Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 14/19/12. The contractual start date was in July 2015. The final report began editorial review in September 2018 and was accepted for publication in April 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Paul Brocklehurst is a member of the National Institute for Health Research (NIHR) Health Services and Delivery Research Funding Committee, the NIHR Health Technology Assessment Prioritisation Committee and the NIHR Academy Doctoral Fellowship Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Brocklehurst et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Context and overview of the report
Introduction
In 2013, our team published a Cochrane Effective Practice and Organisation of Care Group review on the effects of different methods of remuneration on the behaviour of primary care general dental practitioners (GDPs). 1 The aim of this review was to evaluate the effects of different methods of remuneration on the level and mix of activities provided by GDPs and the impact this has on patient outcomes. It concluded that ‘financial incentives within remuneration systems may produce changes to clinical activity undertaken by primary care GDPs. However, the number of included studies is limited and the quality of the evidence from the two included studies was low/very low for all outcomes. Further experimental research in this area is highly recommended’. 1 In medicine, a review of reviews found that serious methodological limitations restricted the completeness and generalisability of the evidence for how changing remuneration systems affected patient care. 2 There was also insufficient evidence to determine the effect of financial incentives on the quality of health care provided. 3
Over the last decade, policy-makers across the UK have acknowledged the need to reform NHS dental contracts. In 2013, a change in the payment system for NHS dental contracts was considered by policy-makers in Northern Ireland, such that GDPs would be paid by a system based on the principles of capitation rather than the predominant fee-for-service (FFS) system. The main reasons behind this initiative were to contain costs, promote prevention rather than treatment of disease, secure access and improve the quality of care provided to NHS dental patients. The Department of Health, Social Services and Public Safety (DHSSPS) in Northern Ireland, in conjunction with the Northern Ireland Health and Social Care Board (NIHSCB), made a commitment to pilot a change in the remuneration system and work collaboratively with the academic team to undertake a rigorous evaluation of the impact of the pilot. This policy initiative provided an opportunity to build on the findings of the Health Services and Delivery Research report (11/1025/04) ‘Determining the optimal model for role substitution in NHS dental services in the UK: a mixed-methods study’4 to undertake a broader-based, economic study to investigate the impact of a change in the remuneration system in Northern Ireland on productivity and the quality of care provided.
Negotiations between the DHSSPS in Northern Ireland and the British Dental Association, the organisation representing the interests of GDPs in Northern Ireland, together with the budgetary constraints of the proposed pilots, had fixed the timetable for the evaluation and the number of practices that would be involved. These negotiations ensured that the payment package for GDPs working under the new financial arrangements (i.e. those in the new pilot) would be based on the total remuneration received under the previous year’s FFS contract.
Working in partnership with the academic team, this provided an opportunity to expand the evaluation and add academic rigour to the process that was originally envisaged by the DHSSPS and the NIHSCB. The outputs of the study would also add to and complement the information emerging from the NHS contract reform pilots in the other home countries. Importantly, this study provides detailed, longitudinal information at a patient level in test and control populations, which has not been possible in other NHS dental contract reform pilots. As the dental pilots in Northern Ireland were to switch from FFS remuneration to a capitation-based payment system and then back to FFS after 12 months, it also provided a unique opportunity to observe and document the scale of effect and issues around implementation and record any unintended consequences of the two changes in remuneration (FFS to capitation, capitation to FFS).
The evidence from the literature would suggest that practitioners respond very quickly to changes in the dental contract to ensure the viability of their practices;5–7 for example, changes to the NHS dental contract in England in 2006 saw an immediate drop in the types of clinical activity that reduced profit margins for practices and an increase in clinical activity in areas where profit margins could be improved. 5,6
Aim and objectives
The aim of the proposed research was to evaluate the impact of a change in the system of provider remuneration on the productivity, quality of care and health outcomes of NHS dental services in Northern Ireland.
The objectives of the research were to:
-
use a difference-in-difference (DiD) approach to measure changes in activity and costs over the different phases of the study in terms of –
-
productivity, as measured by the mean quantity of care delivered per provider
-
service mix, as measured by the proportions of key indicator treatments (these include examination plus scale and polish, radiographs, fillings, root canal treatment, crowns and bridgework)
-
GDPs’ time spent delivering patient care
-
change in cost of care, as measured by the volume of care weighted by the standard item treatment costs
-
co-payment income
-
-
assess GDPs’ and patients’ views about how, why and to what extent the changes in remuneration affect the delivery and quality of care
-
measure changes in patient-reported oral health knowledge, attitudes and behaviour
-
measure changes in patient-rated oral health outcomes and quality of care.
The research programme was co-produced including design and execution by a partnership between academics, policy-makers and senior NHS advisors and managers. The intention was to produce high-quality, stand-alone academic outputs that would contribute to the evidence base but concurrently provide valuable information for policy-makers and senior NHS decision-makers to inform the development of NHS dental services in Northern Ireland.
Research design and project overview
The research was undertaken across three workstreams, using a mixed-methods approach. The first workstream used a DiD design to quantitatively measure the change in service mix, productivity, GDP time spent delivering patient care, changes in the cost of care and changes in co-payment income. The DiD compared the change in outcomes before and after the change in contract model (using matched controls). Control practices were matched according to practice size, rurality and deprivation. For each outcome, an appropriate rate was determined (e.g. number of fillings per 1000 patients seen) and represented on a graph to demonstrate changes to activity levels over time (before and during the pilot). This approach had been adopted before. 6 The DiD analyses were supplemented with additional statistical analyses [interrupted time series (ITS)] on selected variables.
The second workstream used serial cross-sectional surveys of patients in pilot and matched control groups to collect data at each of the three distinct phases (baseline, capitation, reversion) of the programme. This recorded patient-rated and patient-reported measures of oral health status, oral health knowledge, attitudes and behaviour and the quality of care provided.
In the final workstream, qualitative interviews were undertaken with practices’ principals, practice associates, patients and policy-makers in Northern Ireland. The overarching philosophy underpinning the approach was informed by realist evaluation. 8 This approaches policies as ‘theories incarnate’ and seeks to understand ‘what works for whom and under what circumstances’. In other words, whenever a policy is implemented, it is testing a theory about change. Empirical approaches to evaluation seek to establish generative causation (i.e. ‘what’ happens). Realist evaluation also looks at ‘how’ and ‘why’ things happen (i.e. establish successive causation). It starts by making explicit the theories about how the policy might work, identifying the mechanisms through which specific outcomes are hypothesised to occur. This approach was informed by the DiD analyses to understand how and why changes occurred to provide generalisable findings. 9
Structure of the report
This report is arranged in chapters as follows. Chapter 2 provides a review of the literature and describes the historical context of NHS dentistry and the evolution of policy on dental services in Northern Ireland and the other three home countries. Chapter 3 describes the methods and results of the DiD and the additional statistical analyses. Chapter 4 describes the qualitative study of the Northern Ireland pilot and Chapter 5 details the findings from the serial cross-sectional surveys of patients. Chapter 6 integrates all the findings from the empirical work and provides an overview and assessment of the impact of changing provider remuneration on the activity and quality of care provided by NHS GDPs in Northern Ireland. Chapter 7 summarises our conclusions, identifies the limitations of the programme and discusses implications for policy, clinical practice and further research.
Appendices 1–6 provide detailed information on the methods for the matching of control practices, the DiD analyses, the patient questionnaire used in the serial cross-sectional surveys and the analysis for each question in the questionnaire. Appendix 5 also details the data envelopment analysis (DEA) and stochastic frontier modelling (SFM) technical efficiency methods and analyses we completed as per protocol. Appendix 1 also includes a reproduction of the HS45 form, which is used by GDPs in Northern Ireland to claim for the treatments provided during each course of treatment. The contents of these forms returned by GDPs and collated and checked by the Business Services Organisation (BSO) of NIHSCB provided the raw data for the DiD analyses.
Chapter 2 Literature review and policy analysis
Introduction
This chapter provides the context for the programme of research undertaken. It describes the organisation of NHS dentistry in Northern Ireland and the other home countries and the financial incentives present in NHS contracting systems.
Overview of remuneration in dentistry
The payment systems for GDPs working in a primary care environment (‘high-street’ dentistry) are varied, but fall into two broad categories:
-
a retrospective payment system that pays GDPs for every item of clinical activity that they undertake (known as FFS)
-
a prospective payment system that pays GDPs for the number of patients for whom they are providing care (known as per capita or capitation).
Fee for service has been the predominant model for adult service provision across all four nations of the UK, whereas per-capita payments is the predominant model for the provision of NHS care for children in Northern Ireland and Scotland.
Across the UK, non-exempt adult patients pay a substantive element of their cost of treatment (approaching 80%) and this is known as patient charge revenue (PCR). Although the remuneration systems across the UK differ, the process for PCR payments is relatively similar, with the GDP collecting the PCR directly from the patient. The remaining monies (approximately 20% for a completed course of treatment) are paid by the government. For exempt patients (as a result of low income, pregnancy, being nursing mothers or children/young adults up to 18 years of age), the cost of treatment is paid entirely by the government. In Northern Ireland, approaching half of all patients make a financial contribution to their NHS dental treatment.
The GDPs in the UK operate their practices as small businesses and differ from many other health-care professionals in that they take all of the financial risk for service provision. 6,10 They also receive little or no support for initial start-up costs or for the development of their capital infrastructure. As Harris et al. 11 explained, ‘dental practice premises and facilities are owned by the principal(s)/body corporate as capital assets’. The costs involved are then incorporated into the sum of practice overheads and reimbursed through the dental remuneration scheme. The financial risk concerned with falling levels of property value rests solely with the GDP. As a result, GDPs are particularly sensitive to financial incentives within NHS dental contracts. 1,5–7 Changes in the clinical activity of GDPs in the UK following the introduction of new methods of payment in the NHS have been documented. 5,6,12 In addition, unlike primary care physicians, whose predominant function is the management of symptomatic patients or those with chronic conditions, the majority of service delivery in the NHS, in terms of volume of activity, is based on the ‘check-up’ of largely asymptomatic patients. Again, as highlighted by Harris et al. ,11 ‘the independent contractor status of GDPs and GMPs [General Medical Practitioners] has meant both types of practitioners have developed commercial and entrepreneurial as well as professional identities’.
Financial incentives in NHS general dental services
NHS dental contract reform seeks to change the way GDPs are paid in order to influence their behaviour in such a way that it aligns with policy goals. NHS GDPs are part of an altruistic profession, yet much of their activity in primary care is driven by the ‘profit principle’ to maintain the viability of their practices. This is understandable, as dental practices operate as small businesses; with a mixed income of NHS and privately remunerated activity, and unlike general medical practitioners, the majority of a GDP’s NHS income comes from delivering services. As a result, NHS GDPs are sensitive to incentives within the remuneration system. 1,5–7 There are two main systems of remuneration, namely prospective capitation-based systems and retrospective payments for completed activity, or FFS systems. Capitation payments tend to secure effectiveness at the cost of patient selection (GDPs preferentially registering low-need patients and refusing access or even deregistering high-need patients) and undertreatment. 10,13,14 The inherent incentives can be described as ‘service broadening’, providing minimal treatment to as many patients as possible. This contrasts with ‘service deepening’, where inherent incentives can lead to profit maximisation and the delivery of large amounts of treatment to a limited number of patients. The FFS systems incentivise the provision of treatment quantity but often suffer from cost containment problems, supplier-induced demand and the possibility of overtreatment. 10,13,15 There is also a salaried approach to remuneration, which removes the link between income and the level and type of services delivered or the number of patients served. However, this often leads to high costs in patient care. 10
Oral health follows a social gradient, with the poorest experiencing the highest burden of dental disease. 16 However, access to services providing prevention and treatment of oral diseases is often determined by an individual’s ability to pay for services, unless they can be classified as exempt from patient charges. 17 As a result, access to services tends to be greatest in those groups with lowest treatment needs. 18,19 Public funding of health and social care should provide a means of overcoming the divergence between the ability to pay for care and need for care. It offers the opportunity for improving both efficiency (increasing health gains produced from available health-care resources) and equity (removing barriers to access services or the type of care provided that is associated with individuals’ income or wealth). However, reforming public sector-funded contracts of independent contractors to incentivise behaviour to realise the aims of policy-makers, such as increasing efficiency and addressing inequity, is fraught with difficulty. In an evaluation of the 2006 contract reform in England, Whittaker and Birch12 found that the dental reforms had a negative impact on access, with a fall in NHS dental service use among populations with previously good access to NHS care and a move to private practice. This study highlighted the perils of reforming public health-care systems producing unintended consequences, in this case in contrast to a key policy requirement of expanding NHS access; the 2006 dental reforms reduced NHS use among those who previously had good access to care.
Co-payments received via PCR have been a fundamental component of the NHS dental contract since the 1950s, making up a significant proportion of the dental budget. Patient charges are made in exchange for the provision of specific items of treatment. This means of collecting payments from patients, to supplement health-care budgets paid for from general taxation, is a readily understood transaction for patients and the administrative processes to collect PCR are well established in NHS dental practices. Changing the means of collecting PCR, such as monthly direct debits to secure access (as used by insurance schemes and third-party payers) or paying for prevention advice and services (which are perhaps not as tangible as receiving a filling), would be less readily understood by patients and would require significant changes to administrative systems and processes. Any changes to a remuneration system runs the risk of producing a fall in PCR, leading to a gap in the NHS dental budget that the state would have to fill. The level of patient charges can also affect patient behaviour. In the USA, Manning et al. 20 found that dental service use increased as co-payments decreased in a randomised trial of alternative insurance plans, and Parkin and Yule21 found a negative relationship between price and dental care use in Scotland. Little is known about the impact of remuneration system on the efficiency of NHS practices and how they might influence outputs such as access.
Organisation of NHS general dental services until 1990
Dental services were included in the NHS from its inception in 1948. The National Health Service Act 194622 had three key principles: (1) no one would ever have to fear not getting care they needed because they could not afford it, (2) it would be free at the point of delivery and (3) it would be based on clinical need. As a result, NHS dental services across the UK were provided free of charge to the entire population. GDPs were considered to be independent contractors under the National Health Service (General Dental Services Contracts) Regulations 200523 and so could establish their practice anywhere in the UK. GDPs’ general dental services (GDS) remuneration was paid on a FFS basis (i.e. the volume and type of work undertaken) and even though they were self-employed, GDPs were eligible to join the NHS Superannuation Scheme. By 1950, the government had become concerned about the affordability of the new service, given the volume of work being undertaken by NHS GDPs (mostly extractions and fillings) as a consequence of high unmet need and the availability of a new, free service. This resulted in the introduction of the Patient Charge Regulations (1952), which required patients to contribute to the bulk of the cost of treatment according to a Statement of Dental Remuneration (a list of available NHS treatments). 24
Other than minor changes to the Statement of Dental Remuneration, the shape of NHS GDS provision remained relatively static until the introduction of a new contract across the whole of the UK in 1990. For the first time, the NHS dental contract contained an element of prospective payment and patients were required to register with a GDP. The remuneration arrangements for adults and children were split, with adults still treated under FFS arrangements (plus relatively small additional continuing care payments), while the care of children was remunerated on a wholly capitation basis. As a result, approximately 20% of the income for NHS GDPs was based on the number of dental patients registered (on a per-capita basis), rather than simply being based on the volume of activity on a FFS basis. The policy intention of the 1990 contract was to encourage registration and promote prevention and continuity of care, moving service provision away from treating disease to maintaining oral health. 25 However, higher than expected expenditure in the following year led to the government making substantive cuts to NHS dental fees in 1991. The dental profession felt that they were unfairly penalised by this ‘clawback’ and this led many GDPs to feel unhappy with the new NHS system of payment. 26 This triggered a progressive shift towards the provision of privately funded dentistry within the profession, resulting in a growing reduction in the availability of NHS services.
Policy development post 1990
After 1990, influenced by the devolution of NHS health-care policy, the provision of GDS started to become more diverse across the home countries of the UK, with different policy objectives and contracts emerging. The following sections highlight these changes and provide a useful backdrop to the study.
The evolution of a new NHS dental contract in England
By the mid-1990s, difficulties in accessing dental services for NHS patients in England were becoming a political issue and it was increasingly recognised that reform of the NHS contract was necessary. As highlighted by the Bloomfield Report,26 the ‘system of remuneration for GDPs seems to have an inherent leaning towards instability which threatens to undermine the commitment of dental practitioners to the NHS’. In 1997, the NHS (Primary Care) Act enabled the voluntary establishment of personal dental services (PDS) pilot schemes to explore alternative ways of delivering NHS dental services. 27 A key feature of these new contracts was how they were tied to local issues around the need for and access to care through contracting arrangements with NHS primary care trusts (PCTs) in England. For NHS GDPs, the new PDS contracts offered greater flexibility for the provision of services and there was less emphasis on the volume of activity provided. Instead, NHS GDPs were paid on a per-capita basis and rewarded for improving access to care and maintaining oral health for those patients who were already registered. The net effect of paying the PDS schemes was a dramatic reduction in NHS service activity, a fall in the provision of complex treatments and a loss of PCR as a consequence of the fall in activity. 28 The latter development was particularly worrying for the government as patient charges made up a substantial proportion of the budget for NHS dentistry. However, many viewed the PDS pilots as a success, given the focus on addressing the needs of the local population. 28,29
At the turn of the new millennium, ‘Modernising NHS Dentistry’ was introduced in England. 30 Improving access was considered to be the most important policy objective for the government and the new proposals gave PCTs powerful new commissioning tools to improve access to NHS dentistry, to increase the provision of preventative services and to monitor the performance of GDPs. This was further emphasised in ‘Options for Change’ in 2002. 29 However, a tension remained for the government: how could they concomitantly improve access to care and increase the provision of prevention while maintaining PCR, which was reliant on ensuring that activity levels did not fall?
In 2006, a new NHS dental contract was introduced in England and Wales, organised around a local commissioning model. 31 This contract paid GDPs according to activity categorised into three broad bands, rather than the 400 individual treatment items in the Statement of Dental Remuneration, and a new contract currency, units of dental activity (UDAs), was introduced to set activity targets and pay GDPs according to the activity they provided. A band 1 course of treatment attracted 1 UDA and included an examination, radiographs and a simple scale and polish. Band 2 courses of treatment attracted 3 UDAs and included restorations, extractions and root canal treatments, whereas more complex crowns, bridges and dentures attracted 12 UDAs as a band 3 course of treatment. Patient charges were also simplified so that there were only three levels of payment tied to each band of treatment.
For the first time, contracts were raised with practices rather than with individual GDPs. Contracts were held by practice principals (PPs) (equity owing), now referred to in the NHS as providers, and non-equity-holding associate dentists (ADs) were labelled performers. Practice owners raised internal contracts with each of their associates based on a price per UDA delivered. To reflect variation in earnings and activity among NHS practices, the value of a UDA was calculated for each practice individually based on the practice earnings during a reference period, although, for most, this meant that 1 UDA was worth between £20 and £25. Annual activity targets were also set in the form of UDAs required per year, based on historical activity produced during the reference period. One feature of the 2006 NHS contract was that GDPs would receive 3 UDAs for a band 2 course of treatment (i.e. paid the same) if they provided one restoration in a single visit or multiple restorations over a number of visits. In a similar manner, NHS GDPs were also allocated 12 UDAs for one band 3 course of treatment irrespective of whether they provided one crown or multiple crowns within one course of treatment.
A key feature of the 2006 contract was cost containment [i.e. GDPs’ NHS annual activity and revenue were capped at an agreed number of UDAs per year for an agreed price, known as an annual contract value (ACV)]. The NHS GDPs were then paid one-twelfth of their ACV on a monthly basis. As a result, NHS GDPs’ outputs under the new contract in England were constrained and they were penalised if they underperformed (< 96% of their ACV) or overperformed (> 102% of their ACV). Patient registration in the 2006 contract also ceased to exist, along with the contractual responsibility for NHS GDPs to provide continuing care and emergency care for their patients. Instead, this latter responsibility was devolved to PCTs along with the planning and securing of NHS dental services for their locality.
The effect of the 2006 NHS contract change in England was investigated by an earlier National Institute for Health Research Health Services and Delivery Research programme funded study (08/1618/158). Large and abrupt changes in the provision of a number of treatments coincided with the introduction of the 2006 contract. 6 The number of complex treatments provided (root canal treatments, crowns and bridges) fell dramatically whereas the number of extractions rose just as dramatically. This appeared to reflect an increase in those activities that were easier and less costly to perform at the expense of those treatments that were more time intensive or that incurred laboratory costs for making crowns and bridges. The authors concluded that ‘the change in treatment patterns suggests that significant numbers of GDPs [were] attempting to hit their UDA contract targets in the most efficient way possible, i.e. shifting towards treatments where rewards are high relative to costs, as opposed to selecting on the basis of clinical factors’. 6 In addition, McDonald et al. 5 remarked that ‘it is the interests of GDPs, as opposed to patients, which [were] being prioritized’.
In England, the 2006 contract was unpopular with GDPs from the start; a major underlying reason was a loss of autonomy and their accountability to local commissioners. Only 2 years after its introduction, the contract was criticised by the House of Commons Health Select Committee,32 which based its criticism on the four areas that the contract had sought to improve, namely:
-
Patient experience – the contract was criticised for not helping to address the access problem (GDPs could hit their UDA activity targets largely by confining care provision to their long-standing, regularly attending patients) and the simplified patient charging system was also criticised as being insensitive.
-
Clinical quality – the contract did not incentivise preventative care and there were concerns about a fall in the volume of complex treatments such as root canal treatments, crowns and bridges.
-
Local commissioning – there was concern that many PCTs lacked the capacity and expertise to effectively commission dental services.
-
Improving GDPs’ working lives – the UDA activity targets were particularly unpopular with GDPs.
As a result of this criticism, an independent review (the Steele report) was published in 2009. 33 The report emphasised the need for prevention to produce better health outcomes and a standardised approach to patient assessment and disease risk categorisation leading to evidence-based care pathways. To support this approach, a move to a blended contract was envisaged based on capitation, with incentives to provide appropriate levels of activity and to improve quality.
Following the Steele report, in December 2010, the Department of Health and Social Care published NHS Dental Contract: Proposals for Pilots. 34 Three types of pilot were identified to test different remuneration models:
-
Practices were paid on a simple 100% capitation basis.
-
Practices were remunerated based on the number of weighted capitated patients they had; the capitation weighting was calculated according to age, gender and the deprivation status.
-
As per type 2, but with a separately identified budget for higher-cost treatments.
Elements of a reformed contract were piloted in 70 NHS dental practices in England, recruited between July and September 2011, and the findings of the pilots were published in 2014. 34 This report was a service evaluation and a control population was not identified. The evaluation of the oral health assessment, which formed the cornerstone of the new approach to care, was limited to professional and social acceptability. The pilots sought to introduce a new care pathway based on managing disease risk [using a high-, medium- and low-risk scale, the so-called RAG (red, amber, green) rating of risk] through the provision of preventative care. The ability of the associated risk algorithms to correctly classify patients and predict future disease has not been tested. With the methodological limitations, the findings were mainly descriptive in nature. The report acknowledged its methodological limitations, saying that the findings were:
. . . difficult to demonstrate with absolute confidence away from a clinical trial (which the pilots explicitly are not) and we have little or no comparable data from GDS outside the pilot.
Initially, quality was to be measured and incentivised by including quality payments as a small percentage (≈10%) of the practices’ remuneration package. The newly derived, but unvalidated, Dental Quality Outcomes Framework (DQOF) was intended to be used to measure quality and form a ‘pay-for-performance’ element of the remuneration system. However, in the first 2 years of piloting, the DQOF remuneration adjustments were not applied:
. . . due to issues with the robustness of the clinical data on which the DQOF clinical indicators are based.
As there were no control practices, the evaluation was restricted to a simple before-and-after design using a mixed-methods approach. There were significant problems encountered in collecting clinical data from information technology (IT) systems to support the standardised clinical assessment used in the pilot. The evaluation was also hampered by the fact that the collection of data on individual items of treatment ceased with the 2006 contract. Data entry for the new IT systems in the pilot was slow and onerous for practices, resulting in missing data and concerns about data quality. Dental charting data for many patients were incomplete or absent, making it impossible to apply the DQOF financial adjustments. Quantitative data presented in the report were confined to assessing the impact on access and patient experience. 34 Across all of the practices, there was a fall in the total number of unique patients seen for the duration of the pilots, albeit with significant variation across the practices. There was little association between a patient’s recall interval and the assessment of their risk, the ‘6-month check-up’ seemed to be well established and moving to a recall interval dictated by the risk assessment proved difficult. The pilots were generally popular with GDPs and patient satisfaction rates were higher (95.8%) than the national mean for all NHS dental practices of 92.2%, but these universally high scores demonstrate the unresponsive nature of this metric. In addition, baseline satisfaction rates for the pilot practices were not collected.
In 2015, the Department of Health and Social Care introduced a ‘prototype’ remuneration model but without a formally described, a priori evaluation plan. 35 The prototype process was introduced with the stated intention to test whole versions of a possible new contract rather than key elements required to inform the design of a new system (as was the intention in the pilots). Two contract models were tested, both aiming to continue to test the patient pathway based on the standardised assessment and risk stratification model. The UDAs remained as the contract currency to measure and remunerate activity delivery. Two types of prototype were implemented:
-
blend A prototypes included –
-
capitation (band 1)
-
activity (bands 2 and 3).
-
-
blend B prototypes included –
-
capitation (bands 1 and 2)
-
activity (band 3).
-
The intention was for both prototype models to also include remuneration adjustments made according to performance against the DQOF. 36 The prototypes involved greater financial risk for practices than the pilots (in which practices were guaranteed an income matched to a historical reference period); all prototypes were to be able to overdeliver by 2%, but would also have 10% of their contract value at risk if there was underdelivery of patient numbers and UDA activity. The protocol agreement scheme commenced on 1 December 2017. The Department of Health and Social Care and NHS England aimed to recruit a further 20 practices with an anticipated start date of October 2018.
The Department of Health and Social Care published an evaluation of the first year of prototyping in May 2018. 37 The prototype contract models started in spring 2016 and in the evaluation report the prototype practices were described as wave 3 of a larger evaluation programme with pilot practices described as wave 1 (recruited in 2011) and wave 2 (recruited in 2013). The evaluation included practices from all three waves but was problematic in that 40% of the original pilot practices did not continue into the prototype phase. Practices were split into two groups (blends A and B). Although it was originally intended that 10% of the contract value would be based on DQOF performance, it was agreed that for 2016/17 there would be no financial incentives used to try to improve quality. A post hoc matched set of practices working under the 2006 remuneration system was identified to enable comparisons between the prototypes with behaviour under the 2006 system. No methodological details were provided about how the matching was undertaken, nor were any analyses provided that compared the prototype practices with controls. Comparisons were restricted by the limited data available from comparator practices (primarily UDA claims and payments).
Data for the evaluation were provided by the NHS Business Services Authority using the same activity data for the comparator 2006 contract practices. Pilot/prototype practices were also asked to complete a monthly survey; these data were supplemented by two specific surveys undertaken in December 2016–January 2017 and in July–August 2017 to support the evaluation. The report restated the objective of the English Dental Contract Reform Programme:
. . . to maintain or improve access, quality and appropriateness of care and improve oral health, within the current cost envelope, in a way that is financially sustainable for dental practices, patients and commissioners.
However, a hierarchy for these (often competing) requirements was not identified.
The evaluation concluded:
. . . progress has been made in the first year of prototyping on the key issues of improving oral health, providing appropriate care and quality, and maintaining or increasing access to merit continuation of the programme.
The indicators to measure oral health improvement included the number of teeth with dental decay (children and adults) and the basic periodontal examination scores and the number of sextant bleeding sites in adults. Trends in GDP-assessed caries and periodontal risk were also reported but these oral health indicators were reported for the pilot/prototype practices alone and were not compared with the 2006 contract practices.
Appropriateness was assessed by adherence to the care pathway advocated in the Steele review,33 more specifically by the proportions of adults and children in the pilot/prototype practices who had received an oral health assessment. In all prototypes, 87% of adults and 86% of children received an oral health assessment and 99% of prototypes met the DQOF standard for recording an up-to-date medical history. Appropriateness was also assessed by process indicators identifying adherence to the prevention guidelines Delivering Better Oral Health: An Evidence-based Toolkit for Prevention. 38 Prototypes were compared with 2006 contract practices using FP17 (the form used by NHS GDPs to claim for activity provided) data reporting practice-reported adherence to national guidance. 38 For adults, 62% of prototype practices reported delivery of prevention according to the guidelines compared with 56% in 2006 contract practices; for children, these percentages are 60% and 58%, respectively. However, in prototype practices, only 28% of courses of treatment in children reported that fluoride varnish was applied compared with 41% in the 2006 contract practices. This latter finding gave cause for concern and the report recommended that a patient survey should be carried out to determine the impact of prevention advice on patients’ oral health behaviour. It is difficult to see how a survey would add much to the evidence base for the relationship between providing prevention advice and eliciting a sustained change in behaviour. The issue of recall attendance intervals was also considered under appropriateness, and the report recommend that:
. . . a detailed piece of work is undertaken to understand the reasons and rationale from patient and professional perspectives for the approach to implementing the longest recall periods recommended by NICE.
However, there was no mention of the soon-to-be published National Institute for Health Research (NIHR)-funded INTERVAL trial (ISRCTN95933794), the results of which are likely to influence the National Institute for Health and Care Excellence (NICE) guidance on recall intervals. 39
Assessment of the impact of quality in dentistry is limited by the underdeveloped conceptual understanding of quality in dentistry and how to measure it. 40,41 The report identified high percentages of prototype practices meeting all of the DQOF outcome indicator thresholds. The evaluation reported that 97% of patients treated in the prototype system were ‘quite’ or ‘very satisfied’ with NHS dentistry, compared with 96% of patients treated in the 2006 contract system. These very high satisfaction levels may not mean that all NHS services provide a highly satisfactory service; it may reflect that this indicator lacks responsiveness or that patients do not have sufficient knowledge and information to make an informed judgement of the quality of care provided. The report recommended that the development of the DQOF and its application should be continued but without commenting on how research could contribute to this goal.
In the reporting the impact on access and accessibility, these concepts were not defined. Access was measured by the practice capitated patient lists (number of patients attending in a 24-month period), which increased in waves 1 and 2 practices (initial pilot practices), but remained below the expected capitated numbers set in the contracts. During the first year of prototyping in wave 1 practices, attendance in a 24-month period increased from 85% to 88% of the baseline and for wave 2 practices it increased from 89% to 95% of the baseline. During the same period in matched 2006 contracts, access increased from 101% to 103% for wave 1 and stayed constant at 103% for wave 2. The fall in access in the prototypes was attributed to the concentration on embedding and delivering the clinical pathway in the early stages of piloting. Accessibility for patients was measured through a standard patient survey question and 90% of patients who responded reported that ‘the time it took to get an appointment was as soon as necessary’ compared with 91% in the 2006 contract practices. However, this is a limited view of accessibility, applying to only current users of a service and not to individuals living within a practice catchment area who may wish to access the service. This points to the need for clear and agreed definitions of access and accessibility, which are necessary to develop appropriate and valid measures of these important concepts for policy. Value for money was not assessed and the report acknowledged the difficulty of measuring this complex concept and stated that at this early stage there are insufficient ‘steady state’ data to undertake meaningful analysis. There was no elaboration on the measures or possible study designs that could be employed to undertake a rigorous health economic evaluation of different remuneration systems. A significant omission in the report was a lack of analysis of the impact of the pilots on PCR, especially as the objectives of the English Dental Contract Reform Programme include maintaining or improving service and health within the current cost envelope:
. . . in a way that is financially sustainable for dental practices, patients and commissioners.
Sustainability of prototype practices was compared with 2006 system practices using percentage contract achievement and, using this blunt measure, practices in all three waves performed to a similar level as the control 2006 system practices. However, a much broader and deeper means of assessing practice sustainability is required, given the multiple levers practices have at their disposal to improve their productivity and profitability, such as adjusting NHS/private mix and employment of skill mix. 4
The report made two further conclusions. The first was that the clinical model advocated by Steele33 is well accepted by the profession (standardised assessment of risk signposting the following of defined care pathways) but the business model requires further development to improve the sustainability for practices. The second conclusion was that additional practices should be recruited to the programme and randomly allocated to the blends to improve the robustness of evaluation. However, there was no suggestion that evaluation could be commissioned from an external party or that academic expertise could be harnessed to improve ‘the robustness of evaluation’ (contains public sector information licensed under the Open Government Licence v3.0). 37
It has been 10 years since the publication of the Steele report33 and a new English NHS dental contract is still to be implemented. This cautious, incremental approach seems to be reflected across all four of the home countries, which is not surprising given the complexity of the issues involved and the clinical, financial and political risk involved.
The evolution of a new NHS dental contract in Northern Ireland
The development of a new NHS contract in Northern Ireland can be plotted from a review of dental policy over the last 10 years traced through the content of a number of key documents.
In 2004, the DHSSPS produced a ‘Public Attitudes Survey on Oral Health’, which was undertaken to inform the development of an oral health strategy and primary dental care strategy for Northern Ireland (Donncha O’Carolan, NIHSCB, personal communication, 2019). Respondents were asked for their views on dental services, which could potentially form part of the Department’s planned Oral Health Strategy for Northern Ireland. 42 Respondents identified access to basic treatment, better access to emergency treatment and treatment for those with learning disabilities to be the most important aspects of the service that they wanted to see protected and enhanced.
The consultation document An Oral Health Strategy for Northern Ireland was subsequently launched in September 2004 and the final report, The Oral Health Strategy for Northern Ireland, was published in June 2007. 42 Although the main focus of the strategy was public health and community-based preventative interventions, it contained a section on dental services. The strategy reiterated one of the main themes of the Northern Ireland Primary Dental Care Strategy, launched in November 2006, which proposed a shift in the focus of a new GDS contract away from treatment of disease towards prevention. 43 The Oral Health Strategy stressed the need to maintain and improve access to dental services, especially for disadvantaged groups and for individuals with special care needs and implicitly acknowledged the policy aim of modernising dental services but warned about the need to maintain high levels of access and satisfaction in any reform of dental services.
The DHSSPS’s Primary Dental Care Strategy is a key document to help understand policy-makers’ thinking on the need for a new dental contract. 43 In the foreword, the Minister for Health said:
. . . there is widespread agreement that the way that dental services are currently delivered needs to change in order to better meet the needs of patients and GDPs and other oral healthcare professionals.
This sentiment was expanded on in the recommendations section of the strategy, in particular recommendation 10 (paragraph 6.19) stated:
. . . a new Northern Ireland wide GDS primary dental care contract framework will be developed and will provide the basis to commission services to meet local need.
Recommendation 17 (paragraph 8.4) stated that:
. . . any new arrangements should be piloted before rolling out across Northern Ireland.
Many of the recommendations echoed developments in England (see The evolution of a new NHS dental contract in England), which were implemented in the PDS pilots of 2004–6, prior to the introduction of the 2006 English contract. Some of the ideas developed in the early English PDS pilots were translated into the NHS dental contract implemented in England in 2006, in particular an emphasis on local commissioning to make services more responsive to population health needs, a need and desire to secure access to dental services for the whole population, a shift in emphasis from repairing the effects of dental disease to disease prevention and a greater role for skill mix. It was telling that recommendation 16 (paragraph 7.3) of the DHSSPS’s Primary Dental Care Strategy stated:
. . . the new patient charging arrangements in England should be monitored with a view to introducing a similar system in Northern Ireland.
This points to a history of health policy in Northern Ireland tracking developments in England and also an implicit acknowledgement that one of the main difficulties faced by policy-makers designing a new dental contract involves protecting that part of the budget derived from PCR. The 2006 English dental contract gave policy-makers certainty on annual spend for the service while buying a minimum level of access to NHS services (but not necessarily guaranteeing accessibility of NHS dental services particularly for those with greatest need).
Following publication of the DHSSPS’s Primary Dental Care Strategy, the evolution of policy thinking can be traced from the content of the DHSSPS’s Dental Branch Annual Reports from 2006/7 to 2010/11 (no further reports were produced after 2011). 44–48 The 2006/7 report contained a ministerial announcement for a new dental contract and commented on how new contractual arrangements in Northern Ireland would differ significantly from the 2006 contract introduced in England and Wales. 44 More specifically, at that time there was a view that providing a defined range of treatments and rewarding quality would be desirable in a new Northern Ireland contract and that the English UDAs contract currency was not favoured by the profession in Northern Ireland. The report stated:
. . . a commitment to piloting the new contract before rolling out across the region in order that any problems may be addressed and allow GDPs to be familiar with the operational aspects of the contract.
The 2007/8 report commented on a growing concern about access to dental services in Northern Ireland. 45 In response to these concerns, a package of financial support for health service GDPs to increase the registration of NHS patients and a tender for additional dental services were announced in the Northern Ireland Assembly. The report also references a joint communication issued from the DHSSPS and the British Dental Association in September 2007. The key points highlighted in the statement were:
-
The DHSSPS did not intend to introduce the (2006) English contract into Northern Ireland. The DHSSPS was aware of the problems experienced with the new contract in England and intended to learn from these.
-
The contract was to be a block contract but would not be measured in UDAs; alternative remuneration arrangements were to be made.
-
There was to be a strong emphasis on prevention.
-
The new arrangements were to be piloted before rolling out across Northern Ireland.
The report also referenced a commissioned report produced by Professor O’Neill from Queen’s University Belfast to advise on a remuneration model for a new contract. Professor O’Neill’s report advised a blended system of remuneration comprising payment through a block component along with a limited FFS component.
The 2008/9 Dental Branch Annual Report identified access to dental services as ‘very problematic’ and stated that the Minister for Health was prioritising resolution of this problem through ‘a successful outcome to the dental tender’ (contains public sector information licensed under the Open Government Licence v3.0). 46 In May 2009, the Minister for Health awarded a contract to Oasis Dental Care [Oasis Dental Care (Central) Limited, Bupa Dental Care, Bristol, UK], which resulted in the opening of 14 new NHS dental practices, providing care for 57,000 patients, across Northern Ireland operating under pilot PDS contracts (analogous to the pre-2006, wholly capitation-based, English PDS pilots).
Although the access problem dominated policy, work on a new dental contract continued and more details were given about the anticipated remuneration system. A blended payment system was planned, consisting of regular care (capitation) payments along with supplementary item-of-service payments for a clearly defined range of essential services. The Northern Ireland Dental Branch Annual Report of 2008/9 acknowledged publication of the Steele review and noted that the recommendations were consistent with the framework of the proposed Northern Ireland contract. 45
The Northern Ireland Dental Branch Annual Report of 2009/10 provided an update on the initiative to tender for PDS contracts to improve access supported by the £17M injection of funding. 47 More information was again provided on the planned new contract, in particular details about a proposed weighted capitation formula for the proposed capitation element of the blended contract. The weighted capitation formula used to calculate the capitation fee was based on patient age, patient gender, patient socioeconomic position (based on small area measure of deprivation derived from the patient’s postcode) and practice list turnover (as measured by new registrations). It was also anticipated that block payments would be made to reward quality using indicators such as postgraduate qualifications, charter marks, outputs from practice inspections and clinical audit activities.
An update on the proposed pilots of the new contract was provided in the final Dental Branch Annual Report in 2010/11. 48 The NIHSCB began a consultation on the piloting of a new dental contract on 11 October 2010. The consultation ended on 31 January 2011 and was considered by the board of the NIHSCB on 31 March 2011, which supported the outcome of the consultation process, namely that pilot PDS legislation should be used to pilot the elements of the new dental contract.
In 2011, the Patient and Client Council (PCC) published Talking Teeth: Patient Views of General Dental Services in Northern Ireland. 49 The PCC commissioned the report to explore the experiences of patients as they access GDS services and the care that they receive in the NHS. The Chief Dental Officer provided a foreword to the report, which referenced the planned reforms of dental services including proposals to introduce a new dental contract. The report stressed that its findings should be considered within the context of that time and highlighted three key issues:
-
a £17M investment to ease problems of access
-
a planned new general dental contract
-
that economic recession had the potential to change (reduce) public demand for private dental care thereby freeing up capacity in the general dental system for NHS work and potentially helping to alleviate concerns about NHS dental access.
The final point was an interesting comment, as the acute access problem experienced in Northern Ireland was believed to have been caused primarily by GDPs expanding their care in the private sector prior to the financial crisis of 2007/8. The PCC report concluded that patients’ level of satisfaction with dental services was high but that there was confusion about whether or not treatment is provided via an NHS contract or privately. Dental treatment was viewed as expensive and people were worried about the lack of information regarding costs. Access and inequalities in access were concerns and there was support for the suggestion that NHS funding and services should concentrate on a smaller number of basic treatments. A key recommendation was that:
. . . access to basic dental treatments should be prioritised by Health and Social Care.
In June 2016, the NIHSCB produced the report Evaluation of the Oasis Pilot Personal Dental Services (pPDS) Scheme 2010–2015 (Michael Donaldson, NIHSCB personal communication, 2019). The contract started in April 2010 for an initial 3 years then extended for two additional 1-year periods, ending on 31 March 2015. The remuneration system used for these pilots was based on a simple capitation arrangement and co-payments were collected based on the existing item-of-service schedule operated by GDS practices. Capitation payments were based on the 38 GDPs in the programme, each managing a patient list of 1500 patients, resulting in a maximum of 57,000 patients being registered with Oasis Dental Care. Each registered patient attracted an annual fee of £97.74, which resulted in the contract value still being capped at £5.4M per year. A mid-term review of the pPDS was carried out in 2012 by the NIHSCB, approved by the DHSSPS and published in 2013. The mid-term review concluded that access to NHS dentistry had rapidly and significantly improved and that the service appeared to be at least equivalent to the service provided by other (GDS) dental providers in Northern Ireland in terms of the delivery of appropriate, efficient and cost-effective services.
The final report of 2016 concluded that access was not a problem for NHS dentistry in Northern Ireland and that the quality of care provided was comparable to that provided under the GDS. Following completion of the pilot, the 14 Oasis practices moved from pPDS into the GDS. The report commented on the learning, which had been acquired from the pPDS programme to inform the development of a new contract for Northern Ireland, in particular that consideration should be given to how new patients register with dental practices in a capitation-based contract.
The report expressed concerns that it may not be possible to monitor each individual GDP across the whole of Northern Ireland in the same way as the pPDS and that consideration should be given to how monitoring can be scaled up to ensure both quality of care and the level of service provision. There were also concerns that large number of patients who registered with Oasis during the pilot scheme received no treatment. In the pPDS and the GDS, patients aged > 18 years can register with a GDP without ever being examined. The report suggested that by enforcing an examination as part of the registration process more patients might become aware of their treatment needs and receive some preventative advice.
Our research group used data from the pPDS to independently evaluate the effect the newly introduced capitation payment system had on the delivery of primary oral health care and access to services in Northern Ireland. 4,50 This independent study was published in 2017 and treated the pPDS as a natural experiment comparing the NHS treatment claim forms submitted between April 2011 and October 2014 from the 14 Oasis practices with those of a group of matched, control GDS practices, remunerated using the GDS retrospective FFS system. This study could find no evidence of patient selection (excluding high-need patients) in the Oasis practices. However, patients were less likely to visit the GDP and received less treatment when they did attend, compared with those belonging to the control GDS practices. The extent of preventative activity offered did not differ significantly between the two practice groups. The most surprising finding was that there was no difference in PCR between the capitation-remunerated pilot practices and the FFS GDS controls.
In June 2014, the NIHSCB issued a call to all existing GDS practices across Northern Ireland for expressions of interest in participating in wave 1 of the new dental contract pilots and two practices were recruited. This was followed by a similar call in February 2015 for participation in wave 2 of the pilots and a further 11 practices were recruited. The pilot contracts were practice based, not with individual GDPs. The system of remuneration used was based on a form of capitation that was time limited: wave 1 practices would operate under the pilot contract for 18 months and wave 2 practices would operate for 12 months before reverting back to the FFS contract.
In terms of remuneration, the underpinning intention was that practitioners would receive the equivalent gross income during the pilot period that they would have received under the GDS had they maintained their activity and list as per the baseline period (Michael Donaldson and Donncha O’Carolan, NIHSCB, personal communication, 2019). The level and method of payment for patients’ contributions during the pilot would remain the same as for existing GDS patients. The PCR would continue to be collected by the practices and the monthly instalment payments from the BSO to pilot practices would be adjusted to account for the patient charges collected. The risk of any shortfall in patient contributions from the GDPs’ baseline gross income would be carried by the NIHSCB.
In the lead up to the pilots, the Chief Dental Officer posted a policy paper, Current Direction of the New Primary Care (General) Dental Contract, on the DHSSPS website on 6 April 2015, which provided a summary of policy development since 2006 and an update on the thinking of the DHSSPS on the design of the pilots. 51 This paper announced a shift in policy away from the earlier proposed model using a weighted capitation formula supplemented by FFS payments for defined treatments. Instead, the DHSSPS proposed that the latest model for the new dental contract would be calculated through a global sum formula with no routine item-of-service payments. Therefore, the model would be a predominantly simple capitation arrangement with additional payments making up a small proportion of the total remuneration system. It was envisaged that these payments would be determined by developing quality indicators; however, ‘further work on the development of the relevant indicators is necessary’. 51 This change in policy direction was attributed to feedback from GDPs, and was seen as a means of securing a stable budget by avoiding fluctuating costs of FFS claims and producing less complex data returns.
The evolution of a new NHS dental contract in Wales
In Wales, there had been similar dissatisfaction with the 2006 contract, which closely mirrors the current English contract. Dissatisfaction was largely for the same reasons as in England: the 2006 contract did not overtly incentivise prevention or quality and GDPs felt that they were still on a ‘treadmill’, not dissimilar to the treadmill of a FFS system, but with a cap on earnings. In Wales, the pilots for new remuneration systems involved eight dental practices starting in April 2011 and ending in March 2016. 52
Piloting activities could be divided into two phases. In the first phase, two pilots were involved:
-
In the Quality and Outcome Pilot, practices switched their entire GDS contract from the UDA system to a new contract based on a target number of weighted key performance indicators (KPIs), such as number of patients offered a Dental Care Assessment and a capitation target based on the number of unique patients treated in the practice within a defined time period.
-
The Preventive Dental Care for Children and Young People Pilot applied to only patients aged < 18 years. A proportion of each practice’s existing contract value, based on the proportion of children in the practice’s patient profile, was allocated to the pilot. The practices, again, were required to meet a certain number of weighted KPIs, including a capitation target for patients aged < 18 years.
In November 2012, the decision was taken to extend the Quality and Outcome Pilot for an extra year but to discontinue the Preventive Dental Care for Children and Young People Pilot at the end of March 2013, as originally intended. 53 There were some changes to the Quality and Outcome Pilot: a revised Dental Care Assessment was introduced with a disease risk assessment (high, medium or low – similar to England) and the requirement to produce a risk-based preventative care plan for each patient. From April 2015, four of the six pilot practices reverted to their former UDA contract. The remaining two practices were permitted by their health board to continue working under the pilot model into the next financial year, before returning to a UDA contract.
A mixed-methods design was used for the evaluation of the Welsh pilots in a similar approach to that used in England. 49 The main focus was on the qualitative component of the evaluation. Similar findings were reported to those of the evaluation report for the English pilots. The change in remuneration system was universally popular with the GDPs, and there was subjective reporting (by GDPs) of reductions in disease risk and improved oral health of their patients. Like the English pilots, there was a decrease in access: the number of patients seen across all pilot practices ranged between 7% and 10% less than in the year immediately preceding the pilot programme.
Consequently, there was a reduction in PCR: in the first year of the pilot, there was a drop in PCR of over one-third on the previous year under the 2006 arrangements. However, over the 4 years of the pilot, the level of PCR across the practices steadily increased and it was estimated that if the upwards trend in PCR continued at the same rate it would eventually have reached levels similar to the pre-pilot year. However, no time frame was given for when PCR would reach baseline levels. At the end of the pilot programme, the majority of the pilot practices reverted to the 2006 contract and most reported that this has been detrimental to teams and patients, but no specific information was provided on the nature of these detrimental effects. Two of the pilot practices continued as ‘prototype practices’; neither practice is monitored by UDAs but both continue to evolve the model developed in the pilot as part of ‘a learning network’ rather than a formal evaluation.
In August 2017, there was a change of tack in policy. The Welsh Government published Taking Oral Health Improvement and Dental Services Forward in Wales; this policy document outlined the key priorities for NHS dentistry, and contract reform was identified as one of the priorities. 54 The document criticised the current dental contract as being focused on treatment activity and not incentivising needs-led care and prevention. It also criticised the 2006 contract for not encouraging the use of skill mix. The document cited the learning from previous dental pilots in Wales and the ongoing experiences of the dental prototype practices to inform the design of the new programme. Part of this process included the development of a needs and risk assessment tool.
The document did not present a defined ‘new’ dental contract; instead, a more pragmatic approach was advocated to use the flexibility within the existing 2006 contract to produce reform without the need for wholesale change in contract regulations. The document was clear that this approach must be underpinned by robust need and outcome measurement. Examples were given of possible scenarios, such as substituting a proportion of UDAs within a given total contract value by other measures to encourage, for example, a change in care delivery to increase access for high-need groups. This pragmatic approach did not require additional investment and the flexible proportion of the contract could be changed (by negotiation with practices) according to changing population needs.
In February 2018, the Welsh Government announced that from January 2018 a further 23 prototype dental practices would be recruited to expand the flexible adaptation of existing contracts, in particular testing the use of the needs and risk assessment tool. 55 The new Contract Reform programme aims to increase value and quality, based on a needs-based approach to care. Key elements include adopting a needs-led preventive approach to care; extending the use of skill mix as part of prudent dental health care; the acceptance of and provision of care to new patients with higher needs; encouraging ‘well patients’ to attend at longer intervals; and delivering high-quality evidence-based care according to need. As of December 2019, over one-quarter of the dental practices in Wales were participating in the Contract Reform programme.
The evolution of a new NHS dental contract in Scotland
In Scotland, the NHS dental contract has not changed substantially since 1990 and the situation is similar to that in Northern Ireland. The GDPs providing care to children (aged < 18 years) are paid primarily on a capitation basis, receiving a basic monthly fee for the care and treatment of children covering examinations, X-rays, scale and polish, and preventative care. A smaller proportion of the remuneration package for children is made up from additional item-of-service fees for fillings and extractions. The majority of the care provided to adults is still paid on a fee-for-item basis with a small monthly fee payable for the provision of continuing care to registered patients.
In January 2018, the Scottish Government published its Oral Health Improvement Plan, following publication of a consultation exercise in September 2016. 56 The plan is ambitious, setting out 41 actions or commitments to be delivered within a non-specified time scale. Like the other three home countries, there is an aspiration to focus on prevention, highlighting the need for a shift from restorative to preventative dentistry, which was well supported during the consultation exercise.
An explicit action in the plan was to:
. . . change payments to GDPs and introduce a system of monitoring to ensure that all dental practices provide preventive treatment for children.
There was also an aspiration to introduce a preventative system of care for adults by phasing in an oral health risk assessment. This assessment would be more comprehensive than a risk assessment of dental disease alone and would include a comprehensive clinical examination and a discussion about lifestyle choices, including diet, alcohol use and smoking. The patient would receive a personalised care plan based on the outcomes of the assessment.
Interestingly, and in contrast to the other three home nations, there were no plans for scrapping the item-of-service system of remuneration; instead there was an intention to ‘streamline’ item-of-service payments, which would be progressively introduced. NHS health boards would in future take a more proactive approach to the listing of practices, exerting tighter controls on GDPs wishing to set up new practices in their locality. There were also plans to develop a new system to oversee external clinical quality monitoring under the direction of newly appointed directors of dentistry in each health board. The plan echoes the evolutionary approach taken in the prototypes in England and Wales in that:
. . . our intention is to proceed in a progressive manner in order for practices that provide GDS to have the opportunity to plan accordingly whilst maintaining their financial stability.
