Notes
Article history
The research reported here is the product of an HS&DR Evidence Synthesis Centre, contracted to provide rapid evidence syntheses on issues of relevance to the health service, and to inform future HS&DR calls for new research around identified gaps in evidence. Other reviews by the Evidence Synthesis Centres are also available in the HS&DR journal.
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 16/47/22. The contractual start date was in September 2018. The final report began editorial review in October 2018 and was accepted for publication in March 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rob Anderson is a member of the National Institute for Health Research Health Services and Delivery Research (Researcher-Led) Prioritisation Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Nunns et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Hospital use by older adults in the UK
Data from the World Health Organization1 indicate that most people across the world can now expect to live until they are aged ≥ 60 years; this is attributed to reduced mortality from childbirth and childhood illness and to declining mortality for older individuals in low-, middle- and high-income countries. 2,3 In the UK, the number of people aged ≥ 60 years is expected to increase from 14.9 million in 2014 to 21.9 million in 2039, with the life expectancy for men and women being 79.4 and 83.1 years, respectively, in 2017. 4 In England, the number of treatment episodes associated with inpatient and day-case activity recorded in NHS hospitals increased from 12.7 to 16.3 million between 2005/6 and 2015/16. 5 During the same period, there was a steady increase in the number and age of patients admitted to hospitals, with the number of combined elective and emergency admissions of patients aged 60–65 years increasing by 57% and the mean patient age increasing from 49 to 53 years. 5
In 2015/16, the largest number of elective and emergency procedures were conducted in patients aged between 65 and 69 years, with the most common procedures in this age group involving bones and joints, diagnostic tests, rehabilitation, the upper and lower digestive tract, and the eye. 5 Older adults admitted to hospital for elective procedures may present a different patient profile from that of younger adults. For example, they may be more likely to have transport difficulties,6 they may be in poor physical health or living with frailty,7 or they may be socially isolated8 or have living arrangements that require additional support following discharge. 9 Older adult hospital inpatients are also at increased risk of peri- or postoperative complications, such as delirium, falls, hospital-acquired infection, pressure sores, muscle wasting (sarcopenia), loss of mobility, poor nutrition and dehydration, cognitive decline and reduced psychological well-being. 10–17 Admission to hospital may also result in the recognition of a previously unidentified frailty syndrome, which is then destabilised by an inpatient hospital stay,18 leading to additional assessment and/or organisation of follow-up care before the patient can be discharged. Such complications can impede patients’ recovery, increase their length of stay (LOS) in hospital and influence their discharge destination. 13 As a result, care pathways may need to be specialised to allow appropriate and effective care.
Within the current financial climate, NHS hospitals are under increased pressure to maintain or improve their provision of care, and ensure the cost-effective delivery of services. The increased number of hospital admissions in an increasingly ageing population indicates that there are both patient-care and financial drivers of the need to manage the length of time older adults need to spend in hospital.
Length of hospital stay as a key outcome
Optimising the LOS in hospital does not consist solely of reducing the number of days until discharge. Discharge should occur when the patient is physically prepared19 and is involved in the decision to discharge20 and when appropriate post-discharge support is in place. 21 Thus, the LOS in hospital could be considered a composite outcome, encompassing multiple indicators of readiness for discharge.
Although LOS is a key outcome, and the primary focus of this review, the patient care pathway does not end on leaving the hospital, and nor does LOS encompass all aspects of patient recovery and well-being. Measures of additional health resource use, hospital re-admissions and complications, as well as indicators of patient recovery and well-being, are of interest when judging the success of treatment.
Hospital-based multicomponent interventions to manage length of hospital stay for planned admissions
Although identifying the optimal point of discharge can be a challenge for health-care practitioners to address on an individual basis, opportunities exist to streamline organisational processes to enhance recovery after treatment and reduce the risk of complications during hospital stay. Although LOS for patients undergoing high-volume procedures has been decreasing overall, large variation remains among patients undergoing the same procedure across different organisations. 22 A Nuffield Trust report23 indicated that around half of patients experiencing delayed discharge had done so because of factors under the direct control of the hospital. In 2007, the Royal College of Surgeons of England24 suggested separating the organisational structures and processes used for people admitted for elective surgery from those used for emergency admissions in order to establish a more predictable workflow, improve continuity of care and reduce LOS. Planned stays, in contrast to emergency admissions, offer hospitals and the wider health and social care systems a more predictable opportunity to structure the organisation and delivery of their service in order to reduce expected risks and optimise patient recovery.
Several organisational strategies or interventions have been developed and implemented both in the NHS and worldwide with a view to standardising service delivery and improving patient recovery and LOS. Comprehensive geriatric assessment (CGA)25 and enhanced recovery after surgery (ERAS)/enhanced recovery protocols (ERPs)26 are examples of such interventions. These are considered to be ‘multicomponent’ interventions in that they combine several different aspects of care that could be delivered individually otherwise.
ERAS Society guidelines27 exist for multiple planned procedures, aiming to provide explicit recommendations for health-care professionals and detailing the care that a patient should expect to receive throughout their inpatient journey. These include preoperative, intraoperative and postoperative aspects of care. Evidence from several systematic reviews indicates the efficacy of the ERAS/ERP26,28 or ‘care pathways’29 and CGA30,31 approaches in reducing patient LOS, or improving other patient outcomes, among both patients undergoing elective procedures and those undergoing emergency procedures. 32 However, systematic reviews in this area frequently do not specifically address older adults26,32 or common elective procedures requiring overnight hospital stays in this population,28 or focus on only one specific part of the patient care pathway, for example discharge arrangements. 9,33,34 Therefore, there is currently a lack of synthesised evidence examining the effectiveness of all multicomponent interventions to improve recovery and/or reduce LOS in older adults undergoing elective treatments requiring inpatient admission.
Aims and objectives of the review
This review aims to assess the effectiveness of multicomponent organisational interventions that aim to improve or accelerate the recovery of older adults undergoing planned (i.e. ‘elective’, non-emergency) treatments requiring hospital inpatient admission.
We will address two research questions:
-
What is the effectiveness of hospital-based multicomponent interventions in reducing length of inpatient stay in hospitals for older adults following planned admission?
-
What is the cost-effectiveness of hospital-based multicomponent interventions in reducing length of inpatient stay in hospitals for older adults following planned admission?
Chapter 2 Methods
The methods used to identify and select evidence followed best practice. 35–37 A protocol was registered on the PROSPERO database (PROSPERO CRD42017080637).
Search strategy
We identified effectiveness studies by searching bibliographic databases, conducting forwards and backwards citation searching of studies that met the inclusion criteria for the review, inspecting the reference lists of topically similar systematic reviews, carrying out web searches, consulting stakeholders, and contacting authors of potentially relevant conference abstracts. We also identified effectiveness studies via the cost-effectiveness searches. Cost-effectiveness studies were identified in the same way, but with adapted search terms (see below) and by inspecting studies in the effectiveness arm of the review for cost data.
The bibliographic database search strategy for effectiveness studies was developed using MEDLINE (via Ovid) by an information specialist (SB) in consultation with the review team and the stakeholders. Search terms were derived from the titles and abstracts of relevant studies identified from background searches and supplemented with relevant synonyms. The search strategy used controlled headings (e.g. MeSH in MEDLINE) wherever appropriate and free-text terms (i.e. terminology used in the titles and abstracts of studies). A multistranded approach was used to maximise the sensitivity of the search as a result of uncertainty about the specific names of procedures and interventions of interest, as follows.
-
Group 1 terms: included terms for older people and elective procedures commonly undergone by older people. Because we could not be certain of specifying all relevant elective procedures, and nor could we rely on relevant studies describing the population group in the title or abstract, these two sets of terms were combined using the ‘OR’ Boolean operator.
-
Group 2 terms: included generic terms for multimodal interventions for reducing LOS, such as ‘ERAS’ and ‘fast-track’, and common components of these interventions, such as ‘early ambulation’ and ‘nutritional support’. We also included terms that described reducing LOS, using the format ‘length’ adjacent to ‘stay’ adjacent to ‘reducing’. Because we could not be certain of specifying all relevant interventions, we combined the intervention terms and terms that described their intended effect (reducing LOS) using the OR Boolean operator.
The search terms in groups 1 and 2 were combined using the AND Boolean operator and limited using a study type filter. The filter was developed using adapted versions of Royle and Waugh’s38,39 simplified approaches to identifying randomised controlled trials (RCTs), and the Cochrane Effective Practice and Organisation of Care (EPOC) group’s suggested terminology for identifying non-randomised trials, controlled before-after studies and interrupted time series (Paul Miller, EPOC, 23 August 2017, personal communication). We also inspected the titles, abstracts and controlled headings of known relevant studies. No English-language or date filter was used; however, we retrospectively limited the search results to studies published from 2000 to date of searches using the sort by date feature in EndNote (EndNote X7, Thomson Reuters, New York, NY, USA). This date limit was selected because of the increasing prevalence of so-called ‘enhanced recovery pathways’ in the early 2000s, following the work of Kehlet and colleagues. 40–43 In addition, we wanted to limit the extent to which ‘usual care’ could be considered far removed from current day-treatment pathways.
The MEDLINE search strategy was translated for use in a selection of bibliographic databases, chosen because they were felt to be most likely to contain primary studies most relevant to our research questions. The full set of bibliographic databases comprised:
-
MEDLINE (via Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
EMBASE (via Ovid)
-
Health Management Information Consortium (HMIC) (via Ovid)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via The Cochrane Library)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost)
-
Allied and Complementary Medicine Database (AMED) (via EBSCOhost).
We also searched for studies using the Google ScholarTM and GoogleTM (Google, Inc., Mountain View, CA, USA) web search engines. The search strategies for MEDLINE, Google Scholar and Google Search are reproduced in Appendix 1, with the search strategies for other bibliographic databases reported in Report Supplementary Material 1.
The bibliographic database search for the cost-effectiveness review used the same search strategy as above, except the effectiveness study filter was replaced with a cost-effectiveness study filter. The cost-effectiveness filter was derived from a published search for cost-effectiveness studies developed by the team’s information specialist (SB) and refined to meet the specific requirements of our review in discussion with an experienced health economist (RA). 44 The search results were date limited from 2000 to date of search. No English-language filter was applied.
The search strategy was translated for use in an appropriate selection of bibliographic databases, including:
-
MEDLINE (via Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
HMIC (via Ovid)
-
NHS Economic Evaluation Database (NHS EED) (via Ovid) (although this was discontinued in March 2015, it can still be searched as an historical archive).
The results from the bibliographic database searches and Google Scholar were exported to EndNote X7 and deduplicated by manually checking and using the automatic deduplication function. The results from Google Search were copied and pasted into a Microsoft Word (version 14.0; Microsoft Corporation, Redmond, WA, USA) document for screening, as Google Search has no export function.
We carried out forwards and backwards citation searching on all prioritised studies that met the inclusion criteria (as detailed in Study selection). Web of Science (via Clarivate Analytics), Scopus (via Elsevier) and Google Scholar were used for forward citation searching. If a study was not indexed in Web of Science we searched Scopus, and if it was not indexed in Scopus we searched Google Scholar. Backwards citation chasing was conducted manually by inspecting the reference lists of prioritised studies that met the inclusion criteria.
The first authors of relevant conference abstracts from 2014 to date were contacted by e-mail to ascertain whether the study had been subsequently, or was soon to be, published as a journal article. Conference abstracts published before 2014 were not followed up on the assumption that they would have been published as a journal article already. Finally, we screened studies and the publication lists of authors suggested to us by our stakeholders.
Inclusion and exclusion criteria
The following inclusion and exclusion criteria were applied.
Population
Studies were included if patients:
-
were older adults, defined by the mean or median age of study participants being ≥ 60 years, based on the cut-off point agreed by the United Nations1
-
were undergoing planned hospital admission for either surgical or non-surgical procedures/diagnostic tests, for example –
-
hip/knee replacement
-
cardiac surgery
-
oncological surgery.
-
Studies were excluded if patients:
-
were undergoing an unplanned (i.e. non-elective or emergency) admission, as a result of an emergency or acute incident, for example following –
-
hip fracture
-
stroke
-
heart attack
-
acute injury
-
-
were receiving hospital treatment that did not require an overnight stay (e.g. day surgery)
-
had been admitted to psychiatric hospitals
-
had been admitted to hospital for a medical investigation that resulted in an unplanned inpatient stay.
Intervention
The intervention was any multicomponent hospital-based intervention or strategy for patients receiving planned care as an inpatient, which either explicitly aimed to reduce LOS or aimed to improve recovery (or used equivalent language in the aims of the strategy, e.g. ‘accelerate rehabilitation’).
Studies were included if:
-
the intervention had multiple components
-
a pre-treatment assessment was included, as long as there was detail of how the assessment influenced the patient care plan or care pathway in hospital
-
the intervention was deemed to be hospital-led, judged subjectively on the basis of whether the majority, or the core elements, of an intervention took place in hospital and/or were delivered by hospital staff
-
the comparison within the study related to altered care or patient recovery during the hospital stay.
Examples of potentially includable interventions were:
-
ERAS as described by the ERAS Society27
-
ERP
-
the use of a CGA to inform a care pathway
-
multidisciplinary assessment to inform a recovery plan
-
a rehabilitation programme consisting of a variety of exercises
-
multicomponent fast-track surgery programmes.
Studies were excluded if:
-
the intervention focus was surgical technique
-
the intervention was pharmacological, unless it was part of a broader recovery pathway
-
the intervention was focused only on discharge planning or only on pre-treatment assessment (e.g. CGA alone) and did not result in actions affecting the hospital stay
-
the intervention was not hospital-led (e.g. it was a community care programme, a general practitioner (GP) assessment or an intervention based in a nursing home)
-
the intervention had a single component, that is, it featured the administration of only a single dose or bout of an intervention, or it was delivered at a single time point and modality.
Examples of excludable interventions were:
-
early mobilisation in isolation
-
CGA to identify odds of adverse events, without informing a care plan
-
pre-treatment information materials.
Comparator(s)
The comparator was any type of control group or comparator, for example ‘treatment as usual’, ‘usual hospital care’, ‘pre-pathway implementation’ or ‘usual best clinical practice’.
Outcomes
The outcome was any metric of LOS.
Other key outcomes that were of interest, but did not influence a study’s eligibility for inclusion, were:
-
re-admission rates
-
patient-reported outcomes
-
feedback/experiences of patients, carers or clinicians
-
additional health-care use, including re-admission or the use of primary care post discharge
-
incidence of within-hospital or post-discharge complications or harms (e.g. falls, delirium, sarcopenia).
For cost-effectiveness studies, economic outcomes were defined as the amount of resources used or costs incurred directly related to the outcome of interest where a change in that outcome directly resulted in the use of a different amount or different type of health-care resources. In addition to the outcomes extracted listed above, those relevant to research question 2 included:
-
mean per-patient costs (and incremental cost between control and intervention)
-
mean per-patient effectiveness/quality-adjusted life-years (QALYs) (and incremental effectiveness/QALYs between control and intervention)
-
total and mean intervention cost (and comparator)
-
incremental cost-effectiveness ratio (from any included cost-effectiveness or cost–utility analyses
-
net benefit or net monetary benefit (from cost–benefit analyses).
Study design
To answer review question 1, any of the following comparative study designs were included:
-
RCT
-
(non-randomised) controlled clinical trial
-
controlled before-and-after study
-
interrupted time series
-
uncontrolled before-and-after studies.
The study designs included as economic studies were:
-
cost-minimisation analysis
-
cost–consequences analysis
-
cost-effectiveness analysis
-
cost–utility analysis
-
cost–benefit analysis
-
any comparative cost analysis comparing relevant interventions.
Studies were excluded if:
-
methods of calculating cost outcomes were not reported.
Geographical context
Studies were included from any high-income country as defined by the World Bank list of economies. 45 This was to ensure that the studies included in this review were evaluating health systems that were broadly comparable.
Date of publication
The search was restricted to studies published from 2000 to date of search (see Search strategy).
Study selection
The inclusion and exclusion criteria were piloted on a sample of 100 records identified by the database searches by four reviewers (MN, LS, SB and RA) independently. Following discussion, the criteria were refined and applied to the title and abstract of each identified citation independently by two reviewers (LS, MN, SB), with disagreements resolved through discussion. The full text of each potentially relevant paper was obtained and assessed independently for inclusion by two reviewers (SB, MN, LS) using the same method. When necessary, the opinion of a third reviewer was sought (MN, LS, RA, JTC). EndNote software was used to support study selection. A Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA)-style flow chart was produced, detailing the study selection process.
We took the pragmatic step of prioritising the following categories of includable studies for full data extraction and synthesis: (1) RCTs conducted in any high-income country and (2) studies of any of includable trial design and conducted in the UK. This step was taken to allow us to manage the size of the synthesis while ensuring that it was based on the highest quality of evidence available, and to allow us to focus on the most relevant evidence within the UK setting. Only cost-effectiveness evaluations associated with prioritised studies were of interest. Minimal data extraction (study details, design and location; sample size, age and reason for admission; intervention type and key features; comparator type; setting; stages of care affected by the intervention) was carried out for the studies that were not prioritised, and these features were tabulated (see Report Supplementary Material 2, Table 1). Cost-effectiveness studies that were eligible for inclusion but were not based on prioritised effectiveness studies are summarised in Report Supplementary Material 7.
Data extraction
A standardised, piloted data extraction form in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) was used to collect data from each of the prioritised papers. Piloting the form for effectiveness studies involved each reviewer (MN, LS, SB) extracting an included study, which was then checked by another reviewer. Following this, all reviewers discussed ways to improve the form. This was repeated for the cost-effectiveness form by three reviewers (RA, LS and MN).
Data extraction was performed by one reviewer (effectiveness studies: MN, LS, SB; cost-effectiveness studies: RA, LS, MN) and checked by a second (effectiveness studies: LS, MN, SB; cost-effectiveness studies: RA, LS), with disagreements settled through discussion. The following data were extracted where applicable and reported.
Population
Number invited to participate, number randomised/included, dropouts and missing data, age, percentage female, place admitted to, reason for admission, comorbidities, discharge destination, other inclusion or exclusion criteria and any subgroup analysis.
Intervention
Intervention name, aim and description, who delivered the intervention, setting, recipient(s), use of manual/guidelines, frequency of each intervention component, duration of each intervention component and assessment of fidelity.
Comparator
As for intervention.
Outcome
All reported outcomes, as listed in Inclusion and exclusion criteria, Outcomes.
For cost-effectiveness studies, additional data summarising the cost methods used in each paper were extracted.
Quality assessment strategy
The quality of all prioritised studies was independently appraised by two reviewers (LS, MN, SB) using the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies, which is suitable for randomised and non-randomised study designs. 46 Disagreements between reviewers were resolved through discussion. An additional item was considered during quality assessment, namely whether or not LOS was clearly defined. This item was not included when scoring the global quality of each study.
Cost-effectiveness studies were subject to additional appraisal using the Consensus Health Economic Criteria (CHEC) list. 47 Each study was independently appraised by two reviewers (LS and MN) and checked by a third reviewer (RA). Quality assessment informed interpretation of findings, for example by qualifying statistically significant findings in cases of poor study quality, and was not used to exclude studies. Non-prioritised studies were not subject to quality assessment.
Synthesis methods
All studies were grouped into categories based on the anatomical location of the procedure. These groupings were informed by consultation with stakeholders and agreed by two researchers/clinicians (MN and AH) and were as follows: cardiac surgery, colorectal surgery, lower limb arthroplasty, pelvic surgery, thoracic surgery, tumour removal (various locations), upper abdominal surgery, vascular surgery and various surgeries. We expected that this approach would lead to interventions within each group sharing common features, aiding comparison between studies.
Interventions and outcomes were categorised by two researchers (MN and LS). Interventions were classified according to the following broad categories, which were based on terms found in the literature.
-
ERP: an intervention consisting of components at multiple stages of the care pathway (i.e. pre admission; post admission but preoperative; perioperative; postoperative but prior to discharge; post discharge).
-
Prehabilitation: characterised by a focus on preoperative (usually pre-admission) components.
-
Preoperative assessment with care plan (PACP): an assessment prior to hospital admission, with a subsequent care plan for the patient.
-
Rehabilitation: characterised by a focus on postoperative components to improve or speed up recovery, delivered while the patient was still in hospital or when they had been discharged; usually based on physical exercise.
-
Specialist ward: this involved moving the patient to a different location in the hospital, or a ring-fenced ward, with aspects such as restricted opening times, specialist staff or extra infection control measures.
-
Staff mix: the main active ingredient was the provision of particular numbers or types of staff, such as a team of geriatricians, or the provision of extra nurses at key time points.
Comparators were grouped in the same way, with the additional category ‘usual care’ available. The following synthesis was only applied to prioritised studies.
Outcomes were grouped into categories for ease of reporting by two researchers (MN and LS) and confirmed following discussion with stakeholders (JM, AH, DT, CL). Broadly, outcomes were considered as either ‘clinical’ or ‘patient-reported’ in nature. ‘Clinical’ outcomes included those outcomes obtained from patient records or accessed from a database, such as LOS, re-admissions, complications, use of additional care, surgical processes, morbidity and mortality. ‘Patient-reported’ outcomes were considered to be those outcomes that a patient might actively report (although some might be assessed by a third party), such as mental health, quality of life, satisfaction and markers of physical recovery. Outcome categories are defined, with examples, in Appendix 2.
After categorisation and collation of data, effectiveness findings were tabulated and summarised by procedural group. Surgical outcomes were not described but are available on request.
Data processing
Between-group differences were analysed where possible. For continuous outcomes, standardised mean differences were calculated, where possible, to assess the presence and magnitude of any differences between groups. For dichotomous outcomes, odds ratios (ORs) were calculated to assess the relative ‘odds’ of the event occurring in the intervention group, for example re-admissions or complications. Cohen’s d was calculated for continuous outcomes to produce an effect size, with interpretation following Cohen’s guidance (i.e. where d = 0.2 to 0.49, class as ‘small’; where d = 0.5 to 0.79, class as ‘medium’; and where d = 0.8 or above, class as ‘large’). 48 In addition, 95% confidence intervals (CIs) for the effect were calculated using the metan command in Stata (version 14.2, StataCorp, College Station, TX, USA). The mean (non-standardised) difference with 95% CIs was also calculated for the outcome, and the p-value for the difference was obtained using the ttesti command in Stata, using data from the two-tailed analysis.
Where the mean and standard deviation (SD) were not provided for continuous effectiveness outcomes, these were treated as described in Appendix 3 to allow the analysis of as many data as possible from the included studies. Methods for imputing data were taken from Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0,36 except for the method for imputing data from the median and interquartile range, which was taken from the work of Wan and colleagues. 49 The calculations provided by Wan and colleagues also allow imputation to be performed when medians and ranges are provided; however, they are most suitable when data are normally distributed. 49 Based on this, we decided not to report imputed data when medians with ranges were provided because data for outcomes such as LOS were often highly skewed. To investigate the influence of imputed data on pooled effects, we performed sensitivity analyses on LOS meta-analyses using three scenarios: (1) include all data in the analysis, (2) exclude data imputed from medians and ranges and (3) exclude data imputed from medians and any form of variance statistic.
For dichotomous outcomes, ORs were calculated in Microsoft Excel using standard equations described in section 9.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. 36
In addition, the statistical significance of the OR was assessed by calculating a p-value from the z-score for the difference, and ascertaining 95% CIs. CIs were calculated using the following equation:
where p^1 is the proportion of ‘successes’ or occurrence in the experimental group p^2 is the proportion of ‘successes’ or occurrence in the control group; and p^ is the proportion of successes across the two groups combined. P-values were ascertained by referencing Z against normal distribution tables using the function normdist in Microsoft Excel. For the calculation of 95% CIs, we used the method described by Altman and Bland. 50
Throughout, p-values were reported at the levels of < 0.001, < 0.01 and < 0.05, and the actual value if > 0.05. Interpretation of statistical significance was guided by considering the effect size, CI and p-value and whether or not the mean difference between groups was clinically significant, where appropriate.
Random-effects meta-analysis was performed with RCTs where, within a procedure group, studies evaluated the same intervention type (e.g. ERP), evaluated the same comparator type (e.g. (usual care) and reported the same outcomes (e.g. LOS) and from which useable data could be calculated. Forest plots were produced as part of the metan command in Stata. Pooled effects with 95% CIs and p-values were reported. Statistical heterogeneity was assessed using the I2-statistic, with greater values (range 0–100%) indicating greater heterogeneity. 51 Meta-analysis was performed only when all combinable studies were RCTs. Meta-analysis of ORs was performed using log-transformed data.
When multiple outcomes were presented within the same outcome category, for a study included in the meta-analysis, one outcome was chosen as the ‘best representative’. In the case of LOS, this meant the outcome that most closely accounted for the longest portion of the hospital stay, without consideration of re-admissions. For example, ‘total LOS’ would be chosen ahead of ‘postoperative LOS’. LOS including re-admissions was not chosen because of the likelihood that small numbers of re-admissions, and varied reasons for re-admission leading to unpredictable duration of additional stay, were expected to skew data. For complication data, summary or composite outcomes were preferred, rather than incidences of specific complications. For example, ‘total complications’ or ‘patients with complications’ would be preferred to the breakdown of patients with specific complications. When only incidences of individual complications were listed by study authors, a summed outcome was calculated for entry into meta-analysis.
The effectiveness of interventions at reducing LOS and improving other patient outcomes was further explored utilising a narrative synthesis approach based on the methods used by Thomson et al. 52 Within each procedural group, the intervention and outcomes of each study was summarised visually within a table to aid comparison across multiple outcomes and intervention types. The data for each type of intervention within a procedural grouping were examined to see if any differences between the sample, intervention characteristics or study quality could be related to the effectiveness of the intervention. Any characteristics that appeared to differentiate effective interventions from ineffective ones were then compared across different procedural groups where possible. Data provided by RCTs were considered separately from data provided by studies of any design that were conducted in the UK.
Publication bias
Publication bias was assessed across all procedure and intervention categories using a visual inspection of funnel plots (effect size vs. standard error of the effect size) for LOS. This method was used in line with the recommendations of Sterne and Egger. 53 Only standardised mean differences calculated from RCTs were entered into funnel plots. Funnel plots were produced using the metafunnel command in Stata.
Stakeholder and patient and public involvement
Stakeholder and patient and public involvement is described in Chapter 3.
Chapter 3 Stakeholder and patient and public involvement
Stakeholder engagement
Stakeholder involvement was incorporated throughout the review, from development of the protocol to making sense of preliminary results, identifying key messages for dissemination and supporting the preparation of the final report and other outputs. Consultation occurred through a series of individual face-to-face meetings, telephone calls and e-mail correspondence with the following clinical expert advisors:
-
A consultant geriatrician (AH) with expert knowledge in the management of adults with multiple comorbidities and complex needs, frailty syndromes and polypharmacy, and with expertise in achieving successful discharge planning and supportive home-based post-discharge interventions.
-
A consultant urological surgeon in the UK (JM), who was National Clinical Advisor to the UK Department of Health for the Enhanced Recovery Partnership Programme, and chaired the ERAS Guideline Development Group for the British Association of Urological Surgeons and authored the specialty guidelines. Recognised internationally for work in Enhanced Recovery following major urological surgery and has published widely within this field.
-
A clinical lead occupational therapist (CL) in neurology and neurorehabilitation in the UK. His clinical interests include Parkinson’s disease, brain injury and cognitive neuroscience.
-
A deputy chief nurse at Royal Devon and Exeter hospital (DT) with 29 years’ experience in nursing, almost entirely within the surgical specialties, and with clinical understanding of the ERP processes and patient journey during the hospital stay.
Stakeholder engagement is documented and the impact of this involvement on the review is described in Table 1.
| Stage | Stakeholder/PPI involvement | Impact on review |
|---|---|---|
| Protocol development | Individual 60-minute meeting with AH | Development of reviewer understanding of pathway of care for older adults admitted to hospital for planned procedures |
| Preparation of website and advertisement materials (September 2017) | Proofreading of materials by three individuals | Project information for display on project website and advertisement for PPI group sense checked by people aged > 60 years who had experience of planned hospital stay |
| Protocol revisions (October 2017) |
All expert clinical advisors reviewed the draft project protocol by e-mail Telephone conversation with JM |
Revision of review inclusion/exclusion criteria, specifically:
|
| Checking review focus (January 2018) | 1 × 2-hour meeting with four patients/members of the public | Discussion of interventions that the group felt were most important to patients facing a planned hospital stay, which could have an impact on their LOS in hospital, including clear communication, provision of information, provision of transport, receiving medication on discharge and support at home |
| Planning analysis and feedback on search results (June 2018) |
3 × 60-minute meetings with AH, JM and CL individually E-mail correspondence with DT |
Meeting with AH:
|
| 1 × 2-hour meeting with four patients/members of the public | Identification of outcomes that were particularly important to patients, including:
|
|
| Feedback on initial findings (June 2018) | 1 × 2-hour meeting with three patients/members of the public | Identified that patient satisfaction, mental health and quality-of-life outcomes are poorly reported and a research priority. Gave the patient perspective on overnight stays for elective procedures, highlighting a number of factors that the study authors had not considered. These points helped the team to understand gaps in the evidence and provided materials for the discussion section |
| Dissemination activities (August–October 2018) | 2 × members of the public contributed towards a conference abstract |
Provided content used on a poster presentation delivered at the Cochrane Colloquium in Edinburgh, September 2018, reflecting on PPI involvement in the systematic review Co-creation of plain-language summaries for the main report and to use as basic structure for further dissemination materials |
| Reading of draft report (October 2018) | 3 × 1-hour meetings with AH, JM and CL individually | All stakeholders:
|
Patient and public involvement
We met with a group of four adults aged > 60 years for three 2-hour meetings during this review. Each individual had experience of being admitted to hospital overnight for a planned procedure. We planned to learn from their knowledge and experiences to help us identify important outcomes and aspects of care, particularly where they may have been overlooked in the included evidence. We elicited feedback from the group at key stages of the review and aimed to co-produce the plain English summary to maximise readability. The impact of the patient and public involvement on this review is also described in full in Table 1.
Chapter 4 Results
Study selection
This section presents the findings from the effectiveness searches. The cost-effectiveness search results are presented in Synthesis of cost-effectiveness evidence, Study selection.
The PRISMA flow chart in Figure 1 summarises the study selection process. Bibliographic database searches identified 17,150 records and supplementary search methods identified 3501 records. Following the removal of duplicates, a total of 10,448 unique records were screened at title and abstract level. The full texts of 583 papers was sought for further consideration. Of these, 579 full texts were successfully retrieved (99.3%). Following full-text screening, 361 papers were excluded for the reasons specified in Figure 1.
FIGURE 1.
The PRISMA flow chart. NR, not reported; SR, systematic review.
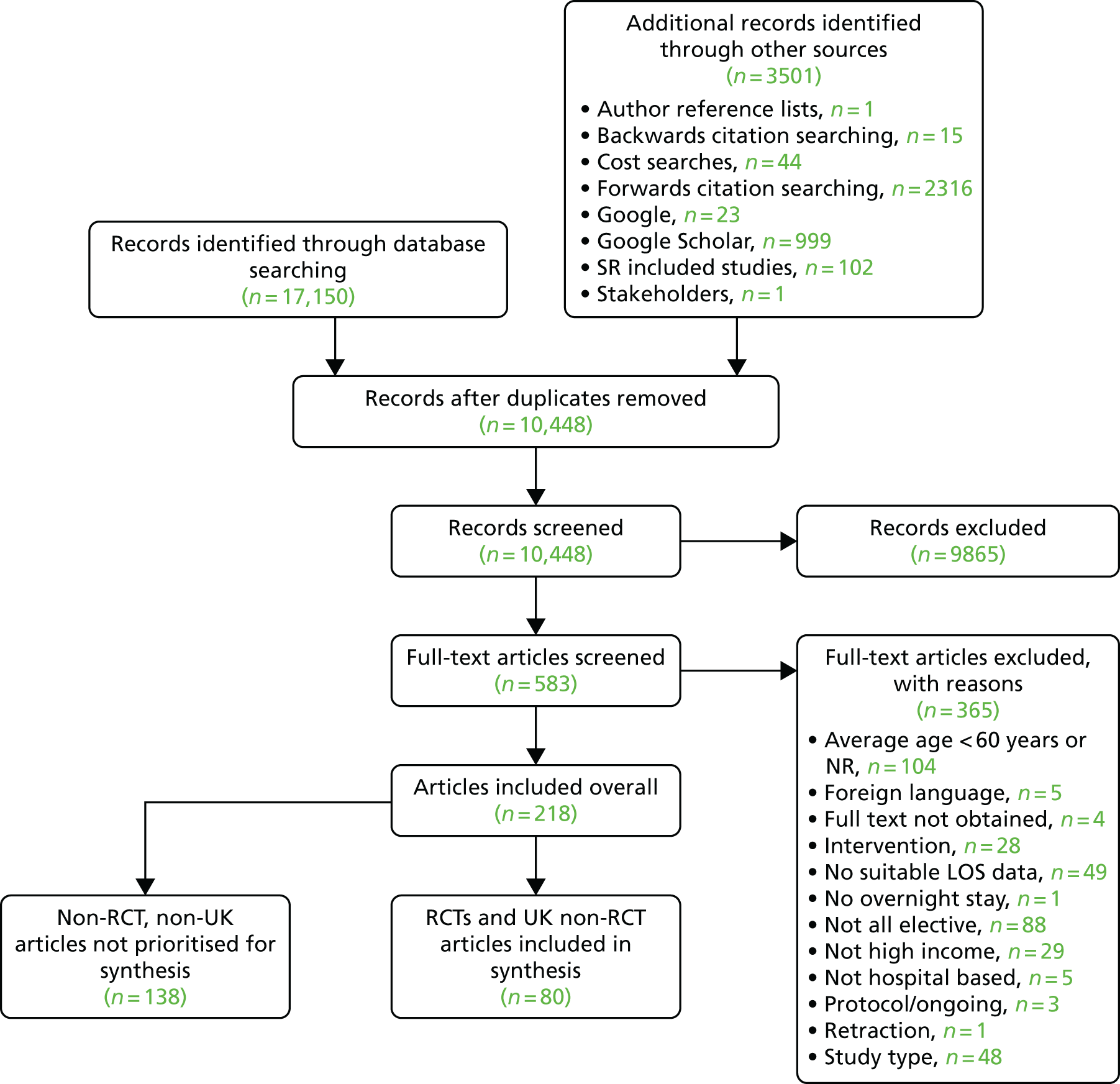
In total, 208 studies, reported in 218 articles, met the inclusion criteria for inclusion in this review (not prioritised,40,54–190 prioritised31,191–269).
As described in Chapter 2, Synthesis methods, only RCTs and studies of any design from the UK were prioritised for quality assessment and further synthesis. Studies that were not RCTs and that were conducted outside the UK are described in Report Supplementary Material 2, including the stages of care targeted by each intervention, along with a brief description of the intervention and comparator. The descriptions in Sample characteristics, Intervention characteristics: prioritised studies and Quality assessment apply only to prioritised studies.
The results from the RCTs conducted in any high-income country are presented in Synthesis of evidence from randomised controlled trials. The results from the studies of any comparative trial design that were conducted in the UK are then presented in Synthesis of evidence from the UK. Evidence within these sections is further broken down by procedural type. Within each procedural subsection, key findings are summarised at the start, followed by a detailed analysis. Full tables of outcome data are located in Report Supplementary Material 5 (RCTs) and Report Supplementary Material 6 (UK evidence).
Sample characteristics
Of the 73 prioritised studies (reported in 80 articles), 39 (40 articles) were conducted in the UK,31,191,192,194,195,197,201,203,205–208,210,212,213,215,216,218,224,226,228–232,236–238,241,244,245,250,253,256,257,259,260,262,263,269 13 of which were RCTs. Thirty-four (41 articles) were RCTs conducted in one of 15 other countries, the most common of which were six studies from Germany,202,217,227,240,246–249,255 five from the Netherlands,204,219–221,265,266,268 four from Denmark196,225,233,234,267 and four from Canada. 193,198,200,214 The remaining studies consisted of 24 uncontrolled before-and-after (UBA) trials191,194,195,197,201,203,206–208,212,216,218,228,230,232,237,238,241,245,250,253,257,262,263 and three controlled trials224,229,259 from the UK.
All of the prioritised articles were published in peer-reviewed journals, apart from one, which was a PhD thesis. 233 The majority of articles (81.5%) were published from 2008 onwards, with 50 (61.7%) published since 2011. 31,191,195,197,199,201–203,205–209,211,212,214,216,219,220,222,225–230,235–243,249,250,252,253,255,257–260,262–265,267,268 Data were collected from 26,365 patients across 73 studies, with a mean number of 366 patients per study, ranging from 21 within a RCT221 to 5319 within a controlled trial229 utilising database sampling. The mean proportion of female participants in studies was 44.9% across 70 studies. Eight studies had an upper age limit for inclusion as follows: 75 years,254,256 80 years235,265,268 and 85 years. 202,243,264 By contrast, nine studies199,204,207,218,220,221,223,242,267 exclusively recruited patients aged ≥ 60 years. Studies explicitly excluded patients who lived with cognitive impairment (n = 6200,218,229,254,261,262), had ‘psychiatric illness’ (n = 6196,202,210,231,254,266), had a history of stroke (n = 2218,259), had ‘mental disability’ (n = 2234,267), had periods of dizziness/confusion (n = 2210,244) or were unable to consent (n = 431,209,226,236). By contrast, seven studies199,200,207,215,218,221,223 selected individuals who were at elevated risk of postoperative complications or who were likely to have complex needs or multimorbidities.
The reasons for admission, according to our broad procedural categories, were lower-limb arthroplasty (n = 25 studies195,196,200,202,206,216,218,221–224,230,234,237,238,241,244,245,253,254,256,261,262,267,269), colorectal surgery (n = 19 studies192,198,203,204,209,211,213,214,229,231,232,235,236,239,242,243,251,252,265), cardiac surgery (n = 8 studies193,208,210,215,255,258,259,266), upper abdominal surgery (n = 8 studies191,201,205,226,227,257,263,264), pelvic surgery (n = 3 studies217,225,250), thoracic surgery (n = 4 studies 197,212,228,248), vascular surgery (n = 2 studies31,247), solid tumour removal at various anatomical sites (n = 1 study220), abdominal surgery (n = 1 study199) and a mix of various different surgeries (n = 1 study207).
Table 2 provides a broad overview of the sample characteristics of studies prioritised for synthesis.
| First author, year, country | Study design | Sample size (n) | Female (%) | Mean age (years) (SD) [range] | Recruitment method | Place admitted to | Comorbidities (intervention vs. comparator) | Inclusion/exclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Abdominal surgery | ||||||||
| Chen 2017,199 Taiwan | RCT | 535 | 43.2 | 74.54 (5.89) [NR] | On admission to gastrointestinal wards | Urban medical centre | Surgery type: total/subtotal gastrectomy, right hemicolectomy, left hemicolectomy/lower anterior resection/anterior resection, pancreaticoduodenectomy, other (open splenectomy, transverse colon partial resection, Hartmann’s procedure with adhesiolysis and bladder lithotripsy, abdominoperineal resection, or laparoscopic debulking surgery), enrolled if expected LOS of > 6 days and aged ≥ 65 years | |
| Cardiac surgery | ||||||||
| Arthur 2000,193 Canada | RCT | 249 | 15 | 62.8 (8.2) [NR] | Waiting lists | General hospital |
Previous myocardial infarction: 52.6% vs. 52.1% Diabetes: 16.4% vs. 25.6% Current smoker: 20.3% vs. 13% Aortocoronary bypasses (mean/median): 2.6/3 vs. 2.6/3 |
First CABG, low-risk surgery date > 10 weeks away. Excluded if had combined CABG and valve surgery, had ejection fractions < 0.40, could not attend exercise classes, or was unable to participate because of physical limitations |
| Fleming 2016,208 UK | UBA | 105 | 27.6 | 67.4 (11.4) [NR] | NR | Teaching hospital |
Mean/SD: NYHA heart failure, 2.2 (0.5) vs. 2.2 (0.5) CCVS angina pectoris, 2.2 (0.5) vs. 2 (0.7) Normal LVF, n = 35 vs. n = 34; impaired LVF, n = 14 vs. n = 11; poor LVF, n = 3 vs. n = 8 Non-insulin-dependent diabetes, n = 10 vs. n = 6 Insulin-dependent diabetes, n = 1 vs. n = 2; receiving treatment for hypertension, n = 30 vs. n = 36; history of myocardial infarctions, n = 22 vs. n = 19 Hypercholesterolemia, n = 33 vs. n = 30; COPD requiring treatment, n = 6 vs. n = 5; history of PONV, n = 4 vs. n = 1 |
Cardiac surgery including CABG, aortic valve replacement and mitral valve/aortic root surgery, as well as redo cardiac surgeries. Excluded: emergency surgery/thoracic procedures |
| Furze 2009,210 UK | RCT | 204 | 19.6 | 64.8 (8.7) [42–83] | Waiting list | Tertiary centre in northern England | NR | CABG, ability to give informed consent. Exclusion criteria: exercise induced arrhythmias, loss of systolic BP of > 20 mmHg during exercise stress testing, unstable angina, score of 4 on the CCVS/NYHA classification for angina/heart failure, current psychiatric problems, dementia, self-reported periods of dizziness/confusion, life-threatening comorbidities, concurrent participation in other research |
| Goodman 2008,215 UK | RCT | 188 | 18.7 | 64.7 (NR) [NR] | NR | Hospital | At least one poorly controlled risk factor (blood pressure 140 mmHg systolic or 80 mmHg diastolic, non-fasting serum cholesterol 4 mmol/l or BMI of 28 kg/m2) | CABG with or without valve surgery, able to understand English, and had specified risk factors needing control (blood pressure 140 mmHg systolic or 80 mmHg diastolic, non-fasting serum cholesterol 4 mmol/l or BMI of 28 kg/m2). Excluded if lived outside designated geographical area or had life-threatening significant non-cardiovascular disease (e.g. cancer) |
| Probst 2014,255 Germany | RCT | 200 | 29 | Median: 65.5 (NR) [IQR 55–72] | Patients screened for inclusion during premedication visit 1 day before surgery, final decision made by anaesthesiologist and cardiac surgeon at end of surgery | University hospital | COPD, 8% vs. 10%; neurological deficit, 9% vs. 5%; peripheral vascular disease, 13 vs. 8%; diabetes mellitus, 25% vs. 31%; renal insufficiency, 6% vs. 14% | CABG, valve surgery or combined CABG/valve surgery haemodynamically stable, normothermic, no bleeding. Excluded if was in cardiogenic shock, was dialysis dependent or had an additive EuroSCORE of > 10, had impaired left ventricular function (ejection fraction < 35%), cardiac assist devices pre or post operation, cardiopulmonary instability post operation, lack of bed in either PACU or ICU |
| Rosenfeldt 2011,258 Australia | RCT | 117 | 26 |
Experimental group: median age 62.5 (NR) [59–68.5] Comparator group: median age 68 (NR) [58–77] |
NR | Public hospital | Diabetes, 20% vs. 29%; previous MI, 28% vs. 31% | CABG. Excluded if urgent/emergency surgery, severe aortic valve stenosis, limited English, NYHA class IV heart failure |
| Salhiyyah 2011,259 UK | CT | 136 | 18.4 | 63.1 (9.0) [40–80] | Invited on admission | General hospital | NR | Cardiac surgery: CABG, valve, atrial septal defect. Excluded if mitral valve replacement, redo grafts/valves, history of cerebrovascular accident, emergency operation, Swan–Ganz catheter, inadequate haemostasis |
| van der Peijl 2004,266 The Netherlands | RCT | 309 | 21.1 | 62.7 (10.2) [NR] | NR | University medical centre | Diabetes mellitus, 19% vs. 19%; COPD 12% vs. 6%; peripheral or CVD, 9% vs. 5%; hypertension, 47% vs. 33%; main stem lesion (> 50%), 20% vs .21% | CABG. Excluded if concomitant surgical procedures, severe comorbidity interfering with daily life, insufficient Dutch language, mental disorders, postoperative complications jeopardising standardised exercise programme |
| Colorectal surgery | ||||||||
| Anderson 2003,192 UK | RCT | 25 | 56 | Median: intervention, 64 [IQR 55–68]; comparator, 68 [IQR 65–75] | Consecutive patients invited to participate at surgical outpatient department | General hospital | Malignant disease, 78.57% vs. 63.64% | Left or right hemicolectomy |
| Carli 2010,198 Canada | RCT | 133 | 42 | 60.5 (15.5) [NR] | Identified by colorectal surgeons | University health centre | NR | Resection of benign or malignant colorectal lesions, or for colonic reconstruction of non-active inflammatory bowel disease, aged > 18 years, receiving preoperative chemo-/radiotherapy. Excluded if had health conditions prohibiting participation in exercise programmes/testing procedures |
| Dronkers 2010,204 The Netherlands | RCT | 42 | 25 | 70 (6.7) [NR] | Referred by gastroenterologist, or surgeon went to outpatient department of physical therapy | General hospital | COPD, 3/21 vs. 3/17; coughing: 2/20 vs. 2/18; diabetes: 8/14 vs. 1/19 | First elective colon surgery for gastric cancer, minimum waiting period of 2 weeks, aged ≥ 60 years, adequate cognitive functioning. Excluded if had heart disease/orthopaedic conditions that impede exercise, had severe systemic illness, had recent embolism, thrombophlebitis, had uncontrolled diabetes (fasting blood glucose of 4400 mg/dl) or was wheelchair-dependent |
| Forsmo 2016,209 Norway | RCT | 324 | 46.3 | Median 65.5 (NR) [19–93] | Contacted on waiting list | University hospital | NR | Elective open or laparoscopic colorectal surgery (including patients with rectal cancer previously treated with pelvic radiation) for malignant or benign disease, aged ≥ 18 years. Excluded if had multivisceral resection planned/ASA grade IV, was pregnant, had emergency operations, had difficulty providing informed consent owing to impaired mental capacity, was unable to adapt to ERAS criteria. Randomised patients were excluded if intended colonic or rectal surgery not performed |
| García-Botello 2011,211 Spain | RCT | 125 | 39 | Median: intervention, 62 [27–85]; comparator, 60 [28–88] | Outpatient care clinics | University hospital | Cancer, n = 46 vs. n = 40; diverticular disease: intervention, n = 10 vs. n = 9, chronic inflammatory bowel disease, n = 5 vs. n = 9 | Elective colorectal surgery requiring colon/rectum resections (including reoperations) using laparotomy or laparoscopy, aged > 18 years, ASA score of 1–4, living in metropolitan area of Valencia, informed consent. Excluded if non-independent daily lifestyle (unable to walk, bathe or eat on own), undergoing emergency surgery |
| Gatt 2005,213 UK | RCT | 39 | 41 | 67 (NR) [59–76] | Consecutive patients on waiting list contacted | General hospital | NR | Colorectal resection, living independently at home. Excluded if was pregnant, was intolerant to probiotics and/or prebiotics, had contraindication to one or more optimisation strategy, had contraindications to early postoperative discharge, had been prescribed medications that may prolong hospital stay, had advanced malignancy on preoperative assessment, had palliative surgery, had emergency surgery, or if there was a failure to perform colonic/rectal resection |
| Gillis 2014,214 Canada | RCT | 89 | 37.7 | 65.9 (11.3) [NR] | Consecutive patients approached at initial office visit with surgeon | University-affiliated tertiary centre |
Ischaemic heart disease: n = 3 (7.5%) vs. n = 2 (5%) Hypertension: n = 8 (21%) vs. n = 12 (31%) Diabetes: n = 3 (7.5%) vs. n = 5 (13%) |
Curative resection of non-metastatic colorectal cancer. Excluded if did not speak English/French or had premorbid conditions that contraindicated exercise |
| Khan 2013,229 UK | CT | 83 | 50.6 | Median: intervention, 75 (NR) [IQR 59–76]; comparator, 64 (NR) [IQR 60–70] | Consecutive patients contacted | District hospital or tertiary referral centre |
Colorectal cancer: n = 1 vs. n = 35 Inflammatory bowel: n = 7 vs. n = 5 Diverticular disease: n = 4 vs. n = 1 |
Elective colorectal surgery. Excluded if no informed consent, inability to complete questionnaires owing to cognitive impairment, poor English comprehension |
| Khoo 2007,231 UK | RCT | 81 | 61 | Median: intervention, 69.3; comparator, 73.0 [overall range 46.3–87.7] | NR | Hospital | NR | Colorectal resection for cancer between May 2003 and October 2004. Excluded if unable to mobilise independently over 100 m at preoperative assessment, contraindications to thoracic epidurals, pre-existing clinical depression, palliative care only, undergoing joint operation involving another surgical specialty |
| King 2006,232 UK | UBA | 146 | 47.9 | 70.8 (11.0) [NR] |
Comparator group: prospectively entered into the multicentre CLASICC trial Experimental group: consecutive patients presenting to a single consultant assessed for eligibility |
Hospital | NR |
Colorectal cancer resection. Historic control: aged > 18 years, suitable for elective colorectal cancer resection, no malignancy within past 5 years, no intestinal obstruction, ability to provide written consent. Excluded if had tumours of transverse colon Prospective cohort inclusion criteria: aged > 18 years, elective resection, no preoperative radiological or clinical evidence of metastases. Patients with transverse colon cancers or with malignancy within last 5 years were included |
| Lee 2011,235 South Korea | RCT | 100 | 44 | 61.2 (7.6) [NR] | NR | University hospital | NR | Laparoscopic resection for colonic tumour, suitable for laparoscopic colonic resection, aged 20–80 years. Excluded if synchronous distant metastasis, intestinal obstruction/perforation, previous major abdominal surgery, severe pulmonary disease/cardiovascular disease |
| Lidder 2013,236 UK | RCT | 57 | 43.9 | Median: intervention, 70 (NR) [IQR 65–78], comparator, 73 (NR) [IQR 63.8–81] | NR | General hospital | NR | Colorectal resection, planned curative resection with primary anastomosis. Excluded if was aged < 18 years, was unable to give informed consent, had frailty,a was participating in another trial, was pregnant, had diabetes, had a preoperative fasting glucose of > 7 mmol/l, was using steroids or immunosuppressants, had history of abnormal gastric emptying, had intestinal obstruction, had concurrent parenteral or enteral nutrition |
| Maggiori 2017,239 France | RCT (multicentre) | 270 | 47.6 | 61.5 (11) [31–90] | NR | Clinic | NR | Laparoscopic resection for colorectal cancer, aged ≥ 18 years, functional capacity of ≥ 4 METS, histologically proven colorectal cancer with curative intent, in absence of evidence of metastatic disease. Excluded if BMI < 18 kg/m2 or > 30 kg/m2, preoperative albumin blood level < 30 g/l, weight loss of > 10% during 6 months preceding surgery, emergency surgery, very low rectal tumours requiring abdominoperineal excision, scheduled sub/total colectomy, scheduled total proctocolectomy, scheduled associated resection of another organ, pregnant, or allergic to ropivacaine, xylocaine, droperidol or ketamine |
| Mari 2014,243 Italy | RCT | 52 | 52 | Median: overall sample, 66 (NR) [29–83] | NR | General hospital | Overall sample: hypertension, 5; BMI close to 30 kg/m2, 2; liver metastasis, 3; multinodular struma, 1; dyslipidaemia, 1 | High anterior resection for benign/oncologic disease – HAR with transanal anastomosis; colorectal laparoscopic surgery, ASA score of 1–3, aged 18–85 years, BMI of < 30 kg/m2, no intestinal diversion |
| Mari 2016,242 Italy | RCT | 83 | 21.7 | 76.5 (NR) [70–85] | NR | General hospital | NR | Colorectal laparoscopic surgery, aged ≥ 70 years, autonomous mobilisation and walking, eligible for laparoscopic technique, ASA score of I to III, with indication for major colorectal surgery |
| Muller 2009,251 Switzerland | RCT | 156 | 49 | Median: intervention, 62 [27–91]; comparator, 59 [39–89] | NR | Four surgical departments in teaching hospitals | Malignant, n = 67 vs. n = 64; benign, n = 9 vs. n = 11 | Open colonic resection with a primary anastomosis, aged > 18 years. Excluded if emergency surgery, contraindication to epidural anaesthesia, scheduled total colectomy or rectum resection, preoperatively immobile patients |
