Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/130/47. The contractual start date was in April 2014. The final report began editorial review in February 2019 and was accepted for publication in September 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Irene J Higginson was a member of the Health Technology Assessment Efficient Study Board (2015–16) and the Health Technology Assessment End of Life Care and Add-on Studies Board (2015–16). Wei Gao was member of the Health Technology Assessment End of Life Care and Add-on Studies Board (2015–16) and the Health Services and Delivery Research Commissioning Board (2009–15).
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Hepgul et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Long-term neurological conditions
Long-term neurological conditions (LTNCs) are a diverse set of conditions resulting from injury or disease of the nervous system that affect individuals for the rest of their lives. This includes long-term progressive conditions, such as idiopathic Parkinson’s disease (IPD), motor neurone disease (MND), multiple sclerosis (MS), progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). Affecting > 200,000 individuals in the UK, these progressive conditions lead to substantial deterioration in quality of life and result in the patient needing lifelong support from health and social care services. 1 Many patients have inadequate symptom control and inadequate psychological and social support, and there is a high burden for family caregivers. 2–5 There are also significant financial burdens to the individual, their families and the NHS, with increasing costs associated with disease progression. 6–8 Despite the progressive nature of these conditions, the scope for improving services to enhance quality of life of affected individuals through the provision of palliative care may be substantial.
Current treatment and service provision
In 2005, the Department of Health and Social Care published the National Service Framework, which set 11 quality requirements to transform the way health and social care services support people with LTNCs and their caregivers. 9 It highlighted the need for integrated care and joined-up services, and made recommendations for the provision of specialist neurology, rehabilitation and palliative care services to support people throughout and to the end of their lives.
However, a National Audit Office report concluded that implementation of the framework has been poor and that although access to neurology services improved, other important indicators of the quality of care for people with neurological conditions worsened. 10 The report highlighted that information and advice to patients and caregivers is inadequate, and ongoing care is fragmented and poorly co-ordinated. Indeed, in the UK and in many other health systems, there is often division among general practitioners, staff working in the community and hospital-based specialists. 11,12 It is increasingly recognised that a hard separation of these functions does not meet the needs of those with long-term conditions; therefore, much of the burden of illness often falls on the community and on lay caregivers. 2,5,13,14 This results in greater negative effects for both patient and caregiver well-being, as well as increased financial burden. 8,15 Attempts have been made to better co-ordinate care through integrative processes, such as joint budgets, governance, information systems, flows of data or case management. 16,17 These may be brought together more formally through different kinds of vertical integration, in which agencies involved at different stages of the care pathway form part of a single organisation or function, as well as horizontal integration of community-based services in examples such as health and social care teams for the frail elderly. 18 Another issue is that multimorbidity is the norm for people with LTNCs. Equally, their spouses or family caregivers may have health conditions that affect their ability to care, and the burden of caring may affect the health of caregivers. 2,13 Palliative care is person rather than disease focused and may have the potential to address these unmet needs.
Palliative care for long-term neurological conditions
Palliative care focuses on improving quality of life through a multidisciplinary approach and has been recognised as a valuable component of care for patients with chronic, life-limiting illnesses. 19–21 The place for palliative care in rapidly fatal neurological conditions is increasingly recognised; however, the evidence is scarce. 22 Indeed, a recent consensus review concluded that there is limited evidence to support any recommendations for the provision of palliative care for progressive neurological disease and that further research is urgently needed. 23 There are three Phase II trials of palliative care for patients with neurological conditions. 24–26 The results of our own Phase II trial of short-term integrated palliative care (SIPC) among 52 patients severely affected by MS found an improvement in pain and a significant reduction in informal caregiver burden at a lower cost and with no harmful effect, compared with standard care. 24 Similarly, in 78 MS patients and their caregivers, a 6-month home-based palliative care service was found to reduce symptom burden compared with usual care. 26 Furthermore, a new 4-month home-based specialist palliative care service for 50 patients with advanced neurodegenerative disorders (MND, MS, IPD, MSA and PSP) found a significant improvement in quality of life and physical symptoms (pain, breathlessness, sleep disturbance and bowel symptoms) across the conditions, although no effect was seen on caregiver burden. 25 We have also previously reported findings from a longitudinal observational study of IPD, MSA and PSP patients demonstrating the profound and complex mix of non-motor and motor symptoms in late stages of disease. 27 Symptoms were highly prevalent in all three conditions and were often unresolved, with half of patients deteriorating over 1 year. Furthermore, palliative problems were predictive of future symptoms, suggesting that an early palliative assessment might help screen for those in need of earlier intervention. 27 However, to the best of our knowledge, there are currently no Phase III trials.
Conceptual framework
People severely affected by LTNCs have many problems and concerns similar to those affected by advanced cancer, including symptoms, psychological needs, and family and caregiver concern. 28 Specialist multiprofessional palliative care teams (MPCTs) successfully improve these problems for cancer patients and are now available widely across the globe. 29,30 The Cochrane handbook outlines31 that, if there is empirical evidence that similar or identical interventions have an impact on other populations, these are quite likely to be effective. Thus, as a starting point, it is reasonable to hypothesise that input from specialist palliative care will help people with LTNCs. Our modelling work demonstrated that people severely affected by MS, Parkinson’s disease-related disorders and MND had many similar symptoms to those affected by advanced cancer,32 with additional problems of loss of care co-ordination. 33–35 These needs are within the remit of specialist palliative care, which offers a holistic approach attending to symptoms, psychological needs and better co-ordination of care. 36 People severely affected by LTNCs often have a longer trajectory of illness than those with advanced cancer and so our modelling found that staff, patient and caregiver groups favoured the idea of specialist palliative care input for a short term, working in a way that was well integrated with existing neurology and rehabilitation services.
Short-term integrated palliative care is modelled on our work to date, following the Medical Research Council guidance for the development and evaluation of complex interventions. 37 This included literature reviews38 and qualitative studies33,34 to determine need and to develop the theoretical underpinning of the service, appraisal of trial methods,32,39 service modelling and a successful Phase II trial randomising 52 patients. 24,29,40
Short-term integrated palliative care is a complex intervention41 in that it:
-
contains several components (assessment, symptom management, future care planning, follow-up visits)
-
aims to change behaviours by those staff delivering the intervention, those providing usual care to this patient group, and, to some extent, patients and families
-
targets patients, families and staff in primary, hospital and voluntary care, thus including different groups and organisational levels
-
has several complex outcomes, including change in symptom management and hospital admissions
-
is tailored to individual patient need and circumstances by those delivering SIPC
-
operates in a context in which there may be some variability between patient groups and settings in the usual care provided to patients with LTNCs; usual care is offered to patients in the intervention and control arms of the trial.
Short-term integrated palliative care could be developed with only small adaptions to existing health-care services. It is much more likely to be possible than other proposed alternatives, such as developing long-term palliative care models. The latter would be difficult to achieve without considerably expanding the number of palliative care specialists, beds and services. In contrast, SIPC builds on and integrates with existing services across the UK and seeks to empower patients, improve symptom control and integrate with existing services, improving their expertise. If found to be effective, the new SIPC service has the potential to be beneficial for a wider range of conditions and in more diverse care settings for patients and their families. This could result in better symptom control and improved quality of life for patients, as well as improved co-ordination of care, more efficient and appropriate use of services, and a reduction in the number of unnecessary emergency admissions at the end of life. This is also in line with other NHS initiatives seeking to move palliative care and discussions about preferences and priorities further upstream and encouraging patients to think about care preferences earlier in their disease trajectory. 42 Understanding whether or not SIPC is clinically effective and cost-effective, and its potential mechanism of action, will help to develop studies in these initiatives. Equally, if the SIPC is not cost-effective in more conditions and in wider settings, the findings will prompt development of customised improvement and modifications in specific LTNCs. 8,43–45
Importance of economic analysis
Health-care costs in the last year of life are high (18–30% of health-care spending), with resource use increasing in the last months of life. 43–45 Despite this, it is known that this expenditure offers poor value, as symptoms often remain uncontrolled. 46 In long-term conditions, including neurological conditions, costs rise with increased disability and as the disease advances. 8 These costs can be unpredictable and can affect caregivers and patients, as well as health and social services. 8 Hospitalisation is a main cost driver of health care and a major public health problem. Indeed, NHS England reports that £750M is spent on urgent and emergency care for patients with neurological conditions, including admissions to hospital, with nearly 4% growth in emergency admissions year on year. 47 Compared with age-matched controls, people with LTNCs, such as MS and Parkinson’s disease, are experiencing higher rates of hospitalisation. 48–50
However, maintaining patients in the community can also be costly to health and social care services. It can also place an increased burden on families and carers. Therefore, it is imperative to evaluate proposed service models in patients with advanced disease to see whether or not they affect health and social care costs. SIPC seeks to alleviate symptoms, prevent symptom escalation, improve care, and help patients and caregivers plan the future care they need, all of which may potentially avoid inappropriate hospitalisation. A full understanding of the cost-effectiveness of SIPC for the NHS is central to decision-making. Despite this need, health economic evaluations of interventions in advanced illness remain rare, especially cost-effectiveness studies. Most studies consider only costs, randomised trials are rare and many studies fail to account for confounding. 51,52
Evaluating a new service model for LTNCs, as well as addressing the concerns for people severely affected by these diseases, develops a potential model of service provision for other long-term diseases in advanced stages. With the ageing population, the predicted rise in the annual number of deaths, the increasing prevalence of long-term conditions and the likely increase in need for palliative care,53,54 it is both highly relevant and timely to robustly test new service models to improve care for this group. This project answers this need and tests an intervention that could be implemented by the current workforce and services.
Research group
This project was led by the Cicely Saunders Institute of Palliative Care, Policy and Rehabilitation at King’s College London, with the following collaborating institutions: The University of Nottingham; Nottingham University Hospitals NHS Trust; Cardiff and Vale University Health Board; University of Sussex; Brighton and Sussex Medical School; The Walton Centre NHS Foundation Trust; Sussex Community NHS Foundation Trust; Ashford and St. Peter’s Hospitals NHS Foundation Trust; Sheffield Teaching Hospitals NHS Foundation Trust and King’s College Hospital NHS Foundation Trust.
Chapter 2 Aim and objectives
Aim
The OPTCARE Neuro trial aimed to determine the clinical effectiveness and cost-effectiveness of SIPC services in improving symptoms, improving selected patient- and caregiver-reported outcomes and reducing hospital utilisation for people severely affected by LTNCs.
Primary objective
To determine the clinical effectiveness and cost-effectiveness of SIPC for people severely affected by LTNCs compared with standard care, according to the primary outcome of reduction in key symptoms at 12 weeks.
Secondary objectives
-
To map current practice and document the services available (and common care pathways) for patients with LTNCs and their caregivers and families in the areas of the study, to better understand variations in normal practice experienced by the control group.
-
To test the feasibility of offering SIPC and the trial methods across five centres, for people severely affected by LTNCs, and to modify the intervention and trial methods accordingly.
-
To determine the clinical effectiveness and cost-effectiveness of SIPC for people severely affected by LTNCs compared with standard care in the secondary outcomes: palliative care needs and other symptoms, patient psychological well-being and quality of life, caregiver burden/positivity and quality of life, improvement in patients’ and caregivers’ satisfaction and communication.
-
To determine the effects of SIPC for people severely affected by LTNCs on hospital admissions, length of hospital stay, emergency attendance and other service use over the trial period.
-
To determine the cost-effectiveness of SIPC for people severely affected by LTNCs.
-
To understand how the change process may work and to identify components of the SIPC that are most valued by patients, their families and caregivers, and other health-care professionals.
-
To determine how the effects change over time, whether or not earlier referral to palliative care affects the subsequent response to palliative care and when assessment or re-referral might be beneficial.
Chapter 3 Design and methods
Study design
This is a mixed-methods study comprising a Phase III, randomised controlled, multicentre, fast-track trial, including assessment of cost-effectiveness and an embedded qualitative component. It is a multicentre evaluation of a complex intervention, following the Medical Research Council guidance for the development and evaluation of complex interventions. 37 This study incorporates:
-
a set-up and feasibility phase to refine recruitment and methods
-
mapping usual care for patients with LTNCs across the different centres (by prior work collecting information about the services, and during the study recording services received at baseline and in the standard care group) to understand the context and baseline variations in practice and resources
-
a randomised controlled trial of SIPC offered from a MPCT, compared with best usual care in terms of outcomes and cost-effectiveness
-
an embedded qualitative component, to explore the ways in which the SIPC affects patients and caregivers, how the change process may work, how SIPC may be improved and to interpret quantitative results
-
a survey of health professionals to understand the impact of SIPC on local practice
-
economic modelling to estimate the NHS and societal resources required for and longer-term impacts of SIPC.
Mapping methods
Parts of the text have been reproduced from van Vliet et al. 55 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The mapping exercise was conducted in eight centres with neurology and palliative care services in the UK. The centres were purposively selected to include our main recruitment centres and other larger centres. The eight centres included different geographical areas and represented both rural and urban areas. Data were provided by the respective neurology and specialist palliative care teams. Questions focused on catchment and population served, service provision and staffing, and integration and relationships. Data were transferred into tables to facilitate comparison between centres.
Survey methods
Parts of the text have been reproduced from Hepgul et al. 56 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Research teams from six trial centres (London, Nottingham, Liverpool, Cardiff, Brighton and Ashford) identified local neurology and palliative care professionals who were then approached via e-mail by the central trial team. Professionals were informed that, by completing the survey, they provided informed consent for use of their anonymised data. The surveys consisted of multiple-choice or open-comment questions, 13 questions for neurology or 10 questions for palliative care, with responses collected using online forms. The survey was launched in July 2015 and closed in April 2016. Data were transferred into tables to facilitate comparison of professional groups and data were explored descriptively.
Randomised trial methods
This was a randomised controlled, multicentre, fast-track, single-blinded trial of SIPC, provided in addition to existing services, compared with standard care. It was conducted and reported in accordance with Consolidated Standards of Reporting Trials (CONSORT)57 and Methods of Researching End of Life Care (MORECare) statements. 58 The economic components were conducted and reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 59
Settings
The trial recruited patients and caregivers from seven centres in the UK (South London, Nottingham, Liverpool, Cardiff, Brighton, Ashford and Sheffield), all with MPCTs and neurology services. In all the centres, neurology services are consultant led with clinical nurse specialists for the relevant conditions. The majority of patient contacts are hospital based, with variable community outreach work. The centres’ respective local areas have networks of palliative care services, including inpatient hospices, community services and hospital support teams. The centres encompass urban, suburban and rural areas, with varying levels of deprivation and ethnic diversity.
Inclusion criteria
Eligible patients were:
-
adults (aged ≥ 18 years) severely affected by advanced or progressive stages of either:
-
MS – patients with either aggressive relapsing disease with rapid development of fixed disability or with advanced primary or secondary progressive disease, often with limitations, such as gait and upper limb function (we did not define referral based on disability, but expected most patients to have an Expanded Disability Status Scale60 of at least 7.5)
-
Parkinsonism and related disorders:
-
MND all stages
-
-
deemed (by referring/usual care clinicians) to have:
-
an unresolved symptom (e.g. pain, breathlessness) that has not responded to usual care
-
at least one of the following: another unresolved other symptom; cognitive problems; complex psychological (depression, anxiety, family concerns) needs; complex social needs; communication/information needs
-
-
able to give informed consent, or their capacity can be enhanced (e.g. with information) so that they can give informed consent, or a personal consultee can be identified and approached to give an opinion on whether or not the patient would have wished to participate
-
living in the catchment area of the SIPC service.
Eligible caregivers were:
-
adults (aged ≥ 18 years) identified by the patient as the person closest to them, usually a family member, close friend, informal caregiver or neighbour
-
able to give informed consent and to complete the questionnaires.
Eligible professionals were:
-
professionals involved in the care of patients with LTNCs
-
professionals (of neurology or palliative care services) who are part of a team involved in the delivery of the OPTCARE Neuro intervention.
Exclusion criteria
Patients who met the inclusion criteria, but:
-
were already receiving specialist palliative care or had done so in the last 6 months
-
lacked capacity and had no family member, friend or informal caregiver willing and available to complete questionnaires about their own and the patient’s symptoms and circumstances.
Recruitment procedure
Potential participants were identified through neurology teams (consultants and clinical nurse specialists) at outpatient clinics. Research nurses liaised directly with these teams, attending clinics when possible to ensure the accuracy of eligibility assessment. Awareness of the trial was raised by:
-
conducting local workshop sessions (e.g. lunchtime seminars) at recruiting centres to explain why the trial is being conducted; equipoise; how to identify and refer patients; and general information on palliative care needs
-
developing posters and flyers detailing the trial, the local research personnel and lead clinicians, to be displayed in appropriate places
-
working with our patient and public involvement (PPI) group, as well as other patient societies and charities.
Identifying clinicians discussed the trial with potential participants and provided written information when possible. If patients were interested and agreed to it, clinicians completed a standard referral form to check that the inclusion criteria were met and this was then sent to the local research teams. The research teams contacted patients by telephone to explain the trial, sent out written information if not already received and subsequently arranged a first visit (after a minimum of 24 hours unless the potential participant wished to waive this period). We also aimed to gather the views of informal caregivers and, when appropriate, asked patients to identify the person nearest to them (such as a family member or informal caregiver) who could also be approached to participate in the trial. If the caregiver was interested and met the inclusion criteria, they were also presented with written information regarding the trial. At the initial visit, researchers provided potential participants (patients and caregivers) the opportunity to further discuss the trial and ask any questions. Following this, written informed consent was obtained and baseline questionnaires administered with patients and their caregivers. As part of the consenting process, researchers discussed the need for the patients to nominate a consultee in case their capacity fluctuated during the course of the trial (see Mental Capacity Act).
If a patient met the inclusion criteria but the clinical team or researcher deemed them (using clinical judgement, in line with local policy guidance) to have reduced capacity, inclusion was discussed with informal caregivers, family members or close friends (in conjunction with the patient if appropriate) to determine the most appropriate person to act as the personal consultee. The research teams contacted the nominated personal consultee to explain the study, provide written information and subsequently arrange a first visit (after a minimum of 24 hours, unless the personal consultee wished to waive this period). At this initial visit, the patient was reassessed and if capacity was confirmed to be insufficient, the consultee was asked to confirm whether or not they believed the patient would like to be included in the trial and provided assent on their behalf. When caregivers provided assent for a patient lacking capacity, they were also asked to provide proxy information about the patient.
Mental Capacity Act
The commonality of cognitive impairment in advanced LTNCs required the inclusion of people with impaired mental capacity in the trial. The Mental Capacity Act62 informed the process of consent for patients lacking capacity. All participants were considered to have capacity unless established otherwise and all practicable steps were taken to enable individuals to decide for themselves if they wished to participate. Capacity was established using the Mental Capacity Act four-step process:
-
The individual is able to understand the information about the study.
-
The individual is able to retain the information (even for a short time).
-
The individual is able to use or weigh up that information.
-
The individual is able to communicate their decision. 62
A process of consent and assent tailored to an individual’s level of capacity and incorporating varying levels of capacity was used. Incorporating different processes of consent and assent has been successfully used in previous studies on end-of-life care. 63–65 For adults lacking capacity, a personal consultee was sought to give an opinion on whether or not the patient would have wanted to participate in the study had they had capacity to indicate this, and whether or not that participation would cause undue distress. 62,65 For adults with impaired capacity who were able to understand, retain and weigh-up information in the moment, a process of consent in the moment was used, with ongoing consent whereby informed consent to participate was reaffirmed prior to each data collection point. 66 If a participant’s capacity declined so that they were no longer able to give informed consent in the moment, researchers followed the procedure for adults lacking capacity as detailed above.
Advance consent was incorporated in the consent process for all patients in anticipation that some may lose capacity over the course of the trial and no longer have capacity to indicate their right to withdraw. The process of advance consent was informed by previous studies with older people65 and on end-of-life care. 67 Participants were asked to indicate if, should they lose capacity in the future, they wished to continue to be involved in the trial and, if yes, asked to nominate a personal consultee. The personal consultee was approached if the participant lost capacity to such an extent that they were no longer able to indicate their right to withdraw or to complete patient-reported outcome measures, requiring instead a proxy informant (e.g. informal or formal caregiver). The procedure of assent for adults lacking capacity was followed to ascertain the personal consultee’s opinion on the individual’s continued participation.
Data collection
Face-to-face visits were undertaken with patients at their location of choice (usually their home). Trained research nurses and researchers assisted, as required, in self-completion of patient and caregiver questionnaires, in accordance with the standardised schedule. Data were collected at baseline and then at 6, 12, 18 and 24 weeks post randomisation. Usually, caregivers self-completed their questionnaires during the patient interview but in some instances questionnaires were returned by post to the local project teams. For adults lacking capacity, baseline and outcome measures were obtained from the informal caregiver interviewer, as above. The use of a proxy informant is common in research on palliative care associated with patients’ advancing illness and deteriorating condition, and on the importance of capturing data at points of deterioration when a patient may most benefit from palliative care, notably the last days of life. In addition, informal caregivers provided the baseline information about the patient’s demographic circumstances and clinical history (e.g. age, educational level, diagnosis, time since diagnosis), as would normally be collected in the patient interview. Outcome assessors were blind to treatment allocation and accommodated separately from the intervention teams.
Outcome measures
The primary outcome was the combined score of eight key symptoms (pain, shortness of breath, nausea, vomiting, constipation, spasms, difficulty sleeping and mouth problems), as measured by the Integrated Palliative care Outcome Scale for Neurological conditions 8 symptom subscale (IPOS Neuro-S8) at 12 weeks. The IPOS Neuro-S8 has been validated among people with LTNCs and found to be responsive to change. 68 The choice of primary outcome was based on the results of our Phase II trial and our modelling work: patients consider these important symptoms in neurological conditions; the SIPC aims to improve several complex symptoms that interact; and these symptoms are often overlooked by existing services but impact on quality of life. Secondary outcomes (all also measured at baseline and 12 weeks) included the following.
Patient outcomes
-
Patients’ palliative needs and symptoms, as measured by the IPOS Neuro, composed of 42 items, for which higher scores indicate more palliative care needs.
-
Patients’ physical symptoms as measured by the Integrated Palliative care Outcome Scale for Neurological conditions 24 symptom subscale (IPOS Neuro-S24) subscale,69 composed of 24 items, for which higher scores indicate more symptom burden.
-
Patients’ psychological and spiritual well-being, information needs and practical issues, as measured by the IPOS Neuro-8 non-physical subscale, composed of eights items, for which higher scores indicate more palliative care needs.
-
Patients’ psychological distress, as measured by the Hospital Anxiety and Depression Scale (HADS),70 composed of two separate subscales for anxiety and depression, each with seven items, for which higher scores indicate more distress.
-
Patients’ satisfaction of care, as measured by the modified FAMCARE-P16,71 composed of 16 items, for which higher scores indicate more satisfaction with care.
-
Patients’ self-efficacy, as measured by the Self-Efficacy for Managing Chronic Disease (SEMCD) Scale,72 composed of six items, for which higher scores indicate more self-efficacy.
-
Safety, adverse events and survival (days from consent to death).
Health economic outcomes and service use
-
Patients’ health-related quality of life and well-being as measured by the EuroQol-5 Dimensions, five-level version (EQ-5D-5L),73 composed of five dimensions plus a visual analogue scale, for which higher scores indicate better quality of life.
-
Hospital admissions, emergency attendances and other health and social care service use, including inpatient, outpatient, home-based services, and tests and diagnostics, as measured by the adapted version of the Client Service Receipt Inventory (CSRI). 74
Caregiver outcomes
-
Caregiver burden as measured by the Zarit Burden Inventory 12 items (ZBI-12),75 composed of 12 items, for which higher scores indicate more burden.
-
Caregiver positivity as measured by the ZBI-12 and positivity,75 composed of eight items, for which higher scores indicate more positivity related to caregiving.
-
Caregiver satisfaction as measured by the modified FAMCARE 2,71,76 composed of 17 items, for which higher scores indicate more carer satisfaction with patient care.
-
Caregiver assessment of patients’ problems and services, as measured by the IPOS Neuro and the CSRI.
Intervention
The SIPC focused on personalised care planning, case management and supporting existing care providers. 17 The SIPC was delivered by existing MPCTs, linked with local neurology and rehabilitation services. MPCTs comprised individuals specifically trained in palliative care from backgrounds in medicine, nursing or social work, together with other allied health professionals. All staff involved in the delivery of the intervention were provided with a standard manual and face-to-face training in advance of the trial commencing. For the purposes of the trial, MPCTs operated a key worker process, in which a specialist team member took initial responsibility for a referred patient.
Frequency and duration of intervention
The length of the intervention was 6–8 weeks from referral. This is broken downs as follows:
-
2 days for first telephone call (from receiving the patient referral).
-
Up to 5 days for first visit (i.e. end of week 1).
-
2–3 weeks for second visit (i.e. end of week 3–4).
-
3–4 weeks for third visit (i.e. end of week 6–8).
Following referral, a key worker (usually a specialist palliative care nurse) contacted the patient within 2 working days to arrange a visit within the next 5 working days to undertake a comprehensive palliative care assessment. As would be standard palliative care practice, at this initial visit a comprehensive palliative care assessment was undertaken, considering both patient and caregiver/family needs. The SIPC manual and training specified the following components to be covered in this assessment:
-
history to include illness understanding
-
completion of IPOS Neuro to aid identification of patient symptoms and needs
-
symptom control and management
-
continuity and co-ordination of care, access to services
-
psychosocial needs
-
information/communication needs
-
practical needs at home
-
decision-making and advance care planning
-
assessment of caregiver and family needs
-
medication review
-
referrals/appointments with other care providers
-
provide information about what is provided through SIPC, along with contact details.
Following this initial assessment, a problem list was generated and prioritised, and a proposed treatment plan agreed with the patient and their family. This may have involved a change in symptom management (e.g. medicine change), contact with other services and/or psychosocial support or counselling. Medicine change recommendations were in liaison with the patient’s general practitioner and/or neurologist, as appropriate, and followed regional and national best practice guidance (e.g. the Palliative Care Formulary77). The treatment plan for each patient was discussed and reviewed at a multiprofessional team meeting, to optimise the management of the patient and caregiver. A summary and action plan were then sent to the patient and all relevant health professionals.
The second contact (face to face or telephone) normally occurred within 2 weeks of the first visit, in order to review and evaluate the proposed plan of care. When appropriate, this included liaison with relevant health professionals for exchange of information, advice and co-ordination of care. The personalised problem list and plan were reviewed and updated, with a copy sent to the patient and all relevant health professionals. The final contact involved a review of outcomes from actions already taken and then discharge to local services, as appropriate. Specialist palliative care is always an individualised service responding to patients’ needs, and SIPC was intended to be the same; therefore, there was some flexibility to adjust to patients’ and families’ individual needs and requirements, and some patients needed prompt support after the first or second visit.
