Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/2003/58. The contractual start date was in January 2013. The final report began editorial review in October 2017 and was accepted for publication in July 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Chiara Bucciarelli-Ducci has received personal fees from Circle Cardiovascular Imaging (Calgary, AB, Canada). Chris A Rogers is a member of the Health Technology Assessment (HTA) Commissioning Board. Barnaby C Reeves reports former membership of the HTA Commissioning Board (from January 2012 to 31 March 2016) and the HTA Efficient Study Designs Board (October–December 2014). He also reports current membership of the HTA Interventional Procedures Methods Group and Systematic Reviews Programme Advisory Group (Systematic Reviews National Institute for Health Research Cochrane Incentive Awards and Systematic Review Advisory Group).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Harris et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and rationale
Cardiovascular magnetic resonance (CMR) is a non-invasive imaging technique that assesses heart structure and function with high spatial and temporal resolution. The use of CMR has increased in recent years in all subgroups of acute coronary syndrome (ACS) patients, including those who activate the primary percutaneous coronary intervention (PPCI) pathway. 1 It is not clear whether or not having CMR influences patient management or reduces the length of hospital stay or the risk of major adverse cardiovascular events (MACEs) in these patients. Systematic reviews from 2014 highlighted the lack of high-quality, adequately powered studies to establish the prognostic value of CMR findings in patients who activate the PPCI pathway. 2,3 Similarly, few studies have assessed how CMR changes patient management, despite the fact that cardiologists believe that CMR brings about important changes in management. 4
Primary percutaneous coronary intervention
Primary percutaneous coronary intervention is the primary therapeutic approach for restoring blood flow to the heart in ACS patients who have had a ST-elevation myocardial infarction (STEMI). Currently, across the UK ≈97% of patients with a STEMI who received any reperfusion treatment were treated with PPCI. 5 Management of the in-hospital stay and follow-up after PPCI is determined by the final infarct size, the presence or absence of comorbidities and patient characteristics. About 10% of patients with ACS who activate the PPCI pathway (i.e. those that present with chest pain and ST-elevation or chest pain and new-onset left bundle branch block on electrocardiogram) are found to have unobstructed coronary arteries on angiography and, therefore, do not receive PPCI. There are no long-term outcome data from well-designed, multicentre trials on CMR use in different subgroups of patients who activate the PPCI pathway. There are also no studies reporting on the cost-effectiveness of CMR in these patients (or indeed patients from other clinical areas), which makes decision-making regarding the provision of CMR services within the NHS difficult.
Subgroups of patients who activate the primary percutaneous coronary intervention pathway and may benefit from cardiovascular magnetic resonance
Two subgroups of patients were identified in the protocol as having the potential to benefit from CMR: (1) patients with multivessel disease and (2) patients with unobstructed coronary arteries on angiography.
It is estimated that 40–65% of the patients presenting with a STEMI have multivessel disease (depending on the baseline characteristics, e.g. age, of the population studied). 6–11 There is ongoing debate about the optimal treatment strategy for these patients. Three main treatment management strategies have been proposed/are currently used: (1) conservative, PPCI of the infarcted artery followed by medical therapy (unless ischaemia occurs), (2) staged, in which only the infarct-related artery is treated acutely and other lesions are treated at a later date and (3) aggressive, percutaneous coronary intervention (PCI) of all significant lesions at the time of PPCI.
The role of CMR in patients with multivessel disease is undefined. CMR allows the volume of ischaemic or poorly perfused myocardium to be quantified but it is not known whether or not access to CMR to plan further revascularisation influences management. Since the Primary percutaneous coronary Intervention Pathway Activation (PIPA) study was commissioned, there has also been a rapid increase in the use of fractional flow reserve (FFR) testing (a guide wire-based procedure done through a standard diagnostic catheter at the time of a coronary angiography) to assess ischaemia; this technology is available to interventional cardiologists in the catheter laboratory. CMR is comparable to FFR for detecting ischaemia; studies have shown excellent diagnostic accuracy of perfusion CMR to detect areas of ischaemia identified by FFR. 12,13 However, while FFR may be appropriate in symptomatic, high-risk patients for whom the benefit of an invasive approach to ischaemia testing may outweigh the risks (i.e. the likelihood of revascularisation is high; therefore, lesions can be revascularised at the same time as the FFR), the benefit is less clear in asymptomatic multivessel disease patients. CMR may, therefore, have a role in selecting patients who will receive the greatest benefit from these invasive procedures. The non-inferiority of CMR in managing patients with multivessel disease is the subject of the magnetic resonance perfusion imaging to guide management of patients with stable coronary artery disease (MR-INFORM) trial. 14
The reported variation in the incidence of normal coronary angiograms in patients activating the PPCI pathway is between 3% and 16%. 15,16 It is in this group that cardiologists believe that CMR has the greatest potential to change patient management. In these patients, the lack of an accurate diagnosis may result in inappropriate treatment and/or follow-up,17,18 associated with a poorer prognosis. 19 A definitive diagnosis of myocardial infarction (MI) or not is also important so that these patients can be managed and followed up appropriately. Studies have shown that CMR can facilitate differential diagnosis in the context of a normal coronary angiogram,20–22 providing a definitive diagnosis (e.g. MI, myocarditis, Takotsubo cardiomyopathy) in 65–90% of these patients. 20,23,24 Although the prognostic implications of non-MI CMR abnormalities are not known, two studies showed that the pattern of CMR abnormalities in myocarditis was predictive of long-term outcome. 25,26
The benefit of CMR to other subgroups of patients who activate the PPCI pathway is less clear. However, given that not all of these patients will have access to centres with dedicated CMR facilities, there is potential for health inequality across the UK, which needs to be identified. Therefore, the population of interest for this study (target population) includes all ACS patients who activate the PPCI pathway, regardless of whether or not they receive PPCI.
Rationale for the study
We wanted to set up a multicentre, prospective cohort study (registry) in ACS patients who activate the PPCI pathway by linking routinely collected data from hospital information systems (HISs) with follow-up data from Hospital Episode Statistics (HES) and the Office for National Statistics (ONS). The aims of the registry were to document CMR activity in patients who activate the PPCI pathway and to provide information on CMR use, uptake over time, patients’ characteristics associated with being referred for CMR, the impact of CMR on patient management (e.g. early discharge of a patient with a normal coronary angiogram or the placement of an additional stent for a patient with multivessel disease) and the prognostic value of CMR after PPCI.
We were uncertain whether or not it would be feasible to set up a registry in this patient population using routinely collected data. In particular, we were uncertain if we could (1) easily identify the eligible population, (2) implement a ‘light-touch’ consent system across multiple hospitals and (3) extract and link data sets from multiple HISs in different hospitals. We also wanted (4) to provide a reliable estimate of the CMR rate in patients who activate the PPCI pathway and (5) to define a ‘proxy’ primary composite outcome, acceptable to cardiologists and other stakeholders (e.g. clinical commissioners) as representing a clinically important change in management as a result of an eligible patient having undergone CMR (e.g. expected to prevent future MACEs). Item number 5 was included so that the registry would be able to assess effectiveness in the medium term. A long-term objective of the registry would be to compare the incidence of MACEs in patients who do or do not have CMR after the index event, but the low frequency of MACEs means that the study would have to accrue over 37,000 subjects to detect a clinically important reduction in the incidence of MACEs with adequate power.
Chapter 2 Aims and objectives
The aim of the study was to evaluate the feasibility of setting up a UK multicentre registry study to document CMR use in patients who activate the PPCI pathway. There were three elements to the study, listed below with the associated objectives.
-
A. To determine whether or not it is feasible to set up a CMR registry in this patient population by linking sources of routinely collected data.
-
Objective A1: to implement consent and establish patient consent rate.
-
Objective A2: to provide evidence of whether or not data linkage and extraction can be carried out across PPCI centres.
-
Objective A3: to determine whether the registry can be compiled from hospital episode data [HES and Patient Episode Database Wales (PEDW)] rather than multiple HISs.
-
-
B. To estimate the proportion of the target population who have CMR at two centres with dedicated CMR facilities and to describe resource use and associated costs of having CMR after PPCI.
-
Objective B1: to estimate the proportion of the target population who get a CMR scan following PPCI pathway activation.
-
Objective B2: to develop simple cost-effectiveness models in relevant subgroups of patients who activate the PPCI pathway in order to identify key drivers of cost-effectiveness for CMR imaging in patients who activate the PPCI pathway.
-
-
C. To identify an outcome measure representing a definitive change in clinical management, conditional on having undergone CMR, that would be credible to cardiologists and other stakeholders as an interim measure of the value added by doing CMR.
-
Objective C1: to define, using formal consensus methods, a treatment/process outcome that will constitute a definitive change in clinical management arising from having undergone CMR.
-
Objective C2: to identify patient subgroups in whom CMR use is indicated using formal consensus methods and illustrative data from the registry.
-
Objective C3: to determine whether or not hospital episode data adequately capture the main changes in clinical management that are identified using formal consensus.
-
Objective C4: to pilot the implementation of a primary outcome that is based on the changes in management, for which consensus was achieved using hospital episode data.
-
Conditional on the success of achieving objectives A, B and C, there was a final objective, D, for which we envisaged that we would define the research objectives and calculate the sample size needed for a cohort study to achieve the aim of evaluating the clinical effectiveness and cost-effectiveness of CMR after PPCI pathway activation, with respect to the outcome identified by objective C.
Changes to objectives during the study
The objectives described above comprise all objectives that were studied rather than the objectives at the outset. The following objectives were added or modified during the course of the study.
With respect to objective A3, we faced several challenges when identifying the eligible population and implementing patient consent in the PPCI centres. These are described in detail in Chapter 3. We therefore wanted to determine whether or not we could identify ACS patients who activate the PPCI pathway in hospital episode data in order to determine whether or not a future registry in this patient population could be set up solely from hospital episode data (Chapter 4, Identifying the eligible study cohort and cardiovascular magnetic resonance exposure in hospital episode data).
With respect to objective B2, we originally planned to identify key cost drivers empirically, by describing resource use and associated costs of having CMR after PPCI pathway activation from HES and PEDW data for participants during follow-up of the cohort. We experienced considerable delays in obtaining HES data, which prevented us from doing this. Therefore, the economists used an alternative approach, seeking secondary sources of data as inputs into economic decision models to identify the key drivers of cost-effectiveness, that is the model parameters that most influence estimates.
With respect to objective C4, we had initially intended to collect follow-up data from each participating hospital to identify the changes in clinical management that could result from CMR (defined through the formal consensus). However, we realised early on that this would not be possible for a proportion of our cohort because many patients whom we recruited had their treatment at the PPCI centres but were quickly repatriated to their local hospital. We therefore decided that following the cohort through hospital episode data would provide more complete data because follow-up data would be available for all patients regardless of which hospital (within England or Wales) they attended after their index admission/procedure.
With respect to objective C3, some of the cardiologists in the research team were sceptical about our ability to pick up potential changes in clinical management resulting from CMR using HES and PEDW data. They highlighted that changes in clinical management may be too subtle to be captured in a data set that records only information about inpatient, outpatient and accident and emergency (A&E) attendances. In contrast, electronic data sources in many hospitals capture a range of additional information, for example prescribing data and patient-level nursing observation data. We therefore designed a questionnaire requesting specific information from the referring cardiologists about how CMR changed management in all patients in our study who were referred for a CMR. We determined whether or not changes in management described in the questionnaire were adequately reflected in hospital episode data [see Chapter 6, Implementation of a primary outcome based on the changes in management for which consensus was achieved (objectives C3 and C4)].
Major delays with the study
The major delay to the study was receiving the HES/ONS linked data set from NHS Digital. Below is a timeline of our data request and the processing of our application:
-
24 June 2013 – application was submitted to NHS Digital. We were told that our application would be processed closer to the time at which we required the data (September 2014), because our request was straightforward and the data set would not take long to assemble.
-
March 2014 – NHS Digital commissioned an independent review into data release and stopped processing all applications.
-
March 2015 – NHS Digital began processing our application.
-
August 2016 – linked HES/ONS data received.
This process added a 23-month delay to the study.
Chapter 3 Methods
Feasibility prospective cohort study (objectives A1, A2, A3 and B1)
Parts of this text have been reproduced from Brierley et al. 27 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Study population
We aimed to approach all ACS patients who activated the PPCI pathway. This population included all patients who underwent an emergency angiogram, whether or not they received PPCI. We included patients if they were aged ≥ 18 years and underwent an emergency angiogram, defined as taking place within 2 hours of arrival at the hospital, unless specified otherwise by local protocols. We excluded patients if they were prisoners or lacked mental capacity to consent. There were no changes to the eligibility criteria during the study recruitment period. The study was reviewed and approved by the National Research Ethics Committee South West-Central Bristol (reference number 12/SW/0326).
Setting
Four NHS hospitals in England and Wales hosting 24/7 PPCI centres took part:27 the Bristol Heart Institute (University Hospitals Bristol NHS Foundation Trust), Leeds General Infirmary (Leeds Teaching Hospitals NHS Trust), Morriston Hospital (Abertawe Bro Morgannwg University Health Board, Swansea) and University Hospital of Wales (Cardiff & Vale University Health Board). Two of these hospitals (Bristol and Leeds) were defined as ‘coronary magnetic resonance (CMR) centres’, that is hospitals that had a dedicated CMR service. The other two hospitals (Swansea and Cardiff) were defined as ‘non-CMR centres’, that is hospitals that did not have access to a dedicated CMR service.
Patient identification and consent
Each hospital implemented its own methods for identifying and consenting patients. Most eligible patients were identified manually (e.g. by checking catheter laboratory records, by checking the local cardiology database or both). One of the participating hospitals set up a database query (including the search terms ‘PPCI’, ‘QueryNot’ and ‘QuerProc’) in the local cardiology database to flag up eligible patients. However, the query was broad and relied on data input by staff in the catheter laboratory, which changed in ‘real-time’. This made identification of some eligible patients difficult. For example, if a patient was originally marked as ‘PPCI’ but was subsequently found to have an unobstructed coronary artery on the angiogram, the ‘PPCI’ label was removed, but no further information was added, necessitating manual searches by local research staff to identify whether or not the patient was eligible.
We gave eligible patients information about the study once they had recovered sufficiently from their angiography and consented them before they were discharged from hospital (see Appendix 1 for a copy of the consent form). In some hospitals, this was often not possible because of quick repatriation to the originating hospital or research staff not being available over the weekend. Patients who could not be approached in person were sent study information and consent forms by post. Postal consents were managed locally at each hospital. We requested consent to use all the information collected by the hospital in relation to the patients’ suspected MI (index admission) and all the information related to subsequent NHS care and vital status in the first 12 months following the index admission. 27
Exposure
Patients were classified as exposed if they received CMR within 10 weeks of the index admission (whether during the index admission or subsequently as an outpatient). CMR had to be documented (i.e. CMR report, including the date of the CMR) in the clinical radiology information system (CRIS) or similar imaging database when the date/time of the CMR was < 10 weeks (70 days) after the date of the index admission.
Data collection: index procedure
We asked each hospital to provide the following data from local HISs for all recruited patients:
-
basic demography [local Patient Administration System (PAS)/British Cardiovascular Intervention Society (BCIS) Central Cardiac Audit Database (CCAD)]
-
clinical characteristics on presentation at the index admission, peri- and post-procedural (PPCI) characteristics {local catheter laboratory database [e.g. Siemens (Munich, Germany) CARDDAS in Bristol]/BCIS-CCAD}
-
echocardiography (ECHO) and CMR reports (local imaging databases, e.g. Picture Archiving and Communication System, CRIS, CRIS in Bristol)
-
biochemistry [local biochemistry databases, e.g. Virtual Pathology Laboratory System/Integrated Clinical Environment (ICE) in Bristol]
-
medications on discharge (local biochemistry database, e.g. ICE in Bristol).
Each hospital had the option to submit a linked data set (i.e. the participating hospital linked data from different HISs within the hospital) or unlinked data extracts from each of the above categories with a unique patient identifier in each extract. We established a data linkage model at Bristol (by liaising with the appropriate HIS managers at University Hospitals Bristol NHS Foundation Trust) and informed the other hospitals that we would undertake linkage at Bristol based on this model, although the decision about how to submit the data was left to each participating hospital. 27
We collected a limited anonymised data set to characterise the eligible patients who were not recruited, that is those who declined to consent or did not respond to an invitation to consent before study recruitment ended. Hospitals were provided with a look-up table containing the Index of Multiple Deprivation (IMD)28 or Welsh Index of Multiple Deprivation (WIMD)29 for English or Welsh postcodes, so that the anonymised data sets could contain information on deprivation, both as scores and ranks, without including postcodes, which might have compromised anonymity. IMD and WIMD are calculated from combined information from several domains (e.g. income, employment, education, health) to produce an overall relative measure of deprivation.
Data collection: follow-up
For subsequent inpatient and outpatient activity in the 12 months following the index admission, we applied to NHS Digital to link our data set with HES (i.e. inpatient, outpatient, A&E and critical care data) for the hospitals in England and to NHS Wales Information Service (NWIS) for PEDW (which collects equivalent information for NHS Wales hospitals) for the hospitals in Wales. Inpatient episode data (HES and PEDW) contain details of hospital admissions (including dates of admission and discharge) and main diagnoses/procedures. Outpatient data contain appointment dates and consultant specialty. A&E data contain dates, methods and sources of arrival at hospital.
NHS Digital routinely links HES with ONS mortality data. Mortality data were unavailable through NWIS, so the two Welsh hospitals provided these data for their patients. Identifiers and evidence of signed consent were submitted via a secure portal to NHS Digital and NWIS; HES, ONS and PEDW data were received with our unique patient identifier embedded within each file for linkage.
Admissions data were provided as data relating to an episode (i.e. admission under one consultant), but we restructured the data to relate to a ‘continuous inpatient spell’ regardless of any transfers to another consultant that may have taken place within the same admission. 30 We created a data set containing the admissions data that included the index admission and the admissions, outpatient, critical care and A&E data in the 12 months after the index admission. This was used to characterise the frequency and duration of outpatient follow-up, unscheduled cardiac-related hospital readmissions and cardiac-related investigations/procedures, such as diagnostic angiography, repeat PCI or cardiac surgery up to 1 year after the index admission.
We assumed that, if patients had any HES/PEDW data (e.g. admissions, outpatient and A&E), data not recorded in any of the data sets were absent (i.e. patient did not have an episode) rather than missing (i.e. data not entered). In addition, for those patients with a CMR within 10 weeks of their index admission, outpatient visits that occurred within this window were excluded from any counts.
Main outcome measures
The main prespecified outcome measures were feasibility parameters (criteria that would have to be met before making the decision to progress to setting up a full registry):
-
patient consent implemented at all four hospitals
-
data linkage and extraction from multiple local HISs achieved for > 90% of consented patients at all four hospitals
-
local data successfully linked with HES and PEDW for > 90% of consented patients at all four hospitals
-
CMR scan requested and carried out for ≥ 10% of patients activating the PPCI pathway in CMR hospitals. 27
Feasibility of compiling the registry from hospital episode data
We tested the feasibility of compiling the registry entirely through hospital episode data. We determined whether or not we could identify the following from hospital episode data:
-
the index event (PPCI or emergency angiography, cohort entry)
-
CMR within 10 weeks of the index event (exposure)
-
relevant subgroups of the population in which CMR may influence clinical management [identified through formal consensus, see Formal consensus study (objectives C1 and C2)]
-
clinical outcomes [MACEs, comprising death, MI, stroke, repeat PCI and a coronary artery bypass graft (CABG)].
We identified events corresponding to the index admission, exposure or outcome in HES and PEDW data sets (inpatient and outpatient) using the International Classification of Diseases, Tenth Edition (ICD-10)31 and the Office of Population Censuses and Surveys (OPCS) classification procedure codes. 32 The index admission was identified from the inpatient data set using the OPCS codes for PCI (K49, K50, K75) and ICD-10 codes for ST-elevation myocardial infarction (STEMI) (I21.0, I21.1, I21.2, I21.3) to identify PPCI patients, and OPCS codes for angiography (K63.3, K63.5 or K63.6) with a method of admission coded as emergency to identify patients who underwent an emergency angiogram. We identified exposure (OPCS code U10.3) in the inpatient and outpatient data sets. We regarded all procedure codes within 1 day of the index admission (PCI and emergency angiography) or subsequent CMR to relate to the same event recorded in our HIS data set. Clinical outcomes were identified using the following codes: ICD-10 I21 for MI and I64 for stroke, and OPCS K49, K50 or K75 for PCI and OPCS K40, K41, K42, K43, K44, K45 or K46 for CABG. A full list of codes is shown in Appendix 2.
Sample size considerations
This was a feasibility study. We aimed to recruit sufficient patients to address the study objectives, not to provide a definitive answer to questions about the clinical effectiveness and cost-effectiveness of CMR in patients who activate the PPCI pathway. None of the objectives was analytic and, therefore, a power-based sample size justification was not appropriate.
Objective A2 and objective B1 required quantitative estimates to be reported. The target sample size of 1600 patients was assessed as sufficient to allow these proportions to be estimated with satisfactory precision. For example, we expected that ≥ 90% of patients who were invited to take part in the study would give consent; the 95% confidence interval (CI) for this proportion would be 88.4% to 91.4%. Similarly, we estimated that 12.5% of the target population would have CMR, although this proportion was less certain. The 95% CIs for proportions 5%, 10% and 15% would be, respectively, 4.0% to 6.2%, 8.6% to 11.6% and 13.3% to 16.8%.
Data analysis
Data were analysed using Stata/IC® version 13 (StataCorp LP, College Station, TX, USA). The analysis of feasibility outcomes was descriptive, with quantitative results expressed as counts, percentages and 95% CIs. Feasibility was assessed by comparing descriptive statistics with the corresponding criteria. We compared patients who did not consent (and for whom we received anonymised data) with those who did consent using standardised mean differences (SMDs). 27,33 We used means and standard deviations (SDs), or medians and interquartile ranges (IQRs), for continuous variables and number (per cent) for categorical variables to report the demographic variables and baseline characteristics of patients recruited from the four hospitals.
Clinical events were expressed as rates per 1000 person-years at risk, along with 95% CIs calculated using the Poisson distribution. Time at risk of death was calculated as the time from index admission to death for those known to have died within 12 months, or the time from index admission to the calendar date on which death data were provided for that site. Any time at risk of death of > 12 months was capped at 12 months.
Time at risk for MI, stroke, repeat PCI and CABG was calculated as the time from index admission to the event, or the time from index admission to the calendar date that follow-up data were provided for that site. If the patient died before experiencing the event, the time at risk of death was time from index admission until death. Any time at risk of death of > 12 months was capped at 12 months.
Formal consensus study (objectives C1 and C2)
Parts of this text have been reproduced from Brierley et al. 27 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Literature review
We searched for studies reporting the impact of CMR on prognosis, patient management and risk stratification in the population of patients who activate the PPCI pathway. We intended to identify studies providing background knowledge to inform the formulation of statements about how CMR could change patient management in specific subgroups and as supporting evidence for the final consensus statements. We did not intend to carry out a formal quantitative systematic review of the effectiveness of CMR in the target population.
We searched without restriction by study design or search terms related to outcomes, so that we could determine the full extent of the literature in this area and identify all studies that used CMR in our population. Thus, the search identified studies that had diverse objectives and measured a range of outcomes (e.g. clinical outcomes, changes in patient management, classifications of patients into prognostic groups, new diagnoses). The search was conducted on MEDLINE, EMBASE, The Cochrane Library, Web of Science (Citations Index and Proceedings) and Bioscience Information Service (BIOSIS) (date range for all searches: 1950 to 16 January 2014). The following search terms were used, both as free text and medical subject headings (MeSH) when possible: ‘acute coronary syndrome’, ‘myocardial infarction’, ‘angioplasty’, ‘percutaneous coronary intervention’ and ‘cardiovascular magnetic resonance imaging’. We applied no restriction on publication date or language. The literature search is shown in Appendix 3.
We included all studies reporting the use of CMR in patients undergoing PPCI or patients who activated the PPCI pathway but did not proceed to PPCI (e.g. had an emergency angiogram). We excluded studies investigating the use of CMR in populations with stable coronary artery disease or patients with non-ST elevation myocardial infarction (NSTEMI), because these patients do not routinely undergo emergency angiography. One researcher triaged the titles and abstracts identified by the search to remove duplicate reports of the same study and studies that did not meet the inclusion criteria. Reference lists of full-text papers were scanned to ensure that no additional studies had been missed during the search.
The broad search identified many different types of study (e.g. diagnostic, prognostic, controlled or uncontrolled, prospective or retrospective, cohort studies) and case reports. They were grouped as follows: (1) studies assessing the prognostic value of a comprehensive CMR scan or specific CMR markers, (2) studies in which CMR was used to ‘diagnose’ a specific feature (e.g. type of MI) or complication arising from the index MI [e.g. left ventricular (LV) thrombus, ventricular septal defect (VSD)], or procedure-related complications, (3) studies in which CMR was used to ‘diagnose’ incidental findings or conditions unrelated to MI, (4) studies in which indices of heart function (e.g. myocardial viability, necrosis, perfusion, myocardium at risk, ischaemia) assessed by CMR and other imaging modalities [e.g. ECHO and single-photon emission computed tomography (SPECT)] were compared and (5) other studies not fitting into any of the categories above.
Formulation of consensus statements
An expert group was convened, consisting of two cardiologists with CMR expertise (CBD and JPG), two interventional cardiologists from sites participating in the feasibility study (SHD and RAA), one cardiac network director (SB), two methodologists (BCR and MP), two statisticians (CAR and JMH), one health economist (SW or EAS) and the study manager (RCB). Draft statements were generated independently by three cardiologists (CBD, JPG and SHD) based on their cardiological expertise and by one methodologist (MP) based on evidence from the literature review. The cardiologists and methodologist had no prior communication with each other. The cardiologists were advised to be as inclusive as possible at this stage and to not be concerned about the strength or quality of the evidence available to support their draft statements.
Three members of the expert group (study manager, RCB; systematic reviewer, MP; and cardiologist with CMR expertise, CBD) collated the statements, organised them according to patient subgroup and standardised the wording of each statement. A 1-day meeting was organised for all members of the group. The structure of the meeting was as follows: an introduction to the aims of the study and the formal consensus process; a review of each statement in turn, considering the relevance, format and wording of each statement; and a discussion about the structure of the survey. Key papers from the literature review were circulated to all members of the group prior to the meeting and these were also considered in the discussions. At the meeting, it was decided that each statement would benefit from a ‘supporting paragraph’, citing key references from the literature review, to provide background information and put the statement in context.
The structure of the process used to generate the statements is summarised in Table 1. We documented all of the discussions that led to the final statements.
| Step | Description |
|---|---|
| Literature review | Published literature was identified on the use of CMR in patients who activate the PPCI pathway and the patient subgroups that may benefit from CMR |
| Generate draft statements | Statements were generated independently in writing by clinical and non-clinical members of the internal group using systematic review and clinical expertise |
| Collate statements | Statements were categorised into patient subgroups and reworded into a standard style |
| Expert group meeting | All members of the expert group convened for a 1-day meeting during which the consensus process was introduced and each statement was discussed in turn. Discussions focused on the relevance, format and wording of each statement, and on how to structure the online survey |
| Generate final list of statements | A final list of statements was produced based on internal group meeting discussions. Each statement was accompanied by a supporting paragraph presenting ‘key’ supporting references from the literature |
Survey design
Statements and supporting paragraphs were worded in a consistent manner and collated in the form of a web-based survey (SurveyMonkey®, Palo Alto, CA, USA) (see Appendix 4). The survey included an introductory page explaining the purpose and layout of the survey and instructions about how to complete it. Each statement was followed by its supporting paragraph that contained links to Portable Document Format (PDF) references. Each statement was accompanied by a nine-point Likert scale asking the respondent to indicate whether or not he/she agreed with the statement (with 1 indicating ‘completely disagree’ and 9 ‘completely agree’). There was also a free-text box for each statement for respondents to comment in and justify their score.
Expert panel
Establishing the expert panel
Clinicians in the working group identified consultant cardiologists with CMR, interventional, ECHO, electrophysiology and heart failure expertise from across the UK (see Acknowledgements). The cardiologists were invited by e-mail to form an expert consensus panel.
Completion of first survey
The expert panel completed the survey independently. Responses were collated and analysed by members of the working group.
Face-to-face meeting
The expert panel attended a face-to-face meeting, chaired by a non-cardiology clinician experienced in facilitating formal consensus panels. The meeting was also attended by non-clinical members of the working group, whose role was to introduce the study, describe the structure of the formal consensus process, provide study-related information and take minutes of the meeting. The expert panel discussed each statement and anonymised responses to the first survey in turn and agreed on modifications to the survey.
Completion of the modified survey
The survey was modified by members of the working group as agreed in the face-to-face meeting (see Appendix 4). 4 The expert panel completed the modified survey independently and rated the statements a second time.
Extension of survey to other UK cardiologists
The survey was extended to other UK cardiologists through the British Cardiovascular Society, which advertised the survey in their monthly newsletter to members (over 2 consecutive months). We offered an incentive to encourage participation: the option of entering a prize draw for an iPad (Apple Inc., Cupertino, CA, USA).
Main outcome measure
A definition of a primary outcome measure (acceptable to cardiologists and other stakeholders) that represents a definitive change in clinical management arising from a patient having CMR.
Criteria for consensus
Data for each statement are shown as medians and IQRs. A median score ≥ 7 and an IQRs 6–9 was considered to be in agreement, or consensus, that the change in management described by the statement was clinically important. These statements were used to identify the patient subgroups perceived to benefit from CMR and define the treatment/process outcome that constitutes a definitive management change as a result of having CMR. A median score ≤ 3 and an IQR 1–3 was considered to be in consensus that the statement did not constitute a clinically important change in management. Data were analysed using Stata/IC version 14.
Implementation of a primary outcome based on the changes in management for which consensus was achieved (objectives C3 and C4)
Patient subgroups
Patient subgroups were identified from HIS data extracts provided by hospitals. When this was not possible, hospital episode data (the admissions data sets) were used to identify the ICD-10 diagnostic codes associated with the index admission.
Manual review of patient notes
We undertook a review of patient notes because of the uncertainty regarding the identification of the subgroup of patients with unobstructed coronary arteries. Forty-seven patients were selected, all identified as having unobstructed arteries in hospital A at the time of the index event. We undertook a thorough review of their patient notes to identify the reason why they activated the PPCI pathway.
Identifying changes in management
We used HES/PEDW inpatient and outpatient data to identify changes in management defined through the formal consensus in the 12 months after the index admission (cohort entry). For those patients who underwent CMR, any outpatient appointments that occurred between the index admission and the date of CMR were excluded. We decided not to use the A&E data set because most of the changes in management identified in the formal consensus were unlikely to occur in the A&E setting.
Cardiologist members of the clinical team identified key clinical ‘events’ that they expected to reflect the important changes in management characterised through the consensus process. For example, new non-ischaemic diagnoses (an important change in management resulting from CMR) were identified as Takotsubo cardiomyopathy, myocarditis, pericarditis, endocarditis and coronary spasm.
We then compiled a list of ICD-10 and OPCS codes representing the key clinical events with the help of a clinical coder. Most changes in management encompassed a number of ICD-10/OPCS codes, for example the use of additional diagnostic tests during follow-up required OPCS codes for positron emission tomography (PET), ECHO, intravascular ultrasound (IVUS) or pressure wire. We identified the changes in management in HES/PEDW for each statement (patient subgroup) separately. The full code list used to identify changes in management is shown in Appendix 2.
Implementation of the consensus statements
We calculated the frequency of all events representing changes in management (e.g. number of new diagnoses, additional diagnostic tests, number of outpatient appointments) in patients who did/did not receive CMR for each patient subgroup in the 12 months following the index admission. We also calculated the time to first event for each change in management (e.g. time to first new diagnosis, time to the first diagnostic test, time to next revascularisation in patients with multivessel disease) and summarised these with medians and IQRs. We did not compare differences between patients who did/did not receive CMR in any of the subgroups because we were interested primarily in the feasibility of identifying relevant events; the number of patients in most subgroups was also small.
Cardiovascular magnetic resonance questionnaire
We determined whether or not the changes in management identified in HES/PEDW accurately reflected the changes in management implemented by the cardiologists who referred patients in the study for CMR. We designed a questionnaire based on the patient subgroups identified in the formal consensus (see Appendix 5) and asked all referring cardiologists to complete it for each patient whom they referred for CMR. The questionnaire asked about whether or not and how CMR changed management (using tick boxes and free text). We used the questionnaire to identify whether or not the changes in management reported by cardiologists were accurately reflected in HES/PEDW, and whether or not there were additional changes in management that could not be picked up from HES/PEDW.
Health economic study (objective B2)
We built two separate cost-effectiveness models to explore the impact of CMR for two patient subgroups: (1) multivessel disease and (2) unobstructed (normal) coronary arteries.
-
Model 1: simulated the costs and effects of introducing CMR for patients with multivessel disease, compared with current practice.
-
Model 2: simulated the costs and effects of introducing CMR for patients with normal coronary arteries, compared with current practice.
From these two models, inferences can be made about the likely drivers of the cost-effectiveness of introducing CMR for all patients who activate the PPCI pathway, because, in practice, this is how CMR might be introduced, and is, therefore, the key issue for policy-makers.
The decision trees model the patient pathway after PPCI pathway activation to 1 year. Each possible patient pathway within the model is associated with a probability, and each pathway incurs costs and outcomes. The analysis was conducted from a NHS and personal social services perspective. 34 Outcomes were measured in quality-adjusted life-years (QALYs), a composite measure of quality and quantity of life.
The structure of the model for patients with multivessel disease is described next, along with the sources of parameter estimates, before the model for patients with normal coronary arteries is considered.
Model 1: patients with multivessel disease
This model was designed to address the question ‘Which is the most cost-effective type of ischaemia testing for patients with multivessel disease who activate the PPCI pathway?’
When the study started, stress ECHO was the ischaemia-testing option commonly used and the study was comparing this with CMR. During the study, the use of FFR increased35 and, therefore, this test was included as an additional arm in the model. Thus, the model compares the cost-effectiveness of three ischaemia testing options: (1) CMR, (2) pressure wire and (3) stress ECHO.
The cohort of patients of interest are those who activate the PPCI pathway, have their index angiography and PPCI, and are identified as having multivessel disease (commonly defined as stenosis of > 50% from the angiogram). This model considers the patient pathway thereafter: whether or not to treat non-culprit lesions with a second revascularisation, the need (or not) for ischaemia testing to help determine the need for the revascularisation of a second vessel and any MACEs that occur. Figure 1 summarises the scenarios in the patient pathways included in the model structure for one arm of the model (CMR).
FIGURE 1.
Scenarios in the patient pathway included in the model structure for patients with multivessel disease.
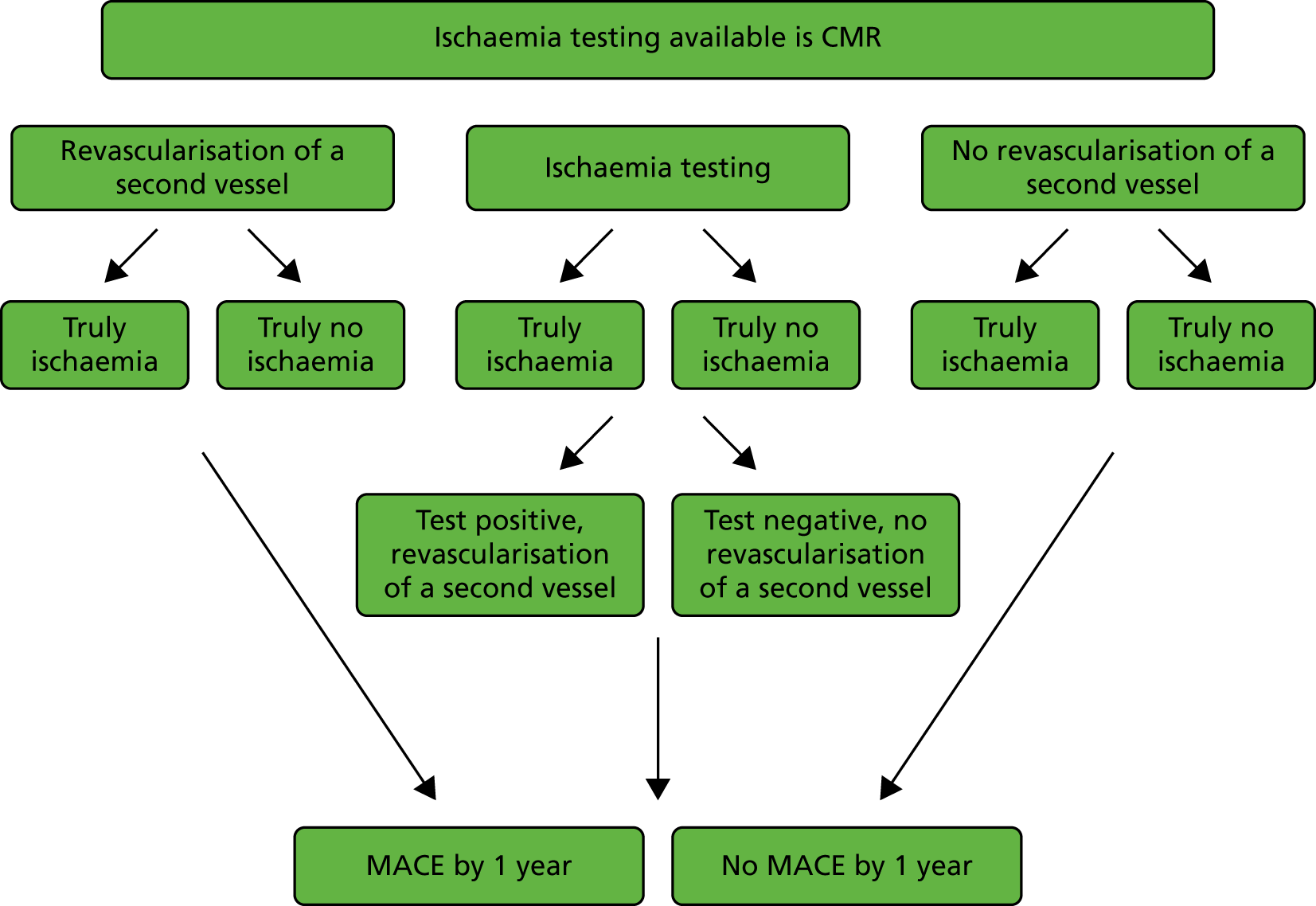
The model structure (shown in detail in Figure 2 splitting into three arms, representing the three different types of ischaemia testing) considered the following: the top branch models the scenario in which the ischaemia-testing option available is CMR, the middle branch models the scenario for pressure wire and the bottom branch models the scenario for stress ECHO. The structure of each of these three main arms is the same for each scenario, but the associated probabilities, costs and outcomes for each arm vary.
