Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 15/137/01. The contractual start date was in May 2017. The final report began editorial review in February 2019 and was accepted for publication in November 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Ian D Maidment was a member of the West Midlands National Institute for Health Research (NIHR) Research for Patient Benefit Committee (January 2014 to December 2018). Geoff Wong is Joint Deputy Chair of the NIHR Health Technology Assessment Programme Prioritisation Committee A. Andrew Booth holds several NIHR committee memberships: NIHR Health Services and Delivery Research (HSDR) Funding Committee (2018 to present), Systematic Reviews Programme Advisory Group (2018 to present) and the Complex Reviews Advisory Group (2015 to 2018). He is also co-director of the NIHR HSDR Evidence Synthesis Centre and a newly awarded NIHR Public Health Research Evidence Synthesis Centre. Sylvia Bailey is a member of Pharmacy Research UK Scientific Advisory Panel.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Maidment et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
I didn’t take all [these medications] at once. It’s gradually built up. You wonder where it’s all going to end.
OP29 – older person
I just feel overwhelmed sometimes about making that decision. It just feels too much sometimes.
C32 – informal carer
You’ve attempted to address your responsibility as a clinician with a responsibility of a budget. And you know, the population side of it. But you’ve also done what you think is best for the individual in front of you. That’s the challenge.
P53 – general practitioner
Overview
The number and proportion of older people in the UK population is increasing. 1,2 In terms of their health, many older people are living with more than one long-term condition. 1,3,4 There are challenges in living with multimorbidity, such as reduced quality of life and increasing amounts of time spent engaging with health and care services. 5–9
Older people are taking increasing numbers of medications to treat multimorbidity. 10 There are burdens11–15 and risks8,16 from medication taking and medication management that fall disproportionately on older people. Medication-related adverse events include both medication errors and side effects. 17–20 These events have been estimated to be responsible for 5700 deaths and cost the UK £750M annually. 21 More recently, definitely avoidable adverse drug reactions have been estimated to cost the NHS £98.5M every year and have contributed to 1708 deaths. 22
Burdens and risks also have an impact on informal carers1,2,23–26 and practitioners involved in supporting older people’s health and care. 3,4,6,8,27–29
These circumstances raise significant challenges for older people, informal carers, and health and care practitioners and services.
In light of these challenges, the MEdication Management in Older people: Realist Approaches Based on Literature and Evaluation (MEMORABLE) study seeks to understand how medication management works and to propose interventions that will contribute to making improvements. 30,31
Older people
In the UK in 2010, more than 10 million people were aged > 65 years. 32 According to the Office for National Statistics:2
The UK population is ageing – around 18.2% of the UK population were aged 65 years or over at mid-2017, compared with 15.9% in 2007; this is projected to grow to 20.7% by 2027.
In 2017, one in five people in the UK population was over the age of 65 years and this is expected to increase to one in four by 2037. Longevity is linked to lifestyle improvements as well as advances in technology and health care. 2
Older people’s health: multimorbidity
As they age, some older people are living with multimorbidities that affect their lives. These are conditions that cannot be cured but can be managed with medication and other treatments. 3 In 2011, just over half of those aged > 65 years resident in the community reported having long-term conditions affecting what they do, day to day. 1 Many older people aged ≥ 75 years are living with two or more long-term conditions. 4
There are challenges for older people who are living with multimorbidity and for their families, practitioners and services. These challenges arise from a reduction in quality of life, increasing difficulties with day-to-day activities and more time spent engaging with and being supported by the health and care system. 5–8
These challenges are exacerbated when they are also associated with increasing cognitive impairment or a diagnosis of dementia. 19,33–36
Medication taking among older people: polypharmacy
As a result of more people living with multimorbidity, older people’s medication use including prescribed and over-the-counter treatments has ‘increased dramatically’ over the last 20 years:10
The number of [older] people taking five or more items quadrupled from 12 to 49%, while the proportion of people who did not take any medication has decreased from around 1 in 5 to 1 in 13.
Gao et al. 10
There can be clinical indications for polypharmacy such as in diabetes mellitus or hypertension for example, or among older people with multimorbidity. 3,37 However, there are also risks, including adverse drug reactions involving side effects and drug interactions6 as well as side effects associated and unique to each different medication. 17–19 For a health service that developed around curing acute, single conditions and treatments, multimorbidity and polypharmacy are emerging as areas of concern, with implications for policy and practice. 27,29,38–44
Burdens and risks
Older people experience burden as a result of their experience of ageing, multimorbidity and polypharmacy. 11–15,42 Polypharmacy, intended to assist with the management of symptoms and conditions, also has risks associated with it, such as drug–drug and drug–disease interactions, inappropriate prescribing, risks of non-adherence to complex drug regimens and medication errors. 7,16–19 Patients should be protected from avoidable harm. 45 Nonetheless, harms continue,20,46,47 leading to significant costs. 21
Informal carers and practitioners
Informally, many older people rely on family carers to manage their medications. 48 The number of informal carers is growing and the demands on them are increasing, according to the Office for National Statistics’ report in 2013:1
In 2011, 14 per cent (1.3 million) of the household population aged 65 and over provided unpaid care (this includes: looking after a partner, older parent, or adult child); this included 6.9 per cent who provided 1-19 hours unpaid care a week, 1.8 per cent who provided 20-49 hours of unpaid care a week, and 5.6 per cent who provided 50 hours or more unpaid care a week.
Formally, within health and care, a range of practitioners provide services to support older people along complex care pathways. 4,6,8 They too face a number of challenges; for example, the evidence base for practitioners can be based on evidence for a single diagnosis41 and services can appear fragmented and communication between them difficult. 3,5,6,9,28
The MEMORABLE study
The MEMORABLE study has been designed and carried out in response to the background outlined above. It uses realist methodology49–51 to understand how, why, for whom and in what circumstances medication management works. With that understanding, MEMORABLE then proposes interventions that will contribute to improvements. To achieve these purposes, its aims and objectives are set out below (taken from the protocol for this study). 30
Aim
To use realist synthesis including primary data collection to develop a framework for a novel multidisciplinary, multiagency intervention(s) to improve medication management in older people who have complex medication regimens and are resident in the community.
Objectives
Underpinning the overall aim were three linked objectives. The second and third objectives, in particular, were closely related. Objective 2 was focused on the key principles and underlying mechanisms, whereas objective 3 was more focused on developing an applied intervention based on the findings from earlier objectives:
-
to understand how and why any potentially relevant interventions to optimise medication management work (or do not work) for particular groups of older people in certain circumstances
-
to synthesise the findings from objective 1 into a realist programme theory of an intervention(s) to support older people living in the community to manage their medication
-
to use realist programme theory developed from objective 2 to inform the development of an intervention(s) to assist older people living in the community to manage their medication.
Chapter 2 sets out the methods by which this research has been carried out, from which the findings are reported in Chapter 3.
Chapter 2 Methods
This chapter sets out the way MEMORABLE was carried out. It describes the research groups that contributed to MEMORABLE, provides an overview of the realist research methodology and sets out the work packages and steps followed in this realist research process. Additional MEMORABLE documents are available at www2.aston.ac.uk/lhs/research/memorable (accessed March 2020), including information on those involved in the project and recording how it progressed (e.g. minutes, newsletters).
Research groups/patient and public involvement
The MEMORABLE study was supported by a number of working groups that provided its governance, management and delivery structure. These are listed below in the order in which they were established, along with details of their membership, activities, outputs and contributions to the study as a whole. These groups enhanced the capacity of the staff employed on MEMORABLE, Dr Ian Maidment (the Chief Investigator) and Dr Sally Lawson (the Research Associate) and enriched the range of experience and expertise available from the start to the end of the study.
Co-applicant Group
This was an informal group of five individuals who were invited by the chief investigator to collaborate on the development of the initial research proposal. Support and advice about the need for the research also came from a patient and public involvement (PPI) event in November 2015 run by Dr Maidment and the research’s PPI lead, Mrs S Bailey. The event involved eight older people and their informal carers.
The Co-applicant Group brought together topic, practice, research, information and public participation interests in medication management. Members collaborated on the development and submission of the MEMORABLE proposal to the National Institute for Health Research (NIHR). They generated the initial Protocol31 and MEMORABLE’s first published paper: Developing a framework for a novel multi-disciplinary, multi-agency intervention(s), to improve medication management in community-dwelling older people on complex medication regimens (MEMORABLE) – a realist synthesis. 30 The Co-applicant Group evolved into the Project Group.
Project Group
With an established membership of nine, this group was the monthly reference point for work on MEMORABLE, responsible for overseeing the project and disseminating the research. Members provided expertise in medication management, medicine, nursing, pharmacy, research and information methodology, and PPI. This group was key to maintaining momentum in the research and assuring delivery of the protocol.
Stakeholder Group
This group had a more fluid membership than the Project Group, including experts by experience, practitioners, managers and researchers. It had an independent lead from within the Project Group, who co-chaired it with the Chief Investigator. It met on five occasions to review key outputs from the research. Each meeting lasted for about 1.5 hours. Meetings were scheduled to fit with the evolution of the research and the need to gain diverse input from multiple perspectives. The group provided advice and feedback to the Project Group on the veracity of the emerging evidence, programme theories and proposed interventions, as well as the dissemination strategy. It had an important role in making sure that recommendations from the research were appropriate, practicable and likely to make a difference for older people, informal carers and practitioners.
Research Team
This small team of five included the chief investigator and the research associate. It was one of the key sources of topic, practice, research and information expertise for MEMORABLE. It met five times to consider discussion papers prepared by the research associate. Meetings were usually face to face and lasted for half a day, with follow-up contact by telephone and e-mail, if needed. It was the forum to engage in extended discussion and debate, and to resolve topic and research issues. The team significantly augmented the expertise available to the chief investigator and research associate, and provided detailed support to and scrutiny of the research, its content and outputs.
Patient and public involvement
While this study was being carried out, the Project Group regularly checked what was being done. The group had a PPI lead who was a co-applicant for the study and who contributed to all meetings, and is a co-author of this report. The PPI lead also provided advice on the way MEMORABLE was engaging with older people and informal carers, including advice on the types and the content of information, as well as facilitating links with potential participants in the research. In addition, the Stakeholder Group provided advice on findings and recommendations as these emerged from the study. This group had several older people and informal carers on it, along with people working in health, care and research. Both groups contributed to the dissemination strategy for MEMORABLE. The researchers valued and benefited from people’s expert, experiential input to the many discussions and debates. However, they also acknowledge the difficulties of ensuring that there is PPI ‘representation’ and the need to consider the workload (volume and time scale) that such a complex study can create for a single PPI lead.
Realist methodology
This section sets out some of the principles of realist methodology49,51–54 with a short account of the way that they were applied in MEMORABLE. Work packages and steps in the research process has the more detailed account of how these principles were followed in practice.
Realist approach
This theory-driven approach helps explain the way complex interventions work in real-world situations. It supports the identification of what works, for whom, why and in which circumstances: the methodology’s four ‘W’s. 49–53,55,56 Thus, it enables researchers to address complexity53 by unpacking the context-sensitive mechanisms that are the generative causal processes found within an intervention. 49,51,57 Realist approaches are interested in the behaviours of those involved, from individuals to groups and organisations; the influential factors that bring about, prevent or modify those behaviours; and the outcomes that result, intended and unintended. One of the purposes of this approach is ‘to improve the thinking that goes into service building’. (Reproduced from Pawson et al. 50 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/.)
As medication management can be understood as a complex intervention,58,59 the characteristics and purpose of realist methodology are particularly well aligned to meeting MEMORABLE’s aims and objectives.
Realist review and realist evaluation
Realist methodology has two arms. The first, realist review, explores data from secondary sources such as published studies and reports60 and the second, realist evaluation, engages with primary data. 61 MEMORABLE was set up as a realist synthesis, combining realist review with realist evaluation. In this approach, knowledge from the literature was analysed with the first-hand experiences of those engaged in medication management, augmenting the potential contribution of both data sets to theorising about how this complex intervention works.
Theory in realist methodology
Two levels of theory are associated with the realist approach: programme theory and substantive theory.
Programme theory is generated within a study to explain what the intervention has been set up to do and how that intervention might work. 51,60 Initial or candidate programme theories developed at the start of a realist research project are then confirmed, refined or refuted through iterations of data synthesis. 50,51,53 Finalised, evidence-based programme theories can inform the development of recommendations or specific proposals. These might involve modifying an existing intervention, or perhaps suggesting a new intervention to try to achieve the outcomes that matter, typically by addressing contextual influences.
In addition to the iterative way programme theories evolve, they have another characteristic which is that they are finally formulated at a ‘middle-range’ level of abstraction. 51 This means that the programme theory is specified in a way that permits empirical testing. Both realist reviews and realist evaluations aim to produce middle-range realist programme theories. Being realist in nature contributes to the transferability of theories to other settings in which that same intervention may be delivered, or even to other interventions. This transferability is based on the assumption that in other settings or interventions the same mechanisms may be in operation. This is the way that realist methodology contributes to the accumulation of knowledge. 55,62 Candidate or initial programme theories for MEMORABLE were drafted at the beginning of the research: see Step 4: Candidate programme theories and substantive theory. Final programme theory for a key stage in medication management is contained in Figure 5.
Underpinning the analytical work on programme theories are the context–mechanism–outcome (CMO) configurations. 51,53,56 Generated from the data, they have explanatory potential in their configured format. It is, therefore, important that they are set out and reported in their entirety rather than disaggregated into lists of single components. 63 CMO configurations contain:
-
Contexts – influential factors that activate people’s responses, enabling or inhibiting their decisions and actions. In MEMORABLE, contexts can include older people’s diagnoses or medication, the interpersonal relationships they have with health and care practitioners as well as system factors such as access to services or information.
-
Mechanisms – the vital but often hidden operational ‘cogs’ that turn in response to the influence of contexts. Mechanisms can include the way people feel, think or act in relation to the resources in an intervention. They are activated by, and sensitive to, contexts. It is the interaction between contexts and mechanisms that leads to outcomes. 51,52,57 In MEMORABLE, mechanisms included older people’s, practitioners’ and informal carers’ diverse responses to what was happening in medication management such as confidence, control, experience and expertise, and mutual trust.
-
Outcomes – the pattern of impacts and effects resulting from the way that contexts and mechanisms interact. In MEMORABLE, these included older people’s and informal carers’ coping experiences, achieving appropriate or inappropriate treatment, following adherent or non-adherent behaviours, quality of life as well as health and care relationships and resource use.
The CMO configurations are generated from, and stay close to, the data and are used to explain causation and therefore why certain outcomes happen under certain contexts within the scope of a programme theory.
Substantive theory is pre-existing, higher-level theory about how phenomena are supposed to work, perhaps domain-specific such as theories of behaviour, learning or change. 64 Identifying and applying substantive theory brings a particular lens to the research, helping with the development of initial programme theories, supporting data analysis and providing analogy. In MEMORABLE, normalisation process theory (NPT)65,66 was chosen as the relevant substantive theory. See Chapter 3, Medication management as implementation, for details of NPT and its application in the research.
Having provided an overview of the research methodology, the next section sets out a more detailed account of the way the methodology was followed in the work packages and the steps by which MEMORABLE was delivered.
Work packages and steps in the research process
This section begins by describing the work packages that were initially set out in the protocol. 31 It then continues with an account of the iterative and flexible way that they were followed in practice, responding to the data and emergent concepts generated within the research.
Work packages
The protocol31 identifies three work packages to be carried out within MEMORABLE, following on from Project Start Up led by the Co-applicant Group, described in Co-applicant Group above. These packages conform with a realist research process,49,51,55 based on the recommended phases for this type of study:50 defining the scope of the review, searching for and appraising the evidence, extracting and synthesising findings, and drawing conclusions and making recommendations. The protocol details each package as follows:
-
Work package 1: realist synthesis (understanding context and mechanism) – this package focuses on the realist review and involves developing the search strategy; selecting and screening articles for their relevance/rigour; analysing data; and developing the candidate or initial programme theories.
-
Work package 2: realist evaluation (exploring mechanism) – this package is about gathering and analysing experiential, narrative data through realist-informed interviews (29 older people and informal carers, and 21 practitioners from health and care, including formal carers).
-
Work package 3: developing framework for intervention(s) and dissemination – this final package involves refining the programme theories; undertaking any additional interviews or searching as required; identifying key mechanisms and related contexts; identifying and developing intervention strategies needed to change contexts and trigger mechanisms; presenting the intervention framework for feedback and further refinement; and dissemination.
Steps associated with work packages in the research process
The MEMORABLE study progressed through seven sequential and partly iterative steps (Figure 1). This figure highlights the non-linear way that the research advanced, with overlaps and iterations, particularly around steps 3, 4 and 5. Such overlaps and iterations are expected in researching a complex intervention such as medication management. 58,59,68
FIGURE 1.
Research process summary: work packages and steps. Reproduced with permission from Maidment et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

Describing this figure, below, steps are grouped by work packages (work package 1: realist synthesis – steps 1–4; work package 2: realist evaluation – step 5; work packages 1 and 2: iterations – steps 3a and b, 4a and 5a and b; and work package 3: developing framework for interventions and dissemination – steps 6–7). The results from this process are presented in Chapter 3.
Work package 1: realist synthesis (understanding context and mechanism)
Step 1: scope of the research
Medication management was scoped during Project Start Up through two strands of work. First, the Co-applicant Group drafted the MEMORABLE protocol,31 setting out the background to the research, highlighting:
-
increases in the number of older people and comorbidity in the population1,2
-
safety concerns and consequences of susceptibility to poor medication management in terms of harm and hospital admission20,21,27,28
-
National Institute for Health and Care Excellence (NICE) guidance identifying the need for a collaborative approach to supporting older people with long-term conditions in managing their medication. 71
Second, just prior to the start of research, a formative Project Group held a day’s facilitated workshop to share knowledge on the principles and practice of realist methodology and to discuss the practicalities of applying them to MEMORABLE. This enabled the group to establish ownership of the planned research and build the team. It also mapped out a simple, tentative medication management process, drawing on the experience and expertise of those present. This mapping process began to theorise about the dynamics and complexity of the intervention and informed subsequent work on the stages of medication management. Although this early informal theorising does not follow the formal progression of the research process set out in Figure 1, it was nonetheless a valuable early contribution to scoping how medication management might work that was gradually refined as data were accumulated; a candidate programme theory in all but name (see Table 5).
Step 2: initial literature search and review
This step involved searching for and reviewing abstracts to broaden the understanding of medication management within MEMORABLE’s aim and objectives. Within a realist review, the aim of the search and synthesis noted by Pawson53 is to identify a body of literature to support ‘explanation building . . . to articulate underlying programme theories’ so that these can be interrogated using the evidence. In MEMORABLE, evidence would be enriched by combining data from the literature and interviews. Accordingly, the literature search was not intended to maximise the number and range of articles on medication management but to be more purposeful in identifying articles with rich data that would best support the generation and exploration of programme theories about what works, for whom, why and in which circumstances. This followed Pawson’s guidance on abstracting policy and theory building, described in detail by Booth et al. 55
The Research Team, which included a qualified information professional, conducted a pilot search to sensitise the team to the literature and to help in determining the scope of the protocol and subsequent review. 52 Details of this pilot search are given in Appendix 1. Once this initial set of literature had been reviewed closely, categorised and coded, the team finalised a search strategy for the initial literature search that would contribute to the review itself.
The initial literature search used terms set out in Table 1, accessing MEDLINE (724 references), CINAHL (Cumulative Index of Nursing and Allied Health Literature) (152 references) and EMBASE (139 references). It included published articles from 1 January 2009 to 31 July 2018. The initial cut-off was chosen because 2009 was the year in which NICE published its clinical guideline on medication adherence. 72 The Research Team had anticipated that systematic reviews published from 2009 onwards would access earlier studies, sufficient to cover the scope of the MEMORABLE realist review.
| Topic | Search terms |
|---|---|
| Medication management |
Medication adherence – polypharmacy – medication management – medicines optimisation – concordance – compliance – adherence – regimen Health services misuse/inappropriate prescribing – drug prescriptions/practice patterns – physicians/inappropriate prescribing – drug utilisation/medication errors – de-prescribing |
| Older people | Age/aged |
| Long-term conditions | Chronic disease |
| Other limitations | English language |
A total of 1018 abstracts were identified, reducing to 974 once duplicates, for example, were removed (Figure 2). 73 Abstracts were then reviewed for inclusion, referral or exclusion by the Research Associate using agreed criteria: population (≥ 60 years, living at home in the community), setting (UK, relevant high-income country), condition (multimorbidity) and interest (medication management). A 10% sample was also reviewed by two members of the Research Team to ensure consistency. A total of 140 articles were agreed for full-text review. The review was piloted by mapping abstracted data using a logic model format74 to identify descriptive accounts of medication management. This contributed to a better understanding of medication management, amplifying and updating the background information in the protocol.
FIGURE 2.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram: medication management. Reproduced with permission from Maidment et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
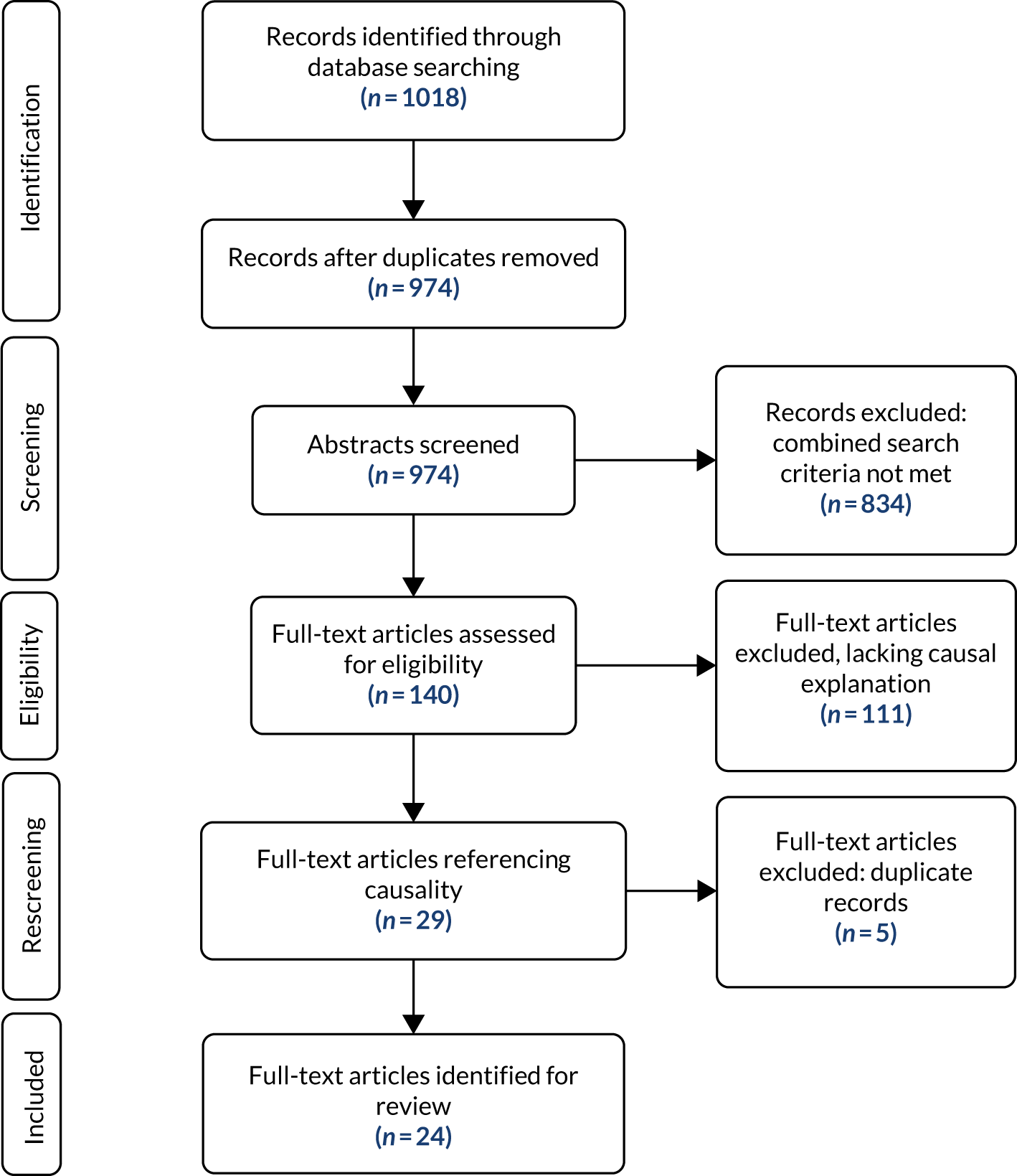
On reviewing the initial data set, the researchers concluded that the breadth and volume of the data explored a range of diverse issues that extended beyond the scope of the immediate needs of the research. More importantly, as a data set, the 140 articles generally lacked explanatory accounts to support the analysis of programme theories. As the research priority was to understand more clearly how medication management might work, it was agreed to focus more closely on a subset of articles containing causal accounts of this intervention. This approach was endorsed by the Project Group.
Step 3: further literature searches and review
Following this decision, the 140 articles were then searched for those containing the terms ‘concept, framework, model or theory’ to identify those articles that might include a logic model, programme theory or a theory of action or change. 74 A total of 29 articles were identified for full-text review and 24 were reviewed following the exclusion of duplicate articles that contained two of the search terms, such as concept and theory.
Articles were analysed and the data were coded into NVivo Pro 11 (QSR International, Warrington, UK). Two approaches were followed. The first used a predetermined coding framework based on the five factors and associated characteristics listed in a World Health Organization (WHO)12 report on adherence to long-term therapies. These factors and characteristics were frequently cited in the literature. The data were structured and reported using the five factors as headings with 27 associated subheadings, listed below to highlight their relevance to MEMORABLE:
-
patient factors – relevant to older people and informal carers: demographics (age, diagnoses-multimorbidity, education, gender, living situation, nationality-culture), independence-dependence-functional ability (dexterity, hearing and sight, mobility, self-care, self-management), knowledge-beliefs-attitudes (about health, about medication, health burden, health literacy), mental health (anxiety, depression, cognitive capacity), physical health
-
medication factors – aligned with polypharmacy: adverse events (including consequences of taking or missing doses, drug–disease interactions, drug–drug interactions, drug–food interactions), medication changes, medication information and instructions, medication name and type, medication number and regimen, packaging, storing, time horizon to benefit
-
health-care provider factors – addressing practitioner and practice issues: challenges, communication and shared decision-making, co-ordination and consistency of care, person-centred care, practitioner roles, trust and confidence
-
health-care system factors – covering health and care organisations: access to dispensers of medication, access to information, access to prescribers, access to review
-
socioeconomic factors – the infrastructural underpinning of health and care organisations: evidence, finance, policy, technology.
However, this initial framework proved unwieldy because coding was constrained to fit with the list of existing factors and terminology, rather than more accurately reflecting the accounts in each text and the causal links between factors identified within and between texts. A different approach was needed. A second analysis coded data directly from the articles. Individual nodes and the eventual node structure emerged, emphasising the meaning and links attributed in each text and across multiple texts. The node summary is set out in Appendix 2. Nodes were then grouped under headings such as diagnoses, medication, health and care relationships, and an analysis framework established (Table 2). This framework combines the headings or factors and the levels at which they operated to ensure that coding was causally structured. In the table, factors are set out as potential contexts, mechanisms or outcomes, by levels, and a working definition formulated for the research. This work made an important contribution to sense-making at this stage and to later analysis.
| Factor in analysis framework | Working definitions in MEMORABLE | |
|---|---|---|
| Potential contexts | ||
| Individual level | Diagnoses: multimorbidity | Two or more long-term conditions |
| Medication: polypharmacy and treatment regimen | Five or more medications or, if fewer, a complex regimen that is proving difficult to manage | |
| Identity and day-to-day life | Older people aged ≥ 60 years, encompassing the individuality of older people and the unique bundle of personal influences on their decisions and actions | |
| Interpersonal level | Health and care relationships and communication | Formal relationships and communication with practitioners |
| Family and social relationships and communication | Informal relationships and communication with significant people in older people’s lives, including informal carers | |
| Institutional/infrastructural level | Health and care system | The provision of services by organisations |
| Support systems and information | Access to support and information services including via third sector or online, and published resources (e.g. patient information leaflet) | |
| Potential mechanisms | ||
| Individual level | Workload | Can be ‘high or increasing’ (e.g. ‘high’: a large number of medications and a complex regimen, or ‘increasing’: the addition of a new medication) or ‘low or decreasing’ (e.g. ‘low’: a few medications with a simple, stable regimen, or ‘decreasing’: further simplification of the regimen) |
| Capacity | Can be ‘high or increasing’ (e.g. ‘high’: knowledge and motivation about medication management or ‘increasing’: gaining knowledge and confidence by experience) or ‘low or decreasing’ (e.g. ‘low’: unable or unwilling to make sense of their medication and how it should be taken, or ‘decreasing’ as a result of an acute exacerbation or loss of informal support) | |
| Burden | The combination of workload and capacity as it impacts on people’s health and well-being. Burden can come from people’s diagnoses, their medication at the individual level, health and care relationships at the interpersonal and the heath and care system at the institutional or infrastructural level | |
| Potential outcomes | ||
| Individual level | Coping experience | The way people are able to manage the burden of medication management in their day-to-day lives |
| Health literacy | People’s knowledge of their health, diagnoses and medication | |
| Appropriate/inappropriate treatment | Whether or not medication is prescribed to meet people’s treatment needs, optimising safety, benefit and cost | |
| Adherence/non-adherence | The degree to which people take their medications as recommended by and agreed with the prescriber | |
| Performance/clinical outcomes | The impact of the medication in terms of the diagnosis or symptoms for which it was prescribed | |
| Quality of life | The impact of taking medication on the older person that accords with their values, goals and preferences | |
| Interpersonal level | Health and care relationships | The interaction between older people or informal carers and practitioners |
| Family and social relationships | The interaction between older people and informal carers as well as a wider network of family and friends | |
| Institutional/infrastructural level | Resources | The operational and strategic goods, services and other outputs provided by organisations |
This analysis was summarised in a discussion paper for a Research Team meeting to inform their work on drafting initial programme theories and reported to the Project Group.
Step 4: candidate programme theories and substantive theory
In this step, the Research Team continued to refine the tentative medication management process developed in step 1 as a theory of action or process. In addition, they drafted three candidate programme theories about medication management; one from the perspective of older people, one from the perspective of informal carers and one from the perspective of practitioners. These three candidate theories were developed from the literature searches and preliminary review, and drew on the Research Team’s own wider expertise and experience. It appeared that the ‘dissonance’ of agendas between different participants might have some traction:
-
Older people – if an older person, living in the community with multiple diagnoses and complex medication regimens, feels that there is a dissonance/difference between their own priorities and those of the practitioners dealing with their medication management, then positive outcomes will not be achieved for and with them.
-
Informal carers – if an informal carer who is providing help with medication management for an older person living in the community with multiple diagnoses and complex medication regimens feels that there is a dissonance/difference between the priorities of the person they care for, themselves or the practitioner dealing with their medication management, then positive outcomes will not be achieved for and with the older person.
-
Practitioners – if a practitioner feels that they have to focus on structural/practice outcomes, then they are not able to accommodate other agendas/priorities, leading to a lack of influence on the knowledge, attitudes, behaviours and outcomes of older people who are living with multiple diagnoses and complex medication regimens in the community.
These theories extended the Research Team’s attempts to explain what works in medication management, for whom, why and in which circumstances to achieve the outcomes that mattered to those involved in the process.
Informed by the data, the simple medication management process that was mapped out informally in step 1 was also revised at this time. In its original format, it appeared to suggest that medication management was a linear process. However, it became increasingly clear that the process model needed to accommodate more of the complexity of potential loops and iterations that people might experience in their day-to-day lives (see Tables 5 and 6). These concepts were then available to discuss with interview participants in work package 2.
Several substantive theories of interest were considered at this stage but none were sufficiently well evidenced from the literature, pending clarification from the interview data.
Work package 2: realist evaluation (exploring mechanisms)
Step 5: interviews and analysis
In order to enrich the data through which the candidate programme theories were being evaluated and to construct CMO configurations by which they might be explored, interviews were carried out with older people (n = 13), health and care practitioners (n = 21) and informal carers (n = 16) (total N = 50). A summary of interviewee profiles are set out in Appendix 3. This work was subject to approval by the sponsor (Aston University), by a regional Research Ethics Committee by proportionate review and by the Health Research Authority (approval issued 26 September 2017: REC reference: 17/EE/3057).
Invitations to participate in the research were issued directly through recruitment sites, such as NHS secondary and primary care organisations, and Join Dementia Research; by purposefully sampling individuals known to the researchers through professional and work contacts; and indirectly through publicity for the launch of the research and an item about MEMORABLE aired on BBC Breakfast that generated interest in taking part [see URL: www.youtube.com/watch?v=rz5UZ3mWx3o&feature=youtu.be (accessed March 2020)].
All recruits received a participant information sheet and consent form to complete before taking part in an interview that generally lasted about 1 hour. Interview schedules for each group followed a realist approach49,75 (see Appendix 4 for annotated details of the common contents). Older people and informal carers were usually interviewed face to face and practitioners most often by telephone. Interviews were audio-taped. The tapes were anonymised and transcribed, the transcripts then being checked for accuracy by the Research Associate. The transcripts were kept on password-protected computers for analysis.
Interviews were designed to enable a purposeful conversation to evolve in which the participant could describe how and why medication management worked for them as a process and to achieve the outcomes that mattered to them. The Research Associate followed the interview schedule using a conversational interviewing style, enabling participants to provide causal accounts of medication management in their day-to-day lives. The approach was nuanced to participants’ experiences and expertise: how older people fitted a complex regimen into their daily routines; how practitioners were directly involved in administering, prescribing or reviewing medication, or indirectly involved by managing staff that undertook those activities; and how informal carers supported increasingly dependent relatives.
Interview data were abstracted for a small number of randomly sampled participants in each group, whether older people, informal carers or practitioners, to trial the analysis framework. This involved the identification of explanatory statements for the factors that they identified as significant from their own experience. When possible, links between factors were identified from the transcripts. These were reviewed, while the recording was listened to, to check accuracy and aid interpretation. Hearing the nuances in the original narrative was found to be valuable, such as the pauses and emphases by which ‘structures and plots’76 could be better understood. Data were initially mapped into an Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet to test the framing of the analysis and the way it might help identify causal patterns. Once verified, this enabled the larger data set to be coded using NVivo.
