|---|---|---|---|---|---|---|
| GM | RoE | ||||||
| Before | After | Difference | Before | After | Difference | ||
| 90-day results | |||||||
| Deaths (n) | 124 | 107 | –17 | 120 | 120 | 0 | –17 |
| LOS (days) | 12 | 8 | –4 | 11 | 9 | –2 | –2 |
| QALYs | 99 | 104 | 4 | 106 | 109 | 3 | 1 |
| Costs (£) | 4,465,323 | 3,532,906 | –932,417 | 3,872,244 | 3,236,413 | –635,831 | –296,586 |
| 10-year results | |||||||
| Deaths (n) | 370 | 353 | –17 | 368 | 356 | –12 | –5 |
| QALYs | 3386 | 2882 | 504 | 2890 | 3388 | 498 | 6 |
| Costs (£) | 40,060,050 | 36,904,129 | –3,155,921 | 39,735,568 | 35,932,607 | –3,802,961 | 647,040 |
| NMB £20,000 per QALY (per patient) (£) | 13,236 | 13,783 | –547 | ||||
| NMB £30,000 per QALY (per patient) (£) | 18,537 | 19,019 | –482 | ||||
FIGURE 17.
Cost-effectiveness acceptability curve of NMB of second reconfigurations in GMB compared with the RoE at 90 days and 10 years (discounted).
Sensitivity analysis
The location that patients are discharged to is a key driver of the 10-year cost-effectiveness of the model. In GMB compared with GMA and the RoE there was an increase from 6% (n = 966) to 9% (n = 319) in the percentage of patients discharged to a non-acute NHS inpatient provider, most commonly a community hospital. No other changes to discharge destination were observed, other than an equal reduction to the number of people discharged home. Following interrogation of the discharge destination, we identified two outlier hospitals and that the majority of the change occurred in the last quarter of 2015/16, potentially owing to incomplete data. As a result we conducted the following sensitivity tests for discharge destination and time to discharge:
-
removal of the two outlier hospitals for both GMA and GMB
-
removing the data for the last quarter of 2015/16
-
holding discharge destination constant from GMA to GMB.
The number and proportion of people discharged to each location in GMA and GMB used for each of the three sensitivity analyses and the base case (values used to generate the results in Analyses of clinical outcomes above) are reported in Table 25. The results of the sensitivity analyses are reported in Table 26. In all three instances, GMB is cost-effective at 90 days compared with the RoE, resulting in less cost for fewer deaths. In analysis 1 and 2, when outliers are removed, GMB is not cost-effective at 10 years compared with the RoE. In analysis 3 (holding discharge destination constant from GMA to GMB), GMB is cost-effective at 10 years at £30,000 WTP for a QALY gained.
| Sensitivity analysis | Discharge location, n (%) | |||
|---|---|---|---|---|
| Home | Publicly funded care home | Private care home | Other NHS inpatient provider | |
| Base case | ||||
| RoE before | 163,531 (81) | 14,406 (7) | 10,562 (5) | 12,715 (6) |
| RoE after | 33,230 (83) | 2710 (7) | 1534 (4) | 2591 (6) |
| GMA | 13,058 (83) | 1209 (8) | 591 (4) | 966 (6) |
| GMB | 2821 (80) | 259 (7) | 112 (3) | 319 (9) |
| Remove outlier hospitals | ||||
| GMA | 9763 (85) | 1060 (9) | 332 (3) | 279 (2) |
| GMB | 1493 (82) | 209 (12) | 55 (3) | 46 (3) |
| Remove data for last quarter of 2015/16 | ||||
| GMA | 13,058 (83) | 1209 (8) | 591 (4) | 966 (6) |
| GMB | 2172 (81) | 220 (8) | 96 (3) | 185 (7) |
| Keep discharge location constant | ||||
| GMA | 13,058 (83) | 1209 (8) | 591 (4) | 966 (6) |
| GMB | 2897 (83) | 268 (8) | 131 (4) | 214 (6) |
| Discharge destination | Region | Difference | DID (compared with RoE in Table 24) | |
|---|---|---|---|---|
| GMA | GMB | |||
| Remove outlier hospitals | ||||
| 90-day deaths (n) | 123 | 106 | –16 | –16 |
| 90-day costs (£) | 4,006,121 | 3,250,940 | –755,181 | –119,350 |
| 10-year QALYs | 2854 | 3312 | 457 | –50 |
| 10-year costs (£) | 40,444,428 | 39,372,913 | –1,071,514 | 2,731,447 |
| NMB (£) | 14,791 | 4228 | ||
| Remove last quarter 2015/2016 | ||||
| 90-day deaths (n) | 123 | 107 | –16 | –16 |
| 90-day costs (£) | 4,247,744 | 3,368,802 | –878,942 | –243,111 |
| 10-year QALYs | 2880 | 3391 | 511 | 4 |
| 10-year costs (£) | 39,904,278 | 37,216,333 | –2,687,945 | 1,115,016 |
| NMB (£) | 18,020 | 998 | ||
| Keep discharge location constant | ||||
| 90-day deaths (n) | 123 | 107 | –16 | –16 |
| 90-day costs (£) | 4,207,833 | 3,175,658 | –1,032,176 | –396,345 |
| 10-year QALYs | 2871 | 3414 | 543 | 36 |
| 10-year costs (£) | 40,077,483 | 36,871,703 | –3,205,780 | 597,181 |
| NMB (£) | 19,492 | –473 | ||
Discussion
Principal findings
At 90 days the reconfigurations in GMB dominated changes that happened in the RoE, with an 88% probability of being cost-effective at a £20,000 WTP for a QALY gained. At 10 years, both GMB and the RoE maintained their cost-savings and decrease in deaths, but there was only a 31% chance that GMB was cost-effective. This may be due to the short maximum of 1-year follow-up available for GMB rather than the changes in GMB not being maintained over longer follow-ups. The discharge destination recorded in HES also has implications for the results. Further work is required to validate the 3% increase in discharge to non-acute NHS inpatient providers given the impact it has on the results. If it is incorrect and is a coding error, GMB is potentially cost-effective at 10 years.
Strengths and weaknesses
The strengths and weaknesses were similar to those of the previous analysis (see Chapter 5). The strengths related to the large national data sets we used and the robust quasi-experiment analytical framework we employed. Limitations were similar to those of the clinical outcomes and interventions analysis (see Chapter 11). Additional limitations were the number of the assumptions made to the model, as discussed in Chapter 5.
The short follow-up duration following the second reconfiguration of stroke care in GM made it challenging to assess the long-term implications of the reconfigurations.
Implications
There was clear evidence that the second reconfigurations of stroke care in GM were cost-effective at 90 days. There was less evidence for this being maintained over 10 years, however. In Chapter 5, we reported that the first reconfiguration of stroke care in GM was cost-effective compared with changes that happened in the RoE over the same time period. This result was driven by the reduction in LOS only. The second reconfiguration of GM included an additional investment of £2.4M as an uplift to the stroke tariff and has resulted in additional reductions in LOS and reductions in mortality in CSC/PSCs (see Chapter 11). At 90 days the additional investment and reconfiguration translated to net cost-savings due to reduced LOS, although the cost-savings were not maintained at 10 years. GMB resulted in additional QALYs at 90 days and 10 years, although the additional health benefit of 6 QALYs per 1000 patients at 10 years was not sufficient to compensate the reduction in cost-savings compared with the RoE over the same time period.
The 3% increase in discharge to non-acute NHS inpatient providers in GMB is of concern, particularly given the implications for the findings. As a result this warrants additional investigation potentially using another data set such as SSNAP.
It is important to note that the cost-savings we modelled were realised by stroke care providers and not commissioners of stroke care services. Commissioners pay for stroke admissions as a tariff per admission, whereby additional costs per bed-day for LOS are incurred only after a trim point. In the case of stroke this was a LOS > 40 days. As a result, commissioners would realise cost-savings only as a result of the reconfigurations (1) if the reconfigurations prevented patients being readmitted for stroke or (2) if a lower percentage of patients were still in hospital after the trim point. We found no evidence that GMB prevented readmissions compared with GMA and changes that occurred in the RoE at the same time. This may be because the follow-up duration was not sufficient to detect a difference. Based on the model there was a reduction in the percentage of patients still in hospital at 41 days in GMB compared with GMA of 3.9% (2.2% of patients still in hospital at 41 days for GMB, compared with 6.1% in GMA). In the RoE over the same time period there was a reduction of 1.7% of patients in hospital at 41 days (2.1% of patients at 41 days after compared with 3.8% before). After the reconfigurations there was a reduction of 888 bed-days per 1000 patients after the trim point in GM compared with 338 in the RoE over the same time period. This translated to a reduction of 540 bed-days following the trim point for GMB compared with that for the RoE. Assuming a cost per bed-day of £200, this was a total cost saving to the commissioner of £108,000 per 1000 strokes for additional bed-days. Assuming 4500 strokes per year in GM, this was still £2M shy of the total investment made.
Chapter 13 Lessons from the planning and implementation of further reconfiguration in Greater Manchester
Overview
What was already known about this subject?
-
Service centralisation in GM led to significant reduction in LOS, but not in mortality, compared with in the RoE. Therefore, further reconfiguration of services was planned and implemented.
-
There is growing evidence about how approaches to leadership, use of feedback, stakeholder engagement and experience of previous changes influence implementation of MSC in acute stroke services (see Chapters 6, 7 and 10).
What this chapter adds
-
The GM acute stroke system – like the rest of the English NHS – faced significant obstacles over the period studied and these affected the time it took to agree a new model, plan it and implement it. Despite these obstacles, change was implemented.
-
Obstacles included turbulence caused by reforms to the NHS and national staffing shortages, which contributed to significant pressures on planning. Post implementation, these factors led to delays in the movement of patients through the system, in finding beds for stroke patients and in discharging stroke patients.
-
Issues relating to leadership and governance, and the use of service and process reviews, including the earlier findings from this study (see Chapter 3) were reported as important in enabling implementation.
-
Leaders of the GMB reconfiguration ‘held the line’ on approaches to implementation (e.g. the timing and degree of phasing of change). A key system enabler post implementation was identified as the Operational Delivery Network (ODN) governance model, funded by the providers, covering the whole stroke pathway. This enabled regular audits and a mechanism to facilitate the system-wide discussions needed to maintain effective system operation. Sources of pressure post implementation included not only staffing but also changes in the nature of workload at CSC, PSCs and DSCs caused by the reconfiguration.
Background
Further reconfiguration of the GM acute stroke pathway (GMB) was implemented from March 2015 (see Chapter 11). This chapter analysed the process of agreeing, planning and implementing this reconfiguration. The Best et al. 5 framework of rules for MSC (see Chapter 6110) has been used to explain the previous centralisation of acute stroke services in GM, showing that feedback may need to be combined with other tools, such as financial incentives and engaging a range of stakeholders, not only physicians. Fraser et al. 203 explored change management roles in the London reconfiguration, suggesting that evidence from clinical research can be used to frame change in a ‘depoliticised’ way. They identified discursive power, emphasising evidence, better patient outcomes and professional support as helpful in generating receptiveness to change. Many authors have emphasised the importance of context when analysing change, pointing out that much evaluative work seeks to eliminate contextual confounders rather than see context as the state into which change must be integrated215 and taken into account. 216 This chapter explored how an analysis of GMB may further develop the understanding of MSC.
Methods
Design
This chapter presents a single case study, based in GM, as described in Chapter 1. Comparison is made with GMA, reported in Chapters 6 and 7, and is discussed in relation to the quantitative data analysis of GMB (see Chapter 11) and sustainability in London (see Chapter 14).
Data
As detailed in Chapter 2, we conducted interviews with 78 stakeholders (governance and service level) and 59 non-participant observations of relevant meetings and events (approximately 120 hours). We also collected > 100 documents (meeting minutes and papers, key local documents, etc.). Data were collected between December 2013 and March 2017 (see Table 2 and Chapter 2, Understanding development, implementation and sustainability), enabling analysis of the 2-year post-implementation qualitative data.
Analysis
We identified those factors that influenced the decision to change, the planning and the implementation approach of GMB, and GMB post implementation. We combined analyses of interviews, meeting notes and documents in order to develop an understanding of MSC in relation to previous evidence (see Chapter 6).
Results
This section describes the findings in relation to three phases of activity in GM (aligned to the framework described in Chapter 7):
-
the ‘to agreement’ phase (decision to change and which model to implement)
-
the ‘planning’ phase (implementation approach)
-
the ‘post-implementation’ phase (implementation outcome).
Table 27 provides details of the activities undertaken up to the implementation date. Nearly 2 years elapsed between the recommendation for further reconfiguration and the formal agreement to the full implementation of the centralised acute stroke care pathway, and it was a further 18 months before the fully centralised pathway was launched: a total of 3.5 years between decision and implementation. We also studied GMB for 24 months post implementation and highlight here the issues relating to the sustainability of the model, which aligned with those identified in London (see Chapter 14).
| Date | Timescale proposals | Governance and leadership | Reviews/feedback | Recommendations/agreements |
|---|---|---|---|---|
| August 2011 | 12-month review of GMA | |||
| October 2011 | EAG recommendation: remove 4-hour cut-off for treatment at a CSC/PSC | |||
| November 2012 | Peer reviews of all SUs (CSC/PSCs/DSCs) | |||
| April 2013 | NHS reforms | |||
| July 2013 | April 2014 proposed for implementation of fully centralised care model. CSC/PSCs had reservations as they were not due to receive financial go-ahead until September/October 2013 | |||
| September 2013 | Association Governing Group (of CCGs) formally agreed full implementation (removing 4-hour limit) | |||
| November 2013 | Programme Manager appointed to manage the planning/implementation | |||
| December 2013 | Gateway Review of process | |||
| January 2014 | Senior Responsible Officer and Chief Financial Officer appointed to act on behalf of all GM CCGs to provide executive leadership and sponsorship for GM reconfiguration | |||
| March 2014 | September 2014 date for implementation of centralised model proposed but considered optimistic | First meeting of Greater Manchester Association of CCGs Governing Group Stroke Implementation Board | ||
| July 2014 | Stroke Implementation Board agreed to work towards an implementation date of January 2015 | Reported at Stroke Implementation Board: finances at CSC and one PSC agreed | ||
| August 2014 | Publication of research relating to mortality and LOS following stroke in GM and London in the BMJ25 | |||
| October 2014 | Clinical Senate review of the plans for the centralised care pathway | |||
| November 2014 | Stroke Implementation Board decision: implementation date to be moved to the end of March 2015 (to allow second PSC to be ready) | Reported at Stroke Implementation Board: finances at the second PSC agreed | ||
| March 2015 | Acute Trust Chief Executives agreed the setting up of a stroke ODN | GM fully centralised acute stroke care pathway launched (30 March 2015) |
To agreement
Activities that were undertaken during the ‘to agreement’ phase are outlined, as well as aspects of governance, which influenced the timescale.
The 12-month review
The 12-month review of GMA, which drew heavily on SINAP data, revealed that only 64% of patients presenting within 4 hours of symptom development were transported to a HASU (CSC/PSC). 32 The review concluded that although progress had been made ‘there is further work still to be done in ensuring that all patients have equitable access to the best quality stroke and TIA services’. 32
The EAG, established in 2008 to carry out an independent review of plans for GMA and oversee provider selection, reconvened in October 2011 to discuss the GM pathway in the light of the review and recommended that there should be further reconfiguration of acute stroke services, with the 4-hour cut-off for transportation to a CSC/PSC removed.
Peer reviews
Following the EAG recommendation, peer reviews were carried out in all SUs (HASUs and DSCs) because commissioners were concerned about the variation in services across GM commented on by the EAG, although some felt that this caused delay:
What needs to be done is further centralisation . . . but the immediate response was 12 months of peer reviews.
GM07(2), SCN
Governance
The reforms of the NHS following the Health and Social Care Act 2012,34 implemented in April 2013, were perceived as resulting in a lack of continuity in leadership, ownership of changes and decision-making, and in the loss of relationships and knowledge. The build-up to this reorganisation, when PCTs were being phased out and CCGs formed (during the ‘to agreement’ phase), affected decision-making:
. . . it was kind of difficult for people to make decisions about a GM-wide thing for about an 18-month period, maybe 2 years until things had settled down.
GM07(2), SCN
The loss of ‘organisational knowledge’ due to impending NHS changes was also noted:
The organisation at the top kept changing and the people changing. So there wasn’t that continual organisation memory you know and that impacted on their ability to make a decision.
GM07(2), SCN
Once new NHS structures were introduced in April 2013, it took time before they were functioning efficiently, contributing to further delays in decision-making. The reconfiguration was finally agreed by the newly formed Greater Manchester Association of Clinical Commissioning Groups Governing Group (AGG) in September 2013, rather than the original plan for agreement in April 2013 by a group disbanded post April. These delays were explained in terms of the ‘newness’ of the organisations and the different composition of the new NHS structures:
I don’t think they [newly formed AGG] were intentionally stalling, it was almost as though they couldn’t make a decision . . . they weren't mature as a group together.
GM02(2), SCN
. . . most GP chairs in CCGs hadn’t had any exposure to the policy and strategy world of the PCTs.
GM18, service commissioner/Implementation Board
Planning
Once agreement was reached in September 2013, formal planning for implementation began, although informal activities had already started. Issues of leadership, governance and feedback were highlighted as influencing process and timescale, with some other specific considerations during planning.
Leadership capacity
Concern was initially expressed about the scale of the changes:
It was kind of assumed that it was a relatively small change, when in fact it was probably as big a change as it had been the first time around.
GM05(3), stroke physician
Project management arrangements were put in place just after the start of the ‘planning’ phase (November 2013) by the AGG, something viewed as pivotal in moving the work forward:
A huge piece of work, it really does need someone who has experience in programme management full time against it, I think it’s a huge risk to not do that.
GM24, SCN
This role was seen as effective and important in supporting the decisions made through the new governance structures, as well as alleviating initial concerns.
Governance
The Stroke Centralisation Implementation Board was established in March 2014, to ensure that the centralisation of acute stroke services was undertaken in line with the agreed timescale and budget. It was accountable through the Chairperson (a new appointment; Senior Responsible Officer for stroke) to the AGG. A Chief Financial Officer was also appointed to act on behalf of all GM CCGs. The Implementation Board was perceived to work well and to involve clinicians in a way that was viewed positively: ‘there has been a culture where clinicians have become a bit marginalised and decisions are being made by the managers’ (GM23, stroke physician).
Feedback from external reviews
During the planning period, two reviews of the GM stroke pathways were undertaken, the findings of which were perceived as supporting the plans. A Gateway Review (which examines projects at key decision points in their life cycle to provide assurance that they can progress successfully to the next stage)217 in December 2013 made recommendations for governance, which were being planned anyway, and was viewed as helpful ‘. . . because we knew what we wanted to happen but sometimes it's better if it comes from somebody else’ [GM02(2), SCN]. A Clinical Senate Review to provide ‘clinical advice with regard to optimising the working of the network model’ (p. 3, reproduced with permission from Greater Manchester, Lancashire and South Cumbria Clinical Senate218) made recommendations regarding the provision of imaging and communication with other change planners, and the review was perceived as lending support to the reconfiguration plans.
Feedback from research findings
The publication of findings of this research (see Chapter 3) was widely attributed as helping to drive the planning of reconfiguration forward in GM and gain agreement on an implementation date:
being able to go to meetings and say to people we’re looking at fifty excess deaths a year . . . because of the publicity . . . because it was a paper and it was a medical journal not just another audit report or just another internal report, I think that has had a significant impact.
GM05(2), stroke physician
It would be extremely difficult to argue for e.g. 3–4 months [slippage] in light of the mortality data . . . which indicates that as many as 16 deaths from stroke could be avoided in that period of time if services were centralised.
Stroke Centralisation Implementation Board meeting, 12 September 2014
Other considerations
At one PSC, the ongoing failure to meet A&E waiting time targets led to nervousness about putting more patients through the department when MSC in GMB was being implemented. The same PSC had carried out financial modelling showing that GMB was more costly for them than the CSC and the other PSC. This led to uncertainty about whether or not this PSC would retain its status as well as to delays in planning by the CSC and other PSC.
The A&E issue was resolved by establishing a ‘stroke specific’ bay in the A&E department, staffed by the PSC, thus minimising impact on the wider A&E department. Following discussions between the CSC and PSCs, including input from the Chief Financial Officer, the modelling issue was resolved, but not until late 2014, which led to the GMB ‘go live’ date being moved back from January to March 2015. This also led to a loss of staff from the CSC who had been employed assuming the January start but who were not needed until March.
Staff morale was affected during this period, with those at DSCs concerned about the potential impact on their roles following reconfiguration, both in terms of losing their jobs and/or skills in acute stroke care. Doubt was also expressed about whether or not it would be possible to recruit sufficient staff – doctors, nurses and therapists – to provide the centralised services.
The delays in agreeing the final launch date meant that the CSC and PSCs were well prepared for launch, although the short time frame between the decision to confirm the second PSC and launch meant that there was a lot of preparation needed in just 3 months.
Implementation: a ‘big bang’ launch
Implementation of GMB took place on 30 March 2015, as a ‘big bang’ rather than a phased launch, as had been the case with GMA (see Chapter 7). Leaders held the line on this approach when phased implementation was proposed, because of the difficulties in phasing presented to the ambulance service and concern about capacity issues if a CSC and one PSC went ‘live’ while the second PSC was not fully operational (‘learning from history’).
Post implementation
Findings are presented here using the same framework for analysis as in Chapter 14, enabling us to compare the post-implementation periods in London and GM.
Enablers of governance: system leadership
Following implementation, a stroke ODN219 – the first in England – was established to support stroke services, as the clinical network no longer had capacity following the 2013 NHS reforms. The clinical network funded this for the first year, with providers taking over subsequently. Within 6 months of implementation, an ODN manager and project co-ordinator started work, and the newly formed ODN Board met. The ODN’s remit was to ‘work collaboratively with its stakeholders to develop high-quality services in terms of patient outcomes and experience across the whole care pathway for stroke’ (GM Stroke ODN, Terms of Reference).
Initially, there was confusion over the ODN’s role and there was a perception that because it was hosted at the CSC site that it might be biased. However, over the first year of its work, many of these worries were allayed, with clinicians becoming supportive of the work:
I think the ODN is a really good sort of go-between, and it’s a way of providing an independent interface for everybody.
GMF20, stroke physician
Most felt that the ODN, or similar, was necessary for the sustainability of the stroke care pathway, although there was uncertainty about how it linked with the evolving GM governance structures following devolution,121,122 whether or not it had any ‘clout’, and whether or not funding would be continued.
Challenges to sustained governance: local and national NHS changes
Changing priorities nationally – ‘stroke isn’t really an NHS priority anymore whereas it clearly was at one time’ [GM05(4), stroke physician] – and locally in GM caused concern. The implications for stroke of ongoing changes to general acute care provision resulting from merger and rationalisation in GM were highlighted, as was the challenge of providing a thrombectomy service in future.
Flow of patients through the hub and spoke model
In order for the stroke pathway to operate optimally, the timely flow of patients through the system is necessary and was a challenge in GMB and London (see Chapter 14).
Transfer of stroke patients to a Comprehensive Stroke Centre/Primary Stroke Centre
Prior to GMB, the local ambulance service called ahead to A&E departments when they were bringing in a suspected stroke patient (within 4 hours of symptom onset). Post implementation, patients within 48 hours of symptom onset were brought to CSC/PSCs, leading to a large increase in the volume of alert calls, which A&E departments were unable to handle. The situation, therefore, changed back to the GMA model in which an alert was radioed ahead only for those stroke patients whose symptoms had commenced within 4 hours. Initially, this resulted in stroke patients whose arrival had not been alerted waiting in A&Es without the knowledge of stroke teams. Local solutions were then developed to ensure that stroke teams saw these patients in a timely manner.
Six months after implementation, following advice from the National Clinical Director for Stroke, processes were changed so that only patients presenting within 48 hours of stroke symptoms were transported to a CSC/PSC, rather than all patients, although this change did not receive universal support. A patient presenting at a DSC within 48 hours of commencement of symptoms might be transferred to a CSC/PSC depending on the clinical presentation. After initial challenges, with staff at DSCs reporting that it could be difficult to contact CSC/PSC staff, the process improved, and audit data indicated that patients were being transferred appropriately.
By March 2016, 86% of stroke patients were being treated at a CSC/PSC and a local audit (by the ODN) indicated that the ambulance service was taking patients appropriately to a CSC/PSC.
Repatriation from a Comprehensive Stroke Centre/Primary Stroke Centre to a District Stroke Centre
Repatriation of stroke patients from a CSC/PSC to a DSC was challenging because of ‘stroke’ beds being used for other patients, or because of the difficulty of discharging people from hospital. DSCs were notified when one of ‘their’ patients was admitted to a CSC/PSC, allowing them to plan to have a bed available, and CSC/PSC staff then contacted DSCs each day with updates, using nhs.net e-mail accounts, supplemented by telephone calls. Responsibility for this contact usually lay with nursing staff, although at the CSC a repatriation co-ordinator was employed. Such contact led to general improvement in communication and the resultant flow of patients:
. . . lots of networking. I’ve then been going over to [CSC/PSC] as well to say, ‘Hello, you probably speak to me on a daily basis’.
GM25, senior nurse, DSC
As timely repatriation from CSC/PSCs was key to maintaining patient flow, a system of financial penalties was instituted: DSCs could be penalised for each day in excess of 72 hours that a patient was unable to move from a CSC/PSC. Some clinicians thought that these penalties were useful as a message to managers about the importance of ‘ring fencing’ stroke beds but, in practice, although financial penalty notices were issued, fines were never paid. Instead, a new system was introduced in 2017/18 whereby money would be ‘top-sliced’ from the DSC tariff using the same criteria.
Discharging patients into the community
Discharging patients could be challenging, partly because ESD provision varied across GM, although this was being addressed through a common specification being agreed across GM, facilitated by the ODN. Also problematic was discharge to social care, as in London:
There are no social care placements, it’s very difficult to get those people out.
GMH12(2), DSC senior nurse
Care provision in a Comprehensive Stroke Centre, Primary Stroke Centre and District Stroke Centre services
As outlined in Chapter 11, achievement of evidence-based clinical indicators either stayed the same or improved in GMB compared with the RoE, and improved over time in GMA compared with GMB. Challenges and enablers are presented here.
Challenges to care provision: system and staffing pressures
The issue of staffing levels continued to cause concern, with staff shortages seen as a threat to the sustainability of the system. This was understood to be a nationwide problem, although there were particular issues with medical staffing at the CSC, with the perception that physicians preferred not to work in a 24/7 service when there were positions at nearby ‘in-hours’ services.
The education of staff about the care pathway was key, including for those outside stroke (e.g. A&E staff and GPs). Staff at SUs delivered training to other staff in their hospitals, and training for GPs was developed through the ODN.
Difficulties arising from numerous trusts operating a single stroke care pathway were highlighted and it was suggested that, to be sustainable, the GM system needed to operate with similar job plans and staffing in all hospitals, with the same terms and conditions. Shared medical rotas were seen as desirable by some (although not all).
Having only one CSC operating 24/7 was perceived as placing significant pressure on it, especially out of hours, because of the volume of patients.
Challenges to care provision: changing workload
The centralised stroke care pathway resulted in changes to the workload and profile at the CSC, PSCs and DSCs. All patients with a suspected stroke were now eligible for CSC/PSC care and, thus, the proportion of admitted patients who were eligible for thrombolysis was smaller than previously. This was viewed as increasing the amount of time in which medical staff became proficient in thrombolysis.
Stroke mimics placed pressure on CSC/PSCs, and, although this had been the case in GMA, the increase in patients through CSC/PSCs increased the number of mimics. These patients were viewed as generally more difficult to manage, as there was no defined care pathway for them. Clinicians spent much time assessing and caring for people who did not ultimately have a stroke diagnosis, and they did not always feel well supported in this work.
The reality for DSCs post implementation was different from the modelling on which planning for change had been based, which assumed that 95% of stroke patients would be admitted to a CSC/PSC (as with London HASUs). Initially, 15–20% of patients were receiving all of their stroke care at a DSC ‘and yet arguably the DSCs now are not designed to cope with those kinds of patients’ (GM28, governance); the implementation of GMB did not result in a loss of beds in PSCs, but it did lead to the loss of, for example, stroke assessment teams. Over time this has become less of an issue because more patients are now going to CSC/PSCs and there is less concern from DSCs.
Enablers of care provision: reviewing care provision and performance
The review of stroke services was carried out primarily by analysis of SSNAP data, which had significant national coverage (and therefore better comparators) not available in GMA. Issues relating to the accurate and complete entry of SSNAP data were identified by the ODN, which ran improvement events for acute and community providers and liaised with the SSNAP team at the Royal College of Physicians over questions that arose. The ODN supported SUs to find the data entry solution that worked best for their organisation.
A year after the implementation of GMB, SSNAP data were described as ‘driving up the quality of the services’ (GM24, SCN). Interviewees spoke about SSNAP data being quite dated by the time results were available, and that with the help of the ODN they were planning to use ‘real-time’ data where possible.
Enablers of care provision: tariffs
In GM ‘unbundled’ local stroke tariffs were developed in order to reimburse both the CSC/PSCs and DSCs involved in a stroke hospital stay, similar to the arrangements for GMA. There was a tariff to cover the first 3 days in a CSC/PSC, and then a second bed-day rate for the DSCs. If the DSCs took a direct admission, they claimed national tariffs. During the initial year of the fully centralised service these tariffs were paid upfront; stroke services did not have to demonstrate that they were meeting particular targets. Future plans are that if performance indicators are not met there may be penalties. This remained different from London, where service standards were linked to financial incentives.
Discussion
Principal findings
The time taken from the original recommendation to further reconfigure stroke services in GM and to obtain formal agreement to do so and implementation of the new model was considerable. However, this was a period of great turbulence in the NHS, and that such system-wide change was achieved in this context may be significant in itself.
Changes in NHS structures and leadership contributed to delays in planning and implementation, with other local changes in context having an impact on planning.
The importance of having a governance structure to oversee and co-ordinate the planning of changes to services was demonstrated. Delays to the planned implementation date meant that the sites were ready on the launch date and there were few teething issues following implementation. This may have led to the achievement of improved outcomes within a year (see Chapter 11).
Challenges identified in GMA, such as dealing with stroke mimics, the repatriation of patients from CSC/PSCs to DSCs and discharging people to the community, continued post implementation. The importance of working collaboratively to find local solutions to these problems was shown, enabled by the governance model, which provided support for regular audits and a mechanism to facilitate the system-wide discussions needed to maintain effective system operation, as well as ensuring that SSNAP data were entered consistently across GM, so that it could be used for reviews. It is likely that the co-ordinating role of the ODN contributed to the improvements in the provision of evidence-based care and the reduction of mortality among those patients treated in a CSC/PSC.
Strengths and weaknesses
Strengths of this analysis included the contemporaneous study of both planning and implementation. A wide range of interviewees participated, including those involved in the planning, governance and review of stroke services and staff from the CSC, both PSCs and two DSCs. Staff from across GM participated in observed meetings, so a wide range of views and experiences were captured. Emerging findings were shared with local stakeholders in order to enhance validity.
Comparison with other studies
The importance of the context215,216 within which changes to stroke services were planned and implemented was evident in this analysis. However, despite this turbulent background, MSC was achieved, with the data showing how the context delayed change, had to be taken into account and was worked around.
As in the Midlands and East of England (see Chapter 10) and London (see Chapter 14), the 2013 NHS reforms disrupted system commissioning and governance. Changes in leadership, both designated and distributed, meant that it was difficult to get decisions made, and there was a consequent loss of knowledge and ownership of the plans for reconfiguration. This suggests that continuity of leadership could be added to designated and distributed leadership as necessary for large-scale transformation,5 given that it will always take place over a period of time.
Feedback was used throughout the ‘to agreement’, ‘planning’ and ‘implementation’ phases. Following the review of GMA, other reviews were carried out, which fed in to the planning process and justified decisions made. Agreement for reconfiguration was based mainly on an analysis of SSNAP data, which continued to be important after implementation, to monitor activity on the stroke pathway. Resource was dedicated to ensuring that staff appreciated the importance of accurate and complete data collection and understood the analysis of the data in relation to their own practice.
Financial penalties were not used in GMA (see Chapters 6 and 7)110,123 but were introduced in relation to delayed repatriation in GMB. However, there were changes to the mechanism used, with plans for ‘top slicing’ of the tariff paid to a DSC if a stroke patient was not repatriated in a timely manner, after a previous method of doing this was not imposed.
The use by leaders of discursive power to promote change,203 with an emphasis on evidence and better patient outcomes, was evident in the planning of GMB. Leaders used SSNAP data and then the publication of research findings in order to keep to the fore the reasons that reconfiguration was necessary.
Attending to history, that is, learning from previous transformations, was evident. A phased approach to implementation was not undertaken, largely because of previous experience in GM.
Despite system-wide disruption resulting from NHS reforms (reducing the extent to which stakeholders could engage) there is evidence that in addition to engaging a range of stakeholders,5,110 ‘ownership’ of the changes was engendered in stakeholders. For example, CCGs funded a project manager, stroke staff championed the changes through training other staff in their locality and provider Trusts funded the ODN beyond its initial year post implementation. ‘Ownership’ can be conceptualised as a step further than engagement, and ultimately as necessary for sustainability.
Implications
-
Major structural reorganisations have been shown to represent a significant obstacle to implementing MSC. However, GMB indicates that it is possible to mitigate or overcome such disruption.
-
Network support organisations (such as the ODN) can facilitate collaboration, service audit and support the development of standards and protocols for organisation and provision of care. Such organisations might maximise their impact if they support the whole patient pathway rather than just the acute phase.
-
MSC benefits from programme management support, within a framework of robust governance, which engages all key stakeholders.
-
Workload profile and the related skill requirements are important during and after change and may be underestimated. Insufficient attention may lead to skills shortages as well as low staff morale.
-
Continuity of leadership is important: steps should be taken to minimise the effect of contextual changes by the involvement of leaders with system-wide authority and collaborations of organisations where possible.
-
Consideration must be given to harmonisation of terms and conditions for staff across the system. Working in a 24/7 service, for example, poses additional requirements for night and weekend working that may not automatically be rewarded equitably and can lead to staff shortages.
Chapter 14 Factors influencing the sustainability of changes in London
Overview
What was already known about this subject?
-
Ongoing sustainability is a key measure by which the ‘success’ of organisational change might be assessed. Sustainability may be influenced by characteristics of the intervention itself, the context in which it was implemented, leadership and processes to support implementation and sustainability.
-
Changes implemented in London led to a significantly higher likelihood of providing evidence-based care (see Chapters 4 and 7), and significantly greater reductions in mortality than elsewhere (see Chapter 3); analysis of data to March 2016 indicates that care provision and outcomes either improved or were sustained (see Chapter 11).
What this chapter adds
-
The London acute stroke system – like the rest of the English NHS – faced significant obstacles over the period studied.
-
Factors identified as obstacles to sustaining organisation and provision of acute stroke care in London included turbulence resulting from the 2013 NHS reforms, perceptions of conflicting national targets (e.g. A&E targets), national staffing shortages and significant pressures on social care services.
-
These factors were felt to have contributed to significant pressures on service provision, for example through delays in the transfer of patients through the system and resultant difficulties in finding beds for stroke patients in HASU and SU wards.
-
Key promoters of sustainability of the system included:
-
the characteristics of the model itself (in particular the service standards linked to the London stroke tariff)
-
processes of sustaining the model, including regular service reviews and use of national audit data
-
leadership of the model (in terms of continuity but also adaptability)
-
the prioritisation of generating and sharing independent evidence was key to ensure ongoing stakeholder ownership.
-
Background
Sustainability is recognised as an important marker of the ‘success’ or otherwise of organisational change (e.g. Chambers et al. 47 suggest that ‘implementation of interventions, which can often require substantial resources, is meaningless without successful long-term use’). However, reviews of the evidence indicate that there is limited evidence in relation to the sustainability of complex organisational change (whether in terms of MSC or not). 41,48,126,220,221 Sustainability has been described in numerous ways, including institutionalisation, embedding, routinisation and normalisation; one review suggests that the concept requires greater clarification. 48 As discussed in Chapter 11, we analysed sustainability in relation to whether clinical outcomes and delivery of evidence-based clinical interventions remained consistent or improved over time, whereby sustainability is defined as ‘the process through which new working methods [e.g. a new referral pathway], performance goals [e.g. improvements in clinical outcomes and delivery of evidence-based clinical interventions] and improvement trajectories are maintained for a period appropriate to a given context’ (italicised examples have been added by the authors). 46
Although there is limited evidence in relation to how MSC is sustained,49 a number of factors are likely to be important, including the intervention itself, the context in which it operates, leadership (both formal and informal) and processes by which the innovation is sustained (e.g. monitoring and feedback, training and decision-making). 48 Recent reviews of evidence and thinking on the issue suggest that sustainability may be seen as a process resulting from a dynamic relationship between such factors. 47,48,215,221–223 Especially important may be the influence of context – whether within a service, across a region or nationally – as changes over time bring new pressures and priorities. 47,48,215
In this chapter, we addressed the following RQs. In which ways were key aspects of the London model sustained? Which factors were influential in this?
In doing so, we aimed to contribute to the understanding of the sustainability of a MSC in a time period that included significant reforms (and disruption) at national and local levels (see Chapters 10 and 13).
