Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/209/27. The contractual start date was in July 2014. The final report began editorial review in April 2018 and was accepted for publication in September 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Bryony Dean Franklin, Ann Blandford and Dominic Furniss report a joint grant from Cerner (North Kansas City, MO, USA). Bryony Dean Franklin reports a grant from the National Institute for Health Research outside the submitted work. Dominic Furniss reports personal fees from Becton, Dickinson and Company/CareFusion (Wokingham, UK) during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Blandford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
This chapter includes material reproduced or adapted from Blandford et al. 1 and Fahmy et al. 2 These articles are distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Infusion devices have been identified as an important source of errors, potentially compromising patient safety. 3 Smart pumps, in which the infusion device is integrated with information systems and drug libraries to set safe limits on medication administration,4 have been advocated as a key technology to block critical medication administration errors. Although such pumps have been widely implemented in the USA, take-up in England has been patchy and, even where smart pumps have been introduced, drug libraries may be only partially implemented. 5
There have been few studies of the role of infusion devices in ensuring safe medication administration practices, and most previous studies have taken place in the USA. 6,7 The overall aim of the study, referred to as ECLIPSE (Exploring the Current Landscape of Intravenous Infusion Practices and Errors), was to better understand current intravenous (IV) infusion practices in England and the possible role of smart pumps in managing patient safety.
Background
We open the background section by summarising what is already known about how infusion pumps are used across health care, the safety of IV medication administration and the role of smart pumps. We then briefly review the background to the analytical perspectives that we used (see Chapter 4). This is followed by an overview of broader perspectives that informed our data gathering and later analyses (namely human factors approaches, different perspectives on safety, and complexity science).
In this report, we use the terms ‘infusion devices’ and ‘pumps’ interchangeably to refer to both volumetric pumps (in which a bag of IV medication is hung on a drip stand and the rate of flow is controlled by the pump) and syringe drivers (in which the medication is prepared in a syringe, and the plunger is depressed at a controlled rate by the syringe driver to deliver the medication).
Intravenous medication practices
Infusion pumps are safety-critical devices in widespread use across many clinical areas: for pain management, delivery of antibiotics and chemotherapy, anaesthesia and fluid management. They are used in many clinical areas, from paediatrics to elderly care, in intensive care, in operating theatres and on general wards. Studies have found that infusion practices vary significantly both between and within hospitals. For example, in a series of observational studies, nurses in an oncology day-care unit were found to follow fairly basic procedures in setting up planned infusions,8 whereas nurses in an intensive care unit (ICU) routinely used advanced functionality, frequently setting up several pumps in parallel to deliver different medications. 9 There is an increasing drive towards standardising devices within organisations, intended to reduce the potential risks associated with staff using a range of different devices or devices configured in different ways, or being required to operate devices that they are not familiar with or have not been trained to use. However, not all clinical areas require the same functionality; for example, Carayon et al. 10 studied how nurses used infusion devices in different areas of the hospital. They compared the tasks actually carried out with the tasks as defined by ward protocol, identifying divergences in practice and highlighting ways in which those divergences increased overall system vulnerability.
Safety of intravenous medication administration
Intravenous medication is essential for many hospital inpatients. However, providing IV therapy is complex, and data suggest that errors are common. 11–13 In a systematic review of UK studies using structured observation of medication administration, errors were found to be five times more likely in IV than non-IV doses. 14 Internationally, published error rates vary from 18% to 173% of IV doses given, in studies using structured observation of medication administration. 15 An international systematic review estimated the probability of making at least one error in the preparation and administration of a dose of IV medication to be 0.73, with most errors occurring at the reconstitution and administration steps. 16 Although many of these errors do not result in patient harm, all can cause anxiety for patients and staff and can reduce patients’ confidence in their care. As a result, the administration of IV medication has been identified as a significant topic of concern by regulators, manufacturers and health-care providers. 3
The potential role of smart infusion pumps
To reduce errors associated with IV infusions, ‘smart pumps’ incorporating dose-error reduction software (DERS) have been widely advocated. 4,17,18 This software checks programmed infusion rates against pre-set limits for each drug and clinical location, using customisable ‘drug libraries’, to reduce the risk of infusion rates that are too high or too low. Limits may be ‘soft’ (in which case they can be over-ridden following confirmation by the clinician) or ‘hard’ (in which case they cannot). Smart pumps may be standalone or integrated with electronic prescribing and/or barcode administration systems, and usually allow administrative data, such as number and types of over-rides, to be downloaded for analysis. Although smart pumps were in use in 68% of US hospitals in 2011,19 a figure now likely to be much higher, their use is not yet as widespread in the UK. 5 Such technology can potentially identify and prevent some kinds of medication errors, but cannot prevent all possible errors. Smart pump use also comes at a cost, both financially and in terms of the changes needed to make their use effective. For instance, Husch et al. 6 carried out a hospital-wide point-prevalence study of errors in IV infusions using standard infusion pumps, and identified infusion rate errors in 37 cases (8% of all infusions) and wrong medication in 14 cases (3%). However, they estimated that only one of these errors would have been prevented using standalone smart pumps. More were judged to be potentially preventable if the pumps were integrated with other hospital systems, such as computerised prescriber order entry (CPOE) and barcode medication administration (BCMA). In a survey study involving 29 hospitals across Canada that either had implemented smart pumps or were in the process of doing so, Trbovich et al. 20 found that respondents did not take the steps necessary to realise the potential safety benefits of pumps; for example, they did not standardise drug concentrations, develop drug libraries, set dosing limits or monitor how pumps were used.
A recent systematic review identified 21 quantitative studies of smart pumps,21 the majority of which studied the over-rides recorded in the smart pump logs and/or used unreliable methods of identifying medication errors and adverse drug events, such as incident reports. The authors concluded that smart pumps can reduce but not eliminate error, and that the picture was far from conclusive. Furthermore, most studies were conducted in the USA; none was from the UK, where systems for prescribing and administering medication differ significantly from those in the USA. 22 For example, nurses play a more active role in preparing IV medication in the UK, and verbal orders are much less common. We therefore know little about the effect on patient safety of using smart pumps in general and nothing about their likely impact in the UK.
Assessing the severity of errors
As well as studying the prevalence of medication errors, their clinical importance must be taken into account when comparing drug distribution systems or assessing the effects of interventions. 23,24 Medication errors range from those with very serious consequences to those that have little or no effect on the patient. Assessing the clinical importance of errors therefore increases the clinical relevance of studies’ findings compared with studies based on prevalence alone. In many studies, actual error outcomes are not known, either because there is no longitudinal patient follow-up or because researchers intervene to prevent errors from causing patient harm. Methods of measuring the potential severity, or clinical importance, of medication errors are therefore needed to evaluate the effectiveness of interventions designed to reduce them.
A systematic review of methods for measuring the clinical importance of prescribing errors identified a wide range of available methods but no comparative studies. 25 No comprehensive review has focused on methods to assess clinical importance of medication administration errors. However, in studies included in a systematic review of the prevalence and nature of medication administration errors, Keers et al. 26 noted that the two most commonly cited severity assessment methods were the US National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) severity index,27 and Dean and Barber’s method. 28 The former is an ordinal scale designed to be used by local staff who would usually have knowledge of actual outcomes and other contextual information, and the latter is an interval scale used by experts using descriptions of the errors without knowledge of their outcomes. In Chapter 3, we describe a comparison of these two methods of assessment to shed further light on the strengths and weaknesses of each.
Systems of practice: intravenous medication administration documentation
Numerous policies and procedures have been put in place at every participating hospital with the aim of reducing the risk of harm during IV infusion administration, and our first analysis presented in Chapter 4 focuses on variations in policy and practice around IV infusion administration.
A 2007 Patient Safety Alert for England and Wales29 made recommendations to reduce errors in injectable medicines, including risk-assessing procedures and products, reviewing protocols, providing technical information and competency-based training, and conducting an annual medicines management audit. It highlighted how procedures should be:
Clearly documented, reflect local circumstances and describe safe practice that all practitioners can reasonably be expected to achieve.
p. 3. 29
Before the ECLIPSE study, to our knowledge, no study had investigated whether or how health-care organisations had responded to this advice or how well these procedures are adhered to in practice.
There has been limited research into procedural and documentation deviations of IV infusion administration. Husch et al. 6 included procedural and documentation errors in their study of 426 IV infusions in a US hospital; this study found two of the most prevalent error types to be no rate on the additive label, affecting 46% of infusions, and patient identification (ID) issues, affecting 13% of infusions. Schnock et al. 7 used a similar method across 10 US hospitals to examine 1164 IV infusions and reported 60% of infusions with an additive label that deviated from policy, 35% of infusions where giving sets were not labelled according to policy, and 0.2% of infusions where the patient had no ID wristband. No previous study has investigated procedural and documentation deviations in the UK and none has explored the surrounding context or possible reasons for the discrepancies identified.
Systems of practice: nursing
Parts of this section are based on Vos et al. 30 © 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CCBY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
The second analysis presented in Chapter 4 focuses on the role of nurses as a source of system resilience. Nurses undergo training, education and lifelong learning in order to provide safe and high-quality care. 31–33 In nursing education, problem-solving is emphasised and is often presented as a systematic process in which one moves from assessment to eventually evaluating the effectiveness of an intervention. 33,34 However, daily nursing practice is often more complex than this linear picture suggests. The planning and implementation of nursing interventions, such as administering IV therapy, requires clinical judgement, and thus an interpretation of the situation by the nurse. 33,34 As a result, actions demand standard approaches to be modified or new approaches to be improvised according to the patient’s response. 34 This flexibility and variability in clinical work can result in a gap between ‘work-as-imagined’ (policy) and ‘work-as-done’ (practice),35–37 but can be paramount to achieving safer practice. 38
In the context of IV therapy, non-compliance with policy is a deviation. However, not all deviations from policy lead to negative consequences for patient care. 39 Larcos et al. 39 have proposed looking at ‘resilience’ in health-care practice to recognise flexibilities that might contradict policy. Examining the safety of IV therapy requires a broader view that also includes focusing on what goes right. The safety of IV therapy might also be influenced by clinical judgement, professionalism and knowledge.
Nurses are a key player in the delivery of high-quality IV therapy to patients. 40 Their decisions and actions contribute to the safety of IV therapy. However, it is unclear how this is accounted for in practice when measuring deviations from policy. One of our analyses (presented in Chapter 4) investigated those situations in IV therapy in which nurses are a source of system resilience in relation to quality and safety of IV practice through their actions and clinical judgement.
Systems of practice: layers of influence
The final analysis presented in Chapter 4 focuses on system adaptability. 41 It draws inspiration from the work of Brand,42 who argues that what makes an artefact adaptable is a design that differentiates between constructional categories according to their likelihood of change, coupling together elements that have the same rate of change into layers that are loosely coupled to other layers that have different change rates. This allows fast-changing layers to ‘slip past’ more stable layers, minimising the scale of impact of change, which makes the artefact easier and less costly to change to accommodate needs. This view is relevant to many designed artefacts that require adaptation and change during their useful lifespan to accommodate new or changing use requirements. This style of thinking can be adapted to view and assess the relative impact of an intervention in one layer on the functioning and performance of the entire artefact or system. The lower (more stable) layers are harder to modify than layers that are higher up in the hierarchy. Changes at the lower layers are also likely to have a greater impact than those at higher ones. The analysis reported in Chapter 4 identifies the layers for IV infusion administration.
Human factors and human error
Building on prior work on human error and IV medication administration, the ECLIPSE study was strongly informed by a human factors perspective. This approach is based on:
-
understanding the factors that contribute to errors occurring
-
focusing on what really happens in practice, which is often different from what is believed to happen
-
taking a ‘systems’ view that puts the person at the centre.
There is an extensive literature on human error, much of it summarised by Reason. 43,44 In brief, Reason identifies two main types of error. The first is mistakes, which occur when people do not have all the information needed to choose an appropriate action, or fail to plan or solve a problem correctly; mistakes can often be reduced by improved training. The second type is slips and lapses, which occur when people intend to do the correct thing, but inadvertently do something else as a result of distraction, fatigue or similar; slips and lapses cannot be eliminated through training, but can often be reduced through the design of tools or systems. Reason points out that the obvious causes of errors often appear at the ‘sharp end’ in the localised actions of an individual (e.g. a nurse programming an infusion pump), but that there are typically many latent failures behind these active failures (e.g. shortage of staff, working practices, local culture). He also distinguishes between errors and violations, with the latter classified into three groups: routine violations (cutting corners), optimising violations (doing something for personal gain) and situational violations (necessary to get the task done – often called workarounds). He argues that different causes of errors and violations require different counter-measures to prevent them or mitigate their effects. Reason focuses on major incidents about which there is general agreement that something went wrong. However, this is not always the case; for example, Furniss et al. 8 document ‘unremarkable errors’: when things did not go entirely according to plan and yet there was no untoward outcome.
Karsh et al. 45 contrast various paradigms for improving patient safety, arguing that, historically, most approaches (such as that presented by Reason44) have focused on minimising harm (by imposing barriers to prevent errors or injuries), and that an alternative human factors perspective is needed. They frame this in terms of designing systems to improve performance, including minimising hazards, and argue that both perspectives (imposing barriers to minimise the chances of bad things happening while also engineering systems to optimise performance) are necessary to improve safety.
There is a growing recognition that formalised descriptions of practices focus on ‘work as imagined’ and often miss the nuances and factors that most influence safety in practice. Blandford et al. 35 discuss this in terms of ‘work as imagined’ (as defined in training or in policies) and ‘work as done’ (as observed in practice). Heeks46 described this as the ‘design–reality gap’, pointing out that systems that do not match their users’ needs provoke workarounds, inefficiencies and sometimes rejection.
Workarounds have been studied extensively in various areas of health care. For example, Koppel et al. 47 provide an account of workarounds in BCMA, identifying 15 categories of workarounds and 31 categories of reasons for people employing workarounds; although workarounds are typically used to get work done in a timely way, they risk compromising patient safety and highlight issues in the broader system of care (in this case, the way in which BCMA has been implemented). Similarly, Debono et al. 48 present a systematic review of workarounds reported in 58 studies relating to nurses’ workarounds in acute-care settings; they highlight the paradox that workarounds can often simultaneously enable and compromise patient care.
Looking beyond the individual interacting with a device or other technology, a human factors systems approach places the person (the user of technology), the equipment and the task within the physical environment and the organisational setting, and accounts for behaviours within that context. This is exemplified in the work of Carayon et al. ,49 who adopt these concepts as foundational for the Systems Engineering Initiative for Patient Safety (SEIPS) model for reasoning about patient safety. They argue that:
Human Factors Engineering interventions that do not consider issues across the whole system, including organisational factors, are unlikely to have significant, sustainable impact on patient safety and quality of care.
Carayon et al. 49
Carayon et al. present SEIPS as an approach for analysing a work context in terms of patient safety. We adopt this approach in one of the analyses reported in Chapter 7.
A complementary perspective that has also been widely applied for understanding health-care systems and health technology is that of distributed cognition. 50 One methodology for applying the theory of distributed cognition, Distributed Cognition for Teams (DiCoT),51 involves analysing a system in terms of physical structure, information flow, how artefacts support cognition, social structures and how a system has evolved over time. DiCoT has been applied to understanding infusion pump use in various settings including intensive care9 and an operating theatre. 52 This perspective informed our data collection in phase 2.
Safety I and Safety II
Traditionally, the literature on safety has focused on barriers to bad things happening (‘Safety I’). As summarised above, it has now been recognised that safety cannot be achieved only by creating barriers, but that performance must also be improved (‘Safety II’). Vincent and Amalberti53 propose a continuum of ways to keep a system safe, or optimising performance. At one end of the continuum they place ultra-adaptive performance, which embraces risk and relies on the skill of an individual; at the other end they place ultra-safe practice, which relies on standardised practices, checklists and policy, based on an expected standard of performance for each role, and limiting scope for individual excellence. Between these extremes they place high-reliability systems that invest trust in the team and manage risks by focusing on team performance and learning. Ultra-safe practices embody a Safety I culture, and high-reliability systems embody a Safety II culture. Although ultra-adaptive systems have a place in health care overall, they rarely play a substantial role in IV medication administration.
We aimed to incorporate a Safety II approach in interpreting our findings. This approach moves away from the traditional focus of classifying all deviations as errors, eliminating error and blaming the human for unreliable processing. Instead, it encourages one to think about deviations in terms of performance variability, how to understand and manage this variability, and that the human component can make positive contributions to safety. 38,53 Safety II maintains that mechanistic performance is inadequate because complex systems are open to surprises, often run in degraded states, and have competing goals and conflicting pressures. So, not all deviations are errors, humans can be a source of success and failure, and errors and discrepancies might point to system issues.
Safety II played an important role in the planning and conduct of our research: (1) it helped us understand that we were sampling everyday work and its consequences; (2) it encouraged us to take a more nuanced approach to any deviations from the medication ordered, classifying some as discrepancies rather than all being errors, as in previous research; (3) it has made us mindful of system resilience, such as where some deviations added to safety; and (4) it encouraged us to engage with local rationality and challenging trade-offs. Safety II advises that one should attend to where the system goes right as well as where it goes wrong. After all, often when safety is working well, it can appear that nothing is happening: there is the absence of incident. However, Safety II defines safety as the presence of something, something that is constantly created, so it makes sense to also look at practices that can potentially contribute to safety that might otherwise be overlooked.
Safety II recommends looking at ‘positive deviations’ in the system. However, ‘positive deviance’ is an established approach in health care that investigates excellence in performance. 54 This involves measuring performance and sampling those practices that are the highest performing. We did not take this approach. Instead, we learnt in earlier phases of our research that clearly defining and measuring IV infusion administration performance is challenging. Data from phases 1 and 2 pointed to something that was more variable and multilevelled than we had previously expected, with multiple contextual dependencies. Following this, therefore, we reviewed the literature on complex adaptive systems (CAS) to better understand how to interpret our findings in terms of complicated and complex systems.
Complexity science and complex adaptable systems
With hindsight, it is now evident that, in framing the ECLIPSE study, the perspective taken was of IV infusion administration as a relatively simple system, centred around the infusion pump. From that perspective, it made sense to ask simple questions around whether ‘smart pumps’ were safer than traditional pumps or gravity feed, and what best practice is in this area. However, our findings cannot readily be distilled into such simple questions or their answers. In the final months of the project, we therefore turned to richer theories that illuminate, and enable new interpretation of, our findings. In particular, prior research on complexity science and CAS provides promising avenues for future research and for interpretation of our existing data.
Complexity science is the science of systems that are difficult to define in simple or structured terms. 55 For example, Holland56 describes CAS as comprising a large number of agents that are diverse in both form (e.g. people and computer systems) and capability (e.g. multidisciplinary teams).
Plsek and Greenhalgh57 argue that behaviour emerges from such systems without being designed ‘top down’ through the local actions and interactions of agents within the system and that it is not generally possible to address problems in such systems by reducing them into a set of simple problems that can be addressed independently. Plsek and Greenhalgh57 propose trying multiple approaches and gradually evolving improved systems over time using techniques such as the plan-do-study-act (PDSA) cycle of quality improvement. 58 Plsek59 also argues that it is not possible to design CAS in detail, but that what needs to be set up are the conditions under which desired outcomes are more likely.
Complex systems are commonly contrasted with simple or complicated systems. A simple system is well understood, it is possible to apply evidence-based best practice, incoming information can be categorised, and an appropriate response can be generated; team members may need to co-ordinate activity, but there are no tight interdependencies. In complicated systems, cause–effect relationships are understood, but may be at-a-distance (in time or space); longitudinal studies may be needed to establish relationships; and team members need to co-operate to achieve desired outcomes. By contrast, in complex systems, cause–effect relationships are non-linear and there are many agents with different roles and relationships, making a conventional style of analysis that decomposes a system into independent subsystems impossible. A complex system includes both simple and complicated problems, but is not reducible to them. Glouberman and Zimmerman60 note that health-care systems are often managed and analysed as if they are complicated when in fact they are complex, and that managers address problems with approaches that are based on rational planning; these do not work as expected because they fail to take account of the complexity of the system.
The same point is made by Braithwaite et al. ,61 who argue that, to make ideas ‘stick’ in health care, it is essential to exploit people’s natural enthusiasms and networks, and that clinicians will ‘become more involved in promoting safer and better care if invited rather than compelled’. Braithwaite et al. 62 argue that, for CAS:
Despite the potential for unpredictability, non-linearity, and even messiness and chaos, there are patterns, behaviours, structures and routines which together define the system, and guide behaviour within it.
Braithwaite et al. ,62 p. 13
The examples they share are largely drawn from large-scale complex systems such as hospitals, and their focus is on the roles of people (particularly networks of people) and the diffusion of practices, with little attention paid to the design of tools or protocols.
Van Beurden et al. 63 summarise the Cynefin framework, which has four principal domains: simple, complicated, complex and chaotic. A fifth domain (disorder) applies when a problem is not well enough understood for it to be classified as one of the first four. They argue that, to effect positive change, it is necessary to develop ‘probes’ so as to ‘sense’ what configurations are most likely to succeed, and then ‘respond’ in ways that amplify effects. The Cynefin framework can be used to describe transitions between different domains as people find out more about what they are dealing with, either in discovering previously unknown information that increases complicatedness and complexity, or by discovering relationships and patterns that can simplify how one perceives the system. 64
Sittig and Singh65 introduce an eight-dimensional model to support reasoning about the design, deployment and evaluation of health information technology (IT) systems based on the perspective of health care as comprising CAS. The eight dimensions are hardware and software infrastructure; clinical content; the user interface; people such as end-users and developers; workflow and communication; internal organisational features such as policies and cultures; external factors such as regulation; and measurement and monitoring (which implicitly includes learning). These eight dimensions serve as a checklist, or set of probes, to ensure appropriate coverage of considerations in analysing the performance of technology in a complex adaptive health-care system.
These ideas of simple, complicated and complex systems, with their different properties and modes of problem-solving, have been applied in various areas of health care and health technology. For example, Greenhalgh et al. 66 have developed a framework to account for the (non-)adoption and spread of digital health interventions based on two orthogonal scales: the simplicity/complexity of the problem and domains of health concern (e.g. the health condition, the technology, the users, the organisational context). Glouberman and Zimmerman60 analyse the Canadian Medicare system as a complex system. Begun et al. 67 take a similar approach to analysing innovations in care delivery in the USA. Sittig and Singh65 focus specifically on health IT systems (e.g. electronic health records). No previous research has explicitly studied DERS or IV infusion administration as a complex system.
Aims and objectives
The aims of the ECLIPSE study were to describe the rates, types, clinical importance and causes of errors involving the infusion of IV medication in English hospitals, and to make recommendations for interventions with greatest potential for reducing harm from these errors.
The more specific objectives were:
-
to describe how IV infusions are administered in a sample of 16 English hospitals, including a specialist cancer hospital and two specialist paediatric hospitals, focusing on differences in terms of nursing practice, equipment, policies and processes, both within and between hospitals
-
to describe the rates, types and clinical importance of errors associated with the following modes of infusion delivery, in critical care, general surgery, general medicine, paediatrics and oncology:
-
gravity administration
-
standard infusion pumps and syringe drivers
-
smart infusion pumps and syringe drivers (with both hard and soft limits).
-
-
to explore variance in the rates, types and clinical importance of errors in relation to:
-
mode of infusion delivery
-
clinical area.
-
-
to explore the causes of the errors that occur and the extent to which innovations in technology or practice, such as the introduction of smart pump technology, electronic prescribing or bar code readers, could have prevented such errors
-
to identify best practices in safe and effective IV medication administration across different hospital contexts, including issues that are important to patients as well as staff
-
to establish how the findings differ from those of an ongoing US study, led by Bates,7 and to explore the reasons for any differences identified
-
to propose recommendations to prevent IV medication errors across different hospital settings in England.
In practice, as discussed below, it was found that the observations on IV medication practices could not be accounted for by considering IV medication administration as a simple or complicated system, but they could be accounted for by viewing it as a CAS. Consequently, research objective 4 was subsequently reframed in those terms, and research objective 7 has been addressed in a more nuanced way (see Chapters 7 and 8) than originally envisaged.
Structure of this report
In Chapter 2, we present the methods applied in the studies reported here; these are organised into two main phases. Phase 1 was a mixed-methods study involving point-prevalence observations supplemented by debrief interviews with the observers and focus groups with key staff members within the participating hospital. Phase 2 comprised in-depth observational study (using qualitative methods) of five of the participating hospitals. Phase 3 of the study involved engagement with stakeholder groups including patients, the public, health professionals, policy-makers and industry, and was conducted in parallel with phases 1 and 2.
The results of the quantitative observational study (phase 1), including further analyses of the quantitative data, are presented in Chapter 3. These results address the main overall aim of the ECLIPSE study, in describing the rates, types and clinical importance of errors involving infusion of IV medication in English hospitals. The analyses presented address objectives 2, 3 and 6 above.
Accounts in terms of systems of practice, based on the qualitative data from debriefs and focus groups in phase 1, are presented in Chapter 4. In this chapter, we present three complementary analyses of the data that focus on emergent themes, namely an analysis of the variations in policy and practice across hospitals (focusing particularly on IV medication procedures and documentation); an analysis of nursing practices, and how expert practices contribute to system resilience; and an analysis of perceived layers of influence. Collectively, these analyses address objective 1.
In Chapter 5, we present further analyses that aimed to explicitly address research objective 4, including further analysis of the phase 1 data and also an analysis of National Reporting and Learning System (NRLS) data to better understand the potential impact of smart pumps in the English NHS. These studies were not in the original protocol, but were added to provide an alternative perspective on the question about the value of smart pumps, at no additional cost to the project.
In Chapter 6, we present findings from the in-depth observational studies (phase 2), focusing on patient perspectives, contributing to objective 5.
In Chapter 7, we present the main findings from the in-depth observational studies (phase 2); in particular, we relate findings to views of IV medication administration as a work system and as a CAS. Findings reported in this chapter contribute to objectives 3, 4 and 5.
In the discussion (see Chapter 8), we draw together key themes from the study around the nature of the challenge, roles for technology, and the novel perspective on IV infusion administration offered by the theory of CAS; we also discuss strengths and limitations of the study, and highlight challenges experienced in conducting it.
In the concluding chapter (see Chapter 9), we highlight the value of informative variability for enhancing patient safety and experience of IV infusion administration, review implications for health care and make recommendations for future research based on our findings in order to address objective 7.
Chapter 2 Methods
This chapter includes material reproduced or adapted from Blandford et al. 1 and Lyons et al. 68 These articles are distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
This chapter is divided into four sections. The first describes the phase 1 mixed-methods study, including the point-prevalence study, debriefs and focus groups, and three supplementary studies. The second section describes the phase 2 methods, comprising deeper ethnographic observations on some wards as well as staff and patient interviews. The third section describes how we engaged with stakeholders during the project, which includes patients and the public, health-care professionals and industry. The final section highlights ethical issues considered in relation to data collection.
Phase 1: mixed-methods study across 16 hospitals
Study design
The study employed a mixed-methods approach bringing together complementary quantitative and qualitative methods. A quantitative point-prevalence study, using observational methods, was used to document the prevalence, types and clinical importance of errors associated with the infusion of IV medication, that is, ‘what’ and ‘how many’ deviations occurred in practice. Qualitative debriefs and focus groups with relevant hospital staff helped explain the findings, in other words ‘why’ the deviations occurred in practice. The design of the point-prevalence study was based closely on that used in a similar US multicentre study that studied general medical, general surgical, medical intensive care and surgical ICUs. 7 This approach, originally developed by Husch et al. ,6 involves trained staff systematically comparing details of each IV infusion in progress at the time of observation with the medication prescribed, to identify any discrepancies. Within the ECLIPSE study, once a preliminary analysis of quantitative data had been conducted, debriefs and focus groups were held with key staff to reflect on the point-prevalence results and relate these to details of hospital IV practices.
Study setting, recruitment and sample selection
The study took place within acute hospitals across England. Expressions of interest were sought from English NHS hospital trusts through the National Institute for Health Research Clinical Research Network, NAMDET and contacts from a previous study. 5 Interested parties were then invited to complete an online survey to provide an overview of their hospital, capacity to take part, infusion pump types and practices. Eighteen NHS trusts responded to the survey, providing information about 26 potential hospital sites. We aimed to include a mix of hospitals that used smart pumps with DERS and those that did not. Few hospital trusts that made use of smart pump technology across all clinical areas responded to the survey, so they were approached separately to maximise the number of observations involving smart pump technology. Hospitals were chosen purposively, with the aim of representing maximum variation in terms of type, size, geographic location, potential indicators of patient safety such as being a Bruce Keogh Trust,69 hospital mortality indexes70 and media reports, and their self-reported use of infusion devices and smart pump technology. Thirteen acute hospitals, two specialist children’s hospitals and one specialist cancer hospital took part in the first phase of the ECLIPSE study. Appendices 1 and 2 summarise the recruitment process and characteristics of each participating trust.
We conducted observations in three clinical areas (general medicine, general surgery and critical care) in 13 hospitals; in eight of these we also conducted observations in paediatrics and oncology day care. Two specialist children’s hospitals collected paediatric data only. One further trust collected oncology day-care data at three hospital sites.
Medication infusions included any medication, fluids, blood products and nutrition administered via an IV infusion, including patient-controlled analgesia (PCA). This slightly extends the focus of previous studies,6,7 which focused on medication and fluids (which are also legally classed as medications), to include other IV administrations. We aimed to include observation of 2100 infusions in total across all study sites to give a confidence interval around a 10% overall error rate across hospitals and clinical areas of 8.7% to 11.3%. 6,17
The debriefs were attended by the two observers and staff who helped to organise the research at the site, such as the local co-ordinator and the local principal investigator. A focus group was held at each site; these were attended by different hospital staff (including ward managers, senior nursing staff, patient safety specialists, medical electronics personnel, trainers, those with responsibility for procurement, and senior managers), so they varied in type and number of attendees. A member of the research team facilitated the debrief and focus group sessions.
Data collection
Point-prevalence study
Data were collected between April 2015 and December 2016. At each trust, two observers (usually a nurse and a pharmacist) employed in the organisation were given half a day of training by the research team to collect data. This training included highlighting the types of deviations to look for, conducting observations in the presence of the research team where possible, and using sample cases to facilitate discussion about classification of deviations identified (including assessing their severity). Observers were also requested to identify and familiarise themselves with relevant local policies and guidelines prior to data collection. Observers then spent one weekday or equivalent collecting data in each clinical area. Although the ward manager was consulted about suitable dates for observation, ward staff were not informed of specific observation dates. However, observation was not covert.
One clinical area could comprise one or more wards. Observers aimed to collect data on all IV infusions being administered at the time of data collection, including drugs, fluids, blood products and nutrition. Bolus doses were excluded, except where a prescribed bolus was given as an infusion, or vice versa. Completed infusions were excluded even if still attached to the patient. Patients were not observed if they were in isolation (because of infection risks), if they were receiving care that would have required interruption, or if they were off the ward.
Observers compared each medication being administered against the prescription and local policies and other guidance, and consulted clinical staff if needed to understand any deviations. Data were recorded using a standardised paper form (see Appendix 3) and subsequently uploaded to a secure web-based tool. 71,72 No patient identifiable data were recorded. Suspected errors were raised with clinical staff so they could be corrected if needed; local reporting practices were then followed.
Debriefs and focus groups
Following observational data collection at each trust, a report was drafted summarising that trust’s data (see Report Supplementary Material 1). This was presented at a debrief meeting with the observers, providing an opportunity to clarify aspects of the policies, practices and deviations observed. These clarifications sometimes led to updates to the data. For example, one site realised that giving set labels in its critical care unit were not compliant with its policy because they did not include the date the infusion was set up; another site initially included some infusions that were completed but were still connected to the patient, so these were subsequently excluded. Focus groups were then conducted with other local stakeholders to contextualise the findings, explore details of policies and practices and reasons for deviations, and discuss implications of the findings. Debriefs and focus groups were audio-recorded and transcribed verbatim. Guides for debrief and focus group sessions can be found in Document A on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hsdr/1220927/#/; accessed 1 March 2019).
Identifying and assessing deviations
The observers at each site recorded any deviations from a prescriber’s written or electronic medication order, the hospital’s IV policy and guidelines, or the manufacturer’s instructions. This included administration of medication to which the patient had a documented allergy or sensitivity, but did not assess other aspects of the clinical appropriateness of the medication order. We also collected data on policy violations and procedural or documentation deviations that may increase the likelihood of medication administration errors occurring. We specified four types of procedural and documentation deviations a priori: (1) giving sets not labelled appropriately; (2) documentation of administration inaccurate or incomplete; (3) infusion additive labels missing, incomplete or incorrect; and (4) patient ID wristbands missing or with incorrect, illegible or missing information. We identified two further types of deviation where policies varied among trusts during the debriefs and focus groups: (5) prescription and administration of IV flushes, and (6) procedures for the double-checking of medication. Finally, we encouraged observers to record any other irregularities, anomalies or workarounds related to the administration. Some of these were grouped together for analysis and formed new categories. Table 1 presents definitions of deviation types.
| Types of deviation | Definition |
|---|---|
| Medication administration deviations (errors and discrepancies) | |
| Unauthorised medication/fluids (no documented order) | Fluids/medications are being administered but no medication order is present. This includes failure to document a verbal order if these are permitted as per hospital policy |
| Wrong medication or fluid | A different fluid/medication/diluent as documented on the IV bag (or bottle/syringe/other container) is being infused from that specified on the medication order or in local guidance |
| Concentration deviation | An amount of a medication in a unit of solution that is different from that prescribed |
| Dose deviation | The same medication but the total dose is different from that prescribed |
| Rate deviation | A different rate is being delivered from that prescribed. Also refers to weight-based rates calculated incorrectly including using a different patient weight from that recorded on the patient’s chart |
| Delay of dose or medication/fluid change | An order to change the medication or rate not carried out within 4 hours of the written medication order, or as per local policy |
| Omitted medication or IV fluids | The medication prescribed was not administered |
| Allergy oversight | Medication is prescribed/administered despite the patient having a documented allergy or sensitivity to the drug concerned |
| Expired drug | The expiry date/time on either the manufacturer’s or the additive label has been exceeded |
| Roller clamp deviation | The roller clamp is not positioned appropriately/correctly |
| Incomplete infusion or delayed completiona | |
| Procedural and documentation deviations (errors and discrepancies) | |
| Patient ID | Either patient has no ID band on wrist or information on their ID band is incorrect |
| Wrong or missing information on additive label | Any incorrect or missing information on the additive label, as required by hospital policy |
| Giving set not tagged according to policy | Tagging or labelling of giving set is different (either missing or incorrect) from that required by hospital policy |
| Documentation of the medication administration | Medication/fluids administered but not documented correctly on chart (e.g. missing signature, start time) |
| Documentation of the medication ordera | Medication/fluids administered based on an incomplete, poorly documented or ambiguous medication order, for example missing signatures or dates, the absence of a specific time to be administered where required, or the absence of clear instructions that a medication should be titrated to clinical need or within certain parameters |
Rating of deviations
Local observers rated each deviation using an adaptation of the NCCMERP severity index. 27 They were also provided with a guidance document that included examples of how to rate errors [see example in Document B on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hsdr/1220927/#/; accessed 1 March 2019); this is the version used at the final training site as the document evolved during the study as new issues came to light].