Patients and caregivers in both arms continued to receive usual care throughout the duration of the trial, regardless of trial arm allocation. This included support from specialist nurses, neurology services (outpatient and inpatient), rehabilitation services, community services, general practitioners, district nurses and social services.
Standardisation and compliance
To understand the delivery of the intervention, all MPCTs completed standardised documentation for each patient, recording the main activities and services provided. Each team was advised to use their own existing paper-based or electronic clinical records, in order not to duplicate work for busy clinical teams; however, they were asked to review their usual documentation to ensure that, as a minimum, they record and report:
-
mode of contact and duration for each contact
-
clinical details and severity of main problems
-
activities performed during contact, plan of care and referrals to other services
-
phase of illness (stable, unstable, etc.)
-
performance status using the Australia-modified Karnofsky Performance Scale (AKPS)
-
level of compliance was using the following classifications: complier (received full intervention as planned), partial complier/erratic user (received some but not all of the intervention, or recommendations not followed), overuser (in frequent contact with the service) and dropout.
Randomisation, blinding and allocation concealment
Following consent and baseline data collection, local research nurses entered the patients’ data and registered each patient on the online database [InferMed MACRO-4 (London, UK)]. The registration process allocated each patient a unique participant identification number, which was used to identify them throughout the course of the trial. Once the patient was registered and the baseline data were verified, the trial manager performed all randomisations centrally using an online randomisation system managed by the King’s College London Clinical Trials Unit (CTU).
Randomisation was performed in a 1 : 1 ratio, at the patient level, with minimisation for centre, primary diagnosis (MS vs. IPD vs. PSP, MSA and MND) and cognitive impairment (capacity vs. impaired or lacking capacity). The trial manager was notified of the trial arm allocation and arranged referrals to the palliative care teams for the intervention accordingly (i.e. immediately or after completion of the 12-week data collection visit). As the randomisation used a dynamic method via a system managed by the CTU, it was not possible for the investigators or the trial manager to know the allocation sequence in advance.
The research nurses conducting the data collection interviews and the trial statistician were blinded to the allocation, but received blinded randomisation confirmation e-mails. The trial manager telephoned patients and/or caregivers to inform them of their trial arm allocation and when they would be contacted by the palliative care team. During this telephone call, they were asked not to reveal their allocation to the research nurses at subsequent visits. This information was also sent to all participants in writing, worded as ‘It is important that you do not tell the researchers which group you have been assigned to. This is important for the information we collect from you’. After the primary end point of 12 weeks, in a small number of cases, research nurses were unblinded in order to conduct the qualitative interviews with patients and caregivers who had received the intervention.
Statistical methods
Sample size
Based on the data from our Phase II MS trial,40 the total required sample size required for five centres was 356 patients. In view of the advanced illness in this patient group, this included allowing for 17% attrition to the primary outcome at 12 weeks, giving 296 patients, or 148 in each arm, with data at both baseline and 12 weeks. The correlation between baseline and the outcome at 12 weeks in the pilot study was 0.55. Using a generalised linear model to adjust for the baseline score, with two-sided alpha = 0.05 and correlation of 0.40, the study will have 80% power for a medium effect size of 0.30. To allow for heterogeneity across conditions and centres, we used conservative figures (e.g. correlation 0.4 rather than 0.55; 17% attrition) to estimate the sample size.
Descriptive analysis
Continuous variables were summarised with descriptive statistics [n, mean, standard deviation (SD), median, minimum and maximum]. Frequency counts and percentage of subjects within each category were provided for categorical data. No significance testing was carried out. Summary tables (descriptive statistics and/or frequency tables) by trial arm were provided for all baseline variables, including demographic and clinical characteristics, cognitive impairment and functional performance status. Summary tables were also provided by those with and without valid primary outcome data (IPOS Neuro-S8 score at 12 weeks) (see Appendix 2).
Missing data
Attrition was summarised in accordance with the MORECare classification as attrition due to death, attrition due to illness or attrition at random. 58 For baseline and outcome data, the number with complete data at both time points is reported. The mechanism of missingness was assumed as missing at random, as bivariate analyses indicated that missingness was associated with patient capacity, age, performance status and ethnicity. Multiple imputation using chained equations was used to impute missing observations. Imputation models included the outcome variable of interest, a binary measure of patient capacity, patient age, patient performance status (as measured by the categorical AKPS measure) and a binary measure of patient ethnicity (white vs. other ethnicities). Twenty imputed values were generated for each variable with missing data, which were then combined as per Rubin’s rule. 78
Effectiveness analysis
Data were analysed on an intention-to-treat basis. The mean scores, mean change scores from baseline to 12 weeks post randomisation and their 95% (for primary outcome – IPOS Neuro-S8) or 99.55% confidence intervals (CIs) (for secondary outcomes) were reported. To account for clustering effects, a generalised linear mixed model (GLMM), with centre as a random effect, adjusting for baseline score of IPOS Neuro-S8 was used. For each of the primary and secondary outcomes, the change score was regressed on to the binary measure of trial arm. The statistical significance value (0.05/11 = 0.0045) for secondary outcomes was adjusted using Bonferroni correction to control for multiple testing.
Sensitivity analysis of effectiveness
Six sensitivity analyses followed for the primary and secondary outcomes. In the first, the analyses were adjusted for ethnicity (as between-arm differences were observed in additional exploratory analysis), using fully imputed data (n = 350). In the second, the two participants who were deemed ineligible for the study post randomisation were excluded; thus, the complete data set totalled 348 patients. The third and fourth sensitivity analyses assessed differences in change scores between trial arms in complete patient and caregiver data, respectively. The fifth sensitivity analysis used complete patient data, if available at both baseline and 12 weeks, and imputed proxy carer data if not. Caregiver data were used only when complete and available, at both baseline and 12 weeks, ensuring that scores were acquired from the same source (patient or caregiver) at both time points. The sixth sensitivity analysis included only patients with MS. For each of the sensitivity analyses, differences between change scores for each trial arm were tested using GLMM, adjusting for baseline scores.
Health economic methods
An economic component was included to assess the cost-effectiveness of SIPC compared with standard care. Cost-effectiveness was assessed by linking data on formal cost differences and outcome measurements differences.
Service use
Participants provided details of services used during the 12 weeks prior to baseline and then for the past 12 weeks post randomisation, using a version of the CSRI. 74 Services included hospital inpatient and outpatient care, primary health care (home care and community care), tests and diagnostics, social care, the provision of aids and home adaptations, and informal care provided by family members and/or friends. The number of contacts with services and, when relevant, the mean length of contacts were documented. The number of hours that family and friends spent providing personal care and help inside and outside the home and in other tasks per week was collected. To better understand the utilisation and associated costs, service items were grouped into health and social care (outpatient, day or community, home, palliative, rehabilitation, primary, social care, and tests and diagnostics) and informal care.
Missing data
For those participants who completed the CSRI, a blank return in the questionnaire on whether or not a participant used a specific service was assumed to indicate no use of that particular service. This followed the structure of CSRI questions. CSRI asks respondents to describe what services or care they have received, to tick when services are received, and then say how often and how much of these they received. It offers a long list of potential options and an open space to describe others. It does not ask ‘yes or no’ for each service in the long list, and the space can be left blank when services are not used. When a service was used, but either the number of contacts or duration of contacts was unknown, the median value from all other valid cases across the whole sample at that time point was used.
Missing data for the outcome variables [IPOS Neuro-S8 and EuroQol-5 Dimensions (EQ-5D) index score] were imputed using multiple imputation with chained equations after examining the associations between missingness and variables of interest such as patient capacity, age, performance status and ethnicity. For those participants who provided outcome data but no CSRI data, total care (health and social care and informal care) costs were also imputed, as described above.
Costs
Costs were calculated, in Great British pounds, by combining resource use data with unit costs obtained from standard sources, such as the NHS Reference Costs 2015 to 201679 or the Unit Costs of Health and Social Care 2016,80 where applicable. The unit costs of a home care worker or a nurse aid were used as a proxy for informal care. We assumed that the CSRI recorded all services provided for the participants, regardless of randomisation, and did not separate the services of the intervention from the rest. The horizon of the analysis is restricted to the trial period and no discounting was considered.
Cost-effectiveness analysis
Cost-effectiveness, from an NHS perspective, was assessed by linking data on health and social care service cost differences and two outcome measurements differences: the primary outcome IPOS Neuro-S8 and EQ-5D index score [quality-adjusted life-year (QALY)]. IPOS Neuro-S8 total score was generated as the sum of eight items, ranging from 0 to 32. For cost-effectiveness analysis, this was rescaled by subtracting from 32 to make higher scores reflect better outcomes. EQ-5D-5L contains five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each of which has five levels (no problems, slight problems, moderate problems, severe problems and extreme problems). These levels are valued 1–5, respectively; however, as these numerals have no arithmetic properties and should not be used as a cardinal score, we calculated an index value using UK value sets for EQ-5D obtained by a crosswalk approach. 81
The mean and SD were examined for formal care costs and the two outcome measurements. Cost-effectiveness of SIPC was assessed by calculating incremental cost-effectiveness ratios (ICERs), using mean changes in formal care costs and outcome measurements, as shown in Equation 1. ICERs smaller than the willingness-to-pay (WTP) threshold (λ = e.g. £20,000, as the threshold used by the National Institute for Health and Care Excellence) indicates that the cost-effectiveness of SIPC. The primary decision criterion for cost-effectiveness of SIPC was whether ICERs were larger or smaller than λ:
To understand the uncertainty around the ICERs, we produced the estimated differences in formal care cost and outcomes by bootstrapping with 1000 replications. Regression approach was used to predict the difference in costs and outcomes: the generalised linear model with a log-link function and the Poisson distribution for cost due to the skewed distribution, and ordinary least squares regressions for EQ-5D index score and IPOS Neuro-S8, after examining the distributions and model specifications. In each regression, baseline values were controlled for. Bootstrapping was conducted using complete cases only. These replications were plotted in cost-effectiveness planes. These planes have four quadrants and combine changes in costs and changes in outcomes: (1) north-east (SIPC is more effective and more costly); (2) north-west (SIPC is less effective and more costly); (3) south-west (SIPC is less effective and less costly); and (4) south-west (SIPC is less costly and more effective). 82 We examined the distribution of 1000 replications on the planes by four quadrants.
Finally, to account for the joint uncertainty of costs and outcomes, we conducted further analysis of the probability of SIPC being cost-effective with a set of WTP thresholds, as well as calculating the incremental net monetary benefit (INMB) of SIPC compared with standard care (details in Appendix 3).
Qualitative methods
Embedded within the trial was a qualitative component conducted concurrently,83 to explore which aspects of SIPC were most valued or had most impact on patients’ and caregivers’ experiences of care, how the change process of SIPC may be working and how the intervention is delivered in practice. The intention was to form a theoretical model of SIPC to inform implementation requirements and processes, and intended outcomes. The embedded qualitative study involved patients, caregivers and health-care staff.
Patient and caregivers
Individual interviews were conducted with participants who received SIPC (at 12 weeks for the intervention group and 24 weeks for the standard care group). We estimated a sample size in each study site of seven patients/caregivers, totalling 35 patients/caregivers. We sought to conduct maximum variation sampling to encompass the conditions eligible for inclusion in the trial. However, given that the trial sample largely comprised MS and IPD patients, this was not practical, and purposive sampling was used. Only participants who had indicated their consent to participate in these qualitative interviews at the initial trial consenting stage were contacted regarding these interviews. Interviews were conducted in participants’ own homes with patients, and caregivers when available, or with caregivers when patients lacked capacity to participate. The interviews were conducted by researchers and research nurses trained in and supervised during qualitative interviews. When possible, a researcher who was not involved in the main trial data collection conducted the interview to minimise the risk of unblinding.
Health-care staff
Focus groups were conducted with health-care staff from the respective centres to explore perceptions of SIPC, processes of SIPC delivery and the local context of service delivery models for patients with neurological conditions. We estimated a sample size of six service providers from each site, totalling 30 providers. Participants were identified by the local research teams for the central King’s College London team, who e-mailed invitations to participate. Eligible participants comprised health professionals involved in delivering the intervention in the respective study site (e.g. specialist nurses, neurologists, allied health professionals). Each group comprised representatives from the respective centres and disciplines involved in the care provision. When individual attendance at a focus group was not possible (e.g. clinical commitments), individual interviews were conducted (either face to face or by telephone) to ensure representation from all centres. The groups were facilitated by a researcher experienced in qualitative research methods and an observer to document, for example, group processes and interactions. All participants provided written informed consent.
Data analysis
Interviews and focus groups were digitally recorded, transcribed verbatim and anonymised prior to analysis. Data analysis drew on Coffey and Atkinson’s84 iterative approach of coding and describing the data, generating categories, through to forming hypotheses and generating theory. We explored the impact of SIPC at three main levels (people and context, processes and tasks, and underpinning theory)85 and sought to identify ways to enhance SIPC and the processes for wider implementation. The analysis approach emphasised theory generation by asking questions about the data and developing emergent lines of thinking to form and question emergent hypotheses. NVivo 11 software (QSR International, Warrington, UK) for qualitative analysis was used for data storage, coding, searching and retrieving, and recording analytical thinking. Quality appraisal sought to ensure systematic and rigorous attention to analysis and reporting by, for example, holding supervisory review meetings to consider the data analysis and emerging findings (held by CE and NH), attention to divergent cases and use of qualitative research software to assist comprehensive reporting, auditability and transparency of the findings.
Patient and public involvement
Patient and public involvement has been an integral part of all our research processes. An independent PPI group was set up specifically for OPTCARE Neuro, comprising both patients and caregivers with lived experience of MS, IPD, MSA and MND. The group advised on the application for ethics approval and the development of all participant materials, as well as the delivery of the trial and the interpretation of the findings. We engaged with our PPI members on multiple levels but predominantly through 3-monthly face-to-face meetings at which we benefited from the expert views of our members to help us prioritise the research questions and ensure that the study was undertaken in a way that was meaningful and relevant to both patients and caregivers. A member of our PPI group was a co-applicant on the funding application and an active member of the Study Steering Committee. We had PPI representation in the Data Monitoring and Ethics Committee, which had oversight and responsibility for the conduct of the trial. We actively involved our PPI members in the interpretation of data, particularly the qualitative components of the study. This provided the study team with valuable insight to understand the data and their relevance for addressing the needs of this patient population.
Engagement
Engagement took place throughout the study. First, charities and patient societies supported and publicised the trial in order to improve recruitment and dissemination. Specifically, the PSP Association, the MS Society and Parkinson’s UK, which all featured details of the trial on their research web pages. The trial was also featured in an issue of the Multiple Sclerosis Trust’s Way Ahead magazine for MS health professionals, and in the PSP Association’s PSP Matters magazine, with two trial participants contributing to the article. In May 2016, the study team hosted a 2-day workshop and conference on palliative care in neurology. The first of these days was a closed meeting for OPTCARE Neuro teams, with representation from principal investigators, research nurses, clinicians and PPI members from all trial centres. A representative from each site gave a brief overview of local progress, and our PPI group also presented on their involvement. It was extremely productive to have all the teams together to exchange ideas and learn from each other. The second day was a conference that focused on clinical components of palliative care for patients with LTNCs, and some interim data from the mapping exercise and survey for professionals were presented. The conference had > 100 registrations and was attended by a mixture of clinicians, researchers, students, PPI members and representatives from charitable organisations and patient societies. The great turnout for the conference and the interest from the audience members highlighted the importance of the OPTCARE Neuro work. Last, throughout the course of the study, we have circulated 6-monthly newsletters to our study contacts, which included palliative care and neurology clinicians, academics, researchers and charities.
Ethics approval and research governance
The trial was conducted in compliance with the principles of the Declaration of Helsinki (2013)86 and the principles of Good Clinical Practice and in accordance with all applicable regulatory requirements, including, but not limited to, the Research Governance Framework and the Mental Capacity Act 2005. 62 The protocol and related documents were submitted for review and approved by the London South East Research Ethics Committee (14/LO/1765).
Chapter 4 Results
Mapping results
Parts of the text have been reproduced from van Vliet et al. 55 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Centres varied in size of catchment areas (39–5840 square miles) and population served (142,000–3,500,000). Neurology and specialist palliative care were often not co-terminous. Service provision for neurology and specialist palliative care also varied; for example, neurology services varied in the number and type of clinics provided, and palliative care services varied in the settings in which they worked. The integration between neurology and palliative care teams varied between centres, but more clearly between diseases. For MS, the integration was limited, with most centres having no formal links. Only two centres broke this trend; one held an 8-weekly multidisciplinary team (MDT) meeting, in which both MS and palliative care teams participated, and the other held a 3-monthly complex problem clinic with palliative care attendance. For MND, a different picture emerged of stronger integration. Most centres either held joint clinics or had a palliative care presence at MDT meetings. At one site, all MND patients were invited to clinics at the local hospice; whereas at another, all patients received a palliative care assessment. Good informal links were reported in one site where the MND and palliative care nurses shared an office, but there were with no joint visits. The least integrated site had no joint clinics and referrals were based on needs. Last, in Parkinson’s disease-related disorders the integration was very mixed. Approximately half of the centres had no joint clinics or formal relationships. Others had 2- to 3-monthly clinics or MDT meetings, with one site having a palliative care presence at weekly clinics. There was a difference between the subsets of diseases, with greater integration for MSA and PSP. The number of neurology patients per annum receiving specialist palliative care reflected these differences in integration (a range of 9–88 patients with MND, a range of 3–5 patients with Parkinson’s disease-related disorders and a range of 0–5 patients with MS).
Survey results
Parts of the text have been reproduced from Hepgul et al. 56 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
The survey received responses from 33 neurology and 26 palliative care professionals (20% response rate). Two-thirds of respondents in both groups had > 10 years of experience in their respective fields. Current levels of collaboration between the two specialties were reported to be ‘good/excellent’ by 36% of neurology professionals and by 58% of palliative care professionals. However, nearly half (45%) of neurology compared with only 12% of palliative care professionals rated current levels of collaboration ‘poor/none’. When asked if there were any particular disease areas for which links were better, both groups reported stronger links for MND. In addition, both professional groups felt that the new SIPC service being trialled would influence future collaborations for the better (65–70% in both groups). Participants were also asked what they thought would be the main barriers for the new SIPC service. The most common barriers identified by neurologists were resources, clinician awareness of services offered, continuing collaborations, and communication between teams beyond the trial and geographical limitations. Similarly, palliative care professionals also identified resources and clinician awareness of services offered (and importantly the appropriateness of referrals they may receive) as barriers. However, the key barrier they identified was that there may be a possible need for longer-term care beyond that offered by the SIPC service. They also drew attention to patients’ perceptions of palliative care as a potential barrier.
Randomised trial results
Participant flow
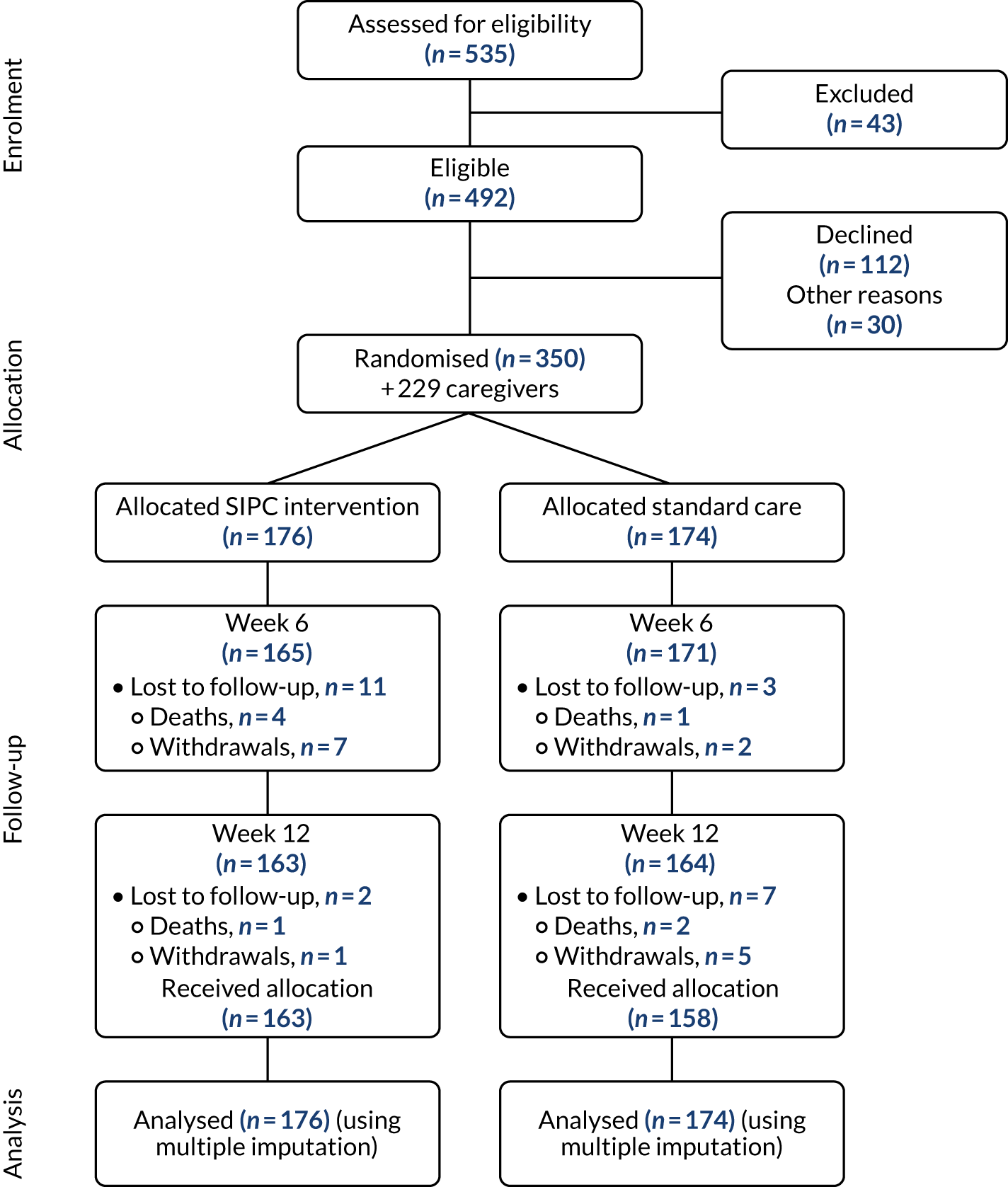
Recruitment began in three centres in April 2015. Two centres opened in July 2015 and November 2015, with an additional two centres opening in February 2016 and September 2016. The total recruitment period was 31 months, ending in November 2017, with all follow-up visits completed in May 2018. One centre, Sheffield, was opened but failed to sustain recruitment and therefore was closed. Other centres’ recruited numbers were broadly reflective of their catchment areas and local populations. Monthly recruitment rates over the course of the recruitment period are presented in Appendix 1. The trial recruited 350 patients living with a LTNC, plus 229 caregivers, with 176 patients in the immediate SIPC intervention arm and 174 in the standard care waiting list control arm. Table 1 details the screening and enrolment by each site and Figure 1 outlines the participant flow up to the primary end point of 12 weeks post randomisation.
| Screening and enrolment | Site | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| London | Liverpool | Nottingham | Cardiff | Brighton | Ashford | Sheffield | ||
| Site opened | April 2015 | April 2015 | April 2015 | July 2015 | November 2015 | February 2016 | September 2016 | |
| Referred | 158 | 69 | 100 | 67 | 71 | 64 | 6 | 535 |
| Not in catchment area | 8 | 1 | 1 | 1 | 5 | 0 | 0 | 16 |
| Already receiving palliative care | 11 | 2 | 0 | 1 | 1 | 6 | 0 | 21 |
| Lacks capacity and no caregiver | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 3 |
| Not meeting diagnostic criteria | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| Declined | 25 | 16 | 19 | 22 | 15 | 12 | 3 | 112 |
| Other | 11 | 2 | 4 | 6 | 0 | 7 | 0 | 30 |
| Enrolled patients (caregivers) | 100 (79) | 48 (37) | 76 (32) | 36 (23) | 50 (31) | 37 (26) | 3 (1) | 350 (229) |
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram showing the flow of participants. Reproduced with permission from Gao et al. 87 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Participant characteristics by trial arm
The baseline characteristics of the sample are presented in Tables 2 and 3 by trial arm. The groups are comparable, except for patient ethnicity, with more patients of ethnicity other than white in the SIPC group (13%) than in the standard care group (5%).