| Pappalardo 2016,252 Italy | RCT | 50 | 48 | 66.65 (NR) [45–83] | NR | NR | Pulmonary, 48% vs. 56%; cardiovascular/hypertension, 64% vs. 60%; diabetes, 24% vs. 20% |
Open extra-peritoneal rectal cancer surgery, January 2009 through December 2013, without a primary derivative stoma with or without a secondary derivative stoma, extraperitoneal tumour location,b cT2–T4 tumours, with or without positive lymph nodes, use of modified FTP, neoadjuvant therapy where indicated (T3–T4 or N+) Excluded if tumours located > 12 cm above the anal verge, cT1 or M1, urgent procedures; had ASA of > 3, operated on with abdominoperineal resection or Hartmann’s procedure, refusing neoadjuvant therapy, refusing or unable to follow FTP, coagulation disorders contraindicating epidural catheter insertion |
| Dhruva Rao 2015,203 UK | UBA | 506 | 43.9 | Median: intervention, 71 (NR); control, 69 (NR) [overall range: 23–93] | Retrospective review of prospectively maintained database | General hospital | NR | Colorectal resections, January 2008–December 2012. Excluded if it was felt patient could not achieve > 50% of targets during counselling or preoperative assessment, requiring postoperative intensive treatment unit management. Patients were withdrawn from programme whenever clinically indicated |
| van Bree 2011265 and Vlug 2011,268 the Netherlands | RCT | 93; 427c | 43.6; 41.5 | 65.2 (9.1) [NR]; 66.5 (8.7) [NR] | Invited to participate | Academic medical centre; three university hospitals, six teaching hospitals | NR; % with comorbidities per group: lap + FT = 71%; open + FT = 59%; lap + standard = 68%; open + standard = 68% | Segmental colectomy for histologically confirmed adenocarcinoma or adenoma without evidence of metastatic disease, aged 40–80 years, ASA status < IV. Excluded if neoadjuvant radiotherapy, prior midline laparotomy, unavailability of a laparoscopic surgeon, emergency surgery, planned stoma |
| Lower limb arthroplasty | ||||||||
| Barlow 2013,195 UK | UBA | 410 | NR | 70.6 (NR) [29–93] | Consecutive patients 6 months before and after implementation of ring-fenced ward | University hospital |
ASA scores (intervention, 214; comparator, 261): Fit and healthy, 27 vs. 21; mild disease not incapacitating, 165 vs. 165; incapacitated by systemic disease, 48 vs. 59; life-threatening disease, 1 vs. 6; moribund, 0 vs. 0 |
Primary lower limb arthroplasty |
| Borgwardt 2009,196 Denmark | RCT | 50 | 55 | 65.6 (NR) [44–86] | Consecutive patients asked to participate | University hospital | NR | UKR, resident in Copenhagen, ASA of I or II, no medical history of GI bleeding, care arranged after discharge. Excluded if major psychiatric disease, incapable of managing own affairs, inflammatory joint disease, neurological/other disease(s) affecting lower limbs, previous major knee surgery |
| Crowe 2003,200 Canada | RCT | 133 | 80 | 68.8 (11.3) [NR] | Consecutive patients asked to participate | Home, physiotherapy clinic, hospital |
Preoperative diagnosis (n): Osteoarthritis, 61 vs. 65; rheumatoid arthritis, 4 vs. 3 Existing comorbidities (n): Hypertension, 33 vs. 35; cardiac, 7 vs. 7, cognitive/psychiatric, 5 vs. 4; stroke/transient ischaemic attacks, 2 vs. 0; other (hiatus hernia, diabetes, asthma, chronic obstructive pulmonary disease, epilepsy, urinary tract disease and chronic lumbar pain), 10 vs. 9 |
Hip/knee arthroplasty, high score on Oxford Questionnaire, coexisting medical conditions, suboptimal social support, requiring home alterations to enable the client to return home. Excluded if functioning well despite joint dysfunction, managing activities of daily living well with good caregiver support, limited English-language skills, marked cognition problems, joint replacement as management for cancer, undergoing revision or second joint replacement < 2 years |
| den Hertog 2012,202 Germany | RCT | 160 | 70.8 | 67.4 (8.11) [40–85] | NR | Non-academic hospital specialising in orthopaedic surgery |
Diagnoses (n): Degenerative arthritis, 72 vs. 72; post-traumatic arthritis, 0 vs. 1; Ahlback’s disease, 2 vs. 0; athritis in knee without surgical procedure, 38 vs. 34 Secondary disorders/concomitant diseases (n): Cardiac, 50 vs. 39; gastrointestinal, 16 vs. 14; allergies, 4 vs. 5; kidney/urinary tract, 2 vs. 4 |
TKA. Excluded if missing informed consent, lack of co-operation capability, ASA score of > 3, RA, cancer, substance abuse, previous major surgery on affected joint, neurological or psychiatric disease, pregnant, participating in other clinical studies |
| Dwyer 2012,206 UK | UBA | 127 | 61.4 | 71.5 (8.8) [48–91] |
Intervention: consecutive patients on an enhanced recovery programme Comparator: data collected retrospectively from patient records |
District hospital | NR | THA |
| Gordon 2011,216 UK | UBA | 847 | 66 | 71 (10.3) [27–98] | Retrospective chart review | General hospital | NR | Hip or knee arthroplasty |
| Harari 2007,218 UK | UBA | 108 | 60 | 74.5 (6.2) [NR] |
Intervention: consecutive patients Historical comparator: consecutive cases reviewed |
Teaching hospital |
% (n): Rheumatoid arthritis, 9.3 (5) vs. 7.4 (4) Ischaemic heart disease, 37.0 (20) vs. 24.1 (13) Heart failure (present/past), 1.9 (1) vs. 3.7 (2) Atrial fibrillation, 14.8 (8) vs. 5.6 (3) Diabetes, 20.4 (11) vs. 13.0 (7) Renal impairment (plasma creatinine > 104 mmol/l), 22.2 (12) vs. 3.7 (2) Hypertension, 80.0 (43) vs. 51.9 (28) Chronic lung disease, 11.1 (6) vs. 7.4 (4) Symptomatic prostate or bladder problems, 35.2 (19) vs. 18.5 (10) Cerebrovascular disease, 7.4 (4) vs. 3.7 (2) |
Orthopaedic hip replacement; aged ≥ 65 years with any of uncontrolled hypertension (blood pressure > 160/90 mmHg); MI in past 2 years; unstable angina, undergoing treatment for heart failure; poorly controlled diabetes; previous stroke; currently taking warfarin; chronic lung disease; poor nutritional status;d ≥ 2 falls from standing height in past year; significant memory problems; history of confusion, known dementia; needs personal help with getting to the toilet, moving from bed to chair, standing up, dressing and walking; likely to need complex discharge package |
| Hoogeboom 2010,221 Netherlands | RCT | 21 | 67 | 76 (4.1) [69–90] | Patients pre-screened by anaesthetist. Eligible patients informed about study and consent obtained by orthopaedic nurse | Hospital | Mean of 1.5 and 1 in experimental and comparator groups; range 0–4 | Primary THA, aged ≥ 70 years, OA of hip, minimum waiting time of 3 weeks, score of 2 on Clinical Frailty Scale. Excluded if unable to communicate or had severe heart disease |
| Huang 2012,222 Taiwan | RCT | 243 | 71.6 | 70.2 (7.3) [NR] | NR | Tertiary hospital | NR | Unilateral, primary TKA for advanced OA, ability to follow rehabilitation programme, interval of 4 weeks between enrolment and time to surgery. Excluded if had inflammatory arthritis or any medical condition where moderate exercise was contraindicated (e.g. heart failure or hypertension), or had bilateral joint replacements |
| Huddleston 2004,223 USA | RCT | 505e | 53.7 | 73.2 (9.6) [NR] | Eligible patients identified during initial outpatient orthopaedic evaluation | Academic medical centre |
n (%): Diabetes, 53 (22.8) vs. 38 (16.0) Congestive heart failure, 13 (5.6) vs. 16 (6.8) CAD, 98 (42.5) vs. 102 (43.0) Dementia, 4 (1.7) vs. 6 (2.1) COPD, 33 (14.2) vs. 30 (12.7) Immunosuppression, 26 (11.2) vs. 34 (14.4) Renal failure or dialysis, 28 (12.0) vs. 25 (10.6) Deep-vein thrombosis/pulmonary embolus, 28 (12.0) vs. 42 (17.7) Cerebrovascular accident/transient ischaemic attack, 24 (10.3) vs. 13 (5.5) Peripheral vascular disease, 8 (3.5) vs. 11 (4.6) |
Primary or revision THA/TKA, at elevated odds for perioperative complications, aged > 75 years with either one or more major comorbid conditions or two or more ‘less disabling’ comorbid conditions considered at elevated odds. Excluded if was aged < 18 years, was a non-US resident or was an inmate of local correctional facility at time of surgery |
| Hunt 2009,224 and Salmon 2013,260 UK | CT (multicentre) | Total across three sites: 579; 560 | 54.3; 58.4 | 67.4 (NR) [23–93]; 67.8 (10.5) [NR] | Consecutive patients attending preoperative assessment clinics by participating surgeons at three centres; additional consecutive patients recruited in hospital 2–5 days postoperatively in one control centre | One of three sites: General Hospital, SWLEOC, University Hospital |
Comorbidities (%) in intervention vs. comparator 1 vs. comparator 2: Hypertension, 47 vs. 45 vs. 51; CAD, 14 vs. 12 vs. 5; COPD, 16 vs. 12 vs. 20; diabetes, 9 vs. 10 vs. 13; thyroid disorders, 10 vs. 4 vs. 3; CVD, 7 vs. 3 vs. 2; GI disease, 14 vs. 14 vs. 10; psychiatric disorders, 6 vs. 3 vs. 7 |
Unilateral primary hip arthroplasty, over 12 months from July 2006 |
| Khan 2014,230 UK | UBA | 6000 procedures in 5319 patients | 46 | 68.5 (10) [NR] | Unselected consecutive arthroplasty procedures | Two sites in same trust | Hypertension, 1409 vs. 936; atrial fibrillation, 162 vs. 143; ischaemic heart disease, 249 vs. 213; insulin-dependent diabetes mellitus, 33 vs. 21; non-insulin-dependent diabetes mellitus, 293 vs. 212; COPD, 133 vs. 87; Alzheimer’s disease, 9 vs. 7 | Total hip and/or total knee arthroplasty. Only patients of ASA grades 1 and 2 were operated on at site 1. Patients of all ASA grades underwent procedures at site 2 |
| Larsen 2008,233,234 Denmark | RCT | 90 | 50.6 | 65 (10) [NR] | Consecutive patients invited to participate in the study | Regional hospital | NR | Primary THA, TKA or UKA. Excluded if had mental disability or severe neurological disease |
| Maempel 2015,238 UK | UBA | 165 | 52.1 | 69.9 (9.7) [NR] | Database examined. All patients under care of senior author were selected | Hospital | NR | Prosthetic total knee replacement, January 2010–April 2013. Excluded if UKR, patellofemoral replacements and revision TKRs |
| Maempel 2016,237 UK | UBA | 1161 | 60.9 | Median age 65 (NR) [IQR 25–94] | Review of patients under care of the senior authors | General hospital | NR | Primary THA, April 2005 to May 2013. Excluded if undergoing THA April-December 2010, simultaneous bilateral THA, transferred from a medical ward for planned semiurgent THA and returned to the medical ward postoperatively, sustained a per prosthetic femoral fracture, requiring further surgery and prolonged rehabilitation |
| Malviya 2011,241 UK | UBA | 4500 | 51 | 68.5 (NR) [NR] | NR | General hospital |
(n): Hypertension, 673 vs. 921; atrial fibrillation, 84 vs. 143; ischaemic heart disease, 113 vs. 211; insulin-dependent diabetes mellitus, 18 vs. 20; non-insulin-dependent diabetes mellitus, 150 vs. 205; COPD, 67 vs. 85; Alzheimer’s disease, 5 vs. 6 |
Primary THR and TKR. Unit 1: exclusively relatively fitter patients (ASA 1 and 2), unit 2: all grades of ASA status. All patients under care of nine surgeons at two units in same hospital. Intervention: first 1500 patients May 2008–November 2009. Comparator: unselected, consecutive series of 3000 patients before introduction of protocol |
| McGregor 2004,244 UK | RCT | 39 | 71.4 | 71.9 (9.3) [51–92] | Via authors’ institution/hospital | Hospital | NR | THA. Excluded if revision or bilateral arthroplasty, previous hip arthroplasty, coexisting morbidity for example history of severe cardiovascular, respiratory, neuromuscular disease, RA, mentally confused, inadequate comprehension of English |
| Mertes 2013,245 UK | UBA | 607f | Overall sample 63.6%; aged ≥ 75 years 72.2% | 70.5 (8.9) [NR] | NR | Hospital | NR | THA or TKA. Excluded if revision arthroplasty, simultaneous bilateral arthroplasty, medically unrelated confounding factors (e.g. diagnosis of a brain tumour in the postoperative period) |
| Pengas 2015,253 UK | UBA | 791 | 48.2 | 67.5 (11.0) [27–92] | NR | Hospital | NR | Hip/knee arthroplasty. Excluded if complex, bilateral/revision arthroplasty, hospital stay of > 10 days due to infection or social circumstances, complications preventing mobilisation |
| Pour 2007,254 USA | RCT | 100 | 46.9 | 60.8 (8.9) [NR] | Consecutive patients screened for inclusion in the study | University hospital | NR | Unilateral THA, aged 18–75 years, underlying diagnosis of OA, informed consent. Excluded if BMI > 30 kg/m2, cognitive impairment/severe psychiatric illness precluding participation in the protocol procedures |
| Reilly 2005,256 UK | RCT | 41 | 41.5 | 63 (NR) [NR] | NR | Nuffield Orthopaedic Centre | NR | UKA, diagnosed with anteromedial OA,g good understanding of procedure, tolerance of large doses of non-steroidal anti-inflammatory drugs, suitable home situation within 25-mile radius, aged ≤ 75 years. Excluded if diagnosis of diabetes/severe respiratory disease/deep-vein thrombosis, previous heart surgery, tri-compartmental arthritis |
| Siggeirsdottir 2005,261 Iceland | RCT | 50 | 52 | 68 (NR) [28–86] | Waiting list. Patients living in another town were also invited to participate | University hospital or general hospital |
Diagnosis: Osteoarthrosis, 24 vs. 21; RA, 1 vs. 1; previous fractures, 2 vs. 0; deformity after Perthes disease, 0 vs. 1 |
Primary hip replacement, diagnosed with OA of hip, RA, primary segmental collapse of femoral head, and sequelae after developmental diseases and hip trauma, living in their own home. Excluded if primary hip fracture, metastatic tumours, dementia |
| Starks 2014,262 UK | UBA | 2128 | 64.5 | 71 (NR) [28–93] | Hospital episode statistics data reviewed | General hospital | NR | Primary joint arthroplasty, August 2007–May 2009. Excluded if cognitive impairment, medical comorbidities requiring ongoing medical supervision during inpatient stay, complex surgery, bilateral arthroplasties, hip resurfacing |
| Vesterby 2017,267 Denmark | RCT | 73 | 46.6 | Median: intervention, 63 (NR) [43–80]; comparator, 64 (NR) [45–84] | Consecutive patients invited | Urban teaching hospital | NR | Primary fast-track elective THR. Excluded if distance to hospital > 60 km, previous hip surgery, mental disability, inability to communicate in Danish, no support person, no internet connection |
| Williamson 2007,269 UK | RCT | 181 | 54 | 70.7 (8.8) [NR] | Waiting list | General hospital | NR | Knee replacement surgery (total, unicondylar, unilateral, bilateral). Excluded if taking anticoagulants; within 2 months of intra-articular steroid injection; experiencing back pain associated with referred leg pain; suffering from ipsilateral OA of the hip; psoriasis or other skin disease in the region of knee; RA, received acupuncture or PT within last year |
| Pelvic surgery | ||||||||
| Arumainayagam 2008,194 UK | UBA | 112 | 23 | 65.9 (NR) [NR] | Retrospective database search | General hospital | NR | Radical cystectomy |
| Gralla 2007,217 and Magheli 2011,240 Germany | RCT | 50 | 0 | 62 (5.9) [NR] | NR | University hospital; hospital | NR | Laparoscopic radical prostatectomy, patients up to ASA III included. Excluded if severe reduced renal function (creatinine levels preoperatively > 1.6 mg/dl) due to analgesic treatment with COX-2 inhibitors; ASA score of IV; use of cytotoxic drugs, immunosuppressants, or anticonvulsives; severe general or nervous system diseases |
| Jensen 2015,225 Denmark | RCT | 129 | 26 | 70.1 (NR) [46–91] | NR | University hospital |
Comorbidity score, n (%): None: 1 (2) vs. 0 1–2 (low): 16 (32) vs. 14 (25) 3–4 (high): 23 (46) vs. 31 (54) ≥ 5 (severe): 10 (20) vs. 12 (21) |
Radical cystectomy |
| Mukhtar 2013,250 UK | UBA | 77 | 22.1 | 68.4 (8.0) [49–85] | NR | Hospital | NR | Radical cystectomy and reconstruction, from October 2007 onwards. No specific inclusion/exclusion criteria |
| Thoracic surgery | ||||||||
| Brunelli 2017,197 UK | UBA | 600 | 59.2 | Median: intervention, 69.7 (NR) [IQR 63–76]; comparator, 68.8 (NR) [IQR 63–75] | Retrospective analysis of a prospectively maintained database | University hospital | Coronary artery disease, 22% vs. 15%; cerebrovascular disease, 4.3% vs. 7.1% | VATS lobectomy or VATS anatomic segmentectomies, included if surgery commenced via a VATS approach but converted to open surgeryh |
| Gatenby 2015,212 UK | UBA | 132 | 30 | 65.1 (NR) [IQR intervention, 13; comparator, 14] | All patients fit for surgery proceeded along intervention pathway as default | Teaching hospital | NR | Open oesophageal and gastric resections (oesophagogastrectomy, total gastrectomy, subtotal gastrectomy). Excluded if not deemed fit enough for oesophagogastric resection surgery |
| Karran 2016,228 UK | UBA | 252 | 23.4 | Median: intervention, 66 (NR) [24–86]; comparator, 65.5 (NR) [42–89] | Retrospective analysis of prospectively maintained database | University hospital |
Tumour site (n): Oesophageal, 64 vs. 53; gastro-oesophageal junction, 35 vs. 13; gastric body, 26 vs. 14; gastric antrum, 29 vs. 11; linitis plastica, 6 vs. 1 |
UGI cancer surgery (including total and subtotal gastrectomy, oesophagostomy) |
| Muehling 2008,248 Germany | RCT | 62 | NR | 66.7 (NR) [NR] | NR | University hospital |
Underlying disease: Non-small cell lung cancer, 25 vs. 19; metastases, 3 vs. 4; carcinoid, 1 vs. 1; aspergilloma, 0 vs. 1; pneumonia, 0 vs. 1; bulla, 0 vs.1; mesothelioma, 0 vs. 1; PECOM,i 1 vs. 0 |
Lung resection, all patients admitted with suspected lung neoplasms with indication for lung resection |
| Surgery to remove tumours (various locations) | ||||||||
| Hempenius 2013220 and 2016,219 the Netherlands | RCT | 297; 260c | 64; 62 | 77.54 (7.21) [NR]; 77.4 (7.3) | NR | University medical centre, medical centre, community hospital | Surgery for solid tumour, aged > 65 years, scheduled for surgery between June 2007 and June 2010 | |
| Upper abdominal surgery | ||||||||
| Abu Hilal 2013,191 UK | UBA | 44 | 54 | Median: 69.3 (NR) [IQR 61–76] | Consecutive patients invited | University hospital |
Diagnosis (n): Ductal adenocarcinoma, 12 vs. 12; duodenal adenocarcinoma, 1 vs. 3; ampullary adenocarcinoma, 2 vs. 6; cholangiocarcinoma, 2 vs. 1; neuroendocrine tumour, 1 vs. 1; intraductal papillary mucinous neoplasm, 1 vs. 1; metastatic cancer, 1 vs. 0 |
Pancreatoduodenectomy. Excluded if total or distal pancreatectomy not eligible for intervention pathway |
| Dasari 2015,201 UK | UBA | 211 | 37 | 64.3 (11.6) [NR] | Review of prospectively maintained database | General hospital |
Reason for resection (n): Colorectal metastases, 60 vs. 61; hepatocellular carcinoma, 4 vs. 17; other, 27 vs. 15; presence of background cirrhosis, 0 vs. 4 |
Liver resection. Excluded if complex procedures, specifically liver donation, associating liver partition with portal vein ligation for staged hepatectomy, concomitant colonic, vascular or a bile duct resection |
| Dunne 2016,205 UK | RCT | 38 | 31.6 | Median: 62 (NR) [IQR 54–69] | All patients referred to service were screened for potential eligibility | University hospital |
Comorbidity (n): Cardiovascular: 10 vs. 8; respiratory: 3 vs. 4; diabetes: 2 vs. 2; renal disease: 1 vs. 0; none: 1 vs. 3; primary tumour: node-positive; 12 vs. 10; adjuvant or neoadjuvant treatment: 11 vs. 7; metastatic presentation; synchronous presentation: 8 vs. 10; extrahepatic metastatic disease: 3 vs. 4; > 3 hepatic metastases; 5 vs. 7; metastasis of > 5 cm in diameter; 7 vs. 6 |
Liver resection, aged > 18 years and able to give informed consent, partake in cycle-based exercise and complete the exercise programme before proposed surgery date, metastases deemed surgically treatable with curative intent, Excluded if known pre-existing chronic liver disease |
| Jones 2013,226 UK | RCT | 104 | 41 | 65.5 (NR) [27–84] | Patients were first approached in the outpatient clinic and given information sheet | Hospital | NR | Open liver resection, all adult patients presenting for procedure eligible. Excluded if entirely laparoscopic operation, needed a second concomitant procedure, inoperable at the time of surgery, unable to consent |
| Kapritsou 2017,227 Greece | RCT | 63 | 39.7 | 60.9 (11.7) [NR] | Potential participants approached the surgical clinic | Oncology hospital | NR | Hepatectomy or pancreatectomy, up to 2 months after cancer diagnosis, ASA classification of I–III, aged > 18 years, normal level of consciousness and communication. Excluded if presence of chronic pain, kidney disease, neuropathy, systemic or chronic treatment with analgesics |
| Richardson 2015,257 UK | UBA | 66 | 56 | Median: intervention, 67 (NR) [IQR 54–70]; comparator, 60 (NR) [IQR 41–70] | Review of prospectively collected database | University hospital |
Diagnoses (%): Adenocarcinoma, 23 vs. 16; acinar cell carcinoma, 0 vs. 2, neuroendocrine tumour, 23 vs. 11; metastatic cancer, 5 vs. 5, lymphoma, 0 vs. 2; intraductal papillary mucinous neoplasms, 9 vs. 0; mucinous cystic neoplasm, 36 vs. 41; pancreatitis, 5 vs. 23 |
Laparoscopic distal/left pancreatectomy, considered for laparoscopic approach. If patients had preoperative evidence of tumour invasion to surrounding organs needing left upper quadrant clearance with multivisceral resection considered for open surgery |
| Sutcliffe 2015,263 UK | UBA | 130 | 41 | Median: intervention, 67 (NR) [18–83]; comparator, 66 (NR) [35–83] | NR | University hospital |
Indication for surgery: Pancreatic ductal adenocarcinoma, 26 vs. 19; ampullary cancer, 19 vs. 21; duodenal cancer, 2 vs. 5; cholangiocarcinoma, 8 vs. 11; neuroendocrine cancer, 3 vs. 3; other malignant tumours, 3 vs. 1; benign disease, 4 vs. 5 |
Pancreaticoduodenectomy |
| Tanaka 2017,264 Japan | RCT | 148 | 31 | 67.5 (NR) [29–85] | NR | Medical college hospital | NR | Gastric cancer surgery, histologically confirmed adenocarcinoma of the stomach for which curative gastrectomy was planned without simultaneous resection of other organs except for the gallbladder, no involvement of the duodenum or oesophagus, aged 20–85 years, sufficient oral intake, ASA score of < 4, no prior chemotherapy/radiotherapy. Excluded if factors that might impede fast recovery, for example pregnant, inflammatory bowel disease, chronic renal disease, severe cardiopulmonary dysfunction, complicated diabetes |
| Various procedures | ||||||||
| Ellis 2012,207 UK | UBA | 313 | 58 | 73 (6.2) [NR] | Assessed for eligibility at pre-admission assessment | General hospital | NR (‘red flags’ raised as part of assessment) | Total hip or knee replacement, other orthopaedic, transurethral resection of prostate, transurethral resection of bladder tumour, other renal, general (GI) surgery. Aged > 65 years, presence of one or more of following ‘red flags’ identified during routine pre-assessment: cognitive problems, mobility concerns, history of falls, difficulties with activities of daily living, concerns regarding home circumstances |
| Vascular surgery | ||||||||
| Muehling 2008,247 2009246 and 2011,249 Germany | RCT | 82; 101; 101c | 7.6; 6.1; 6.1 | Median: intervention, 67 (NR) [40–81]; comparator, 68 (NR) [52–84] | All patients admitted for procedure invited | University hospital | NR | Open repair of infrarenal aortic aneurysm. Excluded if withdrawal of informed consent, clinical signs of infection on admission, contraindications for epidural anaesthesia, neuromuscular disorder that prevented full postoperative physiotherapy, intraoperative suprarenal clamping; thoracoabdominal or juxtarenal aneurysms |
| Partridge 2017,31 UK | RCT | 209 | 23.9 | 75.5 (6.5) [NR] | Potentially eligible patients approached by research nurse or fellow in the vascular surgery outpatient clinic once listed for surgery | Teaching hospital with a tertiary referral practice for vascular arterial surgery |
Diagnoses made at preoperative assessmentj (%): Ischaemic heart disease, 5 vs. 0; cardiac failure, 5 vs. 0; atrial fibrillation, 3 vs. 1; COPD, 14.9 vs. 0; diabetes, 2 vs. 0; CVD, 1 vs. 0; cancer, 2 vs. 0; cognitive impairment, 46.5 vs. 1; chronic kidney disease, 25.7 vs. 0; valve lesion, 8.9 vs. 3; tachyarrhythmia or bradyarrhythmia, 2 vs. 0; Parkinson’s disease, 1 vs. 0 |
Vascular surgery (aorta or lower limb), patients lacking capacity to consent recruited under Sections 30–34 of the Mental Capacity Act |
Intervention characteristics: prioritised studies
Interventions are summarised in Table 3; they are described in detail in subsequent sections of the synthesis and in Report Supplementary Material 3. The key characteristics are summarised here. The most frequently studied intervention category was ERP (n = 49 studies191,192,194,196,197,199,201,203,206,208,209,211–213,216–218,224–232,234–239,241–243,245,247,248,250–252,254,256,257,261–265). Thirteen studies193,198,200,204,205,210,214,215,221,222,244,258,269 evaluated prehab; two studies207,220 evaluated PACPs; four studies202,253,266,267 evaluated rehab; three studies195,255,259 evaluated specialist wards; and one study223 evaluated a staff mix intervention. The most common comparator was standard care or pre-intervention pathway (in UBA designs), although study authors used a range of descriptions (see Table 3).
| First author, year, country | Intervention name in study | Category | Stated aims of intervention | Stages of the care pathway at which intervention elements were delivered | Site | Who was involved in delivery? | Comparator name in study | Outcomes reported (other than LOS) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre admission | Preoperative | Perioperative | Postoperative | Post discharge | ||||||||
| Abdominal surgery | ||||||||||||
| Chen 2017,199 Taiwan | mHELP | ERP | Reduce incidence of delirium and LOS | ✗ | ✗ | Hospital | Trained mHELP nurse | Usual care | Complications | |||
| Cardiac surgery | ||||||||||||
| Arthur 2000,193 Canada | Preoperative Intervention | Prehab | Improve patients’ physical and psychological readiness for surgery, reducing LOS | ✗ | Hospital, phone, patient home | Kinesiologists/exercise specialists nurses, psychologist, family | Usual care | Complications, mental health, QoL | ||||
| Fleming 2016,208 UK | ERACS | ERP | Reduce LOS, improve perioperative outcomes | ✗ | ✗ | ✗ | ✗ | Hospital | NR | Pre-care bundle | Complications, mortality, MoR | |
| Furze 2009,210 UK | HeartOp programme | Prehab | Reduce anxiety and depression, encourage healthy behaviours | ✗ | Outpatient clinic, patient home | Nurse facilitator | Routine nurse counselling/education | Costs, QoL, re-admissions, UAC, mortality, complications, MoR, mental health | ||||
| Goodman 2008,215 UK | ‘Fit for surgery’ nurse-led support and education programme | Prehab | Reduce anxiety, improve control of factors related to postop complications and CHD progression | ✗ | Patient home | Hospital nurses | Standard care | Costs | ||||
| Probst 2014,255 Germany | PACU | Specialist ward | Reduce extubation time and LOS in ICU post surgery | ✗ | ✗ | ✗ | NR | NR | Postoperative care in ICU | Complications, mortality | ||
| Rosenfeldt 2011,258 Australia | Physical conditioning and mental stress reduction | Prehab | Evaluate effect on QoL, rates of postoperative atrial fibrillation, LOS, fitness improvements, stress management | ✗ | Outpatient clinics, patient home | PT, family | Usual care | QoL, mental health | ||||
| Salhiyyah 2011,259 UK | Fast-track programme | Specialist ward | Improve clinical and economic outcomes, shorten ICU stay | ✗ | ✗ | Hospital | NR | ICU for minimum 1 day, transfer to ward | Costs, re-admissions | |||
| van der Peijl 2004,266 the Netherlands | Exercise therapy | Rehab | Facilitate recovery after surgery | ✗ | Hospital | PT | Low frequency exercise programme 1 × day, excluding weekends | MoR | ||||
| Colorectal surgery | ||||||||||||
| Anderson 2003,192 UK | Multimodal optimisation | ERP | Optimise pre-op, perioperative and post-op management | ✗ | ✗ | ✗ | ✗ | General hospital | MDT | Conventional Care | MoR, complications, readmissions | |
| Carli 2010,198 Canada | Bike/strengthening prehabilitation | Prehab | Optimise recovery of functional walking capacity post surgery | ✗ | Patient home | NR | Walking/breathing group | MoR, mental health, QoL, complications | ||||
| Dronkers 2010,204 the Netherlands | Preoperative therapeutic programme | Prehab | Improve physical condition before surgery and improve recovery | ✗ | Outpatient department, patient home | NR | Home-based exercise advice | Complications, QoL, MoR | ||||
| Forsmo 2016,209 Norway | ERAS | ERP | Reduce LOS | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Standard care | Complications, mortality, re-admissions, MoR | |
| García-Botello 2011,211 Spain | Perioperative multimodal rehabilitation protocol | ERP | Improve recovery | ✗ | ✗ | ✗ | ✗ | Outpatient clinic, hospital | MDT, family | TC | Complications, MoR, costs re-admissions | |
| Gatt 2005,213 UK | Multimodal optimisation package | ERP | Reduce surgical stress response, complication, health costs and LOS. Accelerate recovery, without compromising patient safety | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Conventional care | Complications, MoR, mortality, re-admissions, UAC | |
| Gillis 2014,214 Canada | Prehab | Prehab | Reduce LOS | ✗ | Patient home | MDT | Rehab | QoL, MoR, mental health, complications, re-admissions | ||||
| Khan 2013,229 UK | ERAS | ERP | Improve recovery | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Laparoscopic surgery with no ERAS pathway | Re-admissions, complications | |
| Khoo 2007,231 UK |
Multimodal Perioperative management protocol |
ERP | Reduce LOS, improve independence | ✗ | ✗ | ✗ | Hospital | MDT | Control | Complications, MoR, re-admissions, satisfaction, UAC, mortality | ||
| King 2006,232 UK | Enhanced recovery pathway | ERP | Improve clinical outcomes, QoL and costs | ✗ | ✗ | ✗ | Outpatient clinic, hospital | MDT, family | Conventional care | Costs, complications, mortality, re-admissions | ||
| Lee 2011,235 South Korea | Rehab programme | ERP | Reduce LOS | ✗ | ✗ | Hospital | MDT, family | Conventional care | Complications, MoR | |||
| Lidder 2013,236 UK | Carbohydrate and nutritional supplements plus ERAS | ERP | Improve recovery | ✗ | ✗ | Hospital | NR | PrO and PO placebo drinks | Complications, MoR | |||
| Maggiori 2017,239 France | FFT programme | ERP | Decrease morbidity | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Limited fast-track programme | Complications, MoR, mortality | |
| Mari 2014,243 2016, Italy | Fast-track protocol; ERAS group | ERP | Reduce LOS, improve care; Improve immune and nutrition outcomes | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Standard care | Complications, MoR, re-admissions | |
| Muller 2009,251 Switzerland | Fast-track protocol | ERP | Reduce morbidity and complications | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Standard care | Re-admissions, complications | |
| Pappalardo 2016,252 Italy | Fast-track protocol | ERP | Reduce LOS, morbidity, mortality and improve QoL | ✗ | ✗ | Hospital | MDT | Traditional care | Complications, mortality, MoR | |||
| Dhruva Rao 2015,203 UK | ERP | ERP | Reduce LOS | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Pre-ERP: non fast-track | Complications, mortality | |
| van Bree 2011265 and Vlug 2011,268 the Netherlands | Fast-track programme | ERP | Reduce LOS | ✗ | ✗ | ✗ | ✗ | Hospital: multisite | MDT | SC with laparoscopy or open surgery | Complications, MoR, mortality, re-admissions, costs | |
| Lower limb arthroplasty | ||||||||||||
| Barlow 2013,195 UK | Ring-fenced orthopaedic ward | Specialist ward | Reduce LOS | ✗ | Hospital | MDT, family | Prior to ring-fenced ward: general orthopaedic ward | None | ||||
| Borgwardt 2009,196 Denmark | Accelerated recovery programme | ERP | Reduce LOS with good clinical outcomes | ✗ | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Conventional care | MoR, UAC, re-admissions |
| Crowe 2003,200 Canada | Rehab | Prehab | Improve outcomes and recovery, reduce LOS | ✗ | Hospital, clinic, patient home | MDT, family | Usual care | MoR, complications | ||||
| den Hertog 2012202, Germany | Pathway-controlled fast track Rehab | Rehab | Improve recovery | ✗ | Hospital, rehabilitation centre | NR | Standard PO rehabilitation | QoL, complications | ||||
| Dwyer 2012,206 UK | Enhanced Recovery Pathway | ERP | Improve recovery | ✗ | ✗ | ✗ | ✗ | ✗ | Assumed outpatient clinic, hospital | Surgeons, nurses, PTs, OTs, patient as active participant, family | Conventional care | Re-admissions |
| Gordon 2011,216 UK | Rapid recovery programme | ERP | Reduce LOS | ✗ | ✗ | ✗ | ✗ | ✗ | Hospital, outpatient clinic | MDT, family, friend | Pre-rapid recovery programme | None |
| Harari 2007,218 UK | POPS | ERP | Improve access to elective surgery for vulnerable older people | ✗ | ✗ | ✗ | Hospital, clinic, patient home | Consultant geriatrician, nurse specialist in older people, OT, PT, SW | Pre-POPS cohort | Mortality, complications, re-admissions | ||
| Hoogeboom 2010,221 the Netherlands | Preoperative therapeutic exercise | Prehab | Prevent decline of functional health status when waiting for surgery | ✗ | Patient home | NR | Usual PrO/PO care | QoL, MoR, complications | ||||
| Huang 2012,222 Taiwan | Home rehab education programme | Prehab | Improve range of motion of the knee, reduce pain, decrease LOS | ✗ | Patient home | PT | Conventional care | Complications, costs, MoR | ||||
| Huddleston 2004,223 USA | Hospitalist–orthopaedic team (co-management care) | Staff mix | Improve efficiency and quality of care | ✗ | ✗ | Hospital | Hospitalist faculty (no residents), consultative medical specialty teams (faculty and resident) | Standard care | Complications, costs | |||
| Hunt 2009,224 Salmon 2013,260 UK | Rapid discharge policy | ERP | Reduce LOS, maintain functional recovery and QoL | ✗ | ✗ | ✗ | ✗ | ✗ | Hospital, outpatient clinic | MDT | Comparator 1: usual care in large regional centre surgical unit. Comparator 2: usual care in treatment centre | Costs, QoL, MoR, UAC, satisfaction |
| Khan 2014,230 UK | Enhanced recovery programme | ERP | Reduce early mortality after surgery and LOS. Reduce perioperative blood loss, facilitate earlier mobilisation | ✗ | ✗ | ✗ | ✗ | ✗ | Two hospital sites | MDT | Traditional care | Complications, mortality, re-admissions |
| Larsen 2008,234 2008,233 Denmark | Accelerated perioperative care and rehab | ERP | Reduce LOS | ✗ | ✗ | ✗ | ✗ | ✗ | Hospital | MDT, family | Usual care | Costs, QoL, re-admissions |
| Maempel 2015,238 UK | ERP | ERP | Reduce LOS without adversely affecting functional recovery and ROM at 1 year post operation | ✗ | ✗ | ✗ | Hospital | MDT | Pre-ERP | Complications, re-admissions, MoR | ||
| Maempel 2016,237 UK | ERP | ERP | Improve joint function | ✗ | ✗ | ✗ | Outpatient clinic, hospital | MDT | Traditional care | Re-admissions, MoR, mortality | ||
| Malviya 2011,241 UK | Enhanced recovery programme | ERP | Reduce LOS and early complications | ✗ | ✗ | ✗ | Hospital, assumed outpatients clinic | MDT | Traditional protocol | Mortality, complications, re-admissions | ||
| McGregor 2004,244 UK | Preoperative Hip Rehab Advice | Prehab | Aid patient recovery | ✗ | NR | NR | Standard care | QoL, MoR, costs | ||||
| Mertes 2013,245 UK | Integrated care pathway | ERP | Reduce LOS, improve surgery re-admission rate and postoperative LOS | ✗ | ✗ | ✗ | ✗ | ✗ | Outpatient clinic, hospital | MDT, family, carers | Standard PrO treatment | None |
| Pengas 2015,253 UK | Weekend physiotherapy | Rehab | Faster rehabilitation and recovery | ✗ | Hospital | PT assistant | Weekday PT only | MoR | ||||
| Pour 2007,254 USA | Group 1: standard incision, enhanced protocol. Group 2: small incision, enhanced protocol | ERP | To assess influence of intervention on outcomes of total hip arthroplasty | ✗ | ✗ | ✗ | Outpatient clinic, hospital | MDT, family | Group 3: standard incision, standard protocol. Group 4: small incision, standard protocol | Complications, QoL, MoR, mental health | ||
| Reilly 2005,256 UK | Accelerated recovery protocol | ERP | Reduce pain to allow for early mobilisation | ✗ | ✗ | Hospital, patient home | MDT | Standard care | MoR complications, satisfaction, costs | |||
| Siggeirs dottir 2005,261 Iceland | Preoperative education and training programme/rehab and nursing | ERP | Reduce LOS | ✗ | ✗ | ✗ | Outpatient clinic, patient home | PT, OT | Conventional care | Complications, MoR re-admissions | ||
| Starks 2014,262 UK | Enhanced recovery pathway | ERP | Improve patient care, reduce LOS | ✗ | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Pre-ERP | Mortality, re-admissions |
| Vesterby 2017,267 Denmark | Telemedicine support | Rehab | Reduce LOS | ✗ | ✗ | Outpatient clinic, patient home | PT, support persona | Fast track | Re-admissions, UAC | |||
| Williamson 2007,269 UK | Physiotherapy | Prehab | Improve condition before surgery, improve recovery | ✗ | NR | PT | Home exercise | QoL, mental health, MoR | ||||
| Pelvic surgery | ||||||||||||
| Arumainayagam 2008,194 UK | ERP | ERP | Reduce LOS | ✗ | ✗ | Hospital | MDT | Prior to ERP implementation | Complications, MoR, mortality, re-admissions | |||
| Gralla 2007;217 Magheli 2001,240 Germany | Fast track surgery | ERP | Allow hospital discharge 3 days after surgery without additional complication; improve outcomes (pain, mobilisation, recovery of intestinal function), reduce LOS | ✗ | ✗ | ✗ | ✗ | University hospital; hospital | MDT | Conventional care | Complications, MoR, satisfaction, re-admissions | |
| Jensen 2015,225 Denmark | Multi-professional rehab programme | ERP | Reduce LOS | ✗ | ✗ | Hospital, patient home | MDT | Standard care | Complications, MoR, mortality, re-admissions | |||
| Mukhtar 2013,250 UK | ERP | ERP | Improve inpatient hospital stay while ensuring clinical safety | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Pre-ERP | MoR, complications | |
| Thoracic surgery | ||||||||||||
| Brunelli 2017,197 UK | ERP | ERP | Improve outcome benefits | ✗ | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Pre-ERP: TM | Re-admissions, mortality, complications |
| Gatenby 2015,212 UK | ERAS | ERP | Reduced total LOS and stay on CCU | ✗ | ✗ | ✗ | ✗ | ✗ | Teaching Hospital | MDT | Pre-ERP | Mortality, re-admissions |
| Karran 2016,228 UK | Multimodal perioperative pathway | ERP | Improve recovery, reduce LOS, reduce morbidity/mortality | ✗ | ✗ | ✗ | ✗ | Outpatient clinic and hospital | MDT | Pre-ERP | Re-admissions, mortality | |
| Muehling 2008,248 Germany | ERP | ERP | Reduce pulmonary complications | ✗ | ✗ | ✗ | Hospitalb | MDT | Pre-pathway: conservative treatment | Complications | ||
| Surgery to remove tumours (various locations) | ||||||||||||
| Hempenius 2013220 and 2016,219 the Netherlands | LIFE study | PACP | Prevent postoperative delirium | ✗ | University medical centre, teaching hospital, community hospital | Geriatric team | Standard care | Complications, QoL, mortality, re-admissions, mental health | ||||
| Upper abdominal surgery | ||||||||||||
| Abu Hilal 2013,191 UK | ERP | ERP | Assess feasibility and safety of ERP, reduce LOS | ✗ | ✗ | ✗ | Hospital | MDT | Traditional management | MoR, mortality, complications, re-admissions | ||
| Dasari 2015,201 UK | ERP | ERP | Reduce LOS | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Pre-pathway/ERP | Complications, MoR, re-admissions | |
| Dunne 2016,205 UK | Prehabilitation Exercise Programme | Prehab | Improve fitness to improve recovery | ✗ | NR | NR | Standard care | Re-admissions, QoL mental health | ||||
| Jones 2013,226 UK | ERP | ERP | Reduce morbidity and LOS | ✗ | ✗ | ✗ | Hospital | MDT | Standard care | QoL mortality, MoR, complications, re-admissions | ||
| Kapritsou 2017,227 Greece | Fast-track recovery programme | ERP | Improve care, improved management of stress and pain | ✗ | ✗ | Oncology hospital | MDT | Conventional care | Mental health, MoR, complications | |||
| Richardson 2015,257 UK | ERP | ERP | Reduce LOS, maintain patient safety | ✗ | ✗ | ✗ | ✗ | Hospital, outpatient clinic | MDT | Traditional management | Complications, MoR, mortality, costs | |
| Sutcliffe 2015,263 UK | ERP | ERP | Improve clinical outcomes | ✗ | ✗ | ✗ | ✗ | Hospital | MDT | Pre-ERP | Complications, mortality, re-admissions | |
| Tanaka 2017,264 Japan | ERAS | ERP | Reduce LOS, enhance recovery | ✗ | ✗ | ✗ | Hospital | MDT | Conventional care | Complications, MoR, re-admissions, costs | ||
| Various | ||||||||||||
| Ellis 2012,207 UK | Preoperative assessment and care plan | PACP | Identify high-risk patients and give appropriate care | ✗ | Clinic, hospital, patient home as appropriate | Nurse, OT | Pre-programme: routine care | Complications, mental health | ||||
| Vascular surgery | ||||||||||||
| Muehling 2008,247 2009,246 2011,249 Germany | Fast-track regime | ERP | Reduce morbidity and mortality after major surgical procedures | ✗ | ✗ | ✗ | Hospital | MDT | Pre-pathway: conservative treatment | Complications, MoR, mortality, re-admissions | ||
| Partridge 2017,31 UK | Preoperative assessment and care plan | PACP | Optimise care to decrease mortality and morbidity | ✗ | ✗ | Clinic for CGA, hospital for delivery of care plan (assumed) | MDT (geriatrician, CN specialist, SW, OT) depending on patient need, family, carers, GP | Standard PrO assessment | Complications, re-admissions, MoR | |||
Primary intervention delivery sites were at hospital, outpatient clinic, rehabilitation centre or the patient’s home, or a combination of these (see Table 3). The patient usually received the intervention alone, except in 14 studies31,193,195,200,206,211,216,221,232,234,235,245,254,258 where a family member or carer was actively involved in the intervention; in two interventions216,267 that included the involvement of friends or a non-specified support person; in two studies31,245 that involved carers; and in one study31 that involved GPs in the intervention. Ten studies197,209,241,244,247,248,251,261,266,269 did not report the intervention recipient, in which case we assumed that this was just the patient.
Quality assessment
Only two studies,193,270 both of which were RCTs, received a global study quality rating of ‘strong’ using the EPHPP tool. 46 Twenty-six studies,194,196,199,202,205,211,213,215,217,220,222,223,225,227,232,234,235,239,242,243,256,258,264,267–269 of which 24 were RCTs, received a ‘moderate’ quality rating, and the remaining 45 studies191,192,195,197,198,200,201,203,204,206–210,212,214,216,218,221,224,226,228–231,236–238,241,244,245,247,248,250–255,257,259,261–263,266 (22 RCTs) were rated as ‘weak’. Quality ratings are displayed in Table 4, with the full breakdown of scores for each item provided in Report Supplementary Material 4.
| Study (first author and year) | Component rating (1 = strong, 2 = moderate, 3 = weak) | Global rating of paper (strong, no weak ratings; moderate, 1 weak rating; weak, ≥ 2 weak ratings) | ||||||
|---|---|---|---|---|---|---|---|---|
| Selection bias | Study design | Confounders | Blinding of assessors and participants | Data collection methods | Withdrawals and dropouts | Is it clear how LOS is defined (Y/N)?a | ||
| Abdominal surgery | ||||||||
| Chen 2017199 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Cardiac surgery | ||||||||
| Arthur 2000193 | 2 | 1 | 1 | 2 | 1 | 1 | Y | Strong |
| Fleming 2016208 | 2 | 3 | 1 | 2 | 3 | 3 | Y | Weak |
| Furze 2009210 | 3 | 1 | 1 | 2 | 3 | 1 | Y | Weak |
| Goodman 2008215 | 3 | 1 | 1 | 2 | 1 | 1 | Y | Moderate |
| Probst 2014255 | 2 | 1 | 3 | 3 | 1 | 1 | Y | Weak |
| Rosenfeldt 2011258 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Salhiyyah 2011259 | 2 | 3 | 1 | 2 | 3 | 1 | Y | Weak |
| van der Peijl 2004266 | 2 | 1 | 3 | 2 | 3 | 3 | N | Weak |
| Colorectal surgery | ||||||||
| Anderson 2003192 | 2 | 1 | 3 | 3 | 3 | 3 | Y | Weak |
| Carli 2010198 | 2 | 3 | 3 | 2 | 3 | 1 | N | Weak |
| Dronkers 2010204 | 2 | 1 | 3 | 2 | 3 | 1 | N | Weak |
| Forsmo 2016209 | 2 | 1 | 1 | 3 | 3 | 1 | Y | Weak |
| García-Botello 2011211 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Gatt 2005213 | 2 | 1 | 2 | 2 | 3 | 1 | N | Moderate |
| Gillis 2014214 | 2 | 1 | 3 | 2 | 3 | 1 | N | Weak |
| Khan 2013229 | 2 | 3 | 1 | 2 | 3 | 1 | N | Weak |
| Khoo 2007231 | 3 | 1 | 3 | 2 | 3 | 1 | N | Weak |
| King 2006232 | 2 | 3 | 1 | 2 | 1 | 1 | Y | Moderate |
| Lee 2011235 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Lidder 2013236 | 3 | 1 | 1 | 2 | 3 | 3 | N | Weak |
| Maggiori 2017239 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Mari 2016242 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Mari 2016242 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Muller 2009251 | 2 | 1 | 3 | 3 | 3 | 1 | N | Weak |
| Pappalardo 2016252 | 2 | 3 | 1 | 2 | 3 | 3 | Y | Weak |
| Dhruva Rao 2015203 | 2 | 3 | 1 | 2 | 3 | 1 | N | Weak |
| van Bree 2011;265 Vlug 2011268 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Lower limb arthroplasty | ||||||||
| Barlow 2013195 | 2 | 3 | 1 | 2 | 3 | 2 | Y | Weak |
| Borgwardt 2009196 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Crowe 2003200 | 2 | 1 | 3 | 2 | 3 | 1 | N | Weak |
| den Hertog 2012202 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Dwyer 2012206 | 2 | 3 | 1 | 2 | 3 | 1 | Y | Weak |
| Gordon 2011216 | 2 | 3 | 1 | 2 | 3 | 1 | N | Weak |
| Harari 2007218 | 2 | 3 | 1 | 2 | 3 | 1 | N | Weak |
| Hoogeboom 2010221 | 3 | 1 | 1 | 2 | 3 | 1 | N | Weak |
| Huang 2012222 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Huddleston 2004223 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Hunt 2009;224 Salmon 2013260 | 2 | 3 | 1 | 2 | 3 | 1 | Y | Weak |
| Khan 2014230 | 1 | 3 | 3 | 2 | 3 | 1 | N | Weak |
| Larsen 2008234 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Maempel 2015238 | 2 | 3 | 1 | 2 | 3 | 1 | N | Weak |
| Maempel 2016237 | 2 | 3 | 3 | 2 | 3 | 1 | Y | Weak |
| Malviya 2011241 | 2 | 3 | 3 | 2 | 3 | 1 | N | Weak |
| McGregor 2004244 | 2 | 3 | 3 | 2 | 3 | 1 | N | Weak |
| Mertes 2013245 | 2 | 3 | 3 | 2 | 3 | 3 | N | Weak |
| Pengas 2015253 | 2 | 3 | 3 | 2 | 3 | 1 | N | Weak |
| Pour 2007254 | 2 | 1 | 3 | 2 | 3 | 1 | N | Weak |
| Reilly 2005256 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Siggeirsdottir 2005261 | 2 | 1 | 3 | 2 | 3 | 1 | N | Weak |
| Starks 2014262 | 2 | 3 | 1 | 1 | 3 | 1 | N | Weak |
| Vesterby 2017267 | 2 | 1 | 3 | 2 | 1 | 1 | N | Moderate |
| Williamson 2007269 | 2 | 1 | 1 | 2 | 1 | 3 | N | Moderate |
| Pelvic surgery | ||||||||
| Arumainayagam 2008194 | 2 | 3 | 1 | 1 | 1 | 2 | Y | Moderate |
| Gralla 2007;217 Magheli 2011240 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Jensen 2015225 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Mukhtar 2013250 | 2 | 3 | 1 | 1 | 3 | 1 | Y | Weak |
| Thoracic surgery | ||||||||
| Brunelli 2017197 | 2 | 3 | 1 | 1 | 3 | 1 | Y | Weak |
| Gatenby 2015212 | 2 | 3 | 3 | 2 | 3 | 1 | N | Weak |
| Karran 2016228 | 2 | 3 | 1 | 2 | 3 | 1 | N | Weak |
| Muehling 2008248 | 3 | 1 | 1 | 2 | 3 | 1 | N | Weak |
| Surgery to remove tumours | ||||||||
| Hempenius 2013;220 2016219 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Upper abdominal surgery | ||||||||
| Abu Hilal 2013191 | 2 | 3 | 1 | 2 | 3 | 3 | Y | Weak |
| Dasari 2015201 | 2 | 3 | 3 | 2 | 3 | 1 | Y | Weak |
| Dunne 2016205 | 2 | 1 | 1 | 2 | 3 | 1 | N | Moderate |
| Jones 2013226 | 2 | 1 | 3 | 2 | 3 | 1 | Y | Weak |
| Kapritsou 2017227 | 2 | 1 | 1 | 2 | 3 | 1 | Y | Moderate |
| Richardson 2015257 | 2 | 3 | 1 | 2 | 2 | 3 | Y | Weak |
| Sutcliffe 2015263 | 2 | 3 | 1 | 2 | 3 | 1 | N | Weak |
| Tanaka 2017264 | 2 | 1 | 1 | 3 | 1 | 1 | Y | Moderate |
| Various surgeries | ||||||||
| Ellis 2012207 | 2 | 3 | 3 | 2 | 3 | 1 | N | Weak |
| Vascular surgery | ||||||||
| Muehling 2008;247 2009;246 2011249 | 2 | 3 | 1 | 2 | 3 | 1 | N | Weak |
| Partridge 201731 | 1 | 1 | 1 | 2 | 1 | 1 | N | Strong |
The component that contributed most towards the large number of ‘weak’ global ratings was the ‘data collection methods’ item, on which only nine studies193,194,215,232,255,264,267,269,270 received a ‘strong’ rating and one study received a ‘moderate’ rating. 257 To receive a rating of ‘strong’ for this domain, studies had to have discussed the reliability and validity of their primary outcome measure, unless this was well known. In addition, while not contributing towards the global rating of each paper, only 31191–195,197,201,202,206,208–210,215,217,223–227,232,234,235,237,239,250,252,255,257,259,264,268 of the 73 studies prioritised for synthesis (43.2%) clearly defined their LOS outcome. Only two studies229,270 achieved a ‘strong’ rating on the selection bias component, with eight studies205,210,215,221,231,236,238,248 scoring ‘weak’ and the rest scoring ‘moderate’.
Forty-two studies192,193,196,199,200,202,204,205,209–211,213–215,217,220–223,225–227,231,234–236,239,242,243,248,251,254–256,258,261,264,266–270 received a ‘strong’ rating on the study design component, with 31 scoring as ‘weak’. 191,194,195,197,198,201,203,206–208,212,216,218,224,228–230,232,237,238,241,244–247,249,250,252,253,257,259,260,262,263 All of the strongly scoring studies for this component were RCTs. All UBA studies prioritised for inclusion were rated as ‘weak’, as were the two controlled trials. 224,259 Six RCTs198,217,242,244,247,252 received a ‘weak’ rating because randomisation was not described. On the component considering the likelihood of possible confounding of results, the majority of studies were rated as ‘strong’, although one study213 received a rating of ‘moderate’ and 20 were rated as ‘weak’. 192,198,200,201,204,207,212,214,226,230,231,237,241,244,245,251,253–255,261,266,267 Regarding the blinding of outcome assessors and study participants, four studies194,197,250,262 were rated as ‘strong’ and five studies192,209,251,255,264 received a ‘weak’ rating, with the remaining studies ‘moderate’. Studies scored most highly in terms of reporting of numbers and reasons for withdrawal and dropouts. All but 11 studies191,192,194,195,208,236,245,252,257,266,269 were rated as ‘strong’ on this component, including 20 of the 23 UBA studies prioritised for synthesis. 194,195,197,201,203,206,207,212,216,218,228,230,232,237,238,241,250,253,262,263 Two studies194,195 were scored as ‘moderate’ on this item, and 10 studies191,192,208,236,242,245,252,257,266,269 obtained a ‘weak’ rating.
Synthesis of evidence from randomised controlled trials
Interventions to improve recovery from colorectal surgery: randomised controlled trials
There were 17 RCTs (published in 18 papers) evaluating interventions intended to improve recovery and/or reduce LOS for older adults following elective colorectal surgery. Of these, 14 studies192,199,209,211,213,231,235,236,239,242,243,251,252,265,268 evaluated ERP interventions and three198,204,214 trialled prehab. All ERP interventions were compared with usual or standard care, while prehab interventions were compared with walking and breathing exercises,198 rehabilitation214 and home-based exercise advice. 204 The study by Vlug and colleagues268 included comparisons of ERP and standard care in both open and laparoscopic surgery as separate intervention arms.
Four studies were conducted in the UK,192,213,231,236 three were conducted in Italy,242,243,252 two were conducted in each of the Netherlands204,268 and Canada,198,214 and single studies originated from Spain,211 France,239 Switzerland,251 Norway,209 Taiwan199 and South Korea. 235 A total of 3079 people commenced trials, with sample sizes ranging from 25192 to 427. 268 Participants were admitted for hemicolectomy (left and right), resection of malignant lesions, colonic reconstruction, colorectal resection, rectal resection, high anterior resection, colonic resection and extraperitoneal rectal surgery. Across the included studies, the mean proportion of female participants was 44%. Ages within included studies were reported as either median or mean and can be viewed in Table 2.
The two studies by Mari and colleagues242,243 utilised the same intervention with different samples, the latter study focusing on patients of ≥ 70 years of age. van Bree and colleagues265 reported on a small sample of the original 427 participants in the study by Vlug and colleagues268 focusing on recovery of gastrointestinal transit. In this case, we report outcome data from the original sample in the study by Vlug and colleagues268 only, except where van Bree and colleagues265 reported additional, unique outcomes. The study by Chen and colleagues199 included patients undergoing procedures that could be categorised as both colorectal and upper abdominal surgery; we report the data for patients undergoing left and right hemicolectomies as distinct groups in this section, with those undergoing gastrectomy or pancreaticoduodenectomy reported in the upper abdominal section (see Interventions to improve recovery from upper abdominal surgery: randomised controlled trials). 199
Overview: colorectal surgery
Thirteen RCTs evaluated the effectiveness of ERP interventions to improve recovery from colorectal surgery, and three evaluated prehab interventions. This body of evidence is summarised in Table 5. Full details of the effectiveness of interventions in this type of surgery are reported in ERP interventions and Prehab interventions.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Chen 2017,199 Taiwan | 535 | ERP | FM | EON; COMM; EMOB | Oral and nutritional assistance protocol | ◁▷(2/2) | ⇦⇨(2/2) | |||||||||
| García-Botello 2011,211 Spain | 125 | ERP | AEI | PreM; nMBP; nFAST | ANE; FM; WARM PONV | EON; EMOB; nNGT; CATH | ▲ | ◁▷(4/4) | ⇦⇨ | x(6/6) | ||||||
| Gatt 2005,213 UK | 39 | ERP | AEI; PBio | nMBP; CHL; nFAST | ANA; DRA; FM; nNGT; SURG | EON; EMOB | x | ⇦⇨ | ⇦⇨ | ⇦⇨ | x | ⇦⇨ | ||||
| Lee 2011,235 South Korea | 100 | ERP | FM | EON; EMOB; LAX; CATH | Pre admission: involvement of patient and family | x(2/2) | ⇦⇨ |
▲(6/10) ▲(1/10) ◁▷(3/10) |
||||||||
| Maggiori 2017,239 France | 270 | ERP | AEI | CHL; nFAST; PreM | PONV; ANE; FM; WARM | EON; EMOB; ANA | ◁▷(2/2) | ⇦⇨(2/2) | ⇦⇨ | |||||||
| Mari 2014,243 Italy | 52 | ERP | CHL; nFAST | ANE; FM; PONV | ANA; EON; EMOB; nNGT | ▲ | ⇦⇨ | ⇦⇨ | ▲(5/5) | |||||||
| Mari 2016,242 Italy | 83 | ERP | CHL; nFAST | ANE; FM; PONV | EON; EMOB; nNGT; ANA | ▲ | ⇦⇨ |
▲(4/12) ▲(4/12) ◁▷(4/12) |
||||||||
|
van Bree 2011,265 Vlug 2011,268 Netherlands, laparoscopic surgery |
93; 427 | ERP | AIE; EX | CHL; nFAST; PreM | ANA; FM; PONV | EON; EMOB; FM; NUT; LAX; CATH | Guided tour of surgical ward | △(2/2) | ⇦⇨(2/2) | ⇦⇨ | ⇦⇨ |
▲(2/9) ▲(4/9) △(1/9) ◁▷ (2/9) |
||||
| van Bree 2011,265 Vlug 2011,268 Netherlands, open surgery | 93; 427 | ERP | AIE; EX | CHL; nFAST; PreM | ANA; FM; PONV | EON; EMOB; FM; NUT; LAX; CATH | Guided tour of surgical ward | ◁▷(2/2) | ⇦⇨(2/2) | ⇦⇨ | ⇦⇨ |
▲(2/9) ▲(3/9) △(1/9) ◁▷(3/9) |
||||
| Anderson 2003,192 UK | 25 | ERP | AEI; NUT | nMBP; CATH; CHL; nFAST | ANE; ANA; nNGT; SURG | EON; EMOB | ▲ | ⇦⇨ | x |
▲(1/2) ◁▷(1/2) |
||||||
| Forsmo 2016,209 Norway | 324 | ERP | AEI | CHL; PreM | ANE; FM | LAX; ANA; CATH; EON; EMOB | x(2/2) | ⇦⇨(3/3) | ⇦⇨ | ⇦⇨ | x(5/5) | |||||
| Khoo 2007,231 UKa | 81 | ERP | NUT | ANE; FM; nNGT | EON; EMOB; ANA; CATH | x(2/2) | ⬆ | ⇦⇨ | ⇦⇨ | x(3/3) | ⇦⇨(3/3) | |||||
| Lidder 2013,236 UK | 57 | ERP | CHL | EON; NUT | ◁▷ |
⬆(1/4) ⇦⇨(3/4) |
◁▷ | |||||||||
| Muller 2009,251 Switzerland | 156 | ERP | FM; DRA | ANA; EON; FM; NUT | x |
⬆(2/3) ⇦⇨(1/3) |
⇦⇨ | |||||||||
| Pappalardo 2016,252 Italy | 50 | ERP | ANE; AP; TP | EON; EMOB; nNGT; ANA | x | ⇦⇨(3/3) | ⇦⇨ | x(4/4) | ||||||||
| Carli 2010,198 Canada | 133 | Prehab | EX | ◁▷ | ⇦⇨ | ◁▷(2/2) | ◁▷(2/2) | ◁▷ | ||||||||
| Dronkers 2010,204 the Netherlands | 42 | Prehab | AEI; EX | ◁▷ | ⇦⇨ |
▼(1/4) ◁▷(3/4) |
▼(2/7) ◁▷(5/7) |
|||||||||
| Gillis 2014,214 Canada | 89 | Prehab | AEI; EX; NUT; PT | Weekly telephone contact | ◁▷(2/2) | ⇦⇨ | ⇦⇨(2/2) | x | ◁▷(3/3) | ◁▷(8/8) | ||||||
Meta-analysis suggests that ERP interventions are associated with a statistically significant, medium-sized effect on LOS, reducing LOS by a mean of 1.8 days (range 0.0–5.1 days) compared with usual care (based on data from eight comparisons); however, this effect was weaker when a much older outlier study with a small sample size was removed from the analysis. 192 This change in LOS did not lead to negative consequences for other clinical outcomes, as meta-analyses indicated no statistically significant difference overall in the rates of re-admissions (data from seven comparisons) or complications (data from 15 comparisons). Furthermore, there was evidence from meta-analyses of statistically significant improvements on patient-reported outcomes following ERP interventions, including return of bowel function, meeting mobilisation goals earlier and achieving control of pain sooner, although these data always came from three or four studies and there was usually high statistical heterogeneity.
Three ERP intervention studies199,236,239 reported no significant differences in clinical outcomes compared with usual care. The interventions and comparators within two of these studies were atypical. The intervention evaluated by Chen et al. 199 mainly involved a series of tailored postoperative care protocols, added to existing ERP pathways which implemented few ERAS recommendations. For example, mechanical bowel preparation, overnight fasting, routine use of drains and nasogastric tubes were all implemented, in contradiction to ERAS Society guidelines. 271 The intervention evaluated by Lidder et al. 236 involved adding preoperative carbohydrate loading and additional postoperative nutrition to the existing ERP provided to the comparator group. This suggests that these elements alone are not enough to reduce LOS compared with existing standards of care, although caution is required because the studies were low to moderate quality, respectively.