At this point, data from the literature and an increasing number of interviews were being analysed separately, driven by the way the work packages were being progressed in series. The researchers were discussing and reflecting on both data sets in order to refine the process, respond to emerging themes and obtain a better understanding of this complex topic. The Research Associate worked iteratively, carrying out further cycles of purposeful searches and more integrated data analysis to engage more fully with the increasing volume and richness of data being generated.
Work packages 1 and 2: iterations
Steps 3a/5a: further literature searches and review, interviews and evaluation (iteration) – to identify key concepts and processes
Based on access to increasingly extensive data sets, the Research Team identified shared decision-making as an important aspect of the work transacted in the medication management relationships. From this, they were able to develop the understanding of the stages in the medication management process further. They distinguished those stages that were carried out individually by the older person and in which individual decision-making would feature, from those in which the older person interacts with a health or care practitioner through shared decision-making.
Informed by the richness of the data becoming available, discussion and debate in the team resulted in ‘dissonance of agendas’ being discounted as a candidate theory for intervention development. Based on emergent themes, the team acknowledged the increasingly complex work involved in medication management day to day and decreasing capacity from factors such as ageing, disease progression and frailty affecting older people; informal carers’ lack of knowledge and support, or competing demands from work roles; and practitioner time constraints. Informed by their own experiences, discussions with the wider Stakeholder Group supported by data from simple searches on workload capacity, burden emerged as the key potential mechanism of interest, experienced individually and transacted interpersonally.
To inform the way burden might be located in MEMORABLE, a limited search for articles that linked burden to multimorbidity, chronic disease and polypharmacy was conducted. 14,77,78 As part of the analysis, a further nine articles were sourced from those studies, largely kinship and sibling studies. 79–81 In line with MEMORABLE’s primary focus on the experience of older people living with comorbidities and polypharmacy, burden was disaggregated into burden associated with older people’s diagnoses and ‘the lifetime burden of chronic illness’39 as well as ‘treatment-generated disruptions’. 13 The scope of burden was also extended to older people’s interactions with health and care practitioners and systems15,39 to include ‘the workload of healthcare and its impact on patient functioning and wellbeing’. 82 The significance of informal carer and practitioner burden was also acknowledged although these concepts were pursued through narrative data rather than the literature.
Step 4a: theorising (iteration) – substantive theory
Informed by the analysis of selected data and having distinguished the way that older people engage in medication management through their decisions, individual and shared, and actions or behaviours at different but linked stages, the researchers identified NPT65,66 as a substantive theory to guide further analysis of the data. Adopting this theory corresponded with highlighting implementation as a key characteristic of medication management in terms of the work in introducing new medication management practices, and making those practices routine in the day-to-day activities of older people and informal carers.
Steps 3b/5b: further literature searches and review, interviews and evaluation (iteration) – to focus on causality from integrated data sets
Here, the combined data were reviewed and evaluated for causal accounts to prepare for drafting CMO configurations. Starting with the re-review of literature, configurations were set out in NVivo. Each configuration was allocated to a node (n = 59) with associated child nodes, one for each context, mechanism and outcome. Data were then abstracted from the interviews and mapped into the same configurations, some of which were modified in the process and new configurations generated.
Progress on this configuring process highlighted the complexity of medication management. In light of the breadth, volume and quality of data being generated, particularly from the interviews, the researchers agreed to balance the need to understand the breadth of medication management with a more detailed causal analysis focused on the reviewing/reconciling medications (Stage 5) [see Chapter 3, Reviewing/reconciling medication (Stage 5)]. This decision was based on the potential significance of this stage to the overall process for several reasons, including recognition of this stage’s importance in terms of interpersonal decisions and actions, and relevance to Stage 2 in which the other interpersonal work occurred when getting a diagnosis and/or medications. In addition, the researchers could establish links between Stage 5 and Stage 3 in which changes would start to be implemented and Stage 4 in which older people would continue to take their medications over time, and in which, in both stages, non-adherence may emerge as an issue of concern. Finally, the researchers were aware of published policy and guidance on the topic that could clarify standards of practice and their intended impact, causally explained or not.
A key feature of realist approaches to literature searching is the enhancement of the original search with core terms that had not been initially identified as important but whose centrality has emerged during the preliminary literature analysis. 52 Table 3 describes the supplementary searches on medication review (2000–18). Consequently, the discussion paper was informed by a purposive literature search for substantive literature relating to medication review, targeted at the MEDLINE database. A total of 244 review articles were identified and reviewed in relation to different configurations of medication review, published between January 2000 and December 2018. A larger set of non-review articles (3234 references) published over the same period was retrieved as a data set to support exploration of specific issues identified from the initial review subset.
| Search terms | Articles |
|---|---|
| (Search 16) Search “medication review*” OR “medicines use review*” OR “medicines review*” OR (medicine utilization review*) OR (medicine utilisation review*) OR (medicines utilization review*) OR (medicines utilisation review*) Filters: Review; Publication date from 2000/01/01 to 2018/12/31; English | 244 |
| (Search 15) Search “medication review*” OR “medicines use review*” OR “medicines review*” OR (medicine utilization review*) OR (medicine utilisation review*) OR (medicines utilization review*) OR (medicines utilisation review*) Filters: Publication date from 2000/01/01 to 2018/12/31; English | 3234 |
The Research Team chose not to add additional qualifiers beyond the concept of ‘medication review’ for two reasons. First, the population seemed to be self-defining from the terminology (i.e. the large majority of retrieved items referenced an aged population anyway). Second, the list of potentially qualifying terms, such as polypharmacy, would be quite extensive and would be difficult to make sufficiently exhaustive compared with the relative advantage of undertaking this sensitive search.
Informing this step, the Research Team considered a discussion paper written by the Research Associate that listed the full set of CMO configurations. It also included subsets of configurations for reviewing and reconciling medications (Stage 5) and two areas of interest around risk identification and individualised information that had emerged from the data and appeared to have the potential to generate proposed interventions.
In addition to the involvement of the Research Team in these decisions, the Project Group also approved this work, including the focus on reviewing/reconciling medications (Stage 5). The opinions of the Stakeholder Group were also sought on emerging themes: medication management is not ‘one thing’; older people’s goal about what they want from their medication and how, is very important to them; family carers’ awareness of the responsibility and challenges when they support older relatives with their medication; more and more practitioners, in different roles, from different organisations and with different ways of working are involved in medication management. The Stakeholder Group discussions enhanced the way that researchers understood these themes, supporting the analysis.
Work package 3: developing framework for intervention(s) and dissemination
Step 6: final programme theories
Final programme theory for a key stage in medication management is contained in Figure 5, based on both evidence and experiential data.
Step 7: intervention proposals
The intervention proposals emerged from the data, with an evidence and experience base. The Research Team identified the interventions most likely to improve medication management through discussion and debate of the data analysis, themes and concepts. This took place at a face-to-face meeting. Proposed interventions were prioritised as being most likely to bring about improvements in medication management, directly relevant to the expressed needs of older people and informal carers (individualised information) and improving practice (risk identification). They are reported in Chapter 3 and are considered in Chapter 4.
Having completed the account of the method underpinning MEMORABLE, the next chapter sets out the results.
Chapter 3 Findings
Using a realist approach and following the work packages and steps described in Chapter 2, this chapter sets out the findings from the literature and interviews in three sections.
-
Medication management: this section sets out how the data have been analysed and interpreted to develop a series of subprogramme theories about aspects of medication management. These subprogramme theories explain medication management in five ways: as a good practice, as a complex intervention, as implementation, as burden and as experience. The breadth of this work provided an explanatory structure of the complexity of medication management, necessary for the identification and more detailed analysis of Stage 5 as a key and critical stage.
-
Reviewing/reconciling medication: this section is about Stage 5 of medication management in which older people and practitioners are engaged, with or without the support of informal carers, achieving personally meaningful outcomes while enabling medication adherence and medication optimisation. This more in-depth work supported the generation of an explanatory theoretical framework, conceptualised around burden.
-
Proposed interventions: selected for their potential to contribute to the improvement of medication management.
-
Risk identification of older people and informal carers who are not coping with the burden of managing their medications. These are the people who might benefit from an additional, needs-generated intervention such as a review.
-
Individualised information about older people and their unique experiences of complex diagnoses and treatments, developed collaboratively by them, their informal carers if they are actively involved, and practitioners. This would be in an agreed format that encompasses their health, multimorbidities and polypharmacy, situated in their day-to-day lives and reflecting their values, goals and preferences. It would contribute to improved coping and self-management.
-
Reporting MEMORABLE’s findings begins with medication management.
Medication management
I think practice has improved. I think we are, we do more stuff than we used to. I think we sort of follow guidelines a lot better than we used to . . . But you’re sort of, you’re juggling all these things of what the textbook is telling me I should do, what is best for this 90-year-old person who’s on 1001 tablets already, what is the benefit? Actually, is it going to help this individual?
P53 – general practitioner
Medication management is introduced from the perspective of good practice. It is then considered as a complex intervention, as implementation, as burden and as experience. Understanding medication management through the combined analysis of literature and narrative data are pivotal to the research.
Medication management as good practice
Good practice recommendations from medication management policy and guidelines informs practitioners’ work, and the management and commissioning of the services in which they operate. Policy and guidelines are generally aligned with preferred outcomes: adherence,12,72 in which the older person’s actions matches the prescriber’s recommendations, informed by their involvement in and agreement with decisions about their treatment, and optimisation33 that aims to ensure that older people get the best possible outcome from any treatment. Reports from two organisations are noted below, framing medication management practice.
First, a formative WHO12 report endorses a behavioural approach to medication adherence, expressed in an information–motivation–behavioural skills model. Behaviours are characterised by type, frequency, consistency, intensity and/or accuracy. A broad level explanation of behavioural change and adherence is contained in Annex 1 in the WHO report. Good practice is delivered through the practitioner’s clinical and communication competencies, such as providing emotional support (empathy, validation, relationship building); giving information (empowering, tailoring, supporting health literacy); asking adherence-specific questions (monitoring, feedback) and; discussing therapeutic options, negotiation of regimen and discussion of adherence (collaboration, shared decision-making). By pursuing this approach, the practitioner secures the preferred change in the individual. Adherence is however subject to the caveat:
. . . almost everyone has difficulty adhering to medical recommendation, especially when the advice entails self-administered care.
Second, turning to the evolving policy framework for medication management in the UK, 2009–2018, NICE has published relevant guidance on adherence, optimisation and multimorbidity. 33,41,72 It has also issued several linked documents including a guideline and pathway for older people with social care needs,71,83 a flow chart for optimisation,84 and a key therapeutic topic document on multimorbidity and polypharmacy. 29
The NICE guidance on multimorbidity and polypharmacy29,41 acknowledges the complexity of managing future morbidity and mortality. NICE highlight two associated risks: first, in relation to multimorbidity, for which multiple treatments may be recommended although the evidence base is specific to the treatment of single conditions and, second, in relation to polypharmacy, for which benefits decrease and the risk of harm increases with the addition of each medication. NICE also underscore the responsibility of health-care professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient. Good practice interventions accordingly include the need for:
-
an individualised approach to accommodate needs, treatment preferences, lifestyle and concerns through individualised and planned care, assessment and treatment;
-
partnership with shared decision-making to ensure that there are informed discussions and decisions in which the patient experience is understood; and
-
outcomes that include not only clinical aspects of medication safety and effectiveness but also the outcomes that matter to the individual, including improving quality of life and reducing treatment burden.
Despite an extensive, supporting evidence base, the NICE documents do not explain how these good practice interventions might work to enable practitioners to adapt them to local or individual circumstances. 85 Such an approach may appear to support the view that the act of ‘doing’ good practice might suffice. In such circumstances, practitioners could struggle to make informed, individualised decisions and assure good practice within the evidence available, noted by Hughes et al. 86
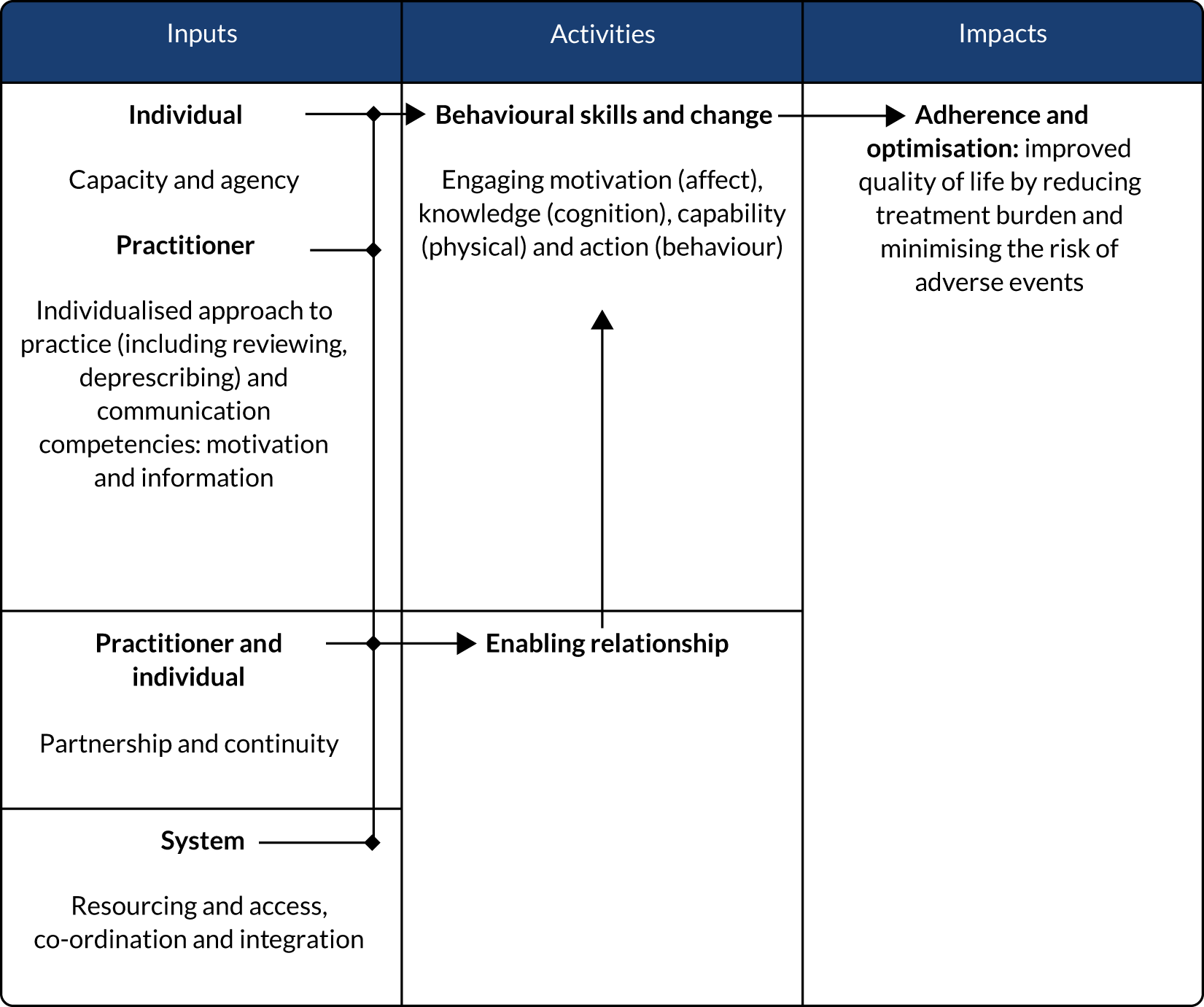
To summarise, key elements of good practice from these strategic reports on medication management have been mapped out in Figure 3, as a logic model: individual, interpersonal and system-level inputs, individual and interpersonal activities, and expected impacts.
Medication management as a complex intervention
Complexity and causality
A total of 24 articles containing rich conceptual data on medication management were identified through screening the initial search results, prioritised because they appeared to have a causal account of how medication management might work (Table 4). They were identified through the presence of the screening terms ‘concept, framework, model or theory’ that might indicate a logic model, programme theory, or a theory of action or change within them. 74 Articles that included more than one of these screening terms were categorised by the Research Team based on their primary focus.
| Authors | Title | Year | Country | Topic | Method |
|---|---|---|---|---|---|
| Concept (n = 4) | |||||
| Cheraghi-Sohi et al.87 | The influence of personal communities on the self-management of medication taking: a wider exploration of medication work | 2015 | UK | Personal communities involved in medication work | Semistructured interviews and the construction of network diagrams |
| Fried et al.88 | A Delphi process to address medication appropriateness for older persons with multiple chronic conditions | 2016 | USA | Translating framework concepts into specific strategies to identify and remediate inappropriate regimens, focusing on deprescribing to reduce medication burden | Modified Delphi process: three rounds of anonymised web-based surveys |
| Naik et al.89 | Patient autonomy for the management of chronic conditions: a two-component re-conceptualization | 2009 | USA | Autonomy: decisional (about treatment) and executive (carry treatment plan out) in the context of multiple conditions | Concept development |
| Upadhyay and Joshi90 | Observation of drug utilization pattern and prevalence of diseases in elderly patients through home medication review | 2011 | India | Medication review by pharmacists | Community-based survey |
| Framework (n = 5) | |||||
| aBartlett Ellis and Welch91 | Medication-taking behaviours in chronic kidney disease with multiple chronic conditions: a meta-ethnographic synthesis of qualitative studies | 2016 | Australia, England and the USA | Medication taking and medication adherence behaviours | Qualitative study review |
| Boskovic et al.92 | Pharmacist competencies and impact of pharmacist intervention on medication adherence: an observational study | 2016 | Croatia | Results of pharmacists intervention on adherence in the community | Observational study |
| Coleman93 | Medication adherence of elderly citizens in retirement homes through a mobile phone adherence monitoring framework (Mpamf) for developing countries: a case study in South Africa | 2014 | South Africa | Intervention development: technology | Case study with qualitative interviews |
| Schuling et al.94 | Deprescribing medication in very elderly patients with multi-morbidity: the view of Dutch general practitioners. A qualitative study | 2012 | Netherlands | Exploring experienced general practitioners’ views on deprescribing and involving older people in these decisions | Qualitative study |
| Yap et al.95 | Medication adherence in the elderly | 2016 | Singapore | Systematic review of the barriers to adherence in the elderly | Case study |
| Model (n = 9) | |||||
| aDoucette et al.96 | Initial development of the Systems Approach to Home Medication Management (SAHMM) model | 2017 | USA | Systems approach to safe and effective home medication management | Model development |
| Hennessey and Suter97 | The Community-Based Transitions Model: one agency’s experience | 2011 | USA | The acquisition and use of health coaching competencies in home care clinicians at health transitions | Model development |
| Jonikas and Mandl98 | Surveillance of medication use: early identification of poor adherence | 2012 | USA | Identification of population level adherence and risk of poor adherence | Model development |
| Khabala et al.99 | Medication Adherence Clubs: a potential solution to managing large numbers of stable patients with multiple chronic diseases in informal settlements | 2015 | Kenya | Assessment of the people’s care through nurse-facilitated Medication Adherence Clubs | Retrospective descriptive study |
| aKucukarslan et al.100 | Exploring patient experiences with prescription medicines to identify unmet patient needs: implications for research and practice | 2012 | Canada and USA | Identification and characterisation of patients’ unmet needs when taking prescribed medication | Grounded theory approach to interview content analysis |
| aMcHorney et al.101 | Structural equation modeling of the proximal-distal continuum of adherence drivers | 2012 | USA | Identification of adherence drivers | Model development |
| Lau et al.102 | Multidimensional factors affecting medication adherence among community-dwelling older adults: a structural-equation-modelling approach | 2017 | China | Multidimensional factors affecting medication adherence: measuring medication adherence, professional-help relationship and self-care abilities | Exploratory cross-sectional approach using interviews and modelling |
| Milani and Lavie103 | Health care 2020: reengineering health care delivery to combat chronic disease | 2015 | USA | Modifying the health-care delivery model to include team-based care in concert with patient-centred technologies | Review |
| Shepherd et al.104 | Health services use and prescription access among uninsured patients managing chronic diseases | 2014 | USA | Identification of relationships between population characteristics, health behaviour and outcomes | Longitudinal quasi-experimental design with convenience sample for assessment and notes review |
| Theory (n = 6) | |||||
| Geryk et al.105 | Medication-related self-management behaviours among arthritis patients: does attentional coping style matter? | 2016 | USA | Coping styles | Internet-based survey |
| Haslbeck and Schaeffer106 | Routines in medication management: the perspective of people with chronic conditions | 2009 | Germany | Routines in medication management along chronic illness trajectory | Semistructured interviews: initial and follow-up |
| Laba et al.107 | Understanding if, how and why non-adherent decisions are made in an Australian community sample: a key to sustaining medication adherence in chronic disease? | 2015 | Australia | Intentional non-adherent decisions and behaviours | Semistructured interviews and theory-informed iterative thematic framework analysis |
| Marks108 | Self-efficacy and arthritis disability: an updated synthesis of the evidence base and its relevance to optimal patient care | 2014 | n/a | Self-efficacy, pain and disability, adherence to therapeutic strategies and outcomes, applicable to assessment and treatment | Review and synthesis |
| Jorge de Sousa Oliveira et al.109 | Interventions to improve medication adherence in aged people with chronic disease – systemic review | 2017 | USA, China, Portugal and Italy | Nursing interventions to improve medication adherence in older people with chronic disease | Systematic review |
| Skolasky et al.110 | Psychometric properties of the patient activation measure among multimorbid older adults | 2011 | USA | Patient activation measure (Hibbard): psychometric properties and model evaluation | Interviews + completion of measure. Cross-sectional and latent class analysis |
Informed by the causal attributions in these articles, relevant factors and combinations of factors were identified and coded into NVivo. The codes are listed in Appendix 2.
This analysis confirms that medication management aligns with the description of complex interventions and their evaluation set out by the Medical Research Council:58,59 several interacting components, the number and difficulties of behaviours among those involved, the number of groups or organisational levels targeted by the intervention, and the number and variability of outcomes.
The following issues emerged from the analysis, associated with the two main groups: older people and practitioners. Some additional articles have been added to the references in Table 4 where these have augmented the understanding of particular factors:
-
Older people: the way they manage the workload13–15 associated with their medications is influenced by their diagnoses, including multimorbidities, symptoms and illness trajectories that overlay ageing processes;11,12,78 the medications they take, including high-risk drugs, doses and complex regimens;89,91,111 and the relationship and interaction with the prescriber. 112–114 Together, these affect behavioural responses such as control, self-efficacy and coping styles87,89,91 through the adoption of routines106,115 that lead to the outcomes that matter to them. Cheraghi-Sohi et al. 87 describe older people’s individuality in the way that they manage their medication: ‘Medication-taking appears to be a personalised, contingent and contextually situated type of work with participants developing highly individualised routines and strategies’.
-
Practitioners in health and care: doctors in particular87,89,103 as well as pharmacists92,94,96 and nurses87,91 are identified as significant in medication management, following a patient-focused care model100,102,103 as part of a health and care system in which there is co-ordination and continuity. 100,103 The immediacy of their work, face to face with older people, is highlighted in prescribing,88,90,91 deprescribing88,94 and information-giving. 91,94,97,100 Establishing a trusted therapeutic relationship89,91,100 enables practitioners to extend their influence over older people’s medication management decisions and actions when they are at home. Lau et al. 102 highlight the importance of practitioners engaging with older people as individuals to enable them to improve the way that they manage their medications: ‘Adherence interventions should be tailored to the needs of a patient to enhance self-management capacity regarding medication use’.
These findings are supported by the interview data reported later in this chapter; see Medication management as experience.
Structuring complexity
Medication management is often described in the literature as a single intervention. However, its real-world complexity has been addressed in MEMORABLE by disaggregating it into stages differentiated by activities and participants (Table 5):
-
Activities – each stage has a distinct group of purposeful activities or work associated with it, such as Stage 1: identifying a problem and Stage 4: continuing to take medications. However, stages are linked and generative so that what happens in one stage informs another. For example, having received a diagnosis and medications in Stage 2, an older person starts to take their new medications in Stage 3 and continues to take them in Stage 4, making them part of their day-to-day routine.
-
Participants – Stages 1, 3 and 4 are where an older person is managing their medications on their own. This is indicated in Table 5 with a ‘(1)’ next to ‘older person’. However, the older person may have the support of an informal carer depending on their particular needs, shown as (1 + 1). Stages 2 and 5 are interpersonal stages where the older person and the practitioner work together, shown as (1 : 1), next to ‘older person and practitioner’. Although informal carers were only identified in association with ‘informal carer burden’ in the screened literature, they have been included in the table to reflect their involvement, as described in the interviews.
| Stage | Stage 1: identifying problem | Stage 2: getting diagnosis and/or medications | Stage 3: starting, changing or stopping medications | Stage 4: continuing to take medications | Stage 5: reviewing/reconciling medications |
|---|---|---|---|---|---|
| Who | Older person (1) | Older person and practitioner (1:1) | Older person (1) | Older person (1) | Older person and practitioner (1 : 1) |
| Older person may be supported by an informal carer (1 + 1) | Older person may be supported by an informal carer (1 + 1) | Older person may be supported by an informal carer (1 + 1) | Older person may be supported by an informal carer (1 + 1) | Older person may be supported by an informal carer (1 + 1) | |
| Doing what | Older person identifies something is wrong | Older person and practitioner agree what is wrong, how to treat it. A prescription is issued and filled | Older person adjusts daily medication routine to include new medication or adjust or omits current medication | Older person fits new routine into day-to-day life |
Practitioner confirms medication safety and efficacy Older person and practitioner agree appropriateness, adherence and fit with day-to-day life |
Stages 2 and 5 are where good practice, discussed previously, is situated. Adherence on the other hand can now be located in Stage 3, as an aspect of a single event when the older person is starting, changing or stopping medications, or when continuing to take them in Stage 4, requiring repetition and consistency. However, Volpp and Mohta116 indicate that the factors that initiate behavioural change are different from those that sustain it although ‘human interaction and social support’ are noted as significant to both.
Developing the stage structure further, Table 6 adds more detail to what is happening in each stage and across stages, locating and linking decision-making and behaviours. The table confirms that the older person (1) is self-managing in Stages 1, 3 and 4, although they may also be benefiting from support with medication management through the involvement of an informal carer (1 + 1). These stages are where the older person engages in individual decision-making to exert control or agency: in Stage 1, when identifying that something is different and potentially problematic, and Stages 3 and 4, when setting up and following routines. However, the emergence of a problem in Stages 3 or 4 may result in a ‘disruption’ loop where the older person reverts to Stage 1 to address a concern.
Interview data confirm that a strong sense of control or striving for control supports adherence and optimisation to get the best from medications that make a tangible difference in older people’s lives. On the other hand, a strong sense of autonomy and weak external influence, such as ambiguity in the advice from the prescriber about the importance of certain medications, could in some instances be linked with an increased likelihood of intentional non-adherence. Media coverage about problems or risks with certain medications, or trusted friends’ stories of their own bad experiences of particular treatments, can compound the weakness of prescriber advice. As a result, older people’s decisions may not always appear consistent as they try to fit medication management with the many other choices and opportunities that they are exposed to on a daily basis. However, non-intentional non-adherence appears to be linked more with occasional forgetfulness or cognitive decline. This suggests that an effective approach to medication management may involve discussing how the older person fits this complex staged work into their day-to-day lives, and copes with it, rather than focusing on more abstract concepts such as adherence.
Turning to the interpersonal stages identified in Table 6, these are where decision-making is shared. The first of these is Stage 2, where practitioners provide a diagnosis and medications. This stage is important for what the practitioner does and communicates in an often time-limited event. Of equal importance is the way these activities draw on older people’s needs and concerns to explain the problem as they perceive it (Stage 1), together with experiences of coping with their medication routines day to day (Stage 4). Adding to the understanding of the way stages link and are generative, work in this stage also feeds forward to influence what an older person does when they are back at home (Stages 3 and 4). This is where older people, sometimes with the support of informal carers, may need to make changes to what they are already doing to fit new medications into their routines and day-to-day lives.
The second interpersonal stage is Stage 5, a key stage in MEMORABLE, where practitioners are reviewing or reconciling medications. This stage is where shared decisions are made between the practitioner and older person about whether or not to continue with the existing medication regimen as it is. In this case, the older person continues to self-manage as before (Stage 4). Practitioners and older people might also decide to make changes to the medication regimen so that the older person needs to adjust what they are currently doing (Stage 3) or, if there is a change in health status that warrants a reassessment, to get a new diagnosis and/or medications (Stage 2). These medication and diagnosis loops are shown in Table 6 that also highlights the interpersonal stages where older people and practitioners are engaged in shared decision-making (Stages 2 and 5) and the other stages where older people are making individual decisions (Stages 1, 3 and 4), distanced from but influenced by engagement with practitioners.
Medication management as implementation
Having established the stages of medication management as a complex intervention, this subsection addresses how medication management can be understood as a form of implementation involving ‘purposive and directed’66 work. As MEMORABLE is using realist methodology, the researchers have drawn on Carl May’s65,66,117 work on NPT and particularly the article by May and Finch. 66 This substantive theory was identified by the researchers during step 4a.
Normalisation process theory articulates the way new practices or activities are introduced and are made routine or sustained through the work that is done by those involved. It identifies four linked areas of work: coherence work to clarify what the new work is, relational work to build and sustain a network of people to carry the work out, operational work to undertake it, and appraisal work to understand the impact of what has been done. Normalisation process theory views this work as purposive, in other words, outcome focused. As a substantive theory on implementation, NPT aligns with medication management as a complex intervention, set out in Structuring complexity. It has been adopted here as an evaluative lens because of the way that it provides a structure and process for understanding the work associated with medication management, integrated across individual, interpersonal and system levels.
May et al. ’s65,66,117 work initially focused on change implementation in organisations. Gallacher et al. ,118 among others, have extended its application, using this theory to explain the workload and capacity issues associated with the experience of stroke. They locate burden work in health-care settings and people’s day-to-day lives. Gallacher et al. ’s article118 is, therefore, referenced here for the way the authors apply NPT to people’s experiences of health care. They also simplify NPT terminology. Accordingly, May et al. ’s terms have been revised as follows: coherence work to ‘sense-making’; relational work to ‘relationships’; operational work to ‘action’; and appraisal work to ‘reflecting’ (associated with older people and informal carers) and ‘monitoring’ (associated with practitioners).
Table 7 builds on Tables 5 and 6. Following the stages of medication management and focusing on the older person and the core relationship between them and the practitioner, this version of the table introduces NPT steps, distinguishing what is happening within and between stages:
-
Stage 1: identifying problem – involving the older person (1) in individual sense-making about the importance of the problem and what to do about it. Drawing on both knowledge and confidence, choosing to act enables the older person to seek help, or not, while reflecting on the appropriateness of that decision and action.
-
Stage 2: getting diagnosis and/or medications – within the relationship between the older person and the practitioner (1 : 1), shared decision-making is located here as an action. This is because shared decision-making is a type of work facilitated by the practitioner and contingent on them providing the time and space for it to occur. The action extends as far as the plan to do something that is subsequently initiated in Stage 3 and continued in Stage 4. The older person may reflect on their experience of the encounter and the practitioner relationship, in that moment and as a longer-term, trusted collaboration. Practitioner monitoring includes the performance elements of the work and the relationship, as well as the anticipated clinical outcomes.
-
Stages 3 and 4: starting, changing or stopping medications and continuing to take medications – these stages are where the older person (1) makes sense of what they are doing in terms of the information that they have been given, what they are currently doing and what needs to be done to start and sustain a new routine. From this, the older person initiates (Stage 3) and follows that routine (Stage 4) and reflects on it, for example thinking about the way the medication makes them feel or the ease with which they can fit the new routine into what they do day to day.
-
Stage 5: reviewing/reconciling medications – as with Stage 2, and the relationship between the older person and the practitioner (1 : 1), shared decision-making located here as an action. Building on Table 6, Table 7 also includes disruption, medication and diagnosis loops discussed previously.
The table highlights the way medication management can be understood using substantive theory as a framework. Within the apparent complexity in this table, patterns of work can be identified that contribute to explaining medication management including:
-
The importance of sense-making and individual and shared decision-making from which actions are generated within medication management. These actions include the way shared decision-making might establish the choice architecture119 for older people’s day-to-day individual decision-making at home.
-
The amount of work older people are doing to start and to continue to take medications as they make them routine, and fit that routine into their day-to-day lives. In this way, they experience and value the control over what they do with medication, as with all other aspects of their lives.
-
The limited but significant opportunities for practitioners to influence older people’s motivation, knowledge and behaviours in the overall medication management process. Generally in NPT, implementation networks and interactions are task-recruited and specific to the introduction of new ways of working after which they may be disbanded. In medication management, although the interactions between older people and practitioners align with Stages 2 and 5, the relationship tends to be more enduring. It is valued for its consistency and qualities such as mutual trust. This can be identified in the table where the final step involves reflection/monitoring. Here, the question in Stages 2 and 5, ‘how did it go’ indicates the work being done to determine satisfaction with the current or recent interaction and also whether or not to confirm continuing trust in the relationship.
With medication management understood as a complex intervention and as implementation, structured and detailed across Tables 5–7, the next subsection explores medication management as burden.
Medication management as burden
Burden was identified as an issue of concern during the research process in step 3a: further literature searches and review (iteration). The Research Team were aware of Gallacher et al. ’s118 research, identifying treatment burden through the workload or demands of health care and people’s capacity or personal resources to ‘manage health problems and follow treatments’, a workload-capacity dynamic that is central to the concept of burden experienced by older people and informal carers. 13–15 A search for articles on burden was, therefore, carried out, linking it with multimorbidity, polypharmacy and chronic disease. Three articles were initially identified: Mohammed et al. ,78 Ridgeway et al. 77 and Sav et al. 14 Several other papers were subsequently identified from included references: Demain et al. ,13 Eton et al. ,82,120 Katusiime et al. ,121 Krska et al. ,122 Shippee et al. 123 and Tran et al. ,124 informing this subsection.