| Variable | Value | All | SIPC | Standard care |
|---|---|---|---|---|
| N | 350 | 176 | 174 | |
| Age (years), mean (SD) | 66.8 (11.8) | 67.3 (10.9) | 66.4 (12.6) | |
| Gender, n (%) | Man | 179 (51.1) | 86 (48.9) | 93 (53.5) |
| Woman | 171 (48.9) | 90 (51.1) | 81 (46.6) | |
| Marital status, n (%) | Single | 35 (10.0) | 16 (9.1) | 19 (10.9) |
| Widowed | 38 (10.9) | 19 (10.8) | 19 (10.9) | |
| Married/civil partner | 231 (66.0) | 114 (64.8) | 117 (67.2) | |
| Divorced/separated | 44 (12.6) | 26 (14.8) | 18 (10.3) | |
| Not done/unknown | 2 (0.6) | 1 (0.6) | 1 (0.6) | |
| Living status, n (%) | Alone | 65 (18.6) | 35 (19.9) | 30 (17.2) |
| With spouse/partner and/or children | 244 (69.7) | 125 (71.0) | 119 (68.4) | |
| With friend(s)/with others | 41 (11.7) | 16 (9.1) | 25 (14.4) | |
| Education, n (%) | No formal education up to lower secondary school | 139 (39.7) | 67 (38.1) | 72 (41.4) |
| Upper secondary to post-secondary vocational qualification | 116 (33.1) | 53 (30.1) | 63 (36.2) | |
| Tertiary education | 91 (26.0) | 55 (31.3) | 36 (20.7) | |
| Not done/missing | 4 (1.1) | 1 (0.6) | 3 (1.7) | |
| Ethnicity, n (%) | White | 316 (90.3) | 166 (94.3) | 150 (86.2) |
| Other ethnic group | 32 (9.1) | 9 (5.1) | 23 (13.2) | |
| Employment, n (%) | No | 340 (97.1) | 173 (98.3) | 167 (96.0) |
| Yes | 10 (2.9) | 3 (1.7) | 7 (4.0) | |
| Feelings towards income, n (%) | Living comfortably on present income | 118 (33.7) | 58 (33.0) | 60 (34.5) |
| Coping on present income | 162 (46.3) | 85 (48.3) | 77 (44.3) | |
| Difficult on present income | 24 (6.9) | 12 (6.8) | 12 (6.9) | |
| Very difficult on present income | 14 (4.0) | 7 (4.0) | 7 (4.0) | |
| Not done/unknown | 32 (9.1) | 14 (8.0) | 18 (10.3) | |
| Diagnosis, n (%) | MS | 148 (42.3) | 74 (42.1) | 74 (42.5) |
| IPD | 140 (40.0) | 71 (40.3) | 69 (39.7) | |
| MSA | 12 (3.4) | 7 (4.0) | 5 (2.9) | |
| PSPa | 27 (7.7) | 13 (7.4) | 14 (8.1) | |
| MND | 23 (6.6) | 11 (6.3) | 12 (6.9) | |
| Years since diagnosis | ||||
| Mean (SD) | 12.3 (10.6) | 12.3 (10.8) | 12.4 (10.4) | |
| Range | 0–56 | 0–56 | 0–46 | |
| Comorbidities, n (%) | No | 99 (28.3) | 42 (23.9) | 57 (32.8) |
| Yes | 251 (71.7) | 134 (76.1) | 117 (67.2) | |
| Patient capacity, n (%) | Consent | 311 (88.9) | 157 (89.2) | 154 (88.5) |
| Personal consultee assent | 39 (11.1) | 19 (10.8) | 20 (11.5) | |
| AKPS, n (%) | Totally bedfast | 7 (2.0) | 3 (1.7) | 4 (2.3) |
| Almost completely bedfast | 10 (2.9) | 5 (2.8) | 5 (2.9) | |
| In bed > 50% of the time | 21 (6.0) | 10 (5.7) | 11 (6.3) | |
| Requires considerable assistance | 170 (48.6) | 77 (43.8) | 93 (53.5) | |
| Requires occasional assistance | 98 (28.0) | 54 (30.7) | 44 (25.3) | |
| Cares for self | 33 (9.4) | 19 (10.8) | 14 (8.1) | |
| Normal activity with effort | 9 (2.6) | 7 (4.0) | 2 (1.2) | |
| Not available/applicable | 1 (0.3) | 1 (0.6) | 0 | |
| Not done | 1 (0.3) | 0 | 1 (0.6) | |
| Variable | Value | All | SIPC | Standard care |
|---|---|---|---|---|
| N | 229 | 121 | 108 | |
| Age (years), mean (SD) | 64.2 (13.3) | 63.3 (13.3) | 65.3 (13.4) | |
| Gender, n (%) | Man | 81 (35.4) | 41 (33.9) | 40 (37.0) |
| Woman | 148 (64.6) | 80 (66.1) | 68 (63.0) | |
| Marital status, n (%) | Single | 14 (6.1) | 7 (5.8) | 7 (6.5) |
| Widowed | 10 (4.4) | 4 (3.3) | 6 (5.6) | |
| Married/civil partner | 200 (87.3) | 109 (90.1) | 91 (84.3) | |
| Divorced/separated | 5 (2.2) | 1 (0.8) | 4 (3.7) | |
| Living status, n (%) | Alone | 9 (3.9) | 4 (3.3) | 5 (4.6) |
| With spouse/partner and/or children | 200 (87.3) | 109 (90.1) | 91 (84.3) | |
| With friends/with others | 20 (8.7) | 8 (6.6) | 12 (11.1) | |
| Relationship to patient, n (%) | Spouse/partner | 177 (77.3) | 97 (80.2) | 80 (74.1) |
| Son/daughter | 29 (12.7) | 17 (14.1) | 12 (11.1) | |
| Other | 23 (10.0) | 0 | 4 (3.7) | |
| Education, n (%) | No formal education up to lower secondary school | 96 (41.9) | 51 (42.2) | 45 (41.7) |
| Upper secondary school to post-secondary vocational qualification | 66 (28.8) | 37 (30.6) | 29 (26.9) | |
| Tertiary education | 62 (27.1) | 30 (24.8) | 32 (29.6) | |
| Not done/missing | 5 (2.2) | 3 (2.5) | 2 (1.9) | |
| Ethnicity, n (%) | White | 211 (92.1) | 113 (93.4) | 98 (90.7) |
| Other ethnic group | 18 (7.9) | 8 (6.6) | 10 (9.3) | |
| Employment, n (%) | No | 162 (70.7) | 86 (71.1) | 76 (70.4) |
| Yes | 67 (29.3) | 35 (28.9) | 32 (29.6) | |
| Illness, n (%) | No | 77 (33.6) | 41 (33.9) | 36 (33.3) |
| Yes | 140 (61.1) | 70 (57.9) | 70 (64.8) | |
Primary analysis of effectiveness
Point estimates and adjusted analyses, using multiply imputed data for the entire sample (n = 350), are presented in Table 4. There were no statistically significant differences between the trial arms for either the primary outcome or any of the secondary outcomes. The primary outcome (IPOS Neuro-S8) fell in both groups between baseline and 12 weeks, with a greater fall (i.e. improvement) in the SIPC group than in the standard care group (–0.78 vs. –0.28). This pattern was consistent for most secondary outcomes. Of note, for some secondary outcomes (indicated with table footnote c), lower scores indicate poorer outcomes. The missing data for the primary outcome due to withdrawal or unknown reasons were 4% at 12 weeks.
| Measure | Time point | All (n = 350) | SIPC (n = 176) | Standard care(n = 174) | p-valuea |
|---|---|---|---|---|---|
| Primary outcome | |||||
| IPOS Neuro-S8, x¯ (CI) | Baseline | 6.93 (6.48 to 7.37) | 6.89 (6.24 to 7.54) | 6.96 (6.34 to 7.58) | |
| 12 weeks | 6.40 (5.93 to 6.86) | 6.11 (5.46 to 6.77) | 6.68 (6.02 to 7.34) | ||
| Change score | –0.53 (–0.90 to –0.16) | –0.78 (–1.29 to –0.26) | –0.28 (–0.82 to 0.26) | 0.14 | |
| Secondary patient outcomesb | |||||
| IPOS Neuro-S24, x¯ (CI) | Baseline | 26.92 (25.14 to 28.71) | 26.69 (24.23 to 29.15) | 27.16 (24.57 to 29.75) | |
| 12 weeks | 25.50 (23.60 to 27.40) | 24.74 (22.10 to 27.37) | 26.27 (23.58 to 28.96) | ||
| Change score | –1.42 (–3.20 to 0.35) | –1.95 (–4.38 to 0.48) | –0.89 (–3.15 to 1.36) | 0.22 | |
| IPOS Neuro-8, x¯ (CI) | Baseline | 11.51 (10.49 to 12.53) | 11.43 (10.07 to 12.79) | 11.58 (10.09 to 13.08) | |
| 12 weeks | 11.19 (10.13 to 12.24) | 10.59 (9.09 to 12.09) | 11.80 (10.34 to 13.26) | ||
| Change score | –0.32 (–1.32 to 0.69) | –0.84 (–2.09 to 0.40) | 0.21 (–1.25 to 1.68) | 0.06 | |
| IPOS Neuro, x¯ (CI) | Baseline | 47.04 (42.49 to 51.59) | 47.36 (41.94 to 52.78) | 46.72 (40.93 to 52.51) | |
| 12 weeks | 43.68 (37.74 to 49.61) | 43.14 (35.28 to 51.00) | 44.22 (37.55 to 50.89) | ||
| Change score | –3.36 (–8.41 to 1.68) | –4.22 (–10.87 to 2.43) | –2.50 (–8.37 to 3.37) | 0.53 | |
| HADS anxiety, x¯ (CI) | Baseline | 7.64 (6.95 to 8.34) | 7.78 (6.78 to 8.77) | 7.51 (6.52 to 8.50) | |
| 12 weeks | 7.51 (6.70 to 8.32) | 7.43 (6.28 to 8.58) | 7.59 (6.53 to 8.66) | ||
| Change score | –0.13 (–0.68 to 0.42) | –0.35 (–1.12 to 0.43) | 0.08 (–0.65 to 0.81) | 0.27 | |
| HADS depression, x¯ (CI) | Baseline | 8.22 (7.61 to 8.84) | 8.13 (7.29 to 8.97) | 8.31 (7.47 to 9.16) | |
| 12 weeks | 8.09 (7.44 to 8.74) | 7.96 (7.03 to 8.88) | 8.22 (7.35 to 9.09) | ||
| Change score | –0.13 (–0.58 to 0.32) | –0.17 (–0.79 to 0.45) | –0.09 (–0.78 to 0.59) | 0.69 | |
| EQ-5D VAS,c x¯ (CI) | Baseline | 52.49 (48.91 to 56.07) | 52.72 (47.91 to 57.53) | 52.25 (47.01 to 57.49) | |
| 12 weeks | 52.23 (48.29 to 56.16) | 53.69 (48.03 to 59.34) | 50.75 (45.36 to 56.14) | ||
| Change score | –0.26 (–4.87 to 4.35) | 0.97 (–5.01 to 6.94) | –1.50 (–8.05 to 5.05) | 0.27 | |
| SEMCD Scale,c x¯ (CI) | Baseline | 5.26 (4.90 to 5.62) | 5.39 (4.89 to 5.89) | 5.13 (4.63 to 5.64) | |
| 12 weeks | 5.11 (4.74 to 5.48) | 5.28 (4.75 to 5.82) | 4.94 (4.41 to 5.47) | ||
| Change score | –0.15 (–0.52 to 0.22) | –0.10 (–0.60 to 0.40) | –0.19 (–0.70 to 0.31) | 0.37 | |
| FAMCARE-P16,c x¯ (CI) | Baseline | 50.32 (47.92 to 52.72) | 50.33 (46.66 to 54.00) | 50.30 (47.08 to 53.53) | |
| 12 weeks | 47.75 (44.81 to 50.69) | 48.08 (43.75 to 52.41) | 47.41 (43.52 to 51.31) | ||
| Change score | –2.57 (–5.12 to –0.03) | –2.26 (–6.05 to 1.53) | –2.89 (–6.23 to 0.45) | 0.70 | |
| Secondary caregiver outcomesb | |||||
| ZBI-12,c x¯ (CI) | Baseline | 18.46 (16.57 to 20.35) | 18.25 (15.59 to 20.90) | 18.68 (16.28 to 21.08) | |
| 12 weeks | 18.76 (16.81 to 20.71) | 18.60 (15.93 to 21.27) | 18.92 (16.28 to 21.55) | ||
| Change score | 0.30 (–0.68 to 1.27) | 0.35 (–0.98 to 1.68) | 0.24 (–1.15 to 1.64) | 0.90 | |
| ZBI positivity,c x¯ (CI) | Baseline | 18.85 (17.66 to 20.03) | 18.97 (17.36 to 20.59) | 18.72 (17.05 to 20.38) | |
| 12 weeks | 18.50 (17.11 to 19.88) | 18.87 (17.08 to 20.67) | 18.12 (16.15 to 20.10) | ||
| Change score | –0.35 (–1.29 to 0.59) | –0.10 (–1.43 to 1.23) | –0.59 (–1.98 to 0.79) | 0.40 | |
| FAMCARE 2,c x¯ (CI) | Baseline | 53.89 (50.79 to 56.99) | 53.81 (49.64 to 57.97) | 53.98 (49.93 to 58.02) | |
| 12 weeks | 53.61 (49.78 to 57.44) | 53.99 (48.92 to 59.07) | 53.23 (48.38 to 58.07) | ||
| Change score | –0.28 (–3.47 to 2.91) | 0.19 (–4.86 to 5.23) | –0.75 (–4.64 to 3.14) | 0.67 | |
Sensitivity analysis 1
Imputed data for the entire sample adjusted for ethnicity are presented in Table 5. These estimates are consistent with those from the primary analyses, in favour of the SIPC arm, although not statistically significant.
| Measure | Time point | All (n = 350) | SIPC (n = 176) | Standard care (n = 174) | p-valuea |
|---|---|---|---|---|---|
| Primary outcome | |||||
| IPOS Neuro-S8, x¯ (CI) | Baseline | 6.93 (6.48 to 7.37) | 6.89 (6.24 to 7.54) | 6.96 (6.34 to 7.58) | |
| 12 weeks | 6.40 (5.93 to 6.86) | 6.11 (5.46 to 6.77) | 6.68 (6.02 to 7.34) | ||
| Change score | –0.53 (–0.90 to –0.16) | –0.78 (–1.29 to –0.26) | –0.28 (–0.82 to 0.26) | 0.13 | |
| Secondary patient outcomesb | |||||
| IPOS Neuro-S24, x¯ (CI) | Baseline | 26.92 (25.69 to 28.15) | 26.69 (24.99 to 28.38) | 27.16 (25.38 to 28.94) | |
| 12 weeks | 25.50 (24.19 to 26.80) | 24.74 (22.92 to 26.55) | 26.27 (24.42 to 28.12) | ||
| Change score | –1.42 (–2.64 to –0.21) | –1.95 (–3.60 to –0.30) | –0.89 (–2.45 to 0.66) | 0.25 | |
| IPOS Neuro-S8, x¯ (CI) | Baseline | 11.51 (10.80 to 12.21) | 11.43 (10.49 to 12.37) | 11.58 (10.55 to 12.62) | |
| 12 weeks | 11.19 (10.46 to 11.92) | 10.59 (9.55 to 11.62) | 11.80 (10.79 to 12.80) | ||
| Change score | –0.32 (–1.01 to 0.37) | –0.84 (–1.70 to 0.01) | 0.21 (–0.79 to 1.22) | 0.07 | |
| IPOS Neuro, x¯ (CI) | Baseline | 47.04 (43.96 to 50.12) | 47.36 (43.66 to 51.06) | 46.72 (42.78 to 50.65) | |
| 12 weeks | 43.68 (39.69 to 47.66) | 43.14 (37.85 to 48.43) | 44.22 (39.69 to 48.75) | ||
| Change score | –3.36 (–6.74 to 0.02) | –4.22 (–8.68 to 0.24) | –2.50 (–6.48 to 1.48) | 0.56 | |
| HADS anxiety, x¯ (CI) | Baseline | 7.64 (7.16 to 8.13) | 7.78 (7.09 to 8.46) | 7.51 (6.83 to 8.19) | |
| 12 weeks | 7.51 (6.96 to 8.07) | 7.43 (6.64 to 8.22) | 7.59 (6.86 to 8.33) | ||
| Change score | –0.13 (–0.51 to 0.25) | –0.35 (–0.88 to 0.19) | 0.08 (–0.42 to 0.59) | 0.28 | |
| HADS depression, x¯ (CI) | Baseline | 8.22 (7.80 to 8.64) | 8.13 (7.55 to 8.71) | 8.31 (7.73 to 8.90) | |
| 12 weeks | 8.09 (7.64 to 8.54) | 7.96 (7.32 to 8.59) | 8.22 (7.62 to 8.82) | ||
| Change score | –0.13 (–0.44 to 0.18) | –0.17 (–0.60 to 0.25) | –0.09 (–0.56 to 0.38) | 0.70 | |
| EQ-5D VAS,c x¯ (CI) | Baseline | 52.49 (50.02 to 54.96) | 52.72 (49.40 to 56.04) | 52.25 (48.64 to 55.86) | |
| 12 weeks | 52.23 (49.52 to 54.93) | 53.69 (49.79 to 57.58) | 50.75 (47.04 to 54.46) | ||
| Change score | –0.26 (–3.43 to 2.91) | 0.97 (–3.15 to 5.08) | –1.50 (–6.00 to 3.00) | 0.31 | |
| SEMCD Scale,c x¯ (CI) | Baseline | 5.26 (5.01 to 5.51) | 5.39 (5.04 to 5.73) | 5.13 (4.78 to 5.48) | |
| 12 weeks | 5.11 (4.86 to 5.37) | 5.28 (4.91 to 5.66) | 4.94 (4.58 to 5.30) | ||
| Change score | –0.15 (–0.40 to 0.11) | –0.10 (–0.45 to 0.25) | –0.19 (–0.54 to 0.15) | 0.38 | |
| FAMCARE-P16,c x¯ (CI) | Baseline | 50.32 (48.66 to 51.97) | 50.33 (47.80 to 52.86) | 50.30 (48.08 to 52.52) | |
| 12 weeks | 47.75 (45.72 to 49.77) | 48.08 (45.10 to 51.06) | 47.41 (44.73 to 50.10) | ||
| Change score | –2.57 (–4.32 to –0.82) | –2.26 (–4.87 to 0.36) | –2.89 (–5.19 to –0.59) | 0.78 | |
| Secondary caregiver outcomesb | |||||
| ZBI-12, x¯ (CI) | Baseline | 18.46 (17.17 to 19.75) | 18.25 (16.43 to 20.06) | 18.68 (17.03 to 20.32) | |
| 12 weeks | 18.76 (17.42 to 20.09) | 18.60 (16.77 to 20.43) | 18.92 (17.11 to 20.72) | ||
| Change score | 0.30 (–0.37 to 0.96) | 0.35 (–0.56 to 1.26) | 0.24 (–0.72 to 1.20) | 0.87 | |
| ZBI positivity,c x¯ (CI) | Baseline | 18.85 (18.03 to 19.66) | 18.97 (17.87 to 20.08) | 18.72 (17.57 to 19.86) | |
| 12 weeks | 18.50 (17.55 to 19.45) | 18.87 (17.64 to 20.10) | 18.12 (16.77 to 19.47) | ||
| Change score | –0.35 (–0.99 to 0.30) | –0.10 (–1.01 to 0.81) | –0.59 (–1.54 to 0.35) | 0.38 | |
| FAMCARE 2,c x¯ (CI) | Baseline | 53.89 (51.77 to 56.01) | 53.81 (50.95 to 56.67) | 53.98 (51.21 to 56.74) | |
| 12 weeks | 53.61 (51.01 to 56.21) | 53.99 (50.52 to 57.46) | 53.23 (49.92 to 56.53) | ||
| Change score | –0.28 (–2.47 to 1.91) | 0.19 (–3.25 to 3.63) | –0.75 (–3.43 to 1.92) | 0.67 | |
Sensitivity analysis 2
Imputed estimates for primary and secondary outcomes for all patients, excluding two who were identified as ineligible for the trial post randomisation (n = 348), are presented in Table 6. These estimates are again consistent with the primary analyses and have the same pattern.
| Measure | Time point | All (n = 348) | SIPC (n = 175) | Standard care (n = 173) | p-valuea |
|---|---|---|---|---|---|
| Primary outcome | |||||
| IPOS Neuro-S8, x¯ (CI) | Baseline | 6.93 (6.45 to 7.40) | 6.83 (6.16 to 7.50) | 7.02 (6.39 to 7.65) | |
| 12 weeks | 6.41 (5.94 to 6.88) | 6.07 (5.42 to 6.71) | 6.76 (6.07 to 7.45) | ||
| Change score | –0.51 (–0.94 to –0.09) | –0.77 (–1.37 to –0.16) | –0.26 (–0.81 to 0.29) | 0.12 | |
| Secondary patient outcomesb | |||||
| IPOS Neuro-S24, x¯ (CI) | Baseline | 26.89 (25.64 to 28.13) | 26.61 (24.90 to 28.32) | 27.17 (25.48 to 28.86) | |
| 12 weeks | 25.45 (24.17 to 26.73) | 24.64 (23.00 to 26.28) | 26.27 (24.38 to 28.17) | ||
| Change score | –1.44 (–2.44 to –0.44) | –1.97 (–3.31 to –0.63) | –0.90 (–2.46 to 0.66) | 0.25 | |
| IPOS Neuro-S8, x¯ (CI) | Baseline | 11.48 (10.79 to 12.17) | 11.52 (10.55 to 12.49) | 11.44 (10.43 to 12.45) | |
| 12 weeks | 11.18 (10.44 to 11.93) | 10.72 (9.62 to 11.83) | 11.65 (10.68 to 12.61) | ||
| Change score | –0.30 (–0.95 to 0.35) | –0.80 (–1.72 to 0.12) | 0.21 (–0.66 to 1.08) | 0.11 | |
| IPOS Neuro, x¯ (CI) | Baseline | 47.51 (44.19 to 50.83) | 47.47 (43.09 to 51.86) | 47.55 (43.62 to 51.48) | |
| 12 weeks | 43.30 (39.92 to 46.67) | 42.53 (38.25 to 46.81) | 44.07 (39.70 to 48.44) | ||
| Change score | –4.21 (–7.54 to –0.89) | –4.94 (–9.39 to –0.49) | –3.48 (–7.95 to 0.99) | 0.59 | |
| HADS anxiety, x¯ (CI) | Baseline | 7.71 (7.22 to 8.20) | 7.81 (7.11 to 8.50) | 7.61 (6.93 to 8.30) | |
| 12 weeks | 7.62 (7.07 to 8.17) | 7.50 (6.70 to 8.30) | 7.75 (7.03 to 8.46) | ||
| Change score | –0.09 (–0.47 to 0.29) | –0.31 (–0.82 to 0.21) | 0.13 (–0.37 to 0.64) | 0.25 | |
| HADS depression, x¯ (CI) | Baseline | 8.23 (7.82 to 8.64) | 8.14 (7.55 to 8.72) | 8.33 (7.76 to 8.89) | |
| 12 weeks | 8.07 (7.59 to 8.54) | 7.91 (7.24 to 8.58) | 8.23 (7.60 to 8.86) | ||
| Change score | –0.16 (–0.51 to 0.19) | –0.23 (–0.71 to 0.25) | –0.09 (–0.58 to 0.39) | 0.57 | |
| EQ-5D VAS,c x¯ (CI) | Baseline | 52.76 (50.26 to 55.26) | 53.14 (49.73 to 56.55) | 52.37 (48.76 to 55.98) | |
| 12 weeks | 51.98 (49.13 to 54.83) | 53.52 (49.57 to 57.46) | 50.42 (46.56 to 54.28) | ||
| Change score | –0.78 (–3.88 to 2.32) | 0.37 (–4.17 to 4.92) | –1.95 (–6.11 to 2.20) | 0.33 | |
| SEMCD Scale,c x¯ (CI) | Baseline | 5.25 (5.00 to 5.50) | 5.37 (5.01 to 5.72) | 5.14 (4.77 to 5.50) | |
| 12 weeks | 5.13 (4.87 to 5.39) | 5.31 (4.95 to 5.66) | 4.95 (4.57 to 5.32) | ||
| Change score | –0.12 (–0.37 to 0.12) | –0.06 (–0.41 to 0.29) | –0.19 (–0.55 to 0.17) | 0.35 | |
| FAMCARE-P16,c x¯ (CI) | Baseline | 50.19 (48.48 to 51.91) | 50.20 (47.74 to 52.67) | 50.18 (47.78 to 52.59) | |
| 12 weeks | 47.73 (45.69 to 49.77) | 48.00 (45.14 to 50.85) | 47.45 (44.57 to 50.33) | ||
| Change score | –2.47 (–4.20 to –0.73) | –2.21 (–4.74 to 0.32) | –2.73 (–5.19 to –0.27) | 0.83 | |
| Secondary caregiver outcomesb | |||||
| ZBI-12, x¯ (CI) | Baseline | 18.48 (17.21 to 19.74) | 18.30 (16.69 to 19.90) | 18.66 (16.83 to 20.49) | |
| 12 weeks | 18.74 (17.34 to 20.15) | 18.58 (16.77 to 20.40) | 18.91 (16.96 to 20.86) | ||
| Change score | 0.27 (–0.40 to 0.93) | 0.28 (–0.60 to 1.17) | 0.25 (–0.67 to 1.16) | 0.95 | |
| ZBI positivity,c x¯ (CI) | Baseline | 18.88 (18.00 to 19.76) | 18.98 (17.85 to 20.11) | 18.77 (17.50 to 20.05) | |
| 12 weeks | 18.48 (17.54 to 19.43) | 18.83 (17.59 to 20.08) | 18.13 (16.85 to 19.41) | ||
| Change score | –0.39 (–0.96 to 0.18) | –0.15 (–1.01 to 0.71) | –0.64 (–1.49 to 0.20) | 0.34 | |
| FAMCARE 2,c x¯ (CI) | Baseline | 53.79 (51.91 to 55.67) | 54.05 (51.32 to 56.78) | 53.53 (50.83 to 56.23) | |
| 12 weeks | 53.32 (50.75 to 55.90) | 53.95 (50.68 to 57.23) | 52.68 (49.01 to 56.36) | ||
| Change score | –0.47 (–2.81 to 1.88) | –0.09 (–3.18 to 2.99) | –0.84 (–3.94 to 2.25) | 0.62 | |
Sensitivity analysis 3
Estimates and analyses for complete patient cases are presented in Table 7. The sample size for those patients who had complete data at both baseline and 12 weeks is reported for each outcome. These sample sizes vary, as different outcomes had different numbers of missing data. Similar to the primary analysis of effectiveness, there were no statistically significant differences between the trial arms for either the primary outcome or most of the secondary outcomes. The change scores for IPOS Neuro-8 (non-symptom items) were significantly different between the trial arms (p = 0.001), with a reduction seen in the SIPC group and an increase in the standard care group. Furthermore, there was a similar pattern of greater favourable change scores in the SIPC group than in the standard care group on most outcomes.