Method
Design
This chapter presents a single case study, based in London and focusing on governance and service levels (see Table 2 and Chapter 2, Understanding development, implementation and sustainability).
Data
For this analysis, we conducted 51 interviews and 24 non-participant observations (approximately 60 hours’ worth), and collected > 100 documents (see Table 2 and Chapter 2, Understanding development, implementation and sustainability). We interviewed a range of stakeholders, including system leaders, commissioners, stroke service staff and service managers; we observed such events as Stroke Clinical Leadership Group (SCLG) meetings and stroke service reviews, and documents collected included meeting papers (e.g. minutes, project updates), service review summaries and local system documents (e.g. updates to the London stroke service standards).
Analysis
To analyse sustainability of the system we were in part guided by the findings presented in Chapter 7, which indicated that the central factors in achieving a significant impact on quality of care and outcomes were: (1) that a high proportion of patients were treated in a HASU and (2) that HASUs had sufficient capacity (in terms of appropriate staff and equipment) to provide evidence-based care. Our analysis was also informed by factors established in the literature as potentially influencing sustainability of complex organisational change, including contextual factors, leadership, and processes to sustain the change. 47–49,126,215,221–223
Results
We present our findings in relation to the aspects of the London stroke model that our previous analyses indicated were important to the effective running of the system, specifically (1) the flow of patients through the system, (2) provision of care in HASUs and SUs and (3) governance of the London acute stroke system. In each case, we considered obstacles to the sustainability of the system as implemented, and factors that acted to support sustainability of the system. Table 28 provides an overview of factors that influenced sustainability of the London acute stroke system.
| Type of influence | Factor | Perceived impact |
|---|---|---|
| Challenges to sustainability | NHS reforms (implemented 2013) |
|
| Staffing pressures |
|
|
| Social services pressures |
|
|
| Facilitators of sustainability | Characteristics of service model |
|
| Processes to sustain model |
|
|
| Generating and sharing independent evidence |
|
Flow of patients through the hub and spoke model
Hub and spoke systems (as in London and GM) rely on the timely transfer of patients through the system. In this section we discuss how the flow of patients through the system was sustained, in terms of (1) direct transfer to a HASU, (2) repatriation from a HASU to a SU and (3) discharge from the acute system to the community.
Transfer of stroke patients to a Hyperacute Stroke Unit
The proportion of patients treated by HASU services was sustained (just under 95% treated in HASU, 2013–16; see Chapter 11). Although SU and ambulance staff both noted occasional resistance from HASU staff to admit potential stroke patients, interviews with system leaders, ambulance services and stroke services indicated few concerns with this aspect of the patient pathway:
. . . our performance against our audit standards and against the national audit standards remain broadly static.
LonAmb01, ambulance service, 2016
Repatriation from a Hyperacute Stroke Unit to a Stroke Unit
Repatriation of stroke patients from a HASU to a SU was seen by many HASU staff as challenging. Staff described frequent delays in transfer, with implications for patient and carer experience and capacity for HASUs to receive new stroke patients. A key factor in repatriation was the HASU and SU agreeing patient transfer: this was often delayed because SUs faced their own difficulties in freeing up beds (in part due to local pressures on SUs to accommodate non-stroke patients, in order to meet the A&E 4-hour target). HASU and SU staff suggested that the abolition of Stroke Clinical Networks meant the loss of an ‘honest broker’ in facilitating repatriation and escalating other interorganisational issues.
The network, I think, played a vital role in umpiring and refereeing relationships between [HASUs and SUs], making sure people were playing by the rules. Whereas now I think people make up and bend the rules in terms of, admission to the HASU, repatriation from the HASU.
LonD02, SU stroke physician, 2015
One HASU that we studied developed a dedicated role of a repatriation nurse, who was responsible for leading these discussions with SUs, and HASU staff reported the importance of forming links with individuals in other services to support negotiation of the repatriation process:
We have got, at every hospital, one definite name that I can call and people go, ‘Oh yes, I’ll get her for you,’ and I can have a sensible conversation.
LonA08, HASU senior nurse, 2017
From 2015, some HASUs explored more systemic responses to address the repatriation challenge. For example, one HASU developed a proposal (approved by the local commissioner) that SUs would be notified that a patient was awaiting transfer; if a bed was not made available within 48 hours of notification the patient would be transferred to the SU’s hospital for care in the Medical Assessment Unit until a SU bed became available. This proposal was discussed at a regular meeting of London stroke service representatives. HASU representatives had diverging views, with some seeing it as a risk to the quality of care provided to stroke patients, whereas others argued that the changes might significantly improve the likelihood of new stroke patients receiving care in a HASU. Several SU representatives felt that the proposal would be a helpful lever to encourage their hospital management to protect SU beds (discussed further in Care provision in Hyperacute Stroke Unit and Stroke Unit services). Although this issue had not been resolved, there appeared to be a growing recognition of the need to address the balance of power in the context of repatriation:
HASUs cannot say no to their local patients coming in with acute stroke . . . whereas we allow the ASUs [Acute Stroke Units] to say no because they’re full, and that's incompatible.
Lon05, stroke physician, 2016
Discharging patients into the community
Similarly, respondents saw discharge to the community as an ongoing, and growing, issue. A positive aspect is that access to ESD improved significantly over the period studied. ESD was a standing work programme of the SCLG, which led to the development of service specifications and an audit of provision of services. ESD provision had been variable for an extended period: although all areas of London commissioned an ESD service, system leaders were aware of significant variations in how ESD was understood and implemented across London. A potential unintended consequence of improved access to ESD services was the impact on patient case-mix treated in SUs; a number of SU staff felt that patients were becoming more complex and more disabled, because less disabled patients were more likely to be discharged directly home via ESD.
A key factor influencing discharge of patients to the community was in terms of depletion of social services. HASU and SU staff reported that they had become less engaged with social services over time; for example, social service representatives had formerly participated in multidisciplinary team meetings, but capacity in the community was latterly no longer available for this.
Over the final year of our study, one sector of London commenced a review of its whole stroke pathway (from HASU to community) to examine whether or not the pathway was appropriately balanced. This was carried out in response to concerns about transfer across the stroke patient pathway and was developed in collaboration with local commissioners and the SCN. Although this activity did not lead to changes to the system during our study, it represents a clear example of a locality attempting to address the patient flow problem with support from local stakeholders and system leaders.
Care provision in Hyperacute Stroke Unit and Stroke Unit services
As described in Chapter 11, the provision of evidence-based clinical interventions either improved or was sustained over our period of study. However, this was achieved in spite of significant pressures faced by the system, in terms of capacity, staff turnover and a national shortage of stroke specialists.
Challenges to sustained care provision: system and staffing pressures
Both HASU and SU staff reported significant pressures on their services, and a view that the number of patients was too great for the staff to care for effectively, potentially as a result of the way in which the service model was designed:
We were having a conversation like every half an hour with a bed manager going, ‘What are you doing?’ . . . A&E consultants screaming at you going, ‘Why are you still accepting stroke patients?’.
LonA08, HASU senior nurse, 2017
We are accommodating a larger number of HASU patients than what we have on our blueprint.
LonC11, HASU manager, 2017
Insufficient bed spaces in wards (in part due to patients awaiting repatriation or discharge) led to an increase in ‘outliers’, patients who were treated not on the wards, but instead on general medical wards and visited by specialists. Interviewees suggested that such patients experienced reduced specialist assessment, therapy and monitoring. Such pressures on services (in terms of bed spaces and physician staffing levels) have led to occasional cases where HASUs have had to go ‘on divert’ (i.e. to not admit acute stroke patients, but instead have them admitted to another HASU):
. . . we were running on locums throughout the summer but then when they became unavailable, there was a period when there were no doctors to cover the nights so the managerial discussion at the time was to go on divert and take the [area] patients to [alternative HASU].
LonC15, HASU physician, 2016
Representatives of HASUs, SUs and ambulance services all reported significant turnover in staff, requiring ongoing recruitment drives. An important obstacle to recruitment was the national shortage of stroke specialists, including medical, nursing and allied health professionals; this has led to a number of services facing longstanding vacancies, including in some cases a lead consultant physician. New staff required the knowledge and skills to operate within the HASU system (and the wider NHS). However, some interviewees indicated that although the necessary training is still provided, it had become more challenging over time, especially in terms of ensuring continuous development of nursing staff. In addition, some staff noted a loss of development support from the stroke network.
Enablers of sustained care provision: service standards linked to financial incentives
As discussed in Chapter 7, the London service standards were seen as important to ensuring that HASUs and SUs were suitably staffed. Our analysis indicated that they were also effective in sustaining HASU and SU settings as specified over the longer term, in terms of staffing levels and prioritisation of stroke within other hospital areas (e.g. A&E and diagnostics).
However, as discussed above, some staff questioned whether or not the specified levels of staffing were sufficient in order to provide care. Furthermore, although there were several reviews of the standards (which were discussed online and in meetings by the SCLG), on each occasion resulting in amendments, it was noted that there were limited advances in addressing substantive staffing issues, especially in the light of emerging priorities, such as 7-day working:
Anything in terms of improvement, refinement on the original model and the original specification has been incredibly hard. Over time we have gradually, gradually increased our junior doctor levels and gradually, gradually increased our consultant working plans, but we haven’t seen any change in weekend therapy, and probably haven’t seen any change in weekend nursing patterns.
LonA03, HASU stroke physician, 2017
Enablers of sustained care provision: reviewing care provision and performance
Linking the standards with the London stroke tariff and assessing compliance through regular service reviews was perceived as vital to sustain services as specified:
There’s an enhanced tariff that we get if we pass. I think if that threat went away, that would be a real loss to us. We need that threat.
LonB03, SU stroke physician, 2016
However, the review process was disrupted significantly by the NHS reforms of 2013 and the loss of Stroke Clinical Networks (discussed further in Challenges to sustained governance: NHS reforms). First, closure of the networks meant loss of capacity to arrange, prepare and conduct the reviews. Second, the need to involve nascent CCGs made the task of arranging meetings much harder. As a result, around the time of the reforms, many services experienced a gap of well over 1 year between reviews.
The national audit (SSNAP) was seen broadly positively by staff as a source of evidence on performance:
The SSNAP data would be the main thing that shows our quality of care: we try really hard to comply really strictly with the collecting of data for the SSNAP, and then we get information for us as a therapy team about how we are performing as therapists and how the rest of the HASU is performing.
LonA12, HASU physiotherapist, 2015
However, there is a general recognition that data gathering is a substantial task, prompting a number of teams to recruit a dedicated staff member to lead on this. Some staff questioned the extent to which SSNAP measures quantity rather than quality, and noted a wider concern about audits – that scoring ‘green’ may force stroke off management’s priorities.
Governance of the London system
As described in Chapters 6 and 7, planning and implementation of the changes in London were influenced significantly by how they were governed. System leadership, the presence of a central source of authority, was important to ensure that a wide range of stakeholders remained engaged in the changes, while the operational support provided by local Stroke Clinical Networks was instrumental in ensuring standards were met across the system.
Challenges to sustained governance: NHS reforms
As discussed elsewhere (above and in Chapters 10 and 13), the NHS reforms implemented in 2013, with the abolition of SHAs, PCTs and Stroke Clinical Networks, introduced significant turbulence in how the NHS in England was commissioned and overseen. Furthermore, all these organisations played a significant part in the governance of the planning, implementation, and initial impact of the changes in London. The loss of these aspects of the London system were felt keenly, in terms of disrupted relationships between services and commissioners:
They got rid of our network so that was part of that, that was massive; secondly bringing in CCGs . . . I'm part of the SCLG, which is the London Stroke Group, and it became clear very quickly that we had no idea who the people with an interest in stroke were within the CCGs.
LonA01, senior stroke nurse, 2016
Enablers of sustained governance: continuity in system leadership
Although the commissioning landscape changed significantly, system leadership was sustained in a number of ways, but with substantially reduced resources. The London Clinical Director (who also became the NHS England National Clinical Director for Stroke) remained in his role based in the SCN, supported by a member of the former Stroke Clinical Network. The SCLG, which drew together representatives of HASUs and SUs from across the region, continued to meet regularly, although with fewer resources to support it (funding for administrative support was threatened at points). The SCLG retained its key functions, continuing to lead on some quality improvement and development projects (often focusing on issues identified by local stroke services as challenging, e.g. variations in community services and ESD, or on new priorities, such as atrial fibrillation and mechanical thrombectomy).
Enablers of sustained governance: adapting leadership style
Following the 2013 reforms, the style of leadership had to change. Leadership roles became more advisory, with less power to influence CCGs:
Now the Strategic Clinical Network, we’re there as an advisory body to the commissioners and that link between clinicians and commissioners, but we don’t have the teeth in the same way and we don’t – because we’re external to the CCGs – gaining access to them is more challenging.
Lon28, SCLG member, 2016
In response to this loss of power, leaders adapted their strategies to support sustainability of the London system. They developed increasingly persuasive arguments (especially drawing on research and audit evidence) to ensure that the key principles of the London model were sustained. A key example of this included the London Clinical Director and network leads actively engaging with nascent CCGs as they developed to ensure that the new commissioners understood the system and its governance arrangements, and the purpose of the tariff. Activities included inviting CCG representatives to an SCLG meeting to discuss the purpose and impact of the London system, engaging CCGs to discuss their participation in stroke service reviews, and discussing such matters as using national audit data as a basis for local key performance indicators (rather than developing new key performance indicators), and encouraging CCGs to commission appropriate ESD services across the region. Underlying these discussions was a prioritisation of using established evidence indicating the value of a pan-London approach to organising acute stroke care.
As discussed earlier, one HASU developed a new repatriation protocol presented to the SCLG as a ‘fait accompli’ and could perhaps be seen as running counter to this pan-London approach. However, the London leadership response was to use this as a prompt for wider discussion about how best to manage a relatively common difficulty with repatriation.
Keeping London working together . . . has enabled us to have at least some elements of the old Strategic Health Authority . . . they’ve continued to operate pretty much really as an SHA but without some of the powers that the SHA previously had.
Lon05, stroke physician, 2016
In terms of adapting the governance of the London system further, a recent development was the commissioning of Operational Leads to take responsibility for sector-level strategic leadership in London from 2016 (drawing on lessons from the ODN set up in GM and seeking to recapture some of the operational capacity provided formerly by the Stroke Clinical Networks).
Enablers of the system overall: evaluating and communicating impact of changes
A further enabler of the sustainability of the London centralisation was that the impact of the changes was communicated actively from the beginning. Indeed, the potential influence of evidence, and the need for measurement and evaluation, was discussed from the planning stages of the London changes in 2008/9. As a result of this approach, various activities were conducted: the stroke networks published reports on improvements in performance of services in regular newsletters, a cost-effectiveness analysis was commissioned and later published and the findings were presented at an event to celebrate the impact of the London changes in late 2011. In addition, the study presented in this report was coproduced with leaders of the changes in London (and GM) as a result of the recognition of the importance of independent evaluation, and the generation of impartial evidence and learning to support further development of improvements in the organisation of stroke care. The findings from our study have been shared actively in a range of settings, including SCLG meetings, and HASU and SU staff meetings.
As a result of the London leadership’s approach to evaluation and sharing of lessons, and prioritisation of demonstrating the impact of the changes, the principle of a centralised ‘hub and spoke’ system of acute stroke care retained strong clinician and management support, with staff referring both to ‘headline’ outcome figures (e.g. the impact on mortality) and the experience of seeing improvements on the ground.
Discussion
Principal findings
The London acute stroke system – like the rest of the English NHS – faced significant obstacles over the period studied. However, this complex system was sustained through a range of factors relating to its design, leadership and management.
Important contextual factors included: turbulence prompted by the 2013 reforms to the NHS in England; national targets (especially the 4-hour A&E target); national staffing shortages (in terms of medical, nursing and allied health professionals); and significant pressures on social care services. These factors contributed to significant pressures on service provision, for example through delays in the transfer of patients through the system and resultant difficulties in finding beds for stroke patients in HASU and SU wards.
The key promoters of sustainability of the system included the characteristics of the model itself (in particular the service standards linked to the London stroke tariff), processes directly aimed at sustaining the model (including regular service reviews and use of national audit data), leadership of the model (achieving a degree of continuity, but also adapting the style of leadership to be more persuasive, for example using evidence to persuade nascent CCGs to engage with the system) and, finally, the prioritisation of generating and sharing independent evidence was key to ensuring ongoing ownership and support of the London model by clinicians, managers and commissioners.
Strengths and weaknesses
This was a rare opportunity to analyse the sustainability of a MSC over several years, drawing on a range of quantitative (see Chapter 11) and qualitative data, assessing sustainability from multiple stakeholder perspectives. This approach reflects recommendations in the literature for optimal assessment of sustainability. 222 The fact that the changes were sustained, despite the loss of several important facilitators (as the result of the NHS reforms implemented in 2013), means that we provide a number of lessons on sustaining change during times of significant turbulence, which should be valuable to planners, managers and clinical leaders in the English NHS and elsewhere.
Our analysis had a number of limitations. First, we sampled only a limited number of HASUs and SUs. However, the governance meetings we observed had representation from across all parts of London, and many governance-level interviewees took a system-wide view of HASUs and SUs; also, several interviewees were based in HASUs or SUs that were not our selected study sites.
Comparison with other studies
This chapter demonstrated clearly the influence of factors identified in the wider organisational change literature on sustainability, suggesting that many of the lessons relating to sustainability or complex change conducted within a single organisation can be applied in relation to MSC.
The characteristics of the innovation (i.e. the service standards linked to the London stroke tariff, measured regularly) were a key facilitator of sustained staffing levels within services across the system. 41,47,48,221,223 However, reported difficulties in adapting standards (e.g. to meet requirements for 7-day working) was in line with the observation of Greenhalgh et al. 49 that ongoing development of a MSC can be challenging. The context in which change is implemented is highly influential, and in the case of MSC the complexity of this relationship grows. Sustainability of a MSC requires ongoing responsiveness to such changes. 47,49,215,221 We found that continuity in leadership was vital to ensure ‘organisational memory’ for the purpose and processes of the London system, but that the ways in which leadership is enacted must adapt to changes in the organisational context. One issue emerging from the pan-London approach, emphasised from the beginning of this change process, is that local adaptations to the system tended to be brought to system leadership for discussion before being put into action, and then used as a basis for consideration of system-wide adaptations. This suggests that following a MSC, once the system reaches a certain stage of maturity, with certain principles established (e.g. the service standards and ‘hub and spoke’ structure), a degree of flexibility may be introduced to permit localised piloting.
Implications
A key implication of this chapter is that, when planning a MSC, there would be clear value in looking beyond its implementation to its long-term sustainability. A range of factors, relating to innovation design, the context in which it is to be introduced, how it is led, and how it is managed and supported, should be considered. Especially important was prioritising the generation of meaningful evidence about the implementation and impact of the MSC: this addressed the question of whether or not the innovation was having the intended effect, but was also fundamental to ongoing dialogue with clinicians, managers, commissioners, patients and the wider public, to ensure ongoing engagement and support of the system.
Chapter 15 Discussion and conclusions
Overview
This research used formative evaluation methods to report and analyse reconfiguration of stroke services and, in doing so, identified lessons to help guide future reconfiguration work in other services. Our RQs were as follows:
-
What are the key processes of and factors influencing the development and implementation of the stroke service reconfigurations?
-
To what extent have system changes delivered process and outcome improvements?
-
Have changes delivered improvements that stakeholders (e.g. commissioners, staff, patients and the public, and reconfiguration leads) think are worthwhile?
-
Have changes delivered value for money?
-
How is service reconfiguration influenced by the wider context of major structural change in the NHS?
In this chapter, we first provide a summary of our principal findings, linking these to our RQs. We then discuss the implications of these findings, the strengths and limitations of our study, the impact our findings have had to date, and propose a future research agenda.
Summary of principal findings
Our analysis of the impact of the centralisation of acute stroke services implemented in 2010 in London and GMA on outcomes (RQ2), including cost-effectiveness (RQ4), found the following:
-
In London, where all patients were eligible for treatment in a HASU, there was a reduction in mortality and LOS.
-
In GMA, where only patients presenting within 4 hours of developing stroke symptoms were eligible for care in a CSC/PSC, there was no impact on mortality but LOS reduced.
-
In London, where almost all patients were treated in a HASU, patients were more likely than elsewhere in the RoE to receive evidence-based care in the first hours following arrival in hospital.
-
GMA’s CSC/PSCs performed as effectively as those in London, and significantly better than London on several important clinical interventions, but treated only 39% of stroke patients. This difference is explained in part by differing eligibility criteria in GMA and London, but also because adherence to the model in GM was lower, with only two-thirds of eligible patients treated in CSC/PSCs.
-
As a result, only patients in London were overall significantly more likely than patients elsewhere in England to receive evidence-based care; stroke patients in GM were overall no more likely to receive evidence-based care in the first hours following arrival in hospital than patients in areas where no equivalent centralisation had taken place.
-
There is a high probability that the centralised models of stroke care in London and GMA implemented in 2010 were cost-effective (RQ4).
-
In London this was as a result of improvements in mortality and morbidity and in GM as a result of reduced cost of stroke care due to reduced LOS.
Our analysis of the impact of the London and GMA changes on patient and carer experiences (RQ3) found the following:
-
Similar experiences were reported from the two regions.
-
Patients and carers on the centralised acute stroke care pathways reported many positive aspects of care.
-
Participants were impressed with emergency services and initial reception at hospital – disquiet about travelling further than a local hospital was allayed by clear explanations.
-
Participants described that they knew who was treating them, were involved in decisions, and had adequate specialist stroke care.
-
Difficulties for families visiting hospitals a distance from home were raised.
-
Repatriation to local hospitals was not always timely, but no detrimental effects were reported.
-
Discharge to the community was viewed less positively.
Our analysis of how the London and GMA changes were implemented (RQ1) found the following:
-
Both system (top-down) and clinical (bottom-up) leadership were necessary to align multiple stakeholders and thus overcome resistance to change. In London, planners were able to ‘hold the line’ on the service model implemented; in GM, where planners attempted to mitigate potential resistance by making decisions through consensus, the model was changed, implementing a ‘4-hour model’, thus meaning that the majority of stroke patients would still be treated in their local stroke service rather than a CSC/PSC.
-
System leadership can –
-
provide authority and power to co-ordinate multiple local stakeholders to agree to change services over a wide area
-
capitalise on clinical leadership to develop further support for the goals of change.
-
-
Change in both areas involved clinical leadership, but whereas system-wide authority was used in London to align stakeholders, it was not applied in GMA.
-
Combining feedback with other tools (e.g. use of audit data) was important to build the case for change and to assess its impact.
-
It was necessary to involve a range of stakeholders beyond physicians in planning MSC.
-
Lay involvement in the changes was enacted through consultation exercises, lay participation in governance structures and elicitation of patients’ perspectives.
-
Interviewees’ views of involvement in these MSCs varied, reflecting different views of involvement per se, and of implicit quality criteria.
-
The value of involvement was found not in its contribution to acute service re-design but in how involvement practices facilitated its implementation. Our analysis identified three types of processes (agitation management, verification and substantiation) through which this was felt to have been achieved.
Our analysis of how approaches to implementing the London and GMA changes influenced care provision and patient outcomes (RQs 1 and 2) found the following:
-
A theory-based framework enabled analysis of the relationships between planning, implementation and outcomes of MSC by linking quantitative outcomes with qualitative findings on processes of change.
-
Referral pathway – in London, where all patients were eligible for HASUs, and all HASUs admitted patients 24/7, the pathway was reported to be more straightforward and inclusive. It was more likely to be understood and followed by both hospital and ambulance staff, maximising the proportion of patients who were treated in a HASU. In GMA, the referral pathway, where only a selection of patients were eligible for treatment in a HASU, was found to be less inclusive and more complex than in London; this reduced the proportion of patients treated in a HASU, in part through limited adherence to the pathway.
-
Phases of implementation – in London, the single launch date was identified as facilitating clear understanding of, and adherence to, the pathway. In GMA, phased implementation caused uncertainty among hospital and ambulance staff, both during and post implementation.
-
Use of service standards linked to financial incentives – in London, standards were linked to financial incentives, and services could not launch until accredited against the standards. This was reported to have increased the likelihood of services having capacity to provide evidence-based care. In GMA, service standards were not linked to incentives and there was no accreditation process, which may have led to greater variation across stroke services.
-
Facilitation – the local stroke network in London was described as providing substantial co-ordination and hands-on facilitation. In GM, the network facilitated implementation by acting as a platform for sharing learning across sites.
Our analysis of how and why changes originally planned for the Midlands and the East of England were not implemented (RQs 1 and 5) found as follows:
-
Several factors known to be associated with the successful implementation of MSC were either absent or severely hampered; this was felt to have influenced progress of this programme.
-
Although system-wide leadership of the programme was evident in the beginning, support from senior leaders in the SHA reduced over time, and, following the NHS reforms implemented in April 2013, the complete loss of this top-down leadership was reported to have made it easier for local commissioners to withdraw than if the SHA had remained.
-
Data were used to build the case for reconfigurations and proposals for MSC. However, local stakeholders did not feel sufficiently engaged in the process, resulting in limited local ownership of recommendations, prompting local areas to repeat similar modelling exercises.
-
The programme sought to engage local networks and stakeholders throughout the review process, but the impending reforms of NHS commissioning made this challenging.
-
The programme used lessons from previous MSC to make their case. However, local stakeholders did not engage with these lessons because they related to work conducted in what were perceived to be very different contexts (i.e. in terms of rurality).
-
Underlying these issues, the NHS reforms implemented in 2013 had a significant influence on the progress of this programme. Key examples of this included (1) disrupting system commissioning and governance across the English NHS, (2) introducing significant distraction throughout the system and (3) limited time to develop reconfiguration proposals because recommendations had to be delivered before the SHA was abolished.
Our analysis of the impact of the implementation of GMB and sustainability of the London changes, in terms of outcomes and clinical interventions (RQ2), including cost-effectiveness (RQ4), found the following:
-
Following implementation of GMB there was a reduction in mortality in GM of approximately 189 fewer deaths per year.
-
However, there were also reductions in mortality in the RoE, meaning that the differences in mortality between GMB and the RoE were not statistically significant.
-
In GMB, there was a reduction in mortality over and above that seen in RoE for people treated in a CSC/PSC (approximately 69 fewer deaths per year).
-
The proportion of patients treated in CSC/PSCs in GMB increased to 86% in 2015/16; this was a higher proportion than in GMA (39%), but still lower than in London (93%).
-
LOS reduced in GMB, resulting in a reduction of around 6750 bed-days per year.
-
There was no significant variation in mortality or LOS over time since the reconfiguration in London, indicating that the reductions in mortality and LOS following centralisation in London were sustained.
-
These patterns were reflected by the analyses of clinical interventions in both areas. Following implementation of GMB, delivery of clinical interventions improved significantly, although it also improved (but generally not to the same degree) in the RoE; in London the delivery of clinical interventions either improved or was sustained.
-
Over 90 days GMB cost £932 less per stroke patient than GMA. This compared with a cost saving of £635 in the RoE over the same time period.
-
GMB also resulted in 4 additional QALYs per 1000 patients over 90 days compared with GMA. In the RoE there were 3 additional QALYs per 1000 patients over the same time period.
-
At 90 days there was an 88% chance that GMB was cost-effective compared with changes that occurred in the RoE over the same time period at a WTP for a QALY of £30,000.
-
At 10 years there was a 39% probability that GMB was cost-effective compared with changes that occurred in the RoE over the same time period at a WTP for a QALY of £30,000. This was driven primarily by an apparent 3% increase in discharge to non-acute NHS inpatient providers according to HES data. Further work is required to validate this and refine other values in the model, potentially with richer data sets such as SSNAP.
Our analysis of the processes of planning and implementation of GMB (RQs 1 and 5) found the following:
-
The GM acute stroke system, like the rest of the English NHS, faced significant obstacles over the period studied and these affected the time it took to agree a new model, plan and implement it. Despite these obstacles, change was implemented.
-
Obstacles included turbulence prompted by reforms to the NHS and national staffing shortages, which contributed to significant pressures on planning. Post implementation, these factors led to delays in the movement of patients through the system, in finding beds for stroke patients and discharging them.
-
Issues relating to leadership and governance, and the use of service and process reviews, including the earlier findings from this study (see Chapter 3) were reported as important in enabling implementation.
-
Leaders of the GMB reconfiguration ‘held the line’ on approaches to implementation (e.g. timing and degree of phasing of change). A key system enabler post implementation was identified as the ODN governance model, funded by the providers, covering the whole stroke pathway. This enabled regular audits and a mechanism to facilitate the system-wide discussions needed to maintain effective system operation. Sources of pressure post implementation included not only staffing but also changes in the nature of the workload at the CSC, PSCs and DSCs due to the reconfiguration.
Our analysis of how the London acute stroke system sustained its performance in relation to clinical outcomes and interventions (RQs 1 and 5) found the following:
-
The London acute stroke system, like the rest of the English NHS, faced significant obstacles over the period studied.
-
Factors identified as obstacles to sustaining organisation and provision of acute stroke care in London included turbulence resulting from the 2013 NHS reforms, perceptions of conflicting national targets (e.g. A&E targets), national staffing shortages and significant pressures on social care services.
-
These factors were felt to have contributed to significant pressures on service provision, for example through delays in the transfer of patients through the system and resultant difficulties in finding beds for stroke patients in HASU and SU wards.
-
Key promoters of sustainability of the system included –
-
the characteristics of the model itself (in particular the service standards linked to the London stroke tariff)
-
processes of sustaining the model, including regular service reviews and use of national audit data
-
leadership of the model (in terms of continuity, but also adaptability)
-
the prioritisation of generating and sharing independent evidence was key to ensuring ongoing stakeholder ownership.
-
Implications of these findings
As part of this study, we have developed a framework for studying MSCs, such as the centralisation of acute stroke services, which has enabled us, first, to link findings on the impact of the changes on outcomes (mortality and LOS) with our findings on the use of evidence-based clinical interventions and, second, to link these quantitative findings (‘What works?’) with qualitative findings on the processes of change (‘How?’). This framework with a summary of the findings is shown in Figure 18.
FIGURE 18.
Summary of findings in relation to a framework for MSC. NSD, no significant difference. Clinical outcomes and cost-effectiveness analyses are based on difference-in-differences analyses of clinical interventions, based on post-centralisation delivery of interventions. Mortality summary was based on risk-adjusted mortality at 90 days. Cost-effectiveness summary was based on results at 10 years. Cost-effectiveness at 90 days was as follows: London (Cost = ↑; QALYs = ↑; NMB < 0), GMA (Cost = ↓; QALYs = ↑; NMB > 0), GMB (Cost = ↓; QALYs = ↑; NMB > 0). ↑, increase; ↓, decrease.
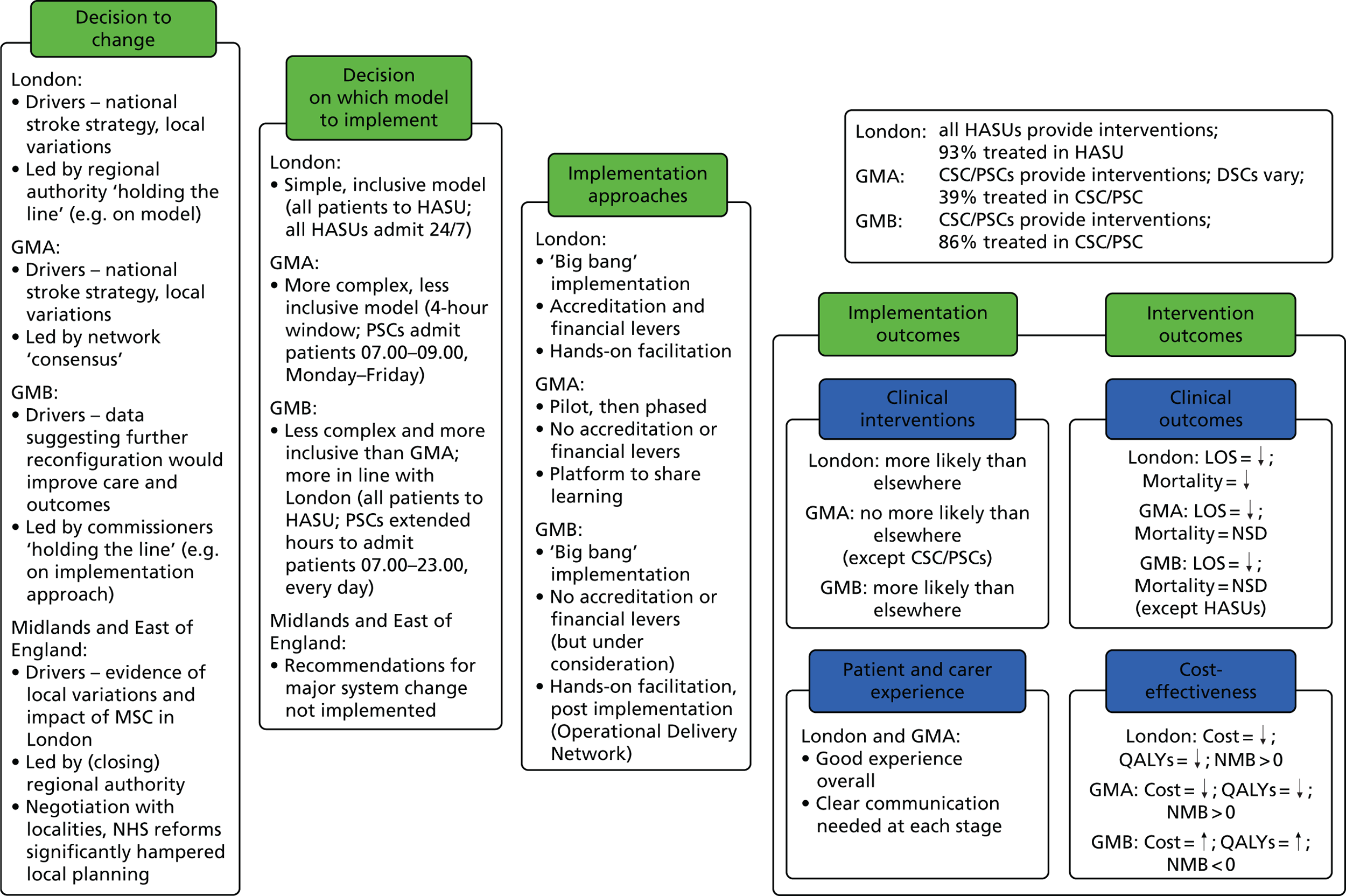
We now discuss implications in relation to the ‘what works’ question (i.e. whether or not our study provides evidence for a particular model of centralising acute stroke services) and in relation to the ‘how’ question (i.e. how MSC, such as centralisation of acute stroke services, can be best implemented).
Implications relating to the ‘what works’ question
Our study provides evidence to support the centralisation of acute stroke services in urban areas, and that this service model should include all stroke patients, not just those suitable for thrombolysis:
-
This is supported by our analysis of the original changes in London and GMA, which found that the London model, in which the majority of patients were treated in a HASU, resulted in reductions in mortality, whereas the GMA model in which most patients were not treated in a CSC/PSC, did not.
-
The finding above was further strengthened by our analysis of the GMB model, in which the majority of patients were treated in a CSC/PSC that showed reductions in mortality.
-
These findings are supported by our analysis of the provision of evidence-based clinical interventions (i.e. that these were more likely to be provided in the London model compared to GMA, and that the provision significantly increased with GMB and was maintained in London). The findings in relation to improvements in the provision of clinical interventions provide some explanation for our findings in relation to improvements in clinical outcomes.
-
Our study suggests that centralisation of acute stroke services can be cost-effective (London and GMA at 10 years, and GMA and GMB at 90 days), predominantly through improved outcomes or reductions in LOS.
Our findings suggest that the centralisation of care provision can offer patients and carers a good experience of care:
-
The disadvantages of travelling further were perceived to be outweighed by the opportunity to receive the best-quality care. The major contributions of this study are highlighting the necessity for clear and understandable information to patients and their families about every stage of the centralised care pathways, in order to maximise their experience of care and highlighting the importance of wide stakeholder engagement in the development and provision of centralised services, so that relevant components of the wider system have sufficient ownership and understanding of system delivery (e.g. the importance of the role of the ambulance service in explaining the centralised pathway on initial contact with stroke patients).
Implications relating to the ‘how’ question
Our findings provide implications for other urban areas considering centralising acute stroke services:
-
In relation to what issues to consider when deciding on the model –
-
Service models should be simple and inclusive to ensure that health-care staff and the public understand the new service and to ensure that all patients who might benefit from specialist care are eligible for treatment in a specialist unit, not just a selection.
-
-
in relation to the processes of implementation –
-
Service standards should be used to ensure that all services are able to provide the best possible care; providers should receive support to achieve standards and ongoing achievement of standards should be linked to financial incentives.
-
The reconfiguration should be implemented ideally as ‘big bang’ (for instance, the London centralisation was launched on a single day) or at least minimise the phases. The timing of these stages should be clearly communicated so that the change is clear, and a smooth transition is achieved.
