Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 16/02/18. The contractual start date was in March 2017. The final report began editorial review in May 2018 and was accepted for publication in January 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Some of the authors of this report were co-authors on studies included in the systematic review but were not involved in the data extraction and quality assessment for these studies. Specifically, one or more authors have been involved with 17 out of 37 publications, across 4 out of 18 services based in London (Irene J Higginson, Wei Gao and Sara Booth), Hull (Sara Booth), Cambridge (Sara Booth, Morag Farquhar and Irene J Higginson) and Munich (Sara Booth). A committee search showed membership of the Health Technology Assessment (HTA) End of Life Care and Add on Studies that ended in February 2016 for Wei Gao, and membership of the Health Services and Delivery Research (HSDR) Commissioned Board 2009–15, HTA Efficient Study Designs 2015–16, HTA End of Life Care and Add on Studies and Service Delivery and Organisation (SDO) Studies Panel Member for Irene J Higginson.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Maddocks et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Chronic breathlessness
Breathlessness (also known as shortness of breath or dyspnoea) is a subjective experience around breathing discomfort that varies according to the sensation and intensity. 1,2 The experience is shaped by multiple interacting factors (i.e. physiological, psychological, social and environmental) and can lead to secondary physiological and behavioural responses. 1,2
Terminology and classification of breathlessness are evolving (Table 1). The terms ‘breathlessness’ and ‘dyspnoea’ are internationally recognised, but their definition does not reflect the nature of breathlessness that is persistent despite optimal disease management. Similarly, ‘episodic’ breathlessness has been used to highlight important spells of breathlessness that often drive hospital use,17 but with less emphasis on its often chronic duration. Terms such as ‘intractable’ and ‘refractory’ breathlessness better capture this feature of breathlessness and how challenging it can be to manage, but can also suggest a complete resistance to treatment, which is not always the case. More recent consensus work16 has since developed a definition of ‘chronic’ breathlessness that better reflects the impact on patient experience and disability, in addition to persistence despite optimal disease management.
| Term | Definition |
|---|---|
| Breathlessness or dyspnoea2 | A subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity |
| Intractable breathlessness3 | Breathlessness that persists despite treatment of the disease |
| Refractory breathlessness4,5 | Breathlessness that persists despite optimal treatment of the underlying condition |
| Chronic breathlessness6 | Episodes of breathlessness lasting > 3 months |
| Chronic refractory breathlessness7–9 | Chronic breathlessness that is refractory to treatments for the underlying condition |
| Episodic breathlessness10–14 | Severe worsening of breathlessness intensity or unpleasantness beyond usual fluctuations13–15 |
| Chronic breathlessness syndrome16 | Breathlessness that persists despite optimal treatment of the underlying pathophysiology and results in disability for the patient |
Given the lack of universal consensus, we use ‘chronic or refractory breathlessness’ in this evidence synthesis. In line with the recent consensus work, this reflects the impact of breathlessness that persists despite optimal management on patients, while also highlighting its relentless nature and how challenging it can be to manage.
Chronic or refractory breathlessness is one of the most common, burdensome and neglected symptoms affecting patients with advanced malignant and non-malignant conditions. 16,18,19 It affects over 2 million people in the UK, including up to 98% of the 1 million people diagnosed with moderate to severe chronic lung disease,20–22 more than half of the ≥ 200,000 people with incurable cancer, 70% of those with lung cancer,23 and half of the 2 million people with chronic heart failure. 23–26 Breathlessness is also found in people with end-stage renal and liver disease, neurological diseases, human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) and many autoimmune diseases. 23–26 The number of people affected by breathlessness will rise globally with population ageing and increasing multimorbidity. 27
Breathlessness can be very frightening for patients and families25,26,28–31 and can have a devastating impact on their lives, severely limiting well-being and quality of life (QoL). 2,32–34 It is associated with considerable anxiety, depression, fear, social isolation, deconditioning and disability,21,28,29,31,35,36 and shortened life expectancy. 37–39 The experience of breathlessness is often compounded by multiple and interacting symptoms including cough, pain, fatigue, anxiety and depression. 25,26,33,36,40,41 For informal carers of people with breathlessness, it can result in disrupted sleep, high levels of stress and caregiver burden,29 fewer positive caring experiences,29 and feelings of being isolated, unsupported by health-care professionals, and ill-prepared for acute exacerbations. 31,35,42,43 The experience of chronic breathlessness tends to increase as the disease progresses;44,45 therefore, it can function as a marker of overall symptom burden and deterioration. 25,46,47
Current treatment and service provision
Chronic breathlessness is one of the most frequent causes of emergency department attendance and prolonged hospital admission, and results in high health, social and informal care costs. 28,29,36,48–52 It was estimated that the total annual cost of respiratory disease in the UK was £11B in 2014, representing approximately 9% of the total economic burden of illness. 53 Despite this, there remain few effective treatments for chronic breathlessness, making it a major challenge in improving palliative and end-of-life care. 18,54–57
There are limited pharmacological treatments for chronic breathlessness43,58 and there are currently no licensed medicines for the treatment of this symptom anywhere in the world. 2,59–62 Systematic reviews of effectiveness and clinical trials are available for opioids, oxygen and benzodiazepines. 3,62–67 Orally administered opioids have been found to be beneficial in the treatment of breathlessness in patients with advanced disease, but the effects are modest or small61,62 and there are concerns regarding adverse cardiac and respiratory effects of long-term use, especially in older people with chronic obstructive pulmonary disease (COPD). 68–71 Oxygen has a clear and accepted role in treating mildly hypoxic patients. 72–74 However, the benefit derived from oxygen in mildly or non-hypoxaemic breathless patients is similar to medical air, and there are limitations to its use (e.g. safety, cost). 67,75,76 Benzodiazepines have been found to have no beneficial effect, with some evidence of possible harm. 58,65 Although there are patient case reports of the effectiveness of antidepressants to treat chronic breathlessness, controlled trials are lacking. 77–79 Both the European Respiratory Society (ERS) and the American Thoracic Society have concluded that there is not a robust evidence base for other pharmacological agents beyond oxygen and opioids. 2,59 In addition, pharmacological treatments do not address the underlying psychosocial problems, which also perpetuate the symptom. 80
Breathlessness can be effectively managed via non-pharmacological treatments that incorporate exercise, education and behavioural interventions. 81–83 Pulmonary or cardiac rehabilitation, a multidisciplinary programme of care comprising exercise-training and education (particularly around self-management) is widely known to improve symptom burden, functional status, physical fitness and health-related QoL. 81,82 However, for those with advanced disease, there are issues with referral, limited uptake and ability to sustain engagement because of social isolation, difficulties with travel, health deterioration, impeding symptoms and potential stigma. 4,28,54,84,85 Therefore, it is important to explore interventions that may work alongside, as a bridge to or, for some, as an alternative to rehabilitation interventions, whereby outcomes may be supported via alternative mechanisms.
Holistic breathlessness services
In response to these challenges with chronic breathlessness management, holistic breathlessness services have emerged for people with advanced disease. 86–88 There is no consensus on the definition of holistic breathlessness services; however, as complex interventions, these services can be described in terms of their setting, structure and content. Core features of holistic breathlessness services include:
-
drawing on multiple specialties, typically with input from multiprofessional palliative care (e.g. doctors, physiotherapists) with or without respiratory medicine, cardiology or oncology
-
delivery by multidisciplinary team members (e.g. physicians, nurses and allied health professionals)
-
use of both pharmacological and non-pharmacological therapies, selected following a holistic assessment of individual patient and caregiver needs (physical, psychological, social and spiritual)
-
emphasising self-management, through use of education and behaviour change techniques
-
targeting improvements in patient and caregiver QoL by reducing the impact of breathlessness and related symptoms on everyday living.
Holistic breathlessness services can be offered in community, outpatient or day hospice settings, and there is variation in the extent to which families or informal carers receive direct support.
Individual studies have reported positive outcomes from these services for patients and carers including higher levels of patient satisfaction, improved self-reported breathlessness mastery, and reduced patient distress, health-care contacts and need for informal care, without increasing the overall cost to the UK NHS. 89–95 One study also suggested a potential survival advantage. 94 Alongside this, international guidelines have advocated early integration of palliative care for people experiencing chronic disease,96,97 for which chronic and/or distressing breathlessness could be a suitable indicator for referral (particularly in non-cancer conditions in which prognostication causes delays54). Yet the evidence base to inform policy and practice is poorly understood.
Scoping and need for evidence synthesis
A scoping search was undertaken to identify the size, nature and range of existing evidence relating to holistic breathlessness services, and this informed the design of the evidence synthesis. In the decade following a positive report from a randomised controlled trial (RCT) of a nurse-led service published in 1999,98 evidence relating to these services was minimal and generally of low quality because it was limited to uncontrolled service evaluations. More robust evidence has emerged in recent years, underscoring the interest and relevance of this topic to health and social care. A scoping search from 2005 onwards identified four RCTs,91,94,95,99,100 as well as a number of prospective cohort studies,101,102 qualitative studies,103–107 narrative reviews or opinion pieces3,7,18,108–122 and consensus statements. 2,56,123,124 No systematic reviews have assessed the clinical effectiveness or cost-effectiveness of these services. Although not exhaustive, the scoping exercise highlighted the need for an evidence synthesis of holistic breathlessness services, to inform clinical practice, policy and research in the future.
Aim and objectives
This project aimed to provide a comprehensive and objective summary of the current available evidence for the clinical effectiveness and cost-effectiveness of holistic breathlessness services for people with advanced malignant and non-malignant disease.
The research objectives were to:
-
describe the available evidence for holistic breathlessness services in terms of the intervention format, content, organisation and context, patient characteristics, study design and quality, and outcomes measured
-
determine the clinical effectiveness of holistic breathlessness services on symptom burden, health status and QoL
-
determine the cost-effectiveness of holistic breathlessness services from patient/caregiver, societal and NHS perspectives
-
examine the acceptability of holistic breathlessness services from the perspective of health-care professionals and patients, considering rates of referral, uptake and adherence, as well as patient experience and satisfaction
-
using individual patient data, examine predictors of treatment response, including characteristics of participants (level of impairment, symptom burden, multimorbidity) and interventions (setting, duration, professional input, delivery)
-
using stakeholder consultation, elicit stakeholders’ priorities for clinical practice, policy and research around holistic breathlessness services, including their role and delivery in relation to cardiac and pulmonary rehabilitation services.
Research group
This project was led by the Cicely Saunders Institute of Palliative Care, Policy and Rehabilitation at King’s College London (KCL), with the following collaborating institutions: University of East Anglia, Royal Brompton & Harefield NHS Trust, University of Cambridge and King’s College Hospital NHS Foundation Trust.
The project advisory group (PAG) included a consortium of international leaders and clinical academics in palliative care and respiratory and rehabilitation research, as well as patient and carer representatives:
-
Dr Matthew Maddocks (Senior Lecturer in Health Services Research and Specialist Physiotherapist, KCL)
-
Professor Irene J Higginson [Professor of Palliative Care and Policy, Scientific Director of Cicely Saunders International and National Institute for Health Research (NIHR) Senior Investigator, KCL]
-
Ms Lisa Jane Brighton (Research assistant, KCL)
-
Dr Wei Gao (Senior Lecturer in statistics and epidemiology, KCL)
-
Dr Deokhee Yi (Health Economist, KCL)
-
Dr Sabrina Bajwah (Consultant and honorary senior lecturer, KCL)
-
Dr Sara Booth (Honorary Consultant and Associate Lecturer, University of Cambridge)
-
Dr Morag Farquhar (Senior Lecturer, University of East Anglia)
-
Dr William D-C Man (Senior Lecturer/Consultant Chest Physician, Imperial College London)
-
Dr Charles Reilly (Consultant Physiotherapist, King’s Health Partners)
-
Ms Lucy Fettes (Specialist Physiotherapist, St Joseph’s Hospice and KCL)
-
Ms Alanah Wilkinson (Research Administrator, KCL)
-
Dr Nicholas Hart (Clinical and Academic Director Lane Fox Respiratory Service, Guy’s & St Thomas’ NHS Foundation Trust/Professor in Respiratory and Critical Care Medicine)
-
Ms India Tunnard (Research Administrator, KCL)
-
Dr Sophie Miller (Specialty Training Registrar, St Christopher’s Hospice)
-
Mr Daniel Marion (patient/carer representative)
-
Ms Lesley Turner (patient/carer representative)
-
Mrs Colleen Ewart (patient/carer representative)
-
Mr Gerry Bennison (patient/carer representative)
-
Ms Margaret Ogden (patient/carer representative)
-
Mrs Sylvia Bailey (patient/carer representative).
Any changes to the protocol were reported to and approved by the PAG.
Chapter 2 Evidence synthesis methods
This project comprised an evidence synthesis of published and unpublished data through systematic review and a secondary analysis of pooled individual patient trial data, undertaken in conjunction with a transparent stakeholder consultation.
Systematic review methods
The systematic review considered quantitative, qualitative and economic studies to examine the clinical effectiveness, cost-effectiveness and acceptability of holistic breathlessness services.
Design and registration
The systematic review and meta-analyses were conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,125 and adhered to guidelines from the Centre for Reviews and Dissemination and Methodological Expectations of Cochrane Intervention Reviews. The review protocol was prospectively registered with PROSPERO (reference number CRD42017057508). 126
Eligibility criteria
The following inclusion criteria were applied.
-
Participants: adults experiencing breathlessness related to advanced disease (described as suffering from breathlessness, dyspnoea, shortness of breath, difficulty breathing, laboured breathing and with advanced stages of diseases with a high prevalence of breathlessness) including, but not limited to, cancer (advanced local or metastatic), chronic respiratory disease [Global initiative for chronic Obstructive Lung Disease (GOLD) stage III or IV/grade C or D], heart failure (New York Heart Association stage III or IV) or progressive neurological conditions. Studies in which ≥ 50% of participants met these definitions were included.
-
Interventions: as there is no standard definition, holistic breathlessness services were defined as services that draw on multiple specialties and disciplines, encompass pharmacological and non-pharmacological interventions selected on the basis of a holistic needs assessment, enrol patients because of their breathlessness (not their diagnosis), emphasise self-management, aim to reduce the perception and impact of breathlessness and related symptoms, and are offered in outpatient, community or day hospice settings.
-
Comparators: all comparators were considered for controlled studies, including no treatment, usual care, an attention control (e.g. a patient support group without a specific focus on breathlessness) or an active control (e.g. alternative service).
-
Outcomes: health outcomes included breathlessness intensity; breathlessness affect and impact;2 anxiety and depression; physical functioning; health status or QoL; and survival. Economic outcomes included formal health and social care service utilisation and costs, unpaid caregiver costs including caregivers’ time off work, quality-adjusted life-years (QALYs) derived from generic QoL measures [e.g. EuroQol-5 Dimensions (EQ-5D)] and perspectives of economic analysis. Acceptability outcomes included patient flow data (uptake, adherence, completion), as well as patient and caregiver perspectives on acceptability, satisfaction and/or experience.
-
Study design: RCTs with a parallel, single-stage or cross-over design, including studies using minimisation, non-randomised studies including prospective and retrospective designs, quantitative and qualitative designs to elicit patient and caregiver satisfaction and experience.
Studies were excluded if they did not specifically target patients with breathlessness, or if interventions that targeted breathlessness used only a single treatment (e.g. physical exercise). Pulmonary rehabilitation and disease-specific services (e.g. integrated respiratory care) were deemed outside the scope of this review. Interventions that exclusively targeted service providers or carers were also excluded. Narrative reviews, opinion papers, case studies and case series with fewer than five participants were excluded.
Search strategy
Electronic searches
The following electronic databases were searched from their respective inceptions up to 2 June 2017:
-
British Nursing Index (1985)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1980)
-
Cochrane Database of Systematic Reviews (CDSR)
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
Database of Abstracts of Reviews of Effects (DARE)
-
EMBASE (1980)
-
MEDLINE (1966)
-
PsycINFO (1985)
-
Science Citation Index Expanded (1985).
The search terms and strategy were developed and piloted with information specialists to ensure broad inclusivity. They were informed by literature scoping, MEDLINE medical subject heading terms and subject filters within specific databases. Subject headings and free-text terms were combined to search for population and intervention terms. The MEDLINE search strategy is shown in Appendix 1.
Hand-searching
To identify additional studies, reference lists of retrieved studies and relevant editorials and reviews, citations, textbooks and voluntary sector materials were searched. We contacted the corresponding authors of retrieved studies and active researchers to identify unpublished data or grey literature arising from meetings or conference proceedings. No language or publication status restrictions were imposed in the selection of evidence reports.
Screening of studies
Potentially eligible reports were imported into bibliographic software Endnote X7 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and duplicates removed. Two researchers (SM and LJB/MM) independently screened all titles and abstracts for relevance, and independently assessed full texts of potentially eligible studies for compliance with the review criteria. Disagreements were resolved by discussion between the screening team and wider team, until consensus on eligibility was reached.
Quality assessment
All included studies were independently assessed by two researchers (LJB and MM) for methodological and reporting quality, using standardised checklists, as outlined below. Information to aid quality assessment was obtained from primary, secondary and protocol articles.
Methodological quality
The Standard Quality Assessment Criteria for Evaluating Primary Research Papers (QualSyst)127 was used to assess the methodological quality of all studies. QualSyst contains two checklists with accompanying manuals to guide systematic quality assessment of quantitative and qualitative studies. For mixed-method studies, both checklists were used for the relevant component of the study and supplemented with three items that were specific to this design from the Mixed Methods Appraisal Tool. 128 Scores were summarised as a percentage score of applicable items.
Randomised controlled trials were also assessed for risk bias using the Cochrane Collaboration’s tool,129 which considers six domains: sequence generation, allocation concealment, blinding of study participants and personnel, completeness of outcome data, selective reporting, and other potential sources of bias. A judgement was made for the level of risk of bias (i.e. low, high or unclear) for each domain.
Methodological quality assessments of economic evaluations were informed by the British Medical Journal checklist for authors and peer-reviewers of economic submissions. 130 Thirty-five items in study design, data collection and analysis and interpretation of results were marked as yes, no, or not clear.
Reporting quality
Established checklists were used to assess the quality of reporting (Table 2).
| Study design | Tool(s) to assess reporting quality |
|---|---|
| RCTs | CONSORT statement131 |
| Pilot/feasibility trials | CONSORT extension for pilot and feasibility studies132 |
| Quasi-experimental | Adaptation of CONSORT statement using applicable items |
| Observational | STROBE statement133 |
| Qualitative | COREQ checklist134 |
| Mixed methods | COREQ plus the most appropriate quantitative checklist |
Quality of the evidence
The quality of the body of evidence for each clinical outcome was rated independently by two members of the research team (MM/LJB and MF) using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach,135 which considers study limitations, consistency of effect, imprecision, indirectness and publication bias. The final grade was reviewed by additional members of the PAG.
We decreased the grade if there was:
-
serious (–1) or very serious (–2) limitation to study quality
-
important inconsistency (–1)
-
some (–1) or major (–2) uncertainty about directness
-
imprecise or sparse data (–1)
-
a high probability of reporting bias (–1).
The following grades of evidence categories were then assigned for each outcome:
-
high – further research is very unlikely to change our confidence in the estimate of effect
-
moderate – further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
-
low – further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
-
very low – any estimate of effect is very uncertain.
Data synthesis/data extraction and analysis
Data on the characteristics of the service, outcomes and study information were extracted from each paper by one researcher (SM/LJB) using a predesigned electronic data capture form. These were checked by a second researcher to ensure rigour (LJB/MM). Authors were contacted if additional information was needed for meta-analyses. To increase validity and ensure comprehensiveness, the analysis and interpretation were reviewed by members of the PAG including researchers, patient/carer representatives and clinicians.
Structure, organisation and delivery/service characteristics
We described the overall content and organisational aspects of services to appraise the degree of consistency or heterogeneity. 136 Data on service characteristics were tabulated with details of the associated studies, including the intervention setting (i.e. home, community, hospital), duration of service involvement and frequency of patient contact, team members by profession/specialty, patient diagnoses, and component interventions. Component interventions were tabulated and summarised narratively.
Clinical effectiveness and cost-effectiveness
Only data from controlled studies were included to estimate effectiveness. Outcomes were analysed as continuous data when possible. Mean differences (MDs) or standardised mean differences between intervention and comparator groups were reported with 95% confidence intervals (CIs). When there were sufficient data and consistent or comparable outcomes, a meta-analysis was performed using random-effects models to estimate the overall direction, size and consistency of effects. Clinical heterogeneity assessed using the I2 statistic to quantify inconsistency across studies and the impact on the meta-analysis. 137 Separate sensitivity analyses were conducted excluding studies with a high risk of bias (< 70% QualSyst score), and removing outliers when substantial heterogeneity (I2 > 75%138) was present. In all cases, individual studies were represented only once within each analysis. We planned funnel plots to assess publication if ≥ 10 studies were included. 139 Additional findings were summarised narratively.
For health economic data, we planned to present types of economic analyses; describe the population, setting and intervention; present effect size and cost-effectiveness results for the high-quality economic evaluations;130 and perform a quantitative synthesis if sufficient data were available.
Patient acceptability and experience
Data were extracted on patient and caregiver perspectives on acceptability, satisfaction and/or experience, as well as process data regarding patient flow (including rates of referral, uptake, adherence to intervention and completion).
For qualitative or mixed-methods studies, all text (including quotations) under the headings of ‘results’ or ‘findings’ were imported verbatim into qualitative data analysis software [NVivo v12 (QSR International, Warrington, UK)]. A thematic synthesis of qualitative data involved three consecutive steps: (1) line-by-line coding of results of all included studies, (2) development of descriptive themes, incorporating themes or codes of primary studies, with particular attention to similarities and differences across and between studies and (3) new development of analytical themes, going beyond presentation of the original data. 140 The final coding frame was reviewed by members of the PAG with a variety of backgrounds (e.g. research, clinical, patient/carer representatives) to increase interpretive rigour. Findings from the quantitative surveys and patient flow data were tabulated and/or summarised narratively.
Responder analysis methods
Design
To identify patient characteristics that predict response to holistic breathlessness services, in terms of patient breathlessness mastery and distress due to breathlessness, we conducted a secondary analysis of pooled individual patient data from three RCTs. 91,94,95 These were a convenience sample of available data through project team members who were involved with, and act as, data custodians for these trials. As a result of collaborations during the trial designs, there was also a high number of variables in common across these data sets to aid pooling.
Data sources and ethics approvals
Trials included Higginson et al. ’s94 2014 trial of a 6-week service with people with advanced disease, Farquhar et al. ’s91 2014 trial of a 2-week service for people with advanced cancer and Farquhar et al. ’s95 2016 trial of a 4-week service for people with non-malignant disease. Participants in each trial had chronic or refractory breathlessness and advanced disease.
Anonymised individual patient data were obtained from the data custodians, and data sets were cleaned and harmonised. This secondary analysis of anonymised data did not require ethics approval. Each of the contributing studies followed appropriate ethics approval procedures (King’s College Hospital reference number 10/H0808/17; Cambridgeshire 2 NHS reference number 08/H0308/157).
Methods
Our study built on methods of a previous analysis of patient predictors of response to opioids. 141 The primary analysis considered an intervention response on Chronic Respiratory Disease Questionnaire (CRQ) mastery, defined by the minimal clinically important difference as an improvement of 0.5. 142 This outcome was selected as it was the primary outcome for the Higginson et al. 94 study, and a secondary outcome in the Farquhar et al. 91,95 studies.
The secondary analysis considered an intervention response on Numerical Rating Scale (NRS) distress due to breathlessness (henceforth, NRS distress), defined as an improvement of 1 point. 143 This was selected for secondary analysis as it was the primary outcome for both Farquhar et al. 91,95 studies, but not measured in the Higginson et al. 94 study.
Candidate variables (and their reference group/possible score ranges) for the primary and secondary analysis included diagnosis (reference group: COPD); predicted forced expiratory volume in 1 second (FEV1) and baseline scores for CRQ dyspnoea; CRQ fatigue; CRQ mastery and CRQ emotional function (1 to 7); EQ-5D Utility Index (–1 to 1) and EQ-5D visual analogue scale (VAS) (0 to 100); Hospital Anxiety and Depression Scale (HADS) anxiety and HADS depression (0 to 21); and NRS average breathlessness in the last 24 hours (0 to 10; henceforth, NRS average). As it was not measured in Higginson et al. ,94 baseline NRS distress (0 to 10) could be included only in the secondary analysis. For all variables, the time point immediately pre intervention for each group was treated as ‘baseline’ (e.g. in the Higginson et al. 94 trial, fast-track participants’ baseline was week 1, wait-list participants’ baseline was week 6).
Variables deemed significantly related to response (p < 0.05) in univariate logistic regression models were included in the multivariate analyses. Multivariate analyses comprised backward stepwise logistic regression, in which variables with the largest non-significant (p > 0.05) p-value in the model were sequentially removed. Dependent variables were assessed for multicollinearity. To control for age, sex (reference group: male) and study of origin (refence group: Farquhar et al. 95), these variables were forced into the multivariate analyses.
To aid interpretation, variables that were significant predictors of response were categorised (when possible, using previously established groupings for that measure). The percentages of participants classified as responders for each baseline category were then tabulated.
Transparent expert consultation methods
Design
Nominal group techniques are commonly used for consultation with stakeholders, but have been criticised for lacking transparency, reliability and opportunities for clarification. 93 Although the Delphi technique overcomes these issues, this method can be time-consuming with multiple rounds of consultation, and the initial content can be shaped by a minority. Transparent expert consultation (TEC) methods have, therefore, been developed in response to these limitations,93,144 and have been successfully used to elicit recommendations in palliative and end-of-life care research. 145–149
Our TEC comprised a stakeholder consultation and follow-up online consensus survey. Like nominal group technique, the stakeholder workshop provided structured opportunities for expression of views by a group of experts. This activity was followed by a wider online consensus survey to rate the suggested priorities (similar to a single-round Delphi technique), enabling rapid consultation of multiple key stakeholders.
Participants
A wide range of stakeholders involved in the provision of care for people with chronic breathlessness were purposively selected and invited to participate in the stakeholder workshop. Service providers and commissioners, voluntary sector organisation representatives, patient and carer representatives, and health and social care practitioners from a range of specialties and professional groups were identified through contact lists of people and organisations that the research team had previously worked with, online literature and website searches, and via recommendations from participants.
The PAG members, workshop participants and those who expressed interested but were unable to attend the stakeholder workshop, were invited to complete the online consensus survey. Additional individuals from groups that were under-represented at the workshop (including service users) were also purposively invited to complete the consensus survey, using the methods described above. All participants were adults with capacity to give informed consent.
Ethics, recruitment and informed consent
Ethics approval for this study was obtained from the KCL research ethics committee (reference number LRS-16/17-4692).
A personal e-mail invitation to the stakeholder workshop was sent to purposively selected stakeholders, including a copy of the information sheet, the consent form and the workshop schedule (see Appendix 2). This provided an opportunity for attendees to consider the study information prior to the day of the workshop, at which time they were asked to provide written consent prior to the recorded discussions and data collection. Participants were reimbursed reasonable travel costs to attend the stakeholder workshop.