Illustrative examples of burden for each stage of medication management are set out in Table 8: Examples of burden: workload and capacity in medication management. A more detailed analysis of burden and coping is set out in Appendix 5, based on Mohammed et al. ,78 Sav et al. ,14 May et al. ,39 Eton et al. 120 and Demain et al.,13 and in Appendix 6, drawing primarily on the work of Katusiime et al. 121 and Krska et al. 122
| Stage | Stage | ||||
|---|---|---|---|---|---|
| 1: identifying problem | 2: getting diagnosis and/or medications | 3: starting, changing or stopping medications | 4: continuing to take medications | 5: reviewing/reconciling medications | |
| Type of burden example | |||||
| Workload examples | Having a sense of what it feels like to be ‘well’ and of sudden or lasting changes that raise concerns | Making and attending appointments | Reading and acting on patient information leaflets, remembering and following prescriber advice | Ordering medications regularly to ensure a constant supply | Organising pre-review tests, attending face-to-face reviews |
| Capacity examples | Knowledge: about comorbidities to be able to identify new symptoms | Motivation: to make and keep appointments, including tests | Flexibility: to adjust medication routines and daily activities | Organisation: to stick to medications routines | Memory: to recall significant details |
Multiple factors inform workload and capacity: individually, these include the stability or instability of diagnoses, side effects from medications or the total medication regimen; interpersonally, having a consistent relationship with a practitioner such as a doctor, pharmacist or nurse that is founded on mutual trust in which the older person feels confident; or at an institutional or infrastructural level, accessing local services (e.g. being able to make convenient or timely appointments, transport or parking) and information.
However, burden is an individual experience, noted by Mohammed et al. :78 ‘patients on the same number of medicines may experience different levels and aspects of medication related burden’. In addition, in as much as workload and capacity change, the experience of burden is not static and people’s response in terms of coping also fluctuates, as set out in Table 9. This table highlights the relationship between increasing/high workload or capacity, and decreasing/low workload or capacity. Decreasing or low workload and increasing or high capacity are likely to be experienced as no burden. Where both workload and capacity are increasing or high, or both are decreasing or low, burden is experienced. In these two burden situations, increasing or high capacity is likely to indicate coping whereas decreasing or low capacity suggests not coping. Finally, increasing or high workload and decreasing or low capacity is associated with high burden and not coping. Here, there are both workload and capacity risks. The experience of low capacity was summarised in a practitioner’s interview:
Generally coping mechanisms, as in psychological, emotional coping. And we get people who, again it’s not necessarily a diagnosis, but personality. They don’t sort of cope with things well and might need a lot of support with relatively minor things. And that’s not a medical diagnosis. But they’re just sort of quite dependent on professionals or support.
P53 – general practitioner
| What capacity does the older person have (individual specific)? | ||
|---|---|---|
Increasing/high capacity: (examples)
|
Decreasing/low capacity: (examples)
|
|
| What is the workload (stage specific)? | ||
Increasing/high workload: (examples)
|
Burden: coping | High burden: not coping – high workload and low capacity risk |
Decreasing/low workload: (examples)
|
No burden: coping | Burden: not coping – low capacity risk |
The way people experience burden-control is described by Mohammed et al. :78 ‘Patients tolerate, control and adapt all events of a medication related burden until the cumulative effect of the burden interference in day-to-day life exceeds their coping threshold and overcomes their controlling capacity’. Older people and informal carers, who are not coping or at risk of not coping, need to be identifiable to practitioners (see Proposed interventions).
Finally, Shippee et al. 123 provide a compelling account of burden as a mechanism through which ‘complicating’ factors impact on outcomes: ‘a functional mechanism by which medical, social, and personal factors translate into the patient’s experience’. Although this use of the term ‘mechanism’ may not align with the way it is understood in realist terms, it does highlight people’s experiences, linking a range of causal influences and outcomes. The Research Team therefore adopted it in this study by conceptualising burden as a potential mechanism of interest. It has been mapped in to the analysis framework in Appendix 7, enhancing the initial framework set out in Table 2. Nonetheless, the Research Team also acknowledge that burden can also be a context or an outcome in the way medication management is explained and experienced, day to day.
Findings set out above have confirmed medication management as a complex intervention, as an implementation process and as burden. The final subsection below addresses the way it is experienced, drawing on the interviews with older people, informal carers and practitioners from health and care.
Medication management as experience
Adherence and optimisation are emphasised as key outcomes in both policy and the literature reported thus far, underscoring a practice-performance perspective. However, participant interviews provided rich and more divergent causal accounts through descriptions of diagnoses, treatments and experiences of managing medicines day to day and, finally, explanations of the way medication management works, or not, for them.
Outcomes from medication management
Based on the realist interest in causality, interviewees’ outcomes are a useful starting point for analysis (Table 10). Data are presented for each group of interviewees: older people were asked about what they wanted from medication management, informal carers were asked about the outcomes for themselves and for the older person they care for, and practitioners were asked about themselves, older people and informal carers.
| Interviewees | Outcomes | |
|---|---|---|
| Older people | Performance/clinical outcomes:Well what I want is for them to maintain the healthy levels of whatever, my glucose or cholesterol . . . And continue in good health, yesOP4 – older personAppropriate treatment:I just want to stay as healthy as I am at the moment. Well, I’d like to be healthier than I am at the moment but actually to be quite honest, I’m pretty goodOP8 – older personThe outcome that I would hope for would be that I don’t need them any moreOP28 – older personQuality of life:I can be normal and go out and do things and play with our grandson and cook meals and live a lifeOP5 – older personJust to remain as active and mentally active as I can be for as long as I can beOP13 – older personI’d like to feel better but I mean they can’t give me a tablet for thatOP21 – older person | |
| Informal carers | For themselves | Performance/clinical outcomes:I hope that it will prevent [my father’s] vascular dementia from getting any worseC9 – informal carerI want them to keep his condition steady . . . I can cope with that . . . And being safeC14 – informal carerAppropriate treatment:In his best interests. I don’t want him to take anything that is irrelevant, just for the sake of it. I just want it to be something that makes his health and his life more comfortable and pain-freeC12 – informal carerQuality of life:I want to be able to keep [my husband] as well as we possibly can for long as we possibly can. Basically it’s a question of maintaining quality of lifeC11 – informal carerIt’s the quality of . . . being able to stay in vibrant relationships and you know enjoy the things that are around you even though there are limits to that [at 95 years old] . . . within the confines of what we are able to offer when we go out or in the garden, you know he is able to enjoy, physically and mentally enjoy the experiencesC46 – informal carer |
| What informal carers think the older person wants | Performance/clinical outcomes:I think what she would like from her medication is the pain goes and she has better mobility, that’s what she would wantC24 – informal carerTo keep her at home. As mobile really. And as functional really. Because. . . we couldn’t cope if she couldn’t get out of bed really, I don’t think I couldC20 – informal carerAppropriate treatment:I don’t think he gives those things a thought to be honest. He takes it because it is prescribed, and if it is prescribed then it’s something he needsC12 – informal carerLess of itC26 – informal carerAdherence/non-adherence:It’s just part of her routine I think now. I think her memory is getting worse, she forgets why she has got to take thingsC20 – informal carerI don’t know that he thinks about it. He just takes them. Sometimes very occasionally look through and ‘oh not all this lot’C32 – informal carerIdeally, it would be not to take it . . . Just to be able to, just take it and forget about itC15 – informal carer | |
| Practitioners | For themselves | Performance/clinical outcomes:No mistakes I suppose... no errors... in the meds reconciliation. So that we are as accurate as we can be when they change care settings, from acute or from the home to us, and then back to homeP1 – senior pharmacistThe best outcome for me with medication management is that our staff made no errorsP38 – community health service managerAppropriate treatment:First and foremost that the patient is on the appropriate medication for them. And that they understand why they are taking it. And are on board and feel it is necessary. And are not getting side effects from it. And then I guess finally that everything is clear and communicated and easy to access in-patient, out-patient whateverP6 – consultant geriatricianPeople are taking medication appropriately. That it helps them. That it’s not a hindrance. And that if it is causing any ill health or harm that we make sure that we can do something about that . . . that it’s helping them to live well and making sure that people have a good quality of lifeP22 – social care managerThe patient still needs and is benefiting from that medication. Overall, it is still hopefully doing more good than it is harm. I want to make sure that it is still a medication that is based on current guidance . . . And also, I think there is a role to play in ensuring that things are cost-effective, in terms of a brand or generic prescribing. That there is an understanding that the patient has the ability to kind of understand why they are taking itP25 – general practitionerAnd that they feel confident about it really, you know, they know what they’re taking and why, and are happy to take it, rather than just saying I take it because the doctor wants me toP33 – specialist nurseQuality of life:People to be able to manage as independently as they want toP18 – clinical pharmacistI want [them] to be happy, I want them to be well. I want them to be able to live their life as well as they can. So if that means them taking their medication regularly then I’m going to sit thereP45 – formal carerI mean patient satisfaction is probably the big driver really, you know. They’ve got to leave here happy that what you’ve come up with is alright for them . . . and making sure people aren’t on unnecessary medications, it’s always nice if you streamline things and you’re on the most effective, fewest most effective medicinesP53 – general practitioner |
| What practitioners think the older person wants | Performance/clinical outcomes:To make sure, I would say, that it’s helping their condition, whatever it isP22 – social care managerThey want to feel better, and they want to, they feel that either the GP’s [general practitioner’s] listening, something’s being doneP33 – specialist nurseTo feel better I suppose . . . not be on stuff that’s sort of pointless and is, you know, lots of preventative stuff that won’t make them feel better, and actually might potentially be making them feel poorlyP53 – general practitionerAppropriate treatment:Most of them want to be on fewer medications. It might also be exploring the route that is acceptable. So, patients often prefer a patch over taking pills. Reviewing whether there are swallowing difficulties, if we can switch onto liquids as opposed to tablets. Things like that can make a big differenceP6 – consultant geriatricianNot to take them. They don’t want to take them. They just don’t want to, which is understandable. But I think I can sympathise with them, you know. You don’t want to be taking 12 tablets every morning, five at lunch, six at tea, 10 at bed, you know . . . you’re rattlingP45 – formal carerSo many people, I don’t think they necessarily want anything . . . I think they’re just so used to be told this is what they need to take . . . People want to have a bit more control over things sometimes, you know what I mean. It feels that they are back in . . . control of it and able to manage itP18 – clinical pharmacistI don’t [know] if I am entirely clear on what they are hoping to get from it. But I kind of imagine or sometimes I sense that it is benefit . . . I guess the other thing is they also want to make sure they are not feeling any worse taking themP25 – general practitioner | |
| What practitioners think informal carers want | Coping experience:Ease of supply to access the medicine from the chemist or delivery by the chemistP1 – senior pharmacistEasy to take, not too many and noticing that it is making a difference. I think they also really want to be listened to . . . They know the person so much better than we do. And we do need to acknowledge thatP6 – consultant geriatricianI guess they want it to be sort of hassle free. So, you know, nobody likes the difficulties that some experience about getting medications on time. I know that some people get a bit fractious when things look different as well, it’s a different colour or it is not the brand I usually haveP25 – general practitionerConvenience I think in terms of, you know, prescribing stuff that’s a bit more straightforwardP53 – general practitionerJust to keep that person as healthy as possible but doing it safely. Informal carers quite often may be a bit concerned about how they’re giving that medication . . . We’ve had some cases where people really don’t want to do it all because of the responsibility of itP18 – clinical pharmacist | |
There is disparity in outcomes based on interests and role. Older people focus on the way medication fits with and adds to their lives by keeping them as well as possible so that they can do what matters to them. Informal carers identify with this on their relatives’ behalf. However, they also express concerns about ensuring that the person they care for receives the best treatment that also maintains or stabilises their health. This may reflect the way the different groups perceive their role and responsibilities and how these are juggled to fit with other demands and priorities in their own lives, juggling made easier when demands are known, stable and predictable. Practitioners’ outcomes are about good practice and are typically role and performance defined, indicating the complex range of factors that inform what they do: maximising benefit, minimising risk, ensuring safety, reducing cost and sustaining relationships. Medical practitioners appear to accommodate the broadest range of interests in their work. Other practitioners appear more limited by the scope of their role, as part of a network of potentially complementary interests in medication management. Practitioners’ perceptions of older people’s outcomes make a distinction between those individuals who are perhaps trusting or passive about taking medication ‘just as the doctor ordered’ and those who are more proactive in seeking to minimise the burden of their regimen. Practitioners also acknowledge the importance of individual outcomes to older people, including the benefits from medication. They also recognise the value older people attribute to the interpersonal, therapeutic relationship and being listened to within it. Aligned with informal carers’ outcomes, practitioners also appear to be aware of carers’ preference for medication management to be ‘hassle free’ as well as a similar need to be listened to in the relationship.
Implementation work in medication management: focusing on older people
This section sets out interviewees’ descriptions of the way older people manage medicines day to day as they seek to achieve outcomes and minimise burden, as well as the influences on what they do in order to build explanations of the way medication management works, or not, for them. Literature on emergent topics is also included for clarification. To aid navigation, the ‘dashboard’ (Table 11) highlights key themes and the illustrative data extracts from narratives, located by box numbers.
| Routines and control: Box 1 | |||
| Adjusting routines to maintain control: Box 2 | |||
| Being informed: Box 3 | Seeking treatment, and personalised advice and support: Box 4 | Practical concerns about medications: Box 5 | Cognitive concerns about medications: Box 6 |
| Future concerns about coping: Box 7 | Disruptions to routines and control – new practitioners: Box 8 | Disruptions to routines and control – transitions: Box 9 | Adherence to routines through others: Box 10 |
Routines and control
Older people and informal carers devise routines to enable them to feel confident and in control (see examples quoted in Box 1). Routines are the habitual behaviours that people adopt to carry out regular daily activities, such as medication taking,115 in order to minimise cognitive demands and achieve consistency. Initiating and sustaining stable routines contributes to burden management by maintaining the balance between workload and capacity. Doing this successfully underscores a sense of self-efficacy and agency119,125 through the experience of being in control: ‘Routines in medication management help people to regain and to maintain control in their everyday life’. 106
I think I’m fairly confident that I’ve got things under control. And that I hope I’ve got future proof systems for the drugs because I’m probably going to be on this regimen for a long time to come. Well, for ever.
OP8 – older person taking a complex regimen
I know what medication I get, I know that I can get it collected every month, and I’m the one who sticks it in the boxes, so I know when to take it . . . I think the process is important and the routine is important – that’s the key bit really.
OP10 – older person living with mild dementia
They come from the pharmacy in their original boxes. You put them into the dosette boxes. And all of the family have them in the dosette boxes to make sure the routine is followed.
C11 – informal carer who cares for their mother, supported by other members of the family
Adjusting routines
Practices and routines do, however, have to be flexible as they need to be adjusted to fit with day-to-day lives. Adjustments can occur when, for example, an older person’s capacity reduces, when there is a mismatch between the quantities of pills provided and the equipment used to simplify medication taking, or when organisational systems are not aligned or change (Box 2). This is confirmed by Haslbeck and Schaeffer:106 ‘Not only a challenge to develop sustainable routines for daily medication management in the first place; they also have to constantly readjust and change them – a task they have to deal with on their own’.
The boxes used to be on the breakfast table and he used to take his tablets out of the box. And then it obviously became apparent at some stage that he couldn’t do that or he wasn’t doing it. So it was easier for me to just take what he needed out of the box, put it in a little dish, and if I put that little dish by his cereal bowl in the morning and his orange juice and whatever else he has, then it was just a natural process for him to take it.
C12 – informal carer enabling her father
So, with the carousel, I mean, it’s good, it’s not perfect, but it’s solved a lot of problems in some respects. It’s by his bedside, but he’s also deaf. So he’s totally deaf in one ear and has partial hearing in the other. So he wears a hearing aid. But of course he doesn’t sleep in the hearing aid, OK? So the alarm goes off [on the carousel]. That wakes mum up. She’s in her hospital profiling bed at the side of dad. When the carers leave mum at night, they leave a walking stick across the bed and she gets the walking stick and prods him until he wakes up and tells him his alarm’s going.
C09 – informal carer describing the night-time routine for her father’s medication taking
Well, I have a box . . . where I put my daily meds in, my tablets. It’s actually a 7-day box but I use it as a 28-day box because it’s got spaces for breakfast, lunch, dinner. So as I load that box . . . it takes two packets to fill the box. But with this thing [in 10’s] it takes nearly three, and you know, that really is a pain. So when I order 3 months’ supply I always get the same quantity of everything but this, you can see how much this is built up.
OP28 – an older person with mild dementia trying to fit pills that are dispensed in 10s into a 7-day box
I had real difficulty over the sharps bins [for two injected medications]. The Cimzia sharps bins, the [supplier] take and they bring me a new one. The Methotrexate ones . . . [the local hospital] stopped dispensing them. So, I was in trouble. Nobody seemed to know how I could get new sharps bins and dispose of them . . . But I can now get that [Methotrexate] sharps bin prescribed by the GP but collected, whether they should do or not, by [the local authority].
OP5 – older person
Being informed
The increase in complex polypharmacy makes it important for older people and informal carers to be informed about their diagnoses and medications (Box 3). Personalised knowledge can then be built into individual decision-making about routines, day to day: ‘to nudge people to make better choices’ (Thaler et al. 119). Individualised information and collaboration with practitioners, based on shared decision-making,42 are important aspects of this transactional work:
I don’t think it’s difficult. And I think I quite understand a lot of my drugs as well which helps. I am quite interested in the drugs in what they do and what they’re for. So because I understand them, that helps. I read the leaflets, yes.
OP19 – older person
It’s just casually batted off during the appointment. And I think that more effort could be made to help somebody that’s got all these conditions to understand not only the risk factor of not taking medication but also what can be done additionally to help you get off that medication.
C26 – informal carer
Maybe as part of the discharge if they’d said OK this one is to do with protecting the lining of the stomach and maybe you want to take it half an hour before. It’s just those little things that would have been helpful.
C15 – informal carer
Patient participation in discussions about medicines and greater involvement in decisions was found to lead to greater subsequent understanding of treatment, adherence, more satisfaction about both the visit and doctor behaviour, and less regret about the treatment decision.
Stevenson et al. 113
Seeking treatment, and personalised advice and support
Where older people believe that they are not coping as well as they would like, based on their perception of and emotional response to symptoms or changes, they seek help and support to regain control and cope better, whether from partners, practitioners or technology. Examples are contained in Box 4.
When I got rheumatoid arthritis, I was terrible. I was in a wheelchair. I couldn’t lift my arms up. I couldn’t open doors. [My husband] had to prop the doors open when he went to work. I couldn’t walk anywhere on my own. And I couldn’t pour kettles and . . . I was really bad. But I’m on the drugs now.
OP05 – older person
You walk into a pharmacy, there’s somebody there, you can have a chat and they’ll help explain. And I guess the language issue obviously helps as well. So, the pharmacy obviously speak the native languages. So you know, in that case, I wouldn’t necessarily have to go with mum and dad to the pharmacy.
C26 – informal carer
A lady that we saw who was in her 60s but was struggling to remember her medication. And when we looked at different things, we realised that she needed something to prompt her. So, when we looked at her daily routine and we talked about different things, it became apparent that the first thing that she did in the day was to go on her computer and play a particular game. So, with the support of her husband, we put a screen saver on the computer so that as soon as she turned it on in the morning, that had a prompt-reminder for her medication. And then she then had to put a password in which was ‘tablets’ . . . And then we made sure that her medication was accessible, near her computer . . . And then, when we did a follow-up a couple of weeks later, we found that this had actually been really successful, and her blood pressure and her blood sugars were much better controlled. And because of this she also felt much better. So she got that positive reinforcement.
P18 – practitioner describing the work they did to put a system in place once they realised that asking her husband to prompt her didn’t work
Practical concerns about medications
Multiple risk factors may impact on routines and control. There are practical issues about being able to distinguish different medications, described in the data extracts below (Box 5).
When you put the two side by side, yes, you can see there is a difference but again, somebody with poor eyesight or slight confusion might not see that.
OP8 – older person
I’ve had three different colours of omeprazole.
C14 – informal carer
They are all little white pills.
C24 – informal carer
Cognitive concerns about medications
Older people may not cope as a result of cognitive decline (Box 6).
Well, he didn’t realise he was in difficulty with it. But he wasn’t taking his meds regularly. But he didn’t know whether he’d taken something or not.
C09 – informal carer
My father was relatively on the ball. He knew what he was doing. But he was becoming frailer and had been very dependent on my mother to help him manage his medication. But she was getting to a point where she couldn’t. But nobody kind of realised and she thought she was still managing.
C11 – informal carer
It might be as simple as, you know, ‘am I supposed to continue this, how many times a day do I take it’ . . . so just the processing of basic instructions of medications.
P53 – general practitioner
George et al. 126 confirm this situation:
Polypharmacy, cognitive and functional decline, inadequate contact with health professionals, depressive symptoms, poor social support and absence of assistance with medication administration are common risk factors for medication non-adherence among community-dwelling elderly patients.
Future concerns about coping
Older people also have concerns about how cognitive decline or changes in the way that they manage routines may affect their capacity to cope in the future (Box 7).
I mean it does worry me as I get older that I won’t be able to cope so well.
OP5 – older person
I’m also trying to put systems in place just in case I get Alzheimer’s. I’m hoping I won’t but you just never know . . . because if you can put systems in place when you are totally compos mentis, that might help if you are less so. Sort of future proofing really.
OP8 – older person
If certain things happen, if I become widowed or I become more demented, then obviously someone has to take over those systems. So it’s my job to make sure the systems are as foolproof as possible, so that any fool can do them so to speak, someone who doesn’t care as much.
OP28 – older person
Disruptions to routines and control: new practitioners and transitions
Older people’s routines can also be disrupted by external factors such as a new practitioner reassessing the need for certain medications (Box 8) or a care transition (Box 9). Both of these can lead to significant adjustments to medication regimes. Disruptions can have positive outcomes, or not.
Since we’ve been at the new place, they called him in for a review. And the doctor’s taken him off two, three, I think, of the regular angina medicine that he’s been taking for quite a long time.
C09 – informal carer
He came to live here more permanently, registered with the doctor here, and then that’s when you know all the, they reviewed all the medication and set him all up, set everything up here. And that’s when everything became much more automatic and less complicated.
C46 – informal carer
Some of our patients come in on so many different tablets that we need to review why they are on them, do they need to continue on them, can we get them down to once daily dosing? . . . Are they actually taking them? Are some drugs causing side effects which are then, other drugs are given for the side effects? So, it is reviewing whether they need them and trying to stop them if they don’t. And you know, statins in 90-year-olds, is it appropriate? . . . But obviously you’ve got to discuss that with the patient when you are making the decision as well, rather than just stopping things without telling them.
P1 – practitioner, senior hospital pharmacist
Especially when they go in and out of hospital and often their medication changes. And there can often be a delay in the message getting back to the pharmacy or the GP. So we often have to chase that up’
C26 – informal carer
Adherence to routines through others
Older people seek to overcome or compensate for physical and some cognitive difficulties with equipment (Table 12 and Box 10). Functional capacity and the ability to cope will vary over a potentially long trajectory of illness and is dependent on factors such as polypharmacy, increasing frailty or cognitive impairment. Practitioners need to be able to identify older people and informal carers struggling to cope. However, when adjustments cease to be effective or if the older person prefers, they may make the decision to share the burden with informal and formal carers, potentially increasing adherence:
| Functional capacity: coping independently to full, formalised support (based on Functional Independence Measure)127 | Stage | |||||
|---|---|---|---|---|---|---|
| 1: identifying problem | 2: getting diagnosis and/or medications | 3: starting, changing or stopping medications | 4: continuing to take medications | 5: reviewing/reconciling medications | ||
| No help: independent/coping | Managing on their own | Coping | Collaborating: sharing decisions and actions | Coping | Coping | Collaborating: sharing decisions and actions |
| Managing safely and in time, with equipment | Coping by, for example, information seeking, using prompts | Coping by filling own pill box | Coping by filling own pill box | |||
| Help: doing more than half the task – coping with increasing help, including increasing formalisation of support | Contributes with support (e.g. verbal prompts, encouragement/set-up, equipment) | Coping with informal carer/formal carer identifying and querying changes in signs or symptoms | Collaborating: sharing decisions and actions with increasing informal carer involvement and increasingly formalised support | Coping by receiving pre-filled pack, informal carer/formal carer reminding/encouraging to take | Collaborating: sharing decisions and actions with increasing informal carer involvement and increasingly formalised support | |
| Contributes with support (i.e. light-touch help) | Coping with informal carer/formal carer setting up medication to take | |||||
| Contributes with support (i.e. some hands-on help) | Increasing informal carer/increasingly formalised support with monitoring multi-morbidities and medications | Increasing informal carer/increasingly formalised support with adhering to medication routines (degrees of institutionalisation/medicalisation) | ||||
| Help: doing less than half the task – dependent on more formalised support | Contributes with a lot of support (i.e. hands-on help) | |||||
| Unable to contribute, receiving full support | ||||||
At home we have a routine that we stick to, as I say, you know, a schedule.
C35 – informal carer
I manage her medication in the sense that we have a dosette box and I discuss with her GP what we’re going to try next or whether or not things seem to be working and then I make up her dosette box every week. And then four times a day, I actually have alarms, I have two alarms for lunchtime and teatime on my iPhone and then that reminds me to get her meds out and stand over her while she takes them.
C43 – informal carer
And the person who is administering the medication is adhering to the principles and administering the medication as prescribed by the relevant individual, i.e. GP or pharmacist.
P60 – practitioner: formal carer
Support from healthcare professionals and informal carers appear to be two independent predictors of medication adherence . . .
Leung et al. 128
Summary: older people
Within the complexity of medication management, routines appear to enable older people to control and cope with balancing workload and capacity: their experience of burden. Setting up, following and adjusting routines to fit with their lives (Stages 3 and 4) as well as resolving disruptions (Stage 1) increase the workload on older people. Information can increase sense-making capacity, and when older people identify problems they may seek personalised information, advice and support with practicalities, mostly through contact with practitioners (Stages 2 and 5), so that they can improve decisions from which action follows (Stages 3 and 4, as well as 1). When they feel that they are no longer coping, older people may regain a sense of control of medication management through sharing aspects or all of the burden with informal or formal carers. This can also help to ensure adherence. Risks to coping can come from practical as well as capacity issues, such as cognitive decline or increasing frailty. Older people may also experience disruptions to routines following contact with new practitioners or at health or care transitions.
Implementation work in medication management: focusing on informal carers
The MEMORABLE study has identified a significant narrative about the experiences of informal carers, summarised below. To aid navigation, the ‘dashboard’, Table 13, highlights key themes and the illustrative data extracts from narratives, located by box numbers.
| Increasing awareness of problems: gradually becoming an informal carer – Box 11 | ||||
| Learning by experience: Box 12 | Contrast of learning by training and ongoing support: formal carers – Box 13 | |||
| Routines and control: Box 14 | Risk awareness and stress: Box 15 | Other medication management work done by informal carers: Box 16 | Role demands and ambiguity: Box 17 | What informal carers give up: Box 18 |
| Seeking recognition: Box 19 | ||||
Increasing awareness of problems: gradually becoming an informal carer
Informal carers take on this role if asked to by an older person or practitioner, or if they become aware that their relative or close friend is no longer coping with the burden of medication management, highlighted by data extracts in Box 11.
Prior to things going wrong, he’d got a routine . . . it started to become obvious to me that there were tablets lying around loose in the kitchen that he should have taken, and he wasn’t sure. Because I was only there once a week, I wasn’t sure whether they’d been there just for that day, whether they’d been there for days . . .
C9 – informal carer
It’s sort of supervisory, to make sure he’s doing it without taking on the total responsibility.
C11 – informal carer
He relies on me for everything.
C14 – informal carer
Typically, this occurs gradually and involving small changes, such as starting to collect medications because it is more convenient or putting out tablets because packaging becomes hard to handle. However, it can also happen more suddenly if there is a disruptive event that adversely impacts on the existing balance of workload and capacity or creates safety concerns, perhaps as a result of an acute infection that causes weakness and confusion.
Learning by experience
Informal carers tend to learn by experience, have little if any access to training, and can end up taking on more responsibility than formal carers (Box 12).
The frustration of the carer that has learnt an awful lot and knows the picture – I’m not a doctor and I’m not a nurse. But I’ve seen a lot and had to do a lot.
C32 – informal carer
I assumed that there would be a lot of information for me and this respiratory nurse, very pleasant, kind of took me through it. And I watched him . . . We left with bags and bags and bags full of stuff . . . I had to swab and check the line. I had to flush it – so I had to fill syringes with saline. And then I had to mix, it was a powder I had to mix with, I can’t remember whether it was saline or what it was . . . And then I had to use a syringe to get that. That then had to go into the line . . . And then I had to flush it out again. I made sure I closed things. And make sure everything’s sterile . . . And I remember I was tired . . . I remember saying to [the respiratory nurse], ‘I remember the sequence of what I had to do’ . . . he put it in writing, just as a letter to the GP I think, that I had demonstrated I could do it. And like hell I’d demonstrated I could do it.
C32 – informal carer
Learning by training and ongoing support: formal carers
The way informal carers learn about the complexities and risks of medication management as they go along is significantly different from the way formal carers are prepared in advance and supported in their role, as part of their service provision work (Box 13).
In terms of medication management for service users and administration, [formal carers] do a full day’s training that is taught, is face to face. And then they’ll have a medication observation whilst they’re out on district. And all staff get that at least once a year, if not twice. And all staff go on a 12-month refresher for medication.
P38 – community service manager
Our medication training is amazing. It’s a full day. You’re there for seven to seven and a half hours. And then once you’ve done your medication training, you actually come back to . . . where you work and . . . you’re observed giving medication 10 times. If the supervisor’s happy, they’ll sign you off. If you’re happy, you’ll be signed off. But if you’re not happy, yeah, for any reason, you will be then put back on 10 more observations . . . as many observations as you like until you’re happy and you’re comfortable to administer medication on your own.
P45 – formal carer
Routines and control: informal carers
As with older people, informal carers rely on routines and making adjustments to them to cope with the burden of medication management, responding to the older person’s changing needs (Box 14).
At home we have a routine that we stick to . . . a schedule, I should say.
C35 – informal carer
What I do now is I’ve got little ramekin pots and I just put all the tablets in there . . . So at night, I set them both up and I give him his evening tablets. But the ones in the morning are ready, for me. Just lift the ramekin up and put it on his table, ready.
C32 – informal carer
So what I’ll do is I’ll take my dad to his appointment. As soon as I’ve dropped him off, l’ll sit down at my mum and dad’s house and I’ll make the notes there and then. Think about what I need to do next. Stick it in [my book] and then I can forget about it.
C15 – informal carer
Risk awareness and stress: informal carers
A lack of expertise and an awareness of risk, anticipating the unknown, adds to the stress of the informal carer role (Box 15).
It’s quite a difficult position to be in as a carer to know whether, what to do, where to go.
C32 – informal carer
And I’m not an expert either . . . I guess it frightens me, you know . . . all these side effects . . . I don’t want to misunderstand the information because I could misinterpret how to take them and I wouldn’t want to put my dad in a dangerous position where I advise him to do something which compromises his health.
C15 – informal carer
Well I don’t think you actually realise because you’re just constantly adapting . . . You kind of get through that week and then all of sudden you think ‘I am so tired’. And you think ‘why am I so tired? Oh, it’s because I was physically exhausted, and mentally, last week just dealing with it.
C12 – informal carer
You can’t help but feel responsible even though I don’t know whether it is my job or not really, is it. Well it is I know, but . . . People assume that you are going to do things you know. They assume that you can do it. They don’t realise that perhaps sometimes you can’t cope . . . I don’t think they realise how much it affects health, caring.
C20 – informal carer
Other medication management work done by informal carers
Being an informal carer involves finding things out independently, being the link between practitioners and joining the system together (Box 16).
[For medication in liquid form to be given via per enteral gastrostomy] But they don’t supply the syringe. It’s ludicrous. And then they’ll say, ‘Well . . . draw the liquid from the cup’. But the syringe is too big for the cup . . . It’s very frustrating, actually, because, as I say, everybody passes the buck and we’re like, ‘We need a syringe’. And, you know, we can’t get one from anywhere.
C35 – informal carer
I have to chase up an awful lot of stuff. So I’m chasing. And I mean, I don’t know what’s available. There’s nobody overseeing it and drawing it together apart from me but without the professional experience or knowledge of what might be available.
C32 – informal carer
Role demands and role ambiguity: informal carers
There is ambiguity in carers’ ‘informal’ role, including barriers to their involvement in practical aspects of medication management, particularly when changes are gradual (Box 17).
It didn’t help that he refused to have them in the blister packs and the chemist won’t put them in the blister packs unless you get his consent.
C14 – informal carer whose husband has dementia
I’ve been in contact with Social Services. But because he’s refusing any help, they won’t send anybody out because he’s got to be engaged fully with the assessment. But I’m going to the GP’s next week with a view to looking at how his memory is and whether he still actually has ‘capacity’ . . . But I’m stuck.
C14 – informal carer whose husband has dementia
The GP knows that they can give me information about my dad over the phone on his behalf [or] ring me to say my dad needs a follow-up review . . . But that’s only recently . . . They did make it clear beforehand that if I needed information on behalf of my dad then I would need to get a letter from my dad to do that . . . I don’t think my dad has formally provided anything in writing to the GP. I think just through me attending the appointments I think they see the value in me being there.
C15 – informal carer whose father has dementia
What informal carers give up
Informal carers give up aspects of their own lives to take on this role (Box 18).
I wasn’t there every day but I was there 4, 5 days a week. I was staying at mum and dad’s for about 3 nights a week or 4 nights a week and then going back [to my home 50 miles away] for 3 nights a week . . .
C9 – informal carer
[Gave up full time work because] they would need my undivided attention to be able to do all of that. And that was because I wanted the best outcome. And I couldn’t trust with all due respect, even though there are people out there, they would actually do this to my satisfaction.
C11 – informal carer for husband and mother, both of whom have dementia
I had to sell my house and move up here to be near him.
C14 – informal carer
Seeking recognition: informal carers
Being an informal carer of an older person living with multimorbidity and polypharmacy is becoming an increasingly expert and demanding role (Box 19). Informal carers seek recognition as an important contributor to medication management, and health and care delivery.
I think any carer, regardless of whether they’re professional or not, should get that [respect from practitioners] because it becomes – you learn so much. You take so much on. You have to listen to the people that are there all the time doing it . . . because there is a massive responsibility managing other people’s medications. It is such an issue.
C11 – informal carer
I know there’s time constraints on everybody. I do understand all that and there’s pressures on budgets and stuff. But I’m just asking for somebody to communicate with me. Talk to me.