In terms of changes to remuneration, the plan recognised the current, mixed economy of item of service, capitation and continuing care payments as a strength and stated that the Scottish Government would:
. . . continue to retain a mix of payments going forward, but the balance of payments will change accordingly.
There were no explicit plans described in the document to evaluate the impact of implementing the ≈40 actions set out in the plan or for how the envisaged changes in remuneration would influence and incentivise the behaviours of GDPs.
Summary
As policy has developed in each of the home countries, there are some common threads emerging from the approaches taken. Primarily, there is a desire to focus on and pay for prevention rather than treatment and to approach this via a standardised assessment to identify and categorise a patient’s risk of oral disease and then formulate a personal prevention plan, which will guide the care provided to patients. This approach presupposes that we have validated tools to categorise a patient’s risk with an acceptable degree of precision and that preventative interventions delivered by GDPs in general practice can effectively mitigate this risk. Indeed, the recent findings of the Northern Ireland Caries Prevention in Practice (NIC-PIP) trial suggest that even with high levels of adherence to practice-delivered prevention regimes, caries prevention delivered in practices is very expensive and limited in its effectiveness. 57 The Improving the Quality of Dentistry (IQuaD) trial has recently evaluated the costs and effects of scale and polish, a long-standing treatment provided to maintain gingival and periodontal health, and could find no evidence that this intervention provides a health benefit to patients, but patients want this treatment provided by the NHS. 58 The NIHR-funded INTERVAL trial will report shortly on the effects of different recall (for dental check-ups) on oral health and will compare intervals decided according to the GDP’s judgement of disease risk with standardised recall periods. 39 The idea of GDPs providing prevention rather than treatment can be traced back to the capitation pilots that led to the 1990 contract; it has always received widespread support across the dental profession and chimes with wider NHS policy, but without hard evidence to demonstrate that the significant costs of this approach for the NHS produce a worthwhile return on investment. These pragmatic trials conducted in dental practices will provide strong evidence about prevention provided by GDPs and what is achievable and how affordable it is for the NHS.
In developing a new remuneration system, there is a tension between this desire to shift the emphasis away from treatment to prevention while maintaining co-payment income. Each country is mindful of the need to secure income from PCR, in Scotland through retaining the largely fee-for-item system and in England and Wales through retaining activity targets as part of a blended contract. In an ideal world, incentives in a blended contract would ensure that essential treatment needs of new and existing patients are provided for and that this activity would generate sufficient co-payment income. This supposition is dependent on GDPs’ assessments of the needs of their patients, which could be influenced in an unpredictable way by the details of a new contract, particularly if there are activity targets to hit as part of a blended arrangement. It also supposes that the future needs of the population will require the activity necessary to maintain co-payment revenue, which is a risky assumption particularly if needs assessment is based on historical activity delivered under existing contracts and incentive structures. The literature strongly suggests that a move from FFS to capitation produces a fall in activity and a consequential fall in co-payment revenues. The literature suggests that a change in remuneration produces a large immediate fall in activity before a gradual increase (bounce back) over time (NIHR Health Services and Delivery Research programme 08/1618/158). The evidence base for the level of activity and the way activity changes (profile of treatments) in response to remuneration changes is underdeveloped, particularly why GDPs change their behaviour and the drivers and restraining factors in relation to behaviour change are poorly understood.
Access to NHS care in each country remains a priority and improvement in quality is also an aspiration, albeit with different approaches to achieve these ends. There is a need to define what is meant by access and accessibility. Gaining access for patients who have regularly attended a practice does not seem to be a significant problem but provision for irregular attenders, with high need, often living in more disadvantaged areas seems to be more problematic, which should be a concern for an NHS service.
Concluding remarks
The preceding sections have highlighted a range of issues relating to the NHS dental contract in Northern Ireland. The evidence base is underdeveloped in terms of how and why GDPs’ behaviour changes as a result of changing remuneration systems and how this behaviour change (often in an unintended way) has an impact at the macro level of the volume and type of care provided for populations and has an impact at a micro level on the care provided for individual patients. The pilot contracts in Northern Ireland offered an opportunity for academics and decision-makers in the NHS to investigate these issues in a co-production partnership. Chapter 3 sets out the research questions prompted by the literature review and the circumstances leading up to the commissioning of the dental contract pilots in Northern Ireland.
Chapter 3 Difference in difference
Introduction
The decision by policy-makers in Northern Ireland to pilot an NHS dental contract for adult care based on capitation, rather than the existing largely FFS payment system, offered an opportunity to undertake a mixed-methods study to investigate the impact of a change in the remuneration system in Northern Ireland on productivity and the quality of care provided. As the dental pilots in Northern Ireland were to switch from FFS remuneration to capitation and then back to FFS after 12 months, it provided a unique opportunity to observe and document the scale of effect, the issues around implementation and any unintended consequences of the two changes in remuneration (FFS to capitation, capitation to FFS). [Although the current NHS dental contract in Northern Ireland is described as FFS in this report, remuneration across the province has three elements: (1) a fee per item of service – approximately 60% of remuneration received by GDPs and the majority of care provided to adults is FFS; (2) capitation and continuing care payments – approximately 20% of remuneration received by GDPs (predominantly children); and (3) practice allowances – approximately 20% of remuneration received by GDPs (and payable to designated dentists or PPs).]
The evidence from the literature would suggest that practitioners respond very quickly to changes in remuneration systems to ensure the viability of their practices. 5–7 Changes to the NHS dental contract in England in 2006 saw an immediate drop in specific areas of clinical activity that reduced profit margins for the practice and an increase in clinical activity in areas where profit margins could be improved. 6,7
The aim of the research was to evaluate the impact of a change in the system of provider remuneration on the productivity, quality of care and health outcomes of NHS dental services in Northern Ireland. The objectives are highlighted in Chapter 2.
Methods
Permissions were granted from the University of Manchester Research Ethics Committee (reference 15236) on 10 June 2015.
Study design
A DiD design was employed to quantitatively measure any change in activity levels across the intervention and control groups in each of the three phases of the study:
-
phase 1 – 12-month baseline period prior to introduction of the new capitation contract in the intervention group of practices (August 2014 to August 2015)
-
phase 2 – 12-month capitation period for the intervention group of practices (August 2015 to August 2016)
-
phase 3 – 12-month period following reversion of the intervention group of practices back to FFS (August 2016 to August 2017).
Figure 1 provides a graphical depiction of the DiD design, which compared the difference in outcomes before and after the change in the contract model in the pilot practices with outcomes in a group of matched controls. 59
FIGURE 1.
The DiD design used in the study.
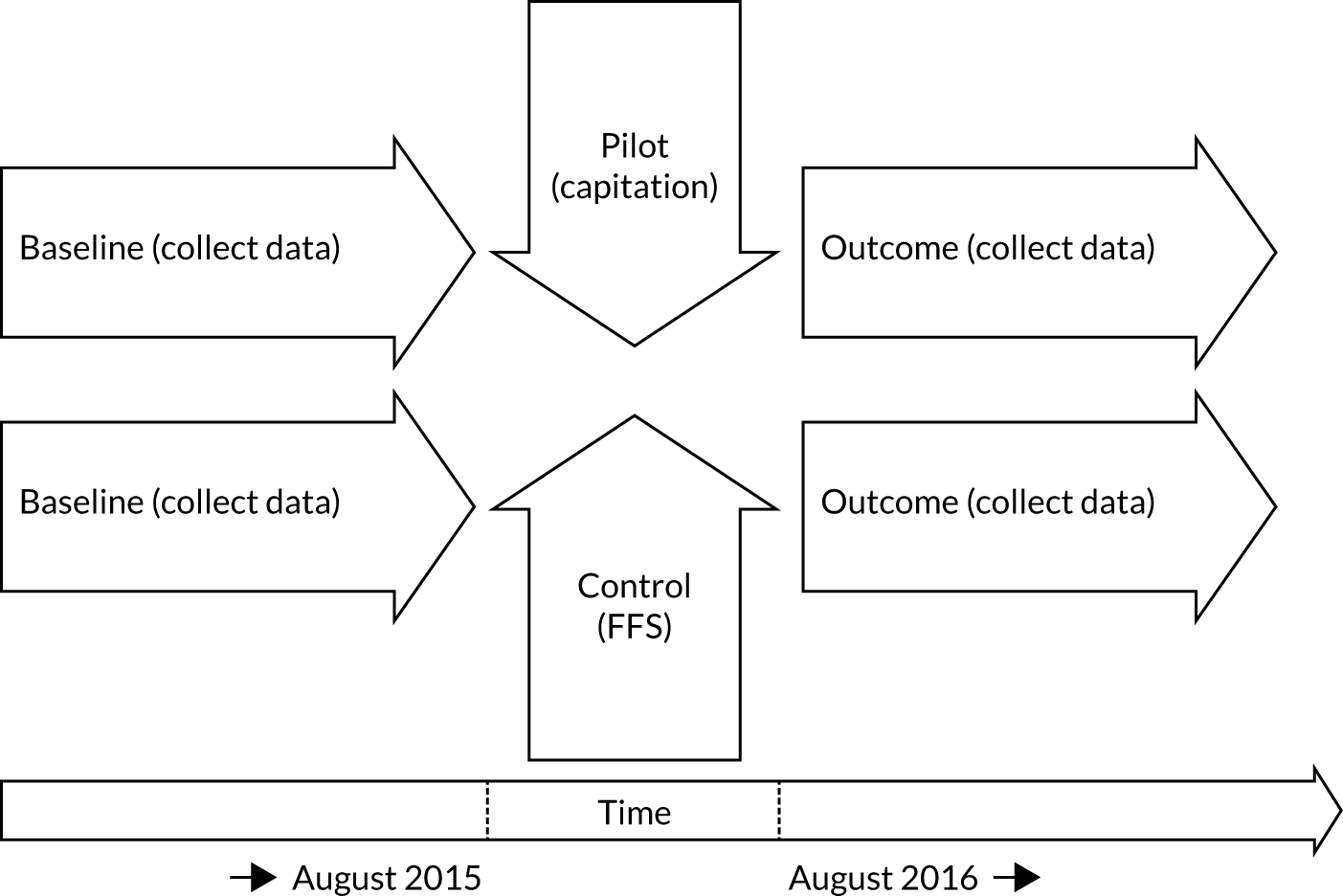
The DiD estimator examined the impact of the contractual change in the intervention practices compared with the control practices at the following points of change:
-
baseline FFS to capitation
-
capitation to reversion FFS
-
baseline FFS to reversion FFS.
Analyses were performed at the practice level. A single outcome measure was used in each DiD model. Clustered standard errors (SEs) were determined to adjust for estimates of the correlation over time. This is because outcomes measured at the patient level (e.g. number of patients, number of treatments delivered to patients) could be influenced over time by a higher-level structure (the practice).
We also used the same DiD approach and outcome measures to analyse activity data at the individual GDP level. The analyses assessed changes in behaviour for the average number of ‘designated GDPs’ in each practice and the average number of ‘other’ GDPs with an NHS contract. A designated GDP is a GDP who is paid practice allowances by the NHS in Northern Ireland. These allowances are to help cover some of the running costs of NHS dental practices. A ‘designated GDP’ is likely to be an equity-owning PP and is labelled as such in the text and results tables. A second analysis was undertaken for all (other) GDPs with an NHS contract who were not ‘designated GDPs’. These were likely to be ADs (non-equity owning) working at the practice and are labelled as such in the text and results tables.
Table 1 describes the outcome measures used in this study. Three broad groups of outcome measures were chosen to assess the impact of the change in remuneration on:
-
access
-
treatment (service mix)
-
finance.
| Outcome label | Description of outcome | Definition of the measure |
|---|---|---|
| Access outcome 1 | Mean number of registrations | This is a measure of the number of registered patients on the practice list. Patients are registered with an NHS GDP. Practice list size is reduced if a registration expires. This occurs if a patient does not receive NHS treatment from their registered GDP in a period of 2 years from their last registration date. Reregistration occurs whenever a patient receives treatment from their registered GDP. Treatment by a different GDP at the same practice does not reduce the size of the practice list, as it transfers the patient registration to that GDP (i.e. a reregistration). Treatment by an NHS GDP at a different practice does reduce the practice list size as it transfers the registration to a GDP at the other practice |
| Access outcome 2 | Mean number of reregistrations | This measure is a proxy for the amount of access to care during the study period for patients who sought access to dental treatment from the practice prior to the start of study. The numerator of this measure is the number of reregistrations for patients on the practice list at the start of the baseline period. Hence, this measure observes only patients who are continuously registered throughout the entire study period of 3 years. Reregistration occurs whenever a patient receives treatment from their registered NHS GDP or an NHS GDP at the same practice. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Access outcome 3 | Mean number of new patients | The numerator is the mean number of patients who joined the practice list in a month and have never appeared on the practice list before. The denominator of this ratio (per 1000 registered patients) is the average number of registrations in each practice over the baseline and intervention study periods. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Access outcome 4 | Proportion of lapsed patients who returned to the practice list | The numerator is the mean number of patients returning to the practice register in 1 month after exit from the practice list. Exit is because of registration expiring after 2 years of no treatment at the practice or because the patient received NHS treatment at a different dental practice. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Access outcome 5 | Mean number of patients lost to the practice | The numerator is the mean number of registrations that exited the practice list in a month and did not return to the list at a later date. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 1 | Mean number of treatments with a gross cost of ≥ £280 | The numerator is the mean number of treatments with a gross remuneration income to the GDP (cost to the NHS) of ≥ £280 per month. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 2 | Mean number of direct restorations | The numerator is the mean number of direct restorations per month. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 3 | Mean number of indirect restorations | The numerator is the mean number of indirect restorations per month. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 4 | Mean number of extractions | The numerator is the mean number of extractions per month. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 5 | Mean number of radiographs | The numerator is the mean number of radiographs per month. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 6 | Mean number of fissure sealants | The numerator is the mean number of fissure sealants per month. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 7 | Mean number of two or more visits periodontal treatments | The numerator is the mean number of two or more visits periodontal treatments per month. This is a course of periodontal treatments that required two or more separate patient visits to the GDP. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 8 | Mean number of root canal treatments | The numerator is the mean number of root canal treatments per month. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 9 | Mean number of treatment plans | The numerator is the mean number of treatment plans per month. A treatment plan is a submitted claim form for a patient, which records a single course of treatment. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Treatment outcome 10 | Mean number of treatment items | The numerator is the mean number of treatment items per month per 1000 registrations. This measure excludes examinations as a treatment item. The denominator of this ratio measure (per 1000 registered patients) is the average number of registrations on the practice list in each month |
| Finance outcome 1 | Mean percentage of patient fee contribution to NHS dental practice income | Patient fee contribution is the gross amount of income from patients paying contributions towards the cost of the NHS care they received. Some patients entitled to free dental care, for example those who are aged < 18 years, are pregnant or have had a baby in the previous 12 months, or are on income support. Income is the amount of NHS gross earnings, excluding any remuneration by way of salary. Gross earnings are mainly from item-of-service payments although they also include continuing care and capitation payments, seniority payments, vocational trainer’s grant and dental service income (but not salary), continuing professional development allowance, clinical audit allowance, maternity payments, paternity payments and adoptive leave payments, sickness payments and peer review scheme payments. The measure does not include block transfers (capitation payment) for participation in the pilot. For the pilot practices/GDPs in the capitation period this measure is the amount of NHS income they would be receiving in item-of-service payments and allowances if they were not enrolled in the pilot |
| Finance outcome 2 | Mean NHS dental practice income | Income is the amount of NHS gross earnings received, excluding any remuneration by way of salary. Gross earnings are mainly from item-of-service payments although they also include continuing care and capitation payments, seniority payments, vocational trainer’s grant and dental service income (but not salary), continuing professional development allowance, clinical audit allowance, maternity payments, paternity payments and adoptive leave payments, sickness payments and peer review scheme payments. The measure does not include block transfers (capitation payment) for participation in the pilot. For the pilot practices/GDPs in the capitation period this measure is the amount of NHS income they would be receiving in item-of-service payments and allowances if they were not enrolled in the pilot |
| Finance outcome 3 | Mean patient contributions | Patient fee contribution is the gross amount of income from patients paying contributions towards the cost of the NHS care they received. Some patients are entitled to free dental care, for example those aged < 18 years, those who are pregnant or have had a baby in the previous 12 months, and those on income support |
The impact on access is important to assess, as one would expect capitation to increase access, with GDPs wishing to ensure that they hit their capitation targets. The outcome measures used to measure patient access to services were different types of registration as a proportion of the total number of patients on the practice list (NHS dental registration lasts for a period of 24 months):
-
mean (total) number of registrations
-
mean number of reregistrations (rollover of existing registrations)
-
mean number of new patients
-
proportion of lapsed patients who returned to the practice list
-
mean number of patients lost to the practice.
The service mix measures were chosen to identify any changes in care provision, which would be important to policy and can be broadly categorised as:
-
complex treatment involving extensive clinical time or work involving a dental laboratory [endodontics, indirect restorations (crowns) and dentures]
-
treatment of established disease – direct restorations (fillings) and extractions
-
preventative care, for example examinations, fissure sealants, radiographs, two-visit periodontal treatments
-
other composite measures of activity (number of treatment plans and treatment items).
The third group of outcome measures were financial in nature and are important from a policy perspective and to dental practices as small businesses. Financial measures included total health service income and revenue from patients’ charges. Data on income from private practice was not available.
The contents of the HS45 form provided the data for access, service activity and financial information; this form is replicated in Appendix 1. This form is the Northern Ireland equivalent of the FP17 form used by the NHS in England. It is a means of claiming payment for any care or treatment detailed in the Statement of Remuneration. 60 Most practices submit their claims for treatment completed electronically and all the information included in the paper HS45 form is replicated in the electronic version. A HS45 or electronic equivalent is submitted every time a health service course of treatment (including examination only) is claimed. The GDPs also usually tick the part 3 ‘reregistration’ element in order to roll over the patient’s registration for a further 24 months.
The BSO of Northern Ireland is responsible for collating and paying claims for activity. The BSO extracted data from the submitted HS45 forms for both intervention and control practices for the analyses. Prior to analysis, the BSO cleaned the data set to:
-
prevent the block transfer of patients between GDPs working in the same practice appearing as new patients
-
identify and separate treatment item ‘tail data’ (treatments that took place towards the end of one study period that were claimed in the following study period)
-
identify large fluctuations over time in treatment activity caused by maternity leave, major business change (newly started practices hiring staff or closing practices transferring patients to other practices) or takeover activity.
The outcome measures for access and service mix were calculated relative to the practice list size (per 1000 patient registrations). This was important because analyses were based on a comparison of group averages (e.g. changes between study periods within and between control and intervention practices). Using proportionate measures gave each practice an equal weight in determining the group average. Measures of activity alone (not relative to practice size) would have given greater weight in the calculations in our analysis to a small number of larger pilot practices.
Population
The population under study was practices with an NHS contract in Northern Ireland. A selection process overseen by the DHSSPS and NIHSCB was undertaken to select the practices in the intervention group (i.e. those whose process of payment would move across to one based on a capitation-type payment). Prior to the evaluation described here, the DHSSPS and NIHSCB had recruited two practices to a pre-pilot stage that started on 13 November 2014 and had run for 6 months. The purpose of this pre-pilot stage was to determine the financial risk (modelling the drop in PCR). This helped the DHSSPS and NIHSCB ensure that the budget for the full pilot was underwritten.
To select the intervention practices, the DHSSPS and NIHSCB arranged evening meetings in November and December 2014 for GDPs to raise awareness about the pilots. Practices expressing an interest in participating attended information evenings held on 2 and 3 March 2015. Offers were made to practices submitting a formal application to participate on 8 May 2015. The inclusion criteria were determined by the NIHSCB and detailed in an expression of interest document. Practices had to:
-
be a provider in one of the following categories –
-
a health and social care trust
-
a GDP whose name is included in a dental list
-
an NIHSCB employee or pilot scheme employee
-
a qualifying body
-
an individual who is providing PDS
-
-
have a commitment to the NHS maintained for the duration of the pilot
-
have full registration with the General Dental Council
-
have appropriate indemnity
-
have completed a vocational training/dental foundation training programme (or have an appropriate exemption).
The final selection of the intervention group was undertaken by an internal NIHSCB panel using the criteria above. Additional criteria that were also considered included practice size (classified as small or large), geography (classified as urban or non-urban) and extent of health service commitment (classified as above or below average). A total of 21 practices submitted expressions of interest to be involved in the pilots: two did not meet the essential criteria – one did not meet the minimum 50% commitment to the NHS and one was unable to provide a service for 37.5 hours per week. A total of 12 practices were selected by the panel and financial templates were produced for all of the selected practices. The selected practices had 2 weeks to consider the expression of interest document and to reply by 22 May 2015; nine practices subsequently signed the service-level agreement to take part in the pilot. These nine practices were added to the two practices that participated in the first-wave practices, making a total of 11 practices who participated in this study. In our original protocol we stated that we would ‘conduct 2 focus groups with dentists involved in the pilot practices to discuss any observed changes in activity and identify areas that dentists feel the simple capitation contract can be improved’. These focus groups did not take place as the DHSSPS/NIHSCB decided that the two pilot practices would be included in the full evaluation. In addition, the DHSSPS/NIHSCB decided to proceed with a simple capitation contract with no changes to that used in the first-wave pilot. These 11 practices and their patients, who were registered during the study period, made up the population who received the intervention. The characteristics of the intervention practices are set out in Tables 3 and 4.
The intervention period lasted 12 months, during which time each practice was required to maintain a health service registered patient list consistent in size and profile with the list registered under the practice on 31 December 2014. The contract stipulated that pilot practices would be closely monitored for any variance outside the tolerance of a decrease in registration of 5%, in which case practices would be asked to address any variance outside the tolerance with the possibility of renegotiation of contract terms and conditions. The level of remuneration under capitation was based on each practitioner’s gross NHS earnings during a reference period, which ran from 1 January 2014 to 31 December 2014. This included appropriate prospective adjustments to reflect and subsequent changes to the Statement of Dental Remuneration made in the period leading up to the start of the pilot. During the pilot, practitioners received their agreed gross income paid in 12 equal monthly instalments. In August 2015, the intervention practices switched to the new capitation contract and returned to FFS 12 months later.
Selection of the control practices
It is not possible to test for the presence of observable characteristics that influence the recorded observations in natural experiments (i.e. there is no certainty that the DiD estimates are free from selection bias). To assess how violations of unconfounded treatment assignment (i.e. selection bias) could affect our study conclusions, we tested the sensitivity of the DiD estimates by using a matched control group of practices with characteristics (which might influence participation in the pilot) that overlapped with the intervention group as closely as possible.
The control group of practices was selected using a two-stage process. The first stage was by stratified random sampling of all NIHSCB practices in Northern Ireland using the following strata: practice list size, proportion of patients who are children, proportion of adults exempt from NHS fees and geographic location of the practice (region size defined by the Northern Ireland Small Areas Code in the 2011 Census geography61). Out of a total of 45 practices initially identified, 15 could not be used because of data inconsistencies, leaving 30 potential control practices. The data inconsistencies were large gaps in the data in some months followed by spikes in the data recorded in later months as the backlog of data records was processed. This occurred because of a change in a computer system that processed dental data sent by practices to the Health and Social Care Board in Northern Ireland. The second stage of selecting control practices involved matching the 11 intervention practices to control practices using a propensity score (PS) approach, which identified 18 matched control practices.
In a DiD model, selection of the intervention group causes the control group to be a poor approximation of the ‘unobservable outcome’ used to estimate a causal effect of treatment in the model, when there are unobserved influences that affect participation in the pilot as well as the outcomes of practices in the intervention group and control group, or both. For example, the standard DiD estimator will have bias in our study if, regardless of the stratified sampling method that was used to (initially) select the control group, the practices that were motivated to join the pilot (intervention practices) have a higher or lower probability of responding to remuneration incentives by altering their health-care provision than a situation in which intervention practices were instead chosen at random and hence were not particularly motivated to join the pilot.
A probit model was used to determine whether or not there were any practice characteristics that influenced the decision to participate in the pilot: 41 observations (baseline) from 30 control practices and 11 intervention practices were tested (Table 2). No statistically significant differences were found between the two groups.
| Explanatory variables | Coefficient | Robust SE | p-value | 95% CI |
|---|---|---|---|---|
| Mean number of registrations in a baseline period month | 0.0002 | 0.0002 | 0.27 | –0.0002 to 0.001 |
| Mean multiple deprivation measure decile (at patient’s current registered address) of patients treated in a baseline period month | –0.45 | 0.3 | 0.16 | –1.1 to 0.2 |
| Mean proportion of treatments that were with women patients in a baseline period month | 12.2 | 13.5 | 0.36 | –14.1 to 38.7 |
| Mean proportion of treatments to patients exempt from fee paying (when treatment was carried out) in a baseline period month | 6.7 | 5.0 | 0.18 | –3.2 to 16.6 |
| Mean proportion of patients (when treatment was carried out) aged ≤ 17 years in a baseline period month | 3.7 | 12.8 | 0.77 | –21.4 to 28.7 |
| Mean proportion of patients (when treatment was carried out) in the age range of 18–39 years in a baseline period month | 5.0 | 6.8 | 0.47 | –8.4 to 18.4 |
| Mean proportion of patients (when treatment was carried out) in the age range of 40–59 years in a baseline period month | 10.8 | 11.1 | 0.33 | –11.0 to 32.7 |
| Pseudo-R2 | 0.23 | |||
| Log-likelihood | –18.9 | |||
Regardless of these findings, selection bias cannot be ruled out in this study if there were unobserved characteristics, which influenced the motivation of practices to participate in the pilot. We examined the plausibility of this possibility by considering whether or not there were fixed differences between the control and intervention practices in the number of different kinds of treatments delivered in the baseline year. The results are presented in Tables 3 and 4.
| Characteristics of practices | Mean intervention | Mean control | Difference | t-statistic | p-value |
|---|---|---|---|---|---|
| Mean number of examination treatments in a baseline period month | 364.53 | 328.9 | 35.63 | 0.39 | 0.70 |
| Mean multiple deprivation measure decile (at patient’s current registered address) of patients treated in a baseline period month | 5.42 | 5.82 | –0.4 | –0.89 | 0.38 |
| Mean proportion of treatments that were with women patients in a baseline period month | 0.56 | 0.55 | 0.01 | 0.28 | 0.78 |
| Mean proportion of treatments to patients exempt from fee paying (when treatment was carried out) in a baseline period month | 0.68 | 0.66 | 0.02 | 0.51 | 0.61 |
| Mean proportion of patients (when treatment was carried out) in the age range of 18–59 years in a baseline period month | 0.76 | 0.74 | 0.02 | 1.26 | 0.21 |
| Mean proportion of patients (when treatment was carried out) aged ≥ 60 years in a baseline period month | 0.23 | 0.25 | –0.02 | –1.38 | 0.17 |
| Baseline variables | Mean control (SE) | Mean intervention (SE) | Difference | 95% CI of difference | p-value |
|---|---|---|---|---|---|
| Mean number of treatments with a gross cost of ≥ £280 in a baseline period month | 3.2 (0.28) | 3.8 (0.46) | –0.55 | –1.75 to 0.65 | 0.37 |
| Mean number of examination treatments in a baseline period month | 330.7 (12.4) | 364.4 (21.3) | –33.7 | –86.8 to 19.4 | 0.21 |
| Mean number of direct restoration treatments in a baseline period month | 230.3 (7.7) | 237 (12.7) | –6.7 | –39.5 to 26.0 | 0.69 |
| Mean number of indirect restoration treatments in a baseline period month | 21.5 (1.0) | 22.1 (1.3) | –0.59 | –4.6 to 3.4 | 0.77 |
| Mean number of extraction treatments in a baseline period month | 33.2 (1.2) | 32.7 (1.7) | 0.50 | –4.7 to 5.7 | 0.85 |
| Mean number of radiograph treatments in a baseline period month | 241.7 (9.6) | 249.9 (16.9) | –8.2 | –49.6 to 33.2 | 0.70 |
| Mean number of fissure sealant treatments in a baseline period month | 12.5 (0.70) | 17.8 (1.2) | –5.3 | –8.3 to –2.3 | < 0.01 |
| Mean number of two or more visit periodontal treatments in a baseline period month | 15.1 (0.85) | 19.1 (1.7) | –4.0 | –7.7 to –0.23 | 0.04 |
| Mean number of root canal treatments in a baseline period month | 16.6 (0.70) | 16.5 (0.89) | 0.15 | –2.8 to 3.1 | 0.92 |
Fissure sealants was the only outcome measure for which the monthly number of treatments delivered during the baseline period differed statistically significantly between intervention and control practices. This suggests that unobservable characteristics that could influence treatment outcomes were likely to be absent or balanced between the two groups. Taken with the evidence of no statistical differences between the groups in the practice characteristics presented in Tables 2–4, it appears that the assignment of practices to the control group was as good as it could be without randomisation. However, the findings in Tables 2–4 are best understood as exploratory work and it is possible that statistically insignificant differences are explained by a low sample size of practices. Further detail about the selection process for the controls can be found in Appendix 2.
In the analysis, we undertook DiD analyses on the initial 30 control practices (henceforward referred to as ‘unmatched controls’) and the 18 matched control practices identified by propensity scoring (henceforward referred to as ‘matched controls’) and compared the difference in outcomes. To avoid unnecessary clutter in the results section, we present the results of the matched controls. The full analysis, which includes the unmatched controls, is in Appendix 3.
Multiple testing
There was a large number of outcome measures (n = 22); this initially included two additional variables to measure different types of fissure sealants, which are not included in this report. These outcome measures used data provided by the BSO, and each outcome measure was a dependent variable in three types of DiD models [i.e. practice level (main analysis), PPs and ADs] that were estimated with and without the use of a PS matching process. Consequently, a large number (n = 132) of DiD estimates (variables) were examined for statistical significance, and the more variables that were tested, the more likely a variable would be statistically significant by chance. Specifically, for the 132 DiD models used, the chance of finding one or more significant differences in the DiD estimator was 1 – (0.95) to the power of 132 or 99.89%, which is the chance that one of the estimators in the models will appear to be significant purely by chance. Šidák and Bonferroni corrections are two methods to counteract the problem of multiple comparisons. For each model, the Šidák and Bonferroni adjustments were calculated (based on the correlation of outcome measure to all other outcomes and the number of observations in that model) and reported in the results tables to identify whether or not either adjustment lowered the critical value of 0.05 to below the p-value of the DiD estimate. If the Šidák and Bonferroni adjustments were above the p-value of the estimate, this indicated that even though the chance of a rare event (incorrectly rejecting a null hypothesis, i.e. making a type I error) had increased because multiple hypotheses were tested, the likelihood of this rare event occurring did not increase to such an extent that the null hypothesis (that the DiD estimate is zero) could no longer be reasonably (i.e. a decision based on the sample distribution of estimate) rejected.
Results of the difference-in-difference analyses
These results for each type of outcome measure (access, service mix and financial) are summarised in the next three sections. All analyses were evaluated using a 0.05 threshold level of statistical significance. A narrative summary of these findings is included below. All figures are expressed per month per 1000 registered patients to adjust for the different size of the practices. Analyses for PPs and ADs are presented in Appendix 4. Findings are presented after adjusting for multiple significance tests undertaken, as appropriate (full details are in Appendix 3).
Results: access outcomes
The difference between intervention and control practices in the number of registered patients significantly increased during the capitation period (p < 0.01) by 1.5 registrations per month per 1000 registered patients (Tables 5 and 6; Figure 2) when compared with baseline and decreased after the capitation period. This was caused by an increase in registrations in the intervention practices. No statistically significant difference was found between FFS at baseline and at reversion.
| Test group | Practice group, study period | Registered patients | Reregistrations | New patients | Lapsed and returned patients | Patients lost to the practice | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Control | Baseline FFS to capitation | 3.14 | 2.93 to 3.35 | 27.55 | 24.29 to 30.81 | 19.81 | 18.73 to 20.90 | 9.72 | 8.83 to 10.61 | 22.25 | 18.92 to 25.59 |
| Capitation to reversion FFS | 2.66 | 2.46 to 2.86 | 31.88 | 29.56 to 34.21 | 20.91 | 18.33 to 23.50 | 12.33 | 11.19 to 13.47 | 28.00 | 20.62 to 35.38 | |
| Baseline FFS to reversion FFS | 2.51 | 2.33 to 2.70 | 38.86 | 36.24 to 41.49 | 15.34 | 14.40 to 16.28 | 19.52 | 17.76 to 21.27 | 15.78 | 14.31 to 17.25 | |
| Intervention | Baseline FFS to capitation | 4.38 | 3.90 to 4.86 | 29.15 | 25.57 to 32.73 | 11.15 | 10.19 to 12.11 | 23.40 | 20.13 to 26.66 | 9.16 | 7.86 to 10.46 |
| Capitation to reversion FFS | 5.15 | 4.64 to 5.65 | 38.73 | 35.05 to 42.40 | 8.46 | 6.88 to 10.04 | 0.60 | 0.45 to 0.74 | 11.67 | 10.32 to 13.01 | |
| Baseline FFS to reversion FFS | 3.52 | 3.13 to 3.91 | 45.75 | 41.63 to 49.87 | 10.49 | 9.29 to 11.70 | 1.22 | 1.03 to 1.42 | 15.90 | 14.51 to 17.29 | |
| Practice group, study period | Registered patients | Reregistrations | New patients | Lapsed and returned patients | Patients lost to the practice | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | 1.45 | < 0.01 | 6.00 | 0.35 | –0.94 | 0.52 | –27.10 | < 0.01 | 1.96 | 0.81 |
| Capitation to reversion FFS | –1.35 | < 0.01 | –3.30 | 0.57 | 6.82 | < 0.01 | –7.82 | < 0.01 | 13.44 | 0.01 |
| Baseline FFS to reversion FFS | 0.13 | 0.82 | 2.94 | 0.49 | 5.66 | < 0.01 | –33.68 | < 0.01 | 15.63 | 0.02 |
FIGURE 2.
Mean number of registered patients.
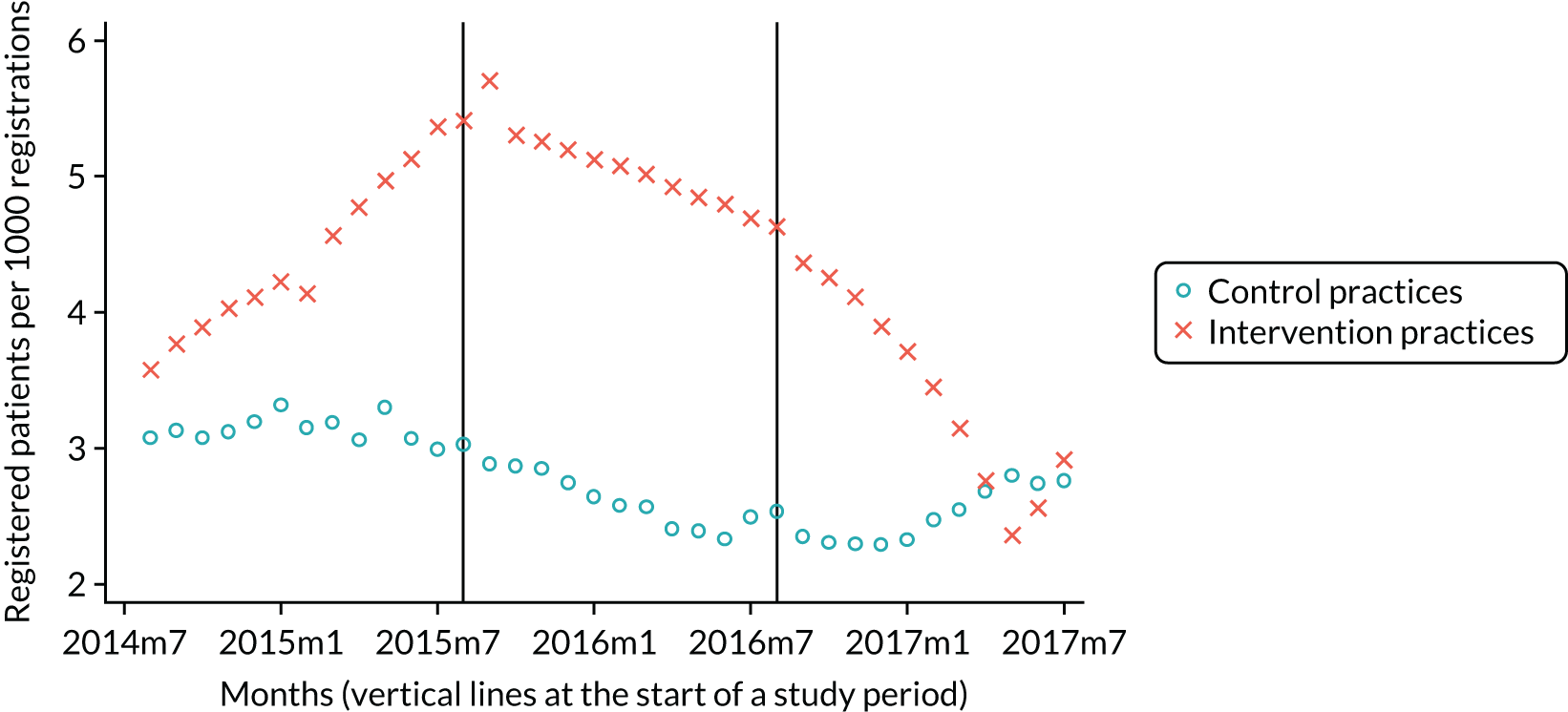
There was no statistically significant difference in the number of patients who were ‘rolled over’ (i.e. when patients already registered with the practice have a new course of treatment and reregister) (see Tables 5 and 6; Figure 3).
FIGURE 3.
Mean number of reregistrations.

The difference between intervention and control practices, in terms of the number of new patients (i.e. the difference in activity between intervention and control practices per month per 1000 registered patients), fell significantly (p < 0.01) between the reversion period and the capitation period, by 6.8 new registrations, and between the reversion period and baseline, by 5.7 new registrations (see Tables 5 and 6; Figure 4). These statistically significant changes were as a result of changes over time in the control group. There was a statistically significant decrease (p < 0.05) in newly registered patients per month in control practices in the reversion period compared with the capitation period, whereas the only statistically significant change in intervention practices was a decrease during the capitation period compared with FFS at baseline (p < 0.05). The non-significant (p > 0.05) difference between intervention and control practices in the number of new patients joining the practice list per month when capitation was compared with the FFS baseline period suggests that the pilot contract did not cause an immediate change in new registrations in intervention practices compared with control practices.
FIGURE 4.
Number of new patients.

The difference between intervention and control practices in the number of lapsed patients was significant and was caused by a reduction of 27.1 registrations per month per 1000 registered patients. This was caused by a reduction in the number of lapsed patients in the intervention group. The difference increased further by 7.8 registrations per month per 1000 registered patients in the reversion FFS period (see Tables 5 and 6; Figure 5). This was because of a comparatively large reduction (p < 0.05) in returning patients, of 22.8 registrations per month per 1000 registered patients, in intervention practices in the capitation period, and there was an increase (p < 0.05) in returning patients in control practices of 2.61 registrations per month per 1000 registered patients. The number of returning patients in control practices increased (p < 0.05) after the capitation period by 7.19 registrations per month per 1000 registered patients, and the increase in intervention practices was much smaller, with 0.62 returning patients per month per 1000 registered patients (p < 0.05). There was a large change of 33.7 returning patients per month per 1000 registered patients (p < 0.05) in the difference between intervention and control practices in the number of registered patients on the practice list per month when the FFS reversion period was compared with the FFS baseline period (see Tables 5 and 6). This suggests that the changes that occurred in the capitation period in intervention practices compared with control practices did persist after the pilot had ended.
FIGURE 5.
Proportion of lapsed patients who returned to the practice list.
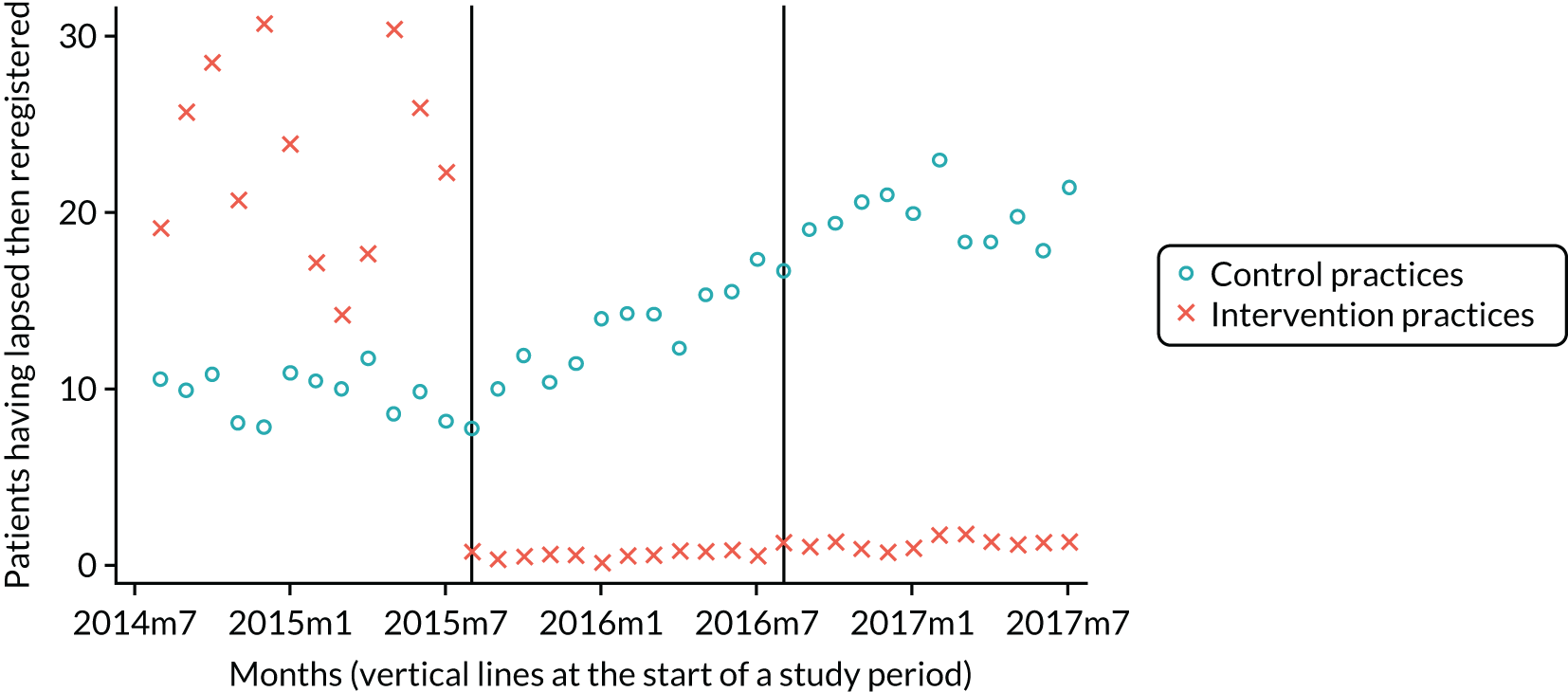
There was no statistically significant change in the difference between intervention and control practices when the capitation period was compared with FFS at baseline, but there was when compared with the FFS reversion period (p = 0.01) and when FFS in the reversion period was compared with FFS during the baseline period (p = 0.02). For an average practice, the difference decreased in the reversion period by 13.4 patients lost to the practice per month per 1000 registered patients when compared with the capitation period, and 15.6 patients lost to the practice per month per 1000 registered patients when compared with FFS at baseline (see Tables 5 and 6; Figure 6). This was as a result of a statistically significant decrease (p < 0.05) in patients lost to the practice in control practices during the reversion period compared with the capitation period, whereas there was a statistically significant increase in patients lost to the practice in intervention practices (p < 0.05). There was not a statistically significant change (p > 0.05) in the per-month number of patients lost to the practice in the control and intervention practices in the capitation period compared with the baseline period.
FIGURE 6.
Number of patients lost to the practice.

Results: treatment outcomes
The difference between intervention and control practices in the mean number of treatments with a gross cost of ≥ £280 per month per 1000 registrations did not significantly change (p > 0.05) between study periods (Tables 7 and 8; Figure 7). There was also no significant difference (p > 0.05) in the number of treatments with a gross cost of ≥ £280 per month per 1000 registrations delivered by PPs in intervention and control practices and no significant difference (p > 0.05) for ADs (see Appendix 4).
| Test group | Practice group, study period | ≥ £280 | Direct restorations | Indirect restorations | Extractions | Radiographs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Control | Baseline FFS to capitation | 3.36 | 2.87 to 3.86 | 220.7 | 211.7 to 229.6 | 15.00 | 14.09 to 15.91 | 30.25 | 28.70 to 31.80 | 151.91 | 144.42 to 159.39 |
| Capitation to reversion FFS | 3.35 | 2.88 to 3.81 | 228.0 | 217.8 to 238.2 | 15.13 | 14.18 to 16.08 | 31.56 | 29.79 to 33.33 | 161.25 | 153.50 to 169.00 | |
| Baseline FFS to reversion FFS | 2.91 | 2.54 to 3.29 | 213.9 | 204.5 to 223.4 | 14.85 | 13.97 to 15.74 | 31.31 | 29.56 to 33.05 | 160.88 | 152.91 to 168.85 | |
| Intervention | Baseline FFS to capitation | 2.40 | 1.80 to 3.00 | 149.2 | 141.4 to 157.1 | 11.52 | 10.50 to 12.55 | 21.71 | 20.25 to 23.18 | 112.70 | 104.48 to 120.92 |
| Capitation to reversion FFS | 1.98 | 1.61 to 2.35 | 105.6 | 99.5 to 111.6 | 8.38 | 7.57 to 9.20 | 18.08 | 17.03 to 19.12 | 86.23 | 80.20 to 92.25 | |
| Baseline FFS to reversion FFS | 1.64 | 1.35 to 1.94 | 153.3 | 145.4 to 161.2 | 10.25 | 9.45 to 11.04 | 20.75 | 19.39 to 22.12 | 118.67 | 109.08 to 128.25 | |
| Practice group, study period | ≥ £280 | Direct restorations | Indirect restorations | Extractions | Radiographs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | –0.56 | 0.17 | –48.98 | < 0.01 | –3.69 | < 0.01 | –6.77 | < 0.01 | –40.93 | < 0.01 |
| Capitation to reversion FFS | 0.01 | 0.97 | 61.95 | < 0.01 | 2.29 | < 0.01 | 5.63 | < 0.01 | 34.13 | < 0.01 |
| Baseline FFS to reversion FFS | –0.63 | 0.25 | 12.98 | 0.24 | –1.40 | 0.11 | –1.15 | 0.50 | –6.80 | 0.52 |
FIGURE 7.
Monthly number of treatments with a gross cost of ≥ £280.
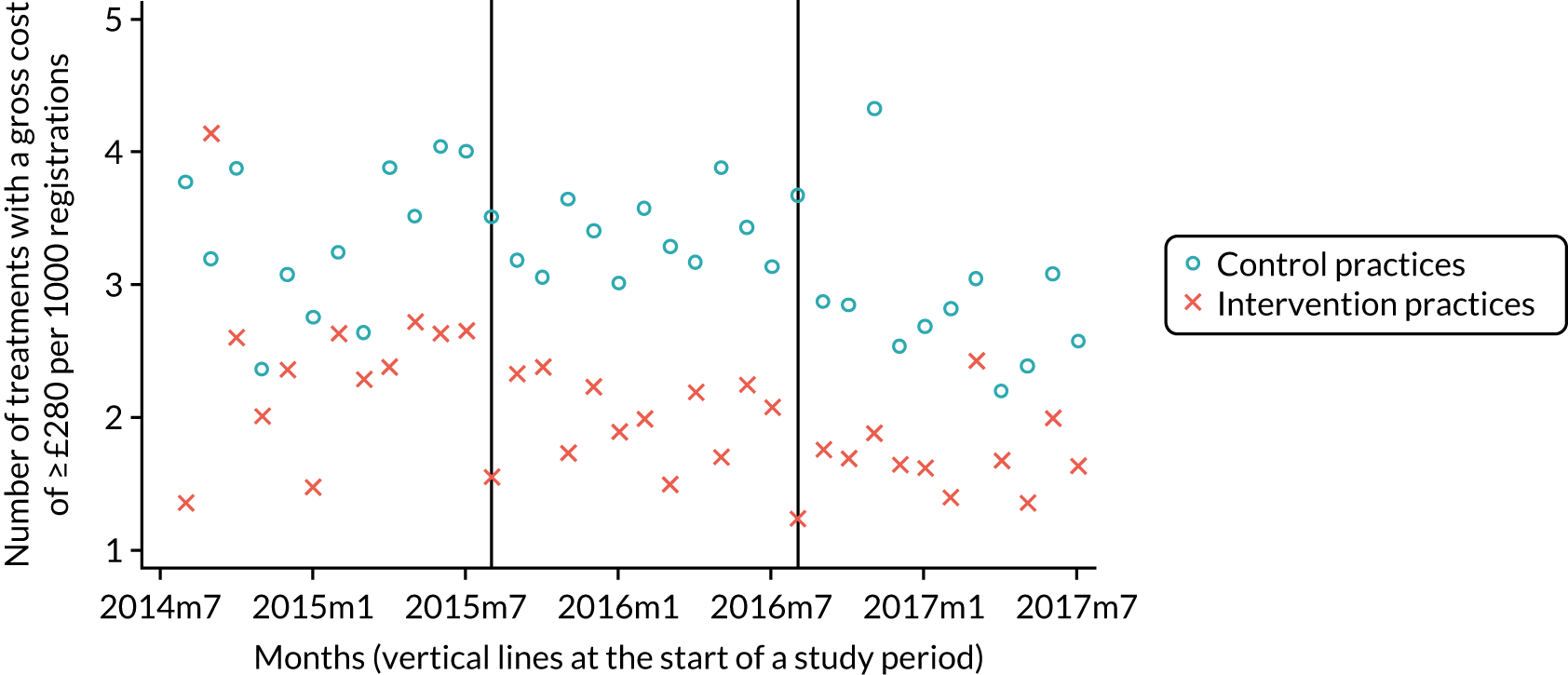
The difference between intervention and control practices in the mean number of direct restoration treatments per month per 1000 registrations changed between the study periods. The difference significantly increased (p < 0.05), with 49.0 fewer direct restoration treatments per month per 1000 registrations. This was caused by a reduction in activity in the intervention practices in the capitation period compared with baseline FFS (see Tables 7 and 8). This difference increased (p < 0.05) by 62.0 direct restoration treatments per month per 1000 registrations in the reversion period compared with capitation (see Tables 7 and 8; Figure 8). There was no evidence of a long-term effect from the pilot because the difference between intervention and control practices was not significant (p > 0.05) when the FFS reversion period was compared with FFS at baseline.
FIGURE 8.
Number of direct restoration treatments.