FIGURE 2.
Model 1 structure: patients with multivessel disease.
Reproduced with permission from Stokes et al. 36 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
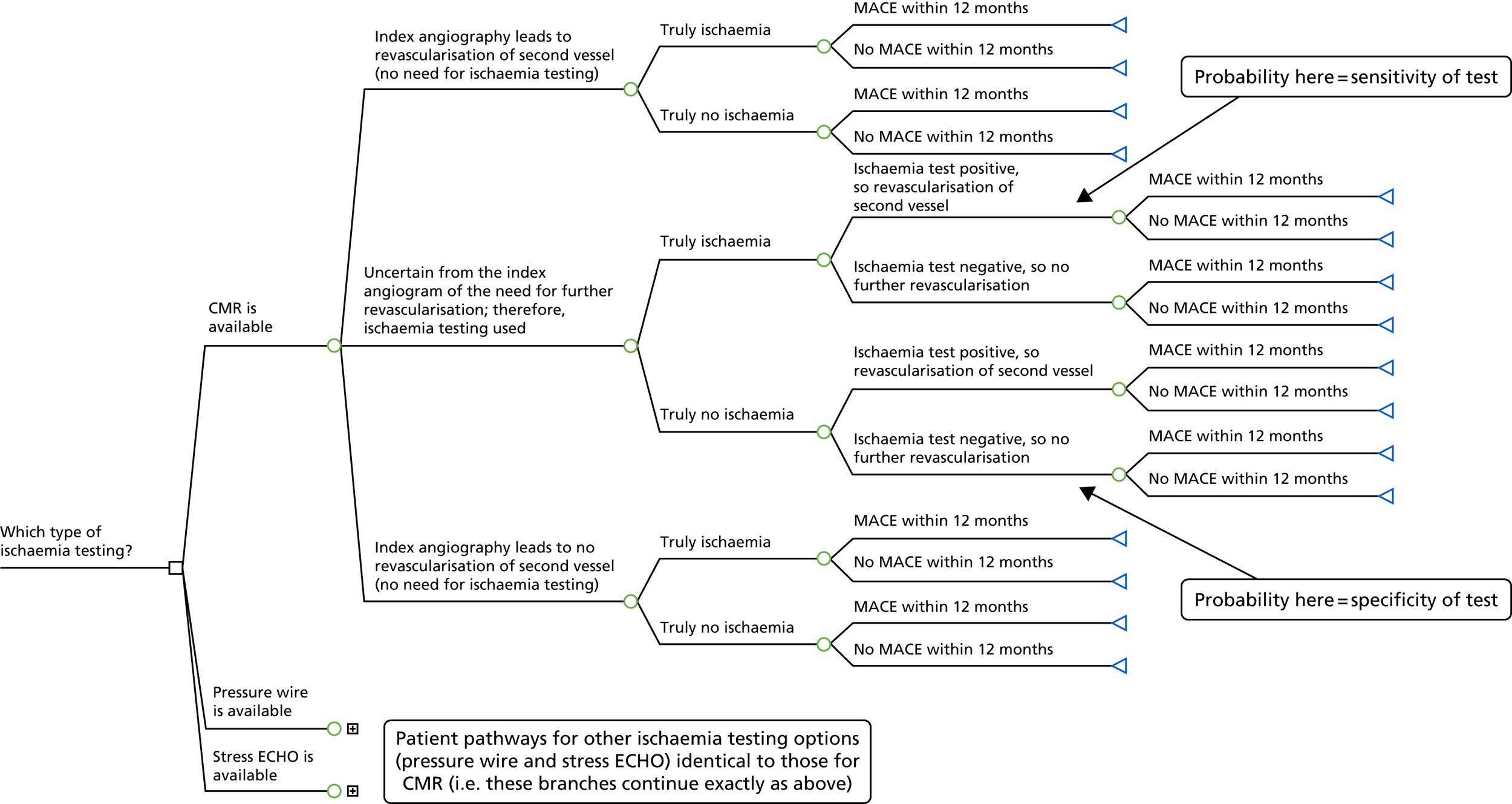
Considering the CMR arm, the decision tree first divides the starting cohort of patients after PPCI in accordance with the results of their index angiography. A clinician may determine that a patient requires or does not require the revascularisation of a second vessel directly from their angiogram, without the need for ischaemia testing; alternatively, there may be uncertainty from the index angiogram of the need for further revascularisation, and, therefore, ischaemia testing is used, in this case CMR. For each of these three categories, patients are then divided by whether or not they truly have ischaemia.
Patients whose index angiography leads to a firm decision on revascularisation are then divided by whether or not they had any MACEs in the 12 months after PPCI. MACE is a composite end point of all-cause mortality, MI, stroke and revascularisation.
Patients for whom ischaemia testing is needed are divided in accordance with whether that test result was positive, and therefore assumed to result in the revascularisation of a second vessel, or negative. Thereafter, patients are divided by whether or not they had a MACE in the 12 months after PPCI.
Although the model structure is the same for each of the ischaemia testing options, there are differences in the timing of events between these arms for patients who have ischaemia testing that leads to a second revascularisation. For CMR and stress ECHO, this is a two-step process, that is a patient has the ischaemia test and then separately has revascularisation of a second vessel. For pressure wire, this all happens in the catheterisation laboratory at the same time, that is a patient has their diagnostic angiography and pressure wire, and, if required, has their PCI at the same time (note that this is entirely separate and subsequent to their index angiography and PPCI). This affects costs, but not the model structure.
Data for the multivessel disease model
Most model parameters were populated with estimates derived from the literature. The following sections describe how transition probabilities, costs and outcomes were estimated to 1 year.
Probabilities for the multivessel disease model
Table 2 shows the probabilities used in the model and their sources. These are the probabilities associated with each branch in the model; probabilities along pathways are multiplied together in the analyses. MEDLINE searching was used to identify relevant papers, and was conducted using MeSH and keywords for each imaging type (e.g. exp/pressure-wire or pressure-wire.mp or fractional flow reserve.mp and exp/myocardial infarction). Any relevant reviews were read in order to identify relevant references, and references and citations of any relevant references were checked for prior or more recent published work.
| Branch of model | Base-case value (positive/total) | Distribution for PSA | Source |
|---|---|---|---|
| For each ischaemia testing option | |||
| Probability that index angiography leads to revascularisation of second vessel | 0.30 (30/100)a | Dirichlet | Expert opinion within the PIPA study team |
| Probability of uncertainty following index angiography and need for ischaemia testing | 0.60 (60/100)a | ||
| Probability that index angiography leads to no revascularisation of second vessel | 0.10 (10/100)a | ||
| Index angiography leads to revascularisation of second vessel | |||
| Probability of true ischaemia | 0.84 (598/709) | Beta | FAME37 |
| Probability of MACE by 12 months, true ischaemia | 0.09 (18/202)b | Beta | Smits et al.38 |
| Probability of MACE by 12 months, truly no ischaemia | 0.13 (54/432)b | Beta | Smits et al.38 |
| Uncertainty following index angiography and need for ischaemia testing | |||
| Probability of true ischaemia | 0.35 (218/620) | Beta | FAME37 |
| Index angiography leads to no revascularisation of second vessel | |||
| Probability of true ischaemia | 0.35 (218/620) | Beta | FAME37 |
| Probability of MACE by 12 months, true ischaemia | 0.31 (71/231)b | Beta | Smits et al.38 |
| Probability of MACE by 12 months, truly no ischaemia | 0.13 (54/432)b | Beta | Smits et al.38 |
| CMR or pressure wire | |||
| Uncertainty following index angiogram and need for ischaemia testing, true ischaemia | |||
| Probability of ischaemia test positive and revascularisation | 1c | Beta | |
| Probability of MACE by 12 months, revascularisation | 0.09 (18/202)b | Beta | Smits et al.38 |
| Probability of MACE by 12 months, no revascularisation | 0.31 (71/231)b,d | Beta | Smits et al.38 |
| Uncertainty following index angiogram and need for ischaemia testing, truly no ischaemia | |||
| Probability of ischaemia test positive and revascularisation | 0c | Beta | |
| Probability of MACE by 12 months, revascularisation | 0.13 (54/432)b,d | Beta | Smits et al.38 |
| Probability of MACE by 12 months, no revascularisation | 0.13 (54/432)b | Beta | Smits et al.38 |
| Stress ECHO | |||
| Uncertainty following index angiogram and need for ischaemia testing, true ischaemia | |||
| Probability of ischaemia test positive and revascularisation | 0.70 (32/46) | Beta | Gurunathan et al.39 |
| Probability of MACE by 12 months, revascularisation | 0.09 (18/202)b | Beta | Smits et al.38 |
| Probability of MACE by 12 months, no revascularisation | 0.31 (71/231)b | Beta | Smits et al.38 |
| Uncertainty following index angiogram and need for ischaemia testing, truly no ischaemia | |||
| Probability of ischaemia test positive and revascularisation | 0.23 (39/171) | Beta | Gurunathan et al.39 |
| Probability of MACE by 12 months, revascularisation | 0.13 (54/432)b | Beta | Smits et al.38 |
| Probability of MACE by 12 months, no revascularisation | 0.13 (54/432)b | Beta | Smits et al.38 |
In the base-case analyses, CMR and pressure wire were treated as reference standards and both were assumed to have 100% sensitivity and specificity. Therefore, the probabilities and outcomes are the same in these arms and they differ only in terms of costs.
The probabilities of MACEs were estimated from Smits et al. ,38 in which MACE was defined as a composite end point of all-cause mortality, non-fatal MI, revascularisation and cerebrovascular events at 12 months. Outcomes for subgroups of patients in the trial were used to estimate MACE for three groups of patients: (1) truly ischaemic patients who had a second revascularisation (p = 0.09), (2) truly ischaemic patients who did not have a second revascularisation (p = 0.31) and (3) truly not ischaemic patients, whether or not they had a second revascularisation (p = 0.13). Based on subgroups of the trial patients, the probability of a MACE at 12 months for truly ischaemic patients who had a second revascularisation was actually lower than for patients without ischaemia. This might seem anomalous, but is a genuine finding. Although the probability estimates for these three subgroups were all based on ≥ 200 patients, only 18 truly ischaemic patients who had a second revascularisation had a MACE; these findings might be an artefact of modest numbers, and larger studies might show different results.
Costs for the multivessel disease model
For each ischaemia testing strategy considered, the costs included were the cost of ischaemia testing (CMR, pressure wire or stress ECHO) if required, the cost of revascularisation of a second vessel, costs associated with adverse events included in MACEs (both the initial inpatient costs and subsequent costs post discharge), costs of cardiac medications and cardiac rehabilitation offered to all patients and additional health-care costs beyond hospital discharge to 1 year, including follow-up outpatient appointments for all patients.
Unit costs were largely obtained from NHS Reference Costs 2015 to 201640 and are shown in Table 3; details of assumptions made in estimating unit costs are also provided in Table 3. NHS Reference Costs provide upper and lower quartiles around mean estimates; the difference between the mean and the upper quartile was captured and used as a crude estimate of standard error (SE) in the probabilistic sensitivity analyses (PSAs) (described in Model analyses). When this was not possible, the SE was assumed to be 20% of the mean cost. All costs were parameterised using a gamma distribution in the PSAs.
| Resource | Unit cost, £ (SE) | Source |
|---|---|---|
| Revascularisation of second vessel (assumed to be PCI) | 2682 (533) | NHS Reference Costs 2015 to 2016.40 Elective Inpatient. Weighted average of codes EY40 and EY41, Standard/Complex Percutaneous Transluminal Coronary Angioplasty |
| CMR | 264 (154) | NHS Reference Costs 2015 to 2016.40 Diagnostic Imaging. Weighted average of all Cardiovascular Magnetic Resonance Imaging Scan codes |
| Angiography and pressure wire | 1340 (374) | NHS Reference Costs 2015 to 2016.40 Day case. Weighted average of code EY42, Complex Cardiac Catheterisation |
| Angiography, pressure wire and revascularisation (as a single catheter laboratory admission) | 2971 (594) | Cost of revascularisation above, and angiography and pressure wire above, minus the cost of an angiography, £1051 (NHS Reference Costs 2015 to 2016.40 Day case. Weighted average of code EY43, Standard Cardiac Catheterisation) |
| Stress ECHO | 182 (3) | NHS Reference Costs 2015 to 2016.40 Outpatient procedures. Cardiology. EY50Z, Complex Echocardiogram |
| MACE | 3251 (650) | The proportion of each component of MACE was taken from Smits et al.38 and a weighted average of the costs of each component was calculated, based on the unit costs below |
| Revascularisation (PCI) | 2682 | As above |
| Revascularisation (CABG) | 10,944 | NHS Reference Costs 2015 to 2016.40 Elective Inpatient. Weighted average of codes ED26, ED27, ED28 Complex/Major/Standard Coronary Artery Bypass Graft |
| MI | 2177 | NHS Reference Costs 2015 to 2016.40 Non-elective long stay. Weighted average of code EB10 A, Actual or Suspected Myocardial Infarction |
| Stroke | 3723 | NHS Reference Costs 2015 to 2016.40 Non-elective long stay. Weighted average of code AA22, Cerebrovascular Accident, Nervous System Infections or Encephalopathy |
| Death | 0 (0) | |
| Cardiac rehabilitation | 364 (73) | Assume offered eight sessions, but only 50% uptake, so cost four sessions (one first and three follow-up appointments, individual costs below) |
| First appointment | 97 | NHS Reference Costs 2015 to 2016.40 Consultant Led. 327, Cardiac Rehabilitation. Non-Admitted Face-to-Face Attendance, First |
| Follow-up appointment | 89 | NHS Reference Costs 2015 to 2016.40 Consultant Led. 327, Cardiac Rehabilitation. Non-Admitted Face-to-Face Attendance, Follow-Up |
| Cardiac medications for treatment of MI (all once daily by mouth). Assumed given for 12 months (or 6 months for patients who died) | ||
| Aspirin (75 mg) | 0.04 | BNF41 |
| Prasugrel (Efient®; Eli Lilly and Company, Indianapolis, IN, USA) (5 mg) | 1.70 | BNF41 |
| Atorvastatin (80 mg) | 0.07 | BNF41 |
| Bisoprolol (2.5 mg) | 0.02 | BNF41 |
| Ramipril (2.5 mg) | 0.04 | BNF41 |
| Total daily cost | 1.87 | |
| Total annual cost | 683 (137) | |
| Outpatient follow-up appointment (assumed to occur at 4–6 weeks and 6 months post discharge) | 122 (22) | NHS Reference Costs 2015 to 2016.40 Consultant-led appointments. Non-Admitted Face-to-Face Attendance, Follow-Up. Cardiology |
| Additional health-care costs to 1 year | 2221 (444) | The Office of Health Economics’ estimate of the annual cost per person of NHS care, inflated to 2015/16 prices using the hospital and community health services inflation index.42,43 For patients who die, costs are assumed to be half of this (£1111) |
| Additional health-care costs to 1 year for those with a MACE | 263 (53) | MI and stroke were assumed to be associated with continuing care costs post discharge. Costs from Greenhalgh et al.44 were inflated to 2015/16 prices.42 Separate costs were provided for disabling and non-disabling stroke. According to Davies et al.,45 58% of strokes are disabling; this percentage was used to weigh the two continuing care costs for stroke. Additional costs to 1 year associated with a MACE were calculated by weighting estimates of the annual costs of MI and stroke by the number of patients with these complications across the number of patients with a MACE, from Smits et al.38 |
Outcomes for the multivessel disease model
Outcomes were measured in QALYs. Estimates of survival and quality of life utility weights were obtained from the literature and combined to estimate the QALYs gained to 1 year. As death is one of the components of MACEs, patients without a MACE are all alive at 1 year in the model. The probability of dying in the first year was taken from the same source as the MACE estimates above, and was 8% for patients with a MACE. 38
The Tufts Cost-effectiveness Analysis Registry46 was searched for appropriate utility weights. This registry is a database of 5655 cost–utility analyses on a wide variety of diseases and treatments and has 21,900 utility weights. Search terms such as ‘primary percutaneous coronary intervention’ and ‘major adverse cardiac events’ were used. Estimates from the Swedish Early Decision reperfusion Study (SWEDES) by Aasa et al. 47 were deemed to be the most appropriate; this study measured health status using the EuroQol-5 Dimensions (EQ-5D) in a group of PPCI patients, and quality weights were obtained using the UK EQ-5D tariff. 47 Patients completed the EQ-5D at three time points: baseline (within 3 days of randomisation, at the end time of index hospitalisation), and at 1 and 12 months. Mean utilities for patients were 0.72, 0.77 and 0.77 at each time point, respectively. Although the patient group included some patients with a MACE, here the estimates are applied to patients without a MACE.
Quality-adjusted life-years were calculated assuming a patient’s utility changed linearly between each time point, so between baseline and 1 month, and between 1 and 12 months. Patients who died were assumed to die midway through the time period (i.e. at 6 months); their utility was assumed to change linearly between time points up to death, and a value of zero was given from death onwards. A QALY estimate for patients without a MACE – who, therefore, were all alive at 1 year – was calculated based on the utilities provided by Aasa et al. 47 For the QALY estimate for patients with a MACE 8% of patients were assumed to die,38 this was used to produce a weighted average for patients alive and dead at 1 year, based on the utility weights and assumptions above. A utility decrement of 0.05 was then applied to this weighted average to reflect the reduced quality of life of patients with a MACE compared with those without a MACE. 48 QALY estimates for patients who had revascularisation of a second vessel were modified to assume their utility for month 1 was repeated for month 2. QALY estimates used in the model are summarised in Table 4. QALYs were parameterised using a beta distribution in the PSAs and a SD of 0.09 was assumed, as used elsewhere. 49
| Patient group | Mean QALYs to 1 year |
|---|---|
| No revascularisation of a second vessel and MACE | 0.686 |
| No revascularisation of a second vessel and no MACE | 0.768 |
| Revascularisation of a second vessel and MACE | 0.684 |
| Revascularisation of a second vessel and no MACE | 0.766 |
Model 2: patients with normal coronary arteries
For a starting cohort of patients with normal coronary arteries, this model compares standard ECHO and CMR with standard ECHO only, and is designed to address the question: ‘Is it cost-effective to introduce CMR in patients with normal coronaries who activate the PPCI pathway?’. For the standard ECHO and CMR arm of the model, Figure 3 summarises the scenarios in the patient pathways included in the model structure.
FIGURE 3.
Scenarios in the patient pathway included in the model structure for patients with normal coronaries.
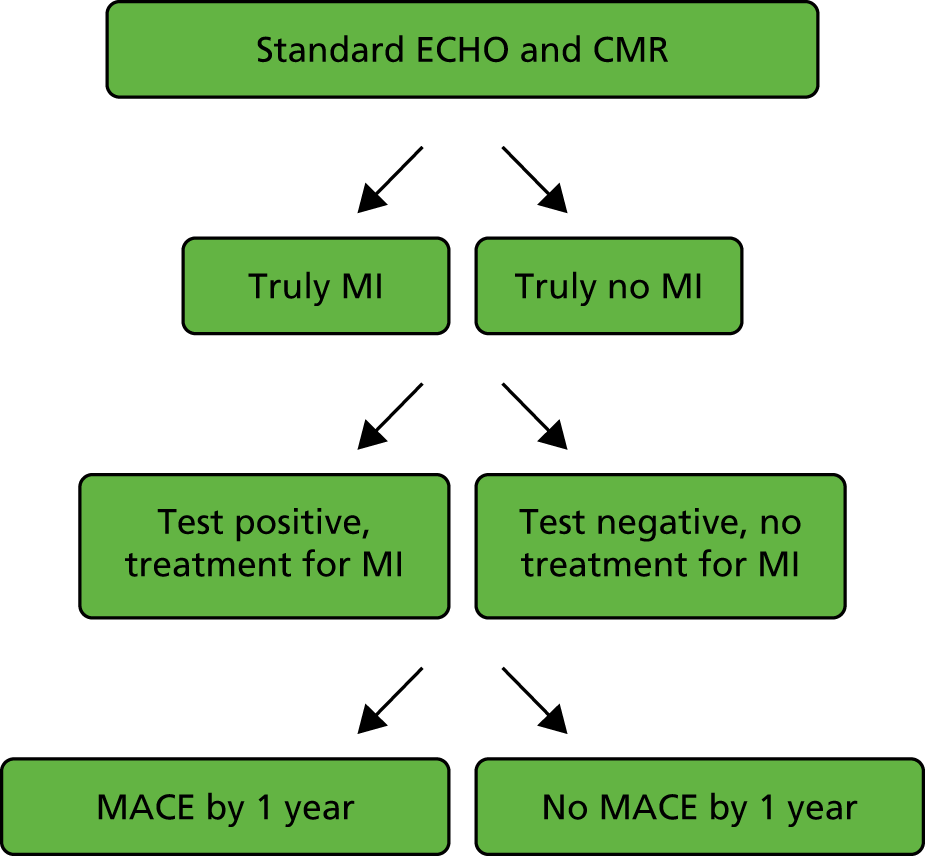
The model structure, shown in detail in Figure 4, is split into two main arms (branches): the top branch represents standard ECHO and CMR and the bottom branch represents standard ECHO only. The structure is the same in each arm, and starts by dividing patients into those who truly did have a MI and those who truly did not. Patients are then divided according to their ischaemia test result. For those with a positive test result, and therefore assumed to have had a MI, treatment for MI in the form of cardiac medications and cardiac rehabilitation will be given. Those with a negative test result will be treated for other causes of their chest pain, and given fewer cardiac medications or none at all. Thereafter, patients are divided into whether or not they had a MACE within 12 months. A MACE is defined in the same way as above: a composite end point of all-cause mortality, MI, stroke and revascularisation.
FIGURE 4.
Model structure for patients with normal coronaries (no blocked arteries).
Reproduced with permission from Stokes et al. 36 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
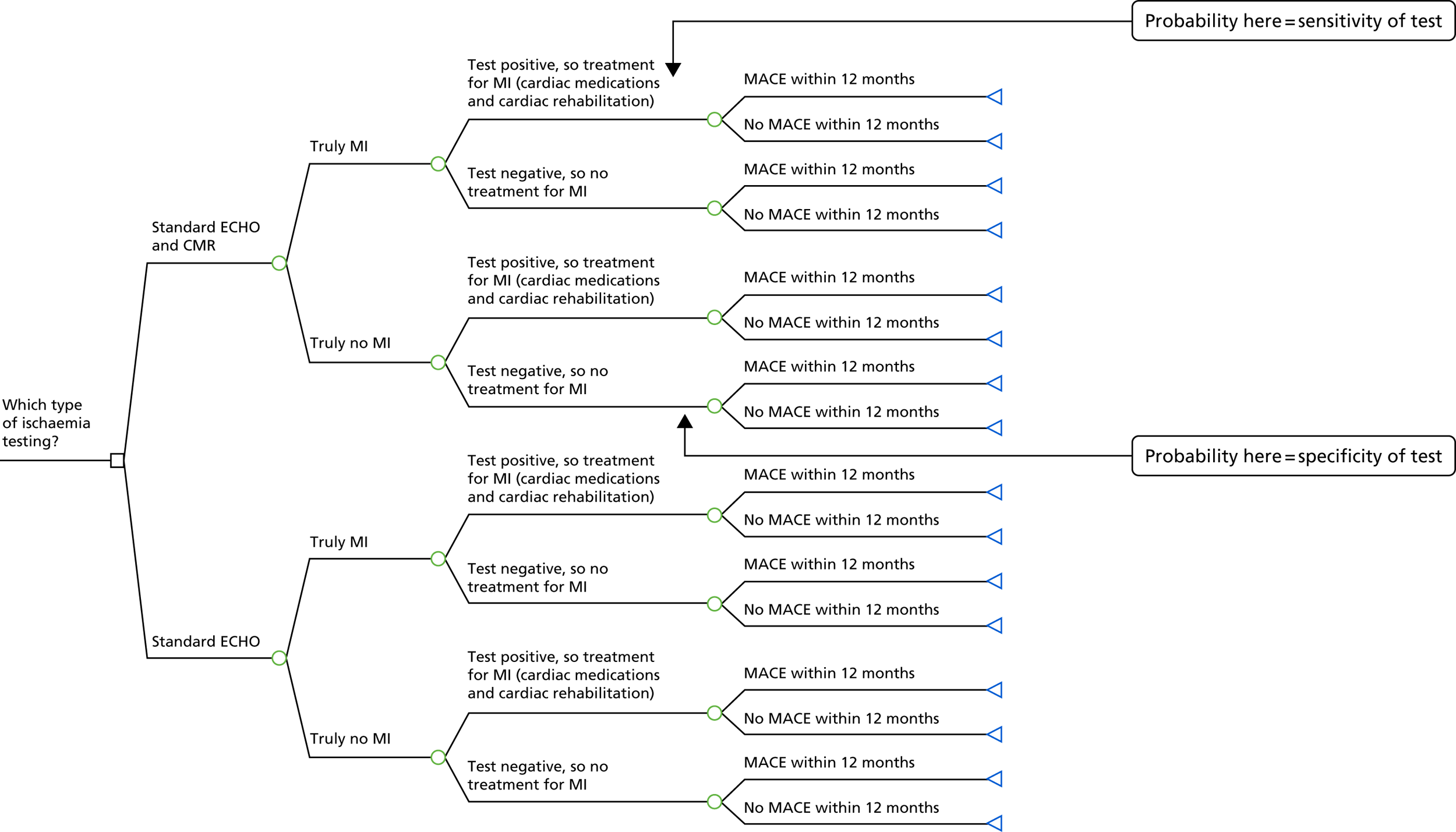
Data for the normal coronaries model
The decision tree for patients with normal coronaries was set up in a similar way to the model for patients with multivessel disease. The following sections describe how transition probabilities, costs and outcomes were estimated to 1 year, the majority of which were derived from the literature.
Probabilities for the normal coronaries model
Table 5 shows the probabilities used in the model and their respective sources. From initial MEDLINE searching, a 2016 review paper by Dastidar et al. 55 identified the first paper to use CMR for diagnosis in patients with normal coronaries,20 and this was confirmed by later reviews. All citations of the Assomull et al. 20 paper were reviewed, and relevant (English) results read. Two meta-analyses were identified,50,56 and the citations of these papers were also reviewed, as were the references of more recent papers. In a final check to ensure that key papers had been identified, MEDLINE searching of keywords, such as ‘myocardial infarction with non-obstructive coronary arteries’, and synonyms was conducted (there are no relevant MeSH terms).
| Branch of model | Base-case value (positive/total) | Distribution for PSA | Source |
|---|---|---|---|
| For both arms | |||
| Probability of truly having had a MI | 0.24 (429/1801) | Beta | Pasupathy et al.50 |
| Standard ECHO and CMR | |||
| Probability of ischaemia test positive and treatment for MI | MI | 1a | Beta | |
| Probability of MACE within 12 months | treatment for MI | 0.21 | Beta | Kang et al.,51 Pathik et al.52 |
| Probability of MACE within 12 months | no treatment for MI | 0.26b | Beta | Kang et al.,51 Pathik et al.52 and Lindahl et al.53 |
| Probability of ischaemia test positive and treatment for MI | no MI | 0a | Beta | |
| Probability of MACE within 12 months | treatment for MI | 0.03b | Beta | Kang et al.,51 Pathik et al.52 |
| Probability of MACE within 12 months | no treatment for MI | 0.03 | Beta | Kang et al.,51 Pathik et al.52 |
| Standard ECHO | |||
| Probability of ischaemia test positive and treatment for MI | MI | 0.47 (25/53)c | Beta | Dastidar et al.54 |
| Probability of MACE within 12 months | treatment for MI | 0.21 | Beta | Kang et al.,51 Pathik et al.52 |
| Probability of MACE within 12 months | no treatment for MI | 0.26 | Beta | Kang et al.,51 Pathik et al.52 and Lindahl et al.53 |
| Probability of ischaemia test positive and treatment for MI | no MI | 0.62 (93/151)c | Beta | Dastidar et al.54 |
| Probability of MACE within 12 months | treatment for MI | 0.03 | Beta | Kang et al.,51 Pathik et al.52 |
| Probability of MACE within 12 months | no treatment for MI | 0.03 | Beta | Kang et al.,51 Pathik et al.52 |
In the base-case analyses, standard ECHO and CMR was treated as a reference standard and assumed to have 100% sensitivity and specificity. Alternative scenarios were considered in sensitivity analyses.
Although there is evidence in the literature of the probability of a MACE, the definition frequently varies and studies follow patients for varying lengths of time. Although mortality at 1 year in patients with normal coronaries is lower than in patients with obstructed vessels,50,57 it is still significant; a recent systematic review estimated this to be 4.7%. 50
For the group as a whole, Kang et al. 51 reported MACEs at 12 months in 7.8% of patients with no or < 50% coronary artery stenosis. Their definition of a MACE included death, MI and ischaemic target vessel revascularisation, but did not include stroke. It should also be noted that, although the study began with 372 patients with no or < 50% coronary artery stenosis, only 126 had follow-up data at 12 months.
Three studies52,58,59 were identified that reported MACE for patients with normal coronaries, broken down by different CMR diagnoses. Pathik et al. 52 reported MACE for 0.27 (7/26) patients diagnosed with MI, and for 0.05 (5/99) patients not diagnosed with MI (diagnosed with myocarditis, cardiomyopathy or normal CMR) over a median of 24 months (n = 125). In the other two studies58,59 of 124 and 87 patients, no MACE outcomes were observed in the non-MI patients. The MACE estimates reported in Pathik et al. 52 were converted to continuous rates (events were assumed to occur evenly over the median 24 months of follow-up). The ratio of events in the MI patients compared with the non-MI patients was six, and was used with the overall estimate of a MACE at 1 year from Kang et al. 51 (0.078), and the estimate of the underlying probability of patients truly having a MI from Pasupathy et al. 50 (0.24), to calculate estimates of the probability of a MACE in patients who truly had a MI and in those who did not have a MI. This generated probability estimates of a MACE of 0.21 for patients with MI, and 0.03 for patients without a MI. However, we needed estimates of MACE based not only on whether or not patients had a MI, but also on whether or not they had treatment for a MI. The estimate generated for patients with MI (0.21) was assumed to apply to patients who had treatment. The hazard ratio reported by Lindahl et al. 53 for a MACE for patients who were taking statins compared with those who were not (0.77) was used to adjust this estimate of a MACE for patients who had a MI and treatment, to give a probability of a MACE for those with MI but without treatment of 0.26. For patients without MI, the probability of a MACE of 0.03 was assumed to apply regardless of whether or not treatment was given. A SE of 0.10 was assumed for the PSA for these probabilities. Although Pathik et al. 52 was the only source found to estimate the ratio of events in MI patients compared with non-MI patients (in patients with normal coronaries), it is important to highlight that the estimated ratio of six raised concerns among the study team as being very high, and that lower values are explored in sensitivity analyses.
The sensitivity and specificity of standard ECHO were estimated from Dastidar et al. 54 The pre-CMR diagnosis reported was treated as the standard ECHO diagnosis, and the post-CMR diagnosis was assumed to be the true underlying diagnosis. It was assumed that those with a pre-CMR diagnosis of MI or uncertain would have been given treatment for MI, and, therefore, patients in these categories were combined for the purposes of the sensitivity and specificity calculations.
Costs for the normal coronaries model
For each ischaemia testing strategy considered, the costs included were the cost of ischaemia testing (standard ECHO, CMR), the cost of treatment for MI in the form of cardiac rehabilitation and medications, the cost of medications for non-MI causes, costs associated with adverse events included in MACEs (both the initial inpatient costs and subsequent costs post discharge), and additional health-care costs beyond hospital discharge to 1 year, including a follow-up appointment for all patients. Unit costs were obtained from national sources40,41 and are shown in Table 6; details of assumptions made in estimating unit costs are also provided in Table 6. As for the unit costs used in the previous model, when mean and upper quartile unit costs were available, the difference was used as a crude estimate of SE in the PSAs, otherwise the SE was assumed to be 20% of the mean cost. Costs were parameterised using a gamma distribution in the PSAs.
| Resource | Unit cost, £ (SE) | Source |
|---|---|---|
| Standard ECHO | 72 (22) | NHS Reference Costs 2015 to 2016.40 Diagnostic Imaging. Outpatient. RD51 A, Simple Echocardiogram, ≥ 19 years |
| CMR | 264 (154) | NHS Reference Costs 2015 to 2016.40 Diagnostic Imaging. Weighted average of all Cardiovascular Magnetic Resonance Imaging Scan codes |
| Cardiac rehabilitation | 364 (73) | Assume offered eight sessions, but only 50% uptake, so cost four sessions (one first and three follow-up appointments, individual costs below) |
| First appointment | 97 | NHS Reference Costs 2015 to 2016.40 Consultant Led. Cardiac Rehabilitation. Non-Admitted Face to Face Attendance, First |
| Follow-up appointment | 89 | NHS Reference Costs 2015 to 2016.40 Consultant Led. Cardiac Rehabilitation. Non-Admitted Face to Face Attendance, Follow-Up |
| Cardiac medications for treatment of MI (all once daily by mouth) | ||
| Aspirin (75 mg) | 0.04 | BNF41 |
| Clopidogrel (75 mg) | 0.05 | BNF41 |
| Atorvastatin (80 mg) | 0.07 | BNF41 |
| Bisoprolol (2.5 mg) | 0.02 | BNF41 |
| Ramipril (2.5 mg) | 0.04 | BNF41 |
| Total daily cost | 0.22 | |
| Total annual cost | 80 (16) | |
| Treatment for non-MI cause (cardiac medications): total daily cost | 0.11 | Assume that half of these patients are taken off cardiac medications above (these medications are given for cardiomyopathy but not for myocarditis) |
| Outpatient follow-up appointment (assumed to occur at 4–6 weeks post discharge) | 122 (22) | NHS Reference Costs 2015 to 2016.40 Consultant-led appointments. Non-Admitted Face to Face Attendance, Follow-Up. Cardiology |
| MACE | 2808 (562) | The proportion of each component of MACE was taken from Pathik et al.,52 and a weighted average of the costs of each component was calculated, based on the unit costs provided in Table 2. Given the small number of MACE events, calculations were based on MI and non-MI patients combined |
| Additional health-care costs to 1 year | 2221 (444) | The Office of Health Economics’ estimate of the annual cost per person of NHS care inflated to 2015/16 prices using the hospital and community health services inflation index.42,43 For patients who die, costs are assumed to be half of this (£1111) |
| Additional health-care costs to 1 year for those with a MACE | 1602 (320) | MI and stroke were assumed to be associated with continuing care costs post discharge. Costs from Greenhalgh et al.44 were inflated to 2015/16 prices.42 Separate costs were provided for disabling and non-disabling stroke. According to Davies et al.45 58% of strokes are disabling; this percentage was used to weigh the two continuing care costs for stroke. Additional costs to 1 year associated with MACE were calculated by weighting estimates of the annual costs of MI and stroke by the number of patients with these complications across the number of patients with MACE, from Pathik et al.52 |
Outcomes for the normal coronaries model
The QALY estimates used in the model are shown in Table 7. Searches of the Tufts Cost-effectiveness Analysis Registry46 did not yield any utility weights that were specific to patients with normal coronaries; therefore, the estimates in Aasa et al. 47 were also used here. The QALY estimate for patients without a MACE who were all alive at 1 year was calculated directly from the utilities in Aasa et al. 47 For the QALY estimate for patients with a MACE, 8% of patients were assumed to die based on the same source as the MACE estimates above;52 this was used to produce a weighted average for patients alive and dead at 1 year, based on the utility weights and assumptions described previously, and a utility decrement of 0.05 was again applied to reflect the reduced quality of life of patients with a MACE. 48 The study team felt that the estimate of the proportion of patients with a MACE who die was low; therefore, a higher proportion (50%) is explored in the sensitivity analyses.
| Patient group | QALYs to 1 year |
|---|---|
| MACE within 12 months | 0.686 |
| No MACE within 12 months | 0.768 |
Model analyses
All analyses were conducted in TreeAge Pro 2013 (TreeAge Software Inc., Williamstown, MA, USA). Costs and QALYs were not discounted, as the time horizon was 1 year. Cost-effectiveness analyses were run, and the expected costs and QALYs associated with each arm (each ischaemia testing strategy) to 1 year were calculated. The incremental cost-effectiveness ratio (ICER) was derived from the difference in costs and QALYs between each ischaemia testing option and current practice. The ICER is the cost per QALY gained of introducing a different form of ischaemia testing compared with current practice.