The ERP intervention evaluated by Vlug and colleagues268 was effective in reducing LOS when a laparoscopic surgical technique was used, but not when an open approach was used, indicating the potential influence of the surgical technique on the effectiveness of an ERP. However, the statistical results for the two studies were marginally different, and the effects for patient-reported outcomes were similar for patients undergoing both types of surgery. It is unclear why the intervention was not more effective than usual care in the study by Maggiori and colleagues,239 except that there were a number of similar pathway elements between the ‘full fast-track’ pathway and the ‘limited fast-track’ pathway that constituted usual care (Table 6).
| ERAS item | Study (first author and year) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aForsmo 2016209 | aLidder 2013236 | Anderson 2003192 | Chen 2017199 | García-Botello 2011211 | Gatt 2005213 | Khoo 2007231 | Lee 2011235 | Maggiori 2017239 | bMari 2014,243 2016242 | Muller 2009251 | Pappalardo 2016252 | van Bree 2011;265 Vlug 2011268 | |
| Preoperative information, education and counselling | E | E | E | E | E, C | E | E, C | E | |||||
| Preoperative optimisation | E | E | E, C | ||||||||||
| Preoperative mechanical bowel preparation avoided | E | E | E | E, C | |||||||||
| Preoperative fasting: clear fluids allowed up to 2 hours and solids up to 6 hours prior to induction of anaesthesia | E, C | E | Ec | E,c Cc | E | E | E,c Cc | E | |||||
| Preoperative carbohydrate treatment | E | E | E | E | Ec | E | E | ||||||
| Pre-anaesthetic medication: no routine long- or short-acting sedatives (colonic); no advantages in using long-acting benzodiazepines, short-acting should not be given to those aged > 60 years (R/P) | E | E | E | ||||||||||
| Prophylaxis against thromboembolism | E, C | E, C | E, C | E | |||||||||
| Antimicrobial prophylaxis and skin preparation | E, C | E, C | E, C | E, C | E, C | E | |||||||
| Standard anaesthetic protocol | E | E, C | E | E, C | E, C | E, C | E | E, C | E | E, C | |||
| PONV prophylaxis | E | E | E | ||||||||||
| Laparoscopy and modifications of surgical access: recommended for colonic surgery | E,d Cd | E,d Cd | E, C | E,e Ce | E,d Cd | E, C | E, C | E, C | E, C | E, C | |||
| Nasogastric intubation: nasogastric tubes not routinely used | E, C | E, C | E | E | E, C | E, C | |||||||
| Nasogastric tubes removed before extubation | E, C | E | E | E | E | E, C | E | E, C | |||||
| Preventing intraoperative hypothermia | E, C | E, C | E, C | E, C | E, C | E, C | |||||||
| Perioperative fluid management: intraoperative fluids guided by flow measurements to optimise cardiac output | E, C | E, C | C | E | E | E | |||||||
| Perioperative fluid management: judicious use of vasopressors | E | E | |||||||||||
| Perioperative fluid management: enteral route for fluids postoperatively should be used as early as possible, and intravenous fluids should be discontinued as soon as is practicable | E | E, C | E | E | E | E | E | E | |||||
| No drainage of peritoneal cavity after colonic anastomosis | E,f C | E | E | E | E, C | ||||||||
| Transurethral catheter (R/P) | |||||||||||||
| Suprapubic catheter (R/P) | E | ||||||||||||
| Urinary drainage: routine transurethral bladder drainage for 1–2 days is recommendedg | E, C | E, C | E, C | E, C | E | ||||||||
| Early removal of bladder catheter | E | E | E | E | E | ||||||||
| Prevention of postoperative ileus: mid-thoracic epidural + laparoscopic surgery (colonic) | E,d Cd | E,d Cd | E | E, C | E | ||||||||
| Prevention of postoperative ileus: fluid overload and nasogastric decompression avoided | E | E | E | E | E | ||||||||
| Prevention of postoperative ileus: chewing gum (R/P), oral magnesium, alvimopan | E | ||||||||||||
| Postoperative laxatives and prokinetics (R/P) | E | E, C | E | E | |||||||||
| Postoperative analgesia. Open surgery: TEA using low-dose local anaesthetic and opioids; laparoscopic: an alternative to TEA is a carefully administered spinal analgesia with a low-dose, long-acting opioid | E, C | E | E, C | E | E, C | E, C | E | E | E | E, C | |||
| Early oral intake | E | E | E | E | E | E | E | E | E | E | E | E | |
| Perioperative nutritional care | E | E | E | E | E | E | E | E | E | E | E | ||
| Postoperative glucose control | |||||||||||||
| Early mobilisation | E | E | E | E | E | E | E | E | E | E, C | E | ||
The evidence for prehab interventions did not indicate a benefit for clinical or patient-reported outcomes, and it is notable that only three RCTs198,204,214 of ‘weak’ quality were identified. There were no significant differences in clinical outcomes for any study, and, although all of these studies measured additional outcomes, only Dronkers and colleagues204 identified a statistically significant difference between groups. Results from the study indicate an increase in the time taken to rise from a chair, a reduction in physical work capacity and an increase in fatigue among patients in the intervention group. 204 This outcome was unexpected, as patients in the prehab group appeared to have performed worse on three outcomes. However, baseline scores for these three measures were similar to post-intervention scores, with near-statistically significant differences between groups. 204 A fairer interpretation of the intervention’s effects may be that it had little influence on these outcomes, rather than a negative impact. As such, caution is needed when interpreting this finding, particularly in the light of the fact that confounders were not controlled for in the study, which received a global quality rating of ‘weak’ overall. With this in mind, the evidence suggests that prehab interventions alone may not convey beneficial or detrimental effects to older adults undergoing elective colorectal surgery.
Elements of prehab (exercise, physiotherapy, nutrition all prior to admission) were present in only three of the ERP interventions,192,213,268 with mixed effectiveness reported. Therefore, little evidence is available to determine whether or not the addition of prehabilitation to ERP interventions might convey benefits on patient recovery.
Caution is advised when interpreting the evidence for colorectal surgery. Study effects should be interpreted alongside detail of the intervention evaluated, as there was considerable heterogeneity in both the content of interventions, and the evidence of effects, as highlighted in Table 5. Furthermore, comparators were often poorly described. Study quality was ‘moderate’ or ‘weak’, with no studies globally rated as ‘strong’.
Enhanced recovery protocol interventions
Components of the ERP interventions and comparators are mapped against ERAS Society recommendations for colonic and rectal/pelvic surgery in Table 6. 272 The full details of both ERP and prehab interventions can be found in Report Supplementary Material 3, Tables 1–4. In summary, the most common ERP components were the provision of preoperative information, education and counselling (8/13 studies); avoidance of lengthy preoperative fasting (8/13); a standardised anaesthetic protocol (10/13); laparoscopic surgery used, where available (10/13); early removal of nasogastric tubes (8/13); thoracic epidural analgesia postoperatively (10/13); early oral intake postoperatively (12/13); perioperative nutritional care (11/13); and early mobilisation (11/13). The least common components were preoperative optimisation (3/13 studies); avoidance of pre-anaesthetic medication (3/13); active prophylaxis against postoperative nausea and vomiting (3/13); judicious use of vasopressors (2/13); transurethral (0/13) or suprapubic catheter (1/13) use; prevention of postoperative ileus with chewing gum, oral magnesium or alvimopan (1/13); and postoperative glucose control (0/13).
Excluding the item for glucose control (specific to patients with diabetes), interventions adhered to a mean number of 14.2 (45.7%) ERAS guideline items (range from 3252 to 24268 items), and comparator groups adhered to a mean number of 4.8 (15.6%) items (range from 0252 to 7211,236,251 items). Interventions branded as ERAS adhered to a mean of 15.5 (50%) of ERAS guideline items, compared with a mean of 13.9 (44.8%) of ERP interventions not branded as ERAS.
In the study by Chen and colleagues,199 the intervention (the Modified Hospital Elder Life Program) integrated relatively few ERAS Society-recommended components, but featured an additional focus on oral and nutritional assistance, with a postoperative communication protocol to facilitate orientation within the hospital environment.
Quality assessment
Seven studies211,213,235,239,242,243,268 were rated as ‘moderate’ overall quality; the rest were rated as ‘weak’. All studies received a rating of ‘weak’ for data collection methods because they failed to describe the reliability and validity of outcome measures. Only six studies192,209,235,239,252,268 clearly defined how LOS had been calculated. The study by Anderson and colleagues192 was rated as ‘weak’ on four domains (confounders, blinding, data collection methods, and withdrawals and dropouts), and five studies198,231,236,251,252 were rated as ‘weak’ on three domains. Seven studies209,211,235,239,242,243,268 scored ratings of ‘strong’ on three domains; in each case, these domains were study design, confounders, and withdrawals and dropouts.
Report Supplementary Material 5, Table 1, displays clinical outcome data from the 14 studies evaluating ERP interventions in older adults undergoing elective colorectal surgery. After imputation, standardised mean differences between groups for LOS were available in 10 group comparisons from eight studies. 192,199,211,236,239,242,243,268 A forest plot displaying the results of meta-analysis of these eight studies is displayed in Figure 2a, showing that ERP interventions are associated with a reduction in LOS when compared with usual care, associated with a medium effect size (d = –0.51, 95% CI –0.78 to –0.24; p < 0.001). Heterogeneity was large and statistically significant for this effect (I2 = 78.9%; p < 0.001), reflecting inconsistency in the evidence. The mean reduction in LOS was 1.8 days (SD 1.5, range 0.0–5.1 days). Examination of Figure 2a suggests that the effect size from the study by Anderson and colleagues192 may be an outlier. Because the study is 8 years older than any other in the analysis, and has the smallest sample size (data from 24 participants), a sensitivity analysis was performed by removing the study from the meta-analysis. The results are shown in the forest plot in Figure 2b, and indicate that there is still a statistically significant reduction in LOS with ERP interventions; however, the effect size reduces marginally (d = –0.45, 95% CI –0.71 to –0.19; p < 0.01) and the reduction in absolute terms decreases slightly to 1.6 days. Sensitivity analysis showing the influence of imputed data on LOS meta-analysis is available in Appendix 4.
FIGURE 2.
(a) Forest plot showing the results of meta-analysis of the effect of ERP vs. usual treatment on LOS following colorectal surgery; (b) forest plot of analysis with Anderson et al. 192 removed. Lap, laparoscopic surgery group; LH, left hemicolectomy/high anterior resection; open, open surgery group; RH, right hemicolectomy.

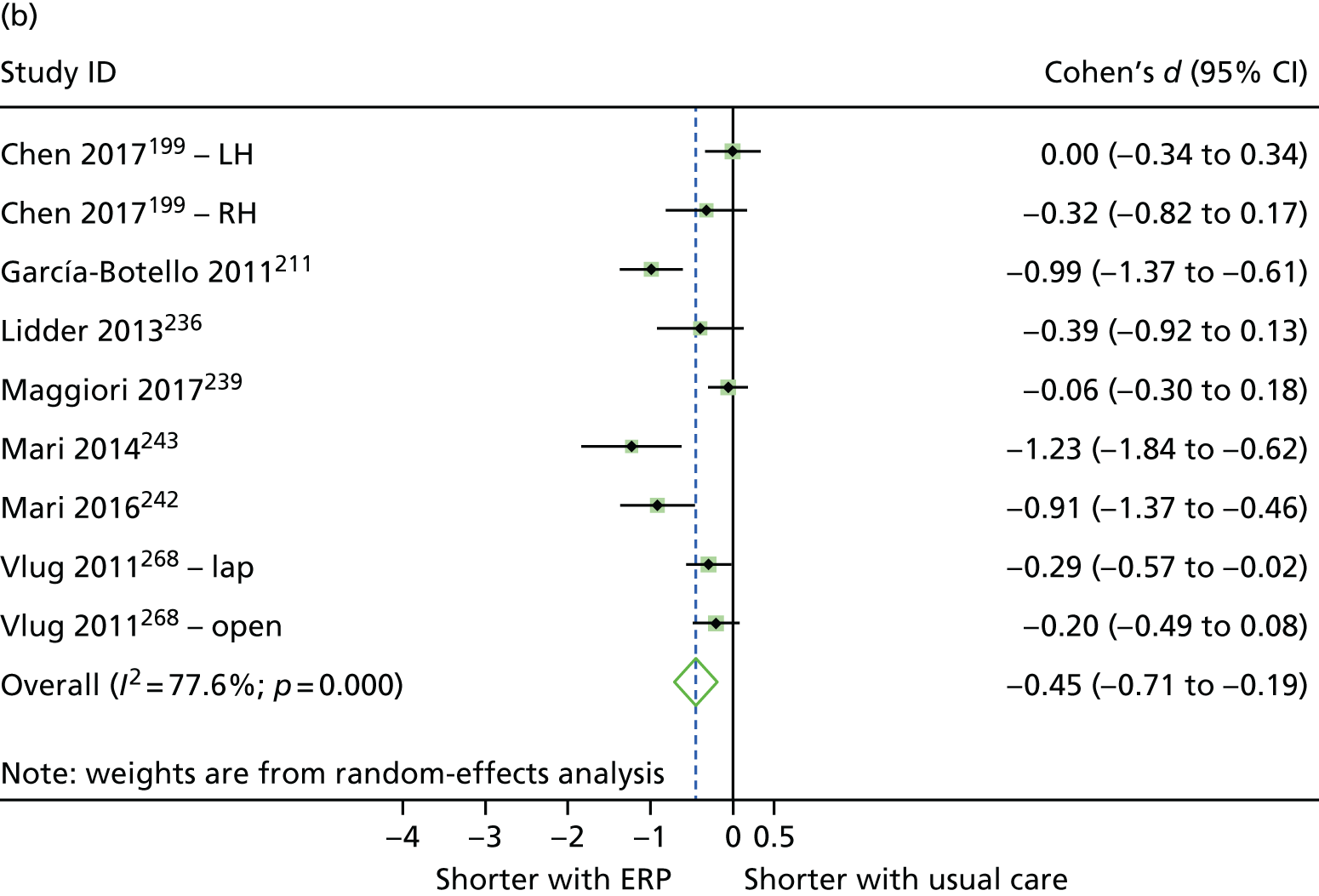
In the studies presenting data that could not be used to calculate standardised mean differences, the median LOS for patients receiving ERP interventions was lower in all cases. 209,213,231,235,251 Pappalardo and colleagues252 reported that patients receiving ERP had been discharged by postoperative day 6, but around half of those receiving usual care had not.
Nine studies, evaluating 10 comparisons, reported readmission rates following ERP interventions. Figure 3 is a forest plot displaying the results of meta-analysis of re-admission data, indicating the odds of re-admission were similar between ERP and usual care (OR 1.19, 95% CI 0.77 to 1.82). Thirteen studies, evaluating 15 comparisons, reported incidence of complications following ERP interventions. Meta-analysis indicates that the odds of experiencing a complication were not statistically significantly different in patients receiving ERP interventions from those receiving usual care (Figure 4), despite the summary OR indicating a trend towards a reduction in odds with ERP (OR 0.82, 95% CI 0.65 to 1.03).
FIGURE 3.
Forest plot showing the results of meta-analysis of the effect of ERP vs. usual treatment on the odds of re-admission following colorectal surgery. Lap, laparoscopic surgery group; open, open surgery group.
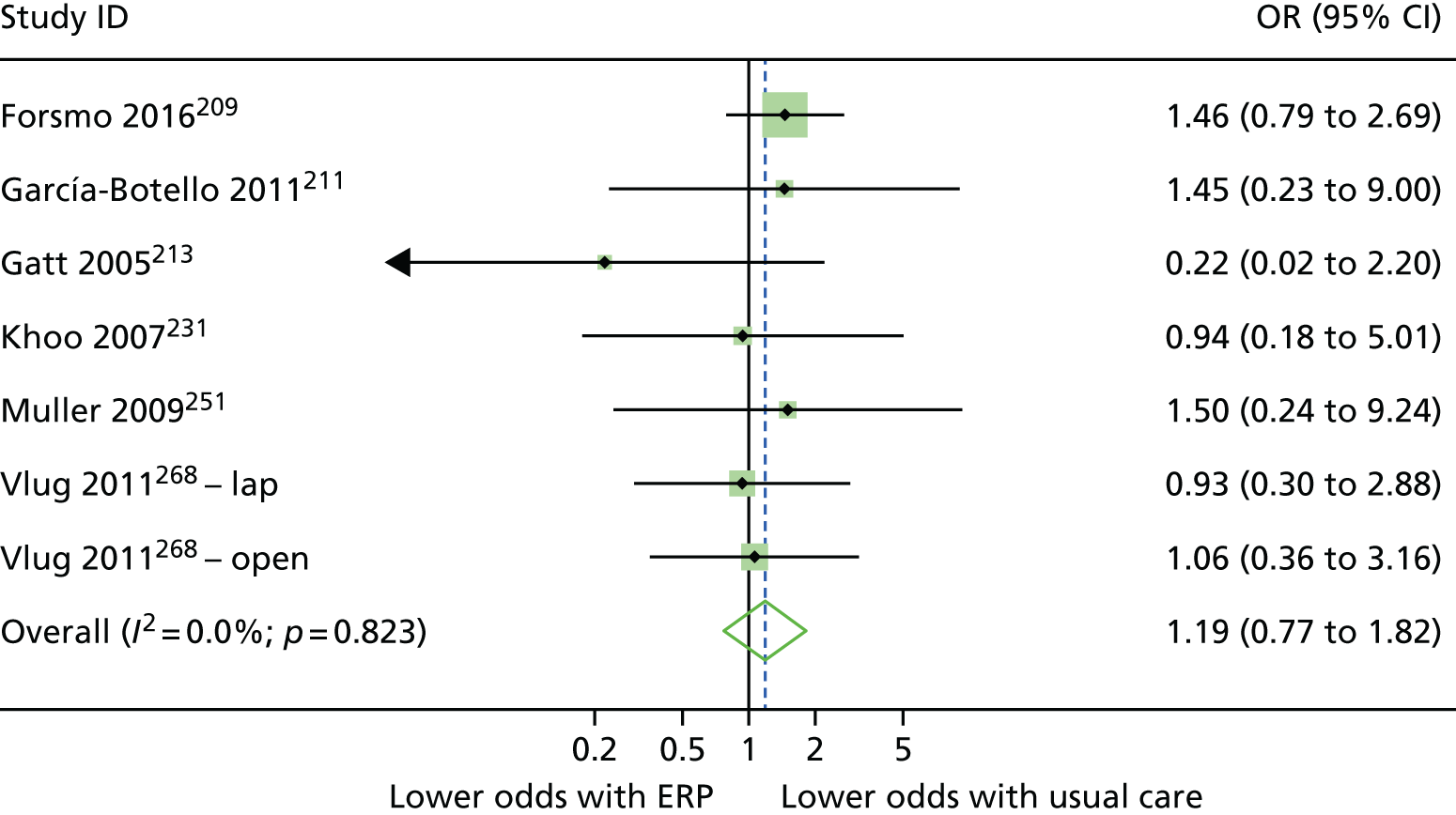
FIGURE 4.
Forest plot showing the results of meta-analysis of the effect of ERP vs. usual treatment on odds of complications following colorectal surgery. AR, anterior resection; lap, laparoscopic surgery group; LAR, lower anterior resection; LH, left hemicolectomy/high anterior resection; open, open surgery group; RH, right hemicolectomy.
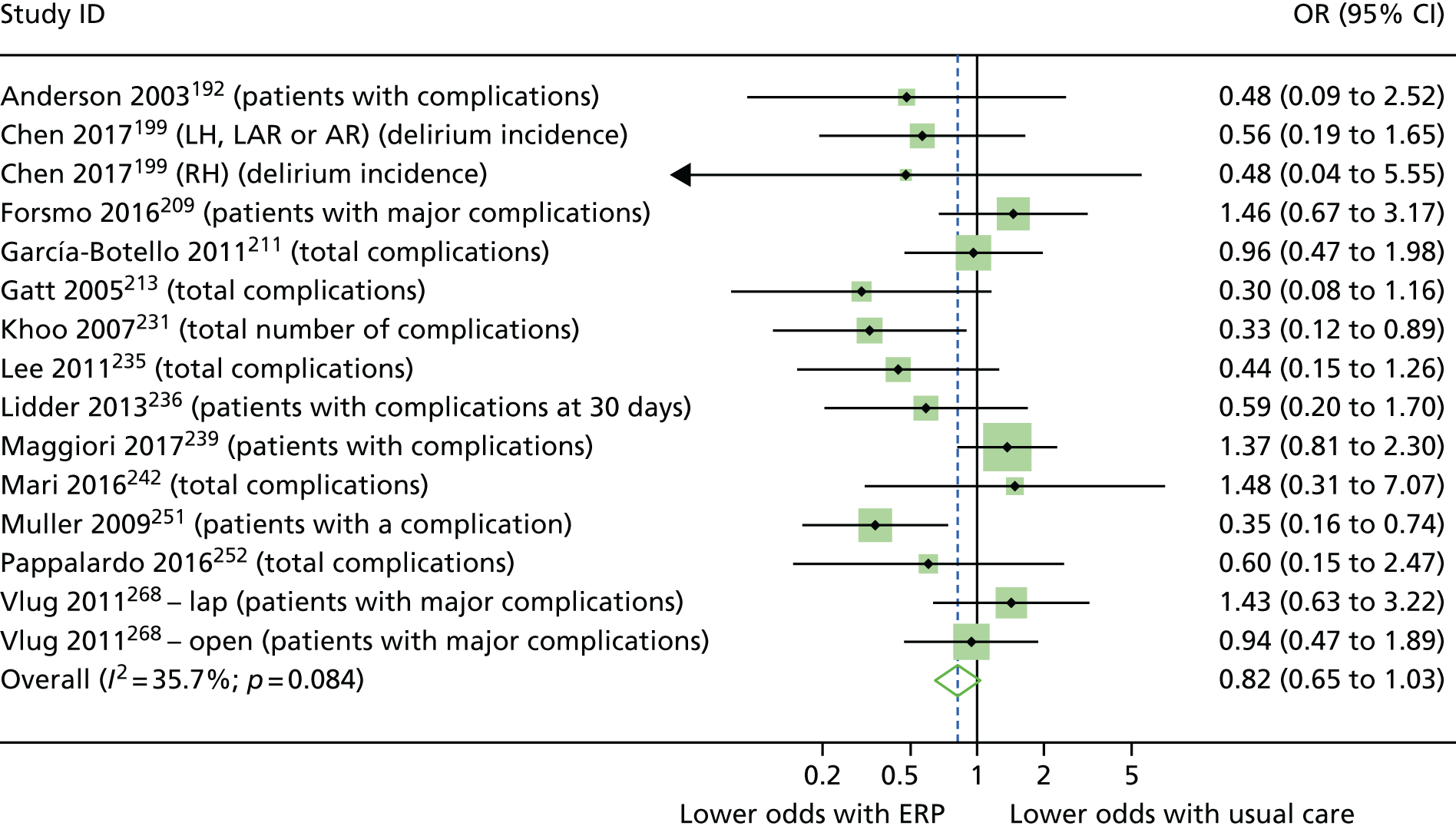
Report Supplementary Material 5, Table 2, displays data for patient-reported outcomes for RCTs trialling ERP interventions to improve recovery from elective colorectal surgery in older adults. Multiple outcomes were reported for markers of recovery across the included studies, allowing several meta-analyses to be performed. In total, five ERP studies192,235,242,243,268 contributed to meta-analyses of the following markers of recovery from surgery: time to first flatus, time to first stool, time to resumption of diet, time to reach mobilisation goals and time to reach pain control goals. There was insufficient data for meta-analysis of mental health or quality-of-life outcomes.
Figure 5a is a forest plot of the effect of ERP interventions on the time to resumption of diet after colorectal surgery, when compared with usual care. Across five group comparisons from four studies,235,242,243,268 there was a statistically significant reduction in time to resumption of diet, with a large effect size (d = –2.07, 95% CI –3.11 to –1.03; p < 0.001). In absolute terms, the mean reduction in time to resumption of diet after surgery was 1.7 days (SD 0.7, range 0.7–2.0 days). In Figure 5b, meta-analysis indicates that there was a medium-sized effect indicating earlier passage of first flatus in participants undergoing ERP (d = –0.64, 95% CI –1.05 to –0.24; p < 0.001), with a mean absolute difference of –0.7 days (SD 0.4, range –0.15 to –1.2 days). The evidence for passage of first stool was similar, occurring 1.4 days earlier with ERP (SD 1.2 days, range 0.3–1.4 days) with Figure 5c indicating a large pooled effect size (d = –1.12, 95% CI –1.81 to –0.43; p < 0.001).
FIGURE 5.
Forest plots displaying meta-analyses of patient-reported outcomes following colorectal surgery. (a) Time to resumption of diet; (b) time to passage of first flatus; (c) time to passage of first stool; (d) time to reach ambulation goals; and (e) time to reach pain reduction of control goals. Lap, laparoscopic surgery group; open, open surgery group.
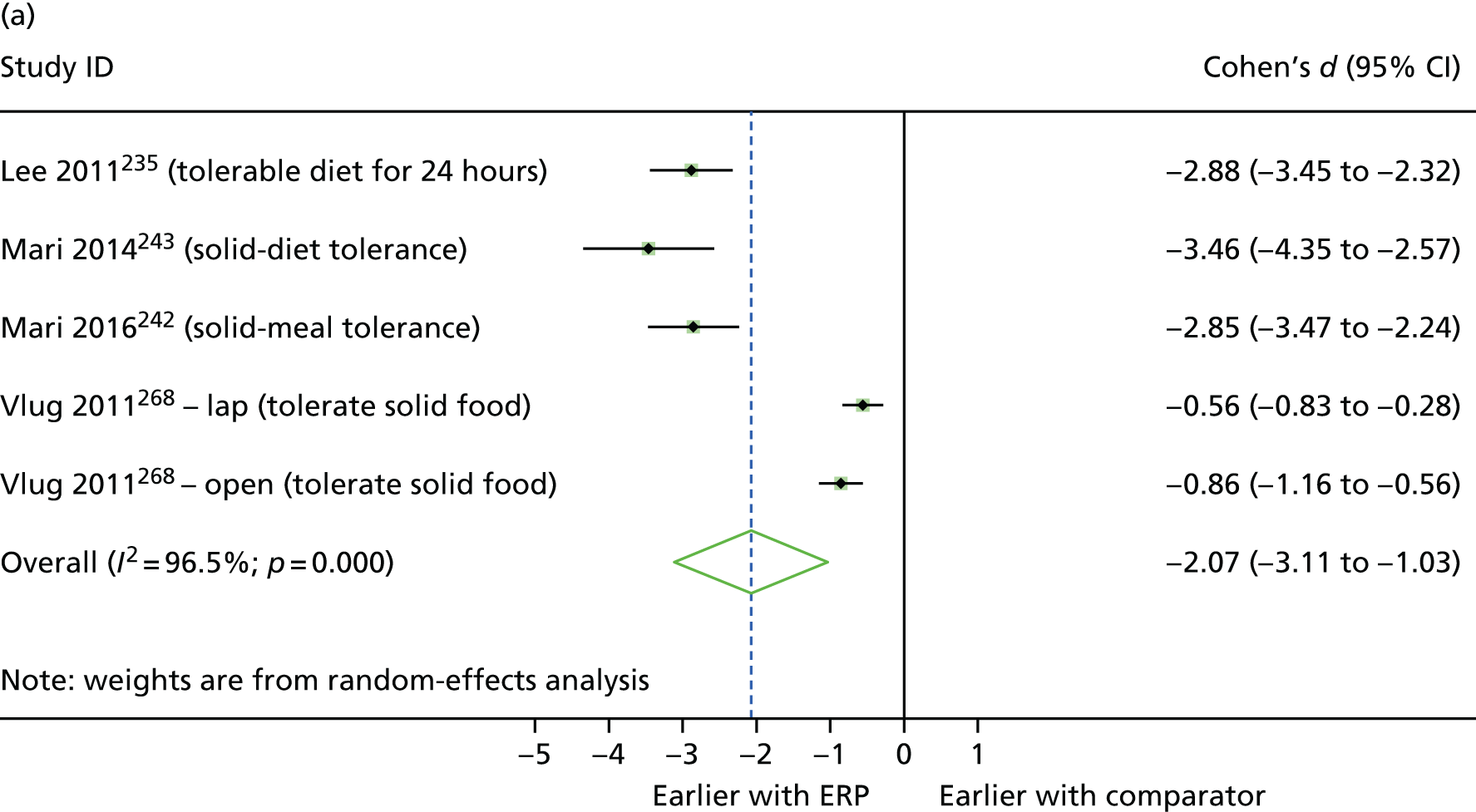
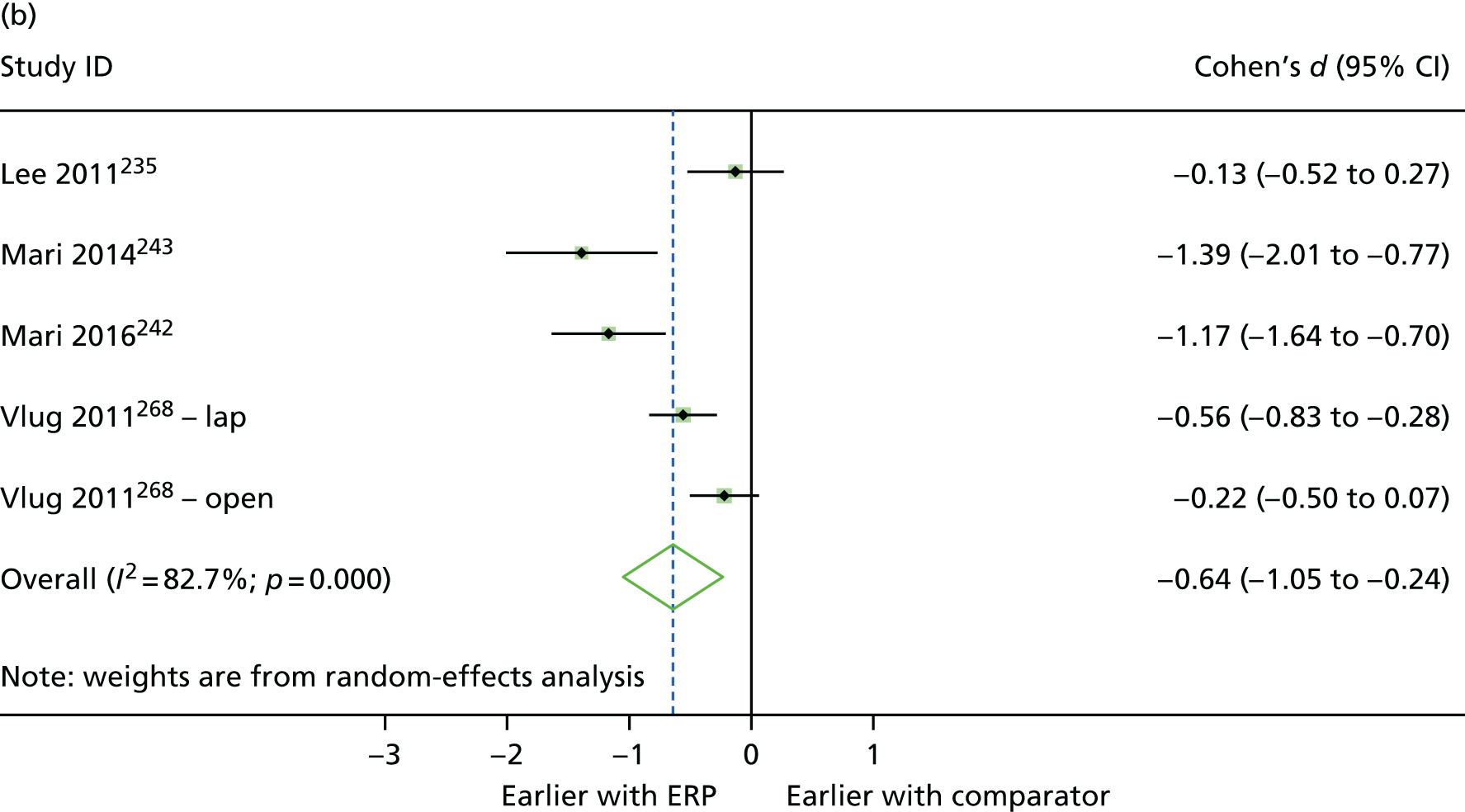
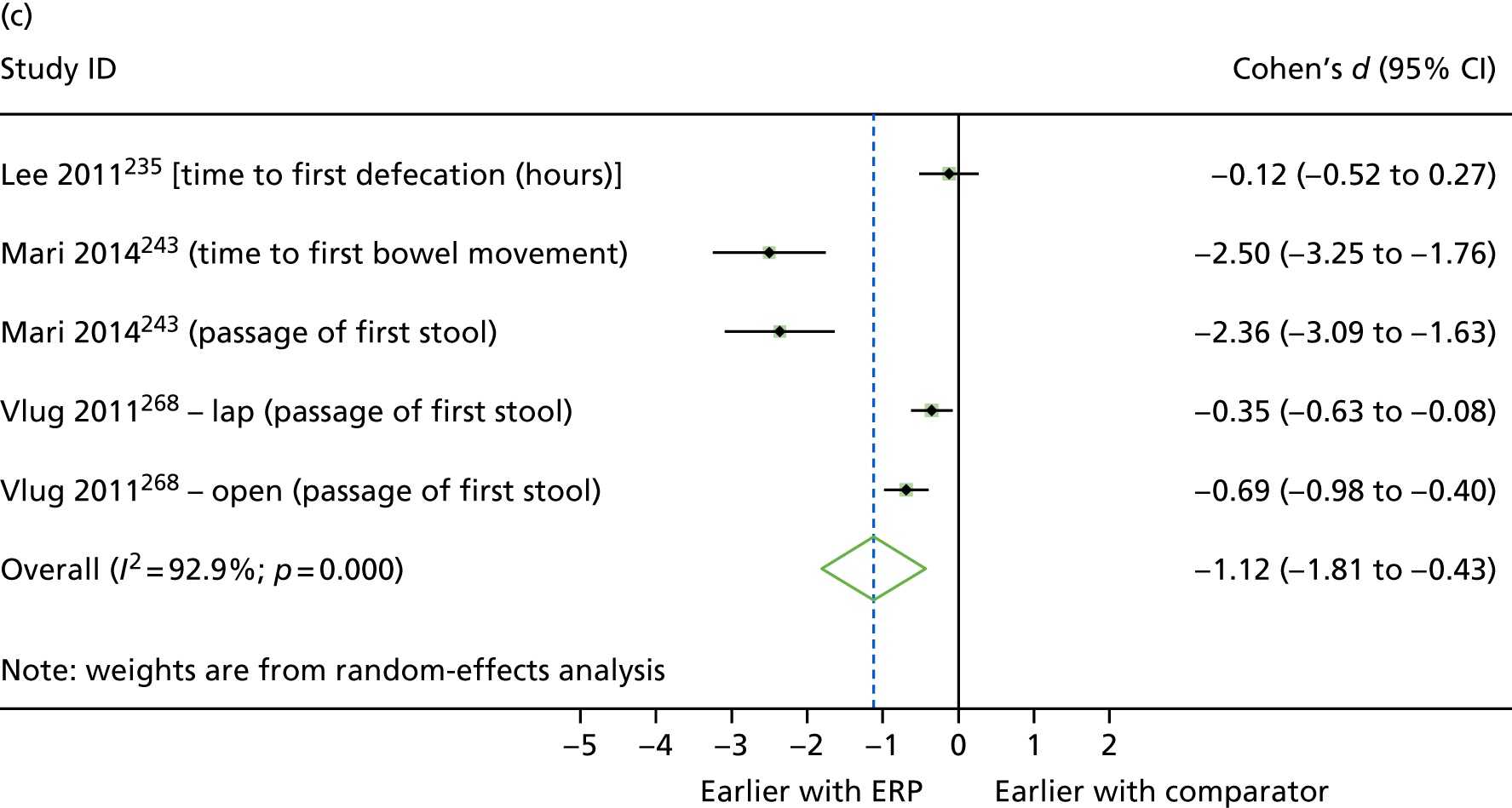
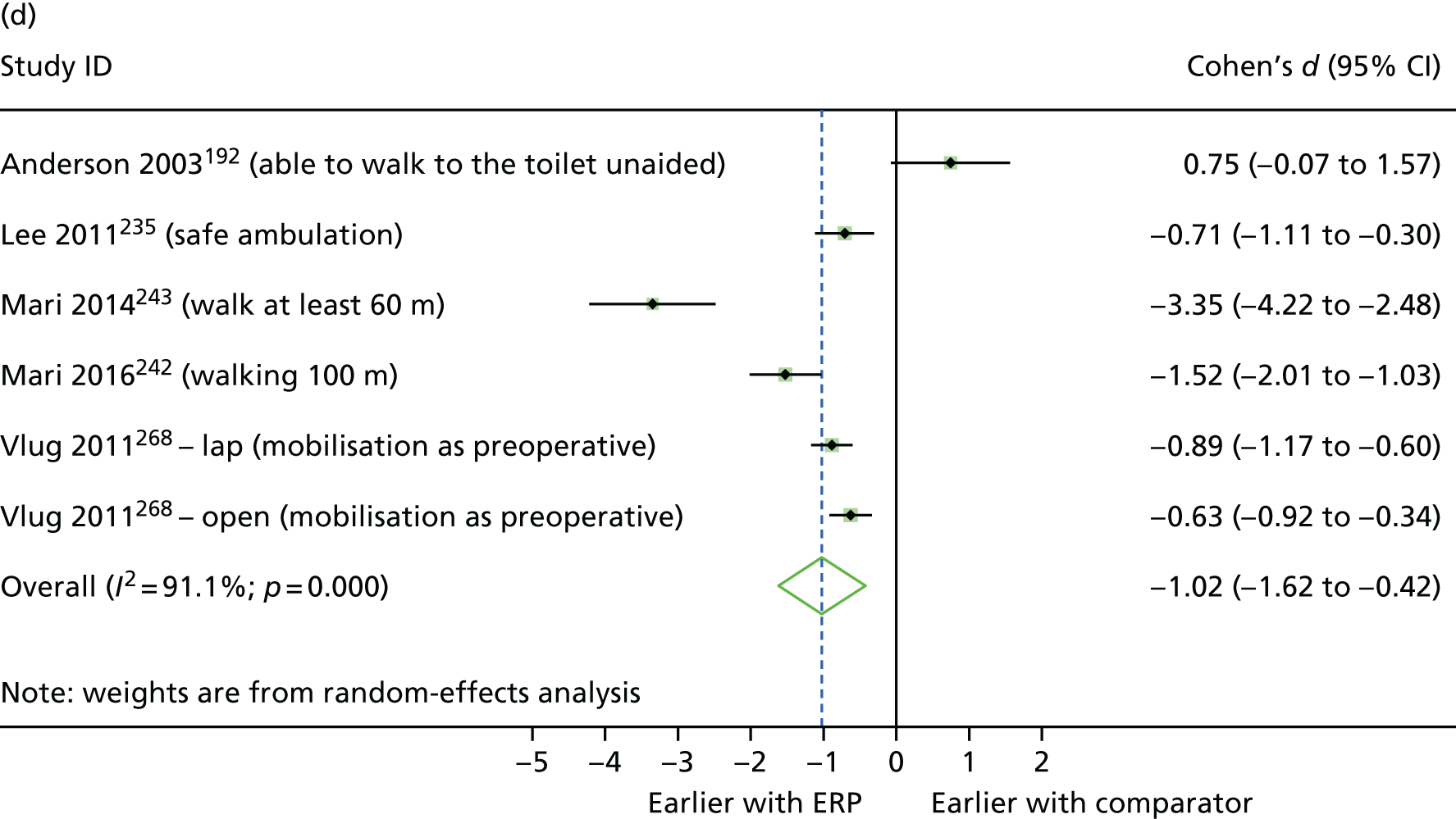

Figure 5d is a forest plot of the effect of ERP interventions on the time to reach ambulation goals following surgery. Goals included walking a target distance, walking safely or independently and reaching preoperative levels of mobilisation. Patients receiving ERP met goals 2.0 days earlier (SD 1.8 days, range 1.3–3.4 days), with a large effect size (d = –1.02, 95% CI –1.62 to –0.42; p < 0.001). Figure 5e shows that there was a small effect size indicating that patients randomised to ERP achieved pain control/goals earlier than those receiving usual care (d = –0.47, 95% CI –0.65 to –0.30; p < 0.001). Pain goals were achieved a mean of 0.8 days earlier (SD 0.2 days, range 0.7–1.0 days).
In studies not reporting analysable data, Forsmo and colleagues209 reported that the median time to passage of first stool and pain control with oral medication occurred earlier in patients receiving ERP, but it took longer to tolerate food without nausea; García-Botello and colleagues211 reported favourable median scores for all outcomes, except for pain scores on postoperative days 2 and 3, which were similar for both groups; Khoo and colleagues231 reported that only 9% of patients randomised to the ERP group felt that they would benefit from a longer inpatient stay, compared with 69% in the usual care group; and Pappalardo and colleagues252 reported similar quality-of-life scores between groups.
Heterogeneity
There was often high and statistically significant statistical heterogeneity in meta-analyses of the effects of ERP on LOS and markers of recovery in patients undergoing elective colorectal surgery, indicating inconsistency between studies. Although heterogeneity was not statistically significant for the meta-analysis of achievement of pain control outcomes (see Figure 5e), it was for other analyses, with I2 values ranging from 77.6% (LOS) to 96.5% (time to resumption of diet), indicating lack of consistency between outcome data. Furthermore, 95% CIs were wide in all cases, suggesting that any underlying true effect of interventions was uncertain. Meta-analyses of complications and re-admissions were associated with low and non-statistically significant heterogeneity.
Prehab interventions
Two204,214 of the three prehab interventions began with dedicated pre-intervention information and education, with Gillis and colleagues214 also including assessments and a booklet to facilitate adherence. All three prehab interventions incorporated a programme of physiotherapy that included multiple weekly sessions and a range of aerobic and strength training exercises. Gillis and colleagues214 supplemented these activities with a review of patient nutrition and subsequent optimisation, visits with a psychologist that included motivation and training to reduce anxiety, and regular telephone support. Participants in the study by Carli and colleagues198 also received weekly telephone support and a home visit. Perioperative care was described only by Gillis and colleagues,214 with all participants receiving the same ERAS protocol. Comparators in the three studies were a walking and breathing exercise group,198 home-based exercise advice204 and postoperative rehabilitation,214 precluding meta-analysis.
Effectiveness of prehab interventions at improving clinical outcomes
Report Supplementary Material 5, Table 3, displays clinical outcome data reported in all studies. There were no statistically significant differences in LOS or complications across the three studies. Only Gillis and colleagues214 reported re-admissions, which were similar between groups. Meta-analysis was not performed because the three studies had different comparators.
Effectiveness of prehab interventions at improving patient-reported outcomes
Report Supplementary Material 5, Table 4, displays data for patient-reported outcomes from the studies trialling prehab interventions to improve recovery following colorectal surgery. A number of outcomes were related to markers of physical recovery, quality of life and mental health. Participants in the prehab group in the study by Dronkers and colleagues204 took 5.4 seconds longer to perform the chair rise test than comparators, associated with a large effect size but wide CIs, leading to uncertainty about the true effect (d = 0.88, 95% CI 0.22 to 1.54; p < 0.01). Participants in the prehab group also had lower physical work capacity (d = –0.79, 95% CI –1.44 to –0.14; p < 0.05) and reported greater fatigue (d = 0.70, 95% CI 0.06 to 1.35; p < 0.05) than their comparators; these differences also had large but uncertain effect sizes. There were no differences between groups on any other outcome across the included studies.
Interventions to improve recovery from lower limb arthroplasty: randomised controlled trials
Thirteen RCTs trialled interventions intended to improve recovery and/or reduce LOS following lower limb arthroplasty. Of these, five studies196,234,254,256,261 compared ERP interventions with usual care; five200,221,222,244,269 trialled prehab versus either usual care200,221,222,244 or home exercise;269 two202,267 compared rehab programmes with usual care and one223 trialled a staff mix intervention versus usual care.
Three studies were conducted in the UK,244,256,269 three were conducted in Denmark,196,234,267 two were conducted in the USA,223,254 and one was conducted in each of Canada,200 Germany,202 the Netherlands,221 Taiwan222 and Iceland. 261
A total of 1686 people commenced trials, with sample sizes ranging from 21221 to 505. 223 Procedures included primary or revision unilateral or total hip or knee arthroplasty. Across the included studies, 58.5% of participants were female.
Overview: lower limb arthroplasty
This body of evidence is summarised below and in Table 7. Full details of the effectiveness of interventions in this type of surgery are reported in Enhanced recovery protocol interventions, Prehab interventions, Rehab interventions and Staff mix interventions.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Borgwardt 2009,196 Denmarka | 50 | ERP | AEI; OT | ANE | ANA | TEL | x | ⇦⇨ | x(5/5) | ⇦⇨(2/2) | ||||||
| Larsen 2008, 234 2008, 2008,233 Denmark | 90 | ERP | AEI; EX; GOAL; NUT; OT; SW | AEI | PONV | EMOB; GOAL; LAX; OT; ANA; EON | PT | ▲ | ⇦⇨ | ▲ | ||||||
| Reilly 2005,256 UK | 41 | ERP | ANA; EMOB; EON | AEI; TEL | x | ⇦⇨(2/2) |
▲(1/5) ◁▷(4/5) |
|||||||||
| Pour 2007,254 USA | 100 | ERP | AEI; PT; OT | ANA | ANE | ANA; DRA; EMOB; PT | x | ⇦⇨ | x(9/9) | x | x(5/5) | |||||
| Siggeirsdottir 2005,261 Iceland | 50 | ERP | AEI; EX | TP | EX; PT; OT | ▲ | ⬆ | ◁▷ |
▲(1/2) x(1/2) |
|||||||
| Huang 2012,222 Taiwan | 243 | Prehab | AEI; EX | ▲ | ⇦⇨(4/4) |
◁▷(2/3) x(1/3) |
||||||||||
| Williamson 2007,269 UK | 181 | Prehab | EX; PT | ◁▷ | ◁▷(3/3) |
▼(1/2) ◁▷(1/2) |
◁▷ | |||||||||
| Crowe 2003,200 Canada | 133 | Prehab | AEI; OT; PT; SoW | Pre-hospital day therapy | △ | ⬆ |
▲(2/7) △(2/7) ◁▷(3/7) |
|||||||||
| Hoogeboom 2010,221 the Netherlands | 21 | Prehab | EX | Spouse/family involved in exercises | x | ⇦⇨ | ◁▷(6/6) | ◁▷ | ||||||||
| McGregor 2004,244 UK | 39 | Prehab | AEI | x | ◁▷(2/2) | ◁▷(4/4) | ||||||||||
| den Hertog 2012,202 Germany | 160 | Rehab | EMOB; MT; PT; SW | x | ⇦⇨ | ▲ | ||||||||||
| Vesterby 2017,267 Denmark | 73 | Rehab | PT | Tele-medicine support | x | ⇦⇨ | ⇦⇨(2/2) | |||||||||
| Huddleston 2004,223 USA | 505b | Staff mix | SM | SM | SM | x |
⬆(2/4) ⇦⇨(2/4) |
|||||||||
Evidence was generally of low quality, and not all studies reported data that could contribute to analyses of effectiveness. Meta-analysis of two studies suggests that ERP interventions were associated with a reduction in LOS of over 3 days compared with usual care, with neither study reporting evidence of detriment to patient recovery or well-being. Similarly, meta-analysis of two studies evaluating prehab suggests that LOS may be reduced by around 2.5 days compared with usual care. There was, however, insufficient evidence to draw further comparisons between interventions in either category.
Complications were reported in eight studies, with evidence of improvement with interventions in three. 200,223,261
There was not enough evidence to allow the evaluation of the effectiveness of rehab or staff mix interventions. In general, lack of useable data for clinical outcomes in eight studies precludes a balanced and critical overview of the evidence.
The prehab intervention evaluated by Crowe and colleagues200 represents the most multidisciplinary intervention, with patients receiving assessment by an occupational therapist, a physiotherapist, a dietitian and a social worker, and with nurse input, within a day-therapy programme. This programme contains both education and physiotherapy components, which is similar to the intervention evaluated by Huang and colleagues,222 although the latter programme was delivered primarily by a physiotherapist. The interventions evaluated by Hoogeboom and colleagues221 and Williamson and colleagues269 constituted a home exercise and a physiotherapy programme, respectively. In the intervention evaluated by McGregor and colleagues,244 although patients were given the opportunity to practise the exercises they were to complete postoperatively, there was no separate physiotherapy or exercise training programme. The effectiveness of prehab in this study may in part be because the comparator group in this study received only minimal information prior to admission, whereas patients in the comparator groups of other studies received more comprehensive education packages. 221,269 A tentative interpretation based on the limited data available may be that an intervention requires both an educational and a physiotherapy component to be effective in reducing LOS. However, the quality of the studies this interpretation is based on was rated as ‘moderate’222,269 and ‘weak’. 200
The four studies199,200,234,261 that provided statistically significant evidence of reduced LOS had different interventions. Intervention components common to these four interventions were preoperative assessment, education or information of differing degrees of intensities; and a pre-admission physical component, either an exercise programme or physiotherapy. However, these components were also present in other interventions that were ineffective at improving outcomes, suggesting that further evidence is required before it is possible to examine components that determine improvements in patient recovery following lower limb arthroplasty.
Enhanced recovery protocol interventions
Components of the ERP interventions and comparators are mapped in Table 8. We used an ERAS-style intervention component mapping approach, despite absence of ERAS Society guidelines for lower limb arthroplasties. The following items were present in at least three of the five studies: pre-admission assessment, education and counselling; preoperative exercises; preoperative assessment by an occupational therapist or a social worker to identify postoperative needs and/or provision of equipment; minimally invasive surgical technique; avoidance of patient-controlled analgesia; avoidance of routine opiate analgesia; early mobilisation protocol; and follow-up support.
| Intervention component | Study (first author and year) | ||||
|---|---|---|---|---|---|
| Borgwardt 2009196 | Larsen 2008234 | Pour 2007254 | Reilly 2005256 | Siggeirsdottir 2005261 | |
| Assessment/education/counselling | E | E | E, C | E | |
| Avoidance of preoperative fasting | |||||
| Preoperative carbohydrate treatment | |||||
| Preoperative exercise | E | E | E | ||
| Optimisation of physical condition prior to surgery | E | E | |||
| Discharge planning: prior to admission | |||||
| Discharge planning after hospital admission | |||||
| Preoperative OT/SW assessment of PO needs and/or equipment provision | E | E | E | ||
| Admission on day of surgery | E | ||||
| Pre- or postoperative antibiotic prophylaxis | E, C | ||||
| Anaesthesia protocol: routine use of spinal or epidural anaesthesia | E, C | E, C | |||
| Anaesthesia protocol: short-acting, opiate avoidance | E | E, C | |||
| Minimally invasive surgical technique | E, C | Ea | E, C | ||
| Wound management protocol | E, C | ||||
| Avoidance of routine urinary catheter | E, C | ||||
| No routine drains | E | ||||
| Avoidance of PCA | E, C | E | E | ||
| Avoidance of routine opiate analgesia | E | E, C | E | ||
| Early mobilisation protocol | E, C | E | E | E | |
| Dedicated post-surgery physiotherapy exercises, supported by PT | E, C | E | |||
| Early resumption of oral intake (fluids, nutrition) | E | E | |||
| PONV prophylaxis | E | ||||
| Laxative use | E | ||||
| Thromboembolic prophylaxis | E, C | E | |||
| Goal-setting with patient | E | ||||
| Patient encouraged to be active participant in own care/disown sick role | E | ||||
| Provision of equipment/medication on discharge | E, C | ||||
| Follow-up support: contact telephone number, telephone call, home visit | E | E | E | ||
| Post-discharge exercise regimen and/or PT support | E | E | E | ||
| Follow-up outpatient/community clinic visit/referrals | E, C | ||||
Quality assessment
Five studies were rated globally as ‘weak’ and eight were rated globally as ‘moderate’. No study was rated as ‘strong’ overall. The study by McGregor and colleagues244 was the only one to be rated as ‘weak’ on three domains: study design, confounders and data collection methods. Only three studies202,223,234 provided a clear definition of their LOS outcomes.
Report Supplementary Material 5, Table 5, displays clinical outcome data for each study trialling an ERP intervention to improve recovery from lower limb arthroplasty. Two studies234,261 provided data from which standardised mean differences could be calculated. Meta-analysis indicates a reduction in LOS of 3.3 days (SD 0.5 days, range 2.9–3.6 days), with a large effect size (d = –1.26, 95% CI –1.62 to –0.89; p < 0.001) (Figure 6). In the three studies196,254,256 reporting LOS variance as range data, thereby precluding secondary analysis, LOS was always shorter in the ERP group. Sensitivity analysis showing the influence of imputed data on LOS meta-analysis is available in Appendix 4.
FIGURE 6.
Forest plot showing the results of meta-analysis of the effect of ERP vs. usual treatment on LOS following lower limb arthroplasty.
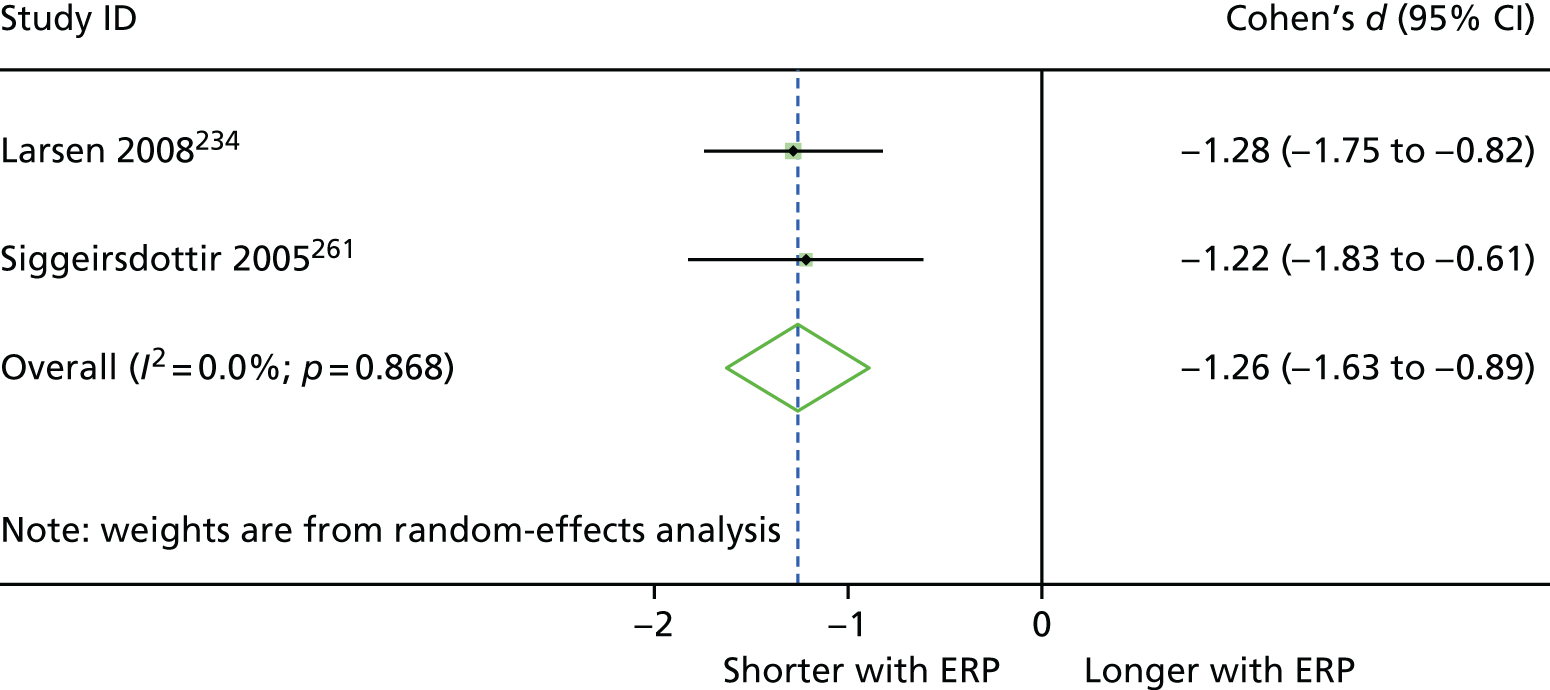
Rates of re-admissions and use of additional support were similar between ERP and usual care, where reported. The odds of experiencing a complication were statistically significantly lower in patients receiving ERP in the study by Siggeirsdottir and colleagues261 (OR 0.25, 95% CI 0.07 to 0.88). The odds of experiencing a complication were similar between groups in the study by Reilly and colleagues. 256 No other studies reported complications.
Report Supplementary Material 5, Table 6, displays patient-reported outcome data for each RCT trialling an ERP intervention to improve recovery from lower limb arthroplasty. Assessments of joint function before discharge were performed in three studies,196,254,256 although standardised mean differences could not be calculated for two of these. 196,254 Reilly and colleagues256 reported that patients receiving ERP had improved knee flexion range of motion, with an increase of around 5°. Although this change was associated with a medium effect size, wide CIs indicate uncertainty about the true effect (d = 0.79, 95% CI 0.16 to 1.43; p < 0.05). There were no differences between groups for other assessments of knee function in this study. 256
Longer-term (up to 6-month follow-up after surgery) assessments of hip and knee function were undertaken using standardised outcomes such as the Harris Hip Score, Oxford Hip Score, Oxford Knee Score, American Knee Society Score, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Figure 7 is a forest plot showing standardised mean differences for these outcomes, as presented in the three studies providing analysable data. 234,256,261
FIGURE 7.