| Measure | Time point | All | SIPC | Standard care | p-valuea |
|---|---|---|---|---|---|
| Primary outcome | |||||
| IPOS Neuro-S8, x¯ (CI) (n = 270) | Baseline | 6.85 (6.38 to 7.32) | 6.68 (6.04 to 7.33) | 7.01 (6.34 to 7.69) | |
| 12 weeks | 6.31 (5.85 to 6.78) | 5.90 (5.28 to 6.52) | 6.72 (6.01 to 7.42) | ||
| Change score | –0.54 (–0.91 to –0.16) | –0.78 (–1.30 to –0.27) | –0.30 (–0.84 to 0.24) | 0.09 | |
| Secondary patient outcomesb | |||||
| IPOS Neuro-S24, x¯ (CI) (n = 235) | Baseline | 26.58 (24.68 to 28.48) | 25.57 (22.94 to 28.20) | 27.57 (24.80 to 30.35) | |
| 12 weeks | 25.0 (23.03 to 26.96) | 23.22 (20.70 to 25.74) | 26.73 (23.75 to 29.72) | ||
| Change score | –1.59 (–3.06 to –0.17) | –2.35 (–4.00 to –0.70) | –0.84 (–3.28 to 1.60) | 0.032 | |
| IPOS Neuro, x¯ (CI) (n = 79) | Baseline | 47.32 (41.35 to 53.28) | 42.22 (34.53 to 49.92) | 51.58 (42.70 to 60.46) | |
| 12 weeks | 42.76 (36.21 to 49.31) | 37.86 (28.88 to 46.85) | 46.86 (37.31 to 56.41) | ||
| Change score | –4.56 (–8.42 to –0.70) | –4.36 (–8.45 to –0.27) | –4.72 (–11.21 to 1.76) | 0.80 | |
| IPOS Neuro-8, x¯ (CI) (n = 246) | Baseline | 11.07 (9.98 to 12.16) | 10.78 (9.36 to 12.20) | 11.34 (9.66 to 13.01) | |
| 12 weeks | 10.70 (9.62 to 11.77) | 9.56 (8.11 to 11.01) | 11.74 (10.17 to 13.31) | ||
| Change score | –0.37 (–1.30 to 0.55) | –1.22 (–2.47 to 0.03) | 0.41 (–0.95 to 1.77) | 0.001 | |
| HADS anxiety, x¯ (CI) (n = 275) | Baseline | 7.45 (6.72 to 8.19) | 7.39 (6.35 to 8.44) | 7.51 (6.49 to 8.56) | |
| 12 weeks | 7.33 (6.57 to 8.09) | 6.95 (5.82 to 8.09) | 7.67 (6.64 to 8.70) | ||
| Change score | –0.13 (–0.64 to 0.39) | –0.44 (–1.18 to 0.30) | 0.16 (–0.56 to 0.88) | 0.07 | |
| HADS depression, x¯ (CI) (n = 275) | Baseline | 8.13 (7.51 to 8.74) | 7.89 (6.99 to 8.79) | 8.35 (7.49 to 9.21) | |
| 12 weeks | 7.96 (7.32 to 8.60) | 7.62 (6.65 to 8.60) | 8.27 (7.42 to 9.13) | ||
| Change score | –0.17 (–0.64 to 0.31) | –0.27 (–0.92 to 0.39) | –0.08 (–0.78 to 0.63) | 0.33 | |
| EQ-5D VAS,c x¯ (CI) (n = 281) | Baseline | 53.14 (49.42 to 56.86) | 54.13 (49.03 to 59.23) | 52.18 (46.68 to 57.68) | |
| 12 weeks | 52.43 (48.71 to 56.16) | 54.72 (49.34 to 60.10) | 50.20 (44.99 to 55.41) | ||
| Change score | –0.71 (–5.05 to 3.63) | 0.59 (–5.63 to 6.81) | –1.98 (–8.15 to 4.20) | 0.11 | |
| SEMCD Scale,c x¯ (CI) (n = 274) | Baseline | 5.34 (4.95 to 5.73) | 5.56 (5.03 to 6.10) | 5.12 (4.55 to 5.69) | |
| 12 weeks | 5.22 (4.84 to 5.59) | 5.50 (4.99 to 6.01) | 4.93 (4.39 to 5.47) | ||
| Change score | –0.13 (–0.47 to 0.22) | –0.06 (–0.54 to 0.42) | –0.19 (–0.69 to 0.31) | 0.12 | |
| FAMCARE-P16,c x¯ (CI) (n = 193) | Baseline | 53.11 (50.38 to 55.84) | 54.05 (50.01 to 58.09) | 52.18 (48.40 to 55.95) | |
| 12 weeks | 52.79 (49.89 to 55.69) | 54.82 (50.70 to 58.95) | 50.77 (46.66 to 54.88) | ||
| Change score | –0.32 (–3.16 to 2.51) | 0.77 (–3.66 to 5.20) | –1.40 (–5.05 to 2.25) | 0.08 | |
Sensitivity analysis 4
Data from caregivers with complete data at both baseline and 12 weeks are presented in Table 8. Results are available only for measures completed by caregivers. Again, there were no statistically significant differences between the trial arms, but there was a pattern of greater, favourable change scores in the SIPC group than in the standard care group on most outcomes.
| Measure | Time point | All | SIPC | Standard care | p-valuea |
|---|---|---|---|---|---|
| Primary outcome | |||||
| IPOS Neuro-S8, x¯ (CI) (n = 175) | Baseline | 7.19 (6.64 to 7.75) | 7.37 (6.58 to 8.15) | 7.01 (6.22 to 7.80) | |
| 12 weeks | 6.20 (5.62 to 6.78) | 6.14 (5.29 to 7.00) | 6.26 (5.45 to 7.07) | ||
| Change score | –0.99 (–1.46 to –0.53) | –1.22 (–1.89 to –0.55) | –0.75 (–1.39 to –0.11) | 0.40 | |
| Secondary patient outcomesb | |||||
| IPOS Neuro-S24, x¯ (CI) (n = 146) | Baseline | 30.96 (28.65 to 33.27) | 30.29 (27.18 to 33.40) | 31.75 (28.16 to 35.33) | |
| 12 weeks | 27.42 (24.74 to 30.10) | 26.30 (22.30 to 30.30) | 28.73 (25.16 to 32.31) | ||
| Change score | –3.54 (–5.51 to –1.57) | –3.99 (–6.80 to –1.18) | –3.01 (–5.85 to –0.18) | 0.34 | |
| IPOS Neuro, x¯ (CI) (n = 73) | Baseline | 55.14 (48.77 to 61.50) | 55.98 (47.06 to 64.89) | 54.06 (44.19 to 63.94) | |
| 12 weeks | 48.79 (42.10 to 55.49) | 49.34 (39.70 to 58.98) | 48.09 (38.12 to 58.07) | ||
| Change score | –6.34 (–10.22 to –2.47) | –6.63 (–12.63 to –0.64) | –5.97 (–10.99 to –0.95) | 0.87 | |
| IPOS Neuro-8, x¯ (CI) (n = 178) | Baseline | 12.75 (11.62 to 13.87) | 12.88 (11.26 to 14.50) | 12.61 (11.00 to 14.22) | |
| 12 weeks | 11.40 (10.17 to 12.63) | 10.73 (9.01 to 12.44) | 12.11 (10.32 to 13.91) | ||
| Change score | –1.34 (–2.31 to –0.38) | –2.15 (–3.51 to –0.80) | –0.49 (–1.86 to 0.87) | 0.011 | |
| Secondary caregiver outcomesb | |||||
| ZBI-12, x¯ (CI) (n = 193) | Baseline | 18.95 (17.04 to 20.87) | 18.62 (15.87 to 21.38) | 19.29 (16.56 to 22.03) | |
| 12 weeks | 19.31 (17.28 to 21.34) | 19.11 (16.15 to 22.08) | 19.52 (16.67 to 22.36) | ||
| Change score | 0.36 (–0.66 to 1.37) | 0.49 (–0.87 to 1.85) | 0.22 (–1.33 to 1.77) | 0.76 | |
| ZBI positivity,c x¯ (CI) (n = 193) | Baseline | 19.03 (17.70 to 20.36) | 19.34 (17.46 to 21.22) | 18.70 (16.76 to 20.64) | |
| 12 weeks | 18.63 (17.25 to 20.01) | 19.37 (17.34 to 21.40) | 17.83 (15.94 to 19.72) | ||
| Change score | –0.40 (–1.42 to 0.62) | 0.03 (–1.43 to 1.49) | –0.87 (–2.32 to 0.58) | 0.11 | |
| FAMCARE 2,c x¯ (CI) (n = 140) | Baseline | 58.29 (55.32 to 61.27) | 60.09 (55.81 to 64.37) | 56.22 (52.06 to 60.37) | |
| 12 weeks | 58.54 (55.52 to 61.57) | 60.21 (55.97 to 64.45) | 56.62 (52.22 to 61.01) | ||
| Change score | 0.25 (–2.81 to 3.31) | 0.12 (–4.50 to 4.74) | 0.40 (–3.70 to 4.50) | 0.34 | |
Sensitivity analysis 5
The fifth sensitivity analysis, in which complete patient data were used, when available, at both baseline and 12 weeks, and proxy data were imputed if patient data were missing but caregiver data were complete and available at both time points, is presented in Table 9. The results remain the same. As seen in sensitivity analysis 3, only the between-group change score for IPOS Neuro 8 reached statistical significance for two-group comparisons (p = 0.003).
| Measure | Time point | All | SIPC | Standard care | p-valuea |
|---|---|---|---|---|---|
| Primary outcome | |||||
| IPOS Neuro-S8, x¯ (CI) (n = 308) | Baseline | 6.81 (6.38 to 7.25) | 6.79 (6.18 to 7.40) | 6.83 (6.20 to 7.46) | |
| 12 weeks | 6.17 (5.74 to 6.61) | 5.84 (5.26 to 6.43) | 6.50 (5.85 to 7.15) | ||
| Change score | –0.64 (–0.99 to –0.29) | –0.95 (–1.44 to –0.46) | –0.33 (–0.83 to 0.17) | 0.05 | |
| Secondary outcomesb | |||||
| IPOS Neuro-S24, x¯ (CI) (n = 278) | Baseline | 27.74 (25.97 to 29.51) | 27.11 (24.57 to 29.64) | 28.37 (25.85 to 30.89) | |
| 12 weeks | 25.85 (24.03 to 27.68) | 24.53 (22.02 to 27.04) | 27.17 (24.50 to 29.84) | ||
| Change score | –1.88 (–3.22 to –0.55) | –2.58 (–4.14 to –1.01) | –1.19 (–3.39 to 1.00) | 0.050 | |
| IPOS Neuro, x¯ (CI) (n = 120) | Baseline | 51.19 (46.28 to 56.10) | 51.11 (43.86 to 58.37) | 51.27 (44.32 to 58.22) | |
| 12 weeks | 46.66 (41.37 to 51.95) | 46.33 (38.51 to 54.15) | 47.00 (39.51 to 54.49) | ||
| Change score | –4.53 (–7.67 to –1.40) | –4.79 (–8.85 to –0.72) | –4.27 (–9.26 to 0.72) | 0.80 | |
| IPOS Neuro-8, x¯ (CI) (n = 290) | Baseline | 11.50 (10.50 to 12.51) | 11.51 (10.15 to 12.87) | 11.50 (9.99 to 13.00) | |
| 12 weeks | 10.91 (9.91 to 11.91) | 10.12 (8.71 to 11.52) | 11.68 (10.27 to 13.09) | ||
| Change score | –0.59 (–1.44 to 0.26) | –1.39 (–2.57 to –0.21) | 0.18 (–1.03 to 1.40) | 0.003 | |
Sensitivity analysis 6
The final sensitivity analysis, using multiply imputed data from patients with MS only, is presented in Table 10. The results are consistent with previous analyses.
| Measure | Time point | All (n = 148) | SIPC (n = 74) | Standard care (n = 74) | p-valuea |
|---|---|---|---|---|---|
| Primary outcome | |||||
| IPOS Neuro-S8, x¯ (CI) | Baseline | 7.09 (6.42 to 7.75) | 7.08 (6.07 to 8.09) | 7.10 (6.24 to 7.96) | |
| 12 weeks | 6.30 (5.56 to 7.05) | 6.25 (5.19 to 7.31) | 6.35 (5.32 to 7.38) | ||
| Change score | –0.79 (–1.33 to –0.24) | –0.83 (–1.66 to 0.00) | –0.75 (–1.49 to 0.00) | 0.88 | |
| Secondary patient outcomesb | |||||
| IPOS Neuro-S24, x¯ (CI) | Baseline | 27.67 (24.85 to 30.50) | 27.61 (23.51 to 31.71) | 27.74 (24.18 to 31.31) | |
| 12 weeks | 24.84 (21.71 to 27.97) | 24.70 (20.63 to 28.76) | 24.98 (20.53 to 29.43) | ||
| Change score | –2.84 (–5.63 to –0.04) | –2.91 (–6.41 to 0.59) | –2.76 (–6.63 to 1.11) | 0.90 | |
| IPOS Neuro-8, x¯ (CI) | Baseline | 11.07 (9.32 to 12.82) | 10.75 (8.25 to 13.26) | 11.38 (8.97 to 13.80) | |
| 12 weeks | 10.30 (8.65 to 11.96) | 9.69 (7.32 to 12.06) | 10.91 (8.54 to 13.29) | ||
| Change score | –0.77 (–2.26 to 0.73) | –1.06 (–3.20 to 1.07) | –0.47 (–2.35 to 1.41) | 0.37 | |
| IPOS Neuro, x¯ (CI) | Baseline | 47.51 (38.16 to 56.87) | 48.13 (36.51 to 59.75) | 46.90 (36.76 to 57.04) | |
| 12 weeks | 43.69 (35.38 to 52.00) | 43.56 (31.56 to 55.56) | 43.83 (34.14 to 53.51) | ||
| Change score | –3.82 (–12.08 to 4.43) | –4.58 (–14.59 to 5.44) | –3.07 (–14.09 to 7.95) | 0.79 | |
| HADS anxiety, x¯ (CI) | Baseline | 7.26 (6.12 to 8.40) | 7.48 (5.88 to 9.07) | 7.04 (5.43 to 8.66) | |
| 12 weeks | 6.88 (5.72 to 8.04) | 6.78 (5.07 to 8.49) | 6.98 (5.41 to 8.54) | ||
| Change score | –0.38 (–1.17 to 0.41) | –0.70 (–1.79 to 0.40) | –0.07 (–1.15 to 1.02) | 0.25 | |
| HADS depression, x¯ (CI) | Baseline | 8.19 (7.22 to 9.17) | 8.38 (6.95 to 9.81) | 8.01 (6.70 to 9.32) | |
| 12 weeks | 7.89 (6.91 to 8.88) | 7.77 (6.27 to 9.26) | 8.01 (6.74 to 9.29) | ||
| Change score | –0.30 (–1.05 to 0.45) | –0.61 (–1.51 to 0.29) | 0.00 (–1.17 to 1.17) | 0.28 | |
| EQ-5D VAS, x¯ (CI) | Baseline | 54.00 (48.36 to 59.64) | 53.32 (45.42 to 61.23) | 54.68 (46.44 to 62.92) | |
| 12 weeks | 52.81 (45.92 to 59.69) | 55.25 (45.87 to 64.63) | 50.36 (41.04 to 59.69) | ||
| Change score | –1.19 (–8.79 to 6.40) | 1.93 (–8.58 to 12.44) | –4.32 (–14.54 to 5.91) | 0.21 | |
| SEMCD Scale, x¯ (CI) | Baseline | 5.54 (4.96 to 6.11) | 5.50 (4.65 to 6.34) | 5.58 (4.79 to 6.37) | |
| 12 weeks | 5.54 (4.95 to 6.12) | 5.65 (4.82 to 6.49) | 5.42 (4.61 to 6.23) | ||
| Change score | 0.00 (–0.50 to 0.50) | 0.16 (–0.55 to 0.86) | –0.16 (–0.89 to 0.57) | 0.38 | |
| FAMCARE-P16, x¯ (CI) | Baseline | 45.93 (41.92 to 49.94) | 45.59 (39.43 to 51.75) | 46.27 (41.05 to 51.50) | |
| 12 weeks | 44.97 (40.36 to 49.57) | 45.82 (39.30 to 52.34) | 44.11 (37.83 to 50.39) | ||
| Change score | –0.96 (–4.85 to 2.93) | 0.23 (–5.27 to 5.73) | –2.16 (–7.43 to 3.11) | 0.37 | |
| Secondary caregiver outcomesb | |||||
| ZBI-12, x¯ (CI) | Baseline | 18.58 (15.56 to 21.61) | 18.47 (14.09 to 22.86) | 18.69 (14.95 to 22.44) | |
| 12 weeks | 18.45 (15.18 to 21.72) | 18.62 (13.94 to 23.30) | 18.28 (14.24 to 22.32) | ||
| Change score | –0.13 (–1.70 to 1.43) | 0.15 (–1.71 to 2.00) | –0.42 (–2.75 to 1.91) | 0.58 | |
| ZBI positivity, x¯ (CI) | Baseline | 19.02 (17.04 to 21.00) | 19.26 (16.49 to 22.04) | 18.77 (16.18 to 21.36) | |
| 12 weeks | 17.95 (15.81 to 20.08) | 18.37 (15.37 to 21.38) | 17.52 (14.85 to 20.19) | ||
| Change score | –1.07 (–2.61 to 0.46) | –0.89 (–2.66 to 0.87) | –1.25 (–3.58 to 1.08) | 0.61 | |
| FAMCARE 2, x¯ (CI) | Baseline | 52.89 (47.95 to 57.83) | 52.94 (46.34 to 59.54) | 52.83 (46.40 to 59.26) | |
| 12 weeks | 49.22 (42.51 to 55.93) | 48.98 (41.22 to 56.75) | 49.45 (40.61 to 58.30) | ||
| Change score | –3.67 (–9.96 to 2.63) | –3.96 (–11.06 to 3.14) | –3.38 (–11.50 to 4.74) | 0.87 | |
Adverse events and attrition
Attrition was summarised using the MORECare classification of attrition due to death, attrition due to illness or attrition at random. The levels of attrition up to the primary end point of 12 weeks were similar in the SIPC group to that of the standard care group (attrition due to death = 5 vs. 3; attrition due to illness = 1 vs. 1; and attrition at random = 7 vs. 6). Within the attrition at random group, reasons for withdrawal included no longer wanting to participate, caregiver/family decision to withdraw and those lost to contact. As presented in Table 11, there were no significant differences in rates of serious adverse events or survival, up to 12 weeks, between the trial arms.
| Serious adverse events | All | SIPC | Standard care | Significance testing |
|---|---|---|---|---|
| Deaths, n | 8 | 5 | 3 | χ2 (1, 350) = 0.49; p = 0.49 |
| Hospitalisation, n | 25 | 13 | 12 | χ2 (1, 350) = 0.03; p = 0.86 |
| Emergency attendance, n | 7 | 2 | 5 | χ2 (1, 350) = 1.35; p = 0.25 |
| Survival in weeks | ||||
| Mean (SD) | 11.7 (1.4) | 11.6 (1.6) | 11.8 (1.2) | t (348) = –1.09; p = 0.28 |
| Range | 0.1–12 | 0.6–12 | 0.1–12 | |
Health economics
Service use
Service use and associated costs per user for the 12 weeks prior to randomisation and the 12 weeks post randomisation are presented in Table 12. The number of people using each different category of service or care and the average costs per user (not per participant in that trial arm) for that category were counted and calculated. At baseline, 40 and 36 participants had used inpatient care in the previous 12 weeks in the SIPC and standard care groups, respectively. By 12 weeks, this had fallen in both groups, to 18 participants in the SIPC group and 25 participants in the standard care group. Outpatient care use also decreased from baseline to 12 weeks in both groups. Changes in use of primary care services were not different between the trial arms and the average cost did not change. The number of patients using palliative care services increased from baseline to 12 weeks in the SIPC group, whereas there was no change in the standard care group. At 12 weeks, fewer patients in the SIPC group used inpatient care and more patients used palliative care services than those in the standard care group. However, a small number of participants in the standard care arm reported receiving palliative care services, which remained unchanged from baseline to 12 weeks. Informal care costs (and the amount of care provided by lay carers and family members) remained broadly similar over the 12 weeks; there was a small reduction in both study arms, with a slightly greater reduction in the SIPC arm, but CIs were wide.
| Measure | Time point | All | SIPC | Standard care | p-valuea | |||
|---|---|---|---|---|---|---|---|---|
| n | x¯ (95% CI) | n | x¯ (95% CI) | n | x¯ (95% CI) | |||
| Inpatient care | Baseline | 76 | 7840 (6649 to 9032) | 40 | 7336 (5904 to 8768) | 36 | 8401 (6391 to 10,411) | |
| 12 weeks | 43 | 7681 (6406 to 8956) | 18 | 7890 (5746 to 10,035) | 25 | 7531 (5839 to 9223) | ||
| Outpatient care | Baseline | 263 | 140 (123 to 157) | 132 | 146 (120 to 170) | 131 | 134 (112 to 157) | |
| 12 weeks | 187 | 157 (126 to 188) | 101 | 148 (108 to 188) | 86 | 168 (118 to 217) | ||
| Day or community care | Baseline | 40 | 941 (682 to 1199) | 22 | 955 (601 to 1310) | 18 | 923 (504 to 1342) | |
| 12 weeks | 47 | 868 (599 to 1138) | 24 | 814 (438 to 1189) | 23 | 925 (508 to 1341) | ||
| Home care | Baseline | 172 | 150 (114 to 186) | 83 | 170 (102 to 238) | 89 | 132 (100 to 163) | |
| 12 weeks | 157 | 142 (104 to 181) | 79 | 144 (80 to 208) | 78 | 140 (95 to 185) | ||
| Palliative care | Baseline | 13 | 196 (–58 to 449) | 5 | 39 (1 to 76) | 8 | 294 (–142 to 730) | |
| 12 weeks | 34 | 436 (283 to 589) | 26 | 415 (253 to 578) | 8 | 503 (32 to 975) | ||
| Rehabilitation | Baseline | 85 | 999 (276 to 1722) | 44 | 1535 (206 to 2864) | 41 | 424 (–63 to 911) | |
| 12 weeks | 68 | 876 (22 to 1730) | 35 | 321 (130 to 512) | 33 | 1465 (–310 to 3239) | ||
| Primary care | Baseline | 184 | 96 (84 to 108) | 94 | 96 (81 to 112) | 90 | 95 (76 to 114) | |
| 12 weeks | 144 | 93 (81 to 105) | 69 | 98 (80 to 116) | 75 | 88 (73 to 103) | ||
| Social care | Baseline | 133 | 980 (644 to 1317) | 60 | 859 (592 to 1126) | 73 | 1080 (501 to 1660) | |
| 12 weeks | 125 | 775 (632 to 918) | 60 | 775 (579 to 971) | 65 | 775 (562 to 988) | ||
| Tests and diagnostics | Baseline | 155 | 131 (90 to 171) | 83 | 114 (77 to 151) | 72 | 150 (73 to 227) | |
| 12 weeks | 120 | 81 (57 to 105) | 56 | 61 (31 to 91) | 64 | 98 (62 to 135) | ||
| Health and social careb | Baseline | 302 | 3152 (2552 to 3752) | 150 | 3237 (2357 to 4117) | 152 | 3068 (2241 to 3894) | |
| 12 weeks | 265 | 2329 (1817 to 2841) | 132 | 2020 (1451 to 2588) | 133 | 2636 (1781 to 3491) | ||
| Informal care | Baseline | 280 | 1061 (914 to 1209) | 137 | 938 (735 to 1,140) | 143 | 1180 (966 to 1394) | |
| 12 weeks | 227 | 933 (796 to 1069) | 108 | 826 (634 to 1,017) | 119 | 1030 (835 to 1225) | ||
| Total carec | Baseline | 309 | 4042 (3433 to 4652) | 155 | 3961 (3061 to 4862) | 154 | 4123 (3293 to 4954) | |
| 12 weeks | 278 | 2982 (2482 to 3481) | 138 | 2578 (2020 to 3136) | 140 | 3380 (2552 to 4207) | ||
| Change in health and social care | Observed | 257 | –828 (–1475 to –180) | 126 | –1170 (–2035 to –306) | 131 | –498 (–1466 to 470) | 0.17 |
| Change in total care | Observed | 274 | –929 (–1561 to –296) | 135 | –1185 (–2044 to –326) | 139 | –680 (–1617 to 257) | 0.11 |
Costs
To better understand the overall health and social care costs, for each category, we calculated average costs across all participants. Table 13 shows the average costs of each service for all trial participants, by trial arm. The average cost of inpatient care at 12 weeks was £899 (95% CI £445 to £1353) in the SIPC group and £1169 (95% CI £677 to £1662) in the standard care group. The reduction in inpatient care costs from baseline to 12 weeks was larger in the SIPC group (–£768) than in the standard care group (–£569). The SIPC arm has lower costs overall, mainly as a result of fewer participants being cared for within inpatient services. The increase in palliative care costs from baseline to 12 weeks was more substantial in the SIPC group (from £1 to £68) than in the standard care group (from £14 to £25).
| Measure | Time point | All | SIPC | Standard care | p-valuea | |||
|---|---|---|---|---|---|---|---|---|
| n | x¯ (95% CI) | n | x¯ (95% CI) | n | x¯ (95% CI) | |||
| Inpatient care | Baseline | 350 | 1702 (1278 to 2127) | 176 | 1667 (1111 to 2223) | 174 | 1738 (1090 to 2387) | |
| 12 weeks | 319 | 1035 (702 to 1369) | 158 | 899 (445 to 1353) | 161 | 1169 (677 to 1662) | ||
| Outpatient care | Baseline | 350 | 105 (91 to 120) | 176 | 109 (88 to 131) | 174 | 101 (82 to 120) | |
| 12 weeks | 319 | 92 (72 to 112) | 158 | 95 (67 to 123) | 161 | 90 (60 to 119) | ||
| Day or community care | Baseline | 350 | 108 (65 to 150) | 176 | 119 (57 to 182) | 174 | 95 (38 to 153) | |
| 12 weeks | 319 | 128 (77 to 179) | 158 | 124 (53 to 194) | 161 | 132 (57 to 207) | ||
| Home care | Baseline | 350 | 74 (54 to 93) | 176 | 80 (46 to 114) | 174 | 67 (48 to 86) | |
| 12 weeks | 319 | 70 (50 to 90) | 158 | 72 (39 to 106) | 161 | 68 (44 to 92) | ||
| Palliative care | Baseline | 350 | 7 (–2 to 16) | 176 | 1 (0 to 2) | 174 | 14 (–5 to 32) | |
| 12 weeks | 319 | 46 (25 to 68) | 158 | 68 (33 to 103) | 161 | 25 (0 to 50) | ||
| Rehabilitation | Baseline | 350 | 243 (64 to 421) | 176 | 384 (47 to 721) | 174 | 100 (–14 to 214) | |
| 12 weeks | 319 | 187 (4 to 369) | 158 | 71 (25 to 117) | 161 | 300 (–60 to 661) | ||
| Primary care | Baseline | 350 | 50 (42 to 58) | 176 | 51 (40 to 62) | 174 | 49 (37 to 61) | |
| 12 weeks | 319 | 42 (35 to 49) | 158 | 43 (32 to 54) | 161 | 41 (31 to 51) | ||
| Social care | Baseline | 350 | 373 (236 to 509) | 176 | 293 (185 to 401) | 174 | 453 (200 to 706) | |
| 12 weeks | 319 | 304 (234 to 373) | 158 | 294 (200 to 388) | 161 | 313 (210 to 416) | ||
| Test and diagnostic | Baseline | 350 | 58 (39 to 77) | 176 | 54 (34 to 73) | 174 | 62 (29 to 95) | |
| 12 weeks | 319 | 30 (21 to 40) | 158 | 22 (10 to 33) | 161 | 39 (23 to 55) | ||
| Health and social careb | Baseline | 350 | 2720 (2190 to 3249) | 176 | 2759 (1991 to 3527) | 174 | 2680 (1942 to 3417) | |
| 12 weeks | 319 | 1935 (1499 to 2370) | 158 | 1687 (1198 to 2176) | 161 | 2177 (1456 to 2899) | ||
| Informal care | Baseline | 350 | 849 (723 to 975) | 176 | 730 (562 to 898) | 174 | 970 (782 to 1158) | |
| 12 weeks | 319 | 664 (556 to 771) | 158 | 564 (421 to 708) | 161 | 761 (601 to 921) | ||
| Total carec | Baseline | 350 | 3569 (3014 to 4123) | 176 | 3489 (2674 to 4304) | 174 | 3650 (2889 to 4410) | |
| 12 weeks | 319 | 2598 (2150 to 3047) | 158 | 2252 (1747 to 2757) | 161 | 2939 (2199 to 3687) | ||
| Change in health and social care | Imputed | 350 | –797 (–1424 to –169) | 176 | –1076 (–1929 to –222) | 174 | –514 (–1448 to 419) | 0.12 |
| Change in total care | Imputed | 350 | –1081 (–1726 to –435) | 176 | –1350 (–2231 to –470) | 174 | –808 (–1746 to 131) | 0.12 |
Taking the results for all data together, there was a decrease in overall health and social care costs (inpatient, community, outpatient, home, palliative, rehabilitation, primary, social care, and tests and diagnostics) from baseline to 12 weeks of –£1076 in the SIPC group and –£514 in the standard care group. The resulting difference across study arms was –£562. This difference is mainly driven by the differences in inpatient care use. Informal care costs were similar between study arms. Correspondingly, total care costs (health and social care plus informal care) also decreased from baseline to 12 weeks by –£1350 in the SIPC group and –£808 in the standard care group, with a resulting difference of –£542 between groups. Overall, it was less expensive to provide care for the SIPC group than to provide standard care. The CIs are wide and individual costs varied greatly. No significant between-group differences were observed for either the change in health and social care costs (p = 0.12) or the change in total care costs (p = 0.12).