-
Our findings also provide implications for other types of MSC. 110
An important contribution of our study was that we were able to combine a qualitative analysis of processes of change with a quantitative analysis of the impact and sustainability of these changes. In doing so in Chapter 6, we extended five lessons for MSC from Best et al. :5
-
Interplay between bottom-up and top-down leadership in achieving MSC – system-wide political authority or governance may be needed to align multiple stakeholders over a large scale and encourage clinical commitment to system-wide improvement goals and thus overcome resistance to change. Continuity of leadership is important, both to implementation and sustainability of change. However, the successful implementation of GMB suggested that MSC can be implemented successfully without making use of system-wide authority. However, we cannot discount the possibility that the previous reconfiguration (GMA) familiarised individuals with the issues and thus facilitated implementation.
-
Combining feedback with other tools (e.g. use of audit data) was important to build the case for change and to assess its impact. Feedback may need to be combined with other tools to encourage behaviour change (e.g. financial incentives).
-
Although learning from previous experiences of change can be a valuable guide to planning and implementing change, contextual factors can be a barrier to implementing these lessons (e.g. local resistance to downgrading or closing services); political authority may be needed to challenge the existing context and enable more radical forms of transformation.
-
There is a need to involve a range of stakeholders in planning MSC (including but not limited to physicians) and have a system-wide governance structure to align their interests.
-
Awareness that the drivers of MSC (e.g. clinical, political, social, financial) influence how different stakeholders’ views come to count during implementation; potential tension between patients’ and others’ perspectives.
The importance of system-wide leadership was illustrated further in our analysis of changes that were not implemented in the Midlands and East of England, following major reform of the NHS in England in 2013 and the removal of a key source of system leadership (SHAs). When this system-wide leadership does not exist or is less effective, we suggest that consideration should be given to how available sources of leadership can be used to enable the joint planning and implementation of MSC. The approaches employed in London to sustain their services (e.g. consistency of leadership, standards linked to financial incentives) and in GM in implementing further reconfiguration during this period (engaging senior commissioners to lead; recreating network capacity to facilitate the new changes) offered some examples of how the turbulence and threat of major structural reform might be mitigated.
Our study adds new empirical knowledge to the relatively small body of literature on involvement of patients and the public in MSC.
This may have wider implications than just for the reconfiguration of acute stroke services. The value of PPI in this case was found not in its contribution to acute service re-design, but in how involvement practices facilitated its implementation. Involvement was seen to have strategic and intrinsic value. Its strategic value lay in facilitating the implementation of a model of care that aimed to deliver evidence-based care to all; its intrinsic value was in the idea of citizen participation in change processes as an end in its own right. The concept of value, rather than impact, may provide greater traction in both the enactment of involvement in MSC and in analyses of involvement practices.
Strengths and limitations
This evaluation was a unique opportunity to study MSC in relation to one of the most significant health-care issues in the UK and elsewhere (stroke). We were able to study the implementation and outcomes of three examples of full implementation of MSC using multiple methods in a number of settings over a significant period of time, and to study contemporaneously cases where change was not implemented. In addition, our study was funded to analyse the impact of the reconfiguration of the acute component of the stroke pathway. Therefore, we did not study community and rehabilitation services, which are important components of the patient pathway.
In terms of our analyses of ‘what works at what cost’ (clinical outcomes, clinical interventions and cost-effectiveness), the main strength was that we analysed large national data sets (HES/ONS/national stroke audits). These allowed us to analyse changes controlling for trends elsewhere in the country and thus assess the ways in which the centralisations contributed to improvements in care provision, outcomes and value for money. There were several limitations:
-
In terms of clinical outcomes, HES data do not include stroke severity (an important predictor of stroke patient mortality), the specific wards in which patients receive care, nor data on other important clinical outcomes, such as disability and QoL. Such databases are also vulnerable to coding errors.
-
In relation to clinical interventions, the nature of the audit changed significantly and had very varied participation over the time period studied. As a result, some of our analyses could only use a limited comparator area (as opposed to the whole of the RoE), or only analyse a limited number of interventions.
-
The cost-effectiveness analyses, in using some of the same data as the outcomes and interventions analyses, faced the same limitations (although we were able to model for disability and QoL by making use of the SLSR).
An important strength in relation to our analyses of ‘development, implementation and sustainability’ is that we had the opportunity to study three cases of implemented MSC (London, GMA and GMB), and use multiple qualitative methods to study changes both retrospectively and contemporaneously. As a result, it was possible for the team to apply a range of theories on planning, implementation and sustainability of MSC to a rich and varied data set. There were several limitations:
-
Two of our analyses were retrospective, limiting the data that could be collected and potentially influencing participant views of the changes, although we sought to minimise this by using a range of data including speaking with a wide range of informants and analysing contemporaneous documents.
-
Within these cases, we were able to study only a selection of examples (e.g. only a sample of services in London and GM, and only a sample of areas covered by the Midlands and East of England review). However, in each analysis we took care to look beyond the local sites to assess the degree to which they reflected wider issues (e.g. by observing oversight groups in London and GM, and by engaging regularly with network representatives to discuss progress of MSC in all areas covered by the Midlands and East of England review).
A key limitation, which applies to both strands of our evaluation, is that all changes that were implemented were conducted in urban areas. Therefore, the degree to which our findings apply to other settings might be limited.
The impact of our study
As outlined in our study protocol, this was a formative evaluation. We have, therefore, taken an active approach to sharing the lessons from our research from the outset, sharing our findings in order to influence service development in the areas studied and nationally over the course of our research (see project webpage: www.journalslibrary.nihr.ac.uk/programmes/hsdr/10100909/). Key examples of engagement activity (beyond conventional academic outlets) include:
-
sharing interim findings annually with our Study Steering Committee (SSC) (which included clinicians, service users, managers, commissioners, the voluntary sector, and academics covering London, GM and the Midlands and East of England) on an annual basis (e.g. we shared interim findings on the impact of London and GMA on clinical outcomes in March 2013, over a year before publication)
-
building an effective working relationship with the Stroke Association
-
presenting findings to clinician groups in London and GM
-
presenting findings to patient representative groups (and coproducing a workshop for the UK Stroke Assembly)
-
producing accessible summaries of our papers (distributed to a list of > 200 stakeholders).
A key lesson for the research team related to dealing with the tension of maintaining the independence of the research with an ethos of co-production. As part of our formative evaluation approach, prior to publication of our findings on the impact of the models in GMA and London on clinical outcomes (see Chapter 3), we engaged actively with stakeholders in both London and GM to facilitate local ownership of the findings. This was more challenging in relation to stakeholders in GM, given that our findings indicated that the GMA changes had not achieved fully their intended impact. However, these findings were used in communications by the NHS in Manchester to justify the further centralisation of acute stroke care (GMB).
As a result, our study has had a significant impact in terms of organisation and provision of services, of national policy and recommendations, and further research, detailed below. In addition, we anticipate that the impact of our research will be ongoing, as evidenced by a number of upcoming dissemination activities (see project webpage: www.journalslibrary.nihr.ac.uk/programmes/hsdr/10100909/).
Impact on service reorganisation
Further reconfiguration in Greater Manchester
Our findings in relation to clinical outcomes (see Chapter 3)25 were cited in the decision to implement further reconfiguration of stroke services in GM; these changes were implemented in March 2015 (GMB). 224 Our analyses of clinical interventions indicate a substantial increase in the proportion of potential stroke patients being treated in a CSC/PSC, from approximately 39% (see Chapter 4)26 to > 80% (see Chapter 11).
Sustainability of acute stroke services in London
The London model is supported by a series of service standards linked to financial incentives (an uplifted tariff when standards are met). These standards have been identified as important both to the implementation and sustainability of the London model, as they are used as the basis of regular quality reviews led by representatives of the Clinical Network, local commissioners and clinicians. The standards were reviewed and a new version published in 2014. The revised and more recent versions cite our work as evidence of the benefits of the use of these standards. 225
Further citations in ‘case for change’ documents for reorganising stroke services
As of August 2017, our research has been cited in six ‘case for change’ documents in support of reorganising acute stroke services (see project webpage: www.journalslibrary.nihr.ac.uk/programmes/hsdr/10100909/).
Impact on national policy and recommendations
Our findings on clinical outcomes25 were presented in the Five Year Forward View as evidence of the ‘compelling case for greater concentration of care’ (p. 23),80 associated guidance on transforming urgent and emergency care services across the English NHS (p. 11)226 and the National Clinical Strategy for Scotland 2016 (pp. 70, 72). 208 Our findings on clinical interventions26 have been cited in the 2016 edition of the National Clinical Guidance for Stroke (pp. 13, 16 and 18)209 as evidence of the benefits of stroke service reorganisation.
Impact on our framework for major system change
Our qualitative findings in relation to the decision to further centralise services in GM and the sustainability of change in London suggested the potential influence of evidence of impact on care and outcomes on the decision to sustain or change a service model. We have, therefore, produced an updated figure (Figure 19) to illustrate feedback from implementation outcomes (C4) and intervention outcomes (C5), to decisions to change (C1), selection of model to be implemented (C2), and implementation approaches used (C3). Revised framework of MSC.
FIGURE 19.
Revised framework of MSC. Light blue shading denotes how key stages of change feed into the progress and outcomes of a given change. Darker green shading denotes how evidence on the outcomes of the different stages of change can feed back into future changes.
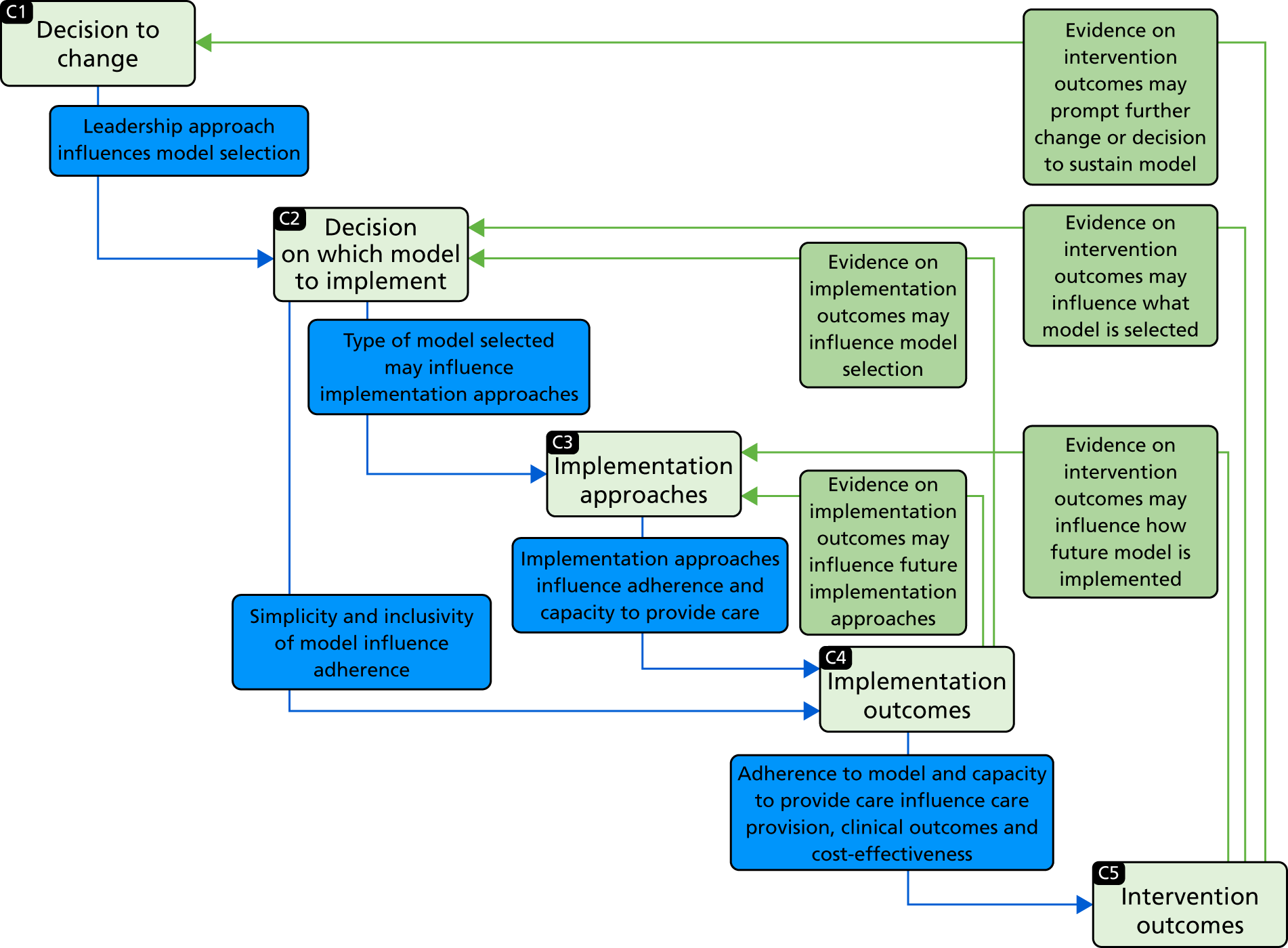
Future research agenda
This research suggests a number of directions for future research. We describe the most important of these below (the order in which these are presented does not imply priority). To date, our findings relate to MSC conducted in one specialty (acute stroke services), and the only changes that were implemented fully were carried out in urban settings (London and GM). To facilitate sharing of lessons to other settings, we believe there is value in terms of evaluating MSCs conducted in other specialties and less urban contexts, and identifying common factors and issues that are particular to different kinds of services and contexts. Examples of potentially valuable areas for evaluation include specialist cancer surgery [which we are now evaluating in London and GM through the HSDR study RESPECT-21 (14/46/19)].
Implementation of mechanical thrombectomy (a procedure that may offer significantly better outcomes for a selection of stroke patients but is currently provided very variably in the UK) is a current focus of NHS England in terms of offering equitable access to high-quality services, but providing this access would require considerable further reconfiguration. This represents an opportunity to examine how lessons from the current study inform future change within the same specialty.
Our evaluation suggests that there may be significant benefits of MSC and we provide lessons on factors that are likely to help influence the planning, implementation and sustainability of such changes. However, there may be value in future research exploring in greater depth some of the factors we identified in greater depth, for instance developing further our understanding of the interplay between top-down and bottom-up leadership, and ways in which teams and individuals adapt (or not) to MSC.
Further analysis of the impact of change would be valuable. The limited time elapsed since GMB (we could study only 1 year of post-GMB patients) suggests that it would be beneficial for further research on and monitoring of the relationship between the proportion of patients treated at a CSC/PSC, delivery of clinical interventions, and reductions in mortality and LOS over a longer period of time. Furthermore, our analysis of impact on disability/health-related QoL was crude and, arguably, disability is more important than mortality as an outcome. The SSNAP audit now collects these data for a high proportion of stroke patients, suggesting that it would be valuable for researchers to be able to access these data linked to other outcomes, including patient mortality. More research is required to understand factors influencing 10-year cost-effectiveness in GMB (specifically the increase in discharge to non-acute NHS inpatient providers), potentially with richer data sets such as SSNAP. Greater analysis of patient and carer experience of centralised services (e.g. during the process of change) is likely to provide valuable lessons on how best to carry out changes.
Acknowledgements
We acknowledge the financial support of the NIHR HSDR programme.
Naomi J Fulop, Simon J Turner and Stephen Morris were partly supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust.
Ruth Boaden and Catherine Perry were partly supported by the NIHR CLAHRC Greater Manchester.
Charles DA Wolfe and Christopher McKevitt were partly supported by the NIHR Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and also by the NIHR CLAHRC South London.
We thank the following for their contributions to this study:
Our Study Steering Committee for their expert advice and support throughout the lifespan of this project (see Appendix 8).
Mr Nanik Pursani for his valuable contributions to our study as patient coinvestigator from 2010 to 2015.
Ms Sally Standley for her valuable contributions to our study in relation to our study of efforts to implement MSC across the Midlands and East of England.
Stroke patient and carer groups in GM (Manchester Patient & Carer and PPI Group) and London (King’s College London Stroke Research Patients and Families Group, Sutton Stroke Support Group, South Islington Stroke Support Group) for their very helpful feedback on our research plans and findings.
Dr Susie Edwards, Dr Andrew Wilshere, Ms Michelle Morton and Ms Chloë Levelle for their valuable contributions to the management and administration of this project.
Finally, we thank our research participants for their invaluable contribution to the project, in particular the stroke services and networks across GM and London where we conducted in-depth data collection.
Contributions of authors
Professor Naomi J Fulop (Professor of Health Care Organisation and Management) was the principal investigator and led the study, provided oversight for all the qualitative analyses and led the analysis explaining outcomes of London and GMA (see Chapter 7) and the discussion (see Chapter 15). She contributed to design and analysis of all aspects of the research and is lead author of the final report.
Dr Angus IG Ramsay (Senior Research Associate) led the analyses of the impact of MSC on delivery of clinical interventions in London, GMA and GMB (see Chapters 4 and 11), efforts to implement MSC across the Midlands and East of England (see Chapter 10) and sustainability in London (see Chapter 14). He contributed to study design, qualitative data collection and quantitative data requests, and qualitative and quantitative analyses.
Ms Rachael M Hunter (Principal Research Associate) led the analyses of cost-effectiveness of London and GMA (see Chapter 5) and cost-effectiveness of GMB (see Chapter 12). She contributed to study design, quantitative data requests and all quantitative analyses.
Professor Christopher McKevitt (Professor of Social Sciences and Health) led the analysis of PPI in London and GMA (see Chapter 8). He contributed to study design and all qualitative and quantitative analyses.
Dr Catherine Perry (Research Fellow) led the analyses of patient and carer experience in London and GMA (see Chapter 9) and planning and implementation of GMB (see Chapter 13). She contributed to study design, qualitative data collection and all qualitative and quantitative analyses.
Dr Simon J Turner (Senior Lecturer) led the analysis of planning and implementation of London and GMA (see Chapter 6). He contributed to study design, qualitative data collection and all qualitative and quantitative analyses.
Professor Ruth Boaden (Professor of Service Operations) led the qualitative research conducted in GM. She contributed to study design and all qualitative and quantitative analyses.
Dr Iliatha Papachristou (Research Associate) contributed to qualitative study design, qualitative data collection and to the patient and carer experience and London sustainability analyses.
Professor Anthony G Rudd (Professor of Stroke Medicine) contributed expert knowledge on organisation and provision of acute stroke services in general, and the planning and implementation of MSC in London and nationally. He contributed to study design and all qualitative and quantitative analyses.
Professor Pippa J Tyrrell (Professor of Stroke Medicine) contributed expert knowledge on the organisation and provision of acute stroke services in general, and the planning and implementation of MSC in GM. She contributed to study design and all qualitative and quantitative analyses.
Professor Charles DA Wolfe (Professor of Public health) contributed expert knowledge on organisation and provision of acute stroke services in general, and the planning and implementation of MSC in London. He contributed to study design and all qualitative and quantitative analyses.
Professor Stephen Morris (Professor of Health Economics) provided oversight for all the quantitative analyses and led the analyses of the impact of MSC on clinical outcomes in London, GMA and GMB (see Chapters 3 and 11). He contributed to study design, quantitative data requests and all qualitative and quantitative analyses.
All authors made critical revisions to the report for important intellectual content and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Publications
Fulop NJ, Boaden R, Hunter R, McKevitt C, Morris S, Pursani N, et al. Innovations in major system reconfiguration in England: a study of the effectiveness, acceptability and processes of implementation of two models of stroke care. Implement Sci 2013;8:5. https://doi.org/10.1186/1748-5908-8-5
Morris S, Hunter RM, Ramsay AIG, Boaden R, McKevitt C, Perry C, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ 2014;349:g4757. https://doi.org/10.1136/bmj.g4757
Ramsay AIG, Morris S, Hoffman A, Hunter RM, Boaden R, McKevitt C, et al. Effects of centralising acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46:2244–51. https://doi.org/10.1161/STROKEAHA.115.009723
Turner S, Ramsay AIG, Perry C, Boaden RJ, McKevitt C, Morris S, et al. Lessons for major system change: centralisation of stroke services in two metropolitan areas of England. J Health Serv Res Policy 2016;21:156–65. https://doi.org/10.1177/1355819615626189
Fulop NJ, Ramsay AIG, Perry C, Boaden R, McKevitt C, Rudd A, et al. Explaining outcomes in major system change: a qualitative study of implementing centralised acute stroke services in two large metropolitan regions in England. Implement Sci 2016;11:80–92. https://doi.org/10.1186/s13012-016-0445-z
McKevitt C, Ramsay AIG, Perry C, Turner SJ, Boaden R, Wolfe CD, Fulop NJ. Patient, carer and public involvement in major system change in acute stroke services: the construction of value. Health Expect 2018;21:685–92. https://doi.org/10.1111/hex.12668
Hunter RM, Fulop NJ, Boaden RJ, McKevitt C, Perry C, Ramsay AIG, et al. The potential role of cost–utility analysis in the decision to implement major system change in acute stroke services in metropolitan areas in England. Health Res Policy Syst 2018;16:23–36. https://doi.org/10.1186/s12961-018-0301-5
Perry C, Papachristou I, Ramsay AIG, Boaden R, McKevitt C, Turner SJ, et al. Patient experience of centralised acute stroke care pathways. Health Expect 2018;21:909–18. https://doi.org/10.1111/hex.12685
Morris S, Ramsay AIG, Boaden R, Hunter RM, McKevitt C, Paley L, et al. Impact and sustainability of centralising acute stroke services in English metropolitan areas: retrospective analysis of hospital episode statistics and stroke national audit data. BMJ 2019;364:l1. https://doi.org/10.1136/bmj.l1
Data-sharing statement
The quantitative data used in this study were obtained by formal application [from NHS Digital (HES/ONS data – data reuse agreement NIC-161339-RC2NB) and the Healthcare Quality Improvement Partnership [Sentinel 2008 and SINAP (Data Sharing Agreement HQIP015a) and SSNAP (Data Sharing Agreement HQIP165)]. The agreements in place for these data do not permit further distribution or sharing. Requests for the relevant data sets must be made directly to NHS Digital or HQIP. All qualitative data generated that can be shared are contained within the report. The nature of the data means that nothing else can be provided. Further information can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Imison C, Sonola L, Honeyman M, Ross S. The Reconfiguration of Clinical Services in the NHS: What is the Evidence?. London: The King’s Fund; 2014.
- Better Care for the Severely Injured. London: RCSE; 2001.
- NHS England’s Business Plan 2014/15–2016/17: Putting Patients First. Leeds: NHS England; 2014.
- Transforming Urgent and Emergency Care Services. Leeds: NHS England; 2013.
- Best A, Greenhalgh T, Lewis S, Saul JE, Carroll S, Bitz J. Large-system transformation in health care: a realist review. Milbank Q 2012;90:421-56. https://doi.org/10.1111/j.1468-0009.2012.00670.x.
- Fulop N, Walters R, Spurgeon P. 6 P . Implementing changes to hospital services: factors influencing the process and ‘results’ of reconfiguration. Health Policy 2012;104:128-35. https://doi.org/10.1016/j.healthpol.2011.05.015.
- Prabhakaran S, O’Neill K, Stein-Spencer L, Walter J, Alberts MJ. Prehospital triage to primary stroke centers and rate of stroke thrombolysis. JAMA Neurol 2013;70:1126-32. https://doi.org/10.1001/jamaneurol.2013.293.
- Smith EE, Dreyer P, Prvu-Bettger J, Abdullah AR, Palmeri G, Goyette L, et al. Stroke center designation can be achieved by small hospitals: the Massachusetts experience. Crit Pathw Cardiol 2008;7:173-7. https://doi.org/10.1097/HPC.0b013e318184e2bc.
- Weir NU, Buchan AM. A study of the workload and effectiveness of a comprehensive acute stroke service. J Neurol Neurosurg Psychiatry 2005;76:863-5. https://doi.org/10.1136/jnnp.2004.053462.
- Lahr MM, Luijckx GJ, Vroomen PC, van der Zee DJ, Buskens E. Proportion of patients treated with thrombolysis in a centralized versus a decentralized acute stroke care setting. Stroke 2012;43:1336-40. https://doi.org/10.1161/STROKEAHA.111.641795.
- Bruins Slot K, Murray V, Boysen G, Berge E. Thrombolytic treatment for stroke in the Scandinavian countries. Acta Neurol Scand 2009;120:270-6. https://doi.org/10.1111/j.1600-0404.2009.01162.x.
- Cadilhac DA, Purvis T, Kilkenny MF, Longworth M, Mohr K, Pollack M, et al. Evaluation of rural stroke services: does implementation of co-ordinators and pathways improve care in rural hospitals?. Stroke 2013;44:2848-53. https://doi.org/10.1161/STROKEAHA.113.001258.
- Greenhalgh T, Robert G, Bate P, Kyriakidou O, Macfarlane F, Peacock R. How to Spread Good Ideas: A Systematic Review of the Literature on Diffusion, Dissemination and Sustainability of Innovations in Health Service Delivery and Organisation. London: National Co-ordinating Centre for NHS Service Delivery and Organisation; 2004.
- Sampalis JS, Denis R, Lavoie A, Fréchette P, Boukas S, Nikolis A, et al. Trauma care regionalization: a process-outcome evaluation. J Trauma 1999;46:565-81. https://doi.org/10.1097/00005373-199904000-00004.
- Mullins RJ, Mann NC. Population-based research assessing the effectiveness of trauma systems. J Trauma 1999;47:59-66. https://doi.org/10.1097/00005373-199909001-00013.
- MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, Egleston BL, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med 2006;354:366-78. https://doi.org/10.1056/NEJMsa052049.
- Grumbach K, Anderson GM, Luft HS, Roos LL, Brook R. Regionalization of cardiac surgery in the United States and Canada. Geographic access, choice, and outcomes. JAMA 1995;274:1282-8. https://doi.org/10.1001/jama.1995.03530160034030.
- Shah V, Warre R, Lee SK. Quality improvement initiatives in neonatal intensive care unit networks: achievements and challenges. Acad Pediatr 2013;13:75-83. https://doi.org/10.1016/j.acap.2013.04.014.
- Mackay J, Mensah GA. The Atlas of Heart Disease and Stroke. Geneva: World Health Organization; 2004.
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics: 2012 Edition. London: British Heart Foundation; 2012.
- Bray BD, Ayis S, Campbell J, Hoffman A, Roughton M, Tyrrell PJ, et al. Associations between the organisation of stroke services, process of care, and mortality in England: prospective cohort study. BMJ 2013;346. https://doi.org/10.1136/bmj.f2827.
- Langhorne P, Fearon P, Ronning OM, Kaste M, Palomaki H, Vemmos K, et al. Stroke unit care benefits patients with intracerebral hemorrhage: systematic review and meta-analysis. Stroke 2013;44:3044-9. https://doi.org/10.1161/STROKEAHA.113.001564.
- National Sentinel Audit of Stroke. London: Royal College of Physicians; 2008.
- National Stroke Strategy. London: Department of Health and Social Care; 2007.
- Morris S, Hunter RM, Ramsay AI, Boaden R, McKevitt C, Perry C, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ 2014;349. https://doi.org/10.1136/bmj.g4757.
- Ramsay AI, Morris S, Hoffman A, Hunter RM, Boaden R, McKevitt C, et al. Effects of centralizing acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46:2244-51. https://doi.org/10.1161/STROKEAHA.115.009723.
- 2011 Census: Usual Resident Population, Local Authorities in England and Wales. Newport: Office for National Statistics; 2012.
- A Framework for Action. London: NHS London; 2007.
- The Shape of Things to Come. London: NHS London; 2009.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Wang Y, Rudd AG, Wolfe CD. Age and ethnic disparities in incidence of stroke over time: the South London Stroke Register. Stroke 2013;44:3298-304. https://doi.org/10.1161/STROKEAHA.113.002604.
- Development of Stroke Services in Greater Manchester: Twelve Month Review. Manchester: GMCCSN; 2011.
- Lintern S. Analysed: Review Aims to Improve Stroke Services in the Midlands and East 2012. www.hsj.co.uk/hsj-local/briefing/midlands-and-east/analysed-review-aims-to-improve-stroke-services-in-the-midlands-and-east/5047149.article (accessed 2 October 2018).
- Health and Social Care Act 2012. London: The Stationery Office; 2012.
- Consulting the Capital. London: NHS London; 2008.
- Fulop N, Boaden R, Hunter R, McKevitt C, Morris S, Pursani N, et al. Innovations in major system reconfiguration in England: a study of the effectiveness, acceptability and processes of implementation of two models of stroke care. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-5.
- Ferlie E, Dopson S, Dopson S, Fitzgerald L. Knowledge to Action? Evidence-based Health Care in Context. Oxford: Oxford University Press; 2005.
- Lemieux-Charles L, Barnsley J, Lemieux-Charles L, Champagne F. Using Knowledge and Evidence in Health Care: Multidisciplinary Perspectives. Toronto, ON: University of Toronto Press; 2005.
- Webster A. Health, Technology, and Society: A Sociological Critique. Basingstoke: Palgrave Macmillan; 2007.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65-76. https://doi.org/10.1007/s10488-010-0319-7.
- Mendel P, Meredith LS, Schoenbaum M, Sherbourne CD, Wells KB. Interventions in organizational and community context: a framework for building evidence on dissemination and implementation in health services research. Adm Policy Ment Health 2008;35:21-37. https://doi.org/10.1007/s10488-007-0144-9.
- Kitson AL, Rycroft-Malone J, Harvey G, McCormack B, Seers K, Titchen A. Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci 2008;3. https://doi.org/10.1186/1748-5908-3-1.
- Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0242-0.
- Ashenfelter O, Card D. Using the longitudinal structure of earnings to estimate the effect of training programs. Rev Econ Stat 1985;67:648-60. https://doi.org/10.2307/1924810.
- Buchanan D, Fitzgerald L, Ketley D, Gollop R, Jones JL, Lamont SS, et al. No going back: a review of the literature on sustaining organizational change. IJMR 2005;7:189-205. https://doi.org/10.1111/j.1468-2370.2005.00111.x.
- Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-117.
- Fleiszer AR, Semenic SE, Ritchie JA, Richer MC, Denis JL. The sustainability of health-care innovations: a concept analysis. J Adv Nurs 2015;71:1484-98. https://doi.org/10.1111/jan.12633.
- Greenhalgh T, Macfarlane F, Barton-Sweeney C, Woodard F. ‘If we build it, will it stay?’ A case study of the sustainability of whole-system change in London. Milbank Q 2012;90:516-47. https://doi.org/10.1111/j.1468-0009.2012.00673.x.
- Yin RK. Case Study Research: Design and Methods. London: Sage Publications; 2009.
- Yin RK. Enhancing the quality of case studies in health services research. Health Serv Res 1999;34.
- Baker GR. The contribution of case study research to knowledge of how to improve quality of care. BMJ Qual Saf 2011;20:i30-5. https://doi.org/10.1136/bmjqs.2010.046490.
- Hunter RM, Davie C, Rudd A, Thompson A, Walker H, Thomson N, et al. Impact on clinical and cost outcomes of a centralized approach to acute stroke care in London: a comparative effectiveness before and after model. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0070420.
- Lahr MM, Luijckx GJ, Vroomen PC, van der Zee DJ, Buskens E. The chain of care enabling tPA treatment in acute ischemic stroke: a comprehensive review of organisational models. J Neurol 2013;260:960-8. https://doi.org/10.1007/s00415-012-6647-7.
- Langhorne P, Lewsey JD, Jhund PS, Gillies M, Chalmers JW, Redpath A, et al. Estimating the impact of stroke unit care in a whole population: an epidemiological study using routine data. J Neurol Neurosurg Psychiatry 2010;81:1301-5. https://doi.org/10.1136/jnnp.2009.195131.
- Sudlow C, Warlow C. Getting the priorities right for stroke care. BMJ 2009;338. https://doi.org/10.1136/bmj.b2083.
- Hospital Episode Statistics. Leeds: NHS Digital; n.d.
- International Classification of Diseases (10th revision). Geneva: World Health Organization; 1990.
- National Clinical Guideline for Stroke, 4th Edition. London: Royal College of Physicians; 2012.
- Linked HES-ONS Mortality Data. Leeds: NHS Digital; 2012.
- Progress in Improving Stroke Care: Department of Health and Social Care, HC 291, Report by Comptroller and Auditor General, Session 2009–10. London: The Stationery Office; 2010.
- 2001 Rural-Urban Classification. Newport: Office for National Statistic; n.d.
- Imbens GW, Wooldridge JM. Recent Developments in the Econometrics of Program Evaluation. Cambridge, MA: National Bureau of Economic Research; 2008.
- Craig P, Cooper C, Gunnell D, Haw S, Lawson K, Macintyre S, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health 2012;66:1182-6. https://doi.org/10.1136/jech-2011-200375.
- Sutton M, Nikolova S, Boaden R, Lester H, McDonald R, Roland M. Reduced mortality with hospital pay for performance in England. N Engl J Med 2012;367:1821-8. https://doi.org/10.1056/NEJMsa1114951.
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. https://doi.org/10.1097/01.mlr.0000182534.19832.83.
- Data.gov.uk . Index of Multiple Deprivation 2004 n.d. http://data.gov.uk/dataset/imd_2004 (accessed 19 June 2017).
- Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197-204. https://doi.org/10.1258/1355819042250249.
- Fonarow GC, Pan W, Saver JL, Smith EE, Reeves MJ, Broderick JP, et al. Comparison of 30-day mortality models for profiling hospital performance in acute ischemic stroke with vs. without adjustment for stroke severity. JAMA 2012;308:257-64. https://doi.org/10.1001/jama.2012.7870.
- SINAP: Latest Results. London: Royal College of Physicians; 2013.
- Cloud G, Hoffman A, Rudd A. Intercollegiate Stroke Working Party . National sentinel stroke audit 1998–2011. Clin Med 2013;13:444-8. https://doi.org/10.7861/clinmedicine.13-5-444.
- SINAP – Combined Quarterly Public Report (Quarters 1–7). London: Royal College of Physicians; 2013.
- Ramelyte M, Virdi G, Fothergill R, Thomson N. Stroke Annual Report: 2011/2012. London: London Ambulance Service NHS Trust; 2012.
- Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489-94. https://doi.org/10.1161/STROKEAHA.110.602615.
- Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 2009;40:394-9. https://doi.org/10.1161/STROKEAHA.108.523209.
- Audebert HJ, Schwamm L. Telestroke: scientific results. Cerebrovasc Dis 2009;27:15-20. https://doi.org/10.1159/000213054.
- Demaerschalk BM, Miley ML, Kiernan TE, Bobrow BJ, Corday DA, Wellik KE, et al. Stroke telemedicine. Mayo Clin Proc 2009;84:53-64. https://doi.org/10.1016/S0025-6196(11)60808-2.
- Silva GS, Farrell S, Shandra E, Viswanathan A, Schwamm LH. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke 2012;43:2078-85. https://doi.org/10.1161/STROKEAHA.111.645861.
- Williams D. Major Reconfiguration Plans Emerging Across England. Southfields: HSJ; n.d.
- Five-Year Forward View. London: NHS England; 2014.
- Stroke Unit Trialists’ Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013;9.
- Norrving B, Adams RJ. Organized stroke care. Stroke 2006;37:326-8. https://doi.org/10.1161/01.STR.0000200554.95094.09.
- Monks T, Pitt M, Stein K, James MA. Hyperacute stroke care and NHS England’s business plan. BMJ 2014;348. https://doi.org/10.1136/bmj.g3049.
- National Sentinel Stroke Audit: Phase II (Clinical Audit) 2008. London: Royal College of Physicians; 2009.
- Rural/Urban Local Authority (LA) Classification (England). Newport: Office for National Statistics; 2009.
- Stroke: Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA). London: NICE; 2008.
- Wardlaw JM, Seymour J, Cairns J, Keir S, Lewis S, Sandercock P. Immediate computed tomography scanning of acute stroke is cost-effective and improves quality of life. Stroke 2004;35:2477-83. https://doi.org/10.1161/01.STR.0000143453.78005.44.
- Best Practice Tariffs and their Impact. London: Audit Commission; 2012.
- McDonald R, Zaidi S, Todd S, Konteh F, Hussain K, Roe J, et al. A Qualitative and Quantitative Evaluation of the Introduction of Best Practice Tariffs. London: Department of Health and Social Care; 2012.
- Rudd AG, Hoffman A, Grant R, Campbell JT, Lowe D. Intercollegiate Working Party for Stroke . Stroke thrombolysis in England, Wales and Northern Ireland: how much do we do and how much do we need?. J Neurol Neurosurg Psychiatry 2011;82:14-9. https://doi.org/10.1136/jnnp.2009.203174.
- Hunter RM, Fulop NJ, Boaden RJ, McKevitt C, Perry C, Ramsay AIG, et al. The potential role of cost-utility analysis in the decision to implement major system change in acute stroke services in metropolitan areas in England. Health Res Policy Syst 2018;16. https://doi.org/10.1186/s12961-018-0301-5.
- Bhattarai N, McMeekin P, Price C, Vale L. Economic evaluations on centralisation of specialised healthcare services: a systematic review of methods. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011214.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Mahoney FI, Barthel D. Functional evaluation: the Barthel index. Maryland State Med J 1965;14:56-61.
- Gordon AL, Franklin M, Bradshaw L, Logan P, Elliott R, Gladman JR. Health status of UK care home residents: a cohort study. Age Ageing 2014;43:97-103. https://doi.org/10.1093/ageing/aft077.
- Stroke Acute Commissioning and Tariff Guidance. London: Commissioning Support for London; 2009.