For the online survey, information regarding its purpose and how the data would be used was included in an attachment to the invitation e-mail and summarised at the start of the survey. Consent for the online survey was presumed through participation. A hard-copy postal consensus survey with a freepost return envelope was available to participants preferring that format.
Procedure
Identifying critical questions
The findings of the systematic review around the service components, clinical effectiveness, cost-effectiveness and acceptability of holistic breathlessness services for people with chronic breathlessness in advanced disease were used to generate critical questions for consideration during the stakeholder workshop. Through discussion of the review findings with the PAG, three critical questions were identified:
-
How do we define and deliver ‘holistic breathlessness services’?
-
How and where can holistic breathlessness services be integrated into current practice?
-
How should the success of holistic breathlessness services be measured/monitored?
Transparent expert consultation workshop
The TEC workshop took place at the Cicely Saunders Institute of Palliative Care, Policy and Rehabilitation, KCL, on October 4 2017. Participants received a pack on arrival containing the information sheet, consent form and schedule for the day.
The workshop format began with whole-group presentations and discussion. The first of these presented results from the systematic review, including data on the make-up or services, common components, preliminary quantitative outcome data and qualitative data on the acceptability of breathlessness services. In addition, evidence was presented by experts on rehabilitation services, care bundles, and supporting informal carers of people with chronic breathlessness (see Appendix 2).
In parallel groups, workshop participants considered one of the three critical questions identified from the review. Participants were allocated to groups to ensure diversity of experience and roles within each. These sessions used a nominal group technique, facilitated by members of the research team using a structured process developed before the workshop (Table 3). This included completion of individual response booklets to gather individual thoughts and suggestions. Parallel-group sessions were also audio-recorded, and scribes recorded the top priorities on flip chart paper for each parallel group and to share with the wider group. 150 A live graphic recording of the whole-group and parallel-group discussions was created by an artist who was present throughout the event. The workshop closed with a summary of the day and information about the follow-on online consensus survey.
| Step | Process |
|---|---|
| Written responses | Participants wrote individual answers to ‘prompt questions’ in response booklets. These were tailored to the critical question each group was focusing on, for example ‘What are the core components of a holistic breathlessness service?’ (group 1); ‘Where should a holistic breathlessness service be based?’ (group 2); and ‘What is the ideal set of outcomes to measure for patients?’ (group 3) |
| Initial reflections | Reflections from this exercise in relation to the critical question were then discussed |
| Individual suggestions | Participants wrote individual suggestions for future practice in their response booklets, with a rationale and indication of relevance for clinical practice, policy and/or research |
| Ranking | Participants were asked to rank each of their own suggestions from highest to lowest |
| Discussion | Participants in turn read out their highest ranked suggestions and rationale, which were discussed by the group. This continued until individual lists were exhausted or time was exceeded149 |
The materials generated from the discussions throughout the day were reviewed and summarised into main themes by one researcher (LJB), including those from whole-group discussions and each parallel group. This was primarily based on the written notes, with reference to the recording when further clarification was needed. The narrative summary of salient and common points was reviewed by members of the research team to ensure an accurate and transparent reflection of the workshop discussions.
Online consensus survey
Participants’ suggestions generated from the workshop, their rational, ranking and grouping, were anonymised and entered in a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet. Two members of the research team (LJB, MM) categorised the statements as relevant to clinical practice, research or policy. For instances in which participants felt that a suggestion was relevant to more than one category, it was assigned to the predominant category. Statements were ranked from most to least important, as reported by participants.
After multiple readings of the material, statements were synthesised and deduplicated. Stakeholders’ suggestions were not retained if they were deemed to be duplicates of other statements generated in this exercise, redundant (e.g. research studies known to have been conducted), unclear, outside the scope (e.g. not specific to chronic breathlessness in advanced disease) and/or ranked as low priority by the participant who wrote it. The graphic and audio-recordings, scribes’ notes and flip chart records were referred to when clarification was needed. Statements retained participants’ original language whenever possible, with amendments only to enhance clarity and avoid inflexible statements (e.g. changing ‘must’ to ‘could’). 145,147
The final list of participants’ suggestions was discussed and refined with the PAG, before being formatted into an online survey. Within the survey, three sets of participants’ suggestions relevant to research, clinical practice and policy were presented, and respondents were asked to indicate their level of agreement with each statement from 1 (strongly disagree) to 9 (strongly agree). Survey respondents were given the opportunity for free-text comments in each section and asked to select their profession/role and area(s) of expertise, with a free-text ‘other’ option if required. 144,149,151 The survey was piloted by clinical, research and patient representative members of the PAG to ensure that it was clear and user-friendly.
The survey ran for a period of 2 weeks, from 12 to 26 February 2018. Potential participants were sent a personalised e-mail invitation, followed by two reminders, to complete the online consensus survey.
Analysis
Survey responses were analysed using descriptive statistics [frequencies, median, interquartile range (IQR), range]. Predetermined categories from previous TECs were used to determine levels of agreement and consensus146,147 (Table 4). Narrative comments were collated within each category and thematically analysed to aid understanding and provide illustrative examples of the issues raised by stakeholders’ suggestions. 152
| Median | IQR | Category |
|---|---|---|
| ≥ 8 | < 2 | Strong agreement/high consensus |
| ≥ 8 | ≥ 2 | Strong agreement/low consensus |
| < 8 to > 6 | < 2 | Moderate agreement/high consensus |
| < 8 to > 6 | ≥ 2 | Moderate agreement/low consensus |
| ≥ 4 to ≤ 6 | < 2 | No agreement/high consensus |
| ≥ 4 to ≤ 6 | ≥ 2 | No agreement/low consensus |
| < 4 to > 2 | < 2 | Moderate disagreement/high consensus |
| < 4 to > 2 | ≥ 2 | Moderate disagreement/low consensus |
| ≤ 2 | < 2 | Strong disagreement/high consensus |
| ≤ 2 | ≥ 2 | Strong disagreement/low consensus |
Patient and public involvement
In addition to the patient and public involvement (PPI) collaborators involved with the original grant application, four additional members were identified and invited to join the research team via the Cicely Saunders Institute Patient and Public Involvement Group. All were given an informal ‘role description’ to introduce them to the Institute, the project and our intentions for PPI.
The aim of PPI throughout this project was to ensure acceptable and appropriate research processes where patient/carers would be participating, include patient/carer voices throughout our consultation with stakeholders, ensure interpretation of findings were grounded in patient/carer experiences, and improve clarity and reach of dissemination.
The PPI involvement in this study used a mixture of face-to-face methods (e.g. inclusion in project advisory meetings) and remote methods (e.g. via telephone and e-mail). Involvement was flexible, with each member being more or less involved at different points in the study, in line with their availability and/or current health. The PPI members were included as members of the project team and invited to all project advisory meetings. They were invited to comment on the plain English summary and study plans, with a particular focus on recruiting PPI members for the TEC and ensuring acceptability of the workshop and online survey methods. They were involved in commenting on study findings, considering the meaning of results, and write-up and dissemination of materials and reports.
We reimbursed PPI members’ expenses and time, in accordance with NIHR INVOLVE guidance. 153 PPI members were supported by the research assistant as a key contact throughout their involvement. At the end of the study, we reflected on PPI involvement using the Guidance for Reporting Involvement of Patients and the Public 2 (GRIPP2) short form and sought feedback from the PPI members in order to share learning from our experiences.
Chapter 3 Systematic review results
Following screening of 3239 unique titles/abstracts and 56 full papers, 37 papers were eligible for inclusion in the review (see Appendix 3 for the PRISMA flow chart). Included papers were published between 1996 and 2017 (27 since 2010) and relate to 18 separate holistic breathlessness services: 12 based in the UK, three in Canada and one each in Australia, Germany and Hong Kong. Thirty-three articles were included in the descriptive synthesis (see Appendix 4).
Data from 12 studies (11 RCTs90,91,94,95,98–100,154–157 and one quasi-experimental design156) of seven different services were included in the quantitative synthesis (see Appendix 4 for a description of all included studies). Five RCTs were designed as pilot/feasibility studies,90,99,155,157 and seven as effectiveness studies. 91,94,95,98,100,154,155 Nine studies90,91,95,98,155–158 compared the services to usual care, and two compared one versus three contacts with a service. 99,100 In one study,90 the control group were not offered training or counselling, but were encouraged to talk freely about their breathlessness and disease.
Nine studies enrolled only cancer patients,90,91,98–100,155–157 two enrolled only patients with non-malignant disease95 or COPD,154 and one study enrolled patients with any advanced disease. 94 Of the 979 total patients recruited (range 2299 to 156100), there were 757 (77.3%) with advanced cancer and 180 (18.4%) with advanced COPD; the remaining participants (4.3%) had other non-malignant diseases, including interstitial lung disease or heart failure. The controlled studies assessed a wide variety of outcome measures (see Appendix 5), the most common being breathlessness intensity,89,90,94,99,154–158 distress due to breathlessness,90,91,95,98,99,155–158 and anxiety and depression. 90,91,94,95,98,155–157
The thematic synthesis included qualitative and quantitative experience data. Qualitative data were from five services, reported across 12 papers,88,90,91,94,95,102–105,159–161 (six mixed-method studies90,91,94,95,102,103,161 and five qualitative studies88,104,105,159,160) (see Appendix 4). Almost all data were from interviews with patients and/or carers,88,90,91,94,95,102,104,105,159,160 except for one using free-text responses to a postal survey103 and one using therapist notes. 161 Altogether, this included data from 167 patients (53.9% with cancer) and up to 49 carers. Quantitative data around satisfaction with, or experiences of, the services were reported in seven studies, which represented the views of 543 patients (60.77% with cancer). 99,100,103,157,161,162,168
Study quality
Quality assessment scores for the studies included in the quantitative synthesis ranged from 35% to 100% (median 90.4%; see Appendix 6). Studies for which only an abstract was available received lower scores. 154–156 All studies were deemed at risk of detection bias and most at risk of performance bias, owing to the nature of the intervention that prohibited patient blinding and relied on primarily self-assessed outcomes (see Appendix 7). Just three studies reported blinding of investigators. 91,94,95
Quality assessment scores for qualitative studies ranged from 40% to 85% (median 70%; see Appendix 8). The majority of studies had clear descriptions of their objectives, with an appropriate design including methods that were clear and systematic. However, the common limitations within the qualitative studies included lack of procedures to establish credibility of the data (e.g. triangulation, member checking), and unclear reporting of the analytic methods. Moreover, none of the included studies demonstrated reflexivity in their reports by reflecting on the impact of the researchers’ own personal characteristics on the data.
For the mixed-methods studies (n = 7), quality assessment items showed that, although this design was always clearly appropriate for the aims of the study, few (n = 2) reflected on the potential limitations of integrating qualitative and quantitative data (see Appendix 9).
For studies containing economic evaluations (n = 4), quality scores ranged from 64% to 77%. Commonly low-scoring items included having a clear statement and justification for the economic viewpoint, information on the evaluation method used, and a discussion around the relevance of productivity changes (see Appendix 10).
Reporting quality
Reporting quality overall was reasonably high for RCTs and pilot/feasibility trials, and generally poorer for quasi-experimental and qualitative studies (Table 5).
| Study design | Reporting quality checklist | Papers assessed (n) | Score median (%) | Score range (%) |
|---|---|---|---|---|
| RCTs | CONSORT statement131 | 5 | 81 | 53–92 |
| Pilot/feasibility trials | CONSORT extension for pilot and feasibility studies132 | 4 | 71 | 31–79 |
| Quasi-experimental | Adaptation of CONSORT statement using applicable items | 4 | 52 | 35–73 |
| Observational | STROBE statement133 | 1 | 84 | – |
| Qualitative | COREQ checklist134 | 10 | 46 | 22–68 |
Across all the experimental designs, there was limited reporting around harms and unintended consequences, or reflection on generalisability of the study findings. For RCTs and quasi-experimental studies, it was often not explicitly stated whether or not important changes had been made to methods and outcomes, or what their processes were for interim analysis and/or stopping guidelines. For quasi-experimental studies, few reported specific objectives or hypotheses, or clarified their primary and secondary outcomes. In the pilot/feasibility studies, papers often failed to define the methods and assessment measures for each study objective, or the criteria they used to judge whether or not to proceed with a definitive trial.
Papers scored with the qualitative reporting checklist were mainly mixed-methods reports, and only three were purely qualitative papers. Information frequently missing included details of the researcher and interviewer, contextual factors surrounding interviews (e.g. prior relationships between researchers and participants, and whether or not others were present during the interview), and elements with the potential to increase rigour (e.g. whether or not field notes were made, and whether or not participants commented on the transcripts and/or findings). These papers also tended not to report interview durations or full coding trees and did not reflect on data saturation or diverse cases/minor themes.
In all cases, papers published prior to the reporting checklists tended to score lower. For full reporting quality scores for each study design, see Appendix 11.
Service characteristics
Services were delivered by doctors, nurses, physiotherapists and occupational therapists, with involvement from the specialisms of palliative care, respiratory care and oncology. Most services (12/18) were short term and usually delivered to people with advanced cancer over a period of 4–6 weeks (range 1–12 weeks) via a mixture of face-to-face and telephone contacts (typically 4–6 contacts, range 1–12 contacts; see Appendix 5).
Services incorporated a wide range of intervention components, including information and education, psychosocial support, self-management strategies and other interventions (Table 6). Components most commonly included in services were breathing techniques (14/18), psychological support (12/18) and relaxation or calming techniques (11/18). A minority of services (≤ 2/18) included acupressure or transcutaneous electrical nerve stimulation, spiritual support, information on sleep hygiene and smoking cessation advice or support.
| Intervention | n | Servicesa |
|---|---|---|
| Information and education | ||
| Education/advice | 9 | 90,94,95,157,162–166 |
| Nutritional advice/support | 3 | 95,164,167 |
| Sleep hygiene | 2 | 95,168 |
| Smoking cessation advice/support | 1 | 95 |
| Written information | 4 | 94,95,164,166 |
| Psychosocial support | ||
| Carer/family support | 5 | 90,94,95,162,166 |
| Psychological support | 12 | 90,94,95,101,154,156,161,162,164–167 |
| Social support | 7 | 90,94,95,154,156,164,166 |
| Spiritual support | 1 | 94 |
| Self-management strategies | ||
| Breathing techniques | 14 | 90,94,95,100,101,154,156,157,161,163,164,166,168,169 |
| Emergency/crisis planning | 3 | 94,95,166 |
| Exercise plans | 5 | 94,95,164,166,170 |
| Handheld fan/water spray | 5 | 94,95,101,164,166 |
| Goal-setting | 4 | 90,95,101,166 |
| Pacing | 8 | 94,95,100,101,161,164,166,167 |
| Positioning | 4 | 94,95,164,166 |
| Relaxation/calming techniques | 11 | 90,94,95,100,101,154,161,164,166,167,169 |
| Other interventions | ||
| Acupressure/transcutaneous electrical nerve stimulation | 2 | 157,170 |
| Occupational aids | 5 | 94,95,164,166,170 |
| Pharmacological review | 4 | 94,95,168,169 |
Clinical effectiveness
Breathlessness severity
Ten studies90,94,98–100,154–157 assessed the severity of breathlessness using one or more of the following measures: VAS, NRS or Borg scores (Table 7). For ‘best breathlessness’, two studies using VAS found a greater improvement in the intervention group than in the control (differences in median change 5.7, p = 0.03,98 and 1.0, p = 0.0290), and three studies with unspecified measures found a significant intervention effect [F(2,44) = 5.30; p = 0.009]156 or no difference (data not reported). 155,156 For ‘worst breathlessness’, one study using VAS found a greater improvement in the intervention group than in the control (difference in median change 3.5; p = 0.0590), whereas no significant differences were found by two studies using NRS (MD –0.35, 95% CI –1.71 to 1.01; p = 0.6194; MD 0.41, 95% CI –0.86 to 1.67; p = 0.53157), one study using VAS (difference in median change 3.8; p = 0.1498) and one with an unspecified measure155 (data not reported). For ‘average breathlessness’, one study using an unspecified measure found a greater improvement in the intervention group than in the control (difference in mean change 1.2155), whereas two studies using NRS did not (MD –0.33, 95% CI –1.28 to 0.62; p = 0.49;94 MD 0.65, 95% CI –0.49 to 1.80; p = 0.26). 157 One study using NRS found no effect on breathlessness on exertion (MD –0.73, 95% CI –1.69 to 0.22; p = 0.13)94 and one study using Borg scale ratings for breathlessness at rest and on exertion found no difference between groups (data not reported). 154 In line with their feasibility study results,99 a powered trial comparing one with three service contacts found no significant difference in NRS worst (MD 0.2, 95% CI −2.31 to 2.97; p = 0.83) or average (MD 0.3, 95% CI –2.00 to 2.62; p = 0.79) breathlessness. 158
| Descriptor | Scale | Timeframe | Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barton et al.99 | Bredin et al.98 | Corner et al.90 | Higginson et al.94 | Johnson et al.158 | aPearce et al.154 | aYates et al.156 | aYates et al.155 | Yorke et al.157 | |||
| Best | VAS | Previous week | ✗ | ||||||||
| Unknown | ✗ | ||||||||||
| Unknown | Unknown | ✗ | ✗ | ||||||||
| Worst | VAS | Previous week | ✗ | ||||||||
| Unknown | ✗ | ||||||||||
| NRS | Previous 24 hours | ✗ | ✗ | ✗ | ✗ | ||||||
| Unknown | Unknown | ✗ | |||||||||
| Average | NRS | – | ✗ | ✗ | ✗ | ||||||
| Previous 24 hours | ✗ | ||||||||||
| Unknown | Unknown | ✗ | |||||||||
| On exertion | Borg | – | ✗ | ||||||||
| NRS | Previous 24 hours | ✗ | |||||||||
| At rest | Borg | – | ✗ | ||||||||
| Now | NRS | – | ✗ | ||||||||
Breathlessness affect
Ten studies90,91,95,98–100,155–157 assessed ‘distress due to breathlessness’ using VAS (range 0–100, higher = worse) or NRS (range 0–10, higher = worse). 91,95 Eight of these studies compared breathlessness services to usual care, three of which155,156 reported no significant difference but did not provide data. Data from five studies90,91,95,98,157 were combined in a meta-analysis (n = 324; Figure 1) that showed significantly lower NRS distress following the intervention than following the control (MD –2.30, 95% CI –4.43 to –0.16; p = 0.03). A sensitivity analysis excluding two outlier studies90,98 resulted in a reduced point estimate of effect and non-significant difference (MD –0.29, 95% CI –1.00 to 0.43; p = 0.43; I2 = 0%). Similar to the finding from the preceding feasibility study,99 a RCT testing service variations found no difference on NRS ‘coping with breathlessness’ (MD –1.7, 95% CI –4.27 to 0.90; p = 0.20),100 and significantly higher NRS distress due to breathlessness following three sessions versus one session (MD 3.9, 95% CI 0.98 to 6.91; p = 0.01). 100
FIGURE 1.
The NRS distress due to breathlessness. df, degrees of freedom; IV, inverse variable. Reproduced with permission from Brighton et al. 92 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
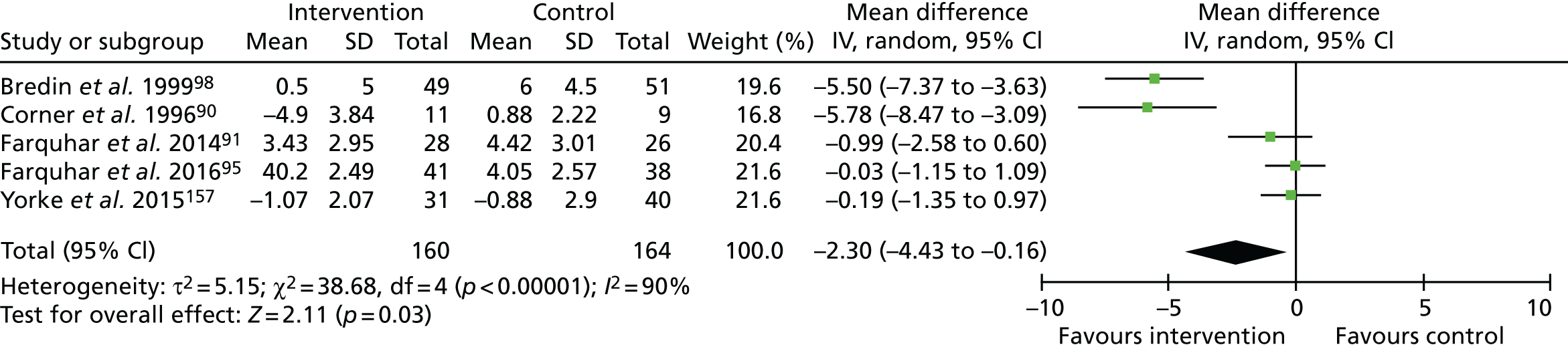
The quality of evidence for distress due to breathlessness was rated as very low. Owing to the lack of blinding of participants or outcome assessors, plus evidence of (or unclear information regarding) attrition bias, the evidence was downgraded for risk of bias. This evidence was also downgraded for inconsistency (owing to wide variation in effect estimates across studies), and imprecision (evidenced by wide variance in point estimates and lower 95% CIs, indicating potentially very little effect).
Four studies91,94,95,154 assessed ‘mastery over breathlessness’ using the CRQ mastery domain. 94,154 A meta-analysis of these data (n = 259; Figure 2) showed a statistically non-significant increase in mastery (range 1–7, higher = better) favouring the intervention (MD 0.23, 95% CI –0.10 to 0.55; p = 0.17). A sensitivity analysis excluding one study,154 deemed to be at a high risk of bias, increased the point estimate of effect (MD 0.30, 95% CI –0.06 to 0.66; p = 0.11). One study found significantly lower mastery scores after three service contacts than after one service contact (MD –0.6, 95% CI –1.06 to –0.11; p = 0.02). 100
FIGURE 2.
The CRQ breathlessness mastery. df, degrees of freedom; IV, inverse variable. Reproduced with permission from Brighton et al. 92 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
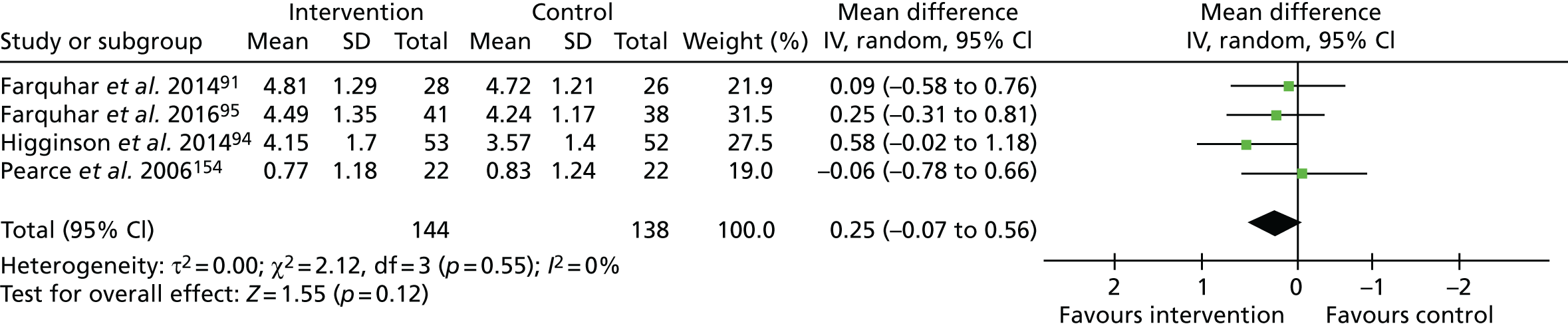
One further study found improved dyspnoea-12 (range 0–36; higher = worse) scores with the intervention than with control (MD 5.19, 95% CI 0.62 to 9.75; p = 0.026). 157
We judged the quality of evidence for mastery to be low. This evidence was downgraded because we deemed the studies to have a high risk of bias due to the lack of blinding of participants or outcome assessors, plus the evidence of (or unclear information regarding) attrition bias. In addition, we downgraded this evidence for imprecision because of the wide variance in point estimates and lower 95% CIs, indicating potentially very little effect.
Psychological outcomes
Seven studies assessed anxiety and depression using HADS. 90,91,94,95,98,155,157 Data from these seven studies (n = 552, Figure 3) showed a statistically non-significant reduction in anxiety scores (range 0–21; higher = worse), favouring the intervention (MD –1.59, 95% CI –3.22 to 0.05; p = 0.06). The sensitivity analysis, excluding one study155 deemed to be at a high risk of bias, increased the point estimate (–1.85, 95% CI –3.76 to 0.06; p = 0.06). A sensitivity analysis removing one outlier study98 resulted in a reduced point estimate but statistically significant group difference (MD –0.66, 95% CI –1.23 to –0.10; p = 0.02; I2 = 0%). No statistical differences in anxiety were reported when comparing one and three contacts. 99,100
FIGURE 3.
The HADS – anxiety. df, degrees of freedom; IV, inverse variable. Reproduced with permission from Brighton et al. 92 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for or any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
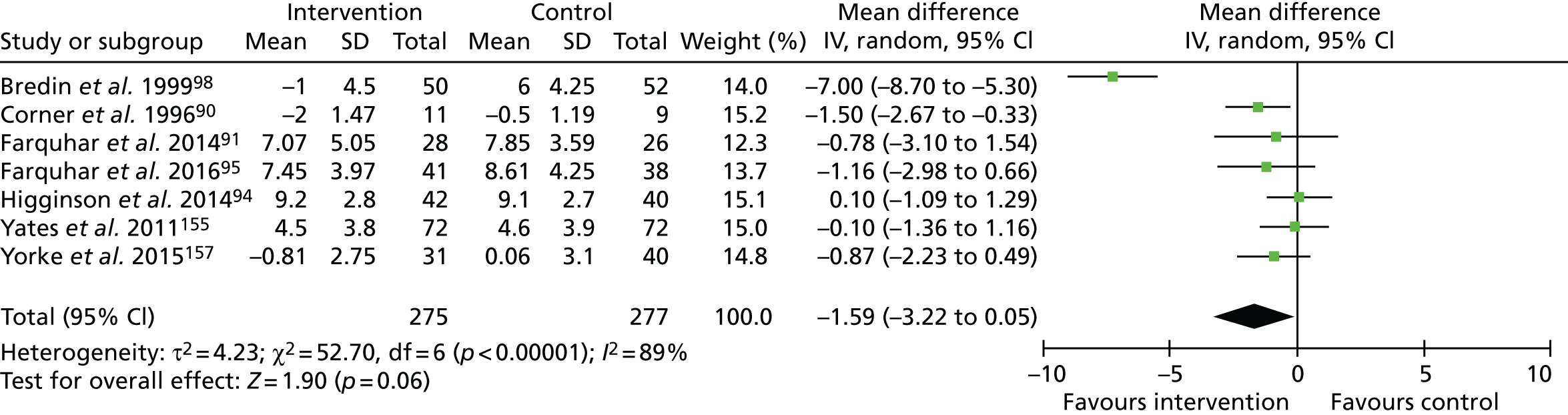
We rated the quality of evidence for anxiety to be very low. This evidence was downgraded because of the risk of bias, including the lack of blinding of participants or outcome assessors, plus the evidence of (or unclear information regarding) attrition bias. We also downgraded for inconsistency (owing to wide variation in effect estimates across studies), and imprecision (evidenced by wide variance in point estimates, and lower 95% CIs indicating potentially very little effect).