Ratings were based on the likelihood of the deviation resulting in patient harm had it not been intercepted, and were used to classify the deviations as discrepancies (rated A1 or A2) or errors (rated from C to I) (Table 2). Throughout, we used the original and adapted NCCMERP severity index as the basis of our thinking. The original NCCMERP index defines category A as ‘circumstances or events that have the capacity to cause error’, while category C is ‘an error occurred that reached the patient but did not cause patient harm’. As noted above, we had to adapt the NCCMERP index to consider whether or not observed deviations had the capacity to cause harm, rather than whether or not they had already done so.
| Harm | Category | Description |
|---|---|---|
| No error | A1 | Discrepancy but no error |
| A2 | Capacity to cause error | |
| Error, no harm | C | An error occurred but is unlikely to cause harm despite reaching the patient |
| D | An error occurred that would be likely to have required increased monitoring and/or intervention to preclude harm | |
| Error, harm | E | An error occurred that would be likely to have caused temporary harm |
| F | An error occurred that would be likely to have caused temporary harm and prolonged hospitalisation | |
| G | An error occurred that would be likely to have contributed to or resulted in permanent harm | |
| H | An error occurred that would be likely to have required intervention to sustain life | |
| Error, death | I | An error occurred that would be likely to have contributed to or resulted in the patient’s death |
On the basis that category A events have the ‘capacity to cause error’, rather than being errors in their own right, we referred to these as ‘discrepancies’ so as to explore nuances in definitions of and reporting of errors. We split this category into two, ‘discrepancy but no error’ and ‘capacity to cause error’, because our pilot sites identified situations in which there were discrepancies that it was agreed would be unlikely to cause an error (see Table 8).
No deviations were rated as B because the study method meant that all deviations reached the patient. There might be an argument that some of the workarounds that were observed were responses to errors that had happened elsewhere in the system, but as we did not conduct observations across the broader system (e.g. actions in prescribing or stock management in pharmacy), we focused our analysis on what was observable at the bedside.
Based on the ratings (see Table 2), we developed and clarified our classifications, recognising that deviations could be either errors or discrepancies, and could occur either in medication administration or in the associated procedural and documentation requirements.
Observers at each trust documented brief descriptions of any deviations identified and provided further qualitative insights during semistructured debriefs once data collection was complete.
Data management and analysis: point-prevalence study
Data cleaning and classification was a highly iterative process as data collectors and the team worked through the many ambiguities and contextual factors that shaped practice.
Data on all deviations were adjusted in response to the debriefs and focus groups held at each trust. Researchers within the research team then collated and analysed these data together. This multisite analysis led to further adjustments in the quantitative data to ensure that they were as consistent as possible between trusts, so that similar deviations that had been rated differently were made consistent. To help with this process, clinicians within the research team reviewed deviations that local observers found difficult to categorise, similar deviations of similar type coded differently by observers and all deviations rated as category D or above. Minor changes were made to classifications of type and severity as needed. Examples of some of the classification heuristics developed within the team and discussions around particular observations are included in Appendix 4.
Error and discrepancy rates were calculated as the proportion of infusions with at least one error or discrepancy, using total opportunities for error (total number of doses administered, plus any omitted doses) as the denominator. Results were presented according to overall error and discrepancy rates, and individual types of errors and discrepancies, grouped into medication administration deviations and procedural/documentation deviations. Variations in deviation rates between clinical areas, delivery modes and infusion types were explored descriptively with their 95% confidence intervals, and chi-squared tests where appropriate. Qualitative data were analysed inductively.
Supplementary analyses
Two supplementary analyses of the data were conducted, comparing the NCCMERP method of assessing severity with an alternative approach, and comparing the findings from England against broadly equivalent data gathered in the preceding US study. 7
Comparison of two methods for assessing the severity of errors
A second method was used to assess the clinical importance of identified errors to explore any differences that may be found. The first method, using the adapted NCCMERP severity index, has been explained above. The second was the Dean and Barber28 method for assessing the severity of medication administration errors, developed and validated in the UK. This involves four experienced health-care professionals assessing each error on a scale of 0–10, where zero represents an error with no potential consequences to the patient and 10 represents an error which would result in death. The mean score across the four judges is then used as an index of severity, which has been shown to be both reliable and valid. Scores of < 3 are considered ‘minor’, those between 3 and 7 are considered ‘moderate’ and those of > 7 are considered ‘severe’. We recruited two experienced clinical pharmacists and two experienced nurses as the four judges. Judges were given a description of each error, blinded to the NCCMERP severity index scores previously allocated, and asked to rate each on the 0–10 scale. If identical errors occurred several times, only one was assessed. Scores from both methods were entered into SPSS (version 21; IBM Corporation, Armonk, NY, USA) for descriptive analysis. A scatterplot was produced to allow visual comparison between the two sets of scores, and the correlation between them was assessed using Spearman’s rank-order correlation.
Comparing findings from the USA and the UK
A comparative analysis was conducted on the findings from phase 1 of this project and a recent similar study from the USA. 7 We developed our protocol from that of this US study, and so the protocols broadly matched. There were differences in the number and type of hospitals that participated in each study; the US hospitals all used smart pumps, whereas these were used for only a proportion of infusions in the UK study; there were slight differences in the clinical areas and type of IV products included; and the US team classified all deviations as errors rather than separating these into discrepancies and errors. Once the separate studies had been completed, key members of both study teams convened to identify themes for comparison and contrast across the two studies. Initially, the well-sorted tool (www.well-sorted.org/) was used to enable all authors to individually identify themes and then collectively agree on key themes; then, through rounds of discussion and writing, themes were honed. Various analyses of the data from both studies were conducted to better understand what factors might be related to differences across countries, what might be due to levels of technological maturity, and what might be a result of other factors.
Developing accounts in terms of systems of practice
Three complementary analyses, drawing on the phase 1 qualitative data, were conducted to better understand the causes underpinning the quantitative data.
Procedural and documentation variations in intravenous infusion administration
The variance in procedural and documentation deviation type and frequency was explored alongside the variance of local policy. Policy requirements were found to be very different in some areas, and so split charts were used to present both the variability of local policy requirements and the rates of deviation against these. Deviation rates were calculated as infusions with at least one deviation against local requirements as the numerator, and the total number of doses administered as the denominator. Debrief and focus group data were analysed inductively, and used to contextualise the quantitative data and provide explanatory detail about issues within and across trusts.
Nurses as a source of system resilience
Nursing behaviour that contributed to system resilience in IV therapy was explored in the debrief and focus group data. The initial inductive analysis involved annotating the transcripts and line-by-line coding. A list of categories and themes was created from the emerging codes. In the final stage, a thorough analysis was conducted of those themes and categories relating to nurses’ behaviour and illustrating system resilience in IV therapy.
Identifying perceived layers of influence
A broad view of influences on IV medication deviations was also explored based on the debrief and focus group data. The analytical steps taken involved:
-
reading to highlight all the interesting elements in the data that relate in some way to IV medication administration (‘generating initial codes’ in thematic analysis73)
-
relating all such codes and constantly comparing them to each other to eliminate redundancies and identify themes (or categories) and establish category/subcategory relationships; this helps compress and summarise the data
-
refining the list of concepts into a more consolidated list
-
relating the concepts to each other and arranging them into related, but ordered, ‘layers’.
Supplementary studies
Two further analyses (not included in the original protocol) were conducted to further explore the role of smart pumps in contributing to patient safety. The first focused on the data from the point prevalence study, the second on data from the NRLS. Findings from these analyses are reported in Chapter 5.
Analysis of the errors identified in phase 1 to explore the role of smart pumps
The quantitative data set from phase 1 provides the opportunity to explore the potential role of smart pumps in preventing or contributing to errors. We therefore conducted a supplementary analysis of these data to identify:
-
the different types of errors identified in infusions given via smart pumps and those given using other types of pump
-
for errors identified in infusions that were not given via a smart pump, the extent to which these errors would have been likely to have been prevented using a smart pump
-
for errors in infusions that were given via a smart pump, the extent to which the use of a smart pump may have contributed to the error.
Each error of NCCMERP severity index category C or above was reviewed by two clinical members of the ECLIPSE study research team (a pharmacist and a nurse) to make a judgement as to whether the error would have been expected to have been prevented using a smart pump or whether a smart pump contributed to the error. Each error was reviewed and classified in discussion between the two researchers.
The following assumptions were made:
-
If using a smart pump, it was assumed that a suitable drug library entry was available and that staff would select and use the correct entry, and that any alerts arising as a result of soft limits would not be over-ridden if they were alerting to an error.
-
The following errors would not be prevented using any kind of smart pump:
-
omission errors, including those due to unavailability of pumps or giving sets, pumps not functioning or staff not knowing how to use the equipment
-
errors concerning patient ID
-
errors involving the administration of expired medication
-
labelling errors
-
delay in starting or completing the infusion.
-
-
The following errors would not be prevented using a standalone smart pump but may be preventable if smart pumps are integrated with electronic prescribing administration systems:
-
giving medication without a corresponding medication order
-
wrong rate errors where the rate was incorrect in relation to what was prescribed for that particular patient, but where the rate administered was within the usual range for the drug concerned
-
wrong rate errors concerning much lower (rather than higher) rates than those prescribed, in drugs for which very low rates are sometimes used in clinical practice.
-
-
For errors involving infusions of drugs or other fluids given via gravity feed where the rate was incorrect because the clamp was set incorrectly, it was assumed that these would be prevented by any pump (not necessarily a smart pump).
For the remaining errors (other wrong rate errors, dose/volume errors, wrong medication, concentration errors, and any other errors), each was reviewed individually with a judgement made on a case-by-case basis. Errors were classified using one of two sets of categories depending on whether or not a smart pump had been in use at the time of the error, as shown in Table 3.
| Smart pump device used? | Categories |
|---|---|
| No or not applicable |
|
| Yes |
|
To increase the rigour of this analysis, members of an expert panel were then invited to assess the preventability of a 25% random sample of the errors detected, stratified by smart pump use, traditional pump use or gravity administration. The panel members were a self-nominated group of 13 drug library experts who either had implemented or were in the process of implementing smart infusion devices in their organisation; they had previously been identified through the medication safety officer network for England to form a National Drug Library Expert Group.
All 13 experts were invited to participate by e-mail. Those who agreed (seven experts) were sent an anonymised sample of the data (listing whether a smart infusion pump, a traditional pump or gravity administration had been used and a description of the error) and requested to complete their assessment of preventability using the same predetermined categories (see Table 3). Participants were also requested to provide any comments or narrative about the errors listed, as well as comments on the assumptions made and the process of assigning categories. Agreement among the panel and between the panel and the research team was assessed descriptively.
Analysis of incidents reported to the National Reporting and Learning System
Having explored the potential role of smart pumps in preventing or contributing to administration errors within the ECLIPSE study point-prevalence data, most of which related to relatively minor errors, we took the opportunity to gain a complementary perspective through analysing data reported to the NRLS. All health-care organisations within England and Wales should report patient safety incidents to the NRLS. Although there is known to be considerable under-reporting in all incident-reporting systems, it is generally believed that serious errors are more likely to be reported. Although it was not part of our original protocol, we decided to conduct this supplementary analysis to complement the ECLIPSE study point-prevalence data. Our objectives were to identify more serious errors involving IV infusions and then to comment on the extent to which these may have been prevented by use of a smart pump or, for those that were given by a smart pump, the extent to which the smart pump may have contributed.
We requested data from the NRLS relating to incidents involving infusion pumps. Following approval by the NRLS and signing of a confidentiality agreement, we obtained anonymised reports for all incidents reported to have occurred between 1 January 2005 and 31 December 2015 that contained search terms relating to infusions or infusion pumps in any of the following fields: ‘description of what happened’ (field IN07), ‘action preventing reoccurrence’ (IN10), ‘apparent causes’ (IN11) and ‘device name’ (DE03). A full list of the search terms used is provided in Appendix 5.
From the data obtained, we selected those incidents rated as being of ‘moderate’ severity or above (moderate, severe or resulting in death; Table 4 provides definitions) that occurred in hospital inpatient settings, excluding operating theatres. We then removed all those relating to subcutaneous or other non-IV infusions; those where an IV infusion pump was mentioned in the report but the patient safety incident was unrelated; those that related to different types of pump, such as intra-aortic balloon pumps; and any others judged to be irrelevant.
| Harm category (relating to the actual harm experienced) | Definitions used within the NRLS |
|---|---|
| Low | Any unexpected or unintended incident that required extra observation or minor treatment and caused minimal harm to one or more persons |
| Moderate | Any unexpected or unintended incident that resulted in further treatment, possible surgical intervention, cancelling of treatment or transfer to another area, and which caused short-term harm to one or more persons |
| Severe | Any unexpected or unintended incident that caused permanent or long-term harm to one or more persons |
| Death | Any unexpected or unintended event that caused the death of one or more persons |
All reports were reviewed by two clinical members of the ECLIPSE study research team plus a third expert medication safety officer with experience of setting up drug libraries. The team discussed each case and made a judgement as to whether the error would have been expected to have been prevented using a smart pump or, for those that were given by a smart pump, the extent to which the smart pump may have contributed.
The same assumptions were made as in the section above, and the remaining errors (other wrong rate errors, dose/volume errors, wrong medication, concentration errors and any other errors) reviewed individually, with a judgement made on a case-by-case basis.
A separate subanalysis was conducted for those errors involving the wrong rate, dose or volume, using the same approach, as these are arguably those most likely to be preventable with a smart pump. To put these data into context, we then estimated the number of infusions given each year in the English NHS. This was done by identifying several sources of relevant data [National Patient Safety Agency (NPSA; now incorporated with NHS improvement) Safer Practice Notice 01: Improving Infusion Device Safety,74 electronic prescribing data from one study site, and IV giving set purchasing data from a second site] and estimating the average number of IV infusion administrations per bed per annum from each of these sources, and then multiplying by the number of beds in English hospitals. 75
Phase 2: in-depth observations and interviews in five hospitals
This phase of the project explored interesting contexts and practices from phase 1 more deeply in order to describe how practices differ and identify what aspects of the sociotechnical system have positive and negative effects on error types and rates. Phase 2 therefore aimed to develop a rich understanding of the factors that influence performance around IV medication administration.
Study design
Phase 2 was a qualitative study based on observations and interviews in five hospitals. Observations included staff administering IV medication and setting up pumps, supplemented by interviews with staff to further understand why certain errors occur within each context. Data gathering and analysis were driven from a human factors and sociotechnical system perspective. As described above, this emphasises how the safety and performance of a system is an emergent property of the ways the people, policy, practices, artefacts and equipment combine within it. The analysis focused on the causes of error, the need for any workarounds in practice, and identifying best practices in safe IV medication administration.
The patient’s role is often neglected in medical care;76 we addressed this by interviewing patients about their IV infusion experiences. These patient-centric findings have the potential to inform practice through better consideration of people’s requirements and preferences.
Study setting, recruitment and sample selection
The phase 1 results were used to identify hospitals and clinical areas for more detailed study in phase 2. These were selected on the basis of the most practically and theoretically interesting, for example where different kinds of errors or practices had been found. We also wanted to maintain a geographic spread and a mix of different smart and non-smart pump use from different manufacturers. Five different hospitals, from five different trusts, were included in phase 2, each with two different clinical areas:
-
site D – adult critical care and oncology day care
-
site G – general medicine and general surgery
-
site H – general medicine and oncology day care
-
site I – paediatric critical care and paediatric general surgery
-
site K – general surgery and paediatric general surgery.
Data gathering took place between October 2016 and December 2017.
In terms of sampling, there were three main strands of data collection at each phase 2 site:
-
Ten days on the wards work shadowing staff, conversations and observations of IV infusion preparation and administration. It was expected that about 40 IV infusions would be observed at each hospital site, which could include bolus doses.
-
Ten interviews with patients to get their perspective on the quality and safety of IV administration. Ethics approval to interview paediatric patients and/or their parents was not sought, which left a total of 35 interviews with adult patients.
-
Ten interviews with staff to discuss IV administration practices, discrepancies, errors, etc. These were stakeholders with some managerial responsibility for IV infusions at the trust, for example the local principal investigator, medical device safety officer, medication safety officer, medical device trainer, someone involved in the procurement of IV pumps, relevant senior nursing staff, relevant ward managers, relevant medical electronics personnel and relevant patient safety specialists.
Data collection
Data collection focused on:
-
establishing how IV infusion preparation and administration worked on the ward, including what triggered this process, what process was followed, what equipment was used, how it worked within the physical space and what issues there were
-
social interactions around technology, including whether patients were involved in the set-up of IV administrations (e.g. what they were told about their medications and how alarms were responded to)
-
what trade-offs and workarounds staff employed to achieve their goals
-
how different technologies such as pumps, barcode readers, CPOE and vital signs monitoring devices were used together, and how each contributed towards patient safety.
Different modes of data collection were used to collect these data, as follows.
-
Mode 1: scheduled interviews with clinical staff. This involved semistructured interviews with clinical staff addressing issues and activities to do with the administration of IV infusions. Interviews were audio-recorded. Document C on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hsdr/1220927/#/; accessed 1 March 2019) presents the topic guide used.
-
Mode 2: informal conversation with staff between tasks and during quieter periods (recorded as field notes). This involved talking to staff when there were naturally occurring breaks and lower quantities of work. This focused on clarifying observations the researcher had made.
-
Mode 3: interviewing patients at the bedside. This involved semistructured interviews with patients around issues and activities to do with the administration of IV infusions. Written consent was obtained. The interview was audio-recorded where possible (n = 24); otherwise, notes were taken (n = 11). All data were anonymised for analysis and subsequent reports. Document D on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hsdr/1220927/#/; accessed 1 March 2019) presents the topic guide used.
-
Mode 4: photographs of medical devices and their situations of use were taken. These facilitated discussion in the phase 2 workshop with representatives from participating sites, but were not a direct focus of analysis.
-
Mode 5: observing ‘everyday’ practice at the wards. This involved observing staff and patients, their workflow and their use of medical devices. The focus was on IV infusions, but in a wider context.
-
Mode 6: shadowing individual members of clinical staff. This involved observing the working practices of clinical staff, particularly focusing on tasks to do with administering IV infusions. This included calculating infusion rates, setting up devices, changing device settings, and performing the closing stages once a device had been finished with.
Extensive field notes were kept from the observational work. Photographs focused on physical spaces and the arrangement of equipment. Audio-recordings were transcribed and anonymised.
Data management and analysis
Consistent with common forms of qualitative data analysis (e.g. grounded theory77 and thematic analysis73), inductive methods were used to explore themes and patterns that emerged from the observational and interview data. Where applicable, we employed relevant theory to gain further insight and give theoretical weight to our analysis.
Data from patient interviews were analysed using a thematic analysis approach. The analysis consisted of a combination of deductive and inductive approaches, guided initially by the framework of the interview topic guide, but also allowing for the emergence of new themes.
The staff-related data were analysed using two complementary perspectives: SEIPS and CAS.
A Systems Engineering Initiative for Patient Safety analysis of the phase 2 observational and interview data
According to Carayon et al. ,49 the process for analysing a work system in terms of the SEIPS model involves five steps:
-
Describing the work system in terms of the organisation, physical environment, technology and tools, people and tasks. The external environment may also influence practices significantly.
-
Aligning this with Donabedian’s78 quality of care model, which links the system to the process of care and the outcome.
-
Identifying how the work system shapes the care process and how that, in turn, influences outcomes.
-
Relating outcomes for patients and professionals.
-
Identifying feedback loops that support organisational learning.
As the focus in this analysis was not to identify new outcomes (which were the focus in phase 1), but to better understand the context behind the observed outcomes, we analysed our observations in terms of the work system (step 1), and then summarised the consequences that emerged in terms of the impact on processes and opportunities for learning.
Analysis in terms of complex systems: best practice, good practices and informative variability
We had three objectives for this part of the study:
-
exploring IV infusion practice as a CAS
-
identifying informative variability by contrasting neighbours
-
exploring the differences between the different clinical areas.
We drew on the literature on CAS to explore whether or not and how it applied to our project. We reflected broadly on our research activities, papers and experiences to explore these relationships. This is in keeping with the ‘all is data’ dictum related to grounded theory. 77 This broadens narrow conceptions of data gathering to include the surrounding conditions in which data are gathered and research is undertaken. These analyses were performed by two authors (DF and GG).
To identify informative variability an analyst (DF) first performed an inductive analysis on the transcribed field notes, staff interviews and patient interviews from three sites (sites D, G and I) using the qualitative data analysis software NVivo (version 11; QSR International, Warrington, UK). Although sociotechnical themes79 informed some of the coding, this was primarily inductive. Deductive coding for positive and negative deviance was performed in parallel. Alongside this, a second analyst (GG) performed an inductive analysis on the transcribed field notes, staff interviews and patient interviews from three sites (sites H, I and K). This included deductive coding of positive deviance. Dominic Furniss extracted examples of positive deviance from both sets of coding and described them in a table. These examples were presented at a workshop attended by representatives from four phase 2 sites (sites D, H, I and K). Feedback from this workshop was noted, and changes were made to clarify and adjust the examples based on that feedback. A further iteration of the table was made so that variability was clearer between individuals, wards and hospital sites, or between different points in time.
To explore the differences in IV infusion administration in the different clinical areas, we summarised their contrasting characteristics, contextual dependencies and key issues.
Engaging with stakeholder groups
The project has benefited from engaging with stakeholders throughout. However, this was particularly useful for the final phase of the project, which focused on synthesising and sharing findings from phases 1 and 2 with stakeholders.
Public and patients
Two workshops involving public and patients were held during the project. The first was near the beginning of the project and comprised a 3-hour workshop involving nine people with experience of IV therapy in the hospital setting. 80 The first part explored their experiences of IV therapy, which informed the research questions that were used in the patient interviews in phase 2. The second part reviewed the patient-facing material for phases 1 and 2. The second workshop was conducted near the end of the project, when people with experience of IV therapy were invited to reflect and comment on our emerging findings. Patient representatives were also included on our advisory group and study steering committee.
Health-care professionals
There were four main ways that we engaged with health-care professionals to get feedback on emerging findings. The first was in the debriefs and focus groups in phase 1, which were much deeper and richer than expected, partly because we were presenting analysis that directly related to their work, so they could engage with it well and provide the local contextual information we were looking for. The second was in a workshop close to the end of the project, when representatives from the phase 2 sites were invited to reflect and comment on the emerging findings from the phase 2 analyses. This worked well, as sites could discuss areas of common interest, bringing different ideas and perspectives. The third involved 10 external talks to health-care professionals about our emerging findings at relevant conferences, meetings, webinars, etc., throughout the project. These were not as effective for engagement as we would have liked, but they contributed towards dissemination. The fourth involved our advisory group, whose membership comprised senior health-care professionals who were very knowledgeable and brought different perspectives to IV infusion administration practices. They provided expert guidance on the conduct and organisation of our research and on our emerging findings at different stages of the project.
Industry
We had planned to have closer engagement with infusion pump manufacturers towards the beginning of the project but our advisory group and study steering committee advised that this should be left until later in the project to counter any threat to the perceived independence of the study. In 2018, we co-organised two closed workshop with representatives of five key manufacturers, from which a confidential report has been produced. Through these events, we shared our findings and engaged in discussions with manufacturers and (hospital-based) user groups on their implications and future priorities in terms of technology developments and policies and practices to support safe IV medication administration.
Ethical issues
This study was approved by a NHS Research Ethics Committee (14/SC/0290) and site-specific research and development approval was obtained from each participating trust. We here outline some of the key ethical considerations made at the two main stages of data collection.
Phase 1: data protection, informed consent and addressing observed errors
At a local level, the point-prevalence study was comparable to an audit of IV medication practice for each site. Written informed consent was obtained from ward managers by the local co-ordinator before including ward areas in the study. Patients and ward staff were offered an information sheet outlining the study. Feedback from a patient and public involvement workshop was incorporated into the patient information sheet and the training of data collectors in how to approach and inform patients and their visitors about the study. As per the protocol approved by the ethics committee, written consent was not required from individual patients or ward staff, and ward staff were not notified of the timing of data gathering, to minimise the likelihood of this influencing their behaviour. Results from individual sites were kept secure and anonymous to other sites.
Written informed consent was obtained from staff taking part in the qualitative interviews. Interviews were audio-recorded and stored on secure password-protected computers before leaving the site. Interviews were transcribed by a professional transcribing service, with due attention to confidentiality, and the transcripts were anonymised.
If an error with potential to cause harm was identified during the point-prevalence study, the local data collectors were instructed to inform the relevant nursing staff caring for the patient in a discreet manner so that remedial action could be taken in line with local procedures. Document E on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hsdr/1220927/#/; accessed 1 March 2019) presents our protocol for dealing with suspected errors.
Phase 2: consent for observations and interviews
This qualitative study involved gathering information on the administration of IV infusions and related work practice through observations, interviews and discussion. There were no interventions or changes to normal medical practice, so there was little additional risk. Clinical staff on the wards were informed about the observations and verbal consent was sought before any close observations were undertaken. Patients on wards where observations were being made were informed of the study verbally and offered a patient information leaflet, as they were not directly involved. Patients and staff taking part in interviews gave written informed consent prior to data collection. Interviews were recorded when possible. In consultation with participating hospitals, it was agreed that it was not appropriate to interview paediatric patients about their IV therapy, and that it would be impractical to arrange interviews with their parents (given resource constraints), so ethics approval was obtained for interviewing adult patients only.
Chapter 3 Phase 1: observed errors and discrepancies in the administration of intravenous infusions
This chapter includes material reproduced or adapted from Fahmy et al.,2 Lyons et al. 68 and Furniss et al. 81 These articles are distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
During the point-prevalence study, 6491 patients were present in the clinical areas observed, of whom 1545 (23.8%) were receiving and/or prescribed an IV infusion at the time of data collection. Data were collected from 1326 (85.8%) of these patients, who were collectively administered and/or prescribed 2008 infusions; as shown in Table 5, this total includes 12 doses that were prescribed but not administered and 76 that were administered but not prescribed.
| Type of deviation | Errors | Discrepancies | |||||
|---|---|---|---|---|---|---|---|
| NCCMERP severity rating | n (% of 2008 infusions) | NCCMERP severity rating | n (% of 2008 infusions) | ||||
| C | D | E | A1 | A2 | |||
| Medication administration | |||||||
| Rate deviation | 65 | 12 | – | 77 (3.8) | 48 | 27 | 75 (3.7) |
| Unauthorised medication | 72 | 3 | – | 75 (3.7) | – | 1 | 1 (0.0) |
| Administration start time discrepancy | 13 | – | – | 13 (0.6) | 31 | 8 | 39 (1.9) |
| Incomplete or delayed completion | 10 | – | – | 10 (0.5) | 4 | 27 | 31 (1.5) |
| Expired drug | 11 | – | – | 11 (0.5) | 1 | 1 | 2 (0.1) |
| Dose discrepancy | 5 | 2 | – | 7 (0.3) | 6 | 6 | 12 (0.6) |
| Wrong drug/fluid/diluent | 11 | – | – | 11 (0.5) | 1 | 1 | 2 (0.1) |
| Omitted medications (not administered at time of data collection) | 2 | 3 | – | 5 (0.2) | 1 | 6 | 7 (0.3) |
| Roller clamp positioned incorrectly or inappropriately | 1 | – | – | 1 (0.0) | – | 10 | 10 (0.5) |
| Concentration discrepancy | – | – | 1 | 1 (0.0) | 7 | 2 | 9 (0.4) |
| Drug library not used or incorrectly used (in the case of smart pumps) | – | – | – | – | – | 67 | 67 (3.3) |
| Allergy oversight | – | – | – | – | 2 | – | 2 (0.1) |
| All medication administration deviations | 190 | 20 | 1 | 211 (10.5) | 101 | 156 | 257 (12.8) |
| Procedure or documentation | |||||||
| Infusion administration set not tagged/labelled correctly | – | – | – | – | – | 537 | 537 (26.8) |
| Documentation of the administration | 1 | – | – | 1 (0.0) | – | 334 | 334 (16.6) |
| Additive label missing or incorrect | 16 | 1 | – | 17 (0.8) | 2 | 200 | 202 (10.1) |
| Patient IDa | 6 | – | – | 6 (0.3) | – | 110 | 110 (5.5) |
| Documentation of the medication order | – | – | – | – | 7 | 31 | 36 (1.8) |
| All procedure or documentation deviations | 23 | 1 | – | 24 (1.2) | 9 | 1212 | 1219 (60.7) |
| Miscellaneous | 4 | 1 | – | 5 (0.2) | 4 | 9 | 13 (0.6) |
| All deviations | 217 | 22 | 1 | 240 (12.0) | 114 | 1377 | 1489 (74.2) |
Frequency, types and potential severity of errors and discrepancies
Overall, 240 errors and 1489 discrepancies were identified across 2008 IV infusions. Table 6 presents the numbers and percentages of infusions and patients affected. Errors affected 11.5% of infusions and 16.5% of patients overall, and discrepancies affected 53.0% and 58.9%, respectively. Table 5 shows the types of deviations observed and their likely harm. Ninety per cent of observed errors were considered unlikely to cause harm despite reaching the patient (NCCMERP category C). Table 7 shows the variation in error and discrepancy rates across clinical areas, delivery methods, smart features used and infusion type. Twenty-two errors (9.5%) were category D and one (0.4%) was category E; these 23 potentially harmful errors represent 1.1% of infusions. Examples in each severity category are presented in Table 8.
| Type of deviation | Infusions (N = 2008), n (%; 95% CI) | Patients (N = 1326), n (%; 95% CI) | ||
|---|---|---|---|---|
| One or more errors (i.e. C–I severity ratings) | One or more discrepancies (i.e. A1 and A2 severity ratings) | One or more errors (i.e. C–I severity ratings) | One or more discrepancies (i.e. A1 and A2 severity ratings) | |
| Medication administration | 207 (10.3; 9.1 to 11.7) | 241 (12.0; 10.6 to 13.5) | 197 (14.9; 13.0 to 16.9) | 222 (16.7; 14.8 to 18.8) |
| Procedural or documentation | 24 (1.2; 0.8 to 1.8) | 953 (47.5; 45.3 to 49.7) | 23 (1.7; 1.1 to 2.6) | 694 (52.3; 49.6 to 55.1) |
| Miscellaneous | 5 (0.2; 0.1 to 0.6) | 13 (0.6; 0.3 to 1.1) | 5 (0.4; 0.2 to 0.9) | 13 (1.0; 0.6 to 1.7) |
| Alla | 231 (11.5; 10.2 to 13.0) | 1065 (53.0; 50.8 to 55.2) | 219 (16.5; 14.6 to 18.6) | 781 (58.9; 56.2 to 61.6) |
| Variation | Infusions (N) | At least one error per infusion (i.e. NCCMERP B–I ratings), n (%, 95% CI) | At least one discrepancy per infusion (i.e. A1 and A2 ratings), n (%) | At least one deviation per infusion, n (%) |
|---|---|---|---|---|
| Clinical area | ||||
| General medicine | 366 | 50 (13.7, 10.2 to 17.2) | 244 (66.7) | 261 (71.3) |
| Paediatrics | 342 | 45 (13.2, 9.6 to 16.8) | 171 (50.0) | 183 (53.5) |
| General surgery | 402 | 51 (12.7, 9.4 to 16.0) | 228 (56.7) | 244 (60.7) |
| Oncology day care | 386 | 49 (12.7, 9.4 to 16.0) | 180 (46.6) | 182 (47.2) |
| Critical care | 512 | 36 (7.0, 4.8 to 9.2) | 242 (47.3) | 250 (48.8) |
| Delivery method | ||||
| Gravity feed | 163 | 35 (21.5, 15.2 to 27.8) | 109 (66.9) | 119 (73.0) |
| Other (e.g. transducer/pressure bag) | 25 | 4 (16.0, 1.6 to 30.4) | 8 (32.0) | 10 (40.0) |
| Volumetric pump | 1364 | 164 (12.0, 10.3 to 13.7) | 723 (53.0) | 759 (55.6) |
| Syringe driver | 375 | 24 (6.4, 3.9 to 8.9) | 187 (49.9) | 193 (51.5) |
| PCA pump | 78 | 4 (5.1, 0.2 to 10.0) | 35 (44.9) | 36 (46.2) |
| Smart pump | ||||
| Pump with no smart features enabled | 1202 | 130 (10.8, 9.0 to 12.6) | 558 (46.4) | 592 (49.3) |
| Smart pump | 640 | 66 (10.3, 7.9 to 12.7) | 395 (61.7) | 406 (63.4) |
| Smart pump used with drug library | 356 | 31 (8.7, 5.8 to 11.6) | 241 (67.7) | 244 (68.5) |
| Smart pump drug library not used, although relevant drug listed | 67 | 11 (16.4, 7.5 to 25.3) | 67 (100) | 67 (100) |
| Smart pump drug library not used, as relevant drug not listed | 215 | 23 (10.7, 6.6 to 14.8) | 85 (39.5) | 93 (43.3) |
| Not known | 2 | 0 | 0 | 0 |
| Infusion type | ||||
| Fluid | 829 | 153 (18.5, 15.9 to 21.1) | 476 (57.4) | 510 (61.5) |
| Blood or blood product | 55 | 5 (9.1, 1.5 to 16.7) | 13 (23.6) | 15 (27.3) |
| Drug | 1012 | 70 (6.9, 5.3 to 8.5) | 507 (50.1) | 524 (51.8) |
| Parenteral nutrition | 102 | 3 (2.9, 0.0 to 6.2) | 59 (57.8) | 61 (59.8) |
| Severity category | Examples |
|---|---|
| E | Patient was administered 2 g of vancomycin diluted in 250 ml of 0.9% sodium chloride. The drug should have been diluted in 500 ml of 0.9% sodium chloride (concentration error: severity category E) and administered over at least 240 minutes. The drug was observed running too fast via gravity feed (rate error: D). The chart had not been signed to confirm that the administration had been double-checked as required (documentation discrepancy: A2). The patient complained of pain and red lumps along arm |
| D |
Piperacillin/tazobactam was prescribed to be given over 3 hours. However, it was given as a bolus over 3–5 minutes, which is the most common way to administer this antibiotic. The nurses presumed that the doctors had made a mistake and corrected it. However, this had been prescribed intentionally after discussions with the consultant, microbiology, pharmacy and the drug manufacturer because of the patient’s poor renal function. This clinical decision was recorded in the patient’s notes but nursing staff had not reviewed these 40 mmol of potassium chloride, rather than the prescribed 20 mmol, was administered together with 10 mmol magnesium sulphate in 0.9% sodium chloride at 1000 ml/hour |
| C |
1 litre of 0.9% sodium chloride with 0.15% potassium chloride was prescribed over 12 hours. The documented start time was 23:25. When observed at 13:00 the following day, the infusion was not running and approximately 150 ml remained. The infusion should have been complete but the pump was not plugged in and the battery was empty A medication order for 20 mcgh of fentanyl stated diluent as 5% dextrose; however, the drug was prepared and administered in 0.9% sodium chloride |
| A2 |
Electronic prescription specified 1 litre of 0.9% sodium chloride over 8 hours. It was started at 02:00 and thus was due to finish at 10:00 but at 09:25 there was still 500 ml to run. The infusion was paused at the time of observation as the patient was receiving an intermittent amoxicillin infusion Hartmann’s solution had been selected in the smart pump’s drug library but the infusion being administered was 0.9% sodium chloride (at the correct rate prescribed) |
| A1 |
The prescribed rate was 250 ml/hour for 123 mg of paclitaxel in 250 ml of 0.9% sodium chloride. However, the final reconstituted volume was 290.5 ml, which was being infused at 290 ml/hour to give the same rate of administration as prescribed Administration of piperacillin/tazobactam was delayed by approximately 30 minutes |
Medication administration deviations
Overall, 427 (21.3%) infusions involved at least one medication administration error (n = 211) or discrepancy (n = 257). The most frequent types of deviation concerned rates and unauthorised medications.