- 2014/2015 National Tariff Payment System: Annex 5A – National Prices. London: NHS England, Monitor; 2014.
- NHS Reference Costs 2013 to 2014. London: Department of Health and Social Care; 2015.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2015.
- Franklin M, Berdunov V, Edmans J, Conroy S, Gladman J, Tanajewski L, et al. Identifying patient-level health and social care costs for older adults discharged from acute medical units in England. Age Ageing 2014;43:703-7. https://doi.org/10.1093/ageing/afu073.
- Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ 2004;13:437-52. https://doi.org/10.1002/hec.864.
- Scott AE, Falkingham J, Rake K. Moves into Residential Care Amongst Older People in Britain n.d.
- Non-Emergency Patient Transport Services. Southampton: NHS South of England Procurement Services; 2015.
- Sheehan J. Pre-Consultation Business Case – Major Trauma and Stroke Services in London. London: NHS London; 2009.
- Boyle S. Economic Case for Change in the System of Stroke Care in Greater Manchester. Manchester: Greater Manchester and Cheshire Cardiac and Stroke Network and Association of Greater Manchester PCTs; 2009.
- Turner M, Barber M, Dodds H, Dennis M, Langhorne P, Macleod MJ. Scottish Stroke Care Audit . The impact of stroke unit care on outcome in a Scottish stroke population, taking into account case mix and selection bias. J Neurol Neurosurg Psychiatry 2015;86:314-18. https://doi.org/10.1136/jnnp-2013-307478.
- Bristow RE, Santillan A, Diaz-Montes TP, Gardner GJ, Giuntoli RL, Meisner BC, et al. Centralization of care for patients with advanced-stage ovarian cancer: a cost-effectiveness analysis. Cancer 2007;109:1513-22. https://doi.org/10.1002/cncr.22561.
- Greving JP, Vernooij F, Heintz AP, van der Graaf Y, Buskens E. Is centralization of ovarian cancer care warranted? A cost-effectiveness analysis. Gynecol Oncol 2009;113:68-74. https://doi.org/10.1016/j.ygyno.2008.12.008.
- Te Ao BJ, Brown PM, Feigin VL, Anderson CS. Are stroke units cost effective? Evidence from a New Zealand stroke incidence and population-based study. Int J Stroke 2012;7:623-30. https://doi.org/10.1111/j.1747-4949.2011.00632.x.
- Turner S, Ramsay A, Perry C, Boaden R, McKevitt C, Morris S, et al. Lessons for major system change: centralization of stroke services in two metropolitan areas of England. J Health Serv Res Policy 2016;21:156-65. https://doi.org/10.1177/1355819615626189.
- Eisenhardt KM, Graebner ME. Theory building from cases: opportunities and challenges. Acad Manag J 2007;50:25-32. https://doi.org/10.5465/amj.2007.24160888.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007;42:1758-72. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
- Timmermans S, Oh H. The continued social transformation of the medical profession. J Health Soc Behav 2010;51:S94-106. https://doi.org/10.1177/0022146510383500.
- Barratt H, Raine R. Hospital service reconfiguration: the battle for hearts and minds. BMJ 2012;344. https://doi.org/10.1136/bmj.e953.
- Tuohy CH. Accidental Logics: The Dynamics of Change in the Health Care Arena in the United States, Britain, and Canada. Oxford: Oxford University Press; 1999.
- Douw K, Nielsen CP, Pedersen CR. Centralising acute stroke care and moving care to the community in a Danish health region: challenges in implementing a stroke care reform. Health Policy 2015;119:1005-10. https://doi.org/10.1016/j.healthpol.2015.05.007.
- Fudge N, Wolfe CD, McKevitt C. Assessing the promise of user involvement in health service development: ethnographic study. BMJ 2008;336:313-17. https://doi.org/10.1136/bmj.39456.552257.BE.
- Thompson J, Bissell P, Cooper CL, Armitage CJ, Barber R. Exploring the impact of patient and public involvement in a cancer research setting. Qual Health Res 2014;24:46-54. https://doi.org/10.1177/1049732313514482.
- Sørensen T, Dyb K, Rygh E, Salvesen R, Thomassen L. A qualitative description of telemedicine for acute stroke care in Norway: technology is not the issue. BMC Health Serv Res 2014;14. https://doi.org/10.1186/s12913-014-0643-9.
- The Functions of GP Commissioning Consortia: A Working Document. London: Department of Health and Social; 2011.
- HM Treasury/Greater Manchester Combined Authority . Greater Manchester Agreement: Devolution to the GMCA &Amp; Transition to a Directly Elected Mayor 2015. www.gov.uk/government/uploads/system/uploads/attachment_data/file/369858/Greater_Manchester_Agreement_i.pdf (accessed 19 June 2017).
- Devolution and Health. London: British Medical Association; 2017.
- Fulop NJ, Ramsay AI, Perry C, Boaden RJ, McKevitt C, Rudd AG, et al. Explaining outcomes in major system change: a qualitative study of implementing centralised acute stroke services in two large metropolitan regions in England. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0445-z.
- Pronovost PJ, Goeschel CA, Marsteller JA, Sexton JB, Pham JC, Berenholtz SM. Framework for patient safety research and improvement. Circulation 2009;119:330-7. https://doi.org/10.1161/CIRCULATIONAHA.107.729848.
- Stetler CB, Legro MW, Wallace CM, Bowman C, Guihan M, Hagedorn H, et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med 2006;21:1-8. https://doi.org/10.1007/s11606-006-0267-9.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. https://doi.org/10.1111/j.0887-378X.2004.00325.x.
- Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map?. J Contin Educ Health Prof 2006;26:13-24. https://doi.org/10.1002/chp.47.
- May C. Towards a general theory of implementation. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-18.
- Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med 2012;43:337-50. https://doi.org/10.1016/j.amepre.2012.05.024.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Qual Saf 2015;24:228-38. https://doi.org/10.1136/bmjqs-2014-003627.
- Foy R, Sales A, Wensing M, Aarons GA, Flottorp S, Kent B, et al. Implementation science: a reappraisal of our journal mission and scope. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0240-2.
- Mays N, Pope C. Rigour and qualitative research. BMJ 1995;311:109-12. https://doi.org/10.1136/bmj.311.6997.109.
- McNulty T, Ferlie E. Process transformation: limitations to radical organizational change within public service organizations. Organ Stud 2004;25:1389-412. https://doi.org/10.1177/0170840604046349.
- McKevitt C, Ramsay AIG, Perry C, Turner SJ, Boaden R, Wolfe CDA, et al. Patient, carer and public involvement in major system change in acute stroke services: the construction of value. Health Expect 2018;21:685-92. https://doi.org/10.1111/hex.12668.
- Rabeharisoa V, Callon M. The involvement of patients’ associations in research. Int Soc Sci J 2002;54:57-63. https://doi.org/10.1111/1468-2451.00359.
- Lehoux P, Daudelin G, Abelson J. The unbearable lightness of citizens within public deliberation processes. Soc Sci Med 2012;74:1843-50. https://doi.org/10.1016/j.socscimed.2012.02.023.
- Dalton J, Chambers D, Harden M, Street A, Parker G, Eastwood A. Service user engagement in health service reconfiguration: a rapid evidence synthesis. J Health Serv Res Policy 2016;21:195-20. https://doi.org/10.1177/1355819615623305.
- Potter DA. ‘Wrong parents’ and ‘right parents’: shared perspectives about citizen participation in policy implementation. Soc Sci Med 2010;70:1705-13. https://doi.org/10.1016/j.socscimed.2010.01.025.
- Cornwall A, Shankland A. Engaging citizens: lessons from building Brazil’s national health system. Soc Sci Med 2008;66:2173-84. https://doi.org/10.1016/j.socscimed.2008.01.038.
- Crawford MJ, Rutter D, Manley C, Weaver T, Bhui K, Fulop N, et al. Systematic review of involving patients in the planning and development of health care. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7375.1263.
- Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy 2002;61:213-36. https://doi.org/10.1016/S0168-8510(01)00214-7.
- Salzburg Global Seminar . Salzburg statement on shared decision making. BMJ 2011;342. https://doi.org/10.1136/bmj.d1745.
- Carman KL, Dardess P, Maurer M, Sofaer S, Adams K, Bechtel C, et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff 2013;32:223-31. https://doi.org/10.1377/hlthaff.2012.1133.
- Gallivan J, Kovacs Burns K, Bellows M, Eigenseher C. The many faces of patient engagement. J Particip Med 2012;4.
- Baggott R. A funny thing happened on the way to the forum? Reforming patient and public involvement in the NHS in England. Public Adm 2005;83:533-51. https://doi.org/10.1111/j.0033-3298.2005.00461.x.
- Martin GP. ‘Ordinary people only’: knowledge, representativeness, and the publics of public participation in healthcare. Sociol Health Illn 2008;30:35-54. https://doi.org/10.1111/j.1467-9566.2007.01027.x.
- Callaghan G, Wistow G. Governance and public involvement in the British National Health Service: understanding difficulties and developments. Soc Sci Med 2006;63:2289-300. https://doi.org/10.1016/j.socscimed.2006.05.023.
- Gradinger F, Britten N, Wyatt K, Froggatt K, Gibson A, Jacoby A, et al. Values associated with public involvement in health and social care research: a narrative review. Health Expect 2015;18:661-75. https://doi.org/10.1111/hex.12158.
- Renedo A, Marston C. Healthcare professionals’ representations of ‘patient and public involvement’ and creation of ‘public participant’ identities: implications for the development of inclusive and bottom-up community participation initiatives. J Community Appl Soc Psychol 2011;21:268-80. https://doi.org/10.1002/casp.1092.
- Veronesi G, Keasey K. Patient and public participation in the English NHS: an assessment of experimental implementation processes. Public Manag Rev 2015;17:543-64. https://doi.org/10.1080/14719037.2013.822526.
- Pizzo E, Doyle C, Matthews R, Barlow J. Patient and public involvement: how much do we spend and what are the benefits?. Health Expect 2015;18:1918-26. https://doi.org/10.1111/hex.12204.
- Gibson A, Britten N, Lynch J. Theoretical directions for an emancipatory concept of patient and public involvement. Health 2012;16:531-47. https://doi.org/10.1177/1363459312438563.
- Ocloo J, Matthews R. From tokenism to empowerment: progressing patient and public involvement in healthcare improvement. BMJ Qual Saf 2016;25:626-32. https://doi.org/10.1136/bmjqs-2015-004839.
- Ward PR, Thompson J, Barber R, Armitage CJ, Boote JD, Cooper CL, et al. Critical perspectives on ‘consumer involvement’ in health research: epistemological dissonance and the know-do gap. J Sociol 2010;46:63-82. https://doi.org/10.1177/1440783309351771.
- Morrison C, Dearden A. Beyond tokenistic participation: using representational artefacts to enable meaningful public participation in health service design. Health Policy 2013;112:179-86. https://doi.org/10.1016/j.healthpol.2013.05.008.
- Li KK, Abelson J, Giacomini M, Contandriopoulos D. Conceptualizing the use of public involvement in health policy decision-making. Soc Sci Med 2015;138:14-21. https://doi.org/10.1016/j.socscimed.2015.05.023.
- Conklin A, Morris Z, Nolte E. What is the evidence base for public involvement in health-care policy?: results of a systematic scoping review. Health Expect 2015;18:153-65. https://doi.org/10.1111/hex.12038.
- Edelman N, Barron D. Evaluation of public involvement in research: time for a major re-think?. J Health Serv Res Policy 2016;21:209-11. https://doi.org/10.1177/1355819615612510.
- Heppollette W. Greater Manchester Integrated Stroke Service: Establishing the Governance Framework to Support Integrated Acute Stroke Care. Greater Manchester: NHS Association of Greater Manchester Primary Care Trusts; 2009.
- The Shape of Things to Come: Developing New, High-Quality Major Trauma and Stroke Services for London – Consultation Analysis. London: Ipsos MORI Social Research Institute; 2009.
- Healthcare for London: Consulting the Capital – Consultation Analysis. London: Ipsos MORI Social Research Institute; 2008.
- Foley C, Droog E, Healy O, McHugh S, Buckley C, Browne JP. Understanding perspectives on major system change: a comparative case study of public engagement and the implementation of urgent and emergency care system reconfiguration. Health Policy 2017;121:800-8. https://doi.org/10.1016/j.healthpol.2017.05.009.
- Martin GP. Representativeness, legitimacy and power in public involvement in health-service management. Soc Sci Med 2008;67:1757-65. https://doi.org/10.1016/j.socscimed.2008.09.024.
- Donaldson LJ. Put the patient in the room, always. Qual Saf Health Care 2008;17:82-3. https://doi.org/10.1136/qshc.2007.025262.
- Milewa T. Local participatory democracy in Britain’s health service: innovation or fragmentation of a universal citizenship?. Soc Policy Adm 2004;38:240-52. https://doi.org/10.1111/j.1467-9515.2004.00388.x.
- NHS England Operations and Delivery . Planning, Assuring and Delivering Service Change for Patients 2013. www.england.nhs.uk/wp-content/uploads/2015/10/plan-ass-deliv-serv-chge.pdf (accessed 14 July 2017).
- Brown P, Zavestoski S, McCormick S, Mayer B, Morello-Frosch R, Gasior Altman R. Embodied health movements: new approaches to social movements in health. Sociol Health Illn 2004;26:50-8. https://doi.org/10.1111/j.1467-9566.2004.00378.x.
- Komporozos-Athanasiou A, Fudge N, Adams M, McKevitt C. Citizen participation as political ritual: towards a sociological theorizing of ‘health citizenship’. Sociology 2018;52:744-61.
- Staniszewska S, Herron-Marx S, Mockford C. Measuring the impact of patient and public involvement: the need for an evidence base. Int J Qual Health Care 2008;20:373-4. https://doi.org/10.1093/intqhc/mzn044.
- Graeber D. It is value that brings universes into being. HAU: J Ethnograph Theor 2013;3:219-43. https://doi.org/10.14318/hau3.2.012.
- Perry C, Papachristou I, Ramsay AIG, Boaden R, McKevitt C, Turner S, et al. Patient experience of centralised acute stroke care pathways. Health Expect 2018;21:909-18. https://doi.org/10.1111/hex.12685.
- Hewitt G, Sims S, Greenwood N, Jones F, Ross F, Harris R. Interprofessional teamwork in stroke care: is it visible or important to patients and carers?. J Interprof Care 2015;29:331-9. https://doi.org/10.3109/13561820.2014.950727.
- Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N. A randomised controlled comparison of alternative strategies in stroke care. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9180.
- Survey of Patients 2005: Stroke. London: Commission for Healthcare Audit and Inspection; 2005.
- Howell E, Graham C, Hoffman A, Lowe D, McKevitt C, Reeves R, et al. Comparison of patients’ assessments of the quality of stroke care with audit findings. Qual Saf Health Care 2007;16:450-5. https://doi.org/10.1136/qshc.2006.022079.
- Asplund K, Jonsson F, Eriksson M, Stegmayr B, Appelros P, Norrving B, et al. Patient dissatisfaction with acute stroke care. Stroke 2009;40:3851-6. https://doi.org/10.1161/STROKEAHA.109.561985.
- Moynihan B, Paul S, Markus HS. User experience of a centralized hyperacute stroke service: a prospective evaluation. Stroke 2013;44:2743-7. https://doi.org/10.1161/STROKEAHA.113.001675.
- Payne S, Jarrett N, Jeffs D. The impact of travel on cancer patients’ experiences of treatment: a literature review. Eur J Cancer Care 2000;9:197-203. https://doi.org/10.1046/j.1365-2354.2000.00225.x.
- Sampson FC, O’Cathain A, Goodacre S. Is primary angioplasty an acceptable alternative to thrombolysis? Quantitative and qualitative study of patient and carer satisfaction. Health Expect 2010;13:350-8. https://doi.org/10.1111/j.1369-7625.2009.00589.x.
- Wolf J, Niederhauser V, Marshburn D, LaVela S. Defining patient experience: a critical decision for healthcare organizations. Patient Exp J 2014;1:3-19.
- Anhang Price R, Elliott MN, Zaslavsky AM, Hays RD, Lehrman WG, Rybowski L, et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev 2014;71:522-54. https://doi.org/10.1177/1077558714541480.
- High Quality Care for All: NHS Next Stage Review Report. London: Department of Health and Social Care; 2008.
- Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-001570.
- Goodrich J, Cornwell J. Seeing the Person in the Patient. London: The King’s Fund; 2008.
- Feeling Better? Improving Patient Experience in Hospital. London: NHS Confederation; 2010.
- Patient Experience in Adult NHS Services. London: NICE; 2012.
- Jones SP, Dickinson HA, Ford GA, Gibson JM, Leathley MJ, McAdam JJ, et al. Callers’ experiences of making emergency calls at the onset of acute stroke: a qualitative study. Emerg Med J 2012;29:502-5. https://doi.org/10.1136/emj.2010.108563.
- Togher FJ, Davy Z, Siriwardena AN. Patients’ and ambulance service clinicians’ experiences of prehospital care for acute myocardial infarction and stroke: a qualitative study. Emerg Med J 2013;30:942-8. https://doi.org/10.1136/emermed-2012-201507.
- Harrison M, Ryan T, Gardiner C, Jones A. Patients’ and carers’ experiences of gaining access to acute stroke care: a qualitative study. Emerg Med J 2013;30:1033-7. https://doi.org/10.1136/emermed-2012-201974.
- Wellwood I, Dennis M, Warlow C. Patients’ and carers’ satisfaction with acute stroke management. Age Ageing 1995;24:519-24. https://doi.org/10.1093/ageing/24.6.519.
- Sadler E, Daniel K, Wolfe CD, McKevitt C. Navigating stroke care: the experiences of younger stroke survivors. Disabil Rehabil 2014;36:1911-17. https://doi.org/10.3109/09638288.2014.882416.
- Pound P, Bury M, Gompertz P, Ebrahim S. Stroke patients’ views on their admission to hospital. BMJ 1995;311:18-22. https://doi.org/10.1136/bmj.311.6996.18.
- Pound P, Tilling K, Rudd AG, Wolfe CD. Does patient satisfaction reflect differences in care received after stroke?. Stroke 1999;30:49-55. https://doi.org/10.1161/01.STR.30.1.49.
- Macduff CN. Stroke patients’ perceptions of hospital nursing care. J Clin Nurs 1998;7:442-50. https://doi.org/10.1046/j.1365-2702.1998.00166.x.
- Morris R, Payne O, Lambert A. Patient, carer and staff experience of a hospital-based stroke service. Int J Qual Health Care 2007;19:105-12. https://doi.org/10.1093/intqhc/mzl073.
- Thomas C, Parry A. Research on users’ views about stroke services: towards an empowerment research paradigm or more of the same?. Physiotherapy 1996;82:6-12. https://doi.org/10.1016/S0031-9406(05)66991-X.
- Pound P, Gompertz P, Ebrahim S. Patients’ satisfaction with stroke services. Clin Rehabil 1994;8:7-17. https://doi.org/10.1177/026921559400800102.
- Ellis-Hill C, Robison J, Wiles R, McPherson K, Hyndman D, Ashburn A. Going home to get on with life: patients’ and carers’ experiences of being discharged from hospital following a stroke. Disabil Rehabil 2009;31:61-72. https://doi.org/10.1080/09638280701775289.
- Rhodes JP, Leathley MJ, Watkins CL, Sharma AK. Stroke patients’ experiences of being admitted to and nursed within a mixed sex environment: a qualitative study. Clin Eff Nurs 2003;7:141-7. https://doi.org/10.1016/j.cein.2003.09.005.
- Payne S, Burton C, Addington-Hall J, Jones A. End-of-life issues in acute stroke care: a qualitative study of the experiences and preferences of patients and families. Palliat Med 2010;24:146-53. https://doi.org/10.1177/0269216309350252.
- Turner S, Goulding L, Denis J-L, McDonald R, Fulop NJ, Raine R, et al. Major system change: a management and organisational research perspective. Health Serv Deliv Res 2016;4.
- Jones L, Exworthy M. Framing in policy processes: a case study from hospital planning in the National Health Service in England. Soc Sci Med 2015;124:196-204. https://doi.org/10.1016/j.socscimed.2014.11.046.
- Fraser A, Baeza JI, Boaz A. ‘Holding the line’: a qualitative study of the role of evidence in early phase decision-making in the reconfiguration of stroke services in London. Health Res Policy Syst 2017;15. https://doi.org/10.1186/s12961-017-0207-7.
- Denis J-L, Dompierre G, Langley A, Rouleau L. Escalating indecision: between reification and strategic ambiguity. Organ Sci 2011;22:225-44. https://doi.org/10.1287/orsc.1090.0501.
- NHS Midlands and East Stroke Review 2012/13 – Legacy Report. Cambridge, Nottingham and Birmingham: NHS Midlands and East; 2013.
- Walshe K, Freeman T. Effectiveness of quality improvement: learning from evaluations. Qual Saf Health Care 2002;11:85-7. https://doi.org/10.1136/qhc.11.1.85.
- Morris S, Ramsay AIG, Boaden R, Hunter RM, McKevitt C, Paley L, et al. Impact and sustainability of centralising acute stroke services in English metropolitan areas: retrospective analysis of hospital episode statistics and stroke national audit data. BMJ 2019;364. https://doi.org/10.1136/bmj.l1.
- A National Clinical Strategy for Scotland. Edinburgh: Scottish Government; 2016.
- National Clinical Guideline for Stroke, 5th Edition. London: Royal College of Physicians; 2016.
- Paul CL, Ryan A, Rose S, Attia JR, Kerr E, Koller C, et al. How can we improve stroke thrombolysis rates? A review of health system factors and approaches associated with thrombolysis administration rates in acute stroke care. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0414-6.
- Dutta D, Hellier K, Obaid M, Deering A. Evaluation of a single centre stroke service reconfiguration – the impact of transition from a combined (acute and rehabilitation) stroke unit to a hyperacute model of stroke care. Future Hosp J 2017;4:99-104. https://doi.org/10.7861/futurehosp.4-2-99.
- Hubert GJ, Meretoja A, Audebert HJ, Tatlisumak T, Zeman F, Boy S, et al. Stroke thrombolysis in a centralized and a decentralized system (Helsinki and Telemedical Project for Integrative Stroke Care Network). Stroke 2016;47:2999-3004. https://doi.org/10.1161/STROKEAHA.116.014258.
- Sentinel Stroke National Audit Programme (SSNAP) Clinical Audit Public Report (December 2016 – March 2017). London: Royal College of Physicians; 2017.
- SSNAP – Clinical Audit August – November 2016 Public Report. London: Royal College of Physicians; 2017.
- May CR, Johnson M, Finch T. Implementation, context and complexity. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0506-3.
- Barratt H, Turner S, Hutchings A, Pizzo E, Hudson E, Briggs T, et al. Mixed methods evaluation of the Getting it Right First Time programme – improvements to NHS orthopaedic care in England: study protocol. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2012-y.
- What are Gateway Reviews?. London: Department of Health and Social Care; 2011.
- An Independent Clinical Review of the Greater Manchester Integrated Stroke Service. Greater Manchester, Lancashire and South Cumbria Clinical Senate; 2014.
- Operational Delivery Networks. London: NHS England; 2014.
- Macfarlane F, Barton-Sweeney C, Woodard F, Greenhalgh T. Achieving and sustaining profound institutional change in healthcare: case study using neo-institutional theory. Soc Sci Med 2013;80:10-8. https://doi.org/10.1016/j.socscimed.2013.01.005.
- Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-17.
- Proctor E, Luke D, Calhoun A, McMillen C, Brownson R, McCrary S, et al. Sustainability of evidence-based healthcare: research agenda, methodological advances, and infrastructure support. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0274-5.
- McEvoy R, Ballini L, Maltoni S, O’Donnell CA, Mair FS, Macfarlane A. A qualitative systematic review of studies using the normalization process theory to research implementation processes. Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-2.
- Greater Manchester Stroke Centralisation – Background Briefing. Manchester: GMCCSN; 2015.
- Stroke Acute Commissioning and Tariff Guidance. London: NHS England; 2014.
- Safer, Faster, Better: Good Practice in Delivering Urgent and Emergency Care – A Guide for Local Health and Social Care Communities. London: NHS England; 2015.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Oliver SR, Rees RW, Clarke-Jones L, Milne R, Oakley AR, Gabbay J, et al. A multidimensional conceptual framework for analysing public involvement in health services research. Health Expect 2008;11:72-84. https://doi.org/10.1111/j.1369-7625.2007.00476.x.
- McKevitt C, Fudge N, Wolfe C. What is involvement in research and what does it achieve? Reflections on a pilot study of the personal costs of stroke. Health Expect 2010;13:86-94. https://doi.org/10.1111/j.1369-7625.2009.00573.x.
Appendix 1 Research governance: ethics and local permissions
| Version | Date | Details |
|---|---|---|
| 1.3 | September 2011 | Initial submission: NRES Committee London-East approved the study, conditional on amendments to recruitment documents |
| 1.4 | October 2011 | Non-substantial amendment: protocol and recruitment documents amended in line with REC conditions |
| 1.5 | March 2012 | Substantial amendment: change in sponsorship of study from King’s College London to University College London (reflected in protocol and recruitment documents) |
| 1.6 | October 2012 | Substantial amendment: data collection commenced in GM and plans to involve service user in data collection in London (changes in data management details reflected in protocol and recruitment documents) |
| 1.7 | February 2013 | Substantial amendment: extension of study to July 2016 to cover Midlands and East of England and further reconfiguration in GM (changes in study details reflected in protocol and recruitment documents) |
| 1.8 | July 2013 | Substantial amendment: addition of ‘key stakeholders’ as a group to be interviewed (reflected in protocol and recruitment documents) |
| 1.9 | November 2015 | Substantial amendment: extension of study to June 2017 to study further reconfiguration in GM and closing Midlands and East of England study (reflected in protocol and recruitment documents) |
| 2.0 | December 2015 | Non-substantial amendment: recruitment documents amended in line with REC conditions |
| Region | NHS provider trusts | NHS commissioners |
|---|---|---|
| GM | 10 | 10 |
| London | 26 | 32 |
| Midlands and East of England | 17 | 14 |
| Total | 53 | 56 |
Appendix 2 Patient and public involvement
Overview
Patient and public involvement informed and enhanced our research throughout the project. Here, we summarise our PPI activity and its impact, guided by the Guidance for Reporting Involvement of Patients and the Public (GRIPP2) checklist. 227
Patient and public involvement approach
In the context of this research study, we viewed PPI as working in collaboration with stroke patients and carers and their representatives. We aimed to benefit from PPI representatives’ unique and important experiential knowledge of services and their perspective as members of the public on changes that can often prompt public resistance. 117,141,228,229 Our PPI approach evolved over the course of the study. In the beginning, it combined having a PPI co-investigator (Mr Nanik Pursani, a stroke service user) as a member of the research team, PPI members of our SSC, and consultation with the King’s Stroke Research Patients and Families Group. Subsequently, following Mr Pursani’s withdrawal from the study in 2015, to ensure ongoing PPI input in our work we engaged with a number of patient representative groups in London and GM. PPI informed and guided all aspects of the study, from the outline application onward, including study design, data analysis and dissemination.
Patient and public involvement activity
Application
Mr Nanik Pursani was a member of the King’s College London Stroke Research Patients and Families Group, and we recruited him to our team as we developed our outline application. He took an active role in the meetings where we planned our application and agreed our RQs, for example arguing for the need to study how PPI influenced MSC (see Chapter 8).
In addition, we discussed our proposal with the King’s College London Stroke Research Patients and Families Group, who agreed that our study focused on issues that were important to stroke patients. Although this group had a lot of experience in PPI, the project was quite technical and with only one element that directly involved patients, and there was direct patient/carer engagement in the development of that element. Importantly, the group understood and confirmed the value of the study because of their concern to see the quality of acute stroke care improve.
Research management and governance
Mr Pursani participated in our monthly study meetings and attended all SSC meetings while he was a member of the project team.
Our SSC initially included two members; when our study was extended to cover planned changes in the Midlands and East of England, we expanded our SSC significantly, and two further service user representatives joined. Patient members of these groups made active contributions to the SSC meetings (detailed in the following sections), and helped us develop our approaches to supporting these meetings, for example how we shared information in advance of the SSC meetings. Over the course of our study we developed a productive working relationship with the Stroke Association, and later in the study two representatives of the organisation joined our SSC.
Study design
To maximise Mr Pursani’s potential contribution to our project, we arranged for him to receive qualitative methods training, including aspects of research design, data collection and data analysis. As the study progressed Mr Pursani contributed to discussions of our overall research strategy. He also commented on interview recruitment documents (information and consent forms) and interview topic guides. A key contribution was as member of the subgroup that led development of the patient and carer experience study (see Chapter 9), as part of which he contributed to the development of interview topic guides and our approach to participant recruitment. Following Mr Pursani’s departure from the project team, we engaged with local patient groups in London (including the King’s College London Stroke Research Patients and Families Group) and GM in order to get patient perspectives on our ongoing research (see project webpage: www.journalslibrary.nihr.ac.uk/programmes/hsdr/10100909/).
Our SSC members commented on the progress of our study and contributed to recommendations for future development of the research, for example our plans for the patient and carer experience analysis (SSC 2012). We also presented the plans for our patient and carer experience analysis to the King’s College London Stroke Research Patients and Families Group, which provided feedback on the topics we would cover in interviews.
Data analysis and interpretation
As a team member, Mr Pursani played a significant part in discussing findings as they developed and commenting on draft manuscripts of articles.
An important function of our SSC meetings was to present interim findings for discussion, and the Chairpersons of these meetings actively requested PPI members’ perspectives on the analyses presented. For example, PPI members provided insights on their experiences suggesting that the purpose of PPI is not clearly explained during consultation exercises (in relation to our PPI in MSC analysis) and factors that might influence patients’ willingness to describe negative experiences of care (in relation to our patient and carer experience analysis).
Dissemination
Mr Pursani contributed to the manuscripts of two of our papers, which were published in BMJ25 and Stroke26 and he is a co-author on these papers. He also contributed to the development of presentations of our findings to patient groups, in terms of clarity and content (e.g. recommending that we use quotations from patients and carers in our presentation on the impact of GMA and London on delivery of clinical interventions). Our SSC PPI member, Judith Williamson, commented on the Plain English summary of our final report, which we have taken into account.
In line with our dissemination strategy, we developed a series of ‘at a glance’ summaries of our published papers, presenting accessible lessons from our research. When engaging with local patient groups in London and GM, we actively sought patient perspectives on how best to share our findings (e.g. which media outlets to use and how best to summarise and present our findings).
As discussed above, our SSC meetings always featured active discussion of progress and ongoing plans for research, and our Chairpersons prioritised PPI input on our dissemination proposals. PPI representatives provided helpful feedback in terms of how we might present our analyses and findings more meaningfully to a general audience.
In 2016, through our strong links with the Stroke Association, we were given the opportunity to present our work at the UK Stroke Assembly (the key conference for stroke patients and families to engage with the latest developments in stroke) [UK Stroke Assembly (North), East Mids Conference Centre, Nottingham, 14 June 2016; and UK Stroke Assembly (South), Alexandra House, Swindon, 8 July 2016]. The research team saw this as an excellent opportunity to share our findings with stroke patients and carers from across the UK, but also, importantly, a chance to collaborate with PPI members of our SSC, who by then had several years’ experience of our research. As a result, we co-designed a workshop with two PPI members of our SSC (Judith Williamson and Norman Phillips), and then copresented the workshop at the two UK Stroke Assembly events, held in Nottingham and Swindon, with each workshop attended by approximately 40 patients and carers.
We have plans to share our findings beyond the end of the project. For example, we plan to share our more recent analyses (including PPI in MSC and patient and carer experience) with patient groups. We also plan to develop accessible summaries of our more recent analyses and distribute these through our dissemination list and network of contacts. We will also share our overall findings through the King’s College London Forward newsletter and the SLSR blog.
Patient and public involvement impact
The key impacts of PPI on our research were as follows:
-
Our research focused on questions that were meaningful and relevant to stroke patients and the public. This was illustrated by the growing interest in our research and feedback from patient and carer audiences in GM, London and nationally.
-
We adapted our approach to research governance, for example ensuring that we distributed SSC meeting documents a week in advance, and providing accessible summaries of the research we planned to present.
-
Our recruitment documentation was made significantly clearer and more accessible thanks to feedback from Mr Pursani.
-
Interview topic guides, especially for the patient and carer experience research, were influenced significantly by input from Mr Pursani, in terms of topics covered and wording used.
-
In terms of dissemination, our papers benefited significantly in terms of focus and interpretation from input from Mr Pursani and our SSC members (including service user members and representatives of the Stroke Association). Our workshop at the UK Stroke Assembly 2016 was enhanced significantly by our PPI collaborators. Judith Williamson, a PPI member of our SSC, commented on the Plain English summary of our final report and we took account of her feedback.
-
In terms of ongoing impact, Judith Williamson and two representatives of the Stroke Association (one of whom was a member of the SSC) have agreed to join the advisory team for an NIHR Knowledge Mobilisation Research Fellowship that is to focus on supporting mobilisation of evidence relating to MSC in stroke services.
Reflections on patient and public involvement
Although our experiences do not necessarily extend how PPI is conceptualised, we feel that our experiences confirm that there are significant benefits of PPI, as detailed above.
As outlined previously, we involved a number of stroke patients and carers over the course of this study. However, we did not involve members of the general public, who might well have had different perspectives on changes of this kind.
Over the course of the study, we came to value encouraging flexible approaches to interactions between PPI representatives and other members of the team. We sought to ensure PPI representatives felt empowered to communicate with the team however they felt most comfortable (e.g. in person, by telephone or by e-mail). We have applied this learning in more recent research projects.
Our approach to PPI evolved over the course of the study, as we shifted our approach to engage with more stroke patient groups. We found value in working closely with individuals (e.g. working with Mr Pursani on articles and with Ms Williamson and Mr Phillips on the UK Stroke Assembly workshop), but equally we found feedback on our research from a wide range of people in our stroke patient groups extremely helpful.
Our developing relationship with a major voluntary sector organisation such as the Stroke Association has been highly beneficial, in terms of increasing our opportunities to involve stroke patients and carers at a national level, beyond the areas we were studying.
Working with PPI representatives and the Stroke Association as we prepared our workshop for the UK Stroke Assembly was an excellent opportunity to learn how to share our findings accessibly and meaningfully.
There were practical challenges that limited the degree to which PPI members contributed to the project. Obtaining research governance permissions for a PPI representative so that he/she could support data collection proved extremely challenging. Particular difficulties related in the first instance to a lack of clarity on the part of Research & Development offices in relation to what permissions were required for someone who was not an employee of an academic or NHS institution (e.g. research passport, honorary research contract or honorary contract), and then the multiple requirements made of the PPI representative in obtaining an honorary contract. Although the honorary contract was finally obtained (the process having taken > 1 year from initial requests in 2012 to the contract being issued in February 2014), we would factor in significant time and effort when planning such activity in future projects. There would be value in bodies such as the NIHR, INVOLVE (www.invo.org.uk/about-involve/; accessed 21 January 2019) and the Health Research Authority considering how involvement of PPI representatives in research activity could be better facilitated and promoted.
In terms of payment, the funding approved for PPI covered only Mr Pursani’s and our SSC PPI representatives’ travel and subsistence, rather than payment for participation in meetings and events. We feel that firmer requirements in relation to suitable remuneration of PPI representatives would facilitate greater sustainability of PPI over the course of lengthy studies such as this. Although INVOLVE provide some examples and advice on remuneration for PPI representatives, clearer guidance and recommendations would be valuable when developing applications for research.
Appendix 3 Supplementary data for Chapter 4: impact of centralisation in London and Greater Manchester A on clinical interventions
This appendix draws on a paper published by Ramsay et al. 26 Effects of centralising acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46(8):2244–51. Permission to reuse this material has been agreed by Wolters Kluwer. Promotional and commercial use of the material in print, digital or mobile device format is prohibited without the permission from the publisher Wolters Kluwer. Please contact permissions@lww.com for further information. No drug/trade name or logo can be included in the same page as the material re-used. Stroke is published on behalf of the American Heart Association, Inc., by Wolters Kluwer. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0) License, which permits use, distribution, and reproduction in any medium, provided that the original work is properly cited, the use is non-commercial, and no modifications or adaptations are made. See https://creativecommons.org/licenses/by-nc-nd/3.0/. Permission to adapt this material has been agreed with Wolters Kluwer.
Process of selecting the comparator area
Table 31 presents participation in Sentinel 2008 (pre centralisation) and SINAP (post centralisation), organised by region (GM, London and RoE).
| Region | Proportion of sites participating in (%) | |
|---|---|---|
| Sentinel 2008 | SINAP | |
| GM | 12/12 (100) | 11/11 (100) |
| London | 30/30 (100) | 24/24 (100) |
| RoE | 147/147 (100) | 96/136 (70.6) |
Sentinel 2008 had high levels of participation across all regions of England, it was estimated that all acute trusts admitting acute stroke patients took part in the audit. In SINAP, the proportion of sites participating across the RoE was considerably lower than in London and GM. Given the different levels of participation in the before and after periods, we concluded that the RoE would not represent a suitable control group for a before-and-after analysis.