Forest plot (without pooled effects) displaying standardised mean differences for quality-of-life outcomes related to lower limb arthroplasty. AKSS, American Knee Society Score; EQ-5D, EuroQol-5 Dimensions.

Improvements were seen on two measures following ERP interventions, the EuroQol-5 Dimensions (EQ-5D) quality-of-life measure234 and the Oxford Hip Score,261 both of which were associated with medium effect sizes but wide CIs (EQ-5D: d = 0.50, 95% CI 0.07 to 0.93, p < 0.05; Oxford Hip Score: d = –0.66, 95% CI –1.24 to –0.07, p < 0.05). The change in Oxford Hip Scores observed by Siggeirsdottir and colleagues261 was five points, which could be enough to change the classification of hip arthritis severity.
For outcomes with no variance data, there were reports of a benefit with ERP interventions for the number of patients with lower limb weakness, the number of patients who were well at 3 months and the walking status of patients at discharge. There was little difference in the ratings of confidence or satisfaction with care at the end of the study by Borgwardt and colleagues,196 despite median scores for all other outcomes appearing favourable for ERP patients. Pour and colleagues254 reported favourable mean scores on a number of outcomes in ERP patients, including quality of life and markers of recovery; however, presentation of only range data precluded further analysis.
Prehab interventions
Five RCTs trialled prehab intervention in patients undergoing lower limb arthroplasty (knee,222,269 hip,221,244 and hip or knee200). Pre-admission education was delivered in two studies. 222,244 Huang and colleagues222 delivered this in the form of an educational group programme 2–4 weeks prior to admission, supplemented with an educational booklet. McGregor and colleagues244 delivered a class in which they provided information about the procedure and rehabilitation process, ensuring that exercises could be performed. They also made provisions for adapting the home environment if required. In the study by Crowe and colleagues,200 participants were assessed by an occupational therapist, a physiotherapist or a nurse, and were assigned tailored strengthening exercises. They also received a home visit to plan environmental changes, were supported financially or loaned equipment to make adaptations if required, received dietary/nutrition support and counselling, and were provided with a telephone number for support.
Pre-admission exercise programmes featured in four of the interventions. 200,221,222,269 Hoogeboom and colleagues221 delivered a 3- to 6-week programme of strength and endurance exercises at their physiotherapy outpatient department, as well as using pedometry to increase general activity and including family in the programme. Similarly, the patients randomised to the intervention group in the study by Williamson and colleagues269 underwent a 6-week programme of lower limb strength and balance training. In the intervention delivered by Huang and colleagues,222 lower limb strength exercises were performed at home, and a telephone call was made to patients 1 week before surgery to discuss the home exercise programme and answer any questions. Participants in the study by Crowe and colleagues200 received their individualised programme of strength and endurance training either in an outpatient physiotherapy clinic or from a physiotherapist at home. Any patients in the study by Crowe and colleagues200 who required multidisciplinary prehab, as determined by baseline assessment, attended a day-care hospital. Full details of these interventions can be found in Report Supplementary Material 3, Table 7.
Two of the studies were rated globally as being of ‘moderate’ quality,222,269 and three were rated as ‘weak’. 200,221,244 Only the study by McGregor and colleagues244 was rated as ‘weak’ in three domains (study design, confounders and data collection methods). None of the studies described clearly how LOS was defined.
Effectiveness of prehab interventions at improving clinical outcomes
The results for clinical outcomes are shown in Report Supplementary Material 5, Table 7. Meta-analysis of two studies in which effect sizes were calculable200,222 indicates that LOS was around 2.5 days shorter with prehab, associated with a medium effect size (d = –0.55, 95% CI –0.77 to –0.28; p < 0.001; Figure 8). Median LOS was 6 days in both groups in the study by Hoogeboom and colleagues221 and mean LOS was 3 days shorter in the prehab group in the study by McGregor and colleagues,244 although it was not possible to calculate effect sizes for these two studies. In the study by Williamson and colleagues,269 LOS was similar for both groups of patients. Sensitivity analysis showing the influence of imputed data on LOS meta-analysis is available in Appendix 4.
FIGURE 8.
Forest plot showing the results of meta-analysis of the effect of prehab interventions vs. usual treatment on LOS following lower limb arthroplasty.
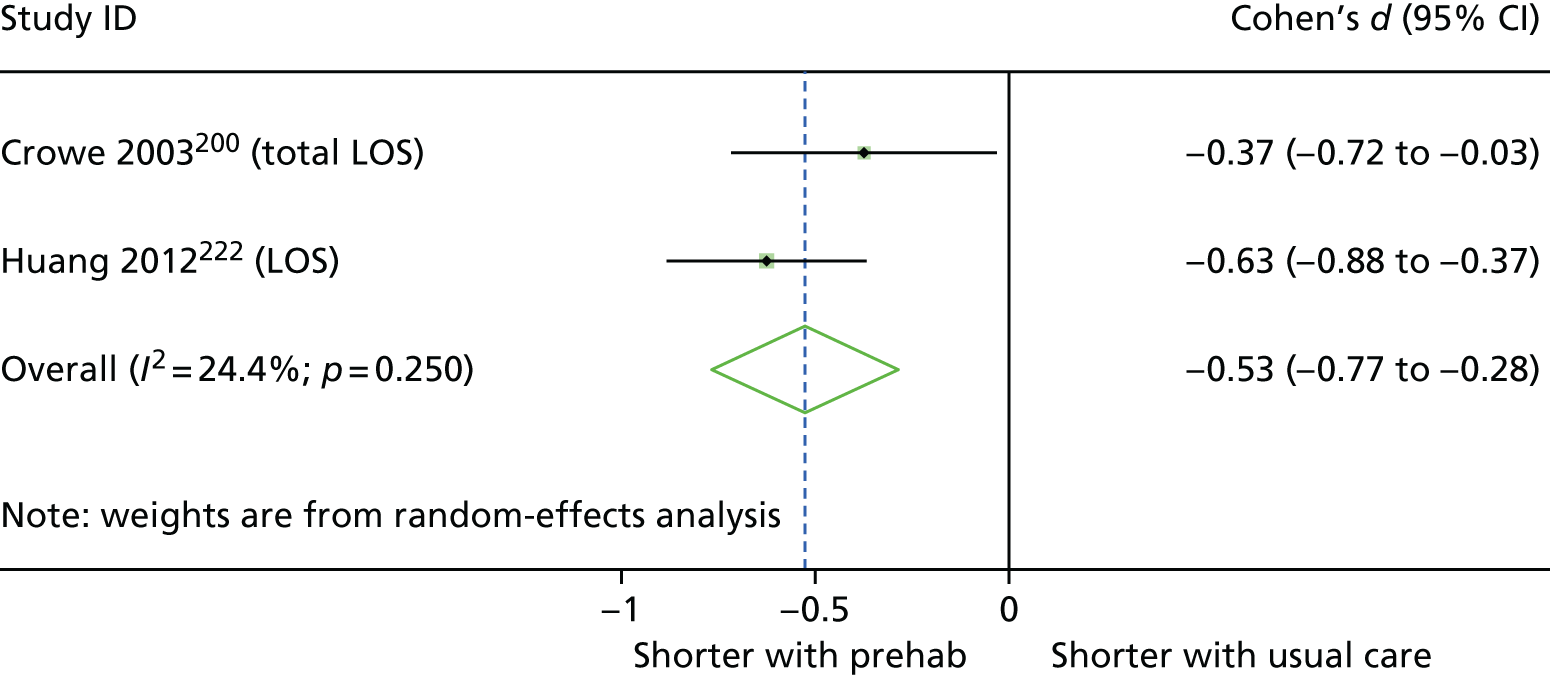
No data were provided for use of additional care, morbidity, mortality or re-admissions. The odds of experiencing complications was statistically significantly lower in the study by Crowe and colleagues,200 with odds 75% lower in the prehab group (OR 0.25, 95% CI 0.10 to 0.64). Complications were also reported in the studies by Hoogeboom and colleagues221 and Huang and colleagues,222 but did not differ statistically between groups. Williamson and colleagues269 reported three complications but did not identify the study group in which these occurred.
Effectiveness of prehab interventions at improving patient-reported outcomes
Report Supplementary Material 5, Table 8, displays results for patient-reported outcomes from RCTs evaluating prehab interventions to improve recovery from lower limb arthroplasty. Crowe and colleagues200 reported that patients randomised to prehab achieved the following discharge criteria sooner than controls: able to get out of bed independently (d = –0.36, 95% CI –0.71 to –0.02; p < 0.05); getting all equipment ready for discharge (d = –0.85, 95% CI –1.20 to –0.49; p < 0.001); having meals planned for discharge (d = –1.32, 95% CI –1.69 to –0.94; p < 0.001); and having all discharge criteria met (d = –0.43, 95% CI –0.77 to –0.08; p < 0.05).
Anxiety was significantly greater in the prehab group in the study by Williamson and colleagues;269 this change was associated with a medium effect size (d = 0.52, 95% CI 0.21 to 0.94; p < 0.01), although scores for both groups were in the ‘normal’ range. 273 Figure 9 is a forest plot displaying standardised mean differences (Cohen’s d) after prehab for a variety of outcomes relating to functional recovery after surgery, quality of life, pain and mental health across included studies.
FIGURE 9.
Forest plot (without pooled effects) displaying effect sizes (Cohen’s d) and 95% CIs for function, quality of life, pain and mental health outcomes from studies trialling prehab outcomes. 6MWT, Six-Minute Walk Test; ADL, Activities of Daily Living; HADS, Hospital Anxiety and Depression Scale; HGS, hand grip strength; LAPAQ, Longitudinal Ageing Study Amsterdam Physical Activity Questionnaire; PWC, physical work capacity; VAS, visual analogue scale.
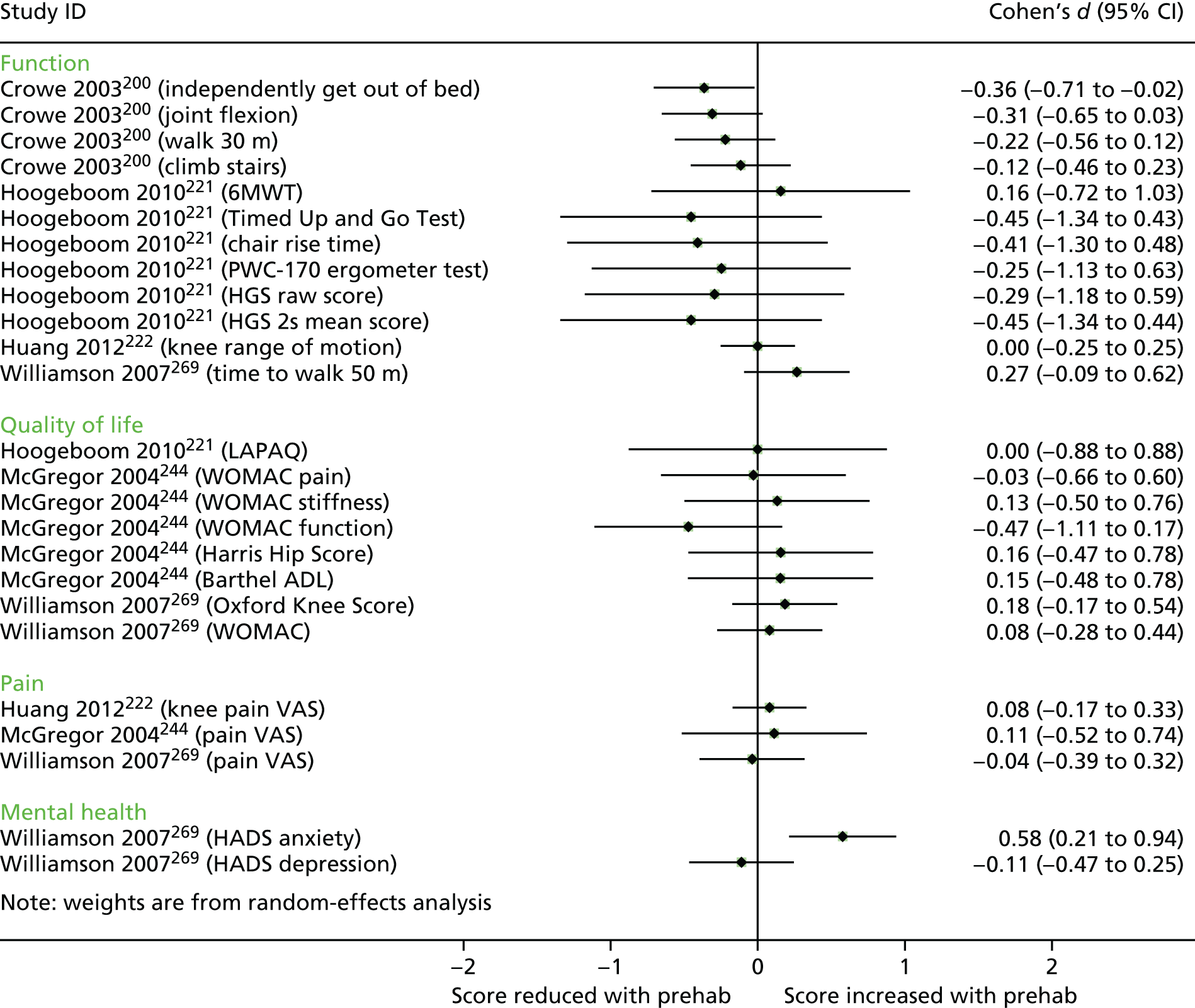
Rehab interventions
Two studies202,267 were RCTs of rehab interventions in patients undergoing lower limb arthroplasty. In the study by den Hertog and colleagues,202 patients in the rehab arm received specialist care in a three-bed care unit, where they were given motivational messages and urged to ‘compete’ with others to meet recovery goals. There was a target discharge day of postoperative day 6. Daily mobilisation goals were set, and 2 hours of physiotherapy were delivered each day in a living room environment. The participants in the intervention arm of the study by Vesterby and colleagues267 received a home visit from a physiotherapist 3 days after their operation, with a video conference 2 and 6 days after the operation. The main component of the intervention was a telemedicine rehabilitation programme, working through a box connected to a television, with interactive comprehensive information about osteoarthritis and guidance on completing exercises and using supplementary aids. Full details of interventions can be found in Report Supplementary Material 3, Table 8.
Effectiveness of rehab interventions
Report Supplementary Material 5, Table 9, displays the results for all outcomes reported in the two rehab intervention studies. Standardised mean differences were not calculable because of the absence of variance data or imputable variance data. However, mean LOS was reported to be 6.5 days shorter in patients receiving rehab intervention in the study by den Hertog and colleagues,202 whereas median LOS was 1 day shorter in the experimental group in the study by Vesterby and colleagues. 267 The number of procedure-related complications was similar in both groups in the study by den Hertog and colleagues. 202
Den Hertog and colleagues202 reported lower scores on the WOMAC measure, with a large effect size (d = –1.52 95% CI –1.89 to –1.14; p < 0.001 – per-protocol analysis). Numbers of unplanned patient telephone calls, hospital visits and re-admissions were similar in both groups in the study by Vesterby and colleagues. 267 Meta-analysis was not possible.
Staff-mix interventions
One study223 trialled a staff mix intervention. The intervention was a hospitalist-orthopaedic team using co-management care. The team comprised hospitalist faculty (no residents) and consultative medical specialty teams (faculty and resident) with a mean length of postgraduate clinical experience of > 6 years. Hospitalists provided postoperative medical care after the surgical team completed initial postoperative orders. In the control group, these duties were undertaken by the regular orthopaedic team. The study was of ‘moderate’ quality overall, offering a clear definition of LOS and rated as ‘weak’ only for data collection methods. 223
Huddleston and colleagues223 collected data on LOS and complications in 469 patients; however, no estimates of variance were reported, precluding the calculation of standardised mean differences. Data are reported in Report Supplementary Material 5, Table 10. LOS was similar between trial arms. The odds of patients experiencing complications was 38% lower in patients in the staff mix intervention (OR 0.62, 95% CI 0.43 to 0.89; p < 0.05). This difference can be largely attributed to the 46% lower odds of minor complications in the intervention group (OR 0.54, 95% CI 0.37 to 0.8; p < 0.01).
Interventions to improve recovery from cardiac surgery: randomised controlled trials
Six RCTs trialled interventions to improve recovery following cardiac surgery. Four compared prehab interventions with usual care193,210,215,258 and there were single studies examining the effectiveness of a specialist ward255 and rehab. 266 The evidence came from the UK,210,215 Canada,193 Germany,255 Australia258 and the Netherlands. 266
A total of 1267 people commenced trials, with sample sizes ranging from 117258 to 309. 266 In all studies, patients were scheduled for coronary artery bypass graft (CABG) surgery, plus in two studies valve surgery or combined CABG and valve surgery was also undertaken. 255,258 Across the included studies, the sample was 21.6% female and 63.7 years old (where mean age was reported).
Quality assessment
One study achieved a global rating of ‘strong’,193 with no ‘weak’ domains. Two further studies were rated as ‘moderate’,215,258 with one weak domain. Goodman and colleagues215 retained < 60% of the recruited participants, and Rosenfeldt and colleagues258 did not report validity or reliability of their primary outcome measure. Of the studies rated as ‘weak’ overall, two were ‘weak’ for two domains (selection bias and data collection methods;210 confounders and blinding255) and one was weak in three. 266 The study by van der Peijl and colleagues266 did not adjust for confounding variables, did not comment on the validity or reliability of their primary outcome, and did not clearly report withdrawals and dropouts. Two of the six studies also failed to clearly define their LOS outcome. 258,266
Overview: cardiac surgery
This body of evidence is summarised below and in Table 9. Full details of the effectiveness of interventions are described in Prehab interventions and Specialist ward and rehabilitation interventions.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Arthur 2000,193 Canada | 249 | Prehab | AEI; EX; REF; TEL | ▲(2/2) | ◁▷ | △ | ||||||||||
| Goodman 2008,215 UK | 188 | Prehab | AEI; MT | ◁▷ | ||||||||||||
| Rosenfeldt 2011,258 Australia | 117 | Prehab | AEI; EX; REF; OT; PSYC | x | ◁▷ | ◁▷ | ||||||||||
| Furze 2009,210 UK | 204 | Prehab | AEI; EX; GOAL; REF; TEL; PSYC | ◁▷ | ⇦⇨ | ⇦⇨ | ⇦⇨ | x | x | ⇦⇨ | ||||||
| Probst 2014,255 Germany | 200 | SW | PreM | ANE; ANA; WARM | ANA; SW | ◁▷ | ⬆ | ⇦⇨ | ||||||||
| van der Peijl 2004,266 the Netherlands | 309 | Rehab | PT; EX | Programme frequency: 2 × day | x | ◁▷(4/4) | ||||||||||
The six RCTs of interventions to improve recovery after elective cardiac surgery in older adults failed to provide conclusive evidence of benefit. Meta-analysis of the four RCTs trialling prehab interventions indicated a small reduction in LOS, compared with usual care; however, two of the three studies included showed no benefit. There were only single studies for rehab and specialist ward interventions. Although there were statistically significant changes on individual measures of quality of life and risk of complications in two studies, more evidence is required to determine whether or not multimodal interventions can convey any benefit in this population.
The only study to indicate a statistically significant reduction in LOS with prehab was conducted by Arthur and colleagues. 193 This Canadian RCT was of ‘strong’ quality with a fairly large sample size (n = 249), and, as such, is the most robust study in the group, although also the oldest. The authors also observed a statistically significant improvement in physical quality of life. 193 The three other prehab programmes210,215,258 had no statistically significant impact on LOS, but did not result in any adverse effects on rates of readmission, use of additional care210 or quality of life. 258
The intervention evaluated by Arthur and colleagues193 was the only one of the four prehab studies to include both an education component and supervised outpatient exercise training as part of its intervention. It was also the only intervention to involve spouses and family members, by informing them of what to expect when they saw the patient after surgery.
Goal-setting with the aim of increasing activity level was incorporated within the education delivered as part of the ‘HeartOp Programme’ evaluated by Furze and colleagues. 210 The studies by both Arthur and colleagues193 and Furze and colleagues210 incorporated telephone follow-up by nurses at regular intervals prior to surgery, which may also have contributed towards improved outcomes.
Perhaps the combination of individual supervision, regular telephone calls and involvement of family members helped motivate patients to engage with the prehab programme in the study by Arthur and colleagues. 193 The other study to integrate face-to-face education and regular patient contact (via motivational interviewing delivered monthly) did not reduce LOS. 215 The absence of ongoing support and/or supervision within this intervention may limit its effectiveness, or motivational interviewing may not be as effective as being motivated by family members.
Prehab interventions
The interventions trialled in the studies by Arthur and colleagues193 and Furze and colleagues210 included extensive preoperative information, education and counselling. Arthur and colleagues193 followed this with individual exercise prescriptions for supervised 90-minute sessions, twice per week in the lead-up to surgery. In the study by Furze and colleagues,210 participants in the prehab arm also received relaxation therapy to reduce stress and advice on postoperative self-management. In the study by Rosenfeldt and colleagues,258 participants in the intervention group received aerobic exercise physiotherapy, aiming to reach four 30-minute sessions per week. Participants also received relaxation therapy from an occupational therapist. The intervention in the study by Goodman and colleagues215 was a series of monthly visits from a cardiac home-care nurse in order to reduce cardiac risks, establish lifestyle changes, provide education and answer questions.
Effectiveness of prehab interventions at improving clinical outcomes
Report Supplementary Material 5, Table 10, displays data for clinical outcomes for each study trialling a prehab intervention. Meta-analysis of the effects of prehab on LOS yielded evidence of a small reduction in LOS with prehab interventions (d = –0.35, 95% CI –0.68 to –0.02; p < 0.05) (Figure 10). In absolute terms, LOS was reduced by a mean of 0.7 days (SD 0.3 days, range 0.5–1 days); however, CIs for the pooled effect approach zero, indicating inconsistency across outcomes. Prehab was associated with a reduction in LOS in the study by Arthur and colleagues193 (d = –0.67, 95% CI –0.92 to –0.41; p < 0.001), but not in any of the other studies. The main influence on LOS in this study came from the time spent in hospital after surgery, which was an average of 1 day shorter in the prehab group. Sensitivity analysis showing the influence of imputed data on LOS meta-analysis is available in Appendix 4.
FIGURE 10.
Forest plot showing the results of meta-analysis of the effect of prehab interventions vs. usual treatment on LOS following cardiac surgery.

Furze and colleagues210 provided information about the use of additional GP and NHS hospital visits in the eight weeks after discharge, with results being similar between groups. Rosenfeldt and colleagues258 reported that median LOS was the same (6 days) in both groups. Only Furze and colleagues210 reported complications or mortality, both of which were similar in both groups.
Effectiveness of prehab interventions at improving patient-reported outcomes
Report Supplementary Material 5, Table 11, displays data for patient-reported outcomes for each study trialling a prehab intervention. The Short Form questionnaire-36 items (SF-36) was utilised by Arthur and colleagues193 and Rosenfeldt and colleagues. 258 Arthur and colleagues193 presented change scores from baseline on various subscales of the SF-36, with significantly greater changes (indicating improvement) in the prehab group than in the usual care group on the physical functioning subscale and the physical composite summary scales; both changes were associated with small effect sizes. There was no statistically significant difference in the change from baseline between groups for any other subscale of the SF-36. In the study by Rosenfeldt and colleagues,258 participants in both groups scored similarly on both physical and mental composite scores. Furze and colleagues210 calculated QALYs as a measure of health-related quality of life and mortality, but, as they evaluated these prior to surgery, we have not reported them here. Goodman and colleagues215 collected data for mental health outcomes but did not report numerical data. The effects for quality-of-life outcomes are displayed in Figure 11.
FIGURE 11.
Forest plot (without pooled effects) displaying the effects of prehab on quality-of-life outcomes in patients receiving cardiac surgery.
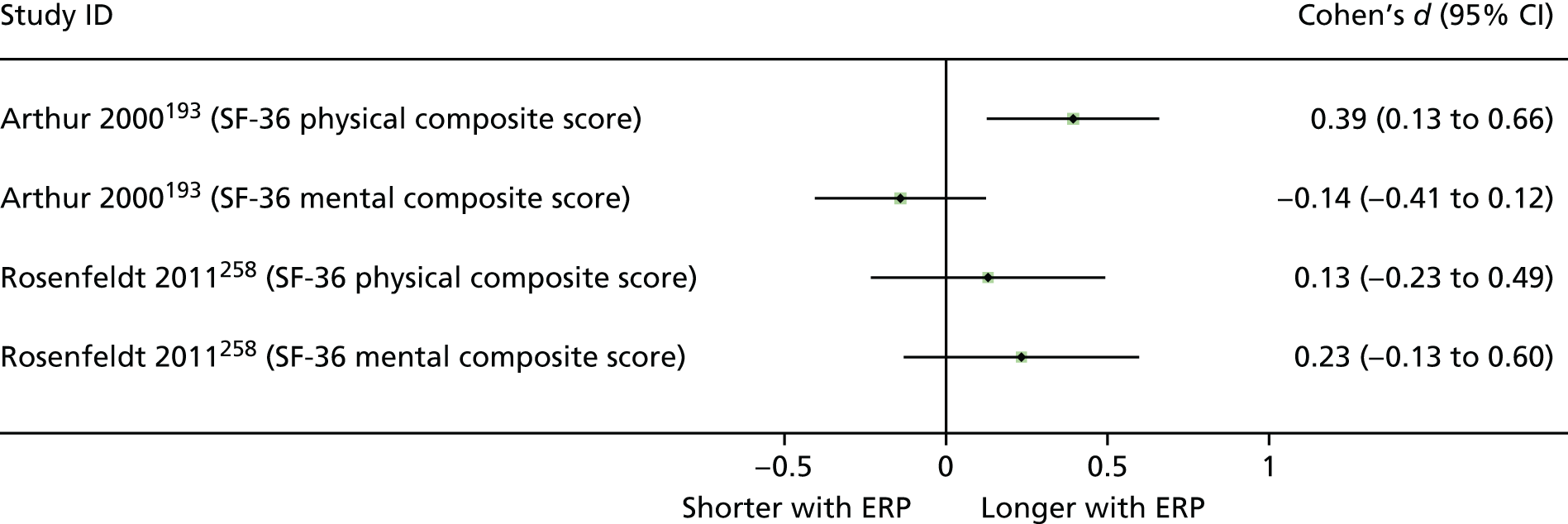
Specialist ward and rehabilitation interventions
Specialist ward and rehabilitation interventions were trialled in one RCT each. The specialist ward in the study by Probst and colleagues255 was a three-bed post-anaesthetic care unit, a patient-to-staff ratio of 1 : 3, limited opening times, strict analgesia regime with pain monitoring, extubation as soon as ready and discharge into a step-down unit. This was compared with the larger conventional unit with 21 beds, where the physician to patient ratio was 12 : 1, staff were less specialised, opening hours were longer, analgesia was liberal, and extubation and discharge were typically delayed.
In the study by van der Peijl and colleagues,266 patients were randomised to high- or low-frequency rehabilitation. In the high-frequency intervention, patients began a progressive exercise programme targeting flexibility, strength, co-ordination, walking and stair climbing. Exercises began the day after surgery and continued at weekends. In the comparator group, exercises were not supervised and were interrupted by the weekend.
The results of these two studies are displayed in Report Supplementary Material 5, Table 12. Despite reduced time in the treatment unit and intermediate care in the intervention group, total LOS was similar in both groups in the study by Probst and colleagues. 255 However, the odds of incurring a complication were statistically significantly lower, by 79%, in the patients randomised to the specialist ward (OR 0.21, 95% CI 0.10 to 0.41).
Median LOS was the same in both groups in the rehabilitation trial by van der Peijl and colleagues,266 and all outcomes related to physical activity and functional independence after surgery were similar between groups.
Interventions to improve recovery from upper abdominal surgery: randomised controlled trials
There were five RCTs199,205,226,227,264 of interventions to improve recovery and/or reduce LOS following upper abdominal surgery. Procedures in these studies were liver resection,205,226 hepatectomy or pancreatectomy,227 pancreaticoduodenectomy199 and gastric cancer surgery. 199,264 Two studies were conducted in the UK,205,226 one was conducted in Taiwan,199 one was conducted in Japan264 and one was conducted in Greece. 227 Four of the studies199,226,227,264 evaluated ERP interventions and one205 evaluated prehab. In all cases, the comparator was standard care. Across the five studies, there were 485 participants, of whom approximately 42% were female. In the study by Chen and colleagues,199 participants were undergoing different abdominal surgeries. The results for those receiving gastrectomy and pancreaticoduodenectomy were treated as separate analyses, both compared with groups of patients receiving usual care for the same procure.
Quality assessment
Three of the studies were rated as ‘moderate’ overall199,227,264 and two were rated as ‘weak’. 205,226 The studies rated as ‘weak’ overall scored the lowest rating across two domains. The study by Dunne and colleagues205 was rated as ‘weak’ for data collection methods and selection bias. Selection bias was rated as ‘weak’ owing to the large number of dropouts. 226 Jones and colleagues226 did not control for important between-group differences. Three studies226,227,264 provided a clear definition of LOS.
Overview: upper abdominal surgery
The evidence for upper abdominal surgery is summarised here and in Table 10. Full details of the effectiveness of interventions can be found in Enhanced recovery protocol interventions and Prehab interventions.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Dunne 2016,205 UK | 38 | Prehab | AEI; EX | ◁▷ | ⇦⇨ | ⇦⇨ | ◁▷ | ⇦⇨(5/5) | ||||||||
| Chen 2017,199 Taiwan | 535 | ERP | FM | EON; COMM; EMOB | Oral and nutritional assistance protocol | ▲(2/4) |
⬆(1/2) ⇦⇨(1/2) |
|||||||||
| Kapritsou 2017,227 Greece | 63 | ERP | nNGT | EMOB | ▲ | ⬆ | ◁▷(5/5) | ◁▷(3/3) | ||||||||
| Tanaka 2017,264 Japan | 148 | ERP | CHL; nFAST | DRA | EON; ANA | ▲(2/2) |
⬆(1/2) ⇦⇨(1/2) |
⇦⇨ |
▲(1/6) △(3/6) ◁▷(2/6) |
|||||||
| Jones 2013,226 UK | 104 | ERP | AEI; NUT | DRA; FM | DRA; CATH; EON; FM; NUT; ANA | ▲ |
⬆(2/4) ⇦⇨(2/4) |
⇦⇨ | ⇦⇨ | ▲ | x | |||||
Meta-analysis of the four RCTs trialling ERP interventions aiming to improve recovery after upper abdominal surgery suggests that LOS can be reduced by around 5 days with ERP compared with usual care. Furthermore, meta-analysis of these four studies199,226,227,264 indicated that the odds of sustaining complications were lower with ERP interventions. Individual studies reported markers of recovery that either improved or remained similar with ERP. There was one trial of a prehab intervention, which led to improvements in some markers of physical fitness, but not in LOS. The successful interventions were heterogeneous, combining multiple components, and, as such, it is difficult to further explore reasons for their success.
Enhanced recovery protocol interventions
Components of the ERP interventions and comparators are mapped against ERAS Society guidelines for liver surgery,274 pancreaticoduodenectomy275 and gastrointestinal surgery253,276–278 in Table 11. The most commonly occurring items were preoperative counselling (2/4 studies); minimal fasting and carbohydrate loading preoperatively (2/4); avoidance of long-acting pre-anaesthetic medication (3/4); early oral intake postoperatively (3/4); early mobilisation (4/4) audit; and feedback (2/4). Usual care in the comparator arms was identical with respect to bowel preparation, pre-anaesthetic medication, antithrombotic and antimicrobial prophylaxis, and the choice of incision. The intervention in the study by Tanaka and colleagues264 was described as an ERAS intervention by the authors. In the study by Chen and colleagues,199 the intervention (the Modified Hospital Elder Life Program) integrated relatively few ERAS Society-recommended components, but featured an additional focus on oral and nutritional assistance, with a postoperative communication protocol to facilitate orientation.
| ERAS item and description | Study (first author and year) | |||
|---|---|---|---|---|
| Chen 2017199 | Jones 2013226 | Kapritsou 2017227 | Tanaka 2017264 | |
| Preoperative counselling: patients should receive routine dedicated preoperative counselling and education before liver surgery | E | E, C | ||
| Preoperative endoscopic biliary drainage should not be undertaken routinely in patients (PD) | ||||
| Preoperative smoking and alcohol consumption: abstinence should be attempted for 1 month prior to surgery (PD) | ||||
| Preoperative nutrition: routine use not warranted, but significantly malnourished patients should be optimised (PD) | ||||
| Perioperative nutrition: patients at risk should receive oral nutritional supplements for 7 days prior to surgery | Ea | |||
| Perioperative oral immunonutrition: limited evidence for use (immunonutrition for 5–7 days perioperatively should be considered – PD) | ||||
| Preoperative fasting and preoperative carbohydrates load: preoperative fasting does not need to exceed 6 hours for solids and 2 hours for liquids. Carbohydrate loading the evening before surgery and 2 hours before the induction of anaesthesia | E | E | ||
| No oral mechanical bowel preparation | E, C | E, C | ||
| Pre-aesthetic medication: long-acting anxiolytic drugs should be avoided. Short-acting anxiolytics may be used to perform regional analgesia prior to the induction of anaesthesia | E, C | E, C | E, C | |
| Antithrombotic prophylaxis | E, C | |||
| Perioperative steroids administration: steroids (methylprednisolone) may be used before hepatectomy in normal liver parenchyma | ||||
| Antimicrobial prophylaxis and skin preparation | E, C | |||
| Incision: the choice of incision is at the surgeon’s discretion | E, C | |||
| Minimally invasive approach: laparoscopic liver resections can be performed by hepatobiliary surgeons experienced in laparoscopic surgery | E,b Cb | E, Cc | ||
| Avoidance of prophylactic nasogastric intubation | E | |||
| Nasogastric tubes removed prior to extubation (PD) | ||||
| Prophylactic abdominal drainage: no recommendation | E, Cd | |||
| Preventing intraoperative hypothermia | E, C | |||
| Early oral intake: eat normal food on POD1. Enteral or parenteral feeding reserved for malnourished patients or those with prolonged fasting due to complications | E,e C | E | ||
| Normal diet after surgery without restrictions, beginning carefully and increasing intake according to tolerance over 3–4 days (PD) | E | |||
| Postoperative glycaemic control (diabetic patients) | ||||
| Avoidance of perianastomotic drains (G) | E, C | |||
| Urinary drainage: suprapubic catheterisation is superior to transurethral catheterisation if used for > 4 days (PD) | ||||
| Prevention of delayed gastric emptying | ||||
| Stimulation of bowel movement: not indicated | E, C | |||
| Stimulation of bowel movement: a multimodal approach with epidural and near-zero fluid balance is recommended, including oral laxatives and chewing gum given postoperatively (PD) | ||||
| Early mobilisation | E | E, Cf | E | E, C |
| Analgesia: routine TEA not recommended in open liver surgery. Wound infusion catheter or intrathecal opiates can be good alternatives combined with multimodal analgesia | ||||
| Pain management: opioid-sparing analgesic strategies, including regional analgesia techniques, should be implemented in context of a multimodal analgesic regimen (G) | E | |||
| Epidural analgesia: mid-thoracic epidurals are recommended, compared with intravenous opioids (PD) | ||||
| Intravenous analgesia: some evidence supports the use of PCA or intravenous lidocaine analgesic methods (PD) | ||||
| Multimodal approach to PONV should be used. Patients should receive PONV prophylaxis with two antiemetic drugs | E, Cg | |||
| Fluid management: maintenance of low central venous pressure with close monitoring. Balanced crystalloid preferred over saline or colloids | E | |||
| Fluid management: perioperative haemodynamic management: maintain fluid homeostasis avoiding fluid excess and organ hypo perfusion (G) | E, C | |||
| Audit: systematic audit | E | E, C | ||
The two studies evaluating ERP pathways with patients undergoing liver surgery226,227 adhered to a mean of 10.5 (45.7%) of the 23 relevant ERAS guidelines, while the comparator groups in these studies adhered to a mean of seven (30.4%) items.
The intervention in the study by Kapritsou and colleagues,227 conducted with patients undergoing hepatectomy or pancreatectomy, adhered to three (9.7%) of the 31 ERAS guideline items relevant to individuals undergoing pancreaticoduodenectomy, as did the comparator group.
The two studies evaluating ERP pathways with patients undergoing gastrointestinal surgery adhered to a mean of six (23%) of the 23 ERAS guideline items, with their comparator groups adhering to a mean of 3.5 items (15.2%). 199,264
The intervention evaluated in the study by Chen and colleagues,199 conducted with patients undergoing a mix of pancreaticoduodenectomy and gastric procedures, adhered to five (17.9%) of the 28 relevant ERAS guideline items. The comparator group adhered to two (7.1%) of the relevant items. 199
Effectiveness of enhanced recovery protocol interventions at improving clinical outcomes
Report Supplementary Material 5, Table 13, displays clinical outcome data for RCTs evaluating ERP interventions to improve recovery from upper abdominal surgery. Meta-analysis of all trials indicated that LOS was a mean of 5.1 days shorter in ERP groups (SD 3.2 days, range 1.2–9.5 days), with a large effect size (d = –1.04, 95% CI –1.55 to –0.53; p < 0.001) (Figure 12). Heterogeneity was high and statistically significant, with wide CIs reflecting uncertainty in the true effect (I2 = 82.8%; p < 0.001).
FIGURE 12.
Forest plot showing the results of meta-analysis of the effect of ERP vs. usual treatment on LOS following upper abdominal surgery. PD, pancreaticoduodenectomy.
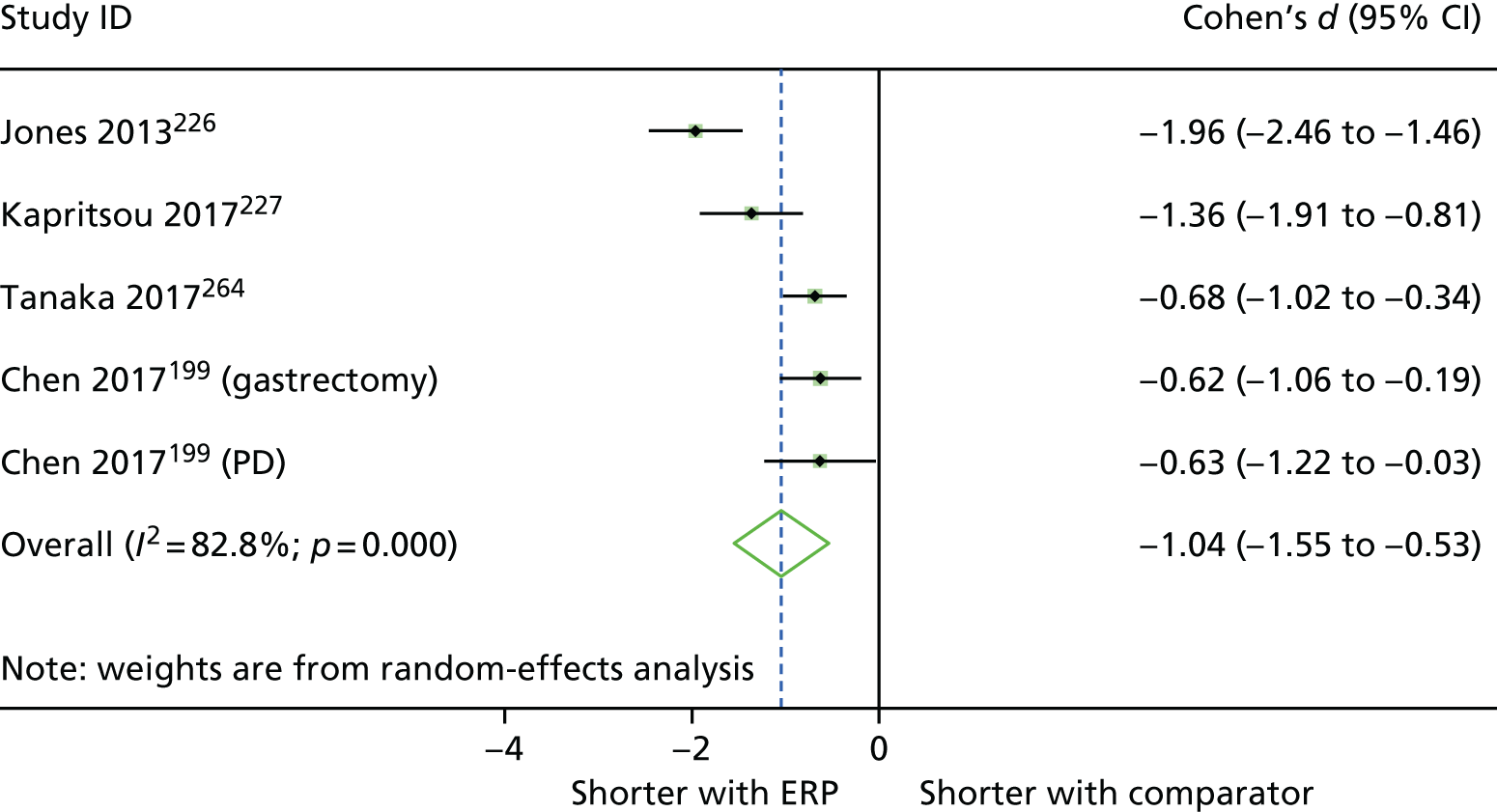
Complications were reported in five groups across four studies,199,226,227,264 with meta-analysis finding that the odds of sustaining complications were 61% lower in patients receiving ERP (OR 0.39, 95% CI 0.24 to 0.64; Figure 13). Although heterogeneity was low and not statistically significant for this effect (I2 = 37.4; p = 0.17), there was a mix of positive and null findings across the included studies. Re-admission rates were similar in the two studies that reported this outcome. 226,264
FIGURE 13.
Forest plot showing the results of meta-analysis of the effect of ERP interventions vs. usual treatment on odds of complications following upper abdominal surgery. C-D, Clavien–Dindo; PD, pancreaticoduodenectomy.
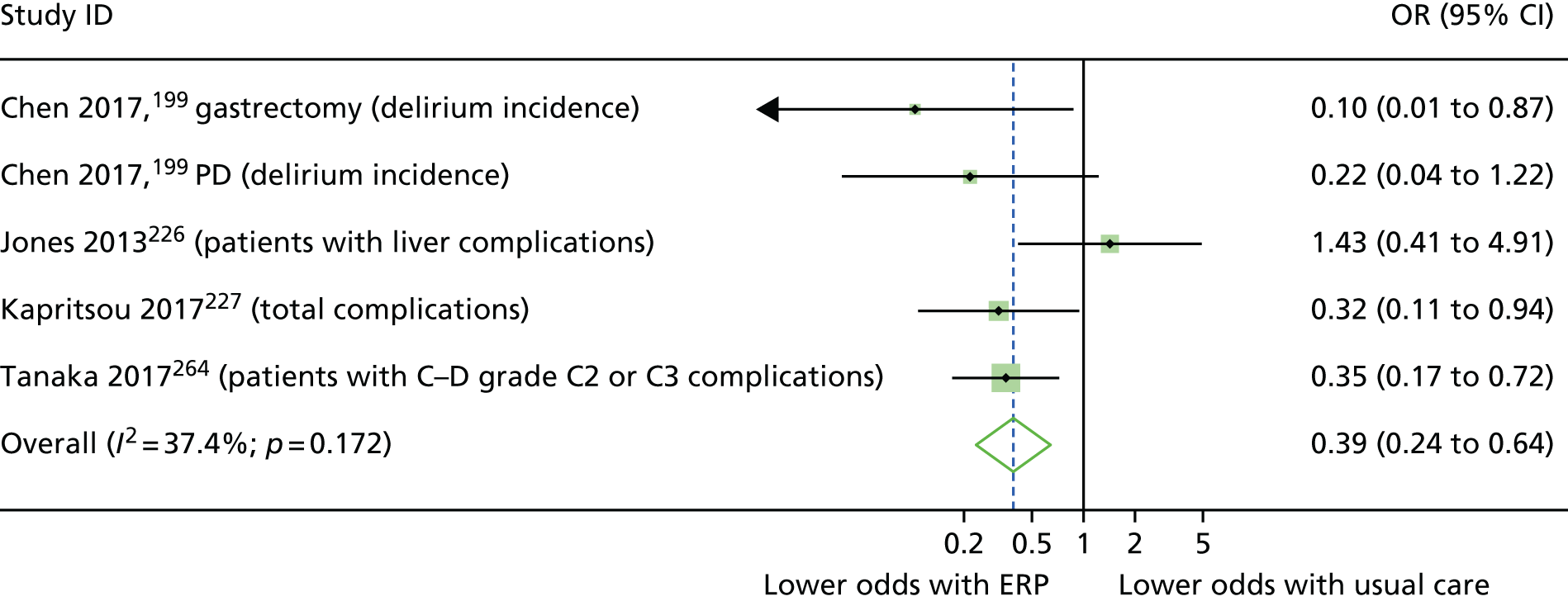
Effectiveness of enhanced recovery protocol interventions at improving patient-reported outcomes
Report Supplementary Material 5, Table 14, displays data for patient-reported outcomes from the studies trialling an ERP intervention to improve recovery from upper abdominal surgery. The study by Jones and colleagues226 found that patients in the ERP arm were medically fit for discharge around 4 days sooner than those receiving standard care, with a very large effect size reported (d = –5.23, 95% CI –6.1 to –4.35; p < 0.001). A range of markers of recovery was reported by Kapritsou and colleagues,227 including pain, sadness, optimism and various stress outcomes, but these did not differ between groups. Tanaka and colleagues264 reported earlier passage of first stool in participants in the ERP group (1 day earlier, d = –0.52, 95% CI –0.85 to –0.18; p < 0.01) but not first flatus. Patients in this study also had greater serum concentration of protein and transthyretin on day 7 following surgery, and experienced less weight loss than patients in the comparator arm at 1 week (0.9% less, d = 0.58, 95% CI 0.24 to 0.92; p < 0.01) and 1 month (1.6% less, d = 0.51, 95% CI 0.17 to 0.84; p < 0.01) after surgery. There was no opportunity to perform meta-analysis as outcomes were not similar between studies.
Prehab interventions
One RCT205 trialled a prehab intervention to improve recovery from upper abdominal surgery, with 35 patients included in the study. Patients in the experimental group performed 4 weeks of exercise sessions tailored to their physical capacity. Outcomes are presented in Report Supplementary Material 5, Table 15. There was no difference in LOS, complications or re-admissions between the groups. The authors evaluated markers of physical conditioning for all participants, also performing a subgroup analysis of ‘high-risk’ patients, as evaluated on study entry. Improvements were seen in a number of these outcomes, associated with large effect sizes, with some benefits also observed in high-risk patients.
Interventions to improve recovery from pelvic surgery, vascular surgery, thoracic surgery and surgery to remove tumours: randomised controlled trials
Several procedure categories featured in only one or two RCTs. These were interventions to improve recovery from pelvic,217,225 vascular31,247 and thoracic surgeries,248 as well as a range of surgeries to remove tumours. 220 Evidence from these studies is summarised below and in Table 12, with full details of intervention effectiveness described in Pelvic surgery, Vascular surgery, Thoracic surgery and Surgery to remove a tumour.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Pelvic surgery | ||||||||||||||||
| Jensen 2015,225 Denmark | 129* | ERP | AEI; PT; EX | EMOB; EX | x | ⇦⇨ | ⇦⇨ | ⇦⇨ | x(4/4) | |||||||
| Gralla 2007;217 Magheli 2001,240 Germany^ | 50 | ERP | nMBP; nFAST | ANE; WARM; PONV | ANA; DRA; EON; EMOB; FM; LAX | AEI | ▲ | ⬆ | ⇦⇨ | ▲ | ||||||
| Vascular surgery | ||||||||||||||||
| Partridge 2017,31 UK | 209 | PACP | AEI | FAPP | Pre-surgery: medications changed and level of care required advised. Post discharge: longer-term GP follow-up | x |
⬆(3/8) ⇦⇨(5/8) |
⇦⇨ | ⇦⇨(2/2) | |||||||
| Muehling 2008,247 2009,246 2011,49 Germany | 82; 101; 101 | ERP | nMBP; nFAST | CATH | CATH; EON; EMOB; ANA | x |
⬆(2/3) ⇦⇨(1/3) |
⇦⇨ | ⇦⇨ | x(3/3) | ||||||
| Thoracic surgery | ||||||||||||||||
| Muehling 2008,248 Germany | 62 | ERP | nFAST | SURG | EON; EMOB; ANA | x | ⇦⇨ | |||||||||
| Surgery to remove tumours | ||||||||||||||||
| Hempenius 2013,220 2016,219 the Netherlands | 297; 260a | PACP | AEI | Screened for delirium 3 × day | x | ⇦⇨(2/2) | ⇦⇨ | ⇦⇨ | ⇦⇨(2/2) |
È(1/13) ⇦⇨(4/13) x(9/13) |
||||||
Overview: interventions to improve recovery from pelvic surgery, vascular surgery, thoracic surgery and surgery to remove tumours
Pelvic surgery
The two RCTs trialling interventions to improve recovery after pelvic surgery investigated the effect of ERPs. Evidence from the two trials was inconclusive regarding the effects of ERP on any of the outcomes presented. The study by Gralla and colleagues217 demonstrated a statistically significant reduction of LOS within the intervention group. There were also statistically significant improvements for complications and markers of recovery in this study. However, the study by Jensen and colleagues,225 which was of higher quality than the German study, did not report any statistically significant differences between groups for any outcomes. 217 One apparent difference between these studies is that the intervention by Gralla and colleagues217 contained 12 active components, compared with the five in the intervention in the study by Jensen and colleagues. 225 However, with only two studies available, the RCT evidence for ERP to improve recovery from pelvic surgery is both limited and inconclusive.
Vascular surgery
Two studies evaluated interventions to improve recovery from vascular surgery. In the study by Muehling and colleagues,247 patients receiving ERP were less likely to experience complications, and there were no re-admissions or deaths in the study. The study was rated as ‘weak’ and the data presented for LOS precluded secondary analysis.
Partridge and colleagues31 evaluated a preoperative assessment that informed a care plan. Although the study was of ‘strong’ quality, and LOS was reported as 2.3 days shorter in the experimental group, a lack of variance data precluded calculations of effect size or statistical difference. The identification of 58 more new comorbidities in the experimental group than in the usual care group may have contributed to the reduced odds of complications and, ultimately, LOS. However, few conclusions about interventions to improve recovery from vascular surgery can be drawn.
Thoracic surgery
Muehling and colleagues248 evaluated an ERP intervention to improve recovery in patients undergoing thoracic surgery. The study was of ‘weak’ quality and outcomes either could not be analysed or did not differ between groups.
Surgery to remove a tumour
One study220 evaluated an intervention to provide supportive care and prevent delirium in patients undergoing various surgeries to remove a tumour. The study was of ‘moderate’ quality and reported on a wide range of outcomes other than LOS. Fewer patients could return to their previous living arrangements, which was an undesirable outcome. No other differences were observed in the study, and LOS could not be analysed.
Interventions to improve recovery from pelvic surgery: randomised controlled trials
There were two RCTs217,225 of ERP interventions to improve recovery following pelvic surgery, reported across three journal articles. One of the studies took place in Germany217 and one took place in Denmark. 225 The study by Gralla and colleagues217 was reported across two papers, with the publication by Magheli and colleagues240 focusing on aspects of recovery, while the core paper focused on LOS and complications. 217 Patients (n = 179) were admitted for radical cystectomy225 or radical prostatectomy;217 they had a mean age of 67.8 years and 81% were male.
The study by Jensen and colleagues225 was rated as ‘moderate’ quality overall, scoring as ‘weak’ only for data collection methods. Across the two papers reporting on the study by Gralla and colleagues,217,240 all domains were rated as ‘strong’ or ‘moderate’ except for data collection methods. LOS was clearly defined in both studies.
Enhanced recovery protocol interventions
Components of the ERP interventions and comparators are mapped against ERAS Society guidelines for pelvic surgery272 and radical cystectomy279 in Table 13. Usual care in the study by Jensen and colleagues225 already included a number of fast-track items; the experimental group underwent additional prehabilitation, rehabilitation and discharge planning in addition to the existing fast-track protocol. In the study by Gralla and colleagues,217 few ERAS items had been implemented into standard care.
| ERAS item | Study (first author and year) | |
|---|---|---|
| Gralla 2007,217 Magheli 2011240 | Jensen 2015225 | |
| Preoperative information, education and counselling | E, C | |
| Preoperative optimisation | E,a C | |
| Preoperative mechanical bowel preparation avoided | Eb | E,b Cb |
| Preoperative fasting: clear fluids allowed up to 2 hours and solids up to 6 hours prior to induction of anaesthesia | ||
| Preoperative carbohydrate treatment for patients without diabetes | E, C | |
| Pre-anaesthetic medication: avoidance of long-acting sedatives | ||
| Prophylaxis against thromboembolism | E, C | |
| Antimicrobial prophylaxis and skin preparation | E, C | E, C |
| Standard anaesthetic protocol | E, C | E, C |
| Epidural analgesia: TEA is superior to systemic opioids. It should be continued for 72 hours | E | E, C |
| PONV prophylaxis | E | E, C |
| Laparoscopic resection recommended for benign disease and rectal cancer (R/P). Minimally invasive approach not recommended outside trial setting (RC) | E, C | E,c Cc |
| Nasogastric intubation: nasogastric tubes not routinely used | E, C | |
| Nasogastric tubes removed before extubation | ||
| Preventing intraoperative hypothermia | E, C | |
| Perioperative fluid management: intraoperative fluids guided by flow measurements to optimise cardiac output (RC) | ||
| Perioperative fluid management: judicious use of vasopressors (RC) | ||
| Transurethral catheter (R/P) | E | |
| Suprapubic catheter (R/P) | ||
| Urinary drainage: transurethral catheter removal on POD1 in low risk patients | ||
| Early removal of bladder catheter | E, C | |
| Prevention of postoperative ileus: chewing gum (R/P), oral magnesium, alvimopan | ||
| Postoperative laxatives and prokinetics (R/P) | E | |
| Early oral intake 4 hours after surgery | E | E, C |
| Early mobilisation | E | E,a C |
| Audit: all patients should be audited for protocol compliance and outcomes | E, C | |
The intervention evaluated by Jensen and colleagues225 adhered to 14 (53.8%) items, the same number as the comparator group, but with additional exercise components. After excluding the two ERAS items exclusive to radical cystectomy, the intervention evaluated by Gralla and colleagues217 adhered to 12 out of the 24 (50%) ERAS items listed in Table 13, and the comparator group adhered to a mean of five (20.8%) items.
Effectiveness of enhanced recovery protocol interventions at improving clinical outcomes
Table 12 displays outcome data for LOS, re-admissions, additional care, mortality, morbidity and complication where reported in both studies. LOS was reduced by 3.1 days in the study by Gralla and colleagues,217 with a large effect size (d = –2.90, 95% CI –3.73 to –2.12; p < .001). In the study by Jensen and colleagues,225 median LOS was the same in both groups.
The odds of experiencing a complication was significantly lower in patients receiving ERP in the study by Gralla and colleagues217 (OR 0.25, 95% CI 0.07 to 0.83), with patients 75% less likely to experience complications. However, the wide CI indicated that this reduction in odds may have been as low as 17% or as high as 93%. ERP and usual care patients experienced similar rates of complications in the study by Jensen and colleagues. 225 Re-admission rates were similar between groups in both studies, and 90-day mortality was similar in the study by Jensen and colleagues. 225
Effectiveness of enhanced recovery protocol interventions at improving patient-reported outcomes
Report Supplementary Material 5, Table 17, displays outcome data for patient-reported outcomes where these were reported. It was possible to calculate standardised mean difference for only one outcome, which was time to first deflation/defecation in the study by Gralla and colleagues. 217 This occurred 0.4 days earlier after ERP than with usual care, associated with a medium effect size (d = –0.61, 95% CI –1.18 to –0.05; p < 0.05).
Descriptive statistics for patient satisfaction were presented by Gralla and colleagues. 217 There were similar distributions of scores for both groups for satisfaction with the LOS, while slightly more patients thought that the perioperative course was better than expected in the ERP group than in the usual care group (17 vs. 13 patients). 217 Jensen and colleagues225 reported postoperative pain scores, suggesting that patients in the ERP group were in more pain than the patients receiving usual care (16% reporting pain as 4 or above vs. 7%). After 90 days, the distribution of scores on the Clavien–Dindo scale was broadly similar between the groups. 225
Interventions to improve recovery from vascular surgery: randomised controlled trials
There were two RCTs31,247 of interventions to improve recovery and/or reduce LOS following vascular surgery. In the German study by Muehling and colleagues,247 82 patients underwent open repair of an infrarenal aortic aneurysm. We report data from the 2009 paper,246 when full recruitment of 101 patients had been completed, and the 2011 paper,249 which contains additional outcomes (incidence of organ failure and development of systemic inflammatory response syndrome). Patients randomised to the experimental arm received an ERP, primarily consisting of no bowel preparation, reduced preoperative fasting, patient-controlled epidural analgesia, enhanced postoperative nutrition and early mobilisation. ERP was compared with usual care.
In the study from the UK by Partridge and colleagues,31 patients were admitted for endovascular/open aortic aneurysm repair or lower limb arterial bypass surgery. Two hundred and nine participants were randomised either to a preoperative CGA leading to an optimised care plan or to standard preoperative assessment. Full details of the interventions trialled in the two vascular surgery studies can be found in Report Supplementary Material 3, Tables 17 and 18.
The study by Partridge and colleagues31 was rated as ‘strong’ overall, with only one rating of ‘moderate’, for blinding of assessors and participants. However, a clear definition of LOS was not provided. The study by Muehling and colleagues,246,247,249 which was reported across three journal articles, achieved a global rating of ‘weak’. LOS was not clearly defined in any of the three papers reporting on the study.