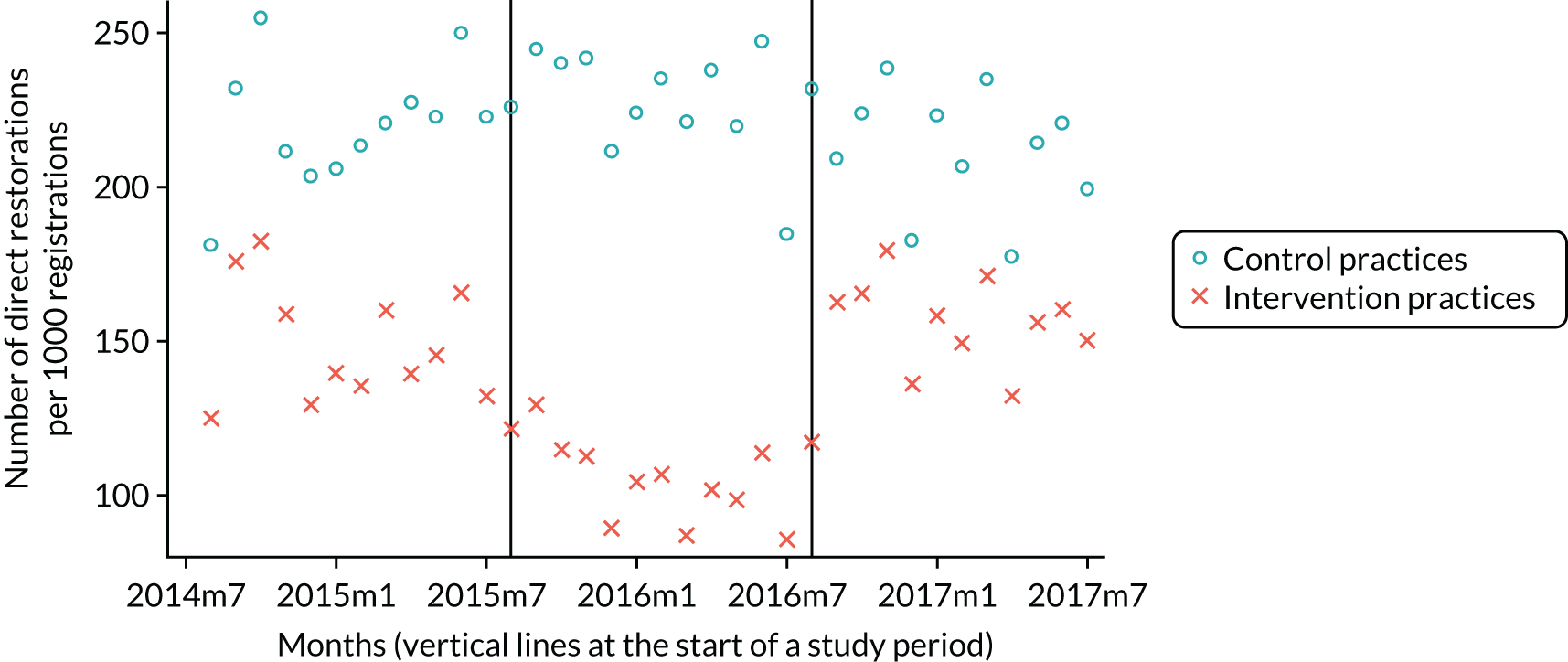
The difference between intervention and control practices in the number of indirect restorations per month per 1000 registrations changed significantly between study periods (see Tables 7 and 8; Figure 9). The difference increased (p < 0.05) and was caused by 3.7 fewer indirect restorations per month per 1000 registrations in the intervention practices in the capitation period compared with baseline FFS; it decreased (p < 0.05) with 2.3 fewer indirect restorations per month per 1000 registrations in the reversion period compared with the capitation period (relative to the control practices). There was no evidence of a long-term effect from the pilot because the difference was not significant (p > 0.05) between intervention and control practices in the FFS reversion period compared with FFS at baseline.
FIGURE 9.
Number of indirect restoration treatments.
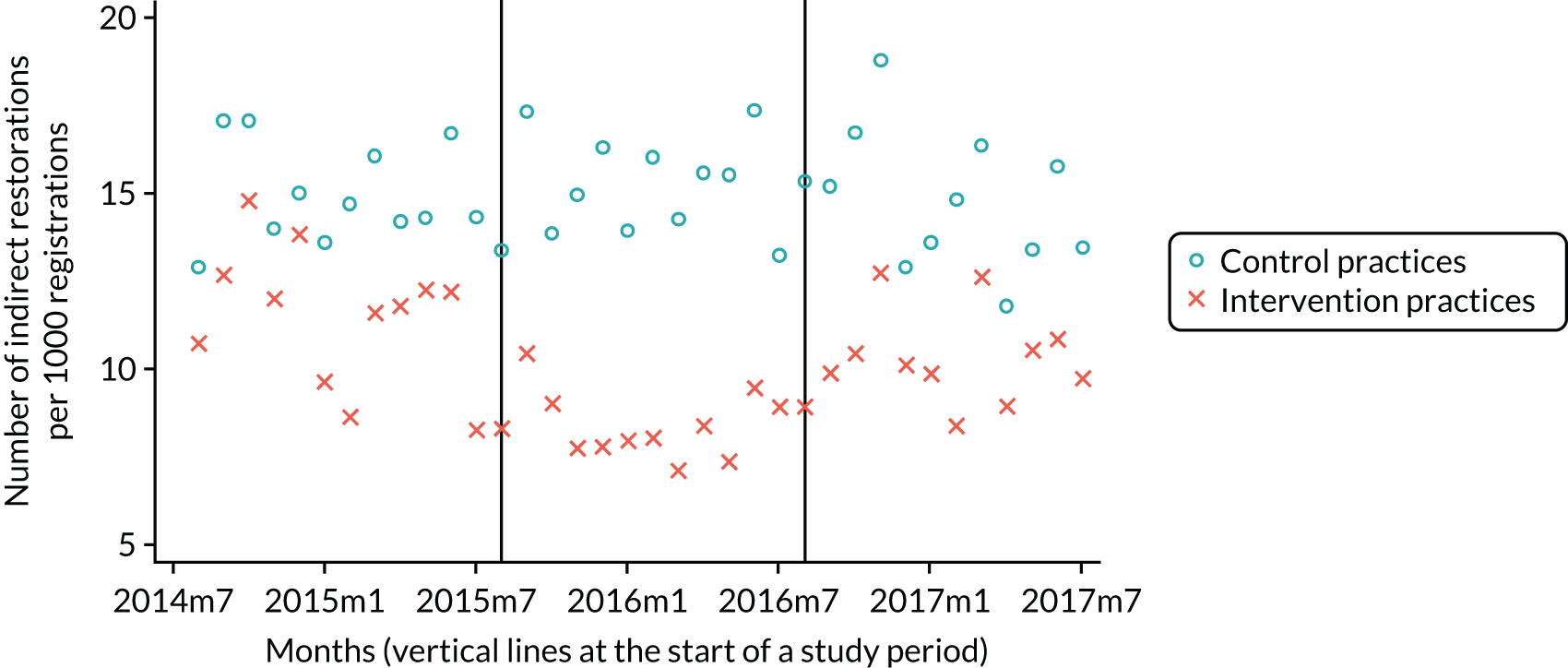
A similar picture was seen for ADs, with the difference increasing (p < 0.05) with 2.8 fewer indirect restorations per month per 1000 registrations being undertaken in the intervention practices in the capitation period compared with baseline FFS, and decreasing (p < 0.05) with 2.8 fewer indirect restorations per month per 1000 registrations in the reversion period compared with the capitation period (see Appendix 4). This was not the case for PPs. There was a statistically significant increase (p < 0.05) of 1.6 in the difference in indirect restorations per month per 1000 registrations delivered by the mean number of PPs in intervention and control practices in the FFS reversion period compared with FFS at baseline.
The DiD analyses identified a significant difference between intervention and control practices in the number of extractions per month per 1000 registrations between study periods. The difference increased (p < 0.05) and was caused by 6.8 fewer extractions per month per 1000 registrations being undertaken. This was caused by a reduction of activity in the intervention practices in the capitation period compared with baseline FFS (see Tables 7 and 8; Figure 10). The difference decreased (p < 0.05) with 5.6 more extractions in the reversion period compared with capitation (see Table 8). Any change in treatment provision did not persist after capitation ceased and there was no significant difference (p > 0.05) between intervention and control practices in the number of extractions per month per 1000 registrations in the FFS reversion period compared with FFS at baseline.
FIGURE 10.
Plot of the monthly number of extraction treatments.
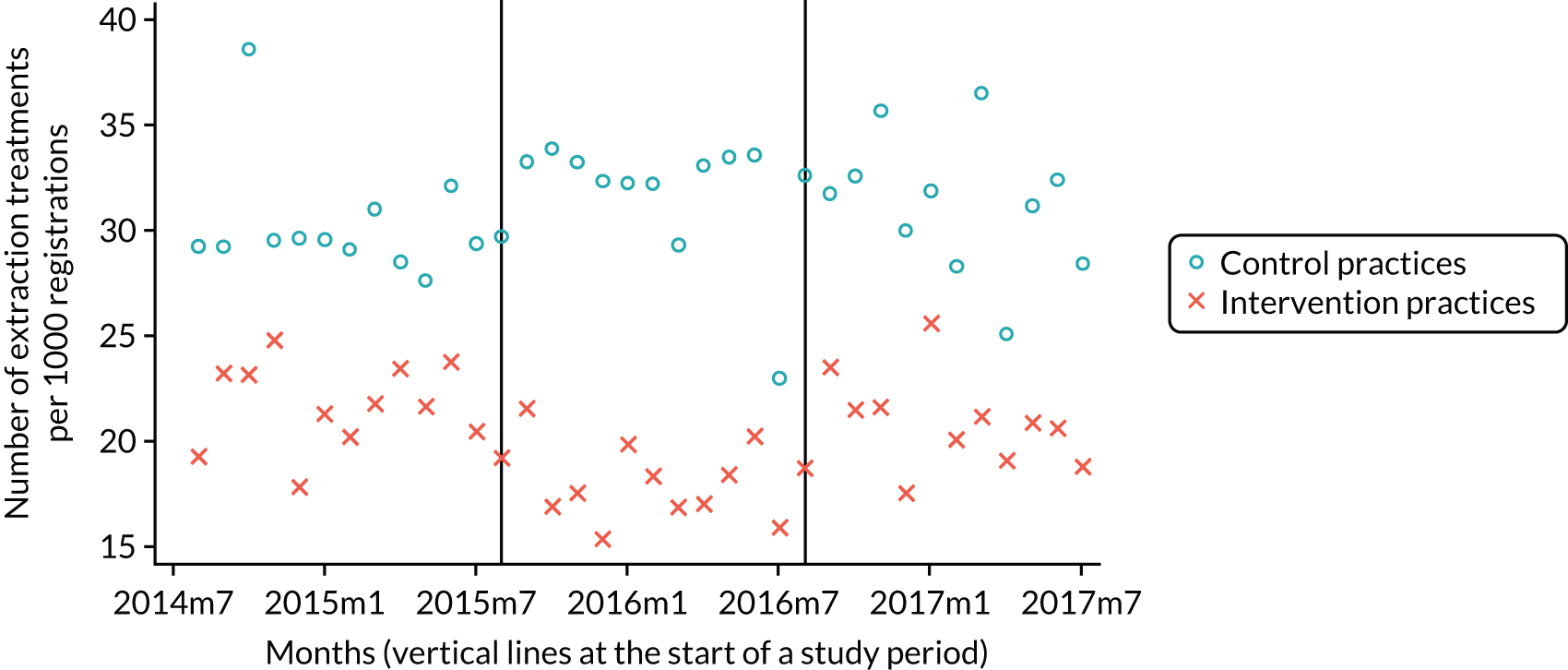
A similar picture was seen in extractions, where the difference compared with baseline FFS increased (p < 0.05) and was caused by five fewer extractions for ADs and increased (p < 0.05) by 3.12 for PPs. During the FFS reversion period, compared with capitation, the situation reversed with the difference decreasing (p < 0.05) by 6.3 for ADs but did not significantly change (p > 0.05) for PPs. There seemed to be a persistent change in practice for PPs with a statistically significant increase (p < 0.05) of 2.42 in the number of extractions per month per 1000 registrations delivered in the FFS reversion period compared with FFS at baseline, which was not found for ADs.
In the capitation period compared with baseline FFS, the difference between intervention and control practices in the number of radiographs significantly increased (p < 0.05), caused by a reduction of 40.9 radiographs per month per 1000 registrations. This was caused by a reduction of activity in the intervention practices in the capitation period compared with baseline FFS (see Tables 7 and 8; Figure 11). This decreased (p < 0.05) by 34.1 in the reversion period compared with capitation (see Tables 7 and 8). There was no evidence of a long-term change in provision as the difference was not significant (p > 0.05) between intervention and control practices in the number of radiographs taken in the FFS reversion period compared with FFS at baseline.
FIGURE 11.
Number of radiograph treatments.
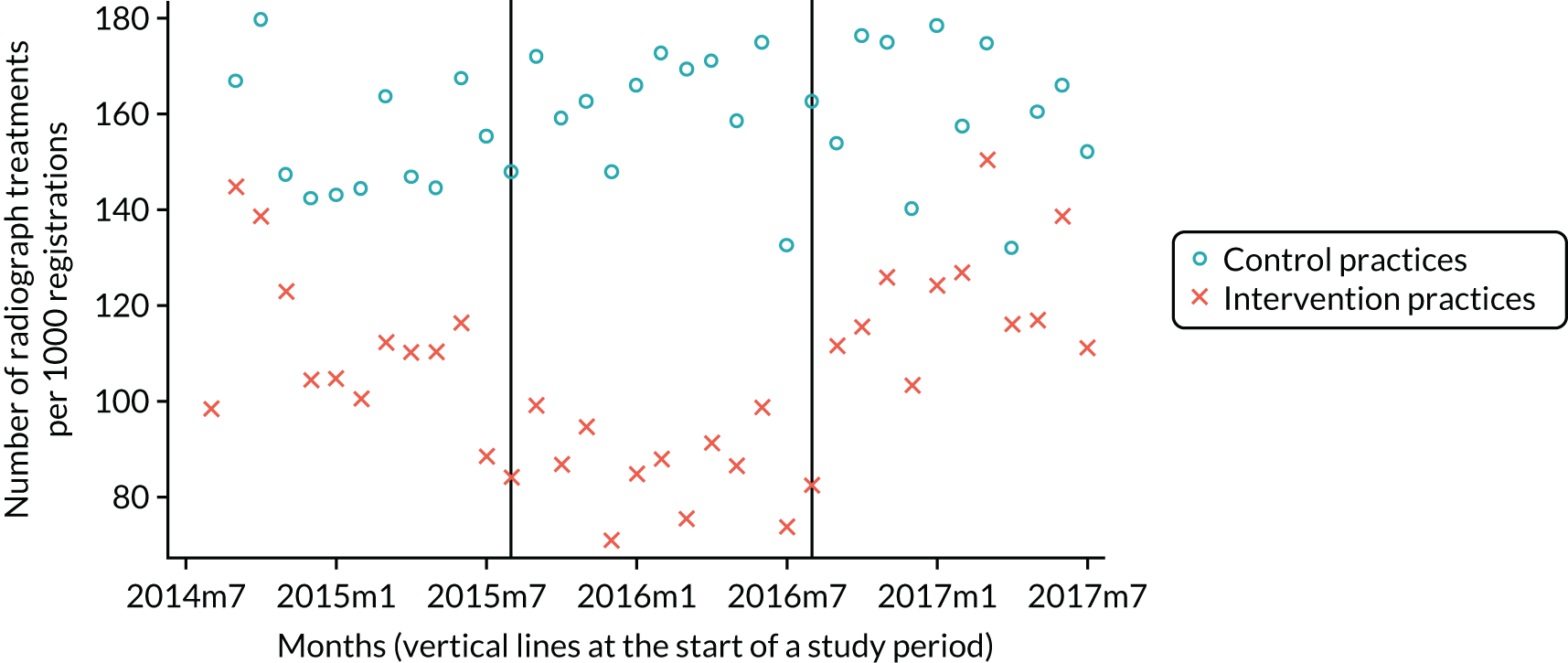
For ADs, the difference in radiographs taken in the capitation period compared with baseline FFS increased (p < 0.05) by 38.0; it also increased significantly (p < 0.05) by 16.3 for PPs (see Appendix 4). When comparing the FFS reversion period with capitation, the difference significantly (p > 0.05) decreased by 38.1 for ADs but did not significantly change (p > 0.05) for PPs.
The difference between the intervention and control group in fissure sealants provided increased significantly (p < 0.05) by 9.3 fissure sealant treatments per month per 1000 registrations in the capitation period compared with baseline FFS and decreased (p < 0.05) by 10.4 in the reversion period compared with the capitation period (Table 10). This was caused by a reduction of activity in the intervention practices in the capitation period compared with baseline FFS (Tables 9 and 10; Figure 12). There was no significant difference (p > 0.05) between intervention and control practices in the number of fissure sealant treatments provided per month in the FFS reversion period compared with FFS at baseline.
| Test group | Practice group, study period | Fissure sealants | Two-visit periodontal treatments | Root canal treatments | Treatment plans | Treatment items | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Control | Baseline FFS to capitation | 24.93 | 21.07 to 28.79 | 10.38 | 9.60 to 11.17 | 12.29 | 11.59 to 12.99 | 307.10 | 294.06 to 320.14 | 819.57 | 791.52 to 847.62 |
| Capitation to reversion FFS | 24.89 | 22.07 to 27.70 | 10.98 | 10.10 to 11.86 | 12.51 | 11.87 to 13.15 | 312.27 | 299.42 to 325.13 | 806.83 | 777.11 to 836.55 | |
| Baseline FFS to reversion FFS | 22.94 | 20.52 to 25.35 | 11.30 | 10.47 to 12.13 | 11.46 | 10.78 to 12.14 | 320.05 | 306.59 to 333.52 | 788.84 | 758.07 to 819.60 | |
| Intervention | Baseline FFS to capitation | 18.34 | 16.24 to 20.44 | 9.11 | 8.06 to 10.16 | 8.45 | 7.78 to 9.12 | 230.02 | 220.59 to 239.45 | 614.91 | 583.73 to 646.09 |
| Capitation to reversion FFS | 8.33 | 6.99 to 9.68 | 4.65 | 3.89 to 5.40 | 6.67 | 5.99 to 7.34 | 209.83 | 201.44 to 218.23 | 450.42 | 429.20 to 471.64 | |
| Baseline FFS to reversion FFS | 17.35 | 15.12 to 19.58 | 8.31 | 7.08 to 9.53 | 7.97 | 7.27 to 8.68 | 237.91 | 229.72 to 246.10 | 592.43 | 567.58 to 617.29 | |
| Practice group, study period | Fissure sealants | Two-visit periodontal treatments | Root canal treatments | Treatment plans | Treatment items | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | –9.34 | 0.01 | –3.81 | 0.01 | –2.65 | < 0.01 | –34.33 | < 0.01 | –174.78 | < 0.01 |
| Capitation to reversion FFS | 10.38 | < 0.01 | 3.45 | 0.02 | 2.37 | < 0.01 | 28.70 | < 0.01 | 173.89 | < 0.01 |
| Baseline FFS to reversion FFS | 1.03 | 0.68 | –0.41 | 0.78 | –0.26 | 0.81 | –5.71 | 0.53 | –0.99 | 0.98 |
FIGURE 12.
Number of fissure sealant treatments.
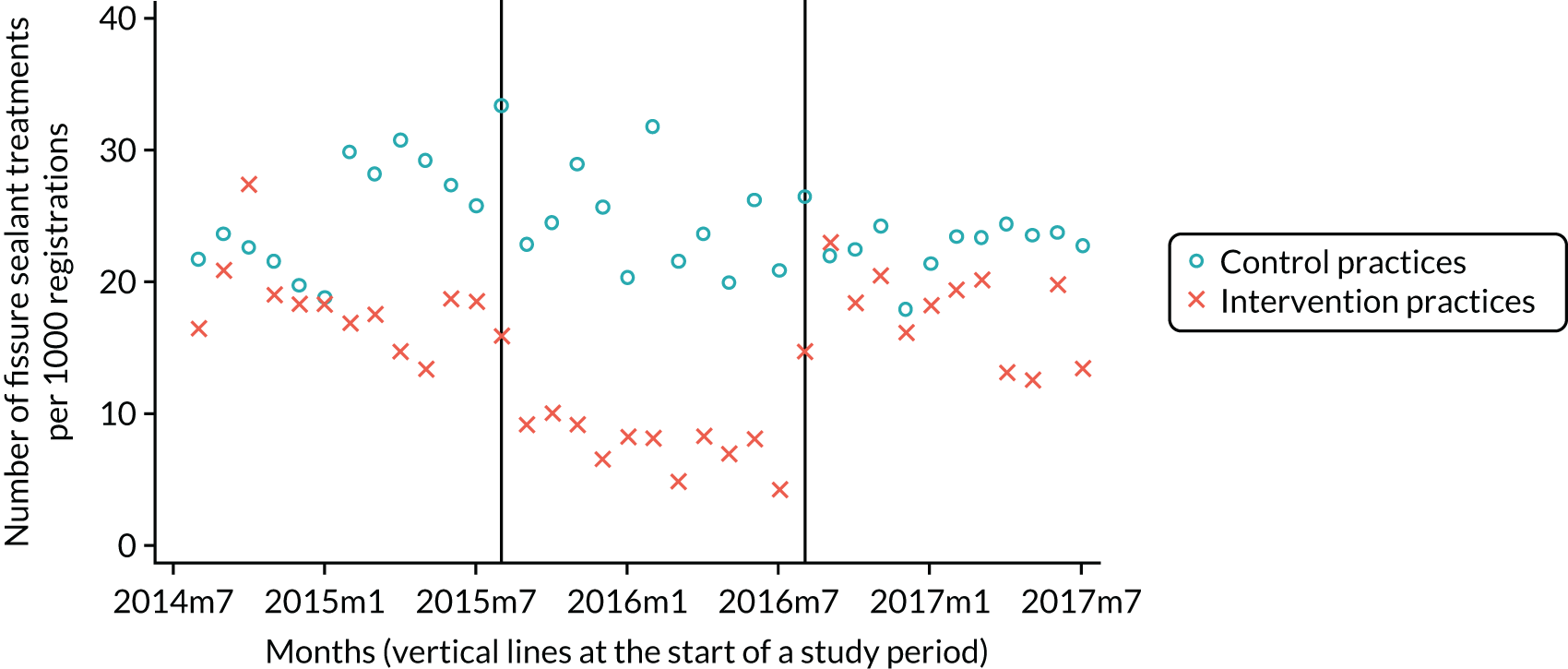
Similar changes were seen for both ADs and PPs (see Appendix 4). The difference in direct restoration treatments in the capitation period compared with baseline FFS increased (p < 0.05), with 56.2 fewer restorations for ADs and 14.3 fewer restorations for PPs. In the FFS reversion period compared with the capitation period, the difference decreased (p < 0.05) with 62.1 fewer restorations for ADs and with 16.2 fewer restorations for PPs.
For ADs, the difference in fissure sealants provided during the capitation period compared with baseline FFS increased (p < 0.05) by 11.3 but this did not significantly change for PPs (see Appendix 4). The difference in fissure sealant treatments provided per month in the FFS reversion period compared with the capitation period decreased (p < 0.05) by 11.1 for ADs but did not significantly change (p > 0.05) for PPs.
The difference between intervention and control practices in the number of two or more visits periodontal treatments provided increased (p < 0.05) by 3.8 during the capitation period compared with baseline FFS and decreased (p < 0.05) by 3.5 in the reversion period compared with the capitation period (see Tables 9 and 10; Figure 13). This was caused by a reduction of activity in the intervention practices in the capitation period compared with baseline FFS (see Tables 9 and 10). There was no evidence of a long-term effect in provision as there was no significant difference (p > 0.05) between intervention and control practices when comparing the FFS reversion period to FFS at baseline.
FIGURE 13.
Number of two or more periodontal treatments.

There was a difference in the pattern of care between ADs and PPs. For ADs, the difference during the capitation period compared with baseline FFS increased significantly (p < 0.05) by 3.8 but it did not significantly change for PPs (see Appendix 4). During the FFS reversion period compared with the capitation period provision, the difference between intervention and control practices significantly decreased (p < 0.05) by 4.1 but it did not significantly change (p > 0.05) for PPs.
The difference in provision of root canal treatments increased (p < 0.05) by 2.7 root canal treatments per month per 1000 registrations during the capitation period compared with baseline FFS and decreased (p < 0.05) by 2.4 in the reversion period compared with the capitation period (see Tables 9 and 10; Figure 14). This was caused by a reduction in activity in the intervention practices in the capitation period compared with baseline FFS (see Table 10). There was no evidence of a long-term effect in provision as a result of the pilot, as the difference was not significant (p > 0.05) between intervention and control practices in the number of root canal treatments provided per month in the FFS reversion period compared with FFS at baseline.
FIGURE 14.
Number of root canal treatments.
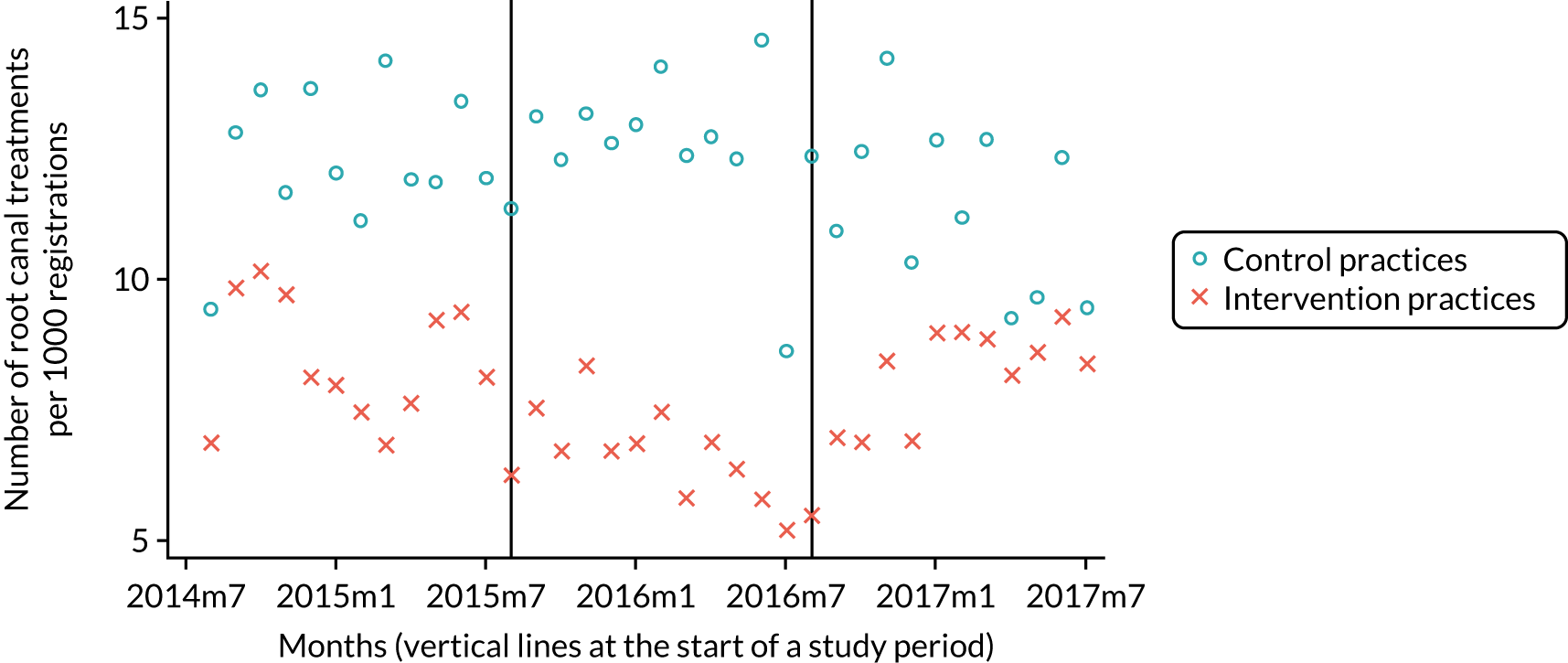
Significant increases in the difference in provision of root canal treatments between intervention and control practices between FFS baseline and the capitation period were observed [2.4 for ADs and 0.9 (p = 0.05) for PPs (see Appendix 4)]. When comparing the FFS reversion period with the capitation period, provision of root canal treatments decreased (p < 0.05) by 2.4 for ADs but did not significantly change for PPs.
The difference increased significantly (p < 0.05) by 34.3 treatment plans per month per 1000 registrations in the capitation period compared with baseline FFS, but decreased significantly (p < 0.05) by 28.7 in the reversion period compared with the capitation period (see Tables 9 and 10). This was caused by a reduction of activity in the intervention practices in the capitation period compared with baseline FFS (see Tables 9 and 10; Figure 15). There was no evidence of a long-term effect as a result of the pilot because the difference was not significant (p > 0.05) between intervention and control practices in the number of treatment plans provided per month in the FFS reversion period compared with the FFS baseline period.
FIGURE 15.
Number of treatment plans.

The difference in treatment plans provided per month in the capitation period compared with baseline FFS increased (p < 0.05) by 39.1 for ADs but did not significantly change (p > 0.05) for PPs (see Appendix 4). Likewise, there was a significant (p > 0.05) difference in the number of treatment plans provided per month by ADs in the FFS reversion period compared with the capitation period, with a decrease of 32.3 treatment plans but no statistically significant change for PPs.
The difference in number of treatment items provided increased significantly (p < 0.05) by 174.8 treatment items during the capitation period compared with baseline FFS and significantly decreased (p < 0.05) by 173.9 in the reversion period compared with the capitation period (see Tables 9 and 10; Figure 16). This was caused by a reduction in activity in the intervention practices in the capitation period compared with baseline FFS (see Table 10). There was no evidence of a long-term effect on the number of treatment items provided from the pilot because there was no significant difference between intervention and control practices in the FFS reversion period compared with FFS at baseline.
FIGURE 16.
Number of treatment items.
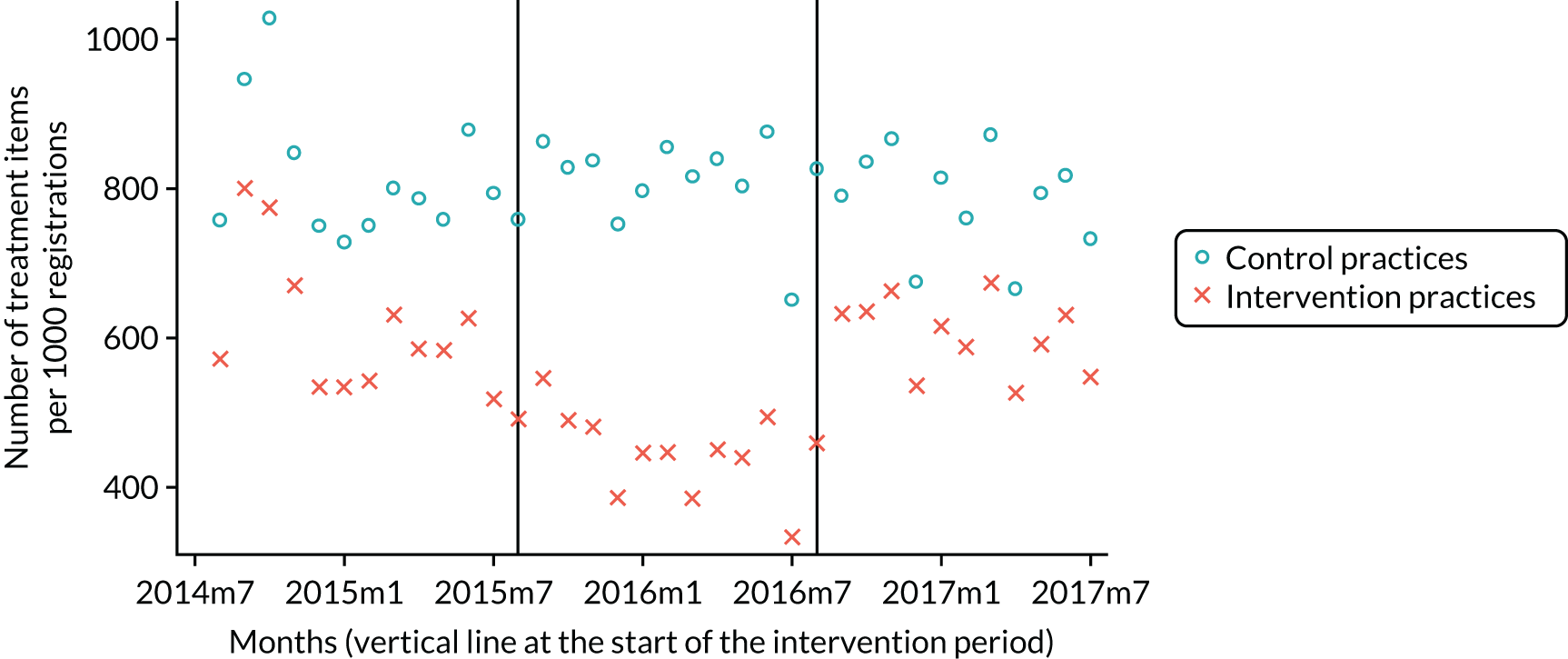
For ADs, the difference between those working in intervention and control practices in terms of the number of treatment items provided in the capitation period compared with baseline FFS increased (p < 0.05) by 174.5; it also increased (p < 0.05) by 70.4 for PPs (see Appendix 4). Corresponding significant decreases in the difference were seen for both ADs (179.0) and PPs (47.0) in the FFS reversion period compared with the capitation period.
Results: financial outcomes
The difference between intervention and control practices in the mean percentage of patient fee contribution to NHS dental practice income per month did not significantly change (p > 0.05) between study periods (Tables 11 and 12; Figure 17).
| Test group | Practice group, study period | Proportion patient fee contribution (%) | NHS dental practice income (£) | Patient contribution (£) | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Control | Baseline FFS to capitation | 41.37 | 40.19 to 42.55 | 20,076.80 | 18,437.20 to 21,716.40 | 8381.50 | 7673.20 to 9089.80 |
| Capitation to reversion FFS | 41.86 | 40.59 to 43.13 | 20,829.40 | 19,192.60 to 22,466.20 | 8917.30 | 8189.50 to 9645.10 | |
| Baseline FFS to reversion FFS | 42.99 | 41.71 to 44.27 | 20,256.10 | 18,663.30 to 21,849.00 | 9011.20 | 8270.40 to 9752.00 | |
| Intervention | Baseline FFS to capitation | 40.10 | 38.39 to 41.81 | 21,353.40 | 19,223.30 to 23,483.4 | 8751.40 | 7744.30 to 9758.50 |
| Capitation to reversion FFS | 41.69 | 39.86 to 43.52 | 16,535.50 | 14,929.00 to 18,141.90 | 7176.70 | 6325.90 to 8027.60 | |
| Baseline FFS to reversion FFS | 42.24 | 40.71 to 43.77 | 20,857.30 | 18,770.90 to 22,943.70 | 9145.40 | 8066.90 to 10,224.00 | |
| Practice group, study period | Proportion patient fee contribution | NHS dental practice income | Patient contribution | |||
|---|---|---|---|---|---|---|
| DiD coefficient (%) | p-value | DiD coefficient (£) | p-value | DiD coefficient (£) | p-value | |
| Baseline FFS to capitation | 0.48 | 0.67 | –5920 | < 0.01 | –2403 | < 0.01 |
| Capitation to reversion FFS | –0.60 | 0.53 | 5248 | < 0.01 | 2028 | < 0.01 |
| Baseline FFS to reversion FFS | –0.11 | 0.91 | –673 | 0.60 | –374 | 0.41 |
FIGURE 17.
Mean percentage of patient fee contribution.
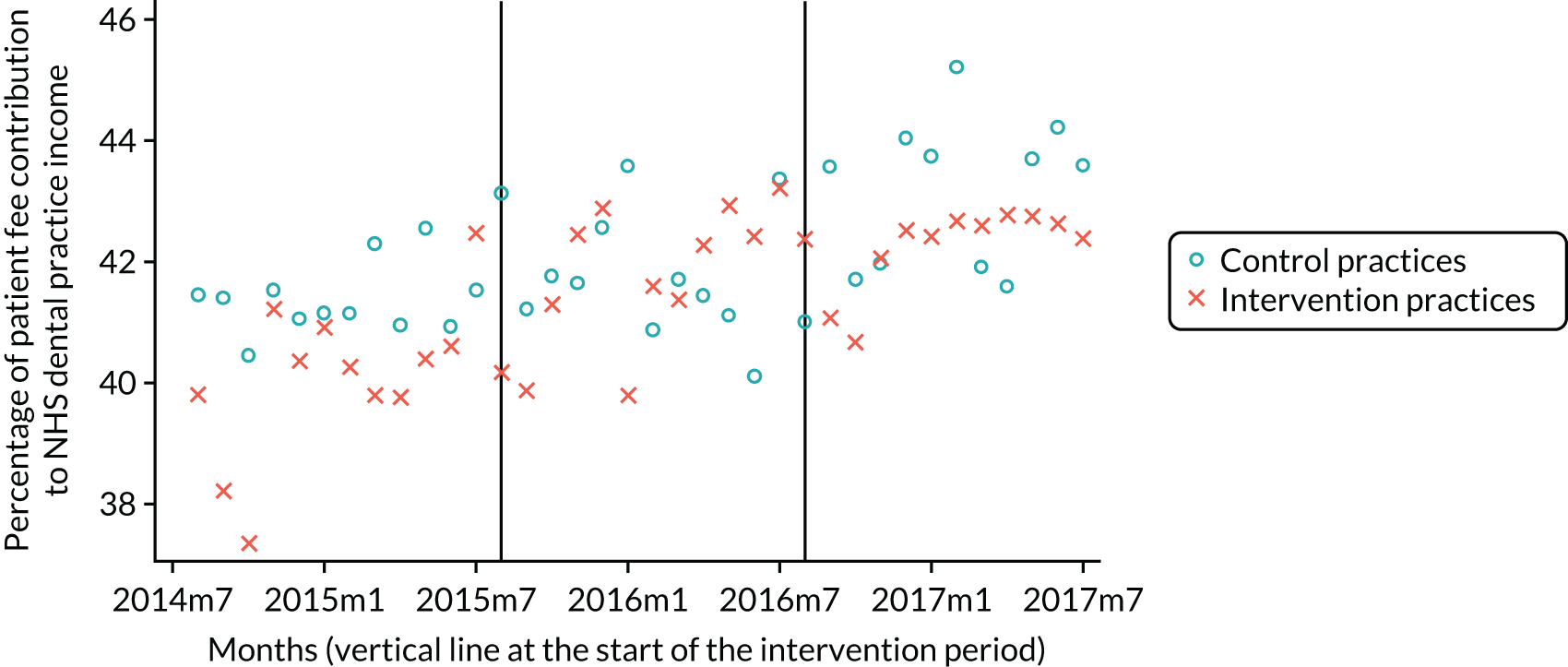
The difference between intervention and control practices in NHS dental practice income per month changed between study periods. The difference in NHS dental practice income per month increased significantly (p < 0.05), by £5920, in the capitation period compared with baseline FFS, and decreased significantly (p < 0.05), by £5248, in the reversion period compared with capitation. This was caused by a reduction of activity in the intervention practices in the capitation period compared with baseline FFS (see Tables 11 and 12; Figure 18). There was no evidence of a long-term effect from the pilot because the difference in NHS dental practice income per month between intervention and control practices was not significantly different (p > 0.05) between the FFS reversion period and the FFS at baseline.
FIGURE 18.
Mean NHS dental practice income.

The difference between intervention and control practices in patient fee contributions per month changed between study periods. The difference in patient fee contributions per month increased significantly (p < 0.05), by £2403, in the capitation period compared with baseline FFS, and decreased significantly (p < 0.05), by £2028, in the reversion period compared with capitation. This was caused by a reduction in activity in the intervention practices in the capitation period compared with baseline FFS (see Tables 11 and 12; Figure 19). There was no evidence of a long-term effect from the pilot because the difference between intervention and control practices in patient fee contributions per month was not significantly different (p > 0.05) between the FFS reversion period and the FFS at baseline.
FIGURE 19.
Mean patient fee contributions.
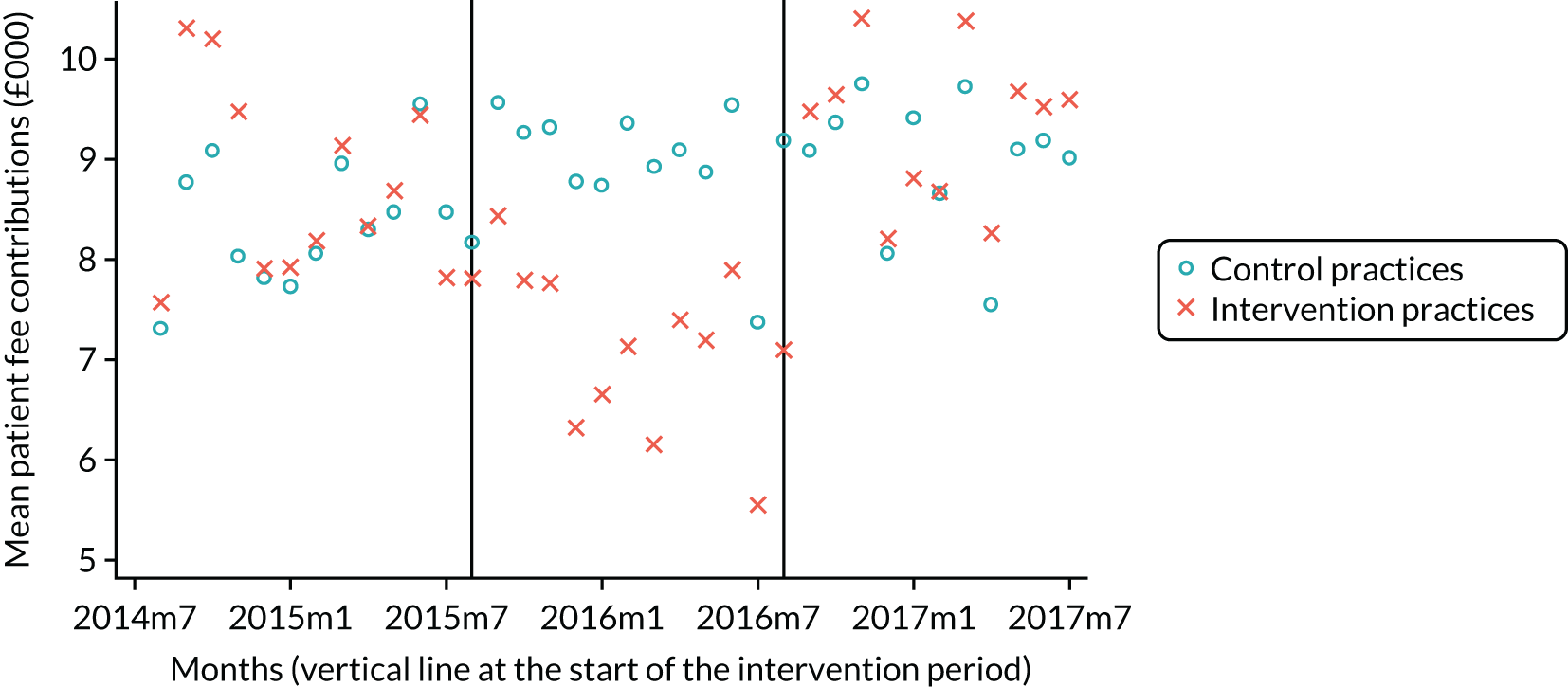
For all of the analyses presented above, no difference is seen between the analyses conducted with matched practices (with PSs) and unmatched practices (see Appendix 3).
Triangulation of difference-in-difference analyses using an interrupted time series analysis
Although the DiD approach is widely used to assess the effect of an intervention in observational studies, the associated statistical inference used to obtain and test the resulting effect estimate relies on quite strong assumptions that are not always applicable in practice. Moulton62 shows that where observations are correlated within groups SEs may be seriously underestimated, resulting in confidence intervals (CIs) for the DiD effect that are too narrow, and an inflated risk of type I error. In other words, the precision of the effect estimate is often overestimated, leading to an increased probability of falsely concluding that an intervention effect is present. This problem is exacerbated when the number of groups considered is small, as in the usual DiD case of four groups (two groups compared at each of two time points), and can persist even where ‘robust’ SE estimation is used, as was the case in this study. 63
One reason for this downwards bias of SE estimates is that the DiD analysis does not account for the possibility that observations within a group may be correlated for reasons other than the presence or absence of the intervention. In the present study, observations within a practice are likely to be correlated; we would expect observations from the same practice to be more similar than observations from different practices on average, meaning that some practices yield observations that are consistently above the group average whereas others are consistently below it. Although matching was used to balance the control and intervention groups in terms of practice size, rurality and deprivation, remaining imbalances in these and other practice-level factors may be expected to contribute to between-practice variation to some degree. We might also expect some correlation over time; observations that are close together are likely to be more similar than observations more distant in time. Although robust SE estimation was used in this study to adjust for within-group correlation, Donald and Lang63 find that the adjustment procedure may itself be unreliable in cases with few groups, as in the usual DiD approach, leading to a downwards bias in SE estimates and increased risk of type I error. 62
In order to investigate the sensitivity of the DiD analysis to within-group correlation, a secondary analysis was performed on four outcomes (from Appendix 3) using an ITS approach. 64,65 This allows for the hierarchical structure of the data: repeated monthly outcome measures are nested within practices, by fitting a linear model for each outcome over time, grouped by practice. A random intercept term was included to allow for correlation between repeated measures within each practice. This means that the observations for each practice are assumed to vary about the practice mean, which may be higher or lower than the overall study mean. An autoregressive (AR3) structure was also used to allow for residual correlation between closer measurements compared with those more distant in time. Discontinuities (i.e. ‘jumps’) are allowed at each transition point (i.e. 12 months indicating the change from FFS to capitation, and again at 24 months for the reversion from capitation to FFS) to represent any sudden change in outcome, and a different slope is permitted for each phase to represent any differences in trend between phases. Furthermore, different jumps at a transition and different slopes on the same phase are allowed for the intervention and control groups. The size of these differences may each be interpreted as a component of the intervention effect, and the simultaneous test that all four coefficients are zero (difference between groups in the intercept at month 12 transition from FFS to capitation; slope from months 12 to 24, the capitation period; intercept at month 24 reversion from capitation to FFS; and slope from months 24 to 36, FFS) was used as the test of the null hypothesis ‘no intervention effect’. However, a broader qualitative interpretation of results may be obtained by examining the effect estimates and CIs, and by comparing the resulting fitted plots for each group. Robust SE estimates were used to compensate for the non-normal distribution of model residuals, due in part to positively skewed outcomes. This was felt to be preferable to transforming outcomes to reduce skew, which would have made interpretation and comparison of results with those found in the DiD approach more difficult.
The four outcomes chosen for the secondary analysis were number of treatments costing ≥ £280 per 1000 registrations, number of direct restorations per 1000 registrations, number of fissure sealants per 1000 registrations and number of items per course of treatment. These were determined a priori to represent a range of interventional and preventative clinical activity and financial domains (Table 13).
| Domain | Baseline FFS to capitation (year 1–year 2) | Capitation (year 2) | Capitation to reversion FFS (year 2–year 3) | Reversion FFS (year 3) | p-value for test H0: all four effects zero | ||||
|---|---|---|---|---|---|---|---|---|---|
| Jump effect | 95% CI | Slope effect | 95% CI | Jump effect | 95% CI | Slope effect | 95% CI | ||
| Treatments ≥ £280 | 0.01 | –1.13 to 1.14 | –0.06 | –0.14 to 0.02 | –0.18 | –0.97 to 0.61 | 0.10 | –0.01 to 0.20 | 0.176 |
| Direct restorations | –25.56 | –55.41 to 4.29 | –0.16 | –2.70 to 2.38 | 30.77 | 0.34 to 61.20 | 3.71 | 1.24 to 6.17 | < 0.001 |
| Fissure sealants | –7.82 | –17.55 to 1.90 | 0.16 | –0.83 to 1.15 | 7.44 | –1.41 to 16.29 | 0.03 | –0.68 to 0.74 | 0.0217 |
| Items per plan | –0.14 | –0.39 to 0.12 | –0.01 | –0.04 to 0.01 | 0.19 | –0.03 to 0.42 | 0.04 | 0.02 to 0.06 | < 0.001 |
Plots are presented for each of the four outcomes considered, showing the observations for the control and intervention practices over time with the fitted model superimposed (Figures 20 and 21). Each fitted line represents one practice; these are parallel within each group (all practices are assumed to follow the same model) but the shape may vary between groups. The spread of the fitted lines within each group indicates the extent of between-practice variation (mean fitted model for each group is shown in red).
FIGURE 20.
The ITS analysis of treatment costs of ≥ £280. (a) Control practices; and (b) intervention practices.

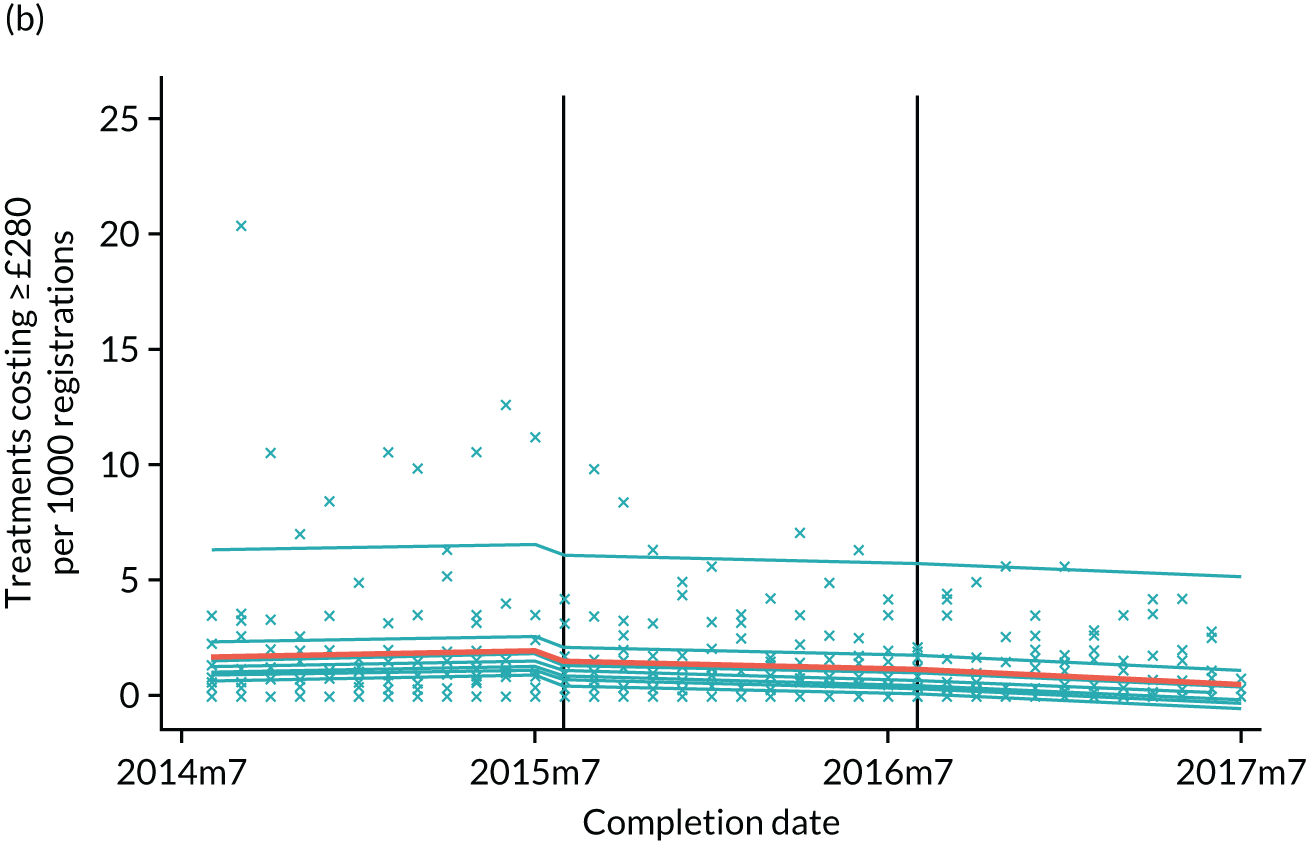
FIGURE 21.