Table 32 presents participation in SINAP by region, in terms of (1) number of cases submitted to SINAP per head of local population and (2) the proportion of organisations submitting data to SINAP.
| Region | Population 2011 | Total cases submitted | Cases per 100,000 | Proportion of sites participating (%) |
|---|---|---|---|---|
| 1. East Midlands | 4,533,222 | 3040 | 67.06 | 4/10 (40) |
| 2. East of England | 5,846,965 | 4261 | 72.88 | 11/17 (64.71) |
| 3. London | 8,173,941 | 16,773 | 205.20 | 24/24 (100) |
| 4. North-east | 2,596,886 | 7877 | 303.32 | 11/11 (100) |
| 5. North-west | 7,052,177 | 24,350 | 345.28 | 27/27 (100) |
| 5a. Greater Manchester | 2,682,500 | 10,295 | 383.78 | 11/11 (100) |
| 5b. Rest of north-west England | 4,369,677 | 14,055 | 321.65 | 16/16 (100) |
| 6. South-east England | 8,634,750 | 8813 | 102.06 | 19/28 (67.86) |
| 7. South-west | 5,288,935 | 7431 | 140.50 | 8/18 (44.44) |
| 8. West Midlands | 5,601,847 | 5659 | 101.02 | 16/20 (80) |
| 9. Yorkshire and the Humber | 5,283,733 | 6680 | 126.43 | 11/16 (68.75) |
| England (total) | 53,012,456 | 84,884 | 160.12 | 131/171 (76.6) |
This analysis shows that GM and London had high levels of participation on both selected criteria, and well above the average for England. The only other areas with similar levels of participation were north-east England and the rest of north-west England (i.e. excluding GM). Based on this analysis it was decided that the data from these areas should be used as the control group in both the pre- and post-centralisation periods of the analyses, with data from other areas excluded.
To ensure comparability with GM and London, both of the cover areas classified by the UK’s ONS as ‘major urban’ (‘districts with either 100,000 people or 50% of their population in urban areas with a population of more than 750,000’85), we limited services in the comparator to those serving populations classified by the ONS as ‘major urban’. This was done by mapping locations of each service in the comparator and selecting those in districts classified as ‘major urban’. Details of cases included and excluded from the analysis are presented in Table 33.
| Region | Sentinel 2008 | SINAP | Total |
|---|---|---|---|
| GM | 653 | 10,295 | 10,948 |
| London | 1541 | 16,773 | 18,314 |
| Urban comparator | 537 | 9044 | 9581 |
| Excluded cases | 7341 | 48,772 | 56,113 |
| Total cases | 10,072 | 84,884 | 94,956 |
Supplementary tables
| Indicator | Region, % likelihood (95% CI) | |||||
|---|---|---|---|---|---|---|
| GM | RoE | |||||
| Overall | CSC/PSCs | DSCs | ||||
| Before | After | After | After | Before | After | |
| Brain scan 3 hours | 21.2 (17.6 to 24.8) | 60.9 (60.0 to 61.8) | 82.4 (81.3 to 83.6) | 46.4 (45.2 to 47.7) | 22.5 (21.6 to 23.3) | 59.0 (58.7 to 59.4) |
| SU 4 hours | 15.9 (13.0 to 18.9) | 53.1 (52.1 to 54.0) | 80.7 (79.6 to 81.6) | 33.2 (32.1 to 34.5) | 18.7 (17.9 to 19.5) | 56.9 (56.5 to 57.2) |
| Brain scan 24 hours | 68.2 (64.6 to 71.9) | 93.0 (92.5 to 93.5) | 97.8 (97.3 to 98.2) | 89.9 (89.2 to 90.7) | 62.1 (61.1 to 63.1) | 91.8 (91.6 to 92.0) |
| Antiplatelets 48 hours | 86.5 (83.9 to 89.2) | 93.9 (93.5 to 94.4) | 97.5 (97.0 to 98.0) | 91.9 (91.2 to 92.5) | 85.4 (84.6 to 86.2) | 91.7 (91.5 to 91.9) |
| Physiotherapist 72 hours | 88.0 (85.4 to 90.5) | 91.7 (91.1 to 92.2) | 93.4 (92.5 to 94.2) | 90.9 (90.2 to 91.6) | 85.0 (84.2 to 85.8) | 92.8 (92.6 to 93.0) |
| Nutrition 72 hours | 88.2 (85.6 to 90.7) | 94.9 (94.5 to 95.3) | 98.1 (97.7 to 98.5) | 92.8 (92.2 to 93.4) | 69.6 (68.6 to 70.6) | 91.4 (91.2 to 91.7) |
| Swallow 72 hours | 85.6 (82.0 to 89.2) | 92.7 (92.2 to 93.1) | 98.1 (97.7 to 98.5) | 89.2 (88.4 to 89.9) | 79.7 (78.6 to 80.8) | 91.1 (90.9 to 91.4) |
| Indicator | Region, % likelihood (95% CI) | |||||
|---|---|---|---|---|---|---|
| London | RoE | |||||
| Overall | HASUs | SUs | ||||
| Before | After | After | After | Before | After | |
| Brain scan 3 hours | 36.1 (33.6 to 38.6) | 72.0 (71.4 to 72.7) | 74.4 (73.8 to 75.1) | 39.1 (36.2 to 42.0) | 19.4 (18.5 to 20.2) | 56.6 (56.2 to 57.0) |
| SU 4 hours | 30.8 (28.4 to 33.1) | 67.9 (67.2 to 68.6) | 70.5 (69.8 to 71.2) | 30.9 (28.1 to 33.8) | 15.7 (14.9 to 16.5) | 54.2 (53.9 to 54.6) |
| Brain scan 24 hours | 77.1 (74.9 to 79.3) | 95.1 (94.8 to 95.4) | 96.1 (95.8 to 96.4) | 82.5 (80.3 to 84.7) | 59.3 (58.2 to 60.4) | 91.3 (91.1 to 91.6) |
| Antiplatelets 48 hours | 94.2 (93.0 to 95.5) | 94.9 (94.6 to 95.3) | 95.4 (95.0 to 95.7) | 89.9 (88.1 to 91.7) | 83.8 (82.9 to 84.6) | 91.6 (91.3 to 91.8) |
| Physiotherapist 72 hours | 90.4 (88.8 to 92.0) | 96.0 (95.7 to 96.4) | 96.6 (96.3 to 96.9) | 88.8 (86.9 to 90.8) | 83.8 (83.0 to 84.7) | 92.1 (91.8 to 92.3) |
| Nutrition 72 hours | 74.6 (72.3 to 76.8) | 98.4 (98.2 to 98.6) | 98.6 (98.5 to 98.8) | 94.6 (93.4 to 95.9) | 70.0 (68.9 to 71.0) | 90.6 (90.3 to 90.8) |
| Swallow 72 hours | 87.3 (85.0 to 89.6) | 98.4 (98.2 to 98.6) | 99.1 (99.0 to 99.3) | 88.8 (86.8 to 90.7) | 77.7 (76.5 to 79.0) | 89.6 (89.3 to 89.8) |
| Intervention | CSC/PSCs after, % likelihood (95% CI) | DSCs after, % likelihood (95% CI) | |||
|---|---|---|---|---|---|
| Overall | Overall | Overall (where onset time available) | ≤ 4 hours | > 4 hours | |
| Brain scan 3 hours | 84.6 (83.6 to 85.6) | 50.7 (49.4 to 51.9) | 53.8 (51.9 to 55.6) | 59.4 (56.7 to 62.1) | 49.2 (46.6 to 51.8) |
| SU 4 hours | 81.4 (80.3 to 82.5) | 34.1 (32.8 to 35.4) | 38.4 (36.5 to 40.4) | 36.6 (33.7 to 39.4) | 40.2 (37.5 to 42.8) |
| Brain scan 24 hours | 98.0 (97.6 to 98.4) | 90.9 (90.2 to 91.5) | 93.4 (92.4 to 94.3) | 94.3 (93.1 to 95.7) | 92.5 (91.1 to 93.9) |
| Antiplatelets 48 hours | 97.6 (97.1 to 98.1) | 92.1 (91.4 to 92.8) | 94.0 (93.1 to 95.0) | 93.3 (91.9 to 94.8) | 94.7 (93.4 to 96.0) |
| Physiotherapist 72 hours | 93.5 (92.7 to 94.4) | 91.1 (90.4 to 91.8) | 92.3 (91.3 to 93.4) | 92.3 (90.7 to 93.9) | 92.4 (91.0 to 93.8) |
| Nutrition 72 hours | 98.3 (97.9 to 98.6) | 93.3 (92.8 to 93.9) | 92.8 (91.9 to 93.8) | 92.7 (91.3 to 94.1) | 93.0 (91.8 to 94.3) |
| Swallow 72 hours | 98.2 (97.9 to 98.6) | 90.1 (89.4 to 90.8) | 91.5 (90.5 to 92.5) | 90.7 (89.2 to 92.3) | 92.3 (91.0 to 93.6) |
| Indicator | HASUs after, % likelihood (95% CI) | ||
|---|---|---|---|
| Overall | ≤ 4 hours | > 4 hours | |
| Brain scan 3 hours | 74.6 (73.8 to 75.3) | 91.4 (90.6 to 92.2) | 68.7 (67.1 to 70.2) |
| SU 4 hours | 69.1 (68.3 to 69.9) | 80.1 (79.8 to 82.0) | 64.7 (63.0 to 66.3) |
| Brain scan 24 hours | 96.2 (95.9 to 96.5) | 98.9 (98.6 to 99.1) | 96.4 (95.7 to 97.0) |
| Antiplatelets 48 hours | 95.3 (94.9 to 95.7) | 94.9 (94.2 to 95.6) | 96.4 (95.7 to 97.1) |
| Physiotherapist 72 hours | 96.0 (95.6 to 96.4) | 95.6 (94.9 to 96.3) | 96.1 (95.3 to 97.0) |
| Nutrition 72 hours | 98.6 (98.4 to 98.8) | 98.7 (98.3 to 99.0) | 99.0 (98.6 to 99.3) |
| Swallow 72 hours | 99.0 (98.8 to 99.1) | 99.2 (98.9 to 99.5) | 99.2 (98.8 to 99.5) |
| Indicator | CSC/PSC after, % likelihood (95% CI) | ||
|---|---|---|---|
| Overall | ≤ 4 hours | > 4 hours | |
| Brain scan 3 hours | 85.5 (84.6 to 86.5) | 92.7 (91.7 to 93.7) | 83.1 (80.9 to 85.2) |
| SU 4 hours | 82.9 (81.8 to 83.9) | 90.1 (89.1 to 91.3) | 83.5 (81.3 to 85.6) |
| Brain scan 24 hours | 98.2 (97.8 to 98.5) | 99.4 (99.1 to 99.7) | 98.2 (97.5 to 99.1) |
| Antiplatelets 48 hours | 97.6 (97.1 to 98.1) | 98.3 (97.7 to 98.9) | 98.2 (97.3 to 99.1) |
| Physiotherapist 72 hours | 93.8 (93.0 to 94.7) | 93.4 (92.1 to 94.6) | 95.6 (94.2 to 97.1) |
| Nutrition 72 hours | 98.5 (98.2 to 98.8) | 98.9 (98.5 to 99.2) | 98.5 (97.9 to 99.2) |
| Swallow 72 hours | 98.6 (98.2 to 98.9) | 99.3 (99.0 to 99.6) | 98.5 (98.0 to 99.2) |
Supplementary figures
Between-hospital variations by area (Greater Manchester, London, comparator, rest of England), before and after centralisation
FIGURE 20.
Proportion of patients undergoing brain scan within 3 hours. (a) GM, pre centralisation; (b) GM, post centralisation; (c) London, pre centralisation; (d) London, post centralisation; (e) comparator, pre centralisation; (f) comparator, post centralisation; (g) RoE, pre centralisation; and (h) RoE, post centralisation. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients).
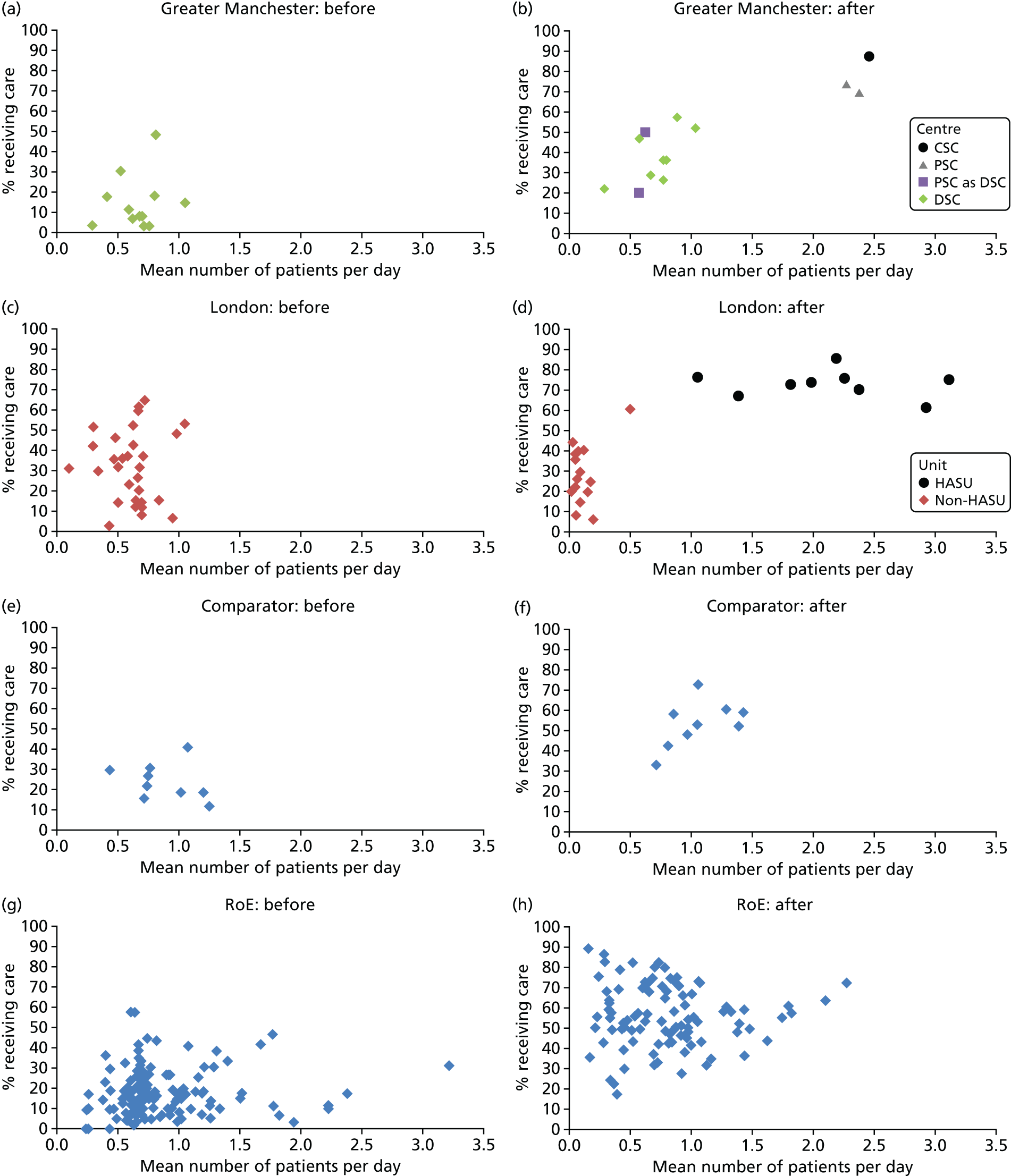
FIGURE 21.
Proportion of patients admitted to SU within 4 hours. (a) GM, pre centralisation; (b) GM, post centralisation; (c) London, pre centralisation; (d) London, post centralisation; (e) comparator, pre centralisation; (f) comparator, post centralisation; (g) RoE, pre centralisation; and (h) RoE, post centralisation. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients).
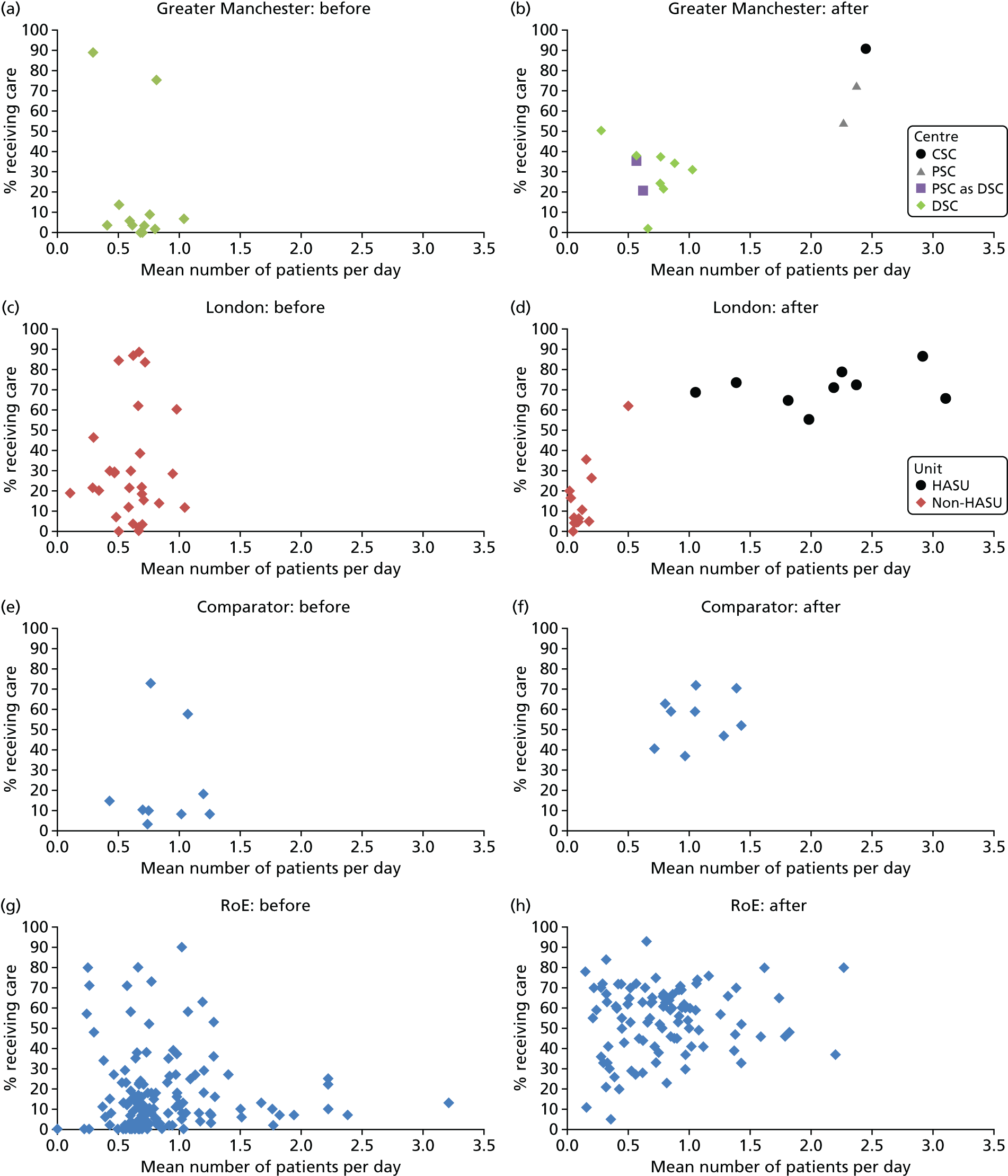
FIGURE 22.
Proportion of patients undergoing brain scan within 24 hours. (a) GM, pre centralisation; (b) GM, post centralisation; (c) London, pre centralisation; (d) London, post centralisation; (e) comparator, pre centralisation; (f) comparator, post centralisation; (g) RoE, pre centralisation; and (h) RoE, post centralisation. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients).
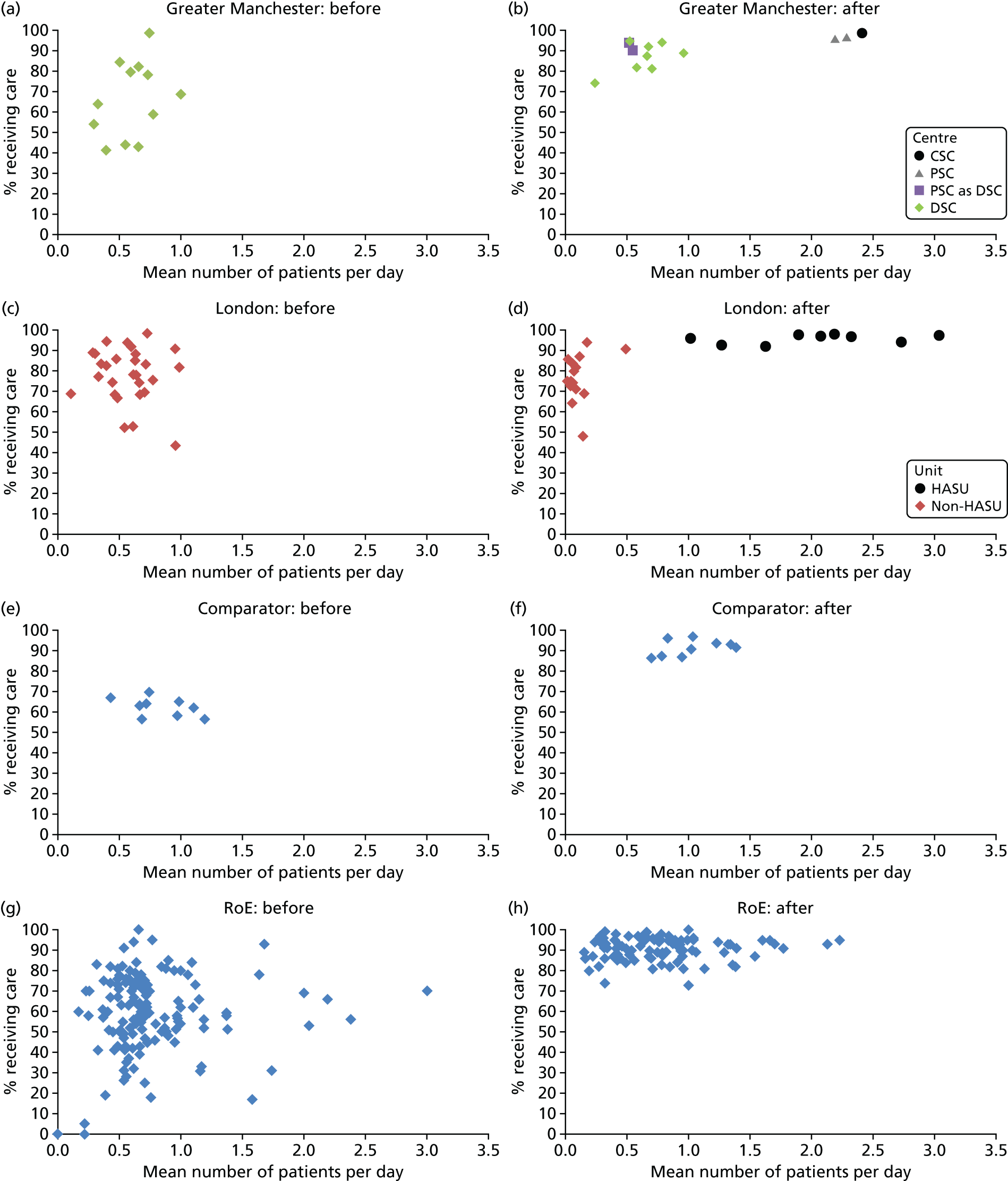
FIGURE 23.
Proportion of patients administered antiplatelets within 48 hours. (a) GM, pre centralisation; (b) GM, post centralisation; (c) London, pre centralisation; (d) London, post centralisation; (e) comparator, pre centralisation; (f) comparator, post centralisation; (g) RoE, pre centralisation; and (h) RoE, post centralisation. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients).
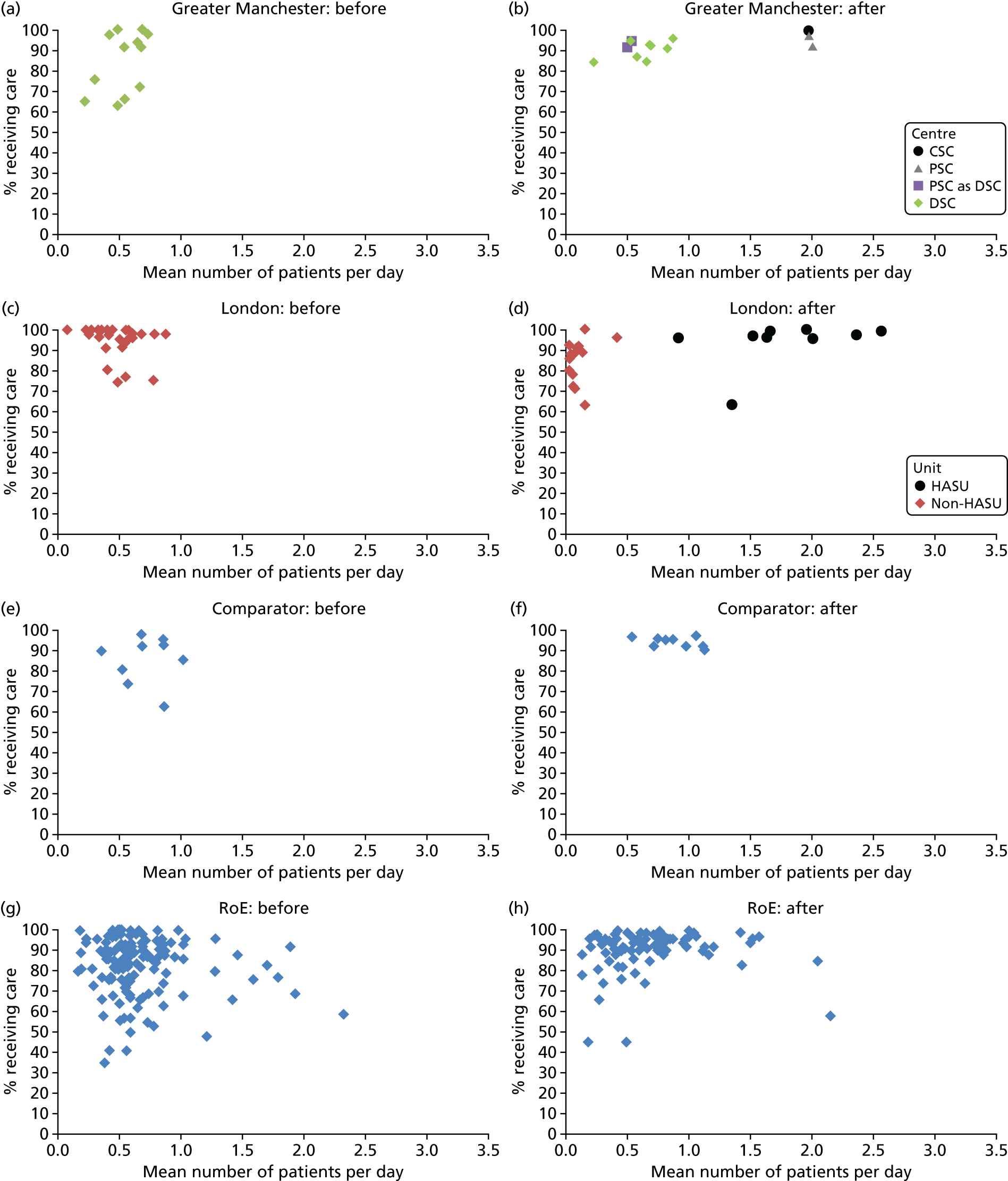
FIGURE 24.
Proportion of patients receiving physiotherapist assessment within 72 hours. (a) GM, pre centralisation; (b) GM, post centralisation; (c) London, pre centralisation; (d) London, post centralisation; (e) comparator, pre centralisation; (f) comparator, post centralisation; (g) RoE, pre centralisation; and (h) RoE, post centralisation. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients).

FIGURE 25.
Proportion of patients receiving nutrition assessment within 72 hours. (a) GM, pre centralisation; (b) GM, post centralisation; (c) London, pre centralisation; (d) London, post centralisation; (e) comparator, pre centralisation; (f) comparator, post centralisation; (g) RoE, pre centralisation; and (h) RoE, post centralisation. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients).
FIGURE 26.
Proportion of patients receiving swallow assessment within 72 hours. (a) GM, pre centralisation; (b) GM, post centralisation; (c) London, pre centralisation; (d) London, post centralisation; (e) comparator, pre centralisation; (f) comparator, post centralisation; (g) RoE, pre centralisation; and (h) RoE, post centralisation. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients).

Appendix 4 Supplementary data for Chapter 5: cost-effectiveness of London and Greater Manchester A
This appendix draws on a paper published as Hunter et al. 91 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Supplementary figures
FIGURE 27.
Ten-year Markov model structure.
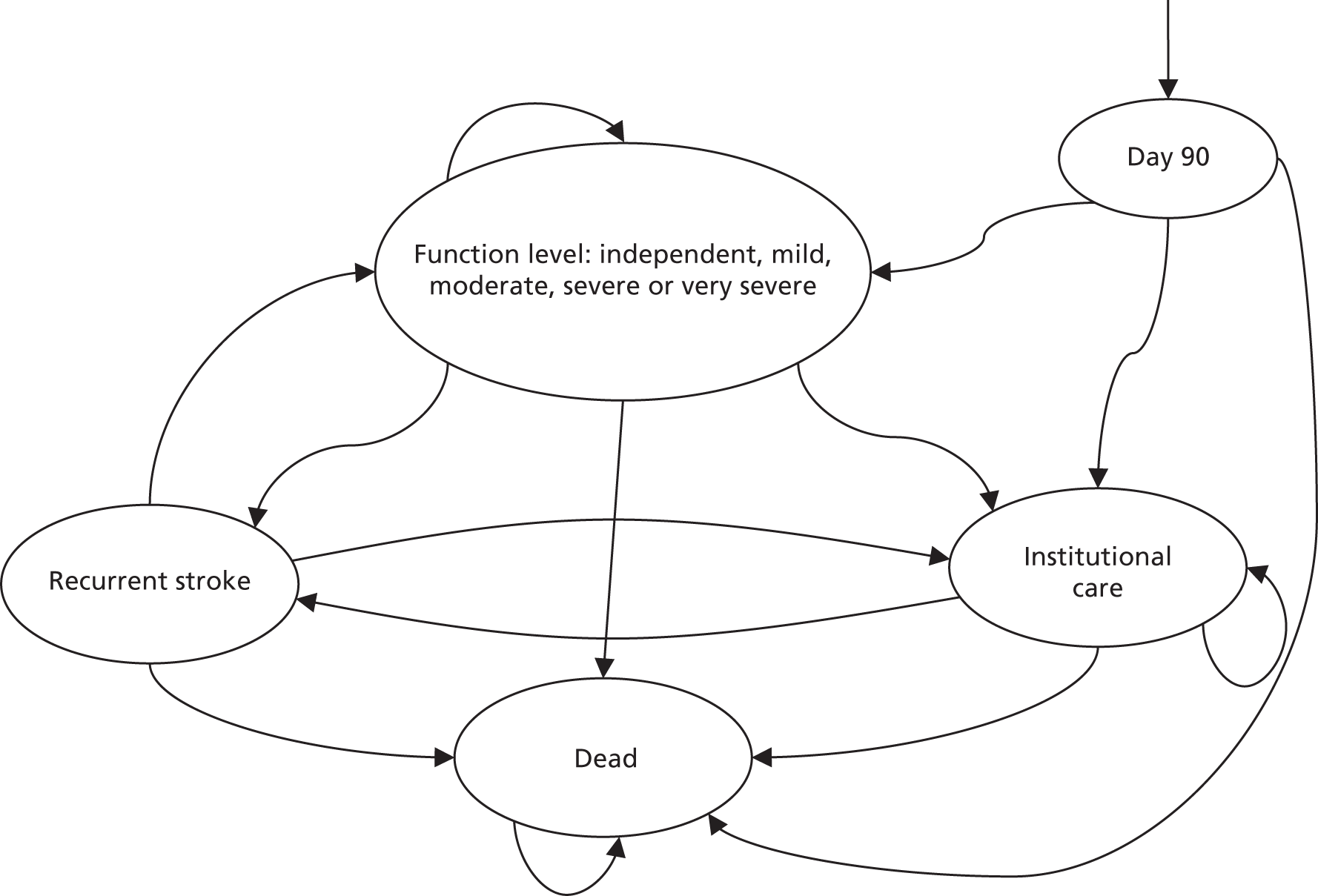
FIGURE 28.
Cost-effectiveness acceptability curve for if improvements in functioning are seen in London and GM but not in the RoE after the reconfigurations.