Rate deviations
Overall, 152 infusions (7.6%) were being administered at a different rate from that prescribed; 77 were classified as errors (rated C or above) and 75 as discrepancies (rated A1 or A2). A large proportion involved order changes that had not been correctly documented and infusions titrated based on the patient’s clinical need or fluid allowance without such titration being prescribed. Three deviations involved prescribed boluses administered as infusions, and one was a prescribed infusion given as a bolus.
Overall, 24 (31%) rate errors occurred in infusions delivered via gravity (without using a pump), despite this method of delivery accounting for just 163 (8.1%) infusions. Of the 12 most serious rate errors (rated D), eight were administered via gravity; these included red blood cells, vancomycin, paracetamol and piperacillin/tazobactam. Many medication orders specified a duration rather than a rate (e.g. over 8 hours). In one case, an infusion was observed running at a very high rate to ‘catch up’: 1 l of Plasma-Lyte® 148 (Baxter Healthcare Ltd, Norfolk, UK) had been prescribed over 24 hours, and at the time of observation, 27 hours after the start time, the rate was set at 500 ml/hour.
Unauthorised medication
Eighty-nine infusions did not have a corresponding medication order. Thirteen were flushes that did not require a medication order according to local policy. Therefore, 76 infusions (3.8%; 75 errors and 1 discrepancy) were judged to be unauthorised. Almost half were fluids used to flush the line, commonly in oncology settings, including 0.9% sodium chloride (n = 29), dextrose (n = 1), Plasma-Lyte (n = 2) and heparin (n = 3). A further seven infusions were of 0.9% sodium chloride administered at low rates to keep the vein open. Twenty infusions were unauthorised repeats of previously prescribed maintenance fluids. Four were administered on verbal orders that had not been documented at the time of observation. Of the remaining 10 unauthorised infusions, seven involved maintenance fluids and three were drugs (calcium folinate, remifentanil and insulin). The remifentanil infusion had been prescribed but had been discontinued, and had not been represcribed after a decision had been made to resedate the patient.
Procedural and documentation deviations
Overall, 961 infusions (47.9%) had at least one procedural or documentation error (n = 24) or discrepancy (n = 1219). Table 5 shows the frequency and severity of different types of procedural and documentation deviations. Non-compliance with hospital requirements for labelling infusion administration sets was most common. Procedural or documentation errors mostly involved unlabelled syringes, or infusions where the label was significantly inaccurate. For example, a patient prescribed 60 mg of pamidronate was being administered an infusion labelled as 30 mg, but staff confirmed that the patient had received the correct dose.
Variation in error and discrepancy rates
Error rates among trusts ranged from 2.7% to 24.4% and discrepancy rates ranged from 13.5% to 100% of infusions (Figure 1). The discrepancy/error correlation is 0.393 (R2 = 0.154), so only 15.4% of the variation in discrepancy rates can be explained by variation in error rates (or vice versa) (Figure 2). Procedural or documentation deviations ranged from 9.9% to 100% of infusions at one trust, reflecting wide variation in hospital policies and how they were enacted (or not) in practice. Some trusts had stringent policy requirements (e.g. trust K), whereas others did not (e.g. trust J), and some had requirements that staff were unaware of in practice (e.g. trusts D and P).
FIGURE 1.
Error and discrepancy rates by trust. Reproduced from Lyons et al. 68 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an open access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

FIGURE 2.
Scatterplot of error and discrepancy rates by trust.
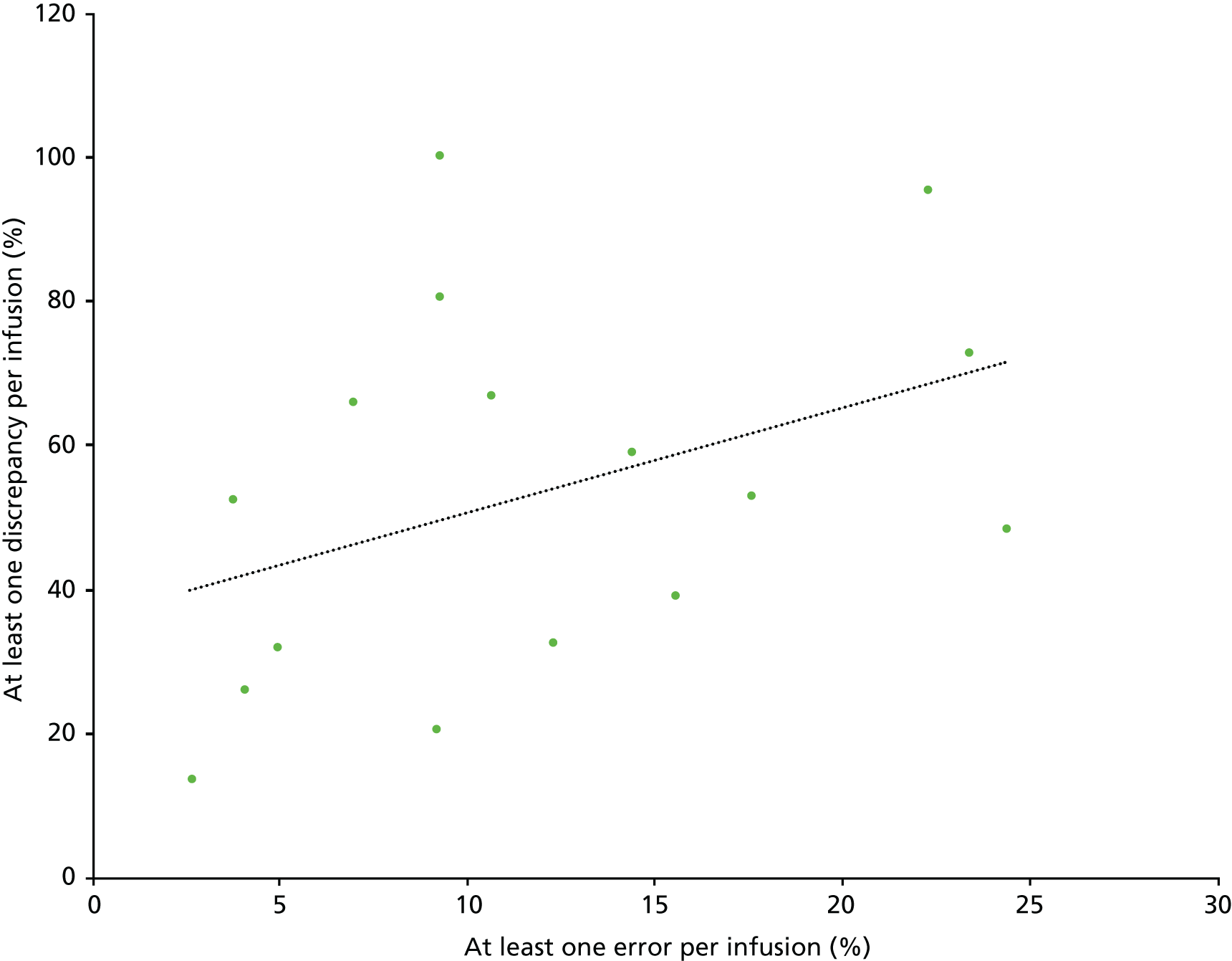
Variation was also evident among clinical areas and different infusion types (see Table 7). Infusions observed in critical care had a significantly lower error rate (7.0%); the error rate for paediatric areas was similar to that for adult non-critical care areas. The lowest discrepancy rate was in oncology day care (46.6%). General medicine had the highest error (13.7%) and discrepancy (66.7%) rates. PCA pumps and syringe drivers had the lowest error rates of 6.4% and 5.1%, respectively, with infusions delivered via gravity having the highest rate (21.5% of 163 infusions). Error rates also varied by the type of medication: maintenance fluids (e.g. 0.9% sodium chloride) had a high error rate (18.5%) compared with drugs (6.9%), blood products (9.1%) and parenteral nutrition (2.9%).
Eleven out of 16 hospital trusts (69%) used smart pumps (i.e. an infusion pump with a drug library and/or DERS enabled) in at least one clinical area. However, just 640 (32%) infusions were administered using a smart pump. Infusions delivered using smart pumps had similar error rates to those using other pumps (10.3% of 640 infusions vs. 10.8% of 1202; p = 0.8). No appropriate entry was available in the drug library for one-third of infusions administered using a smart pump. Out of 424 infusions with a library entry available, this was used in 356 (84%) cases. There was no significant difference in error rates between doses given via a drug library and those given without. Discrepancy rates were higher in infusions delivered using smart pumps (61.7% of 640 infusions) than in those without smart features (46.4% of 1202 infusions; p < 0.001). Sixty-seven discrepancies were identified in the use of a smart pump drug library: 61 where the drug library was bypassed completely and six where the wrong entry was selected. In the latter cases, the correct entry had not been available in the library and an alternative entry had been selected that had the intended effect. For example, a medication order for 1000 ml of Plasma-Lyte 148 to be administered over 8 hours was noted by the observers as ‘used library but chosen Hartmann’s – Plasma-Lyte not available – Plasma-Lyte used in place of Hartmann’s as fluid of choice’.
However, differences in discrepancy rates were more commonly linked with policy requirements for labelling infusions and administration sets at different sites; when discrepancies related to use of a drug library are excluded, the discrepancy rate remains higher in infusions delivered via smart pump (59.2% of 640 infusions).
Although some of the discrepancies identified in our study were deviations from protocols that may have been a result of intentional workarounds, this was not always the case. Some were minor, non-clinically significant variations from what was prescribed that did not meet our definition of a medication administration error (e.g. very minor deviations in flow rate or concentration, or minor delays to maintenance fluids’ start or finish time as a result of their being interrupted for the administration of IV antibiotics), and some were minor documentation discrepancies.
Comparing two methods for assessing errors’ clinical significance
In total, 155 different errors were assessed for clinical significance. Using the NCCMERP severity index method, 137 (88%) were rated C (‘an error occurred but was unlikely to cause harm despite reaching the patient’), 17 (11%) were rated D (‘an error occurred that would be likely to have required increased monitoring’) and one (1%) was rated E (‘an error occurred that would be likely to have caused temporary harm’). Using the Dean and Barber28 method, scores ranged from 0 to 4.75, with a mean of 1.7, with 138 (89%) errors rated minor and 17 (11%) rated moderate. Of the 17 errors rated ‘moderate’ on the Dean and Barber scale, 11 were rated C using the NCCMERP severity index method, five were rated D and one was rated E. Of the 17 errors rated D using the NCCMERP severity index method, 11 were rated minor using the Dean and Barber method, and 6 were rated moderate. Figure 3 presents the scores using each method. Of the 137 errors scored as C using NCCMERP severity index, and the 138 errors classified as minor using the Dean and Barber method, 127 were common to both. Scores from the two methods were significantly but weakly correlated (Spearman’s rank-order correlation = 0.36; p < 0.01).
FIGURE 3.
Dean and Barber28 scores vs. NCCMERP severity index scores. Dean and Barber scores range from 0 to 10, with scores of < 3 considered ‘minor’, those of between 3 and 7 considered ‘moderate’ and those of > 7 considered ‘severe’. C, an error occurred but is unlikely to cause harm despite reaching the patient; D, an error occurred that would be likely to have required increased monitoring and/or intervention to preclude harm; E, an error occurred that would be likely to have caused temporary harm. Some symbols represent more than one error. Reproduced from Furniss et al. 81 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
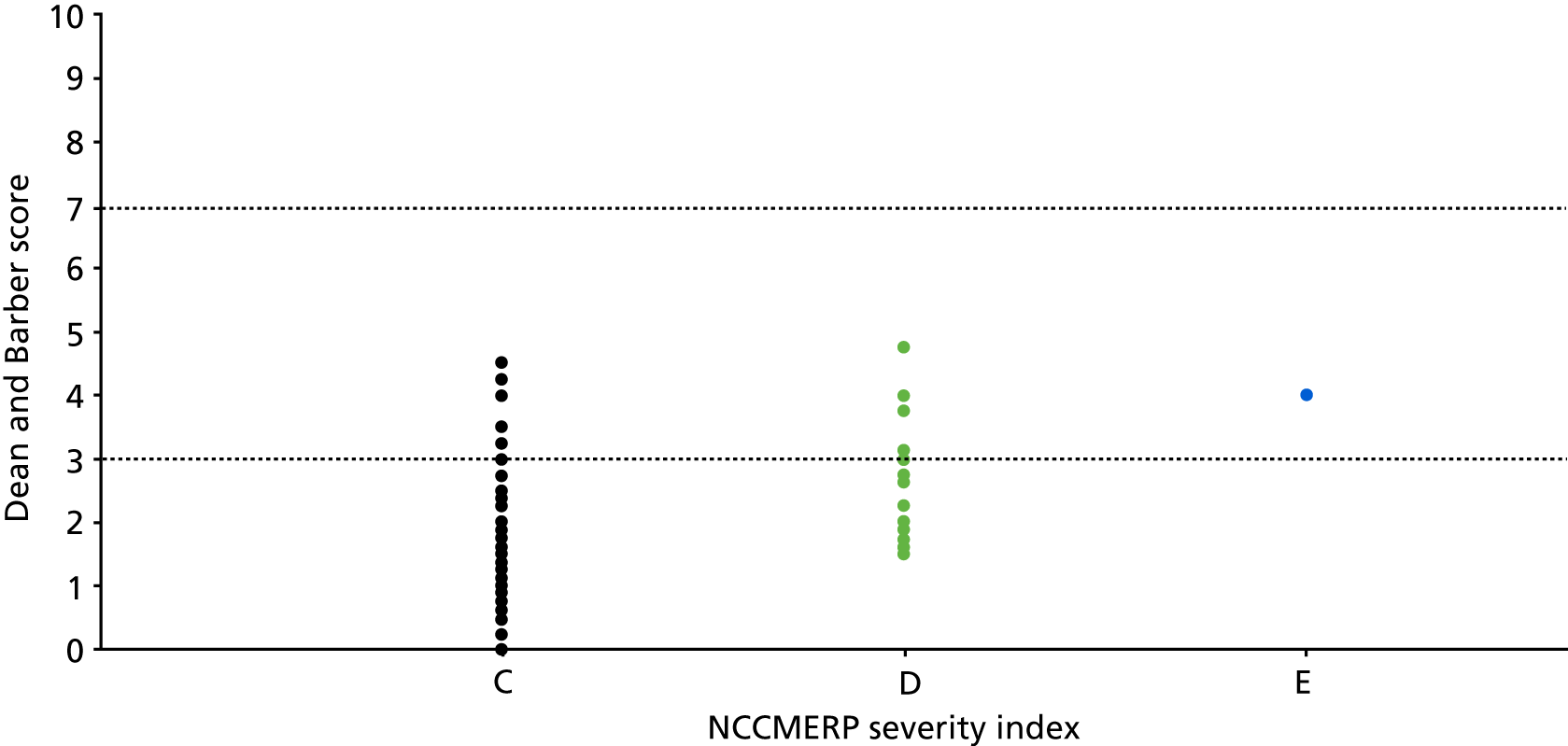
Comparing findings with those of a parallel US study
We present the results of the comparative study under three themes: an overview of the commonalities and differences in the findings from the two study contexts; nuanced differences in methods that became apparent through the process of comparing findings; and differences in contextual factors that were tacitly known prior to the study, but whose significance became apparent during the study.
Commonalities and differences in findings
Table 9 shows a comparison between the USA and England of the different types of error detected.
| Error | Pump type (number of infusions observed) | |||
|---|---|---|---|---|
| USA smart (n = 1164) | England smart (n = 640) | England traditional (n = 1205) | England gravity (n = 163) | |
| Documentation of the order or administration | 6.2 | 16.1 | 17.8 | |
| Label not completed according to policy | 60.1 | 43.1 | 9.6 | 14.7 |
| Tubing not tagged according to policy | 35.0 | 38.9 | 16.9 | 43.6 |
| Unauthorised medication | 24.1 | 4.1 | 4.0 | 1.2 |
| Smart pump or drug library not used | 10.3 | 10.5 | NA | NA |
| Wrong rate | 4.6 | 6.9 | 6.7 | 16.6 |
| Omission of IV medications/fluids | 4.6 | 0.2 | 0.7 | 1.8 |
| Expired drug | 2.1 | 0.3 | 0.9 | 0.0 |
| Wrong dose | 2.0 | 0.9 | 1.1 | 0.0 |
| Delay | 1.2 | 2.2 | 2.4 | 5.5 |
| Pump setting error | 0.5 | NA | NA | NA |
| Wrong fluid/medication | 0.3 | 0.8 | 0.6 | 0.6 |
| Wrong concentration | 0.3 | 0.2 | 0.7 | 0.6 |
| Patient ID error | 0.2 | 2.7 | 7.6 | 4.9 |
| Allergy oversight | 0.1 | 0.0 | 0.1 | 0.6 |
| Incomplete or delayed completion | NA | 0.6 | 2.6 | 3.7 |
| Roller clamp positioned incorrectly | 0.0 | 0.2 | 0.3 | 3.7 |
Documentation errors were not recorded as a separate category in the US study. The figures for labels not being completed and tubing not being tagged according to policy can largely be accounted for by differences in policy between the two countries. There is a more detailed expectation of what should be included on labels in the USA, as specified by the Joint Commission,82 although these guidelines may be interpreted differently across different hospitals. In England, policies are written locally, so there is more substantial variation in policies across study sites.
Patient ID errors were higher in England than in the USA; the main contributory factor is likely to be the widespread use of BCMA in the USA, which makes it more difficult to administer medication without having formally identified the patient.
These findings relate to all deviations observed (some of which were classified as discrepancies in the English study, as discussed above). In practice, the vast majority of errors were classified as A–C according to the adapted NCCMERP severity index (see Table 2). Very few errors (n = 25; 0.79% of all infusions) were classified as D (‘An error occurred that would be likely to have required increased monitoring and/or intervention to preclude harm’) across both the USA and England. Across all 3172 observations, only two (one in the USA and one in England) were assessed to be level E (‘An error occurred that would be likely to have caused temporary harm’). None was rated F (‘An error occurred that would be likely to have caused temporary harm and prolonged hospitalisation’) or higher.
Table 10 summarises the error types for the more serious errors (categories D and E). If no in errors of a particular category occurred, that category is omitted from the table. This shows that the greatest proportion of category D errors featured gravity feed pumps running at the wrong rate. Of the category E errors, one was a delay (USA, smart pump) and one a concentration error (England, gravity administration). No errors were assessed as severe enough to have caused prolonged hospitalisation.
| Error | Pump type (number of infusions observed) | |||
|---|---|---|---|---|
| USA smart (n = 1164) | England smart (n = 640) | England traditional (n = 1205) | England gravity (n = 163) | |
| Label not completed according to policy | 1 | |||
| Unauthorised medication | 2 | 1 | ||
| Wrong rate | 2 | 1 | 3 | 8 |
| Omission of IV medications/fluids | 1 | 1 | 2 | |
| Expired drug | 1 | |||
| Wrong dose | 1 | 1 | ||
| Delay | E | |||
| Wrong concentration | E | |||
Overall, the commonalities across the US and English studies are much more striking than the differences, and the level of technological maturity makes little consistent difference across contexts.
This combined study draws on the observation of 3172 infusion administrations. Across those, as discussed by Lyons et al. ,68 the only clear conclusion is that medication administered by gravity feed is more likely to result in patient harm than medication administered via infusion pumps. Neither the US nor the English study identified any events in which patient safety was significantly compromised, resulting in death or serious harm. It is more likely that such incidents would feature using a different study method that focuses on reports of more serious incidents involving medication administration (see Chapter 5).
The dominant theme across contexts is the variability within and between sites, particularly relating to IV medication administration policy.
Nuanced differences in methods
As noted above, the process of comparing findings across the US and the English study contexts revealed a few unplanned variations in data-gathering methods. These are listed in Table 11.
| Theme | US study | English study |
|---|---|---|
| Assessing delays | 2- to 4-hour delay classed as delay; > 4 hours classed as omission | Delays were assessed contextually, based on discussion between data gatherers and local staff. England guidance is typically that administration should be ‘as soon as possible’, so there is no defined time at which a delay is interpreted as omission |
| Including category B | Counted category B – these were errors that did not reach the patient; in practice, these were all situations where a pump was not connected to a patient but was located near them | Excluded from English study, which focused on errors and discrepancies that reached the patient, so only considered pumps that were connected and running (or should have been) |
| Classification of labelling errors where medication was being delivered | Mostly interpreted as ‘C’, since medication was being delivered, because category C was interpreted as covering any situation in which medication was being administered | For documentation or procedural deviations where the patient was receiving the correct medication these were classified as A2 by the English team |
| Differences in classifications: pump setting errors | Included ‘pump setting error’, which was typically using the wrong drug library or setting up with the wrong pump module. Such a situation would commonly also result in classifications such as ‘wrong rate’ and ‘wrong concentration’ | These errors were classified focusing only on the consequences, such as ‘wrong drug’, ‘wrong rate’ or ‘wrong concentration’ |
| Differences in classifications: incomplete or delayed completion | These were classified as delays or omissions, depending on the length of the delay | A classification of ‘Incomplete or delayed completion’ was introduced to account for instances such as medication administration being suspended (e.g. for patient to have a shower or radiography) |
| Differences in classifications: roller clamp positioned incorrectly | Not included as a specific error type; any instances were classified according to the outcome (e.g. delayed or omitted medication) | Such errors featured occasionally, mostly for gravity feed |
| Differences in classifications: documentation of the order and of the administration | Excluded from study as CPOE enforces provision of complete documentation. Categorised as errors where there was a discrepancy between the order and the administration | Included as specific categories where paper-based documentation was absent or incomplete |
| Nuanced focus of study | Focused on observations as a means to identify areas for improvement, so as to engage in rapid cycles of intervention development and testing | Focused on observations plus debriefs and focus groups to better understand the causes of the errors observed |
Some of the variations are incidental: minor differences in the ways that the study protocol was interpreted across the two studies. For example, some ‘wrong rate’ errors were classified as ‘pump setting’ errors by the US team, where the pump setting was the cause of the wrong rate; conversely, the English team noted roller clamp positioning errors, which would have been classified by the US team as wrong rate errors.
Some variations are also a consequence of the use of different technologies. For example, some kinds of paper documentation omissions observed in England were blocked by CPOE/BMCA technology in the USA.
Differences in contextual factors
There were some key contextual factors across contexts and legislatures that shaped differences in methods and outcomes.
The first is the level of technological maturity across sites. In the USA, all sites had implemented electronic health records, BCMA and CPOE, and IV infusion/medication bags were routinely scanned before administrations. In the English study, some sites had implemented electronic health records, and some had implemented electronic prescribing and medication administration (EPMA). Few hospitals had implemented smart pumps with drug libraries across all clinical areas; more had implemented smart pumps in selected areas (most commonly critical care); others were using traditional pumps and gravity feed. The implementation of electronic health records and EPMA lags behind in England, although it is now progressing rapidly. 83
A second is the use of drug libraries. In the USA, vendors supply drug library packages, but there is nevertheless large variation across hospitals. 84 In England, sites that made use of a drug library reported having constructed it locally; this was typically a pharmacy responsibility.
At a national policy level, in the USA, Joint Commission guidelines drive policy; these guidelines are typically precise, although they may be interpreted differently by different hospitals. In England, the Care Quality Commission (CQC) expects every hospital to have relevant policies, but does not mandate their content.
Finally, in terms of local prescription policies, in the USA, fluids might be given without always requiring a repeated medication order, and KVO administrations may run without a medication order (standing hospital policy); verbal orders are discouraged by the Joint Commission but are not uncommon. In England, there is variation in local policy about what can be given without prescription, KVO practices are rarely reported, and verbal orders are very unusual.
Summary
The study found that approximately 1 in 10 IV infusions involved an error, and 1 in 2 involved a discrepancy. However, few were considered likely to cause patient harm. There was considerable variability in errors, discrepancies, policies and practices among trusts.
Chapter 4 Phase 1: accounts in terms of systems of practice
This chapter includes material reproduced or adapted from Furniss et al. 81 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The information provided by observers revealed some reasons for deviations. Some deviations were simple slips or lapses, such as confusing diluents and forgetting to open roller clamps to start the infusion; others involved a lack of knowledge of policy requirements. Staff also reported deliberate deviations that would benefit patients but conflicted with official rules and formal procedures, for example giving patients fluids that had not yet been prescribed when a doctor was unavailable (i.e. unauthorised fluids) and keeping lines patent (sometimes referred to as KVO) by switching to a low infusion rate in anticipation of another infusion being needed (i.e. rate deviation). There were several instances of inaccurate prescriptions that were ‘corrected’ and administered by nurses without the order being changed prior to administration. However, in one case the administering nurse incorrectly assumed that an unusual prescription was wrong (e.g. piperacillin/tazobactam; see Table 8).
In some instances, staff actively tried to balance risk and efficiency rather than follow procedures mechanistically. For example, staff reported stopping infusions (i.e. delay in completion) when a patient left the ward for investigations so that a nurse did not have to accompany the patient when staffing resources were stretched. In addition, some nurses objected to spending time labelling administration sets and writing batch numbers on additive labels for infusions that would shortly be discarded.
Observers at some trusts reported that collecting the study data provided insights into the reasons for some deviations and helped them identify solutions. For example, at one site where poor compliance with documenting medication administration was recorded, the trust subsequently purchased handheld computers to allow staff to access electronic records when in close proximity to patients.
Overall, the debriefs and focus groups at participating sites yielded a wealth of data on IV medication administration practices and the factors that shape those practices. In this chapter, we report on three separate analyses of these data that deliver complementary causal accounts of the observations reported in Chapter 3. The first focuses on procedural and documentation variations across sites; the second reviews nurses’ roles in contributing to overall system resilience; and the third reviews IV medication administration in terms of layers of influence.
Procedural and documentation variations in intravenous infusion administration
Overall, 961 out of the 2008 infusions observed (47.9%) had at least one procedural or documentation deviation. The prevalence of deviations varied considerably among trusts, affecting between 9.9% and 100% of infusions. Trusts’ deviation profiles also varied, with some having greater numbers of certain deviation types (Table 12).
| Deviation | Trust (number of infusions) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (N = 2008) | A (N = 90) | B (N = 130) | C (N = 131) | D (N = 97) | E (N = 74) | F (N = 282) | G (N = 84) | H (N = 143) | I (N = 139) | J (N = 73) | K (N = 103) | L (N = 114) | M (N = 152) | N (N = 124) | O (N = 111) | P (N = 161) | |
| Overall number of infusions with one or more deviation, n (%) | 961 (47.9) | 33 (36.7) | 69 (53.1) | 55 (42.0) | 76 (78.4) | 34 (45.9) | 84 (29.8) | 53 (63.1) | 90 (62.9) | 68 (48.9) | 13 (17.8) | 96 (93.2) | 22 (19.3) | 15 (9.9) | 77 (62.1) | 15 (13.5) | 161 (100) |
| Procedural deviations | |||||||||||||||||
| Giving set not labelled correctly, n (%) | 537 (26.8) | 7 (7.8) | 12 (9.2) | 3 (2.3) | 70 (72.2) | 79 (28.0) | 52 (61.9) | 67 (46.9) | 86 (83.5) | 161 (100) | |||||||
| No label (where required) | 496 | 7 (7.8) | 12 (9.2) | 3 (2.3) | 70 (72.2) | 74 (26.2) | 52 (61.9) | 64 (44.8) | 86 (83.5) | 128 (79.5) | |||||||
| Incomplete or incorrect label | 41 | 5 (1.8) | 3 (2.1) | 33 (20.5) | |||||||||||||
| Start date | 37 | 3 | 1 | 33 | |||||||||||||
| Discard date | 2 | 2 | |||||||||||||||
| Name of drug/fluid | 2 | 2 | |||||||||||||||
| Other | 5 | 2 | 2 | 1 | |||||||||||||
| Additive label missing or incorrect, n (%) | 219 (10.9) | 18 (20.0) | 24 (18.5) | 22 (16.8) | 9 (9.3) | 6 (8.1) | 12 (4.3) | 1 (1.2) | 8 (5.6) | 23 (16.5) | 4 (5.5) | 27 (26.2) | 3 (2.6) | 8 (5.3) | 37 (29.8) | 2 (1.8) | 15 (9.3) |
| No additive label (where required), n (%) | 15 | 1 (0.8) | 2 (0.7) | 2 (1.4) | 2 (1.4) | 4 (5.5) | 1 (1.0) | 1 (0.7) | 2 (1.6) | ||||||||
| Label obscured (not visible in pump), n (%) | 42 | 1 (1.1) | 2 (1.5) | 3 (4.1) | 3 (1.1) | 7 (5.0) | 15 (14.6) | 1 (0.9) | 2 (1.3) | 7 (5.6) | 1 (0.6) | ||||||
| Incomplete or incorrect additive label, n (%) | 162 | 17 (18.9) | 24 (18.5) | 19 (14.5) | 9 (9.3) | 3 (4.1) | 7 (2.5) | 1 (1.2) | 6 (4.2) | 14 (10.1) | 11 (10.7) | 2 (1.8) | 5 (3.3) | 28 (22.6) | 2 (1.8) | 14 (8.7) | |
| Patient identity (name, hospital number or date of birth) | 45 | 11 | 3 | 10 | 1 | 8 | 10 | 2 | |||||||||
| Time | 57 | 3 | 2 | 6 | 2 | 4 | 3 | 20 | 2 | 9 | 6 | ||||||
| Expiry date | 50 | 4 | 1 | 12 | 25 | 5 | 3 | ||||||||||
| Hung by | 39 | 2 | 2 | 1 | 15 | 3 | 12 | 4 | |||||||||
| Volume | 29 | 1 | 1 | 4 | 1 | 3 | 1 | 16 | 1 | 1 | |||||||
| Date | 33 | 1 | 3 | 20 | 7 | 2 | |||||||||||
| Dose | 23 | 1 | 1 | 2 | 2 | 1 | 11 | 4 | 1 | ||||||||
| Drug name | 12 | 1 | 1 | 5 | 1 | 4 | |||||||||||
| Patient’s location (ward) | 13 | 3 | 9 | 1 | |||||||||||||
| Other | 68 | 24 | 5 | 2 | 1 | 3 | 2 | 2 | 24 | 5 | |||||||
| Patient ID, a n (%) | 116 (5.8%) | 9 (10.0) | 18 (13.8) | 2 (1.5) | 9 (9.3) | 5 (6.8) | 3 (3.6) | 11 (7.7) | 14 (10.1) | 1 (1.4) | 2 (1.9) | 8 (7.0) | 2 (1.3) | 21 (16.9) | 3 (2.7) | 8 (5.0) | |
| Documentation deviations | |||||||||||||||||
| Documentation of the administration, n (%) | 335 (16.7%) | 10 (11.1) | 24 (18.5) | 32 (24.4) | 7 (7.2) | 27 (36.5) | 19 (6.7) | 4 (4.8) | 18 (12.6) | 38 (27.3) | 7 (9.6) | 23 (22.3) | 13 (11.4) | 7 (4.6) | 41 (33.1) | 10 (9.0) | 55 (34.2) |
| Start time incorrectly or not documented, n (%) | 269 | 8 (8.9) | 24 (18.5) | 26 (19.8) | 1 (1.0) | 22 (29.7) | 13 (4.6) | 3 (3.6) | 12 (8.4) | 36 (25.9) | 2 (2.7) | 22 (21.4) | 10 (8.8) | 4 (2.6) | 33 (26.6) | 3 (2.7) | 50 (31.1) |
| Nurse’s signature missing, n (%) | 103 | 8 (8.9) | 1 (0.8) | 23 (17.6) | 1 (1.0) | 2 (0.7) | 3 (3.6) | 5 (3.5) | 8 (5.8) | 1 (1.4) | 10 (9.7) | 8 (7.0) | 25 (20.2) | 8 (5.0) | |||
| Other, n (%) | 75 | 1 (1.1) | 1 (0.8) | 4 (3.1) | 5 (5.2) | 6 (8.1) | 9 (3.2) | 2 (2.4) | 3 (2.1) | 2 (1.4) | 4 (5.5) | 2 (1.9) | 6 (5.3) | 3 (2.0) | 15 (12.1) | 7 (6.3) | 5 (3.1) |
| Documentation of the medication order, b n (%) | 36 (1.8%) | 2 (2.2) | 5 (3.8) | 4 (3.1) | 3 (4.1) | 5 (1.8) | 1 (0.7) | 9 (6.5) | 1 (1.4) | 1 (0.9) | 5 (4.0) | ||||||
Giving set labelling
Deviations in giving set labelling affected 26.8% of infusions. Deviations affected 16.7–100% of infusions that required a giving set label across all trusts (Figure 4). Deviations affecting giving set labelling were common at trusts D, G, H, K and P. Rates of deviation were affected by both the level of detail required by local policy and clinicians’ awareness of that policy. For example, at trust D, infusions in all areas except critical care were generally non-compliant; observers learned that their hospital policy required all IV giving sets to be labelled only in the closing stages of data collection, despite being asked to familiarise themselves with the relevant policy prior to data collection. In their debrief meeting, observers reported that this requirement was part of their peripheral cannula policy and that they had not previously been able to find it. At trust P, giving sets were labelled in critical care only when this was needed to differentiate between drugs, but the trust’s policy explicitly stated that all IV lines must be labelled with the time and date they were connected to the patient; none was labelled with this information. Trust K, which had the most comprehensive giving set labelling requirements, also had a high rate of non-compliance (83.5% of infusions); its policy required all IV lines to be labelled with the name and strength of the medicine, route of administration, diluent and final volume, patient’s name, expiry date and time, and name of practitioner preparing the medicine. Patient Safety Alert 20: Promoting Safer Use of Injectable Medicine29 does not specify whether or not giving sets need to be labelled.
FIGURE 4.