Outcomes
Two outcome measures were used in the economic evaluation: IPOS Neuro-S8 (as presented previously) and EQ-5D index score (or QALY). The mean EQ-5D index score at baseline was 0.27 (95% CI 0.22 to 0.32) in the SIPC group and 0.26 (95% CI 0.21 to 0.31) in the standard care group. At 12 weeks, the mean scores were 0.26 (95% CI 0.21 to 0.32) and 0.23 (95% CI 0.18 to 0.28), respectively. The respective change scores were –0.01 (95% CI –0.05 to 0.03) and –0.03 (95% CI –0.07 to 0.00), indicating that deterioration in quality of life was greater in the standard care group than in the SIPC group. The difference in QALY measured by EQ-5D was not significant between the two groups (p = 0.26).
Cost-effectiveness analysis
Incremental cost-effectiveness ratios were calculated for those for whom both cost data and the outcome measures were available. ICERs were calculated as the ratio of differences in health and social care cost and outcomes (rescaled IPOS Neuro-S8 and EQ-5D index score). Using health and social care cost, in other words, when taking an NHS perspective, the change in cost in the SIPC group between baseline and 12 weeks was –£1076, compared with –£514 in the standard care group, leading to a –£562 between-group difference. For the rescaled IPOS Neuro-S8, the change scores were 0.75 and 0.38, respectively, resulting in a between-group difference of 0.37. For the EQ-5D index score (QALY), as presented above, the respective change scores were –0.01 and –0.03, with a resulting between-group difference of 0.02.
As shown in Table 14, the ICER for IPOS Neuro-S8 was –£1519. As the IPOS Neuro-S8 was rescaled, higher scores correspond to lower symptom burden. The ICER suggests that an improvement of 1 unit in IPOS Neuro-S8 is associated with an on average per patient decrease in health and social care costs of –£1519 compared with standard care. The ICER for EQ-5D index score was –£23,545. With higher scores indicating better quality of life, an improvement of 1 unit (QALY) was less costly in the SIPC group than in the standard care group by £23,545.
| Changes in outcomes and cost between baseline and follow-up at 12 weeks | ICER (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| Δ EQ-5D index score | Δ Rescaled IPOS Neuro-S8 | Δ Cost (£) | EQ-5D index score | IPOS Neuro-S8 | ||||
| SIPC | Standard care | SIPC | Standard care | SIPC | Standard care | |||
| Imputed data (n = 350) | –0.01 | –0.03 | 0.75 | 0.38 | –1076 | –514 | –23,545 | –1519 |
The ICER for health and social care cost and EQ-5D index score was well below our pre-determined threshold of £20,000. Sensitivity analyses conducted in line with the main effectiveness analyses produced consistent results.
Bootstrapping was used to obtain 1000 replications of the ICERs. As presented in Figure 2, when bootstrapped differences in EQ-5D index score and health and social care costs were plotted on cost-effectiveness planes, 74% of replications were found in the fourth quadrant and with rescaled IPOS Neuro-S8 as the outcome measure, 84% of replications were in the fourth quadrant (which represents better outcomes and lower costs in the intervention group than in the standard care group).
FIGURE 2.
Cost-effectiveness planes of outcome measures (a) EQ-5D index score (QALY); and (b) rescaled IPOS Neuro-S8, and health and social care cost.
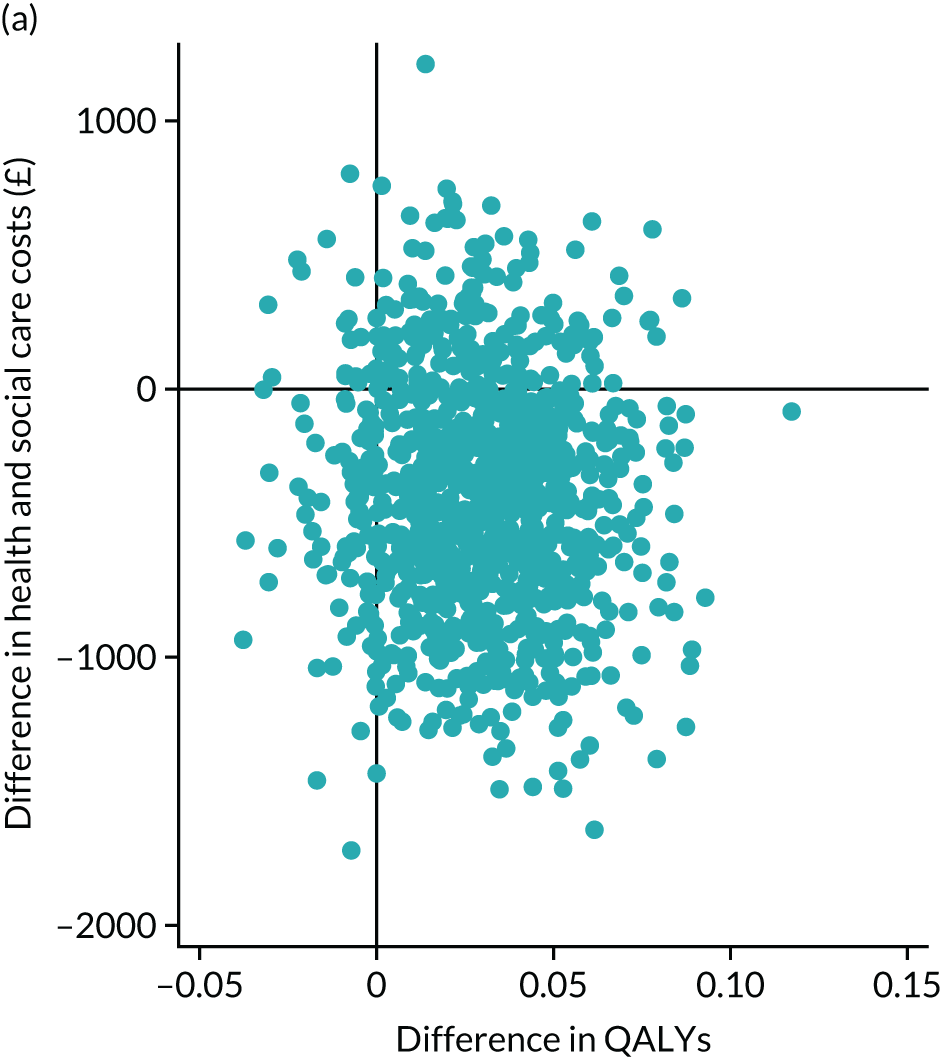
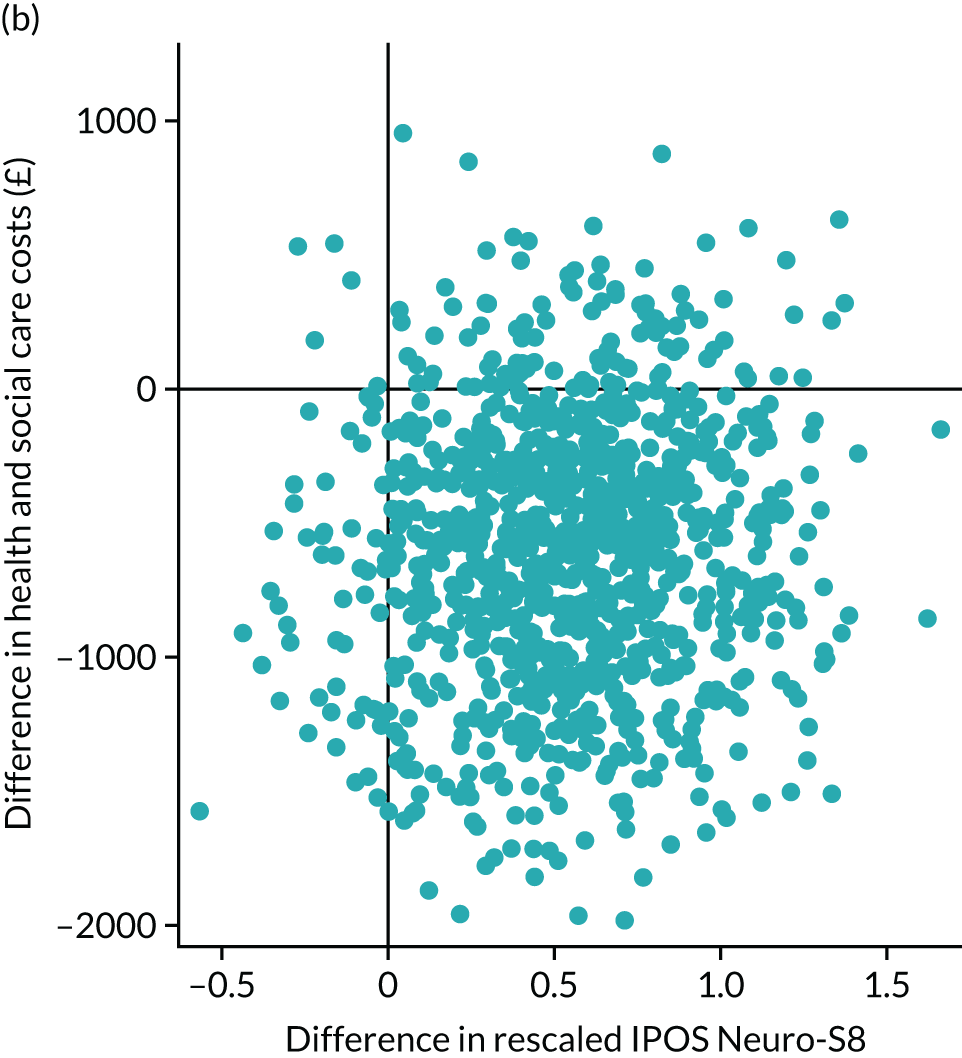
Qualitative findings
Patient and caregiver participants
Twenty-six interviews were carried out with 26 patients and 16 caregivers from three trial centres (London, Brighton and Ashford). Interviews lasted a median of 43 minutes (range 20–93 minutes). Participant demographics are detailed in Table 15. The majority of the interviews were conducted with patients with MS (69%). Most had lived with their condition for a considerable time (mean 13.7 years since diagnosis, SD 10.5 years). Caregivers tended to be younger than the patients (caregivers’ mean age 58.9 years; patients’ mean age 63.5 years), were mainly women (62.5%) and were a spouse or partner (68.8%). Participant characteristics for caregivers who were interviewed are presented in full in Appendix 4.
| Variable | Value | Interviewed patients (N = 26) |
|---|---|---|
| Age (years), mean (SD) | 63.5 (13.5) | |
| Gender, n (%) | Man | 14 (53.8) |
| Woman | 12 (46.2) | |
| Diagnosis, n (%) | MS | 18 (69.2) |
| IPD | 6 (23.1) | |
| PSP | 2 (7.8) | |
| Years since diagnosis | ||
| Mean (SD) | 13.7 (10.5) | |
| Range | 0–38 | |
| Comorbidities, n (%) | No | 5 (19.2) |
| Yes | 21 (80.8) | |
| Patient capacity, n (%) | Consent | 24 (92.3) |
| Personal consultee assent | 2 (7.7) | |
| Baseline IPOS Neuro-S8 | ||
| Mean (SD) | 8.2 (4.1) | |
| Range | 2–17 | |
| Living status, n (%) | Alone | 6 (23.1) |
| With spouse/partner and/or children | 16 (61.5) | |
| With friend(s)/with others | 4 (15.4) | |
| Ethnicity, n (%) | White | 23 (88.5) |
| Other ethnic group | 3 (11.5) | |
| Employment, n (%) | No | 25 (96.2) |
| Yes | 1 (3.8) | |
Health-care participants
Focus groups were conducted with health-care staff involved in delivering SIPC in six of the study centres. Groups comprised six palliative care teams and one neurology team. Additionally, three individual interviews were conducted with members of neurology teams. A total of 43 health-care providers participated. Palliative care team members included consultants in palliative medicine, clinical nurse specialists, occupational therapists, clinical service managers, a chaplain and an administrator. Neurology team members included consultants in neurology and disease-specific clinical nurse specialists.
Overarching themes on the value and impact of short-term integrated palliative care
The value and impact of SIPC, and linkage with key components for delivery, is encompassed in three overarching themes: (1) adapting to losses and building resilience, (2) attending to function, deficits and maintaining stability and (3) enabling caregivers to care. Overall, the themes illustrate the complexity of living with a progressive neurological condition, the daily work of patients and caregivers to accommodate ongoing losses and to adapt to maintain stability in function, and the optimal management of disease and symptoms. The strategies used were typically honed over many years. There were rarely ‘quick fixes’. What was required was attention to the multiple domains of health and interaction between domains, person-centred care to understand priorities and how to enable pursuit, and integrated working across health care to optimise continuity of care and treatment and minimise duplication.
Outcomes were often subtle, the ‘softer things’ of skilled supporting to enable patients and caregivers to manage daily life and live life as well as possible. Skilled support involved skilled listening, appreciation and understanding of the condition, and availability of accessible services responsive to deterioration, to minimise a domino effect of decline, and reviewing care and treatment, to maintain stability. Optimal management required attention to psychological, social and physical concerns: not an either or. The role of the MDT was essential to attend to the multiple domains of health. Interventions were required to attend to points when the patient’s health was unstable (when care and treatment were no longer keeping up with disease progression and symptoms and concerns) or stable (to enable the patient and the caregiver to maintain stability by supporting adaptation, problem-solving and resilience).
Each of the three themes are explored in relation to respective subcategories, with exploration of divergent cases. The intention is to inform theoretical understanding on a model of SIPC within the wider context of health and social care systems of delivery. The overview of the analytical framework on the value and impact of SIPC and linkages with key components for delivery are presented in Appendix 5. The table in Appendix 5 defines respective themes and subcategories and details illustrative quotations.
Theme 1: adapting to losses and building resilience
Patients and caregivers described living with a LTNC as ‘always troublesome’. Adapting to losses and building resilience were key strategies to adjust to increasing disability and declining function. Psychosocial interventions were valued to support resilience and adaptation, and counter feelings of loneliness and isolation, often combined with little understanding of their progressive condition. The theme is formed by two subcategories: (1) care beyond medicines, exploring psychosocial interventions; and (2) asked about everything, focusing on planning future care.
Care beyond medicines
Patient and caregiver concerns comprised multiple interacting components, linkages across health domains, and the ongoing work of adapting to living with a progressive condition and a ‘knowing outcome’ that leads to end of life. Interventions needed to encompass the breadth of actual or potential contributing factors to decline in emotional well-being. Optimal management of physical symptoms, using both pharmacological and non-pharmacological approaches, was often seen as the route to improving emotional well-being:
Well it’s [emotional feelings] sort of linked to my physical feelings really so I didn’t feel like I was ever gonna improve but I have begun to improve. I’m feeling a bit ill and fluey and that sort of winter feeling at the moment, but the massage [sessions from palliative care practitioner] was the start I think of me feeling better, and getting more sleep and feeling more generally well in myself. So it’s a subtle change in some ways but in some ways it’s quite a large thing cos just going to bed was a nightmare, I just couldn’t sleep at all and it was making me more tired and it seemed to make the symptoms worse in the morning and during the day and so I think it’s a good thing that I can actually go to bed and just sleep again since being on this course.
P05182-M
Psychosocial interventions encompassed person-centred care with comprehensive assessment, including social, emotional and physical health, not limited to the disease, and drawing on the skills of the MDT for delivery. Importantly, interventions also included practical support, advocating on patients’ behalf by following up referrals to services or for equipment, financial support reviewing and advocating (e.g. social care benefits), and non-pharmacological interventions of massage and acupuncture, attending to physical and emotional well-being. However, respite care was an area of frustration, seen as a vital resource but with varying provision and quality.
Person-centred care was key to optimal management of psychosocial concerns. The complexity of presentation required careful assessment and understanding of patient and caregiver priorities for care and treatment. Attention needed to focus on empowering patients and caregivers by understanding perspectives and priorities to enable shared decisions about care and treatment. When present, this appeared to build resilience and enable management of conditions, and, when absent, a sense of frustration and anger at the loss of personhood:
I don’t necessarily errm have a huge amount of confidence in them [antidepressants] and actually [palliative care nurse, PC1] agreed that because of the errm TN [trigeminal neuralgia] particularly, errm and because of the carbamazepine that she would be on, increased doses she probably said that actually it probably wouldn’t be recommended in your mum’s case and the GP [general practitioner] also agreed. So errm yeah we discussed it, but we kind of decided that it probably wouldn’t be best for mum . . . But again, it was nice to have someone actually say ‘Actually you’re probably right’. Errm because you kind of don’t get a handbook in going through things like this and you just have to really, it’s a bit of a minefield and you have to kind of make your way through it yourself. And there have been occasions when I’ve thought ‘Oh my God, should she be on them?’ And then we’ll try something and there’ll be nothing.
C01280-F
. . . people are only interested in your medical symptoms and not about your feelings or about your general condition. People are just interested in your drugs and general welfare. So it’s difficult to say. I think that people really didn’t care about you as a person just as a patient, shall we say, if you know that difference. That’s the best way I could say it I think. They didn’t seem to take much interest in what you thought, just whether you could do this or do that.
P06339-F
Assessment and management of psychosocial concerns required an accessible service, ‘it takes away worry knowing I can call’ (C05275-F), and a high level of skill and time to provide the level of support required to explore fears, and the ongoing process of adaptation and resilience, ‘We don’t, we don’t let ourselves get sad and unhappy cos you’ve gotta deal with that . . .’ (C01291-F). For both patients and caregivers, the act of asking, acknowledging and problem-solving was welcomed and valued:
. . . it sort of like makes you feel better if you know what I mean you know when you speak to her [palliative care nurse] and that, you know, you, sort of the help you get and that from them, you know, brilliant really.
C05275-F
However, with increasing complexity of presentation and management, the short-term intervention seemed to limit opportunity to establish rapport and trust, and review and to evaluate the ongoing process of managing psychosocial concerns, such as depression, and reconciling losses and fears about the future:
No, it’s not something we talked about [grieving for my body and the life I had before MS]. I did tell her [the palliative care nurse] that I feel very lonely at times but we didn’t talk about counsellors [that I saw many years ago and that was helpful] or anything like that . . . maybe that is something I should have asked for.
P01348-F
She convinced me to go back on some medications [antidepressants] which I said no to, so she talked and finally persuaded me to go back on those, which I’ve been on quite some time ago then I stopped and so on . . . I had them but was not eager to take them, and after [palliative care nurse, PC1N] handled it differently . . . yeah it made me feel better [after taking them again], but it’s not enough . . . and for this reason, I reject all medications because they’re not intended to make a big help, they just maintain certain level of healthiness, you know.
P01325-M
Asked about everything
Difficult conversations about ‘emotional things’ to adapt to a progressive condition and end of life required trust and rapport. The short-term nature of the intervention confined the time to build this. Sensitive topics were introduced by palliative care practitioners asking about thoughts on the future, but patients and caregivers seemed uncertain about engaging in such conversations. Planning future care for the end of life was marked by uncertainty in ‘not knowing what’s going to take hold’ when living with a LTNC, and fear of increasing disability and loss of capacity. Engagement ranged from ‘not at all’ to ‘I’ve been writing stuff down for years’, as demonstrated in Figure 3.
FIGURE 3.
Engagement with planning future care for the end of life. DNACPR, do not attempt cardiopulmonary resuscitation.

For both patients and caregivers, these conversations were difficult, and when broached by palliative care team members there was a tendency to ‘skirt-around’ talking about the future. Although individuals could see the practical benefits of planning care for the future, fear and uncertainty seemed to inhibit pursuit of such conversations. It was an area that required time for building trust and pacing to the individual:
. . . and I dread the thought of that [what happened to my mother with dementia] happening to me. Being spoken to like a small boy, I, that would just finish me off. I mean I, my mother had her own house. She had dementia, they spoke to her like a little girl, and I thought ‘you’ll not do that to me, speak to me like a child’ because I’m the one that owns this house, I pay the bills and just because I’m not well, whatever which shape or form, I won’t have that. I could be quite a difficult patient I feel as I get older . . . I haven’t got a family so I care for myself and when I can’t do, then maybe I’ll have to think again. But at the moment I don’t want to [think about wishes for care and treatment in the future].
P05258-M
Planning care seemed to focus on the present of managing each day, or confined to practical issues of setting up a power of attorney or making a funeral plan. Advance care planning discussions appeared to be the beginning of a conversation: checking in about what the person thought and offering contact with the palliative care team to discuss in the future:
Yeah I think you have to, everybody with any kind of medical condition you have to talk about things like that [wishes for care and treatment in the future].
Yeah that wasn’t, we hadn’t really thought about things like that before.
Yeah that was beneficial, to think about it . . .
Open our minds a bit I suppose.
. . . of what might happen in the future.
Theme 2: attending to function, deficits and maintaining stability
It was important for patients and caregivers for SIPC to attend to physical needs. This required services to encompass the duality of supporting function to maintain independence, however small, and optimal management of deficits of unstable symptoms amenable to changes to reduce distress. Behind this duality was a background of maintaining stability, requiring daily adaptation and resilience by patients and caregivers to manage a progressive condition with ongoing multiple losses across domains of health. These areas formed two subcategories: (1) little things make a big difference, and (2) maintaining stability.
Little things make a big difference
Optimal management of physical needs required a focus on reducing the distress of unstable symptoms (breathlessness, pain, including spasm, excessive saliva causing dribbling) and on maintaining function and independence. Unstable symptoms ranged from discrete areas to multicomponent across health domains. Interventions enabled stabilising of symptoms, with seemingly small changes in medication making a ‘big difference’ to how symptoms affected well-being. What was important was the timing to intervene early at points of deterioration to prevent a ‘domino effect’ of symptoms:
I mean her mobility, her, her dementia [from primary progressive MS] is all stuff that has kind of deteriorated obviously as we’ve been going along unfortunately errm she’s had issues with her swallowing errm we think there might be a little bit of dysphasia there, that’s actually as we speak being investigated so we’re waiting for a referral for, from speech therapy, errm because she is, she’s losing a lot of weight errm and her appetite is just most of the time really non-existent . . . You can’t do anything when someone’s in pain unfortunately so I think that was, she [palliative care nurse] probably came in at the right time that it you know, it, it couldn’t have been better timing really for us errm it just so happened that at that point in time she was really going through a really tough time with the TN [trigeminal neuralgia]. So, but there’s always gonna be challenges, I don’t think there’s, you know, gonna be a time where everything is necessarily errm you know, staying on a same level, it’s always gonna be progressive because unfortunately that’s the nature of primary progressive MS.
C01280-F
[My husband] was obviously getting embarrassed [dribbling saliva], you know, family came or we wanted, he wouldn’t go out for a meal or anything like that then. No, and again it’s something again, he told the others, and nothing was really, something was suggested but nothing followed through which is this, wasn’t it, the drops . . . But she [palliative care nurse], she done it, she did, you know . . . It’s not, it’s I would say it’s a good 50% better than what it was, isn’t it? . . . He was [also] getting jumpy legs, or what he calls irritable legs and they would jump, he’d be in bed and he’d twitch, keep twitching wouldn’t you? We know it’s part of Parkinson’s but it was, he wasn’t getting any sleep, but at least you get some sleep now don’t you? . . . It’s still there, because you won’t sort of clear it up but it’s, well a good 75% better [following SIPC medication change]. [C01335-F]. 90% [better]. [P01335-M].
Caregiver and patient dyad, C01335-F and P01335-M
Divergent cases illustrated the complexity of symptom management when living with multiple symptoms. Many patients had ‘tried many things’ over time, with, at times, optimal care and treatment having little impact on symptom severity. What mattered to patients and caregivers was ‘to know people are available and willing to try and improve symptom management’. Optimal management required interdisciplinary working across MDTs, and integrated working with main providers of care to maintain continuity of care and treatment. Disrupting continuity of care and treatment, with perceived no benefit or negative effect, increased distress:
My life hasn’t changed [from seeing palliative care nurse] except maybe in the short term, for a bit of aggravation and trouble that she caused me, because I’ve had to put everything back the way it was and I won’t complain about the tiredness again. Because if it’s, if it is the tramadol [analgesia] that caused the tiredness then so be it, I will accept it. But I can’t, as I said to you, I don’t want to be in pain.
P05258-M
Maintaining function through supporting adaptation and problem-solving (e.g. continence, mobility, falls prevention, eating and drinking) was highly valued and felt to support independence. Key components were the involvement of the MDT, notably occupational therapy (OT) to support function, and physiotherapy and complementary therapies to provide non-pharmacological interventions to manage symptoms such as pain, breathlessness and poor sleep. However, when function was seemingly poorly attended to, this was an area of frustration for patients and caregivers. This was often compounded by their long experience in health and social care of long waiting times for assessments, loss of neurological physiotherapy services in the local community, difficulty accessing palliative care services if this had required travelling to day services, or ‘nothing happened’ following referral to community OT or physiotherapy by the palliative care team:
Simple things. And that’s what I think they [the palliative care team] need to educate their self about if they want to help . . . No, with palliative care, that word is to help you, comfortable . . . live life better, a little bit better . . . For her [palliative care nurse] knowledge, we’re not knowledgeable people . . . But she’s got the, the, errrm probably the experience to have walked in anywhere and said to [patient’s name] well you know, he’s sitting there in a chair and he’s like that so what would you think? . . . He hasn’t got no support in his spine, which is, giving him drugs for his pain, but they’re not preventing it . . . See that could have been prevented a long time ago but because of the system, they [palliative care] don’t seem to assess it . . . [he’s] got progressive and, all this, falling over.