C14 – informal carer
I’ve walked out of the appointments feeling really sad, thinking ‘I’m really angry with the way I’ve been treated and the fact that I let it go’ . . . If they [practitioners] could better understand my role, then I think they could make the most of me and make sure that I know what I need to do so that when my dad leaves, he’s going to do what he’s supposed to do.
C15 – informal carer
Summary: informal carers
Within the complexity of medication management, informal carers gradually take on a role in which they are trusted to meet the health and care needs of the older person they support, amid assumptions about their ability to cope. Learning by experience, largely on their own, they take on what can be an increasingly complex workload associated with setting up, following and adjusting medication management routines and tasks (Stages 3 and 4), as well as identifying problems (Stage 1) as the older person’s needs change. They can be pivotal to individualising health and care delivery, linking with practitioners and joining up systems. They make compromises in their own lives to do this. Stress associated with this role derives from factors such as a lack of knowledge, information, training, or support. Amid varying experiences of ambiguity about their role, they seek recognition for the complexity and burden associated with what they do.
This concludes the findings on medication management, highlighting the perspectives of older people, informal carers and practitioners. Medication management is explained as a complex intervention, as implementation through linked and iterative stages, steps and loops, as burden and as experienced. These findings are the platform for a more detailed analysis of the key stage of the medication management process: Stage 5 – reviewing/reconciling medications. Based on these findings, two possible interventions have been generated: the identification of older people at risk in the way they manage their medications and individualised information. These will be presented in Proposed interventions.
Reviewing/reconciling medications (Stage 5)
Medication reviews being a really critical part of good medication management. And, that could be either at points of transition with people going backwards and forwards between different settings, particularly on the return setting, coming back to you in general practice, or on the timed basis, whether that is a 6-monthly or a 12-monthly review that you have built into your system.
P2 – general practitioner
This section starts by exploring good practice in reviewing/reconciling medication, based on policy and the literature, including articles identified through supplementary searches. It then explores reviewing/reconciling medication and burden, synthesising data from the literature and experiential narratives about older people’s, informal carers’ and practitioners’ experiences of this stage. CMO configurations based on this combined data inform the understanding of burden in reviewing/reconciling medications and ways of mitigating it, expressed in a framework (a programme theory).
Reviewing/reconciling medication as good practice
Reviewing/reconciling medications aims to optimise medication management and improve quality of life in older people with long-term conditions requiring complex medication use. 129 This has been identified as Stage 5 in the medication management process, above.
The need for effective interventions to review or reconcile complex medication regimens and their use by older people has increased because of the growth of polypharmacy, the variety of alternative medications per diagnosis and the need to avoid the risks and costs associated with inappropriate treatments:130
The growing number of older adults who are prescribed multiple medications is at increased risk of receiving PIMs (potentially inappropriate medications) and PIRs (potentially inappropriate regimens). A major challenge in their care is the provision of medication reviews to identify these PIMs and PIRs.
Niehoff et al. 131
Scoping good practice
There are three key interventions associated with reviewing/reconciling medications: a medication review, a medication use review (MUR) and medication reconciliation.
First, a medication review: in terms of definitions NICE33 scopes a medication review as:
A structured, critical examination of a person’s medicines with the objective of reaching an agreement with the person about treatment, optimising the impact of medicines, minimising the number of medication-related problems and reducing waste.
There are three accepted levels of medication review based on patient need, set out by Clyne et al. :132
-
level 1: a prescription review – a paper-based, technical review, such as reviewing older people with a less complex medication regimen, stable comorbidities or who are regularly seen and known to practitioners as part of their on-going management
-
level 2: treatment review – a medication review with full clinical notes but not necessarily the person, an enhancement of level 1.
Levels 1 and 2 descriptors are applicable to reconciliations.
-
level 3: clinical medication review – face-to-face review of comorbidities and medication with the older person, such as regular reviews of older people on more complex medication regimens, with unstable comorbidities or who may be regularly seen and known to practitioners as part of their ongoing management but require a more comprehensive assessment of their overall treatment, particularly if there are concerns about not coping, increasing risk or adherence.
Second, a medication use review: carried out by community pharmacists, these are generally considered to be a level 2 review, but with the older person present. The Quality and Outcomes Framework (QOF), the performance and reward system for primary care services in England, currently distinguishes MURs as a complementary optimisation intervention. 133 In these MURs:
The pharmacist reviews the patient’s use of their medicines, ensures they understand how their medicines should be used and why they have been prescribed. The pharmacist will also identify medication problems and will then provide feedback to the prescriber, if appropriate.
Thus, the pharmacist aims to improve the person’s understanding of their medication, identify side effects and problems and agree solutions. This is often seen as a way of improving adherence and reducing waste.
Finally, medication reconciliation: the NICE Medicines Optimisation guideline33 provides the following definition:
Identifying an accurate list of a person’s current medicines and comparing them with the current list in use, recognising any discrepancies, and documenting any changes, thereby resulting in a complete list of medicines, accurately communicated.
Good practice in reviews and reconciliations is informed by guidelines, evidence and experience. NICE guidelines address them as an element of adherence,72 optimisation,33,84 enhancing the management of multimorbidity and polypharmacy. 29,41 Good practice advice includes:
-
aims: adherence;72 optimisation medication management through safe and effective medication use;33 optimising care through the reduction of treatment burden associated with, for example, multiple medications and appointments for tests, consultations and reviews, as well as unplanned care41
-
reviews: regular or as agreed, led by the most appropriate health professional, with technical and therapeutic knowledge as well as communication skills33
-
reconciliations: timed to coincide with health and care transfers, led by an appropriate health practitioner such as a pharmacist, pharmacy technician, nurse or doctor33
-
individualised, person-centred approach33,41 underpinned by shared decision-making33,41
-
practice content: medication list;72 prescribing decisions;72 evidence base for decisions, including using a screening tool for medication appropriateness such as STOPP/START (Screening Tool of Older People’s Prescriptions/Screening Tool to Alert to Right Treatment),35 balancing evidence for single health conditions in multimorbidity;35 addressing prescribing and deprescribing decisions to include medication and non-pharmacological treatments35
-
risk identification by the practitioner: need for adherence support,72 polypharmacy and high-risk medications35
-
older person information elicited by the practitioner: experience of medication use – knowledge, understanding, concerns, needs72 as well as preferences, behaviours and risk,33 including risk aversion;35 importance to them of treatments, health priorities, lifestyle and goals;35 right to involvement in discussions and making informed decisions35
-
outputs: individualised action plan that addresses multimorbidity and subsequent polypharmacy,35 action plan for any non-adherence. 72
Guidelines are supported by a growing body of research evidence on medications reviews129,135–137 and reconciliations. 138–140 Nonetheless, there is ambiguity about the impact of these interventions. On one hand, there is good evidence of the benefits that accrue to individuals including reducing drug–drug interactions, minimising side effects and addressing undertreatment or overtreatment of diagnoses:130,141
Medication reviews can reduce both polypharmacy and the use of inappropriate medications in the elderly. Our findings support these previous findings and highlight the added likelihood of inappropriate prescribing in the elderly and those with lower educational attainment.
Fletcher et al. 135
On the other hand, however, there is less clarity about gains in terms of adherence, quality of life or, at a system level, avoiding hospital admissions or securing cost savings. 129,142
In addition to NICE guidelines, good practice is also framed by professional and task-specific advice, reflecting practitioners’ roles and responsibilities. Examples in use during this research include the General Medical Council’s Good Practice in Prescribing and Managing Medicines and Devices (2013),143 advice from the Nursing and Midwifery Council on medication management144 and record keeping,145 advice from the Royal College of Nursing on medicines management146 and nurse prescribing,147,148 and the Royal Pharmaceutical Society’s good practice guidance on medicines optimisation, 2013. 149
Practice is also informed through more local organisations, including clinical commissioning groups’ policies, formularies, fiscal directives and incentives, as well as employer-related documentation, induction programmes and training packages, including some associated with academic qualifications that aim to ensure standardised, managed delivery and performance. Reviewing the substantial number of locally relevant documents was beyond the resources or needs of this research.
Interpreting and applying good practice
Despite both guidelines and evidence, good practice is open to interpretation, reflected in the experience of one primary care practitioner:
Is a medication review, a GP sitting in the consulting room on their own looking at a list of the patient’s medication? Or is a medication review, the patient sat in front of you and having a discussion about each and every drug, the indication for it and are they getting any side effects?
P25 – general practitioner
According to Huiskes et al. ,129 there is no gold standard for the way individual practitioners deliver reviews and reconciliations: ‘different levels of medication review are applied in daily practice’. This view is confirmed by the previous practitioner:
I think probably as variably as it is done in primary care, it is probably that the variation goes on in secondary care as well.
P25 – general practitioner
In considering when reviews and reconciliation should be carried out, reconciliations are part and parcel of transitional care, particularly when older people move into and out of secondary care. However, the decision on an appropriate level, content and timing of a review appears to be determined by individual practitioners within their own services, balancing the needs of the older person with resource limitations, including their own capacity, and increasing demand:
Time is very pressured. So when people are booked in for their reviews, if you look at the amount of information that you want to capture and outside of a medication review . . . so it is probably blood pressure, weight, height, how much you drink, how much you smoke, giving appropriate lifestyle information, recording a CHAD-VASc or a QRISK [standardised assessment and scoring systems], making sure that blood tests are up to date if they are needed. There is so little time left to kind of do the very, very thorough medication review, you know, the gold standard one.
P25 – general practitioner
In these circumstances, older people’s and informal carers’ experiences of good practice confirm differences in the regularity, purpose and content of reviews, and the scope of the different practitioners undertaking them. Not all those who meet the criteria for regular reviews receive them:
I suppose now, someday, it’ll happen. But I’ve never been to see anybody and they said ‘Oh well, why are you on so much? And why are you doing this? And do you think we should change it or . . .
OP21 – older person
In addition, uncertainty about purpose and access to reviews in particular can create ambiguity around the way in which older people value and subsequently contribute to them. Their value and usefulness may be more limited when they are perceived as a discrete, performance and policy-driven aspect of health system provision, formulaic in their application, rather than an integral, person-centred aspect of their care:
It’s usually 6 months now I think. Something like that . . . my next one is due in April. So I won’t be able to get these [medications] again until I’ve been reviewed in April . . . When they say review, sometimes it’s the pharmacist you see . . . not necessarily a doctor. In fact, well there’s a practice nurse and a practitioner or there’s a pharmacist or a GP and you never know which one you’re going to get . . . I mean if you’re taking things, you ought to be reviewed. But as far as I’m concerned, once a year is enough.
OP29 – older person
This brief exploration of good practice sets out the guidance on what should take place in reviews or reconciliations as well as experiences that highlight some difference in practice. However, this research is concerned with how such interventions work as an aspect of the medication management process. Accordingly, the next subsection reports findings that explore causality in reviews and reconciliations. In particular, it focuses on explaining how burden might be understood and mitigated, as this is fundamental to proposing interventions that are both evidence- and experience-based. It draws on work included in Appendices 5–8.
Reviewing/reconciling medications and burden
Analysis and format of findings
The findings for this stage of medication management have emerged from the continued application of a causal approach to the analysis of subsets of data, in this case centred on burden. Figure 4 sets out the key concepts in the analysis, informed by NPT. The aim is to understand burden in this stage and to identify potential contributions to its mitigation through implementation processes: sense-making, action, reflection/monitoring and relationships.
FIGURE 4.
Structure of the analysis of Stage 5: reviewing/reconciling medications – understanding burden.
Describing reviewing/reconciling medications
It has been established that in Stage 5, reviews are effectively non-acute interventions, proactively instituted by the relevant practitioner. They provide an opportunity to consider older people’s medication management burden: their workload, capacity and coping, extending to a wider review of needs, goals and care. Typically, discussion largely centres on what is happening in Stage 4: continuing to take medications – what are the medications doing, what effect the medications are having, how do they feel they are managing their medications, what would make managing them easier? However, reconciliations are typically more acute interventions to confirm the current medication regimen as a basis for treatment planning, effectively a reactive process whenever there is change or disruption such as at transitions, often from home to hospital and home again. There may be burdens associated with a transition, such as from an acute deterioration in an older person’s overall health or increase in frailty; the stress and anxiety about an acute illness and a hospital admission; or the loss of capacity from learned dependence associated with the sick role and institutionalisation. These may be distinctly different from the underlying burden that an individual has experienced day to day. On this basis, and having set out burden’s explanatory potential in medication management, above, the workload-capacity dynamic of burden is assumed to be transferable to reviews and reconciliations as a key stage.
Describing burden in reviews and reconciliations: from the literature
Good practice guidelines from NICE72 on adherence do not refer to burden and, on optimisation,33 they briefly mention burden in terms of adverse events impacting on the NHS. Guidance on multimorbidity and polypharmacy36 acknowledge both ‘treatment burden’ from multimorbidity and the ‘pill burden’ from polypharmacy. These are generalised references and not specific to this stage in medication management.
Findings from the searched literature on burden have been discussed previously: see Medication management as burden, highlighting important links between workload, capacity, burden, coping and risk, again in generalised terms.
Describing burden in reviews and reconciliations: from interviews
Focusing on reviews and starting with the workload indicative of potential burden, older people and informal carers have described a range of specific tasks associated with this stage, such as:
-
having to make and attend appointments, including travel arrangements
-
in advance of the review, organising blood tests so that results will be available in time
-
preparing for the review by reflecting on their current medication and noting any feedback or concerns that they want to raise
-
contributing to the discussion and decisions, including considering the options and adjustments that will be required when back at home
-
needing to contact the pharmacy about new prescriptions and dispensing adjustments, especially where they have blister packs, disposing of discontinued medications, reading and acting on new patient information leaflets and developing a new routine to accommodate any stops, starts or changes to dosages.
Informal carers may gradually become more involved in these tasks, particularly if the older person is becoming increasingly frail or cognitively impaired. Informal carers may start by providing transport and arranging review appointments. However, they may eventually become a key participant, reflecting their contribution to managing their relative’s or friend’s medications on a day-to-day basis.
The workload for practitioners might include:
-
setting up a recall system
-
generating invitations to attend a review and following up non-responses
-
agreeing a convenient date and time to fit with older people’s travel arrangements and practitioner commitments, such as routine and emergency appointments
-
facilitating discussion and decision-making
-
agreeing plans and next steps
-
communicating outcomes to other practitioners involved in the older person’s medication management
-
acting on any plans such as requesting further blood tests, generating new and cancelling previous prescriptions, and making referrals to other practitioners.
Following on from workload and tasks, the other aspect of burden in this stage is capacity. Capacity can be domain-associated: physical such as dexterity, vision, hearing or stamina/fatigue; affective, particularly the personal motivation and confidence to be engaged in the process; and cognitive, including memory and executive function that are important to effective communication, decision-making and action. Capacity can also be externally generated as part of a ‘compensation’ strategy such as by getting encouragement, help and support through the involvement of informal carers.
Explaining reviewing/reconciling medications and burden
The subsections that follow set out the findings on reviewing/reconciling medications and burden, highlighting key themes that have explanatory value in understanding the complexity of burden in this stage and contributing to the framework in Figure 5. To aid navigation, the ‘dashboard’ (Table 14) highlights themes and the subsections in which they are set out, burden types and key steps.
FIGURE 5.
Summary of findings for Stage 5: reviewing/reconciling medications, mitigating burden.
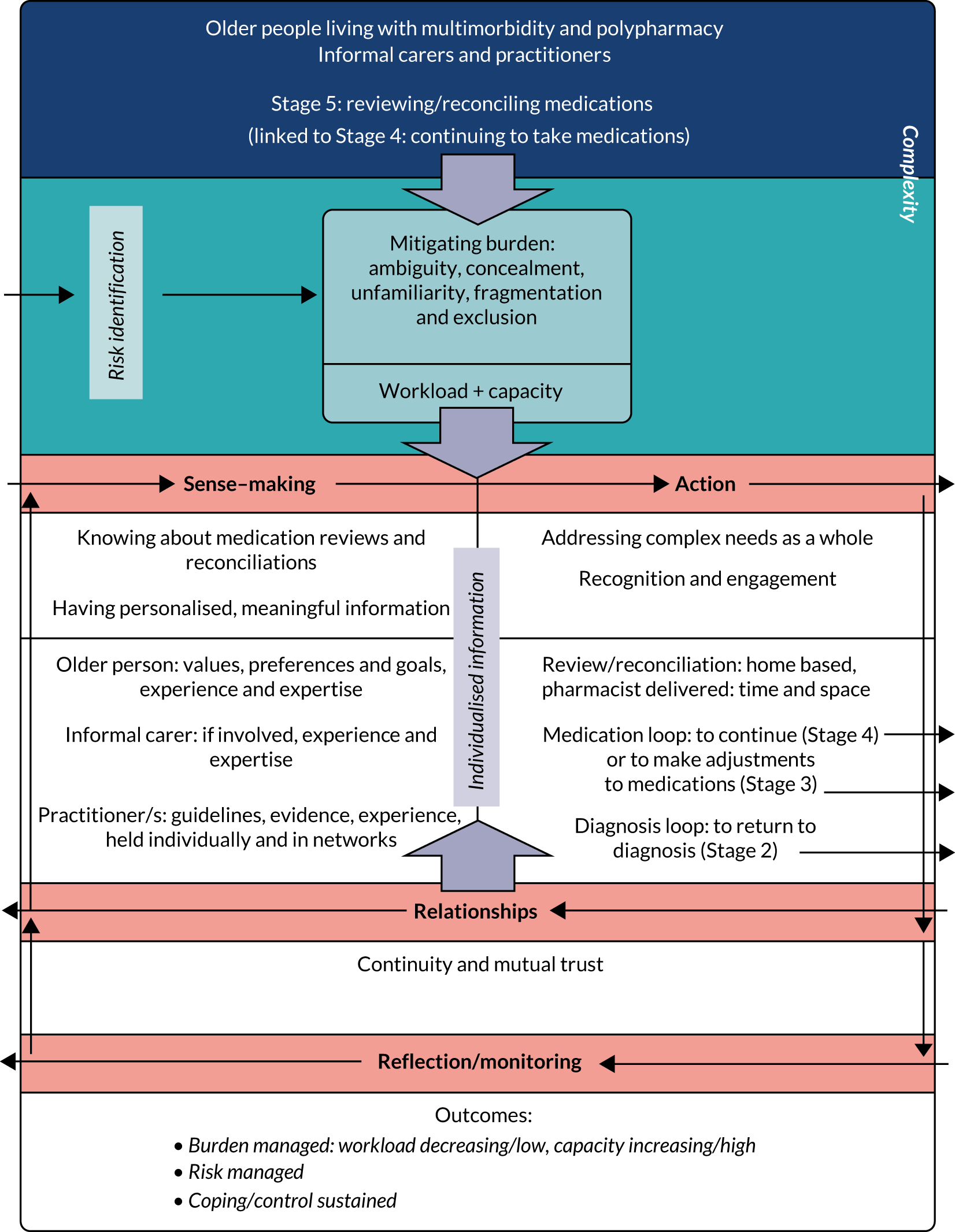
| Key theme | Burden type | Key step |
|---|---|---|
| Knowing about medication reviews and reconciliations: purpose and content, timing, practitioners’ experience and expertise, home-based reviews, reconciliations | Ambiguity | Sense-making |
| Having personalised, meaningful information | Concealment | Sense-making |
| Continuity and mutual trust | Unfamiliarity | Relationships |
| Addressing complex needs as a whole | Fragmentation | Action |
| Recognition and engagement | Exclusion | Action |
Subsections explain each theme, setting out summary CMO configurations evidenced with extracts from the literature, as well as from interviews. CMO configurations are prefixed with RR as an abbreviation for reviews/reconciliations in this stage of medication management. This causal analysis supports the stratification of burden into five types: ambiguity, concealment, unfamiliarity, fragmentation and exclusion, locating these burdens as mechanisms. The researchers acknowledge that if this stratification was applied more widely to the analysis of data, as opposed to identifying the way that the identification of these burdens evolved, burden in general as well as specific burden types could also be contexts or outcomes in more generative explanations of the way medication management stages work.
Illustrative quotations have been selected to provide a rich and insightful example of interviewees’ experiences or because they make a unique but important contribution to understanding the topic. They add to the understanding of each subsection through subtle nuances, often lacking in the literature, that explain the interviewee’s experience of burden, directly or indirectly. The number of data extracts under any configuration does not imply a greater weight of evidence but illustrates its range, highlighting, for example, the difference between the perspectives of older people and practitioners, or the particular experiences of informal carers. CMO configurations contribute to explaining the stage and the burden within it.
Despite focusing on reviewing/reconciling medication (Stage 5), theorising about this stage contributes to confirming, refining or refuting the candidate programme theories on medication management as a whole that were set out at the start of the research (see Step 4: candidate programme theories and substantive theory). Figure 5 summarises the implementation steps within reviewing/reconciling medication (Stage 5) and, for each step, CMO configurations that describe and explain the researchers’ causal claims. Figure 5, therefore, represents a detailed subsection of the research’s emerging programme theory, focused specifically on Stage 5. This will be considered in Chapter 4.
Knowing about medication reviews and reconciliations: ambiguity
Purpose and content of reviews/reconciliations
Validating the work on generic ‘good practice’ set out in Describing burden in reviews and reconciliations: from the literature, the literature highlights the importance of reviews, reconciliations and an individualised approach as a way of influencing older people’s experiences in Stage 4: continuing to take medications:
Patient-focused, structured and collaborative health care service provided in the community setting to optimize patient understanding and quality use of medicines.
Basheti et al. 130
In the process of medication review, healthcare professionals can assess the need for changes in medication, ensure patients’ understanding of the medication regimen and provide review for patients who are at risk, while patients can discuss their perception regarding adverse effects or effectiveness of the prescribed medication.
Leung et al. 128
Reconciliation process at discharge, with individual attention and subsequent monitoring at the patient’s home could ensure appropriate compliance with chronic treatment, with a consequent reduction in medicines errors and adverse effects.
Marmesat et al. 150
Medication improvement strategies to shift from a system repairing overdue maintenance to a more individualized approach . . . related to a patient’s use of medication in the context of his medical condition. For instance individualized medication coaching consults with non-adherent patients or patients experiencing drug-related problems or adverse events.
Huiskes et al. 129
Informed by the literature, work on the initial CMOs identified the way, for example regular medication reviews and transition reconciliations by experienced practitioners optimise medication management by minimising the risk of treatment-related problems [Lit (literature) CMO 05] or when practitioners carry out a medication review in an older person’s home, they are more likely to identify medication-related problems because they understand people’s lived experiences and they take more time (Lit CMO 06).
Interviewees, however, present a less consistent picture of their experiences of reviews, with older people and informal carers describing uncertainty about purpose and content. They struggle to reconcile having a review of their medication, separate to a review of a single diagnosis from the many that they have, such as a diabetic or cardiac review, when they are living with multimorbidity. Some reviews are also perceived as being undertaken to fulfil practitioner/service performance requirements rather than to meet their needs (Box 20).
After a blood test. So it’s a review of your bloods and what I would expect to be a review of medication. I don’t actually think it is a review of medication. It’s a review of your blood test. It’s also apparently now, also a dementia review.
OP10 – older person
Regular reviews of parent’s medications with the GP or pharmacist? No, not particularly . . . because there’s such a high frequency of contact.
C26 – informal carer
Because of his diabetes and that gets reviewed when he has his annual diabetes review . . . specialist nurse . . . to be fair I don’t know how his asthma’s going to be reviewed. I guess that would be done through his GP now.
C15 – informal carer
We get more discussion, support etcetera, and reviewing, through the research program. And that’s when [my husband’s] overall review comes. Because they take bloods forever. They weigh you. They examine you. You have your blood pressure taken. They talk to you. They ask you questions ‘til you’re blue in the face.
OP10 – older person whose husband is participating in research
Getting an appointment with him is more difficult, except when they ring up and say they want me to go and see him and he’s sitting behind his desk fuming because it’s one of these national, government sort of things that he’s got to do.
OP13 – older person
Practitioners appear to recognise the value of medication reviews, if not always their potential (Box 21).
It would make my life a lot easier if everybody was reviewed, every medication had a really good review as to why they are on it. Because that would save so much time . . . you know . . . looking at do they really still need it and what the evidence base is for them still needing it.
P1 – practitioner, senior pharmacist
We had someone the other day . . . I’ve been dealing with seeing every couple of weeks . . . rang the chemist to say, ‘Oh, can you just slow down the delivery of my dosette boxes because I’ve got quite a backlog?’ . . . And he had a months’ worth of dosette boxes that he was sort of picking and choosing from. Some were half empty. Some were full . . . So what we thought was happening and what was actually happening were just very different things.
P53 – general practitioner
People that go out in the community and look at what patients have got. And you know stop re-issuing things that (a) shouldn’t be prescribed anymore and (b) that they have got you know a 10-year supply of. And it just becomes beyond a joke. I mean there is just so much waste . . . We could save millions in the NHS if we just monitored medication properly.
P30 – practitioner, specialist nurse
The evidence highlights ambiguity in the purpose and content of medication reviews, in particular.
Timing of reviews
Box 22 highlights significant variation in the older people's and informal carers' experience of the timing and regularity of reviews. Practitioners also acknowledge potential differences in the interpretation of ‘good practice’ (Box 23). This evidence underscores ambiguity about timing.
Dad has reviews every 3 months . . . by the GP.
C14 – informal carer
It’s a 6-monthly review, yes.
OP19 – older person
The GPs insist once a year that I go and have a review for my rheumatoid arthritis.
OP5 – older person
If she does go to the doctor, which isn’t very often, they probably do do a review then. But it’s not done on a regular basis, I assure you of that.
C35 – informal carer
I can’t remember the last time we had a review.
C12 – informal carer
I suppose I have to be proactive in requesting an annual review.
C46 – informal carer
A regular, by which at least yearly review of patients who are on multiple medications, so that’s usually older patients. It could be by a doctor, pharmacist, nurse with a view to check that all medications are currently still indicated, that there isn’t inconsistent prescribing or multiplication of drugs or prescribing something to counter the side effects of another medication. And just rationalise what’s being prescribed. And confirm that the patient is taking it. And whether they are taking any additional over-the-counter medications as well.
P6 – practitioner, consultant geriatrician
I think it was that medication reviews would generally be about 6 monthly [under QOF]. And also, that would apply to patients taking maybe four or more medications. But if you had less than that then I think the requirement could be kind of annually. And then somewhere along the lines it was actually felt that face to face would be sufficient annually and maybe the 6-month interim, could just be, may not be face to face. So, it may be just sort of the scenario that I described, where you are looking and saying ‘Oh yes, everything is up to date and yes that seems appropriate, we will go on until the next face-to-face review’. And I think that was got rid of in QOF some years ago but I think practices, well certainly our practice, still use that kind of framework in terms of medication reviews currently.
P25 – general practitioner
Practitioner experience and expertise
The combination of professional requirements, experience and expertise required to carry out reviews and reconciliations is available among doctors, nurses, pharmacists and pharmacy technicians. However, evidence exists in the literature of the development of an enhanced role for pharmacists to carry out reviews and reconciliations because of their particular knowledge and skills. However, there is also evidence of significant variation in the range of activities already undertaken by pharmacists in carrying out this work, from simply providing a record of medications dispensed, to more collaborative interventions that directly engage with and support older people and practitioners with complex medication management. 151
Community pharmacists could be more involved in medication review interventions for older adults, with their contribution extending from identification of DRPs (drug related problems) to a more holistic contribution to medication therapy management.
Kallio et al. 151
Interventions involving pharmacists in medicines management, such as medicines reviews (with positive effects on adherence and use, medicines problems and clinical outcomes) and pharmaceutical care services (consultation between pharmacist and patient to resolve medicines problems, develop a care plan and provide follow-up; with positive effects on adherence and knowledge).
Ryan et al. 111
Important role of pharmacists in taking accurate medication history across various hospital transitions. Pharmacists’ increased knowledge and acquaintance with the medications allows them to extend support to other healthcare professionals including doctors and nurses by acquiring precise and complete medication histories from the patients.
Cheema et al. 138
Active interventions by pharmacists including medication reconciliation, tailored patient counselling, and provision of telephonic consultation with patients following hospital discharge were associated with clinically important reduction in medication discrepancies.
Cheema et al. 138
Impacts on cardiovascular disease and diabetes were the most frequently reported clinical outcomes. Pharmacist services in these therapeutic areas may be particularly valuable because warfarin, insulin, oral antiplatelets and oral hypoglycemics are implicated in up to two-thirds of hospitalizations linked to ADEs.
Jokanovic et al. 152
High levels of workload are perceived as a major safety concern in UK general practice, and one of the main policy drivers of pharmacist role development is to free clinical and administrative time for GPs.
McNab et al. 139
Interviews confirm the diversity of practitioners involved in reviews and evidence the increasing contribution of pharmacists, supporting general practitioners (GPs), described in Box 24.
We always see the GP themselves.
OP19 – older person
I have once or twice had reviews of my medication with the pharmacist. Certainly not frequent. The prescriptions always say ‘to be reviewed by the GP’, of course, on such and such a date. And everyone ignores those. So some of those dates are a year late . . . But the pharmacist is supposed to review, and one is supposed to say to a pharmacist ‘I’m feeling a bit funny about this new med’ and they’re supposed to advise you, aren’t they?
OP28 – older person
The pharmacist did pick up on the fact that I was a patient with lots and lots of drugs – and ‘Come and have a chat’.
OP8 – older person
I wouldn’t hesitate to talk to the pharmacist, because they really are very helpful.
C12 – informal carer
Pharmacists in GP practices, I think there is a big role there isn’t there for reviewing medication.
P1 – practitioner, senior pharmacist
We have three practice-based pharmacists . . . CCG [Clinical Commissioning Group] employed . . . they do a number of audits including safety audits . . . they will inform us if there has been a hospital admission that could have been linked to a medication – [they do] a kind of root cause analysis on that . . . But they also have an extended role . . . assist us with medicines reconciliations . . . Also, if there are any clinic letters coming from consultants . . . they also assist in on those changes and liaise with community pharmacists . . . For the housebound patients . . . they have additional expertise in the over 65s.
P25 – practitioner, GP
I give them about a fortnight because as I say I’m due for the review and then the appointment arrives and you never know who you’ll see whether doctor, pharmacist or nurse.
OP29 – older person
The evidence highlights ambiguity in the roles, experience and expertise of those likely to be undertaking medication reviews, in particular potentially exacerbated by workforce innovations in accessing appropriate knowledge and skills in line with the complex needs of older people.
Home-based reviews
Conducting medication reviews in older people’s homes is seen as an effective way of undertaking a comprehensive assessment and planning interventions that fit with older people’s day-to-day lives, as well as being effective for post-discharge reconciliations. Communicating the outcomes with those involved and the wider multidisciplinary team is an important part of the review or reconciliation:
It is necessary to visit patients in their own homes to get the complete representation of a patient’s medication regimen.
Basheti et al. 130
For some patients, the home setting can be a suitable venue for medication review and education after discharge from hospital.
Kogut et al. 140
Older people and informal carers value being reviewed at home, where the reality of managing complex regimens and fitting them into day-to-day lives can be better understood and those at risk identified (Box 25). Home-based reviews appear to be particularly effective for identifying adherence issues.
It would be very nice to be able to access somebody who perhaps will come and see you at home, as I used to do for people for 26 years, if we needed to . . . And sometimes it’s better to have people at home because you’re in your own environment – you feel in control . . . It’s a shame really, because they should come and see how it affects your day-to-day life but they can’t.
OP10 – older person
The relatives are sometimes there when I go out to see a patient at home and can certainly help with, you know, advice as to whether they’re taking what’s prescribed or whether they’re not . . . It certainly helps me though in terms of when they say, ‘Oh this isn’t working, this isn’t working’. ‘Well actually you’re not taking it?’. And little voice pipes up from the kitchen saying, ‘Yes’.
P30 – practitioner, specialist nurse
If we refer somebody to [our practice-based pharmacists], then they will come into the practice, review their medical record and then they will go out to see them in their own home. And then depending on their level of experience, they will come back in and say ‘I think we should stop this, this, this and this’ or ‘I think we should start that’ or ‘We need to get a blood test done on this’ . . . That has been really beneficial because there have been incidents when they have gone out and they have found boxes of stock that would make your eyes water, like 17 salbutamol’s in a cupboard or boxes of insulin in the fridge.
P25 – practitioner, GP
Often, even when [GP’s] are doing home visits, they may not be aware of those adherence issues or patients are very reluctant to open up to them to say that they’re struggling with their medication because you know, they’re the ones that have provided it. Whereas going in a lot more neutral like we do, and with that non-judgmental thing, people tend to open up to you a lot more about how they are or not coping.
P18 – practitioner, clinical pharmacist in a community team
Everybody that’s referred to the service is offered a domiciliary visit if that’s appropriate . . . And do a full review . . . We would still always look at if there’s an adherence issue, if there’s any clinical issues that can be optimised. And also if there’s any cost-saving initiatives that can be done as well . . . We would do that review in the home . . . we did have a gentleman recently who, once we looked in his house with his consent, and looked at the medication quantities in there, he’d obviously struggled with adherence for quite a long time . . . was having very variable blood pressure, we think, because of this. He was having a lot of falls. And whenever the surgery were checking his blood pressure, it was quite high. So they were just adding and adding treatment. But the rest of the time obviously he wasn’t taking it.
P18 – practitioner, clinical pharmacist
The evidence highlights ambiguity between the known benefits of and preferences for home-based medication reviews, and the resources available for this work.
Reconciliations
Reconciliations are purposeful and time specific:
Transitions of care between hospital, GP and care home are points of particular vulnerability to medication error. There is some evidence that a pharmacist review of medication may improve the use of appropriate medication in these circumstances.
The process of medication reconciliation should account for any alterations made in the medications taken by patients and should make sure that patients or their carers have been made aware of these alterations.
Cheema et al. 138
Practitioners acknowledged the contribution of reconciliations to medication management at times of significant risk (Box 26).
We do meds reconciliation when they first come in to us, so we are checking that their medication is correct and tallies up with the GP print out if appropriate or with the print out from the acute trust. Speak to the patients. Try and sometimes reduce the tablet burden, with a view to them going home . . . But we will be reviewing their medication whilst they are in hospital and checking things like compliance, why they are on things.