Variation in giving set label policy and deviations where a label is required among trusts. Reproduced from Furniss et al. 81 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
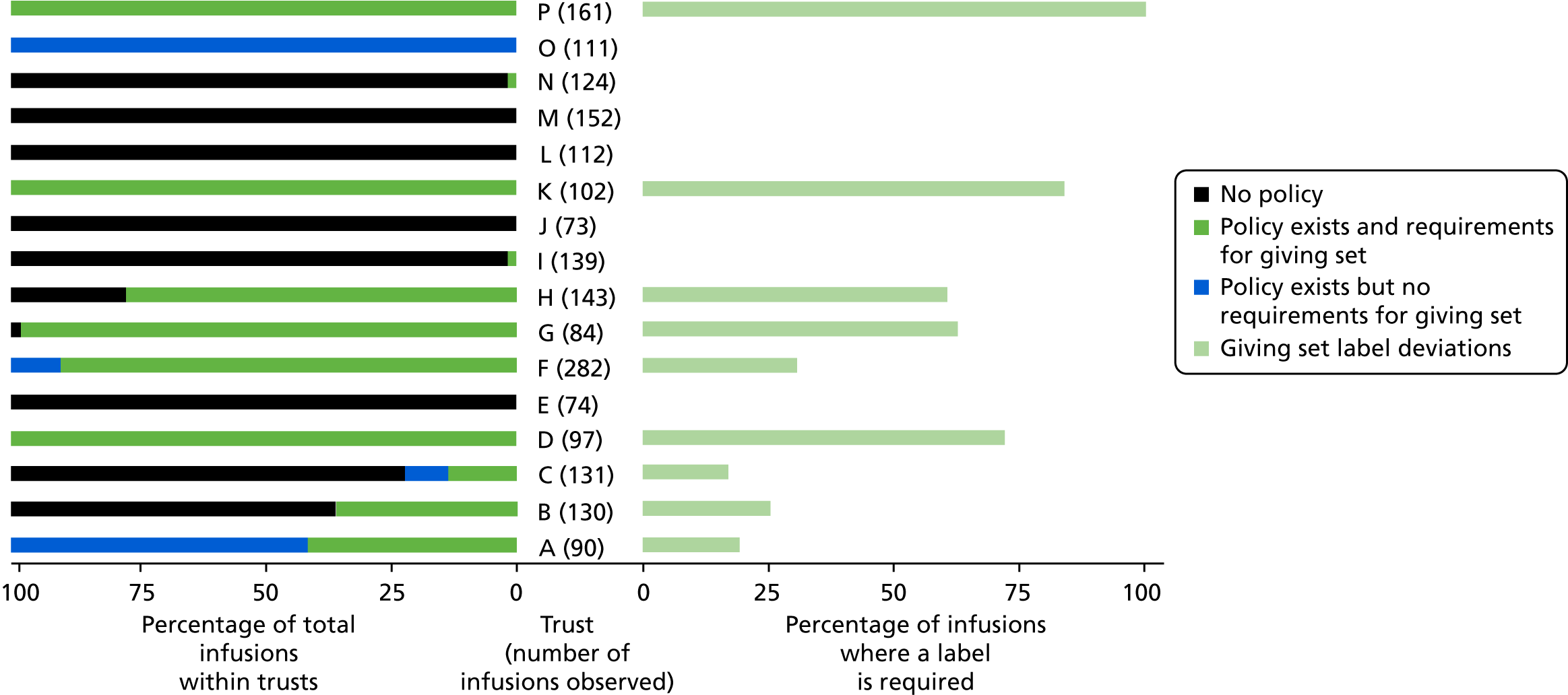
Documentation deviations
Deviations in documenting IV administration affected 16.7% of infusions, ranging from 4.6% to 36.5% across individual trusts (Figure 5). Failure to document the start time was the most common problem. Other less frequent, but potentially more troublesome, issues were discovered during the observations. In some cases, administration was not documented at all. In one case, 20 mmol of potassium chloride in 1 litre of 0.9% sodium chloride was prescribed, but the trust did not stock this formulation. Instead, staff administered two 500-ml infusion bags of 0.9% sodium chloride, with 20 mmol of potassium chloride in one of them. However, poor documentation meant that it was not clear that this prescription had been split across two bags and which was to be given first. Patient Safety Alert 20: Promoting Safer Use of Injectable Medicine29 recommends making a detailed record of the administration as soon as possible after that administration, but does not give more detailed directions.
FIGURE 5.
Policy deviations relating to documentation of medication administration. Reproduced from Furniss et al. 81 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
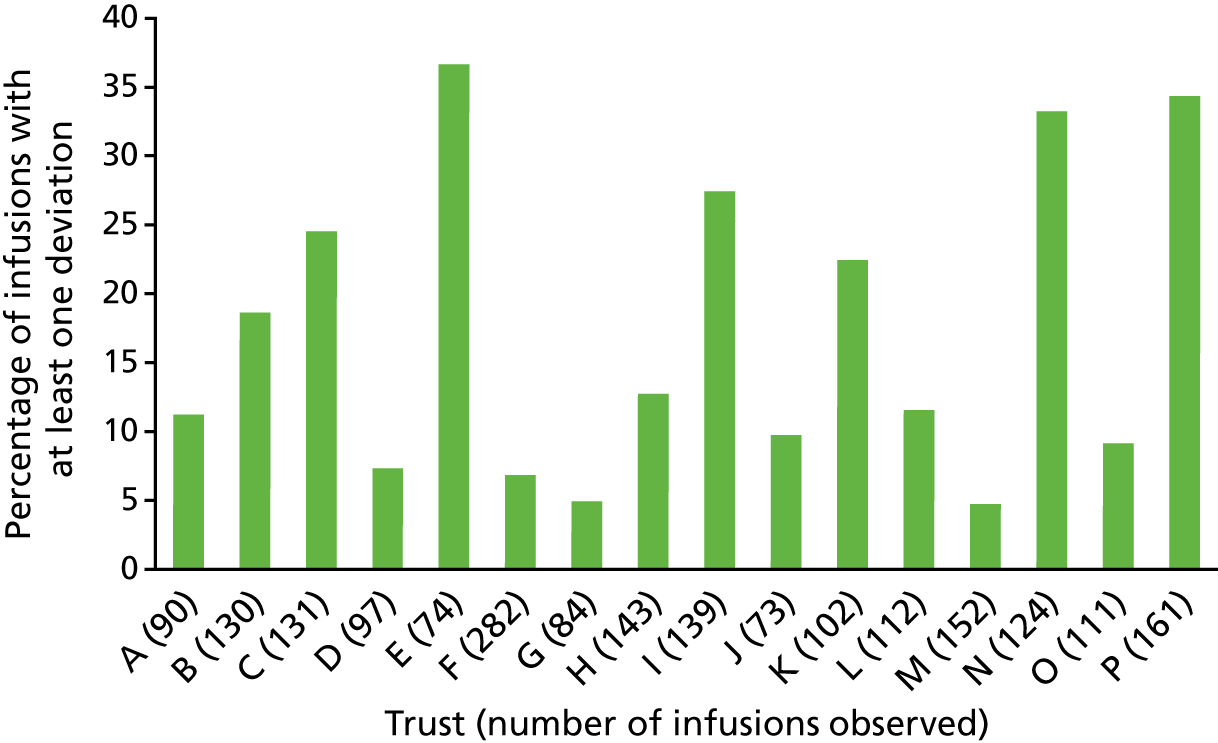
Additive label deviations
Deviations in recording the required details on the additive label affected 10.9% of all infusions. Deviations affected 3.3–74.0% of infusions that required an additive label across trusts, and policy requirements affected different proportions of infusions at different trusts (Figure 6). For example, at trust J, 78% of infusions were standard fluids with no additives, and these did not require a label. Furthermore, not all trusts specified the information required on additive labels in the relevant policy but there seemed to be an implicit expectation in all trusts that nurses should complete all parts of the additive labels. Most additive label deviations were considered low risk by observers and focus group participants, such as missing batch numbers for licensed non-biological medications. As with giving set labelling, trust K had a high deviation rate; its written policy required the most information to be documented on its additive labels: patient name, ward/clinical area, drug, final concentration and volume, administration rate, total amount of drug added to the syringe or bag, batch number and details of the medication added (diluent, date prepared, time prepared, expiry date, expiry time and route of administration). This was more detailed than the Patient Safety Alert 20: Promoting Safer Use of Injectable Medicine29 recommendations of name of medicine, strength, route of administration, diluent and final volume, patient’s name, expiry date and time, and the name of the practitioner preparing the medicine.
FIGURE 6.
Variation in additive label policy and deviations where a label is required among trusts. Reproduced from Furniss et al. 81 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
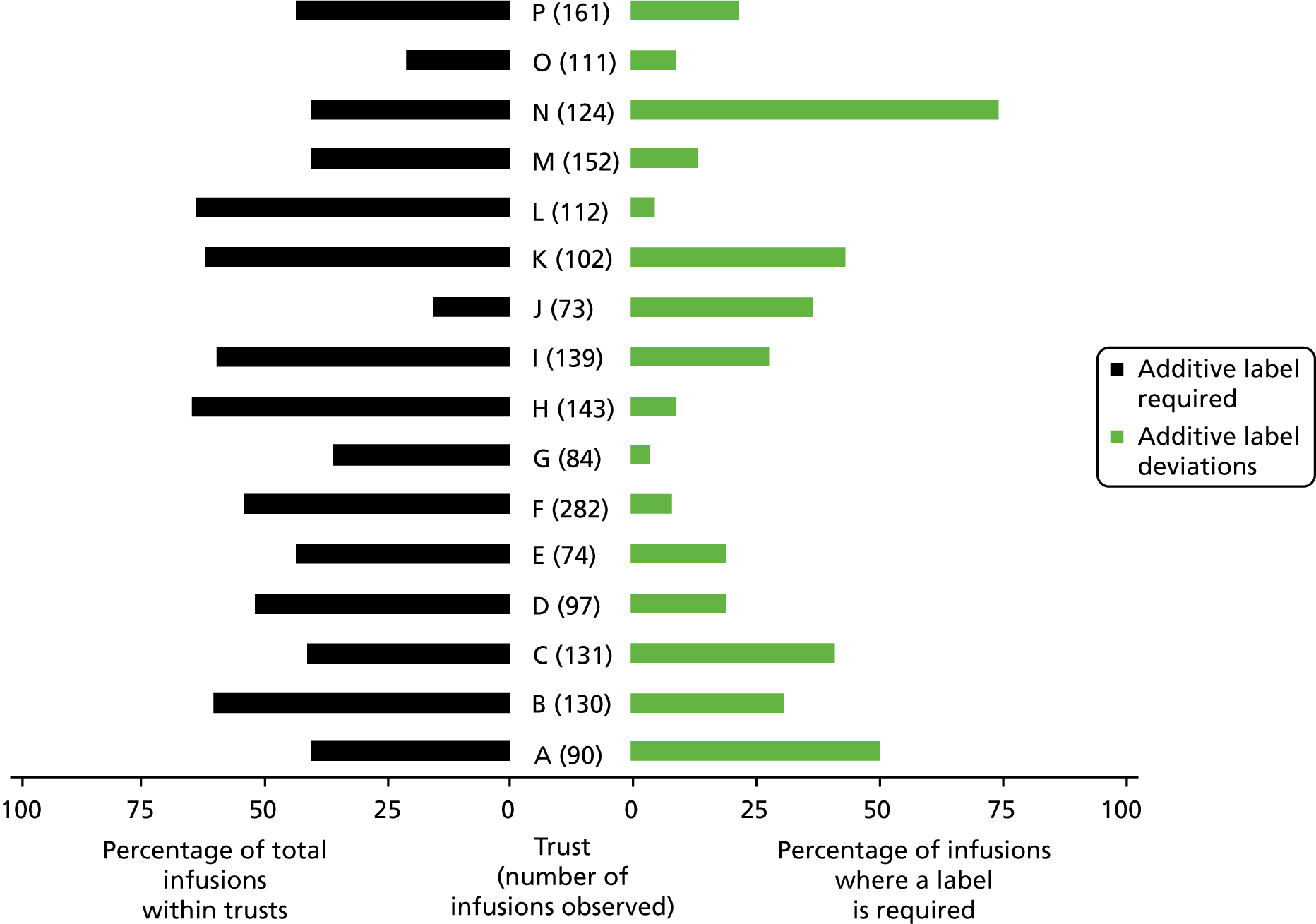
Trust B’s policy required nurses to record the batch number on additive labels. However, nurses in the focus group raised concerns about the utility of doing this, for example for short infusions that would be thrown away after 20 minutes. They suggested that a better place to record batch numbers (if necessary) would be in the patient’s medical records, where this information would be permanent. One nurse suggested that some medications come with a removable batch number sticker that could be stuck in the patient’s notes. Trust D focus group participants said that they had no detailed additive label requirements written into policy, did not expect the batch number to be commonly completed on the label, and wondered if the labels should be redesigned without the section for batch numbers.
Potentially significant deviations included an additive label only marked ‘DEX’, which referred to dexamethasone but could have been confused with dextrose or other drugs; and a completely unlabelled syringe of fentanyl in a syringe driver. Other additive label deviations initially were suspected to be medication errors but, on further investigation, found to be solely documentation issues. For example, observers found a 1000-mg bottle of paracetamol infusing into a patient prescribed 675 mg. However, the nurse reported that they had removed 325 mg before setting up the infusion, so the patient would receive the correct amount. The observers pointed out that the bottle was not labelled to indicate that this had been done, but the nurses said that it was usual practice to remove the excess dose and not label these changes on that ward. Patient Safety Alert 20: Promoting Safer Use of Injectable Medicines29 makes no recommendations for the process of removing excess dose but does recommend that labels are used for medicines prepared in clinical areas and that detailed records of administration are made.
Patient identification deviations
The percentage of infusions with a patient ID deviation varied between clinical areas: general surgery (2.5%), critical care (2.5%), general medicine (5.1%), paediatrics (9.9%) and oncology day care (10.3%). Patient Safety Alert 20: Promoting Safer Use of Injectable Medicines29 recommends that patient ID and details are checked in accordance with local policy. NPSA, Safer Practice Notice 11: Safer Patient Identification85 recommends that all hospital inpatients in acute settings wear ID wristbands.
The deviation rate relating to ID wristbands was 5.8% overall, ranging from 0.0% to 16.9% across trusts (Figure 7). Trust F was fully compliant, which may be because it had prioritised this area and had been auditing this practice prior to our study. Trust C was the only trust whose policy stated that patients receiving IV infusions in oncology day care were not required to wear ID wristbands. At trust B, the oncology day-care manager reported ongoing problems with ID wristband compliance in oncology day care, although local policy required these to be worn. She perceived that it was difficult to change staff behaviour and reported technical problems with the printer used to print the wristbands.
FIGURE 7.
Policy deviations relating to patient ID. Reproduced from Furniss et al. 81 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
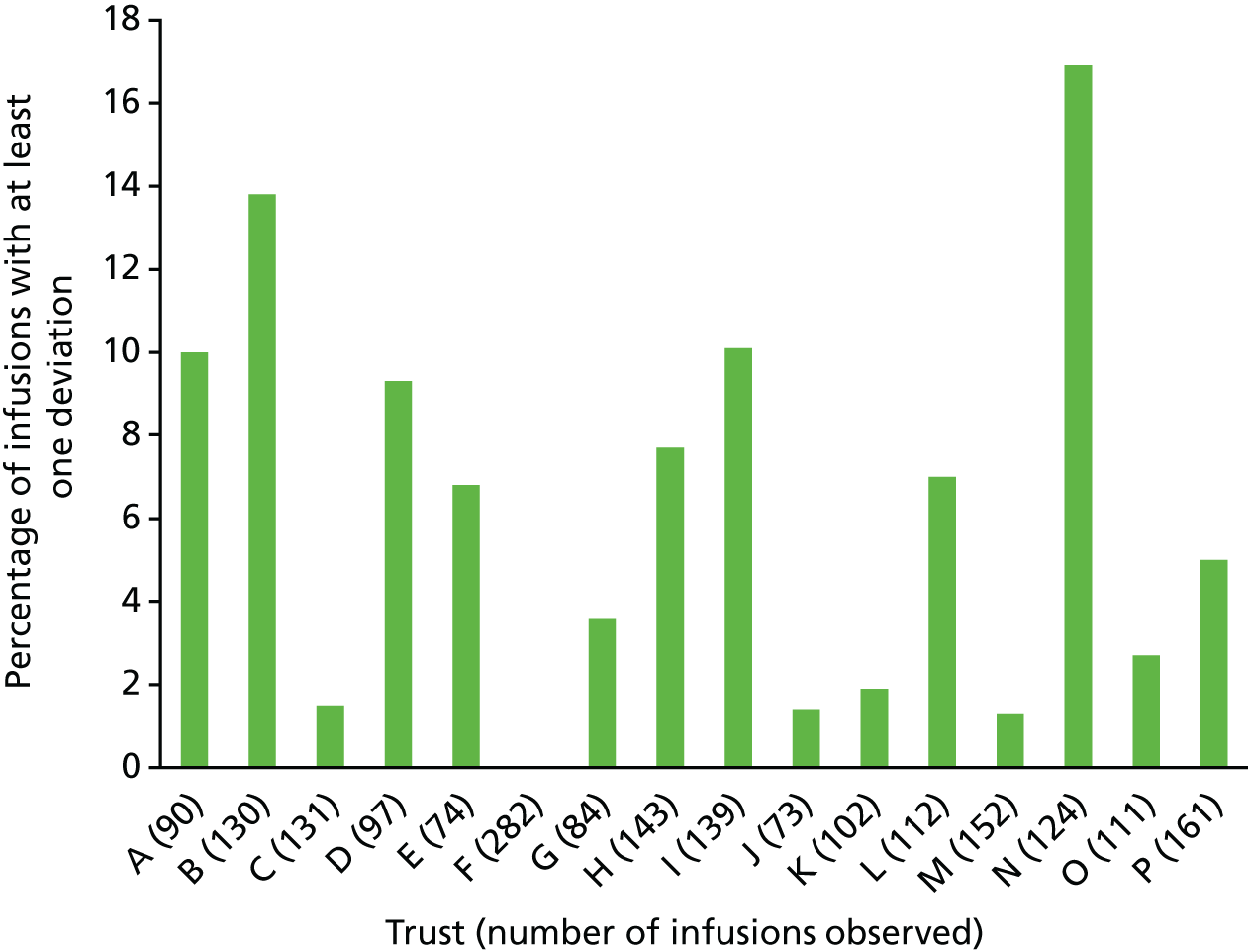
Variability in intravenous flush policies
Most trusts had a patient group direction (PGD) that allowed nurses to administer small-volume flushes (e.g. 1–20 ml of 0.9% sodium chloride) without a patient-specific medication order. However, oncology day-care units sometimes used larger flushes that would need a separate prescription if they fell outside the limits of the PGD (e.g. up to 250 ml or 500 ml across a series of infusions). However, at trust K’s oncology day-care unit, these larger volumes were administered without a prescription or a PGD; this practice was deemed acceptable by the trust’s haematology and oncology care oversight groups. Trust D had an electronic prescribing system that automatically included larger flushes in its chemotherapy regimens, although one flush was observed running but was missing from the medication order.
The issue of whether to flush the whole giving set or just the IV access device arose at a number of trusts. For example, at trust P, a nurse had prepared a 100-ml bag of 0.9% sodium chloride that was not prescribed, and was beyond the 20 ml PGD limit, to flush between giving omeprazole and furosemide infusions. The nurse intended to flush the whole giving set to ensure that the whole dose was administered and to avoid manipulating the connection with the access device. This was noted as unusual practice by trust P observers, who reported that the first giving set would usually be detached, the access device flushed and then a new giving set connected for the second drug. However, some focus group participants recognised that this may lead to partial infusions as some of the dose would remain in the giving set. Patient Safety Alert 20: Promoting Safer Use of Injectable Medicines29 recommends flushing the access device before and after administration.
Variability in double-checking policies
Patient Safety Alert 20: Promoting Safer Use of Injectable Medicines29 recommends double-checking systems (e.g. an independent check or smart pump technology), but it does not go into detail about how these should be done. Different single, double, independent and second checking procedures were required for IV infusions at different trusts. Trusts A and O explicitly permitted single checking for IVs except for specified high-risk drugs, specific situations and controlled drugs. For example, trust O’s policy required staff to double-check before administering chemotherapy. By contrast, trust G’s policy required staff to double-check all stages of preparation and administration from cupboard to bedside, although there was acknowledgement in the focus group that this was not always practical. The wording of double-checking policies at some trusts implied that this was ‘required’, whereas others seemed more flexible, with wording such as ‘where possible’. During the focus group, nurses and pharmacists at trust P recognised that the wording of their policy was ambiguous. The policy was intended to mean that a second clinician signs to confirm that the right patient is receiving the right drug with the correct pump settings, whereas the nursing staff who attended the focus group thought that the second signatory only confirms the contents of the bag or syringe. Trust P’s pharmacy staff also wanted to move away from the concept of a ‘second checker’, as this terminology suggested that it could be a less important and only confirmatory role, and move towards a ‘second administrator’ who was equally accountable and would be expected to carry out a thorough independent check. Trust I was the only trust to have a separate detailed appendix to its main policy that specified what an independent double-check involved.
Summary
Almost one in two infusions had at least one deviation from local policy. Adherence to procedural requirements varied markedly, and it was difficult to make comparisons across sites due to wide variation in local policies, as recently reported in the USA. 7 Most participating trusts had a lower prevalence of deviations relating to additive labels and patient ID than in the hospital studied by Husch et al. ,6 and fewer additive label and giving set labelling deviations than those reported by Schnock et al. 7 We found that the prevalence of deviations involving patient ID wristbands was higher than 0.2% of infusions, as recorded by Schnock et al. 7 The inclusion of oncology day-care units and paediatrics in our study exacerbated this difference because of their deviation rates are higher than those of other clinical areas.
Nurses as a source of system resilience
Parts of this section are based on Vos et al. 30 © 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CCBY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
An overarching theme identified in the debriefs and focus groups conducted in the phase 1 sites was nurses acting as a source of system resilience in relation to supporting the quality and safety of IV infusions. This section reports these findings. During initial inductive analysis, we identified commonalities with the four types of resilience described by Larcos et al. 39 and therefore conducted a deductive analysis based on this framework.
We identified many different types of behaviour on the part of nursing staff that contribute to system resilience, summarised in Table 13. These accounted for three of the resilience themes presented by Larcos et al.:39 anticipatory resilience, responsive resilience and resilience based on past experience. We did not identify examples relating to the remaining theme of attentiveness. Our inductive analysis revealed two further themes: workarounds (usually where policies were perceived to not be fit for purpose) and nurses performing their own informal ‘risk assessments’ in relation to how best to treat individual patients. Each of these five themes is presented in turn.
| Example | Resilience characteristic(s) based on Larcos et al.39 plus additional themes (workarounds and risk assessments) |
|---|---|
| Greater attention is given to medications perceived as being higher risk, such as insulin and chemotherapy |
Anticipatory Nurses performing risk assessment |
| Some nurses perceived that it was less important/risky for patients not to be wearing an ID band if they were oncology day-case patients who could communicate effectively, if they were known to staff or if they received only fluid replacement rather than other medications, and therefore they might choose to focus on getting higher-risk patients to wear them | Nurses performing risk assessment |
| Nurses were resourceful in searching through different charts when patients were transferred from one area of the hospital to another, as any infusions already in progress might be prescribed on different charts or in different systems. Nurses used their clinical judgement in continuing fluids as in the best interest of the patient while waiting for doctors to write a new prescription |
Anticipatory Past experience |
| Nurses sometimes questioned prescribers if they perceived mistakes to have been made in prescribing | Responsive |
| Nurses adjusted their action (e.g. 2 × 500 ml bags of fluid) if the product ordered by the prescriber (e.g. a 1000-ml bag of fluid) was not available | Responsive |
| Nurses may change the concentration of an infusion if they needed to reduce the amount of fluid the patient was receiving |
Responsive Past experience |
| Keeping a medication order active when it is no longer required, such as noradrenaline in critical care, in case the blood pressure falls and the medication needs to be restarted |
Anticipatory Responsive |
| Following verbal orders (e.g. a verbal decision on a ward round to start a medication) although the medication order will not be written until the prescriber has time | Responsive |
| Working around policies perceived to be impractical (e.g. giving small-volume sodium chloride flushes without a medication order, which was perceived to be impractical for them to be individually prescribed) | Workarounds |
| Additive labels not being fully completed, such as with details of batch numbers, if the infusion was due to be up for only a matter of minutes |
Workarounds Nurses performing risk assessment |
| Labelling of giving sets was seen as making practice safer in critical care areas where patients are given multiple infusions, even if not required by local policy |
Anticipatory Responsive Past experience |
| Adjusting the times at which infusions are given in order to space infusions at appropriate intervals when an earlier infusion started or finished late |
Responsive Nurses performing risk assessment |
| Using a blood giving set to run fluids when anticipating that a patient may need blood |
Anticipatory Responsive |
Anticipatory resilience
Larcos et al. 39 define anticipatory resilience as proactively making a decision or taking a course of action that has an expected consequence in a given situation. Such situations were behind some of the deviations discussed in the debriefs and focus groups. For example, nurses were reported as taking action to save time on low-risk infusions so that they could allocate more time to high-risk infusions (e.g. chemotherapy). In other cases, nurses reported making changes to infusion rates if this was judged to be in the best interests of the patient, which could have been classed as a deviation in our quantitative study. Participants suggested that prescribing an infusion with the infusion rate expressed as a range might provide more flexibility to respond to patients’ conditions where appropriate:
You may had it on neonatal intensive care unit or someplace but we do it on ITU [intensive therapy unit] we get a range of an infusion, we’d get one to ten depending on what the patient’s needs are. [. . .] The prescription doesn’t have to get rewritten and the nurse can play around with the medication in the middle.
Site F
Some trusts had detailed policies stating how IV giving sets should be labelled and which information should be included; others did not have such policies in place. Practice in terms of giving set labelling also depended on the ward and the type of infusion (intermittent vs. continuous). Critical care units were often mentioned as areas where nurses used giving set labelling to make practice safer even if there was no requirement to do so:
. . . I think you find on ICU that it is made standardised practice, to have a line tagging in place. [. . .] And it is our policy in critical care, and we always train new nurses to label all the lines. [. . .] Because obviously, wherever you got multi-lumen central lines, etc., etc., you know, obviously you’ll five lines hanging off it [. . .] It’s difficult to identify which is which where it gets near to the patient, so obviously at that point, line tagging is absolutely essential to see what’s on what line going into the central line, etc. [. . .]. However, if there was a patient come to the ward who just wanted infusion by link to a single peripheral cannula, then there probably isn’t such the same kind of need or ethos around line tagging [. . .]
Site D
Where the labelling of giving sets was not common practice, participants revealed that this did not necessarily mean that no information was available about when the giving set needed to be changed. For example, nurses might hand over these details:
Basically there’s other systems for monitoring lines, like [name] has just described, which are preferred to necessarily tagging the lines. So as part of the critical care daily check, lines, as well as the things that they talk about, not just at nursing handovers, but, actually, as part of the main medical ward round, so it’s a discussion point for the care of that patient, and therefore the information is better in the electronic system which is what they’ll be referring to during the ward round [. . .]
Site N
Occasionally, proactive practice when setting up infusions could also cause deviations. For example, the use of blood giving sets for other types of infusion could be considered a deviation, but this may reflect nursing staff anticipating that patients may urgently need blood products.
Responsive behaviour
Responsiveness is defined as reacting effectively when a situation changes. Nurses reported not following a medication order exactly if it was not possible to administer the medication as prescribed. For example, when a prescribed volume of fluid (such as 1000 ml) was not available, nurses described modifying their practice based on what was available (such as giving two bags of 500 ml). This ensured that the patient still received the prescribed treatment, although the medication order was not always updated to reflect what was actually given. Nursing staff perceived that this would result in delays and so they had to make a choice between interrupting or delaying treatment until a valid prescription was available and starting treatment without the medication order precisely matching what was given:
So they did only have 500 mls available? So they needed to give a litre, so they split the prescription, not the prescription, but they split where they signed by 500 mls and they gave 500 mls, so they signed it twice, 500-ml bags against the prescription that is 1 litre. It’s not an error but there’s a potential . . .
Site B
Frequently, patients’ conditions fluctuated, requiring nursing staff to think critically about the medication prescribed. In these cases, improving the safety of IV therapy might mean that a patient no longer needed the drug or amount that was initially prescribed. Safer IV practice in these instances would require nurses to adjust their plan according to the situation, based on their clinical judgement and preferably informing the prescriber, but those actions could lead to the reporting of a deviation (particularly if there was a delay in updating the documentation):
[. . .] the prescription there for [noradrenaline] for example, but if it’s not required we stop it. So in that situation it could be a discrepancy as well because the medication order is signed to be given, but we’re not giving it because of clinical judgment.
Site B
Patients’ clinical conditions also affected nurses’ judgement about how to interpret the prescription, for example by adjusting the concentration of a drug solution:
I think what happened is patient had deteriorated, so rather than getting loads and loads of fluid through the noradrenaline they increased the concentration of the noradrenaline. So rather than giving an 8-mg syringe driver they gave 16-mg syringe driver. So with regards to the prescription, it was still within that . . . um range, but they just increased the concentration of the medication to give them less fluid and so the medication doesn’t run out as quick. So they’re still getting the same dose, but just a more concentrated form [. . .]
Do doctors always prescribe this when it’s double strength or triple strength?
No.
So that’s often a decision that the ITU [intensive care unit] nurse makes, changing the concentration, but the patient’s still getting the same amount of drug.
Site F
There were also other situations in which deviations were identified as a result of nurses acting in the patient’s best interests. These included occasions when one of several infusions was delayed to avoid risks of administering incompatible infusions via the same IV access, when verbal orders were given, or when nurses had requested that the doctor make changes to the documentation but this could not be done:
. . . we saw somebody put up a fluid and there was no prescription for it [. . .] well they’d increased the fluids overnight, overnight there’s skeleton staff, [name] had requested a prescription to be written, but it hadn’t. [. . .] at the moment the surgical team were in theatre, there’s nobody to come and write a prescription [. . .] so what do you do, do you just not put up the fluid that the child needs?
Site I
In addition, patients might have been away from the ward to undergo tests, which led to nurses having to ‘catch up’ with infusions. In situations like this, nurses worked to provide safe IV practice while dealing with competing priorities, all of which could have an impact on the patient’s condition:
So that then you would delay the next dose . . . [. . .] But, you know, then you still try and work to try and get it back on track otherwise you end up totally out of sync. One dose is going to have to be given a little bit earlier then just to get it back on track.
Site C
Prescribing errors were not the focus of our study. However, our data suggest that nursing staff often improved the safety of IV practice by questioning prescriptions if they thought that a mistake might have been made:
[. . .] it’s very clear in our policy that if it doesn’t match [prescription and drug library], then, speak to your ward pharmacist. Speak to a pharmacist or speak to the prescriber to challenge that prescription. It’s very clear in our policy that we don’t just hope for the best.
Site K
Past experience
Past experience is defined as drawing on existing knowledge to influence the sequence and nature of work activities. 39 As well as the current situation in which nurses were working, their approach to the safety of IV therapy seemed to differ according to their familiarity with the infusion, their training and their previous experience.
Often, different medication charts and prescribing systems were used in the same trust, leading to deviations being reported because the observers could not find the relevant chart. If patients had transitioned through the hospital, they might have arrived from another clinical area with IV fluids still running. Nurses had to scrutinise a patient’s documents to find the original prescription in order to provide safe IV therapy:
And you have so many different charts, as well. For instance, you get a kid from [unclear], they have a different drug chart in A&E [accident and emergency], and he goes to [another ward] and gets a different drug chart. Gets worse and goes to ICU, has another different drug chart. Or goes to theatre then to ICU, and you’ve got some drug that are written on some piece of paper in illegible writing. [. . .] You can’t find it. You’re relying on what people have told you beforehand.
Site L
Intravenous medication started in another clinical area might have been prescribed on a different document and/or not yet re-prescribed on the relevant documentation for the new area. If medication was not yet re-prescribed, nurses were left to decide between not providing further IV therapy, and continuing the medication and requesting a new medication order later:
[. . .] what we do have to be careful is where we have different prescribing systems in operation and we’re transferring patients. Quite often we get people saying, for example on acute medical unit that patients haven’t been prescribed fluids in A&E [accident and emergency], but they’re running. And in fact they’re on the central alerting system and not on the orange drug chart. And the patient will get transferred from a paper ward to an electronic with fluids running. The prescription is on the paper but there is no active prescription on electronic, so it looks like that’s not an authorised medication. But in fact it’s an existing prescription on a different prescribing system.
Site K
Workarounds
Our findings suggested that some policies might need to be revised to better align with practice. For example, although verbal orders were not permitted, staff often acknowledged that practice deviated from policy in this respect:
Our medicines policy is perhaps a bit naive in saying we should not do verbal orders. Which is fundamentally what it says at the moment. And then perhaps we do need to go back to revisit where verbal orders are taken, which would be additional, you know.
Site D
Nurses sometimes worked around policies that were perceived to be inefficient or unworkable:
Often we’ll walk around and say can we give another fluid bolus, and we’ll walk off because we’re busy, and we’ll say that’s allowable for two boluses, and then needs a formal signature. And then two further boluses can be given like that, repeatedly. Because we were just finding that [. . .] for the nursing staff to hunt us down to try and get a signature would consume a lot of their time.
Site H
Staff at some trusts had created a workaround for the administration of small-volume flushes such as 10 ml of 0.9% sodium chloride, as staff did not feel it was realistic for these to be individually prescribed. Whereas some trusts used PGDs to allow nurses to administer flushes to pre-defined groups of patients, and some used pre-filled syringes licensed as medical devices rather than medicines, others reported it to be ‘common practice’ to give flushes that were neither prescribed nor covered by a PGD:
Yes. It happens across the trust. [. . .] Because, the trust doesn’t want to pay for the expense of medical devices because obviously it costs [. . .]
Site D
Practically, the number of prescriptions that would have to be written for saline flushes, we did look at this would it be possible to have a patient group [direction], but then we got medical devices that are used for saline flushes and you’ve got saline flushes, so do we have a PGD just for those times that you don’t use a medical device.
Site I
The use of additive labels to specify anything added to an infusion was noted as a mechanism to improve the safety of IV therapy. Some trusts used pre-printed additive labels with blank boxes that had to be completed by the nurse. However, additive labels were not always completed in accordance with the policy. Several reasons were given for incomplete or missing labels, including saving time on low-risk infusions and the relevance of some of the requested information, particularly batch numbers:
So, the batch number should go on the documentation that you keep, whereas putting a batch number on a label that you’re putting a bag up, you give it an hour, then you throw it in the bin. What is the point in that, surely?
Some of my infusions are 10 minutes. So, by the time you . . . Through two pages’ worth of paperwork to get a 10-minute bag up . . .
Site B
In summary, in common with previous studies on workarounds,47,48 it was found that nurses introduce workarounds – many of which then become established practice – to facilitate their work, and often to deal with deficiencies in the broader system of care. Although these are commonly in the patients’ best interests in the short term, many may compromise patient safety in the longer term.
Nurses performing risk assessments
Although incomplete additive labels were not deemed good practice, nurses reported this as a way of freeing up time to allow them to focus in greater detail on higher-risk infusions. On occasion, nurses did not label giving sets, even when there was a policy requiring this. The perceived risk of not labelling giving sets differed depending on the type of infusion (e.g. replacement fluid vs. a specific drug):
I don’t know if they tag all of the fluids necessarily. They tag all of the infusions of drugs because they want to make sure that when they change a syringe or the vial that they’re connecting the right to the right. But I don’t know if they would do it necessarily with IV fluids, which is probably something we need to pick up.