Effectiveness of interventions
Report Supplementary Material 5, Table 18, displays data for all effectiveness outcomes reported in the two studies trialling interventions to improve recovery from vascular surgery. In the study by Muehling and colleagues,246,249 patients receiving ERP were at lower odds of requiring postoperative ventilation (OR 0.11, 95% CI 0.02 to 0.55), experiencing organ failure (OR 0.34, 95% CI 0.13 to 0.88) or developing systemic inflammatory response syndrome (OR 0.39, 95% CI 0.17 to 0.9). There were no re-admissions or deaths in the study. For LOS and markers of recovery, the authors presented only medians and ranges, precluding secondary analysis.
In the study by Partridge and colleagues,31 LOS was reported as 2.3 days shorter in the experimental group; however, lack of variance data precluded calculations of effect size or statistical difference. The CGA identified 58 more new comorbidities than were diagnosed in the group receiving regular preoperative assessment. Postoperatively, odds of delirium (OR 0.39, 95% CI 0.17 to 0.9), cardiac complications (OR 0.24, 95% CI 0.02 to 0.58), and bowel and bladder complications (OR 0.4, 95% CI 0.22 to 0.74) were significantly lower in the experimental group. There were no statistically significant differences in any other outcomes.
Interventions to improve recovery from thoracic surgery: randomised controlled trials
There was one RCT of an intervention aiming to improve recovery following thoracic surgery. 248 The study compared ERP with traditional care among 59 patients undergoing lung resection. Both the ERP and standard care pathways featured preoperative information, education and counselling, preoperative warming and prevention of fluid overload. In addition, patients in the ERP arm received minimal preoperative fasting, a standardised anaesthetic protocol, early oral intake and early mobilisation. Full details of the intervention can be found in Report Supplementary Material 3, Table 19.
Publication bias: randomised controlled trials
Figure 14 is a funnel plot of the data for LOS from 30 comparisons from the 26 RCTs that provided data from which standardised mean differences could be calculated. 192,193,198–200,204,205,210,211,214,215,217,222,226,227,234,236,239,242,243,255,261,264,266,268,269 As the intercept was below zero, this indicates a tendency towards publication of studies showing beneficial effects of the interventions being evaluated. Studies with more robust data were clustered more symmetrically than those with less robust estimates (small sample sizes), which may indicate that there is bias whereby smaller trials with null or negative outcomes are not included in the data.
FIGURE 14.
Funnel plot to assess publication bias in reporting of LOS across all RCTs. Dotted lines indicate pseudo-95% CIs. SMD, standardised mean difference (effect size) for each study; SE of SMD, the standard error of that effect.
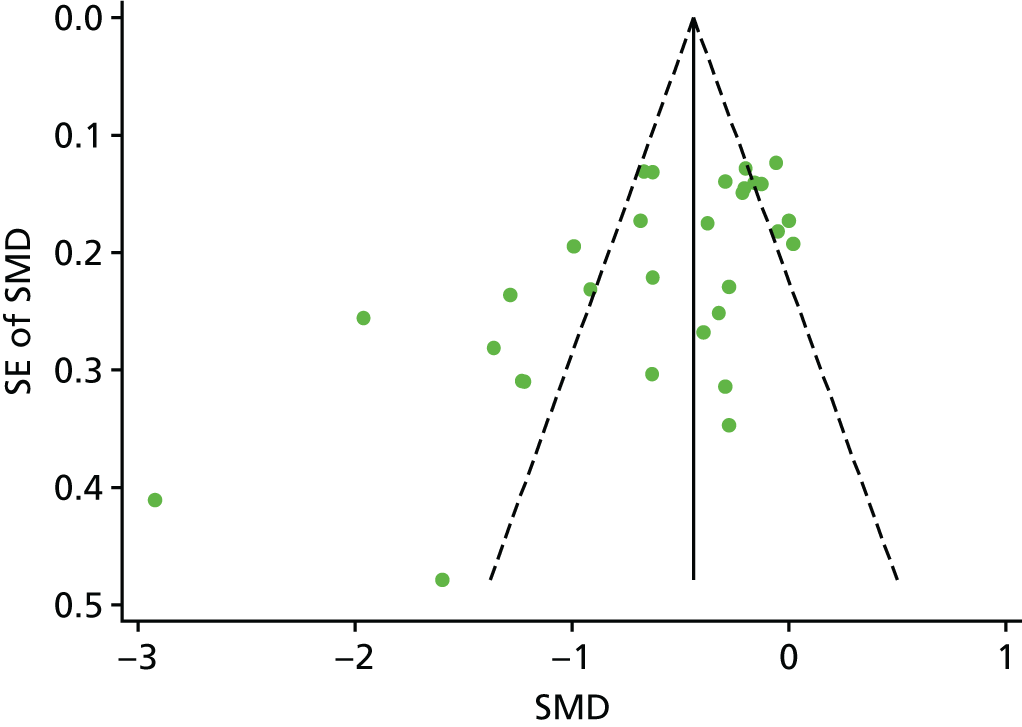
Although publication bias may be suspected in the first instance, it should be noted that interventions were often the implementation of evidence-based protocols to improve recovery from surgery and usually involved additional changes to existing ‘best care’. This may make it unlikely that such interventions would have an adverse effect on LOS, and a negative intercept value could therefore be expected. However, it might be expected that, although interventions ought to lead to reductions in LOS, the influence of such an outcome on risk of complications is less predictable. Therefore, we produced Figure 15, a funnel plot of the data for 36 summary complications outcomes from the 28 RCTs providing such data. 192,198,200,204,205,209,211,213,214,220,222,223,226,227,231,235,236,239,242,246,247,251,252,255,256,261,264,268 Inspection of this funnel plot indicates a symmetrical distribution of studies about the intercept, although this is again in favour of a beneficial effect (lower odds of complications in experimental groups).
FIGURE 15.
Funnel plot to assess publication bias in reporting of complications across all RCTs. Dotted lines indicate pseudo-95% CIs. Log(OR), the log of the OR for each study; SE of log(OR), the standard error of that OR.

In summary, although there is a possibility of publication bias in the evidence from RCTs evaluating interventions to reduce LOS in older adults undergoing elective procedures, the context of the evidence should alleviate concerns that possible unpublished trials with negative results could threaten the robustness of the evidence.
Synthesis of evidence from the UK
Interventions to improve recovery from colorectal surgery: evidence from the UK
Seven studies192,203,213,230–232,236 trialled interventions intended to improve recovery and/or reduce LOS following colorectal surgery in the UK, all of which evaluated ERP interventions compared with usual care. Among these were four RCTs,192,213,231,236 one controlled trial230 and two UBA designs. 203,232 A total of 912 people commenced trials, with sample sizes ranging from 25192 to 506. 203 Only the UBA studies by Dhruva Rao and colleagues203 and King and colleagues232 had sample sizes > 100. Participants were admitted for hemicolectomy (left and right)192 or colorectal resection. 203,213,230,231,236 Across the included studies, approximately half of the participants were female. Sample ages were reported as both medians and means and these can be seen in Table 2.
Overview: colorectal surgery
The evidence from the UK relating to colorectal surgery is summarised here and in Table 14. Full details of the effectiveness of interventions can be found in Enhanced recovery protocol interventions.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Gatt 2005,213 RCT | 39 | ERP | AEI; PBio | nMBP; CHL; nFAST | ANA; DRA; FM; nNGT; SURG | EON; EMOB | x | ⇦⇨ | ⇦⇨ | ⇦⇨ | x | ⇦⇨ | ||||
| King 2006,232 UK, UBA | 146 | ERP | NUT | SURG; FM; nNGT; DRA | DP; EON; EMOB; LAX; ANA; CATH | x(5/5) | ⇦⇨ | ⇦⇨ | ⇦⇨ | |||||||
| Anderson 2003,192 RCT | 25 | ERP | AEI; NUT | nMBP; CATH; CHL; nFAST | ANE; ANA; nNGT; SURG | EON; EMOB | ▲ | ⇦⇨ | ⇦⇨ | x |
▲(1/2) ◁▷(1/2) |
|||||
| Dhruva Rao 2015,203 UK, UBA | 506 | ERP | NUT | CHL; DP; nFAST | ANE; FM; PONV | EON; EMOB; ANA | Pre-admission optimisation | x | ◁▷(2/2) | ◁▷ | ||||||
| Khan 2013,229 UK, CT | 83 | ERP | AEI | CHL; nFAST | ANE; FM; DRA; SURG | EON; EMOB; ANA; CATH; LAX | ▲ | ⇦⇨ | ⇦⇨ | |||||||
| Khoo 2007,231 RCT | 81 | ERP | nFAST | FM; nNGT | EON; EMOB; ANA; CATH | x(2/2) | ⬆ | ⇦⇨ | ⇦⇨ | x(3/3) | ⇦⇨(3/3) | |||||
| Lidder 2013,236 RCT | 57 | ERP | CHL | EON; NUT | ◁▷ |
⬆(1/4) ⇦⇨(3/4) |
◁▷ | |||||||||
Seven studies based in the UK evaluated interventions to improve recovery and/or reduce LOS following colorectal surgery, and all evaluated ERP interventions. The evidence from these studies suggests that ERP interventions may be associated with shorter LOS, with a mean reduction of 2.4 days across three studies reporting data conducive to secondary analysis. 192,230,236 This occurred alongside a trend for the odds of experiencing a complication to be lower. Re-admission rates were similar between groups of patients receiving ERP and usual care, and, although there was a trend for markers of recovery to improve with ERP interventions, such outcomes were not widely reported.
When reported data precluded calculation of standardised mean differences, studies reported favourable effects with ERP. As such, the heterogeneity of interventions (i.e. multiple combinations of multiple components) and homogeneity in results (indicating effectiveness for LOS and little effect on other outcomes) leads to the conclusion that ERP interventions of the nature described in this section may reduce LOS without detrimental effect to other outcomes. However, the causative factors bringing about such an effect are not readily identifiable.
Enhanced recovery protocol interventions
Components of the ERP interventions and comparators are mapped against ERAS Society recommendations for colorectal271 and rectal/pelvic surgery272 in Table 15. The full details of both ERP and prehab interventions can be found in Report Supplementary Material 3, Tables 1–4. In summary, the most common ERP components were the provision of preoperative information, education and counselling (5/7 studies); avoidance of lengthy preoperative fasting (5/7); preoperative carbohydrate treatments (6/7); a standardised anaesthetic protocol (7/7); laparoscopic surgery used where available (5/7); nasogastric tubes not routinely used (5/7); enteral route for fluids and early discontinuation of intravenous fluids postoperatively (5/7); thoracic epidural analgesia postoperatively (7/7); early oral intake postoperatively (7/7); perioperative nutritional care (6/7); and early mobilisation (6/7). The least common components were avoidance of pre-anaesthetic medication (0/7 studies); prophylaxis against thromboembolism (0/7); antimicrobial prophylaxis and skin preparation (1/7); prevention of intraoperative hypothermia (1/7); judicious use of vasopressors (0/7); transurethral (0/7) or suprapubic catheter (0/7) use; prevention of postoperative ileus with chewing gum, oral magnesium or alvimopan (0/7); and postoperative glucose control (0/7) (although this item was a specific allowance for diabetic patients, and no such patients were explicitly reported within the included samples).
| ERAS item | Study (first author and year) | ||||||
|---|---|---|---|---|---|---|---|
| Khan 2013229a | Lidder 2013236a | Anderson 2003192 | Gatt 2005213 | Khoo 2007231 | King 2006232 | Dhruva Rao 2015203 | |
| Preoperative information, education and counselling | E | E | E | E, C | E, C | ||
| Preoperative optimisation | E | E | E | ||||
| Preoperative mechanical bowel preparation avoided | E | E | |||||
| Preoperative fasting: clear fluids allowed up to 2 hours and solids up to 6 hours prior to induction of anaesthesia | E,b C | E | Eb | E,b Cb | E | ||
| Preoperative carbohydrate treatment | E | E | E | E | Ec | E | |
| Pre-anaesthetic medication: no routine long- or short-acting sedatives (colonic); no advantages to using long-acting benzodiazepines, short-acting should not be given to those aged > 60 years (R/P) | |||||||
| Prophylaxis against thromboembolism | |||||||
| Antimicrobial prophylaxis and skin preparation | E, C | ||||||
| Standard anaesthetic protocol | E | E, C | E | E, C | E, C | E, C | E |
| PONV prophylaxis | E | E | |||||
| Laparoscopy and modifications of surgical access: recommended for colonic surgery | E, C | E,d Cd | E, C | E,d Cd | E,d Cd | ||
| Nasogastric intubation: nasogastric tubes not routinely used | E, C | E, C | E | E | E | ||
| Nasogastric tubes removed before extubation | E | E | E | C | |||
| Preventing intraoperative hypothermia | E, C | ||||||
| Perioperative fluid management: intraoperative fluids guided by flow measurements to optimise cardiac output | E, C | C | E | E | |||
| Perioperative fluid management: judicious use of vasopressors | |||||||
| Perioperative fluid management: enteral route for fluids postoperatively should be used as early as possible, and intravenous fluids should be discontinued as soon as is practicable | E | E, C | E | E | E | ||
| No drainage of peritoneal cavity after colonic anastomosis | E | E | E | E | |||
| Transurethral catheter (R/P) | |||||||
| Suprapubic catheter (R/P) | |||||||
| Urinary drainage: routine transurethral bladder drainage for 1–2 days is recommendede | E | E, C | E, C | ||||
| Early removal of bladder catheter | E | E | E | ||||
| Prevention of postoperative ileus: mid-thoracic epidural plus laparoscopic surgery (colonic) | E | E,d Cd | E | Ed | |||
| Prevention of postoperative ileus: fluid overload and nasogastric decompression avoided | E | E | E | ||||
| Prevention of postoperative ileus: chewing gum (R/P), oral magnesium, alvimopan | |||||||
| Postoperative laxatives and prokinetics (R/P) | E | E | |||||
| Postoperative analgesia. Open surgery: TEA using low-dose local anaesthetic and opioids; laparoscopic: an alternative to TEA is a carefully administered spinal analgesia with a low-dose, long-acting opioid | E | E, C | E | E | E, C | E | E |
| Early oral intake | E, C | E | E | E | E | E | E |
| Perioperative nutritional care | E | E | E | E | E | E | |
| Postoperative glucose control | |||||||
| Early mobilisation | E | E | E | E | E | E | |
Excluding the item for glucose control, ERP intervention groups adhered to a mean of just below 14 (45.6%) of the 30 ERAS Society guideline items (range from 11231 to 17232), whereas comparator groups adhered to just over four items (14.9%, range 1213 to 8236). Interventions ‘branded’ by study authors as ERAS adhered to a mean of 10 (33.3%) of ERAS Society guideline items, compared with a mean of 13.8 (45.8%) of ERP interventions not branded as ERAS.
Quality assessment
Studies were rated as either ‘weak’ or ‘moderate’ quality globally. Typically, studies were rated as ‘strong’ for study design if they were RCTs192,213,231,236 and ‘weak’ if they were not. 203,229,232 Studies were commonly rated as ‘moderate’ for selection bias and blinding and ‘weak’ for data collection. Only two studies192,232 clearly described how LOS was defined. Three studies192,231,236 were rated as ‘weak’ on three or more domains.
Report Supplementary Material 6, Table 1, displays data for clinical outcomes for each study trialling ERP interventions to improve recovery from colorectal surgery in the UK. Where it was possible to calculate standardised mean differences for LOS, LOS was significantly shorter in the studies by Anderson and colleagues192 (d = –1.60, 95% CI –2.54 to –0.66; p < 0.001) and Khan and colleagues229 (d = –0.65, 95% CI –1.09 to –0.20; p < 0.01) but not in the study by Lidder and colleagues. 236 The mean reduction in LOS across those three studies was 2.4 days (SD 0.6 days, range 1.9–3.0 days).
In the remaining studies, median LOS was reported as lower with ERP than usual care. 203,213,231,232 Re-admissions were reported in five studies,192,213,229,231,232 and were similar between groups in all.
Complications were reported in seven studies, with results plotted in Figure 16. Although only one study reported a statistically significant reduction in the odds of complications, the point estimates of all seven studies were indicative of a trend in favour of ERP. 231
FIGURE 16.
Forest plot (without pooled effects) showing the results of meta-analysis of the effect of ERP in odds of complications in patients undergoing elective colorectal surgery in the UK.
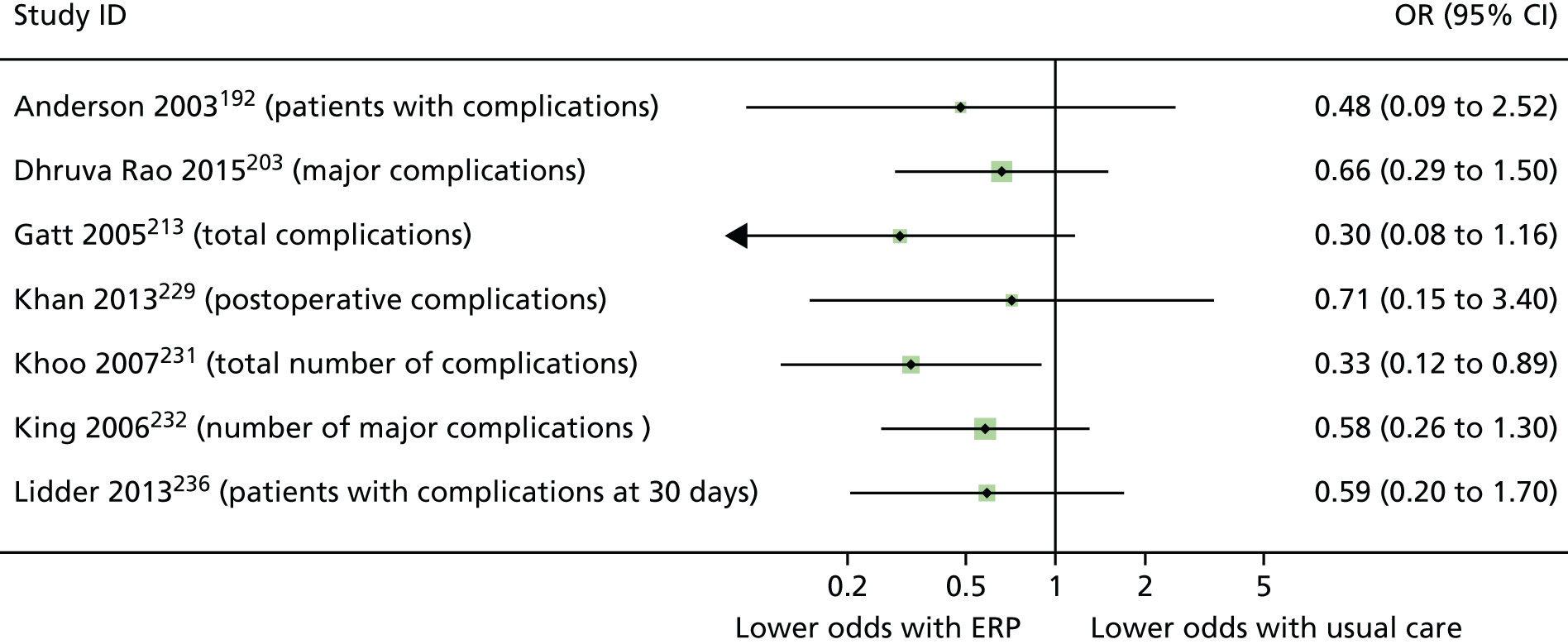
Report Supplementary Material 6, Table 2, displays data for patient reported outcomes for the studies trialling ERP interventions to improve recovery from colorectal surgery in the UK. Gastrointestinal function returned statistically significantly earlier in the ERP group in the study by Anderson and colleagues (d = –1.53, 95% CI –2.43 to –0.66; p < 0.001), but there was no difference in the time until patients could walk to the toilet unaided, despite a medium effect size (d = –0.65, 95% CI –1.46 to 0.16; p = 0.08). 192 Lidder and colleagues236 found no difference in the time taken for patients to be medically fit for discharge.
For other outcomes, where between-group statistics could not be calculated, patients in the ERP group spent longer out of bed on postoperative day 1 in the study by Gatt and colleagues;213 Khoo and colleagues231 reported earlier median time to tolerating solid diet, achieving independent mobility and passage of first stool. In the same study, only 3 out of 35 patients in the ERP group felt that they would have benefited from a longer stay, whereas 24 out of 35 patients receiving usual care would have preferred a longer stay.
Interventions to improve recovery from lower limb arthroplasty: evidence from the UK
There were 15 studies reporting across 16 papers evaluating interventions to improve recovery following lower limb arthroplasty in the UK. Of these, 11 studies206,216,218,224,230,237,238,241,245,256,260,262 compared ERP interventions with usual care; two trialled prehab versus usual care244 or home exercise;269 one trialled a 7-day rehab programme compared with weekday-only rehab253 and one evaluated a specialist ward versus previous usual care. 195
There were three RCTs,244,256,269 two controlled trials224,253 and 10 UBA studies. 195,206,216,218,230,237,238,241,245,262 In the study reported in two papers,224,260 the authors compared outcomes between centres with differing LOS in Belfast, Liverpool and London. The paper by Hunt and colleagues224 reports most outcomes from the study, with the paper by Salmon and colleagues260 focusing on patient satisfaction.
A total of 17,023 participants were included, with sample sizes ranging from 39244 to 5319. 230 Four studies had samples of 100–200 patients,206,218,238,269 five had samples of 400 to 1000216,224,245,253,195 and four had samples of > 1000. 230,237,241,262 Procedures were primary or revision unilateral or total hip or knee arthroplasty.
The full details of all interventions can be found in Report Supplementary Material 3, Tables 5–9.
Overview: lower limb arthroplasty
The evidence regarding interventions to improve recovery from lower limb arthroplasty in older adults in the UK is summarised below and in Table 16. Full details of the effectiveness of interventions are available in Enhanced recovery protocol interventions, Prehab interventions, Rehab interventions and Specialist ward.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Reilly 2005,256 RCT | 41 | ERP | ANA; EMOB; EON | AEI; TEL | x | ⇦⇨(2/2) |
▲(1/5) ◁▷(4/5) |
|||||||||
| Dwyer 2012,206 UBA | 127 | ERP | AEI; DP; NUT; EX; OT; PT | CHL; nFAST; nPreM | ANE; CATH; PONV; TP | ANA; DP; EON; EMOB; EX; PT; NUT; GOAL | TEL; FAPP | Pre-hospital admission: involvement of a family member | x | ⇦⇨ | ||||||
| Gordon 2011,216 UBA | 847 | ERP | AEI; DP; EX; OT | SW | AIE; EMOB; ANA; SW; GOAL | TEL; EX | △ | |||||||||
| Gordon 2011,216 UBA | 847 | ERP | AEI; DP; EX; OT | SW | AIE; EMOB; ANA; SW; GOAL | TEL; EX | Joint replacement school. Family member as ‘coach’. Audio devices in theatre. Senior nurses have discharge authority. Pre-prepared analgesia packs at discharge | △ | ||||||||
| Harari 2007,218 UBA | 108 | ERP | AEI; DP; OT; PT; SoW | EMOB; EON; GOAL | FAPP; REF; TEL | Geriatrician/nurse reviewed patients in wards plus provided staff education | △ |
⬆(5/6) ⇦⇨(1/6) |
⇦⇨ | ⇦⇨ | ||||||
| Hunt 2009,224 Salmon 2013,260 CTa | 579; 560 | ERP | OT; PT | DA | ANA; ANE; SURG | ANA; EMOB; PT; SW | TEL | Discharge planned for POD2. Belfast site vs. Liverpool site | x | ▲ | ◁▷(7/7) | |||||
| Hunt 2009,224 Salmon 2013,260 CTa | 579; 560b | ERP | OT; PT | DA | ANA; ANE; SURG | ANA; EMOB; PT; SW | TEL | Discharge planned for POD2. Belfast site vs. London site | x | △ |
△(1/7) ◁▷(6/7) |
⬆(2/2) | ||||
| Khan 2014,230 UBA | 6000 |
ERP |
AEI | ANA |
ANE FM CATH T |
EMOB FM ANA DP EX LAX |
TEL | x |
⬆(2/7) ⇦⇨(5/7) |
⇦⇨(2/2) | ⇦⇨ | |||||
| Maempel 2015,238 UBA | 165 | ERP | AEI; OT | AP | ANA | x | ⇦⇨(3/3) | ⇦⇨ |
⇦⇨(2/2) x(1/3) |
|||||||
| Maempel 2016,237 UBA | 1161 | ERP | AEI; DP; OT | AP | EMOB; ANA | ▲ | ⇦⇨ | ⇦⇨ | ⇦⇨ | |||||||
| Malviya 2011,241 UBA | 4500 | ERP | AEI; PreM | ANE; FM; CATH | AEI; EMOB; MT; ANA | TEL | x | ⬆(1/1) | ⬆(2/2) | ⇦⇨ | ||||||
| Mertes 2013,245 UBA | 607c | ERP | AEI; DP; PT; OT | AEI; nFAST; TP | AP | AEI; CATH; DRA; DP; EON; EMOB; OT; REF | FAPP | Family involvement in education programme |
▲(1/4) △(1/4) ◁▷(2/4) |
|||||||
| Starks 2014,262 UBA | 2128 | ERP | AEI; DP | nFAST | ANA; ANE; AP; WARM | EMOB; PT; ANA | TEL | Medical optimisation before admission. Promotion of independence and wellness postoperatively |
⬆(2/4) x(2/4) |
⇦⇨(2/2) | ⇦⇨(2/2) | |||||
| Williamson 2007,269 RCT | 181 | Prehab | EX; PT | ◁▷ | ◁▷(3/3) |
▼(1/2) ◁▷(1/2) |
◁▷ | |||||||||
| McGregor 2004,244 RCT | 39 | Prehab | AEI | x | ◁▷(2/2) | ◁▷(4/4) | ||||||||||
| Pengas 2015,253 UBA | 791 | Rehab | PT | Weekends included in programme | ◁▷(2/2) |
△(2/5) x(3/5) |
||||||||||
| Barlow 2013,195 UBA | 410 | SW | SW | ▲(2/2) | ||||||||||||
Of the 11 UK studies evaluating the effectiveness of an ERP intervention in older adults undergoing lower limb arthroplasty, five216,218,237,245,262 provided variance data that allowed for secondary analysis, all demonstrating reduced LOS with the intervention group on at least one outcome measure. These five studies indicated that ERP interventions was associated with a reduction in LOS of between 2.4 and 4.3 days compared with usual care. This change was achieved without detriment to patient recovery or well-being, as demonstrated by eight studies measuring additional outcomes. 206,218,224,230,237,241,256,262 Six studies did not provide variance data to allow for secondary analysis. 206,224,230,238,241,256
There was variation in the magnitude of effect in the two studies by Maempel and colleagues, performed with patients undergoing knee238 and hip237 replacements. Discharge planning before admission was added to the intervention in hip replacement patients, although the greater reduction in LOS may be due to the differences in knee and hip replacement surgeries and their different recovery times.
Evidence for prehab was equivocal, ultimately leading to the conclusion that there was no overall benefit for either LOS or patient recovery beyond that conveyed by usual care. There were just single studies about rehab253 and specialist ward195 interventions, preventing conclusions about their effectiveness.
Enhanced recovery protocol interventions
Components of the ERP interventions and comparators are mapped in Table 17. We used an ERAS-style mapping approach, despite the absence of ERAS Society guidelines for lower limb arthroplasty. The following items were the most common: pre-admission assessment, education and counselling (10/11 studies); preoperative assessment by an occupational therapist or a social worker to identify postoperative needs and/or provision of equipment (7/11); routine use of spinal or epidural anaesthesia (8/11); avoidance of patient-controlled analgesia (9/11); early mobilisation protocol (10/11); dedicated post-surgery physiotherapy, supported by a physiotherapist (PT) (7/11); and follow-up support (8/11).
| Intervention component | Study (first author and year) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dwyer 2012206 | aGordon 2011216 | Harari 2007218 | Hunt 2009;224 Salmon 2013260 | Khan 2014230 | Maempel 2015238 | Maempel 2016237 | Malviya 2011241 | Mertes 2013245 | Reilly 2005256 | Starks 2014262 | |
| Assessment/education/counselling | E | E | E | E, Cb | E | E | E | E | E | E | |
| Avoidance of preoperative fasting | E | E | E | ||||||||
| Preoperative carbohydrate treatment | E | ||||||||||
| Preoperative exercise | Ec | E | E | C2d | |||||||
| Optimisation of physical condition prior to surgery | E | E | E | ||||||||
| Discharge planning: prior to admission | E | E | E | E | E | ||||||
| Discharge planning after hospital admission | E | E | |||||||||
| Pre-treatment OT/SW assessment of PO needs and/or equipment provision | E | E | E | C | E | E | E | ||||
| Admission on day of surgery | E | C1, C2 | E,C | E | E | E | |||||
| Pre- or postoperative antibiotic prophylaxis | E | E | E | E | |||||||
| Anaesthesia protocol: routine use of spinal or epidural anaesthesia | E, C | E, C | E, C2 | E | E, C | E, C | E | E | |||
| Anaesthesia protocol: short-acting, opiate avoidance | E | E, C | E | E | E, C | ||||||
| Minimally invasive surgical technique | E, C | ||||||||||
| Wound management protocol | E | E, C | E | ||||||||
| Avoidance of routine urinary catheter | E | E | |||||||||
| Perioperative fluid management: intraoperative fluids guided by flow measurements to optimise cardiac output | E, C | ||||||||||
| Perioperative fluid management: judicious use of vasopressors | E | E | |||||||||
| No routine drains | E | E, C | |||||||||
| Maintenance of normothermia | E | ||||||||||
| Avoidance of PCA | E | C2 | E | E | E | E | E | E | E | ||
| Avoidance of routine opiate analgesia | E | E | E | E | E | ||||||
| Early mobilisation protocol | E | E | E | E, C1, C2 | E | E, C | E | E | E | E | |
| Dedicated post-surgery physiotherapy exercises, supported by PT | E | E, C1, C2 | E | E | E, C | E | E | ||||
| Early resumption of oral intake (fluids, nutrition) | E | E | E | E | |||||||
| PONV prophylaxis | E | ||||||||||
| Laxative use | E | ||||||||||
| Thromboembolic prophylaxis | E | E, C1, C2 | E | E, C | E, C | ||||||
| Goal-setting with patient | E | E | E | ||||||||
| Patient encouraged to be active participant in own care/disown sick role | E | E | E | E | E | ||||||
| Provision of equipment/medication on discharge | E | E | E | E, C | |||||||
| Follow-up support: contact telephone number, telephone call, home visit | E | E | E | E, C2 | E | E | E | E | |||
| Post-discharge exercise regimen and/or PT support | E | E | |||||||||
| Follow-up outpatient/community clinic visit/referrals | E | E | E, C1, C2 | E | E | ||||||
In the study by Gordon and colleagues,216 two experimental groups were compared with a historical cohort receiving standard care. One experimental group received an ERP and one group received this ERP as well as attending ‘joint recovery school’, where patients received multimodal education about all aspects of care and recovery.
Quality assessment
Two studies256,269 achieved a global rating of ‘moderate’ and the rest were rated as ‘weak’. The studies rated as ‘moderate’ achieved ‘strong’ ratings for study design and confounders. In addition, the study by Reilly and colleagues256 scored ‘strong’ for reporting of withdrawals and dropouts, and the study by Williamson and colleagues269 was rated as ‘strong’ for data collection methods.
All of the ‘weak’ studies were given domain ratings of ‘weak’ for study design and data collection methods. Six studies were rated as ‘weak’ for confounders. 230,237,241,245,253 Only four studies195,206,224,237 offered a clear definition of LOS.
Report Supplementary Material 6, Table 3, displays data for clinical outcomes for each study trialling an ERP intervention to improve recovery from lower limb arthroplasty in a UK hospital. The forest plot in Figure 17 shows standardised mean differences in LOS between ERP and usual care in studies from which these data could be calculated. 216,218,237,245,256 In the seven groups from these five studies, LOS was reduced with ERP by a mean of 2.4 days (SD 1.2 days, range 0.8–4.3 days). Effect sizes were small in five groups,216,218,245 and large in two (–1.35 and –3.0). 237,256 Re-admissions were recorded in six studies,206,218,230,241,256,262 with no statistically significant difference in the odds of re-admission with ERP or usual care for any of the individual studies (Figure 18).
FIGURE 17.
Forest plot (without pooled effects) showing standardised mean differences in LOS between ERP and usual care in patients undergoing lower limb arthroplasty in UK hospitals. JRS, joint replacement school; THA, total hip arthroplasty; TKA, total knee arthroplasty.

FIGURE 18.
Forest plot (without pooled effects) showing the relative odds of being re-admitted for patients receiving ERP or usual care groups when undergoing lower limb arthroplasty in UK hospitals.
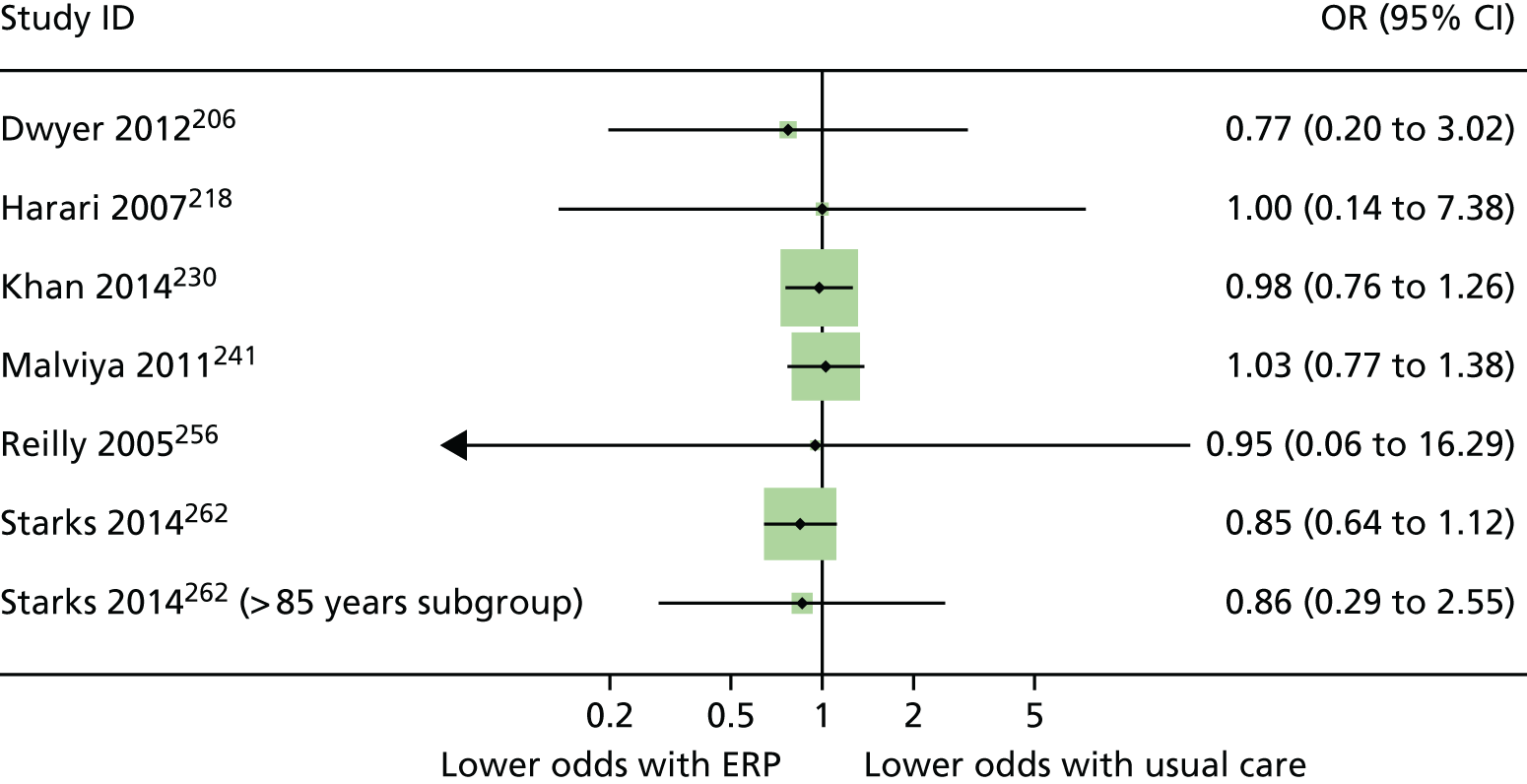
In the study by Gordon and colleagues,216 attendance at the joint replacement school, in addition to ERP, seemed to have greater benefit in hip replacement patients than in knee replacement patients. LOS was reduced by an additional 2.3 days in these knee replacement patients, compared with those who received ERP only.
In the study by Harari and colleagues,218 who evaluated the ‘POPS’ intervention (Proactive care of older people undergoing surgery), LOS was 4.3 days shorter and delayed discharge was 87% less likely in the intervention group (95% CI 0.06 to 0.31). Medical complications (24% fewer in ERP) and delays while waiting for occupational therapists and/or equipment (16.7% fewer in ERP) accounted for the majority of the delays in discharge in the usual care group, with differences between groups being statistically significant for these reasons for delay.
In the studies where standardised mean differences could not be calculated, LOS was always reported as shorter with ERP. 206,224,230,238,241,262 Dwyer and colleagues206 reported that LOS was a mean of 3 days shorter with ERP, with comparable numbers of re-admissions in both groups. Patient data were also stratified by preoperative haemoglobin levels, American Society of Anesthetists scores and body mass index, with similar reductions in LOS for ERP patients regardless of stratum.
In the large UBA study by Khan and colleagues,230 median LOS was 3 days shorter and there were 0.5% fewer myocardial infarctions with ERP, but there were no statistically significant differences in any other outcome compared with the pre-ERP group. Maempel and colleagues conducted separate studies with knee238 and hip237 replacement patients receiving slightly different ERP packages. The study conducted with hip replacement patients237 was considerably larger (n = 1161) than the one conducted with knee replacement patients238 (n = 165). Median LOS was 1 day shorter in the ERP group with knee replacement patients. 238 Rates of dislocation and death in the year following surgery did not differ between groups in the same study. 238 In the 2016 study with hip replacement patients, LOS was 2 days shorter in ERP patients, associated with a large effect size (d = –1.35, 95% CI –1.47 to –1.22; p < 0.001), with no differences between groups for other outcomes. 237
In the large study by Malviya and colleagues,241 mean LOS was 3.7 days shorter in the ERP group, and re-admission rates were similar between groups. Odds of death within both 30 and 90 days of surgery were statistically significantly lower in the ERP group (30 days: OR 0.13, 95% CI 0.02 to 1.01; 90 days: OR 0.24, 95% CI 0.07 to 0.79). However, wide CIs indicate that the odds of death within 30 days was between 98% lower and 1% greater in the ERP group, indicating great uncertainty about the true outcome. 241
Starks and colleagues262 reported mean LOS without variance data, with shorter LOS with ERP in both hip and knee procedures, as well as when considering a subgroup analysis of patients aged > 85 years. The odds of patients experiencing a ‘long LOS’ [defined as those who stayed beyond the national upper quartile LOS for their strata of procedure (8 days for 2007 data and 7 days for 2008 and 2009 data)] was reduced with ERP in all patients (OR 0.18, 95% CI 0.15 to 0.23), and within the subgroup analysis of patients aged > 85 years (OR 0.13, 95% CI 0.07 to 0.25).
Report Supplementary Material 6, Table 4, displays outcome data for patient-reported outcomes from studies evaluating ERP interventions to improve recovery from lower limb arthroplasty in UK hospitals. Assessments of physical recovery, quality of life and patient satisfaction were across the included studies. Where standardised mean differences were calculated, there were no statistically significant differences between outcomes, except an improvement on the Oxford Hip Score in the study by Hunt and colleagues224 for patients in the Belfast centre compared with both control sites. These differences were a mean of 5.1 and 3.3 points on a scale of 12–48, with each category separated by 9 points. There was also a slightly better score on the WOMAC function scale for patients in Belfast than for those in south-west London (d = 0.23, 95% CI 0.02 to 0.44; p < 0.05). In the study by Reilly and colleagues,256 patients in the ERP group demonstrated improved knee function, with 4.9 degrees’ greater knee flexion range of motion than patients receiving usual care. This difference was associated with a medium effect size, although wide CIs indicate the true effect may have ranged from negligible to very large (d = 0.79, 95% CI 0.16 to 1.43; p < 0.05).
Salmon and colleagues260 reported patient satisfaction outcomes from the core study by Hunt and colleagues. 224 More patients in the Belfast centre reported no problems with care than did those in Liverpool (18.8% more) and south-west London (26.5% more). The number reporting no problems with recovery was similar in all three centres.
Prehab interventions
Two RCTs evaluated prehab in patients undergoing knee269 and hip arthroplasty. 244 Between the two studies, there were 220 patients with a mean age of 70.9 ± 8.9 years, of whom 54% were female.
The intervention delivered by McGregor and colleagues244 included a pre-admission class at which staff provided information about the procedure and rehabilitation process and ensured that exercises could be performed effectively by patients. They also made provisions to adapt the home environment, if required. The intervention in the study by Williamson and colleagues269 was a 6-week programme of lower limb strength and balance training. Full descriptions of the interventions are available in Report Supplementary Material 3, Table 7.
Effectiveness of prehab interventions
Data from all outcomes in the two prehab studies are presented in Report Supplementary Material 6, Table 5. There was no difference in LOS between prehab and home exercise in the study by Williamson and colleagues. 269 It was not possible to calculate the effect on LOS in the study by McGregor and colleagues,244 who reported that patients undergoing prehab spent 3 days fewer in hospital than those receiving usual care.
Measures of mental health, quality of life and markers of recovery were assessed. Anxiety was statistically significantly greater in the prehab group in the study by Williamson and colleagues,269 associated with a medium effect size (d = 0.58, 95% CI 0.21 to 0.94; p < 0.001) and a change of 1.8 points on the Hospital Anxiety and Depression Scale anxiety scale (95% CI 0.65 to 3.03). However, anxiety scores in both groups were low and well within the normal range (‘normal’ range being 0–7 points273). There were no differences between groups on any other outcome.
Rehab interventions
One controlled trial evaluated a rehab intervention. 253 The intervention was specifically the incorporation of weekends into the programme, meaning that rehabilitation was uninterrupted and patients could begin without delay. One hundred patients were recruited, with a mean age of 60.8 years, and 47% of them were female.
The study was of low quality, assessed as ‘weak’ on all domains except items relating to selection bias and blinding, for which it received ‘moderate’ scores.
Results are presented in Report Supplementary Material 6, Table 6, with data presented separately for hip and knee patients. LOS was not significantly different between trial arms. Data for patients undergoing knee replacement approached statistical significance but with only a small effect size indicated and a mean difference of less than half a day (d = –0.25, 95% CI –0.51 to 0.0; p = 0.054). In both knee and hip replacement patients, the time to mobilising with two walking sticks was reduced with weekend physiotherapy, by a mean of half a day, associated with small effect sizes. Group numbers were not clearly reported for knee and hip functional assessments and therefore no analysis was performed. 253
Specialist ward
We identified one UBA study195 evaluating a specialist ward intervention in which a ring-fenced orthopaedic ward was introduced into the hospital. The ward had stringent access restrictions and extensive measures to prevent infection or contamination (full details are given in Report Supplementary Material 3, Table 9). Data from patients admitted to this ward were compared with data from patients previously treated on a general orthopaedic ward.
The study achieved a ‘strong’ quality assessment rating for confounders and provided a clear definition of LOS. It was assessed as ‘weak’ for study design and data collection methods and ‘moderate’ for all other items. It was rated as ‘weak’ overall.
Effectiveness data are reported in Report Supplementary Material 6, Table 7. Patients undergoing total hip replacements with recovery in the specialist ward stayed a mean of 1.6 days fewer than those in the standard orthopaedic ward, with a large effect size (d = –1.03, 95% CI –1.32 to –0.74; p < 0.001). Similarly, those patients undergoing total knee replacements with recovery in the specialist ward stayed a mean of 2.2 days fewer than those in the standard orthopaedic ward, with a large effect size (d = –1.02, 95% CI –1.31 to –0.73; p < 0.001).
Although this study indicates that LOS may be reduced with a specialist ward, no outcomes were reported on the wider impact of a shorter stay, for example on complications, recovery or satisfaction with care.
Interventions to improve recovery from cardiac surgery: evidence from the UK
From the UK, we identified two RCTs,210,215 one controlled trial259 and one UBA study208 that evaluated interventions intended to improve recovery following cardiac surgery. Both of the RCTs compared prehab interventions with usual care, 210,215 the controlled trial evaluated the effectiveness of a specialist ward versus usual care259 and the UBA study compared a newly implemented ERP with previous usual care. 208
A total of 633 people commenced trials, with sample sizes ranging from 105208 to 204. 210 In all studies, patients were scheduled for at least CABG surgery, with some patients scheduled for valve surgery or combined CABG and valve surgery. Across the sample, 20.3% of participants were female, and the mean age was 64.8 ± 6.6 years.
Overview: cardiac surgery
Evidence for interventions to improve recovery after cardiac surgery in the UK is summarised here and in Table 18. Full details of the effectiveness of interventions are available in Prehab interventions and Other interventions.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Goodman 2008,215 RCT | 188 | Prehab | AEI | ◁▷ | ||||||||||||
| Furze 2009,210 RCT | 204 | Prehab | AEI; EX; GOAL; REF; TEL | ◁▷ | ⇦⇨ | ⇦⇨ | ⇦⇨ | x | ▲ | ⇦⇨ | ||||||
| Salhiyyah 2011,259 CT | 136 | SW | FM | SW | Extubation criteria, cardiovascular support available. Mechanical ventilation not provided | ◁▷ | ⇦⇨ | |||||||||
| Fleming 2016,208 UBA | 105 | ERP | AEI | CHL; nFAST | FM; ANA | EMOB; LAX | ◁▷ | ⬆(1/7)x(6/7) | ⇦⇨ | ▲(3/12)x(9/12) | ||||||
Only four UK-based studies evaluated interventions to improve recovery from cardiac surgery, distributed across three intervention types. There was no conclusive evidence that any intervention was associated with reduced LOS, despite some findings approaching statistical significance, including meta-analysis of two prehab interventions. Few outcomes were reported with regard to markers of recovery, quality of life or other outcomes not directly related to LOS.
Prehab interventions
The interventions trialled in the RCTs by Furze and colleagues210 included extensive preoperative information, education and counselling. Participants in the prehab arm also received relaxation therapy to reduce stress and advice on postoperative self-management. The intervention in Goodman and colleagues215 was a series of monthly visits from a cardiac home-care nurse to reduce cardiac risks, establish lifestyle changes, provide education and answer questions. The full details of each intervention can be found in Report Supplementary Material 3, Table 10.
Quality assessment
Both studies were allocated ‘strong’ scores regarding study design, confounders, reporting of withdrawals and dropouts, and both reported clearly how LOS was defined. The study by Furze and colleagues210 was ‘weak’ for blinding and data collection tools, and the study by Goodman and colleagues215 was rated as ‘weak’ for study selection but ‘strong’ for data collection tools. All other domains were rated as ‘moderate’ in both studies.
Report Supplementary Material 6, Table 8, displays data for all effectiveness outcomes reported in the two studies trialling a prehab intervention to improve recovery from cardiac surgery in UK hospitals. Meta-analysis of the influence of prehab on LOS yielded evidence of no overall effect, although this result approached statistical significance (Figure 19). Furze and colleagues210 described the use of additional GP and NHS hospital visits in the 8 weeks after discharge, as well as non-fatal cardiac events and deaths; however, there were no statistically significant differences between groups for any of these variables. Goodman and colleagues215 collected data for mental health outcomes but did not report numerical data.
FIGURE 19.
Forest plot showing the results of meta-analysis of the effect of prehab interventions vs. usual treatment on LOS in UK hospitals following cardiac surgery.
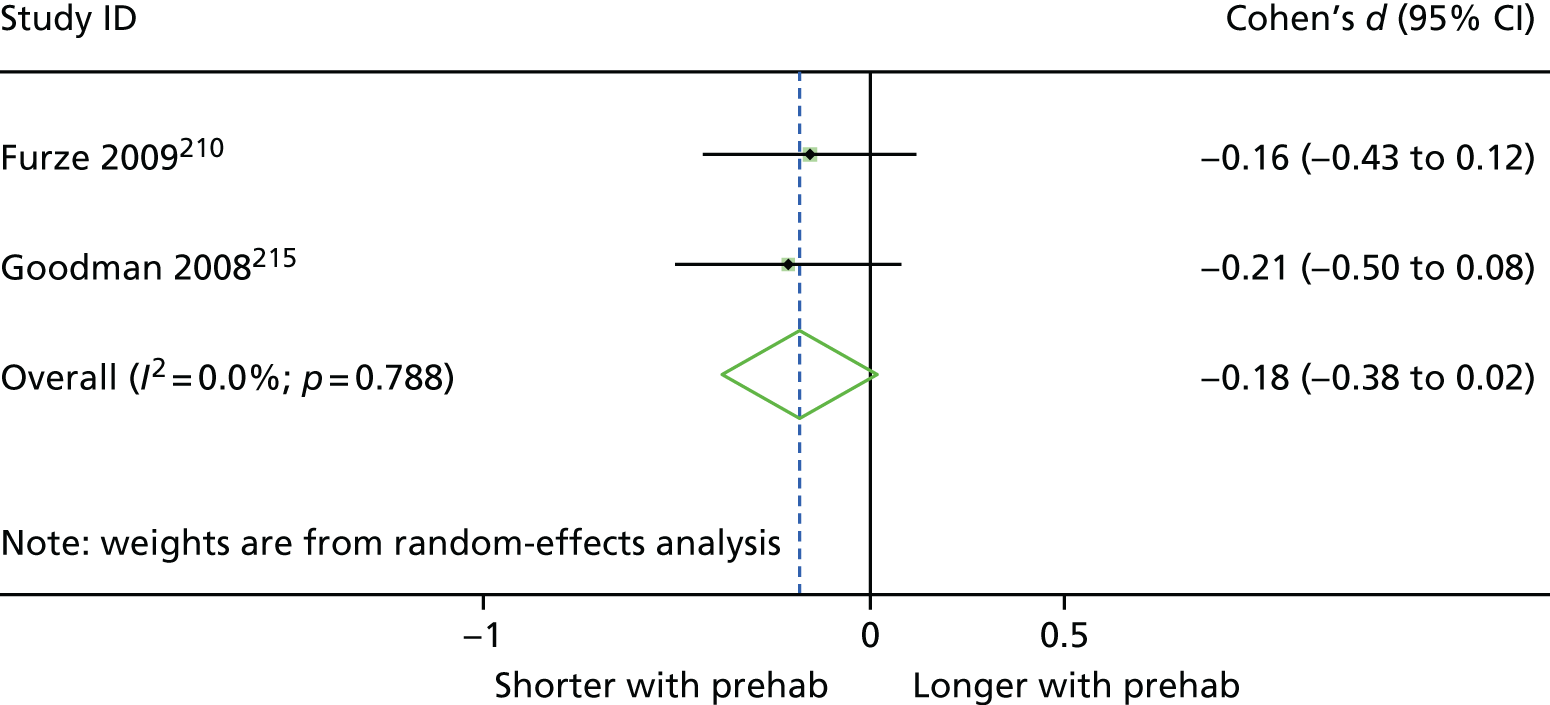
Other interventions
Specialist ward intervention
The specialist ward in the controlled trial by Salhiyyah and colleagues259 was called the theatre recovery unit. The theatre recovery unit had two beds with one-on-one nursing provision, operating between 08.00 and 18.30 on weekdays. Patients were transferred to the theatre recovery unit immediately after surgery and then to the high-dependency progressive care unit on the same day, and then to the general ward. Patients allocated to the comparator arm were transferred to the cardiac intensive care unit immediately after surgery, where they remained for at least 1 day, before moving to the general ward. Full details of the intervention can be found in Report Supplementary Material 3, Table 11.
The study was rated ‘strong’ for confounders and reporting of withdrawals and dropouts. The definition of LOS was reported clearly; however, the study was rated ‘weak’ in terms of study design and data collection tools and therefore ‘weak’ overall.
The effectiveness of the intervention was assessed in terms of overall LOS, time spent in different wards during recovery and complications. Outcomes are presented in Report Supplementary Material 6, Table 9. There was no change in LOS, despite patients in the specialist ward spending significantly less time in the cardiac intensive care unit and intensive care. These patients spent a mean of 5.8 hours in the theatre recovery unit (vs. 0 hours in the usual care group) and 15.4 hours longer in the progressive care unit. Rates of complications were similar in both groups. No indicators of recovery were assessed.
Enhanced recovery protocol intervention
The ERP intervention introduced in the UBA study by Fleming and colleagues208 included the following key components not present in the previous model of care: pre-admission assessment clinic and information; carbohydrate treatment and absence of fasting beyond 2 hours; active prevention of postoperative nausea and vomiting; avoidance of long-acting opioids; early discontinuation of opioid infusion postoperatively; regular oral analgesia after extubation; regular laxatives; early mobilisation; and early enteral nutrition. Full details of the intervention can be found in Report Supplementary Material 3, Table 12.
The study received a global quality rating of ‘weak’, thanks to ‘weak’ ratings for study design, data collection and reporting of withdrawals and dropouts. However, the authors did provide a clear definition of LOS.
Effectiveness of the ERP intervention was assessed in terms of LOS, complications and markers of early physical recovery. Outcomes are presented in Report Supplementary Material 6, Table 9. LOS was not statistically different between the ERP and usual care groups, although there was a small effect size approaching statistical significance (d = –0.37, 95% CI –0.76 to –0.02; p = 0.06). The odds of experiencing a complication were 77% lower in the ERP group, (OR 0.23, 95% CI 0.01 to 0.35). Pain scores on days 1, 2 and 3 after surgery were significantly lower in the ERP cohort, associated with a mean reduction of half a point (on a scale of 1–3) and medium effect sizes (mean effect size –0.63).
Interventions to improve recovery from upper abdominal surgery: evidence from the UK
Six studies investigated the effectiveness of interventions to improve recovery following upper abdominal surgery in the UK, of which two were RCTs205,226 and four were UBA designs. 191,201,257,263 Five studies trialled ERP interventions, 191,201,226,257,263 with one prehab intervention. 205 All interventions were compared with usual care.
Patients in these studies visited hospital for liver resection,201,205,226 pancreatectomy257 or pancreaticoduodenectomy. 191,263 There were 593 participants, with sample sizes ranging from 38205 to 211. 201 Participants were 43.5% female and there was a variety of median and mean ages, as presented in Table 2.
Overview: upper abdominal surgery
Evidence in this area is summarised here and in Table 19. Full details about the effectiveness of interventions can be found in Enhanced recovery protocol interventions and Prehab interventions.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Dunne 2016,205 RCT | 38 | Prehab | AEI | ◁▷ | ⇦⇨ | ◁▷ | ⇦⇨(5/5) | |||||||||
| Abu Hilal,191 2013 UBA | 44 | ERP | nMBP; nFAST | CATH; DRA | EON; EMOB; FM; nNGT; DP | ▲(2/2) | ⇦⇨ | ⇦⇨ | ⇦⇨ |
▲(3/5) ▲(1/5) x(1/5) |
||||||
| Dasari 2015,201 UBA | 211 | ERP | AEI; EX | nFAST; CHL | PONV | EMOB; ANA | ◁▷ | ⇦⇨ | ⇦⇨ | ◁▷ | ||||||
| Jones 2013,226 RCT | 104 | ERP | AEI; NUT | DRA; FM | DRA; CATH; EON; FM; NUT; ANA | ▲ |
⬆(2/4) ⇦⇨(2/4) |
⇦⇨ | ⇦⇨ | ▲ | x | |||||
| Richardson 2015,257 UBA | 66 | ERP | AEI | CHL; TP | PONV; FM | CATH; DRA; DP; EON; EMOB; NGT; ANA | TEL; FAPP | ▲ | ⇦⇨(2/2) | ⇦⇨ | ⇦⇨ |
▲(2/6) ◁▷(2/6) x(3/6) |
||||
| Sutcliffe 2015,263 UBA | 130 | ERP | CHL | DRA | CATH; EON; EMOB | x | ⇦⇨(2/2) | ⇦⇨ | ||||||||
The study was rated ‘weak’ quality overall. The selection bias domain was rated ‘weak’ owing to lack of clarity in the reporting of participant recruitment. Data collection methods were also rated ‘weak’. It was not clear how LOS was defined. 248
Effectiveness of intervention
Report Supplementary Material 5, Table 19, displays data for all outcomes reported in the study by Muehling and colleagues,248 which trialled an ERP intervention to improve recovery from thoracic surgery. There was no difference in the requirement for patients to undergo postoperative ventilation, and the reporting of medians with ranges precluded a statistical comparison of LOS.
One RCT, by Hempenius and colleagues,220 investigated the effectiveness of an intervention to improve recovery in patients undergoing various tumour removal surgeries. Data were obtained for 297 patients in the Netherlands, of whom 64% were female, with a mean age of 77.5. Patients were admitted for solid tumour removal at various hospital sites.
The intervention focused on supportive care and the prevention of postoperative delirium. A preoperative assessment was performed by a specialist geriatric care team, and patients were regularly assessed during the hospital stay, using a checklist to ensure well-being and identify needs. Care plans were formulated as required, with subsequent daily checking to ensure adherence. The intervention was compared with usual care, in which additional geriatric care was provided only at the request of the treating physician.
A related paper by the same authors, published in 2016, focused on long-term outcomes in the study sample, and reported on re-admissions. 219
The study was rated as being ‘moderate’ quality. Study design, confounders and reporting of withdrawals and dropouts achieved scores of ‘strong’ but data collection methods were rated ‘weak’. It was not clear how LOS was defined. 219
Effectiveness of intervention
Report Supplementary Material 5, Table 20, displays data for all outcomes reported in the study by Hempenius and colleagues. 220 Fewer patients in the experimental group were able to return to their preoperative living situation (11.8% fewer). There were no significant differences between groups for any other outcome.