P01 – practitioner, senior pharmacist
[Practice-based pharmacists in primary care] assist us with medicines reconciliations. So if somebody has gone into hospital. Maybe they have had their medicines changed or reviewed in hospital, or quantities need altering etcetera. They pretty much do that.
P25 – practitioner, GP
This subsection highlights ambiguity between the knowledge, practice and experience of medication reviews in particular. Ambiguity covers purpose and content, timing, practitioners’ experience and expertise, home-based reviews and reconciliations. This is explained in the following CMO:
When practitioners carry out medication reviews and reconciliations in ways that are not consistent with guidelines and good practice, integrated within the wider processes of complex health management with older people living with multi-morbidity and polypharmacy (C), individualised (C) and involving their informal carers where relevant (C), they do not engage older people and informal carers effectively in this key intervention that supports their health and wellbeing (O), and waste time and other resources (O), because they generate ambiguity (M).
RR CMO 01
Ambiguity requires clarification of all aspects of reviews and reconciliations set out above, initiated in and contributing to sense-making.
Having personalised, meaningful information: concealment
Practitioners draw on evidence from published sources as well as that gained through continuing professional development opportunities to inform and substantiate their practice. However, there can be tension between guidelines and experience, and the degree to which more powerful practitioner groups or individual practitioners feel confident to adapt them to meet the individual needs of an older person (Box 27), as well as limitations in the scope of some guidelines, such as those only relating to single rather than multiple pathologies.
We’d follow NICE guidance with a view to what medication our elderly patients should be on . . . Then we have got an area prescribing formulary as well, so we’d adhere to that . . .I think we probably just stick to guidelines wouldn’t we more, just because we would want the evidence as to why we have done something . . . other healthcare professionals are more likely to follow guidelines than perhaps the [more senior doctors], just because it is their patient at the end of the day isn’t it and they would expect everyone else probably to justify why they are making the decision and that’s the evidence isn’t it.
P1 – practitioner, senior pharmacist
We have the Medication Administration Policy which is the trust policy and we work to that. And obviously with the NMC [Nursing and Midwifery Council], we have guidelines on the administration of medicines and prescriptions which again is something we draw upon as well. But most of the trust guidelines are in line with the NMC guidelines anyway.
P30 – practitioner, specialist nurse
We’ve got formulary . . . which tells us what we should prescribe. You’ve then got your personal experience and preference of drugs and obviously that’s influenced by I gave this person this drug once and it, they were really ill so I won’t use that again. Sort of like sample size of one . . . And also just sort of less obvious stuff, you know, I’ve used this quite a lot and actually I think I probably do prefer this other one, I find that people respond better to it. And so yeah, the personal experience and preferences. You’ve . . . well obviously you use the various guidelines and NICE and [Clinical Commissioning Group] guidance in terms of in what order to prescribe drugs . . . that sort of pretty much frames what we do.
P53 – practitioner, GP
I do a lot of going to conferences, you know, the primary care conferences – British Geriatrics Society . . . I’ll read information that comes through . . . I give all the NICE guidelines and all those sorts of things out to [staff] . . . I also organise training days . . . We’ll also get people in, so we have clinical meetings every quarter and I try and get a speaker. It’s great for us because it means that we’re constantly updated.
P22 – practitioner, manager of supported care facility
We have got this array of single-disease guidelines and the majority of patients do not have a single disease. They have multiple long-term conditions.
P25 – practitioner, GP
Older people’s and informal carers’ contact with practitioners provides them with an opportunity to receive individualised information that addresses their own combination of multimorbidities and polypharmacy. The exchange of information enables them to be more equally involved in shared decision-making in this stage and potentially influences and informs day-to-day, individual decision-making and actions at home, supporting self-management and coping. The knowledge, understanding and confidence that come from information sharing contributes to increasing control and collaborative capacity, whether sourced from evidence or experience. Conflicting information is disruptive and has an impact on their confidence in practitioners (Box 28).
The one who was adamant about him coming off the lorazepam had this one mind set. And the other one who said ‘Well actually, I think we should keep him on it because I’m quite happy’ . . . The hospital and one GP having one attitude and other GPs having other attitudes . . . 30 or 40 years he’s been on it.
C14 – informal carer
Some of them say don’t take with your antacids. Some say take before food. Some say hours before or minutes before or, so and after . . . And it seems to be regardless of what it actually says from the pharmacy about ‘do take with this or don’t take with that’.
C24 – informal carer
A few older people and informal carers seek information from other sources such as newspapers and websites despite not being sure about quality. In some cases, it is sufficient to feel that the information they find is useful whether or not it is derived from a safe or trustworthy source.
This subsection highlights concealment of information. Findings are summarised in the following CMO:
When practitioners do not provide information in ways that are personalised, meaningful and consistent (C), they do not engage older people and informal carers effectively in this key intervention that supports their health and wellbeing, including self- management (O), and waste time and other resources (O), through concealment (M).
RR CMO 02
Concealment can be addressed through the exchange of information, generic and individualised, ensuring that it is understood and useful, to support sense-making.
Continuity and mutual trust: unfamiliarity
Mutual trust is an emergent property of the relationship between the older person, the informal carer and the practitioner. It is central to practice. 148 Once established, continuity enables trust to be sustained. Furthermore, it engenders loyalty in older people and informal carers towards individual practitioners, especially GPs and their decisions. However, there are also risks that it can also be frustrated or irreconcilably lost.
Trust develops over time, from seeing the same practitioner in whom they are confident, underscoring the importance of continuity of care to practice and practitioner relationships:153–157
The patient–provider relationship is the fundamental starting point for patient centred care delivery. Central to the patient–provider relationship is trust.
Murray and McCrone157
Across diverse clinical settings, patients reported to be more satisfied with treatment, to show more beneficial health behaviours, less symptoms and higher quality of life when they had higher trust in their health care professional.
Birkhäuer et al. 112
Continuity of care is a long-standing feature of healthcare, especially of general practice. It is associated with increased patient satisfaction, increased take-up of health promotion, greater adherence to medical advice and decreased use of hospital services.
Pereira Gray et al. 114
We know that hospital admissions are often undesirable for patients, and there is some evidence that if GP care is managed by the same practitioner over a long period of time, patients have fewer emergency admissions.
Stafford et al. 6
The experience of mutual trust and consistency is described in Box 29.
I just think that they know who you are. They don’t to need to read . . . it’s all very well saying oh yes you’ve got to read this but I mean, they can read what you came for last time yes, but that doesn’t give you the overall picture does it . . . of your patient? I mean if you’re a doctor and you see the same person you know, you pretty well know why they’ve come. So I think it’s better for them.
OP21 – older person
We’ve got at least five GPs, maybe six. It’s a training practice and you can always get an emergency appointment on that day. If you want to see a particular doctor you may have to wait but if you were in an emergency you’ll see anyone, won’t you?
OP19 – older person
In terms of decision-making, you’re the person best placed to make decisions, if you would recognise things that another clinician might not. That whoever it is I’m seeing in front of me just isn’t quite right today . . . normally they would be bright as a button. But I’m seeing them today and I can – they’re just not right, you know? So recognition of what this person’s normally like or how they normally would present. The sort of situations where we’ve been here before . . . If you’ve seen that person a lot you’ll remember that and you’ll remember how you managed it last time. And the medical records don’t give the story. Reading the records isn’t the same as actually going through it and the nuances of sort of experience with this individual, knowing how people respond . . . how to package management plans.
P53 – practitioner, GP
Disruption to services and practitioners is experienced negatively by older people and informal carers, even generating avoidance. This is illustrated in Box 30.
[At the other place I lived] the pharmacist, I’d go in: ‘hello [name], here’s your stuff’ and all the rest of it . . . and they might well have picked up on something unusual. I can’t guarantee it but . . . I always felt they might have done. Whereas here, because it’s a biggish [pharmacy], there’s fluctuating staff . . . unless someone’s checking the paperwork, they might not pick up on something unusual.
OP8 – older person
They didn’t know him [my father] . . . because they have all just recently changed, which is a bit unfortunate . . . And he said ‘They don’t know me. They don’t listen to me. They don’t ask me how I am. So I am not going to sit there and tell them how I am feeling when they know nothing about me’.
C12 – informal carer
At the GP level, it just seems to be churning patients and numbers, you know. There’s so many appointments in the day. There’s only so much time per appointment. You can only discuss one issue at a time. You go in and you spend half of the time trying to work out what happened on the last occasion and who was the last GP to see the patient.
C26 – informal carer
Disruptions in staff and services are also problematic for practitioners, arising from reorganisations, financial cuts and new initiatives, occurring in succession and, increasingly, together (Box 31).
We used to have a woman [smoking cessation] that did the whole of [the city] and she was based at a hospital . . . and she would take all the referrals, and arrange appointments and things like that. But for whatever reason, 3 years ago or so, that funding was pulled and the service was disbanded.
P33 – practitioner, senior community nurse
The feedback that we [a pilot] got everywhere was sort of, ‘Yeah, but in 6 months you’re going to be gone, so what’s the point of us using this service?’. Yeah they’re used to pilots coming and going and things. So actually to have, still, 3 years down the line, still be here they’re like ‘Oh, you’re still going, oh right, we can use this then’ . . . I’ve spoken to other people that have done sort of community work similar to this. It does take a long time to gain those peoples’ sort of trust and you know, respect in the service.
P1 – practitioner, senior pharmacist
We’ve had quite a lot of pharmacy changes. We had a couple of Vanguards. And one of the Vanguard’s involved having a particular company coming in and supply pharmacies into a number of GP practices. So we kind of linked with those initially and now that again has finished now and they’ve all come out. But they’re now trying to, through the CCG [Clinical Commissioning Group], introduce another GP pilot of pharmacies working in the primary care.
P1 – practitioner, senior pharmacist
One CCG [Clinical Commissioning Group] . . . they’re doing, it’s [area wide] . . . they want this all singing, dancing respiratory service [for] admission avoidance and stuff like that. But actually what they’re talking about, rapid response already do [but in the] rapid response team no-one knows anything about it. So they want a service that is already there . . . And actually, patients with comorbidities, if they’re breathless and they’re cardiac, I mean it’s not patients’ responsibility to know whether they’re cardiac or respiratory that’s causing them to be acutely unwell – who do they contact if you’ve got two separate services?
P33 – practitioner, senior community nurse
Initially started with six practices in one network and then expanded to other networks but then since then, as typical in sort of CCGs [Clinical Commissioning Groups], the networks are not networks any more, they’re federations and they’ve changed about three times since.
P1 – practitioner, senior pharmacist
The thing is it’s knowing what facilities and who’s available out there, I mean coming into the community I’m finding people that I didn’t even know, and their roles, existed . . . But then nobody talks and checks upon each other unless you go to sort of a national conference or somebody just happens to find out.
P33 – practitioner, senior community nurse
It’s like we’re sitting on these, doing these sort of workshops. And it’s almost like, God, what goes around comes around . . . we sat and did this 25 years ago. There was certainly a lot of good stuff we did that I think’s got lost over the years . . . And we’ve got those good relationships now but they’re not as close as they were. I think we’ve lost, you know, a lot of good working really.
P38 – practitioner, community service manager
This subsection explores unfamiliarity. Findings are explained in the following CMO:
When there is change and inconsistency in services and practitioners (C), older people, informal carers and practitioners do not establish and sustain mutual trust and confidence that comes from enduring relationships (O), and the foundations for shared decision making (O), as well as the co-ordinated management of complexity and risk (O), because of unfamiliarity (M).
RR CMO 03
Unfamiliarity can be addressed through interpersonal work to build and sustain relationships, supported by systems that ensure space, time and stability in which this work can occur.
Addressing complex needs as a whole: fragmentation
The health and care needs of older people and informal carers living with multimorbidity and polypharmacy are best addressed by a co-ordinated network of practitioners. Responsibility for carrying out medication reviews is increasingly being shared by doctors, nurses, pharmacists and pharmacy technicians across a number of organisations and sectors. Effective medication review is reliant on good interprofessional communication to bridge gaps and reduce any tension between this multiplicity of teams, staff, the older person and any informal carers involved:
Medication reviews, generally performed by physicians, are increasingly involving pharmacist and nurses as well.
Kallio et al. 151
Benefits of face-to-face ‘case conference’ communication between pharmacists and GPs as part of medication reviews.
Jokanovic et al. 152
Value of a multidisciplinary approach to preventing adverse drug events is widely acknowledged. Collaboration and teamwork between patients, general medical practitioners (GPs) and pharmacists is important to achieving the safe and effective use of medications.
Jokanovic et al. 152
Delivery models for health largely support specialisation and centralisation. Models for community teams are emerging that augment the range of knowledge, skills and practices in ways that are more local, integrated and complementary. Nonetheless, there needs to be one practitioner who retains responsibility for oversight. For reasons legal accountability, the primary health role is vested in the GP, although there can be positional ‘deference’ or uncertainty, leading to inertia between primary and secondary care. In addition, older people and informal carers feel that they are not understood nor that their complex needs are addressed as a whole, when they are seen by separate practitioners and across diffuse services114 (Box 32).
I know one person can’t do everything, just to be able to understand right OK, so when I turn up to an appointment they’ll know right my dad’s got diabetes, asthma, and he’s got a heart condition . . . so how does that [new problem] then affect the other conditions? Not just look at that symptom specifically on that day.
C15 – informal carer
You’ll have one specialist for this, one specialist for that, one specialist for something else. And they all add in all of these drugs. The GP then says, ‘Oh no, I can’t change that because the specialist gave them that’.
P22 – practitioner, community service manager
Practitioners adopt ways of working together to overcome diffusion and address complexity (Box 33).
Generally what we would do is we would refer to the GP for [someone identified as not coping and at risk] and request a medication review. And generally the district nurses then work with the GP about how they can do things . . . the actual medication changes would be done through the GP and indeed, the pharmacist that sometimes gets involved as well with this. So it’s sometimes a three-way thing.
P30 – practitioner, specialist nurse
Multidisciplinary team meetings. So myself and the consultant from the hospital, because we’re like secondary care into primary care. We’re doing a pilot project . . . We go through their patients. Like a virtual ward . . . So it is the GP, it’s the consultant, me. We try and get the practice nurse or practice nurses in, and we are trying to get the community case managers in as well, you know, those that do actually the community long-term management for the chronic conditions.
P33 – practitioner, specialist nurse
We’ve got the [service] that covers physiotherapists and occupational therapists. We’ve got community matrons and staff nurses. We’ve got the social workers and then we’ve also got the voluntary sector [for older people, for informal carers]. We’re all in the same office. So of those core ones . . . we have what we call a stand-up meeting every morning . . . I think it’s very much about collaborative working as well . . . even though we’re kind of a really small [pharmacy] service, it’s just trying to make sure that that’s not an isolated service and it feeds into the bigger picture really.
P18 – practitioner, clinical pharmacist
But it’s just everybody talking to everybody isn’t it. And I think the beauty of being in the hospital is that we have easy access to everybody. In a GP environment is not so easy, is it? Hopefully with practice pharmacists, that will improve.
P1 – practitioner, senior pharmacist
The whole idea of health and social care integration which, OK, it’s on the agenda, a government policy to bring that into play. It’s been on the agenda for a long time, although seemingly that hasn’t come to the fruition as yet. But still, I think there is an appetite to do that.
P60 – practitioner, social worker
Access to quality information supports practitioners in reviews and reconciliations, although there are issues with accuracy, delays and the completeness of the picture, partly as a result of disconnected care pathways and practitioners working in silos. This impacts adversely on co-ordination within and across services (Box 34).
It just feels like everybody’s working in isolation but the time it takes to communicate what’s going on with an individual, it almost feels like it’s caused other problems. So it almost feels like you’re causing more harm to my dad by not talking – keeping your communication systems up to date. Because if you don’t know what’s happened and you’re relying on my dad to tell you, you’re not going to get anywhere.
C15 – informal carer whose father has dementia
Patients come in from a variety of sources. So, some would be, come in from the community directly. In which case, if it is via the GP they would usually have a GP summary with a list, which would include a list of medication; that is, acute and long-standing prescriptions. Some have been admitted from the community directly by an advanced nurse practitioner, or a case manager, in which case it is usually a hand-written note which ideally would include a list of medication. A lot of patients also come from the acute sector . . . in which case they come with a discharge letter. We would then routinely try and marry up to check that the information we have got on medication is right.
P6 – practitioner, consultant geriatrician
Changes which we make on in-patient medication would be relayed to the GP via the discharge letter. And, on the back page of our in-patient drug chart, there is a space for recording any changes and then that can just be incorporated in the discharge letter . . . so that the changes continue to happen . . . A good discharge letter would account for those changes and explain . . . for example reduced the dose of the antihypertensive because of the side effects . . . but that is not always the case.
P6 – practitioner, consultant geriatrician
I find that even with the consultants here, who you think would be up for reviewing everything wouldn’t you – And I still think there is reluctance for them to stop anything that the GP started. And I think that the GPs are reluctant to stop anything that the consultant started. And it’s because they don’t know why.
P1 – practitioner, senior pharmacist
I know that sometimes that will just be that they just carried on things, the patient has been a bit ill and they go ‘Well, we just carried that on because the GP has carried it on’. So, there are still opportunities for them as in-patients to have their medications reviewed. But I have spoken to a consultant about it, probably about a year or two ago. And he said ‘Well actually, sometimes there just isn’t the time. Because we try and turn around patients very quickly. And, we just expect it to be picked up again in the community’.
P25 – practitioner, GP
In the UK, there is a political appetite for closer working across health and care being enacted through policy on more integrated delivery systems. However, in practice it appears that economic drivers for efficiency, opportunistic and transient initiatives, and clinical agendas around specialisation may be undermining these aspirations. Practitioners compensate for a lack of integrated health and care by working in formal and informal networks. Older people and informal carers also make significant efforts to navigate and bind disparate practitioners and services together to reflect their needs.
This subsection addresses fragmentation. Findings are explained in the following CMO:
Increasing specialisation and centralisation (C), and extending responsibility for reviewing and reconciling medications to multiple practitioner groups (C), results in older people and informal carers having multiple, discrete contacts with services (O), limiting the way they are understood and their needs addressed as a whole (O), including the co-ordinated management of complexity and risk (O), because of fragmentation (M).
RR CMO 04
Fragmentation can be addressed through relationship and action work at inter- and intra-agency levels to build and sustain operational and strategic networks.
Recognition and engagement: exclusion
As established previously in this report, older people and informal carers develop expertise in the day-to-day management of their health, diagnoses and medications (see Medication management). Their experience and expertise can make a useful contribution to decisions and actions about their health and care. This includes when reviewing and reconciling medications. In this type of interpersonal work, shared decision-making is a well-established part of collaborative working. It is underpinned by a substantial body of evidence42,158–164 and support for its use in practice:33,41,165
Achieving shared decision making depends on building a good relationship in the clinical encounter so that information is shared and patients are supported to deliberate and express their preferences and views during the decision making process.
Elwyn et al. 164
Face to face interactions, permission and space to discuss options, and continuity of patient-professional relationships are key in supporting older people with complex needs to engage in shared decision making.
Bunn et al. 159
Shared decisions based on what is important to each person in terms of treatments, health priorities, lifestyle and goals.
NICE. 41
Older people and informal carers value recognition and engagement in decisions and actions affecting their health and medication (Box 35).
And he sat with me and he, well he said ‘What do you think it might be?’, to me just like that. And I know he wasn’t meaning for me to diagnose myself but what he was sort of saying in a roundabout way was what was are you worried about.
OP19 – older person
We have discussed whether we should look at some of the biologics – that there’s an increased risk of breast cancer if you take it . . . But they know [my rheumatoid arthritis] is progressing because my hands are getting worse. They had wondered – but because of the increased risk, there’s no argument to me. I’d rather have kinky hands than cancer.
OP8 – older person
So the doctor and I will discuss things over the phone, and because we’ve built up a good relationship, trust one another.
C32 – informal carer
You have to listen to the people that are there all the time doing it’.
C11 – informal carer
Practitioners also value their input and ways of securing the involvement of older people and informal carers (Box 36).
Keep the information you tell the patient as basic as you can, in terms that they will understand. I know sometimes it takes a bit of time to get to know the patients doesn’t it and build up a bit of a rapport with them. Which you can if you are seeing them quite regularly.
P1 – practitioner, senior pharmacist
You’re not going to build up a good relationship with that person if you’re spending half an hour bombarding them with information about all their tablets when really they didn’t want to know that . . . we do our reviews, the patient – the person is very much involved in that. And that’s quite a concordant discussion.
P18 – practitioner, clinical pharmacist
So that’s always slightly tricky [best treatment, failed treatment and cost], but I usually involve patients with that in terms of the decision-making.
P53 – practitioner, GP
Box 37 draws on more general evidence about the need for time and space for this interpersonal work to evolve that can be translated to reviews and reconciliations.
So if I said to her I do have a problem, I think it needs some sort of medication. I guess, I might have researched it, she would do whatever I thought, I think. Or she would say ‘Well I’ve found that this one is better in this condition’. She’s good but you know, it’s a partnership I think.
OP28 – older person
With the previous doctor I did eventually sort of build a relationship with the doctor where I felt that he was actually sort of listening to me and accepting that they were reasonable questions I was asking.
C9 – informal carer
I know GPs are constrained but it takes just like 10 seconds for my dad to just get his bit out, it doesn’t take minutes, it only takes a short moment . . . And then we can just then look to try and resolve whatever the problem is. But she just managed it really well. And I felt confident knowing what I need to do to help him going forward as well.
C15 – informal carer
We have all got to speak to the patient haven’t we at the end of the day, and include them in any decisions for anything that is prescribed. Because you might sometimes be surprised about what their priority is compared to what the prescribers priority is . . . I still think old people get a raw deal with an assumption that nobody needs to talk to them about anything or it’s going to be very difficult because they are a bit deaf or they might not understand. Well, have a go.
P1 – practitioner, senior pharmacist
This subsection addresses exclusion. Findings are summarised in the following CMO:
When older people and informal carers are not recognised and engaged with practitioners in shared decisions about their health, diagnoses and medications (C), the individualised basis for decisions is limited (O), older people and informal carers are less likely to apply what has been agreed (O), and their relationship with practitioners is undermined (O), because of exclusion (M).
RR CMO 05
Exclusion can be addressed through action work using shared decision-making to benefit from the individualised expertise and experience of all those most involved.
Summary: burdens in reviewing/reconciling medications
Reviewing and reconciling medications is a key, complex interpersonal stage in medication management. Using a realist approach, MEMORABLE has stratified this complexity by identifying five burdens that could be addressed through mitigation work in this stage, influencing what is done in other stages:
-
The ambiguity burden about reviewing/reconciling medications within medication management – this involves sense-making by clarifying the purpose and content of medication reviews and reconciliations.
-
The concealment burden due to a lack of information-giving that prevents older people and informal carers from understanding, personalising and using what they want or need to know – this is also about sense-making and establishing meaning through information to increase personal efficacy, agency and control.
-
The unfamiliarity burden arising from not seeing the same practitioner consistently – this is about establishing continuity and mutual trust in relationships as the foundations for interpersonal work.
-
The fragmentation burden limits the way older people and informal carers are understood and their needs addressed as a whole when they are seen across a range of services – this is about the importance of inter- and intra-agency collaboration in operational and strategic networks.
-
The exclusion burden when older people and informal carers are not recognised for their experience and expertise, nor fully or effectively engaged in decisions that affect their health and care – this is about action and enacting collaboration through shared decision-making.
These findings are summarised in Figure 5, which highlights several key elements:
-
Identification of those involved in Stage 5: reviewing/reconciling medications – older people, informal carers and practitioners, and their association through multimorbidity and polypharmacy.
-
Complexity is acknowledged and accommodated.
-
Burden issues are highlighted – ambiguity, concealment, unfamiliarity, fragmentation and exclusion.
-
Work to mitigate burden is associated with sense-making, relationships and action as linked implementation steps (NPT) in this and each of the five medication management stages. This includes the way steps interact across stages in the medication management process. These generative links are suggested by arrows that overlap the perimeter of the box.
-
Enduring relationships are emphasised as foundational to the work that is done.
-
Important elements of sense-making and action are highlighted.
-
Reflection/monitoring are included as part of the implementation process, although it was only implied in the data rather than specified.
-
Indicative but important outcomes of burden and risk management, and sustaining coping and control are included.
Based on these findings, the figure also identifies proposed interventions generated within this stage:
-
Risk identification as a point of access from Stage 4: continuing to take medications, signifying its contribution to identifying those who are not coping and should be offered further evaluation or review rather than waiting for a planned review.
-
Individualised information is located centrally to emphasise its importance to sense-making, action and relationships.
Details of the proposed interventions are set out below.
Proposed interventions
This section sets out two proposed interventions from the findings: risk identification and individualised information. They emerged mainly from the experiential data. They were subject to discussion and debate by the Research Team and subsequently endorsed by the Project Group and the Stakeholder Group.
However, before describing these proposed interventions, two issues need to be considered. The first issue is the type of intervention that might be proposed. The researchers acknowledged that, despite having established the complexity of medication management and of the stages involved in it, a complex intervention may not be appropriate or implementable across health and care. The researchers could envisage difficulties with standardisation and fidelity across multiple practitioner groups as well as with trying to introduce another ‘innovation’ to practitioners already overburdenend by initiatives. There would also be unacceptable cost implications attached and sustainability issues. This issue is explained by Santo et al. :166
. . . interventions to improve adherence to medications . . . are mostly complex. The disadvantages of complex interventions are that they are difficult to compare and be replicated, thereby possibly increasing costs and making implementation in routine practice difficult. Our results showed that simple interventions might be as effective as more complex ones.
Thus, discussions in the Research Team initially explored the findings from the analysis of burden, whilst seeking ways to respond to what older people and informal carers were requesting, directly and indirectly. These discussions focused on identifying simple interventions that would potentially have a high impact, such as risk identification.
The researchers also resolved that any proposals needed to:
-
be sensible, practicable and novel
-
address multimorbidity and polypharmacy directly
-
have potential to engage older people, informal carers, practitioners and others in developing these ideas into something practicable, collaboratively
-
have a clear route to introduce such an intervention through which it could be disseminated to those who would benefit most
-
accrue significant gains directly to those involved.
Based on these criteria, risk assessment and a more ambitious proposal for individualised information were agreed.
The second issue involves the level of detail in the specification for each intervention to be included in this study. The consensus among the Research Team, the Project Group and the Stakeholder Group members was that, although the findings substantiated the need for these proposed interventions, and that the need to establish the framework for proposed interventions fully met the research aim, it was beyond the scope of this research to provide a detailed specification for these follow-on studies. Indeed, those involved in these discussions agreed that this work should be undertaken collaboratively by users and beneficiaries as well as by others who might be able to provide expertise to turn proposals into practice and subsequently evaluate the way they work, or not, following a similar realist approach to this work. Therefore, the proposed interventions are briefly described below, at a level of detail sufficient for this research.
Risk identification (linking Stages 4 and 5)
Older people and informal carers who are not coping and are at risk may not be readily visible to practitioners. There needs to be a simple and easily implementable way of identifying older people and informal carers who are not coping and then, having done so, to enable them to access appropriate help and support through a more detailed follow-up, such as a review. Many older people and informal carers have multiple contact points with health and care and therefore, one way of identifying those at risk would be to have simple ‘Whooley-type’ questions [URL: https://whooleyquestions.ucsf.edu (accessed 10 December 2019)] that could be used by any practitioner. These screening questions would be burden focused, one on workload and one on capacity with two ‘no’ answers indicating no significant burden at this time and one or two ‘yes’ answers requiring further evaluation.
Risk identification could be undertaken at any contact point or time in Stage 4, while older people and informal carers are continuing to take medications as a way of fast tracking them to further evaluation, help and support, or to a medication review (Stage 5).
Individualised information (linking Stages 5 and 2 to Stages 1, 3 and 4)
McHorney et al. 101 state: ‘people can only do what they understand’. Despite good information on single diagnoses and single medications, there is limited information, if any, that encompasses the unique combination of multimorbidities that increasing numbers of older people live with, as well as the complex polypharmacy regimens they are prescribed. Older people and informal carers stated that current information does not reflect the uniqueness and complexities that each individual experiences. 167
Learning how to manage this ‘by doing’ is inadequate and fraught with risks, including following routines that could be mistaken in their underlying assumptions about when and how to take certain medications. Making individual decisions on a day-to-day basis, without adequate information, adds to that risk. In addition, the way older people and informal carers have to repeat the same information about themselves to practitioners is burdensome, time-consuming and may inadvertently lead to them not divulging the way that they are actually managing or mismanaging their medications.
In this case, the proposed intervention is for older people and informal carers to have individualised information that they can use and share, tailored to their particular preferences, needs and experiences of living with several long-term conditions and taking many medications. The format, content and ways of using this individualised information would be developed collaboratively by working with those groups most directly involved, as with this research, as well as with others with expertise to turn proposals into practice.
Both proposed interventions would be pursued using realist methodology.
These findings are discussed in Chapter 4.
Chapter 4 Discussion
The aim of MEMORABLE was to undertake a realist synthesis ‘to develop a framework for a novel multi-disciplinary, multi-agency intervention(s), to improve medication management in older people on complex medication regimens resident in the community’. 30 This aim has been met through the combined analysis of literature and narrative data on medication management and subsequently focusing on reviewing/reconciling medications (Stage 5). By theorising about causality, an understanding of what works, for whom, why and in which circumstances was generated: realist methodology’s four ‘W’s. 49–53,55,56 Findings confirm the complexity of medication management, understood through stages and steps, and, furthermore, the complexity of burden as a significant, multifaceted experience within it. This research has established the need for simple, but effective improvements in risk identification by practitioners, and the development of individualised information that reflects the experiences and addresses the day-to-day needs of older people and informal carers living with multimorbidity and polypharmacy.
This chapter draws out salient points from this study that the researchers believe have the potential to inform and improve practice, or warrant further research. Sections cover medication management; reviewing/reconciling medications; proposed interventions: risk identification and individualised information; and the research methodology. Each section considers the results and the implications for practice. The chapter concludes by addressing the strengths and weaknesses of the research.
Medication management (see Chapter 3, Medication management)
Complexity in medication management: stages and steps
Much of the existing literature identifies medication management as a single intervention. Policy and guidelines are often based on good practice. Evaluation is based on describing cause and effect168 rather than explaining real-world complexity. Policy and practice are typically aligned with issues of medication adherence and optimisation, with a behavioural underpinning to achieving both of these aims. The message is that these ‘betterment’ goals, optimisation and adherence, can be secured through various forms of behavioural incentives, disincentives and evidence-based advice.
Advancing this understanding, MEMORABLE views medication management as a complex intervention, aligning with the Medical Research Council58,59 definition, engaging older people, informal carers and diverse practitioners across health and care. Medication management is also an implementation process, operating across five linked stages with four implementation steps and three loops in each stage. This process structure offers a framework for understanding the generative and serial dynamics of real-world complexity within which individual and interpersonal work is carried out (NPT). 65,66
Stages 1, 3 and 4 involve older people doing work on their own: identifying problems (Stage 1); starting, changing or stopping medications (Stage 3); or continuing to take them (Stage 4). In some instances these stages may be supported by an informal carer, typically a family member, or a formal carer, assuring adherence and minimising risk.
Routines are fundamental to what older people and informal carers do, emerging from sense-making, action and reflection, and founded on key relationships, from which they exert agency and control. Routines are flexed to minimise burden and accommodate short-term and longer-term change. Examples of routines include linking medication taking with mealtimes and keeping pills in the kitchen as a prompt, organising regular pharmacy pick-ups as part of trips to the shops or arranging doctor’s appointments well in advance to ensure that older people see their preferred practitioner. Thus, older people and informal carers cope by fitting the practicalities of medication management into their day-to-day lives to achieve the outcomes that matter to them. Medication management plays a part in this, but is not an end in itself; adherence is only part of their narrative. Accordingly, practitioners need to address routines, outcomes, coping and burden in their contacts with older people and informal carers to ensure a purposeful and productive alignment between their agendas and to ‘nudge’ adherence within those discussions.
Difficulties in Stages 3 and 4 can provoke a ‘disruption loop’ when a problem occurs (Stage 1). Sustaining stable medication management, coping and optimisation in Stage 4 appears to be desirable. Frequent jumps from Stage 4 to Stage 1 may indicate a lack of coping and risk, particularly if red flag issues arise in Stage 1 and the older person or informal carer does not contact a practitioner for help and support. Red flags might include consistently not keeping enough tablets at home or stockpiling; muddling up tablets that look similar but which should be taken for different reasons; or avoiding taking particular tablets because of attributing undesirable or inconvenient side effects to them, but not seeking help to identify an alternative. Practitioners need to be able to identify those at risk and provide older people and informal carers with accessible, personalised information that enables them to be confident in determining the significance of problems and how best to resolve them, on their own or by accessing appropriate and timely support.
Stages 2 and 5 are where older people and informal carers engage with practitioners, typically in a clinical or formal setting. These two interpersonal stages are where they are getting a diagnosis and/or medications (Stage 2) and reviewing/reconciling medications (Stage 5). Stage 5 may trigger a ‘medication loop’ to Stage 4 where older people simply continue to take medications as before or to Stage 3 if they are advised either to stop, adjust doses or start something new, which then becomes part of their regimen in Stage 4. In addition, Stage 5 may trigger a ‘diagnosis loop’ where concerns are raised about their health, addressed in Stage 2. Shared decision-making is a key aspect of both stages.
Time-limited, interpersonal contact points are where information about older people’s and informal carers’ experiences of making medication management work day to day is exchanged, shared decisions are made and actions taken that influence what happens in the other three stages. Based on the research findings, shared decision-making provides an opportunity to establish the details and acknowledge the importance of the routines that older people and informal carers follow to feel that they have control over medication management in their lives and to achieve meaningful outcomes. Quality collaboration, with practitioners, optimises older people’s and informal carers’ intrinsic motivation and better informs the individual decisions they make at home. Alongside assuring safety and effectiveness, addressing these issues of ‘fit’ may have greater resonance and leverage in the decisions and actions of older people and informal carers about what medications they take, how and when. Supporting them to make medication management one of the many activities that they choose to engage in, day to day, may prove more enabling than emphasising practice-defined aims of adherence or optimisation.
The MEMORABLE Research Team identified that there were advantages in understanding medication management as a complex, dynamic stage, step and loop implementation process. First, it enabled them to work with its complexity, at a level that was manageable. Second, it provided a structure to identify and characterise key stages, steps and loops in the process, framing the data. Having set out this process early in the research and refining it as the research progressed, it proved to be useful in discussions about the direction of the literature search, in focusing the interviews and in interpreting and applying findings from the data from a consistent perspective.