Site P
Although most participants acknowledged the importance of patients wearing ID wristbands for safe IV therapy, it was perceived that some situations did not allow patients to wear them (e.g. emergency) or made it difficult for them to be issued (e.g. broken printer, limited availability of staff). In these instances, nurses might have to make a decision between breaking the policy stating that an ID wristband was necessary for treatment and not giving a treatment that a patient might urgently need.
. . . there were times in [emergency department] when you’d come for [resuscitation] and you wouldn’t get your ID bands.
Site N
In practice, the risks associated with missing patient ID wristbands were felt to differ depending on the patient. Specifically, risks were perceived to be lower for inpatients who were alert and able to confirm their identity verbally, for ‘known’ oncology outpatients, and for patients receiving fluids rather than other medications:
It all depends what they receive, if they just have fluids it [ID band] probably doesn’t matter, yes, whether it’s the right patient or the wrong patient, unless they have sort of cardiac failure, etc., but you don’t want to give the chemotherapy to the patient or things like that, or any medication which has more implications.
Site B
However, as the following quotation illustrates, such risk assessments may not be appropriate, given that IV iron is also a relatively high-risk medication:
[. . .] we almost never make a chemotherapy error that I know of but we’ll make errors with other things, you know. Because, again, that, sort of, attention to detail and the seriousness of doing, like, you probably would never make an error with insulin, or, you know . . . Because you, you can’t . . . You just can’t make errors with that stuff, but you can make an error with an iron infusion. [. . .]
Site B
Summary
This section has reported our findings related to nurses as a source of system resilience in relation to IV therapy. This may account for some of the more minor deviations identified in our quantitative study that resulted from judgements made by nurses with the aim of improving the safety of IV therapy, often in the context of limited resources and conflicting pressures. These findings also illustrate how the safety of the system appears to rely in part on individual strategies and behaviours that contribute to system resilience.
Perceived layers of influence
Through iterative comparison, and looking for potential relationships between concepts, we constructed a list of key concepts reported by participants:
-
policy and regulation
-
interactions
-
staffing
-
learning
-
risks
-
data content and retrieval
-
clinical practices
-
logistics
-
staff
-
mental models
-
supporting technologies.
Figure 8 is a contextual layers diagram illustrating how the main concepts map on to the contextual layers relevant to IV medication practices. The level of each concept is suggested partly by the qualitative data, and partly by our general understanding of how the health-care system is organised.
FIGURE 8.
A mapping of concepts uncovered to the contextual layers of infusion practices (concepts shown in white text; contextual layers shown in black).
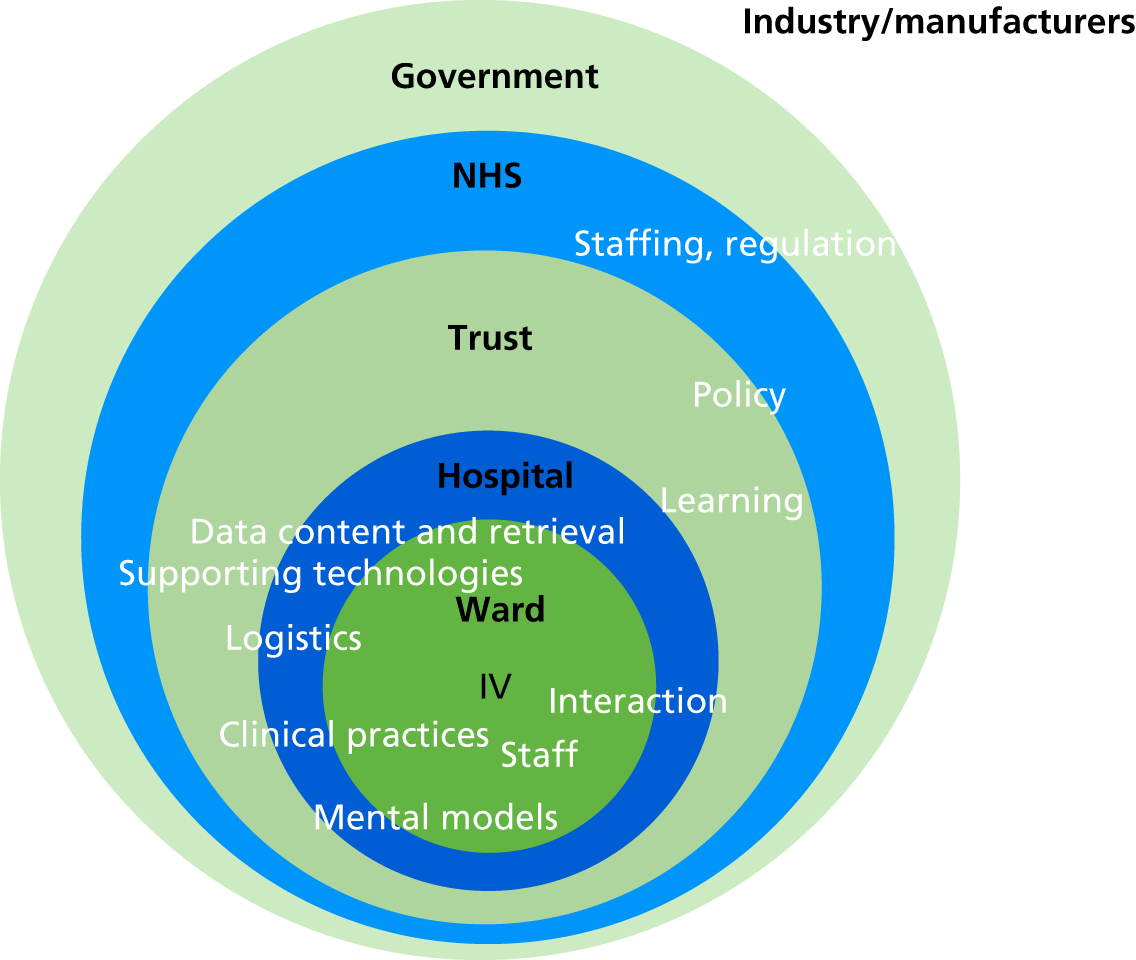
The concepts at the top of the list above, namely policy and regulation (including guidelines and protocols) and interactions (in their wider sense, including staff interactions with artefacts such as devices and documents), had the greatest number of initial ‘codes’, meaning that participants in the debrief and focus groups most frequently referred to those concepts. The density of referencing to the core concept of policy suggests that several other IV domain concepts would be significantly affected by changes to policy.
Health professionals’ awareness of, ease of access to and recall of relevant IV -related policy is likely to influence their compliance. Intervening conditions include staff training and experience and the mental models they have formed (through practice and other factors), as well as working conditions such as pressure of work and the number of patients attended to. The interaction of staff with the wider context – devices, policies and procedures – can raise the visual, cognitive, memory and motor loads placed on them, which may increase the likelihood of errors. Access to relevant data, and the data flow about patients’ conditions and risks, are influential in shaping work practices.
Figure 9 depicts the findings of the qualitative data analysis, but here in terms of the perceived relative impact of the revealed concepts, placing the highest-impact concepts at the base. The impact is deduced from the qualitative data: the more frequently concepts (and their subconcepts) are pointed to in accounting for, or explaining, the phenomena under investigation, the more the concepts may have impact on such phenomena, from the participants’ views. What determines the order of the layers is also the frequency with which the concepts that constitute each were referred to in the debriefs and focus groups. Interaction between staff and technology relate closely to policy. The bottom layers are more ‘core’ to explaining the IV administration deviations identified through the point prevalence study. The hierarchy suggests that the layer encompassing policy, interactions (viewed widely to include interaction with artefacts) and staff (including skills, training and staff loading) is perceived to have the most influence on observed deviations.
FIGURE 9.
Layers of influence in the infusion administration domain.

The next level up features data content and how various items of data, such as policy details, patient data, IV labels, IV timings, drug data and drug charts, are structured, inter-related and retrieved for ease of use and understanding.
Next is the level of detailed clinical practices and medical device usage (including the constraints particular to each make or model of the technology). Clinical practices include drug documentation practices, signing for drugs, interruptions, attaching and detaching tubing, signing on checking drug preparations, and official and unofficial practices (see also Chapters 5 and 7). Supporting technologies include drug libraries and their maintenance (including drug profile design and maintenance), network connectivity and availability.
The layering according to expected impact may be helpful in guiding prioritising of future interventions to influence IV infusion practices. Strategic attention can be given to the most influential layers, but this must be moderated by the timescale and expected cost of implementing changes.
Summary
In this chapter, we have reported on three analyses, each taking a different perspective on the overall system of practice. The first highlighted the variability in procedural and documentation practices around IV infusion administration, illustrating local rationality but global variability; this is consistent with the view of Braithwaite et al. ,62 who argue that complex systems evolve in ways that have local stability, based on local history, resulting in localised communities of practice. The second highlighted ways in which professional nursing practices contribute to overall system resilience; again, this emphasises the roles of skilled professionals in adapting to system perturbations in ways that contribute to system stability. The third highlights the perceived centrality of local and national policies in shaping IV infusion practices; it also shows that clinical staff did not discuss the design of supporting technologies such as smart pumps as much as other factors that affect safety in IV infusion administration. The potential impact of smart pumps is analysed in more detail in the next chapter.
Chapter 5 The potential impact of smart pumps in the English NHS
In this chapter we describe further exploratory analyses of our data, plus a secondary analysis of a data set from the NRLS for England and Wales, to explore the potential impact of smart infusion pumps in preventing errors in the English NHS context.
Further analysis of phase 1 data
The first phase of the ECLIPSE study identified 211 medication administration errors (NCCMERP severity index level C or above) through direct observation of infusions.
Types of errors in smart pumps, traditional pumps and gravity administration
We first conducted a descriptive analysis of the types of medication and documentation errors occurring in those infusions given via smart pump, those given via traditional types of pump and those given via gravity feed. These data are summarised in Table 9 and suggest that documentation errors were less likely with smart pumps, but that labels not being completed or giving sets not being tagged according to policy were more likely in infusions given via smart pumps. It is not clear why this would be the case, but the most likely reason is more stringent policies in trusts using smart pumps (so more opportunities for deviations from those policies). Rate errors were more common in infusions given via gravity, as might be expected, but there was no difference between smart and traditional pumps (see Table 9). Delays in administration were also more common in infusions given via gravity. Patients not wearing ID bands were more likely when infusions were given via traditional pumps, most likely to be a result of the lower prevalence of ID bands in oncology day-care units, where traditional pumps were routinely used. Incomplete or delayed completion of the infusion was less likely in infusions given via smart pumps and, as might be expected, errors involving roller clamps were more common in those given via gravity feed.
Role of smart pumps in preventing or contributing to errors
We then assessed the preventability of these medication errors using smart infusion pumps, or the potential contribution of smart pumps where these were used.
Including the medication administration errors (n = 211) and the miscellaneous errors (n = 5), there were 216 errors of severity level C and above identified across the 16 organisations. Of these 216 errors, 157 were in infusions not given via a smart pump. The remaining 59 were in infusions given via a smart pump.
Preventability of those 157 errors identified in infusions that were not given via a smart pump is summarised in Table 14.
| Preventability with a smart pump | Number (%) of errors |
|---|---|
| Not preventable | 50 (32) |
| Preventable with any pump | 21 (13) |
| Not applicable (blood products or bolus) | 4 (3) |
| Possibly preventable with smart pumps as part of a closed-loop system, with details of the medication order populated from an electronic prescribing system | 80 (51) |
| Possibly preventable with a standalone smart pump depending on limits set in drug library, or with a closed-loop system | 2 (1) |
| Total | 157 (100) |
The two errors judged to be possibly preventable with a standalone smart pump, depending on the limits set in the drug library, were as follows:
-
Dexamethasone was administered over 11 minutes, but the IV guide and prescription both stated that dexamethasone in 100 ml of saline should be given over 15 minutes. This would depend on how the limits were set on a smart pump and whether or not this rate discrepancy would create an alert.
-
Hartmann’s solution was given at 7.5 ml/hour but a rate of 125 ml/hour was prescribed. This would depend on whether or not ‘too low’ rate limits were set on a smart pump – which is unlikely, as even very low rates are sometimes required in clinical practice.
An analysis of the role of smart pumps in preventing or contributing to errors identified in infusions given via a smart pump is summarised in Table 15.
| Role of smart pump | Number (%) of errors |
|---|---|
| No effect of smart pump in contributing or preventing | 8 (14) |
| Different limits may have prevented the error | 1 (2) |
| Smart pump contributed to the error | 2 (3) |
| Possibly preventable with smart pumps as part of a closed-loop system, with details of the medication order populated from an electronic prescribing system | 48 (81) |
| Total | 59 (100) |
Both of the errors to which the smart pump was judged to have contributed were piperacillin/tazobactam being given over 1 hour instead of the recommended 30 minutes, as the drug library did not allow a 30-minute infusion.
The error that may have been prevented by different limits related to atracurium of 500 mg/50 ml prescribed at 0–5 ml per hour being given at 10 ml/hour in a critical care area.
Expert panel assessment
Seven out of the 13 drug library experts approached agreed to participate. A total of 58 errors were sent, of which 33 involved the use of traditional pumps, 16 involved the use of smart pumps and nine involved gravity administration. Many of the panel members did not differentiate between standalone smart pumps and those linked in a closed-loop manner to an electronic prescribing system in their responses.
Of the 33 errors in infusions given using traditional pumps, the overall view of the panel was that 24 (73%) would be preventable using a closed-loop smart pump or a standalone smart pump with suitable limits, and that nine (27%) would not. This contrasts with the research team’s assessment for these 33 errors, that 16 (48%) would be preventable using a closed-loop smart pump with details of the medication order populated from an electronic prescribing system, and 17 (52%) would not be preventable with any type of smart pump.
For the 16 errors given via smart pumps, the overall view of the panel was that nine (56%) would have been prevented using a closed-loop system, in five (31%) cases the smart pump had no effect on preventing or causing the error, and in two (13%) cases different limits would have prevented the error with a standalone smart pump. The smart pump was considered to have contributed to the error in only one instance, by only one of the judges, a case involving administration of morphine in the incorrect diluent. The research team’s assessment was that 11 (69%) would have been preventable only with a closed-loop system and that in five (31%) cases the smart pump had no effect.
Of the nine errors in gravity administrations, the panel’s overall view was that seven (78%) would have been prevented using a smart pump, one (11%) would have been prevented with any pump and one (11%) would not have been prevented. The research team’s view was that six (67%) would have been prevented by any pump, one (11%) would have been prevented with a closed-loop smart pump, one (11%) was not applicable (an infusion of blood) and one (11%) would not have been prevented with any kind of pump.
The level of agreement among the expert panel assessors ranged from 57% to 100% per error; the median agreement was 71%. There was 100% agreement among panel members that 6 out of the 58 errors were possibly preventable using a smart pump, depending on the limits.
The narrative comments provided by the expert panellists were mainly statements of assumptions made when assessing preventability, although one of the panellists included observations about the types of errors that were included in the sample.
Generally, panellists based their decisions on a ‘best-case’ scenario, that is, the smart pumps were set up to optimal limits and functionality and drew data from the electronic prescribing system, with some different assumptions from those made by the research team. For example, one panellist stated that their assessment was based on the assumption that:
where present they are always used – there is no access to other infusion devices; that all drugs administered via a closed loop system are standardised concentrations of pre-prepared bar coded injectables, and that there are no facilities for the preparation of other doses in clinical areas (non-standard concentrations, hand-labelled etc.) i.e. they are ‘closed’ and strictly adhered to.
Panellist 3
There were contrasting views between panellists about the impact on of the type of device on infusions that exceeded the expiry or prescribed duration, or were not prescribed at all:
That the closed loop smart pump system included barcode scanning of medicines (this would have prevented all errors where the infusion was running without a prescription and those where the wrong drug/dose had been added to the bag).
Panellist 2
That the infusions within the electronic prescribing system (within the closed loop system) could be set up with warnings for infusion expiry and that the system would allow you to prescribe single bags of fluid and also prescribe fluid to run continuously (allowing the nurse to set up consecutive fluid bags).
Panellist 4
Smart pumps cannot address a prescription that has not been prescribed.
Smart pumps cannot reduce the likelihood that drugs and fluids are continued longer than is intended.
Smart pumps may have the capability to alarm up to 24hrs after an infusion has commenced as a reminder to change a syringe.
All panellists assumed that smart pumps were programmable with soft and hard limits, and would control the rate of delivery through the library or through linkage with an electronic prescribing system.
The panellists also noted that smart pumps were unlikely to prevent all infusion errors:
Delays in treatment cannot be remedied by smart pump technology.
Panellist 4
A great many of those errors are about the wider systems involved in IV medication use.
Panellist 5
Additional comments from panel members are presented below:
Interesting how few of the problems in [the] sample could have been mitigated by a smart pump . . . was it a truly random sample?
As in everything, there is often no one solution to a problem. i.e. smart pumps are not the whole answer.
It would appear from completing this that smart pumps plus closed loop electronic prescribing probably aren’t the entire solution either.
I had difficulty entering ‘Not Preventable’ on the table – this is probably a psychological issue I have in accepting that an error is ‘Not Preventable’ – there is always contributing factors therefore these must be able to be altered in order to prevent an error happening again- and that elusive ‘acceptable level of risk’ is forever changing.
Interestingly, the research team was more conservative in their assumptions about the extent to which a smart pump would be able to prevent errors, whereas the expert panel made more aspirational assumptions. Akin to a sensitivity analysis, if we assume that the reality lies between the two extremes, then this might suggest that 48–73% of the errors that occurred in traditional infusion pumps could be prevented with a closed-loop smart pump. As many of the panel members did not differentiate between standalone smart pumps and closed-loop smart pumps linked to electronic prescribing, it is not possible to provide a similar sensitivity analysis for standalone smart pumps.
When assessing preventability using smart pumps, neither the research team nor the expert panel considered the challenges of building drug libraries and associated hard and soft limits, and the associated disadvantages of selection errors or alert overload. A further limitation is that most of the expert panel members were at the early stage of smart pump implementation and may not have seen the full consequences (intended and unintended) of using smart pumps in clinical practice.
Analysis of National Reporting and Learning System data
Following cleaning of the NRLS data obtained, 123 reports relating to incidents involving infusion pumps in the inpatient setting were classed by those who reported them as moderate, severe or resulting in death. Of these incidents, four (3%) were reported as resulting in death, 10 (8%) were reported as causing severe harm and 109 (89%) were reported as causing moderate harm.
Of the 123 reports, eight included references to drug libraries or similar terminology that suggested that they involved smart pumps, some of which were PCA pumps; the remaining 115 reports had no evidence of infusions being given via a smart pump.
Errors in infusions that were not given via a smart pump
For those 115 errors reported in which it appears that the infusion was not given via a smart pump, the results are summarised in Table 16. Nine were omission errors due to pump unavailability or rate errors in infusions given by gravity and therefore could have been prevented by the use of any pump. For 59 errors, the infusion was given by a pump, but the error would not be expected to be prevented by a smart pump, such as wrong rate errors, whereby the rate was incorrect for that particular patient, but within the usual range for the drug concerned. It was noted that some errors were attributed to staff pressing the button (intended for the patient’s use) on PCA pumps and others were attributed to pump malfunctions. Overall, 35 errors were judged to have been potentially preventable with a standalone smart pump, depending on the limits set in the drug library. A further nine were potentially preventable with a closed-loop system in which the smart pump rate was set via an electronic prescribing system. In three cases, lack of detail or clarity in the incident report meant that we were unable to draw any conclusions. Equivalent data for the subset of errors involving the incorrect rate, dose or volume are also given in Table 16.
| Preventability with a smart pump | Number (%) of errors (all errors) | Number (%) of errors (those involving rate, dose or volume) |
|---|---|---|
| Preventable with any pump | 9 (8) | 8 (10) |
| Not preventable with a smart | 59 (51) | 30 (36) |
| Possibly preventable with a standalone smart pump depending on limits pump set in drug library (or with a closed-loop system) | 35 (30) | 34 (41) |
| Possibly preventable with a closed-loop smart pump | 9 (8) | 8 (10) |
| Unable to assess | 3 (3) | 3 (4) |
| Total | 115 (100) | 83 (100) |
Analysis of errors identified in infusions that were given via a smart pump
For those eight errors reported in which it appears that the relevant infusion was given via a smart pump, the results are summarised in Table 17.
| Role of smart pump | Number (%) of errors |
|---|---|
| No effect of smart pump in contributing or preventing | 0 |
| Different limits may have prevented the error | 0 |
| Smart pump contributed to the error | 7 (88) |
| Unable to assess | 1 (12) |
| Total | 8 (100) |
In seven cases it appeared that the use of a smart pump had contributed to the error; this largely concerned selection of an incorrect entry from the drug library. In one case, insufficient detail meant that we were unable to assess the case concerned. The seven cases in which the smart pump was judged to have contributed to the error are listed in Box 1. These comprised four ‘wrong dose’ errors and three ‘wrong rate’ errors. All were reported as resulting in moderate harm. In most cases, it seems that the error occurred as a result of the interaction between the user and the infusion pump, rather than either alone.
-
Amiodarone infusion was completed 12 hours earlier than expected. On investigation, it appeared that the infusion rate had been set for the wrong concentration, relating to the dose of amiodarone having been added to a 1000-ml bag rather than a 500-ml bag.
-
Patient-controlled analgesia pump was set up. Patient weighed 59.4 kg, so choice of bolus dose would have been 0.5 mg of morphine. However, the patient had to have 1-mg morphine bolus doses, as the pumps did not have this protocol set up in the library. The patient had 52 mg of morphine in 8 hours and their respiratory rate dropped to 5 breaths per minute, requiring naloxone to reverse the effects of the opiate.
-
Patient-controlled analgesia pump was programmed at 2 mg/1 ml, resulting in the patient receiving a 1-mg bolus of oxycodone every 5 minutes instead of 2 mg as prescribed.
-
Patient-controlled analgesia pump was incorrectly programmed for pethidine instead of morphine, resulting in the wrong dose being received. Patient’s oxygen saturation on air was 81%. They were given oxygen and saturation came up to 97%.
-
Patient-controlled analgesia was set up using morphine protocol instead of pethidine. Morphine was given at 2 mg/ml with a 1-mg bolus and 5-minute lockout. Pethidine was 10 mg/ml with a 10-mg bolus and 5-minute lockout.
-
A new infusion of dopamine was started using the drug library. The patient became tachycardic and hypertensive, and developed an arrhythmia requiring cardiac massage. On checking the dopamine infusion, it was running at 50 ml/hour instead of 5 ml/hour as the dose had been programmed on the pump as 9.6 [mg in the syringe] as opposed to 96 [mg in the syringe]. The reporter noted that the pump had not alarmed to alert to any problems with dosage.
-
Aciclovir infusion was set up. About 10 minutes later, the patient complained of discomfort at the infusion site. On checking the pump, the nurse found that it was set up for diamorphine and cyclizine instead of aciclovir. This resulted in the rate running at 100 ml/hour rather than 2 ml/hour.
To estimate the number of infusions given each year in English NHS hospitals, we identified several sources of relevant data. First, NPSA’s Safer Practice Notice 01: Improving Infusion Device Safety74 refers to 15 million infusions being performed in the NHS every year, although it does not specify whether this relates to England, England and Wales, or the whole of the UK, and does not indicate the source of the data. If we assume that the data relate only to England, where there are 130,000 NHS inpatient beds,75 then this equates to 115 infusions per bed per annum. Second, an analysis of electronic prescribing data from Imperial College Healthcare NHS Trust (1500 non-critical care beds) suggested that somewhere between 1000 and 2000 doses are prescribed to be given by infusion in 1 day, which is equivalent to 533 infusions per bed per annum for non-critical care areas. Third, based on IV giving set purchasing data, one of our study sites estimated that 180,000 IV infusions are given each year within their 500-bed trust (including critical care), equivalent to 360 infusions per bed per annum. Taking the median figure of 360 infusions per bed per annum, we estimate that there are 45 million infusions given per year in English hospital inpatient departments. Our NRLS data comprised 123 reports relating to infusion pumps in the inpatient setting that were classed as moderate or above over an 11-year period, which equates to one report per 4 million infusions. We note that this estimate has numerous limitations in that the number of infusions is a broad estimate, and that the NRLS data include Wales as well as England. Nevertheless, it indicates that reported incidents relating to infusion pumps that are at least of moderate severity are very rare.
Summary
The under-reporting associated with all incident reporting systems means that it is not possible to draw firm quantitative conclusions from these data. We were also limited by lack of information in many of the reports, which made it difficult to determine exactly what had happened. In particular, very few reports specified that a smart pump was used; lack of denominator data means that it is not possible to conclude whether errors were less likely to be reported in infusions given via smart pump, whether smart pumps were not in common use during the period studied, whether some incidents did involve smart pumps but this was not specified in the report, or a combination of these points. It was also noted that, in many cases, the researchers questioned the severity rating given by those reporting the error, with nothing in the incident description suggesting actual patient harm for many of the reports. For example, for two of the four incidents reported to have resulted in death, there was nothing in the description to suggest death (or, indeed, any harm).
However, these data do suggest that up to one-third of NRLS infusion pump incidents of at least moderate severity would have potentially been prevented by a standalone smart pump, with use of a closed-loop system increasing this number to 38%. If analysis is restricted to errors involving the wrong dose, rate or volume, then a higher percentage of 41% were potentially preventable with a standalone smart pump, increasing to 51% with a closed-loop system, although these figures are all based on assumptions around the correct set-up and use of smart pumps and their associated drug libraries. In other cases, the use of a smart pump contributed to errors, usually as a result of selecting the wrong entry in the drug library. It is not possible to calculate a net benefit of smart pumps, as no denominator data are available for the numbers of infusions given by smart pump or other kinds of pump.
Chapter 6 Phase 2: patient perspectives on infusion administration
This chapter contains material reproduced or adapted from Wheeler et al. 86 This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Semistructured interviews were conducted with 35 patients receiving IV medication, from four hospital sites. The ethnic backgrounds of patients were white British/English (n = 28), Pakistani/Indian (n = 3), Pakistani (n = 2), Sri Lankan (n = 1) and black British (n = 1). Twenty patients were male and 15 were female, with an age range of 27–89 years. Patients were receiving IV medication in general medicine (n = 5 patients), general surgery (n = 10), oncology day units (n = 12), intensive care (n = 5) and an acute medical unit (n = 3). Interviews at sites D, H and K were audio-recorded and transcribed verbatim. Interviews at site G (and one at site K) were not audio-recorded and instead the interviewer made notes during and after the interview. Each interview was conducted by one of two researchers face to face in the clinical setting.
Four underlying and interlinked themes emerged from the patient interviews: patients’ knowledge about IV infusions, challenges associated with the process, attitudes towards receiving infusions and feeling safe while receiving them. Each of these is described below in more detail.
Knowledge and information about intravenous infusions
Most patients described a general knowledge of their IV medication administration process, including the medication received and equipment used, although the depth of this knowledge varied widely.
The interviews also suggested variance among patients in their desire for information. Whereas some expressed a personal preference for being kept informed about their treatment, others were less interested or spoke of challenges that made it difficult for them to receive information. This was particularly true for patients who were acutely unwell at the time of infusion, as illustrated by the following patient in general surgery when asked whether they were curious about what was going on:
No. And you know, I was just in so much pain. If these people were saying that this is going to work quicker, I was just go for it . . . I was told . . . I was given as much information as probably it would allow at that time.
Site K_GS_P01
Patients reported that nurses were the main source of information, with variation in information-giving depending on the member of staff:
Some staff will say, I’m giving you this now, which is for something. Other staff just come in, do it, walk off.
Site K_GS_P03
Less frequently, doctors were cited as sources of information, often in situations where the patient’s stay in hospital had been pre-planned, such as when they had come to receive chemotherapy. One patient made a distinction between the ways in which information was delivered by doctors and by nurses:
Actually the doctor, the surgeon told me what was happening, what was going to be done and then the nurse kind of filtered and made it a bit broader. So the surgeon would say you’re going to get a drip, it’s going to be a 5-day course and then the nurse would say this is what’s happening.
Site K_GS_P05
Other sources of information included pharmacists, leaflets, patients’ partners, other patients and the internet.
Despite this variation in understanding, the majority of patients described being satisfied with the amount of information they received. Almost universally, patients also felt comfortable asking health-care professionals questions about their IV infusions:
I’d say 10 [out of 10], I’m quite comfortable. So far, every nurse I’ve had, they’ve been quite approachable, so I’ve never like I can’t ask anything.
Site H_P07
For the small number of patients who did not feel comfortable asking questions or raising issues, all described previous negative experiences with requesting information:
I’d say I wasn’t really given much information about them [the IV infusions], when I did try to ask questions, so it seems as though I was not being taken notice of when I was asking.
Site K_GS_P03
A number of patients, although satisfied with the information they had received verbally from nurses, welcomed the suggestion of being given a written leaflet with information specific to their treatment; they felt that this leaflet could be a helpful addition, as it could be read at a time when they were in a better position to take in the information.
Challenges associated with the intravenous infusion process
When patients were asked about any negative aspects of receiving medication intravenously, three challenges were raised. The most prominent of these was the nuisance of pump alarms:
It’d be all right as long as this alarm thing didn’t keep going off.
Site K_GS_P04
Patients found the frequent alarms both annoying and disruptive, and for those who were inpatients this made sleeping difficult. Patients described how alarms would go off for a number of reasons, including a blockage or backflow, as a warning just before the infusion was due to finish, and when the infusion had actually finished:
Yes the alarms are a bit too much. I understand when there’s an alarm when something’s wrong or something like that. But these go off when it gets near the end, then it goes off at the end. Which to me, it doesn’t really need to, because it’s done its job. You know if there’s a problem, I expect to hear an alarm going off.
Site K_GS_P03
Patients mentioned that it was not just their own alarm sounding that was disruptive, but also those of the other patients in close proximity.
A number of patients also viewed the challenge of frequent alarms from the nurses’ perspective, in that it took them away from their other work as they had to frequently respond to alarms. Patients often felt that there was a delay in the time that it took nurses to come and check on alarms, particularly at night on busy wards. Several patients had suggestions about how the impact of alarms on patients could be reduced, including having systems where alerts went directly to nurses, using visual rather than audible alarms, or involving patients more in monitoring and turning alarms off.
A second frequently mentioned challenge was the lack of mobility associated with receiving an IV infusion. The giving sets attached to the pump were viewed as restrictive, with even the smallest movement of the arm, such as using a mobile phone or reading a book, setting off the alarm. Several patients said that they had had their IV access points moved to their wrist or non-dominant arm and that this had made a big improvement in terms of their being able to keep their arm still:
That is something that for some people their stronger arm is so strong that it’s almost . . . They’re almost kind of imprisoned if the cannula is on that arm.
Site K_GS_PS05
The limiting nature of being attached to an IV infusion had affected several patients’ sleep, with them having become tangled in the giving set. Being unable to freely walk around while attached to an infusion was also frustrating for many, although it was possible to be detached if necessary:
It’s just about mobility really. It’s like, now I’m at the stage where I’m drawing out [prolonging] going to the loo and stuff like that. That’s a bit awkward if I’m sort of tied up. But the guys are great and unhook you if you need to be unhooked and stuff. So it’s more that I don’t want to feel like I’m bothering them too much.
Site D_ICU_P08
A third challenge discussed by patients with a history of receiving infusions was pain and discomfort relating to cannulation:
The worst part is the cannula. The cannula is difficult, because after a while your veins get used so much that they can’t find the vein, and it’s very difficult for them as well. I’ve had to have mine in here, in the side of my hand, because my veins are non-existent now.
Site D_ODC_P05
Attitude towards receiving intravenous drug therapy
For the most part, patients were satisfied with their experiences of receiving IV infusions. In particular, patients frequently described an appreciation of the care received.
Although the occasional negative experience was described, this was often given in the context of the patient’s description of the health-care setting as very busy and with an overworked workforce:
Well the bad time is when I get a really bad allergic reaction, which means that the infusions have been going in too quick but they rectify that straight away. It is again a trial and error, I understand that. You know, there’s not fix.
Site H_P08
Linked to this, patients across all settings approached their infusions with a ‘just get on with it’ attitude. Patients recognised that the treatment they were receiving was necessary and, therefore, accepted what the process entailed. This attitude was illustrated by the patient below when asked if anything could be changed to improve the infusion process:
I don’t think so because I know it’s got to happen. Just looking forward to it coming out really.
Site D_ICU_P09
Feeling safe
Although patients approached their infusions in a ‘just get on with it’ manner, accepting some discomfort as part of this, frequent references were made to considerations regarding their safety. These considerations included behaviour by health-care professionals that contributed to patients feeling either safe or unsafe, and, in a small number of cases, patients’ active behaviour that contributed to their perceived feelings of safety.
As discussed earlier, patients both desired and received varying levels of information about their infusions. A lack of information was described as a source of worry for a number of patients, and this was particularly true for those new to receiving infusions:
I think in the middle of the night when everything’s beeping you sort of panic a bit as a patient that something’s going wrong or it means something serious. So it’s always . . . I think communication’s key really.
Site D_ICU_P10
In a few cases where patients perceived that staff themselves were not informed about how to use pump equipment, this was also described as disconcerting.
However, a level of trust was apparent in most of the health-care professionals patients interacted with in terms of IV infusions. Some patients emphasised this, saying that they did not feel that they needed to know everything about the infusion process:
I’ve got an interest in what I’m doing, but, you know, I don’t want to replicate the doctors. I don’t want to be second guessing the doctors or particularly checking up on them. I trust them but I just have a general interest in what’s happening to me, you know.
Site H_P08
Additionally, several patients reported being happy to receive infusions or have their infusions changed while they were sleeping or while they were unable to see what was happening (because of the limited mobility resulting from being attached to an IV infusion). Patients used language reflecting their feelings towards staff, including ‘expert’ and ‘professional’. This was also demonstrated when patients were asked whether or not they had ever interacted with their infusion pumps themselves; most reported leaving this to the health-care professionals.
Some patients described observing staff behaviours relating to their perceived safety, including not wearing gloves, the extent to which they appeared to pay attention to hygiene and wiping equipment, frequent checking of infusions, and the checking of patient ID wristbands. In one instance, when a patient had previously experienced being mistaken for another patient, she described how both she and her partner were extra vigilant following this.
Summary
Thirty-five patients were interviewed across four hospital sites. Variation was demonstrated in terms of both information received during the infusion process and patients’ desire for information. While patients generally accepted that there would be challenges as part of receiving treatment in a hospital setting and that perhaps this just had to be ‘put up with’, in terms of safety patients did describe an awareness of aspects relating to their treatment. Patients also highlighted challenges, particularly in relation to infusion pump alarms, that would allow for some improvement in their experience if they could be addressed.
Chapter 7 Phase 2: staff perspectives on infusion administration
The observations in phase 2 covered both IV preparation and administration, and also, to some degree, the broader context within which these activities are situated. We present two complementary analyses: first as a work system based on SEIPS49 and then as a CAS.