As this is a feasibility study, it is not the actual ICER and cost-effectiveness results that are the main interest, but rather how the results change in response to sensitivity analyses; this will identify the key drivers of cost-effectiveness that would require detailed measurement and consideration in any subsequent work.
Sensitivity analyses
Both one-way sensitivity analyses and PSAs were conducted. One-way sensitivity analyses were used to assess the sensitivity of the base-case results to key uncertainties in the model to identify the key drivers of cost-effectiveness. Tables 8 and 9 describe the key sensitivity analyses that were conducted for each model. Threshold analyses were also conducted around the costs of CMR, and angiography and pressure wire to establish the cost of the test at which the costs associated with alternative strategies were the same.
| SA | Parameter varied | Base case | Alternative strategies for SA |
|---|---|---|---|
| 1 | Probabilities associated with decision on revascularisation from index angiography | Based on expert opinion | Based on PIPA data |
| Probability that index angiography leads to revascularisation of a second vessel (number of patients with events/total number of patients) | 0.30 (30/100) | 0.11 (78/717) | |
| Probability of uncertainty following index angiography and need for ischaemia testing (number of patients with events/total number of patients) | 0.60 (60/100) | 0.15 (111/717) | |
| Probability that index angiography leads to no revascularisation of second vessel (number of patients with events/total number of patients) | 0.10 (10/100) | 0.74 (528/717) | |
| 2 | Sensitivity and specificity of CMR | 1 and 1 | 0.8 (SE 0.1) for each |
| 3 | Sensitivity and specificity of pressure wire | 1 and 1 | 0.8 (SE 0.1) for each |
| 4 | Sensitivity and specificity of stress ECHO | 0.70 and 0.77 (reported 1 – specificity = 0.23) | Sensitivity: 0.791 (SE 0.008) and 1 – specificity 0.129 (SE 0.007)60 |
| 5 | Probability of MACE for truly ischaemic patients who had a second revascularisation, truly ischaemic patients who did not have a second revascularisation, and truly not ischaemic patients whether or not they had a second revascularisation | 0.09, 0.31, 0.13 | All ± 0.05 |
| 6 | Cost of CMR | £264.00 | ± 20% |
| 7 | Cost of angiography and pressure wire | £1340.00 | ± 20% |
| 8 | QALYs for patients with MACE with and without revascularisation of second vessel | 0.684, 0.686 | Base case –0.2 (0.484, 0.486) |
| 9 | QALYs for patients with no MACE with and without revascularisation of second vessel | 0.766, 0.768 | Base case +0.2 (0.966, 0.968) |
| SA | Parameter varied | Base case | Alternative strategies for SA |
|---|---|---|---|
| 1 | Sensitivity and specificity of standard ECHO plus CMR | 1 and 1 | 0.8 (SE 0.1) for each |
| 2 | Sensitivity and specificity of standard ECHO | 0.47 and 0.38 | 0.7 (SE 0.1) for each |
| 3 | Probability of MACE for patients who had a MI and treatment, for patients who had a MI and no treatment, and for patients without MI regardless of whether they had treatment (ratio of events in MI-to-non-MI group = 4)a | 0.21, 0.26, 0.03 | 0.18, 0.23, 0.05 |
| 4 | Probability of MACE (ratio = 2) | 0.21, 0.26, 0.03 | 0.13, 0.17, 0.06 |
| 5 | Probability of MACE (ratio = 1) | 0.21, 0.26, 0.03 | 0.08, 0.10, 0.08 |
| 6 | Cost of CMR | £264.00 | ± 20% |
| 7 | Cost of additional health-care costs to 1 year for patients with a MACE: increase the proportion who die from 8% to 50% | £1602.00 | £874.00 |
| 8 | QALYs for patients with MACE | 0.686 | Base case –0.2 (0.486) |
| 9 | QALYs for patients with no MACE | 0.768 | Base case 0.2 (0.968) |
| 10 | QALYs for patients with MACE: increase the proportion who die from 8% to 50% | 0.686 | 0.525 |
To quantify the uncertainty around the one-way sensitivity analyses, parameters were assigned distributions (rather than fixed values) so that SEs and CIs could be estimated; this was done in the same way as the PSAs that were conducted. PSAs were used to investigate the joint impact of results of all uncertain parameters in the model simultaneously.
Model parameters were assigned probability distributions to describe the range of plausible values that they could take based on the precision of the estimates available. The type of distribution assigned was matched to the nature of the parameter; for example, a beta distribution lies between zero and one and is, therefore, appropriate for parameterising the probability of two mutually exclusive events. The type of distribution assigned to each parameter was described with the model inputs above.
Randomly selected values from each distribution for each model parameter were simultaneously generated using Monte Carlo simulation; this was repeated 1000 times and the results were calculated for each run of the model, generating a distribution of costs and effects that were used to calculate 95% CIs around the cost and QALY differences between testing strategies, enabling the effect of the parameter uncertainty on results to be investigated.
Changes to study design after commencement of the study
Feasibility prospective cohort study
There were two main changes to the study design, summarised below:
-
We included the option of postal consent to the recruitment protocol, mainly to be able to catch patients who were repatriated quickly to local hospitals after the index procedure.
-
We followed up patients through routinely collected hospital episode data (HES for English hospitals and PEDW for Welsh hospitals). We originally intended to obtain follow-up data for 12 months after the index procedure from each hospital. However, this was not feasible for many patients, given that they were repatriated to/followed up at a different hospital from where they had their index procedure.
Formal consensus study
There were four main changes related to the formal consensus study, summarised below:
-
We had intended to include different stakeholders [e.g. cardiologists, general practitioners (GPs) and patient representatives] in the consensus process. However, as the consensus statements developed it became evident that these were highly specialised and it would have been unreasonable to expect those without cardiology expertise to rate and discuss them. Therefore, the consensus process included only cardiologists.
-
We did not originally intend to give the final survey to cardiologists who did not participate in the consensus process. However, we had fewer than anticipated cardiologists in the consensus panel; therefore, to avoid any criticism of the number of panel members in our study and to prevent the possibility of introducing bias (given the self-selected nature of the panel), we decided to extend the survey to UK cardiologists who did not participate in the formal consensus process.
-
We had originally intended to implement our primary outcome using follow-up data from local HISs. However, given the difficulties of obtaining local follow-up data (highlighted in Feasibility prospective cohort study, point 2), we used HES follow-up data instead.
-
We designed and gave a questionnaire to all cardiologists who referred patients for CMR (in our two centres with dedicated CMR facilities), which requested information about how CMR changed the management of those patients. We determined whether or not changes in management described in the questionnaire were adequately reflected in hospital episode data (see Chapter 6, Implementation of a primary outcome based on the changes in management for which consensus was achieved).
Health economic study
We intended to use data collected as part of the PIPA study, particularly patient episode data from HES and the PEDW. However, because the HES data were not available in time to do this, an alternative approach was required. Secondary sources of data were sought and used as inputs into economic decision models used to identify the key drivers of cost-effectiveness for CMR imaging in patients who activate the PPCI pathway, that is, the model parameters that have the most influence.
Chapter 4 Results for aim A: feasibility of setting up a cardiovascular magnetic resonance registry by linking sources of routinely collected data
Parts of this text have been reproduced from Brierley et al. 27 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Aim A: to determine whether or not it is feasible to set up a cardiovascular magnetic resonance registry in this patient population by linking sources of routinely collected data
-
Objective A1: to implement consent and establish patient consent rate.
-
Objective A2: to provide evidence of whether or not data linkage and extraction can be carried out across PPCI centres.
-
Objective A3: to determine whether or not the registry can be compiled from HES and the PEDW rather than multiple HISs.
The number of data about ‘usual care’ provided by UK NHS hospitals that are being collected and stored electronically in HISs is rapidly increasing. Previous studies have shown that it is possible to use multiple HISs to retrieve and compile these patient-level data into a research database. 61–63 This study tested the feasibility of setting up a large multicentre registry for patients who activate the PPCI pathway by linking data from multiple HISs within several hospitals. If the registry was feasible, we had the longer-term aim of using registry data to evaluate the clinical effectiveness and cost-effectiveness of CMR in this population.
Screening and consent
Standard consent was successfully implemented at all participating hospitals. Across the four participating hospitals, a total of 2462 patients were screened between 13 May 2013 and 4 September 2014. Of these, 1670 (68%) consented to participate in the study. Consent rates ranged from 59% to 74% (an average of 68% across the four hospitals). We investigated various options for obtaining consent during the study: obtaining consent at discharge (rather than straight after the procedure), and an opt-out model on the procedural consent form (adding a paragraph to the procedural consent form explaining that the clinical data collected would be used for research unless patients ‘opted out’ by ticking a box on the form). These were not implemented as they were deemed not feasible.
Detailed screening data were available for only one hospital (hospital A, Figure 5). A total of 1210 patients were screened in hospital A; 856 were identified through the cardiology database ‘query’ that was set up to identify eligible participants for the study and 354 were identified independently by the research nurse team. The other hospitals did not provide detailed screening data. We anticipated that local record linkage between databases held by a hospital would allow patients who gave consent to be distinguished from those who did not. Thus, it was intended that standard aspects of data collection for prospective studies, for example a screening log with reasons for ineligibility and a record of consent, would be implemented in our study as routine reports from the database. In hospital A, we set out to identify eligible patients through a database ‘query’ set up in the local cardiology database. It was hoped that this screening model would be exported to the other participating hospitals. However, when we started recruiting, it became clear that this screening model did not work, as many patients were identified independently of the database query. The other hospitals were, therefore, allowed to implement their own methods of screening, involving a combination of electronic processes (e.g. e-mail alerts or database searches for new potentially eligible patients) and manual processes (e.g. checking admission books) by the research nurses and/or the local principal investigator.
FIGURE 5.
Flow chart of patient recruitment in hospital A.
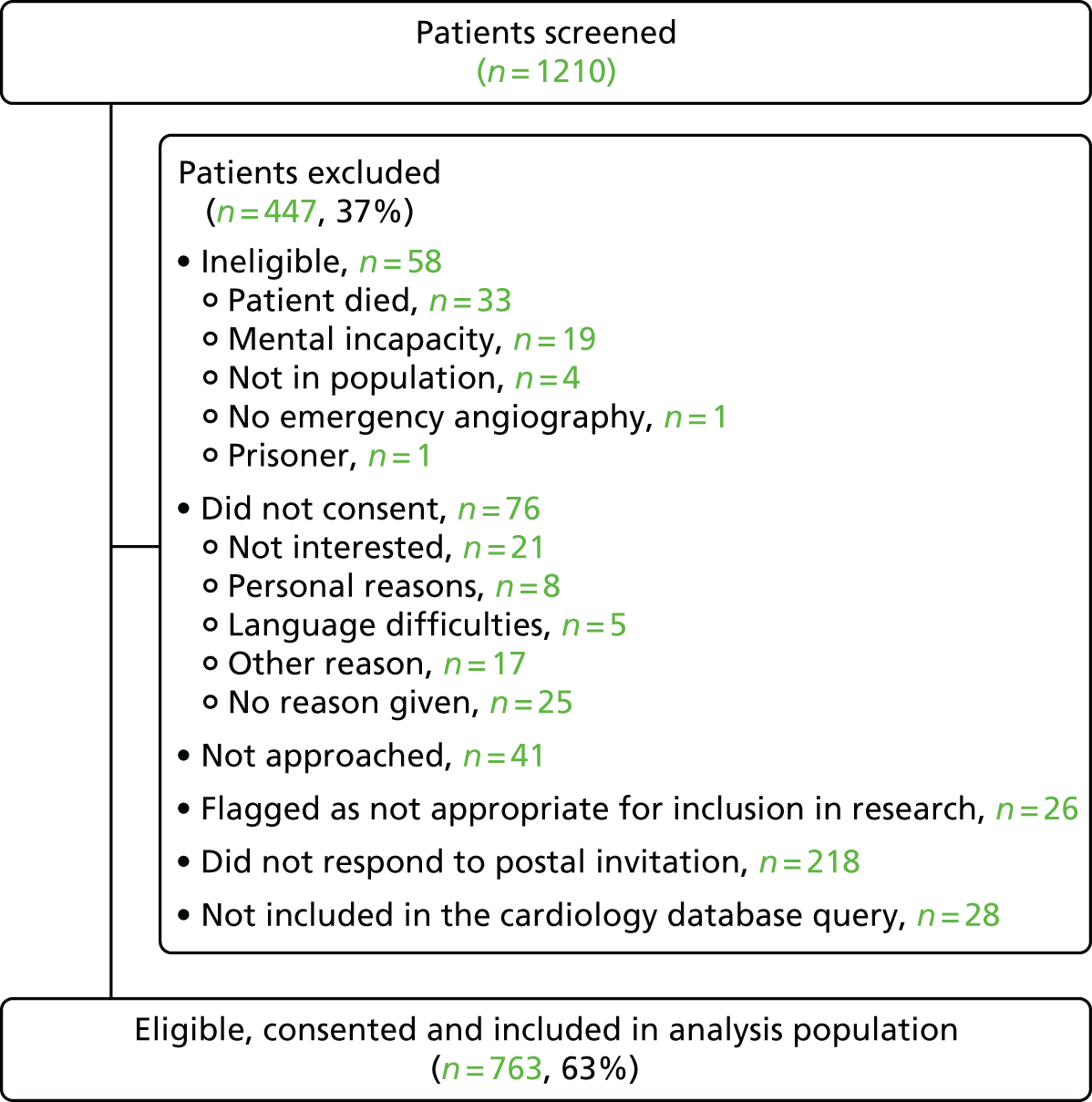
Recruitment
A total of 1670 participants were recruited to the study between May 2013 and September 2014. Cumulative recruitment by hospital is shown in Figure 6. Recruitment over time increased steadily across all hospitals. Hospital A recruited the largest number of patients.
FIGURE 6.
Recruitment over time, by hospital.

Because we did not have screening data for three of the hospitals, we estimated the approximate number of eligible patients over the recruiting period from the data between January and December 2014 provided directly by the BCIS. This registry provides comparative data on the provision of PCI in the UK and describes the quality and patterns of care, the process of care and outcomes for patients from all PCI centres. We extrapolated the number of PPCI procedures carried out during the recruitment period at each of the hospitals from the annual data provided for each hospital. The estimated rate of recruitment at each hospital is shown in Table 10. The average recruitment rate across the four hospitals was 52%, but there was large variation between hospitals (22–86%). These recruitment figures do not take into account (1) patients who did not meet the study eligibility criteria or (2) patients who activated the PPCI pathway but had emergency angiography only (i.e. patients with unobstructed arteries).
| Site | Number of months of recruitment | Estimated number of PPCIsa during recruitment period | Participants recruited to studyb (n) | Estimated % recruited |
|---|---|---|---|---|
| Hospital A | 15.7 | 765 | 655 | 86 |
| Hospital B | 12.5 | 1138 | 246 | 22 |
| Hospital C | 11.2 | 392 | 295 | 75 |
| Hospital D | 13.8 | 532 | 280 | 53 |
| Total | 2827 | 1476 | 52 |
Data linkage and extraction across hospitals
Figure 7 shows the flow chart of data availability. Anonymised data were available for 705 out of the 792 (89%) patients who were identified as eligible but who did not consent or were not approached. Clinical and biochemistry data were most readily available (for > 80% of participants), whereas data on medications were least readily available (for only 44% of participants).
FIGURE 7.
Flow chart of data availability.
a, The number of patients with their index procedure identified in hospital episode data (exact match on day or 1 day out) or inpatient or outpatient or A&E data available in the year following the index procedure. Note that 1612 out of 1670 (97%) patients had their index procedure identified in hospital episode data (exact match on day or 1 day out).
Reproduced from Brierley et al. 27 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
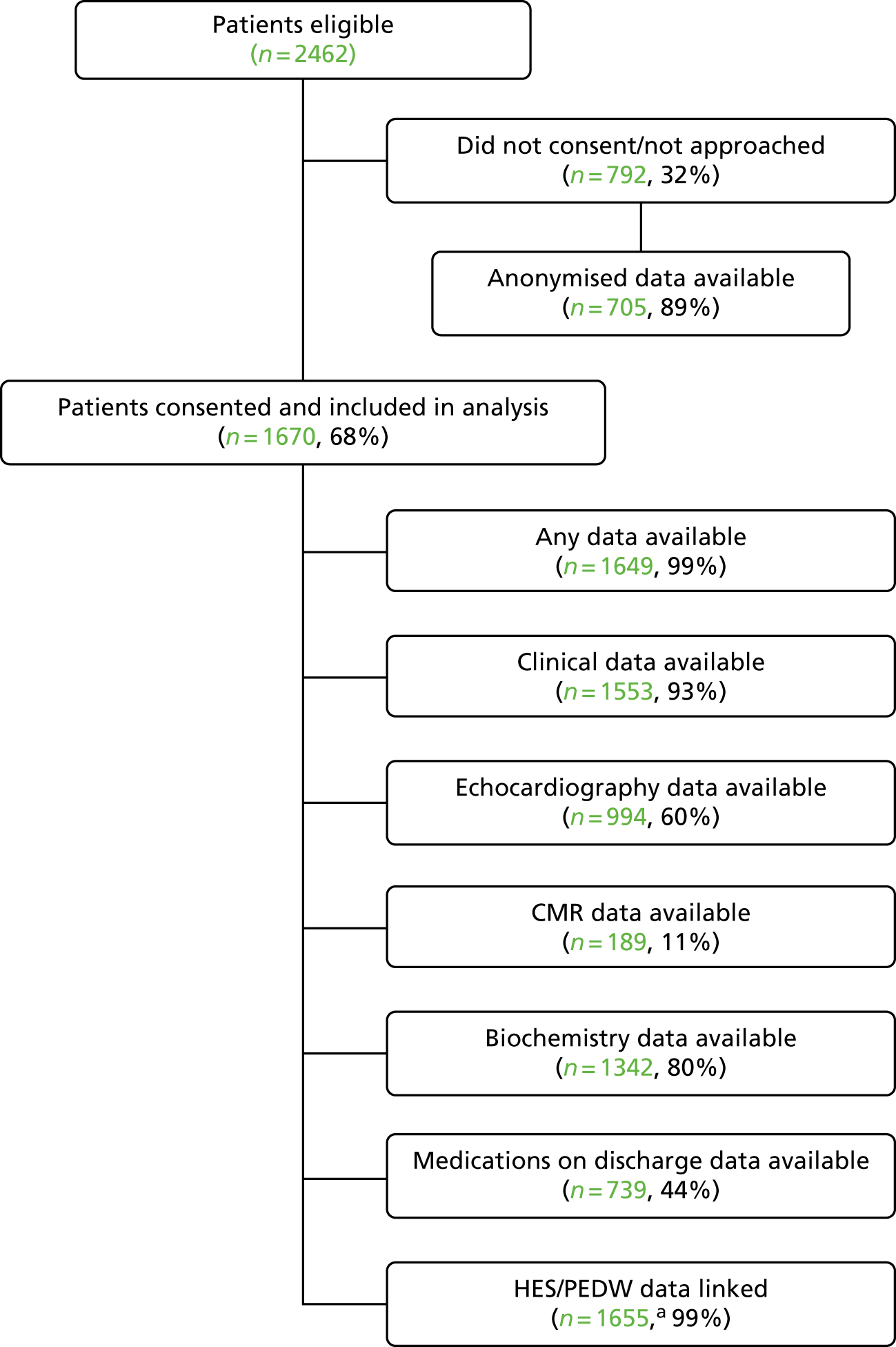
Figure 8 shows the flow chart of data submitted from each hospital. One of the hospitals (hospital B) provided the data in a linked format; linkage was undertaken centrally for the other three hospitals with data linked on a local hospital identifier. Anonymised data were available for > 80% of patients from three hospitals, but for only 50% of patients from one hospital. Clinical data were available for 93% of patients at all hospitals (range 82–98%). The availability of ECHO data was variable; one hospital was unable to submit any ECHO data, one hospital submitted ECHO data for only 34% of patients and two submitted data for > 75% of patients. ECHO data were submitted as free-text reports, rather than coded data. Biochemistry data were available for ≥ 98% of patients from three hospitals; one hospital was unable to submit biochemistry data for any of its patients. Data about medications on discharge were available for 97% of patients from one hospital; the other three hospitals were unable to submit these data. 27 Although we requested biochemistry test results relevant to the cardiovascular disease population [creatinine, full blood count (serial), electrolytes (serial), troponin (admission; 8 hour), cholesterol (on admission), blood glucose (serial) and C-reactive protein (serial)], the three hospitals that provided biochemistry data sent all tests conducted during the specified time frame. However, it was feasible to extract specific test results from the biochemistry data sets and identify specific medications from the medications data sets.
FIGURE 8.
Flow chart of data availability, by hospital. Shading indicates that no data were available.
Reproduced from Brierley et al. 27 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

There were a number of issues with data that were provided by hospitals, such as the inclusion of ‘test’ patients at one hospital, discrepancies between hospitals on the format and naming of the fields (e.g. BCIS fields were provided as: ‘yes/no’, ‘1/2’ or ‘1. yes/2. no’) and the delay in receiving extracts. Once identified, ‘test’ patients were removed at source and discrepancies between formatting and naming of fields were addressed by hardcoding.
Cardiovascular magnetic resonance exposure
Cardiovascular magnetic resonance reports were submitted for all consented patients who received CMR in hospitals A and B. Like the ECHO data, CMR data were in free-text format; the hospital that provided linked data (hospital B) extracted the CMR variables of interest from the free-text reports. Information about whether or not a CMR was ordered for clinical need or research purposes (i.e. patient participating in another research study) was not recorded routinely. From information provided by hospital A, the proportion ordered for clinical need was 67%.
Data linkage with Hospital Episode Statistics and Patient Episode Database Wales
Hospital Episode Statistics, ONS and PEDW data were provided from the first recruitment date across all hospitals (May 2013) up to 30 September 2014 (Swansea), 31 March 2015 (Bristol and Leeds) and 30 April 2015 (Cardiff). Data linkage with HES, PEDW and vital status was achieved for 1655 out of 1670 (99%) patients across all hospitals. The linkage for each HES or PEDW data set is shown in Table 11. No data were supplied for patients for whom the NHS number was not recorded.
| Site | Consented (n) | Patients with, n (%) | |||
|---|---|---|---|---|---|
| Any admissions data | Any outpatient data | Any A&E data | Admissions, outpatient or A&E data | ||
| Hospital Aa | 763 | 757 (99) | 743 (97) | 583 (76) | 758 (99) |
| Hospital B | 272 | 272 (100) | 269 (99) | 196 (72) | 272 (100) |
| Hospital Cb | 320 | 311 (97) | 266 (83) | 264 (83) | 316 (99) |
| Hospital Dc | 315 | 309 (98) | 292 (93) | 191 (61) | 309 (98) |
| Total | 1670 | 1649 (99) | 1570 (94) | 1234 (74) | 1655 (99) |
A further review of the linked data demonstrated that it was possible to identify an admission that matched the index admission for 1554 (93%) patients; this increased to 1612 (97%) patients when the dates of admissions recorded in hospital episode data and the date of the index admission recorded in HISs were allowed to differ by 1 day. For some patients (n = 22, hospital D), it was not possible to identify the index admission in HES because the event date was not recorded in the HIS. This also meant that it was not possible to identify admissions, outpatient appointments or A&E visits in the year after the index admission for these patients. Data availability is shown in Table 12.
| Site | Consented (N) | n (%) | |||
|---|---|---|---|---|---|
| Admission matching index admission date (± 1 day) | Any APC data in year after index admission | Any outpatient data in year after index admission | Any A&E data in year after index admission | ||
| Hospital Aa | 763 | 756 (99) | 342 (45) | 720 (94) | 315 (41) |
| Hospital B | 272 | 269 (99) | 115 (42) | 258 (95) | 116 (43) |
| Hospital Cb | 320 | 295 (92) | 130 (41) | 234 (73) | 119 (37) |
| Hospital Dc | 315 | 292 (93) | 86 (27) | 284 (90) | 89 (28) |
| Total | 1670 | 1612 (97) | 673 (40) | 1496 (90) | 639 (38) |
Identifying the eligible study cohort and cardiovascular magnetic resonance exposure in hospital episode data
We identified 98% (range 92–100% across hospitals) of patients who underwent PPCI and 85% (range 46–99% across hospitals) of patients who had an emergency angiogram but no PCI in hospital episode data.
We could not identify all CMR exposure in HES. Admissions including the date of the CMR were identified for 109 of the 189 patients with CMR recorded in HISs (57%) and a record of CMR was found in the hospital episode data for 49 of these 109 (45%) patients. Patients who had a CMR dated after discharge, based on the HIS, were assumed to have had the CMR as an outpatient (n = 81); only 7 (9%) of these patients had a matching outpatient date in the hospital episode data. None of these seven outpatient episodes included any code for CMR (procedure codes are not well recorded in the HES outpatients’ data set). Data availability is shown in Table 13 and the details of CMR identification in the hospital episode data are shown in Figure 9.
| Site | Consented (N) | n (%) | |||||
|---|---|---|---|---|---|---|---|
| Number (out of N) consented and matched for PPCI index date (CRF and hospital electronic record) | Number (and %) of those in the left adjacent column also identified in national routine data sets (HES/PEDW) | Number (out of N) consented and matched for emergency angiography but without PPCI (CRF and hospital electronic record) | Number (and %) of those in the left adjacent column also identified in national routine data sets (HES/PEDW) | Number (out of N) consented and matched for CMR (CRF and hospital electronic record) | Number (and %) of those in the left adjacent column also identified in national routine data sets (HES/PEDW) | ||
| Hospital A | 763 | 655 | 649 (99) | 108 | 107 (99) | 151 | 49 (32) |
| Hospital B | 272 | 246 | 246 (100) | 26 | 23 (88) | 38 | 6 (16) |
| Hospital C | 320 | 295 | 273 (92) | 25 | 22 (88) | – | – |
| Hospital D | 315 | 280 | 274 (98) | 35 | 16 (46) | – | – |
| Total | 1670 | 1476 | 1444 (98) | 194 | 168 (85) | 189 | 55 (29) |
FIGURE 9.
Details of CMR recording in HES/PEDW data.
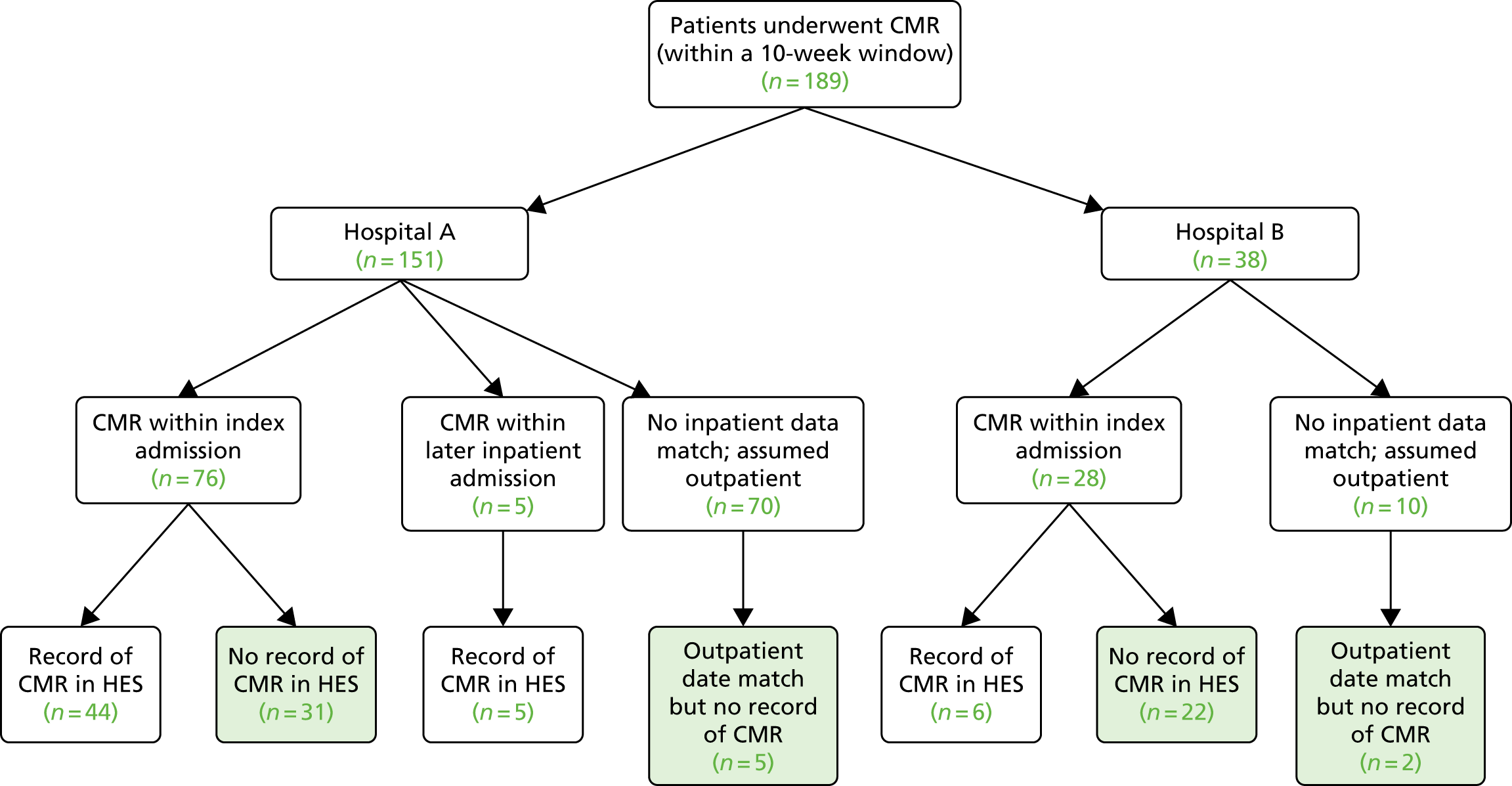
Summary of main findings
Identifying the eligible population
Patients who activated the PPCI pathway and received PCI were easy to identify from catheter laboratory databases, since consistent terminology was used to define these patients. A proportion of these patients recruited in the study were identified independently of these databases. Patients who activated the PPCI pathway but did not receive PPCI were not consistently defined in catheter laboratory databases; these patients required further manual checks by catheter laboratory staff who assisted the research nurses in identifying eligible patients. Some of these patients were screened out, possibly because of (1) misunderstanding by catheter laboratory staff or research nurses over eligibility (especially at the beginning of the study) and (2) the lack of consistent terminology used to describe these patients in catheter laboratory databases, leading to confusion about whether or not to include them.
Implementing consent
We asked all participating hospitals to implement a conventional system of written consent so that we could establish a consent rate and ensure that we had consent to obtain follow-up data from HES and PEDW. We met the feasibility objective of implementing conventional prospective consent at all hospitals. Consent rates ranged from 59% to 74%27 with respect to numbers considered eligible to participate, and from 22% to 86% with respect to approximate BCIS volume in the period of recruitment; these percentages were lower for some hospitals than we had anticipated for a study in which participants have no active involvement. The main reason for the lower than expected consent rate, and the variation between hospitals, was that many patients identified as eligible were missed; PPCI patients are asleep immediately following the procedure, rarely confined to bed after the first 4–6 hours and many are discharged within 24 hours or transferred to referring hospitals. Some of the missed patients activated the PPCI pathway at the weekend, when research nurses were not always available. In some centres, patients did not get admitted to the critical care ward or general ward after the PPCI procedure. We implemented postal consent to invite identified eligible patients who were not approached in hospital, but return rates were low. Some patients were unable to cope with the emergency nature of their condition and the large amount of new information they were given as part of their care; for some, the request to participate in a research study seemed to be yet another thing to think about and it was easier for them to decline to take part.
Data linkage and extraction across hospitals
We did not meet the feasibility criterion for data linkage and extraction from HISs for all of the requested data sets. We prespecified that, for each participating hospital, the data items requested should be available (i.e. extracted from sources and linked) for > 90% of patients. Only one hospital provided all of the requested data sets (anonymised, clinical, ECHO, biochemistry and medication on discharge) for > 80% of eligible (for the anonymised data set) and consented patients. Some hospitals had entire data sets missing: medications on discharge were not available from hospitals B, C and D; information about ECHO tests was not available from hospital B; and biochemistry data were not available from hospital D. There were several reasons for missing data, for example:
-
Some hospitals were unable to extract data from local stand-alone databases that were designed for a specific purpose, for example logging biochemistry requests or storing and presenting test results. 27 Such local databases were typically old and did not have an interface for data extraction; data would have had to be retrieved manually for each patient in turn, rather than submitting a ‘batch’ query. The study was not resourced for this activity. Moreover, obtaining data in this way was considered infeasible for a national registry (as well as time consuming) and hospitals were not requested to do so.
-
Some data (e.g. medications on discharge, bedside ECHO) were not recorded electronically and were available only in paper form (sometimes scanned but not coded/searchable).
-
Non-bedside imaging tests (ECHO and CMR) were available electronically but as free-text reports rather than coded data; variables of interest were impossible to extract from these reports by conventional text-searching methods because of the variability with which the information was recorded. 27
Cardiovascular magnetic resonance exposure
We were able to obtain CMR reports for all consented patients, although imaging reports were largely in free-text format, so individual CMR parameters would have had to be extracted manually. We concluded that this would not be feasible for an extended registry.
Follow-up/outcome data
It would be feasible to obtain 12 months’ follow-up data after the index admission through HES in an extended registry. Admissions (inpatient data set), outpatient appointments and A&E data could be identified for all patients for whom a NHS number was available and who had a date recorded for their index admission. Because we obtained hospital episode data only for patients who we believed to be eligible, we cannot comment on the risk that eligible patients might not have been identified.
Compiling the registry from Hospital Episode Statistics
It is not currently feasible to compile the registry from HES data rather than multiple HISs. Although we could identify the eligible population and obtain follow-up data, we could not identify the CMR exposure within 10 weeks of the index admission. This was mainly because the CMR is often performed at an outpatient clinic and the HES outpatient data quality, particularly for clinical information, is poor (i.e. lack of data on diagnoses or procedures).
Chapter 5 Results for aim B: cohort characteristics, exposure and outcomes, and resource use/cost-effectiveness
Parts of this text have been reproduced from Brierley et al. 27 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Aim B: to estimate the proportion of the target population who have cardiovascular magnetic resonance at two centres with dedicated cardiovascular magnetic resonance facilities and describe resource use and associated costs of having cardiovascular magnetic resonance after primary percutaneous coronary intervention
-
Objective B1: to estimate the proportion of the target population who undergo a CMR following PPCI pathway activation.
-
Objective B2: to describe resource use and associated costs of having CMR after PPCI pathway activation, including cost-effectiveness models.
In Chapter 4, we reported on the feasibility aspects of the study with respect to identifying the eligible population, implementing consent, and data linkage and extraction across sites and with hospital episode data. In this chapter, we report on the quantitative characteristics of the cohort with respect to index admission (baseline) data, follow-up data and the health economic analysis we conducted for this study.
A series of economic decision models were developed to identify the key drivers of cost-effectiveness. As uncertainty surrounds the performance of CMR imaging in practice and its benefit to patients in terms of more optimal treatment, a flexible framework was required to facilitate the assessment of uncertainty around parameter estimates. Models are a simplification of the real world and will never capture every possible scenario associated with an intervention, but the key consequences need to be included when models are conceptualised and structured. 64 It is helpful if there are clinical events driving the models to define branches in the decision trees and associated event probabilities. The models were developed in consultation with clinical experts on the study team.
Two subgroups of patients who activated the PPCI pathway were chosen for the economic decision models: (1) patients with multivessel disease and (2) patients with unobstructed coronary arteries. Both subgroups were identified by formal consensus (see Chapter 6, Patient subgroups) as potentially benefiting from CMR. Patients with multivessel disease are the largest subgroup of patients in the population that activates the PPCI pathway (40–60%), and there is clinical uncertainty about how to treat this subgroup. Patients with normal coronary arteries are a distinct group which, at the outset, the research team expected to benefit most from CMR.