For depression, one study156 reported no difference between groups but did not provide data. Meta-analysis using the six remaining studies (n = 408, Figure 4) showed reduced depression scores (range 0–21, higher = worse) favouring the intervention (MD –1.67, 95% CI –2.52 to –0.81; p < 0.001). No statistical differences in depression were reported when comparing one and three contacts. 99,100
FIGURE 4.
The HADS – depression. df, degrees of freedom; IV, inverse variable. Reproduced with permission from Brighton et al. 92 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
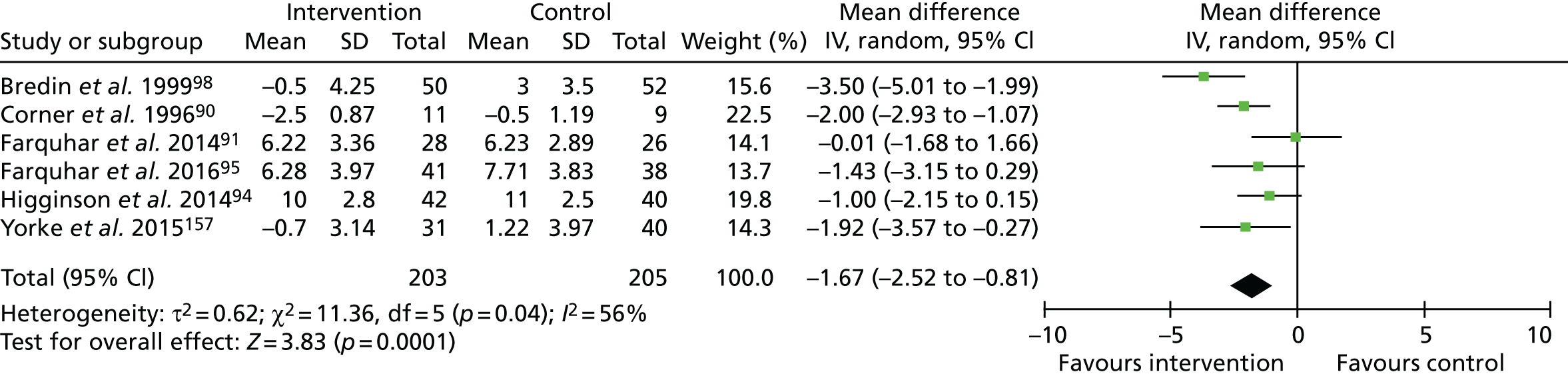
The quality of evidence for depression was judged to be moderate. This evidence was downgraded for risk of bias only, indicated by the lack of blinding of participants or outcome assessors, plus the evidence of (or unclear information regarding) attrition bias.
Three further studies reported no significant differences between the intervention and control groups in ‘psychological symptoms’: two using an unspecified measure (data not reported)155,156 and one using the Rotterdam Symptom Checklist (range 7–28, higher = worse; difference in median change –8; p = 0.21). 98 One study comparing one session with three sessions found no significant difference on CRQ emotion scores (MD –0.09, 95% CI –0.54 to 0.36; p = 0.69). 100
Physical function and health status/quality of life
Five studies90,91,94,95,98 assessed physical function. Two studies found greater improvements following the intervention than in the control participants using the Functional Capacity Scale (range 0–14; higher = better; MD for change 1.25; p < 0.02)90 and World Health Organization (WHO) Performance Status Scale (range 0–5, higher = worse; difference in median change 2, p = 0.02). 98 Three studies observed no difference in functional outcomes between groups assessed using the London Chest Activities of Daily Living Scale (MD –5, 95% CI –12.22 to 1.02; p = 0.10)94 or patient-reported number of times leaving their house (data not reported). 91,95
Seven studies91,94,95,98,100,154,157 included a measure of health status or QoL, none of which found significant differences between groups across the CRQ dyspnoea domain91,94,95,154 (including the comparison between one and three sessions100) or total score,94 EQ-5D index94 or VAS,94,157 and the Rotterdam Symptom Scale QoL domain. 98 Owing to heterogeneous measures, change from baseline and post-intervention scores, and cases of non-normally distributed data, we decided against meta-analysis for these outcomes.
Survival
Two studies reported survival data. 94,98 One found a significant difference in survival (generalised Wilcoxon score of 3.9; p = 0.048) in favour of the intervention. 94 Subgroup analysis found that the difference was driven by participants with non-cancer diagnoses. The remaining study, enrolling only patients with cancer, found no difference in survival across groups (data not reported). 98
Cost-effectiveness
Economic evaluation
Evidence for cost-effectiveness of the interventions was limited and non-conclusive. Four studies of three services were eligible for inclusion,91,94,95 and all used GBP 2011/12 costs. Three studies compared the service with usual care, and one study compared offering one session with offering three sessions within a service.
The perspective of the analysis for Farquhar et al. ’s91 study of their Breathlessness Intervention Service (BIS) was societal by including costs of informal care. Among patients with advanced cancer, total health/social costs including informal care for 8 weeks prior to the baseline assessment were £6137 [standard deviation (SD) £6099] in the BIS group and £5461 (SD £6099) in the usual care group. Costs between baseline and follow-up at 2 weeks were £794 (SD £866) for BIS and £1121 (SD £1635) for usual care. Intervention costs for the BIS were £119 (SD £62). Total costs were £354 lower for the BIS than for usual care (95% CI –£1020 to £246) and incremental QALY gain was 0.0002 (95% CI –0.001 to 0.002) after controlling for baseline. The chance of the BIS having lower total costs and resulting in a greater QALY gain than usual care was 80.9% according to cost-effectiveness planes. There was a 50.9% chance of the BIS being lower than usual care for total costs, and having a greater number of QALYs.
For Farquhar et al. ’s95 study of their BIS, a NHS perspective was used. Among patients with advanced non-malignant disease, total health/social costs for 8 weeks prior to the baseline assessment were £1952 (SD £3290) in the BIS group and £3630 (SD £5588) in the usual care group. Costs between baseline and follow-up at 4 weeks were £1371 (SD £2948) for BIS and £659 (SD £1253) for usual care. Intervention costs for BIS were £156 (SD £80). After adjusting for baseline values, total costs were £799 higher for BIS (95% CI –£237 to £1904) and the BIS group gained 0.003 extra QALYs (95% CI –0.001 to 0.007) than the control group. The cost per QALY for BIS was £266,333. The chance of BIS having lower total costs and resulting in a greater QALY gain than usual care was 7% according to cost-effectiveness planes.
Higginson et al. ’s94 Breathlessness Support Service (BSS) was assessed in terms of hospital inpatient days and formal care costs for 12 weeks prior to the baseline assessment. The cost of formal care was £2911 (SD £2729) for BSS and £3709 (SD £4484) for usual care. Incremental QALY gain between baseline and follow-up at 6 weeks was 0.092 (95% CI –0.23 to 0.04). No more information related to economic evaluation was reported in this paper, but a separate paper51 analysed the same data to measure the cost of care, regardless of randomisation. The total cost for all participants was £11,507 (SD £9911), which was the sum of health-care costs [£2624 (SD £3456)], social care costs [£628 (SD £1132)] and informal care costs [£8254 (SD £8777)]. Increased breathlessness on exertion was associated with higher health-care costs and informal care costs, and having a carer was associated with higher informal care costs.
Johnson et al. ’s158 trial compared one session with three sessions of a breathlessness service from a NHS perspective, including costs of the service in each arm and other health-related resource use costs. For the three-session service, there was a non-significant reduction in overall QALYs (MD –0.006, 95% CI –0.018 to 0.006) and non-significant increase in costs. There was no evidence that the additional costs were offset by lower health-related resource use costs elsewhere. The probability of the single session being cost-effective at a threshold value of £20,000 per QALY was > 80%. 100
Referral, uptake, adherence and adverse events
Of the 11 RCTs included in the analysis, fewer than half reported numbers screened for eligibility. When available, studies reported screening between 53 and 932 people for eligibility, of whom 15% to 48.6% were deemed to be eligible for inclusion. The total number of participants randomised into each trial ranged from 22 to 156. Of the participants randomised, between 50% and 90.8% of participants completed the trial to key outcome stages. Full data including reasons for attrition are described in Table 8. No adverse events were reported.
| Study authors and year | Screened (n) | Eligible, n (%) | Reasons for ineligibility/exclusion | Randomised (n) | Completed (post intervention), n (%) | Adherence |
|---|---|---|---|---|---|---|
| Barton et al. 201099 | 53 | 22 (41.5) | Ineligible included 13 undergoing chemotherapy/radiotherapy, 5 declined, 5 too unwell, 4 prior breathlessness training, 2 unconfirmed malignancy, 1 not breathless, 1 cause of breathlessness treated | 22 | 11 (50.0) | Of 11 allocated to the three-session intervention, 5 dropped out by the fourth assessment (2 died, 2 declined, 1 too unwell). Of the 11 allocated to the single-session intervention, 6 dropped out by the fourth assessment (3 died, 2 too unwell, 1 declined) |
| Bredin et al. 199998 | NR | 119 | 16 patients excluded because of a protocol violation (one centre failed to adhere to the trial protocol and so was excluded on advice of data monitoring committee) | 103 | 60 (58.3) | Of the 52 allocated to the control, 3 refused study after randomisation, 15 withdrawn, 7 died. Of the 51 allocated to intervention, 1 refused study after randomisation, 8 withdrew, 9 died. Of the total 27 who withdrew but did not report an improvement in their breathlessness, 16 withdrew because of deteriorating condition (13 control, 3 intervention) and 4 were unhappy with the arm allocated (3 control, 1 intervention). This left 7 who withdrew for other reasons (2 control, 5 intervention) |
| Corner et al. 199690 | NR | NR | 34 patients had consented to take part in the randomised study when randomisation was stopped in response to requests from medical and nursing staff who felt that they had observed a clear benefit from the intervention strategy | 34 | 20 (58.8) | Of the 19 allocated to intervention and 15 to control, 14 patients withdrew because of deterioration (8 and 6, respectively) |
| Farquhar et al. 201491 | NR | 158 | 81 eligible patients agreed to recruitment visit, 14 of whom died or were withdrawn by service/researcher/patient | 67 | 54 (80.6) | Of the 35 allocated to intervention, 7 were lost to study (5 condition deteriorated, 2 died). Of the 32 allocated to control, 6 were lost to study as their condition deteriorated |
| Farquhar et al. 201695 | NR | 159 | 97 eligible patients agreed to recruitment visit, 10 of whom died or were withdrawn by service/researcher/patient | 87 | 79 (90.8) | Of the 44 allocated to intervention, 3 were lost to study (including 1 death) by time point 3 (conducted 4 weeks after baseline). Of the 43 allocated to control, 5 were lost to study (including 1 death) by time point 3 |
| Higginson et al. 201494 | 216 | 105 (48.6) | 11 did not meet inclusion criteria and an additional 100 were not randomised for other reasons (6 died at time of referral letter receipt, 29 declined to participate, 18 were too ill to participate, 47 unable to be contacted) | 105 | 82 (78.1) | 53 were allocated to intervention and 52 to control. By week 6, 4 died (1 intervention, 3 control), 5 withdrew (no reason) (2 intervention, 3 control), 8 withdrew because of illness (4 intervention, 4 control), and 6 were unable to be contacted (patient often hospitalised or moved away) (4 intervention, 2 control). Attrition to primary outcome was lower than estimated (22% not 40%) and so recruitment was stopped at 105 |
| Johnson et al. 2015158 | 932 | 156 (16.7) | Of the 776 excluded from enrolment, 127 had insufficient shortness of breath, 528 declined (no reason), 38 were unable to give informed consent, 32 had comorbidities/intercurrent illness, 22 required urgent medical intervention, 20 had previous pulmonary rehabilitation, and 9 had other reasons | 156 | 124 (79.5) | 52 were allocated to the three-session intervention and 104 to the single-session, but only 39 and 91 received their respective allocated intervention because 2 from each group withdrew prior; a further 11 did not receive the three-session intervention (2 too unwell, 1 only well enough to receive some, 8 no reason); and another 11 did not receive the single-session [1 did not attend (lost to follow up), 8 no reason, 1 deteriorated before, 1 not reported] |
| Yorke et al. 2015157 | 715 | 107 (15.0) | Of the 608 ineligible, 176 had absence of two or more symptoms or did not have bothersome breathlessness, 40 had poor prognosis, 55 had further treatment, 130 had recent chemotherapy, 128 had other reasons, 74 declined and 5 were reason unknown | 107 | 72 (67.3) | Of the 53 allocated to the intervention, 3 were removed from analysis (2 did not meet eligibility, 1 no baseline data), 7 dropped out during the intervention (4 too unwell, 1 died, 1 shingles, 1 declined) and 12 dropped out post intervention (1 died, 8 declined, 3 lost to follow-up). Of the 54 allocated to the control, 3 were removed from analysis (2 did not meet eligibility, 1 no baseline data) and 10 dropped out (5 died, 4 declined, 1 lost to follow-up) |
| Pearce et al. 2006154 | NR – abstract available only | 51 | NR – abstract available only | |||
| Yates et al. 2007156 | NR – abstract available only | RCT 1: n = 30; RCT 2: n = 57 | NR – abstract available only | |||
| Yates et al. 2011155 | NR – abstract available only | 144 | NR – abstract available only | |||
Experiences
Across the 11 studies with qualitative data90,91,94,95,102–106,159–161 and the five studies with quantitative data99,100,103,157,161,162,168 on participants’ satisfaction, three themes emerged with regard to experiences: valued characteristics, perceived outcomes and challenges to services.
Satisfaction
Five studies assessed overall satisfaction with their service. For one service, 18 out of 21 respondents (86%) stated that they would recommend the clinic that they attended to another breathless patient;168 for another service, 100% of patients reported high satisfaction and would recommend the service. 103 One study reported high patient satisfaction with care but data were not provided. 162 When comparing one versus three sessions with a service, satisfaction with care was higher for those receiving three sessions in the feasibility study (data not provided),99 but there was little difference between the groups in the full trial (MD 0.4). 100
Valued characteristics
Participants valued the education and information-sharing included in the services, particularly in terms of helping them understand their breathlessness, legitimising the interventions being suggested, and providing helpful written resources to refer to (Box 1). The interventions themselves (e.g. techniques for breathing, pacing, positioning, relaxation and using a handheld fan) were praised for their simplicity, portability and perceived effectiveness. The psychosocial support received through the services was also highly valued, providing opportunities for participants to have their experiences listened to and acknowledged, to receive support and reassurance, and to discuss problems beyond their breathlessness.
I, I use that as my reference [patient points to the BSS patient tool kit] all the time [interviewer: do you?]. When I’m having problems I go back and read it to see if I am doing the right thing. I find that very, very helpful.
Man with interstitial lung disease94
Caring and expert service providers
. . . would you like a cup a tea’ [. . .] it’s just human-to-human situation. But that environment makes you: you are in the right place, you know. There is no guessing going on [. . .]. You are gonna get the best of their mind [. . .], and they display that to you.
P01043, man with COPD160
Involving carers
So she did give me some leaders as to what I can do to help, knowing now that he won’t die in one of these sort of situations, so that certainly helped me, and it certainly helped me to realise that, you know, I can probably help him to calm down. So yes, as a carer I think it was a help.
038t3c, non-malignant condition95
Psychological support
I was able to discuss my personal feelings, that you don’t talk to your family about so not to worry them.
69-year-old woman with COPD103
Simple, portable and effective tools
. . . but her telling me that and another thing as well, when I get out of breath, is to put my hand on my tummy . . . puff puff puff . . . and do that, and you know, it’s amazing really, it sounds so pathetic when you say something . . . It is simple, it’s not a thing you’d think of doing.
530t3pc, malignant condition91
Participants’ comments also indicated their appreciation of carers involvement, both to support carers as individuals and to support them in caring for the patient. Overwhelmingly, participants commented on the qualities of the staff delivering the services: they were deemed experts not only in managing breathlessness, but also in providing person-centred care and treating participants with respect and dignity.
Studies that collected quantitative data on the most helpful characteristics of services also reflected these valued characteristics: one study found that talking to a therapist was rated as most helpful, followed by breathing exercises, relaxation techniques and positive thinking;161 another study’s participants felt that the breathing techniques were helpful;157 and a third study found that the leaflets (e.g. managing breathlessness, distraction techniques, positioning techniques, handheld fan information), handheld fan and discussions of crisis management provided at the clinic were most helpful. 103 Of the interventions provided at home, the most helpful were breathing exercises, breathlessness management, relaxation techniques and energy conservation information. The interventions that were reported as not helpful by breathless patients included acupuncture,157 bronchodilators, massage and ‘not fighting it’. 161
Perceived outcomes
In line with quantitative findings on clinical effectiveness, perceived positive outcomes reported in the qualitative data were largely psychological (Box 2). Participants reported an increased understanding of their breathlessness and disease, in a way that normalised their experiences. As a result, participants reported feeling more confident in managing their breathlessness, and it reduced their anxieties and distress. In particular, knowing that breathlessness would not cause death and that they had tools to respond to it was encouraging.
‘. . . the intervention group were able to describe how they were increasing activity and functional levels by using breathing techniques and exploiting the confidence these gave them.’
(Researcher comments)90
‘. . . the blissful thing is, like I’ve said is, you can control your breathing, if you get a bad spell you can work your way through it whereas previously when I was choking I really did not know what to do, how serious it was. Now I realise it’s something I can, I can cope with.’
(Man with interstitial lung disease)94
‘The main benefit deduced from discussions with patients and answers to questionnaires by was that they felt less isolated than they had prior to undertaking the programme.’
(Researcher comment)167
Sensory-perceptual experience
‘It helped me to learn to relax, learn to breathe in a more controlled way.’
(54-year-old man with COPD)103
Symptom impact or burden
‘. . . felt she wouldn’t be able to do the stairs. Went up and down with very little increase in respiratory rate. Flung her arms around my neck and said ‘I never thought I would be able to do that again.’
(Staff comment 11)161
‘. . . because before I would get into a panic when I was breathless, but now I can sit down use my fan, wet my face, read my laminate (breathlessness poem) and I calm down pretty quick.’
(Woman with COPD)94
Having new techniques to actively respond to their breathlessness also led to feeling more ‘in control’ and, in some cases, meant that breathing felt easier. Others felt that the sensation of breathlessness was unchanged, but their reaction to it had changed. Knowing more about the support that exists and is available to them, including the holistic services, also resulted in reduced feelings of isolation. Alongside this, participants reported being more able to maintain and/or increase their daily activities and reported an increased use of self-management strategies.
Challenges for services
Two potential challenges for services were identified (Box 3). First, participants’ accounts showed the importance of motivation to self-manage in the success of the interventions, yet this was sometimes difficult to achieve if the participants did not perceive benefits relatively quickly. Second, some participants had low expectations of what these services, or their component interventions, could achieve. This sometimes resulted in a reluctance to engage with the interventions and/or services.
‘She gave me a fan and told me to, you know, put it on . . . and then blow out. I do try to do it, but I get so out of breath doing it. I give up.’
(Case 013, non-malignant condition)102
‘Hoping that something would help me but a little bit cynical as well . . . I didn’t see how anything could help improve it.’
(03M)105
Chapter 4 Responder analysis results
Description of pooled data
The pooled data set included data for 259 individual patients. Most participants were male (54.3%) and common diagnoses included COPD (49.8%), cancer (34.7%) and interstitial lung disease (10.4%). Table 9 shows the sample baseline characteristics.
| Variable | Higginson et al.,94 mean (SD)/n (%) | Farquhar et al.,91 mean (SD)/n (%) | Farquhar et al.,95 mean (SD)/n (%) | Total, mean (SD)/n (%) |
|---|---|---|---|---|
| Age | 67.0 (9.9) | 68.7 (11.5) | 72.2 (10.0) | 69.2 (10.6) |
| Sex (female) | 44 (41.9) | 40 (60.6) | 34 (39.1) | 118 (45.7) |
| Diagnosis | ||||
| COPD | 55 (52.4%) | – | 74 (85.1) | 129 (49.8) |
| ILD | 19 (18.1%) | – | 8 (9.2) | 27 (10.4) |
| Cancer | 22 (21.0%) | 67 (100%) | 1 (1.1) | 90 (34.7) |
| Other | 9 (8.6%) | – | 4 (4.6) | 13 (5) |
| FEV1% predicted | 46.1 (24.2) | – | 46.2 (21.4) | 46.1 (22.9) |
| NRS | ||||
| Breathlessness (average in the last 24 hours)a | 5.99 (2.07) | 3.93 (1.96) | 4.38 (1.86) | 4.89 (2.16) |
| Distress due to breathlessnessa | – | 4.85 (2.90) | 5.21 (2.74) | 5.06 (2.80) |
| HADS | ||||
| Anxietya | 9.39 (2.90) | 7.36 (3.82) | 8.20 (4.01) | 8.44 (3.64) |
| Depressiona | 10.36 (2.93) | 6.44 (2.79) | 7.23 (3.55) | 8.24 (3.56) |
| EQ-5D | ||||
| VAS | 53.3 (17.8) | 57.8 (18.5) | 54.4 (18.8) | 54.8 (18.4) |
| Utility Index | 0.39 (0.32) | 0.59 (0.24) | 0.50 (0.28) | 0.48 (0.29) |
| CRQ | ||||
| Dyspnoea | 2.25 (0.81) | 3.61 (1.07) | 3.25 (0.96) | 2.95 (1.09) |
| Fatigue | 2.84 (1.38) | 3.30 (1.22) | 3.10 (1.08) | 3.05 (14.25) |
| Emotional function | 3.72 (1.31) | 4.44 (1.06) | 4.09 (1.08) | 4.03 (1.20) |
| Mastery | 3.54 (1.40) | 4.64 (1.17) | 4.04 (1.24) | 3.99 (1.36) |
| Responders | ||||
| Mastery | 36 (51.4) | 17 (34.0) | 44 (59.5) | 97 (50) |
| NRS distress | – | 36 (69.2) | 45 (58.4) | 81 (62.8) |
Predictors of response
Chronic Respiratory Disease Questionnaire mastery
Of the participants for whom the CRQ mastery response could be calculated, half (97/194) were classed as responders. Variables significantly associated with a CRQ mastery response in the univariate analyses included CRQ mastery (p < 0.001), CRQ dyspnoea (p = 0.008), CRQ fatigue (p = 0.009), EQ-5D Utility Index (p = 0.007), EQ-5D VAS (p = 0.014) and NRS average breathlessness in the last 24 hours (p = 0.039) (Table 10).
| Variable | n | OR | 95% CI | p-value |
|---|---|---|---|---|
| Age | 194 | 1.01 | 0.99 to 1.04 | 0.38 |
| HADS anxietya | 189 | 1.05 | 0.97 to 1.14 | 0.21 |
| HADS depressiona | 189 | 1.02 | 0.94 to 1.10 | 0.67 |
| EQ-5D VAS | 193 | 0.98 | 0.96 to 0.996 | 0.01b |
| EQ-5D Utility Index | 194 | 0.24 | 0.08 to 0.68 | 0.01b |
| CRQ dyspnoea | 191 | 0.69 | 0.53 to 0.91 | 0.01b |
| CRQ fatigue | 193 | 0.72 | 0.57 to 0.92 | 0.01b |
| CRQ emotion | 193 | 0.81 | 0.64 to 1.03 | 0.09 |
| CRQ mastery | 194 | 0.55 | 0.43 to 0.71 | < 0.001b |
| NRS average breathlessness in the last 24 hoursa | 193 | 1.15 | 1.01 to 1.32 | 0.04b |
| Sex (female) | 193 | 1.56 | 0.88 to 2.77 | 0.13b |
| Diagnosis (reference COPD) | 194 | |||
| ILD | 0.56 | 0.20 to 1.42 | 0.21 | |
| Cancer | 0.58 | 0.31 to 1.10 | 0.10 | |
| Other | 1.61 | 0.38 to 6.79 | 0.52 | |
| Original study (reference group: Farquhar et al.95) | 194 | |||
| Farquhar et al.91 | 0.35 | 0.17 to 0.74 | 0.01b | |
| Higginson et al.94 | 0.72 | 0.37 to 1.40 | 0.33 | |
| FEV1% predicted | 51 | 1.01 | 0.98 to 1.03 | 0.70 |
Baseline CRQ mastery remained the only significant predictor of intervention response in the multivariate regression when controlling for age, sex and original study. Participants with lower baseline CRQ mastery scores were more likely to respond on this measure [odds ratio (OR) 0.57, 95% CI 0.43 to 0.74; p < 0.001] (Table 11).
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Original study: bFarquhar et al.91 | 0.43 | 0.19 to 1.00 | 0.05 |
| Original study: bHigginson et al.94 | 0.62 | 0.29 to 1.31 | 0.21 |
| Age | 1.02 | 0.99 to 1.05 | 0.13 |
| Sex (female) | 1.68 | 0.87 to 3.24 | 0.12 |
| CRQ mastery | 0.57 | 0.43 to 0.74 | < 0.001c |
Seventy-six per cent (n = 31) of participants with a baseline CRQ mastery score of ≤ 2 responded to the intervention, whereas 53% (n = 49) of those scoring 3–5 and 28% (n = 17) of those with a score of ≥ 5 responded on this measure.
Numeric Rating Scale distress due to breathlessness
Of participants for whom NRS distress response could be calculated, 81 out of 129 (62.8%) were classed as responders. The baseline NRS distress score was the only variable significantly related to distress response in the univariate analysis (p < 0.001) (Table 12).
| Variable | n | OR | 95% CI | p-value |
|---|---|---|---|---|
| Age | 129 | 1.01 | 0.97 to 1.04 | 0.75 |
| HADS anxietya | 126 | 1.03 | 0.94 to 1.12 | 0.58 |
| HADS depressiona | 126 | 0.98 | 0.88 to 1.10 | 0.77 |
| EQ-5D VAS | 129 | 1.00 | 0.98 to 1.02 | 0.78 |
| EQ-5D Index | 129 | 0.79 | 0.20 to 3.17 | 0.74 |
| CRQ dyspnoea | 126 | 0.76 | 0.53 to 1.09 | 0.13 |
| CRQ fatigue | 127 | 0.93 | 0.67 to 1.28 | 0.64 |
| CRQ emotion | 127 | 0.90 | 0.65 to 1.24 | 0.51 |
| CRQ mastery | 127 | 0.77 | 0.58 to 1.04 | 0.09 |
| NRS average breathlessness in the last 24 hoursa | 129 | 1.16 | 0.96 to 1.40 | 0.13 |
| NRS distressa | 129 | 1.52 | 1.28 to 1.80 | < 0.001b |
| Sex (female) | 128 | 1.55 | 0.75 to 3.19 | 0.24 |
| Diagnosis (reference COPD) | ||||
| ILD | 129 | 0.27 | 0.05 to 1.48 | 0.13 |
| Cancer | 129 | 1.54 | 0.72 to 3.32 | 0.27 |
| Other | 129 | 2.00 | 0.20 to 20.29 | 0.56 |
| Original study: cFarquhar et al.91 | 129 | 1.60 | 0.76 to 3.36 | 0.22 |
| FEV1% predicted | 53 | 0.99 | 0.96 to 1.01 | 0.28 |
In the multivariate model, participants with a higher NRS score at baseline were more likely to respond on this measure than those with a low score (OR 1.64, 95% CI 1.35 to 2.03; p < 0.001) (Table 13).
| Variable | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Original study: bFarquhar et al.91 | 2.40 | 0.95 | 6.09 | 0.06 |
| Age | 1.03 | 0.99 | 1.07 | 0.18 |
| Sex (female) | 2.31 | 0.94 | 5.72 | 0.07 |
| NRS distress | 1.64 | 1.35 | 2.03 | < 0.001c |
Eighty-nine per cent of participants (n = 41) with a ‘severe’ (≥ 7) baseline NRS distress score responded to the intervention, whereas 59% (n = 23) of those with ‘moderate’ scores (4–6) and 39% (n = 17) of those with ‘mild’ scores (≤ 3) responded on this measure.
