Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 13/97/12. The contractual start date was in April 2016. The final report began editorial review in May 2018 and was accepted for publication in November 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Carmel Hughes is a member of the Health Services and Delivery Research Commissioned Panel. Mark Loeb has worked for the World Health Organization as a consultant to develop antibiotics for an essential list of medicines and algorithms for appropriate antibiotic use. Martin Underwood is a member of National Institute for Health Research (NIHR) Journals Library Editors Group. He was chairperson of the National Institute for Health and Care Excellence accreditation advisory committee from 2013 until March 2017, for which he received a fee. He is chief investigator or co-investigator on multiple previous and current research grants from NIHR and Arthritis Research UK and is a co-investigator on grants funded by Arthritis Australia, Australian National Health and the Medical Research Council. He has received travel expenses for speaking at conferences from the professional organisations hosting the conferences. He is a director and shareholder of Clinvivo Ltd (Tenterden, UK), which provides electronic data collection for health services research. He is part of an academic partnership with Serco Ltd (Hook, UK) related to return-to-work initiatives. He is an editor of the NIHR journal series, for which he receives a fee. He has accepted an honorarium for advice on Research Excellence Framework submission from Queen Mary University of London. He is co-investigator on an Efficacy and Mechanism Evaluation grant, receiving support in kind from Orthospace Ltd (Caesarea, Israel).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Hughes et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction and background
Introduction
Care homes (with or without nursing) provide care for older people who can no longer live independently. The most frequent acute health-care intervention that care home residents receive is the prescribing of medications. 1 There are serious concerns about the quality of prescribing for care home residents generally, and in particular the prescribing of antimicrobials (antibiotics, antifungals and antivirals). 1 Care homes have been identified as important ‘reservoirs’ of antimicrobial resistance (AMR). 2,3 This has important implications for individual residents, and has broader public health implications due to the development of widespread AMR. A number of prescribing decisions (not just antimicrobials) for care home residents may be made over the telephone,4 and this can lead to medicines-management problems, with erratic medication reviews and prescribing errors.
We have previously shown that Northern Ireland (NI) nursing homes have the highest levels of, and greatest variation in, antimicrobial prescribing among facilities in 20 other European countries/jurisdictions. 5 England was ranked fourth in terms of overall prescribing. 5 Similar findings were reported for residential homes (those facilities that are not required to have qualified nursing staff). 6 Indeed, antimicrobial prescribing in care homes is seen as a global problem, contributing to increasing resistance. 1 There are several policy-level reports that have highlighted this issue, such as Infections and the Rise of Antimicrobial Resistance from the Chief Medical Officer (CMO) in England. 7 The ageing population and increasing requirements for high-quality long-term care are important considerations for the NHS8 and were recognised in the CMO’s report on AMR. 7 This report highlighted ‘the older adult’, with an acknowledgement of this population’s greater vulnerability to infection, which can be exacerbated by living with other older people with risk factors for infection in care homes. The report stated that infections could be managed better, with the appropriate prescribing of antimicrobials being highlighted as an aspect of health care that needed to be tackled in the context of AMR. This theme was echoed in the UK Five Year Antimicrobial Strategy 2013–20189 and in the earlier NI Strategy for Tackling Antimicrobial Resistance (STAR) 2012–2017. 10 These reports emphasised the importance of better stewardship of antimicrobials, which encompasses optimising therapy for individual patients, prevention of overuse, misuse and abuse of antimicrobials and the subsequent minimisation of resistance at both patient and community levels. Education of the health-care workforce was seen as an essential element to draw attention to AMR and appropriate antimicrobial stewardship. 7,9,10
The CMO’s report7 indicated that ‘there is a need to take an international view of this problem [AMR] and work with other nations’ to tackle it. Indeed, there have been a number of international commentaries and studies focusing on antimicrobial prescribing in the care home setting, particularly from North America. Morrill et al. 11 described AMR as a national security threat to the USA and issued a ‘call to action’ to address antimicrobial stewardship in care homes. Thompson et al. 12 noted a high prevalence of antimicrobial prescribing in care homes with nursing in four states in the USA and that there was little documentation to support such prescribing. Scales et al. 13 reported that nurse leaders within care homes and associated medical staff could act as champions for antibiotic stewardship, and Kistler et al. 14 noted that health-care providers may need more support to guide the decision-making process to reduce the excessive use of antimicrobials. Crnich et al. 15 reported that structured assessment, communication between care home staff and prescribers and education about AMR were important facets of interventions that may be effective in supporting antimicrobial stewardship.
Therefore, a more ‘whole-systems’ approach, involving education, diagnosis, treatment and feedback, may help improve practice more broadly. One such study that incorporated a number of these components was conducted by Loeb et al. 16 and focused on prescribing for urinary tract infections (UTIs). In this study, 12 nursing homes in Ontario (Canada) and Idaho (USA) were randomised to receive a multifaceted intervention (based on education and the use of a structured approach to the management of infections) and 12 care homes were allocated to usual care. The intervention consisted of the application of diagnostic (signs and symptoms) and treatment algorithms for UTIs at nursing home level, supported by small group educational interactive sessions for staff, videotapes, written material, outreach visits and face-to-face sessions with physicians. Findings indicated that fewer courses of antimicrobials were prescribed for suspected UTIs in the intervention care homes than in the usual care care homes. No significant differences were found between the intervention and control sites in terms of the total numbers of antimicrobials, admissions to hospitals and mortality.
Rationale for the research
We ran a short feasibility study, based on the original Canadian study,16 in two nursing homes in NI, using some of the same intervention components,17 such as interactive sessions, written material, outreach visits to care homes and educational sessions with general practitioners (GPs), along with the use of algorithms. The intervention was well received by staff and GPs and provided confidence that we could extend this approach on a greater scale. However, this feasibility work was conducted in only two care homes in one region of the UK and focused on one infection (i.e. UTIs), and therefore the relevance of our findings was extremely limited. To extend this research, we considered that it was important to undertake a more comprehensive piece of work that would adapt the intervention that was originally developed for the Canadian care home setting for use in two UK geographic regions in a non-randomised feasibility study, extending the focus to infections common in care homes (including respiratory and skin infections). Importantly, it was also considered essential to address aspects of intervention adaptation, implementation and acceptability by undertaking a process evaluation, which would overarch the conduct of the feasibility study.
At the time of writing this report, a search of trial registries revealed no ongoing intervention studies of this specific topic, although we are aware of one US-based study that is currently recruiting to a trial that will seek to reduce antimicrobial use in nursing home residents with Alzheimer’s disease and other dementias and that will focus on UTIs and lower respiratory tract infections. 18 This study is quite specific in terms of the target population and target infections. The study described in this report is broader in its scope and context (i.e. including care homes with and without nursing care) and is more relevant to the UK setting.
Aims and objectives
Our aim was to evaluate the feasibility and acceptability of a multifaceted intervention on rational prescribing for infections in a non-randomised feasibility study in care homes. The intervention consisted of an educational and management approach, supported by discussion of residents cases. The objectives of the study were as follows:
-
to recruit six care homes – three in NI and three in the West Midlands, England
-
to adapt and develop an intervention (a decision-making algorithm and small group interactive training) originally developed and implemented in Canadian care homes
-
to deliver training in respect of the intervention in the care homes and associated general practices
-
to implement the intervention in the six feasibility care homes
-
to undertake a process evaluation of the non-randomised feasibility phase and test data-collection procedures.
The outcomes that we were interested in for this feasibility study were primarily related to the process evaluation; these included the acceptability of the intervention in terms of recruitment and delivery of training, the feasibility of data collection from a variety of sources, the feasibility of measuring appropriateness of prescribing and a comprehensive overview of the implementation of the intervention. Patient and public involvement (PPI) was embedded in all aspects of the study through our care home representative on the research team and the role of participating care home staff and family members (see Patient and public involvement).
Structure of the report
-
Chapter 1 describes the background to and rationale for the study.
-
Chapter 2 gives an overview of the research approach and methods, providing detail about the study design, data collection and analysis.
-
Chapter 3 focuses on the recruitment of care homes.
-
Chapter 4 describes the adaptation of the intervention, covering the adaptation of the decision-making algorithm and of the small group interactive training materials.
-
Chapter 5 focuses on the implementation of the intervention and the collection and analysis of the associated data.
-
Chapter 6 details the process evaluation that was conducted over the course of the study.
-
Chapter 7 focuses on a survey that was posted to care homes in NI and the West Midlands to assess interest in participating in a larger study.
-
Chapter 8 discusses the key findings from the study, its strengths and limitations, proposals for future research and final conclusions.
Study organisation and oversight
Sponsor
Queen’s University Belfast acted as sponsor and subcontracts were drawn up with the University of Warwick and the Northern Ireland Clinical Trials Unit (NICTU). Indemnity cover was outlined in the letter from the sponsor. The study was led by Carmel Hughes (chief investigator) and a multidisciplinary team of investigators from Queen’s University Belfast, University of Warwick, McMaster University and the NICTU, all of whom had the necessary expertise and experience to undertake the work. The day-to-day running of the 2-year study was undertaken by research fellows based at Queen’s University Belfast (AC) and Warwick (RP), and an intervention developer based at Queen’s University Belfast (CS) oversaw the production of all intervention material.
The funder [the National Institute for Health Research (NIHR)] agreed that we did not require a Data Monitoring and Ethics Committee. Although the proposed research is a feasibility investigation, it has been registered with the International Standard Randomised Controlled Trial Number (ISRCTN) registry (reference 10441831).
Feasibility Study Management Group
The management of the study was overseen by the Feasibility Study Management Group, which consisted of all authors listed on this report, in addition to the manager of the NICTU and other staff from the NICTU as and when required. The Feasibility Study Management Group met on a monthly basis over the course of the study, using teleconference facilities, and all meetings were chaired by the chief investigator at Queen’s University Belfast (CH) or the principal investigator at the University of Warwick (DE). An agenda was prepared in advance of each meeting and circulated to all members; minutes of each meeting were compiled and circulated prior to the next meeting. All agendas and minutes are available to the funder for scrutiny. The Feasibility Study Management Group also met face to face on two occasions (in May 2016 and February 2018). Other ad hoc meetings were held between the chief investigator and other members of the research team to address particular issues. Close attention was paid to progress as assessed against the study timetable and the achievement of key milestones and deliverables. As requested by the funding body, we submitted 6-monthly reports outlining progress and provided other information/data as required. The key milestones for the project were recruitment of care homes; completion of the adaptation of the Canadian intervention model; training in care homes and associated practices; completion of the implementation phase and process evaluation; and analysis and write-up of the study.
Study Steering Committee
An independent Study Steering Committee (SSC) was established and met on two occasions (face to face) over the course of the study (in November 2016 and December 2017). An agenda was prepared in advance of each meeting and circulated to all members; file notes of each meeting were compiled and circulated after the meeting. All agendas and file notes are available for scrutiny by the funder. The role of the SSC was to provide independent oversight of the study as outlined in the study charter, with a particular focus on participant safety, adherence to the protocol, consideration of new information and progress of the study. Professor Catherine Sackley (King’s College London) agreed to act as the independent chairperson. Professor Sackley has experience of care home research and cluster trials. Professor Stephanie Taylor (Queen Mary University of London), Dr Kieran Hand (University of Southampton) and Mr Gordon Kennedy (Research Volunteer, Alzheimer’s Society) also sat on the SSC as independent members. Two members of the research team (CH and DE) attended the two meetings to provide advice and context for the study. SSC members each signed a copy of the charter, and highlighted any conflicts of interest where relevant.
Patient and public involvement
For this feasibility study, we convened an Advisory Group to provide PPI perspectives as well as to contribute to the study design and development. This group was convened with the assistance of the Independent Health & Care Providers, a member organisation that consists of those providing care to vulnerable and older adults. The group was composed of staff and resident family members and it contributed to the drafting of information sheets and related documentation prior to the start of the study. As part of our research team, we had Mr Robert (Bob) Stafford, who is Head of Care and Compliance at Orchard Care Homes. Mr Stafford has responsibility for care compliance across the organisation, which consists of over 100 care homes across the UK. As someone who has direct experience of managing and overseeing care homes, his perspective has been invaluable. He actively participated in all Feasibility Study Management Group meetings and advised on implementation and troubleshooting as and when required. In addition, care home staff contributed to aspects of study conduct, particularly in respect of refinement of data-collection forms, insight into the research process and how the study affected workload.
Ethics considerations and approval
We considered the potential ethics issues for this study very carefully and took advice from a number of organisations. We were advised that the data required for the proposed primary outcome (drug dispensing data) could be obtained without requiring individual resident consent as the data would be available at care home level from community pharmacies and we would not be able to link this back to individual residents. We also needed to collect data from care homes in respect of the resident population and hospitalisations and mortality. In this case, data were extracted, anonymised and/or aggregated by the direct care team (care home staff). We consulted with the Health Research Authority, the Office of Research Ethics Committees Northern Ireland and the Privacy Advisory Committee in NI, which advised that our general approach was acceptable.
The research team have considerable experience of carrying out research within care homes. The team is very aware that a care home is the ‘home’ for each and every resident within it and that care homes are also complex workplaces for the staff. The team liaised closely with the care homes’ managers to ensure minimum disruption to the day-to-day running of the care homes. When possible, researcher visits to the care homes were pre-arranged and the researchers had appropriate training and approvals. Researcher visits were an important part of this study, during which the researcher acted as an ‘observer’. In a setting such as this, non-participant observation is almost impossible as residents and staff may want to interact. The researchers were respectful of residents’ wishes and space and remained in public areas of the care home.
The interviews undertaken with the various stakeholders were held at a time and place to suit participants. In this study, all interviews and focus groups with care home staff took place in the care homes and all interviews with GPs took place in GP surgeries.
The REACH (REduce Antimicrobial prescribing in Care Homes) study was reviewed by Office of Research Ethics Committees Northern Ireland Health and Social Care Research Ethics Committee B and given a favourable opinion (reference 16/NI/0003).
Summary
Care homes for older people are viewed as reservoirs of AMR. Previous research has shown high levels of antimicrobial prescribing in care homes in NI and England. Several national policies have advocated for better antimicrobial stewardship and looking to international examples of how best to manage this issue. A more ‘whole-systems’ approach, involving education, diagnosis, treatment and feedback, may help improve practice more broadly. This informed the conduct of the feasibility study that is described in this report, and which was based on Canadian research that demonstrated that the use of a decision-making algorithm and training sessions with staff led to fewer courses of antimicrobials being prescribed for suspected UTIs. Our study was set up to develop and adapt the Canadian approach to encompass respiratory and soft tissue infections, and addressed aspects of implementation and acceptability in a mixed-methods process evaluation. The study was managed by members of the research team, it had external oversight through a SSC, it had PPI input and all necessary approvals were in place prior to the start of the research.
Chapter 2 Research approach and methods
Introduction
This chapter provides an overview of the research approach and methods, describing the study design, theoretical underpinning, and data collection, management and analysis.
Overview of methods
The REACH study is a non-randomised feasibility study that employed a mixed-methods design. A feasibility study is a piece of research that assesses whether a future main study can be carried out. 19 As a feasibility study, our focus was on the facilitators of or obstacles to implementation of our intervention and to test and adapt our processes to inform a possible larger study. Therefore, our methods and theories were chosen to reflect the focus on our ability to implement the intervention. This study was grounded within the Medical Research Council’s framework for the development of complex interventions. 20
In this section, we present an overview of the methods used within this study and the theoretical frameworks that underpin these. The study consisted of a recruitment phase, adaptation phase, implementation phase and process evaluation, as outlined in Figure 1. PPI was embedded throughout the study via the contributions of Mr Bob Stafford, who was a member of our research team.
FIGURE 1.
Overview of the REACH study.

Recruitment of care homes
We recruited a purposive sample of six care homes meeting specific inclusion criteria; this stage is detailed in Chapter 3.
Adaptation of intervention
We adapted and developed an intervention (a decision-making algorithm and small group interactive training) originally developed and implemented in Canadian care homes. 16 The methods used to adapt the decision-making algorithm included a literature review, a consensus meeting, pre-implementation focus groups with care home staff and relatives of residents, interviews with GPs and ongoing internal review by the study team. The training programme was based on the programme that was delivered in Canada and updated with respect to current practice of related training programmes provided by professional organisations, alongside internal review by the Feasibility Study Management Group. We planned to seek National Institute for Health and Care Excellence (NICE) accreditation for the processes used to develop the intervention material. However, NICE disbanded its Accreditation Advisory Committee in the summer of 2016, so it was no longer possible to seek accreditation. The adaptation of the intervention is fully reported in Chapter 4.
Implementation
We implemented the intervention in the six participating care homes over a 6-month period. This included delivering the training and implementation of the decision-making algorithm. We aimed to collect data on dispensed antimicrobial medicines, use of hospital services, contacts with health and social care professionals, use of the decision-making algorithm, adverse events, hospital episode statistics [from the Hospital Episode Statistics (HES) database] and health economics. We report the implementation of the intervention in Chapter 5.
Process evaluation
We undertook a process evaluation of the feasibility study and explored data-collection procedures. We adopted a mixed-methods approach, using quantitative data from the implementation phase and qualitative data, which included (1) pre-implementation focus groups with care home staff and relatives of residents, and interviews with GPs, (2) observational data during implementation and (3) post-implementation focus groups with care home staff and interviews with REACH Champions (staff who promoted the use of the intervention and provided additional training if required; see Chapter 3, Methods), managers and GPs. We report the process evaluation in Chapter 6.
In order to assess interest in a future larger study, a survey of care homes in NI and the West Midlands was planned, the results of which are reported in Chapter 7.
Normalization process theory
The underpinning theory for this research was normalization process theory. 21,22 This is a sociological theory that aims to explain the social processes that can lead to the routine embedding, or normalization, of a new health organisational practice, focusing on the work that individuals and groups do to enable an intervention to become normalised. Normalization process theory has previously been used to explore the implementation of complex interventions, such as electronic records in maternity units,23 medical revalidation24 and a cardiovascular disease prevention programme in primary care. 25 There are four main constructs to normalization process theory, each of which has four components:26
-
Coherence (individually and collectively the ‘sense-making work’ people do when operationalising new practices):
-
Internalisation – understanding the importance of the problem the new practice addresses.
-
Differentiation – understanding the difference between usual and new practice.
-
Individual specification – understanding the individual tasks and responsibilities around the new practice.
-
Communal specification – building a shared understanding of the collective tasks around the new practice.
-
-
Cognitive participation (the relational work or engagement that people do to build and sustain a community of practice around new practices):
-
Initiation – who is responsible for driving and engaging others in the new practice?
-
Enrolment/buy-in – which stakeholders need to buy-in to it and how can this be done?
-
Legitimation – do participants believe that they can make a legitimate and valid contribution to it?
-
Activation – what actions and procedures are needed to sustain the new practice?
-
-
Collective action (the operational work done to enable the intervention to happen):
-
Interactional workability – what is the interactional work that people do with each other, with artefacts and with other elements of a practice when operationalising it in everyday settings?
-
Relational integration – what is the knowledge work that people do to build accountability and maintain confidence in a set of practices as they use them?
-
Skill set workability – who (with what skill set) is allocated the work as the new practice is operationalised?
-
Contextual integration – what resources or support are available or required to allow the new practice to be operationalised?
-
-
Reflexive monitoring (the formal and informal appraisal and understanding of the new practice or intervention, and how this affects participants and others):
-
Systemisation – how participants determine the usefulness of the new practice for themselves and others.
-
Communal appraisal – how participants work together with different types of information to determine whether or not the new practice works (e.g. formal data analysis meetings or casual collecting of anecdotes).
-
Individual appraisal – how participants individually appraise effects of an intervention on them and their settings (e.g. how the new practice affects an already demanding workload).
-
Reconfiguration – how participants seek to modify the new practice to make it workable in their setting.
-
The general theoretical argument of normalization process theory is that these constructs, and their components, represent a set of generative mechanisms that give structure to the different kinds of individual and collective actions that people do as they respond to a call to implement a new set of practices,26 as staff in care homes were asked to do in this study. These components are not linear, but are in dynamic relationships with each other and with the wider context of the intervention, such as organisational context, structures, social norms, group processes and conventions. 21,22 The elements of this theory are directly applicable to the approach that we have taken with the intervention in this study and provide ‘sensitising concepts’,27 or initial ideas for us to pursue, when reviewing the literature, producing our discussion guides and developing our analysis. In the context of this study, the social organisation of work refers to the decisions staff may take to contact a GP when they suspect that a resident may have an infection.
Data collection
Qualitative data collection
Our primary data collection throughout the study was qualitative. We conducted focus groups with care home staff and relatives of residents and semistructured interviews with GPs as part of the adaptation of the intervention (see Chapter 4). Observation field notes, focus groups (care home staff) and interviews with REACH Champions, care home managers and GPs post intervention were planned and carried out (see Chapter 6).
Quantitative data collection
We developed a protocol to inform pharmacies/dispensaries on the data requirements of the study. Pharmacies that supplied our recruited care homes were asked to provide two anonymised downloads of drugs dispensed to the care home: (1) for the year leading up to our implementation phase and (2) for the 6-month period of implementation. This is reported in Chapter 5.
Screening logs (see Chapter 3), training attendance registers and study case report forms (CRFs) (see Chapters 5 and 6) were developed to capture data associated with intervention implementation. Further data were collected from a care home survey to assess interest in a future larger study (see Chapter 7).
Data analysis
Qualitative data
All interviews and focus group recordings were digitally recorded and transcribed verbatim by an organisation external to Queen’s University Belfast. The transcription process was subject to a non-disclosure agreement. The transcribed data were uploaded into NVivo® 10 (QSR International, Warrington, UK) (a qualitative data analysis software tool) and data analysis was based on the constant comparison method. 27 These methods are fully explained in Chapters 4 and 6.
Quantitative data
Analysis was primarily descriptive, providing an overview of the characteristics of participating care homes and residents. Data on antimicrobial prescribing extracted from community pharmacy computerised records at baseline and at the end of the implementation phase were summarised. We planned to undertake a sample size calculation, estimate the effect size and intraclass correlation coefficient (ICC) from this non-randomised feasibility study, thus informing the parameters for a full study. Subject to the quality of data collected from community pharmacies, if feasible, we had planned to undertake an interrupted time series analysis to explore the trends in the prescription of antimicrobials before and after the intervention.
Data management
All data collected during the study were handled and stored according to relevant legislation and standard operating procedures utilised by Queen’s University Belfast, the University of Warwick and the NICTU. Data were stored on secure servers and access to these data was restricted to authorised personnel. Any data transfer was in accordance with standard operating procedures and required data-sharing agreements to be in place. Study-related documents were made available for internal monitoring and audit activities; this was highlighted in participant information sheets. Study documentation and data will be archived for at least 5 years after completion of the study in accordance with the standard operating procedures of Queen’s University Belfast and the NICTU.
All CRF data returned to the NICTU were dealt with in accordance with its standard operating procedures and accessed only by authorised personnel. A member of the research team checked the data that were entered into a study-specific database designed by the NICTU. After all data had been entered into the database, the original CRFs were securely stored in archiving facilities.
Safety and adverse event management
An adverse event is defined as any untoward medical occurrence in a participant that does not necessarily have a causal relationship with the research treatment/intervention. We did not expect any adverse events related to the intervention in this feasibility study. However, we did expect a large number of reports to be made by staff owing to the nature of this population, but did not attempt to monitor these in real time. Adverse event forms were reviewed when received. Any such events were dealt with in accordance with the NICTU standard operating procedures for safety reporting. We also collected data on hospitalisations and mortality as reported by the care homes through other methods of data collection and monitored these data very carefully. In the original Canadian trial on which this feasibility study is based,16 no differences in admissions to hospitals or mortality between the intervention and control arms were found.
Summary
We have provided a brief overview of the methodologies and theoretical underpinning related to this study. Specific methods for particular aspects of this study will be described in the following chapters.
Chapter 3 Recruitment of care homes
Introduction
In this chapter, we report on the recruitment of care homes. We gave careful consideration to the number and type of care homes required for this feasibility study. Residential care homes are staffed 24 hours a day and provide meals and help with personal care (activities such as washing, dressing and going to the toilet). Nursing homes also provide personal care, but in addition provide specialised nursing care for those who are sick, injured or require regular monitoring, and are required to employ at least one qualified nurse for 24 hours a day. Residential and nursing homes in the UK are collectively known as care homes. We planned to recruit both nursing and residential facilities to this study.
Aim and objectives
Our aim in this phase of the study was to recruit six care homes for participation in this study: three in NI and three in the West Midlands, England.
Methods
The sample size was informed by the research team’s previous experience in care home studies regarding what was considered acceptable for a feasibility study, regarding what would provide the type and quality of data required and to allow us to understand the process and implementation challenges. 4,28,29 We planned to recruit a purposive sample of six care homes: three in NI and three in the West Midlands, with two nursing homes and one residential home in each area. This was to reflect the broad breakdown of nursing home versus residential home numbers in the overall care home sector. The basic inclusion criteria were homes:
-
with/without nursing care, providing 24-hour care for residents aged ≥ 65 years
-
with a minimum of 20 (permanent) residents
-
associated with a small number of general practices (up to four per home providing care for a minimum of 80% of residents within a home)
-
with an exclusive arrangement with one pharmacy for dispensing medications (we also required that the pharmacy used specific dispensing software).
The recruitment process was conducted during April to June 2016. Our recruitment method differed slightly in NI and the West Midlands owing to particular contextual features of each research site. In NI, we compiled a list of care homes from the website of the Regulation and Quality Improvement Authority, the independent body responsible for monitoring and inspecting the availability and quality of health and social care services in NI. We then applied the following inclusion criteria to the Regulation and Quality Improvement Authority list to include care homes:
-
within a 20-mile radius of Belfast (to ensure proximity to Queen’s University Belfast)
-
with ≥ 20 permanent residents
-
providing care for residents aged ≥ 65 years regardless of disability
-
that were not dual registered (i.e. providing nursing and residential services)
-
not owned by health trusts
-
that were members of the Independent Health and Care Providers and any home identified as interested in research.
In the West Midlands, many care homes are part of the NIHR Clinical Research Network (CRN) Enabling Research In Care Homes (ENRICH) programme. 30 The ENRICH programme aims to bring together care home staff, residents and researchers in order to facilitate the design and delivery of research. We asked the CRN to distribute a flyer to these homes, which sought their interest in participating in the REACH study based on the inclusion criteria for the study.
This process identified refined samples of homes in NI and the West Midlands. The research fellows at Queen’s University Belfast and the University of Warwick then randomised their respective lists of nursing and residential homes and, beginning with number 1 on each randomised list, contacted the manager to confirm the initial inclusion criteria at each site. In NI, the survey also applied additional inclusion criteria to identify those homes with four or fewer general practices providing care for a minimum of 80% of residents and with an exclusive arrangement with one pharmacy for dispensing medications. For those homes in each area that met these initial inclusion criteria, we asked the home manager if they were interested in receiving information about how their home could participate in the REACH study.
For those homes that were willing and eligible to participate in the study, we asked the managers to provide contact details of the pharmacy that dispensed medications to their homes. We then contacted these pharmacies to ascertain the type of dispensing software used to ensure that the anonymised dispensing data we required for the study could be downloaded from these systems. We continued contacting care homes and pharmacies until we obtained the requisite number of homes that were fully eligible for the study.
We telephoned the managers of each care home that was eligible and willing to take part in our study, and made arrangements to visit on a mutually convenient date. At this visit, we verbally provided more detail about the study and answered any immediate queries that the managers had. We also provided written information, including an invitation letter, a participant information sheet and a consent form. The participant information sheet informed each home manager of the requirement to identify up to two members of staff (to account for different shifts within the homes) who could act as ‘REACH Champions’: individuals who would be responsible for delivering training to staff unable to attend the original REACH training session. Some managers agreed to take part in the study at this point and completed the consent form. Two homes in NI were part of a group of homes and so the managers of these homes advised that permission to take part in the study should be sought from the management group. The research fellow at Queen’s University Belfast contacted the management group and permission was obtained. The remaining homes were revisited within 1 week and written consent was obtained. The research fellows in each site maintained regular contact with the homes between taking consent and the start of the research processes.
Results
Following screening using the initial sampling criteria, 49 nursing homes and 16 residential homes in NI and seven nursing homes and eight residential homes in the West Midlands were identified (Table 1). This list of homes was randomised, from which the final sample for the feasibility study was recruited, is shown in Table 1.
| Sampling | Number of care homes | |||
|---|---|---|---|---|
| NI | West Midlands | |||
| Nursing homes | Residential homes | Nursing homes | Residential homes | |
| Sampling frame | 261 | 197 | 31 | |
| Initial sampling criteria | ||||
| ≤ 20-mile radius | 127 | 94 | 7a | 8a |
| ≥ 20 residents | 121 | 53 | ||
| Aged ≥ 65 years regardless of disability | 119 | 53 | ||
| Care homes not dual registered as nursing and residential | 81 | 53 | 7b | 8b |
| Care homes not part of health trust | 81 | 37 | 7b | 8b |
| Care homes interested in researchc | 49 | 16 | 7 | 8 |
| Homes randomised for telephone contact | 49 | 16 | 7 | 8 |
| Additional sampling criteriad | ||||
| Homes contacted | 18 | 8 | 4 | 1 |
| More information needed from homes to verify eligibility | 8 | 4 | 0 | 0 |
| Homes not eligible | 7 | 3 | 2 | 0 |
| Homes eligible and not interested | 1 | 0 | 0 | 0 |
| Homes eligible and interested | 2 | 1 | 2 | 1 |
We recruited three homes (two nursing and one residential) in both NI and the West Midlands (Table 2).
| Site | Unique reference | Nursing/residential | Number of beds (resident occupancya) | Number of general practices serving ≥ 80% of residents | Part of chain? |
|---|---|---|---|---|---|
| NI | A | Nursing | 62 (36) | 4 | Yes |
| B | Nursing | 32 (32) | 2 | No | |
| C | Residential | 36 (36) | 1 | Yes | |
| West Midlands | D | Nursing | 56 (42) | 1 | No |
| E | Nursing | 51 (51) | 1 | No | |
| F | Residential | 40 (37) | 1 | Yes |
The number of beds ranged from 32 to 62, with occupancy at almost 100% in all homes apart from home A. In NI, more general practices provided care to the homes, whereas in the English homes, each participating home was served by one practice. Homes varied in ownership, with three being part of a chain and the remaining three being owned by single proprietors. All homes were each associated with one pharmacy that supplied all medication.
Summary
We successfully recruited six homes that met the inclusion criteria for participation in the study. The approach taken in the two geographic sites was largely comparable, but also took into account some contextual differences, such as the role of ENRICH in England. We noted the key characteristics pertaining to homes, such as number of beds and number of general practices associated with the homes.
The six recruited homes then progressed to the adaptation phase of the study, which is described in Chapter 4.
Chapter 4 Adaptation of the intervention
Introduction
In this chapter, we report on the adaptation of the intervention to reduce antimicrobial prescribing. The Canadian study16 had provided ‘proof of concept’ that antimicrobial prescribing could be influenced by a multifaceted intervention consisting of a decision-making algorithm and small group interactive training. However, context in research is important;31 in this case, the difference is between the Canadian care home context and that of the UK. Transposing the intervention from Canada to the UK without any modification was unlikely to be successful. Furthermore, we anticipated that the evidence on management of infections in older people would have developed since the Canadian study was undertaken (the last follow-up was in 2003). 16
Aims and objectives
The aim of this phase of the research was to adapt and develop an intervention (a decision-making algorithm and small group interactive training) originally developed and implemented in Canadian care homes. The objectives were as follows:
-
to undertake a series of rapid reviews encompassing the most up-to-date literature on the management of the three target infections
-
to conduct a consensus meeting, using the nominal group technique, with health-care professionals, to adapt the decision-making algorithm
-
to undertake focus groups and interviews with key stakeholders, including care home staff, family members of residents and GPs, to adapt the decision-making algorithm
-
to develop a training programme outlining the use of the decision-making algorithm, communication techniques between health-care professionals and aspects of the study process
-
to produce an updated and refined decision-making algorithm, which, in conjunction with the training programme, would be used in the implementation phase of the study.
We report on the adaptation of the decision-making algorithm and follow this with a description of the adaptation of the small group interactive training programme.
Methods
Adaptation of the decision-making algorithm
The approaches developed by Loeb et al. in 200132 and 200516 were updated and adapted for UK use through a series of iterative steps: production of a rapid scoping literature review, focus groups with care home staff and family members of residents and semistructured interviews with GPs. As part of discussions with other members of the Feasibility Study Management Group, it was also agreed that a consensus group meeting with health-care professionals would provide further additional input into the development and adaptation of the intervention. The process was also informed by continual iterative internal review and analysis within the research team.
Production of rapid reviews
We undertook a rapid scoping review of the literature to obtain the most up-to-date evidence for the management and diagnosis of UTIs, respiratory tract infections (RTIs) and skin and soft tissue infections (SSTIs) in older people living in care homes. We considered systematic reviews, guidelines, reports, review articles and randomised controlled clinical trials published between 2000 and 2016.
Search methods for identification of studies
We searched the following electronic databases for primary studies: the Cochrane Central Register of Controlled Trials (via The Cochrane Library, 2016, issue 4), MEDLINE (via Ovid, 1946 to May week 1 2016), EMBASE (via Ovid, 1980 to week 41 2016), CINAHL plus EBSCOhost (1980 to May 2016), PubMed (1996 to May 2016) and SCOPUS (1983 to May 2016). We supplemented this with forward citation tracking. In addition, we contacted experts in the field of antimicrobial and geriatric medicine for advice on further potential studies. We conducted a ‘grey’ literature search of NICE, the European Centre for Disease Protection and Control, the Infectious Disease Society of America, the Society for Healthcare Epidemiology of America and NHS Evidence, using the appropriate terminology as applicable. This search focused on identifying guidelines relating to the management, stewardship, initiation, diagnosis and treatment of infections in the care home setting (see Appendix 1).
Screening and review of literature
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database RefWorks (Pro Quest, LLC, Ann Arbor, MI, USA) and removed duplicates. Two review authors (AC and CS) independently examined the remaining references. A reference was included if it was:
-
relevant to the key question ‘what is the most up-to-date evidence for the management of urinary tract, respiratory tract and skin infections and the stewardship of antimicrobials?’
-
a review, guideline or report from a professional organisation or clinical trial
-
published between 2000 and 2016
-
related to the care home setting
-
written in English.
We excluded those articles that clearly did not address the management and diagnosis of the key infections in the care home setting and obtained copies of the full text of potentially relevant references. At least two review authors (CS and AC) independently assessed the eligibility of the full-text papers.
Data extraction and management
Three reviewers (AC, DE and CS) independently extracted data from relevant publications using a predefined extraction table containing the following elements: author and year, setting (i.e. nursing or residential care home), type of publication [i.e. review article, randomised controlled trial (RCT), NICE guideline], population (i.e. older people), infection (i.e. urinary, respiratory or skin/soft tissue infection), objectives, relevant data relating to the target infections from each article and comments (from the reviewers AC, DE and CS), such as whether or not the paper was particularly useful or if it cited the original algorithm by Loeb et al. 16 An article was thought to be particularly useful if it informed the reviewers about new evidence relating to urinary, respiratory or skin/soft tissue infection. This process took place during a 3-day face-to-face meeting between Anne Campbell, David Ellard and Catherine Shaw to discuss and agree discrepancies in their interpretation; those discrepancies that could not be agreed were resolved by an additional author (CH).
An additional meeting was held between Catherine Shaw, Carmel Hughes and Michael Tunney to further discuss the findings from the evidence tables. This involved individual examination of the extracted data alongside group discussion, with the aim of reaching a final conclusion as to which articles should be used to update the algorithm.
The data extracted during this process were used to update the algorithm for the consensus meeting (see the following section) and informed ongoing iterative discussions within the research team during the process of adaptation.
Consensus group meeting
Ethics approval was granted by the School of Pharmacy, Queen’s University Belfast Ethics Committee, to conduct a consensus group meeting using the nominal group technique (reference 022PMY2017). Potential participants were identified through personal networks of members of the research team in Belfast, and were approached by e-mail and given brief details about the consensus group meeting. Participation was voluntary and required written informed consent. All participants received an honorarium for their time, as noted in the relevant information sheet.
Prior to the meeting, each participant was provided with a draft version of the decision-making algorithm as shown in Figure 2, alongside supporting documents (comprising key papers that provided the research team with updated evidence in relation to urinary and respiratory infections in care homes that had been retrieved from the literature search) to allow them to familiarise themselves with the material. No new evidence was found relating to SSTIs (see Adaptation of the decision-making algorithm).
FIGURE 2.
Draft algorithm presented to the consensus group. b.p.m., breaths per minute; COPD, chronic obstructive pulmonary disease.

Nominal group technique
The consensus group meeting format was based around the nominal group technique. 33 Potential benefits of nominal group technique include significant idea generation that may happen through face-to-face discussion and debate, even though it may be in a limited and prestructured format. 34 The research team formulated three nominal questions: (1) how do we best manage UTIs in older people?, (2) how do we best manage RTIs in older people? and (3) how do we best manage SSTIs in older people? These questions were presented to the participants during the face-to-face meeting (Figure 3). Initially, each participant recorded his or her views regarding the nominal questions independently and privately.
FIGURE 3.