Supplementary tables
| Variable | Constant (SE) | β coefficient (SE) | |
|---|---|---|---|
| Before | After | ||
| London 72 hours mortality | |||
| London 72 hours – hospital | –2.547443 (0.1536) | –0.1304453 (0.0892) | –0.3388409 (0.1191) |
| London 72 hours – home before | –1.112563 (0.0291) | –5.498865 (0.206321) | |
| London 72 hours – home after | –0.9000484 (0.044) | –6.268149 (0.33579) | |
| London 72 hours – community hospital before | –1.112563 (0.0291) | –3.914968 (0.238261) | |
| London 72 hours – community hospital after | –0.9000484 (0.044) | –5.442073 (0.57907) | |
| London 30 days mortality | |||
| London 30 days – hospital | –2.196218 (0.12067) | –0.1400739 (0.05099) | –0.4069234 (0.0853) |
| London 30 days – home before | 1.434057 (0.045317) | –6.052406 (0.1111684) | |
| London 30 days – home after | 1.455995 (0.051168) | –6.040282 (0.11063) | |
| London 30 days – nursing home before | 1.434057 (0.045317) | –5.697617 (0.255799) | |
| London 30 days – nursing home after | 1.455995 (0.051168) | –6.091694 (0.32185) | |
| London 30 days – community hospital before | 1.434057 (0.045317) | –4.8455572 (0.158142) | |
| London 30 days – community hospital after | 1.455995 (0.051168) | –4.442539 (0.12421) | |
| London 90 days mortality | |||
| London 90 days – hospital | –2.116191 (0.11408) | –0.1210432 (0.044904) | –0.4217429 (0.0639) |
| London 90 days – home before | 3.735711 (0.118429) | –7.01472 (0.1299083) | |
| London 90 days – home after | 3.272364 (0.106797) | –6.622667 (0.11198) | |
| London 90 days – nursing home before | 3.735711 (0.118429) | –5.945526 (0.1541422) | |
| London 90 days – nursing home after | 3.272364 (0.106797) | –5.449472 (0.14801) | |
| London 90 days – community hospital before | 3.735711 (0.118429) | –6.185021 (0.1540291) | |
| London 90 days – community hospital after | 3.272364 (0.106797) | –5.326356 (0.13123) | |
| RoE (vs. London) 72 hours mortality | |||
| RoE 72 hours – hospital | –2.547443 (0.1536) | 0 | –0.11557 (0.028474) |
| RoE 72 hours – home before | –0.8274124 (0.0127) | –5.915824 (0.102904) | |
| RoE 72 hours – home after | –0.7733679 (0.0150) | –6.168171 (0.11886) | |
| RoE 72 hours – nursing home before | –0.8274124 (0.0127) | –7.27151 (0.5002305) | |
| RoE 72 hours – nursing home after | –0.7733679 (0.0150) | –7.121694 (0.50031) | |
| RoE 72 hours – community hospital before | –0.8274124 (0.0127) | –4.699328 (0.1723066) | |
| RoE 72 hours – community hospital after | –0.7733679 (0.0150) | –5.350084 (0.25072) | |
| RoE (vs. London) 30 days mortality | |||
| RoE 30 days – hospital | –2.196218 (0.12067) | 0 | –0.10559 (0.025677) |
| RoE 30 days – home before | 1.595699 (0.015582) | –5.961341 (0.0351102) | |
| RoE 30 days – home after | 1.790226 (0.019933) | –6.014796 (0.03662) | |
| RoE 30 days – nursing home before | 1.595699 (0.015582) | –5.66156 (0.0696054) | |
| RoE 30 days – nursing home after | 1.790226 (0.019933) | –5.425521 (0.06545) | |
| RoE 30 days – community hospital before | 1.595699 (0.015582) | –4.592778 (0.0530674) | |
| RoE 30 days – community hospital after | 1.790226 (0.019933) | –5.02211 (0.064315) | |
| RoE (vs. London) 90 days mortality | |||
| RoE 90 days – hospital | –2.116191 (0.11408) | 0 | –0.22544 (0.019982) |
| RoE 90 days – home before | 4.030386 (0.044542) | –7.16257 (0.0478558) | |
| RoE 90 days – home after | 4.325614 (0.061488) | –7.408664 (0.33580) | |
| RoE 90 days – nursing home before | 4.030386 (0.044542) | –6.149743 (0.0526994) | |
| RoE 90 days – nursing home after | 4.325614 (0.061488) | –6.262616 (0.06802) | |
| RoE 90 days – community hospital before | 4.030386 (0.044542) | –5.865234 (0.0544597) | |
| RoE 90 days – community hospital after | 4.325614 (0.061488) | –6.268872 (0.0709) | |
| GM 72 hours mortality | |||
| GM 72 hours – hospital | –2.421833 (0.14417) | –0.0163415 (0.072725) | –0.1513003 (0.1442) |
| GM 72 hours – home before | –0.8689113 (0.0747) | –6.182511 (0.7113363) | |
| GM 72 hours – home after | –0.7285307 (0.0561) | –7.156792 (0.70946) | |
| GM 72 hours – community hospital before | –0.8689113 (0.0747) | –3.998623 (1.006607) | |
| GM 72 hours – community hospital after | –0.7285307 (0.0561) | –5.517576 (1.00254) | |
| GM 30 days mortality | |||
| GM 30 days – hospital | –2.015169 (0.11623) | –0.0612502 (0.056943) | –0.2228965 (0.0977) |
| GM 30 days – home before | 1.291925 (0.082786) | –6.084666 (0.2447883) | |
| GM 30 days – home after | 1.792565 (0.075070) | –6.185732 (0.14564) | |
| GM 30 days – nursing home before | 1.291925 (0.082786) | –6.452402 (0.713927) | |
| GM 30 days – nursing home after | 1.792565 (0.075070) | –5.948318 (0.32742) | |
| GM 30 days – community hospital before | 1.291925 (0.082786) | –3.898721 (0.3551919) | |
| GM 30 days – community hospital after | 1.792565 (0.075070) | –4.118781 (0.17175) | |
| GM 90 days mortality | |||
| GM 90 days – hospital | –1.952976 (0.11159) | –0.0042823 (0.063693) | –0.2941545 (0.0843) |
| GM 90 days – home before | 3.471728 (0.199914) | –6.677631 (0.2263131) | |
| GM 90 days – home after | 3.615176 (0.164399) | –6.821608 (0.17903) | |
| GM 90 days – nursing home before | 3.471728 (0.199914) | –5.92642 (0.2800679) | |
| GM 90 days – nursing home after | 3.615176 (0.164399) | –5.604104 (0.20396) | |
| GM 90 days – community hospital before | 3.471728 (0.199914) | –5.127685 (0.3104291) | |
| GM 90 days – community hospital after | 3.615176 (0.164399) | –5.373488 (0.20603) | |
| RoE (vs. GM) 72 hours mortality | |||
| RoE 72 hours – hospital | –2.421833 (0.14417) | 0 | –0.16135 (0.031914) |
| RoE 72 hours – home before | –0.8274124 (0.0127) | –5.915824 (0.102904) | |
| RoE 72 hours – home after | –0.7733679 (0.0150) | –6.168171 (0.11886) | |
| RoE 72 hours – nursing home before | –0.8274124 (0.0127) | –7.27151 (0.5002305) | |
| RoE 72 hours – nursing home after | –0.7733679 (0.0150) | –7.121694 (0.50031) | |
| RoE 72 hours – community hospital before | –0.8274124 (0.0127) | –4.699328 (0.1723066) | |
| RoE 72 hours – community hospital after | –0.7733679 (0.0150) | –5.350084 (0.25072) | |
| RoE (vs. GM) 30 days mortality | |||
| RoE 30 days – hospital | –2.015169 (0.11623) | 0 | –0.26858 (0.0253) |
| RoE 30 days – home before | 1.595699 (0.015582) | –5.961341 (0.0351102) | |
| RoE 30 days – home after | 1.790226 (0.019933) | –6.014796 (0.03662) | |
| RoE 30 days – nursing home before | 1.595699 (0.015582) | –5.66156 (0.0696054) | |
| RoE 30 days – nursing home after | 1.790226 (0.019933) | –5.425521 (0.06545) | |
| RoE 30 days – community hospital before | 1.595699 (0.015582) | –4.592778 (0.0530674) | |
| RoE 30 days – community hospital after | 1.790226 (0.019933) | –5.02211 (0.064315) | |
| RoE (vs. GM) 90 days mortality | |||
| RoE 90 days – hospital | –1.952976 (0.11159) | 0 | –0.30497 (0.023264) |
| RoE 90 days – home before | 4.030386 (0.044542) | –7.16257 (0.0478558) | |
| RoE 90 days – home after | 4.325614 (0.061488) | –7.408664 (0.33580) | |
| RoE 90 days – nursing home before | 4.030386 (0.044542) | –6.149743 (0.0526994) | |
| RoE 90 days – nursing home after | 4.325614 (0.061488) | –6.262616 (0.06802) | |
| RoE 90 days – community hospital before | 4.030386 (0.044542) | –5.865234 (0.0544597) | |
| RoE 90 days – community hospital after | 4.325614 (0.061488) | –6.268872 (0.0709) | |
| Variable | Constant (SE) | β-coefficient (SE) | Gamma | |
|---|---|---|---|---|
| Before | After | |||
| London | ||||
| London – daily probability of discharge home | –2.64098 (0.030461) | 0.045712 (0.009146) | 0.214972 (0.00943) | –0.10706 (0.001542) |
| London – discharge to care home (publicly funded) | –2.64098 (0.030461) | –0.973 (0.0383368) | –0.98968 (0.04183) | –0.10706 (0.001542) |
| London – discharge to care home (privately funded) | –2.64098 (0.030461) | –1.0295 (0.0576309) | –1.07139 (0.059219) | –0.10706 (0.001542) |
| London – discharge to community hospital | –2.64098 (0.030461) | –0.5085 (0.0293949) | –0.1767 (0.027445) | –0.10706 (0.001542) |
| RoE (vs. London) | ||||
| RoE – daily probability of discharge home | –2.64098 (0.030461) | 0 | 0.155154 (0.004678) | –0.10706 (0.001542) |
| RoE – discharge to care home (publicly funded) | –2.64098 (0.030461) | –0.87083 (0.01323) | –0.89047 (0.014033) | –0.10706 (0.001542) |
| RoE – discharge to care home (privately funded) | –2.64098 (0.030461) | –0.99054 (0.013744) | –1.06343 (0.015379) | –0.10706 (0.001542) |
| RoE – discharge to community hospital | –2.64098 (0.030461) | –0.29652 (0.011769) | –0.36854 (0.012613) | –0.10706 (0.001542) |
| GM | ||||
| GM – daily probability of discharge home | –2.64936 (0.034407) | –0.06769 (0.012846) | 0.1702 (0.018182) | –0.10741 (0.001654) |
| GM – discharge to care home (publicly funded) | –2.64936 (0.034407) | –0.8935 (0.074893) | –0.91537 (0.051917) | –0.10741 (0.001654) |
| GM – discharge to care home (privately funded) | –2.64936 (0.034407) | –0.77431 (0.092258) | –0.99686 (0.071256) | –0.10741 (0.001654) |
| GM – discharge to community hospital | –2.64936 (0.034407) | –0.15751 (0.091618) | 0.319658 (0.050619) | –0.10741 (0.001654) |
| RoE (vs. GM) | ||||
| RoE – daily probability of discharge home | –2.64936 (0.034407) | 0 | 0.173084 (0.006077) | –0.10741 (0.001654) |
| RoE – discharge to care home (publicly funded) | –2.64936 (0.034407) | –0.87083 (0.01323) | –0.89047 (0.014033) | –0.10741 (0.001654) |
| RoE – discharge to public care home | –2.64936 (0.034407) | –0.99054 (0.013744) | –1.06343 (0.015379) | –0.10741 (0.001654) |
| RoE – discharge to community hospital | –2.64936 (0.034407) | –0.29652 (0.011769) | –0.36854 (0.012613) | –0.10741 (0.001654) |
| Analysis | Destination, % (n) | |||
|---|---|---|---|---|
| Home | Care home (publicly funded) | Care home (privately funded) | Community hospital | |
| London before | 80 (9848) | 6 (746) | 3 (313) | 11 (1363) |
| London after | 80 (10,023) | 5 (622) | 2 (296) | 13 (1635) |
| Manchester before | 83 (2262) | 7 (198) | 5 (125) | 5 (130) |
| Manchester after | 83 (5220) | 6 (407) | 3 (207) | 8 (511) |
| RoE before | 80 (76,669) | 6 (6261) | 6 (5774) | 8 (8274) |
| RoE after | 80 (74,546) | 6 (5886) | 5 (4874) | 8 (7319) |
| Analysis | Ward (%) | ||||
|---|---|---|---|---|---|
| SU | Medical Assessment Unit | General medical | ITU/CCU | Other | |
| London before | 30 | 64 | 2 | 2 | 2 |
| London after | 87 | 7 | 2 | 2 | 2 |
| Manchester before | 14 | 79 | 2 | 2 | 3 |
| Manchester after | 60 | 34 | 2 | 2 | 3 |
| RoE before | 15 | 78 | 2 | 1 | 3 |
| RoE after | 68 | 26 | 2 | 1 | 3 |
| Movement from | Movement to | Transition probability | Time period/variable type | Source |
|---|---|---|---|---|
| London before | ||||
| Home | Residential care | 0.014 | 90 days to 1 year | SLSR31 |
| Home | Residential care | 0.003 | 1 year to 10 years | SLSR31 |
| Home | Recurrent stroke | 0.022 | Constant | HES57 |
| Home | Recurrent stroke | 0.585 | Gamma | HES57 |
| Home | Dead | 0.042 | Constant | HES57 |
| Home | Dead | 0.459 | Gamma | HES57 |
| Residential care | Recurrent stroke | 0.018 | Constant | HES57 |
| Residential care | Recurrent stroke | 0.585 | Gamma | HES57 |
| Residential care | Dead | 0.085 | Up to end of year 10 | Gordon et al. (2014)95 |
| Recurrent stroke | Home: BI = 20 | 0.300 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 15–19 | 0.244 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 10–14 | 0.131 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 5–9 | 0.075 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 0–4 | 0.094 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Residential care | 0.057 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Dead | 0.100 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Hospital ≥ 90 days | 0.115 | Up to end of year 10 | From 90-day model |
| Hospital | Recurrent stroke | 0.048 | Constant | HES57 |
| Hospital | Recurrent stroke | 0.585 | Gamma | HES57 |
| London after | ||||
| Home | Residential care | 0.014 | 90 days to 1 year | SLSR31 |
| Home | Residential care | 0.003 | 1 year to 10 years | SLSR31 |
| Home | Recurrent stroke | 0.021 | Constant | HES57 |
| Home | Recurrent stroke | 0.585 | Gamma | HES57 |
| Home | Dead | 0.040 | Constant | HES57 |
| Home | Dead | 0.459 | Gamma | HES57 |
| Residential care | Recurrent stroke | 0.018 | Constant | HES57 |
| Residential care | Recurrent stroke | 0.585 | Gamma | HES57 |
| Residential care | Dead | 0.085 | Up to end of year 10 | Gordon et al. (2014)95 |
| Recurrent stroke | Home: BI = 20 | 0.474 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 15–19 | 0.246 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 10–14 | 0.046 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 5–9 | 0.055 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 0–4 | 0.055 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Residential care | 0.052 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Dead | 0.074 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Hospital ≥ 90 days | 0.115 | Up to end of year 10 | From 90-day model |
| Hospital | Recurrent stroke | 0.048 | Constant | HES57 |
| Hospital | Recurrent stroke | 0.585 | Gamma | HES57 |
| GM before | ||||
| Home | Residential care | 0.014 | 90 days to 1 year | SLSR31 |
| Home | Residential care | 0.003 | 1 year to 10 years | SLSR31 |
| Home | Recurrent stroke | 0.022 | Constant | HES57 |
| Home | Recurrent stroke | 0.585 | Gamma | HES57 |
| Home | Dead | 0.045 | Constant | HES57 |
| Home | Dead | 0.459 | Gamma | HES57 |
| Residential care | Recurrent stroke | 0.018 | Constant | HES57 |
| Residential care | Recurrent stroke | 0.585 | Gamma | HES57 |
| Residential care | Dead | 0.085 | Up to end of year 10 | Gordon et al. (2014)95 |
| Recurrent stroke | Home: BI = 20 | 0.285 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 15–19 | 0.231 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 10–14 | 0.125 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 5–9 | 0.071 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 0–4 | 0.089 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Residential care | 0.072 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Dead | 0.127 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Hospital ≥ 90 days | 0.107 | Up to end of year 10 | From 90-day model |
| Hospital | Recurrent stroke | 0.048 | Constant | HES57 |
| Hospital | Recurrent stroke | 0.585 | Gamma | HES57 |
| GM after | ||||
| Home | Residential care | 0.014 | 90 days to 1 year | SLSR31 |
| Home | Residential care | 0.003 | 1 year to 10 years | SLSR31 |
| Home | Recurrent stroke | 0.024 | Constant | HES57 |
| Home | Recurrent stroke | 0.585 | Gamma | HES57 |
| Home | Dead | 0.043 | Constant | HES57 |
| Home | Dead | 0.459 | Gamma | HES57 |
| Residential care | Recurrent stroke | 0.018 | Constant | HES57 |
| Residential care | Recurrent stroke | 0.585 | Gamma | HES57 |
| Residential care | Dead | 0.085 | Up to end of year 10 | Gordon et al. (2014)95 |
| Recurrent stroke | Home: BI = 20 | 0.455 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 15–19 | 0.236 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 10–14 | 0.044 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 5–9 | 0.053 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 0–4 | 0.053 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Residential care | 0.059 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Dead | 0.101 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Hospital ≥ 90 days | 0.107 | Up to end of year 10 | From 90-day model |
| Hospital | Recurrent stroke | 0.048 | Constant | HES57 |
| Hospital | Recurrent stroke | 0.585 | Gamma | HES57 |
| RoE before | ||||
| Home | Residential care | 0.014 | 90 days to 1 year | SLSR31 |
| Home | Residential care | 0.003 | 1 year to 10 years | SLSR31 |
| Home | Recurrent stroke | 0.022 | Constant | HES57 |
| Home | Recurrent stroke | 0.585 | Gamma | HES57 |
| Home | Dead | 0.045 | Constant | HES57 |
| Home | Dead | 0.459 | Gamma | HES57 |
| Residential care | Recurrent stroke | 0.018 | Constant | HES57 |
| Residential care | Recurrent stroke | 0.585 | Gamma | HES57 |
| Residential care | Dead | 0.085 | Up to end of year 10 | Gordon et al. (2014)95 |
| Recurrent stroke | Home: BI = 20 | 0.290 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 15–19 | 0.236 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 10–14 | 0.127 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 5–9 | 0.073 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 0–4 | 0.091 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Residential care | 0.070 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Dead | 0.113 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Hospital ≥ 90 days | 0.065 | Up to end of year 10 | From 90-day model |
| Hospital | Recurrent stroke | 0.048 | Constant | HES57 |
| Hospital | Recurrent stroke | 0.585 | Gamma | HES57 |
| RoE after | ||||
| Home | Residential care | 0.014 | 90 days to 1 year | SLSR31 |
| Home | Residential care | 0.003 | 1 year to 10 years | SLSR31 |
| Home | Recurrent stroke | 0.021 | Constant | HES57 |
| Home | Recurrent stroke | 0.585 | Gamma | HES57 |
| Home | Dead | 0.044 | Constant | HES57 |
| Home | Dead | 0.459 | Gamma | HES57 |
| Residential care | Recurrent stroke | 0.018 | Constant | HES57 |
| Residential care | Recurrent stroke | 0.585 | Gamma | HES57 |
| Residential care | Dead | 0.085 | Up to end of year 10 | Gordon et al. (2014)95 |
| Recurrent stroke | Home: BI = 20 | 0.452 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 15–19 | 0.235 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 10–14 | 0.043 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 5–9 | 0.052 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Home: BI = 0–4 | 0.052 | Up to end of year 10 | SLSR31 |
| Recurrent stroke | Residential care | 0.069 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Dead | 0.096 | Up to end of year 10 | From 90-day model |
| Recurrent stroke | Hospital ≥ 90 days | 0.065 | Up to end of year 10 | From 90-day model |
| Hospital | Recurrent stroke | 0.048 | Constant | HES57 |
| Hospital | Recurrent stroke | 0.585 | Gamma | HES57 |
| Location | Mean | SE |
|---|---|---|
| SU | 0.24 | 0.027932 |
| Medical ward/general ward | 0.266452 | 0.041781 |
| ITU/CCU | 0.01725 | 0.078934 |
| Other | 0.272308 | 0.102945 |
| Community hospital | 0.158667 | 0.062064 |
| Nursing home (public or private) | 0.128947 | 0.056194 |
| Home | 0.557932 | 0.015597 |
| Dead | 0 | |
| Discharge other | 0.324857 | 0.112742 |
| Home: BI = 20 | 0.69 | 0.025 |
| Home: BI = 15–19 | 0.60 | 0.010121 |
| Home: BI = 10–14 | 0.37 | 0.024084 |
| Home: BI = 5–9 | 0.10 | 0.040107 |
| Home: BI = 0–4 | –0.06 | 0.025793 |
| Residential Care | 0.09 | 0.025 |
| Sensitivity analysis | Region | DID | |||||
|---|---|---|---|---|---|---|---|
| London | RoE | ||||||
| Before | After | Difference | Before | After | Difference | ||
| Movements to nursing home per 90 days = 0.9% probability. 10-year results | |||||||
| QALYs | 2832 | 3352 | 520 | 2709 | 3177 | 468 | 52 |
| Costs (£) | 44,506,798 | 43,526,204 | –980,593 | 44,588,151 | 42,514,328 | –2,073,823 | 1,093,230 |
| NMB £30,000 per QALY (£) | 16,567 | 16,102 | 465 | ||||
| Movements to nursing home per 90 days = 0.26% probability. 10-year results | |||||||
| QALYs | 3120 | 3698 | 579 | 2983 | 3505 | 522 | 57 |
| Costs (£) | 34,160,157 | 32,565,792 | –1,594,365 | 34,679,656 | 32,103,072 | –2,576,584 | 982,219 |
| NMB £30,000 per QALY (£) | 18,962 | 18,227 | 735 | ||||
| Additional cost for transfer between hospitals in London | |||||||
| 90-day costs (£) | 5,709,351 | 5,964,428 | 255,077 | 5,500,302 | 4,973,647 | –526,655 | 770,036 |
| QALYs – 10 years | 2928 | 3468 | 541 | 2800 | 3285 | 485 | 55 |
| Costs – 10 years (£) | 39,604,757 | 38,333,585 | –1,271,172 | 39,943,000 | 37,660,758 | –2,282,215 | 1,011,043 |
| NMB £30,000 per QALY (£) | 17,489 | 16,839 | 650 | ||||
| London: 4% ITU admissions before | |||||||
| 90-day costs (£) | 5,835,220 | 5,949,507 | 114,288 | 5,494,071 | 4,966,449 | –527,622 | 641,910 |
| QALYs – 10 years | 2928 | 3468 | 541 | 2800 | 3285 | 485 | 55 |
| Costs – 10 years (£) | 39,747,568 | 38,316,675 | 1,430,892 | 39,935,961 | 37,652,684 | –2,283,277 | 852,385 |
| NMB £30,000 per QALY (£) | 17,650 | 16,840 | 810 | ||||
| HASU costs 50% more per day | |||||||
| 90-day costs (£) | 5,704,557 | 6,562,152 | 857,595 | 5,494,071 | 4,966,449 | –527,622 | 1,997,861 |
| QALYs – 10 years | 2928 | 3468 | 541 | 2800 | 3285 | 485 | 55 |
| Costs – 10 years (£) | 39,599,318 | 39,010,989 | –588,328 | 39,935,961 | 37,652,684 | –2,283,277 | 1,694,949 |
| NMB £30,000 per QALY (£) | 16,806 | 16,840 | –34 | ||||
| Sensitivity analysis | Region | DID | |||||
|---|---|---|---|---|---|---|---|
| GM | RoE | ||||||
| Before | After | Difference | Before | After | Difference | ||
| Movements to nursing home per 90 days = 0.9% probability. 10-year results | |||||||
| QALYs | 2662 | 3177 | 515 | 2658 | 3157 | 499 | 16 |
| Costs (£) | 44,115,237 | 42,120,777 | –1,994,459 | 43,784,539 | 42,204,647 | –1,579,892 | –414,567 |
| NMB £30,000 per QALY (£) | 17,448 | 16,542 | 907 | ||||
| Movements to nursing home per 90 days = 0.26% probability. 10 year results | |||||||
| QALYs | 3120 | 3698 | 579 | 2927 | 3483 | 556 | 18 |
| Costs (£) | 34,354,718 | 31,722,101 | –2,632,617 | 34,062,280 | 31,858,690 | –2,203,589 | –429,028 |
| NMB £30,000 per QALY (£) | 19,847 | 18,881 | 966 | ||||
| GM: 4% ITU admissions before | |||||||
| 90-day costs (£) | 5,724,473 | 5,213,429 | –511,044 | 5,473,849 | 4,905,472 | –532,376 | 21,333 |
| QALYs – 10 years | 2750 | 3286 | 536 | 2747 | 3264 | 517 | 18 |
| Costs – 10 years (£) | 39,685,105 | 37,234,169 | –2,450,936 | 39,220,696 | 37,374,815 | –1,845,881 | –605,055 |
| NMB £30,000 per QALY (£) | 18,521 | 17,367 | 1154 | ||||
Appendix 5 Interview topic guides
London and Greater Manchester A: governance
Background: interviewee
To begin, please tell me:
-
How are/were you involved in stroke services?
-
How were you involved with the reconfiguration?
Background to the reconfiguration
-
What were the catalysts or drivers for the reconfiguration (e.g. national policy and research)?
-
Local drivers and key players – organisations, individuals.
Governing the reconfiguration
-
What groups and individuals led and governed the reconfiguration? (Provide a list?)
-
How were you involved? How much time did you dedicate to this? (e.g. how often did you attend meetings or events? How long were they?)
-
How did these work and work together? (e.g. attendance, focus, inter- and intra-group interactions)?
-
What was the overall timeline for the reconfiguration?
Developing the proposal for change
-
How were you involved in developing the proposal for change? Time dedicated?
-
What were the key influences on how the proposals developed (e.g. groups, individuals)?
-
Who was consulted? How were they consulted (e.g. interviews, focus groups, events, surveys)?
-
Obstacles/enablers – how were these addressed/used?
Agreeing the reconfiguration model
-
How were you involved in developing the reconfiguration model? Time dedicated?
-
What reconfiguration options were developed? What were the key influences (e.g. groups, individuals)?
-
Who was consulted on these, and how?
-
What did you think of the options?
-
How was the final model decided upon?
Implementing the model
-
How were you involved in bringing about the changes? Time dedicated?
-
How were the changes brought about (e.g. in opening/closing services, building capacity)?
-
What groups and individuals were central to implementing the model? How did they work?
-
How were local stakeholders kept up to date on the progress of the reconfiguration (e.g. newsletters, events)?
-
Obstacles/enablers – how were these addressed/used? What were the levers for change?
Outcomes
-
What changes were brought about by the reconfiguration (e.g. organisation, service delivery, partnership working, patient outcomes, costs)?
-
Do you think these changes will be sustained?
-
How were they measured? What capacity was/is dedicated to collecting these data? Are these measures reliable?
-
Do you think the changes were worthwhile? Do other stakeholders feel the same?
-
Would these changes have happened anyway?
Reflections
-
Is there anything you would have done differently, in retrospect?
-
Have you any further comments?
London and Greater Manchester A: service level
Background
To begin, please tell me a little about your background.
-
What is your role in this unit? How long have you worked here?
-
Where have you worked previously, and in what settings? How long have you been involved in stroke care?
Overview of changes
In this evaluation, we are interested in learning about how the reconfiguration of stroke services in London/GM influenced the ways in which care is organised and provided. Thinking back, would you tell me briefly.
-
How did you hear about proposals to reconfigure stroke services across London/GM? What did you think of them? How were you involved in the changes?
-
How did you hear about proposals to change the services provided in this unit/organisation? What did you think of them? How were you involved in these changes?
-
What were services here like, initially? What changes came about as a result of the reconfiguration?
Developing the services
Please tell me about the changes that happened with the reconfiguration.
Examples (with specific issues in parentheses):
-
Processes (e.g. services and therapies, protocols, standard operating procedures) – can you describe the changes that happened here, and what had to be done to support them?
-
Staffing (numbers, rota) – what was done in terms of staffing to support these changes?
-
New roles (e.g. ‘stroke nursing’, or more specialised roles) – have any new roles been created when developing this service as part of reconfiguration, or have new roles developed over time?
-
Skills and training – what kinds of training have you or your colleagues received to support your work in the new services?
-
Becoming part of a wider system – how have things changed in terms of how your service interacts with other parts of the local health system? Other parts of hospital (other SUs, A&E, radiology), other hospitals/units/primary care/ambulance?
-
Governance – have any groups been set up here to oversee and support the changes you’ve mentioned, or to support high-quality care more generally (both within the service and across the whole system)?
General follow-ups
-
What was its purpose? Why was it important?
-
What was the background to this? Whose idea was it, and who was involved in agreeing it? What factors influenced these changes/decisions?
-
How was this developed? How did it work (e.g. how it was led, who was consulted, how was it planned)?
-
How were you involved? How did people work together to develop and implement these changes?
-
What factors made a difference when implementing it? Were there any problems? How were these addressed?
-
What difference did this change make to: provision of care? Patient and carer experience? Outcomes/cost? Culture – inter- and intraprofessional working, communication across the whole system?
-
What did you think of this change? What do other people think? How were these changes measured, and how were these measures agreed?
-
How do other people (colleagues/management/patients and carers) feel about the changes?
-
Are there any further changes that you think would be helpful?
Overall impact of changes
Overall, in what ways do you think the reconfiguration made a difference to stroke services here?
-
Examples: provision of care? Patient and carer experience? Outcomes/cost? Culture – inter- and intraprofessional working, communication across the whole system?
-
Follow-ups: how are these changes measured? How do other people (colleagues/management/patients and carers) feel about the changes?
-
What factors influenced the changes (obstacles/supports)?
-
Can you give me an example of this?
Reflections
Is there anything else you’d like to say about how the changes to services worked?
-
Overall, how would you sum up the impact of the reconfiguration on how services are provided here (and across London/GM)?
-
What have you learned from this experience?
-
Are there other changes you would you like to see happen?
-
Is there anything you’d like to do differently in the future?
London and Greater Manchester A: patient and carer experience
What happened when you had your stroke?
-
How and by whom were services contacted when you had your stroke? (999/NHS Direct/GP/A&E/ambulance)?
-
How long did it take before health services responded to your request for assistance?
-
How and by whom were you and your carer(s) told that you’d had a stroke?
-
Were you and your carer(s) supported by a health-care professional following the diagnosis of a stroke?
-
Did you or your carer(s) feel confident that you were being treated by health-care professionals who knew what to do?
-
Do you and/or your carer(s) think you were admitted to hospital quickly enough?
-
Do you or your carer(s) feel your stroke was diagnosed quickly enough? Why/why not?
What treatments did you receive?
-
For example, thrombolysis/clot-busting drugs/scans?
-
Did health-care professionals make sure you and your carer(s) understood throughout your treatment?
-
Were your health-care options explained clearly to you and your carer(s) (e.g. process, risks, benefits) at the right time?
-
Did you and your carer(s) feel fully involved in the decisions being made? Were your views respected?
-
Did you and your carer(s) feel confident that the professionals overseeing your treatments were sufficiently knowledgeable and experienced in stroke health care? Did you and your carer(s) feel confident that the right decisions had been made?
How did people check how you were doing? What tests did you have?
-
For example, physical, emotional, psychological, nutrition, hydration, swallowing, communication.
-
Who carried out these checks? Were you confident that he/she knew what he/she was doing?
-
How often did they happen? Were they conducted by the same person each time?
What sorts of problems did you have? Did you get enough help with any problems you might have had?
-
For example, eating, swallowing, mobility, speech.
-
Were there any specific health-care needs that you or your carer(s) had that were not addressed by health-care professionals?
-
Did health-care professionals (e.g. doctors, nurses, therapists) introduce themselves to you and your carer(s) and explain what would happen to you?
-
Were you and your carer(s) satisfied and confident in the care you received? Did you feel staff were knowledgeable and competent in managing and delivering your post health-care physical needs?
-
Do you and your carer(s) feel you were treated with dignity and respect?
Did you change ward when you were in hospital?
-
Were you admitted to a stroke specific ward/unit? If not, were you and your carer(s) satisfied with the stroke specialist care you received?
-
How and by whom were you or your carer(s) informed about what was going to happen to you?
-
How and by whom was this explained to you and your carer(s)?
-
Did you or your carer(s) feel you had a say in your post-stroke treatment?
What happened when you were preparing to leave hospital?
-
How far in advance were you or your carer(s) informed that you would be leaving hospital?
-
Was there adequate time for you and your carer(s) to make arrangements for your ongoing care?
-
Did you or your carer(s) feel ready to leave/go home?
-
What concerns did you or your carer(s) have about your continued health-care support at home?
-
Did you feel you or your carer(s) had a say in how your leaving hospital was to be arranged (e.g. consenting to ESD)?
-
Did you or your carer(s) feel suitably informed about sources of support once you had left hospital (e.g. details in joint care plan – re community services, social services, stroke association)?
-
What stroke-related information were you and your carer(s) given on discharge, and did this information include benefits you were entitled to (Disability Living Allowance, Blue Badge, Taxi Card, Freedom Pass)?
-
Were you or your carer(s) given advice on how you might prevent future strokes (e.g. diet, smoking, exercise)?
-
Were you or your carer(s) given sufficient information about your medicines (how to take, side effects)?
What help have you needed since leaving hospital? Have you received this help?
-
For example for your emotional/psychological state of mind, physical abilities.
-
Were services arranged for when you left hospital (e.g. physiotherapy, occupational therapy, speech therapy, mobility, social care)?
-
Was information about your stroke, including medication needed for recovery, shared with your GP?
-
Do you or your carer(s) know who to contact in the event that you needed support?
-
How have community services/social services supported you?
-
Do you feel your needs have been met?
Reflections
-
Was there anything particularly good about your stroke care in hospital and in the community?
-
Was there anything that could be improved, or that you felt was missing in your stroke care in hospital and in the community?
Confirm demographic information: year of birth, hospital(s).
Midlands and East of England: service review
Background
-
How are you involved in stroke care?
-
How have you been involved in the review and reconfiguration processes? (Project Board)?
Background to the reconfiguration
-
How did you hear about proposals to review and reconfigure stroke services across the East of England and the Midlands?
-
What did you and your colleagues think of them?
-
What were the catalysts or drivers for the review and development of reconfiguration plans (e.g. national policy and research, organisations, individuals)?
Governance – how was the review governed?
-
How were you involved? How much time did you dedicate to this (e.g. how often did you attend meetings or events)?
-
How did these groups work and work together (e.g. attendance, focus, inter- and intra-group interactions)?
-
How were decisions made? What was the nature of the discussions? How were they led?
-
Any obstacles to the governance of the review and development of proposals? And supports?
How were you involved in reviewing stroke services and developing the proposal for change? Time dedicated?
-
Please describe these processes (e.g. overall project plan, local reviews and three waves of proposals).
-
What were the key influences on how the proposals developed? (e.g. groups, individuals)
-
How were decisions made? Who was consulted and how? (providers, commissioners, patients?)
-
Obstacles/enablers – how were these addressed/used?
What stage are things at now? How are you involved (currently at consultation stage in Essex)?
-
Current tasks and activities; any issues.
-
What groups/organisations/individuals are involved?
-
Main influences? Obstacles/enablers.
Next steps?
-
What do you see as being the likely progress of the reconfiguration? What factors might influence this?
-
What do you see as the likely supports and obstacles to progress?
-
What differences will the reconfigurations make (e.g. to quality, patient experience)?
Lessons
-
What lessons have you drawn from your experience of the process so far?
Midlands and East of England: governance in local areas
Background
-
Your role – how are you/have you been involved in stroke care?
-
How have you been involved in the review and reconfiguration processes?
Background to the reconfiguration
-
How did you first hear about proposals to review and reconfigure stroke services across the East of England and the Midlands?
-
What did you and your colleagues think of them?
-
What were the catalysts or drivers for the review and development of reconfiguration plans (e.g. national policy and research, organisations, individuals)?
Governance – how was the review governed?
-
How were you involved? How much time did you dedicate to this (e.g. how often did you attend meetings or events)?
-
How did these groups work and work together (e.g. attendance, focus, inter- and intra-group interactions)?
-
How were decisions made? What was the nature of the discussions? How were they led?
-
Any obstacles to the governance of the review and development of proposals? And supports?
The review process and developing the proposal for change
-
How were you involved in reviewing stroke services and developing the proposal for change? Time dedicated?
-
Please describe these processes (e.g. overall project plan? Local reviews and three waves of proposals).
-
What were the key influences on how the proposals developed? (e.g. groups, individuals, London/Manchester)
-
How were decisions made? Who was consulted and how (providers, commissioners, patients)?
-
Obstacles/enablers – how were these addressed/used?
What stage are things at now? How are you involved?
-
Current tasks and activities; any issues.
-
What groups/organisations/individuals are involved?
-
Main influences? Obstacles/enablers (e.g. local/national commissioning and governance, other health-care priorities)?
Next steps? (through to processes of implementation)
-
What do you see as being the likely progress of the reconfiguration? What factors might influence this?
-
What do you see as the likely supports and obstacles to progress?
-
What differences will the reconfigurations make (e.g. to quality, patient experience)?
-
Anything you/others would like to have done differently?
Lessons/reflections
-
What lessons have you drawn from your experience of the process so far?
Midlands and East of England: local commissioners
Background
-
Introduce study (check has information sheet and get consent for recording the interview).
-
Can you tell me about the role of the CCG and what you do within this organisation?
-
How did you become aware of the proposed changes to stroke services in the Midlands and East of England?
-
How have you been involved in this process, both regionally and at a local level?
-
In your view, what are:
-
the main drivers for reconfiguring services?
-
the key processes by which models are being developed and agreed?
-
the relevance of the experiences in London and GM to the changes being proposed in this region? (In what ways does this region differ and what implications might this have for the model’s design?)
-
Progressing changes
-
Which are the main stakeholders you have been discussing the changes with?
-
What has happened thus far in terms of those discussions?
-
What has been your role in those discussions?
-
What is going to happen over the next year (i.e. until March 2014 and beyond)?
-
What appear to be the factors influencing implementation (obstacles and enablers)? (How have you or colleagues been involved in addressing any local issues e.g. potential resistance?)
-
-
What impact have the changes to the commissioning and governance landscape had upon the planning and implementation of the proposed changes?
-
How are these changes to stroke services being considered relative to other priority areas? Do they fit in with other priorities or is there any conflict?
-
What are the main outcomes that you hope the changes will produce? (i.e. for patients, hospitals, service delivery, partnership working, costs, learning)
Greater Manchester B: governance
Background – interviewee
-
To begin, please can you tell me generally about your role, and your involvement with stroke services in GM?
What is the background to the further reconfiguration of stroke services/full implementation of the Manchester model?
-
What do you think has been achieved through the first phase of the reconfiguration of stroke services?
-
Can you tell me about the changes to stroke services that are being planned at the moment?
-
Can you tell me why these changes are happening?
-
What do you/colleagues think about these changes?
-
Do you know what the proposed timescales for reconfiguration are?
How is the current reconfiguration being governed?
-
What groups and individuals are leading and governing the reconfiguration?
-
How are you involved?
-
How do the groups work and work together?
-
What do you think are the main challenges in governing the reconfiguration?
-
Can you say anything about how governance structures are different or similar to those in the previous reconfiguration?
-
Has the reorganisation of the English NHS in 2013 had an impact on the way in which the reconfiguration is governed (explore loss of Stroke Network, etc.)?
What progress has been made so far in agreeing and planning the reconfiguration?
-
How are you involved?
-
Are there/have there been any particular obstacles to planning and preparing for reconfiguration?
-
Are there/have there been any particular ‘enabling’ factors in planning and preparing for reconfiguration, what has helped to move things forward?
-
Who has been consulted about the reconfiguration? How have they been consulted?
-
How are local stakeholders being kept up to date?
-
Relationship to other local initiatives (e.g. Healthier Together).
-
Has the reorganisation of the English NHS in 2013 had an impact on the way in which the reconfiguration has progressed and is being managed?
How do you see the reconfiguration progressing from now?
-
Next steps? What needs to happen?
-
What will your involvement be?
-
Do you perceive any threats to the full implementation of the Manchester model?
Have you any thoughts about the sustainability of the reconfiguration as planned?
-
How will the quality of care be reviewed and maintained?
-
Any key obstacles or enablers?
-
Relationship to other local initiatives (e.g. Healthier Together).
Have you got any reflections on how the reconfiguration of stroke services has been/is being achieved?
-
Is there anything that you think should have been/should be done differently?
-
Anything you have learned from this experience?
-
Any further comments?
Greater Manchester B: service level
Background
-
What is your role? How are you involved in stroke services? For how long?
-
Were you involved with the reconfiguration of Manchester stroke services in 2010? In what way?
-
Were you involved in the more recent reconfiguration of Manchester stroke services in 2015? In what way?
How are services working at present/what services do you provide for people who have had a stroke?
How have services changed here since the 2015 reconfiguration?
Are services working in the way you anticipated?
Has anything become better/more difficult/got worse over time?
-
Unit (HASU/SU) access – ambulance/A&E/other services within hospital.
-
Unit (HASU/SU) care provision (e.g. 24/7), staffing, protocols – stroke mimics, caseload and patient flow.
-
Repatriation: to (SU) – to community, ESD, long-term care.