Chapter 5 Transparent expert consultation results
Stakeholder workshop results
Attendance
The workshop was attended by 37 stakeholders (40 registered and 117 were invited). Most attendees were from the UK (including Bristol, Cambridge, Exeter, Glasgow, Leicester, London, Norwich, Nottingham and York); however, two were visiting the UK from abroad (Australia and the Netherlands) and were able to attend.
Of the 37 event attendees, 33 stakeholders participated in the group work and completed response booklets (there were three groups: one with 8 participants, one with 12 and one with 13). An additional two response booklets were completed by patient representatives who were unable to attend the event but wanted to provide their input. Table 14 shows the characteristics of participants who completed the response booklets.
| Characteristic | Workshop booklets (N = 35),a n (%) |
|---|---|
| Profession/role | |
| Doctor (clinical) | 16 (47) |
| Researcher | 17 (50) |
| Physiotherapist | 4 (10.8) |
| Patient/carer representative | 3 (8.6) |
| Role in charitable organisation | 2 (5.8) |
| Nurse | 2 (5.8) |
| Commissioner | 2 (5.8) |
| Occupational therapist | 1 (2.9) |
| Psychologist | 1 (2.9) |
| Otherb | 2 (5.8) |
| Area of expertise | |
| Lung disease | 16 (47) |
| Palliative care | 17 (50) |
| Research | 13 (38.2) |
| Cancer | 6 (17.6) |
| Patient/carer | 3 (8.6) |
| General practice | 1 (2.9) |
| Heart disease | 6 (5.8) |
| Psychology | 2 (5.8) |
| Geriatrics | 4 (10.8) |
| Otherc | 2 (5.8) |
Prompt question booklet responses
Group 1: how do we define and deliver ‘holistic breathlessness services’?
Participants suggested that to define and deliver these services, different models of care needed to be evaluated for clinical effectiveness and cost-effectiveness. These services need to be evidence based and integrated, with collection of routine data to review access and outcomes. A key component of delivery should be establishing and upskilling a range of clinicians in core breathlessness management skills, and supporting these staff to integrate these skills into their routine practice. Table 15 shows a summary of responses and example quotations.
| Question | Summary of responses | Example quotations |
|---|---|---|
| What are the core components of a holistic breathlessness service? |
Education and self-management skills Comprehensive, person-centred, multidisciplinary team assessments Support (e.g. counselling, groups) Treatment of symptoms/reversible causes |
Education, to understand breathlessness and provide coping strategies to enable patients to function as best they can and to do the things they want to do that are important to them1004Comprehensive assessment exploring patient experience of breathlessness1005 |
| Which staff should lead and contribute to a holistic breathlessness service? |
Multidisciplinary including: GPs, physiotherapists, OTs, nurses, psychologists, respiratory/cardiology/oncology specialists, and palliative care teams Anyone with an interest |
Needs to be ‘joined up’ trained staff1011All – Drs, nurses, physios, OTs, HCAs, [admin], psychologists1009 |
| Who should be referred to holistic breathlessness services, and when? |
Anyone suffering from refractory breathlessness Early in diagnosis When ‘usual treatment’ stops working |
Any condition (advanced progressive disease) causing breathlessness which interferes with the patient’s function or daily life or/and is distressing them1004All breathlessness patients regardless of diagnosis1008 |
| What is the optimal contact time, and over what duration, for a holistic breathlessness service? |
Initial multidisciplinary team assessment of 1–2 hours, plus another 1–3 1-hour appointments Long term/open access Self re-referral Until breathlessness improves using taught self-management techniques |
Once you are assessed as having chronic intractable breathlessness you should never leave the service . . .1010. . . option for patient contact if required1005 |
| Where should a holistic breathlessness service be positioned and delivered? |
Community Access to hospital services if needed Respiratory/cardiology input GP involvement |
The community aspect is very important because that is where the patients experience their breathlessness and where they manage it (at home)1004Where possible, merge with existing services with models of sharing staff expertise1003 |
| What future research is needed around the definition and delivery of holistic breathlessness service? |
Need to identify beneficial components – strong evidence needed Cost-effectiveness |
. . . we need a robust long-term (ideally pooled [internationally]) data from cohort to also examine the impact of these new services1002. . . cost of services vs. effectiveness1001 |
Group 2: how and where can holistic breathlessness services be integrated into current practice?
Participants felt that upskilling clinicians in breathlessness management skills was core to integrating breathlessness services. This should include attention to both the physical and the psychological components of breathlessness, should consider ways to enable self-management, and should not be disease specific. Challenges with service integration and different approaches across different localities were noted. Table 16 shows a summary of responses to each question and example quotations.
| Question | Summary of responses | Example quotations |
|---|---|---|
| Where should a holistic breathlessness service be based? |
Based in the community with hospital access, if required (e.g. home visits, GP surgeries) Wherever appropriate |
An obvious place is primary care. Most GP surgeries have spaces that could be used for group support – whether carer support or education – or psychosocial/specialist nurse facilitated patient support. Ideally these provide the ‘missing piece’ that PR may not be accessing2005 |
| Which services should contribute to a holistic breathlessness service? |
Respiratory, cardiology, palliative care, psychologists, physiotherapist/OT, social work. To a lesser extent rehabilitation and frailty services, community support groups Physical, psychological, social/relational and spiritual aspects need to be considered |
. . . to be truly holistic, there needs to be equal weight given to the physical, psychological, social/relational and spiritual aspects of breathless person’s experience . . .2005 |
| How should people be referred to a holistic breathlessness service? |
Multiple referrals routes rather than one specific place or specialty. Could include GPs, community and rehabilitation services and respiratory and cardiology specialists Self-referral is both advocated by some and explicitly stated as inappropriate by others |
Multiple points of entry so no one is missed . . .2006A holistic service needs to have multiple avenues for referral to allow speedy referral and treatment2007 |
| How do we best raise awareness of a holistic breathlessness service? |
Educating and engaging clinicians (across multiple specialties), commissioners and the public, particularly within relevant local care pathways and existing charity-led initiatives (e.g. patient/carer groups; GP surgeries; PR classes, BLF patient passports, CPD events) Disseminate evidence regarding outcomes and value for money Inclusion in national guidance Earlier focus on supportive care Blogs and social media |
Staff need to be educated about what the service will offer and what benefit it has for patients2007I recently took part in a PPI exercise . . . what struck me was the public’s huge thirst for information about breathlessness and how to self-manage2005 |
| What could help facilitate integration of holistic breathlessness services into current practice? |
Evidence of acceptability and cost-effectiveness Integration of key elements into existing practice (e.g. respiratory and cardiac pathways) and upskilling existing staff. Multidisciplinary working |
Evidence that it is valued by patients and carers2001Identify what elements are already provided in current services . . . identify gaps and add in2003 |
| What future research is needed around integration of holistic breathlessness services into practice? |
What is already being delivered in practice, and consider integration into existing services rather than standalone Identify necessary service elements, influence of patient factors on outcomes, and patient/carer preferences on service design. Considering inclusion of exercise, supportive technology, carer support and longer-term follow-up Timing of referral (e.g. following hospital admission) |
How best to support carers2001Specify necessary service elements2008 |
Group 3: how should the success of holistic breathlessness services be measured/monitored?
Discussions centred around ensuring that outcomes were patient led, clearly mapped to service aims and psychometrically robust. Inclusion (and development) of carer-reported outcomes was also discussed. Participants felt strongly that any approach to measurement should be based on existing successful methods, should be consistent and should be integrated with existing practice. Table 17 shows a summary of responses to each question and example quotations.
| Question | Summary of responses | Example quotations |
|---|---|---|
| What is the ideal set of outcomes to measure for patients? |
Measures should focus on what is important to patients. This may include breathlessness (including mastery of, and distress due to breathlessness), health-related QoL, health status, physical activity, capacity and/or function, psychological and social well-being, patient-centred goals, health-care and medication use, comorbidities (e.g. frailty), acceptability and experiences Measures should be consistent across services and psychometrically validated |
Actually ask the individual what they would like to achieve/happen?3011I think it is key that the outcome measures are there that are identified as important to the patient3001 |
| What is the ideal set of outcomes to measure for carers? | Measures should focus on what is important to carers. This may include health-related QoL, health-care utilisation, psychological and social well-being, carer burden and/or preparedness to care, quality of their relationship with the patient, ability to live with patients’ breathlessness, support needs (e.g. information, financial), acceptability and experiences, and proxy patient outcomes (e.g. breathlessness) | Impact and burden of caring in including physical, psychological and financial. What aspects of the breathlessness intervention has helped them most3001Again, outcomes need to reflect the things that are important to the carer3005 |
| What is the ideal set of service-level variables to monitor? | Measures should be consistent (between and within services) and realistic. This may include interventions delivered, staffing required, referral source, service usage (e.g. uptake, dropouts), service user characteristics (including monitoring equality of access), provider and service user experiences, patient outcomes (including health-care utilisation), and cost-effectiveness | Completion of service use – approach, take up, dropout, completion3007Staff members involved, associated costs, staff members’ views on the services3006 |
| What data will need to be collected to inform commissioning? |
Population needs and access, including equality and diversity implications Patient and carer outcomes and experiences, including health-care utilisation Cost-effectiveness |
Value – either enhanced QoL for same costs or improved outcomes for increased cost3009 |
| What could help facilitate measurement and monitoring of success? |
PPI (e.g. in selection of measures) Use of or integration into routine data collection (e.g. align with existing audits Dedicated training/support/funding for measurement and monitoring Include measurement as part of service contract (e.g. payment by results) Measures should be used consistently across services, psychometrically validated, and timed in order to best capture changes |
Identify a core set of variables and embed the data collection into routine practice3006. . . using the same measurement tools as similar services: consistency, easier to pool data3004 |
| What future research is needed around measurement and monitoring the success of holistic breathlessness services? |
Establishing core service components Identifying predictors of uptake and optimal outcomes Identifying outcomes most important to patients and carers Understanding how best to embed routine data collection in clinical practice Development of carer-reported outcome measures |
Ascertaining what outcomes are most important to the patient and carer, and translating those wishes/wants/desires into outcomes which tick the boxes for commissioners3011 |
Individual suggestions
Across the three parallel groups, stakeholders made 187 individual suggestions in their participant booklets. Most of the participants’ suggestions had implications for research (n = 101), followed by implications for clinical care (n = 76) and then policy (n = 41; multiple categories could be selected). Synthesis of these 187 suggestions resulted in 34 statements for inclusion in the online consensus survey.
Event summary
Throughout the stakeholder workshop, the need for improved collaboration, integrated working and standardisation were strong themes. Stakeholders acknowledged the successful components of existing practices, across multiple specialties and disciplines, which should be developed but not duplicated. Figure 5 shows the graphic recording summarising discussions throughout the workshop.
FIGURE 5.
Graphic recording of stakeholder workshop discussions. Reproduced with permission from Joel Cooper, April 2019.
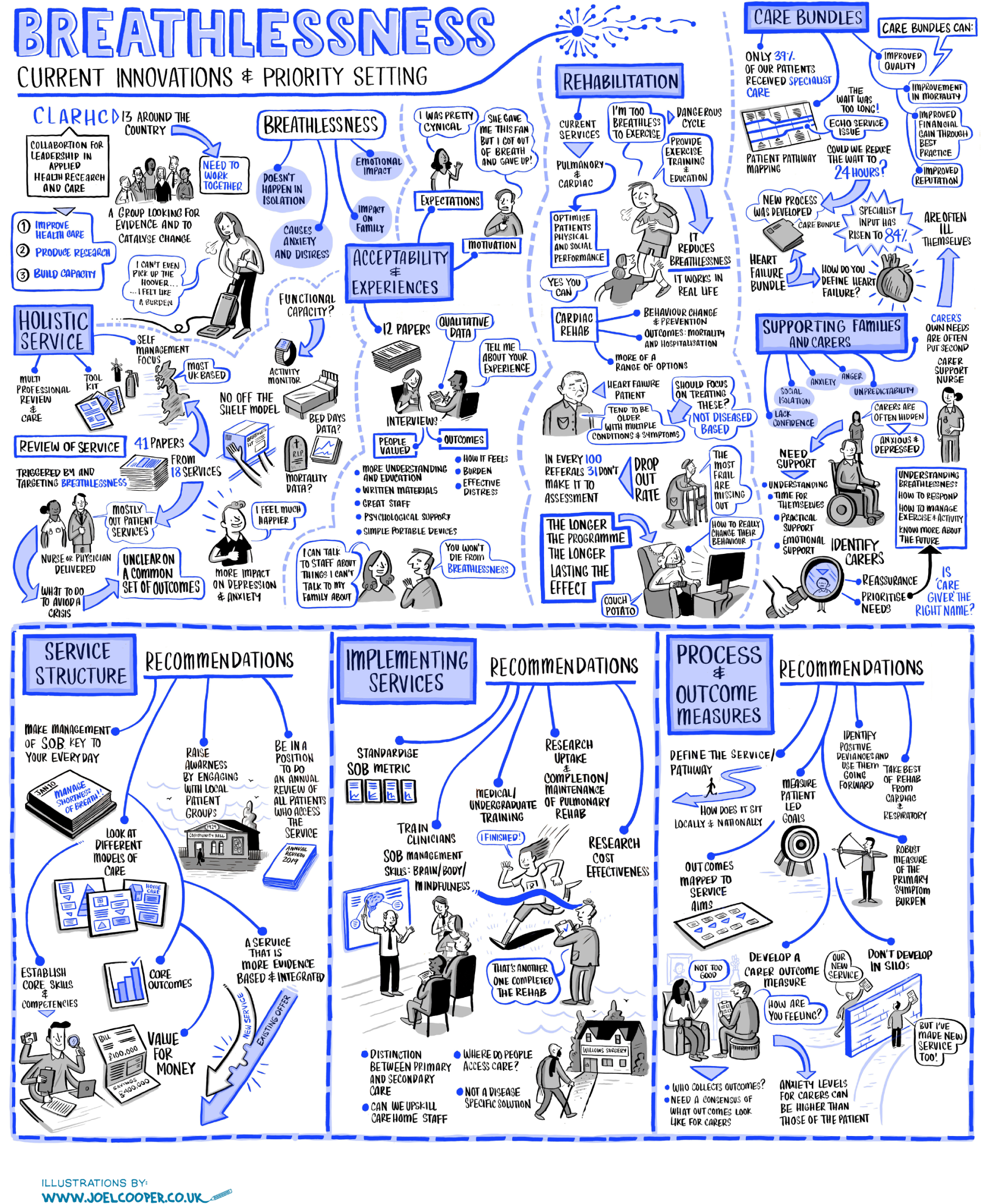
Consensus survey results
Respondents
Those invited to the workshop, plus an additional 43 stakeholders, were invited to take part in the online consensus survey. Of the 160 invited, 72 participated (an additional two participated after receiving an invite forwarded from an originally invited participant), representing a 46% response rate. Seventy-eight invitees did not respond, six were away until after the survey close date, three e-mail addresses were invalid and one person declined. Twenty-six of those who responded to the consensus survey had completed a workshop response booklet. Table 18 shows the characteristics of participants who completed the online consensus survey.
| Characteristic | Online survey (N = 74),a n (%) |
|---|---|
| Profession/role | |
| Doctor (clinical) | 30 (40.5) |
| Researcher | 29 (39.2) |
| Physiotherapist | 11 (14.8) |
| Patient/carer representative | 9 (12.2) |
| Role in charitable organisation | 9 (12.2) |
| Nurse | 7 (9.5) |
| Commissioner | 4 (5.4) |
| Occupational therapist | 0 (0) |
| Psychologist | 2 (2.7) |
| Otherb | 1 (1.4) |
| Area of expertise | |
| Lung disease | 43 (58.1) |
| Palliative care | 29 (39.2) |
| Research | 28 (37.8) |
| Cancer | 12 (16.2) |
| I am a patient/carer | 10 (13.5) |
| General practice | 7 (9.5) |
| Heart disease | 5 (6.8) |
| Psychology | 5 (6.8) |
| Geriatrics | 4 (5.4) |
| Otherc | 4 (5.4) |
Survey responses
Of the 34 final statements from participants included in the online survey, 10 related to clinical practice, eight to policy, and 16 to research. Figure 6 shows boxplots of online consensus survey scores. The majority (n = 20) of participants received strong agreement but low consensus, seven participants received strong agreement and high consensus, six participants received moderate agreement and low consensus, and one participant received moderate agreement and high consensus. No statements were disagreed with. Participants’ priorities for clinical practice were more often met with strong agreement and high consensus, with lowest agreement and consensus being around participants’ priorities for research.
FIGURE 6.
Boxplots of online consensus survey scores. (a) Clinical priorities; (b) policy priorities; and (c) research priorities. Reproduced with permission from Brighton et al. 172 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 License (http://www.creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
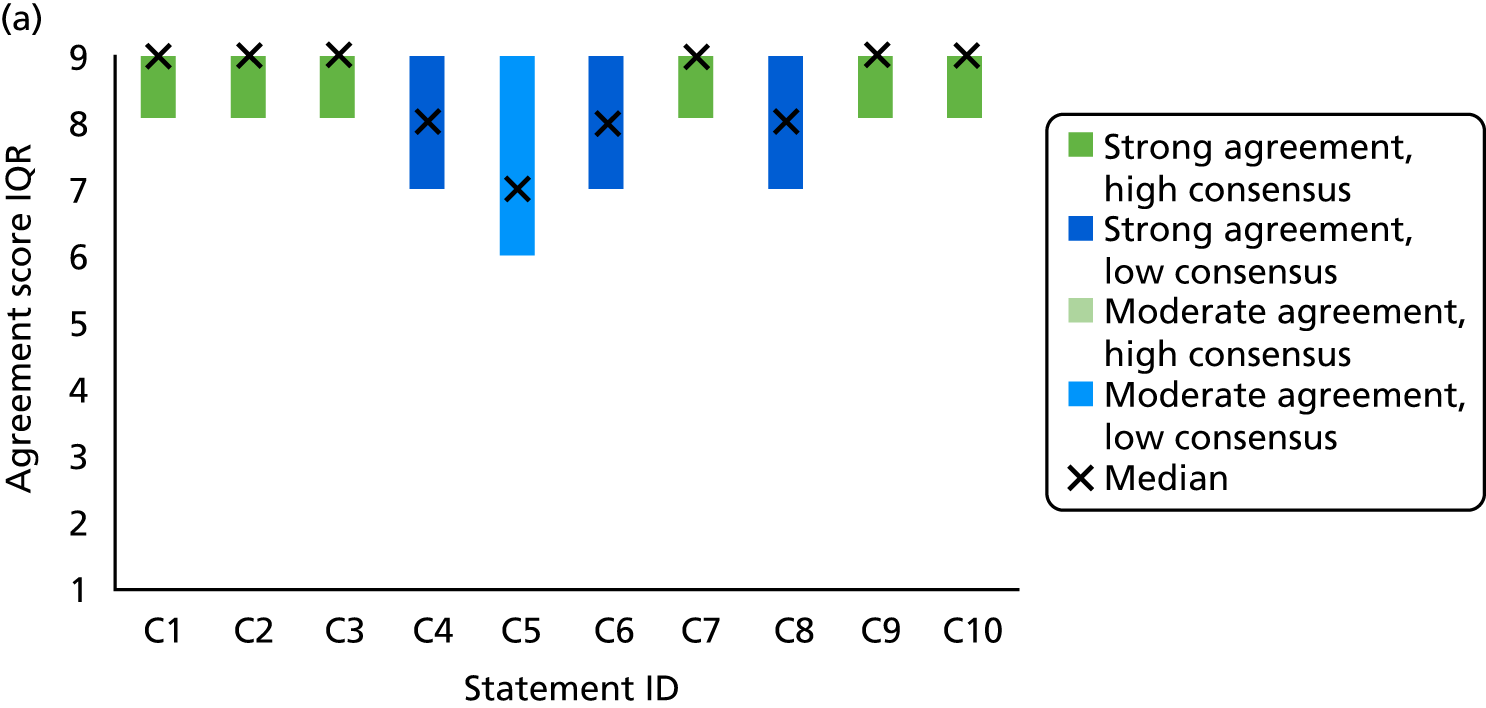
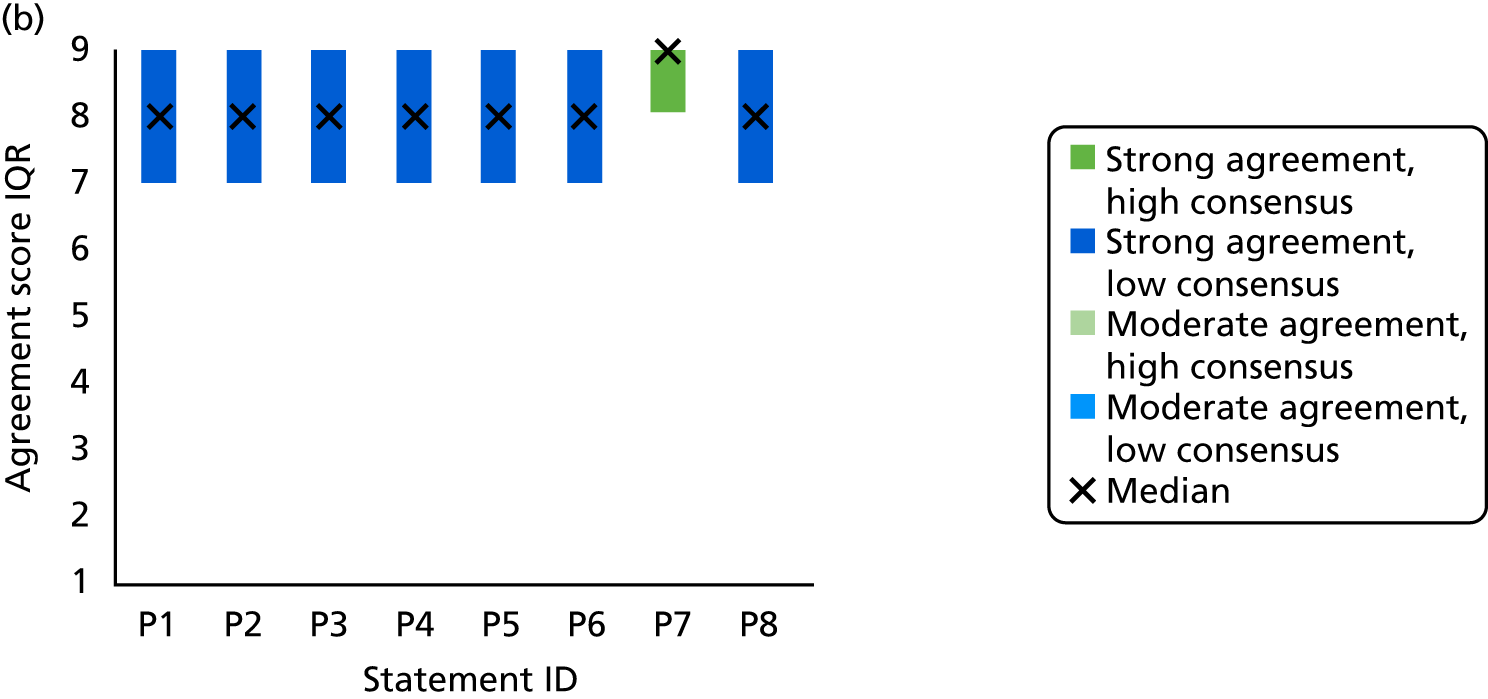
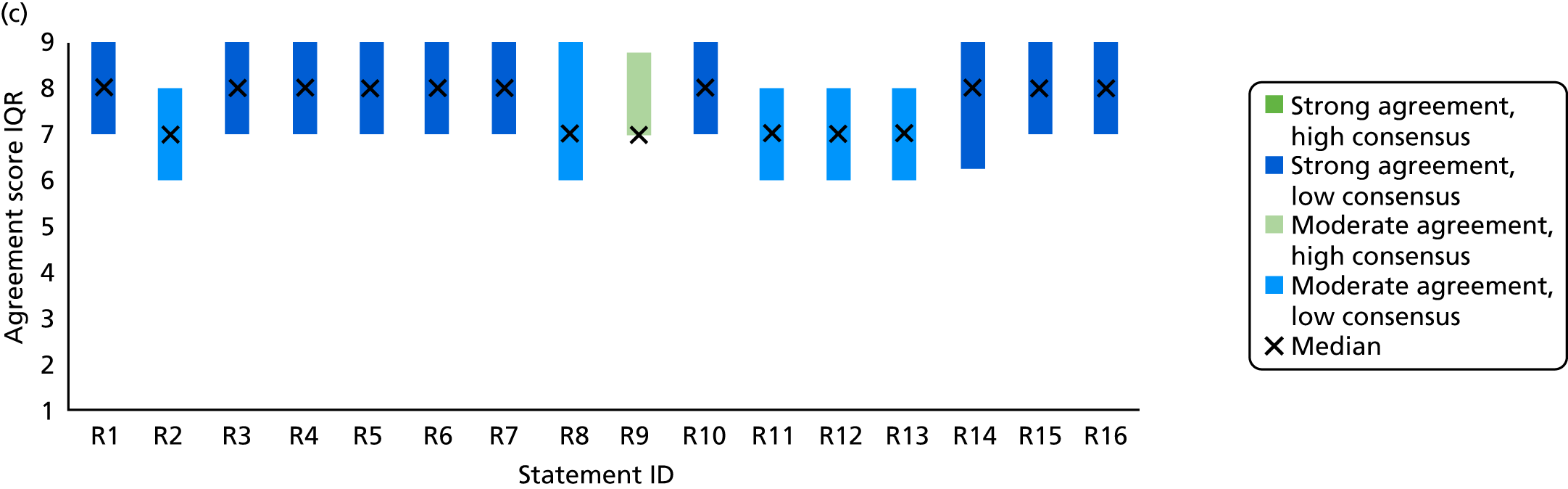
Stakeholders’ priorities for clinical practice
The most strongly supported statements from stakeholders relating to clinical practice were those calling for flexible and accessible person-centred care (C1) and drawing on multiple expertise (C2), with the widest possible coverage geographically and demographically (C3) (Table 19). They also included acknowledgement of the role of informal carers (C7), valuing responding to (and being able to respond to) breathlessness as a symptom in its own right (C9) and sharing breathlessness management skills between other professionals and with informal carers (C10). Free-text consensus survey comments also highlighted the need for multidisciplinary, person-centred, holistic care, which particularly acknowledged psychological concerns:
Health-care professionals should have knowledge of physical and psychological ways to manage breathlessness and should assess these areas. Generally, the psychological impact of breathlessness is ignored.