C01286-F (caregiver and patient dyad, C01286-F and P01286-M)
Maintaining stability
Although they are living with extensive losses and deficits, patients considered themselves ‘stable’ with no concerns, but worked every day to maintain that stability. SIPC was about skilled support to increase awareness and understanding of disease management, symptoms and concerns, and being available for individuals to call for advice and support if things changed. This was a seemingly subtle soft outcome. Some patients and caregivers considered no or little impact on well-being from the involvement of palliative care, with ‘troubles’ the same as always, which they worked continuously to live with and adjust to:
Well the thing is, I don’t, I don’t, I don’t feel like I’m trouble, I’ve got any troubles. I, I obviously I’ve got short memory and . . .
P01286-M
He’s [my husband] got adjusted to his disability . . . but before she came, it was no different to what it was all the time . . . by [Palliative care practitioner, PC1] coming it wasn’t a miracle cure . . . it wasn’t, suddenly she turned up and it . . . all my worries are gonna go and errr all my troubles and worries are just gonna disappear.
C01286-F
Theme 3: enabling caregivers to care
Acknowledging and valuing the work and care given by caregivers meant a lot, with appreciation of someone to talk to and to be reassured of doing a good job. When not acknowledged, this was a source of frustration for caregivers, who wanted to be asked ‘How are you?’, and when they were asked this they were surprised, as there was a tendency for care and attention to focus on the patient. Caregivers expressed feelings of having had to ‘fight for everything’ and to become experts in the person’s care. The ability to call the palliative care team for advice, particularly out of hours, as well as opportunities to talk through care and treatment, were important to empower decision-making:
Errm you’ve either got to be a strong-minded person or just plod along and put up with it which I have. I’ve had a lot of stress, fighting the system for everything. As far as she [the palliative care nurse] was concerned, she was here mostly for [my husband] and not for the person who’s here 24 hours a day, or not 24 hours a day but lives here as his wife and carer. Errm she didn’t say to me ‘Is there anything you think that could be done to help you with caring for your husband?’.
C01286-F
Caregivers indicated a tendency not to ask for help, putting their loved ones before themselves and not allowing themselves to be vulnerable or ‘selfish’ in putting their own needs first. Not acknowledging need for help was complex, linked to fear that asking may trigger thinking that they are unable to cope, ‘Better to keep coping rather than risk downward spiral of thinking I can’t manage’. Without support there was a risk of caregivers eventually breaking psychologically and physically from the strain and worry, particularly when not recognising the limits of their resources and the importance of caring for themselves. This type of ongoing work required trust and rapport for the caregiver, to gradually understand the increasing demands placed on them and how to best manage these. Supporting caregivers who had complex concerns required the continuity of care provided by neurology services, particularly community-based nurse specialists. Working with the palliative care team provided the ‘final prompt or prod’ to enable change:
Well not so much someone to talk to but [Parkinson Clinical Nurse Specialist, NN1], she’s been absolutely brilliant and errm her sort of enthusiasm, I mean she’s been talking to me about giving up work for a long time and kept on saying stop being so stubborn, you know, why can’t you just listen or why can’t you just you know give up and look after [your wife] full time, have quality of life, and I, it came to the stage where it was really difficult in that it was now, it has to happen now and you just sort of errm put it aside, put it aside until we moved, errm where it just suddenly hit me, you know just near the kitchen I thought ‘no I can’t carry on’. I phoned my boss, I said ‘look I’m having a bit of a breakdown here’ . . . he [my boss] said ‘well we’ve actually been waiting, wondering how long you’re going to last for, we’ve been waiting for your breakdown’. I thought ‘thanks’ you know [laughs] but that was done, they were brilliant you know up until I finally left.
C01007-M
Change process and delivery in practice
The overarching themes provided understanding on the outcomes that are important for patients and caregivers, and linkages with key components of models of care to delivery. Important outcomes from SIPC centred on three main areas:
-
Psychosocial well-being of adapting and having the resilience required to live with, and accommodate, multiple ongoing losses leading eventually to end of life.
-
Physical well-being of supporting function to promote independence, optimal management of physical symptoms, with emphasis on person-centred care, pharmacological and non-pharmacological interventions, and accessible services responsive to points of deterioration to minimise a domino effect of decline.
-
Empowering caregivers to care through recognition and valuing of work, shared decision-making and accessible services for advice and support, including practical support.
Complexity was an over-riding feature across the narrative data, surrounding all aspects of care: management of progressive disease; symptoms that appeared refractory, with little response to pharmacological interventions; maintaining independence with increasing disability; adapting to a multitude of losses leading eventually to end of life, with huge uncertainty as to when; and empowering caregivers to continue caring over many years, with increasing demands and losses. Linkage with the outcomes important for patients and caregivers, their experiences of receiving SIPC and the processes of delivery described by practitioners’ informed understanding of the overarching components of a service delivery model for integrated palliative and neurological care. These components include:
-
Comprehensive assessment, encompassing health domains and the priorities for both patients and their caregivers.
-
Person-centred, holistic care, focusing on the person and not the disease alone.
-
MDT working, to provide the expertise for attending to function and deficits of symptoms and concerns across health domains.
-
Integrated working between health-care services, to ensure continuity of care between palliative care and neurological services, and with community and primary care services.
-
Integrated working fostered through reciprocal learning and training between specialties, in order to enhance the provision of palliative care approaches in neurological services and, conversely, expertise in management of advanced neurological disease in palliative care, with joint case review of individuals with complex symptoms and concerns.
Intervention fidelity
Of the 176 patients allocated to receive the SIPC, 173 patients received the intervention. Three patients did not receive the intervention, as two patients withdrew from the trial and one patient could not be contacted by the respective palliative care team following referral. Of the 173 patients who received the intervention, all had an initial face-to-face visit for a comprehensive palliative care assessment. Following this, 152 patients had a second key worker contact (100 via face-to-face meeting and 52 via telephone), and 153 patients had a third key worker contact (91 via face-to-face meeting and 37 via telephone; for 48 patients the type of contact was not recorded). The intervention manual described the core elements to be covered when assessing patients as part of the SIPC, as well as the minimum standards for capturing and reporting delivery of the SIPC intervention. The completion rates of key pro forma documents and core intervention elements are presented in Table 16.
| Variable | Value | SIPC arm |
|---|---|---|
| N | 173 | |
| Number of key contacts received, n (%) | 3 | 126 (72.8) |
| 2 | 27 (15.6) | |
| 1 | 20 (11.6) | |
| Duration of first visits in minutes | ||
| Mean (SD) | 130.9 (75.3) | |
| Range | 40–410 | |
| Duration of intervention in weeks | ||
| Mean (SD) | 7.95 (5.06) | |
| Range | 0–32 | |
| Completion of IPOS Neuro, n (%) | 168 (97.1) | |
| Baseline AKPS recorded, n (%) | 124 (71.7) | |
| Baseline phase of illness recorded, n (%) | 152 (87.9) | |
| Advance care planning discussed, n (%) | Yes | 122 (70.5) |
| No | 25 (14.5) | |
| Not appropriate/patient declined | 26 (15.0) | |
| Discussion in a MDT review, n (%) | 164 (94.8) | |
| Compliance, n (%) | Complier | 120 (69.4) |
| Partial complier | 34 (19.7) | |
| Overuse | 1 (0.6) | |
| Dropout | 4 (2.3) | |
| Not recorded | 14 (8.1) | |
Chapter 5 Discussion and conclusions
Key findings
To the best of our knowledge, this is the largest palliative care trial in people with LTNCs. 87 We found that none of the evaluated primary or secondary outcomes were significantly different between those in the SIPC group and those in the standard care group. However, safety outcomes, including deaths, survival and hospitalisations, were similar between the two groups, as were rates of withdrawals. The health economics data showed that care costs fell in both groups between baseline and 12 weeks, with no statistically significant differences between groups. However, ICERs for EQ-5D index score and (rescaled to match direction of EQ-5D) IPOS Neuro-S8 were –£23,545 and –£1519, respectively, considerably better than our threshold for cost-effectiveness. The bootstrapped point estimates in the cost-effectiveness analysis favoured SIPC, indicating a larger reduction in symptoms on IPOS Neuro-S8 or improvement in EQ-5D along with lower cost. Patients receiving SIPC did show a statistically significant improvement on the primary outcome of eight key physical symptoms (IPOS Neuro-S8) and the secondary outcome of 24 physical symptoms (IPOS Neuro-S24) from baseline to 12 weeks after randomisation. A similar improvement was not seen in the control group. Other than the level of care satisfaction (as measured by FAMCARE-P16), which became worse in the standard care group, there were no statistically significant changes in other patient or caregiver outcomes, for example quality of life, anxiety and distress, caregiver burden and positivity, over 12 weeks, in either group. The qualitative data illustrated the complexity of living with LTNCs for both patients and caregivers, and the value that participants placed on the holistic and timely assessment received through SIPC, with attention and assistance given to psychological, social and physiological concerns. Many symptoms were seen as intractable. The qualitative, mapping and survey data all demonstrate the need and opportunities for increased collaboration and integration between neurology and palliative care services, and the challenges resulting from variation across centres and diseases.
Comparison with literature
Trials evaluating the effectiveness of palliative care for LTNCs are rare, and the existing evidence comes from a small number of feasibility/pilot studies. In comparison, our trial is large and both multicentre and multicondition. The overall pattern of results we see is similar to that of the previous Phase II trial of an early palliative care intervention in people with advanced MS. 24 However, in that trial, at 12 weeks, there was a significant difference in the change score of pain, as well as a significant improvement on caregiver burden, in the early palliative care group compared with the usual care group. In contrast, we have not investigated symptoms individually and we did not observe any improvement on caregiver burden. The latter may not be surprising, as a recent systematic review and meta-analysis highlighted that findings on caregiver burden in palliative care interventions are conflicting. 88 Our qualitative findings iterate this, illuminating understanding on the complexity of caregivers’ sense of legitimacy to receive support and the priority they placed on this. In another Phase II trial26 with 78 MS patients and their caregivers, a 6-month home-based palliative care service was found to significantly reduce symptom burden as compared with usual care (p = 0.047), with a stronger effect at 6 months (effect size = 0.32) than at 3 months (effect size = 0.2). 26 The authors also observed no difference in other outcomes, such as quality of life or caregiver burden. A 4-month home-based specialist palliative care service for 50 patients with advanced neurodegenerative disorders (MND, MS, IPD, MSA and PSP) found a significant improvement in quality of life and physical symptoms (pain, breathlessness, sleep disturbance and bowel symptoms) across the conditions, but, again, no effect was seen for caregiver burden. 25 In contrast with our trial, that trial did not include patients without capacity. Of note, none of the abovementioned trials reported data on intervention fidelity, which is a key factor when evaluating complex interventions. Indeed, implementation fidelity in randomised controlled trials of palliative care for complex interventions is under-recognised and under-reported. 89 In contrast to our trial and the reported trials in neurological condition, studies testing the effectiveness of palliative care interventions for advanced cancer have shown benefit to multidimensional quality-of-life outcomes and survival, but less so for physical symptoms. 36,90,91 Although the prognosis and trajectories of LTNCs are vastly different from cancer and even vary for each type of LTNC. In a trial92 evaluating palliative care for heart failure patients, an improvement on anxiety and depression outcomes, as measured by the HADS, was also reported.
Interpretation
In this trial, we did not detect a difference between trial arms in the primary outcome, although we note that, compared with baseline, the scores on the primary outcome improved in both arms, significantly in the SIPC group and not significantly in the control group. The secondary outcome, IPOS Neuro-S24, followed this pattern, and the significant reduction in patient satisfaction seen in the standard care group further supports the trial findings in favour of SIPC. Compared with baseline, health and social care costs, including inpatient care, community care, outpatient care and home care, were reduced in both arms at 12 weeks. Although there was no significant difference between groups, this reduction in costs was larger in the SIPC group. The ICER suggests that, compared with SIPC, the control arm has a 1 point deterioration in utility at an additional cost of around £1000 per patient. The ICERs for SIPC are well within the usual thresholds for decision-making in the NHS and recommended by the National Institute for Health and Care Excellence (e.g. £20,000 or £50,000, as often used in end-of-life care therapies), making SIPC acceptable as a cost-effective intervention. No harms were identified and, in general, patients and clinicians welcomed the intervention. In addition, crucially, there were no increased costs to informal carers and, if anything, their costs may have been slightly lower in the intervention group.
To base the decision of effectiveness on the statistical significance is a long-recognised misuse of the p-value. The American Statistical Association released a policy statement in 2016 specifically targeting this issue. 93 A comment paper published in Nature earlier this year updated this concern regarding pervasive categorisation on the basis of the p-values. 94 However, through the mapping, survey and qualitative components, we also observed variations in integrated working and delivery of care, and more work is needed to understand the best ways to provide palliative care to this group and how to improve standard care, which varies considerably.
There may be several possible explanations for the non-significant results. The first relates to the outcome measures. The primary outcome measure of eight key symptoms (IPOS Neuro-S8) was selected based on the five symptoms that were most responsive to a palliative care intervention in our Phase II trial and symptom profile data from a longitudinal observational study of late-stage Parkinsonism syndromes. 27,40 In a preliminary psychometric evaluation, the measure exhibited promising psychometric properties. 68 In this trial, some items of the IPOS Neuro-S8, as well as the IPOS Neuro-S24, showed strong floor effects at baseline, indicating that some further refinement of the outcome measures, particularly for a more heterogeneous trial setting like the present one, may be necessary. In our data, the IPOS Neuro-S8, which measured participants’ psychological and spiritual well-being, information needs and practical issues, rather than their symptoms, appears to detect more changes.
The other key question is the match between the patient needs and the interventions offered by the services. Palliative care was first developed mainly for patients with cancer, although it should be noted that early studies of need and problems identified concerns for people with many different diseases, including neurological conditions. 95–97 It may be that palliative care has not sufficiently adapted and developed symptom and other treatments as yet for patients with complex LTNCs. Much research and therapeutic developments in palliative care have focused on cancer pain and other cancer symptoms, rather than on the most prevalent symptoms in this population. These include chronic pain and spasms found in neurological conditions, fatigue, weakness, difficulty sleeping, breathlessness and problems with mobility. 69 These are continuing serious concerns for these patients and their families, and should be the topic of future research. Such research could benefit care widely, and the therapies discovered could be applied by those with neurology and rehabilitation backgrounds. Furthermore, the models of palliative care operation also derived from cancer, and many staff working in palliative care have a cancer training and not a neurological training. Therefore, the models and approaches offered by the services may not have been optimal. It may also be that better targeting of the population likely to benefit from integrated palliative care is needed. Better working and integration between palliative care and neurology is needed to further improve the models of care for people seriously affected by neurological conditions. Such an approach needs to rise beyond specialist-defined boundaries and focus on the best ways of meeting the needs of patients and those important to them.
Additionally, as we used a trial design randomising at the individual level, there may have been some contamination, whereby participants in the standard care group received some components of the SIPC intervention. Indeed, this appears to have been the case, because our economic data found that some people in the standard care arm reported receiving palliative care by 12 weeks, when according to our design they should not have. Referrals to the palliative care teams for the intervention were carefully monitored to avoid contamination, but it seems that this did not fully work. Participation in the trial itself may function as an intervention on some level. This contamination may have occurred through the research contacts, when participants were visited in their homes by friendly, caring researchers (often research nurses), who gave them the time to air their concerns and feel listened to. Indeed, our qualitative findings demonstrate that this was a valued aspect of the holistic assessment received as part of the intervention and may have been mistaken for palliative care in the standard care arm. As such, receiving this to some level through the research contacts probably had benefits for those in the standard care group. In both groups, we saw a reduction in the burden of symptoms, though to a lesser degree in the standard care group; therefore, although the mean change score in the SIPC group for the primary outcome was statistically different from zero, this potential contamination may have further reduced the effect size.
Finally, the SIPC may not have had enough time for the intervention teams to build sufficient rapport with patients and caregivers to address some of the more complex issues, such as advance care planning, supporting caregivers and optimal management of refractory symptoms. Some of the needs identified could be addressed only by referring or signposting to other services (e.g. social services for review of benefits). Therefore, SIPC often had to rely on the actions of other services that it referred to or that were catalysed by SIPC. The time scale of 12 weeks may not have been sufficient to ensure that SIPC could directly act on, or follow up on, its recommendations. It may also be that for some of the longer-term, more intractable, difficulties that it took longer than 12 weeks to see an effect, especially of referrals to other services. We were able to collect longer-term follow-up data, and these will be analysed in future research. Ideally, population-based routine data could be used to augment this analysis. Nevertheless, the few other trials assessing palliative care interventions in this population had similar findings despite varying lengths of intervention (up to 6 months). 24–26
Strengths and limitations
There are several strengths to this study. To the best of our knowledge, this is the largest palliative care trial in a non-cancer population and also the first to include more than one common LTNC. We included patients with cognitive decline who lacked capacity, which is common in neurological conditions, and recruited their caregivers as the proxy to provide data to inform the imputation process. We recruited a large number (n = 229) of caregivers of patients with advanced LTNCs and collected a range of caregiving outcomes, including quality of life, caregiver burden and positivity. The randomisation process appears to be successful. The only imbalanced variable at baseline was ethnicity, with slightly more non-white patients in the standard care group, for which we have adjusted in-group comparisons. Our data quality was high, with much lower attrition and fewer missing data than would be expected in a palliative care trial of this scale. When planning the study, we set the attrition rate at 17%, three times of that in our Phase II MS trial, to accommodate heterogeneities across centres and conditions. The missing data for the primary outcome due to withdrawal or for unknown reasons were 4% at 12 weeks. Although it is impossible to blind the participants and the persons delivering the interventions, research nurses collecting the data were blinded, and the chief investigators and analysis team were kept blinded until the planned analyses were completed. The embedded qualitative component enables us to have a better understanding about the process, the active ingredients and the mechanism of intervention.
We acknowledge that there are some limitations of this study. The sample largely comprised patients with MS and IPD, who tend to have a longer disease course. This is also reflected in the embedded qualitative study, which included mainly patients living with MS. It is possible that the baseline symptom profiles and therefore the subsequent impact and experience of SIPC are different for patients with LTNCs, with a more rapid decline. As mentioned above, the outcome measures used may not have been sensitive enough and did demonstrate some floor effects at baseline for some symptoms. The cost data were based on patient- (or carer-) reported use of services, which relies on their memory of the services received. They may have under- or over-reported some services, and/or may have made some miscategorisations, such as mistaking types of community care or palliative care. It is often difficult for patients to correctly categorise the types of nurses or others visiting them in the community; however, the mistaken categories in community home visiting services would have had little influence on the overall costs. The main driver of costs was hospital admissions, which are less susceptible to miscategorisation as non-admission.
Although every effort was made to standardise the intervention, and our fidelity data show that this was, on the whole, well managed, there were some differences across centres, namely some intervention teams were hospice based and others were hospital based, which led to differences in the make-up of their MDTs, as well as the services and therapies they were able to offer. In addition, the clinical services at baseline and the integration between palliative care and neurology varied across our centres and across diseases. Some patients in the standard care arm reported that they were receiving palliative care at baseline and, although this did not change during the study, this is because either they mistook the research interviews for palliative care or there was contamination, which would have reduced the chances of finding a difference between groups.
Implications
For clinical practice
Our findings have highlighted that patients with LTNCs and their caregivers have significant holistic needs that are currently unmet. Our mapping work demonstrates significant heterogeneity and variance nationally in the way that neurology and palliative care services are working, with several instances in which there is silo working. In addition, there is variance nationally on access to palliative care for different LTNCs. There are key areas in which further work can be taken forward by existing services, to allow collaborative working and sharing of expertise. This includes training to explore advance care planning and end-of-life care, and to facilitate joined-up working between all health and social care systems to deliver optimal holistic care. Training is also required for neurology teams to support comprehensive assessment of patients to best identify those who would benefit from a referral to specialist palliative care services, and to ensure that needs (including social care needs) are not overlooked and appropriate referrals are made.
For policy-makers
Our findings suggest a need for further refinement of SIPC and the provision of holistic, palliative care approaches for this patient group. We found that SIPC provides improvements in patient-reported physical symptoms at a lower cost and without any harmful effects. Even patients in the standard care group have perceived benefits of holistic care approaches through the comprehensive assessment of their needs and concerns by research nurses. The SIPC intervention tested in this trial could be a viable, potentially cost-saving and efficient care model valued by people severely affected by LTNCs. The results from the mapping, survey and qualitative components highlight the urgent need to improve integration between specialties to ensure the continuity of care so valued and desired by patients and their caregivers.
Our findings, especially from the qualitative components, can be used to inform the refinement of SIPC. First, SIPC would need to focus on physical well-being, including symptom management coupled with supporting function, to promote independence, with emphasis on person-centred care and accessible responsive services, especially at points of deterioration. Second, SIPC would need to be able to provide and/or draw in psychosocial support, including mechanisms for adaptation and resilience. Third, a focus on empowering and supporting lay caregivers through recognition and valuing of their work, shared decision-making, and accessible services for advice and support. Easy and timely access to practical support is important. Further research and secondary analysis could explore whether or not any particular models of service (e.g. hospice, community or hospital based), team composition or patient characteristics are associated with benefit.
For research
Our findings identify gaps that future research should focus on:
-
The broader generalisibility of our research findings. It is necessary to evaluate how representative the study sample is of the population the SIPC intended to target.
-
Further testing and refining the triggers and criteria for referral to specialist palliative care for patients with LTNCs.
-
Research to improve symptom management in this population. The qualitative and quantitative data suggested high levels of continuing symptoms that probably need better treatments to be discovered so that care can be improved.
-
Further development and refinement of generic outcome measures that can be used in both research and clinical settings in people with LTNCs.
-
Predictors of high-cost patients with LTNCs, given that we observed some considerable variation in costs.
-
How to monitor patients’ palliative care needs along their disease journey, and identify the right timing for specialist palliative care and the right timing for boosting the intervention.
-
How to implement holistic care approaches, such as SIPC, in wider neurology care settings.
-
Establishing the evidence for longer-term effects of SIPC and determining how the effects change over time, whether or not earlier referral to the palliative care affects the subsequent response to palliative care, and when assessment or rereferral might be beneficial.
On the last point, data collected during the process of routine care, for example electronic health records, have become valuable sources of data for research, as they bear the minimum data collection burden on research participants. There have been an increasing number of applications of routine data to enhance trial designs and facilitate trial implementation. 98–100 Researchers should consider exploiting the potential of routine data sources when addressing research questions, for example those arising from this trial. These may be especially useful in exploring the longer-term effects on health service use.
Conclusions
To the best of our knowledge, this is the largest palliative care trial in people with a variety of LTNCs. Although no significant between-group differences were seen, we have demonstrated that SIPC provides improvements in patient-reported physical symptoms, at a lower cost and without any harmful effects when compared with standard care. However, further work is needed to refine SIPC and the provision of holistic, palliative care approaches for this patient group, with a particular focus on better integration of existing services and specialties, as well as the appropriate timing and criteria for the referral of LTNC patients to specialist palliative care.
Acknowledgements
OPTCARE Neuro investigators
Professor Irene J Higginson (co-chief investigator), Dr Wei Gao (co-chief investigator, trial statistician), Professor Ammar Al-Chalabi, Dr Cynthia Benz, Dr Rachel Burman, Dr Anthony Byrne, Professor K Ray Chaudhuri, Dr Vincent Crosby, Dr Catherine Evans, Professor Matthew Hotopf, Dr Diana Jackson, Professor P Nigel Leigh, Professor Paul McCrone, Dr Fliss Murtagh, Professor Andrew Pickles, Dr Eli Silber, Dr Andrew Wilcock and Professor Carolyn A Young.
Data Monitoring and Ethics Committee
We thank the external members of the Data Monitoring and Ethics Committee, Professor Mike Bennett (chairperson), Professor Gunn Grande, Dr David Oliver, Professor Raymond Voltz and Professor Stephen Walters, for their advice and support.
Study Steering Committee
We thank the external members of the Study Steering Committee, Professor Marie Fallon (chairperson), Dr Cynthia Benz, Professor Mogens Groenvold, Professor William Hollingworth, Dr Denise Howel, Professor Huw Morris, Mr Foster Murphy, Dr Diane Playford, Professor Julia Riley and Professor Jane Seymour, for their advice and support.
Patient and public involvement
Our thanks go to the OPTCARE Neuro PPI members, Dr Cynthia Benz, Mr David Charlton, Mr Colin Fellows, Ms Helen Findlay and Mrs Savita Jain, for their guidance and contributions throughout the study.
Researchers
The team thank all the researchers involved in recruitment, data collection and input: Dr Sarah Awan, Virginia Bray, Evelyne Burssens, Gillian Carey, Rebecca Cloudsdale, Joanna Davies, Mim Evans, Dr Caroline Facey, Lynne Harry, Asmah Hussain, Kate Jones, Paramjote Kaler, Loretta Kerr, Julie Lynch, Cathann Manderson, Jenifer Newton, Caty Pannell, Louise Pate, Maria Preece, Helen Santander, Sarah Schofield, Chifundo Stubbs, Caroline Sunderland, Patricia Thomas, Richard Turner and Dr Liesbeth van Vliet.
Administrative support
Our thanks go to those who provided administrative support: Deborah Tonkin, Zaynah Sheikh, Daniel Gulliford, India Tunnard and Anna Johnston.
Palliative care teams
We offer our appreciation to the palliative care teams and their members who delivered the intervention and provided care for our participants: Marsha Dawkins, Dr Sabrina Bajwah, Connie Jackson, Nottingham University Hospital palliative care team, Martlets Hospice, Queenscourt Hospice, City Hospice, Marie Curie Hospice for Cardiff and the Vale, Ashford and St. Peter’s Hospital palliative care team, Dr Ellie Smith and St. Luke’s Hospice Sheffield.
King’s College London Clinical Trials Unit
The UK Clinical Research Collaboration-registered King’s College London CTU at King’s Health Partners provided the trial database and randomisation systems. We thank the entire King’s College London CTU team, in particular Caroline Murphy, Joanna Kelly and Beverley White-Alao.
Finally, we are extremely grateful to all the participants who supported this study and gave their time so generously.
Contributions of authors
Dr Nilay Hepgul (https://orcid.org/0000-0002-5980-1457) (Research Associate and Trial Manager, King’s College London) managed the study and contributed to data collection, site monitoring, data cleaning, data analysis, interpretation of the data and leading the writing of the report.
Dr Rebecca Wilson (https://orcid.org/0000-0003-4709-7260) (Research Associate, King’s College London) contributed to data cleaning, data analysis, interpretation of the data and leading the writing of the report, especially relating to the analysis of effectiveness.