The REACH consensus group nominal group technique. Reproduced from Hughes et al. 35 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
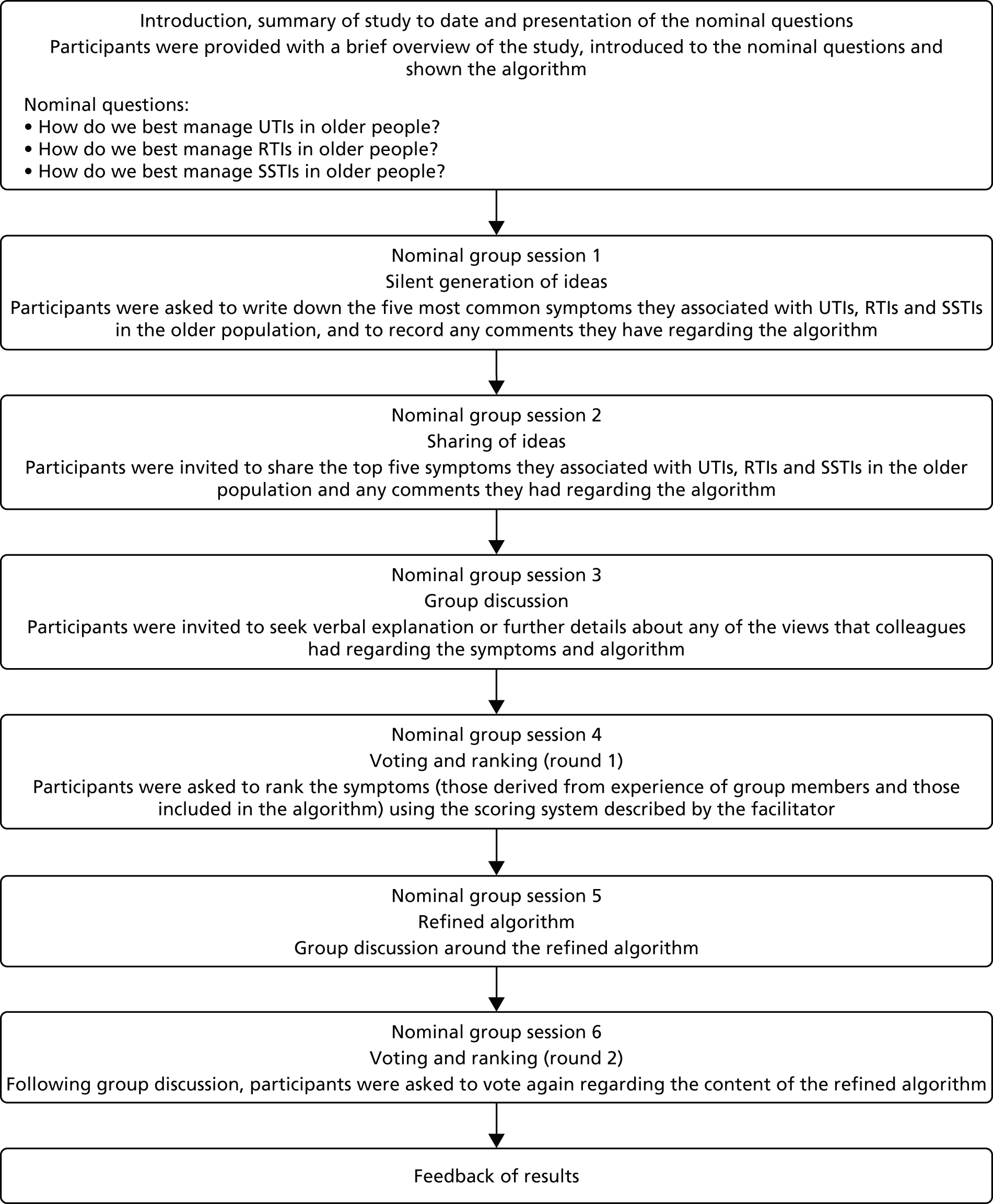
The group members were asked to write down the five most common symptoms they associated with UTIs, RTIs and SSTIs in the older population, and to study the algorithm (see Figure 3, session 1) and supporting evidence. This allowed the participants to refamiliarise themselves with the material. They were then encouraged to share the five most common symptoms they associated with each infection with the rest of the group (see Figure 3, session 2). The facilitator noted these symptoms and any comments on a whiteboard for each member to see. The next step involved group discussion of the symptoms and comments made, and members of the group had the opportunity to seek further explanation from one another if required (see Figure 3, session 3). The symptoms included within the algorithm were discussed. Participants were then asked to rank the top five symptoms of each infection, derived from their personal experience (most common symptoms listed previously) and those included within the algorithm (from the literature), based on their clinical expertise. Participants were provided with a separate table for each infection to guide the ranking of the symptoms and were asked to complete the table by selecting a numerical rating for each one, with the most important receiving a rank of 5 and the least important receiving a rank of 1 (see Figure 3, session 4).
When each participant had ranked their top five symptoms of each infection, the data were entered onto a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet by the facilitator. The ratings for each symptom were summed and item totals were ranked. The facilitator compared the ranked symptoms with those included in the algorithm as presented. If there were any discrepancies between the top-ranked symptoms and those included within the algorithm, the algorithm was amended accordingly and presented back to the group. Further discussion ensued (see Figure 3, session 5) and this was followed by another round of voting and ranking of the symptoms to be included in the algorithm (see Figure 3, session 6), which was amended accordingly again.
Pre-implementation focus groups and interviews
Sampling and recruitment
We aimed to recruit care home staff as users of the intervention and family members of residents, given that family members may be influential in decision-making in relation to the prescribing of antimicrobials. 4 To recruit care home staff and family members of residents prior to the implementation of the intervention, the research fellows at Queen’s University Belfast and the University of Warwick asked the manager in each participating care home for assistance. They provided managers with individual participant information packs for care home staff and family members. Each participant information pack included the following documents: an invitation letter, a participant information sheet and a consent form. The research fellows asked the managers to distribute this information to senior and junior care home staff and family members. The research fellows were available in person, by telephone or by e-mail to provide further explanation. We aimed to recruit 4–12 participants for each focus group36 and to include senior and junior staff in the care home focus groups. The managers of the care homes were not invited to take part in the focus groups so as to prevent the management relationship directing or constraining the group discussion. 36 To recruit GPs, the research fellows approached the practice manager in each general practice associated with the participating care homes. They provided the practice manager with a verbal overview of the study and asked that an invitation letter, information sheet and consent form be sent to a named GP. A follow-up telephone call was made to the GP. All focus group and interview participants received an honorarium for their time, as noted in the relevant information sheet. Participation was voluntary and written consent was obtained from all participants.
Interview and focus group discussion guides
Even though this stage of the study focused on developing and adapting the intervention and did not involve its implementation, we considered the first three constructs of normalization process theory – coherence, cognitive participation and collective action – as these were useful in alerting us to factors that may have an impact on its implementation; hence these constructs were used in the development of topic guides. The fourth construct, reflective appraisal, was not considered useful at this stage given that the intervention was not yet implemented. We used these three constructs to create the following broad working definitions so that normalization process theory could be usefully applied within our study:23–25
-
Making sense (coherence) – how do participants understand the issue of AMR and what is their usual practice?
-
Engagement and commitment (cognitive participation) – what do participants see as necessary to engage staff in the new practice?
-
Facilitating the use of the intervention (collective action) – how do participants envisage the intervention working and what are the factors that may facilitate or inhibit its use?
These three constructs of normalization process theory were used to shape the questions within the discussion guides for focus groups (see Appendix 2 for care home staff and Appendix 3 for family members) and individual interviews (see Appendix 4) that were devised and agreed by members of the research team. For example, both guides included questions about usual practice when a resident was suspected to have an infection.
Conduct of the interviews and focus groups
At the beginning of a focus group or interview, the facilitator assured participants regarding confidentiality, gave a brief background to the study and offered participants an opportunity to ask any questions. We then used questions from the discussion guides, with appropriate prompts, to ask participants about their usual practice when a resident was suspected to have an infection. The participants in the care home staff and family member focus groups were also asked about their understanding of AMR. Next, participants were shown a version of the decision-making algorithm and, using the ‘think–pair–share’ approach,37 were asked to reflect on it for a few moments. We then asked the participants, using appropriate prompts, questions that included how easy the algorithm was to follow; what they perceived to be missing, not needed or confusing; and any concerns they had about the use of the algorithm. This process is described in Box 1.
We distributed the decision-making algorithm to participants and asked them to think briefly about it on their own (1–2 minutes). We then asked them to imagine that they had a resident with a suspected infection and to consider how well they thought the decision-making algorithm would actually work in practice.
Focus groupsWe asked participants to form groups of two or three people to discuss the algorithm (3–4 minutes) and to think about at least one question they would like to ask or one comment they would like to make about it. We then asked each small group of participants about their questions or comments.
Focus groups and interviewsWe proceeded to use the questions and appropriate prompts from the discussion guides to stimulate discussion (25 minutes).
Data analysis
All interviews and focus group recordings were digitally recorded and transcribed verbatim by an external organisation. The transcripts were checked against the recording by the research fellow in each area to ensure accuracy and anonymisation. The transcribed data were uploaded into NVivo® and repeatedly read to increase familiarity with the data. Data analysis was based on the constant comparison method. 27 A selection of focus group and interview transcripts were first open coded inductively, with codes created from the patterns and themes emerging from the data, and an initial coding frame was developed. This thematic coding frame was then applied to subsequent transcripts and iteratively refined as new codes were defined (see Appendix 5 for care home staff and family member interviews and Appendix 6 for GP interviews). We used the framework matrix facility within NVivo® to assist the analytic process. These matrices enabled the research fellows to summarise the text associated with a theme in order to develop a narrative for each of them. These themes were then structured in accordance with key aspects of the decision-making algorithm. A COREQ (consolidated criteria for reporting qualitative research) checklist was completed for this aspect of the study and can be found in Appendix 7.
Internal review by the Feasibility Study Management Group
In addition to the activities outlined in the previous section, the algorithm was also reviewed and refined by the research team through an iterative process. Via the monthly Feasibility Study Management Group meetings that involved all members of the research team, aspects of each draft of the algorithm were discussed and debated. Changes were made by the research team based on results from the literature review, consensus meeting, focus groups and interviews. The final algorithm was agreed on by all members of the research team.
Adaptation and development of training material
The training material was developed at Queen’s University Belfast by Catherine Shaw, with input from members of the research team, based on the learning and results from the adaptation of the decision-making algorithm (see Adaptation of the decision-making algorithm) and the approaches taken by Loeb et al. 16 in the original Canadian study.
We were aware of the different staff categories within care homes (Table 3) and how this would need to be considered in the planning and development of training. The most important differentiating feature was the presence of qualified nurses in care homes with nursing. Other care staff who were not qualified nurses were designated as senior or junior care staff (largely based on their experience), along with activity co-ordinators in English care homes. The role of an activity co-ordinator is to facilitate and support activities in a care home; these may include activities for individual residents, groups of residents or for the whole care home.
| Different categories of staff | Nursing homes | Residential homes |
|---|---|---|
| Nursing staff | ✓ | |
| Senior carers | ✓ | ✓ |
| Junior carers | ✓ | ✓ |
| Activity co-ordinators (English homes) | ✓ | ✓ |
As a result of discussions within the research team, it was decided that the main focus of the training would be those we perceived to be senior staff within the care home, for example nursing staff and senior carers in nursing homes and senior carers in residential homes. These were seen as the staff who would implement the decision-making algorithm. However, we realised that those we perceived to be junior staff (junior carers in each home) also have a role as they are often the carers who will have most contact with residents and will observe if there is a change in their health status. However, they do not have responsibility for contacting GPs. Therefore, we decided that there would be two levels of training, both of which would cover the principles of the project. However, for the junior staff, training would focus on raising awareness of the decision-making algorithm and its use during the study, rather than instructing them on how to use it. It was anticipated that this level of training would be sufficient to enable junior staff to alert more senior staff in the event of a resident with a suspected infection.
The key document that informed the development of the training was the REACH decision-making algorithm. Based on this, careful consideration was given as to what should be incorporated in the training and in what format. Content was informed by material related to AMR, the background to the study, use of the decision-making algorithm, communication skills and illustrative case studies.
Formats considered were:
-
manuals
-
Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentations
-
online platforms
-
video
-
DVD.
Early in the development stage, it was decided that the preferred formats for the initial training were a manual (hereafter known as a study handbook) and a Microsoft PowerPoint presentation. We also recognised that it was not possible for all staff to attend the training session, as care-related activities needed to continue in the care home and night staff may be unable to attend a designated session. We were also aware that turnover of staff can be substantial in care homes (figures range from 19–42% annually). 38,39 Thus, we anticipated that further training would be needed over the course of the study for staff who were unable to attend a training session as well as newly employed staff. Therefore, we decided to also develop the training in DVD and online formats.
Catherine Shaw drew on her previous ethnographic research experience in the care home setting, the skills and knowledge from within Queen’s University Belfast and the research team that had developed similar training materials in the past. 17 It had also been recommended to the chief investigator that there should be a focus on communication skills, particularly between care home staff and GPs (Professor Kevin Brazil, Queen’s University Belfast, 2014, personal communication). The situation–background–assessment–recommendation (SBAR) tool was developed by the NHS Institute for Innovation and Improvement (now incorporated into NHS Improving Quality). This tool provides an easy way for health-care professionals to clarify what information needs to be communicated when making recommendations to other health-care professionals for immediate attention and action. 40
A software package that supports the production of infographics, presentations and flyers (Piktochart®; Bayan Baru, Malaysia) was used to make the training material visually appealing. A short video on AMR was also produced for inclusion in the training material. Each draft of the material was critiqued by Anne Campbell and Carmel Hughes, and a pilot version was delivered to a group of postgraduate students and staff within the School of Pharmacy at Queen’s University Belfast. Feedback from this exercise informed the final content and delivery of the training.
The development of each component is described in more detail in the following sections.
Study handbook and Microsoft PowerPoint presentation
The handbook was the main source of information for the participants; the Microsoft PowerPoint presentation contained all the information included within the study handbook, but in a condensed format that was visually appealing. The introduction in the study handbook and presentation was informed by reference to the literature in respect of AMR, the key signs and symptoms of the three target infections, non-specific indicators such as a change in behaviour and avoidance of an over-reliance on temperature as an indicator of infection in older people and those with dementia. Thereafter, the content of the study handbook and presentation focused on specific aspects of implementation. It was agreed within the research team that the training would also include reference to the key data-collection forms used over the course of the study.
Decision-making algorithm and step-by-step guide on its use
The development of the algorithm is described in this chapter (see Adaptation of the decision-making algorithm). A copy of the decision-making algorithm was provided within the study handbook as a point of reference for care home staff during the training sessions and while the study was ongoing. The step-by-step guide was developed to provide information on each component of the algorithm, including a rationale supporting why certain elements regarding management of infection were included or not included. For example, a common misconception is that change in smell/colour of urine is a valid indicator of UTI, but this was not confirmed/supported within the literature. Thus, an explanation of this was provided alongside the UTI signs and symptoms box. Another misunderstanding regarding infection is the differentiation between bacterial RTIs and viral RTIs; this was also explained in more detail within the guide. It was intended that staff would study this guide during the training session and refer to it throughout the duration of the study to gain clarity or reassurance.
Case scenarios
The research team also considered ways in which the use of the algorithm could be illustrated in a more practical way. This was achieved through the development of three case scenarios (one for each infection). These scenarios were designed to be reflective of everyday life within the care home setting and Catherine Shaw was able to draw on her previous ethnographic experience of studying care homes to produce these scenarios. Each case scenario was divided into two parts. The first part set the scene by providing information about the care home resident and the (mostly non-specific) symptoms with which they presented. This part of the scenario would be used to elicit a description of usual practice by staff during training. The second part of the scenario presented the same information but would then ask staff to apply the decision-making algorithm. The team also developed worked examples of using the decision-making algorithm for inclusion in the study handbook and presentation.
Communication using the situation–background–assessment–recommendation tool
The team referred to the original SBAR tool developed by the NHS Institute for Improvement and Innovation40 to consider how best to include it in the training material. SBAR consists of four standardised stages or ‘prompts’ that help staff to anticipate the information needed by colleagues and to formulate important communications with the right level of detail. This was considered important in the context of care home staff relaying information to GPs in an appropriate manner. As with the use of the decision-making algorithm, the research team developed scenarios that would provide the staff with the opportunity to become familiar with the tool and its use through role play. These scenarios would highlight the situation (identifying the staff member who was calling, from where, in relation to which resident and describing the issue), background (in this case, details about the resident), assessment (signs and symptoms of the suspected infection) and recommendation (what the staff member plans to do and requesting further support if necessary).
Mode of training delivery
The training was designed for face-to-face delivery with groups of staff members using both the presentation and the study handbook, with the latter to be provided to all participants. The format of the training material was designed to be interactive, with the presentation of more didactic information interspersed with a video on AMR and activities such as case studies and role play between participants. It was felt that these interactive activities would illustrate the ‘real-world’ use of the decision-making algorithm and SBAR tool and instil a degree of confidence in staff participating in the study.
Development of alternative modes of delivery
To help support the training (and indeed ongoing training) of care staff, a REACH training video was made by Catherine Shaw, again with support from the research team and colleagues at Queen’s University Belfast. This video was based on the handbook and included short film clips and information from the original Microsoft PowerPoint presentation. The aim of this video was to provide care homes with an alternative training vehicle for refresher training or training of new staff who had not been able to attend the original training sessions. The content was also transferred to a flash drive and online platform.
Results
Adaptation of the decision-making algorithm
Production of rapid reviews
Our searches retrieved 1905 articles. Just under 1500 articles (1487) remained after removal of duplicates, with the addition of another 24 records identified from other sources (hand-searching of other papers), of which 118 were carried through to the next stage. References were included if they met the pre-established criteria outlined in Introduction. The main reason for exclusion of articles at this point was that they did not relate to the older population or the care home setting. Following the 3-day meeting, 38 articles and two guidelines were included and data from these documents were extracted into evidence tables. The main reason for exclusion at this stage was that the article did not specifically relate to the management of urinary, respiratory or skin infection in care home residents. Following a further review of the evidence tables by members of the research team (CS, CH and MT), a further 34 articles were excluded because they did not provide any updated evidence in relation to UTI, RTI or SSTI.
An overview of screening and assessment of all papers/resources is provided in Figure 4.
FIGURE 4.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram outlining the review process for identification of new evidence. Reproduced from Hughes et al. 35 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

The extracted evidence from the remaining six papers is summarised in Table 4.
| Article | Setting | Design | Population and condition | Objective | Relevant information for updating/refining algorithm | Group agreement for updating algorithm |
|---|---|---|---|---|---|---|
| Falcone et al.41 | Community and hospital (includes care home setting) | Review | Older people, pneumonia (community-acquired pneumonia, health-care-associated pneumonia and hospital-acquired pneumonia) | This review sought to produce a summary of therapeutic recommendations on the basis of the most up-to-date clinical and pharmacological data | Signs and symptoms most commonly associated with pneumonia: cough, fever, chills, pleuritic chest pain. Extrapulmonary symptoms such as nausea, vomiting, alternation to sensory stimuli or diarrhoea may also be present. It is important to remember that pneumonia in older patients tends to occur more often with extrapulmonary manifestations. For example, the appearance of a delirium or acute confusion is found in approximately 45% of elderly patients with pneumonia | Agreed to add in extrapulmonary symptoms |
| Juthani-Mehta et al.42 | Nursing home | Prospective observational cohort study | Older people, UTI | To identify, among non-catheterised nursing home residents with clinically suspected UTI, clinical features associated with bacteriuria plus pyuria | The most commonly reported clinical features for suspected UTI in this cohort were change in mental status (39%), change in behaviour (19%), change in character of the urine (i.e. gross haematuria and change in the colour or odour of urine, 15.5%), fever or chills (12.8%) and change in gait or a fall (8.8%). Dysuria, change in character of urine and change in mental status were significantly associated with the combined outcome of bacteriuria plus pyuria. Absence of these clinical features identified residents at low risk of having bacteriuria plus pyuria (25%), and presence of dysuria plus one or both of the other clinical features identified residents at high risk of having bacteriuria plus pyuria (63%) | Change in character of urine (i.e. gross haematuria and change in the colour or odour of urine) was considered but not supported by more recent guidelines |
| SIGN 8843 | All settings | Guideline | Older people, UTI | To provide guidance in the diagnosis and management of suspected UTI in older people |
|
Agreed to add supportive care advice to algorithm |
| Stone et al.44 – updated McGeer et al.45 | Long-term care | Position paper | Older people, infection (general) | To update the 1991 McGeer criteria (infection surveillance definitions for long-term care facilities) using an evidence-based structured review of the literature in addition to consensus opinions from industry leaders including infectious diseases physicians and epidemiologists, infection control specialists, geriatricians, and public health officials | Acute swelling of the testes, epididymis and prostate should be included in surveillance definitions for UTIs as these symptoms are a common complication of UTI in both catheterised and non-catheterised males | Agreed to add acute swelling of testes, epididymis and prostate |
| D’Agata et al.46 | Nursing home | Prospective study | Older people, UTI | To describe the presentation of suspected UTI in nursing home residents with advanced dementia and how they align with minimum criteria to justify antimicrobial treatment | In long-term care residents with dementia, the most common reason for suspected UTI was a change in mental status (44.3%) | Agreed to subdivide the UTI element of the algorithm into two sections to account for two populations within care homes – those with and without dementia; changed in later iterations |
| Rowe47 | Nursing home | Review | Older people, UTI | This review sought to provide an overview of the prevalence, diagnosis and diagnostic challenges, management and prevention of UTI and asymptomatic bacteriuria in older adults | The most commonly reported clinical features for suspected UTI in this cohort were change in mental status (39%), change in behaviour (19%), change in character of the urine (i.e. gross haematuria and change in the colour or odour of urine, 15.5%), fever or chills (12.8%) and change in gait or a fall (8.8%) (Juthani-Mehta et al.42) | Change in character of urine (i.e. gross haematuria and change in the colour or odour of urine) was considered but not supported by UK SIGN guidelines |
Following the data-extraction process and analysis, three separate algorithms were developed: one for each infection of interest (i.e. UTI, RTI and SSTI). The UTI algorithm was developed using the original algorithm by Loeb et al. 16 as a starting point, and updated in accordance with the new evidence identified during the literature review (see Table 4). Thus, the UTI algorithm was based on data from Loeb et al. ,16 Juthani-Mehta et al. ,42 Scottish Intercollegiate Guidelines Network (SIGN) 88 guidelines,43 Stone et al. ,44 and D’Agata et al. 46 The RTI algorithm was informed by the original study undertaken by Loeb et al. 32 (preliminary work leading up to the study by Loeb et al. 16) alongside research by Falcone et al. 41
Because no new evidence was identified from the literature regarding SSTI, it was agreed that the minimum criteria for initiation of antimicrobial therapy for suspected SSTI in long-term care facilities by Loeb et al. 32 should be used. Each decision-making algorithm is described in more detail in the following sections.
Changes made to the urinary tract infection decision-making algorithm
In the first instance, the updated UTI decision-making algorithm was redrafted by the research team into two distinct pathways to account for two populations within care homes: those with and without dementia. This was based on findings from the literature (D’Agata et al. 46) that previous studies did not consider cognitive decline and the effect that this may have on residents with dementia and a suspected UTI. These residents may not experience any specific urinary symptoms, but rather a more pronounced decline in cognition and function. However, following ongoing discussions within the research team, it was agreed that, for the sake of simplicity, only one pathway encompassing residents with or without dementia would be presented.
The updated algorithm was designed to be used in residents who had a raised temperature (> 37.9 °C or > 1.5 °C above baseline) as per Loeb et al. 32 and SIGN 88 guidelines. 43 Swelling/tenderness of testes, epididymis and prostate were additional symptoms to those reported by Loeb et al. ,32 following data published by Stone et al. 44
Changes made to the respiratory tract infection decision-making algorithm
The RTI decision-making algorithm largely mirrored that of Loeb et al. ,32 with the addition of extrapulmonary symptoms as per Falcone et al. 41 Nausea, vomiting, altered response to sensory stimuli and diarrhoea were included in the algorithm as a separate step, provided that these symptoms presented in combination with a cough and a raised temperature. 41
Changes made to the skin and soft tissue infection decision-making algorithm
No changes were made to the approach for SSTI management previously published by Loeb et al. 32 owing to the absence of new evidence.
A combined decision-making algorithm
At this point, the management group decided that it was more practical to combine all three algorithms into a single decision-making algorithm with three ‘paths’ – one for each infection. It was considered that this would be more acceptable to care home staff and was more likely to be used. A single algorithm, encompassing all three infections, updated from evidence extracted from the literature, was then presented to the consensus group (see Figure 2). In all three infections, a supportive care step was added as per the SIGN 88 guidelines. 43 This step instructs the user to continue to monitor the resident, to provide pain relief if necessary and to encourage fluids.
Consensus group meeting
Four participants were approached by e-mail and invited to take part in the study. Three agreed to join the group. The remaining invitee was unable to attend on the day of the consensus group, but suggested an alternative participant who was able to attend. The participants consisted of a hospital consultant (respiratory medicine) with experience of prescribing in older people, a GP, a geriatrician and an expert in microbiology. All had the necessary clinical expertise to comment on the findings from the literature review and the updating and adaptation of the algorithm. The consensus group meeting was convened on 12 September 2016 and was facilitated by a member of the research team (CS).
Following sessions 1 and 3, as outlined in Figure 3, the most common symptoms identified for the three target infections by the consensus group are summarised in Box 2.
-
Urinary frequency.
-
Urinary incontinence.
-
Suprapubic pain.
-
Pain when urinating.
-
Blood in urine.
-
Non-specific feeling of being unwell.
-
Feeling ‘off’.
-
Loin pain.
-
Swelling of testes.
-
Cough.
-
Sputum.
-
Breathlessness.
-
Chest pain.
-
Delirium (not otherwise delirious in normal circumstances).
-
Pain with inspiration.
-
Confusion.
-
Shortness of breath.
-
Rapidly breathing.
-
Fast respiratory rate.
-
Hypoactive delirium.
-
Change in confusion status – more confused than normal.
-
Swelling.
-
Erythema/redness.
-
Heat.
-
Pain.
-
Drainage from area.
-
Hot to touch.
-
Change in colour of area affected.
-
Pus.
The consensus group participants viewed the presented algorithm positively and felt that it had a place within the care home setting, particularly where there was nursing support. Their specific comments on each infection, and how each would be managed using the decision-making algorithm, are summarised in the following sections, reflecting discussions in session 3, as outlined in Figure 3.
Urinary tract infection
-
There were concerns that the ‘two or more’ symptoms requirement suggested that some residents who needed to be seen by a GP would not have a referral. Strong views were expressed by one participant that a resident with one symptom, such as new or increased urinary frequency, would need to be seen.
-
There was discussion regarding whether or not ‘anecdotal evidence’, such as change in character of urine (i.e. smell or colour), could be considered a valid indicator of infection.
-
Confusion was discussed as a non-specific symptom of UTI. All participants felt that confusion should be included within the algorithm as a non-specific sign of any infection (UTI/RTI/SSTI), but it was not necessarily specific to a urinary infection. This is expanded on in the following section when considering RTIs.
Respiratory tract infection
-
Concerns were expressed regarding the use of the algorithm in those with chronic respiratory disease. Such patients may, at any one time, display several symptoms included within the RTI section of Box 2, yet not have an infection.
-
As with the UTI section of Box 2, concerns were expressed by one participant that the ‘two or more’ symptoms requirement meant that some residents would not see a GP.
-
Confusion and delirium were emphasised by most participants as lesser known symptoms of a lower RTI.
-
Chest pain/pain when breathing/pain on inspiration could be renamed dyspnoea.
Skin and soft tissue infection
-
The presence of pus should be included as a standalone symptom.
Following discussion of the algorithm (see Figure 3, session 3), participants continued to debate other aspects of infection management in older care home residents. All participants stated that they would not rely on temperature alone when assessing an older person, particularly in a care home setting.
Most participants considered assessment of pulse/blood pressure/respiratory rate/colour of resident (e.g. pallor) to be more valuable indicators of infection. Rigors was considered to be a very serious presentation and would require medical intervention, and it was recommended that this should be acknowledged in the algorithm. There was extensive discussion regarding the management of infections in residents with specific conditions such as dementia, type 2 diabetes or chronic respiratory disease and whether the algorithm should be applied in such cases or if there should be a specific algorithm for those residents with dementia. However, it was concluded that excluding such residents would render the algorithm essentially redundant as these residents constituted the majority of a care home population. There was also discussion regarding the different types of staff within care homes (with and without nursing) and how non-nursing staff would use the algorithm. However, having a single algorithm was considered to be the most practical option, and feasibility of its application by a range of staff would be tested in the study.
Ranking of the symptoms (see Figure 3, session 4) by participants resulted in the findings that are summarised in Table 5.
| Infection | Symptoms | |
|---|---|---|
| Accepted (ranked score) | Rejected (ranked scores) | |
| UTI |
|
|
| RTI |
|
None |
| SSTI |
|
None |
These findings informed the next iteration of the decision-making algorithm, which was presented to the participants for further comment (see Figure 3, session 5).
Urinary tract infection
It was agreed that symptoms such as urgency, frequency and incontinence should be prefixed by ‘new or worsening’ and that suprapubic pain should be replaced by lower abdominal pain.
Respiratory tract infection
One participant felt that ‘Respiratory rate > 25 (shortness of breath/difficulty breathing/rapid breathing)’ should be presented separately as ‘Respiratory rate > 25 and Shortness of breath/difficulty breathing/rapid breathing’ within the decision-making aid as respiratory rate is a standalone symptom within other algorithms such as those prepared by the British Thoracic Society (e.g. British Guideline on the Management of Asthma48). All participants were in agreement given this additional information.
It was suggested that the term ‘dyspnoea’ might not be understood by staff in residential homes, so it was agreed that this should be replaced by ‘difficulty breathing’.
Skin and soft tissue infection
It was agreed that all symptoms should be prefixed with ‘New or worsening’.
More generally, participants felt that a text box containing the following statement should be added to the overall algorithm:
Please note:
Residents with dementia may not show the usual signs of urinary, chest or skin infection at first. Their symptoms may be more general such as a change in behaviour or functional decline. This may be seen as hyperactivity such as aggression and agitation, or hypoactivity such as appearing withdrawn and loss of appetite.
There was also further discussion regarding the steps preceding the consideration of specific symptoms, and participants suggested the inclusion of observation of other vital signs or symptoms of concern (e.g. rigors) that would highlight the need for medical intervention.
The final round of voting and ranking of symptoms by participants (see Figure 3, session 6) resulted in the findings summarised in Table 6.
| Infection | Symptoms | |
|---|---|---|
| Accepted (ranked score) | Rejected (ranked scores) | |
| UTI |
|
None |
| RTI |
|
Dyspnoea (0) |
| SSTI |
|
None |
These rankings informed the final stage of refinement following on from the consensus group meeting.
Pre-implementation focus groups and interviews
We conducted semistructured face-to-face focus group interviews with care home staff and relatives of residents in a total of six care homes in NI and the West Midlands during September to October 2016. There were 12 focus groups in total: six with care home staff and six with families of residents, with one care home staff and one family focus group in each of the care homes. We conducted semistructured one-to-one interviews with eight GPs during January to March 2017 (five in NI and three in the West Midlands). The interviews in NI were carried out by Anne Campbell and Catherine Shaw, and the interviews in England were carried out by Rachel Potter. All participants gave written informed consent. The numbers participating in each focus group and interview are shown in Table 7.
| Home | Number of participants | ||
|---|---|---|---|
| Care home staff focus groups | Family focus groups | GP interviews | |
| A | 4 | 4 | 1 |
| B | 4 | 5 | 2 |
| C | 8 | 4 | 2 |
| D | 9 | 5 | 1 |
| E | 8 | 8 | 1 |
| F | 8 | 5 | 1 |
| Total | 41 | 28 | 8 |
The focus groups were held in a suitable room in the care homes and participants were provided with refreshments. The duration of these meetings ranged from 50 to 76 minutes with care home staff and from 46 to 71 minutes with family members of residents. The interviews with GPs were conducted in the practice room of the participating GP. The duration of these interviews ranged from 16 to 31 minutes. In the presentation of our findings, all quotations have been given an anonymised label (e.g. A–F represents each participating home) (see Chapter 6, Care homes, for a description of each home). ‘Staff, pre implementation’ indicates that the interview or focus group was conducted with staff pre implementation of the intervention. Other participant groups are family members (‘family’) and GPs.
Here we present the main findings identified from the pre-implementation focus groups with care home staff and relatives of residents and GP interviews that relate to, and informed, the adaptation of the algorithm. The themes that were identified from the analysis were structured in accordance with three key aspects of the decision-making algorithm: initial assessment of the resident, observation of the resident and action by care home staff.
Initial assessment of resident
Care home staff described many examples of new or worsening non-specific symptoms that they thought may indicate that a resident had an infection. Most related to the way in which care home staff observed change in behaviour, such as reduced mobility, increased confusion, agitation or aggression, poor appetite, lethargy, changes to fluid intake and output, not recognising their relative or just ‘not being right’:
Well they’d still be, you’d be going by their mobility, because they’d be off their feet, they’d be shaky, they’d be clammy confused, you know a lot of those symptoms. (. . .) We see a lot of changes in mobility or increased falls when somebody has an infection.
C: staff, pre implementation
They manifest like agitation, anxiety or different from what they are. For example, they are always pleasant to the staff and other patients and their families, they can be different when they come in and then the staff go ‘there’s something wrong, something’s not right’.
A: staff, pre implementation
Some care home participants also felt that the decision-making algorithm should distinguish changes in behaviour for those with and without dementia as many of the examples provided in the algorithm were already present in residents with dementia, therefore making it difficult to notice change. However, the study team felt that it would be difficult to provide a comprehensive list of all examples of change in behaviour suggested by staff as the decision-making algorithm would become illegible. We therefore decided that the examples already provided in the algorithm would remain unchanged, but additional suggestions from the participants would be included and discussed in the training session and accompanying study handbook.
Observation of the resident
Some care home staff considered 37.9 °C too high a threshold and 4 hours too long before contacting the GP. They expressed concern that this may put the resident at risk and suggested a temperature range of 37.3–37.6 °C as being more appropriate to initiate GP contact. However, without exception, staff used temperature to alert them to a possible infection in a resident, in combination with other indicators such as behaviour change, prior knowledge of the resident and vital signs, and sought to reduce temperature with supportive care. The decision-making algorithm already directed staff to look for additional symptoms that may have indicated infection if a resident’s temperature was within this range.
Staff in one nursing home expressed concern about stipulating a time scale before contacting a GP:
I wonder if it will not be safer not to put [the number of] hours because you know if you have this instrument and you want people to follow it and something happens then they said we’ve followed this and it’s your [care home staff] fault.
E: staff, pre implementation
Similarly, family members expressed concern about a 4-hour wait between temperature observations. Family members did not consider temperature a sufficient indicator of infection in older people and were concerned that the algorithm appeared to be over-reliant on temperature as a means of assessment:
I think I would be worried about the 1 to 4 hours I would worry about the time, if the answer is no, I think it should be a shorter period of time. (. . .) Usually an increase in temperature is a good sign that there’s something going on?
A: family, pre implementation
The GPs reported that although having a measurement of temperature as a guide was useful, some older patients may be quite unwell without having a raised temperature and it was not always a reliable indication of infection. One GP expressed concern that staff may feel compelled to follow the algorithm strictly and would wait 12 hours to see if a temperature rise was sustained before contacting the GP. Specific time requirements may be confusing and may lead to accusations of staff not adhering to guidance. Another GP felt that it was important to consider the time at which the temperature was taken, and suggested that this would have implications for requests for out-of-hours visits:
You see there, the over-65 [year-old] with COPD [chronic obstructive pulmonary disease] and delirium is much more clinically urgent than someone with increased frequency of their urine. And yet the end result of that algorithm is phone GP. Now if you phoned the out-of-hours service at 3 o’clock in the morning [for resident with chronic obstructive pulmonary disease] that’s reasonable. If you call the out-of-hours service at 3 o’clock in the morning like that [for resident with increased frequency], that’s unreasonable.
D: GP, pre implementation
Staff and relatives at the residential homes reported that residential home staff were not allowed to measure (‘take’) a resident’s temperature using a thermometer, as it was deemed to be a ‘nursing task’. There is no requirement for nurses to be present in residential homes. Participants reported that they would monitor a resident’s temperature in a number of ways, including feeling the head of the resident, noticing whether or not the resident was sweating or flushed, or whether or not the resident wished to remove clothing to cool down:
See where it says take the resident’s temperature we obviously can’t do that, step 1. (. . .) That’s why we rely wholly on behaviour and that because we don’t have a lot of tools that we are allowed to use. (. . .) We would love to be able to take temperatures and things like that there but company frowns upon it.
C: staff, pre implementation
Although family member participants from residential homes recognised that staff were not clinically trained, they expressed surprise that staff were not able to assess temperature using a thermometer. They queried the possibility of staff doing so, even though it was not part of their normal role:
It would be something to look into, if (. . .) they needed to take the temperature, that they would be able to do it. But you know they are not, they are carers, they are not medical people you know? So whether they would want to take that on is as their everyday work and job?
C: family, pre implementation
Initially, the research team considered changing ‘take resident’s temperature’ to ‘assess resident’s temperature’ to accommodate this practice. However, it was later decided that it would be more useful to train staff to use thermometers in the residential homes.
Care home staff from nursing homes described how they routinely recorded other signs and symptoms alongside temperature if they suspected that a resident might have an infection, such as by measuring pulse, blood pressure and respiration rate, listening to the chest and measuring oxygen saturation levels. Participants in both nursing and residential homes discussed not relying solely on temperature as an indicator of infection and how they considered other signs and symptoms as being important to their decision-making:
We would notice a difference in their mood or the way they are, or confusion would be a big thing with elderly people (. . .) temperature would nearly be the last thing I would take. I would look at all the other things first and then I would take temperature, the GPs will always ask for the temperature.
B: staff, pre implementation
Participants from residential homes described how they would also look for non-clinical indicators of infection, including pallor, tiredness and struggling to breathe.
Again, the study team felt that it would not be useful to include all suggestions as they were not essential criteria in order to move on to the next stage of the decision-making algorithm.
Participants expressed concern that it could be challenging to assess new or increased urinary urgency, frequency or incontinence, blood in urine and lower abdominal pain in care home residents, particularly in those who are incontinent or have dementia. They also noted that they would place more importance on some urinary symptoms than others (e.g. staff would be more concerned about evidence of blood in the urine than about increased urgency or frequency). More common indicators of infection that were suggested included change in smell or colour of urine and dehydrated skin:
Yeah and if someone is incontinent you don’t always know about the urgency of it because with dementia, not everyone can tell you when you need to go so it makes it quite difficult. And with the lower abdominal pain not everyone will tell you if they are in pain.