Characteristics of consented versus non-consented patients
Table 14 shows the baseline characteristics of consented and non-consented patients. The IMD and WIMD scores were higher among those who consented than those who did not (SMDs 0.31 and 0.27, respectively). The percentage of patients who underwent PPCI was also higher among those who consented than those who did not (90% vs. 65%, respectively; SMD 0.70). This imbalance arose primarily from hospital A.
| Characteristic | Consenting patients (n = 1649) | Non-consenting patients (n = 705) | SMDa |
|---|---|---|---|
| Age (years), mean (SD)b | 64 (12.7) | 65 (14.2) | 0.06 |
| Male sex, n/N (%) | 1159/1516 (76) | 481/677 (71) | 0.12 |
| Current smoker, n/N (%) | 478/1473 (32) | 240/602 (40) | 0.16 |
| IMD score, median (IQR)c | 13.4 (7.7–25.8) | 19.9 (10.4–34.9) | 0.31 |
| WIMD rank, median (IQR)d | 891 (459–1370) | 671 (268–1221) | 0.27 |
| Diabetes, n/N (%) | 231/1536 (15) | 134/645 (21) | 0.15 |
| Hypertension, n/N (%) | 601/1455 (41) | 263/663 (40) | 0.03 |
| Previous PCI, n/N (%) | 157/1552 (10) | 73/652 (11) | 0.04 |
| Previous CABG, n/N (%) | 37/1540 (2) | 25/654 (4) | 0.09 |
| Previous MI, n/N (%) | 193/1547 (12) | 101/650 (16) | 0.09 |
| PPCI, n/N (%) | 1307/1452 (90) | 429/664 (65) | 0.70 |
| Length of stay (days), median (IQR)e | 3 (2–4) | 2 (1–4) | 0.13 |
Characteristics of participants by hospital
Table 15 shows the characteristics of participants by hospital. Participants from the four hospitals were similar with respect to demographics, comorbidities and previous cardiac interventions, although rates of hypertension were lower in patients from hospital B than in patients from hospitals A, C and D (18% vs. 41–52%).
| Characteristic | Hospital A (n = 762) | Hospital B (n = 272) | Hospital C (n = 315) | Hospital D (n = 300) |
|---|---|---|---|---|
| Age (years), mean (SD)a | 65.1 (12.7) | 62.9 (12.5) | 63.8 (12.4) | 63.4 (13.0) |
| Male sex, n/N (%) | 512/655 (78) | 183/246 (74) | 238/315 (76) | 226/300 (75) |
| Current smoker, n/N (%) | 191/660 (29) | 95/240 (40) | 95/300 (32) | 97/273 (36) |
| IMD score, median (IQR)b | 12.2 (7.1–21.7) | 20.5 (10.1–39.2) | – | – |
| WIMD rank, median (IQR)c | – | – | 817 (334–1478) | 902 (553–1314.5) |
| Diabetes, n/N (%) | 91/700 (13) | 33/243 (14) | 70/310 (23) | 37/283 (13) |
| Hypertension, n/N (%) | 275/663 (41) | 42/238 (18) | 143/282 (51) | 141/272 (52) |
| Previous PCI, n/N (%) | 71/701 (10) | 35/242 (14) | 27/310 (9) | 24/299 (8) |
| Previous CABG, n/N (%) | 21/704 (3) | 7/243 (3) | 5/308 (2) | 4/285 (1) |
| Previous MI, n/N (%) | 88/707 (12) | 40/240 (17) | 33/312 (11) | 32/288 (11) |
| PPCI, n/N (%) | 597/655 (91) | 220/243 (91) | 267/295 (91) | 223/259 (86) |
| Length of stay (days), median (IQR)d | 3 (2–4) | 2 (1–3) | 3 (1–4) | 2 (1–4) |
A CMR scan was performed (within 10 weeks of the index admission) for 20% of consented patients in one CMR centre and for 14% of consented patients in the second CMR centre. CMR data from hospital A were submitted as free-text reports; individual CMR parameters were difficult to extract electronically from these. Hospital B extracted CMR parameters of interest from the free-text reports manually. The baseline characteristics of patients who did and patients who did not receive CMR are shown in Table 16. Patients referred for CMR were slightly younger than those who did not have CMR. No other differences were noted.
| Characteristic | Patients who had CMR (n = 189) | Patients who did not have CMR (n = 846) | SMDa |
|---|---|---|---|
| Age (years), mean (SD)b | 61.8 (11.7) | 65.1 (12.8) | 0.26 |
| Male sex, n/N (%) | 121/154 (79) | 574/747 (77) | 0.04 |
| Current smoker, n/N (%) | 52/168 (31) | 234/732 (32) | 0.02 |
| IMD score, median (IQR)c | 12.4 (7.5–23.5) | 13.5 (7.8–26.3) | 0.08 |
| Diabetes, n/N (%) | 19/176 (11) | 105/767 (14) | 0.09 |
| Hypertension, n/N (%) | 63/166 (38) | 254/735 (35) | 0.07 |
| Previous PCI, n/N (%) | 18/176 (10) | 88/767 (11) | 0.04 |
| Previous CABG, n/N (%) | 3/176 (2) | 25/771 (3) | 0.09 |
| Previous MI, n/N (%) | 20/174 (11) | 108/773 (14) | 0.07 |
| PPCI, n/N (%) | 140/154 (91) | 677/744 (91) | 0.003 |
| Length of stay (days), median (IQR)d | 2 (2–3) | 3 (2–3) | 0.11 |
Participant follow-up
Table 17 shows the cardiac-related events in the 12 months following the index admission in the entire cohort. Overall, 55 out of 1670 (3%) patients died during follow-up (median age 73 years, IQR 66–82 years). MACE rate in this cohort was 13% (219/1670).
| Event | Number of events, n/N (%) | Person-years of observation | Events per 1000 person-years (95% CI) |
|---|---|---|---|
| Death | 55/1638 (3) | 1536.33 | 35.8 (27.0 to 46.6) |
| MI | 74/1638 (5) | 1376.04 | 53.8 (42.2 to 67.5) |
| Stroke | 2/1638 (0.1) | 1427.12 | 1.4 (0.02 to 5.1) |
| Repeat PCI | 117/1638 (7) | 1347.76 | 86.8 (71.8 to 104.0) |
| CABG | 20/1638 (1) | 1417.25 | 14.1 (8.6 to 21.8) |
| MACE (death, MI, revascularisation) | 219/1638 (13) | 1311.44 | 167.0 (145.6 to 190.6) |
The recorded causes of death for patients who died within 1 year of admission are shown in Appendix 6. Only 32 out of 54 (59%) patients died from causes closely associated with ischaemic heart disease; an additional 2 (4%) died from other heart conditions and 8 out of 54 (15%) patients died of cancer.
Cost-effectiveness models
Multivessel disease
The expected costs and QALYs gained under each of the ischaemia testing options are shown in Table 18 together with the cost-effectiveness results for CMR and pressure wire, each compared with stress ECHO. Comparisons are made with stress ECHO because this was the ischaemia test in use at the time of the study (and is considered the ‘existing’ comparator) and also because CMR and pressure wire were assumed to be equally effective; these strategies differ only in terms of their costs. Based on the point estimates, CMR was slightly less costly and more effective than stress ECHO and, therefore, dominated stress ECHO. Pressure wire was more costly and more effective than stress ECHO: the ICER is considerably higher than the accepted cost-effectiveness threshold of £20,000–30,000 per QALY, and, therefore, would not be considered cost-effective relative to stress ECHO. Pressure wire is convenient for cardiologists if the test identifies the fact that a patient needs revascularisation, as the patient can be treated immediately while in the catheter laboratory, and the cost of the pressure wire in addition to the revascularisation is modest. In the base-case analysis, 35% of patients having ischaemia testing were assumed to truly have ischaemia; the majority of patients are, therefore, having an expensive test without needing further treatment. If a greater proportion of patients undergoing ischaemia testing required revascularisation, it is likely that the estimated cost of a pressure-wire strategy would reduce. It would be possible to use a pressure wire on other vessels at the time of the index angiography and PCI, but it was beyond the scope of this work to consider the likely costs and consequences of such a strategy. Based on the point estimates, pressure wire is dominated by CMR, because CMR is less costly and equally as effective. Cost and QALY differences are small relative to the mean values under each strategy, and none of the differences in costs or QALYs is close to being statistically significant; indeed, the differences in QALYs were tiny. There is considerable uncertainty around these base-case results; however, as is clear from the plots of 1000 simulated cost and QALY differences from the PSA for CMR and pressure wire (each compared with stress ECHO) in Figures 10 and 11. In each figure, there are a large number of points in two or three quadrants of the cost-effectiveness plane. The black dot is the point estimate of the cost and QALY difference, and, in each figure, it is close to the origin.
| Ischaemia testing option | Costs (£), mean (SE) | QALYs, mean (SE) | Difference in costs to stress ECHO, mean (95% CI) | Difference in QALYs, compared with stress ECHO, mean (95% CI) | ICER (£), compared with stress ECHO |
|---|---|---|---|---|---|
| Stress ECHO | 5495 (556) | 0.7564 (0.0545) | |||
| CMR | 5431 (560) | 0.7576 (0.0551) | –64 (–232 to 187) | 0.0012 (–0.0076 to 0.0093) | CMR dominant (–53,563) |
| Pressure wire | 5855 (539) | 0.7576 (0.0551) | 360 (–116 to 844) | 0.0012 (–0.0076 to 0.0093) | 300,216 |
FIGURE 10.
A plot of the 1000 simulated cost and QALY differences from the PSA for CMR vs. stress ECHO.
Reproduced with permission from Stokes et al. 36 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.

FIGURE 11.
A plot of the 1000 simulated cost and QALY differences from the PSA for pressure wire vs. stress ECHO.
Reproduced with permission from Stokes et al. 36 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
For this feasibility study, it is not the cost-effectiveness results per se that are of interest, but rather the way in which they change in response to sensitivity analyses that helps us to determine the key drivers of cost-effectiveness. Table 19 reports the results of the sensitivity analyses around this model. Sensitivity analysis one, around the probabilities of ischaemia testing or a decision to revascularise a second vessel (or not) directly from the index angiography, affects the mean costs and QALYs under each strategy but does not alter the cost-effectiveness results, because these probabilities are the same under each strategy. Sensitivity analyses two and three, around the diagnostic accuracy of CMR and pressure wire, had the most effect on results. When the sensitivity and specificity of CMR were reduced to 80%, CMR became more costly than stress ECHO, rather than dominant, and, at accepted cost-effectiveness thresholds, would not be considered cost-effective relative to stress ECHO. When the sensitivity and specificity of pressure wire were reduced to 80%, conclusions did not alter: pressure wire was still more costly and slightly more effective than stress ECHO, but the ICER quadrupled. The diagnostic accuracy of the ischaemia tests, in particular CMR, are key drivers of cost-effectiveness. Varying the costs of CMR, angiography and pressure wire, and varying QALYs for patients with MACE and those without MACE, did cause cost-effectiveness results to vary, but did not alter cost-effectiveness conclusions.
| SA | Ischaemia testing option | Costs (£), mean (SE) | QALYs, mean (SE) | Difference in costs, compared with stress ECHO, mean (95% CI) | Difference in QALYs, compared with stress ECHO, mean (95% CI) | ICER (£), compared with stress ECHO |
|---|---|---|---|---|---|---|
| Base case | Stress ECHO | 5495 (556) | 0.7564 (0.0545) | |||
| CMR | 5431 (560) | 0.7576 (0.0551) | –64 (–232 to 187) | 0.0012 (–0.0076 to 0.0093) | CMR dominant (–53,563) | |
| Pressure wire | 5855 (539) | 0.7576 (0.0551) | 360 (–116 to 844) | 0.0012 (–0.0076 to 0.0093) | 300,216 | |
| 1. Decision from angiography | Stress ECHO | 4575 (449) | 0.7537 (0.0654) | |||
| CMR | 4558 (500) | 0.7540 (0.0661) | –17 (–62 to 45) | 0.0003 (–0.0022 to 0.0026) | CMR dominant (–53,563) | |
| Pressure wire | 4667 (500) | 0.7540 (0.0661) | 93 (–21 to 214) | 0.0003 (–0.0022 to 0.0026) | 300,216 | |
| 2. CMR test accuracy | Stress ECHO | 5495 (556) | 0.7564 (0.0545) | |||
| CMR | 5558 (600) | 0.7567 (0.0572) | 63 (–202 to 390) | 0.0004 (–0.0119 to 0.0108) | 168,453 | |
| 3. PW test accuracy | Stress ECHO | 5495 (556) | 0.7564 (0.0545) | |||
| Pressure wire | 5944 (568) | 0.7567 (0.0572) | 449 (–18 to 947) | 0.0004 (–0.0119 to 0.0108) | 1,207,875 | |
| 4. ECHO test accuracy | Stress ECHO | 5431 (553) | 0.7568 (0.0555) | |||
| CMR | 5431 (560) | 0.7576 (0.0551) | +0 (–128 to 253) | 0.0008 (–0.0022 to 0.0036) | 201 | |
| Pressure wire | 5855 (539) | 0.7576 (0.0551) | 424 (18 to 888) | 0.0008 (–0.0022 to 0.0036) | 529,193 | |
| 5. MACE +0.05 | Stress ECHO | 5674 (586) | 0.7520 (0.0566) | |||
| CMR | 5610 (580) | 0.7533 (0.0574) | –65 (–233 to 164) | 0.0012 (–0.0078 to 0.0087) | CMR dominant (–53,434) | |
| Pressure wire | 6033 (575) | 0.7533 (0.0574) | 359 (–85 to 831) | 0.0012 (–0.0078 to 0.0087) | 296,587 | |
| 5. MACE –0.05 | Stress ECHO | 5317 (564) | 0.7607 (0.0589) | |||
| CMR | 5252 (565) | 0.7619 (0.0595) | –65 (–244 to 192) | 0.0012 (–0.0081 to 0.0097) | CMR dominant (–53,463) | |
| Pressure wire | 5676 (533) | 0.7619 (0.0595) | 359 (–112 to 830) | 0.0012 (–0.0081 to 0.0097) | 297,408 | |
| 6. CMR cost +20% | Stress ECHO | 5495 (556) | 0.7564 (0.0545) | |||
| CMR | 5463 (556) | 0.7576 (0.0551) | –32 (–218 to 187) | 0.0012 (–0.0076 to 0.0093) | CMR dominant (–27,021) | |
| 6. CMR cost –20% | Stress ECHO | 5495 (556) | 0.7564 (0.0545) | |||
| CMR | 5399 (551) | 0.7576 (0.0551) | –96 (–268 to 159) | 0.0012 (–0.0076 to 0.0093) | CMR dominant (–80,104) | |
| 7. PW cost +20% | Stress ECHO | 5495 (556) | 0.7564 (0.0545) | |||
| Pressure wire | 6015 (560) | 0.7576 (0.0551) | 521 (78 to 992) | 0.0012 (–0.0076 to 0.0093) | 434,425 | |
| 7. PW cost –20% | Stress ECHO | 5495 (556) | 0.7564 (0.0545) | |||
| Pressure wire | 5694 (537) | 0.7576 (0.0551) | 199 (–240 to 622) | 0.0012 (–0.0076 to 0.0093) | 166,007 | |
| 8. QALYs for MACE –0.2 | Stress ECHO | 5495 (556) | 0.7306 (0.0559) | |||
| CMR | 5431 (560) | 0.7346 (0.0565) | –64 (–232 to 187) | 0.0040 (–0.0057 to 0.0117) | CMR dominant (–16,041) | |
| Pressure wire | 5855 (539) | 0.7346 (0.0565) | 360 (–116 to 844) | 0.0040 (–0.0057 to 0.0117) | 89,907 | |
| 9. QALYs for no MACE +0.2 | Stress ECHO | 5495 (556) | 0.9306 (0.0576) | |||
| CMR | 5431 (560) | 0.9346 (0.0587) | –64 (–232 to 187) | 0.0040 (–0.0071 to 0.0104) | CMR dominant (–16,041) | |
| Pressure wire | 5855 (539) | 0.9346 (0.0587) | 360 (–116 to 844) | 0.0040 (–0.0071 to 0.0104) | 89,907 |
An alternative way of assessing the sensitivity of results to the cost of an ischaemia test is to consider how the cost of the test would need to alter for the costs of alternative strategies to be estimated to be equal. In the base-case analyses, the cost of CMR was £264, and a CMR strategy was less costly than a stress ECHO strategy. Threshold analyses around the cost of CMR identified that if the cost rose to £371, there was no difference in the costs between a CMR and a stress ECHO strategy. In the base-case analyses, the cost of angiography and pressure wire was £1340, and a pressure-wire strategy was more expensive than a CMR or stress ECHO strategy. Threshold analyses identified that the cost of angiography and pressure wire would need to reduce to just £250 for there to be no difference in costs between a CMR and a pressure-wire strategy, and to £415 for there to be no difference between the cost of a stress ECHO and a pressure-wire strategy.
Normal (unobstructed) coronary arteries
The expected costs, QALYs and cost-effectiveness results under standard ECHO and CMR compared with standard ECHO alone for patients with normal coronaries are shown in Table 20. The reduction in costs associated with treating fewer patients for MI with the introduction of CMR testing compensated only partially for the additional cost of the CMR test. Mean costs and QALYs gained were higher with standard ECHO and CMR than with standard ECHO alone, and the ICER would not be considered cost-effective at accepted thresholds. There is considerable uncertainty around these base-case results, however, as is clear from the plot of the simulated cost and QALY differences from the PSA in Figure 12. There are a large number of points in three quadrants of the cost-effectiveness plane.
| Ischaemia testing option | Costs (£), mean (SE) | QALYs, mean (SE) | Difference in costs, mean (95% CI) | Difference in QALYs, mean (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Standard ECHO | 3032 (564) | 0.7615 (0.0837) | |||
| Standard ECHO and CMR | 3130 (589) | 0.7620 (0.0844) | 98 (–199 to 488) | 0.0005 (–0.0050 to 0.0077) | 190,114 |
FIGURE 12.
A plot of the 1000 simulated cost and QALY differences from the PSA for standard ECHO and CMR vs. standard ECHO.
Reproduced with permission from Stokes et al. 36 © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
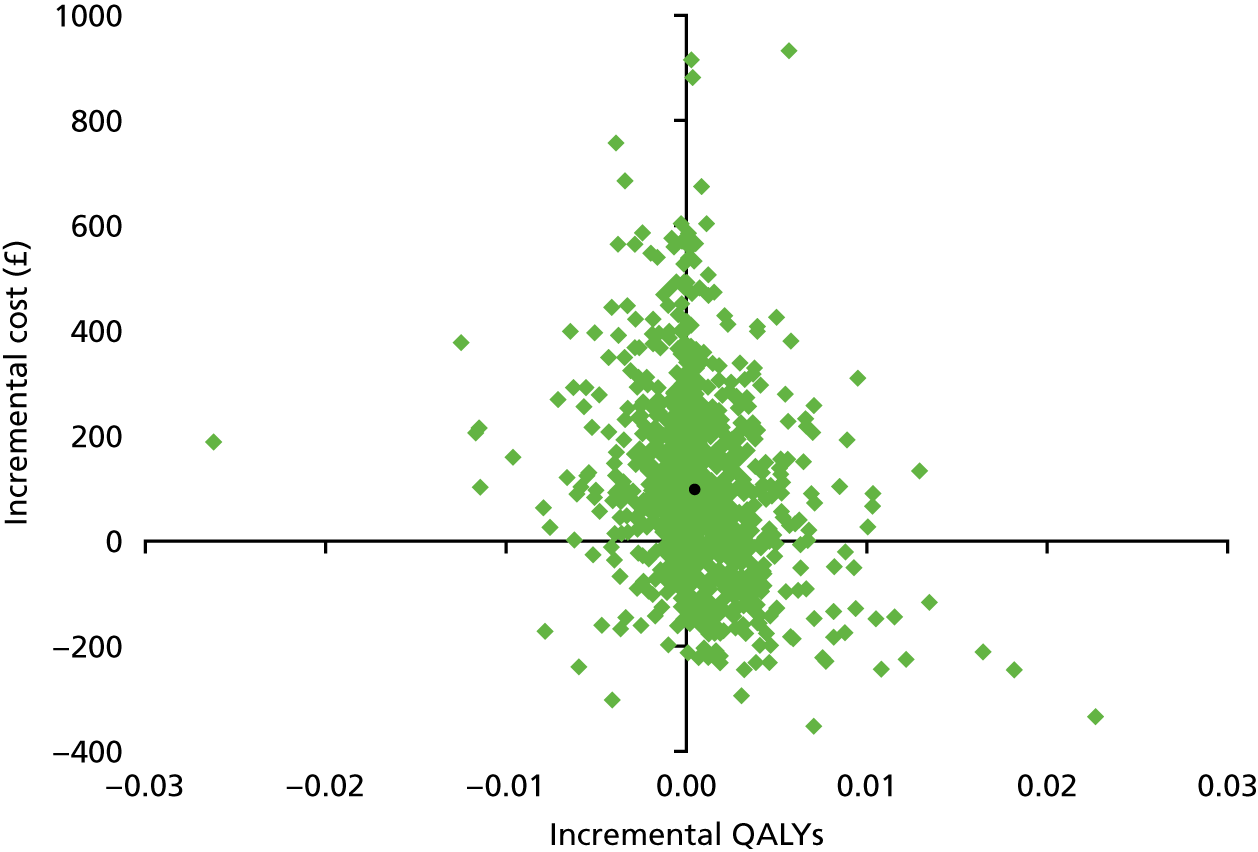
Table 21 reports the results of the sensitivity analyses around this model, designed to help identify the key drivers of cost-effectiveness. Sensitivity analysis one (reducing the diagnostic accuracy of CMR) and sensitivity analysis six (increasing the cost of CMR) had the greatest impact on the mean cost difference between standard ECHO and CMR and standard ECHO alone. Threshold analyses around the cost of CMR (£264 in the base-case analyses) identified that, if the cost reduced to £166, there was no difference in the costs between standard ECHO and CMR and a standard ECHO alone strategy. Sensitivity analyses 8–10, which all directly varied QALYs, had the greatest impact on the difference in QALYs between the arms. However, in all cases, standard ECHO and CMR remained both more costly and more effective than standard ECHO alone, and in none of the sensitivity analyses would standard ECHO and CMR be considered cost-effective at accepted thresholds. These sensitivity analyses suggest that the diagnostic accuracy of CMR is again a key driver of cost-effectiveness. Results are also influenced by the cost of CMR, and the difference between utility values assigned to MACE and no MACE, and the proportion of patients who have MACE, who are assumed to die.
| SA | Ischaemia testing option | Costs (£), mean (SE) | QALYs, mean (SE) | Difference in costs, mean (95% CI) | Difference in QALYs, mean (95% CI) | ICER (£) |
|---|---|---|---|---|---|---|
| Base case | Standard ECHO | 3032 (564) | 0.7615 (0.0837) | |||
| Standard ECHO and CMR | 3130 (589) | 0.7620 (0.0844) | 98 (–199 to 488) | 0.0005 (–0.0050 to 0.0077) | 190,114 | |
| 1. CMR test accuracy | Standard ECHO | 3032 (564) | 0.7615 (0.0837) | |||
| Standard ECHO and CMR | 3183 (591) | 0.7618 (0.0850) | 151 (–105 to 523) | 0.0003 (–0.0026 to 0.0053) | 469,998 | |
| 2. ECHO test accuracy | Standard ECHO | 2945 (561) | 0.7617 (0.0863) | |||
| Standard ECHO and CMR | 3130 (589) | 0.7620 (0.0844) | 185 (–59 to 581) | 0.0003 (–0.0027 to 0.0042) | 631,744 | |
| 3. MACE (ratio = 4) | Standard ECHO | 3067 (541) | 0.7608 (0.0826) | |||
| Standard ECHO and CMR | 3165 (559) | 0.7614 (0.0830) | 98 (–192 to 465) | 0.0005 (–0.0043 to 0.0069) | 190,154 | |
| 4. MACE (ratio = 2) | Standard ECHO | 3043 (573) | 0.7613 (0.0829) | |||
| Standard ECHO and CMR | 3146 (606) | 0.7617 (0.0834) | 104 (–162 to 498) | 0.0004 (–0.0051 to 0.0063) | 250,905 | |
| 5. MACE (ratio = 1) | Standard ECHO | 3046 (579) | 0.7612 (0.0847) | |||
| Standard ECHO and CMR | 3161 (596) | 0.7614 (0.0850) | 115 (–168 to 492) | 0.0002 (–0.0051 to 0.0069) | 554,609 | |
| 6. CMR cost +20% | Standard ECHO | 3032 (564) | 0.7615 (0.0837) | |||
| Standard ECHO and CMR | 3183 (579) | 0.7620 (0.0844) | 151 (–133 to 532) | 0.0005 (–0.0050 to 0.0077) | 292,836 | |
| 6. CMR cost –20% | Standard ECHO | 3032 (564) | 0.7615 (0.0837) | |||
| Standard ECHO and CMR | 3077 (572) | 0.7620 (0.0844) | 45 (–229 to 476) | 0.0005 (–0.0050 to 0.0077) | 87,391 | |
| 7. Reduce MACE costs | Standard ECHO | 2974 (548) | 0.7615 (0.0837) | |||
| Standard ECHO and CMR | 3077 (574) | 0.7620 (0.0844) | 103 (–159 to 502) | 0.0005 (–0.0050 to 0.0077) | 198,992 | |
| 8. QALYs for MACE –0.2 | Standard ECHO | 3032 (564) | 0.7457 (0.0879) | |||
| Standard ECHO and CMR | 3130 (589) | 0.7474 (0.0886) | 98 (–199 to 488) | 0.0018 (–0.0091 to 0.0139) | 55,281 | |
| 9. QALYs for no MACE +0.2 | Standard ECHO | 3032 (564) | 0.9457 (0.0905) | |||
| Standard ECHO and CMR | 3130 (589) | 0.9474 (0.0911) | 98 (–199 to 488) | 0.0018 (–0.0094 to 0.0134) | 55,281 | |
| 10. QALYs for MACE (50% die) | Standard ECHO | 2994 (516) | 0.7488 (0.0859) | |||
| Standard ECHO and CMR | 3095 (542) | 0.7503 (0.0864) | 101 (–180 to 464) | 0.0015 (–0.0090 to 0.0133) | 66,040 |
Summary of main findings
Cohort characteristics and follow-up
Baseline characteristics were reasonably well balanced between participants who consented and those who did not consent in the study, but suggested that patients living in less deprived residential areas were, on average, less willing to take part. Characteristics were better balanced across participants recruited by the four hospitals, and participants who did and those who did not receive a CMR scan. Data completeness was good for most variables. Follow-up data were available for the entire follow-up period (12 months) for only 729 out of 1035 (70%) participants in England and 287 out of 635 (45%) participants in Wales. This is because data were requested soon after recruitment finished and the Welsh data were provided rapidly. The data for English hospitals took much longer to be released, resulting in a longer follow-up period. The limited follow-up was a result of this process being part of the testing feasibility for a full registry; in the context of a registry, the request for these data would be made at an appropriate stage of the study to achieve the desired duration of follow-up.
The proportion of the target population that gets cardiovascular magnetic resonance after activating the primary percutaneous coronary intervention pathway
Although > 10% of patients in hospitals with a dedicated CMR facility were referred for CMR, many of these referrals were for research (patient participating in a research study) rather than clinical purposes. It is not clear whether or not this reflects practice in other hospitals with dedicated CMR facilities. Referral for non-clinical reasons (unless the findings are used to change clinical management) would dilute any observed effect of CMR on outcomes in an extended registry. It was not possible to determine how many CMRs that were done for research purposes were subsequently reviewed by the treating clinician and resulted in changes in patient management.
Cost-effectiveness models
For each of the base-case models, the differences in QALYs between strategies were tiny; therefore, the results are driven largely by the differences in costs, although these were also modest. Sensitivity analyses around the two models identified the diagnostic accuracy of the ischaemia tests as the key driver of cost-effectiveness. In the model for patients with multivessel disease, the costs of ischaemia testing and the QALYs associated with MACE and no MACE exerted an influence on cost-effectiveness results, but not to the same extent as the diagnostic accuracy of CMR and pressure wire. Similarly, in the model for patients with normal coronaries, the cost of CMR, the difference between QALYs associated with MACE and no MACE, and the proportion of patients who have MACE who are assumed to die, exerted an influence on the results.
Chapter 6 Results for aim C: formal consensus to identify clinically important changes in management resulting from cardiovascular magnetic resonance patients who activate the primary percutaneous coronary intervention pathway
Parts of this text have been reproduced from Pufulete et al. 4 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Aim C: to identify an outcome measure representing a definitive change in clinical management, conditional on having had cardiovascular magnetic resonance, that would be credible to cardiologists and other stakeholders as a measure of the ‘value-added’ by doing cardiovascular magnetic resonance
-
Objective C1: to define, using formal consensus methods, a treatment/process outcome that will constitute a definitive change in clinical management arising from having CMR.
-
Objective C2: to identify patient subgroups in whom CMR use is indicated, using formal consensus methods and illustrative data from the registry.
-
Objective C3: to determine whether or not hospital episode data adequately capture the main changes in clinical management identified using formal consensus.
-
Objective C4: to pilot the implementation of a primary outcome based on the changes in management for which consensus was achieved using hospital episode data.
We used a formal consensus method based on the modified nominal group technique66 to identify important changes in management that can be used to define the composite outcome. Formal consensus is a method that combines both research evidence and expert opinion. We used this approach because we knew from a preliminary scoping of the literature that there were few studies that reported the impact of CMR on patient management. 4
We used clinical expertise to define ‘clinical events’ that captured the important changes in management and a clinical coder to identify collaborated with codes (ICD-1031 and the OPCS Classification of Intervention and Procedures32) corresponding to these clinical events. We then searched for these codes in hospital episode data for participants in the cohort study during the 12 months following the index admission (PPCI pathway activation).
Literature review
We initially examined a small number of key reports, reviews and primary studies to determine the extent of the literature reporting how CMR influences patient management. There were few studies that directly reported the impact of CMR on patient management, and none of these related to our population of interest. Figure 13 shows the flow diagram of the systematic review and the different types of studies identified. We identified a total of 171 studies reporting the use of CMR in patients with acute MI who activated the PPCI pathway. There were no studies that directly compared groups of patients having CMR or not with respect to patient management or clinical outcomes in this population.
FIGURE 13.
Flow diagram of the study selection process and types of studies identified.
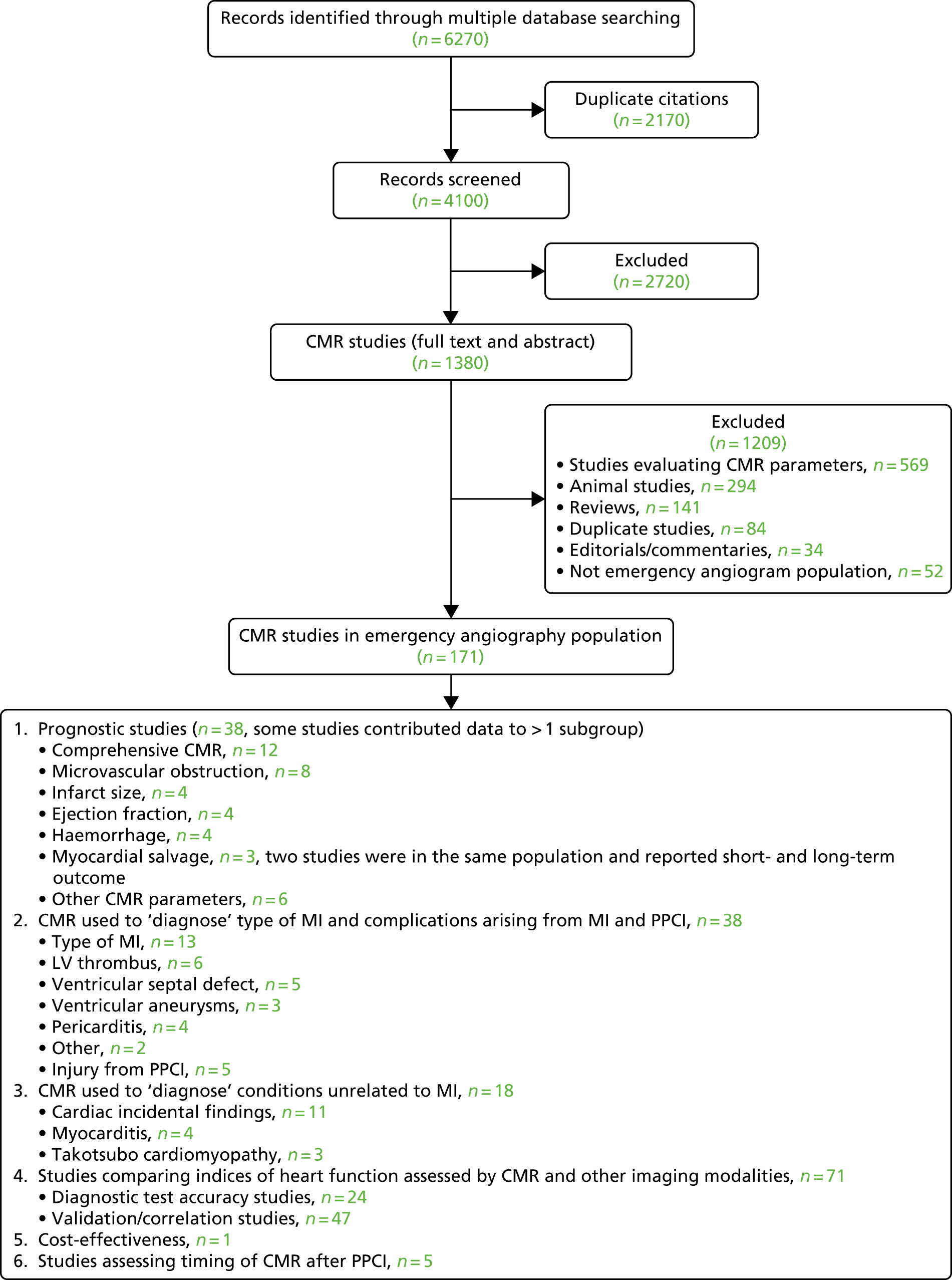
There were 38 prognostic studies assessing the value of CMR in predicting MACEs; 12 did not distinguish the contributing CMR markers, and 26 assessed individual CMR markers of myocardial damage [e.g. microvascular obstruction (MVO), infarct size (IS), LV ejection fraction (EF), myocardial salvage, intramyocardial haemorrhage] as predictors of MACE. These studies did not all evaluate the prognostic value of CMR indices in addition to indices available from alternative imaging modalities; most were small (20–1217 patients, median 257), single centre and heterogeneous in terms of the CMR parameters reported and how these were assessed. Nevertheless, they showed that CMR markers (particularly MVO, IS and myocardial salvage) were consistently associated with MACEs in STEMI patients.
There were 38 studies of the role of CMR in ‘diagnosing’ a specific feature of the index MI or complications arising from the index MI or procedure. CMR was used to investigate the type of MI (i.e. acute, chronic, aborted) in 13 studies, to detect complications of MI [e.g. LV thrombus, ventricular septal defect (VSD) ventricular aneurysms and pericarditis] in 19 studies and to detect injury from PPCI in five studies. Many of these were case reports, some were diagnostic test accuracy studies comparing CMR with ECHO as the reference standard (e.g. diagnosis of thrombus) and some also followed up patients and assessed the prognostic implications of the CMR findings (e.g. aborted MI).
There were 18 studies in which CMR was used to ‘diagnose’ conditions other than MI (e.g. myocarditis and Takotsubo cardiomyopathy in patients with unobstructed arteries on angiography) and incidental cardiac findings. All but two of these were case reports. There were 71 studies in which CMR was compared with one or more other imaging modalities [i.e. ECHO, SPECT, computed tomography (CT), angiography or with standard coronary angiography with respect to indices of heart function (e.g. myocardial viability, necrosis, perfusion, myocardium at risk, ischaemia)]. Twenty-four of these were diagnostic test accuracy studies (those that included a reference standard and provided statistics of test performance, i.e. sensitivity, specificity, positive or negative predictive values, receiver operating characteristic curves) and 47 were validation or correlation studies. Most focused on infarct characteristics and myocardial viability, but the definitions and techniques assessing viability were highly variable for both CMR and the comparator, making it difficult to compare studies.
Formulation of consensus statements
Thirty-seven draft statements were generated by the three cardiologists and one methodologist (see Appendix 7, Table 31). Examples of draft statements, categorised by patient subgroup, are shown in Appendix 7, Table 32. Statements relating to the same patient subgroup/condition (e.g. patients at risk of complications after PPCI who develop LV thrombus) were condensed into one statement. This reduced the number of statements from 37 to 16. All statements were then reworded to standardise their format. Examples of reworded statements are shown in Appendix 7, Table 32. The 16 statements were discussed in turn at the internal group meeting and the following changes were made:
-
The total number of statements was reduced from 16 to 12. Two statements relating to device implantation were condensed into one, three statements were removed because it was felt that their content was covered adequately by other statements and one statement was removed because it was felt that it did not relate to a change in patient management.
-
A supporting paragraph was drafted to put the statement into context, summarise the research evidence and include any key references. These references were chosen to reflect the best available evidence; although, for several statements, the cited studies were small prognostic studies or case series (i.e. the only available evidence).
-
The statements were reworded to clarify the care pathway compared with which the additional benefit of CMR was anticipated (e.g. alternative imaging modalities such as ECHO or SPECT).
The final 12 statements are shown in Table 22 and examples of supporting paragraphs are shown in Appendix 7, Table 33. These 12 statements describe changes in management relating to six patient subgroups:
-
Patients with a poor prognosis or at risk of complications after PPCI [e.g. those who develop LV thrombus, those with low EF who require an implantable cardioverter defibrillator (ICD), those at risk of impending cardiac rupture] whose treatment could potentially be optimised (statements 1, 4, 6, 7 and 9).
-
Patients with a good prognosis after PPCI who could be discharged earlier and followed up less often (statement 2).
-
Patients with multivessel disease, in whom revascularisation for the additional diseased arteries could be optimised (statements 8 and 10).
-
Patients with unobstructed arteries on angiography [who may have had a heart attack that resolved spontaneously (e.g. MI with spontaneous recanalisation/embolic MI) or another condition, such as myocarditis or cardiomyopathy] for whom treatment planning could be optimised based on a definitive diagnosis (statement 5).
-
Patients with an out-of-hospital-cardiac arrest (OHCA) in whom the cause could be definitively identified, leading to appropriate treatment (statement 3).