Dr Deokhee Yi (https://orcid.org/0000-0003-4894-1689) (Health Economist, King’s College London) contributed to data cleaning, data analysis, interpretation of the data and leading the writing of the report, especially relating to health economic components.
Dr Catherine Evans (https://orcid.org/0000-0003-0034-7402) (Senior Clinical Lecturer in Palliative Care, King’s College London; Honorary Nurse Consultant, Sussex Community NHS Foundation Trust) contributed to study design, qualitative data analysis, interpretation of the data and leading the writing of the report, especially relating to qualitative components.
Dr Sabrina Bajwah (https://orcid.org/0000-0001-5338-8107) (Consultant and Honorary Senior Lecturer in Palliative Care, King’s College London) was the site principal investigator and contributed to intervention design, intervention delivery, interpretation of the data and leading the writing of the report, especially relating to intervention fidelity.
Dr Vincent Crosby (Consultant in Palliative Care, Nottingham University Hospitals NHS Foundation Trust) was the site principal investigator and contributed to study design, intervention design, intervention delivery, interpretation of the data and writing of the report, especially relating to intervention fidelity.
Dr Andrew Wilcock (https://orcid.org/0000-0002-3214-1188) (Consultant in Palliative Care, Nottingham University Hospitals NHS Trust) contributed to study design, interpretation of the data and writing of the report.
Dr Fiona Lindsay (https://orcid.org/0000-0003-4223-959X) (Consultant in Palliative Care, Martlets Hospice) was the site principal investigator and contributed to study design, intervention delivery, interpretation of the data and writing of the report.
Dr Anthony Byrne (https://orcid.org/0000-0002-1413-1496) (Consultant in Palliative Care and Clinical Director, Marie Curie Research Centre, Cardiff University) was the site principal investigator and contributed to study design, intervention delivery, interpretation of the data and writing of the report.
Professor Carolyn Young (https://orcid.org/0000-0003-1745-7720) (Consultant Neurologist, The Walton Centre NHS Foundation Trust) was the site principal investigator and contributed to study design, recruitment, interpretation of the data and writing of the report.
Dr Karen Groves (Consultant in Palliative Care, Queenscourt Hospice) contributed to intervention delivery, interpretation of the data and writing of the report.
Dr Clare Smith (https://orcid.org/0000-0002-7754-9769) (Consultant in Palliative Care, Ashford and St Peter’s NHS Foundation Trust) was the site principal investigator and contributed to intervention delivery, interpretation of the data and writing of the report.
Dr Rachel Burman (https://orcid.org/0000-0002-9276-7174) (Consultant in Palliative Care, King’s College Hospital) contributed to study design, interpretation of the data and writing of the report.
Professor K Ray Chaudhuri (https://orcid.org/0000-0003-2815-0505) (Consultant Neurologist, King’s College Hospital) contributed to study design, recruitment, interpretation of the data and writing of the report.
Dr Eli Silber (https://orcid.org/0000-0002-3972-0780) (Consultant Neurologist, King’s College Hospital) contributed to study design, recruitment, interpretation of the data and writing of the report.
Professor Irene J Higginson (https://orcid.org/0000-0002-3687-1313) (Professor of Palliative Care and Policy, King’s College London) was co-chief investigator and led the conception and design of the study, organisation of the conduct of the study, development of study protocol, interpretation of the data and leading the writing of the report, as co-senior author.
Dr Wei Gao (https://orcid.org/0000-0001-8298-3415) (Reader in Statistics and Epidemiology, King’s College London) was co-chief investigator and trial statistician, and led the conception and design of the study, organisation of the conduct of the study, development of the study protocol, statistical analysis plan, data analysis, interpretation of the data and leading the writing of the report, as co-senior author.
All authors approved the final version.
Publications
Gao W, Crosby V, Wilcock A, Burman R, Silber E, Hepgul N, et al. Psychometric properties of a generic, patient-centred palliative care outcome measure of symptom burden for people with progressive long term neurological conditions. PLOS ONE 2016;11:e0165379.
van Vliet LM, Gao W, DiFrancesco D, Crosby V, Wilcock A, Byrne A, et al. How integrated are neurology and palliative care services? Results of a multi-centre mapping exercise. BMC Neurol 2016;16:63.
Ang K, Hepgul N, Gao W, Higginson IJ. Strategies used in improving and assessing the level of reporting of implementation fidelity in randomised controlled trials of palliative care complex interventions: a systematic review. Palliat Med 2018;32:500–16.
Hepgul N, Gao W, Evans CJ, Jackson D, van Vliet LM, Byrne A, et al. Integrating palliative care into neurology services: what do the professionals say? BMJ Support Palliat Care 2018;8:41–4.
Wilson R, Hepgul N, Saha RA, Higginson IJ, Gao W. Symptom dimensions in people affected by long-term neurological conditions: a factor analysis of a patient-centred palliative care outcome symptom scale. Sci Rep 2019;9:4972.
Gao W, Wilson R, Hepgul N, Yi D, Evans C, Bajwah S, et al. Effect of short-term integrated palliative care on patient-reported outcomes among patients with advanced long-term neurological conditions: a randomized clinical trial. JAMA Netw Open 2020;3:e2015061.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Peters M, Fitzpatrick R, Doll H, Playford ED, Jenkinson C. Patients‘ experiences of health and social care in long-term neurological conditions in England: a cross-sectional survey. J Health Serv Res Policy 2013;18:28-33. https://doi.org/10.1258/jhsrp.2012.011176.
- Peters M, Fitzpatrick R, Doll H, Playford ED, Jenkinson C. The impact of perceived lack of support provided by health and social care services to caregivers of people with motor neuron disease. Amyotroph Lateral Scler 2012;13:223-8. https://doi.org/10.3109/17482968.2011.649759.
- Morley D, Dummett S, Peters M, Kelly L, Hewitson P, Dawson J, et al. Factors influencing quality of life in caregivers of people with Parkinson‘s disease and implications for clinical guidelines. Parkinsons Dis 2012;2012. https://doi.org/10.1155/2012/190901.
- Fitzpatrick R, Peters M, Doll H, Harris R, Jenkinson C, Playford D, et al. The Needs and Experiences of Services by Individuals with Long-Term Progressive Neurological Conditions, and their Carers. A Benchmarking Study. London: Queen‘s Printer and Controller of HMSO; 2010.
- Kristjanson LJ, Aoun SM, Oldham L. Palliative care and support for people with neurodegenerative conditions and their carers. Int J Palliat Nurs 2006;12:368-77. https://doi.org/10.12968/ijpn.2006.12.8.368.
- Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. MSCOI Study Group . New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 2017;23:1123-36. https://doi.org/10.1177/1352458517694432.
- Lindgren P, von Campenhausen S, Spottke E, Siebert U, Dodel R. Cost of Parkinson‘s disease in Europe. Eur J Neurol 2005;12:68-73. https://doi.org/10.1111/j.1468-1331.2005.01197.x.
- McCrone P, Payan CA, Knapp M, Ludolph A, Agid Y, Leigh PN, et al. NNIPPS Study Group . The economic costs of progressive supranuclear palsy and multiple system atrophy in France, Germany and the United Kingdom. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0024369.
- Department of Health and Social Care (DHSC) . The National Service Framework for Long-Term Conditions 2005.
- National Audit Office . Services for People With Neurological Conditions 2011.
- Schoen C, Osborn R, How SK, Doty MM, Peugh J. In chronic condition: experiences of patients with complex health care needs, in eight countries, 2008. Health Aff 2009;28:w1-16. https://doi.org/10.1377/hlthaff.28.1.w1.
- Schoen C, Osborn R, Doty MM, Bishop M, Peugh J, Murukutla N. Toward higher-performance health systems: adults‘ health care experiences in seven countries, 2007. Health Aff 2007;26:w717-34. https://doi.org/10.1377/hlthaff.26.6.w717.
- Aoun S, McConigley R, Abernethy A, Currow DC. Caregivers of people with neurodegenerative diseases: profile and unmet needs from a population-based survey in South Australia. J Palliat Med 2010;13:653-61. https://doi.org/10.1089/jpm.2009.0318.
- Wollin JA, Yates PM, Kristjanson LJ. Supportive and palliative care needs identified by multiple sclerosis patients and their families. Int J Palliat Nurs 2006;12:20-6. https://doi.org/10.12968/ijpn.2006.12.1.20392.
- Turner-Stokes L, McCrone P, Jackson DM, Siegert RJ. The Needs and Provision Complexity Scale: a multicentre prospective cohort analysis of met and unmet needs and their cost implications for patients with complex neurological disability. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002353.
- Reilly S, Abell J, Brand C, Hughes J, Berzins K, Challis D. Case management for people with long-term conditions: impact upon emergency admissions and associated length of stay. Prim Health Care Res Dev 2011;12:223-36. https://doi.org/10.1017/S1463423611000028.
- Challis D, Hughes J, Berzins K, Reilly S, Abell J, Stewart K, et al. Implementation of case management in long-term conditions in England: survey and case studies. J Health Serv Res Policy 2011;16:8-13. https://doi.org/10.1258/jhsrp.2010.010078.
- Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet 2012;380:7-9. https://doi.org/10.1016/S0140-6736(12)60482-6.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med 2015;373:747-55. https://doi.org/10.1056/NEJMra1404684.
- Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA 2008;299:1698-709. https://doi.org/10.1001/jama.299.14.1698.
- Siouta N, Van Beek K, van der Eerden ME, Preston N, Hasselaar JG, Hughes S, et al. Integrated palliative care in Europe: a qualitative systematic literature review of empirically-tested models in cancer and chronic disease. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0130-7.
- Turner-Stokes L, Sykes N, Silber E. Guideline Development Group . Long-term neurological conditions: management at the interface between neurology, rehabilitation and palliative care. Clin Med 2008;8:186-91. https://doi.org/10.7861/clinmedicine.8-2-186.
- Oliver DJ, Borasio GD, Caraceni A, de Visser M, Grisold W, Lorenzl S, et al. A consensus review on the development of palliative care for patients with chronic and progressive neurological disease. Eur J Neurol 2016;23:30-8. https://doi.org/10.1111/ene.12889.
- Higginson IJ, McCrone P, Hart SR, Burman R, Silber E, Edmonds PM. Is short-term palliative care cost-effective in multiple sclerosis? A randomized phase II trial. J Pain Symptom Manage 2009;38:816-26. https://doi.org/10.1016/j.jpainsymman.2009.07.002.
- Veronese S, Gallo G, Valle A, Cugno C, Chio A, Calvo A, et al. Specialist palliative care improves the quality of life in advanced neurodegenerative disorders: NE-PAL, a pilot randomised controlled study. BMJ Support Palliat Care 2017;7:164-72. https://doi.org/10.1136/bmjspcare-2014-000788.
- Solari A, Giordano A, Patti F, Grasso MG, Confalonieri P, Palmisano L, et al. Randomized controlled trial of a home-based palliative approach for people with severe multiple sclerosis. Mult Scler 2018;24:663-74. https://doi.org/10.1177/1352458517704078.
- Higginson IJ, Gao W, Saleem TZ, Chaudhuri KR, Burman R, McCrone P, et al. Symptoms and quality of life in late stage Parkinson syndromes: a longitudinal community study of predictive factors. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0046327.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006;31:58-69. https://doi.org/10.1016/j.jpainsymman.2005.06.007.
- Higginson IJ, Hart S, Burman R, Silber E, Saleem T, Edmonds P. Randomised controlled trial of a new palliative care service: Compliance, recruitment and completeness of follow-up. BMC Palliat Care 2008;7. https://doi.org/10.1186/1472-684X-7-7.
- Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families?. Cancer J 2010;16:423-35. https://doi.org/10.1097/PPO.0b013e3181f684e5.
- Page MJ, Cumpston M, Chandler J, Lasserson T, Higgins JPT, Thomas J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated August 2019). Cochrane. 2019.
- Higginson IJ, Hart S, Silber E, Burman R, Edmonds P. Symptom prevalence and severity in people severely affected by multiple sclerosis. J Palliat Care 2006;22:158-65. https://doi.org/10.1177/082585970602200306.
- Edmonds P, Vivat B, Burman R, Silber E, Higginson IJ. ‘Fighting for everything‘: service experiences of people severely affected by multiple sclerosis. Mult Scler 2007;13:660-7. https://doi.org/10.1177/1352458506071789.
- Edmonds P, Vivat B, Burman R, Silber E, Higginson IJ. Loss and change: experiences of people severely affected by multiple sclerosis. Palliat Med 2007;21:101-7. https://doi.org/10.1177/0269216307076333.
- Voltz R, Borasio GD. Palliative therapy in the terminal stage of neurological disease. J Neurol 1997;244:2-10. https://doi.org/10.1007/PL00007721.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. https://doi.org/10.1056/NEJMoa1000678.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Gruenewald DA, Higginson IJ, Vivat B, Edmonds P, Burman RE. Quality of life measures for the palliative care of people severely affected by multiple sclerosis: a systematic review. Mult Scler 2004;10:690-704. https://doi.org/10.1191/1352458504ms1116rr.
- Higginson IJ, Vivat B, Silber E, Saleem T, Burman R, Hart S, et al. Study protocol: delayed intervention randomised controlled trial within the Medical Research Council (MRC) Framework to assess the effectiveness of a new palliative care service. BMC Palliat Care 2006;5. https://doi.org/10.1186/1472-684X-5-7.
- Edmonds P, Hart S, Wei G, Vivat B, Burman R, Silber E, et al. Palliative care for people severely affected by multiple sclerosis: evaluation of a novel palliative care service. Mult Scler 2010;16:627-36. https://doi.org/10.1177/1352458510364632.
- Anderson R. New MRC guidance on evaluating complex interventions. BMJ 2008;337. https://doi.org/10.1136/bmj.a1937.
- Department of Health and Social Care (DHSC) . End of Life Care Strategy – Promoting High Quality Care for All Adults at the End of Life 2008.
- Walker H, Anderson M, Farahati F, Howell D, Librach SL, Husain A, et al. Resource use and costs of end-of-Life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care 2011;27:79-88. https://doi.org/10.1177/082585971102700203.
- Bergman J, Brook RH, Litwin MS. A call to action: improving value by emphasizing patient-centered care at the end of life. JAMA Surg 2013;148:215-16. https://doi.org/10.1001/jamasurg.2013.1568.
- Emanuel EJ, Ash A, Yu W, Gazelle G, Levinsky NG, Saynina O, et al. Managed care, hospice use, site of death, and medical expenditures in the last year of life. Arch Intern Med 2002;162:1722-8. https://doi.org/10.1001/archinte.162.15.1722.
- May P, Normand C, Cassel JB, Del Fabbro E, Fine RL, Menz R, et al. Economics of palliative care for hospitalized adults with serious illness: a meta-analysis. JAMA Intern Med 2018;178:820-9. https://doi.org/10.1001/jamainternmed.2018.0750.
- NHS England . Neurological Conditions. n.d. www.england.nhs.uk/ourwork/clinical-policy/ltc/our-work-on-long-term-conditions/neurological/#_ftn4 (accessed 5 December 2019).
- Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD. Burden of parkinsonism: a population-based study. Mov Disord 2003;18:313-19. https://doi.org/10.1002/mds.10333.
- Karamyan A, Dünser MW, Wiebe DJ, Pilz G, Wipfler P, Chroust V, et al. Critical illness in patients with multiple sclerosis: a matched case-control study. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0155795.
- Marrie RA, Elliott L, Marriott J, Cossoy M, Blanchard J, Tennakoon A, et al. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology 2014;83:929-37. https://doi.org/10.1212/WNL.0000000000000753.
- Smith S, Brick A, O‘Hara S, Normand C. Evidence on the cost and cost-effectiveness of palliative care: a literature review. Palliat Med 2014;28:130-50. https://doi.org/10.1177/0269216313493466.
- Salamanca-Balen N, Seymour J, Caswell G, Whynes D, Tod A. The costs, resource use and cost-effectiveness of clinical nurse specialist-led interventions for patients with palliative care needs: a systematic review of international evidence. Palliat Med 2018;32:447-65. https://doi.org/10.1177/0269216317711570.
- Bone AE, Gomes B, Etkind SN, Verne J, Murtagh FEM, Evans CJ, et al. What is the impact of population ageing on the future provision of end-of-life care? Population-based projections of place of death. Palliat Med 2018;32:329-36. https://doi.org/10.1177/0269216317734435.
- Etkind SN, Bone AE, Gomes B, Lovell N, Evans CJ, Higginson IJ, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0860-2.
- van Vliet LM, Gao W, DiFrancesco D, Crosby V, Wilcock A, Byrne A, et al. How integrated are neurology and palliative care services? Results of a multicentre mapping exercise. BMC Neurol 2016;16. https://doi.org/10.1186/s12883-016-0583-6.
- Hepgul N, Gao W, Evans CJ, Jackson D, van Vliet LM, Byrne A, et al. Integrating palliative care into neurology services: what do the professionals say?. BMJ Support Palliat Care 2018;8:41-4. https://doi.org/10.1136/bmjspcare-2017-001354.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-18.
- Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-111.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-80.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52. https://doi.org/10.1212/WNL.33.11.1444.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-42. https://doi.org/10.1212/WNL.17.5.427.
- Great Britain . Mental Capacity Act 2005 2005.
- Scott S, Jones L, Blanchard MR, Sampson EL. Study protocol: the behaviour and pain in dementia study (BePAID). BMC Geriatr 2011;11. https://doi.org/10.1186/1471-2318-11-61.
- Jones L, Harrington J, Scott S, Davis S, Lord K, Vickerstaff V, et al. CoMPASs: IOn programme (Care Of Memory Problems in Advanced Stages of dementia: Improving Our Knowledge): protocol for a mixed methods study. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-002265.
- Davies K, Collerton JC, Jagger C, Bond J, Barker SA, Edwards J, et al. Engaging the oldest old in research: lessons from the Newcastle 85+ study. BMC Geriatr 2010;10. https://doi.org/10.1186/1471-2318-10-64.
- Dewing J. Participatory research: a method for process consent with persons who have dementia. Dementia 2007;6:11-25. https://doi.org/10.1177/1471301207075625.
- Rees E, Hardy J. Novel consent process for research in dying patients unable to give consent. BMJ 2003;327. https://doi.org/10.1136/bmj.327.7408.198.
- Gao W, Crosby V, Wilcock A, Burman R, Silber E, Hepgul N, et al. Psychometric properties of a generic, patient-centred palliative care outcome measure of symptom burden for people with progressive long term neurological conditions. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0165379.
- Wilson R, Hepgul N, Saha RA, Higginson IJ, Gao W. Symptom dimensions in people affected by long-term neurological conditions: a factor analysis of a patient-centred palliative care outcome symptom scale. Sci Rep 2019;9. https://doi.org/10.1038/s41598-019-41370-3.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Beaumont C, Nekolaichuk C. FAMCARE and FAMCARE-2 Guidelines. Edmonton, AB: Covenant Health; 2012.
- Ritter PL, Lorig K. The English and Spanish Self-Efficacy to Manage Chronic Disease Scale measures were validated using multiple studies. J Clin Epidemiol 2014;67:1265-73. https://doi.org/10.1016/j.jclinepi.2014.06.009.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. Exeter: Gaskell; 2001.
- Higginson IJ, Gao W, Jackson D, Murray J, Harding R. Short-form Zarit Caregiver Burden Interviews were valid in advanced conditions. J Clin Epidemiol 2010;63:535-42. https://doi.org/10.1016/j.jclinepi.2009.06.014.
- Gomes B, McCrone P, Hall S, Koffman J, Higginson IJ. Variations in the quality and costs of end-of-life care, preferences and palliative outcomes for cancer patients by place of death: the QUALYCARE study. BMC Cancer 2010;10. https://doi.org/10.1186/1471-2407-10-400.
- Twycross R, Wilcock A, Howard P. Palliative Care Formulary 5th Edition. Nottingham: Pharmaceutical Press; 2014.
- Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585-98. https://doi.org/10.1002/sim.4780100410.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 5 December 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: University of Kent; 2016.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Klok RM, Postma MJ. Four quadrants of the cost-effectiveness plane: some considerations on the south-west quadrant. Expert Rev Pharmacoecon Outcomes Res 2004;4:599-601. https://doi.org/10.1586/14737167.4.6.599.
- Curry LA, Krumholz HM, O‘Cathain A, Plano Clark VL, Cherlin E, Bradley EH. Mixed methods in biomedical and health services research. Circ Cardiovasc Qual Outcomes 2013;6:119-23. https://doi.org/10.1161/CIRCOUTCOMES.112.967885.
- Coffey A, Atkinson P. Making Sense of Qualitative Data: Complementary Research Strategies. Thousand Oaks, CA: SAGE; 1996.
- Bradley N, Sweeney K, Waterfield M. The health of their nation: how would citizens develop England‘s health strategy?. Br J Gen Pract 1999;49:801-5.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Gao W, Wilson R, Hepgul N, Yi D, Evans C, Bajwah S, et al. Effect of short-term integrated palliative care on patient-reported outcomes among patients with advanced long-term neurological conditions: a randomized clinical trial. JAMA Netw Open 2020;3. https://doi.org/10.1001/jamanetworkopen.2020.15061.
- Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016;316:2104-14. https://doi.org/10.1001/jama.2016.16840.
- Ang K, Hepgul N, Gao W, Higginson IJ. Strategies used in improving and assessing the level of reporting of implementation fidelity in randomised controlled trials of palliative care complex interventions: a systematic review. Palliat Med 2018;32:500-16. https://doi.org/10.1177/0269216317717369.
- Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721-30. https://doi.org/10.1016/S0140-6736(13)62416-2.
- Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741-9. https://doi.org/10.1001/jama.2009.1198.
- Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, et al. Palliative care in heart failure: The PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol 2017;70:331-41. https://doi.org/10.1016/j.jacc.2017.05.030.
- Wasserstein RL, Lazar NA. The ASA‘s statement on p-values: context, process, and purpose. Am Stat 2016;70:129-31. https://doi.org/10.1080/00031305.2016.1154108.
- Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019;567:305-7. https://doi.org/10.1038/d41586-019-00857-9.
- Cartwright A, Hockey L, Anderson JL. Life Before Death. London: Routledge & Kegan Paul; 1973.
- Hinton JM. The physical and mental distress of the dying. Q J Med 1963;32:1-21.
- Hinton JM. Problems in the care of the dying. J Chronic Dis 1964;17:201-5. https://doi.org/10.1016/0021-9681(64)90148-1.
- De Moor G, Sundgren M, Kalra D, Schmidt A, Dugas M, Claerhout B, et al. Using electronic health records for clinical research: the case of the EHR4CR project. J Biomed Inform 2015;53:162-73. https://doi.org/10.1016/j.jbi.2014.10.006.
- Dregan A, van Staa TP, McDermott L, McCann G, Ashworth M, Charlton J, et al. Point-of-care cluster randomized trial in stroke secondary prevention using electronic health records. Stroke 2014;45:2066-71. https://doi.org/10.1161/STROKEAHA.114.005713.
- Gulliford MC, van Staa TP, McDermott L, McCann G, Charlton J, Dregan A. eCRT Research Team . Cluster randomized trials utilizing primary care electronic health records: methodological issues in design, conduct, and analysis (eCRT Study). Trials 2014;15. https://doi.org/10.1186/1745-6215-15-220.
Appendix 1 Cumulative recruitment over the course of the recruitment period

Appendix 2 Demographic and clinical characteristics of patients with and without primary outcome data
| Variable | Value | Complete cases | Missing |
|---|---|---|---|
| N | 270 | 80 | |
| Age (years), mean (SD) | 66.0 (11.6) | 69.6 (11.8) | |
| Gender, n (%) | Man | 137 (50.7) | 42 (52.5) |
| Woman | 133 (49.3) | 38 (47.5) | |
| Marital status, n (%) | Single | 28 (10.4) | 7 (8.8) |
| Widowed | 28 (10.4)) | 10 (12.5) | |
| Married/civil partner | 182 (67.4) | 49 (61.3) | |
| Divorced/separated | 31 (11.5) | 13 (16.3) | |
| Not done/unknown | 1 (0.4) | 1 (1.3) | |
| Living status, n (%) | Alone | 53 (19.6) | 12 (15.0) |
| With spouse/partner and/or children | 188 (69.6) | 56 (70.0) | |
| With friend(s)/with others | 29 (10.7) | 12 (15.0) | |
| Education, n (%) | No formal education up to lower secondary school | 103 (38.2) | 36 (45.0) |
| Upper secondary to post-secondary vocational qualification | 95 (35.2) | 21 (26.3) | |
| Tertiary education | 70 (25.9) | 21 (26.3) | |
| Not done/missing | 2 (0.7) | 2 (2.5) | |
| Feelings towards income, n (%) | Living comfortably on present income | 87 (32.2) | 31 (38.8) |
| Coping on present income | 130 (48.2) | 32 (40.0) | |
| Difficult on present income | 19 (7.0) | 5 (6.3) | |
| Very difficult on present income | 12 (4.4) | 2 (2.5) | |
| Not done/unknown | 22 (8.2) | 10 (12.5) | |
| Employment, n (%) | No | 262 (97.0) | 78 (97.5) |
| Yes | 8 (3.0) | 2 (2.5) | |
| Ethnicity, n (%) | White | 249 (92.2) | 67 (83.8) |
| Other ethnic group | 20 (7.4) | 12 (15.0) | |
| Not done/unknown | 1 (0.4) | 1 (1.3) | |
| Comorbidities, n (%) | No | 76 (28.2) | 23 (28.8) |
| Yes | 194 (71.9) | 57 (71.3) | |
| AKPS, n (%) | Totally bedfast | 5 (1.9) | 2 (2.5) |
| Almost completely bedfast | 3 (1.1) | 7 (8.8) | |
| In bed > 50% of the time | 11 (4.1) | 10 (12.5) | |
| Requires considerable assistance | 127 (47.0) | 43 (53.8) | |
| Requires occasional assistance | 83 (30.7) | 15 (18.8) | |
| Cares for self | 30 (11.1) | 3 (3.8) | |
| Normal activity with effort | 9 (3.3) | 0 | |
| Not available/applicable | 1 (0.4) | 0 | |
| Not done | 1 (0.4) | 0 | |
| Diagnosis, n (%) | MS | 121 (44.8) | 27 (33.8) |
| IPD | 104 (38..5) | 36 (45.0) | |
| MSA | 10 (3.7) | 2 (2.5) | |
| PSPa | 18 (6.7) | 9 (11.3) | |
| MND | 17 (6.3) | 6 (7.5) | |
| Years since diagnosis | 12.8 (10.8) | 10.8 (9.7) | |
| Mean (SD) | 12.8 (10.8) | 10.8 (9.7) | |
| Patient capacity, n (%) | Patient | 270 (100) | 41 (51.3) |
| Personal consultee | 0 | 39 (48.8) | |
Appendix 3 Additional health economic analyses
To further show the uncertainty around the ICERs, we plotted the replications on the cost-effectiveness planes with 95% CIs (Figure 4).