Evidence for ERP interventions came from five studies; four used UBA designs and one was a RCT. The evidence suggests that ERP interventions are associated with a reduction in LOS of around 3 days, although results from individual studies ranged from almost no difference with ERP, to a 5-day reduction. The reduction in LOS was also associated with favourable or non-detrimental effects for other outcomes, including complications and markers of recovery. There was one RCT of a prehab intervention, which led to improvements in some markers of physical fitness, but not in LOS.
Within the ERP interventions evaluated, large beneficial effect sizes for LOS and some markers of recovery were observed in three studies. 191,226,257 Compared with the two studies with less-effective interventions,201,263 these protocols included more additional components than usual care. However, all experimental components were implemented in both highly effective and less effective studies, except discharge planning, which was used in two of the most effective interventions. 191,257 The two studies showing more null effects were the largest two trials, although all five were rated as ‘weak’ overall. Given the heterogeneity of the evidence, it is not possible to make causative associations between intervention components and outcome effectiveness.
Enhanced recovery protocol interventions
Components of the ERP interventions and comparators are mapped against ERAS Society guidelines for liver surgery,274 pancreaticoduodenectomy275 and gastrointestinal surgery276 in Table 20. The most commonly occurring items were: preoperative counselling (4/5 studies); antithrombotic prophylaxis (3/5); antimicrobial prophylaxis and skin preparation (3/5); preventing intraoperative hypothermia (3/5); multimodal approach to postoperative nausea and vomiting (4/5); early oral intake postoperatively (4/5); and early mobilisation (5/5). Usual care in the comparator arms was identical with respect to pre-anaesthetic medication, antithrombotic and antimicrobial prophylaxis, choice of incision, preventing intraoperative hypothermia and use of thoracic epidural analgesia. The full description of each intervention can be found in Report Supplementary Material 3, Table 14.
| ERAS item and description | Study (first author and year) | ||||
|---|---|---|---|---|---|
| Abu Hilal 2013191 (PDE) | Dasari 2015201 (liver) | Jones 2013226 (liver) | Richardson 2015257 (PE) | Sutcliffe 2015263 (PDE) | |
| Patients should receive dedicated preoperative counselling routinely | E | E | E, C | E, C | |
| Preoperative endoscopic biliary drainage should not be undertaken routinely in patients (PD) | |||||
| Preoperative smoking and alcohol consumption: abstinence should be attempted for 1 month before surgery (PD) | |||||
| Patients at risk should receive oral nutritional supplements for 7 days prior to surgery [not warranted unless significantly malnourished (PD)] | Ea | ||||
| Perioperative oral immunonutrition: limited evidence for use (immunonutrition for 5–7 days perioperatively should be considered – PD) | |||||
| Preoperative fasting does not need to exceed 6 hours for solids and 2 hours for liquids. Carbohydrate loading the evening before surgery and 2 hours before the induction of anaesthesia | E | E | |||
| Preoperative treatment with carbohydrates should be given to patients without diabetes (PD) | E | E | |||
| No oral mechanical bowel preparation | Eb | E, C | Eb | ||
| Pre-aesthetic medication: long-acting anxiolytic drugs should be avoided. Short-acting anxiolytics may be used to perform regional analgesia prior to the induction of anaesthesia | E, C | E, C | |||
| Antithrombotic prophylaxis | E, Cc | E, C | E, C | ||
| Perioperative steroids administration: steroids (methylprednisolone) may be used before hepatectomy in normal liver parenchyma | |||||
| Antimicrobial prophylaxis and skin preparation | E, C | E, C | E, C | ||
| Incision: the choice of incision is at the surgeon’s discretion | E, C | E, C | E, C | ||
| Minimally invasive approach: laparoscopic liver resections can be performed by hepatobiliary surgeons experienced in laparoscopic surgery | E, C | ||||
| Avoidance of prophylactic nasogastric intubation | E, C | ||||
| Nasogastric intubation: nasogastric tubes removed early (PD) | E | ||||
| Prophylactic abdominal drainage: no recommendation | E, Cd | ||||
| Preventing intraoperative hypothermia | E, C | E, C | E, C | ||
| Analgesia: routine TEA is not recommended in open liver surgery. Wound infusion catheter or intrathecal opiates can be good alternatives combined with multimodal analgesia | E, C | E, C | |||
| Epidural analgesia: mid-thoracic epidurals are recommended, compared with intravenous opioids (PD) | C | E, C | |||
| Pain management: opioid-sparing analgesic strategies, including regional analgesia techniques, should be implemented in the context of a multimodal analgesic regimen (G) | |||||
| Multimodal approach to PONV should be used. Patients should receive PONV prophylaxis with two antiemetic drugs | E, C | Ee | E,e Ce | E | |
| Fluid balance: near-zero fluid balance, avoiding overload of salt and water, results in improved outcomes (PD) | E | ||||
| Fluid management: maintenance of low central venous pressure with close monitoring. Balanced crystalloid preferred over saline or colloids | E, C | E | |||
| Fluid management: perioperative haemodynamic management – maintain fluid homeostasis, avoiding fluid excess and organ hypoperfusion (G) | |||||
| Early oral intake: eat normal food on POD1. Enteral or parenteral feeding is reserved for malnourished patients or those with prolonged fasting as a result of complications | E | E,f C | E | E | |
| Postoperative glycaemic control (diabetic patients) | |||||
| Avoidance of perianastomotic drains (G) | Eg | E | |||
| Suprapubic catheterisation is superior to transurethral catheterisation if used for > 4 days. Transurethral catheters can be removed safely on POD1 or POD2 unless otherwise indicated (PD) | E, C | ||||
| Prevention of delayed gastric emptying | |||||
| Stimulation of bowel movement: not indicated | |||||
| Early mobilisation | E | E | E, Ch | E | E |
| Audit: systematic audit | E | E, C | |||
Interventions in studies with patients undergoing liver surgery201,226 adhered to a mean of 13.5 (58.7%) of the 23 relevant ERAS guideline items, and comparator groups adhered to a mean of nine (39.1%) items. Interventions with patients undergoing pancreaticoduodenectomy or pancreatectomy191,257,263 adhered to a mean of nine (34.6%) of the relevant 26 ERAS items (range seven263 to 12257), and the comparator group adhered to a mean of four (15.4%) items (range three263 to five191).
Quality assessment
All studies were rated as ‘weak’ globally, having received ratings of ‘weak’ in two or more individual domains. The studies by Abu Hilal and colleagues191 and Dasari and colleagues201 both received three ratings of ‘weak’. Only Jones and colleagues’226 paper avoided a rating of ‘weak’ for study design, and only the study by Richardson and colleagues257 received a ‘moderate’ rating for data collection methods, the rest were ‘weak’. The paper by Sutcliffe and colleagues263 was the only one not to clearly define LOS.
Report Supplementary Material 6, Table 10, displays data for clinical outcomes reported in studies evaluating the effectiveness of ERP interventions at improving recovery in patients undergoing upper abdominal surgery in UK hospitals. LOS was significantly shorter in patients receiving ERP interventions in four of five studies, with only Dasari and colleagues201 reporting a non-statistically significant effect. LOS outcomes are presented in the forest plot in Figure 20. Where LOS was reduced, this was associated with a large effect size. 191,226,257 For the four groups from three studies presenting medians with IQR, the mean reduction in LOS was 3.1 days (range 5.2–0.3 days).
FIGURE 20.
Forest plot showing standardised mean differences between ERP vs. usual care for LOS following upper abdominal surgery in UK hospitals. PWC, patients without complications.

In the four studies that reported re-admissions,191,201,226,263 numbers were similar between groups, with no statistically significant differences identified. Complications were reported in four studies, with three reporting no difference between groups. 191,201,257 However, the odds of experiencing general complications were statistically significantly lower in the study by Jones and colleagues,226 with patients 80% less likely to experience a complication in the ERP group (OR 0.2, 95% CI 0.05 to 0.75). The forest plot in Figure 21 displays outcome data for both complications for the included studies.
FIGURE 21.
Forest plot (without pooled effects) displaying the odds of experiencing complications in patients receiving ERP to improve recovery following upper abdominal surgery in the UK.

Report Supplementary Material 6, Table 11, displays data for patient-reported outcomes from the studies trialling an ERP intervention to improve recovery from upper abdominal surgery in UK hospitals. Abu Hilal and colleagues191 reported that all aspects of early recovery occurred earlier in the ERP group. Patients were medically fit for discharge 3 days earlier when receiving ERP in the study by Jones and colleagues;226 this difference was associated with a large effect size (d = –3.92, 96% CI –4.63 to –3.21; p < 0.001). In the study by Richardson and colleagues,257 there was a mix of findings with regard to markers of early recovery. No markers were achieved later in the ERP group than the usual care group.
Prehab interventions
One RCT trialled a prehab intervention to improve recovery from upper abdominal surgery, with 35 patients included in the study. 205 Patients in the experimental group performed 4 weeks of exercise sessions tailored to their physical capacity.
Outcomes are presented in Report Supplementary Material 6, Table 12. There was no difference in LOS or re-admissions between groups. Dunne and colleagues205 evaluated markers of physical conditioning for all participants, also performing a subgroup analysis of ‘high-risk’ patients, as evaluated on study entry. There were improvements in a number of these outcomes, associated with large effect sizes, with some benefits also observed in high-risk patients.
Interventions to improve recovery from thoracic surgery: evidence from the UK
Three UK studies investigated the effectiveness of interventions to improve recovery from thoracic surgery. 197,212,228 All three studies utilised UBA designs to evaluate the implementation of an ERP, compared with previous standard care. Data from 984 patients were analysed across the three studies, of whom 46.1% were female. Age distribution was provided by medians and means, available in Table 2. Patients were admitted for thorascopic lobectomy,197 oesophageal and gastric cancer surgery212 and upper gastrointestinal cancer surgery (total and subtotal gastrectomy228).
Quality assessment
All three studies were rated as ‘weak’ for study design and data collection and ‘moderate’ for selection bias and reporting of withdrawals and dropouts. Only the paper by Brunelli and colleagues197 clearly reported how LOS was defined or reported that assessors and patients were blinded to group allocation. Gatenby and colleagues212 did not consider confounding variables, whereas Brunelli and colleagues197 reported and controlled for confounders.
Overview: thoracic surgery
Findings for thoracic surgery are summarised here and in Table 21. Full effectiveness details are available in Enhanced recovery protocol interventions.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Brunelli 2017,197 UBA | 600 | ERP | AEI | CHL; AIE; nFAST; MT | FM; ANA; WARM; PONV | AEI; MT; nNGT | TEL | Postoperatively, patients reviewed daily |
◁▷1/2 ⇦⇨1/2 |
⇦⇨ | ⇦⇨ | ⇦⇨3/3 | ||||
| Gatenby 2015,212 UBA | 132 | ERP | AEI; NUT | CHL; nFAST; PreM | CATH; nNGT; FM; SURG; PONV; ANE | DRA; EMOB; NUT; ANA; CATH; PT; EX | AEI; LIA; FAPP; NUT; TEL | x | ⇦⇨ | ⇦⇨ | ⇦⇨ | |||||
| Karran 2016,228 UBA | 252 | ERP | AEI; NUT | AEI; PreM; nFAST | nNGT; CATH; SURG; FM; PONV | DRA; CATH; EMOB; FM; NUT; ANA | Pathway booklets created | ▲3/3 | ⬆ | ⇦⇨ | ⇦⇨3/3 | |||||
Three studies from the UK utilised UBA designs to compare LOS and other clinical outcomes in patients receiving ERP with LOS and other outcoes in those who had previously received standard care. There was a trend for LOS to be reduced with ERP, while evidence for re-admissions and complications was equivocal. There was no evidence of detrimental effects with ERP. Components of ERP that differed between studies included the absence of a pre-hospital nutrition and postoperative social worker input in the study conducted by Brunelli and colleagues197 and lack of preoperative anaesthesia and analgesia, postoperative education/counselling, early oral nutrition and post-discharge telephone follow-up in Karran and colleagues. 228 These two studies demonstrated no statistically significant difference in rates of re-admission between intervention and comparator groups. 197,228 These results indicate that an ERP pathway may have the potential to reduce LOS for older adults undergoing thoracic surgery without affecting rates of re-admission; however, all of the studies evaluating an ERP had a global quality rating of ‘weak’, which prevents firm conclusions being drawn.
Enhanced recovery protocol interventions
Components of the ERP interventions and comparators are mapped in Table 22 against ERAS recommended components for gastrointestinal surgery, in lieu of specific guidelines for ‘thoracic’ surgery. 276 Given the mix of procedures in the included studies, this guideline was deemed most appropriate. In the study by Karran and colleagues,228 three slightly different pathways were evaluated for oesophageal, subtotal gastrectomy and total gastrectomy patients. Full details of the three pathways, and the other ERP interventions for thoracic surgery, can be found in Report Supplementary Material 3, Table 19.
| ERAS item | Study (first author and year) | ||
|---|---|---|---|
| Brunelli 2017197 | Karran 2016228 | Gatenby 2015212a | |
| Risk assessment | E | E | E |
| Preoperative information, education and counselling | E | E | E |
| Preoperative optimisation | E | E | E |
| Preoperative fasting minimised | E | E | E |
| Pre-anaesthetic medication avoided | E | ||
| Preoperative carbohydrate treatment | E | E | E |
| Same day admission | E | ||
| Discharge planning in advance | |||
| Preoperative warming | E | ||
| Prophylaxis against thromboembolism | |||
| Antimicrobial prophylaxis and skin preparation | E, C | ||
| Standard anaesthetic protocol | E, C | E | E |
| PONV prophylaxis | E | E | |
| Minimally invasive surgery | E, C | ||
| Preventing intraoperative hypothermia | E | ||
| Perioperative fluid management: judicious use of vasopressors | |||
| In the absence of surgical losses postoperative intravenous fluid should be discontinued and oral intake (1.5 l/day) encouraged | |||
| 0.9% saline should be avoided and balanced crystalloid solution should be used in the preoperative period. The use of 0.9% saline should be restricted in hypochloraemic and acidotic patients | |||
| Perioperative fluid management: goal-directed | E | E | E |
| Monitoring neuromuscular function | |||
| Reverse neuromuscular blockade | |||
| The inspired fractional concentration of oxygen should be titrated to produce normal arterial oxygen levels and saturations | |||
| 100% inspired oxygen concentrations can be used for pre-oxygenation prior to anaesthesia or for short periods to overcome hypoxia | |||
| Chest drains: early removal | E, C | E | E |
| Measures to prevent postoperative delirium | |||
| Attenuation and treatment of postoperative ileus: multimodal strategies to facilitate return of gastrointestinal function | |||
| Postoperative analgesia: multimodal, avoidance of systemic opioids | E | ||
| Postoperative analgesia: patient-controlled | E, C | E | |
| Prevention of fluid overload | E, C | E | |
| Perioperative nutritional care | E | E | E |
| Early mobilisation: including written information setting daily targets | E, C | E | E |
The following items were common to all three interventions: pre-admission risk assessment, information, education and counselling; preoperative optimisation; minimal preoperative fasting; preoperative carbohydrate treatment; standard anaesthetic protocol; goal-directed fluid management; early removal of chest drains; perioperative nutritional care; and early mobilisation. Gatenby and colleagues212 described their intervention as ERAS.
The interventions evaluated by the studies outlined in Table 22 adhered to a mean of 14 (46.2%) of the 31 ERAS guidelines (range 12228 to 18197). The comparator group in the study by Brunelli and colleagues197 adhered to seven (22.6%) ERAS guidelines. The comparator groups in the studies conducted by Karran and colleagues228 and Gatenby and colleagues212 were not well reported, so adherence to ERAS guidelines was not calculated.
Effectiveness of interventions
Report Supplementary Material 6, Table 13, displays data for all outcomes reported by the three studies evaluating the effectiveness of ERP interventions for improving recovery from thoracic surgery in UK hospitals. LOS was statistically significantly shorter with ERP in the study by Karran and colleagues228 (d = –0.69, 95% CI –0.95 to –0.43; p < 0.001) but did not differ statistically between groups in the study by Brunelli and colleagues. 197 Median LOS was 3 days shorter in the ERP group in the study by Gatenby and colleagues. 212
Re-admissions and mortality were reported in all studies but were not statistically different between groups. The odds of experiencing a Clavien–Dindo grade C3 complication was 57% lower in the recipients of ERP in the study by Karran and colleagues;228 however, the upper CI for this effect was 1.0, suggesting that the true effect may not be statistically significant lower odds. The odds of experiencing complications were similar in the remaining studies. 197,212
Interventions to improve recovery from pelvic surgery, vascular surgery and mixed procedures: evidence from the UK
Interventions to improve recovery from pelvic surgery,194,250 vascular surgery31 and various surgical procedures207 were found in only one or two trials, and these are summarised in this section. Full details of the effectiveness of these interventions can be found in Pelvic surgery, Vascular surgery and Various procedures.
Overview: interventions to improve recovery from pelvic surgery, vascular surgery and mixed procedures
Pelvic surgery
The two studies evaluating interventions to improve recovery after pelvic surgery in the UK194,250 used UBA designs to investigate the impact of implementing an ERP to improve recovery from radical cystectomy. Data suggest that LOS may be reduced with ERP, without detriment to other outcomes. However, the assessment of broader patient outcomes was limited, with no consideration of patient satisfaction, quality of life or mental health, as illustrated in Table 23.
| Study details | Intervention components | Outcome categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year, country (quality assessment) | Sample size | Intervention category | Pre-hospital admission | Preoperative | Perioperative | Postoperative | Post discharge | Other | LOS | Complications | Mortality | Re-admissions | Markers of recovery | Mental health | Quality of life | Use of additional care |
| Pelvic surgery | ||||||||||||||||
| Arumainayagam 2008,194 UBA | 112 | ERP | nMBP; CHL; nFAST | DRA; DP; EON; EMOB | Stoma therapist sees patient. Social circumstances assessed and referrals made if needed. Protocol for stent removal | ▲(2/2) | ⇦⇨(2/2) | ⇦⇨ | ⇦⇨ | x | ||||||
| Mukhtar 2013,250 UBA | 77 | ERP | AEI | CHL; PreM | FM; nNGT; DRA; SURG; WARM | ANA; CATH; EMOB | CNS trained to take final steps in removing ureteric stents and for practical stoma education | x | ⇦⇨ | ▲(3/3) | ||||||
| Vascular surgery | ||||||||||||||||
| Partridge 2017,31 RCT | 209 | PACP | AEI | FAPP | Before surgery: medications changed and level of care required advised. Post discharge: longer-term GP follow-up | x |
⬆(3/8) ⇦⇨(5/8) |
⇦⇨ | ⇦⇨(2/2) | |||||||
| Various procedures | ||||||||||||||||
| Ellis 2012,207 UBA | 313 | PACP | AEI | Referrals made and acted on on basis of assessment | ▲ | ⬆ | ⇦⇨ | |||||||||
Vascular surgery
The single study evaluating a CGA was also a RCT described in Interventions to improve recovery from vascular surgery: randomised controlled trials. 31 The intervention identified 58 more new comorbidities than were diagnosed in the group receiving regular preoperative assessment. The odds of experiencing a complication were lower with the intervention, but there were no statistically significant differences in any other outcomes.
Various procedures
One study evaluated a PACP intervention using a UBA design. 207 Participants were scheduled to undergo various elective procedures, but were all ‘high-risk’ patients. The intervention led to reductions in LOS and odds of experiencing complications, but was of ‘weak’ quality.
Interventions to improve recovery from pelvic surgery: evidence from the UK
Two studies194,250 from the UK sought to evaluate the effectiveness of interventions to improve recovery from pelvic surgery. Both studies evaluated the effectiveness of recently implemented ERP for radical cystectomy using UBA designs. Data from 189 patients were included across the two studies; 22.6% of patients were female and the mean age was 66.9 years.
Quality assessment
Both studies scored ratings of ‘weak’ for study design; however, the study by Arumainayagam and colleagues194 was rated as ‘strong’ for all other areas apart from selection bias (which received a ‘moderate’ rating). The study by Mukhtar and colleagues250 was rated as ‘weak’ for data collection methods, ‘moderate’ for selection bias and ‘strong’ for all other domains.
Components of the ERP interventions and comparators are mapped against ERAS Society guidelines for pelvic surgery272 and radical cystectomy279 in Table 24. In the study by Arumainayagam and colleagues,194 nine ERAS items were implemented in the new pathway, with a focus on post-admission/preoperative items, with early postoperative nutrition and mobilisation. The usual care protocol was similar in terms of perioperative items. In the study by Mukhtar and colleagues,250 12 ERAS recommended items were implemented in their ERP pathway. These included pre-admission information, education and optimisation, perioperative items and postoperative early mobilisation and nutrition. Usual care was not described in the study by Mukhtar and colleagues. 250
| ERAS item | Study (first author and year) | |
|---|---|---|
| Arumainayagam 2008194 | Mukhtar 2013250 | |
| Preoperative information, education and counselling | E | |
| Preoperative optimisation | E | |
| Preoperative mechanical bowel preparation avoided | E | |
| Preoperative fasting: clear fluids allowed up to 2 hours and solids allowed up to 6 hours before the induction of anaesthesia | Ea | |
| Preoperative carbohydrate treatment for patients without diabetes | E | E |
| Pre-anaesthetic medication: avoidance of long-acting sedatives | E | |
| Prophylaxis against thromboembolism | E, C | |
| Antimicrobial prophylaxis and skin preparation | ||
| Standard anaesthetic protocol | E, C | |
| Epidural analgesia: TEA is superior to systemic opioids; it should be continued for 72 hours | E, C | E |
| PONV prophylaxis | E, C | |
| Laparoscopic resection recommended for benign disease and rectal cancer (R/P). Minimally invasive approach not recommended outside trial setting (RC) | E | |
| Nasogastric intubation: nasogastric tubes not routinely used | E | |
| Nasogastric tubes removed before extubation | ||
| Prevention of intraoperative hypothermia | E | |
| Perioperative fluid management: intraoperative fluids guided by flow measurements to optimise cardiac output (RC) | ||
| Perioperative fluid management: judicious use of vasopressors (RC) | ||
| Transurethral catheter (R/P) | ||
| Suprapubic catheter (R/P) | ||
| Urinary drainage: transurethral catheter removal on POD1 in low-risk patients | ||
| Early removal of bladder catheter | E | |
| Prevention of postoperative ileus: chewing gum (R/P), oral magnesium, alvimopan | ||
| Postoperative laxatives and prokinetics (R/P) | ||
| Early oral intake 4 hours after surgery | E | |
| Early mobilisation | E | E |
| Audit: all patients should be audited for protocol compliance and outcomes | E, C | |
Interventions from the two studies involving patients undergoing radical cystectomy194,250 adhered to a mean of 10 (38.5%) out of the 26 ERAS guideline items. The comparator group in the study conducted by Arumainayagam and colleagues194 adhered to a mean of four (15.4%) items. The treatment received by the comparator group in the study by Mukhtar and colleagues250 was not well reported and so the adherence rate was not calculated.
Report Supplementary Material 6, Table 13, displays all outcome data for the two studies evaluating ERP interventions for recovery from pelvic surgery in UK hospitals. Where standardised mean differences were calculable, statistically significant differences were seen between groups, with large effect sizes in all cases. LOS was 4.7 days shorter in the ERP cohort in the study by Arumainayagam and colleagues,194 associated with a large effect size (d = –0.85, 95% CI –1.24 to –0.46; p < 0.001). The numbers of complications, re-admissions and deaths in each group were similar.
In the study by Mukhtar and colleagues,250 median LOS was 1.1 days shorter in the ERP cohort and mean LOS was 1.4 days shorter when an outlier was removed. Standardised mean differences could not be calculated for these outcomes. Markers of recovery were all achieved significantly earlier in the ERP group, associated with very large effect sizes (range –3.43 to –5.66).
Interventions to improve recovery from vascular surgery: evidence from the UK
The single study from the UK investigating the effectiveness of an intervention to improve recovery following vascular surgery was a RCT evaluating a preoperative assessment intervention. 31 Most of the patients in the study were male (24% were female), with a mean age of 75.5 years, and had been admitted for endovascular/open aortic aneurysm repair or lower limb arterial bypass surgery. Two hundred and nine participants were randomised to either preoperative CGA leading to an optimised care plan, or standard preoperative assessment. The intervention is detailed in Report Supplementary Material 3, Table 18.
Quality assessment
The paper was rated as ‘strong’, with only blinding of assessors rated as ‘moderate’. However, it was unclear how LOS was defined.
Report Supplementary Material 6, Table 15, displays outcome data for all effectiveness outcomes in the study by Partridge and colleagues. 31 Mean LOS was 2.3 days shorter in the experimental group. The absence of variance data prevented a calculation of standardised mean difference. The CGA identified 58 more new comorbidities than were diagnosed in the group receiving regular preoperative assessment. Postoperatively, the odds of delirium (OR 0.39, 95% CI 0.17 to 0.9), cardiac complications (OR 0.24, 95% CI 0.02 to 0.58), bowel and bladder complications (OR 0.4, 95% CI 0.22 to 0.74) were statistically significantly lower in the experimental group.
Interventions to improve recovery from various procedures: evidence from the UK
We identified one UBA study that evaluated the value of a PACP intervention in 313 patients undergoing various elective surgeries in a UK hospital. 207 Patients were admitted for total hip or knee replacement, other orthopaedic surgeries, transurethral resection of the prostate or a bladder tumour, other renal surgery and gastrointestinal surgery. Data were not presented by procedure and therefore could not be separated into relevant sections of the report. The study authors sought patients who presented certain ‘red flags’, or risk factors for postoperative complications, during routine assessment. These included cognitive or mobility problems, a history of falls and concerns regarding circumstances at home.
The intervention was an additional comprehensive review process following routine assessment, conducted by a nurse experienced with working with patients living with frailty, and an occupational therapist. If necessary, referral pathways were created to avoid unnecessarily lengthy stays in hospital postoperatively, and to help the discharge process.
Quality assessment
The study was rated as ‘weak’ overall, achieving domain ratings of ‘weak’ for study design, confounders and data collection methods, and ‘moderate’ ratings for selection bias and blinding of assessors. It was unclear how LOS was defined.
Report Supplementary Material 6, Table 16, displays data for all outcomes reported in the study by Ellis and colleagues. 207 LOS was 2.7 days shorter in the intervention group, associated with a medium effect size (d = –0.63, 95% CI –0.86 to –0.41; p < 0.001). Cognitive function was assessed using the Mini Mental State Examination, with scores similar between groups. There were fewer referrals to all agencies in the intervention cohort, except those to carer support workers and ‘other’ agencies not specified.
Synthesis of cost-effectiveness evidence
Study selection
The PRISMA flow chart in Figure 22 summarises the study selection process. Bibliographic database searches identified 2102 records and supplementary search methods identified 891 records. Following the removal of duplicates, there were a total of 2681 unique records that were screened at title and abstract against our inclusion and exclusion criteria. The full texts of 165 papers were sought for further consideration. Of these, 163 (98.8%) full texts were successfully retrieved. Following full-text screening, 108 papers were excluded. In total, 54 studies, reported in 55 articles, met the inclusion criteria. 57,58,67,71,74,81–84,86,91,96,97,100,104–106,109,110,112,115,116,119–121,123,125,131,133,134,145,146,151,159,162,166,179,183,184,186,210,211,215,222–224,232,244,256,257,259,264,268,280,281
FIGURE 22.
The PRISMA flow chart: identification process of economic studies. NR, not reported; SR, systematic review.
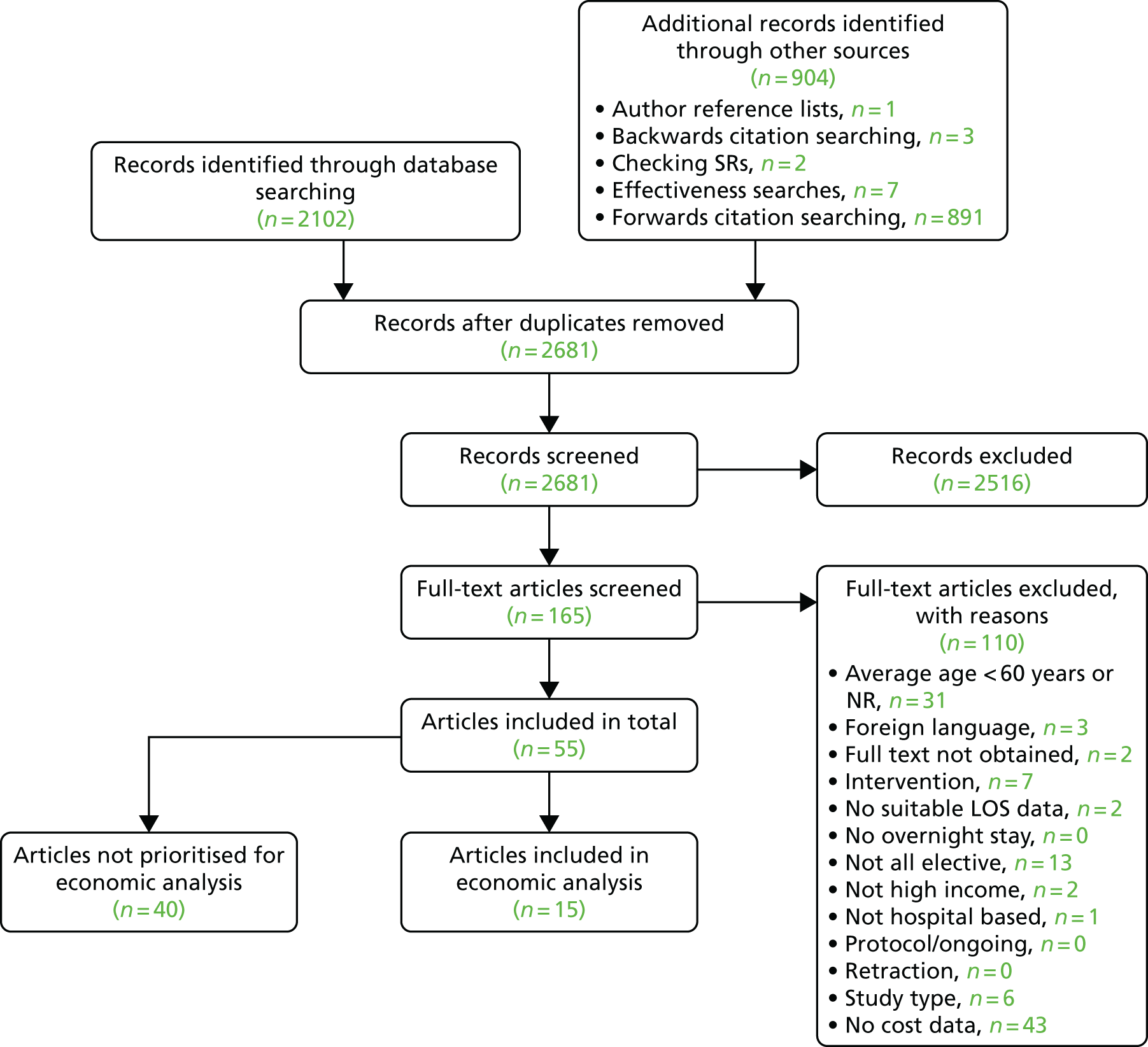
Evaluations of cost-effectiveness not associated with studies that were in the prioritised batch in the effectiveness review are tabulated and described in Report Supplementary Material 7.
Description of included studies
Fifteen studies were synthesised. 210,211,215,222–224,232,244,256,257,259,264,268,280,281 Table 2 provides details of the sample size, mean age and percentage female for each of these studies. Only six of the studies were published after 2010,211,222,257,259,264,268 so the majority of economic evidence is relatively old, stemming from a time when some surgical care was different.
Of the 15 studies, four evaluated interventions that were preoperative only,210,215,222,244 two were postoperative only244,256 and five were delivered outside hospital. 210,215,222,244,281 In terms of the broad type of surgical procedure, seven of the economic studies included patients receiving lower limb arthroplasty,222–224,244,256,280,281 three included patients receiving cardiac surgery,210,215,259 three included patients receiving colorectal surgery211,232,268 and two included patients receiving upper abdominal surgery. 257,264 Interventions are summarised in Table 3 and described in detail in subsequent sections of the synthesis and in Report Supplementary Material 3. Key characteristics are summarised here. The most frequently studied intervention category was ERP (n = 49 studies191,192,194,196,197,199,201,203,206,208,209,211–213,216–218,224–232,234–239,241–243,245,247,248,250–252,254,256,257,261–265). Thirteen studies193,198,200,204,205,210,214,215,221,222,244,258,269 evaluated prehab, two studies207,220 evaluated PACPs, four studies202,253,266,267 evaluated rehab, three studies195,255,259 evaluated specialist wards, and one study223 evaluated a staff mix intervention. The most common comparator was standard care or pre-intervention pathway (in UBA designs), although study authors used a range of descriptions (see Table 3).
Primary intervention delivery sites were at hospital, outpatient clinic, rehabilitation centre, the patient home, or a combination of these (see Table 3). The patient usually received the intervention alone, except in 14 studies31,193,195,200,206,211,216,221,232,234,235,245,254,258 where a family member or carer was actively involved in the intervention; in two interventions216,267 that included the involvement of friends or a non-specified support person; in two studies31,245 that involved carers; and in one study31 that involved GPs in the intervention. Ten studies did not report the intervention recipient, in which case we assumed that this was just the patient. 197,209,241,244,247,248,251,261,266,269 Table 3 shows the characteristics of the interventions and the health system and service settings for the 15 studies contributing towards the economic analysis. The interventions evaluated in each study are described more fully in each of the procedural categories below and in Report Supplementary Material 3.
Only three studies included full economic evaluations (two cost–utility analyses and one cost-effectiveness analysis),210,280,281 the remainder being cost-minimisation analyses,215,244 cost–consequences analyses223,224,232,257,259,264 or cost analyses. 222,256 The last three approaches provided mainly visual juxtaposition of the cost and effectiveness data (e.g. in a single table) without incremental analysis or calculation of cost-effectiveness ratios. The methods used in the 15 included economic evaluations are detailed in Report Supplementary Material 7, Table 2. The quality of the economic studies (as assessed using the 19 questions of the CHEC criteria list47) was extremely varied, meeting between two215,257,259 and 17280 of the quality criteria (Table 25).
| CHEC list item | Broad procedural category | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac surgery | Colorectal surgery | Lower limb arthroplasty | Upper abdominal surgery | ||||||||||||
| Furze 2009210 | Goodman 2008215 | Salhiyyah 2011259 | García-Botello 2011211 | King 2006232 | Vlug 2011268 | Huang 2012222 | Huddleston 2004223 | Hunt 2009224 | Larsen 2009280 | McGregor 2004244 | Reilly 2005256 | Sigurdsson, 2008281 | Richardson 2015257 | Tanaka 2017264 | |
| Is the study population clearly described? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Are competing alternatives clearly described? | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Is a well-defined research question posed in answerable form? | Y | N | N | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y |
| Is the economic study design appropriate to the stated objective? | N | CT | N | N | Y | Y | N | CT | Y | Y | Y | CT | Y | N | CT |
| Is the chosen time horizon appropriate to include relevant costs and consequences? | N | NR | N | NR | Y | N | N | N | Y | Y | Y | NR | Y | N | N |
| Is the actual perspective chosen appropriate? | Y | CT | N | N | Y | N | N | CT | Y | Y | Y | CT | Y | N | CT |
| Are all important and relevant costs for each alternative identified? | N | N | N | N | Y | Y | N | N | Y | Y | Y | N | Y | N | N |
| Are all resources measured appropriately in physical units? | Y | N | N | N | Y | N | N | N | Y | Y | NR | N | Y | N | Y |
| Are resources valued appropriately? | Y | N | N | Y | Y | N | N | N | Y | Y | NR | CT | Y | N | Y |
| Are all important and relevant outcomes for each alternative identified? | Y | N | N | N | NA | Y | NA | NA | Y | Y | N | N | Y | N | N |
| Are all outcomes measured appropriately in physical units? | Y | N | N | NA | NA | N | NA | NA | Y | Ya | Y | N | Y | N | N |
| Are outcomes valued appropriately? | Y | NA | NA | NA | NA | NA | NA | NA | NA | Y | NR | NA | NA | NA | NA |
| Is an incremental analysis of costs and outcomes performed? | Y | N | N | N | N | N | N | N | N | Y | NA | NA | Y | N | N |
| Are all future costs and outcomes discounted appropriately? | NA | NA | NA | NA | NA | NA | N | NA | NA | NA | NA | NA | NA | NA | NA |
| Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Y | N | Y | N | Y | N | N | N | N | Y | N | N | N | N | N |
| Do the [cost-effectiveness] conclusions follow from the data reported? | N | N | N | N | Y | Y | N | Y | Y | Y | Y | Y | Y | N | Y |
| Does the study discuss the generalisability of the results to other settings and patient/client groups? | Y | N | N | N | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y |
| Does the article indicate that there is not potential conflict of interest of study researcher(s) and funder(s)? | N | N | N | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | Y |
| Are ethical and distributional issues discussed appropriately? | N | N | N | N | N | Y | Y | Y | N | Y | N | Y | N | N | Y |
Synthesis of cost-effectiveness evidence
Cost data and primary outcomes for each of the included studies are presented in Report Supplementary Material 7, Table 3, and summarised in each procedural category below.
Cost-effectiveness evidence: cardiac surgery
Of the eight studies evaluating interventions to improve recovery from cardiac surgery, three reported cost outcomes. 210,215,259 All were conducted in the UK. They were based on clinical effectiveness studies judged to be of ‘moderate’215 or ‘weak’210,259 overall quality.
The study by Salhiyyah and colleagues259 was an evaluation of a specialist ward initiative, a ‘fast-track’ care pathway via a ‘theatre recovery unit’ (rather than to the cardiac intensive care unit) and then same-day transfer to an intermediate care unit. By contrast, both of the other two economic studies in cardiac surgery patients evaluated pre-admission prehab interventions to give support and education, intended to reduce anxiety and improve lifestyle. 210,215
The two studies evaluating prehab interventions were a high-quality cost–utility analysis210 and a low-quality and poorly reported cost-minimisation analysis,215 both of which were based on RCTs. The cost-minimisation analysis of the nurse-led programme of support and lifestyle management provided only one sentence of cost methods, but included costs of outpatient, community contacts and home-care contacts (i.e. not just hospital costs). 215 By contrast, the cost–utility analysis of prehab to reduce anxiety or depression and increase physical activity prior to CABG surgery was relatively well described and was conducted and reported according to current best practice standards for cost-effectiveness analyses. This included calculation of both an incremental cost-effectiveness ratio and a cost-effectiveness acceptability curve; however, both were based on only 8 weeks’ follow-up and therefore included only costs while waiting for admission for surgery. This time horizon for the cost study makes this study very different from the other cost studies included in this review: there is no effect on costs of different LOS.
Cost results: prehab
The two studies of preoperative education and support reported contrasting cost outcomes. 210,215 In the lower-quality study, the preoperative programme was associated with statistically significantly lower costs (£1817 lower per patient; 95% CI £3238 to –£396; p < 0.05), which were mostly accounted for by in-hospital costs. 215 Although this represents a considerable cost saving, it must be noted that the study was of low quality, scoring only 2 out of 19 on the CHEC list assessment.
In the cost–utility analysis by Furze and colleagues,210 the mean per-patient cost of the intervention arm was £24.10, or £1.73 higher than routine nurse counselling.
Cost results: specialist ward
The study by Salhiyyah and colleagues259 was a low-quality economic study, based on only the average cost per day in the different units; in addition, crucially, the cost per day of the new theatre recovery unit was assumed to be the same as that of the cardiac intensive care unit. The authors’ estimation of the statistical significance of the cost difference is incorrect, so their conclusion that the fast-track pathway was cheaper than the conventional pathway is flawed.
Cost-effectiveness evidence: colorectal surgery
Of the 19 studies evaluating effectiveness evidence about multicomponent initiatives to enhance recovery after colorectal surgery, only three also reported cost outcomes. 211,232,268 All three studies evaluated changes to care spanning preoperative preparation and support, intraoperative elements and postoperative components to enhance recovery in single hospitals in the UK,232 the Netherlands268 and Spain. 211 They were either cost analyses211 or cost–consequences analyses232,268 reported within the main clinical effectiveness evaluation paper. They were based on clinical effectiveness studies judged to be of ‘moderate’ overall quality.
The highest quality of the three studies (the study from the UK) showed a non-statistically significant lower cost of ERP compared with conventional care (–£671) for patients undergoing colorectal surgery for cancer. 232 This was despite ERP patients having a much lower and statistically significantly fewer postoperative stays (between 48% and 66% of the length of postoperative stay with conventional care, whether including or excluding convalescence stays or re-admissions). This mainly reflects that this study, unlike most in this review, had separate, much lower unit costs for ward hotel costs (£162/day) compared with the per-hour or per-day costs of patients while in theatre, recovery or intensive care.
The other two studies of ERP in colorectal surgery were of lower quality, mainly as a result of poor reporting (in a paper mainly reporting clinical effectiveness methods and results). The study in a single hospital in Spain estimated that those receiving ERP incurred hospital costs that were 55% lower than those for patients receiving traditional surgical care (difference –€1735, 95% CI –€2370 to –€1102; p < 0.001). 211 This is driven purely by the statistically significant 5.1-day-shorter mean LOS and the use of the same unit cost for all days in hospital. Costs associated with complications or re-admissions were not included.
The study by Vlug and colleagues,268 conducted in nine university and teaching hospitals in the Netherlands, evaluated the clinical and hospital cost outcomes of four surgical and care strategies, comparing ERP with standard care in laparoscopic or open surgery. ERP patients had statistically significantly slightly shorter median total LOS or postoperative LOS for comparisons between some groups; however, it was not possible to evaluate the four possible cost comparisons (laparoscopic/open surgery; university/teaching hospital) between fast-track and standard care, because the group numbers were not reported.
Cost-effectiveness evidence: lower limb arthroplasty
Of the 25 studies evaluating effectiveness evidence about multicomponent initiatives to enhance recovery after orthopaedic surgery, seven studies reported cost outcomes. 222–224,244,256,280,281 There were four ERP interventions,224,256,280,281 two studies evaluating prehab222,244 and one staff mix intervention. 223 Studies were diverse in origin, coming from the UK,224,244,256 Denmark,280 Iceland,281 the USA223 and Taiwan. 222 The study by Larsen and colleagues280 was a cost–utility analysis, and the study by McGregor and colleagues244 was a cost-minimisation analysis, with the other five studies being simpler comparative cost analyses or cost–consequences analyses.
The studies were of variable quality, with only the studies by Larsen280 (17/19), Sigurdsson281 (15/19) and Hunt224 (14/19) meeting the majority of the CHEC quality assessment criteria. They were based on clinical effectiveness studies judged to be of ‘moderate’222,223,256,280 or ‘weak’224,244,281 overall quality.
Cost results: prehab
Two studies evaluated both the clinical outcomes and the cost impact of prehab. Huang and colleagues222 evaluated a 4-week programme of strength training and education for total knee arthroplasty patients in Taiwan; McGregor244 evaluated a one-off preoperative physical training class and booklet for total hip arthroplasty patients in the UK. Both gave minimal description of the costing methods and both met fewer than half of the CHEC study quality criteria.
The small (n = 35) randomised UK hip arthroplasty study reported a 17% lower cost in the prehab study group. 244 This was mainly attributed to the 3-day-shorter LOS following the preoperative training and also to this group requiring less occupational therapy. The RCT of 243 hip arthroplasty patients in Taiwan reported a 1.7% lower medical cost in the intervention group (difference –NT$2112; 95% CI –NT$3337 to –NT$886; p < 0.001), which the authors attributed to the shorter mean LOS in the intervention group. 222 However, given the range of LOS in each group, the standard deviations of the mean medical costs in each group seem unusually small, so this finding should be treated with caution.
Cost results: enhanced recovery protocol
Four studies evaluated ERP interventions. 224,256,257,280 Two studies in UK hospitals evaluated the patient outcomes and cost impacts of early discharge and enhanced rehabilitation initiatives: one for patients having primary hip arthroplasty224 and the other for patients having unicompartmental knee arthroplasty. 256 The ERP evaluation reported by Hunt and colleagues224 involved spinal analgesia with sedation intraoperatively, and mobilisation on day 1 post operation, target discharge by post-operation day 3, and other differences from surgical care at two other UK hospitals with different operative and rehabilitation approaches (see Table 3). The ERP intervention evaluated by Reilly and colleagues involved targeted discharge within 24 hours postoperatively, supported by early mobilisation, pain diary and enhanced pain control, a booklet for comprehensive rehabilitation advice and a 24-hour emergency contact telephone number. 256
The study by Hunt and colleagues224 was assessed as a high-quality economic study (meeting 14/19 of CHEC criteria), whereas the study by Reilly and colleagues256 was much lower quality (6/19 CHEC criteria met); it described its cost analysis methods in one 10-word sentence, and the cost differences between the study groups were calculated by multiplying a standard cost per inpatient day (£487) by the difference in mean LOS. The study by Hunt and colleagues,224 comparing hip arthroplasty rehabilitation in three UK hospitals, showed that health-care costs in the hospital with the shortened stay (Belfast, median stay 3 days; cost £4909) were lower than those in the two other hospitals, which had longer median LOS (Liverpool, south-west London, median stay 6 and 5 days; cost £5070 and £5970 – no variance reported). 224 These estimates conceal wide variation in the costs of prosthetic and cements; however, the shortened median stay in Belfast was associated with notably lower costs of hospital staff input, which accounted for between 23% and 34% of the totals cost at each hospital.
Larsen and colleagues280 evaluated an ERP intervention in a regional hospital in Denmark for hip and knee arthroplasty patients, including pre-admission outpatient information visit, surgery on day of admission, controlled fluid and protein consumption, and earlier and more intensive mobilisation, which included a cost–utility analysis (see Report Supplementary Material 3, Table 5, for full details). This Danish cost–utility study was the highest-quality economic study in this review, meeting all but 2 of the 19 CHEC criteria. It was also the only study whose journal paper was devoted exclusively to describing the methods and results of an economic evaluation. The ERP intervention was associated with a statistically significant 3-day shorter LOS, and the study authors reported the intervention to be statistically significantly cheaper than the standard protocol (DKK18,880 less), based on univariate analysis. There were also sustained (12-month) gains in EQ-5D-assessed quality of life for total hip arthroplasty patients undergoing the accelerated protocol, but not for knee arthroplasty patients. The multivariate incremental cost-effectiveness analyses showed that, for total hip arthroplasty, for 98% of the cost and effectiveness probabilistic estimates the ERP both was cheaper and generated more QALYs than usual care. For the same analysis of knee arthroplasty patients, although for 93% of the cost and effectiveness probabilistic estimates ERP was cheaper than usual care, there was no statistically significant or clinically relevant difference in effect.
A cost analysis by Sigurdsson and colleagues281 (linked to the effectiveness paper by Siggeirsdottir261) evaluated an ERP intervention comprising both preoperative education and training and home-based rehab after discharge for total hip arthroplasty patients at two hospitals in Iceland. The costing study was reported in detail in the cost analysis paper and therefore was judged as high quality because it met 15 out of 19 of the CHEC criteria. However, the cost-effectiveness ratios calculated on the basis of between-group differences in gains in the Oxford Hip Score were average cost-effectiveness ratios, which are much less meaningful and useful than cost-effectiveness ratios based on an incremental analysis. 282 Nevertheless, the total care costs of the early discharge with ERP were statistically significantly 28.5% lower than those of conventional care (difference –US$3402, 95% CI –US$5000 to –US$1804; p < 0.001) when including patient costs such as productivity/employment losses, and 30.8% lower when including only health-care costs (difference –US$2543, 95% CI –US$3505 to –US$1581; p < 0.001). This was attributable mostly to differences in inpatient hospital costs, which in turn were strongly driven by the statistically significantly shorter mean LOS with the intervention (difference –3.6, 95% CI –5.29 to –1.91; p < 0.001).
Cost results: staff mix
One study evaluated a staff-mix intervention or ‘medical and surgical comanagement team’ at a tertiary care teaching hospital in the USA, which meant that care before and after surgery was led by general internal medicine faculty and orthopaedic physicians rather than by orthopaedic surgeons. 223 Although the study was based on a RCT, the cost evidence was low quality, with costing methods and results very minimally described, and meeting only 6 of the 19 CHEC criteria.
The staff-mix intervention was associated with no difference in the total cost of care compared with standard orthopaedic surgical care (US$15,373 vs. US$15,283; difference US$90), although this concealed that statistically significantly higher physician costs were compensated for by lower hospital costs.
Cost-effectiveness evidence: upper abdominal surgery
Of the eight studies evaluating effectiveness evidence for multicomponent recovery enhancement interventions in upper abdominal surgery, only two257,264 reported cost outcomes. One study reported cost outcomes as part of a related paper on the implementation, feasibility and safety of the ERP initiative for patients undergoing laparoscopic pancreatectomy in a UK hospital,257 and another reported limited data on cost outcomes for patients undergoing surgery for gastric cancer at a single hospital in Japan. 264
Both studies were classified as cost–consequences analyses and were of relatively low quality in relation to the CHEC criteria for assessing the quality of economic evaluations. In large part this was because the cost methods were minimally described (see Table 25); however, the Japanese study was a larger (n = 148) prospective randomised study, and the UK study was based on a small uncontrolled before (n = 44) and after (n = 22) study. They were based on clinical effectiveness studies judged to be of ‘moderate’264 or ‘weak’257 overall quality.
In terms of the impact of ERP on LOS, the trial in Japan of gastric cancer patients showed postoperative hospital stay 1 day shorter than with usual care (median 9 vs. 10 days; p < 0.001), and the study in UK patients undergoing distal pancreatectomy showed postoperative stay 3 days shorter (median 6 vs. 3 days; p < 0.001). 264
Cost results
In both studies, ERP interventions were associated with statistically significantly lower hospital admission costs,257,264 or total costs including re-admission costs,257 than the usual/previous care protocols (see Report Supplementary Material 7, Table 3). However, for gastric cancer surgical patients in Japan, the median cost of the hospital admission was only 2% lower, so this cost difference is smaller and more uncertain. In the UK study, most of the difference in median cost was from postoperative costs (53% lower with ERP), which reflects that median postoperative LOS was halved after the introduction of the ERP. 257
Summary of cost-effectiveness evidence
It is very difficult to draw firm and widely generalisable conclusions about the cost impact or cost-effectiveness of multicomponent interventions to improve recovery after elective surgery. This is because:
-
The effectiveness evaluations that also presented cost data or cost-effectiveness analyses are a small subset (one-quarter or less) of all of the effectiveness studies relating to a particular type of surgery. In addition, there were no economic studies of interventions in several of the procedural groups.
-
Of the 15 economic studies, only three were full economic evaluations, and only one of these was of sufficient quality. The other 12 studies were comparative cost analyses reported in papers primarily reporting clinical effectiveness, feasibility and safety/complications.
-
There was considerable diversity within the evidence base in terms of anatomical site (colorectal, lower limb, cardiac, upper abdominal), type of procedure (e.g. open vs. laparoscopic, hip vs. knee arthroplasty) and intervention characteristics. There would also be inevitable international differences in hospital organisation and clinical team composition.
-
Two-thirds of the economic studies were of poor quality (meeting between 2 and 10 of the 19 CHEC quality criteria).
With the exception of the four costing studies of ERP for lower limb arthroplasty, it was difficult to identify even a pair of studies that were convincingly similar enough in intervention, care/hospital context and methods to be comparable and, therefore, to allow a plausible synthesis of findings. Furthermore, although most of the economic studies attributed health-care costs to the number of days spent in hospital, few studies:
-
used different unit costs to reflect the relatively lower costs of pre-discharge days compared with surgical or immediately post-surgical days in hospital
-
included costs associated with treating complications or for re-admissions
-
included any non-hospital care costs (e.g. community rehabilitation or primary care costs)
-
had a time horizon of > 3 months post surgery or post admission
-
estimated the additional cost (e.g. additional staff time, different analgesia, nutritional differences) of providing different ERP components or other components of the new care pathways/protocols.
Given the heterogeneity of the evidence, the methodological flaws in calculating costs and the inconsistency of findings, it is not possible to conclude whether or not interventions to improve recovery from planned admissions are more cost-effective than usual care.
Chapter 5 Discussion
Main findings
To our knowledge this systematic review is the first to consider the effectiveness and cost-effectiveness of multicomponent interventions to improve recovery and/or reduce LOS for older adults undergoing elective hospital treatments that require an overnight stay. We identified 208 studies reported across 218 articles evaluating such interventions, of which 73 studies were prioritised for synthesis, including 15 studies reporting costs. The 73 prioritised studies represented the highest-quality evidence available, RCTs from any ‘high-income’ country, as well as the most relevant evidence to the UK context, including a number of RCTs, controlled trials and uncontrolled studies, which often represent the most ethical, if not scientifically rigorous, approach to evaluating the implementation of care pathways in hospital settings.
The prioritised evidence pertained to eight broad types of surgical procedure (cardiac, colorectal, lower limb arthroplasty, pelvic, upper abdominal, thoracic, vascular and various), the most common being either lower limb arthroplasty (34% of studies) or colorectal surgery (25% of studies). The prevalence of evidence within these procedural categories appears to reflect the most common reasons for hospital admission among older adults. 5 Interventions were grouped into six categories (ERP, prehab, rehab, specialist ward, staff mix and PACP), but ERP (67% of studies) and prehab (18% of studies) interventions far outweighed any other category. Overall, the evidence suggests that interventions generally have a beneficial effect on one or more clinical or patient-reported outcomes, and very rarely lead to inferior outcomes for any measure, in a variety of health systems. It is notable, however, that the outcomes reported were almost exclusively concerned with the treatment course from pre-admission to discharge, with few studies reporting outcomes beyond 30-day statistics for mortality, re-admissions and complications.
Despite this observation of general effectiveness (or at least a lack of detrimental effects) with most types of interventions, the evidence is heterogeneous and, thus, limits the generalisability of this statement. The most consistent evidence from international RCTs was for ERP to be associated with reduced LOS after both colorectal surgery and upper abdominal surgery. In patients undergoing colorectal surgery, there was additional evidence that markers of physical recovery before discharge may be improved with ERP. The evidence for reduced LOS after upper abdominal surgery came from five patient groups across four studies, which also indicated that the odds of sustaining complications were reduced with ERP. Evidence from other procedure groups was limited by small numbers of studies and the presentation of data that could not be combined in meta-analyses. In addition to the pooled data for RCTs, there were numerous studies that could not be meta-analysed that provided evidence of improved outcomes with ERP and prehab interventions in particular. There were relatively few studies of other intervention types, limiting the strength of conclusions about their effectiveness, despite a number of single studies reporting positive results.
The evidence from the UK was similarly dominated by evaluations of ERP and, to a lesser extent, prehab interventions for colorectal surgery and lower limb arthroplasty. The evidence for ERP interventions to improve recovery from colorectal surgery came from only seven studies but usually indicated improvement with intervention, or equivalence with usual care for LOS and complications. Thirteen UK-based ERP interventions to improve recovery from lower limb arthroplasty were identified, and although standardised mean differences could not be calculated for nearly half of the studies, the evidence was consistently in favour of ERP in reducing LOS. The seven studies presenting analysable LOS data indicated a mean reduction in LOS of > 4 days, compared with usual care. Some additional outcomes in these studies improved with ERP, but there were few of these outcomes to analyse. As with the RCT-only evidence, studies from the UK showed that LOS may be reduced in upper abdominal surgery with ERP interventions. There was also evidence of markers of recovery improving with ERP.
The effectiveness evidence from the UK and international RCTs showed broadly similar findings and similar distributions of studies between surgical specialties, with UK studies slightly more focused on evaluations of ERP interventions for patients undergoing lower limb arthroplasty. The two types of procedure that accounted for nearly 60% of the evidence (colorectal and lower limb arthroplasty) arguably do not require further evaluations of the effectiveness of ERP interventions. However, the heterogeneity of interventions makes it difficult to identify optimal combinations of components and thus further analysis such as meta-regression may contribute to this understanding. The focus of any future trials in these surgical specialties should, however, be more towards aspects of implementation and scaling up of interventions, assessment of wider patient outcomes such as patient satisfaction and mental health, and consideration of the effect of shorter LOS on the broader health and social care system and on long-term patient outcomes.
It is unclear whether or not the evidence from colorectal surgery and lower limb arthroplasty is transferable to other surgical specialties. Aside from these procedure categories, the evidence for ERP interventions was most positive for upper abdominal surgery, although this was based on only seven studies and with some equivocal findings. There were no other clusters of evidence that could allow comprehensive evaluation of interventions for other surgical specialties; however, there may be evidence-based intervention components that can reasonably be expected to benefit older adults undergoing different procedures. Further research is required to determine this.
Cost-effectiveness evidence was derived from only 15 studies, which were highly heterogeneous in terms of population, intervention and location. Although the general suggestion was that interventions led to cost savings, findings were often the result of basic alignment with daily costs, and not the result of rigorously performed economic evaluations. The majority of cost-effectiveness evidence was of low quality, and, in the light of the aforementioned limitations, may be considered inconclusive with regard to research question 2.
The effectiveness of ERP interventions for optimising LOS without having a detrimental effect on other outcomes is consistent with other systematic reviews that have demonstrated the effectiveness of this type of intervention for surgical patients26 and for patients admitted for elective colorectal surgery. 28 One systematic review283 of RCTs evaluating prehabilitation interventions for individuals undergoing joint-replacement surgery indicated some small effect on postoperative pain, function and mobility but no statistically significant impact on LOS or cost, which is, again, broadly consistent with the findings of this review. The cost-effectiveness findings are less conclusive than those of other recent systematic reviews for ERP in colorectal cancer,116 and various abdominal surgical procedures. 284 These reviews concluded that ERP/ERAS programmes appear to be cost-effective, at least in the short term in relation to hospital costs, but with strong caveats about the lack of inclusion of non-hospital costs, costs to patients, or the cost of providing the ERP itself. Both of these systematic reviews, and our own, clearly show that the research recommendations outlined by Jönsson and Lindgren285 almost 40 years ago have rarely been heeded.