The ITS analysis of direct restorations. (a) Control practices; and (b) intervention practices.
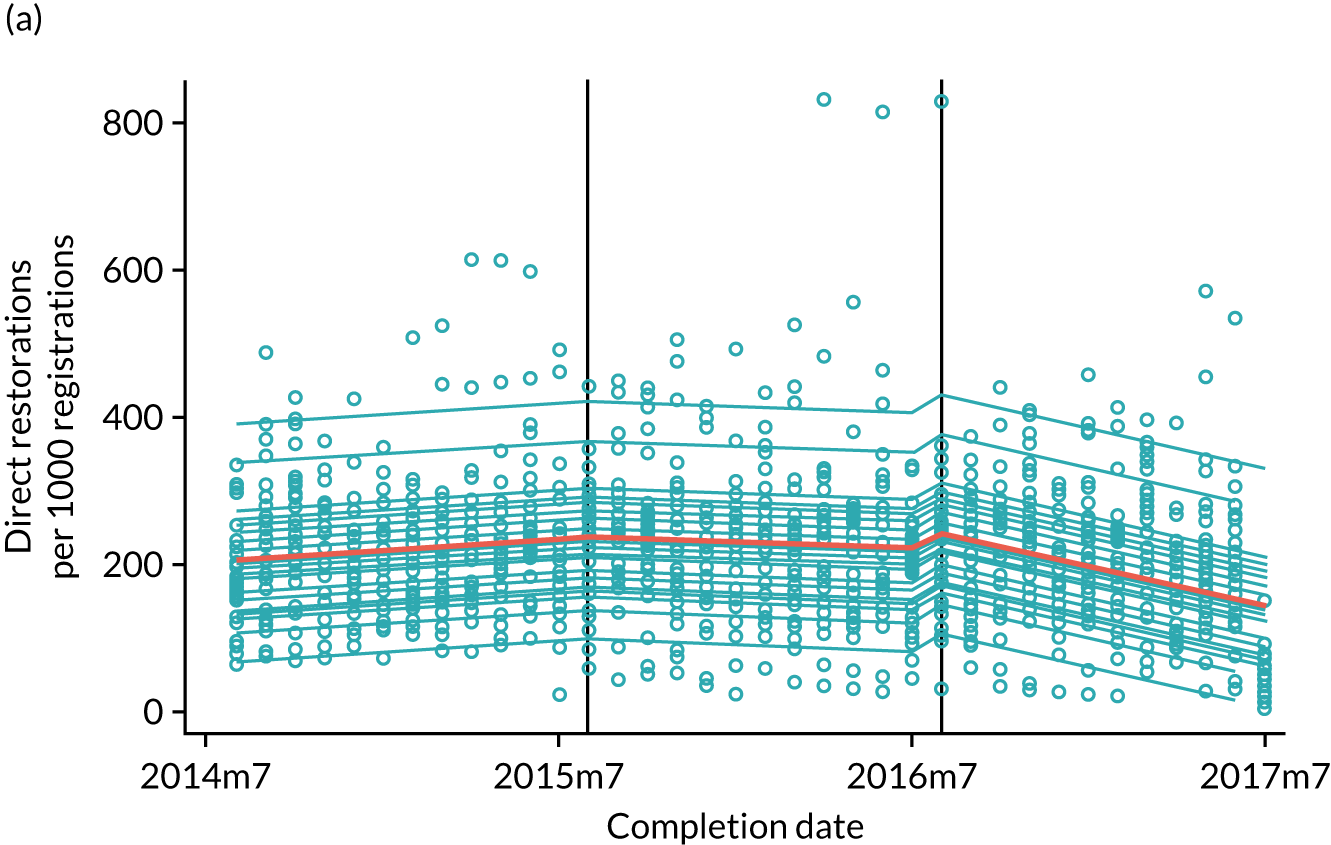
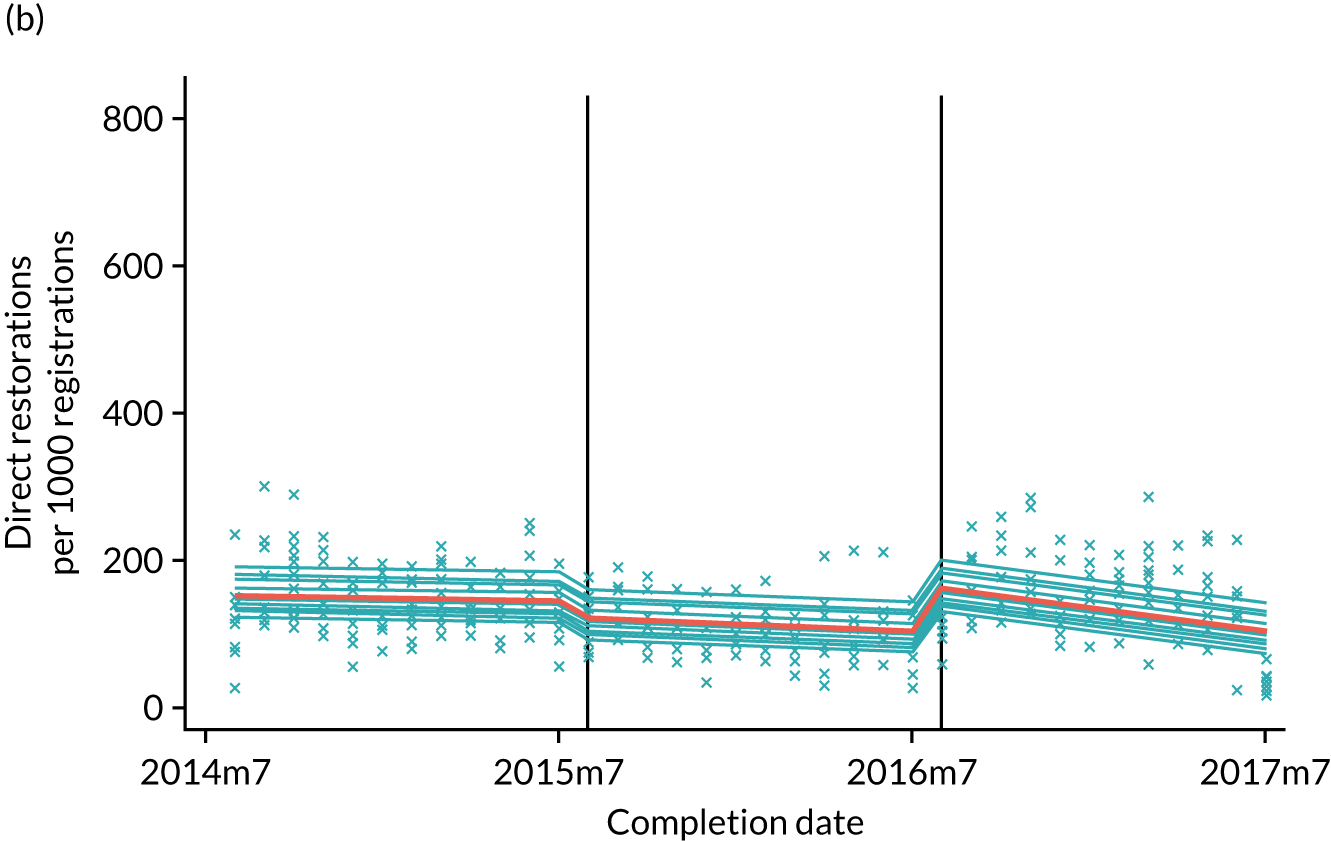
For treatments costing ≥ £280, the fitted model is very similar for both groups, as can be seen in Figure 22. The 95% CI for each jump and slope effect contains zero, and from the simultaneous test that all four effects are equal to zero we find that there is insufficient evidence to conclude that this outcome differs according to group. This conclusion is consistent with the DiD analysis.
FIGURE 22.
The ITS analysis of fissure sealants. (a) Control practices; and (b) intervention practices.


For direct restorations (Figure 23), the fitted models look somewhat different for the control and intervention groups. There is a drop immediately following the introduction of capitation for the intervention group compared with the control group. The estimated difference in jumps here is 25.6 (95% CI –55.41 to 4.29), suggesting that the change in the number of direct restorations performed by practices in the intervention group at the start of capitation was around 26 per 1000 registrations lower on average than the corresponding change in the control group. There was also a positive jump for both groups at the end of year 2, although the increase appears larger in the intervention group. The estimated difference in jumps here is 30.77 (95% CI 0.34 to 61.20), suggesting that the number of direct restorations performed by practices in the intervention group increased by around 31 per 1000 registrations on average than for the control group when FFS was reintroduced. From the simultaneous test that all four effects are equal to zero, we conclude that there is strong evidence of an intervention effect on direct restorations.
FIGURE 23.
The ITS analysis of items per plan. (a) Control practices; and (b) intervention practices.
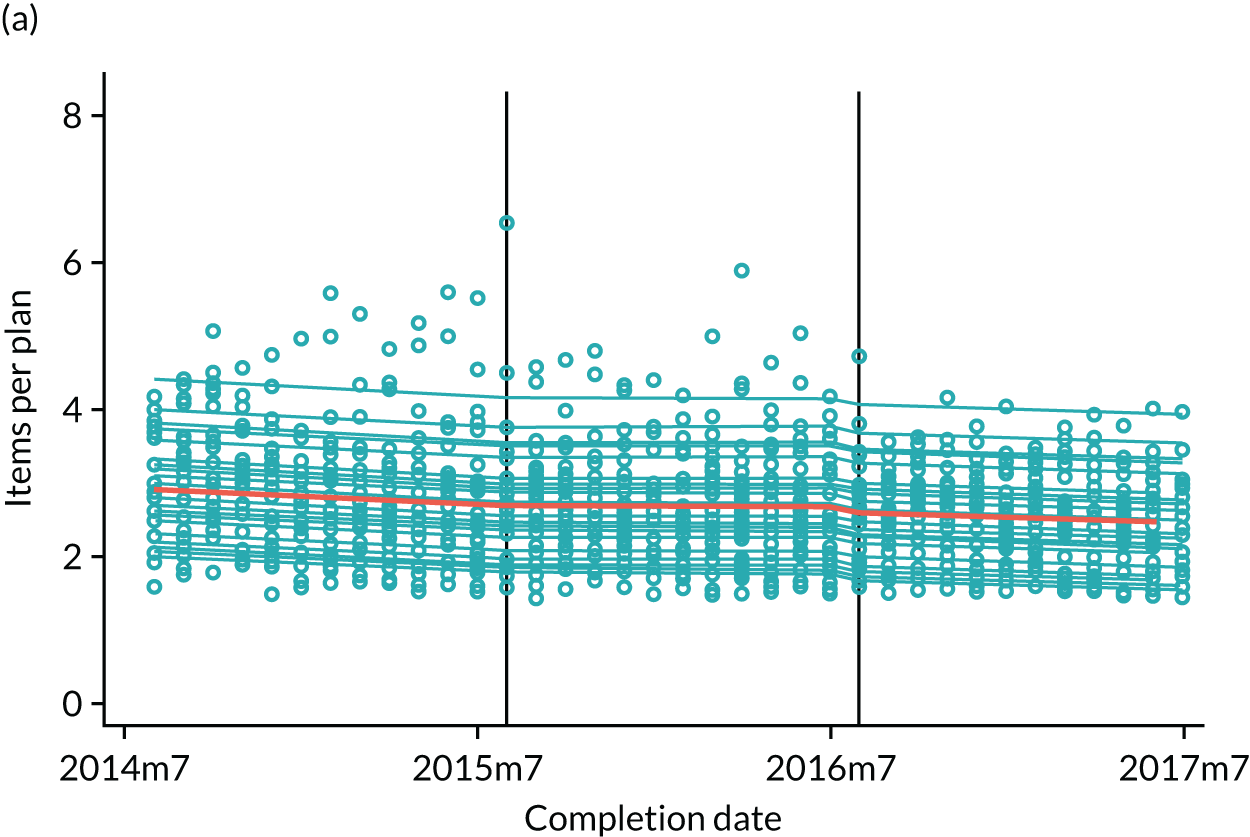
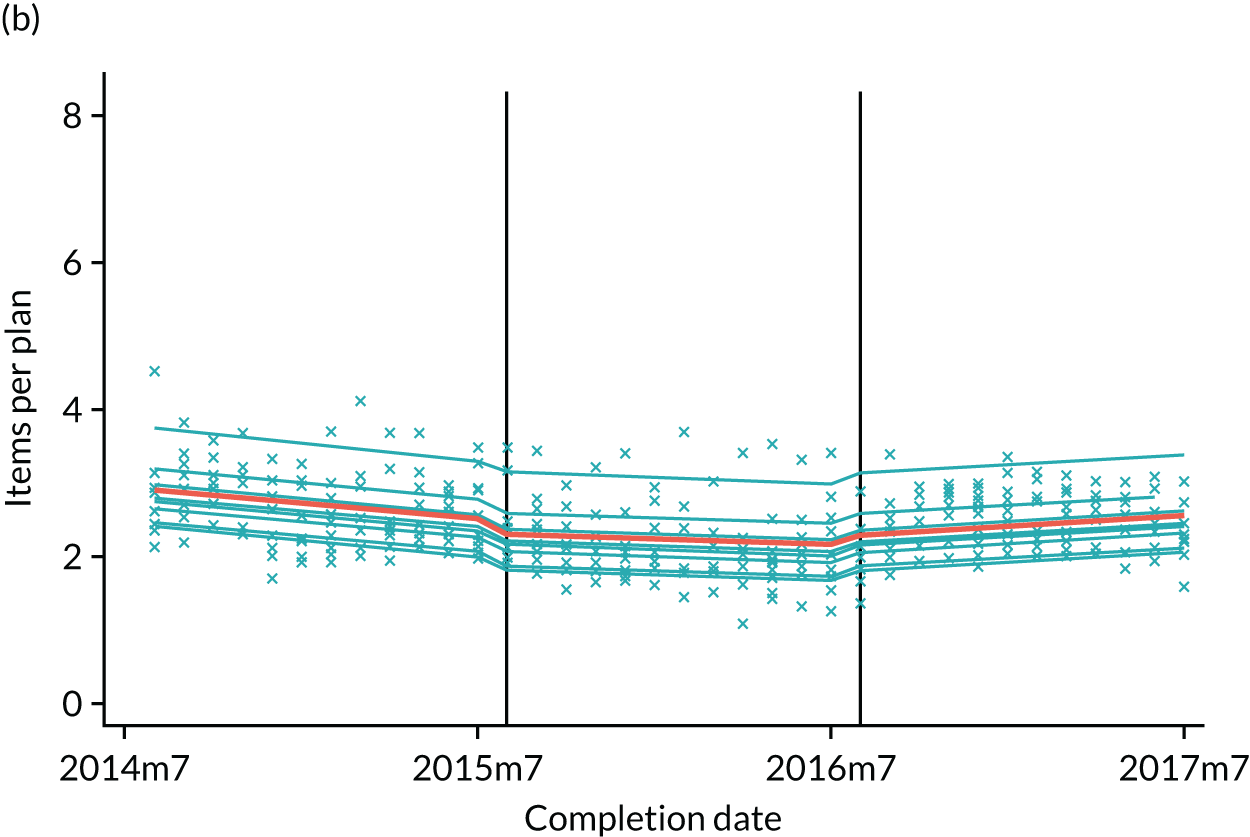
These results are consistent with those found in the DiD analysis in that the same conclusion is reached regarding evidence of an effect, and in the sense that the estimated DiD effects are in the same direction as those found in the ITS analyses. At the start of year 2, the DiD estimate is –51.00 per 1000 registrations, which lies within the CI found here; the DiD estimate for the end of year 2 is 61.79 per 1000 registrations, lying just beyond the upper limit of the CI found here. The larger estimated effect size at both time points found by the DiD analysis is due in part to the issue described above: that with no recognition of within-practice correlation, all variation in outcomes is attributed to group-level factors, including the DiD effect. In the ITS model, by comparison, over half of the total variation is attributed to between-practice variation via the use of the random intercept term, leading to more modest effect size estimates. However, the direction of effect was identical.
Again, for fissure sealants, differences in the fitted models for control and intervention groups are apparent from examining Figure 24 in Appendix 1. There appears to be a slight increase at the start of year 2 for control practices, compared with a drop for intervention practices. The difference is estimated to be –7.82 (95% CI –17.55 to 1.90), suggesting that the change in the number of fissure sealants provided by practices in the intervention group at the start of year 2 was around 8 per 1000 registrations less, on average, than the corresponding change for the control group. A similar-sized, but opposite, effect was found at the end of year 2: a more marked increase in the intervention group compared with the control group. The difference is estimated to be 7.44 (95% CI –1.41 to 16.29), suggesting that the change in the number of fissure sealants provided by practices in the intervention group at the start of year 2 was around 7 per 1000 registrations more on average than the corresponding change for the control group. From the simultaneous test that all four effects are equal to zero, we conclude that there is evidence of an intervention effect on fissure sealants; the test interpreted in isolation would find a statistically significant intervention effect at the 5% level, but cautious interpretation should be considered owing to the multiple testing of the clinical outcomes.
Again, results are consistent with the DiD analysis in finding a negative effect at the start of year 2 (–7.82 compared with the DiD estimate of –9.96) and a positive effect at the transition from year 2 to year 3 (7.44 compared with the DiD estimate of 10.97), suggesting that intervention practices reduced the number of fissure sealant treatments performed during year 2 compared with control practices, and then increased this service at the start of year 3. Again, and for the same reason, effect estimates are somewhat larger for the DiD analysis although this is less marked than for direct restorations. The same conclusion is reached regarding the test for intervention effect; some evidence of an effect is found if the test is interpreted in isolation but after adjustment for multiple testing we cannot reject the null hypothesis of no effect.
For the final outcome considered here (i.e. the number of items per course of treatment), there again appears to be some difference between the fitted models for each group in Figure 25 in Appendix 1. Again, there seems to be a drop at the start of year 2 for the intervention group compared with the control group, followed by a corresponding increase at the end of year 2/start of year 3. However, neither of these jump effects was found to be significantly different from zero by the ITS analysis. Another apparent difference evident in the plots is in the trend for year 3; a slight downwards slope is seen for the control group whereas the gradient is positive over the same period for the intervention group. The estimated difference in slopes is 0.04 (95% CI 0.02 to 0.06), suggesting that the number of items per course of treatment in the intervention group was increasing at a rate of around 0.04 per month more than the corresponding (negative) rate for the control group. In substantive terms, we may interpret this as showing that although any sudden increase at the end of year 2 was slight for the intervention group, a gradual increase was maintained over year 3. From the simultaneous test that all four effects are equal to zero, we conclude that there is strong evidence of an intervention effect on number of items per course of treatment.
Again, results are consistent with the DiD analysis in finding a negative effect at the start of year 2 (–0.14 compared with a DiD estimate of –0.37) and a positive effect at the end of year 2 (0.19 compared with a DiD estimate of 0.46), suggesting that intervention practices reduced the number treatment items per course of treatment during year 2 compared with control practices, and then increased this service at the start of year 3. There is also evidence from the ITS analysis that the number continued to increase more quickly during year 3 in the intervention group than in the control group. Again, and for the same reason, effect estimates are somewhat larger for the DiD analysis. Strong evidence of an intervention effect is found by both analyses.
Overall comparison of difference-in-difference and interrupted time series results
A direct comparison of the two analyses is not straightforward because the two approaches differ in several key ways:
-
The ITS model is fitted simultaneously to the data for the entire study duration. This approach includes the capitation–initial FFS and reversion FFS–capitation comparisons considered by the DiD analysis, but does not directly compare the FFS reversion and initial phases.
-
For each outcome, the ITS model assumes a linear relationship over time, with different slopes allowed for the intervention and control groups in each phase of the study; the DiD analysis does not assume a linear relationship (or any particular functional form), but assumes that the dependencies for the two groups are parallel in each phase.
-
The ITS model uses a random intercept term to allow for each practice’s observations to vary around its own mean, accounting for between-practice variation and within-practice correlation, assumed to be zero by the DiD analysis.
-
The ITS model allows for autocorrelation of observations (that observations closer in time may be more similar than those more distant in time), also assumed to be zero by the DiD analysis.
-
The ITS analysis uses the simultaneous test of the null hypothesis that both jump and both slope coefficients are zero for the study duration as a test of ‘no intervention effect’, whereas the DiD analysis tests separately whether or not the estimated DiD effects are equal to zero at the pairwise comparison of interest.
However, a broad comparison may be made by comparing the ‘jump’ effects estimated at the 12- and 24-month year 1–2 and year 2–3 transition points with the DiD effect estimates for the corresponding periods. These were found to agree in direction, although the ITS estimates were smaller in magnitude with lower estimated precision. All ITS CIs included zero, except for direct restorations at the second transition. This is likely to be as a result of the more realistic treatment of within-practice correlation using random intercepts and autocorrelation, whereas the DiD analysis attributes all variation to group-level factors as described above.
The between-practice variation, as a proportion of the total variation, was found to be between 45% (fissure sealants) and 69% (items per treatment plan), suggesting that the use of random effects is warranted by substantial between-practice variation. The autoregressive (AR3) correlation structure was selected as the best fit for the data from several possibilities including independent errors and autoregressive and moving average structures for lags of between 1 and 5 months, where fit was assessed using the Akaike and Bayesian information criteria.
While the ITS model provides a better fit for the data than the simple linear regression model that underlies the DiD analysis, the statistical power of the ITS analysis to conclude that the estimated jump and slope effects are significantly different from zero is low in this study, which was not designed with this analysis in mind. Using Snijders and Bosker’s methods,66 the power of the ITS analysis to detect a jump effect of the size estimated at the year 1–2 transition point was estimated to be 61% for direct restorations, 38% for fissure sealants and 18% for items per plan for a sample of the size and covariance structure similar to that used here, compared with the 80% power often used as a target when planning a study. This implies a high risk in each case that the 95% CI for a true effect of the size estimated would contain zero. The estimated number of practices required to exclude zero from the resulting CIs ranges from 95 for direct restorations to 150 for items per plan, assuming the same ratio of control to intervention practices, 3 years’ monthly observations and similar distribution of outcomes as seen in this sample. However, more detailed calculations would be needed to plan the required sample size to estimate the full range of effects considered by the DiD analysis, using the ITS approach.
Discussion
The study provides helpful information about the direction of effect seen when a capitation-based contract is introduced. It is likely that a permanent change to capitation would lead to immediate changes of a similar direction and magnitude to those found in the pilot, but that behaviour in terms of access and activity is likely to find an equilibrium somewhere between the FFS and capitation levels recorded in the pilot.
The pilot contract did not appear to cause any reduction in access to care, although it is unclear whether or not this was caused by a concern of GDPs that they would fall below the contract threshold. It also did not dramatically increase access: the mean number of new patients per month per 1000 registered patients for control practices was not statistically different between baseline and capitation periods. A possible explanation was a lack of incentives for practices to increase their list size, perhaps because an average control practice had been operating long enough to grow to an optimal (i.e. profit-maximising) dental practice size, although this does not explain the increase found in the reversion period, which suggests that there was spare capacity to take on new patients. The results also suggest that any drive to expand the practice register (or front-load treatments) prior to the capitation period in intervention practices could have been by finding entirely new patients to treat in the baseline period (this was also suggested in the findings for the number of lapsed patients who returned to the practice list outcome).
The large drop in returning patients in the intervention practices in the capitation phase could be explained by those practices prioritising the recruitment of lapsed patients during the baseline period. This finding is consistent with the finding of an increase in the overall number of registrations in intervention practices in advance of the change to the capitation period. These findings are also consistent with remuneration incentives in the pilot contract. Findings suggest that intervention practices may have been reregistering patients who had not had treatment at the practice for 2 years (and as a consequence their registration lapsed) during the baseline period, thereby freeing up GDP time in the capitation period, and avoiding falling behind the tolerance level for registrations (of 5%) in the capitation period. This ‘freeing up time’ behaviour is also suggested from all treatment outcomes findings, as there was a decrease in activity delivered in intervention practices during the capitation study phase.
The analyses showed a statistically significant reduction in clinical activity, including prevention. The only exception was for the mean number of treatments with a gross cost of ≥ £280. This suggests an overall reduction in activity across the pilot period with no differential selection (i.e. GDPs working in the pilot practices did less ‘across the board’ and did not favour ‘cherry picking’ the provision of more profitable treatments as a result of the change in payment). The lack of a statistical significance between baseline FFS and reversion FFS suggests that GDPs quickly returned to baseline levels of activity (i.e. that the opportunity to practice differently was not sustained). This suggests that financial incentives remain one of the more potent factors behind behaviour change (i.e. original productivity incentives under a FFS system rapidly influence behaviour so much so that activity quickly returns to baseline levels). The further analysis in Appendix 4 appears to show a difference in activity levels between ADs and PPs, with the former being more sensitive to changes in remuneration, perhaps because they are more reliant on NHS income than PPs who have higher private practice incomes. The PPs, as practice owners, also receive approximately 20% of their NHS remuneration in the form of allowances, insulating them further from the predominantly FFS system of remuneration experienced by associates.
Although there was no change in the mean proportion of patient fee contribution to NHS dental practice income per month, there was a statistically significant reduction in the mean NHS dental practice income per month (£5920) and the mean patient charge contributions per month (£2403). This would suggest that the relative mix of fee-paying patients did not change, but there was a reduction in overall income and patient fees, commensurate with the reduction in clinical activity.
What is not known is how practices’ private income changed during the capitation period. There were no statistically significant changes in the financial outcomes when comparing the time period immediately prior to (baseline period) and after (reversion period) the pilot, relative to the control group. This suggests that GDPs returned to their previous level of activity once capitation had ended. This is unsurprising given the rapid return to the same level of baseline activity, once capitation had ended. As highlighted above, these results emphasise the strong relationship between activity and co-payment income with the remuneration system employed.
The fact that no difference was seen in the results between ‘matched’ practices (with PSs) and ‘unmatched’ practices (see Appendix 3) suggests that the findings were not influenced by the choice of the matching process. The use of ITS on selected activity variables was important in order to triangulate the results of the DiD as far as possible. This accounted for variation across practices (random intercept) and the fact that two observations over a short time period are often similar (autocorrelation structure), attributing the remaining variation within practices to the presence or absence of the intervention. The analysis revealed identical directions of effect to the DiD, although the 95% CIs were broadened in all cases. The results from the power analysis of the ITS highlights some of the limitations of this approach for this sample and potentially justifies the use of the DiD for policy-related research when the number of observations can often be limited owing to financial or pragmatic structural constraints. Ultimately, this is a policy-driven piece of research and the DiD is the most appropriate model to use given the data limitation on the number of practices that participated in the pilot. However, the ITS analysis is evidence that a larger sample size is needed to draw a robust conclusion because it is possible that the difference between its findings and the DiD analysis are caused by the DiD-generated CIs being artificially narrow. This is not a limitation unique to this study setting. The assumptions underlying a DiD approach should always be carefully scrutinised, as the smaller SE can lead to erroneous conclusions if care is not exercised. 64,65
Concluding remarks
Overall, the move to a capitation-based payment system appears to suppress clinical activity, including prevention. Equally, GDPs returning to a FFS remuneration system appear to return to levels seen in the baseline period. It is likely that a permanent change to capitation would lead to immediate changes similar to those found in the pilot, but that behaviour in terms of access and activity would find an equilibrium somewhere between the FFS and capitation levels recorded in the pilot. It is not clear whether or not capitation improved access to services, given that GDPs in the pilot may have been wary not to drop below the 5% threshold stipulated in the pilot contract. If increasing access (to those with greatest need) is a policy goal for future contract reform, consideration needs to be given to how best to incentivise opening lists to new patients, rather than setting capitation payment thresholds that can be easily achieved by renewing lapsed registrations. Although the proportion of co-payments as part of overall costs remained the same, the reduction in activity produced a significant fall in PCR. This would be an important consideration for any policy roll-out.
Chapter 4 Qualitative study
Introduction
The introduction of a capitation element to a dental contract has been found to elicit very particular kinds of behaviour; for example, GDPs working under capitation have been found to provide more preventative care. 67–71 They have also been found to reduce invasive dental treatments, extract fewer teeth, place fewer fillings and see patients less often. 67–71 The objectives for this part of the research programme were to assess GDPs’ and patients’ views about how, why and to what extent the changes in remuneration affect the delivery and quality of care.
Methods
Qualitative methods were used to explore these issues, as we needed to understand the nature of both GDPs’ and patients’ experiences resulting from the change in remuneration system. Permissions were granted from the Northern Ireland Research Ethics Committee for the patient interviews (reference 15/NI/0268, 19 January 2016) and University of Manchester Research Ethics Committee for interviews with GDPs and NIHSCB staff (reference 15570, 28 January 2016). Our protocol stated that ‘The overarching philosophy underpinning the approach we have taken is one of Realistic Evaluation’.
It is important to state that the qualitative element of the study was informed by this approach as an overarching philosophy, but we did not aim to produce a fully fledged realistic evaluation.
Recruitment
Purposive sampling was used because of the unique population being studied (GDPs and patients who had participated in the NIHSCB pilot contract). The relevant GDPs were identified by the NIHSCB. The PP and one AD from each participating practice were recruited. Patients were identified by PPs and ADs from participating practices and were given information packs about the study with instructions to contact researchers at the University of Manchester if they were interested in taking part in an interview.
Interview structure and context
The interview questions were designed to explore GDPs’ and patients’ experiences of the shift to capitation and were developed iteratively by the research team. We also sought input from patient and GDP representatives to ensure that the questions asked were meaningful. Interviews were conducted face to face with 11 PPs and were held in private rooms within the dental practice (a staff room, office or empty surgery). Interviews with seven ADs and 14 patients were conducted over the telephone. The interviews varied in length, with PP interviews ranging from 30 to 50 minutes and interviews with the ADs ranging from 20 to 30 minutes. Interviews with patients were much shorter, lasting around 8–15 minutes each. All interviews were recorded and then transcribed verbatim.
Data analysis
Data were collected continuously, starting in March 2016 and ending in July 2017, covering the end periods of phases 2 and 3. We undertook a thematic analysis of individual transcripts, which was informed by realist principles in order to determine ‘what works for whom and under what circumstances’. By using an open-coding technique, in which interview data were examined line by line, we systematically coded and catalogued the transcripts. We recorded the results using NVivo software version 10 (QSR International, Warrington, UK) and sought to identify the mechanisms through which specific outcomes occur.
Results
Overview of sample
The practices in Northern Ireland that were visited by the research team were heterogeneous and were based in a range of urban and rural settings. Some PPs were sole practitioners whereas others had up to 14 ADs working with them. There was a broad mixture of male and females, and ages ranged from ≥ 24 to 60 years.
The patients interviewed ranged in age from ≥ 20 to 80 years and again were broadly an equal mix in terms of gender. All patients had recently attended their NHS dental practice for a routine check-up.
Results: practice principals and associate dentists
Impact of the pilot contract on service and treatment
The majority of PPs, and a number the ADs, were keen to stress that it had been business as usual for the duration of the pilot:
Generally, from our point of view, we’re looking at in that six surgery practice, myself, five associates, working hard up until the pilot came, still working hard now the pilot’s here; same patient base, same patients, same patient demands.
PP1
Well, to be honest day to day hasn’t really changed that much. I’m a single-handed practitioner, 95 per cent plus NHS, roughly 3000 registered patients. So, it’s basically you have got to keep trucking to keep through them, to keep going.
PP4
You’re still doing the same work, the patients are still coming in and their needs haven’t changed at all, they’re still needing fillings or root canal treatments and things.
AD3
I mean, the work’s there; it needs done, you know what I mean. It’s just that – you know, people come in, and they need X, Y and Z done, and so it gets done. Not that I’m aware of. I’ve not really found myself making any sort of conscious adjustments.
AD1
More specifically, when asked about recall strategy or referral rates for example, the majority of PPs and ADs stated that there had been no change. However, when asked about other areas of their service and treatment plans, we discovered that the pilot did affect some change in the majority of practices. Many of the PPs and ADs spoke about having more time, for example:
It has taken the pressure off having to rush through things or juggle a lot of balls in the air at the same time. So I would say it’s given a bit more time for the . . . especially the more complicated things that you feel that you could say, stop, reappoint or take a wee bit longer with a patient who needs it at the time.
PP1
Just getting extra time with patients was the thing I liked, really. You’re not wheeling them out the door because there’s somebody waiting in the waiting room.
PP3
I just think it gave me the opportunity if something was difficult, during the pilot, I would say listen, sclerotic canals, really, really difficult, give them an hour. Book them in for a bit longer, spend a bit more time and then you’re not under pressure to do that.
PP6
You know, if a patient isn’t kind of sure what you’re talking about, you can really spend the time trying to explain different options to them.
AD4
Yeah, the practice took the decision to increase appointments, check-ups from 10 to 20 minutes, so that gave a lot more time in those appointments.
AD5
In terms of the rationale for this, two of the PPs explained:
You’re not under financial constraints to cram in X number of patients every day to make a living.
PP1
I mean, there were things, you know, like, you worked on for a bit longer. You took a wee bit longer with it, ‘cause you could. There wasn’t the same pressure, you know.
PP4
In relation to having more time, a number GDPs believed that the work they carried out under the pilot was of a higher quality:
In terms of our patients I was able to spend a much longer time doing much more quality treatment, I thought.
PP10
I felt I was maybe doing better treatment.
AD7
There is perhaps an indication that, as a result of having more time, some GDPs were doing less, leading to an overall reduction in activity. However, as the evidence below suggests, for other GDPs, having more time provided an opportunity to change their clinical behaviour in a number of ways. One PP and one AD said that the pilot had not had any effect on the time they were able to spend with patients, the rationale being that patients had certain expectations around appointment times that they did not want to disrupt:
The patients are used to a certain sort of level of service, a certain sort of level of . . . you know, if they phone up looking for an appointment, they’re used to a certain sort of period of . . . you know, an acceptable wait. So if you start spending longer with too many people, then that waiting list, as it were, goes up, and where they’re used to being able to get a space . . .
AD1
No, not really. No, you spend what time is necessary regardless of . . .
PP5
With respect to other changes, some GDPs said that the pilot had affected their approach to emergency appointments – either providing the opportunity to introduce them or changing how they are structured within the practice:
Since the start of the pilot we have brought in emergency slots of 20 minutes in the morning and 20 minutes in the afternoon.
AD3
We took the opportunity to lengthen what we did for emergencies. So we have three-quarters of an hour now instead of half an hour.
PP2
A number of PPs and ADs said that the pilot had provided them with more flexibility to discuss or offer private treatment options:
Maybe because you have more time, explain the differences between the health service crown and a private crown, but again there’s no point in pushing it, it’s up to them, there’s no point in you trying to get something into their mouth that they don’t want.
PP3
All the time now, whenever somebody comes in and it’s presumptuous saying that they’re a paying patient, I think, right, well, what maybe could I offer this patient that isn’t available that might be better for them and actually it’s better for me as well which it never was before.
PP4
So I actually, in relation to the private with that, maybe there was more time to talk to patients during the pilot, so they might have consequently become a little bit more private.
PP6
I probably was more aware of making more private options open to the patient, particularly when it came to things like crowns and dentures.
AD3
That has been the main benefit of it, you know, ‘cause with more time and obviously more incentive to sell private.
AD8
Yes, during the pilot there was a wee bit more time for private, yes, whereas now, we are still doing a bit of private but it’s probably to be honest not as much, it’s more health service.
AD10
Although the majority said that this shift towards private was time related, one PP spoke more candidly about the reasoning behind this change, citing the financial opportunities that emerged as a result of being paid on a per-capita basis:
Well, the fact, probably being very mercenary that made you think was financial, that I have already been paid, if you want to be very mercenary about it . . . I’ve already been paid if I put an MO [mesial occlusal] amalgam in and that will cost me the price of the amalgam. If I offer them another restoration should it be a composite inlay, a filling, whatever, I actually, I will get remunerated on top of what I’ve already been remunerated.
PP5
You know there was definitely more with the private opportunities because you were in that mindset that if you did something privately that was a bonus.
AD6
In other practices, however, the pilot was said to have had no significant effect on levels of private dentistry:
We are about 85 per cent mostly health service anyway and we haven’t changed it.
PP2
I wouldn’t say I’m doing any more private but I think you’re still doing as much private on the health service ones as I did before.
AD4
I haven’t really noticed any difference about it. In terms of hours it seems the same; in private, it seems the same.
PP10
No, not at all, not at all. The book’s crammed, you know, no matter what.
AD1
The above quotations highlight a difference in approach to private dentistry that was observed by the research team. In some practices, there was a clear demarcation between NHS and private dentistry – either the GDPs just did NHS work or they had only a small number of exclusively private patients. Moreover, for this group of practitioners, there was a feeling that private and NHS dentistry should not be offered to the same patient: ‘you cannot mix private and NHS’ said PP4. However, other GDPs, namely those who reported an increase in levels of private dentistry, regarded mixing and matching as ‘fair game’, with the introduction of the capitation pilot providing an opportunity to increase their bottom line.
Having more time to focus more on prevention was cited by roughly half of the PPs and ADs as being a significant effect of the pilot, and most framed prevention as the provision of advice:
The type of dentistry that you can do when you’re on the pilot is genuinely more geared towards prevention.
PP6
Yeah, you’d have more time to talk to patients about preventative things. At the moment there’s no fee on the fee scale for talking to people about how to clean their teeth, encouraging them to bring their toothbrush in so you can demonstrate to them, you know and do dental cleaning, all the bits, you know, usually you’re talking away about that stuff while you’re working on the patient, you know, it’s not that we don’t get it in but when you’re drilling away and filling somebody’s tooth in and doing the cleaning you’re chatting away to them and it’s hard for them to concentrate.
PP8
The biggest thing, well for me, I put in a lot of . . . When we were doing the pilot I was doing a lot of, I would say, for patients . . . in terms of patients, it was a lot of dietary advice and all preventative dentistry.
PP10
You can spend ages chatting to kids especially, you know, when they all come in as a family. You can definitely take more time to go over brushing and preventative measures and have a good chat with parents and things like that. I’ve found definitely more time with children and parents.
AD2
Now I’d probably take out the floss, or I’d take out the toothbrush, I’ll show them that and I’ll show them about the exact technique about how they should be brushing, which I just wouldn’t do before.
AD3
However, a proportion said that the pilot had not affected their approach to prevention. This group of GDPs were keen to emphasise that prevention had always been part of the way they did things and that the pilot had not altered this:
Prevention’s always been part of the model. It’s what we just do every day. Is there more time spent on it? I honestly probably couldn’t say that there is, you know?
PP1
Prevention is always something that you try.
AD1
Furthermore, the idea that prevention could be influenced by financial incentives was very much frowned on by one PP:
I haven’t thought more about it because I always do think about it . . . I would be very annoyed if somebody thinks the way you’re paid is the way you’re thinking about the good of your patient, to be honest.
PP5
Impact of the pilot contract on business
A number of the PPs commented on how the pilot had affected their business, emphasising that they thought that the pilot had resulted in less stress on the dental team:
I feel that we’re less stressful this year.
PP2
I think the positive is the depressurisation, in practice there is less anxiety at space in the books from the GDPs, so before there was a lot of jiggling to overbook, squeeze in little appointments, a lot of rejigging just to maximise efficiencies and squeeze a little bit of extra juice out of the lemon and as we’ve worked through the pilot, that has reduced. . . . I think the girls in reception have also noticed that, they’re not getting the same level of stress and hassle.
PP9
I’ve just enjoyed a different way of thinking but definitely I think the staff, GDPs and nurses have all just found the practice a little bit more relaxed over the year.
PP11
Over half of the PPs spoke about the fact that the pilot had given them more financial stability for staff training. This theme had a number of dimensions. In the first two quotations below, the emphasis is about being happier, with staff being trained as a result of the positive effect on the profitability of the practice afforded by capitation. In the third quotation, the PP explains how the pilot created a financial opportunity for more expansive staff development. In the fourth quotation, the PP explains how a reduction in the constant stream of patients associated with FFS reduced pressure on the practice, thereby making staff training easier:
Like I say, we always did that in the practice regardless but am I much happier sitting watching people getting trained now knowing that, you know, the money’s still there, this month the money’s still going to be fine?
PP1
Well, yeah, the staff training and CPD [continuing professional development] generally, yes, you can take an afternoon off easier, because it doesn’t affect your bottom line.
PP4
The main thing that we’ve all got out of it has been allowing all the staff to be getting involved in a lot more training . . . we’ve been sort of like doing our sedation training and the girls started their course in September and sat their exam in March and got the results last month and all three nurses passed first time . . . [B]ecause it was all allocated out every month, you sort of knew what was coming in, you didn’t have to worry about that.
PP8
We didn’t have that constraint on us, that we were always worried about patients. We could actually go away for a . . . away for a course for a day away, up in Newry, which was great. It really helped us.
PP10
Impact of the pilot contract on patients
All but two of the PPs and half of the ADs thought that, on the whole, patients had noticed no difference during the pilot:
Honestly probably say that the patients in the practice here wouldn’t know the difference between what they’ve been in before, what they’re in now, anything else.
PP1
I like to pride myself and think that we would have been looking after patients, and you would give them that courtesy and that time that was required, so I don’t think the patients will have any clue whatsoever.
PP6
I’d be surprised if anybody has noticed a damned thing, to be absolutely honest with you.
AD1
I don’t think they’ve noticed anything different at all.
AD3
Other GDPs, however, thought that their patients had noticed a difference. In both cases it was again to do with having more time, resulting in a superior level of service:
I think they did. I think the big thing was time. They were able to spend more, they knew I wasn’t under a real amount of pressure, force them in and out the door and I think they did notice. There are a few people have talked to me about it.
PP10
Yeah, a few of them have said about it being more thorough, which I suppose is a bad thing on our part, that we weren’t as thorough beforehand, but just about how thorough their exams have been and that we were spending more time going over the oral hygiene and stuff . . .
AD5
So I think patients definitely noticed that you’re not just as rushed. And you’ve more time to point things out to them and says, oh you’re missing a bit of your brushing there, or, you need to floss here better.
PP3
In a similar vein, two PPs agreed that, although they did not think that patients had noticed, they thought that the pilot system had been better for patients. In the first quotation, the suggestion is that the greater emphasis on prevention facilitated by the pilot is more beneficial. In the second quotation, it is the drop in dental activity that was said to have occurred that is perceived to be a benefit:
Now, which is more beneficial for the dental health of that patient? Me trying to get a £12 bloody fissure sealant, or sealant restoration out of that appointment, or me showing that patient how to brush their teeth . . .
PP6
Patients are benefiting from the point of view that they’re not so being, you know, worked on, worked on, worked on, worked on until the point that we’re there almost fatigued or seeing the GDPs, the nurses, things like that.
PP1
Impact of the pilot contract on general dental practitioners
The majority of the principals and associates commented on the income stability and less financial pressure provided by the equal monthly payments that were received under the pilot:
Massive benefit to me as a practice as I know that from one month to the next the income comes in the same, so it gives us a better chance of planning things. Whereas in the past you had sort of good months, bad months, high expenses one month, low income, high income the next month, low expenses.
PP1
What I would say is, it has taken the pressure off slightly, because you don’t constantly have to think right, I have to do X amount of work to get X amount of pounds, you know what I mean. You don’t have that same pressure, yes, you still have to do the work, but it is easier.
PP4
The biggest factor has just been the being able to go away on holiday and not dreading to open the envelope at the end of the following month, you know.
AD1
I think everybody likes the idea of knowing what their wages are going to be at the end of the month, so it’s easier to plan.
AD5
This contrasted dramatically with the income unpredictability and financial pressure that both PPs and ADs experienced coming out of the pilot:
Obviously, we are back down to a fluctuation, things going up, things going down, you work hard one month, the income is maybe low. You work hard, you do something the next month and the income goes up again, you know. So there’s that sort of unpredictability which obviously, you know, GDPs themselves find difficult.
PP1
It took a wee while to build up money again. I got murdered in tax, obviously ‘cause . . . you came off the pilot and then you were starting back up again . . .
PP3
The biggest problem is, I would say, anxiety on the part of the dentist. That’s the owner, me, the two associates because we didn’t know exactly what happened and what was going to happen from a financial point of view. So, there was no point pretending otherwise and financially it was a bigger hit than . . . now we knew there would be a hit, but it was like the recession, it lasted longer and was deeper than people thought.
PP6
There was, you know, at sort of the end of the pilot and then, you know, you were sort of having to start some courses of treatment from zero and, you know, it just took a while to get back up. You know, you wouldn’t have had the normal sort of, you know, rolling on from the previous month sort of thing, you know, as you would under the usual circumstances.
AD2
Yes, well I certainly notice the difference anyway. Yes, it certainly feels like there is more pressure and stress but I think if anything you sort of bring it on yourself. It wouldn’t have to necessarily be like that, but you just, it’s just your mentality changes when you are back on the different system.
AD6
Also the fear of . . . this might sound strange, but the fear of what’s in your pay packet at the end of the month, you don’t know what you’re going to get and since we’ve joined the pilot I’ve bought a house, so I now have the pressure, but I’ve now got a mortgage, and I’m like I have to cover that every month, whereas when we were on the pilot like I knew that I was getting a certain amount every month.
AD5
For other PPs and ADs, there was an acknowledgement that certain financial disincentives had led to them withholding certain treatments or at least deferring them until the capitation pilot had ended, thereby enabling them to perform these treatments in a more cost-effective way. The third quotation below is illustrative of the fact that GDPs are subject to competing demands to provide the best care for their patients, but also to be mindful of their bottom line:
The only thing possibly is there’s no financial incentive to do big courses on people who are exempt, so there’s people who have a mouthful of fillings and maybe need several crowns, and the fillings are kind of doing OK, and you’re just like, oh, that’s a big lab fee, I’ll maybe leave it for a while and see until they really need it.
PP3
But we had kept a lot of the examinations and whatever . . . you know, people were ringing up for a check-up that would have been due in August at . . . halfway through July/August. We, sort of, put them off. We made an excuse and we said, oh he’s away on holiday or whatever, even though I wasn’t.
PP5
If say, for example, someone needed work that involved a lab cost, at this stage of the game you’re, sort of, thinking can it wait till you’re off the pilot so then in effect it’s not costing you money to do it.
AD4
Not necessarily. I tried to see it that the patients were coming, and you’d do what’s needed, and that’s it. I suppose things like long term, if they were, if you thought that they . . . nothing was immediate, you might consider watching it.
AD6
Nevertheless, some PPs were keen to emphasise that their working practices were very much guided by their professional ethics, as opposed to financial incentives:
You know, I mean, basically if someone needs something done, you know, if someone comes in, if they need a crown, they get a crown. If they need new dentures, they get new dentures, I would definitely not say, well, I am not going to do that because I won’t get paid anymore for that, if they need it doing, then that’s fine.
PP4
Furthermore, for two PPs, the weight of patient expectation eventually tempered any potential opportunities that the pilot provided for working differently. For example, when asked whether or not they had restructured their appointment book as a result of the pilot, one PP responded as follows:
Initially I tried to. Then I found that people were having to wait, even by extending one appointment by 5 minutes or something, it did have a knock-on effect so probably as a pilot, as maybe after Christmas I had to come back on that and started just working totally back to taking those extra 5 minutes back . . . I didn’t want to compromise patients having to wait any longer.
PP7
A theme around changing relationships was something that emerged from the interviews with two of the PPs. In the quotations below, the first suggests that capitation results in a more GDP-driven relationship but the second suggests that GDPs may put less pressure on patients under capitation:
Well, it’s probably more dentist driven, isn’t it, if you’re getting capitation you’re thinking, well, if I’m doing a crown and I’m not getting paid for it, it’s included in my capitation and I’ve got a fee for it.
PP3
Pre pilot, what am I doing? I’m going to clean your teeth, because you’re in that chair, and I have to ratchet up money, I have to earn money. The wee denture is rubbing, that’s going to be adjusted, so that’s an adjustment of a denture. I’m going to force you to get your teeth cleaned, because this is what’s going to have to happen. Oh no you can’t. Oh no, you must have them cleaned, it’s very important, you must have them cleaned. The denture’s going to be adjusted.
PP6
In relation to the sense that there was more time to do things under the pilot, two ADs mentioned that they had been afforded the opportunity to develop their clinical skills:
The main thing is the amount of time that I can take to do . . . I mean, I’m not exactly newly qualified but I’m about 5 years qualified and I have just spent the last 18 months, or however long we’ve been on the pilot now, kind of using it as a good opportunity to take my time and really kind of develop my skills without the pressure of the clock ticking, if you know what I mean.
AD2
I think it was a very good . . . it’s nothing to do with you guys, but it was a great opportunity to be able to improve, you could take a bit longer to improve your skills and I definitely think that has translated through now and I definitely see an improvement in certain things that I can do now. But, I think that’s just totally linked to the fact that I had more time before to do that yes.
AD4
Views on capitation payment systems
A number of concerns were raised about the permanent introduction of a pure capitation model. For example, concern that a pure capitation system would risk supervised neglect was mentioned by a number of the GDPs:
What I know is that, you know, GDPs look after patients, they treat patients, they look after them well. Obviously supervised neglect has been reported in the past, so not all GDPs do it the same way, but how do you monitor that? I don’t know.
PP1
Well, my experience of dentistry when there was capitation years ago, was in ‘92 and the fee cut and it was the neglect, it was cavities being charged, and then they weren’t filled and they had to go in for GAs [general anaesthesia], things like that, untreated, that would be, and I would imagine 90 per cent would not be in that, but there’s always one, you know, no matter what profession you’re in.
PP2
If it was a capitation system I would love it because I don’t think I would abuse it but I know that a lot of other people would, and then it wouldn’t work.
AD2
Other GDPs were concerned that a pure capitation system could be problematic if the patient base is unstable or the practice needs to take on a lot of new patients:
Depending, obviously, on what your figure was, to get, but if you were taking a lot of patients at the one time, and they had a very high need, that would obviously be of concern.
PP2
I was talking to a friend who has opened a new practice in [town], and it was before the pilot was coming up, and I said, now, are you going to give the pilot a go, he said no way, he says because we are still actively taking on new patients. I said, we are looking to grow the practice and he says I can’t operate that on a fixed sum.
PP4
I definitely think it should depend on the type of patients, their postcodes and their socioeconomic area, because some patients, no matter what you do, are going to need more work and more treatment than people from better areas.
AD3
In addition, two PPs felt that capitation had the potential to incentivise longer sickness absence, despite not finding any evidence of this so far under the pilot:
I think so, yes, aye I think it would, certainly I mean, the big incentive for an associate under the old system is, they’re not in, no pay.
PP2
I was watching to make sure that everybody was, kind of, maintaining their clinical times, I’ve always had a nervousness of sick leave, that’s always been a slight bone to me and I was worried within this system we would end up having more sick leave, but I don’t think that has happened, probably just through circumstance it hasn’t at all.
PP9
Views on fee-for-service payment systems
The majority of the PPs we spoke to referred to FFS as being like a ‘treadmill’ at some point during the interview, and almost all of them had a sense of trepidation about the return to FFS:
To be honest I’m not really looking forward to it in a way . . .
PP2
I’m not really looking forward to getting back on that again.
PP4
The biggest anxiety, is just because you have to go back to that treadmill type of thing.
PP6
Only one AS mentioned a treadmill, although a sense of trepidation about the return to FFS was also mentioned. More ADs (three in total) said that they were happy or not concerned about the return to FFS, the rationale being that it is a system that they are used to, as well as a perception that FFS rewards hard work:
It’s something I’ve always done, and for this year I’ve known a bit of difference, but it’s been a blip, and I think, to be honest, that we’ll just go back to it and keep doing what I’ve been doing. As I said, when the work comes in, you do it, and it goes back out the door.