F: staff, pre implementation
I don’t think anyone ever tells us that they’ve got that it’s burning. Because a lot of them are already incontinent, they are wearing incontinent pads, you’re not going to see the increased urgency or frequency or increased incontinence, so it’s rare that we actually see blood in the pad when they’ve had a urinary infection. A lot of them can’t tell you if they’ve got a lower abdominal pain OK, you could see the shaking and the rigors but that’s not a symptom that we see often.
D: staff, pre implementation
Because there was insufficient evidence from the literature to support inclusion of a change in smell or colour of urine as an indicator of infection, this was not changed.
Participants who were registered nurses described how they would not expect to observe shaking or rigors in residents with a temperature below 37.9 °C. Evidence from the literature supported a 1.5 °C increase in baseline temperature for UTIs, which may not necessarily be greater than 37.9 °C,32,43 so, again, no changes were made based on the feedback.
Staff in nursing homes described aspects of the algorithm that gave them concern when assessing a RTI, for example that a respiratory rate of > 25 breaths per minute would often warrant emergency assistance (ambulance). There was also concern that residents with RTIs can deteriorate very quickly and waiting 4 hours before contacting GPs would be too long. Staff described how, in their experience, green/yellow sputum may indicate infection and recommended that this should be included in the algorithm. However, evidence did not support this inclusion, so no changes were made.
Staff described how a new or worsening chesty cough was likely to be accompanied by a high temperature, green or blood-tinged sputum, a change in pallor, clamminess and mood. If a resident was exhibiting these signs and symptoms, staff were likely to contact the GP irrespective of what was contained within the algorithm because a resident’s condition – especially a resident with a prior history of RTI – could decline very quickly:
See it’s not mentioning here the sputum, the colour of the sputum because COPD [chronic obstructive pulmonary disease], every patient of COPD has sputum (. . .) you can see a lot from the colour. When it’s infection it’s yellowish, greenish.
A: staff, pre implementation
Staff in residential homes thought that the algorithm largely reflected their usual practice apart from taking temperature and respiratory rate. For the latter, staff discussed how they would be alerted to the resident being more breathless than usual, having a change in colour, experiencing tiredness, having blue lips, struggling to breathe and having reduced mobility. Family member participants reported that although they considered that residential care home staff were competent to observe and test for UTIs, they felt that they were not qualified to do so in the case of RTIs, which would require a GP to assess:
The staff do all the vital signs which is the main things, and as I say some things they can do for UTIs is take a dip, but the like of chest infections or suspected chest infections they have to get a doctor out, they are not qualified to do that, so some of it’s OK and some of it’s not, you have to have a doctor.
A: family, pre implementation
Care home staff from nursing homes described how they would routinely swab when there was pus draining from a wound and that the results from the swab test would be sent to the GP, who would then prescribe an antibiotic. Conversely, GP respondents differed in their views of pus; one thought that it could be potentially confusing to ask those using the decision-making algorithm to contact the GP if pus was draining from a wound, as an antibiotic was not usually required:
If they have pus draining from a wound, we always swab it and send the swab. Always. We would never leave that. (. . .) And then like one was done the other day and it goes back to the GP, the results and then the GP contacts us and then with the antibiotic and then it comes from the pharmacy, so we always swab a pus-y wound.
B: staff, pre implementation
If we get someone with an abscess and we let the, once you let the pus out you don’t usually have to give the antibiotic cover, so it’s more the antibiotics needed here rather than whenever there actually is pus draining.
B: GP, pre implementation
Participants also discussed whether or not they would wait until there were two or more of the other symptoms listed in the algorithm for SSTIs before contacting the GP. Staff in residential homes reported that they would contact a district nurse if they had concerns about a SSTI and the district nurse would contact the GP if needed:
But with ours being residential, if we have any sort of concerns with any of the residents’ skin, we would contact the district nurse to come out, and the district nurse would sort of be the one then to say, if it’s maybe an open wound they would take a swab, or if it’s the likes of say, if it’s the redness, the hot to touch, and they would go back and voice concerns to the GP, and then issue an antibiotic, so the GP would.
C: staff, pre implementation
Well, if there’s a wound, we would do that if it’s localised redness heat or swelling or anything like that where there’s no abrasion or no wound then we go through the GP – if there’s a sore or the skin like moisture lesions we see. Or if it looks like breaking, then we go to through the district nurses.
F: staff, pre implementation
Action by care home staff
Care home staff participants described how they felt that the algorithm generally reflected their usual practice. When a resident had a temperature > 37.9 °C, staff provided supportive care in the form of fluids and paracetamol and rechecked the temperature approximately 2 hours before contacting the GP:
If they are not taking the paracetamol and not taking a drink, I’d be more inclined to contact the GP, because I know I will not get that down, if I can get that down with paracetamol and lots of drinks I would wait, usually.
B: staff, pre implementation
Format of the decision-making algorithm
Participants also discussed aspects of the format of the decision-making algorithm. They suggested that the algorithm should be laminated and preferred A4 size for personal use and A3 size for display. Participants proposed that the decision-making tool could be made more visually appealing and easy to follow with the addition of colour, and this suggestion was incorporated into the final version of the decision-making algorithm. Generally, staff, family members and GPs were happy with the format of the algorithm, but not everyone understood the ‘≤’ symbol used in the algorithm and this was removed in the final version.
Internal review by the Feasibility Study Management Group
As part of the iterative process in the development and adaptation, there were ongoing discussions within the research team. The literature review, the consensus group, focus groups and interviews contributed to these discussions. A further two additional papers49,50 were identified, which informed our thinking regarding the use of baseline temperature to initiate the use of the algorithm. The final version of the decision-making algorithm was structured in accordance with the three distinct stages that had emerged from the findings from the qualitative data collection earlier and provided a step-wise approach to the management of residents. These stages were initial assessment of the resident, observation of the resident and action by care home staff. Each stage is described in the following sections.
Initial assessment of the resident
The research team concluded that the most appropriate way to begin the algorithm was by providing a list of various non-specific signs and symptoms of infection (including change in behaviour of the resident), together with specific signs and symptoms of each of the three infections (see Figure 7). This was because of the emphasis on non-specific indicators as signs of infection in older people within the literature,14,41,46,51,52 expert opinion from the consensus meeting and the opinions of care home staff and family members of care home residents. This approach would ensure that the majority of residents who may be unwell were identified in the first instance (Box 3).
Non-specific: suspected fever, change in behaviour (e.g. delirium, confusion/agitation, unco-operative, reduced mobility/’off legs’, loss of appetite, withdrawn).
AND/OR
Urinary/chest/skin symptoms.
It was agreed that temperature should not be used as a standalone criterion in the initial assessment of the resident (as was the case in the original algorithm by Loeb et al. 16) as older people do not always present with an increase in temperature when they are unwell. 49,50 In addition, an increase of 1.5 °C above the baseline temperature (as denoted in the original algorithm16) was not applicable to the UK setting as baseline temperatures are not routinely recorded in care homes. However, assessment of temperature was included in the observation step to identify residents who may be extremely unwell, and so temperature was included as an additional step, with a value of > 37.9 °C denoting the requirement of additional monitoring. This value was agreed within the research team, in consensus group meetings and focus groups as a temperature that would cause concern and require additional monitoring if presented by a resident. Figure 5 shows the observation section of the algorithm.
FIGURE 5.
Observation of the resident.
Following on from measurement of the resident’s temperature, this stage also incorporated the specific signs and symptoms of each infection. Following internal review by all those contributing to the updating and adaptation of the algorithm, and using a UTI as an example, the final signs and symptoms are outlined in Box 4.
Resident (without catheter) has burning on urination or two or more of the following:
-
new or increased urgency
-
new or increased frequency
-
new or increased incontinence
-
blood in urine
-
lower abdominal pain
-
shaking/rigors.
Action by care home staff
An action stage was added to the end of the algorithm to instruct care home staff on how to proceed, depending on the symptoms presented by the resident. For example, if the resident fulfilled the criteria for a suspected UTI (i.e. two or more symptoms from the list or dysuria alone), the care home staff were instructed to telephone the GP. If the minimum number of symptoms were not present, an additional step instructed the staff member to monitor residents with a temperature between 37.3 °C and 37.9 °C. In this scenario, the algorithm instructed the user to repeat step 1 (taking the resident’s temperature) after 6 hours. Figure 6 shows the ‘action’ stage of the algorithm relating to a UTI.
FIGURE 6.
Action by care home staff.
A copy of the final decision-making algorithm is shown in Figure 7.
FIGURE 7.
Final REACH decision-making algorithm. b.p.m., breaths per minute; COPD, chronic obstructive pulmonary disease. Reproduced from Hughes et al. 35 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
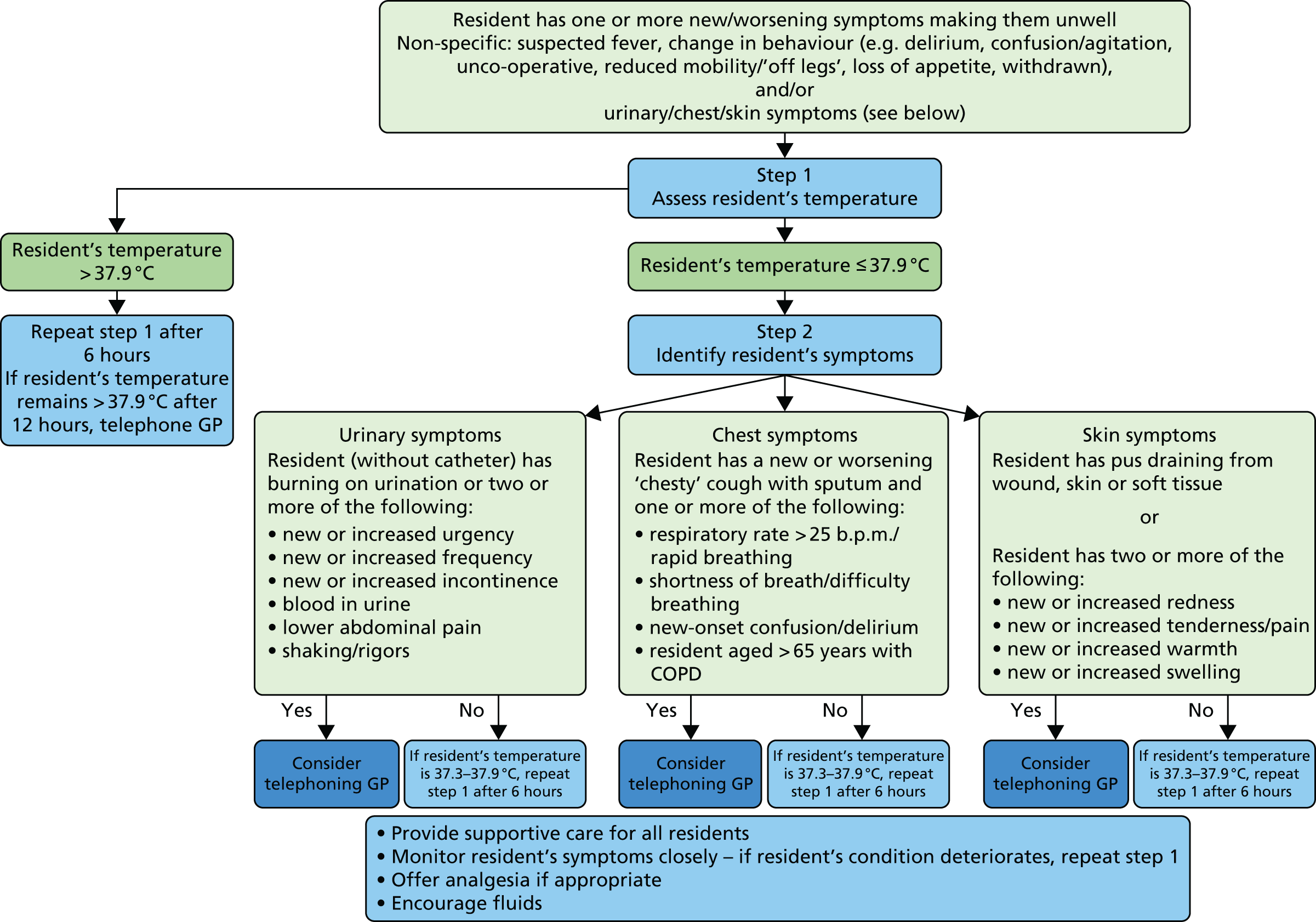
Adaptation and development of training material
A blended learning approach was utilised, incorporating a visual presentation, supporting documentation, opportunities to rehearse the use of the decision-making algorithm and communication skills using case studies and an overview of the data-collection forms.
The main training materials were presented in two formats:
-
a Microsoft PowerPoint presentation
-
a study handbook.
An abbreviated version of the training material was devised for junior staff, which highlighted awareness of the use of the decision-making algorithm, rather than its implementation.
The presentation and study handbook contained the following elements:
-
an overview of AMR, including a short video
-
summarised evidence on the management of infections, including infographics on (1) AMR, (2) each of the infections of interest (UTI, RTI, SSTI), (3) older people and infection and (4) dementia and infection
-
a copy of the finalised decision-making algorithm
-
a step-by-step guide on how to use the algorithm
-
three case scenarios (illustrating the most common infections encountered by care home residents)
-
worked examples of how to use the decision-making algorithm
-
the SBAR tool and how to use it, through illustrative case studies
-
instructions on how to complete the ‘using the decision-making algorithm’ form that was to be used while the study was ongoing.
In order to support ongoing training, in addition to these two formats, we produced a video (lasting 46 minutes) of the training presentation and made this available as a video on a DVD, on a flash drive and on an online platform (www.qub.ac.uk/schools/SchoolofPharmacy/REACHTrainingVideo/).
Summary
In this phase of the study, we set out to adapt and update the decision-making algorithm and training materials developed for the original Canadian study by Loeb et al. 16 for implementation in the feasibility study. This was achieved through a series of rapid reviews of the literature, focusing on identifying new evidence that had been published since the original Canadian study. This fed into the development and adaptation of the decision-making algorithm to encompass management of RTIs and SSTIs in addition to UTIs. A draft version of the decision-making algorithm was presented to, and discussed with, a range of health-care professionals, all of whom had experience of the management of infectious diseases and/or care of older people, as part of a consensus exercise. Further versions were reviewed and discussed in a series of focus groups and interviews with key stakeholders (i.e. care home staff, resident family members and GPs). Continuous iterative review was also undertaken by the research team while the other activities were taking place.
The final agreed version of the decision-making algorithm was included in the training material that was developed to support intervention implementation. The material consisted of a Microsoft PowerPoint presentation and study handbook. The presentation was also available in a number of other formats to facilitate accessibility of training for care home staff.
Chapter 5 Implementation of the intervention
Introduction
This phase of work focused on the implementation of the intervention (training and use of the decision-making algorithm) in the six care homes. Aspects regarding the feasibility of collecting data are discussed in detail in Chapter 6.
Aims and objectives
The aim of this phase of the research was to implement the intervention in the six recruited care homes. The objectives were as follows:
-
to deliver the training programme to care home staff in the participating homes
-
to collect relevant data from a variety of sources to assess aspects of feasibility, including the use of the decision-making algorithm and data pertaining to the dispensing of antimicrobials
-
to measure the resource use and costs associated with the training and implementation.
Methods
Delivering the training programme in participating care homes
The research fellows in NI and the West Midlands (AC and RP) contacted each participating care home to arrange dates and times for the training sessions; this was with either the REACH Champion or the manager of the home or a member of staff nominated by the home. We asked homes to arrange senior training sessions for the nursing staff and senior carers in nursing homes and senior carers in residential homes, and junior training sessions for junior carers, and explained the difference between the two types of training sessions (see Chapter 4, Adaptation and development of training material).
To maximise attendance, we asked staff to arrange four sessions (two senior and two junior) so that as many staff as possible would be able to attend, and to arrange sessions at the times of day that would be most suitable to the care home. For care homes in the West Midlands, we asked, if possible and convenient for the home, for the sessions to be arranged on consecutive days as the trainer (CS) was travelling from NI.
We asked care homes to provide a suitable location for the training sessions that would accommodate the number of staff attending each session. We also asked homes to provide, if possible, a projector and screen that could be used for the presentations, and we arranged a portable projector and screen when this was not possible. The trainer planned to arrive at the care home with sufficient time to set up the training session and to organise the venue in such a way that it facilitated the training event, particularly for discussions and small group tasks.
Maximising attendance at the training
We recognised the importance of trying to embed this new approach to the management of infections and prescribing of antimicrobials in care homes and the importance of engaging staff as fully as possible. Therefore, to further encourage attendance at the training sessions, we offered a £10 voucher to each staff member, along with a certificate of attendance, which would serve as evidence for continuing professional development when required. Both the voucher and the certificate were provided to staff on completion of the training.
Follow-up training
We asked each care home manager to identify up to two members of staff (to account for different shifts within the homes) who could act as REACH Champions (see Chapter 3, Methods) and who would be responsible for delivering training to new staff or those who were unable to attend the original session (see Chapter 4, Adaptation and development of training material). These Champions received the requisite training by the research team and were provided with copies of the REACH training video as a DVD and as a flash drive, plus details of the online platform (see Chapter 4, Adaptation and development of training material).
Temperature training
As identified in the preceding chapter (see Chapter 4, Adaptation of the decision-making algorithm), measurement of temperature was not normally undertaken in residential homes. However, discussions within the research team and advice from the SSC led us to provide this training to staff in the residential homes in this study. We trained staff working in the two residential homes to use thermometers purchased for the study and taught them how to interpret the temperature recording. One of the research fellows (RP), with a nursing qualification, taught the REACH Champion in the West Midlands residential home, who then disseminated the training to the senior care staff. Staff in this home had measured temperature for a previous study and were already confident using the thermometers and interpreting the result. Rachel Potter also trained a small group of staff in the residential home in NI to use the thermometers.
Training for general practitioners
In the original protocol that had been submitted to NIHR, it had been our intention to train GPs for participation in the study. It was anticipated that the training would complement that for care home staff and would focus on the use of the decision-making algorithm and communication with care home staff. However, at the SSC meeting held in November 2016, members of the committee advised us not to include GPs in the training. The view of the committee was that GPs associated with the care homes should be made aware of the study and provided with a copy of the decision-making algorithm that would be used in the care homes. The independent members of the SSC considered that focusing training on both care home staff and GPs would be overly complicated. Therefore, as advised, we did not proceed with GP training, as noted in our progress report in September/October 2017.
Preparing sites for implementation
Prior to implementation in all sites, the research fellows collected baseline data pertaining to the care home with the assistance of the REACH Champion or/and the manager, provided and explained the content of each site file, discussed completion and storage of CRFs and negotiated with the REACH Champion how often to visit the home.
Collection of data from a variety of sources to assess aspects of feasibility
We tested the feasibility of collecting data that would contribute to a primary outcome in a larger study [i.e. antimicrobial dispensing data from community pharmacies/dispensaries in general practices, which could then be converted to defined daily doses (DDDs)]. We were interested in the collection of data relating to hospitalisations, contact with health-care professionals and deaths. These data were important in respect of safety monitoring as it may be hypothesised that a reduction in antimicrobial prescribing may lead to increased numbers of hospitalisations and/or deaths. We were also interested in the resources used in the set-up and delivery of the intervention, and their associated costs. We planned to collect these data from individual care homes and centralised administrative data sources. Most of the quantitative data collected related to processes such as the number of times the decision-making algorithm was used, contact with a GP and GP visits to a care home. Details of the various types of data collection undertaken are outlined in the following sections.
Data collected from the care homes
Owing to the developmental nature of this study, the algorithm used to assist care home staff in decision-making was developed early in the study, and this algorithm dictated the particular data elements to be collected (e.g. type of suspected infection and the action taken by care home staff or GP). Other data to be collected were as follows: baseline characteristics of each home including demographic information on residents, whether or not the decision-making algorithm was used, details of hospital services used, contacts with health and social care professionals and adverse events. We developed five clinical record forms to collect all the required study data:
-
home baseline data form
-
using the decision-making algorithm form
-
use of hospital services form
-
contacts with health and social care professionals form
-
adverse events form.
A copy of each CRF was included in the feasibility study master file. The forms were completed at the care homes, collected/checked by the research fellows at Queen’s University Belfast and the University of Warwick and e-mailed using password protection to the NICTU.
We tested the feasibility of data collection and monitored missing data closely, following up with care home staff and other relevant individuals as to why data may have been missing.
Data collected from community pharmacies
Contact with all of the pharmacies/dispensaries linked to the REACH care homes was made during the adaptation phase of the project to advise pharmacists as to the nature of the study and what would be required in terms of data download and output.
Notifications were sent to the participating pharmacy/organisation requesting the data (as either a letter or an e-mail, dependent on the pharmacy/organisation requirement). For the first download (baseline data), the start date for the baseline search equated to 12 months prior to the date the care home started in the study (i.e. implementation). The end date equated to the date the care home started in the study. For the second download (follow-up), the start date for the search equated to the start date of implementation for the home. The end date corresponded to the date the home completed its 6-month implementation period.
These notifications contained full and clear instructions of the data requirements. These included the locations and names of the homes, the search dates, the output variables (see the list below), the acceptable output styles and instructions of how to securely supply the data to the research team. Pharmacies/organisations were also given instructions on how to invoice the REACH study for the agreed ‘search fee’. The research teams (in NI and England) liaised with the pharmacies/dispensaries to answer questions as necessary. Data-sharing agreements were provided as required.
Antimicrobials were defined as those medicines listed in chapter 5 (infections) of the British National Formulary,53 including antibacterial drugs (see Introduction), antifungal drugs (see Aims and objectives) and selected agents from antiviral drugs (see Methods).
The variables required in the output were as follows:
-
patient/resident number (as assigned by the system)
-
date of prescription
-
drug name
-
drug strength
-
formulation
-
quantity dispensed.
No patient identifiers were to be included in the output. The data were to be supplied in a file format that could be transferred into a Microsoft Excel document, for example comma delimited files (.csv). Transfer of data to the research centre was via the use of encrypted memory sticks or via secure e-mail. The data were checked for inaccuracies or errors (e.g. wrong dates being used). In some cases, the pharmacies were able to filter the data and provide antimicrobial data only, whereas, in others, all dispensing data for participating homes were provided. In such cases, the chief investigator reviewed the data and removed all non-antimicrobial data.
Data collected from administrative sources
For events such as hospitalisations and deaths, we also explored the feasibility of retrieving anonymised resident-level data from large centralised databases (e.g. NHS Digital in England and the various relevant agencies in NI). Our aim was to evaluate if nationally collected data would provide us with robust readily available data in a future trial, thereby lessening the burden of data collection for care home staff.
English data
We planned to make contact with representatives from NHS Digital to discuss the possibility of obtaining centralised HES data for the residents of our study care homes in England (there is a different system in the devolved nations). With the correct permissions, we planned to use the address and postcode of the care home to request anonymised data downloads covering the period of the study.
Northern Irish data
In NI, all health services data (with the exception of primary care data) are held centrally in different databases within the Honest Broker Service, which is under the auspices of the Health and Social Care Business Services Organisation (BSO). 54 All of these databases contain the Health and Care Number, which is the unique identifier within the Health and Social Care services; the Health and Care Number is the primary way of extracting patient-level information from these systems. Once the Health and Care Number for each resident in a care home is known, then all the data in the Honest Broker Service relating to that resident can be ascertained for any period of study. The critical step is therefore to determine the Health and Care Number of each resident in the three care homes during the study period. However, this first required a means of determining who, among the almost 2 million patients on the registration system, were residents in the care homes. This is recognised to be a difficult task. 55,56
The patient registration system for NI (the equivalent of the NHS Central Register) is also held in the BSO. It contains all the usual demographic data relating to patients but does not hold a valid identifier for care home residence. The identification of residents in the study care homes was therefore based on matching the addresses of the three participating NI care homes in the patient registration system with those provided by the organisation responsible for official registration of care homes in NI (Regulation and Quality Improvement Authority). The addresses in the registration system are derived from GP computer systems and it is recognised that these are updated reasonably quickly for patients who enter a care home. 57 However, matching addresses is notoriously difficult as they are often misspelt, abridged or partially incorrect.
The ideal solution and the initial plan was to use the Unique Property Reference Number (UPRN) that is associated with each property in NI and to use this to link the address information in the study and registration data sets. However, this was shown to be impractical as (1) the coverage for addresses in the registration system was much lower than 100% and (2) unexpectedly, analysis demonstrated that one UPRN had been officially assigned to two separate care homes, only one of which was in the study. Therefore, the final algorithm that was used to identify the residents in the three study care homes was based on a combination of care home name, address information and UPRN. This took some time to develop and to validate using systematic clerical checks. Because of the time required to complete this process, the BSO was unable to provide any health services data (e.g. hospitalisations).
Measurement of the resource use and costs associated with the intervention
The research fellows on the study recorded data on the relevant resources and costs involved in the set-up, training and implementation of the REACH intervention prospectively. The costing of intervention was done from the societal perspective to ensure that all costs to both the health service and care homes were fully captured.
In keeping with other studies,58–61 resource use was categorised according to the stage at which it was incurred in the research process, planning and preparation for delivery (stage 1) and delivery itself (stage 2) and included labour, training, intervention materials, equipment and space. Pre-start-up costs (stage 0) associated with the development of the decision-making algorithm for REACH were not be included in the analysis (e.g. literature search, team discussions, designing of materials) as these were too laborious to record and are non-recurring costs. The most recently published unit costs in health and social care were used for costing the time input of staff in the analysis: Unit Costs of Health and Social Care 201762 and the National Minimum Dataset for Social Care for 201763 (Table 8). The trainer in the study was a postdoctoral research fellow employed by Queen’s University Belfast. However, we envisage that if the REACH intervention was rolled out into everyday practice, this role would be undertaken by a health protection nurse (Band 6).
| Staff member | Unit cost (£) | Details (Unit Costs of Health and Social Care 201762) |
|---|---|---|
| Trainer | 44.00 | Cost per hour for a Band 6 nurse (p. 159) |
| Home care manager | 39.00 | Cost per hour (p. 179) |
| Registered nurse | 33.00 | Cost per hour based on an average annual salary of £27,900 taken from the NMDS-SC63 and applying the same assumptions as a home care manager for the calculation of salary oncosts and overheads as provided in the Unit Costs of Health and Social Care 201762 (p. 179) |
| Senior carer | 19.00 | Cost per hour based on an average annual salary of £16,500 taken from the NMDS-SC63 and applying the same assumptions as a home care worker for the calculation of salary oncosts and overheads as provided in the Unit Costs of Health and Social Care 201762 (p. 178) |
| Junior carer | 18.00 | Cost per hour (p. 178) |
Data analysis
Data collected on the delivery of the training
All data were analysed descriptively to provide an overview of the number of staff who attended training sessions, the number of training sessions, who attended and the duration of sessions.
Collection of data from a variety of sources to assess aspects of feasibility
Data collected from the care homes
Statistical analysis was primarily descriptive. This included:
-
baseline characteristics of each home including demographic information of residents
-
use of the decision-making algorithm including type of suspected infection, whether or not the decision-making algorithm was used, action taken by care home/GP and outcome (recovered/died)
-
details of the hospital services used and the outcome (died/returned to home)
-
contacts with health and social care professionals including reason and type of contact
-
adverse event information including hospital admission/discharge and death.
It became apparent that the provision of adverse event information using the adverse event form was problematic in that it was impossible to judge if any reported event could be attributed to the intervention. This was also compounded by the non-randomised nature of the study. Therefore, the use of the adverse event form was discontinued and hospitalisation/death information was recorded on the using the decision-making algorithm form.
Categorical data are presented as number (%), normally distributed data are presented as mean (standard deviation) and ordinal or skewed data are presented as median (interquartile range).
Data collected from community pharmacies
Data were collected on antimicrobial prescribing extracted from community pharmacy computerised records at baseline (relating to 6 or 12 months before the start of the study), and at the end of the implementation phase. This allowed us to estimate the ICC from this non-randomised feasibility study.
We calculated the number of prescriptions dispensed for each individual antimicrobial and the total number of antimicrobials prescribed pre and post implementation. The antimicrobial data were then converted to DDDs (for each item and an overall total) with reference to the World Health Organization data source. 64 All topical products were removed as they do not have assigned DDD values. This analysis was also conducted at the level of each participating home. A further analysis was conducted following removal of all antimicrobials that were not antibiotics (e.g. antifungal agents such as clotrimazole) as antibiotics represent the greatest proportion of antimicrobials prescribed. We also removed any unusual products that were for very specific indications and would be outside the scope of the intervention. These analyses were undertaken to evaluate the total DDDs and the total number of antibiotic prescriptions dispensed per home, the number of residents prescribed antibiotics pre and post implementation of the intervention and the number of prescriptions for antibiotics issued per resident (again pre and post implementation).
We also attempted to assess the appropriateness of antimicrobial prescribing from the dispensing data obtained. Because we did not have access to individual resident-level data, we had no clinical information regarding the nature of infections being treated. Therefore, we consulted local prescribing guidelines in the two jurisdictions65,66 and compared the medications being prescribed for UTIs (the most prevalent infection in care home residents)4 and RTIs with the most frequently prescribed antimicrobials in the care homes.
We had planned to undertake an interrupted time series analysis using the dispensing information obtained from pharmacies/dispensaries, but owing to the quality of these data (small number of homes), we were unable to proceed with this.
Data collected from administrative sources
England
Our primary aim here was to explore if it was feasible to obtain these data. However, we did plan to examine the quality of the data obtainable and how this compared with the data collected in the homes over the course of the study.
Northern Ireland
For the NI homes only, we had planned to compare the data collected using the use of hospital services form with the hospital admissions data held centrally by the various administrative sources (see Data collected from administrative sources – Northern Irish data). This would be dependent on the correct identification of the homes using their UPRN and the subsequent linkage of Health and Care Numbers at that address with the hospitalisation database. We had also planned to compare the number of deaths recorded via the data-collection forms used in the homes with the number of deaths recorded at the address in the General Register Office Register of Deaths. However, as outlined previously, it had not been possible to obtain hospitalisation data.
The analyses used were primarily descriptive, involving frequencies and cross-tabulations relating to residents in the care homes.
Data collected on the resource use and costs associated with the intervention
Costs were presented as cost to the health service, cost to the care home and the total societal cost. The mean cost per nursing home and the mean cost per resident were calculated based on total costs, the number of care homes and the total number of residents at baseline.
Results
Delivery and evaluation of the training
The REACH team delivered training to 87 staff from the six care homes. They delivered 21 training sessions over 35 hours (Table 9). During the implementation period, 14 members of staff (new staff and those not able to attend the initial training) received follow-up training, which was delivered by the REACH Champion or a member of the study team using the training video and study handbook provided to each home (see Chapter 4, Adaptation and development of training material). More information on the training and its delivery can be found in the process evaluation chapter (see Chapter 6, Training for staff). Table 9 also presents details of the number of sessions delivered in each home, including the care home in which the training took place, the date of the training, the number and type of training session (senior or junior), the time and duration of the training session and the number and type of staff who attended each session.
| Care home | Date | Number of sessions | Type of session | Time of day | Duration of sessions (hours) | Type (number) of staff who attended |
|---|---|---|---|---|---|---|
| D | 7 March 2017 | 3 | Senior | 13.00–15.00 | 4.5 |
Nurse (3) Senior carer (3) |
| Junior | 15.00–17.00 | Junior carer (4) | ||||
| 15 March 2017 | Senior | 14.00–15.30 |
Nurse (1) Junior carer (8) |
|||
| E | 8 March 2017 | 2 | Senior | 9.00–11.00 | 4.0 |
Nurse (5) Junior carer (1) |
| 9 March 2017 | Junior | 9.00–11.00 |
Manager (1) Nurse (3) Senior carer (1) Junior carer (4) |
|||
| F | 6 March 2017 | 3 | Senior | 10.30–12.30 | 5.0 |
Deputy manager (1) Senior carer (2) |
| Junior | 14.30–15.30 |
Junior carer (4) Junior carer (4) |
||||
| 13 March 2017 | Senior | 10.30–12.30 |
Senior carer (1) Junior carer (2) |
|||
| A | 22 March 2017 | 5 | Senior | 10.00–12.00 | 9.5 | Nurse (2) |
| 22 March 2017 | Senior | 13.30–15.30 | Nurse (3) | |||
| 29 March 2017 | Senior | 10.00–12.00 |
Nurse (2) Senior carer (1) |
|||
| 29 March 2017 | Senior | 13.30–15.30 | Nurse (4) | |||
| 29 March 2017 | Junior | 15.30–16.00 | Junior carer (4) | |||
| B | 20 March 2017 | 4 | Senior | 14.00–16.00 | 6.0 |
Nurse (2) Senior carer (2) |
| 20 March 2017 | Junior | 18.00–19.00 | Junior carer (1) | |||
| 27 March 2017 | Senior | 14.00–16.00 |
Nurse (4) Senior carer (1) Junior carer (1) |
|||
| 27 March 2017 | Junior | 18.00–19.00 | Junior carer (1) | |||
| C | 23 March 2017 | 4 | Senior | 10.00–12.00 | 6.0 | Senior carer (3) |
| 23 March 2017 | Junior | 13.30–14.30 | Junior carer (4) | |||
| 30 March 2017 | Senior | 10.00–12.00 | Senior carer (2) | |||
| 30 March 2017 | Junior | 13.30–14.30 | Junior carer (2) | |||
| Total | 21 | 35 |
In some cases, it was necessary to combine separate junior and senior training sessions into a single event as insufficient staff were available to attend their designated session. For example, see the session in care home D that took place on 15 March 2017 (see Table 9).
At the end of each of the training sessions, we asked care staff to complete a brief anonymised training evaluation form. Seventy-four forms were completed: 36 from training sessions in NI and 38 from sessions in England. Thirty forms were completed by nurses, 14 were completed by senior carers, 28 were completed by junior carers and two were completed by managers/deputy managers.
We asked staff to rate how relevant the training session was to their work in a care home: 50 (67%) reported that the training was completely relevant to their work, 16 (22%) reported that it was mostly relevant and eight (11%) reported that it was partly relevant. No staff reported that the training was slightly or not at all relevant to their work.
We asked staff to rate the three main topics covered by the training session – AMR; use of the decision-making algorithm (including scenarios) and the SBAR tool (including scenarios) – from very good to very poor. Responses are shown in Table 10. Overall, staff were very positive about the training.
| Rating | AMR (n) | Decision-making algorithm (n) | SBAR (n) |
|---|---|---|---|
| Very good | 57 | 54 | 55 |
| Good | 17 | 20 | 17 |
| Average | 0 | 0 | 0 |
| Poor | 0 | 0 | 0 |
| Very poor | 0 | 0 | 0 |
| Missing | 0 | 0 | 2 |
To understand if the training was pitched at the right level, we asked staff to rate the content of the training session as either too difficult, difficult, straightforward or easy: 18 (24%) reported that it was easy, 52 (70%) reported that it was straightforward, no staff reported that was difficult and three (4%) reported that it was too difficult. There were missing data from one feedback form.
We asked staff, using an open question, which aspects of the training sessions, if any, they found particularly useful; 58 staff provided free-text responses. The aspects of the training that respondents found useful were instruction on how to use the decision-making algorithm (n = 19) and instruction on how to use the SBAR tool (n = 15), scenarios (n = 10), all aspects of the training (n = 9), video (n = 3) presentations (n = 2), sign and symptoms (n = 2) and handbook (n = 2).
Staff were asked, using an open question, to suggest how the training could be improved; there were only five responses. The suggestions were that the training could cover more symptoms of infection, more time could be spent on the decision-making algorithm and more scenarios could have been used in the training. Finally, we asked staff to score their overall assessment of the training session on a scale of 0–9. The mean score was 8.5 (range 6–9), with little difference between scores from nurses and care staff (senior carers and care assistants).