-
Impacts on quality of care, patient outcomes, patient experiences, costs.
-
Patients/carers: satisfied? Do you use data on patient and carer experience? Do you plan to?
-
Governance: has this developed/changed? Still possible to engage a range of professions?
-
Training/development: ongoing training? What kinds? People still able to take part?
-
What do your colleagues think?
-
Influential factors? Have the wider NHS reforms in 2013 been influential?
-
How about interactions with organisations outside your hospital?
-
For example CCGs, local authorities, Clinical Senate, SCN, hospitals, ambulance, networks, and patients, carers and the public?
-
Stroke ODN – Board, HASU Forum, Clinical Effectiveness Group, Sector Forums, special interest subgroups?
-
What is your role in these groups/how do you engage with these groups? Support/feedback? Time dedicated?
-
Obstacles/enablers? What is the impact? How might it improve?
-
Has this changed since the NHS reforms in 2013? Have any other factors been influential?
How does your organisation provide assurance (locally and across GM) that your services provide high-quality care? Has this changed since GM’s stroke services were reconfigured?
-
For example, participation in SSNAP (work of the ODN, local service reviews, local outcome measures).
-
Purpose? How do these work? Who leads? Who participates (including patients, carers and the public)? Are measures reliable?
-
How are you involved? How often do you attend meetings or events? Obstacles/enablers?
-
What factors have played a part in this? Have the wider NHS reforms in 2013 been influential?
What is your view of the sustainability of the GM stroke system in the future (over the next year? Over the next 5 years)?
-
How might things change (number of DSCs)?
-
Any risks/opportunities to develop further (24/7)? Any concerns? Key obstacles or enablers?
-
What do you see as potential factors in this? (e.g. further reforms?) Relationship to other local initiatives (Healthier Together/Devo and transformation plans/single services and chains).
Are there any other lessons from your role (both stroke-related and other) that you’d like to share?
-
Reflections on how one sustains a complex health system, especially during a time of significant organisational change? Key obstacles/enablers?
-
Is there anything you would like to do differently? Anything you have learned? Any further comments?
London sustainability: governance
First, please tell me a little about your background
-
What is your role? How are you involved in stroke services? For how long?
-
Were you involved with the reconfiguration of London stroke services in 2010? In what way?
Which groups and individuals have a responsibility to make sure stroke services in London provide high-quality care? How has this changed since the NHS reforms in 2013?
-
For example CCGs, local authorities, Clinical Senate, SCN, SCLG, Regional Operational Groups, hospitals, ambulance, networks, and patients, carers, and the public).
-
How are you involved? What got you involved? How much time do you dedicate to this?
-
How do these groups work together? Who is involved (including patients, carers, and the public)? Obstacles and enablers?
-
Have any other factors been influential?
How are you (and others, including patients and public) assured that stroke services (locally and across London) provide high-quality care? Has this changed since London’s stroke services were reconfigured?
-
e.g. audit (participation in SSNAP), service reviews.
-
Purpose? How do these work? Who leads? Who participates (including patients, carers and the public)? Are measures reliable?
-
How are you involved? How often do you attend meetings or events? Obstacles/enablers?
-
What factors have played a part in this? Have the wider NHS reforms in 2013 been influential?
Have service improvements been sustained? Why? Why not? Has anything become better or more difficult or got worse over time?
-
HASU access and care (e.g. 24/7), stroke mimics, caseload and patient flow.
-
Transfer to SU/community, ESD, long-term care.
-
Impacts on quality of care, patient outcomes, patient experiences, costs.
-
Are patients and carers satisfied? Do you use data on patient and carer experience? Do you plan to?
-
What do your colleagues think?
-
What factors have played a part in this? Have the wider NHS reforms in 2013 been influential?
What is your view of the sustainability of the London stroke system in the future (over the next year? Over the next five years)? Will your organisation’s role stay the same?
-
How might things change?
-
Any risks/opportunities to develop further (24/7)? Any concerns?
-
What do you see as potential factors in this (e.g. further reforms)?
Are there any other lessons from your role (both stroke-related and other) that you’d like to share?
-
Reflections on how one sustains a complex health system, especially during a time of significant organisational change? Key obstacles/enablers?
-
Is there anything you would like to do differently?
-
Any further comments?
London sustainability: service level
Background
-
What is your role? How are you involved in stroke services? For how long?
-
Were you involved with the reconfiguration of London stroke services in 2010? In what way?
How are services working at present? Has anything become better/more difficult/got worse over time?
-
Unit (HASU/SU) access – ambulance/A&E/other services within hospital.
-
Unit (HASU/SU) care provision (e.g. 24/7), staffing, protocols – stroke mimics, caseload and patient flow.
-
Repatriation: to (SU) - to community, ESD, long-term care.
-
Impacts on quality of care, patient outcomes, patient experiences, costs.
-
Patients/carers: satisfied? Do you use data on patient and carer experience? Do you plan to?
-
Governance: has this developed/changed? Still possible to engage a range of professions?
-
Training/development: ongoing training? What kinds? People still able to take part?
-
What do your colleagues think?
-
Influential factors? Have the wider NHS reforms in 2013 been influential?
How about interactions with organisations outside your service?
-
For example HASU/SU, CCGs, local authorities, Clinical Senate, SCN, SCLG, Regional Operational Groups, hospitals, ambulance, networks, and patients, carers and the public.
-
How do you engage with these groups? How much time do you dedicate to this?
-
Obstacles/enablers? What is their impact? How might it improve?
-
Has this changed since the NHS reforms in 2013? Have any other factors been influential?
How does your organisation provide assurance (locally and across London) that your services provide high-quality care? Has this changed since London’s stroke services were reconfigured?
-
For example participation in audits, local service reviews.
-
Purpose? How do these work? Who leads? Who participates (including patients, carers and the public)? Are measures reliable?
-
How are you involved? How often do you attend meetings or events? Obstacles/enablers?
-
What factors have played a part in this? Have the wider NHS reforms in 2013 been influential?
What is your view of the sustainability of the London stroke system in the future (over the next year? Over the next 5 years)?
-
How might things change?
-
Any risks/opportunities to develop further (24/7)? Any concerns?
-
What do you see as potential factors in this? (e.g. further reforms?)
Are there any other lessons from your role (both stroke-related and other) that you’d like to share?
-
Reflections on how one sustains a complex health system, especially during a time of significant organisational change? Key obstacles/enablers?
-
Is there anything you would like to do differently?
-
Any further comments?
Appendix 6 Supplementary data for Chapter 9: patient and carer experience
This appendix is drawn from a paper by Perry et al. 171 © 2018 The Authors. Health Expectations published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/.
| Main themes | Subthemes |
|---|---|
| Responding to stroke symptoms |
|
| Ambulance service |
|
| Explanation and information |
|
| Person-centred approach |
|
| Availability of therapy |
|
| Patient code | Sex | Age (years) | Carer participated in interview? | Pathway followed | Time between initial hospital contact (discharge imminent) and interview (days) |
|---|---|---|---|---|---|
| LonA01 | Female | 86 | Yes | HASU to local unit | 57 |
| LonA02 | Male | 65 | No | All care at HASU | 13 |
| LonA03 | Male | 38 | Yes | HASU to local unit | 58 |
| LonA04 | Female | 78 | No | All care at HASU | 49 |
| LonA05 | Male | 58 | Yes | HASU to local unit | Not available |
| LonB01 | Male | 62 | No | HASU to local unit | 55 |
| LonB02 | Male | 83 | No | HASU to local unit | 60 |
| LonB03 | Female | 58 | No | Out of area to local unit | 122 |
| LonB04 | Male | Unknown | Yes | All care at local unit | 14 |
| LonB05 | Male | Unknown | No | Out of area to local unit | 41 |
| LonC01 | Male | Unknown | No | All care at HASU | 35 |
| LonC02 | Female | 75 | Yes | All care at HASU | 4 |
| LonC03 | Male | 65 | No | All care at HASU | 8 |
| LonC04 | Male | 51 | Yes | HASU to HASU SU | 20 |
| LonC05 | Female | 56 | No | HASU to HASU SU | 40 |
| LonC06 | Female | 86 | No | All care at HASU | Not available |
| LonD01 | Female | Unknown | Yes | HASU to local unit | 76 |
| LonD02 | Female | 90 | Yes | HASU to local unit | 12 |
| LonD03 | Female | 87 | Yes | HASU to local unit | 15 |
| LonD04 | Male | 72 | No | HASU to local unit | 219 |
| LonD05 | Female | 80 | No | HASU to local unit | 126 |
| GMF01 | Female | 82 | No | HASU to HASU SU | 20 |
| GMF02 | Female | 77 | No | HASU to HASU SU | 126 |
| GMF03 | Female | 41 | No | All care at HASU | 61 |
| GMF04 | Male | 82 | No | All care at HASU | 38 |
| GMG01 | Male | 55 | Yes | All care at HASU | 20 |
| GMG02 | Male | 68 | Yes | All care at HASU | 57 |
| GMH01 | Male | 72 | Yes | All care at local unit | 71 |
| GMH02 | Male | 68 | Yes | HASU to local unit | 20 |
| GMH03 | Male | 52 | Yes | HASU to local unit | 35 |
| GMH04 | Female | 66 | Yes | All care at local unit | 62 |
| GMH05 | Male | 62 | Yes | All care at local unit | 77 |
| GMH06 | Female | 64 | No | HASU to local unit | 35 |
| GMH07 | Female | 55 | No | Local unit to HASU to local unit | 43 |
| GMH08 | Female | 66 | Yes | HASU to local unit | 57 |
| GMH09 | Male | 86 | No | HASU to local unit | 49 |
Appendix 7 Supplementary data for Chapter 11: impact of Greater Manchester B and sustainability of London changes in terms of clinical outcomes and clinical interventions
This appendix draws on Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Figures: clinical outcomes
FIGURE 29.
Probability of mortality at 3 days by region and quarter (London not included). Vertical lines indicate when reconfigurations in GM occurred (April 2010 and April 2015). Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.

FIGURE 30.
Probability of mortality at 30 days by region and quarter (London not included). Vertical lines indicate when reconfigurations in GM occurred (April 2010 and April 2015). Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
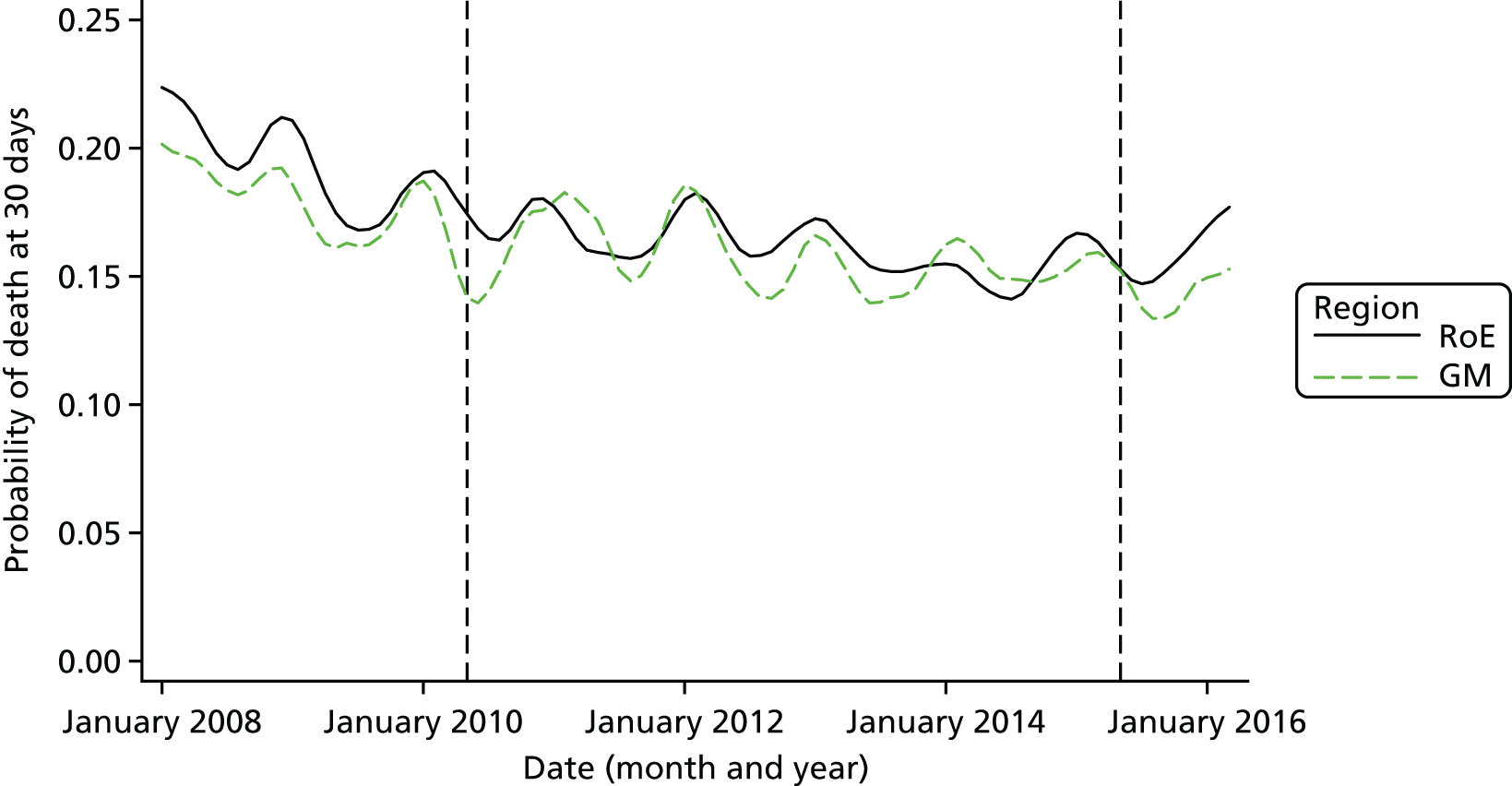
FIGURE 31.
Probability of mortality at 90 days by region and quarter (London not included). Vertical lines indicate when reconfigurations in GM occurred (April 2010 and April 2015). Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
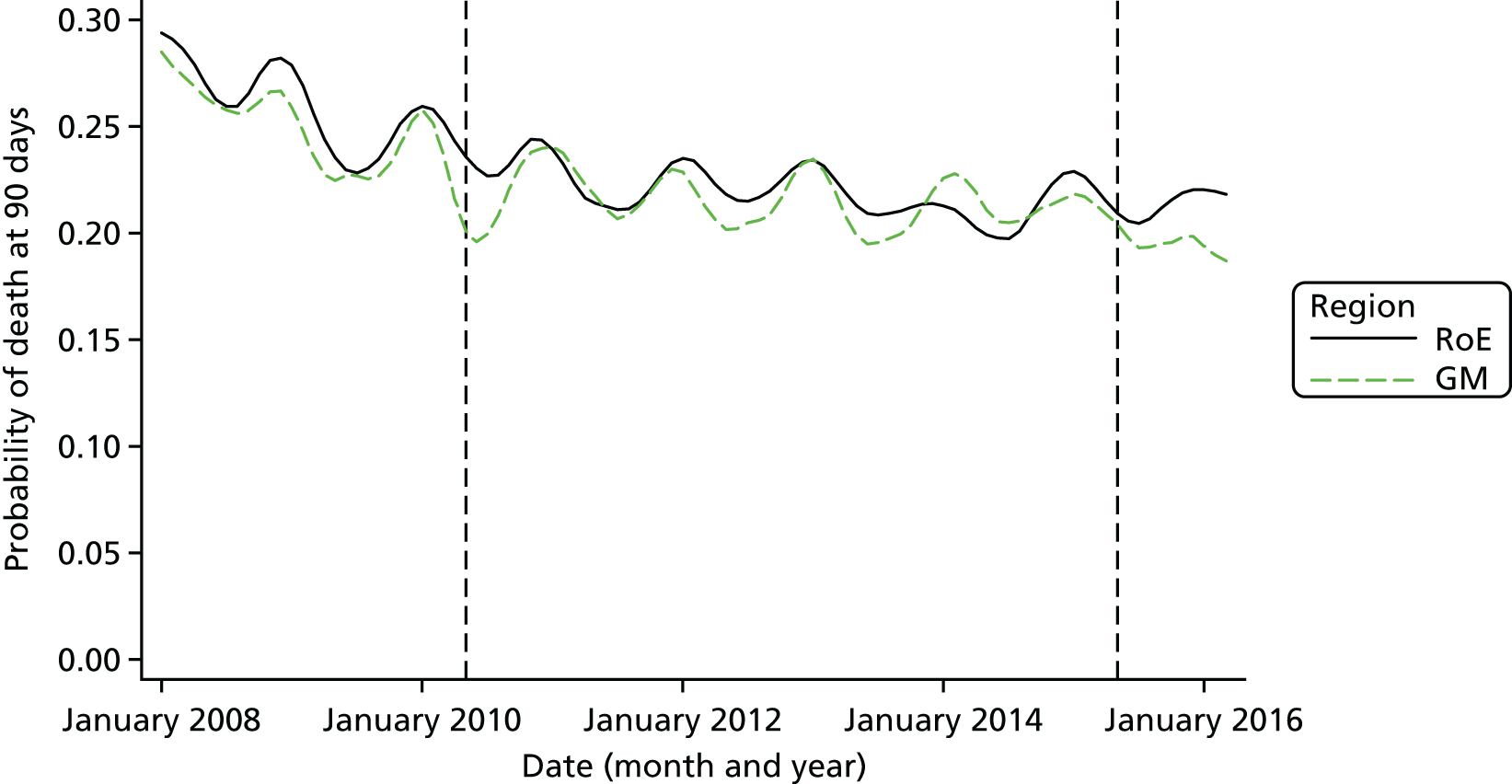
FIGURE 32.
Adjusted trends in mortality at 3 days in London. The vertical line indicates when the reconfiguration in London occurred (July 2010). p-value [under the null hypothesis that the regression coefficient for every month after the reconfiguration (which occurred in July 2010) is the same as the regression coefficient for July 2010] = 0.88. Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
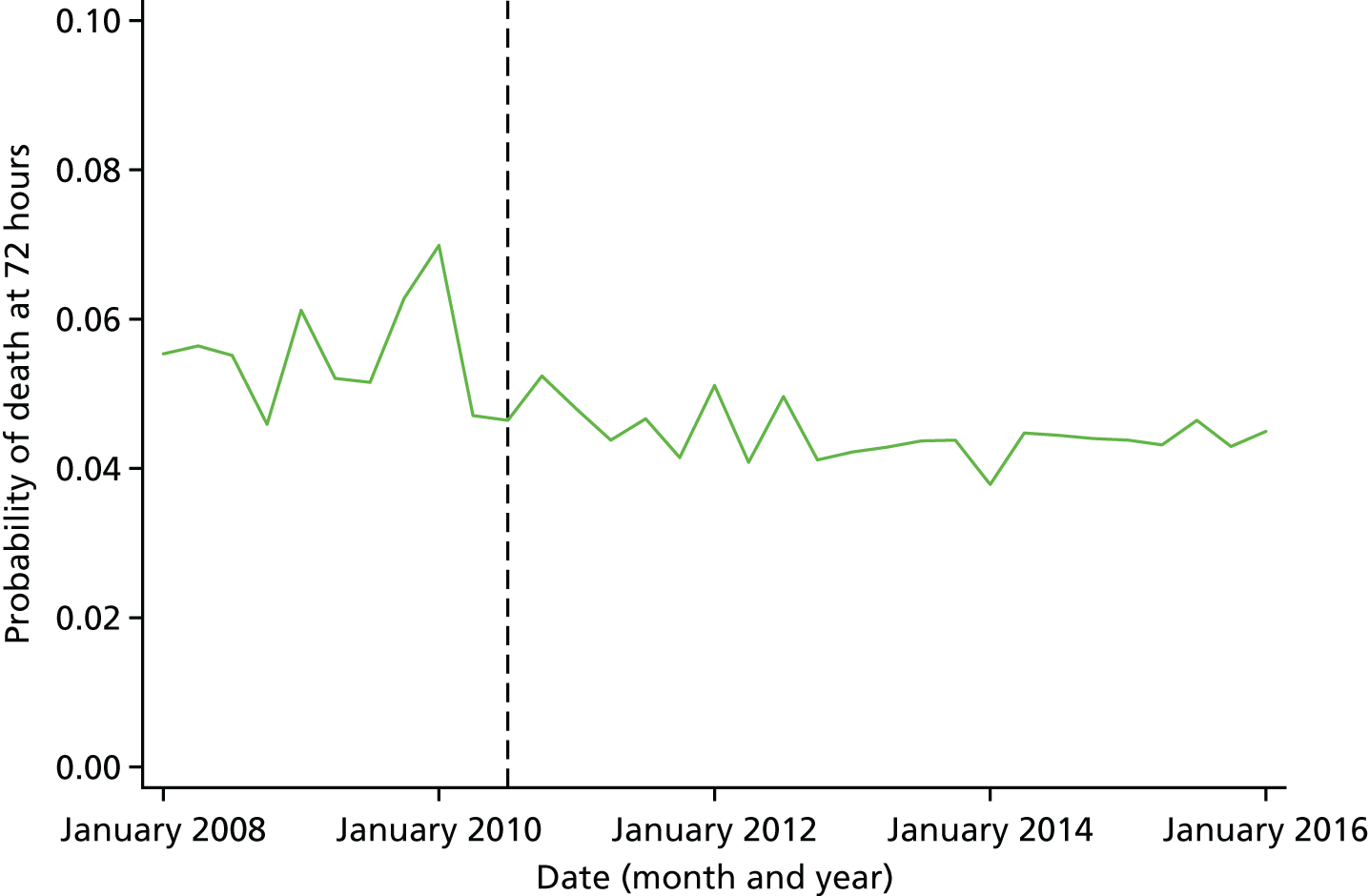
FIGURE 33.
Adjusted trends in mortality at 30 days in London. The vertical line indicates when the reconfiguration in London occurred (July 2010). p-value [under the null hypothesis that the regression coefficient for every month after the reconfiguration (which occurred in July 2010) is the same as the regression coefficient for July 2010] = 0.49. Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
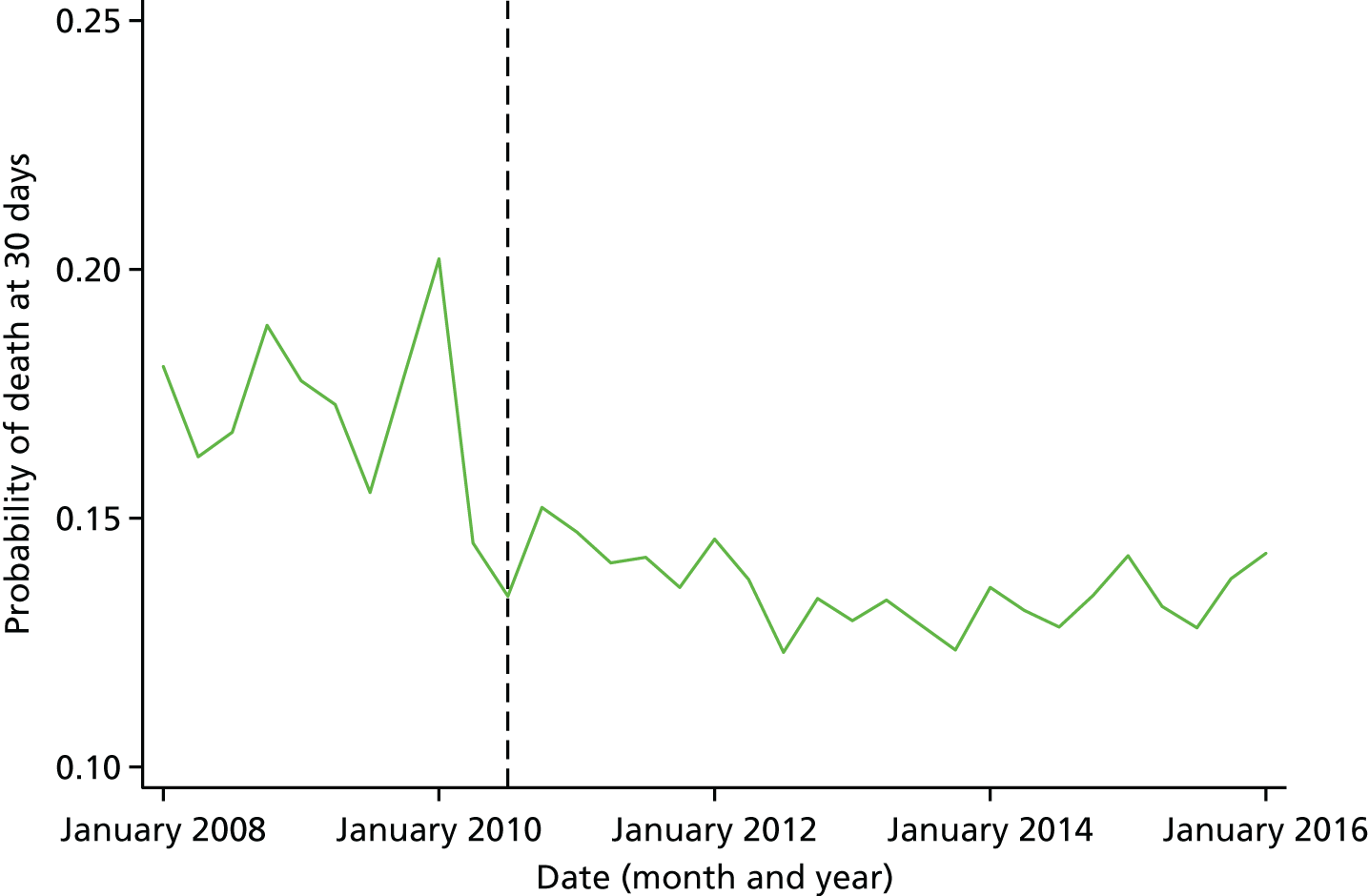
Tables: clinical outcomes
| Stroke type | GMB vs. ‘Before’ | GMC vs. ‘Before’ + GMA | ||
|---|---|---|---|---|
| Marginal effect | p-value | Marginal effect | p-value | |
| All stroke types | ||||
| Mortality at 3 days | –0.7 | 0.09 | –0.5 | 0.16 |
| Mortality at 30 days | –4.0 | < 0.01 | –2.2 | < 0.01 |
| Mortality at 90 days | –7.3 | < 0.01 | –4.2 | < 0.01 |
| Intracerebral haemorrhage (I61) | ||||
| Mortality at 3 days | 4.0 | 0.08 | 1.3 | 0.50 |
| Mortality at 30 days | –1.2 | 0.62 | –0.3 | 0.86 |
| Mortality at 90 days | –4.7 | 0.07 | –2.4 | 0.28 |
| Cerebral infarction (I63) | ||||
| Mortality at 3 days | –0.6 | 0.14 | –0.7 | 0.04 |
| Mortality at 30 days | –3.2 | < 0.01 | –1.9 | < 0.01 |
| Mortality at 90 days | –6.5 | < 0.01 | –4.0 | < 0.01 |
| Stroke, not specified as haemorrhage or infarction (I64) | ||||
| Mortality at 3 days | –2.8 | 0.04 | –1.6 | 0.26 |
| Mortality at 30 days | –7.0 | < 0.01 | –4.5 | 0.02 |
| Mortality at 90 days | –9.6 | < 0.01 | –5.6 | < 0.01 |
| Stroke type | GMB vs. ‘Before’ | GMB vs. ‘Before’ + GMA | ||
|---|---|---|---|---|
| Marginal effect | p-value | Marginal effect | p-value | |
| All stroke types | ||||
| Mortality at 3 days | 0.4 | < 0.01 | 0.4 | < 0.01 |
| Mortality at 30 days | –3.1 | < 0.01 | –1.2 | < 0.01 |
| Mortality at 90 days | –5.7 | < 0.01 | –2.6 | < 0.01 |
| Intracerebral haemorrhage (I61) | ||||
| Mortality at 3 days | 8.6 | < 0.01 | 5.0 | < 0.01 |
| Mortality at 30 days | 4.8 | < 0.01 | 2.7 | < 0.01 |
| Mortality at 90 days | 2.5 | < 0.01 | 1.5 | 0.01 |
| Cerebral infarction (I63) | ||||
| Mortality at 3 days | –0.2 | < 0.01 | –0.2 | 0.09 |
| Mortality at 30 days | –3.3 | < 0.01 | –1.5 | < 0.01 |
| Mortality at 90 days | –6.0 | < 0.01 | –2.9 | < 0.01 |
| Stroke, not specified as haemorrhage or infarction (I64) | ||||
| Mortality at 3 days | –2.6 | < 0.01 | –1.5 | < 0.01 |
| Mortality at 30 days | –6.8 | < 0.01 | –3.6 | < 0.01 |
| Mortality at 90 days | –9.1 | < 0.01 | –5.2 | < 0.01 |
| Region | Mortality (%) | ||
|---|---|---|---|
| 3 days | 30 days | 90 days | |
| RoE (‘Before’) | 6.7 | 19.4 | 26.1 |
| RoE (GMA) | 5.4 | 16.2 | 22.1 |
| RoE (‘Before’ + GMA) | 5.8 | 17.2 | 23.3 |
| RoE (GMB) | 5.3 | 15.8 | 21.3 |
| GM (‘Before’) | 6.3 | 18.1 | 25.2 |
| GM (GMA) | 5.4 | 15.7 | 21.5 |
| GM (‘Before’ + GMA) | 5.7 | 16.5 | 22.7 |
| GM (GMB) | 4.7 | 14.6 | 19.6 |
| Differences | |||
| RoE (GMB minus ‘Before’) | –1.4 | –3.7 | –4.8 |
| GM (GMB minus ‘Before’) | –1.6 | –3.5 | –5.6 |
| RoE [GMB minus (‘Before’ + GMA)] | –0.5 | –1.5 | –2.0 |
| GM [GMB minus (‘Before’ + GMA)] | –1.0 | –1.9 | –3.1 |
| Difference-in-differences | |||
| GMB minus ‘Before’ | –0.2 | 0.2 | –0.8 |
| GMB minus (‘Before’ + GMA) | –0.5 | –0.4 | –1.1 |
| Stroke type | GMB vs. ‘Before’ | GMB vs. ‘Before’ + GMA | ||||
|---|---|---|---|---|---|---|
| Difference-in-differences | 95% CI | p-value | Difference-in-differences | 95% CI | p-value | |
| All stroke types | ||||||
| Mortality at 3 days | –0.9 | –1.7 to –0.1 | 0.03 | –0.9 | –1.6 to –0.2 | 0.02 |
| Mortality at 30 days | –0.5 | –1.8 to 0.8 | 0.45 | –0.8 | –2.0 to 0.3 | 0.15 |
| Mortality at 90 days | –1.0 | –2.5 to 0.4 | 0.17 | –1.3 | –2.7 to 0.01 | 0.05 |
| Intracerebral haemorrhage (I61) | ||||||
| Mortality at 3 days | –3.4 | –7.5 to 0.7 | 0.10 | –3.1 | –6.7 to 0.5 | 0.09 |
| Mortality at 30 days | –5.7 | –10.5 to –0.9 | 0.02 | –3.1 | –7.4 to 1.1 | 0.15 |
| Mortality at 90 days | –6.9 | –11.8 to –1.9 | < 0.01 | –4.1 | –8.4 to 0.2 | 0.06 |
| Cerebral infarction (I63) | ||||||
| Mortality at 3 days | –0.3 | –1.0 to 0.4 | 0.43 | –0.6 | –1.2 to 0.04 | 0.07 |
| Mortality at 30 days | 0.3 | –1.1 to 1.7 | 0.68 | –0.7 | –1.9 to 0.6 | 0.29 |
| Mortality at 90 days | –0.1 | –1.8 to 1.5 | 0.87 | –1.2 | –2.6 to 0.2 | 0.10 |
| Stroke, not specified as haemorrhage or infarction (I64) | ||||||
| Mortality at 3 days | –0.2 | –3.4 to 3.0 | 0.90 | 0.04 | –3.1 to 3.1 | 0.98 |
| Mortality at 30 days | –0.8 | –5.5 to 3.8 | 0.73 | –1.4 | –5.9 to 3.0 | 0.53 |
| Mortality at 90 days | –1.0 | –6.0 to 4.0 | 0.70 | –1.0 | –5.8 to 3.7 | 0.67 |
| Stroke type | GMB vs. ‘Before’ | GMB vs. ‘Before’ + GMA | ||||
|---|---|---|---|---|---|---|
| Difference-in-differences | 95% CI | p-value | Difference-in-differences | 95% CI | p-value | |
| All stroke types | ||||||
| Mortality at 3 days | 0.03 | –1.0 to 1.0 | 0.96 | –0.7 | –1.6 to 0.1 | 0.10 |
| Mortality at 30 days | 0.9 | –0.8 to 2.5 | 0.31 | –0.9 | –2.3 to 0.5 | 0.22 |
| Mortality at 90 days | –0.1 | –2.0 to 1.8 | 0.90 | –1.8 | –3.4 to –0.2 | 0.03 |
| Intracerebral haemorrhage (I61) | ||||||
| Mortality at 3 days | –0.3 | –5.5 to 4.9 | 0.90 | –2.5 | –6.9 to 2.0 | 0.28 |
| Mortality at 30 days | –4.4 | –10.5 to –1.8 | 0.17 | –4.1 | –9.4 to 1.1 | 0.12 |
| Mortality at 90 days | –6.2 | –12.5 to 0.1 | 0.05 | –5.0 | –10.3 to 0.4 | 0.07 |
| Cerebral infarction (I63) | ||||||
| Mortality at 3 days | 0.2 | –0.7 to 1.1 | 0.68 | –0.7 | –1.5 to –0.1 | 0.04 |
| Mortality at 30 days | 1.8 | –0.1 to 3.6 | 0.06 | –1.1 | –2.5 to 0.4 | 0.15 |
| Mortality at 90 days | 1.0 | –1.0 to 3.1 | 0.33 | –1.9 | –3.6 to –0.3 | 0.02 |
| Stroke, not specified as haemorrhage or infarction (I64) | ||||||
| Mortality at 3 days | 4.6 | –0.9 to 10.1 | 0.10 | 4.7 | –0.7 to 10.1 | 0.09 |
| Mortality at 30 days | 5.2 | –2.7 to 13.1 | 0.20 | 5.1 | –2.7 to 12.9 | 0.20 |
| Mortality at 90 days | 4.4 | –4.1 to 12.9 | 0.31 | 4.6 | –3.8 to 12.9 | 0.28 |
| Outcome measure | Region | |||||
|---|---|---|---|---|---|---|
| RoE (excluding London) | GM | |||||
| Before | After ‘A’ | After ‘B’ | Before | After ‘A’ | After ‘B’ | |
| Number of patients | 109,795 | 247,046 | 48,443 | 9412 | 20,390 | 4249 |
| Unadjusted LOS | ||||||
| Mean (days) | 21.0 | 18.1 | 15.7 | 21.7 | 17.4 | 14.4 |
| Stroke type | After ‘B’ vs. ‘Before’ | After ‘B’ vs. ‘Before’ + after ‘A’ | After ‘B’ vs. after ‘A’ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference-in-differences | 95% CI | p-value | Difference-in-differences | 95% CI | p-value | Difference-in-differences | 95% CI | p-value | |
| All stroke types | –4.3 | –5.4 to –3.2 | < 0.01 | –2.2 | –3.3 to –1.3 | < 0.01 | –1.5 | –2.5 to –0.4 | < 0.01 |
| Intracerebral haemorrhage (I61) | –2.1 | –4.5 to 0.4 | 0.10 | –1.4 | –3.6 to 0.7 | 0.19 | –1.2 | –3.3 to 1.0 | 0.29 |
| Cerebral infarction (I63) | –4.5 | –5.7 to –3.2 | < 0.01 | –2.1 | –3.2 to –1.0 | < 0.01 | –1.3 | –2.4 to –0.2 | 0.02 |
| Stroke, not specified as haemorrhage or infarction (I64) | –7.2 | –9.9 to –4.6 | < 0.01 | –5.4 | –8.0 to –2.8 | < 0.01 | –4.4 | –7.0 to –1.8 | < 0.01 |
Figures: clinical interventions
FIGURE 34.