The Research Team acknowledge that one of the tensions in medication management as an intervention is how to view it as a discrete aspect of the wider and complex processes of care involving older people with multimorbidity and polypharmacy,9 to underscore its importance and ensure that appropriate practitioners such as pharmacists are involved and their work co-ordinated. However, it also needs to be integrated within the wider health and care process, acknowledging that medication is increasingly important in the treatment of chronic diseases. To contribute to this, the Research Team recommend that further research is undertaken to extend the application of this stage, step and loop approach to complexity in medication management, underpinning it with a more substantive evidence base and causal account of each stage and extending across stages, to support the development of policy and practice in this field.
Outcomes in medication management
Medication management guidelines emphasise adherence and optimisation as outcomes. The literature on medication management provides an extensive and growing evidence base for both.
The MEMORABLE study’s interviews identify an extended range of outcomes that interviewees attach meaning to or want to achieve. Older people’s outcomes generally appear more diverse, purposeful, functional and qualitative than the role-informed outcomes of practitioners. As the key beneficiaries of medication, there is an embedded friction between minimising the number of medications older people have to take while achieving the effects that enables them to do things that matter to them. There is a reciprocal tension for practitioners as they mediate between criteria in guidelines that inform good practice on which their performance is assessed and the older person’s expressed wishes.
There is some degree of convergence in the outcomes identified by informal carers and practitioners attached to their roles in medication management, perhaps reflecting the way informal carers feel responsible for ‘good practice’ and adherence. There is also divergence in the outcomes informal carers and practitioners identify for others, highlighting the tension between personal and role-based agendas.
Early in the research, the researchers agreed that adherence and optimisation were important practice outcomes in MEMORABLE, acknowledging the importance of taking medications as prescribed and agreed (adherence) to achieve the best outcomes (optimisation). However, the researchers extended the range of outcomes by sourcing them from the literature, set out in the analysis framework (see Table 2 and Appendix 7), and populating them from the research interviews. By asking interviewees about the outcomes that they derived from medication management and then what had brought these about, the researchers identified outcomes occurring at different stages or levels, and evolving over time, as well as identifying the contexts and mechanisms involved. These causal accounts resolved explanatory gaps in the literature and informed the writing of CMO configurations.
The Research Team recommend undertaking further research on outcomes in medication management, involving older people, informal carers, and health and care practitioners. This could contribute to developing a vocabulary and possibly a decision support tool for practitioners to better engage older people and informal carers in discussions about what matters to them and the routines they might use to ensure that these outcomes are achieved. These outcomes would be documented as part of their individualised information.
Informal carers in medication management
Although the main focus in MEMORABLE has been on older people and medication management, the role and contribution of informal carers has emerged as an important topic. MEMORABLE, in line with other research, found that the depth and breadth of support informal carers provide to older people with multimorbidity and polypharmacy is increasing, many taking on a pivotal role and making significant personal sacrifices as a result. 21–25 The outcomes informal carers want from medication management highlight the tension that exists between the responsibility of being a care provider, in which they seek to adhere to the practitioners’ directions, and their identification with the wishes of and for that older person, based on familial ties and personal knowledge.
Informal carers experience role-creep as they become more engaged in the older person’s medication management and a time lag in acquiring the information and the increasingly specialist knowledge and skills that they need, which are largely gained by experience. With many taking on more responsibility and more complex tasks than paid carers, they nonetheless lack equivalent training or support.
Informal carers can also lack recognition from health and care practitioners or within health or care systems, arising from legalities around the older person’s ‘capacity’ as defined by mental health legislation. In circumstances when an assessment of ‘capacity’ may be borderline, an older person may nonetheless be failing to cope with the complexities of medication management. As a result, informal carers may be taking on a significant part of the workload, yet be excluded from key shared decision-making opportunities.
The Research Team recommend that further research is needed on the role of informal carers in supporting medication management. MEMORABLE will publish a paper on the research findings for informal carers to contribute additional evidence to the debate on this issue.
To conclude, medication management is a complex intervention and, therefore, improvement proposals need to engage with that complexity. MEMORABLE has developed a five-stage implementation process, causally focused and evidence and experience based. However, MEMORABLE remains a formative study. Accordingly, the researchers believe that framing medication management as a five-stage, four-step, three-loop process merits further exploration and critique to advance its contribution to research and practice.
Reviewing/reconciling medications [see Chapter 3, Reviewing/reconciling medication (Stage 5)]
Complexity in reviewing/reconciling medications
Reviewing/reconciling medications are the interventions associated with Stage 5 of the medication management process. It is an interpersonal stage where older people and practitioners collaborate, as with Stage 2: getting diagnosis and/or medications. Informal carers may also be involved. It involves regularly reviewing the way in which older people are continuing to take medications (Stage 4) or reconciliation at a health or care transition. As a result of what is decided in Stage 5, older people may continue to take their existing medications (medication loop to Stage 4); they may start, change or stop medications (medication loop to Stage 3); or they may need an assessment for a new diagnosis and medications (diagnosis loop to Stage 2). This process is illustrated in Table 6.
Reviewing/reconciling medications (Stage 5) was selected for more detailed analysis, as it was judged to be a key stage and an exemplar within medication management. This decision was made during the iterations of work packages 1 and 2, steps 3b/5b, based on:
-
the emerging significance of this stage to the overall process
-
its immediate links to Stage 4, where older people are continuing to take their medications and where non-adherence may emerge as an issue of concern
-
the way it is understood through policy and guidance that could clarify standards of good practice and their intended impact, causally explained or not
-
its importance in the way it focuses on interpersonal decisions and actions, and, therefore, its potential relevance to Stage 2, where the other interpersonal work occurs when getting a diagnosis and/or medications.
There are, however, important distinctions between the two interventions that make up this stage: reconciling and reviewing medications.
The first intervention is reconciling medications. It is associated with transitions between settings, the accuracy of medication lists and risk minimisation, such as avoiding drug–drug interactions or prescribing two medications with similar actions for the same or similar symptoms. Medication reconciliations are perceived as more straightforward than medication reviews. In the interviews, prescribers commented on the importance of having accurate information about the medications older people are taking, who prescribed them and why. This information is essential in treatment planning, including rehabilitative work to support older people to regain their independence in managing their medications when they return home from hospital. However, practitioners commented on problems with the quality and speed of communication needed to carry out reconciliations or act on results.
The second intervention is reviewing medications. MEMORABLE has identified three issues in the way medication review is understood and practised.
The first reviewing issue is about ‘what is done and when’. The researchers’ interpretations of the data indicate variation in older people’s and informal carers’ experiences of the content and timing of reviews. There are three accepted levels of medication review based on patient need. Policy, and associated funding, require MURs to be carried out by community pharmacists, with the older person present. Elsewhere, practitioners currently determine which level is most appropriate for any individual, acknowledging the resource limitations in the system.
It appears from the interview data that older people who are coping and whose condition is stable may have 6-monthly or annual reviews reflecting the practitioner’s perception of relatively low risk. Older people with less stable conditions and who are not coping appear to be having reviews as part of regular contact with practitioners. Their formal reviews are then often carried out as level 1 or 2 at which they are not present. 132,169 However, MEMORABLE has established that, despite contact with practitioners as part of ongoing medication management, older people and informal carers who are not coping and are at risk are not being identified or their needs addressed. Risk may be exacerbated by waiting for a planned review. MEMORABLE proposes that older people and informal carers whose day-to-day medication routines are breaking down and who are not coping need to be identified at any point of contact with a health or social care practitioner so that they can be offered further evaluation and support. Risk identification is discussed in Proposed interventions/future research.
The second reviewing issue is about ‘who’. The legal accountability for health care is held by the GP in primary care. However, the development of professional roles and multidisciplinary team working has resulted in pharmacists and nurses taking on additional responsibilities for prescribing and reviewing, building on their specialist knowledge, skills and supplementary training. Specialist nurses contribute to the management of specific long-term conditions, such as respiratory or cardiac, and community matrons support older people and informal carers living with complex long-term conditions in the community.
Pharmacists increasingly work with GPs in reviewing medications. Both the literature and interview data, describe a variety of effective pharmacy service developments in primary care where the specific expertise and experience of pharmacists is valued. Developments do, however, appear to be patchy and sometimes transient, subject to local sign-up and funding uncertainties. With clarification of roles and responsibilities that integrate pharmacists within the primary care team, improved information that gives them access to patient notes, and improved communication systems that would come from co-location, it appears that pharmacists could contribute to more effective service delivery, quality and impact, increasing capacity and reducing workload for GPs. 170,171 Thus, potentially, MEMORABLE found some support for the developing role of practice-based pharmacists.
The third reviewing issue is about ‘where’. There is no need for home-based reviews for all older people with multimorbidity and polypharmacy. However, the potential advantages of reviewing older people and informal carers who are not coping, at home, are clearly made when the focus of the review encompasses the fit between the demands of medication management and older people’s day-to-day lives. Practitioner interviews confirmed the success they have in identifying and resolving risks in the home setting where older people’s and informal carers’ routines can be demonstrated and discussed. It is harder for older people and informal carers to indicate that they are coping, perhaps out of a misplaced loyalty to the practitioner, when there are boxes of pills randomly scattered about the house, a litter of old prescriptions or a muddled store of medications, some of which are long out of date. Support to regain control of the situation and to re-establish routines that fit with day-to-day lives is readily available from practitioners once the issue becomes known. As another aspect of the risk issue, risk identification is discussed in Proposed interventions/future research.
Causality in reviewing/reconciling medications and burden
By focusing in detail on this stage, MEMORABLE has extended the understanding of reviewing/reconciling medications within medication management. It has scoped and identified tasks associated with good practice. Using a realist approach has enabled the researchers to explore how implementation work is done and with what effect.
From the many lenses the researchers could bring to this analysis, they chose to conceptualise burden as a potential mechanism of interest, based on an initial assumption and subsequent validation of the need to mitigate burden in medication management. To do this, they drew on the evidence, experiential accounts and theorising processes within realist methodology, basing analysis on subsets of CMO configurations, and used the additional analytic lens afforded by NPT. The findings on reviewing/reconciling medications therefore have a robust evidence and experience base to inform intervention proposals.
Conceptualising burden as a mechanism of interest has assisted data analysis thus far. In doing this, the Research Team acknowledge that it is also possible to locate burden as an outcome from the work done in any stage, as well as a context to what is being done. The value of this generative, terminological fluidity is acknowledged by Manzano,75 enabling analysis to reflect individual experiences of medication management and changes to those experiences from, for example, acute exacerbations of a particular diagnosis or combination of diagnoses, disease progression or age-related issues such as increasing frailty or cognitive decline.
Realist analysis of the complexity inherent in reviewing/reconciling medications has enabled the researchers to theorise about this stage in ways that are potentially transferable to other stages and circumstances based on the assumption that the same mechanisms may be in operation. In addition, the use of a realist approach has increased the understanding of the influence of contexts–mechanisms on outcomes, potentially contributing to better tailoring of medication management in different contexts.
Understanding burden mitigation in reviewing/reconciling medications
The MEMORABLE study has identified five burdens associated with reviewing/reconciling medications that are experienced primarily by older people but also by their informal carers: burdens of ambiguity, concealment, unfamiliarity, fragmentation and exclusion (see Chapter 3, Reviewing/reconciling medications and burden). Mitigation of these burdens occurs through implementation work, primarily involving sense-making, action and relationships. Reflection/monitoring were not identified directly from the interviews or the literature but are acknowledged as important aspects of implementation in this and all other stages. An absence of data in this aspect of the programme theory would be addressed in further research. Ways of mitigating specific burdens are discussed in the following paragraphs.
Mitigating ambiguity: knowing about medication reviews and reconciliations, sense-making – clarification (RR CMO 01)
The researchers found that the scope of reviews and reconciliations needs to be more clearly and effectively communicated to older people and informal carers, enabling them to prepare and participate more effectively. It would also help older people and informal carers make sense of these interventions in relation to what happens in other stages of medication management so that they can link what is decided in the review or reconciliation with what they do at home. Better general information on reviewing and reconciling medications could be personalised in any invitation sent to older people and informal carers to attend reviews, in particular. This might include questions to think about in advance to help them reflect on their routines and their experiences of the burden of medication management, and what might decrease the work they have to do, or increase their ability to do it, enhancing their feelings of control and coping.
Mitigating concealment: having personalised, meaningful information, sense-making – information (RR CMO 02)
Older people and informal carers struggle to make sense of poor information, particularly when it is impersonal, lacks meaning for them and is inconsistent or conflicting. Generalised information that focuses on a single diagnosis or medication is valued but of limited use when older people’s lives involve multimorbidity, polypharmacy and their unique experiences of both. Overcoming a lack of information adds to their workload, whereas poor information reduces their capacity to engage fully or as they might choose if better informed. It can exacerbate uncertainties when they are managing medications at home, undermining self-management. The use of the internet as an information source is growing, although there are concerns about how to access trustworthy websites and evaluate the information on them. Older people and informal carers are asking for reliable and readily accessible information that helps them to cope. The intervention proposal for individualised information is an attempt to address this issue (see Proposed interventions/future research).
Practitioners also receive poor information, particularly around medications and the reasons for prescribing and deprescribing so they can make sense of the individual they are seeing, and may not have seen before, their diagnoses and treatments. This is made worse by delays in communicating between organisations, further exacerbated by communication by letter or fax. This points to wider information sharing and communication issues across health and care that lie outside the scope of this study. Nonetheless, practitioners may benefit from accessing the individualised information held by older people and informal carers, proposed in Proposed interventions/future research.
Mitigating unfamiliarity: continuity and mutual trust – relationships (RR CMO 03)
Continuity elicits mutual trust and loyalty. It is a desirable in the contacts between older people and practitioners, including informal carers where they are involved. This is particularly the case when sharing decisions about the long-term, often progressive and increasingly complex, nature of the diagnoses, treatments and associated burdens older people are living with. Consistent relationships with GPs in primary care and secondary care consultant physicians, who specialise in the health care of older people, are valued because they retain oversight of this complexity. GPs are seen as having more enduring relationships overall.
Mutual trust is a well-established aspect of interpersonal relationships in health and care according to the literature112,114,153–157 and confirmed in the research interviews. Accessing named practitioners with expert clinical and communication skills, and the sense that they are acting as allies in the best interests of the older person and their informal carers, builds and sustains the trust that is foundational for the effective interpersonal work required for medication optimisation.
In as much as systems permit, older people and informal carers who are struggling with routines and the burden of their medication, and for whom continuity is important, need to be able to identify and access preferred and trusted practitioners, particularly in primary care.
Reflecting on relationships within NPT, it is important to recognise that this substantive theory was initially developed to explain the implementation work associated with new practices; introducing them and making them routine. In NPT, relationships are only established to achieve short-term delivery goals. In Stage 5: reviewing/reconciling medications, MEMORABLE confirms that sustained relationships are key to mutual trust, and to safe and effective medication management across the whole process. This finding also confirms the usefulness of this substantive theory in explaining implementation and the work associated with it.
Mitigating fragmentation: addressing complex needs as a whole, action – collaboration (RR CMO 04)
Serial health and care reorganisations increase practitioners’ workload on sustaining collaborative networks during change. Some practitioners stated that it was difficult to know about new initiatives or where projects have ceased because of funding being withdrawn. Networks break apart, taking with them established, effective and trusted working relationships, the additional capacity they provide, and systems and practices associated with unified and integrated working. These networks are often replaced with increasing specialisation and centralisation, intended to achieve system efficiencies. However, this results in the complexity of older people’s health and care management being distributed across multiple sectors and organisations. Individual practitioners work to bridge these system-initiated discontinuities.
Partnership working between practitioners, systemically supported, brings access to a greater range of knowledge, skills, experience and expertise as a way of addressing the complex needs of older people living with multimorbidity and polypharmacy, as a whole. Longer-term strategic planning and funding streams need to enable this approach in future system reconfigurations, addressing workforce and practice.
Mitigating exclusion: recognition and engagement, action – shared decision-making (RR CMO 05)
Gaining traction as a skill set and evolving as a broader approach to collaborative care, shared decision-making is most effective when it is embedded as a routine behaviour by an expert and experienced practitioner. Older people and informal carers need to be recognised and engaged for the value of their own experience and expertise rather than being defined by their diagnoses or the content of their prescriptions, or left feeling overlooked as an individual when practitioners increasingly focus on single specialisms. MEMORABLE endorses shared decision-making as a way of enacting collaboration. Through it, workload can be reduced by, for example, enhancing effective and meaningful communication in a more mutually trusting therapeutic relationship. In addition, capacity can be increased, because the older person feels more confident in managing their medication.
By facilitating shared decision-making, practitioners can generate benefits in other stages by being able to make more effective adjustments to medication regimens to get a better fit with older people’s and informal carers’ day-to-day routines and not merely deprescribing for adherence;168 increasing motivation through feelings of control; and raising older people’s and informal carers’ confidence to make safer, more informed and effective choices about medications when at home.
Proposed interventions/future research (see Chapter 3, Proposed interventions)
The findings from MEMORABLE are potentially broad ranging. Several lie outside the direct scope or capacity of this study, such as the need to improve the speed and accuracy of communication about older people’s medication regimens between primary, secondary and social care sectors.
Two specific interventions have been proposed from the analysis of the evidence, considered by the Research Team to have practical application that might contribute to an improvement in medication management within a reasonable time frame, mindful of the following caution from a Cochrane Review:111
There are many different potential pathways through which consumers’ use of medicines could be targeted to improve outcomes, and simple interventions may be as effective as complex strategies. However, no single intervention assessed was effective to improve all medicines-use outcomes across all diseases, medicines, populations or settings.
Risk identification
The MEMORABLE study has established the need to identify those who are not coping with medication management and who are at risk. From the literature and the interviews, this intervention should be a brief, simple and targeted risk assessment at any of the multiple points of contact with different practitioners that older people have as part of the medication management process. MEMORABLE found that this screening assessment should focus on identifying who is or is not coping with the burden of medication management, rather than assuming that all older people and informal carers are at risk. This needs to be a simple tool, with questions focused on the workload experienced by older people and informal carers and their capacity to cope with that workload. Such a tool would enable scarce health-care resources to be directed to the older people and informal carers most in need of support.
As it is only possible to outline this proposed intervention in MEMORABLE, it is recommended that scoping the detail and developing, implementing and evaluating a trial of risk identification is progressed through collaborative research to follow this study (MEMORABLE2). Involving all those with an interest, knowledge and skills in this area in realist evaluation would ensure the generation of an evidence and experience base on which development and roll out could be implemented.
Individualised information
The MEMORABLE study found that older people, informal carers and professionals need access to individualised information in an accessible format that can be shared by these key groups. It would provide a reference point to support older people’s and informal carers’ day-to-day decisions and actions, and increase the sense of control that they have over their medications. As a personalised working document, it could include, for example, a short summary of the older person’s values, preferences and goals; integrated and individualised information about their diagnoses and medications, individually and together, including links to trusted resources; their medication routine; key practitioners and contact information. It could be held in paper and electronic format, to be shared and updated as and when required. However, deciding on the most useful content and format would need to be co-created by those involved, with the input of others with expertise in how to make such systems practicable, such as through smart phone app (application) design.
As with the risk identification discussed above, it is recommended that, following co-production principles, future research should scope the detail of the required individual information, and then develop, implement and evaluate the tool, as part of follow-on research. This would be a significant amount of work, including determining lines of responsibility and accountability that would need to be completed before any trial.
Research methodology (see Chapter 2)
This section considers the contribution of the research approach to MEMORABLE, including the implications for researchers or others seeking to use the same approach to understand how complex interventions work.
Combining review and evaluation
The MEMORABLE study is a realist synthesis, integrating a review of the literature with an evaluation of the primary interviews, described in Chapter 2 and Figure 1. This combined approach is gaining popularity among researchers. It enables secondary and primary evidence to be brought together, facilitating the search for and the resolution of resonance and dissonance in their causal explanations of complex interventions. In MEMORABLE, the richness of the interview data was invaluable in supporting explanatory reasoning about the literature.
The MEMORABLE study benefited from the way the synthesis supported understanding of real-world complexity, being theory driven and purposefully evidence generating. In the research, preliminary analysis of the literature was used to inform discussion and debate by the Research Team. This guided further work on the literature review, including extending some of the search activity to address emerging themes and flexing interview schedules to gather interviewees’ opinions on the way medication management was beginning to be understood in the study.
Involvement of the Project Group and Stakeholder Group brought scrutiny and expertise to discussions of progress and the consideration of findings. In this way, the research progressed deductively by considering how the researchers thought medication management might work and then refining those ideas through data, as well as inductively by looking at emerging patterns in the data and generating theory from them. These processes were augmented by accessing the experience and expertise in the Research Team and in both groups to gain consensus on data-informed, causal explanations of patterns in the data. The iterations and associated opportunities for combinations of reasoning across a broad spectrum of experience and expertise provide a robust platform for researching real-world complexity.
Evidence and experiential data
An additional benefit of this combined approach to the research was the added value of interrogating secondary and primary data, moving backwards and forwards between them to ensure that one informed the other. In MEMORABLE, secondary sources provided access to broader, relevant and substantive data whereas primary sources illuminated the subject with finer-grained experiential perspectives.
Interviewing older people, informal carers and practitioners brought real voices directly into the research, informed by years of personal experience and expertise engendered by those experiences. This supported the co-creation of evidence. However, these interviewees were not a sample in research terms but contributed to theorising through their explanatory narratives. Their value was in what they described and explained, rather than who they might represent. In this type of real-world research, gaps can be identified between the way interventions are supposed to work, or are described as working when generic good practice is adhered to, and the way in which individuals actually experience them working in terms of the uniqueness of their own situation. Diverse data contribute directly to the analysis, its structure as well as its content. They also contribute indirectly by constantly challenging the researchers’ assumptions and methodological practices in the steps and iterations within the research process.
Qualitative data have strengthened MEMORABLE’s findings. A range of interviewees were invited to take part, reflecting the breadth of those involved in day-to-day medication management: older people, informal carers, practitioners from all sectors of health and care, including doctors, nurses, pharmacists, pharmacy technicians, social workers, and managers to front-line staff. The structure of the interview schedule enabled interviewees to describe their own experiences of multimorbidity and polypharmacy and to account for the way in which medication management worked for them at that time, and how it might work better in the future.
Approximately 60 hours of audio-recordings were collected and analysed in combination with the literature. From these, 59 initial CMO configurations were developed as well as five additional CMOs explaining burdens in reviewing/reconciling medications, informing a framework for burden mitigation (programme theory). Data saturation was achieved and no new themes were generated from the last two or three interviews. The realist analysis, subsequently conducted, confirmed that data saturation had been obtained.
The MEMORABLE study has accumulated a rich, qualitative data resource. In addition to writing up the research, its methodology and findings, the researchers intend to revisit it with a view to publishing additional articles that explore multiple themes of interest such as perspectives on the emerging role of pharmacists and the experiences of informal carers.
Theorising about causality
Theorising about how interventions work is central to realist methodology. In MEMORABLE, this involved:
-
setting out candidate theories (see Chapter 1, Step 4: candidate programme theories and substantive theory)
-
using substantive theory (NPT) to inform the analysis (see Chapter 3, Medication management as implementation)
-
generating CMO configurations to explicitly and transparently account for the multiplicity of ways interventions work for some people under certain conditions (see Chapter 3, Medication management as experience and Reviewing/reconciling medications and burden and Appendix 8)
-
developing a more narrowly focused final programme theory from a subset of those CMO configurations to explain Stage 5: reviewing/reconciling medications (see Figure 5).
Although substantive theory brings an additional analytic ‘lens’ to the synthesis, theorising in realist methodology is undertaken flexibly. In this way, it supports rather than controls analysis. Programme theory is set out to be confirmed, refined or refuted through the research process. CMO configurations are modifiable as meaning becomes clearer; one person’s outcome can be another person’s context and, even for one individual, contexts, mechanisms and outcomes can shift as the intervention process advances. This can feel messy and daunting.
In MEMORABLE, having a clear process, the work packages and steps by which the research progresses, was important. Process iterations occurred in response to the data with the Research Team mindful of the overall structure and the place of theorising within it. Having a methodology workshop at the start of the research was key to developing a shared knowledge base and an appreciation of realist methodology in the Research Team and Project Group. However, for theorising, having access to high-level realist knowledge and skill in the team and group, and regular meetings where data interpretations and analyses were discussed and debated, has been invaluable and are processes that should be considered by all realist research teams.
Strengths and limitations
Strengths
As far as the Research Team is aware, this is the first realist synthesis of medication management combining realist review and realist evaluation to understanding this key clinical area. As part of the research process, MEMORABLE has developed two key outputs:
-
a five-stage, four-step, three-loop medication management process that provides a structure to address intervention complexity, highlighting the cycles of interactions between individual and interpersonal stages (see Table 5, further developed in Tables 6 and 7)
-
a more detailed and focused programme theory of Stage 5: reviewing/reconciling medications that sets out the way that this key stage can be understood as implementation as a burden, explained by the evidence and informing proposed interventions (see Figure 5).
In addition to these outputs, the researchers have also obtained rich, qualitative data from diverse interest groups: older people, informal carers and health and care practitioners. They have set out an evidence-informed analysis framework to support their use of a realist logic of analysis and integration of data from the literature and interviews. They have also demonstrated the way substantive theory (NPT) has added explanatory value to the analysis. Analysis has generated proposed interventions that are evidence and experience based, and theory informed.
The MEMORABLE study has demonstrated the value of following a clear research process that enacts the research methodology, while maintaining the flexibility to respond to emerging topics of interest. It has also shown how the structures, processes and broad membership of Research Teams and groups supports accountability and transparency in decisions and in the scrutiny of outputs at key stages. This study has also added to practitioner capacity in realist methodology and is already being disseminated as an example of the application of that methodology.
Limitations
Few studies directly address the complexity of medication management as a process and how it works. Therefore, the researchers had to draw more heavily on primary data to validate causal attributions being made in the analysis and in the generation of programme theory. As a formative piece of research, grappling with that complexity proved time-consuming. As a result, the researchers decided that addressing causality across the whole medication management process was likely to be beyond the scope and resources available to them. The researchers had to make judgements on which of the stages of this process were more important to focus on. Analysis was, therefore, not extended or integrated across all five stages of the medication management process. Extending the analysis in the future would build useful knowledge of medication management and the development of evidence- and experience-based interventions that might bring about improvements. In addition, as acknowledged earlier, the framework for the interventions requires further development.
The decision to focus on Stage 5: reviewing/reconciling medications was not taken until part way through the research. This was because the researchers needed to have worked through what data were available to them before identifying that reviewing/reconciling medications (Stage 5) was the key part of the process to focus on. This ‘delay’, caused by a need to refocus a realist review, or evaluation, is a potential challenge for realist researchers and can have an impact on the conduct of a project. In this project, the impact was to delay the search for literature on these interventions and limit the opportunity to focus some of the interview questions on this stage.
Other limitations include that the search was limited to English-language literature, the research focused on non-institutionalised populations and the search had a broad definition of ‘older people’. Furthermore, only a relatively limited number of people with mild dementia were interviewed.
Chapter 5 Conclusions
The MEMORABLE study
The MEMORABLE study has explored the complexity of medication management through a realist approach involving the synthesis of rich data from evidence and experiential accounts. It has set out a five-stage, four-step, three-loop medication management process and, through a more detailed analysis of Stage 5: reviewing/reconciling medications, has identified five burdens experienced by older people and informal carers: ambiguity, concealment, unfamiliarity, fragmentation and exclusion. Drawing these strands together, MEMORABLE has generated an evidence- and experience-based programme theory, set out as a theoretical framework. This framework begins to explain the real-world complexity of medication management as it affects older people, informal carers and practitioners. Informed by the framework, MEMORABLE has proposed two interventions: risk identification and individualised information to assist older people living in the community to manage their medication.
This realist approach, and the theoretical framework and intervention proposals generated through it, demonstrate the way evidence and experience can be synthesised to explain complexity and inform the development of practice.
The MEMORABLE study affirms the place of older people at the centre of medication management, through their experiences and the outcomes that matter to them. The research has underscored their individuality, ingenuity and sense of purpose in the routines that they use to cope with the burdens of living with multimorbidity and polypharmacy.
As the number of older people in the population continues to grow, and, in parallel, multimorbidity and polypharmacy among them, so do the medication management challenges that they experience. These challenges are also experienced by informal carers and the health and care practitioners, services and systems that older people access, underscoring the need for further translational research in this area leading to more effective, evidence- and experience-based interventions to improve outcomes.
The key message for practitioners from MEMORABLE is that medication management for and with older people living with multimorbidity and complex regimens can be very burdensome, for different reasons, even if older people appear to be coping. Informal carers also experience burdens. These burdens are often hidden from practitioners. When prescribers start or change a medication they often think of the side effects that an older person might endure. They must equally consider their actions in terms of burden.
Future research
The researchers recommend three areas of research arising from MEMORABLE.
First, MEMORABLE has established a substantial evidence and experience base to inform two key proposed interventions: risk identification and individualised information. The principles of both interventions have been validated by the Project Group and the Stakeholder Group. Future research should be undertaken to translate these proposed interventions into practice. To be credible, it would need to be collaborative, with design and trialling co-produced with stakeholders.
Second, there needs to be further work to extend the understanding of medication management as a complex intervention and as implementation, pursuing a causal approach to it and on burden mitigation within it. This would extend the understanding of medication management stages, steps and loops by setting out a fully integrated, causally explained process. This is believed to have conceptual as well as practical value.
Third, the researchers see potential in linking to and extending MEMORABLE’s data through a study to clarify the outcomes that older people, informal carers and practitioners want from medication management. Such research would improve understanding of the goals and motivations that inform the decisions and actions of those involved in medication management.
Acknowledgements
General
The researchers would like to express their thanks to all those who took part in research interviews, for their time and for their invaluable contributions to this study.
The researchers would also like to thank the members of the Stakeholder Group for sharing their experience and expertise.
The researchers would like to acknowledge the contributions of recruitment sites (alphabetical order): Aston Research Centre for Healthy Ageing; Birmingham Community Healthcare NHS Foundation Trust; Bournville ExtraCare Village; Eve Hill Medical Practice; Join Dementia Research; Midlands Partnership NHS Foundation Trust; Sheffield City Council (Communities Commissioning).
Contributions of authors
Ian D Maidment (https://orcid.org/0000-0003-4152-9704) (Chief Investigator, Reader in Clinical Pharmacy): responsible for leading and delivering the research, ethics and governance. Lead of the Research Team. Chairperson of the Project Group and co-chairperson of the Stakeholder Group.
Sally Lawson (https://orcid.org/0000-0003-0793-5645) (Research Associate): responsible for delivering the research. Member of the Research Team, the Project Group and attendee at the Stakeholder Group.
Geoff Wong (https://orcid.org/0000-0002-5384-4157) (GP, GP Trainer and Clinical Research Fellow, Realist Approaches): specialist contributions in medicine, research and realist approaches. Member of the Research Team and Project Group.
Andrew Booth (https://orcid.org/0000-0003-4808-3880) (Reader in Evidence Based Information): specialist contributions in information science, literature searches, research and realist approaches. Member of the Research Team and Project Group.
Anne Watson (https://orcid.org/0000-0001-9264-4847) (Medicines Management Research Lead): specialist contributions in pharmacy and research. Co-chairperson of the Stakeholder Group and member of the Project Group. Facilitated site recruitment.
Jane McKeown (https://orcid.org/0000-0003-2920-4931) (Lecturer/Senior Nurse Service User and Carer Involvement): specialist contributions in nursing, research, and PPI. Member of the Project Group. Facilitated recruitment.
Hadar Zaman (https://orcid.org/0000-0002-4252-2822) (Lecturer in Pharmacy Practice): joined MEMORABLE in February 2018, specialist contributions in pharmacy and research. Member of the Research Team and Project Group. Facilitated recruitment.
Judy Mullan (https://orcid.org/0000-0003-3772-7986) (Associate Professor, Director of the Centre for Health Research Illawarra Shoalhaven Population): specialist contributions in pharmacy, research and international perspective. Member of the Project Group.
Sylvia Bailey (Retired Practice Manager with direct experience of challenges associated with medication management in older people): specialist contribution in PPI. Member of the Project Group and PPI lead. Facilitated recruitment.
Publications
Maidment I, Booth A, Mullan J, McKeown J, Bailey S, Wong G. Developing a framework for a novel multi-disciplinary multi-agency intervention(s), to improve medication management in community-dwelling older people on complex medication regimens (MEMORABLE) – a realist synthesis. Syst Rev 2017;6:4–11.
Maidment I, Lawson S, Wong G, Booth A, Watson A, Zaman H, et al. Towards an understanding of the burdens of medication management affecting older people: the MEMORABLE realist synthesis. BMC Geriatr 2020;20:183.
Data-sharing statement
This is a qualitative study and, therefore, the data generated are not suitable for sharing beyond those contained within the report. Further information can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Office for National Statistics . What Does the 2011 Census Tell Us About Older People 2013. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/articles/whatdoesthe2011censustellusaboutolderpeople (accessed September 2018).
- Office for National Statistics . Overview of the UK Population: November 2018 n.d. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation (accessed November 2018).
- Aiden H. Multimorbidity Richmond Group 2018. www.richmondgroupofcharities.org.uk/sites/default/files/multimorbidity_-_understanding_the_challenge.pdf (accessed February 2018).
- Hobbs FD, Baker M, Davies DS. Morbidity matters: challenges for research. Br J Gen Pract 2015;65:e215-6. https://doi.org/10.3399/bjgp15X684301.
- House of Commons Health Committee . Managing the Care of People With Long-Term Conditions 2014. https://publications.parliament.uk/pa/cm201415/cmselect/cmhealth/401/401.pdf (accessed February 2019).
- Stafford M, Steventon A, Thorlby R, Fisher R, Turton C, Deeny S. Briefing: Understanding the Health Care Needs of People with Multiple Health Conditions. London: The Health Foundation; 2018.
- Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ 2015;350. https://doi.org/10.1136/bmj.h176.
- Taskforce on Multiple Conditions . Just One Thing After Another. Living With Multiple Conditions 2018. https://richmondgroupofcharities.org.uk/sites/default/files/final_just_one_thing_after_another_report_-_singles.pdf (accessed February 2019).