A Systems Engineering Initiative for Patient Safety analysis of the phase 2 observational and interview data
Our analysis focuses on describing the work system (step 1 of the SEIPS analysis as described in Chapter 2), and summarising the consequences that emerged, in terms of the impact on processes and opportunities for learning. The focus in this chapter is on understanding the factors that underpin the outcomes that have been presented in Chapters 3, 4 and 6.
The work system
The detailed observations of IV infusion medication preparation and administration were arranged into the categories of organisation, physical environment, technology and tools, tasks and people.
Organisation
Observations focused on the ward level, and broader organisational factors did not feature strongly in the data. Within the ward, two organisational factors that had an impact on the work system were that often wards were understaffed (compared with the notional staffing plan), and that in most cases there was significant staff mobility between wards, and use of contract staff. These factors often put additional strain on all staff, as new staff needed training (often informal) in local procedures and needed to learn about local practices; variability across wards and trusts (discussed above) limited the knowledge that could be transferred from one working context to another.
Physical environment
The physical environment of a ward comprises multiple areas, and the different wards in which observations took place had different general structures. Here we focus on two physical areas of the ward.
First, most wards had a designated IV preparation area. This was often a room labelled ‘treatment room’, ‘clean utility’ or similar. In some wards, the IV preparation area was not labelled. In some cases, IV preparation areas were accessible directly from the corridor, and these had combination locks with numeric keypads. Other IV preparation areas were located such that they could be easily overseen by a receptionist or other staff. In many cases, the space layout and signage meant that it was not possible to detect that an IV preparation was taking place before actually walking into the IV preparation area. In one ward, the IV preparation space was also used as a staff ‘breakout’ space, which included having a radio playing. In other wards, the IV preparation space was very small and frequently cluttered, with limited usable space. Some spaces could not accommodate more than two IV preparations taking place at the same time.
Second, IV administration generally happened at the patient’s bedside. This space was not private, in that other people (particularly the patient’s visitors) were in proximity. This meant that visitors could observe (and interrupt) the work of programming IV pumps; some staff reported that this added to the stress of programming the pumps.
On open wards, audible alarms were frequently heard from many devices including, but not limited to, infusion pumps. As well as making it challenging for staff to distinguish between alarms that required immediate response and those that were less urgent, these caused annoyance or anxiety to patients. Conversely, staff reported that they were sometimes unable to hear alarms because of ambient noise or the remoteness of the pump sounding the alarm.
Technology and tools
The focus of observations was on IV infusion administration. Therefore, a key artefact was the infusion device. Different makes and models were used across the different wards observed, including smart and traditional pumps and syringe drivers.
On certain wards that used smart pumps with a drug library, pumps were designated for a particular medical area and set up with a drug library tailored to that area. At times of peak demand for pumps, there could be problems finding a functioning pump, and the tailored drug libraries limited possibilities for sharing across areas.
It was observed that pumps were sometimes awkward for staff to attach to a stand and to program. Staff had to bend into uncomfortable positions to see the display screen and the keypad. In particular, for pumps with drug libraries, staff reported that the way in which the drug lines were displayed – the choice of abbreviations, and the interplay between spaces and upper- and lower-case letters – sometimes made it difficult to select the correct drug line (each drug can have multiple lines, each with different rates, concentration, etc.).
Other key technologies and tools that supported the work of IV medication administration included the prescription, drugs, drug preparation trays, infusion giving sets, labels and tags, aprons, and computer terminals for access to patient records.
Some staff reported that paper prescription charts were too long to be read in detail, and that the layout did not always make it easy for them to find key information.
Staff reported that changes in medication packaging could cause difficulties. They relied on recognising drugs by their packaging, including size, shape, colour or lettering style as well as the words and numbers.
On some wards, there was a convention that a member of staff would wear an apron to indicate that they were preparing an IV infusion and should not be interrupted. Some wards used red aprons for this purpose and others used blue ones.
Some trusts had adopted conventions on the size and colour (including translucent) of drug preparation trays; some staff expressed preference for the larger trays, but the different colours did not seem to have significance.
Occasionally, it was observed that computer terminals could not be located near the patient’s bed (e.g. because of a weak wireless network signal or the physical difficulty of locating it there). This meant that the nurse setting up the IV infusion pump needed to rely on their memory to program the pump.
Policy documents about IV infusion were also referred to, and the usability and accessibility of this documentation were raised as issues. Staff complained that it was hard to navigate to relevant items on intranet sites. Policies were often described as being ‘long-winded’ and ‘hard to read’, to the extent that they might not be read. Policies were also seen as ‘hard to search’.
Tasks
The task that triggers IV medication administration – namely writing a medication order – was out of the scope of this study, so we focused on the tasks directly related to IV preparation and administration, and the key facilitation tasks that supported that work.
An important early task was locating all of the necessary items for preparing the medication. Although most drugs were stored on the ward, there were occasions when the drugs were unavailable and had to be transferred from another department or from the pharmacy. On some occasions, physical objects, such as drug cabinet keys, syringes or cannulas, could not be found in the expected storage places, requiring staff to search for these. In our phase 1 study, it was also found that there were occasional issues in interpreting or acting on the prescription, for example when an item was simply unavailable and staff had to improvise by using an appropriate substitute.
Once all items had been gathered together, the medication was prepared. Some drug forms (especially powder) were observed to be harder to prepare; for example, they had to be shaken for a long time to dissolve the powder in the diluent.
Pumps needed to be set up and programmed. For some medications, the infusion rate depended on the patient’s age and weight; in these cases, staff reported that the calculation of the rate was complex.
There were requirements for labelling and tube tagging. As discussed in Chapter 4, Procedural and documentation variations in intravenous infusion administration, there were substantial variations in what was required in terms of labelling, tagging and other documentation across wards and sites. In some trusts the label was completed in full, and in others only the basic data, such as drug name and concentration, were required. Approaches to the tagging of giving set lines also varied across trusts, and this was sometimes perceived as unnecessary (e.g. if the giving set was to be thrown away soon after).
When using smart pumps as designed, it was necessary to select the required drug library entry. In some cases, such as when the required drug entry could not be found, people were observed to select ‘drug X’ and then manually set the rate, volume and time. This selection bypassed the safeguards of the DERS.
The double-checking of IV preparations and pump programming by a second person varied widely. In some trusts, only the IV preparation was double-checked, not the pump programming. There was also inconsistency in applying the practice of double-checking. Participants pointed to a certain risk of ‘confirmation bias’ when a second member of staff did the checking, which made it less effective for detecting errors.
The tasks discussed above relate to the preparation and administration of one infusion. Other tasks observed were also relevant to IV infusion management, and these are discussed in the following paragraphs. They included shift handovers, staff training, incident reporting, and maintaining the infusion devices and (in the case of smart pumps) the drug libraries.
Shift handovers took different forms on different wards. In one ward, these involved a meeting in the staff room where departing and arriving staff sat down and exchanged notes (structured according to bed numbers). No systematic recording of notes was observed, and it appeared that there was a lot of reliance on staff to remember details. In another ward, nurses had a special ‘handover’ sheet, and reported on every patient by bed number. Receiving nurses made notes and highlighted text on their own sheets. Some staff reported that IV errors were more common near shift handover time, as staff were anxious to complete outstanding tasks before handing over to the next team.
Staff training was also an important task both for trainers and for those receiving training. Participants reported that training had become more formalised relatively recently. Good training organisation, competency sign-off, follow-up and recertification schemes were identified. An issue highlighted about training was that it took staff away from the ward and from patient care.
When any untoward incident occurred, staff were expected to file an incident report. Completing incident reports via the online incident reporting system was seen as tedious and time-consuming. At one site, if particular types of incident were reported, then the member of staff responsible was expected to undergo retraining, which meant that they would not be available on the ward; staff reported that this might deter them from reporting incidents.
In principle, it is possible (and potentially valuable) to monitor and review pump usage by analysing log data from pumps. This requires downloading data from multiple pumps. In practice, it was found that the logs on the smart pumps were very rarely accessed because of logistical issues with downloading data from multiple pumps. As well as the staff time that this would take, it would be necessary to take pumps out of use for these data to be downloaded, which was an issue when pumps were already in short supply.
Pumps had to be maintained (typically by the clinical engineering department). Practices for pump maintenance and calibration varied across trusts. In some trusts, calibration took place once every year, and in others this happened more or less frequently; in one trust the accuracy was checked every 3 years or when the pump was reported as faulty. This variation could have been as a result of the recommendations from different equipment manufacturers.
For smart pumps, it is necessary to construct and maintain a drug library. Although this was not a focus of observation, staff reported having to form a specific team and devise local procedures and protocols for populating and maintaining their drug libraries.
People
The tasks outlined above highlight a variety of people involved in IV infusion administration in different ways:
-
At the heart of the process is the person (usually a nurse) who prepares the medication and sets up the infusion device. A second individual (also usually a nurse) may double-check the preparation or programming.
-
The person receiving the medication or fluids is the patient; although this person may have no formal role in the tasks, they have an informal role. For example, sometimes the nurse checks identity or allergies with the patient, and the patient is sometimes a source of information about previous medication history, and of vigilance.
-
The prescriber (typically a doctor) has an important, but more remote, role.
-
Trainers and policy-makers have to ensure that training is delivered in a timely way, that it is up to date and is consistent with local and national policy, and that guidelines are appropriate and practicable.
-
Pharmacists check that medication orders are clear, safe and clinically appropriate for the patient, ensure that the necessary medications are available, and prepare selected high-risk infusions in the pharmacy department. In some trusts using smart pumps, they are also responsible for setting up and maintaining the drug library.
-
Clinical engineers are responsible for maintaining equipment.
-
Patient visitors have a minor role; their presence can add stress to staff (e.g. through interruptions) or add resilience to the system through their vigilance.
Environment
This study did not involve an explicit review of the broader environment within which the work of IV infusion administration took place, but key aspects of that environment that became apparent were higher-level policy and regulatory constraints [managed by the CQC and the Medicines and Healthcare products Regulatory Agency (MHRA)]. Another broad factor was resourcing; in particular, wards were frequently short-staffed and also had to manage scarce physical resources, such as suitable infusion pumps.
Summary of consequences and opportunities for learning
As in phase 1 (see Chapter 4), time was identified as a resource to be managed, and also a force that shaped activities. The practice across many wards of prescribing infusions to start at particular times of day created two kinds of bottleneck. First, there was a bottleneck of IV medication preparation, which required staff to be engaged in a resource-intensive, safety-critical task that was concentrated into a short time, often also in a confined space. Second, this practice meant that there were times of peak demand for infusion devices, which were sometimes in short supply. Both of these bottlenecks meant that staff had to manage resources carefully, but this put stress on staff, stress that they often managed by deviating from local policy in order to manage patient safety and well-being (see also Chapter 4).
The fact that downloading pump log data on alerts and over-rides would have meant taking pumps out of active service, as well as taking up staff time, meant that pump logs were not, as currently implemented, a useful source for learning. They have potential to be a more useful source of learning if log data could be downloaded automatically (wirelessly) and unobtrusively, and if analysis of the log data could be done quickly and simply, with the results reported in a way that is immediately meaningful to staff in terms of areas for improvement.
Staff time was also a resource to be managed, and there was a necessary trade-off between a member of staff being available to work on the ward and that member of staff being upskilled through training. This influenced staff attitudes to incident reporting, in particular: they had to consider not only the direct time taken to report an incident (balanced against the perceived value of doing so) but also the knock-on time cost if it resulted in staff being required to undertake additional training in response.
Another factor that emerged repeatedly, particularly in staff interviews, was interruptions. As outlined above, this was an issue that needed to be managed in both preparation and administration of IV infusions. The strategy of wearing a particular coloured apron was not perceived as effective in limiting interruptions, and the design and use of many IV preparation rooms also limited their effectiveness as a space in which people could work without interruption.
Managing quality and safety in complex systems: best practice, good practices and informative variability
Our second analysis views IV infusion administration as a CAS.
Intravenous infusion administration as a complex adaptive system
Our analysis suggests that IV infusion administration is a CAS comprising nurses, pharmacists, doctors and other clinicians, regulators, drug and device manufacturers, incident reporting mechanisms, national policy and local procedures, stickers, trays and trolleys, and other factors and paraphernalia that shape the system. Different histories in hospital trusts and individual wards set the context for current and future actions, for example the risks for some issues may be more salient as a result of previous incidents at a particular hospital, or involving particular people. However, different subissues might be better associated with simple or complicated systems within this wider CAS. For example:
-
A potentially simple issue is advice on labelling IV infusion giving sets. From phase 1 we found that some sites had a high rate of discrepancies because their local policy was to label every giving set. Some nurses felt that it was not worth labelling a giving set for an infusion that would last for only 10–20 minutes. There was broad agreement that there were two reasons for labelling giving sets: (1) when a giving set is to be used for more than 24 hours, the label can help track the time the giving set has been in use, and (2) when a patient has more than one giving set, the label can help differentiate which drug is being given via which giving set. Trusts A and O had local policy that advised labelling giving sets only for continuous infusions and when more than one infusion was in place. This wording better aligns local policy with nurses’ practical concerns, and the two reasons for labelling giving sets. This, therefore, seems a candidate issue for moving towards some form of best practice.
-
A potentially complicated issue relates to site D’s ICU, which had arranged for propofol infusions to be pre-made so that nurses were saved the time of preparing them. Some critical care nurses said that it would also be helpful to have pre-made infusions of noradrenaline, but these infusions would not be purchased because of the cost. The matron reported that pre-made propofol was one of the most expensive drugs, and he thought that pre-made noradrenaline would cost more. He would have liked to make these and other changes, but the trust was already under budgetary pressures. Other trusts might have less severe financial pressures and be able to purchase more pre-made IV preparations. The matron would need to weigh these extra drug costs against the time it would save nursing staff and the added preparation accuracy that comes with pre-made preparations. He or she would also need to consider any additional risks, such as how different concentrations of pre-made noradrenaline are stored and labelled so that they were not confused, and how long these preparations are stable once they have been made up. This is a complicated problem, but the different issues are known and could be considered to find a good solution for this issue.
-
A potentially complex problem is how to effectively learn from incidents. In the workshop for phase 2 sites, some participants reported that they were not satisfied with the quality of reporting of incidents, whereas other participants were generally happy. The form of the report was discussed, as was off-site access, and the benefits of giving fast, individual feedback to the person who completed the incident report. However, it was implied that a ‘culture of reporting’ was a prerequisite for coming to a meaningful understanding of errors and near-misses for learning. There were factors that may discourage staff from reporting errors, such as having to be retrained. One participant reported that a nurse requested an error not be reported because he or she was worried about the repercussions. On reflection, site staff who were not happy with the quality of their incident reports saw it as a simple issue caused by the design of the reporting forms. However, the site that was more satisfied seemed to treat this as a complex issue involving many other dependencies and a positive culture that affected staff motivations and behaviour.
There may also be different perspectives on the same issue, which depends on the scope of variables and the nature of relationships one is able or willing to consider. For example:
-
Double-checking IV infusions could be considered a simple problem as no one is infallible and, intuitively, two checks are better than one. Some nurses reported positive reinforcement, as double-checks had caught errors they might otherwise have missed.
-
Double-checking could be considered a complicated problem because it is not well defined. In phase 1, local policies and procedures varied, as did the staff’s perceptions. For example, the pharmacists who helped draft the policy at site P said that their intention was that nurses who double-checked IV infusions were signing that the right medication had been given to the right patient at the prescribed rate and time; this also included checking the pump settings. However, the nurses presumed that they were only signing that the preparation of the IV infusions was correct, perhaps encouraged by physically signing the additive label. Only site I had detailed advice on what was expected from an independent check. Such issues relate to known unknowns, which can be clarified, defined and tested to assess their effectiveness.
-
Double-checking could be considered a complex problem because nurses adapt their behaviour depending on the informal risk assessments they perform in practice. For example, one nurse was observed casually asking another nurse to check an infusion of paracetamol that they had already hung up, whereas the same nurse carried out a more thorough check of an infusion they had not administered for a while. These decisions are also affected by the culture on the ward and by role models who influence what is seen as acceptable behaviour. Staffing levels also have an influence; for example, it is challenging when there are only two registered nurses on a night shift to carry out full independent checks together for 10–30 infusions. Furthermore, there is a risk that double-checking may cause more errors as a result of the first checker being less diligent because they expect their work to be double-checked. These situated adaptations, cultural influences and feedback loops are complex and hard to track.
Overall, IV infusion practice can be considered a CAS, which may have subcomponents that are influenced by the broader context.
Revealing informative variability by contrasting neighbours
Through the sites that we have engaged with, we have found that IV infusion practice in England is very variable both within and between trusts. Previous phases of our research have alluded to the different types of pumps, policies and procedures at different hospitals. However, variability also emerges from the different interactions that happen in different wards, clinical areas and hospitals. Some of this variability shows how the system, and the people in it, self-organise to try to improve quality and safety or to optimise other aspects of working practices. We illustrate some of this variability in our data by contrasting similar situations across different wards that were observed, or the same ward under different circumstances (e.g. different times or members of staff). We present this under five themes of social factors, artefacts (including technology, medications and equipment), physical space, information flow, and the wider organisational context.
Social factors
The theme of social factors addresses how different people perform in different roles individually and how they work together. Seven examples of contrasting variability are described in the following sections.
The introduction of a safety briefing by critical care consultants
In site D’s ICU, a safety briefing was organised for 10:15 every morning to discuss staffing, issues and learning. This had been introduced after a new member of staff explained what they did in an ICU at a different trust. During one of these meetings, an issue was shared about a batch of gentamicin that could cause allergic reactions in patients. This meant that staff who had not seen the associated memo were forewarned to look out for this. This meeting facilitated teamwork, fast feedback and shared learning. However, the safety briefing intervention seemed vulnerable, as only four out of the six ICU consultants were supportive of this. This might mean that it is less likely to happen or that it will be rushed if it is not supported on any given day.
Nurses’ informal arrangements for double-checking infusions
On wards where extensive double-checking was required, this seemed to work best when nurses paired up and worked in tandem by checking each other’s IVs. This worked well for some nurses on a ward at site G, compared with a nurse on the same ward on a different day who struggled to get anyone to double-check the IV infusions.
Calling on people with particular expertise in cannulation as needed
Certain nurses were known for being particularly good at cannulation and could be called on when a patient was proving difficult to cannulate. This contrasts how different nurses cannulate, and also raises the question about what they are doing differently and whether this tacit or explicit knowledge can be shared.
Employing an intravenous specialist nurse
An IV specialist nurse was funded at site K to help with training in, and deployment and management of, smart pumps and drug libraries, and was involved in dealing with incident reports that fell within her remit. This nurse was funded through the equipment budget, and was employed by the manufacturer rather than the trust. Site K had effectively created this workaround because a business case to employ an IV specialist nurse directly had been rejected. Site G had also tried to employ an IV specialist nurse but had had its business case denied multiple times.
Employing a pharmacy technician to support infusion set-up
A pharmacy technician was funded to help prepare and check IVs on an IV -intensive ward to free nurse time. This had been triggered by staff pressure, staff turnover and complaints from patients that staff did not have time to spend with them, and was having a positive impact on these issues. Site I had introduced this and site K had done something similar. Site D thought that this was unusual. In discussions with phase 2 workshop participants, it became apparent that some health professionals had interpreted current legislation as precluding this possibility.
Different leadership styles
One sister on a general surgery ward had a very strict management style. She was clear about what she expected of her staff (e.g. patients interacting with pumps was forbidden) and about what she expected from double-checking. Another sister on a general medical ward at the same trust reported trying to model acceptable behaviour, and seemed more open to different nurses’ practices and the realities of not being able to do everything as thoroughly as they would have liked. The looser management style may have been better suited to a ward with poorer staffing levels, where the need to trade off thoroughness for efficiency was more pressing. However, this potentially sets the tone of what is and is not acceptable on the ward and influences the ward culture.
A clinical decision hub
An initiative dubbed the ‘clinic hub’ aimed to streamline oncology day unit patient appointments, tests and decisions. This was developed to ensure that the right information was in the right place so that a decision could be made about whether or not a patient would receive anti-cancer treatment. There was a growing sense of frustration and awareness that clinicians were not as well organised as they could be. Patient tests were repeated; doctors had not signed scripts, leading to delays; and many people were making decisions about whether or not patients received anti-cancer treatment. Staff reported that the clinic hub streamlined this, which was different from their previous practice.
Artefacts
The artefacts theme addresses examples of ways in which artefacts were integrated and used across different trusts. Seven examples of contrasting variability are described in the following sections.
Linking smart pumps to the electronic prescribing and medication administration system
Many English ICUs use traditional infusion pumps and paper prescription charts. 9 More modern technologies enable other possibilities. For example, site D had a system that linked its smart pumps to its EPMA system, so that infusion rates could be documented automatically in real time. Here, when the nurse sets up an infusion, they also need to ‘map’ the pump to the patient’s EPMA record so that the rate can be documented against the relevant drug. The EPMA system does not output details of the drug and infusion rate to the pumps, so the nurse has to select the right drug in the drug library and set the infusion rate appropriately. If the pumps are mapped to the patient’s EPMA record correctly, the EPMA system can monitor what the pumps are doing in terms of the rate, volume and any bolus doses, and alert if a drug is being infused too fast, or does not match the electronic prescription for that patient. There were many reported benefits of this system compared with traditional pumps and paper prescription charts:
-
It makes recording medication administration easier for nurses (e.g. in manual systems nurses have to reset pump counters every hour or at 00:00 each day). The EPMA system can also monitor infusion rates and bolus doses to help with monitoring fluid balance.
-
Doctors can monitor drug administration against patient vital signs away from the bedside.
-
The EPMA computers are by the bedside and documenting the administration is as simple as a tick box in some circumstances.
-
The EPMA system does the infusion rate calculations that have to be done manually in traditional systems.
-
Other capabilities of EPMA include listing medications to be given chronologically, providing an easily accessible electronic IV guide and eliminating certain kinds of ambiguity in prescriptions (e.g. related to legibility of handwriting).
However, some staff reported concerns about the long-term impact on their mathematical skills given that the system helps so much. They also reported potential issues with the ‘mapping’ between the pumps and the EPMA system; for example, on occasion nurses may change a drug in the smart pump but not change the mapping so the EPMA system continues to record the administration of a drug that has now been changed. In addition, sometimes the mapping did not work because the hardware could not be plugged together properly, because there were more pumps than could be connected, or because the operating system needed to be updated.
Setting hard or soft limits in the drug library
As described above, smart pumps use DERS, which involves developing a library of drugs with safe dose and rate parameters. These parameters can have soft limits that can be over-ridden by staff or hard limits that do not allow staff to over-ride them. The extra safety provided by the hard and the soft limits depends on how tightly the parameters have been set. One benefit of this in an ICU is that the pump will display the drug when the library has been used, which can be useful when dealing with multiple IV infusions. However, the libraries can be difficult to change, and two sites reported advising staff not to select certain drugs because the library entries for them would not allow a bolus or caused confusion by being in different units from all of the other drugs in the library. The ICUs in sites K, G and D all used smart pumps; many other areas involved in our research did not.
Using standardised concentrations, drugs and doses
The standardising of concentrations varied between clinical areas. Site D’s ICU had previously used 2% propofol and theatres had used 1%, but both areas had been standardised to 1% to avoid any potential confusion. Although the strength of remifentanil still differed between ICU and theatres, the risk and cost equation for this is different from that for propofol. Propofol poses more risk if there is an error, and wastage and cost implications would need to be considered if remifentanil was standardised. This practice at site D’s ICU contrasted with its previous practice.
Site D also had standardised drugs and doses, so staff were more familiar with having a restricted range of options, which reduced complexity and removed extra thinking loops. A consultant in the ICU spoke of these standardising drugs and doses, and of having a set of standard drugs that could be prescribed on admission. This simplified thinking and reduced work and omissions. By contrast, during his training there had been a wide range of drugs and doses that he had found hard to recall.
Using pre-made drugs
Pre-made drugs save nurses time on, and improve the accuracy of, preparation. In site G, nurses complained about the time it took to prepare drugs such as piperacillin-tazobactam (approximately 15 minutes). An agency nurse spoke of her experience at a different hospital, where this drug had come pre-prepared and took less than 2 minutes to retrieve from the fridge.
Propofol was pre-made in site D’s ICU, whereas noradrenaline was not; however, some hospitals did use pre-made noradrenaline. Different strengths of noradrenaline would need to be differentiated and stored appropriately to reduce the risk of confusion. Cost implications would need to be considered alongside nurses’ preparation time and the potential for errors in preparation.
Keeping spare equipment to hand
A nurse was giving three infusions to a patient, including parenteral nutrition. She had brought various paraphernalia with her, including some spare equipment in case something fell on the floor (which had been observed on this ward). The nurse said that she had intentionally brought extra items in case she needed them, to avoid any disruption to the procedure. This was in contrast to the approach of a different nurse on the same ward, who forgot something she needed and had to go back to the treatment room.
Wearing a coloured apron to show that someone should not be interrupted
Red or blue aprons were used in some areas to try to reduce interruptions. The idea was that the apron, which indicated that the wearer should not be disturbed, would deter people from interrupting. The effectiveness of this intervention was questioned, and one nurse at site G said that it could even increase interruptions because people noticed the wearer more. Site I reported that it still had work to do on using its red aprons in terms of trying to foster a culture that valued not disturbing people while they were wearing them. Beyond the more obvious and direct purpose of the intervention, site I thought that using the apron could raise debate and act as a focal point for discussing issues of interruptions and safety.
The ways that medication trays were designed and used
An adequate number of trays need to be available for nurses to carry and organise IV preparation and administration. Nurses at site D’s ICU were observed walking back from the treatment area with their arms full of vials and paraphernalia for infusions. In addition, there was a risk that vials would roll on to the floor by the bed when trays were not used. Some nurses at site D’s ICU reported that there was a shortage of trays, which contrasted with other clinical areas and hospitals we observed.
Trays are key artefacts for physically and cognitively organising IV administration. Site G’s general medical ward had trays that had a small space for a sharps bin at one end. They used this to stand their paper prescription cards in. This displayed the information and made it easier to prepare multiple IVs in trays laid out side by side. This practice at site G’s general medical ward was not observed elsewhere. This contrasts with site D’s oncology day-care unit, where nurses had to try to remember details of the prescription during preparation and administration because the prescription details were on a computer that could not be brought into the treatment room or by the bedside.
An agency nurse at site G spoke about different procedures for cleaning trays. At this site an antibacterial wipe was used for cleaning, but at another hospital hot water was used, which the nurse preferred. She also reported that the other hospital gave clearer advice about what could and could not be put into the tray after it had been cleaned; for example, medication packaging, which is not clean, should not be placed in the tray. Reported practices in the cleaning of trays therefore varied among sites.
Physical space
The physical space theme addresses examples of the ways in which the space and environment differ among trusts. Two examples of contrasting variability are described in the following sections.
How drugs were stored
Site G’s medication safety officer spoke about how the site aimed to remove high-risk drugs from wards and put in tighter controls when necessary. For example, it was reviewing how lignocaine infusions were stored because these looked very similar to bags of 0.9% sodium chloride and, in some instances, were stored alongside them.
Areas designated as quiet areas or no-interruption zones
Treatment rooms for preparing and double-checking drugs were intended to be quiet. Along with site I’s other initiatives to reduce interruptions, treatment rooms were to be quiet areas where nurses could prepare infusions undisturbed. However, these areas were not always quiet. Site I’s quiet treatment rooms contrasted with one at site K where a radio was playing.
Site I had prescribing areas in its paediatric ICU in which people were discouraged from interrupting, and there were prescribing areas on the ward designated no-interruption zones. This seemed unique to this area and contrasts with most of the other wards we observed.
Information flow
The information flow theme addresses examples of the ways in which tasks are organised and information is processed across different people, artefacts and technologies at different trusts. Examples of contrasting variability are described in the following sections.
Prescribing and administering flushes
Site I did not prescribe flushes at the time of observation but its new e-prescribing software automatically added this when necessary. Site D’s oncology day-care unit also worked in this way. However, site K’s oncology day-care unit did not prescribe large flushes. Other sites used PGDs to administer flushes that had not been prescribed. Site G had just issued new guidance that meant that only registered nurses could double-check and administer small flushes, including those used to cannulate patients. There was a wide variety of contrasting practices relating to flushes between different sites. An efficient and practical way to handle the prescription and administration of flushes, which conforms to current legislation and guidance, might reduce unnecessary variations.
Thorough double-check
Double-checking before chemotherapy was very thorough at site D, which included checking the place in the patient’s cycle, and their previous dose, weight and blood results. This meticulous double-check could include additional details, such as reminding the administering nurse to tell the patient not to take ibuprofen with a certain regimen. This contrasted with the approach to infusions that were perceived to be less risky; for example, in a different clinical area, one nurse asked another nurse to check an infusion of paracetamol that they had already started.
Continuity of care: patients
Continuity of care seemed better in some areas than others. Site D’s oncology day-care unit seemed to have good continuity of care, as nurses looked after the same patient all day, and tried to be allocated that patient when they returned days or weeks later for more anti-cancer treatment. Site G’s general medical ward had communication issues, as one patient’s request for an IV infusion rather than a bolus had not been conveyed to three different sets of nurses who all came to give it to her on the same day.
Continuity of care: omissions
The sister on site G’s general surgical ward reported that her staff had changed to long shifts, which she believed had contributed to a reduction in omissions. This was a contrast between the old and new shift patterns on site G’s general surgical ward.
Asynchronous learning
Site D’s ICU had developed ways for staff who were away from the ward to learn about key issues regardless: issues were noted in a communication book that was left in the nurses’ common room, and these were also e-mailed to staff. This was noted only on site D’s ICU, in contrast to other areas.
Patient involvement: allergy check
Patients were sometimes asked about allergies in case information on this had been missed from the documentation. One nurse had asked a patient about their allergies and reported this to be good practice. The patient, who had been on the ward for many weeks, seemed surprised that the nurse had asked, which suggested that this was not usual practice. Later in the shift, the same nurse did not ask a different patient about their allergies. Many other nurses were also observed not asking this, which also suggested that asking this question was not routine.
Patient identity checked systematically
Staff on site G’s general surgery ward seemed to be particularly consistent in double-checking patient ID for every infusion, even if patients had been resident on the ward for months (patients even joked to the observer about the staff’s thoroughness). For example, two nurses checked a patient’s ID, administered an infusion, realised that they needed to administer a second infusion, returned with that promptly and carried out another ID check on the patient they had just left.
Senior management monitoring
In the staff interviews, a sister reported trying to audit five sets of notes per day on her ward to check that there were no inaccuracies or omissions in the documentation. On another ward, a matron reported keeping an eye out for how nurses prepared and checked their infusions when they were on the ward. This shows some adaptive capacity in how senior staff monitor and control what is happening on the wards. Some of this quality checking seems vague and informal, such as ‘keeping an eye out’, and some seems more targeted, such as ‘five sets of notes’. These two forms of senior management auditing and checking were different, so they can choose what issues need their attention. These differences in practice draw attention to the role of senior management in monitoring and maintaining standards on their ward, and also in trying to find out about how work is carried out in documenting, preparing and administering infusions.
Tailored intravenous medication guide
An IV guide had been provided by the pharmacy on a paediatric unit to provide extra information on preparation. The more general IV guides that were in widespread use on adult wards were seen as inadequate for paediatrics, and even the more formal published children’s IV guides were still seen as maturing or not appropriate for the patients at site I. Staff working on site I’s paediatric surgical ward reported that the level of detail in this specialist IV guide provided great support. It included directions for every medicine that they would give on the ward, including what and how much to reconstitute it with, what rate to give it at, and how many milligrams were in a millilitre once it had been reconstituted. For example, if staff were dealing with volumes or doses below a millilitre, the guide would explain how to make it up. The monographs were also updated with drug-specific safety information when this came out. This type of detailed guide that was tailored by in-house pharmacists was not observed or salient in other trusts we observed, which included a different paediatric surgical ward.
The wider organisational context
Two examples of contrasting variability in the wider organisational context are described below.
Safety culture
Some sites seemed to invest more in safety than others. Some sites seemed to have a proactive safety programme with ongoing initiatives, such as ‘we’re having a focus this month on medication’. There was also awareness of raising debate and issues around safety (e.g. using the red apron as a focus). This active and continued investment at site I contrasted with another site that mentioned a declined business case for an IV specialist nurse and a disbanded IV therapy group, and that had previously had practice educators to organise PGDs and support for staff.
Leadership and learning culture
Site D’s ICU appeared to have a good learning culture as it had introduced a safety briefing, in which staff talked about the issues around incidents rather than about who was involved. This facilitated fast feedback and learning, had asynchronous communication so that nurses who were not on shift could also learn, and had a mini risk assessment tool that focused on system issues regarding an incident rather than on the individual involved. The site also included human factors in the leadership training for their senior nurses. By contrast, at another site, when an incident happened, the nurses involved were isolated quickly and required to reflect on why they had not met expected standards of care. A senior nurse there said that the mindset did not exist for broader systems thinking.
Summary of informative variability
These examples of informative variability highlight the diversity of interactions involved in the system of IV infusion administration. Elements of the social theme emphasise the importance of how people are organised that goes beyond the nurse carrying out the administration. These self-organising networks of people can influence learning, monitoring and the smooth and safe flow of work in the system. Elements in the artefact theme emphasise the broad role of information technology, medications and equipment design in shaping work and in influencing risks and outcomes. The physical theme emphasises the need for an environment that actively tries to reduce interruptions and removes unnecessary risks. The information flow theme emphasises different procedures that can have an impact on the learning, checking and monitoring of the infusion process, including the potential disruptions caused by poor continuity of care. The wider organisational elements include safety culture, learning culture and leadership.
The variability presented here serves to demonstrate the different ways in which IV infusion administration is organised. We do not claim that all of these are positive or relevant in all contexts; however, practitioners may consider their potential relevance to their own practices.
One size does not fit all: context is everything
During the project we engaged with different clinical areas. At one level, IV infusion practice might not seem very different between these areas; after all, in each area a medication is prescribed, prepared and administered following the local policies and procedures. However, there are particular characteristics and contextual dependencies that affect emergent behaviour in the different clinical areas. We reflect on typical staffing levels, patient dependency, treatment timescales, key IV infusion practice characteristics, involvement of different clinicians, and key issues for each area in the following sections.
Intensive care units
Critical care is often relatively well staffed because it has the most unwell patients in the hospital and one-to-one patient care is common. Nurses typically manage 5–15 infusions per patient and have greater autonomy to administer drugs within prescribed parameters, for example titrating noradrenaline to keep the patient’s heart rate and blood pressure at the right levels. There can be a closer working relationship with doctors and other clinicians than in general wards because the patients are so unwell and the timescales are short. Decisions about minutes and hours matter, so doctors are generally readily available and close to hand. Owing to the flexibility nurses have in administrating and titrating drugs, doctors need to review what has been administered to infer the patient’s changing condition, that is, the fluctuating administration record is important for doctors to interrogate, whereas on normal wards there is a closer match between the prescription chart and the medication administration record. Decisions made in relation to ICU may differ from those made in the rest of the hospital, and the ICU may have different infusion pumps. In this area, patients are generally unconscious and receiving life-supporting interventions, and patient safety is key as high-risk drugs are used on high-risk patients. Confusing lines and drugs might be an issue that arises from the numbers of drugs given and lines in use; lines can be tangled. In addition, taking patients to and from surgery and imaging can be complicated, as life-supporting equipment and infusions are disconnected and adjusted to facilitate the move.