Evaluation of data-collection feasibility
Care home data
Data were collected on the characteristics of the six participating care homes (Table 11). The number of beds ranged from 32 to 62 at baseline and occupancy ranged from 58% to 100%. Care home A was undergoing refurbishment over the course of the study and one floor within the home was not available, hence the reduced occupancy. Over three-quarters of all residents were female. The age range for male residents was 63–96 years, whereas for females the range was 57–103 years. The majority of all staff in both types of care home were junior carers. Half of care homes belonged to national chains/groups, and only one home reported having a policy relating to AMR.
| Characteristics | NI | England | Range | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Care home | Care home | |||||||
| A | B | C | D | E | F | |||
| Type of home | Nursing | Nursing | Residential | Nursing | Nursing | Residential | – | – |
| Registered to provide care for residents with dementia? | Yes | No | Yes | Yes | Yes | Yes | ||
| Number of beds in home (n) | 62 | 32 | 36 | 56 | 51 | 40 | 32–62 | 277 |
| Reported total occupancy at baseline, n (%) | 36 (58) | 28 (88) | 36 (100) | 42 (75) | 51 (100) | 37 (93) | 26–51 | 230 (83) |
| Males, n (%) | 12 (33) | 8 (36) | 5 (14) | 12 (29) | 13 (25) | 5 (16) | 5–13 | 55 (24) |
| Females, n (%) | 24 (67) | 18 (64) | 31 (86) | 30 (71) | 38 (75) | 31 (84) | 18–38 | 172 (76) |
| Age of residents (years) | ||||||||
| Males (median) | 75.5 | 83 | 80 | 82 | 86 | 82 | – | |
| Males (range) | 65–89 | 73–95 | 71–93 | 63–96 | 71–95 | 74–89 | 63–96 | – |
| Females (median) | 85.5 | 88 | 87 | 92 | 89.5 | 85 | ||
| Females (range) | 67–99 | 70–97 | 75–103 | 68–99 | 57–98 | 66–97 | 57–103 | |
| Total reported staffing at baseline (n) | 67 | 39 | 18 | 53 | 38 | 50 | 265 | |
| Manager/deputy manager | 2 | 1 | 2 | 1 | 1 | 2 | 9 | |
| Nurses | 15 | 8 | 0 | 9 | 10 | 0 | 42 | |
| Senior carer | 2 | 3 | 3 | 3 | 5 | 9 | 25 | |
| Junior carer | 30 | 18 | 7 | 27 | 22 | 39 | 143 | |
| Ancillary staff/others | 18 | 9 | 6 | 13 | 0 | 0 | 46 | |
| Home is part of a chain or national group? | Yes | No | Yes | No | No | Yes | ||
| Home has a policy relating to AMR? | No | No | Yes | No | No | No | ||
The decision-making algorithm and its use in the care homes
Five of the six care homes recorded using the decision-making algorithm on a study-specific CRF; the sixth home was unable to complete these forms primarily owing to staffing shortages and a safeguarding issue within the home that prevented them from engaging fully with the study. The decision-making algorithm form was completed 135 times across five care homes during the 6-month implementation phase of the study. On 81 out of 135 completed CRFs (60%), staff reported using the decision-making algorithm (Table 12). We do not have data on frequency of use of the algorithm, or where training might have affected decision-making, when a CRF was not completed. Data in this section are likely to represent only an unknown proportion of actual activity.
| Items completed on the decision-making algorithm form | n (%) |
|---|---|
| Use of decision-making algorithm (N = 135 forms completed) | |
| Yes | 81 (60) |
| Missing (neither box ticked) | 1 (1) |
| No | 53 (39) |
| Type of suspected infection (N = 135) | |
| Urinary tract | 66 (49) |
| Respiratory tract | 38 (28) |
| Skin and soft tissue | 11 (8) |
| Both urinary and respiratory tract | 4 (3) |
| Do not know | 10 (7) |
| Other specified | 6 (4) |
| Biliary sepsis | 1 |
| Broad spectrum between mouth and urine infection | 1 |
| Eye infection | 1 |
| Swollen gum inspection | 1 |
| Vaginal thrush | 1 |
| Resident blood test indicates an infection, source not known, GP prescribed antibiotic | 1 |
Urinary tract infections were the commonest suspected infection in residents (49%), with RTIs ranked second (28%). The most frequently reported reasons for not using the tool were that other tests were carried out (14/53) or the resident was too unwell (13/53). Of note, staff reported that in five cases (5/53) relatives either intervened or became involved in the decision-making process.
Table 13 shows that from the 135 forms completed by staff, 274 actions were initiated. The most commonly reported action was to contact the GP (39%), followed by continuing to monitor the resident or provide supportive care (33%). Conducting additional tests was the most reported ‘other’ action (21/60); of note, junior staff reported their findings to senior staff on seven occasions (7/60). There were 186 actions taken by the GP when contacted. In 24% of these cases, the GP visited the resident. In 52% of such cases, GPs prescribed an antibiotic. Of the 25 ‘other actions’ taken by the GP, eight related to residents being sent to the emergency department. There were 101 records reporting on outcomes at 2 weeks post suspected infection. In most cases, the resident had recovered (59%) and 15% were admitted to hospital. Of the ‘other’ outcomes at 2 weeks, five out of 20 residents were missing data, four out of 20 residents were still in hospital and four out of 20 residents were still on antibiotics.
| Records | n (%) |
|---|---|
| Actions taken by care home staff (N = 274) | |
| Continued to monitor/provide supportive care | 89 (32.5) |
| Contacted the GP | 106 (38.7) |
| Over-ruled the decision aid | 18 (6.6) |
| No action taken | 1 (0.4) |
| Other | 60 (21.9) |
| Actions taken by GP (N = 186) | |
| The GP visited the resident at the care home | 44 (23.7) |
| The GP prescribed antibiotics | 97 (52.2) |
| The GP advised to continue to monitor and/or provide supportive carea | 20 (10.8) |
| Other | 25 (13.4) |
| Outcome 2 weeks after suspected infection (N = 101) | |
| Resident recovered | 60 (59.4) |
| Resident was prescribed further antibiotics by the GP | 5 (5) |
| Resident admitted to hospital | 15 (14.8) |
| Resident died | 1 (1) |
| Other | 20 (19.8) |
Figure 8 displays the pathway from the decision-making algorithm being used, GP contacted, antibiotics prescribed to the outcome after 2 weeks. The figure illustrates the decision-making process as it was recorded by the staff. At each step, there is a ‘yes/no’ pathway until an outcome is achieved. If the extreme left-hand pathway is followed from when the decision-making algorithm was used, an infection was suspected and the GP was contacted (64 times), an antimicrobial was prescribed in most cases (57 times). If a pathway was followed in which the decision-making algorithm was used, and the GP was not contacted (17 times), an antimicrobial was less likely to have been prescribed (prescribed twice).
FIGURE 8.
Flow chart of the use of the decision-making algorithm to an outcome after 2 weeks.
The effect of the decision-making algorithm is borne out further by the data presented in Table 14. From the 81 cases in which the algorithm was used, 27% (n = 22) did not receive an antimicrobial, compared with 73% (n = 59) who did (see Table 14). Even with these small numbers, the trend was in the expected direction [i.e. when the decision-making algorithm was used, there were fewer cases of antimicrobial prescribing (73%) than when the algorithm was not used (76%)].
| Use of tool? | Total | ||
|---|---|---|---|
| No | Yes | ||
| Antimicrobial prescribed? | |||
| No | |||
| Cases (n) | 13 | 22 | 35 |
| % of use of tool | 24.5 | 27.2 | 26.1 |
| Yes | |||
| Cases (n) | 40 | 59 | 99 |
| % of use of tool | 75.5 | 72.8 | 73.9 |
| Total | |||
| Cases (n) | 53 | 81 | 134 |
| % of use of tool | 100.0 | 100.0 | 100.0 |
Use of health and social services data
The use of health and social care services by residents over the 6-month implementation phase was collected using two forms for completion by care home staff: the use of hospital services form and the contacts with health and social care professionals form. The function of this exercise was to inform the design of a full cost-effectiveness analysis in a future definitive trial. We wanted to establish if the forms were fit for purpose (i.e. did they collect the information that we needed?) and if they were easily completed by care home staff or if they were burdensome. We also wanted to establish the type of resources used most frequently by residents so that future versions of the data-collection forms could be refined. Comments from staff on data collection are provided in Chapter 6.
Hospital services
In total, 127 hospital events involving the use of 151 services were recorded on the use of hospital services form from five out of the six homes. No data were collected from the English residential home (F) as they were reported to be too difficult/inaccurate to collect retrospectively; in addition, this home had faced a number of difficulties during the study. In only 22 of the hospital events an infection was suspected, and half of these were RTIs (Table 15).
| Suspected infection | Frequency |
|---|---|
| RTI | 11 |
| UTI | 5 |
| Sepsis | 2 |
| Unspecified | 4 |
| Total | 22 |
The majority of hospital services were outpatient appointments (84/151); however, none of these or the day procedures were due to a suspected infection (Table 16). In total, 34 hospital admissions (three following an outpatient appointment, 21 from accident and emergency visits and 10 direct admissions) were reported and only one of these was a planned admission. Most of the accident and emergency admissions were associated with an infection (18/21). The outcome from the hospital admission was recorded in 33 out of 34 cases, with the majority (25/33) returning to the care home. The mean length of stay associated with a hospital admission was 6.8 days; for those admissions in which an infection was suspected, it was longer (7.9 days). One resident who was admitted to hospital with a suspected infection (sepsis) later died in hospital.
| Hospital service used | Number of residents (n = 151) | Cases in which infection was suspected (n = 22) |
|---|---|---|
| Outpatient appointment | 81 | 0 |
| Outpatient appointment leading to admission | 3 | 0 |
| Day procedures | 5 | 0 |
| Accident and emergency visit | 7 | 1 |
| Accident and emergency visit leading to admission | 21 | 18 |
| Direct admissionsa | 10 | 3 |
| Outcome from all admissions (n = 34) | ||
| Returned to care | 25 | 16 |
| Moved elsewhere | 3 | 3 |
| Died | 3 | 1 |
| Otherb | 2 | 1 |
| Missing | 1 | 0 |
| Mean length of stay (days) (95% CI) | 6.8 (4.8 to 8.7) | 7.9 (5.4 to 10.3) |
Contacts with health and social care professionals
In total, 1540 contacts with health-care professionals were recorded on the contacts with health and social care professionals form from all six care homes. In only 145 cases, this was due to a suspected infection (Table 17). A total of 2220 residents were involved in these contacts. Nurses were the most frequently contacted health-care professional, with 584 contacts recorded, of which 544 involved a visit to the care home. However, in only seven of these contacts was an infection implicated.
| Resource used | Number of contacts | Cases in which infection was suspected | Number of out-of-hours contacts | Total number of residents involved | Mean number of residents involved per contact (range) |
|---|---|---|---|---|---|
| GP telephone call | 257 | 68 | 24 | 261 | 1.0 (1–3) |
| GP visit at homea | 210 | 65 | 22 | 601 | 2.9 (1–29) |
| GP clinic visit | 9 | 0 | 0 | 9 | 1.0 (1–1) |
| Nurse telephone call | 37 | 3 | 0 | 39 | 1.1 (1–2) |
| Nurse visit at homeb | 544 | 4 | 5 | 599 | 1.1 (1–5) |
| Nurse clinic visit | 5 | 0 | 0 | 5 | 1.0 (1–1) |
| Other telephone call | 178 | 2 | 5 | 216 | 1.2 (1–36) |
| Other visit at home | 279 | 3 | 2 | 466 | 1.7 (1–30) |
| Other clinic visit | 21 | 0 | 0 | 24 | 1.2 (1–2) |
| Total | 1540 | 145 | 58 | 2220 |
There were fewer contacts with GPs (n = 476) than with nurses, but GPs tended to see multiple patients per visit, with 210 home visits involving a total of 610 patients, and these visits were more frequently associated with a suspected infection (133/476).
The type of nurse varied considerably (Table 18), with the vast majority of nurse contacts made by district nurses. There were also nurse contacts recorded that were associated with NHS continuing health-care assessments; this is an organised visit related to the provision of resident health care and not connected to a specific health event.
| Nurse type | Number of contacts |
|---|---|
| District | 469 |
| Continuing health-care assessment | 29 |
| Tissue viability | 25 |
| Diabetic | 13 |
| Mental health | 13 |
| Practice | 11 |
| Palliative | 8 |
| Not specified | 5 |
| Incontinence | 3 |
| Respiratory | 3 |
| Clinical nurse facilitator | 2 |
| Care home nursing team | 2 |
| Test results | 2 |
| Renal | 1 |
| Total | 584 |
There were 478 contacts recorded with other health-care professionals (Table 19), but an infection was suspected in just five of these contacts. Again, some contacts could be regarded as inappropriately recorded (e.g. telephone calls to obtain test results or medication and contacts with hospital and ambulance services that should have been recorded on the hospital services use form).
| Other health-care professional | Total number |
|---|---|
| Social worker | 173 |
| Speech and language therapist | 75 |
| Chiropodist/podiatrist | 57 |
| Dietitian | 56 |
| Physiotherapist | 30 |
| Psychiatrist/psychologist | 26 |
| Optician | 23 |
| Pharmacist | 10 |
| Ambulance | 9 |
| Audiologist | 7 |
| Test results/medication orders | 5 |
| Care home on-call manager | 4 |
| Intermediate care team | 1 |
| Hospital | 1 |
| Unspecified | 1 |
| Total | 478 |
Community pharmacy dispensing data
Based on the data supplied by the community pharmacies, the number of antimicrobial items dispensed for residents in the participating care homes was summarised, together with a calculation of the total number of DDDs of antimicrobials dispensed, as shown in Table 20. The most frequently prescribed antimicrobials were nitrofurantoin (n = 84 items pre implementation; n = 82 items post implementation), trimethoprim (n = 71 items pre implementation; n = 46 items post implementation) and amoxicillin (n = 59 items pre implementation; n = 56 items post implementation). Comparing the antimicrobial prescribing against the NI Management of Infection Guidelines for Primary and Secondary Care (2016)65 and the Community Antibiotic Guidelines for Common Infections in Adults (2017) produced by the Coventry and Warwickshire Area Prescribing Committee66 suggests that the prescribing patterns were broadly in line with recommendations for UTIs and RTIs.
| Antimicrobial agent | Total number of prescriptions | Total number of DDDs | ||||
|---|---|---|---|---|---|---|
| Before intervention (6 monthsa) | After intervention | Change (%) | Before intervention (6 monthsa) | After intervention | Change (%) | |
| Nitrofurantoin | 84 | 82 | –2 (2) | 544 | 531 | –13 (2) |
| Trimethoprim | 71 | 46 | –25 (35) | 401 | 244 | –157 (39) |
| Amoxicillin | 59 | 56 | –3 (5) | 501 | 518 | +17 (3) |
| Doxycycline | 53 | 45 | –8 (15) | 612 | 445 | –167 (27) |
| Flucloxacillin | 35 | 23 | –12 (34) | 223 | 142 | –81 (36) |
| Cefalexin | 20 | 13 | –7 (35) | 76 | 53 | –23 (30) |
| Co-amoxiclav | 18 | 13 | –5 (28) | 149 | 95 | –54 (36) |
| Clarithromycin | 15 | 25 | +10 (67) | 161 | 328 | +167 (104) |
| Ciprofloxacin | 8 | 8 | 0 (0) | 35 | 40 | +5 (14) |
| Erythromycin (includes ethyl succinate) | 4 | 4 | 0 (0) | 31 | 33 | +2 (6) |
| Pivmecillinam | 11 | 12 | +1 (9) | 41 | 48 | +7 (17) |
| Others | 5 | 7 | –2 (40) | 74 | 82 | +8 (11) |
| Total | 383 | 334 | –49 (13) | 2848 | 2559 | –289 (10) |
The total number of prescriptions dispensed in the 6 months prior to the intervention was 383, whereas 334 prescriptions were dispensed post intervention, equating to a reduction of 49 prescriptions (13% reduction). In terms of DDDs, the pre-implementation total was 2848, whereas the post-implementation total was 2559, a reduction of 289 DDDs (10% reduction). As highlighted in Table 20, there was a reduction in the prescribing of trimethoprim, flucloxacillin and cephalexin. The prescribing of nitrofurantoin remained relatively unchanged, whereas clarithromycin prescribing increased post implementation. These changes were somewhat reflected in the DDDs prescribed for each drug. From the data, we could see that approximately 30 nitrofurantoin prescriptions pre intervention had been written for a quantity of 30 tablets; in the case of trimethoprim, there were prescriptions written for 30 × 100-mg tablets. These prescribing courses may suggest prophylaxis of UTIs rather than treatment.
Table 21 summarises data at the care home level. In three homes, there was a reduction in both the number of antimicrobial items and the number of DDDs. In two care homes, there was a slight increase in the number of items (homes A and E). The home designated F demonstrated a larger increase (an increase of 20 prescriptions post intervention), but this home had experienced a number of difficulties over the course of the study and we have concerns about the completeness of the data.
| Home | Total number of prescriptions | Total number of DDDs | ||||
|---|---|---|---|---|---|---|
| Before intervention (6 monthsa) | After intervention | Change (%) | Before intervention (6 monthsa) | After intervention | Change (%) | |
| A | 77 | 78 | +2 (2) | 548 | 595 | +48 (9) |
| B | 69 | 49 | –20 (28) | 691 | 479 | –212 (31) |
| C | 68 | 44 | –24 (35) | 433 | 276 | –156 (36) |
| D | 68 | 34 | –34 (50) | 464 | 280 | –184 (40) |
| E | 82 | 89 | +7 (9) | 572 | 640 | +68 (12) |
| F | 21 | 40 | +20 (95) | 142 | 289 | +147 (103) |
| Total | 383 | 334 | –49 (13) | 2848 | 2559 | –289 (10) |
| Mean | 63.8 (SD 22.0) | 55.7 (SD 22.4) | –8.2 (95% CI –29.7 to 13.4) | 474.7 (SD 186.6) | 426.5 (SD 167.1) | –48.2 (95% CI –209.2 to 112.8) |
Following removal of all non-antibiotic products [in addition to unusual drugs such as rifaximin (Targaxan®)], analysis at the resident level, as shown in Table 22, reveals the number of residents who were prescribed antimicrobials 12 months prior to the intervention being implemented and 6 months post implementation.
| Home | Number of residents prescribed antimicrobials (average occupancy) | |
|---|---|---|
| 12 months pre implementation | 6 months post implementation | |
| A | 52 (45)a | 30 (35) |
| B | 32 (32) | 21 (32) |
| C | 33 (36) | 16 (36) |
| D | 40 (53.5) | 19 (53.5) |
| E | 45 (51) | 19 (51) |
| F | 21 (37) | 24 (37) |
| Total | 223 (254.5) | 125 (244.5) |
Analysis at the resident level, as shown in Table 23 [again, with the removal of non-antibiotic products and rifaximin (Targaxan)] illustrates the number of prescriptions issued per resident pre and post implementation. We were unable to collect data pertaining to the number of residents pre and post implementation who had not been prescribed any antimicrobial.
| Number of antimicrobial prescriptions | Residents in receipt of an antimicrobial prescription, n (%) | |
|---|---|---|
| Pre implementation to 12 months | Post implementation to 6 months | |
| 1 | 81 (36) | 52 (42) |
| 2 | 47 (21) | 33 (26) |
| 3 | 20 (9) | 12 (10) |
| 4 | 21 (9) | 11 (9) |
| 5 | 14 (6) | 3 (2) |
| 6 | 6 (3) | 3 (2) |
| 7 | 8 (4) | 4 (3) |
| 8 | 5 (2) | 0 (0) |
| 9 | 4 (2) | 2 (2) |
| ≥ 10 | 17 (8) | 5 (4) |
| Total | 223 (100) | 125 (100) |
Calculation of the ICC revealed that it was 0.11 [95% confidence interval (CI) 0.00 to 0.24] at baseline, 0.05 (95% CI 0.00 to 0.13) post implementation and 0.09 (95% CI 0.00 to 0.24) overall.
Administrative data
English data
In England, we approached NHS Digital to discuss if we could obtain anonymised HES data for the residents in the three care homes in England. At the outset of this project, NHS Digital was going through considerable organisational changes and this initially proved to be a barrier to engagement. Our enquiries focused on identifying an approach to obtain HES data on all of the residents in the three English care homes covering the period of the study. As our study did not have resident-level consent, we had hoped that the NHS database could be searched against the addresses and postcodes of the care homes. This would give three sets of data relating to each of the homes. However, NHS Digital informed us that a search based on a postcode and address was not possible on their system. Our discussions have been ongoing and it now seems that we may be able to obtain pooled anonymous data through using the residents’ NHS Number. As noted, we did not have ethics approval for individual consent so we would have to seek permissions from the Confidentiality Advisory Group. Resident NHS Numbers can be found in two places in the study: the first is within the records maintained by the care homes and the second is at the pharmacy/dispensary that supplies the home with its medicines. We would require someone (e.g. care home manager) who has the appropriate permissions to access the residents’ NHS Numbers, collate them and send these numbers to NHS Digital, who would then supply the anonymised data for that particular care home.
Northern Irish data
The algorithms developed within the patient registration system by the Health and Social Care BSO were used to identify the number of residents in each of the three care homes in NI at the start of the study. These are presented in Table 24 along with the number of residents ascertained by data collection undertaken by the research fellows and staff within the homes.
| Care home | Number of residents | |
|---|---|---|
| Data collected during study | Data collected from administrative dataa | |
| A | 36 | 35 |
| B | 28b | 24 |
| C | 36 | 34 |
As can be seen, there is a reasonably close correlation between the numbers of residents with data derived from the administrative data systems for two care homes and those with data derived from data collection over the course of the study. It appears, however, that the algorithm tended to under-record residents, perhaps because it erred on the side of caution/selectivity rather than sensitivity. According to the data collected directly during the study, there was one additional resident recorded in care home A, four additional residents in care home B and two additional residents in care home C, compared with data collected from administrative sources; it should be noted, however, that care home B included two respite residents who would not have been detected by the administrative data system, which registers only permanent residents as temporary addresses are not recorded. Some further discrepancies would be expected as a result of slight differences in the timing of the dates of the data collection during the study and extraction from the registration systems. The patient registration system, which depends on changes of address at the GP surgery, will always lag behind ‘real-time data’.
Table 25 shows some of the additional information garnered from the administrative data systems. This includes a tally of all residents entering the three care homes in NI during the 6 months of implementation, producing the total number of residents for the study period; the total number of deaths (all causes) of residents (n = 15) in these homes was extracted, again during the same period.
| Care home | Total number of residents (1 May to 31 October 2017) | Number of deaths (1 May to 31 October 2017) (all causes) |
|---|---|---|
| A | 39 | 6 |
| B | 33 | 7 |
| C | 42 | 2 |
Resource use and costs associated with the intervention
A breakdown of the resources and costs associated with the REACH intervention is presented in Table 26. The costs of flash drives and the online platform were not included as these were provided free of charge by Queen’s University Belfast. This would have little impact on the cost per resident as flash drives are low-cost items and, if the intervention were rolled out in practice, the training material could be uploaded to online training platforms offered by many care home providers. The mean cost to the care home was £699 (£18 per resident), all of which was associated with staff attendance at training. The mean cost to the health service was £570 (£18 per resident), with costs being associated with intervention materials, equipment and trainer input. From a societal perspective, the mean cost per care home was £1269 (£33 per resident).
| Resource use | Unit cost (£) | Number of units | Cost to health service (£) | Cost to care homes (£) | Cost details |
|---|---|---|---|---|---|
| Planning and preparation for delivery (stage 1) | |||||
| Intervention materials/equipment | |||||
| Training booklets | n/a | 330 | 990 | Cost for 330 booklets | |
| Other intervention materials | n/a | n/a | 130 | Cost for nine A4, nine A3 posters and 300 A4 laminated copies of the decision-making algorithm | |
| Video | 60 | 1 | 60 | ||
| DVD | |||||
| Recording training sessions and voiceover | 45 | 1 | 45 | ||
| Editing voiceover to graphics, rendering out and creating DVD covers | 135 | 1 | 135 | ||
| Recording video | 5 | 6 | 30 | One DVD provided per care home | |
| Intervention training | |||||
| Trainer | 44 | 35 | 1540 | Based on 35 hours of formal face-to-face training sessions | |
| Trainer travel | 0.56 | 609 | 341 | Based on an average round trip of 29 miles, 21 training sessions and 56 pence per mile | |
| Home care manager | 39 | 4 | 260 | Based on the number of each staff type attending face-to-face formal training (initial and follow-up). Sessions lasted, on average, 1 hour and 40 minutes based on 21 sessions lasting a total of 35 hours | |
| Registered nurse | 33 | 35 | 1925 | ||
| Senior carer | 19 | 17 | 538 | ||
| Junior carer | 18 | 45 | 1350 | ||
| Thermometer training | |||||
| Home care manager | 39 | 1 | 10 | Based on 15 minutes of additional training provided to only residential home staff at initial training | |
| Senior carer | 19 | 8 | 38 | ||
| Junior carer | 18 | 16 | 72 | ||
| Implementation (stage 2) | |||||
| Thermometers | 2 | 35 | 70 | Thermometers provided to the residential homes only | |
| Thermometer consumables | 1 | 81 | 81 | Includes 2000 thermometer tip covers | |
| Total | 3422 | 4193 | |||
| Mean cost per home | 570 | 699 | Based on six nursing homes | ||
| Mean cost per resident | 15 | 18 | Based on 230 residents in six homes at baseline | ||
| Totals | |||||
| Total societal cost | 7615 | ||||
| Mean societal cost per home | 1269 | Based on six nursing homes | |||
| Mean societal cost per resident | 33 | Based on 230 residents in six homes at baseline | |||
Summary
The intervention (training and use of the decision-making algorithm) was implemented in the six participating care homes. Data were collected from a number of sources. Analysis of the data provided insight into the use of the decision-making algorithm, use of hospital services and contact with other health-care professionals. Dispensing data were summarised to provide the number of antimicrobial items dispensed over different time periods and converted to DDDs, and then used in subsequent analysis. Very few administrative data were available owing to logistical difficulties. Resource use and costs associated with the intervention were calculated.
Chapter 6 Process evaluation
Introduction
In this chapter, we present the process evaluation. Our process evaluation was adapted from and based around a Medical Research Council framework67 for such evaluations and was inclusive of a number of key components for process evaluations proposed by Steckler and Linnan. 68 The components chosen for this study reflect that this was a feasibility study and that our main interest was in evaluating the feasibility of a number of aspects of the study, for example the ability to recruit, deliver (implement) and evaluate an intervention in care homes in NI and England.
As this was a feasibility study, it was not appropriate for us to consider fidelity as a component of this process evaluation. Should a larger trial be conducted in the future, a full evaluation of the fidelity of the intervention would be undertaken.
As outlined in Chapter 2, normalization process theory was the underpinning theory within this study. Normalization process theory is a middle-range theory that can underpin process evaluations of complex interventions in health care. 22 Middle-range theory is an approach to sociological theorising aimed at integrating theory and empirical research. 69 We have used normalization process theory to evaluate the development and implementation of the intervention. Normalization process theory is not a methodology or how-to-do guide to research and it is not prescriptive or rigid; rather, it is a theoretical device that is meant to be used in a flexible and active way, allowing us to both direct and explain the everyday and critical course of the project. 22,70,71 In the description of the methods, we outline how normalization process theory was used within this process evaluation.
Aims and objectives
The aims of the process evaluation were:
-
to comprehensively describe the implementation of this intervention, including the facilitators of and barriers to implementation
-
to develop a set of recommendations regarding the intervention to inform its implementation on a wider scale.
The objectives were:
-
to monitor implementation processes (e.g. recruitment, development of the intervention, delivery of the intervention and acceptability/use of the intervention in practice)
-
to undertake ethnographic observations in the care homes to understand current practice and to explore possible changes due to the intervention
-
to carry out in-depth interviews/focus groups with a sample of care home staff, care managers and other stakeholders (e.g. GPs).
Methods
We used a mixed-methods approach, combining qualitative and quantitative data72,73 to facilitate exploration of apparent discrepancies between findings. 74,75 The principal data-collection method was qualitative (e.g. interviews, focus groups and observational field notes), complemented and illuminated by the quantitative data (e.g. recruitment to focus groups, training sessions provided and attendances at sessions), providing an in-depth and breadth of understanding of the study processes and the implementation of the intervention into practice. We used normalization process theory (see Chapter 2, Normalization process theory) as the theoretical underpinning to our methodological and analytical approach in this study. As this was a feasibility study, all six care homes were included in the process evaluation. We have outlined the components of process evaluation we explored in the study (Table 27).
| Component | Definition |
|---|---|
| Context | Aspects of the larger social, political and economic environment that may influence implementation |
| Reach | The proportion of the intended target audience that participates in the intervention |
| Dose delivered | The number or amount of intended units of each intervention or each component delivered or provided |
| Dose received | The extent to which participants actively engage with and interact with the recommended resources |
In the following sections, we outline the procedures used to gather process evaluation data.
Pre-/post-implementation interviews, focus groups and questionnaires
With the co-operation of our study care home managers, staff who may have been involved in decision-making relating to a resident’s well-being were invited to take part in a qualitative study. This generally involved nurses and senior care staff in nursing homes and senior and junior carers in residential homes. In order to maximise input from the staff, we arranged focus groups. The first of these was during the adaptation phase of the study and the second was post implementation.
Pre-implementation focus groups and interviews
The first set of focus groups (during the adaptation phase, as reported in Chapter 4) had two purposes: (1) staff and family members of residents were given the opportunity to be involved in the adaptation of the decision-making algorithm (the intervention) and (2) to explore normal practice within the homes in relation to the actions of staff when they suspected that a resident had an infection. GPs were interviewed to explore their usual practice in respect of the management of infections in care home residents and their opinion of the decision-making algorithm.
Interview/focus group discussion guides were developed based on the four key constructs from normalization process theory (our theoretical underpinning21) (see Appendix 2 for care home staff, Appendix 3 for family members and Appendix 4 for GPs).
Post-implementation focus groups and interviews
Following implementation, we conducted focus groups with care home staff and interviews with REACH Champions and home managers to explore their experience of the training for the study, implementing the intervention, completing the study paperwork and facilitators of and barriers to implementing a larger study; in addition, we asked care home managers why they had decided to take part in the study. We also sent a brief e-mail questionnaire to the eight GPs who took part in the pre-implementation interviews to explore their experience of staff using the decision-making tool over the duration of the implementation phase. Interviews and focus groups were conducted within the care homes at a time convenient to all concerned, and they were facilitated by the study research fellows. All participants provided written informed consent and each was given an honorarium for taking part in the study, as outlined in the relevant participant information sheets.
Interviews and focus groups were recorded and transcribed verbatim.
Observations
The key requirement for the process evaluation during the implementation phase was to undertake ethnographic-type observations in the care homes to understand current practice and to explore possible changes due to the intervention. We defined ethnographic-type observations as collecting data through informal conversations with staff and observations of activities related to the implementation of the intervention and study-related processes throughout the 6-month implementation period.
The research fellows (AC and RP) telephoned and visited the care homes regularly over the 6-month period to encourage and support staff to implement the intervention and to collect data from the homes, and they used an ethnographic approach to understand current practice and explore possible changes due to the intervention.
At subsequent visits, the research fellow met with the REACH Champion (or another member of staff if the REACH Champion was not available) to collect the use of decision-making algorithm forms, check completion and, where possible, discuss each case for which the form was completed. The research fellows also asked about any ‘missed opportunities’ when a resident presented with a possible infection and a form had not been completed. In addition, the research fellows spent time in the care homes to observe handover times or other staff meetings when decisions around management of infections and contact with the GP may have been discussed. Field notes were made at each visit, recording observations, conversations and activities related to the implementation of the decision-making algorithm and study-related processes.
The visits were arranged at the convenience of the home and included a range of times (e.g. at handover, in mornings or in afternoons). We produced a document to aid these observations, highlighting activities to identify and note. Thus, items potentially recorded in field notes included:
-
date and time
-
conversations with staff about using the decision-making algorithm
-
conversations with staff about REACH paperwork
-
general interactions with staff/residents/relatives
-
observation of the use of the algorithm in practice
-
conversations with health-care professionals (e.g. GPs) about using the decision-making algorithm or antimicrobial prescribing issues.
Field notes were transcribed and stored on a password-protected computer and uploaded into NVivo® for data management and analysis, which is detailed in Qualitative data analysis.
Routine data collection
Table 28 outlines the data sources used for routine data collection. Although the qualitative aspects of the process evaluation dominated the data that were collected, Table 28 highlights that many of the key components were measured from quantitative records.
| Component | Data source | Data description |
|---|---|---|
| Context | Home baseline data-collection form | Demographics of homes and residents |
| Reach (care staff) |
|
|
| Dose delivered | Record of formal initial and follow-up training sessions |
|
| Dose received |
|
|
Records were drawn together and summarised for analyses.
Data analysis
Qualitative data analysis
All interviews and focus group recordings were digitally recorded and transcribed verbatim by an external organisation. The transcripts were checked against the recording by the research fellow in each area to ensure accuracy and anonymisation. The transcribed data, along with the field notes from observations throughout implementation, were uploaded into NVivo® for data management and analysis, and were repeatedly read to increase familiarity with the data. Data analysis was based on the constant comparison method. 27 A selection of focus group transcripts were first open coded inductively, with codes created from the patterns and themes emerging from the data, and an initial coding frame was developed (see Appendix 8). This coding frame was then applied to subsequent transcripts and iteratively refined as new codes were defined. Field note data were similarly analysed and a coding frame developed (see Appendix 9). These codes were then deductively mapped to the a priori concepts and components of normalization process theory. We used the framework matrix facility within NVivo® to assist the analytic process. These matrices enabled each research fellow to summarise each piece of text associated with a code. A COREQ checklist was completed for this aspect of the study and can be found in Appendix 10.
Researcher bias was minimised through regular cross-checking of data and findings by the members of the research team. Quotations have been used as exemplars of key points.
Quantitative data analysis
The quantitative data were appropriately summarised and presented as descriptive statistics in tables and charts as appropriate to the data.
Results
Care homes
The following sections contain brief descriptions of the care homes included in the study, which provide a basic context for the settings in this study.
Northern Ireland
Home A was a nursing home registered to provide personal and nursing care for up to 62 elderly persons with a range of conditions or needs, including dementia. The home was purpose built and situated in a residential area. It was close to shops, amenities and public transport and was owned and managed by a national not-for-profit organisation. The home was associated with a large number of general practices (up to 15, although four practices provided care for 80% of residents), which reflected its Belfast location. Most medications were provided by a national pharmacy chain.
Home B was a nursing home registered to provide personal and nursing care for up to 32 elderly persons with a range of conditions or needs. The home was a large converted house built on approximately 1 acre of grounds, was situated on the coast road, with views out to sea, about 1 mile from the town centre. The home was privately owned and managed. Most GP services were provided by two practices and medications were supplied by one local pharmacy.
Home C was a residential non-nursing home registered to provide accommodation for up to 36 elderly persons, including people living with dementia. The home was purpose built and had been specifically designed for the needs of elderly residents. The home was in an urban area with nearby shops and amenities, and was a short distance from the town centre. The home was owned and managed by a national not-for-profit organisation. GP services were provided by a single practice and a national pharmacy chain supplied the home’s medication.
England
Home D was a nursing home registered to provide accommodation, nursing and personal care for up to 56 older people who may have dementia. The home was a converted property situated in the heart of a small Warwickshire village. The home was privately owned and run. The home was supported by a named GP from a single local practice and most medications were provided by the practice dispensary.
Home E was a nursing home registered to provide personal and nursing care for up to 51 older people, including people living with dementia. The home was divided into two separate floors, with older frail residents on the first floor and the dementia unit on the ground floor. A converted property, the home was situated in large grounds/garden surrounded by fields in a rural area, approximately 1 mile from the nearest village. The home was privately owned and managed. The home was supported by a named GP from a single local practice and most medications were provided by the practice dispensary.
Home F was a residential non-nursing home registered to provide accommodation for up to 40 older people with dementia care needs. The purpose-built home was divided into four units, each unit consisting of bedrooms, a lounge, a dining area and a kitchenette. The home was in an urban area approximately 1 mile (1.6 km) from a city centre. The home was managed by a national not-for-profit organisation. The home was supported by a named GP from a single local practice and most medications were provided by a national pharmacy chain.
Training for staff
Dose delivered (delivery of the training)
Training took place in all six care homes participating in the study. A total of 21 training sessions took place, lasting a total of 35 hours (see Table 9 and Chapter 5, Delivery and evaluation of the training). In general, the research fellows noted few problems with arranging training within the homes. However, there were a number of anticipated challenges, such as locating rooms that would enable the training to be delivered without interruption and the lack of audio-visual equipment in the care homes. Generally, suitable spaces were found and we provided the audio-visual equipment.
Dose received (staff trained)
The total number of care staff involved in the initial face-to-face training was 87 (including 22 night staff). The total number of staff involved in follow-up training was 14 (including two night staff). Follow-up training was conducted by either a member of the research team (one session in care home A and one session in care home D) or a REACH Champion using the training DVD (two sessions in care home C). The numbers and grades of staff receiving initial and follow-up training are shown in Table 29. From the baseline data presented in Chapter 5, there was a total of 219 care staff, of whom 101 were trained (46%). As the intervention was targeted at the nurses in nursing homes and senior carers in residential homes, it was important to include these staff in the training sessions, and this was achieved. We trained 35 out of 42 nurses, equating to 83% of all available nurses, and 17 out of 25 senior care staff (68%).
| Grade of care staffa | Number of staff | Baseline care staff numbers | Percentage of care staff trained | ||
|---|---|---|---|---|---|
| Initial formal training (night staff) | Follow-up formal training, new staff trained (night staff) | Total number trained (night staff) | |||
| Nurse | 29 (10) | 6 (2) | 35 (12) | 42 | 83 |
| Senior care assistant | 16 (4) | 1 (0) | 17 (4) | 25 | 68 |
| Junior care assistant/activity co-ordinator | 40 (8) | 5 (0) | 45 (8) | 143 | 31 |
| Manager/deputy manager | 2 (0) | 2 (0) | 4 (0) | 9 | 44 |
| Total | 87 (22) | 14 (2) | 101(24) | 219 | 46 |
Pre-/post-intervention interviews, focus groups and observations
Pre implementation, we conducted semistructured face-to-face focus group interviews with care home staff in six care homes during September to October 2016 and eight interviews with GPs during January to March 2017 (reported in detail in Chapter 4). Post implementation, we conducted semistructured face-to-face focus group interviews with care home staff in the six care homes during October to November 2017. There were six focus groups in total, with one focus group conducted in each care home. We also conducted semistructured face-to-face interviews with REACH Champions and managers of homes during October to December 2017. There were 11 interviews in total, six with REACH Champions and five with managers; one manager had left the care home at the time of the interviews. Of the eight GPs who took part in the pre-implementation interviews, seven were invited to take part in a brief e-mailed questionnaire (one GP had left the practice). None of the GPs responded to the invitation. The focus groups and interviews were carried out by Anne Campbell in NI and by Rachel Potter in England. All participants provided written informed consent. The numbers of participants in each focus group and interview are shown in Table 30.
| Care home | Number of care home staff focus groups (number of participants) | Number of interviews with REACH Champions | Number of interviews with home managers | Number of GP questionnaires |
|---|---|---|---|---|
| A | 1 (n = 5) | 1 | 1 | 0 |
| B | 1 (n = 4) | 1 | 1 | 0 |
| C | 1 (n = 2) | 1 | 1 | 0 |
| D | 1 (n = 6) | 1 | 1 | 0 |
| E | 1 (n = 6) | 1 | 1 | 0 |
| F | 1 (n = 3) | 1 | 0 | 0 |
| Total | 6 (n = 26) | 6 | 5 | 0 |
The focus groups and interviews were conducted in a suitable room in the care homes. The duration of the focus groups with care home staff ranged from 27 to 61 minutes. The duration of the interviews ranged from 26 to 71 minutes with REACH Champions and from 12 to 27 minutes with managers. In the presentation of our findings, all quotations have been given an anonymised label (e.g. A–F represents each participating home) (see Care homes for a description of each home); ‘staff, post implementation’ indicates that the interview or focus group was conducted post implementation. Other participant groups are family members (‘family’) and GPs.