Limitations of the evidence
Study authors defined LOS in a number of ways, such as the time from admission to discharge, the length of time spent in hospital after surgery or the total time in hospital with re-admissions included. However, > 40% of studies did not offer a clear definition of LOS, limiting comparability between studies. Furthermore, only 12% of studies addressed either the reliability or the validity of the primary outcome. In many cases, it could be supposed that data obtained from a database are reliable and valid; however, without descriptions of the definition of LOS, or of how data were recorded, we chose not to make this assumption. We note that a generous approach to this item would have resulted in 20 more studies achieving a global rating of ‘moderate’ or ‘strong’. 196,199,202,205,211,213,217,220,222,223,225,227,234,235,239,242,243,256,258,268 Additional clarification about data collection tools and definitions of LOS would benefit future studies in this area.
Comparator groups were often poorly reported. It was frequently difficult to determine which intervention components were present only in the intervention group, and often the comparator was not described at all. This limits the extent to which the effectiveness of intervention components can be determined.
Studies almost exclusively failed to consider longer-term patient outcomes. Only two studies investigated outcomes at 12 months, with the vast majority of studies ending their consideration of patient outcomes 30 days after discharge. Although this may be understandable in many cases, as a result of the specific aims of studies, it means that the wider impact of reduced LOS is unknown in this body of evidence. Given that we sought any related or ‘sibling’ articles for all included studies, we are confident that there is a dearth of such research. Although earlier discharge from hospital may be desirable, the impact of this on other health and social care services must be examined, particularly in the wider context of the NHS. Only six studies assessed the use of additional care (follow-up appointments, GP visits, etc.) after discharge. In addition, we sought information on discharge destination, but this was reported very rarely. Ideally, the patient should be followed over the course of at least 1 month following discharge and ideally for 3–6 months, with assessments of their outcomes, such as quality of life, mental health, satisfaction with surgical outcomes and physical activity, as relevant to the procedure, and with documentation of their health and social care resource use.
Non-clinical patient outcomes were not measured routinely. The evidence was dominated by evaluations of ERP interventions, which were heavily focused on perioperative components, with a lack of consideration of broader patient outcomes. In particular, measures of mental health, quality of life and satisfaction with care were scant, whereas short-term markers of physical recovery were often included. Non-ERP studies tended to give greater attention to these outcomes, possibly because interventions such as prehab, rehab and PACPs focus more on non-perioperative aspects of patient care, and thus seek to measure effectiveness in those terms. There may be mutual opportunities for learning for ERP and non-ERP interventionists alike, where aspects of prehab and PACP in particular offer promise of improvement of patient care, and could be integrated into existing pathways. The evidence for non-ERP interventions is limited and requires further study.
Around one-third of studies presented LOS without variance data, or with range data that could not be used to calculate standardised mean differences. Although median and range data provide a valuable metric, range data are often highly skewed by long LOS, and, as such, additional variance statistics can give readers extra insight. For example, this type of data is useful to systematic reviewers examining a collective body of evidence with a view to providing a cohesive/comprehensive message to organisations developing health policy recommendations. In addition, one metric that would enhance the interpretation of LOS data, particularly when high upper ranges are reported, is the number of patients experiencing ‘long LOS’, as reported by Starks and colleagues. 262 These data are available from hospital records and should be considered in future studies.
Studies frequently deliberately excluded patients who were over a certain age or who had complex, or potentially complex, needs, with a number of studies also choosing to exclude ‘outliers’ or patients who experienced severe complications. This reduces the validity of the evidence, as patients with complex needs are a regular occurrence in public health-care systems. By contrast, a small number of studies (n = 7199,200,207,215,218,221,223) selected individuals who were at greatest risk of postoperative complications, or who were likely to have complex needs or multimorbidities. For example, very few studies mentioned delirium, despite its prevalence and negative sequelae among older adults undergoing elective procedures. One reason for this was the choice in a number of studies (n = 1831,196,200,202,209,210,226,229,231,234,236,244,254,259,261,262,266,267) to exclude patients who had risk factors for delirium, such as cognitive impairment. It could be considered that studies that embrace the complexity of typical patients offer more valuable evidence about real-world situations and manage the likely confounders that introduce variation in LOS.
Certain surgical specialties or procedures requiring elective inpatient admission were not identified. For example, no studies evaluated neurosurgical or neurorehabilitation pathways for planned neurosurgery procedures, despite efforts to develop these in areas such as the south-west of the UK. This particular area has relatively low patient volume and a mix of elective and acute patients within surgical units, compared with other surgical specialties. This means that effectiveness evaluations of rehabilitation pathways were unlikely to meet our inclusion criteria. As such, we recognise that there is likely to be useful research into elective procedures that did not fit the remit of this review. A significant body of high-quality cost-effectiveness evidence, directly based on high-quality effectiveness studies, would have been a valuable facet of the evaluation of interventions in this review. However, the lack of high-quality cost-effectiveness evidence limits the conclusions that can be made.
Strengths and limitations of this review
We included studies published in 2000 or later to focus on organisational strategies that are currently used in health-care organisations in high-income economies. However, it could be argued that this limit was too generous, with data collection in some included studies taking place in the 1990s. As > 80% of articles were published within the past 10 years, this might have been a more prudent date restriction.
A strength of this review was that we included non-RCT study designs, acknowledging an ethical dilemma researchers face about whether or not to randomise patients to care that may be suboptimal. However, we were unable to consider non-randomised trials from outside the UK because we had insufficient resources to carry out a review on such a scale. As a result, there may be useful international evidence that we have not synthesised. However, the studies utilising non-randomised trial designs included in the UK tranche are vulnerable to selection bias and influence from confounding variables across different participant groups.
It was also beyond the scope of this review to conduct any additional analysis, such as metaregression. This might have allowed further exploration of the particular intervention components associated with successful ERP interventions in terms of reducing LOS and improving other outcomes for patients undergoing colorectal surgery or lower limb arthroplasty. It could also be beneficial to conduct subgroup analysis by considering whether the findings differ among ‘the oldest-old’, people with various comorbidities or people living with frailty.
Although we contacted the authors of studies that had missing data, we did not do this when only median and range outcomes were presented. Given the number of studies falling into this category, such an approach would have demanded significant extra resources, and we felt that this was not justifiable.
To our knowledge, this is the first systematic review to bring together and evaluate evidence about multicomponent interventions of any type, aiming to improve recovery and reduce LOS following elective procedures in older adults. We used best practice methods to identify, select, appraise and synthesise the evidence and have incorporated the views of both clinical experts in the field and patients with experience throughout the review process. Our findings are based on both the highest-quality and the most relevant evidence to the UK audience, which was identified using extensive search methods.
Chapter 6 Conclusions
Overall, the evidence in this review suggests that ERP and, to an extent, prehab interventions, may help optimise the LOS of older adults in hospital following admission for a planned procedure. In general, these interventions appear to improve recovery from surgical procedures, often by reducing LOS without increasing the odds of complications or re-admissions, as well as improving or maintaining other clinical and patient-reported outcomes. These findings were also shown across other procedure and intervention categories that we identified, with the UK research base broadly echoing the findings from the RCT evidence.
Little evidence was available to evaluate the cost-effectiveness of interventions, coming from 15 studies and being of generally poor quality, highlighting the need for economic evaluations to be incorporated alongside any further evaluations of organisational interventions to optimise hospital LOS for older adults.
We believe that further studies evaluating the effectiveness of implementing full protocols for enhanced recovery in colorectal surgery and lower limb arthroplasty are not warranted, although other surgical specialties, such as cardiac, vascular and lower abdominal surgeries, still lack evidence of this. Evidence of the effectiveness of other types of intervention within these specialties is also scarce, including rehabilitation, staff-mix and specialist ward interventions. There is a case for future research to move towards a focus on the processes of implementing or scaling up interventions, which should include mixed-methods approaches.
Patient outcomes and longer-term implications were rarely considered within the body of research in this review and should be brought into focus in future studies. This is necessary to fully evaluate the impact of hospital-based organisational strategies to optimise LOS on patient experience and community health and social care services.
Implications for further research
Further research is required in the following areas:
-
More evidence is needed about under-represented interventions, such as rehabilitation, staff-mix interventions and enhanced preoperative assessments, as well as about how they may integrate with existing ERPs to maximise effect.
-
More evidence is needed to determine the effectiveness and transferability of interventions outside the areas of colorectal surgery and lower limb arthroplasty. Some surgical specialties or reasons for planned admission were not represented in the evidence at all.
-
Studies should include the long-term follow-up of patient outcomes for at least 1 month after surgery, and ideally for 3–6 months, with additional investigation of the implications for society, such as the use of extra resources, need for support, and use of social care.
-
More studies evaluating intervention effectiveness should embrace (rather than screen out) the likely complexities of the older adult population in order to improve the treatment of suboptimal conditions.
-
Further research evaluating the effectiveness of ERPs for colorectal surgery and lower limb arthroplasty should be stepped down. However, evidence is needed about how to effectively implement such protocols and scale up across surgical specialties. This may require the consideration of qualitative experiences of the patients and staff involved in the implementation of such protocols.
-
Further research is needed on the patient experience of a shorter stay in hospital. This may involve interviewing patients treated within the organisational interventions identified by this review and exploring their experiences of the care they received both in hospital and post discharge. A systematic review of existing primary qualitative approaches may help to identify where further primary research is required.
-
Robust evidence about the cost-effectiveness of interventions is required, and may be particularly valuable in the context of a financially pressured NHS.
Implications for clinical practice
Acceptance is growing of the need to provide standardised approaches to surgical care. Unwarranted variation is thought to have an impact on both clinical outcomes and patient safety. Additionally, the cost of health care is growing exponentially, while health-care budgets have failed to keep pace. Enhanced recovery programmes afford the opportunity to improve the quality of clinical care while reducing health-care costs.
Organisational initiatives to accelerate recovery and/or reduce LOS have been widely implemented in Europe and the UK, as well as further afield under the label of ‘quality improvement’ in North America. However, significant variation in LOS exists for procedures across the UK, and the extent to which such interventions are embedded and standardised between and within hospital sites in the UK is not known. 22
No core configuration of intervention components emerges for the populations included in this review. Indeed, despite variation in individual elements, similar benefits are realised, suggesting that endeavours to find the ‘ideal protocol’ are less important than the act of whole-team engagement in scrutinising and measuring the patient pathway. The lack of detrimental outcomes as a result of these interventions further supports this notion. A gap in understanding is the level of compliance with which the interventions identified within this review are implemented.
Warranted variation in protocols may exist where the elements within enhanced recovery pathways need to be adapted to the needs of patients, for example those with complex needs. We note that only a handful of studies embraced the challenge of patients with complex needs such as multimorbidity, frailty or dementia. This may be because these patients are deemed unsuitable for non-critical surgical intervention; however, even in life-saving situations, minimal guidance remains about how to adapt recovery pathways for these patients.
The findings of this review support the use of multicomponent interventions, particularly ERP, to improve clinical outcomes for patients undergoing colorectal surgery and lower limb arthroplasty, while there are suggestions of similar benefits for other types of surgery. However, we are unable to comment with certainty about the effects of interventions on patient-reported outcomes, such as quality of life, or on costs, and recommend further research on these aspects.
Acknowledgements
We would like to thank Sue Whiffin for administrative support and proofreading, Jenny Lowe for administrative support, Obi Ukoumunne and Jaime Peters for advice about statistics, Rebecca Abbott for proofreading and comments on draft work, Caroline Farmer for comments on draft work and Jude Fewings for advice on neurorehabilitation research.
Contributions of authors
Michael Nunns (https://orcid.org/0000-0001-5500-0911) was involved in all stages of the review, including direction/conception, planning searches, screening, data extraction, critical appraisal, synthesis and write-up. He led the data analysis and synthesis and led clinical expert involvement. He was the key author of the final manuscript.
Liz Shaw (https://orcid.org/0000-0002-6092-5019) was involved in all stages of the review, including direction/conception, planning searches, screening, data extraction, critical appraisal, synthesis and write-up. She led the PPI involvement and was a key author of the final manuscript.
Simon Briscoe (https://orcid.org/0000-0002-6982-4521) was involved in direction/conception, designing and conducting database and supplementary searches, screening, data extraction, critical appraisal and write-up. He also managed the reference library.
Jo Thompson Coon (https://orcid.org/0000-0002-5161-0234) guided the direction of the review, advised on all stages of conducting the review, and critically read and edited all sections of the report.
Anthony Hemsley (https://orcid.org/0000-0002-5595-4528) helped guide the direction of the report, contributed to synthesis and interpretation, provided links to expert clinical advisors, and critically read and edited all sections of the report.
John S McGrath (https://orcid.org/0000-0001-9416-9912) helped guide the direction of the report, contributed to synthesis and interpretation, and critically read and edited all sections of the report.
Christopher J Lovegrove (https://orcid.org/0000-0003-2530-1988) helped guide the direction of the report, contributed to synthesis and interpretation, and critically read and edited all sections of the report.
David Thomas (https://orcid.org/0000-0002-5166-0565) helped guide the direction of the report and critically read and edited the report.
Rob Anderson (https://orcid.org/0000-0002-3523-8559) guided the direction of the review, advised on all stages of conducting the review, critically read and edited all sections of the report, and led the cost-effectiveness synthesis and write-up. He is the guarantor of the report.
Data-sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- World Health Organization . World Report on Ageing and Health 2015.
- Bloom DE. 7 billion and counting. Science 2011;333:562-9. https://doi.org/10.1126/science.1209290.
- United Nations . World Economic and Social Survey 2007: Development in an Ageing World 2007.
- NHS Confederation . NHS Statistics, Facts and Figures 2017. www.nhsconfed.org/resources/key-statistics-on-the-nhs (accessed 20 September 2018).
- NHS Digital . Hospital Admitted Patient Care Activity, 2015–16 2016. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2015-16 (accessed 30 August 2018).
- Age UK . Painful Journeys: Why Getting to Hospital Appointments Is a Major Issue for Older People n.d. www.ageuk.org.uk/contentassets/7354623c9df1491a84cc34ef46105647/painful_journeys_campaignreport.pdf (accessed 29 October 2019).
- Bakker FC, Robben SH, Olde Rikkert MG. Effects of hospital-wide interventions to improve care for frail older inpatients: a systematic review. BMJ Qual Saf 2011;20:680-91. https://doi.org/10.1136/bmjqs.2010.047183.
- Age UK . Loneliness and Isolation Evidence Review n.d. www.ageuk.org.uk/documents/en-gb/for-professionals/evidence_review_loneliness_and_isolation.pdf?dtrk=true (accessed 29 October 2019).
- Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD000313.pub4.
- Buurman BM, Hoogerduijn JG, de Haan RJ, Abu-Hanna A, Lagaay AM, Verhaar HJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0026951.
- Elia M, Zellipour L, Stratton RJ. To screen or not to screen for adult malnutrition?. Clin Nutr 2005;24:867-84. https://doi.org/10.1016/j.clnu.2005.03.004.
- Healey F, Oliver D. Preventing falls and injury in hospitals. The evidence for interventions. Health Care Risk Rep 2006;June:12-7.
- Lewis R, Edwards N. Improving Length of Stay: What Can Hospitals Do?. London: Nuffield Trust; 2015.
- Lim SC, Doshi V, Castasus B, Lim JK, Mamun K. Factors causing delay in discharge of elderly patients in an acute care hospital. Ann Acad Med Singap 2006;35:27-32.
- National Ageing Research Institute . An Analysis of Research on Preventing Falls and Falls Injury in Older People: Community, Residential Care and Hospital Settings 2004.
- Vanderwee K, Clays E, Bocquaert I, Gobert M, Folens B, Defloor T. Malnutrition and associated factors in elderly hospital patients: a Belgian cross-sectional, multi-centre study. Clin Nutr 2010;29:469-76. https://doi.org/10.1016/j.clnu.2009.12.013.
- Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc 2011;59:266-73. https://doi.org/10.1111/j.1532-5415.2010.03276.x.
- NIHR Dissemination Centre . Comprehensive Care: Older People Living With Frailty in Hospitals 2017.
- National Audit Office . Discharging Older Patients from Hospital 2016.
- Enhanced Recovery Partnership Programme . Delivering Enhanced Recovery: Helping Patients to Get Better Sooner After Surgery 2010.
- National Institute for Health and Care Excellence . Transition Between Inpatient Hospital Settings and Community or Care Home Settings for Adults With Social Care Needs 2015.
- ACT Academy . Reducing Length of Stay 2018.
- Nuffield Trust . Reducing Length of Stay 2014. www.nuffieldtrust.org.uk/event/reducing-length-of-stay (accessed 3 September 2018).
- Royal College of Surgeons of England . Separating Emergency and Elective Surgical Care: Recommendations for Practice 2007.
- McCue P, Parker S, Conroy S, Bardley M, Roberts H, Kennedy S. How Best to Deliver Comprehensive Geriatric Assessment (CGA) on a Hospital Wide Basis: An Umbrella Review 2015. www.crd.york.ac.uk/prospero/display_record.php?RecordID=19159 (accessed 30 August 2018).
- Lau CS, Chamberlain RS. Enhanced recovery after surgery programs improve patient outcomes and recovery: a meta-analysis. World J Surg 2017;41:899-913. https://doi.org/10.1007/s00268-016-3807-4.
- ERAS Society . List of Guidelines n.d. http://erassociety.org/guidelines/list-of-guidelines/ (accessed 20 September 2018).
- Paton F, Chambers D, Wilson P, Eastwood A, Craig D, Fox D, et al. Effectiveness and implementation of enhanced recovery after surgery programmes: a rapid evidence synthesis. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005015.
- Rotter T, Kugler J, Koch R, Gothe H, Twork S, van Oostrum JM, et al. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs and patient outcomes. BMC Health Serv Res 2008;8. https://doi.org/10.1186/1472–6963-8-265.
- Ellis G, Langhorne P. Comprehensive geriatric assessment for older hospital patients. Br Med Bull 2004;71:45-59. https://doi.org/10.1093/bmb/ldh033.
- Partridge JS, Harari D, Martin FC, Peacock JL, Bell R, Mohammed A, et al. Randomized clinical trial of comprehensive geriatric assessment and optimization in vascular surgery. Br J Surg 2017;104:679-87. https://doi.org/10.1002/bjs.10459.
- Miani C, Ball S, Pitchforth E, Exley J, King S, Roland M, et al. Organisational interventions to reduce length of stay in hospital: a rapid evidence assessment. Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02520.
- Hyde CJ, Robert IE, Sinclair AJ. The effects of supporting discharge from hospital to home in older people. Age Ageing 2000;29:271-9. https://doi.org/10.1093/ageing/29.3.271.
- Parker SG, Peet SM, McPherson A, Cannaby AM, Abrams K, Baker R, et al. A systematic review of discharge arrangements for older people. Health Technol Assess 2002;6. https://doi.org/10.3310/hta6040.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews In Health Care 2008.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011.
- Royle P, Waugh N. A simplified search strategy for identifying randomised controlled trials for systematic reviews of health care interventions: a comparison with more exhaustive strategies. BMC Med Res Methodol 2005;5. https://doi.org/10.1186/1471-2288-5-23.
- Royle PL, Waugh NR. Making literature searches easier: a rapid and sensitive search filter for retrieving randomized controlled trials from PubMed. Diabet Med 2007;24:308-11. https://doi.org/10.1111/j.1464-5491.2007.02046.x.
- Basse L, Hjort Jakobsen D, Billesbølle P, Werner M, Kehlet H. A clinical pathway to accelerate recovery after colonic resection. Ann Surg 2000;232:51-7. https://doi.org/10.1097/00000658-200007000-00008.
- Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003;362:1921-8. https://doi.org/10.1016/S0140-6736(03)14966-5.
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. https://doi.org/10.1016/S0002-9610(02)00866-8.
- Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001;322:473-6. https://doi.org/10.1136/bmj.322.7284.473.
- Crathorne L, Huxley N, Haasova M, Snowsill T, Jones-Hughes T, Hoyle M, et al. The effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating cancer treatment-induced anaemia (including review of technology appraisal no. 142): a systematic review and economic model. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20130.
- World Bank List of Economies (June 2017) n.d. http://iccmoot.com/wp-content/uploads/2017/07/World-Bank-List-of-Economies.pdf (accessed 20 September 2018).
- Effective Public Health Practice Project . Quality Assessment Tool For Quantitative Studies 1998. https://merst.ca/ephpp/ (accessed 29 October 2019).
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5. https://doi.org/10.1017/S0266462305050324.
- Cohen J. A power primer. Psychol Bull 1992;112:155-9. https://doi.org/10.1037/0033-2909.112.1.155.
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-135.
- Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ 2011;343. https://doi.org/10.1136/bmj.d2090.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. https://doi.org/10.1136/bmj.327.7414.557.
- Thomson H, Thomas S, Sellstrom E, Petticrew M. Housing improvements for health and associated socio-economic outcomes. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD008657.pub2.
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046-55. https://doi.org/10.1016/S0895-4356(01)00377-8.
- Abou-Haidar H, Abourbih S, Braganza D, Qaoud TA, Lee L, Carli F, et al. Enhanced recovery pathway for radical prostatectomy: implementation and evaluation in a universal healthcare system. Can Urol Assoc J 2014;8:418-23. https://doi.org/10.5489/cuaj.2114.
- Alvis BD, King AB, Pandharipande PP, Weavind LM, Avila K, Leisy PJ, et al. Creation and execution of a novel anesthesia perioperative care service at a Veterans Affairs Hospital. Anesth Analg 2017;125:1526-31. https://doi.org/10.1213/ANE.0000000000001930.
- Andersen J, Hjort-Jakobsen D, Christiansen PS, Kehlet H. Readmission rates after a planned hospital stay of 2 versus 3 days in fast-track colonic surgery. Br J Surg 2007;94:890-3. https://doi.org/10.1002/bjs.5669.
- Aragon D, Burton V, Byers JF, Cohen M. The effect of a critical pathway on patients’ outcomes after carotid endarterectomy. Am J Crit Care 2002;11:250-8.
- Arana M, Harper L, Qin H, Mabrey J. Reducing length of stay, direct cost, and readmissions in total joint arthroplasty patients with an outcomes manager-led interprofessional team. Orthop Nurs 2017;36:279-84. https://doi.org/10.1097/NOR.0000000000000366.
- Archibald LH, Ott MJ, Gale CM, Zhang J, Peters MS, Stroud GK. Enhanced recovery after colon surgery in a community hospital system. Dis Colon Rectum 2011;54:840-5. https://doi.org/10.1007/DCR.0b013e31821645bd.
- Arroyo A, Ramirez JM, Callejo D, Viñas X, Maeso S, Cabezali R, et al. Influence of size and complexity of the hospitals in an enhanced recovery programme for colorectal resection. Int J Colorectal Dis 2012;27:1637-44. https://doi.org/10.1007/s00384-012-1497-4.
- Auyong DB, Allen CJ, Pahang JA, Clabeaux JJ, MacDonald KM, Hanson NA. Reduced length of hospitalization in primary total knee arthroplasty patients using an updated Enhanced Recovery After Orthopedic Surgery (ERAS) Pathway. J Arthroplasty 2015;30:1705-9. https://doi.org/10.1016/j.arth.2015.05.007.
- Aviles C, Hockenberry M, Vrochides D, Iannitti D, Cochran A, Tezber K, et al. Perioperative care implementation: evidence-based practice for patients with pancreaticoduodenectomy using the Enhanced Recovery After Surgery Guidelines. Clin J Oncol Nurs 2017;21:466-72. https://doi.org/10.1188/17.CJON.466-472.
- Baack Kukreja JE, Kiernan M, Schempp B, Siebert A, Hontar A, Nelson B, et al. Quality Improvement in Cystectomy Care with Enhanced Recovery (QUICCER) study. BJU Int 2017;119:38-49. https://doi.org/10.1111/bju.13521.
- Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreatico-duodenectomy reduces delayed gastric emptying. Br J Surg 2008;95:1387-93. https://doi.org/10.1002/bjs.6324.
- Barber C, Fraser JF, Mendez GG, Bradley B, Loftus TJ, Jacofsky DJ. The Halo effect: an unintended benefit of care pathways. J Knee Surg 2017;30:264-8. https://doi.org/10.1055/s-0036-1584577.
- Basse L, Thorbøl JE, Løssl K, Kehlet H. Colonic surgery with accelerated rehabilitation or conventional care. Dis Colon Rectum 2004;47:271-7. https://doi.org/10.1007/s10350-003-0055-0.
- Batsis JA, Naessens JM, Keegan MT, Huddleston PM, Wagie AE, Huddleston JM. Resource utilization of total knee arthroplasty patients cared for on specialty orthopedic surgery units. J Hosp Med 2008;3:218-27. https://doi.org/10.1002/jhm.299.
- Blind PJ, Andersson B, Tingstedt B, Bergenfeldt M, Andersson R, Lindell G, et al. Fast-track program for liver resection – factors prolonging length of stay. Hepatogastroenterology 2014;61:2340-4.
- Braga M, Pecorelli N, Ariotti R, Capretti G, Greco M, Balzano G, et al. Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J Surg 2014;38:2960-6. https://doi.org/10.1007/s00268-014-2653-5.
- Brodner G, Van Aken H, Hertle L, Fobker M, Von Eckardstein A, Goeters C, et al. Multimodal perioperative management – combining thoracic epidural analgesia, forced mobilization, and oral nutrition – reduces hormonal and metabolic stress and improves convalescence after major urologic surgery. Anesth Analg 2001;92:1594-600. https://doi.org/10.1097/00000539-200106000-00049.
- Brunenberg DE, van Steyn MJ, Sluimer JC, Bekebrede LL, Bulstra SK, Joore MA. Joint recovery programme versus usual care: an economic evaluation of a clinical pathway for joint replacement surgery. Med Care 2005;43:1018-26. https://doi.org/10.1097/01.mlr.0000178266.75744.35.
- Cakir H, van Stijn MF, Lopes Cardozo AM, Langenhorst BL, Schreurs WH, van der Ploeg TJ, et al. Adherence to enhanced recovery after surgery and length of stay after colonic resection. Colorectal Dis 2013;15:1019-25. https://doi.org/10.1111/codi.12200.
- Cerruto MA, De Marco V, D’Elia C, Bizzotto L, De Marchi D, Cavalleri S, et al. Fast track surgery to reduce short-term complications following radical cystectomy and intestinal urinary diversion with Vescica Ileale Padovana neobladder: proposal for a tailored enhanced recovery protocol and preliminary report from a pilot study. Urol Int 2014;92:41-9. https://doi.org/10.1159/000351312.
- Chang PL, Wang TM, Huang ST, Hsieh ML, Chuang YC, Chang CH. Improvement of health outcomes after continued implementation of a clinical pathway for radical nephrectomy. World J Urol 2000;18:417-21. https://doi.org/10.1007/s003450000158.
- Chen CC, Chen CN, Lai IR, Huang GH, Saczynski JS, Inouye SK. Effects of a modified Hospital Elder Life Program on frailty in individuals undergoing major elective abdominal surgery. J Am Geriatr Soc 2014;62:261-8. https://doi.org/10.1111/jgs.12651.
- Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011;213:245-52. https://doi.org/10.1016/j.jamcollsurg.2011.05.004.
- Chindhy SA, Edwards NM, Rajamanickam V, Lushaj EB, Lozonschi L, De Oliveira NC, et al. Acuity adaptable patient care unit system shortens length of stay and improves outcomes in adult cardiac surgery: University of Wisconsin experience. Eur J Cardiothorac Surg 2014;46:49-54. https://doi.org/10.1093/ejcts/ezt582.
- Christelis N, Wallace S, Sage CE, Babitu U, Liew S, Dugal J, et al. An enhanced recovery after surgery program for hip and knee arthroplasty. Med J Aust 2015;202:363-8. https://doi.org/10.5694/mja14.00601.
- Christensen HK, Thaysen HV, Rodt SÅ, Carlsson P, Laurberg S. Short hospital stay and low complication rate are possible with a fully implemented fast-track model after elective colonic surgery. Eur Surg Res 2011;46:156-61. https://doi.org/10.1159/000324406.
- Collins JW, Adding C, Hosseini A, Nyberg T, Pini G, Dey L, et al. Introducing an enhanced recovery programme to an established totally intracorporeal robot-assisted radical cystectomy service. Scand J Urol 2016;50:39-46. https://doi.org/10.3109/21681805.2015.1076514.
- Cullen J, Bramley D, Armstrong D, Butler L, Rouse P, Ashton T. Increasing productivity, reducing cost and improving quality in elective surgery in New Zealand: the Waitemata District Health Board joint arthroplasty pilot. Intern Med J 2012;42:620-6. https://doi.org/10.1111/j.1445–5994.2012.02815.x.
- Cunningham KE, Zenati MS, Petrie JR, Steve JL, Hogg ME, Zeh HJ, et al. A policy of omitting an intensive care unit stay after robotic pancreaticoduodenectomy is safe and cost-effective. J Surg Res 2016;204:8-14. https://doi.org/10.1016/j.jss.2016.04.023.
- Duplantier NL, Briski DC, Luce LT, Meyer MS, Ochsner JL, Chimento GF. The effects of a hospitalist comanagement model for joint arthroplasty patients in a teaching facility. J Arthroplasty 2016;31:567-72. https://doi.org/10.1016/j.arth.2015.10.010.
- Ehrlich A, Kellokumpu S, Wagner B, Kautiainen H, Kellokumpu I. Comparison of laparoscopic and open colonic resection within fast-track and traditional perioperative care pathways: clinical outcomes and in-hospital costs. Scand J Surg 2015;104:211-18. https://doi.org/10.1177/1457496914557016.
- El Baz N, Middel B, van Dijk JP, Boonstra PW, Reijneveld SA. Coronary artery bypass graft (CABG) surgery patients in a clinical pathway gained less in health-related quality of life as compared with patients who undergo CABG in a conventional-care plan. J Eval Clin Pract 2009;15:498-505. https://doi.org/10.1111/j.1365-2753.2008.01051.x.
- Emaminia A, Corcoran PC, Siegenthaler MP, Means M, Rasmussen S, Krause L, et al. The universal bed model for patient care improves outcome and lowers cost in cardiac surgery. J Thorac Cardiovasc Surg 2012;143:475-81. https://doi.org/10.1016/j.jtcvs.2011.10.001.
- Ender J, Borger MA, Scholz M, Funkat AK, Anwar N, Sommer M, et al. Cardiac surgery fast-track treatment in a postanesthetic care unit: six-month results of the Leipzig fast-track concept. Anesthesiology 2008;109:61-6. https://doi.org/10.1097/ALN.0b013e31817881b3.
- Esteban F, Cerdan FJ, Garcia-Alonso M, Sanz-Lopez R, Arroyo A, Ramirez JM, et al. A multicentre comparison of a fast track or conventional postoperative protocol following laparoscopic or open elective surgery for colorectal cancer surgery. Colorectal Dis 2014;16:134-40. https://doi.org/10.1111/codi.12472.
- Feo CV, Lanzara S, Sortini D, Ragazzi R, De Pinto M, Pansini GC, et al. Fast track postoperative management after elective colorectal surgery: a controlled trail. Am Surg 2009;75:1247-51.
- Galbraith JG, Fenelon C, Gibbons J, Kelly GA, Bennett D. Enhanced recovery in lower limb arthroplasty in the Irish setting. Ir J Med Sci 2017;186:687-91. https://doi.org/10.1007/s11845-017-1571-6.
- Garfinkle R, Boutros M, Ghitulescu G, Vasilevsky CA, Charlebois P, Liberman S, et al. Clinical and economic impact of an enhanced recovery pathway for open and laparoscopic rectal surgery. J Laparoendosc Adv Surg Tech A 2018;28:811-18. https://doi.org/10.1089/lap.2017.0677.
- Geltzeiler CB, Rotramel A, Wilson C, Deng L, Whiteford MH, Frankhouse J. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg 2014;149:955-61. https://doi.org/10.1001/jamasurg.2014.675.
- Glassou EN, Pedersen AB, Hansen TB. Risk of re-admission, reoperation, and mortality within 90 days of total hip and knee arthroplasty in fast-track departments in Denmark from 2005 to 2011. Acta Orthop 2014;85:493-500. https://doi.org/10.3109/17453674.2014.942586.
- Gouvas N, Gogos-Pappas G, Tsimogiannis K, Tsimoyiannis E, Dervenis C, Xynos E. Implementation of fast-track protocols in open and laparoscopic sphincter-preserving rectal cancer surgery: a multicenter, comparative, prospective, non-randomized study. Dig Surg 2012;29:301-9. https://doi.org/10.1159/000342554.
- Gwynne-Jones DP, Martin G, Crane C. Enhanced recovery after surgery for hip and knee replacements. Orthop Nurs 2017;36:203-10. https://doi.org/10.1097/NOR.0000000000000351.
- Hansen TB, Bredtoft HK, Larsen K. Preoperative physical optimization in fast-track hip and knee arthroplasty. Dan Med J 2012;59.
- Healy WL, Iorio R, Ko J, Appleby D, Lemos DW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am 2002;84:348-53. https://doi.org/10.2106/00004623-200203000-00003.
- Hennon MW, Kothari A, Maloney JD, Weigel T. Implementation of an acuity adaptable patient care unit is associated with improved outcomes after major pulmonary resections. J Surg Res 2011;170:e17-21. https://doi.org/10.1016/j.jss.2011.04.029.
- Hjort Jakobsen D, Sonne E, Basse L, Bisgaard T, Kehlet H. Convalescence after colonic resection with fast-track versus conventional care. Scand J Surg 2004;93:24-8. https://doi.org/10.1177/145749690409300105.
- Ho DM, Huo MH. Are critical pathways and implant standardization programs effective in reducing costs in total knee replacement operations?. J Am Coll Surg 2007;205:97-100. https://doi.org/10.1016/j.jamcollsurg.2007.03.009.
- Huibers CJ, de Roos MA, Ong KH. The effect of the introduction of the ERAS protocol in laparoscopic total mesorectal excision for rectal cancer. Int J Colorectal Dis 2012;27:751-7. https://doi.org/10.1007/s00384-011-1385-3.
- Indrakusuma R, Dunker MS, Peetoom JJ, Schreurs WH. Evaluation of preoperative geriatric assessment of elderly patients with colorectal carcinoma. A retrospective study. Eur J Surg Oncol 2015;41:21-7. https://doi.org/10.1016/j.ejso.2014.09.005.
- Jakobsen DH, Sonne E, Andreasen J, Kehlet H. Convalescence after colonic surgery with fast-track vs conventional care. Colorectal Dis 2006;8:683-7. https://doi.org/10.1111/j.1463-1318.2006.00995.x.
- Joliat GR, Labgaa I, Hübner M, Blanc C, Griesser AC, Schäfer M, et al. Cost-benefit analysis of the Implementation of an enhanced recovery program in liver surgery. World J Surg 2016;40:2441-50. https://doi.org/10.1007/s00268-016-3582-2.
- Joliat GR, Labgaa I, Petermann D, Hübner M, Griesser AC, Demartines N, et al. Cost–benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br J Surg 2015;102:1676-83. https://doi.org/10.1002/bjs.9957.
- Kagedan DJ, Devitt KS, Tremblay St-Germain A, Ramjaun A, Cleary SP, Wei AC. The economics of recovery after pancreatic surgery: detailed cost minimization analysis of an enhanced recovery program. HPB 2017;19:1026-33. https://doi.org/10.1016/j.hpb.2017.07.013.
- Kaibori M, Matsui K, Ishizaki M, Iida H, Yoshii K, Asano H, et al. Effects of implementing an ‘enhanced recovery after surgery’ program on patients undergoing resection of hepatocellular carcinoma. Surg Today 2017;47:42-51. https://doi.org/10.1007/s00595-016-1344-2.
- Keane C, Savage S, McFarlane K, Seigne R, Robertson G, Eglinton T. Enhanced recovery after surgery versus conventional care in colonic and rectal surgery. ANZ J Surg 2012;82:697-703. https://doi.org/10.1111/j.1445-2197.2012.06139.x.
- Kennedy EP, Rosato EL, Sauter PK, Rosenberg LM, Doria C, Marino IR, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution – the first step in multidisciplinary team building. J Am Coll Surg 2007;204:917-23. https://doi.org/10.1016/j.jamcollsurg.2007.01.057.
- Kim HE, Kim YH, Song KB, Chung YS, Hwang S, Lee YJ, et al. Impact of critical pathway implementation on hospital stay and costs in patients undergoing pancreaticoduodenectomy. Korean J Hepatobiliary Pancreat Surg 2014;18:14-20. https://doi.org/10.14701/kjhbps.2014.18.1.14.
- Kobayashi S, Ooshima R, Koizumi S, Katayama M, Sakurai J, Watanabe T, et al. Perioperative care with fast-track management in patients undergoing pancreaticoduodenectomy. World J Surg 2014;38:2430-7. https://doi.org/10.1007/s00268-014-2548-5.
- Krummenauer F, Guenther KP, Kirschner S. Cost-effectiveness of total knee arthroplasty from a health care providers’ perspective before and after introduction of an interdisciplinary clinical pathway – is investment always improvement?. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-338.
- Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop 2008;79:624-30. https://doi.org/10.1080/17453670810016632.
- Larsen K, Hvass KE, Hansen TB, Thomsen PB, Søballe K. Effectiveness of accelerated perioperative care and rehabilitation intervention compared to current intervention after hip and knee arthroplasty. A before-after trial of 247 patients with a 3-month follow-up. BMC Musculoskelet Disord 2008;9. https://doi.org/10.1186/1471-2474-9-59.
- Lee L, Li C, Robert N, Latimer E, Carli F, Mulder DS, et al. Economic impact of an enhanced recovery pathway for oesophagectomy. Br J Surg 2013;100:1326-34. https://doi.org/10.1002/bjs.9224.
- Lee L, Mata J, Ghitulescu GA, Boutros M, Charlebois P, Stein B, et al. Cost-effectiveness of enhanced recovery versus conventional perioperative management for colorectal surgery. Ann Surg 2015;262:1026-33. https://doi.org/10.1097/SLA.0000000000001019.
- Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013;27:1072-82. https://doi.org/10.1007/s00464-012-2560-5.
- Li C, Ferri LE, Mulder DS, Ncuti A, Neville A, Lee L, et al. An enhanced recovery pathway decreases duration of stay after esophagectomy. Surgery 2012;152:606-14. https://doi.org/10.1016/j.surg.2012.07.021.
- Lin PC, Hung SH, Wu HF, Hsu HC, Chu CY, Su SJ. The effects of a care map for total knee replacement patients. J Clin Nurs 2011;20:3119-27. https://doi.org/10.1111/j.1365-2702.2011.03804.x.
- Lin YK, Su JY, Lin GT, Tien YC, Chien SS, Lin CJ, et al. Impact of a clinical pathway for total knee arthroplasty. Kaohsiung J Med Sci 2002;18:134-40.
- Loftus T, Agee C, Jaffe R, Tao J, Jacofsky DJ. A simplified pathway for total knee arthroplasty improves outcomes. J Knee Surg 2014;27:221-8. https://doi.org/10.1055/s-0033-1360657.
- Madani A, Fiore JF, Wang Y, Bejjani J, Sivakumaran L, Mata J, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-908. https://doi.org/10.1016/j.surg.2015.04.046.
- Marcantuono R, Gutsche J, Burke-Julien M, Anwaruddin S, Augoustides JG, Jones D, et al. Rationale, development, implementation, and initial results of a fast track protocol for transfemoral transcatheter aortic valve replacement (TAVR). Catheter Cardiovasc Interv 2015;85:648-54. https://doi.org/10.1002/ccd.25749.
- Martin TD, Lorenz T, Ferraro J, Chagin K, Lampman RM, Emery KL, et al. Newly implemented enhanced recovery pathway positively impacts hospital length of stay. Surg Endosc 2016;30:4019-28. https://doi.org/10.1007/s00464-015-4714-8.
- Maruyama R, Miyake T, Kojo M, Aoki Y, Suemitsu R, Okamoto T, et al. Establishment of a clinical pathway as an effective tool to reduce hospitalization and charges after video-assisted thoracoscopic pulmonary resection. Jpn J Thorac Cardiovasc Surg 2006;54:387-90. https://doi.org/10.1007/s11748-006-0014-5.
- Marx C, Rasmussen T, Jakobsen DH, Ottosen C, Lundvall L, Ottesen B, et al. The effect of accelerated rehabilitation on recovery after surgery for ovarian malignancy. Acta Obstet Gynecol Scand 2006;85:488-92. https://doi.org/10.1080/00016340500408325.
- Melbert RB, Kimmins MH, Isler JT, Billingham RP, Lawton D, Salvadalena G, et al. Use of a critical pathway for colon resections. J Gastrointest Surg 2002;6:745-52. https://doi.org/10.1016/S1091-255X(02)00038-0.
- Mohn AC, Bernardshaw SV, Ristesund SM, Hovde Hansen PE, Røkke O. Enhanced recovery after colorectal surgery. Results from a prospective observational two-centre study. Scand J Surg 2009;98:155-9. https://doi.org/10.1177/145749690909800305.
- Moon MC, Abdoh A, Hamilton GA, Lindsay WG, Duke PC, Pascoe EA, et al. Safety and efficacy of fast track in patients undergoing coronary artery bypass surgery. J Card Surg 2001;16:319-26. https://doi.org/10.1111/j.1540-8191.2001.tb00528.x.
- Morales Soriano R, Esteve Pérez N, Tejada Gavela S, Cuadrado García Á, Rodríguez Pino JC, Morón Canis JM, et al. Outcomes of an enhanced recovery after surgery programme for pancreaticoduodenectomy. Cir Esp 2015;93:509-15. https://doi.org/10.1016/j.ciresp.2015.04.009.
- Nabhani J, Ahmadi H, Schuckman AK, Cai J, Miranda G, Djaladat H, et al. Cost analysis of the enhanced recovery after surgery protocol in patients undergoing radical cystectomy for bladder cancer. Eur Urol Focus 2016;2:92-6. https://doi.org/10.1016/j.euf.2015.06.009.
- Nagata Y, Masuda A, Suzuki Y. Impact of a clinical pathway in cases of transurethral resection of the prostate. Tokai J Exp Clin Med 2007;32:54-8.
- Nelson G, Kiyang LN, Chuck A, Thanh NX, Gramlich LM. Cost impact analysis of enhanced recovery after surgery program implementation in Alberta colon cancer patients. Curr Oncol 2016;23:e221-7. https://doi.org/10.3747/co.23.2980.
- Nelson G, Kiyang LN, Crumley ET, Chuck A, Nguyen T, Faris P, et al. Implementation of Enhanced Recovery After Surgery (ERAS) across a provincial healthcare system: the ERAS Alberta Colorectal Surgery Experience. World J Surg 2016;40:1092-103. https://doi.org/10.1007/s00268-016-3472-7.
- Nikfarjam M, Weinberg L, Low N, Fink MA, Muralidharan V, Houli N, et al. A fast track recovery program significantly reduces hospital length of stay following uncomplicated pancreaticoduodenectomy. JOP 2013;14:63-70. https://doi.org/10.6092/1590–8577/1223.
- Nussbaum DP, Penne K, Stinnett SS, Speicher PJ, Cocieru A, Blazer DG, et al. A standardized care plan is associated with shorter hospital length of stay in patients undergoing pancreaticoduodenectomy. J Surg Res 2015;193:237-45. https://doi.org/10.1016/j.jss.2014.06.036.
- Nygren J, Hausel J, Kehlet H, Revhaug A, Lassen K, Dejong C, et al. A comparison in five European centres of case mix, clinical management and outcomes following either conventional or fast-track perioperative care in colorectal surgery. Clin Nutr 2005;24:455-61. https://doi.org/10.1016/j.clnu.2005.02.003.
- Nygren J, Soop M, Thorell A, Hausel J, Ljungqvist O. ERAS Group . An enhanced-recovery protocol improves outcome after colorectal resection already during the first year: a single-centre experience in 168 consecutive patients. Dis Colon Rectum 2009;52:978-85. https://doi.org/10.1007/DCR.0b013e31819f1416.
- Okamura K, Nojiri Y, Tanaka Y, Nagae H, Arai Y, Matsuda T, et al. Changes in perioperative management of radical prostatectomy using clinical pathways according to a standardized care plan: a multi-institutional study. Int J Urol 2013;20:337-43. https://doi.org/10.1111/j.1442-2042.2012.03191.x.
- Oldmeadow LB, McBurney H, Robertson VJ, Kimmel L, Elliott B. Targeted postoperative care improves discharge outcome after hip or knee arthroplasty. Arch Phys Med Rehabil 2004;85:1424-7. https://doi.org/10.1016/j.apmr.2003.12.028.
- Olsson LE, Hansson E, Ekman I. Evaluation of person-centred care after hip replacement-a controlled before-and-after study on the effects of fear of movement and self-efficacy compared to standard care. BMC Nurs 2016;15. https://doi.org/10.1186/s12912-016-0173-3.
- Olsson LE, Karlsson J, Berg U, Kärrholm J, Hansson E. Person-centred care compared with standardized care for patients undergoing total hip arthroplasty – a quasi-experimental study. J Orthop Surg Res 2014;9. https://doi.org/10.1186/s13018-014-0095-2.
- Opasich C, Patrignani A, Mazza A, Gualco A, Cobelli F, Pinna GD. An elderly-centered, personalized, physiotherapy program early after cardiac surgery. Eur J Cardiovasc Prev Rehabil 2010;17:582-7. https://doi.org/10.1097/HJR.0b013e3283394977.
- Ota H, Ikenaga M, Hasegawa J, Murata K, Miyake Y, Mizushima T, et al. Safety and efficacy of an ‘enhanced recovery after surgery’ protocol for patients undergoing colon cancer surgery: a multi-institutional controlled study. Surg Today 2017;47:668-75. https://doi.org/10.1007/s00595-016-1423-4.
- Ovaere S, Boscart I, Parmentier I, Steelant PJ, Gabriel T, Allewaert J, et al. The effectiveness of a clinical pathway in liver surgery: a case–control study. J Gastrointest Surg 2018;22:684-94. https://doi.org/10.1007/s11605-017-3653-1.
- Paci P, Madani A, Lee L, Mata J, Mulder DS, Spicer J, et al. Economic impact of an enhanced recovery pathway for lung resection. Ann Thorac Surg 2017;104:950-7. https://doi.org/10.1016/j.athoracsur.2017.05.085.
- Pape B, Thiessen PS, Jakobsen F, Hansen TB. Interprofessional collaboration may pay off: introducing a collaborative approach in an orthopaedic ward. J Interprof Care 2013;27:496-500. https://doi.org/10.3109/13561820.2013.808611.
- Pearson S, Moraw I, Maddern GJ. Clinical pathway management of total knee arthroplasty: a retrospective comparative study. Aust N Z J Surg 2000;70:351-4. https://doi.org/10.1046/j.1440-1622.2000.01819.x.
- Pearson SD, Kleefield SF, Soukop JR, Cook EF, Lee TH. Critical pathways intervention to reduce length of hospital stay. Am J Med 2001;110:175-80. https://doi.org/10.1016/S0002-9343(00)00705-1.
- Pecorelli N, Capretti G, Balzano G, Castoldi R, Maspero M, Beretta L, et al. Enhanced recovery pathway in patients undergoing distal pancreatectomy: a case-matched study. HPB 2017;19:270-8. https://doi.org/10.1016/j.hpb.2016.10.014.
- Pędziwiatr M, Wierdak M, Nowakowski M, Pisarska M, Stanek M, Kisielewski M, et al. Cost minimization analysis of laparoscopic surgery for colorectal cancer within the enhanced recovery after surgery (ERAS) protocol: a single-centre, case-matched study. Wideochir Inne Tech Maloinwazyjne 2016;11:14-21. https://doi.org/10.5114/wiitm.2016.58617.
- Pellegrino L, Lois F, Remue C, Forget P, Crispin B, Leonard D, et al. Insights into fast-track colon surgery: a plea for a tailored program. Surg Endosc 2013;27:1178-85. https://doi.org/10.1007/s00464-012-2572-1.
- Persson B, Carringer M, Andrén O, Andersson SO, Carlsson J, Ljungqvist O. Initial experiences with the enhanced recovery after surgery (ERAS) protocol in open radical cystectomy. Scand J Urol 2015;49:302-7. https://doi.org/10.3109/21681805.2015.1004641.
- Poon JT, Fan JK, Lo OS, Law WL. Enhanced recovery program in laparoscopic colectomy for cancer. Int J Colorectal Dis 2011;26:71-7. https://doi.org/10.1007/s00384-010-1059-6.
- Raphael M, Jaeger M, van Vlymen J. Easily adoptable total joint arthroplasty program allows discharge home in two days. Can J Anaesth 2011;58:902-10. https://doi.org/10.1007/s12630-011-9565-8.
- Raue W, Haase O, Junghans T, Scharfenberg M, Müller JM, Schwenk W. ‘Fast-track’ multimodal rehabilitation program improves outcome after laparoscopic sigmoidectomy: a controlled prospective evaluation. Surg Endosc 2004;18:1463-8. https://doi.org/10.1007/s00464-003-9238-y.
- Renkawitz T, Rieder T, Handel M, Koller M, Drescher J, Bonnlaender G, et al. Comparison of two accelerated clinical pathways – after total knee replacement how fast can we really go?. Clin Rehabil 2010;24:230-9. https://doi.org/10.1177/0269215509353267.
- Rivas JG, Gregorio SAY, Ledo JC, Orejon RU, Doslada P, Sebastian J, et al. Early recovery protocol in patients undergoing laparoscopic radical cystectomy. Urol Sci 2017;28:2-5. https://doi.org/10.1016/j.urols.2015.01.006.
- Roulin D, Donadini A, Gander S, Griesser AC, Blanc C, Hübner M, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg 2013;100:1108-14. https://doi.org/10.1002/bjs.9184.
- Saar M, Ohlmann CH, Siemer S, Lehmann J, Becker F, Stöckle M, et al. Fast-track rehabilitation after robot-assisted laparoscopic cystectomy accelerates postoperative recovery. BJU Int 2013;112:E99-106. https://doi.org/10.1111/j.1464-410X.2012.11473.x.
- Salati M, Brunelli A, Xiumè F, Refai M, Pompili C, Sabbatini A. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardiothorac Surg 2012;41:1083-7. https://doi.org/10.1093/ejcts/ezr171.
- Salvans S, Gil-Egea MJ, Pera M, Lorente L, Cots F, Pascual M, et al. Multimodal rehabilitation program in elective colorectal surgery: Impact on hospital costs. Cir Esp 2013;91:638-44. https://doi.org/10.1016/j.ciresp.2013.01.010.
- Sammour T, Zargar-Shoshtari K, Bhat A, Kahokehr A, Hill AG. A programme of Enhanced Recovery After Surgery (ERAS) is a cost-effective intervention in elective colonic surgery. N Z Med J 2010;123:61-70.
- Savikko J, Ilmakunnas M, Mäkisalo H, Nordin A, Isoniemi H. Enhanced recovery protocol after liver resection. Br J Surg 2015;102:1526-32. https://doi.org/10.1002/bjs.9912.
- Schwarzbach MH, Ronellenfitsch U, Wang Q, Rössner ED, Denz C, Post S, et al. Effects of a clinical pathway for video-assisted thoracoscopic surgery (VATS) on quality and cost of care. Langenbecks Arch Surg 2010;395:333-40. https://doi.org/10.1007/s00423-009-0507-7.
- Shargall Y, Hanna WC, Schneider L, Schieman C, Finley CJ, Tran A, et al. The Integrated Comprehensive Care Program: a novel home care initiative after major thoracic surgery. Semin Thorac Cardiovasc Surg 2016;28:574-82. https://doi.org/10.1053/j.semtcvs.2015.12.003.
- So JB, Lim ZL, Lin HA, Ti TK. Reduction of hospital stay and cost after the implementation of a clinical pathway for radical gastrectomy for gastric cancer. Gastric Cancer 2008;11:81-5. https://doi.org/10.1007/s10120-008-0458-7.
- Stephen AE, Berger DL. Shortened length of stay and hospital cost reduction with implementation of an accelerated clinical care pathway after elective colon resection. Surgery 2003;133:277-82. https://doi.org/10.1067/msy.2003.19.
- Stowers MD, Manuopangai L, Hill AG, Gray JR, Coleman B, Munro JT. Enhanced recovery after surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg 2016;86:475-9. https://doi.org/10.1111/ans.13538.
- Sugi M, Matsuda T, Yoshida T, Taniguchi H, Mishima T, Yanishi M, et al. Introduction of an enhanced recovery after surgery protocol for robot-assisted laparoscopic radical prostatectomy. Urol Int 2017;99:194-200. https://doi.org/10.1159/000457805.
- Talatzko S, Deprey SM, Hager N. Comprehensive facility-wide approach improves outcomes after lower extremity surgical arthroplasty in an acute care hospital. J Healthc Qual 2014;36:17-2. https://doi.org/10.1111/jhq.12000.
- Tarin T, Feifer A, Kimm S, Chen L, Sjoberg D, Coleman J, et al. Impact of a common clinical pathway on length of hospital stay in patients undergoing open and minimally invasive kidney surgery. J Urol 2014;191:1225-30. https://doi.org/10.1016/j.juro.2013.11.030.
- Teeuwen PH, Bleichrodt RP, de Jong PJ, van Goor H, Bremers AJ. Enhanced recovery after surgery versus conventional perioperative care in rectal surgery. Dis Colon Rectum 2011;54:833-9. https://doi.org/10.1007/DCR.0b013e318216067d.
- Thanh NX, Chuck AW, Wasylak T, Lawrence J, Faris P, Ljungqvist O, et al. An economic evaluation of the Enhanced Recovery After Surgery (ERAS) multisite implementation program for colorectal surgery in Alberta. Can J Surg 2016;59:415-21. https://doi.org/10.1503/cjs.006716.
- Thomas K. Clinical pathway for hip and knee arthroplasty. Physiotherapy 2003;89:603-9. https://doi.org/10.1016/S0031-9406(05)60059-4.
- van Dam RM, Hendry PO, Coolsen MM, Bemelmans MH, Lassen K, Revhaug A, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg 2008;95:969-75. https://doi.org/10.1002/bjs.6227.
- van der Kolk M, van den Boogaard M, Becking-Verhaar F, Custers H, van der Hoeven H, Pickkers P, et al. Implementation and evaluation of a clinical pathway for pancreaticoduodenectomy procedures: a prospective cohort study. J Gastrointest Surg 2017;21:1428-41. https://doi.org/10.1007/s11605-017-3459-1.
- Vanhaecht K, Sermeus W, Tuerlinckx G, Witters I, Vandenneucker H, Bellemans J. Development of a clinical pathway for total knee arthroplasty and the effect on length of stay and in-hospital functional outcome. Acta Orthop Belg 2005;71:439-44.
- Vanounou T, Pratt W, Fischer JE, Vollmer CM, Callery MP. Deviation-based cost modeling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg 2007;204:570-9. https://doi.org/10.1016/j.jamcollsurg.2007.01.025.
- Vignali A, Elmore U, Cossu A, Lemma M, Calì B, de Nardi P, et al. Enhanced recovery after surgery (ERAS) pathway vs traditional care in laparoscopic rectal resection: a single-center experience. Tech Coloproctol 2016;20:559-66. https://doi.org/10.1007/s10151-016-1497-4.
- Walter FL, Bass N, Bock G, Markel DC. Success of clinical pathways for total joint arthroplasty in a community hospital. Clin Orthop Relat Res 2007;457:133-7. https://doi.org/10.1097/01.blo.0000246567.88585.0a.
- Wichmann MW, Eben R, Angele MK, Brandenburg F, Goetz AE, Jauch KW. Fast-track rehabilitation in elective colorectal surgery patients: a prospective clinical and immunological single-centre study. ANZ J Surg 2007;77:502-7. https://doi.org/10.1111/j.1445-2197.2007.04138.x.
- Wilches C, Sulbarán JD, Fernández JE, Gisbert JM, Bausili JM, Pelfort X. Fast-track recovery technique applied to primary total hip and knee replacement surgery. Analysis of costs and complications. Rev Esp Cir Ortop Traumatol 2017;61:111-16. https://doi.org/10.1016/j.recote.2017.02.004.
- Williamsson C, Karlsson N, Sturesson C, Lindell G, Andersson R, Tingstedt B. Impact of a fast-track surgery programme for pancreaticoduodenectomy. Br J Surg 2015;102:1133-41. https://doi.org/10.1002/bjs.9856.
- Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, et al. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer 2012;15:34-41. https://doi.org/10.1007/s10120-011-0057-x.
- Yanatori M, Tomita S, Miura Y, Ueno Y. Feasibility of the fast-track recovery program after cardiac surgery in Japan. Gen Thorac Cardiovasc Surg 2007;55:445-9. https://doi.org/10.1007/s11748-007-0162-2.
- Yueh B, Weaver EM, Bradley EH, Krumholz HM, Heagerty P, Conley A, et al. A critical evaluation of critical pathways in head and neck cancer. Arch Otolaryngol Head Neck Surg 2003;129:89-95. https://doi.org/10.1001/archotol.129.1.89.
- Yui R, Satoi S, Toyokawa H, Yanagimoto H, Yamamoto T, Hirooka S, et al. Less morbidity after introduction of a new departmental policy for patients who undergo open distal pancreatectomy. J Hepatobiliary Pancreat Sci 2014;21:72-7. https://doi.org/10.1002/jhbp.4.
- Zargar-Shoshtari K, Connolly AB, Israel LH, Hill AG. Fast-track surgery may reduce complications following major colonic surgery. Dis Colon Rectum 2008;51:1633-40. https://doi.org/10.1007/s10350-008-9386-1.
- Zargar-Shoshtari K, Paddison JS, Booth RJ, Hill AG. A prospective study on the influence of a fast-track program on postoperative fatigue and functional recovery after major colonic surgery. J Surg Res 2009;154:330-5. https://doi.org/10.1016/j.jss.2008.06.023.