AD1
I think I’m quite happy about it because I feel like I’m doing more work now than before the pilot but at least when you were busy when you were not on the pilot, there was a reward for it. There’s no incentive to work harder when you’re on the pilot but that’s the way I feel about it.
AD4
In expressing other views about FFS, two PPs said that they thought that FFS encourages overzealous dentistry and one said that it is an unsuitable system of remuneration:
I have no problem at all in saying it, the dental contract, item per service, in my opinion, is not fit for purpose, it’s just not. It encourages overzealous dentistry. I have always had that opinion. But it’s how I pay the bills, sort of thing.
PP6
We try not to drill and fill as much we used to. We try to watch small lesions . . . but under the present system, there’s a disincentive to do things like that.
PP11
Two of the associates were critical of the current FFS structure. In one instance, this was because of a perception that the level of remuneration for certain treatments was unfair owing to the amount of time taken to perform them. In the other instance, it was the time taken to receive payments that was the main cause for complaint:
Denture repairs – if somebody comes in and they get a repair, if they come in, get the impression for their repair and then come back and have to come in to get it fitted, you’re literally working for nothing. By the time the lab fee comes off, and then the amount of time that you’re spending with that patient, you’re getting pennies for it.
AD1
Yeah, it was just taking ages. Now from my point of view I wasn’t still completing treatment, but sometimes, I don’t know, payments just take ages to go through for no reason, or for reasons that you never know.
AD3
Views on the pilot contract
Although the majority of GDPs were, in the main, positive about the pilot, certain criticisms were raised, particularly in relation to the issue of laboratory fees:
I would say if they were going to run it long term, they would need to have built in some allowance for lab work.
PP4
We did have situations where new patients arrived in the practice and so they were never registered and they required laboratory work and each practitioner just picked that up as a negative hit to them. So it certainly would be a disincentive to register a patient who you knew in fact you were going to have to treat this patient, you were going to get no additional money for it and better than that you were actually going to lose money because you were providing this treatment that incurred lab costs.
PP9
I had one guy, for example, came in and he was needing quite a lot of lab work, and I’m think . . . you know, you find yourself thinking, well, if I was to take you on, I’m having to pay for all this lab work, and I’m getting nothing different in return.
AD1
I suppose the one thing, it makes you think twice when you’re doing lab work because that’s coming off your money at the end of the day, so if I make 10 crowns this month, that’s a lot of money off my wage at the end of the month, which if I only made two . . .
AD3
Moreover, a number of PPs said that they thought that the pilot had been too short:
It’s such a short time frame that you can’t see how does it change behaviour really.
PP1
We know it’s only a year, so you’re not going to do a major reorganisation because you know that if you do you’re going to have to reorganise all the way back again.
PP3
I think had the pilot run for more than a year, I actually think that would have been more useful, significantly more useful, because I think a year pilot is . . . you can see the end of it very quickly.
PP9
Views on the future of dentistry
We asked all of the PPs and ADs how they would choose to remunerate GDPs if they were in charge of dentistry in Northern Ireland. The majority of PPs and half of the ADs said that they would favour a mix between FFS and capitation:
So is it some sort of blended system where you have a certain amount given, you know?
PP1
I think a mix of some sort, yes. It certainly is very nice having security of capitation but there has to be . . . I think it’s good business to have some sort of incentive and some feeling of if you work harder you will be better remunerated.
PP5
I think a mix is the way that it needs to be. You know, I think there needs to be a significant capitation level to give us even a sporting chance of having the time to talk to people.
AD1
I would say a mix. I think capitation would . . . as lovely as it sounds, I don’t think, you know . . . I think I really love my job and I’m in it for the right reasons. If it was a capitation system I would love it because I don’t think I would abuse it but I know that a lot of other people would, and then it wouldn’t work.
AD2
Some GDPs were more specific here and advocated the need for a core service:
Obviously one of the major pushes with change to contracts is that there’s only a certain amount of money to go round. There’s an argument that if you only have a certain amount of money should you only expect a certain amount of service as in at the moment we’re working on SDR [Statement of Dental Remuneration], which is vast.
PP1
A core service I think, very limited, yeah, definitely core service.
AD5
Yeah, have a core service and you’re just getting paid for that, and then other treatment, separate treatments, have fee for item.
AD7
Two PPs and one AD, however, said that they believed that a pure capitation model was the answer:
I would be purely capitation based. From my experience and from what . . . you know, chatting to my other colleagues that are on this pilot, yeah, definitely . . .
PP10
I think to a certain extent, if there was a good, you know, that we were salaried and it was capitation, I do think that a lot of GDPs would appreciate that. And it would . . . but it would need to be a good contract.
AD6
Views on the length of the pilot contract
A number of PPs said that they would consider role substitution if the pilot had been longer than 1 year or if capitation was permanent.
Probably longer time frames, could you see therapists, hygienists, nurses with special qualifications doing more within a practice? Yeah, the crystal ball would probably say, yes.
PP1
The hygienist comes in and she operates privately, she just comes in and operates privately, but if it was possible to provide health service through a therapist or something that would be something we would look at.
PP2
Although the pilot itself did not really affect recall times, some GDPs said that they would consider changes if they were operating under a permanent capitation model:
I think, long term, if you were going to be in the capitation long term, I think we would probably put more patients onto yearly and 2-yearly recall.
PP2
I think that could be tinkered with . . . you know, maybe patients who have a lower caries rate could be put back a little bit longer.
PP11
Yeah, I mean, I suppose if you’re in a capitation model there’s an argument to saying, well, we could maybe see low-risk patients every 9 months . . .
AD7
Putting an even greater emphasis on prevention was mentioned by three PPs:
Possibly spend a lot more time on preventative things about patient education, stuff that wasn’t covered . . .
PP3
I think you would be going more towards prevention, obviously, because it is in your interest to be doing that. And you would probably structure it more around that.
PP4
A further two PPs said that they would have more opportunity to focus on and accommodate private dentistry:
If we were involved in a capitation for a longer period, it would enable us to spread among the GDPs, if they were doing private work, they would have a session set aside specifically for that, but there still would be health service going on with the other GDPs. So that would allow us to rearrange the practice . . .
PP2
I think you’d be pushing your private more I think, but because our patients have been going so long, they’d just be going, why you doing this, you know what I mean, suddenly.
PP3
Motivation for action
During the course of our interviews, a number of PPs and ADs spoke about what motivates them as GDPs, with a number of themes emerging here. The desire to provide high-quality care and keep patients happy was mentioned by two PPs:
You, kind of . . . you want somebody else to look at you say, oh that guy’s good. You know, and that’s pride in your work. I think nearly everybody has that in whatever you’re doing . . .
PP3
[A] lot of it is just keeping people happy, doing good-quality work and actually thinking about the money.
PP5
One PP, although mentioning their desire to make a profit, also saw money-making as connected to a wider responsibility to support their staff:
To come away with nothing at the end of the year . . . I have to make a profit . . . We all have an understanding that without the practice being sort of running properly, working well, that there’s a number of staff whose, like if their mortgages and their welfare rely on us doing things right.
PP1
For other PPs, profit-making did not feature as a driving force for practising dentistry and running business was seen merely as a means to an end:
I think what varies most perhaps is, you know, some will say I’m not interested in making a profit, I’m not interested in the business, whereas other GDPs would say, well no I am interested in that . . . I mean I don’t need to make £50 or £60,000 more, you know, that sort of thing, that would be – that’s where I would be on that.
PP2
That is not the main driving force in the speed at which you . . . and how stressful your day is. It’s focusing on keeping the people happy.
PP5
But I mean, yeah, to be honest, 100 per cent, my practice is not driven the way it should be if it was a business model. Number 1, I don’t have a website, number 2, I’m not pushing the high profit type of treatments. I’m not pushing . . . patients have been coming here for 28 years . . .
PP6
So . . . and I . . . it’s the dentistry I love, it’s not run as a business or, you know, the business is just a means to an end.
PP10
Other PPs spoke about the competing concerns that GDPs have to juggle roles, as both business owners and health-care professionals, in particular the tension between adherence to and compliance with their professional ethics versus business acumen and entrepreneurship:
If you’re going by the letter of the health service and you want to sleep in your bed at night, you do what the health service asks you to. So, you do more root treatments. You do your crowns and you do non-metal . . . metal crowns at the back of the mouth even though you probably only making maybe £5 on them. ‘Cause that’s part of the treatment. But you will try and upsell your own private stuff and say, well it could be a white crown. And this is what it is. It’s metal-free, da, da, da. That’s part of your sales technique.
PP3
I would spend the time talking to people and still would do that, but on the pilot you felt as if you were getting paid to give that preventative chat. When you’re not on the pilot, you know you’re not getting paid, but you still want to give that.
PP6
And I’m in a situation now where I’m at times I’m having to be a bit of a salesman and that sort of, you know, when you’re a health professional, a health professional and a salesman are two sort of, you know, competing heads and I must admit I find it hard.
AD2
So there definitely is a path between the finances and the ethics and I think ethics always wins you know it always kind of . . . that’s sort of what the main . . . that would guide you but definitely in the back of your head you’re thinking about the financial side of things.
AD4
Despite the desire to exploit opportunities for financial gain, or to maintain cash flow, the impact of being situated in their local community was mentioned as a constraining factor by a number of PPs and ADs:
People know who . . . exactly who I am. So if I walk in to Costcutter [Costcutter Supermarkets Group, York, UK] across the road and they go, that guy there he just charged me £300 and it fell apart. You’re not going to . . . you know, word spreads.
PP6
Well yeah, I couldn’t go to the local pub. Everyone knows me, right? I can’t go down the street for a sandwich or I get asked . . . You know, that’s how small a community we are . . . It’s not like a city like, you know, they wouldn’t see that patient. I will see that patient on a Friday evening. Probably I’ll see them out in the street here. So that’s . . . You have to cut your cloth the same, you know, really. And for me, obviously I treat people with respect. You always treat people with respect but it’s different in, I’m sure, in a big, very busy practice. It’s just in and out and that’s it. You don’t see that person again. I will see them, you know.
PP10
And you’ve got to be, you know, you’ve got to be in a situation that if anybody chats to you in the street, again, you’ve got to be able to look them in the eye and say, yep, you know, I remember what I did for you. And I did it to the best of my ability and I did what I thought was the best for you.
AD2
Results: key stakeholders in the Northern Ireland Health and Social Care Board
By conducting a set of brief interviews with project leads and senior management at the NIHSCB, we gained further insight into the way in which GDPs responded to the contract change.
Some staff mentioned the tendency for some GDPs to exploit loopholes and opportunities within contracts. There was also the recognition that GDPs’ motivations do vary and that they have to juggle the tension between adherence to their professional ethics on one hand versus business acumen and entrepreneurship on the other:
I think, well the first one obviously is you need . . . you’ve got to be careful if you’re completely, you know, changing it. You’ve got to think, are there loopholes, or are there, you know, ways around this, because these are our businesses at the end of the day, and I think we found that out very early on . . . GDPs generally, I think, just want to play by the rules. But, you know, I think if you make the rules too complicated, like our current SDR [Statement of Dental Remuneration], it, kind of, encourages that, maybe, behaviour . . . [I]t was such a diverse group of practices, and characters, and you did expect everybody to respond differently, I think. So, I did expect that, sort of, varied response that we’ve got.
NIHSCB1
There was a lot of rumour that [pilot] practices were advertising on the radio, extra private sessions and, but whenever, I suppose, it’s very difficult from the board’s perspective to monitor that because we have no insight into what their private work is.
NIHSCB4
I mean, the GDPs are shrewd, by and large. I mean, they’re business people, ultimately. And, I’m sure they’ve, some practices more than others, I mean some have probably just slept walked straight into this, and taken it completely at face value, and made the most of it, and thought, oh, this is great, you know, and just reacted to it. Whereas, others, I think, have been proactively basically reading the rules. OK, now just before we get into this, now this is what they’re saying, before we get into this game, let’s understand the rules of this game, how we can make this work for us. And, they are maybe the ones who more quickly started to do things differently. Maybe started to stretch out recalls, thinking, you know, that well, I mean there’s no point spending our time doing a lot of treatment here on patients that were not going to get paid for it, you know, whilst we could get paid for it down the far end deferring the treatments. You know, make it, allowing a little bit more space for their private. All those sorts of angles, you know.
NIHSCB5
I suppose inevitably we all thought that GDPs would reduce their activity. But the other side of that is, you know, the other human bit of it is that there are GDPs out there who want to do the best for their patients . . . I think you’ve a spectrum as well. You’ve one or two who will have looked at the system and thought, how can I maximise it? And that’s good . . . I think a lot of them, it is quality of care as well. I wouldn’t say they don’t care about the quality level. They care very deeply about the quality, but it’s how do you get the quality? And where is the line between the NHS and private? So if I’m not making enough on the NHS, the NHS is not giving me enough, I go private.
NIHSCB6
The idea that GDPs were situated in a particular local community and that this may place constraints on opportunistic behaviour was also mentioned:
There is such a range of practices within Northern Ireland, and different locations. You know, a small single-handed practice in a country area is so different to a large, you know, practice in a, sort of, thriving area in a city.
NIHSCB1
It’s still quite small, Northern Ireland, and people feel a dedication to their patients. So, their patients are their patients for the long term. Their reputation is really important. That’s word of mouth. So, you’re not going to short change, you know, patients, just because you can in a pilot, you know, you’re just not going to do that.
NIHSCB2
Staff commented on what they saw as the future of dentistry in Northern Ireland. The need for a core service was mentioned by some, with cost containment being achieved by a reduction in the range of NHS treatments to be offered. The need to incentivise quality and prevention was also mentioned, as was support for some form of capitation and in one instance a local innovation fund:
I still think the SDR [Statement of Dental Remuneration] is not a bad thing, but I think it needs simplified. I think there needs to be a core . . . well paid, but core things that people should be provided. So, you know, somebody should be always be got out of pain, an extraction, a simple filling, but I think . . . I do think that GDPs should be paid more . . . So, pay them better for less things, and then people can still get all those other things like endodontics and, you know, bridge work, but they pay a private fee for it. And, I think that’s very similar to most other aspects of life.
NIHSCB1
I think I probably would have a central payment, but I would have incentivising additional payments for equality, and for areas like prevention. I’d also have, kind of, an innovation, and almost a grant-based aspect to it, where people have flexibility to apply things differently in their own context on their own site that might work well for them.
NIHSCB2
I think I’m a convert now to a capitation-type model. There may well be extra quality payments involved. There may be some, say, laboratory items that may well have to be fee per item, but the vast majority . . . I think, would be capitation based. You know, I’m a fan now of that system, especially because if you wish to carry out some private dentistry you still can.
NIHSCB3
Going by the feedback, even in the old surgery pilot when we visited a lot of practices and speaking to people face to face, the feeling that I get that what the profession would like is a core service and then other things set outside that . . . So, I suppose, like, your general drilling and filling and anything that you need in terms of maintaining your oral health. But anything . . . crowns and things like that, that could be seen to be just purely from a cosmetic point of view, you want a white filling, you know, you’d be paying a separate fee for that.
NIHSCB4
I think something like capitation has to be in there, because it’s cost containment and no matter what way you look at it, it has to be cost contained. I think that it’s also looking at the administration side of it. I would be much more keen for the capitation to have some sort of quality indicators within that as well, you know, to be paid on quality indicators so that some sort of . . .
NIHSCB6
Finally, the observation that some GDPs might have been telling us what we want to hear regarding their experience of the pilot was mentioned by one staff member:
You see, and that’s, I mean, it’s interesting all of this, because, on the other of it there is a bit of a game of chess going on here, you know, and I would say in the evaluation that there’s always this, sort of, level in their head, that there’s the right answer, that there’s the truth, and a good answer to tell you. And, you know, exactly what happens is hard to know, and you can never really confirm what has gone on. But, you know, we know the hard data that’s, you know, it’s hard to argue against, does seem to suggest that there’s less prevention. And, I sometimes wonder, you know, is there an element of this where the GDPs genuinely believe that they are providing more prevention, and that this is all effective. And, you know, it’s almost a deep-seated, almost like a faith, issue, which they are able to separate out from their rational brain, when maybe they do think that they’ve looked at numbers, and went, gee, you know what, I’m probably not doing as much preventive interventions as I thought I was. I don’t know.
NIHSCB5
Results: views of participating patients
Experience of the dental surgery
All patients spoke positively about the experience of attending their dental surgery, with many of them commenting on the pleasant environment and friendly atmosphere:
It’s a lovely environment when you walk in. It’s bright and it’s clean.
P3
It is really a nice facility, you know, the treatment rooms are always spotless, it is a really nice surgery.
P12
They really make you feel at ease right from reception, right through the girls, the hygienists and the dentist, they’re lovely.
P1
It’s always a friendly chat whenever you go in.
P14
All patients said that they had been able to arrange their dental appointments easily and were able to access their GDP at a time and date that was convenient for them:
She just sent me a text message and I contact her there to make an appointment. So I just contact her, like, and made appointments for the following day no problem.
P3
Yeah, they do late nights so it’s convenient.
P4
They usually send out a wee text message, so it was just a matter of phoning them up and saying whatever day suits me best, with work and that . . .
P6
Experience of the general dental practitioner’s treatment
During the course of the interviews, we asked patients several times whether or not they had noticed any changes in their dental care during the course of the pilot. They all said that they had not noticed anything:
No, no, everything is just normal.
P2
No, it just continues on just the same. There’s no difference, no change.
P3
No. No, nothing that I can note.
P6
To be honest, no, they’ve always been very good, like I’ve been going there from when I was a child, so I haven’t noticed anything significantly different, like, you know, they’re always quick to get an appointment and things like that, so I haven’t noticed anything more so, if you know what I mean?
P8
I don’t think there’s anything outstanding from my point of view that has been different.
P12
However, on the return back to FFS, one patient noticed that their bill was presented in a different way:
Yeah, I was just, one thing would be that we definitely now get a breakdown of prices and, you know, an estimated cost. Like I’ve had treatment earlier this year and it was, there was an estimated cost of what it would be, which is good, you know.
P1
Furthermore, they also commented on some private treatment they had been offered during the course of the pilot:
Yes, yes, she offered to replace one of the, it was one of the fillings that she had to do. It was, apparently I grind my teeth and then one was broken and she was going to repair it, and she did offer to do it with white, which was, I think it was, it was over about twice the price of the, you know, doing it with the grey, or you know. And, I said, look would you be able to see it and she said no, it’s in the back. So, I said no just, just don’t do it with the white then, you know.
P1
However, the majority of patients had no sense that GDPs’ offers around private treatment had been affected by the pilot:
No, no, not any more or any less, no, just the same.
P2
No, he’s never mentioned that.
P3
It’s never been . . . it’s never been pushed, it’s never been mentioned.
P9
I think that I actually asked him about it, and he said it really, like, I mean at the time, if we wanted to take out a private plan, like, fair enough, but he didn’t need it as such . . .
P10
Well, it’s always available, you know? Leaflets . . . I get e-mails from my GDPs, but I’ve never been directly offered it as such, you know . . .
P13
Another patient mentioned that they had been switched to a 9-month interval for their check-up during the pilot and that this interval had been maintained on the return to FFS:
Look, I’m 65, and he thought they were in reasonably good nick, everything considered, and he asked me would I be happy with a 9-month interval, and I said look . . . if you think 9 months is appropriate, and we agreed on that. So the last time it was a 9-month interval, and my next . . . my next appointment now is for 9 months from then.
P9
All patients said that they were happy with the amount of time that the GDP was able to spend with them. They also all said that the GDP provided good feedback and that they felt involved in their treatment:
I’m happy enough. He does his job and does it efficiently and that’s it. There’s no hurrying up or getting you in and getting you out, type of thing, you know. It’s normally a relaxed atmosphere and very professional.
P3
Yeah. Definitely, you know, previous experiences with different GDPs, I feel the dentist I’m with at the minute takes a lot of time to – on a personal level as well – to ask how you are . . .
P6
No, I never feel rushed. If I have had a question about something then they’re happy to answer it, you know, so no I never feel like I’m being rushed out or anything, no.
P13
No, it seems as if he’s doing a thorough job, a thorough check-up, and just, you know, it’s the appropriate length of time for what I would be in for.
P14
I’ve always felt like he has examined my mouth thoroughly, that he’s . . . he says are you sure that this is OK. Yeah, I don’t have any complaints about him at all in all honesty.
P10
Actually she allowed me to look at the screen where they had taken an X-ray and she allowed me to, you know, where she was talking about things and she pointed it out on the screen, which was nice to be able to see.
P1
I don’t feel rushed. I mean, I know . . . I do like how they keep their times. They are good at keeping their times. If I have my appointment at quarter past 11 I’m rarely seen, you know, very seldom after that. For people who work and I think it’s good that . . . you know, I’m not there for a leisurely afternoon. I just want in, I want the treatment done and I want out and that’s really always the service I’ve got . . .
P15
However, one patient mentioned that they thought that the GDP was able to spend more time with them during the pilot and then at their next appointment in the return to FFS:
It’s probably a wee bit longer, you know? They’d probably have more time to spend with you as a patient, because sometimes I had noticed previously they’d be quite busy, practices, so they were possibly seeing different patients in different rooms, and they’d be in and out, but I have noticed in the last couple of visits, they would be in with me more so than going to another patient as well.
P8
There was a strong degree of trust among the majority of patients (13 out of 14) and a willingness to always accept the GDP’s advice. Similarly, the majority of patients (12 out of 14) said that they felt that they had never been offered any unnecessary treatment:
I trust him entirely. Whatever treatment he recommends I let him go ahead and do it. He knows what he’s doing.
P3
Yeah, definitely, yeah, a lot of trust there, yeah.
P8
No, he would not be in the business of trying to do work which isn’t necessary in all honestly he wouldn’t . . .
P9
Yeah, yeah, yeah. I kind of know them all now, you know, so it’s just familiar for me taking advice like that. They’re professional, you take their advice, so, yeah.
P15
We asked all patients specifically about prevention, which the majority of patients said they received on a regular basis – particularly brushing, flossing and dietary advice:
Usually he would give me a wee demonstration, just the previous 6 months and this time he’d really went into great care with just flossing, ‘cause I wasn’t really getting the knack of it.
P6
So they would always kind of like, you know, give you a bit of advice on, you know, what kind of brushing methods and how often you should be and mouthwash and, you know, using floss, and things like that.
P8
He tells me to floss and he tells me not to drink Coke [Coca-Cola®, The Coca-Cola Company, Atlanta, GA, USA]. He would advise me on what’s good to drink and what’s not. I drink a lot of Diet Coke®, I used to drink a lot of Coke but he told me, listen, [name of patient], I can’t stop you drinking Coke but the best thing to do would be to drink Diet Coke, it’s bad but it’s not as bad, you know.
P11
I suppose only if there was an issue, like because I did have a bit of bleeding in my gums and just talked to me about the importance of flossing, really, and I remember asking for a bit of advice around using an electric toothbrush and whether it would be better to do that or not, and getting a bit of advice about the best way to hold the toothbrush to make sure my teeth were getting cleaned properly.
P13
A number of patients were keen to emphasise the fact that, in their experience, the GDP appeared to favour prevention, as opposed to treatment, more specifically that they were not inclined to rush into treatment. In the second quotation, there is also perhaps a suggestion that attitudes towards prevention had recently changed. Although not acknowledged by the patient, this could have been affected by the pilot and tallies with what a number of principals and associates said about their approach to treatment during this period:
He would recommend interdental cleaning things, you know things like that and if you’ve any questions like . . . he’s very good at saving your teeth, I have a back tooth that him and I joke about it, it’s held in with cement. But he’ll say to me it’s still there and it’s not giving any harm so we’ve agreed while it doesn’t give me any bother we’ll just leave it alone. But he’s very good in terms of advising you and not being invasive if you know what I mean . . .
P9
Uh-huh. He’s very much on the preventative side, he’s very much on advising you what you shouldn’t do as much as . . .
P10
One patient, however, said that prevention was not a priority:
They don’t really do an awful lot of that at the practice that I go to.
P15
The majority of patients (10 out of 14) said that they were happy with the cost of their dental care. However, one patient felt that treatment was perhaps too expensive:
Not really, no. It’s been the same, check-ups are the same price it’s just if you have extra it goes up slightly, you know. I think it is quite competitively priced for, you know, compared to other GDPs.
P1
I’m happy with the cost, you know. It’s reasonable enough, I can’t complain.
P3
I’m quite happy with . . . it’s very reasonable, yeah.
P6
I could absolutely not complain about it in all honesty and my wife would feel the same way.
P9
It is reasonable, surely, yeah.
P11
I think it’s reasonable for, you know, just to ensure that my teeth are being maintained and healthy, and I just feel like the appointment, it’s not a huge amount, and I think it’s a really small amount to pay just to make sure that everything’s OK, you know . . .
P13
Possibly it’s a wee bit expensive the treatment, yeah, it’s quite expensive but, touch wood, I’ve never had to get anything.
P4
Yeah, I did think it was going to be more expensive for whatever it was I was getting done the last day but, no, it was just the usual price, so I was happy enough.
P14
The last time when I was in, it was £18 for a check-up and a clean, so I think that’s cheap. I’ve got glasses and my glasses are like hundreds of pounds.
P15
Discussion
The results outlined above provide an understanding of GDPs’ and patients’ views about how changes in remuneration during the pilot affected the delivery and quality of dental care.
Only 14 patients were recruited and interviewed, as data saturation was reached very quickly. One limitation here is that the dentists recruited the patients to be interviewed and one might suspect that it is easier to recruit satisfied, long-standing patients for interview. It would appear that long-standing patients are very loyal to their dentist and we got the impression that something would have to go badly wrong for a regularly attending patient to express views of dissatisfaction. The views of patients were not very illuminating and perhaps this reflects the complexities of assessing quality in dentistry. In addition, patients’ lack of knowledge about dental disease and its effective treatment and their limited understanding of the ‘what, why and how’ of the care provided during each course of treatment will hamper patients making an informed judgement about the quality of the dental care they receive. Although the pilot appeared to have very little impact on patients, its effect on GDPs was significant.
On one hand, the results reaffirm some of the well-described themes in the literature, for example that capitation encourages more preventative care. 56–59,62 However, some of the results appear to contradict the prevailing wisdom about what happens to GDPs’ behaviour under capitation. Whereas some GDPs felt that their activity levels had dropped during the pilot, others maintained that there had been no change. Some GDPs spoke openly about having the opportunity to conduct more private dentistry, whereas others stated that their levels of private dentistry remained the same. Although some claimed to have adopted a more preventative approach, others stated that their approach to prevention was unaffected. Moreover, some GDPs spoke very candidly about the fact that they had taken advantage of certain loopholes and opportunities for the purposes of financial gain, whereas others almost took offence at the suggestion that this constituted acceptable behaviour.
What then explains this tension and why did the pilot appear to affect GDPs’ behaviour in different ways? The answer may be linked to the fact that GDPs are affected by a number of competing interests that may lead individuals to behave differently, despite being provided with the same kind of incentive. These competing interests include a responsibility to keep their practice going for the benefit of patients and staff and serving people and providing quality care, as well as running their business profitably. 11,72 This was also highlighted by Harris et al. 11 and Harris and Holt. 73 How individual GDPs balance these interests varies, but in all likelihood this balance will be affected by the social and institutional structures in which they operate, their interpersonal relationships as well as their own particular wilfulness. 74 There are a number of observations that we can make about these factors.
First, not all GDPs work within the same institutional structures. Since the establishment of the NHS General Dental Service, GDPs have been independent contractors to the NHS, owning their premises, employing their own staff and paying their expenses from their income. NHS contractual terms permit practices to provide a mixture of NHS and private care. On average, GDPs spend 75% of their time providing services to the NHS. 73 The majority of GDPs in the UK work in professional partnerships alongside other GDPs, although one-third are single-handed practices where just one GDP owns the practice and provides all of the care. 75 In addition, as part of the NHS changes made in 2006, the government made it easier for practices to be owned by external commercial organisations. This has recently given rise to several large commercial chains, which now provide dental care under a corporate model. 76
Hence, structural arrangements regarding the delivery of primary care dentistry in the UK are mixed. Crucially, these different structural arrangements give rise to different institutional logics, in other words ‘the predominating beliefs that create connections and a common purpose allowing those within a field a sense of grounding, orthodoxy and habituated normalcy’. 63 Dentistry appears to be governed by a range of at least three kinds of institutional logics: a professional clinical ethic, a business-minded approach and commercial opportunism, in which care appears to be commoditised. 11 Although the dominance of one logic over another is likely to vary from practice to practice, we can imagine these logics mapping roughly onto the different kinds of institutional structures described above. As such, commercial opportunism may be more heavily associated with the large commercial chains and the attachment to a professional clinical ethic (as the operative decision-making concern) may be more common in smaller practices, especially the single-handed practice embedded in a local community with a long history of providing care.
Second, although institutional structures and their associated logics affect individual behaviour, there is also the role of human agency to consider, in particular our purported ability to employ reflexive self-monitoring, in which we choose a course of action from various alternatives. 77 In other words, we have the capacity to judge what we should do in a particular set of circumstances and then act on such a judgement, if we so wish. 78 Consequently, we are able to accept or reject the influence of a particular institutional logic and behave in contrast to the prevailing wisdom. For example, not all GDPs in a commercially orientated practice will necessarily follow the dictates of commercialism all of the time. Moreover, the acceptance or rejection of a particular institutional logic might not be as deliberate as this. There are many things that we do as individuals out of habit or as an emotional response to immediate circumstances, as opposed to as a result of a considered judgement. This may also affect the ability of a prevailing institutional logic to determine individuals’ behaviour.
Finally, the relationships GDPs have with each other (with both their immediate colleagues and professional peers) mean that there is always the potential for someone to govern and affect the actions of another. 59 The preferences of a PP may influence an AD’s clinical behaviour, but, equally, a PP’s relationship with those around them may generate a sense of responsibility for keeping a practice going, for the sake of the staff they employ, and influence their decision-making in certain situations. Furthermore, the relationship GDPs have with their patients also provides additional context as GDPs operate under the influence of different social networks. 63 GDPs in an inner-city practice with a high throughput of patients might have a very different relationship with those patients compared with a GDP operating in a rural setting, whose patients may even be their neighbours and friends. Consequently, the depth of these particular community ties might make them more or less likely to view their patients as a commodity that could be exploited for financial reasons.
The complex interplay of these different factors were subject to a concurrent rapid realist review, drawing on our realist informed methodology. 79 As highlighted here, the findings suggest that changing the way in which GDPs are paid results in complex and nuanced behaviour. Three modified programme theories were developed, which align to the discussion above:
-
GDPs are affected by a number of competing interests, including a responsibility to keep their practice going for the benefit of patients and staff, serving people and providing good-quality care and running their business profitably. The balance between these interests may shift over time as a result of increased commercial necessity. Entrepreneurialism is not just connected to profit maximisation, but also to the need to maintain cash flow.
-
Where loopholes, opportunities and perverse financial incentives exist, exploitation may occur in order to prevent financial loss or to bring about financial gain. Although this exploitation could be linked to self-interest, it could also be driven by the desire to keep the practice going for the sake of patients and staff.
-
The existence of professional and social networks, professional norms and the presence of a strong professional ethic may constrain the impact of opportunism.
Concluding remarks
Qualitative findings provide a useful counterpoint to the aggregated findings of the DiD analyses. The results suggest that even among the relatively small number of practices taking part in the pilot contract, the views expressed about the pilot and remuneration systems for dentistry were broad and varied. Although GDPs can be acutely sensitive to incentives within dental contracts, the subtle contextual variations and their associated causal mechanisms mean that not all GDPs will necessarily behave in the same way when the terms of their remuneration are altered.
Policy-makers will be interested in the aggregated impact of change on the health and perceptions of the population of service users and on the overall impact on budgets. Should a capitation payment system be introduced, commissioners charged with the responsibility of managing individual contracts will want to identify outlying practices and individual GDPs who are providing suboptimal care. The qualitative results presented in this chapter provide useful insight in this regard.
Chapter 5 Patient questionnaire
Introduction
Given the difficulty of collecting meaningful clinical outcome measures within a short (3-year) study period and given that the pilot period was only 1 year in length, the research team used a questionnaire to capture any changes in the care provided to patients from their own perspective. The objectives for this part of the research programme were to:
-
measure changes in patient-reported oral health knowledge, attitudes and behaviour
-
measure changes in patient-rated oral health outcomes and quality of care.
A questionnaire was iteratively developed with a patient and public involvement (PPI) group in order to understand how patients of dental practices think about and comprehend issues around dental care. Two focus groups were organised by the PCC and the NIHSCB (n = 7 and n = 8), which were designed to capture appropriate questions to include. Initially, the research team asked open-ended questions around three broad areas specified a priori in the research protocol:
-
quality of care provided
-
oral health knowledge, attitudes and behaviour
-
oral health outcomes.
In addition, the research team presented the results of a separate study undertaken by the team, which had collected the important domains of quality. 80 Each domain was discussed separately and suitable questions from a Northern Ireland perspective were developed with the PPI group, capturing the feedback from the group. At the end of the second focus group, a set of questions was produced and agreed on.
Methods
Permissions were granted by the Northern Ireland Research Ethics Committee (reference 15/NI/0167) on 5 August 2015.
Sampling strategy
The sampling strategy, decided prior to recruitment, was to avoid the sampling error (possible error that stems solely from the fact that data are collected from a sample rather than from every single member of the population) by obtaining a large number of responses collected in each study phase. To reduce bias (potentially caused by a systematic difference between responders and the target population), the PPI group and policy stakeholders (senior staff at the BSO and the NIHSCB) were consulted on a sampling frame that could include all potential representatives of a heterogeneous population. The aim of the sampling frame was to balance, before the analysis began, the demographic and socioeconomic characteristics of patients attending intervention and control practices.
Questionnaires were distributed to patients registered with practices in the intervention and control practices at each study phase (pre pilot, during the pilot and post pilot). The first part of the sampling frame was designed to ensure the adequacy of the analysis by reducing the influence of selection bias on questionnaire answers and on differences in the response rate between intervention and control practices and between study phases. The approach taken was to select for each pilot practice five matched control practices from all dental practices with an NHS contract in Northern Ireland. The five control practices were those most similar to each pilot practice based on matching criteria of practice size, proportion of registered patients exempt from fees, proportion of registered patients who were adults and rural/urban status. Matching took place during the baseline study phase for all three study phases, hence there was no change in the intervention and control practices between study periods. The benefit of this matching approach was that it decreased the likelihood that an analysis of the questionnaire was influenced by the effect different practice characteristics (e.g. small practices vs. large practices) may have had on patients’ opinions of the health care they received. Consequently, findings of a difference in the answers given by respondents in each practice group between study phases can be attributed to changes in the remuneration conditions of intervention practices between study phases rather than differences in practice characteristics.
The next step of the sampling frame was to ensure that the patients who responded to the questionnaire were representative of the registered population of Northern Ireland in domains correlated with the oral health of the population. All eligible (registered) patients in each study phase were subdivided by dental practice group (control or intervention practice group) and then further subdivided into three domains used to stratify the sample: exemption from NHS charges (i.e. a proxy for socioeconomic status), gender and age. This meant that there were 12 strata in each practice group: (1) exempt (from NHS charges), male, aged 18–39 years, (2) exempt, male, aged 40–59 years, (3) exempt, male, aged ≥ 60 years, (4) exempt, female, aged 18–39 years, (5) exempt, female, aged 40–59 years, (6) exempt, female, aged ≥ 60 years, (7) non-exempt, male, aged 18–39 years, (8) non-exempt, male, aged 40–59 years, (9) non-exempt, male, aged ≥ 60 years, (10) non-exempt, female, aged 18–39 years, (11) non-exempt, female, aged 40–59 years, and (12) non-exempt, female, aged ≥ 60 years.
Questionnaire packs (letter of invitation, patient information sheet, questionnaire and Freepost return envelopes to the workplace of the research team) were sent out in strata for the practice groups at each phase. These were sent to eligible patients (registered at each practice) selected at random from each stratum of patients in their practice group using contact information recorded by the practice (and available to the BSO).
Questionnaire distribution
There were 9000 questionnaire packs (4500 for patients in intervention practices and 4500 for patients in control practices), which were sent out in waves of 250. The first wave of questionnaires consisted of an equal number of patients from each stratum in each practice group. In subsequent waves, invitations were not distributed equally across all strata. They were targeted differently, with a greater proportion of the questionnaire packs distributed to strata with low response rates. This approach reduced the overall response rate but had the benefit of producing similar numbers of responders in each stratum to ensure that certain strata were not under-represented.
Data analysis
Difference in difference models were estimated using individual patient responses to the questionnaire to evaluate whether or not there was a change over time in the difference between registered patients in control practices and pilot practices in their responses to the questionnaire. The disadvantage of using each question as a dependent variable in a DiD model was that the answers to a proportion of questions were on a categorical scale. An interval-dependent variable (binary or categorical) requires an assumption that the interval space between each ordered response category is an equal distance apart. This was clearly not the case for a number of the questions on the questionnaire; for example, ‘How often do you use dental floss?’ has ordered responses that are not equally spaced periods of time, namely every day, every other day, two/three times a week, less frequently or never. Athey and Imbens81 provide an overview of the problem.
One way of avoiding the problem of non-interval variables is to collapse the response categories into a dichotomous variable. Regression with a proportions measure (e.g. proportion of patient responses that answered ‘everyday’ to a question on the frequency of brushing teeth) avoids the need for assumptions about equal intervals because it involves creating a dichotomous variable from a continuous measure, and hence the coefficient has a meaningful interpretation. The sensitivity of our results to the equal interval assumption was tested by estimating DiD models with dependent variables that were the proportion of respondents in each sample strata who answered in a ‘relevant’ response category. This approach was used only as a sensitivity check because the number of observations available for analysis was reduced from the number of patient responses in the two study periods being compared (approximately 1200 observations when capitation is compared with baseline) to 48 observations: 12 (the number of strata) pilot practice observations and 12 (the number of strata) control practice observations in each study phase. ‘Relevant’ response categories were decided by examining the distribution of responses to a question and, where necessary, combining categories to create a proportions measure that has the least skew in its distribution. These models were estimated without matching methods and control variables because the proportions measure was already created from a similar population of patients (all from the same sampling strata: similar in terms of age, exemption status and gender). For a given survey question, the statistical significance of the DiD estimator did not change from being non-significant to statistically significant (at typical threshold levels of 1%, 5% and 10%) when the dependent variable was changed from an interval to a proportions measure, and a statistically significant result did not become non-significant. This suggests that the statistical significance of the DiD results was not affected by the assumption of equal proportions.
Results
In total, 1215 patients in pilot practices responded (347 in the baseline period, 316 in the intervention period and 552 in the reversion period) and 1187 patients in control practices responded (313 in the baseline period, 348 in the intervention period and 526 in the reversion period), with an overall response rate of 26.7%. The number of responses in strata (in each practice group for each study period) ranged from 6 to 51 responses, with the mean number of responses across all strata being 33.4 and the median number of responses being 28.2. The full results are presented in Appendix 6.
Only three items on the questionnaire showed a statistically significant difference between the intervention practices and the matched controls:
-
‘How long did you have to wait for your health service routine check-up?’ significantly increased (p < 0.01) for patients in pilot practices compared with patients in control practices.
-
‘Yes’ to having had a radiograph taken at their last check-up significantly decreased (p < 0.03) for patients in pilot practices compared with patients in control practices.
-
‘Yes’ to having been treated by a dental hygienist at their last check-up significantly increased (p < 0.04) for patients in pilot practices compared with patients in control practices.
The remaining 27 questions used in the questionnaire showed no difference in the ‘quality’ of care provided, patients’ oral health knowledge, attitudes and behaviour or oral health outcomes.
Discussion
The difference in responses from patients in intervention practices and patients in control practices to the question ‘How long did you have to wait for your health service routine check-up?’ significantly increased in the FFS reversion period compared with the baseline FFS period. This was also the case for the probability that patients would respond ‘yes’ to having been treated by a dental hygienist at their last check-up. These results suggest that patients’ perceived waiting time for a check-up increases when GDPs are remunerated using a capitation system (see Chapter 4). Similarly, the chance of being treated by a dental hygienist increased for patients in the intervention practices more than for patients in control practices, suggesting a greater use of skill mix under capitation.
The difference between patient responders in intervention practices and patient responders in control practices in the probability that they would respond ‘yes’ to having had a radiograph taken at their last check-up significantly increased between FFS at baseline and the capitation period. This result suggests that one of the immediate effects of the pilot contract is that fewer radiographs were taken under capitation than under FFS, something that was also identified in the DiD analyses (see Chapter 3). Patients did not identify reductions in other treatments, even though the DiD analyses identified across-the-board reductions in treatment provision. This could be explained by the readily apparent (to patients) difference in having a radiograph compared with other less identifiable dental treatments.
There were a large number of variables included in the analyses of the questionnaire items, and the more variables that are tested, the more likely a variable is to be statistically significant. Specifically, for the 30 DiD models used, the chance of finding one or more significant differences in the DiD estimator is 1 – (0.95) to the power of 30 or 78.54%, which is the chance that one of the estimators in the models will appear to be statistically significant purely by chance. Šidák and Bonferroni corrections were used to counteract the problem of multiple comparisons. In the Šidák adjustment, the critical value of 0.05 is lowered to 0.00171, whereas for the Bonferroni’s adjustment, it is lowered to 0.00167. No DiD estimators were found to be statistically significant after one adjustment from Šidák’s method and after one adjustment from Bonferroni’s method.
Concluding remarks
Using a questionnaire designed by patients to identify meaningful changes in (1) the quality of care provided, (2) oral health knowledge, attitudes and behaviour, and (3) oral health outcomes, there were only three observed differences between the patients in the intervention group and those in the control group. There is some evidence that a contract based on a capitation model increased waiting times and skill mix, and resulted in fewer radiographs. However, other items in the questionnaire showed no change; this may be a reflection of the limitations of the measuring instrument used and the paucity of validated measures to assess quality of care in dentistry.
Chapter 6 Discussion
Summary of main findings
The aim of the research was to evaluate the impact of a change in the system of provider remuneration on the productivity, quality of care and health outcomes of NHS primary care dental services in Northern Ireland. The study provides useful, contemporary information on the behaviour of GDPs working in the NHS in response to a change in remuneration. The results are timely in that, at the time of writing this report, all four of the home countries are actively considering changes to the NHS dental contract, and the results could inform the design and development of the new contracts. From an international perspective, the study is important as it adds to the limited evidence base on how changes in remuneration influence the behaviour of dental clinicians and service providers. 1 The key findings are summarised below:
-
GDPs in the pilot practices recruited sufficient patients to make sure that they met the anticipated capitation targets in order to secure their regular monthly payments (akin to capitation).
-
The pilot appeared to guarantee a minimum level of access to patients registered with the practice. However, there was no increase in new patients registered with the practices and there were concerns that registration did not guarantee rapid access for an appointment.
-
A significant change in practice behaviour happened immediately and consistently at each transition period of the pilot.
-
Activity was depressed ‘across the board’ and there was no evidence of ‘cherry picking’ treatments that were more profitable to provide.
-
Treatments incurring additional costs (e.g. laboratory fees) were also depressed.
-
The ITS analyses showed that although practices all moved in the same direction in response to the variation, there was large variation between practices in the size of the effect produced by the intervention.
-
PCR reduced significantly, with a mean reduction of ≈£2500 per practice per month. If this was replicated across the province, it would be a reduction in the budget of £11M (PCR for 2016–17 was £23,176,605).
-
There was a rapid return to baseline levels on reversion from capitation to FFS, so much so that there was no significant difference between FFS activity before or after the pilot.
-
There was no evidence that capitation promoted evidence-based preventative treatment (as recorded on the activity forms, e.g. fissure sealants).
-
There were no major changes in the perceived quality of the service from a patient’s perspective other than a reported difficulty in obtaining access for appointments.
The interviews with GDPs revealed a more nuanced picture of the perceived effects of the change in remuneration on GDPs’ behaviour than the quantitative workstream did. The following issues highlight the key findings from the qualitative workstream:
-
Different behaviours were evident, influenced by the variance in organisational structures within the pilot practices.
-
The capitation model was preferred by PPs, as it was seen to provide more time for managing the activities of the practice.
-
Associates were less keen on the capitation model and perceived themselves to be at financial risk if the capitation model was introduced, as contracts would be held at practice level, not by individual GDPs.
-
The reduction in NHS activity provided greater opportunities for sessions to be devoted to private treatment and increase income, primarily for PPs.
-
NHS capitation payments introduced a sense of value and entitlement in PPs. The capitation payment was seen by some principals as a ‘retainer fee’ for keeping their doors open to NHS patients.
What have we added to the evidence base?
The decision by policy-makers in Northern Ireland to pilot an NHS dental contract based on capitation, rather than the predominantly FFS payment system, offered an opportunity to undertake a mixed-methods study to investigate the impact of a change in the remuneration system in Northern Ireland on productivity and the quality of care provided.
This study makes a valuable contemporary contribution to the literature and provides a more comprehensive evaluation of the pragmatic effects of changes to remuneration on GDPs’ behaviour by employing a mixed-methods approach and by including a significant health economics component in the study design.
The results from this study reinforce the evidence from the literature that GDPs respond markedly and quickly to changes in how they are remunerated in terms of both the volume of care provided and how they provide care. 1,5,6 The study shows that a change from FFS to capitation produces a significant and almost immediate drop in activity, without a noticeable drop in patient-perceived quality. However, our understanding and measurement of quality in dentistry is underdeveloped and it is not surprising that a change in quality was not evident given that the 2006 change in contract in England resulted in large reductions in specific treatments (e.g. root fillings, crowns and bridgework) and an increase in the number of extractions without any perceptible increase in service users’ concerns. 40,41 The immediate changes seen over a 1-year period in this study are unlikely to remain static for the long term. The qualitative data showed that in many practices, particularly those in rural communities, the GDPs had a strong sense of providing an essential service that was embedded in the local community and, although a short-term reduction in activity for a pilot running for a defined period could produce short-term financial benefits, it is likely that behaviour would change and reach an equilibrium in the long term in response to pressures resulting from patients’ ongoing needs and expectations.
This study makes a unique contribution to the literature to demonstrate that, even after experiencing a period of remuneration via capitation, once remuneration changes back to FFS the behaviour of GDPs rapidly reverts to the same level of activity as prior to the introduction of capitation. This finding suggests that practices retain an ‘organisational memory’ and a practice can very quickly return to a system with which it had been working in the recent past. However, longer periods of working under a capitation system, particularly when there could be personnel changes in the practice, could mean that the rapid return to previous practices and activity profiles under FFS may not be as straightforward, and working under capitation for longer periods may have longer-lasting effects.
Strengths and limitations of the research
This was a large study providing contemporary evidence about how GDPs working in the NHS react to a change in remuneration. Their behaviour in terms of the registration of NHS patients to their services and the treatments provided was compared with a large, well-matched group of control practices. The study was pragmatic in that a ‘real-world’ service was being provided to patients as the evaluation progressed, so the outcomes observed could be expected if the intervention was rolled out across the NHS. Although the intervention practices knew that they were being observed, their behaviour changed consistently and markedly, which could be criticised for maximising short-term gain, suggesting that response bias did not have a major influence on the outcomes. The registration and activity outcome measures relied on data collected by NHS bodies, so the source data had high validity and reliability; the NHS has comprehensive, well-tested data audit systems to check these data sets because these data are used to claim and pay GDPs’ remuneration. The project was completed in partnership between independent academic researchers, the DHSSPS and the NIHSCB, and represents a good example of co-production of new knowledge. Throughout the project there was close collaboration between academics, NHS commissioners and Department of Health and Social Care staff. No restrictions were placed on the NHS staff or the academic team on reporting the findings of the study.
The design of the programme using mixed methods was a strength; qualitative methods provided an understanding of why some of the changes in activity had occurred. Comparisons were made between the intervention practices and ‘matched’ practices (with PSs) and ‘unmatched’ practices (see Appendix 3). Little difference was found across the groups, suggesting that the findings were not influenced by the choice of the matching process. Equally, the use of the ITS on selected activity variables was important in order to triangulate the results of the DiD. The analysis revealed identical directions of effect, although the 95% CIs were broadened in all cases (although the results from the power analysis of the ITS highlights some of the limitations of this approach for the budget-limited sample size used in this project), justifying the use of the DiD for this project. The qualitative methods also identified that there was a large range of views and approaches to providing care between and within practices, something that would not have been apparent if a solely quantitative design had been employed.
There were a number of limitations of the study. The main limitation was that the intervention (capitation) lasted for a short time (1 year) and the practices knew that they would revert back to a FFS contract at the end of this time period. Therefore, any changes in behaviour were influenced by the impermanent nature of the intervention and an opportunity to maximise short-term gain. It is likely that a permanent change to capitation would lead to immediate changes similar to those found in the pilot, but that behaviour in terms of access and activity would find an equilibrium somewhere between the FFS and capitation levels recorded in the pilot. However, this supposition would need to be tested through long-term evaluation. Ideally, the project would have randomised practices into intervention groups and control groups. This was not possible, as the DHSSPS had agreed a process of recruitment to the pilots with the profession, and randomisation was seen as unattractive to practices and a barrier to recruitment. The DHSSPS was also aware that if randomisation was employed they would have to seek expressions of interest to participate in the pilot from all practices in Northern Ireland, and that practices, as small businesses, would carefully weigh up the potential advantages and disadvantages of participating.
A potential criticism would be that the intervention was too simplistic, using a solely capitation arrangement rather than, for example, a more complex blended mix of capitation and incentives to encourage activity used in the English pilots/prototypes. Capitation arrangements have the advantage of cost containment, whereas a blended intervention (e.g. based primarily on capitation but with incentives to stimulate activity and quality) would have produced additional problems and limitations. It would have been difficult to identify the optimal blended approach to test empirically and a greater problem would be identifying what specific elements of a blended approach would be responsible for inducing a change in behaviour. Given the limited number of practices available, a comparison of capitation and FFS using quantitative and qualitative methodologies was considered to provide the most useful approach to provide useful information to inform policy.
The voluntary nature of the selection process calls into question the representativeness of the intervention group of practices. The DHSSPS was aware of this issue and as a consequence wanted to include practices with a broad range of characteristics that would influence how care is provided. Practices were selected from those who had submitted an expression of interest based on a pragmatic mixture of being representative (assessed on practice characteristics) but also affordable. Affordability dictated the number of practices that were involved in the study, given the PCR reduction and the additional resources required from the NIHSCB to oversee the pilot. Ideally, a larger number of practices would have been involved, and the sample size determined by a formal sample size calculation. But the post hoc ITS power calculation suggested that many more practices would be needed if this analytical approach was to be employed. The DiD approach used was more pragmatic and better suited to the number of practices recruited, with policy-makers mindful of the potential drop in PCR during the capitation phase of the study. This concern was fuelled by a 24% drop in PCR experienced by the two practices involved in wave 1 of the pilot. Given the restrictions on the number of practices and the DHSSPS’s wish to have broad practice representation, the design of the study was the best compromise possible. The process of matching controls was based on key variables identified by the research team, DHSSPS staff and NIHSCB staff as having important influence on the provision of care. However, because randomisation was not possible, unknown confounders may have had an influence on the analyses.
The qualitative element could be criticised for the ‘thematic analysis’ providing largely descriptive subheadings rather than a conceptual post hoc realist interpretation of ‘thematic analysis’. We think there is a balance to be struck with the kind of study we report here. In order to make it policy and practice relevant to the DHSSPS, the NIHSCB and dentists, the themes we identify are practice focused rather than simply descriptive. Given the real-world nature of this evaluation, there is a need to provide policy-makers and practitioners with outputs that they can actually work with in terms of understanding GDPs’ experience of the pilot; this calls for something practical, not conceptual.