-
Patients with incidental cardiac (e.g. mitral regurgitation, pericardial effusion, valve disease, papillary rupture, congenital cardiac anomalies) and extra-cardiac findings (e.g. pleural effusion, lung mass, mediastinal lymph adenopathy), in whom appropriate treatment could be started (statements 11 and 12).
| Statement number | Statement | Patient subgroup |
|---|---|---|
| 1 | Compared with ECHO, CMR after PPCI allows patients with CMR markers that indicate a poor prognosis (e.g. impaired LV function, large infarct size, MVO) to be followed up more appropriately and undergo more aggressive medical therapy for secondary prevention | Poor prognosis/risk of complications after PPCI |
| 2 | Compared with ECHO, CMR after PPCI allows patients with CMR markers that indicate a good prognosis (e.g. normal LV function, high myocardial salvage, no MVO, no residual ischaemia) to be discharged earlier and followed up less frequently | Good prognosis after PPCI |
| 3 | Compared with ECHO, CMR better identifies the cause of OHCA (e.g. large MI, arrhythmogenic right ventricular cardiomyopathy, aberrant coronary arteries, hypertrophic cardiomyopathy) to optimise further treatment for the patient (e.g. defibrillator for primary arrhythmia or PCI) or family members | Out-of-hospital cardiac arrest |
| 4 | CMR after PPCI identifies patients at high risk of having a ventricular septal defect or impending cardiac rupture, who may require a ventricular patch or other urgent cardiac surgery, and guides the optimal management of these patients | Poor prognosis/risk of complications after PPCI |
| 5 | In patients with a ‘normal’ (unobstructed) coronary angiogram, CMR can differentiate patients who have had a MI with spontaneous reperfusion or distal embolisation from patients with a non-ischaemic diagnosis (e.g. myocarditis, Takotsubo cardiomyopathy, aortic dissection), resulting in a patient treatment plan appropriate for the definitive diagnosis | Unobstructed arteries on angiogram |
| 6 | Compared with ECHO, CMR after PPCI better identifies patients at high risk of sudden cardiac death who would benefit most from an implantable cardiac device (e.g. ICD or CRT) | Poor prognosis/risk of complications after PPCI |
| 7 | Compared with ECHO, CMR after PPCI better identifies patients who would not benefit from cardiac resynchronisation therapy | Poor prognosis/risk of complications after PPCI |
| 8 | Compared with stress ECHO or SPECT, CMR after PPCI in patients with multivessel disease better assesses ischaemia and viability of the myocardium to optimise the revascularisation strategy for the patient and avoid additional diagnostic tests | Multivessel disease |
| 9 | Compared with ECHO, CMR after PPCI better identifies patients with LV thrombus for treatment with anticoagulation therapy | Poor prognosis/risk of complications after PPCI |
| 10 | Compared with other imaging modalities, in patients with multivessel disease, CMR after PPCI better identifies the artery that caused the MI and guides the subsequent treatment plan in relation to additional revascularisation (e.g. PCI or coronary artery bypass graft surgery) | Multivessel disease |
| 11 | Compared with ECHO, CMR after PPCI better identifies incidental cardiac findings that may need further investigation and treatment | Incidental cardiac findings |
| 12 | Compared with ECHO, CMR after PPCI identifies significant incidental non-cardiac findings (e.g. oesophageal and lung tumours, pulmonary embolus, aortic aneurysm) that may need further investigation and treatment | Incidental non-cardiac findings |
The expert panel
Nineteen consultant cardiologists were invited to participate in the consensus process. Seven cardiologists (37%) agreed to participate. Of these, two had CMR, three had interventional, one had ECHO and one had heart failure expertise. All seven cardiologists completed the first survey independently.
First survey
Figure 14 shows the distribution of responses for the first survey. There was consensus for three of the 12 statements (25%) regarding the change in management being clinically important: statement 3, relating to the ability of CMR to identify the cause of OHCA and, therefore, optimise treatment for the patient; statement 5, relating to the ability of CMR to provide a definitive diagnosis in patients found to have unobstructed arteries on angiography; and statement 9, relating to the ability of CMR to identify patients with LV thrombus and initiate treatment with anticoagulation therapy. There was no consensus for any statement that the change in management was not clinically important.
FIGURE 14.
Median and IQR for the 12 statements in the first survey (n = 7). Boxes represent the median and IQR and the whiskers represent the upper and lower adjacent values (upper quartile + 1.5*IQR, and lower quartile – 1.5*IQR). 67 Dots represent extreme values. Statements 3, 5 and 9 were considered to be in consensus (median score ≥ 7 and IQR 6–9).

Examples of respondents’ comments to the first survey regarding individual statements are shown in Appendix 7, Table 34. Consensus was sought on the importance of the change in management and most respondents commented on this issue. However, some respondents also considered other factors when rating statements, for example the quality of the supporting evidence, the proportion of patients likely to benefit, the ability of the NHS to provide a service in line with the statement and whether or not the cost of CMR justified the perceived benefit. 4
Face-to-face meeting
The face-to-face meeting was attended by six of the seven cardiologists and three non-clinical members of the initial working group. As a result of the face-to-face meeting, the survey was modified as follows:
-
The number of statements was reduced from 12 to 10. Statement 10 (relating to patients with multivessel disease) was removed because cardiologists felt that this statement overlapped significantly with statement 8. Statements 11 and 12 (relating to incidental cardiac and non-cardiac findings) were combined into one statement.
-
Respondents were asked to rate five aspects of each statement, organised hierarchically – (1) whether or not CMR is better than the comparator (e.g. ECHO), (2) whether or not the information from CMR leads to a change in management, (3) whether or not the change in management is clinically important (i.e. likely to reduce risk of MACEs in the long term), (4) whether or not the change in management is likely to reduce NHS costs in the long term and (5) whether or not the anticipated benefit was sufficiently large to make CMR cost-effective among the patients in whom it would be indicated. Consensus was based on the distribution of responses to questions 1–3 of each statement (i.e. median ≥ 7 and IQR 6–9 for clinically important changes in management, and median ≤ 3 and IQR 1–3 for questions 1, 2 and 3). Responses to questions 1–3 were used because question 1 was constructed to interrogate the respondent’s appraisal of the evidence, question 2 was constructed to determine whether or not the respondent believed the NHS service delivery changed as a result of CMR and question 3 was constructed to determine whether or not the respondent believed that any change recognised in the response to question 2 was clinically important. Discussions in the meeting revealed that consideration of costs and cost-effectiveness for the NHS influenced some panel members’ responses to the initial survey. Questions 4 and 5 were added to isolate these aspects of consideration and separate them from the question of change in management, which was the aim of the consensus process.
-
The free-text box in which respondents could justify their score was removed.
The modified survey is shown in Table 23.
| Statement number | Statement |
|---|---|
| 1 | The following statements relate to the ability of CMR to identify patients who have a poor prognosis after PPCI:
|
| 2 | The following statements relate to the ability of CMR to identify patients who have a good prognosis after PPCI:
|
| 3 | The following statements relate to the ability of CMR to identify the causes of OHCA in patients who undergo an emergency angiogram:
|
| 4 | The following statements relate to the ability of CMR to identify patients with VSD after MI:
|
| 5 | The following statements relate to the ability of CMR to differentiate MI from other diagnoses in patients found to have unobstructed coronary arteries on emergency angiography:
|
| 6 | The following statements relate to the ability of CMR to identify patients at high risk of sudden cardiac death after PPCI who would benefit most from an ICD:
|
| 7 | The following statements relate to the ability of CMR to identify patients who would not benefit from CRT after PPCI:
|
| 8 | The following statements relate to the ability of CMR to assess ischaemia and viability in patients with multivessel disease:
|
| 9 | The following statements relate to the ability of CMR to identify patients with post-infarct LV thrombus:
|
| 10 | The following statements relate to the ability of CMR to detect incidental cardiac and non-cardiac findings if offered routinely to patients who undergo an emergency angiogram:
|
Modified survey
Fifty-four cardiologists (including five of the cardiologists who attended the formal consensus meeting) completed the survey. There was consensus that five of the 10 statements (50%) described clinically important changes in management (Figure 15). These were statements 3, 5 and 9 (which were in consensus in the first survey) and two additional statements: statement 1, relating to the ability of CMR to identify patients who have a poor prognosis after PPCI, and statement 8, relating to the ability of CMR to assess ischaemia and viability in patients with multivessel disease. There was no consensus that any of the other statements described a change in management that was not clinically important. 4 The final statements from the modified survey are in Appendix 8.
FIGURE 15.
Median and IQR for the 10 statements in the modified survey (n = 54). Boxes represent the median and IQR and the whiskers represent the upper and lower adjacent values (upper quartile + 1.5*IQR, and lower quartile – 1.5*IQR). 67 Dots represent extreme values.
Reproduced from Pufulete et al. 4 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
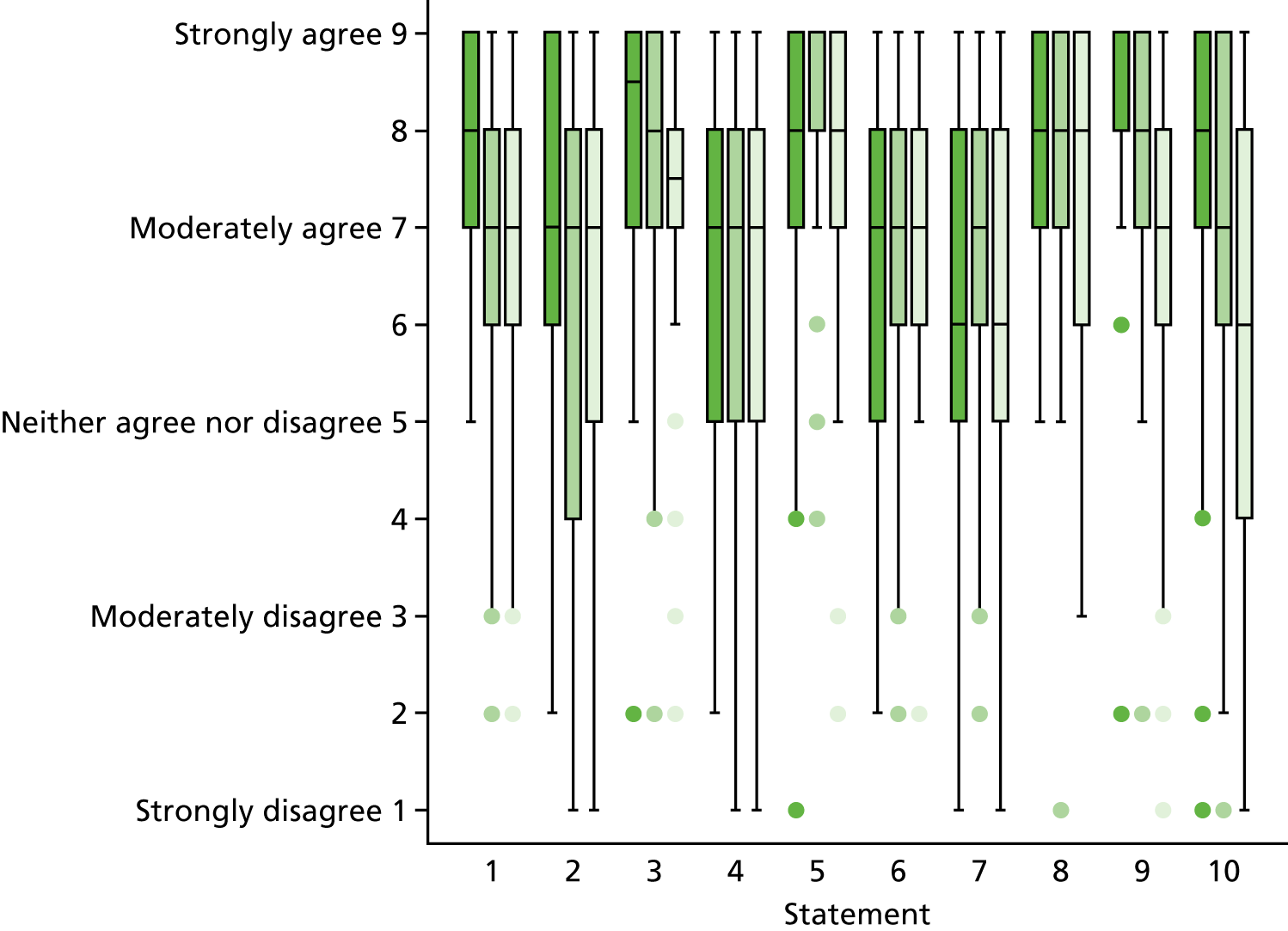
Implementation of a primary outcome based on the changes in management for which consensus was achieved
Patient subgroups
We identified five patient subgroups (Table 24) for which there was consensus that CMR changed management in a clinically important way, either from HISs or from HES/PEDW.
-
Patients who underwent PPCI could be identified both from HISs and from HES/PEDW.
-
Patients with unobstructed arteries could not be directly identified from either HIS or HES, but they were indirectly identified from HIS (consented into study and underwent angiography but had no data in BCIS) and from HES/PEDW (had a record for angiography but no record for PCI).
-
Patients with multivessel disease could only be identified reliably from HISs; the presence of disease in multiple vessels is not coded by ICD-10 but might be inferred from multiple procedure codes in HES/PEDW for coronary revascularisation (PCI or CABG).
-
Patients who experienced OHCA, and PPCI patients who went on to develop LV thrombus, could be identified only from HES/PEDW.
| Subgroup | Data source | Definition |
|---|---|---|
| PPCI | BCIS | BCIS data recorded at site |
| Multivessel disease | BCIS | Two or more vessels with > 50% stenosis (pre PCI) |
| Unobstructed coronary arteries | BCIS | BCIS data not recorded |
| OHCA | HES/PEDW | Index admission includes HES/PEDW diagnosis I46 ‘Cardiac arrest’ |
| LV thrombus | HES/PEDW | Index admission includes HES/PEDW diagnosis I23.6 ‘Thrombosis of atrium, auricular appendage and ventricle as current complications following acute myocardial infarction’ |
The proportions of patients in each subgroup (across all hospitals and by hospital) are shown in Table 25. Across all hospitals, 89% of patients underwent PPCI, 11% were found to have unobstructed coronary arteries and 44% of patients had multivessel disease. The proportions of patients in each subgroup were similar between hospitals, except for patients with multivessel disease (19% in hospital D vs. 48%, 51% and 53% in hospitals A, B and C, respectively).
| Patient subgroup | Hospital, n (%) | Total (N = 1655), n (%) | |||
|---|---|---|---|---|---|
| A (N = 758) | B (N = 272) | C (N = 316) | D (N = 309) | ||
| PPCI | 651 (86) | 246 (90) | 291 (92) | 279 (90) | 1467 (89) |
| Multivessel disease | 361 (48) | 138 (51) | 169 (53) | 58 (19) | 726 (44) |
| LV thrombus after PPCI | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) |
| Unobstructed coronary arteries | 107 (14) | 26 (10) | 25 (8) | 30 (10) | 188 (11) |
| OHCA | 57 (8) | 22 (8) | 16 (5) | 14 (5) | 109 (7) |
Manual review of patient notes
We conducted a manual review of patient notes because of the uncertainty regarding the identification of the unobstructed coronary arteries subgroup from HISs. Only 38 out of 47 (81%) patients were identified as having unobstructed coronary arteries in the manual review of patient notes (see Appendix 9). Of the remaining nine patients, six had a culprit lesion identified (therefore, the likely reason for their emergency angiography was expected to be MI), and the reason for the coronary angiography in the other three patients was unknown.
We compared the diagnoses from the patient notes with the primary diagnoses recorded in HES for the index admission (see Appendix 9). Four of the 38 patients identified as having unobstructed coronary arteries in their medical notes had ‘myocardial infraction’ as a primary diagnosis in HES. Of the six patients who had a culprit lesion identified in their medical notes, only two had ‘myocardial infraction’ recorded as their primary diagnosis in HES; two had a diagnosis of ‘chronic ischaemic heart disease’, one had a diagnosis of ‘pain in throat and chest’ and one had a diagnosis of ‘atrial fibrillation and flutter’.
Identifying changes in management in hospital episode data
The important changes in management identified from the statements in consensus, the patient subgroups these changes in management refer to, the HES/PEDW data sources used to identify them and the events identified from hospital episode data sources in the 12 months of follow-up after the index admission are shown in Appendix 10. Medication data are not available in hospital episode data; therefore, any changes in medication during follow-up could not be identified. This was particularly relevant for the LV thrombus after PPCI subgroup, for which the start of anticoagulation therapy was identified as the important change in management. All relevant non-ischaemic diagnoses were identifiable in hospital episode admissions data, as were procedures, such as implantation of devices, repeat revascularisation and additional diagnostic tests. In contrast, we could not identify these from the outpatient data set because ICD-10 and OPCS codes associated with each outpatient episode were missing for a large proportion of patients (25% of patients had missing diagnoses codes, 74% of patients had missing procedure codes and 74% of patients had ‘unknown and unspecified causes of morbidity’ as diagnosis). This will lead to an underestimation of diagnostic tests in the cohort, because most diagnostic tests are carried out on an outpatient basis. However, we could identify the frequency of outpatient appointments under the cardiology specialty.
Implementation of the consensus statements
The number of events representing changes in management in the relevant patient subgroups, identified in HES/PEDW up to 12 months after the index admission by CMR status are shown in Table 26. The frequency of events in patients who did and in those who did not have CMR was similar across all subgroups. Patients who had CMR had, on average, more outpatient appointments across all subgroups.
| Patient subgroup | n (%) | Cardiology outpatient appointments, median (IQR) | ||||
|---|---|---|---|---|---|---|
| New diagnoses (non-ischaemic, Takotsubo, myocarditis, pericarditis, endocarditis, coronary spasm) | Changes in medication | Additional diagnostic tests (PET, ECHO, IVUS, pressure wire, radionuclide angiocardiography, CT, angiography) | Implantation of devices (CRT or ICD) | Revascularisation (PCI or CABG) within 3 months | ||
| PPCI | ||||||
| CMR (N = 152) | 0 (0) | No data available | 16 (11) | 0 (0) | N/A | 2 (1–3) |
| No CMR (N = 1312) | 10 (1) | No data available | 129 (10) | 6 (0.5) | N/A | 1 (1–2) |
| Multivessel disease | ||||||
| CMR (N = 104) | 0 (0) | No data available | 11 (11) | 0 (0) | 11 (11) | 2 (1–3) |
| No CMR (N = 622) | 4 (1) | No data available | 61 (10) | 4 (1) | 64 (10) | 1 (1–2) |
| LV thrombus after PPCI | ||||||
| CMR (N = 1) | 0 (0) | No data available | 0 (0) | 0 (0) | N/A | 3 (3–3) |
| No CMR (N = 0) | 0 (0) | No data available | 0 (0) | 0 (0) | N/A | – |
| Unobstructed coronary arteries | ||||||
| CMR (N = 35) | 1 (3) | No data available | 6 (17) | 1 (3) | N/A | 2 (1–3) |
| No CMR (N = 139) | 1 (1) | No data available | 12 (9) | 3 (2) | N/A | 1 (0–2) |
| OHCA | ||||||
| CMR (N = 14) | 0 (0) | No data available | 2 (14) | 0 (0) | N/A | 1 (1–3) |
| No CMR (N = 95) | 1 (1) | No data available | 10 (11) | 5 (5) | N/A | 1 (1–2) |
Table 27 shows the time to first event for each change in management and for all subgroups, by CMR status. Except for patients with unobstructed coronary arteries, patients in other subgroups who did not have CMR had an admission for an additional diagnostic test earlier than patients who had CMR. In the subgroup of patients with unobstructed coronary arteries, patients who did not have CMR had an admission related to a new non-ischaemic diagnosis earlier, and an admission for an additional diagnostic test and implantation of a device later than patients who did get CMR. In the PPCI/multivessel disease subgroups, time to first cardiology outpatient appointment was earlier for patients who did not have CMR than for patients who had CMR.
| Patient subgroup | Number of days until event after the index admission, median (IQR) | |||||
|---|---|---|---|---|---|---|
| New diagnoses (non-ischaemic, Takotsubo, myocarditis, pericarditis, endocarditis, coronary spasm) | Changes in medication | Additional diagnostic tests (PET, ECHO, IVUS, pressure wire, radionuclide angiocardiography, CT, angiography) | Implantation of devices (CRT or ICD) | Revascularisation (PCI or CABG) within 3 months | Cardiology outpatient appointments | |
| PPCI | ||||||
| CMR (N = 152) | – | No data available | 107 (50–240) | – | N/A | 64 (34–99) |
| No CMR (N = 1312) | 16 (8–20) | No data available | 74 (27–171) | 147 (48–171) | N/A | 55 (33–95) |
| Multivessel disease | ||||||
| CMR (N = 104) | – | No data available | 85 (50–221) | – | 99 (50–180) | 76 (52–116) |
| No CMR (N = 622) | 13 (8–110) | No data available | 62 (24–130) | 147 (84–169) | 66 (39–122) | 57 (33–95) |
| LV thrombus after PPCI | ||||||
| CMR (N = 1) | – | No data available | – | – | N/A | 66 (66–66) |
| No CMR (N = 0) | – | No data available | – | – | N/A | – |
| Unobstructed coronary arteries | ||||||
| CMR (N = 35) | 358 (358–358) | No data available | 93 (58–140) | 78 (78–78) | N/A | 67 (52–121) |
| No CMR (N = 139) | 180 (180–180) | No data available | 123 (78–284) | 245 (71–262) | N/A | 67 (44–106) |
| OHCA | ||||||
| CMR (N = 14) | – | No data available | 110 (79–141) | – | N/A | 65 (61–104) |
| No CMR (N = 95) | 48 (48–48) | No data available | 62 (48–93) | 126 (71–167) | N/A | 64 (41–99) |
The frequency of events in CMR versus non-CMR hospitals is shown in Table 28. On average, patients in CMR centres had more outpatient appointments than patients in non-CMR centres. New diagnoses, additional diagnostic tests, implantation of devices and additional revascularisation in patients with multivessel disease were more frequent in CMR hospitals than in non-CMR hospitals. Time to the first event in CMR versus non-CMR centres for all events and by patient subgroup are shown in Table 29. In CMR centres, patients in most subgroups had an admission for an additional diagnostic test and implantation of a device later than patients in non-CMR centres. Time to first cardiology outpatient appointment was earlier for patients in CMR centres across all subgroups.
| Patient subgroup | n (%) | Cardiology outpatient appointments, median (IQR) | ||||
|---|---|---|---|---|---|---|
| New diagnoses (non-ischaemic, Takotsubo, myocarditis, pericarditis, endocarditis, coronary spasm) | Changes in medication | Additional diagnostic tests (PET, ECHO, IVUS, pressure wire, radionuclide angiocardiography, CT, angiography) | Implantation of devices (CRT or ICD) | Revascularisation (PCI or CABG) within 3 months | ||
| PPCI | ||||||
| CMR centres (N = 897) | 6 (1) | No data available | 112 (12) | 5 (1) | N/A | 2 (1–3) |
| Non-CMR centres (N = 567) | 4 (1) | No data available | 33 (6) | 1 (0.2) | N/A | 1 (0–1) |
| Multivessel disease | ||||||
| CMR centres (N = 499) | 4 (1) | No data available | 61 (12) | 4 (1) | 53 (11) | 2 (1–3) |
| Non-CMR centres (N = 227) | 0 | No data available | 11 (5) | 0 | 22 (10) | 1 (0–1) |
| LV thrombus after PPCI | ||||||
| CMR centres (N = 1) | 0 | No data available | 0 | 0 | N/A | 3 (3–3) |
| Non-CMR centres (N = 0) | – | No data available | – | – | N/A | – |
| Unobstructed coronary arteries | ||||||
| CMR centres (N = 133) | 2 (2) | No data available | 16 (12) | 4 (3) | N/A | 1 (0–2) |
| Non-CMR centres (N = 41) | 0 | No data available | 2 (5) | 0 | N/A | 0 (0–1) |
| OHCA | ||||||
| CMR centres (N = 79) | 0 | No data available | 9 (11) | 4 (5) | N/A | 2 (1–3) |
| Non-CMR centres (N = 30) | 1 (3) | No data available | 3 (10) | 1 (3) | N/A | 1 (0–1) |
| Patient subgroup | Number of days until event after the index admission, median (IQR) | |||||
|---|---|---|---|---|---|---|
| New diagnoses (non-ischaemic, Takotsubo, myocarditis, pericarditis, endocarditis, coronary spasm) | Changes in medication | Additional diagnostic tests (PET, ECHO, IVUS, pressure wire, radionuclide angiocardiography, CT, angiography) | Implantation of devices (CRT or ICD) | Revascularisation (PCI or CABG) within 3 months | Cardiology outpatient appointments | |
| PPCI | ||||||
| CMR centres (N = 897) | 14 (8–17) | No data available | 79 (33–205) | 167 (126–171) | N/A | 46 (30–81) |
| Non-CMR centres (N = 567) | 20 (14–34) | No data available | 45 (24–116) | 48 (48–48) | N/A | 90 (57–126) |
| Multivessel disease | ||||||
| CMR centres (N = 499) | 13 (8–110) | No data available | 69 (32–159) | 147 (84–169) | 71 (41–151) | 52 (32–87) |
| Non-CMR centres (N = 227) | – | No data available | 59 (15–122) | – | 63 (43–88) | 95 (68–136) |
| LV thrombus after PPCI | ||||||
| CMR centres (N = 1) | – | No data available | 0 | 0 | N/A | 66 (66–66) |
| Non-CMR centres (N = 0) | – | No data available | – | – | N/A | – |
| Unobstructed coronary arteries | ||||||
| CMR centres (N = 133) | 269 (180–358) | No data available | 103 (68–205) | 162 (75–254) | N/A | 65 (46–103) |
| Non-CMR centres (N = 41) | – | No data available | 184 (69–299) | – | N/A | 89 (54–133) |
| OHCA | ||||||
| CMR centres (N = 79) | – | No data available | 79 (62–141) | 147 (99–169) | N/A | 63 (42–95) |
| Non-CMR centres (N = 30) | 48 (48–48) | No data available | 48 (5–74) | 48 (48–48) | N/A | 86 (52–105) |
Cardiovascular magnetic resonance imaging questionnaire
The changes in management identified by the CMR questionnaire and from HES/PEDW data are shown in Table 30. Changes in management identified by the CMR questionnaire could also be identified in hospital episode data, although there were clearly some gaps in the data we had available to us, namely: (1) poor coding of outpatient hospital episode data (diagnoses), (2) failure to obtain medication data and (3) poor coding of diagnostic tests and investigations (both acute care and outpatient data sets).
| Change in management | n/N (%) | |
|---|---|---|
| CMR questionnaire (n = 187) | HIS or HES (n = 185) | |
| Risk stratification after PPCI | ||
| Patients with markers of a good prognosis | 102/160 (64) | Not possible to identify |
| Patients with markers of a poor prognosis | 58/162 (36) | |
| Change in management? | 55/123 (45) | Yes, potentially identifiable in HES by considering the frequency and timing of events representing changes in management |
| Patients with multivessel disease | 121/165 (73) | 104/185 (56) |
| Change in management (additional diagnostic tests)? | 18/111 (16) | 11/104 (11) |
| Difficult to identify all diagnostic tests because procedure codes were not well recorded in HES/PEDW outpatient data | ||
| Change in management (did CMR optimise revascularisation)? | 95/120 (79) | Yes, potentially identifiable in HES by considering timing of revascularisation and frequency of additional diagnostic tests |
| Patients with LV thrombus | 9/187 (5) | 2/185 (1) |
| Change in management (anticoagulation therapy)? | 5/9 (60) | Not possible to identify in HES as medications not available |
| Potentially possible to identify in HIS if medications on discharge data are provided | ||
| Patients with unobstructed arteries | 19/183 (10) | 35/185 (19) |
| New diagnosis? | 13/16 (81) | 1/35 (3) |
| What were the diagnoses? |
|
|
| Change in management? | 15/19 (79) | Yes, potentially identifiable |
| Patients with OHCA | 24/180 (13) | 14/185 (8) |
| Cause identified by CMR? | 19/24 (79) | 0/14 (new diagnosis recorded) |
| Change in management? | 11/24 (46) | Yes, potentially identifiable |
Summary of main findings
We identified five subgroups of ACS patients who activate the PPCI pathway and for whom there was consensus that CMR changes patient management in a clinically important way: (1) patients who have an OHCA (≈7% of those who activate the PPCI pathway),69 (2) patients who have a ‘normal’ (unobstructed) coronary angiogram (≈10% of those who activate the PPCI pathway),15,16 (3) patients who develop LV thrombus (3% overall and 9% in patients with anterior STEMI),70 (4) patients who have multivessel disease (between 40% and 65% of those who undergo PPCI)7,10,71 and (5) patients in whom CMR markers indicate a poor prognosis (up to 60% of patients after STEMI). 2,72 There was consensus about the first three subgroups in both rounds of the survey, and consensus was reached about the remaining two subgroups in the modified survey. These results suggest that CMR benefits a large proportion of patients who activate the PPCI pathway. 4 The population investigated was generally representative of the population of patients who activate the PPCI pathway; 11% of patients did not undergo PPCI and were categorised as having unobstructed coronary arteries, 44% of patients had multivessel disease and 7% of patients had an OHCA. Fewer patients than reported in the literature were identified as having LV thrombus (0.3% in our data vs. 3% reported in PPCI populations).
Two subgroups, patients with OHCA and patients who developed LV thrombus after PPCI, could not be identified from HIS data submitted by hospitals but could be identified in the HES/PEDW admissions data relating to the index admission. We defined patients as having unobstructed coronary arteries if they had no BCIS data, because we did not have other information in our HIS data sets for positive identification of this subgroup. We conducted a manual review of these patients’ medical notes because we were uncertain whether or not all patients identified as such truly had unobstructed coronary arteries. The majority of patients (81%) were defined as having unobstructed arteries from their medical notes, although 13% did have a culprit lesion identified, suggesting that the most likely reason that these patients activated the PPCI pathway was MI. We also compared the diagnosis from medical notes with the primary diagnosis in HES for all patients in the unobstructed coronary artery subgroup. Again, there were discrepancies, with some patients who were identified as having unobstructed coronary arteries in their medical notes having a primary diagnosis of MI. We conclude that some patients were incorrectly classified as having unobstructed coronary arteries from HIS, and that classification of this patient subgroup is likely to vary depending on the data source used (medical notes, HIS or hospital episode data).
Some important changes in management identified through the formal consensus (e.g. additional revascularisation in the multivessel disease group, implantation of devices) were readily identifiable in HES/PEDW admission data sets. Some changes in management (e.g. changes in medication) were unavailable because medications data are not available in HES/PEDW. Other changes in management (e.g. additional diagnostic tests) were likely to be underestimated because a large proportion of diagnostic tests are performed on an outpatient basis but procedure codes are not well recorded in outpatient data sets.
When implementing the consensus statements, we considered both the frequency of the events representing the important changes in management and time to the first event. This is because, for some changes in management (e.g. additional revascularisation for multivessel disease patients or a new diagnosis in patients with unobstructed coronary arteries), it is the timing to the event that represents the main change in management resulting from CMR. Although there were differences by CMR status in both the frequency and timing of events for all patient subgroups, we chose not to interpret these because of the small numbers involved and because our main objective was to test the feasibility of implementing the consensus statements using hospital episode data.
The CMR questionnaire highlighted several gaps in hospital episode data (i.e. lack of medications data and poor coding of diagnostic tests and investigations). Without the former data, it would be difficult to characterise patients at baseline (index admission), which is important for identifying and controlling for, confounding, and without the latter data we would be unlikely to have reasonable sensitivity for important changes in management during follow-up in a registry.
Chapter 7 Discussion
Main findings: study results
Parts of this text have been reproduced from Brierley et al. 27 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Feasibility of setting up a registry using hospital information system data
The outcome measures of the feasibility prospective cohort study were feasibility parameters to establish if (1) consent can be implemented at all four hospitals, (2) data linkage and extraction from multiple HISs can be achieved for > 90% of consented patients at all hospitals, (3) local data can be successfully linked with hospital episode data for > 90% of consented patients at all hospitals and (4) the proportion of patients activating the PPCI pathway who get a CMR scan is ≥ 10% in hospitals with dedicated CMR facilities.
The results suggest that the registry is feasible with respect to points 1, 3 and 4, albeit with some caveats, but not point 2. We found that recruitment and consenting of the patient population (using conventional consent), and linkage of local HIS data with hospital episode/vital status data are feasible. However, we experienced problems with identifying and approaching all eligible patients across all sites and we could not obtain all of the requested data from all hospitals. We had envisaged that it would be relatively straightforward to identify patients meeting the eligibility criteria (i.e. patients who activated the PPCI pathway, including those who had an emergency angiogram but did not receive PCI) consecutively, given the emergency nature of PPCI pathway activation. Indeed, we chose this population for a national CMR registry using routinely collected data because, at the outset, we expected that this population would be easy to identify from hospital databases. Although patients who received PPCI were easy to identify from catheter laboratory databases, not all eligible patients were identified in this way and many were identified manually by the research nurses.
The numbers of PPCI patients identified as eligible and approached at each hospital were lower than the numbers of PPCIs reported to BCIS by each hospital over the recruiting period (varying from 22% to 86% across the four hospitals; see Table 10). Some PPCI patients were missed because they were quickly repatriated to their local referring hospital. Other patients who arrived at the PPCI centre via A&E were not always classified as PPCI (e.g. patients who experienced out-of-hospital cardiac arrest), resulting in many of them being missed.
Patients who activated the PPCI pathway but had an emergency angiogram without PPCI (i.e. those with unobstructed coronary arteries) were difficult to identify from catheter laboratory databases. Contrary to expectation, there was no data field in any database capturing emergency admission to the catheter laboratory. These patients were also not described consistently in HIS databases and there was misunderstanding over eligibility (especially at the beginning of the study) by catheter laboratory staff and research nurses. Therefore, it is unclear what proportion of these patients was identified at each site during the recruiting period and how the proportion may have changed over time.
We met the feasibility objective of implementing conventional prospective consent at all hospitals, although consent rates were lower than anticipated for a study in which participants have no active involvement. The main reason for this was that many patients identified as eligible were missed (because of short hospital stay, no nurse cover at weekends, quick repatriation to referring hospitals, mobile inpatient population, etc.). There was also the issue of competing studies in the hospitals, including the perception that interventional studies should be given priority over observational studies. We relied on postal consent to invite patients identified as eligible and who were not approached in hospital, but return rates were low and we did not have the resources to send reminders or make telephone calls.
We explored alternative methods of obtaining consent as part of the feasibility study. We considered applying for approval to recruit patients without consent [‘presumed consent’, section 251 (of the NHS Act 200676) exemption] but concluded that this proposal would be unacceptable legally, given that, in principle, it is possible for patients to be asked to give consent for their data to be used for the study. 73 Exemptions are granted only on a temporary basis (pending implementation of consent) or when consent is impossible, not just infeasible. We considered the option of requesting consent at the same time as requesting procedural consent (e.g. by adding a paragraph to the standard NHS form, either opt-in or opt-out consent), although the emergency nature of the procedure means that written procedural consent is not always obtained and verbal assent is often recorded by hand in the patient notes. 27 Furthermore, we were unsuccessful previously when trying to introduce a research consent step into usual care; at the lead hospital, we could not get permission to alter the standard NHS consent form for one specialty and it was impossible to coordinate a change in the written procedural consent across all specialties. Although we were aware of at least one instance when a lead consultant had been able to integrate an opt-out consent model (to allow the use of routinely collected data for research) in the procedural consent form, this instance related to a diagnostic rather than an interventional context. The former setting has a more structured environment with fewer consultants and possibly less time pressure and we considered that an analogous process would be difficult to replicate in the PPCI environment, in which patients are critically ill and staff are under constant pressure to free beds for incoming patients.
Most prospective registries in the UK require explicit patient consent (e.g. National Joint Registry,74 Inflammatory Bowel Disease registry75), using stand-alone consent forms either with/without an approved ‘short text’ for obtaining consent that can be added to local consent forms. Participating hospitals are responsible for obtaining and recording patient consent with their uploads. However, the issue of missing information on consent is recognised and the National Joint Registry, for example, has dispensation [section 251 (of the NHS Act 200676) exemption] to record patient details when the indication for patient consent is ‘not recorded’, although this dispensation is granted on an annual review basis. Other registries, such as the cardiac audits under the umbrella of the National Institute for Cardiovascular Outcomes Research (NICOR) [which includes the Myocardial Ischaemia National Audit Project (MINAP) and the BCIS National Audit of Percutaneous Coronary Intervention (NAPCI)], have exemption under section 251 of the NHS Act 200676 to use patient information for medical research without consent. We cannot discern a rationale for the inconsistency in the consent arrangements for existing registries. Therefore, we are uncertain whether or not a future registry would be granted a section 251 (of the NHS Act 200676) exemption, as it is manifestly possible to implement prospective consent, as evidenced by some but not all existing registries.