The defining characteristics of the ICUs observed include managing multiple IV infusions with many lines and close co-ordination among medical staff within short timeframes. Many IV infusions need to be managed in parallel.
Oncology day-care units
Oncology day care involves anti-cancer treatment for outpatients, who are typically mobile, and come in for 1–8 hours in any given day, depending on their treatment. Nurses typically look after about five patients per day and give pre-medications (to prepare the patient) and anti-cancer drugs in a serial fashion (i.e. one infusion after another with large flushes in between). There might be 4–8 different infusions per patient. Nurses are specially trained to deliver anti-cancer treatments, which can be highly toxic. Before the start of treatment, nurses need to check the patient’s weight and health to ascertain whether they are well enough to receive treatment. Borderline cases will involve advice from the doctor. Doctors may be difficult to contact when advice is needed (e.g. they might be in clinics). Some drugs can cost thousands of pounds, some may expire in only 2 hours, some are light-sensitive, and some might lead to the need for plastic surgery if extravasation occurs. There needs to be a close working relationship with the aseptic unit, where many anti-cancer drugs are made up. High pressure and miscommunication between the oncology day-care unit and the aseptic unit can lead to hours of delays for patients, which can be highly frustrating given their condition and transport arrangements.
The defining characteristics include highly toxic drugs being delivered to outpatients. Effective communication with the aseptic unit is required so that drugs are prepared in a timely way. Many IV infusions are delivered in a serial fashion.
Surgical wards and general medical wards
Nurses typically look after 5–15 patients each, depending on staffing levels and levels of patient dependency. The number of IV infusions they carry out can vary from 0 to 20, and if these all need to be made up and double-checked, and their administration noted, this can give a heavy workload on top of other duties. In addition, when there are shorter shifts, there can be issues with continuity of care. Many different professionals contribute to patient care; some of these are based on the ward, but others move around the hospital. On IV infusion rounds, nurses might make up many different infusions for different patients at the same time. There are frequent interruptions and multitasking on the wards. Different wards might have their own nuances; for example, patients might be on a general surgical ward for one-off treatment, either planned or unplanned, whereas a respiratory ward might have patients with chronic conditions who make frequent visits to hospital and are knowledgeable about their condition and treatment.
The defining characteristics include that each nurse is looking after many different patients and all of their associated needs, of which IV infusion rounds are only a small part. Generally, fewer IV infusions are administered than in ICU and oncology day-care units. Many IV infusions can be prepared in parallel but delivered to different patients.
Paediatrics
Children’s fluids and medications often have to be adjusted to account for their smaller weight and size. This can lead to more frequent complicated calculations for IV infusions than one might find on an adult ward. There might also be challenges in preparing small doses of medication that are diluted multiple times.
The defining characteristics are as above, depending on the area of paediatrics, but generally calculations can be much more complicated.
Chapter 8 Discussion
In previous chapters, we have presented both quantitative and qualitative findings from our mixed-methods studies on both errors and the contextual factors that shape the practices that sometimes lead to errors. In this chapter, we discuss our findings under the following headings:
-
Is there a problem to be addressed?
-
Can smart pumps make IV medication administration safer?
-
The language of safety and complexity.
-
Our journey towards understanding IV infusion administration as a CAS.
-
Rethinking IV infusion administration as a CAS.
We close the discussion by reflecting on some of the challenges experienced in conducting this study and highlighting the key limitations of the work.
Is there a problem to be addressed?
As discussed in Chapter 1, there is a widespread view that IV infusion administration is fraught with errors that affect patient safety. Our data do not support this view, and neither does the most recent published study from the USA. 87 This might have been true in the days when IV infusion administrations were delivered via gravity feed, but we have searched for evidence that errors in IV infusion administration are resulting in patient harm and have found little. In practice, we identified only one error likely to lead to patient harm out of 2008 observations, and Schnock et al. 7,87 have reported similarly small numbers.
To give a realistic view of error rates, and in the spirit of the descriptors used within the NCCMERP severity index, we have reported discrepancies (categories A1 and A2) separately from errors (categories C and above). This means that our reported error rate is lower than that of previous studies, particularly those emerging from the USA, in which all deviations have been classed as errors. To compare studies, it is necessary to compare error rates as reported in most earlier studies with the overall deviation rates reported here. However, we believe that our approach of representing discrepancies separately, and discussing the complexity of infusion administration, gives a more useful benchmark against which to compare the effects of future interventions on error rates.
The relatively low reported error rate might be a limitation of our primary research methods, which inevitably focused attention on ‘unremarkable errors’. 8 Some health professionals have memories of unfortunate incidents where patients did suffer harm from IV therapy (e.g. the death of Denise Melanson in Canada88). However, we established a data-sharing agreement with NHS Improvement to identify incident reports in the NRLS in which IV infusion administration was implicated in patient harm. As reported in Chapter 5, the rate of reported harm was also low in that corpus of reports. There is no reliable figure for the number of IV infusions administered annually in England, but if our estimate of 45 million is accurate to the order of magnitude, then four reported deaths in 11 years of NRLS data equates to approximately one death in 108 IV infusion administrations. This number is not highly reliable (quality of incident reporting, uncertainty around whether two incidents reported were indeed deaths, uncertainty in the number of IV infusions delivered, etc.), but it does suggest that there is no significant national problem of errors in IV infusion administration resulting in significant patient harm. In the UK, there were 0.34 fatalities per 108 vehicle kilometres in 2015;89 therefore, based on the evidence available, the number of deaths related to IV infusion administration is comparable with the number of deaths in road traffic accidents associated with a vehicle driving 3 kilometres.
This rate of harm is worthy of headlines if one believes that health-care performance should be perfect at all times, and that no error or deviation from established clinical practice is acceptable. Like researchers who take a Safety II or CAS perspective (see Chapter 1), who argue that health care cannot be reduced to a set of simple soluble problems, Nieva and Sorra90 argue that health care is inherently risky, and that it:
Must abandon the philosophy of requiring perfect, error free performance from individuals and focus, instead, on designing systems for safety.
Nieva and Sorra90
This includes accepting that errors are inevitable, and developing systems that can learn – not just from past incidents, but also from identifying latent threats and learning from practices elsewhere (see Chapter 7).
Arguably, the current level of safety has been achieved through vigilance, system improvements, and the diligence and professionalism of staff who manage their work within a CAS to maintain patient safety and well-being (see Chapters 4 and 7). Future technologies and policies, therefore, need to support staff in doing this work effectively.
Can smart pumps make intravenous infusion administration safer?
As discussed in previous chapters, neither our own data nor the data from the US study7,87 make a compelling case for smart infusion pumps being inherently safer than traditional pumps.
Through the process of trying to identify which deviations smart pumps would eliminate and which new possible deviations they introduce, we have recognised how much would depend on the way in which smart pumps are set up and used in practice. For example, drugs such as insulin, furosemide and heparin have a wide range of possible infusion rates in clinical practice, depending on the patient’s clinical condition. Any limits set on a smart pump would therefore probably be quite wide, which means that even quite large rate deviation errors for an individual patient may not be picked up. An alternative strategy might be to program the drug library with multiple entries for the same drug to reflect these different clinical uses, but this introduces a further challenge of making it easy for the person programming the pump to select the correct entry.
Strategies such as introducing pre-made infusions to reduce the time preparing infusions on the ward and to ensure standardisation of concentrations may be appropriate for some drugs, and under certain circumstances, as discussed in Chapter 7. However, as with many other interventions, this is not an ‘instant fix’ for all medications and all situations, and it comes at a cost that may not always be proportionate to the benefits.
Preventability also depends a lot on local practice: how standardised/protocol-driven local practice is, and whether lower as well as upper infusion rate limits are set on smart pumps in practice, as well as the culture and usage of drug libraries, such as whether their use is mandated or optional. It is beyond the scope of the ECLIPSE study to determine what an optimum configuration might look like in relation to use of alerts and limits, and the optimum would vary significantly across clinical contexts.
In Figure 10, we present a smart pump logic model that illustrates conditions that have to be in place for a smart pump to realise its potential in preventing medication errors, together with an indication of how these conditions do not always hold in practice. However, as discussed in Chapter 5, smart pumps also address only certain classes of error, and cannot eliminate all errors in IV medication administration. There is greater potential for smart pumps integrated with EPMA and related technologies to detect and prevent more errors than standalone smart pumps, but the US study7 does not provide strong evidence that even this reduced errors in a way that improved patient safety. It may be that future developments in smart pumps will deliver real value in patient care by reducing stress on staff and improving the patient experience. This might include seamless integration with EPMA and related technologies, alarms that better support the work of staff and improve the experience of patients, improved user interfaces and consistency across pump interaction designs so that staff can transfer their knowledge more easily as they move across sites, and the easy download of pump data in a form that is readily interpreted and actionable. However, this remains conjecture, because it is not possible to test these assumptions at present.
FIGURE 10.
A smart pump logic model.

Our journey towards understanding intravenous infusion administration as a complex adaptive system
As outlined in Chapter 1, we initially framed the study in terminology and concepts that were, and remain, prevalent in both the literature and common discourse. There remains a widespread assumption that, done ‘right’, smart pumps will improve patient safety, and that the key challenge is to identify what ‘right’ looks like. Yet, as discussed above, the findings from our study do not support such a conclusion. This has forced us to take a journey that enables us, now, to make better sense of our findings.
The concepts of Safety II played a critical role in the research: (1) they helped us understand that we were sampling everyday work and its consequences; (2) they encouraged us to take a more nuanced approach to any deviations from the medication ordered, classifying some as discrepancies rather than all as errors as in previous research on this theme; (3) they made us mindful of system resilience (e.g. where some deviations add to safety); and (4) they encouraged us to engage with local rationality and challenging trade-offs.
On reflection, we can track our pathway through the Cynefin Framework domains64 associated with our findings:
-
Simple domain – the research-as-proposed aimed to elicit ‘best practices’ and to determine whether or not smart pumps significantly contributed to safety. An unrecognised assumption behind the aim is that the problem can be reduced to a simple one with clear causes and effects.
-
Disorder domain – results from the first phase of our research showed much more variability than anticipated; the type of system we were dealing with was not clear.
-
Chaotic domain – some health-care professionals overseeing the project remarked that the system was chaotic because no manageable patterns presented themselves in the data.
-
Complicated domain – ‘good practices’ were sought in focus groups and observational studies.
-
Complex domain – emergence in the system became ever more apparent as multilayered dependencies were observed in ethnographic studies and through engagement with stakeholders.
Enhancing our mixed-methods approach, and our multidisciplinary background, Safety II has helped us to explore IV infusion administration safety ‘as done’. The Cynefin Framework helped guide us towards recognising the nature of the system we are dealing with, which affects the suitability of different interventions. Both areas rely on complexity theory; this theoretical perspective shows promise for further exploration in this field.
Rethinking intravenous infusion administration as a complex adaptive system
Our current view of IV medication administration as a complex domain is supported by recent work by Schnock et al. ,87 who introduced an infusion safety intervention bundle into their participating hospitals. This comprised eight interventions to address areas of IV labelling and tube tagging (e.g. standardised labels), administering unauthorised medications (e.g. standardised verbal order recommendations) and smart pump use errors (e.g. drug library use compliance monitoring). They found that different components of the bundle were adopted and adapted by different hospitals, and that local contextual factors influenced the uptake and effectiveness of the interventions. They recognised that the process of introducing the interventions (which involved staff engagement and education) was itself an important component of the intervention and, conversely, that the interventions were an important vehicle for effecting change. Despite this, the changes in error rates in pre and post tests were statistically significant in only a small proportion of cases. These authors’ experience supports the view that IV therapy is made up of complex, interacting sub-systems that interoperate in different ways in different contexts, and that what is ‘best’ has to fit the context (and will evolve over time).
If we accept this view that IV infusion therapy is a CAS, then the established literature on CAS60,62 repeatedly points out the futility of trying to reduce it to a set of independently addressable problems. This is consistent with the view of Vincent and Amalberti,53 particularly in their distinction between high reliability and ultra-safe systems. The introduction of smart pumps, and the interventions introduced by Schnock et al. ,87 if used with a ‘checklist mentality’, risk trying to push IV infusion administration towards the ultra-safe end of the spectrum, when all the evidence we have gathered in the ECLIPSE study points in the other direction, towards IV infusion administration being a high-reliability system whereby safety is maintained through the professionalism of teams, not through the imposition of barriers. This does not mean that localised interventions are ineffective, but that they should be treated as ‘probes’63,65 to better understand what does and does not work in a particular context, and what might work well in the future. Others91,92 have discussed CPOE as a complex system; we have not identified any previous studies that discussed IV infusion administration in these terms.
This perspective also challenges established views of the role of design in managing safety. Regulatory approval processes for Class III medical devices, such as infusion pumps, require manufacturers to specify how these will be used in practice, and the US Food and Drugs Administration human factors guidance93 also lays out expectations of usability testing for interactive medical devices (including infusion pumps). The ECLIPSE study has highlighted variability in practice, which is not entirely compatible with this regulatory approach. Once a technology (e.g. an infusion pump) is in use, as discussed in Chapter 3, the Joint Commission in the USA sets out clearly defined guidelines on IV infusion administration practices. All of these expectations and requirements are framed as if IV infusion therapy is a simple (or, at worst, complicated) system. Regulation and oversight in the UK (e.g. involving the MHRA and the CQC) is less prescriptive than that in the USA, and is thus – at least in principle – more accommodating of the view of IV therapy as a CAS. Our study findings highlight the importance of balancing standardisation with flexibility.
In our study (in contrast to Schnock et al. 7), we have distinguished between discrepancies and errors, depending on severity. Many of the discrepancies were either routine violations,44 aimed at managing workload in time-pressured situations, or situational violations44 (i.e. workarounds), generally aimed at managing patient care to deliver the best possible patient outcomes. This is consistent with the view of Braithwaite et al. 62 that, despite their apparent potential for chaos, CAS in practice self-regulate through the actions of people who develop structures and routines that create stability and acceptable performance within the system. The challenge with CAS is to ensure that performance remains acceptable over time and, where possible, improves, as external pressures change. Achieving this requires minimising hazards and optimising performance to improve safety.
Our studies across different contexts of use have highlighted the huge diversity of ways in which infusion devices are used. It is likely that, had we extended the study into areas such as anaesthesia or emergency care, or even outside the hospital into home use94 or use in extreme environments such as air ambulances or field hospitals, we would have identified even greater variation in the work systems and the contexts in which IV infusion therapy is used. As discussed in Chapter 7, ‘one size does not fit all’, and it is important to be able to configure pumps according to local practices (while also maintaining consistency of use to facilitate transfers in learning), and to set policies according to local contexts. Again, this highlights the complexity of the various modes of use of what is superficially a simple (if safety-critical) device and task.
When we started, we had envisaged being able to make clear design and policy recommendations at the end of the ECLIPSE project. The kinds of recommendations that we now make are different from the ones that we had envisaged. It is not possible to design the emergent behaviours of CAS; what is needed is to set up the conditions for success, and to adopt strategies to continually discover the factors that contribute to success in local contexts. Plsek and Greenhalgh57 suggest that this involves experimenting with multiple approaches and gradually evolving systems over time (they suggest PDSA cycles as an approach for this). Van Beurden et al. 63 suggest developing ‘probes’ to identify the configurations most likely to succeed, and then finding ways to amplify their effects. Westbrook et al. 92 propose a methodology for developing an evaluation model focusing on key aspects of complexity. An approach that we have developed, based on our findings, is to identify areas of informative variability so that practitioners can review configurations of IV practice that could bring positive benefits (see Chapter 7).
When the process revolves around a set of technologies (as listed in Chapter 7) that are difficult to adapt, as a result of regulatory and other pressures, it might seem an impossible challenge to optimise the design of both technologies and policies. The findings from the ECLIPSE study suggest that, because practice is complex and deviations are so varied, technology is not a panacea. The technology has to support and enhance the work of IV infusion administration, working effectively with the policies, practices and broader learning culture that most directly contribute to patient safety and experience.
Challenges experienced in executing the study
The ECLIPSE study was originally planned as a 3-year study, but a 9-month funded extension was granted to allow for delays that were outside the control of the project team. These included factors that can affect any research project. For example, whereas one of the two researchers was with the project throughout (apart from two periods of paternity leave), the second post was occupied by four individuals, each of whom had an unavoidable initial learning curve (this was partially compensated for by all of the departing researchers remaining engaged with the project and continuing to deliver on outstanding commitments after they had left). Of greater significance were the challenges experienced in gathering data in NHS hospitals and engaging with those hospitals throughout the project.
Phase 1 had been initially planned to be completed in November 2015. This phase involved setting up local research and development approval and subcontracts with 16 hospitals as well as completing data collection at each. We experienced significant delays in getting approvals and contracts put in place with most of our participating hospitals. Each hospital had a different internal process for approving research projects and setting up contracts, and many were reliant on a particular individual within the hospital, resulting in varying levels of availability and engagement. Although at each participating site a key individual or team had expressed enthusiasm about participating, those people did not necessarily have any influence over their internal approval processes. The variable delays meant that the planned co-ordinated training had to be conducted separately at each participating site. This, together with the time spent by researchers on progressing every contract independently, was a huge drain on resources.
Two sites were particularly slow, and required significant intervention to get subcontracts set up. Around the same time, there was a major change in the process for obtaining ethics approval for studies in England (involving the new Health Research Authority oversight); this added new delays, as procedures at the last two sites that had been in progress under the previous process had to be redone under the new process. These delays were exacerbated as one of our planned observers went on maternity leave, so the site had to organise a new observer, who then had to be trained; at that site, dates had been reserved so that the study could be completed in May 2016, but the cumulative delays resulted in data collection being delayed to November of that year. Although we managed the delays as best we could (e.g. obtaining NHS Research Ethics Committee approval for phases 2 and 3 of the project, and recruiting sites to participate in phase 2), they put significant demands on the project team in chasing contracts, rescheduling training sessions and so on. The research in phases 2 and 3 was significantly less dependent on external organisations, so the delays experienced were much more minor (and, correspondingly, less disruptive).
The phase 1 study ought to have been low risk from ethical and project risk points of view; it was based closely on a protocol used in the USA, and we had obtained firm expressions of interest in participating in the study from around 25 NHS trusts before we submitted the application. The delay did not arise from ethical concerns about the study; the issue lay in local research and development approval processes across multiple NHS trusts and reliance on particular individuals who were not invested in the study and, in many cases, were overloaded. We believe that the fact that phase 1 did not involve recruiting participants in a way that would have contributed to recruitment figures in the National Institute for Health Research portfolio was also a barrier for some sites. We do not believe that the changes in approval processes that were introduced part-way through this study would have ameliorated the situation in terms of creating greater consistency or bringing the slowest up to the efficiency of the fastest. As far as we are aware, this remains a challenge for any future studies that aim to deliver generalisable findings on clinical practice across a significant number of NHS sites in England.
Limitations
Although we aimed for a purposive sample of hospitals, aiming for maximum variability but with some bias towards those already using smart pumps, hospitals had to agree to take part in this study, and it is possible that they differed in some way from hospitals that did not respond to the earlier open invitation to participate or that chose not to take part. In 2017, there were 135 acute non-specialist hospital trusts and 17 acute specialist hospital trusts in England,95 so participating hospitals represent 10% of all English hospital trusts. As shown in Appendix 2, there is a slight regional bias in our participating hospitals towards London; this is because particular hospitals satisfied some of our inclusion criteria (e.g. using smart pumps across all clinical areas and having multiple sites delivering oncology day care), and we considered these to be more important than having proportional representation across all regions of England. Nevertheless, given that the findings from the US study are broadly comparable with those from this English study, we do not believe that this makes our findings any less representative than they would have been with any other selection of 16 sites.
There might also have been volunteer bias in the patients who agreed to participate. Unavoidably, we had to work with patients who were sufficiently well at the time of the interviews, and also who were able to communicate effectively in English, but we are not aware of any other biases other than those that are naturally imposed by the ethics requirement to obtain informed consent. We do not believe that the requirement to obtain informed consent skewed our sample significantly, although the other requirement (level of illness and fluency in English) might have.
Adopting a mixed-methods approach provided a rich understanding of IV infusion errors and the contexts in which they occur.
There are advantages and disadvantages of using local observers versus standardised observers from a research team. Employing local data collectors may have allowed for less conspicuous observation and reduced the likelihood that nurses modified their behaviour on observation days. However, using local staff may have resulted in some interobserver variability or institutional blindness to local poor practice; variability was minimised as far as possible by using two observers from different professional backgrounds at each site, by providing training, and by the subsequent review of data by the multidisciplinary research team. Resource limitations and confidentiality agreements precluded the measurement of interobserver reliability across sites.
The timing of data collection at each trust depended on local approvals and staff availability. Both daily and seasonal variation in factors such as staffing levels and workload may have affected deviation rates; our results provide a snapshot of current practice. All observations took place during the day, mirroring the design of previous studies on which we based ours; this removes one potential confounding factor when comparing findings across studies and across sites, but does mean that we do not have data on practices at night or at weekends, when staffing and workload may differ. We focused on infusions running at the time of observation and will therefore have underestimated the overall medication administration error rate. The observation of prescribing, dispensing, preparation and setting up infusions is likely to have revealed further errors. 13 Errors already identified and corrected by smart pumps or a double-check by another staff member prior to our observations would also not have been captured using our methodology. Ward managers were aware that the study was investigating medication administration errors and discrepancies, so it is possible that nurses changed their behaviour on observation days. However, the observation dates were not publicised in advance and nurses were not directly observed, so the impact is likely to have been minimal.
Three different researchers were involved in the phase 2 observational studies, and the period for data analysis from this phase was shorter than planned, owing to delays in obtaining access to the final sites. Furthermore, our conceptual understanding of our findings and their significance evolved significantly in the final few months of the project. For reasons discussed elsewhere, it has not been possible to make or test recommendations for simple interventions to improve patient safety in IV infusion administration, or to make recommendations about investment in smart pumps. The key insight – that IV infusion administration has to be understood as part of a CAS – has made it impossible to propose specific recommendations to prevent IV medication errors across different hospital settings within England, which was the last of our initial objectives.
Chapter 9 Conclusions and recommendations
The question of how to administer IV infusions safely is complex:96 it is not simply a case of ‘this pump or that one?’ or ‘this protocol or that one?’. Nevertheless, it is an important question about which the NHS needs reliable information. Therefore, the aims of the ECLIPSE study were to describe the rates, types, clinical importance and causes of errors involving infusion of IV medication in English hospitals, and to make recommendations for interventions to minimise harm from errors identified. Our findings, based on a mixed-methods approach, suggest that although minor errors and discrepancies in IV infusion administration are very common, more serious errors are rare. We found ‘potentially harmful’ errors in about 1 in 100 infusions, but among all 2008 observed infusions, we did not identify any judged likely to prolong hospital stay or result in long-term harm. Our findings identify considerable complexity and wide variation in working practices, local policies and the use of infusion pumps. Staff often developed practices that enabled them to work around systemic challenges, and deviations from medication orders and local policies were sometimes made for efficiency or to respond to patient need. Given this complexity, the low rate of serious errors is perhaps surprising.
However, our data also identify factors that appear to affect the safety of infusion administration, with types and prevalence of deviations varying widely among hospital trusts. Our quantitative data suggest that the use of infusion pumps is safer than the use of gravity administration, while the use of smart infusion pumps does not seem to confer additional advantages. Error rates were lower in critical care than in other clinical areas, possibly as a result of more specialist training, a higher nurse-to-patient ratio and intensive patient monitoring. There was no evidence of a relationship between error rates and discrepancy rates, with our data suggesting that discrepancies were often due to detailed policy requirements that may not be achievable in practice, rather than arising from unsafe practices that may also lead to errors. Our qualitative findings suggest many other factors likely to affect infusion safety, including local culture and practice, the interactions between people and technology, and the ways in which pumps are set up and used in practice. Viewing the infusion of IV medication as a CAS highlights the importance of this complex interplay between policies, practices, staff and technology.
We next highlight the implications for practice and for future research arising from this study.
Implications for health-care practice
We summarise implications under four themes: findings relevant to national policy; findings regarding the use of infusion pumps and related technologies; findings addressed in (local) policy and practice; and findings relating to the role of the patient as the recipient of IV infusion therapy.
Implications for national policy
We identified a high degree of variability in trust policies to support IV administration, both within and between hospitals. Although some variation is likely to be appropriate based on the needs of different clinical settings, our findings suggest that there may be scope for greater standardisation to support consistent approaches. This would be likely to benefit patients who expect the same standards of care across the NHS, as well as staff who may move between hospitals.
-
Our data suggest that national advice and guidance may be helpful in some areas, such as whether or not small-volume flushes need to be prescribed and, if so, how; labelling requirements for IV infusions and giving sets; and requirements around double-checking, if any, at each stage of preparation and administration.
In line with research in other areas of health-care practice, our qualitative data suggest that there is variation between hospital trusts in the culture of learning from errors and deviations.
-
Our data suggest that practices around the preparation and administration of IV infusions would benefit from being included in ongoing efforts to encourage a positive culture around reporting and learning from errors.
-
In line with earlier work on CAS in health care, our data show that IV infusion administration cannot be readily reduced to simple problems that have simple solutions (e.g. introducing smart pumps), and that a more nuanced approach to improving safety is likely to deliver greater benefits, including sharing best practices across and within NHS trusts. Examples of practices that had been adopted in some trusts are employing a pharmacy technician to help nurses with IV preparation and administration, introducing a linked pump and EPMA system, removing a radio from the treatment room and trying to improve learning mechanisms and a safety culture.
Use of infusion pumps and other technologies
Although smart pumps are often advocated as making IV infusions safer in health-care practice, our findings do not support this, with error rates similar for infusions given via smart pumps and those given via traditional pumps. Although smart pumps may have a role in preventing very rare and serious errors, there was no difference in the prevalence or types of error identified between infusions given via smart pumps and those given via traditional pumps in our study. Instead, our findings suggest that the use of any pump is safer than relying on gravity administration. Ensuring that sufficient numbers of traditional pumps are available, taking into account the peaks and troughs of demand at different times of day, may therefore represent a better use of resources than purchasing smart pumps. Our findings suggest that:
-
gravity administration introduces vulnerabilities, particularly for safety-critical infusions
-
a shortage of pumps forces staff to find workarounds, particularly when there are peaks and troughs of demand at different times of day
-
there is not yet clear evidence that the costs of implementing standalone smart pumps, including the ongoing costs such as maintaining the drug library, always compare favourably with the costs of alternatives, such as traditional pumps or developing and maintaining integrated smart pumps (interoperating with EPMA, etc.), or with the costs of other patient safety initiatives.
When smart pumps are used, our data suggest a number of associated challenges. In particular, drug libraries are time-consuming to construct and difficult to manage, and were sometimes found to be out of date. In some cases, pump memories were insufficient to permit comprehensive drug libraries to be loaded. One-third of smart pumps observed in our study offered no advantage over standard pumps because of incomplete drug libraries. Additional problems were found to arise when a change in supplier resulted in changes in the strengths or volumes of the products concerned. Error prevention is likely to depend on exactly how drug libraries, and the associated limits for hard and soft alerts, are set up and used. Our findings also suggest that benefits and efficiencies may be possible through collaboration in setting up and sharing drug libraries, associated experiences and good practice.
-
Our findings suggest that health-care organisations may benefit from collaborating, ideally on a national basis, to share practice in the set-up and use of drug libraries. Existing networks, such as those for medication safety officers or medical device safety officers, could support such an activity.
-
Health-care organisations might review whether drug libraries should include all fluids or only those that are considered high risk (based on agreed criteria).
As well as the challenges with smart pump drug libraries as above, our observations and interviews highlighted some poorly defined user interfaces and displays on infusion pumps, which created challenges for staff in setting up and using them. We also found that when smart pumps were used, organisations were rarely downloading or analysing pump log data, as this was perceived as too difficult and/or time-consuming.
-
Our findings suggest that future procurement decisions for IV pumps could usefully include user testing and comparing experiences at other hospitals as well as, for smart pumps, fully exploring memory capabilities for drug libraries, the ease with which these can be set up and updated, and the ease with which pump log data can be downloaded and analysed to deliver actionable insights.
An analysis of our phase 1 quantitative data and of data obtained from the NRLS suggests that many additional errors may be preventable by using systems in which smart pumps are integrated with EPMA systems. Site D’s ICU had such a system but we do not have enough data to determine whether it was statistically significantly different from ICUs that did not have this system. While cautious about making assumptions about the capabilities of integrated systems, and recognising that these systems are likely to bring their own challenges, it seems likely that the potential for smart pumps to improve patient safety may be more fully realised with integrated systems.
-
There is some evidence to suggest that the use of systems integrating EPMA systems and smart pumps might be suitable in the English NHS. Further research is needed to evaluate whether or not, and under what circumstances, their potential is borne out in practice.
The advent of EPMA systems, while having many potential benefits, was identified as also introducing some challenges in allowing simultaneous access to the three elements required for safe administration: the medication order, the medication and the patient. With EPMA, details of the medication order may be on a computer that nurses cannot take to the patient’s bedside when infusions are being set up. A similar issue arises if a computer is not available in the room being used to prepare IV infusions. We also identified issues with interoperability, in that nurses sometimes had to ‘translate’ medication details between EPMA, smart pump libraries and the medication itself if each used different terminology.
-
Our data suggest that health-care organisations using EPMA could usefully consider how best to make details of the medication order available at the bedside and in treatment rooms.
Local policy and practice
Our data suggest that procedural and documentation deviations sometimes represent discrepancies between official policy and everyday practice. In some cases, therefore, it may be more beneficial for managing risk to have policies that better reflect the realities of existing practice than to enforce compliance with policies that are not achievable in clinical practice. For example, policies allowing the administration of small-volume 0.9% sodium chloride flushes without a medication order in specific circumstances or for specific patient groups, an approach already in place in many trusts, could be introduced in hospitals where unprescribed flushes are currently accepted as standard practice by clinical staff but are not permitted according to local policy.
-
There is evidence that IV infusion policies work best when they are aligned with the realities of practice. This may best be achieved by ensuring that they are written with input from practising clinical staff. One specific example is that realistic policies are needed in relation to small volumes of 0.9% sodium chloride as a flush to avoid these having to be prescribed individually.
We identified locally appropriate practices, such as greater involvement of pharmacy staff in preparing and administering IV infusions, and employment of a specialist infusion pump nurse to assist with managing and updating drug libraries, training and liaison with the pump manufacturer. Staff at some sites had interpreted current legislation and the guidance of professional bodies as precluding the administration of medication by health-care professionals other than doctors and nurses.
-
Evidence from the ECLIPSE study highlights the potential value of greater sharing of good and novel practices around IV infusions for others to consider adopting or adapting. As above, this could be done through existing networks, such as those for medication safety officers or medical device safety officers.
As well as in their content, considerable variation was identified in terms of the style, length and readability of policy documents relating to IV infusions, many of which were difficult to locate on trust intranet systems and/or difficult to read. These problems were observed to lead to policy documents not being used or not being followed as intended.
-
Finding from the ECLIPSE study suggest that health-care organisations would benefit from reviewing how policies and associated guidance documents are accessed, indexed, structured and written, with input from end-users to ensure that they are useful, usable and used in practice.
-
It might be valuable to develop and disseminate standards on best practice in developing policy documents and making these easily accessible to staff.
We noted that the physical space in which health-care professionals work shapes their activities. For example, we identified instances when rooms used to prepare infusions were multipurpose and cramped, and had doors that did not work.
-
Findings from the ECLIPSE study suggest that when wards are renovated or new facilities are designed, work practices might be better supported if these are taken into account in the design in order to aid access to all equipment needed while minimising distractions during the preparation and administration of medication.
Patient involvement
Although patients generally felt that their care in relation to IV infusions was safe, some suggested that their role in providing key information was not always drawn on by health-care professionals. Frequent pump alarms were a source of anxiety as well as interrupting patients’ sleep, adversely affecting patients’ experiences. Although inpatients are generally advised not to interact with infusion pumps, the resulting irritation from frequent alarms means that some do interact with them. Some patients also expressed an interest in being able to view what medications they are prescribed and why, which may be more challenging to provide with EPMA than with paper medication charts. Evidence from our study indicates that there are potential benefits from:
-
Ensuring that patients are informed and involved with their infusion therapy to the extent that they wish to be, and that the patient is recognised as a key source of information about their previous treatments and any associated problems.
-
Informing patients of the purpose of pump alarms and what to do when the alarms sound. There may also be benefits in reviewing the settings of pump alarms so that these are configured in a way that is appropriate for the patient.
-
Considering how to best support patient involvement and access to information about their inpatient medication, particularly for hospitals using EPMA.
Recommendations for future research
In order of priority, we would recommend the following:
-
Further in-depth investigation of IV infusion therapy as a CAS, focusing on how to better support reasoning about technology design and the implementation of policies and learning systems that are fit for purpose, and how to extend research on implementation science to incorporate technology development and deployment within CAS. This would ideally include:
-
the full process, including prescribing and patient ID as well as IV infusion preparation and administration
-
the broader sociotechnical system (e.g. training, clinical engineering and incident reporting)
-
the environment, including national organisations such as the CQC and the MHRA and their roles in shaping IV therapy technologies and practices
-
novel approaches to improving IV therapy based on best practice techniques for CAS (e.g. drawing from improvement science)
-
an investigation into possible learning mechanisms for best practice, involving groupings such as medication safety officers, medical device safety officers and NAMDET.
-
-
The evaluation of interventions to increase the safety of IV infusion administration, using suitable study designs and sufficient sample sizes to detect rare and more serious errors. Such research should also include cost-effectiveness, unintended consequences of the interventions, and consideration of whether certain interventions are more effective in specific clinical areas. Interventions could include:
-
technological interventions such as closed-loop systems that integrate smart pumps with EPMA and/or BCMA
-
interventions based on audit and feedback
-
improved usability and utility of smart pump logs, and interventions based on an analysis of smart pump log data on the alerts fired and over-ridden
-
making different use of staff skill mix in preparation and administration of IV infusions, such as use of pharmacy staff and specialist IV nurses
-
use of pre-made infusions
-
different types of user interface on pumps used for IV infusions.
-
-
Research is needed into how best to design infusion pump alarms and configure infusion pump alarm settings to ensure that health-care staff are alerted when action is needed while not overburdening patients (or staff) with frequent alarms.
-
Extravasation, and a potential link with infusion pump settings, was raised several times in interviews by both staff and patients. Although outside the scope of the present study, this is an area for further work on the safety and quality of IV infusions.
Acknowledgements
We are grateful to the many people and organisations who contributed to this research and without whom it would have been impossible. These include the data collectors and site co-ordinators at all 16 participating hospital trusts, the patient and public involvement groups, and all individuals who agreed to be observed or interviewed. We particularly wish to acknowledge the contributions of the following.
Pat Baird (international member of the Advisory Board) made the initial introduction between the team and Brigham and Women’s Hospital, which was just launching its study on which the ECLIPSE study protocol is based.