Ideally, the study would have used health outcome measures, such as dental caries or periodontal disease, as the primary outcome measure. However, using these measures would require a much longer follow-up time and would have required the services of external clinical examiners. This would have made the project unaffordable. It could be argued that the activity measures used in the project are more meaningful to patients; for example, the NIHR Health Technology Assessment REFleCt trial’s82 primary outcome measure is whether or not a patient receives dental treatment because of caries. This was determined by PPI, and patients felt that avoidance of invasive dental treatment (fillings, extractions) was the most important issue for them. Similarly, access has been identified by patients as a key requirement of services. 81 These two issues of activity volume and access are important to policy-makers in providing an affordable NHS service. Measurement of quality, although important for policy-makers, is difficult because our understanding of quality and its measurement in dentistry is underdeveloped compared with primary medical care. A lack of discriminative power of the quality measures used in the questionnaire developed for this study may account for the failure to identify differences between test and control groups. In addition, the response rate to the quality questionnaire distributed to patients was very low (26.7%) and, although a quota-sampling approach was used, the results reported could have been subject to non-response bias if the views of responders were systematically different from those of non-responders. It is also possible that the effects of non-response could be different in patients attending intervention and control practices, biasing the comparison of their views of the services they use.
Chapter 7 Conclusions
Possible considerations for future policy
There was a rapid and clinically significant fall in the quantity of care delivered following the transition from FFS to capitation. This was evident when examining individual treatment items or composite indicators such as the number of courses of treatment and the mean number of items per course of treatment provided. An equally rapid reversion of activity to baseline levels was seen in the return from capitation to FFS. The behaviour of ADs was more sensitive to the change in NHS remuneration than that of PPs. There was evidence of between-practice variation in response to the intervention, although the direction of the response to change was consistent across all practices.
There was evidence of practices ‘front-loading’ their registered population during the baseline period to ensure that they hit capitation requirements. Overall, capitation resulted in a small rise in registrations. The small increase in registrations was primarily achieved through reregistering patients of the practices whose registrations had lapsed. Importantly, the number of new patients registered was negligible.
There was an ‘across-the-board’ depression in activity and no appreciable change in service mix. There was no evidence of an increase in the provision of preventative care, such as examinations, fissure sealants, periodontal treatment and radiographs, at the expense of invasive treatments, such as fillings, crowns or extractions, in the transition from FFS to capitation.
The fall in activity freed up GDPs’ time, which was evident in the responses given in the qualitative interviews. There was evidence of variation in how this time was spent; some GDPs reported that it enabled them to spend more time giving preventative advice or on the management and administration of the practices. Others reported that the freed-up time was used to provide more privately funded care.
Using FFS costings, the fall in the volume of care under capitation resulted in an overall fall in NHS dental practice income per month of £5920. Following the change back from FFS to capitation, mean NHS dental practice income per month quickly returned to baseline levels.
There was no change in the proportion of practice income derived from patient fee contributions following the change from FFS to capitation. However, under capitation there was a policy-significant reduction in PCR, with a mean fall of £2403 per practice per month. If this fall was replicated across Northern Ireland, it would result in a reduction in the NHS dental budget of £11M or approximately 8%. PCR rapidly returned to baseline levels following the reversion from capitation to FFS.
Among GDPs, there was evidence of a large variation in how the change from FFS to capitation affected behaviour and the care provided to patients. There was a tension evident between professional ethics and the pressures of running a small business. Multiple factors, such as social and institutional structures in which the GDPs operate, their interpersonal relationships with colleagues and patients, and their local community, as well as their own personal and professional goals, all influenced how they reacted to a change in remuneration to a greater or lesser degree. These findings suggest that GDPs will not necessarily behave in the same way in response to a change in their remuneration.
A difference was evident between PPs and ADs. Capitation was preferred by PPs as it was seen to provide more time for managing the activities of the practice and provided greater opportunities to provide private treatment and, therefore, increase income. Some PPs saw the capitation payments as a ‘retainer fee’ for keeping their doors open to NHS patients. ADs were less keen on the capitation model and perceived themselves to be at financial risk if a capitation model was introduced, as NHS contracts would be held at practice level by PPs, not by individual GDPs.
The interviews with patients revealed high levels of satisfaction with the care they received and a high degree of trust in their GDP among the majority of patients. Universally, patients reported that they had not noticed any difference in the care they received under FFS and capitation.
There were no statistically significant differences recorded in patients’ oral health knowledge, attitudes and behaviour associated with the change from FFS to capitation or reversion from capitation to FFS.
Patients reported no significant differences in oral health outcomes associated with the change from FFS to capitation or the reversion from capitation to FFS (although the response rate was relatively low). However, statistically significant changes were evident in indicators related to the quality of care provided. Patients reported that under capitation they had to wait longer for an NHS check-up, had fewer radiographs taken and were more likely to be seen by a dental hygienist.
The change in behaviour of GDPs, although seeking to generally maximise an advantage in terms of income or freeing up time, is not predictable in its nature, nor are the behavioural changes we detected uniformly adopted to the same degree by individual clinicians or dental practices. The impact of a change in remuneration will vary depending on an individual clinician’s attitudes and circumstances and local context, particularly the culture and leadership within different dental practices.
There is no doubt that a change from FFS to capitation will produce an overall fall in activity and, in a co-payment system, such as in the NHS, a concomitant fall in income. Although a change in remuneration from FFS to capitation will produce a predicable abrupt reduction in activity, the variation between individual GDPs and practices make it difficult to predict the size of this change. This makes planning for policy-makers difficult, and suggests that a large, abrupt, and difficult-to-reverse change in policy would be clinically and financially risky. This is in addition to the greater risk of a breakdown in trust between the dental profession and the NHS/Department of Health and Social Care by the introduction of a contract that could be seen as unfair and not conducive to enabling GDPs to provide (as they see it) optimal care for their patients, something that resulted from the introduction of the English 2006 NHS contract. 7
The variation between practices also suggests that a ‘one size fits all’ approach to contract reform using remuneration as a sole means of changing practitioners’ behaviour is too blunt an instrument to meet all (often conflicting) policy goals. Furthermore, designing a contract to primarily restrict the excesses of a minority of clinicians or practices whose prime goal is to maximise income is likely to disadvantage the majority of practices (and their patients), who are striving to provide the best possible care to their patients to high standards of professional integrity. This suggests that multiple levers, not just remuneration, should be considered in bringing about a desired change in behaviour in a clinical workforce with a high degree of autonomy.
This study has important implications for NHS policy-makers and dental care providers in the design of dental services and incentive structures in all four home countries. It demonstrated the complexity of producing a new NHS dental contract that is seen as fair and fit for purpose by the majority of GDPs, but also will have the built-in incentives to influence behaviour in such a way that the goals of policy-makers are achieved. This latter point is probably the most important factor to consider when designing structures to incentivise behaviour. The policy review in the literature review identified a number of common goals in each of the home countries for a new dental contract; these can be summarised as incentivising prevention, incentivising quality, maintaining and improving access, maintaining current income levels from co-payments and ensuring that the profession is content and will work with any new arrangements. The implications of the findings of each of these goals is considered in turn.
Incentivising prevention
A change from FFS to capitation does generally incentivise prevention. In this study, items of preventative care, such as fissure sealants, periodontal treatment and the provision of radiographs, reduced along with all other treatments during the capitation period. However, this may have been as a result of the limited duration of the intervention. A more fundamental question is ‘should promoting prevention in primary dental care remain the sole priority?’. This was one of the reasons cited for the change from FFS to capitation in children in the 1990 NHS contract. However, the recent evidence shows that caries prevention provided solely in dental practice can have limited effectiveness, when compared with community-based programmes. 52,53
Incentivising quality
Incentives to improve quality were not included in the intervention. Any investigation into the impact of changes to the organisation and delivery of dental health services on quality will be severely hampered by a lack of understanding of what quality means in primary dental care and how to measure it. The questionnaire used in the study was designed by our PPI group, but may have been limited by this lack of understanding and the indicators used may have lacked responsiveness. This seems to be the case with current patient satisfaction indicators. The results from the GDP patient survey showed that in January to March 2017, 50% of respondents had a very good experience and 35% had a fairly good experience at their GDP’s practice and only 4% had fairly poor and 3% had very poor experiences. At face value, it would seem that the majority of dental services are generally providing a very high standard of care. Alternatively, this could be a result of a measure with poor responsiveness or patients having little knowledge or understanding to enable them to judge what constitutes high-quality care. If the dental profession and NHS policy-makers are not certain of what constitutes good-quality dental care and how to measure it, it is unlikely that the majority of patients are equipped to provide an informed opinion.
Concerns have been voiced that a simple capitation arrangement could lead to ‘supervised neglect’, whereby GDPs receive their capitation fees but neglect to provide essential treatment for patients. The results of this study and studies in the literature1 show that activity is depressed under capitation but the qualitative element of this study shows that many GDPs see themselves as part of the local community with a responsibility to look after the dental needs of that community, which brings with it a sense of community accountability that would check unprofessional behaviour. Concerns about ‘supervised neglect’ would also be tempered by previous studies that suggest that treatment is delayed rather than withheld. 7 However, the Referral Dental Service in Northern Ireland did inspect completed courses of treatment on patients and reviewed records in each practice as part of the ongoing monitoring. No supervised neglect was observed. From a public health point of view, especially with an ageing population for whom self-care of heavily restored dentitions is a future concern, incentivising less activity is probably better than artificially stimulating activity through arbitrary activity targets (in blended contracts) aimed in part to ensure that PCR levels are maintained.
Maintaining and improving access
Registrations increased prior to the introduction of the capitation period to ensure that practices met capitation targets. The impact of the change in remuneration on registration was negligible. We estimated the impact of the introduction of capitation on registration across the whole of Northern Ireland, by applying our results to all NHS practices in Northern Ireland. Using the DiD result for practice list size, the scaled-up effect of the introduction of capitation across Northern Ireland would result in an increase in the number of registered patients in the population by 365. In April 2017, there were 1,198,286 dental-registered patients across Northern Ireland.
One of the attractive elements of a capitation-based system for commissioners is that it secures a minimum amount of access, as measured by patient registration. However, the research findings question how we should measure access. Registration is one way to measure access to NHS care, but for patients this does not seem to be a meaningful measure, as patients reported that they could not get timely access to their GDP when they needed it. One possible downside of a capitation-only contract would be potentially blocking access to care for unregistered patients who may have high needs, and would, therefore, represent a financial risk to practices operating under a capitation system if they were to register them. In this study, expansion of the list size of practices to ensure that capitation targets were met was done largely by encouraging patients with lapsed registrations to reregister. This tactic reduced the risk for practices, as previously registered patients would be known to the practice and would be likely to have low treatment needs, as opposed to new patients who may require a large amount of treatment, the costs of which would have to be met solely from the capitation fees that they generate. If a new contract is to be primarily based on capitation, careful thought needs to be given to how those with greatest need can access care. Providing care for a geographically defined population rather than a historically registered population and making time (and resource) provision through dedicated sessions for unregistered patients may need to be considered as options.
Maintaining income from co-payments
One of the key concerns about moving from a FFS to a capitation-based system is a significant fall in PCR, leaving a gap in the NHS dental budget. This study reported a fall in PCR that was immediate and substantial. If capitation was introduced in Northern Ireland, the impact on the annual NHS dental budget can be estimated by extrapolating our findings to all NHS dental practices. The DiD result for the patient fee contributions showed a reduction by £2403 per practice per month when capitation was compared with baseline FFS. If this figure were multiplied by the number of months in a year and the number of NHS dental practices operating in Northern Ireland (387 in April 2017), capitation would reduce the gross annual co-payment income received by the NHS by £11,159,532, which equates to approximately 8% of the dental budget.
The fall in PCR is worrying if policy-makers want to realise the benefits of capitation (a capped and predicable budget, attractive to the dental profession, removal of incentives for early intervention and possible overtreatment). Although there may be a degree of ‘bounce-back’, PCR rising after an initial abrupt fall because of GDPs delaying treatment, there will be a net fall in co-payment revenue when compared with a FFS system. A key question for policy-makers is how to mitigate this fall in the overall budget. A number of options are available. They could simply take the hit, making good the drop in income by an expansion in the NHS dental budget; this option is unlikely to be attractive in the current and foreseeable economic environment. One simple way of correcting this shortfall would be to increase patient charges. In effect, this happened overnight in England with the introduction of the 2006 contract: a check-up under FFS was £9, whereas the charge for a band 1 course of treatment was £19, an 111% increase, with little public outcry. Our data estimate that, based on the average patient contribution revenue received by control practices per month scaled up to all practices in Northern Ireland, a 27.62% increase in patient charges would be needed to prevent a fall in PCR affecting the overall budget. This assumes that an increase in price would not produce a downturn in demand for NHS treatments; this scenario did not happen in England.
Another option would be to implement a blended contract, in which the bulk of remuneration is based on capitation with a proportion retained as FFS to stimulate activity, as in the English prototypes. The problem with a blended contract is how to calculate the size of the FFS component; this calculation runs the risk of being too focused on artificially generating activity and, hence, income at the expense of encouraging a needs-led approach to care. A more radical option would be to remove co-payments entirely and provide a capitation-based system with an attenuated ‘core’ service funded entirely within the current state-funded component of the dental budget. This could be politically difficult, but if access to a defined list of treatments essential to secure dental health were freely available, and more complex and cosmetic treatments were offered privately, such a system might be attractive. The PCC document Talking Teeth: Patient Views of General Dental Services in Northern Ireland49 suggests that this option merits further investigation.
Ensuring that the profession is content with a capitation-based system
The study demonstrated that capitation is popular with PPs and, therefore, senior members of the profession, but was seen as a threat to non-equity-holding associates. Capitation provided greater opportunities for GDPs to upsell private treatment, which may not be detrimental to NHS care if the focus of privately funded care is largely on cosmetic treatments (e.g. crowns or treatments that patients demand but that have a poor evidence base, such as scale and polish). However, in any new system, patients and the profession need clarity about what treatments are available via the NHS and what treatments are not. One of the possible downsides of a poorly monitored and managed capitation-based system is that NHS care could be marginalised by the upselling of care provided on a private basis that should be available on the NHS. Capitation was less attractive for associates as under a capitation system their contracts would be with practices, not with the NIHSCB. This could reduce their bargaining power, putting downward pressure on wages and possibly reducing job security.
Impact of service redesign on workforce planning
Changing a dental contract will influence the volume and service mix of dental treatments provided and consideration needs to be given to how to provide the resulting service mix in the most efficient way. A capitation-based system incentivises service broadening, providing fewer and a simpler range of treatments to more people. This would suggest a larger role for skill mix and role substitution. Service planning and workforce planning should be conducted simultaneously, and simply replacing like with like in terms of current skill mix is unlikely to meet the evolving needs of the population using the service in the future. A more sophisticated, needs-based approach to workforce planning is one way of addressing this issue.
In conclusion, it is unlikely that a single (complex) NHS dental contract can meet all of the required policy objectives: secure a minimum level of access, provide a predictable capped budget, encourage prevention, ensure that good-quality care is provided, secure sufficient income from PCR and ensure that patients and GDPs are happy with a new system. Policy-makers need a range of tools to both monitor and influence behaviour prospectively in any new system; any contractual change also needs to have built-in flexibility to change as population needs change.
Flexibility to include performance management within a capitation contract may provide GDPs with the autonomy they desire while also enabling policy-makers to ensure that population needs are addressed. The need for performance management was demonstrated in this study by the large between-practice variation in outcome measures. Performance management does not need to be labour intensive; it could be informatics driven by benchmarking practices against agreed metrics using existing activity monitoring and payment systems in Northern Ireland. Simplifying the new NHS dental contract, rather than relying on a complex blended contract, could avoid the need for new complex IT systems that England has been struggling with. The dental reference officer function could also be used to support a peer-reviewed system of quality improvement.
Implications for practice
The research has implications for the amount and type of clinical care provided and for the way dental practices are managed if there is a change from a FFS to a capitation model of remuneration.
The change from FFS to capitation resulted in a fall in treatment activity across the board; therefore, dentists are likely to be more reluctant to intervene at an early stage in the disease process and focus their efforts on trying to prevent disease or arrest the disease process in its early stages. Fewer complex treatments such as crowns and bridgework would be provided because dentists would carry the costs of laboratory fees. In addition, clinicians are likely to be more selective about identifying which patients are most likely to benefit from time-consuming interventions, such as multiroot endodontics. This might be seen as a negative consequence of a change in remuneration, but a less invasive approach to dentistry might lead to fewer short- and long-term adverse outcomes for patients, and is probably more in tune with the declining dental treatment needs of the UK population. 82 The historical concern about capitation payment models is that patients will not receive the most appropriate treatment they need at the most appropriate time. The qualitative findings of this study suggest that the majority of dentists will respond appropriately and meet the needs of their patients in a timely fashion. There will be variation in behaviour between dentists and between practices, which is to be expected as a result of context and variation in clinical judgement between practices and dentists. However, internal (personal ethics) and external (peer and community influence) pressures reduce the risk of ‘supervised neglect’.
In terms of managing dental practices, a switch to capitation should result in freeing up time. This additional time could be usefully used in a number of ways, such as addressing the increasing administration and regulatory burden on practices, providing dedicated time to support training in quality improvement and service development, such as the expansion of emergency care reported by two practices in the qualitative element of the project. Providing fewer and simpler treatments logically points to a greater deployment of skill mix, whereas more complex treatments warrant the development of skills among existing GDPs and the development of specialist centres where these treatments can be provided on referral.
The development of the service will depend on the broader terms and conditions of a reformed contract, not just the system of remuneration. The contractual expectations of the commissioners and the budget provided to practices plus the way performance is monitored and policed will all influence the way clinical care is provided and how dental practices evolve in the future.
Recommendations for further research
In undertaking this research, a number of very significant gaps in knowledge have been unearthed, both in the review of policy development (see Chapter 2) and in the empirical research we have conducted. To follow are suggestions for undertaking further research to inform and support the NHS in its quest to provide evidenced-based, affordable dental care that meets the needs of the population. These suggestions for research are academically driven and not an attempt to recommend changes to policy. The following research questions have been prioritised by the research team.
What should be the aims and limitations of NHS dental services?
This would require a consensus view on what NHS services should aim to provide and what they will not provide in the context of changing population needs, developing health technologies and pressure on public sector budgets. Issues such as reducing the levels of disease in children, the needs of a growing, predominantly dentate elderly population and concentration of the dental disease burden in disadvantaged communities remain public health concerns.
Is less more?
Does a more restrained approach to provision of dental restorative treatment, encouraged by capitation, provide greater long-term population health gains and savings for the NHS than an incentivised approach to intervention? In the context of a growing elderly, dentate population this question is growing in importance.
Does paying general dental practitioners for prevention provide an attractive return on investment for the NHS?
The idea of paying GDPs to provide prevention has been accepted since the 1990 NHS contract and is supported by policy in each of the home countries. However, with emerging evidence, perhaps this concept now needs to be challenged. Should GDPs make the main contribution to prevention, on a patient-by-patient basis, within the NHS? This has been a long-standing assumption, but HTA trials (e.g. Northern Ireland Caries Prevention In Practice and the Improving the Quality of Dentistry trials) are starting to challenge this notion. 57,58 Greater use of skill mix in practice and community-orientated approaches to care could help augment existing population-based interventions and provide more continuity, rather than focusing entirely on practice-based interventions delivered by dentists.
What should the patient pay for?
Given the increasing pressure on NHS budgets, is there a case for restricting dental care to a list of essential treatments necessary to secure health? This question raises a number of additional questions. How could or should such a list be decided? What impact would the introduction of a ‘core service’ have on the population’s oral health? What effect would the introduction of a core service have on health inequalities?
What additional interventions can supplement changes in remuneration systems to achieve policy goals?
Research is required to develop and test supplementary interventions that can work in a complementary way alongside a remuneration system to achieve desired goals, for example the quality improvement interventions discussed above, but also efficient performance monitoring and management methods, perhaps using informatics approaches to automatically identify outliers deviating from predefined expected patterns of service delivery.
Research into workforce planning
Changing the payment system changes service provision, which changes the workforce needs of the service. Currently the NHS has an unstructured approach to dental workforce planning that is not aligned to the needs of the system or, more importantly, the needs of the population. Research is required into the development of different workforce planning models and their impact on productivity and overall costs, accounting for role substitution, shifting work patterns and expectations.
How do you measure and improve quality in dentistry?
Wanting to incentivise quality is a common thread for policy-makers, but research into quality in dentistry is 20 years behind primary medical care. Dentistry has some fundamental differences to services provided by general practitioners: patients are encouraged to attend regularly and asymptomatically, it is still a largely surgical discipline and there is a large cosmetic element to the care provided. In England, the quality indicators included in the DQOF have been chosen arbitrarily and have not been validated. Research is needed to understand quality (including patient safety) in the dental context, how to measure it with validity and reliability, and how to improve it. Learning health systems are being developed to support continuous service improvement; it would seem timely to investigate an informatics-supported approach to dental service improvement in the NHS.
Concluding remarks
Some of the issues described above are addressed by currently commissioned NIHR projects. 83 There is a need for national oversight of evidence production in dentistry and a means of pooling and considering information as it emerges. This would require a co-production partnership between academics and service commissioners. This project provides a good example of a co-production approach to new knowledge.
Acknowledgements
The research team would like to thank all of the key stakeholders in the DHSSPS, including Simon Reid (Chief Dental Officer), Bryan Dooley and all the project leads in the NIHSCB (Jeni Rosborough, Christine McIlvenny, Chris Young and Diane McKillen). We would like to thank Donald Burden for chairing the Steering Group, Seamus Killough for representing the British Dental Association and Paul Schofield from the PCC (at the early stage of the project). Paul McAlister was a key member from the BSO, who collated and provided the clinical activity data for the DiD workstream that led to Chapter 3.
Contributions of authors
Paul Brocklehurst (https://orcid.org/0000-0003-1878-9030) (Professor of Health Services Research) obtained the funds and led the project, provided dental public health input and co-authored the report.
Martin Tickle (https://orcid.org/0000-0001-5348-5441) (Professor of Dental Public Health) coauthored the report.
Stephen Birch (https://orcid.org/0000-0002-1372-0440) (Professor of Health Economics) provided oversight for the DiD workstream.
Ruth McDonald (https://orcid.org/0000-0002-3488-4209) (Professor of Health Science Research and Policy) oversaw the qualitative study with GDPs and patients.
Tanya Walsh (https://orcid.org/0000-0001-7003-8854) (Professor of Healthcare Evaluation) oversaw the ITS triangulation.
Tom Lloyd Goodwin (https://orcid.org/0000-0002-4013-0809) (Research Fellow) recorded and analysed the qualitative study with GDPs and patients and drafted Chapter 4.
Harry Hill undertook the detailed analysis of the activity and questionnaire data and also drafted Chapters 3 and 5.
Elizabeth Howarth (https://orcid.org/0000-0003-3462-3946) (Research Associate) analysed the ITS triangulation.
Michael Donaldson (https://orcid.org/0000-0003-4838-9804) provided expertise in his role as senior stakeholder from the NIHSCB and contributed to Chapters 6 and 7.
Donncha O’Carolan (Dental Public Health) provided expertise in his role as senior stakeholder from the NIHSCB and contributed to Chapters 6 and 7.
Sandy Fitzpatrick was a key member from the BSO, who collated and provided the clinical activity data for the DiD workstream that led to Chapter 3.
Gillian McCrory was a key member from the BSO, who collated and provided the clinical activity data for the DiD workstream that led to Chapter 3.
Carolyn Slee acted as our PPI representative throughout the project, contributing to the Steering Group, the plain English summary and the dissemination strategy.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Brocklehurst P, Price J, Glenny AM, Tickle M, Birch S, Mertz E, et al. The effect of different methods of remuneration on the behaviour of primary care dentists. Cochrane Database Syst Rev 2013;11. https://doi.org/10.1002/14651858.CD009853.pub2.
- Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev 2011;7. https://doi.org/10.1002/14651858.CD009255.
- Scott A, Sivey P, Ait Ouakrim D, Willenberg L, Naccarella L, Furler J, et al. The effect of financial incentives on the quality of health care provided by primary care physicians. Cochrane Database Syst Rev 2011;9. https://doi.org/10.1002/14651858.CD008451.pub2.
- Brocklehurst P, Birch S, McDonald R, Hill H, O’Malley L, Macey R, et al. Determining the optimal model for role substitution in NHS dental services in the UK: a mixed-methods study. Health Serv Deliv Res 2016;4. https://doi.org/10.3310/hsdr04220.
- McDonald R, Cheraghi-Sohi S, Sanders C, Tickle M. Changes to financial incentives in English dentistry 2006-2009: a qualitative study. Community Dent Oral Epidemiol 2012;40:468-73. https://doi.org/10.1111/j.1600-0528.2012.00687.x.
- Tickle M, McDonald R, Franklin J, Aggarwal VR, Milsom K, Reeves D. Paying for the wrong kind of performance? Financial incentives and behaviour changes in National Health Service dentistry 1992-2009. Community Dent Oral Epidemiol 2011;39:465-73. https://doi.org/10.1111/j.1600-0528.2011.00622.x.
- Coventry P, Holloway PJ, Lennon MA, Mellor AC, Worthington HV. A trial of a capitation system of payment for the treatment of children in the General Dental Service. Final report. Dental Health Services Research Unit, University of Manchester. September, 1989. Community Dent Health 1989;6:1-63.
- Pawson R, Tilley N. Realistic Evaluation. London: SAGE Publications Ltd; 1997.
- Byng R, Norman I, Redfern S. Using realistic evaluation to evaluate a practice-level intervention to improve primary healthcare for patients with long-term mental illness. Evaluation 2005;11:69-93. https://doi.org/10.1177/1356389005053198.
- Grytten J. Models for financing dental services. A review. Community Dent Health 2005;22:75-8.
- Harris R, Mosedale S, Garner J, Perkins E. What factors influence the use of contracts in the context of NHS dental practice? A systematic review of theory and logic model. Soc Sci Med 2014;108:54-9. https://doi.org/10.1016/j.socscimed.2014.01.032.
- Whittaker W, Birch S. Provider incentives and access to dental care: evaluating NHS reforms in England. Soc Sci Med 2012;75:2515-21. https://doi.org/10.1016/j.socscimed.2012.09.035.
- Gosden T, Forland F, Kristiansen IS, Sutton M, Leese B, Giuffrida A, et al. Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database Syst Rev 2000;3. https://doi.org/10.1002/14651858.CD002215.
- Ellis RP, McGuire TG. Supply-side and demand-side cost sharing in health care. J Econ Perspect 1993;7:135-51. https://doi.org/10.1257/jep.7.4.135.
- Birch S. The identification of supplier-inducement in a fixed price system of health care provision. The case of dentistry in the United Kingdom. J Health Econ 1988;7:129-50. https://doi.org/10.1016/0167-6296(88)90012-4.
- Tickle M, Brown P, Blinkhorn A, Jenner T. Comparing the ability of different area measures of socioeconomic status to segment a population according to caries prevalence. Community Dent Health 2000;17:138-44.
- Leake JL, Birch S. Public policy and the market for dental services. Community Dent Oral Epidemiol 2008;36:287-95. https://doi.org/10.1111/j.1600-0528.2008.00438.x.
- Hart JT. The inverse care law. Lancet 1971;1:405-12. https://doi.org/10.1016/S0140-6736(71)92410-X.
- Tchicaya A, Lorentz N. Socioeconomic inequalities in the non-use of dental care in Europe. Int J Equity Health 2014;13. https://doi.org/10.1186/1475-9276-13-7.
- Manning WG, Bailit HL, Benjamin B, Newhouse JP. The demand for dental care. Evidence from a randomized trial in health insurance. J Am Dent Assoc 1985;110:895-902.
- Parkin D, Yule B. Patients charges and the demand for dental care in Scotland, 1962–81. Appl Econ 1988;20:229-42. https://doi.org/10.1080/00036848800000007.
- Great Britain . The National Health Service Act, 1946 n.d. www.legislation.gov.uk/ukpga/1946/81/pdfs/ukpga_19460081_en.pdf (accessed 4 October 2019).
- Great Britain . The National Health Service General Dental Services Regulations 2005. www.legislation.gov.uk/uksi/2005/3361/pdfs/uksi_20053361_en.pdf (accessed 4 October 2019).
- Great Britain . National Health Service (Charges for Dental Treatment) Regulations 1952 1952.
- The Health Departments of England, Scotland, Wales and Northern Ireland . The New Contract – An Operating Manual for Dentists 1990.
- Bloomfield K. Fundamental Review of Dental Remuneration. London: Department of Health; 1992.
- Great Britain . National Health Service (Primary Care) Act 1997 1997.
- Goodwin N, Morris AJ, Hill KB, McLeod HS, Burke FJ, Hall AC. National evaluation of personal dental services (PDS) pilots: main findings and policy implications. Br Dent J 2003;195:640-3. https://doi.org/10.1038/sj.bdj.4810781.
- Pitts NB. NHS dentistry: Options for Change in context – a personal overview of a landmark document and what it could mean for the future of dental services. Br Dent J 2003;195:631-5. https://doi.org/10.1038/sj.bdj.4810779.
- Department of Health and Social Care . Modernising NHS Dentistry – Implementing the NHS Plan 2000.
- Great Britain . The National Health Service (General Dental Services Contracts) Regulations 2005 2005. www.legislation.gov.uk/uksi/2005/3361/contents/made (accessed 1 September 2018).
- House of Commons Health Select Committee . Dental Services – Fifth Report of Session 2007–08 2008.
- Steele J. NHS Dental Services in England. London: Department of Health and Social Care; 2009.
- Department of Health and Social Care . NHS Dental Contract Pilots – Learning After First Two Years of Piloting 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/282760/Dental_contract_pilots_evidence_and_learning_report.pdf (accessed 1 September 2018).
- Department of Health and Social Care . Dental Quality Outcomes Framework: 2016 to 2017 n.d. www.gov.uk/government/publications/dental-quality-and-outcomes-framework (accessed 1 September 2018).
- Department of Health and Social Care . Dental Contract Reform: Prototypes 2015. www.gov.uk/government/uploads/system/uploads/attachment_data/file/395384/Reform_Document.pdf (accessed 1 September 2018).
- Department of Health and Social Care . Dental Contract Reform: Evaluation of the First Year of Prototyping 2016–17 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/709555/evaluation-report-2016-2017.pdf (accessed 4 October 2019).
- Department of Health and Social Care . Delivering Better Oral Health: An Evidence-Based Toolkit for Prevention 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachme nt_data/file/605266/Delivering_better_oral_health.pdf (accessed 1 September 2018).
- Clarkson JE, Pitts NB, Bonetti D, Boyers D, Braid H, Elford R, et al. INTERVAL (investigation of NICE technologies for enabling risk-variable-adjusted-length) dental recalls trial: a multicentre randomised controlled trial investigating the best dental recall interval for optimum, cost-effective maintenance of oral health in dentate adults attending dental primary care. BMC Oral Health 2018;18. https://doi.org/10.1186/s12903-018-0587-2.
- Campbell S, Tickle M. What is quality primary dental care?. Br Dent J 2013;215:135-9. https://doi.org/10.1038/sj.bdj.2013.740.
- Tickle M, Campbell S. How do we measure quality in primary dental care?. Br Dent J 2013;215:183-7. https://doi.org/10.1038/sj.bdj.2013.789.
- Department of Health (Northern Ireland) (DH) . The Oral Health Strategy for Northern Ireland 2007. www.health-ni.gov.uk/publications/oral-health-strategy- northern-ireland-june-2007 (accessed 1 September 2018).
- Department of Health and Social Care . Primary Dental Care Strategy 2006. www.health-ni.gov.uk/publications/primary-dental-care-strategy-2006 (accessed 1 September 2018).
- Department of Health (Northern Ireland) (DH) . Dental Branch Annual Report. November 2007 n.d. https://wayback.archive-it.org/11112/20100918235903/http://www.dhsspsni.gov.uk/print/2007-2.pdf (accessed 1 September 2018).
- Department of Health (Northern Ireland) (DH) . Dental Branch Annual Report. December 2008 n.d. https://wayback.archive-it.org/11112/20100918235752/http://www.dhsspsni.gov.uk/print/dental_branch_annual_report_2007_2008.pdf (accessed 1 September 2018).
- Department of Health (Northern Ireland) (DH) . Dental Branch Annual Report. February 2009 n.d. http://webarchive.proni.gov.uk/20120831034147/http://www.dhsspsni.gov.uk/print/in dex/dental/dental-pubs.htm (accessed 1 September 2018).
- Department of Health (Northern Ireland) (DH) . Dental Branch Annual Report. May 2010 n.d. http://webarchive.proni.gov.uk/20120831034147/http://www.dhsspsni.gov.uk/print/in dex/dental/dental-pubs.htm (accessed 1 September 2018).
- Department of Health (Northern Ireland) (DH) . Dental Branch Annual Report. May 2010 n.d. http://webarchive.proni.gov.uk/20120831034147/http://www.dhsspsni.gov.uk/print/in dex/dental/dental-pubs.htm (accessed 1 September 2018).
- Patient and Client Council (PCC) . Talking Teeth: Patient Views of General Dental Services in Northern Ireland 2011. https://patientclientcouncil.hscni.net/download/19/reports/555/talking-teeth-patient-views-of-general-dental-services-in-northern-ireland.pdf (accessed 14 January 2020).
- Hill H, Birch S, Tickle M, McDonald R, Donaldson M, O’Carolan D, et al. Does capitation affect the delivery of oral healthcare and access to services? Evidence from a pilot contact in Northern Ireland. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2117-3.
- Department of Health and Social Care . Current Direction of the New Primary Care (General) Dental Contract 2006. www.health-ni.gov.uk/articles/dental-contracts (accessed 1 September 2018).
- Public Health Wales (PHW) . Welsh Dental Pilots Programme 2017. www.wales.nhs.uk/sitesplus/888/page/66924 (accessed 1 September 2018).
- Public Health Wales (PHW) . Future Plans for the Continuation and Development of the Welsh Dental Pilot Programme 2017.
- Welsh Government . Taking Oral Health Improvement and Dental Services Forward in Wales 2017. https://gov.wales/topics/health/professionals/dental/publication/information/dental-health/?lang=en (accessed 1 September 2018).
- Welsh Government . Dental Contract Reform and Expanding New Ways of Working for NHS Dental Practices 2017. https://gov.wales/about/cabinet/cabinetstatements/2018/dentalcontractreform/?lang=en (accessed 1 September 2018).
- Scottish Government . Oral Health Improvement Plan 2018. www.gov.scot/Publications/2018/01/9275 (accessed 1 September 2018).
- Tickle M, O’Neill C, Donaldson M, Birch S, Noble S, Killough S, et al. A randomised controlled trial to measure the effects and costs of a dental caries prevention regime for young children attending primary care dental services: the Northern Ireland Caries Prevention In Practice (NIC-PIP) trial. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20710.
- Ramsay CR, Clarkson JE, Duncan A, Lamont TJ, Heasman PA, Boyers D, et al. Improving the Quality of Dentistry (IQuaD): a cluster factorial randomised controlled trial comparing the effectiveness and cost-benefit of oral hygiene advice and/or periodontal instrumentation with routine care for the prevention and management of periodontal disease in dentate adults attending dental primary care. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22380.
- Hutchison B, Birch S, Hurley J, Lomas J, Stratford-Devai F. Do physician-payment mechanisms affect hospital utilization? A study of health service organizations in Ontario. CMAJ 1996;154:653-61.
- Statement of Dental Remuneration (Northern Ireland) n.d. www.hscbusiness.hscni.net/pdf/STATEMENT%20OF%20DENTAL%20REMUNERATION%202017-18%20-%20replacement%20pages%20to%20Det%20I%20-M. pdf (accessed 4 October 2019).
- Northern Ireland Statistics and Research Agency . Northern Ireland Small Areas n.d. www.nisra.gov.uk/support/geography/northern-ireland-small-areas (accessed 4 October 2019).
- Moulton BR. An illustration of a pitfall in estimating the effects of aggregate variables on micro units. The Review of Economics and Statistics 1990;72:334-8. https://doi.org/10.2307/2109724.
- Donald SG, Lang K. Inference with difference-in-differences and other panel data. The Review of Economics and Statistics 2007;89:221-3. https://doi.org/10.1162/rest.89.2.221.
- Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015;350. https://doi.org/10.1136/bmj.h2750.
- Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299-30. https://doi.org/10.1046/j.1365-2710.2002.00430.x.
- Snijders TAB, Bosker RJ. Standard errors and sample sizes for two-level research. J Educat Stats 1993;18:237-59. https://doi.org/10.3102/10769986018003237.
- Mellor AC, Blinkhorn AS, Hassall DC, Holloway PJ, Worthington HV. An assessment of capitation in the General Dental Service contract 2. Patterns of treatment provided to regularly attending patients. Br Dent J 1997;182:460-4. https://doi.org/10.1038/sj.bdj.4809413.
- Mellor AC, Coventry P, Worthington HV, Holloway PJ, Lennon MA. The capitation study. 3. The views of participating dentists and the profession. Br Dent J 1990;168:303-5. https://doi.org/10.1038/sj.bdj.4807170.
- Blinkhorn AS, Hassall DC, Holloway PJ, Mellor AC, Worthington HV. An assessment of capitation in the new General Dental Service contract. Community Dent Health 1996;13:3-20.
- Holloway PJ, Lennon MA, Mellor AC, Coventry P, Worthington HV. The capitation study. 1. Does capitation encourage ‘supervised neglect’?. Br Dent J 1990;168:119-21. https://doi.org/10.1038/sj.bdj.4807099.
- Rosen HM, Sussman RA, Sussman EJ. Capitation in dentistry: a quasi-experimental evaluation. Med Care 1977;15:228-40. https://doi.org/10.1097/00005650-197703000-00004.
- Taylor-Gooby P, Sylvester A, Calnan M, Manley G. Knights, knaves and gnashers: professional values and private dentistry. J Soc Pol 2000;29:375-95. https://doi.org/10.1017/S0047279400005997.
- Harris R, Holt R. Interacting institutional logics in general dental practice. Soc Sci Med 2013;94:63-70. https://doi.org/10.1016/j.socscimed.2013.05.038.
- Bhaskar R. A Realist Theory Of Science. London: Routledge; 2013.
- Kravitz AS, Bullock A, Cowpe J. Manual of Dental Practice. Edition 5.1 n.d. www.omd.pt/content/uploads/2017/12/ced-manual-2015-completo.pdf (accessed 1 September 2018).
- Watson M. Are Corporate Bodies Good For Dentistry? n.d. www.dentistry.co.uk/2016/11/21/are-corporate-bodies-good-for-dentistry/ (accessed 1 September 2018).
- Bhaskar R. The Possibility of Naturalism: A Philosophical Critique of the Contemporary Human Sciences. London: Routledge; 2014.
- Donagan A. Human Ends and Human Actions: An Exploration in St. Thomas’s Treatment. Milwaukee, WI: Marquette University Press; 1985.
- Goodwin TL, Brocklehurst PR, Hall E, McDonald R, Tickle M, Williams L. How, and why, does capitation affect general dental practitioners’ behaviour? A rapid realist review. Br J Healthcare Manag 2018;24:505-13. https://doi.org/10.12968/bjhc.2018.24.10.505.
- Tickle M, O’ Malley L, Brocklehurst P, Glenny AM, Walsh T, Campbell S. A national survey of the public’s views on quality in dental care. Br Dent J 2015;219. https://doi.org/10.1038/sj.bdj.2015.595.
- Athey S, Imbens G. Identification and inference in nonlinear difference-in-differences models. Econometrica 2006;74:431-97. https://doi.org/10.1111/j.1468-0262.2006.00668.x.
- NIHR . NIHR HTA 16 23 01 A Randomised Controlled Trial to Evaluate the Effectiveness and Cost Benefit of Prescribing High Dose FLuoride Toothpaste in Preventing and Treating DEntal Caries in High-Risk Older AdulTs (REFleCt Trial) n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/162301/#/ (accessed 1 September 2018).
- NIHR . NIHR HTA 06 35 05 INTERVAL Dental Recalls Trial (Investigation of NICE Technologies for Enabling Risk-Variable-Adjusted-Length Dental Recalls Trial) – A Feasibility Study and Follow On n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/063505/#/ (accessed 1 September 2018).
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33-8. https://doi.org/10.2307/2683903.
- Jacobs R. Alternative methods to examine hospital efficiency: data envelopment analysis and stochastic frontier analysis. Health Care Manag Sci 2001;4:103-15. https://doi.org/10.1023/A:1011453526849.
- Smith PC. Data Envelopment Analysis in Health Care: An Introductory Note. York: Centre for Health Economics, University of York; 1998.
Appendix 1 The HS45 form
Reproduced with permission from Health and Social Care Business Services Organisation.
The form below highlights what GDPs working in Northern Ireland complete to record treatment activity.
Appendix 2 Matching process for the control groups
In the matching process, control practices are found that are similar to the intervention practices in all relevant characteristics that may explain participation in the pilot. There are a wide variety of matching methods available, and it is important to choose an approach that yields the best balance. We found that the best balance was achieved using a PS approach (i.e. the probability of a practice participating in the pilot given observed characteristics X). As with all matching procedures used to eliminate selection bias, matching by PS assumes that selection occurs through observed X or unobserved factors highly correlated with X. Therefore, we include all available covariates in the PS specification and, to ensure that we chose variables that are unaffected by the introduction of the pilot contract (or the anticipation of it), we used units of the variables measured before the capitation period. We chose a probit model to estimate PS as, compared with linear probability models (i.e. ordinary least squares regression), predictions are not outside the [0; 1] bounds of probabilities for a binary dependent variable (treatment or control group) that is highly skewed (few intervention practices compared with control practices). This is detailed in Table 3.
After PSs were estimated, we tried a variety of methods including approaches that (1) produce the smallest standardised difference of means across the largest number of variables related to participation, (2) minimise the standardised difference of means of a few variables particularly strong in association with participation and (3) produce the smallest number of large standardised differences of means. The first approach was found to have the closest overlap with the intervention practices on the number of treatments delivered and variables that are associated with participation. We found that one advantage of this approach is that an adequate number of control practices were matched to treated practices even after a large number of matching variables was used (all those strongly and weakly associated with participation). This is explained by the large pool of available control practices for the matching method to select from and because, even before matching took place, there were no large mean differences between intervention and control practices on any of the matching variables. This is detailed in Table 4.
We found that kernel matching at a kernel function bandwidth of 0.06 achieved the smallest standardised difference of means across the largest number of variables related to participation. Kernel matching is an approach that uses the weighted averages of all individuals in the control group to construct the counterfactual outcome, and we found that it yielded the lowest variance in the DiD estimator because it used information from all practices. It is important with this approach to check that the lower variance is not the result of using poor-quality information (bad matches even if such observations are given ‘low’ weights in model estimation because of their low PS) that may bias the DiD coefficient. This was not the case in our sample because the unmatched control group and intervention group had similar practice characteristics and because there was ‘common support’ in the distribution of PS between the control and intervention groups. In other words, practices had the same PS values in both groups so no practices in the control group had a PS below the minimum or above the maximum PS values in the intervention group (and vice versa for intervention practices and the minimum and maximum PSs in the control group). As we found no bad matches (all control group practices were in a region of PS overlap), the outcome variable(s) is independent of assignment to the intervention group conditional on the PS. This means that the DiD estimator has no selection bias if selection occurs from only characteristics of practices that we used to calculate the PS.
A further reason for matching to all observations is that we found that matching to a random sample of control practices did not constitute a balanced control group. This is probably because the unmatched control practices were not from a random sample of all Northern Ireland practices. As we could not achieve representativeness in the matched sample (generalisability of the control group to all Northern Ireland practices), we instead chose a matching method for improved comparability to the intervention group, although the lack of representativeness is not likely to be as large as typically observed in other (non-randomised) natural experiment studies. This is because no practices were excluded in the matched control group and the unmatched control group practices were chosen randomly within strata.
Table 14 shows the characteristics of the matched control group compared with the intervention group. There was no statistical difference in practice characteristics between the groups in the unmatched and matched samples. The standardised percentage bias was used to assess the degree to which the sample differs in practice characteristics. The standardised percentage bias is the percentage difference of the sample means in the intervention and control (full or matched) subsamples as a percentage of the square root of the mean of the sample variances in the intervention and control groups (Rosenbaum and Rubin, 198584). The mean standardised bias across all variables was reduced from 38% in the unmatched sample to 7% in the matched sample. The close similarity in practice characteristics between intervention and matched control practices is also shown by the absence of an association of any practice characteristics with the decision of practices to join the pilot contract (pseudo-R2 from the probit estimation was 0.01). Two other measures were used to assess the reduction in bias from matching. Rubin’s B is the absolute standardised difference of the means of the linear index of the PS in the intervention and (matched) control group, and Rubin’s R is the ratio of intervention to (matched) control variances of the PS index. The consistent findings from these tests of no selection bias in the matched sample is unsurprising because there was a large pool of potential controls.
| Variable | Unmatched/matched | Intervention | Control | % bias | t-statistic | p-value |
|---|---|---|---|---|---|---|
| Mean number of registrations in a baseline period month | Unmatched | 2235.7 | 1536.4 | 59.4 | 1.80 | 0.08 |
| Matched | 2235.7 | 2066.5 | 18.8 | 0.39 | 0.70 | |
| Mean multiple deprivation measure decile (at patient’s current registered address) of patients treated | Unmatched | 5.4 | 6.0 | –46.0 | –1.32 | 0.19 |
| Matched | 5.5 | 5.5 | –2.1 | –0.04 | 0.97 | |
| Mean proportion of treatments to female patients in a baseline period month | Unmatched | 0.56 | 0.56 | 23.2 | 0.59 | 0.56 |
| Matched | 0.56 | 0.56 | 8.3 | 0.33 | 0.74 | |
| Mean proportion of treatments to patients exempt from fee paying (when treatment was carried out) | Unmatched | 0.69 | 0.65 | 32.5 | 0.84 | 0.40 |
| Matched | 0.69 | 0.70 | –7.6 | –0.20 | 0.85 | |
| Mean proportion of patients (when treatment was carried out) aged ≤ 17 years, in a baseline period month | Unmatched | 0.008 | 0.02 | –31.7 | –0.77 | 0.44 |
| Matched | 0.008 | 0.01 | 2.9 | –0.08 | 0.93 | |
| Mean proportion of patients (when treatment was carried out) in the age range of 18 to 39 years | Unmatched | 0.38 | 0.38 | 5.0 | 0.13 | 0.89 |
| Matched | 0.38 | 0.38 | –5.5 | –0.13 | 0.90 | |
| Mean proportion of patients (when treatment was carried out) in the age range of 40 to 59 years | Unmatched | 0.38 | 0.36 | 68.3 | 1.71 | 0.10 |
| Matched | 0.38 | 0.38 | 3.6 | 0.10 | 0.92 | |
| Pseudo-R2 from probit estimation | Unmatched | 0.23 | ||||
| Matched | 0.02 | |||||
| Mean bias | Unmatched | 38.0 | ||||
| Matched | 7.0 | |||||
| Rubin’s B and Rubin’s R | Unmatched |
B = 103.7 R = 0.39 |
||||
| Matched |
B = 24.4 R = 0.75 |
|||||
| Number of observations used in matching equation | 516 monthly observations (baseline) from 30 control practices and 11 intervention practices | |||||
| Number of observations used in analysis (with or without matching) | 1032 monthly observations (baseline and capitation period) from 30 control practices and 11 intervention practices | |||||
Appendix 3 Additional difference-in-difference analyses at the practice level
Given the restrictions to the number of tables and figures that can be presented, this appendix details the full DiD analysis, including a comparison of matched and unmatched controls (Tables 15–31). All figures are expressed per month per 1000 registered patients, to adjust for the different sizes of the practices. No difference is seen between the analyses conducted with matched practices (with PSs) and unmatched practices.