A possible solution to the challenge of obtaining consent in the usual care setting would be to implement changes in NHS information technology (IT) administrative systems (ideally at the national level) to allow a record of verbal patient consent to be recorded in patients’ electronic notes (e.g. a ‘consent module’). The use of a system embedded within the NHS administrative systems could be applied more broadly to all registry or similar projects seeking to exploit routinely collected data, so that patients could be easily identified as having given or not given consent, having withdrawn consent, or not having been asked for consent. It could also capture whether or not the patient consents to all data being used and if not, which data are specifically excluded from the consent. However, we are uncertain whether or not verbal patient consent recorded in this way would comply with NHS Digital’s requirement for ‘explicit’ patient consent (the legal basis for accessing HES/ONS data). 27,77
Although patients and the public are broadly supportive of the use of electronic patient data for research,78–81 there are concerns about potential breaches of privacy and the misuse of health data. 82 The public response to the care.data public consulation83 highlighted important challenges in terms of patient confidence and trust in how electronic health records are used in medical research. 84 Newer consent models have been proposed, including a dynamic consent model, which would allow patients to control consent electronically over time and receive information about the uses of their data and up-to-date lay summaries of research projects that have used their data. 85 However, such a model would require investment in and maintenance of an electronic infrastructure, which would be difficult in the current economic climate of the NHS. Others have called for more adaptive governance models depending on the particular circumstances of the proposed research. 86 It was beyond the scope of this study to explore these possibilities for obtaining consent. 27 The difficulties we experienced in identifying consecutive eligible patients and consenting those who were identified in the emergency setting of activating the PPCI pathway highlight that the conventional consent model that we successfully implemented would not be feasible for a national registry.
We did not meet the feasibility criterion for data linkage and extraction (the target was that this should be achieved for > 90% of patients at all four hospitals) from HIS for all the requested data sets. Reasons for missing data included the following: difficulties in extracting data from local stand-alone databases, some of which were old and did not have an interface for data extraction; some data (e.g. medications on discharge, bedside ECHO) were not recorded electronically; imaging data were available electronically but only as free-text reports, making individual parameters of interest difficult to extract. The main impacts for a registry of failing to obtain these HISs is the inability to characterise the registry population in detail, to adjust any comparisons for patients’ characteristics and to describe changes in these data over time (potential indicators of the consequences of important changes in management).
We had initially envisaged establishing a data linkage and extraction model in one hospital and using this to guide data extraction at the other hospitals. We also envisaged that this model would be automated, with minimal IT support beyond the set-up phase. However, this was not possible because HIS for each data set differed across hospitals and different hospitals provided different levels of start-up IT support for the research project. 27 We budgeted for only a small amount of funding (£5000) to each hospital to help with this aspect of the study. Data linkage requires expertise in several areas, including knowledge of the databases to be linked and skills in creating linkage programmes. This expertise was variable across hospitals and in-depth knowledge was sometimes lacking; this was perhaps not surprising given the diversity of the databases involved, and the fact that some of these were old and were being phased out. 27 We relied on each hospital identifying key individuals to help with this aspect of the study. In addition, even in hospital A, the highest priority for the IT team was to maintain the integrity and functionality of the clinical care systems; in this context, it was difficult for them to prioritise our request to support this research project, even with the funding that was available.
The complexity of data retrieval from multiple HISs in secondary care in the NHS has been highlighted previously. 61 The government’s vision for the NHS, outlined in the National Information Board’s complex Personalised Health and Care 2020 framework,87 is that it will be an entirely digital organisation by 2020. Although such a system, if properly implemented, should provide benefits for research as well as patient care, it is an ambitious goal given the current financial and operational pressures within NHS organisations. The earlier (and equally ambitious) National Programme for IT, which aimed to create a single electronic care record for patients, connect primary and secondary care IT systems and provide a single IT platform for health professionals, was dismantled, mainly because IT companies could not provide solutions to match the ambition. 27,88 Despite such programmes, our experience from this study has highlighted the poor-quality electronic infrastructure in hospitals and the multitude of clinical databases that are not amenable to ‘interoperability’ (i.e. not needing extensive customisation or permission from the database vendor to provide data flow). Full interoperability for HISs across the majority of hospitals, therefore, still appears to be a distant goal. 89
We met the feasibility criterion of having > 10% of our consented population being referred for CMR in hospitals with a dedicated CMR facility. However, many of these referrals were for research (patient participating in a research study) rather than clinical purposes. For example, in the lead hospital, 33% of all referrals were for research. It is not clear whether or not this reflects practice in other hospitals with dedicated CMR facilities. Referral for non-clinical reasons (unless the findings are used to change clinical management) would dilute any observed effect of CMR on outcomes in an extended registry. Another challenge was that imaging reports were largely in free-text format, so individual CMR parameters would have to be extracted manually, which would not be feasible for an extended registry. A registry assessing the prognostic value of CMR, including individual CMR parameters, such as contractile function, myocardial oedema, MVO, intracardiac thrombus, myocardial haemorrhage and myocardial scar, would allow for an assessment of the value added of each parameter on patient outcome. Similarly, ECHO reports were also in free-text format, and bedside ECHO reports were missing entirely from our data sets because these are not routinely uploaded to electronic databases.
We made the decision to obtain data about follow-up from hospital episode data rather than from the source data submitted by participating hospitals, which are used to compile the hospital episode data. Using hospital episode data has two advantages: (1) follow-up episodes of care can be linked to index admissions, wherever they occur (not just in the participating hospitals) and (2) the organisations compiling hospital episode data apply established data management processes to improve data quality. We met the feasibility objective with respect to linkage of index admission data with hospital episode data. Linkage was achieved for 99% of patients. We failed to achieve linkage for the remainder mainly because of missing identifiers (NHS numbers) in the data sets submitted by participating hospitals. We had originally envisaged collecting information from local HISs on outcomes in the 12 months following the index procedure. However, we would have been unable to capture these data on every patient included in the study because many patients were discharged to, and followed up in, different hospitals, and it would have been difficult to liaise with and obtain data from multiple sites.
Is it feasible to set up a national registry from hospital episode data?
We explored the feasibility of setting up the registry retrospectively entirely by using linked, routinely collected data sets [e.g. hospital episode data, NHS England’s Diagnostic Imaging Dataset (DID) and national audits, such as the BCIS’ NAPCI90]. Setting up the registry in this way would avoid the need to obtain patient consent. This would be deemed appropriate on the basis that all of the information made available would be anonymised.
We were able to identify patients who activate the PPCI pathway in hospital episode data using the relevant procedure codes. We identified 98% of patients in our cohort who underwent PPCI and 85% of patients who underwent emergency angiography without PPCI. We could not, however, identify the CMR exposure in hospital episode data for the majority of study participants who had a CMR scan. This is because CMR is carried out mainly as an outpatient investigation and the hospital episode outpatient data sets do not contain details of the exact nature of the outpatient visit (only the specialty), so the reason for outpatient visits coded under the cardiology specialty was unclear. One option to consider would be the use of NHS England’s DID to identify CMR exposure. DID is a central collection of detailed information about diagnostic imaging tests carried out on NHS patients, extracted from local radiology information systems and submitted monthly to NHS England. 91 The main driver for the creation of the DID was to assess how the use of diagnostic imaging could contribute to the early diagnosis of cancer (in particular, GP direct access to these tests). As such, DID has already been linked to cancer registry data, but not other national audits, and there could be substantial difficulties/delays in applying for linkage permissions with, for example, the BCIS’ NAPCI data set. 90 Although DID captures information about referral source and patient type, details of the test (e.g. type of test and body site), demographic information (e.g. GP-registered practice, patient postcode, ethnicity, gender and date of birth), plus items about waiting times for each diagnostic imaging event (it does not store the images themselves, the individual parameters measured by the test or the outcomes/diagnoses related to the test). DID could not, therefore, be used to assess the prognostic value of individual components of a CMR scan, or the extent to which a CMR scan altered patient management. Nevertheless, it should allow ascertainment of CMR exposure.
Economic evaluation
We developed a series of economic decision models to identify the key drivers of cost-effectiveness. To our knowledge, this is the first time the key drivers of the cost-effectiveness of introducing CMR have been considered. We chose two patient subgroups for our models: patients with multivessel disease and patients with unobstructed coronary arteries. Both of these subgroups were identified as potentially benefiting from CMR in the formal consensus study.
Our results showed that, for each of the base-case models, the differences in QALYs between strategies were very small and, therefore, the results were largely driven by the differences in costs, although mean differences in cost for different strategies were modest. Sensitivity analyses around the two models identified the diagnostic accuracy of the ischaemia tests as the key driver of cost-effectiveness. In the model for patients with multivessel disease, the costs of ischaemia testing and the QALYs associated with MACEs and no MACEs exerted an influence on cost-effectiveness results, but not to the same extent as the diagnostic accuracy of CMR and pressure wire. Similarly, in the model for patients with unobstructed coronary arteries, the cost of CMR, the difference between QALYs associated with MACEs and no MACEs, and the proportion of patients who have MACEs who are assumed to die exerted an influence on the results; however, the better diagnostic accuracy of CMR and standard ECHO combined (the reference standard in the base case) compared with standard ECHO only was more important.
The key drivers of cost-effectiveness highlighted by each model are similar, and, therefore, it is likely that if CMR was introduced for our entire eligible population (patients who activate the PPCI pathway), it would be these parameters that would need to be measured accurately in any future study. This work highlights the importance of having accurate information about the diagnostic accuracy of different tests, as estimates for these parameters are such key determinants of cost-effectiveness. We have identified some of the key unknowns in terms of costs and outcomes in the population of patients who activate the PPCI pathway, which can inform future studies to ensure that important variables are captured in sufficient detail.
Identifying important changes in management resulting from cardiovascular magnetic resonance and the subgroups of patients to which these refer
Parts of this text have been reproduced from Pufulete et al. 4 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
A key objective of our feasibility study was to define a primary composite outcome, acceptable to cardiologists and other stakeholders (e.g. clinical commissioners) as representing a clinically important change in management (e.g. expected to prevent future MACEs) as a result of an eligible patient having had CMR, that could be used for the registry in the medium term. Although such a primary outcome is not typical, it would allow evidence to be obtained more quickly to inform decisions about implementing CMR than would be possible for an evaluation based on MACEs. In the long term, we envisaged that the primary outcome for the registry would be MACEs, but addressing relevant research questions using the registry would need data for a large number of patients. For example, in order to have 90% power to detect a 1.2% difference in MACEs (4.8% vs. 6.0%) between those having CMR and those not, at the 5% level of statistical significance, and assuming 10% of patients who activate the PPCI pathway receive CMR and 90% do not, the registry would require > 37,000 patients. This represents 20 hospitals, with an average of 500 patients giving consent per year per site for > 3.5 years. We expected that important changes in management would be much more frequent than MACEs, allowing the effectiveness of CMR with respect to this outcome to be evaluated more quickly with data from fewer patients.
We used a formal consensus approach because there were no studies in the literature that reported the impact of CMR in our study population, reflecting the fact that changes in clinical management are difficult to use as end points in trials. This is because some important changes in clinical management are not easily observed92 and the causal relationship between changes in management and improved outcomes is not well defined. Imaging has been identified as one of the key drivers of increased health-care costs; therefore, the adoption of an imaging modality in a new clinical context should be supported by evidence of its benefit to specific patient groups or clinical scenarios. Such evidence is difficult to obtain in practice.
We identified five subgroups of ACS patients who activate the PPCI pathway for whom there was consensus that CMR changes patient management in a clinically important way: (1) patients who have an OHCA (≈7% of those who activate the PPCI pathway),69 (2) patients who have a ‘normal’ (unobstructed) coronary angiogram (≈10% of those who activate the PPCI pathway),15,16 (3) patients who develop LV thrombus (3% overall and 9% in patients with anterior STEMI),70 (4) patients who have multivessel disease (between 40% and 65% of those who undergo PPCI)7,10,71 and (5) patients in whom CMR markers indicate a poor prognosis (up to 60% of patients after STEMI). 2,72 There was consensus about the first three subgroups in both rounds of the survey, whereas consensus was reached about the other two subgroups in the modified survey. These results suggest that CMR benefits a large proportion of patients who activate the PPCI pathway.
Cardiologists who participated in our research agreed that CMR is superior to ECHO in establishing a diagnosis in patients who survive an OHCA, which has implications for treatment and prognosis. Despite this view, there are few studies that have reported the role of CMR in managing these patients. A retrospective case series of 54 OHCA survivors showed that CMR diagnosed the cause (ischaemic or non-ischaemic cardiomyopathy) in 40 (74%) of the survivors. 93
The cardiologists also agreed that CMR can provide a definitive diagnosis (e.g. acute MI, acute myocarditis and cardiomyopathy, especially Takotsubo cardiomyopathy) in patients with ACS and unobstructed coronary arteries. Evidence for the role of CMR in patients with ACS and unobstructed coronary arteries also comes from small retrospective case series. These suggest that CMR provides a definitive diagnosis in 80–90% of these patients. 20,23,94 Without access to CMR, the management of these patients is variable in clinical practice, which may have long-term implications. A 2015 systematic review50 showed that the overall all-cause mortality in patients presenting with suspected MI and unobstructed coronary arteries was ≈5% at 12 months.
The cardiologists agreed that CMR identifies LV thrombus better than ECHO, which allows more patients to be identified and treated appropriately. Accurate identification of a LV thrombus is important because it often directs subsequent anticoagulation therapy to prevent embolic events. A 2015 systematic review,95 published after we conducted the formal consensus study, showed that late gadolinium enhancement (LGE) CMR is the most accurate modality for detecting LV thrombus, with 88% sensitivity and 99% specificity (compared with standard ECHO, which had 24–33% sensitivity and 94–95% specificity, and contrast ECHO, which had 23–61% sensitivity and 96–99% specificity), although most of the included studies in this review did not use a pathological or surgical gold standard for the detection of LV thrombus.
There was agreement among cardiologists that CMR-based testing of ischaemia after PPCI would be likely to optimise the revascularisation strategy for patients with multivessel coronary disease, although they acknowledged that there is no evidence to support the view that a CMR-based revascularisation strategy would improve outcomes. Patients with multivessel disease have a two-fold increase in MACEs compared with patients with single-vessel disease. 96 There is continuing debate about the benefits of complete versus single lesion revascularisation during the index PPCI admission. 97 Despite recent evidence from randomised controlled trials (RCTs) showing improved clinical outcomes when complete revascularisation is undertaken at PPCI,98 current American99 and European100 revascularisation guidelines for acute MI recommend revascularisation only of the infarct-related artery at PPCI in patients with multivessel disease.
There was considerable debate about using CMR to identify patients with a poor prognosis after PPCI. Although there was agreement that CMR parameters of cardiac function (e.g. impaired LV function, large infarct size, MVO) are useful for risk stratification after STEMI, there was disagreement about whether or not this would lead to a management change. Some cardiologists felt that there would be no changes to prescribing for secondary prevention because all patients should receive aggressive secondary prevention in accordance with guidelines. It was acknowledged, however, that CMR markers are prognostic for outcome;4 two recent meta-analyses of prognostic studies (conducted after this formal consensus study) showed an increased risk of MACE, by 13–15% for every 10% decrease in EF assessed by CMR,2 and in patients with MVO assessed by CMR [odds ratio (OR) 2.60, 95% CI 1.68 to 4.02 and OR 4.30, 95% CI 2.19 to 8.43, depending on the method of MVO assessment]. 3
We could not identify all patient subgroups from HIS data sets. Patients with LV thrombus and OHCA were identifiable only in hospital episode data, from ICD-10 codes for these diagnoses assigned to the index admission. Patients with unobstructed arteries could not be positively identified from HIS; we identified them by their exclusion from the hospital BCIS’ NAPCI data sets (as only patients who have a stent inserted, i.e. have PPCI, or have had an attempt to have a stent inserted, are included in BCIS’ NAPCI). Given the uncertainty we faced when trying to identify this population prospectively (see Feasibility of setting up a registry using hospital information system data) and the initial confusion over eligibility at the beginning of our recruitment period, we decided to review the medical notes (representing the reference standard) for a subset of these patients to determine the proportion classified correctly according to our definition of ‘unobstructed coronary arteries’ (exclusion from BCIS’ NAPCI). Although we classified 81% of patients correctly, some patients we identified as having unobstructed arteries were identified as having a culprit lesion in the manual review of patients’ notes. Furthermore, there was also a degree of disagreement between medical notes and the primary diagnosis recorded in hospital episode data for the index admission of these patients, with 11% of patients who were identified as having unobstructed coronary arteries in their medical notes having a primary diagnosis of MI in HES. Our investigation using medical notes highlights a degree of misclassification of this subgroup of patients, although this is not surprising given the uncertainty in diagnosis during the index admission.
Implementation of a primary outcome based on the changes in management for which consensus was achieved using hospital episode data
Parts of this text have been reproduced with permission from Pufulete et al. 68 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
There was consensus that CMR changes patient management in a clinically important way for five subgroups of ACS patients. For each group, we listed ‘activities’ (e.g. new diagnoses or procedures, additional diagnostic tests, changes in medication, outpatient appointments) that reflected a change in management. Activities were identified from the relevant consensus statements and by looking at care pathways, when available, to confirm that we captured all aspects of patient management in these activities. We then compiled a list of the relevant ICD-10 diagnosis codes and OPCS procedure codes (with help from clinicians on the study team and a dedicated HES coder). We identified these codes in hospital episode data for participants up to 12 months following the index procedure.
We were able to identify all of the activities that we identified as representing changes in management in the hospital episode data. Therefore, we conclude that it would be feasible to look for differences between patients who have and those who do not have CMR within ACS subgroups in terms of frequency and time to first event for some of the activities. We recognise that some events that were listed as representing a change in management (e.g. cardiology outpatient appointment, changes in medication) are not immediately amenable to inclusion in a composite outcome for a time-to-event model.
This approach to constructing a proxy outcome reflects our original concept and appears to be feasible when the important changes in management are hypothesised to cause a change in the same direction in all events/activities included in a composite outcome for a subgroup. However, through the consensus process, it became apparent that some of the hypothesised changes in management arising from CMR will not necessarily result in a unidirectional change in the frequency or rate of an activity. In several instances, an important change in management was more appropriate targeting of a treatment activity to the patients who were likely to benefit most, without necessarily any change in the overall frequency of the activity. For example, in patients with multivessel disease, CMR may result in better (i.e. more cost-effective) targeting of additional revascularisation. In these situations, a simple evaluation of the frequency or rate of a proxy outcome would not detect any value of CMR to patients and the NHS.
We were unable to identify some important changes in management that could have arisen from CMR. There are no medication records in hospital episode data, so changes in drug therapy could not be identified. Initiation, withdrawal or changes in medication represented an important change in management for all of our patient subgroups and, in particular, patients with LV thrombus, for whom anticoagulation therapy is the main change in management. A potential solution to this problem is the linkage of HIS and hospital episode data with data from the Clinical Practice Research Datalink (CPRD), a primary care data set that has detailed prescription information. However, because not all general practices contribute data to CPRD, medications data would be available for only a proportion of patients in a registry.
We were also unable to identify additional tests or procedures carried out in the outpatient department because most outpatient episodes in the HES outpatient data set do not have diagnostic/procedure codes assigned to them. We are uncertain about the extent to which DID, recently added to the HES portfolio of data sets, may improve this situation. Finally, hospital episode data alone may not capture all activity related to changes in management as some patients will be seen multiple times by their GP or in private facilities. However, as the extent of after care in private facilities in the UK is minimal, it is unlikely to invalidate a future comparison in a registry.
Patient and public involvement and engagement
The chairperson of the Study Steering Committee (SSC) was a patient representative. We also included on the SSC a representative from the British Heart Foundation (the Area Development Manager). We felt that it was particularly important to have members of the public and a previous patient on the SSC because one of the main objectives of the feasibility study was to investigate the feasibility of consenting patients into a registry.
We sought the views of both of our lay members about various aspects of the study at all stages. For example, at the first meeting, we explored the issue of consent. Both our lay members suggested exploring the possibility of not having consent for the study and confirmed that they did not think using ‘assumed consent’ would be a problem if it was implemented in routine practice. We did this (as described in Feasibility of setting up a registry using hospital information system data) but concluded that this viewpoint, although prevalent among patients and the public, is not currently consistent with the data protection law. 101 When implementing our conventional consent method, we also asked patients who had been in a similar position to review the patient information leaflet and consent form to ensure that the information was not excessive but covered all the points that patients would want to know. This input was very useful and helped to guide some changes during the finalisation of these documents for the Research Ethics Committee submission.
We had planned to hold a focus group with patients and the public to engage with them about developing the primary outcome as part of the formal consensus process. The lay representatives on the SSC were consulted at length about this, including reviewing questionnaires that had been drafted for the consensus meetings. They concluded that, to form an opinion about the importance of the changes in management for which consensus was obtained, lay people would require an understanding in terms of technical knowledge and recent experience and, therefore, it was not reasonable to expect lay people to comment on or refine the outputs from the consensus process. Throughout the project, the lay representatives on the SSC (and, in particular, the SSC chairperson) expressed dismay about the lack of availability of HIS and hospital episode data and the delays this caused to the study. Accepting that our application for these data was held up by the one-off event of the moratorium in processing applications at NHS Digital (see Chapter 2, Major delays with the study), lay representatives were surprised that the plan for implementing new governance processes did not appear to have considered how applications in progress should be handled. The ruling from NHS Digital and ONS that the wording of the patient information leaflet was not compliant with the new guidance, after we had completed recruitment, seemed Kafkaesque102 from the perspective of the researcher team.
Strengths and limitations of the study
Feasibility prospective cohort study
We established a successful collaboration among four hospitals and exceeded the recruitment target for the study of 1600 patients. We also identified the main challenges to establishing a prospective registry in the way we had envisaged: identifying the eligible population is difficult to do from local HISs; the conventional consent model fails to capture a sizeable proportion of the eligible population and would be costly to implement; and, most importantly, the required local data from HISs are not uniformly available/exportable. 27
We did not identify or test a more efficient method to obtain consent. The methods used by hospitals for the study are almost certainly not feasible to implement in the long term for a registry, and are likely to result in varying completeness across hospitals. However, the barriers to any form of ‘presumed consent’ or ‘opt out’ seemed insurmountable without a long-term plan for implementing consent.
We are aware that CMR may benefit other groups of ACS patients (e.g. those with NSTEMI). However, we considered at the outset that it would be impossible to identify consecutive patients presenting with a broader diagnosis of ACS but not requiring emergency coronary angiography; being able to define and ascertain the entire eligible population is a key requirement of a high-quality database. This was a particularly important consideration given that the proposed registry was based on the premise of linking information collected in the course of usual care, avoiding the time and cost burden of primary data collection. 27 Even so, we still encountered difficulties in identifying from electronic records the group of patients with unobstructed coronary arteries in our eligible population.
We concluded that we could identify our eligible population in hospital episode data, based on the fact that we identified the index admission for most patients recruited into our study. However, because we had only hospital episode data for patients in the cohort, we cannot comment on or quantify the risk that an algorithm designed to identify such patients from hospital episode data could also identify some patients who would have been ineligible for the registry. Our impression from exploring codes assigned to hospital episode data for index admissions is that this risk is low and is likely to reduce as the quality of hospital episode data improves. The only way to quantify the risk would have been to request anonymised linkage of all HISs and hospital episode data for all cardiology admissions during the study period, with identification of the study cohort participants as a subgroup.
A major limitation is the length of time it took to complete the study. The main reason for this was because our application for HES data coincided with the moratorium on data requests by NHS Digital (formerly known as the Health and Social Care Information Centre), which resulted in a delay of 23 months in processing our application. This meant that the study took > 4.5 years to complete, whereas we had projected 1.5 years for completion. During this time, it is likely that data availability and the quality of the data will have changed/improved, given the rapidly evolving NHS IT systems/platforms. During this time, new diagnostic tests for detecting ischaemia (e.g. pressure wire), directly competing with CMR, were introduced and rapidly adopted by some cardiologists. These changes highlight the need for new research questions; therefore, it is likely that the aims and objectives for a national registry now would be different from those we proposed when we set up this study. For example, it may be more relevant to compare ischaemia testing using pressure wire with CMR, rather than to compare CMR with no CMR, which we proposed for our registry. We have tried to adapt the study to the current circumstances, for example with respect to the cost-effectiveness models, but have been unable to do anything about the changes in practice or the historic nature of the data collected.
Economic evaluation
There is considerable uncertainty around the majority of parameter estimates in both models. In the model for patients with unobstructed coronary arteries, the probability of patients truly having a MI was obtained from the results of a systematic review,50 but all other probability estimates in both models are based on single papers, often on small or modest sample sizes. There was only sparse evidence found in the literature for many of the probability estimates required. This was particularly true for the probabilities of MACEs for patients who truly did and those who did not have a MI (and had appropriate treatment or not), in the model for patients with unobstructed coronary arteries. Members of the study team were concerned that the estimated ratio of MACEs in MI patients compared with non-MI patients (in patients with unobstructed coronary arteries) found in Pathik et al. 52 was very high; this is why we investigated this parameter in the sensitivity analyses.
Even when papers reported on the correct patient group and outcomes, studies may have been conducted in other countries, where practices may differ from those in the UK. For example, Smits et al. 38 specifically reported numbers that could be used to estimate the probability of a MACE by 1 year for those truly with ischaemia separately for those who were and those who were not revascularised (see Figures 1 and 2, and Chapter 5, Cost-effectiveness models); however, this study was conducted in Europe and Asia, with no UK centres included. Occasionally, estimates from a different patient group were the only estimates available. For example, in the model for patients with multivessel disease, the diagnostic accuracy of stress ECHO (its sensitivity and specificity) was taken from a paper considering the diagnosis of significant coronary artery disease, rather than a PPCI population, and estimates are based on the number of vessels rather than the number of patients. 39
In the base-case analyses for multivessel disease, CMR and pressure wire were treated as reference standards, and both were assumed to have 100% sensitivity and specificity. There are suggestions in the literature that this is reasonable; for example, Emrich et al. 103 found that CMR correctly identified all 20 out of 125 patients with MI in their study. The assumption of equal diagnostic accuracy is critical, because the sensitivity analyses showed how diagnostic accuracy of the ischaemia tests was the key driver of cost-effectiveness. Similarly for the unobstructed coronary arteries model, reducing the diagnostic accuracy of CMR plus standard ECHO compared with standard ECHO alone (sensitivity analysis 1) doubled the ICER.
Individual patient data on resource use were not available from hospital episode data in time to be used in these analyses; resource use associated with each of the patient pathways was, therefore, estimated based on discussions with the PIPA study team and costs were largely obtained from NHS Reference Costs. 40 These methods have allowed standard care pathways to be costed but will have underestimated the variability between individual patients and their actual patient pathways.
As well as the costs of initial ischaemia testing (if applicable), and any revascularisation, we included the costs of follow-up outpatient appointments, medications and rehabilitation over the first year. We also included additional health-care costs to 1 year based on the Office of Health Economics’ estimate of the annual cost per person of NHS care. 43 It is recognised that there will be an element of double counting here, as the costs of ischaemia testing, revascularisation and subsequent costs will overlap with these annual costs, but this will be the same for all strategies and should not affect the comparison of costs between strategies. These annual costs were included to account for differences in costs for patients who died; these costs were halved for patients with a MACE who died by 1 year.
Utility estimates for a PPCI population were found in the literature, but there was uncertainty around the utility decrement for patients with MACEs compared with those without MACEs. A decrement of 0.05 was assumed for patients with a MACE compared with those without a MACE. 48 Although this decrement has been used by others, the original reference for this decrement implies that it was a sensitivity analysis, and was not based on any primary data. 104 Utility estimates for patients with unobstructed coronary arteries were not found in the Tufts Cost-effectiveness Analysis Registry,46 and a recent paper measuring quality of life in patients with MI with unobstructed coronary arteries reported that no studies measuring quality of life in these patients have previously been conducted. 105 This study measured quality of life using the SF-36 at 3 months post acute event and found similar findings in patients with unobstructed coronary arteries and a control group of MI patients with coronary heart disease, suggesting that the estimates used in our model for patients with unobstructed coronary arteries are reasonable.
Formal consensus study
Parts of this text have been reproduced from Pufulete et al. 4 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
A key strength of the study was the use of a formal consensus approach, based on a systematic search of the literature as well as expert opinion. The nominal group technique is one of the four well-established methodologies for formal consensus [the other three are (1) the Delphi method, (2) the Research ANd Development Corporation (RAND)/University of California, Los Angeles Appropriateness Method (RAM) and (3) the National Institutes’ of Health consensus development conference methodology]. 4,106,107 Our literature review included all study designs to determine the size and nature of the evidence base and highlight research gaps. The search was systematic and extensive, including five databases and published abstracts. Four cardiologists contributed expert opinion. Two are UK/European CMR experts. The process provided evidence for the content validity of each statement. All cardiologists who drafted the statements proposed the same patient subgroups as potentially benefiting from CMR. There was generally good agreement between draft statements generated by cardiologists and the non-clinical reviewer.
There were no disagreements in the group meeting regarding the potential management change in each of the patient subgroups identified. Similarly, the studies identified in our literature review (both prognostic and diagnostic) were consistent in their findings. The majority of prognostic studies reported that CMR markers such as EF, MVO and haemorrhage were associated with MACEs, although most did not evaluate the prognostic value of CMR indices in addition to indices available from alternative imaging modalities. The evidence supporting many of the statements came from small, retrospective case series. There were no prospective studies in the literature that reported the impact of CMR on clinical management in our population or whether or not any changes in management affected patient outcomes.
The main features of a formal consensus method are anonymity (i.e. statements were rated free from peer-group pressure) and iterative feedback (i.e. participants could adjust their initial rating based on the feedback of the group rating). We described the supporting evidence for each statement, identified via the literature review, and fed back qualitative (i.e. panel members’ comments) and quantitative (i.e. group median ratings of each statement) information at the face-to-face meeting. We also included a varied group of cardiologists from different specialties in order to encompass diverse perspectives. 4
A potential limitation of this study is that we did not perform any formal quality assessment of primary studies. We made this decision for two reasons. First, the purpose of the review was to identify the evidence base in order to inform the consensus process, not to use the literature to quantify the effectiveness of CMR or its diagnostic or prognostic value. Second, no single assessment tool would have been appropriate for the diverse studies that were identified, hence assessments would not have been comparable. Therefore, we have not referred to our overall review as being ‘systematic’ (only the search), although its conduct closely reflected established guidelines for systematic reviews and we believe that other researchers following the same steps would replicate its findings.
The number of panel members in our study was lower than the recommended 8–12 members for a consensus panel. 107 Nevertheless, smaller groups are preferable to larger ones because, although having more group members increases the reliability of group judgement, large groups reduce the ability to elicit potentially important contributions from every member of the panel. 107 To overcome any criticism of the number of panel members in the study and to prevent the possibility of introducing bias (given the self-selected nature of the panel), we extended the survey to UK cardiologists who did not participate in the formal consensus process. Apart from one statement (statement 1, relating to the ability of CMR to identify patients with a poor prognosis after PPCI), the same statements were in consensus when the survey was completed by cardiologists external to the consensus process. This indicates that the process was robust and the survey statements were clear and unambiguous.
A further limitation of the study was that we did not include other stakeholders (e.g. GPs and patient representatives) in the consensus process. 108 Although we had originally planned to do so (see Patient and public involvement and engagement), we decided that the specialised nature of the statements would not be easily understood by those without cardiology expertise. 4 We did, however, hold a separate meeting (outside the consensus process) with a GP commissioner and the director of the Southwest Cardiac and Stroke Network. The conclusion of the meeting was that, given that the cohort is an emergency population and the consensus process identified that a large proportion of the population would potentially benefit from scanning, it would be better to offer CMR to the whole population rather than complicating the patient management strategy by scanning only subsets of patients.
Lessons for the future
Future studies attempting to answer research questions through linkage of routinely collected data sets (at the local and national level) should consider the following:
-
Timeliness – a study must be reported within a reasonable time frame given the rapidly changing environment (e.g. NHS IT systems, introduction of new diagnostic tests).
-
Data availability in hospitals – the HIS available at each hospital and the level of IT support available to provide the data need to be investigated thoroughly; funding is likely to be required to support data extraction.
-
Data sources in hospitals – the relevant data sources at each hospital need to be identified before starting a study. For example, we decided to use BCIS/MINAP submission data sets instead of catheter laboratory databases after the study started; this caused considerable confusion and difficulties over patient eligibility, given that BCIS/MINAP could provide data only for patients who had STEMI/received PPCI and not patients with unobstructed coronary arteries.
-
Hospital episode data – these data are made available (after ‘cleaning’) by NHS Digital (or Information Services Division Scotland, PEDW or Northern Ireland Department of Health) for whole financial years. Our failure to appreciate this meant that only ≈67% of patients had a whole 1 year of follow-up.
Implications for practice
Our experience highlights the need to assess ‘feasibility’ before attempting to set up a new registry. Although some of the pitfalls we experienced may, in hindsight, be viewed as resulting from a lack of ‘preparedness’, our experience is not unique. Registries (e.g. national audits) that have managed to overcome issues with data collection and quality have achieved this through a lengthy process, necessitating many iterations (and a feedback loop) before attaining any quality targets. This process requires considerable investment, infrastructure and commitment at the national level, which would be difficult to achieve in a research setting. 4 The process also has drawbacks; for example, iterations add to the complexity of creating a data set that is consistent over time and that takes into account changes in the methods of collecting data, which may result in concomitant inadvertent changes in definitions of the data.
We consider the following as desirable developments to make it more feasible to construct a registry from routinely collected data:
-
More uniform implementation of IT across acute NHS trusts (e.g. a shortlist of ‘approved’ database suppliers with compatible features, including features to facilitate data extraction for secondary purposes).
-
Lighter touch governance when anonymisation is done centrally. Access to patient data is likely to remain a challenge, particularly with the introduction of the General Data Protection Regulation (GDPR). 109,110 The lack of clarity over the safe and ethical secondary use of routinely collected data is currently a major barrier preventing access to routine data for research.
-
A method for obtaining consent that is practicable to implement in the busy setting of the NHS. For example, this could involve an opt-out model of consent or presumed consent, combined with a national publicity campaign promoting the benefits of using routine data in research. Opt-out models of consent are currently very difficult to implement because of the specific, and changing, nature of the instructions for informing affected people. The apparent failure to make provision for analyses of historic and ongoing data sets, when data collection started before changes in law, is particularly wasteful and frustrating for researchers.
-
Efficient provision of routine data by data guardians, for example NHS Digital.
Future research recommendations
The present study highlighted the difficulties of implementing a conventional consent process for a registry that recruits patients who undergo an emergency procedure and have a short hospital stay. An important area of future research should, therefore, be to identify and test a more efficient method of obtaining consent for similar studies in similar settings that need to link data sources. This issue should be approached at a national rather than a local level, and involve detailed discussions with all relevant stakeholders. Critically, clarification is required about whether or not it is possible, given the recent debates about data sharing and the changes to information governance policies at NHS Digital,111 to obtain approval (under section 251 of the NHS Act 200676) to use patient information for medical research and link national data sets without consent. Currently, there is discrepancy between UK national registries, with many implementing prospective consent,74,75 whereas others, such as the national cardiac audits managed by NICOR, operate on the basis of section 25176 exemptions.
Alternatively, we need to test the feasibility of conducting the study using linked, anonymised national data sets (e.g. hospital episode data, BCIS NAPCI, DID, CPRD for medications). This would avoid the need for obtaining individual patient consent or section 25176 exemption, as data are anonymised when made available to researchers. Such a linked data set would be able to capture all the eligible population and could use MACEs as an outcome, although there may still be regulatory hurdles in obtaining permission to link these data sets and CPRD captures data for only a small proportion of the UK population.
There has been a rapid adoption of pressure-wire testing for detecting ischaemia during this study despite a lack of evidence of benefit over CMR. Our cost-effectiveness models have highlighted that the diagnostic accuracies of the CMR versus pressure-wire ischaemia tests are key drivers of the relative cost-effectiveness of management strategies based on these tests, compared with stress ECHO or each other. Therefore, future research is needed to quantify the relative diagnostic accuracy of CMR and pressure wire versus stress ECHO, and versus each other, for ischaemia testing. The fact that both CMR and pressure-wire testing are regarded as reference standards in clinical practice and no superior standard is recognised may mean that the cost-effectiveness of CMR versus pressure wire can be tested with respect to effectiveness only in a large RCT.
Chapter 8 Conclusion
We conclude that it is not currently feasible to set up a multicentre registry in the way that we had envisaged. We did not identify all patients who were eligible for the study: patients with unobstructed arteries (i.e. those that had an emergency angiogram but did not receive PPCI) were difficult to identify from catheter laboratory databases. We successfully consented patients but obtaining individual, opt-in consent would not be feasible for a national registry. We explored several consent models (e.g. opt-out on procedural consent form and standard consent at discharge) but none was implemented because of logistic difficulties. Linkage of data from HISs with hospital episode data was feasible, but data from HISs are not uniformly available/exportable. Information about whether or not participants had had CMR in CMR hospitals was obtained successfully from HISs, although some referrals for CMR were for research rather than clinical purposes. 27
In the cost-effectiveness models, the differences in QALYs between strategies were very small, and the results were driven largely by the differences in costs. Sensitivity analyses around the two models identified the diagnostic accuracy of the ischaemia tests as the key driver of cost-effectiveness.
We defined five subgroups of patients who activate the PPCI pathway for whom there was consensus that CMR changes patient management in a clinically important way. 4 All subgroups could be identified in either the local HIS data set, hospital episode data or both. It is feasible to identify some, but not all, important changes in management in follow-up hospital episode data. The main constraints on identifying important changes in management were as follows: (1) outpatient hospital episode data were poorly coded with respect to diagnosis, (2) medication data were not available at baseline or follow-up (preventing inspection of prescription of/changes in medication after the index event) and (3) data about diagnostic investigations were poorly coded in both acute care and outpatient data sets. More research would be needed to develop a specific composite outcome for a patient subgroup. 68
Any multicentre registry set up in this patient population would need to be able to capture new and emerging diagnostic/interventional technologies. During the study, priority for CMR decreased because of the introduction of pressure-wire testing for diagnosing ischaemia in patients with multivessel disease. However, it is not clear whether or not pressure-wire testing is the optimal management strategy from the perspective of health services. This question could potentially be answered in a national registry, given that the use of pressure wire is (currently) driven largely by the preferences of interventional cardiologists rather than patient characteristics. However, we are uncertain whether or not CMR is used sufficiently to allow this question to be addressed.