In the following sections (our findings from the focus group, interview and observational field notes), qualitative data are largely presented around the four main components of normalization process theory: (1) making sense (coherence), (2) engagement and commitment (cognitive participation), (3) facilitating the use of the intervention (collective action) and (4) the value of the intervention (reflexive monitoring).
Making sense (coherence)
We report here on how participants understood the problem the intervention aimed to address, how they perceived their use of the intervention to affect this problem and how they understood that what they were being asked to do differed from their usual practice.
Making sense of the problem of antimicrobial resistance and how the intervention addresses it
Before implementing the intervention, we asked care home staff and family member participants in focus groups what they knew about AMR. Although some family members reported not knowing anything about AMR, they recognised terms such as ‘MRSA’ (meticillin-resistant Staphylococcus aureus) and ‘superbugs’, associated these with harm and how they may be acquired through hospital stays or poor hygiene practices. For care home staff and family members with some knowledge of AMR, understanding varied from believing that it was bacteria that became resistant to antibiotics to, conversely, individuals who developed resistance. Participants also discussed who, or what, they felt was to blame for the growing problem of AMR, including too easy access to antibiotics, either by GPs overprescribing or by patients accessing antibiotics through the internet; patient expectations that antibiotics will help recovery from illness; and patients’ non-adherence to advice on treatment duration:
Antimicrobial resistance is a patient developing resistance with the antibiotic, that’s been prescribed. Due to probably not complying with the 7-day course and just stopping or something happened within the 7-day course.
A: staff, pre implementation
Likewise, participants in post-implementation focus groups and interviews expressed varied views of AMR, again attributing resistance to either bacteria or individuals. They also expressed their understanding that antibiotics were becoming less effective and new or stronger ones were needed, that GPs should prescribe the right antibiotic for the right infection and that reducing antibiotic use would reduce AMR in residents. Staff reported how the training associated with the intervention led them to reflect on their own personal use of antibiotics and improved their knowledge of the local and global AMR problem:
What I liked about [the training] was knowing that it is a global problem, that the effects of overprescribing of antibiotics is affecting us without us really realising because we tend to ask the GP for antibiotics for everything that we think that is happening to the resident, (. . .) which will be difficult later on in life because then you won’t have anything to fight the bacteria.
E: Champion, post implementation
Some participants discussed how taking part in the study and using the decision-making algorithm made them focus more on preventative measures before contacting the GP, which, in turn, could influence antibiotic prescribing. However, others thought that use of the algorithm would not influence prescribing for infections and the problem of AMR. Although participants recognised that the algorithm may have a local care home-level impact on prescribing, it would not have a wider impact without a change in attitude in the general public. It was also believed that GPs would continue to prescribe antibiotics for those residents presenting as unwell and who had a past history of infection in order to avoid the risk of rapid deterioration:
These elderly people can become quite toxic within 6 hours. The sooner we can get them treated, then the better. (. . .) I don’t think it will change the prescribing for possible infections.
B: manager, post implementation
Engagement and commitment (cognitive participation)
We present here what participants viewed as necessary for staff to become engaged in implementing the intervention, notably the role of the REACH Champion and the manager of the home in driving the intervention and engaging others and the actions and procedures needed to sustain the new practice. We describe the main facilitators and challenges for each of these.
The role of managers and REACH Champions in driving the implementation
Managers of care homes reported a number of motivations for taking part in the study. These were largely associated with being part of a solution to the problem of AMR and staff gaining empowering knowledge to improve the quality of care for residents:
I always feel anything to empower us, to give us more knowledge and to participate, to improve quality is worth it for us.
E: manager, post implementation
On agreeing to take part in the study, the manager of each care home was asked to appoint a REACH Champion (see Chapter 3, Methods). All six homes appointed at least one Champion (two were appointed in one residential home in NI), all of whom were either nurses or deputy managers/nurses in nursing homes, or senior carers in residential homes. In most care homes, the manager appointed the REACH Champion with their agreement. In some homes, there were challenges in appointing a Champion. In one nursing home in England, the first REACH Champion resigned shortly after the start of the study and the replacement Champion initially appeared reluctant to take on the role. In one small nursing home in NI with many part-time staff, the manager reported difficulties in appointing someone to this role:
The problem is, I have link nurses for lots of different topics and I have run out of people to give responsibility to and [name] didn’t actually want to take it on either, but I mean somebody had to do it.
B: manager, post implementation
In post-implementation interviews, the appointed Champions reported what they liked about the role; this included being part of a solution to an important problem, being more aware about the level of infection within a care home and what the outcomes were, training staff in the intervention, having the increased responsibility of helping staff become engaged in the intervention and doing something different from their routine work:
It’s nice to do something different apart from the routine, then it gives you something else to think about.
E: Champion, post implementation
The Champions and staff also described a range of tactics used to engage staff. These included challenging staff at opportune moments to consider if it was really necessary to contact the GP immediately. Tactics also included discussing the algorithm and corresponding documentation in formal situations, such as handover times, regular staff meetings and in casual break-time conversations:
[REACH Champion] helped us with the meetings with you, or to ask us ‘[name], what you’ve done, do you think of things like that?’. Or just to remind us all the time when we are sitting and talking.
A: staff, post implementation
Another important tactic employed by the Champions were ‘friendly reminders’ to use the algorithm, complete the paperwork and of the researcher’s regular visits to the home:
A friendly reminder [laughs] (. . .) I brought the box out and said, ‘Do you remember about that wee form you have to fill in?’. (. . .) I obviously tell them when you’re coming in and why you’re in. And if they want any information on it or they need any help with it, I’m there to help.
C: Champion, post implementation
One factor that appeared to help Champions engage staff was the support of the manager in giving dedicated time for the role and being interested and engaged with the study by, for example, highlighting the study at staff meetings and with individual staff. Flexibility of the research team in fitting into the schedule of the Champions was also important:
The manager would be supportive here. Anything that needs done, she would put out a reminder. You can talk to her and say to her, ‘these staff aren’t really doing this’. Or we’ve also had staff meetings where she’s brought it up (. . .) I think if she maybe wasn’t, some of the staff would be like, ‘well, you’re just asking me to do more work’. It could be that sort of element to it rather than, ‘well, no actually this is a whole team effort’.
C: Champion, post implementation
Matron is very supportive [and] gave me time to do things and then you’re [researcher] very flexible when I’m unable to [meet].
E: Champion, post implementation
However, the pressures of everyday work in some care homes meant that such support was not always in place. Many of the challenges voiced by Champions and managers across the sites centred on the burden of completing the paperwork associated with the study. Most Champions discussed how they would have liked more engagement from staff in this task, although they recognised that there was a limit to how much more they could demand of already busy staff. Champions expressed their frustration with either the continual request for staff to complete the paperwork or their own, at times, detailed review of handover sheets, home diaries, patient charts or progress notes to obtain the information required:
So, it’s chasing people up, making them fill in the forms and getting to remember who filled it in or who it was because the name of the person wasn’t on it. Sometimes you go ‘who is this filled in for?’. You have to remember who it was written for and what the outcome was.
B: Champion, post implementation
Findings from the observational data also indicated the facilitating function of the REACH Champion, especially one who was enthusiastic and organised regarding the study. In addition, the frequent and regular visits by the researcher were seen to be encouraging to both the Champion and staff, emphasising the importance of the study and providing some support for the Champion:
The REACH Champion says it’s very good for me [researcher] to be there on a regular basis as it backs up the importance of the study to staff, my time there is fully concentrating on evaluating the intervention, her time with staff includes focus on our study along with hundreds of other things.
A: observations, month 1
Actions and procedures needed to secure engagement and commitment
Participants in the post-implementation focus groups and interviews discussed the importance of the initial REACH training (see Chapter 4, Adaptation and development of training material, and Chapter 5, Delivering the training programme in participating care homes and Delivery and evaluation of the training) in providing insight into the problem of AMR, explaining how the algorithm worked and highlighting what was expected of them throughout the duration of the study:
In the training, we got an idea because you give the examples, the case studies, and we discussed it and we found out how we are going to do it through REACH.
E: staff, post implementation
Staff in residential homes, who were previously not allowed to monitor temperature using thermometers (see Chapter 4, Adaptation of the decision-making algorithm), described how the training they received to do this (see Chapter 5, Delivering the training programme in participating care homes) was very helpful in communicating with the GP:
[The Champion] said they were discovering, just as they learned in the REACH training, that older people may not have a high temperature. Most residents they suspected of having an infection did not have a high temperature and instead usually had a low temperature. She says that the GP always ask if the resident has a temperature and now they are able to say what it is instead of just saying that the resident is warm or clammy.
C: observations, month 6
We anticipated that further training would be needed through the implementation period for those staff unable to attend the initial training or any new staff, and had provided a DVD and online platform for this (see Chapter 5, Delivering the training programme in participating care homes); the training session was also made available on a flash drive. Only one REACH Champion conducted training with staff using the flash drive and reported particular difficulties regarding getting the device to work and how staff appeared disinterested and lacked knowledge:
I think some of the younger care staff got quite disinterested towards the end of the training [DVD], and if I was asking the questions about it, they’d maybe switched off at this point. Some of them maybe didn’t understand what antimicrobials were.
C: Champion, post implementation
We provided a study handbook to be used alongside the initial training (see Chapter 4, Adaptation and development of training material). Many staff reported this as useful in helping address the different learning styles of staff and to use in conjunction with the initial training presentation. In addition, some staff described it as a good alternative to the face-to-face training, which could be referred to throughout the study and a useful resource for new staff:
The study handbook was given to each staff member to have it for consultation at home, and I do believe it will be well used afterwards. Any time that someone has a question or a doubt, they can go and check the book.
A: Champion, post implementation
However, other staff discussed how they did not consult the study handbook after the initial training, finding the laminated decision-making algorithm and the SBAR tool (see Chapter 4, Adaptation and development of training material) to be of more value. Some care staff in nursing homes reported how the content of the study handbook, like the training, was not relevant to them:
I was just having a nosey through it. (. . .) It was quite interesting [but] we wouldn’t really take anything to do with any of that side, so I just read it and that’s it.
D: staff, post implementation
The delivery of the training was welcomed by participants, who mostly considered it to be pitched at the right level for different types of staff, with an appropriate focus on each aspect of the decision-making algorithm. Staff described how they particularly liked the interactive case studies. They also described how the training made them confident that the algorithm would be straightforward to use. Most staff felt that the duration of the training was appropriate and made it feasible for staff to attend:
It didn’t take us off the floor for long as well. So, it’s easier for more people to attend when it’s that sort of time. When it’s longer, it’s harder and you get less people.
F: staff, post implementation
Although some participants discussed how they thought that the training did not require any changes, others described a variety of potential changes. This included having a greater focus on alerting staff to symptoms of infection rather than on the study processes, and provision of a follow-up training session in order to test the knowledge of staff:
So just a little follow-up to find out from everyone, more interactive, how did you apply that training?
E: manager, post implementation
Staff in residential homes suggested that it would be useful to have training on how to deal with relatives’ concerns or to include relatives in the training, especially relatives of residents prone to infection. They discussed their discomfiture, not being nurses, in attempting to persuade relatives to ‘wait and see’ when they demanded antibiotics as soon as the resident showed any sign of being unwell. This was especially in anticipation of either a response from the relative of ‘well, you don’t know, you’re not a nurse’ (C: Champion, post implementation) or accusations of blame if the resident became more ill because of the perceived insufficient or wrong action by staff. An alternative to such training was to provide relatives with more insight into the study through displaying the algorithm on an accessible noticeboard or by making them aware that antibiotics were not always needed and that other actions (e.g. providing pain relief) may be sufficient:
I think maybe if families had maybe more of an insight into this, because I know we have it in our office and that, but maybe if it was on the noticeboard for them to refer to as well.
C: staff, post implementation
Staff in focus groups also discussed the usefulness of having joint REACH training sessions with GPs, perceiving this to be a way for both sets of professionals to understand each other’s actions and concerns. Staff also discussed how best to include night staff in the training, including providing training to these staff just before they came on duty, who could then deliver similar training to colleagues.
Facilitating the use of the REACH intervention (collective action)
We report here on participants’ descriptions of how the decision-making algorithm interacted with other important elements of their daily practice: the interaction of the intervention with the existing knowledge and experience of staff, interaction with colleagues, interactions with GPs and interaction with residents and their relatives.
Interaction of the intervention with existing knowledge and experience of staff
Staff described how the intervention interacted with their knowledge and experience regarding change in behaviour of the resident and prior knowledge of the resident, and around each of the infections.
Participants reported a variety of changes in behaviour that usually alerted them to the possibility that a resident may have had an infection; these included confusion, unsteady on feet, change in eating or drinking patterns, wanting to stay in their room, hallucinations, agitation, scratching genital area and poor behaviour (e.g. bad language):
Hallucinations, a resident talking alone, more agitated, not eating, urine very concentrated, scratching genital area, these sort of things.
A: Champion, post implementation
Participants in both nursing and residential homes described the importance of their prior knowledge of the resident, or that of the resident’s family in helping them notice anything unusual and giving them a ‘gut instinct’ alert to a potential infection; this included knowledge of their body, behaviour and history of infections. The information from this acquired familiarity affected whether or not and the extent to which they used the decision-making algorithm to help them decide whether or not to contact a GP. This knowledge could take precedence over the algorithm or other sources of information such as results of a urinalysis. For example, staff would contact the GP immediately for those residents with a history of septicaemia and who had a high temperature – or, in contrast, delay contacting the GP for those residents for whom their prior knowledge meant that they were able to attribute symptoms to another potential cause:
With [resident], we had a history of going into septicaemia very quickly. You don’t take a risk like waiting for the symptoms to come again (. . .) we didn’t get the chance to look at the aid.
E: staff, post implementation
The resident was noted as having smelly urine and a positive dipstick result (++) – but in this case, the resident was known to usually have ESBL [extended-spectrum beta-lactamase] bacteria in her urine which meant she usually had smelly urine and so this didn’t cause concern. A note was made in the diary to monitor her the next morning and the GP was not subsequently contacted.
B: observations, month 4
Participants described how this prior knowledge was particularly important when dealing with a GP who did not know the resident and that they would try to communicate this information to the GP. The decision-making algorithm was perceived to be of more value in situations in which staff lacked this prior knowledge of the resident (e.g. when there was either a new member of staff or a new resident):
This would probably be handy for somebody, a new resident coming in that we don’t know so much, and you could probably use it in that respect. But for residents that we’ve had here long term, you sort of know them, you know their ways, you know their character.
C: staff, post implementation
Participants described how the decision-making algorithm influenced their assessment and management of UTIs and their decision to contact the GP. Some reported how they were now less likely to rely on foul-smelling or strong-coloured urine or a urinalysis as indicators of infection and that they were more alert to the symptoms indicated on the algorithm:
It [the algorithm] does give like wee pointers as to what exactly you’re looking for. I know before, like we would have like the colour and the smell, especially for urine and that, which we were talking about and then said it’s not actually an indicator that there is an infection present, whereas these are.
C: Champion, post implementation
We are lessening that time we are phoning the GP because we are not prompted just with that urine dipstick. Unlike before, that is the first thing that I usually just check and then it is like that is your cue to talk to the doctor and now you just think maybe I have to keep an eye and then just check for any other symptoms.
E: staff, post implementation
However, participants reported that although UTIs were the most common infection encountered in care home residents, this was the one infection for which they found the decision-making algorithm most challenging to use. One reason for this was that the urinary symptoms stated in the algorithm (new or increased frequency, urgency or incontinence, blood in urine and lower abdominal pain) were not applicable for many residents in nursing and residential homes, particularly those residents with dementia who were also incontinent. Participants described how they needed to be more vigilant for these residents, usually ringing the GP quickly to avert the risk of these residents deteriorating quickly. Residents with dementia were described as not able to express symptoms of burning urination or abdominal pain and, in addition, for those who were incontinent, the frequency or urgency of urination could not be measured. Therefore, staff were more likely to ignore the decision-making algorithm for these residents as many of the UTI symptoms were deemed irrelevant. Instead, they relied on other information, such as the status of a resident as one with dementia, increased confusion and, when possible, indicators such as concentrated or strong-smelling urine and results from a dipstick analysis, to decide whether or not to telephone the GP:
Because new or increased urgency, someone wears a pad and doesn’t express their needs, we don’t know. Increased frequency, the same. Increased incontinence, they are already incontinent. Blood in urines, yes, we can see. Lower abdominal pain, they can complain. So, we have already here on a list more than half, they cannot be applied to our residents because they are not mobile and they are already incontinent. So, that’s why the dipstick is on the picture sometimes, I think.
A: Champion, post implementation
For those residents who were neither incontinent nor had dementia, participants also described alternative symptoms they used to help decide whether or not they should call a GP if they suspected that a resident had a UTI, including strong-smelling urine and a change in colour of the urine:
You see, it’s not on that, you see, but now when we see a resident and the smell of urine is really powerful and the colour has changed, there’s nothing here [on the algorithm] it’s only blood in urine. You can see that it’s a UTI but then you cannot know, so, you wait, right? So, we wait but then nothing like here, blood in urine, no shaking or rigors, nothing.
A: staff, post implementation
In addition, participants in both nursing and residential homes reported alternative sources of information they sought to help their assessment of whether or not to contact the GP, one of which was to try and get a urine sample. Participants in residential homes described how the GP would always expect staff to have tried to obtain a urine sample before being contacted; in one residential home, it was company policy to send a urine sample following a family request:
It is just sort of protocol for us to do it now. A sample helps and that’s what they [GP] would say. Say we couldn’t get a urine sample from a particular resident, and you would ring the GP and say they seem symptomatic. They will ask for a urine sample, so it is always best to get one in the first place and send it down.
C: staff, post implementation
Instead of, or in addition to, sending a urine sample to a laboratory, many participants reported regularly using a reagent dipstick strip to test the urine (urinalysis) when they suspected that a resident had a UTI. The results of this, from the perspective of staff, provided additional information to help decide whether to contact the GP or to send a sample to the practice or laboratory. Although one nursing home in England had a policy of not using a dipstick for residents aged over 65 years, most participants reported how difficult it was not to continue with urinalysis as it appeared to be an intrinsic part of their usual practice and that this often led to them overruling the algorithm. One participant also discussed how not testing the urine conflicted with other artefacts of practice, such as a falls algorithm that instructed staff to test urine after a resident had fallen and showed signs of confusion:
But with urinary symptoms, I really have found it difficult. We had to overrule a lot of times, because the staff did the dipstick. And then we have signs, and we have nothing on the algorithm about the dipstick.
A: Champion, post implementation
The REACH Champion began to talk about a ‘falls algorithm’ which she had become aware of in another setting. (. . .) She said if an elderly resident has a fall and showing signs of confusion then staff are obligated to test their urine.
A: observations, month 4
Participants expressed concern that confusion was not one of the urinary symptoms included in the decision-making algorithm because they considered it a common indicator of UTI and more important than for a RTI:
Well, if somebody usually presents with maybe confusion because they’re elderly, they’re more confused than normal, they might have a temperature, but not always. Their urine would be foul-smelling, which would be a big one for us rather than temperature because if you’re confused and you’ve foul-smelling urine, then usually it’d be, that’s what it is. And we would give them lots of fluid to drink and then phone the GP if it doesn’t settle.
B: Champion, post implementation
One participant expressed particular concern regarding the case of a resident with dementia who deteriorated after they followed the decision-making algorithm. This resident did not have a raised temperature or two or more urinary symptoms, but did have hallucinations:
No, I wasn’t satisfied because even the resident didn’t . . . so he wasn’t fulfilling all the steps from one like no temperature and things like that, but he was hallucinating, he wasn’t well, you could see the deterioration. But yet I was waiting, you know more, to follow the steps, but then I left that person for 1 day to suffer. Then the next day, he was worse because I followed the steps.
A: staff, post implementation
Participants in one care home questioned why the intervention was not also aimed at residents with catheters as they are at an increased risk of UTIs:
I think it’s just bizarre for us not to consider those who are on catheter because they’re the ones who are more prone to UTIs than those who are not.
E: Champion, post implementation
Participants in nursing and residential homes reported a range of symptoms that they observed when a resident had a suspected RTI, including increased breathing, coughing, chestiness, being clammy and sweaty, productive sputum, confusion, raised temperature and a history of RTI. Most participants perceived the decision-making algorithm to work well for RTIs, when compared with other infections such as UTI. This was because it reflected the symptoms they usually looked for and because it fitted in with other aspects of their practice; for example, some nurses explained how the RTI part of the algorithm was similar to the Modified Early Warning System,76 a tool they used to monitor change in residents. Participants who were nurses reported finding RTIs easier to manage than UTIs because they perceived there to be more actions to take, including using nebulisers, encouraging fluids and simple linctus and monitoring observations:
Like I said to you on the chest infection, it was necessary only to look for one or two and it was there, and it really worked. Like it was really, really good the way it was written in here. So, we could really rely on it.
A: staff, post implementation
Participants in residential homes described how they would usually observe a resident’s skin or wound for inflammation, redness, tenderness, pain, swelling and warmth if they suspected that a resident had a skin infection. Rather than contacting a GP, their usual practice was to first contact a district nurse, who would then assess the resident and advise on how to manage the skin or wound and whether or not the GP should be contacted. Participants who were nurses explained that they would usually manage suspected skin infections themselves or seek the advice of a tissue viability nurse if needed. Usual management would include applying topical treatments and taking a swab when appropriate, and seeking antibiotics only if systemic symptoms were present. They described how the symptoms indicated on the decision-making algorithm largely reflected their usual practice:
Well, that’s fine, I mean they are all appropriate and we would have found two or three of those in every one, yes. That was fine, it was right on what we would do.
B: staff, post implementation
Participants who were nurses described how, at times, they used their clinical judgement rather than the algorithm to inform their decision-making (e.g. if a resident had type 2 diabetes or there was a visible increase in the size of a wound):
We have one case because the wound was healing and then we noted that there is more exudates and the wound is getting bigger around the area, so we did consider infection for that resident and then when the doctor checked and then the swab came back as positive for an infection.
E: staff, post implementation
In general, participants reported that they used the decision-making algorithm less for SSTIs than for other infections. This was because SSTIs were not common, the symptoms were very easy to observe and they were able to act quickly to prevent them from getting worse:
With the skin symptoms, I don’t think we did [use the algorithm]. Usually when we have a wound they don’t really get worse. We tend to catch them very early and then we try to prevent them from getting worse.
B: staff, post implementation
Interaction of the intervention with colleagues
Although participants in all of the care homes discussed the importance of junior staff (senior and junior care staff in nursing homes and junior care staff in residential homes) noticing when a resident may be ill, there were contrasting views regarding whether or not junior staff used the decision-making algorithm to report their concerns to senior staff. For example, some senior care assistants in nursing homes discussed how they did not use the decision-making algorithm to communicate their concerns about a resident, whereas, conversely, some junior care staff in residential homes described how they did use the algorithm for this purpose:
I am not going to read this [the algorithm], go to this to see ‘oh, should I report this to the nurse or not?’. I automatically will report everything to the nurse if you know what I mean. I won’t go down the list and say ‘do I need to report this to the nurse because he has these symptoms’. (. . .) It makes no difference to me what this says here, whether I report things or not.
B: staff, post implementation
I think with care staff, yes, I think they’re able to let us know the different symptoms and what’s happening, how often is it, is there an urgency, the frequency, is there just the one symptom, are they just unwell today, and in what way, what symptoms have they actually got, that they’re able just to look at this and say, ‘this is what’s wrong’.
C: Champion, post implementation
Interaction of the intervention with general practitioners
As part of the intervention, we also asked care home staff to use the SBAR tool (see Chapter 4, Adaptation and development of training material). Many participants in nursing homes reported prior use of SBAR, either as part of local initiatives or as part of their student nurse training. Most care staff in nursing homes reported that they did not use the SBAR tool because it was not part of their role to contact the GP, although some junior staff in residential homes thought that they may use it when senior staff were busy. For those participants across all homes who had not used SBAR before, they felt that it was similar to their usual practice. For those participants whose role included communicating with the GP, they reported that SBAR made this task easier as it helped them present the required and relevant information in a logical way. Although most participants did not report any difficulty using the SBAR tool and found it easy to follow, one reported challenge was using it in an emergency while the tool was still unfamiliar:
At first, you are not used to it, because you are thinking while you’re talking. So, it doesn’t come naturally, and it can be tricky when you are phoning in an emergency situation.
E: staff, post implementation
Observational data showed how the SBAR was perceived to be more useful when communicating with a GP who was unfamiliar with the resident and how the order of communicating key aspects of information was re-arranged when in contact with out-of-hours GP services:
REACH Champion had used [SBAR] recently for out-of-hours [name of centre] and says that in this context the background comes first before the situation – this is because this is what is asked in the initial contact with [name of centre].
C: observations, month 4
Participants reported the ways in which using the decision-making algorithm strengthened their communication with the GP when they suspected that a resident had an infection. In one way, the algorithm worked as a type of justification ‘checklist’ – justifying the contact with a GP when they perceived the resident to have sufficient symptoms in accordance with an evidenced-based algorithm or justifying delaying calling if sufficient symptoms were not evident. Equally, it provided justification to the GP that there were enough symptoms to warrant the call and that they were not wasting the GP’s time:
It gives you like I say, a checkpoint of ‘well these are all the symptoms’, or ‘you don’t have any symptoms why are you ringing the GP then’. (. . .) And the study’s being done into it that these are signs and symptoms that we’re supposed to be looking for (. . .) you’ve got, as I say, as a bit of a backup, that you can be confident in what you’re saying to them.
C: Champion, post implementation
The algorithm also served as a tool to persuade GPs to visit the resident and to give staff more ‘vocabulary’ of what to say and a structure for saying it:
It gave us more vocabulary, more points, more bullet points, more objective. You don’t say to the GP ‘I think the person has an infection’. No, no ‘this person, we are phoning, she has respiratory breathings over 25 [breaths per minute], is chesty, has history of . . .’. We will know exactly the important words to tell the GP.
A: Champion, post implementation
Interaction of the intervention in communication with residents or relatives
Participants described how they were required to comply with a resident’s demand for action, such as contacting the GP or a conducting a dipstick analysis, when the resident themselves suspected that they may have had an infection. Such a demand was more usual among residents who were retired health professionals:
A resident who has capacity and she was a nurse and she asks for a dipstick because she thinks that she has urinary infection. It doesn’t matter what is this [algorithm]. You just go and make a dipstick and she has right. She complains, just has pain when passing the urine, just one symptom, not more. But she wants ‘I want to make a dipstick, I think I have urinary infection’. You can’t say no. You make, the dipstick show leukocyte and everything. The doctor prescribe antibiotic for her.
A: staff, post implementation
Participants reported that they did not usually show the decision-making algorithm to residents, perceiving that they would not understand it, nor did they usually show it to relatives. They described how they were required to notify relatives when a resident was suspected of having an infection and to check whether or not they were in agreement regarding any proposed action. Participants also described how sometimes, conversely, it was a family member who informed them that a resident might have had an infection, usually by noticing a change in behaviour. Some participants who were nurses described how relatives were often confident to leave the decision to contact the GP to them, especially if the nurse appeared confident in the proposed action or if the relative had previously advised that they did not want the resident to go to hospital:
I did mention to the family that we perhaps weren’t going to go down the antibiotic route. We were just going to give him fluids and see how it goes (. . .) and they seemed fine with that, yes, and as it turns out, he hadn’t got a chest infection and it seems to have cleared a bit by itself anyway.
D: Champion, post implementation
Other participants, despite recognising the place of the relative’s expert knowledge in decisions about the care of the resident, described how they found it difficult to persuade relatives that a decision to monitor the resident, provide supportive care and delay contacting a GP represented a legitimate action and should not be perceived as neglect. They discussed how they were required to comply with a relative’s wishes and how this sometimes meant overruling the algorithm:
The families don’t know about decision aid tool, (. . .) but sometimes, we can get pressured for antibiotics. If they understand the person is unwell, ‘why are they not on antibiotic?’. [We say] ‘Sometimes it’s not needed or sometimes it will harm more than benefit’. (. . .) Yes, families want the treatment. No treatment may appear as no action that ‘nothing is being done’, and nothing as being done is, emptiness, it’s lack of action, it’s neglect almost.
A: Champion, post implementation
[The REACH Champion] discussed how family members are very influential in staffs’ decision to contact the GP still and the challenge of dealing with relatives in care homes.
E: observations, set-up visit
Value of the intervention (reflexive monitoring)
In addition to the participants understanding why the decision-making algorithm was introduced and what they were being asked to do with it, what was needed for them to become engaged with it and how they used the algorithm in practice, we describe how they determined the usefulness of the intervention for different types of staff and the ways in which they modified it to make it workable in their practice.
Determining the usefulness of the intervention and for which staff
With regard to how staff perceived the value of the decision-making algorithm, participants were divided regarding which staff, with which skill set and responsibility, could legitimately use it. Nursing staff in nursing homes and senior carers in residential homes, who had responsibility for contacting the GP, discussed how the algorithm acted as a reference for symptoms of infection and actions to take. They perceived it to be particularly useful whenever there were elements of unfamiliarity, for example new or inexperienced senior staff, more experienced staff dealing with a new resident, or when dealing with unfamiliar GPs. Participants in residential care homes discussed how they thought that the algorithm was useful for senior and junior care staff and for new staff. For senior staff, it provided a point of reference for symptoms of infection and it was used by junior staff to help them decide when to raise concerns with a senior carer when they felt that a resident might be unwell:
I think it’s good for say new members of staff and senior staff, like senior carers, because it gives them a reference to look at and to know what kind of symptoms, maybe if they don’t have the experience. It’s also good for [junior] care staff that they’re not just coming and saying, ‘Hmm, there’s something not right here’.
C: Champion, post implementation
However, some senior and junior care staff in nursing homes reported that the algorithm was of no value to them. They emphasised that because their role was limited to reporting concerns to the nurse, a tool to help nurses know when to contact the GP was seen as not only irrelevant to them but also beyond their usual, and preferred, responsibility:
Because we [senior care staff] don’t really deal with GPs or anything; I don’t really know how it can really work for us.
B: staff, post implementation
This perceived lack of value in, or indeed legitimacy of, care staff using the algorithm was echoed by a nursing home manager. In addition, one REACH Champion described how senior and junior care staff in her home lacked both initiative and competence to be able to use the decision-making algorithm:
[Care staff] have to report everything to the nurse, so it’s the nurses’ decision at the end of it all. You know it’s not as if the care staff member is going to decide right, ‘I’ll follow that decision aid and I’ll not tell the nurse until later on’. That would be wrong of them.
B: manager, post implementation
I don’t see that happening here in this care home. Perhaps in another care home where we have younger staff, more alert perhaps. Then they would come to me and say ‘we have a resident with two symptoms and no temperature, maybe it’s . . .’ (. . .) They don’t have that much initiative.
A: Champion, post implementation
Another reason why care staff in nursing homes were perceived not to use the decision-making algorithm was that they were limited in number and had limited time; this was compounded by the demands of attending to the care needs of residents in nursing homes, which were perceived as greater than the needs of residents in residential homes:
I don’t think the care staff will have the time honest. It’s only us [nurses] who can do it (. . .) and like our residents really need help in here with everything.
B: staff, post implementation
In addition, although some nursing home participants discussed the benefits of care staff attending training, they thought that this may be resented by staff as being an additional workload and stressful:
[The care staff] will say ‘argh, another thing to do for us, more work for us’ (. . .) because they feel like they are really pressured about the amount of work.
A: staff, post implementation
In contrast to the perceived lack of utility of the decision-making algorithm by some care staff in nursing homes, others discussed how using the algorithm was very important. One reason for this was that care staff, as front-line staff, knew the residents very well and were able to report any small change in the resident’s status to the nursing staff:
As carers we know our residents quite well so, if you’re noticing something that’s not right with them and you’ve got this kind of tool to use, then you can actually say this is the symptoms they’re showing, so you can always refer back to it and check symptoms and then pass it on to the nurse.
D: staff, post implementation
Participants differed in their views of whether or not junior staff (senior and junior care staff in nursing homes and junior care staff in residential homes) should have been included in the training. Some emphasised how ensuring that more nurses took part in the training, or compelling them to, may have increased their engagement with the study and encouraged them to take more responsibility for implementing the intervention rather than leaving this to the REACH Champion:
I think you should have ignored [care assistants] and made sure all the nurses were here (. . .) if they weren’t here on the day they probably should have been made to come in for the meeting and we should have all have been explained to and told it was all our responsibility, individually (. . .) if you don’t attend the thing, you’re less engaged in it.
B: Champion, post implementation
Despite this, staff reported how including junior staff (senior and junior care staff in nursing homes and junior care staff in residential homes) in the training would enable them to have greater understanding of important aspects of the care of residents and ease the workload of senior staff.
Finally, although staff recognised that the training for using the decision-making algorithm was good in theory, some found it difficult to put into practice, either because of time pressures or because of particular situations:
Well, theoretically, it’s [the training] good and advantageous, but I think sometimes because of our function, a bit busy, we cannot really implement in awkward situations. Like what we learn, we’re [not] going to always put into action.
A: staff, post implementation
Modifying the intervention to make it workable
Regarding the training aspect of the intervention, some REACH Champions described reasons why they did not use the DVD, flash drive, or online platform – preferring the convenience of a quick informal explanation to staff of how to use the decision-making algorithm compared with the time needed to watch the DVD:
No [laughs] we never had time to sit down and do that. ‘Cos the other thing is, in the offices is where you are. So, therefore it takes 2 minutes to explain it, 5 minutes to explain it and people understand it. But to go and say ‘go and watch this’, nobody gets time to go and watch that and they will not do it on their lunch break.
B: Champion, post implementation
In some care homes, although there was no formal follow-up training, participants described a variety of informal learning around the decision-making algorithm. This included having the algorithm displayed in convenient locations, such as the office where staff gathered, general discussion with colleagues and the REACH Champion either talking staff through the algorithm in the office where it was displayed or by regularly reminding them, for example at morning handovers. Some junior care staff described their lack of engagement with aspects of such informal learning and perceived it not to be relevant to them:
It was just the chart thing was pointed out [in the office]. We were told to look at it a couple of times (. . .). It’s nothing really to do with me because I am not a nurse and I don’t take anything to do with that side of things. I just read it and that was it.
B: staff, post implementation
Burden of data collection
Contact with health and social professionals and use of hospital services forms
In NI care homes, staff were initially asked to complete the contact with health and social professionals form (hereafter known as the contact form) each day to record contacts with health and social care professionals and the use of hospital services form (hereafter known as the hospital services form) each time a resident used a hospital service. In the two nursing homes in NI, staff reported finding the contact form too detailed and time-consuming to complete:
It was very detailed and too much time-consuming, too much information that I hadn’t felt was relevant for the study.
A: Champion, post implementation
The homes found the hospital services form easier to complete, but made suggestions to improve its format. The study team subsequently revised both forms and these changes were well received by staff. However, staff still reported completion of the forms to be time-consuming and burdensome, they did not always remember to complete the forms and they thought that the forms duplicated information that they already routinely recorded:
I didn’t feel I felt I got all the information that there was so much completion of forms to be done. I think maybe I didn’t listen to you clearly enough. I imagined it would just be for specific infections, whereas it was for lots of things, including hospital appointments and GP calls to the home and all sorts of involvement in it. Whether that wasn’t made clear at the outset or whether I didn’t take the whole thing on board . . .
B: manager, post implementation
In two homes in England, the research fellow worked with the REACH Champion to complete the contact form and the hospital services form retrospectively, usually every 2 weeks. This appeared to work well. Data were collected from notes from routine doctors’ visits and the care home diary in which key events were recorded. In one care home, the REACH Champion created a monitoring form on which details regarding telephone contacts with health and social professionals were extracted from the residents’ care plans in advance of the research fellow’s visit:
I didn’t find it very hard. We’re always organised, once we got around how to properly fill up the forms it was quite easy for us. In the beginning we were having difficulty on what to put where, what data to collect, but once we got used to it I already know which paperwork I need to prepare every time you come here, which dates I need to prepare, from which date up until what date. It wasn’t very hard, actually.
E: Champion, post implementation
Later in the data-collection process, care homes in NI also completed the forms retrospectively every 2 weeks with the support of the research fellow and found this preferable to daily completion, which was burdensome. One care home preferred collecting the data on a monthly basis because there seemed to be relatively few data to collect. However, when data collection was attempted retrospectively after a 6-month period (because the home was too busy to engage in the implementation phase), the research fellow was unable to collect accurate data retrospectively for this duration; some data had already been archived and were therefore difficult to access.
Using the decision-making algorithm form
Staff reported that using the decision-making algorithm form (hereafter known as the algorithm form) was generally straightforward to understand and that the structure of the form was easy to follow. However, completion was often challenging, particularly when staff were busy and data collection for a research study was not considered a priority:
Here is only one nurse and three carers and if there is an ill patient, there could be another two or three ill patients, and that nurse has all the documentation, all the decision-making to do and for her to write up in the patients’ notes to contact families, to contact GP, to record all that, organise scripts and then take another 10 minutes to fill in one of those forms, it is a lot added on and I am not sure for what benefit.
B: manager, post implementation
Staff understood the importance of completing the algorithm form for the study, but considered that it duplicated information already routinely recorded by the care home and therefore added to their workload and burden of documentation. A manager in one care home described how when staff forgot to complete the algorithm form, they would have to review residents’ notes to locate the information needed to complete the forms, which was time-consuming and inconvenient:
I think they found sometimes they didn’t remember to do it and when they did do it, I think they found it a bit of a nuisance because there is so much other writing to do. And I know it was changed a bit but I think they just felt it was another form to fill in, but it is not really going to help the decision on the client’s treatment. Particularly if they didn’t remember to do it, and then they had to backtrack and with having quite a lot of part-time staff, they maybe weren’t here for like 7 or 8 days and then suddenly remembered ‘oh, I forgot to fill in that form and I have to start to look back’. I think some of them found it a bit of a nuisance.