Participant 5026
Important to take a holistic view of breathlessness and support the wider psychological, social and practical implications.
Participant 5002
| Participants’ priorities for clinical practice when working with people with chronic breathlessness in advanced disease (statement ID) | Median (IQR)a |
|---|---|
| Strong agreement, high consensus | |
| Ensure breathlessness services are person-centred and flexible in terms of delivery (e.g. appointment location, time, and duration) (C1) | 9 (8–9) |
| Ensure breathlessness services are cross-cutting, drawing on relevant expertise from multiple disciplines, professions and providers (C2) | 9 (8–9) |
| Work towards ensuring that breathlessness services have the widest possible geographical coverage and access (e.g. travelling communities, people who are homeless, people living in care/nursing homes) (C3) | 9 (8–9) |
| Acknowledge family and/or informal carers within breathlessness services and, when appropriate, actively encourage their participation in education and in management of the patient’s breathlessness (C7) | 9 (8–9) |
| Value symptom management in its own right, and be able to deliver, or refer patients for, breathlessness interventions (C9) | 9 (8–9) |
| Share breathlessness management skills with other health and social care professionals, and informal carers (C10) | 9 (8–9) |
| Strong agreement, low consensus | |
| Define clear referral criteria for breathlessness services (e.g. limiting breathlessness that persists despite optional management of underlying disease) and share these with potential referrers (C4) | 8 (7–9) |
| Use multiple strategies to raise awareness of breathlessness services among potential referrers and the public (e.g. by engaging with professional bodies, charities or patient groups) (C6) | 8 (7–9) |
| Be alert to, and respond to, under-recognised related issues (e.g. sleep, intimacy) (C8) | 8 (7–9) |
| Moderate agreement, low consensus | |
| Consider providing the option for patients to self-refer to breathlessness services (C5) | 7 (6–9) |
Despite strong agreement, there was low consensus around defining referral criteria (C4), using multiple strategies to raise awareness of breathlessness (C6) and responding to under-recognised related issues (C8). One respondent noted that referral structures should not be too rigid, as this may be a barrier when presentation to the service is atypical:
I have stated strongly agree for the need for clear referral criteria. However, referral criteria should be inclusive and aim to capture all those limited by breathlessness. Rigid referral criteria risk excluding people who don’t present typically.
Participant 2003
The most contentious statement was around the option for patients to self-refer to services (C5); comments revealed concerns around self-referral and ensuring joined up health-care, ensuring medical record access and ensuring that medications are maximised and reversible conditions ruled out. Additional comments also noted that the role of the wider community should be considered.
Stakeholders’ priorities for policy
The most strongly supported of participants’ statements relating to policy was recognition of informal carers in terms of their role, importance and support needs (P7) (Table 20). The remaining statements received high overall agreement but low consensus. The comments highlighted the contention over the utility of mapping (P1, P3) and national audit (P4, P5), questioning their benefit to day-to-day practice. Others felt that this was a necessary first step:
Needs assessment and mapping would logically precede further service development (avoid duplication) and quality standards/audit should come as evidence expands.
Participant 5047
| Participants’ priorities for policy relating to people with chronic breathlessness in advanced disease (statement ID) | Median (IQR)a |
|---|---|
| Strong agreement, high consensus | |
| Recognise informal carers in terms of their role, importance, and support needs (P7) | 9 (8–9) |
| Strong agreement, low consensus | |
| Complete a needs assessment around breathlessness, map it to current service provision and consider areas for service improvement (P1) | 8 (7–9) |
| Prioritise supporting development of breathlessness-triggered services, which span all stages of multiple diseases and conditions (P2) | 8 (7–9) |
| Map how breathlessness services could sit within existing care provision and plans, to avoid duplication (P3) | 8 (7–9) |
| Agree, publish, and review breathlessness service quality standards as new evidence accumulates (P4) | 8 (7–9) |
| Establish an audit programme for breathlessness services to track impact of services nationally or internationally (P5) | 8 (7–9) |
| Increase public awareness and/or education around breathlessness (e.g. as a sign of disease vs. normal exertional symptom) (P6) | 8 (7–9) |
| Provide all health and social care staff with education around breathlessness and its management, ideally starting during vocational and/or undergraduate training and continuing throughout professional lives (P8) | 8 (7–9) |
Multiple comments stressed the importance of the priority area of education, to increase awareness among the general public as well as health and social care professionals (P6, P8), particularly to support existing services. It was also suggested that breathlessness-triggered services (P2) should be developed through adapting existing services rather than introducing something new, and that this might be achieved by educating and upskilling care staff:
Re. policy – there are so many symptoms and diseases that compete for limited resources, so this is challenging. But educating all health professionals from undergrad onwards is probably the most important issue to change practice long term.
Participant 1002
One respondent commented that the policy statements were health-care focused, despite people with breathlessness spending most of their time outside the health services. It was suggested that work might be undertaken to understand the role of psychological approaches (e.g. cognitive–behavioural therapies), social care and communities in supporting patients with breathlessness and their carers.
Stakeholders’ priorities for research
None of the participants’ statements relating to research received high agreement and high consensus (Table 21). The need for economic modelling of breathlessness services (R9) was the only statement receiving high consensus (and moderate agreement). This gap in evidence was reflected in the limited economic data available from studies and services included in the systematic review.
| Participants’ priorities for policy relating to people with chronic breathlessness in advanced disease (statement ID) | Median (IQR)a |
|---|---|
| Strong agreement, low consensus | |
| Explore optimal delivery methods of service provider education for breathlessness assessment and management (R16) | 9 (7–9) |
| Understand the impact of breathlessness and associated factors (e.g. fatigue or isolation) on health and social care service use and costs (R1) | 8 (7–9) |
| Determine medium- to long-term effects of breathlessness services using follow-up assessments beyond completion of the intervention (R4) | 8 (7–9) |
| Examine and understand models of integrated working between breathlessness services and other providers (e.g. palliative, respiratory, primary, social care) (R5) | 8 (7–9) |
| Assess the clinical effectiveness and cost-effectiveness of breathlessness services for people unable to engage in cardiac/respiratory rehabilitation services (R6) | 8 (7–9) |
| Assess the clinical effectiveness and cost-effectiveness of breathlessness services for people who have had their first unplanned hospital admission related to breathlessness (R7) | 8 (7–9) |
| Assess the clinical effectiveness and cost-effectiveness of the following components within breathlessness services: carer-focused interventions (R10) | 8 (7–9) |
| Assess need for service provider education around breathlessness (R15) | 8 (7–9) |
| Establish a core set of outcome measures for clinical practice and research, incorporating validated patient and carer measures (R3) | 8 (7–9) |
| Complete economic modelling (including cost-effectiveness studies) of breathlessness services, which should include health and societal perspectives (R14) | 8 (6.25–9) |
| Moderate agreement, high consensus | |
| Assess the clinical effectiveness and cost-effectiveness of the following components within breathlessness services: structured exercise training (R9) | 7 (7–8.75) |
| Moderate agreement, low consensus | |
| Assess the clinical effectiveness and cost-effectiveness of breathlessness services for care/nursing home residents (R8) | 7 (6–9) |
| Convene a representative group of funders/commissioners to establish the type of outcomes they would need to see for breathlessness services (R2) | 7 (6–8) |
| Assess the clinical effectiveness and cost-effectiveness of the following components within breathlessness services: telehealth (e.g. virtual multidisciplinary team meetings, video resources for patients/carers) (R11) | 7 (6–8) |
| Assess the clinical effectiveness and cost-effectiveness of the value of the following variations of breathlessness services: as an adjunct to existing services (e.g. pulmonary rehabilitation) (R12) | 7 (6–8) |
| Assess the clinical effectiveness and cost-effectiveness of the value of the following variations of breathlessness services: group vs. individual delivery (R13) | 7 (6–8) |
The remaining statements from participants received low consensus, with strong (R1, R3–7, R10, R14–16) or moderate (R2, R8, R11–13) agreement. Free-text responses to the research statements suggested that the low consensus may result, in part, from inadequate definitions of the population of interest (e.g. for people having unplanned admissions due to breathlessness: R7) or insufficient justification for the area of research (e.g. effectiveness for care/nursing home residents: R8). Stakeholders also indicated that they had assigned lower agreement when they believed that the existing knowledge base was already well understood (e.g. the impact of breathlessness: R1):
I rated those low of which I believe there is already clear understanding, not because they are unimportant. Understanding impact for example – this is well documented – the focus now needs to be on solutions.
Participant 5009
It was emphasised that this evidence should be built on to develop effective solutions to the issues already identified. Proposed avenues for future research included identifying the best way to support informal carers; the role of charity, voluntary and community groups; involving patients in service development; and the role of psychology and emotion:
More research into the role of anxiety/emotions/health behaviours and beliefs and breathless, and psychosocial interventions to positively affect these factors.
Participant 2005
Chapter 6 Discussion and conclusion
Key findings
This project aimed provide a comprehensive and objective summary of available evidence for the clinical effectiveness and cost-effectiveness of holistic services for people with advanced malignant and non-malignant disease and chronic or refractory breathlessness. The systematic review found evidence of a positive effect on the affective components of breathlessness, and on psychological health outcomes, including depression. There was limited evidence that services led to a measurable change in overall health status or QoL, and mixed evidence regarding an effect on physical function. Evidence for cost-effectiveness was limited to four studies and was inconclusive. Services were heterogeneous in their content and delivery, but most were short term (4–6 weeks) and involved a limited number of contacts with health professionals (four to six face-to-face or telephone contacts). Patients and their informal carers valued services, particularly highlighting the provision of dignified, person-centred care from expert staff; education and information-sharing; and useful self-management interventions that were simple and portable, such as the handheld fan. The responder analysis revealed that baseline mastery and distress due to breathlessness were strong predictors of response to holistic breathlessness services. The other patient characteristics that we considered, including diagnosis, lung function, breathlessness severity, and QoL, were not associated with treatment outcome.
Considering these findings in the context of current service provision, the TEC elicited evidence-based priorities from stakeholders in this field in relation to research, clinical practice and policy around the provision, delivery and development of holistic services for chronic or refractory breathlessness. Participants showed strongest agreement and consensus on statements centred around improving access to symptom-focused, person-centred, multiprofessional care, as well as on statements regarding carers accessing and sharing breathlessness management knowledge and skills. Stakeholders who participated also called for clinical practice and policy to acknowledge and value the role of informal carers in supporting people with advanced disease and chronic breathlessness.
Strengths and limitations
Systematic review
For the systematic review, registration of the protocol and the systematic and comprehensive search across multiple databases (inclusive of grey literature), with no exclusions by publication year or language, ensured that there was a high level of transparency and representativeness. The review eligibility and quality assessments were conducted independently by two researchers. Furthermore, multiple stakeholders, including researchers, clinicians and patient/carer representatives, contributed to both the analysis and the interpretation of the review data. The integration of qualitative and quantitative studies was another strength of this work and allowed for a comprehensive evaluation of service experiences and outcomes. Recipients of services reported health benefits in line with those found with established tools, again highlighting an effect on affective and psychological domains of health.
The review also had some limitations. The meta-analyses combined data from services with varying structure, delivery and recipients, and there was evidence of statistical heterogeneity. We completed sensitivity analyses in response to this, but the overall data set was moderate in size and these sensitivity analyses inevitably compromised the precision of the effect estimates. Effect estimates may also have been inflated by lack of blinding of study personnel, and low expectations from participants in studies for which a fast-track design was not employed. Moreover, we did not assess for statistical evidence of publication bias given the low number of studies, and there was some evidence of selective reporting where study authors did not provide data for statistically non-significant findings. Our estimates therefore do not include these data. In addition, challenges with inconsistent use of and/or unclear reporting of outcome measures sometimes precluded meta-analysis (e.g. for breathlessness intensity). Evidence around cost-effectiveness was also limited by a lack of studies with an economic component.
Qualitative data were predominantly drawn from two UK services91,94,95 and patients who had fully engaged with the services. Less is understood about the experiences of the carers, the patients using these services internationally and those who dropped out and who perhaps might report less benefit. Finally, although limiting our review to studies with people with advanced disease was reflective of the current evidence base, it is important to acknowledge that limiting service access based on disease severity may limit reach and not serve those with distressing breathlessness during early stages of disease. For this reason, and in the light of our findings, we advocate access to these services based on the presence of distressing chronic or refractory breathlessness, rather than disease severity.
When interpreting the data, we note that some of the study group were co-authors on included studies. These individuals were not involved in any data extraction or quality assessment of these studies, providing a level of objectivity and impartiality. However, we acknowledge that influences of motivated reasoning (e.g. confirmation biases) have the potential to influence all stages of research, from deciding research questions to presentation of findings. 173 As such, team discussions encompassing those who were co-authors (4 out of the 12 report authors) and were not co-authors on previous studies of breathlessness services were important in balancing existing expertise and new insights, in addition to continually exposing our findings to feedback from diverse audiences (see Dissemination strategy).
Responder analysis
A strength of this work was that individual patient data were pooled from three high-quality RCTs. The combined data set enabled a more powerful analysis to be undertaken than would have been possible via study-level meta-analysis. However, it did mean that the analysis was limited to variables common across the three data sets. For this reason, we were unable to test some potentially important patient factors (e.g. multimorbidity, functional status) and intervention characteristics (e.g. setting, duration, professional input), which could have been important moderators of effect. There were fewer cases available for the secondary analysis of NRS distress due to breathlessness, as this was included in only two out of the three data sets, reducing statistical power. Finally, it is unclear to what extent the primary finding reflects regression to the mean;174 however, the magnitude of the effect observed following the breathlessness services suggests that this is not the only contributing factor. The finding that response was not influenced by other patient characteristics may relate to the baseline scores of the sample. For example, baseline scores for anxiety and depression were mild across the included studies175 and, therefore, the analysis may not have tested for influences of higher levels of psychological distress. Conversely, this finding could relate to the tailored nature of the services, which should consider each patient based on their physical, psychological, social and spiritual profile.
Transparent stakeholder consultation
The diversity of stakeholders who contributed to the workshop and consensus survey was a strength of the TEC process; participants included service providers and commissioners, voluntary sector organisations, patient and carer representatives, as well as health and social care practitioners from a range of specialties and professional groups. Although this diversity of knowledge of existing research may have led to lower consensus on participants’ statements relating to research, it also led to generation of a wide variety of suggestions for areas in need of further evidence. The priorities elicited from participants are also closely linked to emerging evidence as a result of the workshop structure, where this information was presented at the start for reflection and discussion. For example, participants’ suggestions around understanding the optimal models, economic modelling and medium- to long-term effects of breathlessness services, relate directly to findings from our systematic review, while recognition and inclusion of informal carers drew from evidence presented around their role and its impact.
It is also important to note that the suggestions made by our participants may be most applicable to health and social care in UK settings, as most people in the stakeholder consultation process were based in UK universities and NHS secondary care settings. There was also a higher number of participants with roles as a researcher or doctor, but these were also the participants for whom having more than one role was most common (e.g. 69% of researchers were also health-care professionals; 47% of doctors had additional roles in research, charities and/or commissioning). A smaller number of service user representatives attended the workshop than we had anticipated. This suggests that this engagement method may be less suited to people living with chronic breathlessness and/or with caring responsibilities and may have biased the types of priorities elicited from participants. However, more than 10 out of 74 (14%) respondents to the survey identified as a patient/carer. In addition, patient/carer representatives were members of the PAG and helped with the synthesis of the suggestions generated from the workshop participants in this role. This included working closely together to ensure that the participants’ statements were clear and understandable for people with a range of professional and personal expertise.
Having a clear and structured workshop process ensured that there was efficient collection of the attendees’ views. We mitigated the risk of bias inherent in face-to-face consultation techniques, whereby some participants may contribute to discussions more than others, by providing the opportunity to submit individual written suggestions in the response booklets. These individual responses were the primary focus in generating content for the consensus survey. A full Delphi process176 or having additional consultation rounds may have provided more opportunity to refine participants’ statements, but the TEC technique utilised the multiple forms of data collected at the workshop (including the scribe notes, graphic recording, response booklets and audio-recordings, which enabled the rapid synthesis and revision of participants’ statements). Although this was substantially undertaken by two researchers (MM and LJB), the full list of original suggestions had been shared with the PAG for transparency. Crucially, individual suggestions from participants were removed or modified only in line with the reasons outlined above (e.g. duplicates, low priority) and not on the basis of controversy or creativity. This method resulted in generally high levels of agreement and consensus, particularly around participants’ priorities for clinical practice. The consensus survey response rate was limited, although a high proportion of those completing the workshop booklets participated (74%) and the overall response rate was similar to studies that have previously utilised this method. 146–148 Importantly, all key stakeholder groups were represented.
Implications
For clinical practice
To our knowledge, this evidence synthesis offers the first systematic review in this field. The bias of clinical effect towards improved psychological health is consistent with the primary focus of holistic services to support people with advanced disease to live with breathlessness rather than to take this symptom away. The wider effect on psychological health may have been achieved through management of breathlessness, but also through addressing concurrent symptoms and the receipt of expert holistic care that put the person before their disease. The effect sizes from our meta-analyses (point estimates: distress –0.57, mastery 0.25, anxiety –0.45, depression –0.55) are larger than those achieved with psychological therapies and self-management programmes in similar populations, and more comparable to pulmonary rehabilitation. 86,87. For NRS distress and HADS anxiety and depression, the MDs also represent a minimum clinically important difference (> 1 and > 1.5, respectively). 143,177 Although few measurable effects were identified for physical function, we acknowledge the qualitative data from patients and carers that captured feelings of expanding horizons, for example being able to independently complete activities of daily living. The diverse nature of improvements in physical function may be more readily captured using individualised measures, such as goal attainment scaling. 178
The interface between holistic services for breathlessness and pulmonary rehabilitation is an important consideration for people with chronic respiratory disease. Based on current evidence, we do not view holistic breathlessness services as a replacement for pulmonary rehabilitation, which is a highly effective and underutilised intervention. 81 However, these services may have a role for people who remain highly symptomatic despite completing pulmonary rehabilitation, or as a bridge for people who decline it, including those recently hospitalised with an acute exacerbation of disease. Holistic breathlessness services may also provide an additional opportunity for health gains in those unable to complete programmes with a major exercise component (e.g. in cases for which breathlessness limits exercise to an intensity associated with a training response). 28,84,179 With high levels of health service use in breathless patients and limited resource, evidence-based factors to prioritise access based on likelihood of benefit may be useful. When services are already in place or being established, our responder analysis suggests that there is merit in focusing efforts on patients with poor psychological health relating to their breathlessness.
Stakeholders’ priorities for clinical practice elicited through our TEC with strongest consensus called for flexible and accessible person-centred care, drawing on multiple expertise, and with the widest possible coverage. Stakeholders advocated for acknowledgement of the role of informal carers, valuing and being able to respond to breathlessness as a symptom in its own right, and sharing breathlessness management skills between other professionals and with informal carers. Free-text survey comments from participants underscored the need for person-centred, holistic care that acknowledged both psychological and emotional concerns. This fits with findings from the systematic review in terms of therapeutic components most valued by patients (e.g. tailored education, psychological support) and clinical benefit relating to anxiety and depression. The promotion of joint working around breathlessness was also suggested in a previous consultation focused on combined rehabilitation services for people living with COPD and heart failure. 180 This and other elements of stakeholders’ statements relating to clinical practice are in line with a palliative care approach of person-centred care, multidisciplinary input and inclusion of informal carers in the unit of care. 54 Working to build links with, or learn from, palliative care may be an efficient way to facilitate working in line with their priorities.
For policy
Recent international guidelines advocate for early integration of palliative care in people experiencing chronic disease. 96,97 Chronic or refractory breathlessness may serve as an appropriate referral indicator, especially in non-cancer conditions for which the unpredictable course of disease and difficulty predicting survival are barriers to timely palliative care referral and receipt. 54 Indeed, a symptom-triggered approach should reach more people than approaches based on prognostication.
Stakeholders’ priorities relating to policy elicited through our TEC particularly supported the recognition of informal carers in terms of their role, importance and support needs. When appropriate, this extended to supporting and encouraging their participation in health care. This is supported by recent evidence demonstrating the substantial contribution of informal carers to people with advanced disease and chronic breathlessness,35 including that their contribution saves approximately two-thirds of what would otherwise be formal care costs,51 and the impact that this has on their own health and well-being. 51,181
Our participants also stressed the importance of education, both to increase awareness among the general public and health and social care professionals, and to upskill professionals within existing services. Multiple stakeholders suggested that breathlessness services should be developed by adapting existing services rather than introducing something new. They noted that educating professionals in social care and communities about supporting people with breathlessness should also have value because, despite the high utilisation, people with breathlessness spend most of their time outside health services.
For research
The need for economic modelling of breathlessness services was the only research recommendation that received high consensus in our stakeholder consultation. This gap in evidence was reflected in the limited economic data available from studies included in the systematic review. The heterogeneity of services with respect to staffing, structure and content highlights a concurrent need to determine the most effective service delivery models. Although the patient and carer experience data highlight valued components (e.g. simple, portable interventions; involvement of carers), additional work is needed to understand which components of holistic services are most effective and how these might be integrated into, or alongside, the existing models of care. Consistency in the use of outcome measures in future studies would permit more detailed evaluations of comparative clinical effectiveness and cost-effectiveness. Considering our findings around the impact of these services on NRS distress and CRQ mastery, but the shorter administration format of the former, consistently using NRS distress due to breathlessness to measure service outcomes could be a good starting point. Alongside this, it is crucial to acknowledge that service users should value services and be willing to access them. Understanding what makes services attractive and acceptable to service users could help better serve patients and provide evidence to inform more efficient resource allocation. Discrete choice experiments could be used to identify which ‘attributes’ would be prioritised and preferred by patients and carers.
The remaining research recommendations generated by participants received strong or moderate agreement, but low consensus. Although this, in part, may relate to inadequate definitions or justifications for the populations and/or settings, and differing knowledge bases of contributing stakeholders around existing evidence, it may also reflect the diversity of issues that are in need of better evidence and the differing opinions over which issues to prioritise. Overall, participants emphasised that research should focus on developing effective solutions rather than revisiting the problem. Additional proposed topics for research included the role of psychosocial factors in breathlessness management, enhancing community support and testing the optimal ways to support informal carers of patients with breathlessness.
Dissemination strategy
Summary of outputs and dissemination methods
Through the systematic review, we have disseminated evidence on symptom-triggered services as a means to access timely palliative care, especially in non-cancer conditions, and have provided evidence on the effectiveness of holistic breathlessness services. The responder analysis allowed for shared knowledge on patient characteristics that moderated treatment response from services. Furthermore, our participants were able to share consensus- and evidence-based priorities relating to clinical practice, policy and research.
We have shared findings via open-access publications in scientific journals. 92,171,172 Plain English summaries have been completed for each study component. 182,183 This project has been presented at several meetings to varied audiences (see List 1: presentations) and additional abstracts have been submitted [e.g. to the ERS International Congress (https://erscongress.org/)]. Throughout this project we have engaged with PPI representatives to provide feedback and inform the project from an external perspective. Furthermore, the Cicely Saunders Institute ‘Open House’ and ‘Conversation Starter’ events allowed for information-sharing with members of the public. To extend our reach further, information on our stakeholder event has been published in online news pieces in various local and international platforms (see List 2: online news articles). To enable engagement with a wider audience, we will share our publications, lay summaries and the full report via several social media platforms, including an online journal club on Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com).
Our stakeholder workshop allowed for education of professional bodies and lead service managers through presentation of relevant findings. We have shared the findings of this project directly with public bodies and policy-makers through submission to the Taskforce for Lung Health call for evidence. 184 We may have further opportunities to present findings at the Knowledge Exchange Seminar held in the Cicely Saunders Institute to educate colleagues, and at PPI workshop events to provide information to patient and public representatives (both events will have a rehabilitation theme).
List 1: presentations
We gave four internal presentations, in addition to the presentation given at the stakeholder consultation workshop. We also gave presentations at a breathlessness research interest group, the Collaboration for Leadership in Applied Health Research and Care (CLAHRC) South London award ceremony and the ERS International Congress. In addition to these presentations, we presented this research to second-year nursing students as a part of their undergraduate programme.
Reflections on patient and public involvement
We believe that this project benefited greatly from PPI, with multiple positive impacts in keeping with our aims.
Ensuring acceptable and appropriate research processes
For the stakeholder consultation, involvement of our PPI members in reviewing the workshop groupwork processes and refining the final list of statements from stakeholders ensured that these materials were clear and readable by participants from all backgrounds. Their help with piloting and redesigning the online survey to be more engaging and user-friendly was also important; for example, adding a background image and adding an option to be contacted by the researcher if patient/carer representatives wanted assistance completing the survey. This seemed to make these processes more acceptable to participants, including patient/carer representatives (who formed > 10% of our online survey respondents).
Including patient/carer voices throughout the consultation with stakeholders
The PPI members have been an important part of including patient/carer voices throughout our consultation, not only by improving the acceptability of our research methods, but also by suggesting additional patient/carer stakeholders to invite, and, through their own participation, by completing the workshop booklets and online consultation survey.
Ensuring interpretation of findings were grounded in patient/carer experiences
Having PPI members included across all project meetings ensured that there were opportunities to relate emerging findings to their real-life experiences, whether this was by highlighting inconsistencies in clinical practice or highlighting important issues related to breathlessness (e.g. relationships, including intimacy). Including a PPI member in the thematic analysis of the systematic review data ensured that the findings were not limited to researcher interpretations. This included raising the importance of reassurance for patients with breathlessness in how they are already self-managing, the need for psychological support that goes beyond just breathlessness, and the complexities of who is a ‘patient’ and who is a ‘carer’ when both may have substantial support needs.
Improving clarity and reach of dissemination
Our PPI group’s help with dissemination so far, commenting on the systematic review paper92 and co-authoring the stakeholder paper,172 has ensured increased reporting clarity. In our project meetings, they have also suggested additional ideas for disseminations to increase reach (e.g. through the NIHR INVOLVE network), and have agreed to help write plain English summaries to share alongside publications as they are accepted.
Patient and public involvement member reflections
Box 4 provides direct quotations taken from our PPI members reflecting on their work on the project and with the research team. Our PPI members said that they appreciated the opportunity to get involved in a variety of tasks and being given specific training (including one-to-one sessions) when required. They acknowledged that some of the more technical research methods could be difficult to understand at times but appreciated that the research team took time to explain and respond to questions about this during and outside meetings. Overall, they commented that they felt valued as part of the project team, leading to more rewarding and satisfying involvement.