Risk-adjusted likelihood of patients receiving clinical interventions in GM. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients). h, hours; SaLT, Speech and Language Therapist; tPA, tissue plasminogen activator. Scan 60, brain scan within 60 minutes of clock start; Scan 180, brain scan within 180 minutes of clock start; Scan 24 hours, brain scan within 24 hours of clock start. Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
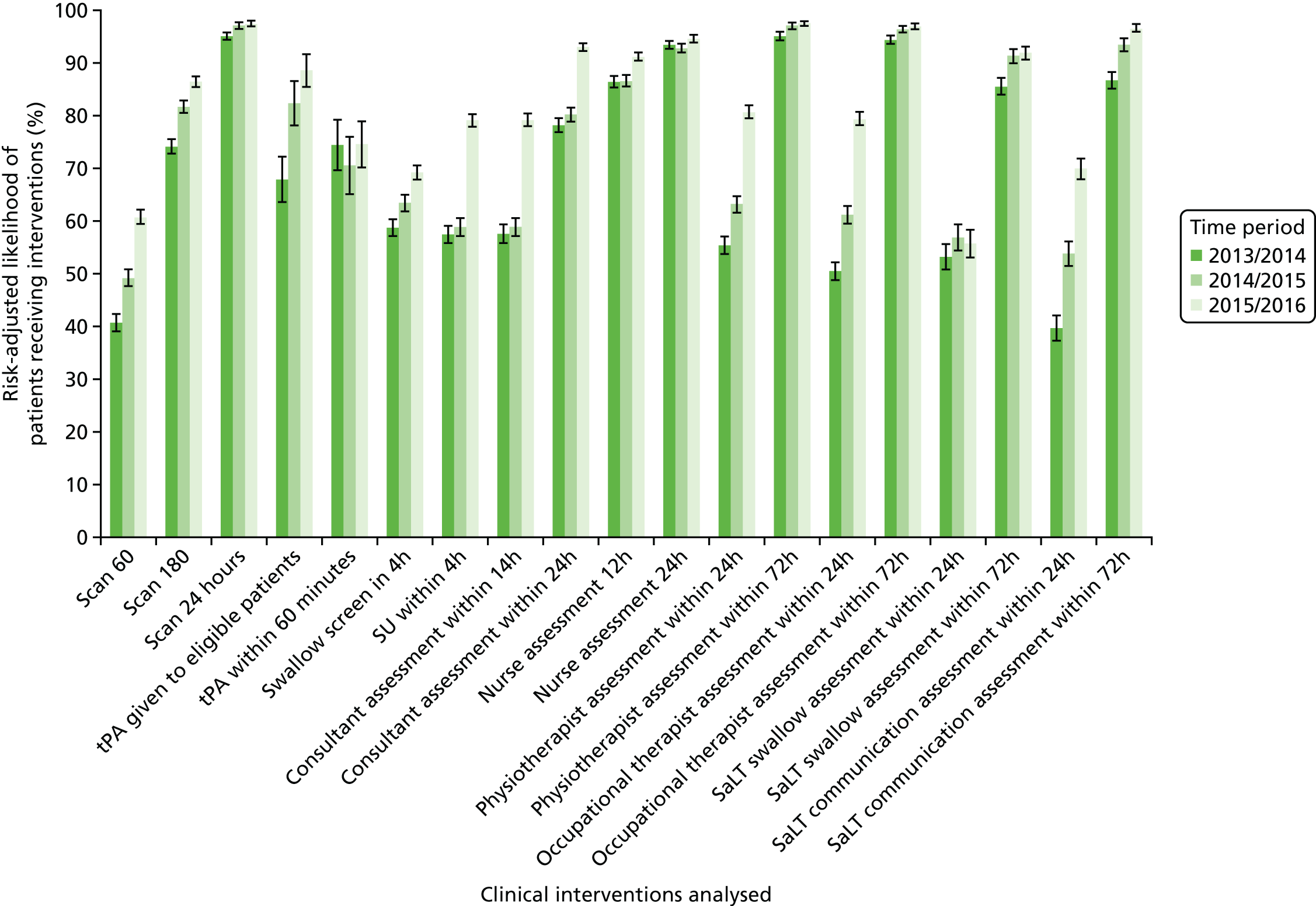
FIGURE 35.
Risk-adjusted likelihood of patients receiving clinical interventions in GM HASUs. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients). h, hours; SaLT, Speech and Language Therapist; tPA, tissue plasminogen activator. Scan 60, brain scan within 60 minutes of clock start; Scan 180, brain scan within 180 minutes of clock start; Scan 24 hours, brain scan within 24 hours of clock start. Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.
FIGURE 36.
Risk-adjusted likelihood of patients receiving clinical interventions in RoE. All measures reflect time from ‘clock start’ (i.e. when patient first arrives at hospital or when symptoms are identified in in-patients). h, hours; SaLT, Speech and Language Therapist; tPA, tissue plasminogen activator. Scan 60, brain scan within 60 minutes of clock start; Scan 180, brain scan within 180 minutes of clock start; Scan 24 hours, brain scan within 24 hours of clock start. Adapted from Morris et al. 207 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. Figure title and numbering updated for report.

Tables: clinical interventions
| Characteristic | Region | Comparator | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | London | |||||||||||
| Ramsay et al. (2015)26 | Time period | Ramsay et al. (2015)26 | Time period | Ramsay et al. (2015)26 | Time period | |||||||
| 2013/14 | 2014/15 | 2015/16 | 2013/14 | 2014/15 | 2015/16 | 2013/14 | 2014/15 | 2015/16 | ||||
| N | 10,295 | 3877 | 3729 | 4480 | 16,533 | 8131 | 8150 | 8000 | 9044 | 55,262 | 62,824 | 63,667 |
| Age | ||||||||||||
| Mean age (years) | 73.2 | 73.5 | 73.7 | 73.5 | 72.7 | 72.4 | 72.5 | 72.5 | 73.6 | 75.3 | 75.2 | 75.2 |
| Aged > 75 years (n) | 2077 | 2041 | 2401 | 4276 | 4257 | 4182 | 32,960 | 37,285 | 37,478 | |||
| Aged > 75 years (%) | 50 | 53.57 | 54.73 | 53.59 | 50 | 52.59 | 52.23 | 52.28 | 51 | 59.6 | 59.4 | 58.9 |
| Sex | ||||||||||||
| Female (n) | 1974 | 1819 | 2208 | 3969 | 3905 | 3832 | 28,009 | 31,628 | 31,552 | |||
| Female (%) | 51 | 50.92 | 48.78 | 49.29 | 49 | 48.81 | 47.91 | 47.9 | 51 | 50.7 | 50.3 | 49.6 |
| Stroke type | ||||||||||||
| Primary infarction (n) | 3393 | 3277 | 3946 | 6970 | 7063 | 6862 | 48,734 | 55,072 | 55,476 | |||
| Primary infarction (%) | 87 | 88.38 | 88.57 | 88.46 | 86 | 88.15 | 87.97 | 86.73 | 89 | 89.4 | 88.5 | 87.8 |
| Haemorrhagic (n) | 446 | 423 | 515 | 937 | 966 | 1050 | 5772 | 7174 | 7732 | |||
| Haemorrhagic (%) | 13 | 11.62 | 11.43 | 11.54 | 14 | 11.85 | 12.03 | 13.27 | 11 | 10.6 | 11.5 | 12.2 |
| Where stroke happened | ||||||||||||
| Admitted from outside hospital (n) | 3608 | 3485 | 4195 | 7792 | 7799 | 7671 | 52,236 | 59,269 | 60,168 | |||
| Admitted from outside hospital (%) | 90 | 93.06 | 93.46 | 93.64 | 95 | 95.83 | 95.69 | 95.89 | 94 | 94.5 | 94.3 | 94.5 |
| Treatment in HASU | ||||||||||||
| Total treated in HASU (n) | 2205 | 2389 | 3849 | 7713 | 7668 | 7522 | – | – | – | – | ||
| Total treated in HASU (%) | 39 | 56.9 | 64.1 | 85.9 | 93 | 94.9 | 94.1 | 94.0 | – | – | – | – |
| Reaching hospital in ≤ 4 hours (n) | 1254 | 1156 | 1322 | 3060 | 3203 | 3162 | – | 21,515 | 24,381 | 24,248 | ||
| Reaching hospital in ≤ 4 hours (%) | 61.0 | 59.2 | 53.8 | 59.3 | 59.8 | 59.1 | – | 64.0 | 62.8 | 60.8 | ||
| Reaching hospital in ≤ 48 hours (n) | 1175 | 1077 | 1435 | 2521 | 2190 | 2082 | 17,318 | 20,050 | 19,452 | |||
| Reaching hospital in ≤ 48 hours (%) | 98.4 | 98.3 | 97.8 | 97.8 | 97.7 | 97.8 | 97.7 | 97.9 | 98.1 | |||
| Treated in HASU if arrive in ≤ 4 hours (n) | 991 | 982 | 1246 | 2999 | 3147 | 3111 | – | – | – | – | ||
| Treated in HASU if arrive in ≤ 4 hours (%) | 67 | 79.0 | 85.0 | 94.3 | 96 | 98.0 | 98.3 | 98.4 | – | – | – | – |
| Thrombolysis | ||||||||||||
| Infarct patients classed as eligible (n) | 420 | 297 | 382 | 1432 | 1344 | 1284 | 7889 | 8103 | 7573 | |||
| Infarct patients classed as eligible (%) | 12.4 | 9.1 | 9.7 | 20.5 | 19.0 | 18.7 | 16.2 | 14.7 | 13.7 | |||
| Infarct patients receiving tPA (n) | 274 | 244 | 337 | 1331 | 1267 | 1134 | 6145 | 7138 | 6986 | |||
| Total receiving tPA (% infarct patients) | 8.1 | 7.4 | 8.5 | 19.1 | 17.9 | 16.5 | 12.6 | 13.0 | 12.6 | |||
| Total receiving tPA (% eligible patients) | 65.2 | 82.2 | 88.2 | 93.0 | 94.3 | 88.3 | 77.9 | 88.1 | 92.3 | |||
| Worst level of consciousness | ||||||||||||
| Alert (n) | 3230 | 3078 | 3814 | 6829 | 6755 | 6713 | 45,988 | 52,317 | 53,309 | |||
| Alert (%) | 74 | 83.31 | 82.54 | 85.13 | 78 | 83.99 | 82.88 | 83.91 | 75 | 83.2 | 83.3 | 83.7 |
| Verbally arousable (n) | 331 | 349 | 396 | 795 | 825 | 768 | 5374 | 6108 | 6068 | |||
| Verbally arousable (%) | 15 | 8.54 | 9.36 | 8.84 | 15 | 9.78 | 10.12 | 9.6 | 11 | 9.7 | 9.7 | 9.5 |
| Not alert (n) | 181 | 195 | 166 | 317 | 379 | 338 | 2183 | 2585 | 2490 | |||
| Not alert (%) | 4 | 4.67 | 5.23 | 3.71 | 3 | 3.9 | 4.65 | 4.23 | 9 | 4.0 | 4.1 | 3.9 |
| Totally unresponsive (n) | 135 | 107 | 104 | 190 | 191 | 181 | 1717 | 1814 | 1800 | |||
| Totally unresponsive (%) | 7 | 3.48 | 2.87 | 2.32 | 4 | 2.34 | 2.34 | 2.26 | 12 | 3.1 | 2.9 | 2.8 |
| Intervention | Ramsay et al. (2015)26 (n/N) | Time period (n/N) | ||
|---|---|---|---|---|
| 2013/14 | 2014/15 | 2015/16 | ||
| Scan 60 minutes | 1490/3839 (38.8%) | 1758/3700 (47.5%) | 2627/4461 (58.9%) | |
| Scan 180 minutes | 5785/10,295 (56%) | 2803/3839 (73.0%) | 2998/3700 (81.0%) | 3822/4461 (85.7%) |
| Scan 24 hours | 8814/9563 (92%) | 3642/3839 (94.9%) | 3587/3700 (97.0%) | 4340/4461 (97.3%) |
| tPA to eligible patients | 274/420 (65.2%) | 244/297 (82.2%) | 337/382 (88.2%) | |
| tPA within 60 minutes | 197/274 (71.9%) | 165/244 (67.6%) | 243/337 (72.1%) | |
| Swallow screen in 4 hours | 2050/3544 (57.8%) | 2128/3399 (62.6%) | 2843/4145 (68.6%) | |
| SU within 4 hours | 4982/10,295 (48%) | 2414/3870 (62.4%) | 2347/3724 (63.0%) | 3232/4470 (72.3%) |
| Consultant assessment 14 hours | 1978/3428 (57.7%) | 1977/3346 (59.1%) | 3356/4234 (79.3%) | |
| Consultant assessment 24 hours | 2650/3428 (77.3%) | 2654/3346 (79.3%) | 3925/4234 (92.7%) | |
| Nurse assessment 12 hours | 3136/3664 (85.6%) | 3057/3562 (85.8%) | 3917/4320 (90.7%) | |
| Nurse assessment 24 hours | 3409/3664 (93.0%) | 3289/3562 (92.3%) | 4073/4320 (94.3%) | |
| Physiotherapist assessment 24 hours | 1927/3498 (55.1%) | 2141/3415 (62.7%) | 3342/4155 (80.4%) | |
| Physiotherapist assessment 72 hours | 8030/8867 (91%) | 3315/3498 (94.8%) | 3308/3415 (96.9%) | 4047/4155 (97.4%) |
| Occupational therapist 24 hours | 1720/3460 (49.7%) | 2059/3419 (60.2%) | 3308/4199 (78.8%) | |
| Occupational therapist 72 hours | 3255/3460 (94.1%) | 3290/3419 (96.2%) | 4063/4199 (96.8%) | |
| SaLT swallow 24 hours | 848/1611 (52.6%) | 853/1516 (56.3%) | 805/1471 (54.7%) | |
| SaLT swallow 72 hours | 8521/9388 (91%) | 1349/1611 (83.7%) | 1374/1516 (90.6%) | 1342/1471 (91.2%) |
| SaLT communication 24 hours | 591/1559 (37.9%) | 907/1738 (52.2%) | 1579/2310 (68.4%) | |
| SaLT communication 72 hours | 1328/1559 (85.2%) | 1611/1738 (92.7%) | 2222/2310 (96.2%) | |
| Intervention | Time period (n/N) | ||
|---|---|---|---|
| 2013/14 | 2014/15 | 2015/16 | |
| Scan 60 minutes | 1147/1882 (61.0%) | 1361/1948 (69.9%) | 2472/3741 (66.1%) |
| Scan 180 minutes | 1650/1882 (87.7%) | 1773/1948 (91.0%) | 3421/3741 (91.5%) |
| Scan 24 hours | 1851/1882 (98.4%) | 1929/1948 (99.0%) | 3703/3741 (99.0%) |
| tPA to eligible patients | 270/286 (94.4%) | 244/271 (90.0%) | 336/339 (99.1%) |
| tPA within 60 minutes | 194/270 (71.9%) | 165/244 (67.6%) | 243/336 (72.3%) |
| Swallow screen in 4 hours | 1157/1674 (69.1%) | 1262/1727 (73.1%) | 2633/3505 (75.1%) |
| SU within 4 hours | 1563/1886 (82.9%) | 1613/1954 (82.6%) | 3107/3748 (82.9%) |
| Consultant assessment 14 hours | 1535/1826 (84.1%) | 1558/1900 (82.0%) | 3204/3734 (85.8%) |
| Consultant assessment 24 hours | 1709/1826 (93.6%) | 1776/1900 (93.5%) | 3644/3734 (97.6%) |
| Nurse assessment 12 hours | 1746/1841 (94.8%) | 1812/1923 (94.2%) | 3608/3727 (96.8%) |
| Nurse assessment 24 hours | 1786/1841 (97.0%) | 1853/1923 (96.4%) | 3650/3727 (97.9%) |
| Physiotherapist assessment 24 hours | 822/1658 (49.6%) | 1123/1786 (62.9%) | 3065/3525 (87.0%) |
| Physiotherapist assessment 72 hours | 1579/1658 (95.2%) | 1759/1786 (98.5%) | 3498/3525 (99.2%) |
| Occupational therapist 24 hours | 742/1645 (45.1%) | 1125/1793 (62.7%) | 3068/3582 (85.7%) |
| Occupational therapist 72 hours | 1555/1645 (94.5%) | 1750/1793 (97.6%) | 3542/3582 (98.9%) |
| SaLT swallow 24 hours | 378/712 (53.1%) | 485/769 (63.1%) | 704/1176 (59.9%) |
| SaLT swallow 72 hours | 586/712 (82.3%) | 713/769 (92.7%) | 1118/1176 (95.1%) |
| SaLT communication 24 hours | 307/722 (42.5%) | 564/939 (60.1%) | 1491/2052 (72.7%) |
| SaLT communication 72 hours | 628/722 (87.0%) | 897/939 (95.5%) | 2013/2052 (98.1%) |
| Intervention | Ramsay et al. (2015)26 (n/N) | Time period (n/N) | ||
|---|---|---|---|---|
| 2013/14 | 2014/15 | 2015/16 | ||
| Scan 60 minutes | 4524/7907 (57.2%) | 4733/8029 (59.0%) | 4738/7912 (59.9%) | |
| Scan 180 minutes | 11,614/16,553 (70%) | 6324/7907 (80.0%) | 6571/8029 (81.8%) | 6508/7912 (82.3%) |
| Scan 24 hours | 14,895/15,679 (95%) | 7695/7907 (97.3%) | 7805/8029 (97.2%) | 7709/7912 (97.4%) |
| tPA to eligible patients | 1331/1432 (93.0%) | 1267/1344 (94.3%) | 1134/1284 (88.3%) | |
| tPA within 60 minutes | 1104/1331 (83.0%) | 1059/1267 (83.6%) | 915/1134 (80.7%) | |
| Swallow screen in 4 hours | 5068/7718 (65.7%) | 5220/7645 (68.3%) | 5369/7606 (70.6%) | |
| SU within 4 hours | 11,360/16,553 (69%) | 4993/8112 (61.6%) | 4861/8121 (59.9%) | 4806/7973 (60.3%) |
| Consultant assessment 14 hours | 3932/7821 (50.3%) | 4197/7873 (53.3%) | 3906/7662 (51.0%) | |
| Consultant assessment 24 hours | 7014/7821 (89.7%) | 7118/7873 (90.4%) | 6797/7662 (88.7%) | |
| Nurse assessment 12 hours | 7078/7767 (91.1%) | 7259/7810 (92.9%) | 7178/7678 (93.5%) | |
| Nurse assessment 24 hours | 7443/7767 (95.8%) | 7485/7810 (95.8%) | 7398/7678 (96.4%) | |
| Physiotherapist assessment 24 hours | 4062/7064 (57.5%) | 4315/7001 (61.6%) | 4498/6970 (64.5%) | |
| Physiotherapist assessment 72 hours | 14,190/14,760 (96%) | 6712/7064 (95.0%) | 6753/7002 (96.4%) | 6670/6970 (95.7%) |
| Occupational therapist 24 hours | 3386/6766 (50.0%) | 3871/6840 (56.6%) | 4179/6856 (61.0%) | |
| Occupational therapist 72 hours | 6161/6766 (91.1%) | 6464/6840 (94.5%) | 6443/6856 (94.0%) | |
| SaLT swallow 24 hours | 1798/3279 (54.8%) | 1857/3403 (54.6%) | 1600/3104 (51.6%) | |
| SaLT swallow 72 hours | 15,431/15,684 (98%) | 2924/3279 (89.2%) | 3114/3403 (91.5%) | 2728/3104 (87.9%) |
| SaLT communication 24 hours | 2269/4933 (46.0%) | 2343/4755 (49.3%) | 2308/4640 (49.7%) | |
| SaLT communication 72 hours | 4411/4933 (89.4%) | 4410/4755 (92.7%) | 4204/4641 (90.6%) | |
| Intervention | Time period (n/N) | ||
|---|---|---|---|
| 2013/14 | 2014/15 | 2015/16 | |
| Scan 60 minutes | 22,462/54,506 (41.2%) | 26,801/62,246 (43.1%) | 29,160/63,208 (46.1%) |
| Scan 180 minutes | 38,036/54,506 (69.8%) | 45,452/62,246 (73.0%) | 48,277/63,208 (76.4%) |
| Scan 24 hours | 51,592/54,506 (94.7%) | 59,642/62,246 (95.8%) | 61,054/63,208 (96.6%) |
| tPA to eligible patients | 6145/7889 (77.9%) | 7138/8103 (88.1%) | 6986/7573 (92.3%) |
| tPA within 60 minutes | 2938/6145 (47.8%) | 3739/7138 (52.4%) | 3901/6986 (55.8%) |
| Swallow screen in 4 hours | 30,570/51,864 (58.9%) | 37,564/59,396 (63.2%) | 40,643/60,458 (67.2%) |
| SU within 4 hours | 33,057/55,069 (60.0%) | 36,585/62,605 (58.4%) | 37,599/63,441 (59.3%) |
| Consultant assessment 14 hours | 25,968/50,422 (51.5%) | 30,354/57,600 (52.7%) | 31,758/59,179 (53.7%) |
| Consultant assessment 24 hours | 40,379/50,422 (80.1%) | 47,114/57,600 (81.8%) | 49,576/59,179 (83.8%) |
| Nurse assessment 12 hours | 44,033/51,244 (85.9%) | 51,042/58,378 (87.4%) | 52,874/59,613 (88.7%) |
| Nurse assessment 24 hours | 47,616/51,244 (92.9%) | 54,651/58,378 (93.6%) | 56,214/59,613 (94.3%) |
| Physiotherapist assessment 24 hours | 26,988/48,588 (55.5%) | 31,076/54,942 (56.6%) | 33,301/56,297 (59.2%) |
| Physiotherapist assessment 72 hours | 45,375/48,589 (93.4%) | 51,115/54,943 (93.0%) | 52,621/56,297 (93.5%) |
| Occupational therapist 24 hours | 20,314/45,341 (44.8%) | 24,265/52,170 (46.5%) | 27,830/54,192 (51.4%) |
| Occupational therapist 72 hours | 39,408/45,341 (86.9%) | 45,731/52,171 (87.7%) | 48,304/54,194 (89.1%) |
| SaLT swallow 24 hours | 11,245/23,586 (47.7%) | 12,416/25,219 (49.2%) | 13,535/24,983 (54.2%) |
| SaLT swallow 72 hours | 18,199/23,588 (77.2%) | 20,405/25,220 (80.9%) | 21,103/24,983 (84.5%) |
| SaLT communication 24 hours | 7997/23,674 (33.8%) | 9620/26,621 (36.1%) | 11,196/27,529 (40.7%) |
| SaLT communication 72 hours | 18,049/23,674 (76.2%) | 21,207/26,621 (79.7%) | 23,148/27,529 (84.1%) |
| Intervention | Risk-adjusted proportions (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| GM | Comparator | ||||||
| Ramsay et al. (2015)26 | 2013/14 | 2014/15 | 2015/16 | 2013/14 | 2014/15 | 2015/16 | |
| Scan 60 minutes | 62.0 (59.9 to 64.2) | 70.9 (68.9 to 72.8) | 67.6 (66.1 to 69.0) | 43.0 (42.6 to 43.4) | 44.8 (44.4 to 45.2) | 47.6 (47.3 to 48.0) | |
| Scan 180 minutes | 85.5 (84.6 to 86.5) | 87.8 (86.4 to 89.3) | 91.2 (90.0 to 92.5) | 91.7 (90.9 to 92.6) | 70.9 (70.6 to 71.3) | 74.0 (73.7 to 74.3) | 77.0 (76.7 to 77.3) |
| Scan 24 hours | 98.2 (97.8 to 98.5) | 98.3 (97.7 to 98.9) | 99.0 (98.6 to 99.5) | 99.0 (98.7 to 99.3) | 94.9 (94.8 to 95.1) | 96.0 (95.8 to 96.1) | 96.7 (96.6 to 96.8) |
| tPA to eligible patients | 94.5 (91.9 to 97.1) | 90.0 (86.5 to 93.6) | 99.1 (98.1 to 100) | 80.6 (79.8 to 81.3) | 89.1 (88.5 to 89.7) | 91.8 (91.3 to 92.4) | |
| tPA within 60 minutes | 74.3 (69.4 to 79.2) | 70.5 (65.1 to 75.8) | 74.7 (70.4 to 79.1) | 53.1 (52.0 to 54.2) | 57.1 (56.0 to 58.1) | 59.7 (58.6 to 60.7) | |
| Swallow screen in 4 hours | 69.0 (66.8 to 71.2) | 73.3 (71.2 to 75.3) | 75.0 (73.5 to 76.4) | 59.7 (59.3 to 60.1) | 63.9 (63.5 to 64.2) | 67.7 (67.3 to 68.0) | |
| SU within 4 hours | 82.9 (81.8 to 83.9) | 82.5 (80.8 to 84.2) | 82.0 (80.3 to 83.7) | 82.0 (80.8 to 83.2) | 51.3 (50.9 to 51.7) | 52.8 (52.4 to 53.2) | 53.4 (53.0 to 53.7) |
| Consultant assessment 14 hours | 83.9 (82.2 to 85.6) | 81.7 (80.0 to 83.5) | 85.6 (84.5 to 86.8) | 50.6 (50.2 to 51.0) | 52.1 (51.7 to 52.5) | 52.6 (52.2 to 53.0) | |
| Consultant assessment 24 hours | 93.8 (92.7 to 94.8) | 93.6 (92.6 to 94.7) | 97.7 (97.2 to 98.1) | 81.1 (80.8 to 81.4) | 82.8 (82.5 to 83.1) | 84.3 (84.1 to 84.6) | |
| Nurse assessment 12 hours | 94.9 (93.9 to 95.9) | 94.3 (93.3 to 95.3) | 96.8 (96.3 to 97.4) | 86.5 (86.2 to 86.7) | 88.1 (87.8 to 88.3) | 89.3 (89.0 to 89.5) | |
| Nurse assessment 24 hours | 97.0 (96.2 to 97.7) | 96.4 (95.5 to 97.2) | 97.9 (97.5 to 98.4) | 93.2 (93.0 to 93.4) | 93.9 (93.7 to 94.0) | 94.5 (94.4 to 94.7) | |
| Physiotherapist assessment 24 hours | 49.4 (47.0 to 51.8) | 63.0 (60.8 to 65.2) | 86.8 (85.7 to 87.9) | 55.6 (55.2 to 56.0) | 57.1 (56.7 to 57.4) | 59.7 (59.3 to 60.1) | |
| Physiotherapist assessment 72 hours | 93.8 (93.0 to 94.7) | 95.3 (94.3 to 96.3) | 98.5 (98.0 to 99.1) | 99.2 (99.0 to 99.5) | 93.6 (93.4 to 93.8) | 93.4 (93.2 to 93.6) | 93.7 (93.5 to 93.9) |
| Occupational therapist 24 hours | 45.5 (43.1 to 47.9) | 63.3 (61.1 to 65.5) | 85.8 (84.6 to 86.9) | 45.2 (44.7 to 45.6) | 47.5 (47.1 to 47.9) | 52.3 (51.9 to 52.7) | |
| Occupational therapist 72 hours | 94.7 (93.6 to 95.7) | 97.7 (97.0 to 98.4) | 98.9 (98.6 to 99.2) | 87.3 (87.1 to 87.6) | 88.5 (88.2 to 88.7) | 89.7 (89.4 to 89.9) | |
| SaLT swallow 24 hours | 52.8 (49.2 to 56.5) | 62.9 (59.5 to 66.3) | 59.8 (57.0 to 62.6) | 48.4 (47.8 to 49.0) | 49.9 (49.3 to 50.5) | 54.0 (53.4 to 54.6) | |
| SaLT swallow 72 hours | 98.6 (98.2 to 98.9) | 83.8 (81.2 to 86.4) | 93.0 (91.2 to 94.7) | 95.3 (94.1 to 96.5) | 78.8 (78.3 to 79.3) | 82.2 (81.7 to 82.6) | 84.9 (84.5 to 85.3) |
| SaLT communication 24 hours | 43.8 (40.2 to 47.4) | 61.4 (58.3 to 64.5) | 73.6 (71.8 to 75.5) | 35.5 (34.9 to 36.1) | 38.0 (37.5 to 38.6) | 42.0 (41.5 to 42.6) | |
| SaLT communication 72 hours | 88.0 (85.7 to 90.3) | 95.9 (94.6 to 97.1) | 98.3 (97.7 to 98.8) | 78.3 (77.8 to 78.7) | 81.6 (81.2 to 82.0) | 85.1 (84.7 to 85.5) | |
| Intervention | Risk-adjusted proportions (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| London | Comparator | ||||||
| Ramsay et al. (2015)26 | 2013/14 | 2014/15 | 2015/16 | 2013/14 | 2014/15 | 2015/16 | |
| Scan 60 minutes | 57.3 (56.2 to 58.3) | 58.9 (57.9 to 60.0) | 59.9 (58.8 to 61.0) | 41.0 (40.6 to 41.4) | 43.3 (43.0 to 43.7) | 47.0 (46.6 to 47.3) | |
| Scan 180 minutes | 66.3 (65.6 to 67.1) | 80.0 (79.1 to 80.9) | 81.8 (81.0 to 82.6) | 82.2 (81.4 to 83.1) | 69.9 (69.6 to 70.3) | 73.5 (73.2 to 73.9) | 77.0 (76.7 to 77.3) |
| Scan 24 hours | 95.2 (94.8 to 95.5) | 97.2 (96.9 to 97.6) | 97.1 (96.8 to 97.5) | 97.3 (97.0 to 97.7) | 94.7 (94.5 to 94.9) | 95.9 (95.8 to 96.1) | 96.6 (96.5 to 96.8) |
| tPA to eligible patients | 92.9 (91.6 to 94.2) | 94.4 (93.2 to 95.7) | 88.6 (86.9 to 90.3) | 77.9 (77.0 to 78.8) | 87.9 (87.2 to 88.6) | 92.2 (91.6 to 92.8) | |
| tPA within 60 minutes | 83.0 (81.0 to 85.0) | 83.9 (81.9 to 85.9) | 80.8 (78.6 to 83.1) | 48.7 (47.5 to 49.9) | 52.9 (51.7 to 54.0) | 56.4 (55.3 to 57.6) | |
| Swallow screen in 4 hours | 65.1 (64.1 to 66.2) | 68.1 (67.1 to 69.2) | 70.6 (69.6 to 71.6) | 59.0 (58.5 to 59.4) | 63.3 (62.9 to 63.7) | 67.4 (67.0 to 67.8) | |
| SU within 4 hours | 66.3 (65.6 to 67.1) | 61.1 (60.0 to 62.1) | 59.8 (58.7 to 60.8) | 60.3 (59.2 to 61.3) | 60.2 (59.8 to 60.6) | 58.8 (58.4 to 59.2) | 60.1 (59.8 to 60.5) |
| Consultant assessment 14 hours | 50.3 (49.2 to 51.5) | 53.7 (52.6 to 54.8) | 51.3 (50.1 to 52.4) | 51.8 (51.4 to 52.2) | 53.1 (52.7 to 53.5) | 55.3 (54.9 to 55.7) | |
| Consultant assessment 24 hours | 89.3 (88.6 to 90.0) | 90.2 (89.6 to 90.9) | 88.5 (87.8 to 89.2) | 79.9 (79.5 to 80.2) | 81.7 (81.4 to 82.0) | 84.4 (84.1 to 84.7) | |
| Nurse assessment 12 hours | 90.8 (90.1 to 91.4) | 92.7 (92.1 to 93.2) | 93.3 (92.7 to 93.8) | 85.9 (85.6 to 86.2) | 87.4 (87.1 to 87.7) | 88.9 (88.7 to 89.1) | |
| Nurse assessment 24 hours | 95.5 (95.1 to 96.0) | 95.6 (95.2 to 96.1) | 96.2 (95.7 to 96.6) | 92.9 (92.7 to 93.2) | 93.6 (93.4 to 93.8) | 94.3 (94.2 to 94.5) | |
| Physiotherapist assessment 24 hours | 57.4 (56.2 to 58.5) | 61.7 (60.5 to 62.8) | 64.7 (63.6 to 65.8) | 55.4 (55.0 to 55.8) | 56.9 (56.5 to 57.3) | 60.5 (60.1 to 60.9) | |
| Physiotherapist assessment 72 hours | 95.4 (95.0 to 95.8) | 95.0 (94.5 to 95.5) | 96.5 (96.0 to 96.9) | 95.8 (95.3 to 96.2) | 93.5 (93.3 to 93.7) | 93.3 (93.1 to 93.5) | 93.7 (93.5 to 93.9) |
| Occupational therapist 24 hours | 50.3 (49.1 to 51.4) | 57.0 (55.8 to 58.1) | 61.5 (60.3 to 62.6) | 44.9 (44.5 to 45.3) | 47.3 (46.9 to 47.7) | 53.1 (52.7 to 53.5) | |
| Occupational therapist 72 hours | 91.1 (90.5 to 91.8) | 94.6 (94.1 to 95.1) | 94.1 (93.6 to 94.7) | 87.4 (87.1 to 87.7) | 88.2 (88.0 to 88.5) | 89.6 (89.4 to 89.9) | |
| SaLT swallow 24 hours | 53.9 (52.2 to 55.7) | 54.1 (52.5 to 55.8) | 51.5 (49.8 to 53.3) | 48.0 (47.4 to 48.6) | 49.8 (49.2 to 50.4) | 54.4 (53.8 to 55.0) | |
| SaLT swallow 72 hours | 98.2 (97.9 to 98.4) | 89.5 (88.5 to 90.6) | 91.8 (90.9 to 92.7) | 88.4 (87.3 to 89.5) | 77.8 (77.3 to 78.3) | 81.5 (81.1 to 82.0) | 84.8 (84.4 to 85.3) |
| SaLT communication 24 hours | 46.4 (45.0 to 47.8) | 50.1 (48.7 to 51.5) | 50.7 (49.2 to 52.1) | 34.0 (33.4 to 34.6) | 37.1 (36.5 to 37.6) | 42.5 (42.0 to 43.1) | |
| SaLT communication 72 hours | 89.7 (88.8 to 90.5) | 93.0 (92.3 to 93.7) | 91.0 (90.2 to 91.8) | 76.9 (76.4 to 77.4) | 80.5 (80.0 to 80.9) | 84.9 (84.5 to 85.3) | |
Appendix 8 Study Steering Committee membership
Study Steering Committee as of 2017
-
Sir Roger Boyle (Chairperson).
-
Audrey Bowen (Professor of Neuropsychological Rehabilitation, University of Manchester).
-
Helen Cutting (Senior Project Manager at Strategic Clinical Networks, NHS England, London region).
-
David Davis (Clinical Informatics Advisor, Medical Directorate, NHS England).
-
Warren Heppolette (Strategic Director, Health & Social Care Reform, Greater Manchester).
-
Peter Huskinson (National Commercial Director, Specialised Commissioning, NHS England).
-
Sue Jowett (Reader in Health Economics, University of Birmingham).
-
Allan Kitt (Chief Officer, NHS South West Lincolnshire CCG).
-
Mark MacDonald (Senior Policy & Influencing Officer, Stroke Association).
-
Russell Mannion (Professor of Health Systems, Health Services Management Centre, University of Birmingham).
-
Ruth McDonald (Professor of Health Science Research and Policy, University of Manchester).
-
Peter Moore (Regional Director, Stroke Association, north-east England).
-
John Murray (lay member, London Stroke Research Network).
-
Anita Patel (Professor of Health Economics, Queen Mary University of London).
-
Norman Phillips (service user representative).
-
Sarah Rickard (Manager, Greater Manchester Stroke Operational Delivery Network).
-
Rod Sheaff (Professor of Health Services Research, University of Plymouth).
-
Dawn Smith (Chief Operating Officer, NHS Nottingham City CCG).
-
Sally Standley (Director, Cambridge University Health Partners).
-
Paul Trevatt (London Strategic Clinical Network Lead, Cardiovascular, End of Life Care).
-
Judith Williamson (lay member, NIHR Senior Leadership Team).
-
Rob Wilson (Associate Director for West Midlands Strategic Clinical Networks and Senate, West Midlands Cardiovascular SCN Manager).
Former members of the Steering Committee
-
Karen Helliwell.
-
Jo James.
-
Candy Jeffries.
-
Gareth Jones.
-
Sallie Mills-Lewis.
-
Andy Mitchell.
-
Tony O’Brien.
-
Liz Pope.
-
Corrine Ralph.
-
Janet Ratcliffe.
-
Julie Rigby.
-
Tony Whitfield.
List of abbreviations
- A&E
- accident and emergency
- AGG
- Greater Manchester Association of Clinical Commissioning Groups Governing Group
- C
- component
- CCG
- Clinical Commissioning Group
- CI
- confidence interval
- CLAHRC
- Collaboration for Leadership in Applied Health Research and Care
- CSC
- Comprehensive Stroke Centre
- DES
- discrete event simulation
- DSC
- District Stroke Centre
- EAG
- External Advisory Group
- EEAG
- External Expert Advisory Group
- ESD
- early supported discharge
- GLM
- generalised linear model
- GM
- Greater Manchester
- GMA
- Greater Manchester A
- GMB
- Greater Manchester B
- GMCCSN
- Greater Manchester and Cheshire Cardiac and Stroke Network
- GP
- general practitioner
- HASU
- Hyperacute Stroke Unit
- HES
- Hospital Episode Statistics
- HSDR
- Health Services and Delivery Research
- ICD-10
- International Classification of Diseases, 10th Revision
- IMD
- Index of Multiple Deprivation
- ITU
- Intensive Therapy Unit
- LOS
- length of stay
- MSC
- major system change
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NIHSS
- National Institutes of Health Stroke Scale
- NMB
- net monetary benefit
- ODN
- Operational Delivery Network
- ONS
- Office for National Statistics
- PCT
- Primary Care Trust
- PPI
- patient and public involvement
- PSC
- Primary Stroke Centre
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RoE
- rest of England
- RQ
- research question
- SCLG
- Stroke Clinical Leadership Group
- SCN
- Strategic Clinical Network
- SHA
- Strategic Health Authority
- SINAP
- Stroke Improvement National Audit Programme
- SLSR
- South London Stroke Register
- SSC
- Study Steering Commitee
- SSNAP
- Sentinel Stroke National Audit Programme
- SU
- Stroke Unit
- TIA
- transient ischaemic attack
- WTP
- willingness to pay