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | 1.24 | 0.36 | 3.45 | < 0.01b |
| 18a | 1.45 | 0.39 | 3.69 | < 0.01b | |
| Capitation to reversion FFS | 30 | –1.48 | 0.30 | 4.86 | < 0.01b |
| 18a | –1.35 | 0.34 | 3.93 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | –0.24 | 0.50 | 0.47 | 0.64 |
| 18a | 0.13 | 0.55 | 0.24 | 0.82 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | 5.24 | 4.89 | 1.07 | 0.29 |
| 18a | 6.00 | 6.33 | 0.95 | 0.35 | |
| Capitation to reversion FFS | 30 | 0.04 | 4.06 | 0.01 | 0.99 |
| 18a | –3.30 | 5.67 | 0.58 | 0.57 | |
| Baseline FFS to reversion FFS | 30 | 5.29 | 3.98 | 1.33 | 0.19 |
| 18a | 2.94 | 4.22 | 0.70 | 0.49 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –3.79 | 2.68 | 1.42 | 0.16 |
| 18a | –0.94 | 1.46 | 0.65 | 0.52 | |
| Capitation to reversion FFS | 30 | 7.61 | 2.17 | 3.50 | < 0.01 |
| 18a | 6.82 | 1.40 | 4.89 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | 3.82 | 1.38 | 2.77 | 0.01 |
| 18a | 5.66 | 1.22 | 4.64 | < 0.01b |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –25.41 | 2.87 | 8.87 | < 0.01b |
| 18a | –27.10 | 3.07 | 8.83 | < 0.01b | |
| Capitation to reversion FFS | 30 | –6.56 | 1.71 | 3.83 | < 0.01b |
| 18a | –7.82 | 2.06 | 3.79 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | –31.97 | 3.18 | 10.05 | < 0.01b |
| 18a | –33.68 | 3.53 | 9.55 | < 0.01b |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –3.24 | 4.75 | 0.68 | 0.50 |
| 18a | 1.96 | 8.18 | 0.24 | 0.81 | |
| Capitation to reversion FFS | 30 | 16.45 | 4.36 | 3.77 | < 0.01 |
| 18a | 13.44 | 5.04 | 2.67 | 0.01 | |
| Baseline FFS to reversion FFS | 30 | 13.21 | 3.70 | 3.57 | < 0.01 |
| 18a | 15.63 | 6.37 | 2.45 | 0.02 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –0.41 | 0.45 | 0.90 | 0.38 |
| 18a | –0.56 | 0.40 | 1.41 | 0.17 | |
| Capitation to reversion FFS | 30 | 0.10 | 0.35 | 0.27 | 0.79 |
| 18a | 0.01 | 0.29 | 0.04 | 0.97 | |
| Baseline FFS to reversion FFS | 30 | –0.31 | 0.62 | 0.50 | 0.62 |
| 18a | –0.63 | 0.54 | 1.17 | 0.25 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –51.00 | 15.34 | 3.33 | < 0.01b |
| 18a | –48.98 | 13.80 | 3.55 | < 0.01b | |
| Capitation to reversion FFS | 30 | 61.79 | 12.81 | 4.82 | < 0.01b |
| 18a | 61.95 | 12.32 | 5.03 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | 10.79 | 11.23 | 0.96 | 0.34 |
| 18a | 12.98 | 10.71 | 1.21 | 0.24 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –3.27 | 0.96 | 3.42 | < 0.01b |
| 18a | –3.69 | 0.85 | 4.33 | < 0.01b | |
| Capitation to reversion FFS | 30 | 2.14 | 0.66 | 3.23 | < 0.01b |
| 18a | 2.29 | 0.69 | 3.32 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | –1.13 | 1.02 | 1.11 | 0.28 |
| 18a | –1.40 | 0.85 | 1.64 | 0.11 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –4.95 | 2.12 | 2.33 | 0.03 |
| 18a | –6.77 | 1.82 | 3.72 | < 0.01 | |
| Capitation to reversion FFS | 30 | 2.93 | 1.29 | 2.27 | 0.03 |
| 18a | 5.63 | 1.34 | 4.21 | < 0.01 | |
| Baseline FFS to reversion FFS | 30 | –2.02 | 2.05 | 0.99 | 0.33 |
| 18a | –1.15 | 1.69 | 0.68 | 0.50 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –35.81 | 9.00 | 3.98 | < 0.01b |
| 18a | –40.93 | 9.38 | 4.36 | < 0.01b | |
| Capitation to reversion FFS | 30 | 32.80 | 10.46 | 3.14 | < 0.01b |
| 18a | 34.13 | 11.01 | 3.10 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | –3.01 | 10.32 | 0.29 | 0.77 |
| 18a | –6.80 | 10.38 | 0.66 | 0.52 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –9.96 | 4.03 | 2.47 | 0.02 |
| 18a | –9.34 | 3.50 | 2.67 | 0.01 | |
| Capitation to reversion FFS | 30 | 10.97 | 3.45 | 3.18 | < 0.01 |
| 18a | 10.38 | 3.36 | 3.09 | < 0.01 | |
| Baseline FFS to reversion FFS | 30 | 1.01 | 3.10 | 0.33 | 0.75 |
| 18a | 1.03 | 2.46 | 0.42 | 0.68 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –5.06 | 1.49 | 3.40 | < 0.01 |
| 18a | –3.81 | 1.34 | 2.85 | 0.01 | |
| Capitation to reversion FFS | 30 | 3.34 | 1.56 | 2.15 | 0.04 |
| 18a | 3.45 | 1.44 | 2.39 | 0.02 | |
| Baseline FFS to reversion FFS | 30 | –1.72 | 1.47 | 1.17 | 0.25 |
| 18a | –0.41 | 1.43 | 0.29 | 0.78 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –2.00 | 0.63 | 3.19 | < 0.01b |
| 18a | –2.65 | 0.71 | 3.71 | < 0.01b | |
| Capitation to reversion FFS | 30 | 2.36 | 0.67 | 3.52 | < 0.01b |
| 18a | 2.37 | 0.68 | 3.50 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | 0.36 | 0.83 | 0.43 | 0.67 |
| 18a | –0.26 | 1.04 | 0.25 | 0.81 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –151.75 | 44.53 | 3.41 | < 0.01b |
| 18a | –174.78 | 41.42 | 4.22 | < 0.01b | |
| Capitation to reversion FFS | 30 | 160.00 | 32.94 | 4.86 | < 0.01b |
| 18a | 173.89 | 33.08 | 5.26 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | 8.25 | 38.55 | 0.21 | 0.83 |
| 18a | –0.99 | 31.44 | 0.03 | 0.98 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –0.37 | 0.07 | 5.15 | < 0.01b |
| 18a | –0.42 | 0.07 | 5.98 | < 0.01b | |
| Capitation to reversion FFS | 30 | 0.46 | 0.12 | 3.94 | < 0.01b |
| 18a | 0.41 | 0.11 | 3.78 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | 0.08 | 0.10 | 0.86 | 0.40 |
| 18a | –0.01 | 0.08 | 0.09 | 0.93 |
| DiD model | Number of control practices | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | 1.10 | 1.16 | 0.95 | 0.35 |
| 18a | 0.48 | 1.12 | 0.43 | 0.67 | |
| Capitation to reversion FFS | 30 | –0.58 | 0.96 | 0.60 | 0.55 |
| 18a | –0.60 | 0.93 | 0.64 | 0.53 | |
| Baseline FFS to reversion FFS | 30 | 0.52 | 1.12 | 0.47 | 0.64 |
| 18a | –0.11 | 1.00 | 0.11 | 0.91 |
| DiD model | Number of control practices | DiD coefficient (£) | SE (£) | t-statistic | p-value |
|---|---|---|---|---|---|
| Baseline FFS to capitation | 30 | –5570 | 1508 | 3.69 | < 0.01 |
| 18a | –5920 | 1612 | 3.67 | < 0.01b | |
| Capitation to reversion FFS | 30 | 4895 | 1450 | 3.37 | < 0.01 |
| 18a | 5248 | 1488 | 3.53 | < 0.01b | |
| Baseline FFS to reversion FFS | 30 | –675 | 1118 | 0.60 | 0.55 |
| 18a | –673 | 1264 | 0.53 | 0.60 |
Appendix 4 Difference-in-difference analyses of practice principals and associate dentists
Further analyses were undertaken on the results, determining the differences between PPs and ADs. The results are expressed per month per 1000 registrations.
There was no significant difference in the difference between the number of treatments with a gross cost of ≥ £280 delivered by PPs compared with ADs (Table 32; Figures 24 and 25).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –0.004 | 0.24 | 0.02 | 0.99 |
| Capitation to reversion FFS | –0.37 | 0.31 | 1.18 | 0.25 | |
| Baseline FFS to reversion FFS | –0.43 | 0.32 | 1.35 | 0.19 | |
| ADs | Baseline FFS to capitation | –0.70 | 0.54 | 1.29 | 0.21 |
| Capitation to reversion FFS | 0.07 | 0.33 | 0.21 | 0.84 | |
| Baseline FFS to reversion FFS | –0.65 | 0.76 | 0.85 | 0.40 |
FIGURE 24.
Number of treatments with a gross cost of ≥ £280 (PPs).
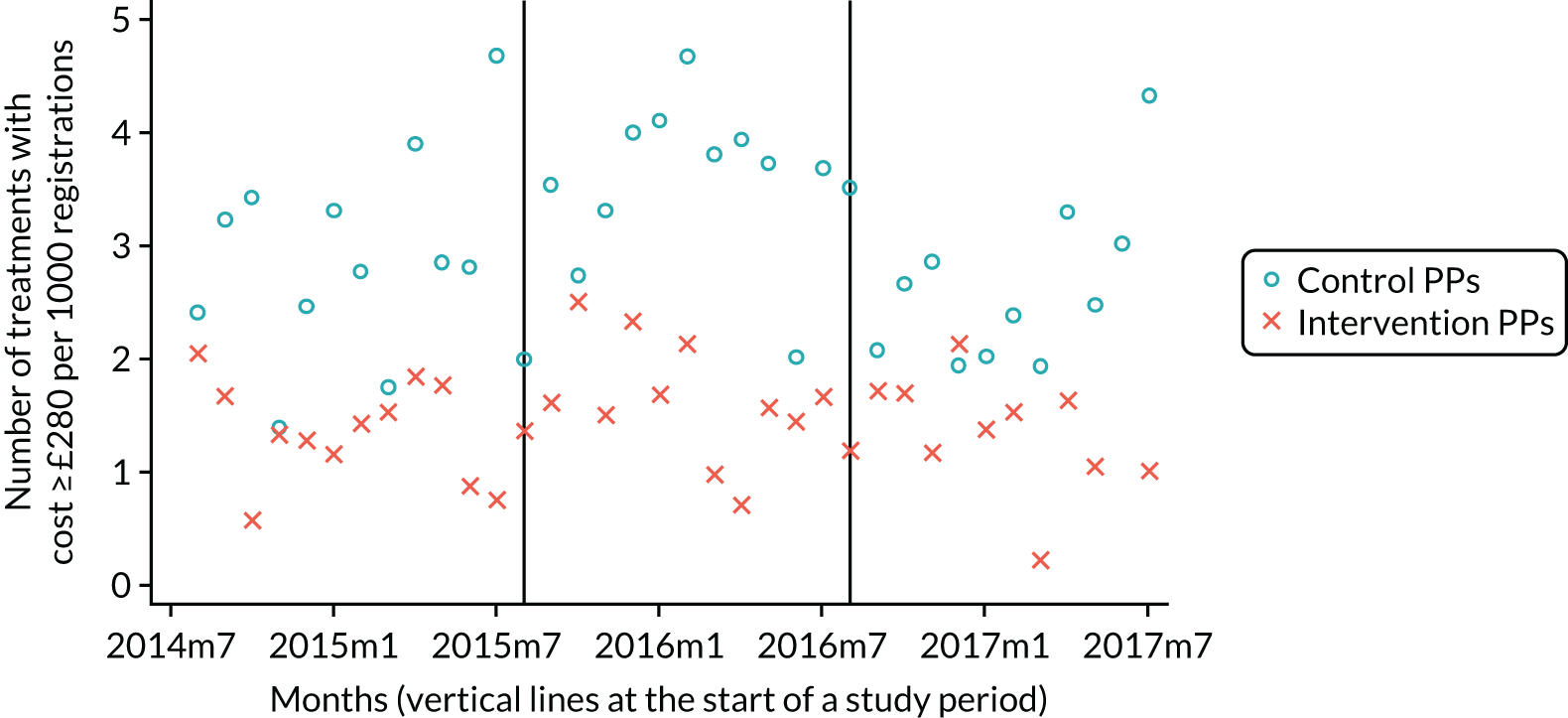
FIGURE 25.
Number of treatments with a gross cost of ≥ £280 (ADs).

Fewer direct restorations were placed by ADs in the capitation period (difference in the difference of 56.2) and this increased on reversion to FFS (difference in the difference of 62.12). PPs followed a similar trend (Table 33; Figures 26 and 27).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –14.27 | 6.52 | 2.19 | 0.04 |
| Capitation to reversion FFS | 16.15 | 6.99 | 2.31 | 0.03 | |
| Baseline FFS to reversion FFS | 1.88 | 4.54 | 0.41 | 0.68 | |
| ADs | Baseline FFS to capitation | –56.17 | 15.56 | 3.61 | < 0.01 |
| Capitation to reversion FFS | 62.12 | 15.82 | 3.93 | < 0.01 | |
| Baseline FFS to reversion FFS | 5.95 | 14.02 | 0.42 | 0.68 |
FIGURE 26.
Number of direct restoration treatments (PPs).
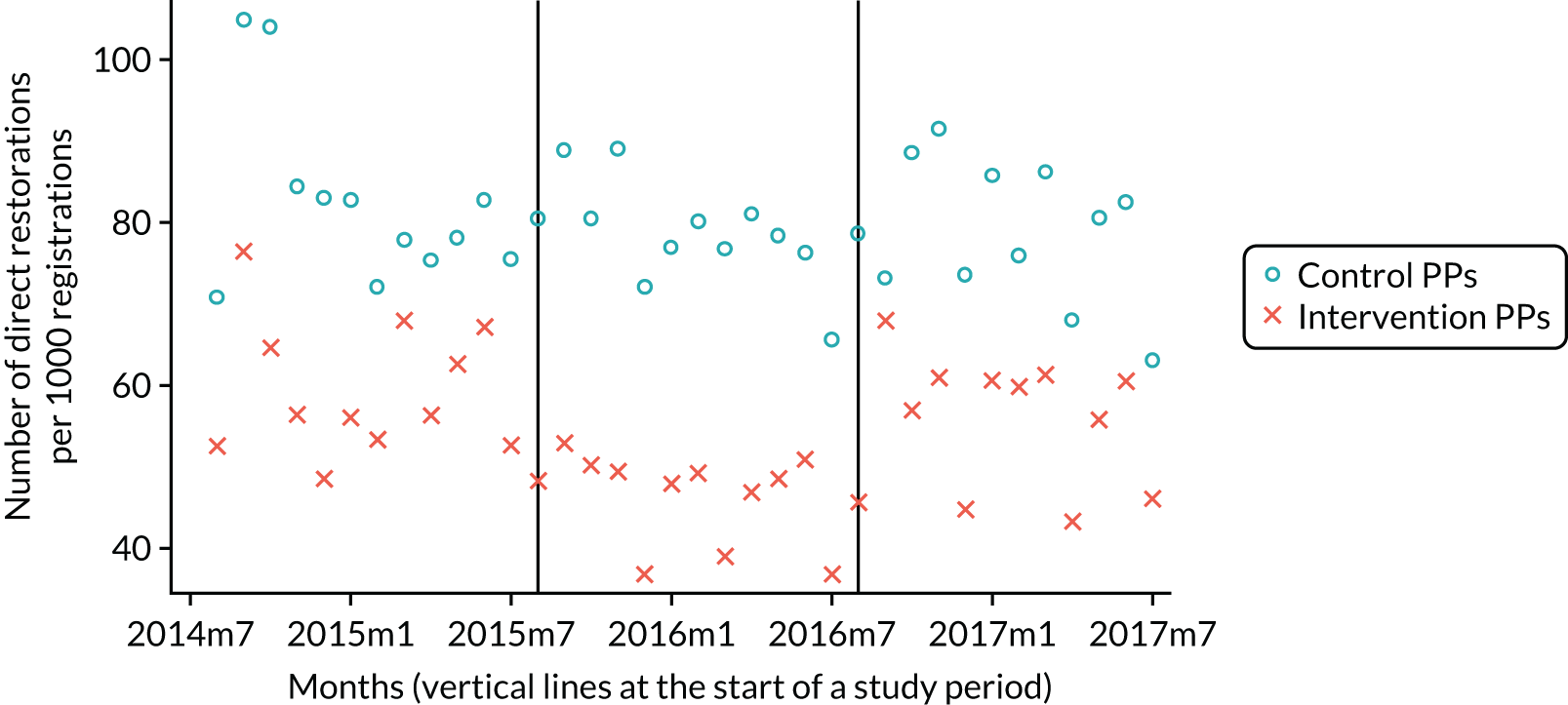
FIGURE 27.
Number of direct restoration treatments (ADs).

There were 2.8 fewer indirect restorations placed by ADs during the pilot period (Table 34; Figures 28 and 29).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –1.41 | 0.77 | 1.84 | 0.08 |
| Capitation to reversion FFS | –1.41 | 0.77 | 1.84 | 0.08 | |
| Baseline FFS to reversion FFS | –1.41 | 0.77 | 1.84 | 0.08 | |
| ADs | Baseline FFS to capitation | –2.79 | 1.01 | 2.78 | 0.01 |
| Capitation to reversion FFS | –2.79 | 1.01 | 2.78 | 0.01 | |
| Baseline FFS to reversion FFS | –2.79 | 1.01 | 2.78 | 0.01 |
FIGURE 28.
Number of indirect restoration treatments (PPs).

FIGURE 29.
Number of indirect restoration treatments (ADs).
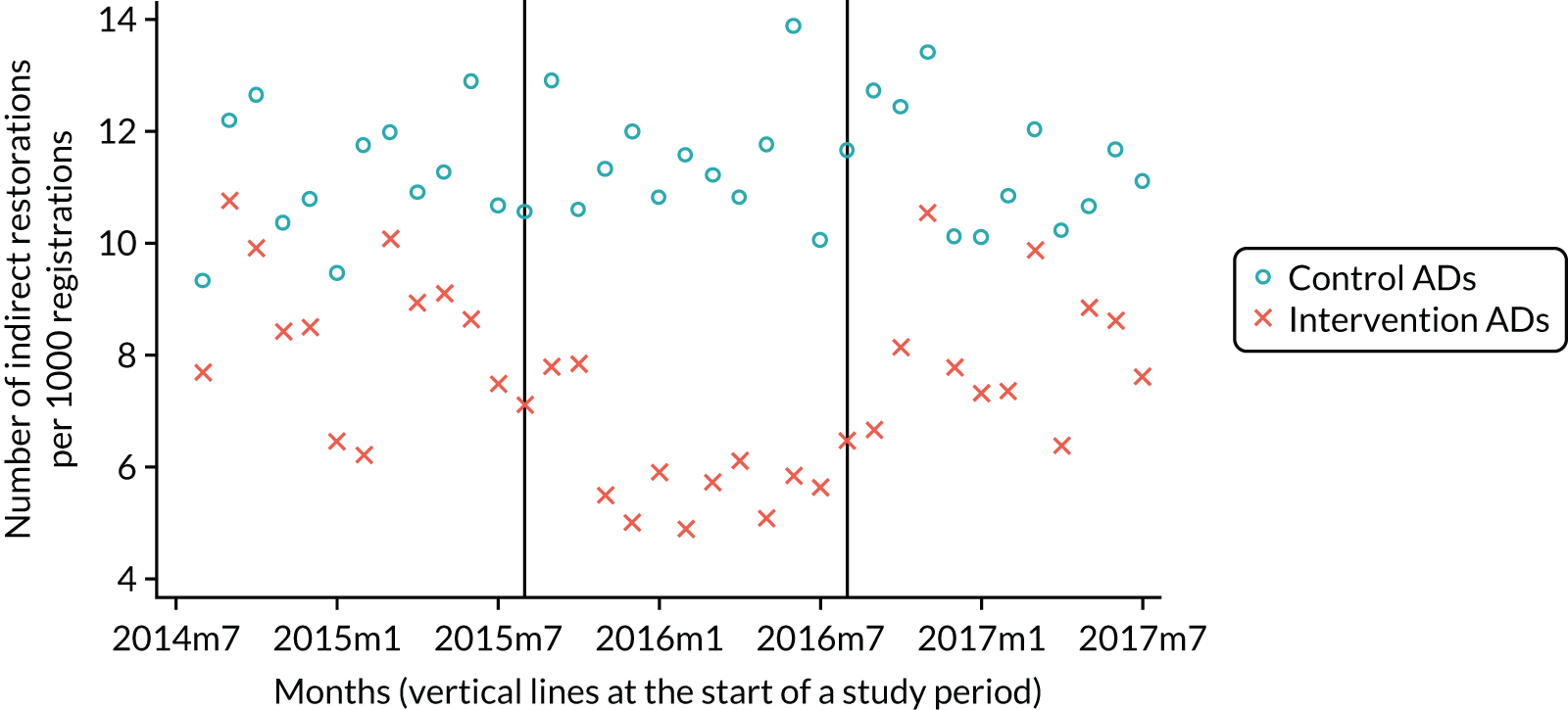
Fewer extractions were undertaken by both PPs (difference in the difference of 3.1) and ADs (difference in the difference of –5.0) as they moved from baseline FFS to capitation. This trend reversed for ADs on reversion to FFS (Table 35; Figures 30 and 31).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –3.12 | 1.37 | 2.27 | 0.03 |
| Capitation to reversion FFS | –3.12 | 1.37 | 2.27 | 0.03 | |
| Baseline FFS to reversion FFS | –2.42 | 1.18 | 2.05 | 0.05 | |
| ADs | Baseline FFS to capitation | –5.00 | 2.06 | 2.43 | 0.02 |
| Capitation to reversion FFS | 6.34 | 1.89 | 3.35 | < 0.01 | |
| Baseline FFS to reversion FFS | 1.35 | 1.93 | 0.70 | 0.49 |
FIGURE 30.
Number of extractions (PPs).
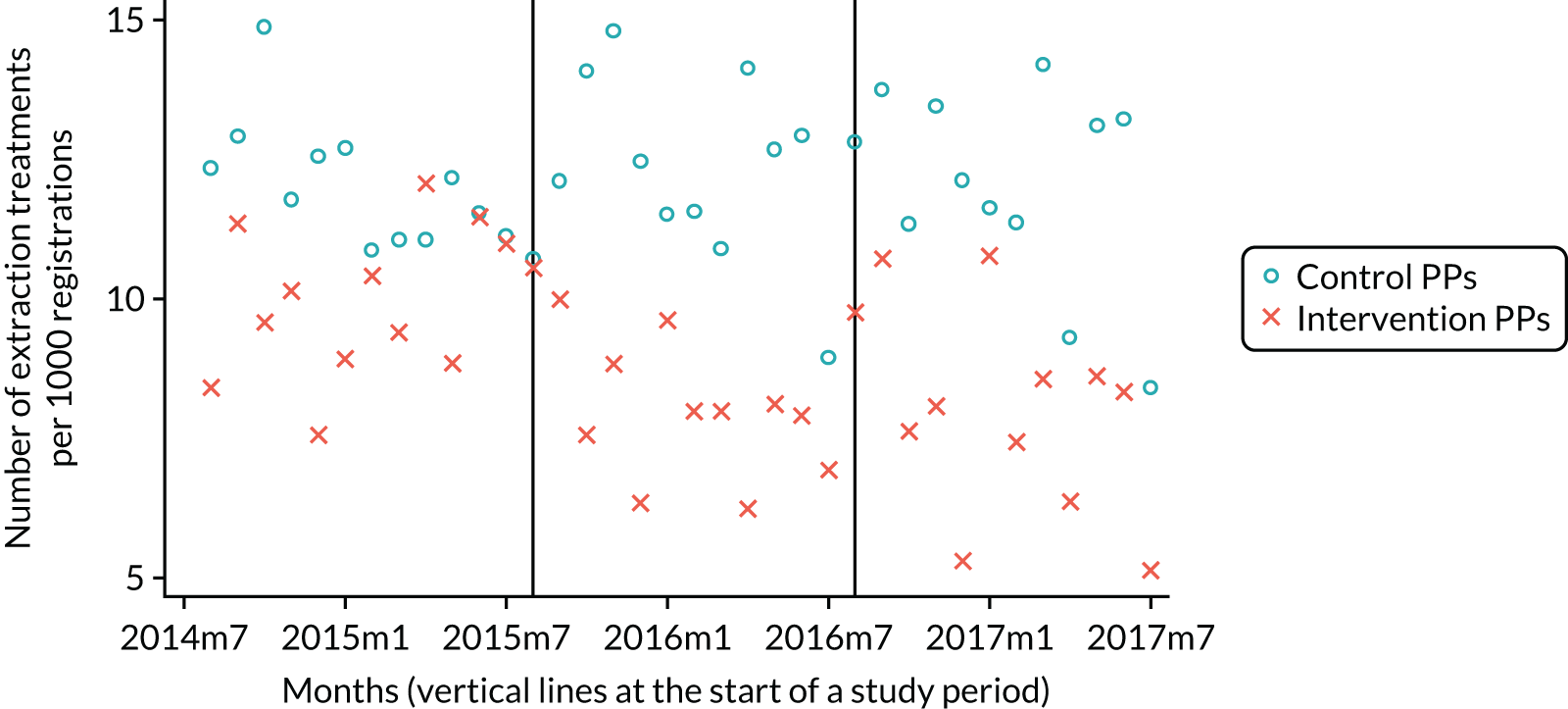
FIGURE 31.
Number of extractions (ADs).
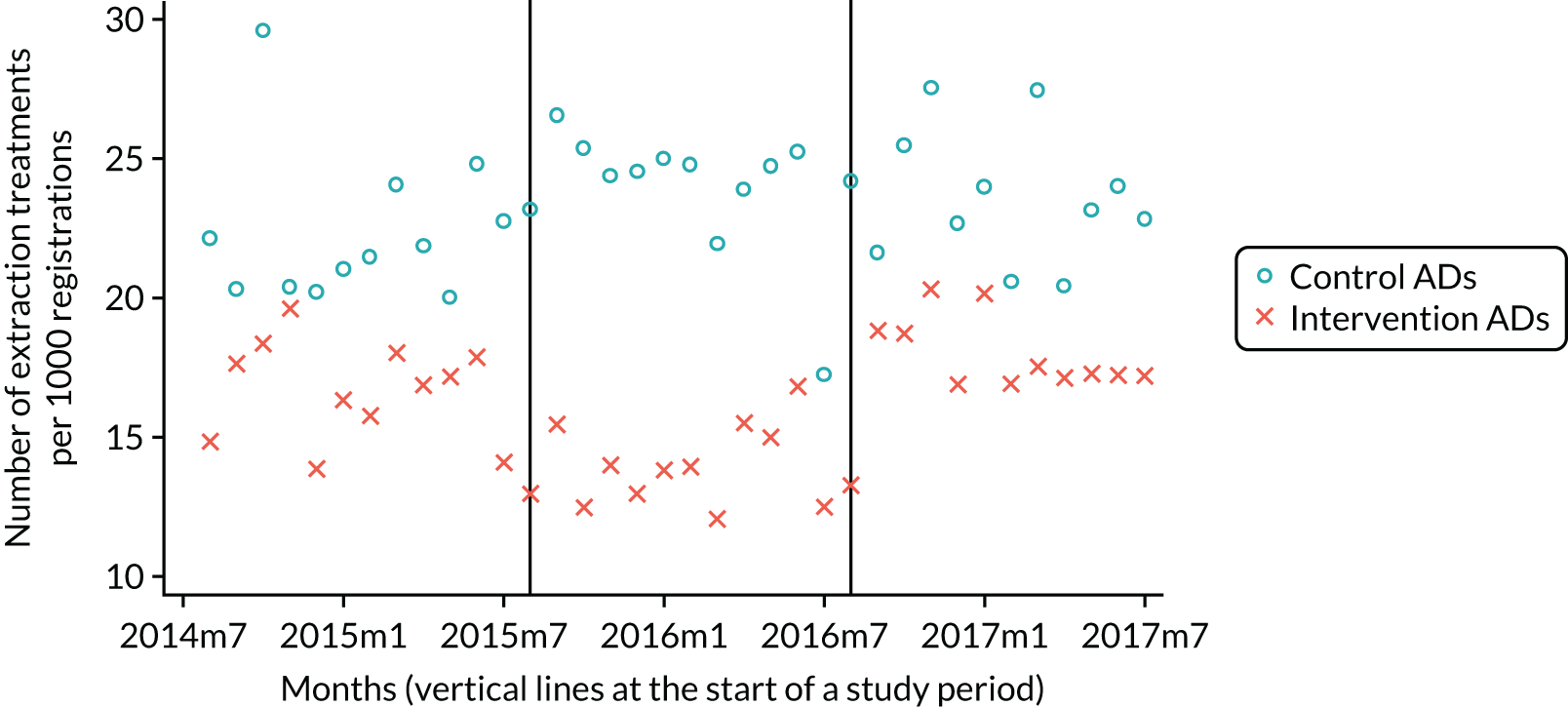
Both PPs and ADs delivered fewer radiographs as they moved from baseline FFS to capitation. This trend reversed for ADs as they moved back to FFS (Table 36; Figures 32 and 33).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –16.25 | 5.00 | 3.25 | < 0.01 |
| Capitation to reversion FFS | 8.45 | 4.97 | 1.70 | 0.10 | |
| Baseline FFS to reversion FFS | –7.86 | 5.12 | 1.54 | 0.14 | |
| ADs | Baseline FFS to capitation | –37.97 | 12.14 | 3.13 | < 0.01 |
| Capitation to reversion FFS | 38.05 | 14.38 | 2.65 | 0.02 | |
| Baseline FFS to reversion FFS | 0.08 | 11.51 | 0.01 | 0.99 |
FIGURE 32.
Number of radiographs (PPs).

FIGURE 33.
Number of radiographs (ADs).
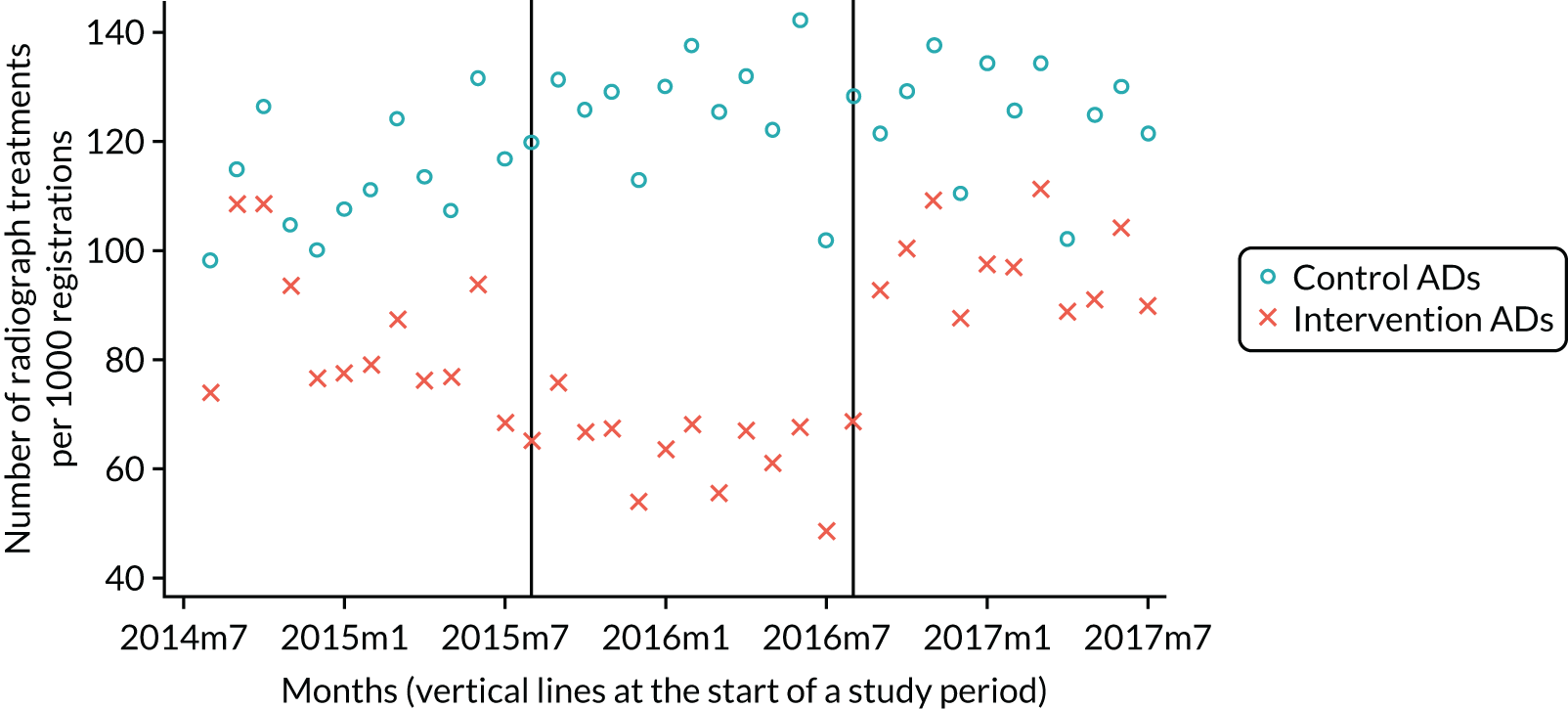
The ADs delivered fewer fissure sealants as they moved from baseline FFS to capitation. This trend reversed for ADs as they moved back to FFS (Table 37; Figures 34 and 35).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –1.45 | 1.77 | 0.82 | 0.42 |
| Capitation to reversion FFS | 1.86 | 2.38 | 0.78 | 0.44 | |
| Baseline FFS to reversion FFS | 0.19 | 2.24 | 0.08 | 0.93 | |
| ADs | Baseline FFS to capitation | –11.29 | 4.06 | 2.78 | 0.01 |
| Capitation to reversion FFS | 11.09 | 3.13 | 3.54 | < 0.01 | |
| Baseline FFS to reversion FFS | –0.12 | 2.61 | 0.05 | 0.96 |
FIGURE 34.
Number of fissure sealants (PPs).
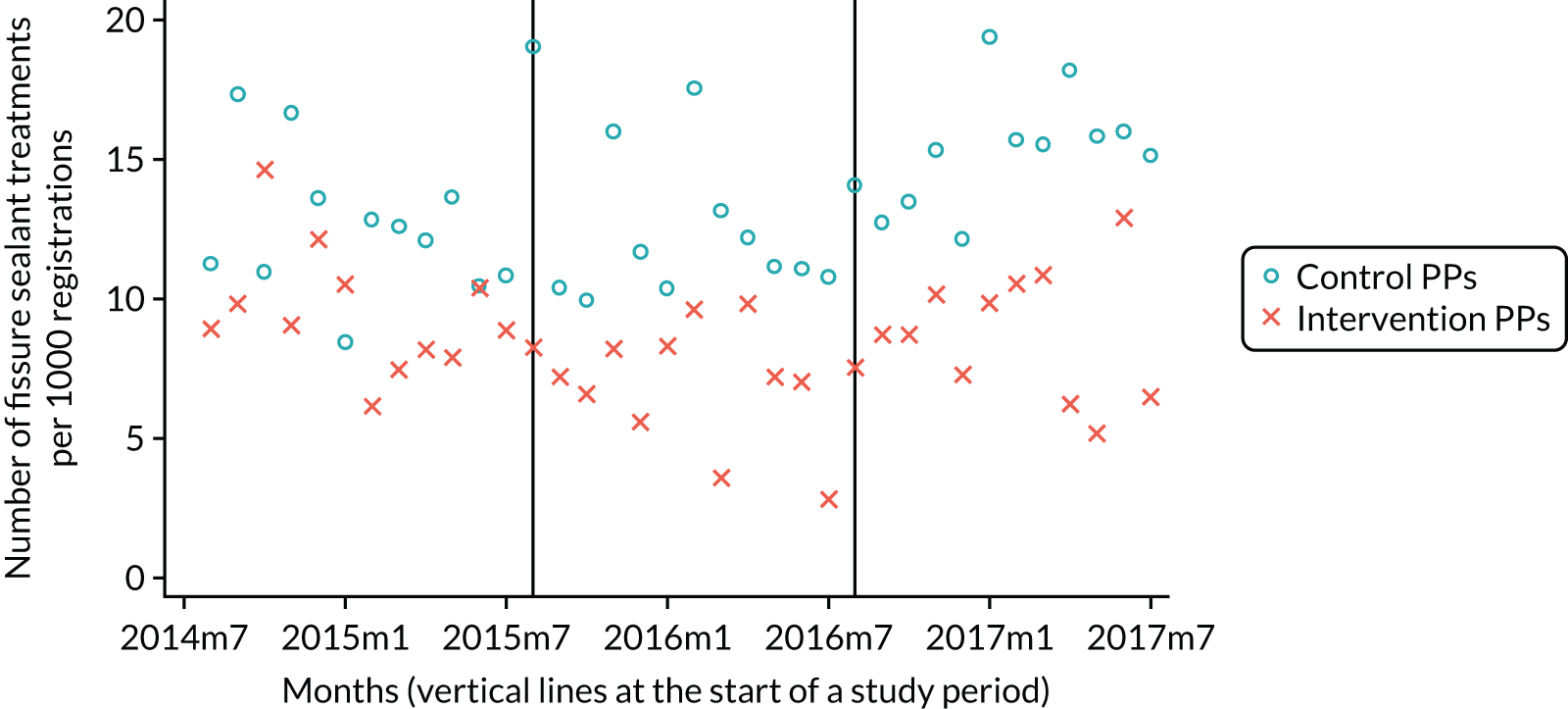
FIGURE 35.
Number of fissure sealants (ADs).
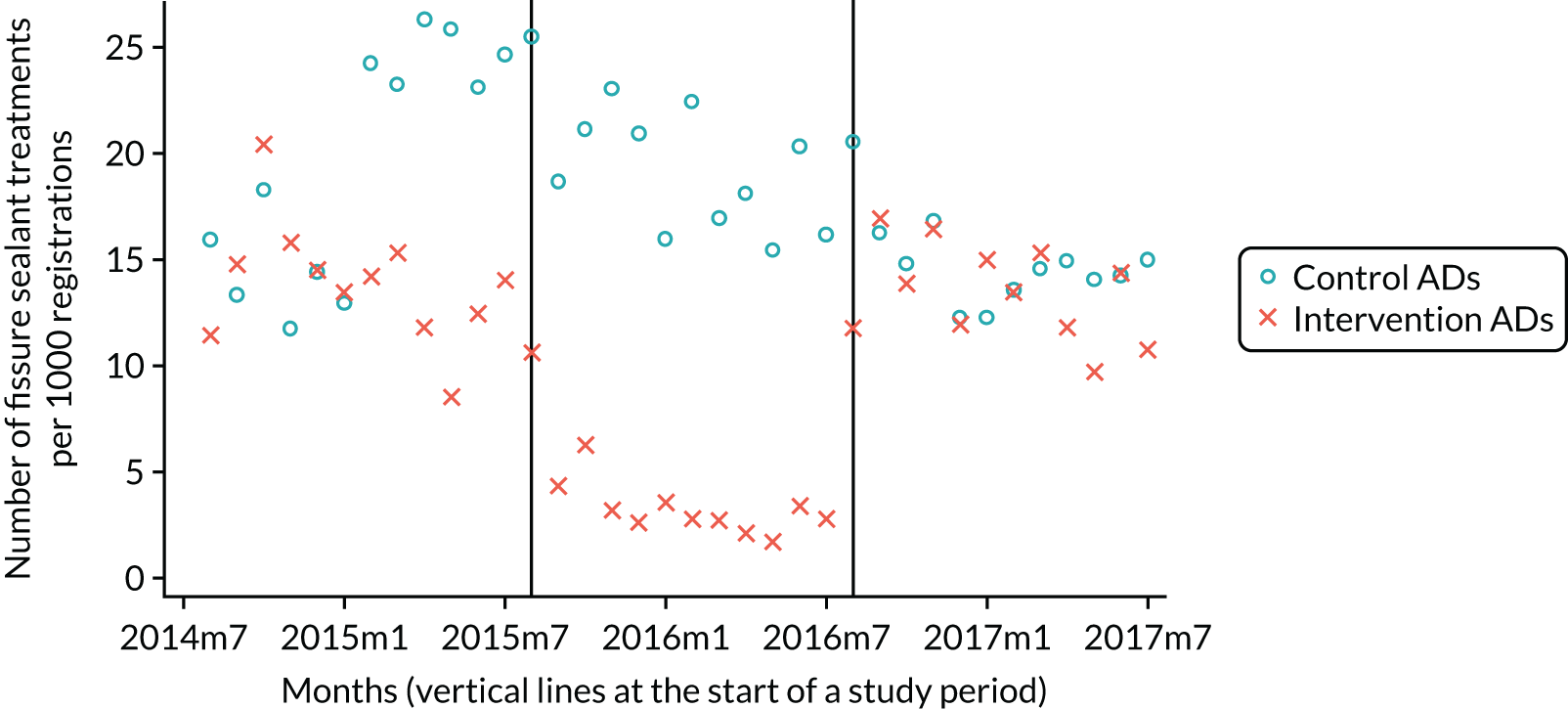
The ADs delivered fewer two-visit periodontal treatments as they moved from baseline FFS to capitation. Again, this trend reversed for ADs as they moved back to FFS (Table 38; Figures 36 and 37).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –1.25 | 0.80 | 1.56 | 0.13 |
| Capitation to reversion FFS | 0.29 | 0.48 | 0.60 | 0.55 | |
| Baseline FFS to reversion FFS | –0.63 | 0.72 | 0.87 | 0.39 | |
| ADs | Baseline FFS to capitation | –3.77 | 1.63 | 2.32 | 0.03 |
| Capitation to reversion FFS | 4.05 | 1.72 | 2.36 | 0.03 | |
| Baseline FFS to reversion FFS | 0.28 | 1.62 | 0.17 | 0.87 |
FIGURE 36.
Number of two-visit periodontal treatments (PPs).
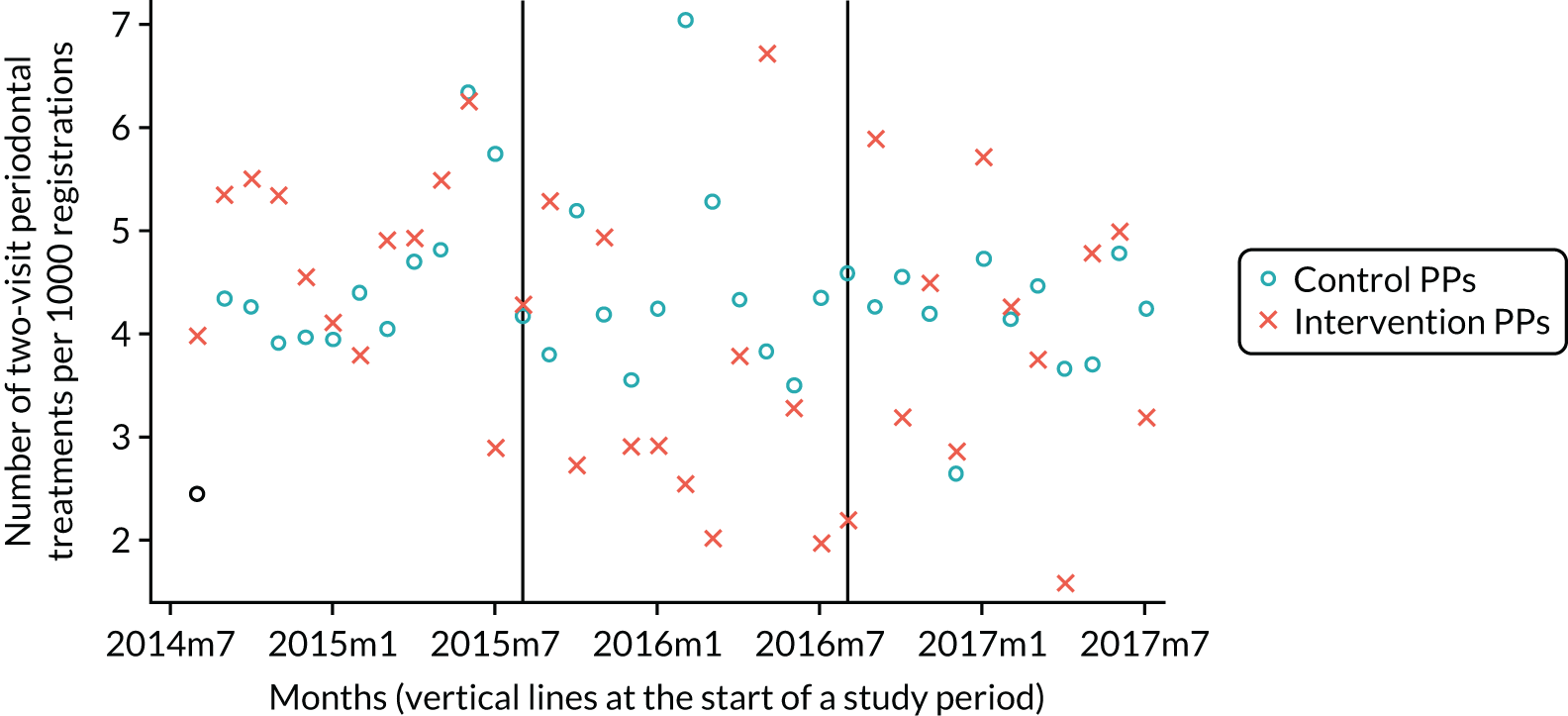
FIGURE 37.
Number of two-visit periodontal treatments (ADs).
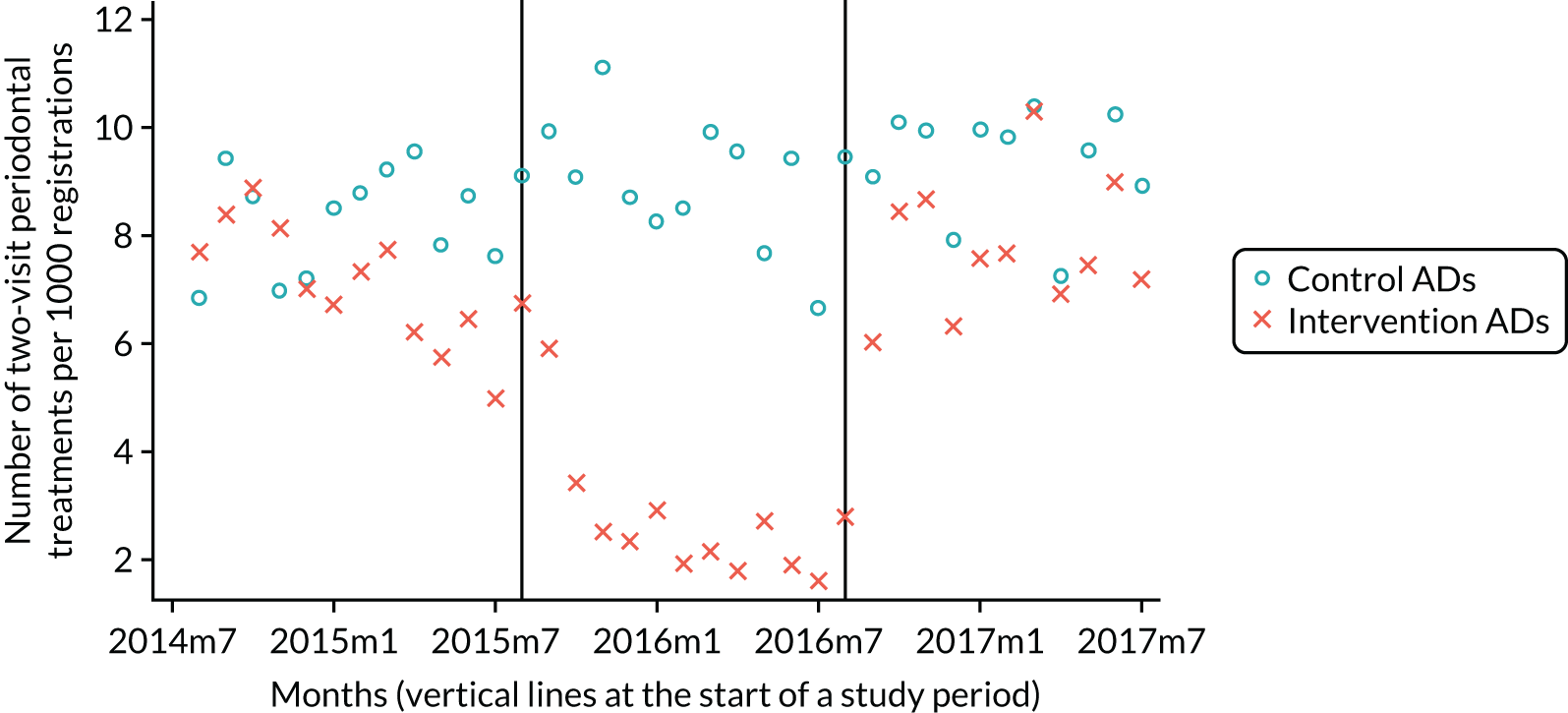
Both PPs and ADs undertook fewer root canal treatments as they moved from baseline FFS to capitation. Again, this trend reversed for ADs as they moved back to FFS (Table 39; Figures 38 and 39).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –0.91 | 0.45 | 2.03 | 0.05 |
| Capitation to reversion FFS | 0.40 | 0.65 | 0.61 | 0.55 | |
| Baseline FFS to reversion FFS | –0.52 | 0.67 | 0.78 | 0.44 | |
| ADs | Baseline FFS to capitation | –2.35 | 0.73 | 3.23 | < 0.01 |
| Capitation to reversion FFS | 2.39 | 0.66 | 3.61 | < 0.01 | |
| Baseline FFS to reversion FFS | 0.03 | 0.63 | 0.05 | 0.96 |
FIGURE 38.
Number of root canal treatments (PPs).
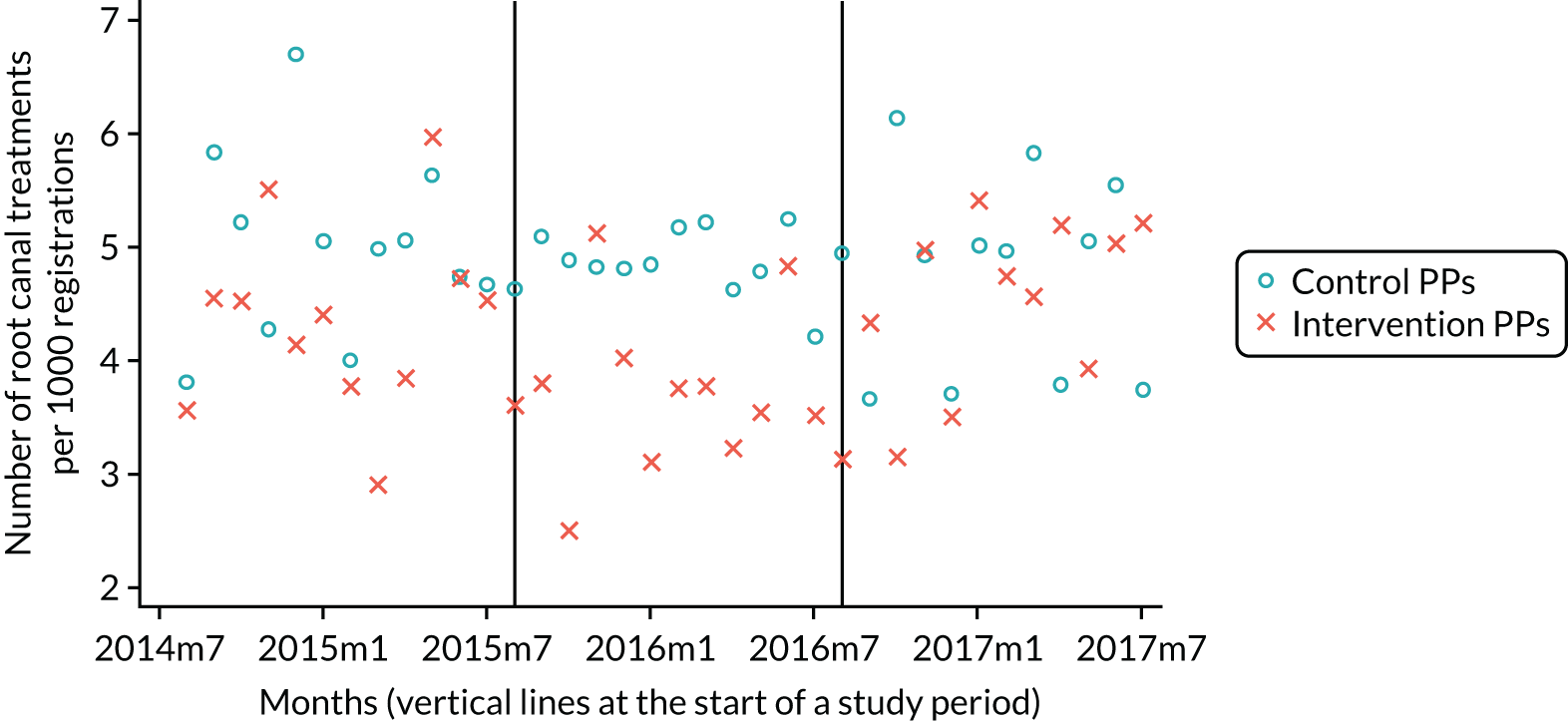
FIGURE 39.
Number of root canal treatments (ADs).