There was ample opportunity to be involved in everything and I was amazed at the scope of opportunities – numerous and so varied. I have had experience of doing first time one-off tasks, e.g. thematic analysis on which Lisa gave me one-to-one training. This was a major step forward in my personal development.
Margaret Ogden
[The researchers] encourage questions/explanations and are excellent mentors, helping to develop each contributor’s learning experience.
Colleen Ewart
The collaborative approach and feeling a valued member of the team and not a ‘bottom on a seat for box-ticking purposes’ brings its own rewards and satisfaction to all team members, including PPI members.
Sylvia Bailey
Conclusions
In people with advanced disease, holistic services for chronic or refractory breathlessness led to improvements in psychological aspects of breathlessness and health. Services are heterogeneous in their content and delivery, but are highly valued by patients and families, who appreciate the tailored education around breathlessness; the provision of simple, portable self-management interventions; and the expert staff who provide person-centred, dignified care. Clinical response to these services is more likely in patients with low levels of mastery and high levels of distress related to their breathlessness at baseline, but is not influenced by breathlessness intensity, diagnosis, lung function or health status. When services have limited resources, prioritising patients based on levels of mastery or distress may be appropriate.
Stakeholders for this population agreed on the importance of improved access to person-centred, multiprofessional care, and support for carers to provide or access breathlessness management interventions. Future research should test the optimal models of care and educational strategies to address stakeholders priorities, plus understand how to embed the core therapeutic components of these services into routine clinical practice and health-care systems. Chronic or refractory breathlessness may serve as useful referral indicator for timely referral and receipt of palliative care, especially in non-cancer conditions for which poor prognostication causes unhelpful delays.
Acknowledgements
We extend our thanks to our team of patient/carer representatives who have provided their reflections and guidance throughout this project. Thank you to Margaret Ogden and Charlie Reilly for providing their feedback on the qualitative analysis for the systematic review. Thank you to our colleagues at the Collaboration for Leadership in Applied Health Research and Care North West London and South London for helping organise the stakeholder workshop. Thanks go to Lucy Fettes, Jo Bayly and Natasha Lovell for scribing during the group work, and to Joel Cooper (www.joelcooper.co.uk) for capturing the day in a graphic recording. Finally, we thank all who participated in the stakeholder workshop, including those who agreed to be named here: Sabrina Bajwah, Noel Baxter, Jo Bayly, James Beattie, Kate Binnie, Sara Booth, Lisa J Brighton, Julie Burkin, John Cleland, Patrick Doherty, Sarah Elkin, Rachael Evans, Colleen Ewart, Morag Farquhar, Kate Flemming, Marjolein Gysels, Georgia Hardavella, Irene J Higginson, Natasha Lovell, Rachel Macklin, Matthew Maddocks, Vince Mak, Finbarr Martin, Dionne Matthew, Sophie Miller, Margaret Ogden, Charlie Reilly, Louise Restrick, Natasha Smallwood, Wei Gao, Jenny Wingham and Trish Winn. Thank you also to Colleen Ewart, Sylvia Bailey and Margaret Ogden for their contributions to the stakeholder consultation paper.
Contributions of authors
Dr Matthew Maddocks (Senior Lecturer in Health Services Research and Specialist Physiotherapist, KCL) designed and led the study, obtained funding and takes overall responsibility for the content of the report. Matthew Maddocks contributed to the eligibility and quality assessment, analysis and interpretation for the review, analysis and interpretation for the responder analysis, stakeholder workshop data collection and facilitation, analysing and refining stakeholder statements, and interpreting the consensus survey results. He also contributed to drafting, revising and approving the final report.
Ms Lisa Jane Brighton (Research assistant, KCL) contributed to the study design, systematic review searches, eligibility and quality assessment, data extraction, analysis and interpretation, as well as the cleaning, analysing and interpreting of the findings for the responder analysis. She also contributed to the stakeholder workshop data collection and facilitation, analysing and refining stakeholder statements, and the interpretation of the consensus survey results. She also contributed to drafting, revising and approving the final report.
Dr Morag Farquhar (Senior Lecturer, University of East Anglia) contributed to the study design, systematic review and responder analysis, data analysis and interpretation, stakeholder workshop facilitation, analysing and refining stakeholder statements, and the interpretation of the consensus survey results. She also contributed to drafting, revising and approving the final report.
Dr Sara Booth (Honorary consultant and Associate Lecturer, University of Cambridge) contributed to the study design, systematic review and responder analysis results interpretation, stakeholder workshop facilitation, analysing and refining stakeholder statements, and interpreting the consensus survey results. She also contributed to drafting, revising and approving the final report.
Dr Sophie Miller (Specialty Training Registrar, St Christopher’s Hospice) contributed to the study design; systematic review searches, eligibility and quality assessment; data extraction, analysis and interpretation; analysing and refining the stakeholder workshop statements; and interpreting the consensus survey results. She also contributed to drafting, revising and approving the final report.
Ms Lara Klass (Research administrator, KCL) contributed to the systematic review data extraction, analysis and interpretation. She also contributed to drafting, revising and approving the final report.
Ms India Tunnard (Research administrator, KCL) contributed to the study design; systematic review data extraction, analysis and interpretation; stakeholder workshop data collection, analysing and refining the stakeholder statements; and interpreting the consensus survey results, She also contributed to drafting, revising and approving the final report.
Dr Deokhee Yi (Health Economist, KCL) contribute to the study design, systematic review data extraction, analysis and interpretation; interpretation of the responder analysis findings; and analysing and refining the stakeholder workshop statements interpreting the consensus survey results. She also contributed to drafting, revising and approving the final report.
Dr Wei Gao (Senior Lecturer in statistics and epidemiology, KCL) contributed to the study design, interpretation of systematic review findings, analysis and interpretation for the responder analysis, analysing and refining the stakeholder workshop statements and interpreting the consensus survey results. She also contributed to drafting, revising and approving the final report.
Dr Sabrina Bajwah (Consultant and honorary senior lecturer, KCL) contributed to the study design, interpretation of systematic review and responder analysis findings, stakeholder workshop facilitation, analysing and refining stakeholder statements and interpreting the consensus survey results. She also contributed to drafting, revising and approving the final report.
Dr William D-C Man (Senior Lecturer/Consultant Chest Physician, Imperial College London) contributed to the study design, interpretation of systematic review and responder analysis findings, analysing and refining stakeholder workshop statements and interpreting the consensus survey results. He also contributed to drafting, revising and approving the final report.
Professor Irene J Higginson (Professor of Palliative Care and Policy, Scientific Director of Cicely Saunders International and NIHR Senior Investigator, KCL) designed the study, obtained funding and takes overall responsibility for the content of the report. Irene J Higginson contributed to the eligibility assessment and interpretation for the review, analysis and interpretation for the responder analysis, analysing and refining stakeholder workshop statements, and interpreting the consensus survey results. She also contributed to drafting, revising and approving the final report.
Publications
Maddocks M, Lovell N, Booth S, Man WD, Higginson IJ. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet 2017;390:988–1002.
Brighton LJ, Gao W, Farquhar M, Booth S, Bajwah S, Man WDC, et al. Predicting outcomes following holistic breathlessness services: a pooled analysis of individual patient data. Palliat Med 2019;33:462–6.
Brighton LJ, Miller S, Farquhar M, Booth S, Yi D, Gao W, et al. Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax 2019;74:270–81.
Brighton LJ, Tunnard I, Farquhar M, Booth S, Yi D, Gao W, et al. Recommendations for services for people with living with chronic breathlessness in advanced disease: results of a transparent expert consultation. Chron Resp Dis 2019;16:1–12.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Meek PM, Schwartzstein RM, Adams L, Altose MD, Breslin EH, Carrieri-Kohlman V, et al. Dyspnea: mechanisms, assessment, and management: a consensus statement. Am J Resp Crit Care Med 1999;159:321-40. https://doi.org/10.1164/ajrccm.159.1.ats898.
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012;185:435-52. https://doi.org/10.1164/rccm.201111-2042ST.
- Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nat Clin Pract Oncol 2008;5:90-100. https://doi.org/10.1038/ncponc1034.
- Booth S, Bausewein C, Higginson I, Moosavi SH. Pharmacological treatment of refractory breathlessness. Expert Rev Respir Med 2009;3:21-36. https://doi.org/10.1586/17476348.3.1.21.
- Horton R, Rocker G. Contemporary issues in refractory dyspnoea in advanced chronic obstructive pulmonary disease. Curr Opin Support Palliat Care 2010;4:56-62. https://doi.org/10.1097/SPC.0b013e328338c1c6.
- Bowden JA, To TH, Abernethy AP, Currow DC. Predictors of chronic breathlessness: a large population study. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-33.
- Currow D, Johnson M, White P, Abernethy A. Evidence-based intervention for chronic refractory breathlessness: practical therapies that make a difference. Br J Gen Pract 2013;63:609-10. https://doi.org/10.3399/bjgp13X674611.
- Currow DC, Quinn S, Greene A, Bull J, Johnson MJ, Abernethy AP. The longitudinal pattern of response when morphine is used to treat chronic refractory dyspnea. J Palliat Med 2013;16:881-6. https://doi.org/10.1089/jpm.2012.0591.
- Johnson MJ, Currow DC. Chronic refractory breathlessness is a distinct clinical syndrome. Curr Opin Support Palliat Care 2015;9:203-5. https://doi.org/10.1097/SPC.0000000000000150.
- Simon ST, Higginson IJ, Benalia H, Gysels M, Murtagh FE, Spicer J, et al. Episodic and continuous breathlessness: a new categorization of breathlessness. J Pain Symptom Manage 2013;45:1019-29. https://doi.org/10.1016/j.jpainsymman.2012.06.008.
- Simon ST, Higginson IJ, Benalia H, Gysels M, Murtagh FE, Spicer J, et al. Episodes of breathlessness: types and patterns – a qualitative study exploring experiences of patients with advanced diseases. Palliat Med 2013;27:524-32. https://doi.org/10.1177/0269216313480255.
- Simon ST, Bausewein C, Schildmann E, Higginson IJ, Magnussen H, Scheve C, et al. Episodic breathlessness in patients with advanced disease: a systematic review. J Pain Symptom Manage 2013;45:561-78. https://doi.org/10.1016/j.jpainsymman.2012.02.022.
- Linde P, Hanke G, Voltz R, Simon ST. Unpredictable episodic breathlessness in patients with advanced chronic obstructive pulmonary disease and lung cancer: a qualitative study. Support Care Cancer 2018;26:1097-104. https://doi.org/10.1007/s00520-017-3928-9.
- Mercadante S, Fusco F, Caruselli A, Cartoni C, Masedu F, Valenti M, et al. Background and episodic breathlessness in advanced cancer patients followed at home. Curr Med Res Opin 2017;33:155-60. https://doi.org/10.1080/03007995.2016.1240668.
- Mercadante S. Episodic breathlessness in patients with advanced cancer: characteristics and management. Drugs 2018;78:543-7. https://doi.org/10.1007/s40265-018-0879-5.
- Johnson MJ, Yorke J, Hansen-Flaschen J, Lansing R, Ekström M, Similowski T, et al. Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J 2017;49. https://doi.org/10.1183/13993003.02277-2016.
- Weingärtner V, Scheve C, Gerdes V, Schwarz-Eywill M, Prenzel R, Otremba B, et al. Characteristics of episodic breathlessness as reported by patients with advanced chronic obstructive pulmonary disease and lung cancer: results of a descriptive cohort study. Palliat Med 2015;29:420-8. https://doi.org/10.1177/0269216314563428.
- Currow DC, Abernethy AP, Allcroft P, Banzett RB, Bausewein C, Booth S, et al. The need to research refractory breathlessness. Eur Respir J 2016;47:342-3. https://doi.org/10.1183/13993003.00653-2015.
- Moens K, Higginson IJ, Harding R. EURO IMPACT . Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage 2014;48:660-77. https://doi.org/10.1016/j.jpainsymman.2013.11.009.
- Elkington H, White P, Addington-Hall J, Higgs R, Edmonds P. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med 2005;19:485-91. https://doi.org/10.1191/0269216305pm1056oa.
- Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage 2009;38:115-23. https://doi.org/10.1016/j.jpainsymman.2008.07.006.
- Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199-203. https://doi.org/10.1164/ajrccm.157.1.9704130.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006;31:58-69. https://doi.org/10.1016/j.jpainsymman.2005.06.007.
- Kavalieratos D, Kamal AH, Abernethy AP, Biddle AK, Carey TS, Dev S, et al. Comparing unmet needs between community-based palliative care patients with heart failure and patients with cancer. J Palliat Med 2014;17:475-81. https://doi.org/10.1089/jpm.2013.0526.
- Janssen DJ, Spruit MA, Uszko-Lencer NH, Schols JM, Wouters EF. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med 2011;14:735-43. https://doi.org/10.1089/jpm.2010.0479.
- Janssen DJ, Wouters EF, Schols JM, Spruit MA. Self-perceived symptoms and care needs of patients with severe to very severe chronic obstructive pulmonary disease, congestive heart failure or chronic renal failure and its consequences for their closest relatives: the research protocol. BMC Palliat Care 2008;7. https://doi.org/10.1186/1472-684X-7-5.
- Etkind SN, Bone AE, Gomes B, Lovell N, Evans CJ, Higginson IJ, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0860-2.
- Gysels MH, Higginson IJ. The lived experience of breathlessness and its implications for care: a qualitative comparison in cancer, COPD, heart failure and MND. BMC Palliat Care 2011;10. https://doi.org/10.1186/1472-684X-10-15.
- Malik FA, Gysels M, Higginson IJ. Living with breathlessness: a survey of caregivers of breathless patients with lung cancer or heart failure. Palliat Med 2013;27:647-56. https://doi.org/10.1177/0269216313488812.
- Janssen DJA, Spruit MA, Schols JMGA, Wouters EFM. A call for high-quality advance care planning in outpatients with severe COPD or chronic heart failure. Chest 2011;139:1081-8. https://doi.org/10.1378/chest.10-1753.
- Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliat Support Care 2003;1:337-44. https://doi.org/10.1017/S1478951503030499.
- Dorman S, Jolley C, Abernethy A, Currow D, Johnson M, Farquhar M, et al. Researching breathlessness in palliative care: consensus statement of the National Cancer Research Institute Palliative Care Breathlessness Subgroup. Palliat Med 2009;23:213-27. https://doi.org/10.1177/0269216309102520.
- Dudgeon DJ. Managing dyspnea and cough. Hematol Oncol Clin North Am 2002;16:557-77. https://doi.org/10.1016/S0889-8588(02)00019-9.
- Currow DC, Dal Grande E, Ferreira D, Johnson MJ, McCaffrey N, Ekström M. Chronic breathlessness associated with poorer physical and mental health-related quality of life (SF-12) across all adult age groups. Thorax 2017;72:1151-3. https://doi.org/10.1136/thoraxjnl-2016-209908.
- Farquhar M. Carers and breathlessness. Curr Opin Support Palliat Care 2017;11:165-73. https://doi.org/10.1097/SPC.0000000000000281.
- Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med 2010;13:1109-18. https://doi.org/10.1089/jpm.2010.0068.
- Frostad A, Søyseth V, Andersen A, Gulsvik A. Respiratory symptoms as predictors of all-cause mortality in an urban community: a 30-year follow-up. J Intern Med 2006;259:520-9. https://doi.org/10.1111/j.1365-2796.2006.01631.x.
- Frostad A, Søyseth V, Haldorsen T, Andersen A, Gulsvik A. Impact of respiratory symptoms on lung cancer: 30-year follow-up of an urban population. Lung Cancer 2008;60:22-30. https://doi.org/10.1016/j.lungcan.2007.09.002.
- Frostad A, Soyseth V, Haldorsen T, Andersen A, Gulsvik A. Respiratory symptoms and long-term cardiovascular mortality. Respir Med 2007;101:2289-96. https://doi.org/10.1016/j.rmed.2007.06.023.
- Beynon T, Gomes B, Murtagh FE, Glucksman E, Parfitt A, Burman R, et al. How common are palliative care needs among older people who die in the emergency department?. BMJ Support Palliat Care 2011;1:184-8. https://doi.org/10.1136/bmjspcare.2009.090019rep.
- Kane PM, Vinen K, Murtagh FE. Palliative care for advanced renal disease: a summary of the evidence and future direction. Palliat Med 2013;27:817-21. https://doi.org/10.1177/0269216313491796.
- Gysels MH, Higginson IJ. Caring for a person in advanced illness and suffering from breathlessness at home: threats and resources. Palliat Support Care 2009;7:153-62. https://doi.org/10.1017/S1478951509000200.
- Verberkt CA, van den Beuken-van Everdingen MHJ, Schols JMGA, Datla S, Dirksen CD, Johnson MJ, et al. Respiratory adverse effects of opioids for breathlessness: a systematic review and meta-analysis. Eur Respir J 2017;50. https://doi.org/10.1183/13993003.01153-2017.
- Seow H, Barbera L, Sutradhar R, Howell D, Dudgeon D, Atzema C, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol 2011;29:1151-8. https://doi.org/10.1200/JCO.2010.30.7173.
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage 2010;39:680-90. https://doi.org/10.1016/j.jpainsymman.2009.09.017.
- Maguire R, Stoddart K, Flowers P, McPhelim J, Kearney N. An interpretative phenomenological analysis of the lived experience of multiple concurrent symptoms in patients with lung cancer: a contribution to the study of symptom clusters. Eur J Oncol Nurs 2014;18:310-15. https://doi.org/10.1016/j.ejon.2014.02.004.
- Bentsen SB, Gundersen D, Assmus J, Bringsvor H, Berland A. Multiple symptoms in patients with chronic obstructive pulmonary disease in Norway. Nurs Health Sci 2013;15:292-9. https://doi.org/10.1111/nhs.12031.
- Bone AE, Gao W, Gomes B, Sleeman KE, Maddocks M, Wright J, et al. Factors associated with transition from community settings to hospital as place of death for adults aged 75 and older: a population-based mortality follow-back survey. J Am Geriatr Soc 2016;64:2210-17. https://doi.org/10.1111/jgs.14442.
- Currow DC, Johnson MJ. Chronic breathlessness: silent and deadly. Curr Opin Support Palliat Care 2016;10:221-2. https://doi.org/10.1097/SPC.0000000000000231.
- Siegel M, Bigelow S. Palliative care symptom management in the emergency department: the ABC’s of symptom management for the emergency physician. J Emerg Med 2018;54:25-32. https://doi.org/10.1016/j.jemermed.2017.08.004.
- Dzingina MD, Reilly CC, Bausewein C, Jolley CJ, Moxham J, McCrone P, et al. Variations in the cost of formal and informal health care for patients with advanced chronic disease and refractory breathlessness: a cross-sectional secondary analysis. Palliat Med 2017;31:369-77. https://doi.org/10.1177/0269216317690994.
- Hutchinson A, Pickering A, Williams P, Bland JM, Johnson MJ. Breathlessness and presentation to the emergency department: a survey and clinical record review. BMC Pulm Med 2017;17. https://doi.org/10.1186/s12890-017-0396-4.
- Trueman D, Woodcock F, Hancock E. Estimating the Economic Burden of Respiratory Illness in the UK 2017. www.blf.org.uk/policy/economic-burden (accessed April 2019).
- Maddocks M, Lovell N, Booth S, Man WD, Higginson IJ. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet 2017;390:988-1002. https://doi.org/10.1016/S0140-6736(17)32127-X.
- Ecenarro PS, Iguiñiz MI, Tejada SP, Malanda NM, Imizcoz MA, Marlasca LA, et al. Management of COPD in end-of-life care by Spanish pulmonologists. COPD 2018;15:171-6. https://doi.org/10.1080/15412555.2018.1441274.
- Marciniuk DD, Goodridge D, Hernandez P, Rocker G, Balter M, Bailey P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J 2011;18:69-78. https://doi.org/10.1155/2011/745047.
- Rocker GM, Simpson AC, Horton R. Palliative care in advanced lung disease: the challenge of integrating palliation into everyday care. Chest 2015;148:801-9. https://doi.org/10.1378/chest.14-2593.
- Simon ST, Higginson IJ, Booth S, Harding R, Weingärtner V, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev 2016;10. https://doi.org/10.1002/14651858.CD007354.pub3.
- Johnson M, Barbetta C, Currow D, Maddocks M, McDonald V, Mahadeva R, et al. Palliative Care in Respiratory Disease. ERS Monograph. 73. Sheffield: ERS; 2016.
- Rocker GM, Simpson AC, Horton R, Sinuff T, Demmons J, Hernandez P, et al. Joanne Young BHSc . Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomes. CMAJ Open 2013;1:E27-36. https://doi.org/10.9778/cmajo.20120031.
- Ekström M, Bajwah S, Bland JM, Currow DC, Hussain J, Johnson MJ. One evidence base; three stories: do opioids relieve chronic breathlessness?. Thorax 2018;73:88-90. https://doi.org/10.1136/thoraxjnl-2016-209868.
- Barnes H, McDonald J, Smallwood N, Manser R. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD011008.pub2.
- Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax 2002;57:939-44. https://doi.org/10.1136/thorax.57.11.939.
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol 2012;51:996-1008. https://doi.org/10.3109/0284186X.2012.709638.
- Simon ST, Higginson IJ, Booth S, Harding R, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev 2010;1. https://doi.org/10.1002/14651858.CD007354.pub2.
- Simon ST, Köskeroglu P, Gaertner J, Voltz R. Fentanyl for the relief of refractory breathlessness: a systematic review. J Pain Symptom Manage 2013;46:874-86. https://doi.org/10.1016/j.jpainsymman.2013.02.019.
- Abernethy AP, McDonald CF, Frith PA, Clark K, Herndon JE, Marcello J, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010;376:784-93. https://doi.org/10.1016/S0140-6736(10)61115-4.
- Vozoris NT, O’Donnell DE, Bell C, Gill SS, Rochon PA. Incident opioid use is associated with risk of respiratory harm in non-palliative COPD. Eur Respir J 2017;49. https://doi.org/10.1183/13993003.02529-2016.
- Vozoris NT, Wang X, Austin PC, Lee DS, Stephenson AL, O’Donnell DE, et al. Adverse cardiac events associated with incident opioid drug use among older adults with COPD. Eur J Clin Pharmacol 2017;73:1287-95. https://doi.org/10.1007/s00228-017-2278-3.
- Rocker G, Bourbeau J, Downar J. The new ‘opioid crisis’: scientific bias, media attention, and potential harms for patients with refractory dyspnea. J Palliat Med 2018;21:120-2. https://doi.org/10.1089/jpm.2017.0619.
- Downar J, Colman R, Horton R, Hernandez P, Rocker G. Opioids in COPD: a cause of death or a marker of illness severity?. Eur Respir J 2016;48:1520-1. https://doi.org/10.1183/13993003.01443-2016.
- Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD004769.pub2.
- Uronis HE, Ekström MP, Currow DC, McCrory DC, Samsa GP, Abernethy AP. Oxygen for relief of dyspnoea in people with chronic obstructive pulmonary disease who would not qualify for home oxygen: a systematic review and meta-analysis. Thorax 2015;70:492-4. https://doi.org/10.1136/thoraxjnl-2014-205720.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD006429.pub3.
- Rocker G. Harms of overoxygenation in patients with exacerbation of chronic obstructive pulmonary disease. CMAJ 2017;189:E762-E763. https://doi.org/10.1503/cmaj.170196.
- Booth S, Kelly MJ, Cox NP, Adams L, Guz A. Does oxygen help dyspnea in patients with cancer?. Am J Respir Crit Care Med 1996;153:1515-18. https://doi.org/10.1164/ajrccm.153.5.8630595.
- Smoller JW, Pollack MH, Otto MW, Rosenbaum JF, Kradin RL. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med 1996;154:6-17. https://doi.org/10.1164/ajrccm.154.1.8680700.
- Smoller JW, Pollack MH, Systrom D, Kradin RL. Sertraline effects on dyspnea in patients with obstructive airways disease. Psychosomatics 1998;39:24-9. https://doi.org/10.1016/S0033-3182(98)71377-5.
- Papp LA, Weiss JR, Greenberg HE, Rifkin A, Scharf SM, Gorman JM, et al. Sertraline for chronic obstructive pulmonary disease and comorbid anxiety and mood disorders. Am J Psychiatry 1995;152. https://doi.org/10.1176/ajp.152.10.1531a.
- Spathis A, Booth S, Moffat C, Hurst R, Ryan R, Chin C, et al. The Breathing, Thinking, Functioning clinical model: a proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim Care Respir Med 2017;27. https://doi.org/10.1038/s41533-017-0024-z.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD003793.pub3.
- Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal H, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD003331.pub4.
- Bausewein C, Booth S, Gysels M, Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev 2008;2. https://doi.org/10.1002/14651858.CD005623.pub2.
- Brown TM, Hernandez AF, Bittner V, Cannon CP, Ellrodt G, Liang L, et al. Predictors of cardiac rehabilitation referral in coronary artery disease patients: findings from the American Heart Association’s Get With The Guidelines Program. J Am Coll Cardiol 2009;54:515-21. https://doi.org/10.1016/j.jacc.2009.02.080.
- Maddocks M, Kon SS, Canavan JL, Jones SE, Nolan CM, Labey A, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016;71:988-95. https://doi.org/10.1136/thoraxjnl-2016-208460.
- Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000;355:362-8. https://doi.org/10.1016/S0140-6736(99)07042-7.
- Coventry PA, Bower P, Keyworth C, Kenning C, Knopp J, Garrett C, et al. The effect of complex interventions on depression and anxiety in chronic obstructive pulmonary disease: systematic review and meta-analysis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0060532.
- Booth S, Farquhar M, Gysels M, Bausewein C, Higginson IJ. The impact of a breathlessness intervention service (BIS) on the lives of patients with intractable dyspnea: a qualitative phase 1 study. Palliat Support Care 2006;4:287-93. https://doi.org/10.1017/S1478951506060366.
- Rocker GM, Verma JY. ‘INSPIRED’ COPD Outreach Program™: doing the right things right. Clin Invest Med 2014;37:E311-9. https://doi.org/10.25011/cim.v37i5.22011.
- Corner J, Plant H, A’Hern R, Bailey C. Non-pharmacological intervention for breathlessness in lung cancer. Palliat Med 1996;10:299-305. https://doi.org/10.1177/026921639601000405.
- Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0194-2.
- Brighton LJ, Miller S, Farquhar M, Booth S, Yi D, Gao W, et al. Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax 2019;74:270-81. https://doi.org/10.1136/thoraxjnl-2018-211589.
- Raine R, Sanderson C, Black N. Developing clinical guidelines: a challenge to current methods. BMJ 2005;331:631-3. https://doi.org/10.1136/bmj.331.7517.631.
- Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014;2:979-87. https://doi.org/10.1016/S2213-2600(14)70226-7.
- Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, et al. The clinical and cost effectiveness of a Breathlessness Intervention Service for patients with advanced non-malignant disease and their informal carers: mixed findings of a mixed method randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1304-6.
- Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:96-112. https://doi.org/10.1200/JCO.2016.70.1474.