Acknowledgements
We would like to thank the local research teams that contributed to the study, including Ruth Bowles, Jo Roberts, Alan Davies, Helen Kingsland, Linda Wadey, Petra Bijsterveld, Kathryn Somers, Richard Gillott, Lesley Davies, Stephen Hiles, Stephen Morris and Clare Fagan. We would also like to thank Danielle Bargo, for contributions towards the health economic models, and Alice Redfern, who assisted with the literature searching for the economic models. In addition, we would like to thank the SSC, including John Walsh, the chairperson of the committee, and our colleagues Lynn Cook, Peter Brindle, Emma Hopkins and Andreas Baumbach for their contributions to the research. We would also like to acknowledge the contribution made by the expert cardiology panel for the consensus process.
This study was designed and delivered in collaboration with the Clinical Trials and Evaluation Unit, a UK Clinical Research Collaboration-registered clinical trials unit that, as part of the Bristol Trials Centre, is in receipt of National Institute for Health Research clinical trials unit support funding.
Contributions of authors
Jessica M Harris undertook the data handling, linkage and analysis, and prepared the tables and figures for the report.
Rachel C Brierley conducted the study and conceived some aspects of data collection from participating hospitals.
Maria Pufulete designed and conducted the study, determined the structure of the consensus process, conducted the literature review and wrote the report.
Chiara Bucciarelli-Ducci conceived the original research question and provided clinical and CMR expertise.
Elizabeth A Stokes carried out the health economic analyses and wrote the health economics sections of the report.
John P Greenwood was principal investigator at one of the recruiting hospitals, provided cardiology and CMR expertise and was a member of the working group in the consensus process.
Stephen H Dorman was principal investigator at one of the recruiting hospitals, provided cardiology expertise and was a member of the working group in the consensus process.
Richard A Anderson was principal investigator at one of the recruiting hospitals, provided cardiology expertise and was a member of the working group in the consensus process.
Chris A Rogers provided guidance with respect to statistics and study design.
Sarah Wordsworth provided guidance and oversaw the health economics analysis.
Sunita Berry was a member of the working group in the consensus process and provided expertise with respect to NHS commissioning of services.
Barnaby C Reeves was chief investigator with overall responsibility for the study, designed the study and provided strategic direction with the interpretation of study results.
Publications
Pufulete M, Brierley RC, Bucciarelli-Ducci C, Greenwood JP, Dorman S, Anderson RA, et al. Formal consensus to identify clinically important changes in management resulting from the use of cardiovascular magnetic resonance (CMR) in patients who activate the primary percutaneous coronary intervention (PPCI) pathway. BMJ Open 2017;7:e014627.
Brierley RC, Pufulete M, Harris J, Bucciarelli-Ducci C, Greenwood JP, Dorman S, et al. Developing a UK registry to investigate the role of cardiovascular magnetic resonance (CMR) in patients who activate the primary percutaneous coronary intervention (PPCI) pathway: a multicentre, feasibility study linking routinely collected electronic patient data. BMJ Open 2018;8:e018987.
Stokes A, Doble B, Pufulete M, Reeves BC, Bucciarelli-Ducci C, Dorman S, et al. Cardiovascular magnetic resonance in emergency patients with multi-vessel disease or unobstructed coronary arteries: a cost-effectiveness analysis in the UK. BMJ Open 2019;9:e025700.
Pufulete M, Harris J, Dorman S, Cook L, Bucciarelli-Ducci C, Greenwood JP, et al. Feasibility of identifying important changes in care management resulting from cardiovascular magnetic resonance (CMR) using Hospital Episode Statistics data. BMC Med Res Methodol 2019; in press.
Data-sharing statement
Anonymised individual patient data (excluding hospital episode/vital status linked data) may be available for secondary research, conditional on assurance from the secondary researcher that the proposed use of the data is compliant with the Medical Research Council Policy on Data Preservation and Sharing regarding scientific quality, ethics requirements and value for money. All data requests should be submitted to the corresponding author for consideration and access may be granted following review. The hospital episode data/vital status data cannot be shared further due to conditions imposed by NHS Digital in the original data-sharing agreement that covered initial data release.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Antony R, Daghem M, McCann GP, Daghem S, Moon J, Pennell DJ, et al. Cardiovascular magnetic resonance activity in the United Kingdom: a survey on behalf of the British Society of Cardiovascular Magnetic Resonance. J Cardiovasc Magn Reson 2011;13. https://doi.org/10.1186/1532-429X-13-57.
- El Aidi H, Adams A, Moons KG, Den Ruijter HM, Mali WP, Doevendans PA, et al. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol 2014;63:1031-45. https://doi.org/10.1016/j.jacc.2013.11.048.
- Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2014;7:940-52. https://doi.org/10.1016/j.jcmg.2014.06.012.
- Pufulete M, Brierley RC, Bucciarelli-Ducci C, Greenwood JP, Dorman S, Anderson RA, et al. Formal consensus to identify clinically important changes in management resulting from the use of cardiovascular magnetic resonance (CMR) in patients who activate the primary percutaneous coronary intervention (PPCI) pathway. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014627.
- National Institute for Cardiovascular Outcomes Research. Myocardial Ischaemia National Audit Project n.d. www.ucl.ac.uk/nicor/audits/minap/documents/annual_reports/minap-report-2013 (accessed 17 January 2019).
- Cardarelli F, Bellasi A, Ou FS, Shaw LJ, Veledar E, Roe MT, et al. Combined impact of age and estimated glomerular filtration rate on in-hospital mortality after percutaneous coronary intervention for acute myocardial infarction (from the American College of Cardiology National Cardiovascular Data Registry). Am J Cardiol 2009;103:766-71. https://doi.org/10.1016/j.amjcard.2008.11.033.
- Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med 2000;343:915-22. https://doi.org/10.1056/NEJM200009283431303.
- Lee JH, Park HS, Chae SC, Cho Y, Yang DH, Jeong MH, et al. Predictors of six-month major adverse cardiac events in 30-day survivors after acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry). Am J Cardiol 2009;104:182-9. https://doi.org/10.1016/j.amjcard.2009.03.010.
- Rasoul S, Ottervanger JP, de Boer MJ, Dambrink JH, Hoorntje JC, Marcel Gosselink AT, et al. Predictors of 30-day and 1-year mortality after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis 2009;20:415-21. https://doi.org/10.1097/MCA.0b013e32832e5c4c.
- Toma M, Buller CE, Westerhout CM, Fu Y, O’Neill WW, Holmes DR, et al. Non-culprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: insights from the APEX-AMI trial. Eur Heart J 2010;31:1701-7. https://doi.org/10.1093/eurheartj/ehq129.
- Vlaar PJ, Mahmoud KD, Holmes DR, van Valkenhoef G, Hillege HL, van der Horst IC, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: a pairwise and network meta-analysis. J Am Coll Cardiol 2011;58:692-703. https://doi.org/10.1016/j.jacc.2011.03.046.
- Lockie T, Ishida M, Perera D, Chiribiri A, De Silva K, Kozerke S, et al. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve. J Am Coll Cardiol 2011;57:70-5. https://doi.org/10.1016/j.jacc.2010.09.019.
- Watkins S, McGeoch R, Lyne J, Steedman T, Good R, McLaughlin MJ, et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation 2009;120:2207-13. https://doi.org/10.1161/CIRCULATIONAHA.109.872358.
- ClinicalTrials.gov . MR INFORM – MR Perfusion Imaging to Guide Management of Patients With Stable Coronary Artery Disease n.d. https://clinicaltrials.gov/ct2/show/NCT01236807 (accessed April 2018).
- Larsen AI, Galbraith PD, Ghali WA, Norris CM, Graham MM, Knudtson ML. APPROACH Investigators . Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol 2005;95:261-3. https://doi.org/10.1016/j.amjcard.2004.09.014.
- Meryon I, Patel N, Millane T, Varma C. Normal coronary angiography and primary percutaneous coronary intervention for ST elevation myocardial infarction: a literature review and audit findings. Int J Clin Pract 2010;64:1245-51. https://doi.org/10.1111/j.1742-1241.2010.02394.x.
- Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest 2004;125:1877-84. https://doi.org/10.1378/chest.125.5.1877.
- Sarda L, Colin P, Boccara F, Daou D, Lebtahi R, Faraggi M, et al. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol 2001;37:786-92. https://doi.org/10.1016/S0735-1097(00)01201-8.
- Dokainish H, Pillai M, Murphy SA, DiBattiste PM, Schweiger MJ, Lotfi A, et al. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS-TIMI-18 substudy. J Am Coll Cardiol 2005;45:19-24. https://doi.org/10.1016/j.jacc.2004.09.056.
- Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J 2007;28:1242-9. https://doi.org/10.1093/eurheartj/ehm113.
- Codreanu A, Djaballah W, Angioi M, Ethevenot G, Moulin F, Felblinger J, et al. Detection of myocarditis by contrast-enhanced MRI in patients presenting with acute coronary syndrome but no coronary stenosis. J Magn Reson Imaging 2007;25:957-64. https://doi.org/10.1002/jmri.20897.
- Laissy JP, Hyafil F, Feldman LJ, Juliard JM, Schouman-Claeys E, Steg PG, et al. Differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology 2005;237:75-82. https://doi.org/10.1148/radiol.2371041322.
- Leurent G, Langella B, Fougerou C, Lentz PA, Larralde A, Bedossa M, et al. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch Cardiovasc Dis 2011;104:161-70. https://doi.org/10.1016/j.acvd.2011.01.005.
- Stensaeth KH, Fossum E, Hoffmann P, Mangschau A, Klow NE. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST-elevation myocardial infarction and normal coronary arteries. Int J Cardiovasc Imaging 2011;27:355-65. https://doi.org/10.1007/s10554-010-9671-7.
- Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006;114:1581-90. https://doi.org/10.1161/CIRCULATIONAHA.105.606509.
- Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol 2012;59:1604-15. https://doi.org/10.1016/j.jacc.2012.01.007.
- Brierley RC, Pufulete M, Harris J, Bucciarelli-Ducci C, Greenwood JP, Dorman S, et al. Developing a UK registry to investigate the role of cardiovascular magnetic resonance (CMR) in patients who activate the primary percutaneous coronary intervention (PPCI) pathway: a multicentre, feasibility study linking routinely collected electronic patient data. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-018987.
- NHS Reference Costs. London: Department of Health and Social Care; 2014.
- Welsh Index of Multiple Deprivation. Cardiff: Welsh Government; 2011.
- NHS Digital . Methodology to Create Provider and CIP Spells from HES APC Data 2014. http://content.digital.nhs.uk/media/11859/Provider-Spells-Methodology/pdf/Spells_Methodology.pdf (accessed 16 January 2019).
- International Statistical Classification of Diseases and Related Health n.d. https://icd.who.int/browse10/2016/en (accessed 20 December 2018).
- OPCS Classification of Interventions and Procedures version 4 n.d. www.datadictionary.nhs.uk/web_site_content/supporting_information/clinical_coding/opcs_classification_of_interventions_and_procedures.asp (accessed 20 December 2018).
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. https://doi.org/10.1002/sim.3697.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- The Pressure Wire Fractional Flow Reserve Measurement System for Coronary Artery Disease. London: NICE; 2014.
- Stokes A, Doble B, Pufulete M, Reeves BC, Bucciarelli-Ducci C, Dorman S, et al. Cardiovascular magnetic resonance in emergency patients with multi-vessel disease or unobstructed coronary arteries: a cost-effectiveness analysis in the UK. BMJ Open 2019;9.
- Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816-21. https://doi.org/10.1016/j.jacc.2009.11.096.
- Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med 2017;376:1234-44. https://doi.org/10.1056/NEJMoa1701067.
- Gurunathan S, Young G, Parsons G, Karogiannis N, Vamvakidou A, Elghamaz A, et al. 132 diagnostic accuracy of stress echocardiography compared with invasive coronary angiography with fractional flow reserve for the diagnosis of haemodynamically significant CAD in patients with known or suspected CAD. Heart 2016;102:A94-5. https://doi.org/10.1136/heartjnl-2016-309890.132.
- NHS Reference Costs 2015 to 2016. London: Department of Health and Social Care; 2016.
- British National Formulary. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2017.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Hawe E, Cockcroft L. OHE Guide to UK Health and Health Care Statistics. London: Office of Health Economics; 2013.
- Greenhalgh J, Bagust A, Boland A, Martin Saborido C, Oyee J, Blundell M, et al. Clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events (review of Technology Appraisal No. 90): a systematic review and economic analysis. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15310.
- Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10440.
- Tufts Medical Center . Cost-Effectiveness Analysis (CEA) Registry n.d. http://healtheconomics.tuftsmedicalcenter.org/cear4/Home.aspx (accessed 6 April 2017).
- Aasa M, Henriksson M, Dellborg M, Grip L, Herlitz J, Levin LA, et al. Cost and health outcome of primary percutaneous coronary intervention versus thrombolysis in acute ST-segment elevation myocardial infarction: results of the Swedish Early Decision reperfusion Study (SWEDES) trial. Am Heart J 2010;160:322-8. https://doi.org/10.1016/j.ahj.2010.05.008.
- Nam J, Briggs A, Layland J, Oldroyd KG, Curzen N, Sood A, et al. Fractional flow reserve (FFR) versus angiography in guiding management to optimise outcomes in non-ST segment elevation myocardial infarction (FAMOUS-NSTEMI) developmental trial: cost-effectiveness using a mixed-trial and model-based methods. Cost Eff Resour Alloc 2015;13. https://doi.org/10.1186/s12962-015-0045-9.
- Palmer S, Sculpher M, Philips Z, Robinson M, Ginnelly L, Bakhai A, et al. Management of non-ST-elevation acute coronary syndromes: how cost-effective are glycoprotein IIb/IIIA antagonists in the UK National Health Service?. Int J Cardiol 2005;100:229-40. https://doi.org/10.1016/j.ijcard.2004.08.042.
- Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015;131:861-70. https://doi.org/10.1161/CIRCULATIONAHA.114.011201.
- Kang WY, Jeong MH, Ahn YK, Kim JH, Chae SC, Kim YJ, et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe?. Int J Cardiol 2011;146:207-12. https://doi.org/10.1016/j.ijcard.2009.07.001.
- Pathik B, Raman B, Mohd Amin NH, Mahadavan D, Rajendran S, McGavigan AD, et al. Troponin-positive chest pain with unobstructed coronary arteries: incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2016;17:1146-52. https://doi.org/10.1093/ehjci/jev289.
- Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjöld A, Gard A, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017;135:1481-9. https://doi.org/10.1161/CIRCULATIONAHA.116.026336.
- Dastidar AG, Rodrigues JC, Johnson TW, De Garate E, Singhal P, Baritussio A, et al. Myocardial infarction with nonobstructed coronary arteries: impact of CMR early after presentation. JACC Cardiovasc Imaging 2017;10:1204-6. https://doi.org/10.1016/j.jcmg.2016.11.010.
- Dastidar AG, Rodrigues JC, Baritussio A, Bucciarelli-Ducci C. MRI in the assessment of ischaemic heart disease. Heart 2016;102:239-52. https://doi.org/10.1136/heartjnl-2014-306963.
- Tornvall P, Gerbaud E, Behaghel A, Chopard R, Collste O, Laraudogoitia E, et al. Myocarditis or ‘true’ infarction by cardiac magnetic resonance in patients with a clinical diagnosis of myocardial infarction without obstructive coronary disease: a meta-analysis of individual patient data. Atherosclerosis 2015;241:87-91. https://doi.org/10.1016/j.atherosclerosis.2015.04.816.
- Wang ZJ, Zhang LL, Elmariah S, Han HY, Zhou YJ. Prevalence and prognosis of nonobstructive coronary artery disease in patients undergoing coronary angiography or coronary computed tomography angiography: a meta-analysis. Mayo Clin Proc 2017;92:329-46. https://doi.org/10.1016/j.mayocp.2016.11.016.
- Chopard R, Jehl J, Dutheil J, Genon VD, Seronde MF, Kastler B, et al. Evolution of acute coronary syndrome with normal coronary arteries and normal cardiac magnetic resonance imaging. Arch Cardiovasc Dis 2011;104:509-17. https://doi.org/10.1016/j.acvd.2011.05.004.
- Gerbaud E, Harcaut E, Coste P, Erickson M, Lederlin M, Labèque JN, et al. Cardiac magnetic resonance imaging for the diagnosis of patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Int J Cardiovasc Imaging 2012;28:783-94. https://doi.org/10.1007/s10554-011-9879-1.
- Heijenbrok-Kal MH, Fleischmann KE, Hunink MG. Stress echocardiography, stress single-photon-emission computed tomography and electron beam computed tomography for the assessment of coronary artery disease: a meta-analysis of diagnostic performance. Am Heart J 2007;154:415-23.
- Bettencourt-Silva J, De La Iglesia B, Donell S, Rayward-Smith V. On creating a patient-centric database from multiple Hospital Information Systems. Methods Inf Med 2012;51:210-20. https://doi.org/10.3414/ME10-01-0069.
- García Álvarez L, Aylin P, Tian J, King C, Catchpole M, Hassall S, et al. Data linkage between existing health-care databases to support hospital epidemiology. J Hosp Infect 2011;79:231-5. https://doi.org/10.1016/j.jhin.2011.06.016.
- Torres V, Cerda M, Knaup P, Löpprich M. Assessment of automatically exported clinical data from a Hospital Information System for clinical research in multiple myeloma. Stud Health Technol Inform 2016;228:332-6.
- Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. ISPOR-SMDM Modeling Good Research Practices Task Force. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 2. Value Health 2012;15:804-11. https://doi.org/10.1016/j.jval.2012.06.016.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Lawrence Erlbaum Associates, Publishers; 1988.
- Rycroft-Malone J. Formal consensus: the development of a national clinical guideline. Qual Health Care 2001;10:238-44. https://doi.org/10.1136/qhc.0100238.
- Tukey JW. Exploratory Data Analysis. Reading, MA: Addison–Wesley; 1977.
- Pufulete M, Harris J, Dorman S, Cook L, Bucciarelli-Ducci C, Greenwood JP, et al. Feasibility of identifying important changes in care management resulting from cardiovascular magnetic resonance (CMR) using Hospital Episode Statistics data. BMC Med Res Methodol 2019.
- Choudry FA, Weerackody RP, Timmis AD, Wragg A, Mathur A, Sporton S, et al. Importance of primary percutaneous coronary intervention for reducing mortality in ST-elevation myocardial infarction complicated by out-of-hospital cardiac arrest. Eur Heart J Acute Cardiovasc Care 2015;4:378-85. https://doi.org/10.1177/2048872614555990.
- Robinson AA, Jain A, Gentry M, McNamara RL. Left ventricular thrombi after STEMI in the primary PCI era: a systematic review and meta-analysis. Int J Cardiol 2016;221:554-9. https://doi.org/10.1016/j.ijcard.2016.07.069.
- Corpus RA, House JA, Marso SP, Grantham JA, Huber KC, Laster SB, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J 2004;148:493-500. https://doi.org/10.1016/j.ahj.2004.03.051.
- van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging 2014;7:930-9. https://doi.org/10.1016/j.jcmg.2014.05.010.
- NHS Health Research Authority . Confidentiality Advisory Group 2018. www.hra.nhs.uk/about-the-hra/our-committees/section-251/ (accessed 16 January 2019).
- National Joint Registry . Patient Consent 2017. www.njrcentre.org.uk/njrcentre/Healthcareproviders/Collectingdata/Patientconsent/tabid/101/Default.aspx (accessed 16 January 2019).
- Inflammatory Bowel Disease (IBD) Registry . Consent and Patient Information Materials 2018. http://ibdregistry.org.uk/consent-materials/ (accessed 16 January 2019).
- Great Britain . National Health Service Act 2006 2006.
- NHS Digital n.d. Obtaining-consent-for-patient-identifiable-and-or-sensitive-data-V2-050613/pdf/obtaining_consent_for_patient_identifiable_and_or_sensitive_data_V2_050613.pdf (accessed April 2018).
- Ipsos Mori . Public Support for Research in the NHS 2011. www.ipsos-mori.com/researchpublications/researcharchive/2811/Public-support-for-research-in-the-NHS.aspx (accessed 16 January 2019).
- Campbell B, Thomson H, Slater J, Coward C, Wyatt K, Sweeney K. Extracting information from hospital records: what patients think about consent. Qual Saf Health Care 2007;16:404-8. https://doi.org/10.1136/qshc.2006.020313.
- Kass NE, Natowicz MR, Hull SC, Faden RR, Plantinga L, Gostin LO, et al. The use of medical records in research: what do patients want?. J Law Med Ethics 2003;31:429-33. https://doi.org/10.1111/j.1748-720X.2003.tb00105.x.
- Xafis V. The acceptability of conducting data linkage research without obtaining consent: lay people’s views and justifications. BMC Med Ethics 2015;16. https://doi.org/10.1186/s12910-015-0070-4.
- Simon SR, Evans JS, Benjamin A, Delano D, Bates DW. Patients’ attitudes toward electronic health information exchange: qualitative study. J Med Internet Res 2009;11. https://doi.org/10.2196/jmir.1164.
- NHS England . NHS England Sets Out the Next Steps of Public Awareness About Care.Data 2013. www.england.nhs.uk/ourwork/tsd/care-data/ (accessed 16 January 2019).
- Goldacre B. The NHS plan to share our medical data can save lives – but must be done right. The Guardian 2014. www.theguardian.com/society/2014/feb/21/nhs-plan-share-medical-data-save-lives (accessed 16 January 2019).
- Williams H, Spencer K, Sanders C, Lund D, Whitley EA, Kaye J, et al. Dynamic consent: a possible solution to improve patient confidence and trust in how electronic patient records are used in medical research. JMIR Med Inform 2015;3. https://doi.org/10.2196/medinform.3525.
- Laurie G, Ainsworth J, Cunningham J, Dobbs C, Jones KH, Kalra D, et al. On moving targets and magic bullets: can the UK lead the way with responsible data linkage for health research?. Int J Med Inform 2015;84:933-40. https://doi.org/10.1016/j.ijmedinf.2015.08.011.
- National Information Board . Personalised Health and Care 2020 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/384650/NIB_Report.pdf (accessed 16 January 2019).
- Justinia T. The UK’s National Programme for IT: why was it dismantled?. Health Serv Manage Res 2017;30:2-9. https://doi.org/10.1177/0951484816662492.
- Gliklich RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes: A User’s Guide [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014.
- Ludman PF. BCIS Audit Returns Adult Interventional Procedures Jan 2014 to Dec 2014 2015. www.bcis.org.uk/wp-content/uploads/2017/01/BCIS-audit-2014.pdf (accessed 16 January 2019).
- NHS England . Diagnostic Imaging Dataset n.d. www.england.nhs.uk/statistics/statistical-work-areas/diagnostic-imaging-dataset/ (accessed 16 January 2019).
- Royal College of Surgeons n.d. www.rcseng.ac.uk/search/#SearchTerm=pre-operative (accessed February 2019).
- Dastidar AG, Ahmed A, McAlindon E, Lawton C, Manghat N, Hamilton M, et al. Incremental value of cardiovascular magnetic resonance imaging in patients surviving non-traumatic out-of-hospital cardiac arrest: a tertiary UK centre experience. Heart 2014;100:A79-80. https://doi.org/10.1136/heartjnl-2014-306118.134.
- Monney PA, Sekhri N, Burchell T, Knight C, Davies C, Deaner A, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart 2011;97:1312-18. https://doi.org/10.1136/hrt.2010.204818.
- Roifman I, Connelly KA, Wright GA, Wijeysundera HC. Echocardiography vs. cardiac magnetic resonance imaging for the diagnosis of left ventricular thrombus: a systematic review. Can J Cardiol 2015;31:785-91. https://doi.org/10.1016/j.cjca.2015.01.011.
- Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J 2007;28:1709-16. https://doi.org/10.1093/eurheartj/ehm184.
- Ruggieri A, Piraino D, Dendramis G, Cortese B, Carella M, Buccheri D, et al. STEMI patients and nonculprit lesions: to treat or not to treat? And when? A review of most recent literature. Catheter Cardiovasc Interv 2015;87:1258-68. https://doi.org/10.1002/ccd.26236.
- El-Hayek GE, Gershlick AH, Hong MK, Casso Dominguez A, Banning A, Afshar AE, et al. Meta-analysis of randomized controlled trials comparing multivessel versus culprit-only revascularization for patients with ST-segment elevation myocardial infarction and multivessel disease undergoing primary percutaneous coronary intervention. Am J Cardiol 2015;115:1481-6. https://doi.org/10.1016/j.amjcard.2015.02.046.
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78-140. https://doi.org/10.1016/j.jacc.2012.11.019.
- Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619. https://doi.org/10.1093/eurheartj/ehs215.
- Great Britain . Data Protection Act 1998 1998.
- Kafka F. Metamorphosis, and other stories. London: Penguin Modern Classics; 1961.
- Emrich T, Emrich K, Abegunewardene N, Oberholzer K, Dueber C, Muenzel T, et al. Cardiac MR enables diagnosis in 90% of patients with acute chest pain, elevated biomarkers and unobstructed coronary arteries. Br J Radiol 2015;88. https://doi.org/10.1259/bjr.20150025.
- Palmer S, Sculpher M, Philips Z, Robinson M, Ginnelly L, Bakhai A, et al. A Cost-Effectiveness Model Comparing Alternative Management Strategies for the Use of Glycoprotein IIbIIIa Antagonists in Non-ST-Elevation Acute Coronary Syndrome. Report to the National Institute for Clinical Excellence 2002.
- Daniel M, Agewall S, Caidahl K, Collste O, Ekenbäck C, Frick M, et al. Effect of myocardial infarction with nonobstructive coronary arteries on physical capacity and quality of life. Am J Cardiol 2017;120:341-6. https://doi.org/10.1016/j.amjcard.2017.05.001.
- Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum 2011;41:95-105. https://doi.org/10.1016/j.semarthrit.2010.12.001.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2.
- Hutchings A, Raine R. A systematic review of factors affecting the judgments produced by formal consensus development methods in health care. J Health Serv Res Policy 2006;11:172-9. https://doi.org/10.1258/135581906777641659.
- Regulation (EU) 2016/679 of the European Parliament and of the Council n.d. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32016R0679&from=EN (accessed January 2019).
- European Commission . Data Protection n.d. https://ec.europa.eu/info/law/law-topic/data-protection_en (accessed January 2019).
- NHS Digital . Data Access Request Service (DARS) n.d. https://digital.nhs.uk/services/data-access-request-service-dars (accessed 16 January 2019).
- Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol 2012;59:1799-808. https://doi.org/10.1016/j.jacc.2012.01.037.
- Eitel I, Desch S, de Waha S, Fuernau G, Gutberlet M, Schuler G, et al. Long-term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart 2011;97:2038-45. https://doi.org/10.1136/heartjnl-2011-300098.
- Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, et al. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol 2010;55:2470-9. https://doi.org/10.1016/j.jacc.2010.01.049.
- Klug G, Mayr A, Schenk S, Esterhammer R, Schocke M, Nocker M, et al. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14. https://doi.org/10.1186/1532-429X-14-46.
- Wong TC, Piehler K, Puntil KS, Moguillansky D, Meier CG, Lacomis JM, et al. Effectiveness of late gadolinium enhancement to improve outcomes prediction in patients referred for cardiovascular magnetic resonance after echocardiography. J Cardiovasc Magn Reson 2013;15. https://doi.org/10.1186/1532-429X-15-6.
- Parsai C, O’Hanlon R, Prasad SK, Mohiaddin RH. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson 2012;14. https://doi.org/10.1186/1532-429x-14-54.
- Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2012;5:370-7. https://doi.org/10.1016/j.jcmg.2011.11.021.
- Artis NJ, Thomson J, Plein S, Greenwood JP. Percutaneous closure of postinfarction ventricular septal defect: cardiac magnetic resonance-guided case selection and postprocedure evaluation. Can J Cardiol 2011;27:869.e3-5. https://doi.org/10.1016/j.cjca.2011.01.018.
- Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004;110:3760-5. https://doi.org/10.1161/01.CIR.0000150390.04704.B7.
- Joshi SB, Connelly KA, Jimenez-Juan L, Hansen M, Kirpalani A, Dorian P, et al. Potential clinical impact of cardiovascular magnetic resonance assessment of ejection fraction on eligibility for cardioverter defibrillator implantation. J Cardiovasc Magn Reson 2012;14. https://doi.org/10.1186/1532-429X-14-69.
- Alexandre J, Saloux E, Dugué AE, Lebon A, Lemaitre A, Roule V, et al. Scar extent evaluated by late gadolinium enhancement CMR: a powerful predictor of long-term appropriate ICD therapy in patients with coronary artery disease. J Cardiovasc Magn Reson 2013;15. https://doi.org/10.1186/1532-429X-15-12.
- Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F, et al. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2011;13. https://doi.org/10.1186/1532-429x-13-29.
- Leclercq C, Kass DA. Retiming the failing heart: principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol 2002;39:194-201. https://doi.org/10.1016/S0735-1097(01)01747-8.
- Bilchick KC, Dimaano V, Wu KC, Helm RH, Weiss RG, Lima JA, et al. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging 2008;1:561-8. https://doi.org/10.1016/j.jcmg.2008.04.013.
- Ellims AH, Pfluger H, Elsik M, Butler MJ, Hare JL, Taylor AJ. Utility of cardiac magnetic resonance imaging, echocardiography and electrocardiography for the prediction of clinical response and long-term survival following cardiac resynchronisation therapy. Int J Cardiovasc Imaging 2013;29:1303-11. https://doi.org/10.1007/s10554-013-0215-9.
- Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J 2007;153:105-12. https://doi.org/10.1016/j.ahj.2006.10.015.
- White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol 2006;48:1953-60. https://doi.org/10.1016/j.jacc.2006.07.046.
- Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol 2013;62:826-38. https://doi.org/10.1016/j.jacc.2013.03.080.
- Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453-60. https://doi.org/10.1016/S0140-6736(11)61335-4.
- Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, et al. Superior diagnostic performance of perfusion-cardiovascular magnetic resonance versus SPECT to detect coronary artery disease: the secondary endpoints of the multicenter multivendor MR-IMPACT II (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial). J Cardiovasc Magn Reson 2012;14. https://doi.org/10.1186/1532-429X-14-61.
- Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation 1999;99:763-70. https://doi.org/10.1161/01.CIR.99.6.763.
- Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart 2012;98:1743-9. https://doi.org/10.1136/heartjnl-2012-301962.
- Weinsaft JW, Kim RJ, Ross M, Krauser D, Manoushagian S, LaBounty TM, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009;2:969-79. https://doi.org/10.1016/j.jcmg.2009.03.017.
- Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006;152:75-84. https://doi.org/10.1016/j.ahj.2005.08.021.
- Wong DT, Leung MC, Das R, Liew GY, Williams K, Dundon BK, et al. Diagnostic accuracy of adenosine stress cardiovascular magnetic resonance following acute ST-segment elevation myocardial infarction post primary angioplasty. J Cardiovasc Magn Reson 2011;13. https://doi.org/10.1186/1532-429X-13-62.
- Doesch C, Seeger A, Doering J, Herdeg C, Burgstahler C, Claussen CD, et al. Risk stratification by adenosine stress cardiac magnetic resonance in patients with coronary artery stenoses of intermediate angiographic severity. JACC Cardiovasc Imaging 2009;2:424-33. https://doi.org/10.1016/j.jcmg.2008.11.017.
- Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2462-508. https://doi.org/10.1161/CIR.0b013e3181d44a8f.
- Hoffmann U, Globits S, Schima W, Loewe C, Puig S, Oberhuber G, et al. Usefulness of magnetic resonance imaging of cardiac and paracardiac masses. Am J Cardiol 2003;92:890-5. https://doi.org/10.1016/S0002-9149(03)00911-1.
- Chan PG, Smith MP, Hauser TH, Yeon SB, Appelbaum E, Rofsky NM, et al. Noncardiac pathology on clinical cardiac magnetic resonance imaging. JACC Cardiovasc Imaging 2009;2:980-6. https://doi.org/10.1016/j.jcmg.2009.04.014.
- McKenna DA, Laxpati M, Colletti PM. The prevalence of incidental findings at cardiac MRI. Open Cardiovasc Med J 2008;2:20-5. https://doi.org/10.2174/1874192400802010020.
- Wyttenbach R, Médioni N, Santini P, Vock P, Szucs-Farkas Z. Extracardiac findings detected by cardiac magnetic resonance imaging. Eur Radiol 2012;22:1295-302. https://doi.org/10.1007/s00330-011-2369-y.
Appendix 1 Participant consent form
Appendix 2 Office of Population Censuses and Surveys and International Classification of Diseases, Tenth Edition, codes list
| OPCS procedure diagnosis codes | |
|---|---|
| CMR | |
| U10.3 | Cardiac magnetic resonance imaging |
| PCI | |
| K49 | Transluminal balloon angioplasty of coronary artery |
| K50 | Other therapeutic transluminal operations on coronary artery |
| K75 | Percutaneous transluminal balloon angioplasty and insertion of stent into coronary artery |
| CABG | |
| K40 | Saphenous vein graft replacement of coronary artery |
| K41 | Other autograft replacement of coronary artery |
| K42 | Allograft replacement of coronary artery |
| K43 | Prosthetic replacement of coronary artery |
| K44 | Other replacement of coronary artery |
| K45 | Connection of thoracic artery to coronary artery |
| K46 | Other bypass of coronary artery |
| Angiography, no PCI | |
| K63.3 | Angiocardiography of left side of heart NEC |
| K63.5 | Coronary arteriography using single catheter |
| K63.6 | Coronary arteriography NEC |
| PET | |
| U10.4 | Myocardial positron emission tomography |
| U21.3 | Positron emission tomography NEC |
| U36.2 | Positron emission tomography with computed tomography NEC |
| ECHO | |
| K58.5 | Transluminal intracardiac echocardiography |
| U20.1 | Transthoracic echocardiography |
| U20.2 | Transoesophageal echocardiography |
| U20.3 | Intravascular echocardiography |
| U20.4 | Epicardial echocardiography |
| U20.5 | Stress echocardiography |
| IVUS | |
| K51.2 | Intravascular ultrasound of coronary artery |
| L726 | Intravascular ultrasound of artery NEC |
| Pressure wire | |
| K63.4- K63.6 AND | Coronary arteriography |
| K51.8 AND | Other specified diagnostic transluminal operations on coronary artery |
| Y44.2 AND | Monitoring of pressure in organ NOC |
| Y53.- | Approach to organ under image control |
| Radionuclide angiocardiography | |
| U10.5 | Radionuclide angiocardiography |
| Computed tomography angiography | |
| U10.2 | Cardiac computed tomography angiography |
| Single photon emission computed tomography | |
| U21.4 | Single photon emission computed tomography NEC |
| Implantation of ICD | |
| K59 | Cardioverter defibrillator introduced through the vein |
| K72 | Other cardioverter defibrillator |
| Implantation of CRT | |
| K60.7 | Implantation of intravenous biventricular cardiac pacemaker system |
| K61.7 | Implantation of biventricular cardiac pacemaker system |
| K59.6 | Implantation of cardioverter defibrillator using three electrode leads |
| ICD-10 diagnosis codes | |
| OHCA | |
| I46 | Cardiac arrest |
| LV thrombus | |
| I23.6 | Thrombosis of atrium, auricular appendage and ventricle as current complications following acute myocardial infarction |
| STEMI | |
| I21.0 | Acute transmural myocardial infarction of anterior wall |
| I21.1 | Acute transmural myocardial infarction of inferior wall |
| I21.2 | Acute transmural myocardial infarction of other sites |
| I21.3 | Acute transmural myocardial infarction of unspecified site |
| Takotsubo cardiomyopathy | |
| I42.8 AND | Other cardiomyopathies |
| F43.8 | Other reactions to severe stress |
| Myocarditis | |
| I51.4 | Myocarditis, unspecified |
| Pericarditis | |
| I30 | Acute pericarditis |
| I31.0 | Chronic adhesive pericarditis |
| I31.1 | Chronic constrictive pericarditis |
| I31.9 | Disease of pericardium, unspecified |
| I32.0 | Pericarditis in bacterial diseases classified elsewhere |
| I32.1 | Pericarditis in other infectious and parasitic diseases classified elsewhere |
| I32.8 | Pericarditis in other diseases classified elsewhere |
| I01.0 | Acute rheumatic pericarditis |
| I02.0 | Rheumatic chorea with heart involvement |
| I09.2 | Chronic rheumatic pericarditis |
| Endocarditis | |
| I33.9 | Acute endocarditis, unspecified |
| Coronary spasm | |
| I20.1 | Angina pectoris with documented spasm |
| MI | |
| I21 | Acute myocardial infarction |
| Stroke | |
| I64 | Stroke, not specified as haemorrhage or infarction |
Appendix 3 Literature search
MEDLINE (via OvidSP)
Date range searched: 1950 to 16 January 2014.
Date searched: 16 January 2014.