Anna Cox (co-investigator, Professor of Human–Computer Interaction) jointly conceived and designed the phase 1 study and co-authored key papers from that phase.
Ioanna Iacovides co-authored the original proposal and helped to set up the phase 1 study. She co-authored two key papers from the ECLIPSE study. 1,68
Imogen Lyons was a Research Fellow on the ECLIPSE study (December 2014–July 2016). She managed the phase 1 study with Dominic Furniss, and led on the quantitative analysis of the point prevalence data. She co-authored key papers from the ECLIPSE study. 1,68,80,81 She was unable to review this report as a result of pressure of other work, and is therefore not listed as an author.
Jolien Vos participated in the ECLIPSE study as a Research Fellow (September 2016–May 2017). She conducted an analysis of the phase 1 qualitative data (see Chapter 4) and an observational study at one phase 2 site.
David W Bates (MD, MSc), Kumiko O Schnock (RN, PhD) and Patricia C Dykes [RN, PhD (BWH)] shared their research protocol, training materials and REDCap data gathering tool and also gave their time and expertise generously to ensure that the ECLIPSE study phase 1 point-prevalence data gathering and analysis was carried out to the same high standard as in their own study. They co-authored two key papers from the ECLIPSE study with the England team1,68 and we are working together on an international comparison paper (based on findings presented in Chapter 3).
The Study Steering Committee, expertly chaired by John Williams and comprising Glenys Davies, Lisa Dougherty, Chris Frerk, Allan Hackshaw, Katharina Hauck and Sarah Sharples. The Study Steering Committee gave invaluable guidance to ensure that the ECLIPSE study team stayed on track and prioritised appropriately.
The Advisory Group, expertly chaired by Ann Jacklin and comprising Nick Barber, Susan Keeling, Linda Murdoch, Stephen Tomlin, John Trow and Pat Baird, who engaged and challenged us, shared their expertise unstintingly, and helped us reinterpret our data in more insightful ways.
Colleagues and students who worked with the team on particular studies, including Helen Bell,1 Emma Callewaert (NRLS study), Sandra Fahmy and Sara Garfield,2 Yogini Jani (analyses in Chapter 5), Carly Wheeler86 (analysis of patient data), and other experts who contributed to the analyses in Chapter 6.
Contributions of authors
Ann Blandford (Professor of Human–Computer Interaction) was lead author of the original proposal, led the programme of work jointly with Bryony Dean Franklin, jointly designed all studies and oversaw the qualitative analyses of observational and interview data. She led the authorship of the final manuscript.
Dominic Furniss (Senior Research Fellow, Human Factors) co-authored the original proposal, led both phase 1 and phase 2 studies, led public participation workshops, acted as project manager, and co-authored and approved the final manuscript.
Galal H Galal-Edeen (Senior Research Fellow, Human–Computer Interaction) (from May 2017) conducted an analysis of the phase 1 qualitative data, conducted two of the phase 2 observational studies, and contributed to and approved the final manuscript.
Gill Chumbley (Consultant Nurse, Pain Management) contributed throughout the project, particularly to the design of the phase 1 study, to the cleaning and interpretation of the phase 1 data, and to the analysis of ‘nurses as a source of system resilience’. She read and approved the final manuscript.
Li Wei (Senior Lecturer of Epidemiology and Medical Statistics) contributed her expertise in statistics throughout the project, particularly in interpreting phase 1 data. She read and approved the final manuscript.
Astrid Mayer (Consultant Oncologist) contributed her clinical expertise throughout the project, particularly in interpreting phase 1 data. She read and approved the final manuscript.
Bryony Dean Franklin (Professor of Medication Safety) led the programme of work jointly with Ann Blandford, jointly designed all studies, and oversaw the quantitative analyses of observational data and data obtained from the NRLS. She co-authored and approved the final manuscript.
Publications
Blandford A, Furniss D, Lyons I, Chumbley G, Iacovides I, Wei L, et al. Exploring the Current Landscape of Intravenous Infusion Practices and Errors (ECLIPSE): protocol for a mixed-methods observational study. BMJ Open 2016;6:e009777.
Furniss D, Iacovides I, Lyons I, Blandford A, Franklin BD. Patient and public involvement in patient safety research: a workshop to review patient information, minimise psychological risk and inform research. Res Involve Engage 2016;2:19.
Fahmy S, Garfield S, Furniss D, Blandford A, Franklin BD. A comparison of two methods of assessing the potential clinical importance of medication errors. Safety Health 2018;4:3.
Furniss D, Lyons I, Franklin BD, Mayer A, Chumbley G, Wei L, et al. Procedural and documentation variations in intravenous infusion administration: a mixed methods study of policy and practice across 16 hospital trusts in England. BMC Health Serv Res 2018;18:270.
Lyons I, Furniss D, Blandford A, Chumbley G, Iacovides I, Wei L, et al. Errors and discrepancies in the administration of intravenous infusions: a mixed methods multihospital observational study. BMJ Qual Saf 2018;27:892–901.
Furniss D, Franklin BD, Blandford A. The devil is in the detail: how a closed-loop documentation system for IV infusion administration contributes to and compromises patient safety [published online ahead of print 15 April 2019]. Health Informatics J 2019.
Wheeler C, Furniss D, Galal-Edeen G, Blandford A, Franklin BD. Patients’ perspectives on the quality and safety of intravenous infusions: a qualitative study [published online ahead of print 30 April 2019]. J Patient Experience 2019.
Furniss D, Garfield S, Husson F, Blandford A, Franklin BD. Distributed Cognition: Understanding Complex Sociotechnical Informatics. In de Keizer N, Scott P, Georgiou A, editors. Applied Interdisciplinary Theory in Health Informatics: A Knowledge Base for Practitioners. Amsterdam: IOS Press; 2019. pp. 75–86.
Vos J, Franklin BD, Chumbley G, Galal-Edeen GH, Furniss D, Blandford A. Nurses as a source of system-level resilience: secondary analysis of qualitative data from a study of intravenous infusion safety in English hospitals. Int J Nurs Stud 2020;102:103468.
Data-sharing statement
The anonymised data set for the ECLIPSE study phase 1 (point prevalence) data has been published in the University College London repository: https://doi.org/10.5522/04/7951511.v1 Any queries should be submitted to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Blandford A, Furniss D, Lyons I, Chumbley G, Iacovides I, Wei L, et al. Exploring the Current Landscape of Intravenous Infusion Practices and Errors (ECLIPSE): protocol for a mixed-methods observational study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009777.
- Fahmy S, Garfield S, Furniss D, Blandford A, Franklin BD. A comparison of two methods of assessing the potential clinical importance of medication errors. Safety Health 2018;4. https://doi.org/10.1186/s40886-018-0071-3.
- Association for the Advancement of Medical Instrumentation/US Food and Drug Administration . Infusing Patients Safely: Priority Issues from the AAMI FDA Infusion Device Summit, 2010 n.d. http://tinyurl.com/46l7ynq (accessed 29 July 2015).
- Murdoch LJ, Cameron VL. Smart infusion technology: a minimum safety standard for intensive care?. Br J Nurs 2008;17:630-6. https://doi.org/10.12968/bjon.2008.17.10.29476.
- Iacovides I, Blandford A, Cox A, Franklin BD, Lee P, Vincent CJ. Infusion device standardisation and dose error reduction software. Br J Nurs 2014;23.
- Husch M, Sullivan C, Rooney D, Barnard C, Fotis M, Clarke J, et al. Insights from the sharp end of intravenous medication errors: implications for infusion pump technology. Qual Saf Health Care 2005;14:80-6. https://doi.org/10.1136/qshc.2004.011957.
- Schnock KO, Dykes PC, Albert J, Ariosto D, Call R, Cameron C, et al. The frequency of intravenous medication administration errors related to smart infusion pumps: a multihospital observational study. BMJ Qual Saf 2017;26:131-40. https://doi.org/10.1136/bmjqs-2015-004465.
- Furniss D, Blandford A, Mayer A. Unremarkable Errors: Low-Level Disturbances in Infusion Pump Use n.d. https://dl.acm.org/citation.cfm?id=2305353 (accessed 10 May 2019).
- Rajkomar A, Blandford A. Understanding infusion administration in the ICU through distributed cognition. J Biomed Inform 2012;45:580-90. https://doi.org/10.1016/j.jbi.2012.02.003.
- Carayon P, Hundt AS, Wetterneck TB. Nurses’ acceptance of Smart IV pump technology. Int J Med Inform 2010;79:401-11. https://doi.org/10.1016/j.ijmedinf.2010.02.001.
- Cousins DH, Sabatier B, Begue D, Schmitt C, Hoppe-Tichy T. Medication errors in intravenous drug preparation and administration: a multicentre audit in the UK, Germany and France. Qual Saf Health Care 2005;14:190-5. https://doi.org/10.1136/qshc.2003.006676.
- Taxis K, Barber N. Ethnographic study of incidence and severity of intravenous drug errors. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7391.684.
- Taxis K, Barber N. Causes of intravenous medication errors: an ethnographic study. Qual Saf Health Care 2003;12:343-7. https://doi.org/10.1136/qhc.12.5.343.
- McLeod MC, Barber N, Franklin BD. Methodological variations and their effects on reported medication administration error rates. BMJ Qual Saf 2013;22:278-89. https://doi.org/10.1136/bmjqs-2012-001330.
- Franklin BD, Taxis K, Barber N. Parenteral drug errors. Reported error rates are likely to be underestimation. BMJ 2009;338. https://doi.org/10.1136/bmj.b1814.
- McDowell SE, Mt-Isa S, Ashby D, Ferner RE. Where errors occur in the preparation and administration of intravenous medicines: a systematic review and Bayesian analysis. BMJ Qual Saf Health Care 2010;19:341-5.
- Department of Health and Social Care . An Organisation With a Memory 2000.
- Institute of Medicine . To Err Is Human: Building a Safer Health System. Report of the Committee on Quality of Health Care in America, Institute of Medicine 2000.
- Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: dispensing and administration – 2011. Am J Health Syst Pharm 2012;69:768-85. https://doi.org/10.2146/ajhp110735.
- Trbovich PL, Cafazzo JA, Easty AC. Implementation and optimization of smart infusion systems: are we reaping the safety benefits?. J Healthc Qual 2013;35:33-40. https://doi.org/10.1111/j.1945-1474.2011.00175.x.
- Ohashi K, Dalleur O, Dykes PC, Bates DW. Benefits and risks of using smart pumps to reduce medication error rates: a systematic review. Drug Saf 2014;37:1011-20. https://doi.org/10.1007/s40264-014-0232-1.
- Brock TP, Franklin BD. Differences in pharmacy terminology and practice between the United Kingdom and the United States. Am J Health Syst Pharm 2007;64:1541-6. https://doi.org/10.2146/ajhp060444.
- Uzych L. Medication errors – beyond frequency. Am J Health Syst Pharm 1996;53:1079-80. https://doi.org/10.1093/ajhp/53.9.1079.
- American Society of Hospital Pharmacists . ASHP guidelines on preventing medication errors in hospitals. Am J Hosp Pharm 1993;50:305-14.
- Garfield S, Reynolds M, Dermont L, Franklin BD. Measuring the severity of prescribing errors: a systematic review. Drug Saf 2013;36:1151-7. https://doi.org/10.1007/s40264-013-0092-0.
- Keers RN, Williams SD, Cooke J, Ashcroft DM. Prevalence and nature of medication administration errors in health care settings: a systematic review of direct observational evidence. Ann Pharmacother 2013;47:237-56. https://doi.org/10.1345/aph.1R147.
- National Coordinating Council for Medication Error Reporting and Prevention . Types of Medication Errors 2001. www.nccmerp.org/types-medication-errors (accessed 28 July 2015).
- Dean BS, Barber ND. A validated, reliable method of scoring the severity of medication errors. Am J Health Syst Pharm 1999;56:57-62. https://doi.org/10.1093/ajhp/56.1.57.
- NPSA . Patient Safety Alert 20: Promoting Safer Use of Injectable Medicines 2007. https://webarchive.nationalarchives.gov.uk/20171030131058/http://www.nrls.npsa.nhs.uk/resources/type/alerts/?entryid45=59812&p=3 (accessed 25 April 2017).
- Vos J, Franklin BD, Chumbley G, Galal-Edeen GH, Furniss D, Blandford A. Nurses as a source of system-level resilience: secondary analysis of qualitative data from a study of intravenous infusion safety in English hospitals. Int J Nurs Stud 2020;102. https://doi.org/10.1016/j.ijnurstu.2019.103468.
- Gopee N. Facilitating the implementation of lifelong learning in nursing. Br J Nurs 2005;14:761-7. https://doi.org/10.12968/bjon.2005.14.14.18553.
- ten Hoeve Y, Jansen G, Roodbol P. The nursing profession: public image, self-concept and professional identity. A discussion paper. J Adv Nurs 2014;70:295-309. https://doi.org/10.1111/jan.12177.
- Tucker AL, Spear SJ. Operational failures and interruptions in hospital nursing. Health Serv Res 2006;41:643-62. https://doi.org/10.1111/j.1475-6773.2006.00502.x.
- Tanner CA. Thinking like a nurse: a research-based model of clinical judgment in nursing. J Nurs Educ 2006;45:204-11.
- Blandford A, Furniss D, Vincent C. Patient safety and interactive medical devices: realigning work as imagined and work as done. Clin Risk 2014;20:107-10. https://doi.org/10.1177/1356262214556550.
- Hollnagel E. Why WAI Is Different from WAD n.d.
- Braithwaite J, Wears RL, Hollnagel E. Resilient Health Care, Volume 3: Reconciling Work-as-imagined and Work-as-done. Boca Raton, FL: CRC Press; 2016.
- Hollnagel E, Wears RL, Braithwaite J. From Safety-I to Safety-II: A White Paper. The Resilient Health Care Net 2015.
- Larcos G, Prgomet M, Georgiou A, Westbrook J. A work observation study of nuclear medicine technologists: interruptions, resilience and implications for patient safety. BMJ Qual Saf 2017;26:466-74. https://doi.org/10.1136/bmjqs-2016-005846.
- Dougherty L, Lamb J. Intravenous Therapy in Nursing Practice. Oxford: Blackwell Publishing; 2008.
- Maccari A, Galal GH, Bosch J, Gentleman M, Hofmeister C, Kuusela J. Software Architecture. Boston, MA: Springer; 2002.
- Brand S. How Buildings Learn: What Happens After They’re Built. London: Phoenix Illustrated; 1994.
- Reason J. Human Error. Cambridge: Cambridge University Press; 1990.
- Reason J. Understanding adverse events: human factors. Qual Health Care 1995;4:80-9. https://doi.org/10.1136/qshc.4.2.80.
- Karsh BT, Holden RJ, Alper SJ, Or CK. A human factors engineering paradigm for patient safety: designing to support the performance of the healthcare professional. Qual Saf Health Care 2006;15:i59-65. https://doi.org/10.1136/qshc.2005.015974.
- Heeks R. Health information systems: failure, success and improvisation. Int J Med Inform 2006;75:125-37. https://doi.org/10.1016/j.ijmedinf.2005.07.024.
- Koppel R, Wetterneck T, Telles JL, Karsh BT. Workarounds to barcode medication administration systems: their occurrences, causes, and threats to patient safety. J Am Med Inform Assoc 2008;15:408-23. https://doi.org/10.1197/jamia.M2616.
- Debono DS, Greenfield D, Travaglia JF, Long JC, Black D, Johnson J, et al. Nurses’ workarounds in acute healthcare settings: a scoping review. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-175.
- Carayon P, Wetterneck TB, Rivera-Rodriguez AJ, Hundt AS, Hoonakker P, Holden R, et al. Human factors systems approach to healthcare quality and patient safety. Appl Ergon 2014;45:14-25. https://doi.org/10.1016/j.apergo.2013.04.023.
- Hollan J, Hutchins E, Kirsh D. Distributed cognition: toward a new foundation for human-computer interaction research. ACM Trans Comp Hum Interact (TOCHI) 2000;7:174-96. https://doi.org/10.1145/353485.353487.
- Furniss D, Blandford A. Understanding emergency medical dispatch in terms of distributed cognition: a case study. Ergonomics 2006;49:1174-203. https://doi.org/10.1080/00140130600612663.
- Berndt E, Furniss D, Blandford A. Learning contextual inquiry and distributed cognition: a case study on technology use in anaesthesia. Cognition Technol Work 2015;17:431-49. https://doi.org/10.1007/s10111-014-0314-y.
- Vincent C, Amalberti R. Safer Healthcare: Strategies for the Real World. New York, NY: Springer International Publishing; 2016.
- Lawton R, Taylor N, Clay-Williams R, Braithwaite J. Positive deviance: a different approach to achieving patient safety. BMJ Qual Saf 2014;23:880-3. https://doi.org/10.1136/bmjqs-2014-003115.
- Waldrop MM. Complexity: The Emerging Science at the Edge of Order and Chaos. New York, NY: Simon and Schuster; 1993.
- Holland JH. Hidden Order: How Adaptation Builds Complexity. Reading, MA: Helix Books; 1995.
- Plsek PE, Greenhalgh T. Complexity science: the challenge of complexity in health care. BMJ 2001;323:625-8. https://doi.org/10.1136/bmj.323.7313.625.
- Berwick DM. Developing and testing changes in delivery of care. Ann Intern Med 1998;128:651-6. https://doi.org/10.7326/0003-4819-128-8-199804150-00009.
- Plsek P. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- Glouberman S, Zimmerman B. Complicated and complex systems: what would successful reform of Medicare look like?. Romanow Pap 2002;2:21-53.
- Braithwaite J, Runciman WB, Merry AF. Towards safer, better healthcare: harnessing the natural properties of complex sociotechnical systems. Qual Saf Health Care 2009;18:37-41. https://doi.org/10.1136/qshc.2007.023317.
- Braithwaite J, Churruca K, Ellis LA, Long J, Clay-Williams R, Damen N, et al. Complexity Science in Healthcare – Aspirations, Approaches, Applications and Accomplishments: A White Paper 2017.
- Van Beurden EK, Kia AM, Zask A, Dietrich U, Rose L. Making sense in a complex landscape: how the Cynefin framework from complex adaptive systems theory can inform health promotion practice. Health Promot Int 2013;28:73-8. https://doi.org/10.1093/heapro/dar089.
- Snowden DJ, Boone ME. A leader’s framework for decision making. Harv Bus Rev 2007;85:68-76.
- Sittig DF, Singh H. A new sociotechnical model for studying health information technology in complex adaptive healthcare systems. Qual Saf Health Care 2010;19:i68-74. https://doi.org/10.1136/qshc.2010.042085.
- Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.8775.
- Begun JW, Zimmerman B, Dooley K. Health care organizations as complex adaptive systems. Adv Health Care Organ Theory 2003;253.
- Lyons I, Furniss D, Blandford A, Chumbley G, Iacovides I, Wei L, et al. Errors and discrepancies in the administration of intravenous infusions: a mixed methods multihospital observational study. BMJ Qual Saf 2018;27:892-901. https://doi.org/10.1136/bmjqs-2017-007476.
- NHS England . News: NHS England’s Sir Bruce Keogh Sets Out Plan to Drive Seven-Day Services Across the NHS 2013. www.nhs.uk/NHSEngland/bruce-keogh-review/Pages/published-reports.aspx (accessed 29 Jul 2015).
- NHS Digital . Summary Hospital-Level Mortality Indicator n.d. www.hscic.gov.uk/SHMI (accessed 28 July 2015).
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- REDCap n.d. http://project-redcap.org/ (accessed 28 July 2015).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- NPSA . Safer Practice Notice 01: Improving Infusion Device Safety 2004. www.nrls.npsa.nhs.uk/EasySiteWeb/getresource.axd?AssetID=59977&type=full&servicetype=Attachment (accessed 25 April 2017).
- NHS England . Bed Availability and Occupancy Data – Overnight n.d. www.england.nhs.uk/statistics/statistical-work-areas/bed-availability-and-occupancy/bed-data-overnight/ (accessed April 2018).
- Vincent CA, Coulter A. Patient safety: what about the patient?. Qual Saf Health Care 2002;11:76-80. https://doi.org/10.1136/qhc.11.1.76.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Research. London: Sage Publications Ltd; 2006.
- Donabedian A. The quality of medical care. Science 1978;200:856-64. https://doi.org/10.1126/science.417400.
- Furniss D, Masci P, Curzon P, Mayer A, Blandford A. Exploring medical device design and use through layers of distributed cognition: how a glucometer is coupled with its context. J Biomed Inform 2015;53:330-41. https://doi.org/10.1016/j.jbi.2014.12.006.
- Furniss D, Iacovides I, Lyons I, Blandford A, Franklin BD. Patient and public involvement in patient safety research: a workshop to review patient information, minimise psychological risk and inform research. Res Involv Engagem 2016;2. https://doi.org/10.1186/s40900-016-0035-x.
- Furniss D, Lyons I, Franklin BD, Mayer A, Chumbley G, Wei L, et al. Procedural and documentation variations in intravenous infusion administration: a mixed methods study of policy and practice across 16 hospital trusts in England. BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-3025-x.
- The Joint Commission . Medication Labelling – IV Solutions Retrieved from Stock Supply n.d. www.jointcommission.org/standards_information/jcfaqdetails.aspx?StandardsFAQId=1743 (accessed 9 April 2018).
- Armstrong S. Hospitals that are leading the way to a digital future. BMJ 2017;356. https://doi.org/10.1136/bmj.j1366.
- Bates DW, Vanderveen T, Seger D, Yamaga C, Rothschild J. Variability in intravenous medication practices: implications for medication safety. Jt Comm J Qual Patient Saf 2005;31:203-10. https://doi.org/10.1016/S1553-7250(05)31026-9.
- NPSA . Safer Practice Notice 11: Safer Patient Identification 2005. www.nrls.npsa.nhs.uk/EasySiteWeb/getresource.axd?AssetID=60032&type=full&servicetype=Attachment (accessed 9 February 2018).
- Wheeler C, Furniss D, Galal-Edeen G, Blandford A, Franklin BD. Patients’ perspectives on the quality and safety of intravenous infusions: a qualitative study. J Patient Experience 2019.
- Schnock KO, Dykes PC, Albert J, Ariosto D, Cameron C, Carroll DL, et al. A multi-hospital before-after observational study using a point-prevalence approach with an infusion safety intervention bundle to reduce intravenous medication administration errors. Drug Saf 2018;41:591-602. https://doi.org/10.1007/s40264-018-0637-3.
- ISMP Canada . Fluorouracil Incident Root Cause Analysis 2007. www.ismp-canada.org/download/reports/FluorouracilIncidentMay2007.pdf (accessed 9 April 2018).
- International Transport Forum . Road Safety Annual Report 2017 2017.
- Nieva VF, Sorra J. Safety culture assessment: a tool for improving patient safety in healthcare organizations. Qual Saf Health Care 2003;12:ii17-23. https://doi.org/10.1136/qhc.12.suppl_2.ii17.
- Carpenter JD, Gorman PN. What’s so special about medications: a pharmacist’s observations from the POE study. Proc AMIA Symposium 2001.
- Westbrook JI, Braithwaite J, Georgiou A, Ampt A, Creswick N, Coiera E, et al. Multimethod evaluation of information and communication technologies in health in the context of wicked problems and sociotechnical theory. J Am Med Inform Assoc 2007;14:746-55. https://doi.org/10.1197/jamia.M2462.
- US Food and Drug Administration . Applying Human Factors and Usability Engineering to Medical Devices: Guidance for Industry and Food and Drug Administration Staff 2016.
- Lyons I, Blandford A. Safer healthcare at home: Detecting, correcting and learning from incidents involving infusion devices. Appl Ergon 2018;67:104-14. https://doi.org/10.1016/j.apergo.2017.09.010.
- NHS Confederation . NHS Statistics, Facts and Figures n.d. www.nhsconfed.org/resources/key-statistics-on-the-nhs (accessed 9 April 2018).
- Sims N, Kinnealey M, Hampton R, Fishman G, DeMonaco H. The MGH Textbook of Anesthetic Equipment. Philadelphia, PA: Saunders Elsevier; 2010.
Appendix 1 Recruitment flow chart
FIGURE 11.
Recruitment flow chart. The shading highlights the accepted sites.

Appendix 2 Participating site characteristics
To maintain the confidentiality of participating trusts, regions are described broadly: South includes South West, South Central, South East, and East of England (an area which includes approximately 54 acute and specialist hospital trusts); Midlands includes East and West Midlands (approximately 22 hospital trusts); North includes North East, North West and Yorkshire and Humber (approximately 44 hospital trusts); and London includes approximately 27 hospital trusts.
| Attribute | Trust | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | |
| Region | London | London | South | South | South | South | South | North | Midlands | South | London | North | London | London | London | North |
| Hospital type | TH | TH | DGH | DGH | DGH | DGH | DGH | DGH | CH | DGH | TH | CH | TH | TH | OC | TH |
| Number of beds (approximately) | 500 | 850 | 350 | 505 | 380 | 550 | 575 | 760 | 360 | 290 | 1000 | 150 | 600400 | 850 | 270 | 750 |
| Clinical areas included | GM, GS, CC | GM, GS, CC, P, O | GM, GS, CC, P, O | GM, GS, CC, P, O | GM, GS, CC | GM, GS, CC, P, O | GM, GS, CC | GM, GS, CC, P, O | P | GM, GS, CC | GM, GS, CC, P, O | P | GM, GS, CC, P, O | GM, GS, CC, P, O | O | GM, GS, CC |
| Days of data collection | 3 | 5 | 5 | 5 | 3 | 8 | 3 | 5 | 6 | 3 | 5 | 5 | 5 | 5 | 6 | 5 |
| Data collection period | April–May 2015 | June–October 2015 | August 2015 | June–December 2015 | August 2015 | September 2015–March 2016 | June–October 2015 | September 2015 | August 2015–March 2016 | September 2015 | February–April 2016 | October 2015–February 2016 | March–May 2016 | January 2016 | August–December 2016 | September–October 2016 |
| Variety of pump brands used |
Brand 1 Brand 4 Brand 9 |
Brand 2 Brand 8 |
Brand 2 Brand 4 Brand 9 |
Brand 2 Brand 5 |
Brand 6 | Brand 4 |
Brand 1 Brand 2 Brand 7 Brand 9 |
Brand 1 Brand 4 Brand 9 |
Brand 3 Brand 4 Brand 8 |
Brand 1 Brand 2 |
Brand 4 Brand 7 |
Brand 4 Brand 5 |
Brand 4 Brand 7 |
Brand 2 Brand 10 |
Brand 1 Brand 9 |
Brand 2 Brand 5 Brand 7 |
| Clinical areas in which smart pumps used (pump types) | None | CC (Vol, Syr) | CC (Vol, Syr), GS (PCA), P (PCA) | CC (Vol, Syr), GS (PCA) | None | None | CC (Vol, Syr), GM (Vol, Syr), GS (Vol) | CC (Vol, Syr), GM (Vol) | CC (Syr), GM (Vol, Syr), GS (Syr) | All (PCA) | All | All (Syr) | All areas | GS (PCA) | None |
CC GM GS |
| CPOE | None* | Some areas | Some areas (O) | Some areas | Some areas (CC, GM) | None | None | Some areas (O, GS) | Some areas | None | Some areas | None | Some areas (O) | Yes | Some areas (O) | Yes |
Appendix 3 The REDCap observation form
Appendix 4 Data cleaning examples
Example extract from the data cleaning heuristics
Unauthorised medication/no documented medication order
| Error/discrepancy details | Proposed rating |
|---|---|
|
Verbal orders Policies typically give very specific details about the circumstances in which verbal orders are allowed and how these should be documented. Often observations lack sufficient information to determine if they have met these criteria; in these circumstances, defer to observers ratings |
|
| Verbal order allowed by policy and documented correctly | No error or discrepancy |
| Verbal order not documented according to policy | C error |
| Flushes |
If local policy/PGD does not require a prescription – no error or discrepancy Flushes where there is no local policy to indicate a prescription is not required, even where this is considered routine practice – C error |
| Unauthorised repeat of a previously prescribed medication/fluid | C error – may be higher rating depending on drug, etc. |
| Keeping the vein open | C error, unless covered by local policy |
| Medication/fluid for which there is no documented prescription | Error – rating dependent on drug, patient condition, etc. Defer to observer’s ratings unless details contradict |
Example discussion about the ratings of a specific deviation
The following is an extract from documented discussion among members of the ECLIPSE study team on the ratings for all deviations where the rating was not straightforward or easily resolved:
Paediatric ward. Potassium 10 mmol and 5% dextrose & 0.9% sodium chloride. Prescribed rate 21 ml/hour but actual rate 26 ml/hour, as instructed by doctor verbally during ward round. Currently rated A1. Researcher asked site to get back to us about their policy on verbal orders. However, I presume they did not as this has not been updated. It does not appear to be covered in their injectable medicines policy. If they do not have policy on verbal orders I would be inclined to rate this a C rate error. However, the only information we have is the local observers’ rating as A1 so I think we might need to go with that? i.e. they either have a policy or is it not considered an error locally.
Action: Unanimous to rate this as a C error. Agreement with Investigator who commented “In all trusts I know of, VOs are forbidden except in an emergency.”
Appendix 5 Search terms used to obtain National Reporting and Learning System data set
Search terms
-
/\bAbbott\b/i
-
/\bAlaris\b/i
-
/\bArcomed\b/i
-
/\bArgus\b/i
-
/\bAscor\b/i
-
/\bAtom(\s)medical\b/i
-
/\bB(\s)Braun\b/i
-
/\bBaxa\b/i
-
/\bBaxter(\s)Colleague\b/i
-
/\bBaxter(\s)Ipump\b/i
-
/\bBodyguard\b/i
-
/\bBraun\b/i
-
/\bCADD(-|\s)Legacy\b/i
-
/\bCADD(-|\s)Prizm\b/i
-
/\bCADD(-|\s)Solis\b/i
-
/\bCarefusion\b/i
-
/\bMcKinley\b/i
-
/\bCME\b/i
-
/\bCMExpress\b/i
-
/\bCodan(\s)argus\b/i
-
/\bCurlin\b/i
-
/\bDai(\s)wha\b/i
-
/\bDelphi\b/i
-
/\bDeltec\b/i
-
/\bEden\b/i
-
/\bEureka\b/i
-
/\bFoures\b/i
-
/\bFresenius(\s)Kabi\b/i
-
/\bGemini\b/i
-
/\bGrasby\b/i
-
/\bgraseby\b/i
-
/\bGreen(\s)pump\b/i
-
/\bHospira\b/i
-
/\bInfusa\b/i
-
/\bInfusion(\s)pump\b/i
-
/\bIradimed\b/i
-
/\bIvac\b/i
-
/\bIvantage\b/i
-
/\bJMS\b/i
-
/\bLMA\b/i
-
/\bM16\b/i
-
/\bMedifusion\b/i
-
/\bMedima\b/i
-
/\bMedis\b/i
-
/\bMedrad\b/i
-
/\bMicrel\b/i
-
/\bMicrofuse\b/i
-
/\bMicropump\b/i
-
/\bMoog(-|\s)?aitecs\b/i
-
/\bMP100\b/i
-
/\bMP101\b/i
-
/\bMPdaily\b/i
-
/\bOmnifuse\b/i
-
/\bPainsmart\b/i
-
/\bPCA\b/i
-
/\bPEGA\b/i
-
/\bPega\b/i
-
/\bPhoenix\b/i
-
/\bPump\b/i
-
/\bSamtronic\b/i
-
/\bSigma\b/i
-
/\bSmiths(\s)MS\b/i
-
/\bSummit\b/i
-
/\bSyramed\b/i
-
/\bSyringe(\s)driver\b/i
-
/\bSyringe(\s)pump\b/i
-
/\bT34\b/i
-
/\bTerumo\b/i
-
/\bUniversal(\s)medical(\s)technolog/i
-
/\bVen(n)?er(\s)medical\b/i
-
/\bVolumed\b/i
-
/\bVolumetric(\s)infusion(\s)pump\b/i
-
/\bWalkmed\b/i
-
/\bZimed\b/i
-
/\bZol(l)?(\s)medical\b/i
-
/\bVTBI\b/i
-
/\bvolume(\s)to(\s)be(\s)infused/i
-
/\binfusion(\s)rate/i
-
/\bpressed\b/i
-
/\bbutton/i
-
/\bdevice/i
-
/\bprogrammed\b/i
-
/\bdisplay/i
-
/\binterface/i
-
/\bmiscalculation/i
-
/\bcalculated/i
-
/\bcalculation/i
-
/\bover(-|\s)?infus(?:ed|ion)/i
-
/\bunder(-|\s)?infus(?:ed|ion)/i
-
/\broller(\s)clamp/i
-
/\buser(\s)error/i
-
/\bfree(\s)flow/i
-
/\balarm\b/i
-
/\bover(-|\s)?dose/i
-
/\bunder(-|\s)?dose/I
Glossary
- Barcode medication administration
- A system for checking both the identity of the patient and the medication before administering the medication.
- Bolus
- The administration of a small volume of medication over a short, specific time (typically 3–5 minutes).
- Computerised physician (or prescriber) order entry
- The term used in the USA for electronic prescribing.
- Deviation (the ECLIPSE study definition)
- Any variation between the medication order, plus local policy and guidelines, and what was observed being administered.
- Discrepancy (the ECLIPSE study definition)
- Any deviation (see Deviation) that was judged by the observers not to be an error and as unlikely to result in any patient harm. Discrepancies are classified as A1 or A2 in the National Coordinating Council for Medication Error Reporting and Prevention severity index (see Table 2).
- Error (the ECLIPSE study definition)
- Any deviation (see Deviation) that was judged by the observers to be an error. Errors are classified as C or above in the National Coordinating Council for Medication Error Reporting and Prevention severity index (see Table 2).
- Giving set
- Tubing used to transport intravenous medication from the bag or bottle to the patient, with the flow rate controlled by an infusion pump or a roller clamp (for gravity administration).
- Keep vein open
- Running medication at a very low rate (commonly referred to as ‘keeping the line patent’ in the UK).
List of abbreviations
- BCMA
- barcode medication administration
- CAS
- complex adaptive system
- CPOE
- computerised prescriber order entry
- CQC
- Care Quality Commission
- DERS
- dose-error reduction software
- DiCoT
- Distributed Cognition for Teamwork
- ECLIPSE
- Exploring the Current Landscape of Intravenous Infusion Practices and Errors
- EPMA
- electronic prescribing and medication administration
- ICU
- intensive care unit
- ID
- identification
- IT
- information technology
- IV
- intravenous
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NAMDET
- National Association of Medication Device Educators and Trainers
- NCCMERP
- National Coordinating Council for Medication Error Reporting and Prevention
- NPSA
- National Patient Safety Agency
- NRLS
- National Reporting and Learning System
- PCA
- patient-controlled analgesia
- PDSA
- plan-do-study-act
- PGD
- patient group direction
- SEIPS
- Systems Engineering Initiative for Patient Safety
Notes
-
Example report back to hospital from phase 1 study
Supplementary material can be found on the NIHR Journals Library report project page (www.journalslibrary.nihr.ac.uk/programmes/hsdr/1220927/#/documentation).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.