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 2017;195:557-82. https://doi.org/10.1164/rccm.201701-0218PP.
- Bredin M, Corner J, Krishnasamy M, Plant H, Bailey C, A’Hern R. Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancer. BMJ 1999;318:901-4. https://doi.org/10.1136/bmj.318.7188.901.
- Barton R, English A, Nabb S, Rigby AS, Johnson MJ. A randomised trial of high vs. low intensity training in breathing techniques for breathless patients with malignant lung disease: a feasibility study. Lung Cancer 2010;70:313-19. https://doi.org/10.1016/j.lungcan.2010.03.007.
- Chin CA, Butcher HH, Spathis A, Ryan R, Johnson M, Pattinson K, et al. What’s trending in breathlessness research? Proceedings from the 8th Annual Meeting of the Breathlessness Research Interest Group. 8th Annual Meeting of the Breathlessness Research Interest Group. Progress in Palliative Care 2015;23:326-30. https://doi.org/10.1179/1743291X15Y.0000000005.
- Connors S, Graham S, Peel T. An evaluation of a physiotherapy led non-pharmacological breathlessness programme for patients with intrathoracic malignancy. Palliat Med 2007;21:285-7. https://doi.org/10.1177/0269216307079172.
- Farquhar M, Higginson IJ, Fagan P, Booth S. Results of a pilot investigation into a complex intervention for breathlessness in advanced chronic obstructive pulmonary disease (COPD): brief report. Palliat Support Care 2010;8:143-9. https://doi.org/10.1017/S1478951509990897.
- Reilly CC, Bausewein C, Pannell C, Moxham J, Jolley CJ, Higginson IJ. Patients’ experiences of a new integrated breathlessness support service for patients with refractory breathlessness: results of a postal survey. Palliat Med 2016;30:313-22. https://doi.org/10.1177/0269216315600103.
- Gysels M, Reilly CC, Jolley CJ, Pannell C, Spoorendonk F, Bellas H, et al. How does a new breathlessness support service affect patients?. Eur Respir J 2015;46:1515-18. https://doi.org/10.1183/13993003.00751-2015.
- Wood H, Connors S, Dogan S, Peel T. Individual experiences and impacts of a physiotherapist-led, non-pharmacological breathlessness programme for patients with intrathoracic malignancy: a qualitative study. Palliat Med 2013;27:499-507. https://doi.org/10.1177/0269216312464093.
- Booth S. Improving research methodology in breathlessness: a meeting convened by the MRC clinical trials unit and the Cicely Saunders Foundation. Palliat Med 2006;20:219-20. https://doi.org/10.1191/0269216306pm1132xx.
- Moore S, Wells M, Plant H, Fuller F, Wright M, Corner J. Nurse specialist led follow-up in lung cancer: the experience of developing and delivering a new model of care. Eur J Oncol Nurs 2006;10:364-77. https://doi.org/10.1016/j.ejon.2006.01.007.
- Kamal AH, Maguire JM, Wheeler JL, Currow DC, Abernethy AP. Dyspnea review for the palliative care professional: treatment goals and therapeutic options. J Palliat Med 2012;15:106-14. https://doi.org/10.1089/jpm.2011.0110.
- Abernethy AP, Uronis HE, Wheeler JL, Currow DC. Management of dyspnea in patients with chronic obstructive pulmonary disease. Wien Med Wochenschr 2009;159:583-90. https://doi.org/10.1007/s10354-009-0727-z.
- Booth S, Moffat C, Burkin J, Galbraith S, Bausewein C. Nonpharmacological interventions for breathlessness. Curr Opin Support Palliat Care 2011;5:77-86. https://doi.org/10.1097/SPC.0b013e3283460c93.
- Wiseman R, Rowett D, Allcroft P, Abernethy A, Currow DC. Chronic refractory dyspnoea – evidence based management. Aust Fam Physician 2013;42:137-40.
- Currow DC, Higginson IJ, Johnson MJ. Breathlessness – current and emerging mechanisms, measurement and management: a discussion from an European Association of Palliative Care workshop. Palliat Med 2013;27:932-8. https://doi.org/10.1177/0269216313493819.
- Thomas S, Bausewein C, Higginson I, Booth S. Breathlessness in cancer patients – implications, management and challenges. Eur J Oncol Nurs 2011;15:459-69. https://doi.org/10.1016/j.ejon.2010.11.013.
- Yates P, Zhao I. Update on complex nonpharmacological interventions for breathlessness. Curr Opin Support Palliat Care 2012;6:144-52. https://doi.org/10.1097/SPC.0b013e3283536413.
- Simon ST, Bausewein C. Management of refractory breathlessness in patients with advanced cancer. Wien Med Wochenschr 2009;159:591-8. https://doi.org/10.1007/s10354-009-0728-y.
- Laviolette L, Laveneziana P. ERS Research Seminar Faculty . Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J 2014;43:1750-62. https://doi.org/10.1183/09031936.00092613.
- Donesky D. Management of acute breathlessness in the person with chronic refractory breathlessness. Curr Opin Support Palliat Care 2015;9:212-16. https://doi.org/10.1097/SPC.0000000000000153.
- Uronis HE, Currow DC, Abernethy AP. Palliative management of refractory dyspnea in COPD. Int J Chron Obstruct Pulmon Dis 2006;1:289-304. https://doi.org/10.2147/copd.2006.1.3.289.
- Jassem E, Kozielski J, Górecka D, Krakowiak P, Krajnik M, Słomiński JM. Integrated care for patients with advanced chronic obstructive pulmonary disease: a new approach to organization. Pol Arch Med Wewn 2010;120:423-8.
- Yorke J, Brettle A, Molassiotis A. Nonpharmacological interventions for managing respiratory symptoms in lung cancer. Chron Respir Dis 2012;9:117-29. https://doi.org/10.1177/1479972312441632.
- Ekström MP, Abernethy AP, Currow DC. The management of chronic breathlessness in patients with advanced and terminal illness. BMJ 2015;350. https://doi.org/10.1136/bmj.g7617.
- Johnson MJ, Oxberry SG. The management of dyspnoea in chronic heart failure. Curr Opin Support Palliat Care 2010;4:63-8. https://doi.org/10.1097/SPC.0b013e32833929aa.
- Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V, Curtis JR, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010;137:674-91. https://doi.org/10.1378/chest.09-1543.
- Mularski RA, Reinke LF, Carrieri-Kohlman V, Fischer MD, Campbell ML, Rocker G, et al. An official American Thoracic Society workshop report: assessment and palliative management of dyspnea crisis. Ann Am Thorac Soc 2013;10:S98-106. https://doi.org/10.1513/AnnalsATS.201306-169ST.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9. https://doi.org/10.7326/0003-4819-151-4-200908180-00135.
- Maddocks M, Higginson IJ, Farquhar M, Yi D, Gao W, Bajwah S, et al. Protocol: Holistic Services for Refractory Breathlessness in Advanced Malignant and Non-Malignant Disease. 2017. www.crd.york.ac.uk/PROSPERO/display_record.php?ID%20=%20CRD42017057508 (accessed 26 January 2018).
- Kmet LM, Lee RC, Cook LS. Standard Quality Assessment Criteria for Evaluating Primary Research Papers From a Variety of Fields. Edmonton, AB: Alberta Heritage Foundation for Medical Research; 2004.
- Pluye P, Robert E, Cargo M, Bartlett G, O’Cathain A, Griffiths F, et al. Proposal: A Mixed Methods Appraisal Tool for Systematic Mixed Studies Reviews. 2011. http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/84371689/MMAT%202011%20criteria%20and%20tutorial%202011-06-29updated2014.08.21.pdf (accessed 2 June 2017).
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-18.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. PAFS consensus group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355. https://doi.org/10.1136/bmj.i5239.
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007;147:W163-94. https://doi.org/10.7326/0003-4819-147-8-200710160-00010-w1.
- Tong A, Sainsbury P, Craig J. COnsolidated criteria for REporting Qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. GRADE Working Group . What is ‘quality of evidence’ and why is it important to clinicians?. BMJ 2008;336:995-8. https://doi.org/10.1136/bmj.39490.551019.BE.
- Spruit MA, Pitta F, Garvey C, ZuWallack RL, Roberts CM, Collins EG, et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J 2014;43:1326-37. https://doi.org/10.1183/09031936.00145613.
- Gagnier JJ, Morgenstern H, Altman DG, Berlin J, Chang S, McCulloch P, et al. Consensus-based recommendations for investigating clinical heterogeneity in systematic reviews. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-106.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0: 9.5.2 Identifying and Measuring Heterogeneity. 2011. http://handbook-5-1.cochrane.org/ (accessed 19 January 2017).
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0: 10.4 Detecting Reporting Biases. 2011. http://handbook-5-1.cochrane.org/ (accessed 19 January 2017).
- Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8. https://doi.org/10.1186/1471-2288-8-45.
- Johnson MJ, Bland JM, Oxberry SG, Abernethy AP, Currow DC. Opioids for chronic refractory breathlessness: patient predictors of beneficial response. Eur Respir J 2013;42:758-66. https://doi.org/10.1183/09031936.00139812.
- Schünemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ). COPD 2005;2:81-9. https://doi.org/10.1081/COPD-200050651.
- Ekström M, Currow DC, Johnson MJ. Outcome measurement of refractory breathlessness: endpoints and important differences. Curr Opin Support Palliat Care 2015;9:238-43. https://doi.org/10.1097/SPC.0000000000000147.
- Yardley L, Beyer N, Hauer K, McKee K, Ballinger C, Todd C. Recommendations for promoting the engagement of older people in activities to prevent falls. Qual Saf Health Care 2007;16:230-4. https://doi.org/10.1136/qshc.2006.019802.
- Evans CJ, Harding R, Higginson IJ. MORECare . ‘Best practice’ in developing and evaluating palliative and end-of-life care services: a meta-synthesis of research methods for the MORECare project. Palliat Med 2013;27:885-98. https://doi.org/10.1177/0269216312467489.
- Farquhar M, Preston N, Evans CJ, Grande G, Short V, Benalia H, et al. Mixed methods research in the development and evaluation of complex interventions in palliative and end-of-life care: report on the MORECare consensus exercise. J Palliat Med 2013;16:1550-60. https://doi.org/10.1089/jpm.2012.0572.
- Gysels M, Evans CJ, Lewis P, Speck P, Benalia H, Preston NJ, et al. MORECare research methods guidance development: recommendations for ethical issues in palliative and end-of-life care research. Palliat Med 2013;27:908-17. https://doi.org/10.1177/0269216313488018.
- Preston NJ, Fayers P, Walters SJ, Pilling M, Grande GE, Short V, et al. Recommendations for managing missing data, attrition and response shift in palliative and end-of-life care research: part of the MORECare research method guidance on statistical issues. Palliat Med 2013;27:899-907. https://doi.org/10.1177/0269216313486952.
- Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-111.
- Evans CJ, Benalia H, Preston NJ, Grande G, Gysels M, Short V, et al. The selection and use of outcome measures in palliative and end-of-life care research: the MORECare International Consensus Workshop. J Pain Symptom Manage 2013;46:925-37. https://doi.org/10.1016/j.jpainsymman.2013.01.010.
- Stevinson C, Preston N, Todd C. Cancer Experiences Collaborative (CECo) . Defining priorities in prognostication research: results of a consensus workshop. Palliat Med 2010;24:462-8. https://doi.org/10.1177/0269216310368452.
- Garcia J, Evans J, Reshaw M. ‘Is there anything else you would like to tell us’ – methodological issues in the use of free-text comments from postal surveys. Quality Quantity 2004;38:113-25. https://doi.org/10.1023/B:QUQU.0000019394.78970.df.
- Budgeting for Involvement: Practical Advice on Budgeting for Actively Involving the Public in Research Studies. London: Mental Health Research Network, and Eastleigh: INVOLVE; 2013.
- Pearce L, MacLeod V, Baker A, Grant A, Dotesio-Eyes N, Sydor M, et al. Randomised controlled trial of nurse-led breathlessness intervention to improve the management of breathlessness in patients with chronic obstructive pulmonary disease at a district general hospital. Thorax 2006;61.
- Yates P, Hardy J, Kwun F, Brunelli V, Zhao I, Skerman H, et al. A randomised controlled trial of a nonpharmacological intervention for dyspnoea. Asia-Pacific J Clin Oncol 2011;7.
- Yates P, White E, Skerman H. Evaluating alternate approaches for delivering non-pharmacological interventions for dyspnea in patients with lung cancer. Las Vegas, NV: Oncology Nursing Society 32nd Annual Congress; April 24–27 2007. Oncology Nursing Forum 2007;34.
- Yorke J, Lloyd-Williams M, Smith J, Blackhall F, Harle A, Warden J, et al. Management of the respiratory distress symptom cluster in lung cancer: a randomised controlled feasibility trial. Support Care Cancer 2015;23:3373-84. https://doi.org/10.1007/s00520-015-2810-x.
- Johnson MJ, Kanaan M, Richardson G, Nabb S, Torgerson D, English A, et al. A randomised controlled trial of three or one breathing technique training sessions for breathlessness in people with malignant lung disease. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0453-x.
- Farquhar M, Higginson IJ, Booth S. Modelling the carer support component of a complex intervention for breathlessness in advanced disease. Palliat Med 2010;24:S167-8.
- Gysels M, Reilly CC, Jolley CJ, Pannell C, Spoorendonk F, Moxham J, et al. Dignity through integrated symptom management: lessons from the breathlessness support service. J Pain Symptom Manage 2016;52:515-24. https://doi.org/10.1016/j.jpainsymman.2016.04.010.
- Hately J, Laurence V, Scott A, Baker R, Thomas P. Breathlessness clinics within specialist palliative care settings can improve the quality of life and functional capacity of patients with lung cancer. Palliat Med 2003;17:410-17. https://doi.org/10.1191/0269216303pm752oa.
- Ung YC, Evans W, Gatto A, Gollnow A, Martelli-Reid L, Kiteley C, et al. Implementing dyspnea management: a quality improvement project for patients with lung cancer in Ontario, Canada. J Thoracic Oncol 2013;8.
- Ahmadi L, Das Gupta T, Lilien T, Winterhoff M, Gibson L, Chakraborty A, et al. Breathe easy: a pilot project for dyspnea management in lung cancer patients. J Thoracic Oncol 2011;6:S367-8.
- Chan WL, Ng CW, Lee C, Cheng P, Cheung KW, Siu WK, et al. Effective management of breathlessness in advanced cancer patients with a program-based, multidisciplinary approach: the ‘SOB Program’ in Hong Kong. J Pain Symptom Manage 2016;51:623-7.e2. https://doi.org/10.1016/j.jpainsymman.2015.10.022.
- Kachuik L, Amjadi K. An inter-professional dyspnea management clinic: collaborating to manage shortness of breath. J Thoracic Oncol 2011;6:S579-80.
- Bausewein C, Schunk M, Schumacher P, Dittmer J, Bolzani A, Booth S. Breathlessness services as a new model of support for patients with respiratory disease. Chron Respir Dis 2018;15:48-59. https://doi.org/10.1177/1479972317721557.
- Scullion J, Henry C. A multidisciplinary approach to managing breathlessness in lung cancer. Int J Palliat Nurs 1998;4:65-9. https://doi.org/10.12968/ijpn.1998.4.2.9121.
- Goffin JR, Kerigan A, Russell SA, Ellis K, Edwards D, Young L, et al. Assessment of a dyspnea clinic for patients with thoracic malignancies. J Clin Oncol 2014;32. https://doi.org/10.1200/jco.2014.32.15_suppl.e20667.
- McMahon D, McAleer C, O’Leary N. Retrospective study of referrals to a specialist palliative care breathlessness management programme Our Lady’s Hospice and Care Services (OLH&CS) Dublin and of patient outcomes. Palliat Med 2016;30.
- Douglas L, English A, Obita GP, Hart SP, Crooks MG. Breath-taking outcomes: evaluation of a specialist breathlessness clinic. Thorax 2016;71. https://doi.org/10.1136/thoraxjnl-2016-209333.372.
- Brighton LJ, Gao W, Farquhar M, Booth S, Bajwah S, Man WD, et al. Predicting outcomes following holistic breathlessness services: a pooled analysis of individual patient data. Palliat Med 2019;33:462-6. https://doi.org/10.1177/0269216319830299.
- Brighton LJ, Tunnard I, Farquhar M, Booth S, Yi D, Gao W, et al. Recommendations for services for people living with chronic breathlessness in advanced disease: results of a transparent expert consultation. Chron Resp Dis 2019.
- Jussim L, Crawford JT, Anglin SM, Stevens ST, Duarte JL. Interpretations and methods: towards a more effectively self-correcting social psychology. J Experiment Social Psychol 2016;66:116-33. https://doi.org/10.1016/j.jesp.2015.10.003.
- Linden A. Assessing regression to the mean effects in health care initiatives. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-119.
- Stern AF. The hospital anxiety and depression scale. Occup Med 2014;64:393-4. https://doi.org/10.1093/occmed/kqu024.
- Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf Manag 2004;42:15-29. https://doi.org/10.1016/j.im.2003.11.002.
- Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008;6. https://doi.org/10.1186/1477-7525-6-46.
- Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil 2009;23:362-70. https://doi.org/10.1177/0269215508101742.
- Wedzicha JA, Bestall JC, Garrod R, Garnham R, Paul EA, Jones PW. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J 1998;12:363-9. https://doi.org/10.1183/09031936.98.12020363.
- Man WD, Chowdhury F, Taylor RS, Evans RA, Doherty P, Singh SJ, et al. Building consensus for provision of breathlessness rehabilitation for patients with chronic obstructive pulmonary disease and chronic heart failure. Chron Respir Dis 2016;13:229-39. https://doi.org/10.1177/1479972316642363.
- Farquhar M. Assessing carer needs in chronic obstructive pulmonary disease. Chron Respir Dis 2018;15:26-35. https://doi.org/10.1177/1479972317719086.
- Using a Holistic Approach to Reduce Distress Due to Breathlessness in People With Advanced Disease. Vilvoorde: EAPC; 2018.
- On SAGE Insight: New Recommendations for Chronic Breathlessness Care. Thousand Oaks, CA: SAGE Publishing; 2019.
- British Lung Foundation (BLF) . Task Force for Lung Health 2019. www.blf.org.uk/taskforce/evidence (accessed April 2019).
- CLAHRC South London . Workshop Generates Recommendations for Improving Services for People Living With Breathlessness 2017 n.d. www.clahrc-southlondon.nihr.ac.uk/news/2017/workshop-generates-recommendations-improving-services-people-living-breathlessness (accessed April 2019).
- CLAHRC North West London . Breathlessness: Current Innovations and Priority Setting Event 2017. https://clahrcnwlblog.wordpress.com/2017/10/20/breathlessness_crossclahrc_innovations/ (accessed April 2019).
- Bredin M. Multicentre randomized controlled trial of a nursing intervention for breathlessness in patients with lung cancer: update of study progress. Eur J Oncol Nurs 1998;2:176-7. https://doi.org/10.1016/S1462-3889(98)81276-3.
- Farquhar MC, Higginson IJ, Fagan P, Booth S. The feasibility of a single-blinded fast-track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. BMC Palliat Care 2009;8. https://doi.org/10.1186/1472-684X-8-9.
- Booth S, Moffat C, Farquhar M, Higginson IJ, Burkin J. Developing a breathlessness intervention service for patients with palliative and supportive care needs, irrespective of diagnosis. J Palliat Care 2011;27:28-36. https://doi.org/10.1177/082585971102700106.
- Farquhar MC, Prevost AT, McCrone P, Higginson IJ, Gray J, Brafman-Kennedy B, et al. Study protocol: Phase III single-blinded fast-track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-130.
- Booth S. Cambridge Breathlessness Intervention Service (CBIS). Prog Palliat Care 2013;21:224-8. https://doi.org/10.1179/1743291X13Y.0000000058.
- Bausewein C, Jolley C, Moxham J, Reilly CC, Kelly J, Bellas H, et al. Feasibility of a new out-patient breathlessness support service. Palliat Med 2012;26.
- Bausewein C, Jolley C, Reilly C, Lobo P, Kelly J, Bellas H, et al. Development, effectiveness and cost-effectiveness of a new out-patient Breathlessness Support Service: study protocol of a Phase III fast-track randomised controlled trial. BMC Pulm Med 2012;12. https://doi.org/10.1186/1471-2466-12-58.
- Schunk M, Berger U, Le L, Mansmann U, Rehfuess E, Seidl H, et al. A mixed methods study for the evaluation of a breathlessness support service in Germany: research protocol. Palliat Med 2016;30.
Appendix 1 The MEDLINE search strategy
Reproduced with permission from Brighton et al. 92 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
MEDLINE (via OvidSP)
Date range searched: inception to 2 June 2017.
Date searched: 2 June 2017.
Search strategy
-
exp Palliative Care/
-
exp Terminal Care/
-
exp Terminally Ill/
-
exp Hospices/
-
((advanc* or progressiv* or agressiv* or end) adj2 (diagnos* or diseas* or illnes* or cancer* or malignan* or stage* or dementia* or failure* or heart*)).tw.
-
(last adj3 life).tw.
-
(Advanced disease or Cancer or Intrathoracic malignancy or Lung cancer or Chronic obstructive pulmonary disease or Non-malignant disease).tw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7
-
exp nursing/
-
exp physical therapy modalities/
-
exp occupational therapy/
-
(Multiprofessional or Multidisciplinary or Holistic or Complex intervention or Non-pharmacological intervention or non-pharmacological management or non-pharmacological or physiotherap* or Nurs* or Occupational therap*).tw.
-
dyspn?ea.tw.
-
(short* adj2 breath).tw.
-
(urge* adj2 breath*).tw.
-
breathless*.tw.
-
((labo?red or difficult* or small) adj3 breath*).tw.
-
((respirat* or breath*) adj3 (distress* or comfort* or discomfort*)).tw.
-
(air adj3 (hunger or starve* or need* or gasp* or pant*)).tw.
-
suffocat*.tw.
-
unsatisf* inspiration.tw.
-
exp Dyspnea/
-
(breathlessness intervention service or breathlessness support service).tw.