Search strategy
-
exp Myocardial Infarction/ (142,973)
-
myocardial infarct$.tw. (134,547)
-
heart attack$.tw. (3601)
-
MI.tw. (25,881)
-
ami.tw. (12,166)
-
stemi.tw. (3663)
-
heart infarct$.tw. (705)
-
Acute Coronary Syndrome/ (6784)
-
acute coronary syndrome$.tw. (15,521)
-
ACS.tw. (9320)
-
or/1-10 (210,783)
-
exp angioplasty/ (53,568)
-
angioplasty.tw. (34,003)
-
percutaneous coronary intervention$.tw. (14,406)
-
exp Percutaneous Coronary Intervention/ (34,668)
-
PCI.tw. (11,157)
-
PPCI.tw. (366)
-
exp Stents/ (50,784)
-
stent$.tw. (56,427)
-
Myocardial Revascularization/ (9079)
-
revasculari$.tw. (37,853)
-
reperfused.tw. (5688)
-
reperfusion.tw. (55,775)
-
or/12-23 (191,056)
-
exp Magnetic Resonance Imaging/ (295,774)
-
Magnetic resonance imag$.tw. (122,420)
-
Cardiovascular magnetic resonance.tw. (1765)
-
cardiac magnetic resonance.tw. (3179)
-
MRI.tw. (121,901)
-
CMR.tw. (3035)
-
or/25-30 (344,849)
-
24 and 31 (5713)
-
11 and 32 (1318)
-
exp animals/ not humans/ (3,863,199)
-
33 not 34 (1051)
EMBASE
Date range searched: 1980 to 16 January 2014.
Date searched: 16 January 2014.
Search strategy
-
exp heart infarction/ (261,299)
-
myocardial infarct$.tw. (185,732)
-
heart attack$.tw. (4991)
-
MI.tw. (45,694)
-
ami.tw. (19,697)
-
stemi.tw. (10,463)
-
heart infarct$.tw. (1450)
-
exp Acute Coronary Syndrome/ (26,244)
-
acute coronary syndrome$.tw. (28,004)
-
ACS.tw. (17,708)
-
or/1-10 (337,561)
-
exp angioplasty/ (67,762)
-
angioplasty.tw. (47,179)
-
percutaneous coronary intervention$.tw. (27,203)
-
exp Percutaneous Coronary Intervention/ (59,160)
-
PCI.tw. (26,664)
-
PPCI.tw. (1281)
-
Stent/ (64,591)
-
exp cardiovascular stent/ (34,380)
-
stent$.tw. (94,492)
-
heart muscle revascularization/ (21,848)
-
revasculari$.tw. (56,445)
-
reperfused.tw. (7304)
-
reperfusion.tw. (77,453)
-
or/12-24 (298,341)
-
exp nuclear magnetic resonance imaging/ (524,106)
-
Magnetic resonance imag$.tw. (164,672)
-
Cardiovascular magnetic resonance.tw. (3414)
-
cardiac magnetic resonance.tw. (6341)
-
MRI.tw. (203,793)
-
CMR.tw. (7673)
-
or/26-31 (563,445)
-
25 and 32 (13,408)
-
11 and 33 (3451)
-
(exp animal/ or nonhuman/) not exp humans/ (5,173,875)
-
34 not 35 (3048)
-
limit 36 to embase (2864)
The Cochrane Library
Date range searched: 1950 to 16 January 2014.
Date searched: 16 January 2014.
Search strategy
-
#1 MeSH descriptor: [Myocardial Infarction] explode all trees
-
#2 MeSH descriptor: [Acute Coronary Syndrome] explode all trees
-
#3 myocardial next infarct*
-
#4 heart next attack*
-
#5 MI
-
#6 AMI
-
#7 STEMI
-
#8 heart next infarct*
-
#9 ‘acute coronary syndrome*’
-
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
-
#11 MeSH descriptor: [Angioplasty] explode all trees
-
#12 MeSH descriptor: [Percutaneous Coronary Intervention] explode all trees
-
#13 MeSH descriptor: [Stents] explode all trees
-
#14 MeSH descriptor: [Myocardial Revascularization] this term only
-
#15 angioplasty
-
#16 ‘percutaneous coronary intervention*’
-
#17 PCI or PPCI
-
#18 stent*
-
#19 revasculari*
-
#20 reperfused
-
#21 reperfusion
-
#22 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21
-
#23 MeSH descriptor: [Magnetic Resonance Imaging] explode all trees
-
#24 ‘Magnetic resonance imag*’
-
#25 ‘Cardiovascular magnetic resonance’
-
#26 ‘cardiac magnetic resonance’
-
#27 MRI or CMR
-
#28 #23 or #24 or #25 or #26 or #27
-
#29 #11 and #22 and #28
Web of Science
Date range searched: 1950 to 16 January 2014.
Date searched: 16 January 2014.
Search strategy
-
# 4 1,351 #3 AND #2 AND #1
-
# 3 218,389 TOPIC: (Angioplasty or “percutaneous coronary intervention*” or PCI or PPCI or stent* or revasulari* or reperfused or reperfusion)
-
# 2 257,465 TOPIC: (“Magnetic Resonance Imag*” or “Cardiovascular magnetic resonance” or “ cardiac magnetic resonance” or MRI or CMR)
-
# 1 273,481 TOPIC: (“myocardial infarct*” or “heart attack*” or “heart infarct*” or “acute coronary syndrome*” or MI or AMI or STEMI or ACS)
Bioscience Information Service (BIOSIS)
Date range searched: 1950 to 16 January 2014.
Date searched: 16 January 2014.
Search strategy
-
# 6 873 #5 AND #4
-
# 5 TAXONOMIC DATA: (human not (animal* not human))
-
# 4 #3 AND #2 AND #1
-
# 3 TOPIC: (Angioplasty or “percutaneous coronary intervention*” or PCI or PPCI or stent* or revasulari* or reperfused or reperfusion)
-
# 2 TOPIC: (“Magnetic Resonance Imag*” or “Cardiovascular magnetic resonance” or “ cardiac magnetic resonance” or MRI or CMR)
-
# 1 TOPIC: (“myocardial infarct*” or “heart attack*” or “heart infarct*” or “acute coronary syndrome*” or MI or AMI or STEMI or ACS)
Appendix 4 First survey (statements, supporting paragraphs and references)
Benefit to patients and the NHS of cardiac magnetic resonance imaging after primary percutaneous coronary intervention pathway activation
Thank you for agreeing to complete this survey. We expect the survey to take about 20 minutes of your time. You are asked to read and rate 12 statements relating to the potential impact of CMR in changing the management of patients in a clinically important way. The patient population under consideration includes all patients who activate the PPCI pathway. Please rate your agreement with each statement on the scale provided. Each statement has a supporting paragraph that provides background information and links to full-text references. We believe that we have used the best available evidence but we are aware that the evidence is of variable quality.
In order to progress through the survey, please use the following navigation buttons:
Click the Next button to go to the next page.
Click the Previous button to return to the previous page.
Click the Number buttons in the text to access the full references.
Click the Submit button to submit the survey at the end.
Statement 1. Compared with echocardiography, cardiovascular magnetic resonance after primary percutaneous coronary a intervention allows patients with cardiovascular magnetic resonance markers that indicate a poor prognosis (e.g. impaired left ventricular function, large infarct size, microvascular obstruction) to be followed up more appropriately and undergo more aggressive medical therapy for secondary prevention
Accurate assessment of infarct characteristics is important for risk stratification after PPCI. CMR can quantify in a single scan all cardiac markers relevant to PPCI outcomes, with high reproducibility and accuracy. CMR has high spatial and temporal resolution and is superior to ECHO for measuring LV volumes and EF. 112 Additionally, CMR markers, such as infarct size, MVO and myocardial salvage, have been shown to have long-term prognostic value. 113–115 LGE CMR has added prognostic value over ECHO. 116
Statement 2. Compared with echocardiography, cardiovascular magnetic resonance after primary percutaneous coronary intervention allows patients with cardiovascular magnetic resonance markers that indicate a good prognosis (e.g. normal left ventricular function, high myocardial salvage, no microvascular obstruction, no residual ischaemia) to be discharged earlier and followed up less frequently
Cardiovascular magnetic resonance measures several markers (i.e. infarct size, myocardial salvage index, MVO, myocardial oedema and haemorrhage) that cannot be measured by ECHO. These markers can be used to predict cardiac remodelling and prognosis after MI,113–115 determine the optimal therapeutic pathway for each patient and prevent over- and under-treatment.
Statement 3. Compared with echocardiography, cardiovascular magnetic resonance better identifies the cause of out-of-hospital cardiac arrest (e.g. large myocardial infarction, arrhythmogenic right ventricular cardiomyopathy, aberrant coronary arteries, hypertrophic cardiomyopathy) to optimise further treatment for the patient (e.g. defibrillator for primary arrhythmia or percutaneous coronary intervention) or family members
Out-of-hospital cardiac arrest affects about 60,000 people in the UK each year. Currently, hospital survival is about 32%. Early identification of the cause is essential to improve survival. Causes of OHCA include MI (40–90%) and inherited cardiomyopathies, such as arrhythmogenic right ventricular cardiomyopathy (ARVC) and hypertrophic cardiomyopathy (HCM). Unlike ECHO, CMR allows in vivo tissue characterisation, which differentiates scarring resulting from MI from other causes of focal fibrosis (e.g. observed in non-ischaemic cardiomyopathies such as ARVC and HCM). 117 LGE on CMR can predict serious cardiac complications in patients with HCM (e.g. all-cause death, cardiac death and death from heart failure),118 and identifies patients who may need more aggressive medical and device therapy (e.g. renin-aldosterone system inhibition for prevention of heart failure or ICD placement for primary prevention of sudden cardiac death).
Statement 4. Cardiovascular magnetic resonance after primary percutaneous coronary intervention identifies patients at high risk of having ventricular septal defect or impending cardiac rupture, who may require ventricular patch or other urgent cardiac surgery, and guides the optimal management of these patients
Ventricular septal defect (VSD) and LV free wall rupture are rare complications of MI, occurring in < 1% of PPCI patients, usually within 1 week of the infarct. Mortality ranges between 50% and 80%. Diagnosis can be difficult to make and usually requires multimodality imaging (e.g. ECHO, ventriculography, CT, CMR) before surgical repair. Because of its high spatial resolution, CMR can be used to clarify the detailed structure of these lesions. CMR accurately identifies the location, size and tissue margins of VSD and is useful for detecting apical defects, which are not easily identified by ECHO. CMR measurements may also be used to determine the size of the ventricular patch required to close the VSD, which avoids the inflation of a sizing balloon in friable infarcted tissue. 119 These features, together with other CMR markers of damage (e.g. infarct size, MVO, LV dysfunction) are useful for guiding optimal management of patients with post-infarct VSD. Similarly, CMR can differentiate between the different types of impending free wall rupture (i.e. acute, subacute and chronic), which may be helpful in planning surgical management. 119
Statement 5. In patients with a ‘normal’ (unobstructed) coronary angiogram, cardiovascular magnetic resonance can differentiate patients who have had a myocardial infarction with spontaneous reperfusion or distal embolisation from patients with a non-ischaemic diagnosis (e.g. myocarditis, Takotsubo cardiomyopathy, aortic dissection), resulting in a patient treatment plan appropriate for the definitive diagnosis
The incidence of unobstructed coronary angiogram in patients who activate the PPCI pathway is between 5% and 12%. In these patients, the lack of an accurate diagnosis may result in inappropriate or unnecessary treatment and/or follow-up and a poorer prognosis. 19 CMR facilitates differential diagnosis in the context of an unobstructed coronary angiogram, providing a definitive diagnosis (e.g. myocardial infarction, myocarditis, Takotsubo cardiomyopathy) in 65–90% of these patients. 20,23 In patients with myocarditis, LGE on CMR may predict long-term adverse outcomes. 26 In the context of MI without an angiographic lesion, CMR can locate the culprit infarct-related artery in patients with spontaneous reperfusion or with distal embolisation.
Statement 6. Compared with echocardiography, cardiovascular magnetic resonance after primary percutaneous coronary intervention better identifies patients at high risk of sudden cardiac death who would benefit most from an implantable cardiac device (e.g. implantable cardiac defibrillator, cardiac resynchronisation therapy)
About 6% of heart attack patients subsequently die suddenly from a presumed cardiac cause. Current guidelines recommend the use of ICD to prevent sudden cardiac death in patients who have a low LV EF after a heart attack. EF is used in clinical practice to make decisions about ICD implantation, but it has a low predictive value and many patients with an ICD will never benefit from it. 120 EF is most commonly measured by ECHO, but CMR is now considered the gold standard for EF measurement because it is more reproducible than ECHO. 112 CMR has been shown to be better than ECHO for selecting patients for ICD implantation when strict EF thresholds are used to guide implantation. 121 The extent of myocardial scar characterised by LGE CMR may also be used to predict whether or not ICD implantation is appropriate in this patient group. 122 Furthermore, LGE CMR can guide placement of the LV lead away from scarred myocardium, which results in a better clinical outcome after cardiac resynchronisation therapy (CRT). 123
Statement 7. Compared with echocardiography, cardiovascular magnetic resonance after primary percutaneous coronary intervention better identifies patients who would not benefit from cardiac resynchronisation therapy
Cardiac resynchronisation therapy (or biventricular pacing) uses a specialised pacemaker to resynchronise the beating of the two ventricles by pacing both simultaneously, which improves the contraction of the left ventricle and the overall efficiency of the heart. It is used in patients with systolic ventricular dysfunction and heart failure. However, about 30% of patients who meet the inclusion criteria for CRT do not respond to it. 124 CMR assessment of mechanical dyssynchrony and myocardial scar provides additional value over ECHO for identifying responsive patients. 125,126 Myocardial scar is an important feature of non-response to CRT127,128 and LGE CMR accurately differentiates between transmural, mid-myocardial, epicardial and subendocardial scar.
Statement 8. Compared with stress echocardiography or single-photon emission computed tomography, cardiovascular magnetic resonance after primary percutaneous coronary intervention in patients with multivessel disease better assesses ischaemia and viability of the myocardium to optimise the revascularisation strategy for the patient and avoid additional diagnostic tests
Between 40% and 65% of the patients who activate the PPCI pathway have multivessel disease. Adequate assessment of residual ischaemia in non-culprit arteries post PPCI is important for effective management because this patient group has an adverse prognosis. Various techniques are currently used to assess the need for additional revascularisation, including stress ECHO, SPECT and stress-perfusion CMR. Stress CMR has excellent prognostic value129 and better diagnostic accuracy than SPECT130,131 or stress ECHO132 for detecting angiographically significant coronary artery disease.
Statement 9. Compared with echocardiography, cardiovascular magnetic resonance after primary percutaneous coronary intervention better identifies patients with left ventricular thrombus for treatment with anticoagulation therapy
Left ventricular thrombus is a serious complication of acute MI. It increases the risk of thromboembolic events, particularly stroke. LV thrombus develops in up to 10% of patients with anterior wall infarctions after PPCI. Although ECHO is most commonly used to detect LV thrombus and to assess its shape and size, between 10% and 46% of echocardiograms are inconclusive. 133 LGE CMR is considered the gold standard for detecting LV thrombus, because it detects thrombus based on tissue characteristics rather than anatomic appearance. Fewer thrombi are detected by contrast-ECHO than by LGE CMR. 134 CMR has a higher sensitivity (88%) than contrast (61%) and non-contrast (< 33%) ECHO. 134,135
Statement 10. Compared with other imaging modalities, in patients with multivessel disease, cardiovascular magnetic resonance after primary percutaneous coronary intervention better identifies the artery that caused the myocardial infarction and guides the subsequent treatment plan in relation to additional revascularisation (percutaneous coronary intervention or coronary artery bypass graft surgery)
In patients with multivessel disease presenting with acute MI, the culprit artery is not always easy to identify as more than one artery could be responsible. CMR has higher spatial resolution than SPECT, allowing easy identification of the infarct-related artery and non-culprit territory stenosis in PPCI patients,136 and, hence, appropriate risk-stratification of patients with multivessel disease for planning their future treatment. 137
Statement 11. Compared with echocardiography, cardiovascular magnetic resonance after primary percutaneous coronary intervention better identifies incidental cardiac findings that may need further investigation and treatment
Compared with ECHO, CMR provides versatile imaging planes, superior tissue contrast and advanced tissue characterisation, allowing a comprehensive assessment of cardiac anatomy, function and flow, and imaging of the great vessels (including venous return), the pericardium and suspected cardiac tumours. 138 As such, CMR can identify congenital coronary anomalies, cardiac masses, coronary artery aneurysms, valvular heart disease, thoracic aortic disease, and so forth. CMR can adequately differentiate benign from malignant tumours in the heart. 139
Statement 12. Compared with echocardiography, cardiovascular magnetic resonance after primary percutaneous coronary intervention identifies significant incidental non-cardiac findings (e.g. oesophageal and lung tumours, pulmonary embolus, aortic aneurysm) that may need further investigation and treatment
Unlike ECHO, which provides limited imaging of mediastinal and extra-cardiac structures, CMR images a substantial part of the thorax and abdomen in the field of view, which may potentially contain non-cardiac abnormalities. The prevalence of non-cardiac findings on CMR is up to 80% (with up to 30% of these representing potentially significant findings), depending on the characteristics of the population examined. 140–142
Appendix 5 Cardiovascular magnetic resonance questionnaire for cardiologists
Appendix 6 Recorded causes of death for deaths within 1 year of index admission
| Cause of death | n (%) |
|---|---|
| Acute MI, unspecified | 14 (26) |
| Acute or chronic renal failure | 1 (2) |
| Aortic (valve) stenosis | 1 (2) |
| Atrial fibrillation and flutter | 1 (2) |
| Bronchopneumonia | 1 (2) |
| Cardiogenic shock | 1 (2) |
| Chronic ischaemic heart disease, unspecified | 5 (9) |
| Community-acquired pneumonia | 1 (2) |
| Congestive cardiac failure | 2 (4) |
| Heart failure | 1 (2) |
| Intentional self-harm by hanging, strangulation and suffocation | 1 (2) |
| Intentional self-harm by other specified means | 1 (2) |
| Intercerebral bleed | 1 (2) |
| Ischaemic bowel | 1 (2) |
| Ischaemic cardiomyopathy | 2 (4) |
| Ischaemic colitis | 1 (2) |
| Ischaemic heart disease | 2 (4) |
| Malignant neoplasm of bronchus and lung | 3 (6) |
| Malignant neoplasm of other and unspecified parts of mouth | 1 (2) |
| Metastatic adenocarcinoma of lung | 1 (2) |
| Multiple myeloma | 1 (2) |
| MI | 4 (7) |
| Other interstitial pulmonary diseases with fibrosis | 1 (2) |
| Pulmonary embolism | 1 (2) |
| Pulmonary embolism without mention of acute cor pulmonale | 1 (2) |
| Severe LV failure | 1 (2) |
| Small-cell lung cancer | 1 (2) |
| Squamous cell carcinoma of the lung | 1 (2) |
| Urinary tract infection, site not specified | 1 (2) |
| Total | 54 |
Appendix 7 Supporting tables for consensus process
| Number | Statement |
|---|---|
| Coronary artery disease positive | |
| 1 | Multivessel disease – CMR assessment of ischaemia and viability to guide further PCI either as inpatient or outpatient |
| 2 | Multivessel disease – CMR assessment of ischaemia and viability to guide CABG |
| 3 | PPCI with discordance between ECG and angiographic findings? New/old infarct? Territory of infarct? Was the culprit artery successfully treated? |
| 4 | Chronic multivessel disease with no clear culprit lesion? Clarify diagnosis/guide further therapy |
| 5 | Assess the significance of bystander coronary artery disease (which will influence the need of further elective PCI to other vessels) |
| 6 | CMR can differentiate between acute and chronic MI (important when the cause of cardiac dysfunction is unclear despite angiography or ultrasound) |
| 7 | In patients with multivessel disease in whom the culprit artery cannot be identified, CMR can identify the infarct-related artery and guide (1) targeted PCI and (2) viability of CABG |
| 8 | Assessment of LV function to guide AICD implantation (primary prevention) |
| 9 | CMR is more effective than ECHO at identifying STEMI patients who are at risk of sudden arrhythmic death (consequence: early implantation of a cardioverter defibrillator?) |
| 10 | OHCA with ambiguous anatomy? Primary arrhythmia or acute MI – defibrillation or revascularisation? |
| 11 | Assessment of LV thrombus to guide possible anticoagulation |
| 12 | CMR can accurately diagnose LV thrombus (unlike ECHO) (consequence: possible anti-coagulation therapy with vitamin K antagonist?) |
| 13 | Assessment of pericardial effusion |
| 14 | CMR markers: MVO, oedema, scar size, haemorrhage, etc. Good prognostic indicators but no/little current evidence that they are used to change patient management at the moment |
| 15 | Impact on the length of stay (if CMR does not show very poor EF, very large area of MVO, impending cardiac rupture) |
| 16 | CMR can accurately diagnose LV thrombus (consequence: possible anti-coagulation therapy with vitamin K antagonist?) |
| 17 | CMR can identify impending cardiac rupture (consequence: cardiac operation or ventricular patch if needed) |
| 18 | More aggressive medical treatment in those with large infarcts and large MVO? |
| 19 | Closer medical follow-ups in those with large infarcts and large MVO? |
| 20 | Potential to change/optimise/maximise pharmacological treatment |
| 21 | Assessment of LV function to guide more aggressive medical therapy/secondary prevention |
| 22 | CMR has prognostic importance – follow-up patients in clinic more or less often depending on how good or bad the CMR is. Secondary prevention needed? |
| 23 | Identify complications of acute MI (e.g. impending cardiac rupture and act on it, e.g. surgery) |
| Coronary artery disease negative | |
| 24 | In STEMI patients with atypical angiographic characteristics (in whom the culprit artery cannot be identified) CMR may identify the MI location and infarct-related artery (consequence: revascularisation of appropriate artery) |
| 25 | PPCI with ‘normal’ coronaries – is it infarct with spontaneous reperfusion? |
| 26 | PPCI with ‘normal’ coronaries – is it myopericarditis? |
| 27 | PPCI with ‘normal’ coronaries – is it cardiomyopathy (dilated, hypertrophic, Takotsubo, etc.)? |
| 28 | In patients with chest pain, elevated troponins and unobstructed coronary arteries, CMR can rule out any significant pathology (e.g. patients with spontaneous reperfusion) (consequence: early discharge? Any follow-up for these patients?) |
| 29 | Equivocal ST elevation and angiographic findings? Alternative diagnosis e.g. pulmonary emobolus, aortic dissection |
| 30 | PPCI with ‘normal’ coronaries – impact on the length of stay (early discharge) |
| 31 | PPCI with ‘normal’ coronaries – impact on medical treatment and no secondary prevention medications |
| 32 | PPCI with ‘normal’ coronaries – impact on the patients’ quality of life (of having this diagnosis as opposed to the diagnosis of a MI) (impact on insurance, mortgage, etc.) |
| 33 | Impact on the length of hospital stay (shorter bed stay if CMR does not show major abnormalities) |
| Incidental findings | |
| 34 | Assessment of other incidental valvular heart disease findings |
| 35 | Assessment of mitral regurgitation – is it ischaemic, dilated annulus, native valve disease? |
| 36 | Incidental findings on CMR (e.g. valve disease, congenital anomalies) that will be subsequently treated/monitored? |
| 37 | Potential for CMR to be a ‘one-stop shop’ for imaging procedures |
| Subgroup | Draft statements | Reworded statements |
|---|---|---|
| Unobstructed arteries on angiogram | PPCI with ‘normal’ coronaries – is it infarct with spontaneous reperfusion? | In patients with a normal coronary angiogram, CMR can identify a heart attack with spontaneous reperfusion or distal embolisation for treatment with medical therapy for secondary prevention |
| Equivocal ST elevation and angiographic findings? Alternative diagnosis, for example pulmonary embolus, aortic dissection? | ||
| PPCI with ‘normal’ coronaries – is it cardiomyopathy (e.g. dilated, hypertrophic, Takotsubo) | In patients with a normal coronary angiogram, CMR can provide a new diagnosis (e.g. myocarditis, Takotsubo cardiomyopathy, aortic dissection) resulting in a different patient treatment plan | |
| PPCI with ‘normal’ coronaries – is it myopericarditis? | ||
| Multivessel disease | Multivessel disease – CMR assessment of ischaemia and viability to guide further PCI either as inpatient or outpatient | In patients with multivessel disease, CMR after PPCI assesses ischaemia and viability of the heart tissue to optimise the treatment for the patient |
| Assess the significance of bystander coronary artery disease (which will influence the need of further elective PCI to other vessels) | In patients with multivessel disease, CMR after angiography identifies the artery that caused the heart attack, and guides the appropriate therapy (PCI or CABG surgery) | |
| Chronic multivessel disease with no clear culprit lesion? Clarify diagnosis/guide further therapy | ||
| LV thrombus after MI | Assessment of LV thrombus to guide possible anticoagulation | CMR after PPCI clearly identifies patients with LV thrombus for treatment with anticoagulation therapy |
| CMR can accurately diagnose LV thrombus (unlike ECHO) |
| Statement 5 | The incidence of normal coronary angiogram in patients who activate the PPCI pathway is between 5% and 12%. In these patients, the lack of an accurate diagnosis may result in inappropriate or unnecessary treatment and/or follow-up and poorer prognosis.19 CMR facilitates differential diagnosis in the context of a normal coronary angiogram, providing a definitive diagnosis (e.g. MI, myocarditis, Takotsubo cardiomyopathy) in 65–90% of these patients.20,23 In patients with myocarditis, LGE on CMR may predict long-term adverse outcomes.20,23 In the context of MI without an angiographic lesion, CMR can locate the culprit infarct-related artery in patients with spontaneous reperfusion or with distal embolisation |
| Statement 8 | Between 40% and 65% of the patients who activate the PPCI pathway have multivessel disease. Adequate assessment of residual ischaemia in non-culprit arteries post PPCI is important for effective management because this patient group has an adverse prognosis. Various techniques are currently used to assess the need for additional revascularisation, including stress ECHO, SPECT and stress-perfusion CMR. Stress CMR has excellent prognostic value129 and better diagnostic accuracy than SPECT130,131 or stress ECHO132 for detecting angiographically significant coronary artery disease |
| Statement 9 | LV thrombus is a serious complication of acute MI. It increases risk of thromboembolic events, particularly stroke. LV thrombus develops in up to 10% of patients with anterior wall infarctions after PPCI. Although ECHO is most commonly used to detect LV thrombus and to assess its shape and size, between 10% and 46% of echocardiograms are inconclusive.133 LGE CMR is considered the gold standard for detecting LV thrombus, because it detects thrombus based on tissue characteristics rather than anatomic appearance. Fewer thrombi are detected by contrast-ECHO than by LGE CMR.134 CMR has a higher sensitivity (88%) than contrast (61%) and non-contrast (< 33%) ECHO134,135 |
| Statement 3 | Importance of management changeCMR differentiates causes of cardiomyopathy (IHD vs. not). We use it regularly in patients post OHCAI have seen many cases of OHCA in which the use of CMR has made a diagnosis or significantly altered the diagnosis. Diagnostic refinement in the light of CMR findings also frequently results in changes in drug or device treatment, and often highlights the need for family screening. The improved anatomical, morphological and tissue characterisation that CMR allows drives the benefit of CMR over ECHO and allows better identification of the cause of out-of-hospital arrest |
| Quality of supporting evidenceI don’t think it has been proved that clinical outcomes are better with a CMR strategyCMR is unlikely to alter the immediate management of these patients. However, if the cause is unclear after ECHO and enzymes, then CMR is helpful but I am not aware of any studies specifically assessing this | |
| Statement 5 | Importance of management changeAgree. Unobstructed coronaries with elevated troponin need diagnostic resolution and CMR is helpfulThese patients are difficult to manage and often given incorrect diagnosis and especially different theories by different doctors during same admissionI have direct experience of the benefit of CMR in this area. Therefore, I strongly agree with statement 5. CMR with LGE is especially useful and I have seen many instances when CMR after acute MI with ‘normal’ coronary arteries has helped diagnosis, differentiating between distal vessel occlusion, LV clot, myocarditis and Takotsubo cardiomyopathy. The results of CMR in this patient group have also directly affected my management of this group of patients including drugs used and length of stayMRI is clinically useful in this situation in my experience and occasionally makes a very useful change to management |
| Quality of supporting evidenceUnfortunately, again no RCT to indicate benefit of CMR | |
| Statement 9 | Importance of management changeThrombus is poorly assessed by ECHO with many inconclusive reports in my experience locallyI strongly agree with this statement as I have experience of many patients in which ECHO demonstrated no LV thrombus that was subsequently found on CMR. This personal experience correlates with the studies quoted in the text of the statement |
| Quality of supporting evidenceThis statement is true but whether this leads to better patient outcomes is uncertain | |
| Statement 1 | Importance of management changeAll patients who have suffered an acute MI and subsequently undergone PPCI should receive aggressive secondary prevention. Thus, even though CMR can help refine prognosis, I do not feel that this additional information would lead to any significant changes to prescribing for secondary prevention. Enhanced confidence in CMR findings (compared to ECHO) and refinement of prognosis with respect to infarct size, MVO and MSI may help physicians discharge/follow up patients more appropriatelyAll patients should have aggressive secondary prevention |
| Quality of supporting evidenceWhilst I agree with the evidence presented, there is no evidence to support the assertion the CMR findings lead to better outcomes for patients, as there have been no trials assessing thisI don’t think it has been proved that CMR compared with ECHO leads to improved patient outcomesWhile MVO and MSI are markers of prognosis, LV systolic function remains the most important prognostic factor, which can be assessed with ECHO. We need interventions based on CMR parameters which improve prognosis | |
| Cost of CMR in relation to perceived benefitAgree, but not sure current restricted availability and high cost and only modest anticipated change in clinical action justifies wholesale change from ECHO that also predicts risk well | |
| Statement 2 | Importance of management changePPCI patients are already discharged early and often have little follow-upCMR has more reproducible results than ECHO for measuring cardiac function. When this reproducibility is combined with additional measurements that can further indicate good prognosis, this should aid physicians’ confidence in earlier discharge and appropriate timing of follow-upAlso agree, but if good LV on ECHO and no clinical heart failure then discharge is safe. Unaware if that can be accelerated further by CMR |
| Quality of supporting evidenceI don’t think it has been proved that clinical outcomes are better with a CMR strategyAlthough this could be used in clinical practice, there are no studies that I am aware [of] that have assessed such a strategy | |
| Statement 8 | Importance of management changeI’d accept perfusion scanning or DSE as adequate tests for ischaemia and would really only specifically request CMR if there were additional diagnostic questionsTotal revascularisation at one sitting with FFR guidance may render this unnecessaryStress CMR in my experience is better than SPECT or stress ECHO. This is because the improved prognostic and diagnostic accuracy helps physicians manage, with confidence, non-significant coronary disease medically rather than invasively. There are also additional benefits of CMR, for example definition of scar for CRT implant or for VT ablation. It can be helpful to ‘archive’ this information for latter use if the patient is going to receive a device such as an ICD that may preclude latter CMR scanning |
| Quality of supporting evidenceEvidence shows improved diagnostic accuracy compared to SPECT and DSE, but not in this specific cohortThe evidence for ischaemia testing in the PPCI era does not really exist. There are no studies comparing MRI in this context with other modalities and definitely no RCT comparing CMR versus another modality |
Appendix 8 Modified survey (statements, supporting paragraphs and references)
Appendix 9 Primary diagnoses in medical notes and Hospital Episode Statistics admissions data set for patients initially identified as having ‘unobstructed coronary arteries’ from hospital A
| Summary from medical notes | Primary diagnoses (HES) | Frequency |
|---|---|---|
| Culprit lesion found, PCI | Pain in throat and chest | 1 |
| Culprit lesion found, no PCI | Acute MI | 1 |
| Culprit lesion found, no PCI | Acute MI and stroke, not specified as haemorrhage or infarction | 1 |
| Culprit lesion found, no PCI | Atrial fibrillation and flutter | 1 |
| Culprit lesion found, no PCI | Chronic ischaemic heart disease | 2 |
| Other/unknown problem | Cardiac arrest | 1 |
| Other/unknown problem | Pain in throat and chest | 2 |
| Unobstructed arteries | Other diseases of pericardium | 1 |
| Unobstructed arteries | Pain in throat and chest | 1 |
| Unobstructed arteries | Syncope and collapse | 1 |
| Unobstructed arteries and other cardiac cause | Acute MI | 1 |
| Unobstructed arteries and other cardiac cause | Acute MI and other diseases of pericardium | 1 |
| Unobstructed arteries and other cardiac cause | Acute pericarditis | 1 |
| Unobstructed arteries and other cardiac cause | Atrial fibrillation and flutter | 3 |
| Unobstructed arteries and other cardiac cause | Atrial fibrillation and flutter and other cardiac arrhythmias | 1 |
| Unobstructed arteries and other cardiac cause | Atrioventricular and left bundle-branch block | 1 |
| Unobstructed arteries and other cardiac cause | Chronic ischaemic heart disease | 1 |
| Unobstructed arteries and other cardiac cause | Non-rheumatic aortic valve disorders | 1 |
| Unobstructed arteries and other cardiac cause | Other diseases of pericardium | 4 |
| Unobstructed arteries and other cardiac cause | Other diseases of pericardium and atrial fibrillation and flutter | 1 |
| Unobstructed arteries and other cardiac cause | Pain in throat and chest | 3 |
| Unobstructed arteries and other cardiac cause | Paroxysmal tachycardia | 1 |
| Unobstructed arteries and other cardiac cause | Pneumonia, organism unspecified and acute renal failure | 1 |
| Unobstructed arteries and other cardiac cause | Pulmonary embolism | 1 |
| Unobstructed arteries and other/unknown problem | Acute MI | 2 |
| Unobstructed arteries and other/unknown problem | Chronic ischaemic heart disease | 1 |
| Unobstructed arteries and other/unknown problem | Other chronic obstructive pulmonary disease | 1 |
| Unobstructed arteries and other/unknown problem | Pain in throat and chest | 5 |
| Unobstructed arteries and other/unknown problem | Pain in throat and chest and acute MI | 1 |
| Unobstructed arteries and other/unknown problem | Pain in throat and chest and cerebral infarction | 1 |
| Unobstructed arteries and other/unknown problem | Pneumonia, organism unspecified | 2 |
| Unobstructed arteries and other/unknown problem | Syncope and collapse | 1 |
Appendix 10 Data sources and definitions used for identifying changes in management up to 12 months after the index admission
| Important change in management resulting from CMR (identified in formal consensus) | Patient subgroup | Data source | ICD-10 diagnosis codes and OPCS procedure codes (up to 12 months after the index admission) |
|---|---|---|---|
| New diagnosis (non-ischaemic) | Unobstructed coronary arteries | HES/PEDW admission data | Any record of the following:
|
| Changes in medication | All subgroups | Not available | Not available |
| Additional diagnostic tests | All subgroups | HES/PEDW admission data and outpatient data | Any record of the following:
|
| Implantation of devices |
|
HES/PEDW admission data | Any record of the following:
|
| Revascularisation (PCI or CABG) within 3 months | Multivessel disease | HES/PEDW admission data | Any record of the following (up to 3 months after the index admission):
|
| Frequency of cardiology outpatient appointments | All subgroups | HES/PEDW outpatient data | Rate of outpatient visits attended (count of visits divided by follow-up time) where the treatment specialty in which the consultant responsible was working during the period of care was cardiology (code = 320) |
List of abbreviations
- ACS
- acute coronary syndrome
- A&E
- accident and emergency
- ARVC
- arrhythmogenic right ventricular cardiomyopathy
- BCIS
- British Cardiovascular Intervention Society
- CABG
- coronary artery bypass graft
- CCAD
- Central Cardiac Audit Database
- CI
- confidence interval
- CMR
- cardiovascular magnetic resonance
- CPRD
- Clinical Practice Research Datalink
- CRIS
- clinical radiology information system
- CRT
- cardiac resynchronisation therapy
- CT
- computed tomography
- DID
- Diagnostic Imaging Dataset
- ECHO
- echocardiography
- EF
- ejection fraction
- EQ-5D
- EuroQol-5 Dimensions
- FFR
- fractional flow reserve
- GP
- general practitioner
- HCM
- hypertrophic cardiomyopathy
- HES
- Hospital Episode Statistics
- HIS
- hospital information system
- ICD
- implantable cardioverter defibrillator
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICE
- Integrated Clinical Environment
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IS
- infarct size
- IT
- information technology
- IVUS
- intravascular ultrasound
- LGE
- late gadolinium enhancement
- LV
- left ventricular
- MACE
- major adverse cardiovascular event
- MeSH
- medical subject headings
- MI
- myocardial infarction
- MINAP
- Myocardial Ischaemia National Audit Project
- MVO
- microvascular obstruction
- NAPCI
- National Audit of Percutaneous Coronary Intervention
- NICOR
- National Institute for Cardiovascular Outcomes Research
- NSTEMI
- non-ST elevation myocardial infarction
- NWIS
- NHS Wales Information Service
- OHCA
- out-of-hospital cardiac arrest
- ONS
- Office for National Statistics
- OPCS
- Office of Population Censuses and Surveys
- OR
- odds ratio
- PAS
- Patient Administration System
- PCI
- percutaneous coronary intervention
- PEDW
- Patient Episode Database Wales
- PET
- positron emission tomography
- PIPA
- Primary percutaneous coronary Intervention Pathway Activation
- PPCI
- primary percutaneous coronary intervention
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SMD
- standardised mean difference
- SPECT
- single-photon emission computed tomography
- SSC
- Study Steering Committee
- STEMI
- ST-elevation myocardial infarction
- VSD
- ventricular septal defect
- WIMD
- Welsh Index of Multiple Deprivation