B: manager, post implementation
Similarly, staff reported that it could be challenging and time-consuming to complete the final section of the form, which recorded what happens to the resident 2 weeks after the infection was first suspected. The nurse on duty at the time may not have been available and other staff would have to seek information from alternative sources:
Also, what happens with the resident in 2 weeks, it’s a bit difficult to go back in that because the nurse who filled in the initial form might not be here or might not be in on that 2-week timing. And I am sure when you came to check up, quite a lot of those weren’t completed because going back to things. I just feel in a way it is a bit more of a time-consuming exercise, that maybe we don’t really need to help us.
B: manager, post implementation
One REACH Champion also reported experiencing additional burden to workload and frustration when the research fellow visited the home and other staff had not completed the algorithm forms. This required her to review handover sheets and care plans to complete the forms retrospectively:
I think just whenever you visited and there was no forms filled in from the other staff, I think that was a bit frustrating. Because maybe it was only [name] filling them in for that week and there was no other seniors filling them in? I think that’s quite annoying because then we have to look back on the handovers and progress notes to see if there’s any contact been made, and it was us who had to fill them in then.
C: Champion, post implementation
Suggestions to improve the completion of forms included making the forms accessible and shorter for staff to complete. It was also suggested that staff could provide brief details that could then be added to by a ‘study administrator’:
. . . they just jot down for whom they use this tool and then afterwards an administrator can come in for the research study and complete that, because everything will be in the records of the patient or the resident.
E: manager, post implementation
Summary
The process evaluation explored the implementation of the intervention presented in Chapter 5. As this was a feasibility study, the process evaluation provided valuable information about how the intervention was implemented and, indeed, to what extent it was implemented.
Our results have demonstrated that we could recruit care homes and train staff to use the decision-making algorithm, and there was some evidence of a level of implementation of the intervention. The process evaluation has identified a number of challenges and suggestions that should be considered in a future study.
Chapter 7 Survey of care homes
Introduction
In this study, we explored the views of care home managers, or the individuals who they designated, on the REACH training programme and intervention and the likelihood of recruitment to a future trial by conducting a short postal survey with all care home managers in NI and the West Midlands, England.
Aim and objectives
Our aim was to assess the likelihood of care home participation in a future trial evaluating the effectiveness of the REACH intervention to reduce antimicrobial prescribing in care home residents and to gain care home managers’ views on the REACH training programme and intervention.
Ethics approval
This study was approved by members of the School of Pharmacy Ethics Committee at Queen’s University Belfast (reference 022PMY2017).
Methods
In order to fulfil the aims and objectives of this study, quantitative methodology, employing a brief postal questionnaire survey, was used. Using surveys as a research tool is advantageous when the desired outcome is to gather information from a large sample within a relatively short time frame. 77 In addition, respondents had the opportunity to provide free-text responses.
Inclusion criteria and sample size
Our sampling frame was all 446 care homes (nursing and residential) for people aged ≥ 65 years in NI and 1040 such homes in the West Midlands region of England. Care homes were identified from publicly available databases: the Regulation Quality and Improvement Authority in NI and the Care Quality Commission in England. We included all those care homes with at least 20 residents, homes not dual registered as nursing and residential homes and homes not part of a health trust. We excluded the three homes in each site (two nursing and one residential) that had taken part in the REACH feasibility study. The sampling process and final sample targeted are outlined in Table 31. Questionnaires were posted to the registered care homes in NI and the West Midlands and addressed to the manager.
| Sequence of the sampling process | Number of care homes | |||
|---|---|---|---|---|
| NI | West Midlands | |||
| Nursing home | Residential home | Nursing home | Residential home | |
| Sampling frame | 249a | 197a | 274b | 766b |
| Homes with ≥ 20 beds | 239 | 97 | 237 | 303 |
| Homes with residents aged ≥ 65 years | 221 | 81 | 225 | 286 |
| Homes not part of a health trust | 221 | 61 | 225 | 286 |
| Homes not dual registered as nursing and residential | 169 | 61 | 214 | 286 |
| Homes that had not already participated in REACH | 167 | 60 | 212 | 285 |
| Final sample | 167 | 60 | 212 | 285 |
| Total | 227 | 497 | ||
With surveys, a high response rate is crucial to ensure the validity of the study findings. As participation in the study was voluntary, where possible we made efforts to maximise the response rate. This included giving careful attention to the content and design of the questionnaire. The survey content was initially informed by the results of the REACH feasibility study and then developed and refined following discussions within the research team. Following this, the survey was piloted with three managers for comprehensibility and validity in achieving the research aims. Our efforts to maximise the response rate also included giving consideration to the timing of survey mailings (e.g. avoiding holidays).
Distribution of survey material
For the initial mailing (January 2018), each sampled home was sent a pack containing a short cover letter of invitation on study headed notepaper, a participant information sheet and a questionnaire with a pre-paid return envelope. The cover letter gave a brief background to the survey stating that we were interested in the views of care home managers about a study to reduce antimicrobial prescribing in care homes for older people. The participant information sheet outlined the background to the REACH feasibility study and a proposal to undertake a larger study. The information sheet provided an overview of the proposed larger study, including its duration, the commitment required by homes and staff, the training session and using the decision-making algorithm. The survey consisted of seven questions:
-
Do you think a study like the one described in the participant information sheet would be welcomed by care home staff in your home?
-
Do you think an education and training programme about antimicrobial prescribing would be helpful to care home staff in your home?
-
Do you think 2 hours is a reasonable time for staff to be able to attend an education and training programme?
-
Do you think it is feasible for all care staff in your home to attend an education and training programme if it was provided at different times of the day?
-
Do you think it would be useful for care staff to use a decision-making aid to help them decide when to contact the GP if they suspect that a resident has an infection?
-
In principle, would your care home be prepared to take part in a study like this?
-
Do you have any other comments you wish to add?
The response options to questions 1–6 were ‘yes’, ‘no’ or ‘don’t know’, with the further option of providing additional comments. Question 7 invited participants to provide any additional comments.
As completion of the questionnaire was voluntary for all potential respondents, informed consent for participation in the study was assumed on receipt of a completed questionnaire. A specific date for completion was highlighted within the invitation letter to encourage response. Assurances of anonymity were detailed in the covering letter. After a period of approximately 3 weeks from the initial mailing, a reminder letter was posted alongside a second copy of the questionnaire to encourage participation from those who had not responded. It was made clear in the reminder letter that those who had already responded need not complete the questionnaire again. Repeated mailings including such reminders are a recognised facilitator of improved questionnaire response rates. 78
Data analysis
Responses were entered into IBM SPSS® Statistics version 20 (IBM Corporation, Armonk, NY, USA) statistical analysis software. All respondents were given a unique identifier to ensure anonymity when reporting any free-text responses provided. The content of such responses were imported into NVivo® for analysis of recurring themes. Descriptive statistical analysis (e.g. frequencies, means and medians) were used to report findings from closed and multiple-response questions. As with all studies using questionnaires, a level of missing data was to be expected; however, it was hoped that this would be minimised through the process of piloting and refining the questionnaires. Where there were missing response data, they were coded as such and omitted from the final analysis.
Results
In total, 167 care homes (23%) responded to the survey; the response rate for care homes in NI was 47% (n = 107) and the response rate for English care homes was 12% (n = 60). Over half of responders (n = 89) provided additional free-text comments in response to one or more of the questions.
From those who responded, 83% (80% of care homes in NI and 88% of English care homes) indicated that they would welcome such a study and 6% (6% of care homes in NI and 7% of English care homes) indicated that they would not (11% did not know; 14% of care homes in NI and 5% of English care homes). Free-text comments suggested that respondents thought it an important area to research, although managers felt that some staff may be more interested than others. Some respondents expressed reservations that care homes already use a range of decision-making aids and guidelines and had limited time to commit to research.
There was good support for an education and training programme on antimicrobial prescribing (91%; 91% of care homes in NI and 90% of English care homes), and this was reflected by free-text comments advocating the benefits of any training for staff and increasing the awareness of the subject area:
Most care staff including myself as the home manager would benefit from more training as we have little knowledge about this subject.
There were also a number of comments expressing concern that care staff have limited control over what GPs prescribe and that either the education and training programme should be aimed at GPs rather than care staff or GPs should be included in the training programme. Similarly, there was concern that some families insist that GPs are contacted in order to prescribe antibiotics and, therefore, respondents thought that families should also be offered the programme:
I feel it would be beneficial to have GPs on board to avoid prescribing antibiotics when patients are asymptomatic.
Some GPs are reluctant to give [antibiotics] as client does not require, but families insist so they need the training more as they are unwilling to listen.
Most of the respondents (90%; 91% of care homes in NI and 87% of English care homes) reported that 2 hours was a reasonable period of time for staff to attend such a programme. Among those who did not think that this was reasonable and provided free-text comments, most suggested that a 1-hour training session would be more appropriate and that care staff could have shorter sessions than nursing staff.
Approximately three-quarters of respondents (73% of care homes in NI and 80% of English care homes) indicated that it was feasible for staff to attend an education and training programme if it were provided at different times of the day, but 19% (20% of care homes in NI and 17% of English care homes) did not think that it was feasible for all staff in the home to attend. Free-text comments suggested that shift patterns, annual leave, sickness, difficulty taking staff away from direct care and relying heavily on agency staff made it difficult for all staff to attend. Some respondents suggested that it was necessary only for senior staff and managers to attend the training. Respondents reported that they currently provided training sessions at 14.00–16.00 and 06.00–08.00 to allow both day and night staff to attend, and suggested timetabling sessions weekly for a few weeks to maximise attendance:
Ideally I’d like to say yes, however experience proves that lots of things can happen throughout the day, and therefore limit staff availability.
In terms of using a ‘decision-making aid’ to help staff decide when to contact the GP if they suspected that a resident had an infection, 88% of respondents thought that it would be useful (87% of care homes in NI and 88% of English care homes) and 9% (10% of care homes in NI and 9% of English care homes) did not think that it would be useful; 3% (3% of care homes in NI and 3% of English care homes) did not know. Comments regarding the usefulness of the ‘decision-making aid’ included support for the use of evidence-based tools to give staff confidence in decision-making:
The care staff would report concerns to the deputy manager. I think it would be great for them to take more responsibility and take action in a resident’s best interest.
A number of managers reported that staff already used tools for the assessment of UTIs and considered additional tools unnecessary:
My own nurses express concern about the number of tools already in place. Paperwork has become excessive. The practical, observant and experienced nurses suggest tool overload.
The most common comment was that the decision-making tool would be useful only if GPs and families were also supportive:
If the GPs are also in agreement as many GPs are still reluctant to listen to qualified nurses who know the patients.
One manager expressed concern based on participation in a previous study that decision-making aids can be too simplistic for residents in care homes who have complex conditions and that they do not take into consideration the knowledge nurses use to assess change in residents with dementia who are unable to verbalise their symptoms.
Overall, 79% of care homes (78% of care homes in NI and 82% of English care homes) were, in principle, prepared to participate in a study such as that described in the participant information sheet that was provided with the questionnaire. Free-text comments suggested that managers were keen to be involved in research, felt the subject area to be important and welcomed training for staff:
I believe it is an essential area of study and the home and myself would be happy to participate.
Some respondents, however, commented that despite the undoubted benefits of research, current staffing levels would prevent the care homes from taking part:
Depending on how much time it would involve as staff are already very busy.
Summary
A survey was conducted in a sample of care homes in NI and the West Midlands to gauge interest in a larger study. The overall response rate was 23%. There was interest and support for a larger study and recognition of the importance of AMR, and the opportunity for training was welcomed. Concerns were expressed regarding time commitment and the need to involve GPs and family members.
Chapter 8 Discussion
Introduction
This chapter summarises and discusses the findings from each phase of the study in order to directly address the aims and objectives of the research. The overall aim was to evaluate the feasibility and acceptability of a multifaceted intervention on rational prescribing for infections in a non-randomised feasibility study in care homes. The objectives related to recruitment of homes, adapting and developing the intervention (a decision-making algorithm and small group interactive training), implementing the intervention, undertaking a detailed process evaluation of the non-randomised feasibility phase and testing data-collection procedures. We discuss these issues in subsequent sections in this chapter, in addition to the findings of a survey in care homes that was undertaken in order to gauge interest in a larger study.
Recruitment
We successfully recruited six care homes that met the inclusion criteria. The approach taken in the two geographic sites was largely comparable, but also took into account some contextual differences, notably the role of ENRICH in England; there is no equivalent organisation in NI. In NI, we also restricted the geographic area from which care homes were recruited to reduce the amount of travelling that would be required to be undertaken by research staff. It was at this point in the sampling approach that many homes were excluded in NI. Therefore, a greater sample of care homes would be available from which to recruit if the requirement of geographic proximity to Belfast was lifted. We had also excluded care homes that were dual registered (i.e. providing nursing and residential services) as we wished to explore the feasibility of this intervention in the residential setting compared with the nursing home environment. Dual registration may have confounded this assessment. It had also been decided, a priori, to exclude trust-owned homes; almost all nursing homes in NI are privately owned, but this is not the case with residential facilities. Applying this criterion ensured a degree of comparability between nursing and residential homes. Interest in research was gauged by a care home being a member of an advocacy organisation in NI or a member of ENRICH in England. Further attrition was noted at this point in the NI sample.
The number of beds in the care homes ranged from 32 to 86, with almost 100% occupancy in all of the homes apart from one in NI. In the case of this one care home, one floor containing 22 beds was closed for refurbishment from December 2016 to December 2017.
The number of general practices providing care to each home reflects the organisation of primary care services in care homes in different parts of the UK. In NI, residents will often remain registered with the general practice that they attended prior to admission to a care home. In England, residents, on admission to a care home, will often register with a practice that has an exclusive arrangement with the home. Hence, care homes in NI tend to be associated with more general practices, and this was reflected in the characteristics of the participating homes. 79 All care homes were associated with only one community pharmacy, which also reflects usual practice. 80
The sampling and recruitment process achieved the aim as we recruited the requisite number of homes. The inclusion criteria do not appear to have been unduly restrictive, apart from geographic proximity to Belfast in NI. This would be removed in the case of a larger study, providing a larger sample from which to recruit. We could also consider focusing on care homes that have an interest in research. Clearly, this can be an advantage, but may also introduce an element of bias in selection. However, if appropriate support could be provided to care homes in a larger study, this may also increase the sample available for recruitment. Findings from the survey reported in Chapter 7 suggested that there was interest and support from care home managers for a larger study.
Adaptation and development of the intervention
In this phase of the study, we set out to adapt and update the decision-making algorithm and training material developed for the original Canadian study by Loeb et al. 16 for implementation in the feasibility study. This was achieved through a series of rapid reviews of the literature, a consensus exercise, a series of focus groups and interviews, and continuous iterative review undertaken by the research team. This comprehensive approach ensured that a robust and rigorous approach was taken to updating of all material, while also navigating through the tension between published evidence and ingrained clinical practice. This phase also generated a huge number of data, which represented a challenge in terms of synthesis and producing an updated decision-making algorithm that would be practical and feasible to use in a busy care home environment. Box 5 illustrates the key components of the original Canadian intervention compared with the components of REACH.
-
Focus on UTIs, with one algorithm focusing on diagnosis and the second algorithm focusing on treatment. Diagnostic algorithm initiated with temperature monitoring and observation of symptoms; treatment algorithm initiated with results of urine culture.
-
Differentiation between catheterised and non-catheterised patients.
-
Presence of specific symptoms in catheterised/non-catheterised patients dictated if antibiotics were to be prescribed.
-
Training, based on case scenarios demonstrating the use of the algorithm, delivered to nurses and care assistants. These small group sessions were taped and used to supplement training for other and new staff. Visits made to doctors to explain the use of the algorithm in the context of the case scenarios.
-
Focus on UTIs, RTIs and SSTIs. Single algorithm (decision-making algorithm), which began with assessment of common symptoms and monitoring of temperature.
-
Assessment of infection-specific (UTI, RTI, SSTI) symptoms.
-
Dependent on symptoms, contact GP or monitor temperature.
-
Supportive care (fluids, analgesia) recommended for all residents.
-
Training consisting of Microsoft PowerPoint presentation and study handbook, containing information on AMR (video), the decision-making algorithm, case scenarios, SBAR tool and how to complete study documentation. Training delivered in sessions to care home staff only. A video of a training session was provided on a DVD, a flash drive and an online platform.
In terms of the rapid reviews, it was striking that there were relatively few new publications in the field of managing the three key infections. The reason for the exclusion of most papers was that they did not relate to the older population or the care home setting, or that they did not offer any new evidence regarding management of the three target infections. Six papers that met the inclusion criteria were consulted and used in the updating of the decision-making algorithm. Most focused on UTIs, which may not be surprising as this is the most common infection type in care home residents. 4 There was no new evidence regarding the management of SSTIs. The most recent papers had been published in 2013. 46,47 The lack of more recent papers may be due to there being a relative paucity of research in the care home population and the difficulty in establishing definitive diagnostic criteria for infection in this population. Three papers referred to change in mental status as being important in the identification of UTIs;42,46,47 however, this is difficult in a population in which cognitive impairment is highly prevalent. This led to discussion within the research team regarding whether or not two management ‘pathways’ should be presented for UTIs (i.e. one for those with dementia and one for those without dementia). However, it was agreed that it would be more straightforward and practical for staff for a single ‘pathway’ to be presented. This was also the view in respect of three separate algorithms for the three target infections. Again, to facilitate ease of use, it was agreed that a combined algorithm, with a common starting point, followed by three ‘pathways’ for each of the infections would be the preferred form of presentation.
The consensus exercise was not part of the original application submitted to NIHR. The decision to conduct such an exercise arose through discussions within the research team, and was seen as an additional method to assist us in deciding on the content of the updated decision-making algorithm. Separate ethics approval for the consensus exercise was obtained and granted. The participants came from a range of backgrounds, but all had experience of the care of older people and/or management of infectious diseases. The consensus approach allowed for a consideration of the key symptoms that could be included when updating the algorithm. Temperature was identified as important, but its interpretation was seen as problematic in the care home population as a rise in temperature was not always indicative of infection. 49,50 As had been identified in the literature, confusion and cognitive impairment were recognised as difficult in care home residents, and the participants in the consensus exercise recommended that staff should be aware of changes in behaviour in residents as these may be suggestive of infection rather that confusion per se. It was also the view of the participants that a single decision-making algorithm with three ‘pathways’ for the three target infections was the most practical approach for use in the implementation phase.
The qualitative aspects of this phase (focus groups and interviews) generated rich and complex data, which were managed through careful analysis. The participants included key stakeholders: a range of staff from care homes, GPs and family members. All could see the value in the use of the decision-making algorithm, and, in many cases, care home staff reported that it reflected their usual practice. The findings also highlighted aspects that had been previously raised through the rapid reviews and the consensus exercise: the challenges presented by confusion in this population and concerns regarding the interpretation of temperature. There was the additional challenge in that staff in residential care homes did not usually measure temperature as this was viewed as a ‘nursing’ task. However, this was overcome by providing the necessary training to these staff, as outlined in Chapter 5. Many of the participants also suggested a range of other symptoms that they thought should be included in the decision-making algorithm. However, the inclusion of all suggestions would have produced an illegible and probably unmanageable tool to use. There were also interesting contrasts between evidence and usual practice informed by experience. Care home staff frequently reported on the smell of urine as being indicative of infection, but SIGN, in particular, had not included this within its most recent publication. 43 Other publications (albeit ones that did not meet the criteria for inclusion in the rapid review) have highlighted this symptom as being problematic in terms of evidence for a UTI. For example, Midthun et al. 81 evaluated freshly voided urine from care home residents for the presence of odour compared with urine analysis and culture results. The positive predictive value of odour was 54% for bacteriuria and only 28% for situations in which pyuria was present with bacteriuria.
Involving key stakeholders was challenging as it elicited a huge body of data and views that contradicted published evidence, which is discussed further later in this section. However, it may have engendered a sense of ownership of the decision-making algorithm and encouraged its use during the implementation phase of the study. A number of publications have highlighted the importance of the role of co-creation in terms of promoting practice change and generating ownership of proposed changes. Goeman et al. 82 showed the value of a co-creation approach in developing a model of care for dementia support in culturally and linguistically diverse communities. This required input from community aged-care services, consumer advocacy organisations and ethnic community group representatives to develop and refine the dementia model of care. In contrast, Beck et al. 83 reported that nursing home managers did not view advance care planning as part of their role, with lack of ownership having an impact on current practice behaviours. Similarly, a broad range of health-care professionals who were interviewed regarding delivering appropriate care to patients with multimorbidity and polypharmacy did not use or were unaware of structured approaches, such as decision aids, to support activities in improving care. This was partly attributed to the participants avoiding ownership of multimorbidity management. 84
The rapid reviews, consensus exercise, focus groups and interviews contributed to the ongoing discussions and work of the research team, which was refining and updating the decision-making algorithm on an iterative basis. This was not a linear process, as a number of the activities were overlapping. The 3-day meeting to undertake data extraction from papers and to identify relevant papers took place in June 2016; the consensus exercise took place in September 2016 and the interviews and focus groups took place during September 2016 to March 2017. As data emerged from these activities, the decision-making algorithm was refined, altered and refined again until the final version, as shown in Figure 7, was produced. This was a culmination of discussion within the research team, as it attempted to produce a tool that would have relevance in the unique context of a care home and reflect practice as far as possible while incorporating the most up-to-date evidence. This was particularly challenging as data emerging, notably from the staff focus groups, reflected practice that was often not supported by evidence, as previously highlighted. This has been seen in other examples of practice in care homes (e.g. person-centred interventions in care homes). Fossey et al. 85 found that despite a range of evidence-based support materials to promote person-centred care, many interventions that were being employed did not meet recognised quality standards, and few had been evaluated in trials. In the management of residents with dementia who displayed symptoms of agitation, the role of non-pharmacological interventions was advocated based on evidence, but for consistent and long-term implementation, staff training was required. 86 Training has been identified as a means by which care home staff can become empowered in their roles, to facilitate clinical reasoning and critical thinking. 87 Therefore, as part of this phase of the project, we developed a comprehensive training programme that would suit a range of staff and provide a number of modes of delivery.
Although conventional in format (a presentation and study manual), we tried to make the material as interactive as possible. Evidence underpinning the content of the algorithm was incorporated into slides and text, highlighting the key points and where referral to a GP would be required. Case scenarios were also included and would provide staff with an opportunity to compare and contrast practice with and without the use of the decision-making algorithm, and with and without the use of the SBAR tool. In order to facilitate as many staff as possible, other modes of delivery of the presentation were also made available. Access to up-to-date and easily accessible information was found to be important in supporting hospital-based nurses in antimicrobial stewardship programmes,4,88 and required the development of a specific app (application). We supplied the training material in a number of formats to facilitate access, and staff were also given a copy of the study handbook.
In the original submission to NIHR, we had planned to involve GPs in intervention implementation. However, based on the advice of the independent members of the SSC, we did not proceed with this. The SSC were of the view that training GPs and care home staff would have been overly complicated in terms of intervention delivery. We consider that this was an appropriate course of action for this study. The focus of the study was on the care home and its staff, as staff instigate contact with a practice when an infection was suspected. 4 We are aware that GPs currently issue most prescriptions for residents within the care home settings and would be logical targets for an intervention. GPs did have an opportunity to contribute their views on the decision-making algorithm, and were informed that the study would be taking place in homes to which they provided care. However, as reported in the process evaluation and the survey to care homes (described in Chapters 6 and 7, respectively), staff thought that GPs and, indeed, family members should be involved in the training aspect of a future study. How this would be implemented would need to be carefully considered, in view of the scale of a larger project and the time and resources required.
Implementation
The implementation of the intervention included monitoring training attendance and the use of the decision-making algorithm. In addition, we also set out to collect data pertaining to a number of key outcomes, notably the acceptability of the intervention in terms of recruitment and delivery of training, feasibility of data collection from a variety of sources, the feasibility of measuring appropriateness of prescribing and a comprehensive overview of the implementation of the intervention. We achieved many of these outcomes, but there is still uncertainty regarding data collection from a variety of sources, notably from administrative databases, and the feasibility of measuring appropriateness of prescribing.
The training was well attended, with a total of 87 staff from the six care homes receiving training from the REACH team, which delivered 21 training sessions over 35 hours. Providing the training in the alternative format of a DVD was useful for those staff who were unable to attend scheduled sessions in the homes, and this was overseen by the REACH Champions.
Data collection from various sources
The research team was able to collect basic demographic data pertaining to care homes, and this was relatively straightforward to do. However, we also asked staff to collect data in relation to the use of the decision-making algorithm and use of health-care services; in the case of the former, we were interested in how often the decision-making algorithm was used, actions taken and if not used, why it was not used. First, we obtained useful process information but recognise that this was a burden on staff; this was identified in the process evaluation (see Chapter 6). Second, we also asked staff to collect data on the use of health services to help inform the design of a cost-effectiveness analysis that would be part of a larger study. Again, this represented a burden on staff and we had hoped that using administrative data would negate the need for staff collecting such data (see later in this section). For example, most outpatient visits were not associated with infections, so it is unlikely that collecting such data in the future would be useful. However, a useful output from this activity was the involvement of staff in helping revise the data-collection forms. Staff insight in terms of the format of the form and terminology to guide data collection was invaluable.
We were able to access antimicrobial dispensing data from community pharmacies or dispensing practices as we had planned to do. We attempted to standardise the format of the data by providing pharmacies/dispensaries with a standard operating procedure to follow, but because of the differences in some of the systems used, there were still some variations in the format of presentation. In some cases, it was not possible for the data to be filtered to provide antimicrobial data only; therefore, for some care homes, all data pertaining to residents were supplied and these were reviewed by the chief investigator (a qualified pharmacist) to remove any irrelevant medicines. The data were then converted to DDDs. We also calculated the total number of prescriptions for antimicrobials. Some further rationalisation of the data was undertaken by removing medicines that were not antibacterials (e.g. antifungal agents) and unusual drugs, such as rifaximin (Targaxan), which is indicated for the prevention of recurrent hepatic encephalopathy and would be beyond the scope and focus of the intervention.
Overall, there appeared to be a reduction in the number of prescriptions for antimicrobials and this was also reflected in the DDD calculations, although the differences pre and post intervention were small and do not demonstrate effectiveness. At the care home level, the effect was more variable, although the increase in the number of prescriptions post intervention was small. We were also able to calculate the number of residents who were dispensed antimicrobials pre (12 months) and post intervention, together with the number of prescriptions that were issued per resident (again, pre and post implementation). However, we were unable to collect data on the number of residents who did not receive any prescriptions for antimicrobials. As was noted in Chapter 5, some prescribing may have been for the prophylaxis of UTIs, and our intervention had not been targeted specifically at prophylaxis. This may need to be considered in any future work. We were able to calculate an ICC based on the DDD data.
We have shown that we can extract and analyse dispensing data for residents in care homes. However, what was limiting with respect to these data was the lack of unique resident identifier and the inability to track residents pre and post implementation of the intervention. Because we did not have resident-level data, we were unable to access relevant clinical information that would have allowed us to assess the appropriateness of prescribing. The most frequently prescribed antibiotics for residents in the care homes were trimethoprim and nitrofurantoin, which are first-line choices for UTIs, followed by amoxicillin, which is the first-line treatment for many RTIs [e.g. sinusitis, infective exacerbation of chronic obstructive pulmonary disease (COPD)]. Comparing the antimicrobial prescribing in the six homes against local guidelines65,66 suggested that the prescribing was broadly consistent with what was being recommended by these guidelines.
Although we have some proof-of-concept evidence that our intervention does reduce antibiotic prescribing, we are unable to conduct a sample size calculation for any main trial with confidence. In terms of defining a primary outcome for any main trial, it may be that the number of antibiotic prescriptions per resident would be a more suitable primary outcome than DDDs of antibiotics per resident, although the total amount of antibiotic dispensed remains an important measure. Although we can estimate antibiotic prescribing per resident, our denominator is uncertain and we cannot reliably link prescribing back to individuals. To do this, we will need to obtain permission to access resident-specific data. The sample size estimate for any main trial will need to be grounded in the existing literature. It would be feasible once we have relevant permissions to examine historical resident-specific data from participating homes to confirm the appropriateness of sample size early in the lifetime of the study.
This does raise the issue of the most appropriate outcome to be used in a future, larger definitive trial. A systematic review89 has extracted the range of outcomes that have been used in RCTs that have focused on antimicrobial stewardship in care homes for older people, which are shown in Table 32.
| Paper | Outcomes |
|---|---|
| Naughton et al.90 | Primary:
|
| Loeb et al.16 | Primary:
|
| Monette et al.91 | Primary:
|
| Pettersson et al.92 | Primary:
|
| Fleet et al.93 | Primary:
|
There is a debate as to what is the most appropriate outcome measure to use in such studies. In Table 32, the focus was usually on some aspect of antimicrobial/antibiotic use, which was defined in a number of different ways, such as number of prescriptions, a specific type of antibacterial (e.g. quinolones, nitrofurantoin or DDDs). We had selected the latter as our measure of antimicrobial use, as reported in Chapter 5.
In the UK, the Advisory Committee on Antimicrobial Prescribing, Resistance and Healthcare Associated Infection has debated the relative merits of DDDs versus ‘prescriptions’ as numerator units for antibiotic consumption and has agreed on ‘prescriptions’ or ‘prescription items’ as the numerator of choice (Dr Kieran Hand, University of Southampton, 2017, personal communication). This was on the basis of evidence that suggested that the relationship between resistance and the number of antibiotic courses (equating to number of prescriptions or prescription items) was stronger than the relationship between resistance and the antibiotic dose. 94,95 The evidence indicates that prescription items as a numerator is not confounded by variability in dosing conventions or course length and, therefore, more accurately represents numbers of patients exposed to antibiotics. If we were to proceed to a full trial, this would need further consideration.
Accessing data from the administrative data sources proved to be particularly challenging, as was reported in Chapter 5. This was largely attributed to re-organisation within NHS Digital and the inability to conduct searches without access to a resident’s NHS Number, which we did not have. This was also an issue for accessing NI data, which would have been easier if we had been able to access records of the Health and Care Number (equivalent to the NHS Number) for each resident. This challenge raises issues regarding how a definitive trial would be designed. In the feasibility study, we had opted not to seek individual consent from residents because we were testing the feasibility of accessing data at a home level. To proceed with a definitive trial may require individual consent from residents or an ‘opt-in’ approach to participation to allow us to collect resident-level data. We recognise that this will be time-consuming and potentially resource-intensive, but this would appear to be the most practical approach to data collection. One of the co-investigators who worked on this present study has been involved in other research in which an ‘opt-out’ approach has been adopted for seeking consent (i.e. data will be used unless there is an instruction that the participant wishes to actively ‘opt-out’). The research in question is deemed to be of low risk for participants (Dr Ashley Agus, NICTU, 2018, personal communication). This could be considered for a larger study of this intervention. At present, we cannot be certain of access to large administrative data sources. This will require consideration if we were to proceed to a larger study.
We were able to identify and enumerate the various elements that contributed to the resources used and costs incurred as a result of the intervention. The costing was done from a societal standpoint, to incorporate costs from both the health service and the care homes, which are often considered to sit outside publicly funded services. The mean cost per care home was £1269 (£33 per resident). A full RCT with appropriate embedded economic analysis would be required to confirm if the REACH intervention is cost-effective.
Process evaluation
The process evaluation aims were to comprehensively describe the implementation of this intervention, including facilitators and barriers, and to develop a set of transferable principles regarding the intervention to inform its implementation on a wider scale. Our purpose in this feasibility study was to generate data that would help us decide if we should proceed to a larger randomised study. Indicators for success or failure in this regard related to our ability to recruit care homes (discussed in Chapter 3), engage with and train staff in the homes, implement an intervention and collect data. Process evaluation results, in general, reveal that we were successful in meeting these indicators. In Chapter 3, we demonstrated that we recruited the requisite number of care homes in both NI and England. The care homes were in various settings, both rural and urban, with residents reflecting the general mix of residents in care homes across NI and England. 96
We also delivered a training package to a substantial number of the key staff in these homes and provided information about AMR and possible antimicrobial overuse in an older person care setting. This also provided an opportunity to introduce them to the updated and refined decision-making algorithm, which was then used in the implementation phase of the study. Feedback on the training shows that it was generally well received. A larger study of longer duration may require more frequent training to ensure continued engagement with the use of the decision-making algorithm.
The results of the normalization process theory analysis gave us an interesting insight into the implementation of the decision-making algorithm into practice over a 6-month period.
In the construct ‘making sense’ (coherence), the findings demonstrated a range of views. Pre-intervention interviews with relatives and some of the care staff reflect the findings of a number of recent publications, with some participants not understanding the problem (AMR) and others being very knowledgeable. 97 This suggests that more work may need to be done within the homes and with relatives to raise awareness and knowledge. Interviews post intervention revealed that staff were more aware of the issues around AMR and could see that there was a need to ensure that GPs prescribed an antimicrobial only when it was needed. They also noted that the decision-making algorithm was useful in the care home but they were unsure if it would change how GPs prescribed.
The analysis revealed that ‘engagement and commitment’ (cognitive participation) was generally high. Care home managers felt that being involved helped to empower the staff to increase their knowledge for the benefit of the residents. Empowerment is the process of enabling others to do something, to make them feel free to act on their own judgement and to trust their own decisions. 98 Empowerment contributes to each carer’s sense of worth and inspires greater aspirations. 99,100 A number of previous studies have been undertaken on ‘structural empowerment’, defined as the presence of social structures in the workplace that enable people to accomplish their work in meaningful ways. 98,101 It appears from our results that the intervention conferred a sense of empowerment among the carers and nurses. Evidence shows that a sense of empowerment can facilitate the implementation of beneficial practices. 102–104
Appointing a REACH Champion in each care home was a challenge for two of our six care homes, but, generally, our analysis suggested that REACH Champions were very important in helping to ensure the engagement and commitment of the staff in the homes. Little is specifically reported about the effectiveness of such champions in supporting the implementation of study interventions, but they are seen as invaluable in some research settings. 105 Problems were also reported regarding having time to engage in the research activities (e.g. completing paperwork) when staff were very busy with day-to-day care activities. This burden will need to be reviewed for any future study. Our training was seen to be very useful for most staff in terms of securing engagement, but some nurses did suggest that they were aware of all of the issues and others felt that we should not have included junior care staff in our training. Although acknowledging these views, we stand by our inclusive approach as we clearly demonstrated the need for increased knowledge of AMR. Including junior staff who cared for residents in the training appeared to raise their awareness to alert a senior member of staff if they observed a significant change in a resident’s status, and this was demonstrated in the implementation phase. Training in care homes is challenging but can bring about changes to practice. 106
In ‘facilitating the use of the REACH intervention’ (collective action), our results were somewhat inconsistent. There was evidence that many staff were implementing the decision-making algorithm but others were not. In some cases, staff forgot to refer to it; in others, staff reported not having time to use it. In the case of the latter, this was partly related to staff having to complete additional forms if they used the decision-making algorithm. It seems that REACH provided access to learning for the teams within the care homes, but implementation was mediated by the context of working in a care home environment, as seen in other studies. 107 Finding the time to participate in an intervention and complete associated paperwork is a challenge that is encountered in many studies; other studies have also found that such tasks conflict with day-to-day resident care activities. 108
The staff were very willing to provide feedback on the decision-making algorithm, particularly with regard to some of the symptoms that had been included following the adaptation and development phase. Issues included not seeing or being able to identify particular symptoms that we had identified as evidence based. Some staff also noted that their own knowledge of the resident (through close contact from delivering care) was important and, therefore, they did not use the decision-making algorithm. Interestingly, a study undertaken in a nursing home setting109 concurs with our finding that staff knowledge of residents was the main driver for decisions about their care; research evidence did not feature highly. If, as some of the findings suggest, our evidence-based tool was being ignored because it did not reflect staff’s everyday practice, we may need to consider how best to embed and convey evidence-based practice in training. The issue is complex as staff do know their residents well and this is invaluable in recognising the onset of new symptoms, but this should not preclude the use of the decision-making algorithm in making an informed decision. We also have to be cognisant of the comments provided by staff relating to the evidence-based symptoms within the decision-making algorithm that were seldom seen or difficult to observe in a care home population.
Some staff seemed to be a little confused regarding how the decision-making algorithm was to be used. There appeared to be a number of staff who did not realise that any member of staff could be involved with the recognition that a resident was unwell (through the use of the ‘trigger’ symptoms at the start of the decision-making algorithm). Instead, they considered that subsequent actions were dependent on their role, with the more junior staff relaying findings to a senior member of staff. In all of the care homes in this study, if a GP contact was indicated, this was initiated by the senior staff (nurse, carer or manager). Some staff noted that working through the decision-making algorithm helped them when communicating with relatives as it was a clear pathway documenting their decision process. However, some staff felt that relatives would overrule decisions they had made. Medical situations involving decisions with relatives about ill residents are very challenging. Trust and confidence can be seriously challenged and conflicts may arise. 110,111 Van Keer et al. 112 suggest that families may lack understanding of a prognosis and have unrealistic expectations, perhaps based on media misinformation. This is a complex issue as conflict between staff and relatives is likely to have an impact on the care of the resident. In terms of testing the REACH intervention further, engaging with family members on the topic of AMR and the use of the decision-making algorithm may improve knowledge and acceptance. For staff, training on how to handle discussions with relatives may also be useful.