-
9 or 10 or 11 or 12
-
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
-
24 and 25
-
23 or 26
-
8 and 27
Appendix 2 Schedule for stakeholder workshop
Appendix 3 The PRISMA flow chart
FIGURE 7.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. a, One article reported results from two studies. Reproduced with permission from Brighton et al. 92 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
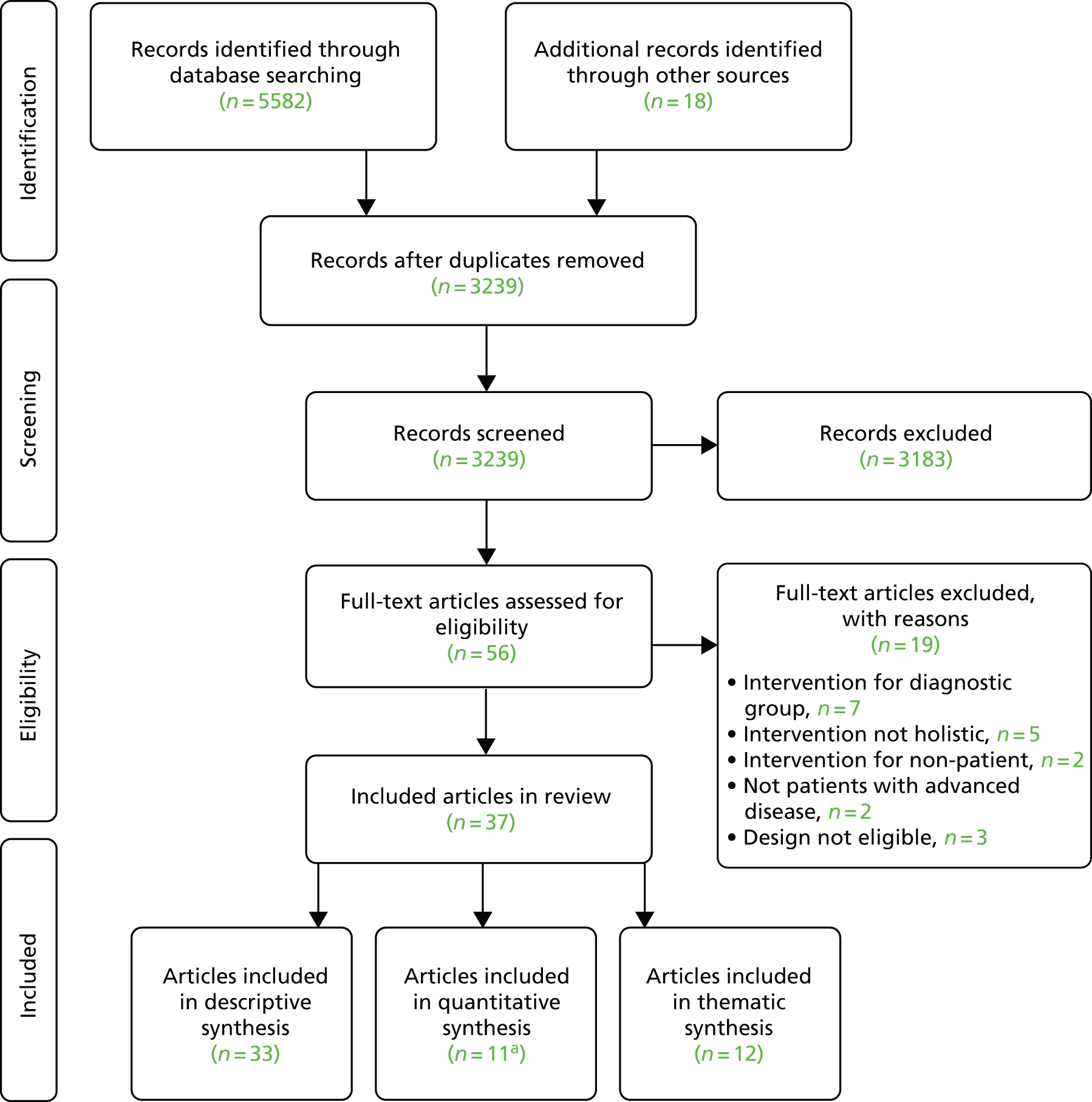
Appendix 4 Description of included services and studies
| Service description | Studies included in synthesis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Service | Country | Conditions | Discipline/staff | Contacts | Duration | Author | Design | Effectiveness data | Experience data | ||
| n | Quality (%) | n | Quality (%) | ||||||||
| aAhmadi et al.163 | Canada | Lung cancer |
Nursing Occupational therapy Social work Palliative care (doctors) Oncology (doctors) |
2–3 face to face 1 class Telephone contact for opioid follow-up if needed |
4–6 weeks | ||||||
| Chan et al.164 | Hong Kong | Cancer |
Occupational therapy Physiotherapy Home-care nurses Palliative care (doctors, nurses) |
Inpatients: daily face to face, then 2 post discharge Home care: 3 face to face, then 6 weekly if needed ≥ 1 telephone calls |
4 weeks | ||||||
| Connors et al.101,105 | UK | Intrathoracic malignancy | Palliative and respiratory (physiotherapist) | 5 face to face | 1–8 weeks | Wood et al.105 | Qualitative study | – | 9 | 85 | |
| Corner et al.90,98,187 | UK | Lung cancer or mesothelioma | Trained nurse research practitioners working alongside respiratory clinics |
3–8 clinic visits 3 or 4 telephone calls |
8–12 weeks | Corner et al.90 | Mixed-methods RCT (pilot) | 34 | 88 | 20 | 60 |
| Bredin et al.98 | RCT | 102 | 81 | – | – | ||||||
| aDouglas et al.170 | UK | Cancer and non-cancer | Respiratory physiotherapist | 1–3 clinic visits | 1–4 weeks | ||||||
| Farquhar et al.88,91,95,102,159,188–191 | UK | Cancer and non-cancer |
Occupational therapy Physiotherapy Palliative care (doctor) Access to: respiratory medicine, psychologist |
2–4 home visits 3 or 4 telephone calls |
4–8 weeks | Booth et al.88 | Qualitative study | – | – | 19 | 85 |
| aFarquhar et al.159 | Qualitative study | – | – | Missing | 40 | ||||||
| Farquhar et al.102 | Mixed-methods before–after study (pilot) | – | – | 13 | 45 | ||||||
| Farquhar et al.91 | Mixed-methods RCT | 67 | 100 | 20 | 85 | ||||||
| Farquhar et al.95 | Mixed-methods RCT | 87 | 100 | 20 | 80 | ||||||
| aGoffin et al.168 | Canada | Intrathoracic malignancy |
Oncology (doctor) Palliative care (doctor) Respiratory (therapist, doctor) Nursing |
1 clinic visit, follow-up needed | |||||||
| Hately et al.161 | UK | Lung cancer or mesothelioma | Clinic run by specialist palliative care physiotherapist | 3 clinic visits | 4–6 weeks | Hately et al.161 | Uncontrolled mixed-method study | – | – | 30 | 50 |
| Higginson et al.94,103,104,160,192,193 | UK | Cancer and non-cancer |
Physiotherapy Occupational therapy Palliative care (nurse, social worker, doctor) Respiratory care (doctor) |
2 clinic visits 1 home visit 3 or 4 telephone calls |
6 weeks | Higginson et al.94 | Mixed-methods RCT | 105 | 100 | 20 | 70 |
| Gysels et al.104,160 | Qualitative | – | – | 20 | 80 | ||||||
| Reilly et al.103 | Cross-sectional postal survey | – | – | 25 | 70 | ||||||
| Johnson et al.99,158 | UK | Lung cancer |
Varied by site; could include: Physiotherapy Occupational therapy Oncology (nurse) Palliative care |
1 face to face vs. 3 face to face Both with 1 telephone call |
2–4 weeks | Barton et al.99 | Feasibility RCT | 22 | 92 | – | – |
| Johnson et al.158 | RCT | 156 | 92 | – | – | ||||||
| aKachuik and Amjadi165 | Canada | Lung cancer |
Physician Nurse Occupational therapist Respiratory therapist Social worker Above with oncology and palliative care expertise |
Clinic visits as needed | |||||||
| aMcMahon et al.169 | Ireland | Idiopathic pulmonary fibrosis or COPD |
Advanced nurse practitioner led Physiotherapist Occupational therapist |
4–6 weeks | |||||||
| aPearce et al.154 | UK | COPD |
COPD nurse Physiotherapy Occupational therapy |
4 clinic visits | 4 weeks | aPearce et al.154 | RCT | 51 | 54 | – | – |
| Schunk et al.166,194 | Germany | Cancer and non-cancer |
Palliative care consultants Respiratory physicians Physiotherapists Access to psychologists, social workers and nurses |
2 clinic visits 4 home visits Telephone calls as needed |
6 weeks | ||||||
| Scullion and Henry167 | UK | Lung cancer |
Oncology (nurse) Physiotherapy Occupational therapy Dietitian |
4 group sessions | 4 weeks | ||||||
| aUng et al.162 | Canada | Lung cancer | Multidisciplinary team, including a ‘clinical champion’, tailored by local services | Precise methodology left to individual cancer centres | |||||||
| aYates et al.155,156 | Australia | Lung cancer | Nurse led | 4 face to face or telephone | 4 weeks | aYates et al. 156 | Quasi-experimental (pilot) and RCT (pilot) | 30 and 57 | 35 and 46 | – | – |
| aYates et al.155 | RCT | 144 | 69 | – | – | ||||||
| Yorke et al.157 | UK | Lung cancer |
Specialist nurses Physiotherapists Complementary therapists |
2 face to face 1 telephone call |
4 weeks | Yorke et al.157 | RCT (feasibility) | 107 | 92 | – | – |
Appendix 5 Outcomes measured in controlled studies
| Outcomes | Study | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barton et al.99 | Bredin et al.98 | Corner et al.90 | Farquhar et al.91 | Farquhar et al.95 | Higginson et al.94 | Johnson et al.158 | aPearce et al.154 | aYates et al.156 (quasi-experimental) | aYates et al.156 (RCT) | aYates et al.155 | Yorke et al.157 | ||
| Breathlessness | |||||||||||||
| Intensity (best/worst/average) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 10 | ||
| Distress | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 10 | ||
| Mastery | ✗ | ✗ | ✗ | ✗ | ✗ | 5 | |||||||
| Other symptoms | |||||||||||||
| Anxiety/depression | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 9 | |||
| Cough | ✗ | 1 | |||||||||||
| Fatigue | ✗ | ✗ | ✗ | 3 | |||||||||
| General | ✗ | ✗ | ✗ | 3 | |||||||||
| Functioning | ✗ | ✗ | ✗ | ✗ | ✗ | 5 | |||||||
| QoL | ✗ | ✗ | ✗ | ✗ | ✗ | 5 | |||||||
| Service use | ✗ | ✗ | ✗ | ✗ | ✗ | 5 | |||||||
| Survival | ✗ | ✗ | 2 | ||||||||||
| Carer distress/burden | ✗ | ✗ | 2 | ||||||||||
Appendix 6 The QualSyst quality assessment: quantitative
| Author | 1. Objective | 2. Design | 3. Subject selection | 4. Subject characteristics | 5. Random allocation | 6. Blinding investigators | 7. Blinding subjects | 8. Outcomes | 9. Sample size | 10. Analysis | 11. Estimate of variance | 12. Confounding | 13. Results | 14. Conclusion | Total score (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barton et al.99 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 92 |
| Bredin et al.98 | Yes | Yes | Partial | Yes | Yes | No | NA | Yes | Yes | Partial | Yes | Yes | Yes | Partial | 81 |
| Corner et al.90 | Yes | Yes | Yes | Yes | Partial | No | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 88 |
| Farquhar et al.91 | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100 |
| Farquhar et al.95 | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100 |
| Higginson et al.94 | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100 |
| Johnson et al.158 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 92 |
| aPearce et al.154 | Partial | Yes | No | No | Partial | No | NA | Yes | Partial | Partial | Yes | Partial | Partial | Yes | 54 |
| aYates et al.156 | |||||||||||||||
| Quasi-experimental study | Yes | Yes | No | No | No | No | NA | No | Partial | Partial | No | No | Partial | Yes | 35 |
| RCT | Yes | Yes | No | No | Partial | No | NA | No | Partial | Partial | No | Yes | Partial | Yes | 46 |
| aYates et al.155 | Yes | Yes | Partial | Partial | Partial | No | NA | Partial | Yes | Partial | Yes | Yes | Partial | Yes | 69 |
| Yorke et al.157 | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 92 |
Appendix 7 Risk of bias
Reproduced with permission from Brighton et al. 92 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.

Appendix 8 The QualSyst quality assessment: qualitative
| Author | 1. Objective | 2. Design | 3. Context | 4. Connection to literature | 5. Sampling | 6. Data collection | 7. Analysis | 8. Credibility | 9. Conclusions | 10. Reflexivity | Total score (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Booth et al.88 | Partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 84 |
| Corner et al.90 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | No | 60 |
| Farquhar et al.91 | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Yes | Yes | No | 85 |
| Farquhar et al.95 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | 80 |
| aFarquhar et al.159 | Yes | Yes | Partial | Partial | No | Partial | No | No | Partial | No | 40 |
| Farquhar et al.102 | Partial | Partial | Yes | Yes | Partial | No | No | No | Yes | No | 45 |
| Gysels et al.104,160 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | 80 |
| Hately et al.161 | Partial | Yes | Yes | Partial | Partial | Partial | Partial | No | Partial | No | 50 |
| Higginson et al.94 | No | Partial | Yes | Partial | Yes | Yes | Yes | Yes | Yes | No | 70 |
| Reilly et al.103 | Yes | Partial | Yes | Yes | Yes | Yes | Partial | No | Yes | No | 70 |
| Wood et al.105 | Yes | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Yes | No | 85 |
Appendix 9 Mixed-methods quality assessment
| Study | Appropriate mixed-methods design | Relevant integration of qualitative and quantitative data | Considers limitations of integration (e.g. divergence) | Total score (%) |
|---|---|---|---|---|
| Corner et al.90 | Yes | No | No | 33 |
| Farquhar et al.91 | Yes | Yes | No | 67 |
| Farquhar et al.95 | Yes | Yes | Yes | 100 |
| Higginson et al.94 | Yes | Yes | No | 67 |
| Farquhar et al.102 | Yes | Yes | No | 67 |
| Hately et al.161 | Yes | Yes | Yes | 100 |
| Reilly et al.103 | Yes | Yes | No | 67 |
Appendix 10 Quality assessment of economic evaluations
| Item | Farquhar et al.91 | Farquhar et al.95 | Higginson et al.94 | Johnson et al.158 |
|---|---|---|---|---|
| 1. The research question is stated | ✓ | ✓ | ✓ | ✓ |
| 2. The economic importance of the research question is stated | ✓ | ✓ | ✓ | |
| 3. The viewpoint(s) of the analysis are clearly stated and justified | ? | ? | ||
| 4. The rationale for choosing the alternative programmes or interventions compared is stated | ✓ | ✓ | ✓ | ✓ |
| 5. The alternatives being compared are clearly described | ✓ | ✓ | ✓ | ✓ |
| 6. The form of economic evaluation used is stated | ✓ | ✓ | ✓ | ✓ |
| 7. The choice of form of economic evaluation is justified in relation to the questions addressed | ||||
| 8. The source(s) of effectiveness estimates used are stated | ✓ | ✓ | ✓ | ✓ |
| 9. Details of the design and results of effectiveness study are given | ✓ | ✓ | ✓ | ✓ |
| 10. The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | ✓ | ✓ | ✓ |
| 11. Methods to value health states and other benefits are stated | ✓ | ✓ | ✓ | ✓ |
| 12. Details of the subjects from whom valuations were obtained are given | ✓ | ✓ | ✓ | ✓ |
| 13. Productivity changes (if included) are reported separately | NA | NA | NA | |
| 14. The relevance of productivity changes to the study question is discussed | ||||
| 15. Quantities of resources are reported separately from their unit costs | ✓ | ✓ | ✓ | |
| 16. Methods for the estimation of quantities and unit costs are described | ✓ | ✓ | ✓ | ? |
| 17. Currency and price data are recorded | ✓ | ✓ | ✓ | |
| 18. Details of currency of price adjustments for inflation or currency conversion are given | ✓ | |||
| 19. Details of any model used are given | NA | NA | NA | NA |
| 20. The choice of model used and the key parameters on which it is based are justified | NA | NA | NA | NA |
| 21. Time horizon of costs and benefits is stated | ✓ | ✓ | ✓ | ✓ |
| 22. The discount rate(s) is stated | NA | NA | NA | NA |
| 23. The choice of rate(s) is justified | NA | NA | NA | NA |
| 24. An explanation is given if costs or benefits are not discounted | NA | NA | ||
| 25. Details of statistical tests and confidence intervals are given for stochastic data | ? | ✓ | ? | |
| 26. The approach to sensitivity analysis is given | ✓ | NA | NA | ✓ |
| 27. The choice of variables for sensitivity analysis is justified | NA | NA | ✓ | |
| 28. The ranges over which the variables are varied are stated | NA | NA | ✓ | |
| 29. Relevant alternatives are compared | ✓ | ✓ | ✓ | ✓ |
| 30. Incremental analysis is reported | ✓ | ✓ | ||
| 31. Major outcomes are presented in a disaggregated as well as aggregated form | ✓ | ✓ | ✓ | |
| 32. The answer to the study question is given | ✓ | ✓ | ✓ | ✓ |
| 33. Conclusions follow from the data reported | ✓ | ✓ | ✓ | ✓ |
| 34. Conclusions are accompanied by the appropriate caveats | ✓ | ✓ | ✓ | ✓ |
| Total score (%) | 72 | 77 | 77 | 64 |
Appendix 11 Reporting quality
The PRISMA checklist (for randomised controlled trials)
| Item | Bredin et al.98 | aFarquhar et al.91 | aFarquhar et al.95 | aHigginson et al.94 | Johnson et al.158 |
|---|---|---|---|---|---|
| 1a Identification as RCT in title | ✓ | ✓ | ✓ | ✓ | ✓ |
| 1b Structured summary | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2a Rationale | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2b Specific objectives/hypotheses | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3a Description of trial design | ✓ | ✓ | ✓ | ✓ | |
| 3b Important changes to methods | ✓ | ✓ | |||
| 4a Eligibility criteria for participants | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4b Settings/locations where the data were collected | ✓ | ✓ | ✓ | ✓ | |
| 5 The interventions for each group | ✓ | ✓ | ✓ | ✓ | |
| 6a Defined primary and secondary outcome measures | ✓ | ✓ | ✓ | ✓ | |
| 6b Any changes to trial outcomes | ✓ | ||||
| 7a How sample size was determined | ✓ | ✓ | ✓ | ✓ | ✓ |
| 7b Interim analyses and stopping guidelines | |||||
| 8a Method used to generate the random allocation | ✓ | ✓ | ✓ | ✓ | |
| 8b Type of randomisation | ✓ | ✓ | ✓ | ✓ | |
| 9 Mechanism used to implement random allocation | ✓ | ✓ | ✓ | ||
| 10 Who generated the random allocation, enrolled participants, assigned participants to interventions | ✓ | ✓ | ✓ | ✓ | ✓ |
| 11a If done, who was blinded | ✓ | ✓ | ✓ | ✓ | |
| 11b If relevant, description of the similarity of interventions | ✓ | ✓ | ✓ | ||
| 12a Statistical methods used | ✓ | ✓ | ✓ | ✓ | ✓ |
| 12b Methods for additional analyses | ✓ | ✓ | ✓ | ✓ | ✓ |
| 13a Numbers of participants randomly assigned, received intended treatment, and analysed | ✓ | ✓ | ✓ | ✓ | ✓ |
| 13b Losses and exclusions after randomisation, | ✓ | ✓ | ✓ | ✓ | ✓ |
| 14a Dates defining the periods of recruitment and follow-up | ✓ | ✓ | ✓ | ✓ | |
| 14b Why the trial ended or was stopped | |||||
| 15 A table showing baseline characteristics | ✓ | ✓ | ✓ | ✓ | ✓ |
| 16 Number of participants included in each analysis | ✓ | ✓ | ✓ | ✓ | ✓ |
| 17a Estimated effect size and its precision | ✓ | ✓ | ✓ | ✓ | ✓ |
| 17b For binary outcomes, absolute and relative effect sizes | NA | NA | NA | NA | |
| 18 Results of any other analyses performed | ✓ | ✓ | ✓ | ✓ | ✓ |
| 19 All important harms or unintended effects in each group | ✓ | ✓ | |||
| 20 Trial limitations | ✓ | ✓ | ✓ | ✓ | ✓ |
| 21 Generalisability | ✓ | ✓ | |||
| 22 Interpretation consistent with results | ✓ | ✓ | ✓ | ✓ | ✓ |
| 23 Registration number and name of trial registry | ✓ | ✓ | ✓ | ✓ | |
| 24 Where the full trial protocol can be accessed | ✓ | ✓ | ✓ | ||
| 25 Sources of funding and other support | ✓ | ✓ | ✓ | ✓ | ✓ |
| Checklist items reported (%) | 53 | 81 | 78 | 92 | 89 |
Modified PRISMA checklist (for quasi-experimental studies)
| Item | Chan et al.164 | Connors et al.101 | aFarquhar et al.102 | aHately et al.161 |
|---|---|---|---|---|
| 1b Structured summary | ✓ | ✓ | ||
| 2a Rationale | ✓ | ✓ | ✓ | ✓ |
| 2b Specific objectives/hypotheses | ||||
| 3a Description of trial design | ✓ | ✓ | ||
| 3b Important changes to methods | NA | |||
| 4a Eligibility criteria for participants | ✓ | ✓ | ✓ | ✓ |
| 4b Settings/locations where the data were collected | ✓ | ✓ | ||
| 5 The interventions for each group | ✓ | ✓ | ||
| 6a Defined primary and secondary outcome measures | ✓ | |||
| 6b Any changes to trial outcomes | NA | |||
| 7a How sample size was determined | ✓ | |||
| 7b Interim analyses and stopping guidelines | NA | |||
| 11a If done, who was blinded | NA | NA | NA | NA |
| 11b If relevant, description of the similarity of interventions | NA | NA | NA | NA |
| 12a Statistical methods used | NA | NA | ✓ | |
| 12b Methods for additional analyses | NA | NA | NA | NA |
| 13a Numbers of participants randomly assigned, received intended treatment, and analysed | NA | ✓ | ✓ | ✓ |
| 13b Losses and exclusions | NA | ✓ | ✓ | ✓ |
| 14a Dates defining the periods of recruitment and follow-up | ✓ | ✓ | ||
| 14b Why the trial ended or was stopped | NA | ✓ | ||
| 15 A table showing baseline characteristics | ✓ | ✓ | ✓ | |
| 16 Number of participants included in each analysis | ✓ | ✓ | ✓ | ✓ |
| 17a Estimated effect size and its precision | ✓ | ✓ | ✓ | ✓ |
| 17b For binary outcomes, absolute and relative effect sizes | NA | NA | NA | NA |
| 18 Results of any other analyses performed | NA | NA | NA | NA |
| 19 All important harms or unintended effects in each group | ||||
| 20 Trial limitations | ✓ | ✓ | ||
| 21 Generalisability | ✓ | ✓ | ||
| 22 Interpretation consistent with results | ✓ | ✓ | ✓ | |
| 23 Registration number and name of trial registry | NA | ✓ | ||
| 24 Where the full trial protocol can be accessed | NA | ✓ | ||
| 25 Sources of funding and other support | ✓ | ✓ | ✓ | |
| Checklist items reported (%) | 63 | 35 | 73 | 41 |
The PRISMA checklist (for pilot and feasibility studies)
| Item | aCorner et al.90 | Farquhar et al.190 | Barton et al.99 | Yorke et al.157 |
|---|---|---|---|---|
| 1a Identification as a pilot or feasibility trial | ✓ | ✓ | ✓ | |
| 1b Structured summary | ✓ | ✓ | ✓ | ✓ |
| 2a Rationale | ✓ | ✓ | ✓ | ✓ |
| 2b Specific objectives/research questions | ✓ | ✓ | ✓ | |
| 3a Description of pilot trial design | ✓ | ✓ | ✓ | |
| 3b Important changes to methods | ✓ | ✓ | ||
| 4a Eligibility criteria for participants | ✓ | ✓ | ✓ | ✓ |
| 4b Settings and locations where the data were collected | ✓ | ✓ | ||
| 4c How participants were identified and consented | ✓ | ✓ | ||
| 5 The interventions for each group | ✓ | ✓ | ✓ | ✓ |
| 6a Defined assessments to address each objective | ✓ | |||
| 6b Any changes to assessments | ✓ | |||
| 6c Prespecified criteria used to judge whether/how, to proceed with future definitive trial | ||||
| 7a Rationale for numbers in the pilot trial | ✓ | ✓ | ✓ | |
| 8a Method used to generate the random allocation | ✓ | ✓ | ||
| 8b Type of randomisation | ✓ | ✓ | ✓ | |
| 9 Mechanism used to implement the random allocation | ✓ | ✓ | ||
| 10 Who generated the random allocation, enrolled participants, assigned participants to interventions | ✓ | ✓ | ✓ | |
| 11a If done, who was blinded | ✓ | ✓ | NA | |
| 11b If relevant, description of the similarity of interventions | ✓ | ✓ | ✓ | |
| 12 Methods used to address each objective | ✓ | |||
| 13a Numbers of participants randomly assigned, received intended treatment, and analysed | ✓ | ✓ | ✓ | |
| 13b Losses and exclusions after randomisation | ✓ | ✓ | ✓ | ✓ |
| 14a Dates defining the periods of recruitment and follow-up | ✓ | ✓ | ✓ | |
| 14b Why the pilot trial ended or was stopped | ✓ | |||
| 15 A table showing baseline characteristics | ✓ | ✓ | ✓ | ✓ |
| 16 Number of participants included in each analysis | ✓ | ✓ | ✓ | ✓ |
| 17 Results including expressions of uncertainty | ✓ | NA | ✓ | ✓ |
| 18 Results of any other analyses | ✓ | ✓ | ✓ | |
| 19 All important harms or unintended effects in each group | ||||
| 19a Other important unintended consequences | ||||
| 20 Pilot trial limitations | ✓ | ✓ | ||
| 21 Generalisability | ✓ | |||
| 22 Interpretation consistent with objectives and findings | ✓ | ✓ | ✓ | ✓ |
| 22a Implications for progression from pilot to future definitive trial, including any proposed amendments | ✓ | ✓ | ✓ | ✓ |
| 23 Registration number and name of trial registry | ✓ | ✓ | ||
| 24 Where the pilot trial protocol can be accessed | ||||
| 25 Sources of funding and other support | ✓ | ✓ | ✓ | ✓ |
| 26 Ethics approval | ✓ | ✓ | ✓ | |
| Checklist item reported (%) | 31 | 79 | 74 | 68 |
The STROBE checklist (for observational studies)
| Item | aReilly et al.103 |
|---|---|
| 1 (a) Indicate the study’s design | |
| 1 (b) Provide in the abstract an informative and balanced summary | ✓ |
| 2 Explain the rationale | ✓ |
| 3 State specific objectives/hypotheses | ✓ |
| 4 Present key elements of study design | ✓ |
| 5 Describe the setting, locations, and relevant dates | ✓ |
| 6 (a) Eligibility criteria, sources and selection of participants | ✓ |
| 6 (b) For matched studies, give matching criteria | NA |
| 7 Define outcomes, exposures, predictors, confounders, modifiers | ✓ |
| 8 Give sources of data and details of methods of assessment | ✓ |
| 9 Describe any efforts to address potential sources of bias | |
| 10 Explain how the study size was arrived at | ✓ |
| 11 Explain how variables were handled in the analyses | ✓ |
| 12 (a) Describe all statistical methods | ✓ |
| 12 (b) Describe methods used to examine subgroups/interactions | NA |
| 12 (c) Explain how missing data were addressed | |
| 12 (d) Explain how loss to follow-up/matching was addressed | NA |
| 12 (e) Describe any sensitivity analyses | NA |
| 13 (a) Report numbers of individuals at each stage of study | ✓ |
| 13 (b) Give reasons for non-participation at each stage | ✓ |
| 13 (c) Consider use of a flow diagram | |
| 14 (a) Give characteristics of study participants | ✓ |
| 14 (b) Indicate number of participants with missing data | ✓ |
| 14 (c) Summarise follow-up time | NA |
| 15 Outcome data: numbers of outcome events/summary measures | ✓ |
| 16 (a) Give unadjusted and adjusted estimates and their precision | NA |
| 16 (b) Report category boundaries for grouped continuous variables | NA |
| 16 (c) Consider translating relative risk into absolute risk | NA |
| 17 Report other analyses done | NA |
| 18 Summarise key results with reference to study objectives | ✓ |
| 19 Discuss limitations of the study | ✓ |
| 20 Give a cautious overall interpretation of results | ✓ |
| 21 Discuss the generalisability of the study results | ✓ |
| 22 Give the source of funding and the role of the funders | ✓ |
| Checklist items reported (%) | 84 |
The COREQ checklist (for qualitative studies)
| Item | aCorner et al.90 | aFarquhar et al.91 | aFarquhar et al.95 | aHigginson et al.94 | aFarquhar et al.102 | Gysels et al.160 | aHately et al.161 | aReilly et al.103 | Wood et al.105 | Booth et al.88 |
|---|---|---|---|---|---|---|---|---|---|---|
| Which author/s conducted the interview/focus group | ✓ | ✓ | NA | ✓ | ||||||
| Researcher’s credentials | NA | |||||||||
| Researcher occupation at the time of the study | ✓ | ✓ | ✓ | NA | ✓ | |||||
| Researcher gender | NA | ✓ | ||||||||
| Experience or training of the researcher | ✓ | ✓ | ✓ | NA | ||||||
| Whether a relationship was established prior to study | ✓ | NA | ||||||||
| What participants knew about the researcher | NA | |||||||||
| Characteristics of the interviewer/facilitator | NA | |||||||||
| Methodological orientation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| How participants were selected | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| How participants were approached | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| How many participants there were in the study | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| How many people refused to participate/dropped out | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Where the data was collected | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Whether anyone else was present | NA | ✓ | ||||||||
| Important characteristics of the sample | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Whether questions, prompts, guides were provided | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Whether repeat interviews carried out | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ||
| Whether they used audio or visual recording | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ||
| Whether field notes were made | ✓ | ✓ | NA | |||||||
| Duration of the interviews or focus group | NA | |||||||||
| Data saturation discussed | ✓ | |||||||||
| Whether transcripts were returned for comment | NA | |||||||||
| How many data coders coded the data | ✓ | ✓ | ✓ | |||||||
| Description of the coding tree | ✓ | |||||||||
| Themes identified in advance or derived from the data | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Software used to manage the data | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Whether participants provided feedback on the findings | ✓ | |||||||||
| Identified participant quotations to illustrate findings | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Consistency between the data presented and findings | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Major themes clearly presented in the findings | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Description of diverse cases/minor themes | ||||||||||
| Checklist items reported (%) | 22 | 50 | 50 | 41 | 38 | 41 | 41 | 68 | 63 | 50 |
List of abbreviations
- BIS
- Breathlessness Intervention Service
- BSS
- Breathlessness Support Service
- CI
- confidence interval
- CLAHRC
- Collaboration for Leadership in Applied Health Research and Care
- COPD
- chronic obstructive pulmonary disease
- CRQ
- Chronic Respiratory Disease Questionnaire
- EQ-5D
- EuroQol-5 Dimensions
- ERS
- European Respiratory Society
- FEV1
- forced expiratory volume in 1 second
- HADS
- Hospital Anxiety and Depression Scale
- IQR
- interquartile range
- KCL
- King’s College London
- MD
- mean difference
- NIHR
- National Institute for Health Research
- NRS
- Numeric Rating Scale
- OR
- odds ratio
- PAG
- project advisory group
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QualSyst
- Standard Quality Assessment Criteria for Evaluating Primary Research Papers
- RCT
- randomised controlled trial
- SD
- standard deviation
- TEC
- transparent expert consultation
- VAS
- visual analogue scale