- Parker G, Corden A, Heaton J. Synthesis and Conceptual Analysis of the SDO Programme’s Research on Continuity of Care 2010. www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1813-248_V01.pdf (accessed July 2019).
- Gao L, Maidment I, Matthews FE, Robinson L, Brayne C. Medical Research Council Cognitive Function and Ageing Study . Medication usage change in older people (65+) in England over 20 years: findings from CFAS I and CFAS II. Age Ageing 2018;47:220-5. https://doi.org/10.1093/ageing/afx158.
- Baxter R, Hastings N, Law A, Glass EJ. World Report on Ageing and Health 2015. www.who.int/ageing/events/world-report-2015-%0Alaunch/en (accessed November 2018).
- Sabate E. Adherence to Long-Term Therapies: Evidence for Action World Health Organization 2003 n.d. www.who.int/chp/knowledge/publications/adherence_report/en/%0A (accessed November 2018).
- Demain S, Gonçalves AC, Areia C, Oliveira R, Marcos AJ, Marques A, et al. Living with, managing and minimising treatment burden in long term conditions: a systematic review of qualitative research. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0125457.
- Sav A, King MA, Whitty JA, Kendall E, McMillan SS, Kelly F, et al. Burden of treatment for chronic illness: a concept analysis and review of the literature. Health Expect 2015;18:312-24. https://doi.org/10.1111/hex.12046.
- Tran VT, Barnes C, Montori VM, Falissard B, Ravaud P. Taxonomy of the burden of treatment: a multi-country web-based qualitative study of patients with chronic conditions. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0356-x.
- Centre for Reviews and Dissemination . Reducing Harm from Polypharmacy in Older People. Improvement Academy 2017. www.improvementacademy.org/documents/resources/effectiveness_matters/Effectiveness%20Matters%20August%202017.pdf (accessed February 2019).
- Maidment ID, Haw C, Stubbs J, Fox C, Katona C, Franklin BD. Medication errors in older people with mental health problems: a review. Int J Geriatr Psychiatry 2008;23:564-73. https://doi.org/10.1002/gps.1943.
- Peklar J, O’Halloran AM, Maidment ID, Henman MC, Kenny RA, Kos M. Sedative load and frailty among community-dwelling population aged ≥ 65 years. J Am Med Dir Assoc 2015;16:282-9. https://doi.org/10.1016/j.jamda.2014.10.010.
- Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc 2011;59:1477-83. https://doi.org/10.1111/j.1532-5415.2011.03491.x.
- National Patient Safety Agency . The Fourth Report from the Patient Safety Observatory. Safety in Doses: Medication Safety Incidents in the NHS. 2007. https://nationalpatientsafetysuite.vctms.co.uk/assets/content/35497/story_content/external_files/NRLS-0486-safety-in-doses-PSO-2007-v1.pdf.pdf (accessed February 2019).
- Frontier Economics . Exploring the Costs of Unsafe Care in the NHS 2014. www.frontier-economics.com/documents/2014/10/exploring-the-costs-of-unsafe-care-in-the-nhs-frontier-report-2-2-2-2.pdf (accessed November 2019).
- Elliott RA, Camacho E, Campbell F, Jankovic D, Martyn St James M, Kaltenthaler E, et al. Prevalence and Economic Burden of Medication Errors in the NHS in England: Rapid Evidence Synthesis and Economic Analysis of the Prevalence and Burden of Medication Error in the UK 2018. www.eepru.org.uk/wp-content/uploads/2018/02/medication-error-report-revised-final.2-22022018.pdf (accessed February 2019).
- Gillespie R, Mullan J, Harrison L. Managing medications: the role of informal caregivers of older adults and people living with dementia. A review of the literature. J Clin Nurs 2014;23:3296-308. https://doi.org/10.1111/jocn.12519.
- Poland F, Mapes S, Pinnock H, Katona C, Sorensen S, Fox C, et al. Perspectives of carers on medication management in dementia: lessons from collaboratively developing a research proposal. BMC Res Notes 2014;7. https://doi.org/10.1186/1756-0500-7-463.
- Aston L, Hilton A, Moutela T, Shaw R, Maidment I. Exploring the evidence base for how people with dementia and their informal carers manage their medication in the community: a mixed studies review. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0638-6.
- Maidment ID, Aston L, Moutela T, Fox CG, Hilton A. A qualitative study exploring medication management in people with dementia living in the community and the potential role of the community pharmacist. Health Expect 2017;20:929-42. https://doi.org/10.1111/hex.12534.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. https://doi.org/10.1016/S0140-6736(12)60240-2.
- Health Service Journal/Serco Commission . Hospital Care for Frail Older People 2014. www.hsj.co.uk/download?ac=1292263 (accessed February 2019).
- NICE . Multimorbidity and Polypharmacy (KTT18) 2017:1-5. www.nice.org.uk/guidance/ktt18/resources/multimorbidity-and-polypharmacy-58757959453381 (accessed January 2019).
- Maidment I, Booth A, Mullan J, McKeown J, Bailey S, Wong G. Developing a framework for a novel multi-disciplinary, multi-agency intervention(s), to improve medication management in community-dwelling older people on complex medication regimens (MEMORABLE) – a realist synthesis. Syst Rev 2017;6. https://doi.org/10.1186/s13643-017-0528-1.
- Maidment I, Lawson S. Developing a Novel Multi-Disciplinary, Multi-Agency Intervention, to Improve Medication Management in Older People on Complex Medication Regimens Resident in the Community 2018. www2.aston.ac.uk/lhs/research/memorable (accessed November 2018).
- www.parliament.uk . The Ageing Population: Key Issues for the 2010 Parliament n.d. www.parliament.uk/business/publications/research/key-issues-for-the-new-parliament/value-for-money-in-public-services/the-ageing-population (accessed February 2019).
- NICE . Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. NICE Guideline 5 2015. www.nice.org.uk/guidance/ng5/resources/medicines-optimisation-the-safe-and-effective-use-of-medicines-to-enable-the-best-possible-outcomes-pdf-51041805253 (accessed January 2019).
- Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet 2007;370:185-91. https://doi.org/10.1016/S0140-6736(07)61092-7.
- British Geriatrics Society . Fit for Frailty Part 1 Consensus Best Practice Guidance for the Care of Older People Living in Community and Outpatient Settings 2017. www.bgs.org.uk/campaigns/fff/fff_full.pdf (accessed February 2019).
- Gillespie RJ, Harrison L, Mullan J. Deprescribing medications for older adults in the primary care context: a mixed studies review. Health Sci Rep 2018;1. https://doi.org/10.1002/hsr2.45.
- Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2018;9. https://doi.org/10.1002/14651858.CD008165.pub4.
- Banerjee S. Multimorbidity – older adults need health care that can count past one. Lancet 2015;385:587-9. https://doi.org/10.1016/S0140-6736(14)61596-8.
- May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ 2009;339. https://doi.org/10.1136/bmj.b2803.
- Palmer K, Marengoni A, Forjaz MJ, Jureviciene E, Laatikainen T, Mammarella F, et al. Multimorbidity care model: Recommendations from the consensus meeting of the Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS). Health Policy 2018;122:4-11. https://doi.org/10.1016/j.healthpol.2017.09.006.
- NICE . Multimorbidity: Clinical Assessment and Management 2016. www.nice.org.uk/guidance/ng56/resources/multimorbidity-clinical-assessment-and-management-pdf-1837516654789 (accessed January 2019).
- Hoffmann T, Jansen J, Glasziou P. The importance and challenges of shared decision making in older people with multimorbidity. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002530.
- World Health Organization . Global Patient Safety Challenge: Medication Without Harm. WHO Global Patient Safety Challenge 2017. http://apps.who.int/iris/bitstream/10665/255263/1/WHO-HIS-SDS-2017.6-eng.pdf?ua=1&ua=1 (accessed July 2019).
- Horne R, Weinman J, Barber N, Elliott R. Concordance, Adherence and Compliance in Medicine Taking. Report for the National Co-Ordinating Centre for NHS Service Delivery and Organisation R &Amp; D (NCCSDO) 2005:1-331. www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1412-076_V01.pdf (accessed January 2019).
- Francis R. Report of the Mid Staffordshire NHS Foundation Trust Public Inquiry. Executive Summary 2013. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/279124/0947.pdf (accessed February 2019).
- World Health Organization . Medication Errors. Technical Series on Safer Primary Care 2016. https://doi.org/10.1097/01.NURSE.0000524761.58624.1f (accessed February 2019).
- NHS Improvement . Patient Safety Review and Response Report 2018. https://improvement.nhs.uk/documents/2526/Patient_Safety_Review_and_Response_Report_Apr-Sept_2017.pdf (accessed February 2019).
- Office for National Statistics . Living Longer: Fitting It All in – Working, Caring and Health in Later Life 2018. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/articles/livinglongerhowourpopulationischangingandwhyitmatters/2019-03-15 (accessed March 2019).
- Pawson R, Tilley N. Realistic Evaluation. London: SAGE Publications Ltd; 1997.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist Synthesis: An Introduction 2004. www.researchgate.net/publication/228855827_Realist_Synthesis_An_Introduction (accessed November 2018).
- Wong G, Westhorp G, Pawson R, Greenhalgh T. Realist Synthesis: RAMESES Training Materials 2013. www.ramesesproject.org/media/Realist_reviews_training_materials.pdf (accessed November 2018).
- Pawson R. Evidence-based Policy: A Realist Perspective. London: SAGE Publications Ltd; 2006.
- Pawson R. The Science of Evaluation: A Realist Manifesto. London: SAGE Publications Ltd; 2013.
- Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-33.
- Booth A, Wright JM, Briscoe S, Emmel N, Greenhalgh J, Manzano A, et al. Doing Realist Research. London: SAGE Publications Ltd; 2018.
- Westhorp G. Realist Evaluation: An Overview Report from an Expert Seminar 2011. www.alnap.org/help-library/realist-evaluation-an-overview-report-from-an-expert-seminar-with-dr-gill-westhorp (accessed November 2018).
- Dalkin SM, Greenhalgh J, Jones D, Cunningham B, Lhussier M. What’s in a mechanism? Development of a key concept in realist evaluation. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0237-x.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. J Adv Nurs 2013;69:1005-22. https://doi.org/10.1111/jan.12095.
- Wong G, Westhorp G, Manzano A, Greenhalgh J, Jagosh J, Greenhalgh T. RAMESES II reporting standards for realist evaluations. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0643-1.
- Abbott A. Reconceptualizing knowledge accumulation in sociology. Am Sociol 2006;37:57-66. https://doi.org/10.1007/s12108-006-1005-9.
- Pawson R, Manzano-Santaella A. A Realist Diagnostic Workshop. Evaluation 2012;18:176-91. https://doi.org/10.1177/1356389012440912.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. ‘Psychological Theory’ Group. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. https://doi.org/10.1136/qshc.2004.011155.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Maidment I, Lawson S, Wong G, Booth A, Watson A, Zaman H, et al. Towards an understanding of the burdens of medication management affecting older people: the MEMORABLE realist synthesis. BMC Geriatr 2020;20. https://doi.org/10.1186/s12877-020-01568-x.
- Plsek PE, Greenhalgh T. Complexity science: the challenge of complexity in health care. BMJ 2001;323:625-8. https://doi.org/10.1136/bmj.323.7313.625.
- National Voices . Supporting Self-Management. A Summary of the Evidence n.d. www.nationalvoices.org.uk/publications/our-publications/supporting-self-management (accessed November 2018).
- Burstow Commission . Key to Care n.d. www.lgiu.org.uk/2014/12/02/key-to-care-report-of-the-burstow-commission-on-the-future-of-the-home-care-workforce (accessed November 2018).
- NICE . Older People With Social Care Needs and Multiple Long-Term Conditions 2015. www.nice.org.uk/guidance/ng22 (accessed November 2018).
- NICE . Medicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence. Clinical Guideline 76 2009. www.nice.org.uk/guidance/cg76 (accessed November 2018).
- Moher D, Liberati A, Tetzlaff J, Altman DG. the PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Patton MQ. Utilization-Focused Evaluation. London: SAGE Publications Ltd; 2008.
- Manzano A. The craft of interviewing in realist evaluation. Evaluation 2016;22:342-60. https://doi.org/10.1177/1356389016638615.
- Brinkman S, Kvale S. Interviews: Learning the Craft of Qualitative Research Interviewing. London: SAGE Publications Ltd; 2015.
- Ridgeway JL, Egginton JS, Tiedje K, Linzer M, Boehm D, Poplau S, et al. Factors that lessen the burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Prefer Adherence 2014;8:339-51. https://doi.org/10.2147/PPA.S58014.
- Mohammed MA, Moles RJ, Chen TF. Medication-related burden and patients’ lived experience with medicine: a systematic review and metasynthesis of qualitative studies. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010035.
- Booth A. Unpacking your literature search toolbox: on search styles and tactics. Health Info Libr J 2008;25:313-17. https://doi.org/10.1111/j.1471-1842.2008.00825.x.
- Booth A, Harris J, Croot E, Springett J, Campbell F, Wilkins E. Towards a methodology for cluster searching to provide conceptual and contextual ‘richness’ for systematic reviews of complex interventions: case study (CLUSTER). BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-118.
- Booth A, Carroll C. Systematic searching for theory to inform systematic reviews: is it feasible? Is it desirable?. Heal Inf Libr J 2015;32:220-35.
- Eton DT, Elraiyah TA, Yost KJ, Ridgeway JL, Johnson A, Egginton JS, et al. A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Relat Outcome Meas 2013;4:7-20. https://doi.org/10.2147/PROM.S44694.
- NICE . Managing Medicines for People Receiving Social Care in the Community Overview – NICE Pathways 2017. https://pathways.nice.org.uk/pathways/managing-medicines-for-people-receiving-social-care-in-the-community (accessed January 2019).
- NICE . Medicines Optimisation Overview – NICE Pathways 2015:1-12. https://pathways.nice.org.uk/pathways/medicines-optimisation#content=view-node%3Anodes-self-management-plan (accessed January 2019).
- Patton DE, Hughes CM, Cadogan CA, Ryan CA. Theory-based interventions to improve medication adherence in older adults prescribed polypharmacy: a systematic review. Drugs Aging 2017;34:97-113. https://doi.org/10.1007/s40266-016-0426-6.
- Hughes LD, McMurdo MET, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing 2013;42:62-9. https://doi.org/10.1093/ageing/afs100.
- Cheraghi-Sohi S, Jeffries M, Stevenson F, Ashcroft DM, Carr M, Oliver K, et al. The influence of personal communities on the self-management of medication taking: a wider exploration of medicine work. Chronic Illn 2015;11:77-92. https://doi.org/10.1177/1742395314537841.
- Fried TR, Niehoff K, Tjia J, Redeker N, Goldstein MK. A Delphi process to address medication appropriateness for older persons with multiple chronic conditions. BMC Geriatr 2016;16. https://doi.org/10.1186/s12877-016-0240-3.
- Naik AD, Dyer CB, Kunik ME, McCullough LB. Patient autonomy for the management of chronic conditions: a two-component re-conceptualization. Am J Bioeth 2009;9:23-30. https://doi.org/10.1080/15265160802654111.
- Upadhyay J, Joshi Y. Observation of drug utilization pattern and prevalence of diseases in elderly patients through home medication review. Asian J Pharm Clin Res 2011;4:143-5. www.embase.com/search/results?subaction=viewrecord&from=export&id=L361820478%0Ahttp://www.ajpcr.com/Vol4Issue1/240.pdf%0Ahttps://resolver.library.uq.edu.au/?sid=EMBASE&issn=09742441&id=doi:&atitle=Observation+of+drug+utilization+pattern+and+prevale (accessed January 2019).
- Bartlett Ellis RJ, Welch JL. Medication-taking behaviours in chronic kidney disease with multiple chronic conditions: a meta-ethnographic synthesis of qualitative studies. J Clin Nurs 2017;26:586-98. https://doi.org/10.1111/jocn.13588.
- Boskovic J, Mestrovic A, Leppée M, Bago M, Sostar Z, Naletilic D. Pharmacist competences and impact of pharmacist intervention on medication adherence: an observational study. Psychiatr Danub 2016;28:420-7.
- Coleman A. Medication adherence of elderly citizens in retirement homes through a mobile phone adherence monitoring framework (Mpamf) for developing countries: a case study in South Africa. Indian J Pharm Educ Res 2014;48:6-11. https://doi.org/10.5530/ijper.48.3.2.
- Schuling J, Gebben H, Veehof LJ, Haaijer-Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract 2012;13. https://doi.org/10.1186/1471-2296-13-56.
- Yap AF, Thirumoorthy T, Kwan YH. Medication adherence in the elderly. J Clin Gerontol Geriatr 2016;7:64-7. https://doi.org/10.1016/j.jcgg.2015.05.001.
- Doucette WR, Vinel S, Pennathur P. Initial development of the Systems Approach to Home Medication Management (SAHMM) model. Res Social Adm Pharm 2017;13:39-47. https://doi.org/10.1016/j.sapharm.2015.12.013.
- Hennessey B, Suter P. The community-based transitions model: one agency’s experience. Home Healthc Nurse 2011;29:218-30. https://doi.org/10.1097/NHH.0b013e318211986d.
- Jonikas MA, Mandl KD. Surveillance of medication use: early identification of poor adherence. J Am Med Inform Assoc 2012;19:649-54. https://doi.org/10.1136/amiajnl-2011-000416.
- Khabala KB, Edwards JK, Baruani B, Sirengo M, Musembi P, Kosgei RJ, et al. Medication Adherence Clubs: a potential solution to managing large numbers of stable patients with multiple chronic diseases in informal settlements. Trop Med Int Health 2015;20:1265-70. https://doi.org/10.1111/tmi.12539.
- Kucukarslan SN, Lewis NJ, Shimp LA, Gaither CA, Lane DC, Baumer AL. Exploring patient experiences with prescription medicines to identify unmet patient needs: implications for research and practice. Res Social Adm Pharm 2012;8:321-32. https://doi.org/10.1016/j.sapharm.2011.08.003.
- McHorney CA, Zhang NJ, Stump T, Zhao X. Structural equation modeling of the proximal-distal continuum of adherence drivers. Patient Prefer Adherence 2012;6:789-804. https://doi.org/10.2147/PPA.S36535.
- Lau Y, Htun TP, Chan KS, Klainin-Yobas P. Multidimensional factors affecting medication adherence among community-dwelling older adults: a structural-equation-modeling approach. J Public Heal 2017;25:113-22. https://doi.org/10.1007/s10389-016-0764-1.
- Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med 2015;128:337-43. https://doi.org/10.1016/j.amjmed.2014.10.047.
- Shepherd JG, Locke E, Zhang Q, Maihafer G. Health services use and prescription access among uninsured patients managing chronic diseases. J Community Health 2014;39:572-83. https://doi.org/10.1007/s10900-013-9799-1.
- Geryk LL, Blalock SJ, DeVellis RF, Jordan JM, Han PK, Carpenter DM. Medication-related self-management behaviors among arthritis patients: does attentional coping style matter?. Open Rheumatol J 2016;10:60-7. https://doi.org/10.2174/1874312901610010060.
- Haslbeck JW, Schaeffer D. Routines in medication management: the perspective of people with chronic conditions. Chronic Illn 2009;5:184-96. https://doi.org/10.1177/1742395309339873.
- Laba TL, Lehnbom E, Brien JA, Jan S. Understanding if, how and why non-adherent decisions are made in an Australian community sample: a key to sustaining medication adherence in chronic disease?. Res Social Adm Pharm 2015;11:154-62. https://doi.org/10.1016/j.sapharm.2014.06.006.
- Marks R. Self-efficacy and arthritis disability: an updated synthesis of the evidence base and its relevance to optimal patient care. Health Psychol Open 2014;1. https://doi.org/10.1177/2055102914564582.
- Jorge de Sousa Oliveira C, José H, Castro Caldas A. Interventions to improve medication adherence in aged people with chronic disease – systematic review. Univers J Public Heal 2017;5:25-31. https://doi.org/10.13189/ujph.2017.050104.
- Skolasky RL, Green AF, Scharfstein D, Boult C, Reider L, Wegener ST. Psychometric properties of the patient activation measure among multimorbid older adults. Health Serv Res 2011;46:457-78. https://doi.org/10.1111/j.1475-6773.2010.01210.x.
- Ryan R, Santesso N, Hill S, Lowe D, Kaufman C, Grimshaw J. Consumer-oriented interventions for evidence-based prescribing and medicines use: an overview of systematic reviews. Cochrane Database Syst Rev 2011;5. https://doi.org/10.1002/14651858.CD007768.pub2.
- Birkhäuer J, Gaab J, Kossowsky J, Hasler S, Krummenacher P, Werner C, et al. Trust in the health care professional and health outcome: A meta-analysis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0170988.
- Stevenson FA, Cox K, Britten N, Dundar Y. A systematic review of the research on communication between patients and health care professionals about medicines: the consequences for concordance. Health Expect 2004;7:235-45. https://doi.org/10.1111/j.1369-7625.2004.00281.x.
- Pereira Gray DJ, Sidaway-Lee K, White E, Thorne A, Evans PH. Continuity of care with doctors-a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-021161.
- Sanders MJ, Van Oss T. Using daily routines to promote medication adherence in older adults. Am J Occup Ther 2013;67:91-9. https://doi.org/10.5014/ajot.2013.005033.
- Volpp KG, Mohta NS. What creates behavior change may not sustain it. NEJM Catal 2018. https://catalyst.nejm.org/survey-sustaining-behavior-change.
- May C. Agency and implementation: understanding the embedding of healthcare innovations in practice. Soc Sci Med 2013;78:26-33. https://doi.org/10.1016/j.socscimed.2012.11.021.
- Gallacher KI, May CR, Langhorne P, Mair FS. A conceptual model of treatment burden and patient capacity in stroke. BMC Fam Pract 2018;19. https://doi.org/10.1186/s12875-017-0691-4.
- Thaler RH, Sunstein CR, Balz JP, Eldar S. Choice Architecture. The Behavioural Foundations of Public Policy 2014:428-39. https://doi.org/10.2139/ssrn.2536504.
- Eton DT, Yost KJ, Lai JS, Ridgeway JL, Egginton JS, Rosedahl JK, et al. Development and validation of the Patient Experience with Treatment and Self-management (PETS): a patient-reported measure of treatment burden. Qual Life Res 2017;26:489-503. https://doi.org/10.1007/s11136-016-1397-0.
- Katusiime B, Corlett SA, Krska J. Development and validation of a revised instrument to measure burden of long-term medicines use: the Living with Medicines Questionnaire version 3. Patient Relat Outcome Meas 2018;9:155-68. https://doi.org/10.2147/PROM.S151143.
- Krska J, Katusiime B, Corlett SA. Validation of an instrument to measure patients’ experiences of medicine use: the Living with Medicines Questionnaire. Patient Prefer Adherence 2017;11:671-9. https://doi.org/10.2147/PPA.S126647.
- Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol 2012;65:1041-51. https://doi.org/10.1016/j.jclinepi.2012.05.005.
- Tran VT, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med 2014;12. https://doi.org/10.1186/1741-7015-12-109.
- Bandura A. Self-efficacy mechanism in human agency. Am Psychol 1982;37:122-47. https://pdfs.semanticscholar.org/8bee/c556fe7a650120544a99e9e063eb8fcd987b.pdf (accessed January 2019).
- George J, Elliott RA, Stewart DC. A systematic review of interventions to improve medication taking in elderly patients prescribed multiple medications. Drugs Aging 2008;25:307-24. https://doi.org/10.2165/00002512-200825040-00004.
- Uniform Data System for Medical Rehabilitation . The FIM® Instrument: Its Background, Structure, and Usefulness 2014. www.udsmr.org/Documents/The_FIM_Instrument_Background_Structure_and_Usefulness.pdf (accessed February 2019).
- Leung DY, Bai X, Leung AY, Liu BC, Chi I. Prevalence of medication adherence and its associated factors among community-dwelling Chinese older adults in Hong Kong. Geriatr Gerontol Int 2015;15:789-96. https://doi.org/10.1111/ggi.12342.
- Huiskes VJ, Burger DM, van den Ende CH, van den Bemt BJ. Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials. BMC Fam Pract 2017;18. https://doi.org/10.1186/s12875-016-0577-x.
- Basheti IA, Rizik M, Bulatova NR. Home medication management review in outpatients with alarming health issues in Jordan: a randomized control trial. J Pharm Heal Serv Res 2018;9:91-100. https://doi.org/10.1111/jphs.12213.
- Niehoff KM, Rajeevan N, Charpentier PA, Miller PL, Goldstein MK, Fried TR. Development of the Tool to Reduce Inappropriate Medications (TRIM): a clinical decision support system to improve medication prescribing for older adults. Pharmacotherapy 2016;36:694-701. https://doi.org/10.1002/phar.1751.
- Clyne W, Blenkinsopp A, Seal R. A Guide to Medication Review 2008 n.d. www.cff.org.br/userfiles/52%20-%20CLYNE%20W%20A%20guide%20to%20medication%20review%202008.pdf (accessed January 2019).
- Lee CY, George J, Elliott RA, Stewart K. Prevalence of medication-related risk factors among retirement village residents: a cross-sectional survey. Age Ageing 2010;39:581-7. https://doi.org/10.1093/ageing/afq079.
- Pharmaceutical Services Negotiating Committee (PSNC) . PSNC Briefing 038 17: A Summary of Literature Relating to Medicines Use Reviews (June 2017) n.d. https://psnc.org.uk/wp-content/uploads/2013/04/PSNC-Briefing-038.17-A-summary-of-literature-relating-to-MURs.pdf (accessed January 2019).
- Fletcher J, Hogg W, Farrell B, Woodend K, Dahrouge S, Lemelin J, et al. Effect of nurse practitioner and pharmacist counseling on inappropriate medication use in family practice. Can Fam Physician 2012;58:862-8.
- Centre for Policy on Ageing . The Effectiveness of Community Pharmacy Medication (Medicine Use) Reviews: Rapid Review 2014. www.cpa.org.uk/information/reviews/CPA-Rapid-Review-Community-Pharmacy-Medication-Reviews.pdf (accessed November 2018).
- Coane S, Payne R. Carrying out a structured medication review. Prescriber 2016;27:22-6. http://doi.org/wiley.com/10.1002/psb.1426.
- Cheema E, Alhomoud FK, Kinsara ASA, Alsiddik J, Barnawi MH, Al-Muwallad MA, et al. The impact of pharmacists-led medicines reconciliation on healthcare outcomes in secondary care: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0193510.
- McNab D, Bowie P, Ross A, MacWalter G, Ryan M, Morrison J. Systematic review and meta-analysis of the effectiveness of pharmacist-led medication reconciliation in the community after hospital discharge. BMJ Qual Saf 2018;27:308-20. https://doi.org/10.1136/bmjqs-2017-007087.
- Kogut SJ, Goldstein E, Charbonneau C, Jackson A, Patry G. Improving medication management after a hospitalization with pharmacist home visits and electronic personal health records: an observational study. Drug Healthc Patient Saf 2014;6:1-6. https://doi.org/10.2147/DHPS.S56574.
- Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010003.
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD000011.pub4.
- General Medical Council (GMC) . Good Practice in Prescribing and Managing Medicines and Devices (2013) n.d. www.gmc-uk.org/Prescribing_guidance.pdf_59055247.pdf (accessed January 2019).
- Nursing and Midwifery Council . Standards For Medicines Management n.d. www.nmc.org.uk/standards/standards-for-post-registration/standards-for-medicines-management (accessed January 2019).
- Nursing and Midwifery Council . Standards of Record Keeping for Proficiency Nurse and Midwife Prescribers 2006. www.nmc.org.uk/globalassets/sitedocuments/standards/nmc-standards-proficiency-nurse-and-midwife-prescribers.pdf (accessed February 2019).
- Royal College of Nursing . Better Medicines Management Advice for Nursing Staff and Patients 2013;10. www.rcn.org.uk/__data/assets/pdf_file/0018/518130/004393.pdf (accessed January 2019).
- Royal College of Nursing . Nurse Prescribing: RCN Fact Sheet 2014. www.rcn.org.uk/professional-development/publications/pub-004393 (accessed January 2019).
- Royal College of Nursing . Non-Medical Prescribing. Advice Guides. Royal College of Nursing Types of Nurse Prescriber Nurse Independent Prescribers and Controlled Regulations 2018. www.rcn.org.uk/get-help/rcn-advice/non-medical-prescribers (accessed January 2019).
- Royal Pharmaceutical Society . Medicines Optimisation: Helping Patients to Make the Most of Medicines. Good Practice Guidance for Healthcare Professionals in England n.d. https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Policy/helping-patients-make-the-most-of-their-medicines.pdf (accessed January 2019).
- Marmesat B, Gallego M, Fernandez I, . System for improving adherence in polymedicated patients. Eur J Hosp Pharm 2014;21. https://doi.org/10.1136/ejhpharm-2013-000436.404.
- Kallio SE, Kiiski A, Airaksinen MSA, Mäntylä AT, Kumpusalo-Vauhkonen AEJ, Järvensivu TP, et al. Community pharmacists’ contribution to medication reviews for older adults: a systematic review. J Am Geriatr Soc 2018;66:1613-20. https://doi.org/10.1111/jgs.15416.
- Jokanovic N, Tan EC, Sudhakaran S, Kirkpatrick CM, Dooley MJ, Ryan-Atwood TE, et al. Pharmacist-led medication review in community settings: an overview of systematic reviews. Res Social Adm Pharm 2017;13:661-85. https://doi.org/10.1016/j.sapharm.2016.08.005.
- Rolfe A, Cash-Gibson L, Car J, Sheikh A, McKinstry B. Interventions for improving patients’ trust in doctors and groups of doctors. Cochrane Database Syst Rev 2014;3. https://doi.org/10.1002/14651858.CD004134.pub3.
- Piette JD, Heisler M, Krein S, Kerr EA. The role of patient-physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med 2005;165:1749-55. https://doi.org/10.1001/archinte.165.15.1749.
- Maidment ID, Brown P, Calnan M. An exploratory study of the role of trust in medication management within mental health services. Int J Clin Pharm 2011;33:614-20. https://doi.org/10.1007/s11096-011-9510-5.
- Brennan N, Barnes R, Calnan M, Corrigan O, Dieppe P, Entwistle V. Trust in the health-care provider-patient relationship: a systematic mapping review of the evidence base. Int J Qual Health Care 2013;25:682-8. https://doi.org/10.1093/intqhc/mzt063.
- Murray B, McCrone S. An integrative review of promoting trust in the patient-primary care provider relationship. J Adv Nurs 2015;71:3-23. https://doi.org/10.1111/jan.12502.
- Reeve J, Britten N, Byng R, Fleming J, Heaton J, Krska J. Identifying enablers and barriers to individually tailored prescribing: a survey of healthcare professionals in the UK. BMC Fam Pract 2018;19. https://doi.org/10.1186/s12875-017-0705-2.
- Bunn F, Goodman C, Russell B, Wilson P, Manthorpe J, Rait G, et al. Supporting shared decision making for older people with multiple health and social care needs: a realist synthesis. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0853-9.
- Coulter A, Edwards A, Entwistle V, Kramer G, Nye A, Thomson R, et al. Shared decision making in the UK: moving towards wider uptake. Z Evid Fortbild Qual Gesundhwes 2017:123-4. https://doi.org/10.1016/j.zefq.2017.05.010.
- Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns 2012;86:9-18. https://doi.org/10.1016/j.pec.2011.02.004.
- Daly RL, Bunn F, Goodman C. Shared decision-making for people living with dementia in extended care settings: a systematic review. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-018977.
- Elwyn G, Durand MA, Song J, Aarts J, Barr PJ, Berger Z, et al. A three-talk model for shared decision making: multistage consultation process. BMJ 2017;359. https://doi.org/10.1136/bmj.j4891.
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27:1361-7. https://doi.org/10.1007/s11606-012-2077-6.
- Joseph-Williams N, Lloyd A, Edwards A, Stobbart L, Tomson D, Macphail S, et al. Implementing shared decision making in the NHS: lessons from the MAGIC programme. BMJ 2017;357. https://doi.org/10.1136/bmj.j1744.
- Santo K, Kirkendall S, Laba TL, Thakkar J, Webster R, Chalmers J, et al. Interventions to improve medication adherence in coronary disease patients: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol 2016;23:1065-76. https://doi.org/10.1177/2047487316638501.
- Young A, Tordoff J, Smith A. ‘What do patients want?’ Tailoring medicines information to meet patients’ needs. Res Social Adm Pharm 2017;13:1186-90. https://doi.org/10.1016/j.sapharm.2016.10.006.
- Ulley J, Harrop D, Ali A, Alton S, Fowler Davis S. Deprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: a systematic review. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1031-4.
- Duncan P, Cabral C, McCahon D, Guthrie B, Ridd MJ. Efficiency versus thoroughness in medication review: a qualitative interview study in UK primary care. Br J Gen Pract 2019;69:e190-e198. https://doi.org/10.3399/bjgp19X701321.
- Bradley F, Seston E, Mannall C, Cutts C. Evolution of the general practice pharmacist’s role in England: a longitudinal study. Br J Gen Pr 2018;68:e727-e734. https://doi.org/10.3399/bjgp18X698849.
- Karampatakis GD, Ryan K, Patel N, Lau WM, Stretch G. How do pharmacists in English general practices identify their impact? An exploratory qualitative study of measurement problems. BMC Health Serv Res 2019;19. https://doi.org/10.1186/s12913-018-3842-y.
Appendix 1 Pilot search
Overview
The information specialist (AB) and the research associate (SL) carried out a pilot search on MEDLINE, using the following combined search terms (including a cut-off date of 2009 and in English):
-
‘aged’ (65 years >) and ‘aged, 80 and over’ (Protocol 60 years >);
-
‘medication management, medicines optimisation/optimisation, concordance, compliance/patient compliance, adherence, health services misuse or inappropriate prescribing, deprescribing’;
-
‘chronic disease’; and
-
‘regime or regimen/regimes or regimens’.
There were 160 articles identified.
The research associate read all article abstracts. This provided a ‘sense’ of the data while adding to her understanding of the research topic. The task was then for her to identify and report:
-
relevant articles – by date and by content
-
additional terms not included in the pilot search
-
sift criteria to be used in future searches, as well as a data extraction form based on those criteria.
Initial analysis and results
These are summarised below.