Our normalization process theory analysis of the ‘value of the intervention’ (reflexive monitoring) reflected a more negative outcome than in the other constructs. Although most participants believed that it was a good idea, operationalising it was more problematic. Some reported that junior staff lacked the skills and competence to use the tool and some nursing staff considered that it was beyond the accepted role of some levels of staff. Again, the workload issue of time associated with intervention implementation and documentation was highlighted. There was, however, evidence that some staff fully understood how to implement the decision-making algorithm and how and when to refer to a senior member of staff. The problems of task orientation and a strict division of labour are increasing concerns for many workplaces, including nursing homes. 113 Task orientation can be defined as work that is highly focused and prioritised on the completion of tasks, without adequately considering the outcomes of the tasks completed. 113 For example, in a care home, dining might be completed at a designated time and baths are given when time allows, but these do not necessarily take place in reference to the individual needs of the residents. Rather, these activities take place in accordance with a work schedule that breaks down care into discreet tasks. 114 Task orientation is in marked contrast to relational work. Relational work involves co-operative task sharing, more flexible regulations, limited restrictions on work and increased work autonomy. 115 Relational work is beneficial to workers through stress reduction and beneficial to residents through improvements in their care. 115 Therefore, we need to consider and address these issues before trying to implement REACH on a larger scale.
When presenting normalization process theory, May and Finch21 note that there is an interplay and non-linear relationship between the four constructs. Studies using normalization process theory, when identifying challenges or crucial drivers in the implementation, highlight just one of the constructs. 116–118 Something we did not see in our study was a lack of coherence, which is often cited as an important challenge in the implementation (i.e. the intervention does not make sense or is met with conflicting attitudes). 116,117 In this study, reflexive monitoring highlighted a number of challenges; although REACH was valued by staff, it was not fully implemented. In a feasibility study, this may be expected as the implementation period is short, whereas in other studies, such periods have been longer, allowing for a new practice to be normalised. 97 It has been argued that for practices to become accepted, integrated, and sustained in day-to-day work, they must be experienced as dealing effectively with real everyday problems. 119
Limitations to this study are addressed elsewhere in the report; however, it is important to note here that only five of the six homes fully embraced the implementation of the intervention. The residential home in England experienced a number of problems during the study period, which made it difficult to achieve complete engagement. Staff were trained but they did not record the use of the decision-making algorithm, in contrast to the other homes.
A future study should consider the items in Table 33, which are based on our findings, presented against components of normalization process theory, to help overcome some of the issues identified from this feasibility study. These could help to ensure the success of implementation on a larger scale.
| Normalization process theory component | Facilitator |
|---|---|
| Coherence |
|
| Cognitive participation |
|
| Collective action |
|
| Reflexive monitoring |
|
Care home survey
A postal survey was undertaken with a sample of care homes in NI and the West Midlands to assess the likelihood of care home participation in a future trial evaluating the effectiveness of the REACH intervention. The overall response rate was 23%, but there was a marked difference in the response rates between the NI and West Midlands samples, which were 47% and 12%, respectively. It is unclear why there was this differential as the questionnaire was quite short (seven questions), requiring ‘yes’ or ‘no’ responses, with free-text answers being optional. Therefore, responses should be considered in the light of this overall response rate, recognising that there will be an element of response bias, and that those who did respond are likely to be those who are interested in this topic. Moreover, to some extent, the positive results would support this. It was encouraging to note that those who did respond were generally supportive of a larger study, recognising the importance of the topic. Reflecting some of the findings from the process evaluation, a number of respondents suggested that the training should be extended to GPs and, indeed, family members. Most agreed that the duration of training (2 hours) was a reasonable period of time but highlighted the importance of flexibility in scheduling of training to maximise attendance. Respondents also considered that a ‘decision-making aid’ was useful but highlighted the range of tools that were already being employed within care homes. Overall, 79% of respondents were, in principle, prepared to participate in a future study. This is encouraging, and provides some evidence that we would have a sample from which to recruit to a larger study.
Reflections on patient and public involvement
This was a challenging study and PPI helped us navigate through some of the difficulties, particularly in respect of data collection and its impact on staff.
A group that had been convened in advance of the study advised on the content of study documentation before submission for ethics approval. The input received ensured that the language was clear and unambiguous. We had hoped to be able to involve residents in this process, but this could not be achieved as the Independent Health and Care Providers were unable to identify anyone willing to take part.
Mr Bob Stafford was a member of the research team and actively participated in all meetings. His insight into care home practice helped us approach issues with staff as to how to balance the demands of the study with the immediate demands of everyday work.
In many ways, the participating care home staff acted as research partners, particularly in the adaptation of the intervention and its implementation. It was a challenge in respect of the development of the decision-making algorithm as the evidence did not reflect usual practice. As outlined in the section Process evaluation, data collection was seen as a burden for staff, but some did actively engage in helping to refine the data-collection forms or devised systems that would make aspects of the study more practical for them. These types of innovations are useful when considering how best to engage with care home staff in future studies.
Strengths and limitations of the research
The study has a number of strengths that should be acknowledged:
-
We have developed and updated a decision-making algorithm to help guide care home staff in the management of the three most common infections observed in this environment. The algorithm was generally well received by staff, as was the accompanying training programme.
-
Stakeholder involvement, particularly in the development and updating of the algorithm, was comprehensive and generated rich data and important insights, particularly from care home staff who would use the decision-making algorithm.
-
Staff appeared to recognise the importance of AMR in the context of their everyday work and were enthusiastic about implementation of the intervention.
-
The process evaluation generated a rich and wide-ranging understanding of the facilitators of and barriers to implementation in a busy care home environment.
-
We were able to confirm many aspects of feasibility relating to recruitment, data collection and implementation.
-
The results of the survey to a sample of care homes in NI and England suggests support for a larger study.
However, there are a number of limitations that need to be taken into account when considering our findings:
-
This was a feasibility study and no assessment of efficacy of the intervention can be made.
-
The feasibility study and process evaluation took place in six care homes, with three homes in each jurisdiction. Therefore, all findings must be interpreted in the light of this small sample. It is also likely that a number of these homes were highly motivated to participate, and so cannot be considered representative of UK facilities.
-
Although the process that we undertook to develop and adapt the intervention (decision-making algorithm and training) was extensive and comprehensive, the number of participants in the consensus exercise was small (n = 4). The focus groups and interviews were extremely valuable in generating rich data to contribute to the adaptation process, but caveats associated with qualitative work must be considered (i.e. findings may not be generalisable and, as noted, the participants were drawn from a small number of homes and practices). However, careful consideration was given to reflexivity and standard approaches to data analysis and interpretation.
-
Not all staff attended the training sessions provided in their respective care homes. However, the availability of other modes of delivery, such as the DVD, was useful.
-
Data collection was challenging. The lack of a unique patient identifier prevented the tracking of individual residents and collection of resident-level data. It also prevented us from accessing administrative data, notably in the English context. It was also noted that data collection was perceived to be a burden by staff. We attempted to streamline this as far as possible. We cannot be confident that we have a complete record of the use of the decision-making algorithm. We wanted to collect these data as a measure of process, but in any future larger study, it is unlikely that we would collect such data.
-
Staff struggled with reconciling the evidence presented in the training and the guidance within the decision-making algorithm with usual practice, which led to the algorithm being over-ruled in a number of cases. This applied more often in the management of UTIs. However, staff also reported that the decision-making algorithm broadly reflected practice.
-
General practitioners did not participate in any post-implementation interviews, so we are unable to comment on how they perceived the intervention. They were also not the focus of the intervention (following advice from the independent SSC). We recognise the role that GPs play in the prescribing of antimicrobials, but previous research has shown that care home staff initiate the prescribing process through contact with GPs.
-
The response rate to the survey to gauge interest in a larger study was 23%. Therefore, the findings need to be interpreted carefully.
Implications for practice
This was a feasibility study to assess various elements of research methodology and possible progression to a larger trial, so implications for practice at this stage are somewhat limited. However, the following points are worthy of consideration:
-
Training for care home staff was an important aspect of this feasibility study and would also be a key part of a definitive trial. Being able to integrate training into everyday practice and shift patterns was a challenge in the study, and would also appear to be difficult outside a research context. More generally, care home organisations should consider how best to provide and facilitate training events and opportunities for their staff to ensure that their practice is up to date and evidence based.
-
It was accepted practice in care homes without nursing not to measure temperature; this would have been challenging for the implementation of the intervention. However, we obtained agreement from the management of such care homes to allow us to train staff to undertake this task during the course of the study. Allowing this to be part of everyday practice in care homes without nursing would be beneficial for staff (and indeed residents) outside the research context.
Recommendations for future research
As a result of these findings, we consider that we have achieved many of the objectives that were initially established for this study. We have demonstrated that we can recruit homes, oversee implementation and collect data. However, there are a number of key issues that need to be highlighted to allow a future study to proceed:
-
We need to consider how best to minimise the data-collection burden on care home staff. Over the course of the data collection, forms were refined and it may be possible to further refine, or indeed omit, data collection for some variables. It may also be possible for research staff to undertake data collection (with the appropriate approvals to do so) in consultation with care home staff.
-
There is a need to obtain resident-level data from care homes and other sources. Consideration regarding obtaining individual consent or employing an ‘opt-out’ approach may be the best course of action to obtain the data that would be needed. General use of administrative data sources is being advocated by research funding bodies, but this was very difficult to achieve in this study, as has been previously described.
-
The content and focus of the intervention may need to be reviewed in the light of antimicrobial use for prophylaxis in the case of UTIs, and how evidence-based management of UTIs can be best promoted in UK care homes.
-
Further consideration and guidance should be produced in respect of the most appropriate outcome measure to assess the effects of antimicrobial stewardship interventions, with a focus on a ‘prescribing outcome’.
-
Progressing to a full RCT could be considered, but only with the inclusion of an internal randomised pilot study and the application of robust progression rules. Further consideration should also be given to whether or not GPs should be targets for the intervention and how best to engage all care home staff in the implementation of the intervention approach.
Final conclusions
Based on our findings, we draw the following conclusions:
-
We have demonstrated feasibility in respect of recruitment, data collection and implementation of the intervention, although challenges remain with respect to accessing centralised administrative data.
-
Stakeholder involvement in the adaptation and development of the intervention was challenging, but it was also valuable as it provided an important perspective and may have engendered a sense of ownership of the intervention, particularly among care home staff.
-
We were able to collect dispensing data and health economic data, all of which would be critical for a larger study.
-
The intervention appeared to be broadly acceptable to care home staff, and could be integrated into everyday practice; measurement of temperature in care homes without nursing would need to be considered if a larger study was to be undertaken.
-
A larger definitive trial, using a cluster randomised design, with inclusion of an internal pilot study and appropriate progression criteria may be considered. However, individual resident consent may be the best option in order to obtain the data required.
Acknowledgements
We are extremely grateful for the contribution of all study participants including relatives, care home staff, consensus group participants and GPs. In particular, we are grateful to the REACH Champions for their support throughout the study. We would like to thank members of the SSC for their invaluable contributions: Professor Catherine Sackley, Professor Stephanie Taylor, Dr Kieran Hand and Mr Gordon Kennedy. We would also like to thank Leigh-Ann McCrum, Melinda Hornyak, Nuala Hannaway and Mark Wilson at the NICTU for their contribution to the study.
Contributions of authors
Carmel Hughes (Professor of Primary Care Pharmacy, Queen’s University Belfast) was the chief investigator, led the study design, oversaw the whole study and is lead author of the report.
David Ellard (Principal Research Fellow, Warwick Clinical Trials Unit, University of Warwick) was principal investigator for the study in England. He was involved in the conception and development of the study, was responsible for oversight for the English part of the study and for the process evaluation, contributed significantly to the development of the protocol and the study’s processes and paperwork and was involved in analyses and interpretation of results. He contributed to the writing of the final report.
Anne Campbell (formerly Research Fellow, Queen’s University Belfast, during the course of the study, subsequently Research Associate, Imperial College London) had day-to-day responsibility for project management in NI and was involved in all aspects of the study. This included recruitment of care homes, recruitment for and conducting of pre- and post-intervention focus groups and interviews, rapid review of the literature, organisation of training of care home staff, observation visits and support of care home staff with all aspects of research activity during implementation, data analysis and administration of the care home survey. She was a co-author of the report.
Rachel Potter (Senior Research Fellow, University of Warwick) had day-to-day responsibility for management of the study in the West Midlands and was involved in all aspects of the study, including conducting the focus groups and interviews, organisation of the training of care home staff, observation visits, support of care home staff with all aspects of research activity, data collection pre, post and throughout the implementation of the intervention, administration of the care home survey and analysis She was a co-author of the report.
Catherine Shaw (Research Fellow, Queen’s University Belfast) conducted rapid review of the literature, updated and expanded the algorithm, developed and delivered training material and was a co-author of the report.
Evie Gardner (Head of Statistics, NI Clinical Trials Unit) was a co-applicant, conducted the statistical analysis and prepared the results for publication, and was a co-author of the report.
Ashley Agus (Health Economist, NI Clinical Trials Unit) was a co-applicant, conducted the economic analysis, the analysis of health service use and prepared the results for publication, and was a co-author of the report.
Dermot O’Reilly (Senior Clinical Lecturer, Queen’s University Belfast) led on the aspects of the study related to the potential use of administrative data in the monitoring of study outcomes and reviewed the final report for critical academic content.
Martin Underwood (Professor, Warwick Clinical Trials Unit, University of Warwick) was part of the team that developed the proposal, he contributed to data collection and analysis and he reviewed the final report for critical academic content.
Mark Loeb (Professor, Infectious Diseases, McMaster University) advised on the conduct of the study.
Bob Stafford (Group Head of Care, Orchard Care Homes) was a co-applicant, provided PPI input from a care home operator’s perspective and was a co-author of the report.
Michael Tunney (Professor of Clinical Pharmacy, Queen’s University Belfast) was a co-applicant, he advised on the conduct of the study and he reviewed the final report for critical academic content.
Publications
Potter R, Campbell A, Ellard DR, Shaw C, Gardner E, Agus A, et al. A multifaceted intervention to reduce antimicrobial prescribing in care homes: a process evaluation of a non-randomised feasibility study. BMJ Open 2019;9:e032185.
Hughes CM, Ellard DR, Campbell A, Potter R, Shaw C, Gardner E, et al. Developing evidence-based guidance for assessment of suspected infections in care home residents. BMC Geriatr 2020;20:59.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Hughes CM, Tunney MM. Improving prescribing of antibiotics in long-term care: resistant to change?. JAMA Intern Med 2013;173:682-3. https://doi.org/10.1001/jamainternmed.2013.4077.
- Gilpin DF, Tunney MM, Baldwin N, Hughes C. Long-term care for older adults: reservoirs of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci. Geriatrics and Aging 2009;12:483-90.
- Yang M, Vleck K, Bellantoni M, Sood G. Telephone survey of infection-control and antibiotic stewardship practices in long-term care facilities in Maryland. J Am Med Dir Assoc 2016;17:491-4. https://doi.org/10.1016/j.jamda.2015.12.018.
- Schweizer AK, Hughes CM, Macauley DC, O’Neill C. Managing urinary tract infections in nursing homes: a qualitative assessment. Pharm World Sci 2005;27:159-65. https://doi.org/10.1007/s11096-005-1191-5.
- McClean P, Hughes C, Tunney M, Goossens H, Jans B. European Surveillance of Antimicrobial Consumption (ESAC) nursing home project group . Antimicrobial prescribing in European nursing homes. J Antimicrob Chemother 2011;66:1609-16. https://doi.org/10.1093/jac/dkr183.
- McClean P, Tunney M, Gilpin D, Parsons C, Hughes C. Antimicrobial prescribing in residential homes. J Antimicrob Chemother 2012;67:1781-90. https://doi.org/10.1093/jac/dks085.
- Davies S. Annual Report of the Chief Medical Office, Volume Two, 2011. Infections and the Rise of Antimicrobial Resistance 2013.
- Sackley CM, van den Berg ME, Lett K, Patel S, Hollands K, Wright CC, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: a cluster randomised controlled trial. BMJ 2009;339. https://doi.org/10.1136/bmj.b3123.
- Department of Health and Social Care, Department of Environment, Food and Rural Affairs . UK Five Year Antimicrobial Strategy 2013–2018 2013.
- Department of Health, Social Services and Public Safety . Strategy for Tackling Antimicrobial Resistance 2012. www.health-ni.gov.uk/publications/strategy-and-guidelines-tackling-antimicrobial-resistance (accessed 12 April 2018).
- Morrill HJ, Caffrey AR, Jump RL, Dosa D, LaPlante KL. Antimicrobial stewardship in long-term care facilities: a call to action. J Am Med Dir Assoc 2016;17:183.e1-16. https://doi.org/10.1016/j.jamda.2015.11.013.
- Thompson ND, LaPlace L, Epstein L, Thompson D, Dumyati G, Concannon C, et al. Prevalence of antimicrobial use and opportunities to improve prescribing practices in U.S. nursing homes. J Am Med Dir Assoc 2016;17:1151-3. https://doi.org/10.1016/j.jamda.2016.08.013.
- Scales K, Zimmerman S, Reed D, Beeber AS, Kistler CE, Preisser JS, et al. Nurse and medical provider perspectives on antibiotic stewardship in nursing homes. J Am Geriatr Soc 2017;65:165-71. https://doi.org/10.1111/jgs.14504.
- Kistler CE, Zimmerman S, Scales K, Ward K, Weber D, Reed D, et al. The antibiotic prescribing pathway for presumed urinary tract infections in nursing home residents. J Am Geriatr Soc 2017;65:1719-25. https://doi.org/10.1111/jgs.14857.
- Crnich CJ, Jump R, Trautner B, Sloane PD, Mody L. Optimizing antibiotic stewardship in nursing homes: a narrative review and recommendations for improvement. Drugs Aging 2015;32:699-716. https://doi.org/10.1007/s40266-015-0292-7.
- Loeb M, Brazil K, Lohfeld L, McGeer A, Simor A, Stevenson K, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ 2005;331. https://doi.org/10.1136/bmj.38602.586343.55.
- McClean P. Antimicrobial Prescribing and Infection Control in Care Homes for Older People 2012.
- US National Library of Medicine . Trial to Reduce Antimicrobial Use in Nursing Home Residents With Alzheimer’s Disease and Other Dementias (TRAIN-AD) n.d. https://clinicaltrials.gov/ct2/show/NCT03244917 (accessed 27 March 2018).
- Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0150205.
- Medical Research Council . Developing and Evaluating Complex Interventions: New Guidance 2015. www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/ (accessed 13 January 2015).
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- Scantlebury A, Sheard L, Watt I, Cairns P, Wright J, Adamson J. Exploring the implementation of an electronic record into a maternity unit: a qualitative study using normalisation process theory. BMC Med Inform Decis Mak 2017;17. https://doi.org/10.1186/s12911-016-0406-0.
- Tazzyman A, Ferguson J, Hillier C, Boyd A, Tredinnick-Rowe J, Archer J, et al. The implementation of medical revalidation: an assessment using normalisation process theory. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2710-5.
- Volker N, Williams LT, Davey RC, Cochrane T, Clancy T. Implementation of cardiovascular disease prevention in primary health care: enhancing understanding using normalisation process theory. BMC Fam Pract 2017;18. https://doi.org/10.1186/s12875-017-0580-x.
- May C, Rapley T, Mair FS, Treweek S, Murray E, Ballini L, et al. Normalization Process Theory On-Line Users’ Manual, Toolkit and NoMAD Instrument 2015. www.normalizationprocess.org (accessed 16 November 2017).
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: Sage Publications Ltd; 2006.
- Underwood M, Lamb SE, Eldridge S, Sheehan B, Slowther AM, Spencer A, et al. Exercise for depression in elderly residents of care homes: a cluster-randomised controlled trial. Lancet 2013;382:41-9. https://doi.org/10.1016/S0140-6736(13)60649-2.
- Ellard DR, Thorogood M, Underwood M, Seale C, Taylor SJ. Whole home exercise intervention for depression in older care home residents (the OPERA study): a process evaluation. BMC Med 2014;12. https://doi.org/10.1186/1741-7015-12-1.
- National Institute for Health Research . Enabling Research In Care Homes 2018. https://enrich.nihr.ac.uk/ (accessed 3 April 2018).
- Hughes C, Tunney M, Bradley MC. Infection control strategies for preventing the transmission of meticillin-resistant Staphylococcus aureus (MRSA) in nursing homes for older people. Cochrane Database Syst Rev 2013;11. https://doi.org/10.1002/14651858.CD006354.pub4.
- Loeb M, Bentley DW, Bradley S, Crossley K, Garibaldi R, Gantz N, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: results of a consensus conference. Infect Control Hosp Epidemiol 2001;22:120-4. https://doi.org/10.1086/501875.
- Van de Ven AH, Delbeq AL. The effectiveness of nominal, delphi and interacting group decisionmaking processes. Acad Manage J 1974;17:605-21. https://doi.org/10.2307/255641.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2. https://doi.org/10.3310/hta2030.
- Hughes CM, Ellard DR, Campbell A, Potter R, Shaw C, Gardner E, et al. Developing evidence-based guidance for assessment of suspected infections in care home residents. BMC Geriatr 2020;20.
- Tonkiss F, Seale C. Researching Society and Culture. London: Sage; 2012.
- Lyman F, Anderson AS. Mainstreaming Digest. College Park, MD: University of Maryland College of Education; 1981.
- Lievesley N, Crosby G, Bowman C. The Changing Role of Care Homes. London: BUPA and Centre for Policy on Ageing; 2011.
- Thompson J, Cook G, Duschinsky R. ‘I feel like a salesperson’: the effect of multiple-source care funding on the experiences and views of nursing home nurses in England. Nurs Inq 2015;22:168-77. https://doi.org/10.1111/nin.12066.
- NHS Institute for Improvement and Innovation . SBAR Communication Tool – Situation, Background, Assessment, Recommendation 2018. https://improvement.nhs.uk/resources/sbar-communication-tool/ (accessed 22 March 2018).
- Falcone M, Blasi F, Menichetti F, Pea F, Violi F. Pneumonia in frail older patients: an up to date. Intern Emerg Med 2012;7:415-24. https://doi.org/10.1007/s11739-012-0796-7.
- Juthani-Mehta M, Quagliarello V, Perrelli E, Towle V, Van Ness PH, Tinetti M. Clinical features to identify urinary tract infection in nursing home residents: a cohort study. J Am Geriatr Soc 2009;57:963-70. https://doi.org/10.1111/j.1532-5415.2009.02227.x.
- SIGN . Management of Suspected Bacterial Urinary Tract Infection in Adults 2012. www.sign.ac.uk/sign-88-management-of-suspected-bacterial-urinary-tract-infection-in-adults.html (accessed 3 April 2018).
- Stone ND, Ashraf MS, Calder J, Crnich CJ, Crossley K, Drinka PJ, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol 2012;33:965-77. https://doi.org/10.1086/667743.
- McGeer A, Campbell B, Emori TG, Hierholzer WJ, Jackson MM, Nicolle LE, et al. Definitions of infection surveillance in long-term care facilities. Am J Infect Control 1991;19:1-7. https://doi.org/10.1016/0196-6553(91)90154-5.
- D’Agata E, Loeb MB, Mitchell SL. Challenges in assessing nursing home residents with advanced dementia for suspected urinary tract infections. J Am Geriatr Soc 2013;61:62-6. https://doi.org/10.1111/jgs.12070.
- Rowe TA, Juthani-Mehta M. Urinary tract infection in older adults. J Aging Health 2013;9. https://doi.org/10.2217/ahe.13.38.
- British Thoracic Society and SIGN . British Guideline on the Management of Asthma 2016. www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/ (accessed 3 April 2018).
- Sund-Levander M, Forsberg C, Wahren LK. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand J Caring Sci 2002;16:122-8. https://doi.org/10.1046/j.1471-6712.2002.00069.x.
- Waalen J, Buxbaum JN. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J Gerontol A Biol Sci Med Sci 2011;66:487-92. https://doi.org/10.1093/gerona/glr001.
- Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis 2004;4:112-24. https://doi.org/10.1016/S1473-3099(04)00931-4.
- Faverio P, Aliberti S, Bellelli G, Suigo G, Lonni S, Pesci A, et al. The management of community-acquired pneumonia in the elderly. Eur J Intern Med 2014;25:312-19. https://doi.org/10.1016/j.ejim.2013.12.001.
- British National Formulary 67. London: BMJ Group and Pharmaceutical Press; 2014.
- Health and Social Care Business Services Organisation . Honest Broker Service 2018. www.hscbusiness.hscni.net/services/2454.htm (accessed 3 April 2018).
- Maguire A, Hughes C, Cardwell C, O’Reilly D. Psychotropic medications and the transition into care: a national data linkage study. J Am Geriatr Soc 2013;61:215-21. https://doi.org/10.1111/jgs.12101.
- Burton JK, Guthrie B. Identifying who lives in a care home – a challenge to be conquered. Age Ageing 2018;47:322-3. https://doi.org/10.1093/ageing/afx200.
- Housley G, Lewis S, Usman A, Gordon AL, Shaw DE. Accurate identification of hospital admissions from care homes; development and validation of an automated algorithm. Age Ageing 2017;47:387-91. https://doi.org/10.1093/ageing/afx182.
- Sumnall H, Agus A, Cole J, Doherty P, Foxcroft D, Harvey S, et al. Steps Towards Alcohol Misuse Prevention Programme (STAMPP): a school- and community-based cluster randomised controlled trial. Public Health Res 2017;5. https://doi.org/10.3310/phr05020.
- Lohan M, Aventin A, Maguire L, Curran R, McDowell C, Agus A, et al. Increasing boys’ and girls’ intentions to avoid teenage pregnancy: a cluster randomised controlled feasibility trial of an interactive video drama-based intervention in post-primary schools in Northern Ireland. Public Health Res 2017;5. https://doi.org/10.3310/phr05010.
- Hollingworth W, Cohen D, Hawkins J, Hughes RA, Moore LA, Holliday JC, et al. Reducing smoking in adolescents: cost-effectiveness results from the cluster randomized ASSIST (A Stop Smoking In Schools Trial). Nicotine Tob Res 2012;14:161-8. https://doi.org/10.1093/ntr/ntr155.
- Mytton J, Ingram J, Manns S, Stevens T, Mulvaney C, Blair P, et al. The feasibility of using a parenting programme for the prevention of unintentional home injuries in the under-fives: a cluster randomised controlled trial. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18030.
- Curtis LB, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Skills for Care . National Minimum Dataset for Social Care 2017. www.nmds-sc-online.org.uk/content/About.aspx (accessed 3 April 2018).
- World Health Organization Collaborating Centre for Drug Statistics . ATC/DDD/Index/2018 n.d. www.whocc.no/atc_ddd_index/ (accessed 3 April 2018).
- NI Management of Infection Guidelines for Primary and Secondary Care. Health and Social Care NI; 2016.
- Munthali PG. Community Antibiotic Guidelines for Common Infections in Adults (2017) 2017. www.coventrywarksapc.nhs.uk/mf.ashx?ID=a7ff5ff9-034a-4d77-afcc-d4a7c2db3ecf (accessed 5 April 2018).
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Steckler A, Linnan L. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Merton R. On Theoretical Sociology: Five Essays Old and New. New York, NY: Free Press; 1967.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- May CR, Mair FS, Dowrick CF, Finch TL. Process evaluation for complex interventions in primary care: understanding trials using the normalization process model. BMC Fam Pract 2007;8. https://doi.org/10.1186/1471-2296-8-42.
- Bryman A. Integrating quantitative and qualitative research: how is it done?. Qual Res 2006;6:97-113. https://doi.org/10.1177/1468794106058877.
- Pope C, Mays N. Reaching the parts other methods cannot reach: an introduction to qualitative methods in health and health services research. BMJ 1995;311:42-5. https://doi.org/10.1136/bmj.311.6996.42.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. https://doi.org/10.1136/bmj.332.7538.413.
- Oakley A, Strange V, Stephenson J, Forrest S, Monteiro H. Evaluating processes: a case study of a randomized controlled trial of sex education. Evaluation 2004;10:440-62. https://doi.org/10.1177/1356389004050220.
- Morgan RJ, Wright MM. In defence of early warning scores. Br J Anaesth 2007;99:747-8. https://doi.org/10.1093/bja/aem286.
- Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care 2003;15:261-6. https://doi.org/10.1093/intqhc/mzg031.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324. https://doi.org/10.1136/bmj.324.7347.1183.
- Patterson SM, Hughes CM, Crealey G, Cardwell C, Lapane KL. An evaluation of an adapted U.S. model of pharmaceutical care to improve psychoactive prescribing for nursing home residents in Northern Ireland (Fleetwood Northern Ireland study). J Am Geriatr Soc 2010;58:44-53. https://doi.org/10.1111/j.1532-5415.2009.02617.x.
- Schweizer AK, Hughes CM. Providing pharmacy services to care homes in Northern Ireland: a survey of community pharmacists’ views. Pharm World Sci 2004;26:346-52. https://doi.org/10.1007/s11096-004-1409-y.
- Midthun SJ, Paur R, Lindseth G. Urinary tract infections. Does the smell really tell?. J Gerontol Nurs 2004;30:4-9. https://doi.org/10.3928/0098-9134-20040601-04.
- Goeman D, King J, Koch S. Development of a model of dementia support and pathway for culturally and linguistically diverse communities using co-creation and participatory action research. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-013064.
- Beck ER, McIlfatrick S, Hasson F, Leavey G. Nursing home manager’s knowledge, attitudes and beliefs about advance care planning for people with dementia in long-term care settings: a cross-sectional survey. J Clin Nurs 2017;26:2633-45. https://doi.org/10.1111/jocn.13690.
- McNamara KP, Breken BD, Alzubaidi HT, Bell JS, Dunbar JA, Walker C, et al. Health professional perspectives on the management of multimorbidity and polypharmacy for older patients in Australia. Age Ageing 2017;46:291-9. https://doi.org/10.1093/ageing/afw200.
- Fossey J, Masson S, Stafford J, Lawrence V, Corbett A, Ballard C. The disconnect between evidence and practice: a systematic review of person-centred interventions and training manuals for care home staff working with people with dementia. Int J Geriatr Psychiatry 2014;29:797-80. https://doi.org/10.1002/gps.4072.
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry 2014;205:436-42. https://doi.org/10.1192/bjp.bp.113.141119.
- Blekken LE, Nakrem S, Gjeilo KH, Norton C, Mørkved S, Vinsnes AG. Feasibility, acceptability, and adherence of two educational programs for care staff concerning nursing home patients’ fecal incontinence: a pilot study preceding a cluster-randomized controlled trial. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0263-8.
- Wentzel J, van Velsen L, van Limburg M, de Jong N, Karreman J, Hendrix R, et al. Participatory eHealth development to support nurses in antimicrobial stewardship. BMC Med Inform Decis Mak 2014;14. https://doi.org/10.1186/1472-6947-14-45.
- Nguyen HQ, Tunney MM, Hughes CM. Interventions to improve antimicrobial stewardship for older people in care homes: a systematic review. Drugs Aging 2019;36:355-69. https://doi.org/10.1007/s40266-019-00637-0.
- Naughton BJ, Mylotte JM, Ramadan F, Karuza J, Priore RL. Antibiotic use, hospital admissions, and mortality before and after implementing guidelines for nursing home-acquired pneumonia. J Am Geriatr Soc 2001;49:1020-4. https://doi.org/10.1046/j.1532-5415.2001.49203.x.
- Monette J, Miller MA, Monette M, Laurier C, Boivin JF, Sourial N, et al. Effect of an educational intervention on optimizing antibiotic prescribing in long-term care facilities. J Am Geriatr Soc 2007;55:1231-5. https://doi.org/10.1111/j.1532-5415.2007.01250.x.
- Pettersson E, Vernby A, Mölstad S, Lundborg CS. Can a multifaceted educational intervention targeting both nurses and physicians change the prescribing of antibiotics to nursing home residents? A cluster randomized controlled trial. J Antimicrob Chemother 2011;66:2659-66. https://doi.org/10.1093/jac/dkr312.
- Fleet E, Gopal Rao G, Patel B, Cookson B, Charlett A, Bowman C, et al. Impact of implementation of a novel antimicrobial stewardship tool on antibiotic use in nursing homes: a prospective cluster randomized control pilot study. J Antimicrob Chemother 2014;69:2265-73. https://doi.org/10.1093/jac/dku115.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340. https://doi.org/10.1136/bmj.c2096.
- Opatowski L, Mandel J, Varon E, Boëlle PY, Temime L, Guillemot D. Antibiotic dose impact on resistance selection in the community: a mathematical model of beta-lactams and Streptococcus pneumoniae dynamics. Antimicrob Agents Chemother 2010;54:2330-7. https://doi.org/10.1128/AAC.00331-09.
- Laing W. Care of Elderly People UK Market Survey 2012 13 Report 2013.
- McCullough AR, Parekh S, Rathbone J, Del Mar CB, Hoffmann TC. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother 2016;71:27-33. https://doi.org/10.1093/jac/dkv310.
- Laschinger HK, Finegan J, Shamian J. The impact of workplace empowerment, organizational trust on staff nurses’ work satisfaction and organizational commitment. Health Care Manage Rev 2001;26:7-23. https://doi.org/10.1097/00004010-200107000-00002.
- Covey SR. Principle-Centred Leadership. New York, NY: Simon & Schuster; 1991.
- Spence Laschinger HK. Effect of empowerment on professional practice environments, work satisfaction, and patient care quality: further testing the Nursing Worklife Model. J Nurs Care Qual 2008;23:322-30. https://doi.org/10.1097/01.NCQ.0000318028.67910.6b.
- Manojlovich M, Spence Laschinger HK. The relationship of empowerment and selected personality characteristics to nursing job satisfaction. J Nurs Adm 2002;32:586-95. https://doi.org/10.1097/00005110-200211000-00006.
- Anthony MK, Standing TS, Glick J, Duffy M, Paschall F, Sauer MR, et al. Leadership and nurse retention: the pivotal role of nurse managers. J Nurs Adm 2005;35:146-55. https://doi.org/10.1097/00005110-200503000-00008.
- Rokstad AM, Vatne S, Engedal K, Selbæk G. The role of leadership in the implementation of person-centred care using Dementia Care Mapping: a study in three nursing homes. J Nurs Manag 2015;23:15-26. https://doi.org/10.1111/jonm.12072.
- Killett A, Burns D, Kelly F, Brooker D, Bowes A, La Fontaine J, et al. Digging deep: how organisational culture affects care home residents’ experiences. Ageing Soc 2014;36:160-88. https://doi.org/10.1017/S0144686X14001111.
- Oduola S, Wykes T, Robotham D, Craig TKJ. What is the impact of research champions on integrating research in mental health clinical practice? A quasi-experimental study in South London, UK. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016107.
- Low LF, Fletcher J, Goodenough B, Jeon YH, Etherton-Beer C, MacAndrew M, et al. A systematic review of interventions to change staff care practices in order to improve resident outcomes in nursing homes. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0140711.
- Fuller AU, Unwin L, Rainbird H, Fuller A, Munro A. Workplace Learning in Context. London: Routledge; 2004.
- Bridges J, May C, Fuller A, Griffiths P, Wigley W, Gould L, et al. Optimising impact and sustainability: a qualitative process evaluation of a complex intervention targeted at compassionate care. BMJ Qual Saf 2017;26:970-7. https://doi.org/10.1136/bmjqs-2017-006702.
- Perry L, Bellchambers H, Howie A, Moxey A, Parkinson L, Capra S, et al. Examination of the utility of the promoting action on research implementation in health services framework for implementation of evidence based practice in residential aged care settings. J Adv Nurs 2011;67:2139-50. https://doi.org/10.1111/j.1365-2648.2011.05655.x.
- Azoulay E, Timsit JF, Sprung CL, Soares M, Rusinová K, Lafabrie A, et al. Prevalence and factors of intensive care unit conflicts: the conflicus study. Am J Respir Crit Care Med 2009;180:853-60. https://doi.org/10.1164/rccm.200810-1614OC.
- Studdert DM, Mello MM, Burns JP, Puopolo AL, Galper BZ, Truog RD, et al. Conflict in the care of patients with prolonged stay in the ICU: types, sources, and predictors. Intensive Care Med 2003;29:1489-97. https://doi.org/10.1007/s00134-003-1853-5.
- Van Keer RL, Deschepper R, Francke AL, Huyghens L, Bilsen J. Conflicts between healthcare professionals and families of a multi-ethnic patient population during critical care: an ethnographic study. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-1158-4.
- Syed I, Daly T, Armstrong P, Lowndes R, Chadoin M, Naidoo V. How do work hierarchies and strict divisions of labour impact care workers’ experiences of health and safety? Case studies of long term care in Toronto. J Nurs Home Res Sci 2016;2:41-9.
- Daly T, Szebehely M. Unheard voices, unmapped terrain: care work in long-term residential care for older people in Canada and Sweden. Int J Soc Welf 2012;21:139-48. https://doi.org/10.1111/j.1468-2397.2011.00806.x.
- Daly T, Struthers J, Müller B, Taylor D, Goldmann M, Doupe M, et al. Prescriptive or interpretive regulation at the frontlines of care work in the ‘Three Worlds’ of Canada, Germany and Norway. Labour 2016;77:37-71. https://doi.org/10.1353/llt.2016.0029.
- Bamford C, Heaven B, May C, Moynihan P. Implementing nutrition guidelines for older people in residential care homes: a qualitative study using Normalization Process Theory. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-106.
- Lloyd A, Joseph-Williams N, Edwards A, Rix A, Elwyn G. Patchy ‘coherence’: using normalization process theory to evaluate a multi-faceted shared decision making implementation program (MAGIC). Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-102.
- Gask L, Bower P, Lovell K, Escott D, Archer J, Gilbody S, et al. What work has to be done to implement collaborative care for depression? Process evaluation of a trial utilizing the Normalization Process Model. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-15.
- Røsstad T, Garåsen H, Steinsbekk A, Håland E, Kristoffersen L, Grimsmo A. Implementing a care pathway for elderly patients, a comparative qualitative process evaluation in primary care. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-0751-1.
Appendix 1 Search strategy for MEDLINE
Database: Ovid MEDLINE(R)
Date range searched: 1946 to April week 3 2016.
Date searched: 19 April 2016.