- Abu Hilal M, Di Fabio F, Badran A, Alsaati H, Clarke H, Fecher I, et al. Implementation of enhanced recovery programme after pancreatoduodenectomy: a single-centre UK pilot study. Pancreatology 2013;13:58-62. https://doi.org/10.1016/j.pan.2012.11.312.
- Anderson AD, McNaught CE, MacFie J, Tring I, Barker P, Mitchell CJ. Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg 2003;90:1497-504. https://doi.org/10.1002/bjs.4371.
- Arthur HM, Daniels C, McKelvie R, Hirsh J, Rush B. Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann Intern Med 2000;133:253-62. https://doi.org/10.7326/0003-4819-133-4-200008150-00007.
- Arumainayagam N, McGrath J, Jefferson KP, Gillatt DA. Introduction of an enhanced recovery protocol for radical cystectomy. BJU Int 2008;101:698-701. https://doi.org/10.1111/j.1464–410X.2007.07319.x.
- Barlow D, Masud S, Rhee SJ, Ganapathi M, Andrews G. The effect of the creation of a ring-fenced orthopaedic ward on length of stay for elective arthroplasty patients. Surgeon 2013;11:82-6. https://doi.org/10.1016/j.surge.2012.03.001.
- Borgwardt L, Zerahn B, Bliddal H, Christiansen C, Sylvest J, Borgwardt A. Similar clinical outcome after unicompartmental knee arthroplasty using a conventional or accelerated care program: a randomized, controlled study of 40 patients. Acta Orthop 2009;80:334-7. https://doi.org/10.3109/17453670903035559.
- Brunelli A, Thomas C, Dinesh P, Lumb A. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. https://doi.org/10.1016/j.jtcvs.2017.06.037.
- Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, et al. Randomised clinical trial of prehabilitation in colorectal surgery. Br J Surg 2010;97:1187-97. https://doi.org/10.1002/bjs.7102.
- Chen CC, Li HC, Liang JT, Lai IR, Purnomo JDT, Yang YT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017;152:827-34. https://doi.org/10.1001/jamasurg.2017.1083.
- Crowe J, Henderson J. Pre-arthroplasty rehabilitation is effective in reducing hospital stay. Can J Occup Ther 2003;70:88-96. https://doi.org/10.1177/000841740307000204.
- Dasari BV, Rahman R, Khan S, Bennett D, Hodson J, Isaac J, et al. Safety and feasibility of an enhanced recovery pathway after a liver resection: prospective cohort study. HPB 2015;17:700-6. https://doi.org/10.1111/hpb.12447.
- den Hertog A, Gliesche K, Timm J, Mühlbauer B, Zebrowski S. Pathway-controlled fast-track rehabilitation after total knee arthroplasty: a randomized prospective clinical study evaluating the recovery pattern, drug consumption, and length of stay. Arch Orthop Trauma Surg 2012;132:1153-63. https://doi.org/10.1007/s00402-012-1528-1.
- Dhruva Rao PK, Howells S, Haray PN. Does an enhanced recovery programme add value to laparoscopic colorectal resections?. Int J Colorectal Dis 2015;30:1473-7. https://doi.org/10.1007/s00384-015-2320-9.
- Dronkers JJ, Lamberts H, Reutelingsperger IM, Naber RH, Dronkers-Landman CM, Veldman A, et al. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin Rehabil 2010;24:614-22. https://doi.org/10.1177/0269215509358941.
- Dunne DF, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 2016;103:504-12. https://doi.org/10.1002/bjs.10096.
- Dwyer AJ, Tarassoli P, Thomas W, Porter P. Enhanced recovery program in total hip arthroplasty. Indian J Orthop 2012;46:407-12. https://doi.org/10.4103/0019-5413.98829.
- Ellis G, Spiers M, Coutts S, Fairburn P, McCracken L. Preoperative assessment in the elderly: evaluation of a new clinical service. Scott Med J 2012;57:212-16. https://doi.org/10.1258/smj.2012.012120.
- Fleming IO, Garratt C, Guha R, Desai J, Chaubey S, Wang Y, et al. Aggregation of marginal gains in cardiac surgery: feasibility of a perioperative care bundle for enhanced recovery in cardiac surgical patients. J Cardiothorac Vasc Anesth 2016;30:665-70. https://doi.org/10.1053/j.jvca.2016.01.017.
- Forsmo HM, Pfeffer F, Rasdal A, Østgaard G, Mohn AC, Körner H, et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis 2016;18:603-11. https://doi.org/10.1111/codi.13253.
- Furze G, Dumville JC, Miles JN, Irvine K, Thompson DR, Lewin RJ. ‘Prehabilitation’ prior to CABG surgery improves physical functioning and depression. Int J Cardiol 2009;132:51-8. https://doi.org/10.1016/j.ijcard.2008.06.001.
- García-Botello S, Cánovas de Lucas R, Tornero C, Escamilla B, Espí-Macías A, Esclapez-Valero P, et al. Implementation of a perioperative multimodal rehabilitation protocol in elective colorectal surgery. A prospective randomised controlled study. Cir Esp 2011;89:159-66. https://doi.org/10.1016/j.ciresp.2010.12.004.
- Gatenby PA, Shaw C, Hine C, Scholtes S, Koutra M, Andrew H, et al. Retrospective cohort study of an enhanced recovery programme in oesophageal and gastric cancer surgery. Ann R Coll Surg Engl 2015;97:502-7. https://doi.org/10.1308/003588415X14181254789880.
- Gatt M, Anderson AD, Reddy BS, Hayward-Sampson P, Tring IC, MacFie J. Randomized clinical trial of multimodal optimization of surgical care in patients undergoing major colonic resection. Br J Surg 2005;92:1354-62. https://doi.org/10.1002/bjs.5187.
- Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anaesthesiology 2014;121:937-47. https://doi.org/10.1097/ALN.0000000000000393.
- Goodman H, Parsons A, Davison J, Preedy M, Peters E, Shuldham C, et al. A randomised controlled trial to evaluate a nurse-led programme of support and lifestyle management for patients awaiting cardiac surgery ‘Fit for surgery: Fit for life’ study. Eur J Cardiovasc Nurs 2008;7:189-95. https://doi.org/10.1016/j.ejcnurse.2007.11.001.
- Gordon D, Malhas A, Goubran A, Subramanian P, Messer C, Houlihan-Burne D. Implementing the Rapid Recovery Program in primary hip and knee arthroplasty in a UK state run hospital. Eur J Orthop Surg Tr 2011;21:151-8. https://doi.org/10.1007/s00590-010-0690-9.
- Gralla O, Haas F, Knoll N, Hadzidiakos D, Tullmann M, Romer A, et al. Fast-track surgery in laparoscopic radical prostatectomy: basic principles. World J Urol 2007;25:185-91. https://doi.org/10.1007/s00345-006-0139-2.
- Harari D, Hopper A, Dhesi J, Babic-Illman G, Lockwood L, Martin F. Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing 2007;36:190-6. https://doi.org/10.1093/ageing/afl163.
- Hempenius L, Slaets JP, van Asselt D, de Bock TH, Wiggers T, van Leeuwen BL. Long term outcomes of a geriatric liaison intervention in frail elderly cancer patients. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0143364.
- Hempenius L, Slaets JP, van Asselt D, de Bock GH, Wiggers T, van Leeuwen BL. Outcomes of a geriatric liaison intervention to prevent the development of postoperative delirium in frail elderly cancer patients: report on a multicentre, randomized, controlled trial. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0064834.
- Hoogeboom TJ, Dronkers JJ, van den Ende CH, Oosting E, van Meeteren NL. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: a randomized pilot trial. Clin Rehabil 2010;24:901-10. https://doi.org/10.1177/0269215510371427.
- Huang SW, Chen PH, Chou YH. Effects of a preoperative simplified home rehabilitation education program on length of stay of total knee arthroplasty patients. Orthop Traumatol Surg Res 2012;98:259-64. https://doi.org/10.1016/j.otsr.2011.12.004.
- Huddleston JM, Long KH, Naessens JM, Vanness D, Larson D, Trousdale R, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med 2004;141:28-3. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
- Hunt GR, Crealey G, Murthy BV, Hall GM, Constantine P, O’Brien S, et al. The consequences of early discharge after hip arthroplasty for patient outcomes and health care costs: comparison of three centres with differing durations of stay. Clin Rehabil 2009;23:1067-77. https://doi.org/10.1177/0269215509339000.
- Jensen BT, Petersen AK, Jensen JB, Laustsen S, Borre M. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: a prospective randomized controlled trial. Scand J Urol 2015;49:133-41. https://doi.org/10.3109/21681805.2014.967810.
- Jones C, Kelliher L, Dickinson M, Riga A, Worthington T, Scott MJ, et al. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg 2013;100:1015-24. https://doi.org/10.1002/bjs.9165.
- Kapritsou M, Papathanassoglou ED, Bozas E, Korkolis DP, Konstantinou EA, Kaklamanos I, et al. Comparative evaluation of pain, stress, neuropeptide Y, ACTH, and cortisol levels between a conventional postoperative care protocol and a fast-track recovery program in patients undergoing major abdominal surgery. Biol Res Nurs 2017;19:180-9. https://doi.org/10.1177/1099800416682617.
- Karran A, Wheat J, Chan D, Blake P, Barlow R, Lewis WG. Propensity score analysis of an enhanced recovery programme in upper gastrointestinal cancer surgery. World J Surg 2016;40:1645-54. https://doi.org/10.1007/s00268-016-3473-6.
- Khan SA, Ullah S, Ahmed J, Wilson TR, McNaught C, Hartley J, et al. Influence of enhanced recovery after surgery pathways and laparoscopic surgery on health-related quality of life. Colorectal Dis 2013;15:900-7. https://doi.org/10.1111/codi.12191.
- Khan SK, Malviya A, Muller SD, Carluke I, Partington PF, Emmerson KP, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6000 consecutive procedures. Acta Orthop 2014;85:26-31. https://doi.org/10.3109/17453674.2013.874925.
- Khoo CK, Vickery CJ, Forsyth N, Vinall NS, Eyre-Brook IA. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg 2007;245:867-72. https://doi.org/10.1097/01.sla.0000259219.08209.36.
- King PM, Blazeby JM, Ewings P, Longman RJ, Kipling RM, Franks PJ, et al. The influence of an enhanced recovery programme on clinical outcomes, costs and quality of life after surgery for colorectal cancer. Colorectal Dis 2006;8:506-13. https://doi.org/10.1111/j.1463-1318.2006.00963.x.
- Larsen K. Efficacy, Effectiveness, and Efficiency of Accelerated Perioperative Care and Rehabilitation Intervention After Hip and Knee Arthroplasty 2008.
- Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop 2008;79:149-59. https://doi.org/10.1080/17453670710014923.
- Lee TG, Kang SB, Kim DW, Hong S, Heo SC, Park KJ. Comparison of early mobilization and diet rehabilitation program with conventional care after laparoscopic colon surgery: a prospective randomized controlled trial. Dis Colon Rectum 2011;54:21-8. https://doi.org/10.1007/DCR.0b013e3181fcdb3e.
- Lidder P, Thomas S, Fleming S, Hosie K, Shaw S, Lewis S. A randomized placebo controlled trial of preoperative carbohydrate drinks and early postoperative nutritional supplement drinks in colorectal surgery. Colorectal Dis 2013;15:737-45. https://doi.org/10.1111/codi.12130.
- Maempel JF, Clement ND, Ballantyne JA, Dunstan E. Enhanced recovery programmes after total hip arthroplasty can result in reduced length of hospital stay without compromising functional outcome. Bone Joint J 2016;98–B:475-82. https://doi.org/10.1302/0301-620X.98B4.36243.
- Maempel JF, Walmsley PJ. Enhanced recovery programmes can reduce length of stay after total knee replacement without sacrificing functional outcome at one year. Ann R Coll Surg Engl 2015;97:563-7. https://doi.org/10.1308/rcsann.2015.0016.
- Maggiori L, Rullier E, Lefevre JH, Régimbeau JM, Berdah S, Karoui M, et al. Does a combination of laparoscopic approach and full fast track multimodal management decrease postoperative morbidity?: A multicenter randomized controlled trial. Ann Surg 2017;266:729-37. https://doi.org/10.1097/SLA.0000000000002394.
- Magheli A, Knoll N, Lein M, Hinz S, Kempkensteffen C, Gralla O. Impact of fast-track postoperative care on intestinal function, pain, and length of hospital stay after laparoscopic radical prostatectomy. J Endourol 2011;25:1143-7. https://doi.org/10.1089/end.2011.0020.
- Malviya A, Martin K, Harper I, Muller SD, Emmerson KP, Partington PF, et al. Enhanced recovery program for hip and knee replacement reduces death rate. Acta Orthop 2011;82:577-81. https://doi.org/10.3109/17453674.2011.618911.
- Mari G, Costanzi A, Crippa J, Falbo R, Miranda A, Rossi M, et al. Surgical stress reduction in elderly patients undergoing elective colorectal laparoscopic surgery within an ERAS protocol. Chirurgia 2016;111:476-80. https://doi.org/10.21614/chirurgia.111.6.476.
- Mari GM, Costanzi A, Maggioni D, Origi M, Ferrari GC, De Martini P, et al. Fast-track versus standard care in laparoscopic high anterior resection: a prospective randomised-controlled trial. Surg Laparosc Endosc Percutan Tech 2014;24:118-21. https://doi.org/10.1097/SLE.0b013e3182a50e3a.
- McGregor AH, Rylands H, Owen A, Doré CJ, Hughes SP. Does preoperative hip rehabilitation advice improve recovery and patient satisfaction?. J Arthroplasty 2004;19:464-8. https://doi.org/10.1016/j.arth.2003.12.074.
- Mertes SC, Raut S, Khanduja V. Integrated care pathways in lower-limb arthroplasty: are they effective in reducing length of hospital stay?. Int Orthop 2013;37:1157-63. https://doi.org/10.1007/s00264-013-1829-1.
- Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder-Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J Surg 2009;33:577-85. https://doi.org/10.1007/s00268-008-9892-2.
- Muehling BM, Halter G, Lang G, Schelzig H, Steffen P, Wagner F, et al. Prospective randomized controlled trial to evaluate ‘fast-track’ elective open infrarenal aneurysm repair. Langenbecks Arch Surg 2008;393:281-7. https://doi.org/10.1007/s00423-008-0284-8.
- Muehling BM, Halter GL, Schelzig H, Meierhenrich R, Steffen P, Sunder-Plassmann L, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. https://doi.org/10.1016/j.ejcts.2008.04.009.
- Muehling BM, Ortlieb L, Oberhuber A, Orend KH. Fast track management reduces the systemic inflammatory response and organ failure following elective infrarenal aortic aneurysm repair. Interact Cardiovasc Thorac Surg 2011;12:784-8. https://doi.org/10.1510/icvts.2010.262337.
- Mukhtar S, Ayres BE, Issa R, Swinn MJ, Perry MJ. Challenging boundaries: an enhanced recovery programme for radical cystectomy. Ann R Coll Surg Engl 2013;95:200-6. https://doi.org/10.1308/003588413X13511609957579.
- Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N. Zurich Fast Track Study Group . A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology 2009;136:842-7. https://doi.org/10.1053/j.gastro.2008.10.030.
- Pappalardo G, Coiro S, De Lucia F, Giannella A, Ruffolo F, Frattaroli FM. Open sphincter-preserving surgery of extraperitoneal rectal cancer without primary stoma and fast track protocol. G Chir 2016;37:257-61. https://doi.org/10.11138/gchir/2016.37.6.257.
- Pengas IP, Khan WS, Bennett CA, Rankin KS. Impact of weekend physiotherapy service on the cost-effectiveness of elective orthopaedic hip and knee arthroplasty. Open Orthop J 2015;9:515-19. https://doi.org/10.2174/1874325001509010515.
- Pour AE, Parvizi J, Sharkey PF, Hozack WJ, Rothman RH. Minimally invasive hip arthroplasty: what role does patient preconditioning play?. J Bone Joint Surg Am 2007;89:1920-7. https://doi.org/10.2106/00004623-200709000-00005.
- Probst S, Cech C, Haentschel D, Scholz M, Ender J. A specialized post anaesthetic care unit improves fast-track management in cardiac surgery: a prospective randomized trial. Crit Care 2014;18. https://doi.org/10.1186/s13054-014-0468-2.
- Reilly KA, Beard DJ, Barker KL, Dodd CA, Price AJ, Murray DW. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty – a randomised controlled trial. Knee 2005;12:351-7. https://doi.org/10.1016/j.knee.2005.01.002.
- Richardson J, Di Fabio F, Clarke H, Bajalan M, Davids J, Abu Hilal M. Implementation of enhanced recovery programme for laparoscopic distal pancreatectomy: feasibility, safety and cost analysis. Pancreatology 2015;15:185-90. https://doi.org/10.1016/j.pan.2015.01.002.
- Rosenfeldt F, Braun L, Spitzer O, Bradley S, Shepherd J, Bailey M, et al. Physical conditioning and mental stress reduction – a randomised trial in patients undergoing cardiac surgery. BMC Complement Altern Med 2011;11. https://doi.org/10.1186/1472-6882-11-20.
- Salhiyyah K, Elsobky S, Raja S, Attia R, Brazier J, Cooper GJ. A clinical and economic evaluation of fast-track recovery after cardiac surgery. Heart Surg Forum 2011;14:E330-4. https://doi.org/10.1532/HSF98.20111029.
- Salmon P, Hunt GR, Murthy BV, O’Brien S, Beverland D, Lynch MC, et al. Patient evaluation of early discharge after hip arthroplasty: development of a measure and comparison of three centres with differing durations of stay. Clin Rehabil 2013;27:854-63. https://doi.org/10.1177/0269215513481686.
- Siggeirsdottir K, Olafsson O, Jonsson H, Iwarsson S, Gudnason V, Jonsson BY. Short hospital stay augmented with education and home-based rehabilitation improves function and quality of life after hip replacement: randomized study of 50 patients with 6 months of follow-up. Acta Orthop 2005;76:555-62. https://doi.org/10.1080/17453670510041565.
- Starks I, Wainwright TW, Lewis J, Lloyd J, Middleton RG. Older patients have the most to gain from orthopaedic enhanced recovery programmes. Age Ageing 2014;43:642-8. https://doi.org/10.1093/ageing/afu014.
- Sutcliffe RP, Hamoui M, Isaac J, Marudanayagam R, Mirza DF, Muiesan P, et al. Implementation of an enhanced recovery pathway after pancreaticoduodenectomy in patients with low drain fluid amylase. World J Surg 2015;39:2023-30. https://doi.org/10.1007/s00268–015-3051-3.
- Tanaka R, Lee SW, Kawai M, Tashiro K, Kawashima S, Kagota S, et al. Protocol for enhanced recovery after surgery improves short-term outcomes for patients with gastric cancer: a randomized clinical trial. Gastric Cancer 2017;20:861-71. https://doi.org/10.1007/s10120-016-0686–1.
- van Bree SH, van Bree S, Vlug MS, Vlug M, Bemelman WA, Bemelman W, et al. Faster recovery of gastrointestinal transit after laparoscopy and fast-track care in patients undergoing colonic surgery. Gastroenterology 2011;141:872-80.e1. https://doi.org/10.1053/j.gastro.2011.05.034.
- van der Peijl ID, Vliet Vlieland TP, Versteegh MI, Lok JJ, Munneke M, Dion RA. Exercise therapy after coronary artery bypass graft surgery: a randomized comparison of a high and low frequency exercise therapy program. Ann Thorac Surg 2004;77:1535-41. https://doi.org/10.1016/j.athoracsur.2003.10.091.
- Vesterby MS, Pedersen PU, Laursen M, Mikkelsen S, Larsen J, Søballe K, et al. Telemedicine support shortens length of stay after fast-track hip replacement. Acta Orthop 2017;88:41-7. https://doi.org/10.1080/17453674.2016.1256939.
- Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868-75. https://doi.org/10.1097/SLA.0b013e31821fd1ce.
- Williamson L, Wyatt MR, Yein K, Melton JT. Severe knee osteoarthritis: a randomized controlled trial of acupuncture, physiotherapy (supervised exercise) and standard management for patients awaiting knee replacement. Rheumatology 2007;46:1445-9. https://doi.org/10.1093/rheumatology/kem119.
- Partridge JS, Harari D, Martin FC, Dhesi JK. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia 2014;69:8-16. https://doi.org/10.1111/anae.12494.
- Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013;37:259-84. https://doi.org/10.1007/s00268-012-1772-0.
- Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013;37:285-30. https://doi.org/10.1007/s00268-012-1787-6.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CH, et al. Guidelines for perioperative care for liver surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 2016;40:2425-40. https://doi.org/10.1007/s00268-016-3700-1.
- Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:240-58. https://doi.org/10.1007/s00268-012-1771-1.
- Feldheiser A, Aziz O, Baldini G, Cox BP, Fearon KC, Feldman LS, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 2016;60:289-334. https://doi.org/10.1111/aas.12651.
- Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg 2014;101:1209-29. https://doi.org/10.1002/bjs.9582.
- Scott MJ, Baldini G, Fearon KC, Feldheiser A, Feldman LS, Gan TJ, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand 2015;59:1212-31. https://doi.org/10.1111/aas.12601.
- Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(®)) society recommendations. Clin Nutr 2013;32:879-87. https://doi.org/10.1016/j.clnu.2013.09.014.
- Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am 2009;91:761-72. https://doi.org/10.2106/JBJS.G.01472.
- Sigurdsson E, Siggeirsdottir K, Jonsson H, Gudnason V, Matthiasson T, Jonsson BY. Early discharge and home intervention reduces unit costs after total hip replacement: results of a cost analysis in a randomized study. Int J Health Care Finance Econ 2008;8:181-92. https://doi.org/10.1007/s10754-008-9036-0.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009857.
- Stowers MD, Lemanu DP, Hill AG. Health economics in Enhanced Recovery After Surgery programs. Can J Anaesth 2015;62:219-30. https://doi.org/10.1007/s12630-014-0272-0.
- Jönsson B, Lindgren B. Five common fallacies in estimating the economic gains of early discharge. Soc Sci Med Med Econ 1980;14:27-33. https://doi.org/10.1016/0160-7995(80)90005-2.
Appendix 1 Literature search strategies
Bibliographic databases
Database: MEDLINE.
Host: Ovid.
Date parameters: 1946 to August week 5 2017.
Date searched: 12 September 2017.
Searcher: SB.
Hits: 4227.
Search strategy
-
((older or frail or elderly) adj2 (person* or people or patient* or population* or adult*)).tw.
-
geriatric*.tw.
-
*aged/
-
*”Aged, 80 and over”/
-
*frail elderly/
-
*Geriatrics/
-
or/1-6
-
((eye* or sclera or iris or retina or cataract or ophthalmol*) adj3 (surgery or surgical* or procedur*)).tw.
-
exp *ophthalmologic surgical procedures/
-
((heart or cardiac or coronary) adj3 (surgery or surgical* or procedur* or transplant* or angiography or angioplasty or bypass)).tw.
-
(aortic adj3 (replacement or surgery or surgical* or procedur*)).tw.
-
(carotid adj3 endarterectomy).tw.
-
((arterial or artery or arteries) adj3 (bypass or surgery or surgical* or angioplasty or embolectomy)).tw.
-
*coronary artery bypass/
-
((urinary or urologic* or genitourinary or bladder or prostate) adj3 (surgery or surgical* or procedur*)).tw.
-
(urethrotomy or prostatectomy).tw.
-
exp *Urologic Surgical Procedures/
-
(meningioma* adj3 (surgery or surgical* or procedur*)).tw.
-
craniotomy.tw.
-
*craniotomy/
-
((lung or thoracic or thorax or cardiothoracic or pulmonary or chest or diaphragm) adj3 (surgery or surgical* or resection* or procedur*)).tw.
-
(thoracotomy or pneumonectomy).tw.
-
*Thoracic Surgery/
-
(‘bile duct’ adj3 (resection* or surgery or surgical* or procedur*)).tw.
-
((pancreas or pancreatic) adj3 (surgery or surgical* or resection* or procedur*)).tw.
-
(pancreatectomy or pancreaticoduodenectomy).tw.
-
*Pancreatectomy/
-
“endovascular aortic aneurysm repair*”.tw.
-
”endovascular abdominal aneurysm repair*”.tw.
-
((hip or knee or “lower limb*”) adj3 (replacement* or restructur* or arthroplasty or hemiarthroplasty or surgery or surgical* or procedur*)).tw.
-
*arthroplasty, replacement, hip/
-
*arthroplasty, replacement, knee/
-
or/8-32
-
7 or 33
-
(“enhanced recovery after” adj3 surgery).tw.
-
ERAS.tw.
-
((enhanced or early or earlier) adj3 (recovery or mobili?ation or ambulation or rehab*)).tw.
-
ERP.tw.
-
(“proactive care” adj2 “older people”).tw.
-
POPS.tw.
-
(“fast track” adj3 (surgery or surgical* or program* or management or “patient care”)).tw.
-
(multimodal adj3 (rehab* or perioperative or postoperative or “post operative” or optimi?ation or care or convalesc*)).tw.
-
(optimal adj2 (“preoperative assessment” or “preoperative management”)).tw.
-
((accelerated or optimi?ed or rapid or “fast track”) adj3 (care or rehab* or recovery or mobili?ation or ambulation or convalesc*)).tw.
-
((improved or improving) adj2 recovery).tw.
-
“comprehensive geriatric assessment*”.tw.
-
“short acting an?esthetic*”.tw.
-
((integrated or managed) adj1 “care pathway*”).tw.
-
((multidisciplinary or “multi disciplinary”) adj1 assessment*).tw.
-
((physiotherap* or exercise*) adj3 (augment* or increas* or “higher frequency”)).tw.
-
(“pressure ulcer*” adj3 “risk assessment”).tw.
-
((nutrition* or feed* or eat*) adj3 support*).tw.
-
*Nutritional Support/
-
“supported discharge”.tw.
-
or/35-54
-
((length or duration) adj4 stay adj8 (reduce* or reduction* or reducing or shorter or shortening or “positive effect*” or prolong* or increas* or decreas* or improve* or improving or “patient outcome*” or ‘clinical outcome*’ or ‘clinical indicator*’ or ‘outcome measure*’)).tw.
-
(hospital* adj3 stay adj8 (reduce* or reduction* or reducing or shorter or shortening or ‘positive effect’ or prolong* or increas* or decreas* or improve or improving or “patient outcome*” or “clinical outcome*” or “clinical indicator*” or “outcome measure*”)).tw.
-
(time adj3 discharg*).tw.
-
*”Length of Stay”/
-
or/56-59
-
55 or 60
-
randomi?ed.tw.
-
rct*.tw.
-
trial*.tw.
-
((single or doubl* or tripl* or treb*) and (blind* or mask*)).tw.
-
(“4 arm” or “four arm”).tw.
-
((before adj4 after) or “BA stud*” or “CBA stud*”).tw.
-
(“pre post” or “pre test*” or pretest* or posttest* or “post test*” or (pre adj3 post)).tw.
-
(interrupt* adj2 “time series”).tw.
-
(“time points” adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month* or hour* or day* or “more than”)).tw.
-
((“quasi experiment*” or quasiexperiment* or “quasi random*” or quasirandom* or “quasi control*” or quasicontrol*) adj3 (method* or stud* or design*)).tw.
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
or/62-73
-
34 and 61 and 74
Bibliographic database search results
| Database | Number of results |
|---|---|
| MEDLINE | 4227 |
| MEDLINE In-Process & Other Non-Indexed Citations | 459 |
| HMIC | 69 |
| EMBASE | 7194 |
| CENTRAL | 3603 |
| CINAHL | 1422 |
| AMED | 176 |
| Total number of results | 17,150 |
| Duplicate results | 7875 |
| Total number of unique results | 9275 |
| Total number of unique results from 2000 to date | 8038 |
Web search engine searches
Google Scholar
Data parameters: not applicable.
Date searched: 20 September 2017.
Searcher: SB.
Hits: Google Scholar results are limited to 999.
Search strategy
(older OR frail OR elderly OR geriatric) (reduce OR reducing OR improve OR improving) (“length of stay” OR “duration of stay”) trial*
Notes: number of records screened following application of date limit (2000 to date of search) and de-duplication against bibliographic database results = 694.
Data parameters: search activity based search results = off.
Date searched: 21 November 2017.
Searcher: SB.
Hits: we copied and pasted the first 100 results into a Microsoft Word document for screening.
Search strategy
(older OR frail OR elderly OR geriatric) (reduce OR reducing OR improve OR improving) (“length of stay” OR “duration of stay”) trial*
Notes: number of records screened following application of date limit (2000 to date of search) and de-duplication against bibliographic database results = 23.
Forwards citation chasing
Citation index: Web of Science (Core Collection); Scopus; Google Scholar.
Date searched: 14 and 15 December 2017.
Searcher: SB.
Search strategy
SB searched for studies identified by bibliographic databases that met the inclusion criteria of our review in Web of Science. If a study was indexed in Web of Science, SB exported the citations to EndNote. If a study was not indexed in Web of Science, SB searched for it in Scopus; if it was not indexed in Scopus, SB searched for it in Google Scholar.
| Number of citations | |
|---|---|
| Total citations | 2316 |
| Duplicate citations (including duplicates with bibliographic database and Google Scholar results) | 785 |
| Unique results | 1531 |
Appendix 2 Definitions of outcome categories
| Outcome category | Description of category | Examples of outcomes presented |
|---|---|---|
| Additional care | Need for additional care after discharge | Additional GP visits; 30-day emergency visits; patient called ward |
| Complications | Complications occurring during or after surgery and reported as such by study authors. In reporting complications, we prioritised summary outcomes over specific complications | Summary outcomes: number of major complications; number of patients presenting more than one complication; reoperations; comprehensive complication index. Specific complications: wound infections; ileus; myocardial infarction; death during initial hospital stay |
| LOS | Any markers of duration of stay within hospital | Total LOS; postoperative LOS; stay in intensive care unit; total LOS, including 30-day re-admissions |
| Markers of recovery | Markers of physical recovery after surgery, prior to discharge | Hand grip strength; gut function; 6-Minute Walk Test; time to first bowel movement; pain; timed up and go test; C-reactive protein levels |
| Mental health | Assessments of mental health | Hospital Anxiety and Depression Scale; SF-36 mental subscale |
| Morbidity | Morbidity after discharge | Morbidity within 30 days; morbidity within 90 days |
| Mortality | Incidence of death after treatment | Mortality within 30 days; mortality within 90 days |
| Patient satisfaction | Assessment of patient satisfaction or feedback | Patient satisfaction questionnaire |
| Quality of life | Any outcomes that either are explicitly quality-of-life measures, or relate to the impact of surgery on daily living | SF-36; EORTC quality-of-life questionnaire; markers of physical activity; gastrointestinal quality-of-life index; physical work capacity; WOMAC, Harris Hip Score |
| Re-admissions | Additional stay in hospital following initial discharge | Re-admissions within 30 days; need for reoperation |
| Surgical | Indicators of surgical performance and markers of implementation of the surgical protocol | Duration of surgery; blood loss; time at which intravenous fluids discontinued; fluid given; time catheterised; urinary output |
Appendix 3 Data treatment
| Data provided | Method to obtain mean | Method to obtain SD |
|---|---|---|
| Mean with SD | Not required | Not required |
| Mean with standard error | Not required | SD=SE×n |
| Mean with 95% CI | Not required |
SD=n×(UpperCI−LowerCI)/2t-distribution Note: t-distribution assumed to be 3.92 for total samples over n = 122, otherwise calculated manually |
| Median with range | Do not impute, describe in text | Do not impute, describe in text |
| Median with interquartile rangea | x¯=∑(median, q1,q3)3 | SD=q3−q12Ø−1(0.75n−0.125n+0.25) |
| Mean difference with CI, p-value | Cannot impute group means; report mean difference in outcome table instead |
First calculate SE for difference in means: SE=Upper limit−lower limitt-distribution Then calculate SD from SE: SD=SE1nE+1nC Finally, apply SD to both groups before calculation of standardised mean difference Note: t-distribution assumed to be 3.92 for total samples over n = 122, otherwise calculated manually |
Appendix 4 Sensitivity analyses for length of stay meta-analyses
The following forest plots show meta-analysis results when different levels of data imputation are performed, and the result included in the analyses. In the main report, we chose not to use data imputed from medians and ranges because upper ranges often highly skew the data. For comparison, we provide forest plots that include ‘all imputable outcomes’, meaning that data from medians and ranges are included. We also display meta-analyses performed with only the studies that provided mean and standard deviation, where possible.
FIGURE 23.
Forest plot showing the results of meta-analysis of the effect of ERP interventions on LOS following colorectal surgery, compared with usual treatment. All imputable outcomes are included. Lap, laparoscopic surgery group; LH, left hemicolectomy/high anterior resection; open, open surgery group; RH, right hemicolectomy.
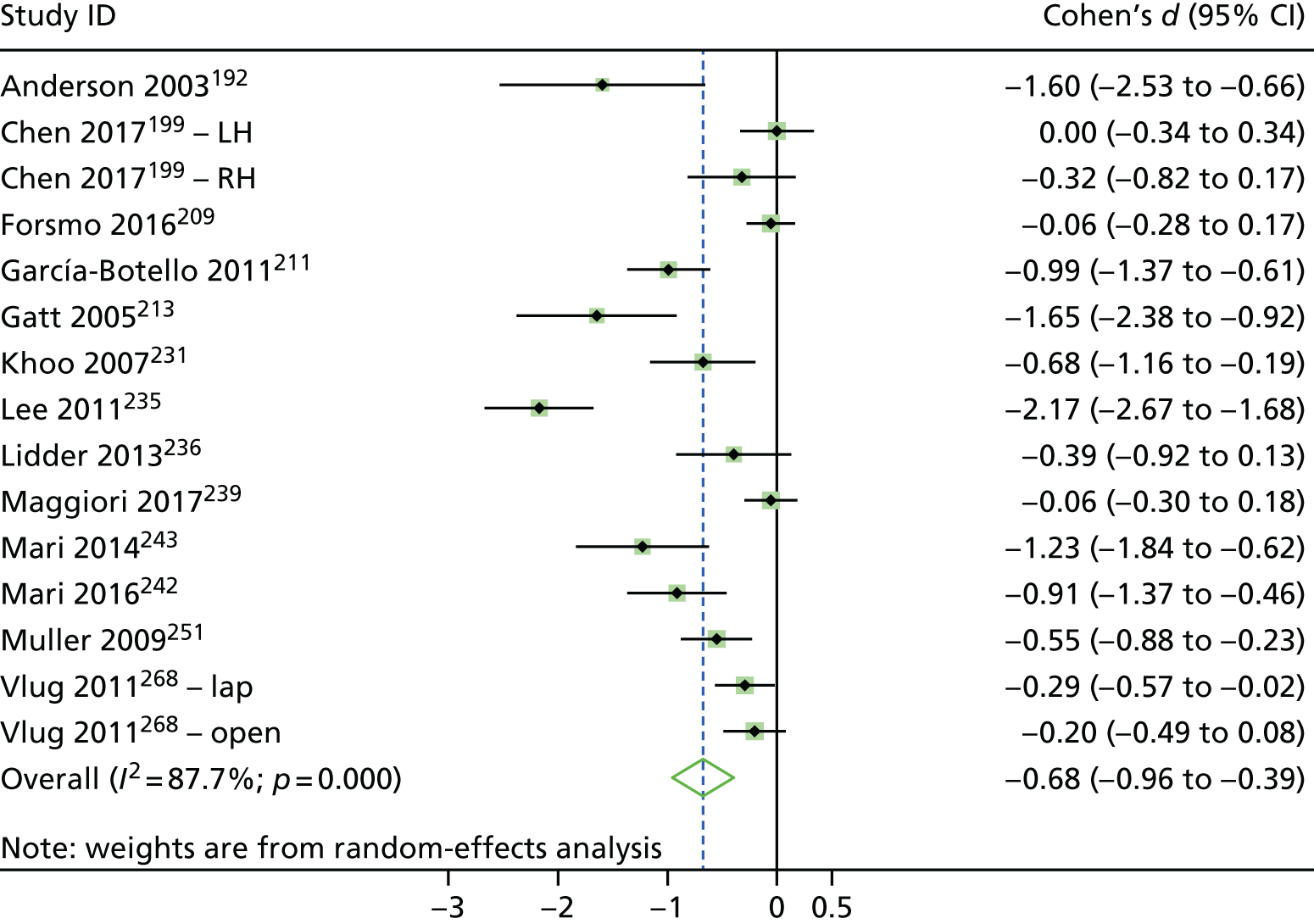
FIGURE 24.
Forest plot showing the results of meta-analysis of the effect of ERP interventions on LOS following colorectal surgery, compared with usual treatment. Data imputed from medians and ranges are removed (i.e. this is the method used in the main report). Lap, laparoscopic surgery group; open, open surgery group.

FIGURE 25.
Forest plot showing the results of meta-analysis of the effect of ERP interventions on LOS following colorectal surgery, compared with usual treatment. Only studies providing means and standard deviations for LOS are included.

FIGURE 26.
Forest plot showing the results of meta-analysis of the effect of ERP interventions on LOS following lower limb arthroplasty, compared with usual treatment. All imputable outcomes are included.
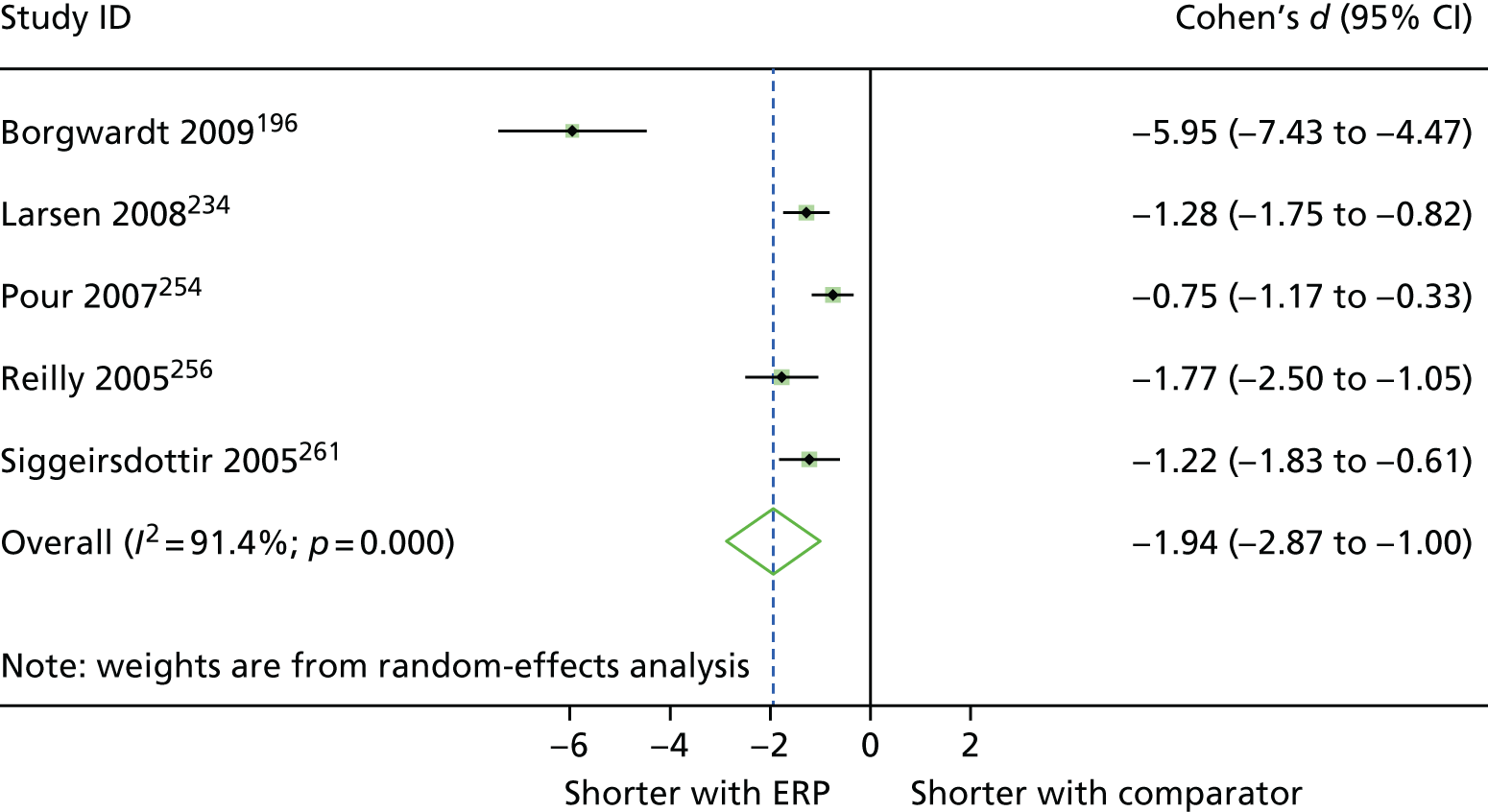
FIGURE 27.
Forest plot showing the results of meta-analysis of the effect of ERP interventions on LOS following lower limb arthroplasty, compared with usual treatment. Only studies providing means and standard deviations for LOS are included (this is the method used in the main report).

FIGURE 28.
Forest plot showing the results of meta-analysis of the effect of prehab interventions on LOS following lower limb arthroplasty, compared with usual treatment. All imputable outcomes are included.

FIGURE 29.
Forest plot showing the results of meta-analysis of the effect of prehab interventions on LOS following lower limb arthroplasty, compared with usual treatment. Only studies providing means and standard deviations for LOS are included (this is the method used in the main report).
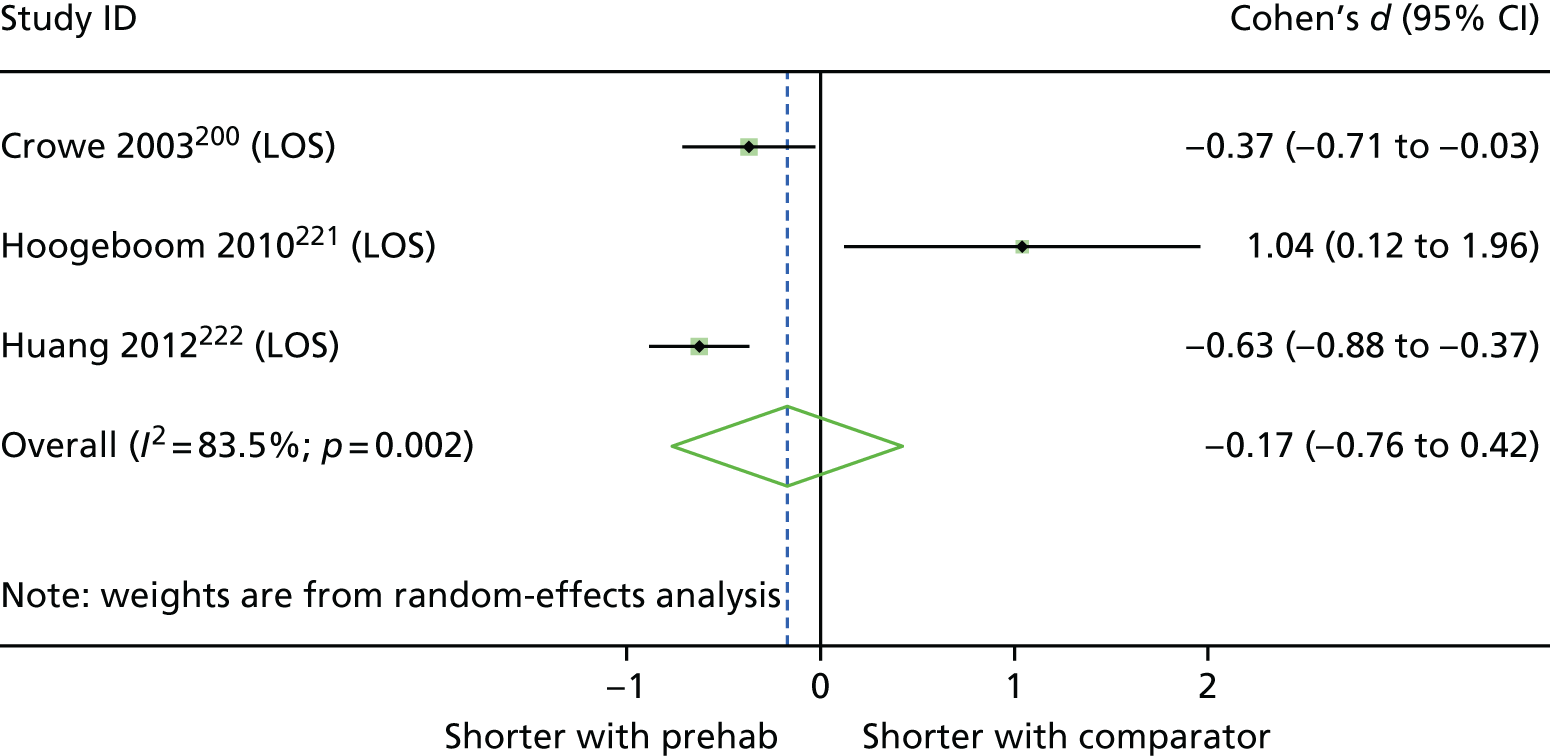
FIGURE 30.
Forest plot showing the results of meta-analysis of the effect of prehab interventions on LOS following cardiac surgery, compared with usual treatment. All imputable outcomes are included.
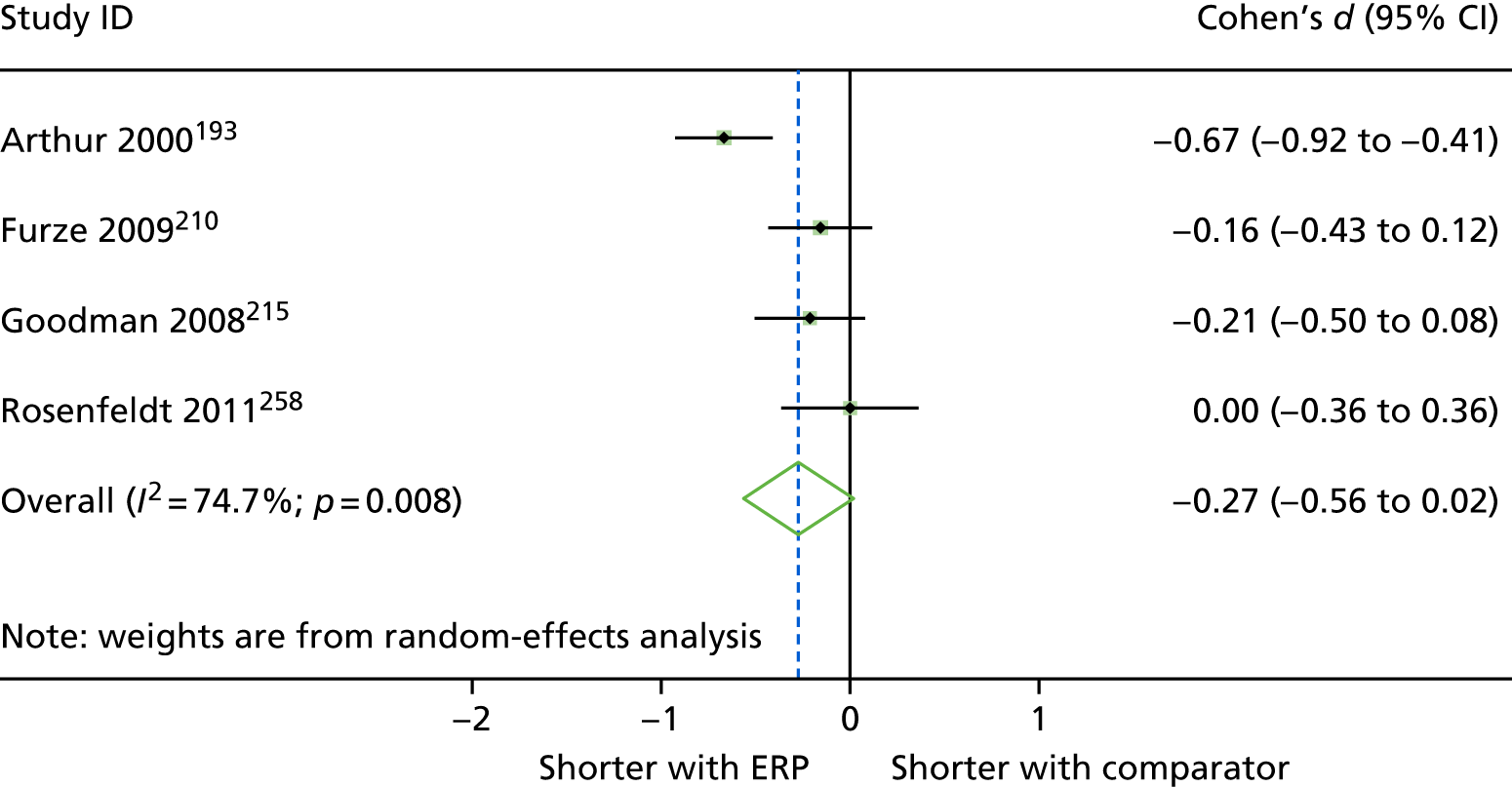
FIGURE 31.
Forest plot showing the results of meta-analysis of the effect of prehab interventions on LOS following cardiac surgery, compared with usual treatment. Data imputed from medians and ranges are removed (i.e. this is the method used in the main report).
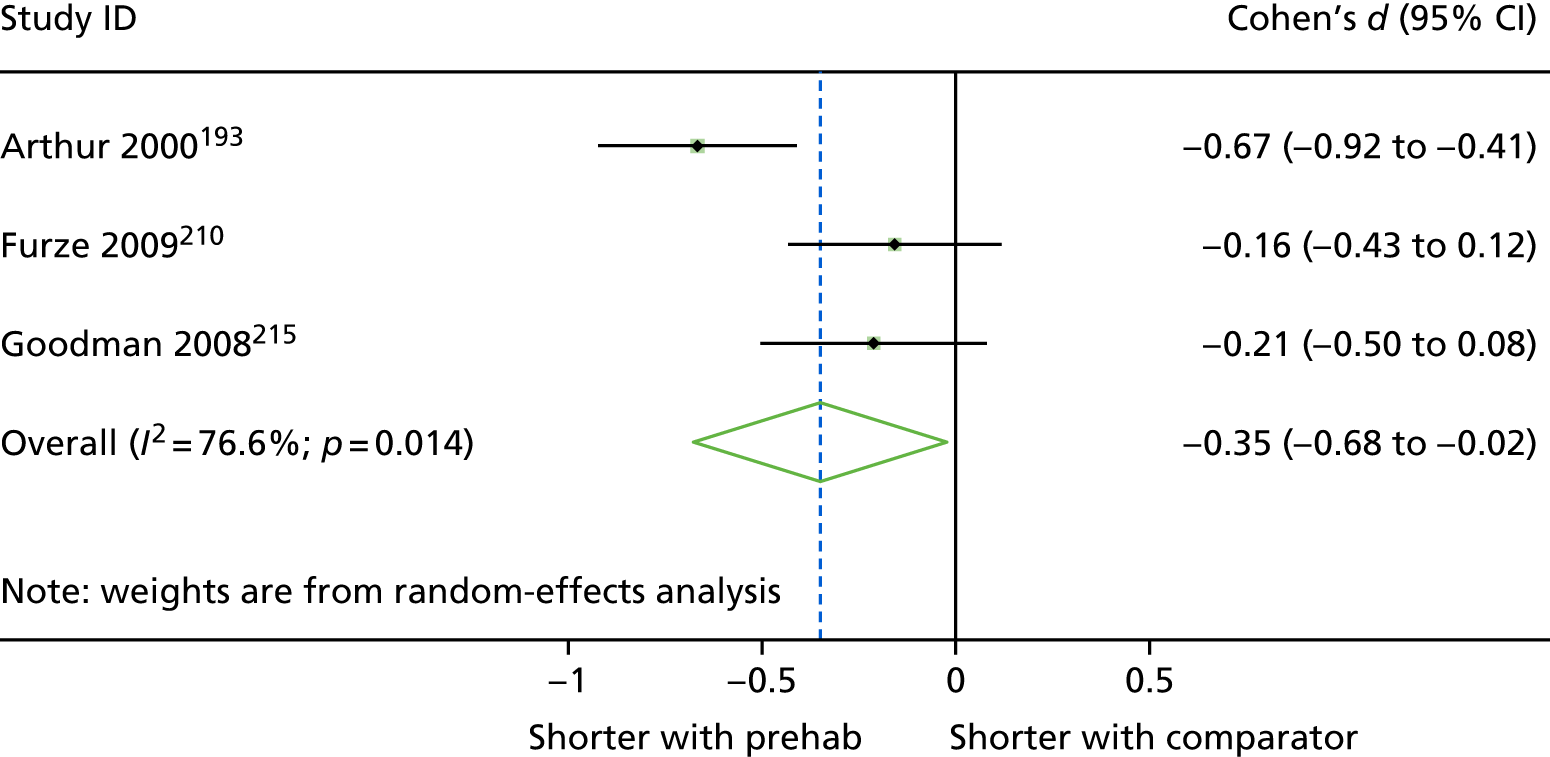
Glossary
- Abdominal
- Relating to the abdomen, that is, the anatomical region between the thorax (chest) and pelvis, including the stomach, small and large intestines, pancreas, liver and gallbladder.
- Cardiac
- Relating to the physiology of the heart.
- Care pathway (or patient care pathway)
- Separated into five distinct phases in relation to a hospital admission: pre admission; after admission but before treatment; perioperative/during treatment; postoperative but before discharge; and post discharge.
- Colorectal
- Relating to the physiology of the rectum, anus and colon.
- Comprehensive geriatric assessment
- A multidisciplinary diagnostic and treatment process that identifies the medical, psychosocial and functional limitations of an older person. The aim of a Comprehensive Geriatric Assessment is to develop a co-ordinated and integrated plan for the needs of the patient.
- Enhanced recovery after surgery
- A multidisciplinary approach to caring for surgical patients involving surgeons, anaesthetists, nurses and allied health professionals. Enhanced recovery after surgery programmes typically follow a protocol involving preoperative assessment, minimally invasive surgery wherever possible, and a structured approach to postoperative care. The aims of enhanced recovery after surgery include improving patient experience and reducing postoperative complications and hospital length of stay. It is associated with the ERAS Society.
- Enhanced recovery protocol (or programme or pathway)
- A multicomponent intervention that includes the delivery of health-care components at multiple stages of the patient care pathway.
- Hospitalist
- A physician in the USA who specialises in the general medical care of hospitalised patients, both within hospital and in related outpatient care.
- Inpatient
- A person admitted to hospital for at least one night.
- Kinesiologist
- A care professional with training in kinesiology, which is the study of human body movements, performance and function, combining knowledge from biomechanics, anatomy, physiology, psychology and neuroscience. It is not a licensed or officially recognised profession in most countries.
- Length of stay
- The time a patient stays in hospital, usually measured in days.
- Lower limb arthroplasty
- The surgical reconstruction or replacement of joints of the lower limb, most commonly the hip or knee.
- Multicomponent intervention
- An intervention that has two or more components that could otherwise be delivered as independent interventions.
- Pedometry
- The measurement of distance (usually walking distance within a given time) or another physical activity using a pedometer.
- Pelvic
- Relating to the pelvis, that is, the lower part of the torso between the abdomen and thighs.
- Prehabilitation (or ‘prehab’)
- The process of preparing a patient for a medical intervention such as a surgical procedure. This can involve physical strengthening, making dietary changes or engaging with learning materials. The aim of prehabilitation is to optimise the patient’s physical health and well-being before a medical intervention with a view to facilitating a rapid recovery after the intervention.
- Rehabilitation (or ‘rehab’)
- The assisted process of recovery following a medical intervention. Rehabilitation can involve physical, occupational and mental health therapies that aim to improve a patient’s post-treatment recovery.
- Resident
- In hospital systems in the USA and some other countries, a qualified doctor or physician who is undergoing postgraduate training (e.g. in a particular medical specialty) and practises medicine in that setting under the supervision of more senior, fully qualified, clinicians.
- Staff mix
- In a hospital setting, the organised deployment of various clinical and non-clinical staff roles with the aim of optimising patient care, including reducing length of hospital stay.
- Thoracic
- Relating to the anatomical region of the chest (or thorax), in particular the heart and lungs.
- Upper abdominal
- Relating to the upper abdomen, that is, the anatomical region containing the stomach, spleen, pancreas, kidneys, liver and gallbladder.
- Vascular
- Relating to the system of vessels that move fluids around the body, including the arteries, veins, lymph vessels and lymph nodes.
List of abbreviations
- AMED
- Allied and Complementary Medicine Database
- CABG
- coronary artery bypass graft
- CGA
- comprehensive geriatric assessment
- CHEC
- Consensus Health Economic Criteria
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- EPHPP
- Effective Public Health Practice Project
- EQ-5D
- EuroQol-5 Dimensions
- ERAS
- enhanced recovery after surgery
- ERP
- enhanced recovery protocol
- GP
- general practitioner
- HMIC
- Health Management Information Consortium
- LOS
- length of stay
- MeSH
- medical subject heading
- OR
- odds ratio
- PACP
- preoperative assessment with care plan
- PRISMA
- Preferred Reporting Items for Systematic reviews and Meta Analyses
- PT
- physiotherapist
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- UBA
- uncontrolled before-and-after
- WHO
- World Health Organization
- WOMAC
- Western Ontario and McMaster Universities Osteoarthritis Index
Notes
-
Clinical effectiveness search strategies for other databases
-
Non-prioritised studies eligible for inclusion in the effectiveness arm of the review
-
Tables providing full descriptions of interventions used within each study within each procedural group
-
Full quality assessment for prioritised effectiveness studies
-
Tables of results for evidence from randomised controlled trials
-
Tables of results for evidence from the UK
-
Tables for cost-effectiveness arm of the review
Supplementary material can be found on the NIHR Journals Library report project page (www.journalslibrary.nihr.ac.uk/programmes/hsdr/164722/#/documentation).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