FIGURE 4.
Cost-effectiveness planes of outcome measures (a) EQ-5D index score (QALY); and (b) rescaled IPOS Neuro-S8, and health and social care cost with 95% CIs.
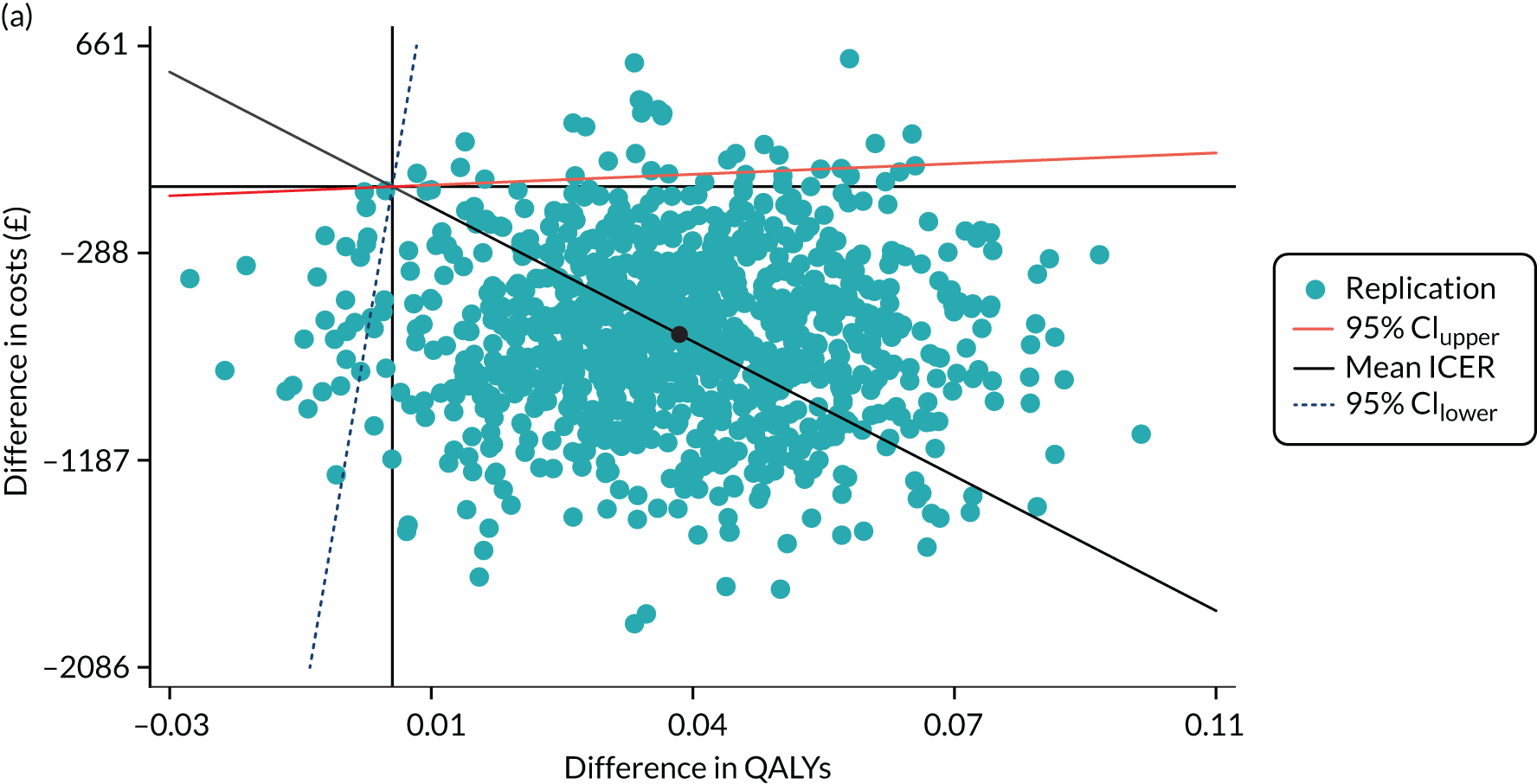
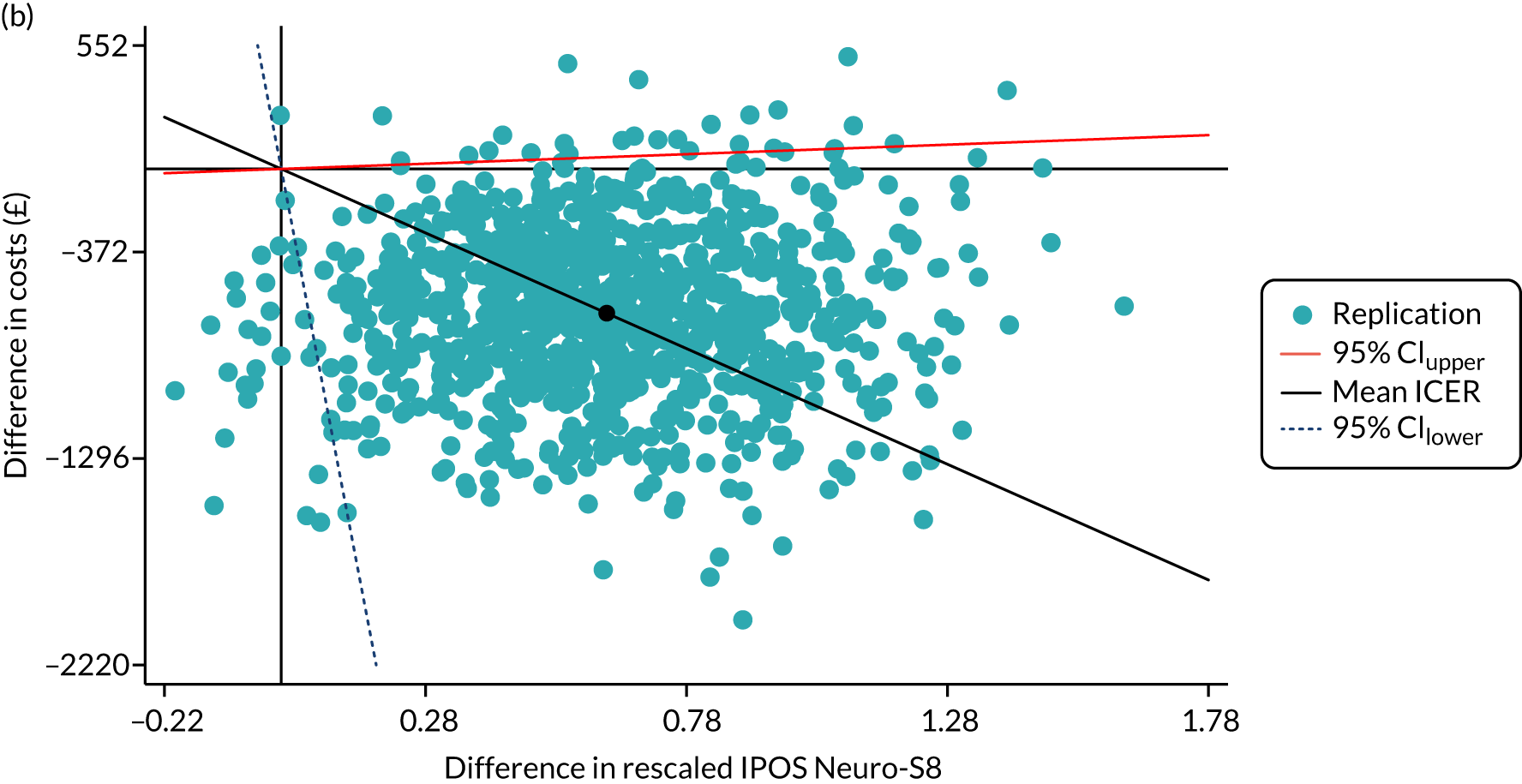
To account for the joint uncertainty around the costs and outcomes, we calculated the probability of SIPC being cost-effective compared with standard care, with reference to a set of maximum acceptable ceiling ratios or WTP thresholds (λ). These probabilities were drawn as a cost-effectiveness acceptability curve. The cost-effectiveness acceptability curves (Figure 5) show that SIPC was more cost-effective than standard care, as measured by either EQ-5D index score or rescaled IPOS Neuro-S8.
FIGURE 5.
Cost-effectiveness acceptability curves of SIPC compared with standard care, using outcome measures (a) EQ-5D index score/QALY; and (b) rescaled IPOS Neuro-S8, and health and social care cost.

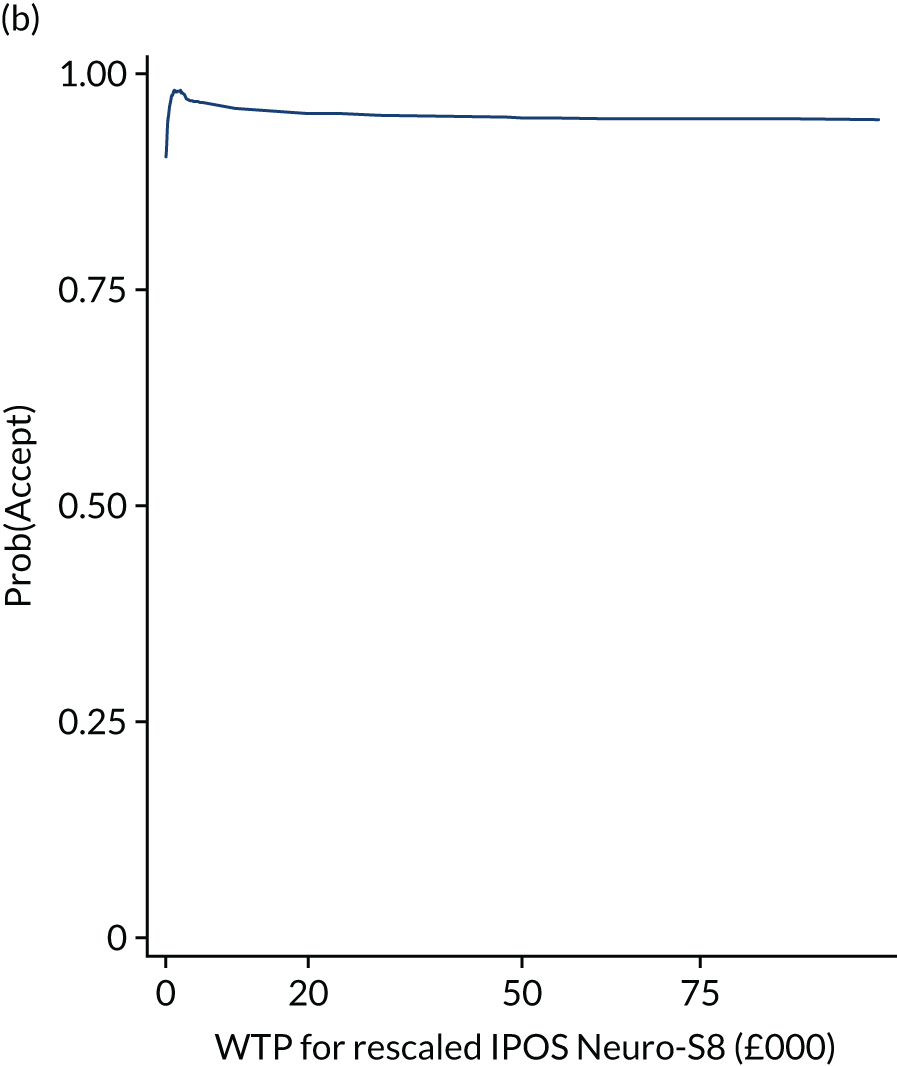
The INMB of SIPC and standard care at a range of WTP thresholds, was generated using Equation 2, in which λ is a given WTP threshold. The INMB of SIPC relative to standard care at various WTP thresholds was plotted:
The INMB of SIPC compared with standard care was positive (Figure 6), with wider 95% CIs as the WTP threshold became larger. The INMB of SIPC using IPOS Neuro-S8 needs to be interpreted with caution, as there has been no agreement on the WTP for 1 unit of the measurement.
FIGURE 6.
Incremental net monetary benefit of SIPC compared with standard care, using outcomes (a) EQ-5D index score/QALY; and (b) rescaled IPOS Neuro-S8, and health and social care cost.
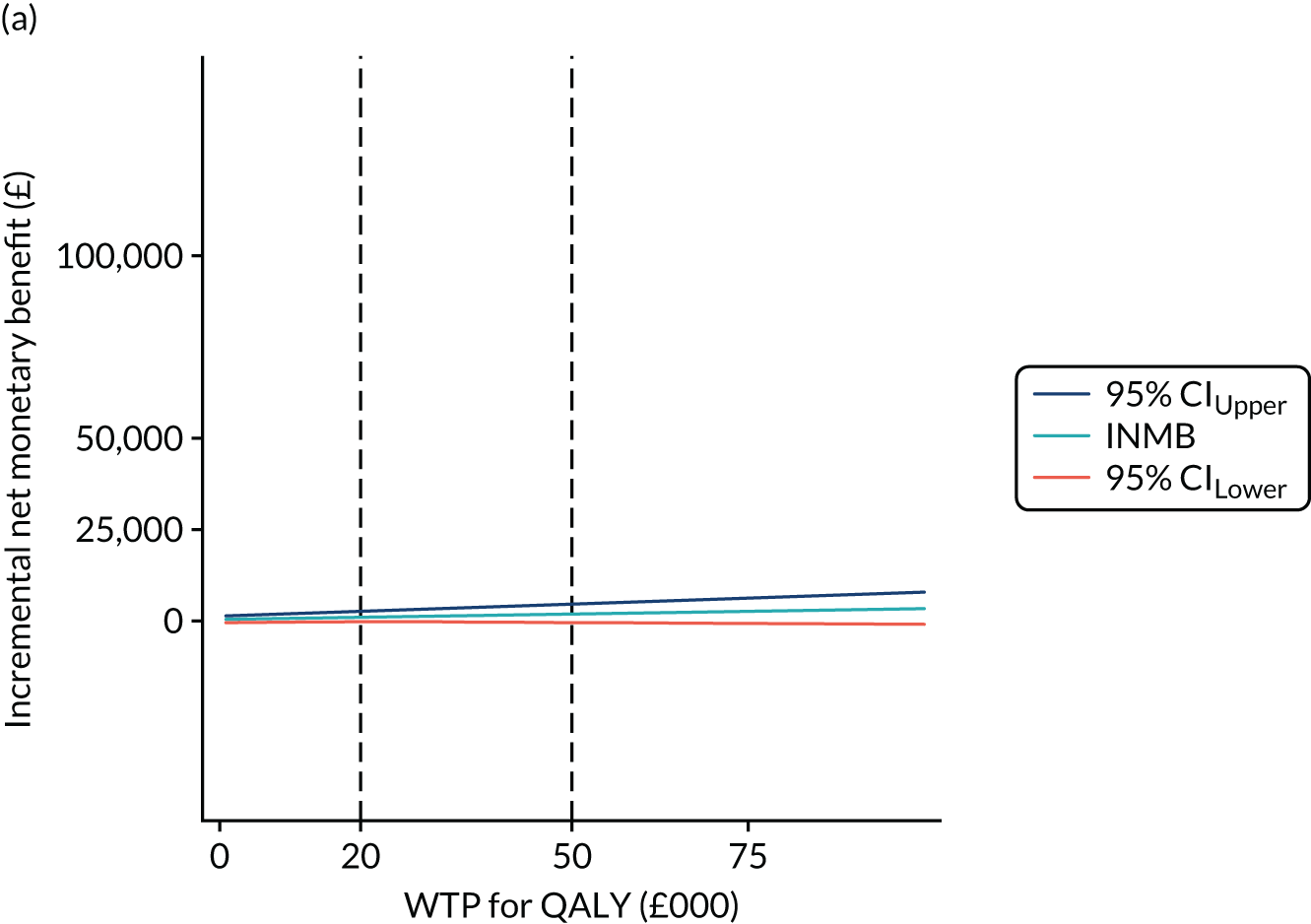
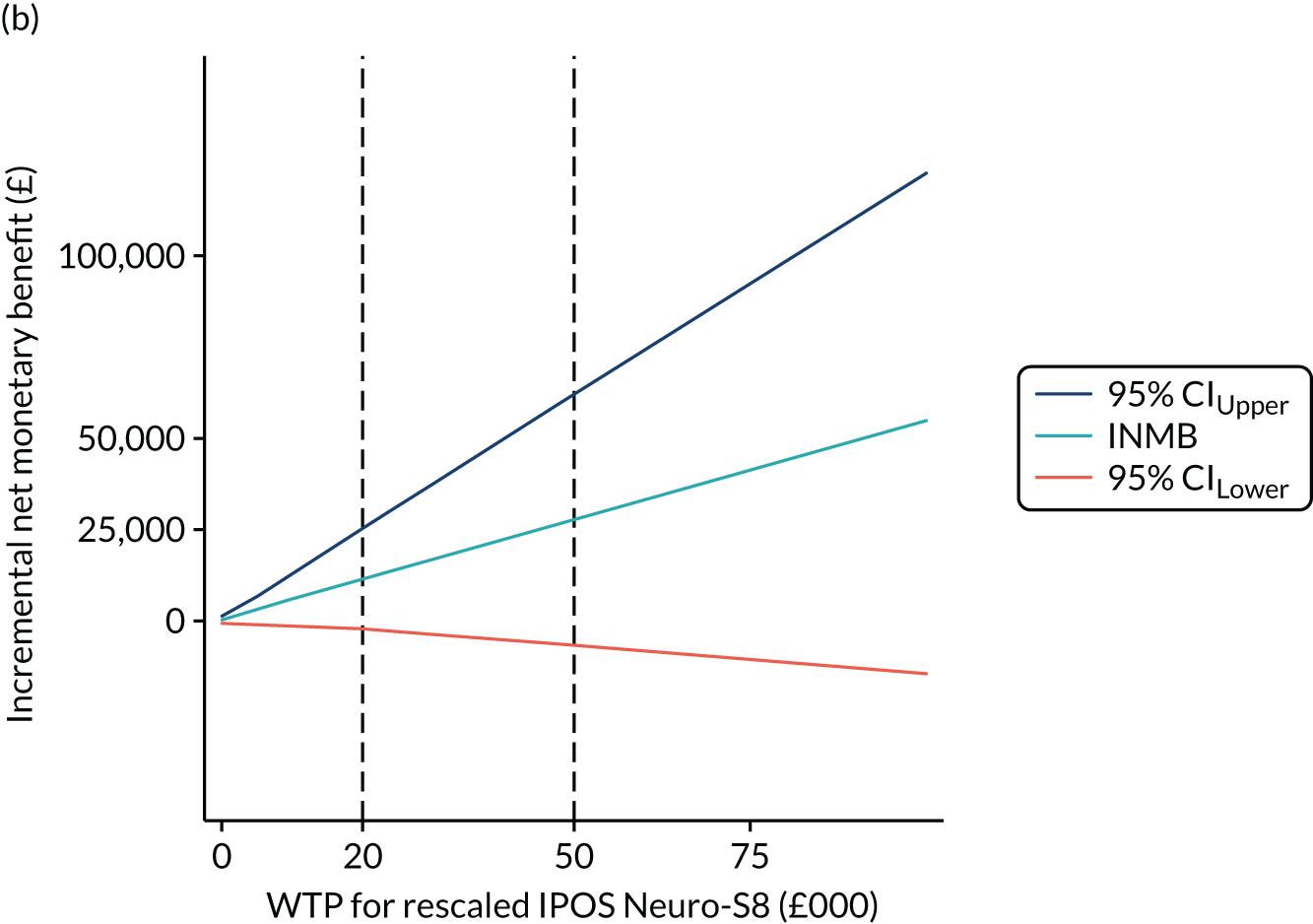
Overall, these further analyses are consistent with the findings from the main analysis. The INMB obtained can be used to present the benefit of SIPC compared with standard care as a monetary benefit from the intervention.
Appendix 4 Caregiver demographics for qualitative interview participants
| Variable | Value | Interviewed caregivers (N = 16) |
|---|---|---|
| Age (years), mean (SD) | 58.9 (14.7) | |
| Gender, n (%) | Man | 6 (37.5) |
| Woman | 10 (62.5) | |
| Relationship to patient, n (%) | Spouse/partner | 11 (68.8) |
| Son/daughter | 3 (18.8) | |
| Other | 2 (12.5) | |
| Ethnicity, n (%) | White | 15 (93.7) |
| Other ethnic group | 1 (6.3) | |
| Employment, n (%) | No | 10 (62.5) |
| Yes | 6 (37.5) | |
| Illness, n (%) | No | 7 (43.8) |
| Yes | 9 (56.2) | |
| Baseline ZBI-12 | ||
| Mean (SD) | 20.9 (9.5) | |
| Range | 0–41 | |
Appendix 5 Analytical framework on the value and impact of short-term integrated palliative care
| Theme/definition | Subcategory | Definition | Illustrative quotation |
|---|---|---|---|
| Adapting to losses and building resilience: key strategies for adjusting to increasing disability, declining function and nearness to end of life | Care beyond medicines |
Psychosocial interventions of skilled support, valuing and appreciating what life is like, and practical support. Psychosocial interventions were valued to support resilience and adaptation, and counter feelings of loneliness However, with increasing complexity of psychosocial needs and the short-term nature of the intervention limited opportunity to build sufficient trust and rapport to enable patients and caregivers to engage in difficult conversations |
We [the palliative care nurse and I] did talk about it [feeling lonely and down sometimes]. MS can be very frightening and lonely and you know I’m grieving for my body and the life I had. I know this is still my house, but it doesn’t feel like my house anymore. I do get quite down sometimes and then I think who can I talk to but then I use meditation to help me with that and to stay positive. I did see a counsellor many years ago and that was helpful so maybe that is something I should have asked for [from the palliative care nurse]Patient P01348-F |
| Asked about everything |
Planning future care for end-of-life experiences, expectations and impact Engagement ranged from ‘not all’ to ‘I’ve been writing stuff down for years’ Engagement was marked by uncertainty in ‘not knowing what’s going to take hold’ and fear of increasing disability and loss of capacity The SIPC intervention seemed to be the start of a conversation |
. . . she [the palliative care nurse] did say ‘Have you thought about the future?’ and what your plans are for the future and stuff like that. I mean, I know that a lot people do recommend that you make plans and you think about what’s gonna happen when she becomes more dependent. Errm you know, how you’re gonna cope as a family, what kind of errm, what you’re gonna do really. I’m really of the thought that you can’t really plan too much when it comes to something like MS, dementia because you just don’t know when things are gonna take hold. I mean things have taken hold and we’re still copingCaregiver C01280-F | |
| Attend to function, deficits and maintaining stability: optimising function and independence, and managing physical deficits and concerns | Little things that make a big differenceP0139-M |
Optimal management of unstable symptoms to reduce distress (e.g. breathlessness, pain) Key components for impact:Maintaining function and independence by supporting adaptation and problem-solving (e.g. continence, mobility, falls prevention, eating and drinking) Key components were:Lack of attention to function an area of frustration for patients and carers |
Well it’s [emotional concerns] sort of linked to my physical feelings really so I didn’t feel like I was ever gonna improve but I have begun to improve. I’m feeling a bit ill and fluey and that sort of winter feeling at the moment, but the massage [SIPC therapist] was the start I think of me feeling better, and getting more sleep and feeling more generally well in myself. So it’s a subtle change in some ways but in some ways it’s quite a large thing cos just going to bed was a nightmare, I just couldn’t sleep at all and it was making me more tired and it seemed to make the symptoms worse in the morning and during the day and so I think it’s a good thing that I can actually go to bed and just sleep again since being on this course [of massage and change to medication for spasm]Patient P05182-MWell when I discussed my incontinence [with palliative care Clinical Nurse Specialist], which is difficult to discuss with a guy [my husband], it’s nice to talk to a lady about that sort of thing, I find that very helpful. She gave me some nice pointers as to what to do and how to overcome certain things which was very good you know because in the end though he does a lot for me, I love him [husband] but you can’t talk about some certain things, it’s too personal you know . . .Patient P01007-F |
| Maintain stability |
Although living with extensive losses and deficits, individuals considered themselves ‘stable’ and as working continuously to maintain stability SIPC is about skilled support, by increasing awareness and understanding of management of symptoms and concerns. For some, there was no impact from involvement of SIPC. No difference felt from symptoms and concerns as used to living with them and equipment already in place to maintain independence |
Errm wouldn’t say make a difference but she [palliative care nurse], she you know explained errm certain things how things would be helped in certain ways and that. Errm . . . well just really just sort of [pause] like with his errm bowel movement and that to give you know, if it’s really bad, giving him Imodium [loperamide] every now and again which could possibly helpCaregiver C05275-F | |
| Enabling carers to care: empowering carers | Enabling carers to care |
Recognising the role of caregivers, valuing and acknowledging their work Complexity of caring with a tendency to put the person before themselves Not asking for help, but also frustrated when their needs are not considered Supporting caregivers from simple intervention of acknowledging and valuing, through to complex ongoing process requiring continuity of care |
Whilst I don’t think errm, there’s not really a lot that she can do for me errm, that I can think of because I tend to sort of manage, you know. I manage as best I know how, you know errm, but I think it’s the fact that she’s, her intervention, you know, irrespective of what she was or wasn’t able to do for us, I think meant a lotCaregiver C01319-F |
List of abbreviations
- AKPS
- Australia-modified Karnofsky Performance Scale
- CI
- confidence interval
- CSRI
- Client Service Receipt Inventory
- CTU
- Clinical Trials Unit
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GLMM
- generalised linear mixed model
- HADS
- Hospital Anxiety and Depression Scale
- ICER
- incremental cost-effectiveness ratio
- INMB
- incremental net monetary benefit
- IPD
- idiopathic Parkinson’s disease
- IPOS Neuro
- Integrated Palliative care Outcome Scale for Neurological conditions
- IPOS Neuro-S8
- Integrated Palliative care Outcome Scale for Neurological conditions 8 symptom subscale
- IPOS Neuro-S24
- Integrated Palliative care Outcome Scale for Neurological conditions 24 symptom subscale
- LTNC
- long-term neurological condition
- MDT
- multidisciplinary team
- MND
- motor neurone disease
- MORECare
- Methods of Researching End of Life Care
- MPCT
- multiprofessional palliative care team
- MS
- multiple sclerosis
- MSA
- multiple system atrophy
- OT
- occupational therapy
- PPI
- patient and public involvement
- PSP
- progressive supranuclear palsy
- QALY
- quality-adjusted life-year
- SD
- standard deviation
- SEMCD
- Self-Efficacy for Managing Chronic Disease
- SIPC
- short-term integrated palliative care
- WTP
- willingness to pay
- ZBI-12
- Zarit Burden Inventory 12 items