Search strategy
-
antimicrobial.mp. (96,403)
-
antibiotic.mp. (151,007)
-
antifungal.mp. (58,082)
-
antibacterial.mp. (45,237)
-
1 or 2 or 3 or 4 (308,910)
-
urinary tract infection.mp. (16,191)
-
uti.mp. (5722)
-
respiratory infection.mp. (6430)
-
skin infection.mp. (1520)
-
pneumonia.mp. (116,336)
-
asymptomatic bacteriuria.mp. (1348)
-
cellulitis.mp. (10,150)
-
6 or 7 or 8 or 9 or 10 or 11 or 12 (150,279)
-
criteria.mp. (376,464)
-
guidelines.mp. (263,300)
-
recommendations.mp. (136,786)
-
standards.mp. (128,852)
-
measures.mp. (483,550)
-
principles.mp. (103,717)
-
benchmarks.mp. (3686)
-
advice.mp. (32,797)
-
procedures.mp. (667,184)
-
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 (1,950,459)
-
5 and 13 and 23 (4145)
-
long-term care.mp. (30,424)
-
residential care.mp. (2095)
-
nursing care.mp. (44,503)
-
care home.mp. (1155)
-
nursing home.mp. (16,288)
-
residential home.mp. (245)
-
25 or 26 or 27 or 28 or 29 or 30 (90,348)
-
elderly.mp. (181,994)
-
old.mp. (731,499)
-
old-age.mp. (21,572)
-
aged.mp. (4,361,343)
-
32 or 33 or 34 or 35 (4,825,458)
-
31 and 36 (36,708)
-
24 and 37 (114)
-
antimicrobial.mp. (96,403)
-
antibiotic.mp. or Anti-Bacterial Agents/ (347,706)
-
Anti-Bacterial Agents/ or Antifungal Agents/ or antifungal.mp. (321,324)
-
Anti-Bacterial Agents/ or Anti-Infective Agents/ or antibacterial.mp. (325,160)
-
39 or 40 or 41 or 42 (480,524)
-
urinary tract.mp. or Urinary Tract/ (78,786)
-
urinary tract infection.mp. or Urinary Tract Infections/ (40,697)
-
respiratory.mp. or Respiratory Tract Infections/ (436,116)
-
Skin/ or Skin Diseases, Viral/ or Staphylococcal Skin Infections/ or Skin Diseases, Bacterial/ or Skin Diseases, Infectious/ or skin.mp. (619,278)
-
skin infection.mp. or Staphylococcal Skin Infections/ (3455)
-
Pneumonia, Bacterial/ or Pneumonia, Pneumococcal/ or pneumonia.mp. or Pneumonia/ or Pneumonia, Viral/ or Pneumonia, Staphylococcal/ (116,336)
-
Aged/ or Urinary Tract Infections/ or Bacteriuria/ or Adult/ or asymptomatic
-
bacteriuria.mp. or Pyelonephritis/ or Escherichia coli Infections/ (5,308,045)
-
Cellulitis/ or cellulitis.mp. (10,150)
-
criteria.mp. (376,464)
-
guidelines.mp. or Guideline/ or Practice Guideline/ (278,358)
-
recommendations.mp. (136,786)
-
standards.mp. (128,852)
-
measures.mp. (483,550)
-
principles.mp. (103,717)
-
benchmarks.mp. (3686)
-
advice.mp. (32,797)
-
procedures.mp. or Methods/ (891,899)
-
52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 (2,179,882)
-
44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (6,093,419)
-
long-term care.mp. or Long-Term Care/ (30,424)
-
Homes for the Aged/ or Aged/ or residential care.mp. or Long-Term Care/ or Nursing Homes/ (2,548,076)
-
nursing care.mp. or Nursing Care/ (44,503)
-
care home.mp. or Home Care Services/ (30,242)
-
63 or 64 or 65 or 66 (2,608,042)
-
elderly.mp. or Aged/ (2,555,768)
-
old.mp. (731,499)
-
Aged/ or Aging/ or ‘Aged, 80 and over’/ or old-age.mp. (2,683,502)
-
aged.mp. or ‘Aged, 80 and over’/ or Homes for the Aged/ or Aged/ or Health Services for the Aged/ (4,361,343)
-
68 or 69 or 70 or 71 (4,906,184)
-
43 and 61 and 62 and 67 and 72 (9453)
-
limit 73 to (English language and ‘review articles’ and humans and yr=‘2000 - Current’ and (‘review’ or systematic reviews)) (373)
Appendix 2 The REACH topic guide: pre-implementation care home staff focus group version 1
Housekeeping (15 minutes)
-
Collect consent and arrange for copies to be given to participants (if not already done).
-
Get contact details of participants so that payment can be made (if not already done).
-
Introduce ground rules for focus group regarding (1) practicalities (need to record session, role of researcher in guiding discussion but allowing participants to take up the discussion in their own terms, importance of not talking over one another and encouraging people to voice their opinions) and (2) ethics considerations (seeking agreement regarding voicing disagreements in a reasonable way and maintaining confidentiality outside the group).
Introduction (5 minutes)
-
Introduce study and give very brief overview of decision-making aid and data-collection forms that will be used by staff in the study (mention the training they will receive). Make clear that the decision aid is used to help care home staff decide whether or not to contact the GP when they think a resident might have an infection.
-
Check whether participants have any questions before beginning focus group and recording.
-
Begin recording.
Questions (approximately 70 minutes)
Knowledge of antimicrobial resistance (5–10 minutes)
1. What do you know about antimicrobial resistance?
Prompt (if AMR appears not to be a familiar term).
‘Antimicrobial resistance’ may be too much of a technical term, are terms such as ‘superbugs’ or ‘MRSA’ more familiar to you? Tell me more about what you know about these.
Usual practice (10–15 minutes)
2. Tell me what usually happens when you think a resident might have an infection. Take me through it step by step. (Stress we’d like to know what actually happens rather than what they think should happen and who and what is involved in the process.)
Prompts
2.1. What symptoms or signs make you think a resident might have an infection?
2.2. What do you usually do next? [e.g. take temperature (how – oral/ear/rectal), speak to colleague/GP, consult guidelines, order urine culture, dipstick]
2.3. And then what? Anything else?
2.4. Tell me about any notes you take when you suspect a resident has an infection. What information do you usually record? How do you do this and where do you keep these notes (e.g. hard copies or electronic)? How do you usually hand over these notes?
2.5. What else is important when you’re concerned about whether a resident might have an infection and be prescribed antibiotics or not? (e.g. your knowledge of the resident, pressure from relatives, the GP, care home staff, manager)
Decision aid (approximately 30 minutes)
3. Think–pair–share 1: using the decision aid
-
Give very brief introduction to decision aid.
-
Distribute decision aid and have participants think briefly about on own (1–2 minutes). Ask them to imagine they have a resident with a suspected infection in front of them and to consider how well they think the decision aid would actually work in practice.
-
Allow participants to form groups of 2–3 people to discuss tool (3–4 minutes) (ask them to think about at least one question they’d like to ask, or one comment they’d like to make about it).
-
Re-group and in larger group ask about their question/comment. Then use the following questions to prompt discussion (25 minutes).
Prompts
3.1. Who do you think will use this decision aid?
3.2. Where do you think it will used? What format should we provide it in (e.g. A4 or A3 size laminated poster – where should the decision aid be displayed or kept)?
3.3. Is the tool easy to follow? In what ways?
3.4. Are you confident that using the aid will help decision-making around when to contact the GP and what to say to the GP? In what ways will it help?
3.5. In what particular circumstances do you think it will be easy to use the aid? (e.g. with particular residents, infections, times of day/year, when working with particular staff, with particular GPs, with particular support)? Why is this?
3.6. What’s missing? What’s not needed? What’s confusing? (e.g. symptoms/signs)?
3.7. What concerns do you have about using it?
3.8. In what circumstances do you think the decision aid will be difficult to use or that it might not be used (e.g. with particular residents, infections, times of day/year, when working with particular staff, with particular GPs, with particular support)? Why is this?
3.9. Still thinking about using the aid – who, or what, do you think is essential to help make sure that it will actually work in practice (e.g. training needs, managerial support, support from GPs, residents, families)? Anyone/anything else?
-
You have mentioned X (particular person/need/support raised by participants), tell me more about why X is so important?
-
What difficulties do you see if you do not have X? How would you get around these?
3.10. How different do you think using the aid will be from what you normally do? (e.g. your activities and relationships with others)
3.11. Is there anything else you would like to say about the decision aid?
Data-collection form (approximately 20 minutes)
4. I’ve already asked you about any notes you take when you think a resident might have an infection, but do you usually collect any other information? If so, tell me more about what type of information you collect and how you do that.
Think–pair–share 2: using the decision aid data-collection form
Imagine you have to fill out this form, think about how well this would work in practice.
-
Distribute data-collection form and have participants think briefly about on own (1–2 minutes).
-
Ask participants to form groups of 2–3 to discuss and share thoughts (3–4 minutes).
-
Re-group and use the following questions to prompt discussion in larger group.
Prompts
4.1. Who will complete this form? Will more than one person have to contribute to completing it?
4.2. When and where do you think it will be completed?
4.3. How will you keep track of this form in the midst of all the other forms you have?
4.4. Where will it best be kept before and after it is completed?
4.5. How easy is the form to follow?
4.6. What particular difficulties do you foresee in using the form? Why is this?
-
e.g. with particular residents, infections, busy times of day, when working with particular staff)?
4.7. What changes would you make to make it easier for you to complete or fit in with your usual practice?
4.8. Is there anything else you would like to say about this data-collection form?
Appendix 3 The REACH topic guide: pre-implementation family member focus group version 1
Housekeeping (15 minutes)
-
Collect consent and arrange for copies to be given to participants (if not already done).
-
Get contact details of participants so that payment can be made (if not already done).
-
Introduce ground rules for focus group regarding (1) practicalities (need to record session, role of researcher in guiding discussion but allowing participants to take up the discussion in their own terms, importance of not talking over one another and encouraging people to voice their opinions) and (2) ethics considerations (seeking agreement regarding voicing disagreements in a reasonable way and maintaining confidentiality outside the group).
Introduction (5 minutes)
-
Introduce study background, making clear that the decision aid is used to help care home staff decide whether or not to contact the GP when they think a resident might have an infection.
-
Check whether participants have any questions before beginning focus group and recording.
-
Begin recording.
Questions (70 minutes)
Knowledge of antimicrobial resistance (10 minutes)
1. What do you know about antimicrobial resistance?
Prompt (if AMR appears not to be a familiar term).
‘Antimicrobial resistance’ may be too much of a technical term, are terms such as ‘superbugs’ or ‘MRSA’ more familiar to you? Tell me more about what you know about these.
I’d like to show you a short video which talks about the massive concern about the high level of prescribing of antibiotics, it particularly focuses on the risks to the general population (show first 1.02 minutes of video https://youtu.be/7PhmyNBWGik).
The video gives you an idea of the public health risks from AMR but there are also risks to individuals and these are particularly important for older people living in care homes. Over the past few decades several studies have shown that there is substantial use of antibiotics in care homes but that only between 49–62% is prescribed appropriately. This includes prescriptions for residents who are thought to have a UTI or RTI but who don’t. Older people who are treated with antibiotics are at increased risk of adverse reactions including, for example, Clostridium difficile-associated diarrhoea. What are your views about that?
Usual practice (10 minutes)
2. Thinking about your relatives here at the home, what usually happens when you or the care home staff think they might have an infection?
Prompts
-
How do you become aware that they might have an infection, e.g. do the care home staff talk to you about a possible infection in your relative, or do you feel you can talk to staff if you think your relative has an infection?
-
What actions do you expect staff to take if your relative has a potential infection?
-
What usually happens next? And then what?
3. What are you usually concerned about when you discover your relative might have an infection?
Prompts
-
What concerns do you usually have if your relative is not prescribed an antibiotic?
Decision aid (approximately 30 minutes)
Think–pair–share: the decision aid
-
Give very brief introduction to decision-making aid.
-
Distribute decision-making aid and have participants think briefly about on own (1–2 minutes).
-
Allow participants to form groups of 2–3 people to discuss tool (3–4 minutes) (ask them to think about at least one question they’d like to ask about it, or one comment they’d like to make).
-
Re-group and in larger group ask each small group about their question/comment. Then use the following questions to prompt discussion (25 minutes).
4. What do you think about care home staff using this aid when making decisions about infections in the care home?
5. How different do you think using the aid will be from what staff normally do?
6. What concerns do you have about care home staff using this aid?
Prompt
-
What concerns do you have if your relative is not prescribed an antibiotic by the GP as a result of care home staff using this aid?
7. What do we need to do to deal with your concerns?
8. Finally, and before we bring the discussion to a close, is there anything else you would like to say about the decision-making aid?
Appendix 4 The REACH topic guide: pre-implementation general practitioner interviews version 1
1. Usual practice
1.1. How do you usually find out about a resident of a care home who may have an infection?
1.2. How do you think care home staff currently assess whether a resident has an infection that needs an antibiotic? [Prompt: what signs or symptoms do they prioritise? (e.g. temperature, behaviour change, etc.) What signs or symptoms would you like them to prioritise?]
1.3. How do care home staff usually convey their assessment of the resident to you? [Prompt: do they use any particular method of communicating this assessment to you e.g. SBAR? (Situation: Background: Assessment: Recommendation.)]
1.4. What influence do care home staff or family members have on any decision you might make regarding prescribing antibiotics for residents of care homes?
1.5. What else influences any decision you make regarding prescribing antibiotics for residents of care homes? (Prompt: knowledge of the resident and their history of infection; whether you are able to make a visit to the resident.)
2. Using the decision aid (show aid and let think briefly about it on own)
2.1. Imagine the care home staff have a resident with a suspected infection. How do you think the decision aid will actually work in practice?
2.2. How easy or difficult is it to follow?
2.3. In which ways do you think using the decision aid will help staff decide when to contact the GP?
2.4. In which ways do you think using the decision aid will help staff convey their assessment of the resident to you?
2.5. Which staff do you think would use the aid? (e.g. nurses, senior care assistants, junior care assistants).
2.6. Are there particular circumstances in which you think it will be easier or more difficult to use the tool than others (e.g. with particular residents, infections, times of day/year)? Why?
2.7. Do you foresee any problems in using this tool during an outbreak of a respiratory virus? What changes could we make to the aid to prevent this?
2.8. Is there anything missing? (Prompt: symptoms/signs. Anything confusing?)
2.9. What concerns do you have about care home staff using it?
2.10. How do you think using the decision aid will change how care home staff usually assess whether a resident has an infection that needs an antibiotic?
2.11. Is there anything else you would like to say about the decision-making tool?
Appendix 5 The REACH pre-implementation care home staff and family member focus groups coding frame
| 1. Knowledge of AMR |
| 1.1 Resistance attributed to an individual’s immune system |
| 1.2 Resistance attributed to mutation of bacteria |
| 1.3 Impact of resistance on individuals and population |
| 1.4 Infection control |
| 1.5 Function of antibiotics |
| 1.6 How you get resistant bacteria |
| 1.7 Blame and context for resistance or overprescribing |
| 1.8 No knowledge |
| 1.9 Other |
| 2. Usual practice |
| 2.001 No signs |
| 2.01 Signs – temperature |
| 2.02 Signs – change in behaviour |
| 2.03 Signs – history or prior knowledge of resident |
| 2.04 Signs – vital and other |
| 2.05 Signs – RTI |
| 2.06 Signs – SSTI |
| 2.07 Signs – UTI |
| 2.08 Tests – blood |
| 2.09 Tests – sputum |
| 2.10 Tests – urine |
| 2.11 Actions – GP HCP Interactions |
| 2.12 Actions – monitoring the resident |
| 2.13 Actions – consulting with staff and guidelines |
| 2.14 Special case – palliative care |
| 2.15 Special case – dementia |
| 2.16 Special case – COPD |
| 2.17 Special case – residential home |
| 2.18 Frequency of infections |
| 2.19 Recording information |
| 2.20 Handover |
| 2.21 Family and CHS interaction |
| 2.22 Family concerns or expectations |
| 2.23 Time of noticing suspected infection |
| 2.24 Problems in assessing |
| 2.25 Other |
| 3. Using the decision aid |
| 3.01 Signs – temperature |
| 3.02 Signs – change in behaviour |
| 3.03 Signs – history or prior knowledge of resident |
| 3.04 Signs – vital and other |
| 3.05 Signs – RTI |
| 3.06 Signs – SSTI |
| 3.07 Signs – UTI |
| 3.08 Tests |
| 3.09 Special case – COPD |
| 3.10 Special case – dementia |
| 3.11 Special case – diabetes |
| 3.12 Special case – incontinence |
| 3.13 Special case – residential homes |
| 3.14 Actions – monitoring or providing supportive care |
| 3.15 Actions – GP HCP interaction |
| 3.16 Actions – consulting other staff or guidelines |
| 3.17 Actions – handover |
| 3.18 Comparisons with usual practice |
| 3.19 Ease of following aid |
| 3.20 Format and location of aid |
| 3.21 Who will use the aid |
| 3.22 Concern – if not prescribed an antibiotic |
| 3.23 Concern – blame and accountability |
| 3.24 Concern – need for evidence-based algorithm |
| 3.25 Concern – CHS and family interaction |
| 3.26. When would not use |
| 3.27 Support needed to use aid |
| 3.28 No concerns |
| 3.29 Confidence in using |
| 3.30 Benefits |
| 3.31 Other |
| 4. Using the data-collection form |
| 4.1 Benefits |
| 4.2 Ease of following or completion |
| 4.3 Format |
| 4.4 Potential changes |
| 4.5 What’s confusing |
| 4.6 Where completed and kept |
| 4.7 Who would complete it |
| 4.8 When would it be completed |
| 4.9 Other |
Appendix 6 The REACH pre-implementation general practitioner interviews coding frame
| 1. Usual practice |
| 1.1 How GPs find out about an infection |
| 1.1.1 CHS faxing antibiotic prescription sheet |
| 1.1.2 CHS phoning GP surgery |
| 1.1.3 CHS phoning ‘out of hours’ |
| 1.1.4 CHS requesting GP visit |
| 1.1.5 GPs doing regular rounds of CHs |
| 1.1.6 GPs receiving positive result of MSU |
| 1.2 GPs views of how CHS currently assess a resident |
| 1.2.1 Signs and symptoms GPs would like CHS to prioritise |
| 1.2.2 Signs and symptoms prioritised by CHS |
| 1.2.3 Which CHS usually assess resident |
| 1.3 GPs views of how CHS usually convey assessment |
| 1.3.1 CHS being disjointed or unstructured in conveying |
| 1.3.2 CHS being good at conveying |
| 1.3.3 CHS not using SBAR |
| 1.3.4 GPs requiring CHS to use an antibiotic prescribing sheet |
| 1.3.5 GPs using electronic patient record to complement CHS assessment |
| 1.4 Influences of CHS or families on prescribing decisions |
| 1.4.1 Pressure from CHS |
| 1.4.2 Pressure from families |
| 1.5 Other influences on prescribing |
| 1.5.1 Appropriateness of treating palliative residents |
| 1.5.2 C. diff prevalence and/or quantity of antibiotics recently prescribed |
| 1.5.3 Considering comorbidities, previous history of infection, current signs and symptoms |
| 1.5.4 Local guidelines |
| 1.5.5 Visits to resident |
| 1.5.6 Wanting to keep resident out of hospital |
| 2. Using the decision aid |
| 2.01 How DA envisaged to work in practice |
| 2.01.1 Concerns about how DA works in practice |
| 2.01.1.1 Concern that DA places too high expectation on staff |
| 2.01.1.2 Concerns around assessing residents with dementia |
| 2.01.1.3 Concerns around CHS having good recording and handover skills |
| 2.01.1.4 Concerns around getting uniform approach |
| 2.01.1.5 Concerns around temperature |
| 2.01.2 Cutting down on CHS phone calls to GPs |
| 2.01.3 Depends on culture of home and experience of CHS |
| 2.01.4 Enabling CHS to follow a logical structure to assessing residents |
| 2.01.5 Importance of CHS being able to take temperature |
| 2.02 How easy or difficult is DA to follow |
| 2.02.1 Easy to follow |
| 2.02.2 Potentially some difficulty |
| 2.03 How will DA help staff know when to contact GP |
| 2.03.1 Concerns around how staff will know when to contact GP |
| 2.03.2 Giving a uniform approach and preventing knee jerk calls to GP |
| 2.03.3 Helping staff identify relevant symptoms |
| 2.04 How will DA help staff convey assessment |
| 2.04.1 Helping staff to be more specific and succinct |
| 2.05 Which staff will use DA |
| 2.05.1 Junior and senior staff |
| 2.05.2 Nursing staff in nursing homes |
| 2.06 Particular ease or difficulties of using DA |
| 2.06.1 Difficulties diurnal or seasonal |
| 2.06.2 Difficulties with particular comorbidities |
| 2.06.3 Difficulties with temperature |
| 2.06.4 Ease or difficulties with particular infections |
| 2.06.5 No foreseen difficulties |
| 2.07 Particular problems during outbreak of respiratory virus |
| 2.07.1 Increased workload for CHS in recording demands of DA |
| 2.07.2 Solutions |
| 2.08 What’s missing or confusing |
| 2.08.1 Confusing |
| 2.08.2 Missing |
| 2.09 Concerns about staff using DA |
| 2.09.1 No concerns |
| 2.09.2 Personal or organisational risk of litigation |
| 2.09.3 Practically burdensome for CHS to build DA into daily work |
| 2.09.4 Specific concerns around lack of nursing expertise of residential CHS |
| 2.09.5 Specified time delays in monitoring may cause harm |
| 2.10 Envisaged changes in how staff assess resident |
| 2.10.1 Depending on training provided to residential CHS |
| 2.10.2 Enabling CHS to be more structured in assessing resident and conveying this to GP |
| 2.11 Anything else |
| 2.11.1 Being involved in similar interventions before |
| 2.11.2 Changing format |
| 2.11.3 Differences between nursing and residential |
| 2.11.4 Thinking it useful for GP to have DA |
| 2.11.5 Welcoming the DA |
Appendix 7 Completed COREQ checklist (adaptation of intervention)
| Domain 1: research team and reflexivity | ||
|---|---|---|
| Personal characteristics | ||
| 1. Interviewer/facilitator | Which author(s) conducted the interview or focus group? | One researcher carried out the interviews and focus groups in the West Midlands (RP). In NI, one researcher carried out the interviews (AC) and two researchers carried out the focus groups (AC and CS) |
| 2. Credentials | What were the researcher’s credentials? (e.g. PhD, MD) | AC and RP each have a PhD. CS submitted her PhD within 5 months of the focus groups |
| 3. Occupation | What was their occupation at the time of the study? | AC, CS and RP were research fellows |
| 4. Gender | Was the researcher male or female? | AC, CS and RP were female |
| 5. Experience and training | What experience or training did the researcher have? | AC and RP had both undertaken training in qualitative research methodologies and had previous experience of this methodology |
| Relationship with participants | ||
| 6. Relationship established | Was a relationship established prior to study commencement? | No prior relationship was established between the researchers and participants |
| 7. Participant knowledge of the interviewer | What did the participants know about the researcher? (e.g. personal goals, reasons for doing the research) | Participants knew where the researchers worked and the purpose of the research |
| 8. Interviewer characteristics | What characteristics were reported about the interviewer/facilitator? (e.g. bias, assumptions, reasons and interests in the research topic) | The three researchers had an interest in the research. RP and CS had previously worked in care homes research |
| Domain 2: study design | ||
| Theoretical framework | ||
| 9. Methodological orientation and theory | What methodological orientation was stated to underpin the study? (e.g. grounded theory, discourse analysis, ethnography, phenomenology, content analysis) | The study was underpinned by normalization process theory. Analysis was based on the constant comparison method |
| Participant selection | ||
| 10. Sampling | How were participants selected? (e.g. purposive, convenience, consecutive, snowball) | A purposive sample of care homes (six in total, four with nursing and two without nursing, with three in NI and three in the West Midlands) had been recruited. The researchers contacted the managers of each home and asked for assistance in recruiting care home staff and family members. Researchers also asked care home managers for assistance in identifying GPs who provided care to their residents in each home |
| 11. Method of approach | How were participants approached? (e.g. face to face, telephone, mail, e-mail) | Care home staff, family members and GPs were posted letters of invitation, an information sheet and a consent form. A follow-up telephone call was made to GPs who had been approached |
| 12. Sample size | How many participants were in the study? | Forty-one care home staff took part in six focus groups (n = 4–9 participants), 28 family members took part in six focus groups (n = 4–8 participants) and eight GPs participated in interviews |
| 13. Non-participation | How many people refused to participate or dropped out? Reasons? | Numbers of refusals were not recorded |
| Setting | ||
| 14. Setting of data collection | Where was the data collected? (e.g. home, clinic, workplace) | Focus groups with care homes staff and family members and interviews with staff took place in care homes and interviews with GPs took place in general practices |
| 15. Presence of non-participants | Was anyone else present besides the participants and researchers? | Only the researchers were present |
| 16. Description of sample | What are the important characteristics of the sample? (e.g. demographic data, date) | No other demographic information was collected about participants |
| Data collection | ||
| 17. Interview guide | Were questions, prompts, guides provided by the authors? Was it pilot tested? | Topic guides (for care home staff, family members and GPs) with prompts was developed and used during the sessions. These were extensively reviewed within the research team |
| 18. Repeat interviews | Were repeat interviews carried out? If yes, how many? | No repeat focus group sessions/interviews were required |
| 19. Audio/visual recording | Did the research use audio or visual recording to collect the data? | Interviews and focus group sessions were audio-recorded |
| 20. Field notes | Were field notes made during and/or after the interview or focus group? | No |
| 21. Duration | What was the duration of the interviews or focus group? | Focus group duration ranged from 46 to 71 minutes and interviews ranged in duration from 16 to 31 minutes |
| 22. Data saturation | Was data saturation discussed? | Data saturation was not discussed |
| 23. Transcripts returned | Were transcripts returned to participants for comment and/or correction? | Transcripts were not returned to participants |
| Domain 3: analysis and findings | ||
| Data analysis | ||
| 24. Number of data coders | How many data coders coded the data? | Two researchers (AC and RP) independently coded the data |
| 25. Description of the coding tree | Did authors provide a description of the coding tree? | A coding frame was provided in Appendices 5 and 6 |
| 26. Derivation of themes | Were themes identified in advance or derived from the data? | Themes were derived from the data |
| 27. Software | What software, if applicable, was used to manage the data? | NVivo 10 |
| 28. Participant checking | Did participants provide feedback on the findings? | No |
| Reporting | ||
| 29. Quotations presented | Were participant quotations presented to illustrate the themes/findings? Was each quotation identified? (e.g. participant number) | Quotations have been presented throughout Chapter 4, with participant codes assigned to all participants and used against quotations |
| 30. Data and findings consistent | Was there consistency between the data presented and the findings? |
We endeavoured to report the study findings in a clear, consistent manner in order to accurately reflect the data that have been collected Yes, major themes are clearly presented in Chapter 4, Adaptation of the decision-making algorithm All data relating to the development of the algorithm is presented in section Chapter 4, Adaptation of the decision-making algorithm |
| 31. Clarity of major themes | Were major themes clearly presented in the findings? | |
| 32. Clarity of minor themes | Is there a description of diverse cases or discussion of minor themes? | |
Appendix 8 The REACH post-implementation focus groups with care home staff and interviews with managers and REACH Champions coding frame
| Post-implementation focus groups and interviews |
|---|
| 01. First thoughts |
| 02. Why managers chose to take part |
| 03. Training |
| 04. Study handbook |
| 05. Using the DA |
| Aspects of context (e.g. small, residential or nursing home) |
| Change in residents behaviour box |
| Comparing different infections |
| Continuing to monitor box |
| Ease of following, any changes |
| General |
| Impact on practice |
| Interaction with colleagues |
| Interaction with family |
| Interaction with GPs |
| Out of hours |
| Interaction with residents |
| Location or format of DA |
| Modifying the DA |
| Not using DA |
| Overruling |
| Prior knowledge of resident |
| Residents with dementia |
| RTI box |
| SSTI box |
| Sufficiency of symptoms indicated on DA |
| Temperature box |
| Use by night staff |
| UTI box |
| Who should use the DA |
| 06. Value of and being a REACH Champion |
| 07. Completing paperwork |
| CHSCP & UHS |
| UDAF – changes made or needed |
| UDAF – duplicating for home |
| UDAF – extent of burden of completing forms |
| UDAF – location |
| UDAF – who completes, when and where |
| 08. SBAR |
| 09. Challenges and facilitators to implementing in this and future study |
| Challenges |
| Facilitators |
| 10. Knowledge of AMR and making a difference to it |
| 11. Final thoughts |
| Usual documenting practice |
Appendix 9 The REACH post-implementation care home staff observational data coding frame
| Observations |
|---|
| 0.01. General conversations and observations |
| Barriers around new practice or study |
| Beliefs about the value of the practice or study |
| Facilitators around new practice or study |
| Management of data collection and study documents |
| Method issues |
| Other |
| 0.02. Using DA and completing Using DA form |
| Burden or not of using aid and data collection |
| Burden |
| No burden |
| Chatting with other colleagues |
| Continue to monitor issues |
| Copying UDAF for home |
| Date and time issues |
| Dementia issues |
| Enthusiasm or motivators or their lack |
| Enthusiasm, motivators |
| Lack of enthusiasm or motivators |
| External context |
| Infections – RTI |
| Infections – SSTI |
| Infections – UTI |
| Interactional workability – with others, objects, elements of practice |
| Interactions with family members |
| Interactions with GP |
| Interactions with urine tests |
| With objects |
| Location and format of DA |
| Methods issues |
| Missing data |
| Other |
| ‘Other actions’ |
| Other completion |
| Out of hours |
| Potential adverse event |
| Professional norms and roles |
| REACH changing usual practice or not |
| Remembering and forgetting |
| Resident identifiers |
| Staff handbook |
| Temperature issues |
| Using DA or not |
| DA not used |
| DA used |
| Overruling DA |
| Uncertain use of DA |
| Who completes UDAF and when |
| 0.03. Contacts with health and social care professionals |
| Lack of engagement by staff to complete |
| Methods of collecting data |
| Operationalising new CHSCP |
| Operationalising old CHSCP |
| Operationalising staff produced CHSCP |
| Perceived accuracy of data |
| 0.04. Use of hospital services |
| Methods of collecting data |
| Operationalising various UHS forms |
| 0.05. Routinely recorded data |
| Constraints and facilitators of routine data |
| Data |
| Handover sheets |
| Home diary |
| Other |
| Residents files, care plans, daily evaluation sheets |
| 0.06. Adverse events |
| Linking forms |
| 0.07. SBAR |
| Differences between old and new practice |
| Enthusiasm or its lack |
| Location |
| Operationalising SBAR |
| 0.08. Training |
| Further RC led training |
| Further researcher led training |
| Lack of training regarding data collection |
| Technical issues |
| Training record |
| Training requested for UTI issues |
| 0.09. Study handbook |
| 0.10. Use of catheter |
| 0.12. Handover |
| 0.13. Temperature |
| 0.14. Linking forms |
| 0.15. Questions or actions for follow-up research team |
| 0.16. Times of arrival and departure |
| 0.17. Other |
| Baseline information |
| Definitions |
| Focus groups post implementation |
| NPT |
| Cognitive participation – relational work |
| Activation |
| Initiation and enrolment, buy-in |
| Legitimation |
| Coherence – Sense-making work |
| Communal specification |
| Differentiation |
| Individual specification |
| Internalisation |
| Collective action – operational work |
| Contextual integration |
| Material resources |
| Professional or social norms |
| Interactional workability |
| With colleagues |
| With elements of practice |
| With families |
| With GPs |
| With other objects |
| With study document objects |
| With urine tests |
| Relational integration |
| Skill set workability |
| Reflexive monitoring – appraisal work |
| Communal appraisal |
| Individual appraisal |
| Reconfiguration – plasticity or, and elasticity |
| Systemisation |
| Resources – roles, rules, norms, objects – available to agents |
Appendix 10 Completed COREQ checklist (post implementation)
| Domain 1: research team and reflexivity | ||
|---|---|---|
| Personal characteristics | ||
| 1. Interviewer/facilitator | Which author(s) conducted the interview or focus group? | One researcher carried out the interviews and focus groups in NI (AC) and the West Midlands (RP) |
| 2. Credentials | What were the researcher’s credentials? (e.g. PhD, MD) | AC and RP each have a PhD |
| 3. Occupation | What was their occupation at the time of the study? | AC and RP were research fellows |
| 4. Gender | Was the researcher male or female? | AC and RP were female |
| 5. Experience and training | What experience or training did the researcher have? | AC and RP had both undertaken training in qualitative research methodologies and had previous experience of this methodology |
| Relationship with participants | ||
| 6. Relationship established | Was a relationship established prior to study commencement? | These interviews and focus groups were conducted following the implementation of the intervention. Participants would have known the researchers who had been in regular contact and had visited the homes over the course of the study |
| 7. Participant knowledge of the interviewer | What did the participants know about the researcher? (e.g. personal goals, reasons for doing the research) | Participants knew where the researchers worked and the purpose of the research |
| 8. Interviewer characteristics | What characteristics were reported about the interviewer/facilitator? (e.g. bias, assumptions, reasons and interests in the research topic) | Both researchers had an interest in the research. RP had previously worked in care homes research |
| Domain 2: study design | ||
| Theoretical framework | ||
| 9. Methodological orientation and theory | What methodological orientation was stated to underpin the study? (e.g. grounded theory, discourse analysis, ethnography, phenomenology, content analysis) | The study was underpinned by normalization process theory. Analysis was based on the constant comparison method |
| Participant selection | ||
| 10. Sampling | How were participants selected? (e.g. purposive, convenience, consecutive, snowball) | A purposive sample of care homes (six in total, four with nursing and two without nursing, with three in NI and three in the West Midlands) had been recruited. The researchers contacted the managers of each home and asked for assistance in recruiting care home staff to participate in focus groups following implementation of the intervention. Care home managers and REACH Champions in each home were asked to participate in post-implementation interviews |
| 11. Method of approach | How were participants approached? (e.g. face to face, telephone, mail, e-mail) | Care home staff were provided with letters of invitation, an information sheet and a consent form by the care home managers (supplied by the researchers). The care home managers and REACH Champions were approached in person to participate in interviews |
| 12. Sample size | How many participants were in the study? | Twenty-six care home staff took part in six focus groups (n = 2–6 participants); six REACH Champions and five managers participated in interviews |
| 13. Non-participation | How many people refused to participate or dropped out? Reasons? | Numbers of refusals were not recorded |
| Setting | ||
| 14. Setting of data collection | Where was the data collected? (e.g. home, clinic, workplace) | Focus groups and interviews took place in care homes |
| 15. Presence of non-participants | Was anyone else present besides the participants and researchers? | Only the researchers were present |
| 16. Description of sample | What are the important characteristics of the sample? (e.g. demographic data, date) | No other demographic information was collected about participants |
| Data collection | ||
| 17. Interview guide | Were questions, prompts, guides provided by the authors? Was it pilot tested? | Topic guides with prompts were developed and used during the sessions. These were extensively reviewed within the research team |
| 18. Repeat interviews | Were repeat interviews carried out? If yes, how many? | No repeat focus group sessions/interviews were required |
| 19. Audio/visual recording | Did the research use audio or visual recording to collect the data? | Interviews and focus group sessions were audio-recorded |
| 20. Field notes | Were field notes made during and/or after the interview or focus group? | Field notes were taken over the course of the implementation phase in relation to observations made by the research fellows during their regular visits to the care homes |
| 21. Duration | What was the duration of the interviews or focus group? | Focus group duration ranged from 27 to 61 minutes; interviews with REACH Champions ranged in duration from 26 to 71 minutes, and interviews with managers ranged in duration from 12 to 27 minutes |
| 22. Data saturation | Was data saturation discussed? | Data saturation was not discussed |
| 23. Transcripts returned | Were transcripts returned to participants for comment and/or correction? | Transcripts were not returned to participants |
| Domain 3: analysis and findings | ||
| Data analysis | ||
| 24. Number of data coders | How many data coders coded the data? | Two researchers (AC and RP) independently coded the data |
| 25. Description of the coding tree | Did authors provide a description of the coding tree? | A coding frame was provided in Appendices 8 and 9 |
| 26. Derivation of themes | Were themes identified in advance or derived from the data? | Themes were derived from the data |
| 27. Software | What software, if applicable, was used to manage the data? | NVivo 10 |
| 28. Participant checking | Did participants provide feedback on the findings? | |
| Reporting | ||
| 29. Quotations presented | Were participant quotations presented to illustrate the themes/findings? Was each quotation identified? (e.g. participant number) | Quotations have been presented throughout Chapter 6, with participant codes assigned to all participants and used against quotations |
| 30. Data and findings consistent | Was there consistency between the data presented and the findings? | We endeavoured to report the study findings in a clear, consistent manner in order to accurately reflect the data that have been collected |
| 31. Clarity of major themes | Were major themes clearly presented in the findings? | |
| 32. Clarity of minor themes | Is there a description of diverse cases or discussion of minor themes? | |
List of abbreviations
- AMR
- antimicrobial resistance
- BSO
- Business Services Organisation
- CI
- confidence interval
- CMO
- Chief Medical Officer
- COPD
- chronic obstructive pulmonary disease
- COREQ
- consolidated criteria for reporting qualitative research
- CRF
- case report form
- CRN
- Clinical Research Network
- DDD
- defined daily dose
- DVD
- digital versatile disc
- ENRICH
- Enabling Research In Care Homes
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- ICC
- intraclass correlation coefficient
- NI
- Northern Ireland
- NICE
- National Institute for Health and Care Excellence
- NICTU
- Northern Ireland Clinical Trials Unit
- NIHR
- National Institute for Health Research
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- REACH
- REduce Antimicrobial prescribing in Care Homes
- RTI
- respiratory tract infection
- SBAR
- situation–background–assessment–recommendation
- SIGN
- Scottish Intercollegiate Guidelines Network
- SSC
- Study Steering Committee
- SSTI
- skin and soft tissue infection
- UPRN
- Unique Property Reference Number
- UTI
- urinary tract infection