Relevant articles: by date and content
| Records | N | Criteria |
|---|---|---|
| Abstracts identified through pilot search: MEDLINE on 10 July 2017 | 160 | Search criteria listed in Overview |
| Screened by date (2009>): records removed (n = 92) | ||
| Screened by relevance (excluding ‘not relevant’ because of e.g. focus on the action of medications, other treatments/interventions, protocol/study design only, duplicate records): records removed (n = 33) | ||
| Remaining records | 35 | |
Additional terms identified from the remaining records
The 35 abstracts were then mapped onto an Excel database (AB/SL Search Database, tabbed Pilot Search), identifying the record number from the search, author, title, topic, design, number of subjects and country.
The contents of each abstract were then analysed for additional terms that added to the scope of the original four search terms noted above or because they appeared to have explanatory or causal significance in relation to the research topic. These were identified in the content of the abstract and the key word listing. As a result, the four combined search terms were expanded to 56, and grouped in three ways: those associated with individual experiences, whether older people and carers, or practitioners, and those associated with health and care practice and systems that were often highlighted in the articles’ recommendations (as below).
The following bullet points provide a sense of the data, setting out the top three clustered terms occurring in each group:
-
Individual experiences (older people and carers)
-
High risk patients/Health status and complications/Chronic diseases/Chronic conditions/Long-term conditions/Co-morbidity/Multimorbidity/Progressive/Diagnoses/Other health conditions/Disability/Chronic illness trajectory/Restrictive health care conditions (n = 32)
-
Medication adherence – nonadherence: (n = 22)
-
Complex medication regimens/Dosing frequency/Schedules of medication-taking events and Pill burden/Frequent changes/New medications/Inconvenience/Taking and timing adherence/Adjusting routines (n = 21).
-
-
Individual experiences (practitioners)
-
Drug therapy (unnecessary, ineffective, incomplete)/Dosage/Pharmaceutical preparations/Medication therapy management (n = 6)
-
Discussion/Shared decision-making/Effective communication/Decision-making/Time/Priority reconciliation/Health professions interest-disinterest in balancing diagnosis, regimen and life (n = 6)
-
Collaborative approaches/Patient centred care (n = 5).
-
-
Health and care practice and systems
-
Person centred approach/Decision support systems/Patient care/Personal support/Patient–provider relationship/Teach-back methods/Coaching (n = 6)
-
General practitioners/Physicians practice patterns/Socio-economic influences/Doctor-patient/Physicians role (n = 6)
-
Community pharmacist/Professional role/Pharmacist (n = 4).
-
Sift terms and a data extraction form
Based on this analysis, an indicative (rather than definitive) list of terms is proposed below, identifying those associated with inclusion as well as exclusion.
Under inclusion, these are grouped under core terms and supplementary terms. The core terms reflect the scope of the research to ensure that abstract content aligns with the research topic. This frames the search. The supplementary terms encompass the different levels, interests and perspectives within the research: individual older people and carers, individual practitioners, and health and care practice and systems. Applying these permutations enabled further searches to explore different aspects of the literature, following separate but integrated lines of thinking about this complex topic (e.g. core terms + combined older person/carer perspective terms to explore their perspective and experiences; or core terms + shared decision making and decision aids/decision support systems; or core terms + deprescribing to focus on particular interventions that might be included in the final research output, i.e. the framework to support improved medication management).
Inclusion [based on the pilot search: note not all MeSH (medical subject heading) terms]
Core terms
-
aged; aged, 80 and over; independent living; home/community
-
chronic disease; comorbidity; long-term conditions; multimorbidity
-
polypharmacy; multiple prescribed medications
-
complex medication regimens; schedules of medication-taking events
-
medication management; medication compliance; medication adherence; medication concordance; medicines optimisation; patient compliance.
Supplementary terms: individual – older person/carer perspective
-
patient acceptance of health care; health literacy; health belief model; belief about medications
-
self-care; self-management; self-administration; medication knowledge
-
physical/cognitive/emotional capacity; motivation; intentional/unintentional nonadherence
-
social support (family/friends); interpersonal relationships
-
treatment burden/pill burden; medication related health regimens; lifestyle changes
-
patient reported adverse reactions/events/intolerance; drug/treatment related side effects; safety
-
patient goals/outcomes; lived experience; life style; sense of normality; goal conflict with clinicians
-
medication-related A&E visits/hospital admissions or re-admissions/length of stay.
Supplementary terms: individual – practitioner perspective
-
evidence based medicine; practice guidelines/guideline directed interventions; cumulative treatment recommendations; risk benefit comparison
-
patient centred care; shared decision-making; decision aids/decision support systems; patient information/education; coaching; practitioner/professional role/relationships
-
medication errors; inappropriate prescribing/dosing
-
Home Medication Review; home visits; telephone interviews
-
deprescribing
-
health related quality of life; treatment outcome/adverse health outcomes; outcome data
-
general practitioner; practitioner practice patterns
-
nursing staff/nurse practitioners; community matron
-
community pharmacist; pharmacist
-
home care/aides.
Supplementary terms: health and care practice and systems perspective
-
continuity of care (records, plans, medication, team approach); transitions; medication reconciliation
-
telehealth; personal health applications.
Exclusion
-
< 2009
-
aged below 60 years or mixed age cohorts that include ages below 60 years
-
limited-generalisable race/ethnic groupings to England, for example Asian American/Pacific Islander, Hispanic, Mexican American
-
in-patient or in-care settings
-
non-medication interventions, for example screening programmes
-
single medication or treatment, or assessment or trial protocols or evaluations
-
cost, funding or insurance determinants of health behaviours in privately funded health-care systems e.g. cost-related non-adherence/Medicare/Medicaid beneficiaries/USA
-
duplicate records.
A data extraction form was set up by the research associate combining the record identifiers (i.e. the record number from the search, author, title, topic, design, number of subjects and country) with inclusion terms, once these terms had been agreed within the Research Team. This was used to inform the analysis of outputs from searches within work package 1.
As each search was undertaken, identifiers for each abstract were listed on the data extraction form and the occurrence of core and supplementary terms recorded where these were identified in the body of the abstract or as key words at the end of it. Associated terms identified in the abstract that enhanced the understanding of the listed terms were assigned to existing terms to which they relate. New terms were highlighted so that they could be assimilated into further searches as necessary or used to inform the development of programme theory/ies and CMO configurations.
Further terms were identified through the expertise and experience available through the Research Team Project Group, the work of the steering group or through the interviews in work package 2. These were added to the evolving list in order to clarify, for example, the contribution of secondary or social care, or to refine or focus the search on selected factors that warrant exploration (e.g. specifically medication review) because of their criticality to the causal processes within medication management for and with this group of older people. These cumulative, iterative processes were aligned with accepted approaches to realist methodology and served to enrich the findings.
Next steps
Once agreed, the preliminary list of terms was circulated to the rest of the Research Team Project Group for information and comment.
Appendix 2 Node summary (research process step 3: further literature searches and review)
| Nodes\\Health and Care |
|---|
| Nodes\\Health and Care\Access |
| Nodes\\Health and Care\Beliefs about patients |
| Nodes\\Health and Care\Care co-ordination Care Planning Team based infrastructure Continuity |
| Nodes\\Health and Care\Care model Patient focus Personalisation |
| Nodes\\Health and Care\Coaching Behavioural interventions |
| Nodes\\Health and Care\Doctor GP |
| Nodes\\Health and Care\Healthcare practitioners system organisation |
| Nodes\\Health and Care\Information giving |
| Nodes\\Health and Care\Nurse |
| Nodes\\Health and Care\Pharmacist |
| Nodes\\Health and Care\Prescribing Deprescribing |
| Nodes\\Health and Care\Psychosocial support |
| Nodes\\Health and Care\Quality Safety |
| Nodes\\Health and Care\Relationships Trust Respect |
| Nodes\\Health and Care\Remote monitoring of meds and diagnosis |
| Nodes\\Health and Care\Risk assessment Decision support tools |
| Nodes\\Health and Care\Social worker Care manager |
| Nodes\\Health and Care\Time and expertise Experience and expertise Competence Professionalism |
| Nodes\\Health and Care\Timing |
| Nodes\\Health and Care\Treatment choices |
| Nodes\\Health and Care\Workforce learning and development |
| Nodes\\Health and Care\Workload |
| Nodes\\Medication Management |
| Nodes\\Medication Management\1 Identifying problem |
| Nodes\\Medication Management\2 Getting medication |
| Nodes\\Medication Management\3 Starting medication |
| Nodes\\Medication Management\4 Continuing to take medication |
| Nodes\\Medication Management\5 Reviewing medication |
| Nodes\\Medication Management\6 Changing or stopping medication |
| Nodes\\Medication Management\Adherence and non-adherence |
| Nodes\\Medication Management\Interventions |
| Nodes\\Medication Management\Specific medications |
| Nodes\\Older people |
| Nodes\\Older people\Ageing and age |
| Nodes\\Older people\Beliefs about medicines, diagnoses, health |
| Nodes\\Older people\Beliefs about self + Identity |
| Nodes\\Older people\Informal carer burden |
| Nodes\\Older people\Coping styles Control Self efficacy Self-management |
| Nodes\\Older people\Decision making Autonomous SDM Collaboration |
| Nodes\\Older people\Diagnoses Symptoms Illness trajectories Comorbidities |
| Nodes\\Older people\Drug alternatives |
| Nodes\\Older people\Drug dosage Regimes |
| Nodes\\Older people\Drug interactions Side effects Drug burden |
| Nodes\\Older people\Drug packaging Drug labelling Drug information |
| Nodes\\Older people\Functional status Dexterity Swallow Cognition Emotion |
| Nodes\\Older people\Health and care experiences |
| Nodes\\Older people\Health burden Illness and medication work Self-rated health status |
| Nodes\\Older people\Health literacy Expert patient Health behaviours |
| Nodes\\Older people\Help seeking |
| Nodes\\Older people\Home and social environment |
| Nodes\\Older people\Information seeking |
| Nodes\\Older people\Outcomes Goals Goal directed behaviour Benefit |
| Nodes\\Older people\Relationship and interaction with doctor-prescriber |
| Nodes\\Older people\Relationship and interaction with formal carers |
| Nodes\\Older people\Relationship and interaction with nurse |
| Nodes\\Older people\Relationship and interaction with pharmacist |
| Nodes\\Older people\Relationship and interaction with social networks |
| Nodes\\Older people\Relationship and interaction with spouse partner family friends |
| Nodes\\Older people\Remembering Reminders |
| Nodes\\Older people\Routines Roles Everyday life |
| Nodes\\Older people\Self-monitoring Self reporting |
| Nodes\\Older people\Social norms |
| Nodes\\Older people\Socio-demographic characteristics Culture |
| Nodes\\Older people\Technology Tools |
| Nodes\\Older people\Transitions Change Certainty-uncertainty |
| Nodes\\Other External Factors |
| Nodes\\Other External Factors\Evidence Guidelines Standards Protocols |
| Nodes\\Other External Factors\Policy Finance |
Appendix 3 Interviewee profiles
| Category | Characteristic |
|---|---|
| Older people (n = 13) |
Age: range 60–84 years, mean 73 years Male: 7; female: 6 Living with mild dementia (and living with informal carer – spouse): 2 Number of diagnoses (multimorbidity): range 3–6, mean 4 Number of different medications (polypharmacy): range 5–15, mean 9 |
| Informal carers (n = 16) |
Male: 5, female: 11 Black, Asian, and minority ethnic: 4 Caring for someone living with memory problems/dementia: 7 Caring for more than one person living with dementia: 2 |
| Practitioners (n = 21) |
Doctors: 2 consultant geriatricians, 2 GPs Nurses: 5, including a nurse manager, specialist nurses, community matrons Pharmacists: 3, including head of service, a clinical pharmacist, pharmacy technician Social worker: 1 Care providers: 7, including managers, assessors, team leaders and front-line staff Strategic manager (health and care): 1 |
Appendix 4 Interview schedules: common content of the interview schedules (research process step 5: interviews and evaluation)
-
Participant identifiers: confirming participant and interview details, including unique code used to anonymise the participant.
-
Baseline demographic data: (for monitoring purposes) including ethnicity.
-
Pen portrait: home circumstances, community circumstances, access to and use of technology, functional status relevant to medication management – sight, hearing, swallow, dexterity, mobility (indoor/outdoor), health and medication.
-
Finding out about the general experience of medication management:
Process of managing medication:
-
(exploring the intervention): understanding of medication management (reference to five stages), key people involved and doing what
-
(exploring what works, for whom, why and in which circumstances): what currently makes medication management easier/more difficult (with how and why for explanatory detail), what would make it better or easier in the future (solution focus, with how and why for explanatory detail)?
Outcomes from taking medication:
-
(generating explanatory accounts, working back from outcomes): what are the outcomes that matter to that individual, are they achieved, who or what makes it easier/more difficult to achieve those outcomes (doing what, how and why for explanatory detail, anything else), who or what would make it better or easier in the future to achieve those outcomes (solution focus, with how and why for explanatory detail, anything else)?
-
Eliciting responses to explaining experiences of medication management: (responses to candidate programme theories): ‘You have told us about your experiences of . . .’ (summarise to bridge to programme theory) > ‘In the Research Team we have been thinking about . . .’ (describe programme theory in detail appropriate to that individual and their experiences) > ‘What do you think about this (summary title of programme theory)? When does it affect you most (enable discussion of detail: what, who, why and in which circumstances)?’
-
Anything else arising from the interview.
Note that the schedules for informal carers and practitioners shared this common content but were flexed:
-
Informal carers provided data on themselves as well as the person they cared for.
-
Practitioners scoped their role, expertise and experience, and what informed their practice and provided data on themselves and the older people and/or informal carers they work with.
Appendix 5 Initial mapping of burden: from a Research Team working paper
Appendix 6 Dimensions of burden: workload and capacity
| Burden | Dimensions | Contexts | ||
|---|---|---|---|---|
| Workload | Increasing burden (increasing/high workload) | Day-to-day life: life revolves around using my medicines and affects daily tasks, social relationships, social or leisure activities, driving, sexual life (increases workload) | Medication: polypharmacy and treatment characteristics | |
| Side effects: bothersome, interfere with day-to-day life, sometimes worse than the problem (increases workload) | ||||
| Practicalities: participant identifiers – confirming getting prescriptions from doctor/pharmacist (increases workload) | Health and care system | |||
| Practicalities: regimen needs planning and thought, difficulties using medicines (increases workload) | Identity and day-to-day life | |||
| Participant identifiers: confirming payment, choice between essentials and medicines (increases workload) | ||||
| Decreasing burden (decreasing/low workload) | Greater likelihood of coping | Patient–doctor relationships and communication about medicines: me and my medicines known enough by my health professionals, listened to by doctor and concerns taken seriously, have enough information, trust (reduces workload) | Health and care relationships and communication | |
| Practicalities: easy to keep to regimen, timing (reduces workload) | Identity and day-to-day life | |||
| Autonomy/control of medicines use: choice of whether to take or not, can vary dose/times (reduces workload) | ||||
| Capacity | Decreasing burden (increasing/high capacity) | Medication effectiveness: medicines working, live up to expectations, prevent condition getting worse, side effects worth the benefits, able to live life as wanted, satisfied (increases capacity) | Medication: polypharmacy/treatment characteristics | |
| Increasing burden (decreasing/low capacity) | General concerns about medicines: long-term damage, interactions with other medicines/alcohol, reliance, polypharmacy, lack of information/choice (reduces capacity) | Identity and day-to-day life | ||
| Side effects: adversely affect my well-being (reduces capacity) | ||||
| Practicalities: concerns about forgetting (reduces capacity) | ||||
| Cost-related burden: worry about paying (reduces capacity) | ||||
Appendix 7 Revised analysis framework conceptualising burden as a potential mechanism
Appendix 8 Initial list of context–mechanism–outcome configurations from the literature and interviews with older people, practitioners and informal carers
| Literature | ||
|---|---|---|
| Literature CMOs | C + M > O short form | Source |
| Lit CMO 01 Older people are at increased risk of an adverse drug event (O) when they are taking multiple medications and certain high-risk drugs (C) because of age-related changes (M) | Polypharmacy and high risk drugs + physiology of ageing > increased risk | Lit, OP |
| Lit CMO 02 Older people who have informal carer or formal carer support with managing their medications (C) are more likely to be adherent (O) because they have given control to the person that supports them (M) | Support + control > adherence | Lit, C |
| Lit CMO 03 Individual practitioners who are treating older people with multimorbidity following disease-specific guidelines (C) are more likely to contribute to polypharmacy (O) because of adherence to evidence-based rather than person-centred practice (M) | Guidelines + evidence than person > polypharmacy | Lit, P |
| Lit CMO 04 When practitioners carry out a medication review using an evidence-based review tool (C), they are more likely to identify and discontinue high-risk medications and simplify regimens (O) because they are confident making decisions (M) | Evidence-based tool + confidence > deprescribing/simplifying | Lit, P |
| Lit CMO 05 Regular medication reviews and transition reconciliations by experienced practitioners (C) optimises medication management (O) by minimising the risk of treatment-related problems (M) | Regular reviews and experienced practitioner + risk minimisation > optimisation | Lit, P |
| Lit CMO 06 When practitioners carry out a medication review in an older person’s home (C), they are more likely to identify medication-related problems (O) because they understand people’s lived experiences and they take more time (M) | Home-based review + understanding and time > identify problems | Lit, P |
| Lit CMO 07 Pharmacists carrying out a home medication review (C) are more likely to identify and resolve medication-related problems (O) because of their particular expertise and experience (M) | Pharmacist and home-based review + expertise and experience > identify and resolve problems | Lit, P |
| Lit CMO 08 Practitioners, older people and informal carers are likely to make better decisions about medication, strengthen their relationships and achieve continuity of care (O) when they collaborate through shared decision-making (C) because of mutual trust (M) | Collaboration and shared decision making + mutual trust > better decisions, relationships and continuity | Lit, OP, P, C |
| Lit CMO 09 Information technology used by older people, informal carers and practitioners (C) improves access, information sharing and support (O) by reinforcing communication (M) | Information technology + reinforcing communication > improves access, sharing, support | Lit, OP, P, C |
| Lit CMO 10 Equipment and technology used by older people, informal carers and practitioners (C) helps them to cope with managing their medications (O) by supporting what they do (M) | Equipment and technology + as support > coping | Lit, OP, P, C |
| Older people | ||
| Older people CMOs | C + M > O short form | Source |
| OP CMO 01 Older people access health-care services and practitioners (O) when they think a health problem or medication is disrupting their day-to-day lives and threatening their independence (C) because they want to get back in control (M) | Problem + want control > access health and care (help seeking) | OP |
| OP CMO 02 When older people and informal carers are informed about their illnesses and medications (C), they are more likely to use them correctly and less likely to experience negative emotions such as worry or distress (O) because they feel more in control (M) | Information + in control > correct use/reduced worry | OP |
| OP CMO 03 When older people want to get on with their lives (C), they will take those medications (O) that they believe will keep them well and help them achieve their goals (M) | Get on with life + belief/confidence > take medications | OP |
| OP CMO 04 When older people on a complex medication regimen are informed about their medications (C), they are more likely to make them part of their day-to-day routines and less likely to forget them (O) because they feel more in control (M) | Information + in control > make routine/less forgetting | OP |
| OP CMO 05 Older people and informal carers are less likely to feel burdened by their medication regimen (O) when they have a routine (C) because they feel confident and in control (M) | Routine + in control/confidence > less burden | OP, C |
| OP CMO 06 Older people and informal carers cope with the practicalities of taking their medications (O) by making adjustments (C), because they want a better fit with their lives (M) | Making adjustments + better fit > coping | OP, C |
| OP CMO 07 When medications look the same (colour, shape) but have different purposes (C), older people and informal carers are at risk (O) because it is confusing (M) | Medications similar appearance/purpose + confusing > at risk | OP |
| OP CMO 08 Older people with comorbidities question taking medication (O) when they are free of symptoms (C) because they feel well (M) | Symptom free + feel well > question taking medications | OP |
| OP CMO 09 When older people have multiple medications (C), they want to reduce the number they take (O) because the medications would be easier to remember and less difficult to fit in, day to day (M) | Polypharmacy + easier > reduce | OP |
| OP CMO 10 When older people have cognitive problems (C), they may not be aware they are not managing their medications (O) because they forget or get confused (M) | Cognitive problems + forget/confused > unaware not managing | OP |
| OP CMO 11 Older people are more likely to worry about not coping in the future (O) if they anticipate losing capacity (dementia, functional, bereavement) (C) because they fear losing control (M) | Loss of capacity + fear losing control > worry about not coping | OP |
| OP CMO 12 When older people and informal carers trust the practitioners they see consistently (C), they are less likely to worry about their illnesses and medications (O) because they feel understood and supported (M) | Practitioner trust and consistency + feel understood and supported > less worry | OP, C |
| OP CMO 13 Older people’s and informal carers’ trust in practitioners is reduced (O) when practitioners provide inconsistent, conflicting or lack of information or advice (C) because they struggle to make sense of it (M) | Poor information or advice + struggle to make sense > reduced trust in practitioner | OP, C |
| OP CMO 14 When older people are seen by a new practitioner (C), medication is likely to be changed (O) because they are assessed rather than reviewed (M) | New practitioner + assessment rather than review > medications changed | OP |
| OP CMO 15 When older people and informal carers have to have multiple health and care contacts across different sites, services, teams and practitioners (C), they find this an additional burden (O) because it takes time and effort (M) | Fragmentation services, teams and practitioners + time and effort > additional burden | OP, C |
| OP CMO 16 Older people and informal carers are likely to cope better (O) when there is consistency and stability in services, practitioners and medications (C), because they feel in control and can rely on them and their own routines (M) | Consistency and stability services, practitioners and medications + control and reliance > coping | OP, C |
| OP CMO 17 Older people and informal carers are likely to experience fewer negative emotions such as distress (O) when they have confidence in their local health and care services (C) because they feel they can rely on them (M) | Confidence in services + reliance > reduced worry | OP, C |
| Informal carers | ||
| Informal carers (C) | C + M > O short form | Source |
| C CMO 01 Family members take on the role of informal carer (O) when they notice problems with medication management (forgetting, safety) (C), because they feel responsible and want to reduce risk (M) | Problems + feel responsibility/risk reduction > become informal carer | C |
| C CMO 02 When there are problems with medication management and family members take responsibility for it (C), older people and practitioners agree (O) because they are trusted as carers (M) | Problems and informal carer take responsibility + trusted as informal carers > older person and practitioner agree | C |
| C CMO 03 When informal carers identify problems and gaps (C), they increasingly manage medication, health and care (O) because they respond to what is needed (M) | Problems and gaps + respond to needs > increasingly manage medications, health and care | C |
| C CMO 04 When a relative is losing competence but retains capacity (C), the informal carer’s work with them and practitioners is made more difficult (O) because they lack status to act in their best interest (M) | Older person losing competence but has capacity + lack carer status > work with older person and practitioner more difficult | C |
| C CMO 05 Informal carers are under constant stress (O) when they are dealing with changes in their relative and medications (C), because they need to remember, monitor frequently and make adjustments | Older person and medications changes + need to remember, monitor and adjust > stress | C |
| C CMO 06 When informal carers lack formal training (C), they become expert (O) because they learn by experience (M) | Lack training + learn by experience > expert | C |
| C CMO 07 To manage complex medication regimens (O) informal carers devise their own routines and systems (C) so they feel in control (M) | Devise routines and systems + feel in control > manage complex regimens | C |
| C CMO 08 Informal carers seek support from practitioners and services (O) when they want to optimise treatment (C), because they need to feel confident about what they do (M) | To optimise treatment + need to feel confident > seek support from practitioner | C |
| C CMO 09 Carer stress is made worse (O) when they are dealing with changes in their relative and medications (C), because of delays in practitioners responding or unhelpful responses (M) | Older person and medications changes + practitioner delays and unhelpful responses > stress | C |
| C CMO 10 Informal carers challenge practitioners (O) when they treat the diagnosis and not the person (C), because they can see the bigger picture and the risks (M) | Practitioner treats diagnosis not person + informal carer sees big picture and risk > challenge practitioner | C |
| C CMO 11 When informal carers look after their relatives (C), they become their advocates (O) because they have personal knowledge and developing expertise (M) | Become informal carer + personal knowledge and developing expertise > become advocates | C |
| C CMO 12 To manage difficult situations (C), informal carers take risks with medication (O) because they feel desperate and do not know what else to do (M) | Manage difficulties + desperate + lack alternatives > take risks | C |
| C CMO 13 Informal carers can feel undervalued and unsupported (O) when they are expected to cope (C), because their role, responsibilities and needs are not recognised and systems are unclear (M) | Expected to cope + not recognised and systems unclear > feel undervalued and unsupported | C |
| C CMO 14 When family members take on the role of informal carer for a relative with complex needs (C), they give up aspects of their own lives (O) because the demands on them are so great (M) | Become informal carer + high demands > give up aspects of own lives | C |
| C CMO 15 When they look after their relatives (C), informal carers are expected to do more than formal carers to manage medications (O) because their role is informal, unregulated and untrained (M) | Become informal carer + informal, unregulated and untrained > expected to do more than carers | C |
| C CMO 16 Informal carers are more likely to worry about their relative being alone or in the future (O) if they anticipate not being available (C), because they recognise the significance of their role (M) | Loss of availability + recognise role significance > worry about older person not coping | C |
| Practitioners | ||
| Practitioner CMOs | C + M > O short form | Source |
| P CMO 01 Older people who might be at risk with their medication management (C) become visible to health and care practitioners (O) when they experience a crisis (M) | Older person at risk + crisis > visibility to practitioners | P |
| P CMO 02 Practitioners assess and provide an intervention (O) when older people are not coping (C), because of the need to minimise the risk of harm and maximise independence (M) | Older person not coping + risk minimisation > intervention | P |
| P CMO 03 When practitioners assess and provide an intervention (C), they draw on evidence and experience (M) to optimise their practice (O) | Intervention + evidence and experience > optimise practice | P |
| P CMO 04 When older people need help with medication management (C), practitioners work with formal or informal multidisciplinary teams and networks (O) because they want to maximise expertise for a complex issue (M) | Older person not coping + formal and informal networks > manage complexity | P |
| P CMO 05 When practitioners have access to timely, accurate information (C), they are more likely to provide better assessments and interventions (O) because they can be more confident about the basis for making decisions (M) | Information + crucial to work > better interventions | P |
| P CMO 06 When practitioners make time and space for older people and informal carers (C), they are more likely to provide better assessments and interventions (O) because they can collaborate to find solutions to problems (M) | Time and space + collaborate and shared decision-making > better interventions | P |
| P CMO 07 Carers take responsibility for medication taking (O) when older people are not coping (C), because of the need to minimise risk of harm (M) | Older person not coping + risk minimisation > carers take responsibility | P |
| P CMO 08 When carers and nurses assist with medication taking (C), older people are more likely to adhere (O) because they maintain the routine and motivation (M) | Formal assistance + maintain routine and motivation > older person adherence | P |
| P CMO 09 When carers assist with medication taking (C), older people are more likely to be engaged with what they are doing (O) because they invest time and effort (M) | Formal assistance + time and effort > older people engagement | P |
| P CMO 10 Carers take responsibility for collecting medications (O) when older people or informal carers agree because they are not coping (C) as part of their job to ensure a constant supply (M) | Older person or informal carer not coping + need to ensure supply > carers collect | P |
| P CMO 11 Practitioners provide auto-ordering and third party requests for medication (O) when older people choose or are not coping (C), as part of their job to ensure a constant supply (M) | Older person or informal carer not coping + need to ensure supply > auto-ordering and third party requests | P |
| P CMO 12 Practitioners provide medications in blister packs (O) when older people or informal carers choose or are not coping (C), to simplify taking them (M) | Older person or informal carer + simplify taking > blister packs | P |
| P CMO 13 Practitioners provide home delivery of medications (O) when older people or informal carers choose or are not coping (C), as part of their job to ensure a constant supply (M) | Older person or informal carer + need to ensure supply > home delivery | P |
| P CMO 14 Practitioners and informal carers are likely to cope better (O) when there is consistency and stability in services and practitioners (C), because they are less distracted by fragmentation and change (M) | Service and practitioner consistency and stability + less distracted by fragmentation and change > practitioner and informal carer coping | P |
| P CMO 15 Hospital admission and discharge (C) threatens older people’s ability to cope with their medication (O) because it involves changes in what they take and disruption of their routines (M) | Hospital admission and discharge + change and disruption of routines > threatens coping | P, OP |
| P CMO 16 When older people have several long-term conditions (C), they need to see the same practitioner (O) to deal with complexity and manage risk (M) | Older person with comorbidities + complexity > need practitioner consistency | P |
Glossary
- Adverse drug event
- An unintended harm from taking medication.
- Burden
- People’s experience of the workload associated with an intervention and how it is balanced, or not, with their capacity to undertake that work, for example taking medication as prescribed, and remembering the instructions (e.g. which medication and how many tablets to be taken when and how), and making this part of day-to-day routines. (This is linked to Capacity and Workload.)
- Capacity
- The resources a person can draw on, for example physical, cognitive, affective, to undertake a task or series of tasks. (This is linked to Workload as part of Burden and Coping.) Note that this is not capacity under the Mental Health Act.
- Carer
- See Formal carer and Informal carer.
- Context
- The circumstances or influences that activate or inhibit the responses of those involved in an intervention. The responses are part of the mechanism. (This is linked to Mechanism and Outcome.)
- Context–mechanism–outcome configurations
- The association of causal components that explain what works in an intervention (see Intervention below) for who, why and in which circumstances.
- Coping
- The experience of managing burden, its workload and capacity requirements, within people’s day-to-day lives.
- Formal carer
- A trained and managed practitioner who is paid to provide care support, including support with medication management.
- Implementation
- The ‘purposive and directed’ work by which a process, practice, programme or project is brought about. (May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535–54.
- Informal carer
- Typically a family member who provides support with medication management as part of their informal care role. May also include neighbours and friends.
- Intervention
- A process, practice, programme or project that provides resources to which participants respond. In MEdication Management in Older people: Realist Approaches Based on Literature and Evaluation (MEMORABLE), the interventions are medication management (overall) and reviewing/reconciling medication (as a specific stage in medication management).
- Mechanism
- The responses of those involved in an intervention to the resources or opportunities offered by that intervention. Responses may include thoughts, feelings or actions. They are activated or inhibited by circumstances or contexts that then have an effect. (This is linked to Context and Outcome.)
- Medication adherence
- ‘The extent to which the patient’s action matches the agreed recommendations’ [National Institute for Health and Care Excellence (NICE). Medicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence. Clinical Guideline 76. London: NICE; 2009; © NICE 2009. Medicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence. Available from www.nice.org.uk/guidance/cg76. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication] and informed by their involvement in and agreement with decisions about their treatment.
- Medication management (see also Stages of medication management)
- In MEMORABLE, what older people do to deal with medication and make it part of their day-to-day lives, with or without the support of informal carers. In addition, what practitioners do as part of a complex process of care to support older people with the treatment of their co-morbidities and maintain their health and well-being.
- Medication optimisation
- ‘A person-centred approach to safe and effective medicines use, to ensure people obtain the best possible outcomes from their medicines’ (NICE. Medicines Optimisation: the Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. NICE Guideline 5. 2015; © NICE 2009. Medicines Optimisation: the Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. Available from www.nice.org.uk/guidance/ng5. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication). Optimisation involves shared decision-making by engaging with evidence-based medicine as well as individual needs, preferences and values.
- Medication reconciliation
- At a care transition, ‘identifying an accurate list of a person’s current medicines and comparing them with the current list in use, recognising any discrepancies, and documenting any changes, thereby resulting in a complete list of medicines, accurately communicated’ (NICE. Medicines Optimisation: the Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. NICE Guideline 5. 2015; © NICE 2009. Medicines Optimisation: the Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. Available from www.nice.org.uk/guidance/ng5. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication).
- Medication review
- ‘A structured, critical examination of a person’s medicines with the objective of reaching an agreement with the person about treatment, optimising the impact of medicines, minimising the number of medication-related problems and reducing waste’ (NICE. Medicines Optimisation: the Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. NICE Guideline 5. 2015; © NICE 2009. Medicines Optimisation: the Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. Available from www.nice.org.uk/guidance/ng5. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication).
- Multimorbidity
- Two or more long-term conditions occurring together.
- National Institute for Health Research
- The research arm of the NHS, funded by the Department of Health and Social Care, which aims to improve the health and wealth of the nation through research.
- Normalisation process theory
- A substantive theory that explains how changes are introduced and made routine by those involved.
- Older people
- In MEMORABLE, people aged > 60 years who are living at home.
- Outcome
- The pattern of results, effects or impacts generated by contexts and mechanisms acting together. These may occur in the short or longer term, and be expected or unexpected (e.g. an improved coping experience, an enhanced relationship with general practitioner or pharmacist). (This is linked to Context and Mechanism.)
- Output
- An intervention product (e.g. a prescription, the report of a medication review).
- Patient and public involvement
- The engagement of service users as active partners and advisors in the research process.
- Polypharmacy
- In MEMORABLE, scoped as taking five or more medications or, if less, having a complex regimen. Note that polypharmacy may be appropriate or inappropriate.
- Practitioner
- A trained employee in the health or care sector providing services to an older person. They may or may not be professionally registered to practice.
- Programme theory
- A theory about how an intervention is intended to work, often written as if–then statements. In MEMORABLE, programme theories were ‘candidate’ at the start of the research and ‘final’ when they had been revised through the synthesis of the literature review with the evaluation of narrative data.
- Stages of medication management
- In MEMORABLE, the five stages by which medication management can be understood: Stage 1 – identifying a problem; Stage 2 – getting diagnosis and/or medications; Stage 3 – starting, changing or stopping medications; Stage 4 – continuing to take medications; and Stage 5 – reviewing/reconciling medications. Some stages involve the older person on their own, sometimes with support from an informal carer; some involve the older person, again on occasion with informal carer support, and a practitioner.
- Workload
- The tasks or series of tasks associated with an activity. (This is linked to Capacity as part of Burden and Coping.)
List of abbreviations
- CMO
- context–mechanism–outcome
- GP
- general practitioner
- MEMORABLE
- MEdication Management in Older people: Realist Approaches Based on Literature and Evaluation
- MUR
- medication use review
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- PPI
- patient and public involvement
- QOF
- Quality and Outcomes Framework
- RR
- reviews/reconciliations
- WHO
- World Health Organization