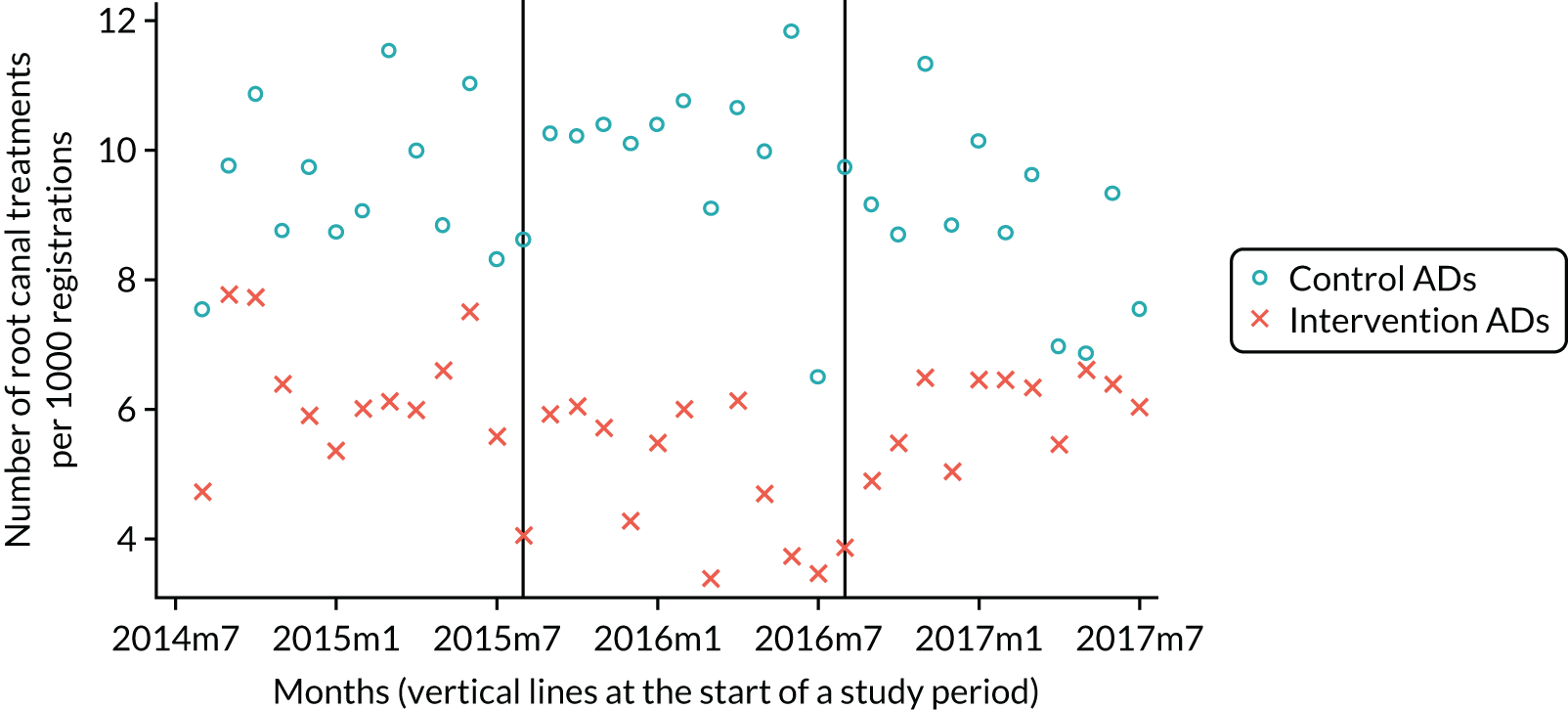
The ADs provided fewer treatment plans and undertook fewer root canal treatments as they moved from baseline FFS to capitation. Again, this trend reversed for ADs as they moved back to FFS (Table 40; Figures 40 and 41).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –10.07 | 6.66 | 1.51 | 0.14 |
| Capitation to reversion FFS | 8.44 | 5.18 | 1.63 | 0.11 | |
| Baseline FFS to reversion FFS | –1.63 | 7.48 | 0.22 | 0.83 | |
| ADs | Baseline FFS to capitation | –39.10 | 12.11 | 3.23 | < 0.01 |
| Capitation to reversion FFS | 32.33 | 9.53 | 3.39 | < 0.01 | |
| Baseline FFS to reversion FFS | –6.77 | 10.34 | 0.65 | 0.52 |
FIGURE 40.
Number of treatment plans (PPs).
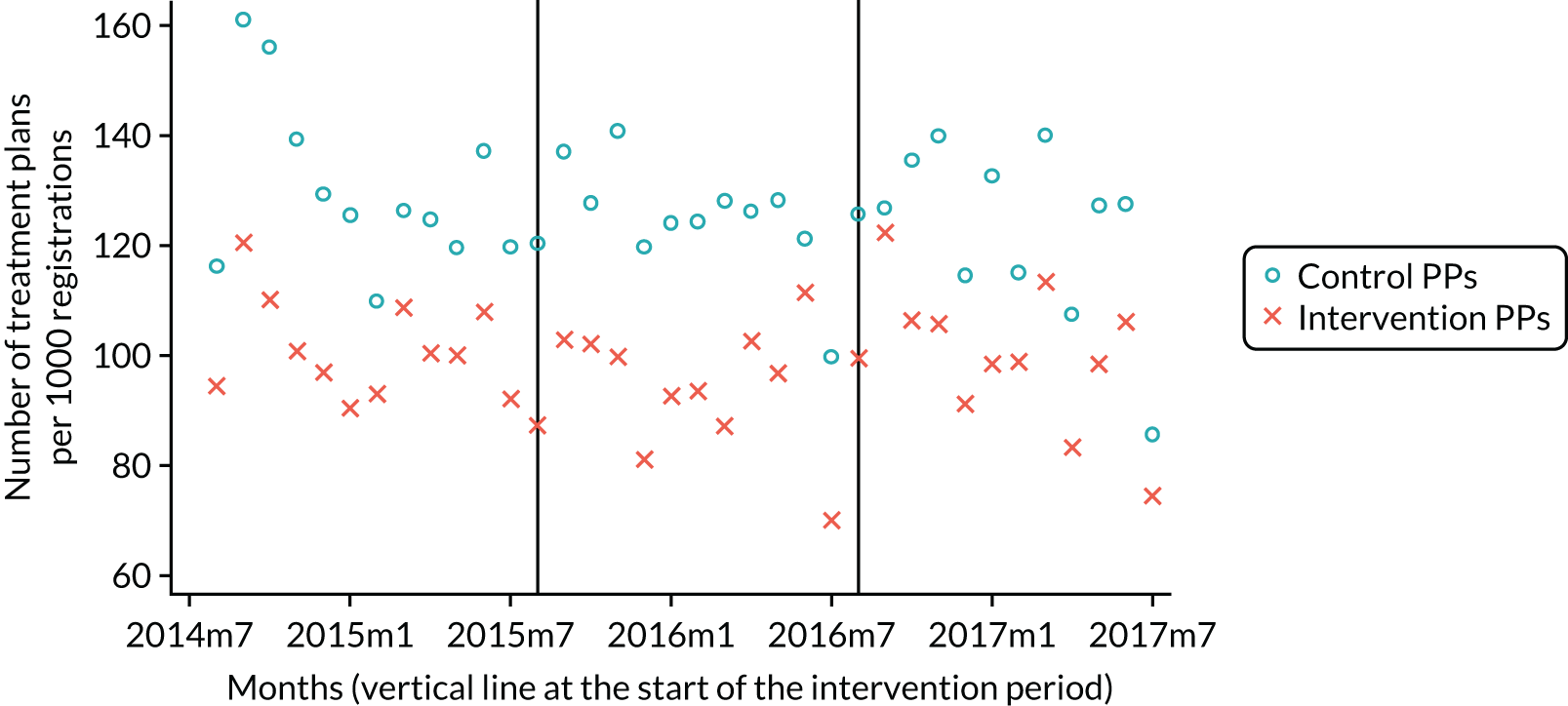
FIGURE 41.
Number of treatment plans (ADs).
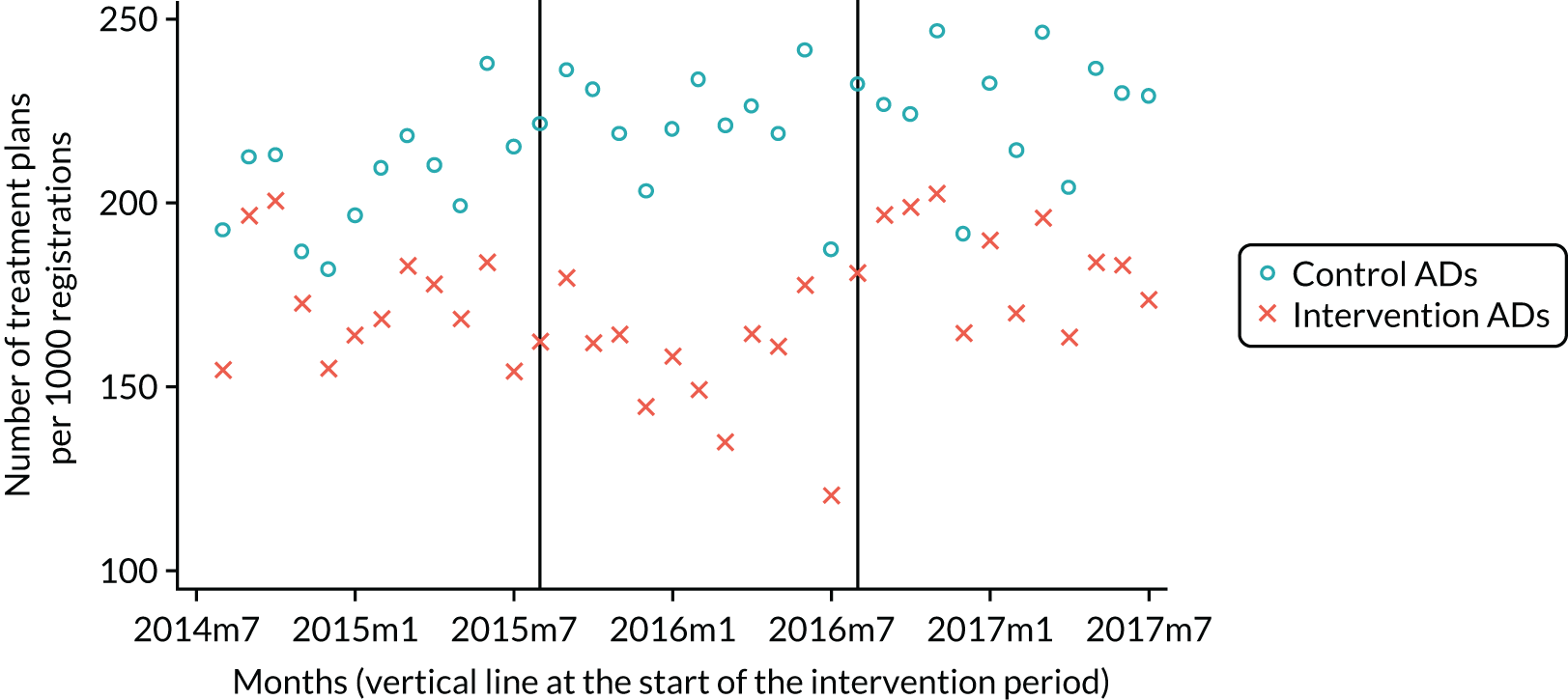
Both PPs and ADs provided fewer treatment items per plan as they moved from baseline FFS to capitation. Again, this trend reversed for both PPs and ADs as they moved back to FFS (Table 41; Figures 42 and 43).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –70.41 | 25.87 | 2.72 | 0.01 |
| Capitation to reversion FFS | 47.02 | 18.71 | 2.51 | 0.02 | |
| Baseline FFS to reversion FFS | –23.46 | 18.13 | 1.29 | 0.21 | |
| ADs | Baseline FFS to capitation | –174.51 | 48.31 | 3.61 | < 0.01 |
| Capitation to reversion FFS | 179.01 | 43.72 | 4.09 | < 0.01 | |
| Baseline FFS to reversion FFS | 4.50 | 38.26 | 0.12 | 0.91 |
FIGURE 42.
Number of treatment items (PPs).
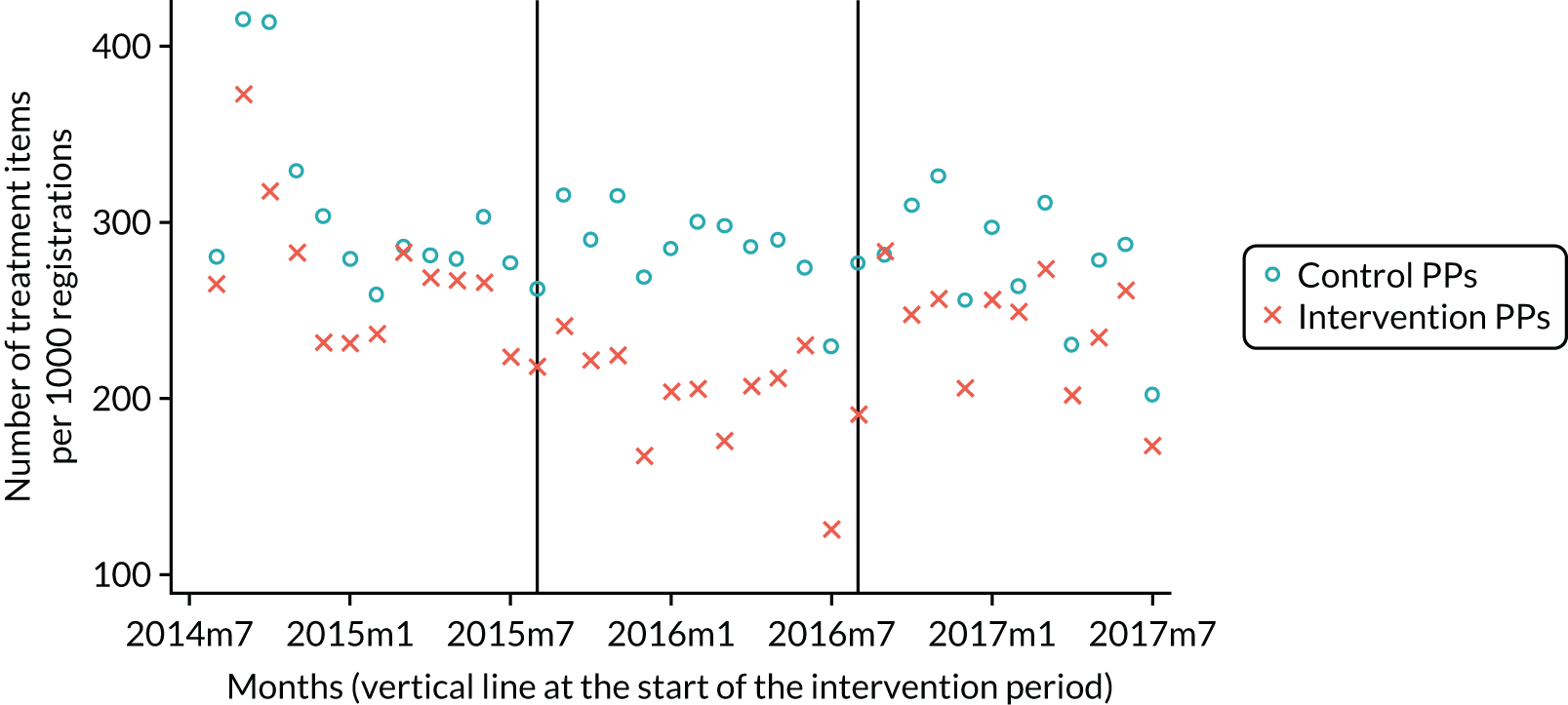
FIGURE 43.
Number of treatment items (ADs).
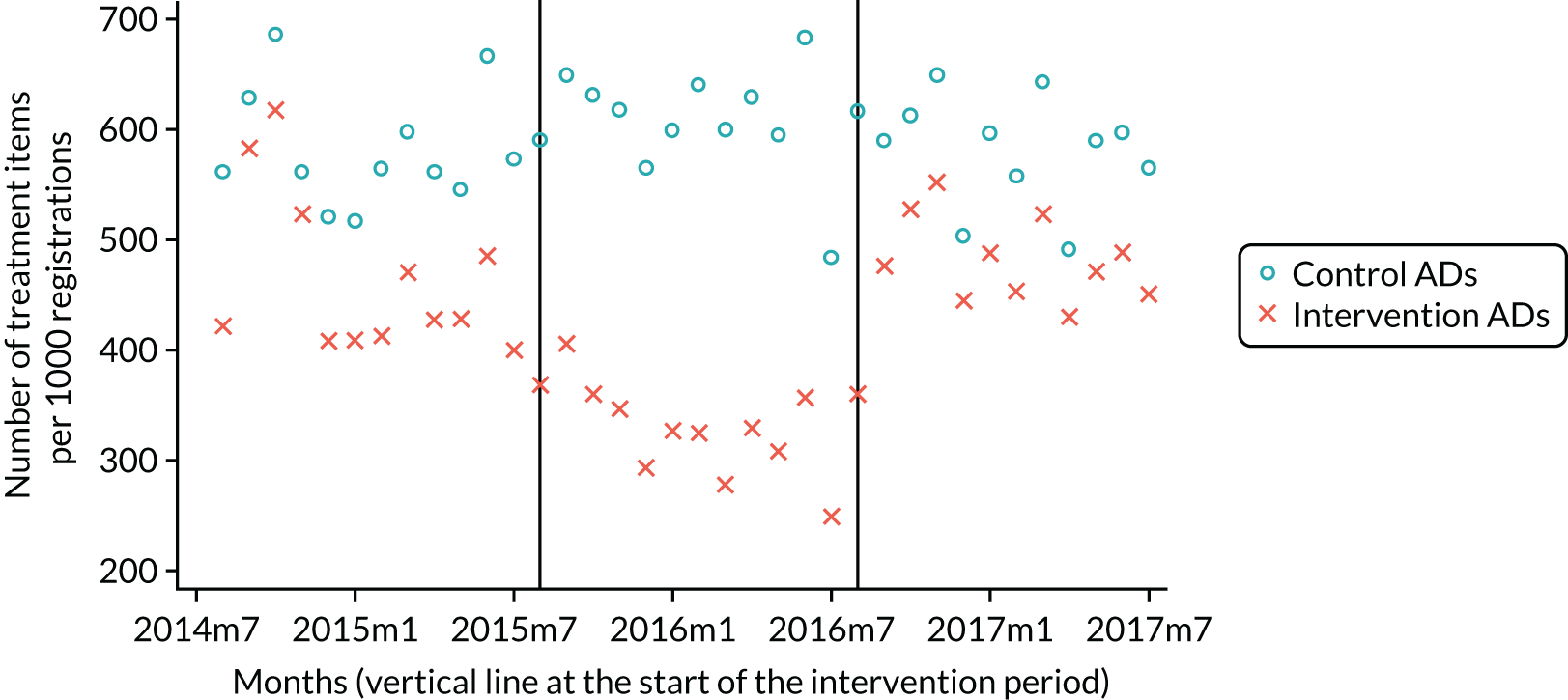
No significant difference was found for either PPs or ADs in relation to the proportion of patient fee contribution (Table 42; Figures 44 and 45).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | 2.09 | 1.59 | 1.32 | 0.20 |
| Capitation to reversion FFS | –1.97 | 1.12 | 1.77 | 0.09 | |
| Baseline FFS to reversion FFS | 0.12 | 1.54 | 0.08 | 0.94 | |
| ADs | Baseline FFS to capitation | –0.76 | 1.46 | 0.52 | 0.61 |
| Capitation to reversion FFS | –0.43 | 1.32 | 0.33 | 0.75 | |
| Baseline FFS to reversion FFS | –1.19 | 1.25 | 0.95 | 0.35 |
FIGURE 44.
Percentage of patient fee contribution (PPs).

FIGURE 45.
Percentage of patient fee contribution (ADs).
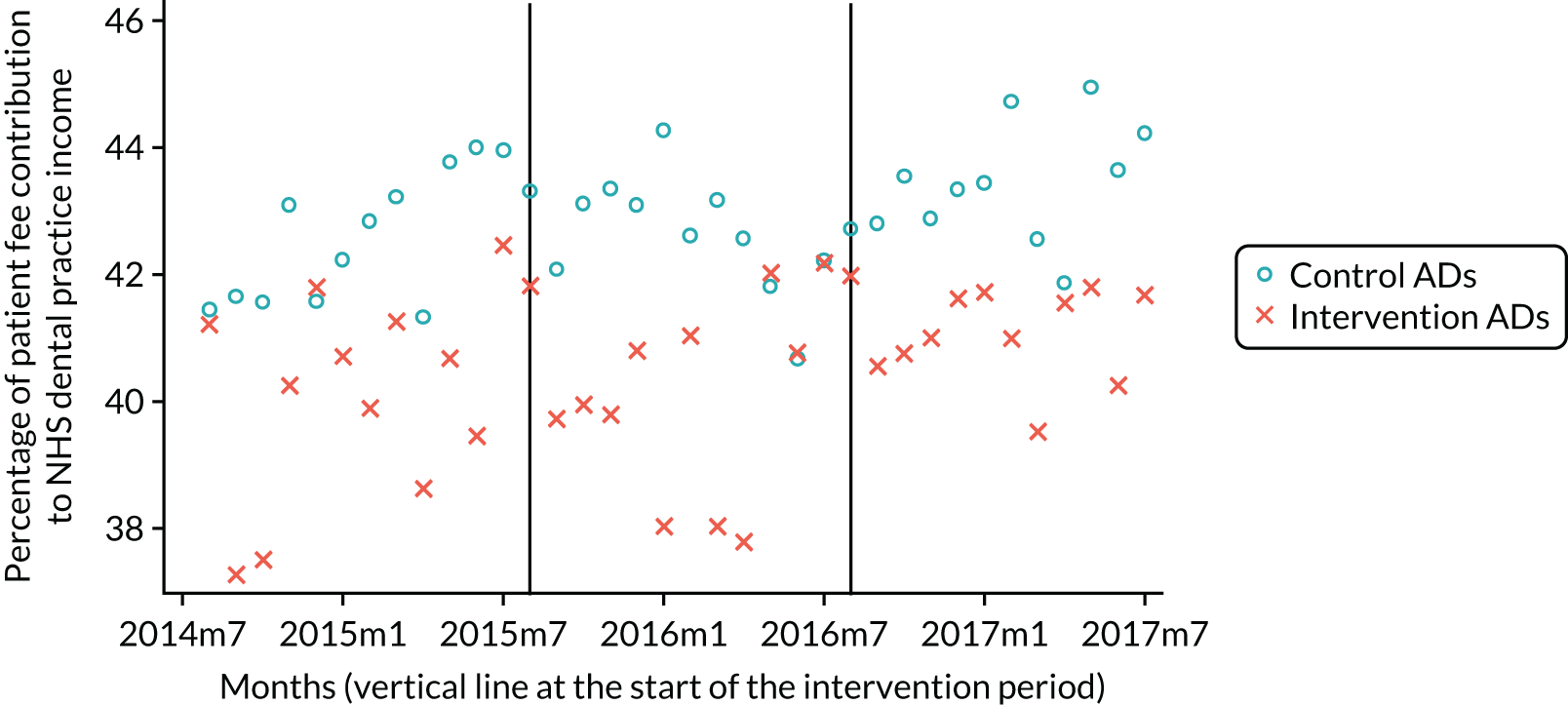
Both PPs and ADs dental practice income fell as they moved from baseline FFS to capitation. Again, this trend reversed for both PPs and ADs as they moved back to FFS (Table 43; Figures 46 and 47).
| Clinical provider | DiD model | DiD coefficient | SE | t-statistic | p-value |
|---|---|---|---|---|---|
| PPs | Baseline FFS to capitation | –1490 | 376 | 3.96 | < 0.01 |
| Capitation to reversion FFS | 706 | 351 | 2.01 | 0.05 | |
| Baseline FFS to reversion FFS | –785 | 390 | 2.01 | 0.05 | |
| ADs | Baseline FFS to capitation | –6566 | 1839 | 3.57 | < 0.01 |
| Capitation to reversion FFS | 5941 | 2012 | 2.95 | 0.01 | |
| Baseline FFS to reversion FFS | –624 | 1768 | 0.35 | 0.73 |
FIGURE 46.
Mean dental practice income per month (PPs).

FIGURE 47.
Mean dental practice income per month (ADs).
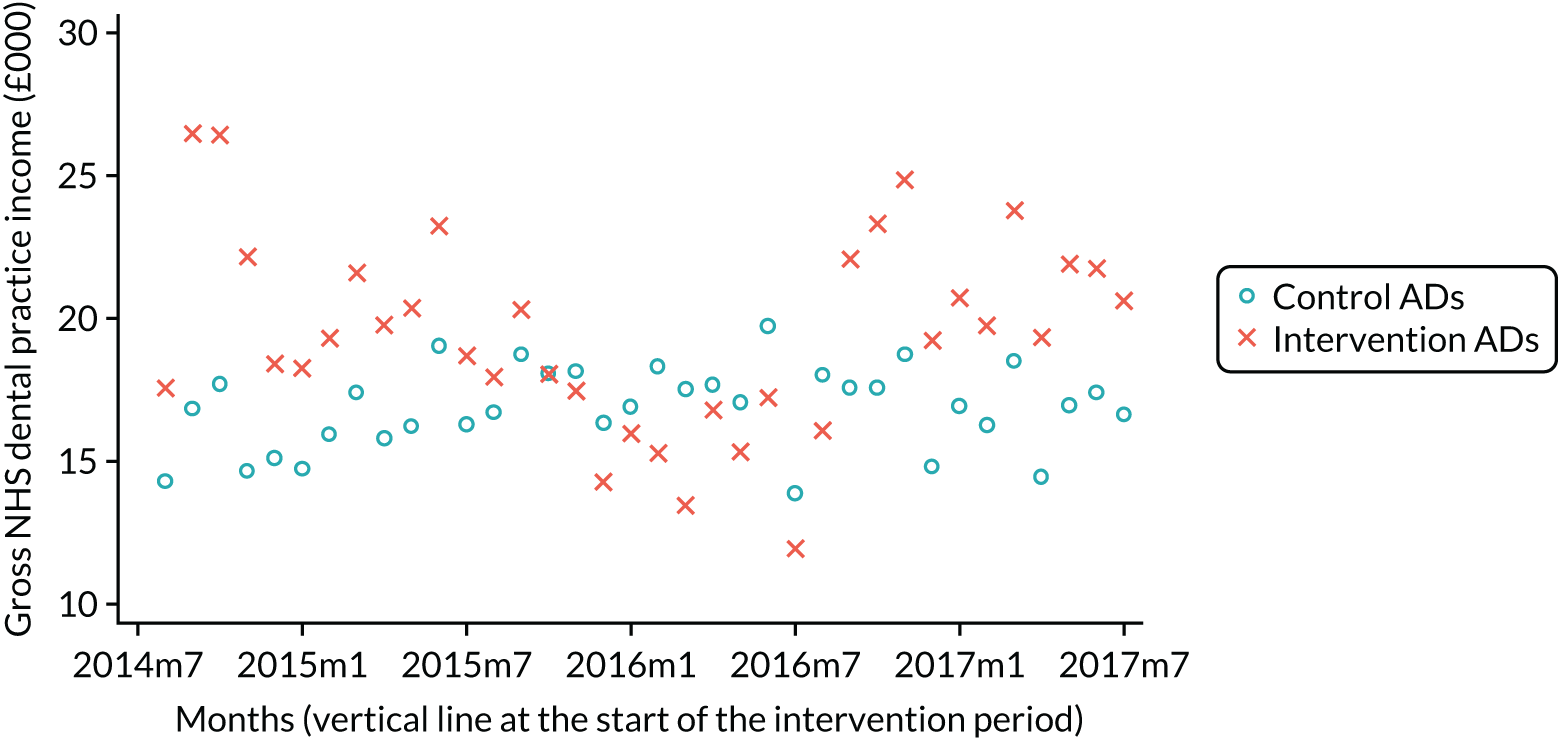
Appendix 5 Per-protocol data envelopment analysis and stochastic frontier modelling analyses
Introduction
We aimed to estimate the level of technical efficiency of practices and the change in technical efficiency associated with the change in the provider remuneration method. The rationale for producing these estimations was to provide decision-makers with important information to inform commissioning decisions about provider remuneration. Specifically, the study results could be used to set performance targets for a capitation system, for example if we find that, without any reduction in quality of care, practices that move to a capitation system typically see 15% fewer patients (with the same staffing level and inputs). In addition, identifying the operating practices associated with the highest levels of technical efficiency in each remuneration method and those that see the greatest improvement in technical efficiency from the change in FFS to capitation remuneration could be used to establish a guide to ‘best practice’ for others to emulate.
Data envelopment analysis methodology
Technical efficiency determines the maximum output possible from the available resources (labour, capital and consumables). Data envelopment analysis identifies the prevailing level of outputs that can be produced by a given level of inputs and so determines which substitutive model lies closest to the production possibilities frontier. 85 Data envelopment analysis is a non-parametric technique that uses a linear-segmented efficiency frontier based on best practice and a linear programming methodology. 86 It requires few assumptions to be satisfied and is considered to be a highly flexible approach that has been used in a range of pragmatic health applications. 85 Unlike parametric techniques, DEA can determine the relative efficiency of different models and can be undertaken without explicitly specifying the formal relations between inputs and outputs a priori. 85
Efficiency in DEA is defined as the ratio of the weighted sum of outputs to its weighted sum of inputs. 86 The weights are specific to each unit so that 0 ≤ model 0 ≤ 1 and a value of unity implies complete technical efficiency relative to the other models under scrutiny. Because the weights are not known a priori, they are calculated from the efficiency frontier by comparing one model with another. 86 Data envelopment analysis computes all possible sets of weights that satisfy all constraints and produces the highest efficiency score. This will be stated as a mathematical linear programming problem by constraining the numerator (output) of the efficiency ratio to be equal to 1 and minimising the weighted input. 86 The model then computes the factor Z needed to reduce the input of model 0 to a frontier formed by the remaining models and will be efficient if Z equals 1. This composite unit provides targets for the inefficient unit and Z represents the maximum inputs in a service specification that maintains current output. 86 The approach is non-parametric because the location and shape of the efficiency frontier is anchored to observations rather than estimated from parameters, such as the level of inputs practices have used. The distance of a practice unit from the frontier is a measure of technical inefficiency.
The output of each practice was measured by the average number of treatments delivered during each study period (1 year). Data envelopment analysis requires the selection of the ‘orientation’ to be adopted for estimating technical efficiency scores. An input-orientated model estimates the amount by which inputs can be proportionally reduced, without loss of outputs. Output orientation is an estimation of the amount by which outputs can be proportionally expanded, without additional inputs. Selection of orientation depends on the precise research question. In this analysis, a separate DEA model was estimated for each type of output and both types of orientation. All DEA models were estimated to allow for variable returns to scale in the production of health care.
Stochastic frontier modelling methodology
A limitation of DEA is that it assumes that if an NHS dental practice can produce a certain level of output using specific input levels, another NHS dental practice of equal scale should be capable of doing the same. This might not be the case if, for example, the frontier of most efficient practices is reached because of unique local contextual circumstances (e.g. a practice has established itself as being able to employ or refer to one of a limited number of specialist dental practices) or because of measurement error in the values of input or output variables. Stochastic frontier modelling helps overcome this limitation by estimating the frontier with statistical regression methods rather than fixing the frontier from extreme point observations.
Stochastic frontier modelling was used to compute the technical efficiency scores of each pilot practice present in the baseline and capitation study phases (n = 8). t-tests comparing group means were used to evaluate if there was a statistically significant change in pilot practices’ efficiency scores from one study period to another. A Cobb–Douglas function form was used for the SFM after a more flexible functional form (translog) was rejected in a joint chi-squared test of the coefficients on the additional translog variables. The input variables were the logarithm of the number of surgeries and staff time worked (weekly number of completed dentist sessions with NHS patients, weekly number of completed dental care professional sessions with NHS). The output of the practice was the logarithm of the average number of treatments delivered during a study phase (1 year) and a separate SFM model was estimated for each type of output.
Results
The results of the DEA and SFM analyses are presented in Tables 44–46.
| Description | Appointments with GDPs | Appointments with DCPs | Number of surgeries |
|---|---|---|---|
|
Mean in baseline FFS period (SD) Number of observations |
228.0 (152) 8 |
31.2 (77) 8 |
3.7 (2.5) 8 |
|
Mean in capitation period (SD) Number of observations |
262.8 (140) 11 |
7.9 (78) 11 |
3.7 (2.5) 11 |
|
Mean in reversion FFS period (SD) Number of observations |
313.9 (186) 11 |
49.2 (97) 11 |
3.7 (2.5) 11 |
|
Mean difference: baseline to capitation (95% CI) p-value of difference Number of observations |
5.37 (–20.0 to 30.7) 0.63 8 |
5.4 (–6.8 to 17.7) 0.33 8 |
0 – 8 |
|
Mean difference: capitation to reversion FFS (95% CI) p-value of difference Number of observations |
–34.1 (–98.9 to 30.7) 0.26 11 |
–7.4 (–18.5 to 3.6) 0.16 11 |
0 – 11 |
|
Mean difference: baseline FFS and reversion FFS (95% CI) p-value of difference Number of observations |
–53.3 (–126.4 to 19.8) 0.12 8 |
0 – 8 |
0 – 8 |
| Output measure | Input orientation | Output orientation | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean difference (p-value) | Mean (SD) | Mean difference (p-value) | |||
| Baseline | Capitation | Baseline | Capitation | |||
| Examinations | 0.92 (0.14) | 0.94 (0.08) | 0.02 (0.74) | 0.88 (0.14) | 0.93 (0.10) | 0.05 (0.45) |
| Direct restorations | 0.95 (0.10) | 0.92 (0.16) | –0.03 (0.63) | 0.84 (0.21) | 0.80 (0.25) | –0.04 (0.66) |
| Indirect restorations | 0.91 (0.20) | 0.91 (0.19) | 0.0004 (0.93) | 0.89 (0.39) | 0.88 (0.38) | –0.01 (0.26) |
| Extractions | 0.96 (0.08) | 0.97 (0.07) | 0.004 (0.23) | 0.91 (0.19) | 0.91 (0.18) | 0.003 (0.50) |
| Radiographs | 0.90 (0.18) | 0.91 (0.17) | 0.005 (0.26) | 0.83 (0.24) | 0.82 (0.24) | –0.006 (0.39) |
| Fissure sealants | 0.88 (0.18) | 0.88 (0.18) | 0.002 (0.33) | 0.82 (0.41) | 0.81 (0.37) | –0.02 (0.22) |
| Periodontal | 0.82 (0.26) | 0.82 (0.26) | 0.001 (0.86) | 0.72 (0.26) | 0.71 (0.26) | –0.006 (0.51) |
| Root canals | 0.82 (0.26) | 0.82 (0.26) | 0.00003 (0.99) | 0.76 (0.26) | 0.76 (0.27) | –0.008 (0.12) |
| Cost of ≥ £280 | 0.69 (0.34) | 0.69 (0.34) | 0.003 (0.49) | 0.59 (0.58) | 0.62 (0.61) | 0.03 (0.16) |
| All treatments | 0.94 (0.11) | 0.95 (0.10) | 0.006 (0.26) | 0.91 (0.14) | 0.91 (0.13) | –0.003 (0.92) |
| Output measure | Efficiency scores | ||
|---|---|---|---|
| Mean (SD) | Mean difference (p-value) | ||
| Baseline | Capitation | ||
| Examinations | 0.99 (0.00004) | 0.88 (0.036) | –0.12 (< 0.001) |
| Direct restorations | 0.81 (0.163) | 0.78 (0.279) | –0.027 (0.70) |
| Indirect restorations | 0.61 (0.280) | 0.56 (0.326) | –0.047 (0.38) |
| Extractions | 0.998 (0.00001) | 0.996 (0.00004) | –0.003 (< 0.001) |
| Radiographs | 0.76 (0.200) | 0.88 (0.029) | 0.118 (0.11) |
| Fissure sealants | 0.997 (0.00002) | 0.993 (0.00006) | 0.004 (< 0.001) |
| Periodontal | 0.69 (0.326) | 0.62 (0.209) | –0.075 (0.24) |
| Root canals | 0.66 (0.242) | 0.60 (0.317) | –0.060 (0.165) |
| Cost of ≥ £280 | 0.989 (p < 0.001) | 0.989 (0.0001) | 0.0004 (< 0.001) |
| All treatments | 0.99 (0.00004) | 0.96 (0.005) | –0.041 (< 0.001) |
Discussion
Technical efficiency is a comparative measure of how well a decision-making unit deploys inputs to produce outputs, as opposed to the production possibility frontier, which is the maximum output that can be achieved for a particular level and mix of inputs. As the DEA efficiency frontier is based on best observable practice, it is only an approximation of the true unobserved efficiency frontier. In other words, it can tell how efficient a practice is compared with its peers but not compared with a theoretical maximum. The advantage of DEA is that it can accommodate the multiple inputs and multiple outputs of dental health-care production more easily than regression analysis, as non-biased estimation requires specific distributional forms (usually normally distributed) and there is the potential for correlation-based problems such as multicollinearity.
The results of the DEA found no statistically significant difference between the amount of surgery time that was allocated to GDPs or dental care professionals between FFS at baseline and capitation, capitation and reversion to FFS and baseline FFS and reversion FFS (see Tables 45 and 46). This largely relates to the fact that DEA constructs a relative technical efficiency frontier and so requires a number of data points to do this. With such a small number of observations, the relative difference across these is minimised; however, the SFM did show a statistically significant reduction in technical efficiency in examinations, extractions and fissure sealants, treatments that cost ≥ £280 and across all treatments. This suggests that, overall, practices were less efficient during the capitation period.
These results do have to be viewed with some caution because of the small number of observations in the DEA model and the limitations associated with the SFM approach. In SFM, efficiency scores (as measured by regression residuals) reflect a combination of relative efficiency, measurement error in the dependent variable, and statistical noise. Moreover, it does not calculate a productive frontier that corresponds to the theoretical notion of a production function but, instead, estimates a fitted ‘average’ function that provides no direct quantitative information on productive inefficiency in the sample.
Appendix 6 Per-question analysis of the questionnaire
No statistical significance was found between the pilot and control practices in questions 1–4b at any time period (Tables 47 and 48).
| Practice group, study period | Question 1 | Question 2 | Question 3 | Question 4a | Question 4b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Intervention: baseline FFS to capitation | 1.39 | 1.32 to 1.45 | 2.24 | 2.13 to 2.35 | 0.96 | 0.94 to 0.98 | 0.57 | 0.52 to 0.62 | 0.24 | 0.19 to 0.28 |
| Intervention: capitation to reversion FFS | 1.26 | 1.20 to 1.33 | 2.32 | 2.20 to 2.44 | 0.95 | 0.92 to 0.97 | 0.58 | 0.53 to 0.64 | 0.21 | 0.16 to 0.25 |
| Intervention: baseline FFS to reversion FFS | 1.13 | 1.10 to 1.17 | 1.95 | 1.86 to 2.04 | 0.96 | 0.95 to 0.98 | 0.62 | 0.58 to 0.66 | 0.25 | 0.22 to 0.29 |
| Control: baseline FFS to capitation | 1.39 | 1.32 to 1.45 | 2.25 | 2.14 to 2.36 | 0.97 | 1.01 to 1.05 | 0.59 | 0.54 to 0.65 | 0.29 | 0.24 to 0.35 |
| Control: capitation to reversion FFS | 1.27 | 1.21 to 1.32 | 2.38 | 2.28 to 2.49 | 0.95 | 1.02 to 1.07 | 0.63 | 0.57 to 0.68 | 0.30 | 0.25 to 0.35 |
| Control: baseline FFS to reversion FFS | 1.20 | 1.17 to 1.24 | 2.21 | 2.12 to 2.30 | 0.97 | 0.96 to 0.98 | 0.61 | 0.57 to 0.65 | 0.30 | 0.26 to 0.34 |
| Practice group, study period | Question 1 | Question 2 | Question 3 | Question 4a | Question 4b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | 0.00 | 0.95 | 0.07 | 0.57 | 0.00 | 0.86 | 0.02 | 0.79 | 0.03 | 0.58 |
| Capitation to reversion FFS | 0.07 | 0.16 | 0.20 | 0.06 | 0.00 | 0.95 | –0.05 | 0.32 | –0.05 | 0.29 |
| Baseline FFS to reversion FFS | 0.03 | 0.20 | 0.13 | 0.01 | 0.00 | 0.78 | –0.02 | 0.44 | –0.01 | 0.67 |
The only statistically significant difference was found in question 5 ‘At your last health service routine check-up, did you have any X-rays taken?’ (Tables 49 and 50). This was found between baseline FFS to capitation and from capitation to reversion FFS.
| Practice group, study period | Question 4c | Question 4d | Question 4e | Question 4f | Question 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Intervention: baseline FFS to capitation | 0.05 | 0.03 to 0.08 | 0.06 | 0.03 to 0.08 | 0.017 | 0.003 to 0.031 | 0.17 | 0.13 to 0.21 | 0.26 | 0.21 to 0.30 |
| Intervention: capitation to reversion FFS | 0.03 | 0.01 to 0.05 | 0.05 | 0.02 to 0.07 | 0.010 | –0.001 to 0.020 | 0.19 | 0.15 to 0.23 | 0.31 | 0.26 to 0.36 |
| Intervention: baseline FFS to reversion FFS | 0.04 | 0.02 to 0.06 | 0.06 | 0.04 to 0.09 | 0.027 | 0.012 to 0.04 | 0.16 | 0.12 to 0.19 | 0.27 | 0.23 to 0.30 |
| Control: baseline FFS to capitation | 0.04 | 0.02 to 0.07 | 0.04 | 0.02 to 0.06 | 0.019 | 0.004 to 0.034 | 0.15 | 0.11 to 0.18 | 0.28 | 0.23 to 0.33 |
| Control: capitation to reversion FFS | 0.06 | 0.04 to 0.09 | 0.04 | 0.02 to 0.07 | 0.017 | 0.004 to 0.031 | 0.19 | 0.15 to 0.23 | 0.22 | 0.18 to 0.27 |
| Control: baseline FFS to reversion FFS | 0.05 | 0.03 to 0.06 | 0.06 | 0.04 to 0.08 | 0.018 | 0.007 to 0.03 | 0.15 | 0.12 to 0.18 | 0.28 | 0.24 to 0.31 |
| Practice group, study period | Question 4c | Question 4d | Question 4e | Question 4f | Question 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | 0.03 | 0.16 | 0.01 | 0.80 | 0.00 | 0.76 | 0.02 | 0.60 | –0.11 | 0.03 |
| Capitation to reversion FFS | –0.02 | 0.26 | 0.00 | 0.91 | –0.02 | 0.20 | 0.00 | 0.97 | 0.10 | 0.03 |
| Baseline FFS to reversion FFS | 0.00 | 0.68 | 0.00 | 0.99 | –0.01 | 0.35 | 0.01 | 0.45 | –0.01 | 0.80 |
No statistical significance was found between the pilot and control practices in questions 6a–e at any time period (Tables 51 and 52).
| Practice group, study period | Question 6a | Question 6b | Question 6c | Question 6d | Question 6e | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Intervention: baseline FFS to capitation | 0.31 | 0.26 to 0.36 | 0.06 | 0.04 to 0.09 | 0.05 | 0.03 to 0.08 | 0.09 | 0.06 to 0.12 | 0.07 | 0.04 to 0.10 |
| Intervention: capitation to reversion FFS | 0.31 | 0.26 to 0.36 | 0.09 | 0.05 to 0.12 | 0.06 | 0.03 to 0.08 | 0.08 | 0.05 to 0.11 | 0.05 | 0.03 to 0.08 |
| Intevention: baseline FFS to reversion FFS | 0.29 | 0.26 to 0.33 | 0.08 | 0.05 to 0.10 | 0.03 | 0.02 to 0.05 | 0.06 | 0.04 to 0.08 | 0.03 | 0.02 to 0.05 |
| Control: baseline FFS to capitation | 0.35 | 0.40 to 0.40 | 0.06 | 0.03 to 0.09 | 0.04 | 0.02 to 0.06 | 0.07 | 0.04 to 0.09 | 0.05 | 0.03 to 0.07 |
| Control: capitation to reversion FFS | 0.32 | 0.27 to 0.37 | 0.04 | 0.02 to 0.07 | 0.03 | 0.01 to 0.05 | 0.08 | 0.05 to 0.11 | 0.03 | 0.01 to 0.05 |
| Control: baseline FFS to reversion FFS | 0.30 | 0.26 to 0.34 | 0.06 | 0.04 to 0.08 | 0.04 | 0.03 to 0.06 | 0.05 | 0.03 to 0.06 | 0.05 | 0.03 to 0.07 |
| Practice group, study period | Question 6a | Question 6b | Question 6c | Question 6d | Question 6e | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | –0.03 | 0.59 | –0.05 | 0.09 | –0.01 | 0.54 | 0.02 | 0.52 | 0.00 | 0.85 |
| Capitation to reversion FFS | –0.01 | 0.78 | 0.03 | 0.31 | 0.04 | 0.07 | –0.01 | 0.67 | 0.04 | 0.07 |
| Baseline FFS to reversion FFS | –0.02 | 0.39 | –0.01 | 0.43 | 0.01 | 0.33 | 0.01 | 0.71 | 0.02 | 0.14 |
The only statistically significant difference was found in question 7 ‘As well as seeing the dentist, were you treated by either of the following staff? (dental nurse, dental hygienist)’ (Tables 53 and 54). This was found between baseline FFS to reversion FFS.
| Practice group, study period | Question 7 | Question 8 | Question 9 | Question 10 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Intervention: baseline FFS to capitation | 0.13 | 0.10 to 0.17 | 0.60 | 0.55 to 0.65 | 0.09 | 0.06 to 0.12 | 4.54 | 4.47 to 4.62 |
| Intervention: capitation to reversion FFS | 0.13 | 0.10 to 0.17 | 0.62 | 0.56 to 0.67 | 0.10 | 0.07 to 0.14 | 4.50 | 4.41 to 4.59 |
| Intervention: baseline FFS to reversion FFS | 0.12 | 0.09 to 0.15 | 0.56 | 0.52 to 0.61 | 0.08 | 0.05 to 0.01 | 4.59 | 4.52 to 4.65 |
| Control: baseline FFS to capitation | 0.06 | 0.03 to 0.09 | 0.54 | 0.48 to 0.59 | 0.07 | 0.04 to 0.10 | 4.65 | 4.58 to 4.72 |
| Control: capitation to reversion FFS | 0.09 | 0.06 to 0.12 | 0.54 | 0.49 to 0.60 | 0.07 | 0.04 to 0.09 | 4.64 | 4.57 to 4.71 |
| Control: baseline FFS to reversion FFS | 0.11 | 0.08 to 0.13 | 0.53 | 0.49 to 0.57 | 0.05 | 0.03 to 0.07 | 4.75 | 4.70 to 4.79 |
| Practice group, study period | Question 7 | Question 8 | Question 9 | Question 10 | ||||
|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | 0.03 | 0.42 | 0.02 | 0.71 | –0.02 | 0.57 | 0.038 | 0.63 |
| Capitation to reversion FFS | 0.03 | 0.31 | 0.04 | 0.32 | 0.01 | 0.60 | 0.009 | 0.90 |
| Baseline FFS to reversion FFS | 0.03 | 0.04 | 0.03 | 0.15 | 0.00 | 0.87 | 0.023 | 0.48 |
No statistical significance was found between the pilot and control practices in questions 11–15 at any time period (Tables 55 and 56).
| Practice group, study period | Question 11 | Question 12 | Question 13 | Question 14 | Question 15 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Intervention: baseline FFS to capitation | 4.60 | 4.53 to 4.67 | 4.53 | 4.46 to 4.60 | 0.09 | 0.06 to 0.12 | 0.02 | 0.00 to 0.03 | 3.87 | 3.80 to 3.94 |
| Intervention: capitation to reversion FFS | 4.60 | 4.52 to 4.68 | 4.49 | 1.43 to 1.59 | 0.10 | 0.07 to 0.14 | 0.02 | 0.00 to 0.04 | 3.90 | 3.82 to 3.98 |
| Intervention: baseline FFS to reversion FFS | 4.69 | 4.63 to 4.75 | 4.61 | 4.55 to 4.67 | 0.10 | 0.07 to 0.12 | 0.01 | 0.00 to 0.02 | 3.91 | 3.86 to 3.97 |
| Control: baseline FFS to capitation | 4.71 | 4.63 to 4.77 | 4.62 | 4.55 to 4.70 | 0.09 | 0.05 to 0.12 | 0.01 | –0.00 to 0.02 | 3.85 | 3.78 to 3.92 |
| Control: capitation to reversion FFS | 4.67 | 4.60 to 4.74 | 4.59 | 4.52 to 4.67 | 0.09 | 0.06 to 0.12 | 0.002 | –0.00 to 0.01 | 3.89 | 3.82 to 3.96 |
| Control: baseline FFS to reversion FFS | 4.74 | 4.69 to 4.79 | 4.67 | 4.62 to 4.73 | 0.11 | 0.09 to 0.14 | 0.02 | 0.01 to 0.03 | 3.89 | 3.84 to 3.95 |
| Practice group, study period | Question 11 | Question 12 | Question 13 | Question 14 | Question 15 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | –0.04 | 0.60 | 0.01 | 0.88 | –0.00 | 0.96 | –0.01 | 0.45 | 0.03 | 0.71 |
| Capitation to reversion FFS | –0.02 | 0.77 | –0.04 | 0.51 | 0.03 | 0.35 | 0.02 | 0.06 | 0.01 | 0.92 |
| Baseline FFS to reversion FFS | –0.03 | 0.36 | –0.02 | 0.58 | 0.01 | 0.43 | 0.01 | 0.31 | 0.02 | 0.60 |
No statistical significance was found between the pilot and control practices in questions 16–20 at any time period (Tables 57 and 58).
| Practice group, study period | Question 16 | Question 17 | Question 18 | Question 19 | Question 20 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Intervention: baseline FFS to capitation | 2.36 | 2.20 to 2.52 | 2.67 | 2.58 to 2.76 | 0.93 | 0.90 to 0.96 | 0.89 | 0.85 to 0.92 | 0.29 | 0.24 to 0.34 |
| Intervention: capitation to reversion FFS | 2.32 | 2.16 to 2.48 | 2.76 | 2.67 to 2.86 | 0.95 | 0.92 to 0.97 | 0.89 | 0.86 to 0.93 | 0.27 | 0.22 to 0.32 |
| Intervention: baseline FFS to reversion FFS | 2.40 | 2.26 to 2.53 | 2.77 | 2.69 to 2.84 | 0.94 | 0.92 to 0.96 | 0.92 | 0.90 to 0.95 | 0.22 | 0.18 to 0.26 |
| Control: baseline FFS to capitation | 2.28 | 2.12 to 2.44 | 2.71 | 2.61 to 2.81 | 0.96 | 0.94 to 0.98 | 0.90 | 0.86 to 0.93 | 0.24 | 0.19 to 0.29 |
| Control: capitation to reversion FFS | 2.28 | 2.12 to 2.43 | 2.79 | 2.69 to 2.88 | 0.95 | 0.92 to 0.97 | 0.89 | 0.86 to 0.92 | 0.26 | 0.21 to 0.31 |
| Control: baseline FFS to reversion FFS | 2.39 | 2.26 to 2.51 | 2.80 | 2.72 to 2.87 | 0.93 | 0.90 to 0.95 | 0.90 | 0.87 to 0.93 | 0.22 | 0.19 to 0.26 |
| Practice group, study period | Question 16 | Question 17 | Question 18 | Question 19 | Question 20 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | DiD coefficient | p-value | |
| Baseline FFS to capitation | 0.06 | 0.71 | –0.03 | 0.72 | –0.03 | 0.28 | –0.01 | 0.77 | –0.03 | 0.56 |
| Capitation to reversion FFS | 0.05 | 0.74 | 0.02 | 0.77 | –0.02 | 0.42 | –0.02 | 0.45 | 0.02 | 0.66 |
| Baseline FFS to reversion FFS | 0.05 | 0.50 | 0.00 | 0.93 | –0.02 | 0.06 | –0.02 | 0.27 | 0.03 | 0.25 |
List of abbreviations
- ACV
- annual contract value
- AD
- associate dentist
- BSO
- Business Services Organisation
- CI
- confidence interval
- DEA
- data envelopment analysis
- DHSSPS
- Department of Health, Social Services and Public Safety
- DiD
- difference in difference
- DQOF
- Dental Quality Outcomes Framework
- FFS
- fee for service
- GDP
- general dental practitioner
- GDS
- general dental services
- IT
- information technology
- ITS
- interrupted time series
- KPI
- key performance indicator
- NIHR
- National Institute for Health Research
- NIHSCB
- Northern Ireland Health and Social Care Board
- PCC
- Patient and Client Council
- PCR
- patient charge revenue
- PCT
- primary care trust
- PDS
- personal dental services
- PP
- practice principal
- pPDS
- pilot personal dental services
- PPI
- patient and public involvement
- PS
- propensity score
- SE
- standard error
- SFM
- stochastic frontier modelling
- UDA
- unit of dental activity
